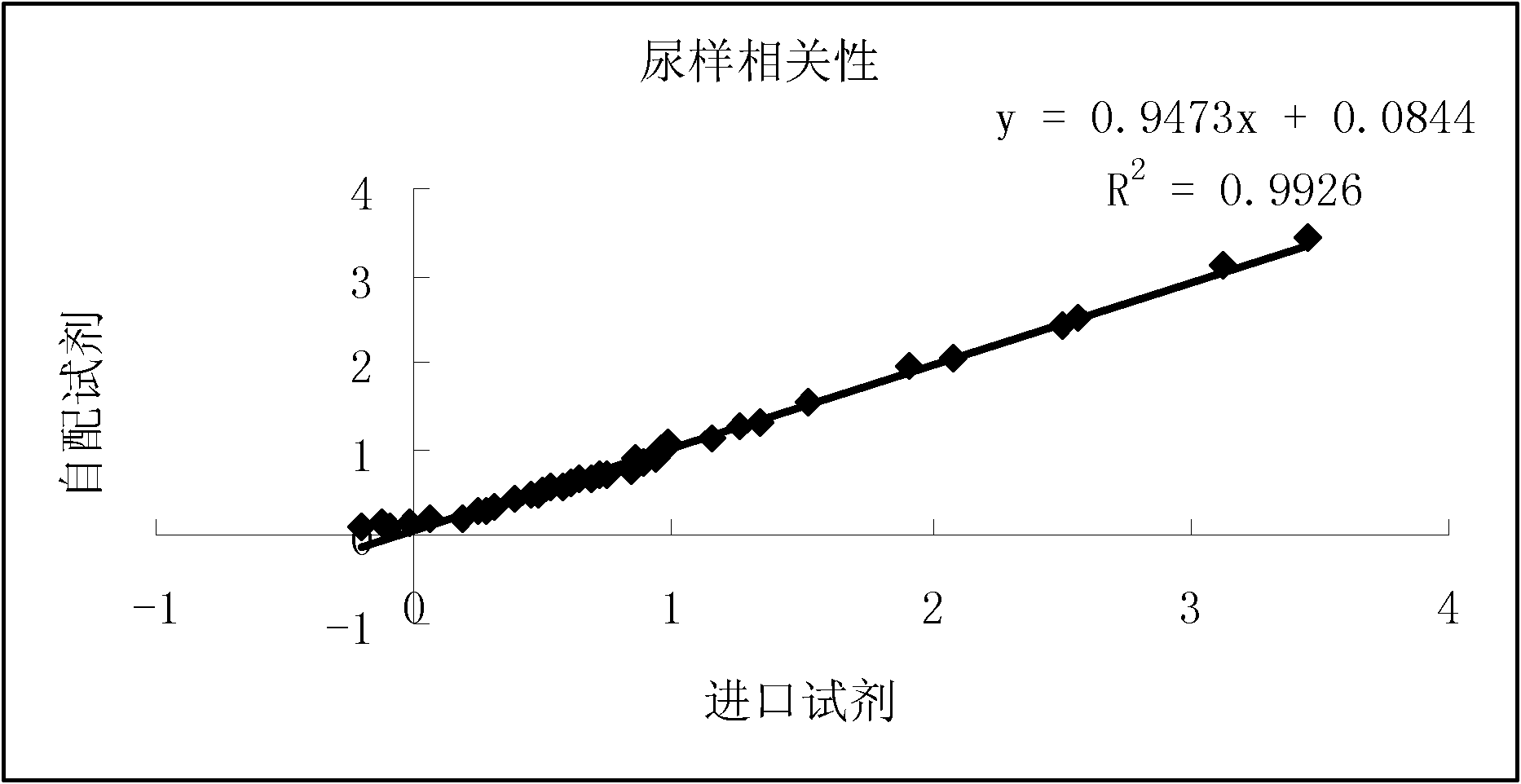
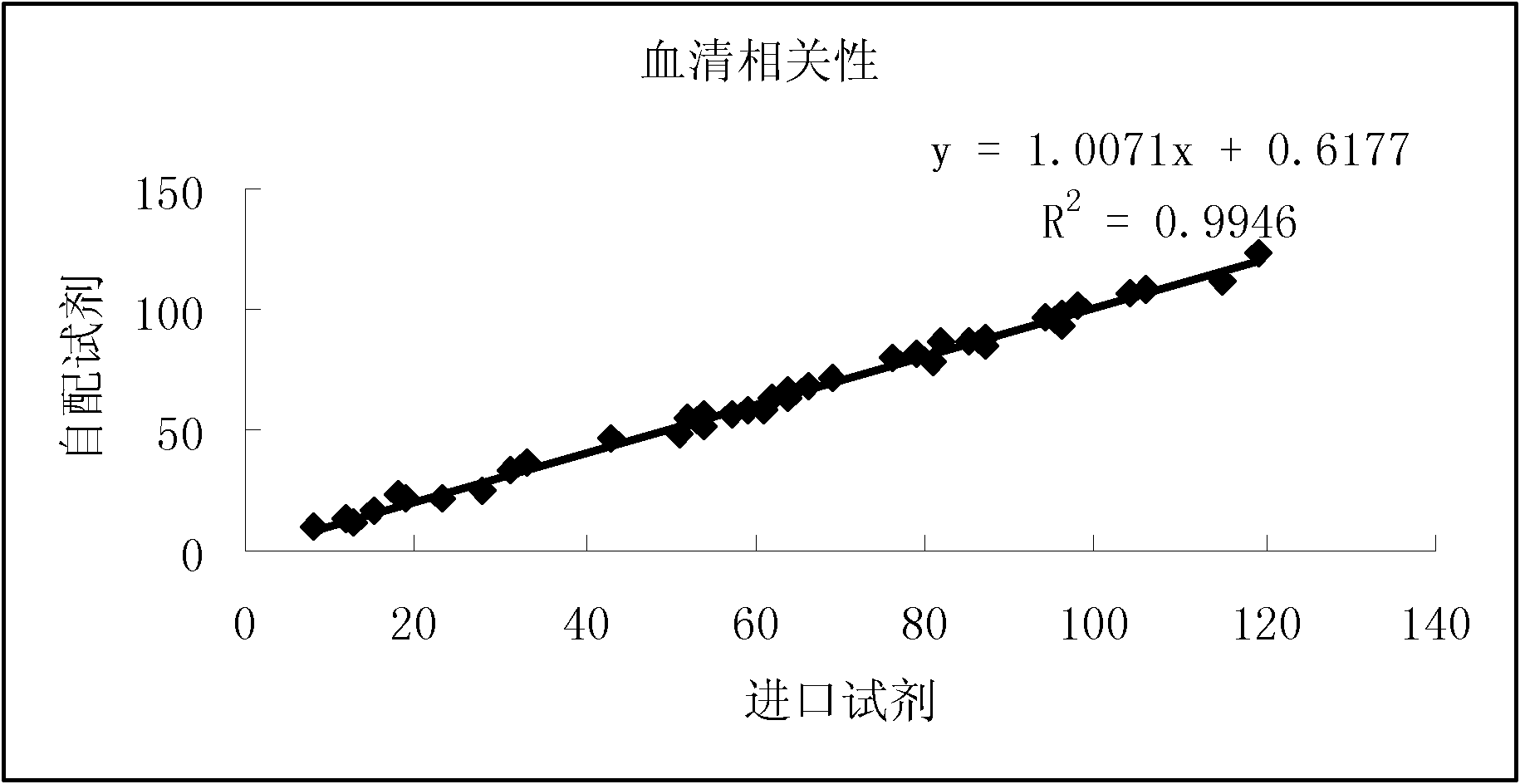
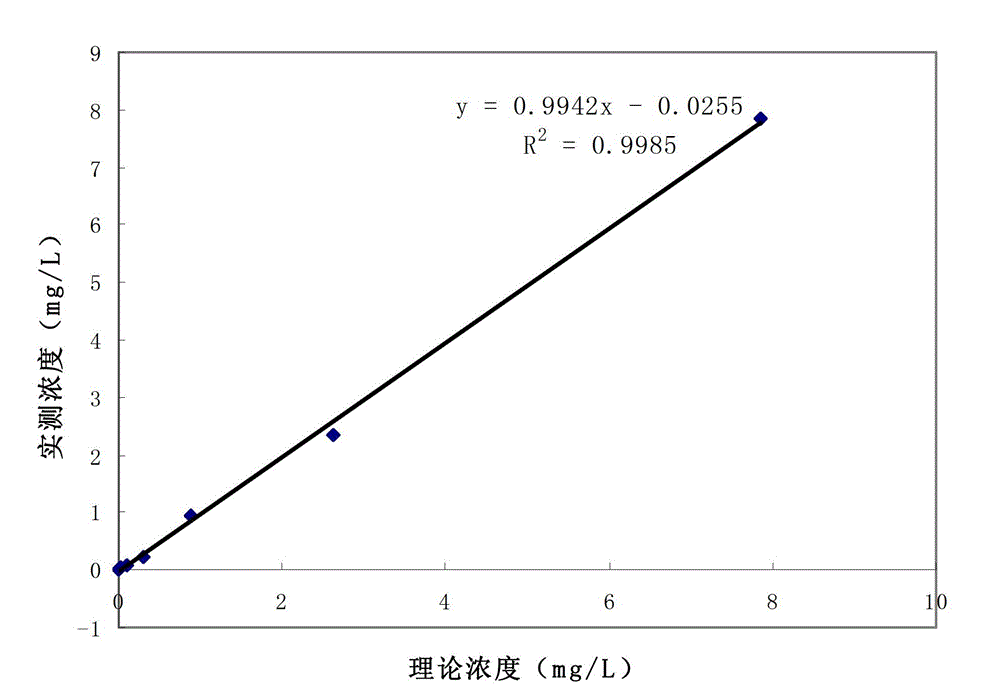
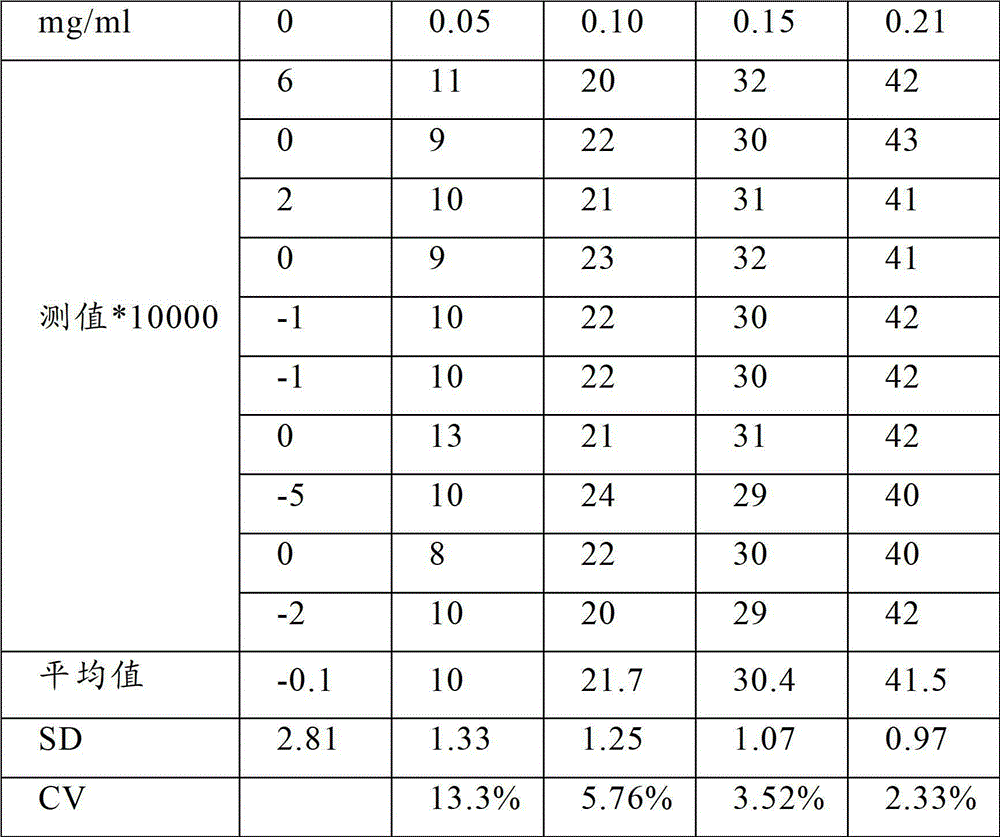
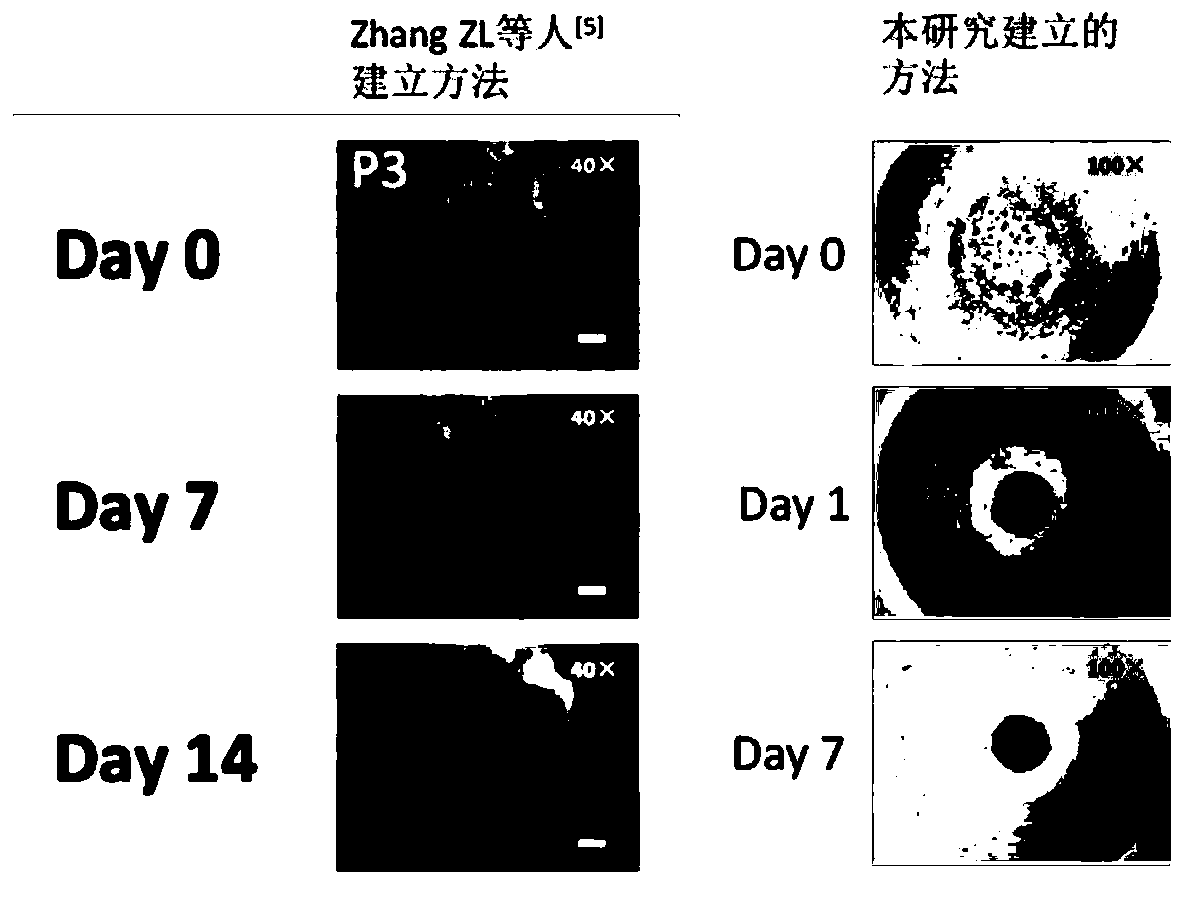
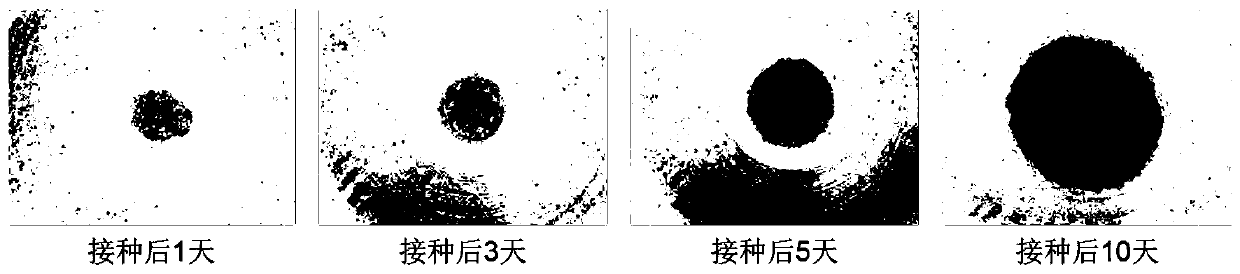
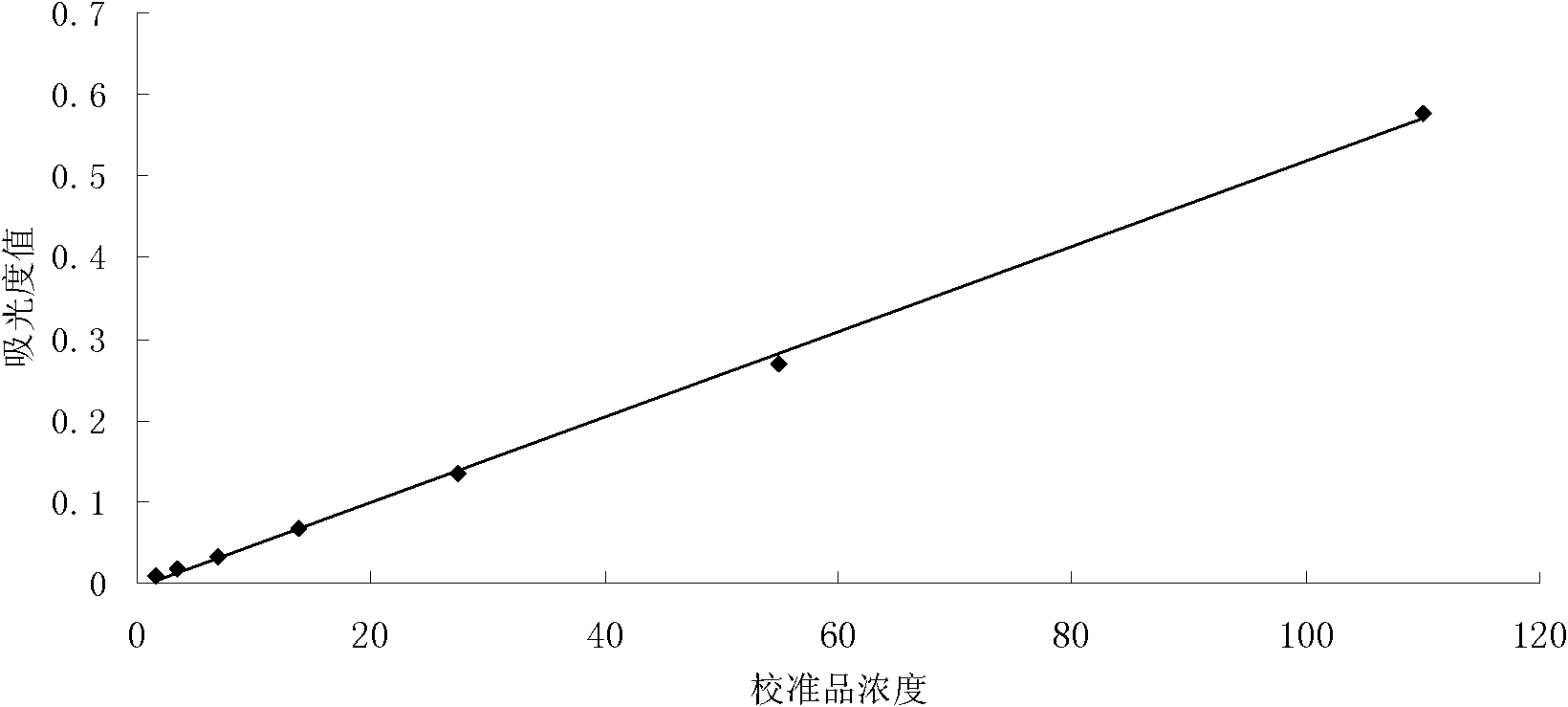
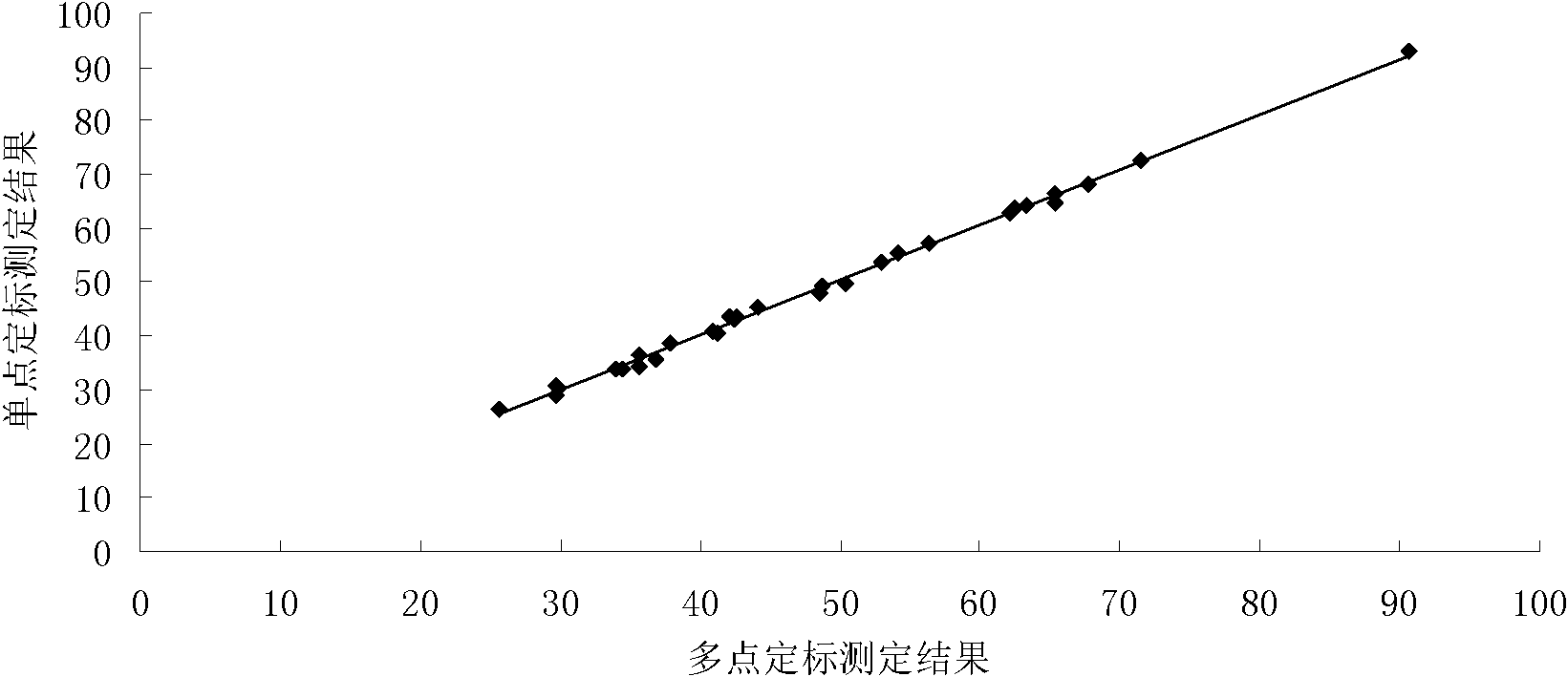
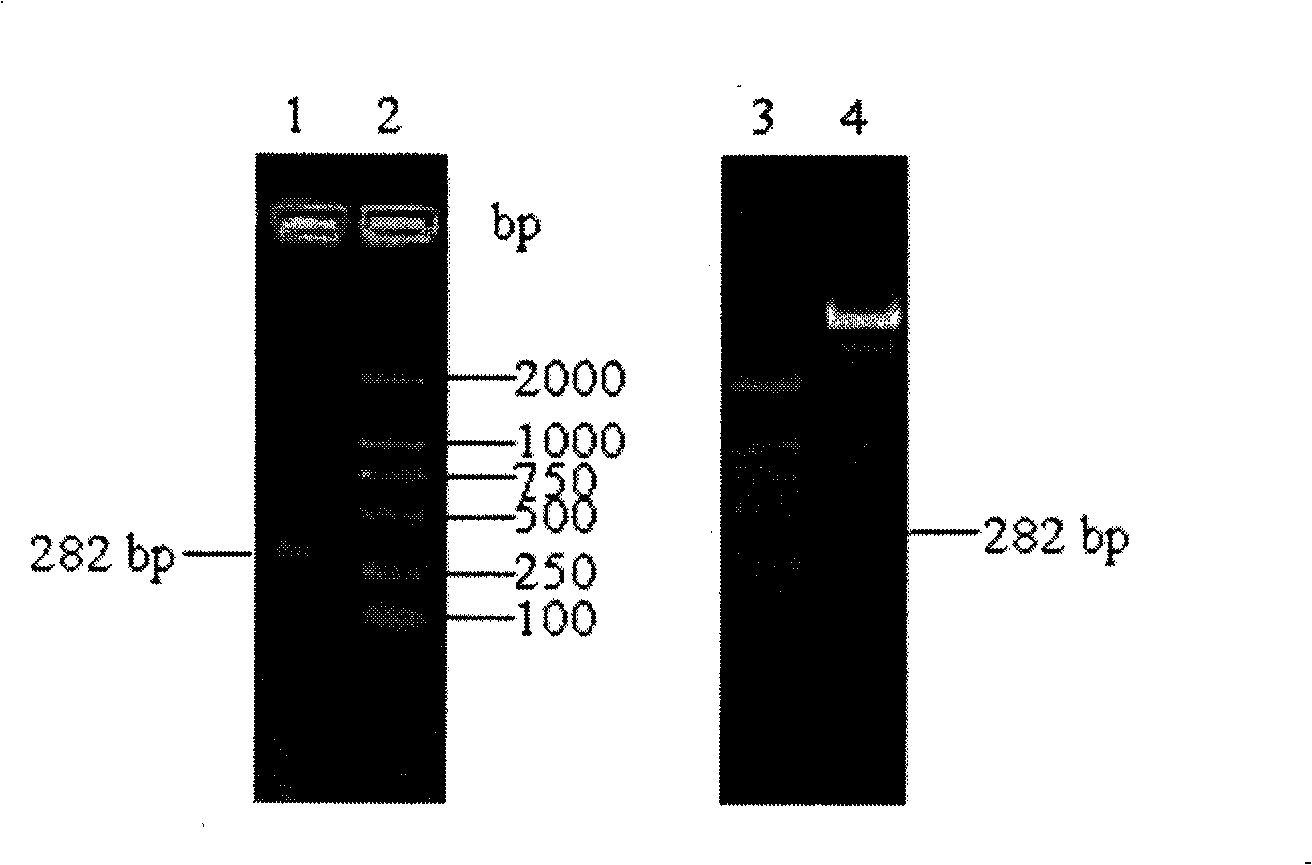
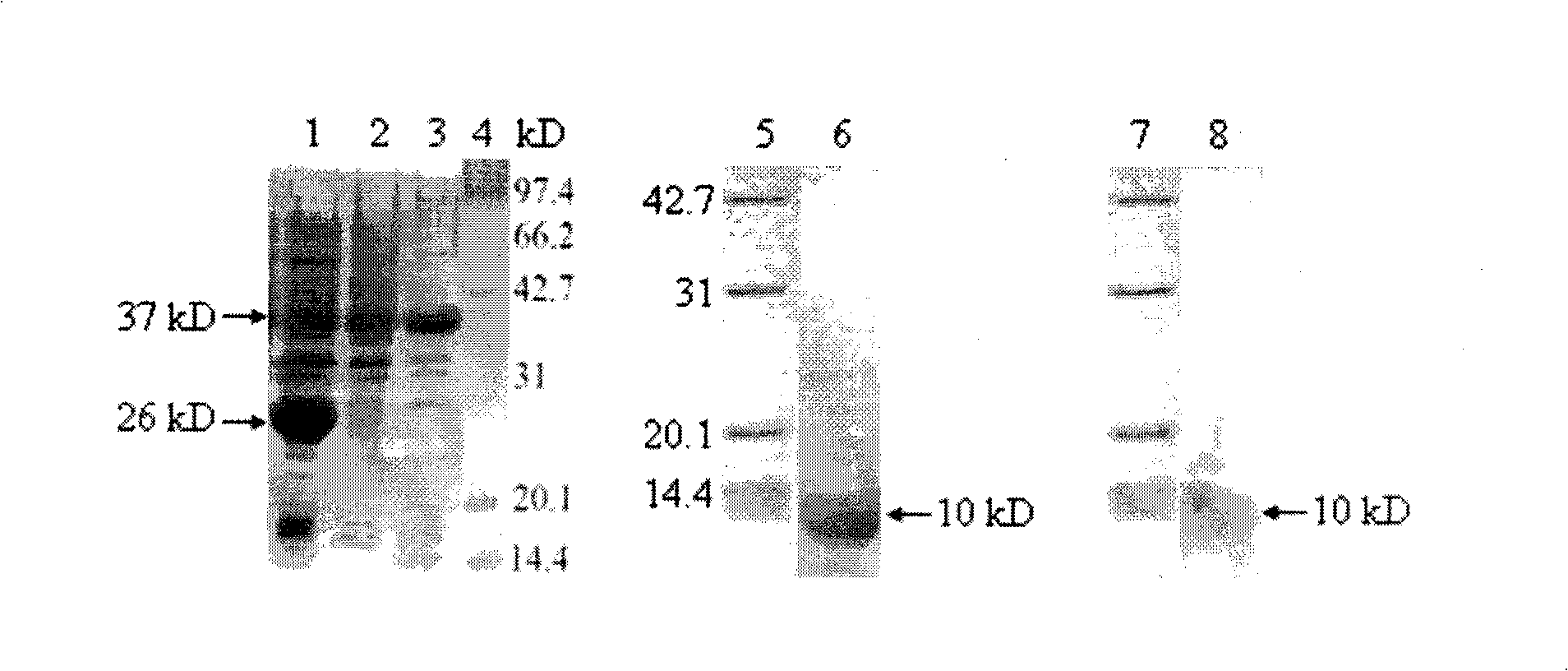
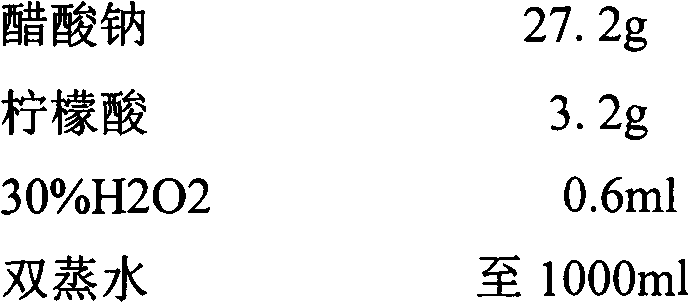Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
229 results about "Double antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
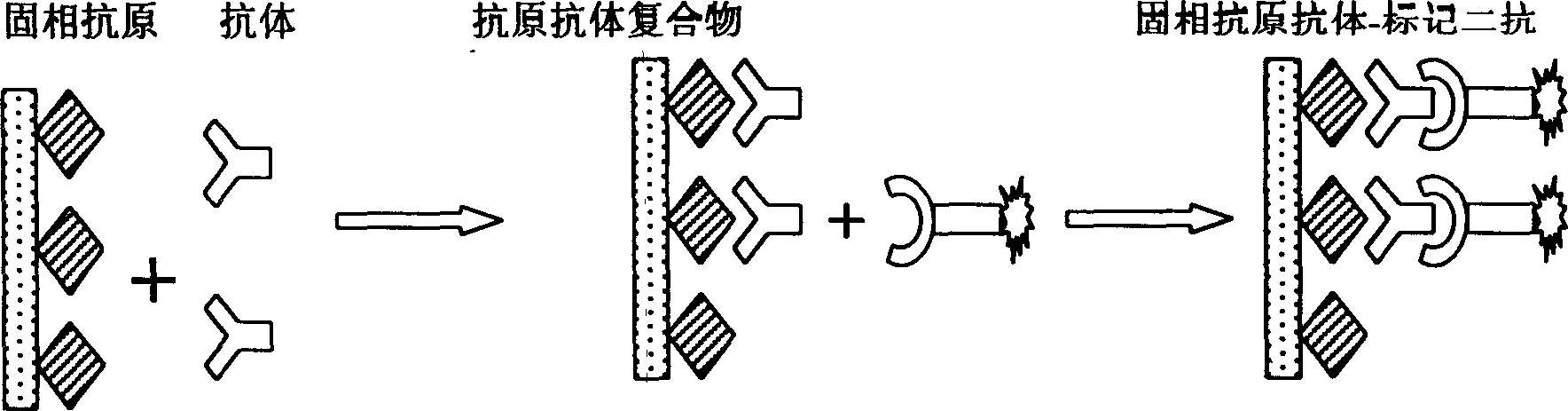
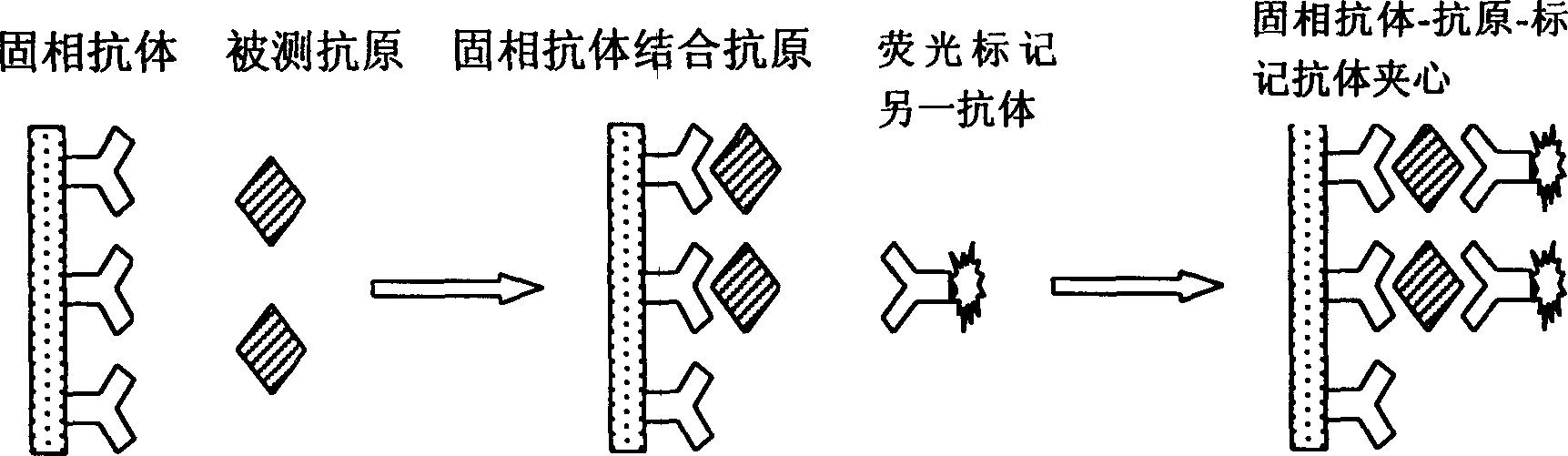
Double antibody precipitation. (redirected from double antibody immunoassay) a method of separating antibody-bound antigen (for example, insulin) from free antigen by precipitating the former with antibody specific for immunoglobulin. Synonym(s): double antibody immunoassay, double antibody method.
Electrochemical immunodetection method
InactiveCN102226779AHigh sensitivityAmino richChemiluminescene/bioluminescenceNanotechnologyDouble antibodyLower limit
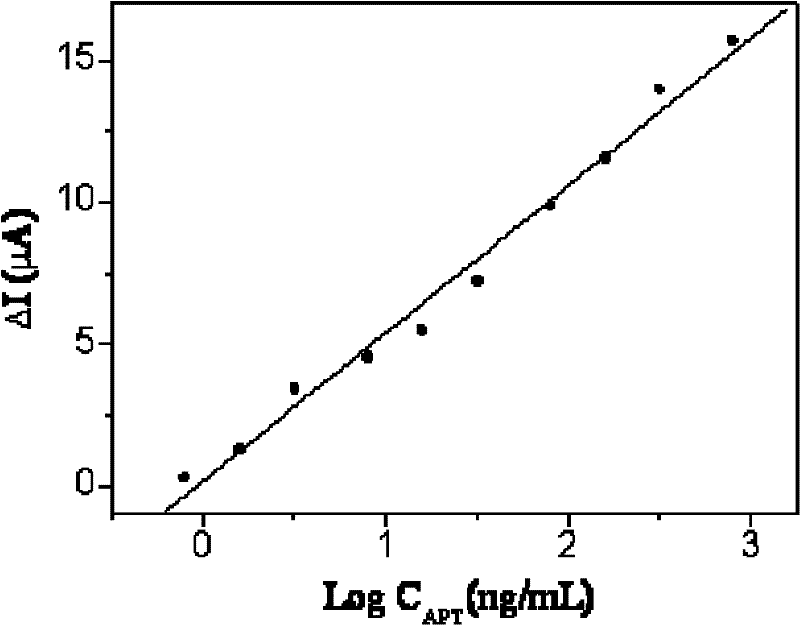
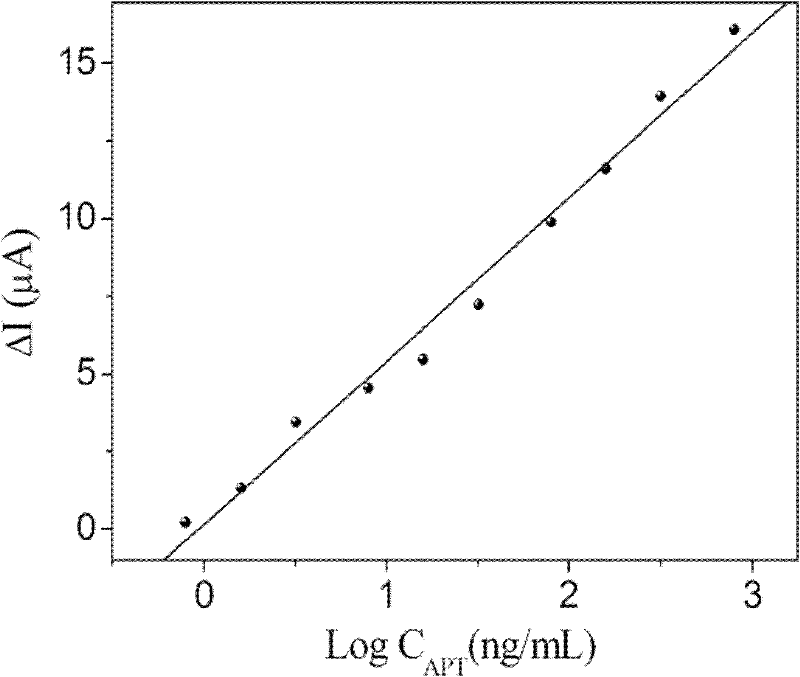
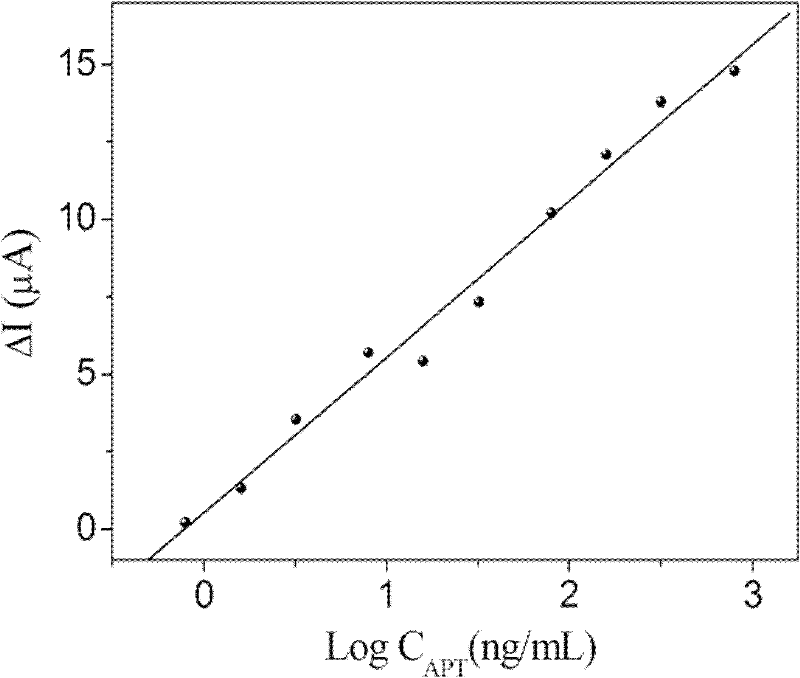
The invention discloses an electrochemical immunodetection method based on a thionine-graphene nano composite double coated with nano platinum chitosan. According to the invention, double antibody sandwiched method is adopted, an abnormal prothrombin(APT) antibody supported on the surface of electrodes is subject to an immunization reaction with an abnormal prothrombin(APT) in a sample solution, then combines with the coupling object of the thionine-graphene nano composite double coated with nano platinum chitosan and the abnormal prothrombin(APT). Based on the electrochemical activity of thethionine-graphene nano composite double coated with nano platinum chitosan, peak current value of reduction peak of cyclic voltammetry is detected so as to further detect the concentration of the abnormal prothrombin in the sample under test. The method has the linear response range of 0.8-800ng / ml, the lower limit of the detection of 0.23ng / ml, with good specificity and high sensitivity, and is significant to the diagnosis of the abnormal prothrombin(APT).
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Double antibody latex enhanced retinol binding protein detection kit
The invention relates to a double antibody latex enhanced retinol binding protein detection kit. More specifically, the invention discloses a double antibody coating latex enhanced immunoturbidimetry kit for detecting retinol binding protein. The kit contains a reagent 1, a reagent 2 and a calibrator. Paring monoclonal antibody A and B are respectively coated on latex particles. Coated antibody is bonded with RBP to be detected, and a plurality of bonders are aggregated together to form detectable turbidity change. The detection kit provided by the invention has high sensitivity, can be used to detect urine samples and serum samples and can be used as nutritive index to detect RBP in serum. Simultaneously, through the detection of RBP in urine, the kit is sensitive to the damage degree of renal proximal tubule.
Owner:BEIJING STRONG BIOTECH INC
Kit for performing retinol binding protein detection by using latex turbidimetry
InactiveCN102944679AHigh detection sensitivityGuaranteed SensitivityColor/spectral properties measurementsHydrogenTurbidimetry
The invention relates to the technical field of biotechnology, and particularly discloses a kit for detecting retinol binding protein content by using latex immunoturbidimetry. The kit comprises a reagent R1, a reagent R2 and a standard product, wherein the reagent R1 is buffer solution with pH (Potential of Hydrogen) value of 6-9; the reagent R2 is latex reagent coated by anti-retinol binding protein double antibodies; and the standard product is retinol binding protein solution with pH value of 5-8. According to the kit, the retinol binding protein content in a sample can be detected by using the latex immunoturbidimetry; the sensitivity is high and can reach 0.042 mg / L; and the kit has the advantages of high stability, easiness and quickness in operation, high specificity, low probability of interference, accurate quantification and broad application prospect.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
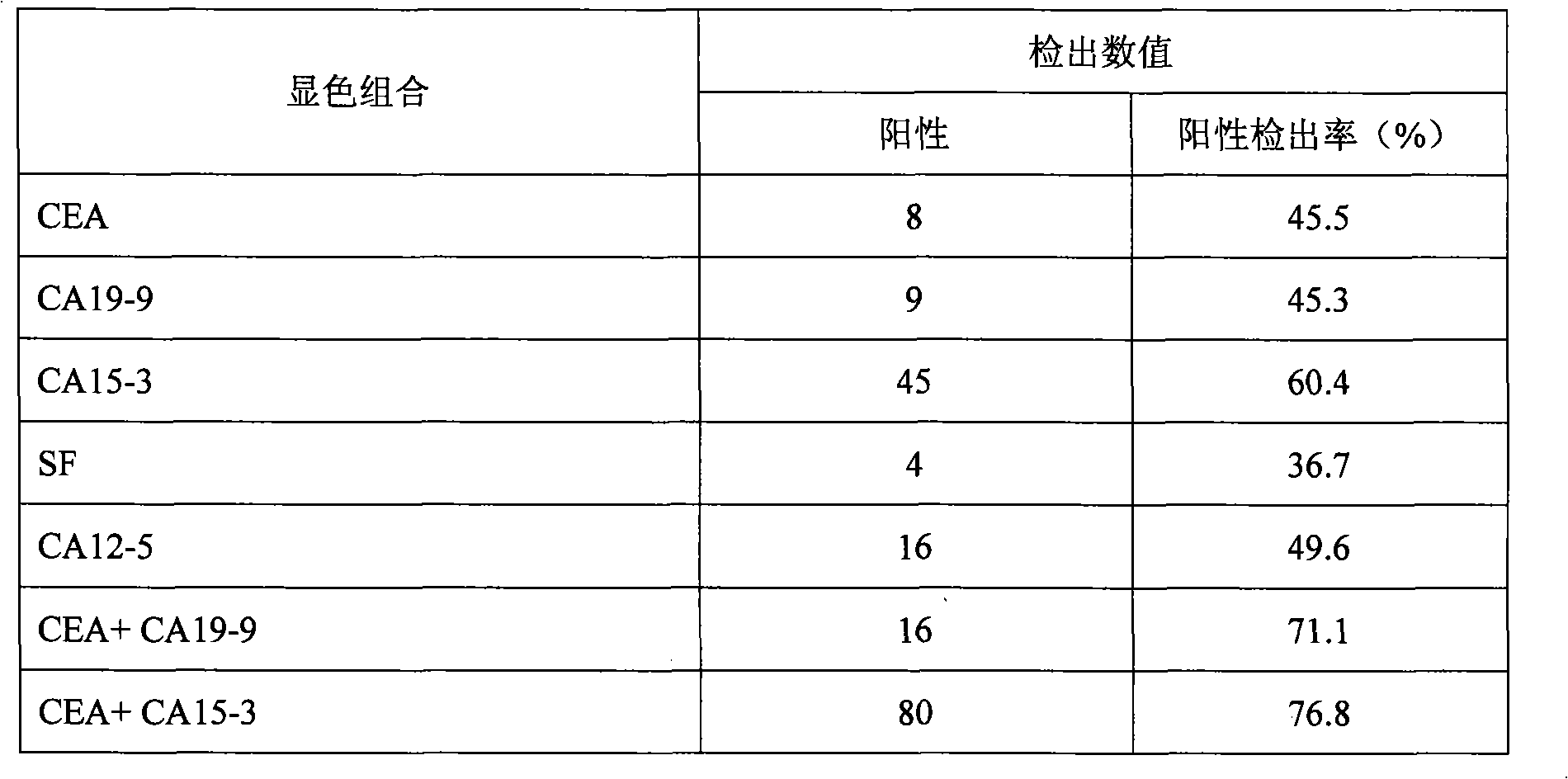
CA15-3, CEA, CA19-9, CA12-5, SF mammary cancer colloidal gold five joint inspection diagnostic reagent kit
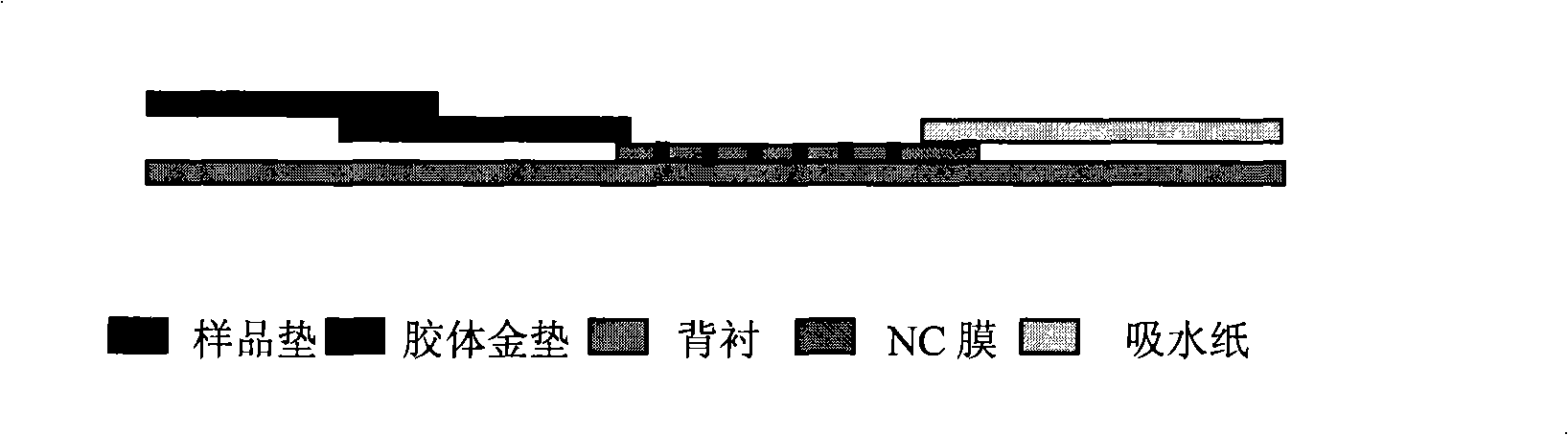
The invention discloses a diagnosis kit for breast cancer and preparation method thereof. Five kinds of tumor markers for diagnosis of breast cancer such as CA15-3, CEA, CA19-9, CA12-5, SF are detected together at the same test paper and using immunity chromatography technique the monoclonal antibodies for CA15-3, CEA, CA19-9, CA12-5, SF are fixed on the cellulose nitrate film, at the same time the five kinds of monoclonal antibodies are marked by gold particles of different size and the said gold antibodies are mixed to produce gold affixture pad, finally the gold affixture pad is assembled with a sample treating pad to form a test paper. The said kit quickly test the five kinds of antigens in human whole blood or serum, plasm using double antibody sandwiching method, so as to conquer many defects of previously separation detecting method, only one drop of blood can detect five tumor markers and the detection result is more direct and the sensitivity and specificity of detection is greatly increased.
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
Anti-Golgi apparatus protein monoclonal antibody and use
InactiveCN101407544AHigh sensitivityQuantitatively accurateImmunoglobulins against animals/humansFermentationSerum igeSerum samples
The invention relates to an anti-Golgi protein antibody and an application thereof. The invention recombines human GP73 protein immunity animal to obtain an anti-GP73 polyclonal antibody and a monoclonal antibody which specifically aims at GP73 and builds a plurality of methods for detecting GP73 in clinical tissue sections and serum samples, such as immunohistochemical stain and double antibody sandwiched ELISA method and the like. Tests prove that the polyclonal and monoclonal antibodies can be used for preparing a plurality of GP73 detecting agents of different detecting methods.
Owner:曹伯良 +1
Specific culture medium for lung tumor organ and stentless 3D culturing method
ActiveCN110592022AStrong cell stemnessRetain heterogeneityCulture processCell culture active agentsY-27632HEPES
The invention discloses a specific culture medium for a lung tumor organ and a stentless 3D culturing method. The specific culture medium is prepared from the following components: FBS, double antibody, N-2, Noggin, B-27, EGF, FGF-10, Y-27632, A 83-01, SB202190, N-acetylcysteine, HEPES, Glutamax, IGF-1, hydrocortisone and Advanced DMEM / F12. The culturing method comprises the following steps: adding a tumor cell into a low serum culture medium, re-suspending the tumor cell, inoculating the tumor cell into a culture vessel, adding the specific culture medium into the culture vessel, changing thespecific culture medium once a day, and performing culturing until an organoid is formed. According to the culture medium and culturing method, a tumor organoid can quickly generate, can be stably cultured for a long time, is regular in spheroid form and has uniform and controllable size, and the heterogeneity of a tumor tissue of a patient can be well maintained in vitro.
Owner:浙江弘瑞医疗科技有限公司
Double antibody complex retinol-binding protein assay kit
A retinol-binding protein assay kit in the market at present has good specificity and insufficient sensitivity or has high sensitivity and poor specificity because the purity of antibodies is insufficient or the potency cannot meet the requirement. The invention provides a double antibody complex retinol-binding protein assay kit, which consists of three parts, namely, a reagent R1, a reagent R2 and calibration materials. The reagent R1 is a phosphate buffer system which consists of phosphate buffer solution with the pH of 7.2 to 7.6, polyethylene glycol 6000-8000 and ethylene diamine tetraacetic acid; the reagent R2 is antibody solution which consists of mouse anti-human monoclonal antibody, rabbit anti-human polyclonal antibody, phosphate buffer solution with the pH of 7.2 to 7.6 and the ethylene diamine tetraacetic acid. By adopting a complex antibody of the polyclonal antibody and monoclonal antibody, the sensitivity and high linearity are guaranteed, and the accuracy of the measured result is greatly improved.
Owner:浙江康特生物科技有限公司
Human oophoroma tumor marker HE4 enzymoimmunoassay kit
InactiveCN101525614ASave foreign exchangeImprove stabilityMicroorganism based processesFermentationElisa kitPeroxidase
The invention discloses a human oophoroma tumor marker HE4 enzymoimmunoassay kit, a preparation method and an application thereof. A PCR overlapping method is adopted to fully manually synthesize an HE 4 gene by a synthesized HE4 gene prime; the gene is constructed into a Rho GEX-4T1 expression vector for protein expression; the expressed HE4 fusion protein is restricted by enzyme and purified to obtain an HE4 purified product which immunes animals to obtain a polyclonal antibody and a monoclonal antibody; a rabbit antihuman HE4 polyclonal antibody is used to wrap and close an ELISA plate; the rabbit polyclonal antibody is used as a capture antibody, rat antihuman HE4 monoclonal antibody labeled by horseradish peroxidase is used as a detection antibody so as to be assembled into a double-antibody sandwiched ELISA kit for detecting HE4. The kit has the advantages of high detection sensitivity and high specificity, is suitable for the early diagnosis of oophoroma and provides oophoroma patients for curative effect observation and prognosis judgment with an easy, convenient, rapid, economical and reliable detection method.
Owner:大连美亿德生物科技有限公司
Method for removing cells from human amnion
ActiveCN103114073AEfficient and thorough removalSo as not to damageArtificial cell constructsVertebrate cellsCell-Extracellular MatrixPhosphate
The invention discloses a method for removing cells from a human amnion. The method is characterized by comprising the following steps of: obtaining a clean semitransparent amnion, rinsing the semitransparent amnion by using a PBS (Phosphate Buffer Solution), soaking the semitransparent amnion in a comprehensive PBS liquid, shocking, rinsing the amnion by using the PBS after the amnion is taken out, successively soaking the amnion in steapsin / PBS liquid and deoxyribonuclease / PBS liquid for treatment, rinsing the amnion by using double-antibody / PBS liquid, taking out the decellularized amnion and putting the decellularized amnion in the PBS for preservation and standby. The method has the advantages that the cells are removed from the amnion in a manner that a surfactant is combined with steapsin and deoxyribonuclease, the cells can be prompted to be completely and efficiently removed from the amnion due to the combined use of the steapsin and the deoxyribonuclease, and meanwhile, the extracellular matrix is reserved to the maximum and is enabled to be free from damage.
Owner:杭州恩格生物医疗科技有限公司
Test paper strip for detecting clostridium difficile toxin A and toxin B colloidal gold, method for making same and applications
ActiveCN101363867ASave manpower and material resourcesThe result is clear and easy to distinguishMaterial analysisCelluloseClostridium difficile infections
The invention provides a test strip for rapid detection of clostridium difficile toxins A and B. A monoclonal antibody or polyclonal antibody of the clostridium difficile toxin A, a monoclonal antibody or polyclonal antibody of the clostridium difficile toxin B, and a quality control double-antibody IgG coat a nitrate cellulose film (NC film), and a membrane chromatography double antibody sandwich method is adopted to detect the clostridium difficile toxins A and B in a specimen in combination with a monoclonal antibody of colloidal gold labeled clostridium difficile toxins A and B. The test strip is simple in operation, convenient, and fast, and has the advantages of no requirements of special instruments and special training, clear and identified result, and easy popularization. The test strip is suitable for base course, site detection and epidemiological investigation, and has auxiliary effect on the diagnosis of clostridium difficile toxin infection.
Owner:辽宁迪浩生物科技有限公司
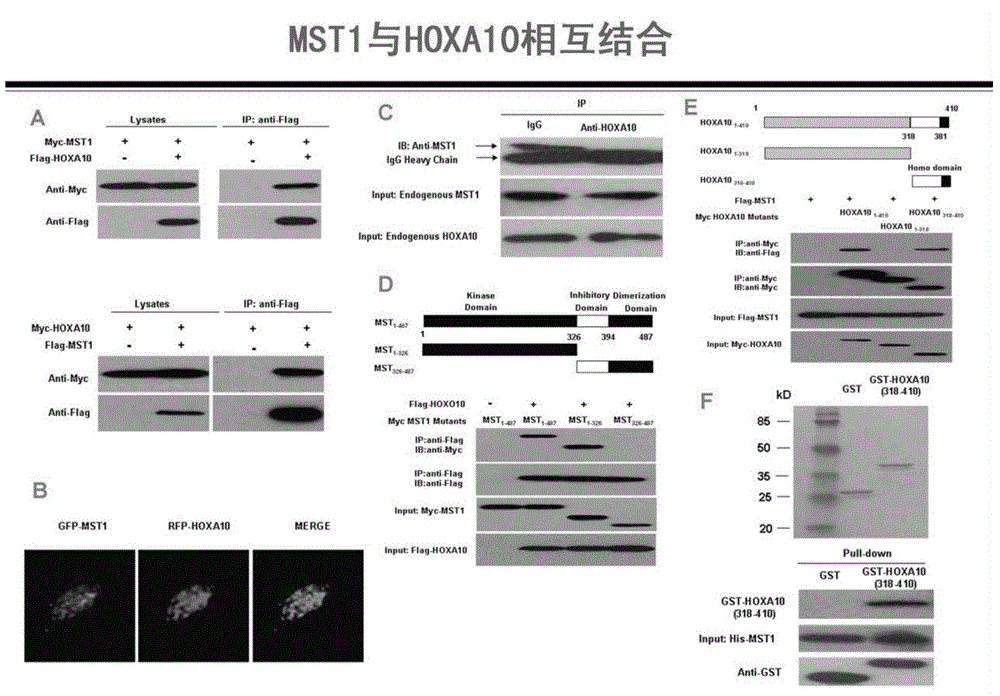
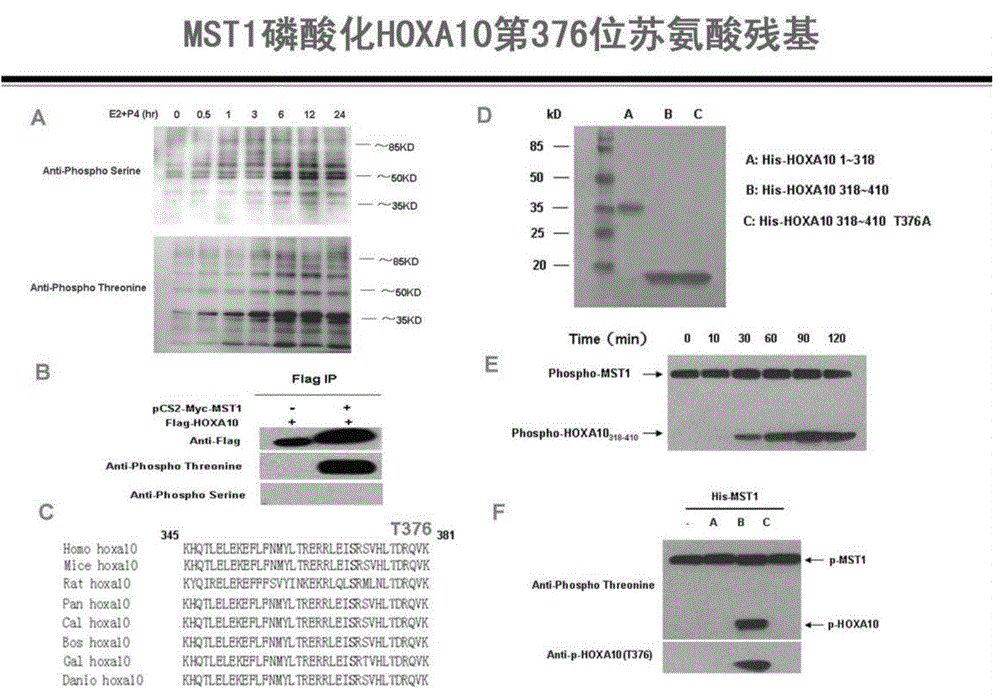
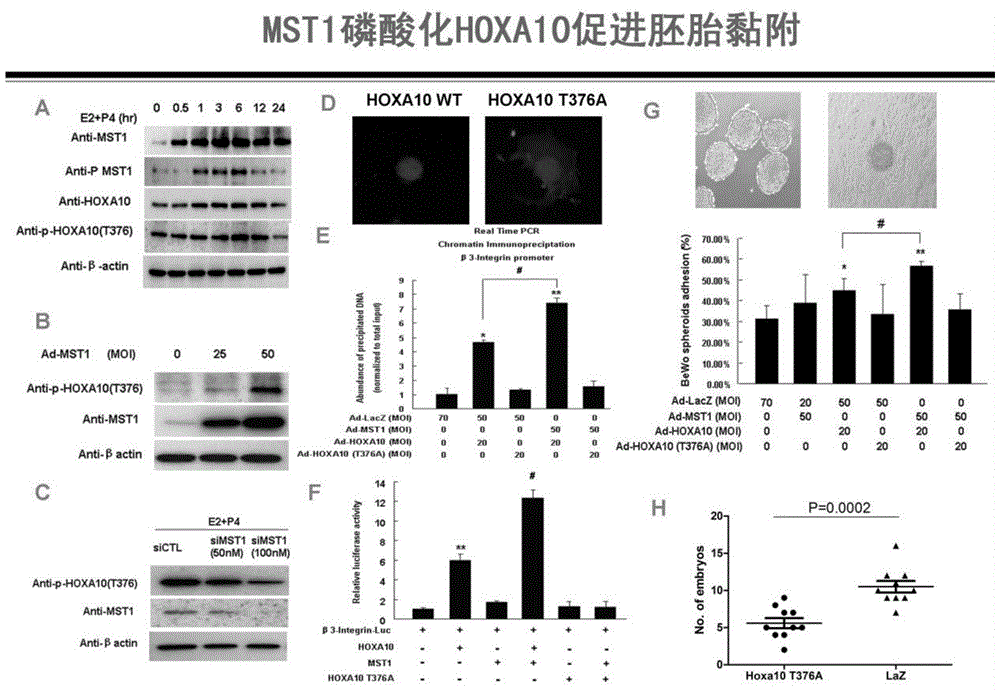
Method for detecting endometrial receptivity through MST1 and phosphorylated MST1
ActiveCN105988002AQuick and easy expressionEasy and fast retouching levelMaterial analysisCarcinoma cell linePhosphorylation
The invention provides a method for detecting endometrial receptivity through MST1 and phosphorylated MST1. The method comprises the steps that a human endometrial carcinoma cell line Ishikawa and HEK293T cells are kept and cultured in a 10% FBS and mycillin containing double-antibody DMEM / F12 culture solution; an immunoblotting experiment is carried out; co-immunoprecipitation is carried out; immunohistochemistry is carried out; adjustment of MST1 on the transcriptional activity of HOXA10 is analyzed through chromosome co-immunoprecipitation (ChIP) / PCRs; human choriocarcinoma cell (BeWo) sphere adhering experiments are carried out. According to the method, the expression level of MST1 and MST1 phosphorylation modification in endometrial tissue is detected conveniently and fast, and the receptivity state of the endometrium can be directly reflected.
Owner:江苏华朵生物科技有限公司
Human skin epidermal cell culture medium and application thereof
InactiveCN105950542AShorten the timeMeet the needs of clinical treatmentCulture processEpidermal cells/skin cellsSerum free mediaCell culture media
The invention provides a human skin epidermal cell culture medium and application thereof. The culture medium is prepared by the following steps: mixing a K-SFM (keratinocyte serum-free medium), a DMEM (dulbecco's modified eagle medium) and a F12 culture medium according to the ratio of 2:1:1, and adding a BPE (bovine pituitary extract), an EGF (epidermal growth factor), an SCGF (stem cell growth factor), an FGF (fibroblast growth factor), a TGF-beta (transforming growth factor-beta), a VEGF (vascular endothelial growth factor), CaCl2, glutamine and a double-antibody. The culture medium is free of serum, and can be used for human skin epidermal cell primary culture and subculture. The human skin epidermal cell culture medium can obtain abundant epidermal cells by in-vitro culture by using only a small amount of patient skin tissues. Compared with the traditional epidermal cell culture medium, the human skin epidermal cell culture medium provided by the invention greatly shortens the time required by amplifying the same cell count, and satisfies the demands for clinical therapy.
Owner:JINAN PANSHENG BIOTECH
Erythroculter ilishaeformis spermatogonia stem cell separation and culture method
InactiveCN104830756AGood retention propertiesPromote proliferationGerm cellsSterile environmentErythroculter ilishaeformis
The invention relates to an erythroculter ilishaeformis spermatogonia stem cell separation and culture method. The method comprises the following steps: (1) collecting spermatic tissues: collecting spermary of erythroculter ilishaeformis with an age of seven months in a sterile environment, then rinsing spermary in PBS containing double-antibody, and finally grinding the spermary to disperse the spermary tissues; (2) digesting and separating spermatogonia stem cell: adding IV type collagenase (0.1%) into the dispersed spermary tissues, subjecting the digestive fluid to centrifugal separation, collecting the precipitate, digesting the precipitate by trypsin (0.25%), stopping the digestion by a culture medium containing FBS (10%), and collecting the cells; (3) carrying out primary culture of spermatogonia stem cell: using a DMEM / F12 complete medium containing cell factors to re-suspend the cells obtained in the step (2), adjusting the cell concentration, then paving the suspension liquid on a 24-hole cell culture plate coated by gelatin, and culturing the cells at a temperature of 26 DEG C. Trough the provided method, the in-vitro growth of primary cells of erythroculter ilishaeformis spermatogonia stem cell becomes easier, and an effective cell platform is provided for the research on reproduction and growth of erythroculter ilishaeformis.
Owner:HUZHOU TEACHERS COLLEGE
Isolated culture method of porcine fat stem cells
InactiveCN104673745AEasy to isolate and cultureStrong proliferative growth abilitySkeletal/connective tissue cellsAnimal sciencePhosphate
The invention discloses an isolated culture method of porcine fat stem cells, which comprises the following steps: extracting porcine subcutaneous fat tissues, washing the fat tissues with a double-antibody-containing PBS (phosphate buffer solution), adding a I-type collagenase digestive juice into the fat tissues for digestion to obtain porcine fat stem cells, and culturing in an EGF-containing serum-free culture medium. The porcine fat stem cell isolated culture method can reduce the influence of blood and other impurities in the fat tissue source on the enzymolysis fat and shorten the fat enzymolysis time of the I-type collagenase; the serum-free culture medium is utilized to culture the primary cells; and proper growth factors are added into the culture medium, so that the porcine fat stem cells can be better subjected to isolated culture and maintain high proliferation and growth capacity, thereby establishing a porcine fat stem primary isolated culture method conforming to the Standard Specification.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Influenza A,B virus antigen colloidal gold combined detection test paper
InactiveCN1904615ASave the procedure of secondary inspectionThe result is clear and easy to distinguishMaterial analysis by observing effect on chemical indicatorTest sampleColloid
The invention supplies a rapid testing tape that could simultaneously test A, B virus antigen. It covers FluA-McAb, FluB-McAb and IgG on NC film, compounds FluA-McAb, FluB-McAb marked by colloidal gold, simultaneously tests A, B virus antigen in tested sample by using film chromatography double antibody cream filling method. The invention could decrease cockamamie process, and could gain result in 10-15 minutes. It is suitable to hospital and the research for large scale epidemiology to handle sporadic affair.
Owner:BEIJING ZHUANGDI HAOHE BIOMEDICINE SCI & TECH
Irrigating solution, enzymatic hydrolysate and method for isolating placenta hematopoietic stem cells
ActiveCN105368780ASimple componentsGuaranteed survival rateBlood/immune system cellsHydroxyethyl starchPenicillin
The invention discloses an irrigating solution, an enzymatic hydrolysate and a method for isolating placenta hematopoietic stem cells. The irrigating solution consists of a penicillin-streptomycin double-antibody, phentolamine, vitamin C and PBS. The enzymatic hydrolysate consists of a basal culture medium, collagenase VIII, pancreatin, 1*heparin sodium, phentolamine, vitamin C and Z-VAD-FMK. The method comprises the following steps: irrigating and cleaning a preprocessed placenta with the irrigating solution; irrigating the enzymatic hydrolysate from placental artery until the enzymatic hydrolysate flows out of placental vein, ligating placental artery and vein and standing by; loosening the placental artery and vein and irrigating the irrigating solution from the artery, collecting all liquid from the vein, centrifuging and reserving cell sediments, and recovering and purifying mononuclear cells containing the hematopoietic stem cells sequentially by virtue of a hydroxyethyl starch method and a red blood cell lysis method; re-suspending and obtaining the placenta hematopoietic stem cells. The irrigating solution, the enzymatic hydrolysate and the method disclosed by the invention can significantly improve separation and extraction efficiencies, and a great amount of cells are obtained; the hematopoietic stem cells are high in content, high in survival rate and quite low in cell pollution rate.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Preparation method of hemizygote CAPRI cells
ActiveCN103966164ASolve the problem that the training cannot be carried outHas broad-spectrum antitumor effectDead animal preservationBlood/immune system cellsCell expansionDouble antibody
The invention relates to a preparation method of hemizygote CAPRI cells. The method comprises the following concrete steps: (1) obtaining peripheral blood of a patient and a hemizygote donor, and separating and purifying a peripheral blood mononuclear cell (PBMC); (2) preparing serum from hemizygote donor plasma; (3) activating the PBMC; (4) expanding the CAPRI cells; (5) harvesting the CAPRI cells; (6) cryopreserving the CAPRI cells. In addition, an early cell treatment step and a double-antibody loading method are added to increase the number of CAPRI cells and improve the tumor killing capability. According to the preparation method provided by the invention, the problem that the CAPRI culture cannot be carried out due to factors of relatively poor condition and the like of the patient in the prior art is solved, the generated cells have a broad-spectrum tumor-killing effect, the PBMC has good cell expansion and activation capability due to the early cell treatment step, and the tumor cell killing effect of the CAPRI cells is improved due to the double-antibody loading mode; the advantages of the CAPRI cells are brought into full play, so that cells from the patient fully cooperate with cells from the hemizygote donor, and the blank in the prior art is filled; moreover, the reactivation capability of cryopreserved cells is greatly improved due to cell cryopreserving liquid provided in the method.
Owner:王盛 +1
Human polycystic albumen -1 quantitative determination kit
The invention relates to a reagent box to quantitatively detect multivesicular protein-1 content in human body fluid, using anti-human multivesicular protein-1 N-end monoclonal antibody and anti-human mulitvesicular protein-1 N-end polyclonal antibody, adopting immunologic technique to establish a double-antibody filled enzyme linked immunosorbent assay (ELISA) method of quantitatively detecting body fluid and multivesicular protein-1 content in tissue cracking liquid. Comparing the difference in multivesicular protein-1 content in body fluid, judge if the patient has autosomal dominant hereditary nephropathy and has an important reference value in clinic diagnosis of this disease and provides an effective route to prevent and cure this disease as soon as possible.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Type 1 diabetes related antigen-antibody simultaneous detection egg white slice
InactiveCN1448724AAccurate identificationEasy to formulateBiological testingSpecific antibodyTreatment regimen
Owner:GENETECH BIOTECH SHANGHAI
Method for separating and culturing mouse synovial cells
InactiveCN107475179AHigh purityA large amountCell dissociation methodsArtificial cell constructsBALB/cSynovial Cell
The invention provides a method for isolating and cultivating mouse cells, which includes the following steps: (1) sterilizing tissue processing equipment; (2) selecting male BALB / c mice aged 1 to 2 months, killing them by breaking their necks, and using surgical scissors. Separate the synovial layer and fibrous layer of the joint capsule, then remove the synovial layer tissue, put the tissue into pre-cooled sterile PBS solution containing double antibody and wash it several times to remove blood stains and fatty tissue; (3) Cut the tissue into pieces, and use Digest with preheated type II collagenase and trypsin respectively; (4) Stop digestion, centrifuge, and discard the supernatant; (5) Inoculate into the coated culture flask by manual adhesion method; (6) After incubation for 1 to 2 hours, Cultivate in a 37°C, 5% CO2 environment; (7) Cell morphology observation; (8) Cell subculture; (9) Cell identification. The method for isolating and culturing mouse synovial cells provided by the invention is simple to operate, has a large number of cells obtained, and has a high survival rate. It is an ideal primary method for isolating and cultivating mouse synovial cells and provides reliable results for experiments. Cell resources.
Owner:JIANGYIN CHI SCI
Immunochromatographic test strip, and making method and detection method thereof
ActiveCN104714008ASimple and fast operationSimple structureMaterial analysisHigh concentrationCellulose
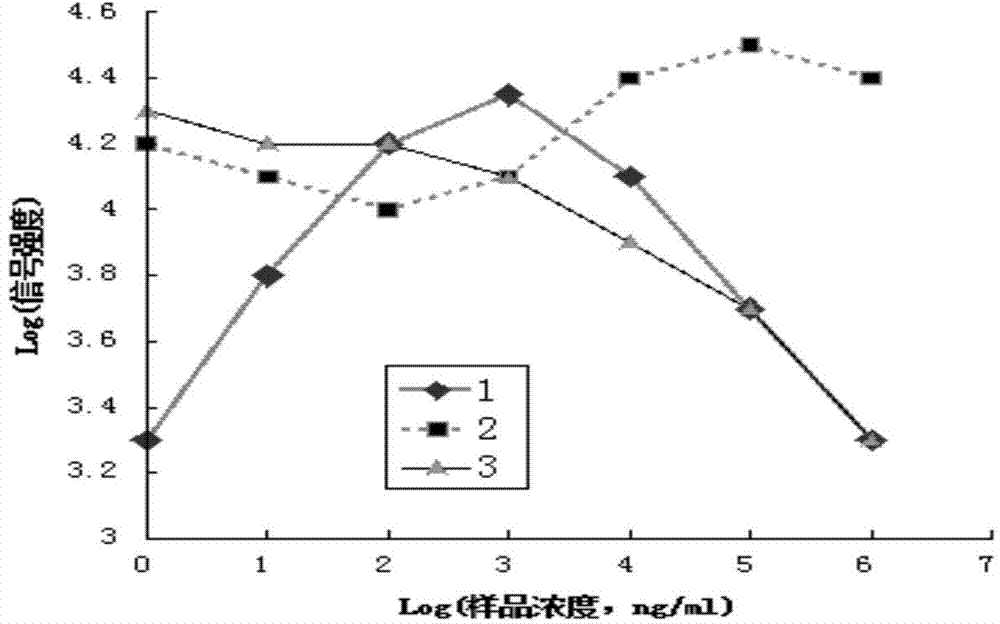
The invention provides an immunochromatographic test strip. The test strip comprises a sample pad, a binding pad, a cellulose nitrate membrane, an absorbent pad and a backing pad, the cellulose nitrate membrane comprises a detection line and a quality control line, and the cellulose nitrate membrane also comprises a double antibody line. The double antibody line is arranged between the detection line and the quality control line. The invention also provides a making method and a detection method of the immunochromatographic test strip. The immunochromatographic test strip reserves the advantages of simple operation, simple structure and low cost of traditional immunochromatographic test strips, effectively avoids the false negative phenomenon appearing in the detection of high concentration of a substance to be tested of traditional test strips, effectively improves the detection accuracy of immunochromatography, and enlarges the linear detection range; and the test strip comprehensively utilizes the signal intensities of the above lines in a display area and the relationship among the signal intensities of the three lines not limited to the signal intensity of the detection line, so the test strip can be used to accurately determine the concentration of the substance to be tested, and the sensitivity and the specificity of immunochromatographic detection are greatly improved.
Owner:SHANGHAI JIAO TONG UNIV
Method for separating and extracting hUC-MSC (human Umbilical Cord mesenchymal stem cells) from wharton jelly tissue of umbilical cord
InactiveCN105462919AReduce incubation timeImprove securityCell dissociation methodsCulture processSerum free mediaUmbilical cord tissue
The invention provides a method for rapidly separating and extracting hUC-MSC (human Umbilical Cord mesenchymal stem cells). The method comprises the following steps: taking the freshly collected umbilical cord tissue of a healthy newborn baby, carrying out on-ice transportation on the freshly collected umbilical cord tissue in umbilical cord storage transportation liquid containing double antibodies, carrying out cleaning and disinfection by adopting 75% alcohol and normal saline, removing blood vessels, carrying out blunt dissection on wharton jelly, carrying out mechanical pulverization, treating the obtained product I by adopting red blood cell lysis buffer for 3 min, digesting the obtained product II by adopting IV collagenase, screening the obtained product III by adopting a 100-200-mesh sieve, carrying out suspension culture on the obtained product IV by adopting a serum-free medium, wherein the liquid is changed every 3-5 days, taking supernatant, detecting cell pollution, after the adherent rate in a plate reaches 30-70%, carrying out trypsinization, carrying out centrifugation, collecting cells, carrying out passage amplification, carrying out merging when the cell merging rate reaches 90% or above, collecting the cells, carrying out cryopreservation on the cells, and detecting the biological characteristics of hUC-MSC.
Owner:郭镭 +1
Rapid honey detection test strip, and preparation method and application thereof
InactiveCN107462713AReduce manufacturing costEasy to useMaterial analysisHoney samplesPolyclonal antibodies
The invention discloses a rapid honey detection test strip, and a preparation method and application thereof. The preparation method includes (1), preparing and purifying polyclonal antibodies SP1 and SP2 capable of specifically recognizing MRJP1; (2), preparing a collaurum solution; (3), coating a nitrocellulose membrane with a quality control line (line C) and a detection line (line T); (4), assembling the rapid honey double-antibody sandwich detection test strip taking the bee endogenous protein MRJP1 as a biomarker; (5), preparing a honey detection sample. After separated and purified protein in the honey sample reacts with the gold-labeled test strip, authenticity of the honey sample can be judged according to color development conditions of the line T. The gold-labeled honey double-antibody test strip is applicable to detection of fake honey pretend by artificial syrup, and a new reliable rapid detection method is provided for quality and authenticity detection of honey.
Owner:ZHEJIANG UNIV
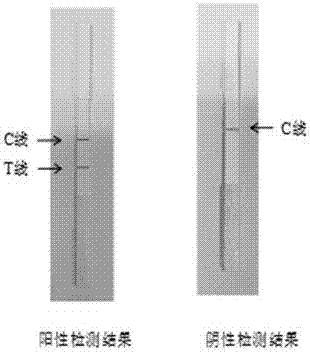
Test paper strip for rapidly detecting morbilli and rubella virus IgG antibody colloidal gold
ActiveCN101363856AHigh sensitivityImprove featuresMaterial analysisRubulavirus InfectionsSpecific igg
The invention provides a test strip for simultaneous detection of measles and rubella virus specific IgG antibodies, which comprises a reaction film and a conjugate release pad. The reaction film has a detection band simultaneously coated with measles virus H antigen and rubella virus E1 specific antigen, and a quality control band coated with double-antibody IgG. The conjugate release pad is coated with colloidal gold labeled anti-human IgG. The test strip is simple in operation, convenient, and fast, and has the advantages of no requirements of special instruments and special training, clear and identified result, and easy popularization. The test strip is suitable for base and site detection and epidemiological investigation, has auxiliary and differential diagnosis effects on measles and rubella virus infection, and can be used for the immune effect observation after vaccination.
Owner:辽宁迪浩生物科技有限公司
EV71 (human enterovirus 71) antigen enzyme-linked reaction detection kit and its preparation method
The invention relates to the biotechnical field, concretely relates to a virus antigen content detection kit and its preparation method, and more concretely relates to an enzyme-linked reaction kit for qualitatively and quantitatively detecting the EV71 virus antigen content, and its preparation method. The double-antibody enzyme-linked reaction detection kit adopted in the invention and its preparation method have the characteristics of good specificity, high sensitivity, convenience, fastness, economy and the like.
Owner:BEIJING HUAWEI BRAVOBIO
Combination detection reagent for detecting schistosomiasis and detecting method thereof
InactiveCN1700007AImprove standardizationEasy specificityTransferasesImmunoglobulinsAntigenGlutathione S-transferase
The invention relates to a combined medium and method for testing snail fever. It has the following characters: the medium contains recombination antigen mixture and recombination snail signal protein (rSj14-3-3) monoclonal antibody, and recombination antigen is mixed as recombination snail signal protein (rSj14-3-3) and recombination snail glutathione-s-transferase (rSjGST). The test operation is separately according to indirect enzyme immunosorbent test and double antibody bedded texture method. The invention adopts the two advantage diagnosis antigen molecule and its corresponding monoclonal antigen to form combined testing medium box.
Owner:ANHUI MEDICAL UNIV
Separation culture method of primary hepatocyte of jian carp
InactiveCN104293731APromote growthReduce in quantityVertebrate cellsArtificial cell constructsExperimental methodsDigestion Treatment
The invention discloses a separation culture method of the primary hepatocyte of a jian carp. The separation culture method comprises the following steps: selecting a healthy jian carp without injury, collecting blood from the caudal vein of the jian carp, then sterilizing the surface of a fish body, placing into a super clean bench, and aseptically collecting the liver of the jian carp; rinsing by using a PBS solution which contains double antibodies, adding a trypsin digestion solution, wherein the temperature of digestion treatment is 26-28 DEG C, and the time of the digestion treatment is 15-20 minutes; after digestion is finished, adding to a culture medium A to finish the digestion, filtering the trypsin digestion solution by using a filter screen of 200 meshes, and collecting filter liquor; and respectively centrifugalizing 50 grams of the filter liquor and 30 grams of the filter liquor for 5 minutes, then washing twice by using a culture medium B, removing a supernatant to obtain a precipitation, namely an extracted hepatocyte, preparing a cell suspension, and planking for culture. The experimental method disclosed by the invention is simple and fast and can save the separation time to a great extent. The separation time adopted by an experiment is about 40 minutes, and the obtained hepatocyte has the advantages of good growth condition, small erythrocyte quantity and cell survival rate of about 95%.
Owner:FRESHWATER FISHERIES RES CENT OF CHINESE ACAD OF FISHERY SCI
Foot-and-mouth disease vaccine host cell protein double-antibody sandwiched enzyme-linked immunosorbent assay kit as well as using method thereof
ActiveCN103792366AEasy and sufficient emulsificationAvoid cross influenceSerum immunoglobulinsImmunoglobulins against animals/humansElisa kitDiluent
The invention relates to a double-antibody sandwiched enzyme-linked immunosorbent assay kit for detecting a foot-and-mouth disease vaccine host cell protein. The double-antibody sandwiched enzyme-linked immunosorbent assay kit comprises a standard substance, a 96-pore elisa plate coated with a rabbit anti-BHK21 cell protein polyclonal antibody, horse radish Peroxidase labeled goat-anti-rabbit IgG, an anti-BHK21 cell protein antibody fluid, a diluent, a confining liquid, a washing liquid, a stop liquid 2M H2SO4 and a developing liquid, wherein the standard substance is a baby hamster kidney cell protein; the coated antibody and the detection antibody are both rabbit anti-BHK21 cell protein polyclonal antibodies. The coated antibody quantity in the detection kit provided by the invention is more stable and homogeneous; trace antigens are emulsified more simply, conveniently and fully; a binding antibody and a detection antibody are the same antibody and are more convenient, rapid and efficient for manufacturing the kit; the cross influence among different antibodies is prevented.
Owner:内蒙古必威安泰生物科技有限公司
Quantitative determination kit for neutralizing antibodies of virus and application thereof
The invention belongs to the field of a biotechnology and particularly relates to a quantitative determination kit for neutralizing antibodies of virus and application of the quantitative determination kit to detection of the neutralizing antibodies of virus of severe fever with thrombocytopenia syndrome. Virus NP proteins of the severe fever with thrombocytopenia syndrome are subjected to prokaryotic expression to prepare a rat source monoclonal antibody or polyclonal antibody aiming at NP proteins. The tissue cell half infection amount (TCIF50) of determined viruses is measured by detecting the NP proteins through an enzyme linked immunosorbent assay. A specimen to be detected and the equal amount of virus liquid are mixed and inoculated with cells. The monoclonal antibody or the polyclonal antibody of the NP proteins is monoclonal antibody; a specimen neutralizing antibody valence is detected by a double-antibody enzyme linked immunosorbent assay. The quantitative determination kit can be applied to the aspects of clinical immune effect evaluation of severe fever with thrombocytopenia syndrome vaccines, blood serum epidemiologic studies of the severe fever with thrombocytopenia syndrome of crowds or faunas, in-vitro valence determination and estimation of the manually-prepared virus-neutralizing antibodies of the severe fever with thrombocytopenia syndrome. The method provided by the invention has the characteristics of sensitivity, rapidness, specificity, high flux and the like.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
HIV-1 p24 antigen acridine ester chemiluminescence immune analyse detecting method
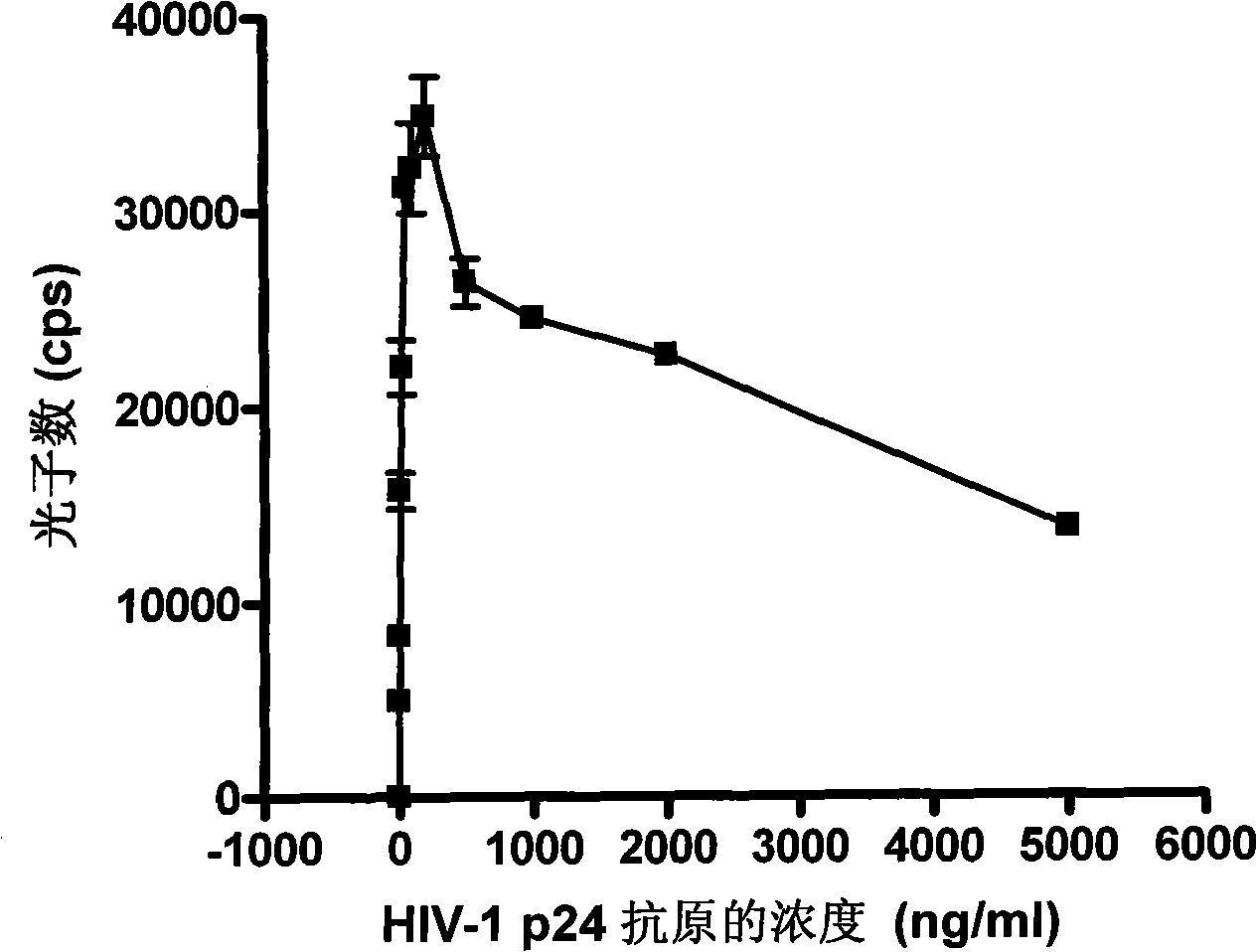
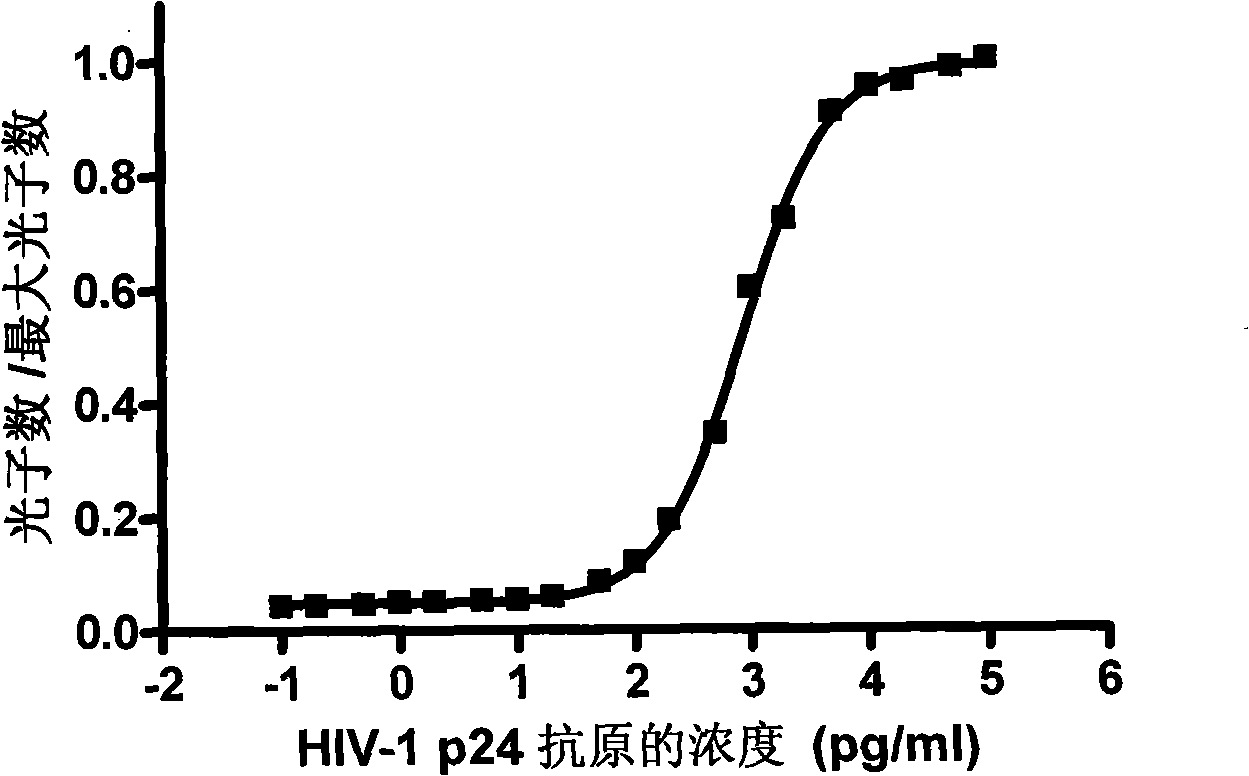
InactiveCN101281196AShorten the timeReduce stepsChemiluminescene/bioluminescenceAntigen testingSodium hydroxide
HIV-1 p24 antigen acridine ester chemiluminescence immunity analyzing testing method pertains to the clinical blood testing analysis method technique field. The prior HIV-1 p24 antigen testing method has problems of low sensitivity, rigmarole operations and the like. The invention uses acridine ester series compounds to mark the HIV-1 p24 antibody, adopts a double antibody / antigen sandwich method to execute immunity combination between the p24 antigen-antibody; uses excitant hydrogen peroxide, nitric acid, Triton X-100, and sodium hydrate to excite acridine ester series compounds to execute chemiluminescence reaction; and tests the number of photon to execute a qualitative and / or quantitative analysis. The invention has good correlativity with the general ELISA testing method, which has a correlation coefficient of 0.951 at the instance of P no more than 0.01; has high sensitivity, wherein, the testing limit is 0.5pg / ml; has wide detection range in 5-6 magnitude order; and the invention also has advantages of short reaction time, easier operation and the like.
Owner:BEIJING UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com