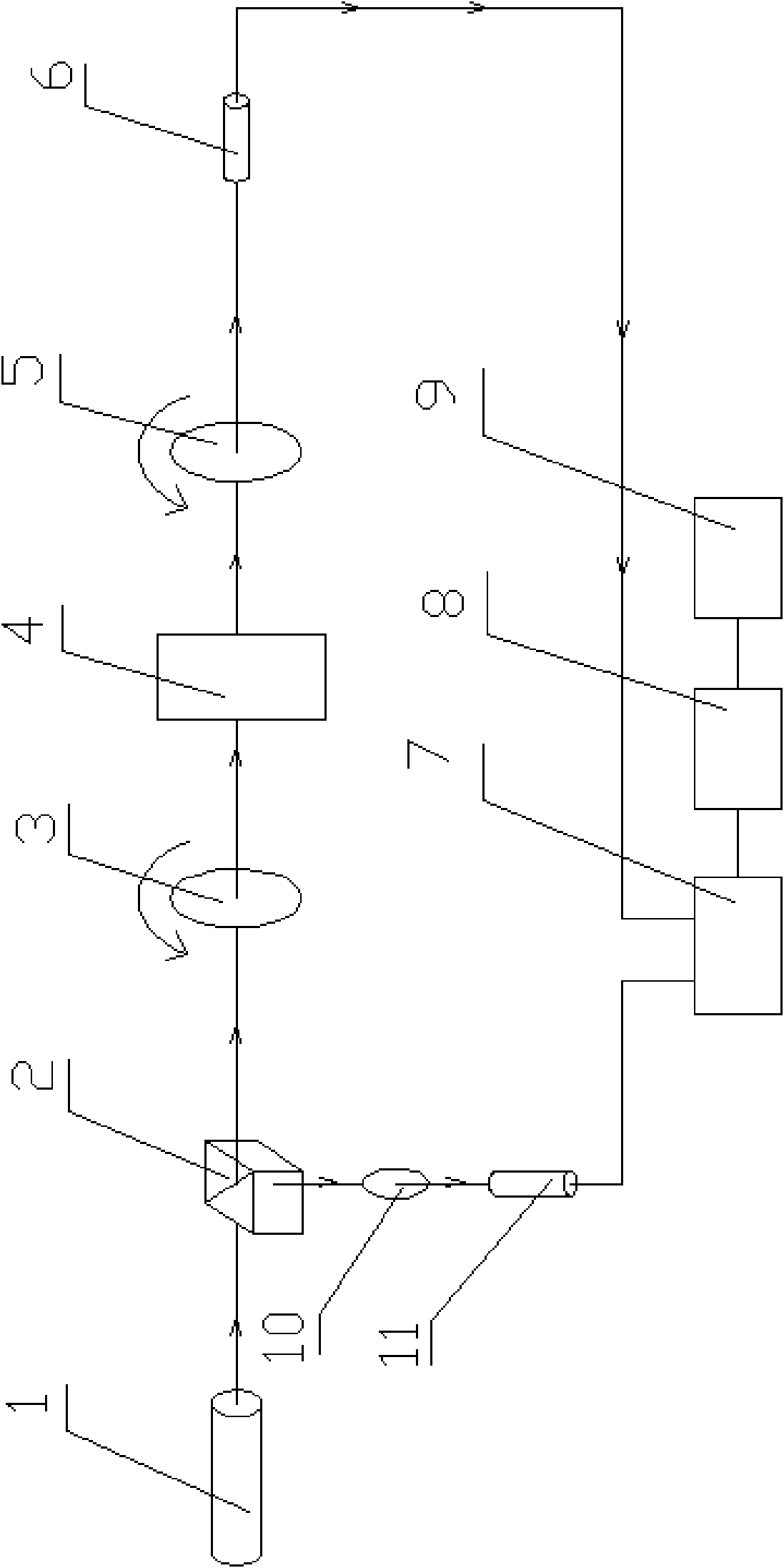
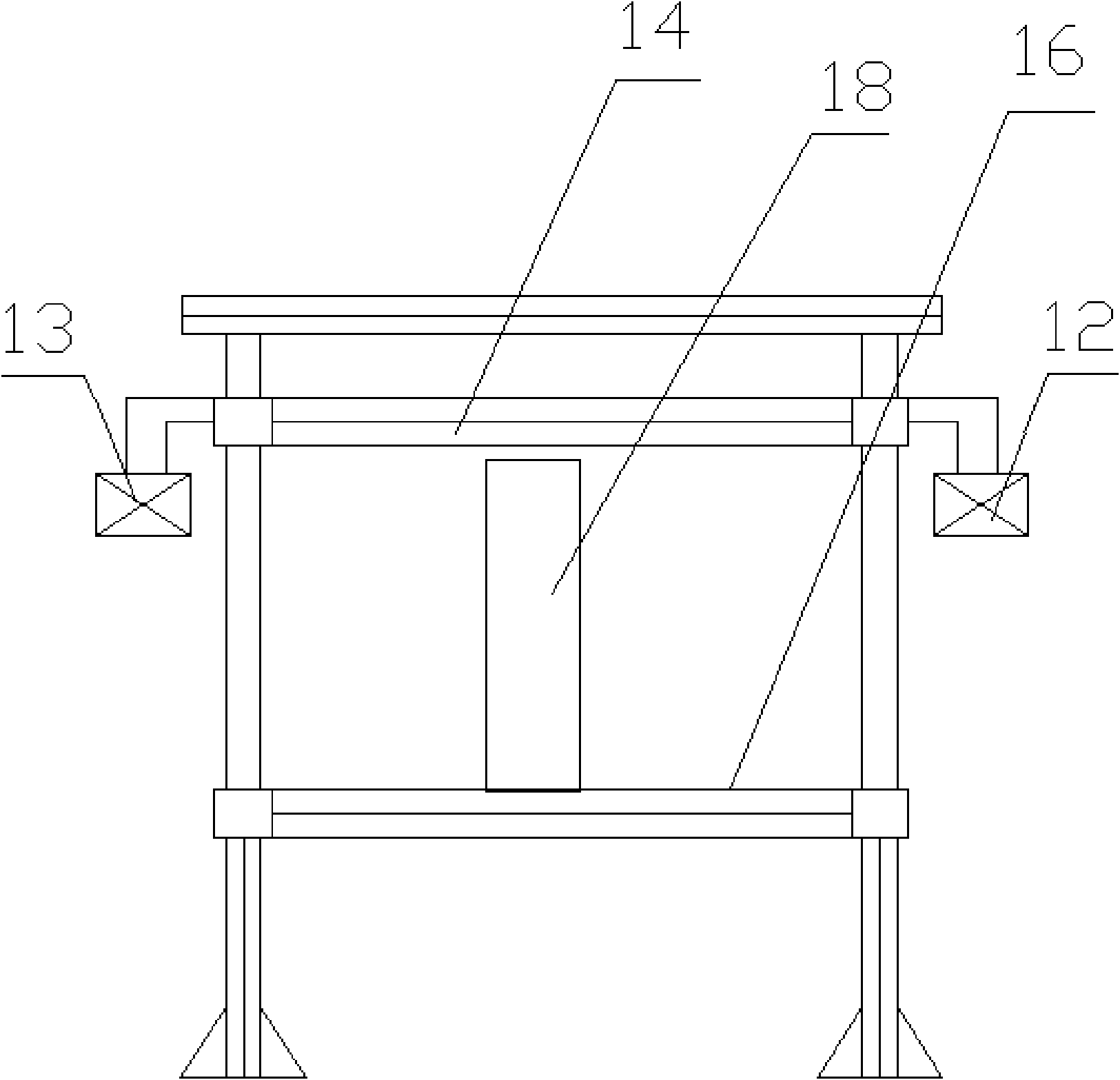
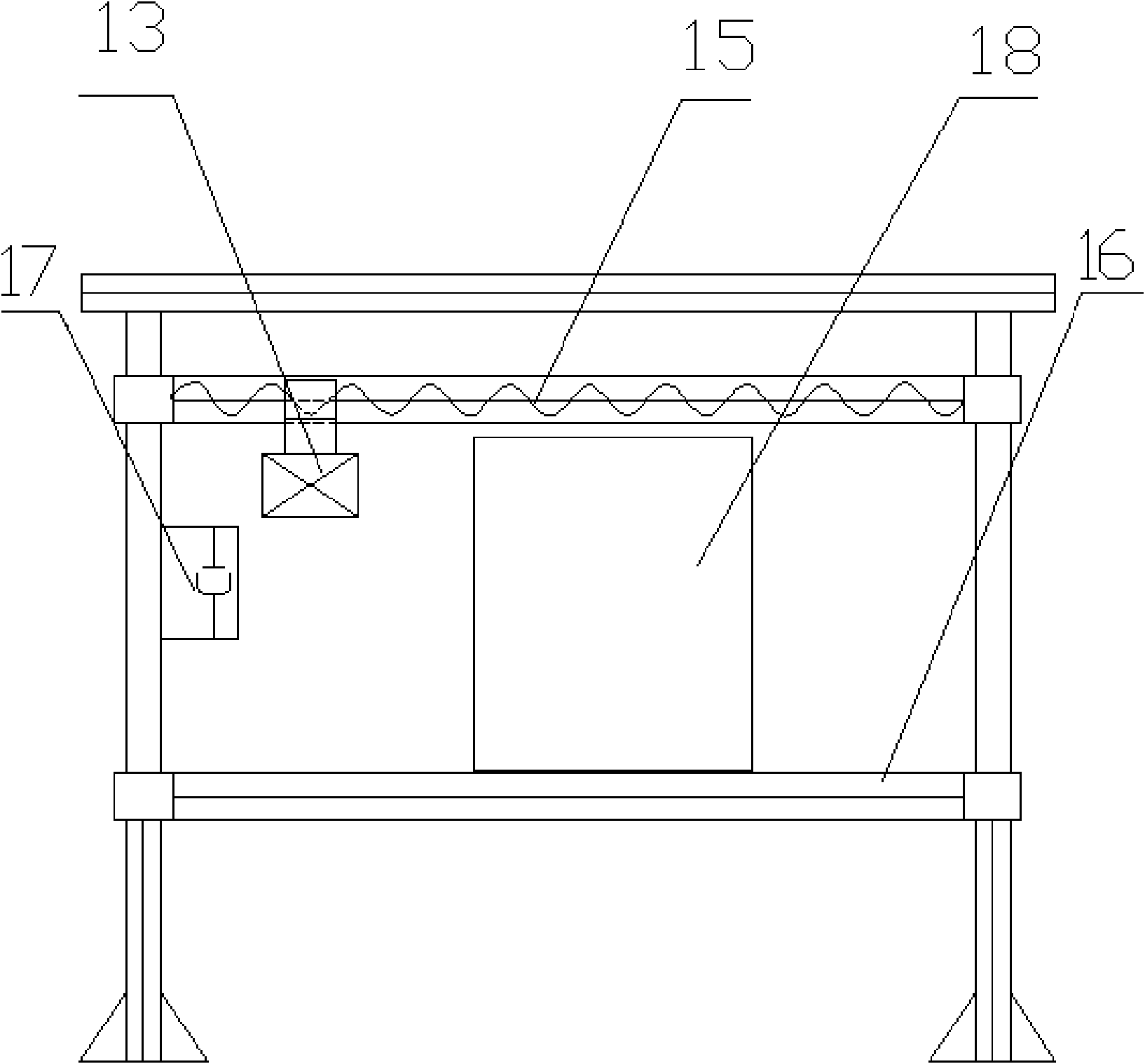
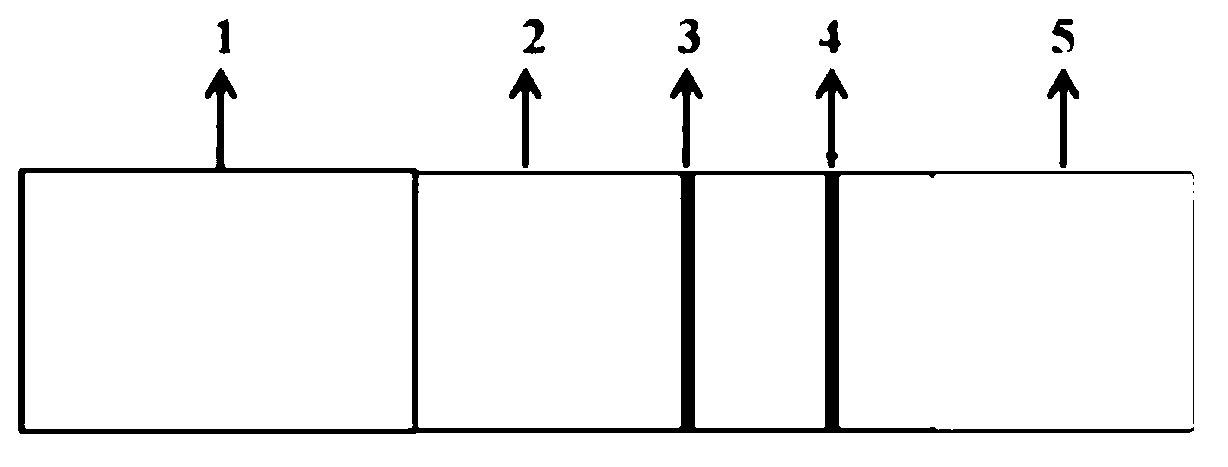
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2622 results about "Quantitative determination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Quantitative analysis refers to the determination of how much of a given component is present in a sample. The quantity may be expressed in terms of mass, concentration, or relative abundance of one or all components of a sample.
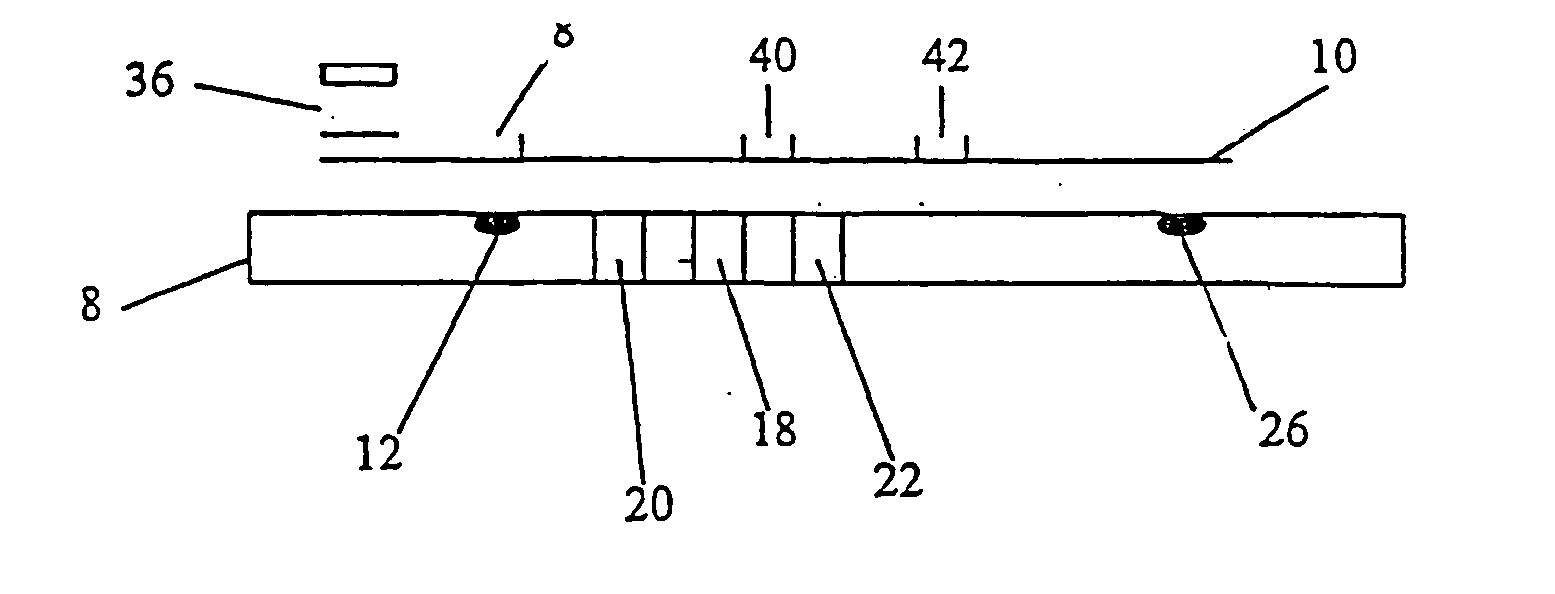
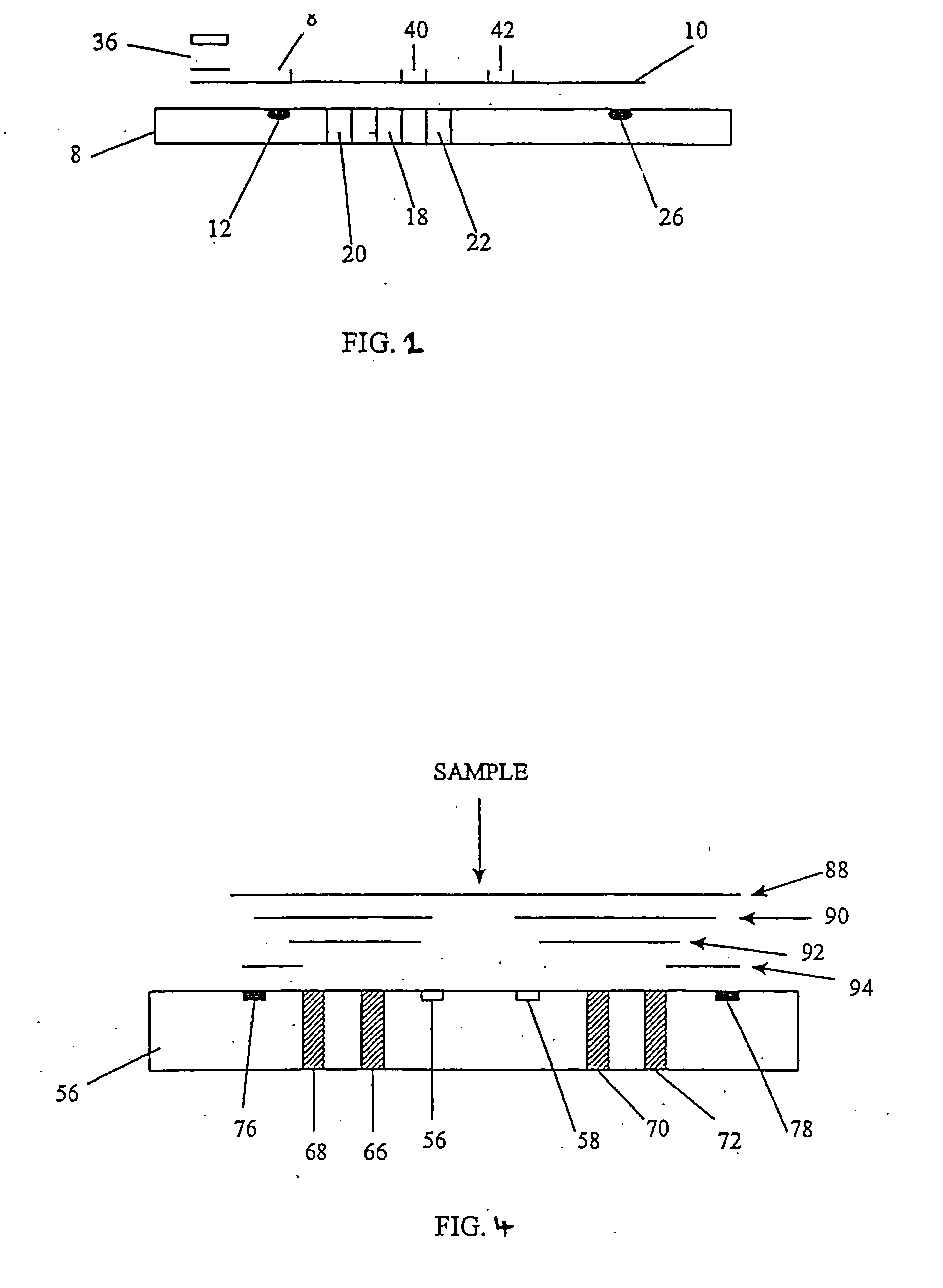
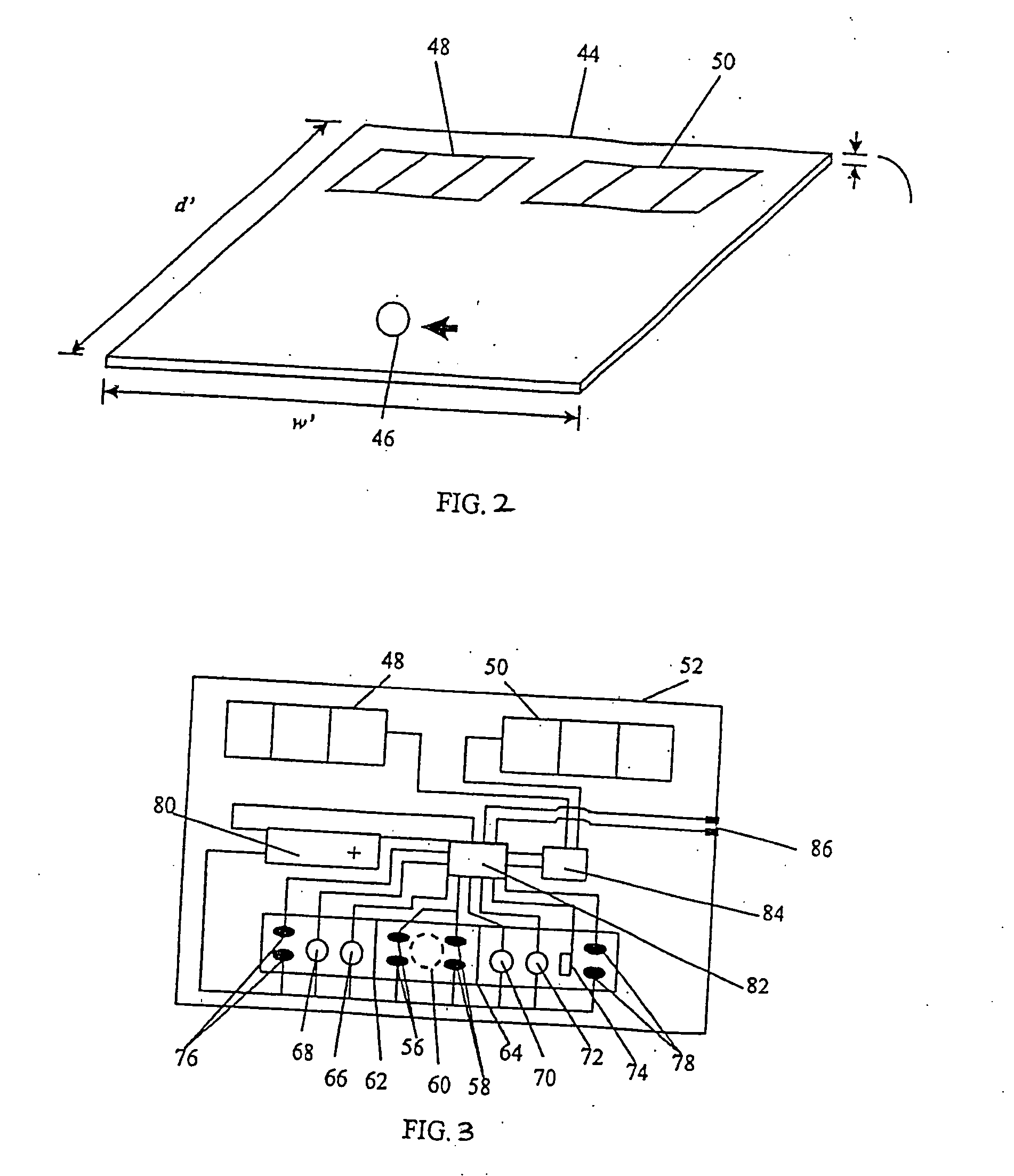
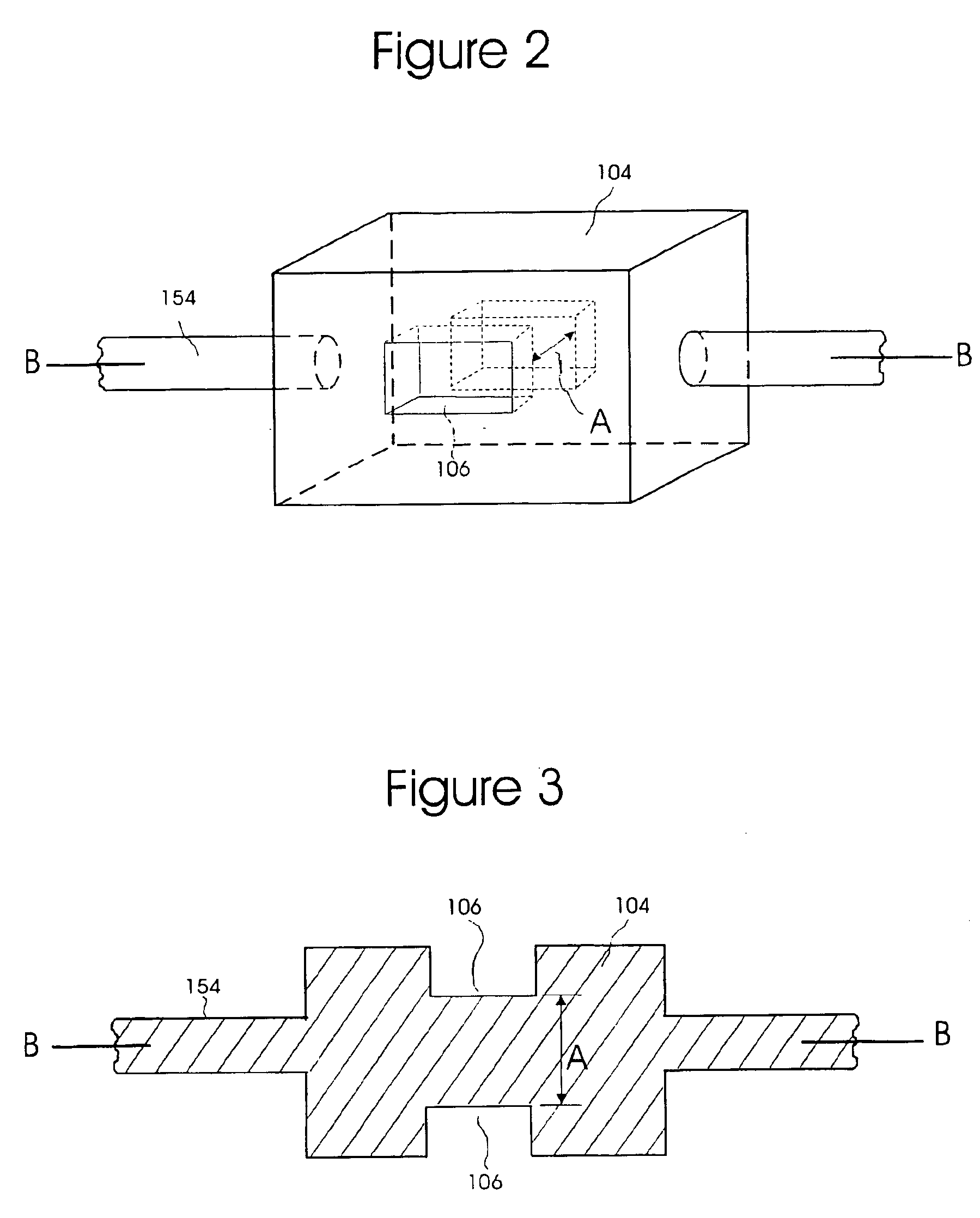
Biosensor, method of forming thin-film electrode, and method and apparatus for quantitative determination
InactiveUS6875327B1Improve convenienceImprove securityImmobilised enzymesBioreactor/fermenter combinationsThin film electrodeQuantitative determination
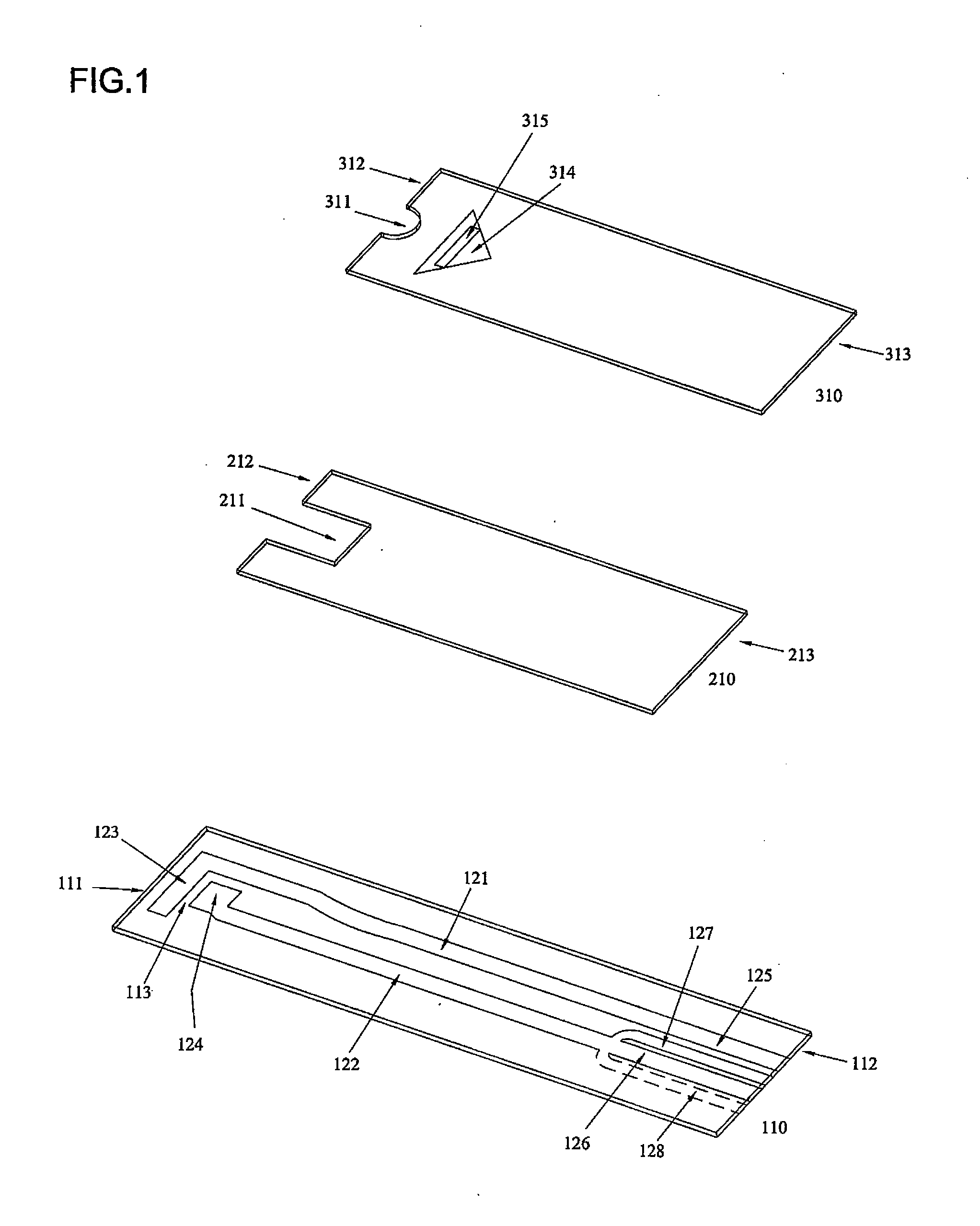
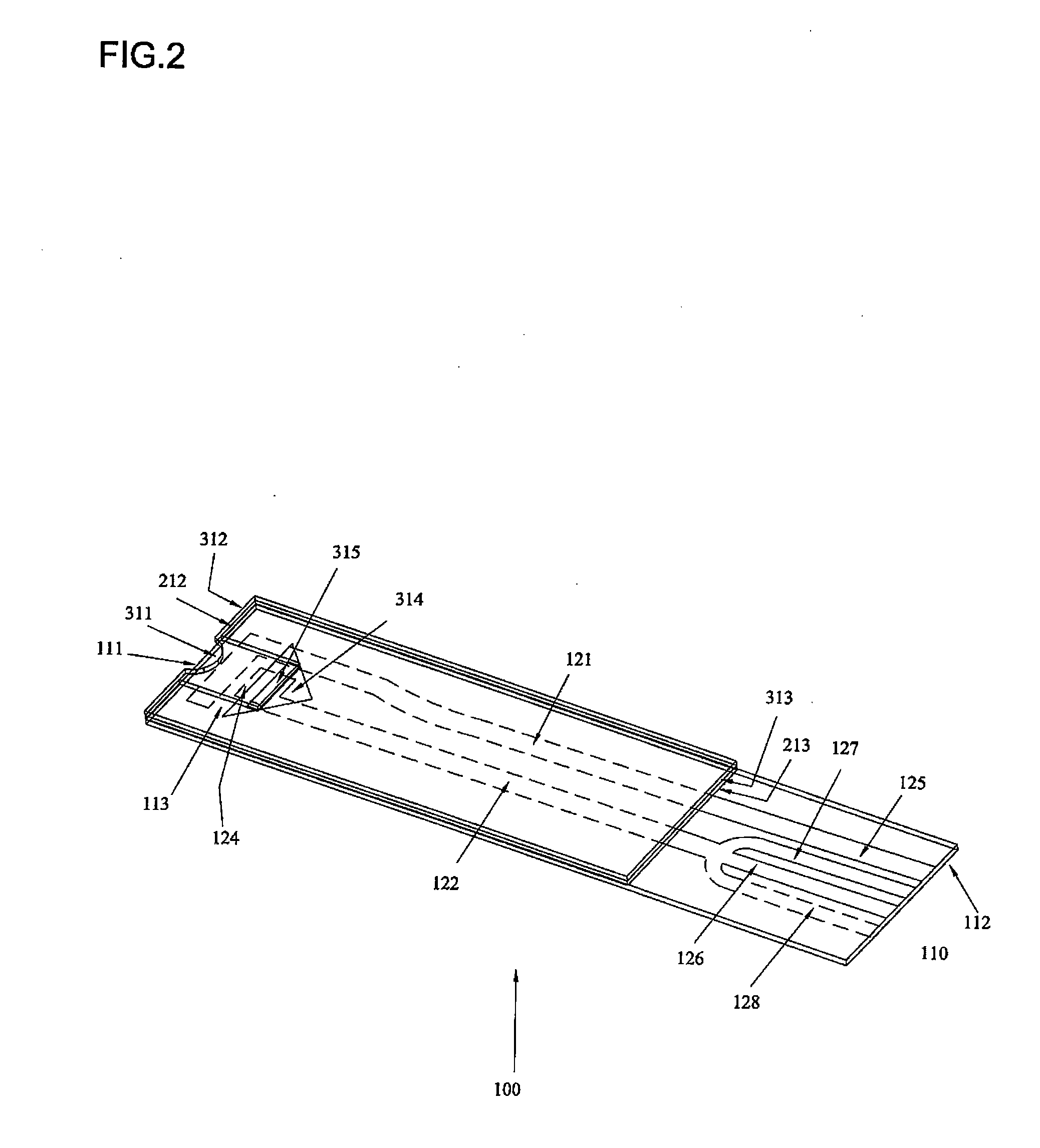
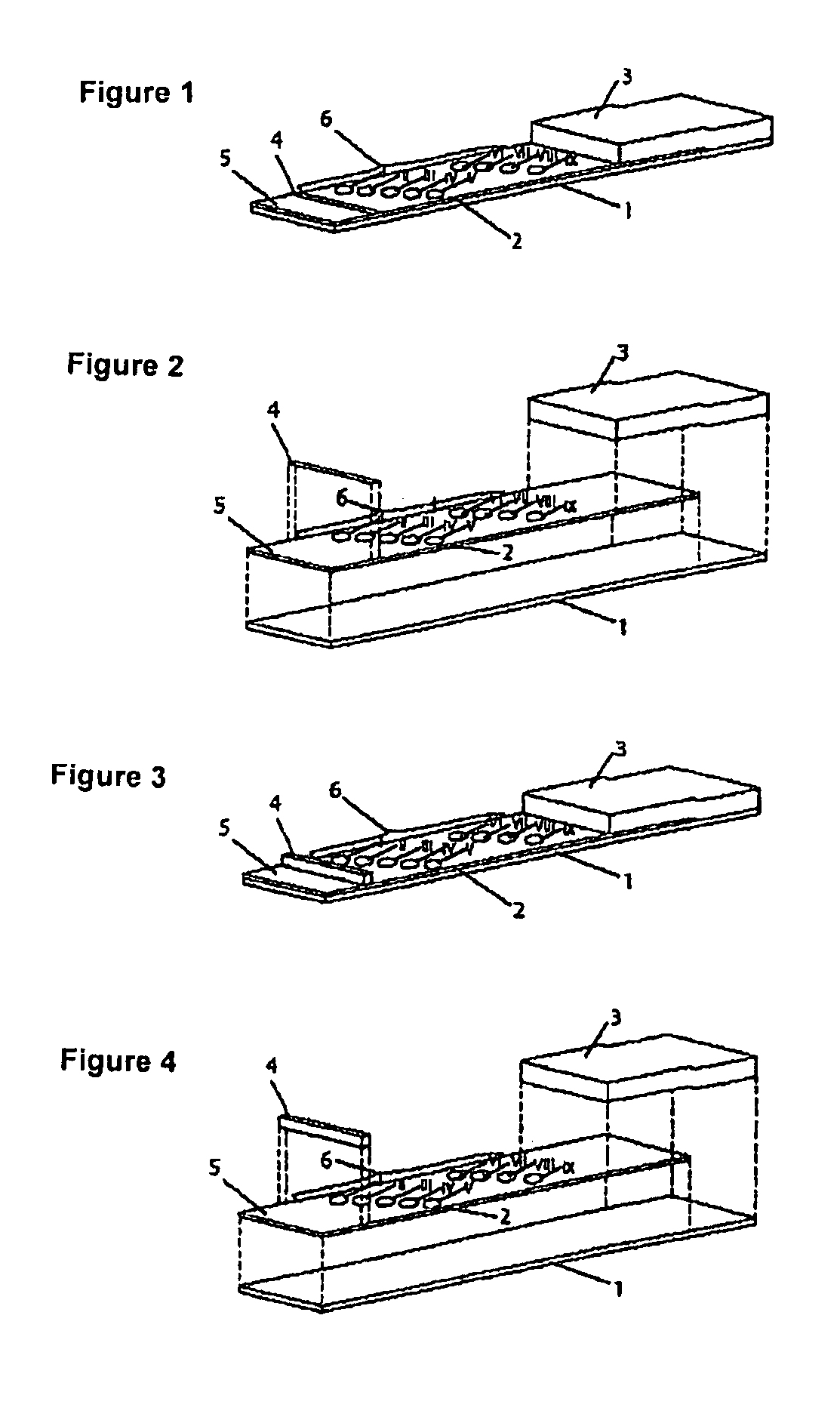
The invention provides a biosensor comprising a support; a conductive layer composed of a electrical conductive material such as a noble metal, for example gold or palladium, and carbon; slits parallel to and perpendicular to the side of the support; working, counter, and detecting electrodes; a spacer which covers the working, counter, and detecting electrodes on the support; a rectangular cutout in the spacer forming a specimen supply path; an inlet to the specimen supply path; a reagent layer formed by applying a reagent containing an enzyme to the working, counter, and detecting electrodes, which are exposed through the cutout in the spacer; and a cover over the spacer. The biosensor can be formed by a simple method, and provides a uniform reagent layer on the electrodes regardless of the reagent composition.
Owner:PHC HLDG CORP
Method and apparatus for electrochemical detection
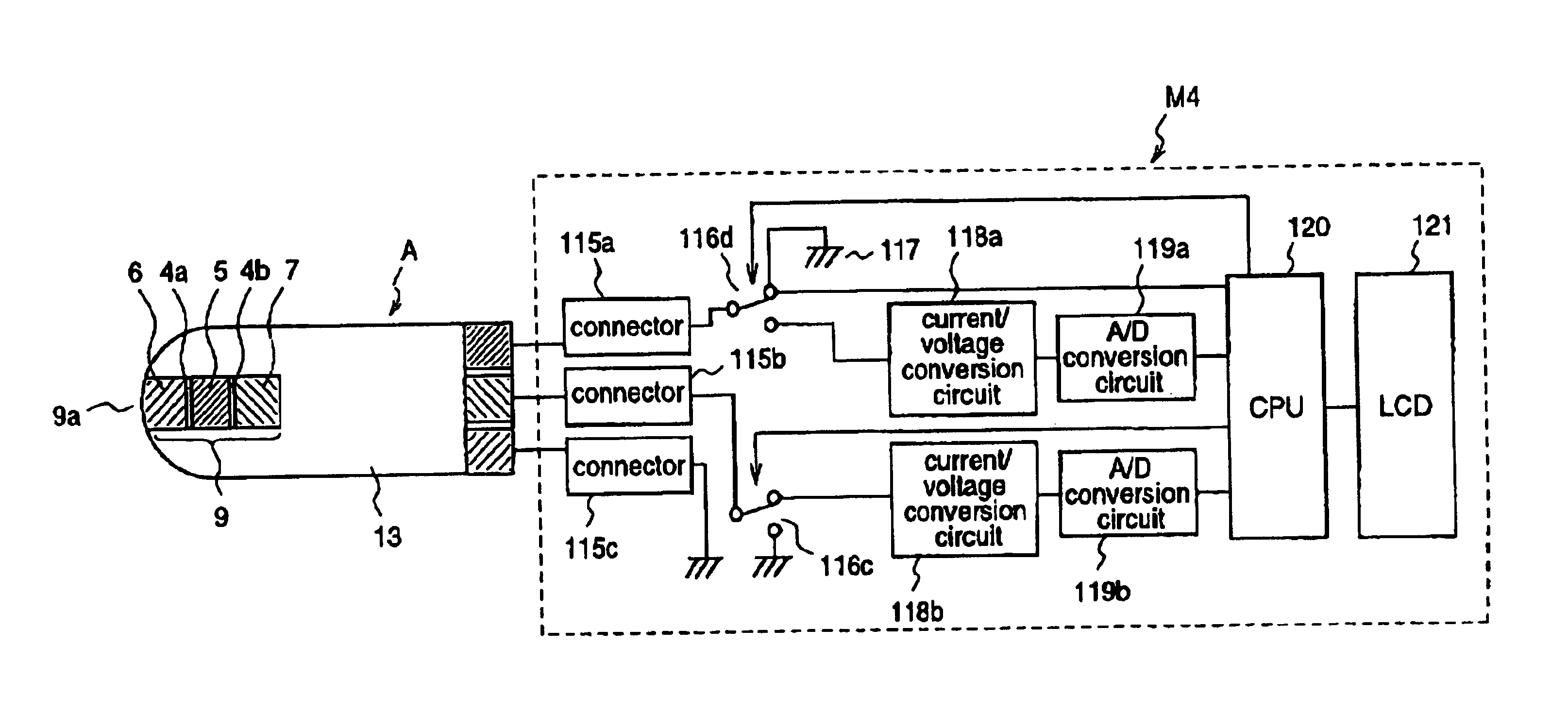
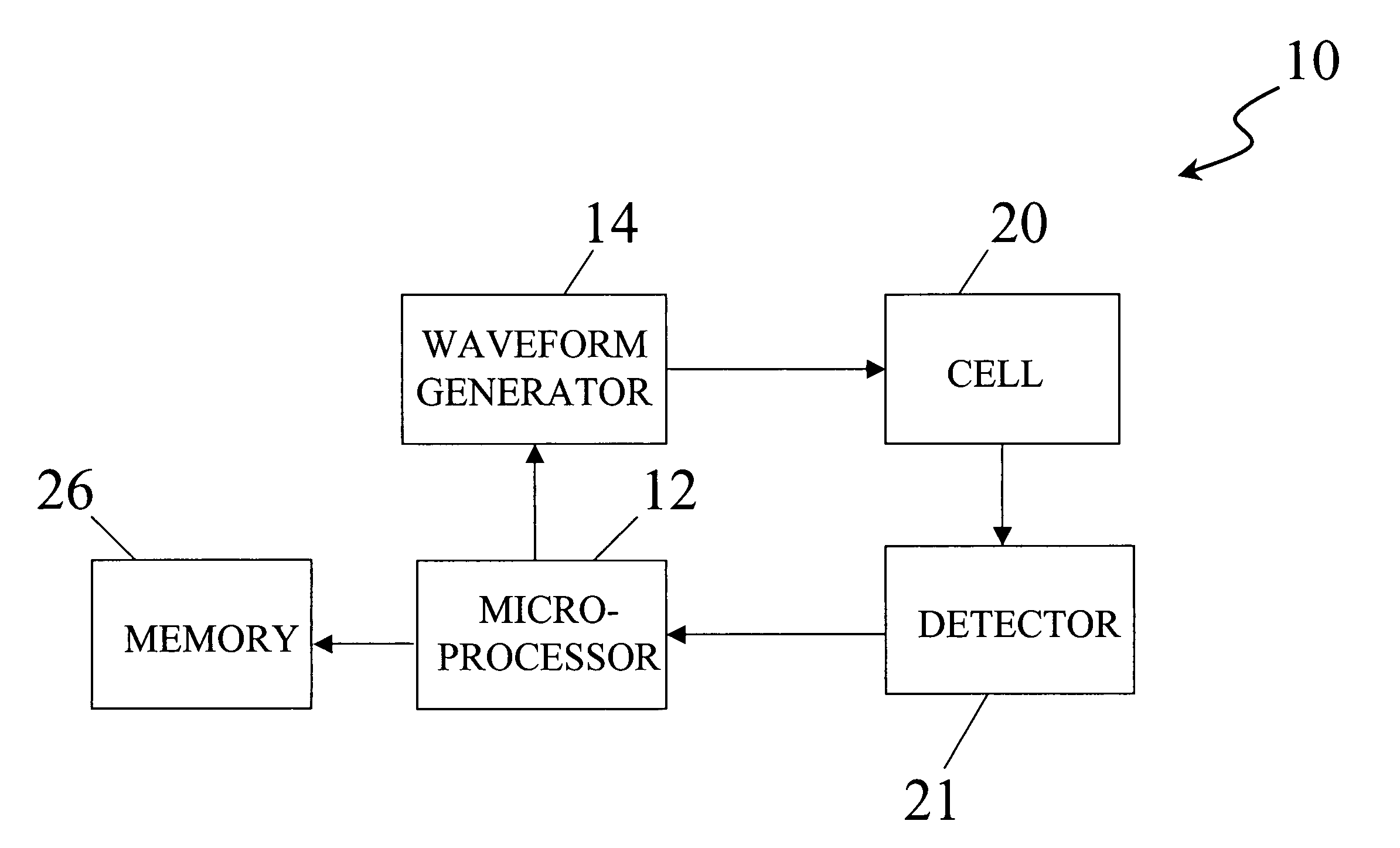
InactiveUS20060016698A1Enhance electrochemical reactionHigh signal resolutionImmobilised enzymesBioreactor/fermenter combinationsAnalyteQuantitative determination
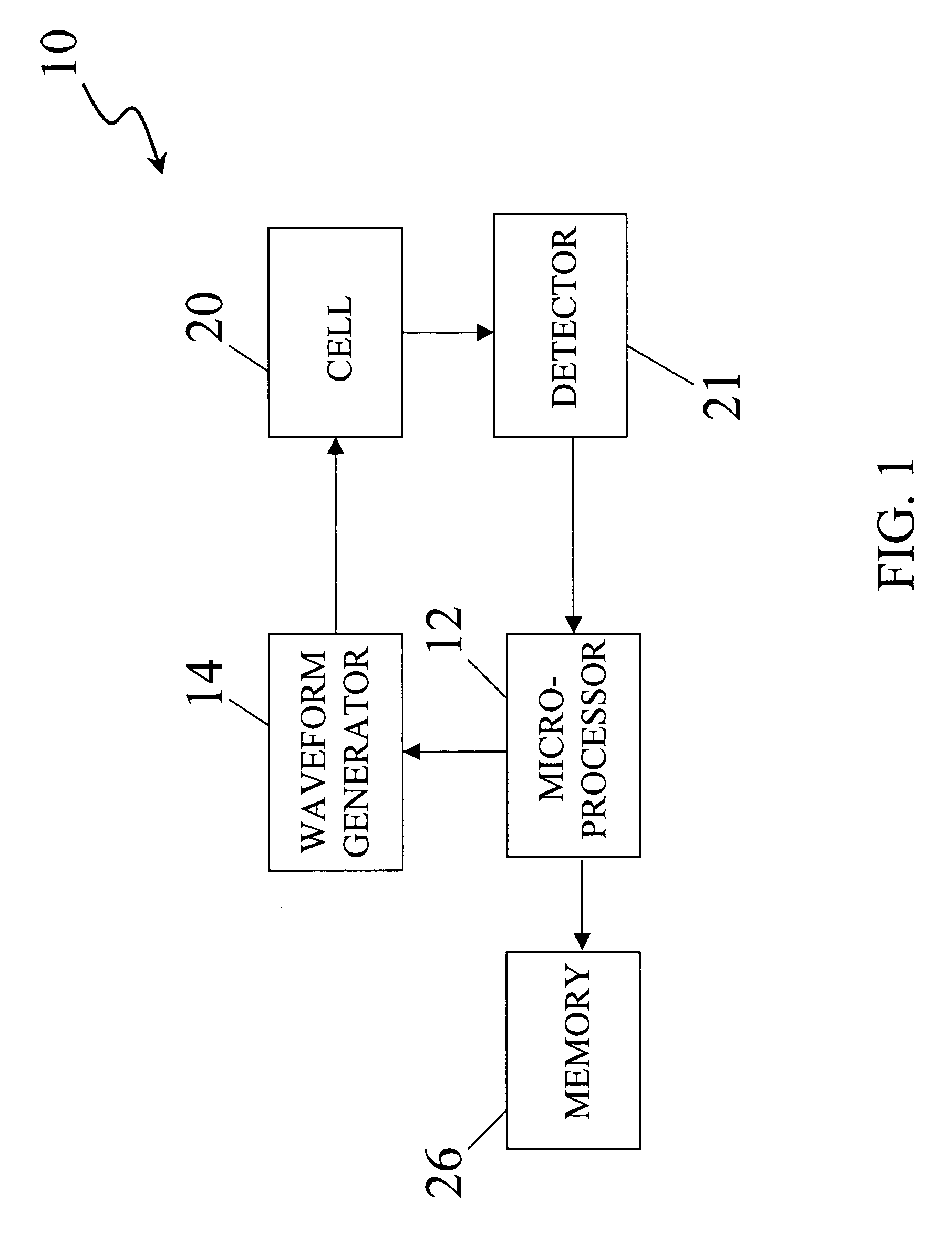
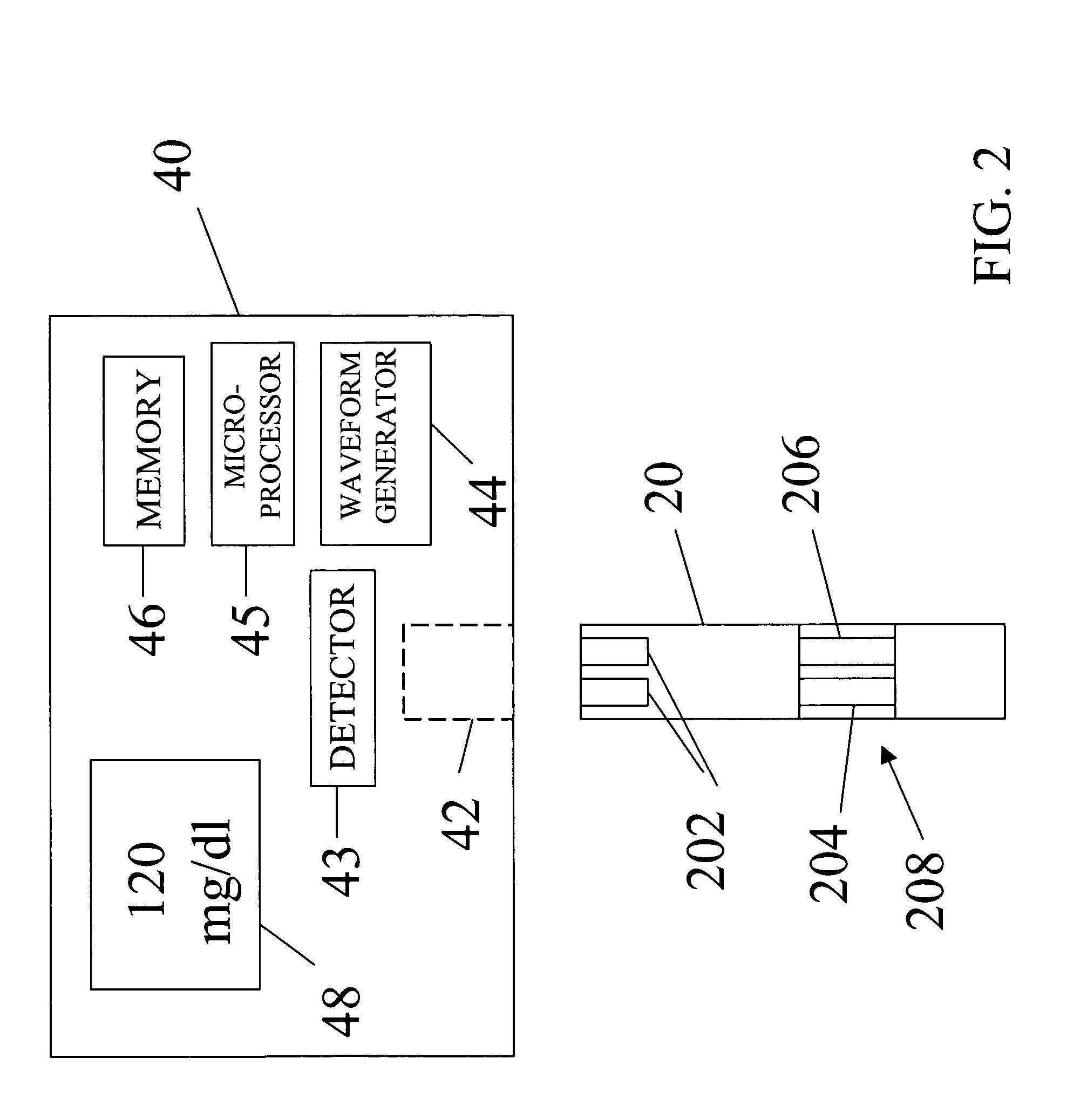
An apparatus for quantitatively determining an analyte in a sample fluid includes a holder for holding an electrochemical cell that includes a catalyst, a waveform generator for generating a potential profile having a voltage bias and an alternating part, a detector for detecting a current signal for a period of measuring time through the electrochemical cell, a memory for storing the current signal, and a processor for correlating the current signals with the concentration of the analyte.
Owner:LEE CHIH KUNG +1
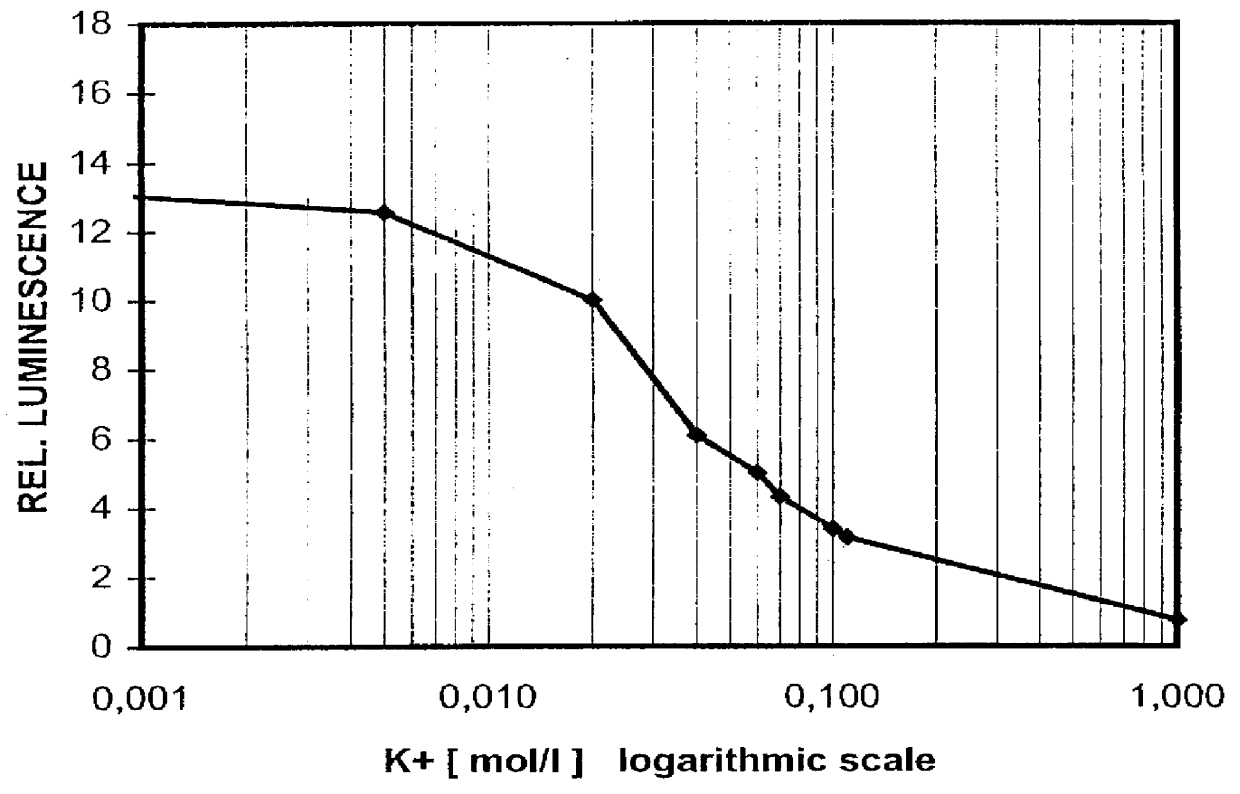
Luminescence-optical method and sensor layer for quantitative determination of at least one chemical component of a gaseous or liquid sample
InactiveUS6046055AEasy to produceMaterial analysis by observing effect on chemical indicatorChemiluminescene/bioluminescenceEnergy transferLuminophore
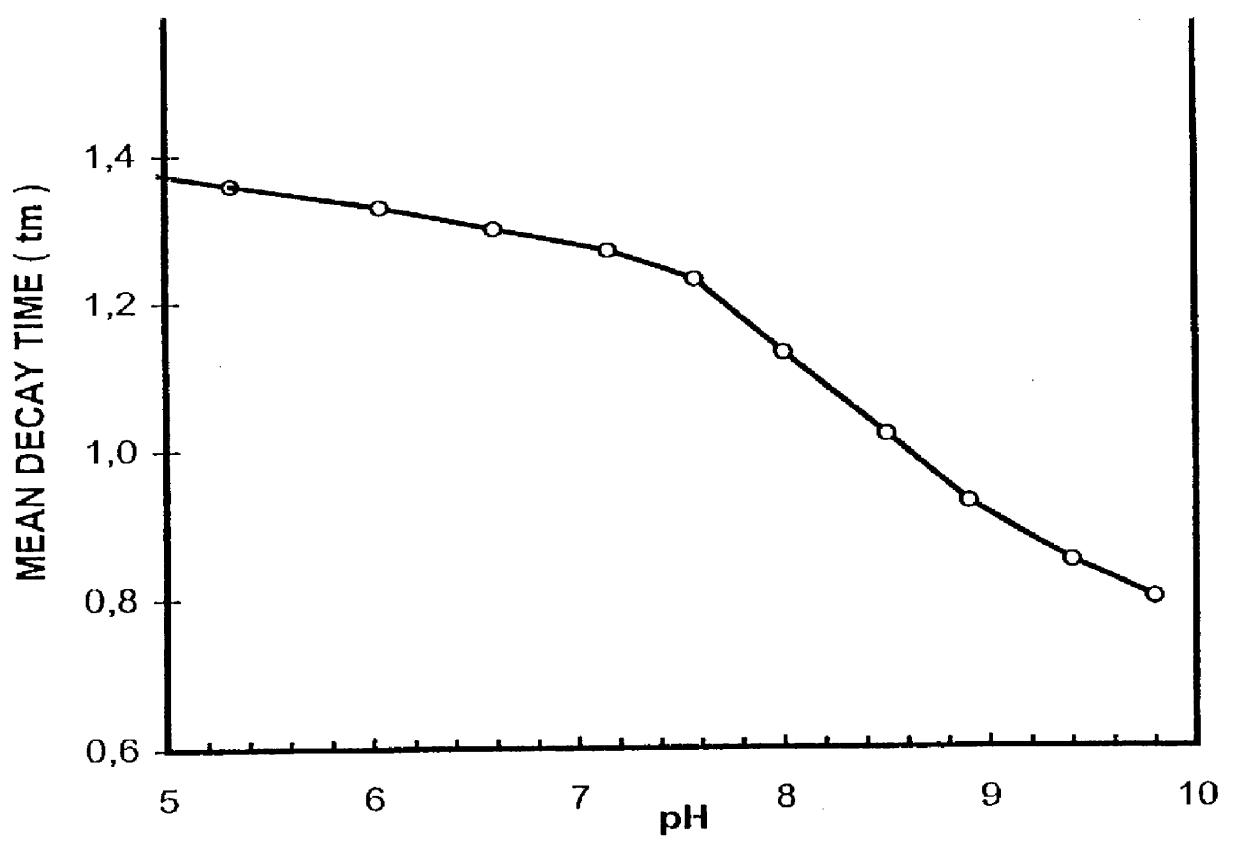
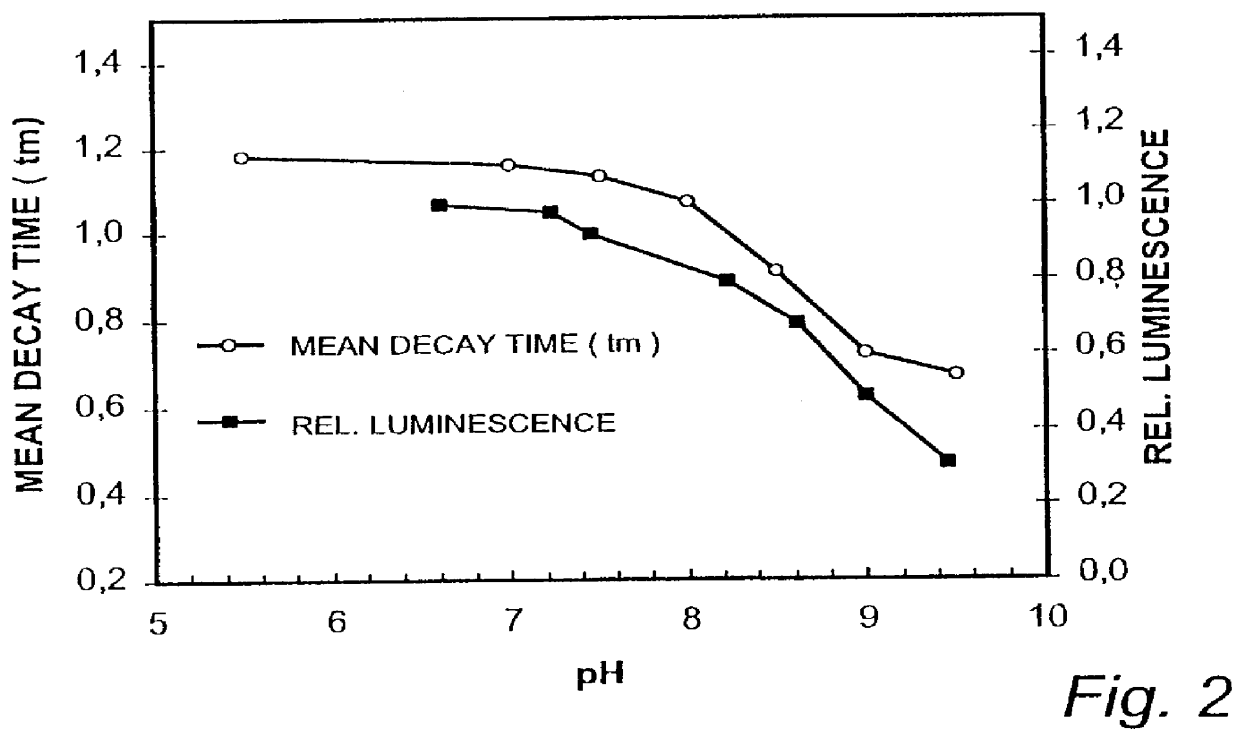
In a sensor layer for quantitative determination of at least one chemical component in a gaseous or liquid sample medium containing a chromophore which is directly or indirectly responsive to the component being determined by changing its absorption spectrum, and a luminophore which is not responsive to the component being determined, where there is an at least partial overlap between the emission spectrum of the luminophore and the absorption spectrum of the chromophore, and where the energy transfer between luminophore and chromophore produces a measurable change in at least one luminescence characteristic of the luminophore, the luminophore L and the chromophore GAMMA are ionic substances with differing electrical charges, which are incorporated in a matrix material that is permeable to the chemical component being determined.
Owner:AVL MEDICAL INSTR
Chemical analysis apparatus and method
InactiveUS6020147ABioreactor/fermenter combinationsAnalysis using chemical indicatorsAnalyteQuantitative determination
A method and apparatus for the quantitative determination of an analyte in a liquid employs a liquid-permeable solid medium defining a liquid flow path. The medium includes a number of reactant-containing reaction zones spaced apart along the flow path and in which reaction occurs with the analyte or an analyte derivative (e.g., a labeled analyte) to result in the formation of a predetermined product. Detector means are employed to detect analyte, analyte derivative, reactant or predetermined product in the reaction zones, the number of such zones in which such detection occurs indicating the amount of analyte in the liquid.
Owner:SURMODICS INC
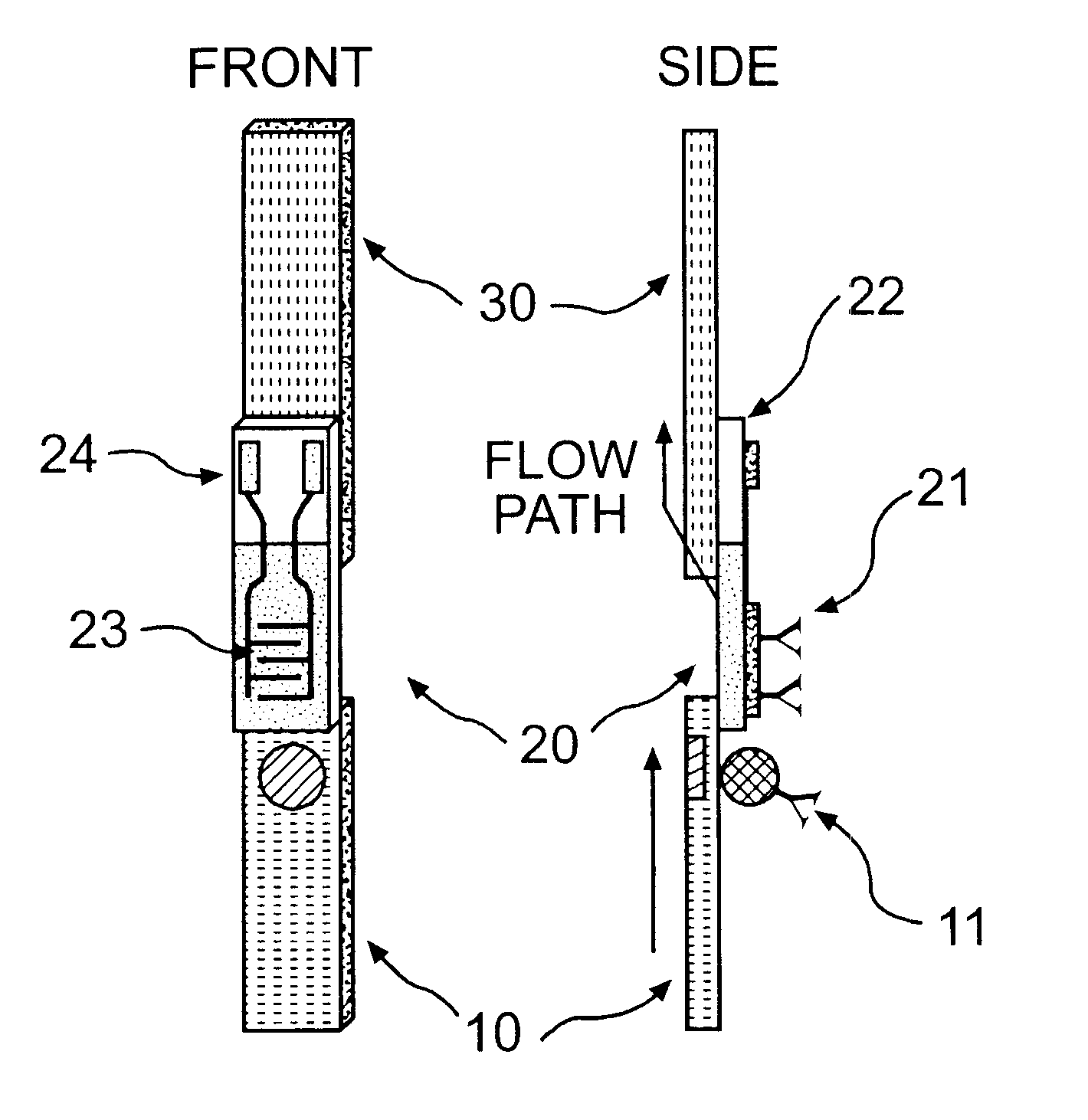
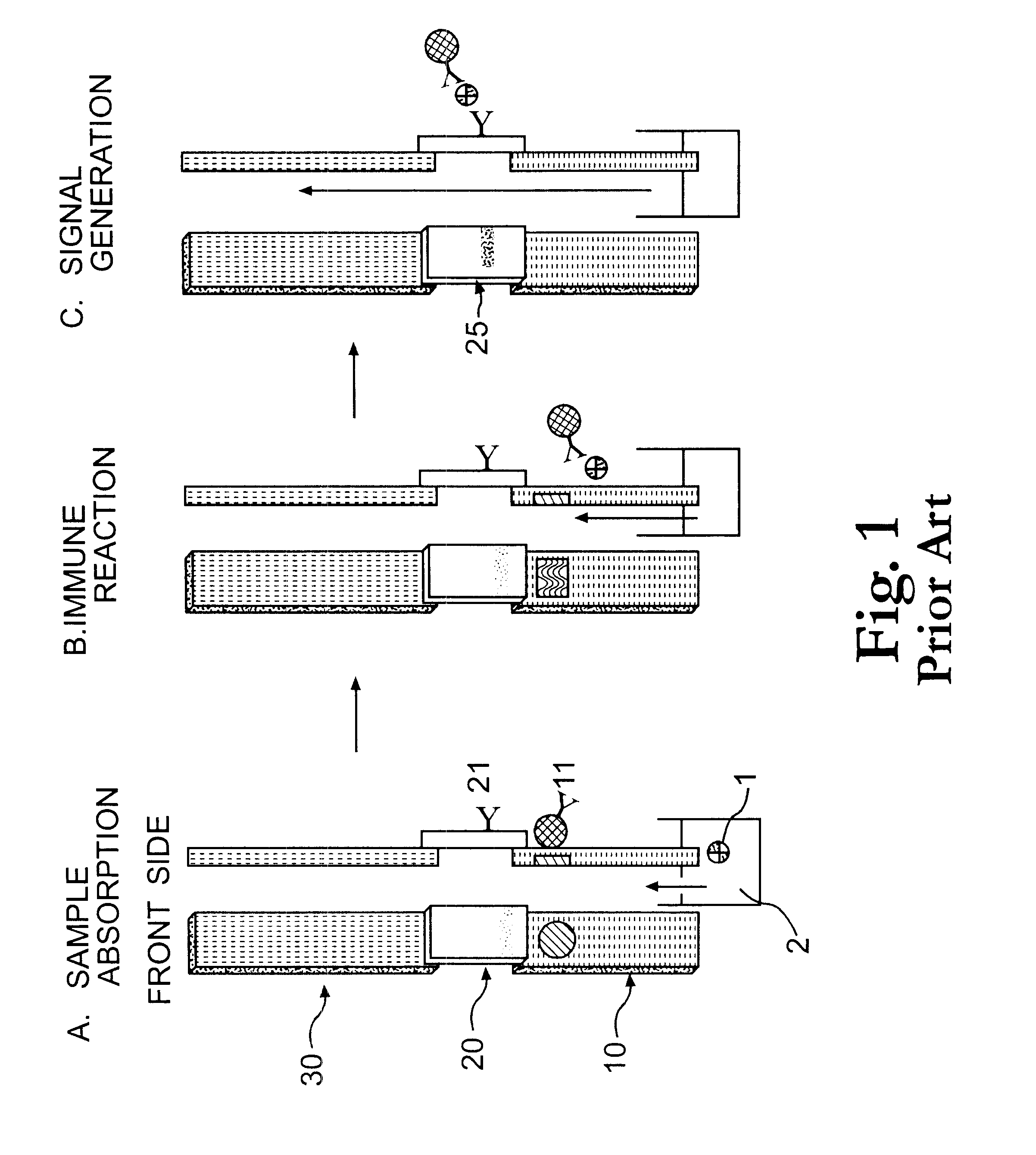
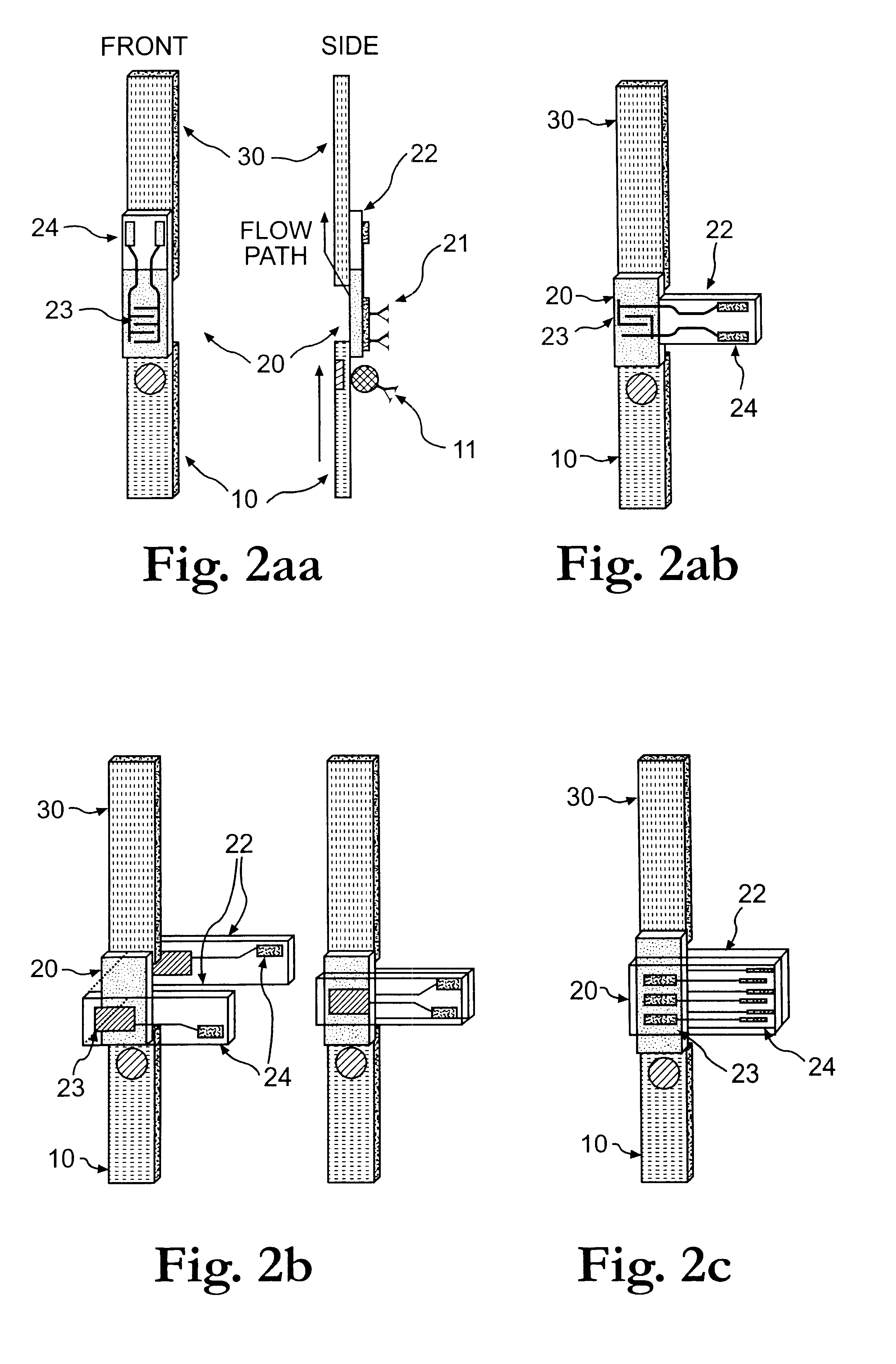
Electrochemical membrane strip biosensor
The present invention is directed to the development of a biosensor based on the immuno-chromatographic method that can provide an assay speed and convenience required for point-of-care (the doctor's office and emergency room) testing or home-version diagnosis. Though certain physical symptoms, such as pregnancy and ovulation, or bacterial infection may be identified by a qualitative analysis for the presence of indicating substances, most analytes for clinical investigation demand their concentrations known in specimens. Therefore, the inventors of the present invention have developed a novel biosensor by combining the immuno-chromatographic method and the electric conductivity detection technology so that on-site quantitative determination at the points of care or at home may be carried out.
Owner:BIODIGIT LAB CORP
Method and apparatus for quantitative microimaging
InactiveUS20120224053A1Easy to operateSimple designMaterial analysis by optical meansLaboratory glasswaresQuantitative determinationAnalytical chemistry
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Electrochemical test strip for multi-functional biosensor
InactiveUS20070193882A1Reduce the air bubbles trappedImmobilised enzymesBioreactor/fermenter combinationsSmall sampleAnalyte
The present invention provides an electrochemical biosensor test strip used for quantitative determination of an analyte in a liquid sample. Methods of fabricating the test strip and reagent formulas are also provided. Improvements in electrochemical test strips are required to detect the presence of a compound in a liquid mixture using a smaller sample size with increased accuracy. The present invention was developed to be user-friendly, decrease sample requirements and decrease analyzing time while increasing the reproducibility and accuracy of the electrochemical test strip.
Owner:VISGENEER
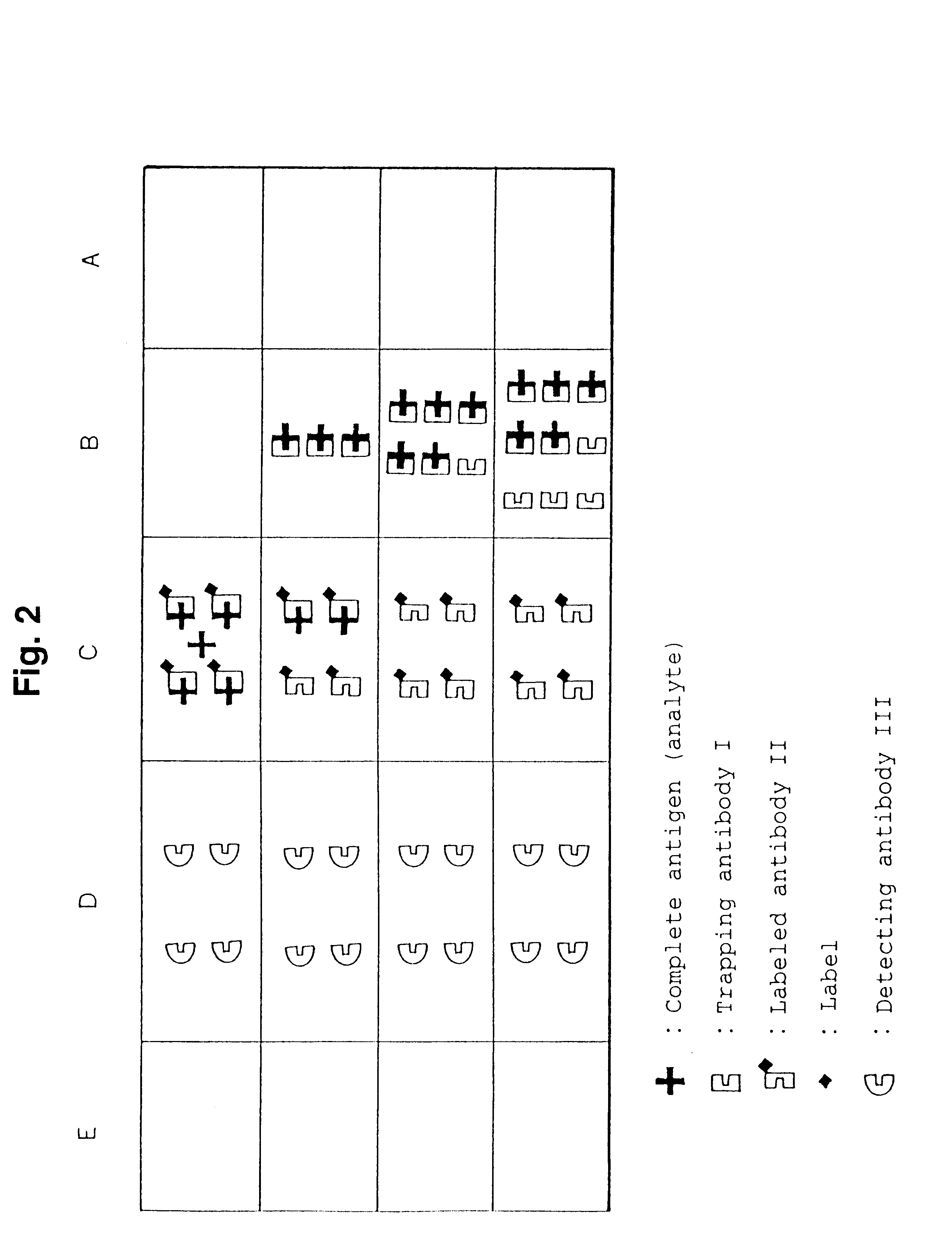
Simple immunochemical semi-quantitative assay method and apparatus
InactiveUS6177281B1Easy to adjustHigh sensitivityBioreactor/fermenter combinationsAnalysis using chemical indicatorsAnalyteQuantitative determination
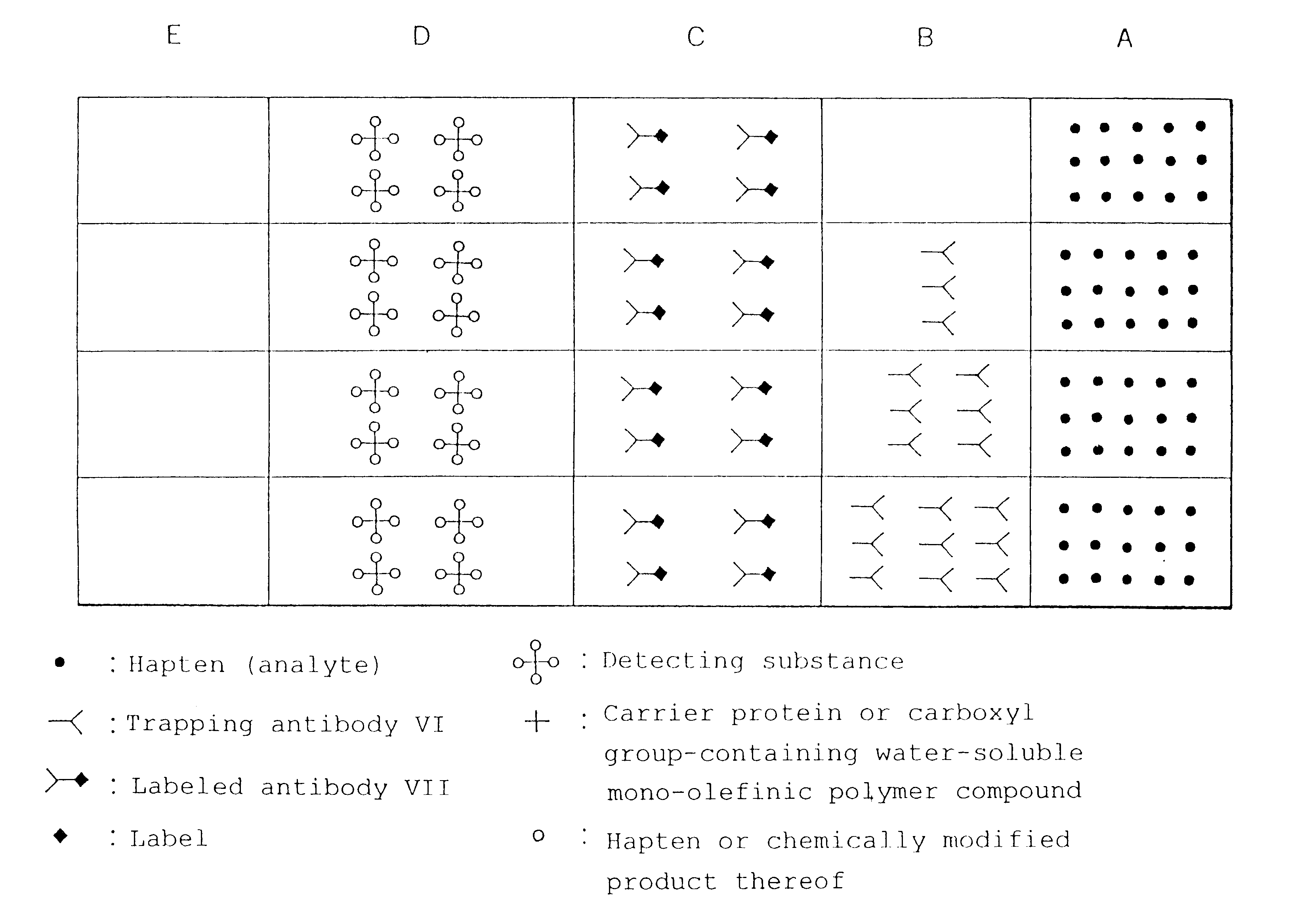
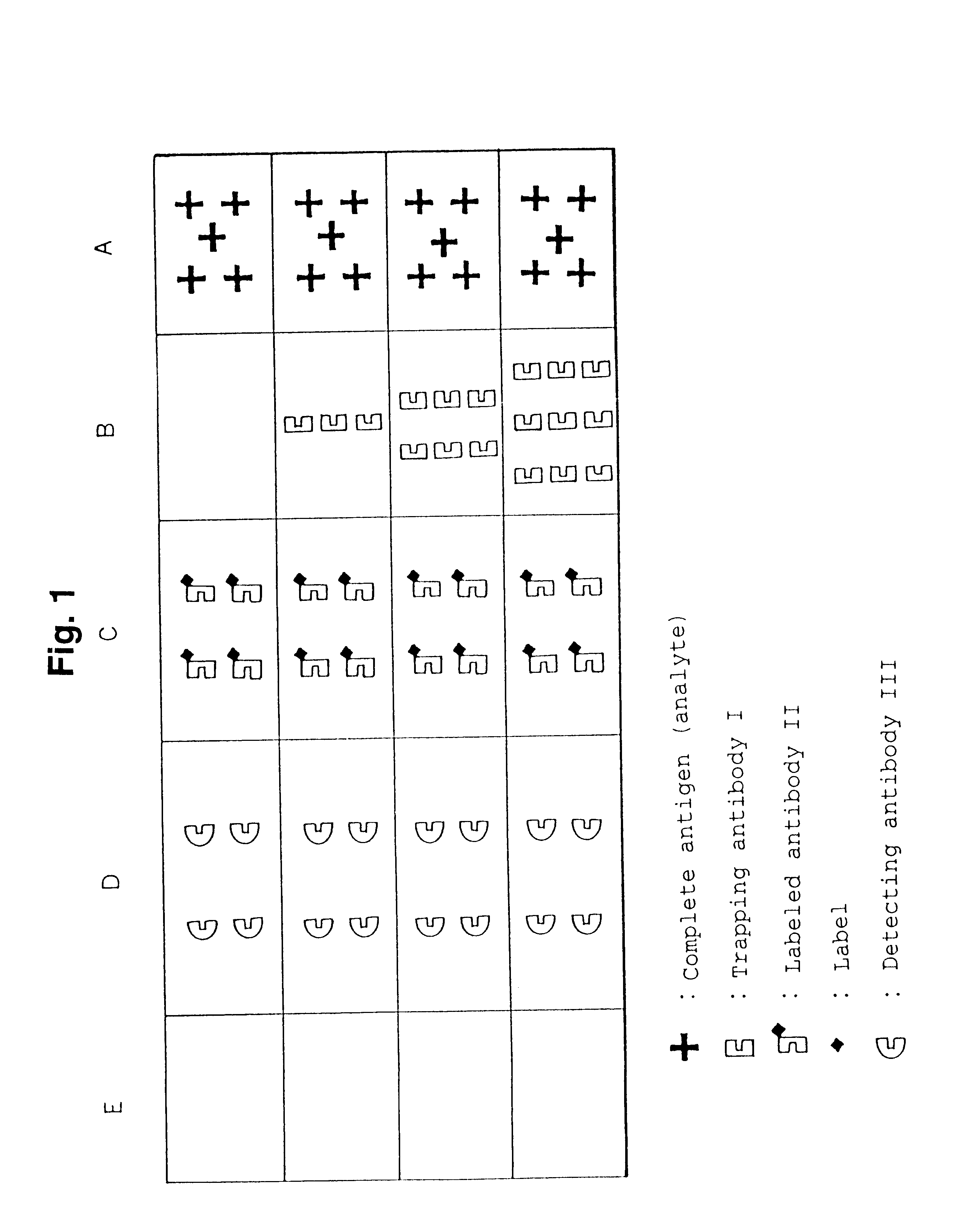
The present invention relates to a simple immunochemical semi-quantitative assay method according to chromatography, which comprises trapping a certain amount of an analyte in a sample with a predetermined amount of a fixed antibody for the analyte before qualitative analysis of the analyte, the certain amount corresponding to the amount of the fixed antibody, and thereby decreasing a concentration of the analyte to be subjected to subsequent immunochemical qualitative determination, and an apparatus therefor.
Owner:TEIKOKU HORMONE MFG
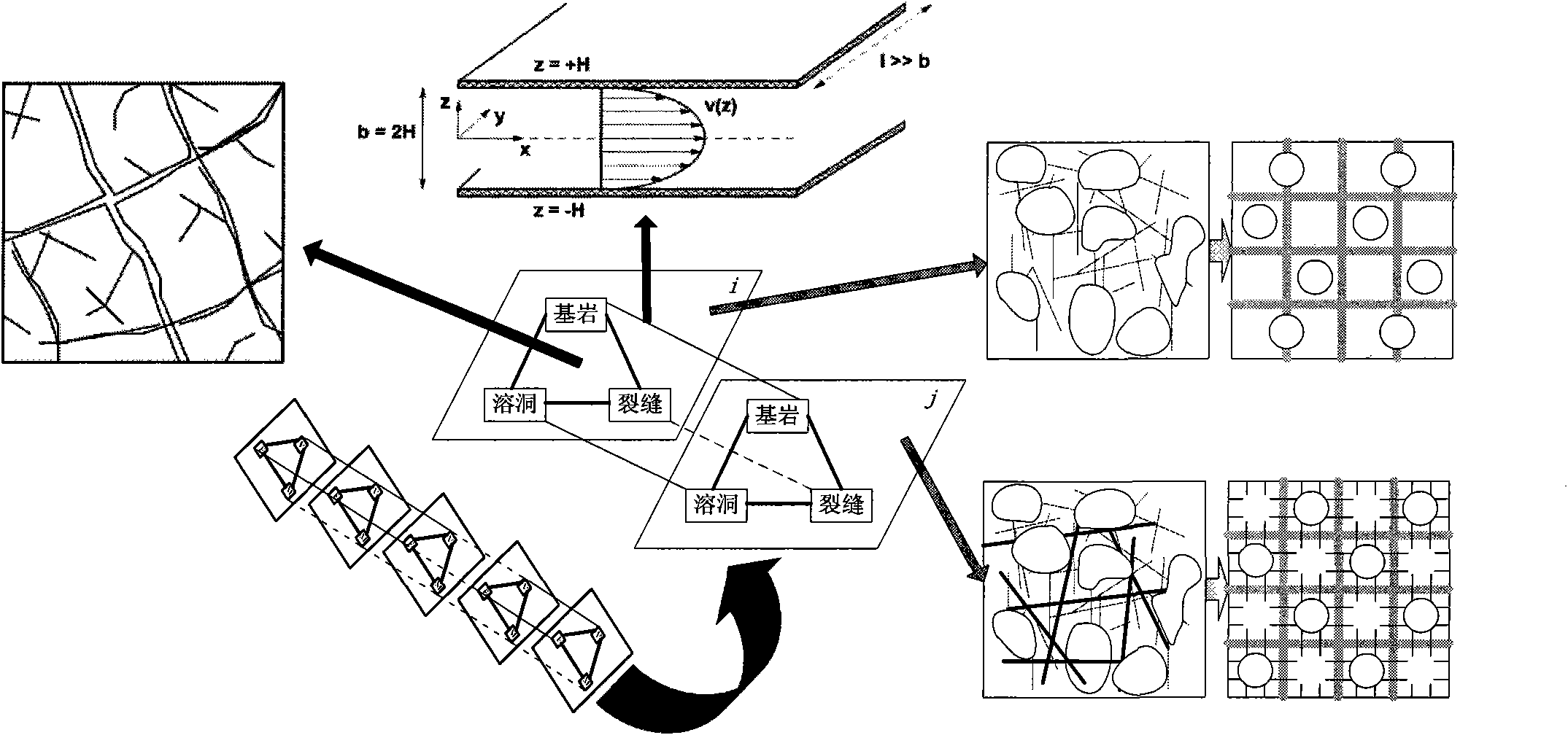
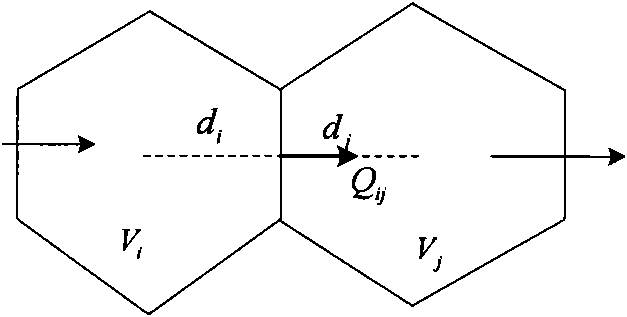
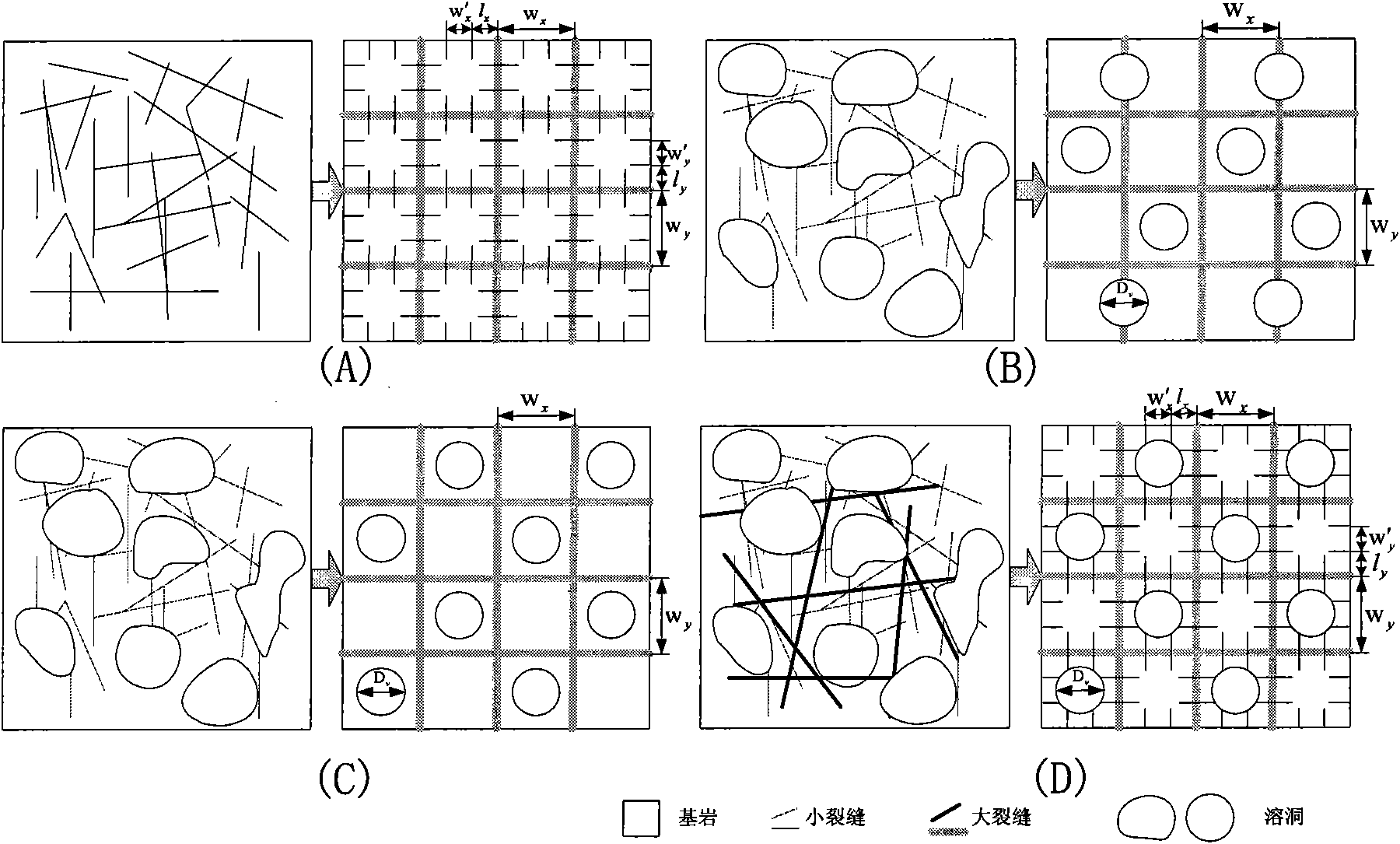
Method for analyzing remaining oil distribution of fractured-vuggy reservoir
ActiveCN102339339AScientific and rational developmentAchieve scientific descriptionSpecial data processing applicationsQuantitative determinationOil field
The invention provides a method for analyzing remaining oil distribution of a fractured-vuggy reservoir, belonging to the fields of numerical reservoir simulation and oil-gas field development. In the method, a complex medium consisting of a cave medium, a crack medium and a pore medium is partitioned into a plurality of space unit blocks in a space field; each block consists of V, F and M units which represent a cave, a crack and a substrate in the block respectively and constitute a V-F-M model; the flow of a multi-phase fluid in the complex medium is described by the motion of a fluid among the units in each block and the motion of a fluid among the units of different blocks; and the flow of the fluid among the units can be considered as infiltration flow, pipe flow or laminar flow between parallel walls, Darcy flow or non-Darcy flow. According to the method, scientific description and accurate numerical simulation of the fractured-vuggy reservoir are realized, and technical foundations are laid for the alignment of the remaining oil distribution position of the fractured-vuggy reservoir with a numerical simulation technology, quantitative determination of the reserves abundance of the reservoir, scientific and reasonable development of oil fields provided with the reservoir and final increase in the recovery ratio.
Owner:CHINA PETROLEUM & CHEM CORP +1
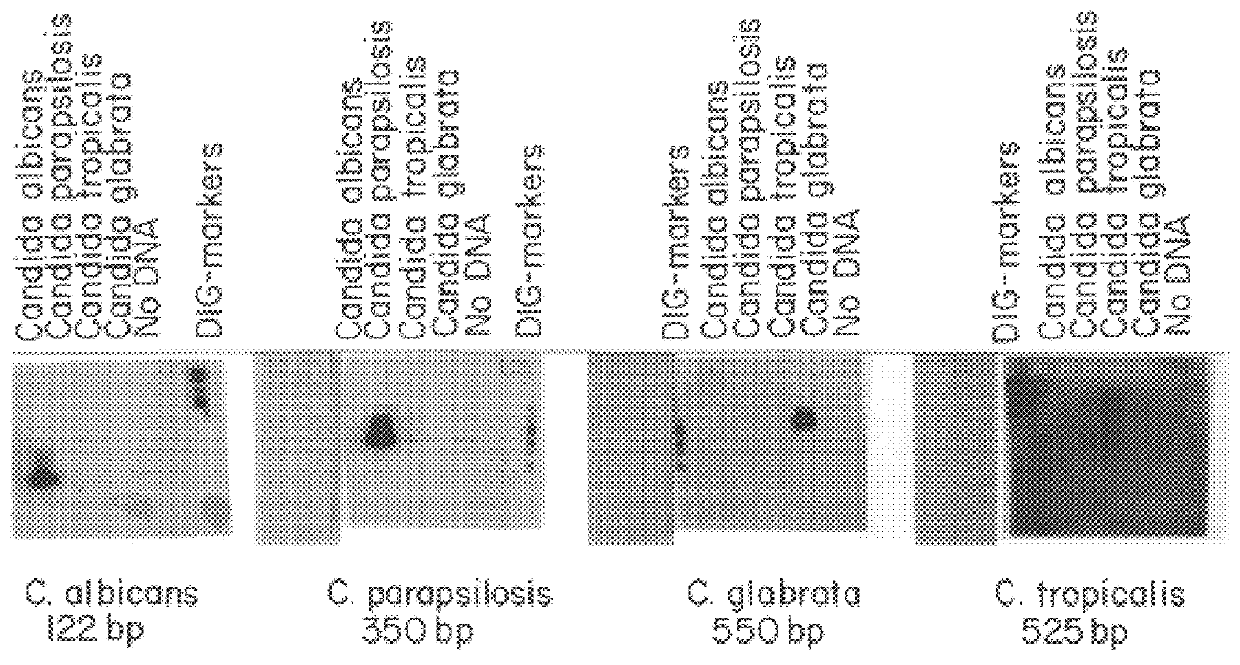
PCR identification and quantification of important Candida species
InactiveUS6017699AConvenient amountHigh sensitivitySugar derivativesMicrobiological testing/measurementQuantitative determinationCombined use
The subject invention relates to a set of DNA primers which, when utilized in conjunction with the polymerase chain reaction (PCR) assay, can amplify and speciate DNA from five medically important Candida species. Furthermore, the PCR amplified products, generated by the primers, can also be used to create species specific probes which can also detect and confirm the five species of Candida. Thus, the present invention allows for early diagnosis and treatment of an infection. The assay is useful in the context of monitoring antifungal treatment regimens, screening potential antifungal agents, and similar applications requiring quantitative determinations.
Owner:UNIV OF PITTSBURGH THE
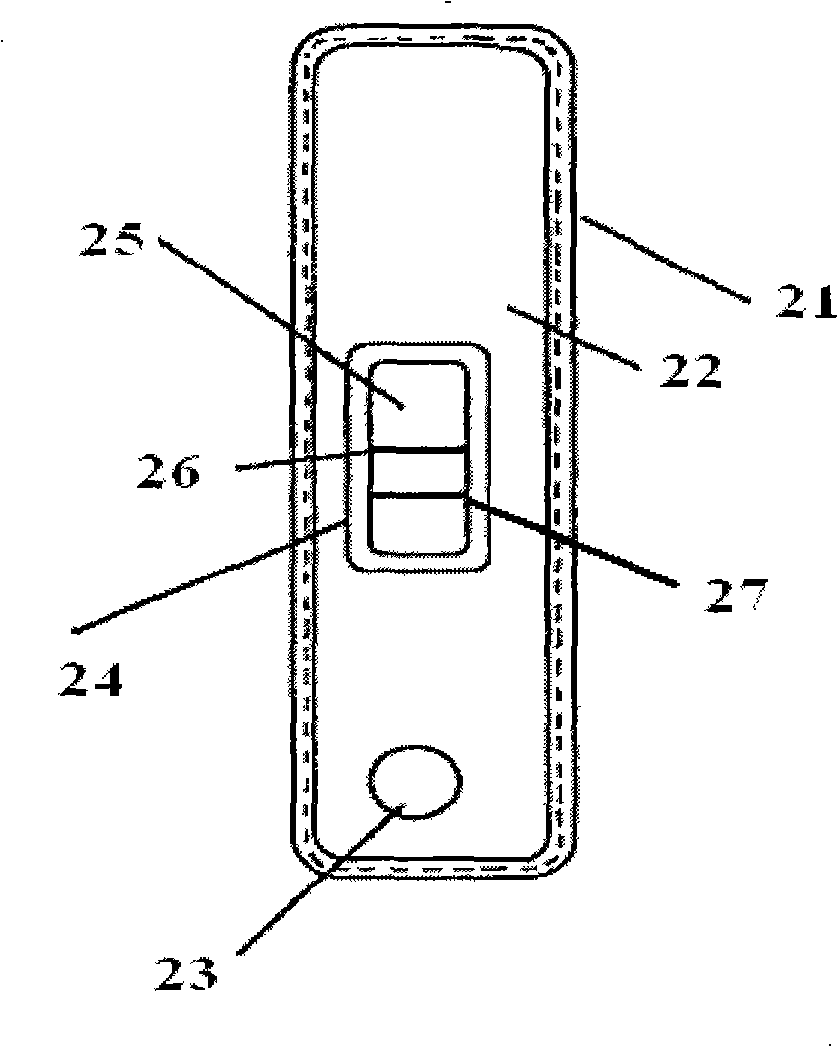
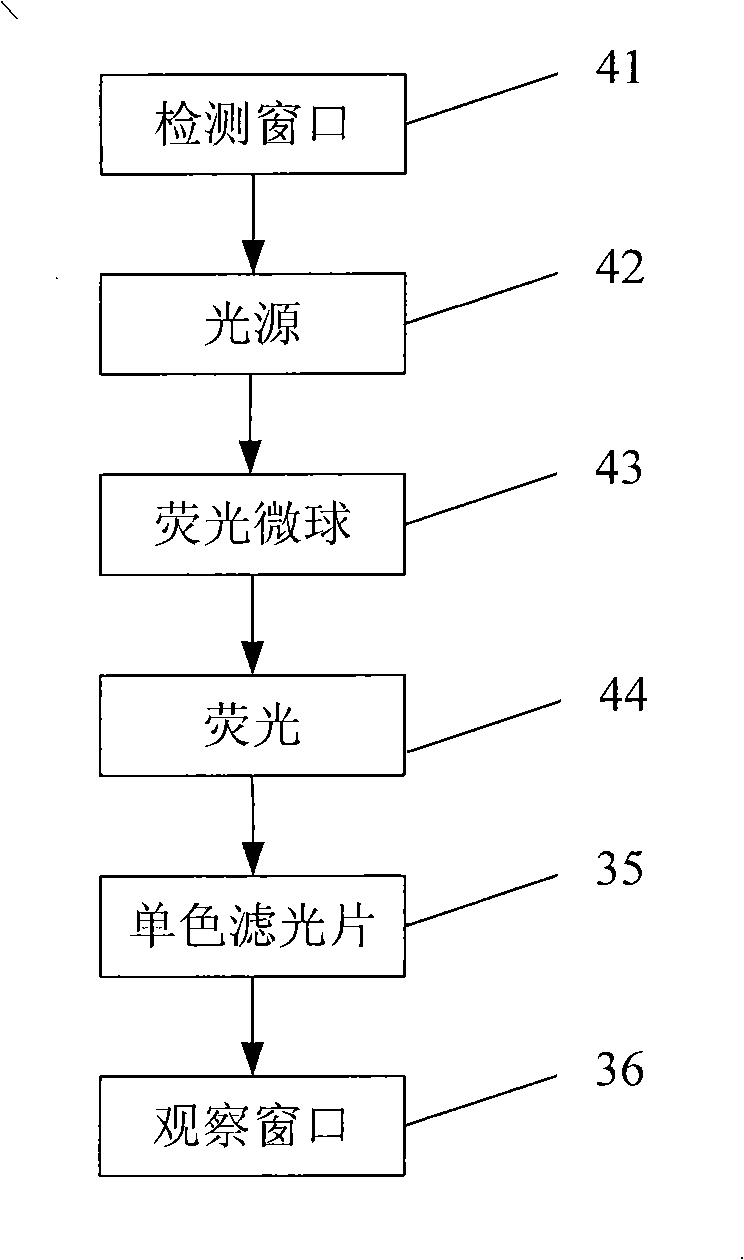
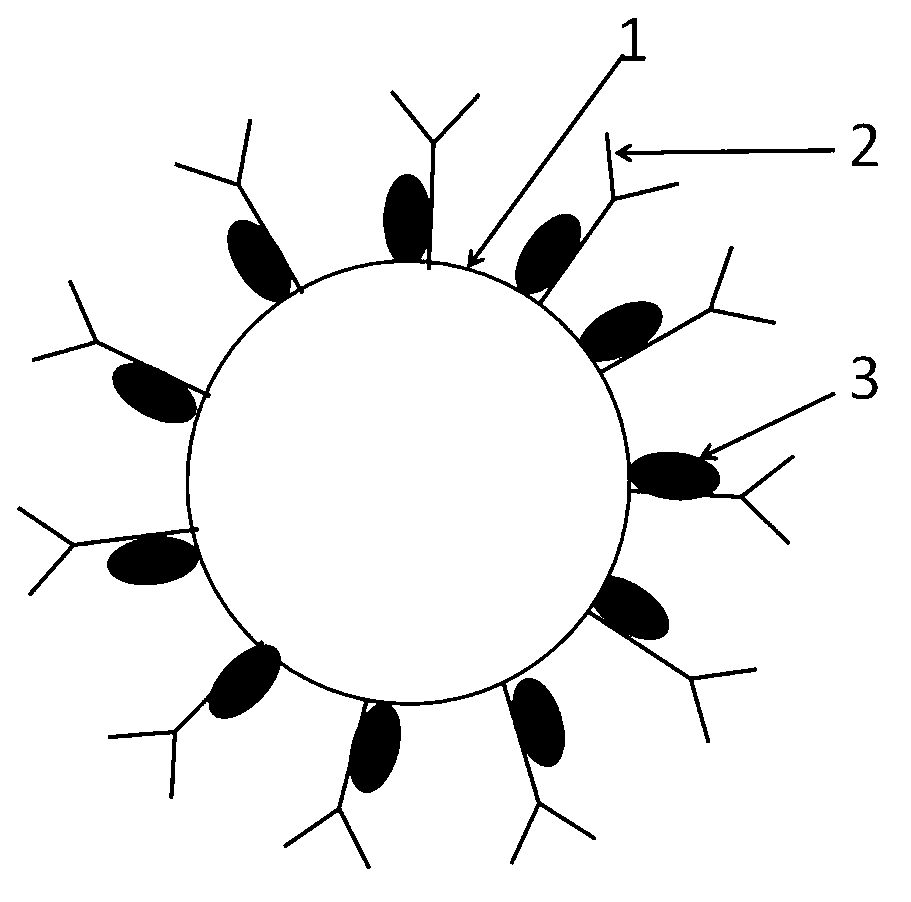
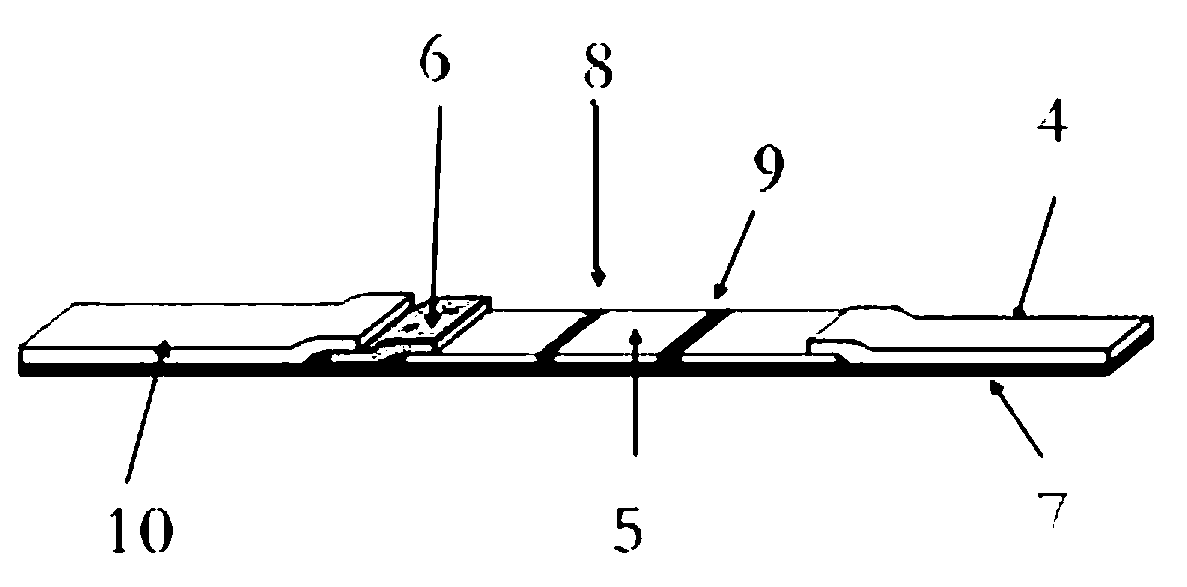
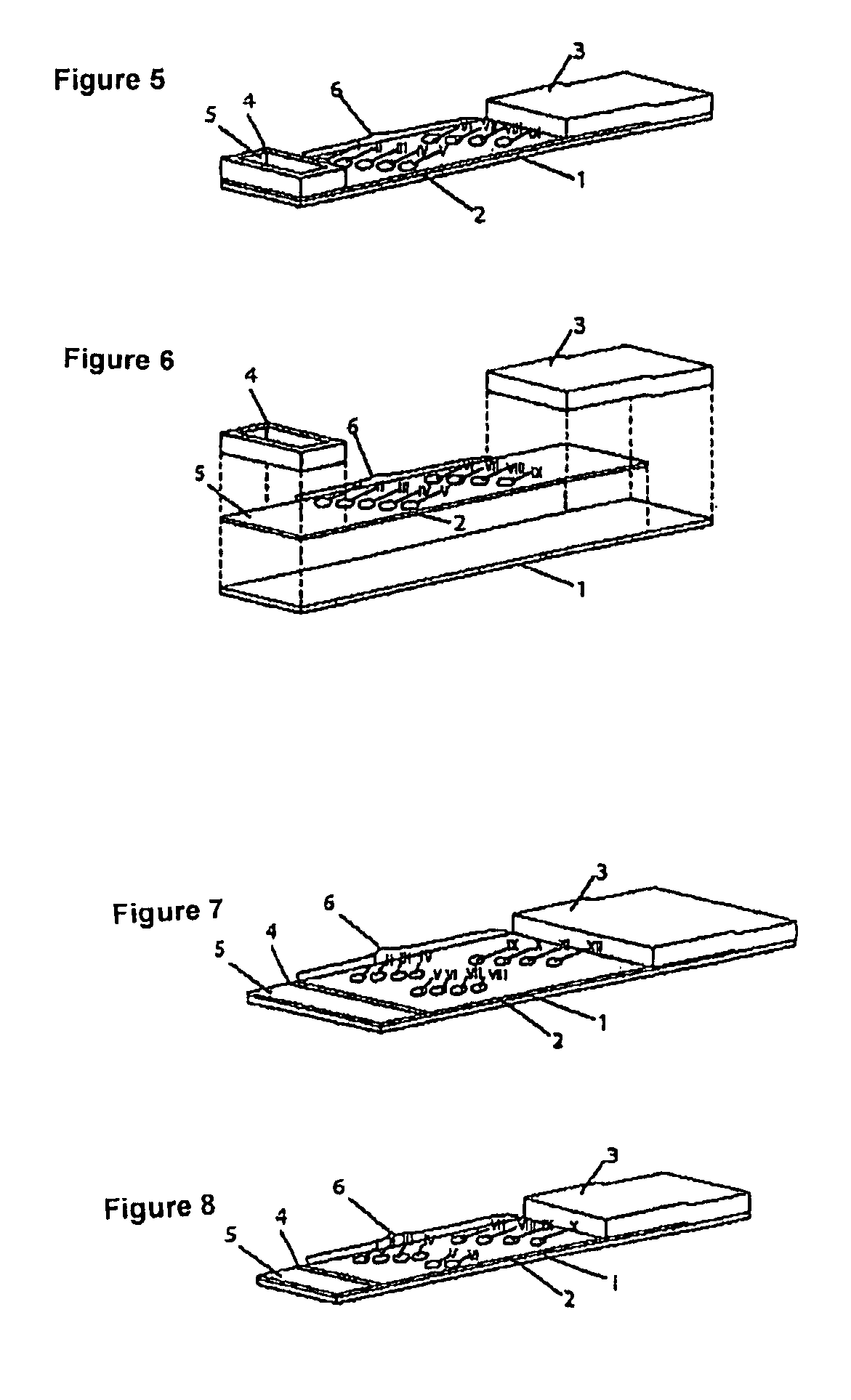
Method for producing fluorescent microballoons immune chromatography test paper stripe and quantitative determination method
ActiveCN101493460ASimple and fast operationHigh sensitivityFluorescence/phosphorescenceFiberCompound organic
The invention discloses a method for preparing fluorescent microspheres immunochromatographic test paper strip and quantitative detection method. The invention takes the luminous nano-particles of dual-structure silicon dioxide compound organic dye as a marker, uses the immunochromatographic technology for preparing fluorescent microspheres immunochromatographic test paper strip, and then prepares a detection card which consists of a sample pad, a glass fiber membrane, a nitrocellulose membrane and absorbent paper, wherein the nitrocellulose membrane is fixedly provided with a detection line and a quality control line. In the detection process, the best excitation light souce of fluorescent microspheres is used for excitation; after the emitted fluorescence passes through a filter, a CCD scanning technology or fiber-optic technology is used for collecting, accumulating or multiplicating the emitted spectra which is then converted into a numerical signal; then the measured fluorescence intensity of the detection line is multiplied by a correction coefficient, and later the corrected fluorescence intensity is substituted in a standard curve which is preset in a fluorescence analyzer; and finally, the concentration of an object to be measured in the sample can be automatically calculated and obtained by the fluorescence analyzer. The invention has high sensitivity, accurate quantization and easy operation.
Owner:江西中德生物工程股份有限公司
Immunochromatography quantitative determination reagent based on near infrared fluorescence nanoparticle markers
ActiveCN103197074AImprove chromatography propertiesHigh detection sensitivityMaterial analysisMicrosphereChemical products
The invention relates to a method based on near infrared fluorescence molecules and nano particle marks as well as an immunochromatography quantitative detection reagent based on near infrared fluorescence nanoparticles. According to the invention, infrared fluorescence is connected with nanoparticles to prepare an immunochromatography test strip based on the near infrared fluorescence nanoparticles. During the detection, an infrared light scanner is utilized, and a quality control line and a sample line are respectively scanned by utilizing near infrared light; and after the fluorescence intensity of a detection line is rectified by utilizing the fluorescence intensity of a quality control line, and a standard curve in a fluorescence analyzer is taken in, the concentration of a to-be-detected matter in a sample can be analyzed and detected. Compared with the direct connection of the near infrared fluorescence molecules and detecting molecules, the near infrared fluorescence nanoparticle immunochromatography improves the detection flexibility obviously, and the lowers the background fluorescence intensity. The marking method and the reagent can be applied to microorganism detection, food safety detection, poison detection as well as rapid dangerous chemical product detection.
Owner:BEIJING RUNBO FUDE BIOLOGICAL TECH DEV
Lanthanide Chelates and Use Thereof in Bioanalysis
InactiveUS20110136242A1Improve stabilityGood spectral characteristicsChemiluminescene/bioluminescenceGroup 3/13 element organic compoundsFluorescenceQuantitative determination
Novel chemical compounds, with application in fluorometric analytical methods, for qualitative and quantitative determination of biomolecules. The aim of the invention is to identify and prove the suitability of such compounds. Said aim is achieved with compounds of formula (1) where R′ is an antenna function, R2 is a chelate forming agent, containing a coordinated lanthanide(III)ion, X is —OH or a group with affinity for the biomolecule, bonded to a carboxylate group of the chelate forming agent by means of an amide bond and Y is —H or a group with affinity for the biomolecule, coupled to the antenna function.
Owner:SENSIENT IMAGING TECH GMBH
Pipette, apparatus and kit for light measurement and method
InactiveUS20120224179A1Sure easyPrevent leakageTransmissivity measurementsBurettes/pipettesEntry pointQuantitative determination
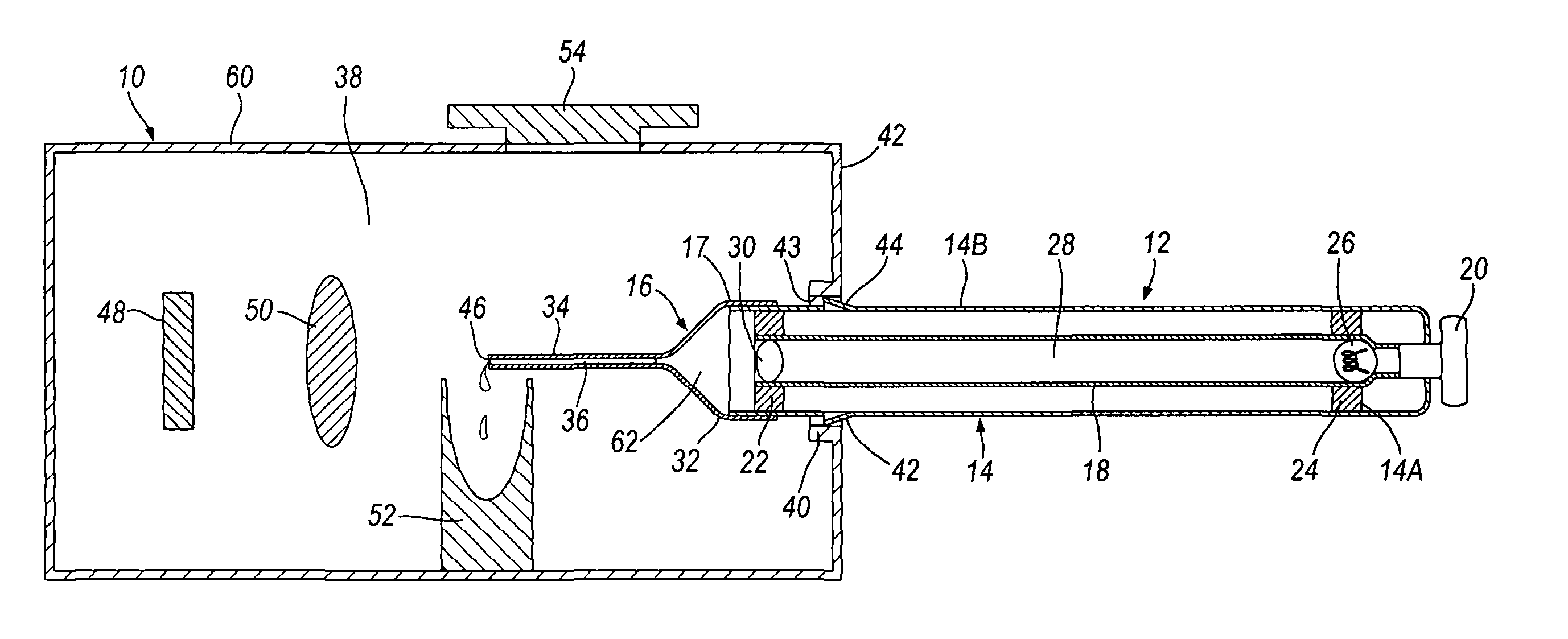
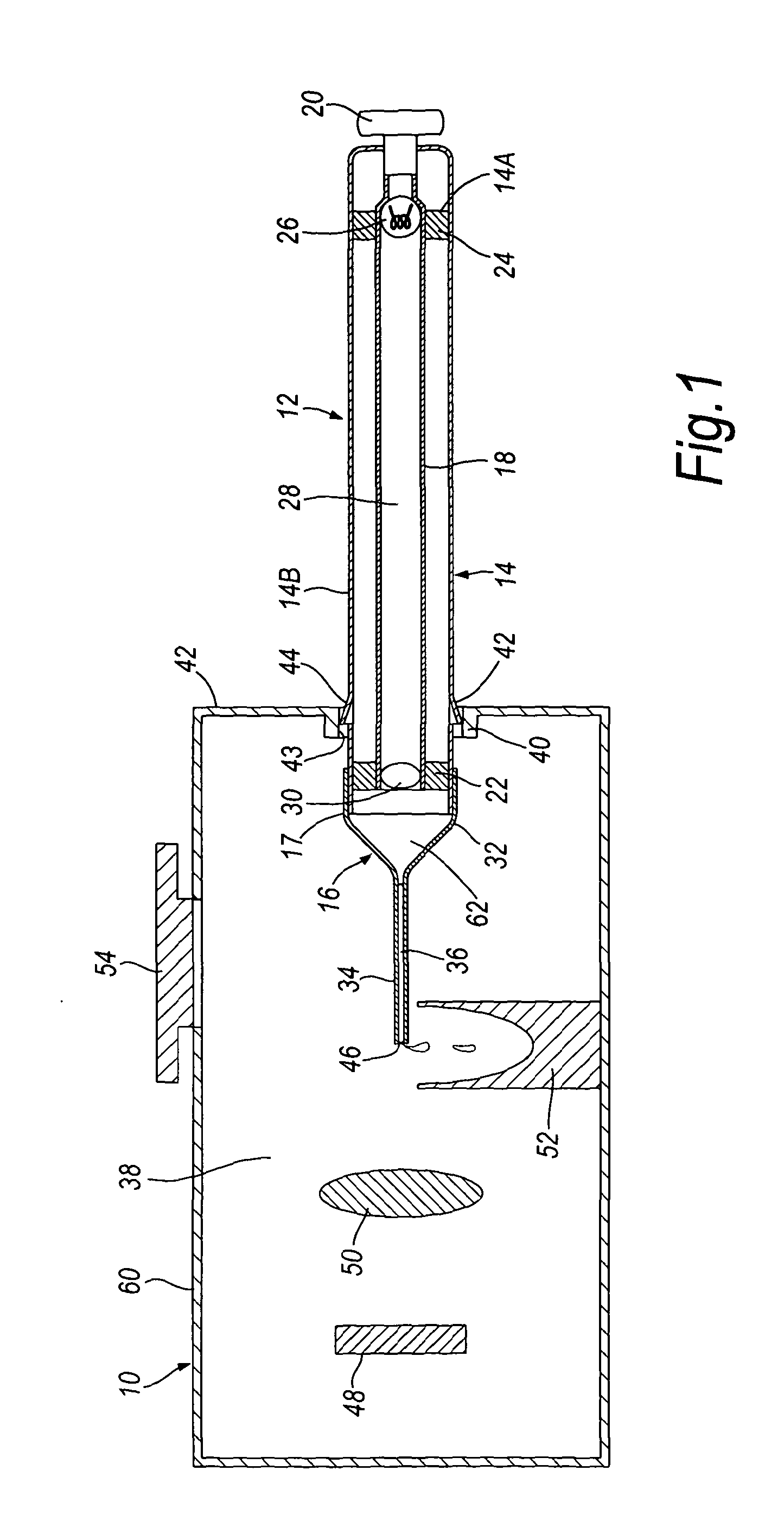
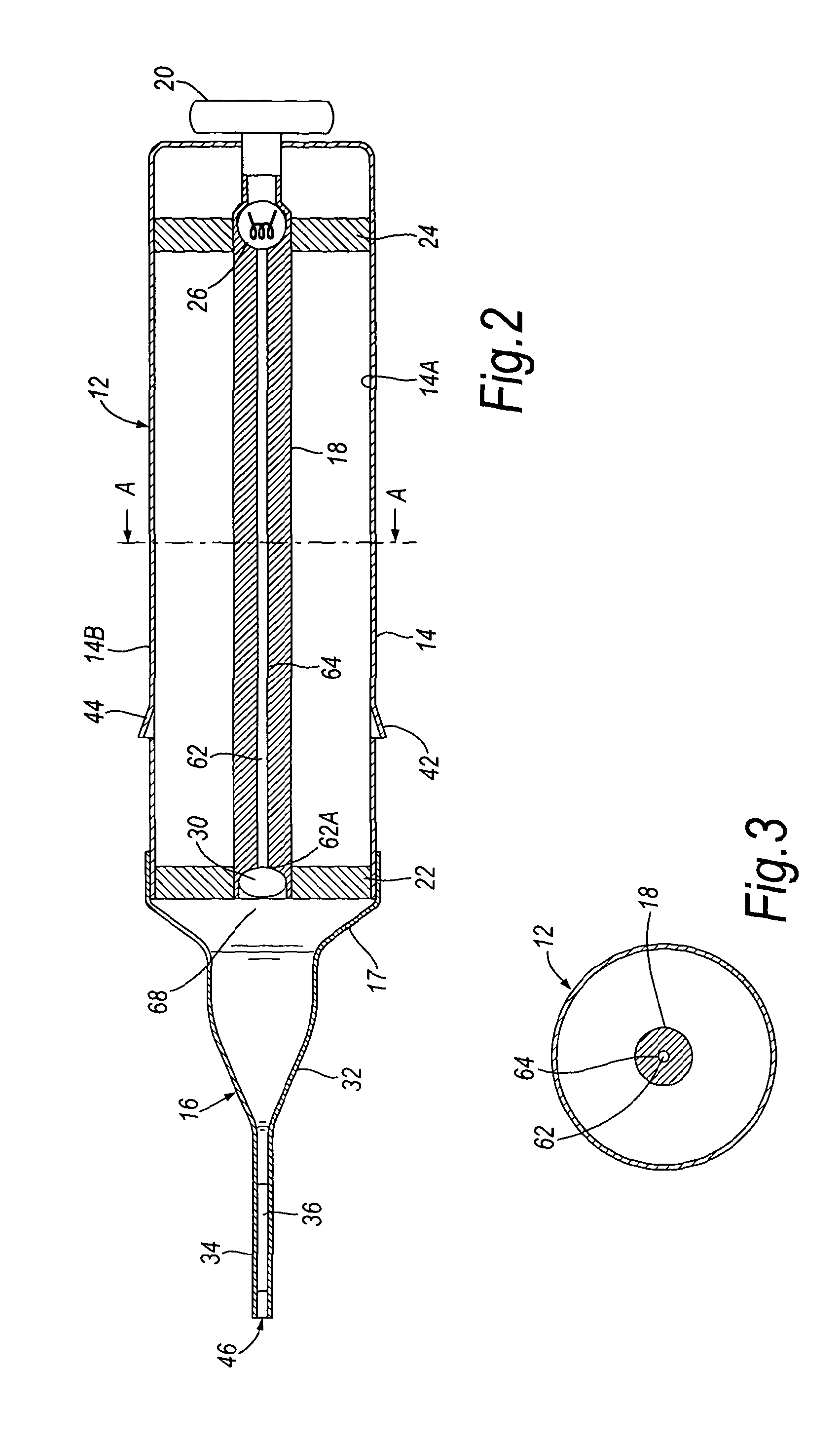
The present invention relates to a pipette (12) comprising a body portion (14) for aspirating a fluid sample (36) into a pipette tip (16) when attached thereto, wherein the body portion (14) comprises at least one light source (26), or an entry point for light from at least one light source, providing an optical path of light that passes through the sample (36) in a direction essentially along the longitudinal axis of the pipette tip (16). A method for measuring light output from a fluid sample (36), comprises providing a pipette tip (14) or capillary tube having first and second open ends (46, 68) with the sample (36) to be analysed contained therein, and detecting light output from an open end (46) of the pipette tip (16) or capillary tube. An apparatus and kit are also described. The pipette, apparatus, kit and method allow the accurate analysis and quantitative determination of minute amounts of biological samples.
Owner:PAGE BRIAN
Device for simultaneously carrying out blood group determination, serum cross-check and antibody detection test
ActiveUS7745228B2Practical and easily mannerFunction increaseBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteQuantitative determination
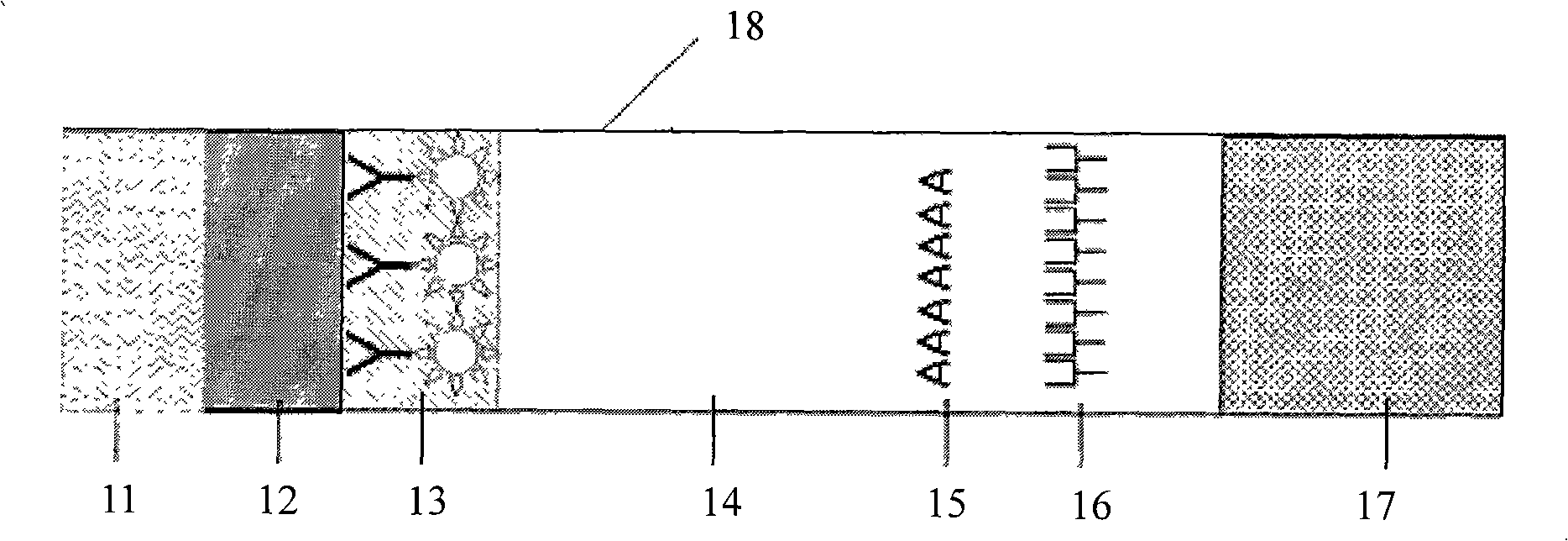
This invention relates to a device for the simultaneous qualitative or quantitative determination of several analytes in a liquid sample. The device comprises a membrane with a charging zone, for the application of the liquid sample, at least two indicator zones which can interact with the analyte(s) and at least one absorption region, which accepts the fluid after passing through the indicator zones, whereby the indicator zones lie between the charging zone and an absorption region, characterized in that the flow directions (flow tracks) are essentially parallel from the application zone through each indicator zone to an absorption region and at least two different flow tracks are present. The invention further relates to a method for the determination of several analytes or derivatives thereof in a liquid sample, comprising: application of the sample to the charging zone of a membrane of the device, whereby said sample is present in sufficient amounts to permit the sample fluid to flow in the direction of the absorption region through the indicator zones and to permit the analytes or derivatives thereof in the liquid sample to form a complex in the indicator zone.
Owner:GRIFOLS DIAGNOSTIC SOLUTIONS INC
Biochip and biochip kit, and method of producing the same and method of using the same
InactiveUS20060252044A1Improve efficiencyShort timeBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteQuantitative determination
There are provided a biochip and a biochip kit, in which a target contained in an analyte is reacted with a probe with high efficiency in a short time, B / F separation efficiency is high, and high-sensitive quantitative determination and detection can be realized, and a production process thereof, and a method for reacting a target contained in an analyte with a probe, and, for example, separation and fractionation method and a detection and identification method for a target contained in an analyte, using the biochip kit. The biochip according to the present invention comprises a well(s) provided with a filter comprising straight pores, with a uniform pore diameter, provided at uniform pore spacings. A dispersion with probe-supported particles dispersed therein is contained in the well, and an analyte is placed in the well(s) to react the analyte with the probe-supported particles. A solution such as an analyte solution can be introduced into or discharged from the well through the filter.
Owner:JSR CORPORATIOON +1
Quantitative assay for low abundance molecules
InactiveUS7074586B1Bioreactor/fermenter combinationsBiological substance pretreatmentsQuantitative determinationBioinformatics
A method for quantitatively assaying one or more target molecules in a sample uses a nucleic acid aptamer that is specific for each target molecule. A quantitative replicative procedure is used to determine a quantity of aptamer specific for each molecule.
Owner:SOURCE PRECISION MEDICINE INC
Quantitative assay with extended dynamic range
InactiveUS20060019404A1Material analysis by observing effect on chemical indicatorBiological testingAnalyteChemical composition
An efficient design for an expanded dynamic range in a lateral flow one step assay for the detection of an analyte in a biological sample is disclosed. The device comprises a multiple strip design, each constructed of four zones; a sample receiving zone, a sample treatment zone, a labeling zone, and a capture zone. The sample containing analyte is accepted in the sample receiving zone in the form of blood, serum, plasma, or urine. It is then carried into the sample treatment zone where it is rendered compatible with the chemistries of the assay strip. The treated sample then flows into the labeling zone where it interacts with visible particles that are coupled to analyte specific binding proteins. The flow continues, carrying the labeled analyte into the capture zone where it is immobilized in specific regions with analyte specific binding proteins. Excess flow is absorbed in an absorbent zone that is in contact with the capture zone. A positive result is interpreted by detection of the visible particles in the specified regions of the capture zone.
Owner:BAYER HEALTHCARE LLC
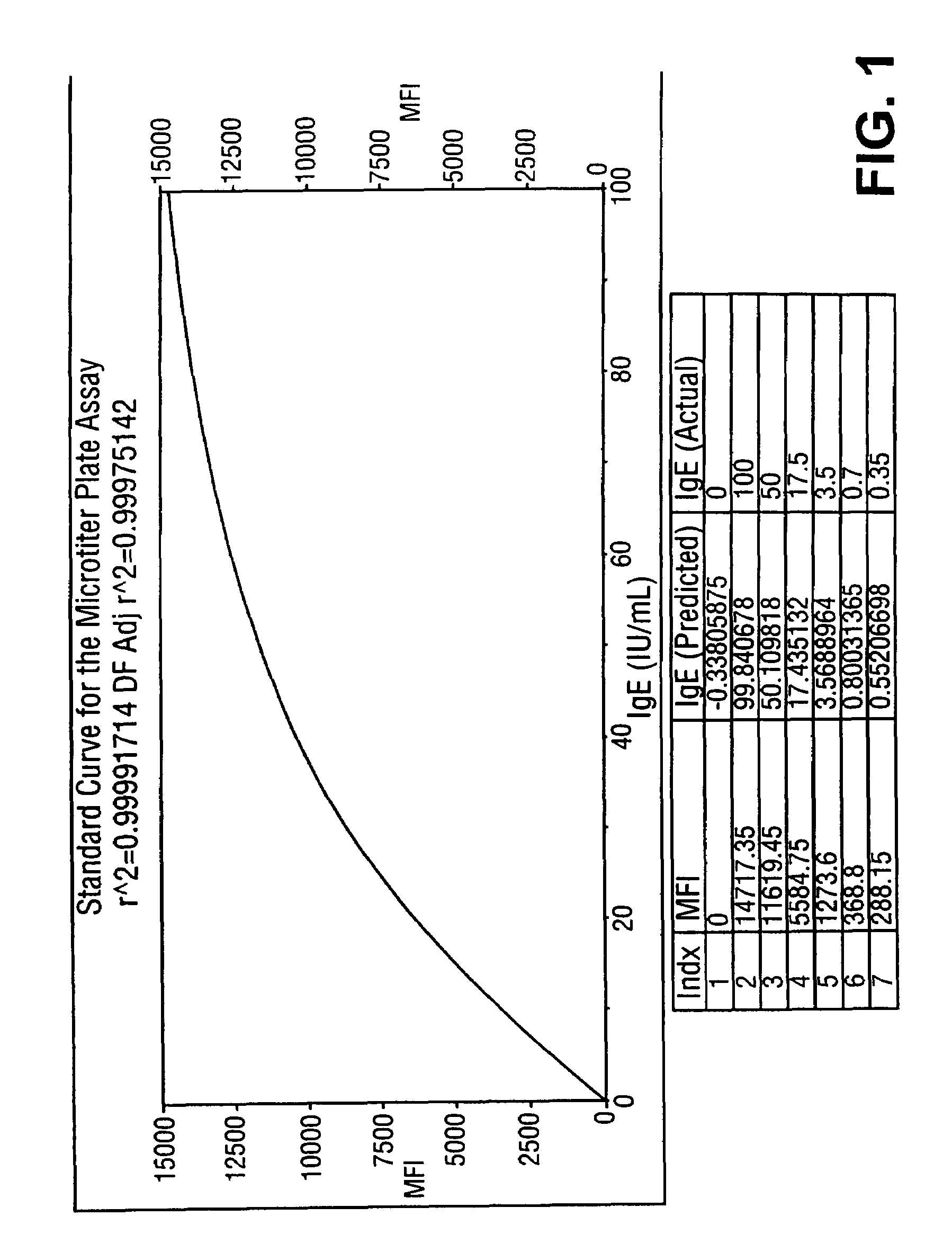
Homogeneous immunoassays for multiple allergens
InactiveUS7491553B2Chemiluminescene/bioluminescenceBiological testingQuantitative determinationMicroparticle
A homogeneous immunoassay method and system for quantitative determination of total immunoglobulin E and specific antibody levels to a plurality of allergens, in which a relatively small sampling of blood is required. The method utilizes relatively small microparticles in aqueous suspension. The immunoassay procedure is an immunometric sandwich procedure preferably utilizing biotin-streptavidin signal amplification techniques and R-phycoerytherin fluorescent labels.
Owner:IMMUNETECH
Emissive compositions with internal standard and related techniques
InactiveUS20080085566A1Chemiluminescene/bioluminescenceSynthetic resin layered productsAnalyteQuantitative determination
The present invention provides materials, devices, and methods related to determination of an analyte. In some embodiments, an analyte may be determined by monitoring, for example, a change in an optical signal of a luminescent material (e.g., particle) upon exposure to an analyte. The present invention may be particularly advantageous in that some embodiments may comprise an emissive species useful as an internal reference standard. Methods of the invention may also be useful in the quantitative determination of an analyte. In some cases, the present invention may allow for selective determination of an analyte.
Owner:MASSACHUSETTS INST OF TECH
Analyte test system using non-enzymatic analyte recognition elements
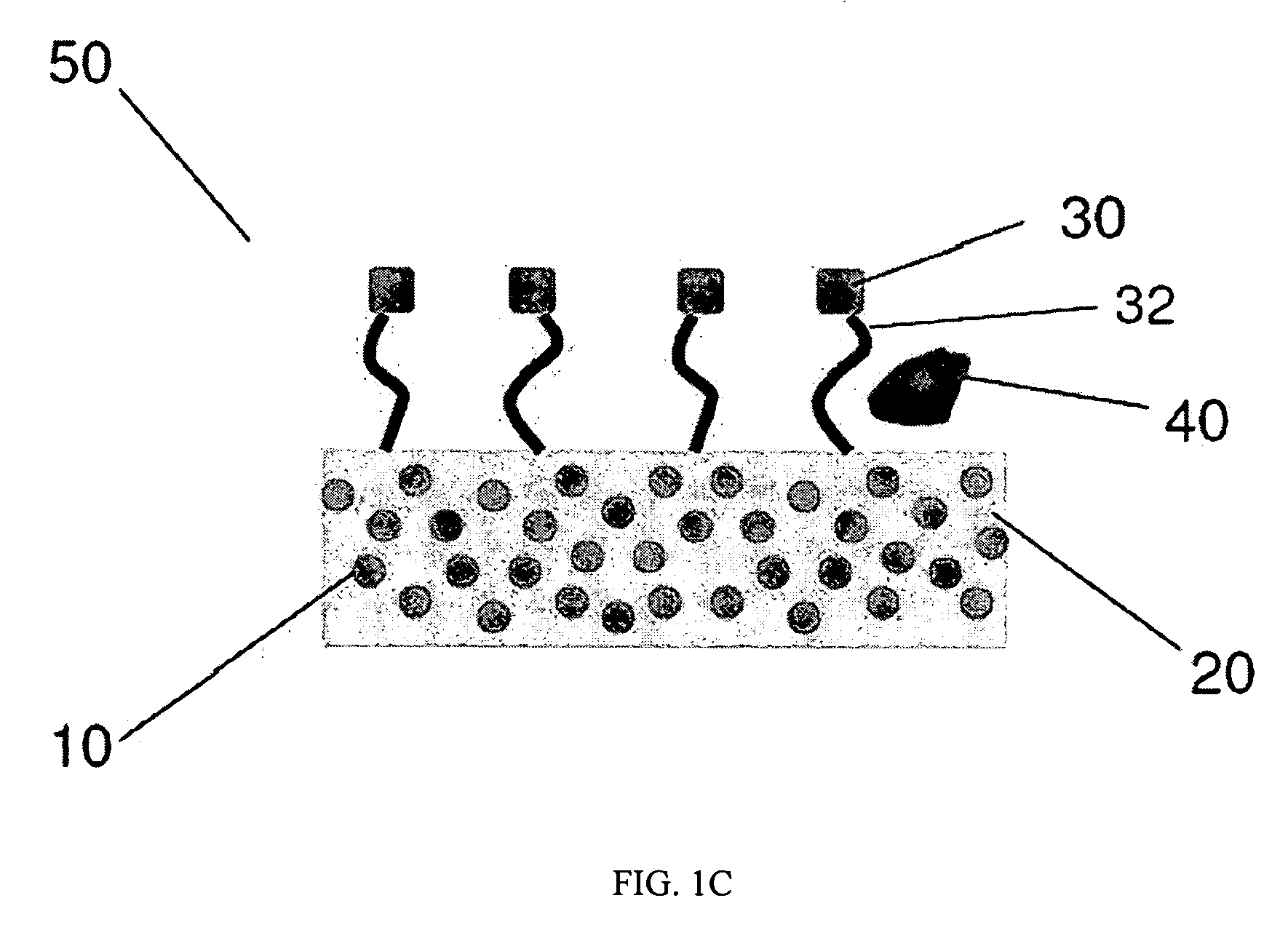
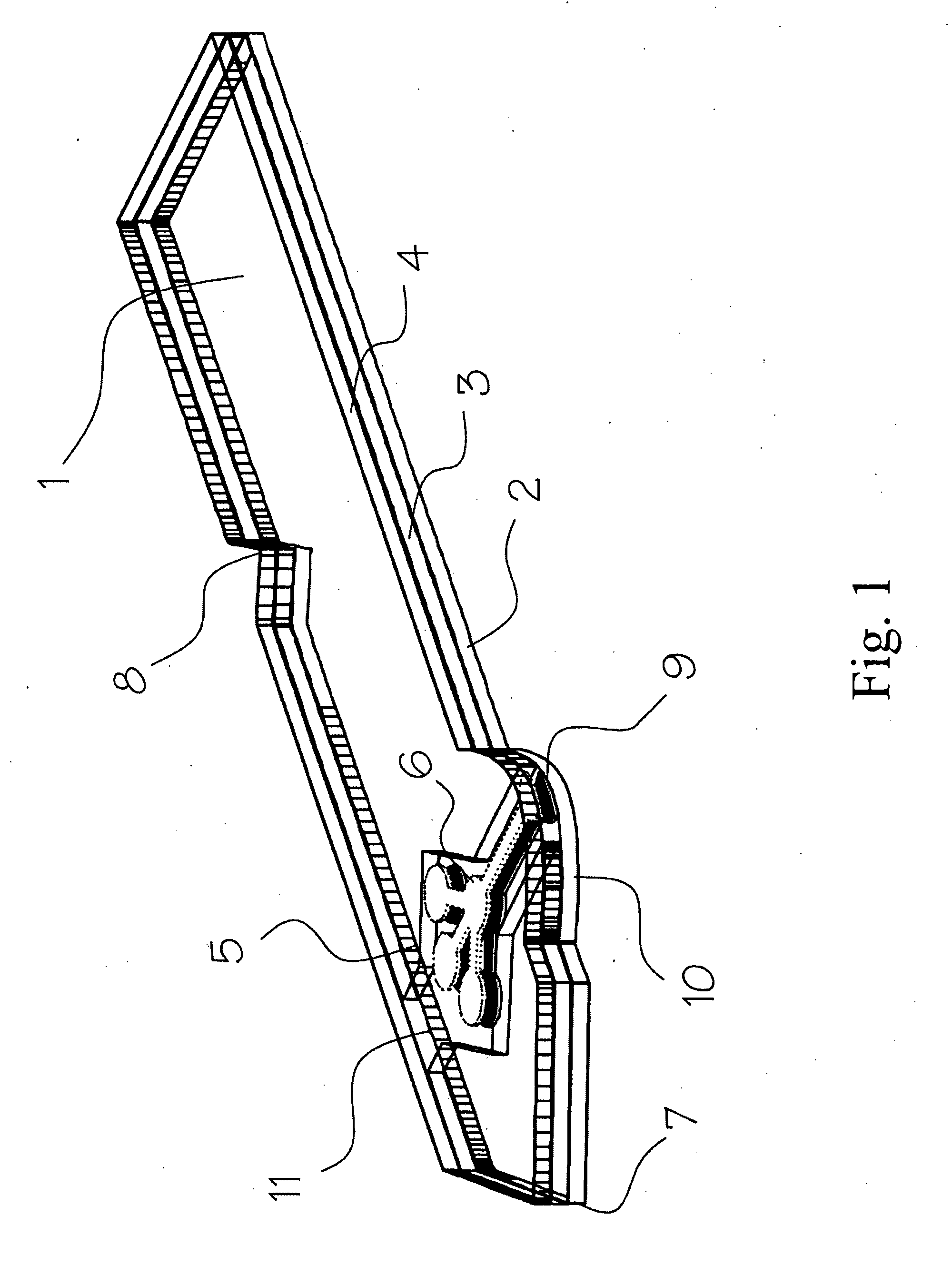
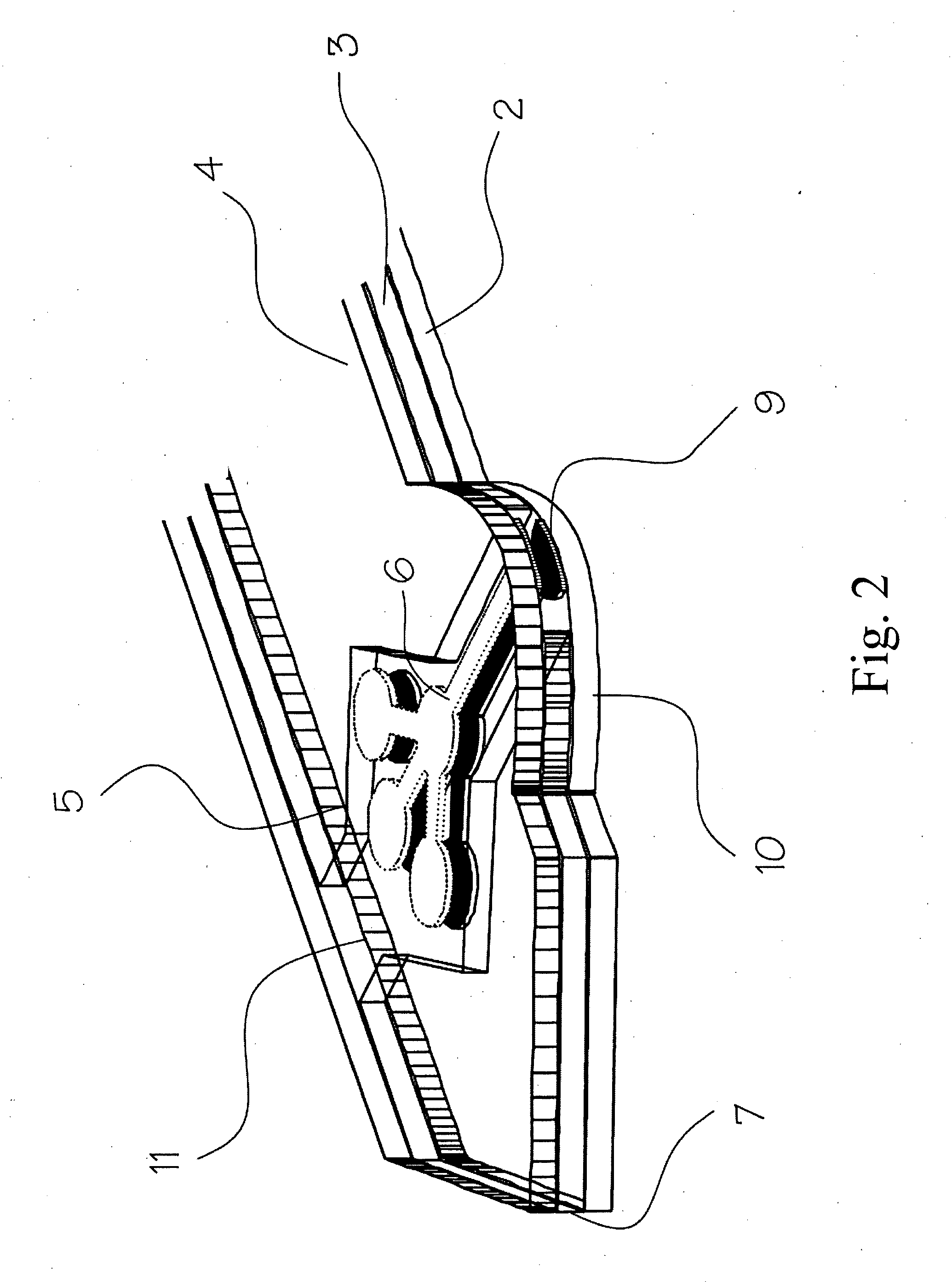
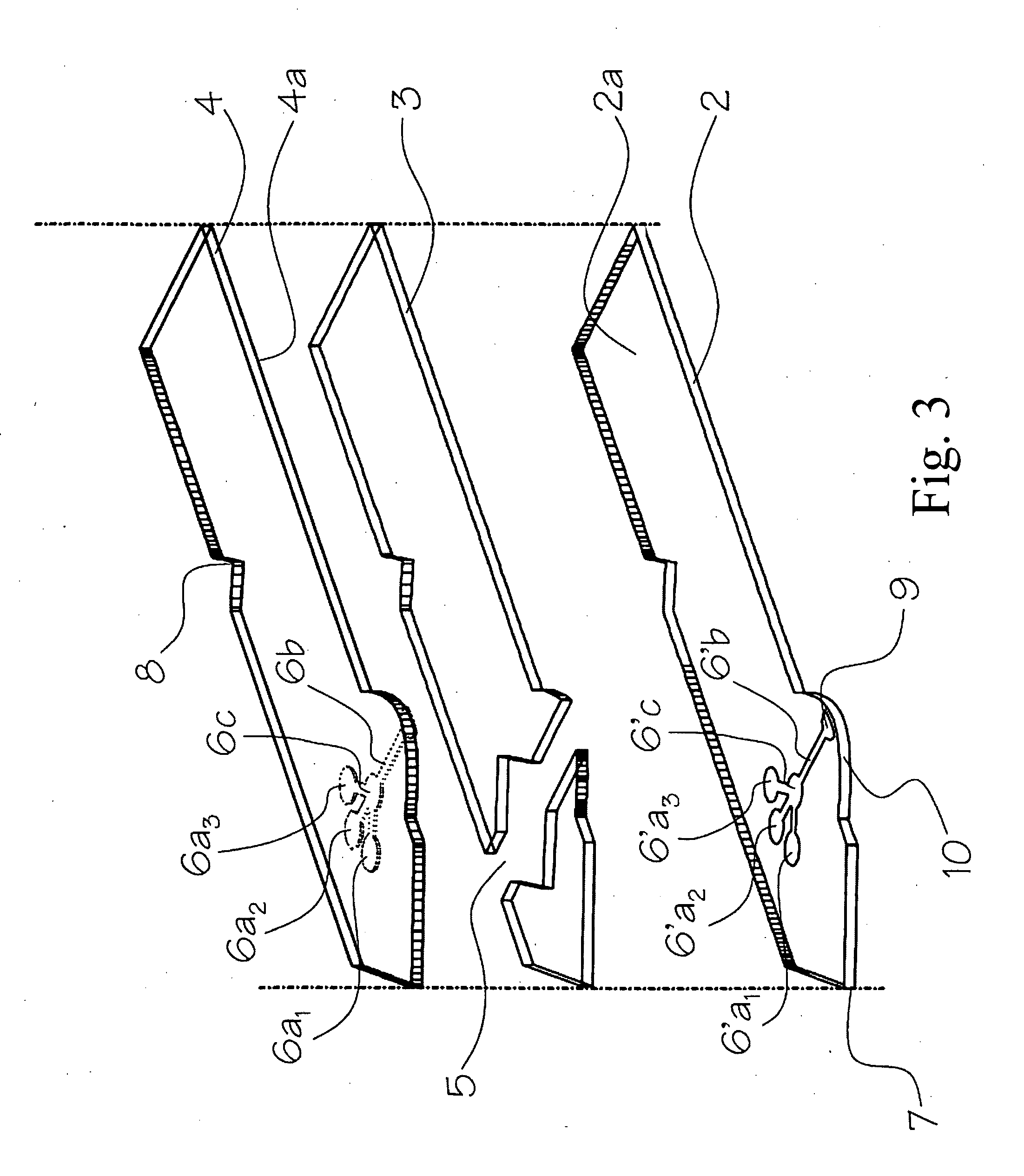
InactiveUS20100035245A1Simple wayCheap productionBioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careTarget analysis
An analyte test element for the qualitative and / or quantitative determination of at least one analyte in a physiological or aqueous sample fluid having a first surface (2a) and a second surface (4a) in a predetermined distance opposite from each other, said both surfaces are provided with two substantially equivalent patterns forming areas of high and low surface energy which are aligned mostly congruent, whereby the areas of high surface energy (6, 6′) create a sample distribution system with at least two detection areas (6a, 6′a), said at least one of the detection areas (6a, 6′a) of the first and second surfaces (2a, 4a) is provided with at least one non-enzymatic recognition element (32). The analyte test element is suitable for analyte test systems evaluating the affinity reaction between an analyte of interest and a recognition element and therefore provides a suitable test system to perform immunoassays, receptor-assays, or other affinity assays with a simple test element containing qualitative or quantitative calibration mechanisms suitable for point of care and home settings.
Owner:EGOMEDICAL TECH
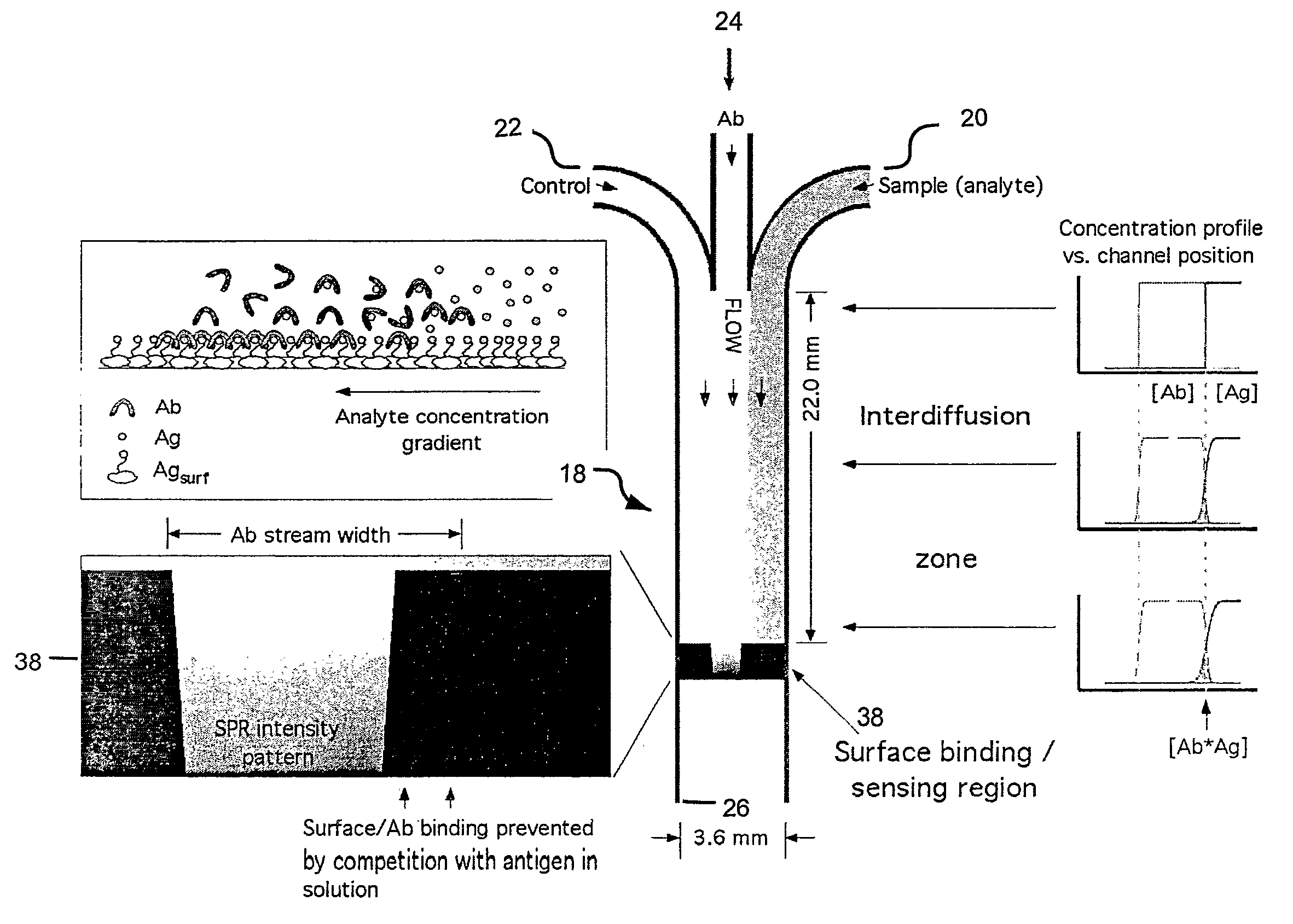
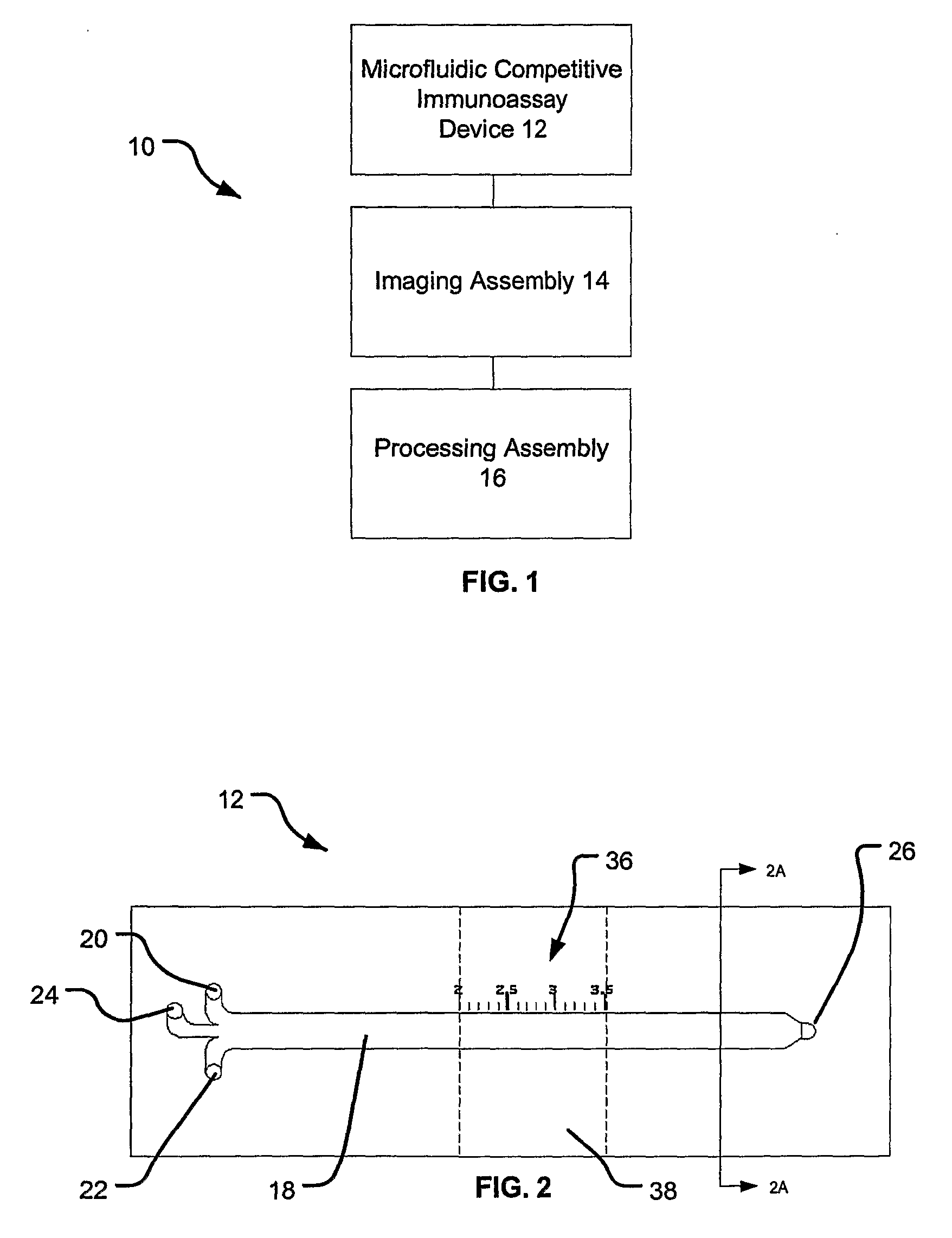
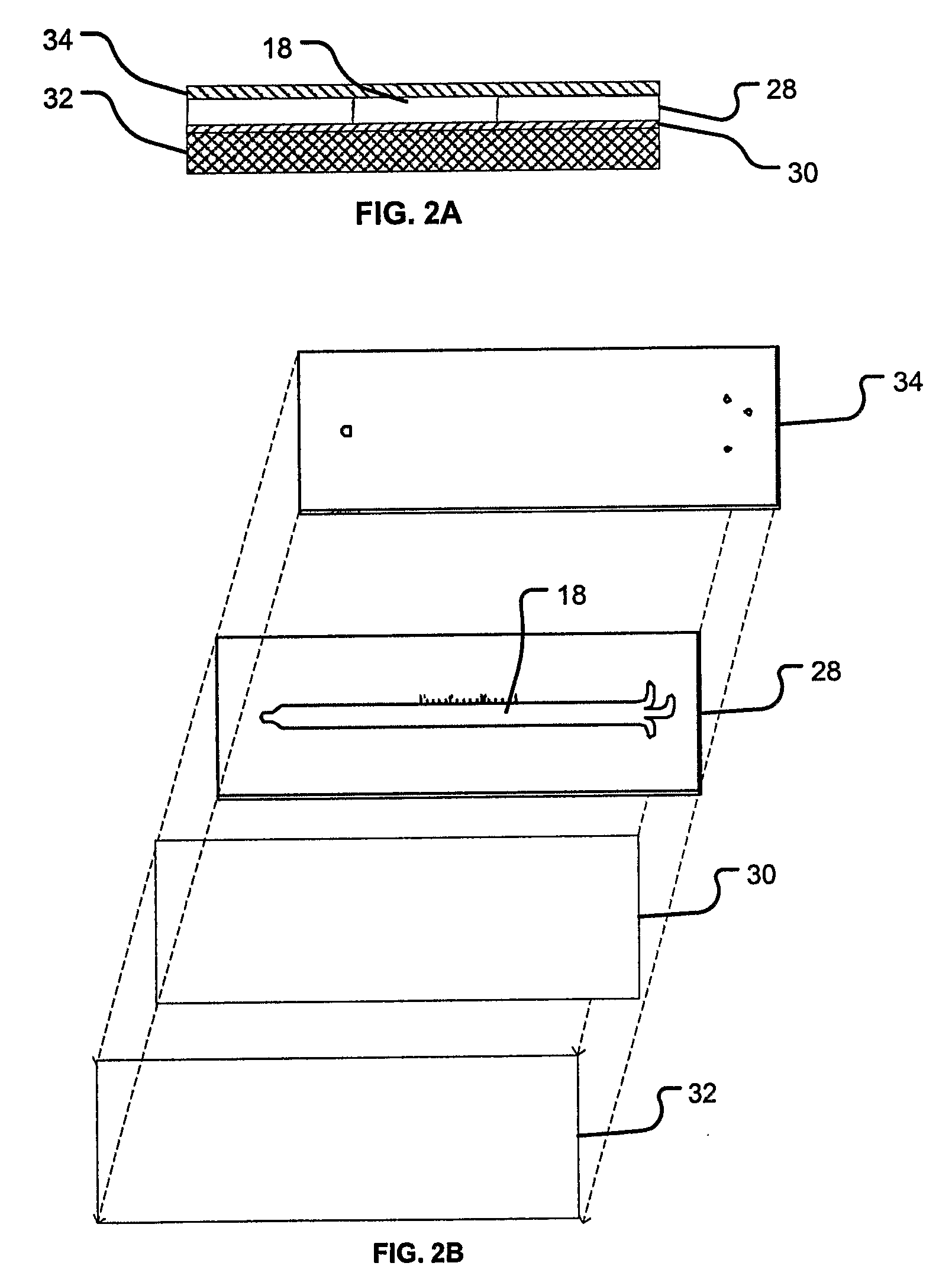
Rapid Microfluidic Assay for Quantitative Measurement of Interactions Among One or More Analytes
InactiveUS20080014575A1Rapid and quantitative measurementShorten the timeBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteAssay
The invention provides microfluidic competitive immunoassay devices and assay methods for rapid, quantitative measurement of binding interactions between analytes and the quantitative determination of an amount (e.g., concentration) of the analyte in an unknown sample.
Owner:UNIV OF WASHINGTON
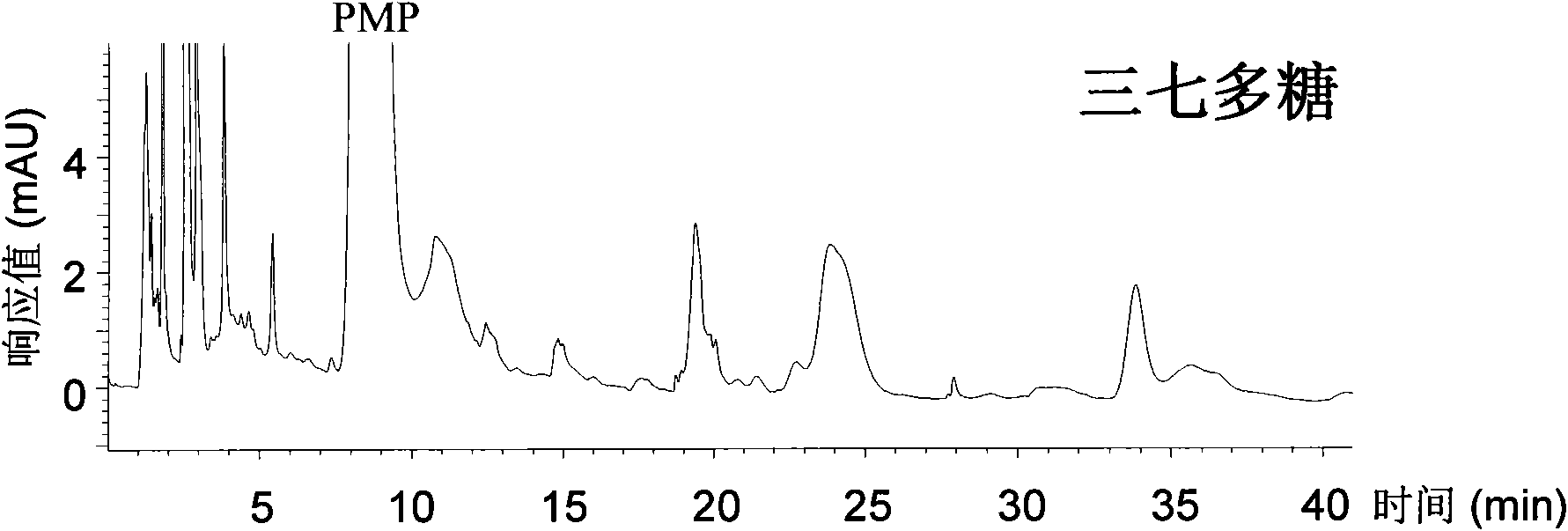
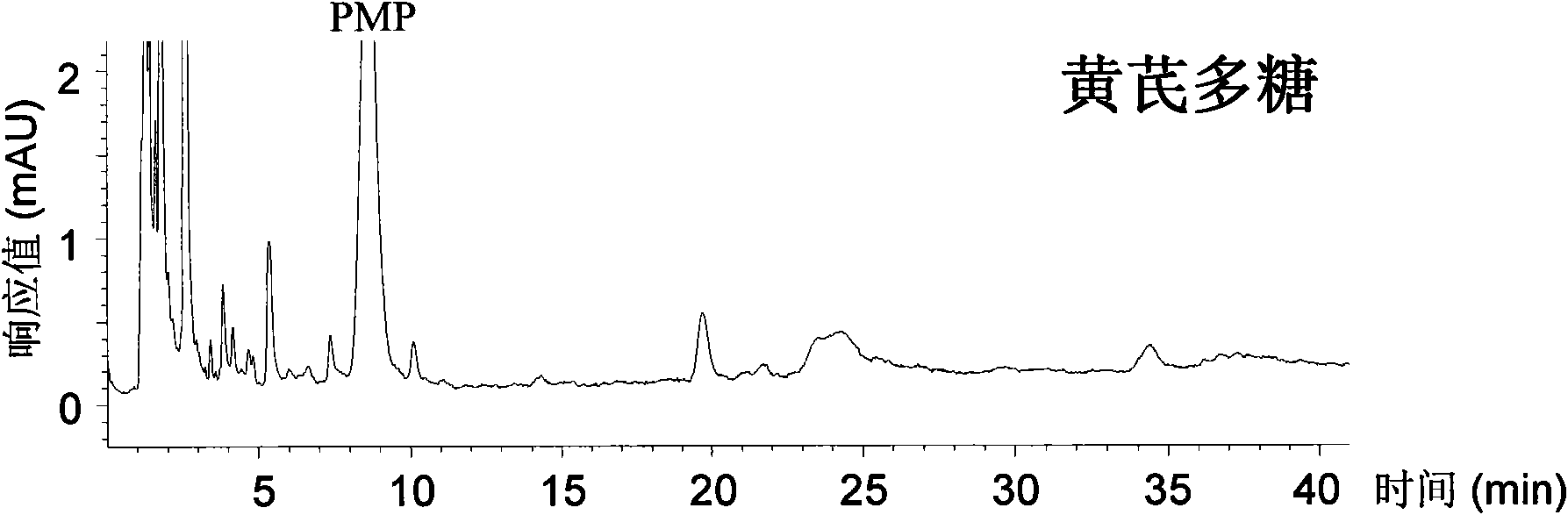
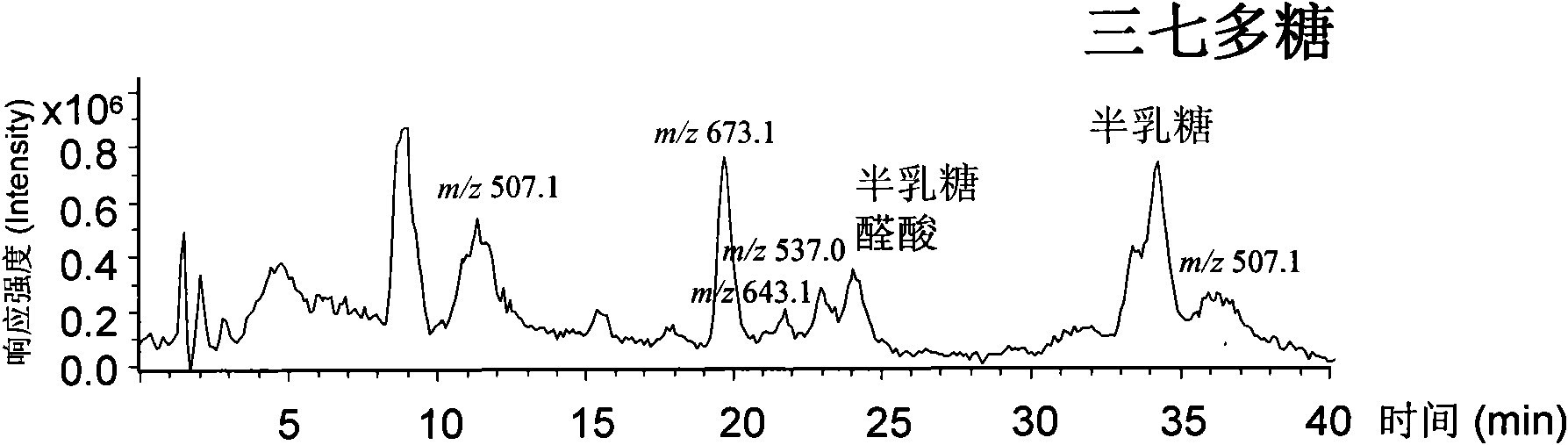
Qualitative and quantitative analysis method for polyoses
ActiveCN101539550AEasy to separateImprove featuresComponent separationMaterial analysis by observing effect on chemical indicatorAdditive ingredientUronic acid
The invention relates to a qualitative and quantitative analysis method for polyoses, which comprises the following steps: (1) the zymohydrolysis and the verification of the polyoses: measuring the content of a polyoses water solution by a colorimetric method; (2) comparing the change of a polyoses mapping before and after zymohydrolysis by HPSEC-ELSD analysis; (3) saccharide ingredient direct HPLC analysis of a polyoses zymohydrolysis solution or HPLC analysis after pre-column derivatization: identifying polyoses mapping and chromatogram peaks by taking a standard monosaccharose, a uronic acid, a disaccharide, and the like as contrasts and taking a chromatogram peak mass-to-charge ratio as reference, and using stable characteristic sections as a quantitative analysis index; and (4) realizing the qualitative and quantitative analysis of the polyoses by establishing polyoses zymohydrolysis responding characteristics and polyoses zymohydrolysis mapping which are based on structural information through the independent or combining application of the steps. The qualitative and quantitative analysis method can be used for the identification and quantitative measurement of the polyoses of traditional Chinese medicine, such as panax, panax pseudoginseng, gen-seng, aweto, cordyceps, ganoderma lucidum, fomes japonica, milk veteh, angelica, and the like and provides an effective method for controlling the quality of the polyoses and the products thereof.
Owner:李绍平
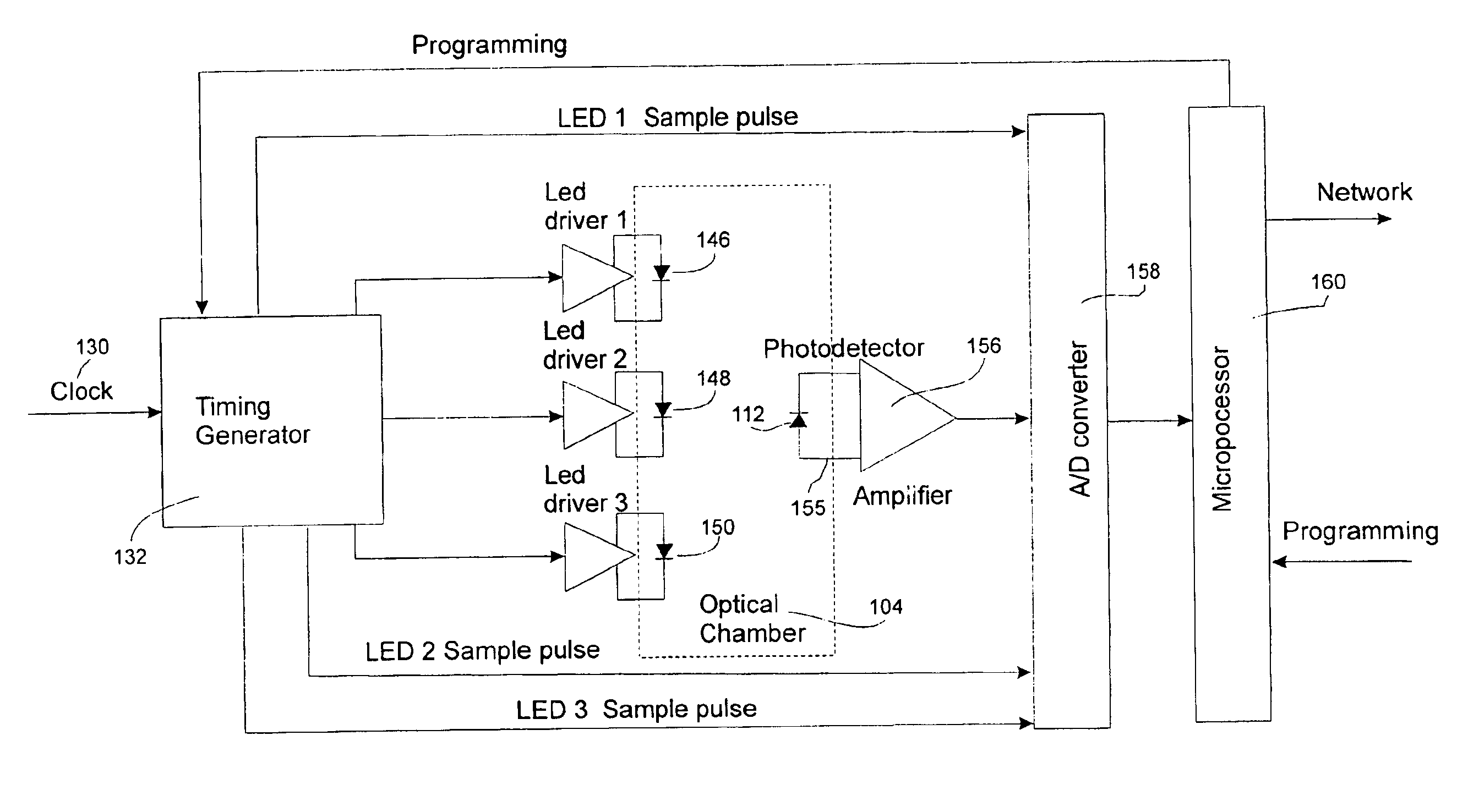
Device and method for qualitative and quantitative determination of intravenous fluid components
InactiveUS6847899B2Minimize and eliminate errorConvenient careOptical radiation measurementDrug and medicationsMedicineQuantitative determination
A device and process for preventing medical errors due to the improper administration of an intravenously delivered medication includes the spectroscopic analysis of intravenous fluid components. An emission source and detector are placed adjacent to the intravenous tubing of an administration set to generate signals for spectroscopic analysis. The signals are processed to identify the medication and, in certain embodiments of the invention, can determine the medication's concentration. In a preferred embodiment, the emission source, detector, and hardware and software for the spectroscopic analysis are placed in an infusion pump.
Owner:DEAN ALLGEYER M D
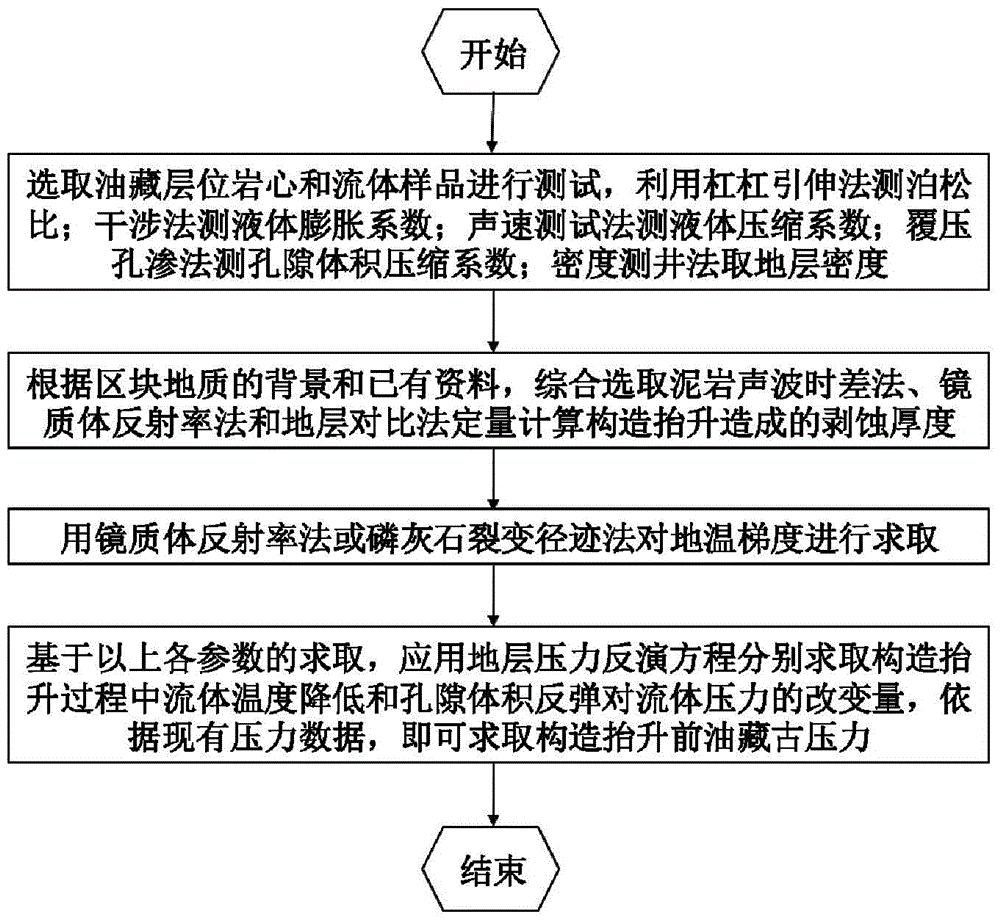
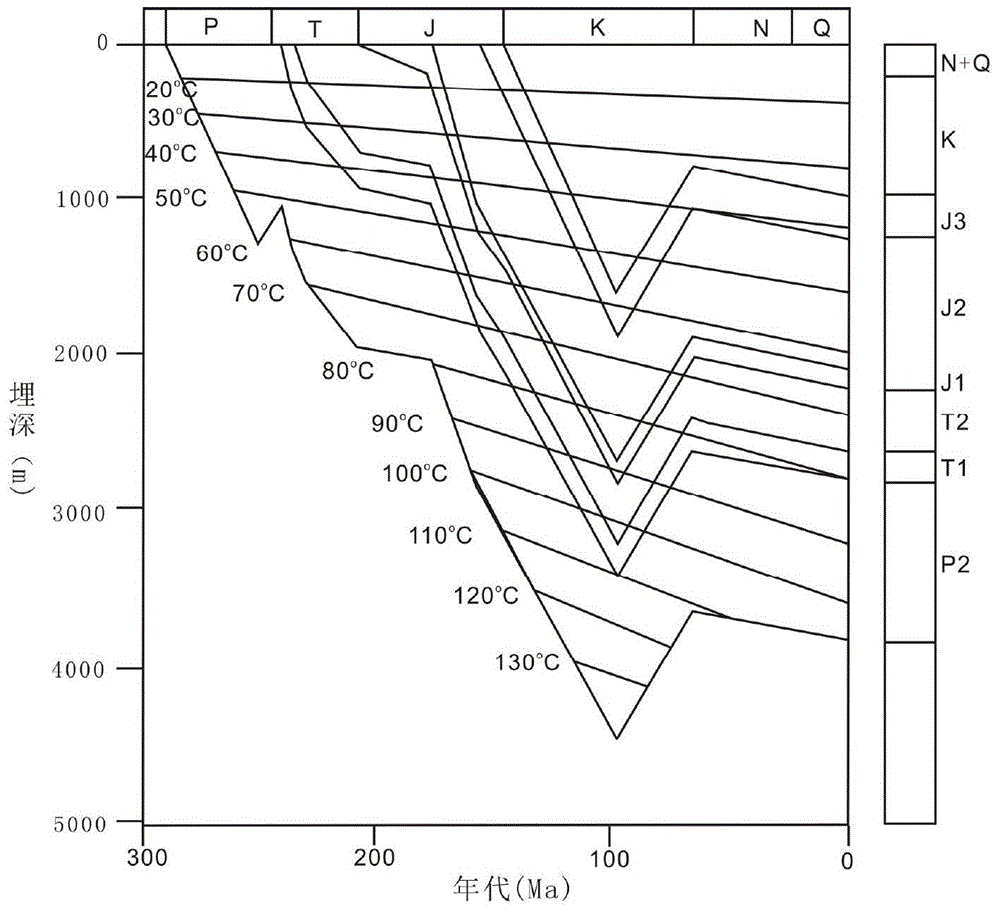
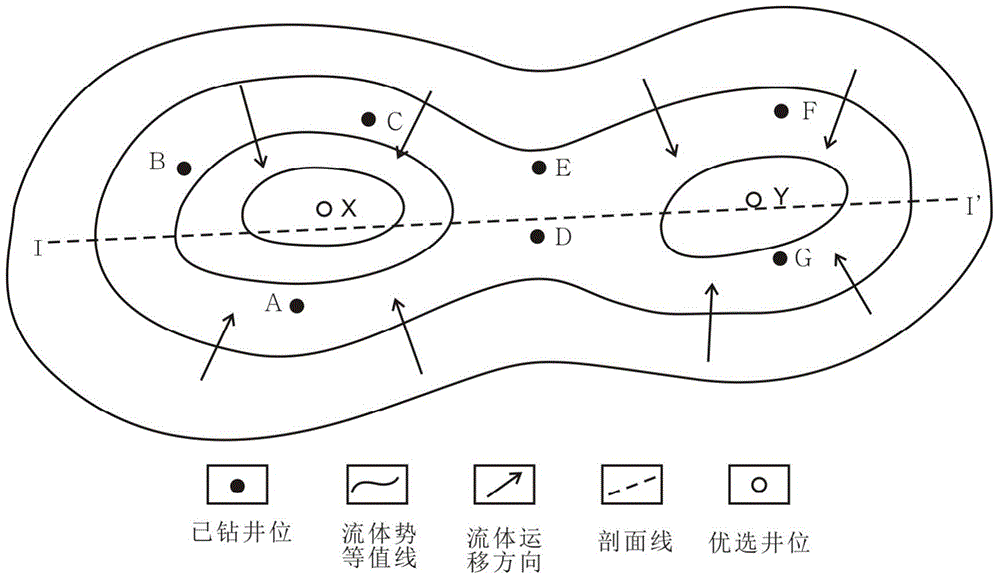
Paleopressure quantitative inversion detection method of oil reservoir
The invention discloses a paleopressure quantitative inversion detection method of an oil reservoir. The method comprises the following steps: detecting parameters of a core and a liquid sample at the oil reservoir; generating an erosion-thickness based oil reservoir burial history and geotemperature history simulation processed by a data device; then quantitatively detecting the paleopressure before tectonic movement. According to the paleopressure quantitative retrieval detection method of the oil reservoir, the pressure variation caused by tectonic uplift is acquired on the basis of actually-detected data and a liquid state equation and in light of strata burial history simulation and erosion thickness restoration, and the paleopressure of the oil reservoir before tectonic movement can be quantitatively inverted according to the current actually-detected oil reservoir pressure. With the adoption of the inversion method, the palaeozoic oil-gas migration-accumulation direction can be acquired to guide the selection of a better target region for oil-gas exploration, namely, the strata pressure of an adjacent area without being subjected to drilling exploration is forecasted and the drilling process is optimized according to the current oil reservoir pressure data of a drilled area, and thereby, the technical problems like diameter expansion of mudstone, collapse of well wall, and dead pressure at oil reservoir can be effectively handled.
Owner:CHINA UNIV OF GEOSCIENCES (BEIJING)
Device and method for measuring stress of optical glass
ActiveCN101592537ASize relaxedEasy to measureForce measurement by measuring optical property variationMaterial analysis by optical meansStress measuresMeasurement device
The invention provides a stress measuring device with higher precision, which can measure the size, direction and distribution of the stress of optical glass. The stress measuring device of the optical glass comprises a communicated light path interferometer and a stander, wherein the stander comprises a frame and a plane scanning test platform arranged on the frame; and a laser receiving unit and a laser emitting unit of the communicated light path interferometer are arranged on the stander, and can synchronously move front and back, and up and down. The stress measuring device achieves the quantitive measurement of the stress size and the stress uniformity of the optical glass; in addition, the precision is greatly improved, the resolution reaches 0.01nm, and the size of the measurable optical glass is greatly widened. The stress measuring device can better measure the stress optical coefficient of the optical glass; and compared with the other polarized stress meter measuring devices with weight application, the measuring precisions of the pressure and the stress of load are obviously improved, and the measuring precision of the stress optical coefficient can reach 0.03*10<-12> / Pa.
Owner:CDGM OPTICAL GLASS
Peroxide and chlorine test strip
InactiveUS6087089AAddressing Insufficient SensitivityAccurate and reliable quantitative determinationPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesMicrobiological testing/measurementHigh concentrationCellulose
A composition, method, and test device for quantitatively determining the oxidant concentration of a test sample are disclosed. The test device includes a test pad having a suitable carrier matrix incorporating an indicator reagent composition capable of interacting with an oxidant to produce a detectable and measurable response for oxidant over a wide concentration range of oxidant. An indicator reagent composition contains: (a) an iodide salt, (b) a buffer, and (c) a water-soluble polymer, preferably a cellulose-based polymer. An indicator reagent composition is incorporated into a carrier matrix, like filter paper, to provide a test pad useful in a dry phase oxidant assay of a test sample, especially for test samples containing a high concentration of oxidant, like chlorine or a peroxide.
Owner:INTEGRATED BIOMEDICAL TECH INC AN INDIANA
Aflatoxin B1 flow lag immunization time distinguishing fluorescence rapid-detection kit and application thereof
The invention relates to an aflatoxin B1 flow lag immunization time distinguishing fluorescence rapid-detection kit and an application thereof. The kit comprises a fluorescent test strip and a sample reaction bottle containing an europium-labeled anti-aflatoxin B1 monoclonal antibody lyophilized product, wherein the fluorescent test strip comprises a cardboard, a water absorption pad, a detection pad and a sample pad are sequentially pasted on one surface of the cardboard from top to bottom, adjacent pads are connected at the connection in an overlapping manner, the detection pad treats a cellulose nitrate membrane as a base pad, a transverse quality control line and a detection line are arranged on the cellulose nitrate membrane from top to bottom, the quality control line is coated with a rabbit anti-mouse polyclonal antibody, and the detection line is coated with an aflatoxin B1 bovine serum albumin conjugate; and the anti-aflatoxin B1 monoclonal antibody is secreted by a hybridoma cell strain having a preservation number of CCTCC NO.C201015. The kit can be used for the quantitative determination of the content of the aflatoxin B1, and has the advantages of simple operation, rapidness and high accuracy.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Sensor for luminescense-optical determination of an analyte
InactiveUS7067320B2High Luminous Quantum EfficiencyLong luminescence decay timeAnalysis using chemical indicatorsChemical analysis using titrationAnalyteLuminophore
The invention relates to an optochemical sensor functioning in accordance with the FRET-principle and exhibiting an acceptor (chromophore or luminophore) responsive to an analyte contained in a sample medium as well as a donor (luminophore), characterized in that acceptor and donor are located in separate chemical phases, whereby the phase containing the donor is essentially impermeable to the sample medium or to components of the sample medium affecting the luminescence characteristics of the luminophore. The invention further relates to a method for qualitative and / or quantitative determination of at least one analyte and / or component of a gaseous or liquid measuring medium according to the FRET-principle, characterized by the use of the sensor according to the invention.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Hybrid phase lateral flow assay
ActiveUS7632687B2Increased binding surface areaHigher immobilizationBioreactor/fermenter combinationsCompound screeningAnalyteQuantitative determination
The invention relates to devices for performing single step assays for the determination of the presence or absence of an analyte in a liquid sample, and methods of determining the presence or absence of such analytes using such devices. Devices disclosed comprise a labeled analyte-binding reagent reversibly-immobilized on a non-porous solid material, which solid material is in physical contact with a dry porous carrier bearing an immobilized analyte-binding reagent. Also provided are quantitative assay devices.
Owner:QUIDEL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com