Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3009 results about "Hybridoma cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
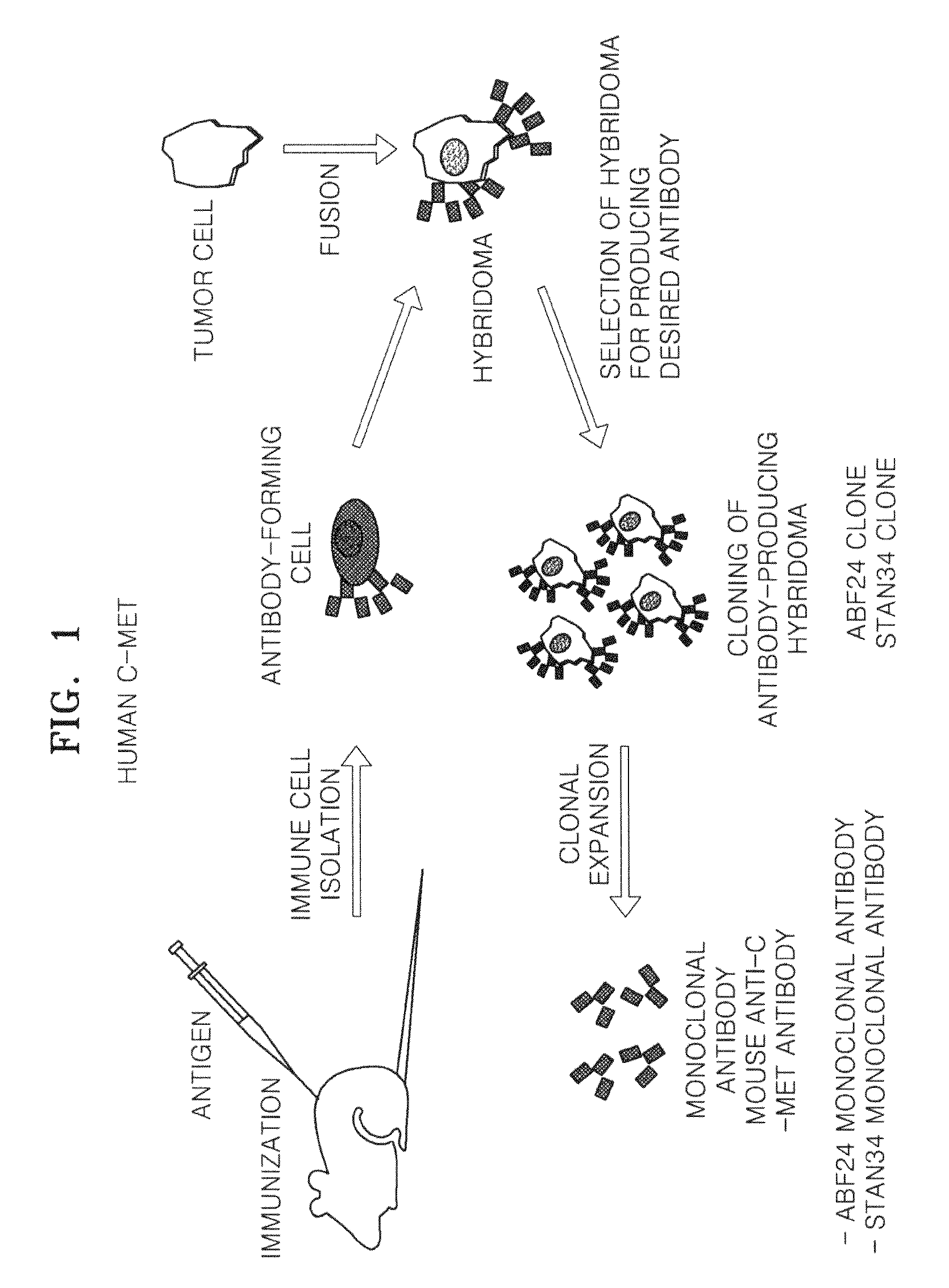
Definition of hybridoma. : a hybrid cell produced by the fusion of an antibody-producing lymphocyte with a tumor cell and used to culture continuously a specific monoclonal antibody.
Production of proteins by cell culture
InactiveUS6413746B1High protein yieldReduce cell viabilityImmunoglobulins against blood group antigensPeptide/protein ingredients3D cell cultureBiochemistry
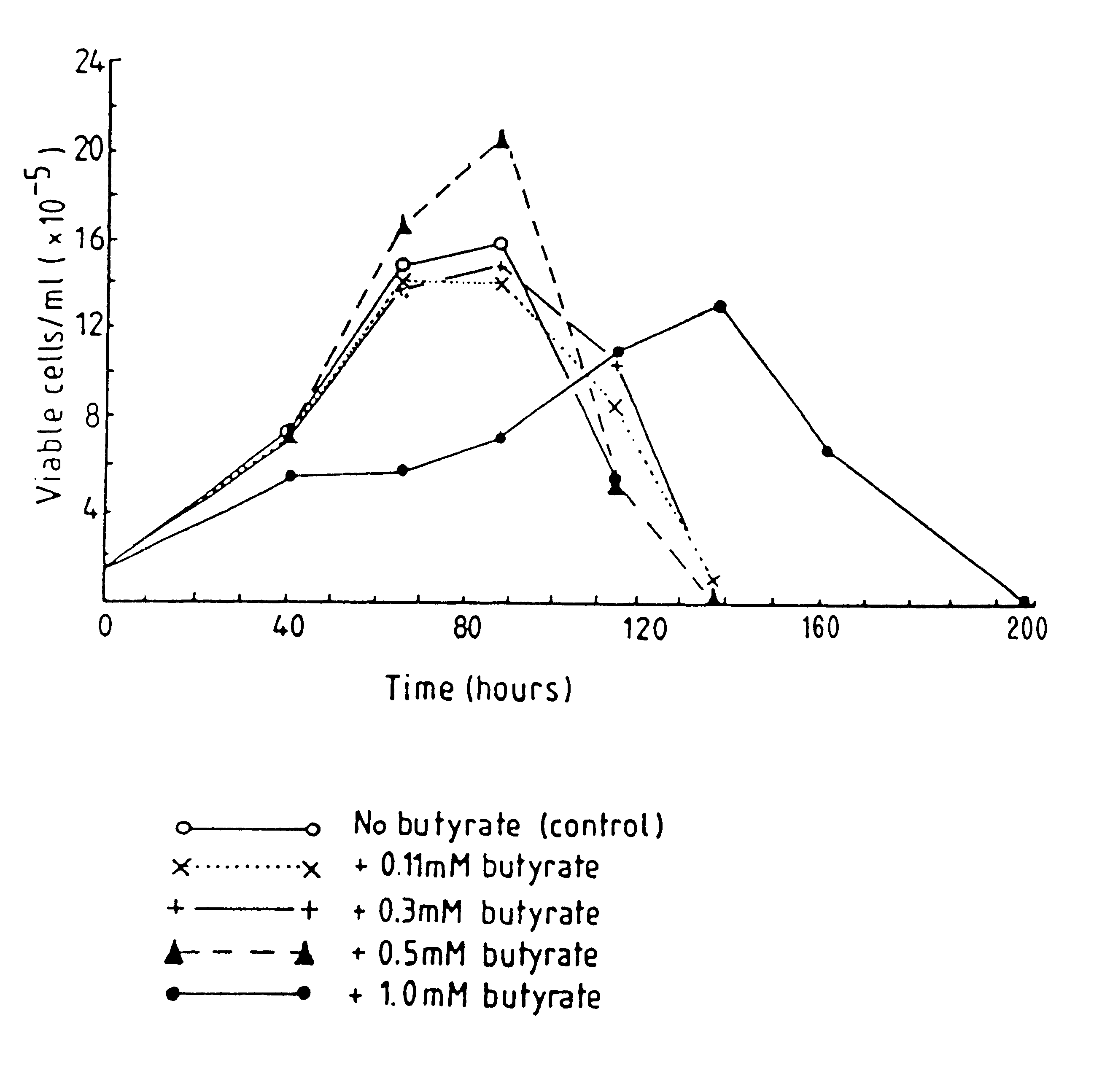
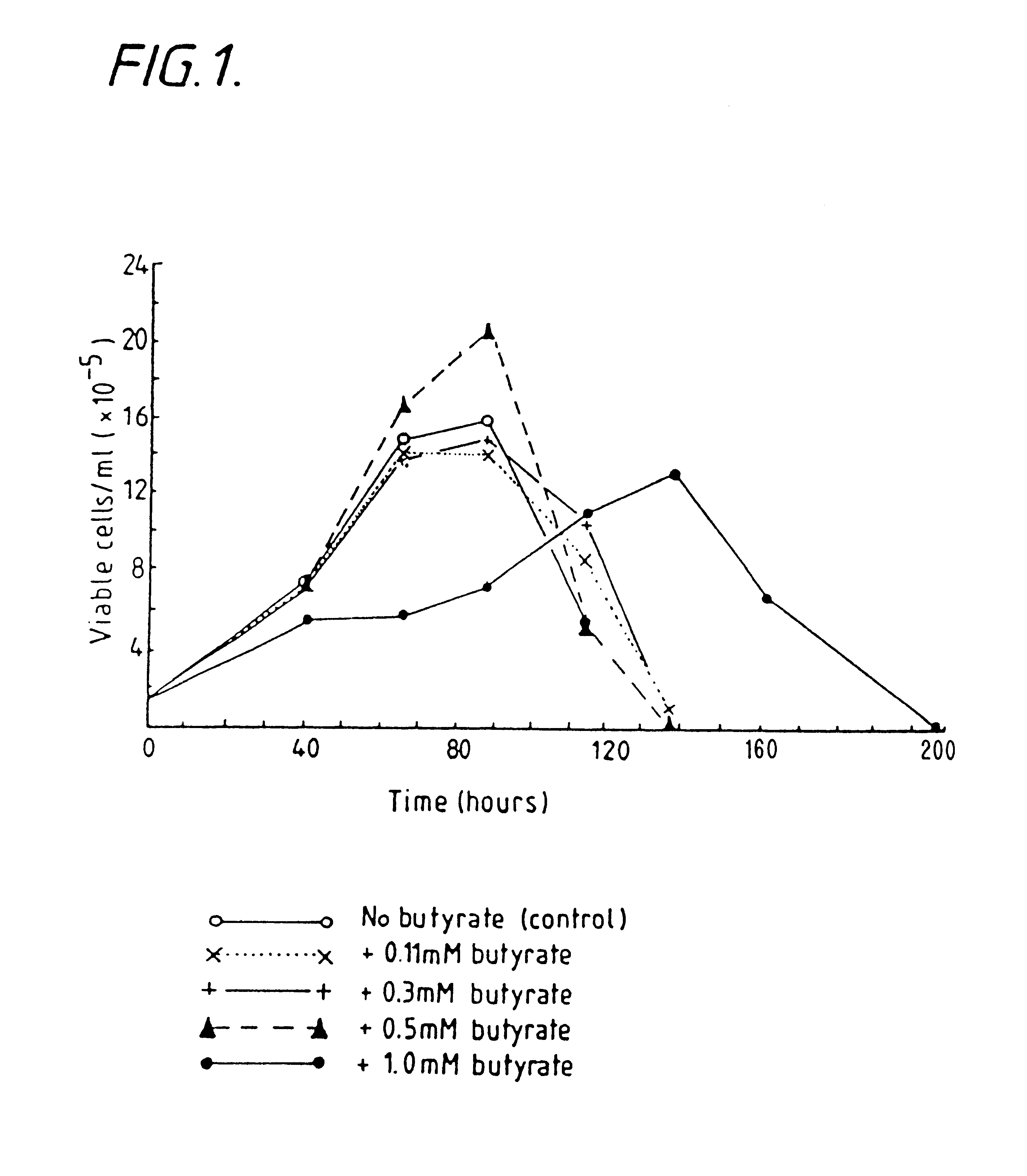
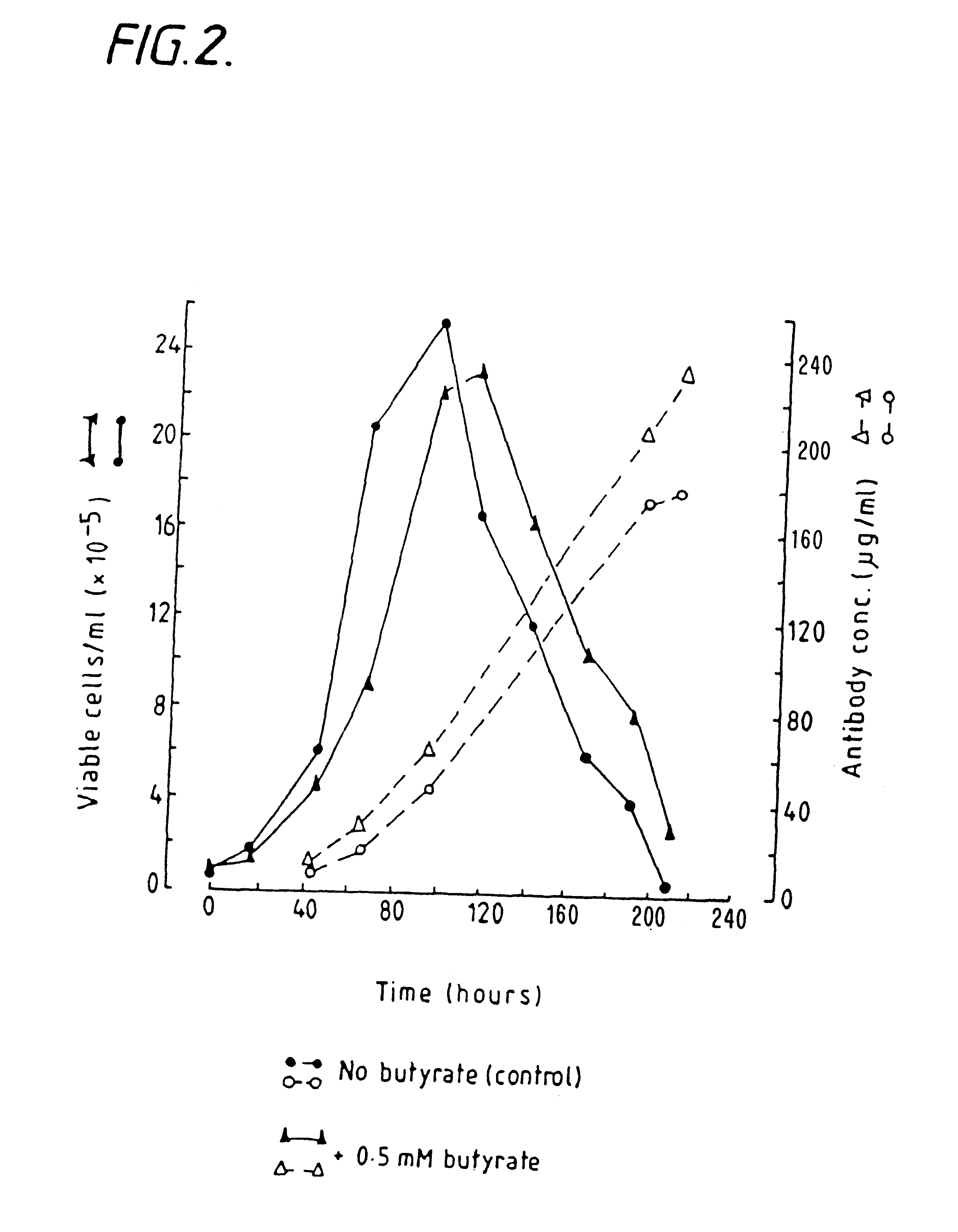
Methods for obtaining a protein by culture of hybridoma cells, wherein said protein is an immunoglobulin, are disclosed. The methods involve culturing animal hybridoma cells in continuous presence of an alkanoic acid or salt thereof, which enhances protein production, wherein said alkanoic acid or salt thereof is present at 2 concentration range of 0.1 mM to 200 mM.
Owner:LONZA LTD
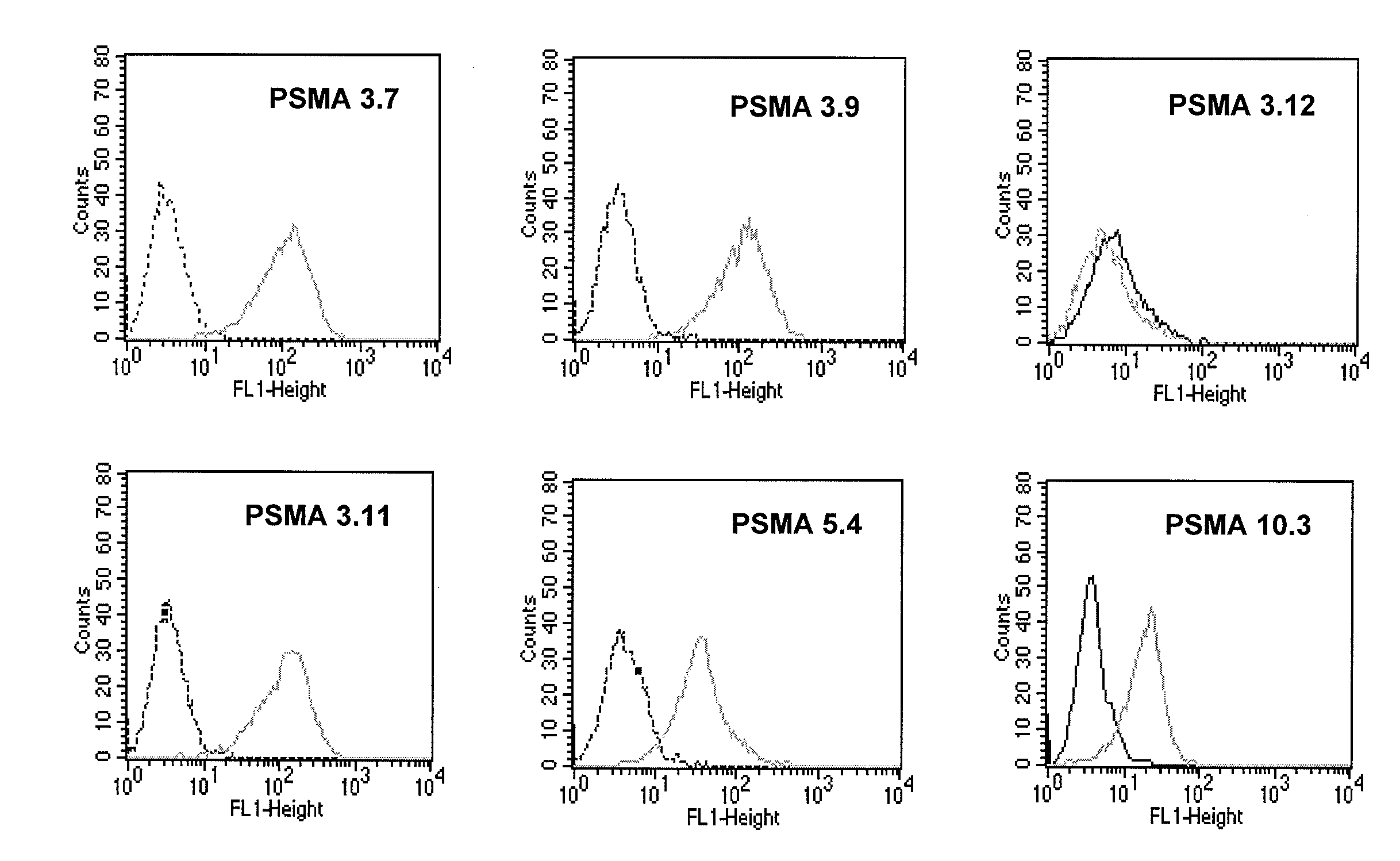
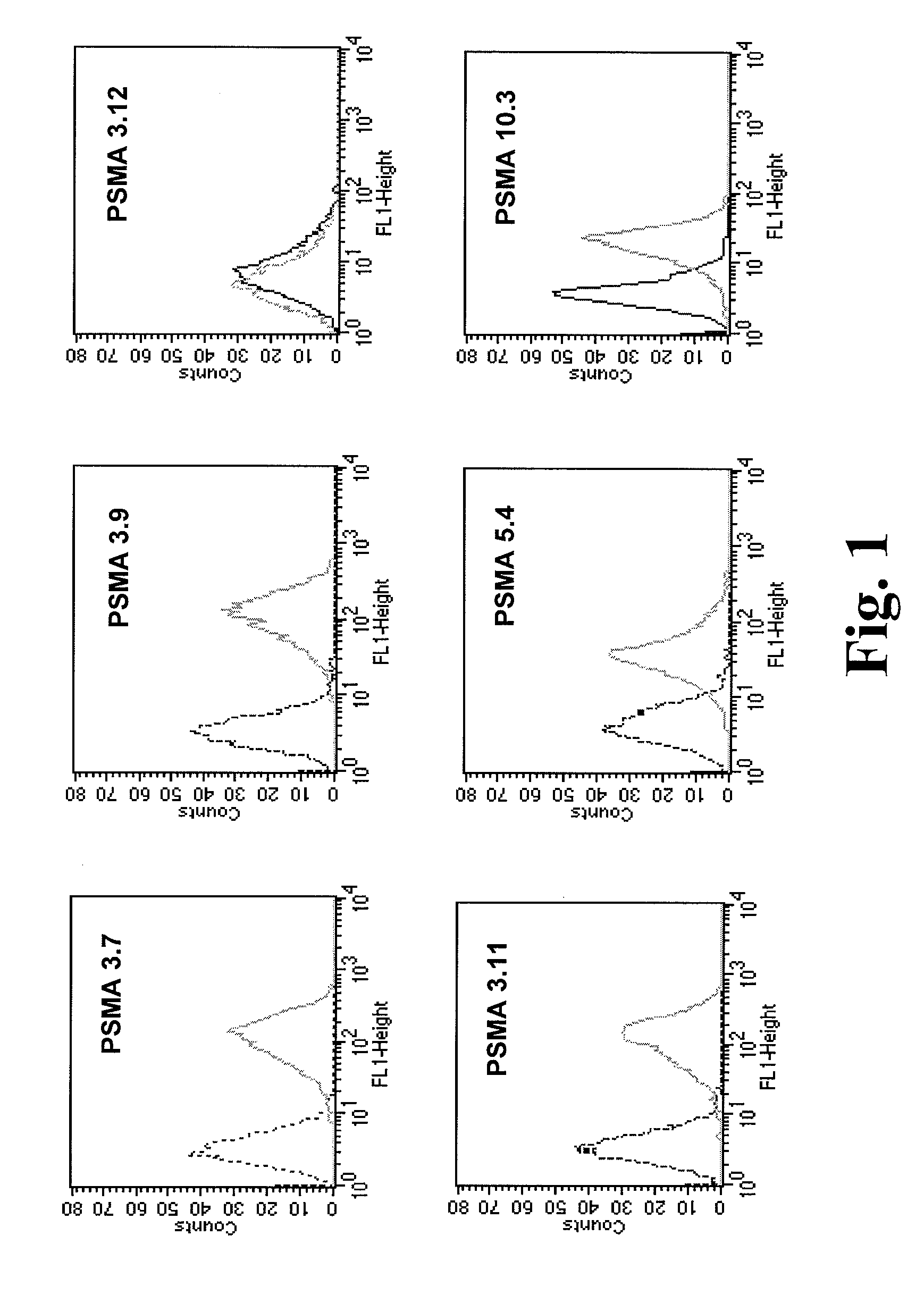
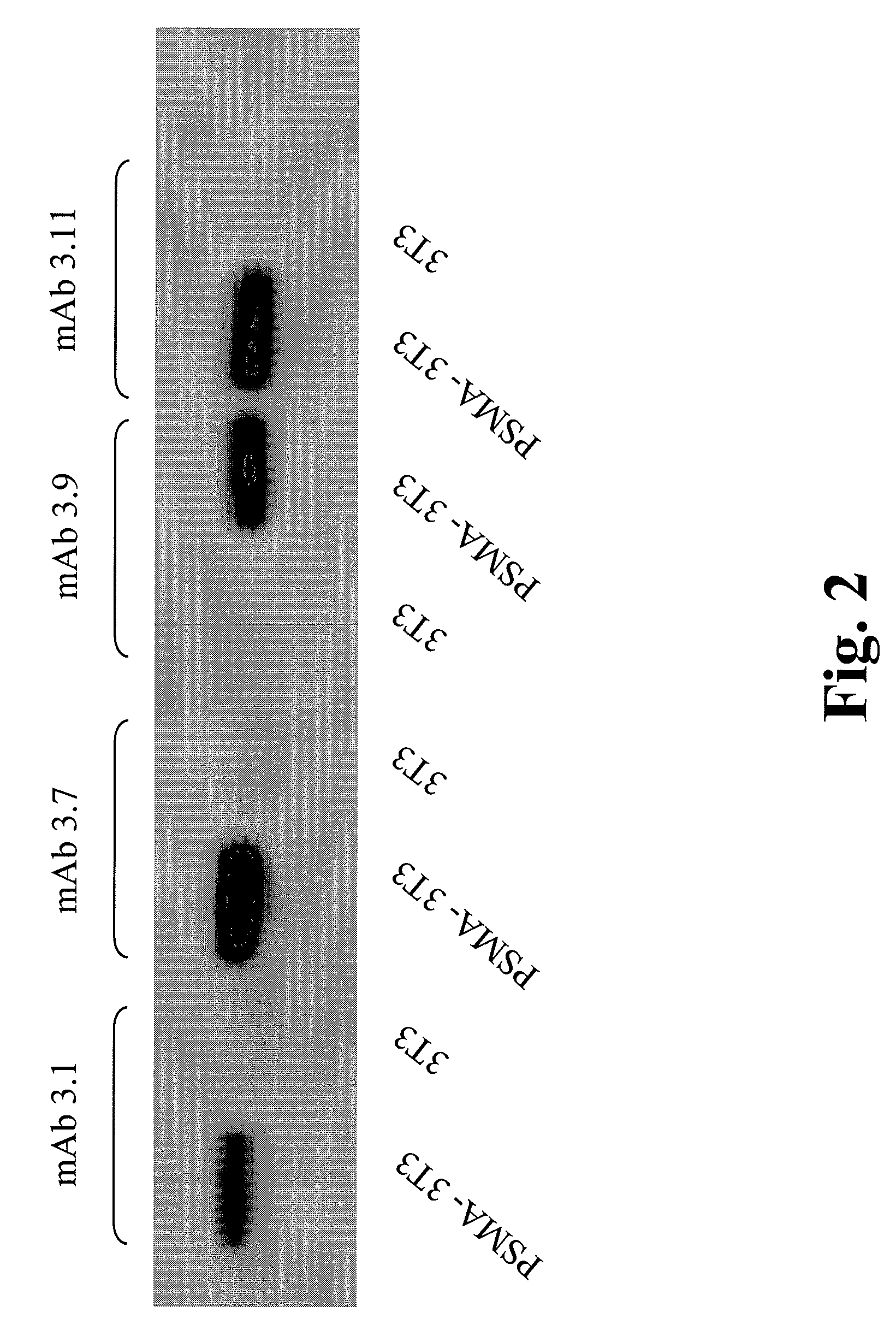
Monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen
The present invention relates to monoclonal antibodies that bind to the extracellular domain of prostate-specific membrane antigen (PSMA), hybridoma cell lines producing the antibodies, and methods of using such antibodies for diagnosis and treatment of cancer. In particular, thirty-five monoclonal antibodies reactive with PSMA expressed on the cell surface are exemplified. Additionally, the present invention relates to a novel protein variant (PSM') of PSMA detected by a number of the antibodies of the invention. The hydrolase activity of PSMA and PSM' allows the use of an immunoenzymatic assay for their detection.
Owner:ER SQUIBB & SONS INC
Monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen
InactiveUS6962981B1VirusesCell receptors/surface-antigens/surface-determinantsImmunoenzymatic assayAntigen
The present invention relates to monoclonal antibodies that bind to the extracellular domain of prostate-specific membrane antigen (PSMA), hybridoma cell lines producing the antibodies, and methods of using such antibodies for diagnosis and treatment of cancer. In particular, thirty-five monoclonal antibodies reactive with PSMA expressed on the cell surface are exemplified. Additionally, the present invention relates to a novel protein variant (PSM′) of the PSMA detected by a number of the antibodies of the invention. The hydrolase activity of PSMA and PSM′ allows the use of an immunoenzymatic assay for their detection.
Owner:ER SQUIBB & SONS INC
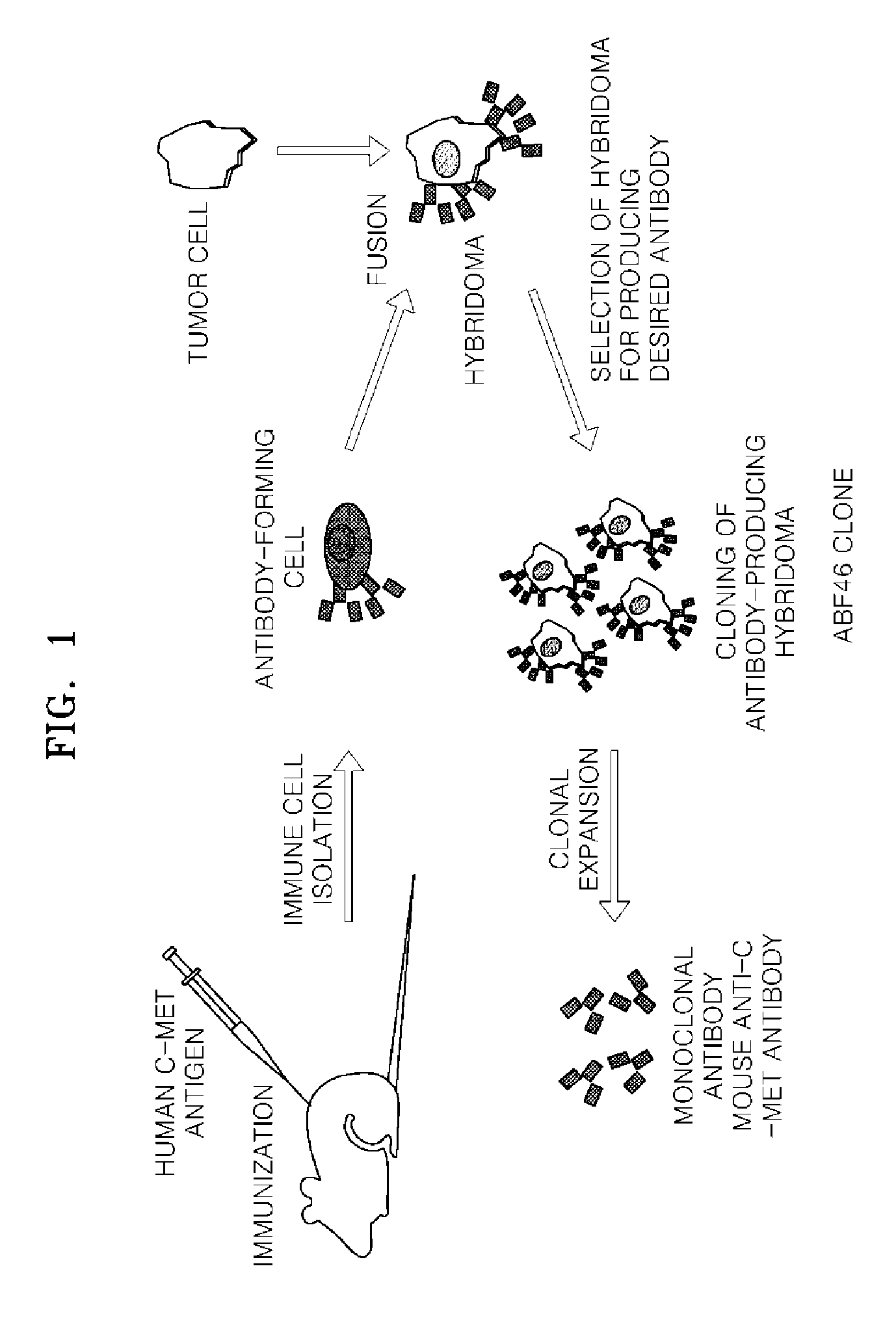
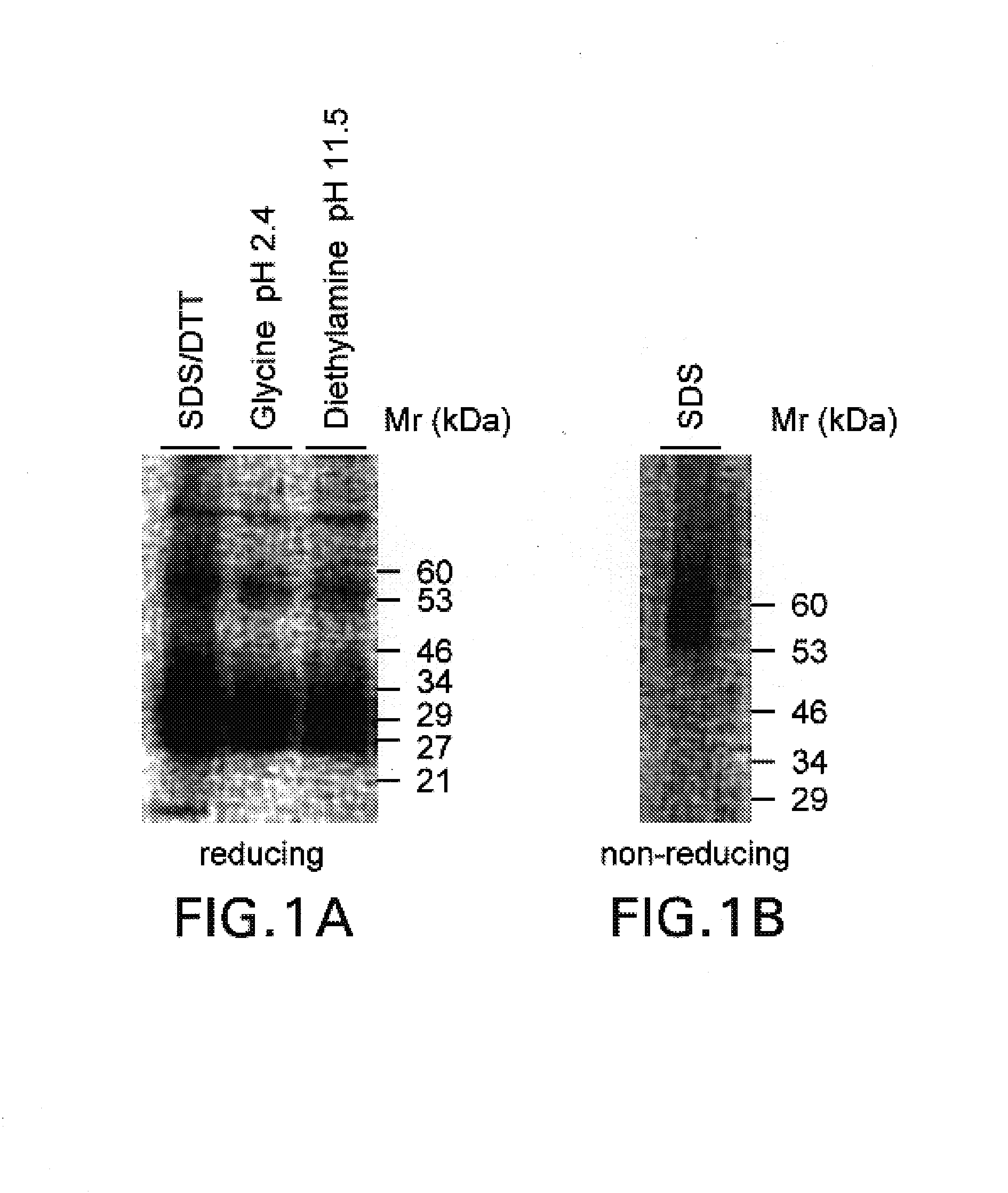
Antibody specifically binding to c-met and use thereof
Antibodies specifically binding to c-Met protein, hybridoma cell lines, and compositions comprising the antibodies are disclosed herein. Methods of making and using the antibodies and compositions are also disclosed.
Owner:SAMSUNG ELECTRONICS CO LTD
Compositions of PSMA antibodies
InactiveUS20080286284A1Effectiveness of treatmentOrganic active ingredientsFungiAntigen Binding FragmentCancers diagnosis
The invention includes antibodies or antigen-binding fragments thereof which bind specifically to conformational epitopes on the extracellular domain of PSMA, compositions containing one or a combination of such antibodies or antigen-binding fragments thereof, hybridoma cell lines that produce the antibodies, and methods of using the antibodies or antigen-binding fragments thereof for cancer diagnosis and treatment. The invention also includes oligomeric forms of PSMA proteins, compositions comprising the multimers, and antibodies that selectively bind to the multimers.
Owner:AMGEN FREMONT INC +1
Isolation of proteins
InactiveUS20050176122A1Other chemical processesSolid sorbent liquid separationSpecial classCarboxylic acid
Owner:UPFRONT CHROMATOGRAPHY
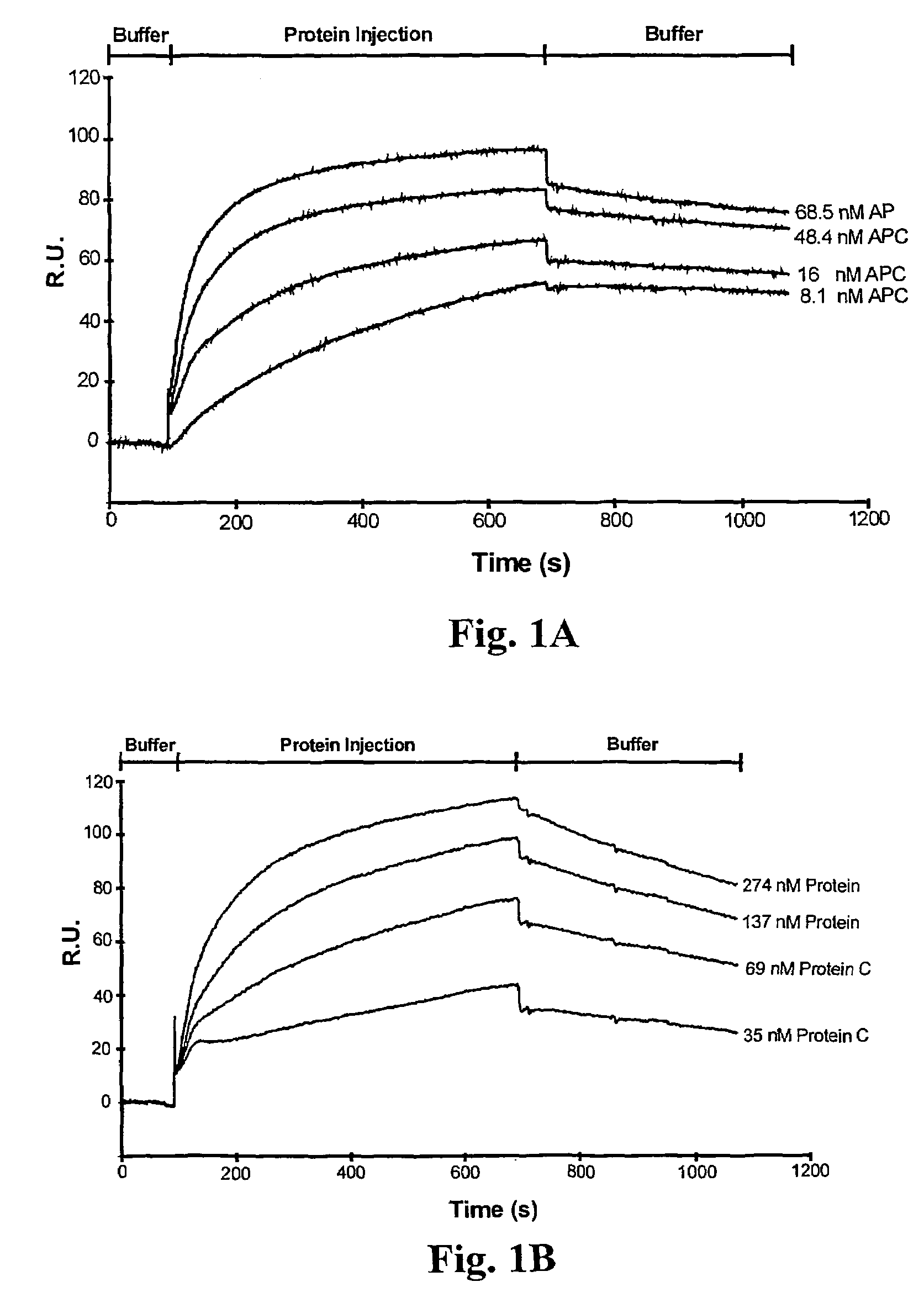
Assay for rapid detection of human activated protein C and highly specific monoclonal antibody therefor
InactiveUS6989241B2High selectivityStrong specificityAnimal cellsMicrobiological testing/measurementProtein activationHybridoma cell
Owner:OKLAHOMA MEDICAL RES FOUND
Isolation of proteins
InactiveUS6919436B2Other chemical processesSolid sorbent liquid separationSpecial classCarboxylic acid
The present invention relates to a novel method for the isolation or purification of immunoglobulins (a special class of proteins) from a solution containing immunoglobulins, e.g. hybridoma cell culture supernatants, animal plasma or sera, or colostrum. The method includes the use of a minimum of salts, such as lyotropic salts, in the binding process and preferably also the use of small amounts of organic solvents in the elution process. The solid phase matrices, preferably epichlorohydrin activiated agarose matricees, are functionalised with mono- or bicyclic aromatic or heteroaromatic ligands (molecular weight: at the most 500 Dalton) which, preferably, comprises an acidic substituent, e.g. a carboxylic acid. The matrices utilised show excellent properties in a “Standard Immunoglobulin Binding Test” and in a “Monoclonal Antibody Array Binding Test” with respect to binding efficiency and purity, and are stable in 1M NaOH.
Owner:UPFRONT CHROMATOGRAPHY
Human SARS-CoV-2 monoclonal antibody and preparation method and application thereof
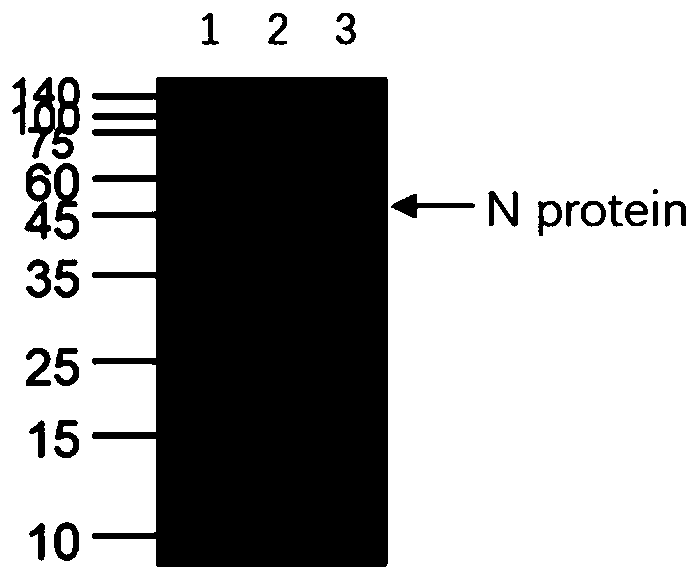
PendingCN111153991AHigh affinityStrong specificitySsRNA viruses positive-senseVirus peptidesBALB/cMonoclonal antibody agent
The invention discloses a human SARS-CoV-2 monoclonal antibody. The preparation method of the human SARS-CoV-2 monoclonal antibody comprises the steps: adopting SARS-CoV Nucleocapsid recombinant protein as immunogen, immunizing BALB / c mice, performing fusion and subcloning on spleen cells and myeloma cells of mice, then performing a large amount of repeated screening and domestication of cell lines through commercialized products SARS-CoV-2 Nucleocapsid and MERS Nucleocapsid so as to obtain a hybridoma cell line capable of secreting the SARS-CoV-2-resistant N monoclonal antibody with high affinity and high specificity finally and successfully, and finally performing ascites preparation and purification so as to obtain the monoclonal antibody, wherein the amino acid sequence of the SARS-CoVNucleocapsid recombinant protein is shown in SEQ ID No. 1. The invention also discloses application of the monoclonal antibody in preparation of SARS-CoV-2 virus detection products and preparation ofdrugs for inhibiting the SARS-CoV-2 viruses. The monoclonal antibody can be used for detecting the SARS-CoV-2 in human throat swabs / pulmonary secretions and other samples by using a double-antibody sandwich method, and can be applied to diagnosis and prevention and control of SARS-CoV-2 virus infection and scientific researches of viruses and other study.
Owner:BEIJING BIOSYNTHESIS BIOTECH
Treatment and diagnosis of prostate cancer
InactiveUS7666425B1Effective diagnosisEffective treatmentPeptide/protein ingredientsMicrobiological testing/measurementAntigenProstatic epithelium
The present invention is directed to the use of antibodies or binding portions thereof, probes, ligands, or other biological agents which either recognize an extracellular domain of prostate specific membrane antigen or bind to and are internalized with prostate specific membrane antigen. These biological agents can be labeled and used for detection of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof. They also can be used alone or bound to a substance effective to ablate or kill such cells as a therapy for prostate cancer. Also disclosed are four hybridoma cell lines, each of which produces a monoclonal antibody recognizing extracellular domains of prostate specific membrane antigens of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof.
Owner:CORNELL RES FOUNDATION INC
PSMA antibodies and protein multimers
The invention includes antibodies or antigen-binding fragments thereof which bind specifically to conformational epitopes on the extracellular domain of PSMA, compositions containing one or a combination of such antibodies or antigen-binding fragments thereof, hybridoma cell lines that produce the antibodies, and methods of using the antibodies or antigen-binding fragments thereof for cancer diagnosis and treatment. The invention also includes oligomeric forms of PSMA proteins, compositions comprising the multimers, and antibodies that selectively bind to the multimers.
Owner:PSMA DEV COMPANY
Treatment and diagnosis of cancer
InactiveUS7163680B2Effective diagnosisEffective treatmentBiological material analysisNanomedicineAntigenVascular endothelium
Owner:CORNELL RES FOUNDATION INC
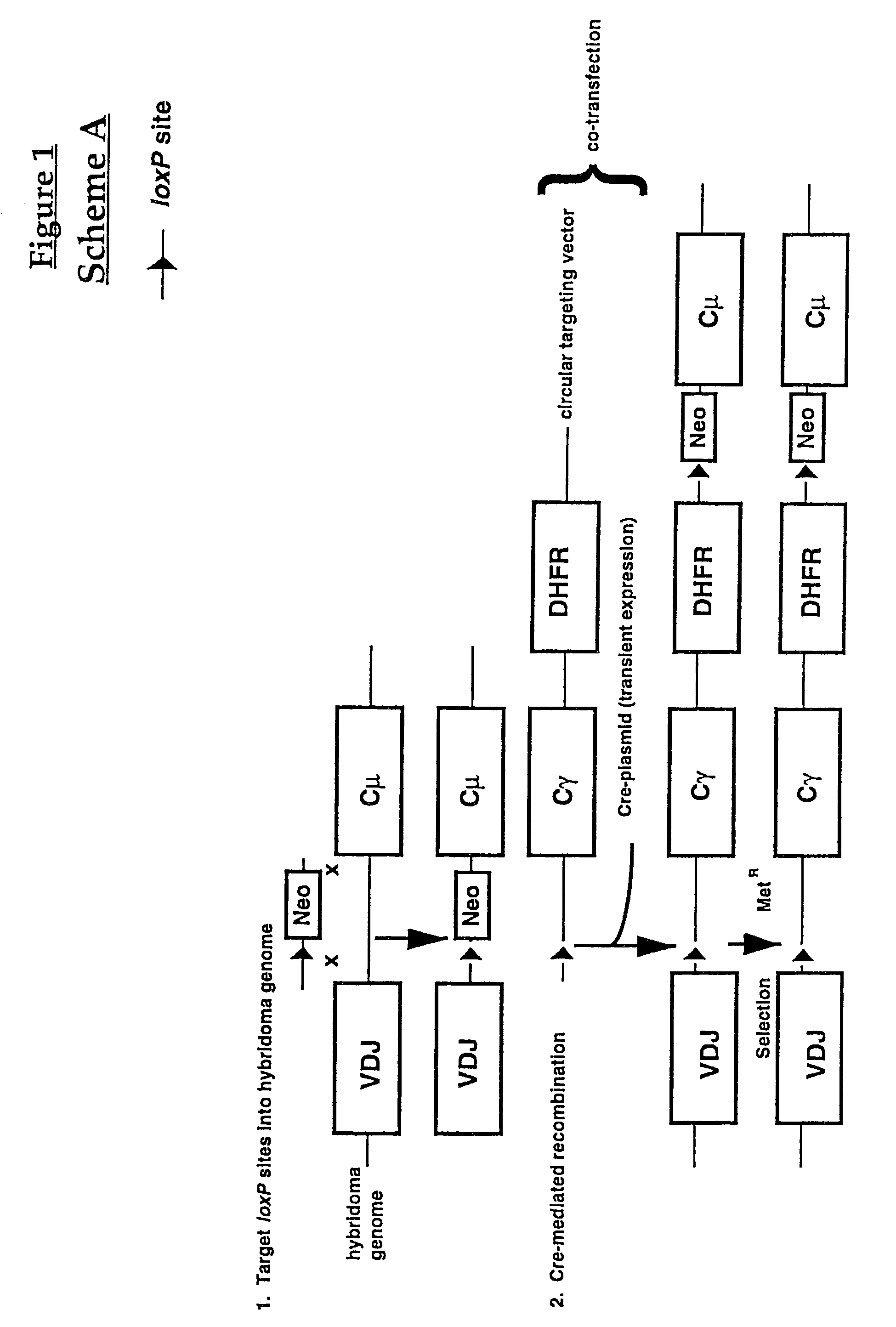
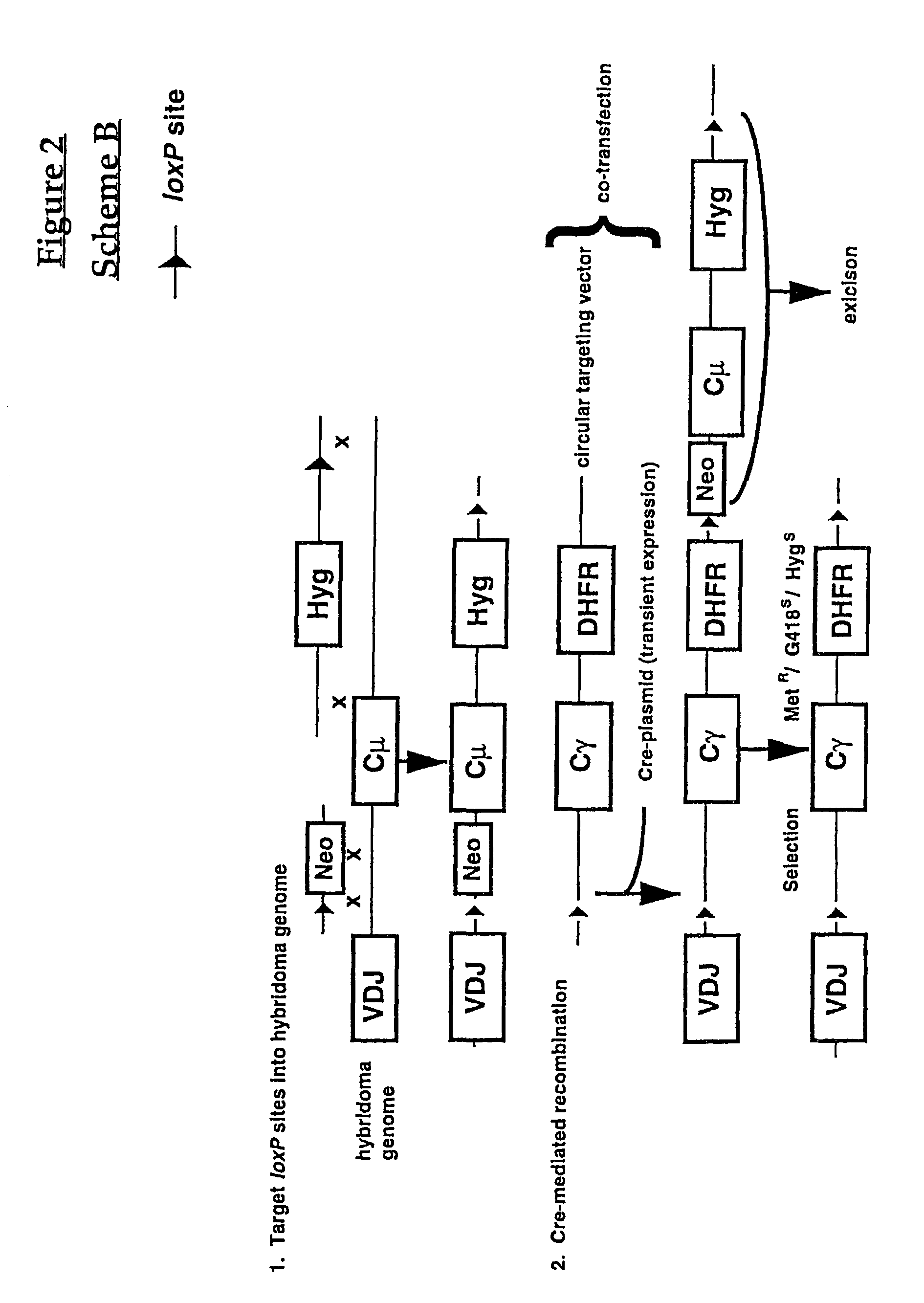
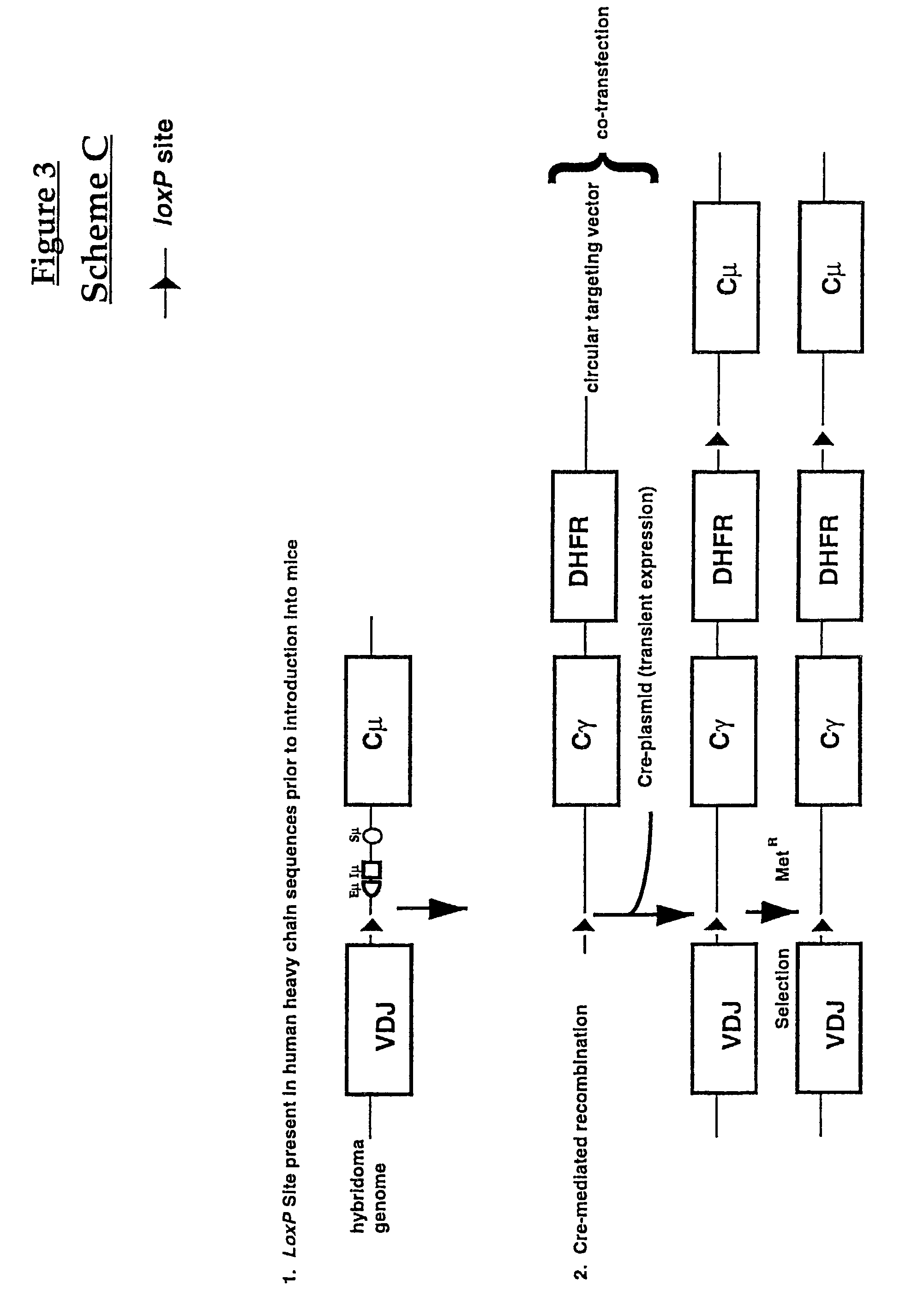
Production of antibodies using cre-mediated site-specific recombination
InactiveUS7145056B2Improve recombination efficiencyAnimal cellsSugar derivativesSite-specific recombinationAntibody-Producing Cells
A method to produce a cell expressing an antibody from a genomic sequence of the cell comprising a modified immunoglobulin locus using Cre-mediated site-specific recombination is disclosed. The method involves first transfecting an antibody-producing cell with a homology-targeting vector comprising a lox site and a targeting sequence homologous to a first DNA sequence adjacent to the region of the immunoglobulin loci of the genomic sequence which is to be converted to a modified region, so the first lox site is inserted into the genomic sequence via site-specific homologous recombination. Then the cell is transfected with a lox-targeting vector comprising a second lox site suitable for Cre-mediated recombination with the integrated lox site and a modifying sequence to convert the region of the immunoglobulin loci to the modified region. This conversion is performed by interacting the lox sites with Cre in vivo, so that the modifying sequence inserts into the genomic sequence via Cre-mediated site-specific recombination of the lox sites. Also disclosed are a form of the method used to produce a cell expressing a modified antibody molecule using Cre-mediated site-specific recombination, and antibody-producing cells obtainable by the disclosed methods. Class-switching modifications of human antibodies produced in murine hybridoma cells are exemplified.
Owner:JAPAN TOBACCO INC +1
Therapeutic methods for benzodiazepine derivatives
This invention provides an antibody with high affinity for single-stranded DNA, low or no affinity for double-stranded DNA, and capable of specifically binding a DNA hairpin and the hybridoma cell lines which produces these monoclonal antibodies. A chimeric mouse comprising these hybridoma cell lines and a histocompatible mouse is further provided. A method for screening for an agent which will inhibit anti-DNA antibody.DNA binding. One such agent is a benzodiazepine derivative. This invention therefore provides a method of inhibiting the binding of an anti-DNA antibody to its DNA ligand in a sample by contacting the sample with an effective amount of a benzodiazepine derivative.
Owner:RGT UNIV OF MICHIGAN
Antibody specifically binding to c-Met and methods of use
Antibodies specifically binding to c-Met protein, hybridoma cell lines, and compositions comprising the antibodies are disclosed herein. Methods of making and using the antibodies and compositions are also disclosed.
Owner:SAMSUNG ELECTRONICS CO LTD
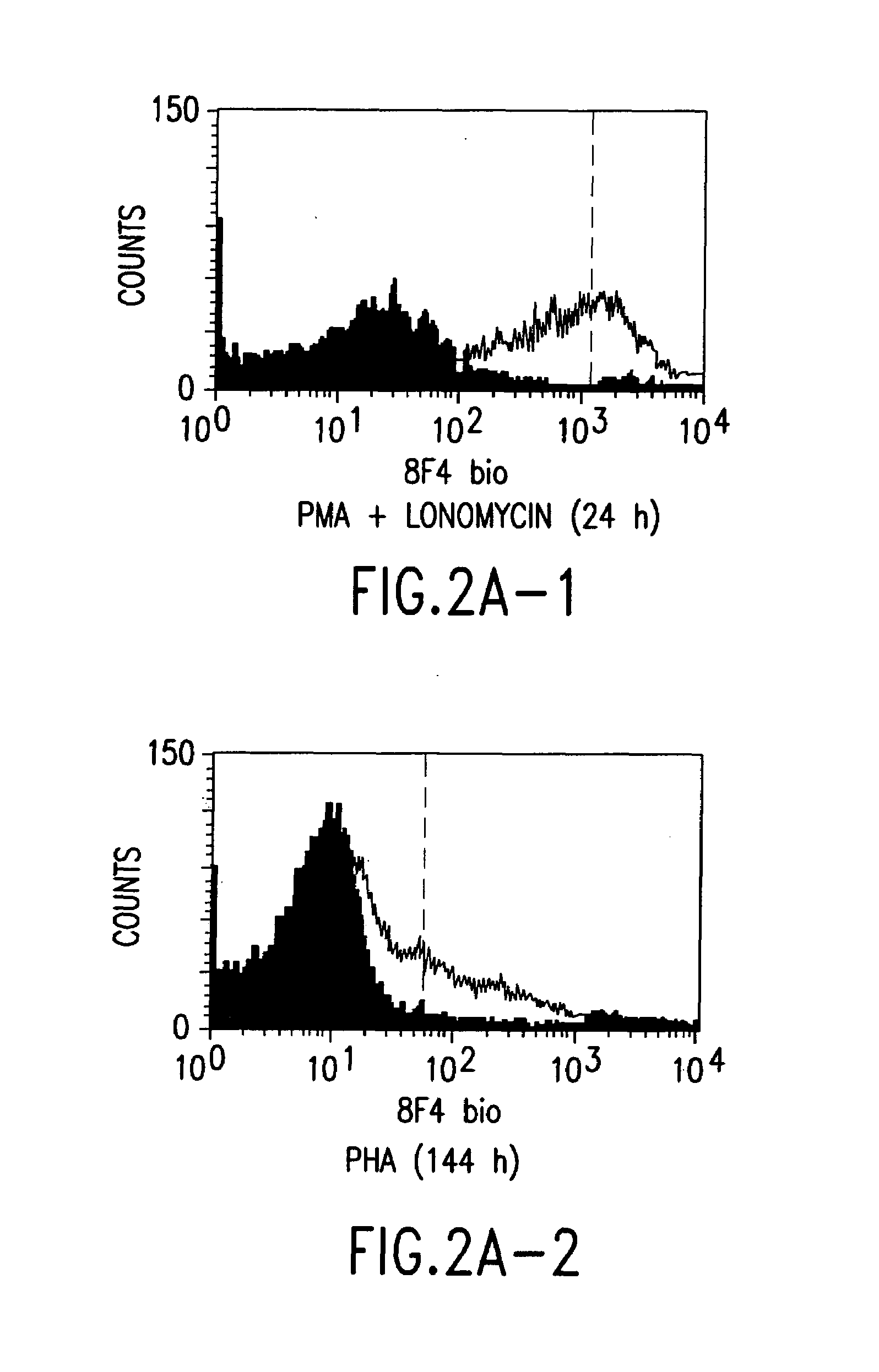
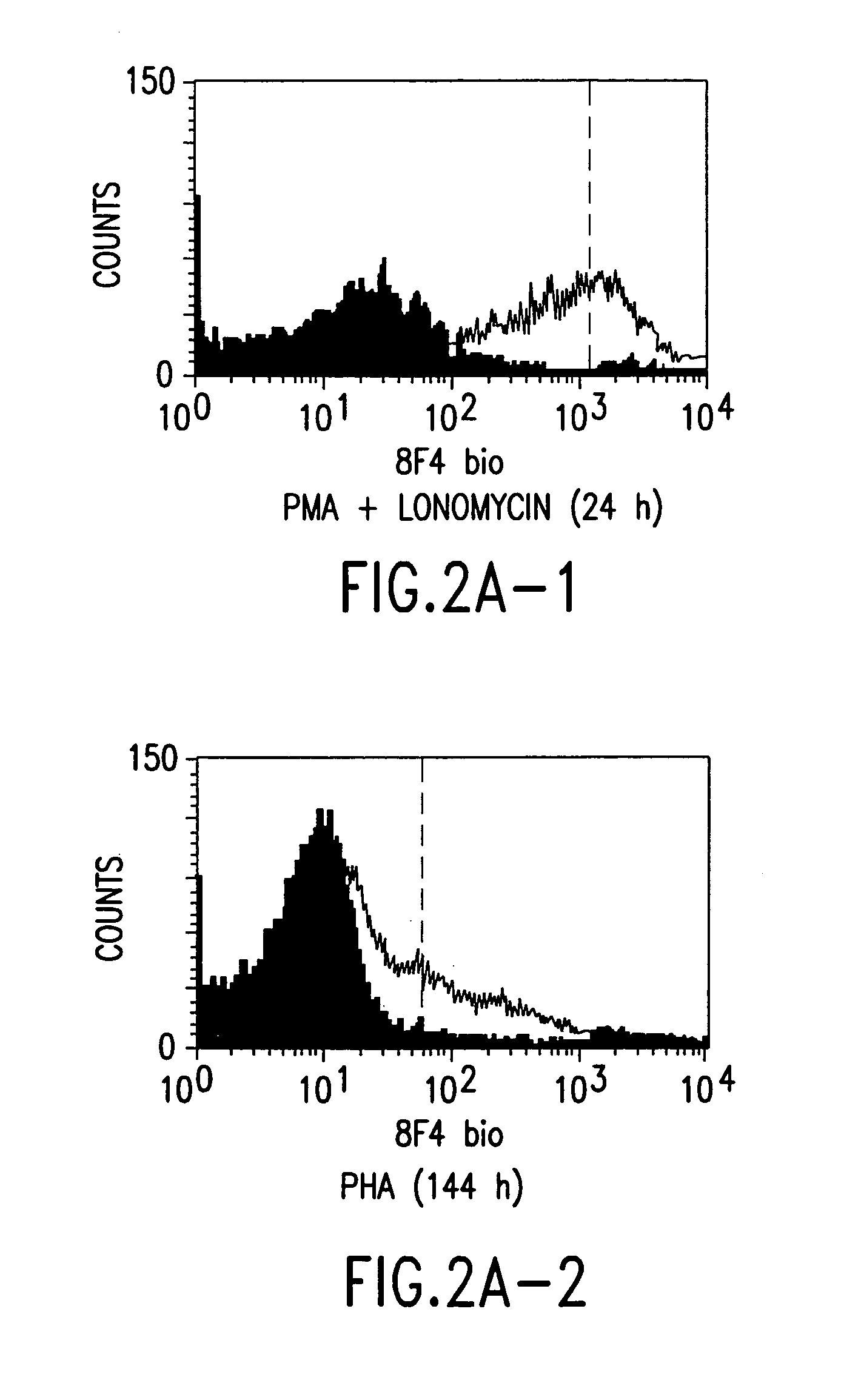
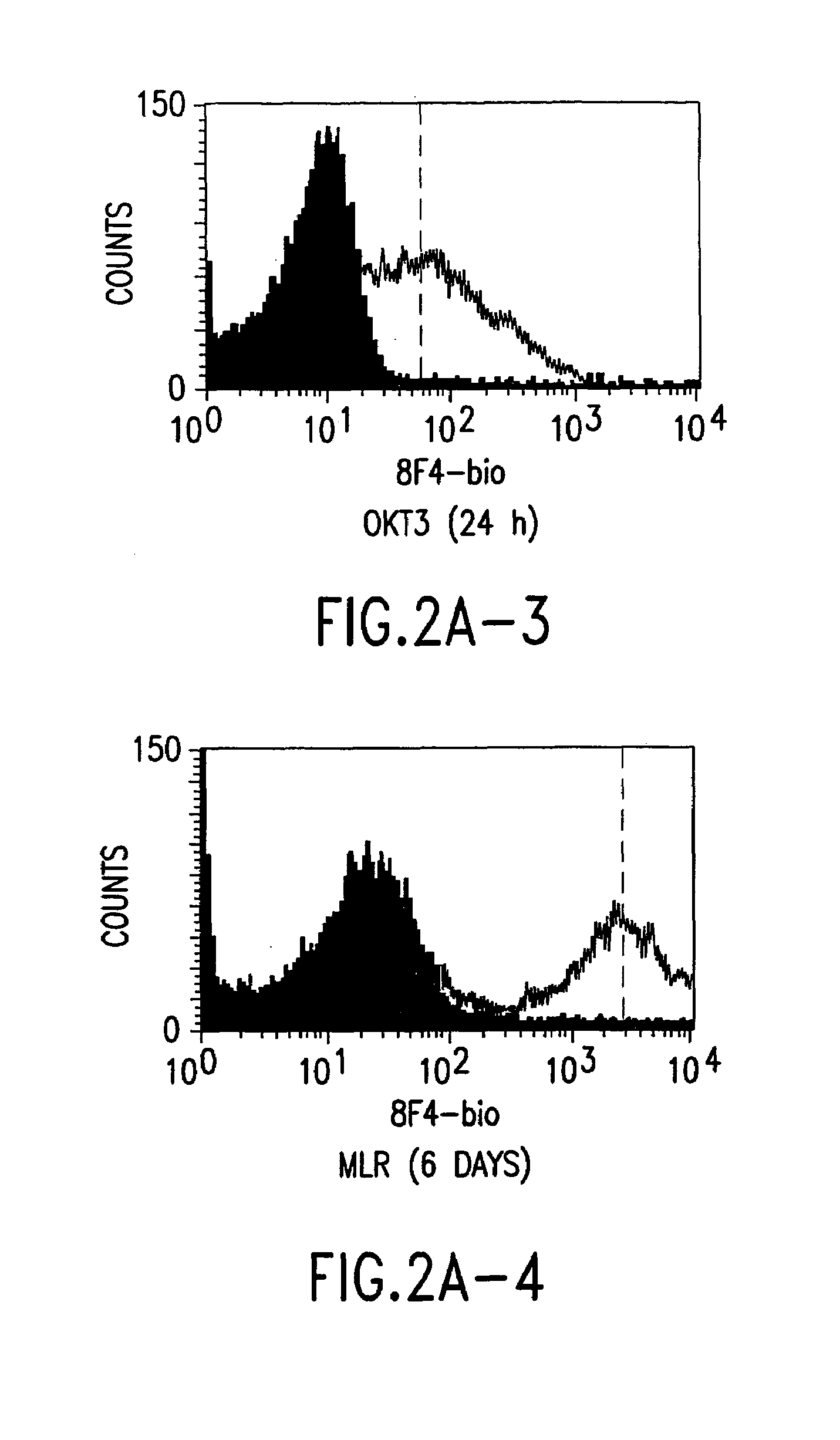
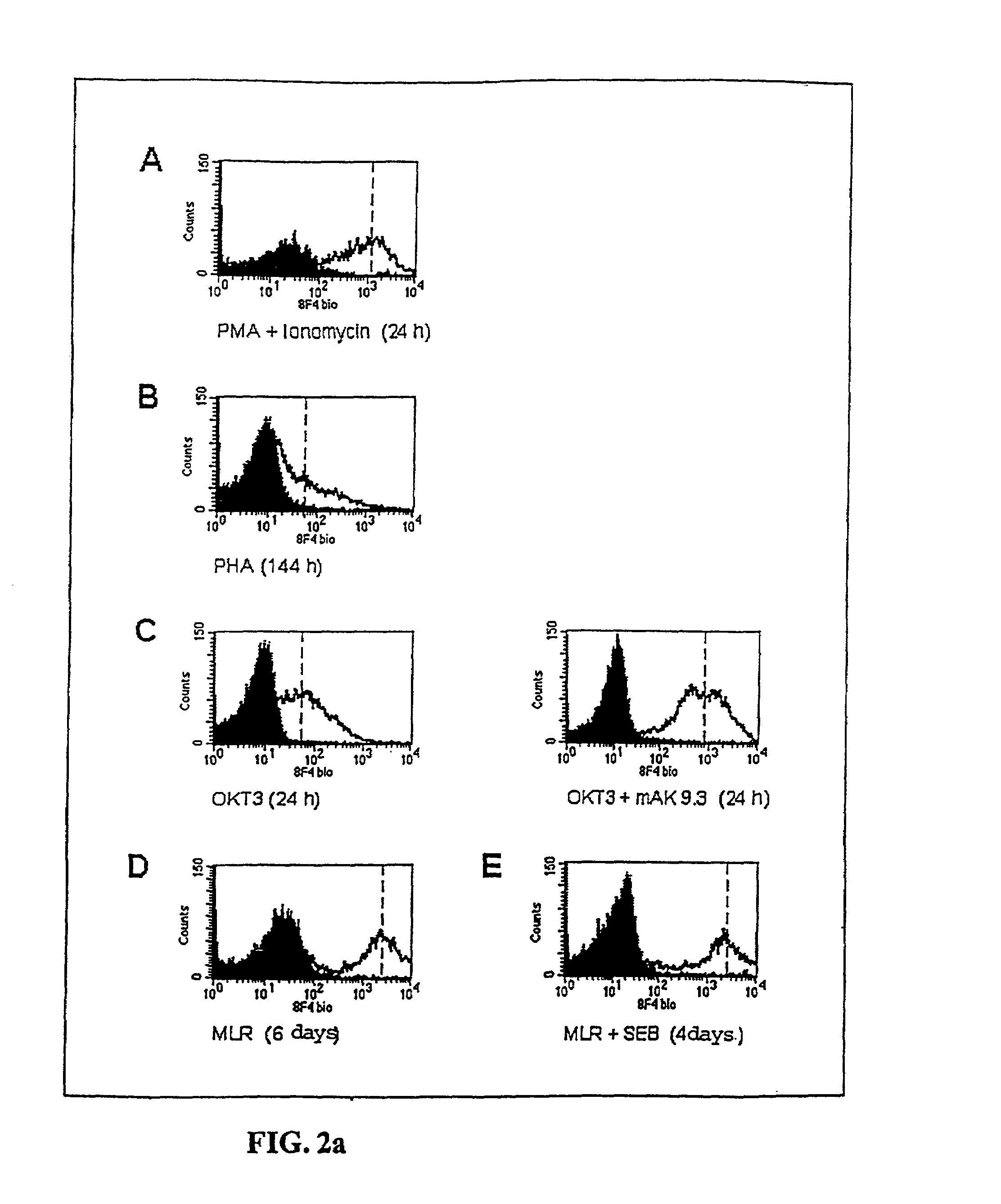
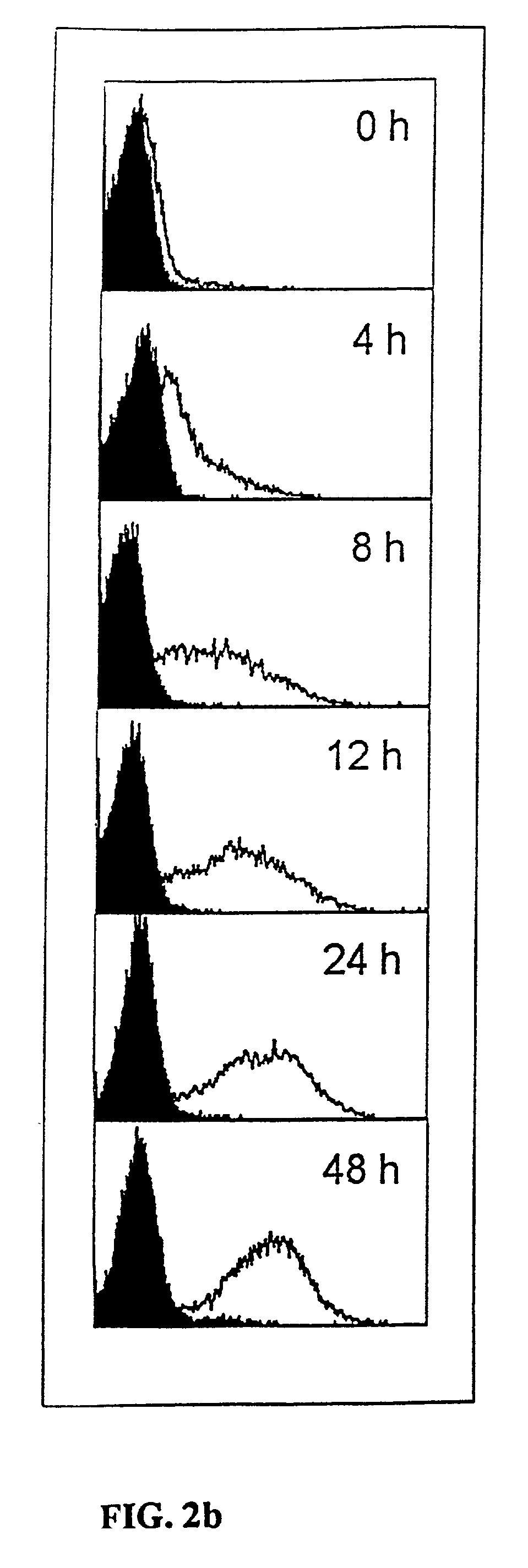
Anti-human T-cell costimulating polypeptide monoclonal antibodies
A polypeptide (8F4 molecule) with a T-cell costimulating biological activity is disclosed, as well as monoclonal antibodies against said 8F4 molecule and hybridoma cells which produce the monoclonal antibodies, the use as medicaments of substances which inhibit the biological activity of the disclosed 8F4 polypeptide, in particular monoclonal antibodies, natural or synthetic ligands, agonists or antagonists, in particular for preventing or treating diseases which involve the immune system, the use of said 8F4 molecule or cells containing said 8F4 molecule as medicaments, in particular for preventing or treating diseases which involve the immune system, and the use of substances which specifically recognize the disclosed polypeptide, in particular monoclonal antibodies, natural or synthetic ligands, agonists or antagonists, for diagnosing diseases which involve the immune system.
Owner:BUNDESREPUBLIK DEUT IETZVERTRETEN DURCH DAS ROBERT KOCH INST VERTRETEN DURCH SEINEN PRASI
Treatment and diagnosis of prostate cancer
InactiveUS7112412B1Effective diagnosisEffective treatmentPeptide/protein ingredientsMicrobiological testing/measurementAntigenProstatic epithelium
The present invention is directed to the use of antibodies or binding portions thereof, probes, ligands, or other biological agents which either recognize an extracellular domain of prostate specific membrane antigen or bind to and are internalized with prostate specific membrane antigen. These biological agents can be labeled and used for detection of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof. They also can be used alone or bound to a substance effective to ablate or kill such cells as a therapy for prostate cancer. Also disclosed are four hybridoma cell lines, each of which produces a monoclonal antibody recognizing extracellular domains of prostate specific membrane antigens of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof.
Owner:CORNELL RES FOUNDATION INC
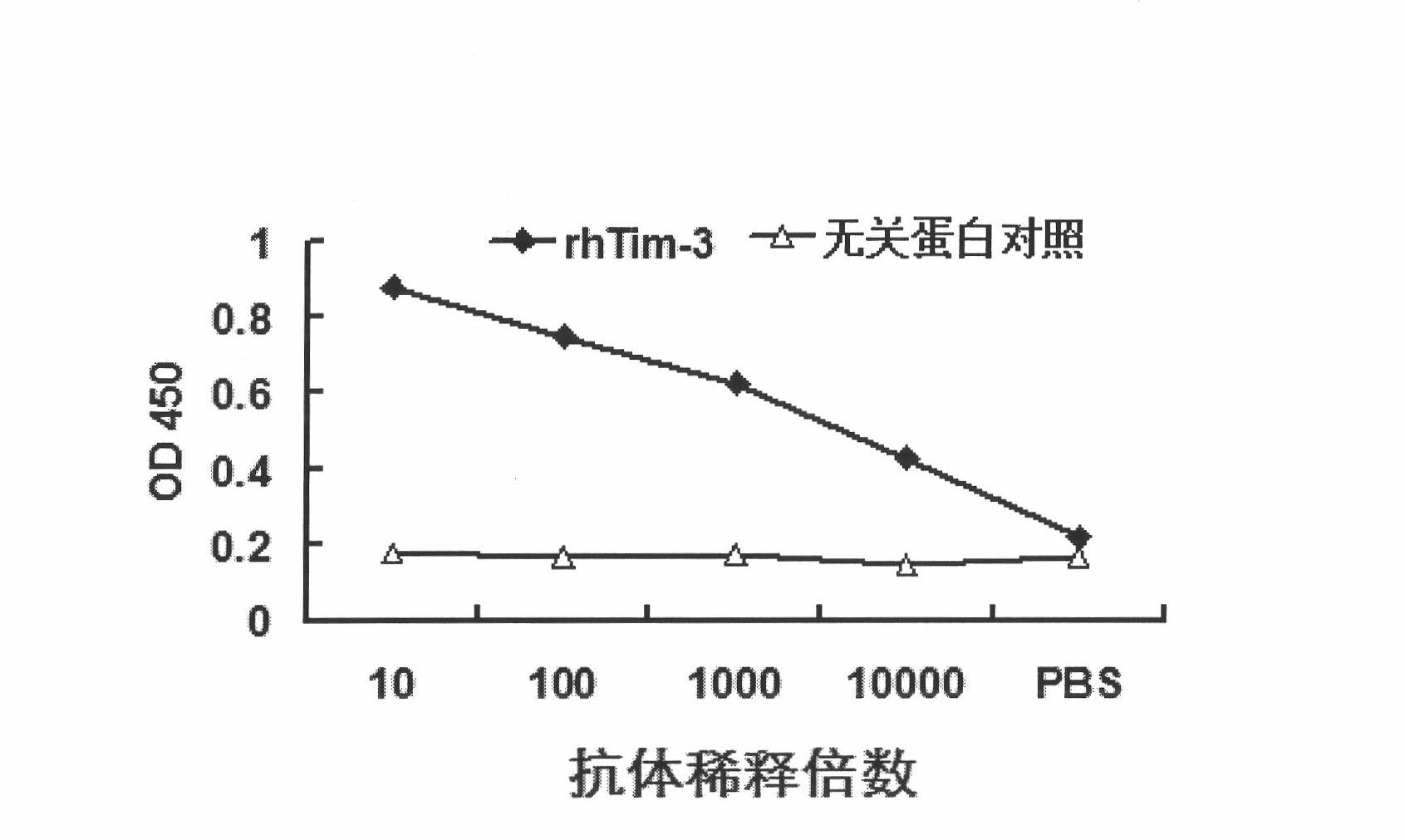
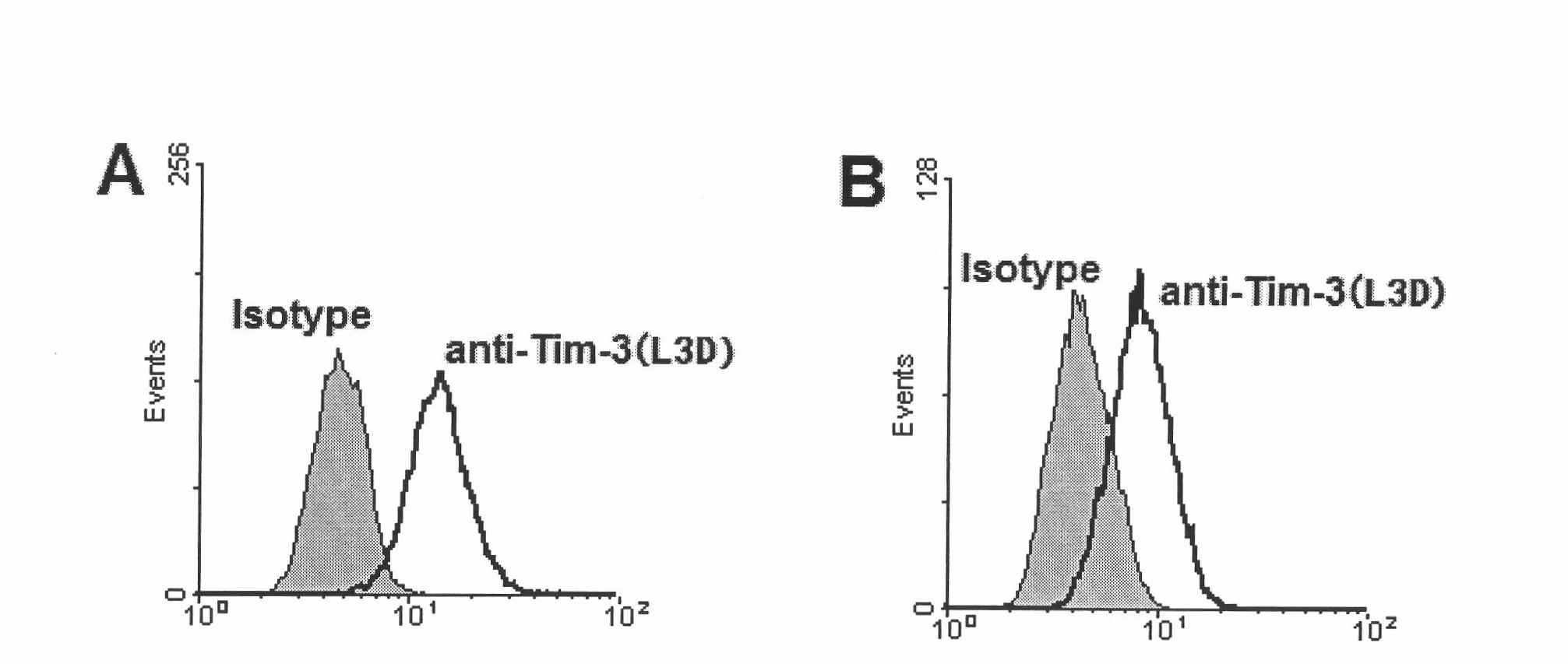
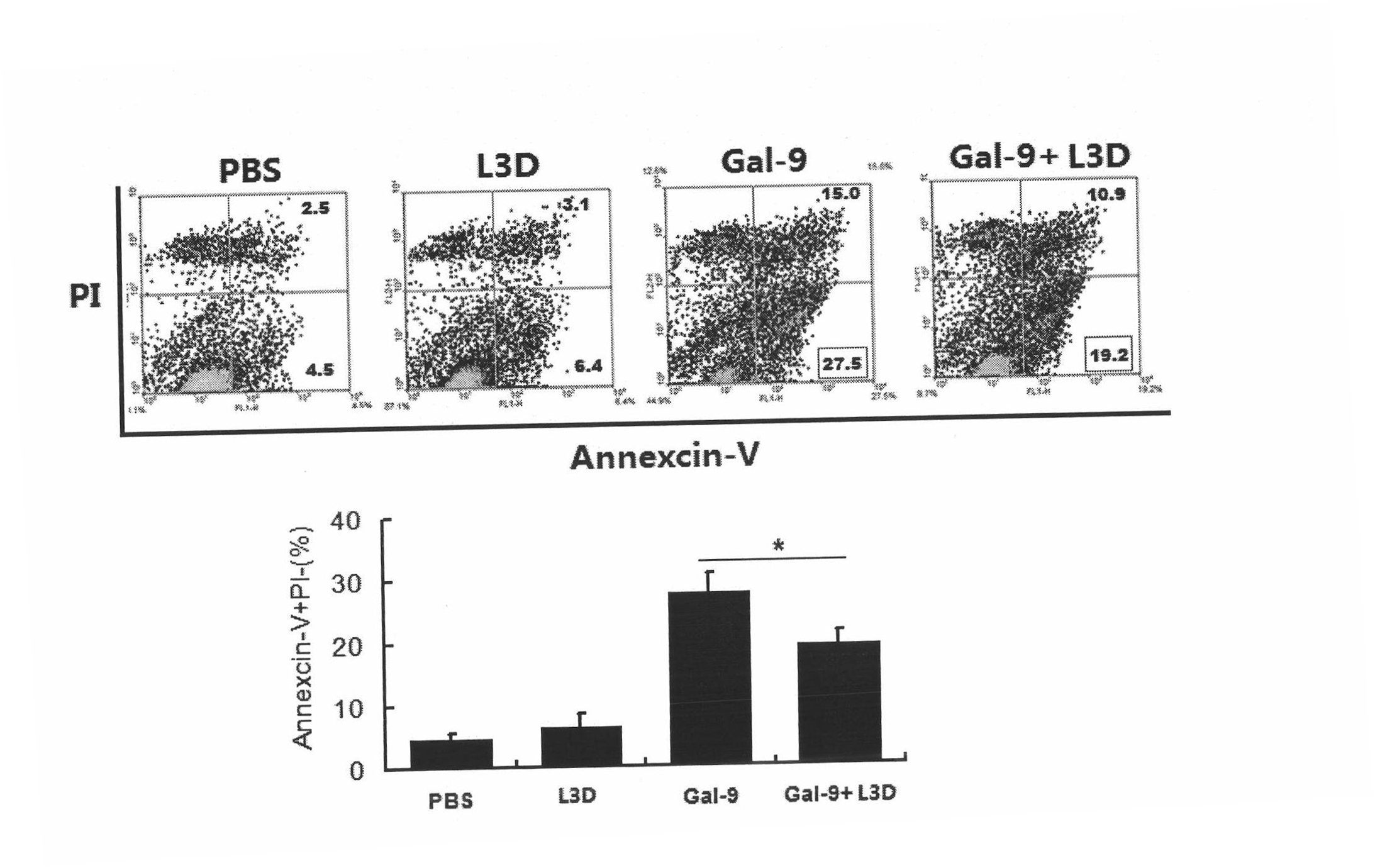
Anti-human Tim-3 neutralized monoclonal antibody L3D and application thereof
The invention discloses an anti-human Tim-3 neutralized monoclonal antibody L3D and an application thereof. Antibody light and heavy chain variable region genes are cloned from a prepared anti-human Tim-3 neutralized monoclonal antibody L3D hybridoma cell; and the obtained light chain and heavy chain variable region genes can be used for encoding a monoclonal antibody variable region. Based on the light and heavy chain variable region genes of the monoclonal antibody, a plurality of small molecular genetic engineering antibodies can be constructed and expressed. Polypeptides or proteins encoded on the basis of the genes can be cross-linked with a plurality of bioactive molecules, so that a Tim-3 expression level detection reagent is prepared for diagnosing and treating diseases caused by abnormal expression of Tim-3.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Methods of modulating T lymphocyte costimulation with an antibody to an 8F4 polypeptide
A polypeptide (8F4 molecule) with a T-cell costimulating biological activity is disclosed, as well as monoclonal antibodies against said 8F4 molecule and hybridoma cells which produce the monoclonal antibodies, the use as medicaments of substances which inhibit the biological activity of the disclosed 8F4 polypeptide, in particular monoclonal antibodies, natural or synthetic ligands, agonists or antagonists, in particular for preventing or treating diseases which involve the immune system, the use of said 8F4 molecule or cells containing said 8F4 molecule as medicaments, in particular for preventing or treating diseases which involve the immune system, and the use of substances which specifically recognize the disclosed polypeptide, in particular monoclonal antibodies, natural or synthetic ligands, agonists or antagonists, for diagnosing diseases which involve the immune system.
Owner:BUNDESREPUBLIK DEUTSCHLAND
Hybridoma cell strain ST03, anti-aflatoxin biosynthesis precursor ST monoclonal antibody and application thereof
ActiveCN103849604ANo cross reactionHigh sensitivityTissue cultureImmunoglobulins against fungi/algae/lichensAbzymeAflatoxin biosynthesis
The invention relates to a hybridoma cell strain ST03, an anti-aflatoxin biosynthesis precursor ST monoclonal antibody and application thereof. The hybridoma cell strain ST03 with the preservation number of CCTCCNO.C2013187 can be used for preparing high-valence anti-aflatoxin biosynthesis precursor ST monoclonal antibody, wherein valence measured by an enzyme linked immunosorbent assay (ELISA) method of the anti-aflatoxin biosynthesis precursor ST mouse ascites antibody can reach 6.4*10<5>. The anti-aflatoxin biosynthesis precursor ST monoclonal antibody is high in sensitivity, has 50% inhibition concentration IC50 of 0.36 ng / mL on the aflatoxin biosynthesis precursor ST, has no cross reaction with the aflatoxin B1, aflatoxin B2, aflatoxin G1 and G2, and can be applied to measuring content of the aflatoxin biosynthesis precursor ST.
Owner:OIL CROPS RES INST CHINESE ACAD OF AGRI SCI
Hybridoma cell line 1C11 and anti-aflatoxin general monoclonal antibody generated by same as well as applications thereof
ActiveCN101993855AHigh sensitivityHigh practical application valueMicroorganism based processesTissue cultureCell strainHybridoma cell
The invention provides a hybridoma cell line 1C11 and an anti-aflatoxin general monoclonal antibody secreted by the same as well as the applications thereof. The hybridoma cell line 1C11 can be used for preparing a high-titer aflatoxin antibody, and a mouse hydroperitoneum antibody is measured to reach 5.12*106 by using an ELISA (Enzyme-Linked Immunosorbent Assay). The anti-aflatoxin general monoclonal antibody has high sensitivity, respectively reaches the IC50 (50% inhibiting concentration) of aflatoxin B1, B2, G1 and G2 to be 1.2, 1.3, 2.2 and 18.0 pg / mL, is the antibody with highest sensitivity among currently reported four aflatoxin antibodies, is used for measuring the total aflatoxin amounts, i.e. the total amounts of the aflatoxin B1, B2, G1 and G2 and has great practical application values.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
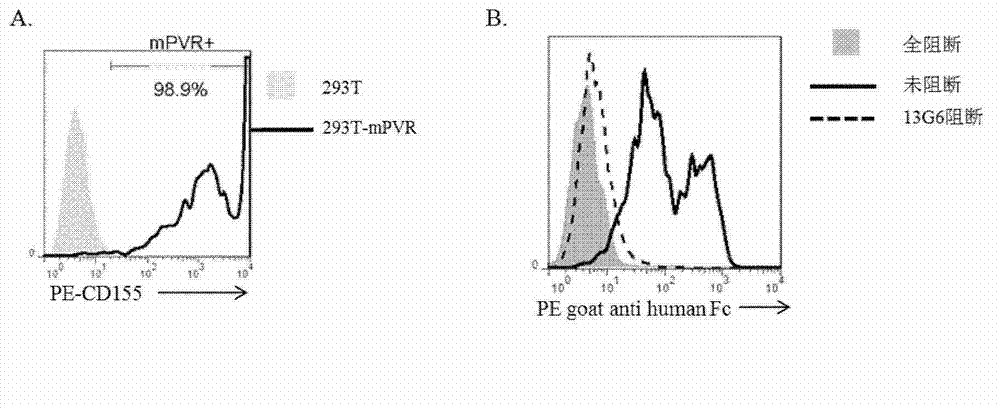
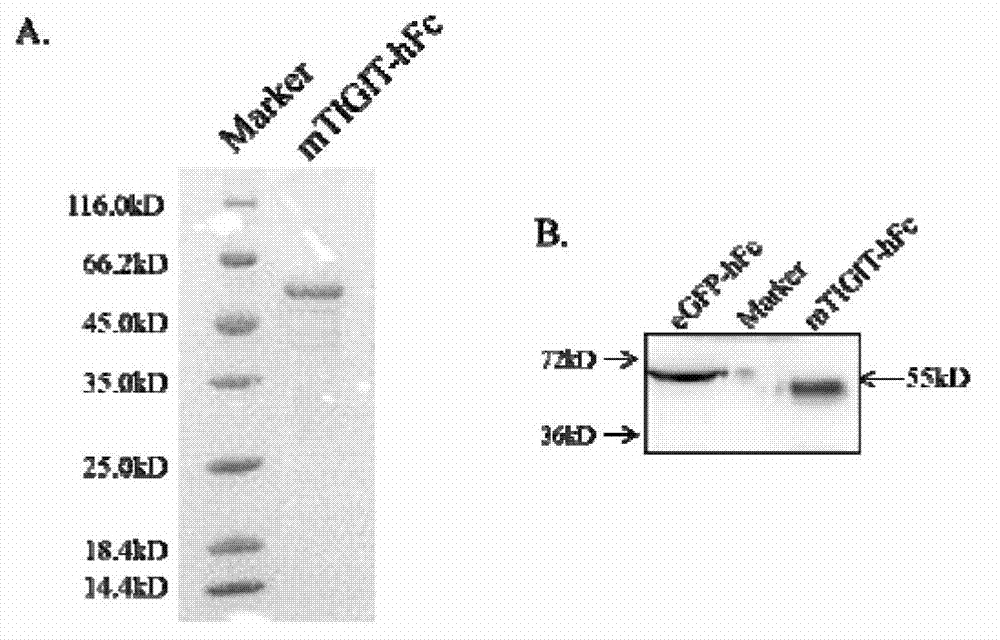
Specific anti-mouse TIGIT monoclonal antibody and preparation method, identification and application thereof
ActiveCN103073644AHigh potencyStrong specificityImmunoglobulins against cell receptors/antigens/surface-determinantsTissue cultureWestern blotPoliovirus Receptor
The invention relates to an anti-mouse TIGIT (T cell Ig and ITIM domain), and a hybridoma cell strain mTIGIT-mAb-13G6 (the preservation NO: CCTCC NO: C201299) for producing the monoclonal antibody, and further relates to a preparation method and the application of the monoclonal antibody. The monoclonal antibody can efficiently prevent combination of mTIGIT and mPVR (poliovirus receptor), and can be used for Western blot, ELISA and Flow Cytometry for mTIGIT molecule detection.
Owner:IMMUNOPHARMACEUTIC INST OF HEFEI RUIDA CO LTD
Methods for treatment of asthmatic disorders using a monoclonal antibody to 8F4 polypeptide
The present invention relates to methods and compositions for the prevention and treatment and prevention of immune system disorders, including cancer, AIDS, asthmatic disorders, autoimmune diseases, organ transplant rejection and chronic viral diseases such as HCV or HBV infections. The therapeutic methods of the invention comprise administering molecules that modulate the activity of 8F4, thereby modulating costimulation of T cells. The present invention further provides monoclonal antibodies against the 8F4 molecule and hybridoma cells which produce said monoclonal antibodies. Pharmaceutical compositions comprising molecules that modulate the activity of 8F4 are also provided.
Owner:BUNDESREPUBLIK DEUTSCHLAND
H5 subtype avian flu virus hemagglutinin protein monoclonal antibody, and its preparing method and use
This invention relates to a monoclonal antibody capable of combining with H5 subtype avian influenza virus HA protein specifically, the hybridoma cell line secreting said antibody and a preparing method. The invention also relates to a serial test kit for testing H5 subtype avian influenza virus by the antibody and a bit of said antibody in the test sample of the virus and its usage in treatment.
Owner:XIAMEN UNIV
Monoclonal antibody specific for the extracellular domain of prostate specific membrane antigen
InactiveUS7476513B2VirusesIn-vivo radioactive preparationsImmunoenzymatic assayProstate specific membrane
The present invention relates to monoclonal antibodies that bind to the extracellular domain of prostate-specific membrane antigen (PSMA), hybridoma cell lines producing the antibodies, and methods of using such antibodies for diagnosis and treatment of cancer. In particular, thirty-five monoclonal antibodies reactive with PSMA expressed on the cell surface are exemplified. Additionally, the present invention relates to a novel protein variant (PSM′) of PSMA detected by a number of the antibodies of the invention. The hydrolase activity of PSMA and PSM′ allows the use of an immunoenzymatic assay for their detection.
Owner:ER SQUIBB & SONS INC
Aflatoxin B1 flow lag immunization time distinguishing fluorescence rapid-detection kit and application thereof
The invention relates to an aflatoxin B1 flow lag immunization time distinguishing fluorescence rapid-detection kit and an application thereof. The kit comprises a fluorescent test strip and a sample reaction bottle containing an europium-labeled anti-aflatoxin B1 monoclonal antibody lyophilized product, wherein the fluorescent test strip comprises a cardboard, a water absorption pad, a detection pad and a sample pad are sequentially pasted on one surface of the cardboard from top to bottom, adjacent pads are connected at the connection in an overlapping manner, the detection pad treats a cellulose nitrate membrane as a base pad, a transverse quality control line and a detection line are arranged on the cellulose nitrate membrane from top to bottom, the quality control line is coated with a rabbit anti-mouse polyclonal antibody, and the detection line is coated with an aflatoxin B1 bovine serum albumin conjugate; and the anti-aflatoxin B1 monoclonal antibody is secreted by a hybridoma cell strain having a preservation number of CCTCC NO.C201015. The kit can be used for the quantitative determination of the content of the aflatoxin B1, and has the advantages of simple operation, rapidness and high accuracy.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Monoclonal antibodies binding to avian influenza virus subtype h5 haemagglutinin and use thereof
InactiveUS20090068637A1Bioreactor/fermenter combinationsBiological substance pretreatmentsHemagglutininAvian influenza virus
The present application provides monoclonal antibodies that specifically bind to the hemagglutinin of avian influenza virus subtype H5, as well as monoclonal antibodies capable of blocking at least 50% of the hemagglutinin binding activity of these monoclonal antibodies. Such antibodies are useful, for example, in the detection, diagnosis, prevention, and treatment of avian influenza virus. Also provided herein are hybridoma cell lines, isolated nucleic acid molecules, and short peptides related to the monoclonal antibodies provided herein, and pharmaceutical compositions and kits containing the monoclonal antibodies provided herein.
Owner:HX DIAGNOSTICS INC
Antigenic GM-CSF peptides and antibodies to GM-CSF
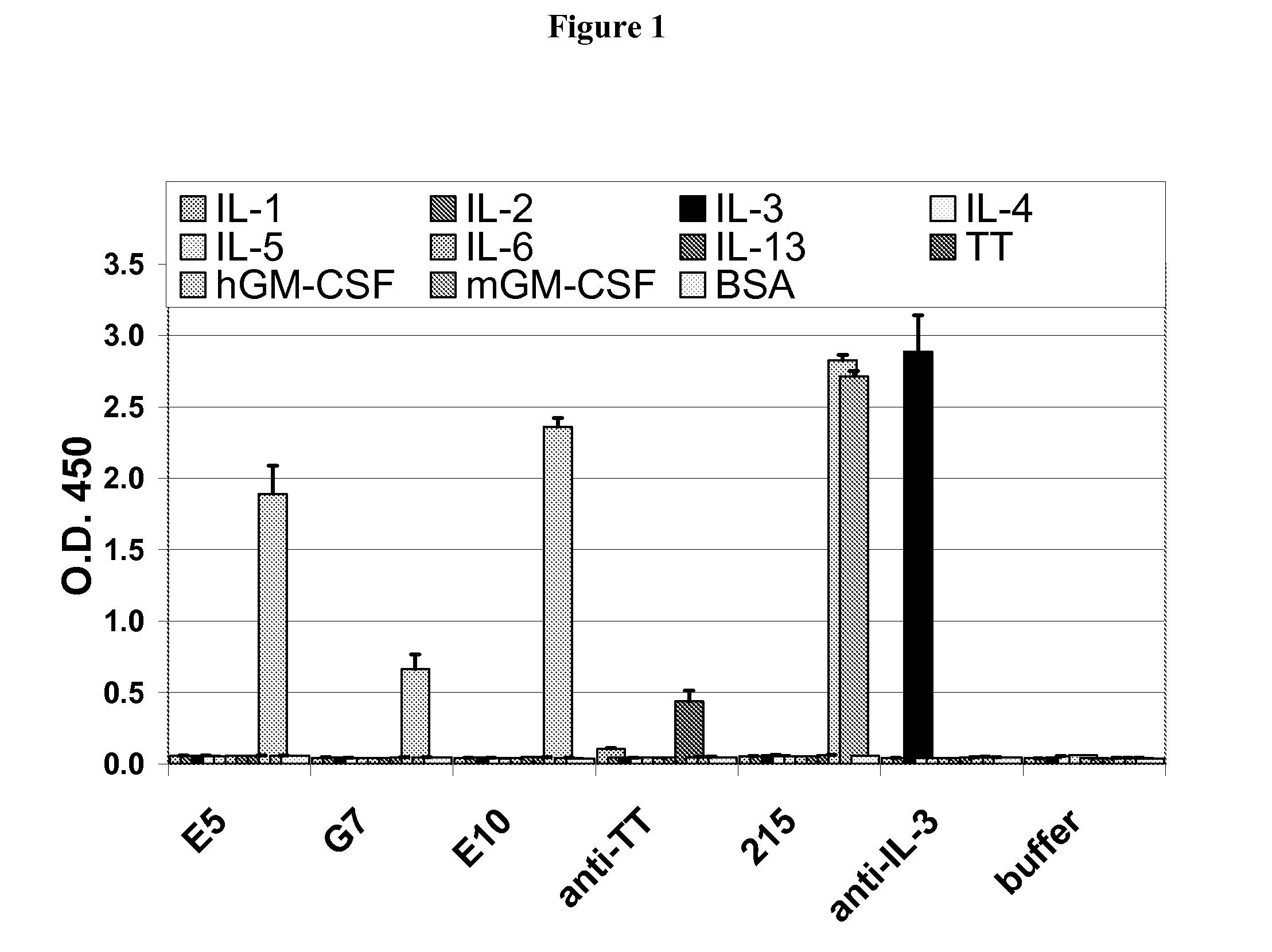
Hybridoma lines that secrete human monoclonal antibodies with high binding specificity and biological activity, particularly neutralizing activity against granulocyte-macrophage colony stimulating factor, and methods of generating the hybridoma lines are provided. Target antigens and epitopes are also provided. The antibodies may be used in therapeutic methods, for example in the treatment of cancer, infectious disease, or autoimmune disease.
Owner:EISAI INC
Antibodies directed against binding-associated epitopes
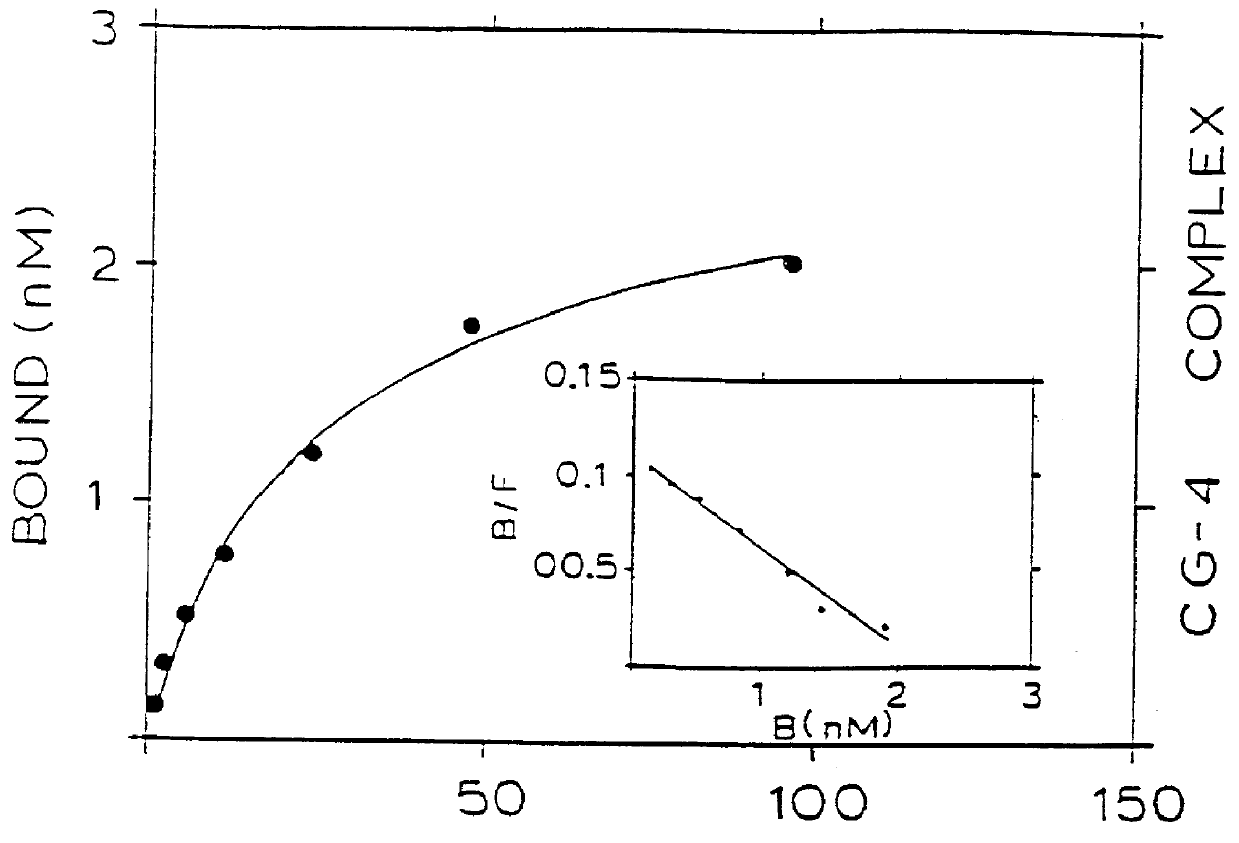
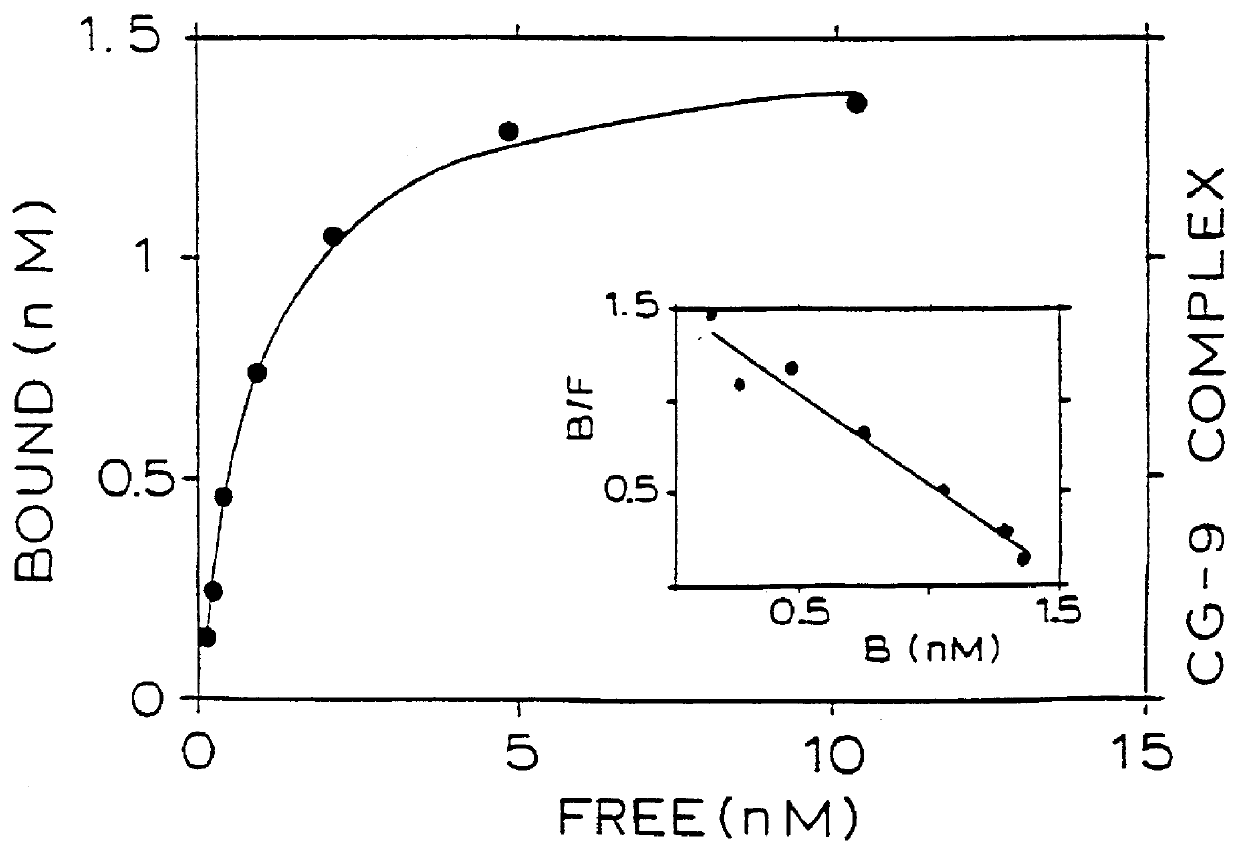
Binding of two members of a binding couple reveals epitopes which are revealed only after binding and the monoclonal antibody secreted from the hybridoma cell line CG-10 directed against these epitopes bind to the bound couple at a significantly higher affinity than their binding affinity to either of the two members themselves when not bound to one another.
Owner:RAMOT AT TEL AVIV UNIV LTD
Method for Accelerating Somatic Mutations and use Thereof in Proteomics
The invention relates to a method of accelerating the induction of somatic mutations in vitro. The inventive method comprises the expression of at least one cDNA expressing a modified version of the AID gene in the cells to be mutated, in culture conditions and a medium that are suited thereto, said modified version resulting from an AID gene in which the three hydrophobic amino acids, leu189, phe193 and leu196, have been replaced by means of alanine mutations in each case. The invention can be used to induce mutations in Burkitt's lymphoma BL2. The invention can also be used to induce mutations in the immunoglobulin genes of immortalised antibody-producing cells, such as mouse hybridoma cells, human hybridoma cells or human B-cell lines immortalised by the Epstein-Barr virus (EBV).
Owner:INST NECKER
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com