Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
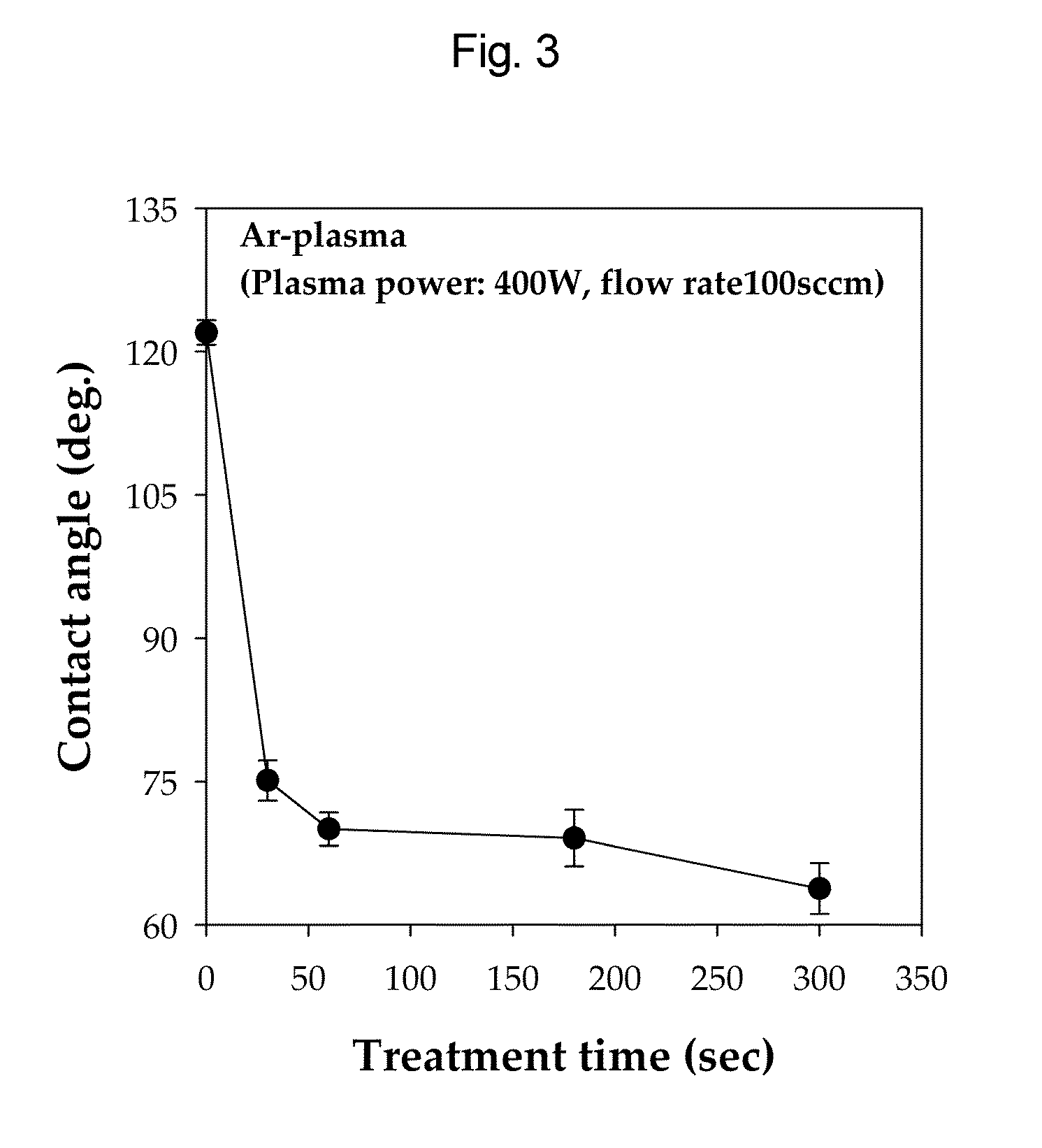
1067 results about "Western blot" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
<ul><li>A positive test indicates presence of antibody/other specific protein and a negative test indicates its absence.</li><li>In case of infections, a positive test is commonly a result of disease. However, in certain conditions such as agammaglobulinemia where Western blotting is performed to detect normal proteins (BTK protein), absence could be the abnormal result.</li><li>The doctor reviews the results and discusses it.</li></ul>
Apparatus for and method of processing biological samples
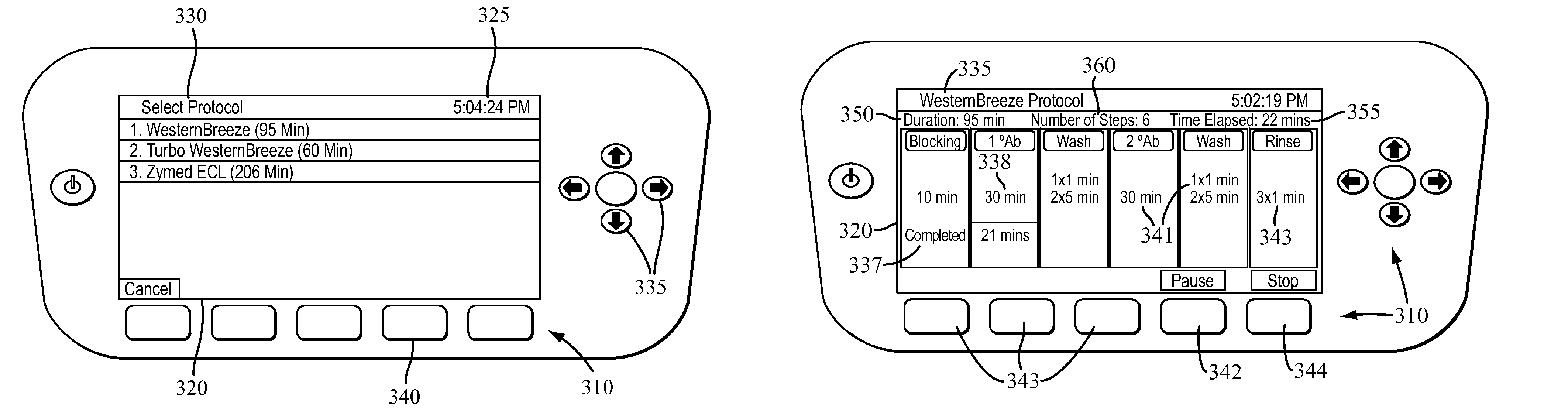
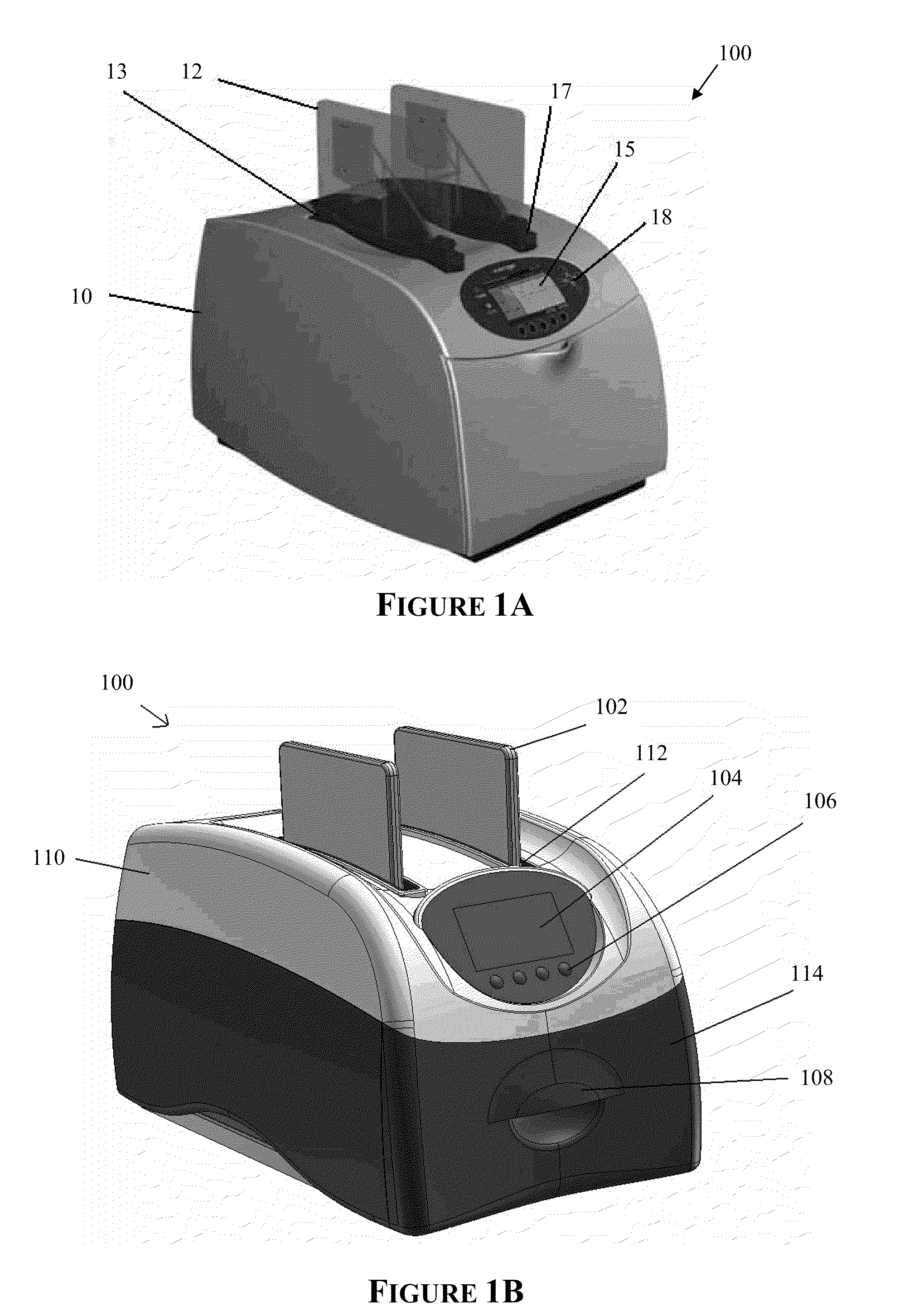
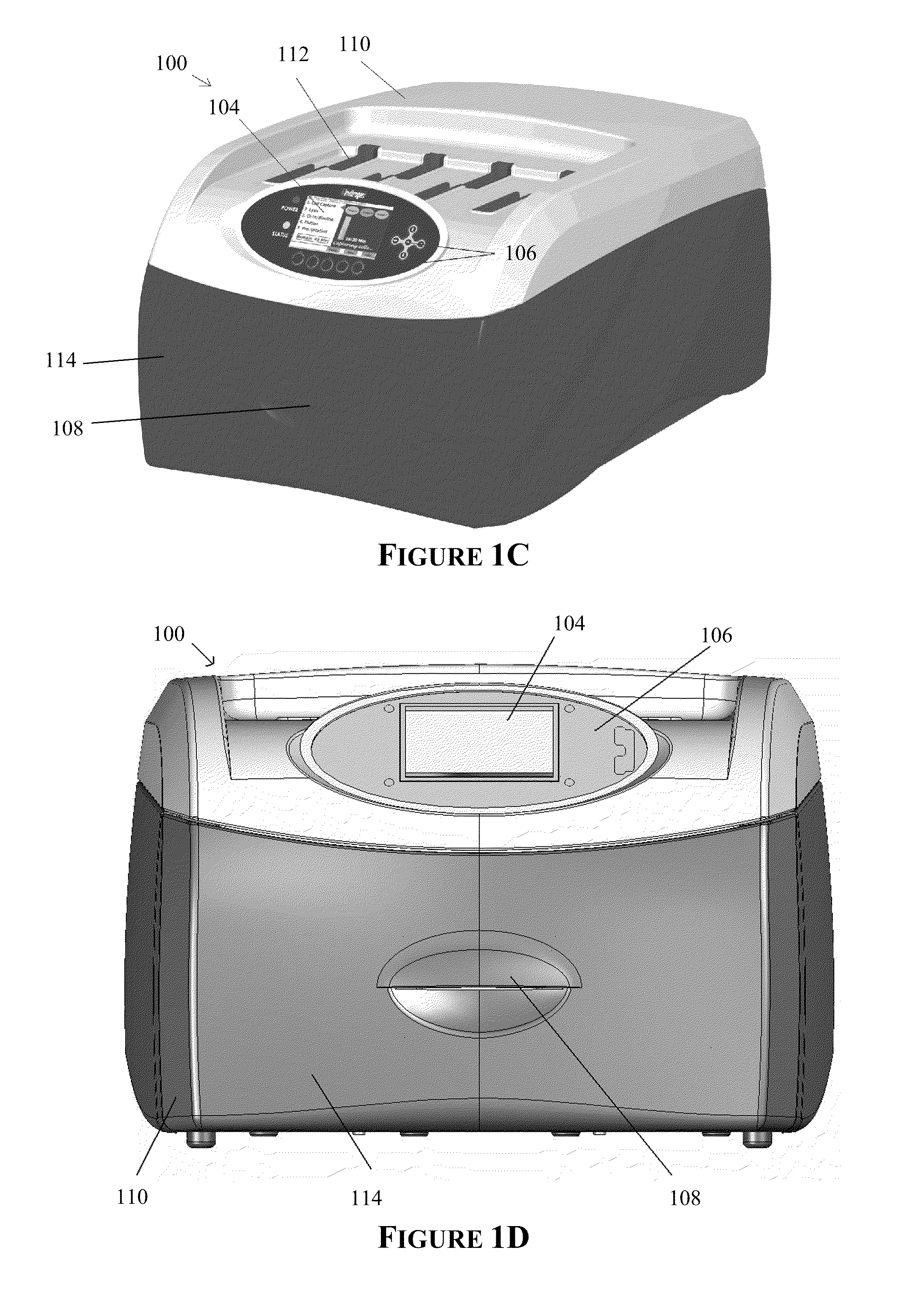
The present invention provides systems, devices, apparatuses and methods for automated bioprocessing. Examples of protocols and bioprocessing procedures suitable for the present invention include but are not limited to: immunoprecipitation, chromatin immunoprecipitation, recombinant protein isolation, nucleic acid separation and isolation, protein labeling, separation and isolation, cell separation and isolation, food safety analysis and automatic bead based separation. In some embodiments, the invention provides automated systems, automated devices, automated cartridges and automated methods of western blot processing. Other embodiments include automated systems, automated devices, automated cartridges and automated methods for separation, preparation and purification of nucleic acids, such as DNA or RNA or fragments thereof, including plasmid DNA, genomic DNA, bacterial DNA, viral DNA and any other DNA, and for automated systems, automated devices, automated cartridges and automated methods for processing, separation and purification of proteins, peptides and the like.
Owner:LIFE TECH CORP
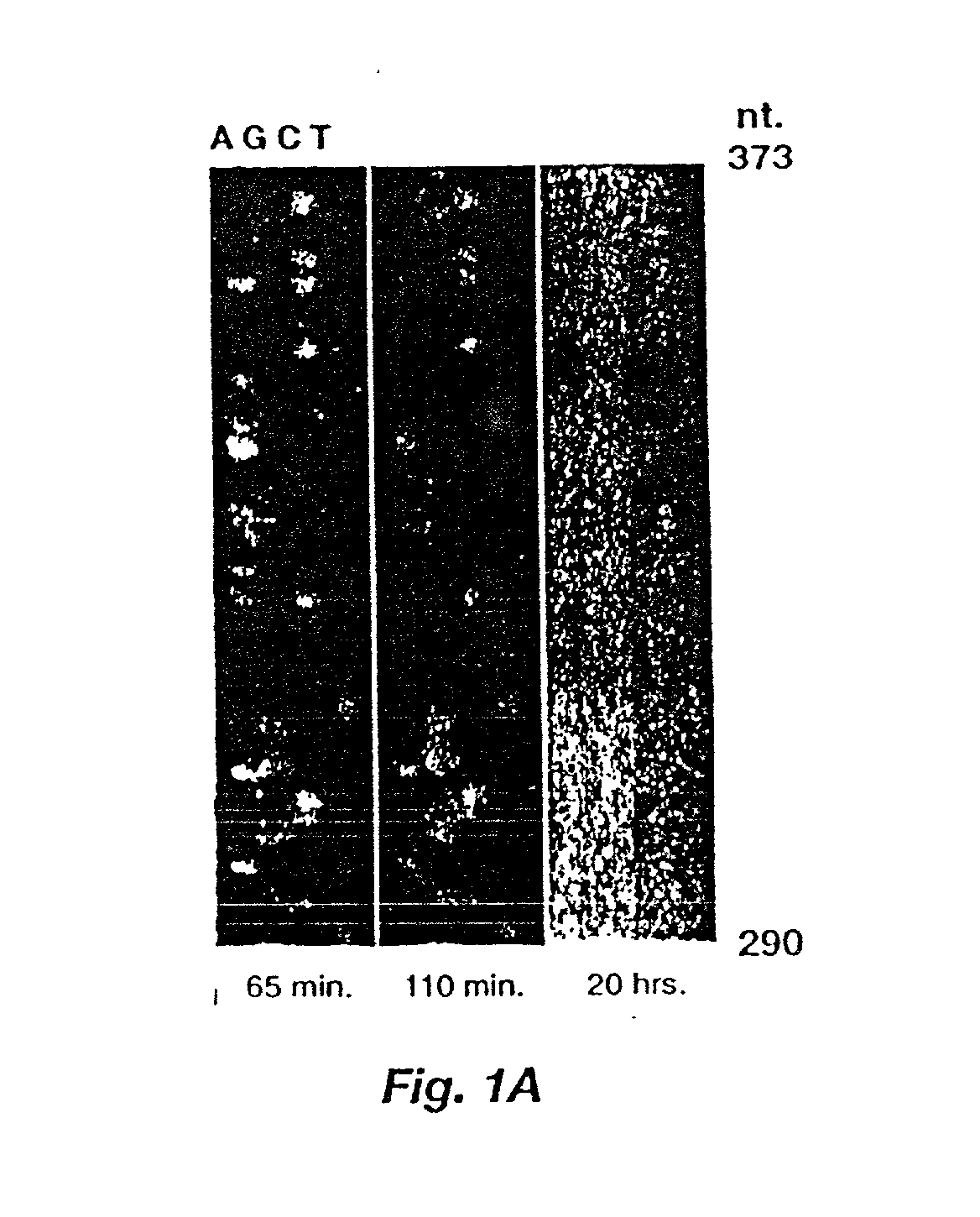
Automated hybridization/imaging device for fluorescent multiplex DNA sequencing
InactiveUS20020012910A1Easy to disassembleBioreactor/fermenter combinationsSludge treatmentHybridization probeSize fractionated
A method is disclosed for automated multiplex sequencing of DNA with an integrated automated imaging hybridization chamber system. This system comprises an hybridization chamber device for mounting a membrane containing size-fractionated multiplex sequencing reaction products, apparatus for fluid delivery to the chamber device, imaging apparatus for light delivery to the membrane and image recording of fluorescence emanating from the membrane while in the chamber device, and programmable controller apparatus for controlling operation of the system. The multiplex reaction products are hybridized with a probe, then an enzyme (such as alkaline phosphatase) is bound to a binding moiety on the probe, and a fluorogenic substrate (such as a benzothiazole derivative) is introduced into the chamber device by the fluid delivery apparatus. The enzyme converts the fluorogenic substrate into a fluorescent product which, when illuminated in the chamber device with a beam of light from the imaging apparatus, excites fluorescence of the fluorescent product to produce a pattern of hybridization. The pattern of hybridization is imaged by a CCD camera component of the imaging apparatus to obtain a series of digital signals. These signals are converted by the controller apparatus into a string of nucleotides corresponding to the nucleotide sequence an automated sequence reader. The method and apparatus are also applicable to other membrane-based applications such as colony and plaque hybridization and Southern, Northern, and Western blots.
Owner:WEISS ROBERT B +6
Soluble Form of Carbonic Anhydrase IX (s-CA IX), Assays to Detect s-CA IX, CA IX's Coexpression with HER-2/neu/c-erbB-2, and CA IX-Specific Monoclonal Antibodies to Non-Immunodominant Epitopes
InactiveUS20080176310A1Improve efficiencyIncrease resourcesOxidoreductasesFermentationKilodaltonWestern blot
Disclosed herein is the discovery of a soluble MN / CA IX (s-CA IX) in body fluids, such as, urine and serum. Said s-CA IX comprises the extracellular domain of CA IX or portions thereof. The predominant s-CA IX species is the extracellular domain comprising a proteoglycan-like (PG) domain and carbonic anhydrase (CA) domain, and having a molecular weight of about 50 / 54 kilodaltons (kd) upon Western blot. A smaller s-CA IX form of about 20 to about 30 kd comprising the CA domain or parts thereof, not linked to the PG domain, has also been found in body fluids. Diagnostic / prognostic methods for precancer and cancer that detect or detect and quantitate said s-CA IX in body fluids, are described. Also disclosed herein is the coexpression of CA IX and HER-2 / neu / c-erbB-2 that provides parallel, alternative and potentially synergistic diagnostic / prognostic and therapeutic strategies for precancer and cancer. Further disclosed are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, preferably monoclonal antibodies and immunoreactive fragments and engineered variants thereof. Such new MN / CA IX-specific antibodies, fragments and variants are useful diagnostically / prognostically and therapeutically for cancer and precancer. Particularly preferred are the new monoclonal antibodies, fragments and variants that are specific for the non-immunodominant epitopes of MN / CA IX, which antibodies are, among other uses, useful to detect soluble MN / CA IX (s-CA IX) in body fluids, alone but preferably in combination with antibodies specific to the immunodominant epitopes of MN / CA IX, for example, in a sandwich assay.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
Soluble Form of Carbonic Anhydrase IX (s-CA IX), Assays to Detect s-CA IX, CA IX's Coexpression with Her-2/neu/c-erbB-2, and CA IX-Specific Monoclonal Antibodies to Non-Immunodominant Epitopes
InactiveUS20080177046A1Good curative effectImprove efficiencyImmunoglobulins against animals/humansBiological material analysisKilodaltonWestern blot
Disclosed herein is the discovery of a soluble MN / CA IX (s-CA IX) in body fluids, such as, urine and serum. Said s-CA IX comprises the extracellular domain of CA IX or portions thereof. The Predominant s-CA IX species is the extracellular domain comprising a proteoglycan-like (PG) domain and carbonic anhydrase (CA) domain, and having a molecular weight of about 50 / 54 kilodaltons (kd) upon Western blot. A smaller s-CA IX form of about 20 to about 30 kd comprising the CA domain or parts thereof, not linked to the PG domain, has also been found in body fluids. Diagnostic / prognostic methods for precancer and cancer that detect or detect and quantitate said s-CA IX in body fluids, are described. Also disclosed herein is the coexpression of CA IX and HER-2 / neu / c-erbB-2 that provides parallel, alternative and potentially synergistic diagnostic / prognostic and therapeutic strategies for precancer and cancer. Further disclosed are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, preferably monoclonal antibodies and immunoreactive fragments and engineered variants thereof. Such new MN / CA IX-specific antibodies, fragments and variants are useful diagnostically / prognostically and therapeutically for cancer and precancer. Particularly preferred are the new monoclonal antibodies, fragments and variants that are specific for the non-immunodominant epitopes of MN / CA IX, which antibodies are, among other uses, useful to detect soluble MN / CA IX (s-CA IX) in body fluids, alone but preferably in combination with antibodies specific to the immunodominant epitopes of MN / CA IX, for example, in a sandwich assay.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
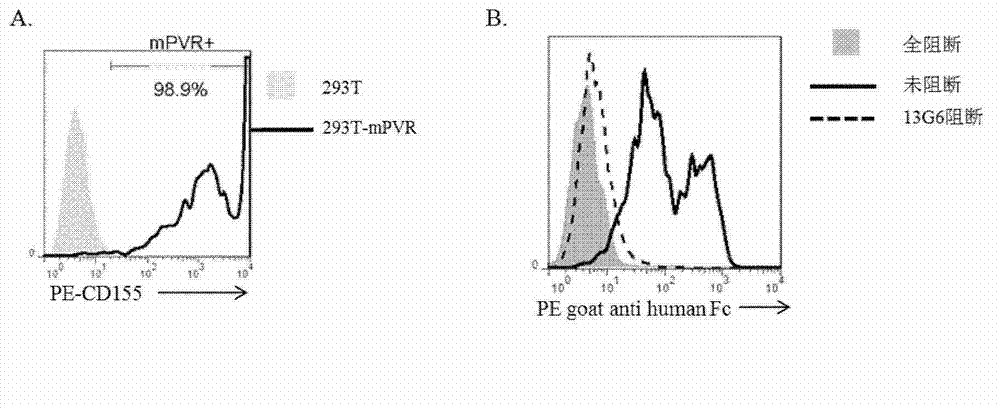
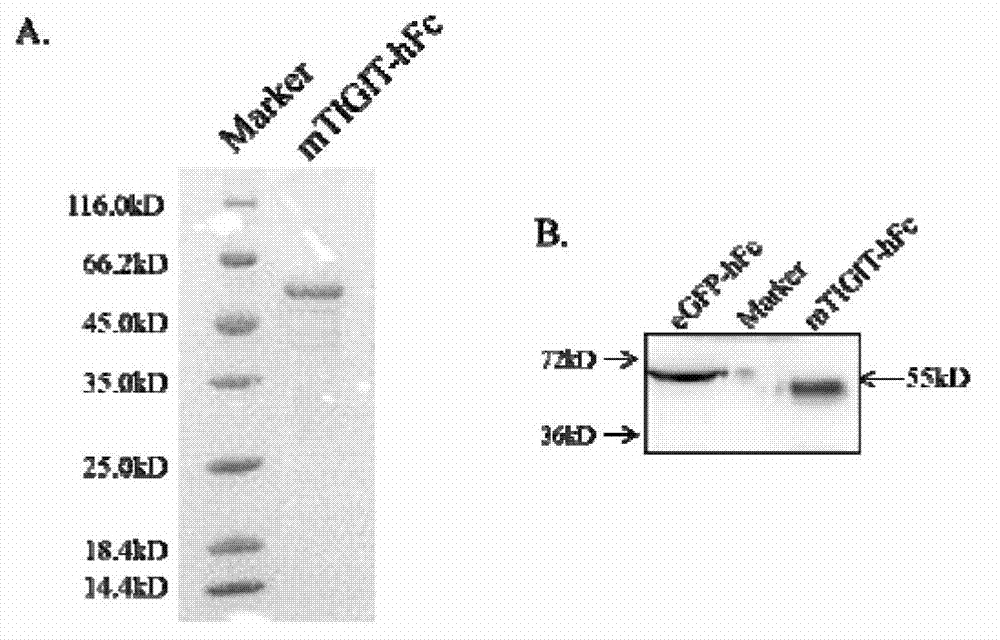
Specific anti-mouse TIGIT monoclonal antibody and preparation method, identification and application thereof
ActiveCN103073644AHigh potencyStrong specificityImmunoglobulins against cell receptors/antigens/surface-determinantsTissue cultureWestern blotPoliovirus Receptor
The invention relates to an anti-mouse TIGIT (T cell Ig and ITIM domain), and a hybridoma cell strain mTIGIT-mAb-13G6 (the preservation NO: CCTCC NO: C201299) for producing the monoclonal antibody, and further relates to a preparation method and the application of the monoclonal antibody. The monoclonal antibody can efficiently prevent combination of mTIGIT and mPVR (poliovirus receptor), and can be used for Western blot, ELISA and Flow Cytometry for mTIGIT molecule detection.
Owner:IMMUNOPHARMACEUTIC INST OF HEFEI RUIDA CO LTD
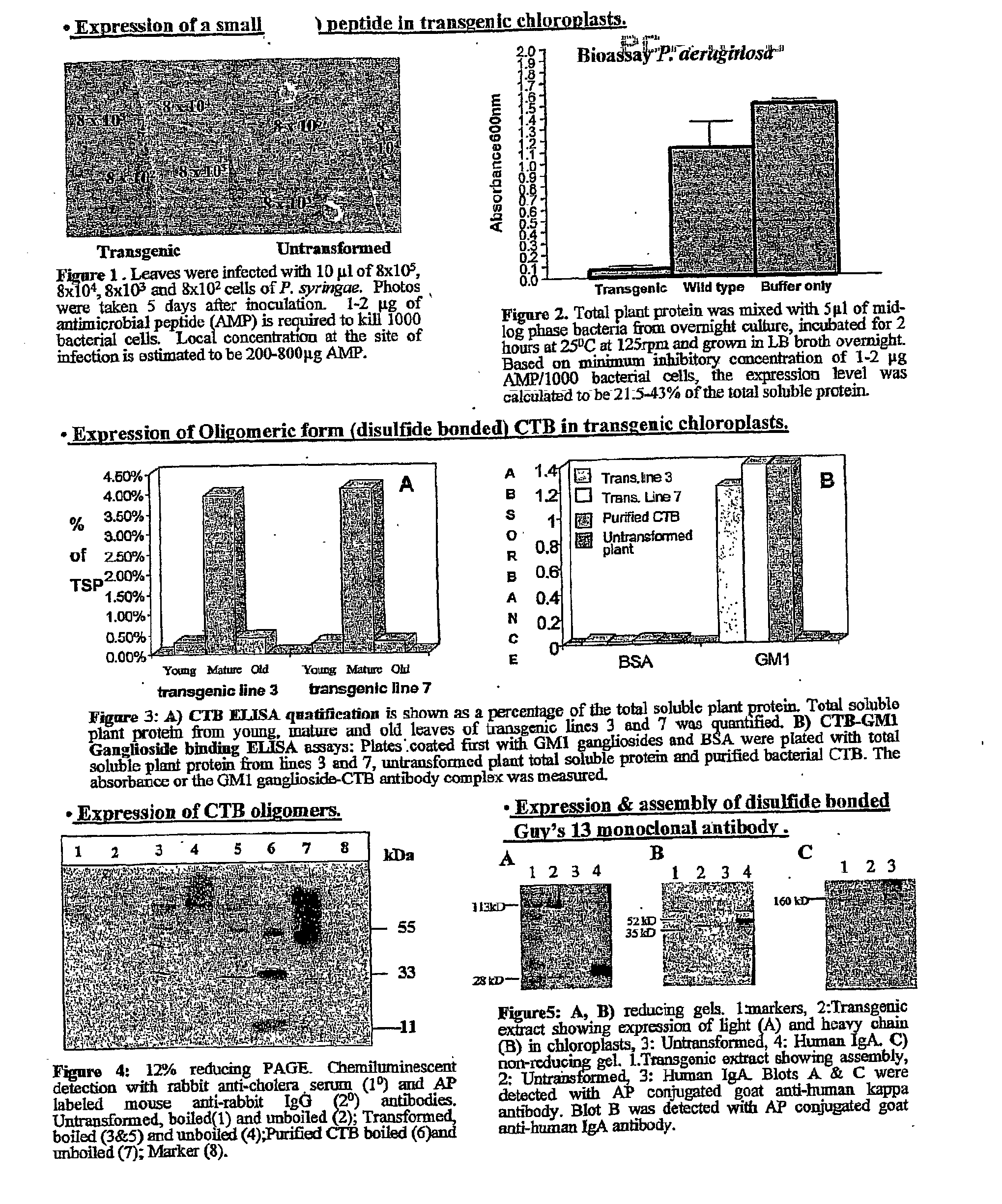
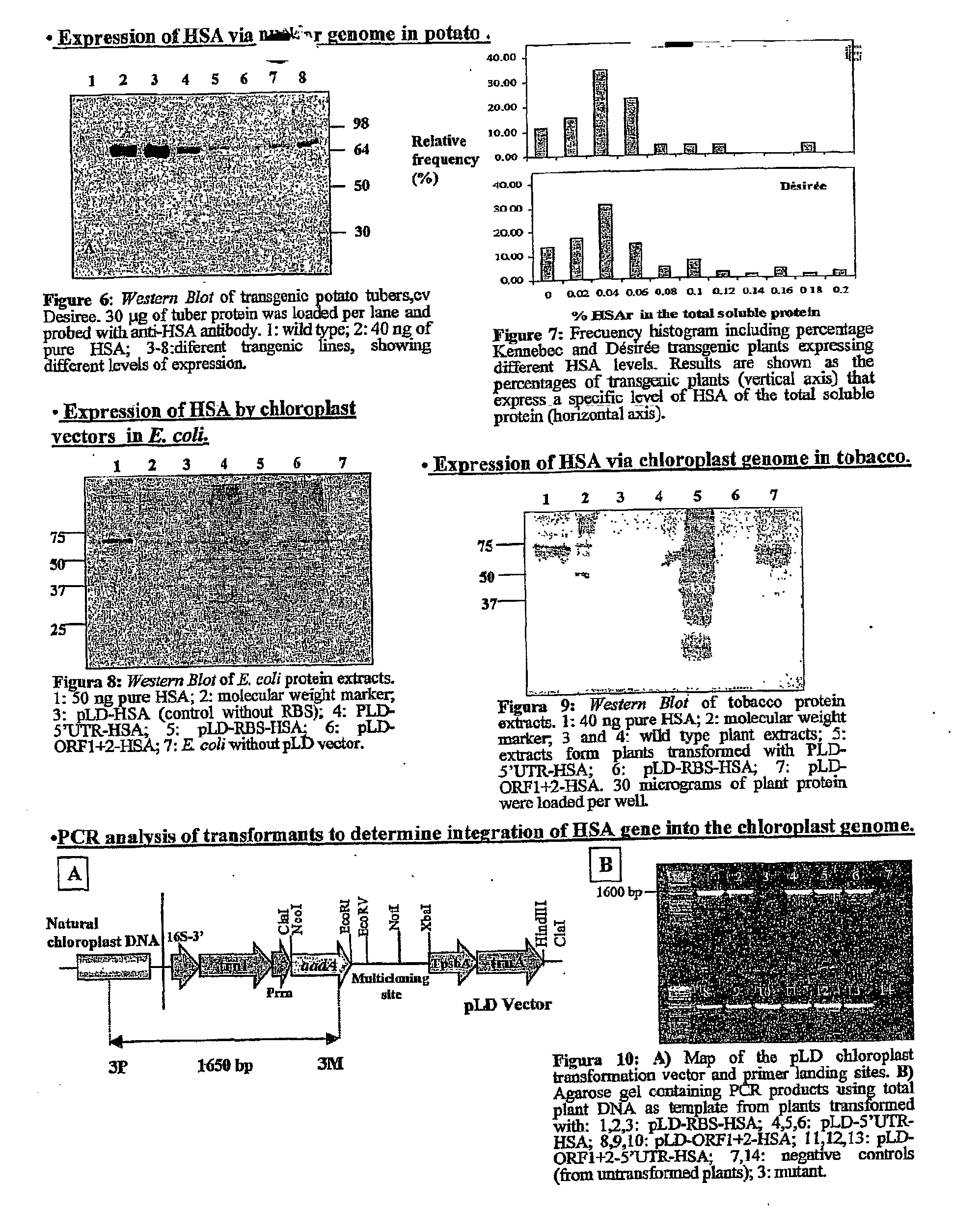
Pharmaceutical proteins, human therapeutics, human serum albumin, insulin, native cholera toxic b submitted on transgenic plastids
InactiveUS20030204864A1Eliminate needLarge biomassBiocidePeptide/protein ingredientsEscherichia coliInsulin-like growth factor
Transgenic chloroplast technology could provide a viable solution to the production of Insulin-like Growth Factor I (IGF-I), Human Serum Albumin (HSA), or interferons (IFN) because of hyper-expression capabilities, ability to fold and process eukaryotic proteins with disulfide bridges (thereby eliminating the need for expensive post-purification processing). Tobacco is an ideal choice because of its large biomass, ease of scale-up (million seeds per plant), genetic manipulation and impending need to explore alternate uses for this hazardous crop. Therefore, all three human proteins will be expressed as follows: a) Develop recombinant DNA vectors for enhanced expression via tobacco chloroplast genomes b) generate transgenic plants c) characterize transgenic expression of proteins or fusion proteins using molecular and biochemical methods d) large scale purification of therapeutic proteins from transgenic tobacco and comparison of current purification / processing methods in E. coli or yeast e) Characterization and comparison of therapeutic proteins (yield, purity, functionality) produced in yeast or E. coli with transgenic tobacco f) animal testing and pre-clinical trials for effectiveness of the therapeutic proteins. Mass production of affordable vaccines can be achieved by genetically engineering plants to produce recombinant proteins that are candidate vaccine antigens. The B subunits of Enteroxigenic E. coli (LTB) and cholera toxin of Vibrio cholerae (CTB) are examples of such antigens. When the native LTB gene was expressed via the tobacco nuclear genome, LTB accumulated at levels less than 0.01% of the total soluble leaf protein. Production of effective levels of LTB in plants, required extensive codon modification. Amplification of an unmodified CTB coding sequence in chloroplasts, up to 10,000 copies per cell, resulted in the accumulation of up to 4.1% of total soluble tobacco leaf protein as oligomers (about 410 fold higher expression levels than that of the unmodified LTB gene). PCR and Southern blot analyses confirmed stable integration of the CTB gene into the chloroplast genome. Western blot analysis showed that chloroplast synthesized CTB assembled into oligomers and was antigenically identical to purified native CTB. Also, GM1,-ganglioside binding assays confirmed that chloroplast synthesized CTB binds to the intestinal membrane receptor of cholera toxin, indicating correct folding and disulfide bond formation within the chloroplast. In contrast to stunted nuclear transgenic plants, chloroplast transgenic plants were morphologically indistinguishable from untransformed plants, when CTB was constitutively expressed. The introduced gene was stably inherited in the subsequent generation as confirmed by PCR and Southern blot analyses. Incrased production of an efficient transmucosal carrier molecule and delivery system, like CTB, in transgenic chloroplasts makes plant based oral vaccines and fusion proteins with CTB needing oral administration a much more practical approach.
Owner:AUBURN UNIV +1
Construction method of cell model for detecting pyrogens, cell model and pyrogen detection kit
ActiveCN106148286AImprove stabilityIncreased sensitivityCell receptors/surface-antigens/surface-determinantsCulture processWestern blotCytokine
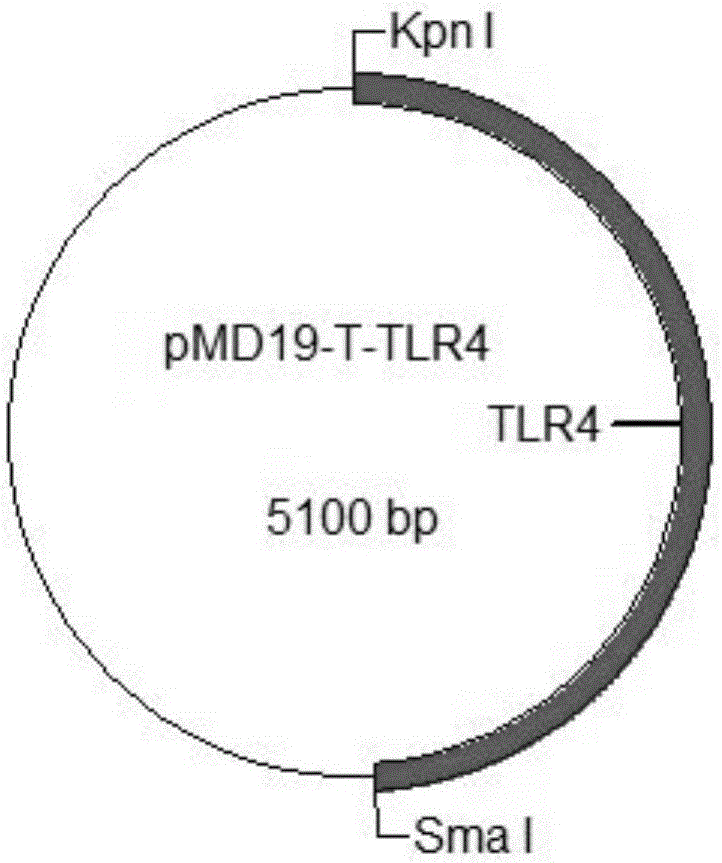
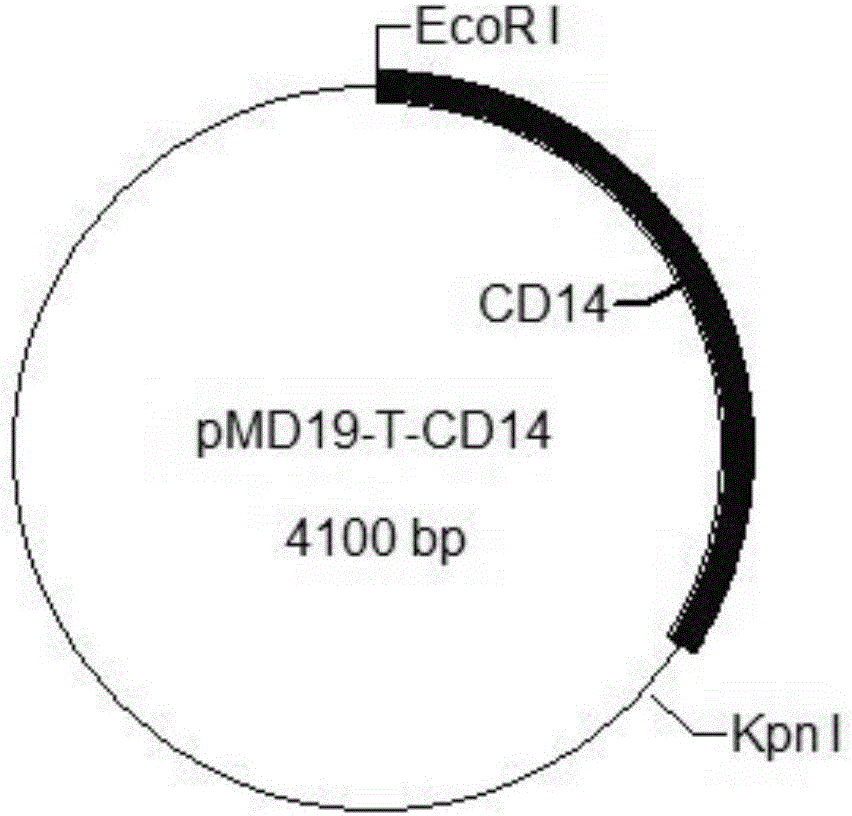
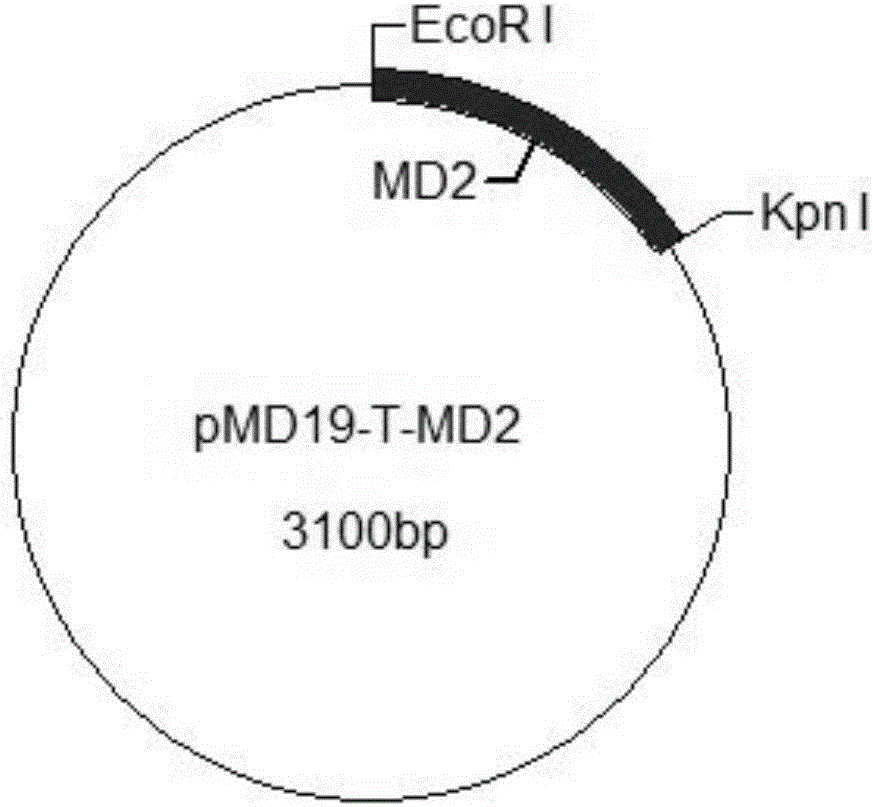
The invention provides a construction method of a cell model for detecting pyrogens, the cell model and a pyrogen detection kit. The cell model utilizes specific locations of CRISPR / CAS9 induced genomes to form double-bond fission, TLR4 and CD14-MD2 are knocked into two chromosomes of a cell line respectively by the aid of the homologous recombination repair principle, green fluorescence GFP and red fluorescence RFP are respectively used for tracing finally successfully constructed TLR4 / CD14 / MD2 fixed-point knocked-in fluorescent tracer cell models, and the LPS stimulating cell model can detect release of IL-6 and TNF-a cytokines by means of ELISA, Western Blot, mass spectrum and immunomagnetic beads. The cell model is good in stability and high in sensitivity, and the lowest detectable limit can reach 0.005EU / mL and is far lower than 0.025EU / mL of the Tachypleus Amebocyte Lysate method.
Owner:牛刚
Methods and kit for diagnosing tick borne illnesses
InactiveUS20060194267A1High sensitivityStrong specificityImmobilised enzymesAnimal cellsWestern blotDecorin
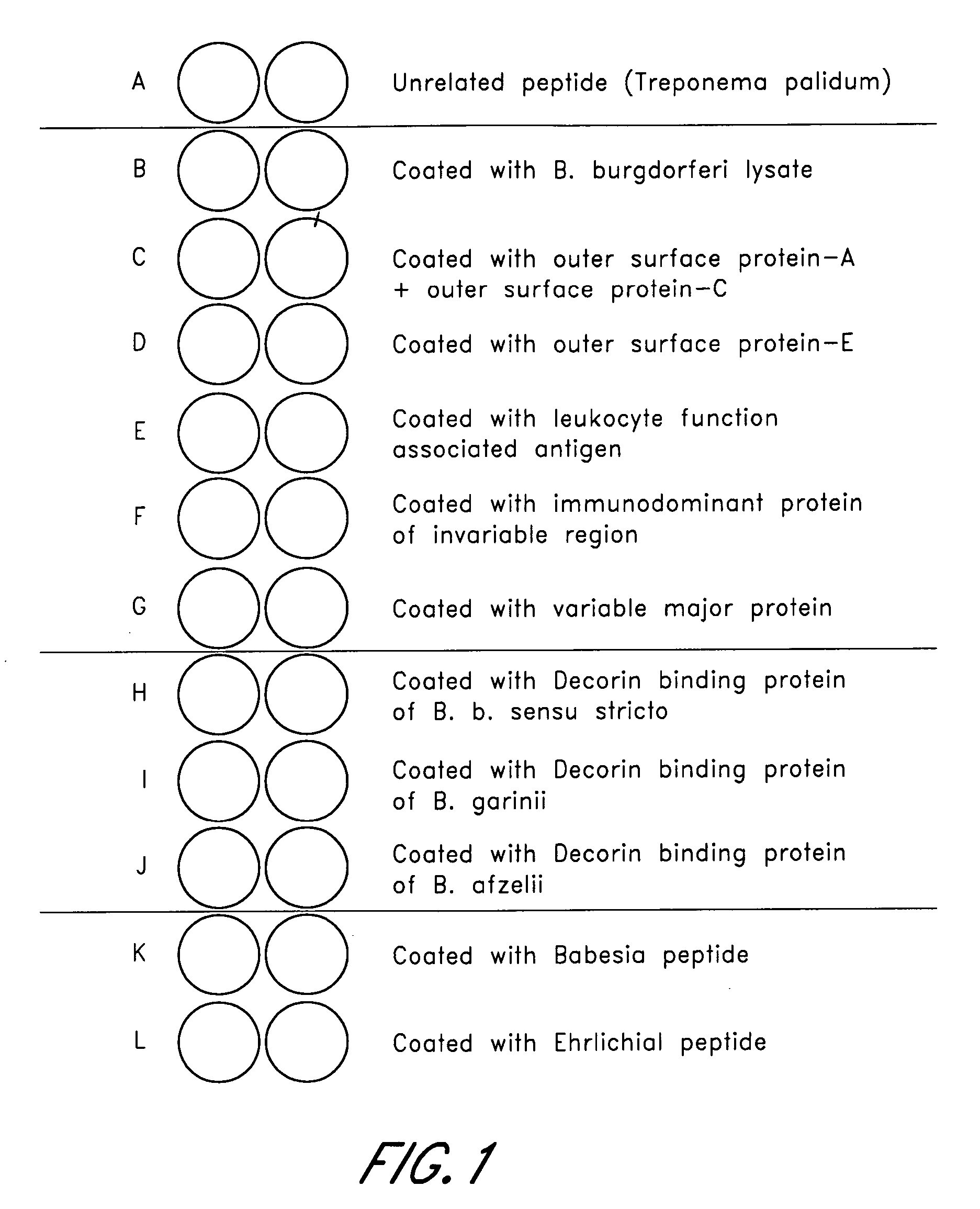
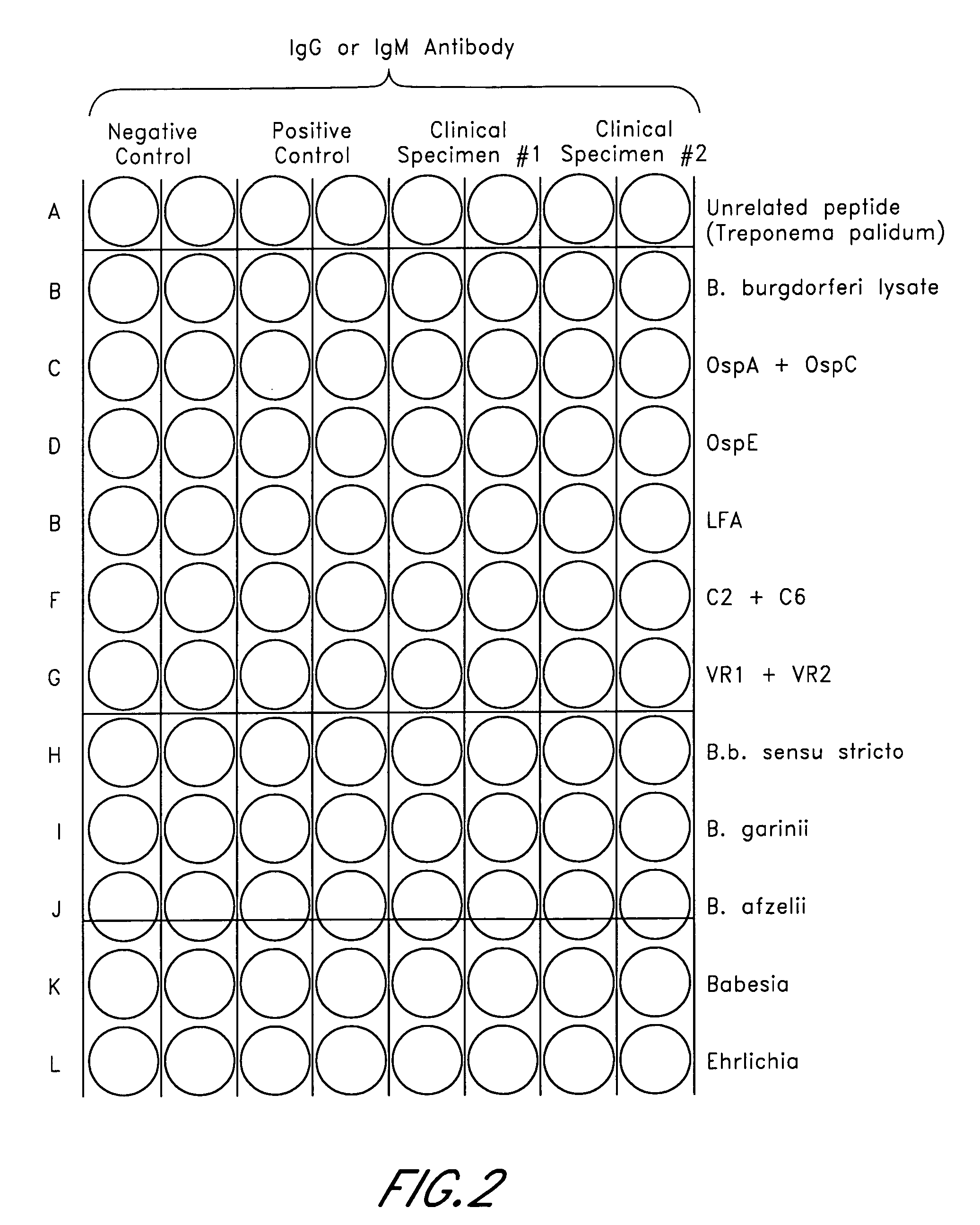
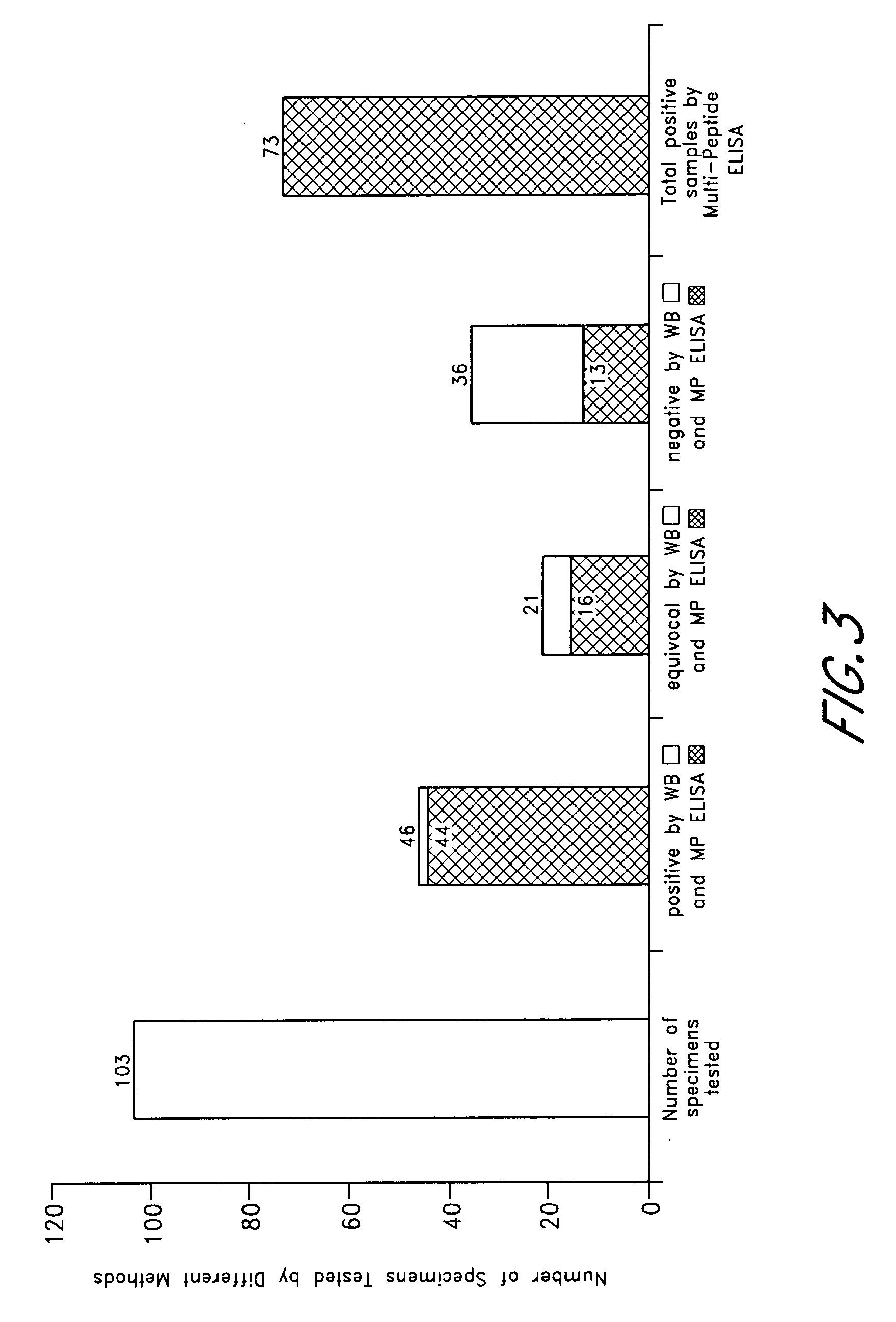
ELISA, Western Blot, and a peptide-based ELISA were applied to clinical specimens from patients with clinical symptoms of tick borne diseases, including Lyme disease. Peptides from different components of Borrelia during different cycles, including peptides from outer surface protein, leukocyte function associated antigens, immunodominant antigens, variable major proteins, and peptides from decorin-binding proteins of Borrelial subspecies (B. sensu stricto. B. afzelii, B. garinii) were used. Antibodies against specific peptides from Babesia and Ehrlichia were also measured.
Owner:IMMUNOSCI LAB
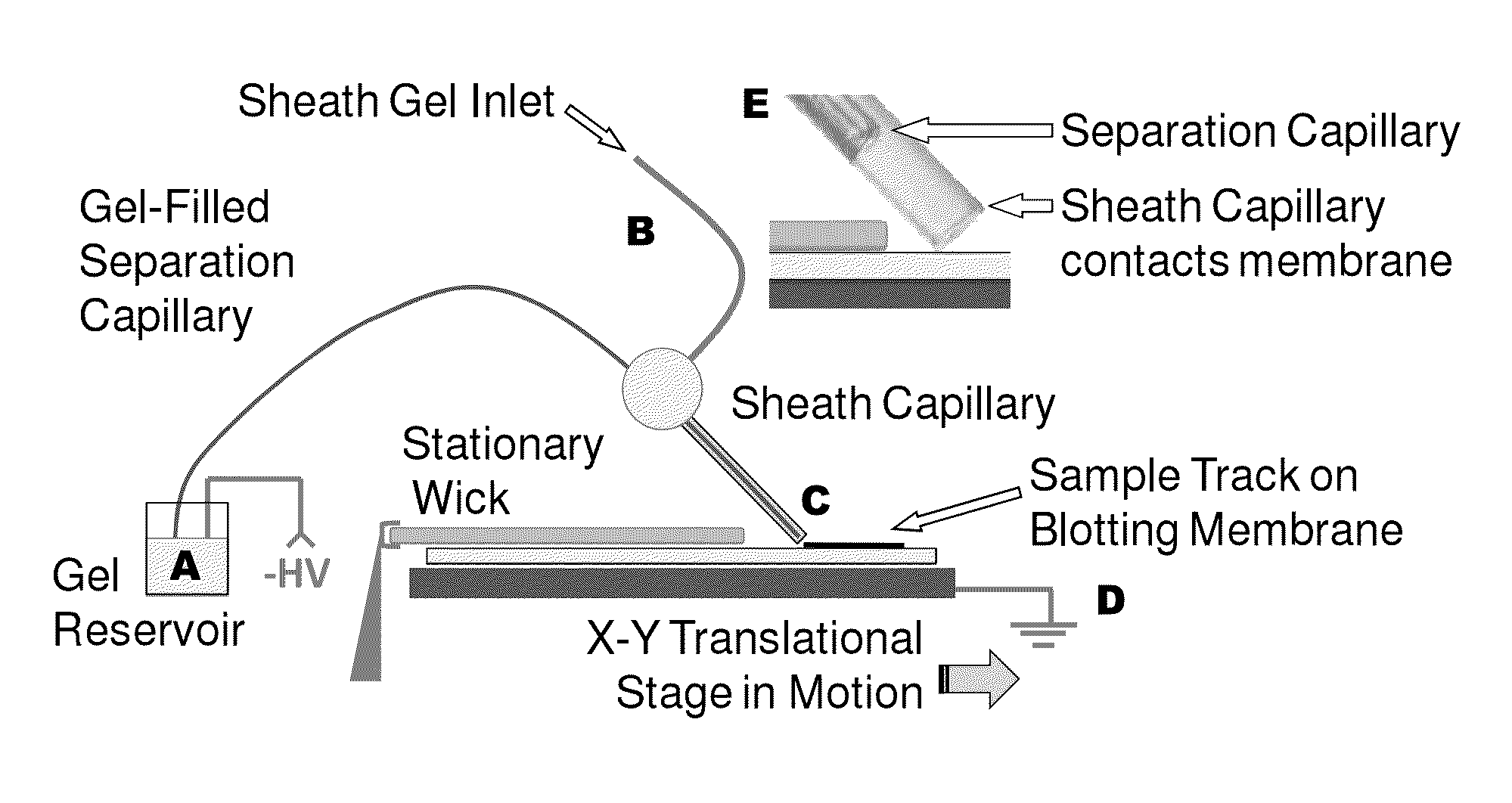
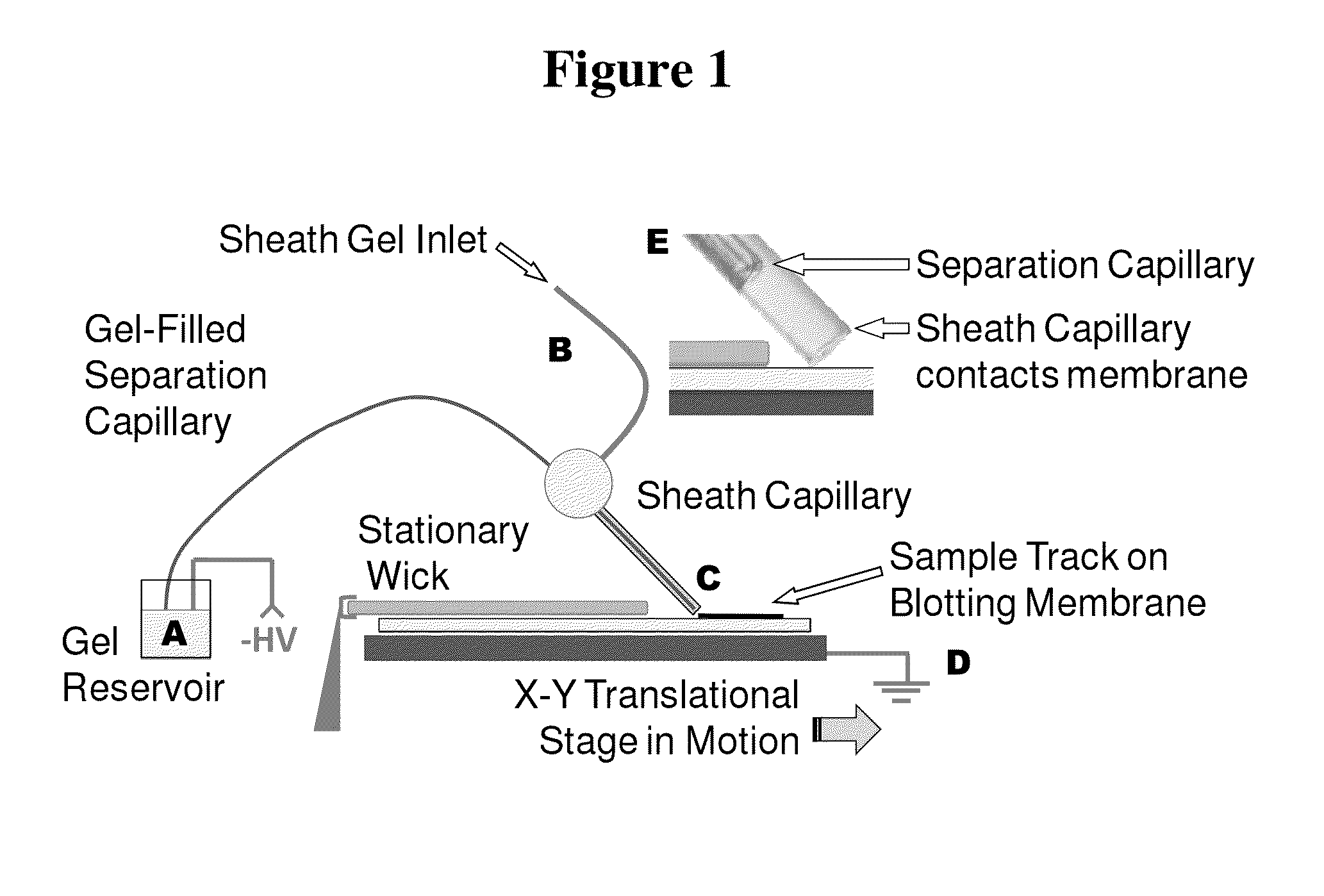
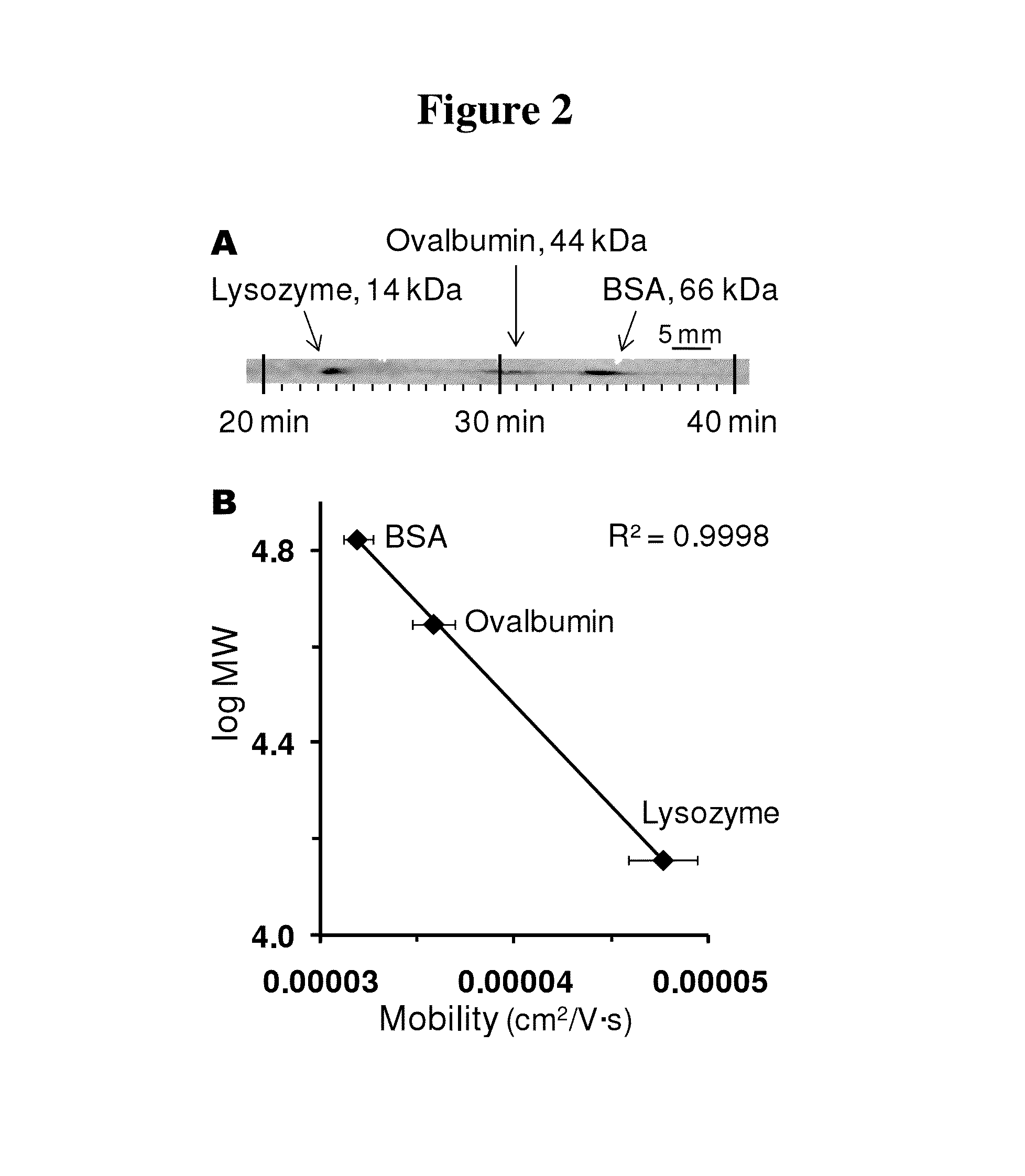
Microscale western blot
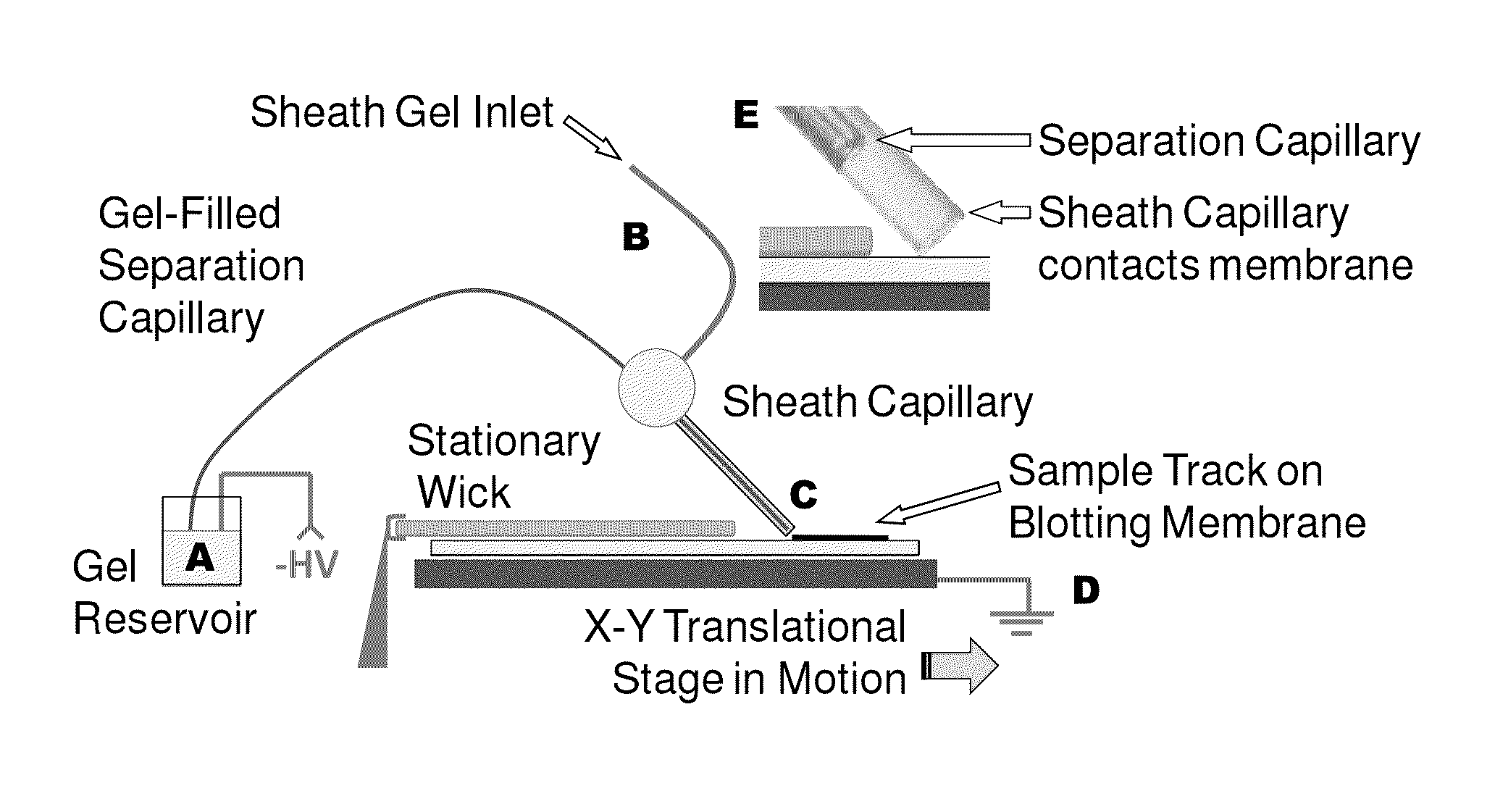
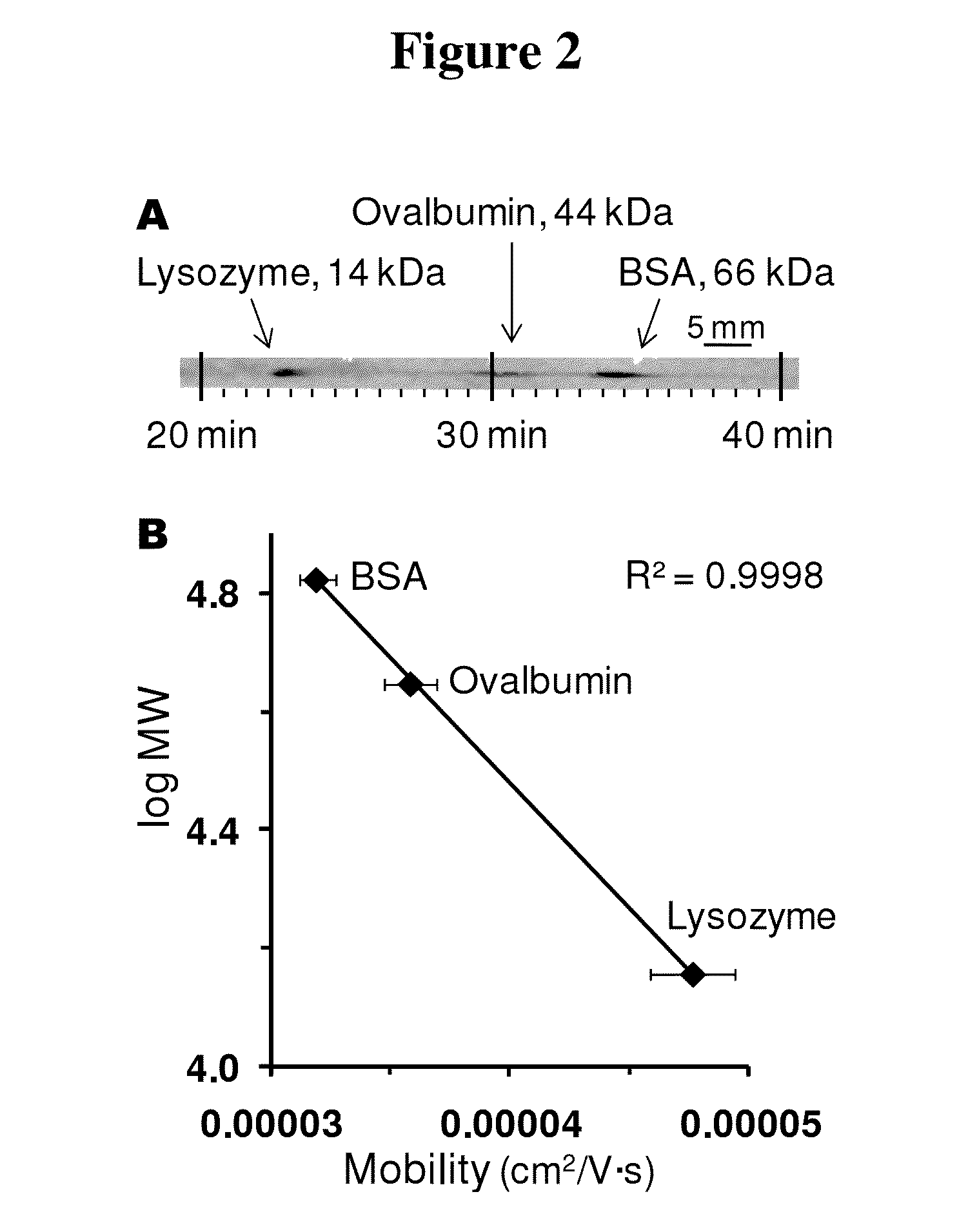
ActiveUS20130213811A1Improve bindingStable electrophoresisSludge treatmentVolume/mass flow measurementElectrophoresesCapillary electrophoresis
Systems and methods are provided for integrating the electrophoresis and blotting of samples. A capillary electrophoresis and blotting system allows concomitant electrophoretic separation and blotting to provide a rapid and simplified process. A microfluidic electrophoresis and blotting system provides electrophoretic separation in a microfluidic channel followed by electroblotting from the microfluidic channel to also provide a rapid and simplified process. These systems and methods can be used to assay smaller amounts of sample in less time than conventional processes, including conventional Western blotting techniques.
Owner:RGT UNIV OF MICHIGAN
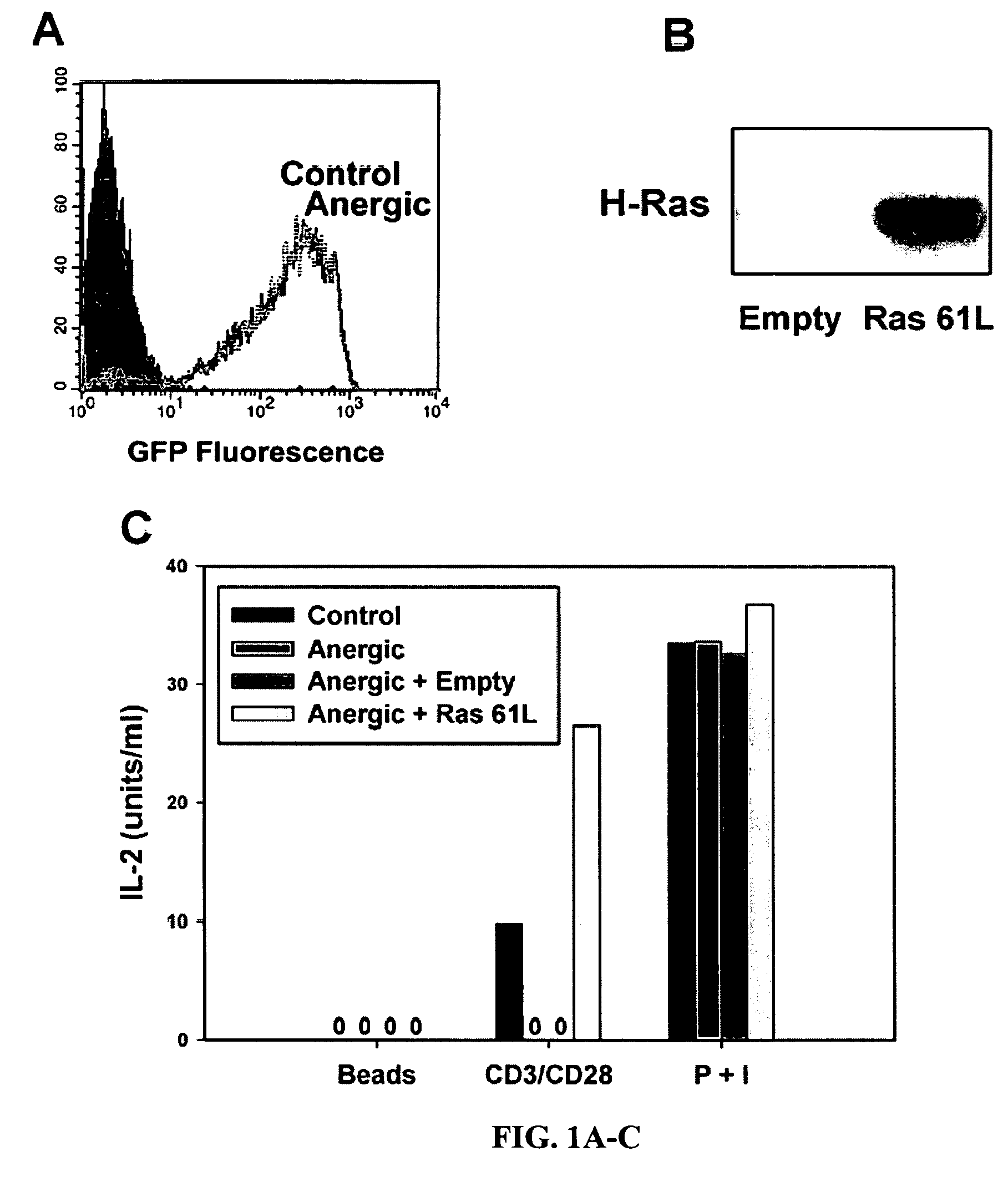
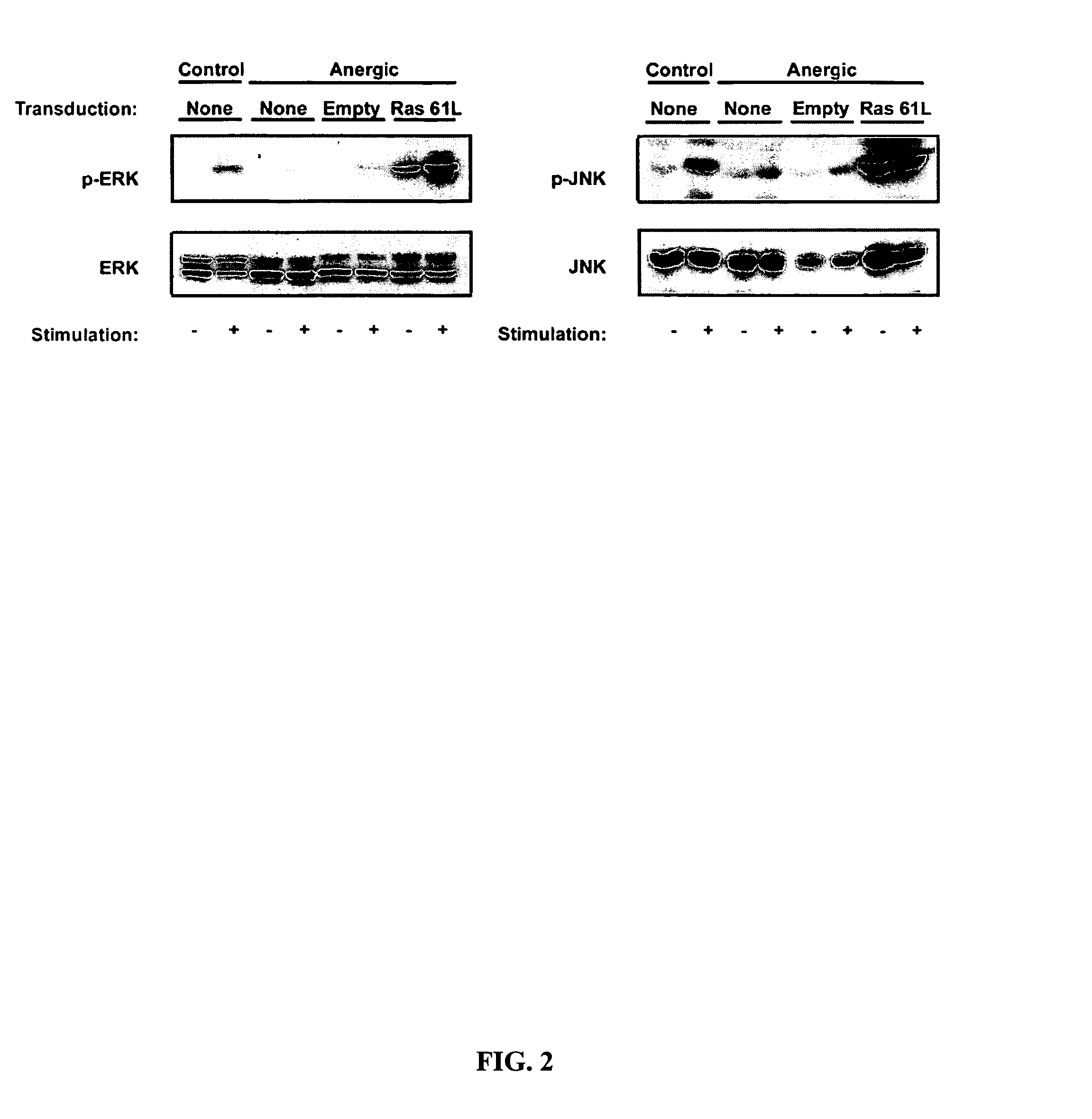
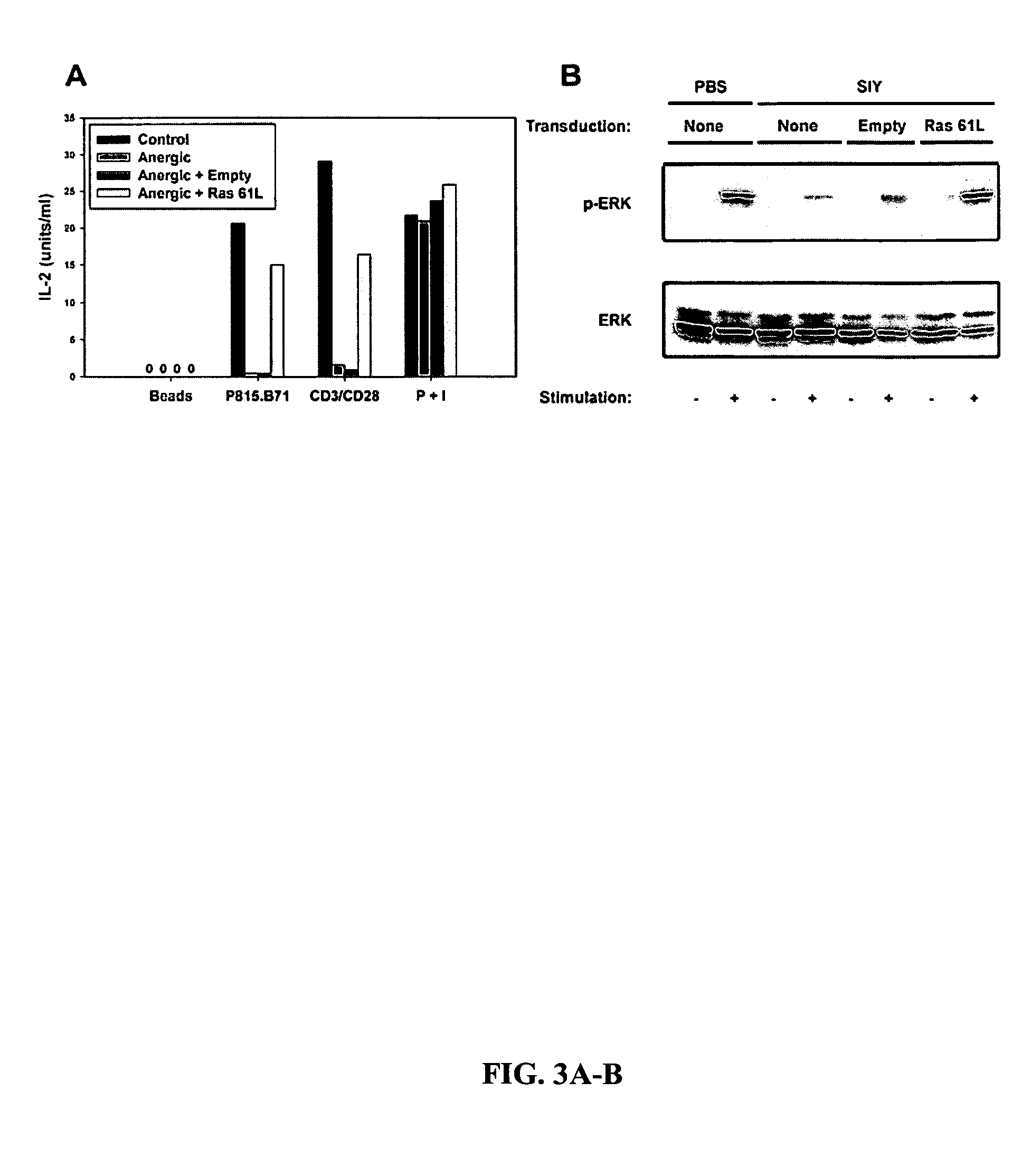
T cell anergy is reversed by active Ras and regulated by diacylglycerol kinase
ActiveUS20050266510A1Wide applicationMicrobiological testing/measurementBiological testingWestern blotIl 2 production
T cell anergy has been correlated with defective Ras signaling. However, neither a causal relationship nor the mechanism of Ras hypoactivation have been established. Using adenoviral transduction of CAR Tg T cells to enable genetic manipulation in nonproliferating cells, we show that Ras61L restores IL-2 production and MAP kinase signaling in T cells anergized in vitro or in vivo. A gene array screen revealed upregulated diacylglycerol kinase (DGK) in the anergic state, which was confirmed by RT-PCR and Western blot analysis. A DGK inhibitor significantly restored IL-2 production by anergic cells. Our data support a causal role for DGK and defective Ras signaling in T cell anergy.
Owner:CHICAGO UNIV OF THE
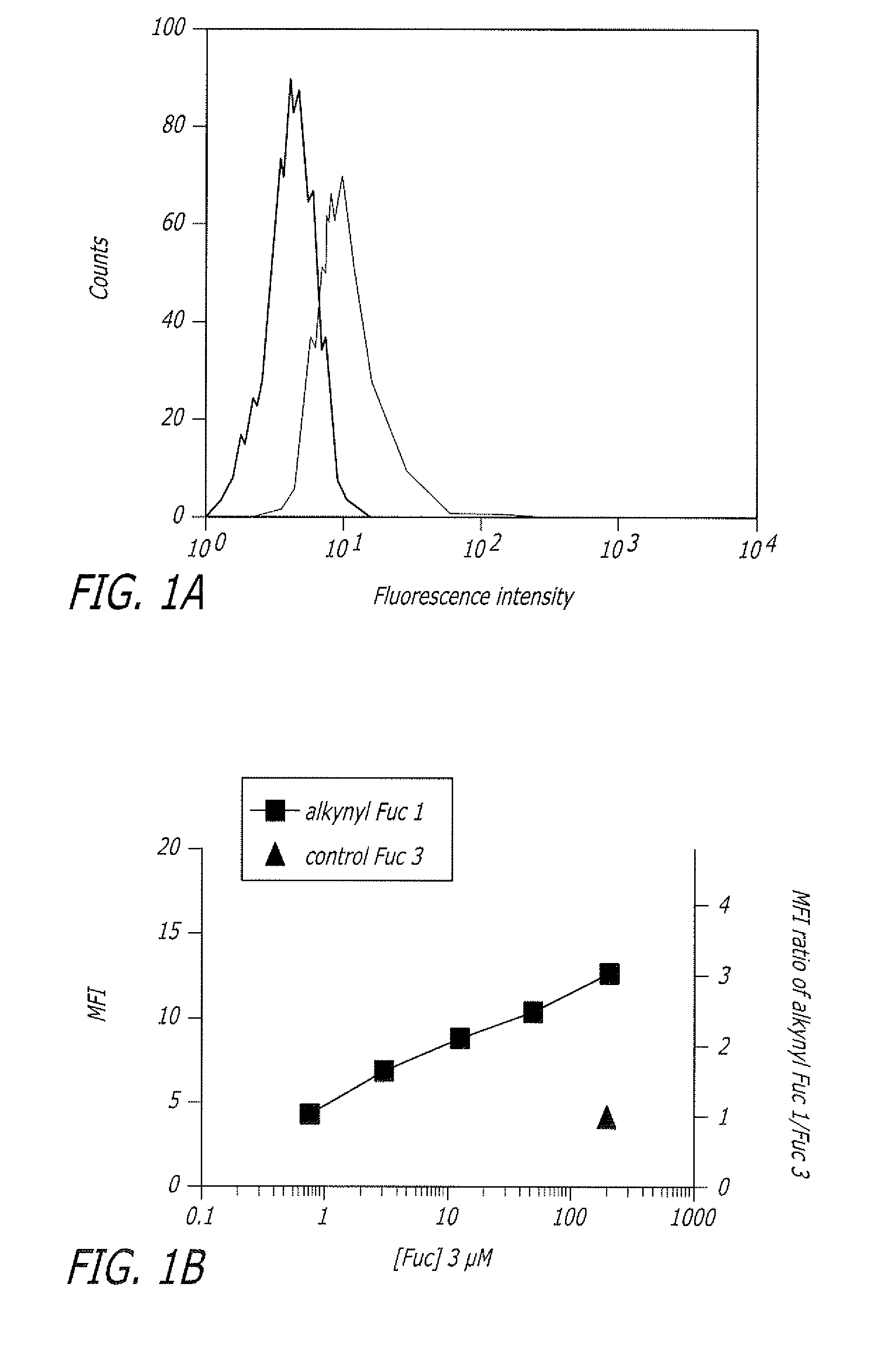
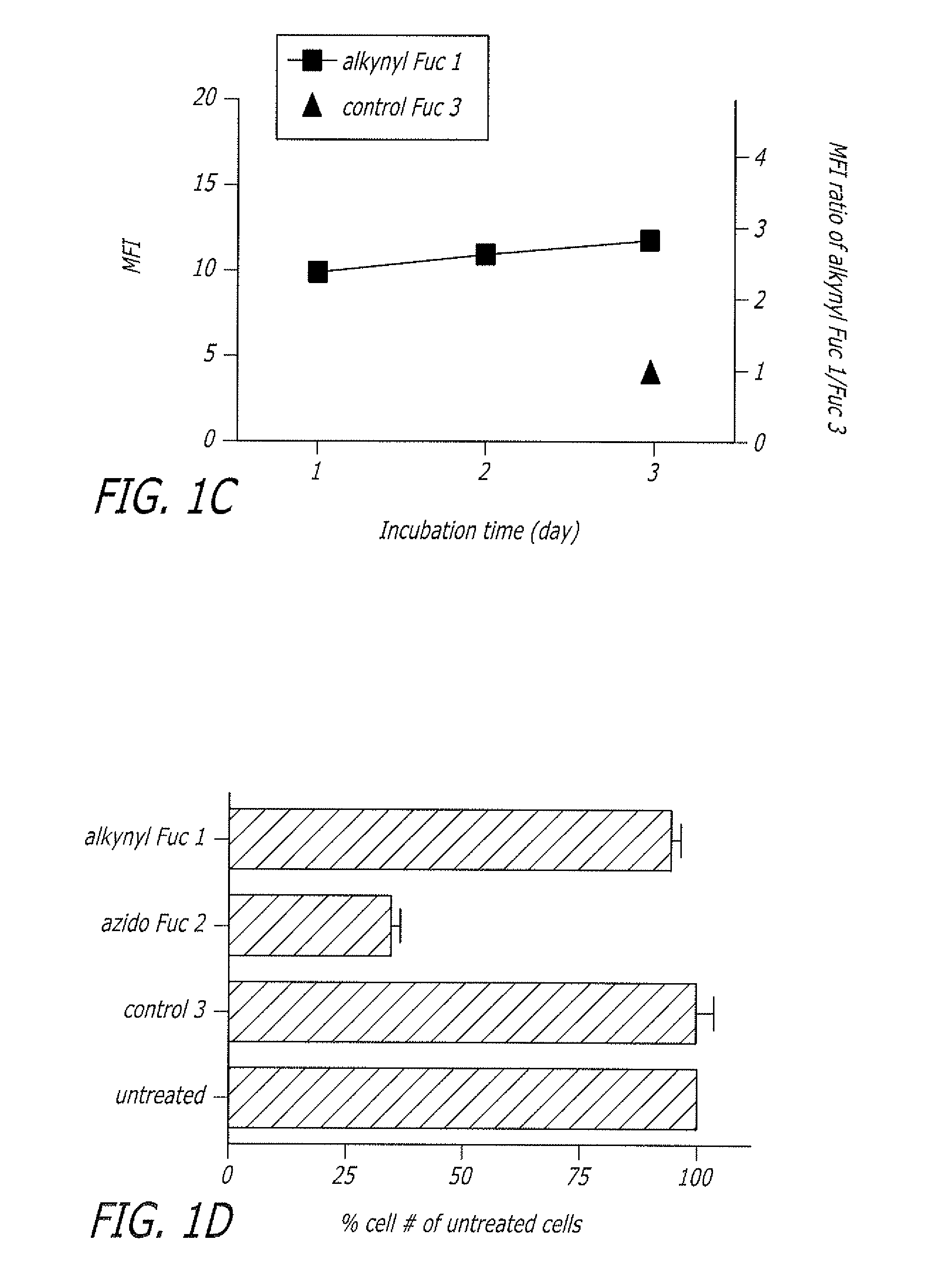
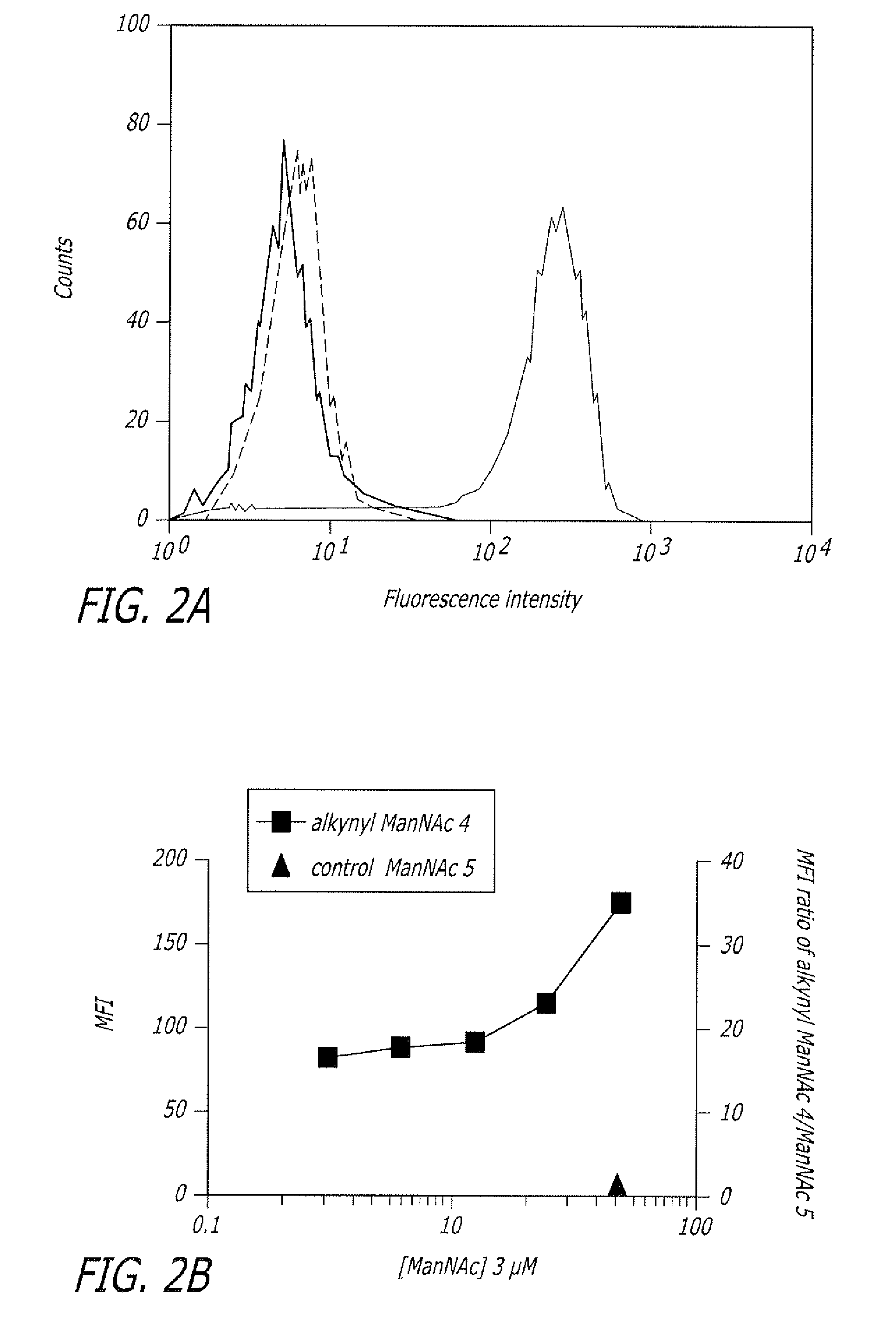
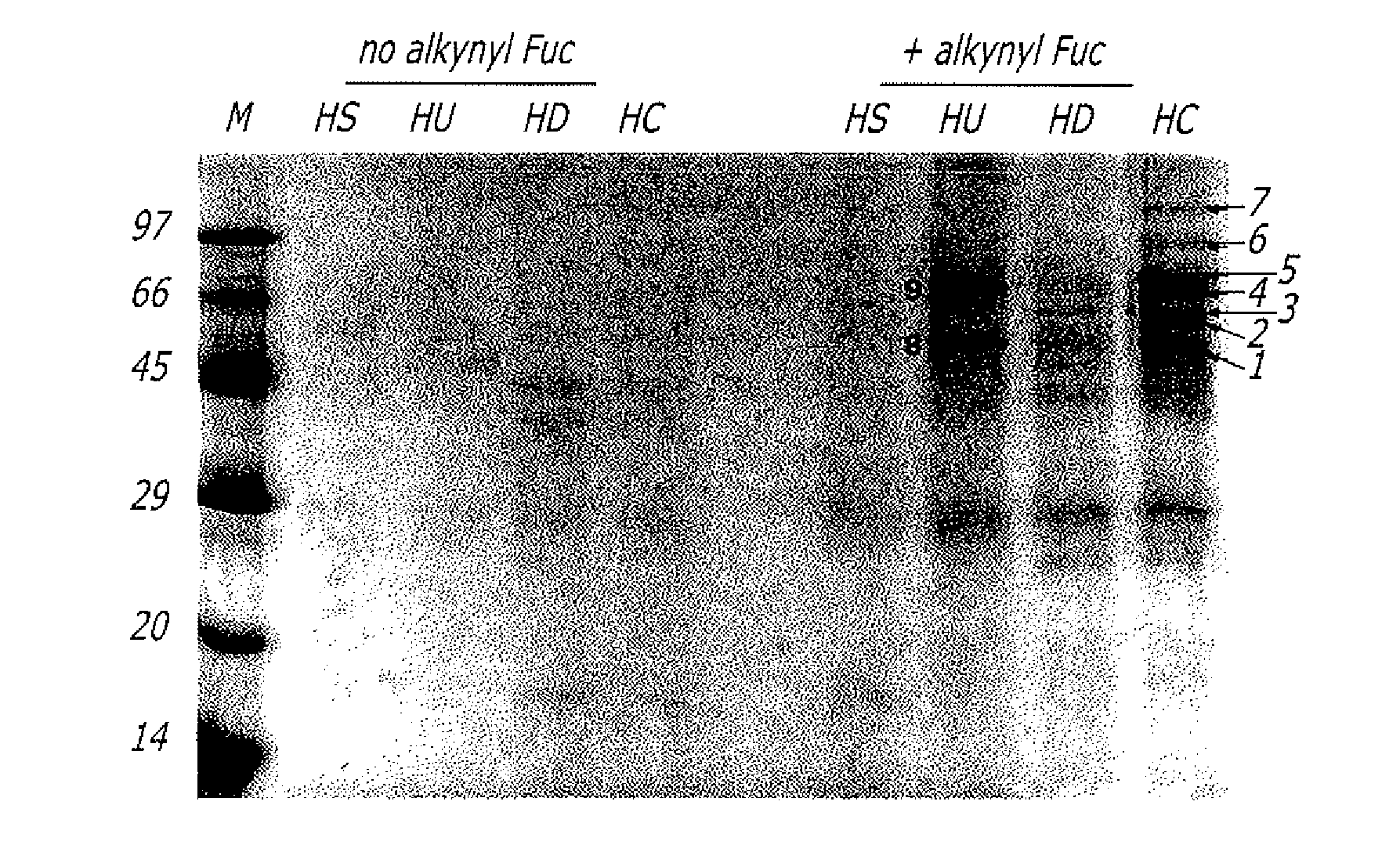
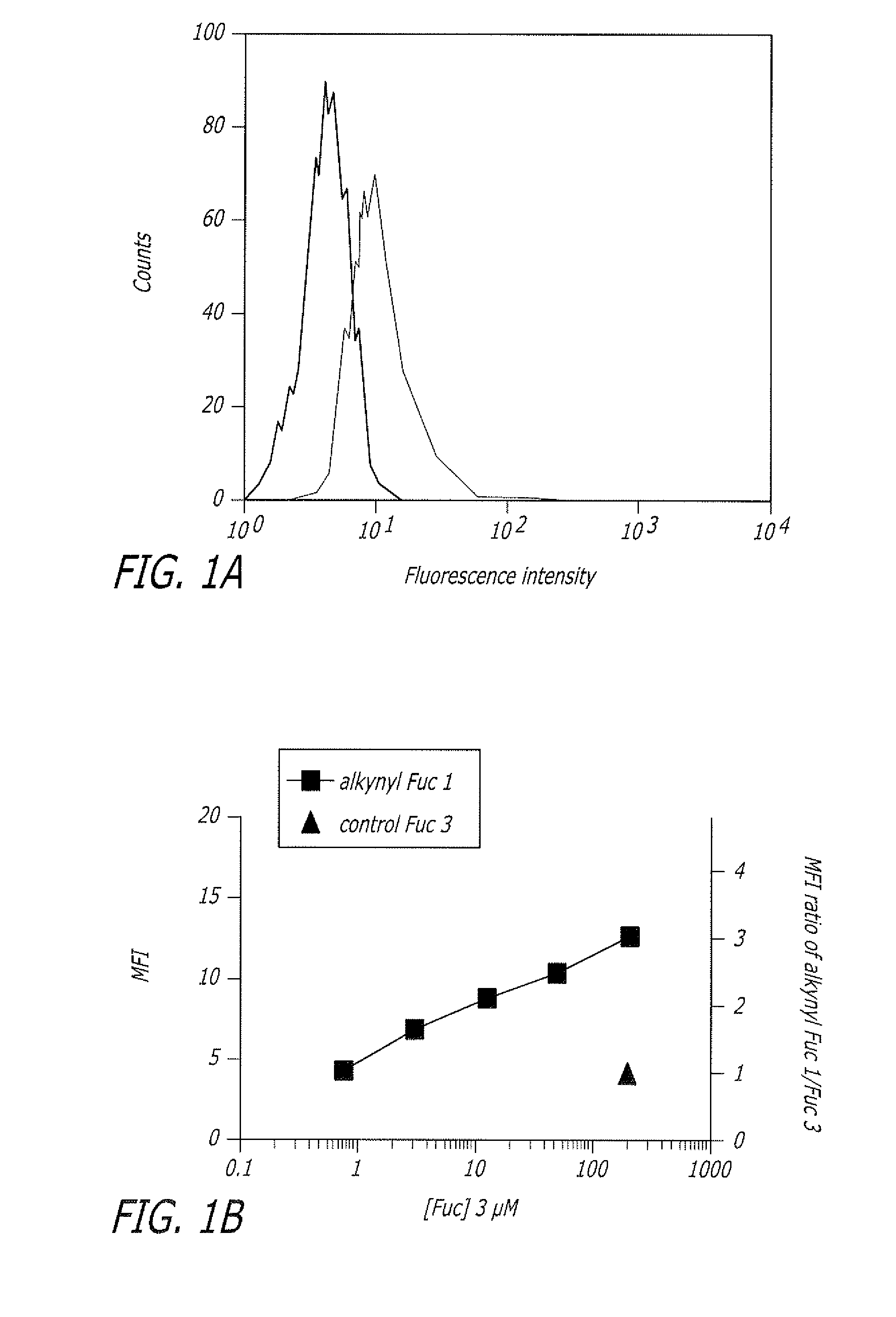
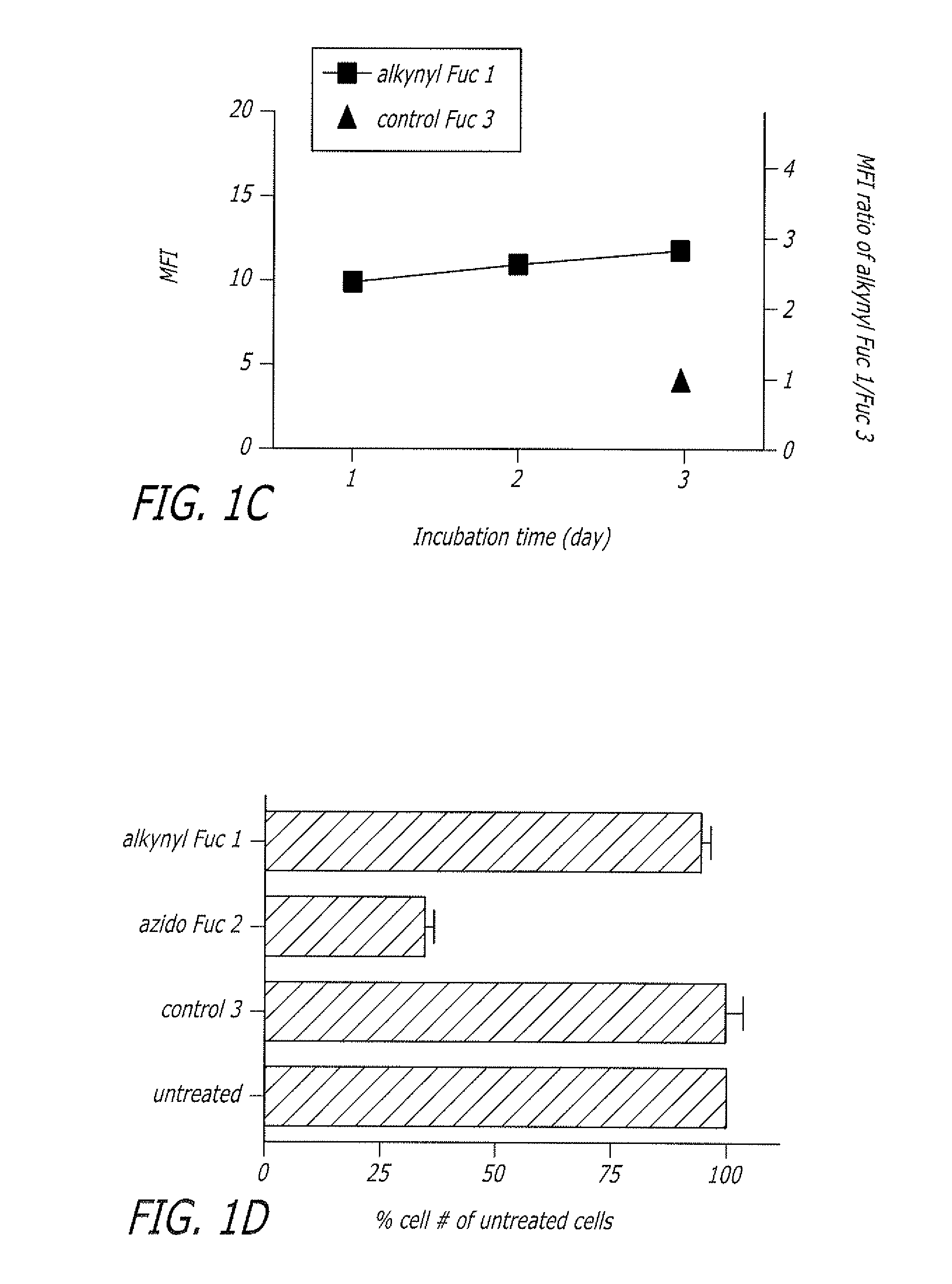
Alkynyl sugar analogs for the labeling and visualization of glycoconjugates in cells
ActiveUS7960139B2Increased toxicitySugar derivativesMicrobiological testing/measurementFluorescenceWestern blot
The present disclosure relates to a method for metabolic oligosaccharide engineering that incorporates derivatized alkyne-bearing sugar analogs as “tags” into cellular glycoconjugates. The disclosed method incorporates alkynyl derivatized Fuc and alkynyl derivatized ManNAc sugars into a cellular glycoconjugate. A chemical probe comprising an azide group and a visual probe or a fluorogenic probe is used to label the alkyne-derivatized sugar-tagged glycoconjugate. In one aspect, the chemical probe binds covalently to the alkynyl group by Cu(I)-catalyzed [3+2] azide-alkyne cycloaddition and is visualized at the cell surface, intracellularly, or in a cellular extract. The labeled glycoconjugate is capable of detection by flow cytometry, SDS-PAGE, Western blot, ELISA or confocal microscopy, and mass spectrometry.
Owner:ACAD SINIC
Alkynyl sugar analogs for the labeling and visualization of glycoconjugates in cells
ActiveUS20080268468A1Increased toxicitySugar derivativesMicrobiological testing/measurementFluorescenceCycloaddition
The present disclosure relates to a method for metabolic oligosaccharide engineering that incorporates derivatized alkyne-bearing sugar analogs as “tags” into cellular glycoconjugates. The disclosed method incorporates alkynyl derivatized Fuc and alkynyl derivatized ManNAc sugars into a cellular glycoconjugate. A chemical probe comprising an azide group and a visual probe or a fluorogenic probe is used to label the alkyne-derivatized sugar-tagged glycoconjugate. In one aspect, the chemical probe binds covalently to the alkynyl group by Cu(I)-catalyzed [3+2] azide-alkyne cycloaddition and is visualized at the cell surface, intracellularly, or in a cellular extract. The labeled glycoconjugate is capable of detection by flow cytometry, SDS-PAGE, Western blot, ELISA or confocal microscopy, and mass spectrometry.
Owner:ACAD SINIC
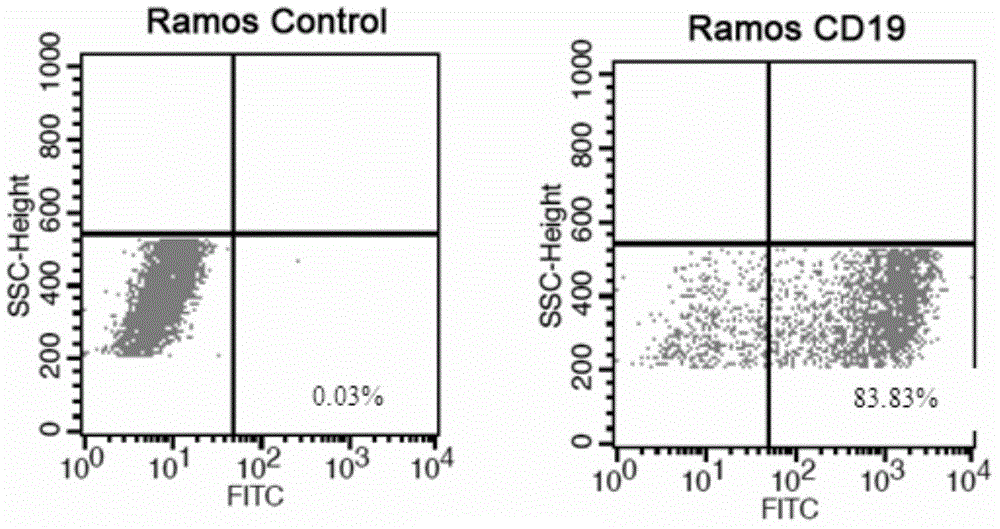
Anti-CD19 monoclonal antibody and preparation method thereof
ActiveCN105837689AEfficient identificationHigh affinityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsProtein targetWestern blot
The invention provides an anti-CD19 monoclonal antibody, and a preparation method and an application thereof. The anti-CD19 monoclonal antibody can effectively recognize cells with CD19 highly expressed, and can be successfully used for Western Blot detection. An affinity test shows that the monoclonal antibody has the extremely high affinity to target protein.
Owner:PERSONGEN ANKE CELLULAR THERAPEUTICS CO LTD
Treatment of inflammatory disease
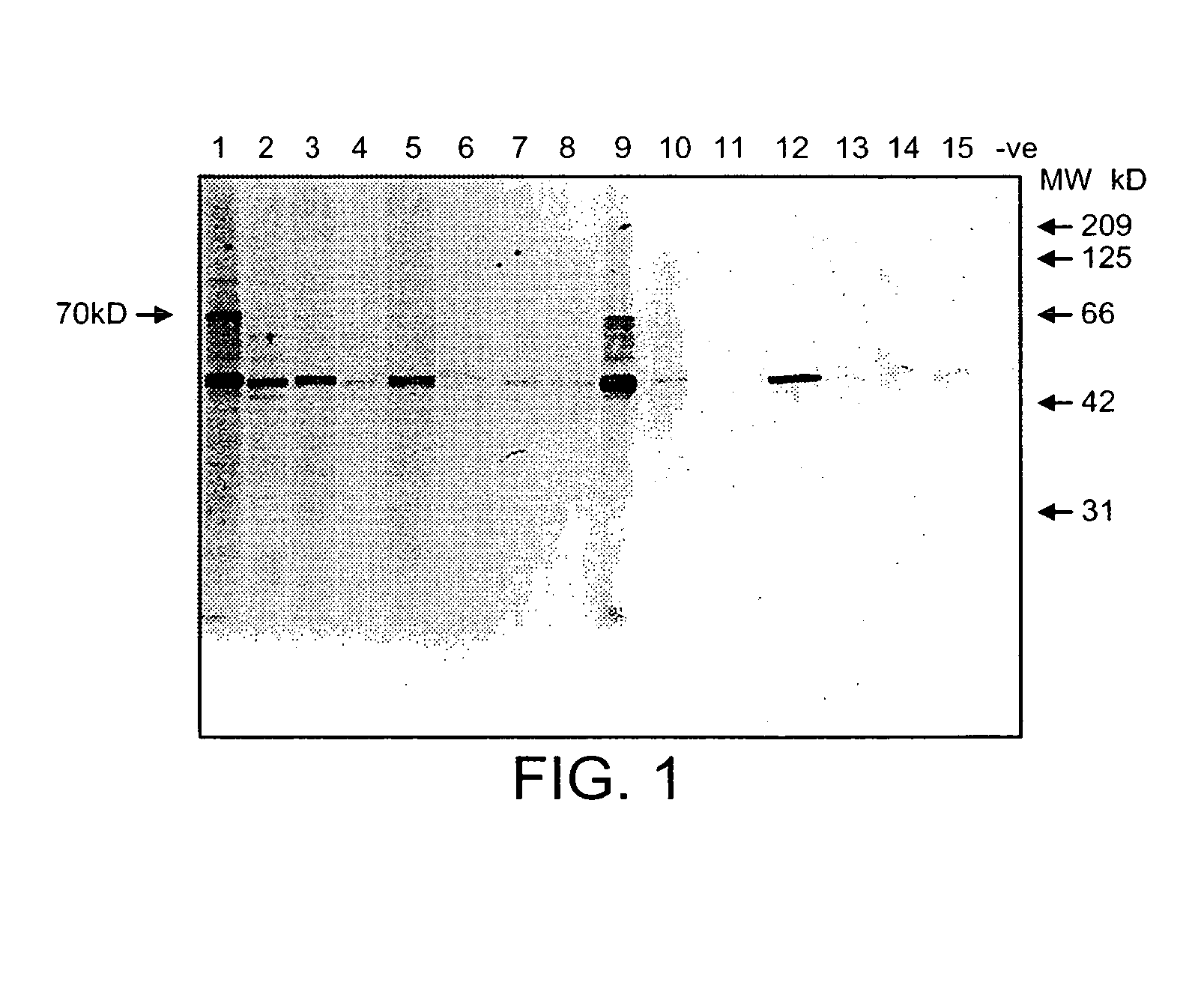
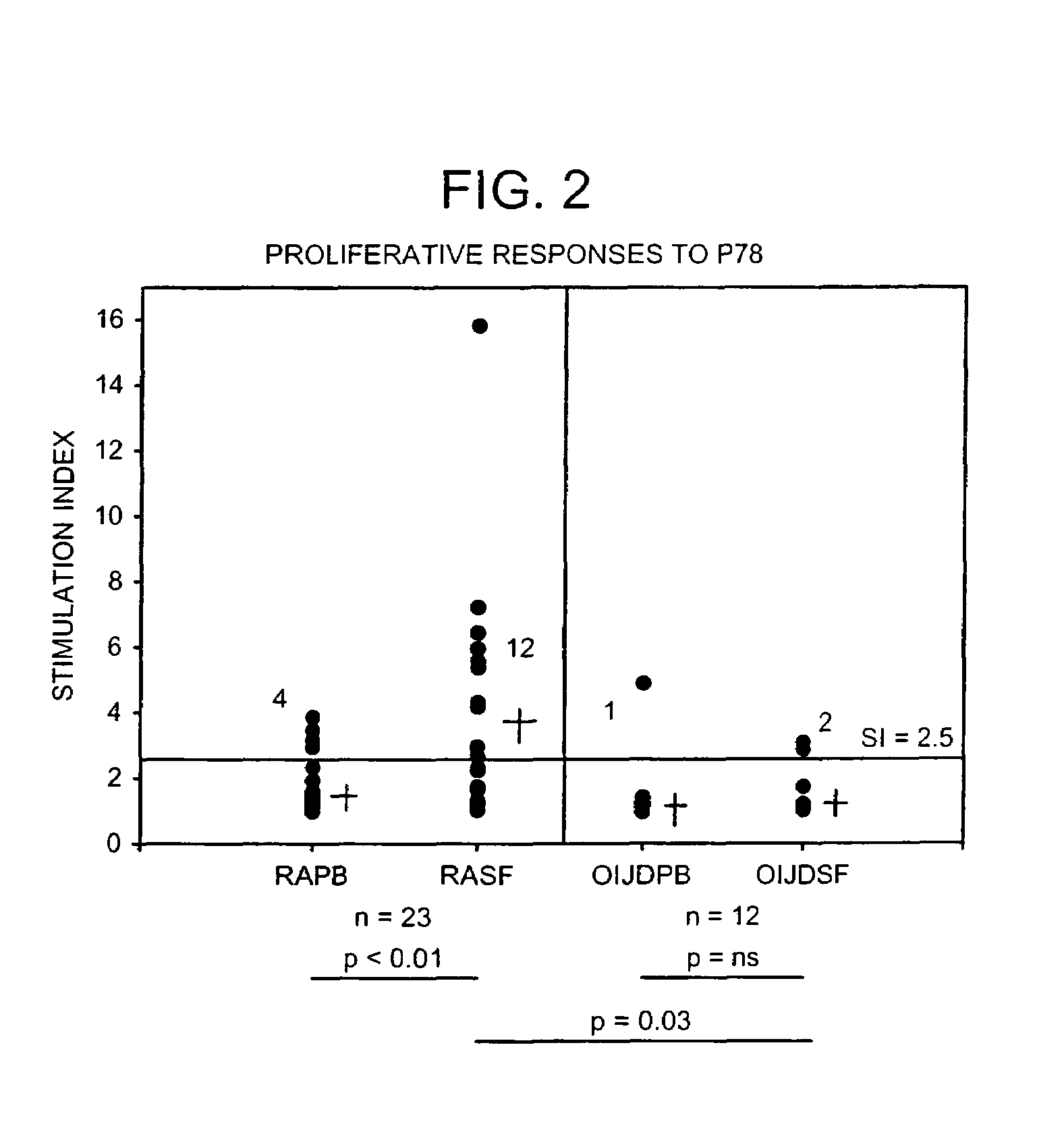
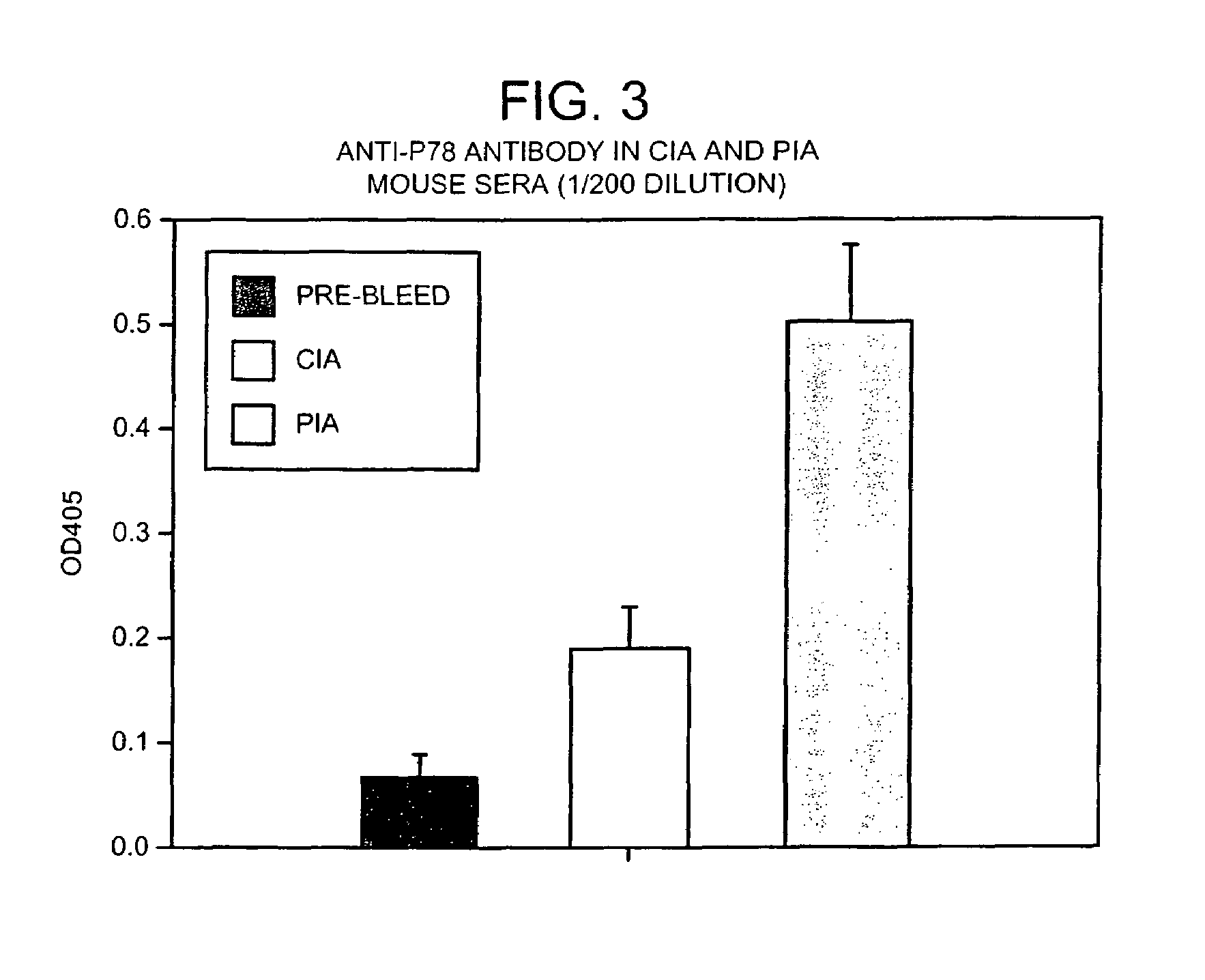
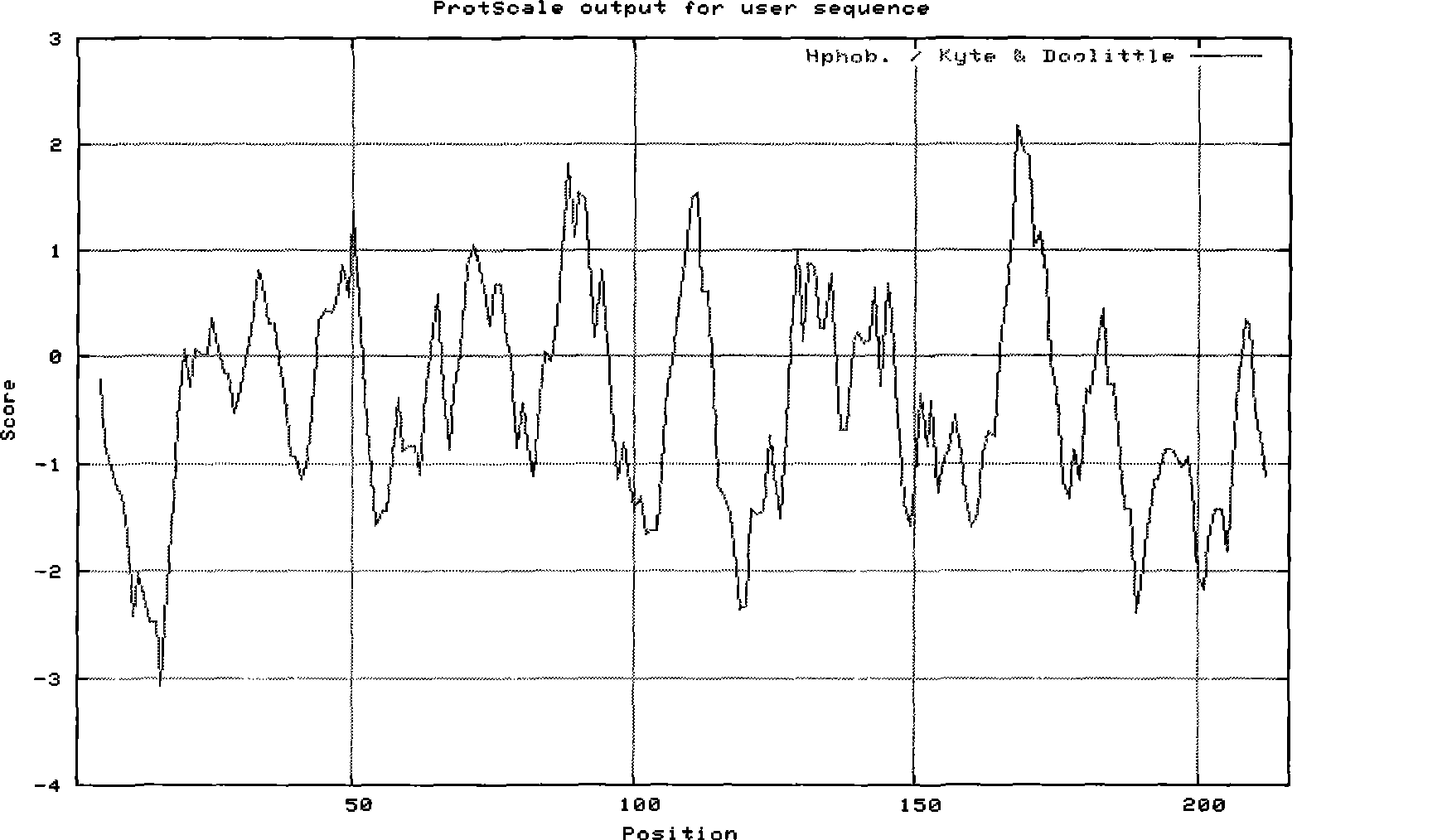
The immunoglobulin heavy chain binding protein BiP(GRP78), or a fragment thereof, provides a reagent for indicating the presence of rheumatoid arthritis (RA). The protein used is a recombinant BiP(GRP78) having a defined sequence. A prognostic and / or diagnostic test for RA may be an ELISA or a Western Blot that utilizes the BiP(GRP78) protein or a fragment thereof. Also disclosed are a method of testing for RA using BiP(GRP78) or a cDNA sequence coding therefor. Further disclosed is a method of treating RA by the BiP(GRP78) protein or corresponding cDNA.
Owner:KINGS COLLEGE LONDON
Cloning, expression and application of eimeria tenella protein disulfide isomerase gene
InactiveCN101418309AGenetic material ingredientsRecombinant DNA-technologyEscherichia coliEmoia loyaltiensis
The invention discloses an E.tenella protein disulfide linkage isomerase gene EtPDI (Clone ID is BW1-E06,and the Genbank accession number of is EF552214). The gene is connected with a procaryon expression vector pGEX-4T-2; a procaryon expression recombination plasmid pGEX-4T-EtPDI is constructed and is expressed in a colibacillus system; and most of the expressed recombining protein exists in a soluble form. The recombining protein 4T-EtPDI is purified to carry out SPS-PAGE and is transferred to a PVDF film; and antiserum of E.tenella oocyst oral immunized chicken is used as first resistance and goat anti-chicken IgG is used as second resistance to carry out Western-blot analysis, thereby indicating that the gene has certain antigen. The gene is used for preparing an anti-chicken coccidiosis drug and an anti-chicken coccidiosis vaccine.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
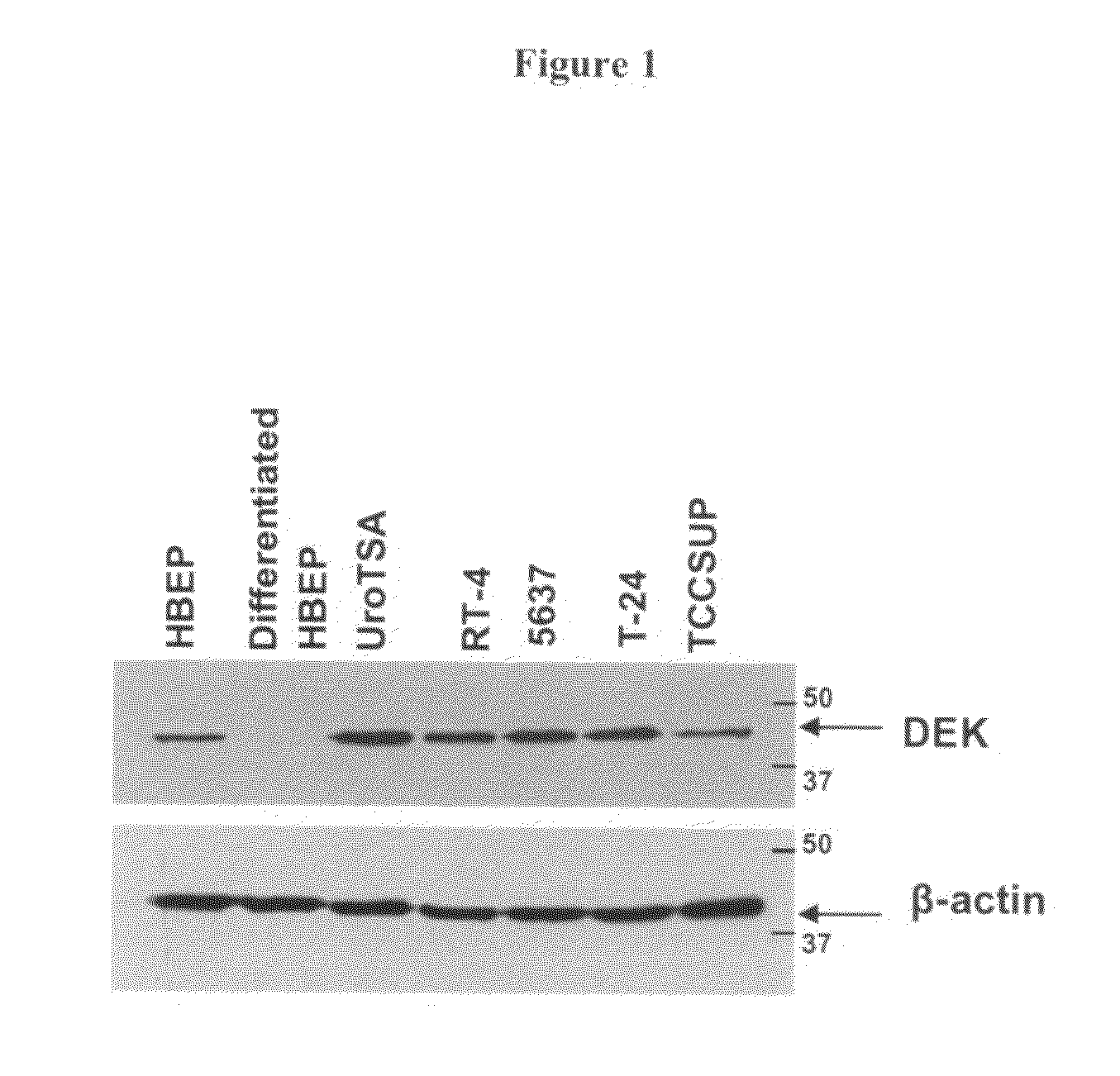
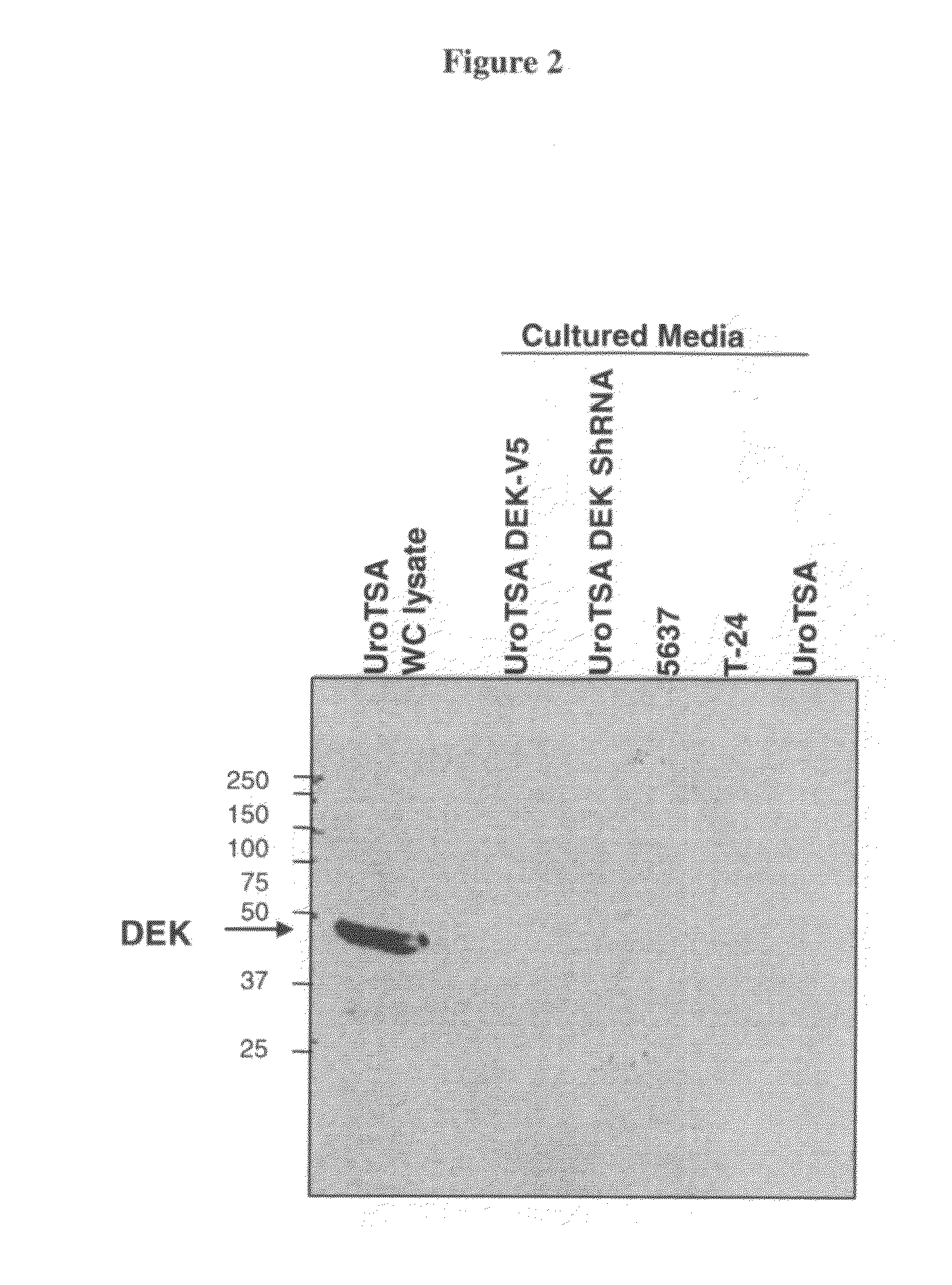
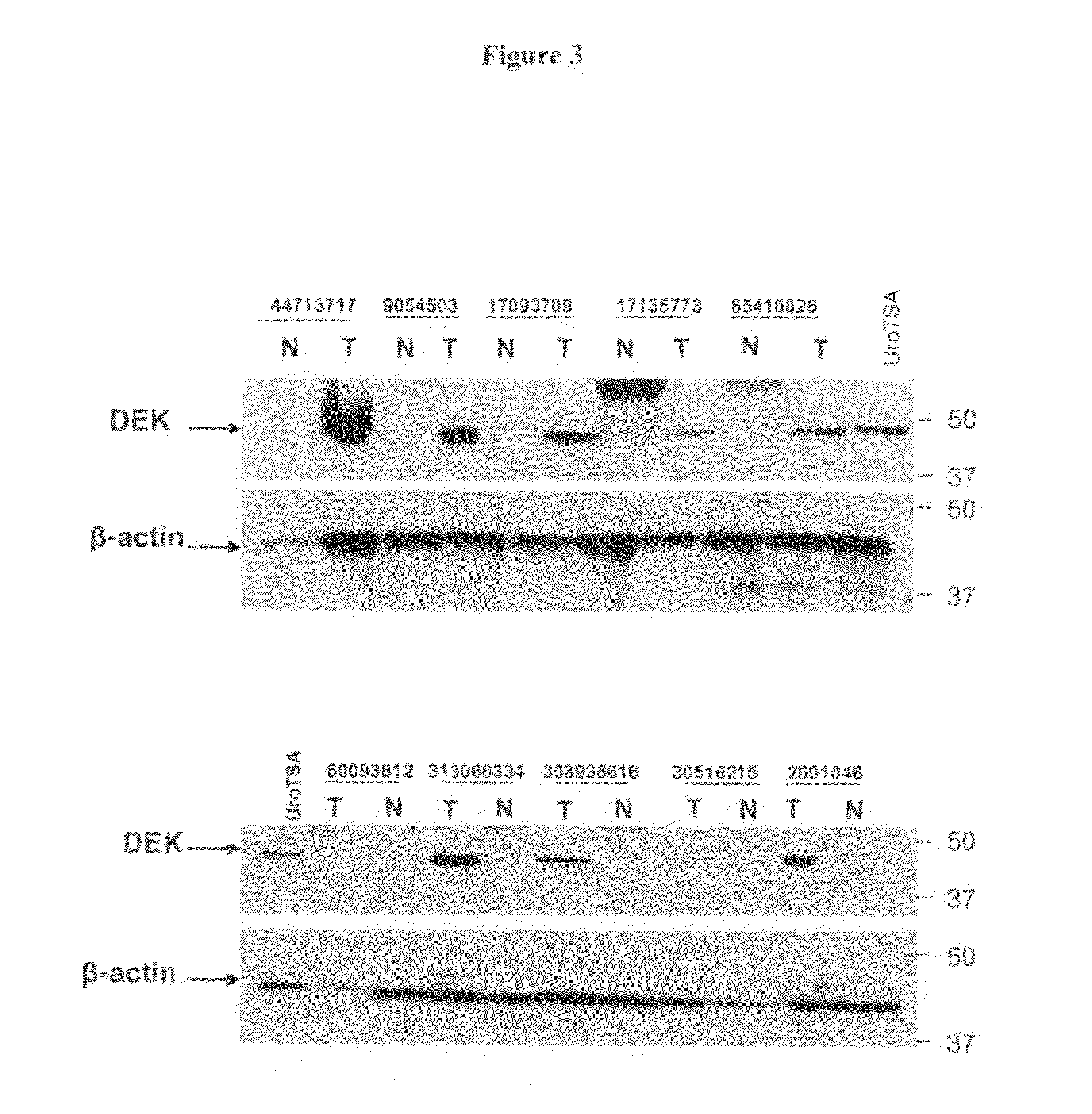
DEK as a urine based biomarker for bladder cancer
The present invention is directed to a method of detecting DEK protein in a urine sample. Methods and compositions are provided herein for detecting and diagnosing bladder cancer by chemical-induced precipitation of urine proteins, followed by filtration-induced concentration and Western blot analysis to specifically detect DEK protein. The present method permits specific detection of DEK protein in urine as a biomarker for bladder cancer in humans.
Owner:MEDICAL DIAGNOSTIC LAB
T cell anergy is reversed by active Ras and regulated by diacylglycerol kinase
ActiveUS7381401B2Wide applicationMicrobiological testing/measurementBiological testingWestern blotIl 2 production
T cell anergy has been correlated with defective Ras signaling. However, neither a causal relationship nor the mechanism of Ras hypoactivation have been established. Using adenoviral transduction of CAR Tg T cells to enable genetic manipulation in nonproliferating cells, we show that Ras61L restores IL-2 production and MAP kinase signaling in T cells anergized in vitro or in vivo. A gene array screen revealed upregulated diacylglycerol kinase (DGK) in the anergic state, which was confirmed by RT-PCR and Western blot analysis. A DGK inhibitor significantly restored IL-2 production by anergic cells. Our data support a causal role for DGK and defective Ras signaling in T cell anergy.
Owner:CHICAGO UNIV OF THE
Western blot kit for detecting antibody of autoimmune disease and preparation method thereof
ActiveCN103105489AOvercome the cumbersome operation of individual detection one by oneImprove accuracyMaterial analysisAntigenAnti-mitochondrial antibody
The invention provides a western blot kit for detecting the antibody of autoimmune disease and a preparation method of the western blot kit, and relates to a western blot kit for detecting related antibodies of various autoimmune diseases, aiming at overcoming the technical defect that a western blot product is unavailable for testing and screening various autoimmune diseases in the prior art. The nitrocellulose membrane or the nylon membrane contains at least two parallel detection lines coated by at least two of ten natural antigens or recombinant antigens, i.e. dsDNA (deoxyribonucleic acid), Sm / RNP (ribonucleoprotein), CCP (critical compression pressure), SSA (sulfosalicylic acid), SSB (single-strand binding protein), GAD (glutamic acid decarboxylase), ICA (islet cell antibody), IA-2A (islet cell), TG (triglyceride) and AMA-M2 (anti-mitochondrial antibody), a high-concentration quality control band, a median-concentration quality control band and a low-concentration quality control band. The deficiency of the detection sensitivity and the specificity of the single autoantibody can be overcome, the operating complexity for independently detecting the related autoantibody of various diseases one by one can be overcome, and the detection efficiency and the result judging accuracy degree can be greatly improved.
Owner:SHENZHEN YHLO BIOTECH
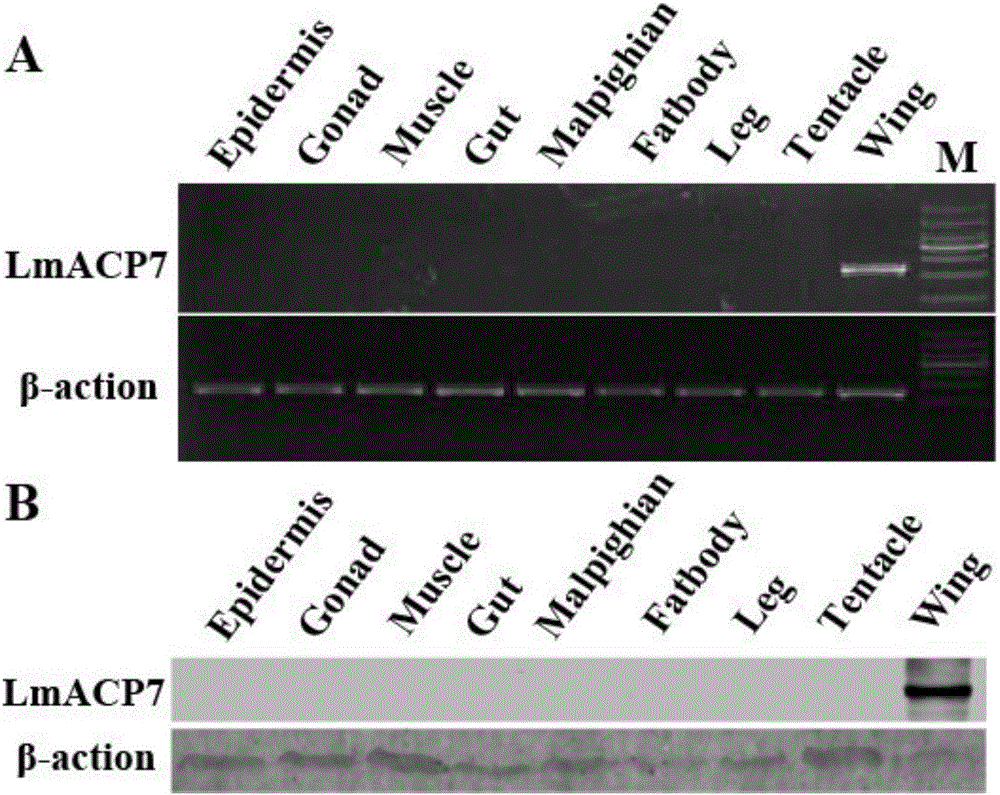
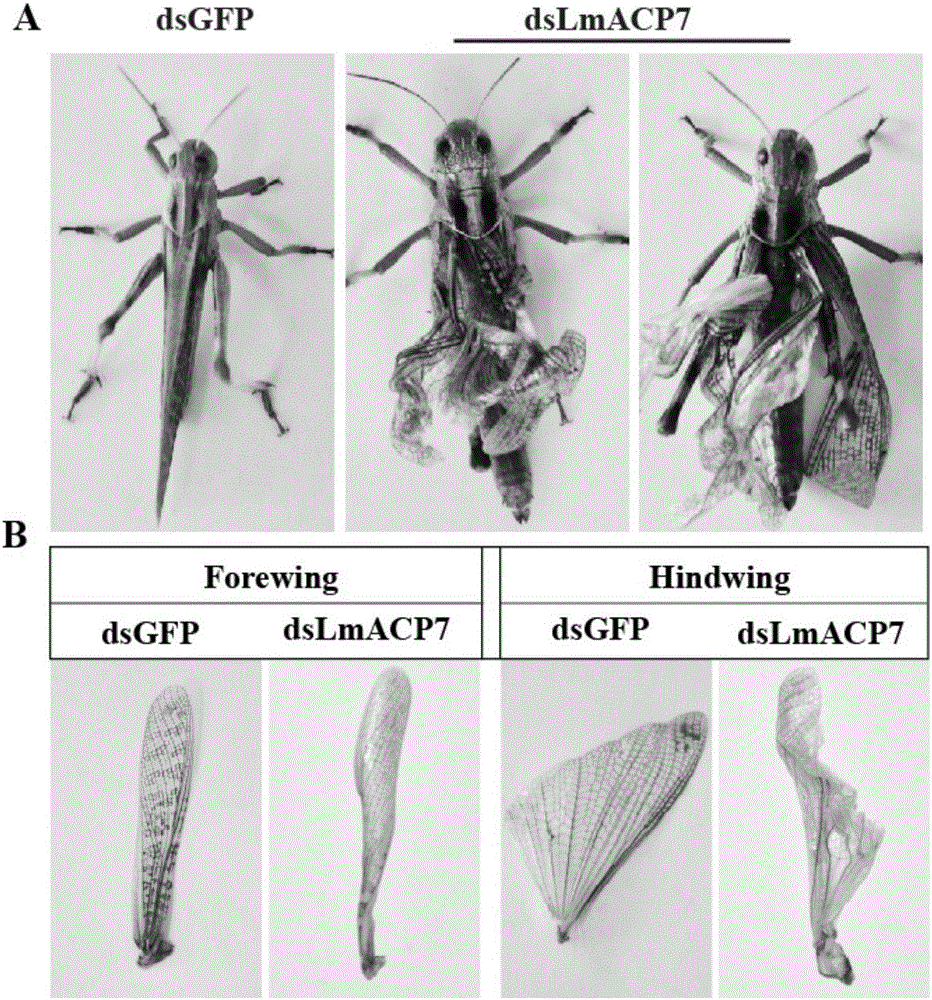
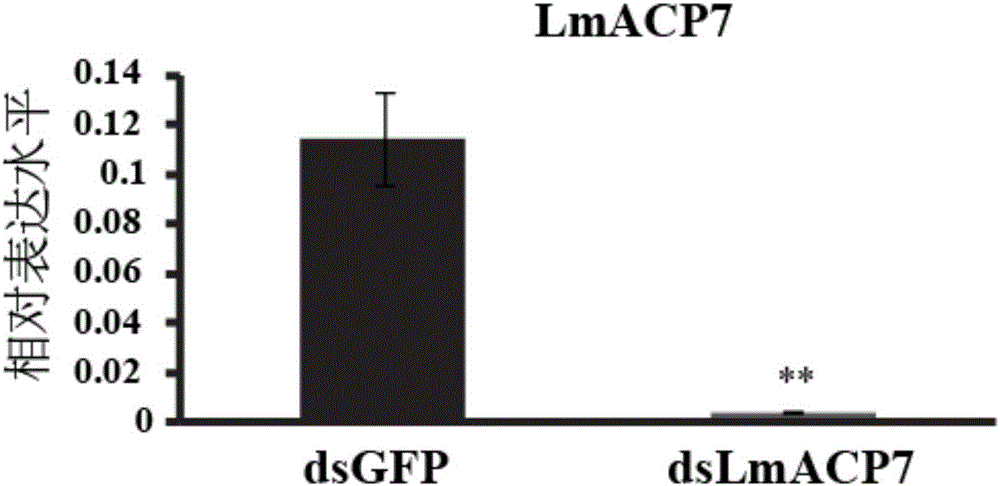
Migratory locust wing specific cuticle protein gene and application of dsRNA thereof
The invention provides a migratory locust wing specific cuticle protein gene and application of dsRNA thereof. DsRNA of the gene can silence the specific cuticle protein gene, so that migratory locust wings grow abnormally and migratory locust finally die.Concretely, through cloning and sequencing migratory locust cuticle protein gene, the wing cuticle protein gene with the sequence being SEQ ID NO:1 is obtained, and RT-PCR and western blot results show specific existence of the wing cuticle protein gene in migratory locust wing tissue; then a gene segment with the sequence being SEQ ID NO:2 is selected and used for double-stranded RNA (dsRNA) synthesis. After the gene dsRNA is injected into body cavities of migratory locust nymphs, wings of adult nymphs grow abnormally after molting, are manifested to grow with malformation and shrinkage and finally the migratory locusts die.A new target is provided for insect prevention and treatment based on RNA interference.
Owner:SHANXI UNIV
Chemiluminiscence diagnostic kit for sensitization allergens and preparation method thereof
InactiveCN103033611AHigh sensitivityGood repeatabilityChemiluminescene/bioluminescenceSorbentWestern blot
The invention provides a chemiluminiscence diagnostic kit for sensitization allergens and a preparation method thereof. The diagnostic kit comprises a microporous plate coated with the sensitization allergens, a horseradish peroxidase-labeled anti-human IgE (immunoglobulin e) antibody, chemiluminiscence substrate solutions A and B, and a washing solution, wherein the sensitization allergens comprise one or more of an insect allergen, a pollen allergen, a fungus allergen and a food allergen. According to the diagnostic kit, the difference of the sensitivities of different proteins in one diagnostic kit is overcome, and the detection sensitivity of each allergen is enhanced to the maximum extent by repeatedly testing and adjusting the coating amount of the allergens, the concentration of the enzyme-labeled antibody and the formulae and the concentrations of the chemiluminiscence substrate solutions. The chemiluminiscence diagnostic kit is higher in detection sensitivity, safe, reliable, simple and convenient to operate and low in cost in comparison with the ELISA (enzyme linked immunosorbent assay), western-blot and RAST (radioallergo-sorbent test) diagnostic kits for the allergens.
Owner:北京新华联协和药业有限责任公司
Recombinant porcine epidemic diarrhea virus (PEDV) lactococcus lactis and its construction method and use
InactiveCN103074291AGood immune protectionPromote absorptionBacteriaMicroorganism based processesProtective antigenWestern blot
Owner:DALIAN UNIVERSITY
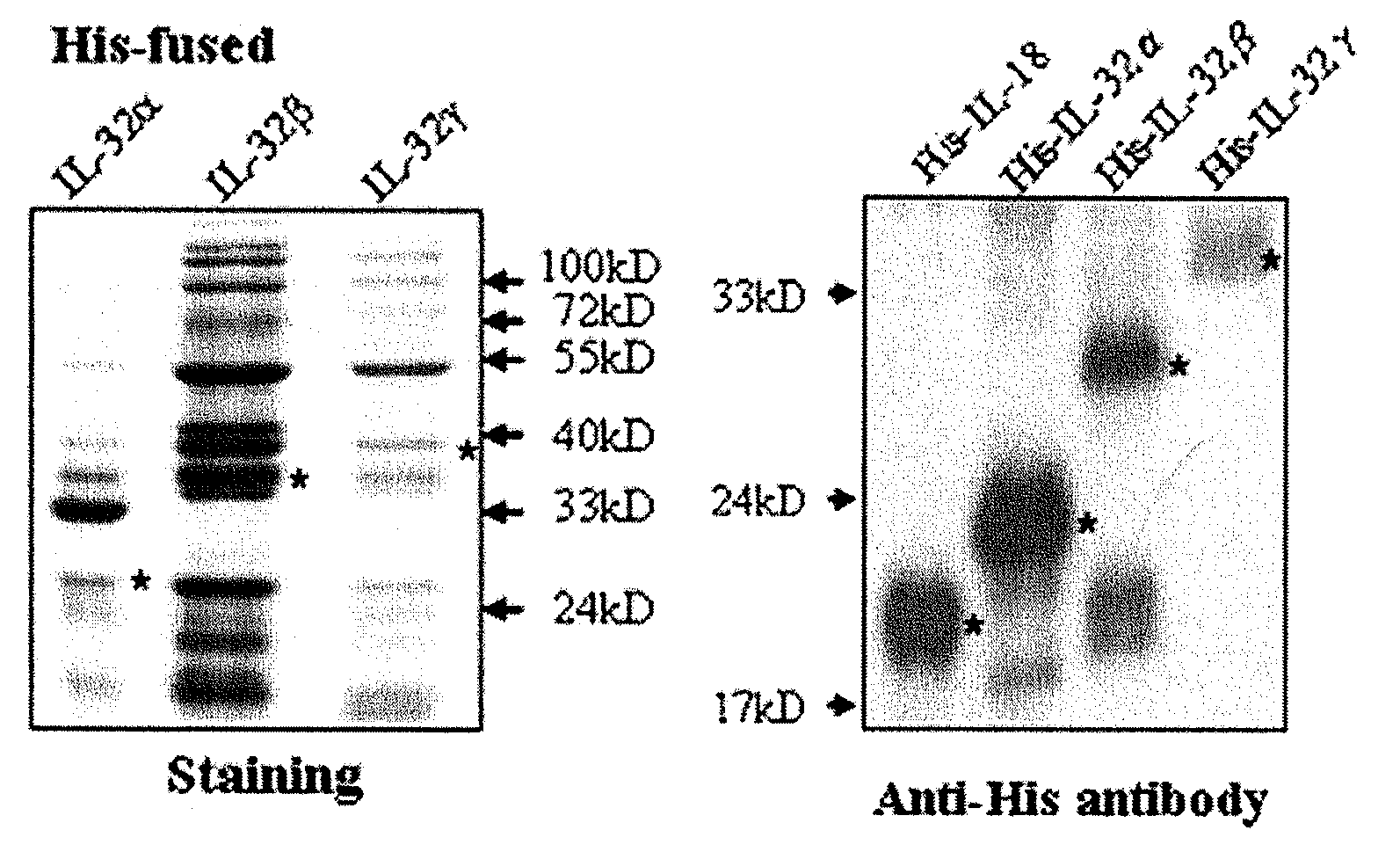
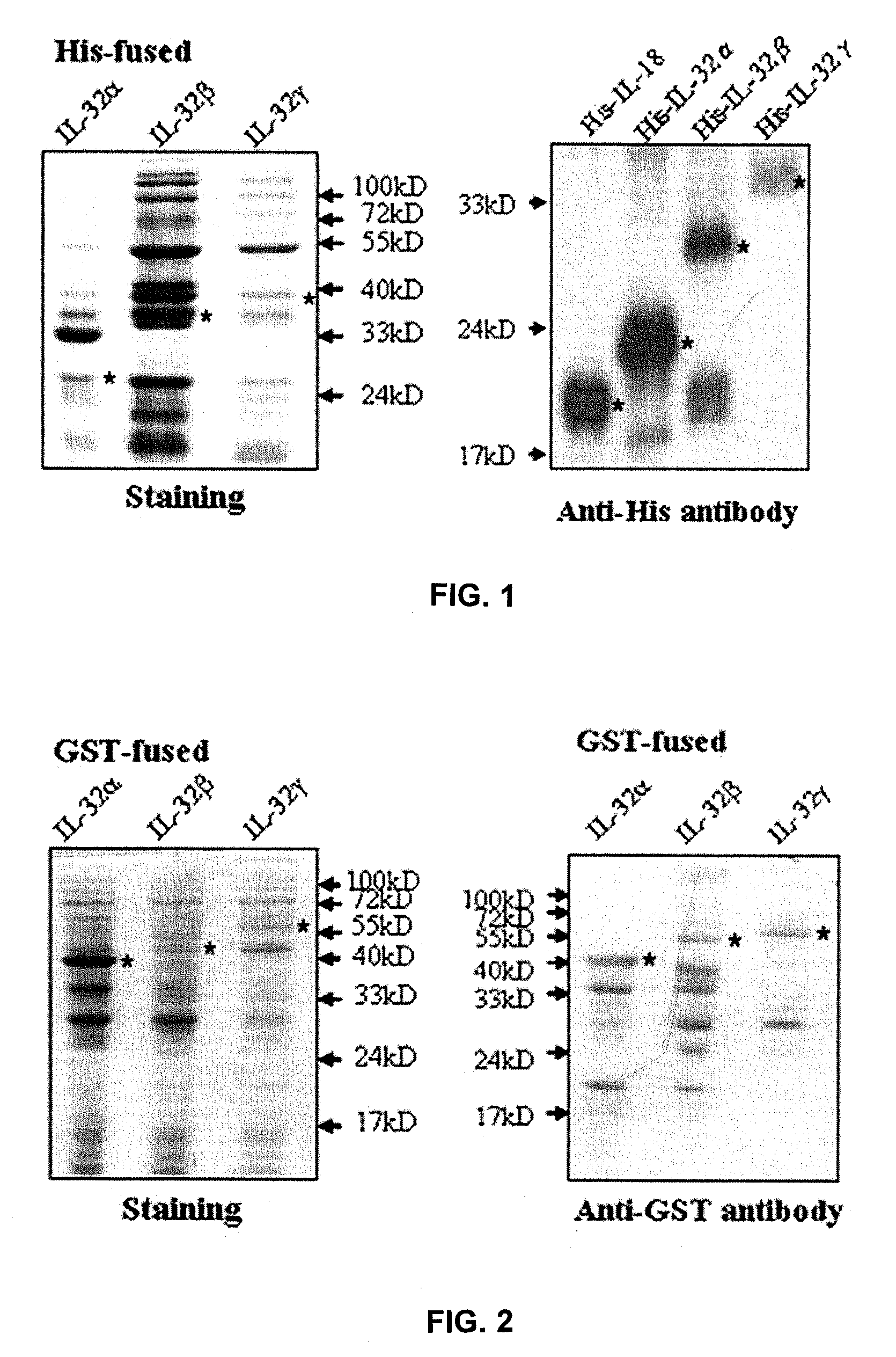
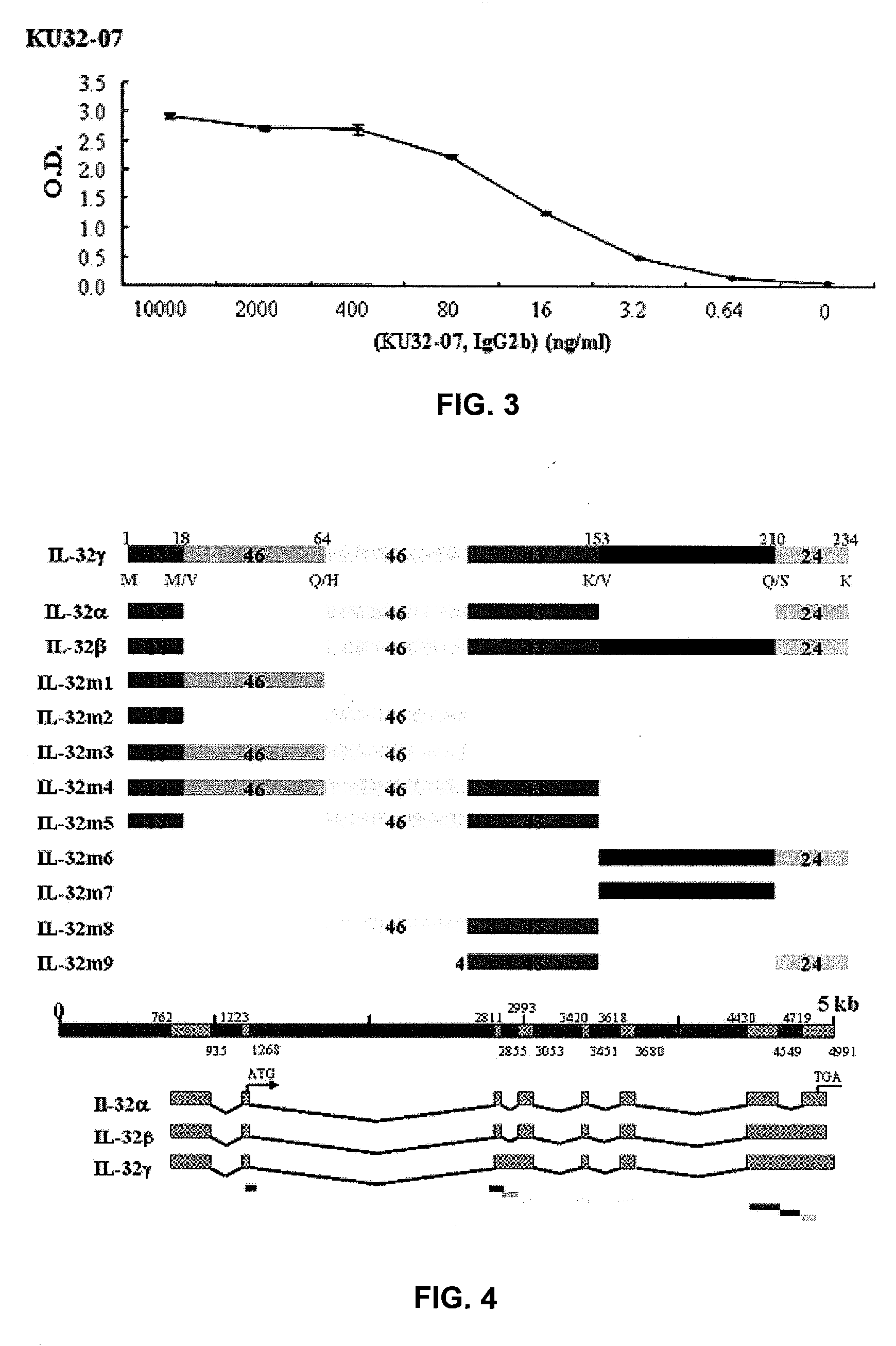
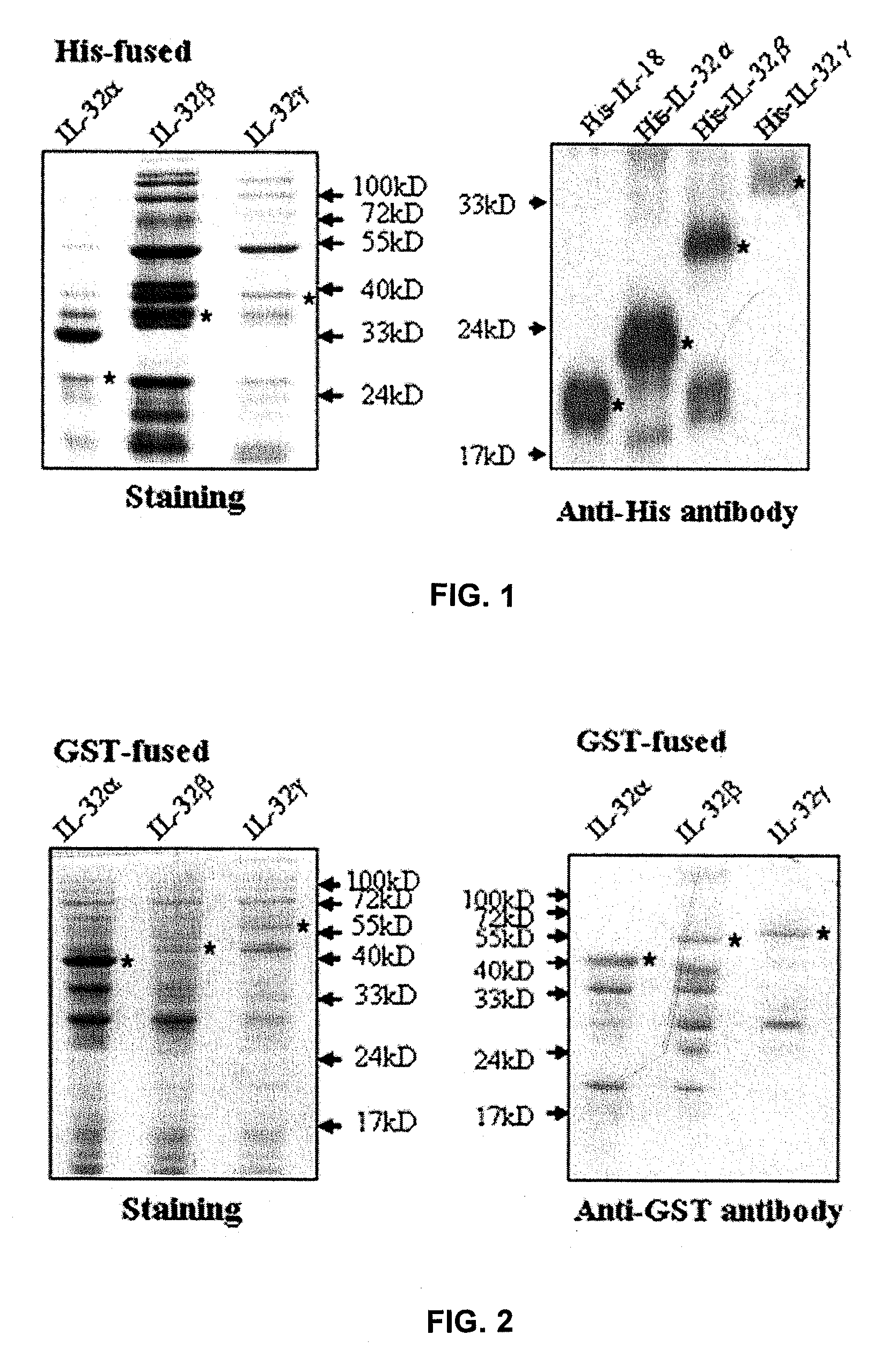
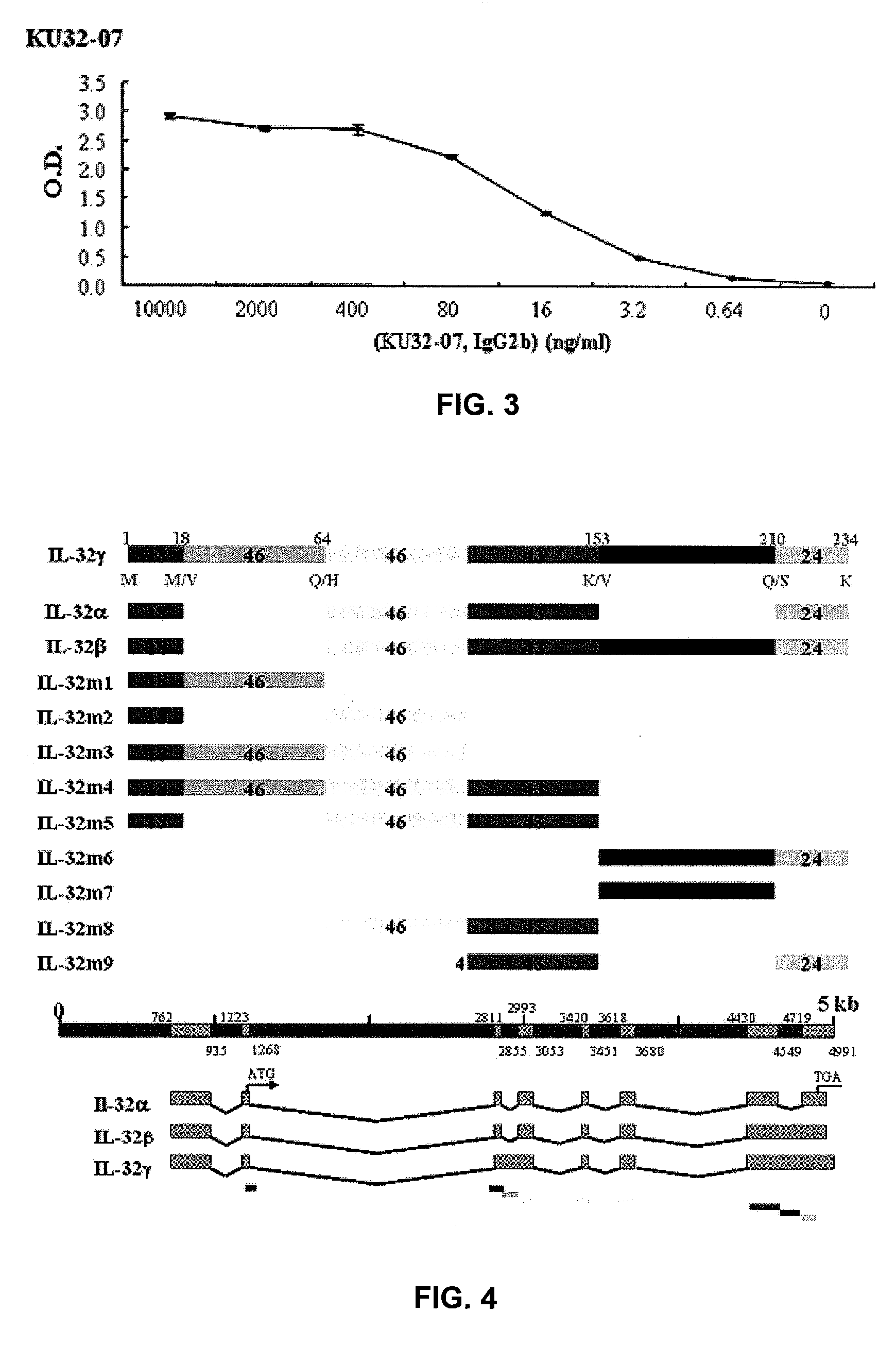
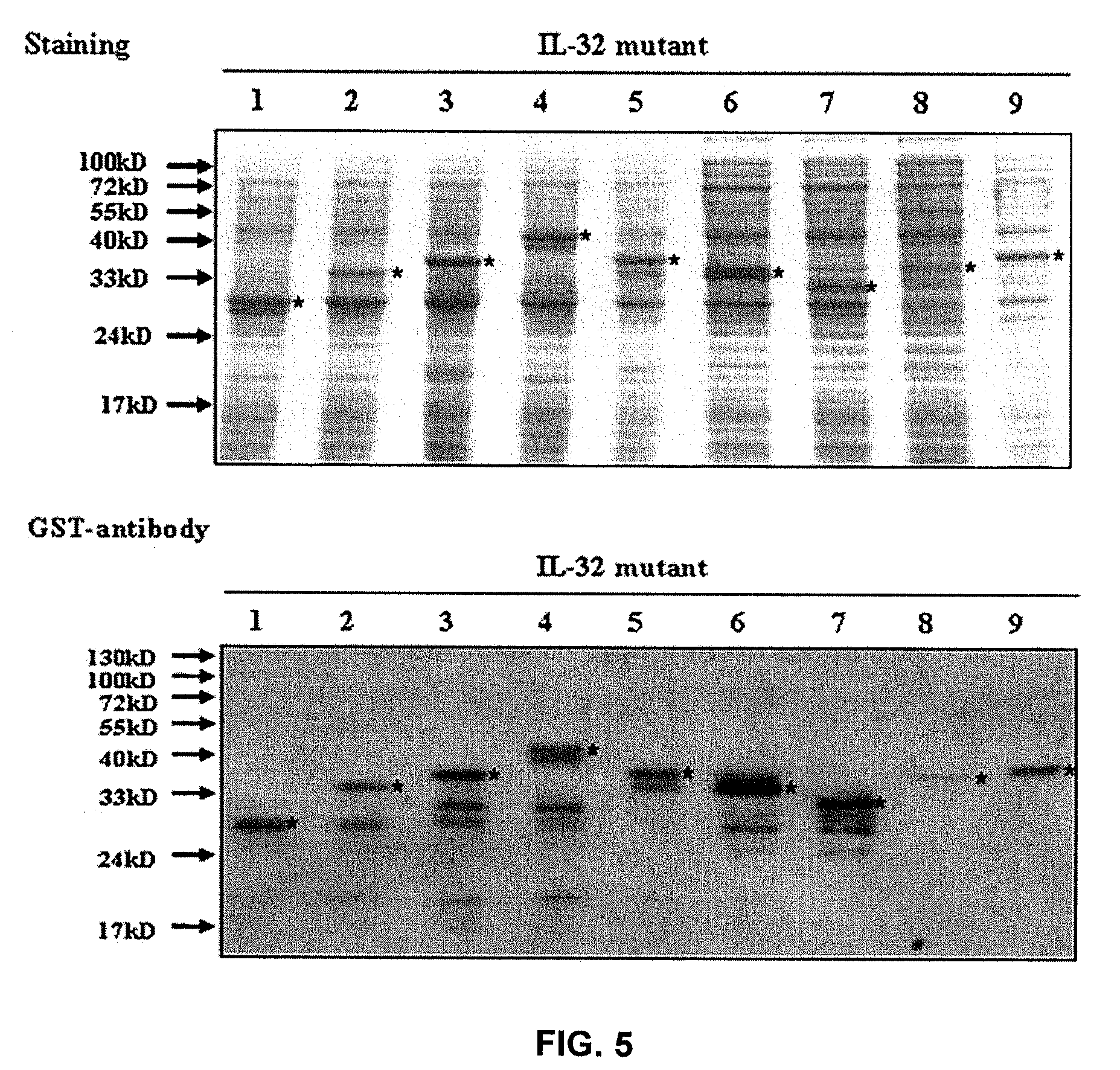
Il-32 monoclonal antibodies and uses thereof
The present invention provides monoclonal antibodies specific for interleukin-32 (IL-32, previously referred to as “natural killer cell transcript 4” or “NK4”) and hybridomas secreting monoclonal antibodies specific for IL-32. Also provided are diagnostic methods and kits (e.g., ELISA, Western blot, etc.) which utilize monoclonal antibodies specific for IL-32.
Owner:KONKUK UNIV IND COOP CORP
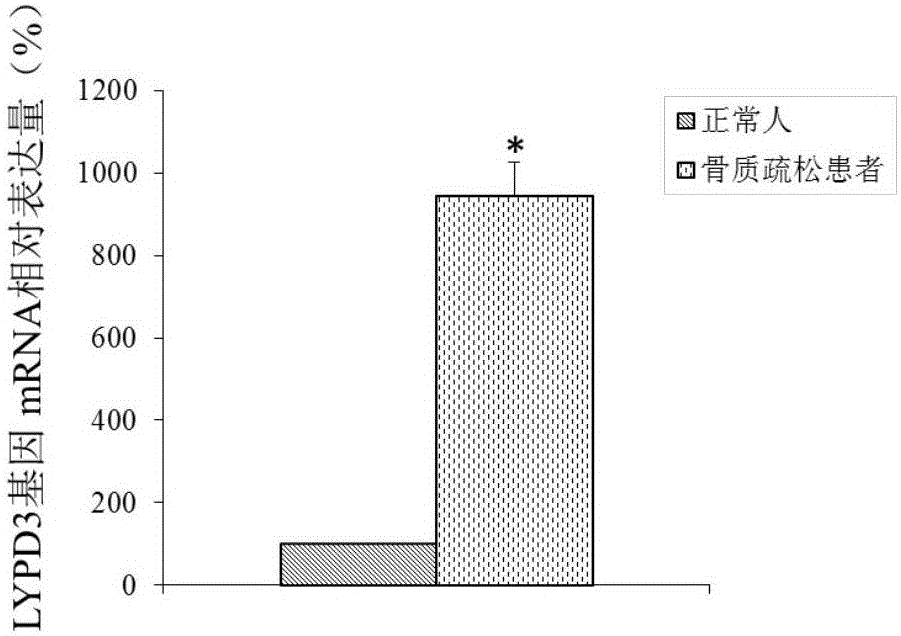
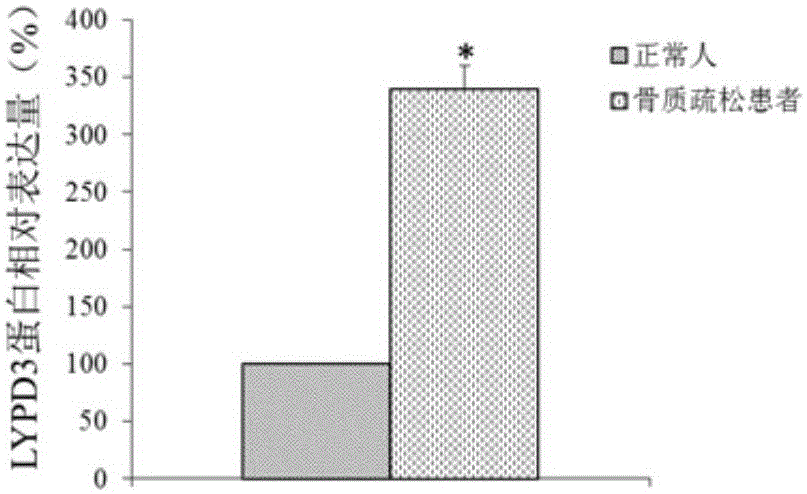
Molecular marker for osteoporosis and application of marker
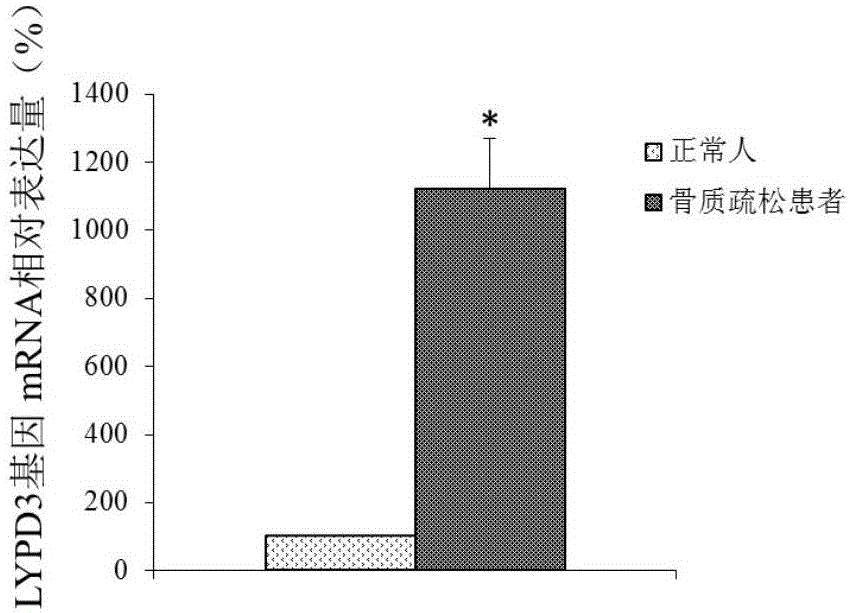
ActiveCN105132575AAchieve early diagnosisReduce mortalityOrganic active ingredientsMicrobiological testing/measurementBone tissueWestern blot
The invention discloses an LYPD3 gene and an expression product thereof both of which can serve as molecular markers for diagnosing osteoporosis. According to the LYPD3 and the expression product disclosed by the invention, a QPCR (quantitative polymerase chain reaction) experiment and a Western blot experiment provide that the LYPD3 gene has expression difference in blood or bone tissues of a normal person and an osteoporosis patient and can be used as an index for early diagnosis of the osteoporosis. In addition, the invention also discloses the LYPD3 gene and the expression product thereof both of which can serve as targets for diagnosing the osteoporosis and be used for guiding the research and development of novel medicaments.
Owner:GUAN BOJIAN BIOTECH CO LTD
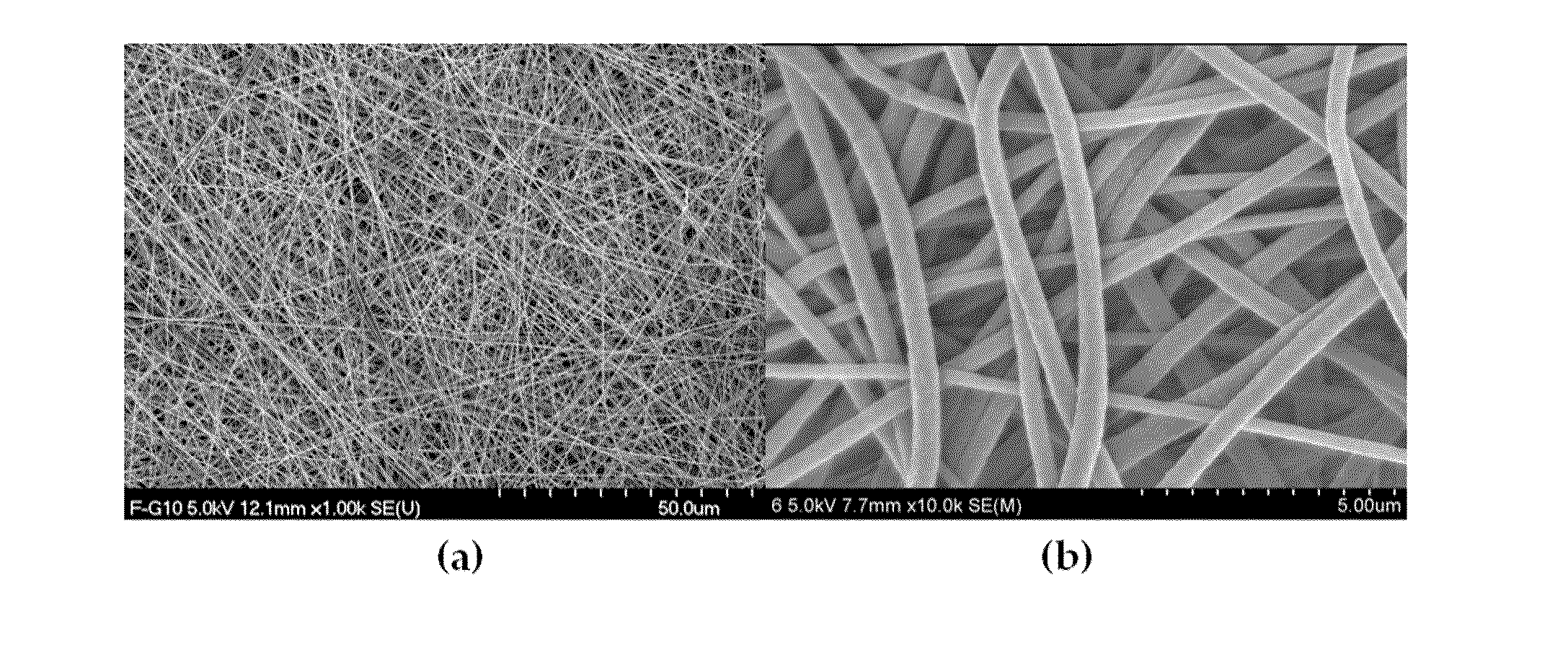
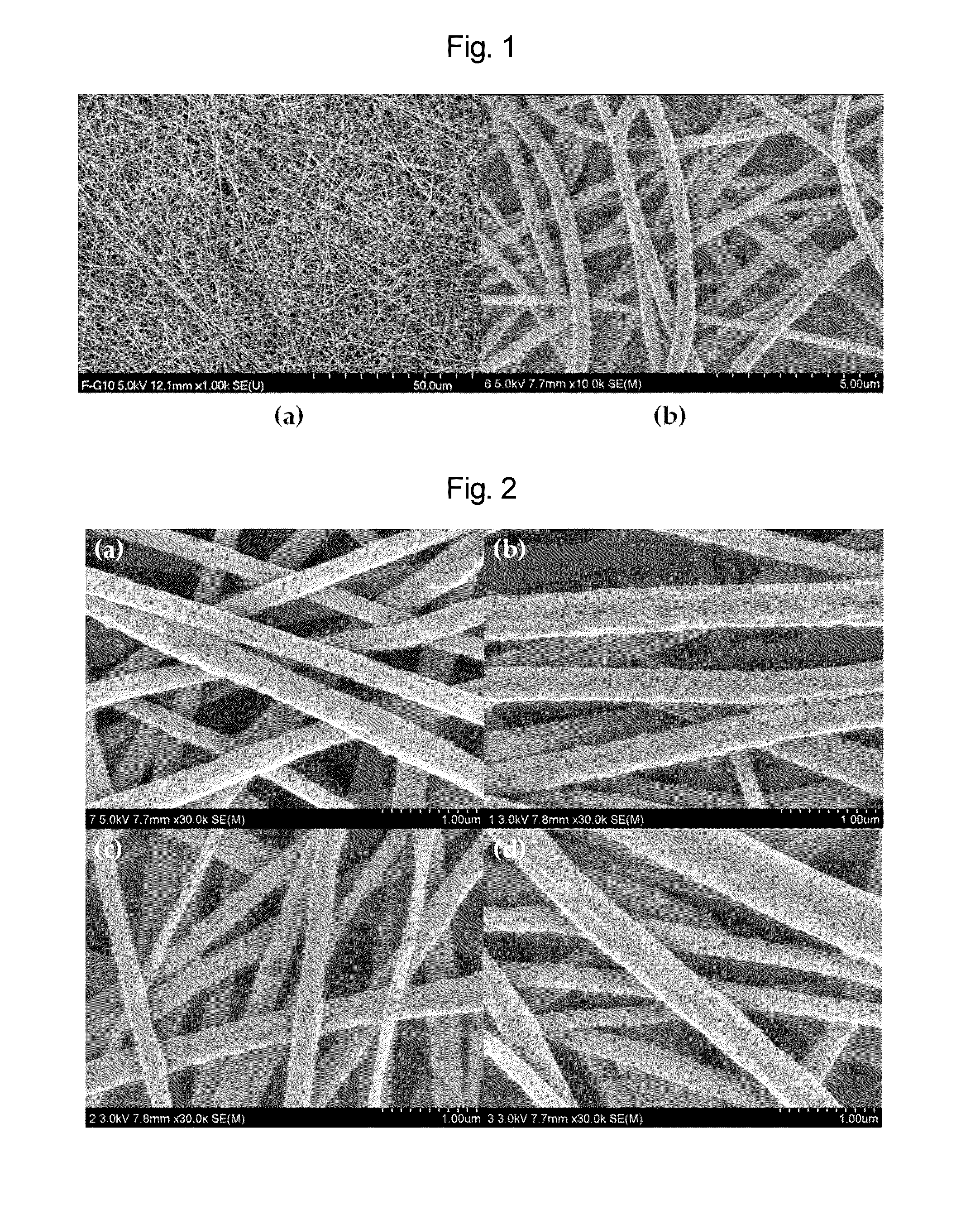
Nano-fibered membrane for western blot and manufacturing method of the same
ActiveUS20110076197A1Maximize surface areaHigh sensitivityMembranesSemi-permeable membranesPolymer scienceHydrophobic polymer
The present invention relates to a membrane for Western blotting which has a three-dimensional open pore structure, an average pore diameter of 0.1-1.0 μm and a thickness of 30-200 μm, wherein the membrane for Western blotting is manufactured by subjecting nanofibers having an average fiber diameter of 50-1000 nm, obtained by electrospinning, to a hot-plate calendering process, and a method for manufacturing the same. The method comprises the steps of: dissolving a hydrophobic material in a solvent to prepare a spinning solution; subjecting the spinning solution to a spinning process to obtain a hydrophobic polymer nanofiber web; and calendering the obtained nanofiber web to obtain a membrane for Western blotting.
Owner:AMOLIFESCI CO LTD
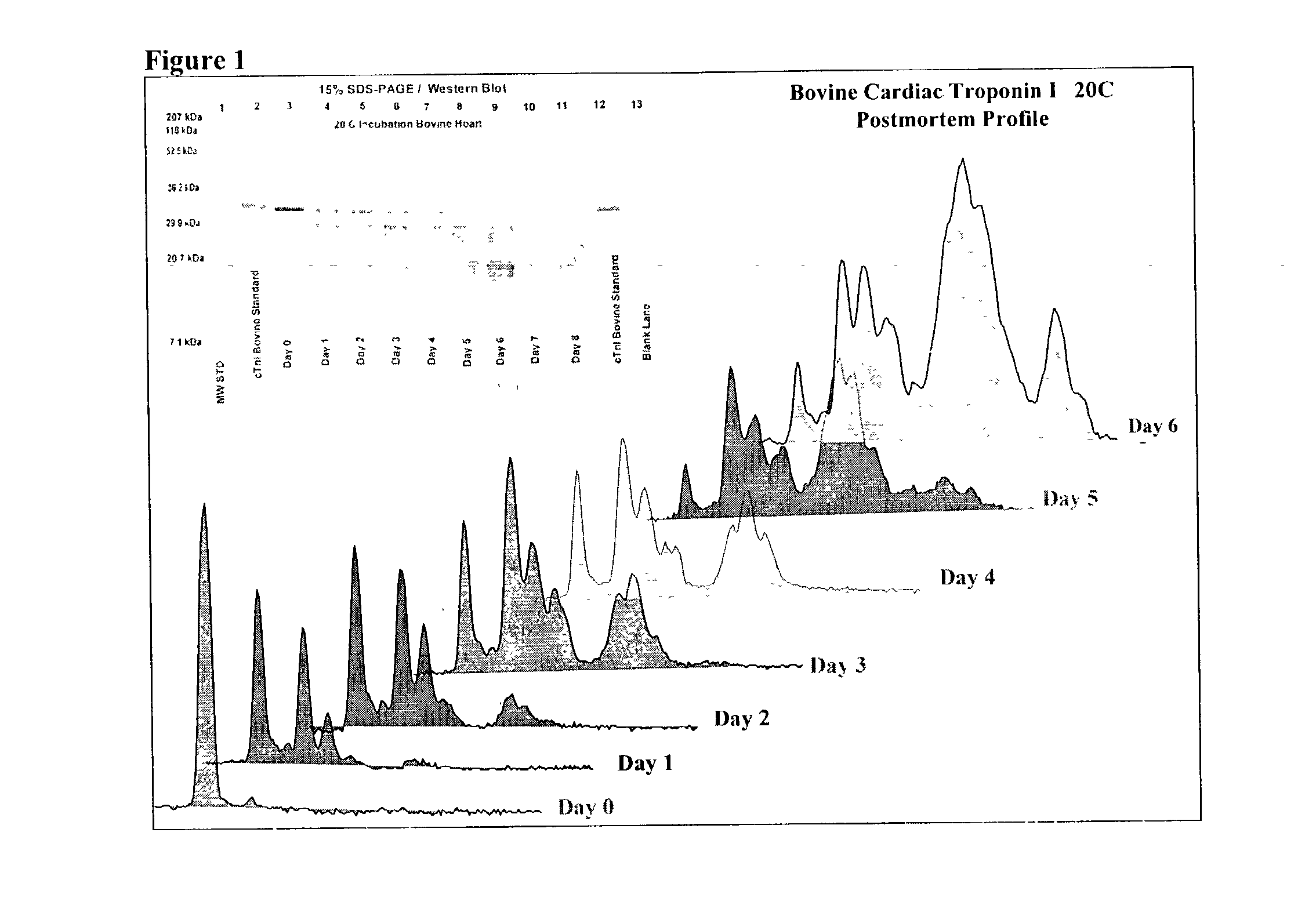
Method of determining time of death
A method of determining time of death is disclosed. Cardiac troponin I (cTnI) is the most specific marker for the determination of myocardial infarction (MI). Cardiac troponin I has a distinctive temporal degradation profile after death, which is key to its use as a time of death marker in forensic medicine. The method consists of sampling cardiac tissue, extracting a cardiac protein from a homogenate via methods which may include the magnetic microparticles or magnetic microparticles linked to anti-cTnI antibodies, eluting the proteins, separating proteins by electrophoresis, transferring by western blot the different bands of proteins to paper. A comparison / analysis of the concentration and kinetics of degradation of the protein at a given temperature(s) against known standards after time of death provides an accurate measurement of the time of death. The present invention is more accurate and reliable than the rudimentary time of death techniques currently used by medical examiners.
Owner:SABUCEDO ALBERT J +1
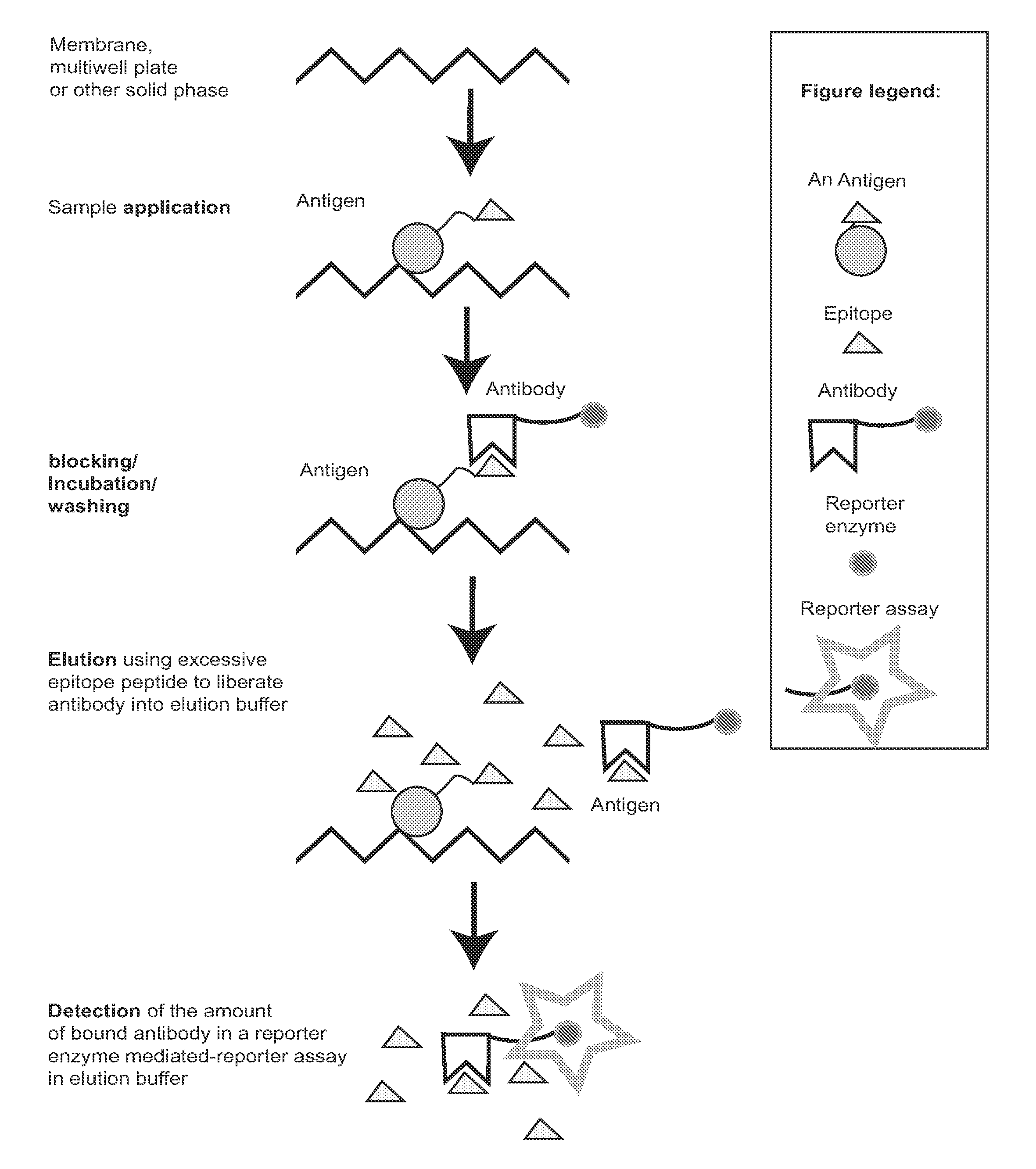
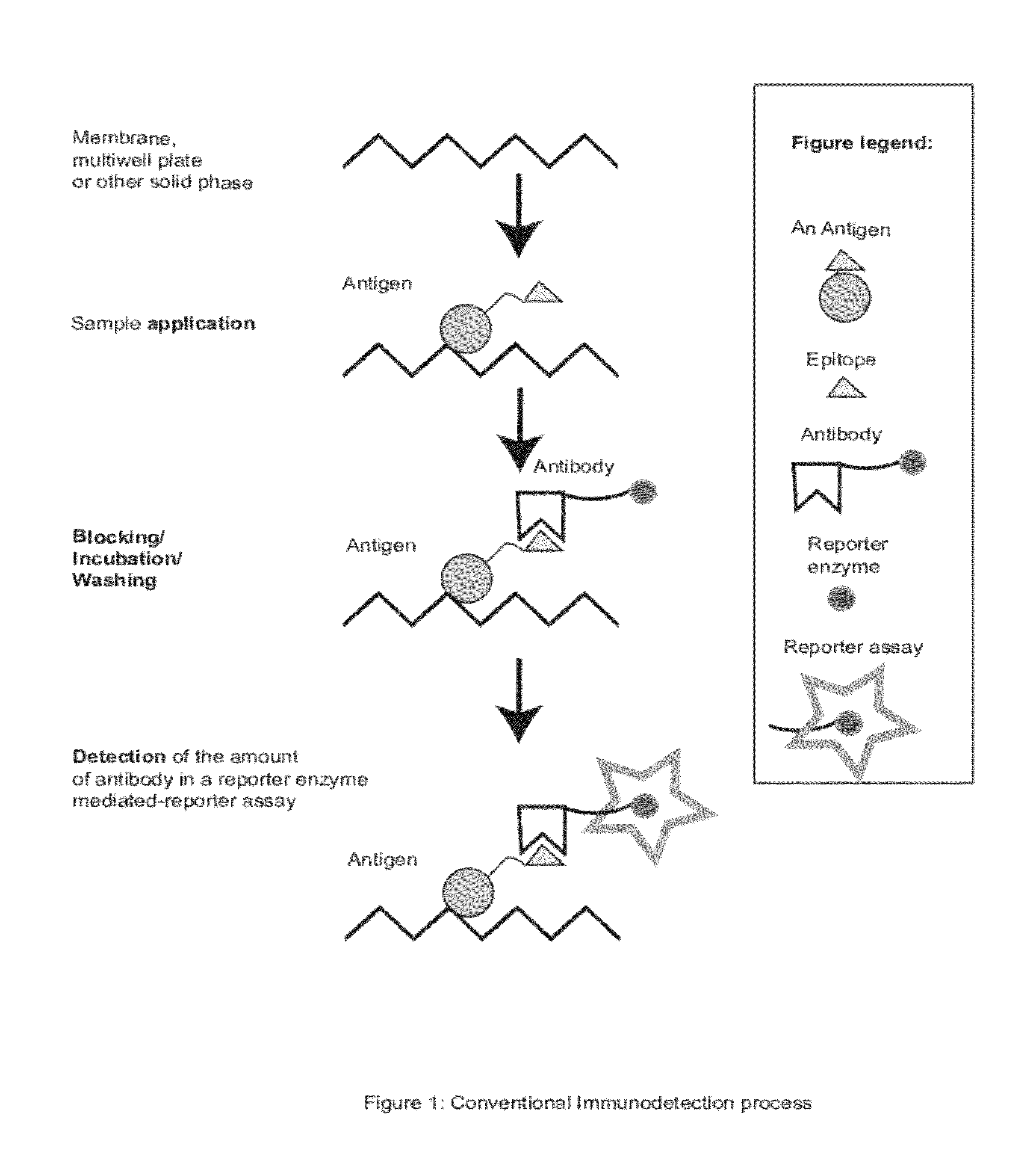
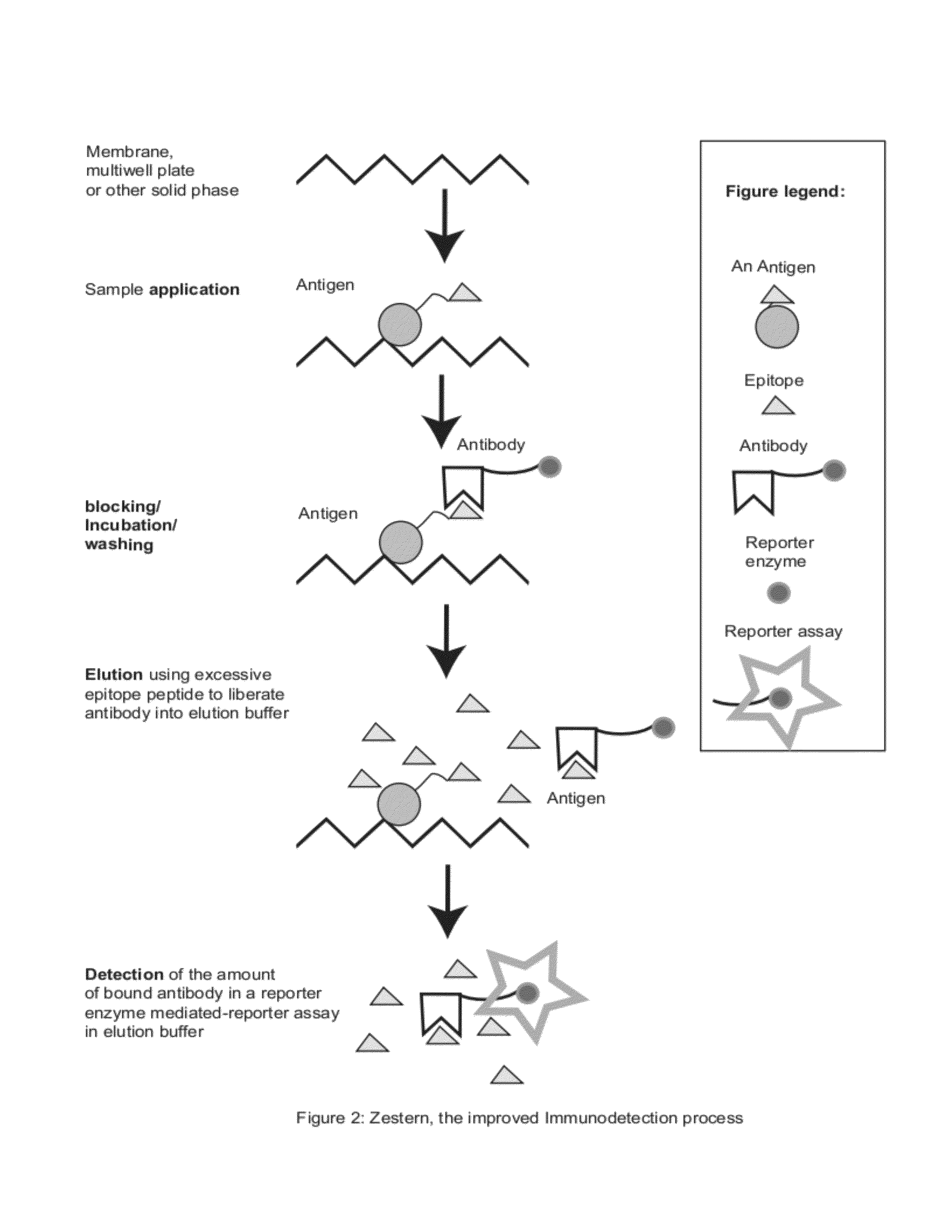
Zestern, a simple, fast, specific and quantifiable improvement of immunodetection process
InactiveUS8293487B1Strong specificityHigh detection specificityBiological testingProtein Microarray AnalysisElution
The present invention provides an improved, simple and quantifiable process of immunodetection with improved specificity, allowing for its large-scale applications in clinical, pharmaceutical and biomedical studies and diagnostics applications. Compared with traditional immunodetection methods, this invention eliminates gel separation and transfer steps, and the results can be directly quantified with improved specificity. This invention adds one elution step in a typical immunodetection process, where bound immunocomplex is exposed to elution solution containing excessive amount of antigen or part of antigen in single or multiple copies within one molecule, to liberate bound antibody labeled with reporter enzyme into solution for direct quantification. This invention can be particularly useful to improve both the efficiency and accuracy of Western blot, Dot blot, ELISA and protein microarray analysis in multiwell plate format. It also allows for the automation of protein analysis for clinical, pharmaceutical, and biomedical applications and their diagnostics applications.
Owner:ZHANG JIANDI +1
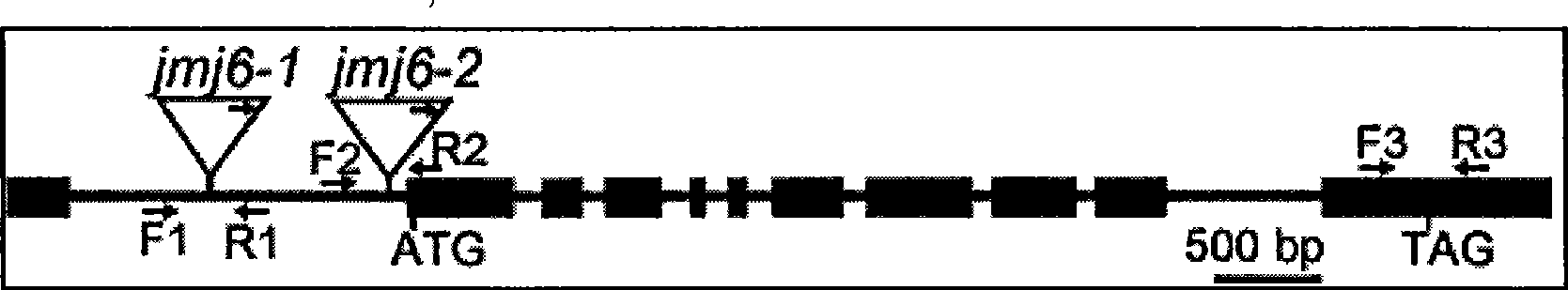
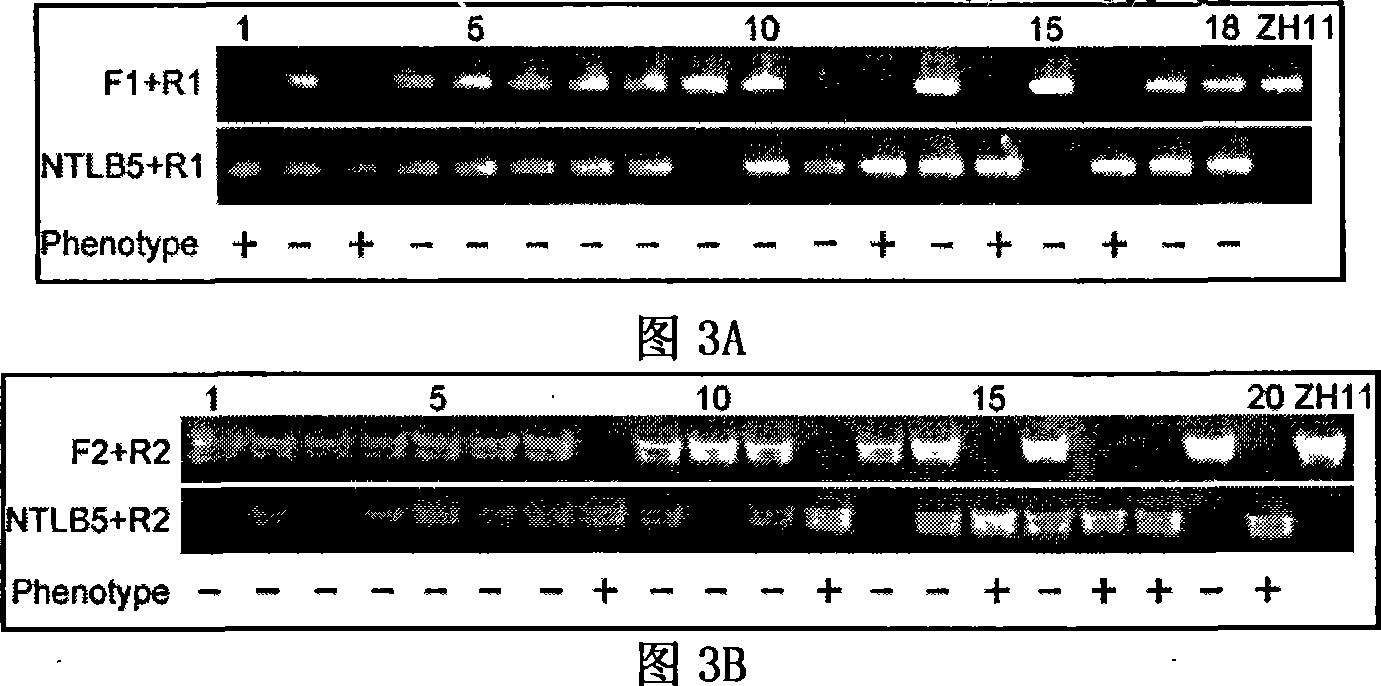
Histone demethylase gene OsJMJ706 of rice, encoding protein thereof and applications
The invention belongs to the technical field of plant gene engineering, and more particularly relates to a paddy rice histone demethyl methylase gene, coded protein and the application thereof. The invention provides a new paddy rice histone demethyl methylase gene OsJMJ706, due to inactivation, heteroplasia of rice flowerthe, heteroplasia or deficit of internal and external glumes, as well as lessening of seeds can be caused on the gene, and by being detected by the method of western blot, the gene is proved to have enzyme activity for removing histone H3K9me3 and H3K9me2 locus methylate. The invention provides nucleic acid sequence and protein sequence of the gene as well as the application thereof.
Owner:HUAZHONG AGRI UNIV
Microscale western blot
ActiveUS9182371B2Improve bindingStable electrophoresisMaterial analysis by electric/magnetic meansCapillary electrophoresisElectrophoresis
Systems and methods are provided for integrating the electrophoresis and blotting of samples. A capillary electrophoresis and blotting system allows concomitant electrophoretic separation and blotting to provide a rapid and simplified process. A microfluidic electrophoresis and blotting system provides electrophoretic separation in a microfluidic channel followed by electroblotting from the microfluidic channel to also provide a rapid and simplified process. These systems and methods can be used to assay smaller amounts of sample in less time than conventional processes, including conventional Western blotting techniques.
Owner:RGT UNIV OF MICHIGAN
IL-32 monoclonal antibodies and uses thereof
Owner:KONKUK UNIV IND COOP CORP
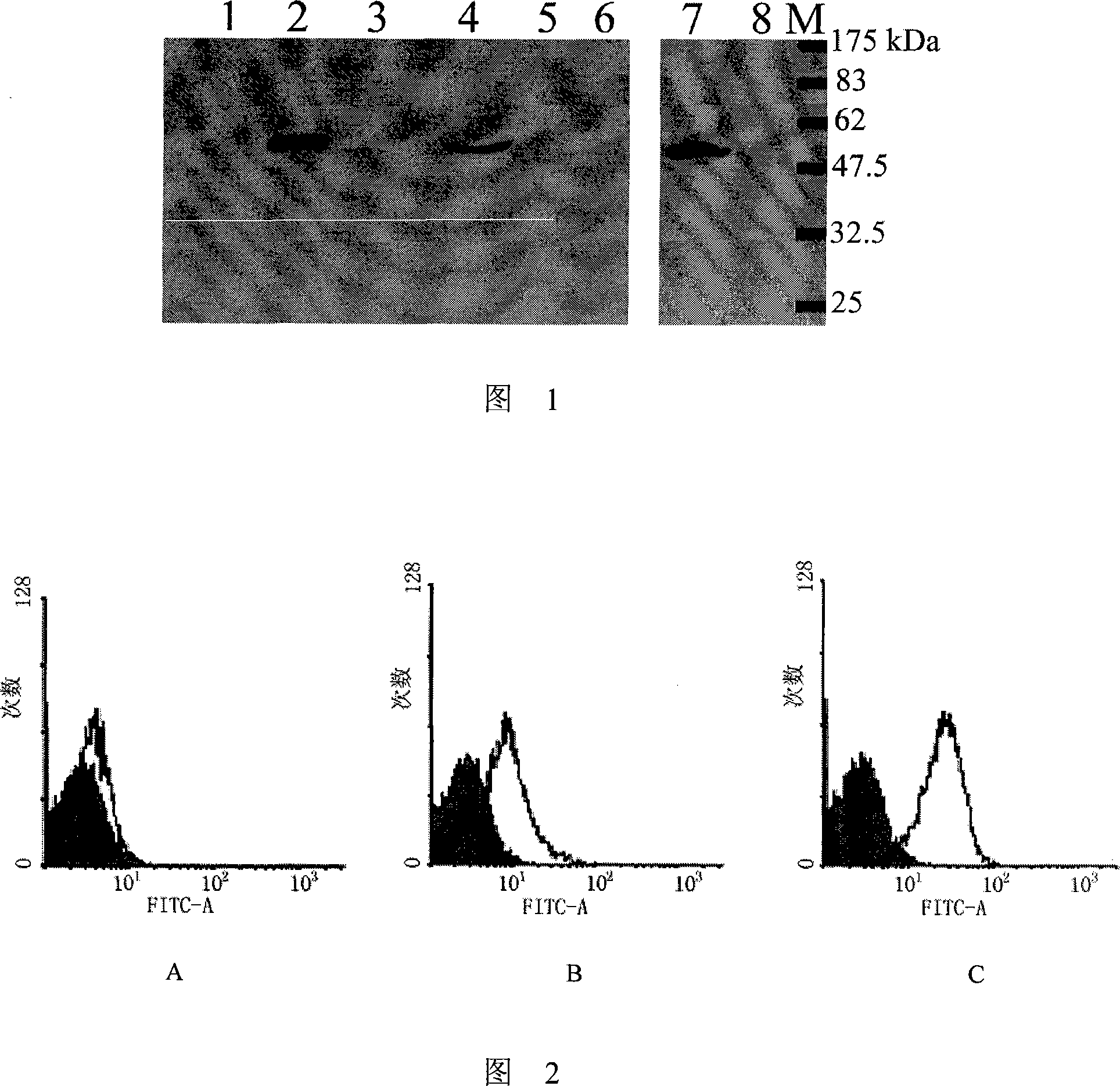
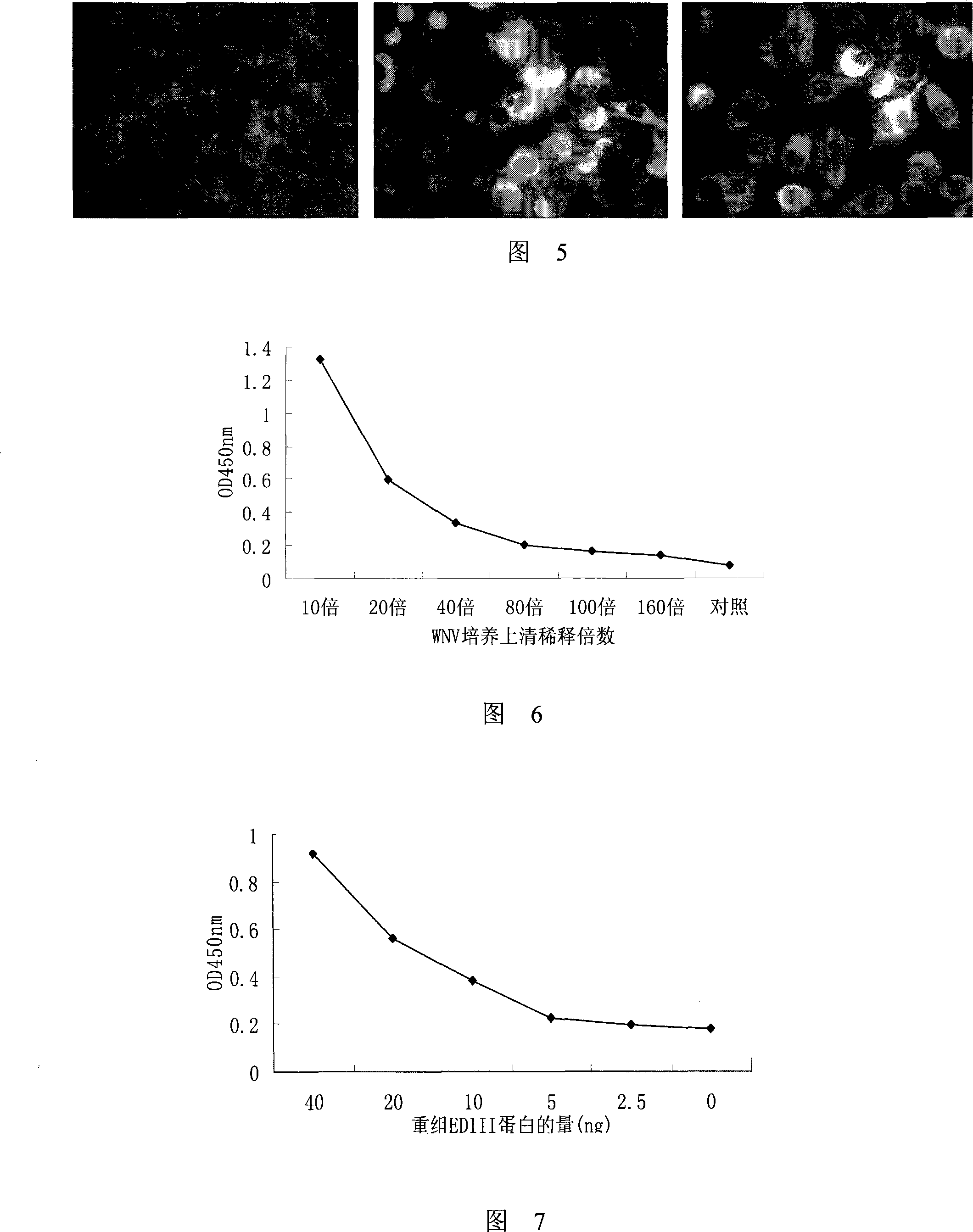
Monoclonal antibody of membrane protein E for resisting West Nile virus and application thereof
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com