Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1531 results about "Azide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Azide is the anion with the formula N⁻₃. It is the conjugate base of hydrazoic acid (HN₃). N⁻₃ is a linear anion that is isoelectronic with CO₂, NCO⁻, N₂O, NO⁺₂ and NCF. Per valence bond theory, azide can be described by several resonance structures; an important one being . Azide is also a functional group in organic chemistry, RN₃.
Precursor source mixtures
A precursor source mixture useful for CVD or ALD of a film comprising: at least one precursor composed of an element selected from the group consisting of Li, Na, K, Rb, Cs, Fr, Be, Mg, Ti, Zr, Hf, Sc, Y, La, V, Nb, Ta, Cr, Mo, W, Mn, Re, Fe, Ru, Os, Co, Rh, Ir, Ni, Pd, Pt, Cu, Ag, Au, Zn, Cd, Hg, B, Al, Ga, In, Tl, Si, Ge, Sn, Pb, As, P, Sb and Bi, to which is bound at least one ligand selected from the group consisting of hydride, alkyl, alkenyl, cycloalkenyl, aryl, alkyne, carbonyl, amido, imido, hydrazido, phosphido, nitrosyl, nitryl, nitrate, nitrile, halide, azide, alkoxy, siloxy, silyl, and halogenated, sulfonated or silyated derivatives thereof, which is dissolved, emulsified or suspended in an inert liquid selected from the group consisting of aliphatic hydrocarbons, aromatic hydrocarbons, alcohols, ethers, aldehydes, ketones, acids, phenols, esters, amines, alkylnitrile, halogenated hydrocarbons, silyated hydrocarbons, thioethers, amines, cyanates, isocyanates, thiocyanates, silicone oils, nitroalkyl, alkylnitrate, and mixtures thereof. The precursor source mixture may be a solution, emulsion or suspension and may consist of a mixture of solid, liquid and gas phases which are distributed throughout the mixture.
Owner:GLOBALFOUNDRIES INC
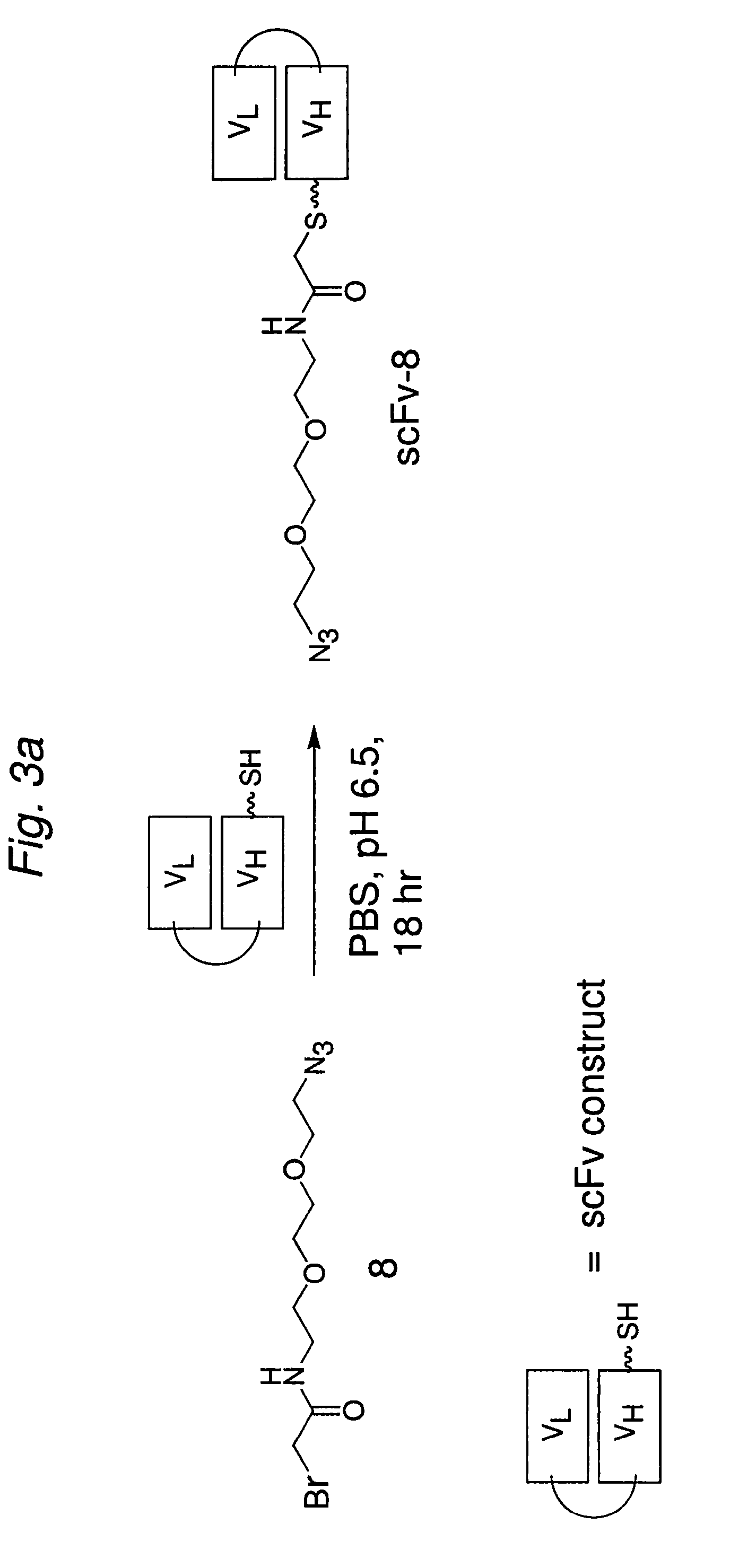
Construction of a Multivalent SCFV Through Alkyne-Azide 1,3-Dipolar Cycloaddition
The present invention provides for a practical, universal and efficient method to ligate two large macromolecules (e.g., proteins) using the alkyne-azide 1,3-dipolar cycloaddition reaction to produce a conjugated macromolecule, such as a multivalent scFv. The present invention also provides for conjugate macromolecules comprising a plurality of macromolecule components cross-linked through at least one linking group comprising at least one 1,2,3-triazole moiety, wherein at least 50 percent of the macromolecule components in the conjugate macromolecule has only one site available for cross-linking.
Owner:RGT UNIV OF CALIFORNIA
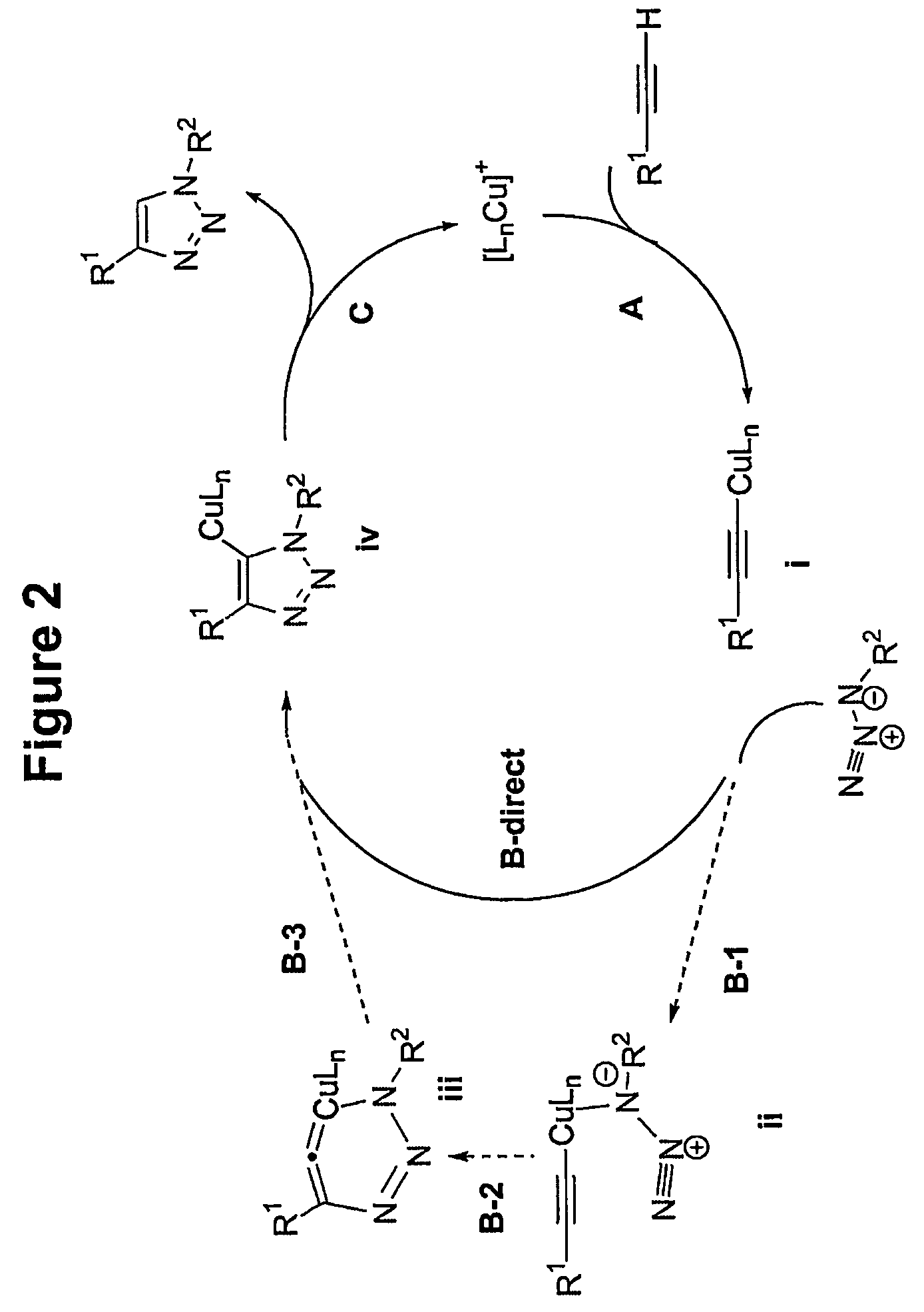
Copper-catalysed ligation of azides and acetylenes
InactiveUS7763736B2Organic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsChemical ligationAqueous alcohol
A copper catalyzed click chemistry ligation process is employed to bind azides and terminal acetylenes to provide 1,4-disubstituted 1,2,3-triazole triazoles. The process comprises contacting an organic azide and a terminal alkyne with a source of reactive Cu(I) ion for a time sufficient to form by cycloaddition a 1,4-disubstituted 1,2,3-triazole. The source of reactive Cu(I) ion can be, for example, a Cu(I) salt or copper metal. The process is preferably carried out in a solvent, such as an aqueous alcohol. Optionally, the process can be performed in a solvent that comprises a ligand for Cu(I) and an amine.
Owner:TETARD INC
Compositions and methods for modification of biomolecules
The present invention provides modified cycloalkyne compounds; and method of use of such compounds in modifying biomolecules. The present invention features a cycloaddition reaction that can be carried out under physiological conditions. In general, the invention involves reacting a modified cycloalkyne with an azide moiety on a target biomolecule, generating a covalently modified biomolecule. The selectivity of the reaction and its compatibility with aqueous environments provide for its application in vivo (e.g., on the cell surface or intracellularly) and in vitro (e.g., synthesis of peptides and other polymers, production of modified (e.g., labeled) amino acids).
Owner:RGT UNIV OF CALIFORNIA
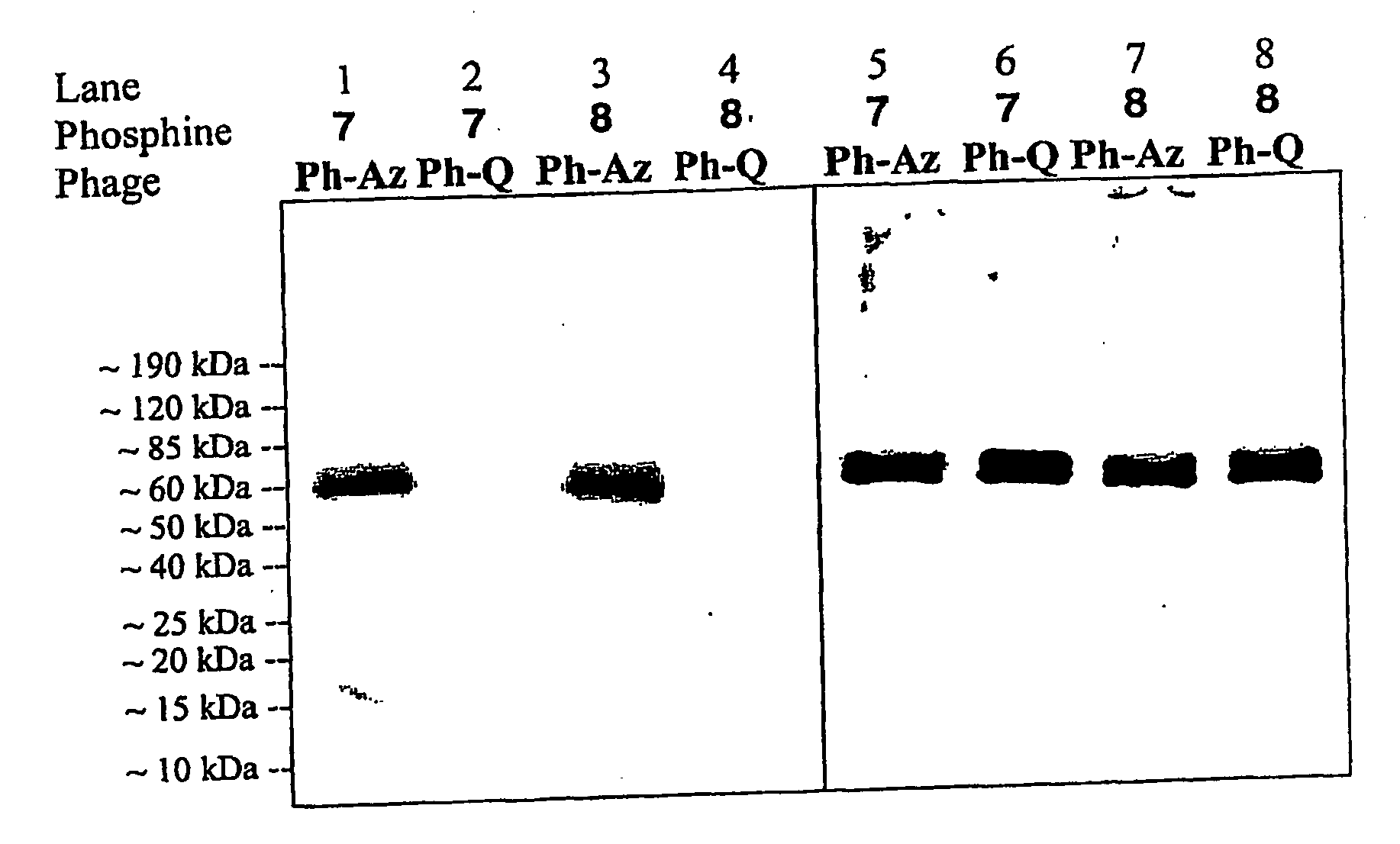
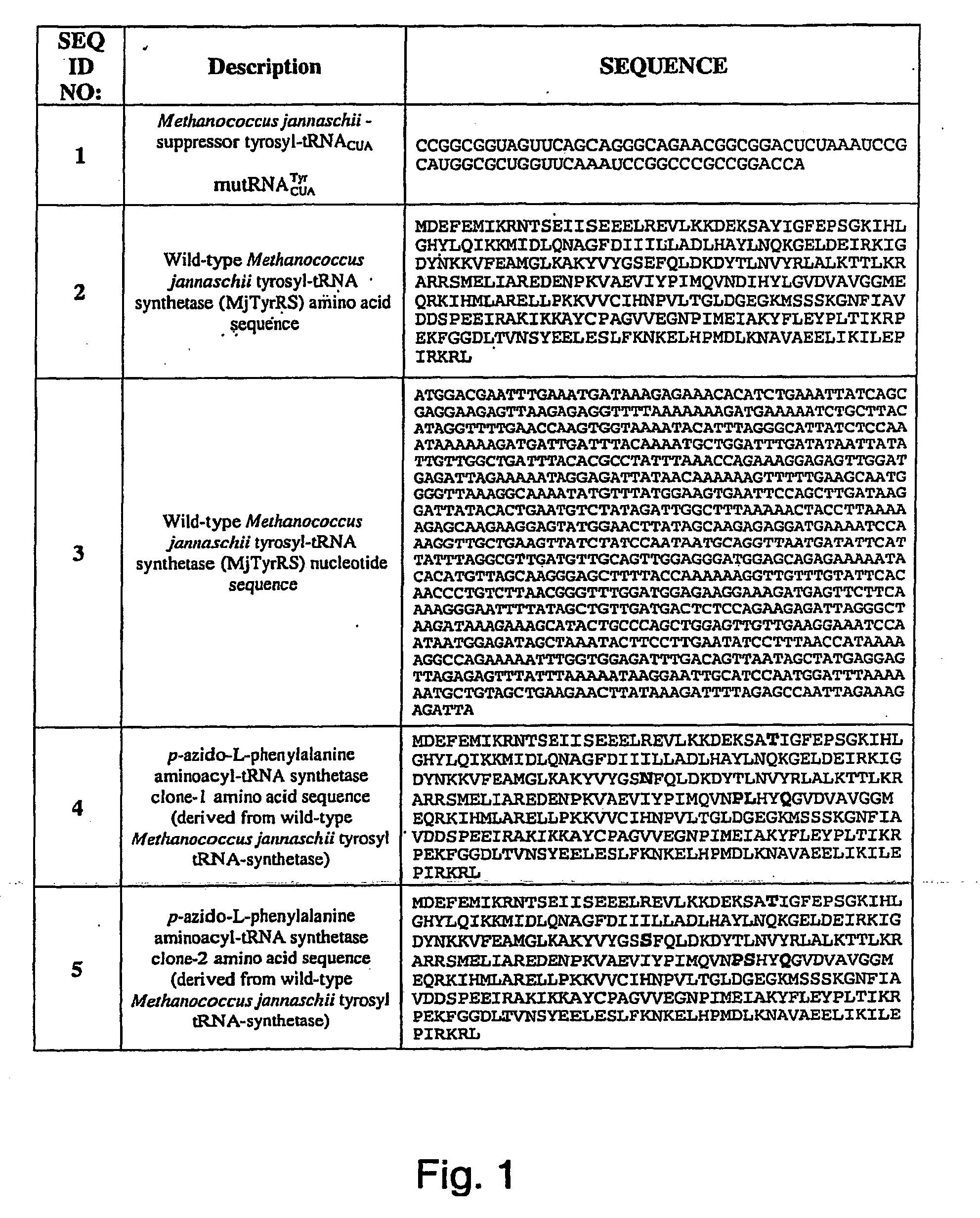
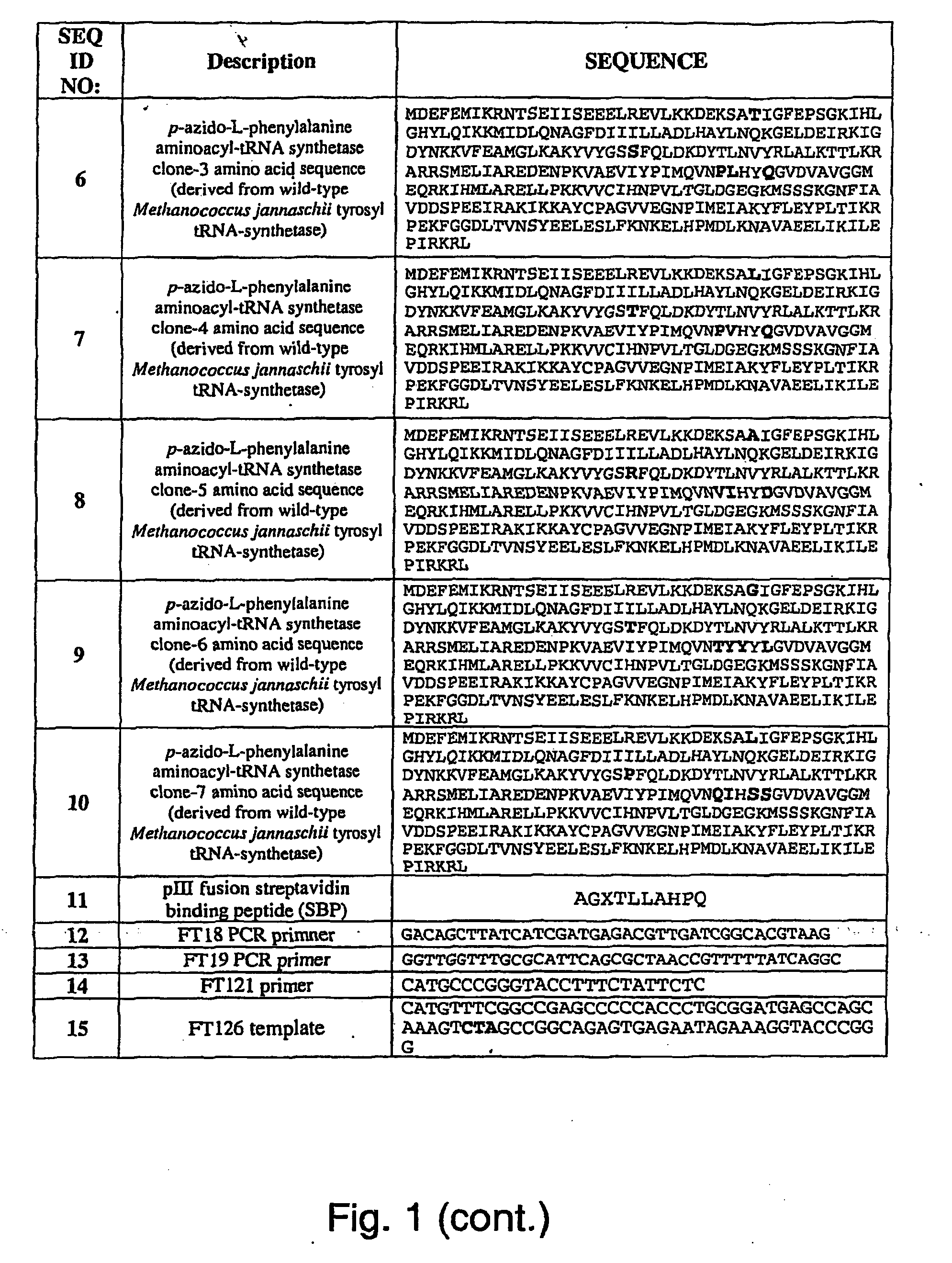
Selective posttranslational modification of phage-displayed polypeptides
ActiveUS20070178448A1Easy to detectEasy to quantifyAntibody mimetics/scaffoldsVirus peptidesArylCycloaddition
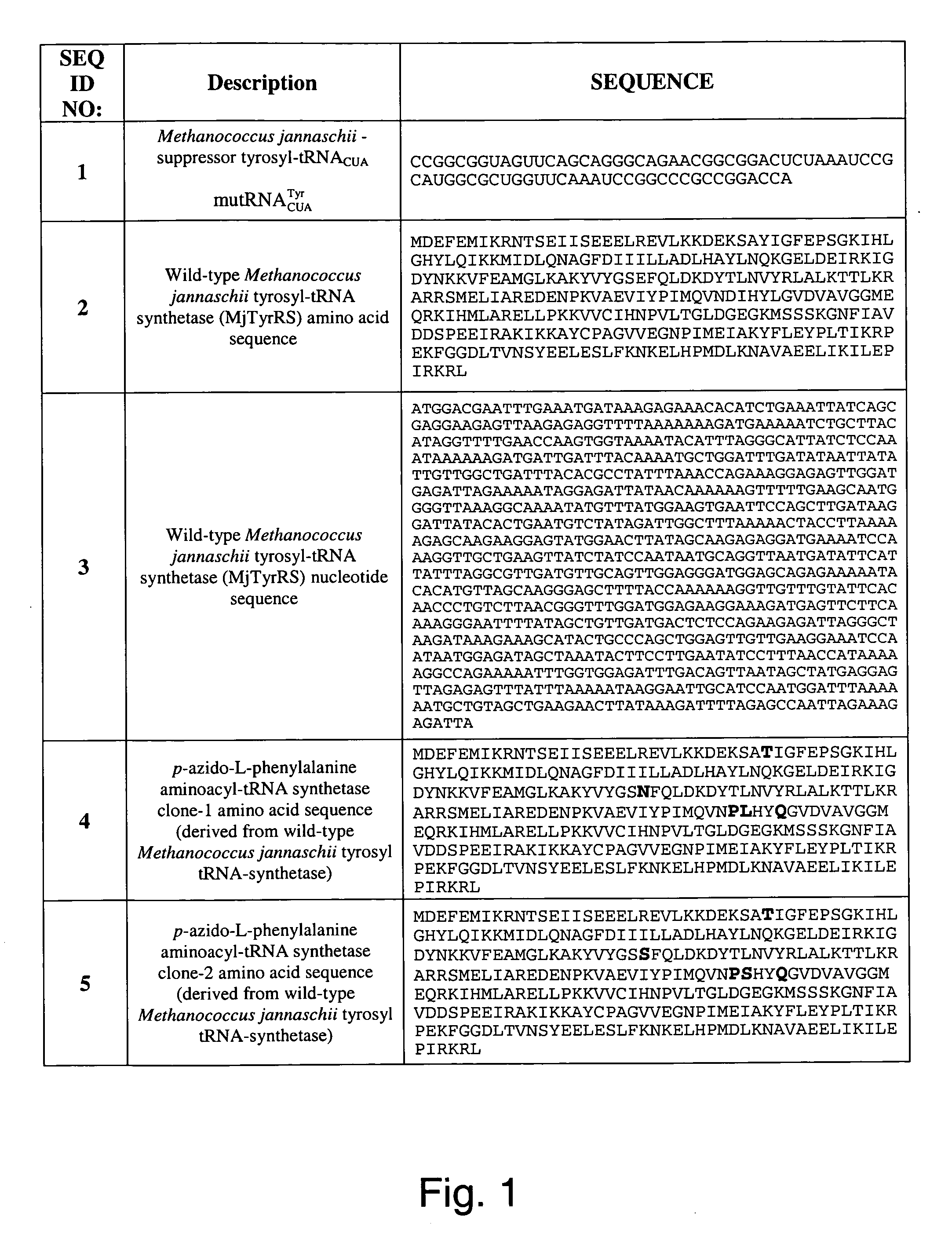
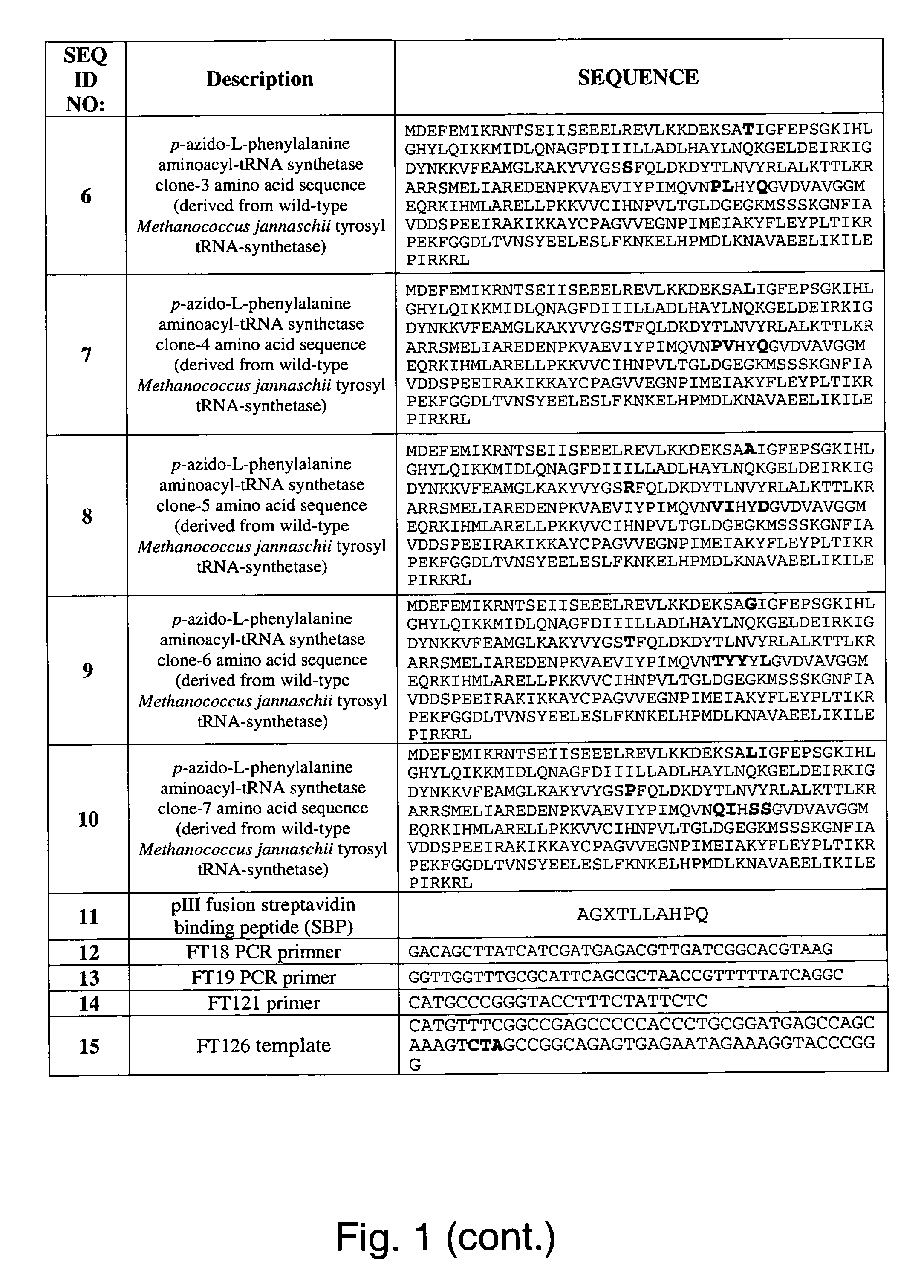
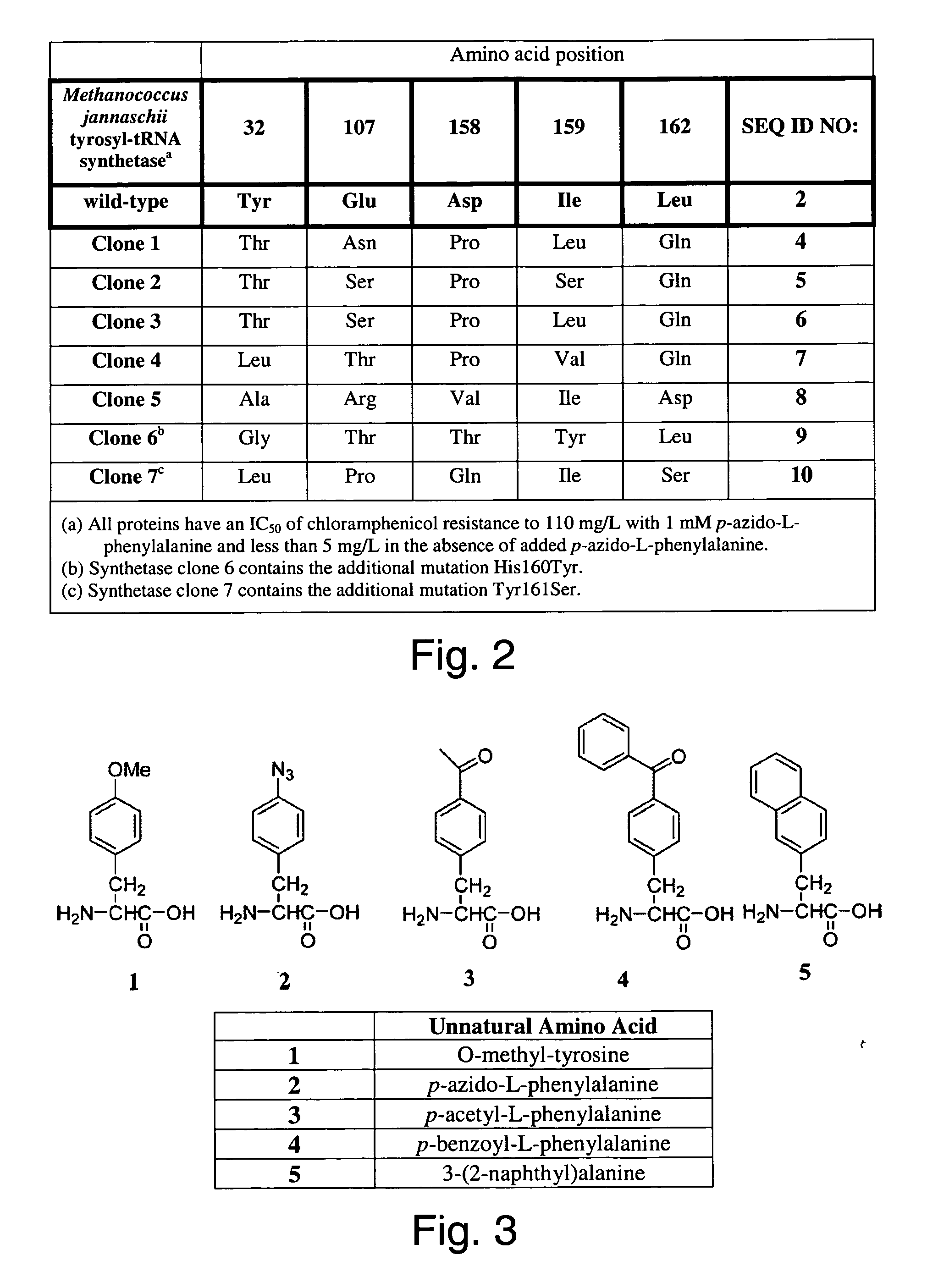
The invention relates to posttranslational modification of phage-displayed polypeptides. These displayed polypeptides comprise at least one unnatural amino acid, e.g., an aryl-azide amino acid such as p-azido-L-phenylalanine, or an alkynyl-amino acid such as para-propargyloxyphenylalanine, which are incorporated into the phage-displayed fusion polypeptide at a selected position by using an in vivo orthogonal translation system comprising a suitable orthogonal aminoacyl-tRNA synthetase and a suitable orthogonal tRNA species. These unnatural amino acids advantageously provide targets for posttranslational modifications such as azide-alkyne [3+2] cycloaddition reactions and Staudinger modifications.
Owner:THE SCRIPPS RES INST
Phosphinoamidite carboxylates and analogs thereof in the synthesis of oligonucleotides having reduced internucleotide charge
InactiveUS7067641B2Reduced internucleotide chargeImprove stabilitySugar derivativesOrganic chemistry methodsHydrogenHalogen
Nucleoside phosphinoamidite carboxylates and analogs are provided that have the structure of formula (III)wherein A is hydrogen, hydroxyl, lower alkoxy, lower alkoxy-substituted lower alkoxy, halogen, SH, NH2, azide or DL wherein D is O, S or NH and L is a heteroatom-protecting group, unsubstituted hydrocarbyl, substituted hydrocarbyl, heteroatom-containing hydrocarbyl, or substituted heteroatom-containing hydrocarbyl; B is a nucleobase; and one of R11 and R12 is a blocking group and the other is (IV) or (VI)in which W, X, Y, Z, R1 and n are as defined herein.
Owner:AGILENT TECH INC
Labeling and detection of nucleic acids
ActiveUS20080050731A1Increase temperatureAnalysis using chemical indicatorsSugar derivativesAzideNucleic acid
Provided in certain embodiments are new methods for forming azido modified nucleic acid conjugates of reporter molecules, carrier molecules or solid support. In other embodiments are provided methods for enzymatically labeling nucleic acids with an azide group.
Owner:LIFE TECH CORP
Artificial corneal implant
A material that can be applied as implants designed to artificially replace or augment the cornea, such as an artificial cornea, corneal onlay, or corneal inlay (intrastromal lens) is provided. The artificial corneal implant has a double network hydrogel with a first network interpenetrated with a second network. The first network and the second network are based on biocompatible polymers. At least one of the network polymers is based on a hydrophilic polymer. The artificial cornea or implant has epithelialization promoting biomolecules that are covalently linked to the surface of the double network hydrogel using an azide-active-ester chemical linker. Corneal epithelial cells or cornea-derived cells are adhered to the biomolecules. The double network has a physiologic diffusion coefficient to allow passage of nutrients to the adhered cells.
Owner:SANTA CLARA UNIVERSITY
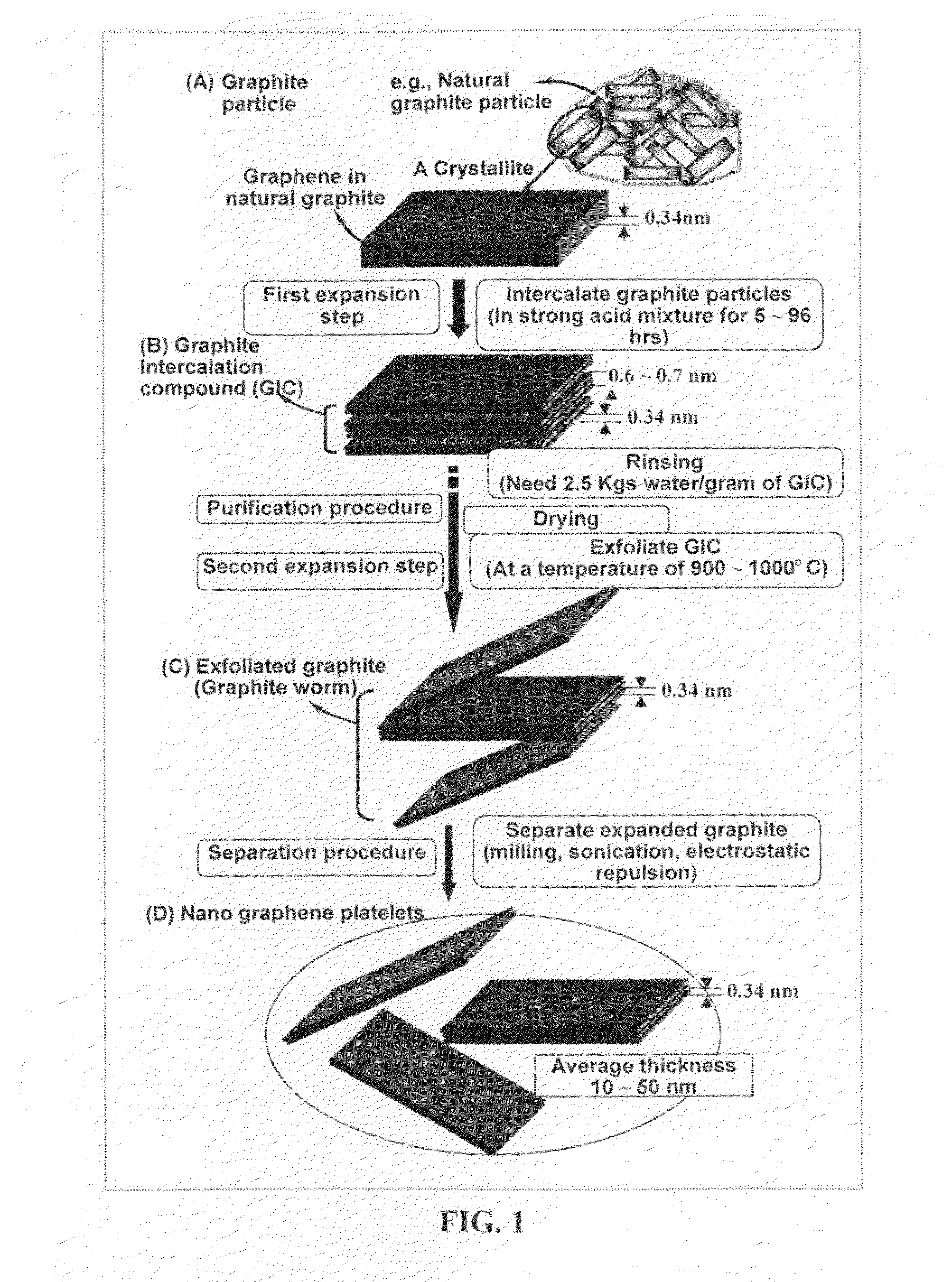
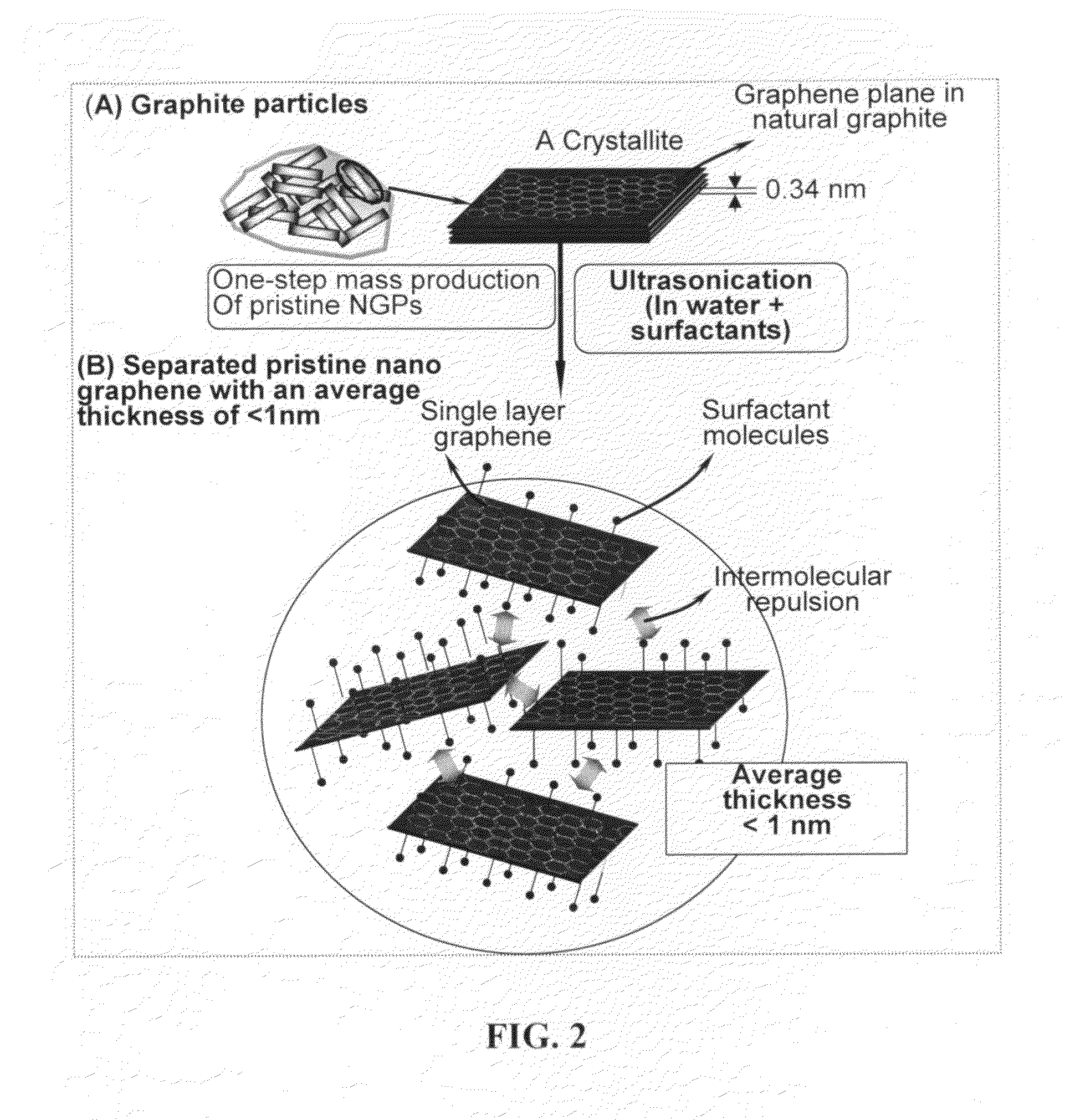
Production of chemically functionalized nano graphene materials
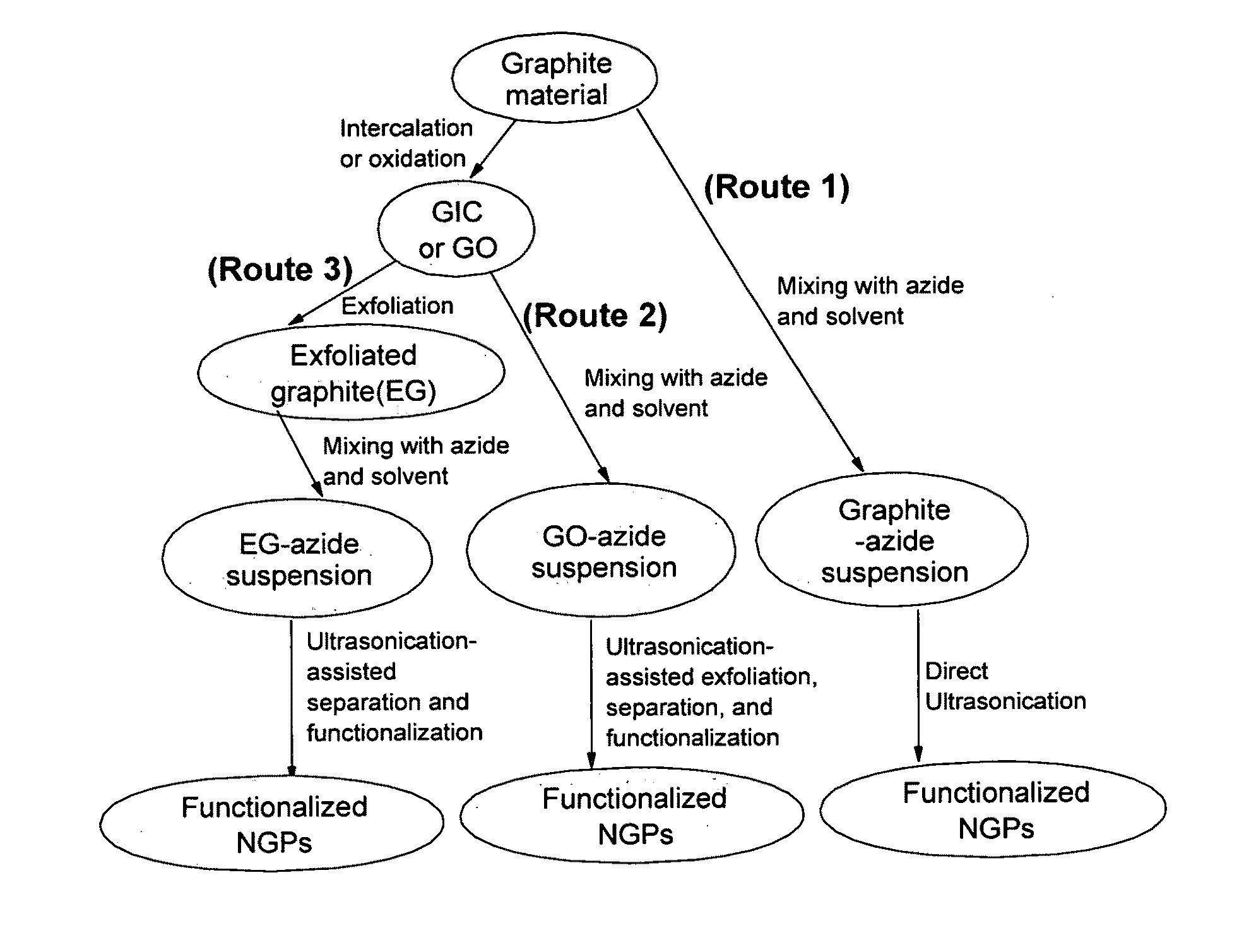
Provided in this invention is a process for producing chemically functionalized nano graphene materials, known as nano graphene platelets (NGPs), graphene nano sheets, or graphene nano ribbons. Subsequently, a polymer can be grafted to a functional group of the resulting functionalized NGPs. In one preferred embodiment, the process comprises (A) dispersing a pristine graphite material and an azide or bi-radical compound in a liquid medium comprising to form a suspension; and (B) subjecting the suspension to direct ultrasonication with to ultrasonic waves of a desired intensity or power level for a length of time sufficient to produce nano graphene platelets and to enable a chemical reaction to occur between the nano graphene platelets and the azide or bi-radical compound to produce the functionalized nano graphene material. Concurrent production and functionalization of NGPs directly from pristine graphitic materials can be achieved in one step and in the same reactor.
Owner:GLOBAL GRAPHENE GRP INC
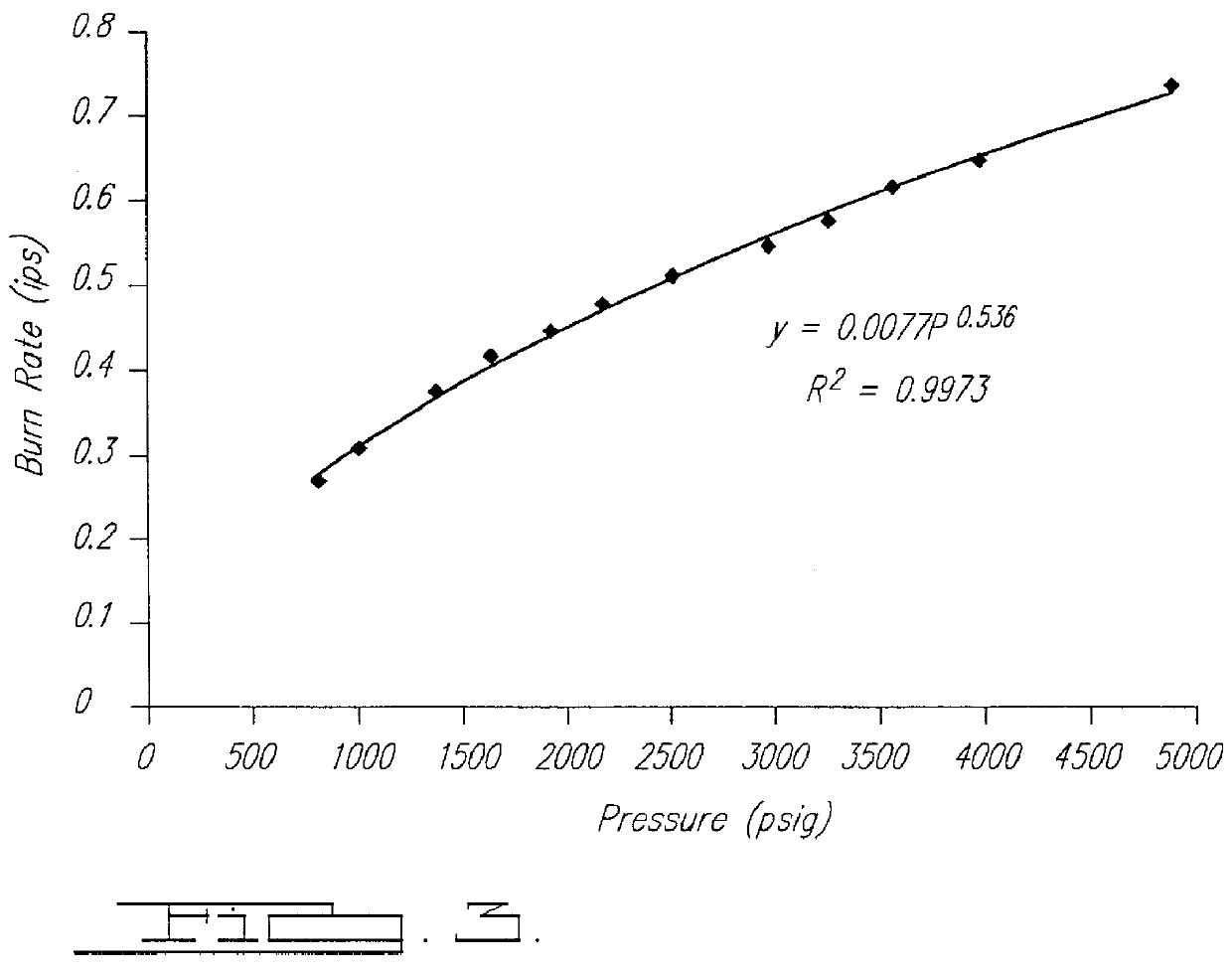
Smokeless gas generant compositions
Thermally stable gas generant compositions incorporate a combination of one or more primary nonazide high-nitrogen fuels selected from a group including tetrazoles, bitetrazoles, and triazoles, and salts thereof; and one or more secondary nonazide high nitrogen fuels selected from azodicarbonamide and hydrazodicarbonamide. The primary and secondary fuels are combined with phase-stabilized ammonium nitrate that when combusted, results in a greater yield of gaseous products per mass unit of gas generant, a reduced yield of solid combustion products, lower combustion temperatures, and acceptable burn rates, thermal stability, and ballistic properties. These compositions are especially suitable for inflating air bags in passenger-restraint devices.
Owner:AUTOMOTIVE SYST LAB
Compositions and methods for modification of biomolecules
The present invention provides modified cycloalkyne compounds; and method of use of such compounds in modifying biomolecules. The present invention features a cycloaddition reaction that can be carried out under physiological conditions. In general, the invention involves reacting a modified cycloalkyne with an azide moiety on a target biomolecule, generating a covalently modified biomolecule. The selectivity of the reaction and its compatibility with aqueous environments provide for its application in vivo (e.g., on the cell surface or intracellularly) and in vitro (e.g., synthesis of peptides and other polymers, production of modified (e.g., labeled) amino acids).
Owner:RGT UNIV OF CALIFORNIA
Compositions and methods for modification of biomolecules
The present invention provides modified cycloalkyne compounds; and method of use of such compounds in modifying biomolecules. The present invention features a cycloaddition reaction that can be carried out under physiological conditions. In general, the invention involves reacting a modified cycloalkyne with an azide moiety on a target biomolecule, generating a covalently modified biomolecule. The selectivity of the reaction and its compatibility with aqueous environments provide for its application in vivo (e.g., on the cell surface or intracellularly) and in vitro (e.g., synthesis of peptides and other polymers, production of modified (e.g., labeled) amino acids).
Owner:RGT UNIV OF CALIFORNIA
Labeling and detection of post translationally modified proteins
Provided in certain embodiments are new methods for forming azido modified biomolecule conjugates of reporter molecules, carrier molecules or solid support. In other embodiments are provided methods for enzymatically labeling a biomolecules with an azide group.
Owner:LIFE TECH CORP
Process and apparatus for growing a crystalline gallium-containing nitride using an azide mineralizer
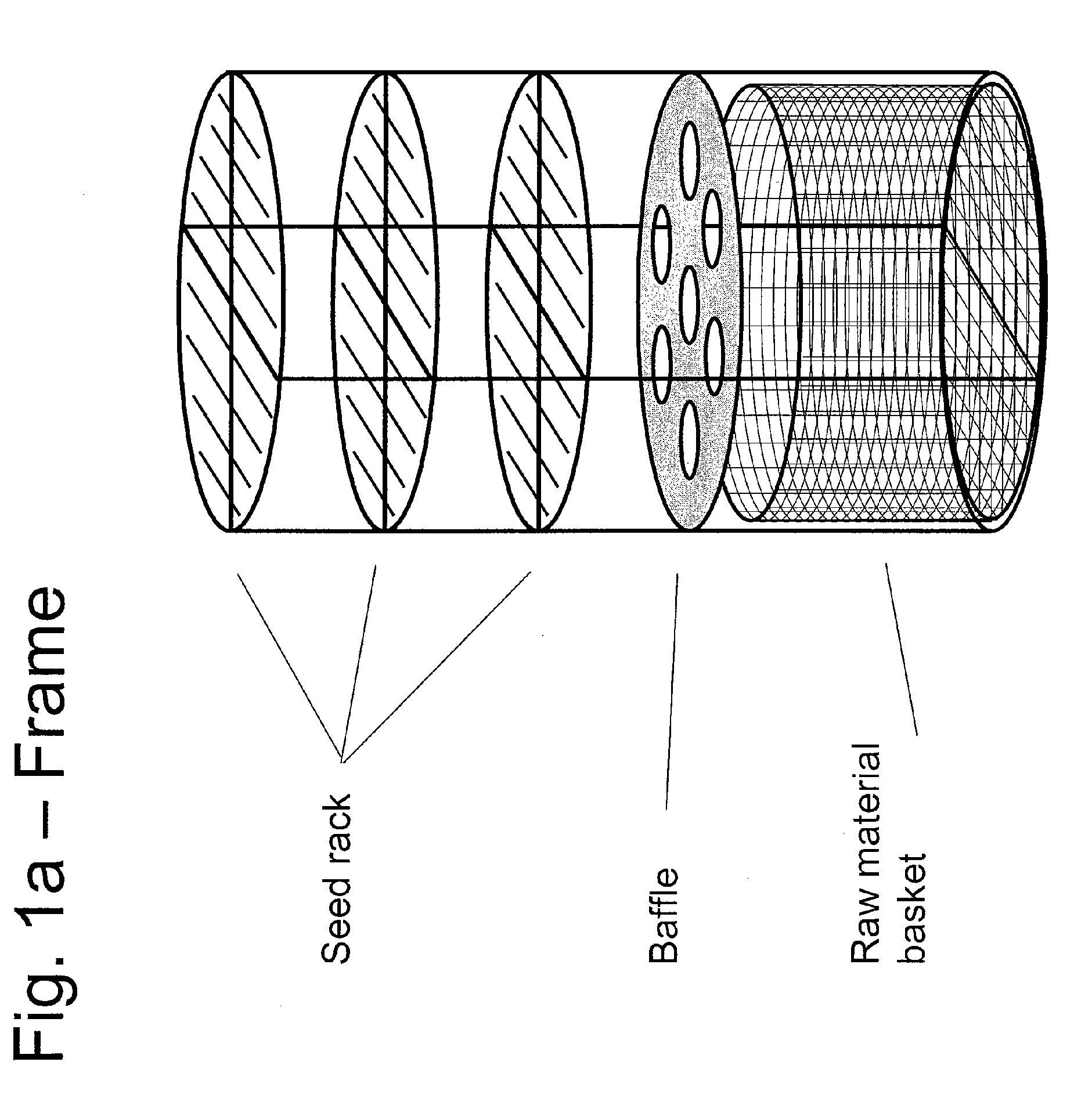
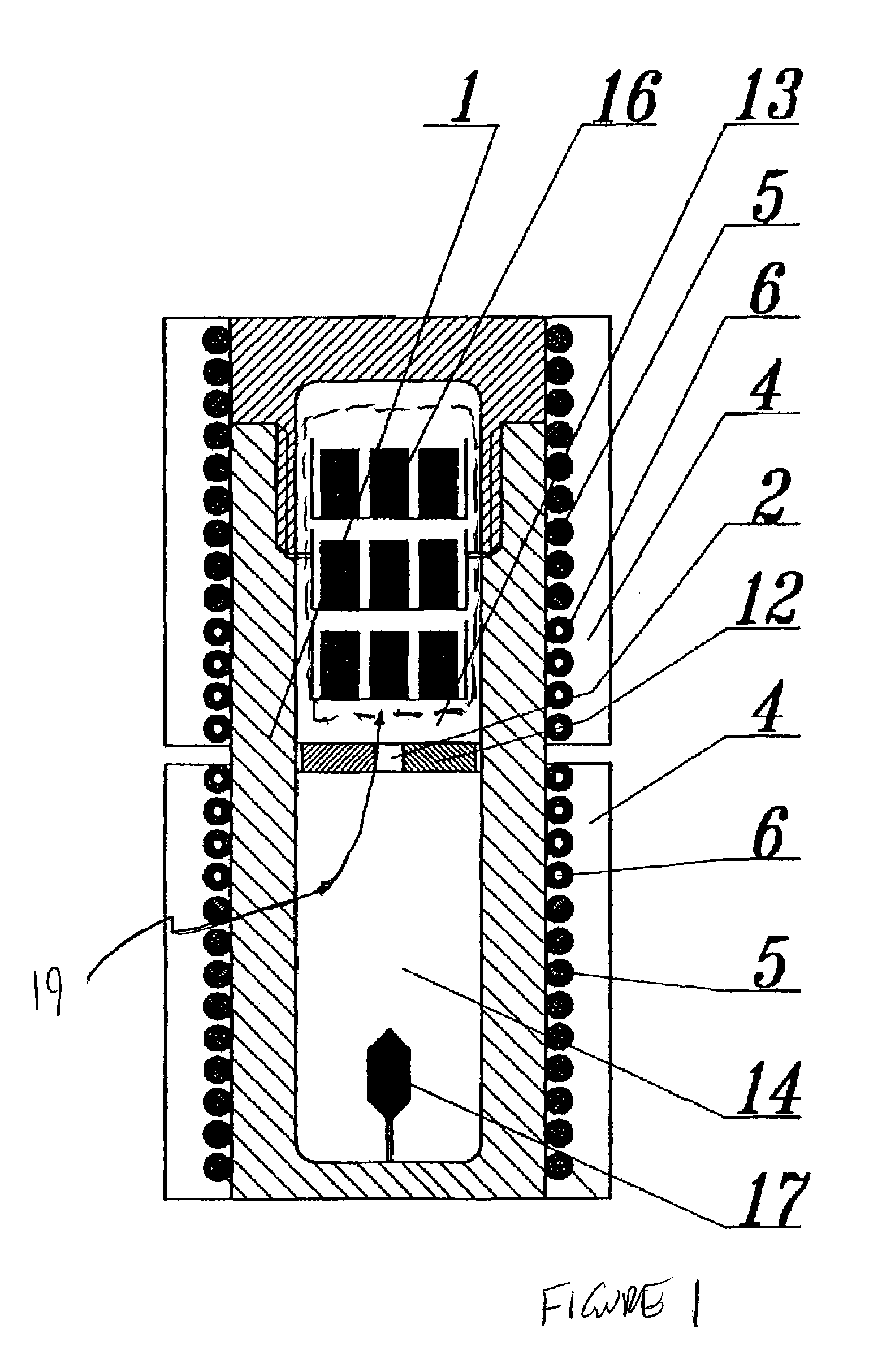
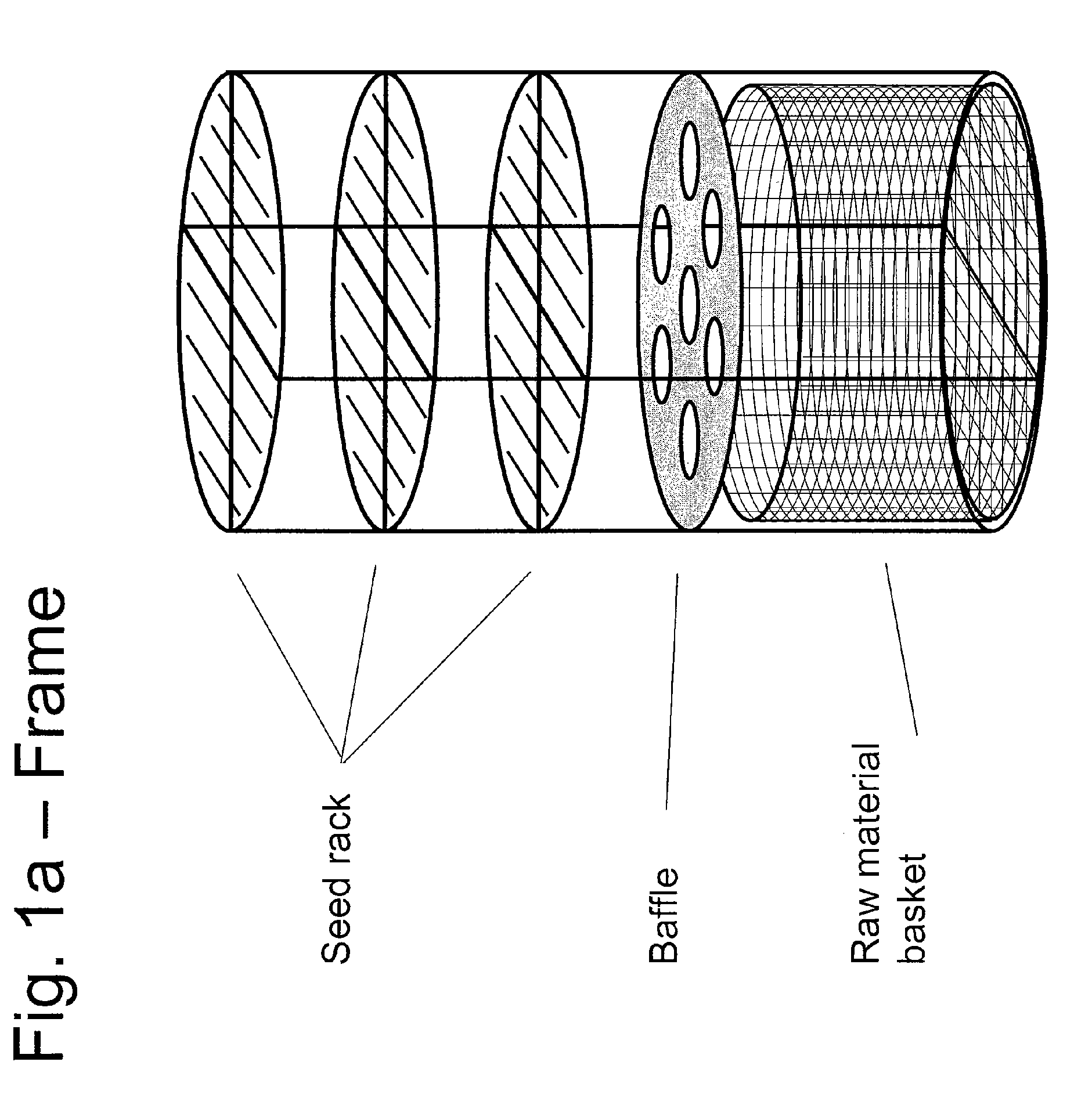
ActiveUS20100031874A1Increase in sizeCost effectivePolycrystalline material growthFrom normal temperature solutionsDopantCost effectiveness
An apparatus and associated method for large-scale manufacturing of gallium nitride is provided. The apparatus comprises a large diameter autoclave and a raw material basket. Methods include metered addition of dopants in the raw material and control of the atmosphere during crystal growth. The apparatus and methods are scalable up to very large volumes and are cost effective.
Owner:SLT TECH
Selective Posttranslational Modification of Phage-Displayed Polypeptides
ActiveUS20090137424A1High selectivityPreserves viral infectivityAntibody mimetics/scaffoldsMicrobiological testing/measurementArylCycloaddition
The invention relates to posttranslational modification of phage-displayed polypeptides. These displayed polypeptides comprise at least one unnatural amino acid, e.g., an aryl-azide amino acid such asp-azido-L-phenylalanine, or an alkynyl-amino acid such as para-propargyloxyphenylalanine, which are incorporated into the phage-displayed fusion polypeptide at a selected position by using an in vivo orthogonal translation system comprising a suitable orthogonal aminoacyl-tRNA synthetase and a suitable orthogonal tRNA species. These unnatural amino acids advantageously provide targets for posttranslational modifications such as azide-alkyne [3+2]cycloaddition reactions and Staudinger modifications.
Owner:THE SCRIPPS RES INST
Gas generating agent composition and gas generator
InactiveUS6517647B1Improve thermal stabilityImprove reliabilityNon-explosive desensitisers/phlegmatisersPressure gas generationSorbentDecomposition
A gas generating composition having an improved thermal stability of a fuel is obtained.A gas generating compostion comprising a gas generating agent containing a non-sodium azide compound fuel and an oxidizing agent, and an adsorbent. Since substances accelerating decomposition of a fuel, such as radicals generating by decomposition of the fuel are adsorbed and kept on the adsorbent, further decomposition of the fuel is inhabited, so that the thermal stability is increased.
Owner:DAICEL CHEM IND LTD
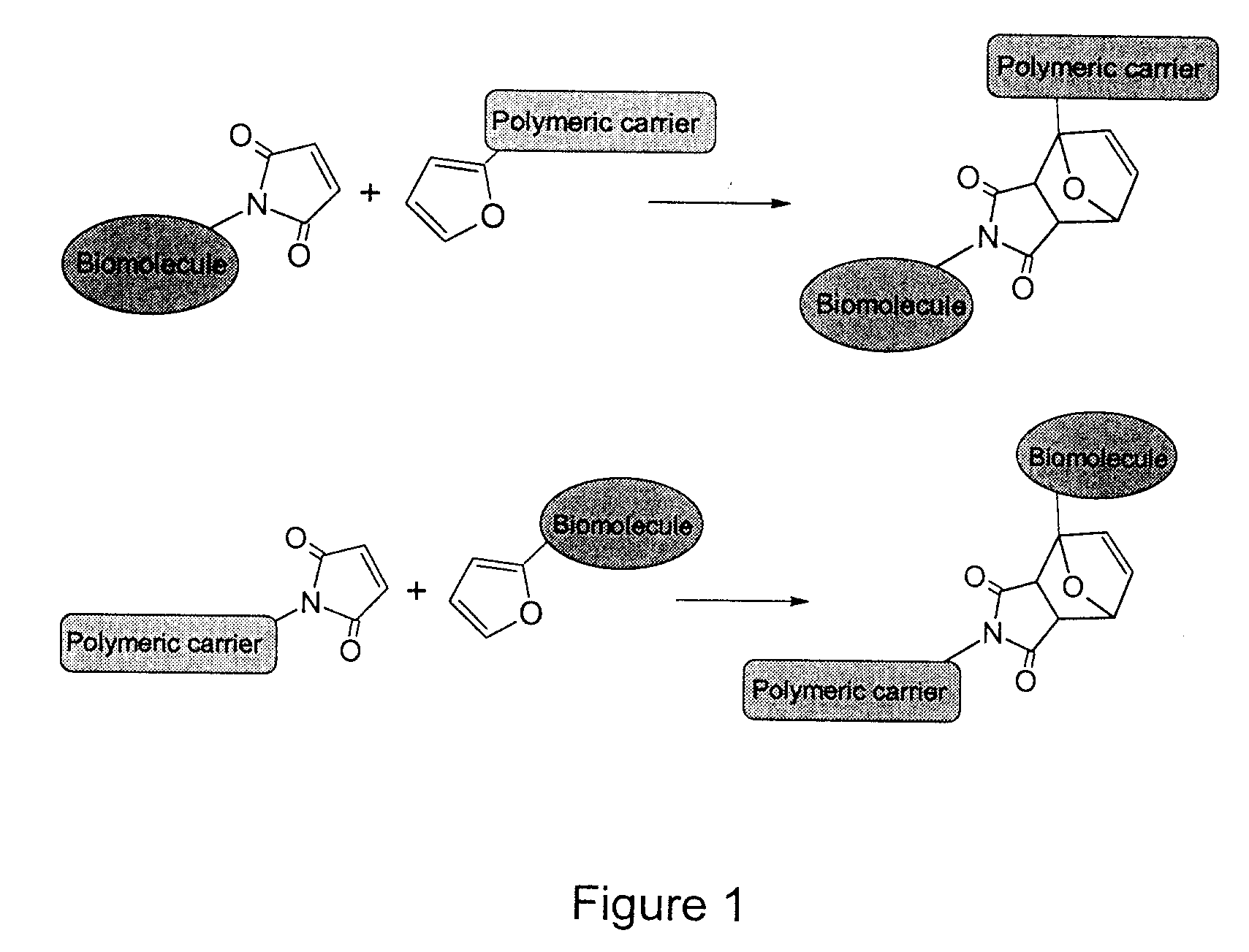
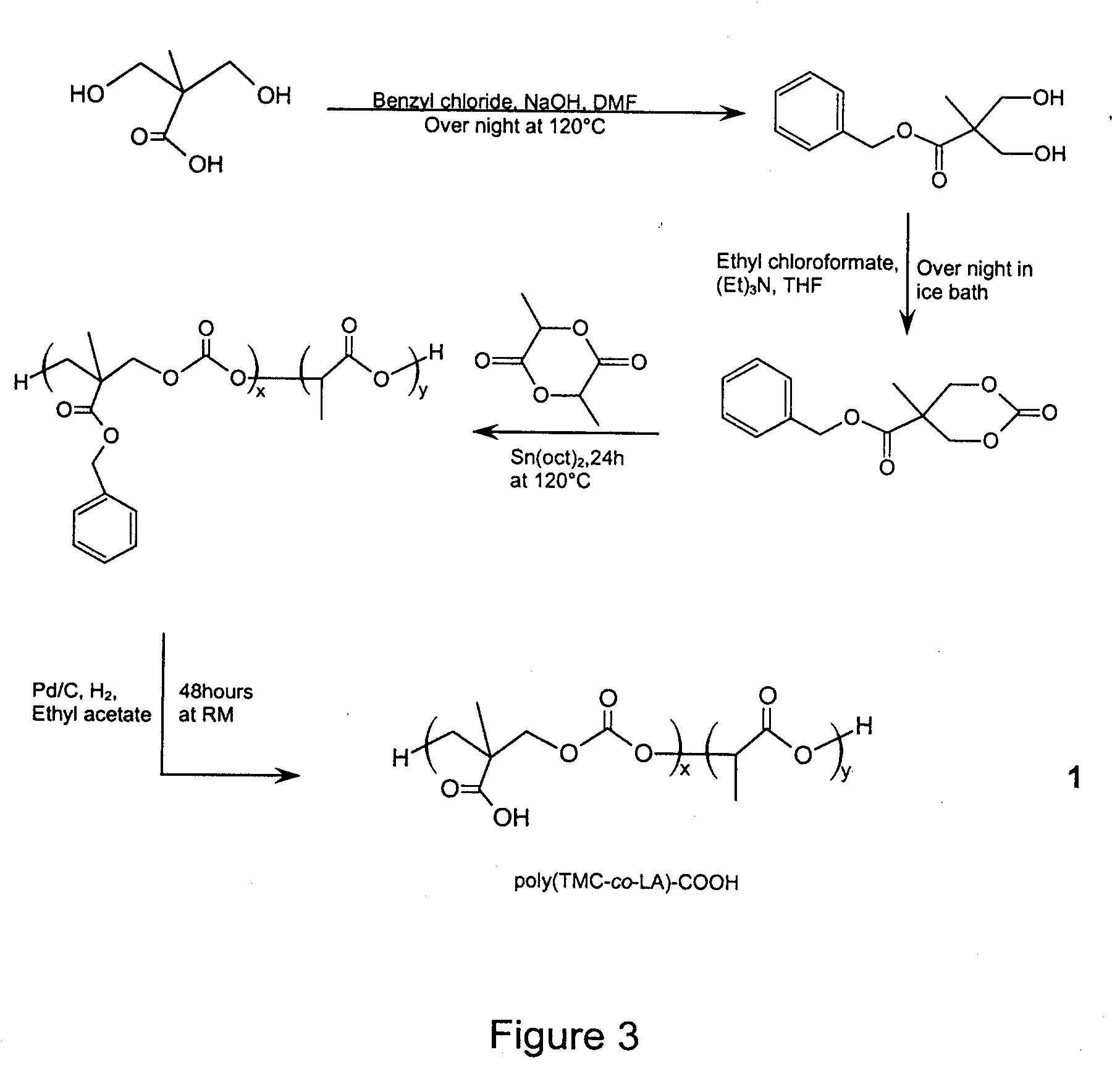
Method of Biomolecule Immobilization On Polymers Using Click-Type Chemistry
ActiveUS20090297609A1Improve efficiencyProcess environmental protectionBiocideOrganic active ingredientsFuranAlkyne
The present invention provides a method for the covalent immobilization of biomolecules on polymers for delivery of the biomolecules, which has the advantage of being simple, highly efficient, environmentally friendly and free of side products relative to traditional immobilization techniques. The invention provides a modified micro / nanoparticle system, which uses a functionalized polymer formed into micro or nanoparticles to bind a molecule to the particles using uses facile chemistry, the Diels-Alder cycloaddition between a diene and a dienophile with the polymer being functionalized with one of them and the molecule with the other, or the Huisgen 1,3-dipolar cycloaddition between a terminal alkyne and an azide to bind the molecule to the particle. The molecules and / or other therapeutic agents may be encapsulated within the polymer particles for intravenous therapeutic delivery. The invention also provides a novel synthetic biodegradable polymer, a furan / alkyne-functionalized poly(trimethylene carbonate) (PTMC)-based polymer, whose composition can be designed to meet the defined physical and chemical property requirements. In one example, the particle system self-aggregates from functionalized PTMC-based copolymers containing poly(ethylene glycol) (PEG) segments. The composition of the copolymers can be designed to meet various particle system requirements, including size, thermodynamic stability, surface PEG density, drug encapsulation capacity and biomolecule immobilization capacity.
Owner:SHOICHET MOLLY S +2
Method for producing a microfabricated atomic vapor cell
The present invention relates to a method for producing a microfabricated atomic vapor cell, including a step of forming at least one cavity in a substrate and closing the cavity at one side. The method further includes: —a step of depositing a solution including an alkali metal azide dissolved in at least one of its solvents, —a step of evaporating such solvent for forming a recrystallized alkali metal azide, —a step of decomposing the recrystallized alkali metal azide in an alkali metal and nitrogen, such alkali metal depositing in the cavity of the substrate.
Owner:CSEM CENT SUISSE DELECTRONIQUE & DE MICROTECHNIQUE SA RECH & DEV
Chemoselective ligation
InactiveUS20050148032A1Esterified saccharide compoundsSugar derivativesIn vivoChemoselective ligation
The present invention features a chemoselective ligation reaction that can be carried out under physiological conditions. In general, the invention involves condensation of a specifically engineered phosphine, which can provide for formation of an amide bond between the two reactive partners resulting in a final product comprising a phosphine moiety, or which can be engineered to comprise a cleavable linker so that a substituent of the phosphine is transferred to the azide, releasing an oxidized phosphine byproduct and producing a native amide bond in the final product. The selectivity of the reaction and its compatibility with aqueous environments provides for its application in vivo (e.g., on the cell surface or intracellularly) and in vitro (e.g., synthesis of peptides and other polymers, production of modified (e.g., labeled) amino acids).
Owner:RGT UNIV OF CALIFORNIA
Haloacetamide and azide substituted compounds and methods of use thereof
InactiveUS20050137172A1Effective treatmentPrevent cancerBiocideOrganic chemistryCancer preventionMetabolite
The present invention relates to a novel class of anti-cancer compounds, which contain a haloacetamide or azide moiety and are, in one embodiment, alkylating agents. These agents, either alone or in a composition, are useful for treating cancer, preventing cancer, delaying the progression of cancer, treating and / or preventing the recurrence of cancer, suppressing, inhibiting or reducing the incidence of cancer, or inducing apoptosis in a cancer cell. Accordingly, the present invention provides a) methods of treating cancer in a subject; b) methods of preventing cancer in a subject; c) methods of delaying the progression of cancer in a subject; d) methods of treating the recurrence of cancer in a subject; e) methods of preventing the recurrence of cancer in a subject; f) methods of suppressing, inhibiting or reducing the incidence of cancer in a subject; and g) methods of inducing apoptosis in a cancer cell; by administering to the subject an anti-cancer compound of the present invention or an analog or metabolite thereof, its N-oxide, ester, pharmaceutically acceptable salt, hydrate, or any combination thereof as described herein.
Owner:DALTON JAMES +3
Chemoselective ligation
The present invention features a chemoselective ligation reaction that can be carried out under physiological conditions. In general, the invention involves condensation of a specifically engineered phosphine, which can provide for formation of an amide bond between the two reactive partners resulting in a final product comprising a phosphine moiety, or which can be engineered to comprise a cleavable linker so that a substituent of the phosphine is transferred to the azide, releasing an oxidized phosphine byproduct and producing a native amide bond in the final product. The selectivity of the reaction and its compatibility with aqueous environments provides for its application in vivo (e.g., on the cell surface or intracellularly) and in vitro (e.g., synthesis of peptides and other polymers, production of modified (e.g., labeled) amino acids).
Owner:RGT UNIV OF CALIFORNIA
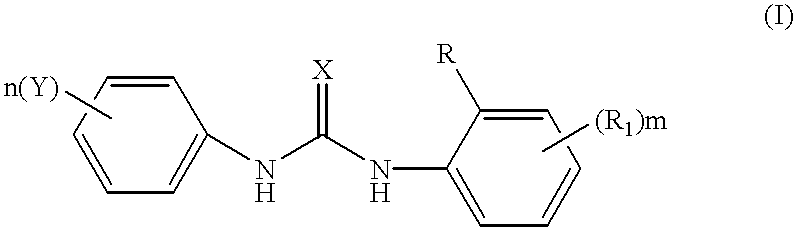
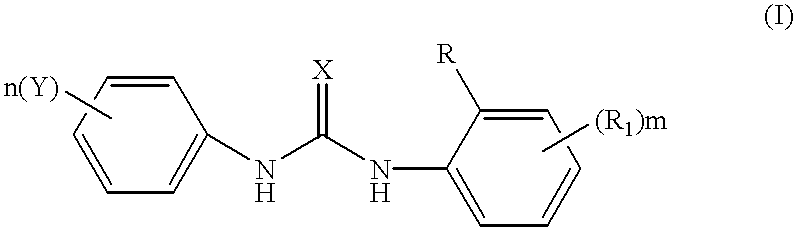
Phenyl urea antagonists of the IL-8 receptor
This invention relates to novel compounds of Formula (I), and a novel use of these compounds in treating chemokine mediated diseases, wherein the chemokine binds to an IL-8 a or b receptor. Compounds of Formula (1) are represented by the structure:wherein interalia, X is oxygen or sulfur;R is any functional moiety having an ionizable hydrogen and a pKa of 10 or less;R1 is independently selected from hydrogen; halogen; nitro; cyano; C1-C10 alkyl; halosubstituted C1-10 alkyl; C2-10 alkenyl; C1-10 alkoxy; halosubstituted C1-10 alkoxy; azide; (CR8R8)qS(O)tR4; hydroxy; hydroxy substituted C1-4 alkyl; aryl; aryl C1-4 alkyl; aryl C2-10 alkenyl; aryloxy; aryl C1-4 alkyloxy; heteroaryl; heteroarylalkyl; heteroaryl C2-10 alkenyl; heteroaryl C1-4 alkyloxy; heterocyclic, heterocyclic C1-4 alkyl; heterocyclic C1-4 alkyloxy; heterocyclic C2-10 alkenyl;q is 0 or an integer having a value of 1 to 10; n is an integer having a value of 1 to 3;m is an integer having a value of 1 to 3;n is an integer having a value of 1 to 3;Y is hydrogen; halogen; nitro; cyano; halosubstituted C1-10 alkyl; C1-10 alkyl; C2-10 alkenyl; C1-10 alkoxy; halosubstituted C1-1- alkoxy; azide; (CR8R8)qS(O)tR4, (CR8R8)qOR4; hydroxy; hydroxy substituted C1-4 alkyl; aryl; aryl C1-4 alkyl; aryloxy; aryC1-4 alkyloxy; aryl C2-10 alkenyl; heteroaryl; heteroarylalkyl; heteroaryl C1-4 alkyloxy; heteroaryl C2-10 alkenyl; heterocyclic, heterocyclic C1-4 alkyl; heterocyclic C2-10 alkenyl;or a pharmaceutically acceptable salt thereof.
Owner:GLAXO SMITHKLINE LLC
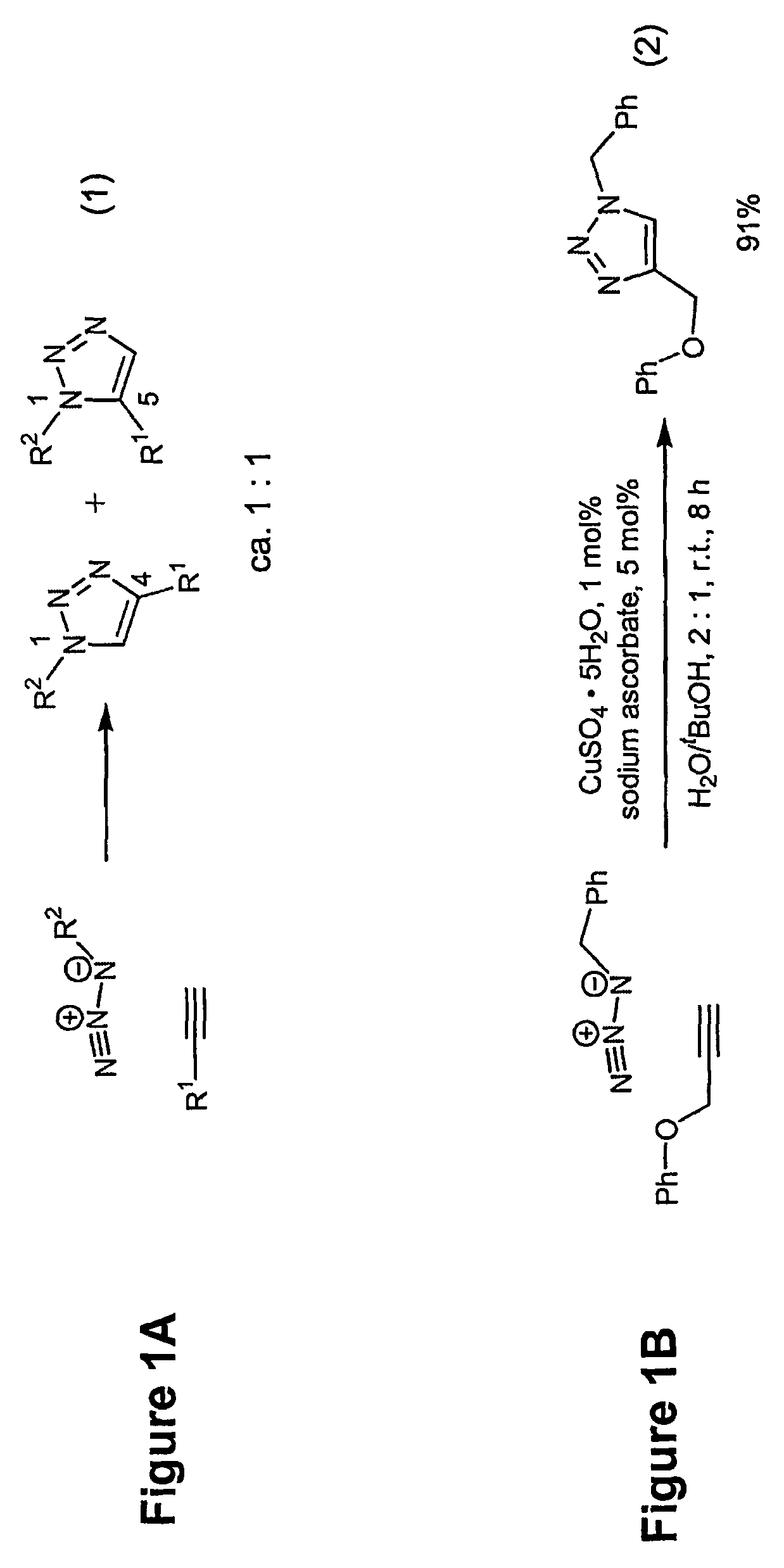
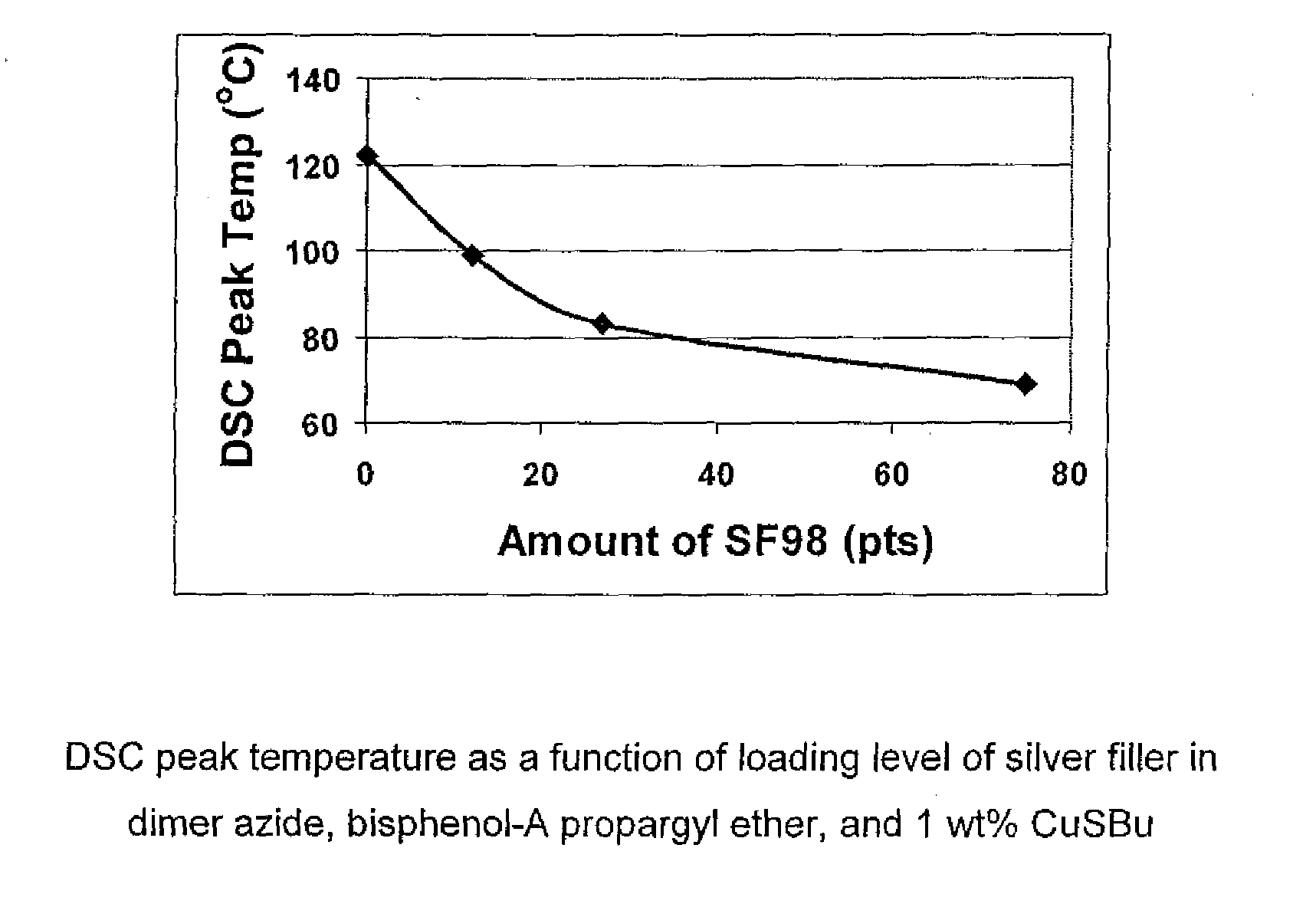
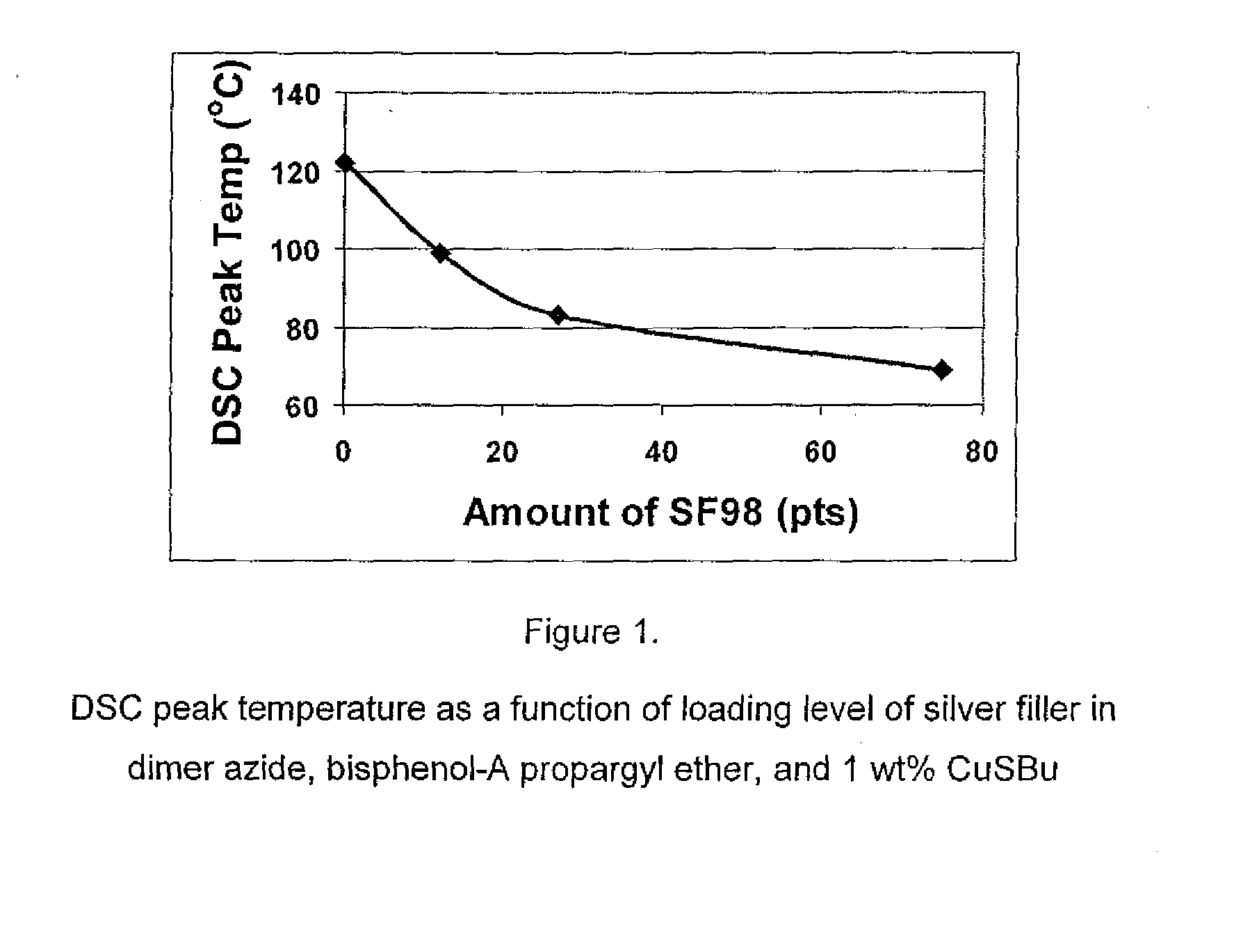
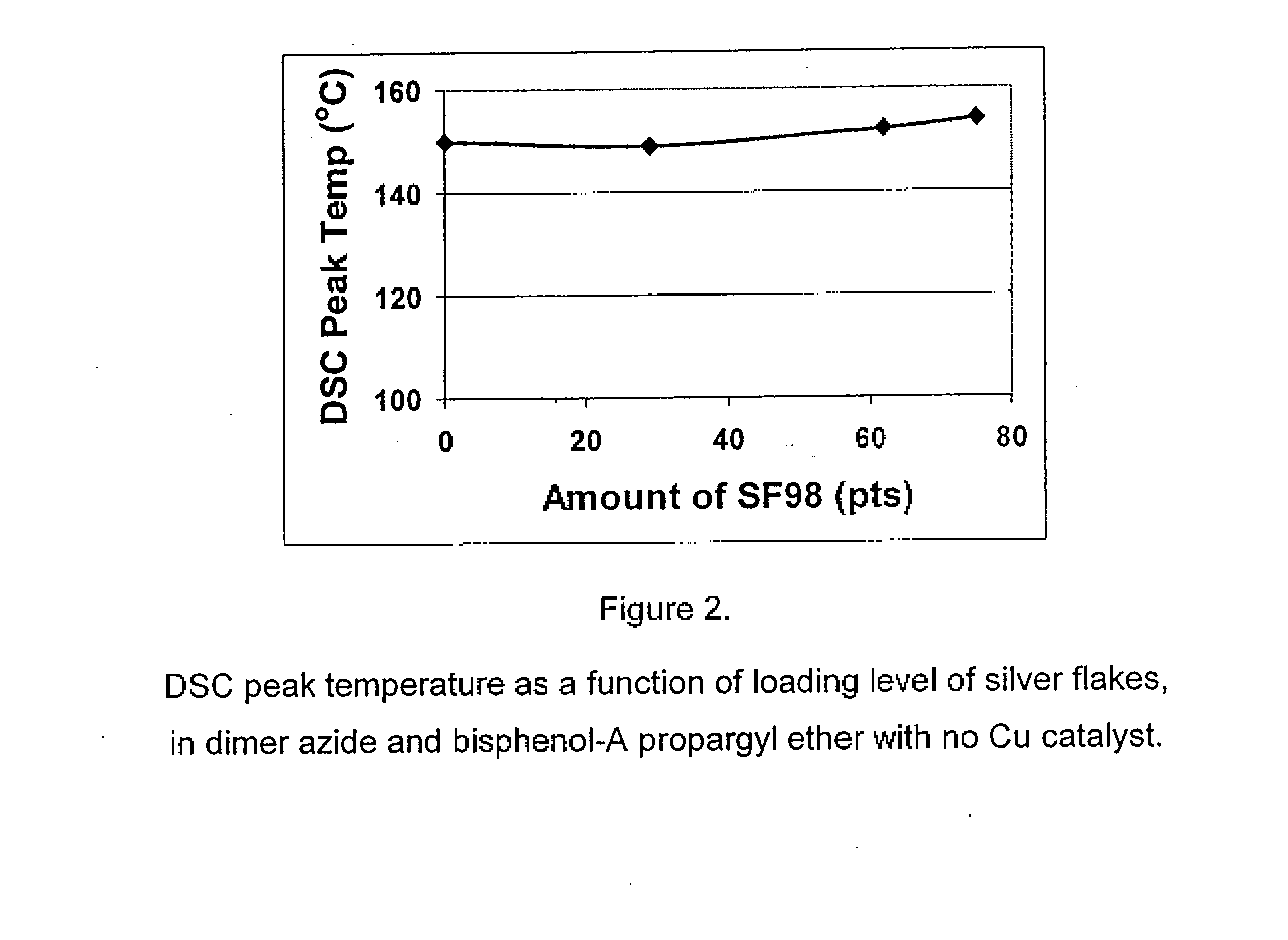
1,3-dipolar cycloaddition of azides to alkynes
InactiveUS20100121022A1Decreased DSC peak temperatureIncrease loadAdhesivesOligomer1,3-Dipolar cycloaddition
Owner:HENKEL KGAA
Bioadhesive Composition Formed Using Click Chemistry
The present disclosure provides compositions which may be utilized as adhesives or sealants in medical and surgical applications. The compositions may, in embodiments, be formed from the cycloaddition reaction of a first component possessing at least one azide group with a second component possessing at least one alkyne group.
Owner:TYCO HEALTHCARE GRP LP
Process and apparatus for growing a crystalline gallium-containing nitride using an azide mineralizer
ActiveUS8323405B2Increase in sizeCost effectivePolycrystalline material growthFrom normal temperature solutionsDopantGallium nitride
An apparatus and associated method for large-scale manufacturing of gallium nitride is provided. The apparatus comprises a large diameter autoclave and a raw material basket. Methods include metered addition of dopants in the raw material and control of the atmosphere during crystal growth. The apparatus and methods are scalable up to very large volumes and are cost effective.
Owner:SLT TECH
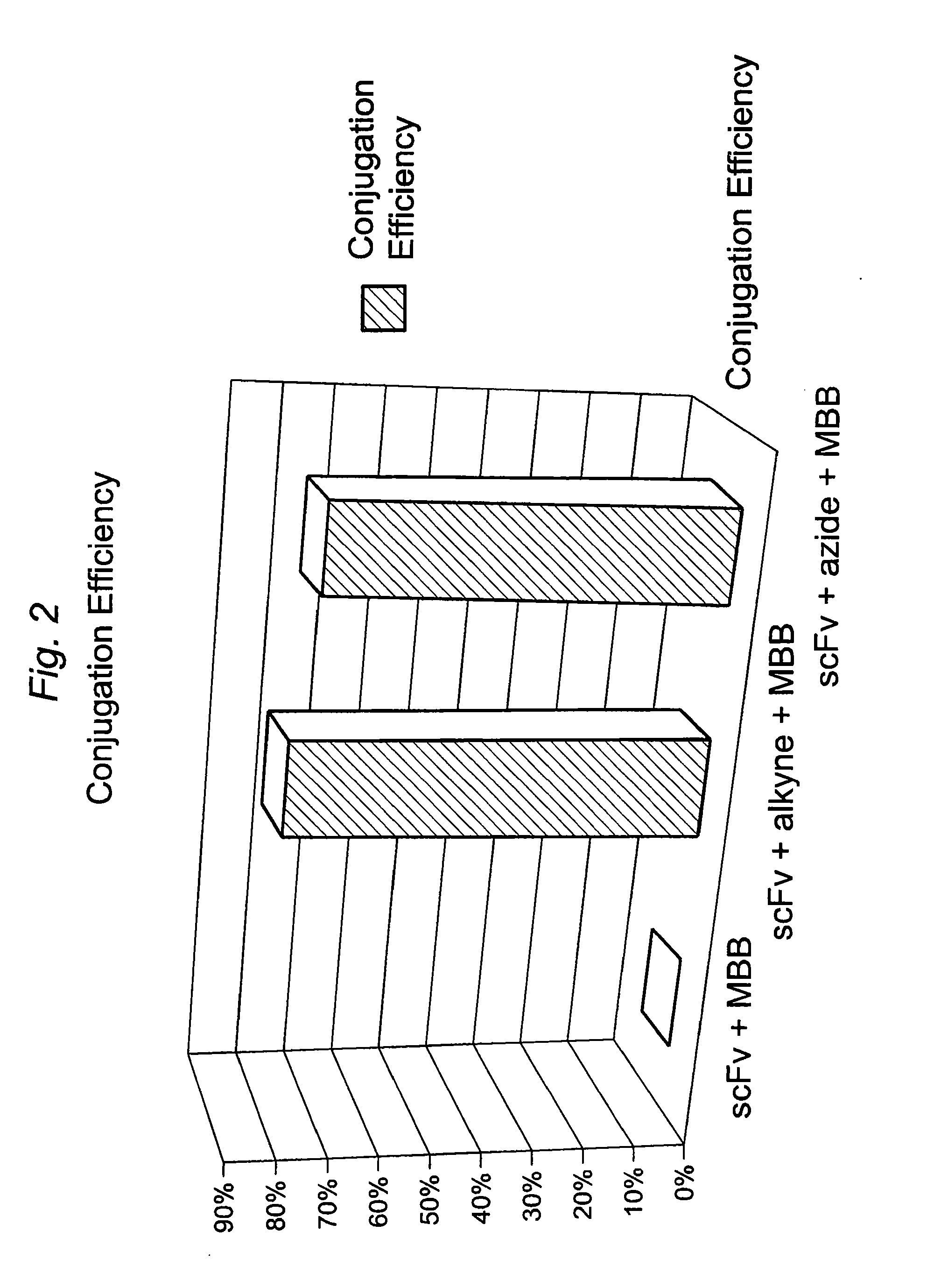
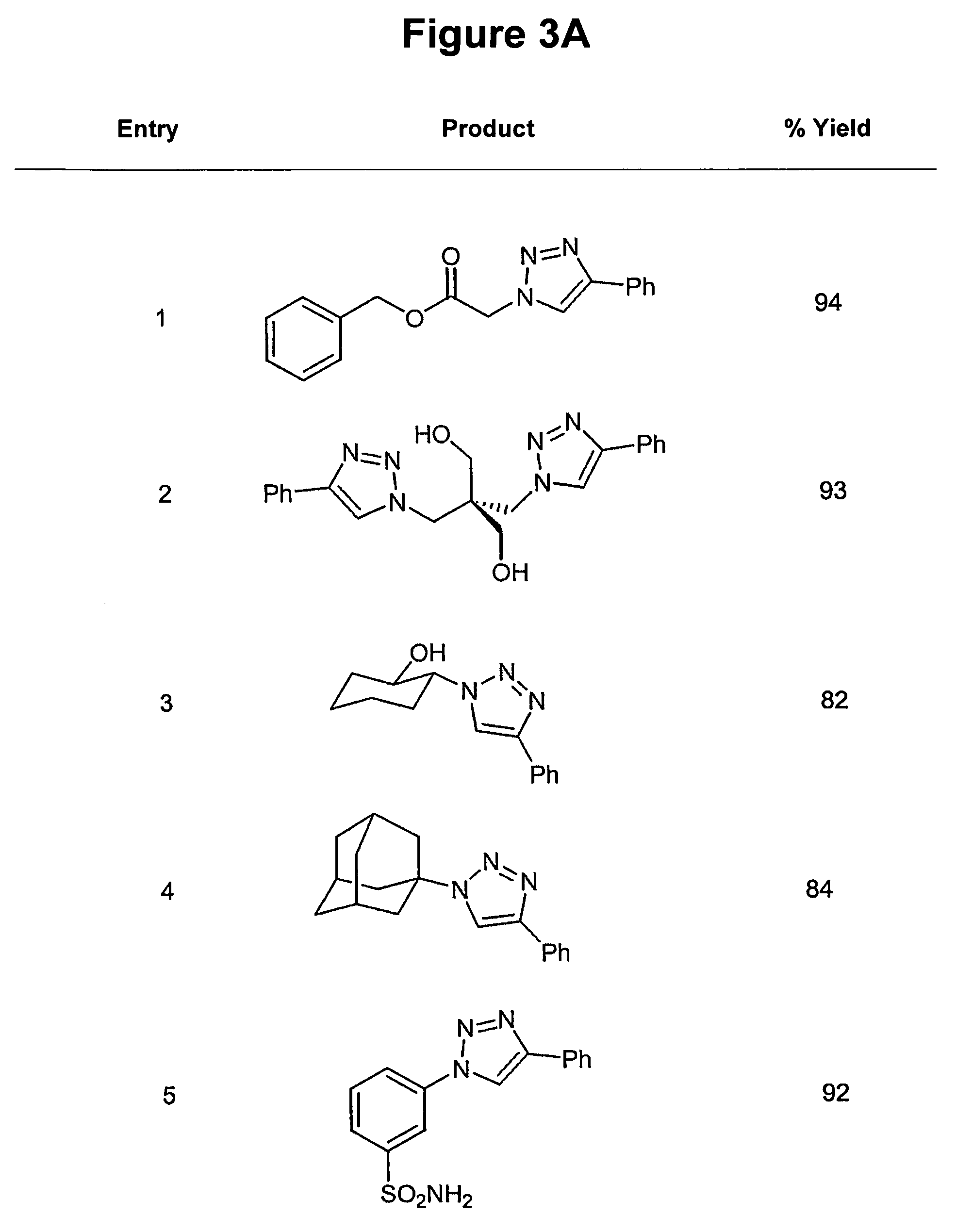
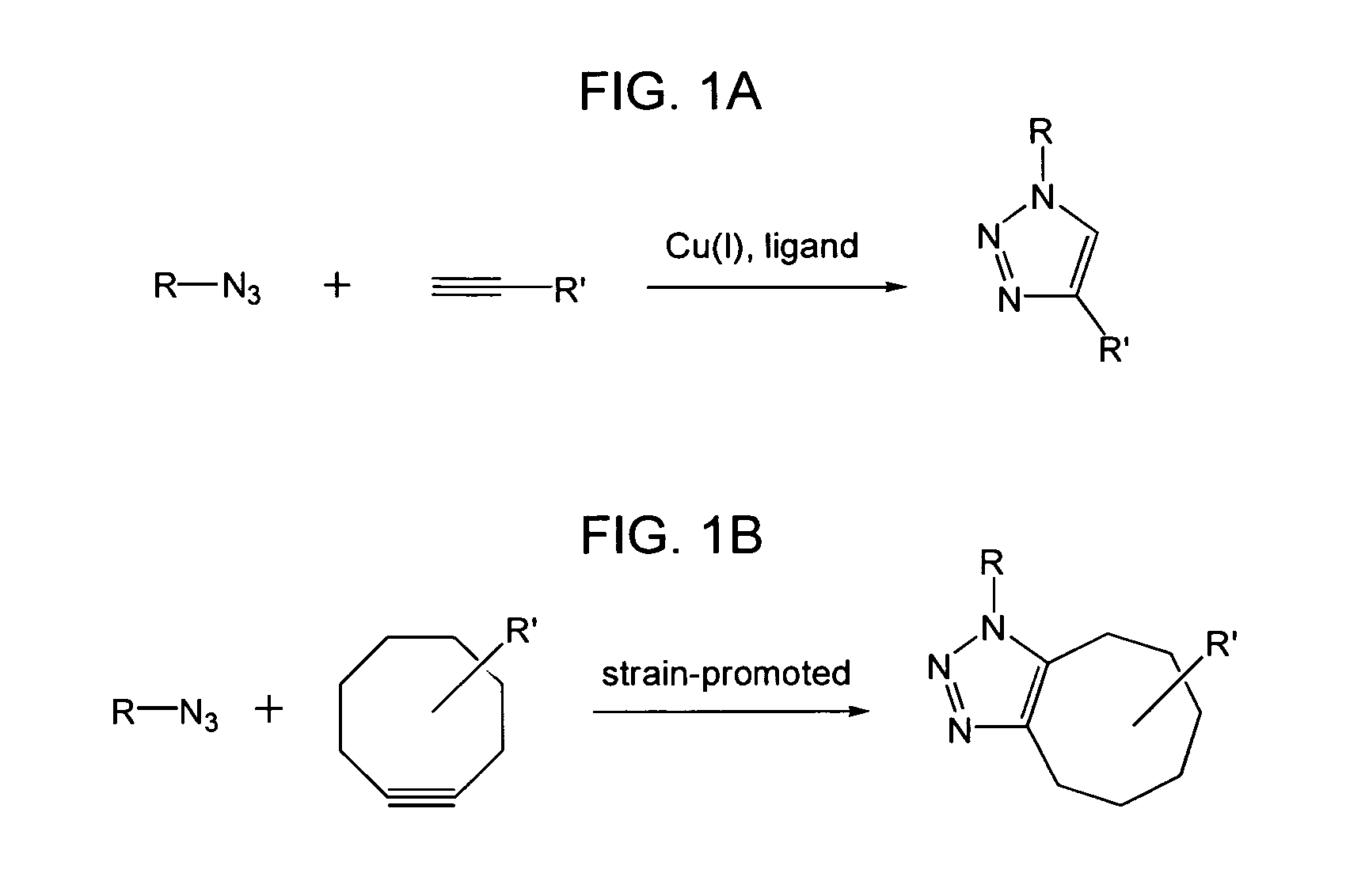
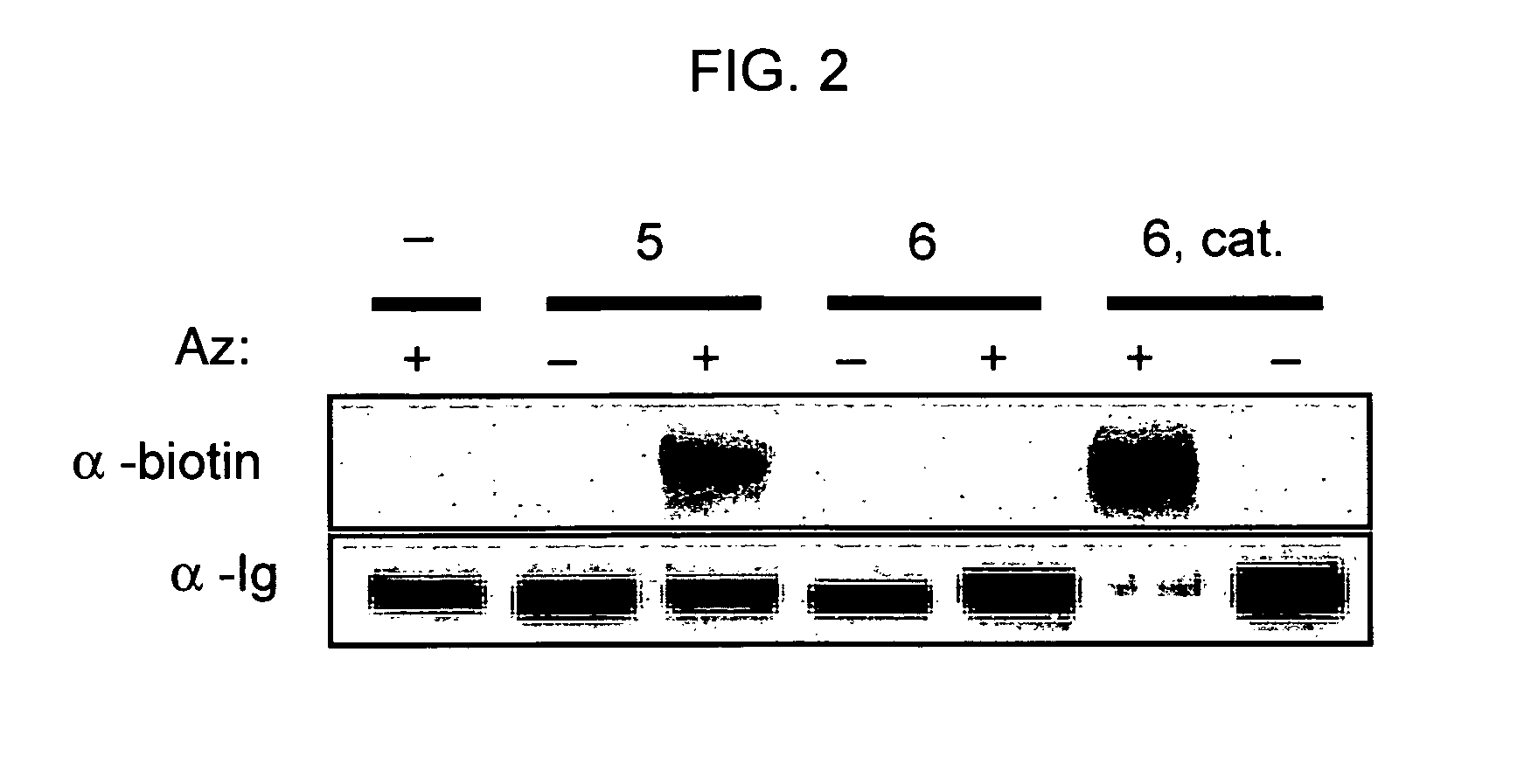
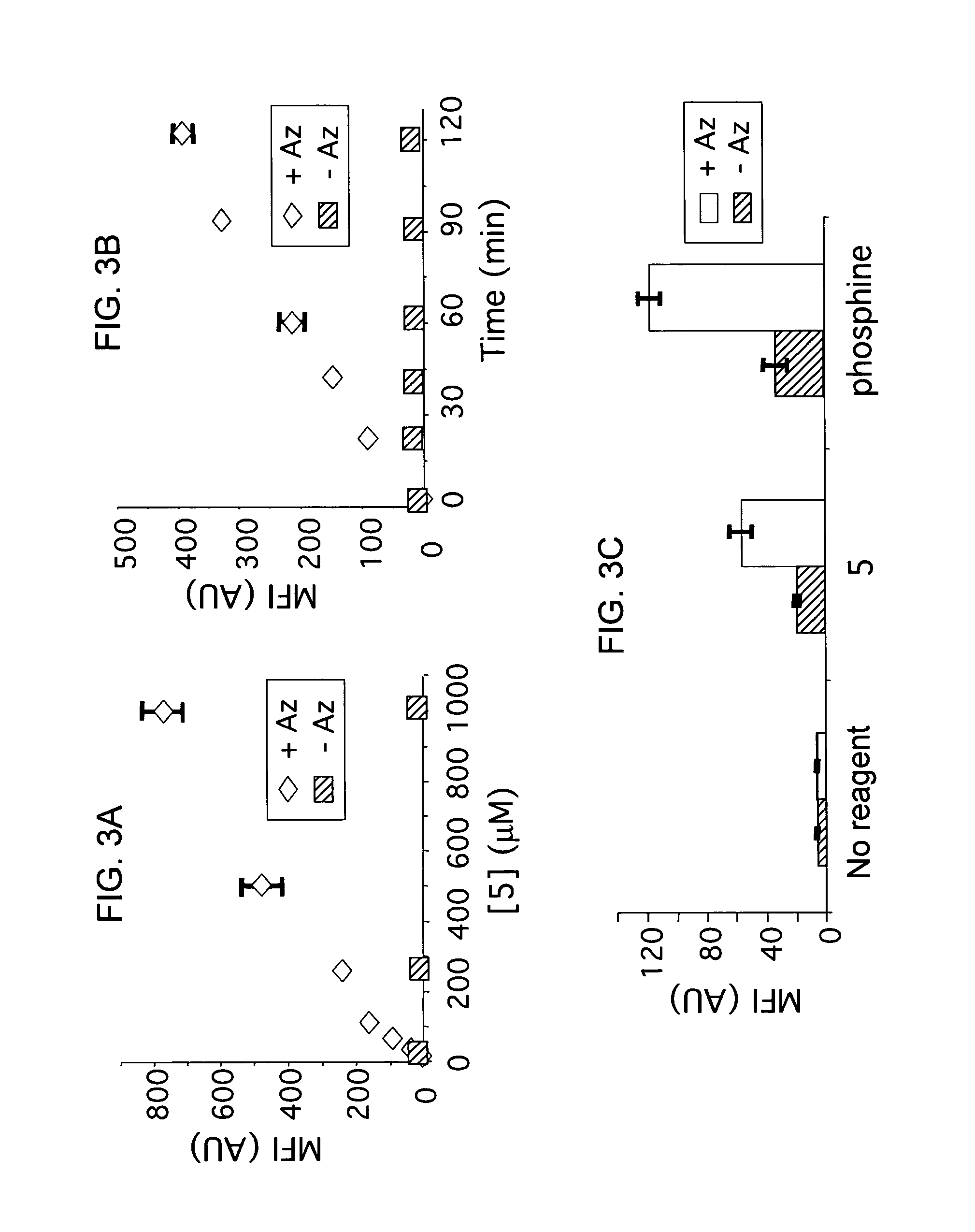
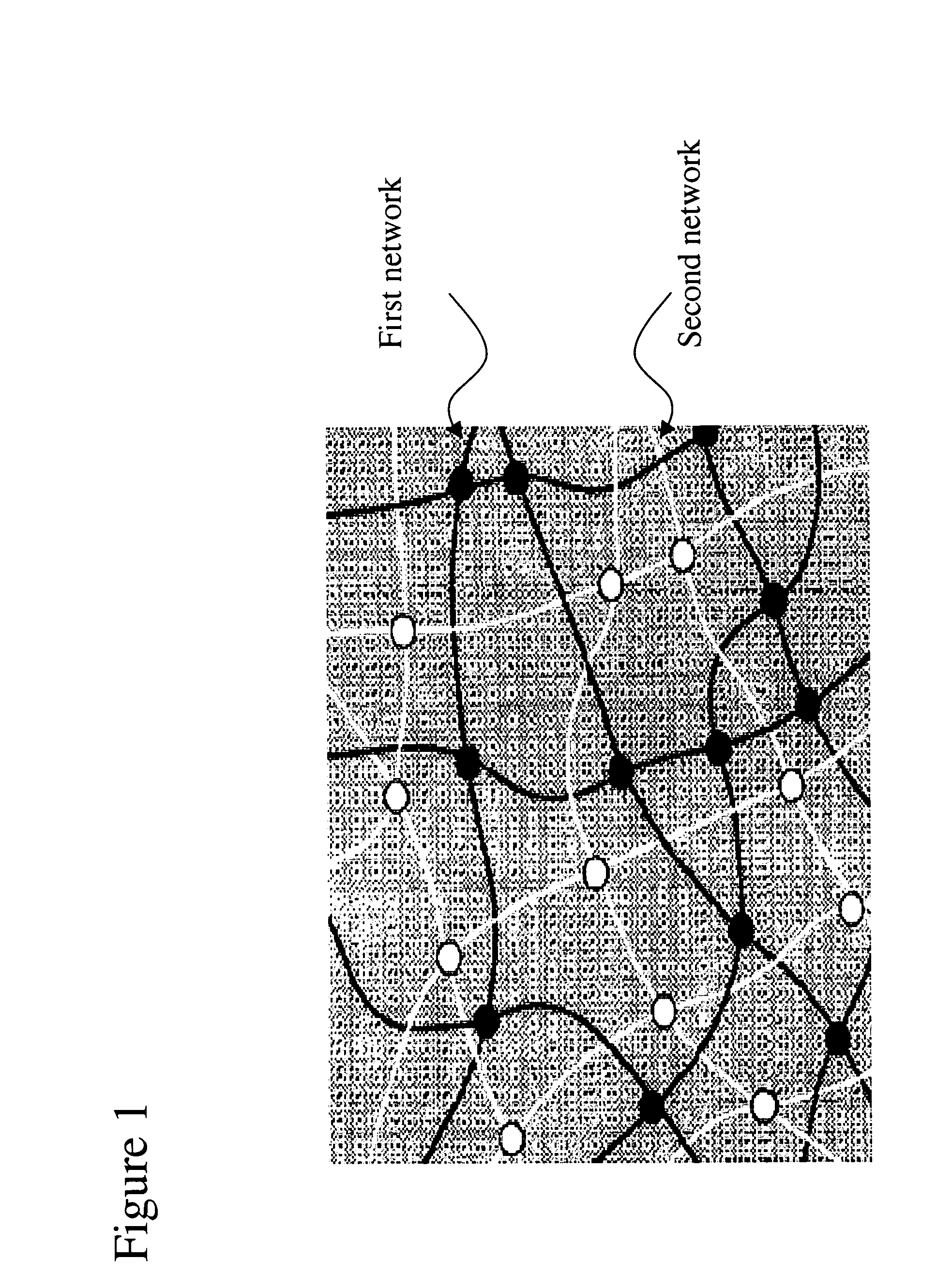
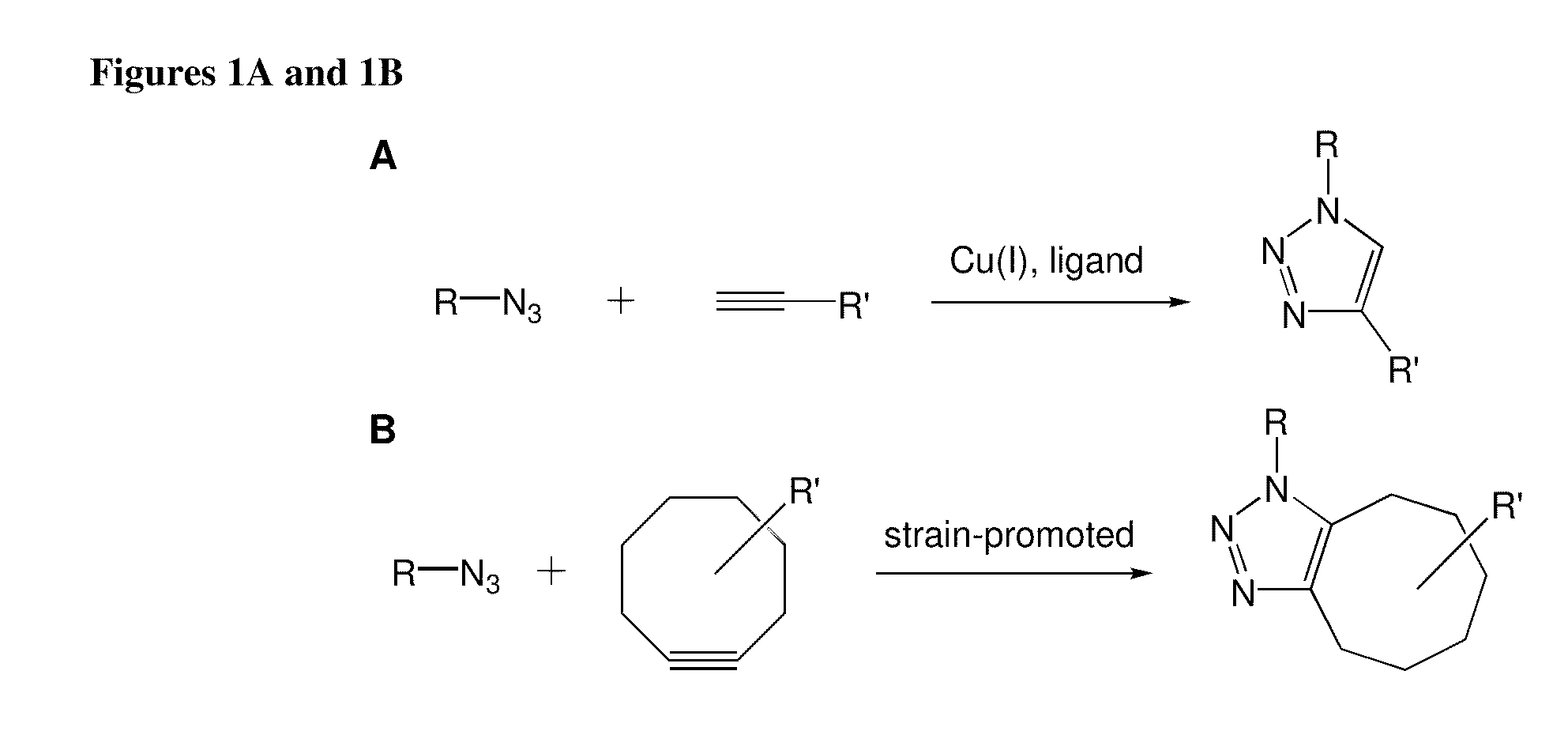
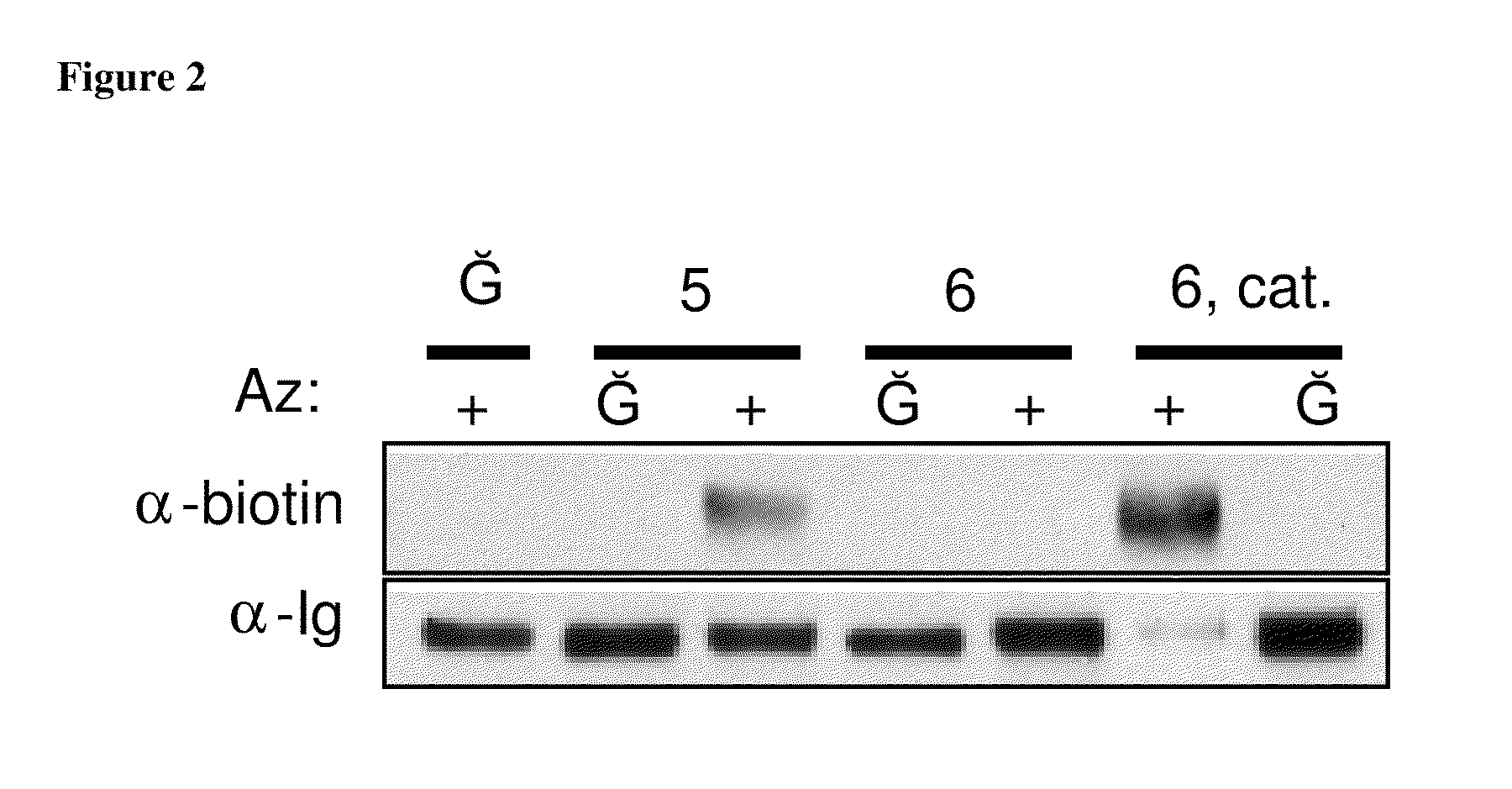
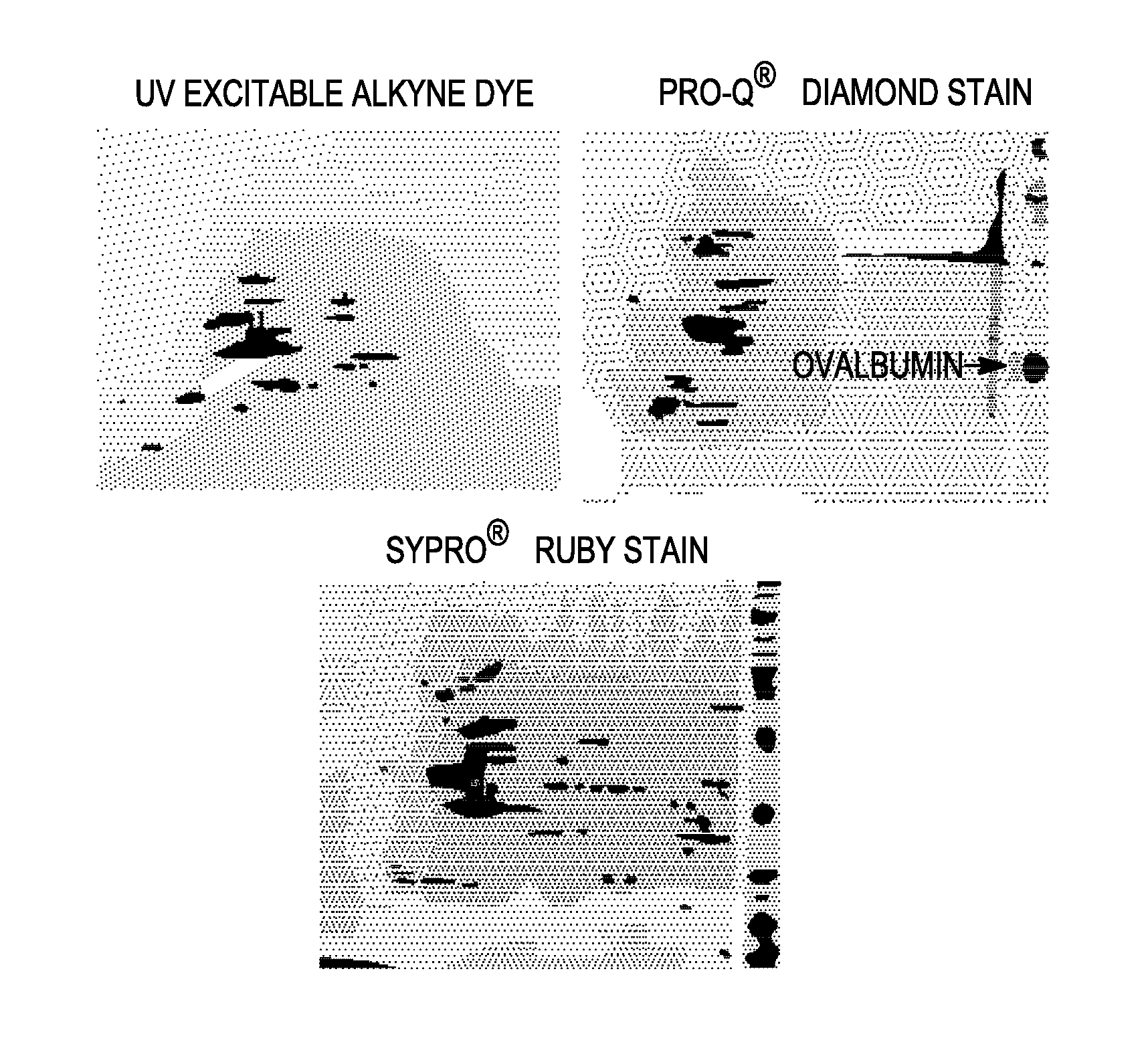
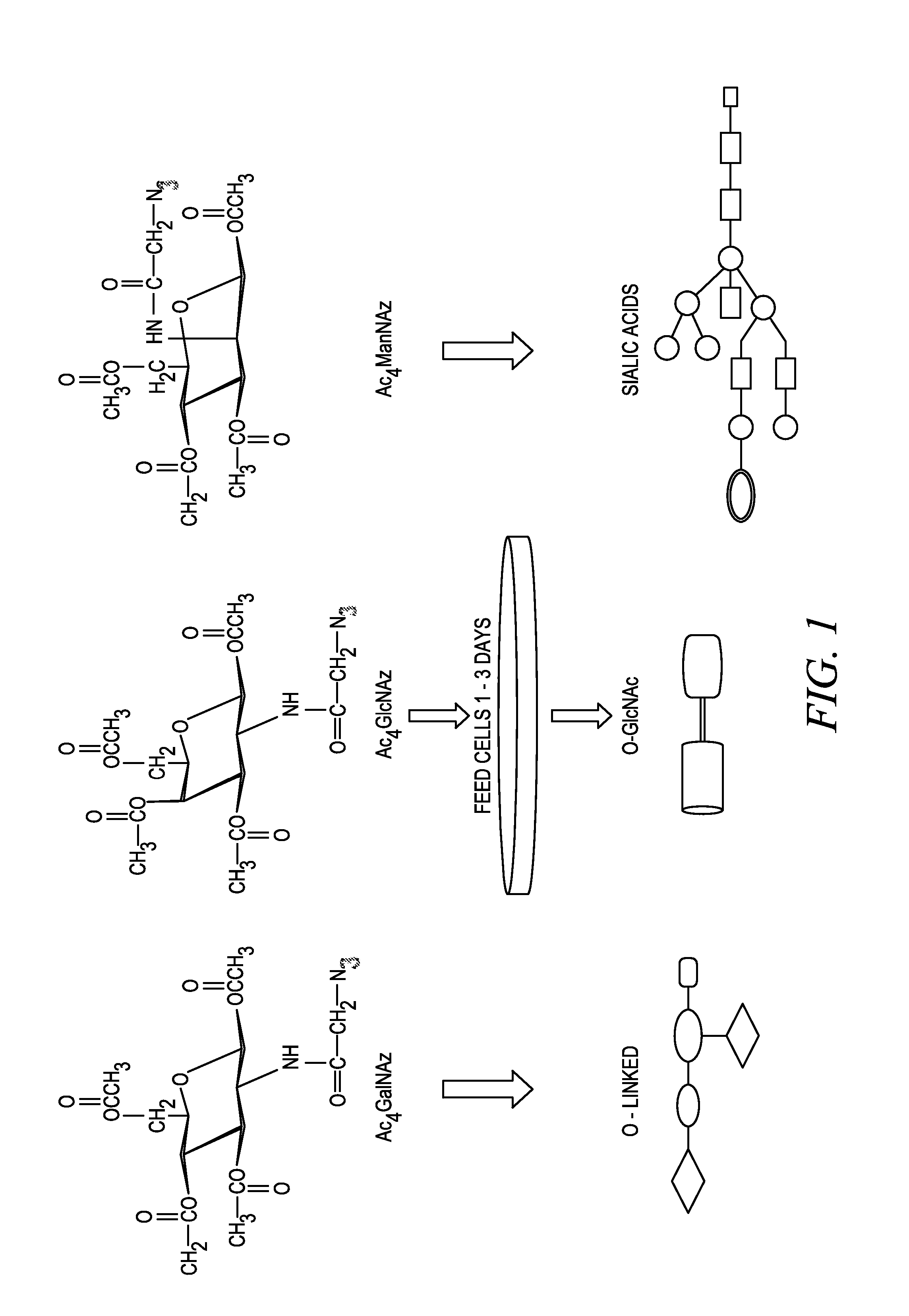
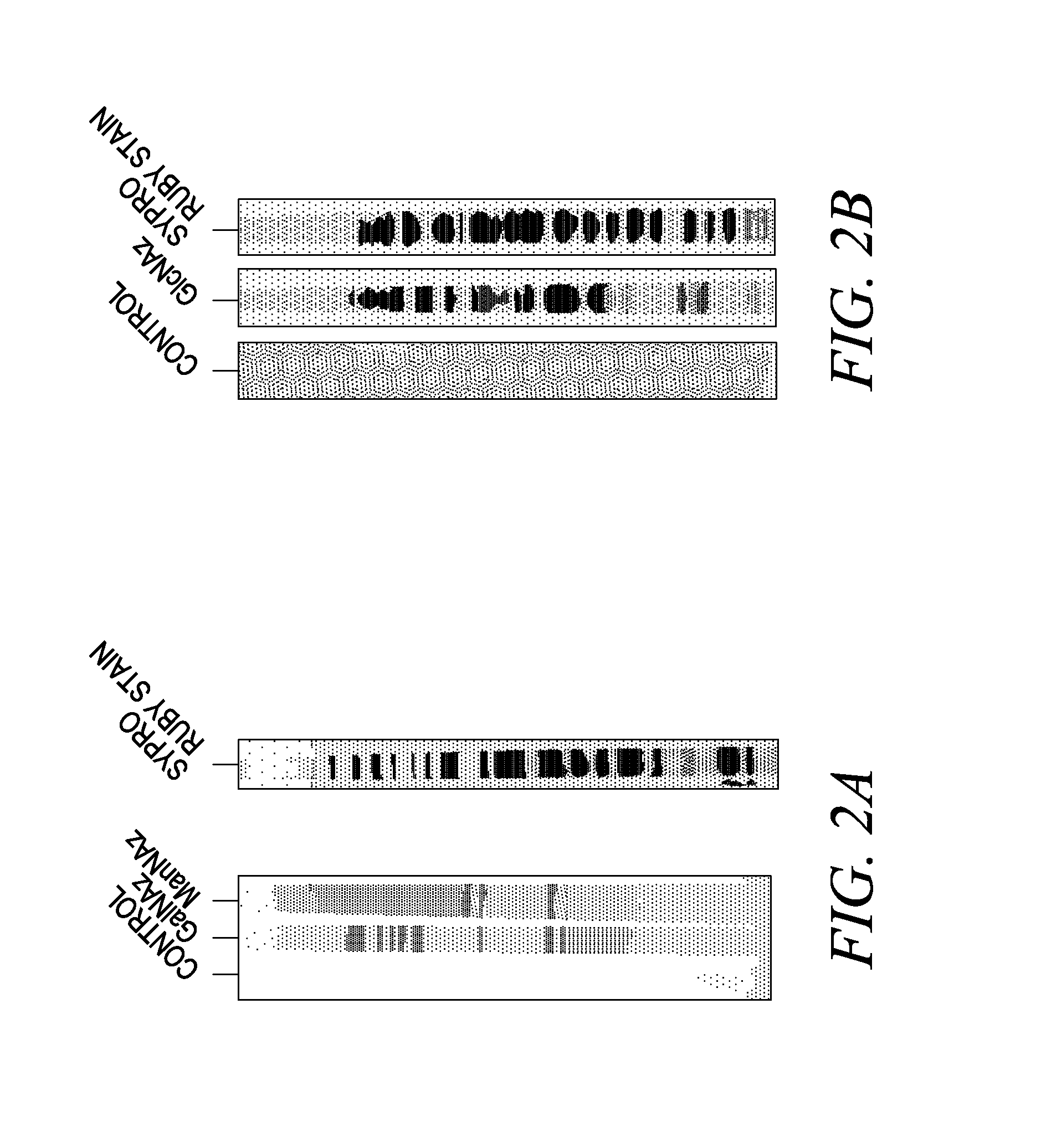
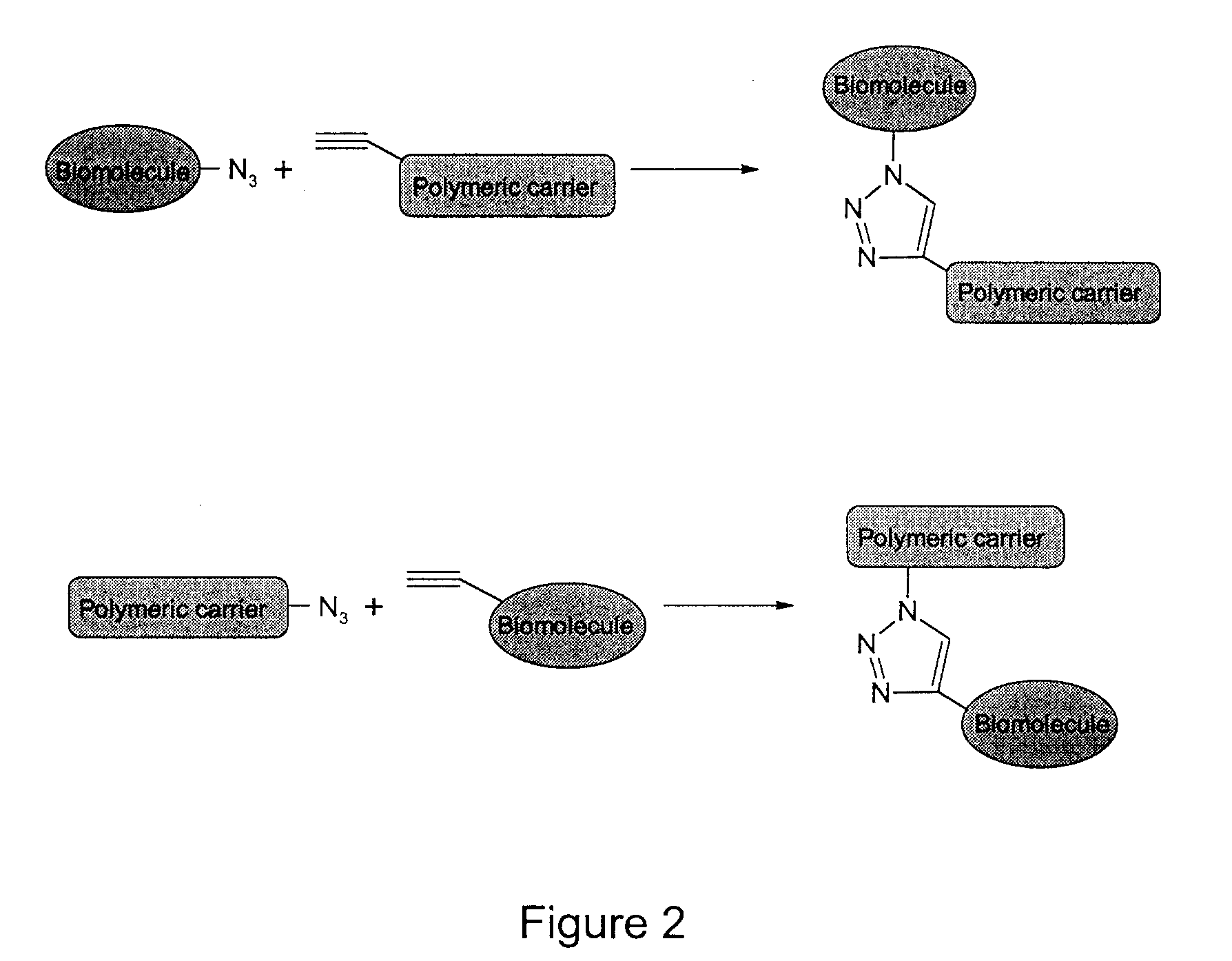
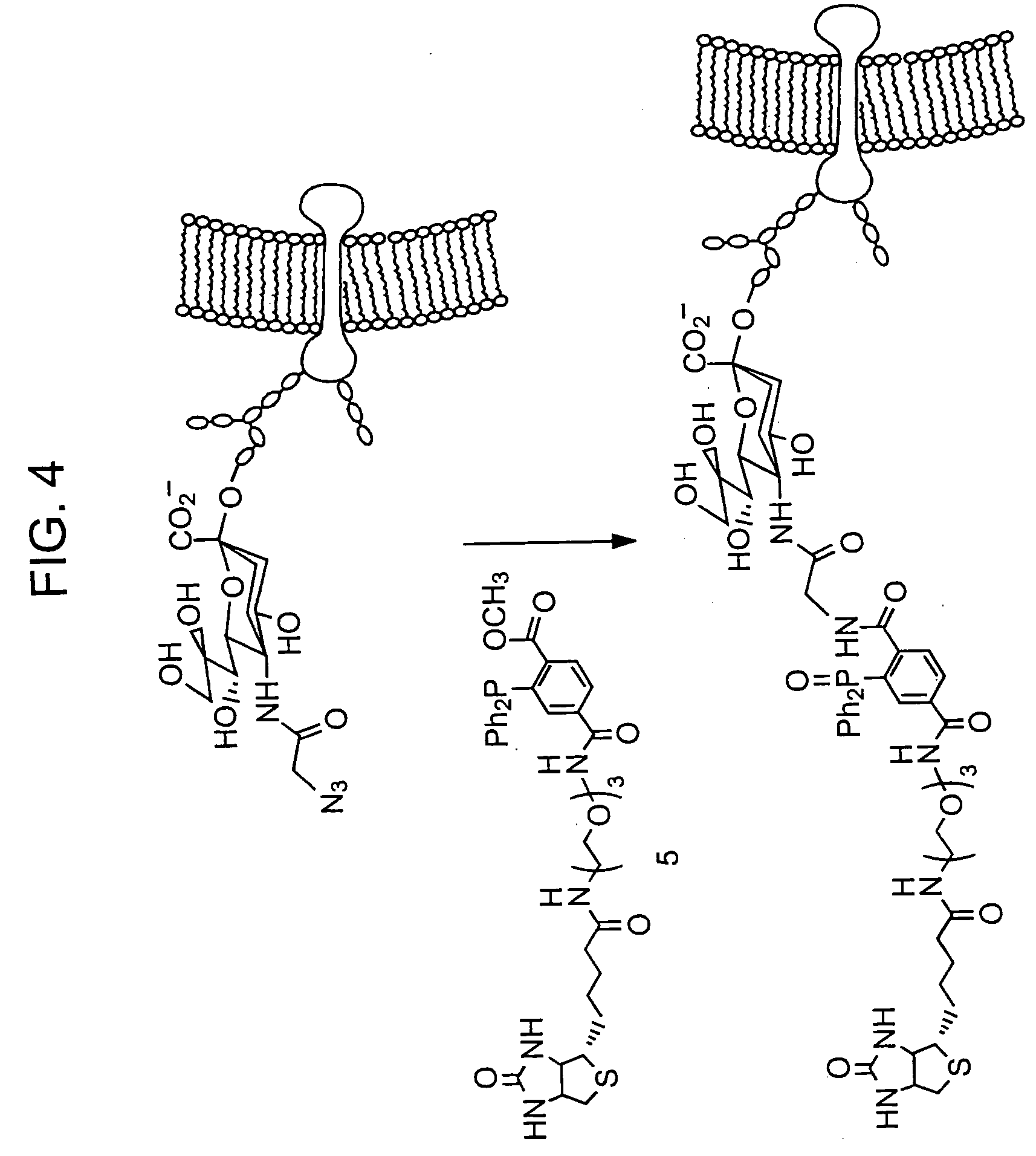
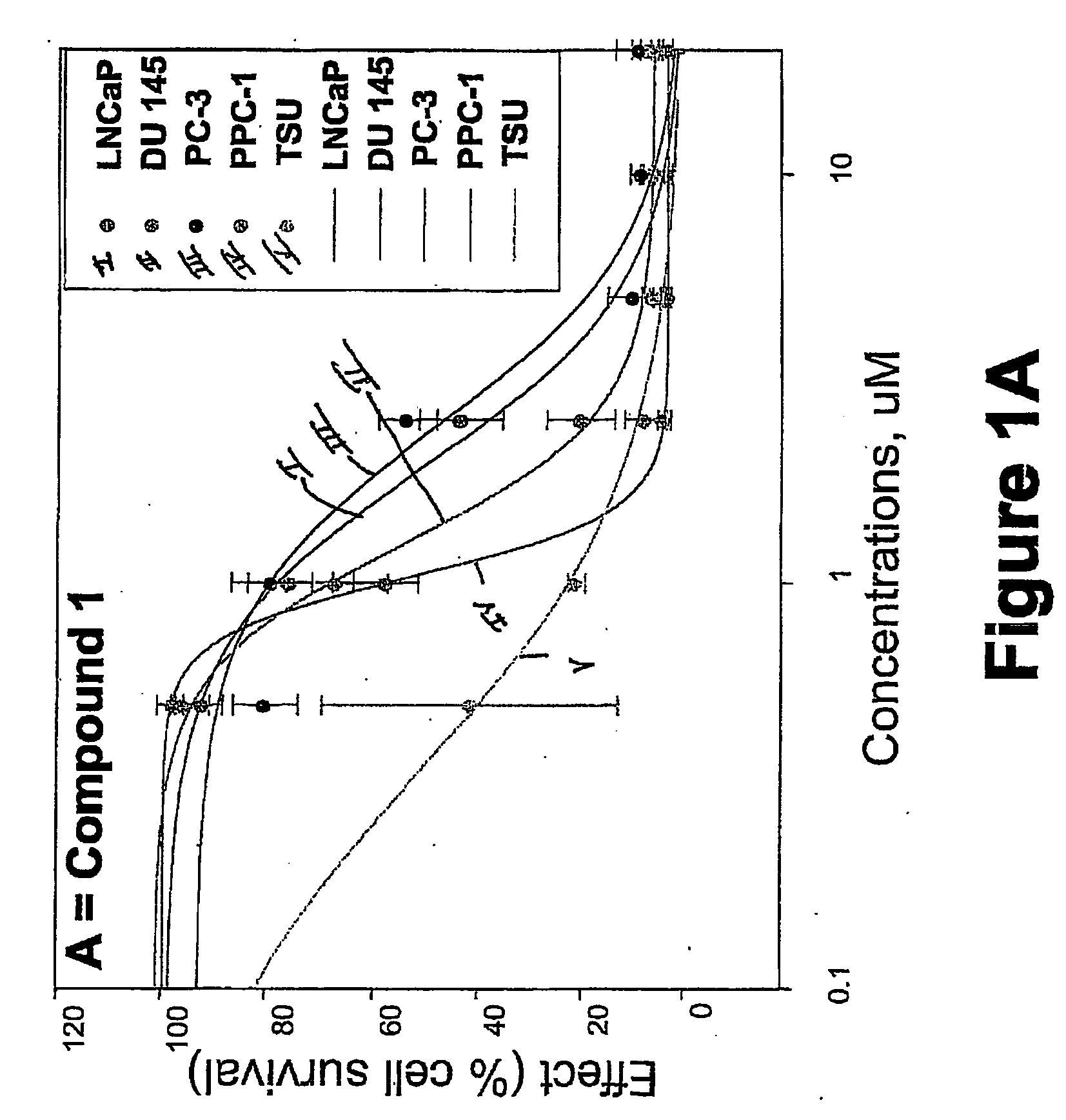
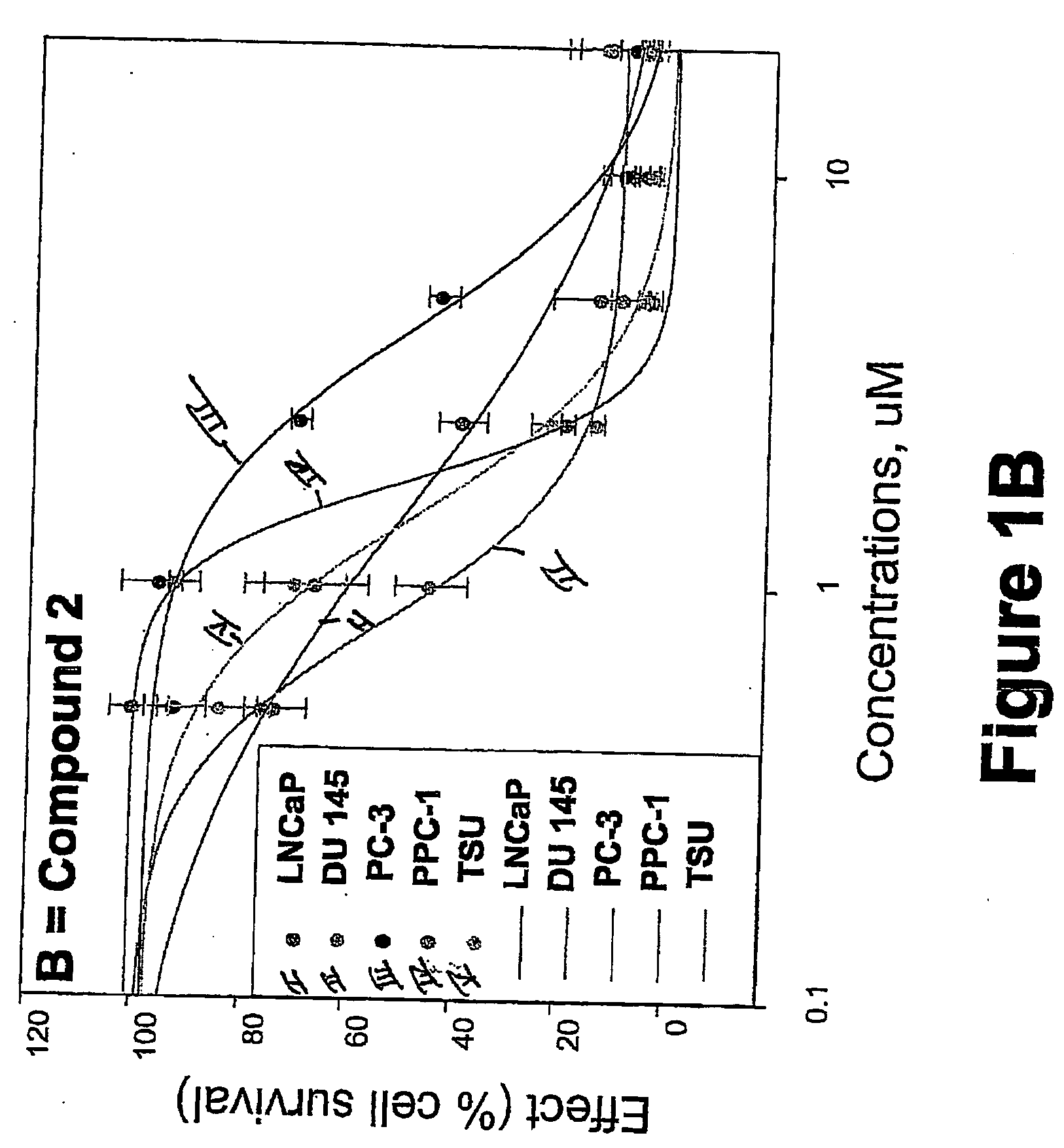
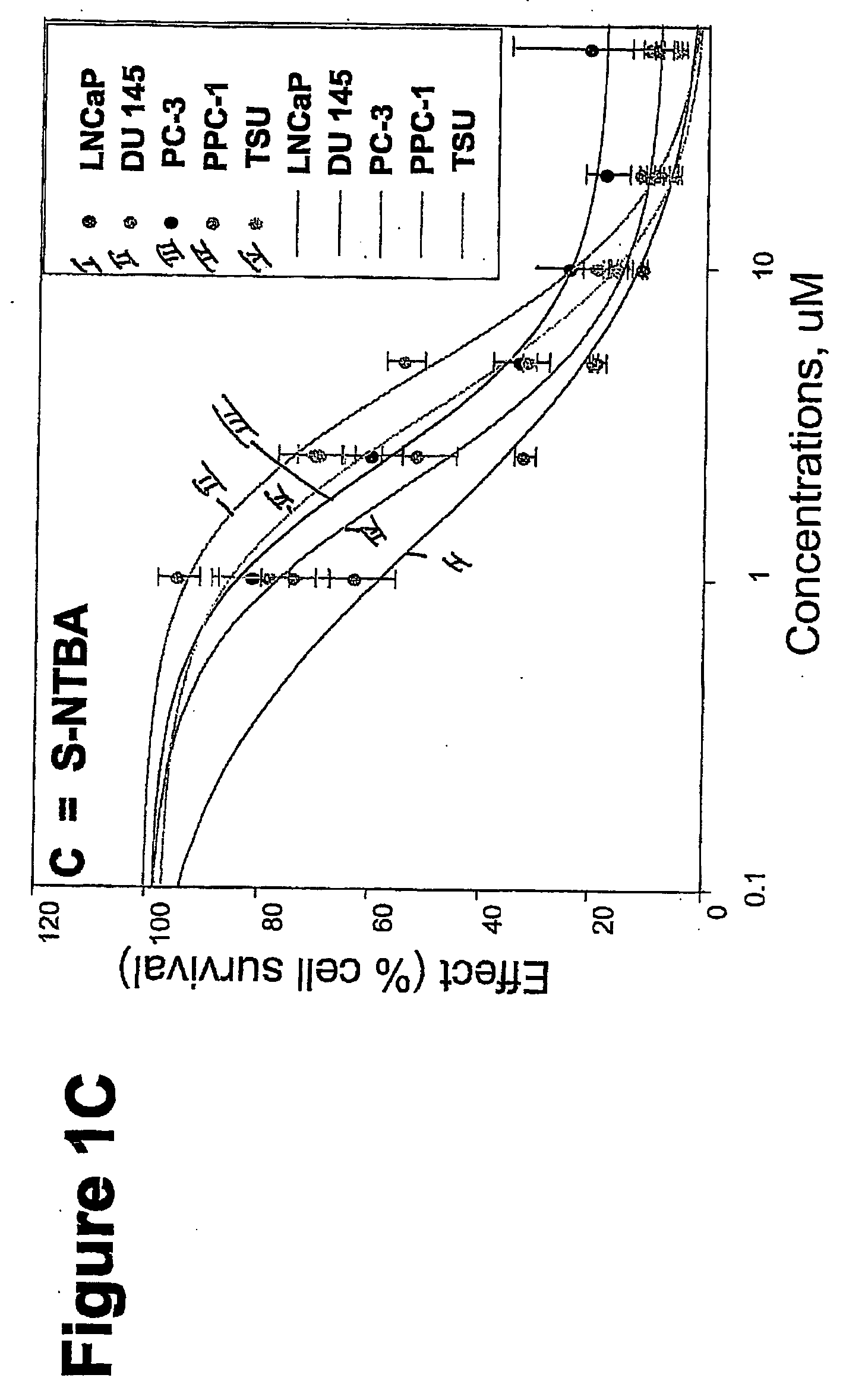
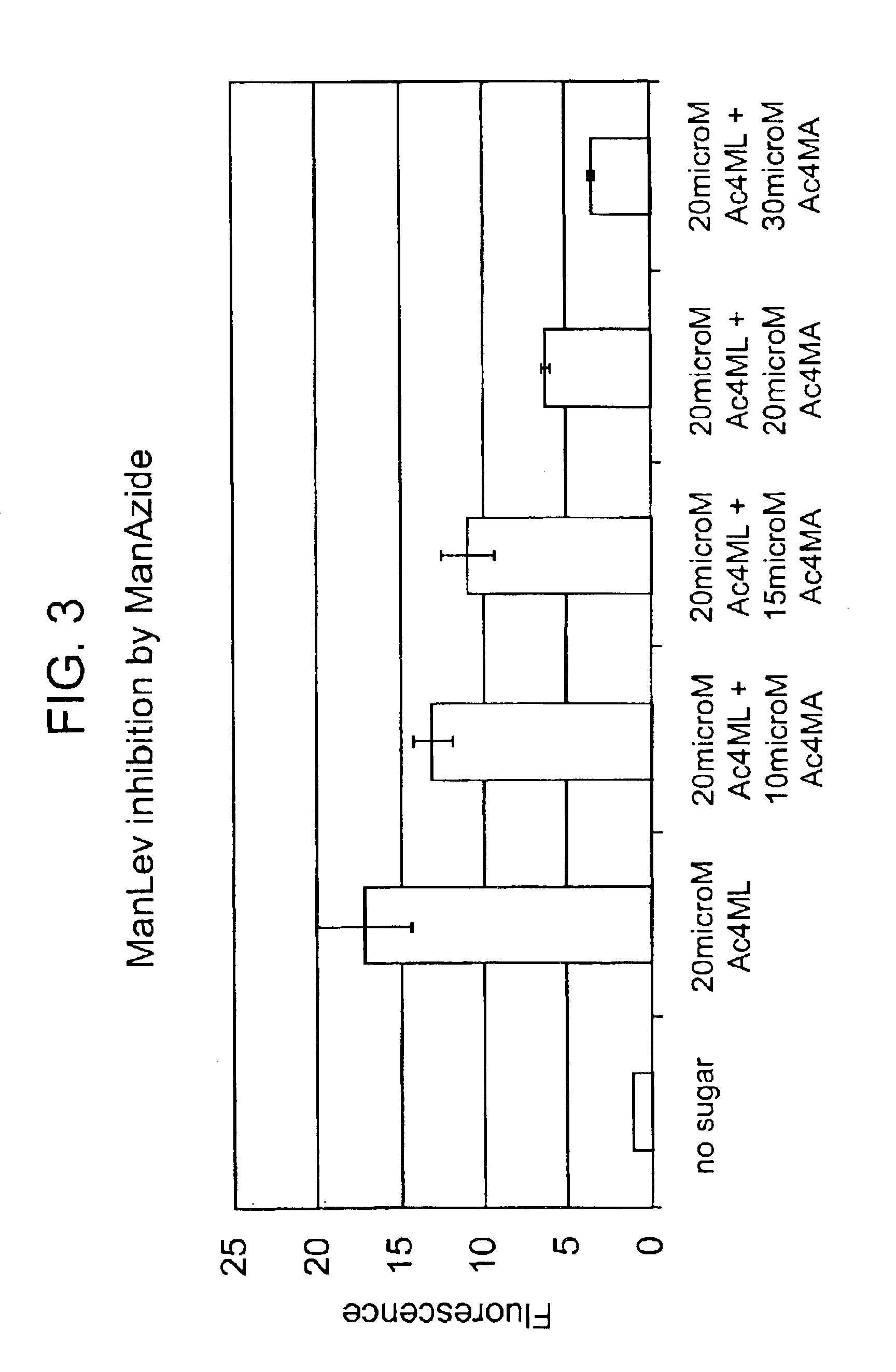
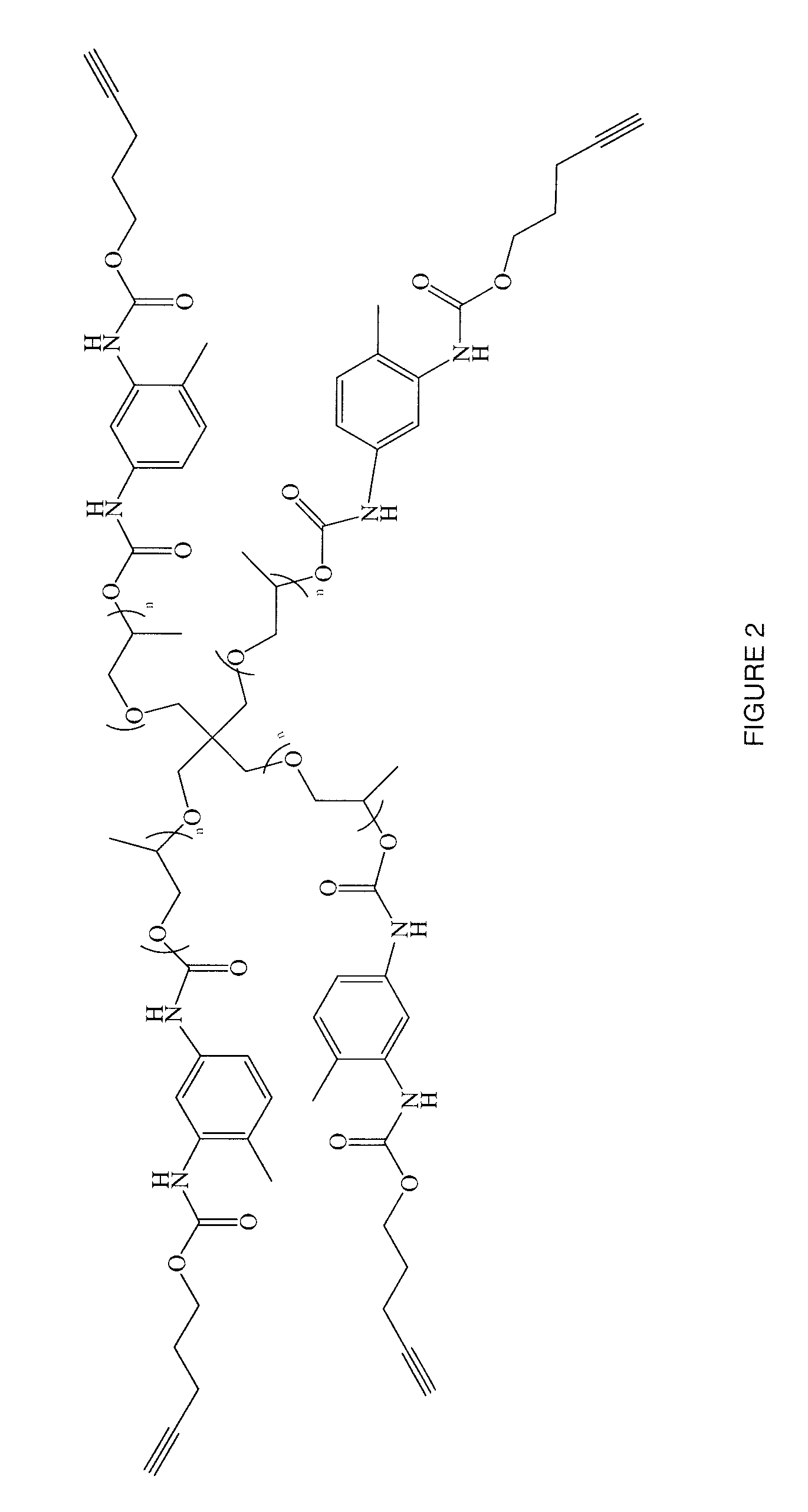
Targeted Imaging And/Or Therapy Using The [3+2] Azide-Alkyne Cycloaddition
InactiveUS20080267878A1NanomedicineRadioactive preparation carriersBiotin-streptavidin complexMolecular imaging
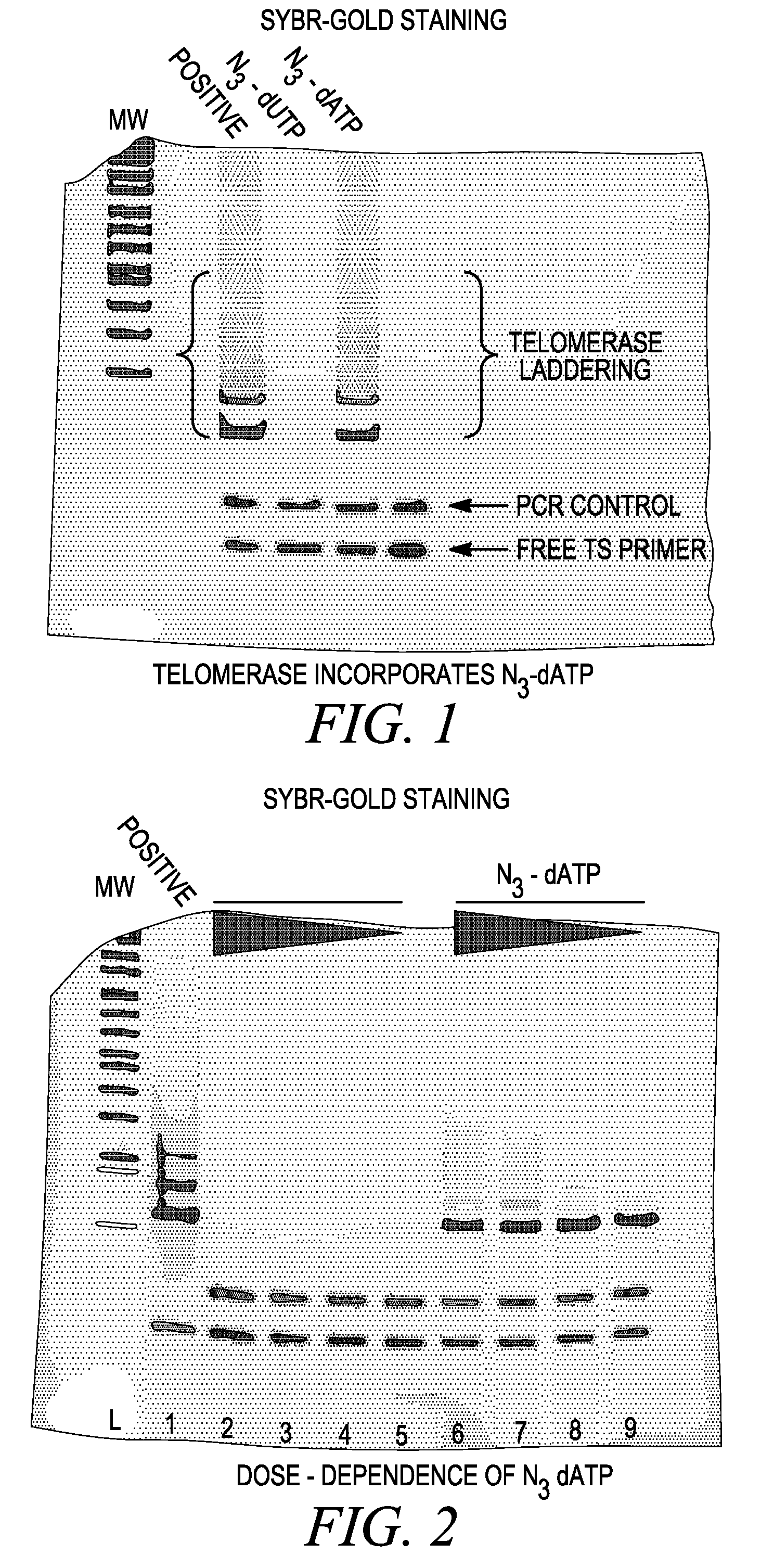
The use of a selective chemical and bioorthogonal reaction providing a covalent ligation such as the [3+2] cycloaddition, in targeted molecular imaging and therapy is presented, more specifically with interesting applications for pre-targeted imaging or therapy. Current pre-targeted imaging is hampered by the fact that it relies solely on natural / biological targeting constructs (biotin / streptavidin). Size considerations and limitations associated with their endogenous nature severely limit the number of applications. The present invention describes how the use of an abiotic, bio-orthogonal reaction which forms a stable adduct under physiological conditions, by way of a small or undetectable bond, can overcome these limitations.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Oligonucleotide ligation
ActiveUS20130046084A1Efficient methodImprove thermal stabilitySugar derivativesSugar derivatives preparationSynthesis methodsAlkyne
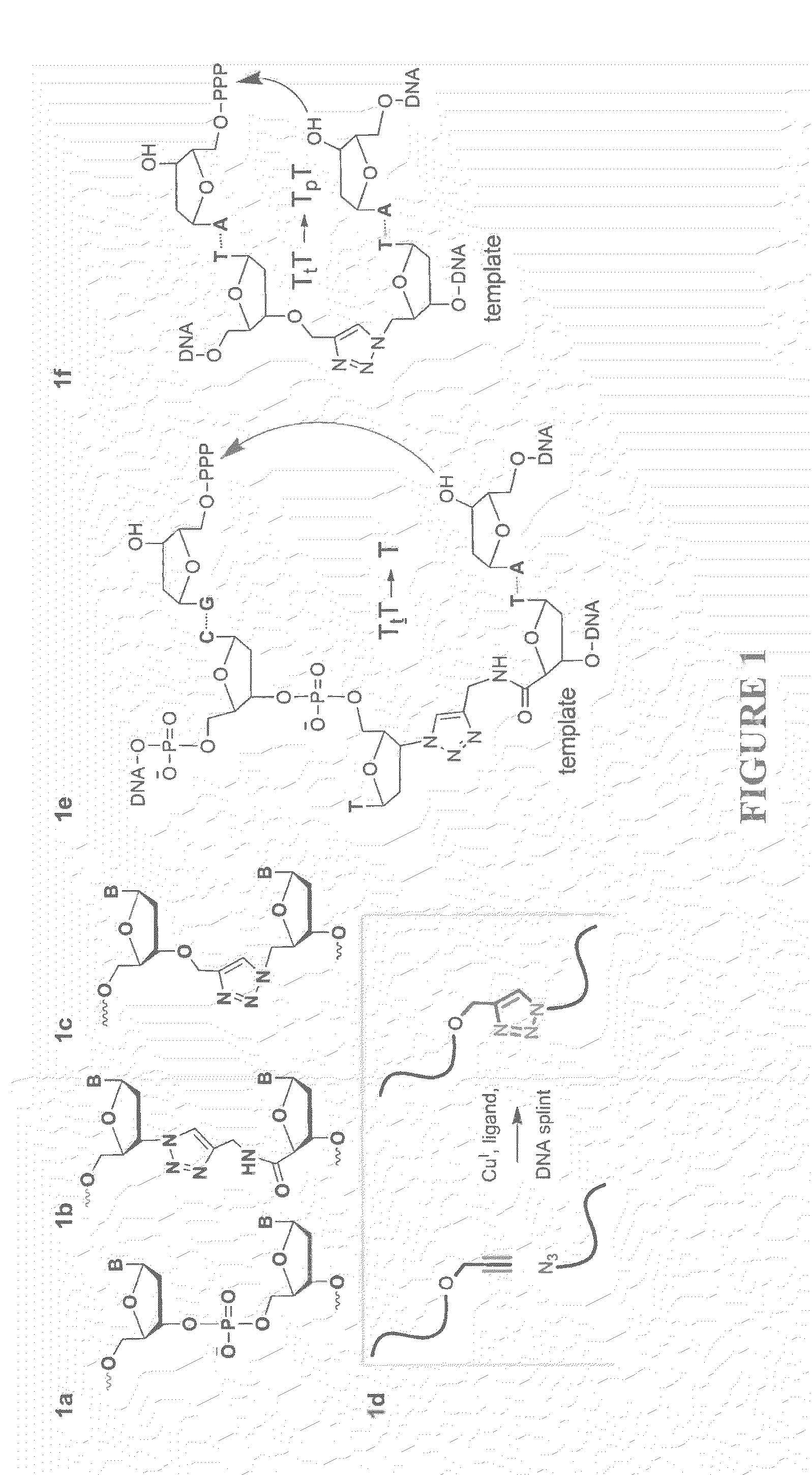
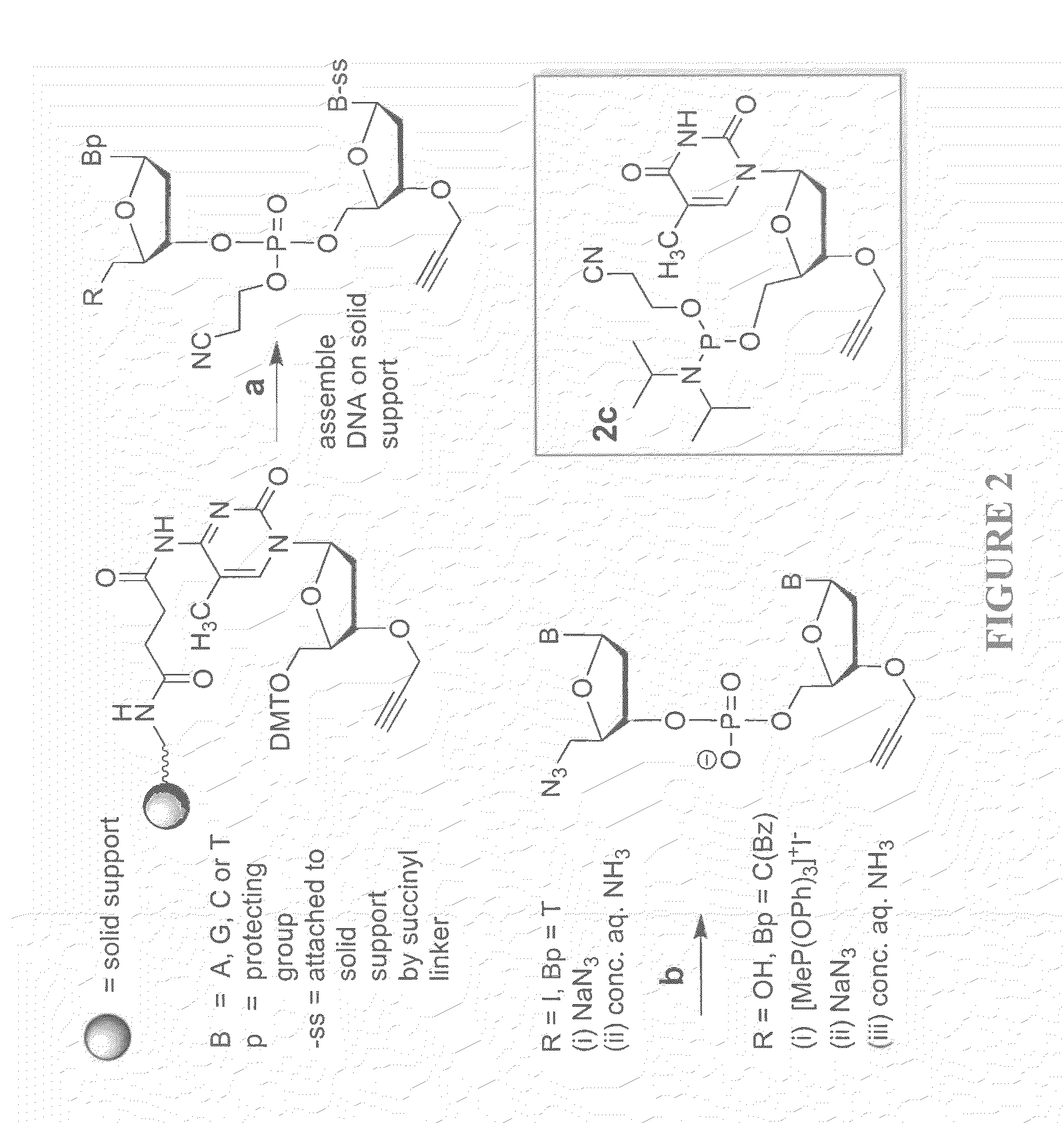
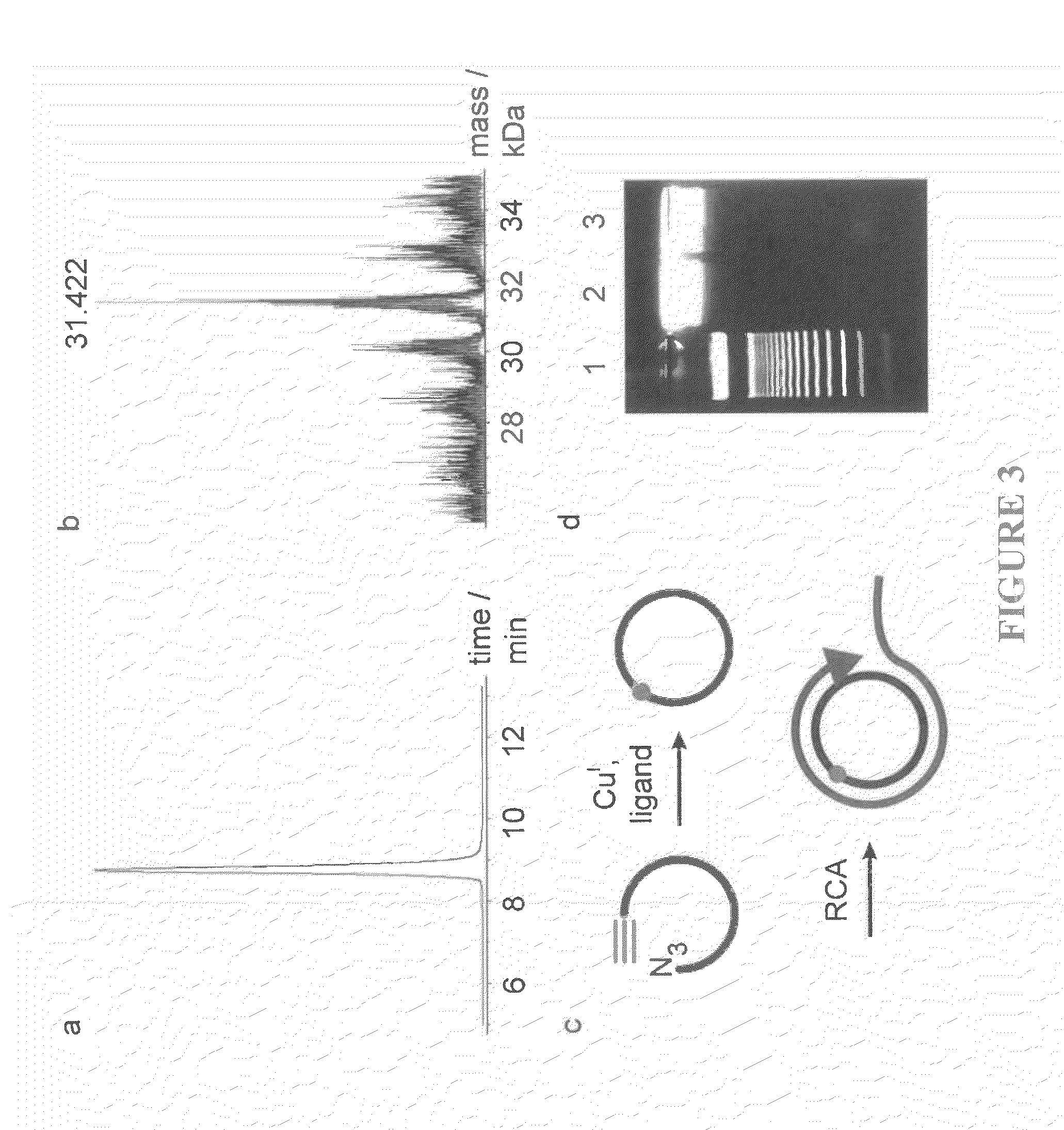
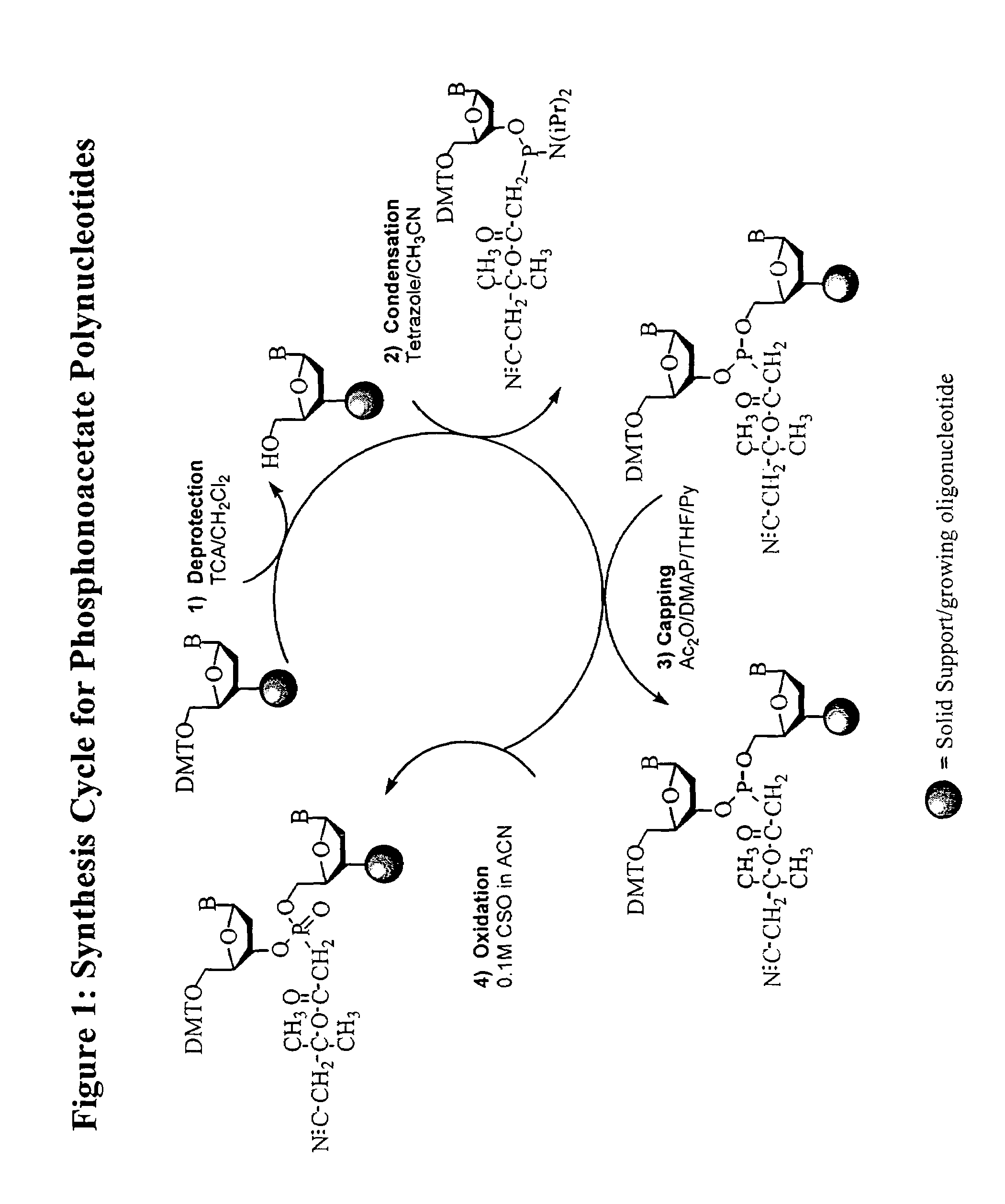
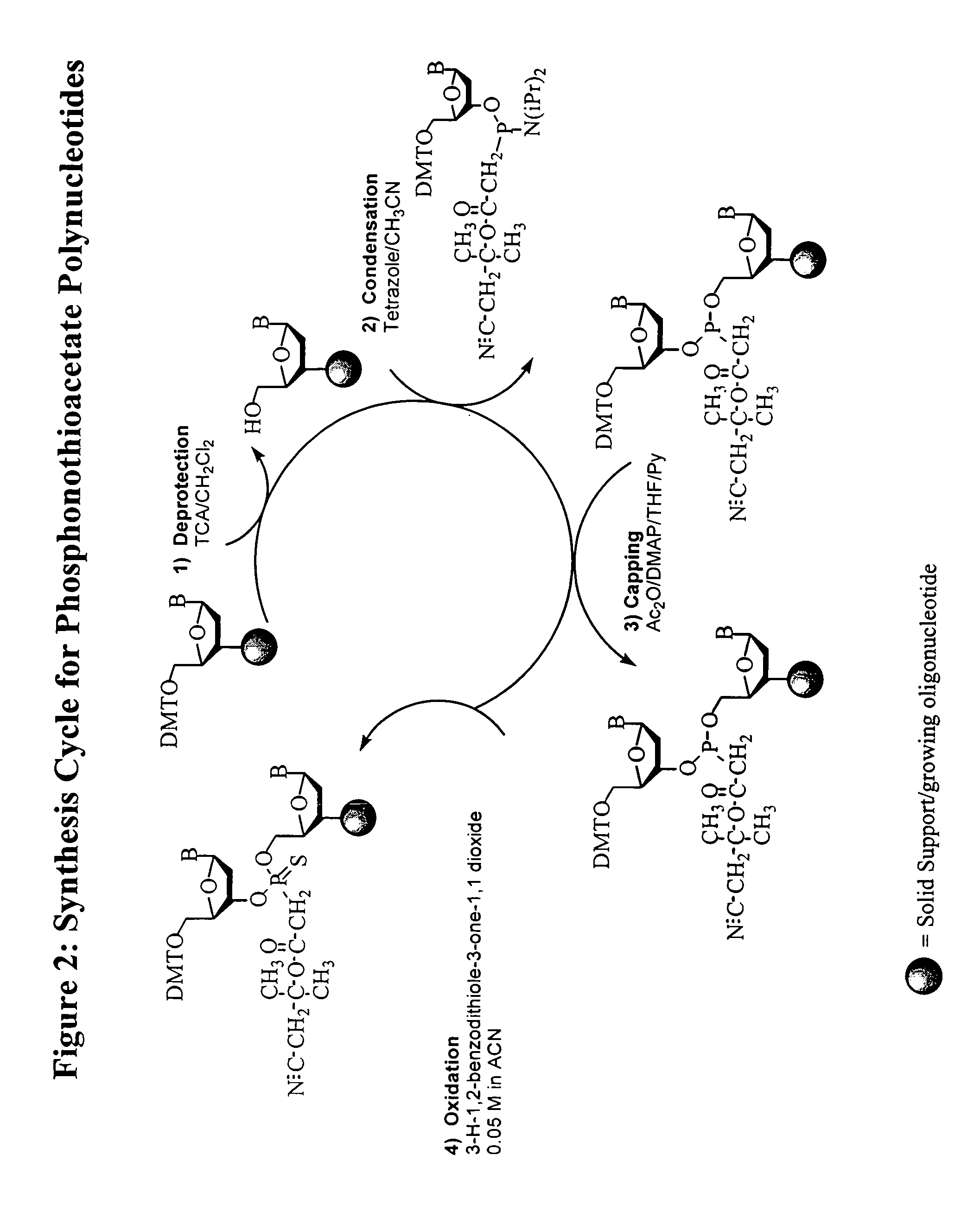
Oligonucleotide chemistry is central to the advancement of core technologies such as DNA sequencing, forensic and genetic analysis and has impacted greatly on the discipline of molecular biology. Oligonucleotides and their analogues are essential tools in these areas. They are often produced by automated solid-phase phosphoramidite synthesis but it is difficult to synthesize long DNA and RNA sequences by this method. Methods are proposed for ligating oligonucleotides together, in particular the use of an azide-alkyne coupling reaction to ligate the backbones of oligonucleotides together to form longer oligonucleotides that can be synthesized using current phosphoramidite synthesis methods.
Owner:ATDBIO LTD
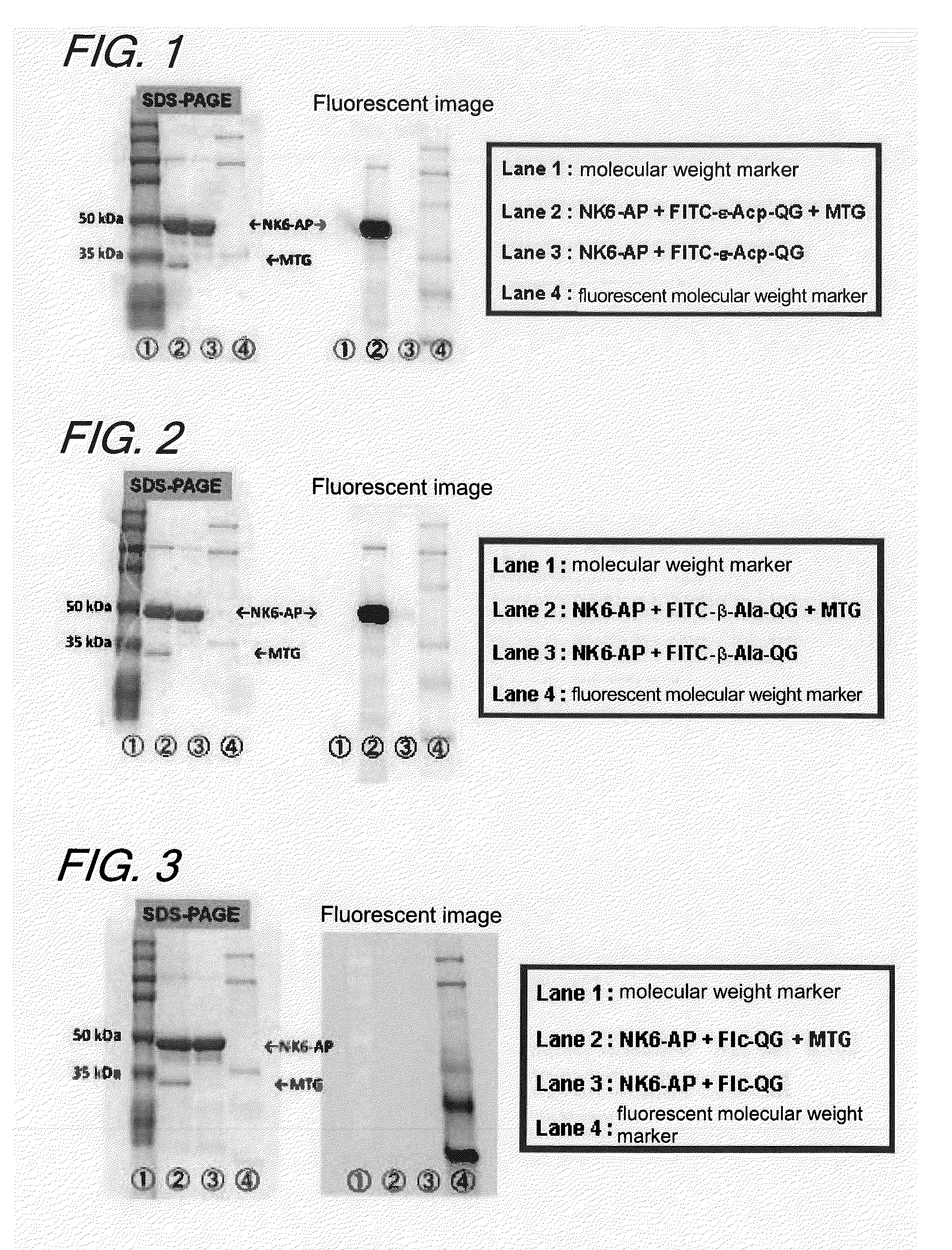
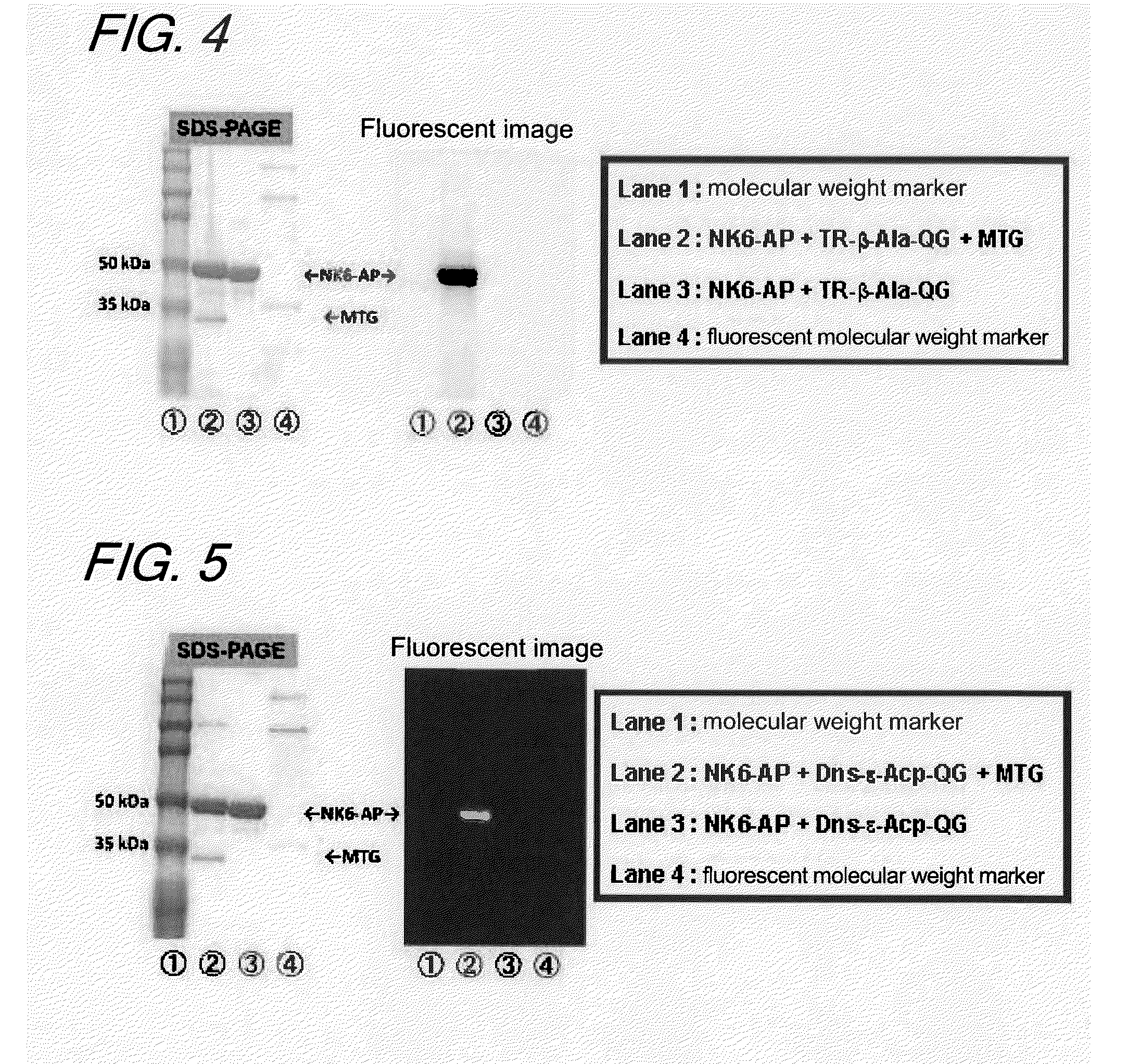
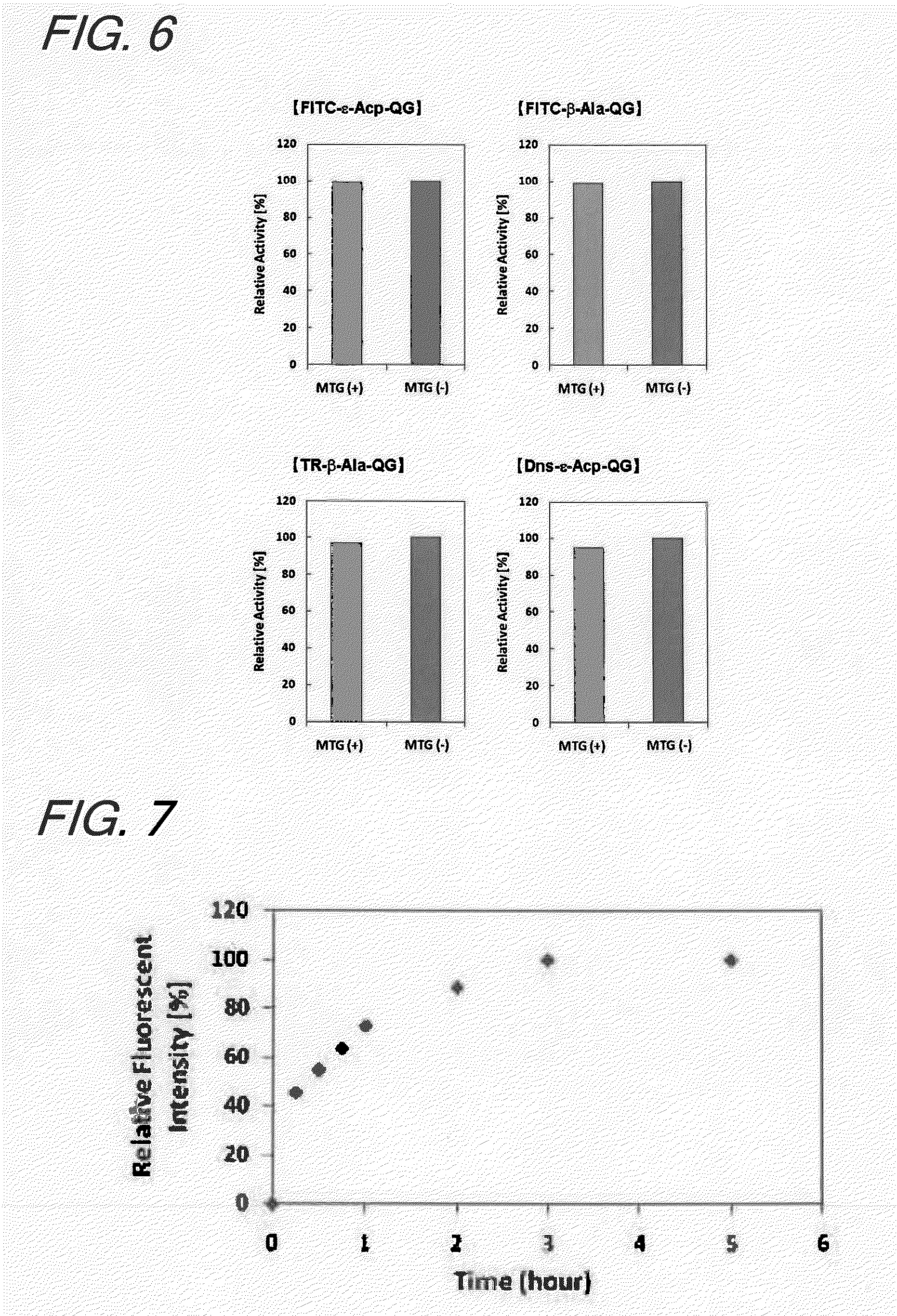
Enzyme substrate for labeling of protein
The present invention provides a substrate of TGase represented by (fluorescent group)-(linker)-(a portion containing a Gln residue capable of recognition by transglutaminase (TGase))-R, wherein the fluorescent group is fluorescein isothiocyanate (FITC), Texas Red (TE) or dansyl (Dns) or a group derived therefrom; the linker is a group represented by —NH—(CH2)n—CO— (n is an integer of 1 to 6); the portion containing a Gln residue capable of recognition by TGase is a group derived from a peptide selected from among QG and the like; and R is a hydroxyl group, or biotin, nucleic acid, azide, alkyne, maleimide or cyclopentadiene, or a group derived therefrom.
Owner:KYUSHU UNIV
Positive photosensitive polyimide resin composition
InactiveUS6875554B2Low viscosityDiazo compound compositionsPhotosensitive materials for photomechanical apparatusOrganic groupPolyamic acid
A positive photosensitive polyimide resin composition comprising:(a) a solvent-soluble polyimide comprised of from 1 to 100 mol % of a bivalent organic group derived from a diamine, that has at least one solvent solubilizing functional group, the polyimide having a reduced viscosity ranging from 0.05 to 5.0 dl / g,(b) a photosensitive orthoquinonediazide compound, and(c) from 0.1 to 50 wt %, based on the total weight of all polymers of the composition, of a component (c1) or (c2), wherein:component (c1) is a solvent-soluble polyimide comprised of from 1 to 100 mol % of a bivalent organic group derived from a diamine, that has at least one functional group selected from the group consisting of a long chain alkyl group having at least 6 carbon atoms and a fluorinated alkyl group, the polyimide having a reduced viscosity ranging from 0.05 to 5.0 dl / g, andcomponent (c2) is a polyamic acid comprised of from 1 to 100 mol % of a bivalent organic group derived from a diamine, that has at least one functional group selected from the group consisting of a long chain alkyl group having at least 6 carbon atoms and a fluorinated alkyl group, the polyimide having a reduced viscosity ranging from 0.05 to 5.0 dl / g.
Owner:NISSAN CHEM IND LTD
Cyclodextrin chiral chromatogram fixed phase and preparation method thereof
InactiveCN101306354AHigh column efficiencyHigh selectivityOther chemical processesAzirineChemical reaction
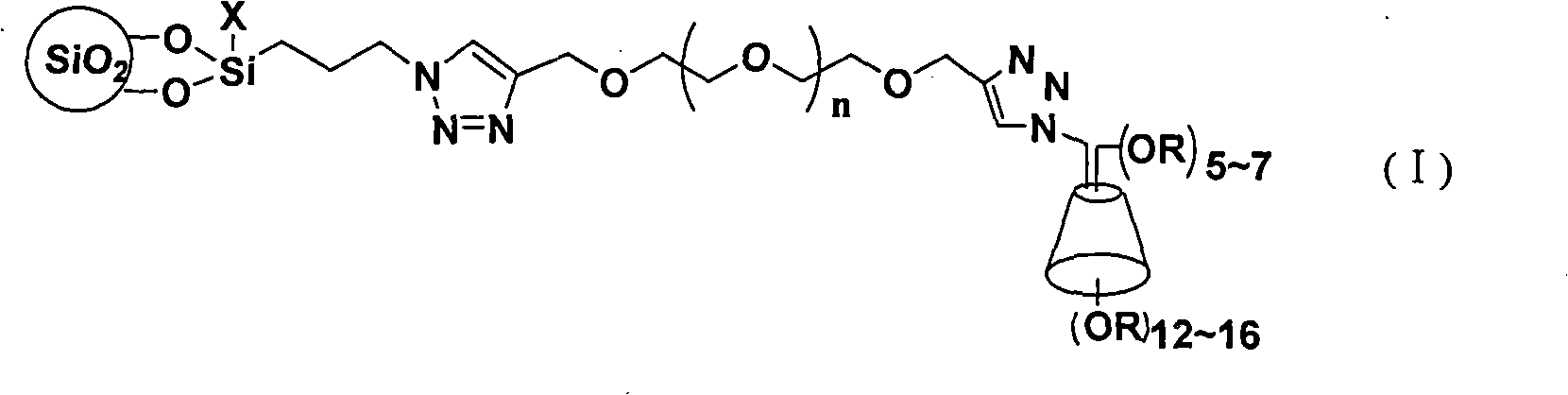
The invention discloses a cyclodextrin chiral stationary phase, the structure of which is shown in the general formula (I), wherein X is -OCH3 or -OCH2CH3, n is equal to 1-7, and R is -H, -CH3, -COCH3, -COC6H5 and -CONHC6H5. The preparation method of the stationary phase comprises the following steps: a silane coupling agent, sodium azide and a catalyst are added into an organic solvent, then spheroidal silicon is added for preparing azide silica gel derivant; oligomeric ethylene glycol, sodium hydride and propargyl bromide are added into tetrahydrofuran for preparing bialkynyl oligomeric ethylene glycol; monosubstituted nascent and derivative cyclodextrin containing azid groups is prepared; finally, the click chemistry reaction method is used for bonding the cyclodextrin. The cyclodextrin chiral stationary phase has the advantages that the selectivity of the bonding reaction is high, and the surface bonded amount is large; the chiral separation ability is strong, thereby being especially suitable for the chiral separation of a high efficiency liquid chromatography in the reversed-phase mode; the preparation method is simple and has less steps, the bonding reaction is the click chemistry reaction, the reaction condition is mild, and the reaction is carried out in the water solution.
Owner:EAST CHINA UNIV OF SCI & TECH
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




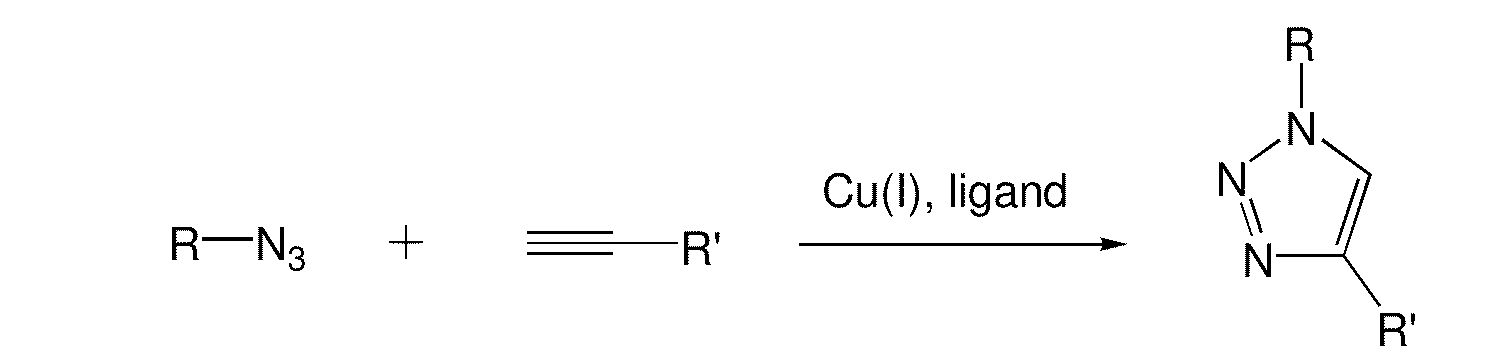






![Targeted Imaging And/Or Therapy Using The [3+2] Azide-Alkyne Cycloaddition Targeted Imaging And/Or Therapy Using The [3+2] Azide-Alkyne Cycloaddition](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f254bdb1-9433-4d65-8b75-909a30cbb9ed/US20080267878A1-20081030-D00000.png)
![Targeted Imaging And/Or Therapy Using The [3+2] Azide-Alkyne Cycloaddition Targeted Imaging And/Or Therapy Using The [3+2] Azide-Alkyne Cycloaddition](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f254bdb1-9433-4d65-8b75-909a30cbb9ed/US20080267878A1-20081030-D00001.png)
![Targeted Imaging And/Or Therapy Using The [3+2] Azide-Alkyne Cycloaddition Targeted Imaging And/Or Therapy Using The [3+2] Azide-Alkyne Cycloaddition](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f254bdb1-9433-4d65-8b75-909a30cbb9ed/US20080267878A1-20081030-D00002.png)