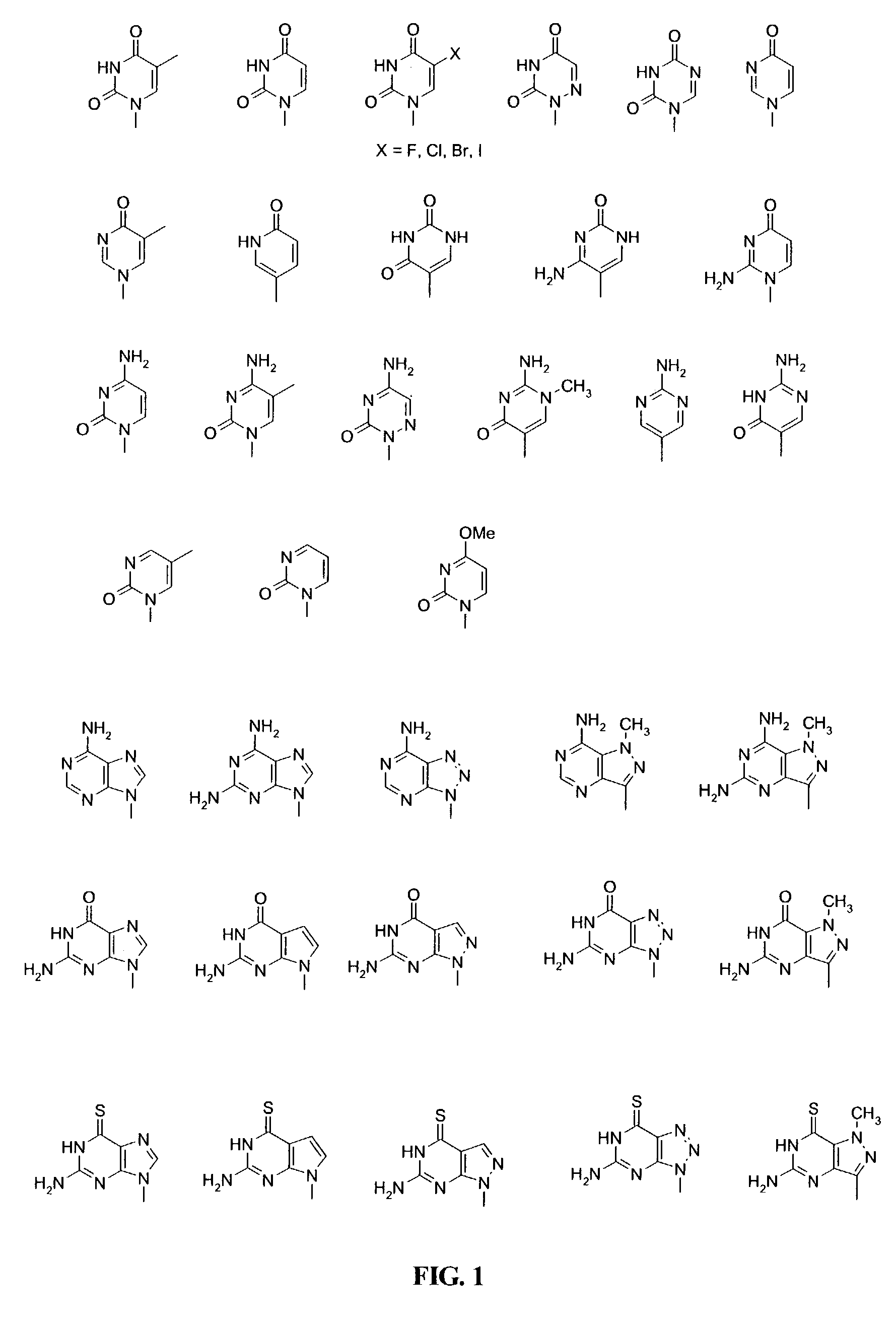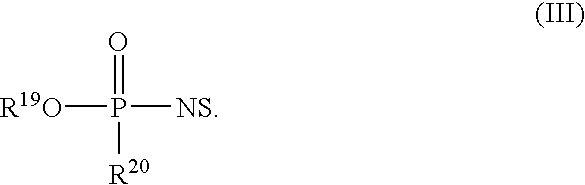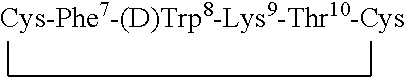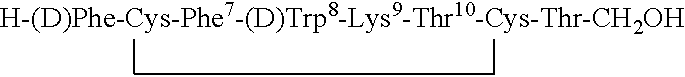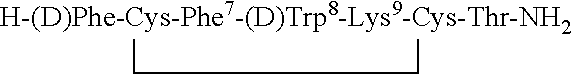Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3922 results about "Protecting group" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
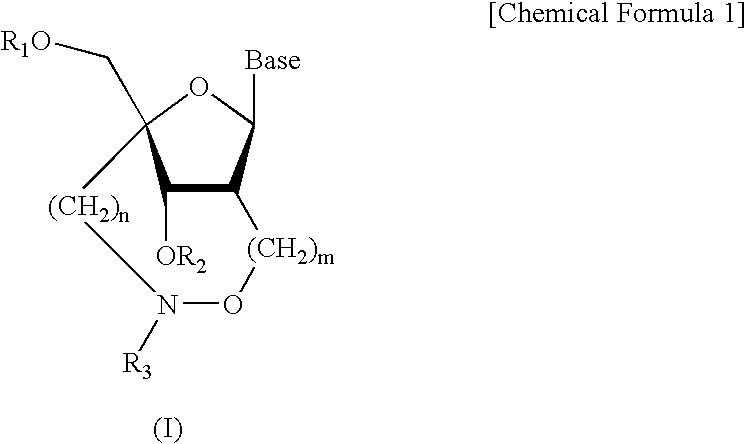
Artificial nucleic acids of n-o bond crosslinkage type
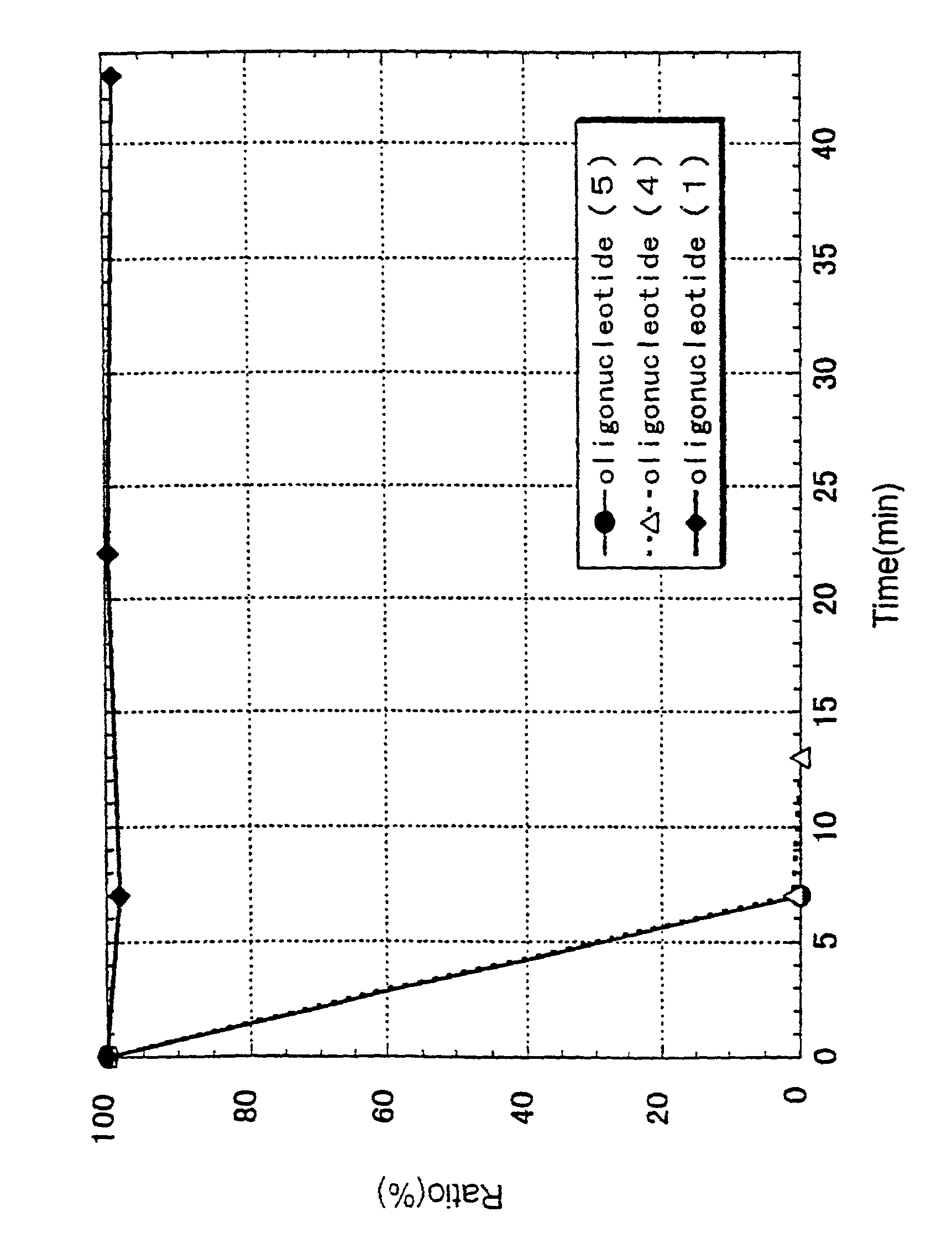
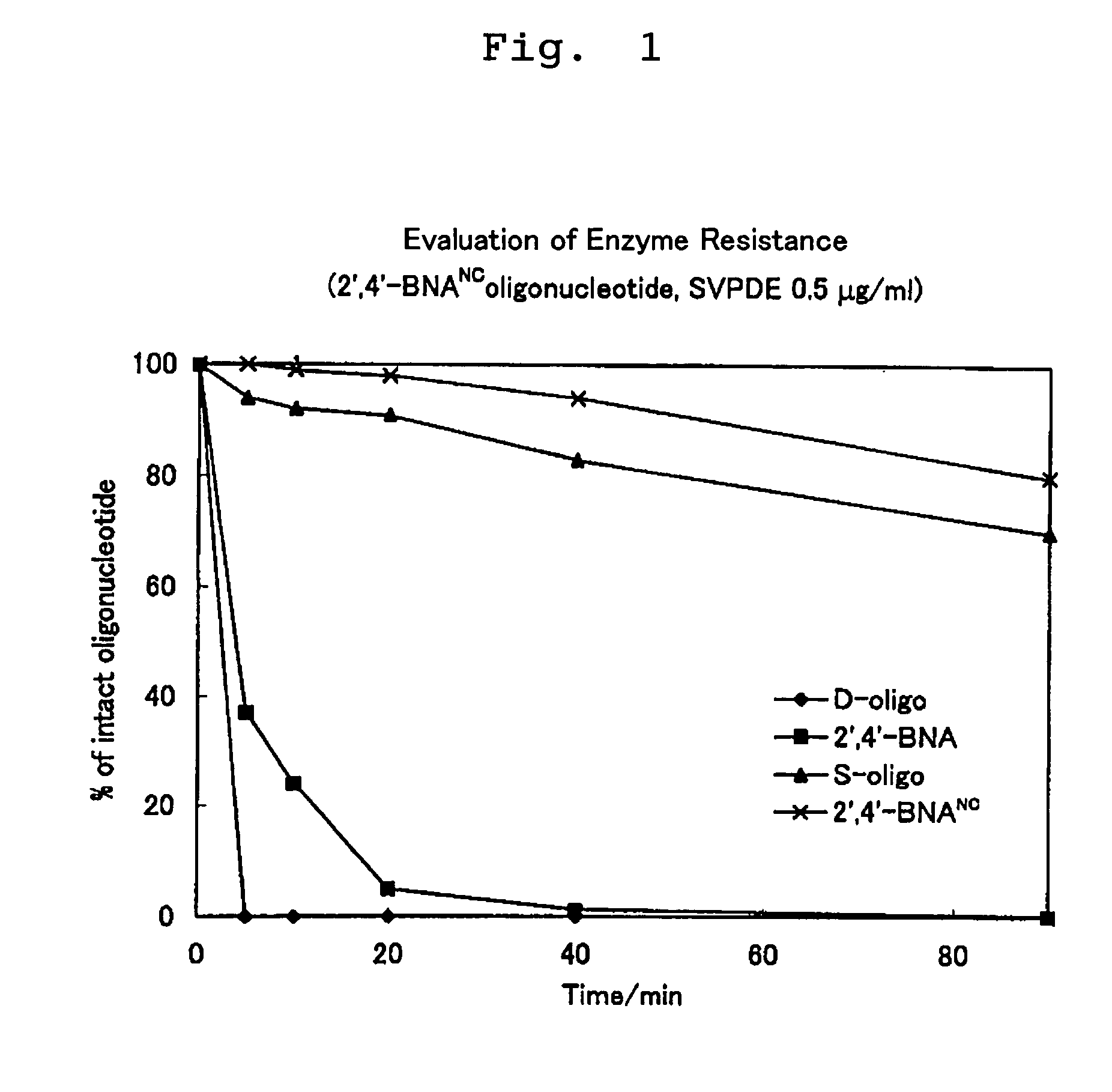
ActiveUS7427672B2High sensitivity analysisConfirmed its usefulnessBiocideSugar derivativesSingle strandDouble strand
Owner:RIKEN GENESIS
Polymer sacrificial light absorbing structure and method
InactiveUS6876017B2From solid stateSemiconductor/solid-state device detailsSolubilityLithographic artist
Method and structure for optimizing dual damascene patterning with polymeric dielectric materials are disclosed. Certain embodiments of the invention comprise polymeric sacrificial light absorbing materials (“polymer SLAM”) functionalized to have a controllable solubility switch wherein such polymeric materials have substantially the same etch rate as conventionally utilized polymeric dielectric materials, and subsequent to chemical modification of solubility-modifying protecting groups comprising the SLAM materials by thermal treatment or in-situ generation of an acid, such SLAM materials become soluble in weak bases, such as those conventionally utilized to remove materials in lithography treatments.
Owner:INTEL CORP
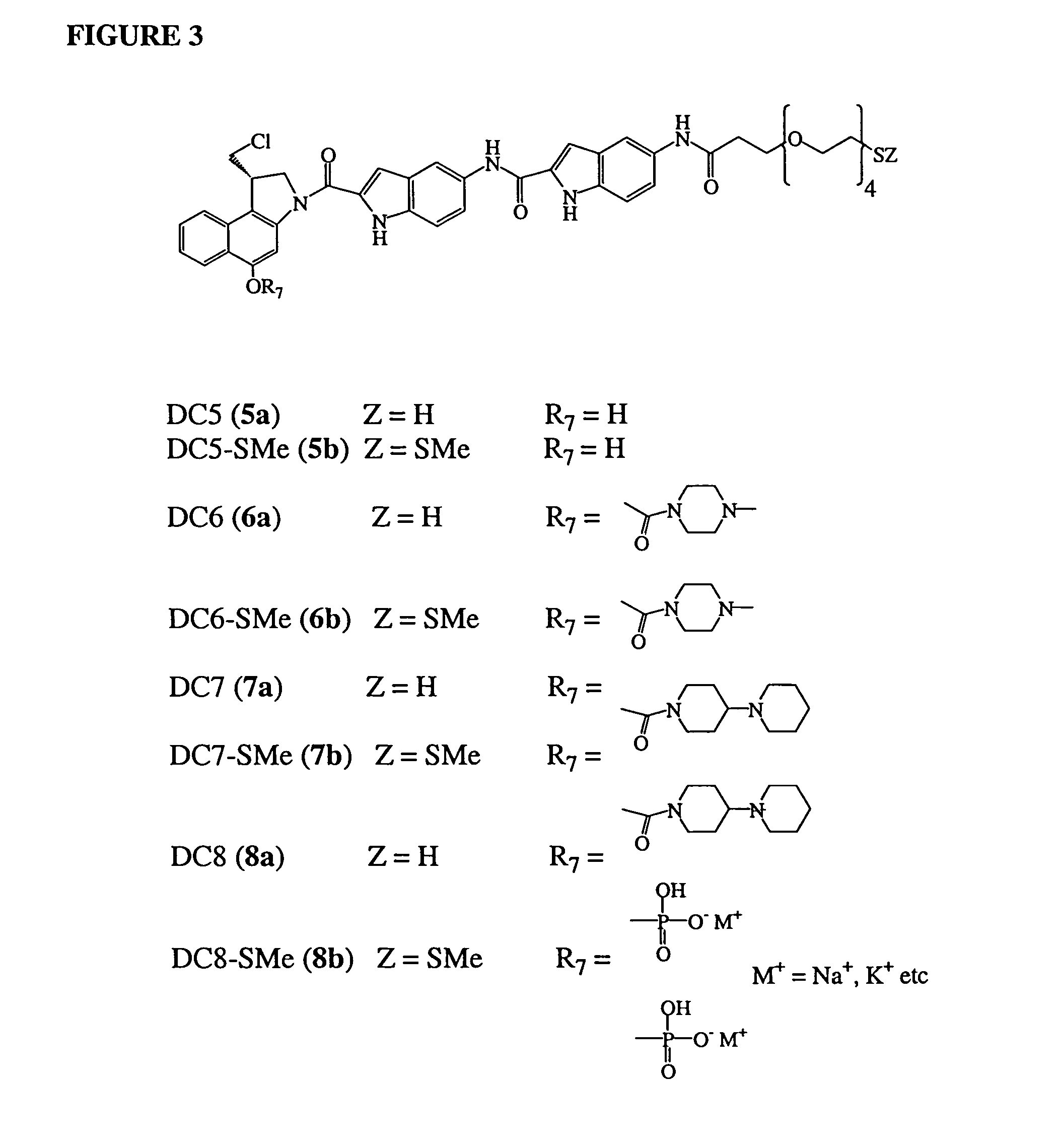
Pyrrolobenzodiazepines as key intermediates in the synthesis of dimeric cytotoxic pyrrolobenzodiazepines
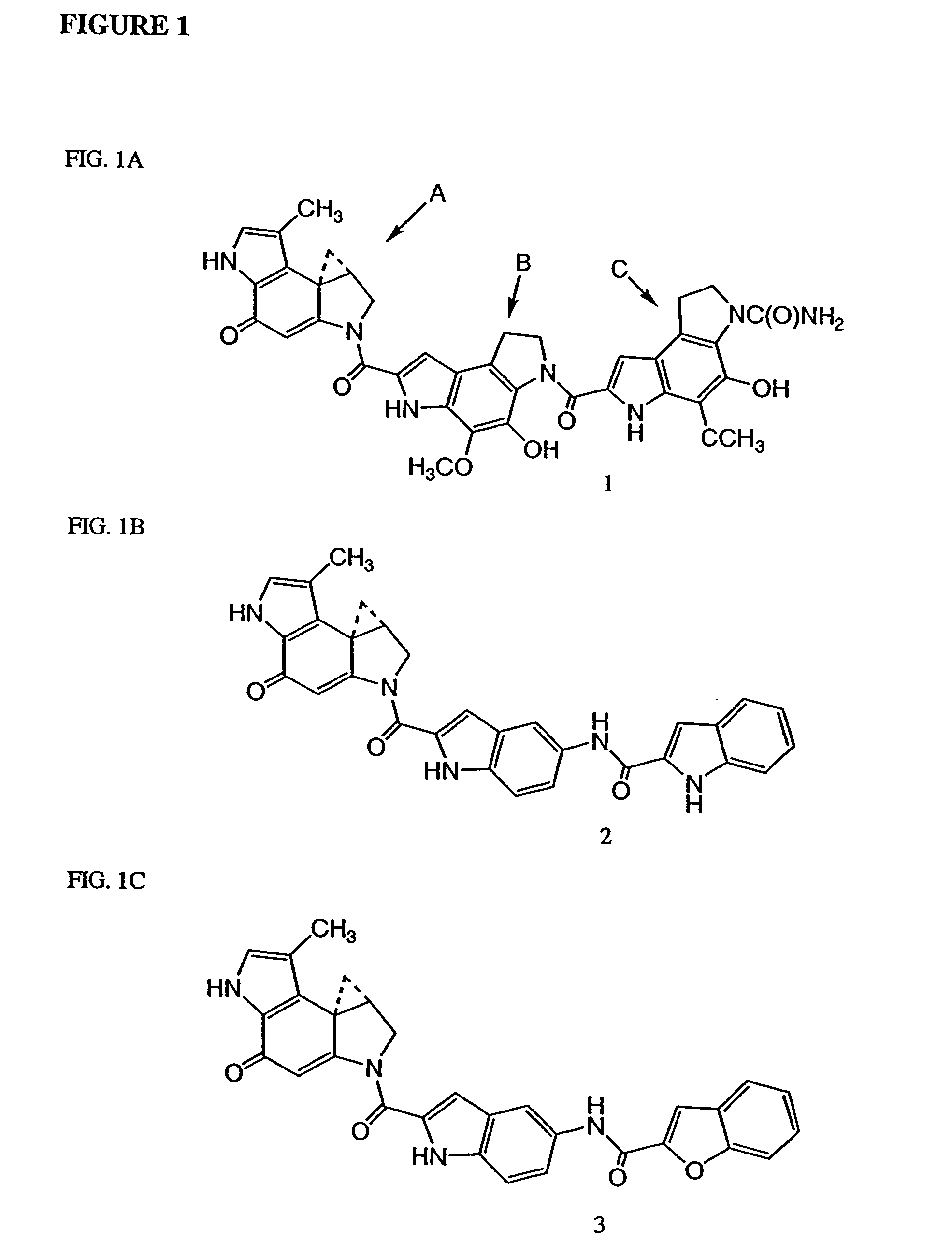
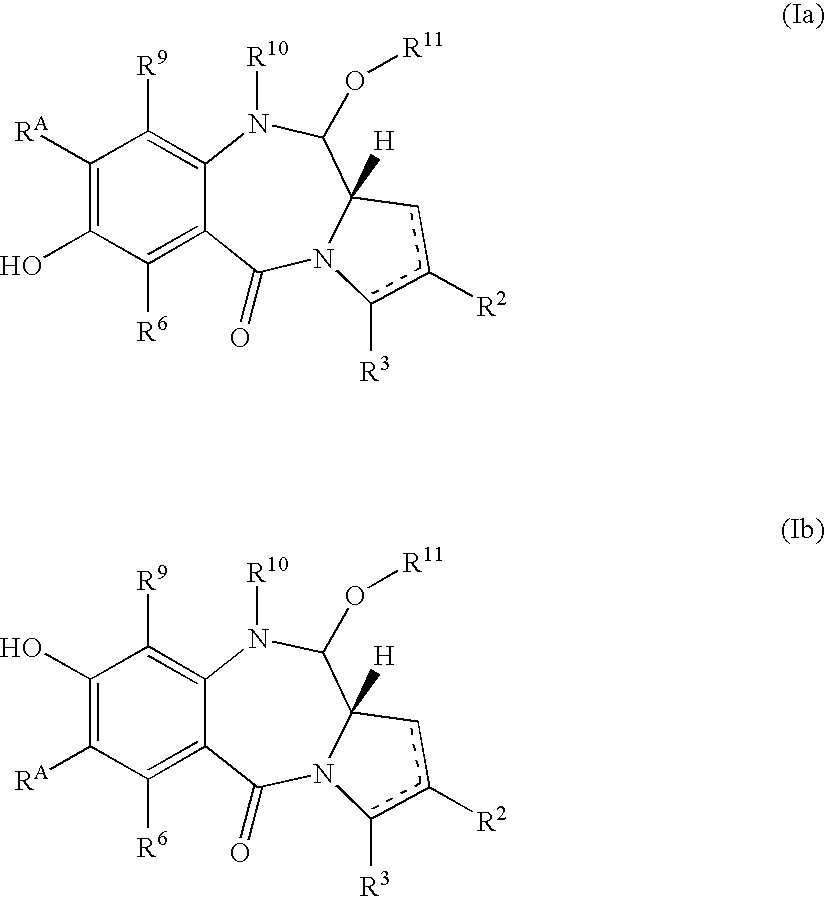
Compounds and a method of synthesis of compounds of formula (Ia) or (Ib): and salts, solvates, and chemically protected forms thereof, wherein the dotted lines indicate the optional presence of a double bond between C1 and C2 or C2 and C3; R2 and R3 are independently selected from —H, ═O, ═CH2, —CN, —R, OR, halo, ═CH—R, O—SO2—R, CO2R and COR; R10 is a carbamate-based nitrogen protecting group; and R11 is an oxygen protecting group.
Owner:MEDIMMUNE LTD
Pyrrolobenzodiazepines
A compound of formula (I) and salts and solvates thereof, wherein: R10 is a nitrogen protecting group and R11 is either OH or O—R12, wherein R12 is an oxygen protecting group, or R10 and R11 together form a double bond between N10 and C11; and R10′ and R11′ are selected from the same options as R10 and R11 respectively.
Owner:MEDIMMUNE LTD
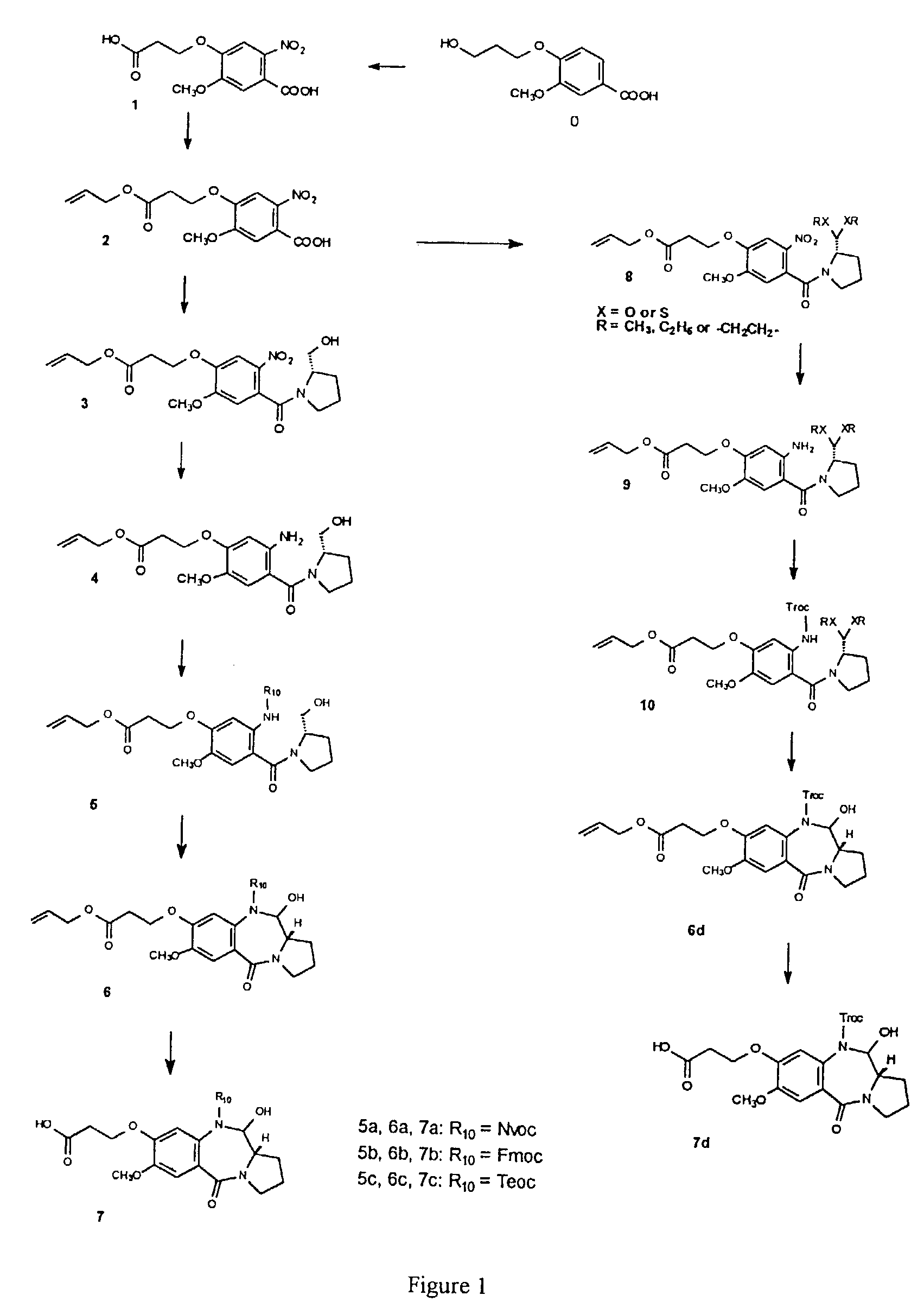
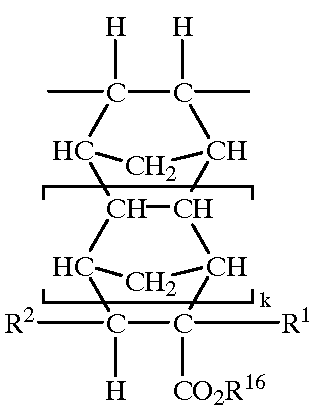
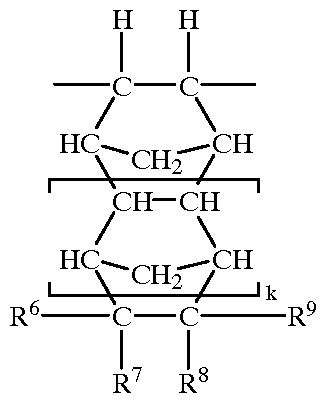
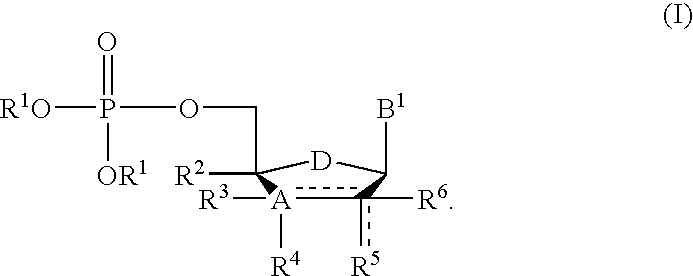
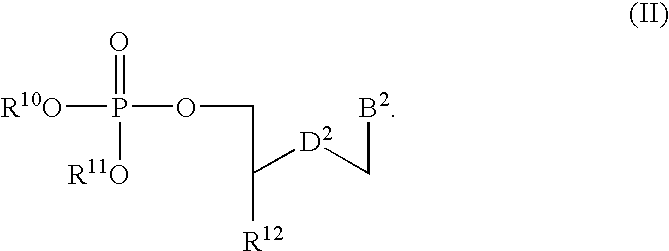
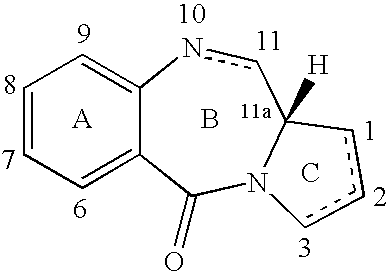
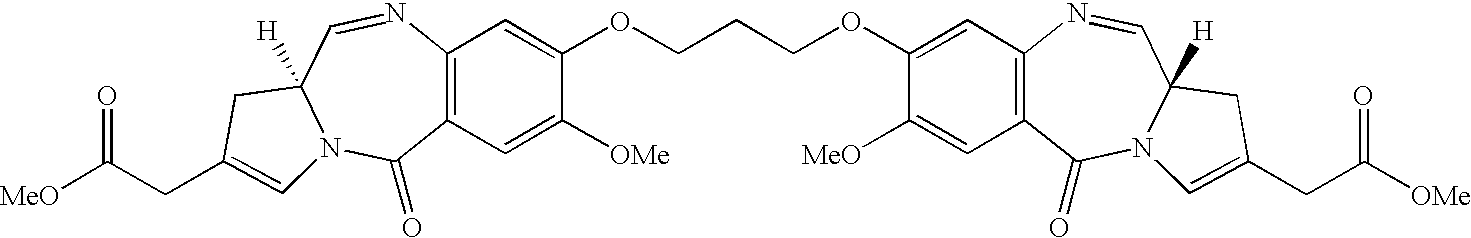
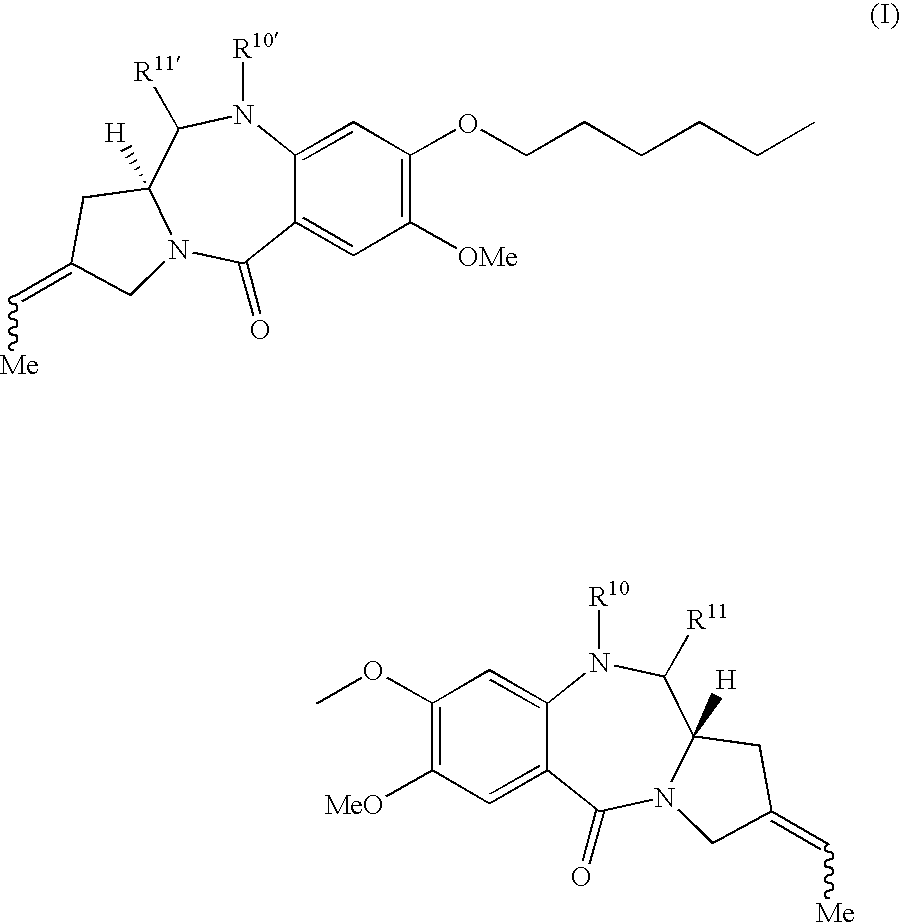
11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines
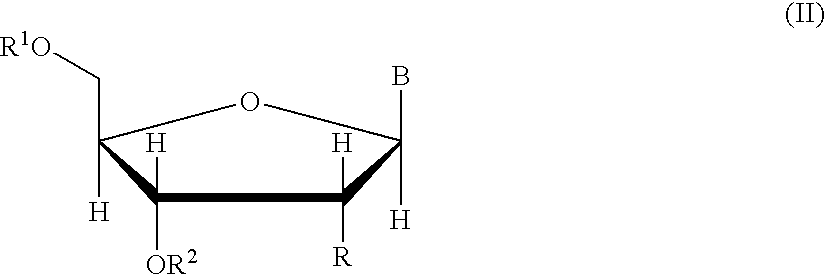
The present inventors have developed a key intermediate for the production of C2 substituted PBDs, which has a leaving group at the C2 position, a carbamate protecting group at the N10 position and a protected hydroxy group at the C11 position. In a first aspect, the present invention comprises a compound with a the formula (I), wherein: R10 is a carbamate-based nitrogen protecting group; R11 is an oxygen protecting group; and R2 is a labile leaving group. In a further aspect, the present invention comprises a method of synthesising a compound of formula (III), or a solvate thereof, from a compound of formula (I) as defined in the first aspect, R16 is either O—R11, wherein R11 is as defined in the first aspect, or OH, or R10 and R16 together form a double bond between N10 and C11; and R15 is R. The other substituents are defined in the claims. Further aspects of the present invention relate to compounds of formula (III) (including solvates thereof when R10 and R16 form a double bond between N10 and C11, and pharmaceutical salts thereof), pharmaceutical compositions comprising these, and their use in the manufacture of a medicament for the treatment of a proliferative disease.
Owner:MEDIMMUNE LTD
Nucleoside and oligonucleotide analogues
InactiveUS7335765B2Reduce incidenceImprove stabilityOrganic active ingredientsAntipyreticHydrogen atomHalogen
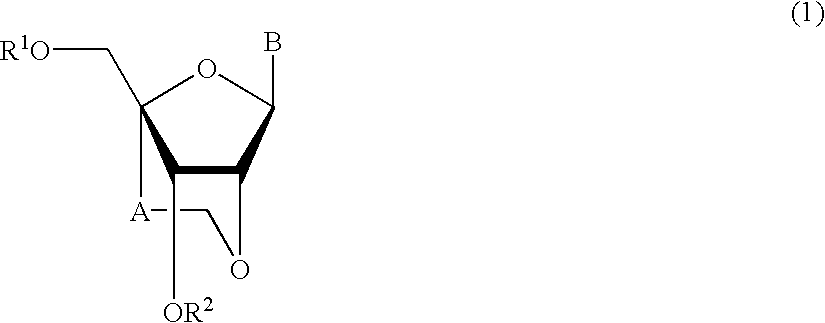
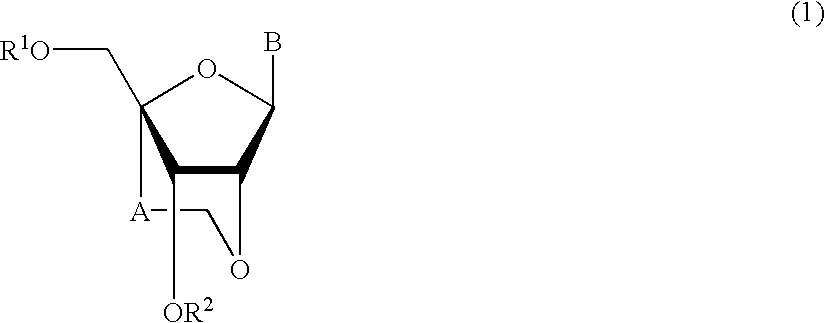
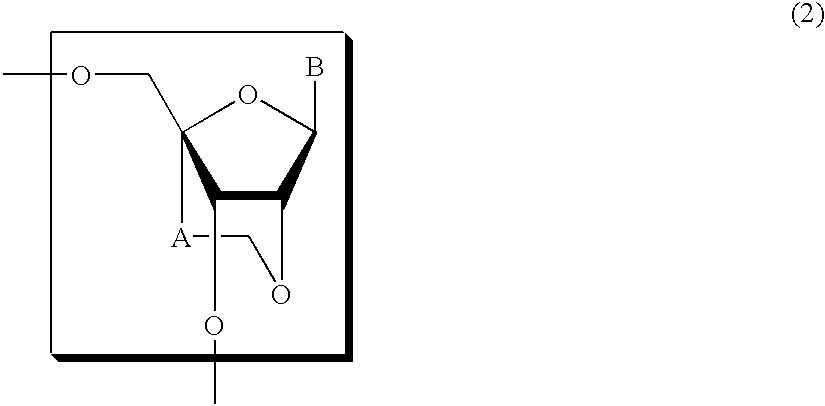
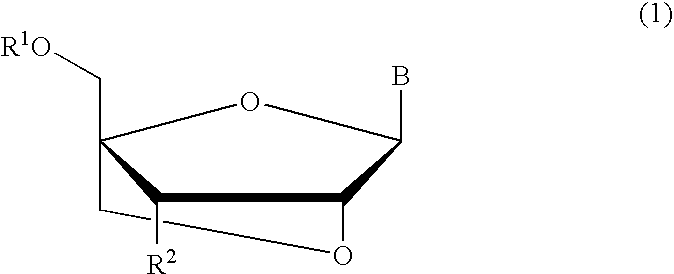
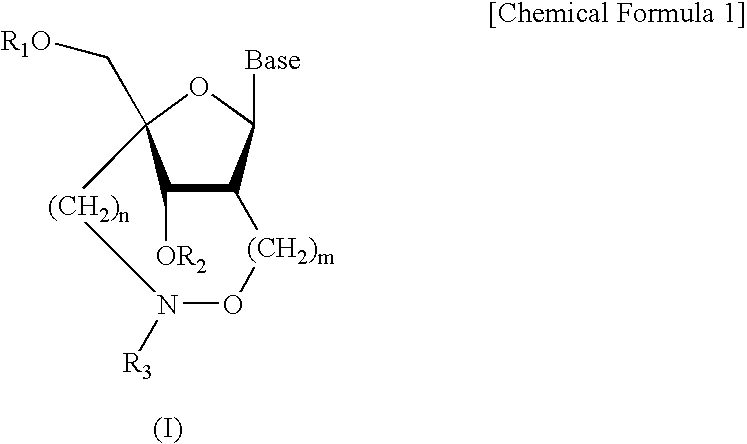
A compound of the formula (1):wherein R1 and R2 are the same or different and represent a hydrogen atom, a hydroxyl protecting group, a phosphate group, or —P(R3)R4, wherein R3 and R4 are the same or different and represent a hydroxyl group, an amino group, an alkoxy group having from 1 to 4 carbon atoms, a cyanoalkoxy group having from 1 to 5 carbon atoms or an amino group substituted by an alkyl group having from 1 to 4 carbon atoms; A represents an alkylene group having from 1 to 4 carbon atoms and B represents a purin-9-yl group, a 2-oxo-pyrimidin-1-yl group, a substituted purin-9-yl group or a substituted 2-oxo-pyrimidin-1-yl group having a substituent α selected from the group consisting of a hydroxyl group which may be protected, an alkoxy group having from 1 to 4 carbon atoms, a mercapto group which may be protected, an alkylthio group having from 1 to 4 carbon atoms, an alkoxy group having from 1 to 4 carbon atoms, an amino group which may be protected, a mono- or di-alkylamino group which may be substituted by an alkyl group having from 1 to 4 carbon atoms, an alkyl group having from 1 to 4 carbon atoms and a halogen atom; or a salt thereof.
Owner:DAIICHI SANKYO CO LTD
PNA monomer and precursor
InactiveUS7022851B2Improve efficiencyImprove convenienceSugar derivativesPeptide/protein ingredientsBase JOligomer
This application relates to monomers of the general formula (I) for the preparation of PNA (peptide nucleic acid) oligomers and provides method for the synthesis of both predefined sequence PNA oligomers and random sequence PNA oligomers: whereinR1, R2, R3, R4, R5 is independently H, halogen, C1–C4 alkyl, nitro, nitrile, C1–C4 alkoxy, halogenated C1–C4 alkyl, or halogenated C1–C4 alkoxy, wherein at least one of R1, R3, and R5 is nitro;R6 is H or protected or unprotected side chain of natural or unnatural α-amino acid; andB is a natural or unnatural nucleobase, wherein when said nucleobase has an exocyclic amino function, said function is protected by protecting group which is labile to acids but stable to weak to medium bases.
Owner:PANAGENE INC
PNA monomer and precursor
ActiveUS7211668B2Improve efficiencyImprove convenienceOrganic active ingredientsSugar derivativesSide chainNucleobase
Owner:PANAGENE INC
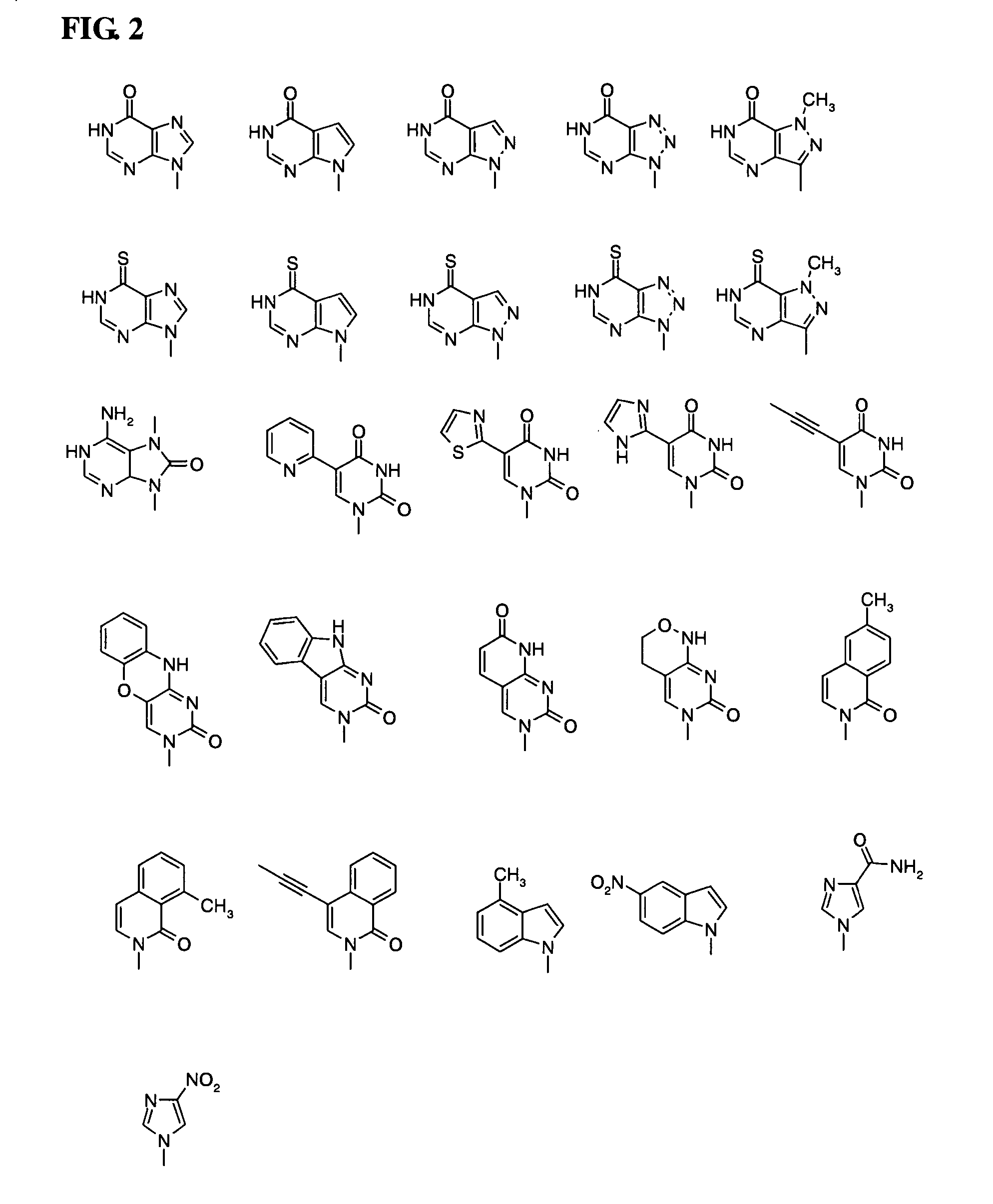
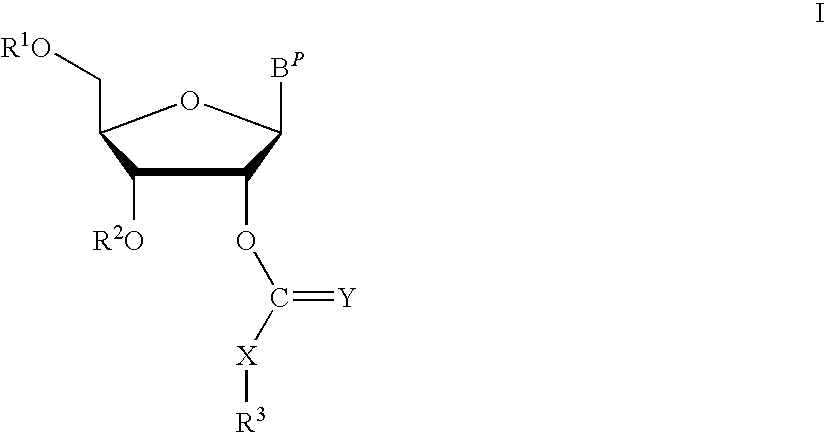
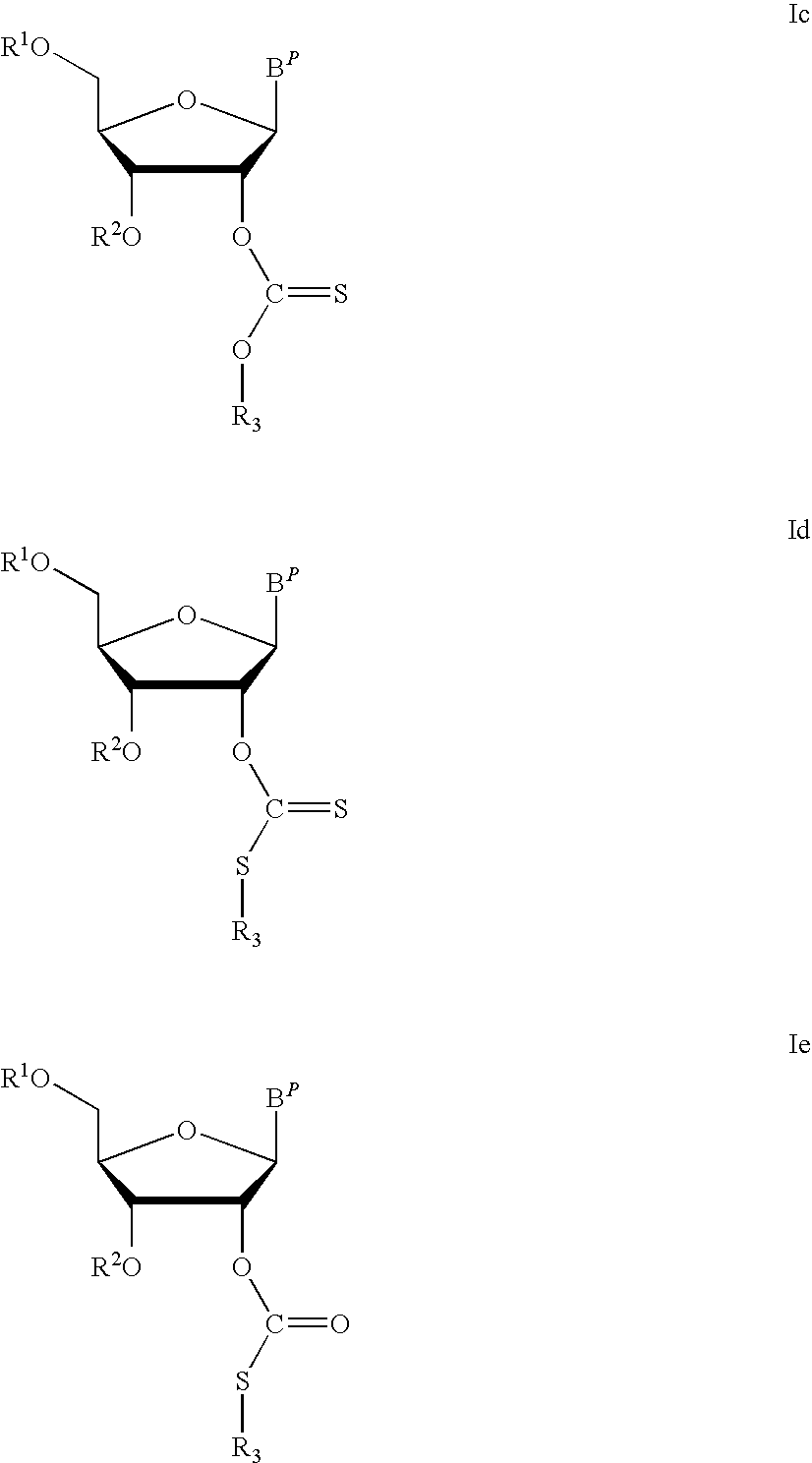
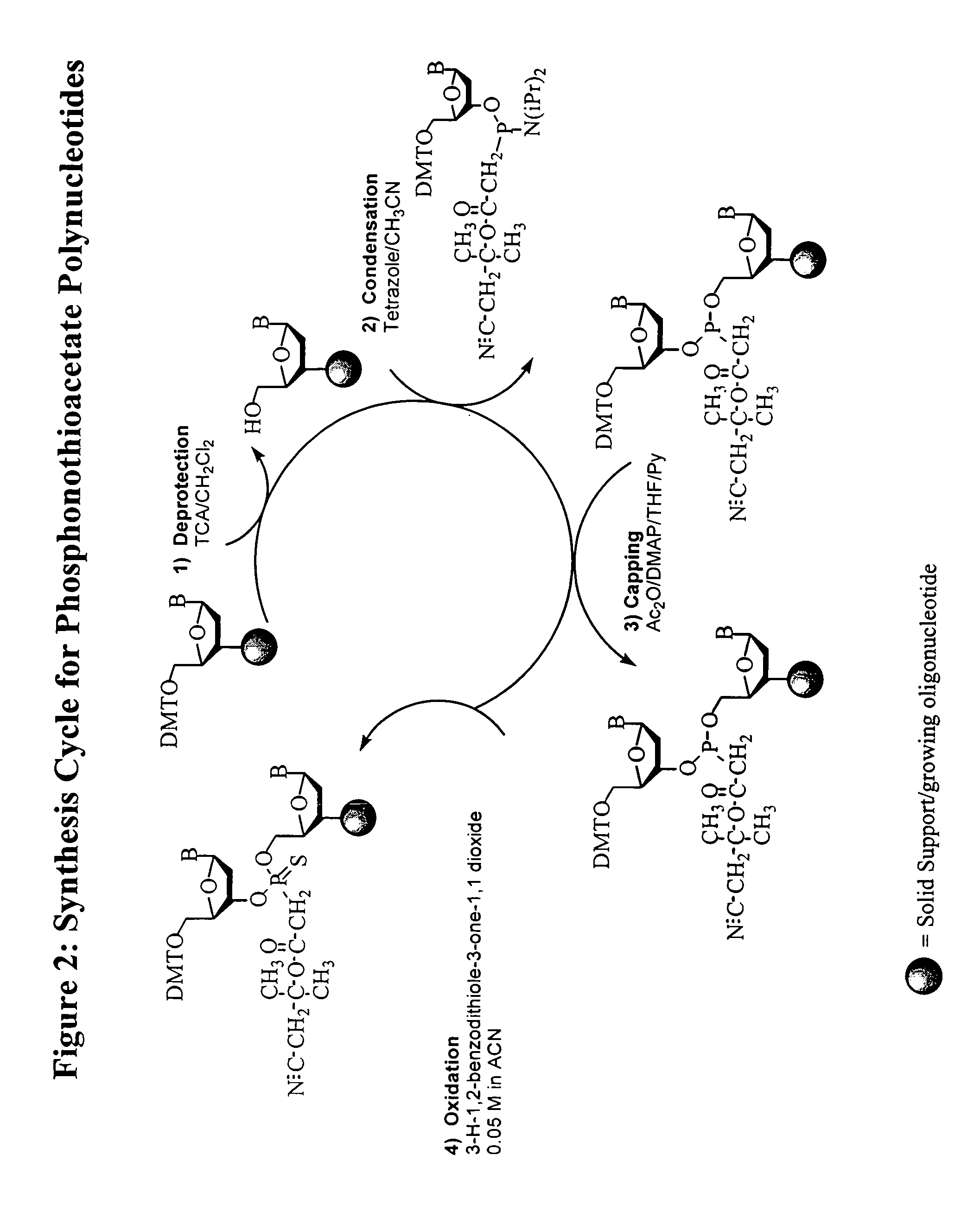
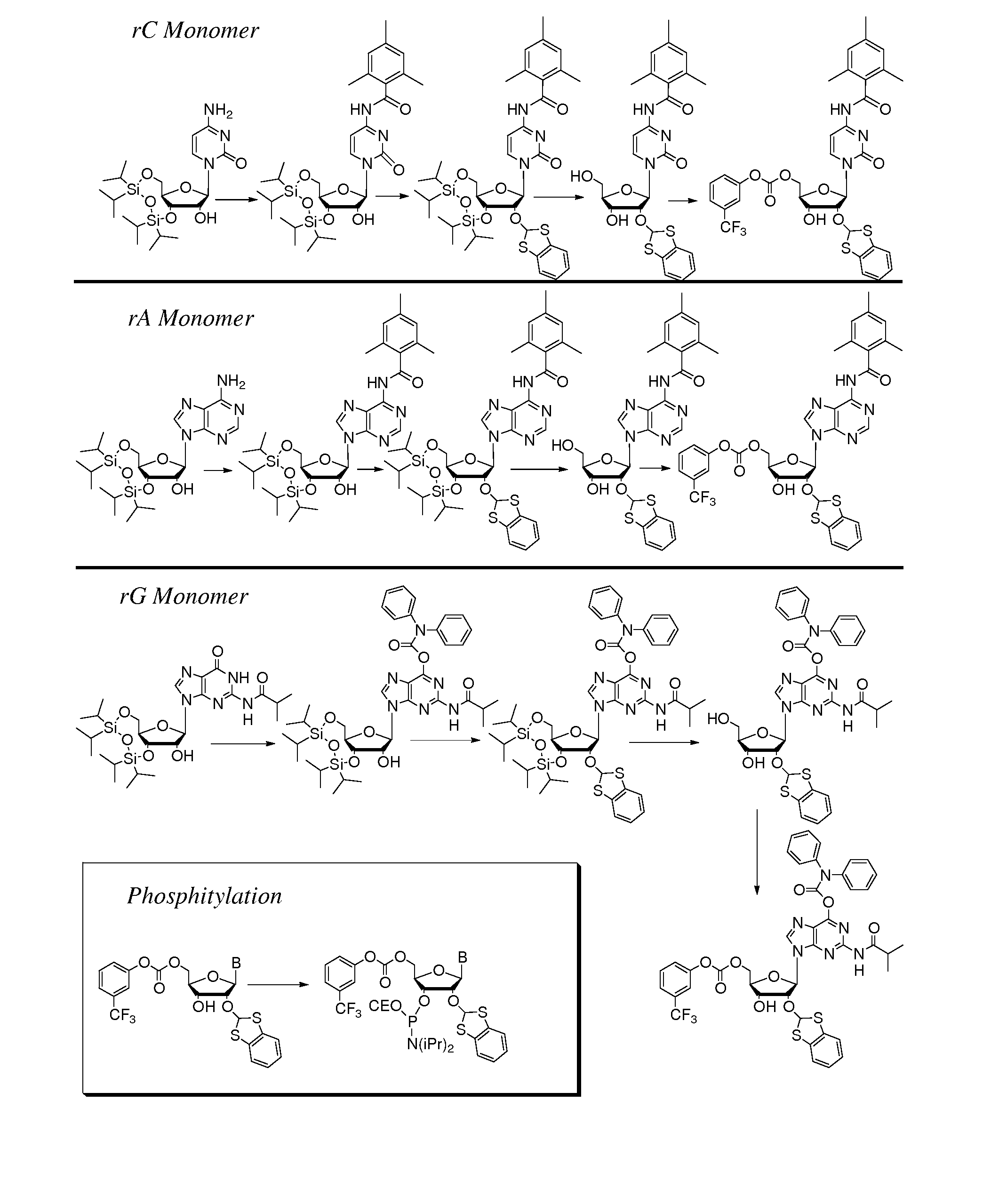
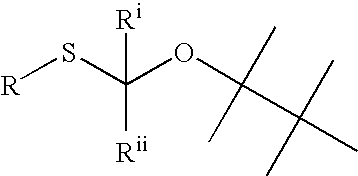
Thiocarbon-protecting groups for RNA synthesis
ActiveUS8202983B2Esterified saccharide compoundsOrganic active ingredientsProtecting groupMedicinal chemistry
Aspects of the invention include 2′ protected nucleoside monomers that are protected at the 2′ site with thiocarbon protecting groups. Thiocarbon protecting groups of interest include thiocarbonate, thionocarbonate, dithiocarbonate groups, as well as thionocarbamate protecting groups. Aspects of the invention further include nucleic acids that include the protecting groups of the invention, as well as methods of synthesizing nucleic acids using the protecting groups of the invention.
Owner:AGILENT TECH INC +1
Phosphinoamidite carboxylates and analogs thereof in the synthesis of oligonucleotides having reduced internucleotide charge
InactiveUS7067641B2Reduced internucleotide chargeImprove stabilitySugar derivativesOrganic chemistry methodsHydrogenHalogen
Nucleoside phosphinoamidite carboxylates and analogs are provided that have the structure of formula (III)wherein A is hydrogen, hydroxyl, lower alkoxy, lower alkoxy-substituted lower alkoxy, halogen, SH, NH2, azide or DL wherein D is O, S or NH and L is a heteroatom-protecting group, unsubstituted hydrocarbyl, substituted hydrocarbyl, heteroatom-containing hydrocarbyl, or substituted heteroatom-containing hydrocarbyl; B is a nucleobase; and one of R11 and R12 is a blocking group and the other is (IV) or (VI)in which W, X, Y, Z, R1 and n are as defined herein.
Owner:AGILENT TECH INC
2′-silyl containing thiocarbonate protecting groups for RNA synthesis
ActiveUS7999087B2Avoid lostEfficient couplingSugar derivativesBulk chemical productionSilyleneProtecting group
Nucleoside monomers, nucleic acids, e.g., oligonucleotides and polynucleotides, methods of making each, methods of deprotecting each, and the like are disclosed herein. Aspects of the invention include 2′ silyl containing thiocarbonate protecting groups. Corresponding compositions and methods are provided.
Owner:UNIV OF COLORADO THE REGENTS OF +1
Prodrugs of CC-1065 analogs
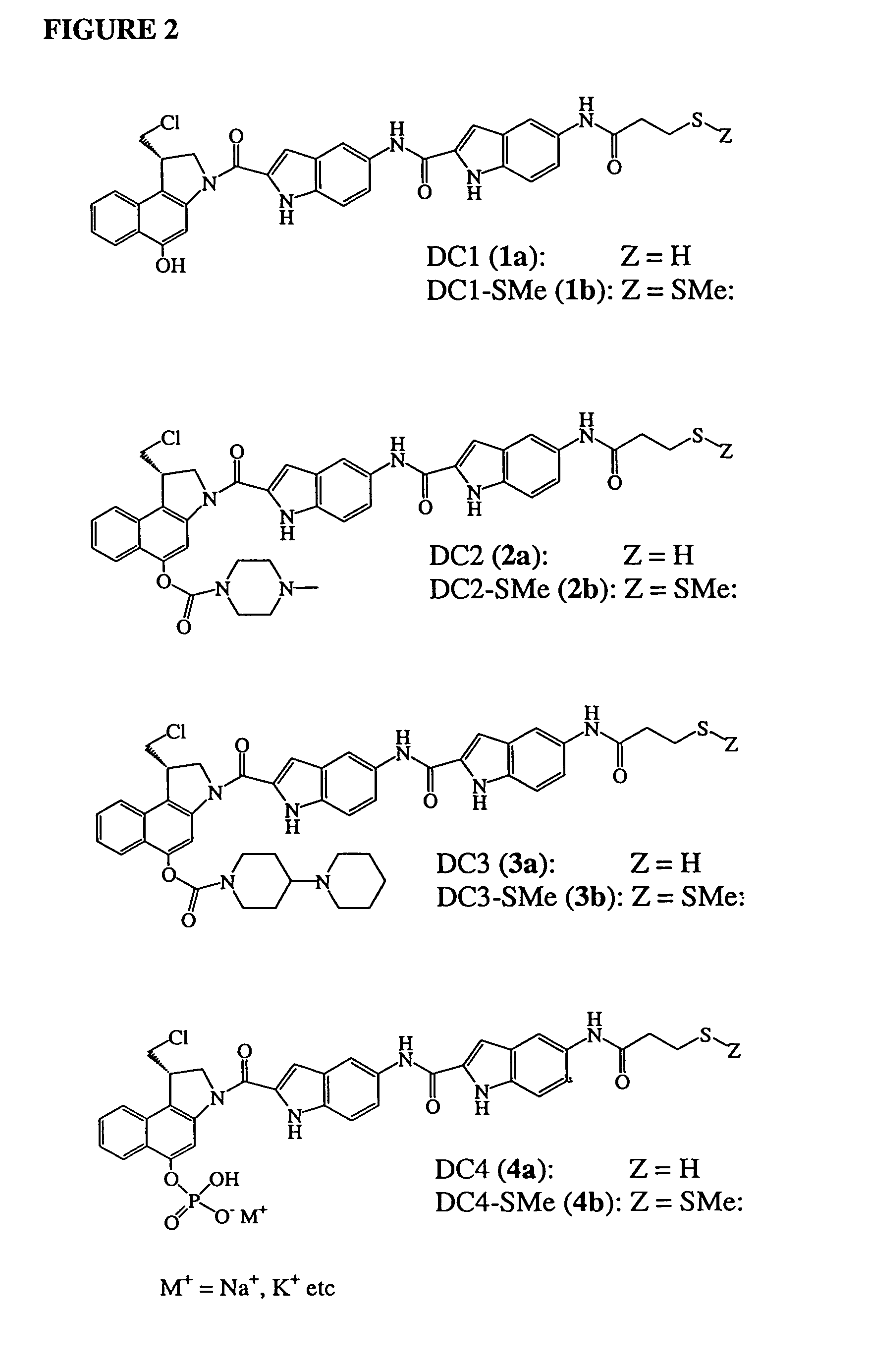
InactiveUS7049316B2Improve stabilityGood water solubilityBiocideGroup 5/15 element organic compoundsSolubilityCarbamate
Prodrugs of analogs of the anti-tumor antibiotic CC-1065 having a cleavable protective group such as a piperazino carbamate, a 4-piperidino-piperidino carbamate or a phosphate, in which the protecting group confers enhanced water solubility and stability upon the prodrug, and in which the prodrug also has a moiety, such as a disulfide, that can conjugate to a cell binding reagent such as an antibody. The therapeutic use of such prodrug conjugates is also described; such prodrugs of cytotoxic agents have therapeutic use because they can deliver cytotoxic prodrugs to a specific cell population for enzymatic conversion to cytoxic drugs in a targeted fashion.
Owner:IMMUNOGEN INC
Library of compounds comprising pyrrolobenzodiazepine moieties
InactiveUS7704924B2Easily brokenPrevent premature cleavageAntibacterial agentsOrganic active ingredientsNitrogenDouble bond
A compound of formula (IV): O is a solid support; L is a linking group or a single bond; X′ is selected from CO, NH, S, or O; A is O, S, NH, or a single bond; R2 and R3 are independently selected from: H, R, OH, OR, ═O, ═CH—R, ═CH2, CH2—CO2R, CH2—CO2H, CH2—SO2R, O—SO2R, CO2R, COR, CN and there is optionally a double bond between C1 and C2 or C2 and C3; R6, R7, and R9 are independently selected from H, R, OH, OR, halo, nitro, amino, Me3Sn; R11 is either H or R; Q is S, O or NH; R10 is a nitrogen protecting group; and Y is a divalent group such that HY═R, and other related compounds and collections of compounds.
Owner:MEDIMMUNE LTD
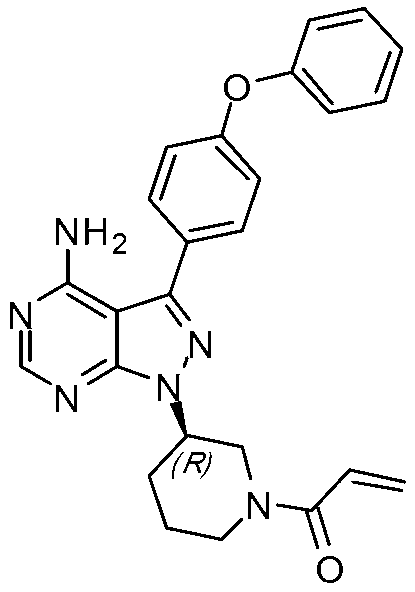
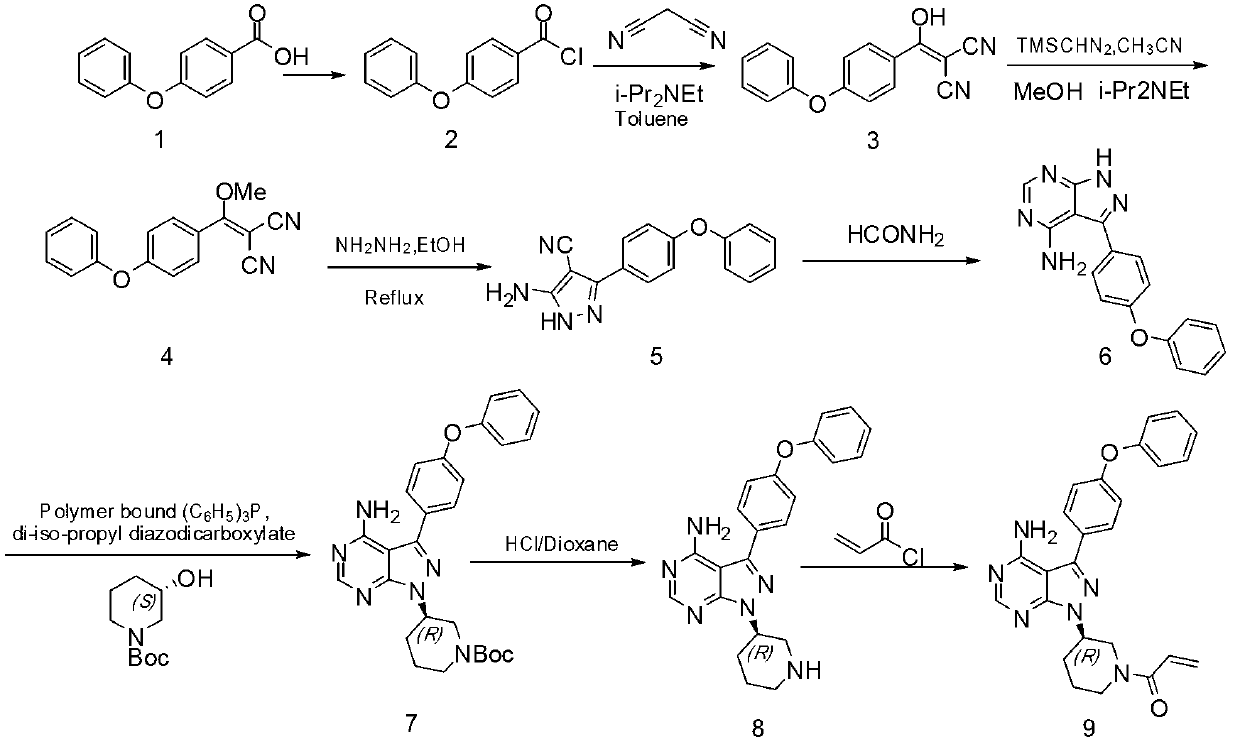
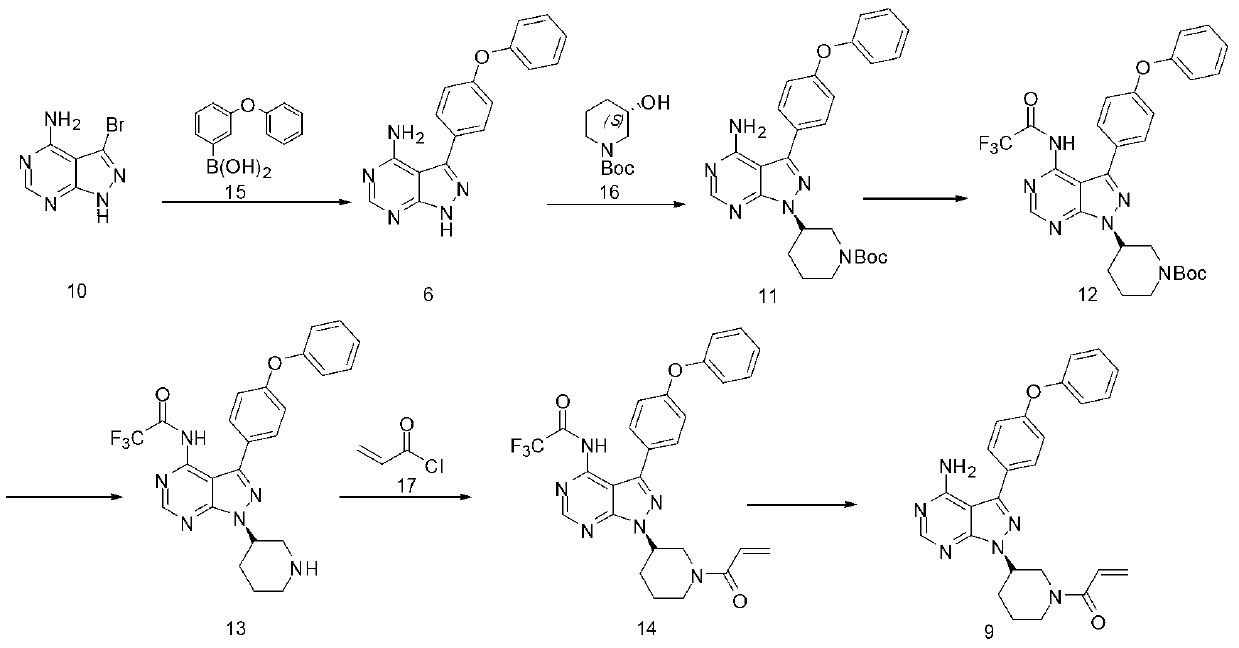
Method for synthesizing tyrosine kinase inhibitor PCI-32765
InactiveCN103121999ASuppress generationSmall side effectsOrganic chemistryBulk chemical productionSide effectCompound 17
The invention discloses a method for synthesizing tyrosine kinase inhibitor PCI-32765. The method comprises the following steps of: 1) carrying out a coupled reaction between a compound 10 and a compound 15 to obtain a compound 6; 2) reacting the compound 6 with a compound 16 to obtain a compound 11 in the presence of a more ideal catalyst; 3) protecting the compound 11 to obtain a compound 12; 4) carrying out selective de-protection on the compound 12 to obtain a compound 13; 5) attacking the compound 13 which has only one position which can be attacked by a compound 17 to obtain a very pure compound 14; and 6) removing protecting groups, thus obtaining the PCI-32765. By the method, tyrosine kinase which serves as a main receptor signal of B cells can be inhibited, and the tyrosine kinase inhibitor PCI-32765 is not only capable of inhabiting the generation of hemocyte with little toxicity and side effects, but also is moderate in reaction conditions, simple to operate, convenient to purify, low in cost, environment-friendly, and suitable for large scale production.
Owner:SUZHOU DEVI PHARMA TECH CO LTD
Protecting groups for RNA synthesis
Aspects of the invention include 2′ protected nucleoside monomers that are protected at the 2′ site with orthoester-type protecting groups. The 2′ protected monomers also include a second, aryl carbonate-type, protecting group. Aspects of the invention further include nucleic acids that include the protecting groups of the invention, as well as methods of synthesizing nucleic acids using the protecting groups of the invention.
Owner:UNIV OF COLORADO THE REGENTS OF +1
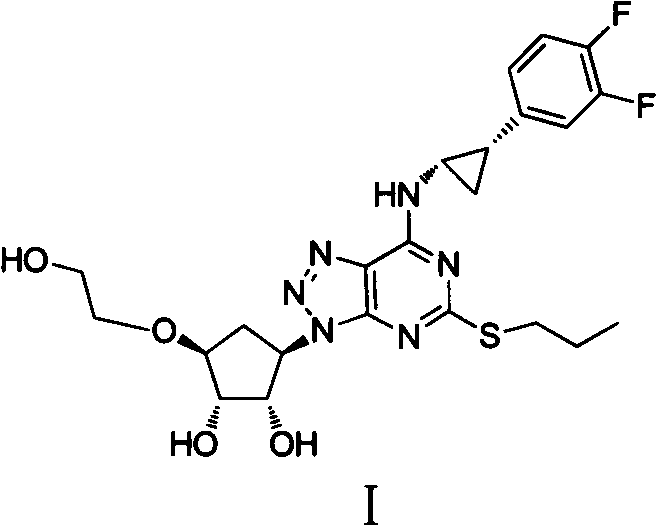
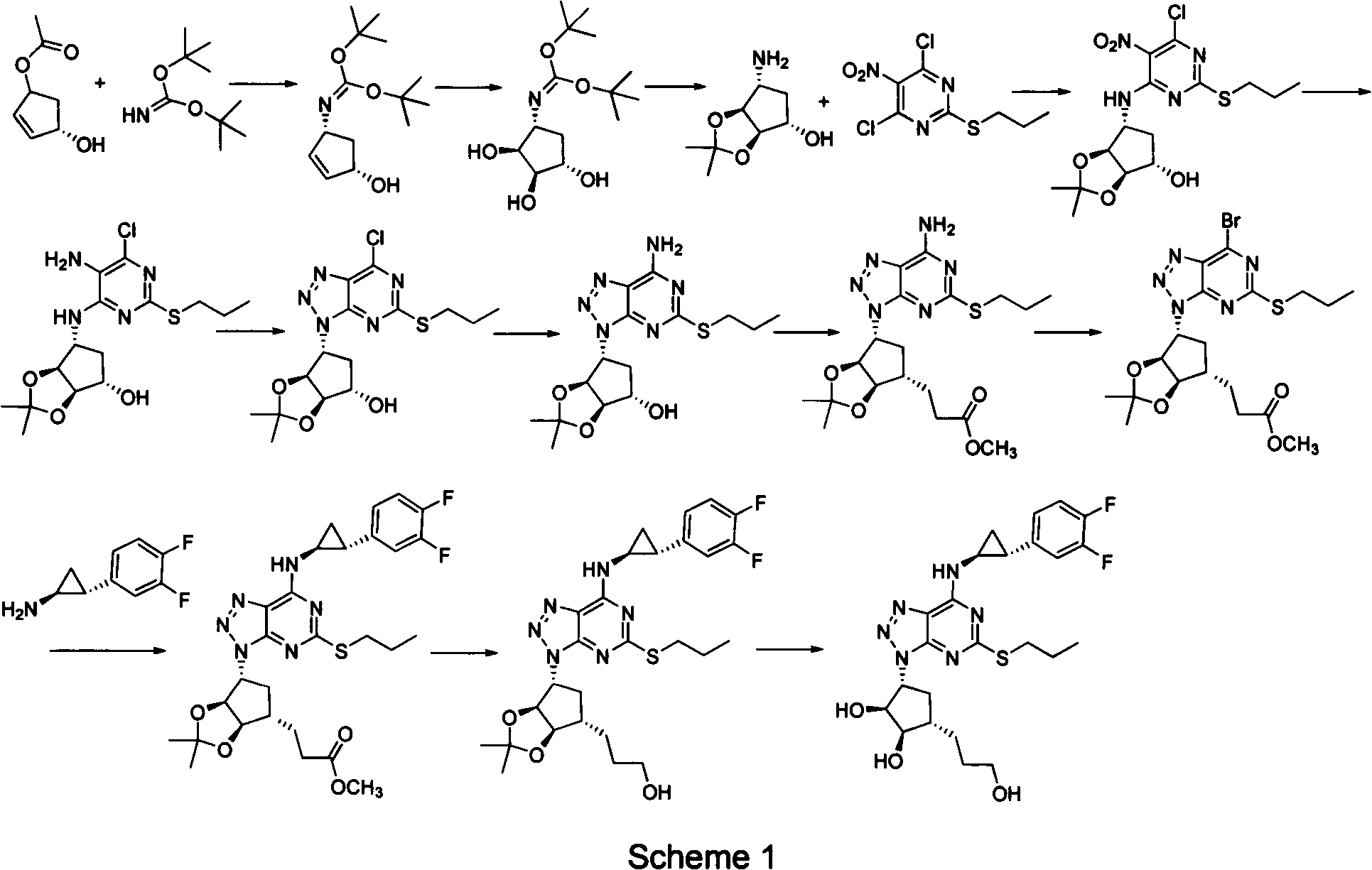
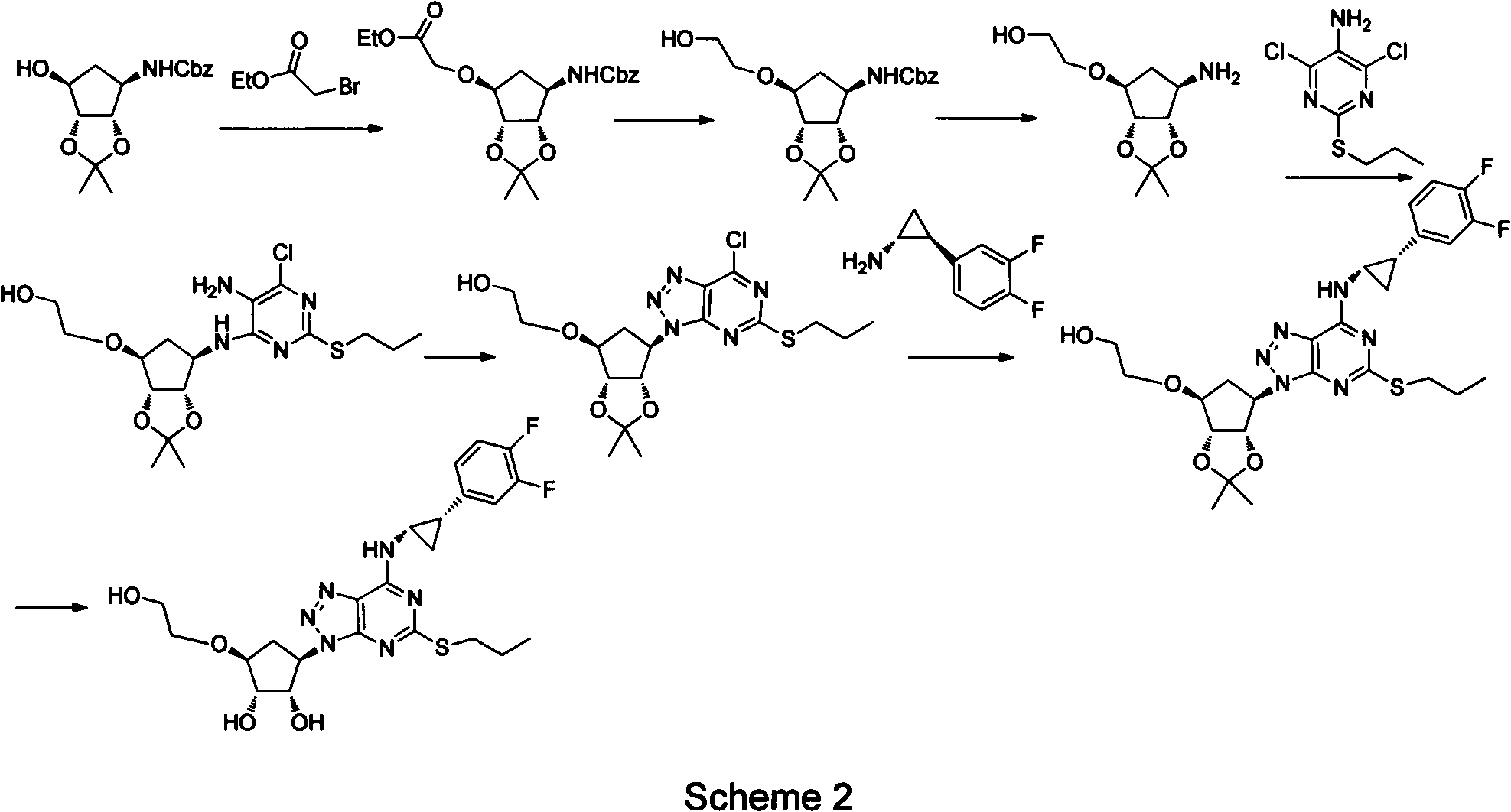
Preparation method of ticagrelor
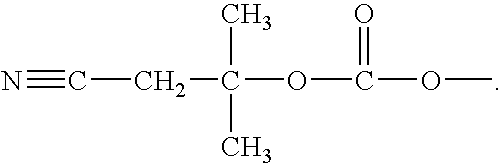
ActiveCN102675321AHigh yieldReduce manufacturing costOrganic chemistryBulk chemical productionNitriteTicagrelor
The invention provides a preparation method of ticagrelor, belonging to the technical field of medicine manufacturing. According to the method, a compound VII is taken as a raw material, and the method comprises the steps of: carrying out a nucleophilic substitution reaction on the raw material to obtain a compound VI; hydrogenating the VI, removing carbamazepine (Cbz) protection to obtain a compound V; carrying out a reaction on the V and 4, 6-dichloro-2-(allyl sulfide)-5-amio-pyrimidine to obtain a compound IV; carrying out a reaction on the IV and nitrite of alkali metal to obtain a compound III; carrying out a reaction on the III and (1R, 2S)-2-(3, 4-difluoro phenyl) cyclopropylamine to obtain a compound II; and finally, removing protecting group of the II to obtain a compound I.
Owner:SHANGHAI HAOYUAN CHEMEXPRESS
Ester compounds, polymers, resist composition and patterning process
A novel ester compound having an alkylcycloalkyl or alkylcycloalkenyl group as the protective group is provided as well as a polymer comprising units of the ester compound. The polymer is used as a base resin to formulate a resist composition having a higher sensitivity, resolution and etching resistance than conventional resist compositions.
Owner:SHIN ETSU CHEM IND CO LTD
Protected nucleotide analogs
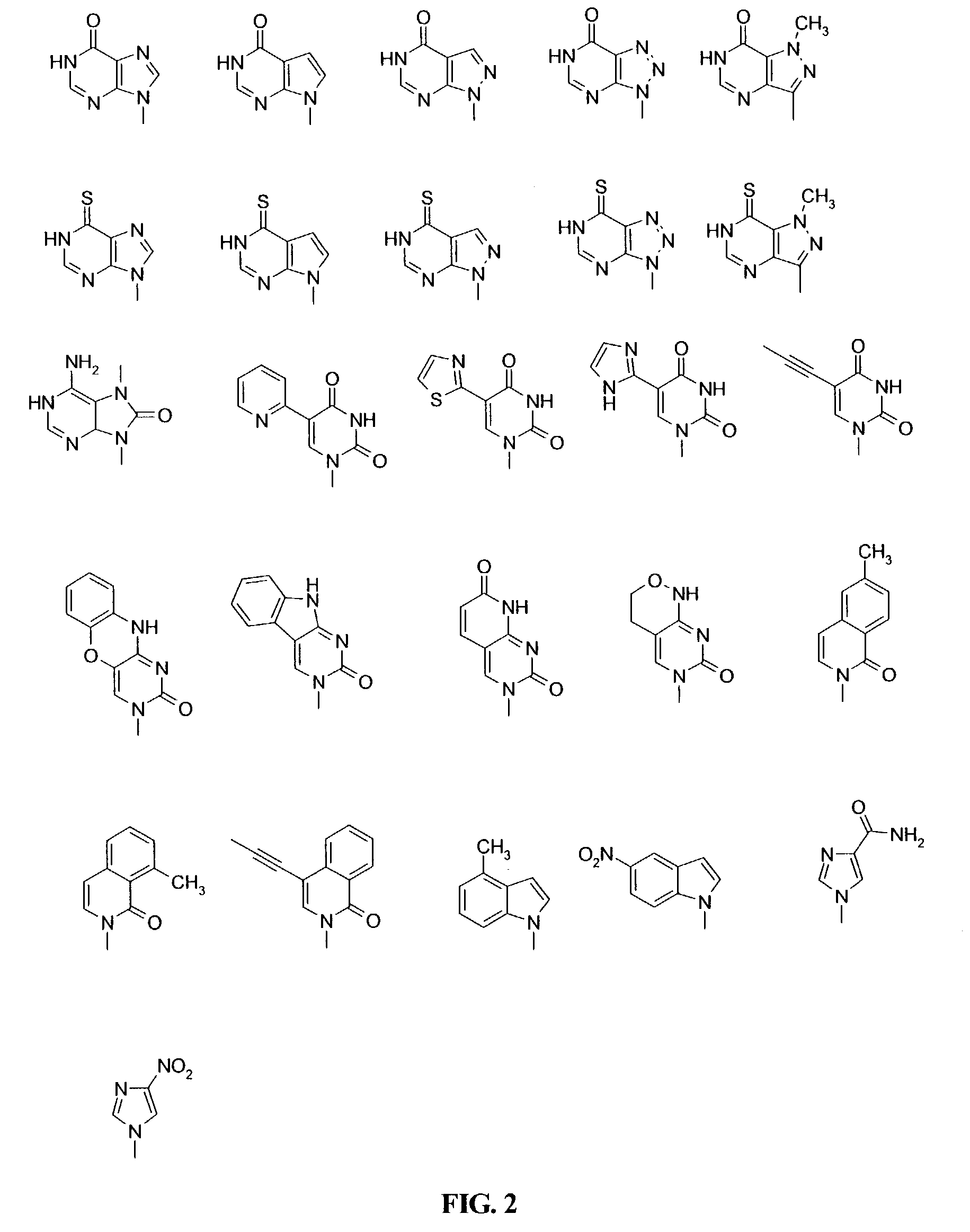
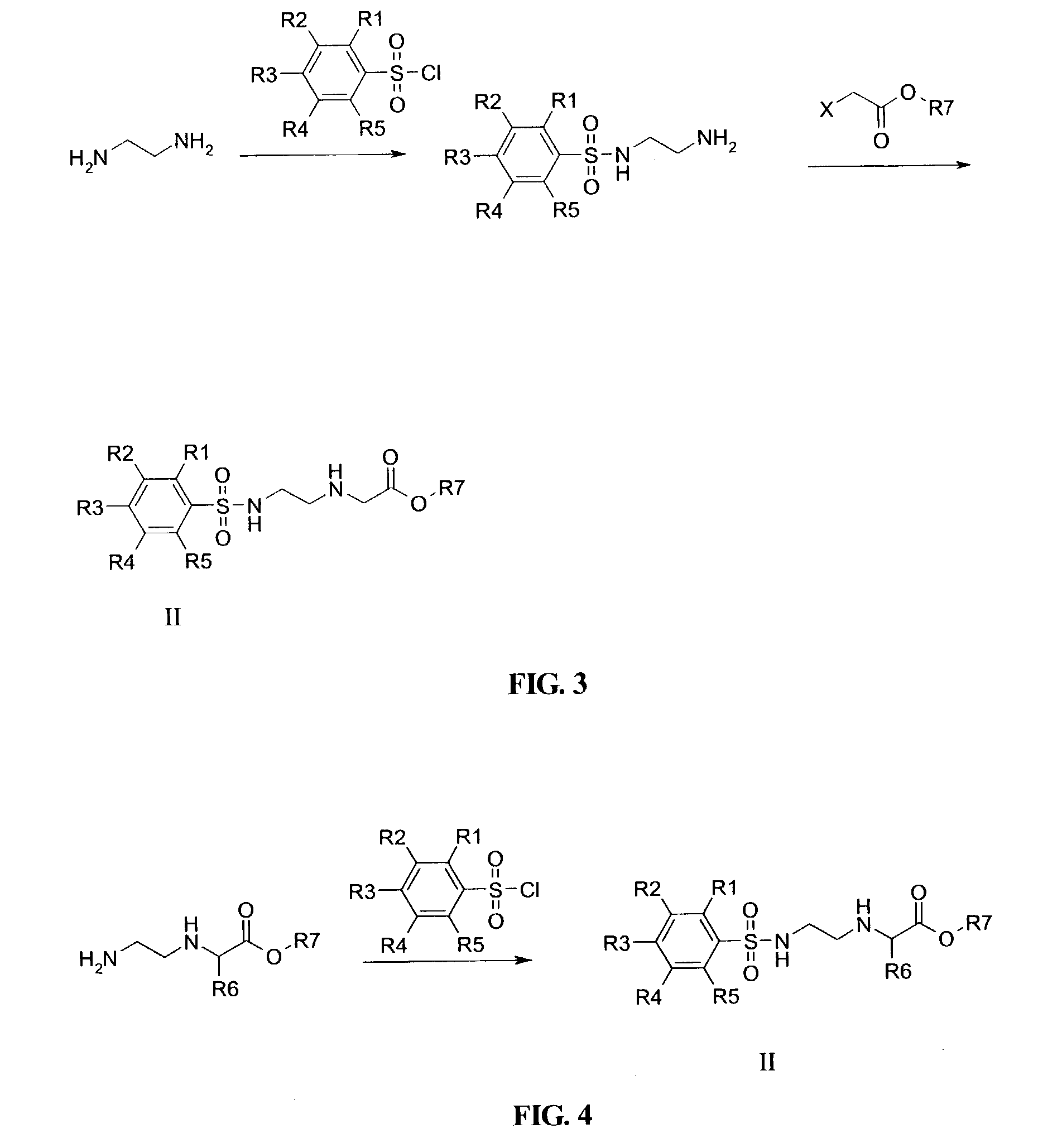
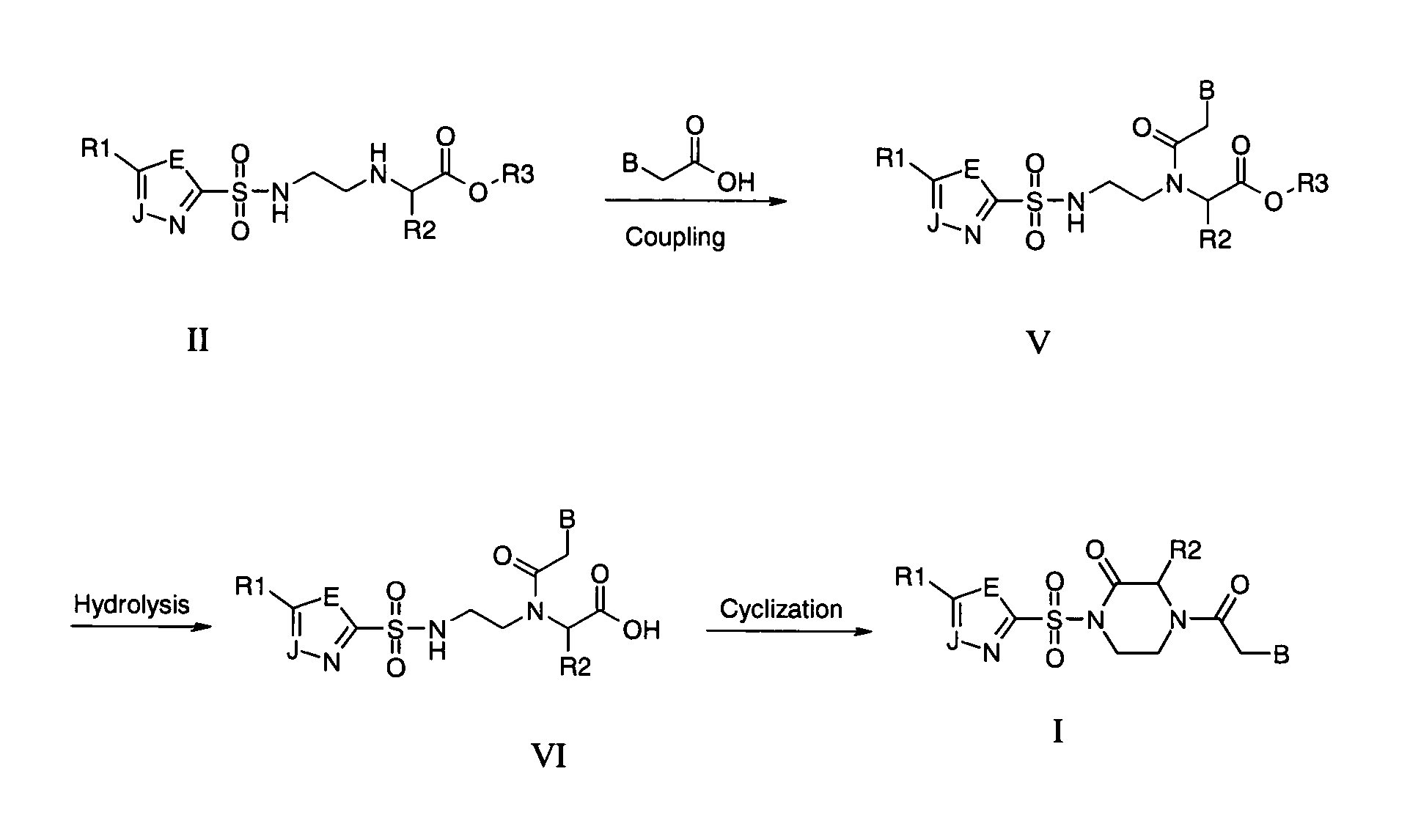
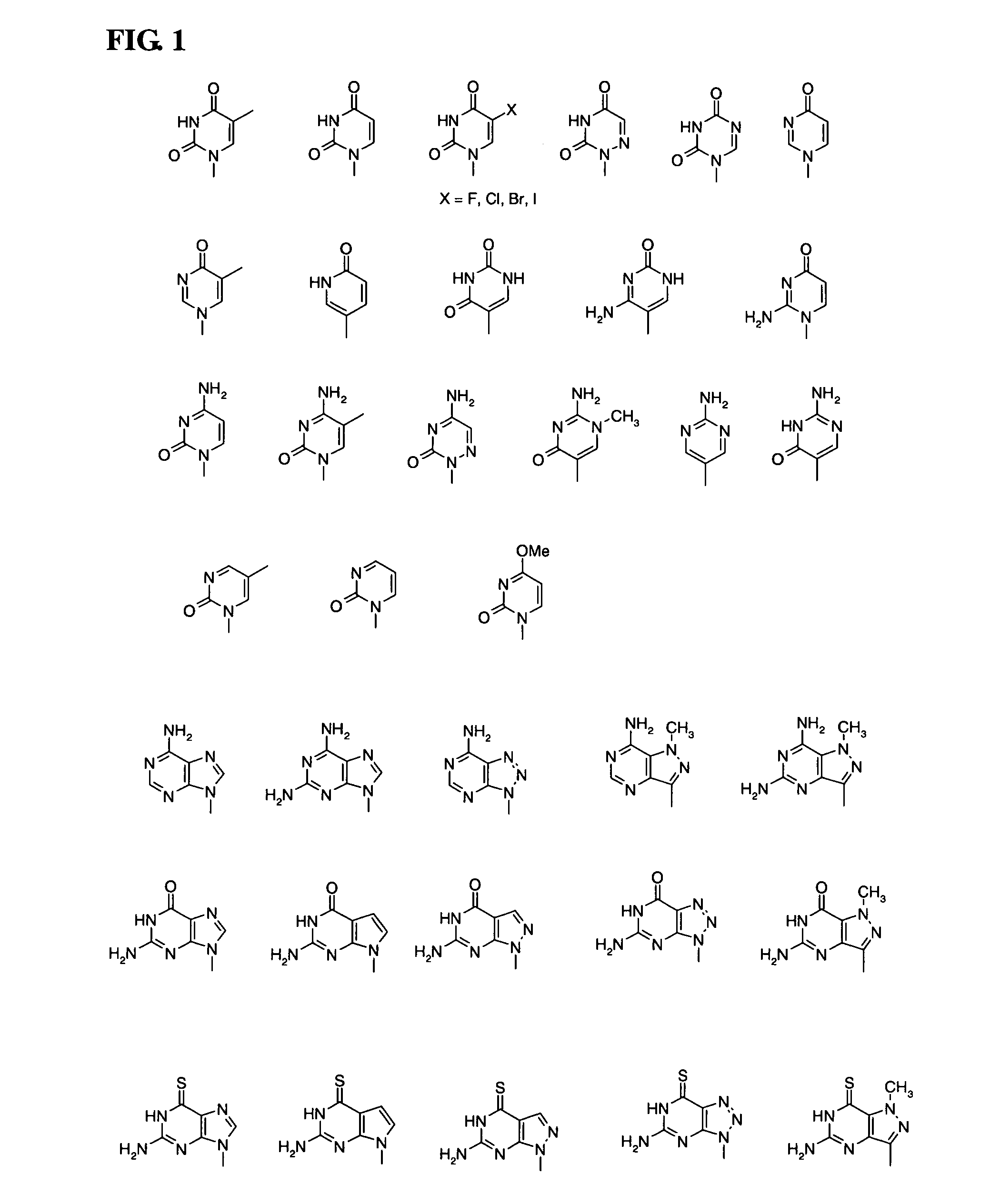
Disclosed herein are nucleotide analogs with one or more protecting groups, methods of synthesizing nucleotide analogs with one or more protecting groups and methods of treating diseases and / or conditions such as viral infections, cancer, and / or parasitic diseases with the nucleotide analogs with one or more protecting groups.
Owner:ALIOS BIOPHARMA INC
Bicyclonucleoside analogues
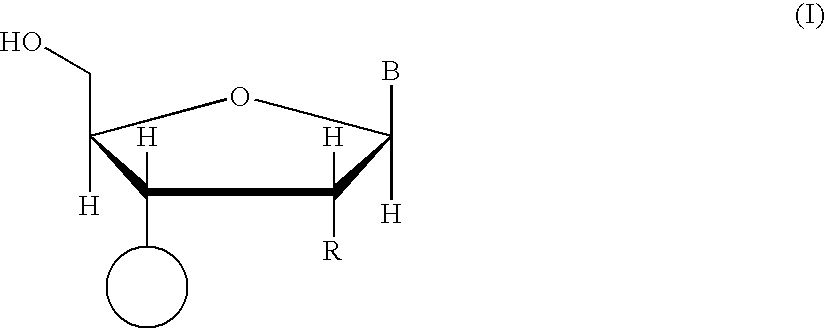
Bicyclonucleoside analogues which exhibit anti-AIDS activity and intermediates to produce oligonucleotide analogues which have anti-sense or anti-gene activity as well as in vivo stability. Compounds of the following formula (1) or their pharmaceutically acceptable salts.wherein R1 represents a hydrogen atom or a protecting group for a hydroxy group, R2 represents an azido group or an optionally protected amino group or the like, B represents a purin-9-yl or a 2-oxo-1,2-dihydropyrimidin-1-yl group, which are optionally substituted with substituents selected from the group consisting of a halogen atom, an alkyl group having 1–6 carbon atoms, a hydroxy group, a mercapto group and an amino group.
Owner:IMANISHI TAKESHI
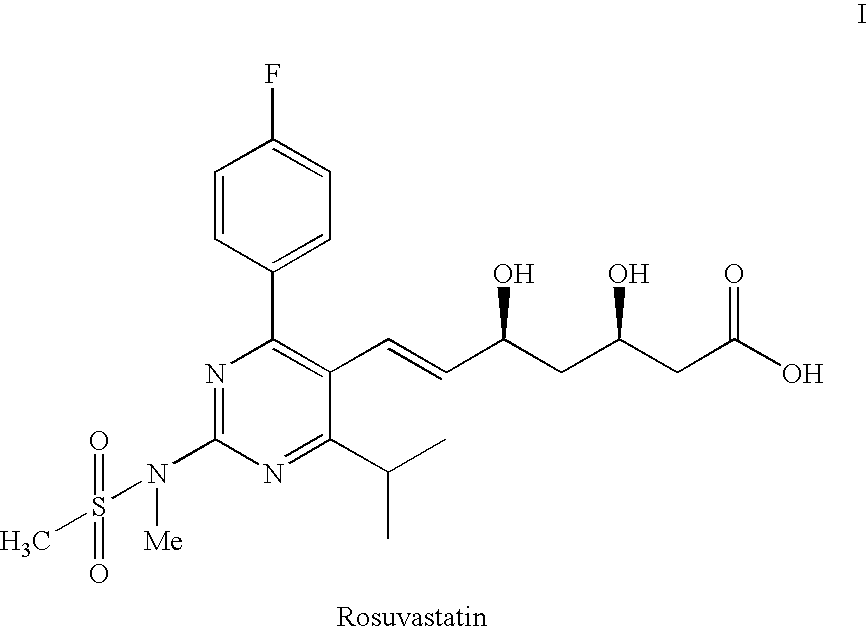
Process for the preparation of pyrimidine derivatives
ActiveUS20050124639A1Easier and economical productionSimple reaction conditionsBiocideOrganic active ingredientsOxygenAldehyde
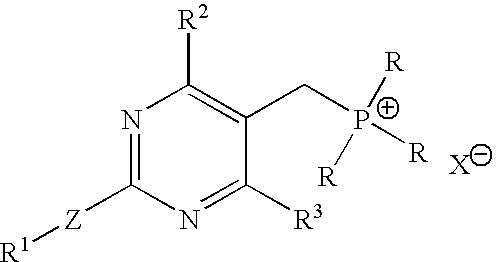
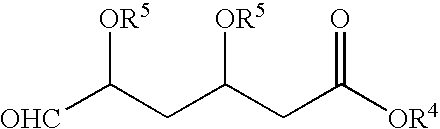
An improved process for the preparation of pyrimidine derivatives is provided comprising reacting a Wittig reagent of the general formula wherein R is an alkyl of from 1 to 10 carbon atoms, aryl or arylalkyl, R1 is a substituted or unsubstituted hydrocarbon group, R2 and R3 are the same or different and are hydrogen or a substituted or unsubstituted hydrocarbon group; Z is sulfur, oxygen, sulfonyl, or imino which may be substituted by formyl, acetyl, propionyl, butyryl, isobutyryl, valeryl, isovaleryl, amino substituted by sulfonyl or alkylsulfonyl, and sulfonyl substituted by alkyl, amino or alkylamino and X is a halogen; with an aldehyde of the general formula wherein R4 is hydrogen, a lower alkyl or a cation capable of forming a non-toxic pharmaceutically acceptable salt and each R5 are the same or different and are hydrogen or a hydrolyzable protecting group, or each R5, together with the oxygen atom to which each is bonded, form a hydrolyzable cyclic protecting group, or each R5 is bonded to the same substituent which is bonded to each oxygen atom to form a hydrolyzable protecting group; in the presence of a base.
Owner:GLENMARK LIFE SCI LTD
Conformationally constrained backbone cyclized peptide analogs
Novel backbone cyclized peptide analogs are formed by means of bridging groups attached via the alpha nitrogens of amino acid derivatives to provide novel non-peptidic linkages. Novel building units disclosed are Nα(ω-functionalized) amino acids constructed to include a spacer and a terminal functional group. One or more of these Nα(ω-functionalized) amino acids are incorporated into a peptide sequence, preferably during solid phase peptide synthesis. The reactive terminal functional groups are protected by specific protecting groups that can be selectively removed to effect either backbone-to-backbone or backbone-to-side chain cyclizations. The invention is specifically exemplified by backbone cyclized bradykinin antagonists having biological activity. Further embodiments of the invention are somatostatin analogs having one or two ring structures involving backbone cyclization.
Owner:DEVELOGEN ISRAEL
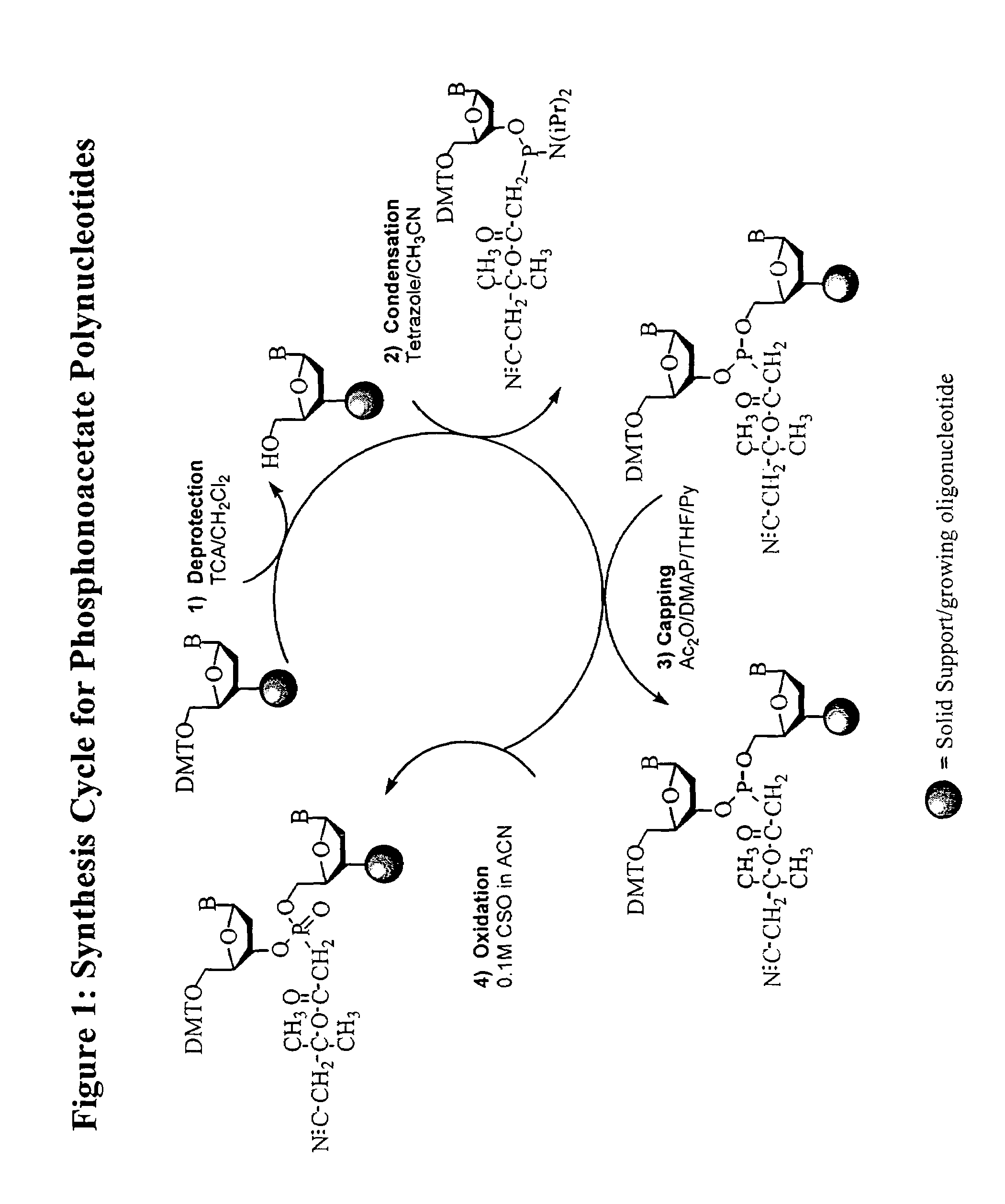
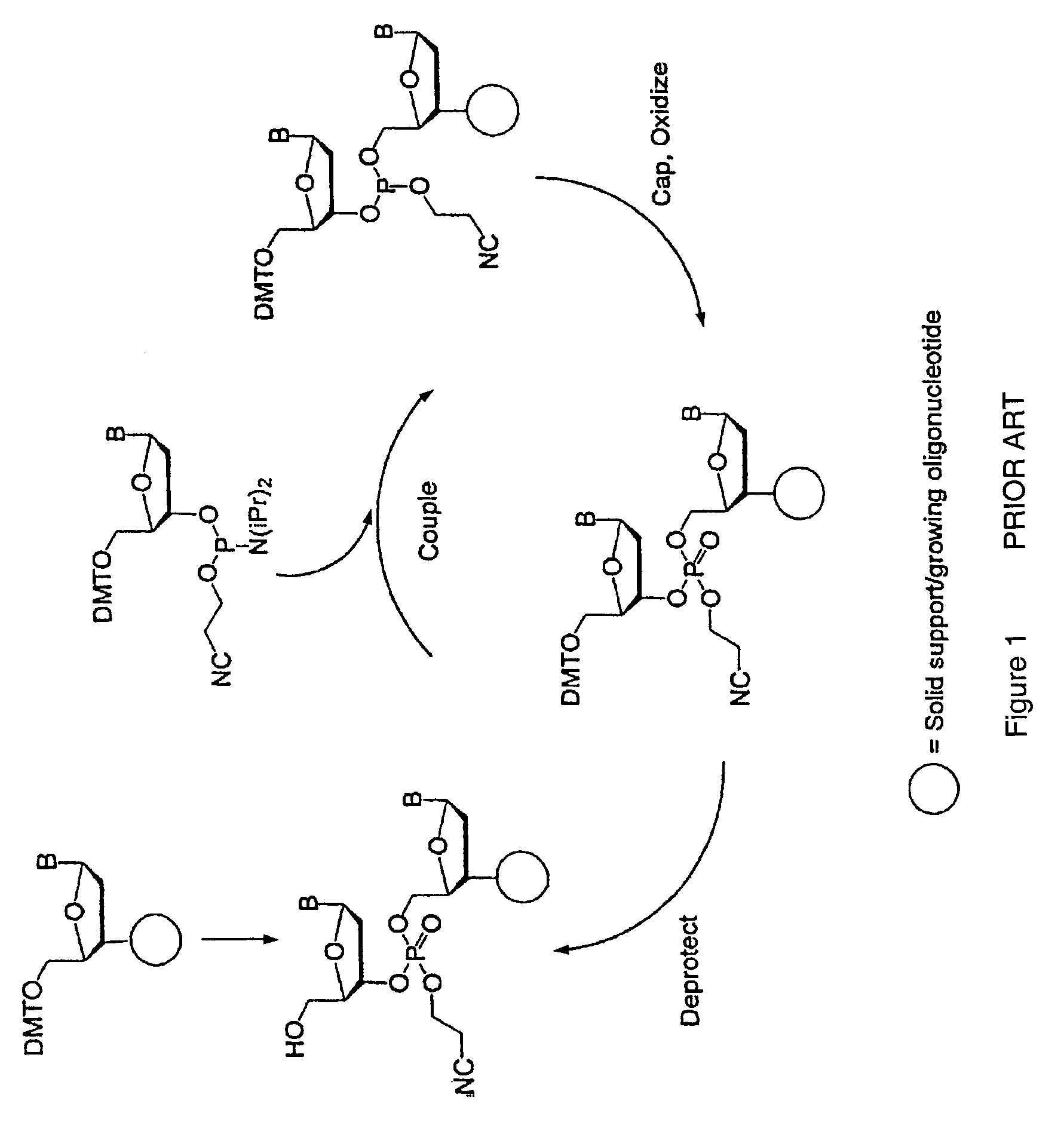
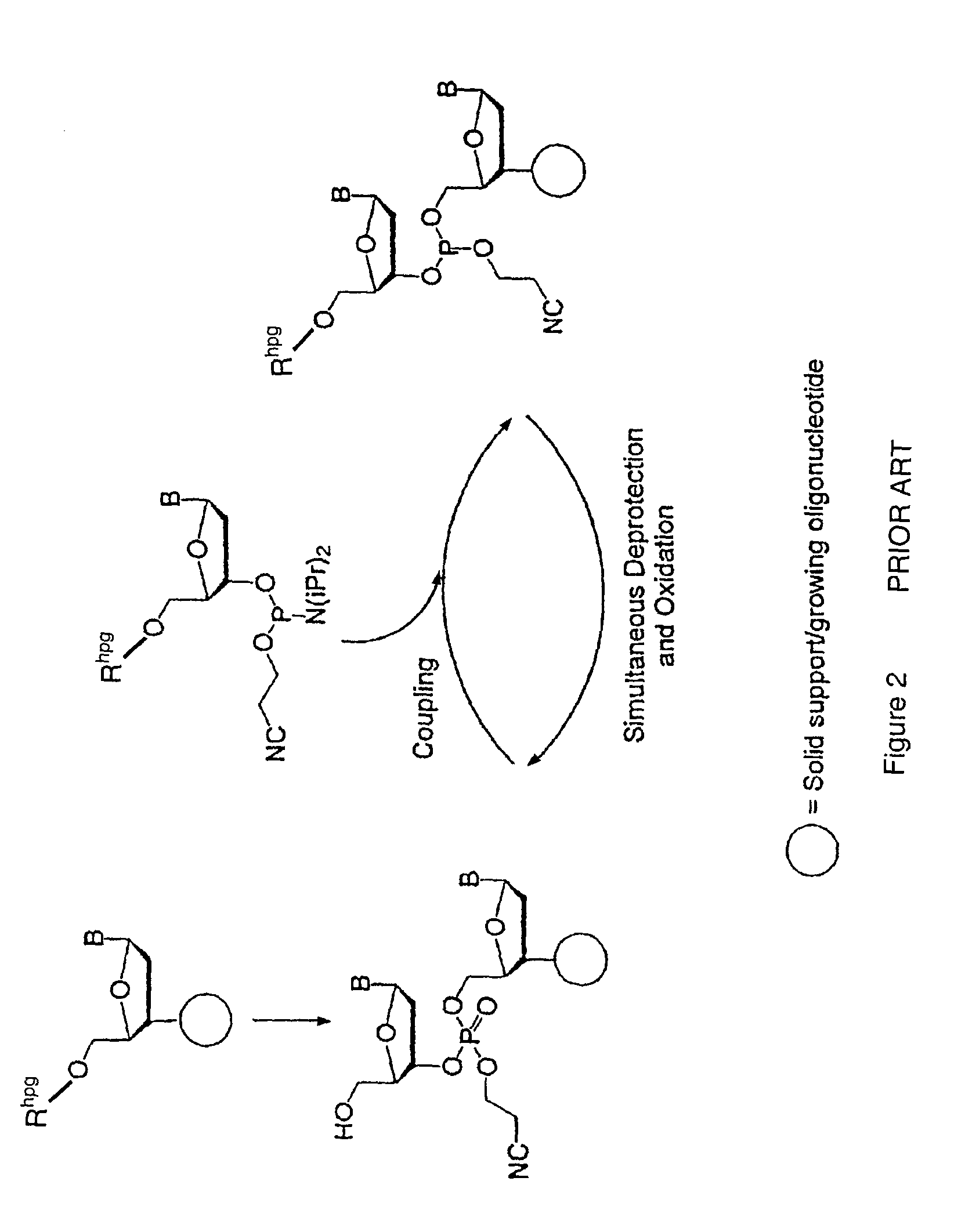
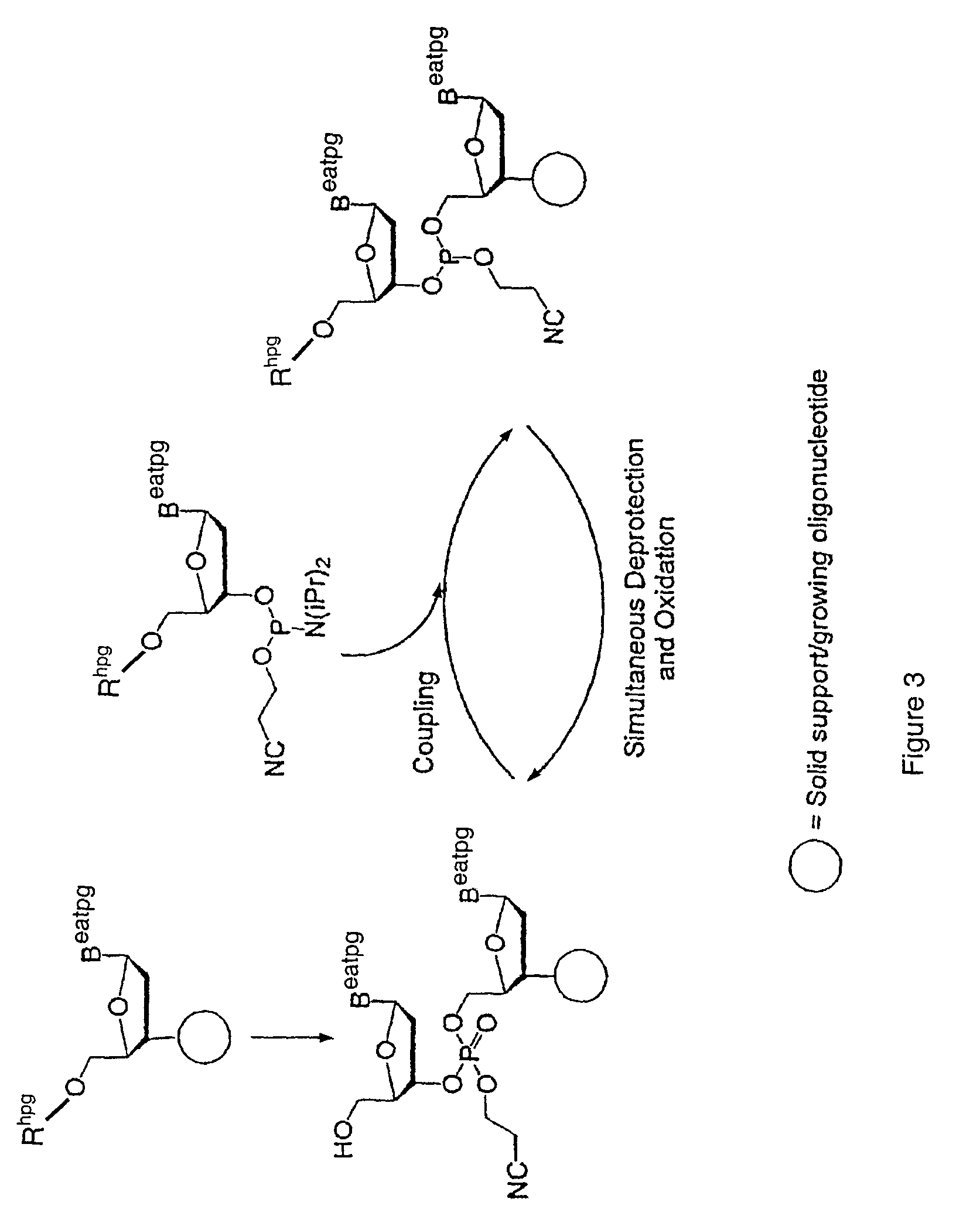
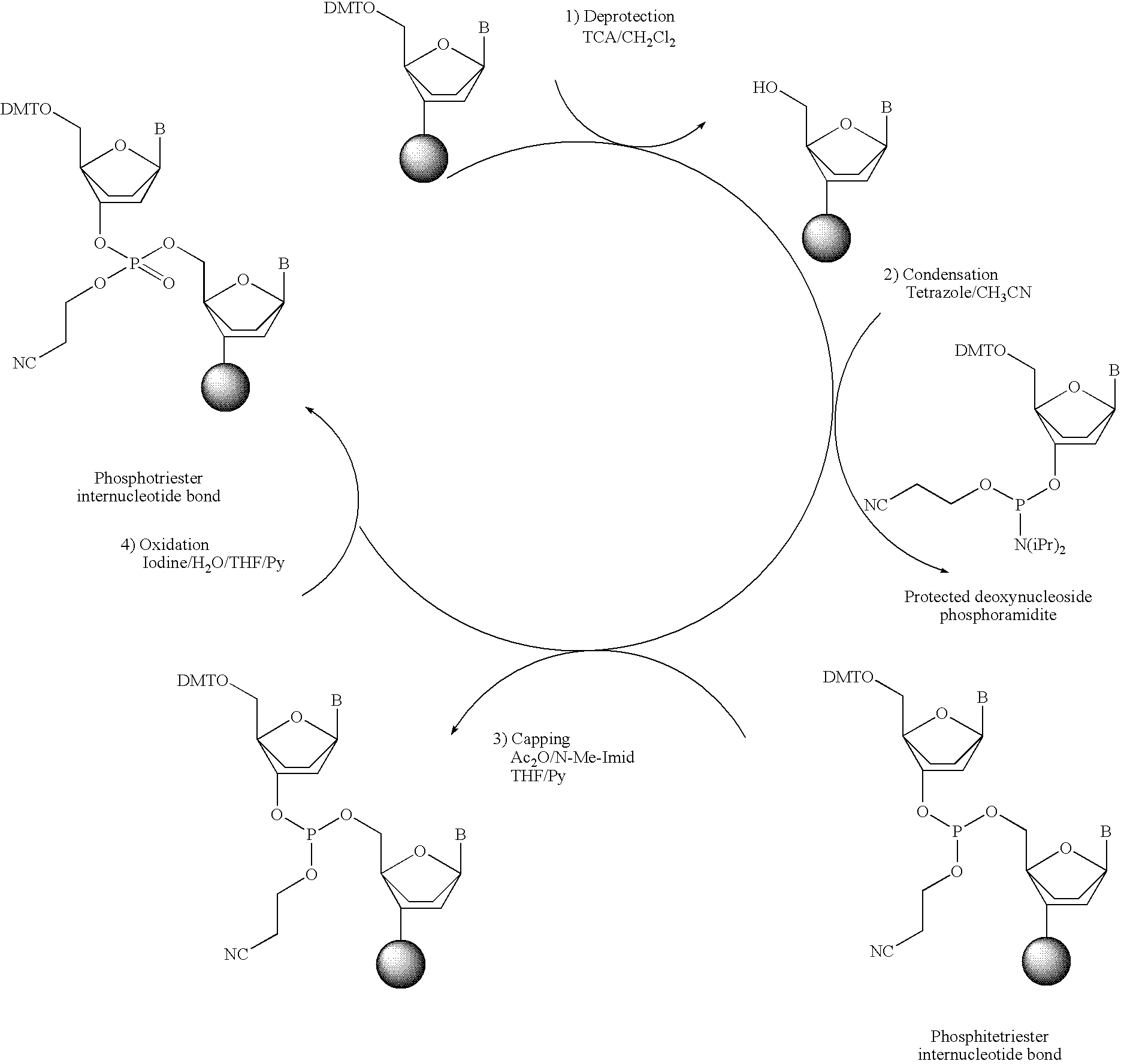
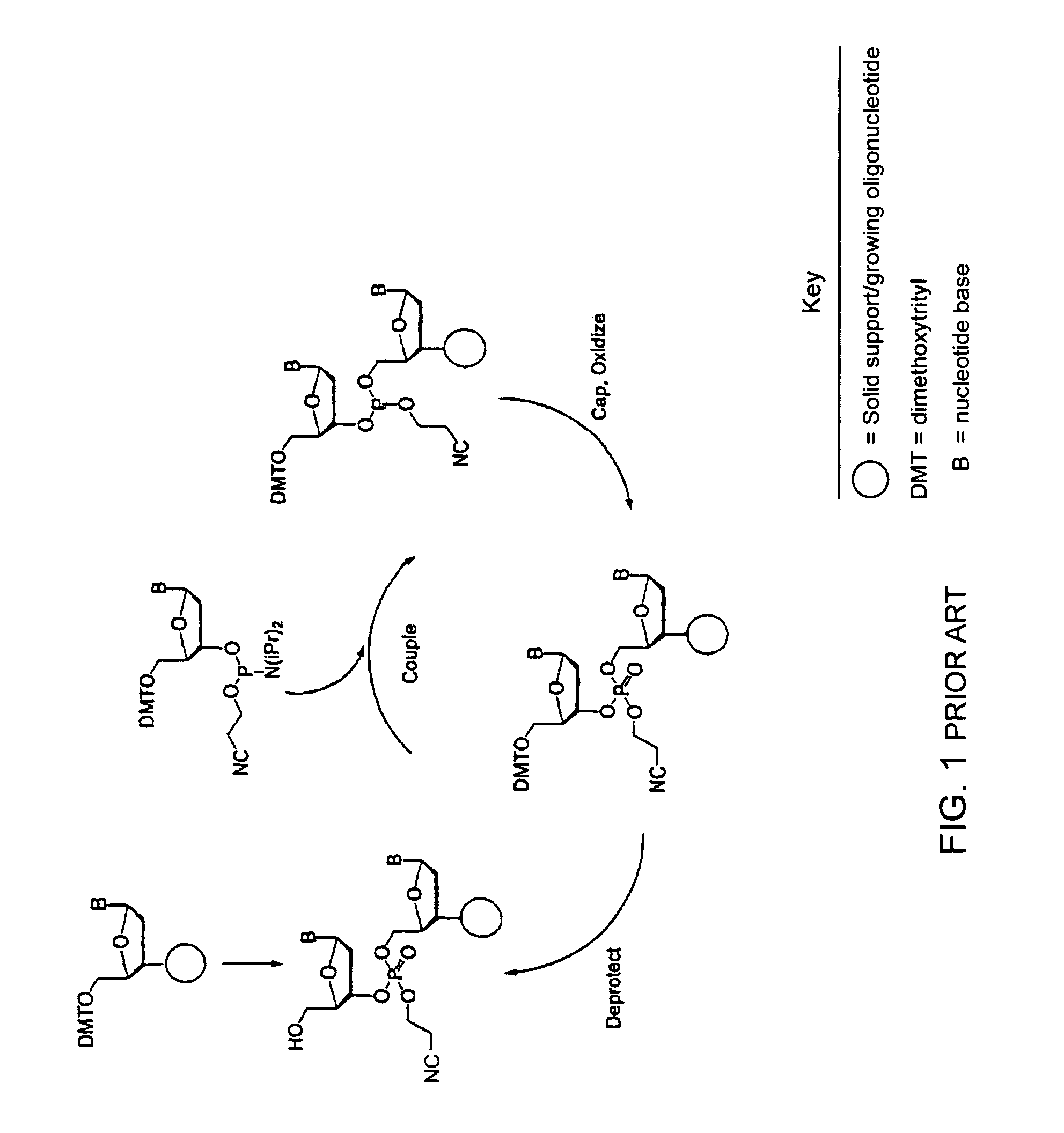
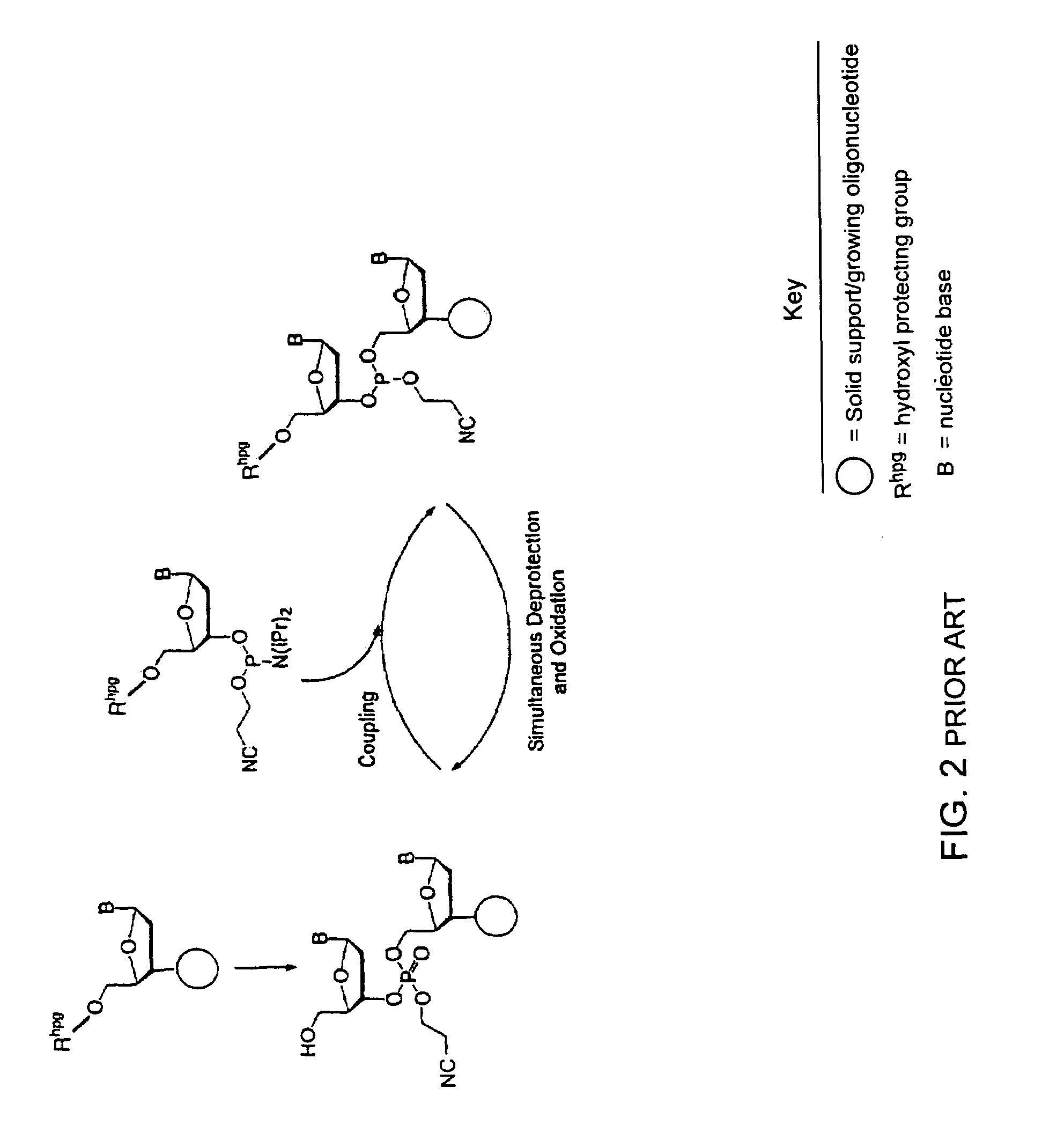
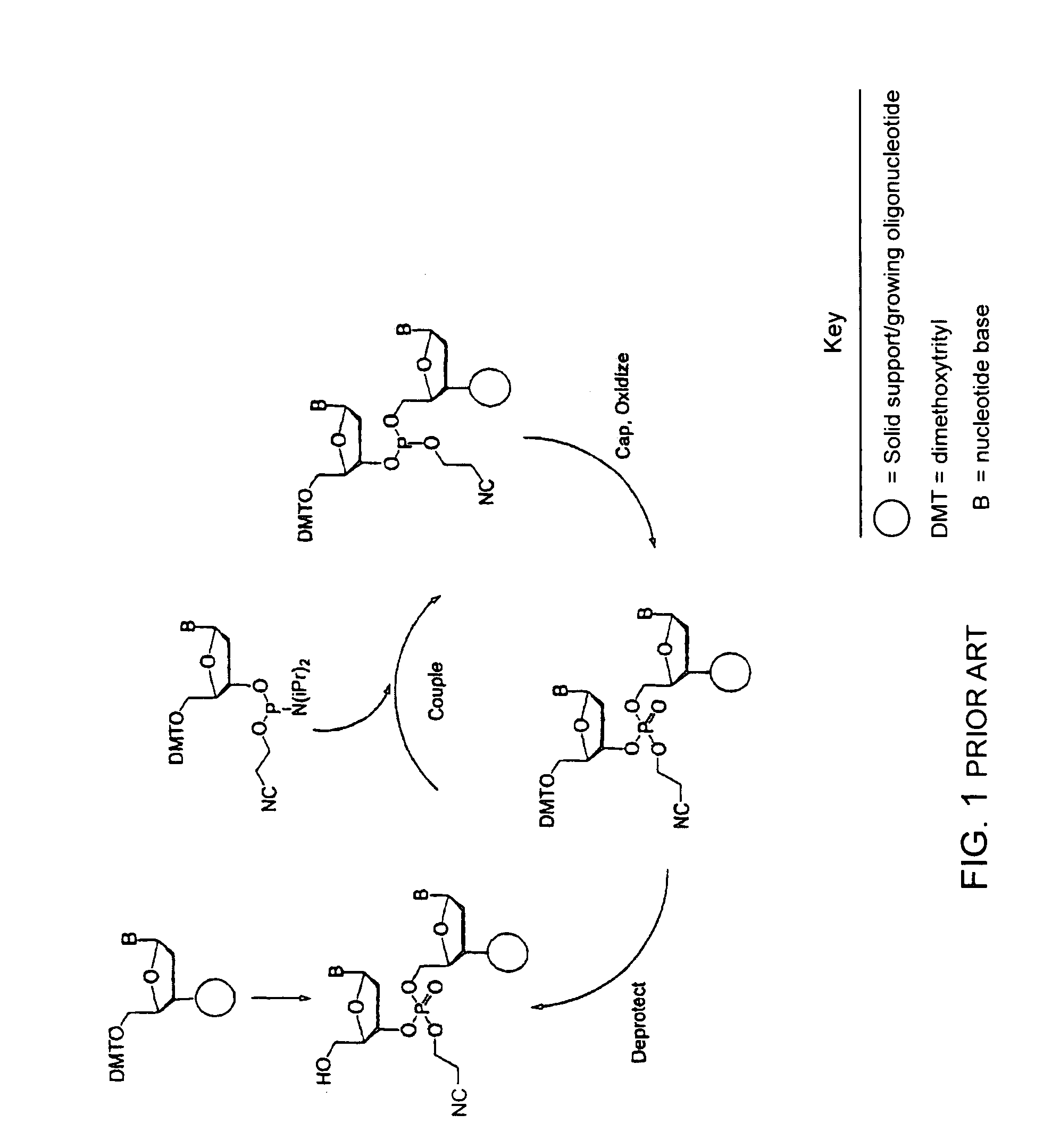
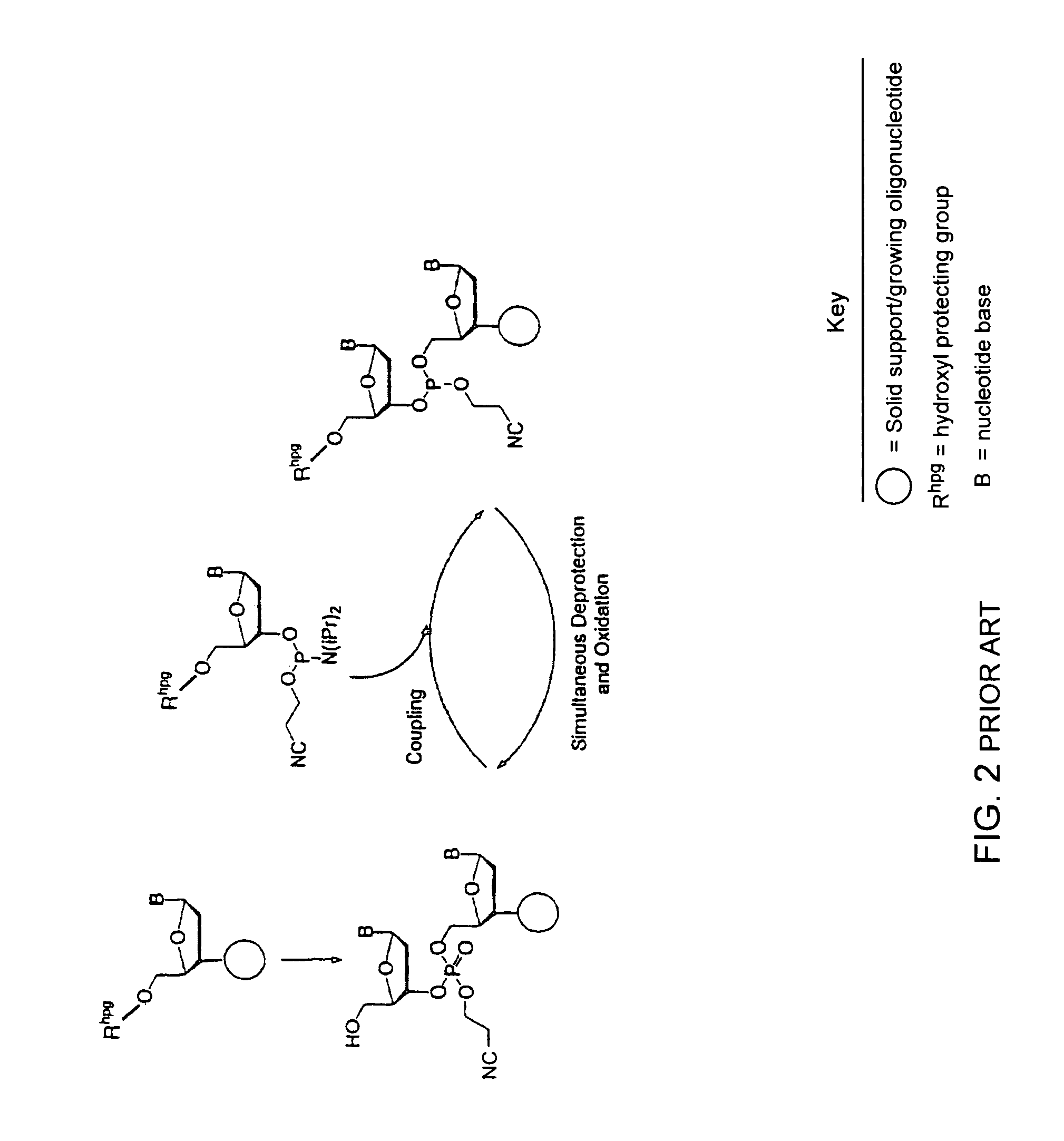
Synthesis of polynucleotides using combined oxidation/deprotection chemistry
A method of synthesizing a polynucleotide which can, for example, be used during fabrication of an array. A second nucleoside is coupled to a first nucleoside through a phosphite linkage, with the second nucleoside having a hydroxyl protecting group that is a non-carbonate protecting group. The product of the foregoing step is exposed to a composition which both oxidizes the formed phosphite to a phosphate and deprotects the protected hydroxyl of the coupled nucleoside. The method has particular application to fabricating an addressable array of polynucleotides on a substrate which carries substrate bound moieties each with a hydroxyl group.
Owner:UNIV OF COLORADO THE REGENTS OF +1
Generating protein pro-drugs using reversible PPG linkages
InactiveUS20050079155A1Reduce and block activityEliminate side effectsPeptide/protein ingredientsPharmaceutical non-active ingredientsSide effectProtecting group
The invention relates to methods for modulating the side effects, including immunogenicity, of a protein therapeutic via derivatization with a protein protecting group using reversible or labile linkages.
Owner:XENCOR
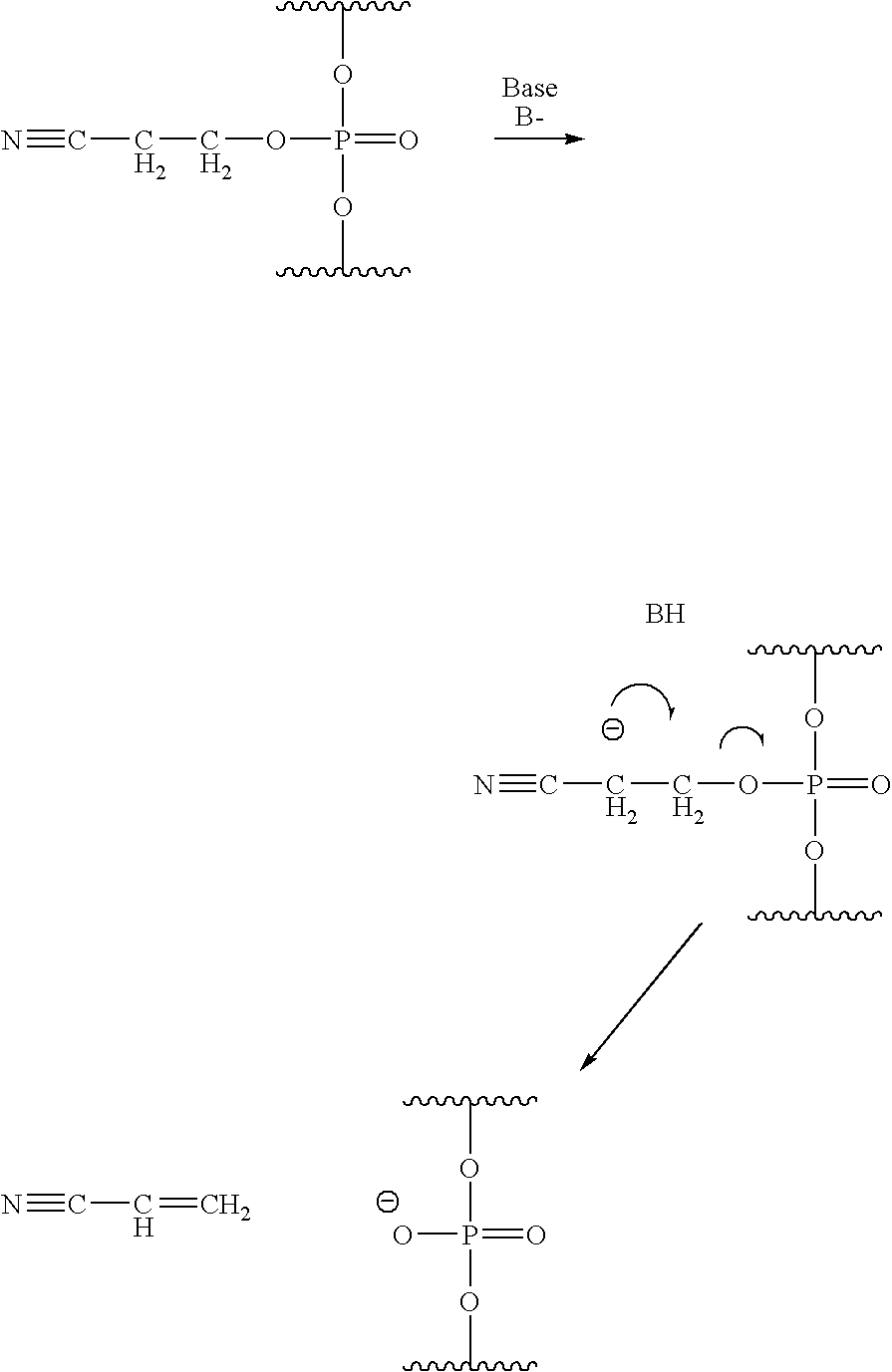
Methods of synthesizing oligonucleotides using carbonate protecting groups and alpha-effect nucleophile deprotection
The invention provides methods for synthesizing oligonucleotides using nucleoside monomers having carbonate protected hydroxyl groups that are deprotected with α-effect nucleophiles. The α-effect nucleophile irreversibly cleave the carbonate protecting groups while simultaneously oxidizing the internucleotide phosphite triester linkage to a phosphodiester linkage. The procedure may be carried out in aqueous solution at neutral to mildly basic pH. The method eliminates the need for separate deprotection and oxidation steps, and, since the use of acid to remove protecting groups is unnecessary, acid-induced depurination is avoided. Fluorescent or other readily detectable carbonate protecting groups can be used, enabling monitoring of individual reaction steps during oligonucleotide synthesis. The invention is particularly useful in the highly parallel, microscale synthesis of oligonucleotides.
Owner:AGILENT TECH INC +1
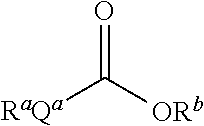
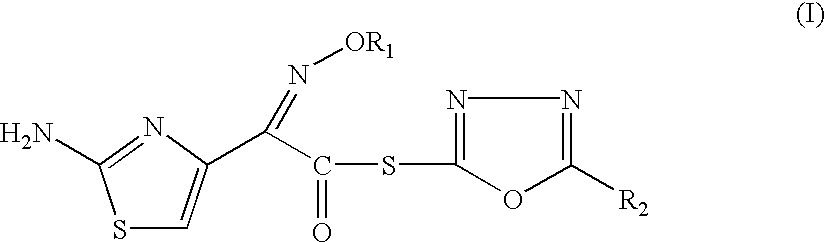
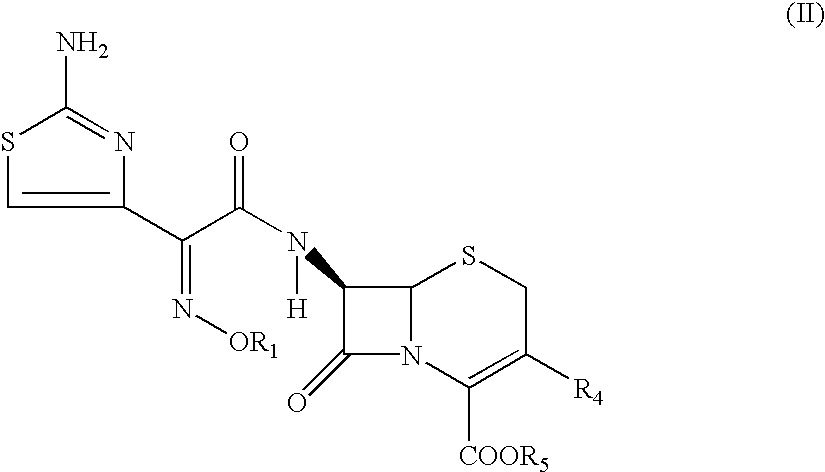
Thioester derivatives of thiazolyl acetic acid and their use in the preparation of cephalosporin compounds
InactiveUS6388070B1High purityHigh yieldOrganic chemistryBulk chemical productionAcetic acidHydrogen
The present invention provides novel thioester derivatives of thiazolyl acetic acid of the general formula (I),wherein, R1 represents H, trityl, CH3, or CRaRbCOOR3, in which Ra and Rb, independently of one another, represents hydrogen or methyl and R3 represents H or C1-C7 alkyl; and R2 represents C1-C4 alkyl or phenyl. The invention also provides a method for preparation of the thioester derivatives and reaction of the thioester derivatives with cephem carboxylic acids to produce cephalosporin antibiotic compounds having general formula (II),wherein, R1 represents H, trityl, CH3, or CRaRbCOOR3, in which Ra and Rb, independently of one another, represents hydrogen or methyl and R3 represents H or C1-C7 alkyl; R4 is CH3, -CH=CH2, CH2OCH3, CH2OCOCH3,and R5 is H or a salt or a carboxylic protecting group, comprising,acylating a compound of formula (III),wherein, R4 and R5 are defined as above, and R6 is H or trimethylsilyl;with a compound of formula (I).
Owner:ORCHID CHEM & PHARM LTD
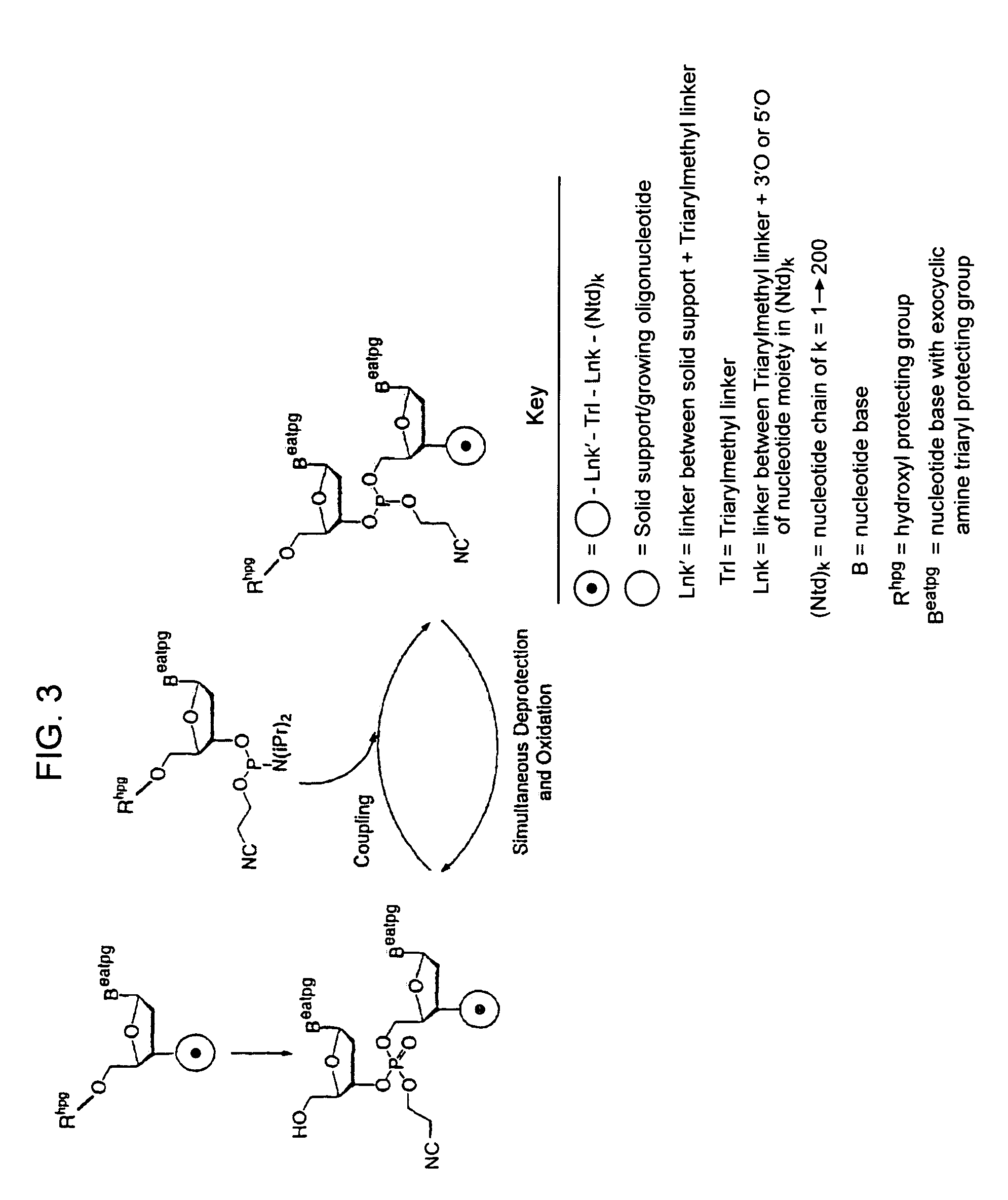
Exocyclic amine triaryl methyl protecting groups in two-step polynucleotide synthesis
Precursors for use in the synthesis of polynucleotides and methods of using the precursors in synthesizing polynucleotides are disclosed. The precursors include a heterocyclic base having an exocyclic amine group and a substituted or unsubstituted triaryl methyl protecting group bound to the exocyclic amine group.
Owner:AGILENT TECH INC +1
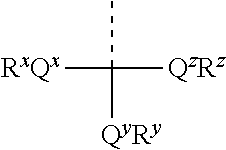
Phosphorus protecting groups
Compounds having a phosphorus group structure of:wherein:R is alkyl, modified lower alkyl; andRi and Ri are each independently H, alkyl, modified alkyl, or aryl; are provided. Also provided are polynucleotide compositions that include these compounds and methods of using the compounds in synthesis of the same.
Owner:UNIV OF COLORADO THE REGENTS OF +1
Method for polynucleotide synthesis
InactiveUS7417139B2Undesirable side reactionSugar derivativesPeptide/protein ingredientsHydrogenSynthesis methods
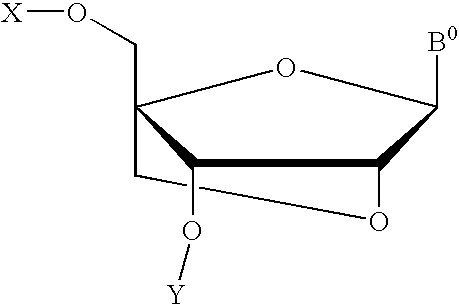
Methods of forming an internucleotide bond are disclosed. Such methods find use in synthesis of polynucleotides. The method involves contacting a functionalized support with a precursor having an exocyclic amine triaryl methyl protecting group under conditions and for a time sufficient to result in internucleotide bond formation. The functionalized support includes a solid support, a triaryl methyl linker group, and a nucleoside moiety having a reactive site hydroxyl, the nucleoside moiety attached to the solid support via the triaryl methyl linker group. In particular embodiments, the precursor has the structure:wherein:O and H represent oxygen and hydrogen, respectivelyR1 is hydrido, hydroxyl, protected hydroxyl, lower alkyl, modified lower alkyl, or alkoxy,one of R2 or R3 is a hydroxyl protecting group; and the other of R2 or R3 is a reactive group capable of reacting with the reactive site hydroxyl,Base is a heterocyclic base having an exocyclic amine group, andTram is the exocyclic amine triaryl methyl protecting group.
Owner:UNIV OF COLORADO THE REGENTS OF +1
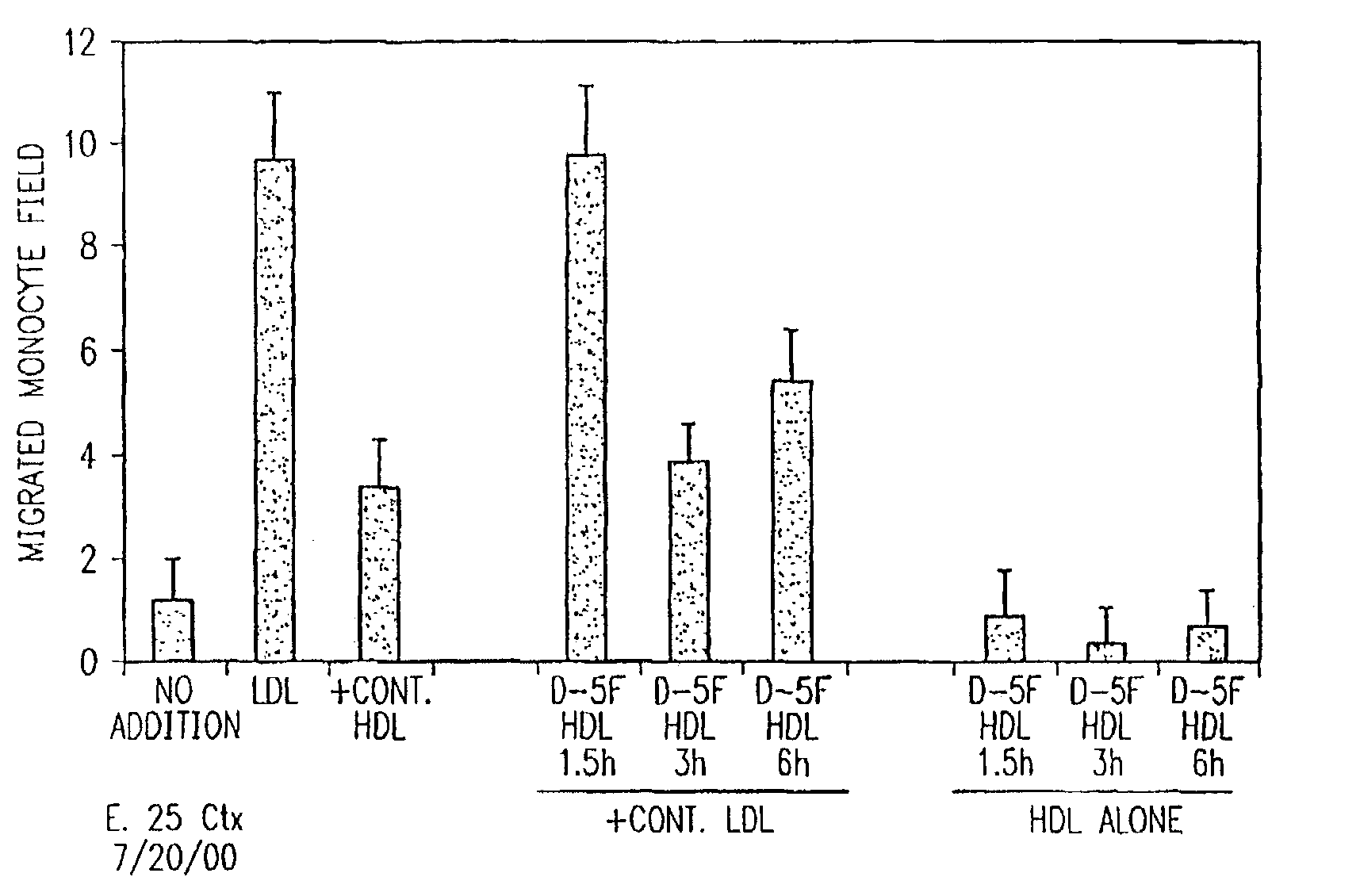
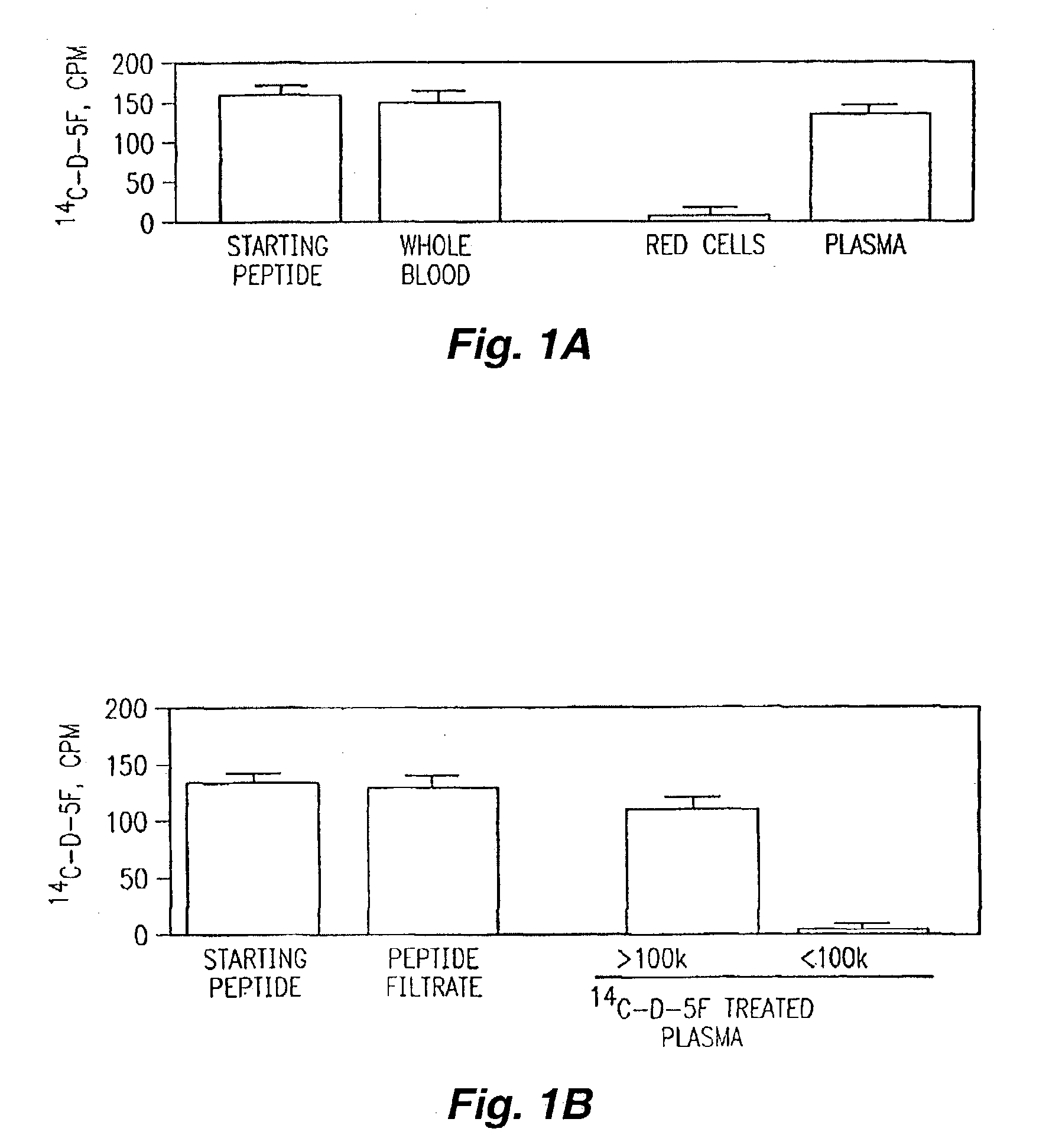
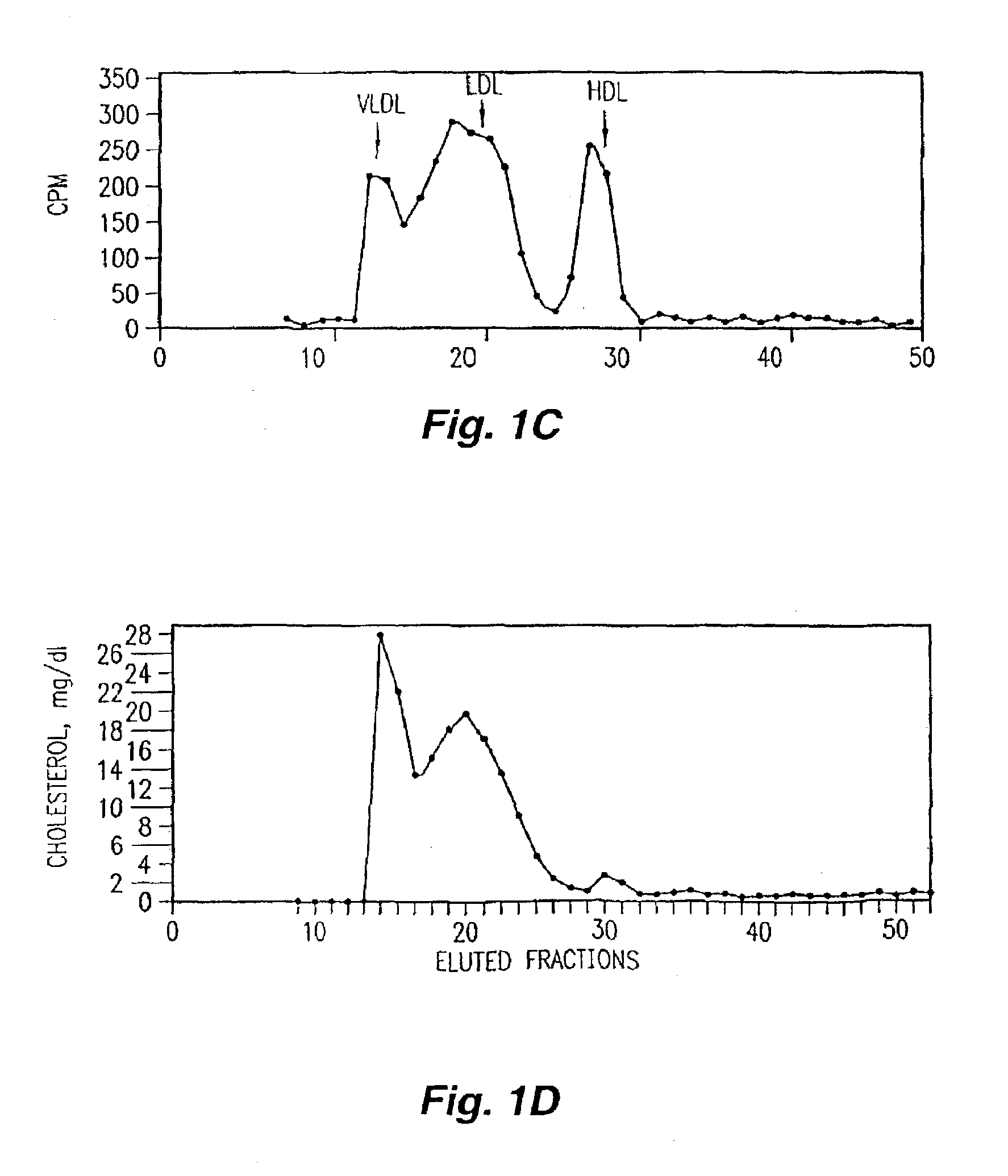
Orally administered peptides to ameliorate atherosclerosis
InactiveUS7144862B2Readily taken up and deliveredMany symptomAntibacterial agentsBiocideAmphipathic helixMedicine
This invention provides novel peptides that ameliorate one or more symptoms of atherosclerosis. In certain embodiments, the peptide comprises an amino acid sequence that ranges in length from about 10 up to about 30 amino acids, that comprises at least one class A amphipathic helix, that bears at least one protecting group, that protects a phospholipid against oxidation by an oxidizing agent; and that is not the D-18A peptide. The peptides are highly stable and readily administered via an oral route.
Owner:UAB RES FOUND +1
Precursors for two-step polynucleotide synthesis
Precursors for use in the synthesis of polynucleotides are disclosed. The precursors include a heterocyclic base having an exocyclic amine group and a substituted or unsubstituted triaryl methyl protecting group bound to the exocyclic amine group. In particular embodiments, the precursor has the structure:wherein:O and H represent oxygen and hydrogen, respectively,R1 is hydrido, hydroxyl, protected hydroxyl, lower alkyl, modified lower alkyl, or alkoxy,one of R2 or R3 is a hydroxyl protecting group; and the other of R2 or R3 is a reactive group capable of reacting with a reactive site hydroxyl,Base is a heterocyclic base having an exocyclic amine group, andTram is a triaryl methyl group having the structure (V)wherein the broken line represents a bond to the amino nitrogen of the exocyclic amine group, and R4, R5 and R6 are independently selected from unsubstituted or substituted aryl groups, provided that at least one of R4, R5, and R6 is an aryl group other than phenyl and other than substituted phenyl.
Owner:AGILENT TECH INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines 11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dfe0bdc2-9bfe-4984-a8af-93415b268a91/US07741319-20100622-C00001.png)
![11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines 11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dfe0bdc2-9bfe-4984-a8af-93415b268a91/US07741319-20100622-C00002.png)
![11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines 11-hydroxy-5h-pyrrolo[2,1-c][1,4] benzodiazepin-5-one derivatives as key intermediates for the preparation of c2 substituted pyrrolobenzodiazepines](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dfe0bdc2-9bfe-4984-a8af-93415b268a91/US07741319-20100622-C00003.png)