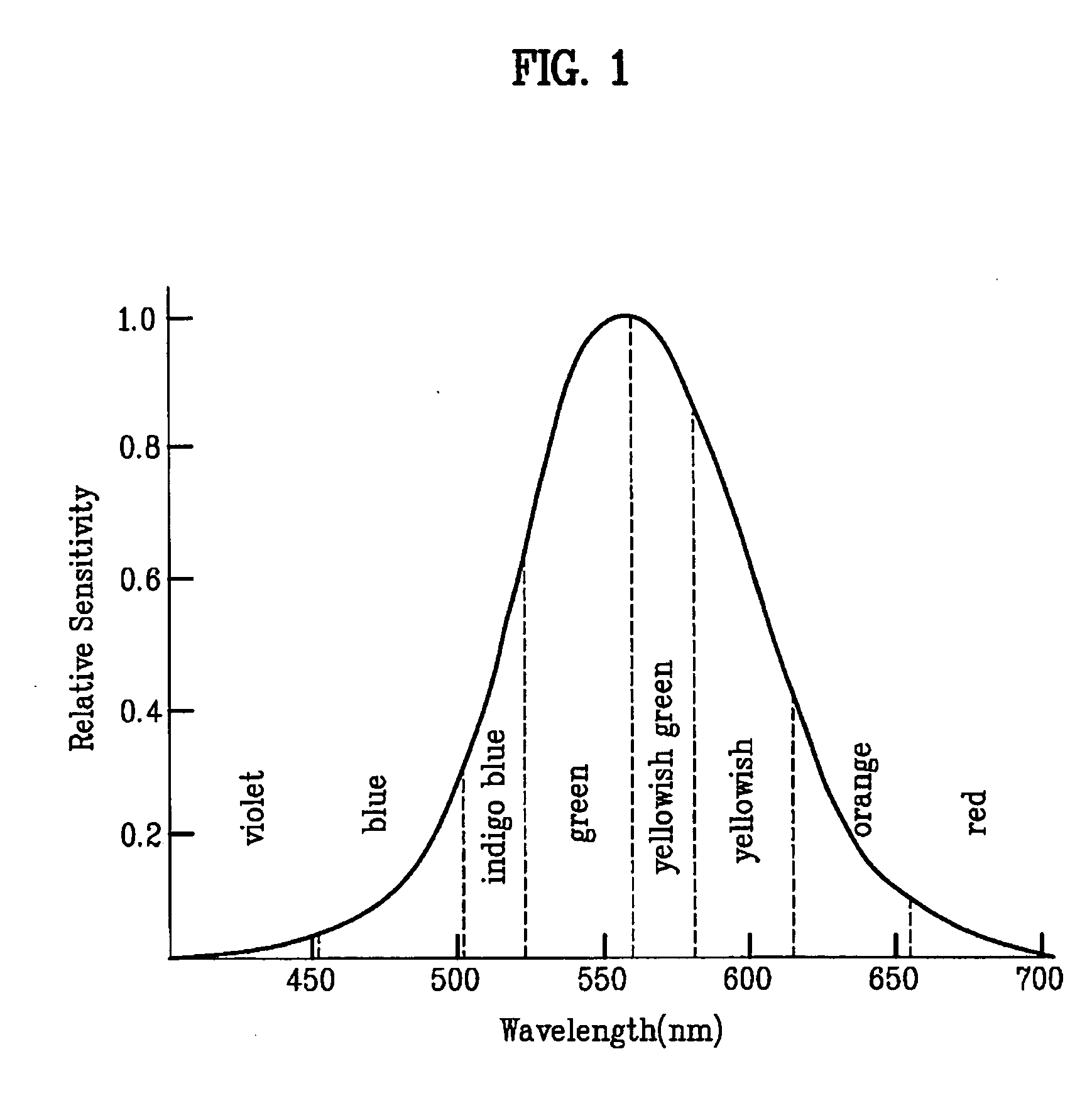
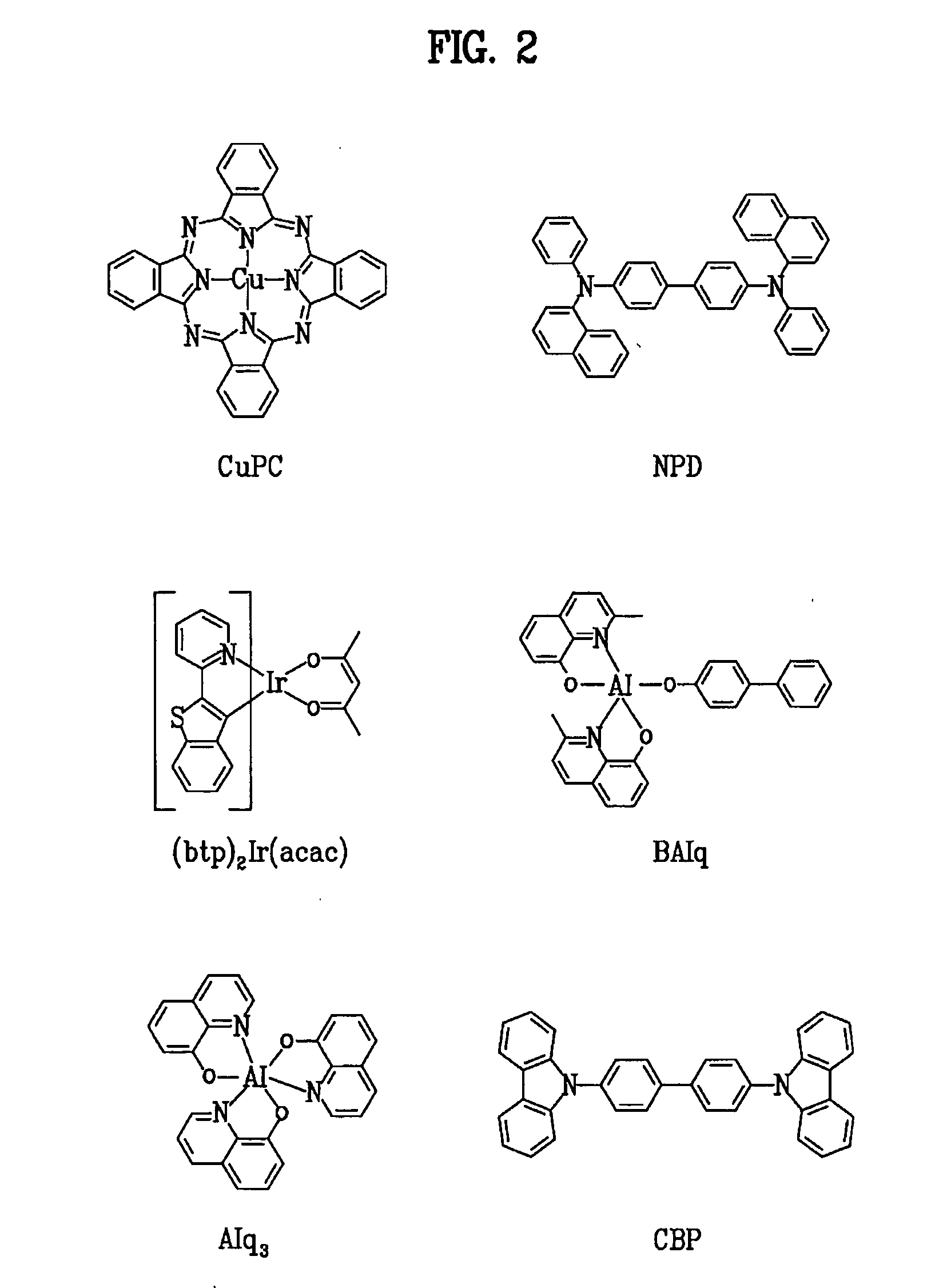
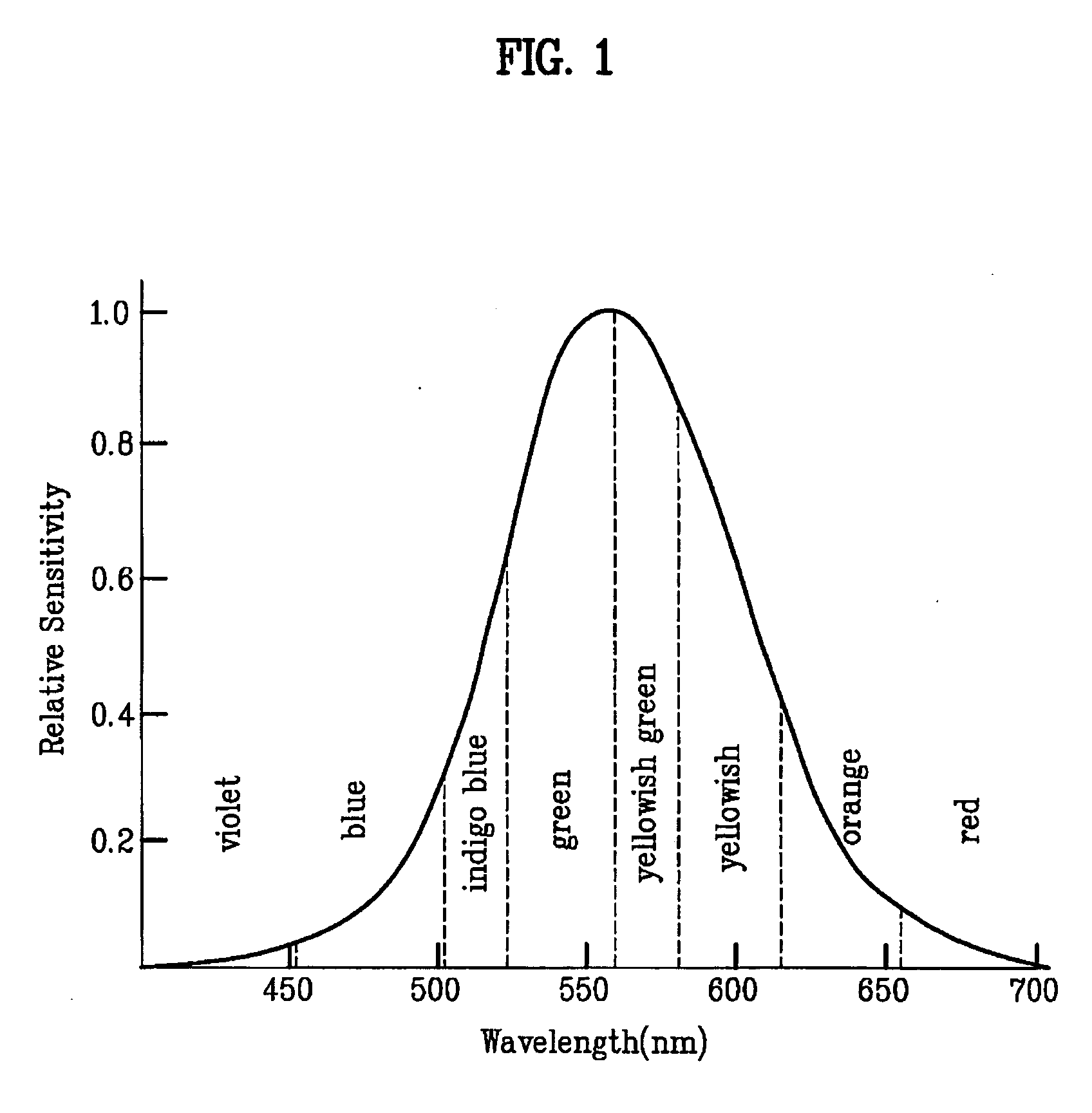
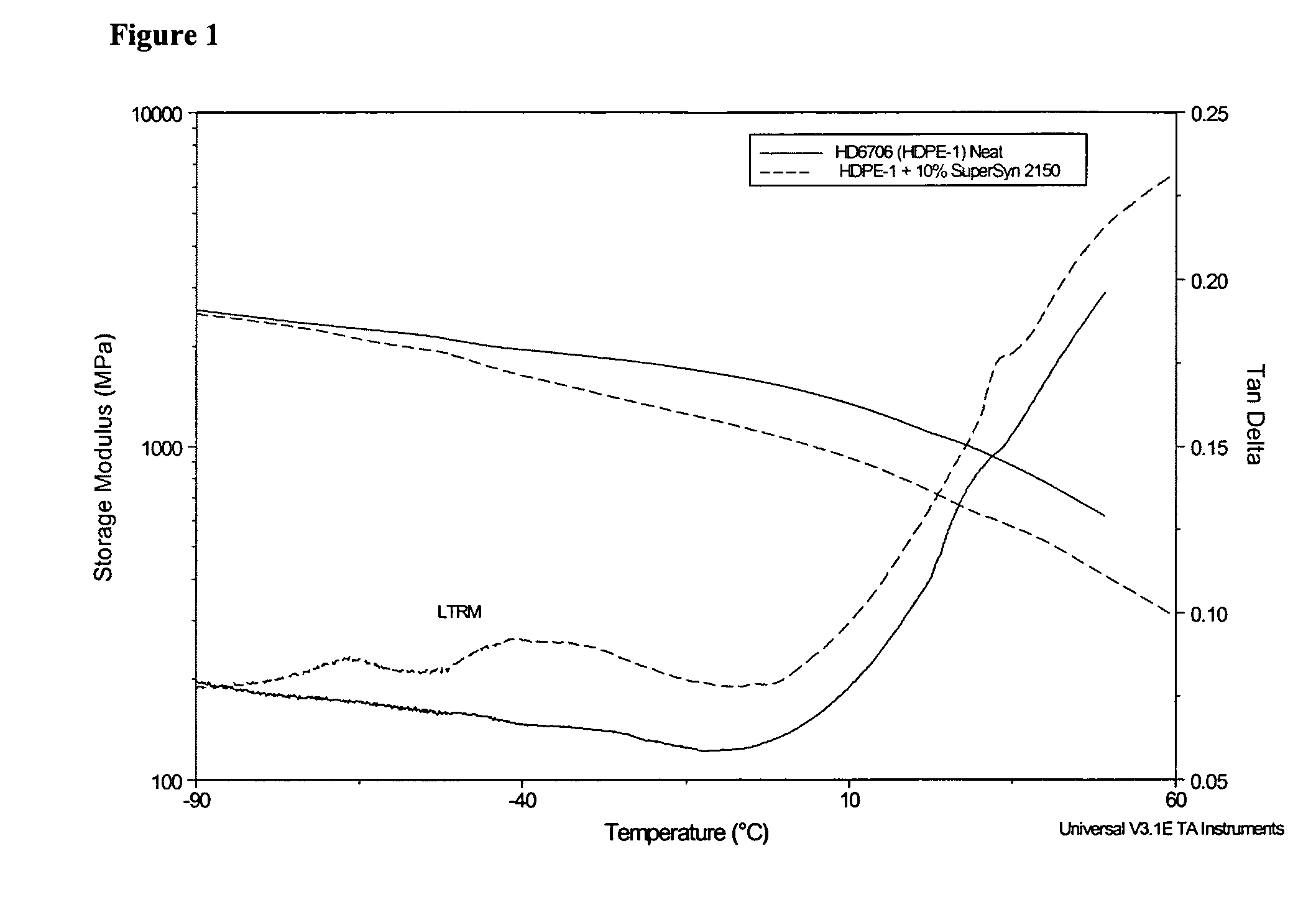
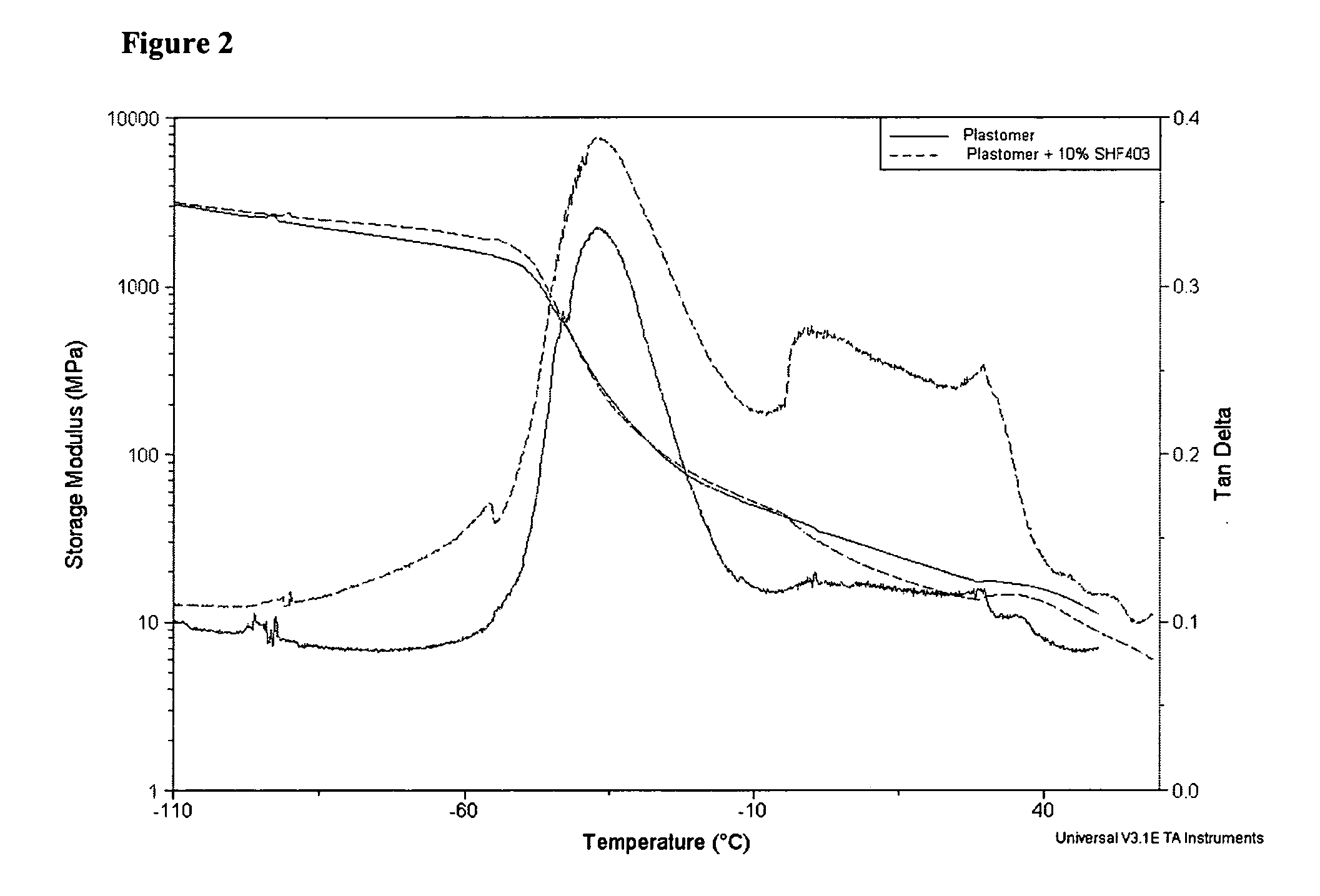
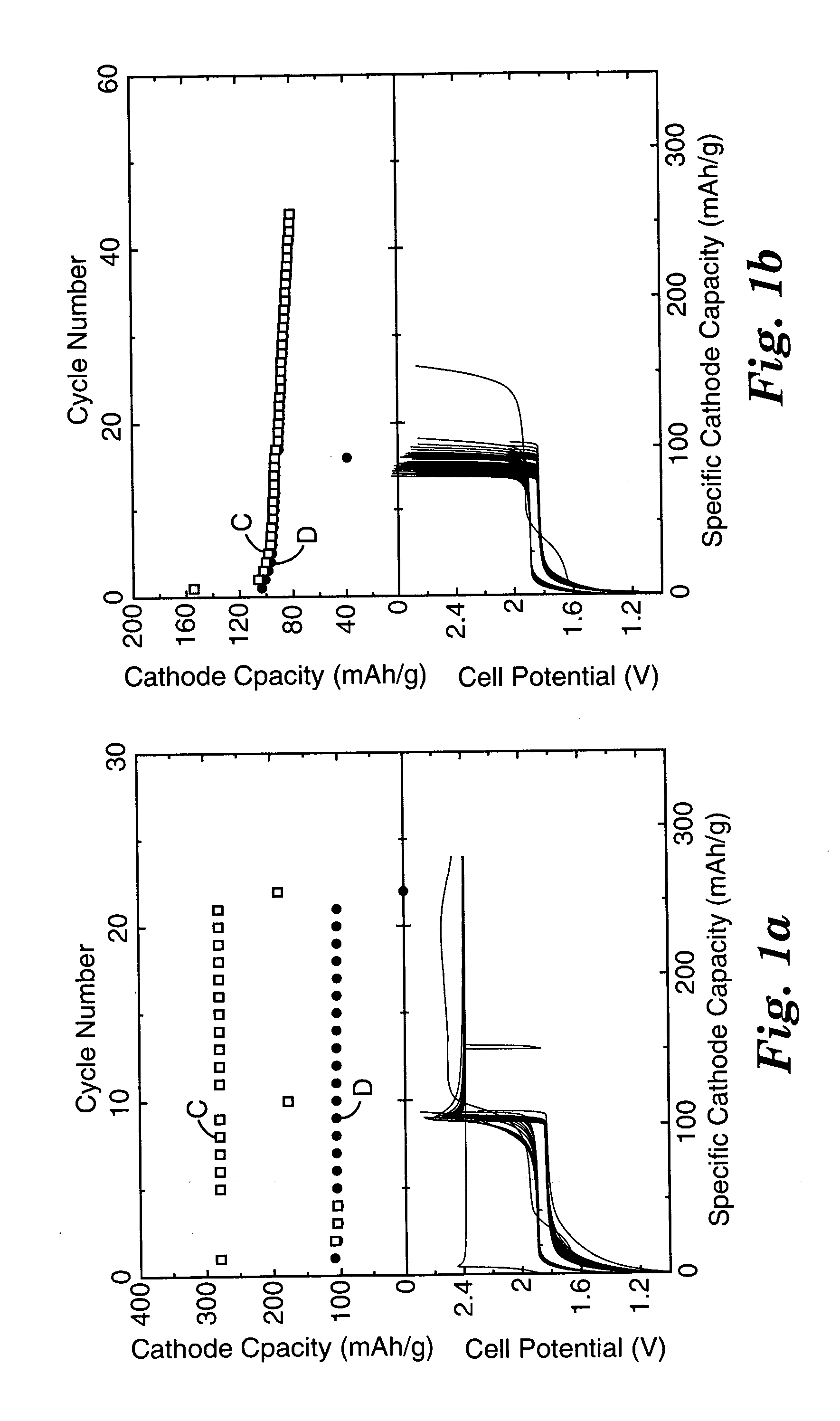
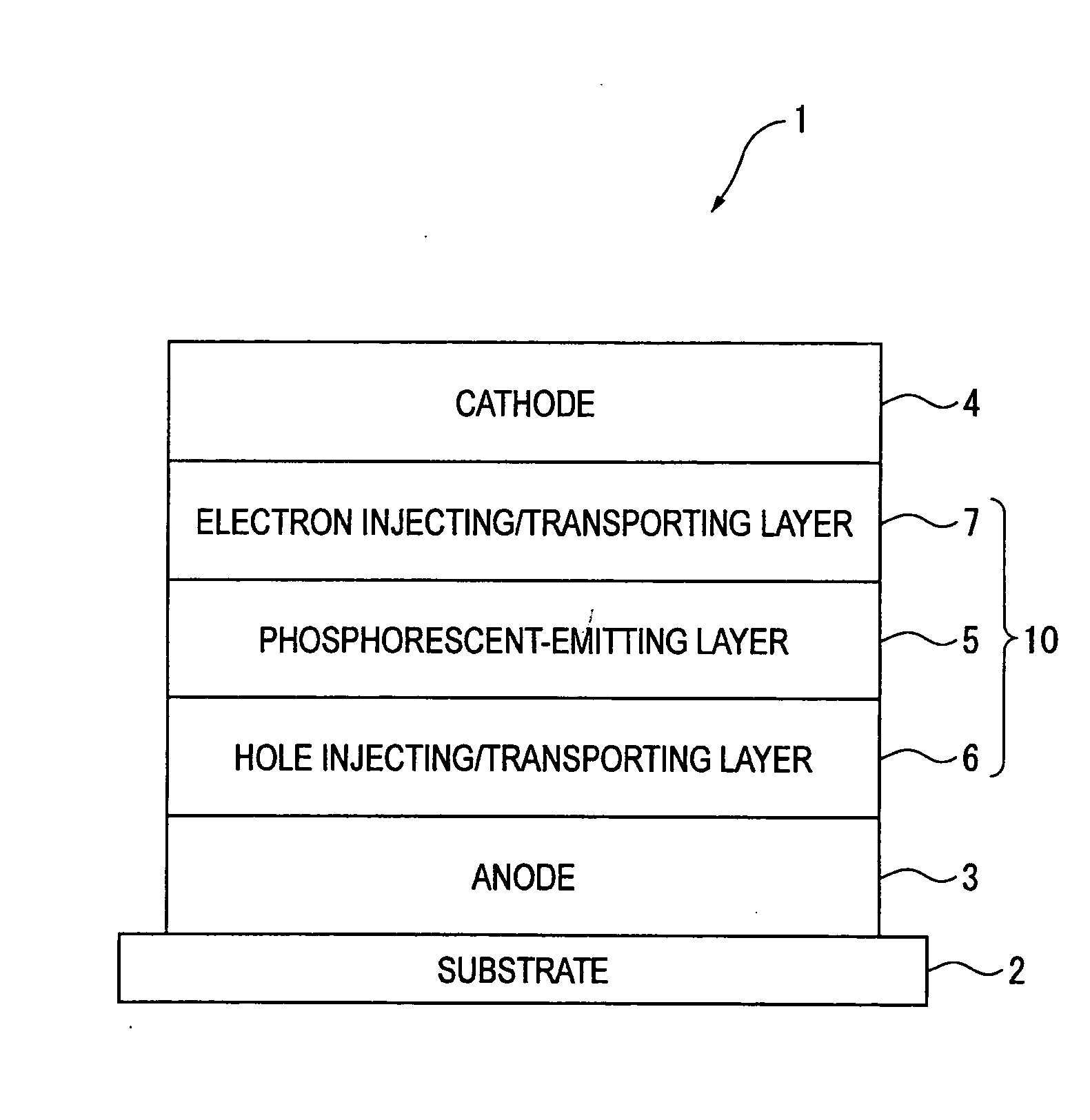
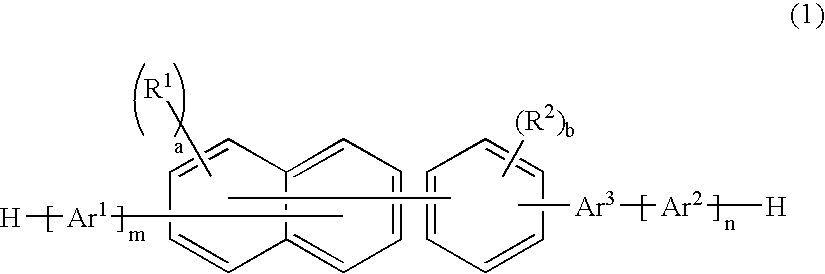
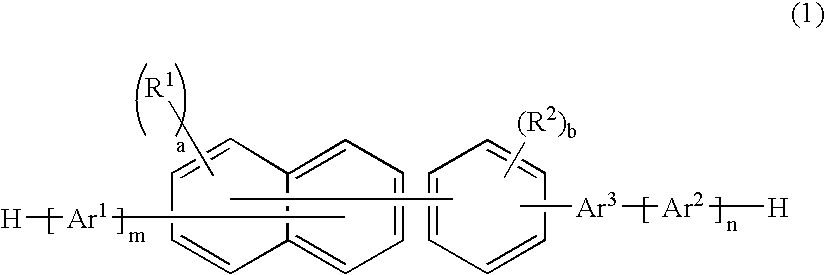
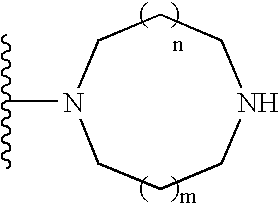
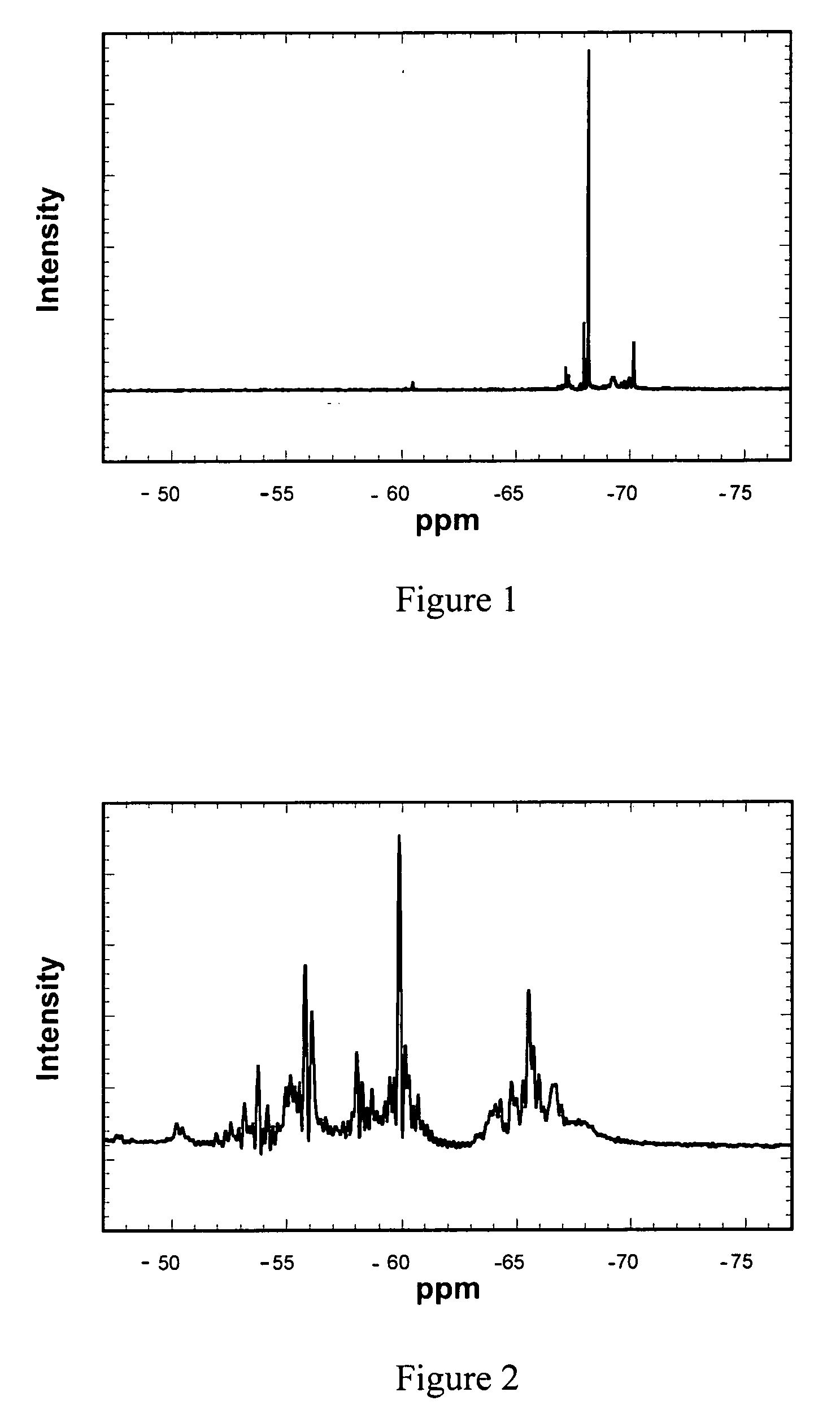
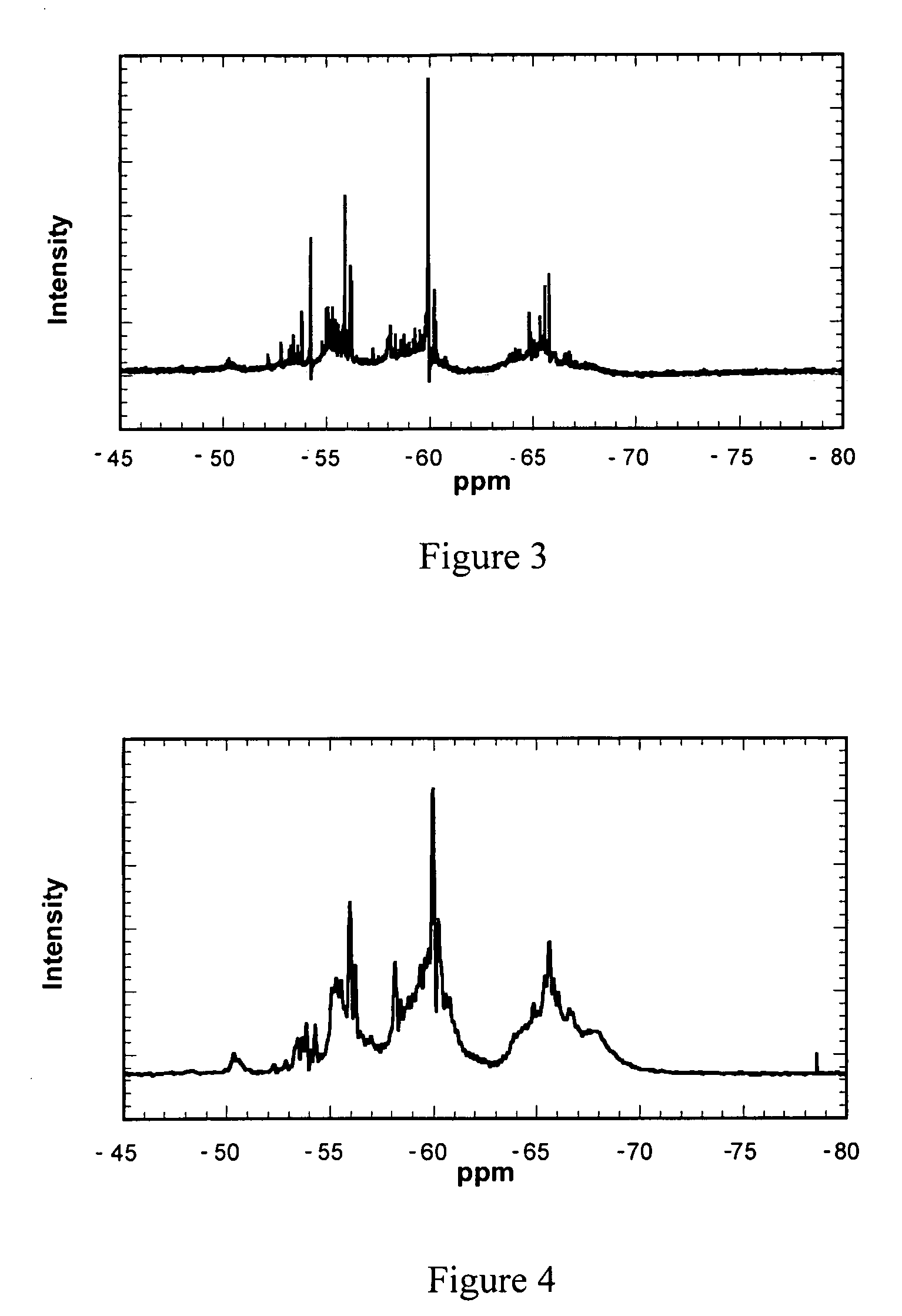
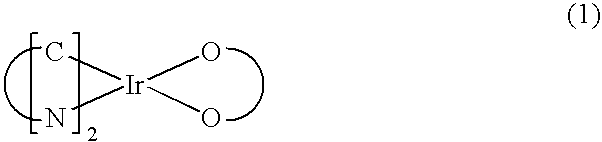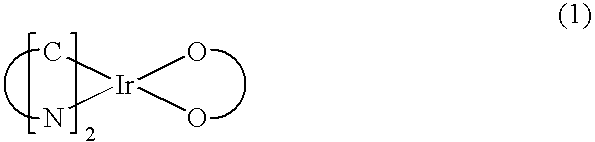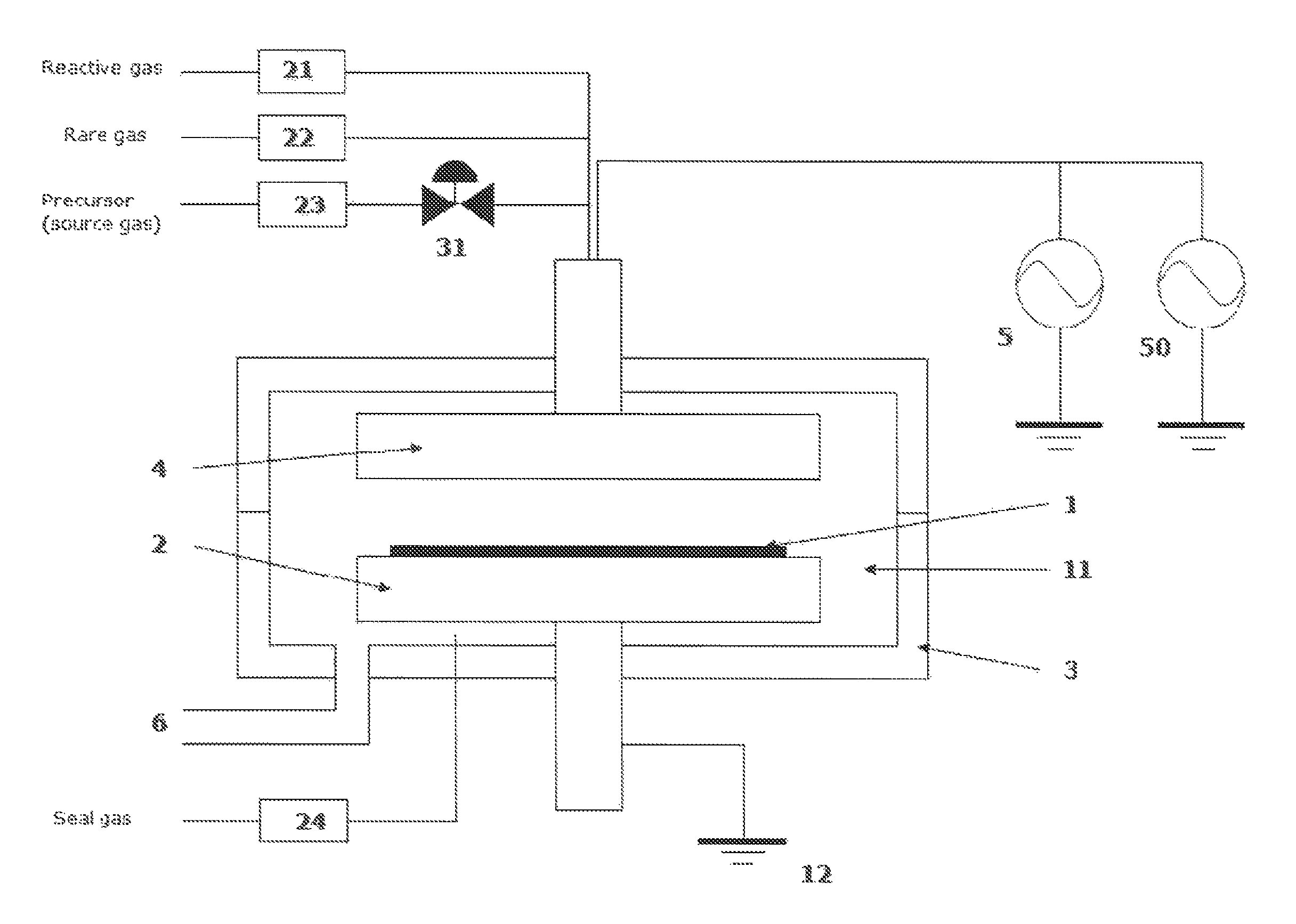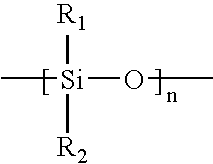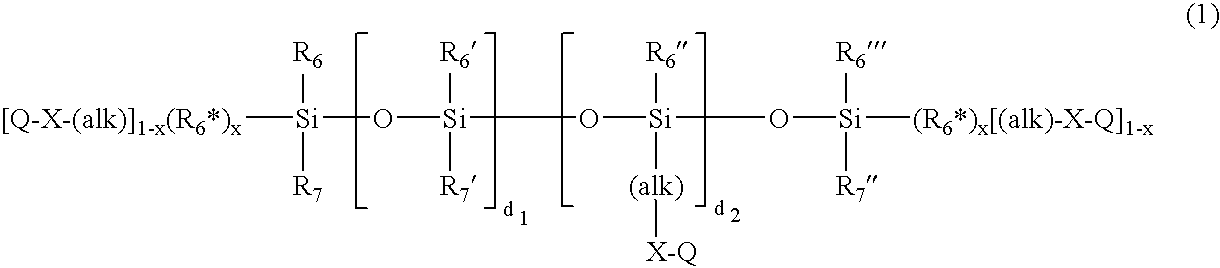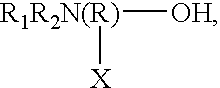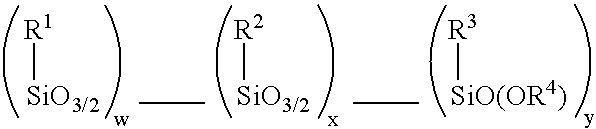Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
8301 results about "Alkoxy group" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In chemistry, the alkoxy group is an alkyl (carbon and hydrogen chain) group singularly bonded to oxygen; thus R–O. The range of alkoxy group is vast, the simplest being methoxy (CH₃O–). An ethoxy group (CH₃CH₂O–) is found in the organic compound ethyl phenyl ether, C₆H₅OCH₂CH₃ which is also known as ethoxybenzene. Related to alkoxy groups are aryloxy groups, which have an aryl group singular bonded to oxygen such as the phenoxy group (C₆H₅O–).
Polyester process
A process for the preparation of an unsaturated polyester which comprises (i) reacting an organic diol with a cyclic akylene carbonate in the presence of a first catalyst to thereby form a polyalkoxy diol, and (ii) optionally adding thereto a further amount of cyclic alkylene carbonate in the presence of a second catalyst, and (iii) subsequently polycondensing the resulting mixture with a dicarboxylic acid.
Owner:XEROX CORP
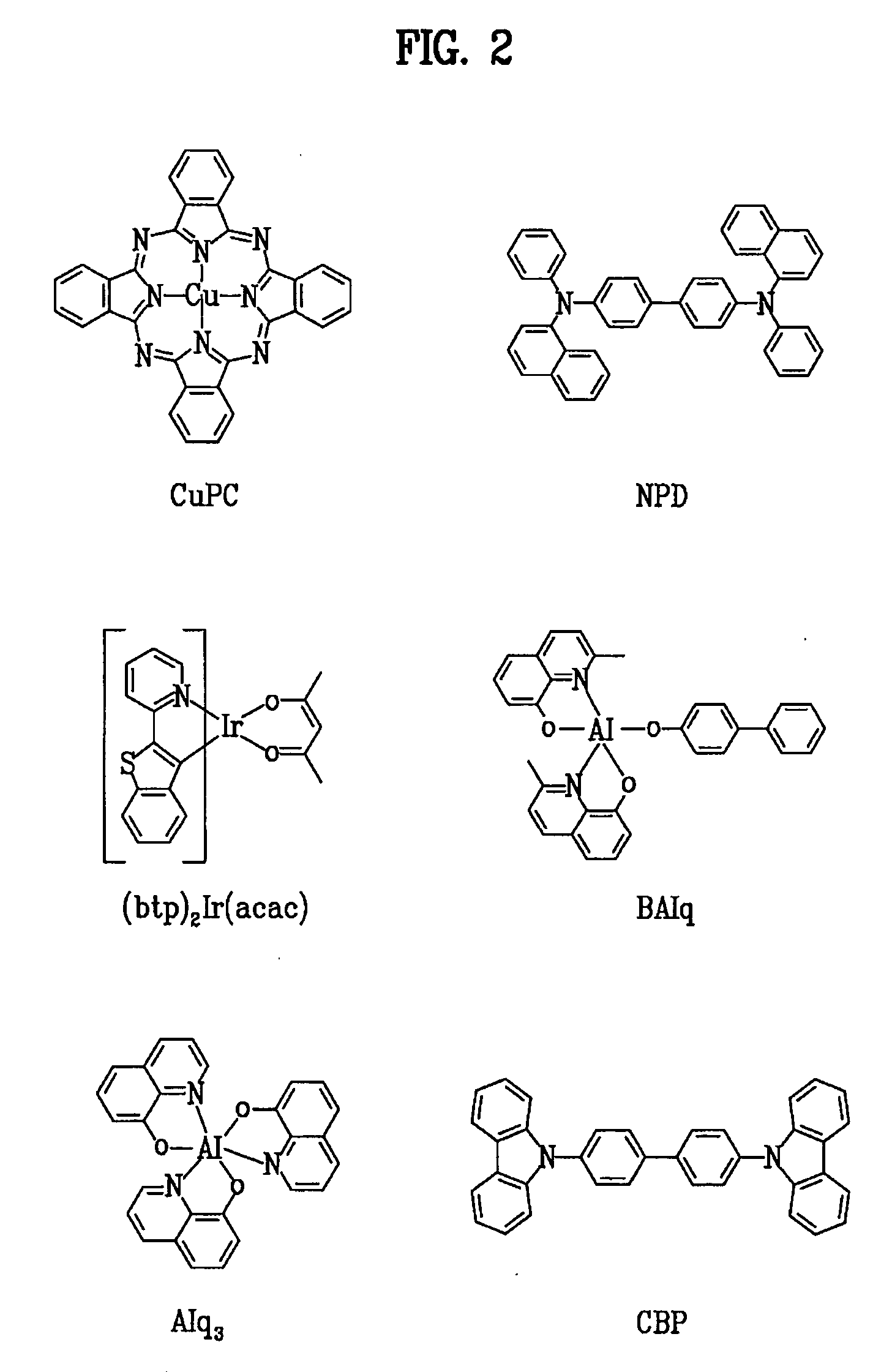
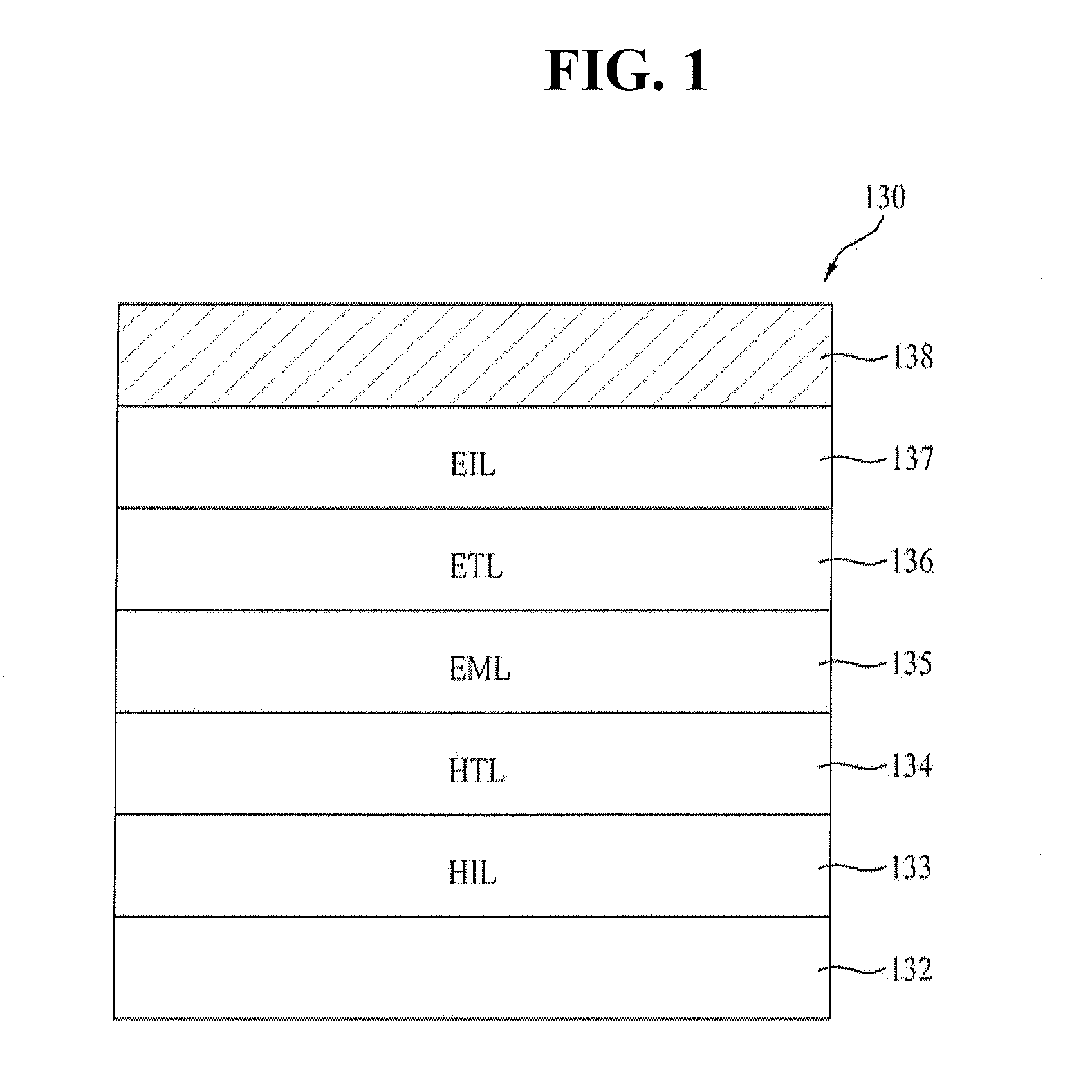
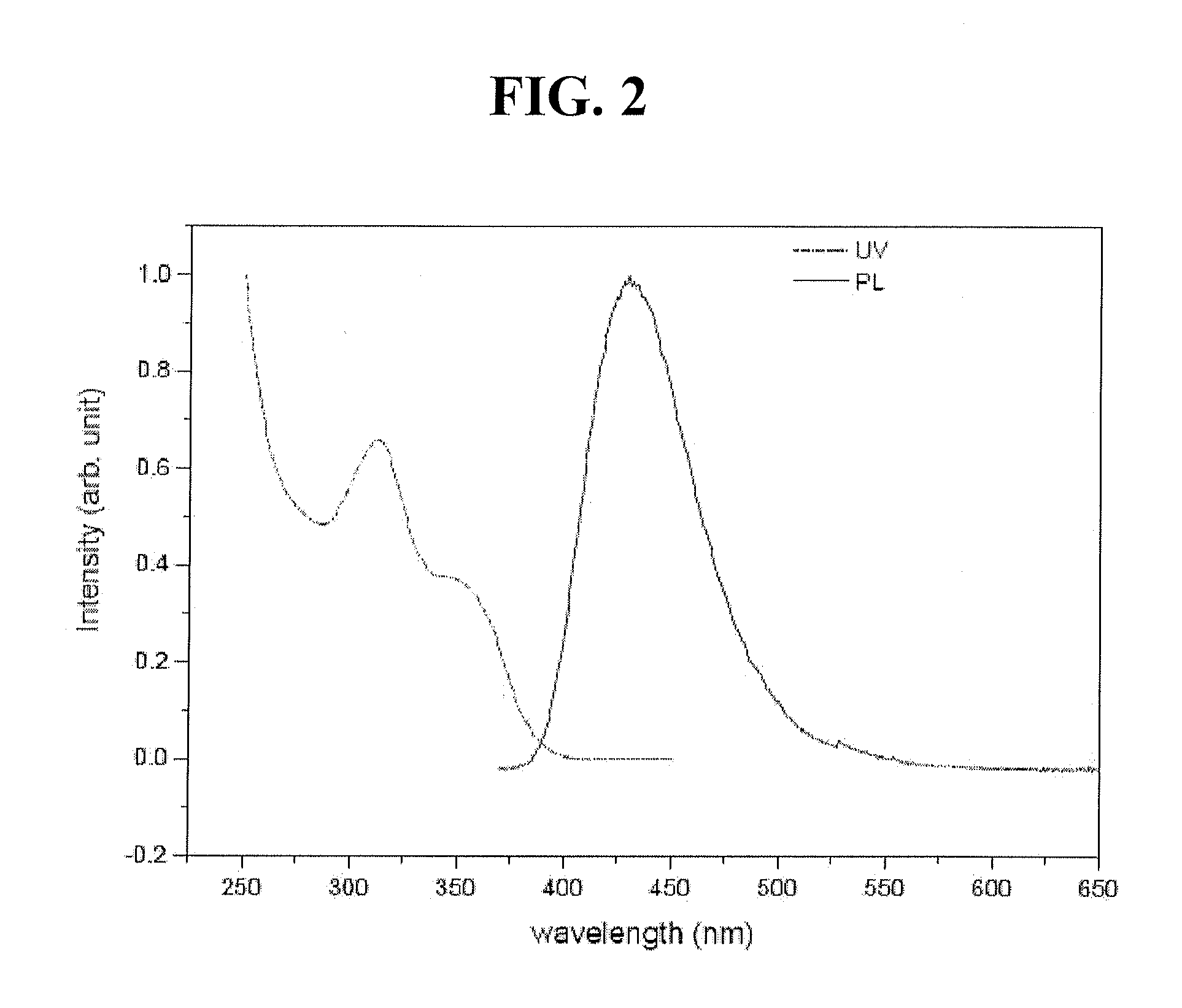
Red phosphorescent compounds and organic electroluminescent devices using the same
ActiveUS20070104980A1High color purityLong life-timeIndium organic compoundsDischarge tube luminescnet screensDopantHydrogen
Disclosed herein are red phosphorescent compounds of the following Formulas 1 to 4: wherein is R1, R2 and R3 are independently a C1-C4 alkyl group, R4, R5, R6 and R7 are independently selected from hydrogen, C1-C4 alkyl groups and C1-C4 alkoxy groups, and is selected from 2,4-pentanedione, 2,2,6,6,-tetramethylheptane-3,5-dione, 1,3-propanedione, 1,3-butanedione, 3,5-heptanedione, 1,1,1-trifluoro-2,4-pentanedione, 1,1,1,5,5,5-hexafluoro-2,4-pentanedione, and 2,2-dimethyl-3,5-hexanedione; wherein is R1 and R2 are independently selected from C1-C4 alkyl groups and C1-C4 alkoxy groups, R3, R4, R5 and R6 are independently selected from hydrogen, C1-C4 alkyl groups and C1-C4 alkoxy groups, and is selected from 2,4-pentanedione, 2,2,6,6,-tetramethylheptane-3,5-dione, 1,3-propanedione, 1,3-butanedione, 3,5-heptanedione, 1,1,1-trifluoro-2,4-pentanedione, 1,1,1,5,5,5-hexafluoro-2,4-pentanedione and 2,2-dimethyl-3,5-hexanedione; wherein is R1 and R2 are independently selected from C1-C4 alkyl groups and C1-C4 alkoxy groups, R3, R4, R5 and R6 are independently selected from hydrogen, C1-C4 alkyl groups and C1-C4 alkoxy groups, and is selected from 2,4-pentanedione, 2,2,6,6,-tetramethylheptane-3,5-dione, 1,3-propanedione, 1,3-butanedione, 3,5-heptanedione, 1,1,1-trifluoro-2,4-pentanedione, 1,1,1,5,5,5-hexafluoro-2,4-pentanedione and 2,2-dimethyl-3,5-hexanedione; and wherein is R1 and R2 are independently selected from C1-C4 alkyl groups and C1-C4 alkoxy groups, R3, R4, R5 and R6 are independently selected from hydrogen, C1-C4 alkyl groups and C1-C4 alkoxy groups, and is selected from 2,4-pentanedione, 2,2,6,6,-tetramethylheptane-3,5-dione, 1,3-propanedione, 1,3-butanedione, 3,5-heptanedione, 1,1,1-trifluoro-2,4-pentanedione, 1,1,1,5,5,5-hexafluoro-2,4-pentanedione and 2,2-dimethyl-3,5-hexanedione. Further disclosed herein is an organic electroluminescent (EL) device comprising an anode, a hole injecting layer, a hole transport layer, a light-emitting layer, an electron transport layer, an electron injecting layer, and a cathode laminated in this order wherein one of the red phosphorescent compounds is used as a dopant of the light-emitting layer.
Owner:LG DISPLAY CO LTD
Red phosphorescent compound and organic electroluminescent device using the same
ActiveUS20070104979A1High color purityLong life-timeIndium organic compoundsDischarge tube luminescnet screensDopantHydrogen
Disclosed herein is a red phosphorescent compound of the following Formula 1: wherein is R1 is a C1-C4 alkoxy group, R2, R3, R4 and R5 are independently selected from hydrogen, C1-C4 alkyl groups and C1-C4 alkoxy groups, and is selected from 2,4-pentanedione, 2,2,6,6,-tetramethylheptane-3,5-dione, 1,3-propanedione, 1,3-butanedione, 3,5-heptanedione, 1,1,1-trifluoro-2,4-pentanedione, 1,1,1,5,5,5-hexafluoro-2,4-pentanedione, and 2,2-dimethyl-3,5-hexanedione. Further disclosed herein is an organic electroluminescent (EL) device comprising an anode, a hole injecting layer, a hole transport layer, a light-emitting layer, an electron transport layer, an electron injecting layer, and a cathode laminated in this order wherein one of the red phosphorescent compounds is used as a dopant of the light-emitting layer.
Owner:LG DISPLAY CO LTD
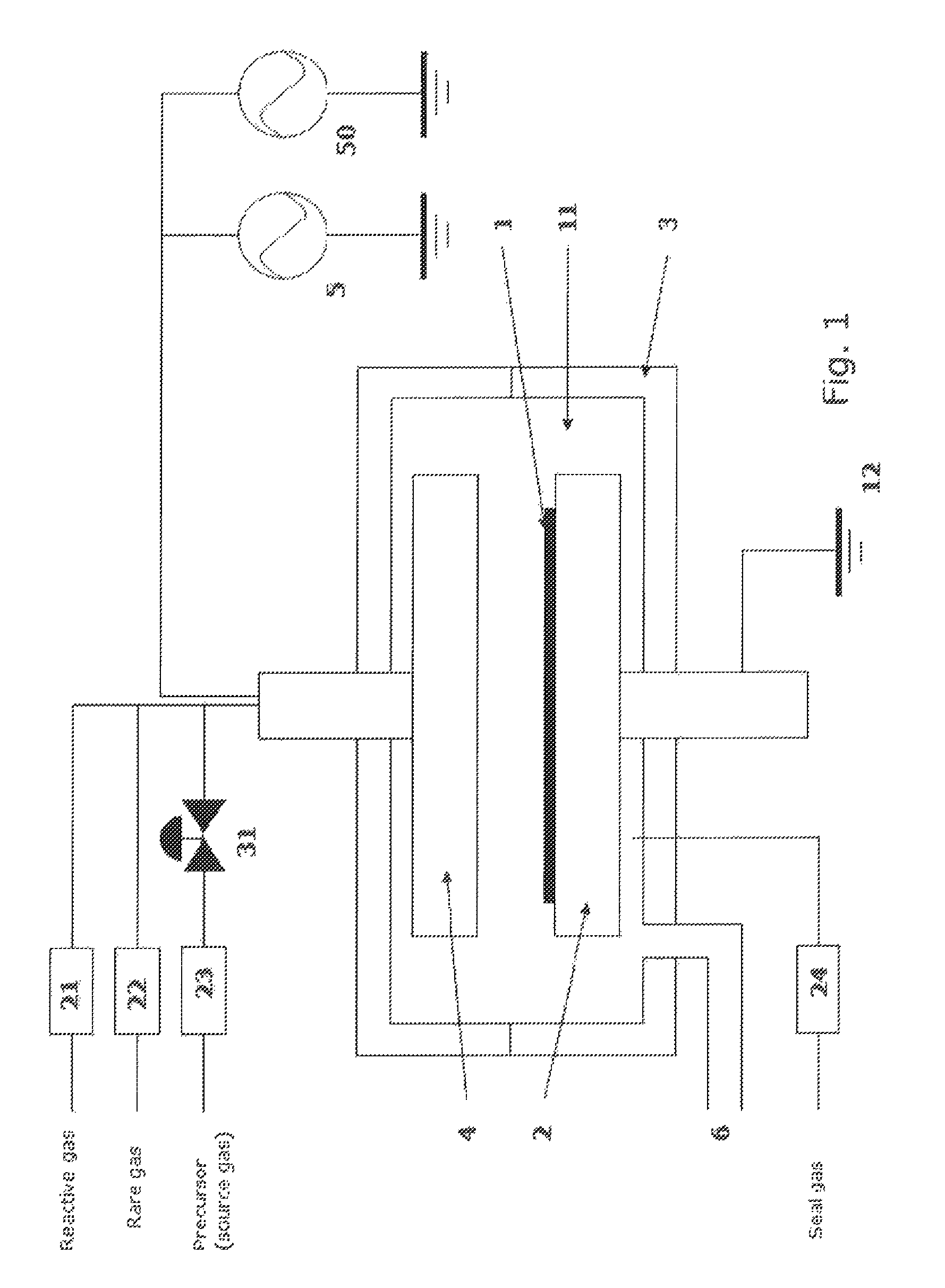
Method for forming aluminum oxide film using Al compound containing alkyl group and alkoxy or alkylamine group
ActiveUS8784950B2Safety with regard to handling and storage of the precursor can be ensuredDamage is causedChemical vapor deposition coatingPlasma techniqueProduct gasPhotochemistry
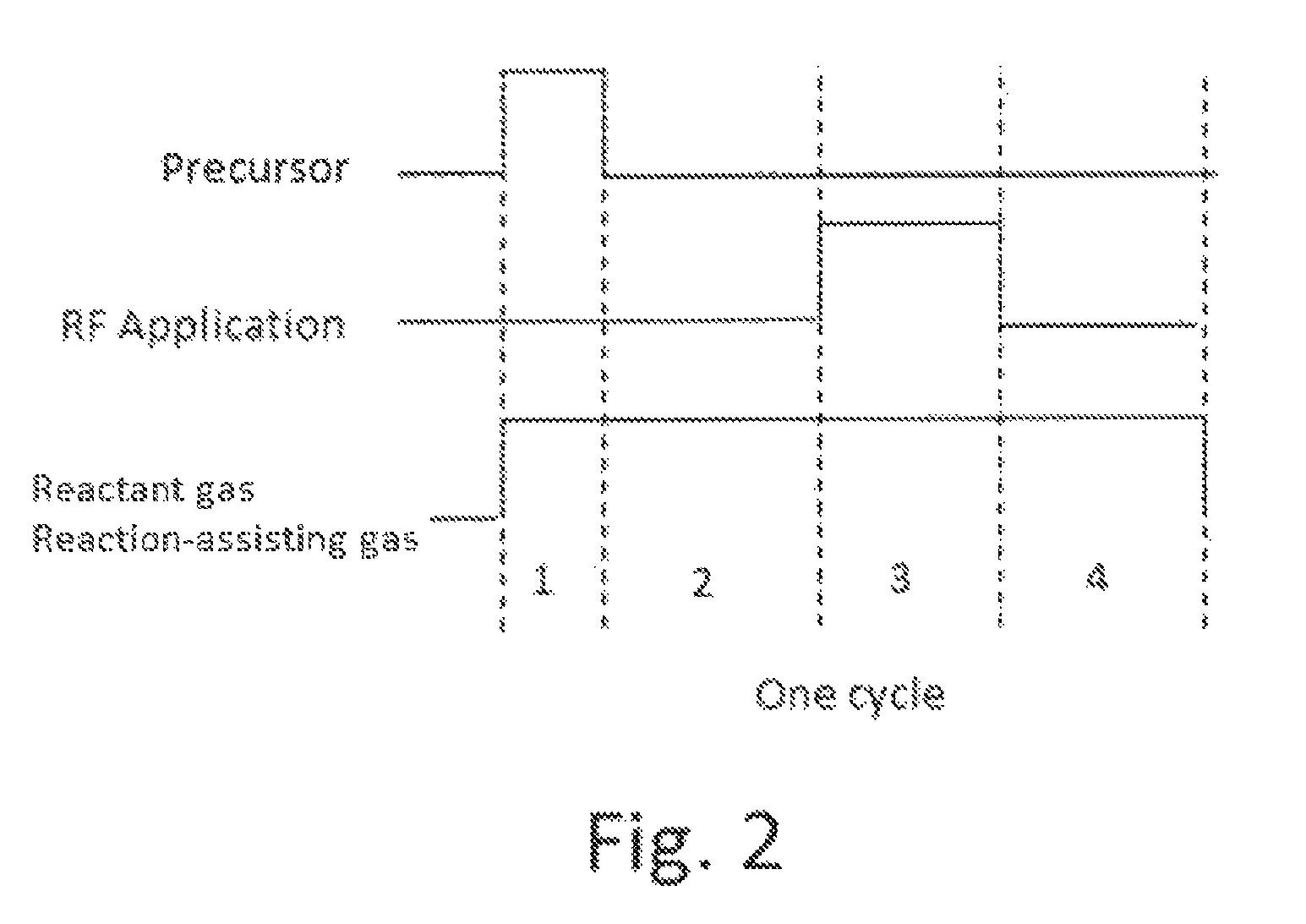
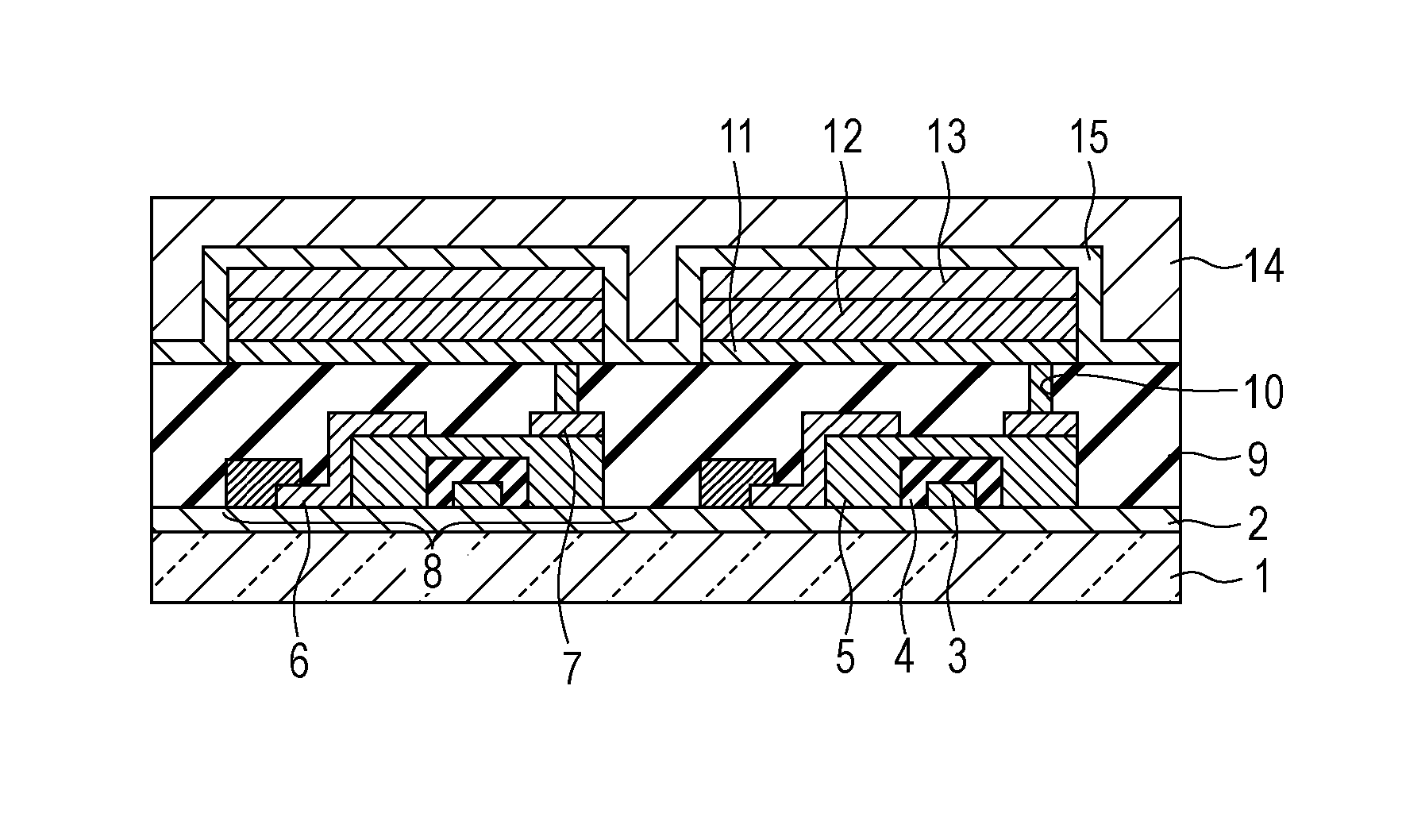
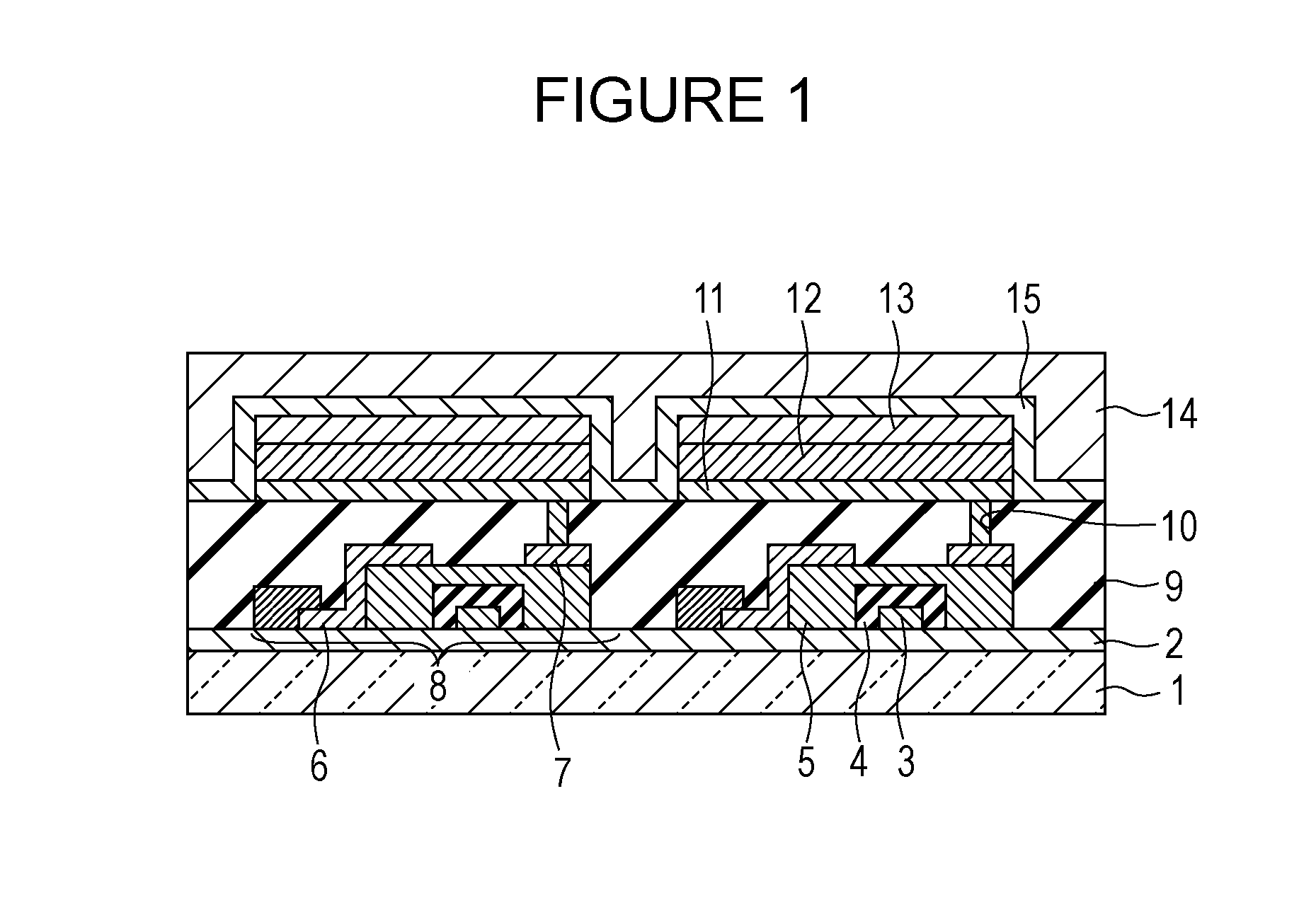
A method for forming a conformal film of aluminum oxide on a substrate having a patterned underlying layer by PEALD includes: adsorbing an Al precursor containing an Al—C bond and an Al—O—C or Al—N—C bond; providing an oxidizing gas and an inert gas; applying RF power to the reactant gas and the reaction-assisting gas to react the adsorbed precursor with the reactant gas on the surface, thereby forming a conformal film of aluminum oxide on the patterned underlying layer of the substrate, wherein the substrate is kept at a temperature of about 200° C. or lower.
Owner:ASM IP HLDG BV
New condensed polycyclic compound and organic light-emitting element using the same
InactiveUS20130175519A1Improve light emission efficiencyReduce the driving voltageOrganic chemistryOrganic compound preparationPolycyclic compoundAryl
The present invention provides a stable new condensed polycyclic compound which is not likely to form a molecular association. In addition, the present invention also provides an organic light-emitting element having a high light-emitting efficiency and a low drive voltage. In the condensed polycyclic compound in Claim 1 represented by the general formula [1], R1, R2 and R5 are each independently selected from a hydrogen atom, an alkyl group having 1 to 4 carbon atoms, an aryl group, and a heterocyclic group. R3 and R4 each represent an alkyl group having 1 to 4 carbon atoms. The aryl group and the heterocyclic group each may have at least one of an alkyl group, an aralkyl group, an aryl group, a heterocyclic group, an amino group, and an alkoxy group as a substituent.
Owner:CANON KK
Modified polyethylene compositions
The present invention relates to a composition comprising more than 25 weight % (based on the weight of the composition) of one or more ethylene polymers having an Mw of 20,000 g / mole or more and at least 0.1 weight % of a liquid hydrocarbon modifier where the modifier has: 1) a viscosity index of 120 or more, and 2) an kinematic viscosity of 3 to 3000 cSt at 100° C., and 3) a pour point of −10° C. or less, and 4) a flash point of 200° C. or more; and wherein the modifier contains less than 5 weight % of functional groups selected from hydroxide, aryls, substituted aryls, halogens, alkoxys, carboxylates, esters, acrylates, oxygen, nitrogen, and carboxyl, based upon the weight of the modifier.
Owner:EXXONMOBIL CHEM PAT INC
Novel Polymers
ActiveUS20080015315A1Sufficient amountReduce the amount requiredOrganic compound preparationCarboxylic acid esters preparationHydrophilic monomerPolymer science
The invention relates to novel crosslinkable copolymers which are obtainable by (a) copolymerizing at least two different hydrophilic monomers selected from the group consisting of N,N-dimethyl acrylamide (DMA), 2-hydroxyethyl acrylate (HEA), glycidyl methacrylate (GMA), N-vinylpyrrolidone (NVP), acrylic acid (AA) and a C1-C4-alkoxy polyethylene glycol (meth)acrylate having a weight average molecular weight of from 200 to 1500, and at least one crosslinker comprising two or more ethylenically unsaturated double bonds in the presence of a chain transfer agent having a functional group; and (b) reacting one or more functional groups of the resulting copolymer with an organic compound having an ethylenically unsaturated group.
Owner:ALCON INC
Nucleoside and oligonucleotide analogues
InactiveUS7335765B2Reduce incidenceImprove stabilityOrganic active ingredientsAntipyreticHydrogen atomHalogen
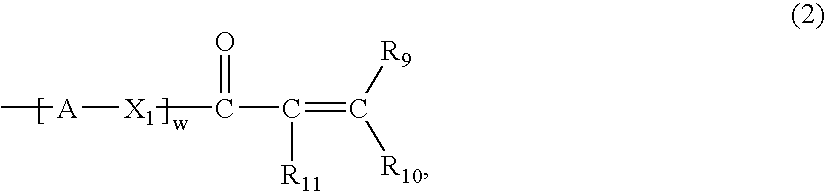
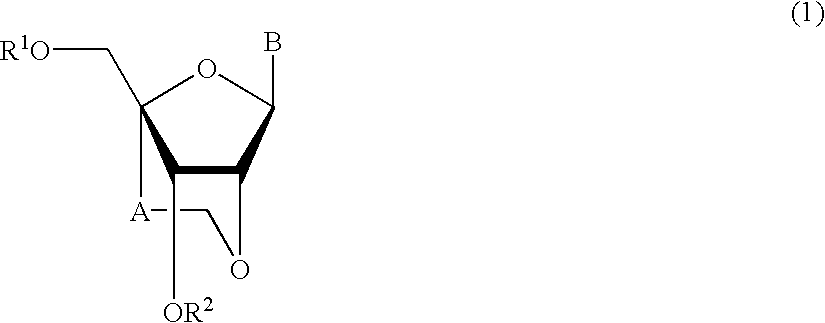
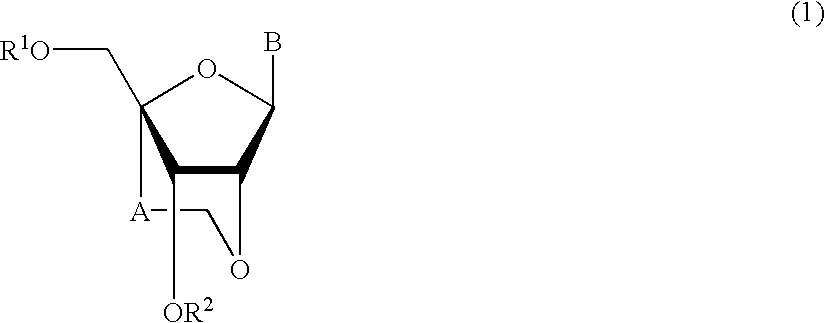
A compound of the formula (1):wherein R1 and R2 are the same or different and represent a hydrogen atom, a hydroxyl protecting group, a phosphate group, or —P(R3)R4, wherein R3 and R4 are the same or different and represent a hydroxyl group, an amino group, an alkoxy group having from 1 to 4 carbon atoms, a cyanoalkoxy group having from 1 to 5 carbon atoms or an amino group substituted by an alkyl group having from 1 to 4 carbon atoms; A represents an alkylene group having from 1 to 4 carbon atoms and B represents a purin-9-yl group, a 2-oxo-pyrimidin-1-yl group, a substituted purin-9-yl group or a substituted 2-oxo-pyrimidin-1-yl group having a substituent α selected from the group consisting of a hydroxyl group which may be protected, an alkoxy group having from 1 to 4 carbon atoms, a mercapto group which may be protected, an alkylthio group having from 1 to 4 carbon atoms, an alkoxy group having from 1 to 4 carbon atoms, an amino group which may be protected, a mono- or di-alkylamino group which may be substituted by an alkyl group having from 1 to 4 carbon atoms, an alkyl group having from 1 to 4 carbon atoms and a halogen atom; or a salt thereof.
Owner:DAIICHI SANKYO CO LTD
8-Oxoadenine Compound
InactiveUS20070225303A1Preventing systemic adverse effectReduce compoundingAntibacterial agentsBiocideDisease8-oxoadenine
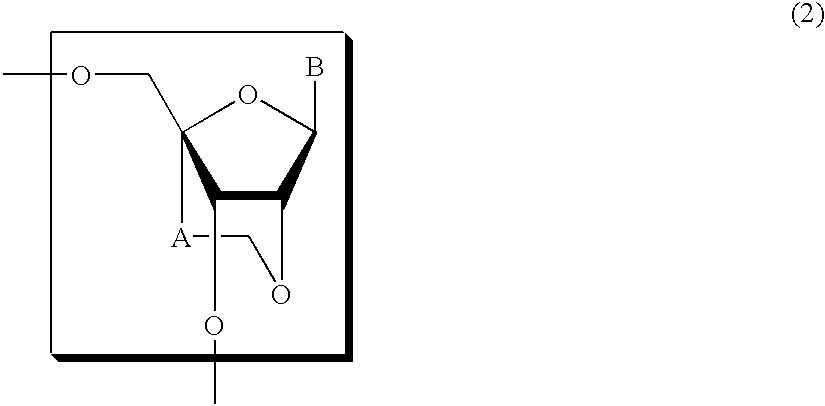
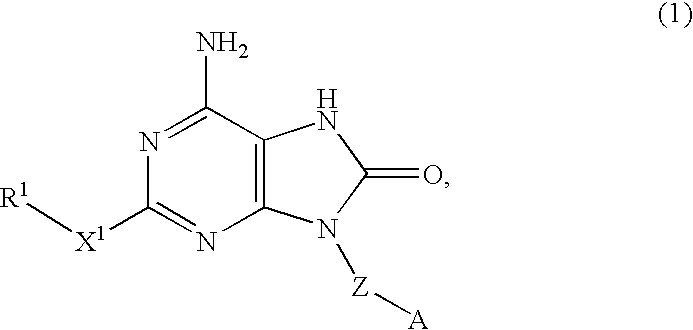
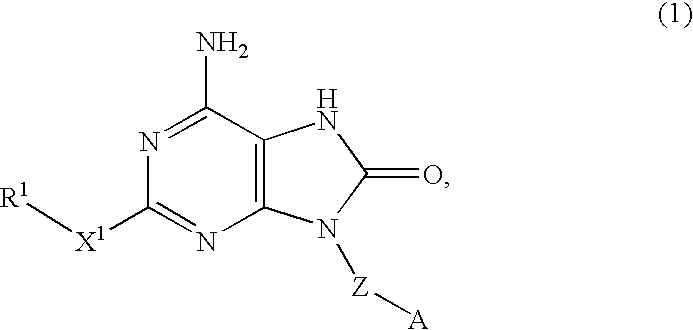
An 8-oxoadenine compound useful as an immuno-modulator having specific activity against Th1 / Th2, specifically a prophylactic and therapeutic agent for a topical application for allergic diseases, viral diseases and cancers, which is represented by the following formula (1): wherein A is a group of a formula represented by the formula (2): wherein R2 is a substituted or unsubstituted alkyl group and so on, R3 is hydrogen atom or an alkyl group, R is a halogen atom and so on, n is 0˜2, X1 is oxygen atom, Z is straight or branched chain alkylene, and R1 is an alkyl group which is optionally substituted by hydroxy group, an alkoxy group, alkoxycarbonyl group and so on, or its pharmaceutically acceptable salt.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD +1
Photoelectric conversion device and method for producing same
InactiveUS20020040728A1Promote conversionPrevent deterioration and volatilityLight-sensitive devicesDeferred-action cellsArylHydrogen atom
A method for producing a photoelectric conversion device comprising a conductive support and a photosensitive layer containing a semiconductor fine particle on which a dye is adsorbed, wherein the semiconductor fine particle is treated with a compound represented by the following general formula (I): wherein X represents an oxygen atom, a sulfur atom, a selenium atom or NY, in which Y represents a hydrogen atom, an aliphatic hydrocarbon group, a hydroxyl group or an alkoxy group; R1, R2, R3 and R4 independently represent a hydrogen atom, an aliphatic hydrocarbon group, an aryl group, a heterocyclic group, -N(R5)(R6), -C(=O)R7, -C(=S)R8, -SO2R9 or -OR10; R5 and R6 few independently have the same meaning as the R1, R2, R3 and R4; R7, R8 and R9 independently represent a hydrogen atom, an aliphatic hydrocarbon group, an aryl group, a heterocyclic group, -N(R5)(R6), -OR10 or -SR11; and R10 and R11 independently represent a hydrogen atom or an aliphatic hydrocarbon group.
Owner:FUJIFILM CORP
Organometallic complex and light emitting element, light emitting device, and electronic device using the organometallic complex
InactiveUS20070244320A1High light emitting efficiencyReduce power consumptionGroup 5/15 element organic compoundsSolid-state devicesArylHydrogen
An organometallic complex having a structure represented by the following general formula (G1) is provided. (In the formula, A represents an aromatic hydrocarbon group having 6 to 25 carbon atoms. Further, Z represents any one of hydrogen, an alkyl group having 1 to 4 carbon atoms, an alkoxy group having 1 to 4 carbon atoms, or an aryl group having 6 to 25 carbon atoms. In addition, Ar1 represents an aryl group having 6 to 25 carbon atoms. R1 represents any one of hydrogen, an alkyl group having 1 to 4 carbon atoms, or an alkoxy group having 1 to 4 carbon atoms. Further, M is a central metal and represents an element belonging to Group 9 or Group 10.)
Owner:SEMICON ENERGY LAB CO LTD
Blue phosphorescent compound and organic electroluminescent device using the same
Disclosed are a blue phosphorescent compound with a high color purity and a high efficiency, and an organic electroluminescent device using the same. The blue phosphorescent compound is represented by the following Formula:wherein R1 to R5 are each independently hydrogen (H), fluorine (F), chlorine (Cl), bromine (Br), a cyano group, a C1 to C6 alkyl group, a C1 to C6 alkoxy group, a C6 to C20 substituted or unsubstituted aromatic group, a C5 to C20 substituted or unsubstituted heterocyclic group, a C1 to C6 amine group, a C6 to C20 aromatic-substituted amine group, or a C5 to C20 heterocycle-substituted amine group, X is selected from nitrogen (N), oxygen (O), phosphorous (P) and sulfur (S) atoms, at least one of A1, A2, A3 and A4 is nitrogen (N), and the remaining are selected from hydrogen (H)-substituted carbon, and alkyl or alkoxy-substituted carbon, L is a monodentate or bidentate ligand and n is 1 to 3.
Owner:LG DISPLAY CO LTD
Microcapsule containing core material and method for producing the same
A microcapsule containing core material and a capsule wall, in which the capsule wall of the microcapsule comprises:organopolysiloxane synthesized by polycondensing one or more compounds represented by the general formula (II):wherein, m represents an integer from 1 to 4; n represents an integer from 0 to 3; m+n<=4; R represents an organic group in which a carbon atom is directly connected to a silicone atom, and when n is greater than 1, each of the R groups may be the same or different; and Y represents at least one group selected from the group consisting of an alkoxy group, hydrogen and siloxy group, and when (4-m-n) is greater than 1, each of the groups Y may be the same or different; provided that the compound (B) comprises at least one compound of formula(II) wherein m=2 or 3 and at least one of R group possesses affinity for at least one of a continuous phase and a dispersed phase;a method for producing the microcapsule; anda use of the microcapseile, for example for cosmetics are provided.
Owner:SEIWA KASEI CO JP
Diol-derived organofunctional silane and compositions containing same
Described are diol-derived organofunctional silanes in which the silanes contain cyclic and bridged alkoxy groups derived from hydrocarbon-based diols and methods for the preparation of the silanes. Also described are rubber compositions containing the diol-derived organofunctional silanes, methods for the preparation of the rubber compositions and articles of manufacture containing the rubber compositions, in particular, automotive tires and components thereof.
Owner:GENERAL ELECTRIC CO
Method of improving the permeability of an underground petroleum-containing formation
InactiveUS6972274B1Restore permeabilityEasy injectionOther chemical processesJoints with sealing surfacesAmmonium compoundsAlcohol
The present invention generally relates to a method and a composition useful in restoring the permeability of a porous underground petroleum containing formation. Restoring permeability to the formation enhances the injectivity thereby accelerating petroleum recovery. The method of the invention comprises treating said formation with a composition which comprises a combination of at least one nonionic compound, preferably an alkoxylated alcohol, with at least one cationic compound, preferably a quaternary ammonium compound, in an amount effective to improve the permeability of the formation. The composition may also optionally contain an alkyl glycoside.
Owner:AKZO NOBEL CHEM INT BV
Naphtholactams and lactones as bone morphogenetic protein active agents
InactiveUS6030967AHigh toxicological thresholdReduce riskBiocideOrganic chemistryActive agentCarbon chain
PCT No. PCT / JP97 / 02858 Sec. 371 Date Oct. 30, 1997 Sec. 102(e) Date Oct. 30, 1997 PCT Filed Aug. 19, 1997 PCT Pub. No. WO98 / 07705 PCT Pub. Date Feb. 26, 1998A compound of the formula: wherein Q is an optionally substituted carbon atom or N(O)p wherein p is 0 or 1; Y is an optionally substituted methylene group, S(O)q wherein q is an integer of 0 to 2, or an optionally substituted imino group; Z1 is a C1-3 alkylene group which may have an oxo group or a thioxo group and may contain etherified oxygen or sulfur within the carbon chain; Z2 is an optionally substituted C1-3 alkylene group; Ar is an optionally substituted carbocyclic group or an optionally substituted heterocyclic group; one of R1 and R2 is a hydrogen atom, a halogen atom, a hydroxyl group, an optionally substituted lower alkyl group, or an optionally substituted lower alkoxy group; the other is a halogen atom, a hydroxyl group, an optionally substituted lower alkyl group, or an optionally substituted lower alkoxy group; or R1 and R2 taken together with adjacent -c=c- form a ring; and ring A is a benzene ring which may be substituted in addition to R1 and R2; or a salt thereof.
Owner:TAKEDA PHARMA CO LTD
Organometallic complex, and light-emitting element, light-emitting device and electronic device including the organometallic compex
ActiveUS20080160345A1Improve emission efficiencySolve low luminous efficiencyGroup 5/15 element organic compoundsSolid-state devicesArylHalogen
An object is to provide an organometallic complex that can emit red light. Another object is to provide an organometallic complex having high emission efficiency. Still another object is to provide an organometallic complex that can emit red light with high luminous efficiency. The present invention provides an organometallic complex having a structure represented by the following general formula (G1′).In the formula, Ar represents an aryl group having 6 to 25 carbon atoms; R1 represents any one of hydrogen, an alkyl group having 1 to 4 carbon atoms, and an alkoxy group having 1 to 4 carbon atoms; R2 to R8 each represent any one of hydrogen, an alkyl group having 1 to 4 carbon atoms, an alkoxy group having 1 to 4 carbon atoms, and a halogen group; at least one of pairs R3 and R4, R4 and R5, and R5 and R6 may be bound to each other to form a ring; and M represents a central metal of Group 9 elements and Group 10 elements.
Owner:SEMICON ENERGY LAB CO LTD
Active materials for photoelectric devices and devices that use the materials
A conjugated polymer has a repeated unit having the structure of formula (I)wherein A1, A2, R1 and R2 are independently selected from the group consisting of a proton, an alkyl group comprising up to 18 carbon atoms, an alkoxy group comprising up to 18 carbon atoms, cyano, nitro, aryls and substituted aryls, and wherein Ar is selected from the group consisting of ethenylene, ethynylene, monocyclic arylene, bicyclic arylene, polycyclic arylene, monocyclic heteroarylene, bicyclic heteroarylene, polycyclic heteroarylene, and one to five such groups one of fused or linked.
Owner:RGT UNIV OF CALIFORNIA
Ink-jet printing ink compositions having superior smear-fastness
InactiveUS6417249B1Improve printing effectGood printing performanceInksCoatingsPolyesterPolymer science
Specific core-shell binders and additives for use in ink-jet printing ink compositions are provided. One class of specific core / shell binders has the general formula [AmBnC'p]x, where A and B are hydrophobic components in which A exhibits a glass transition temperature Tg between about -150° and +25° C. and B exhibits a glass transition temperature greater than 25° C., C' is a component that forms a hydrophilic or water-soluble component in the polymer chain, and has an ionic or non-ionic structure, m<30 wt %, n>40 wt %, and p<30 wt %, with the total of m+n+p=100 wt %, and x=1 to 100,000. The molecular weight (weight average) of the polymer is between about 1,000 and 2,000,000. The polymers useful in the practice of the invention are prepared by emulsifying the monomers and then conducting a free-radical polymerization in water. The foregoing binder polymer is used in conjunction with additives comprising either (a) amine alcohols having the general formulawhere R1 and R2 are independently selected from the group consisting of hydrogen, alkyl, alkoxy, aryl, and phenoxy, R is alkyl, X is selected from the group consisting of hydrogen, alkyl, aryl, -OH, -COOH, -CHO, and substituted groups or (b) organic acids (water-soluble or water-dispersive), including polymeric acids. Other additives include amines, polyalcohols, polyamines, and polyesters. In the ink compositions of the present invention, the ratio of binder (1) to colorant (pigment) is greater than 1 to 10. The concentration of the additive is within the range of 0.005 to 50 wt %. The general ink formulation comprises: 5 to 50 wt % water-miscible solvent; 0.5 to 10 wt % colorant; 0.005 to 50 wt % additive; and water.
Owner:HEWLETT PACKARD DEV CO LP
Organometallic Complex and Light-Emitting Element, Light-Emitting Device, and Electronic Device Using the Same
InactiveUS20100145044A1Easy to synthesizeSharp emission spectrumGroup 5/15 element organic compoundsSolid-state devicesHydrogenAlkoxy group
An organometallic complex having a structure represented by the general formula (G1) is synthesized and applied to a light-emitting element.In the formula, R3 represents either an alkyl group having 1 to 4 carbon atoms, an alkoxy group having 1 to 4 carbon atoms, or an alkoxycarbonyl group having 1 to 5 carbon atoms; R2 and R3 each show either hydrogen or an alkyl group 1 to 4 carbon atoms; Ar represents an arylene group having 6 to 25 carbon atoms; M is a center metal selected from Group 9 element and Group 10 element.
Owner:SEMICON ENERGY LAB CO LTD
Redox shuttle for rechargeable lithium-ion cell
InactiveUS20050221196A1Excellent repeated overcharge stabilityNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsLithiumAlkoxy group
A redox chemical shuttle comprising an aromatic compound substituted with at least one tertiary carbon organic group and at least one alkoxy group (for example, 2,5-di-tert-butyl-1,4-dimethoxybenzene) provides repeated overcharge protection in rechargeable lithium-ion cells.
Owner:3M INNOVATIVE PROPERTIES CO
Naphthalene derivative, material for organic electroluminescence device, and organic electroluminescence device using the same
InactiveUS20090008607A1Excellent in luminous efficiency and heat resistance and lifetimeImprove efficiencyOrganic chemistryElectrical apparatusAlkoxy groupOrganic electroluminescence
A naphthalene derivative represented by the following formula (1) is provided. In the formula (1), Ar1 and Ar2 each represent an aromatic hydrocarbon cyclic group having 6 to 18 carbon atoms forming a ring. The aromatic hydrocarbon cyclic group has none of anthracene skeleton, pyrene skeleton, aceanthrylene skeleton and naphthacene skeleton. Ar3 represents a benzene skeleton or a naphthalene skeleton. R1 and R2 each represent an alkyl group, a cycloalkyl group, an alkoxy group, a cyano group, a silyl group or a halogen atom. a and b each represent an integer in a range of 0 to 4. m and n each represent an integer in a range of 1 to 4.
Owner:IDEMITSU KOSAN CO LTD
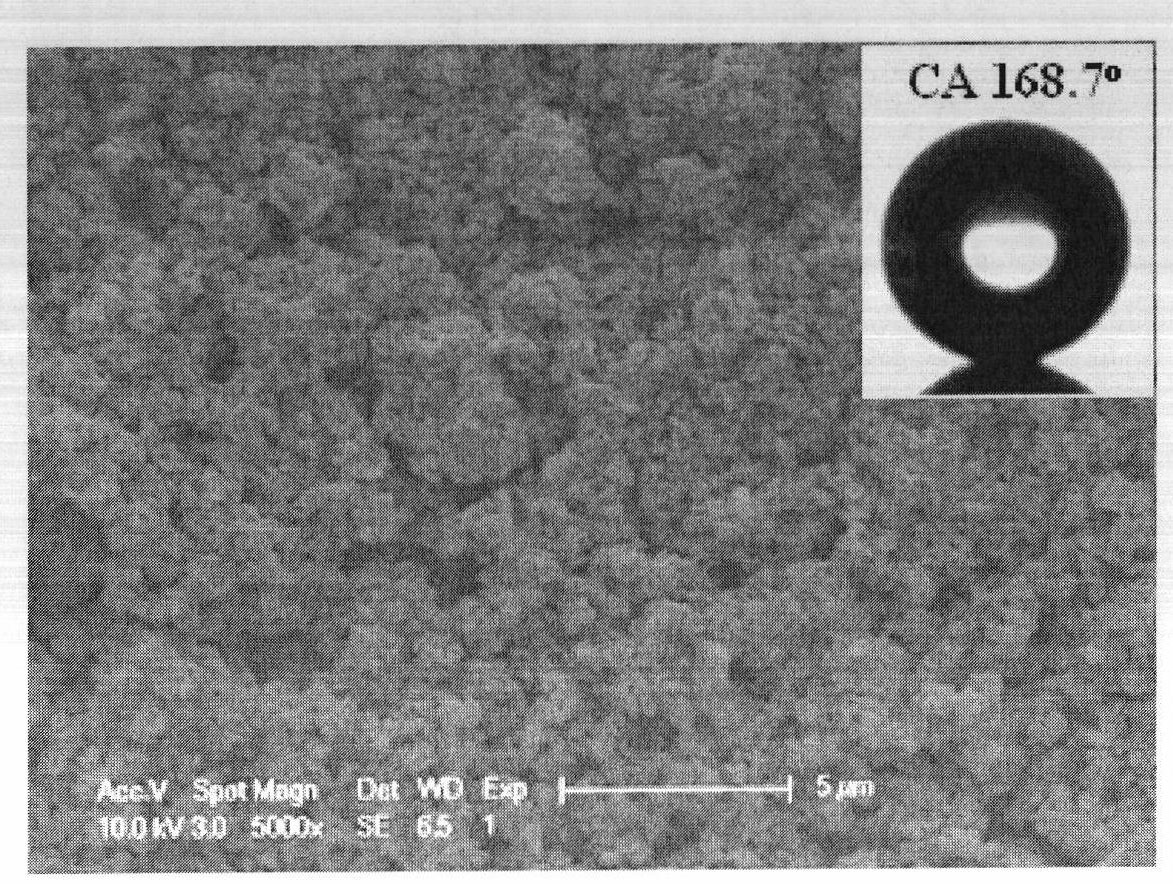
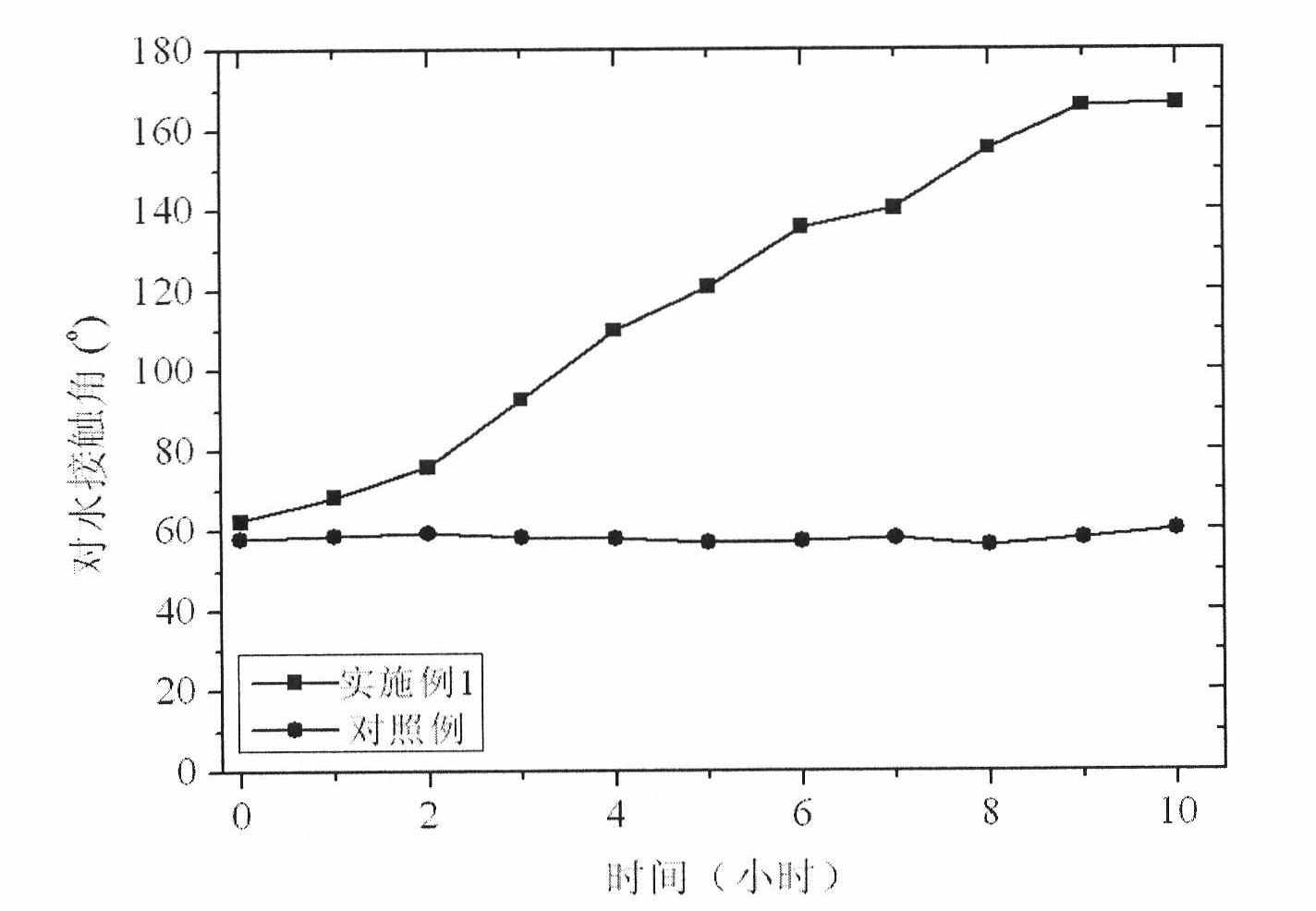
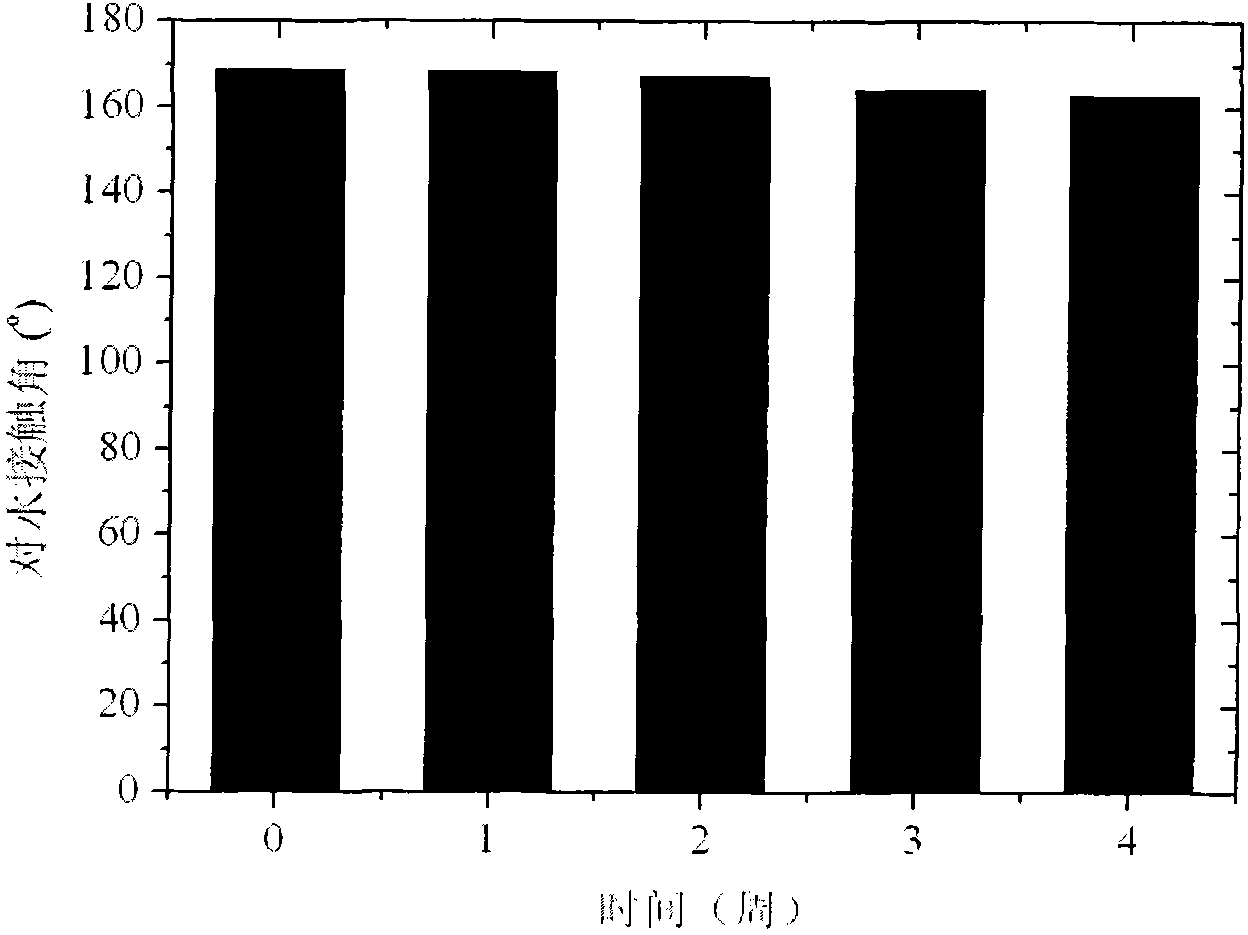
High-durability super-hydrophobic self-cleaning coating material and preparation method thereof
InactiveCN101962514AHas the following advantages: (1) cleanlinessHas the following advantages: (1) has the functionAntifouling/underwater paintsPaints with biocidesDouble bondDimethyl siloxane
The invention belongs to the technical field of a new chemical material, and in particular relates to a high-durability super-hydrophobic self-cleaning coating material and a preparation method thereof. The coating material of the invention is prepared by curing and drying nanoparticles with photo-catalytic activity, a low-surface-free-energy polymer and a cross-linking agent at the room temperature, wherein the low-surface-free-energy polymer consists of one or more of polysiloxane fluoride, dimethyl silicone polymer and polyphenylene methyl siloxane, which contain active groups, such as hydroxyl alkoxy group, carbon-carbon double bond, silanol group, siloxy group, and the like; the cross-linking agent is hydrogen-containing silicone oil or aminosilane; and the mass content of the photo-catalytic nanoparticles in the coating ranges from 10 to 60 percent. The coating is formed into a micro-nanostructure by nanoparticle self-organization; a super-hydrophobic self-cleaning coating with lotus effect is prepared from the coating and a cross-linked filming matrix with low surface energy; the persistence of a lotus-shaped super-hydrophobic characteristic of the coating is realized by using the photo-catalytic decomposition characteristic of an organic pollutant for the nanoparticles; and thus the material is suitable for large-area construction and has high weathering resistance andprominent self-cleaning characteristic.
Owner:FUDAN UNIV
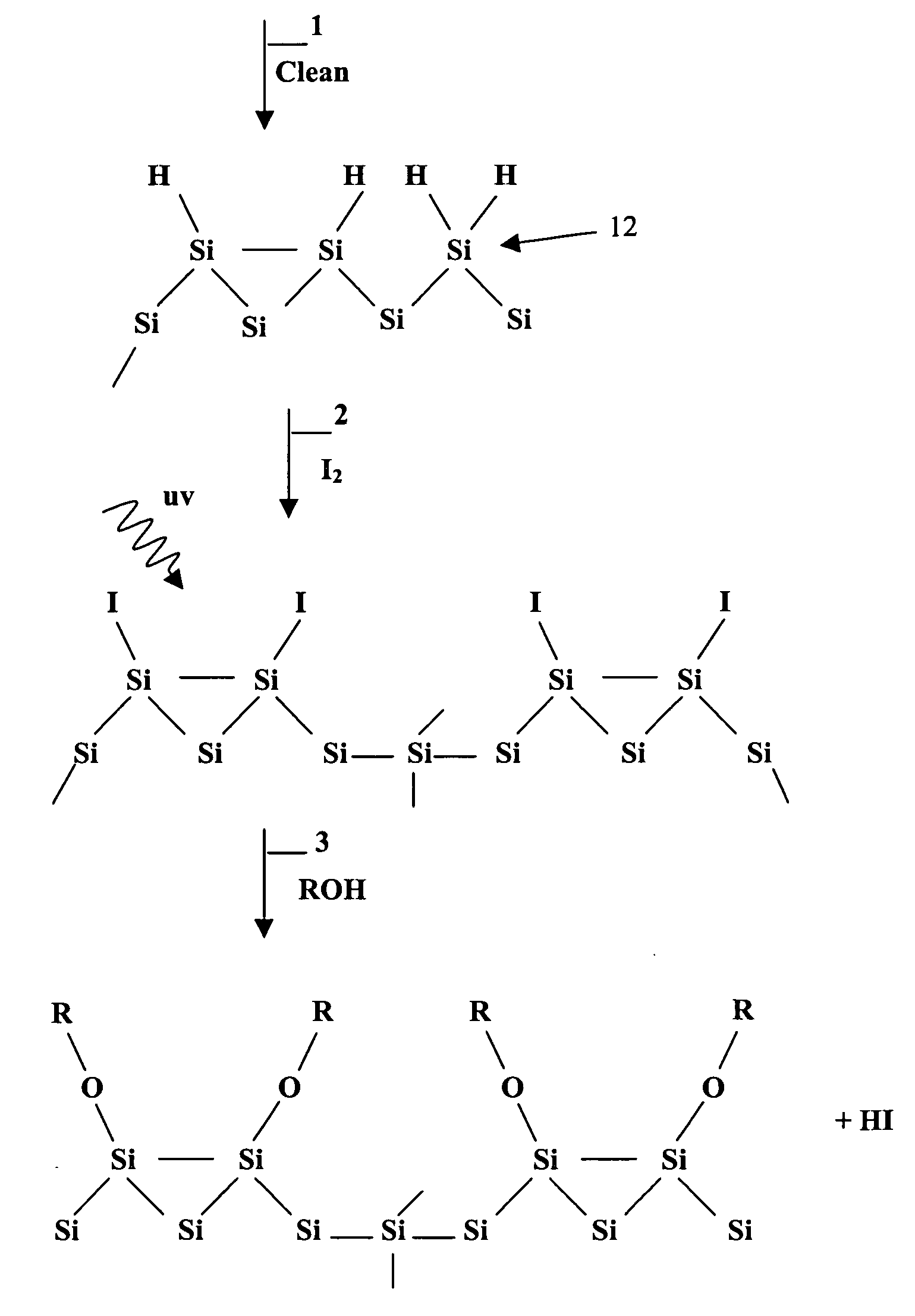
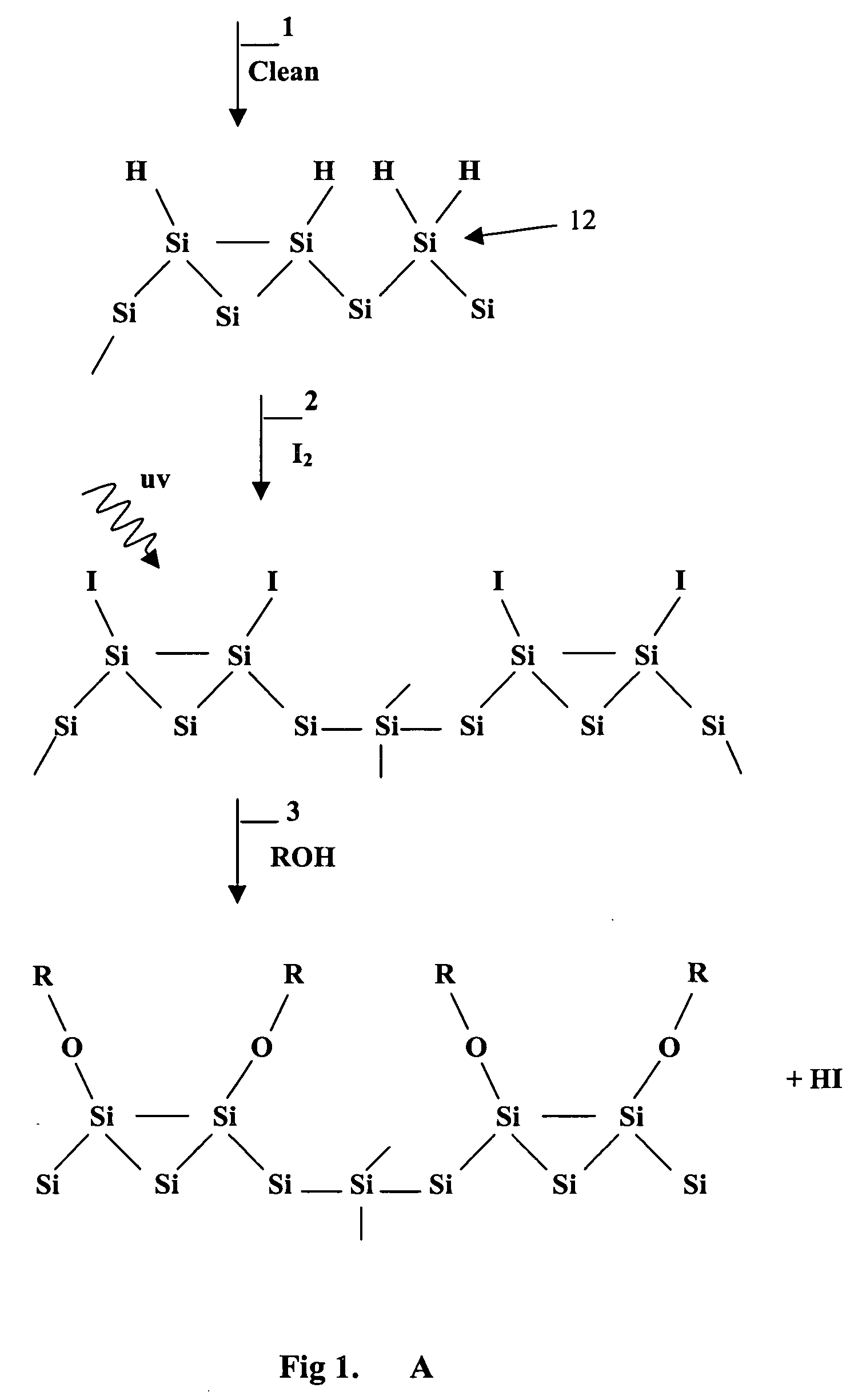
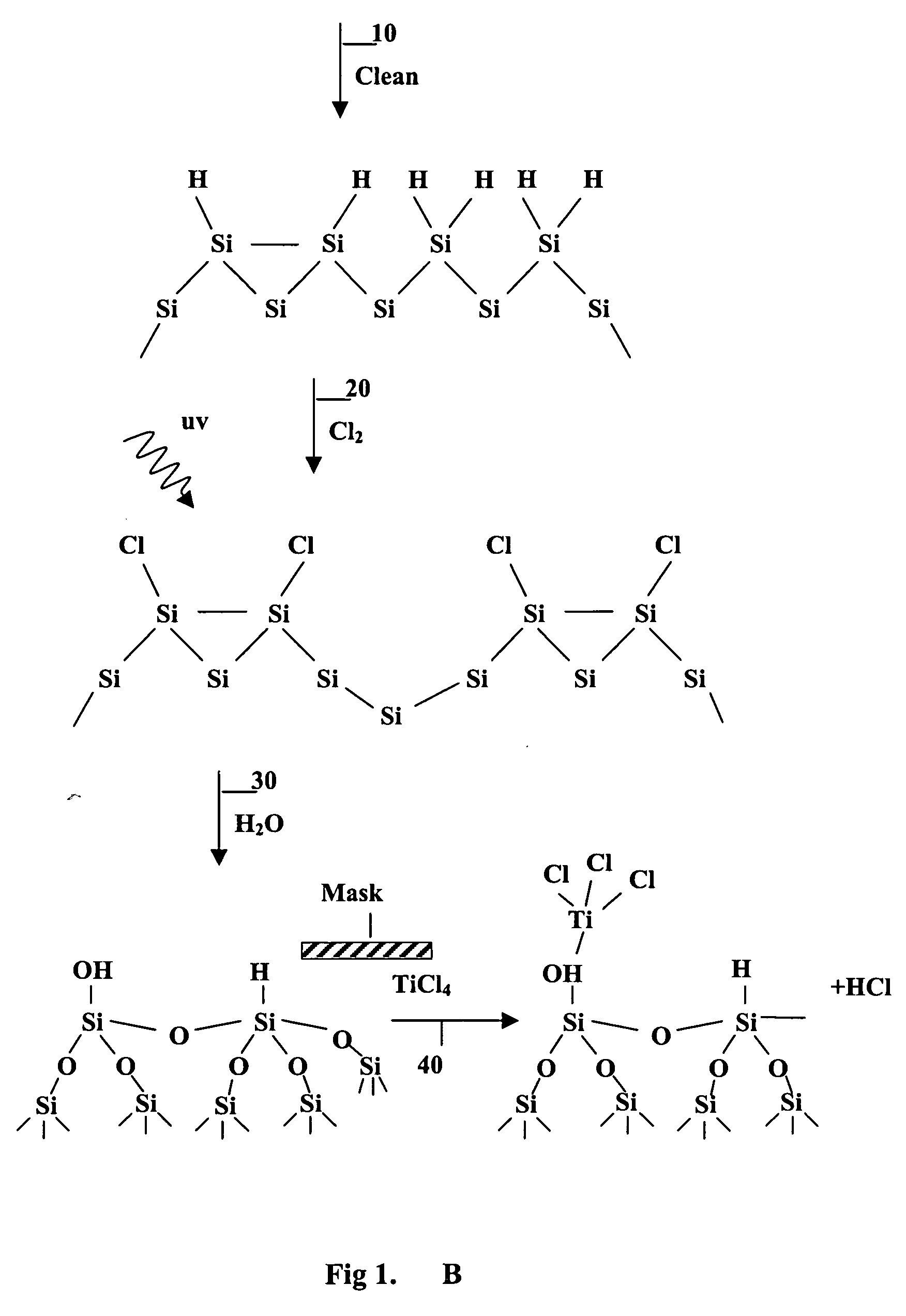
Surface manipulation and selective deposition processes using adsorbed halogen atoms
InactiveUS20060199399A1Saving process stepReduce environmental impactSemiconductor/solid-state device manufacturingChemical vapor deposition coatingElectrical conductorGas phase
The present invention provides a surface preparation process using adsorbed halogen. The halogen is applied in a gas phase with UV light. The adsorbed halogen is subsequently modified in another gas phase reaction. The halogen may be reacted with water to form a hydroxyl-bearing Si—O monolayer that forms a layer for subsequent metal deposition. In one aspect the halogen layer is reacted with an alkyl or alkoxy of the formula R-OH to form a passivation layer. By replacing hydrogen atom termination with alkoxy (e.g.methoxy termination, —OCH3). The selective deposition process can be used for passivating and depositing thin metal films on material surfaces composed of any combination of the group consisting of semiconductors, conductors, insulators, and the like.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
Cyclic diol-derived blocked mercaptofunctional silane compositions
InactiveUS20050245753A1Reduce volatilityReducing VOC levelSilicon organic compoundsSilanesAlkoxy group
Diol derived blocked mercaptofunctional silane compositions in which the silanes comprise cyclic and bridged alkoxy groups derived from hydrocarbon-based diols and processes for their preparation are provided. Also provided are rubber compositions comprising the cyclic diol-derived blocked mercaptofunctional silanes, processes for their preparation and articles of manufacture comprising the rubber compositions, in particular, automotive tires and components thereof.
Owner:GENERAL ELECTRIC CO
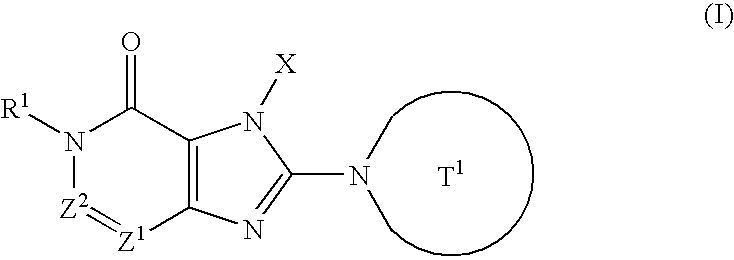
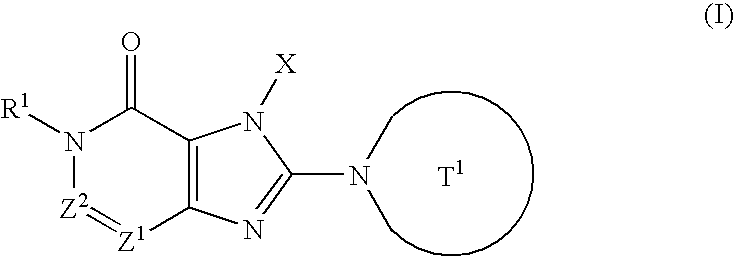
Condensed imidazole derivatives
InactiveUS20060063787A1Excellent DPPIV-inhibiting activityBiocideOrganic chemistryHydrogen atomAlkoxy group
The present invention is related to compounds represented by the following formula, or salts or hydrates thereof wherein, T1 represents a 4- to 12-membered heterocyclic group containing one or two nitrogen atoms in the ring, which is a monocyclic or bicyclic structure that may have one or more substituents; X represents a C1-6 alkyl group which may have one or more substituents, or such; Z1 and Z2 each independently represent a nitrogen atom or a group represented by the formula —CR2—; R1 and R2 independently represent a hydrogen atom, a C1-6 alkyl group which may have one or more substituents, or a C1-6 alkoxy group which may have one or more substituents, or such. These are novel compounds that exhibit an excellent DPPIV-inhibiting activity.
Owner:EISIA R&D MANAGEMENT CO LTD
Compounding silica-reinforced rubber with low volatile organic compound (VOC) emission
ActiveUS20060217473A1Enhanced rubber reinforcementEnhanced interactionSilicon organic compoundsSynthetic resin layered productsAlcoholReinforced rubber
Alkoxy-modified silsesquioxane compounds are described. The alkoxy-modified silsesquioxane compounds contain an alkoxysilane group that participates in an alkoxysilane-silica reaction as a silica dispersing agent in rubber, with the release of zero to about 0.1% by weight of the rubber of volatile organic compounds (VOC), especially alcohol, during compounding and further processing. Further described are methods for making alkoxy-modified silsesquioxanes, methods for making vulcanizable rubber compounds containing alkoxy-modified silsesquioxanes, vulcanizable rubber compounds containing alkoxy-modified silsesquioxanes, and pneumatic tires comprising a component that contains alkoxy-modified silsesquioxanes.
Owner:BRIDGESTONE CORP
Method for preparing alkoxyl magnesium solid particles
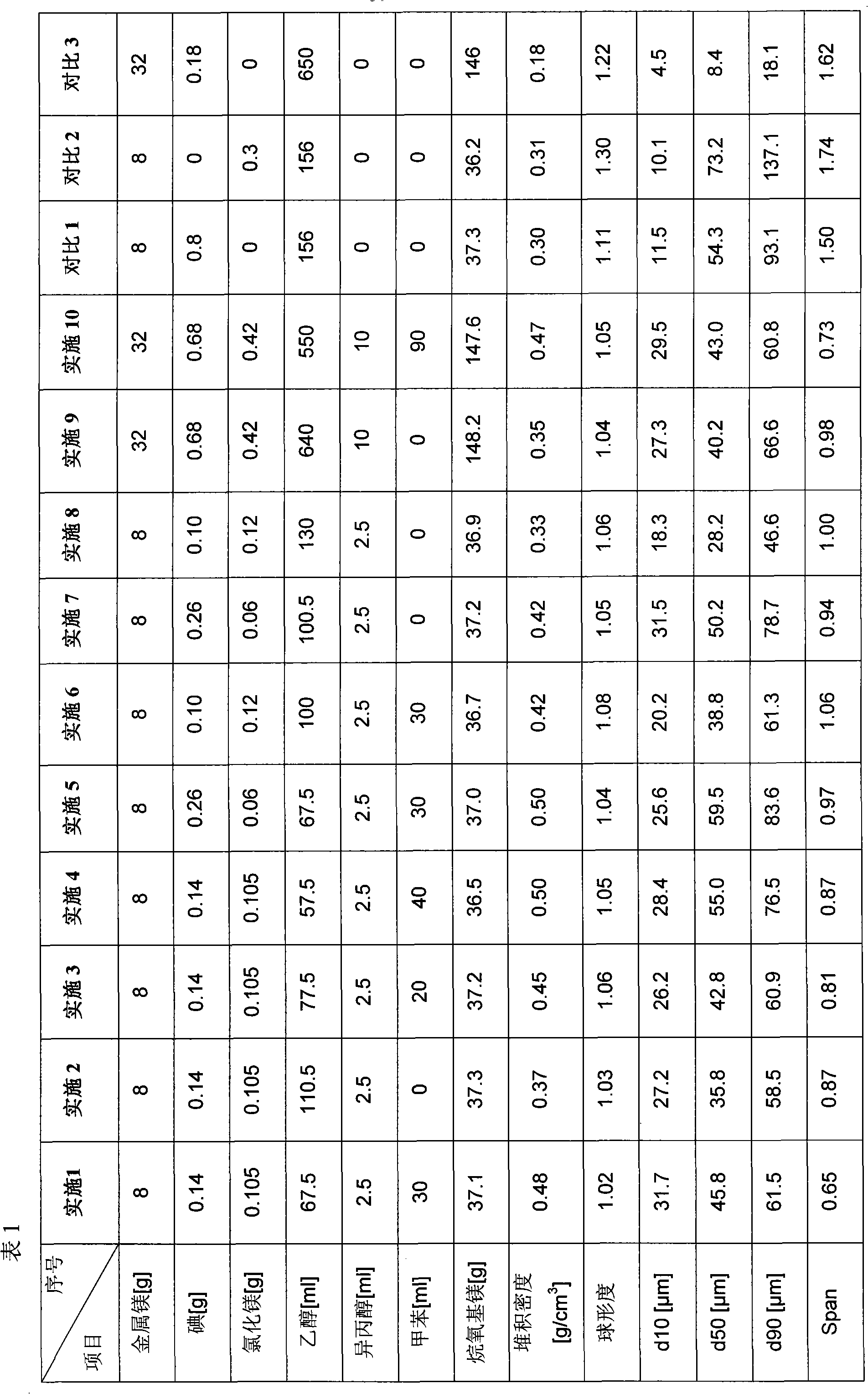
InactiveCN101906017AReduce dosageReduce manufacturing costPreparation of metal alcoholatesAlcoholPhysical chemistry
The invention provides a method for preparing spherical alkoxyl magnesium particles with excellent properties. In the method, magnesium, alcohol and a halogenating agent are reacted at a reflux temperature, so that the alkoxyl magnesium particles are obtained. The alkoxyl magnesium particles have the advantages of higher bulk density, good particle shape and particular suitability for serving as a catalyst carrier of an olefin polymerization reaction.
Owner:CHINA PETROLEUM & CHEM CORP +1
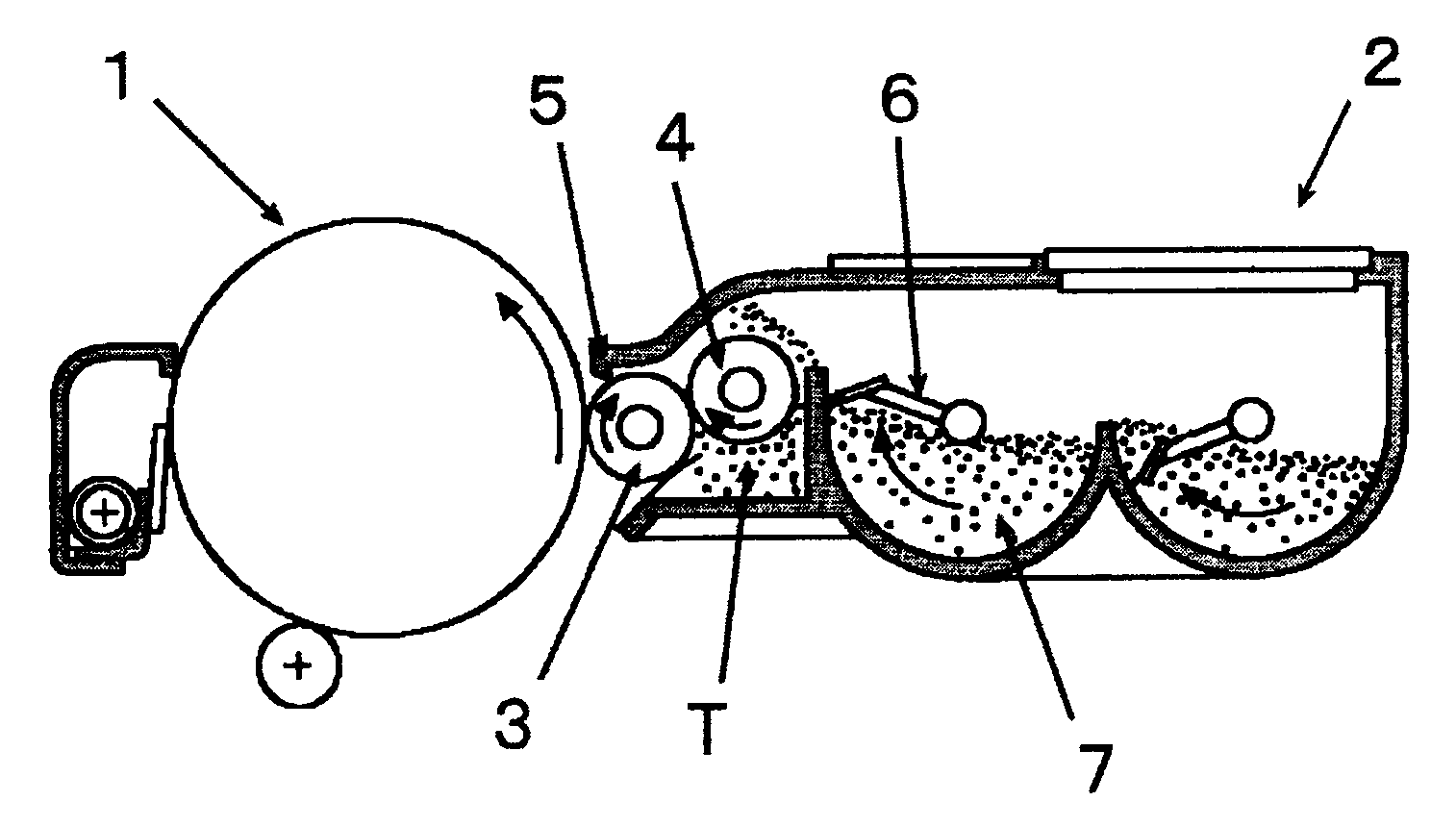
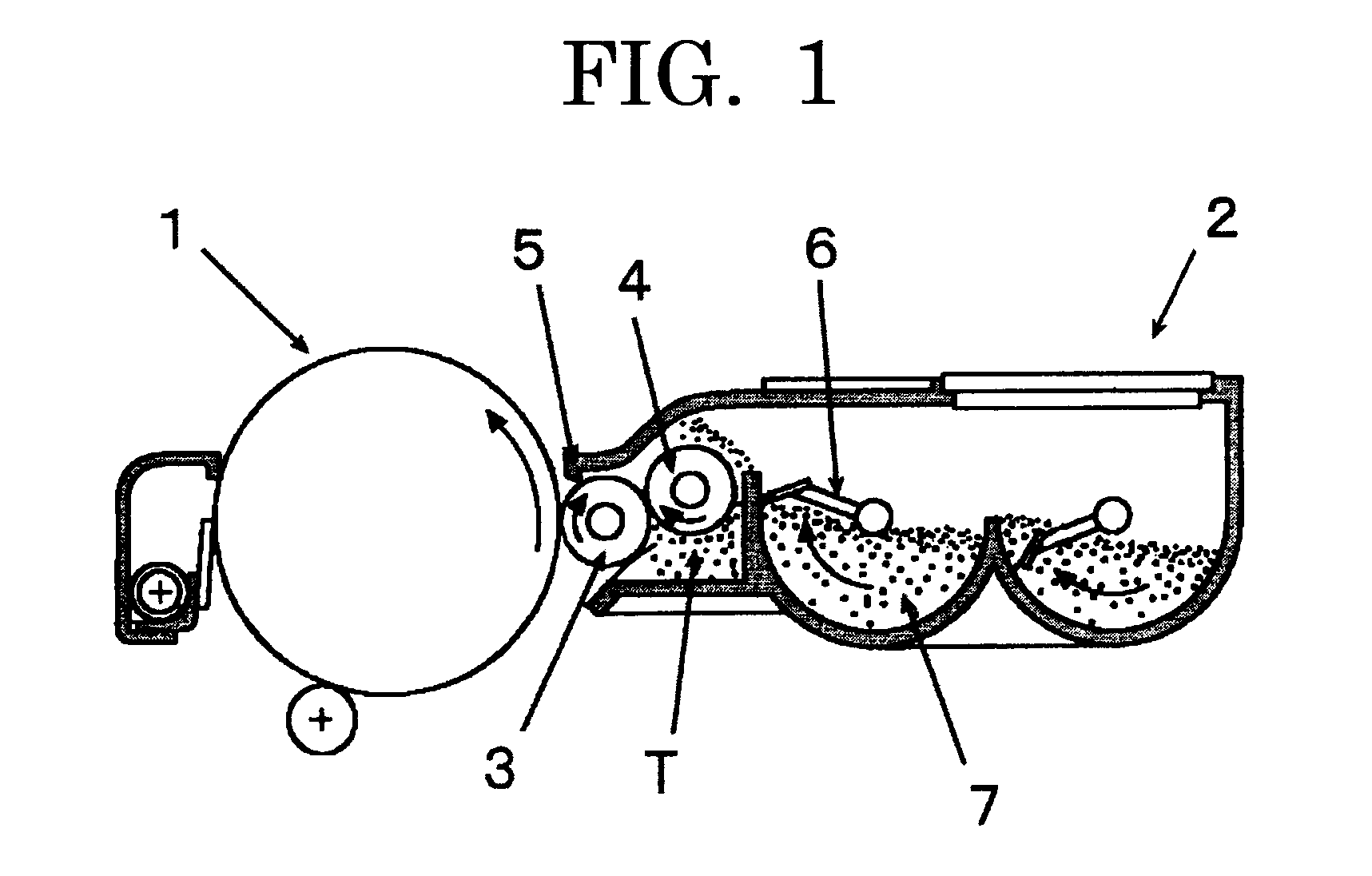
Toner and image forming method
InactiveUS20070207399A1Improve stabilityUniformly and efficiently chargingElectrographic process apparatusDevelopersImage transferLatent image
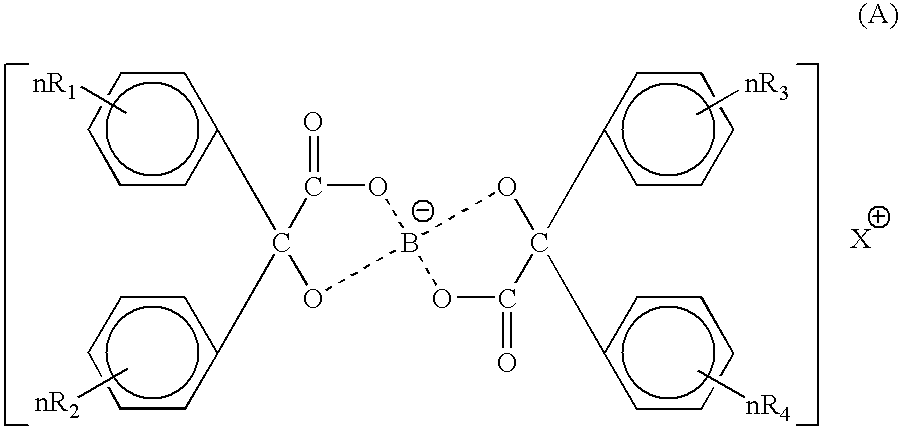
Toner for electrostatic charge development wherein no scumming occurs, and toner leakage caused by charge defect of the toner on a developing roller can be inhibited, and an excellent image stability is obtained is provided.The toner used for an image forming method having a latent electrostatic image forming step of forming a latent electrostatic image on a latent electrostatic image bearing member primarily charged, a developing step of developing the latent electrostatic image by each toner which multiple developing devices have to form a toner image on the latent electrostatic image bearing member, a transferring step of transferring the toner image with respective colors formed on the latent electrostatic image bearing member onto a recording material and a fixing step of fixing the toner image transferred onto the recording material, wherein the toner comprises a colorant and a resin and contains an organic boron compound represented by a following chemical formula (A) as a charge controlling agent, further the toner is treated with an inorganic fine particle and at least one of the inorganic particles is a magnesium silicate compound represented by a following general formula [2] is provided.wherein X is an alkali metal, R1, R2, R3 or R4 each represents a hydrogen atom, an alkyl group having 1 to 4 carbon atoms, an alkoxy group having 1 to 4 carbon atoms, or a halogen atom.MgxSiyO(x+2y) [2]wherein x and y are integers.
Owner:RICOH KK
Process for preparing alpha -hydroxy acids using microorganism and novel microorganism
PCT No. PCT / JP97 / 00578 Sec. 371 Date Aug. 11, 1998 Sec. 102(e) Date Aug. 11, 1998 PCT Filed Feb. 27, 1997 PCT Pub. No. WO97 / 32030 PCT Pub. Date Sep. 4, 1997A process for preparing alpha -hydroxy acids represented by the general formula (II): RCH(OH)COOH (wherein R represents a hydrogen atom, an optionally substituted C1-C6 alkyl group, an optionally substituted C2-C6 alkenyl group, an optionally substituted C1-C6 alkoxy group, an optionally substituted aryl group, an optionally substituted aryloxy group, or an optionally substituted heterocyclic group) by allowing a microorganism to act on alpha -hydroxy nitriles (I): RCH(OH)CN (wherein R is as defined above) to hydrolyze and convert the alpha -hydroxy nitrites to alpha -hydroxy acids (II), wherein the alpha -hydroxy acids (II) are produced and accumulated in an aqueous solvent by a microorganism having the concentration resistance to the alpha -hydroxy nitrites (I) and / or alpha -hydroxy acids (II) and durability preferably in the presence of a cyanide, and harvested. According to this process, the use of the microorganism having the concentration resistance to the alpha -hydroxy nitriles (I) and / or alpha -hydroxy acids (II) and durability high enough to permit the activity to persist for a long period of time enables alpha -hydroxy acids (II) to be accumulated in high concentrations and cell bodies to be repeatedly used, and hence enables alpha -hydroxy acids (II) to be efficiently prepared. The addition of a cyanide to the reaction system results in more efficient preparation of alpha -hydroxy acids (II).
Owner:NIPPON SODA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com