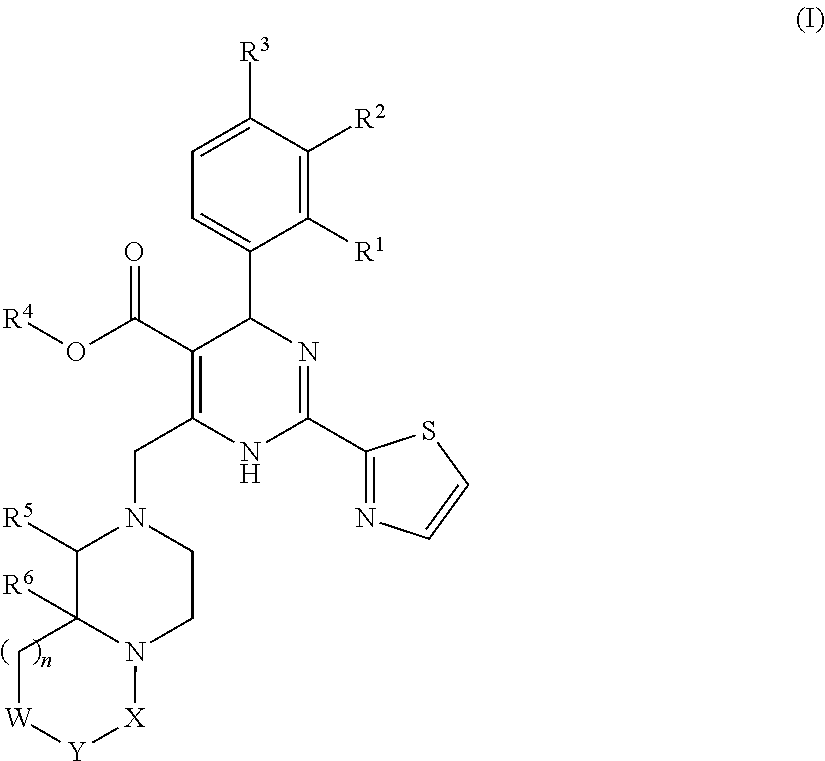
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
23978 results about "Aryl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
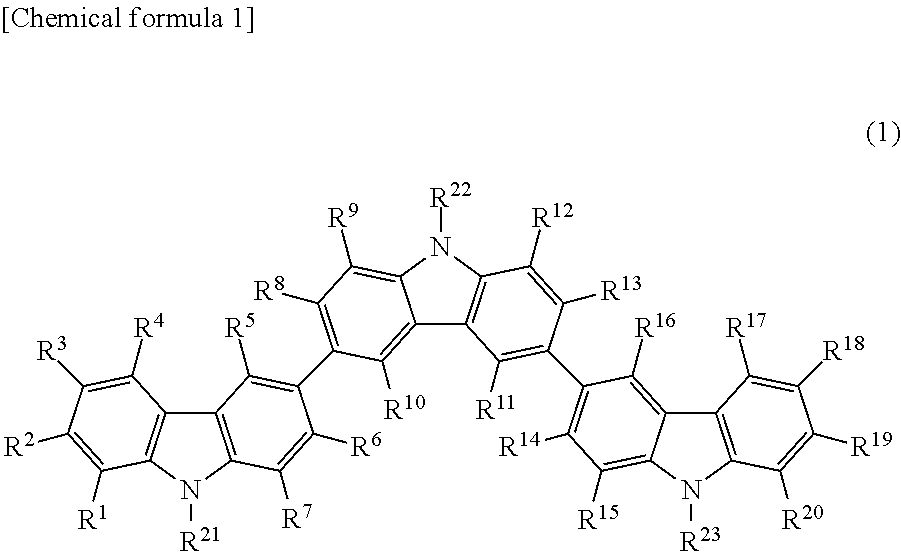
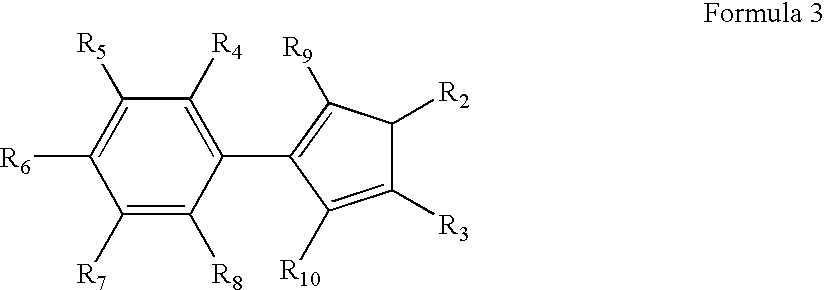
In the context of organic molecules, aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as a placeholder for the aryl group in chemical structure diagrams.
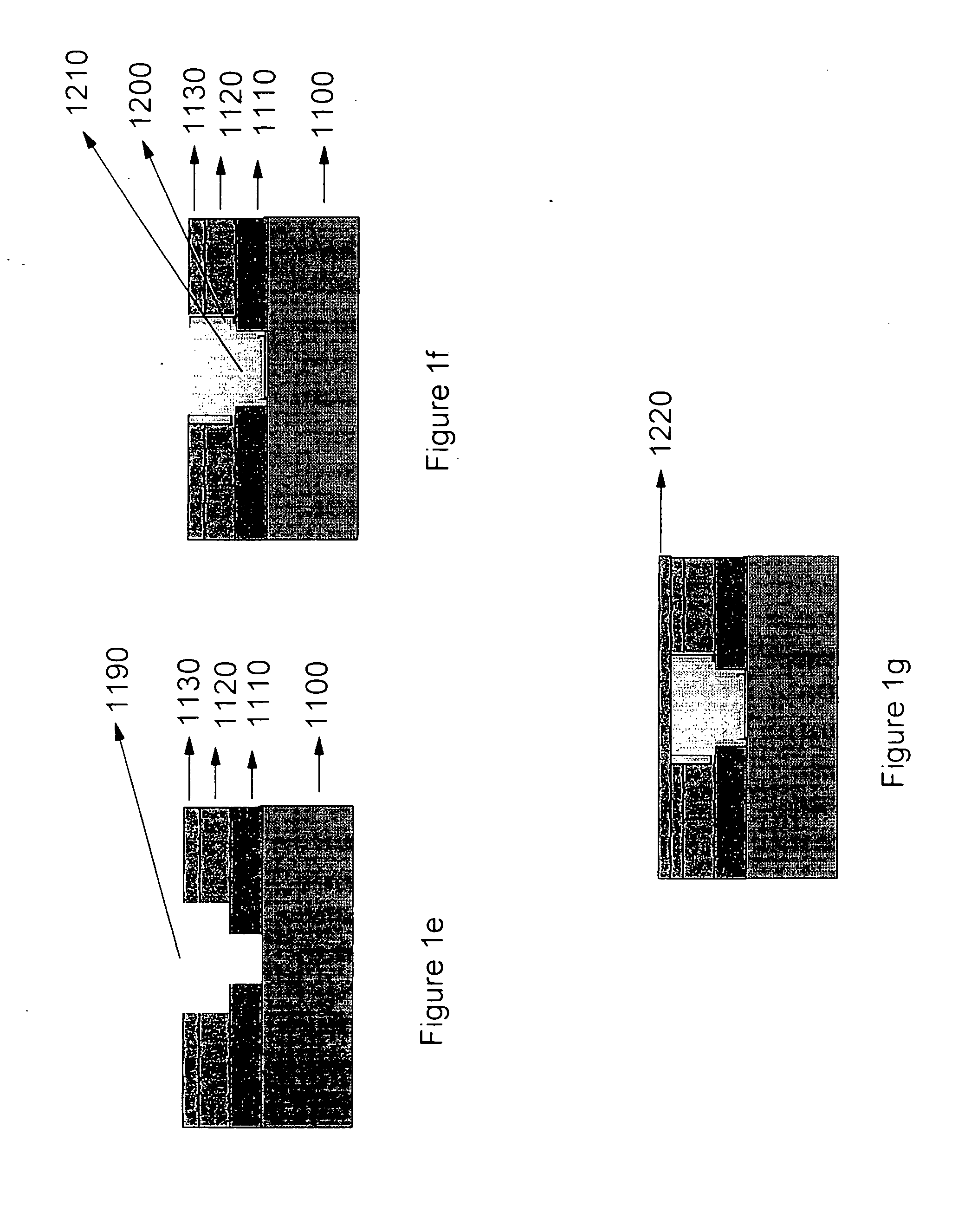
Aromatic amine derivative and organic electroluminescence device using the same
ActiveUS20080124572A1Improve efficiencyImprove recombination efficiencyOrganic chemistrySolid-state devicesArylCarbazole
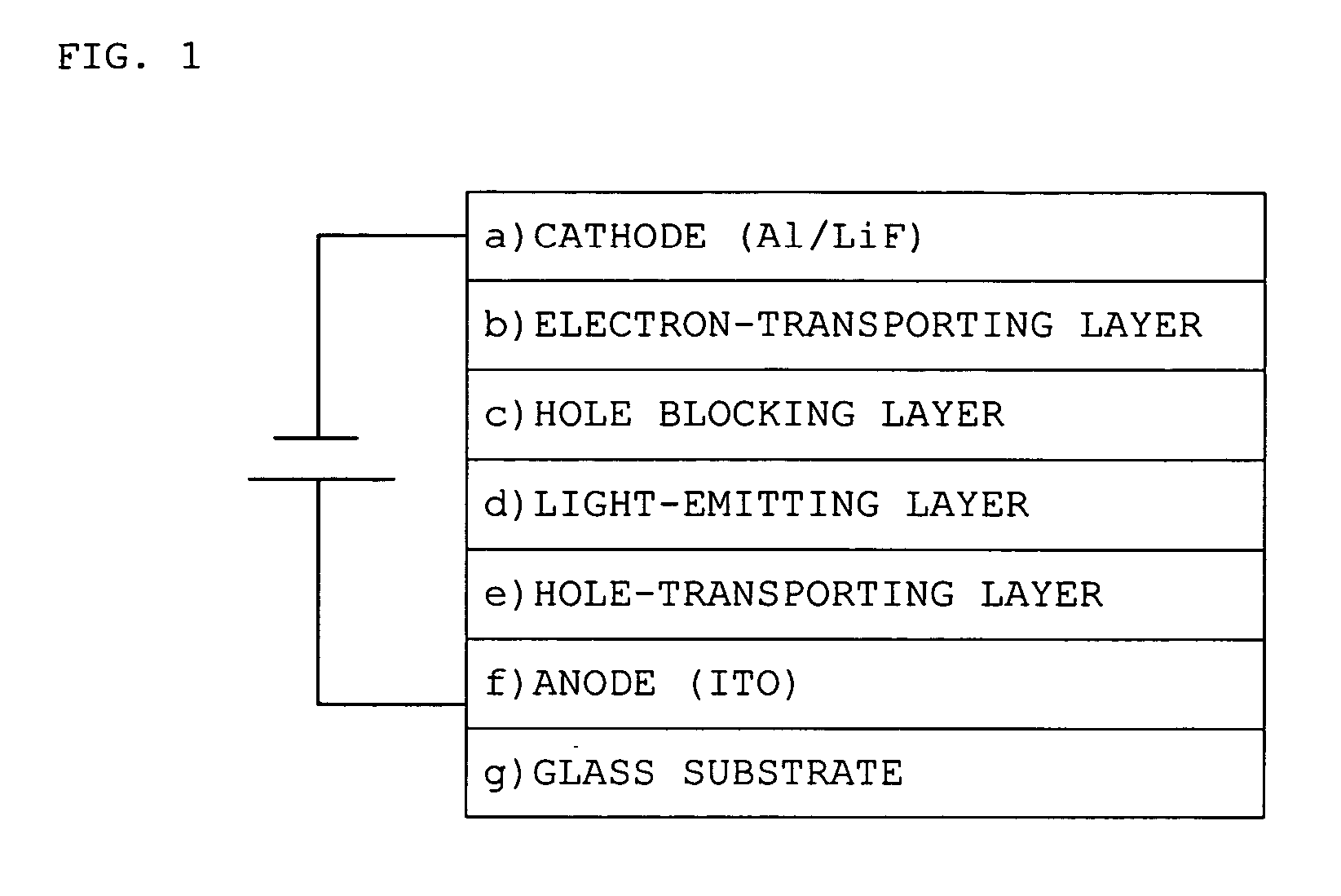
An aromatic amine derivative with a specific structure having a carbazole skeleton to which a diarylamino group bonds via a bonding group. An organic electroluminescence device which is composed of one or more organic thin film layers including at least one light emitting layer sandwiched between a cathode and an anode, wherein at least one of the organic thin film layers contains the aromatic amine derivative singly or as its mixture component. Organic electroluminescence devices with enhanced efficiency of light emission and a compound realizing the devices are provided.
Owner:IDEMITSU KOSAN CO LTD
Adhesive formulations
ActiveUS8349987B2Organic non-macromolecular adhesiveSynthetic polymeric active ingredientsArylPolymer science
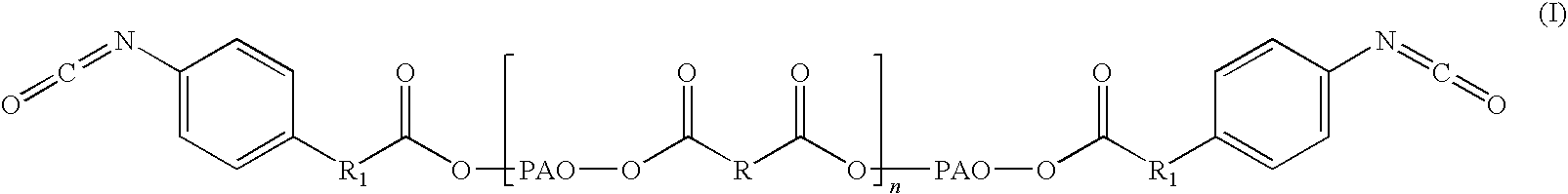
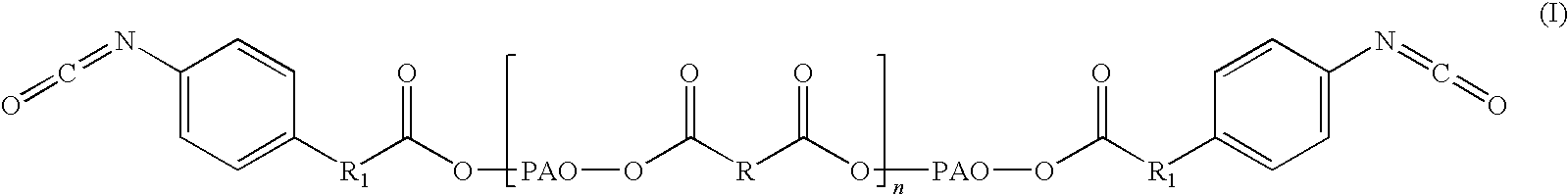
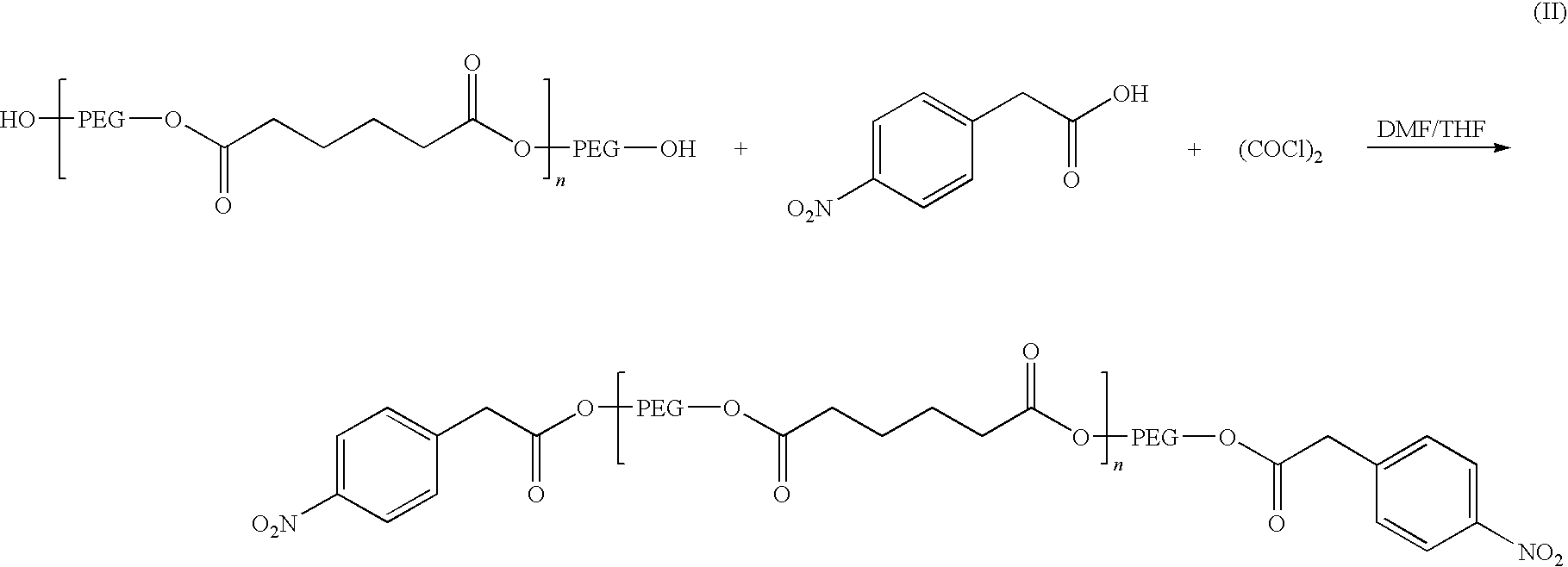
The disclosure relates to biocompatible components useful for forming compositions for use as medical / surgical synthetic adhesives and sealants. Biocompatible components of the present disclosure may include a polymeric polyol core, which may be treated with a nitroaryl compound to form a nitro ester. The resulting nitro ester groups may be reduced to form amino groups which, in turn, may be treated to form isocyanate groups. The resulting isocyanate may then be reacted with a second component to form adhesive and / or sealant compositions.
Owner:COVIDIEN LP
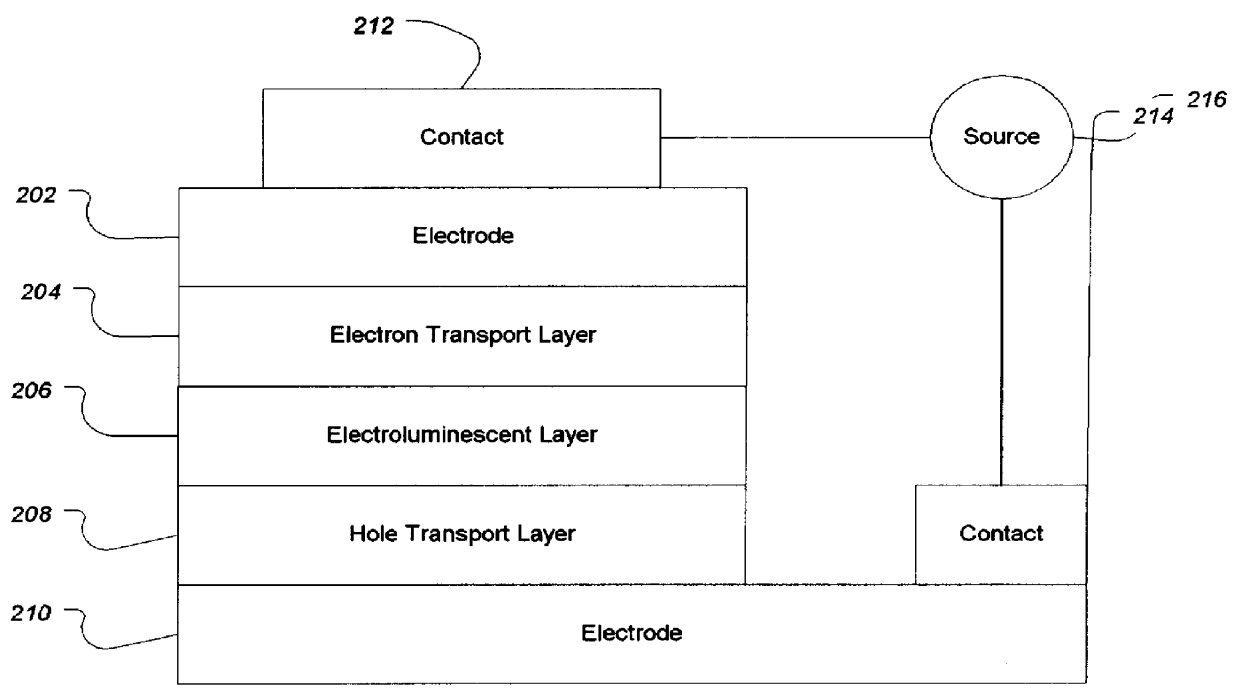
Electroluminescent (EL) devices
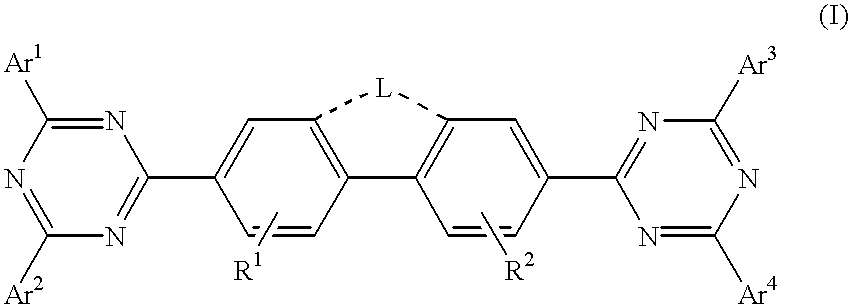
InactiveUS6225467B1Improve efficiencyIncreased durabilitySilicon organic compoundsElectroluminescent light sourcesArylHalogen
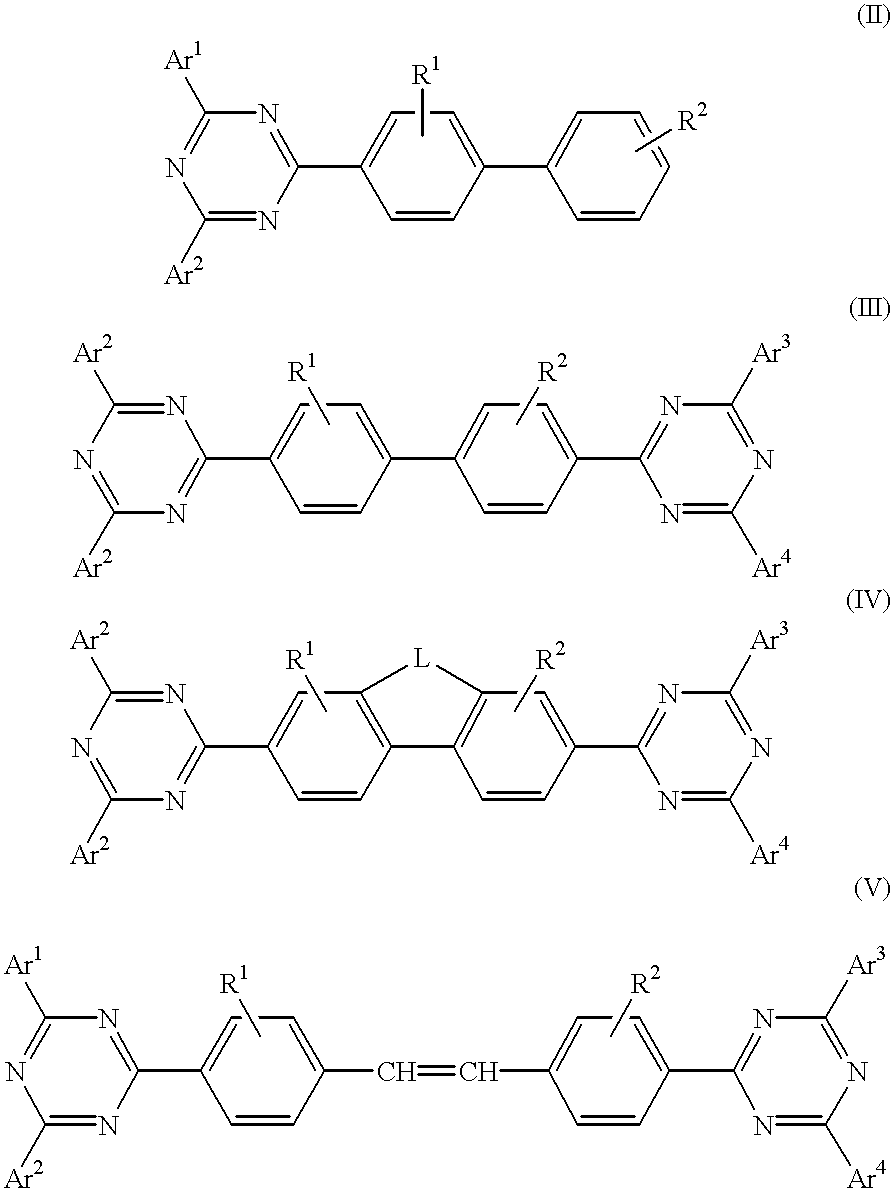
The triazinewherein Ar1, Ar2, Ar3, and Ar4 are each independently an aryl; R1 and R2 are substituents selected from the group consisting of hydrogen, an alkyl, an aryl, an alkoxy, a halogen atom, and a cyano; R3 and R4 are each a divalent group L selected from the group consisting of -C(R'R'')-, alkylene, an oxygen atom, a sulfur atom, and -Si(R'R'')-, wherein R' and R'' are selected from the group consisting of hydrogen, alkyl, alkoxy, and aryl.
Owner:LG DISPLAY CO LTD
Organic electroluminescence device and material for organic electroluminescence device
ActiveUS20090045730A1Improve efficiencyLong life-timeOrganic chemistryDischarge tube luminescnet screensOrganic filmBenzo(c)phenanthrene
An organic electroluminescence device includes: a cathode; an anode; and a single-layered or multilayered organic thin-film layer provided between the cathode and the anode. The organic thin-film layer includes at least one emitting layer. The at least one emitting layer contains at least one phosphorescent material and a host material represented by the following formula (1).Ra-Ar1-Rb (1)In the formula, Ar1, Ra and Rb each represent a substituted or unsubstituted benzene ring or a condensed aromatic hydrocarbon ring selected from a substituted or unsubstituted naphthalene ring, a substituted or unsubstituted chrysene ring, a substituted or unsubstituted fluoranthene ring, a substituted or unsubstituted phenanthrene ring, a substituted or unsubstituted benzophenanthrene ring, a substituted or unsubstituted dibenzophenanthrene ring, a substituted or unsubstituted triphenylene ring, a substituted or unsubstituted benzo[a]triphenylene ring, a substituted or unsubstituted benzochrysene ring, a substituted or unsubstituted benzo[b]fluoranthene ring and a substituted or unsubstituted picene ring. Substituents for Ra and Rb are not aryl groups.
Owner:IDEMITSU KOSAN CO LTD
Precursor source mixtures
A precursor source mixture useful for CVD or ALD of a film comprising: at least one precursor composed of an element selected from the group consisting of Li, Na, K, Rb, Cs, Fr, Be, Mg, Ti, Zr, Hf, Sc, Y, La, V, Nb, Ta, Cr, Mo, W, Mn, Re, Fe, Ru, Os, Co, Rh, Ir, Ni, Pd, Pt, Cu, Ag, Au, Zn, Cd, Hg, B, Al, Ga, In, Tl, Si, Ge, Sn, Pb, As, P, Sb and Bi, to which is bound at least one ligand selected from the group consisting of hydride, alkyl, alkenyl, cycloalkenyl, aryl, alkyne, carbonyl, amido, imido, hydrazido, phosphido, nitrosyl, nitryl, nitrate, nitrile, halide, azide, alkoxy, siloxy, silyl, and halogenated, sulfonated or silyated derivatives thereof, which is dissolved, emulsified or suspended in an inert liquid selected from the group consisting of aliphatic hydrocarbons, aromatic hydrocarbons, alcohols, ethers, aldehydes, ketones, acids, phenols, esters, amines, alkylnitrile, halogenated hydrocarbons, silyated hydrocarbons, thioethers, amines, cyanates, isocyanates, thiocyanates, silicone oils, nitroalkyl, alkylnitrate, and mixtures thereof. The precursor source mixture may be a solution, emulsion or suspension and may consist of a mixture of solid, liquid and gas phases which are distributed throughout the mixture.
Owner:GLOBALFOUNDRIES INC
Platinum complex and light emitting device
ActiveUS20070103060A1Enhanced glowSolve low luminous efficiencyDischarge tube luminescnet screensElectroluminescent light sourcesOxygenLight emission
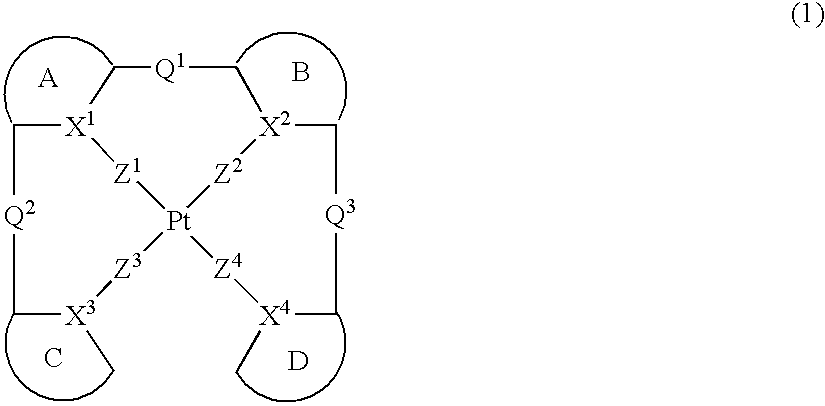
Provision of a novel platinum complex which is useful as a material for a light-emitting device of good light emission characteristic and light emission efficiency, and a novel light-emitting material that may be utilized in various fields. A platinum complex represented by the following general formula (1): (in which two rings of ring A, ring B, ring C, and ring D represent nitrogen-containing heterocyclic rings which may have a substituent and the remaining two rings of them represent aryl rings or hetero aryl rings which may have a substituent, the ring A and the ring B, the ring A and the ring C or / and the ring B and the rind D may form condensed rings. Two of X1, X2, X3, and X4 represent nitrogen atoms coordination bonded to a platinum atom and the remaining two of them represent carbon atoms or nitrogen atoms. Q1, Q2, and Q3 each represents a bond, oxygen atom, sulfur atom or bivalent group, two of Z1, Z2, Z3, and Z4 represent coordination bonds, and the remaining two of them represent covalent bonds, oxygen atoms or sulfur atoms), and a light-emitting device containing the platinum complex.
Owner:TAKASAGO INTERNATIONAL CORPORATION
Organic electroluminescent device using aryl amine derivative containing heterocycle
An organic electroluminescent device including: an anode, a cathode, an emitting layer formed of an organic compound and interposed between the cathode and the anode, and two or more layers provided in a hole-injecting / hole-transporting region between the anode and the emitting layer; of the layers which are provided in the hole-injecting / hole-transporting region, a layer which is in contact with the emitting layer containing a compound represented by the formula (1); and of the layers which are provided in the hole-injecting / hole-transporting region, a layer which is interposed between the anode and the layer which is in contact with the emitting layer containing an amine derivative represented by the formula (2).
Owner:IDEMITSU KOSAN CO LTD +1
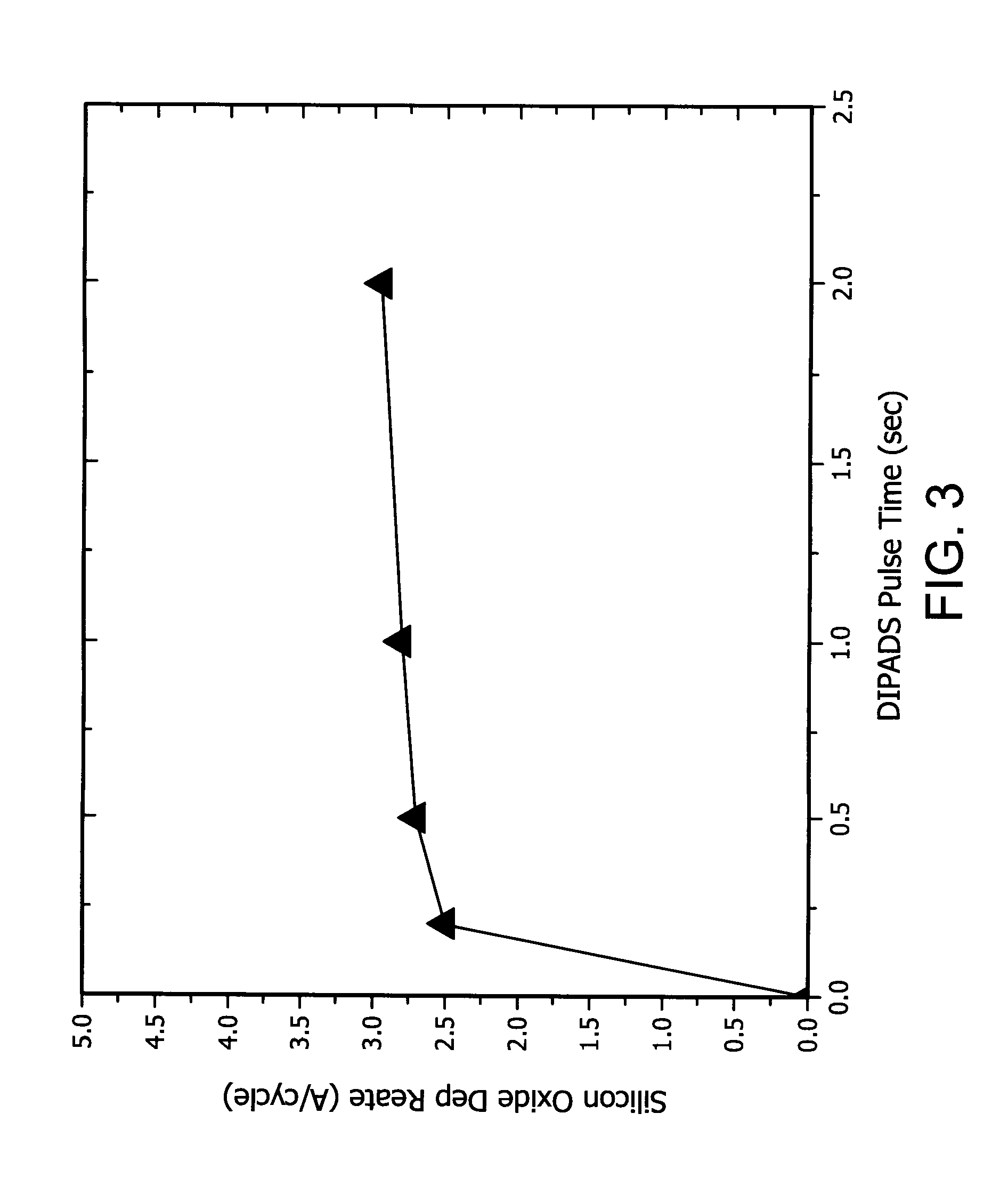
Organoaminodisilane precursors and methods for depositing films comprising same
Described herein are precursors and methods for forming silicon-containing films. In one aspect, there is provided a precursor of Formula I:wherein R1 is selected from linear or branched C3 to C10 alkyl group, linear or branched C3 to C10 alkenyl group, linear or branched C3 to C10 alkynyl group, C1 to C6 dialkylamino group, electron withdrawing group, and C6 to C10 aryl group; R2 is selected from hydrogen, linear or branched C1 to C10 alkyl group, linear or branched C3 to C6 alkenyl group, linear or branched C3 to C6 alkynyl group, C1 to C6 dialkylamino group, C6 to C10 aryl group, linear or branched C1 to C6 fluorinated alkyl group, electron withdrawing group, and C4 to C10 aryl group; optionally wherein R1 and R2 are linked together to form ring selected from substituted or unsubstituted aromatic ring or substituted or unsubstituted aliphatic ring; and n=1 or 2.
Owner:VERSUM MATERIALS US LLC
Heterocyclic compounds
InactiveUS6329381B1Excellent interferon biosynthesis inducing activityInhibition thicknessAntibacterial agentsBiocideBULK ACTIVE INGREDIENTInterferon inducer
The present invention relates to a heterocyclic compound of the following general formula (I):wherein X is sulfur atom, oxygen atom or -NR3- (R3 may form a heterocyclic ring or a substituted heterocyclic ring with R1 via the nitrogen atom),R1 is alkyl group, substituted alkyl group, aryl group, substituted aryl group, heterocyclic group or substituted heterocyclic group, andR2 is hydrogen atom, halogen atom etc.;or its pharmaceutically acceptable salt and interferon inducers, antiviral agents, anticancer agents and therapeutic agents for immunologic diseases comprising the compound (I) or its pharmaceutically acceptable salt as active ingredients.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
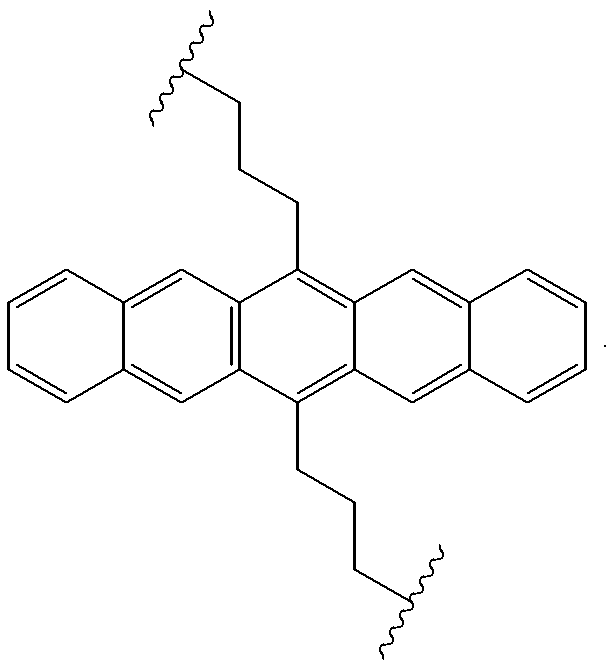
New condensed polycyclic compound and organic light-emitting element using the same
InactiveUS20130175519A1Improve light emission efficiencyReduce the driving voltageOrganic chemistryOrganic compound preparationPolycyclic compoundAryl
The present invention provides a stable new condensed polycyclic compound which is not likely to form a molecular association. In addition, the present invention also provides an organic light-emitting element having a high light-emitting efficiency and a low drive voltage. In the condensed polycyclic compound in Claim 1 represented by the general formula [1], R1, R2 and R5 are each independently selected from a hydrogen atom, an alkyl group having 1 to 4 carbon atoms, an aryl group, and a heterocyclic group. R3 and R4 each represent an alkyl group having 1 to 4 carbon atoms. The aryl group and the heterocyclic group each may have at least one of an alkyl group, an aralkyl group, an aryl group, a heterocyclic group, an amino group, and an alkoxy group as a substituent.
Owner:CANON KK
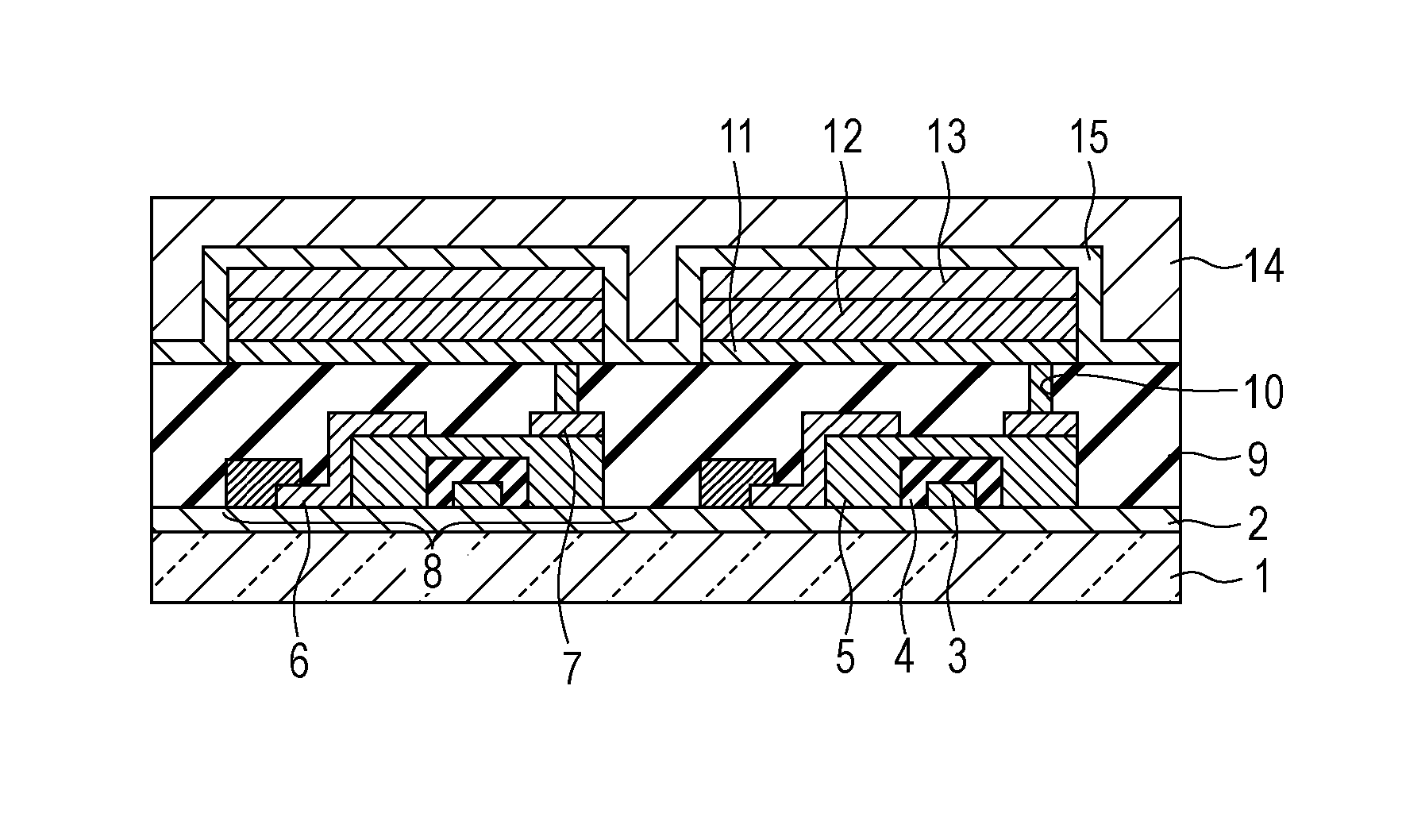
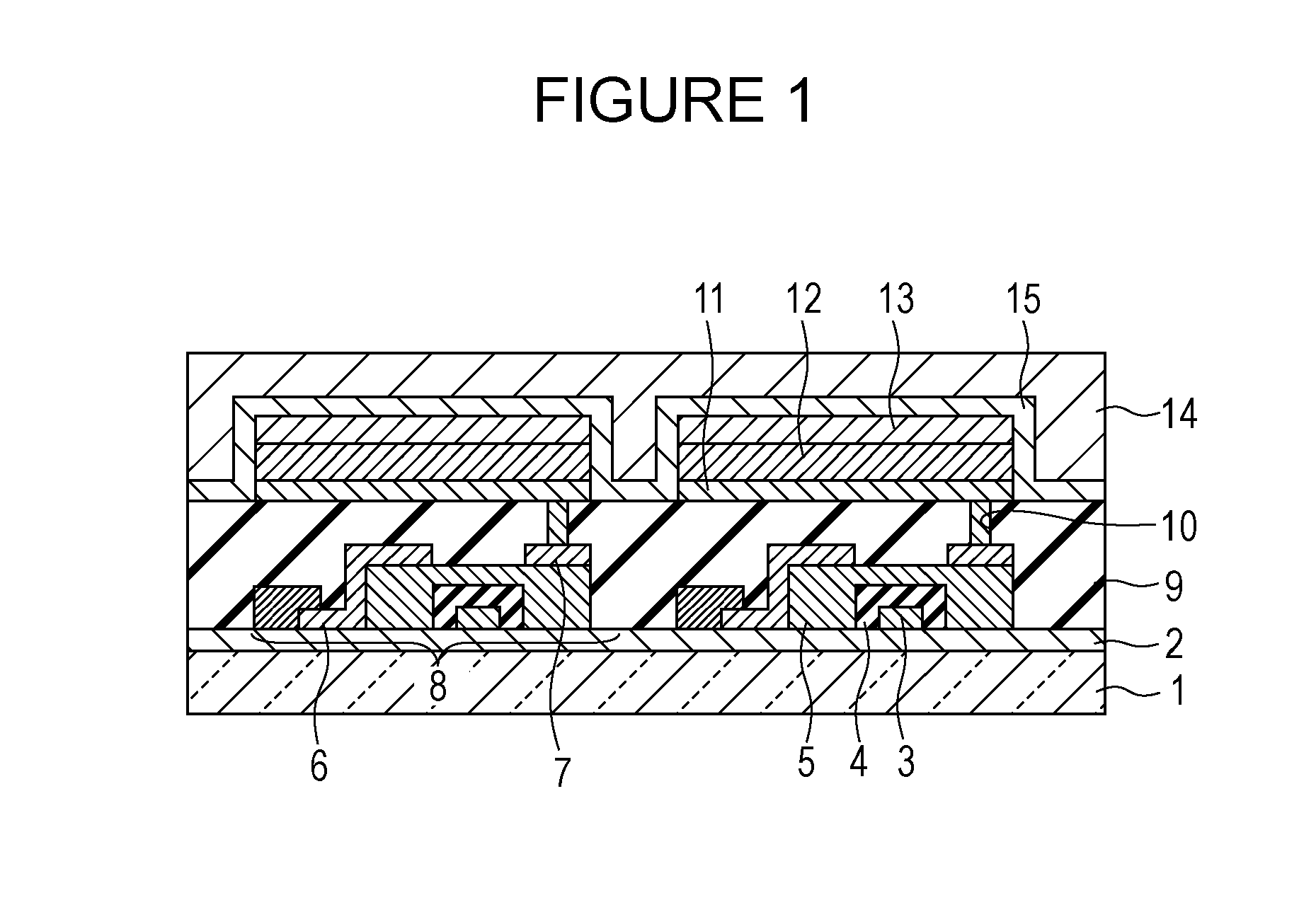
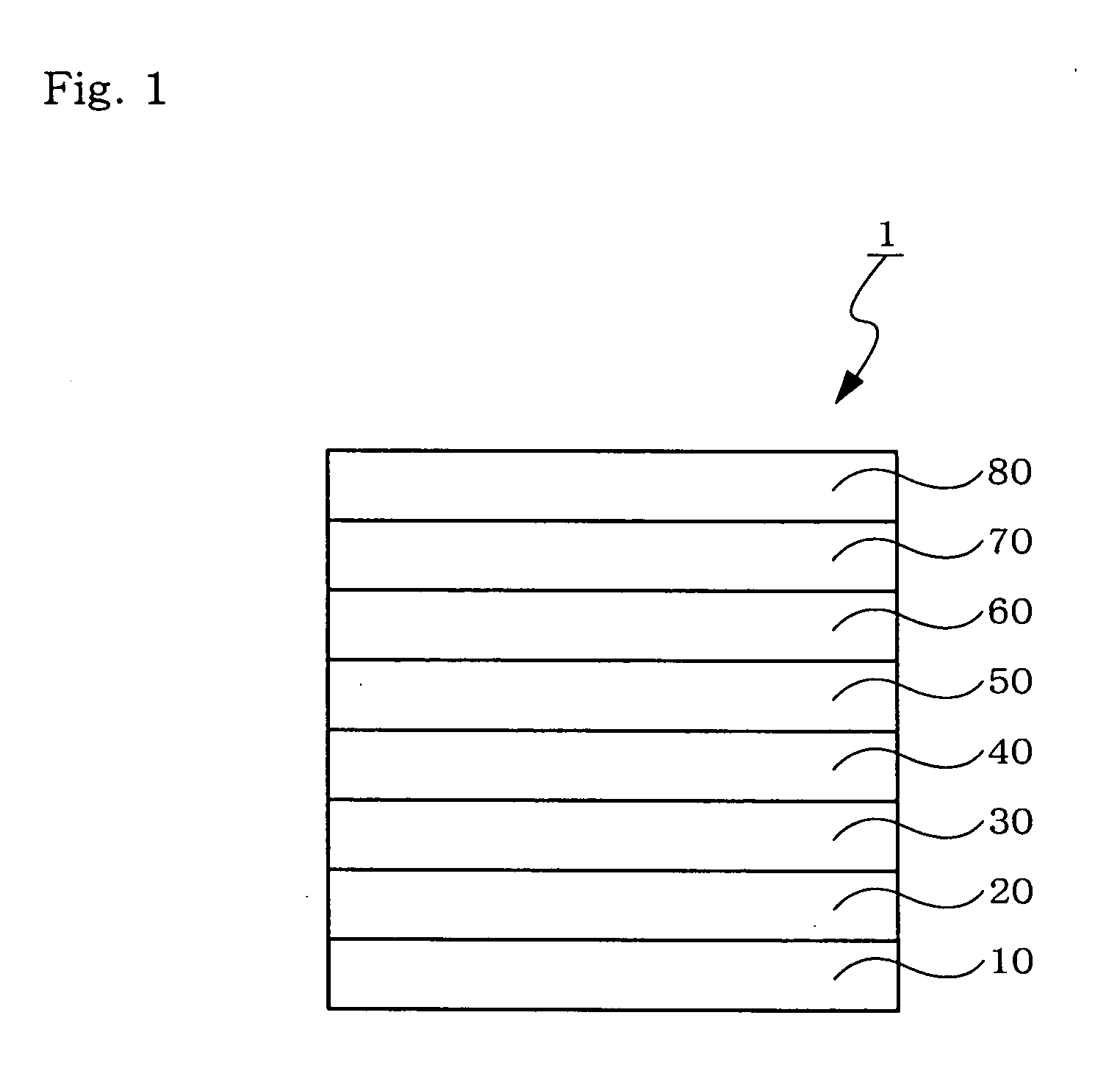
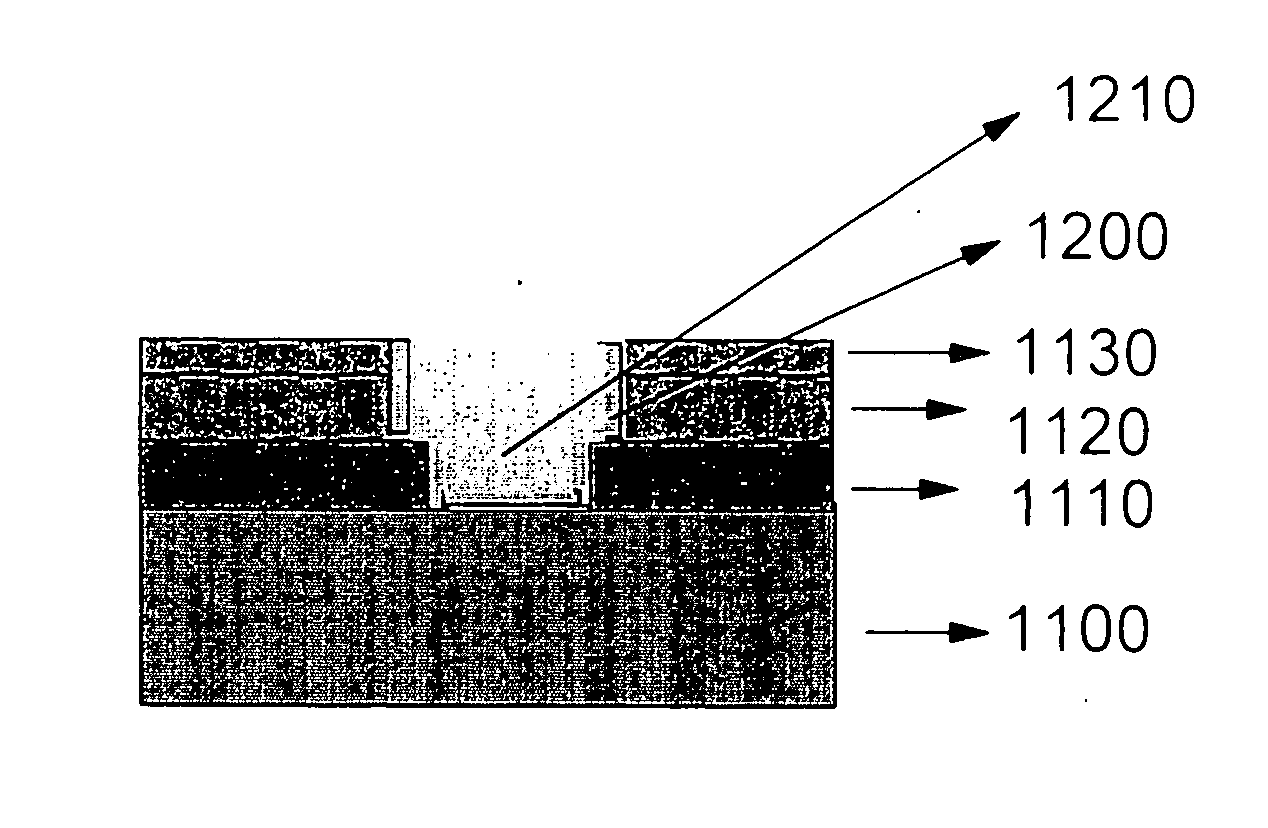
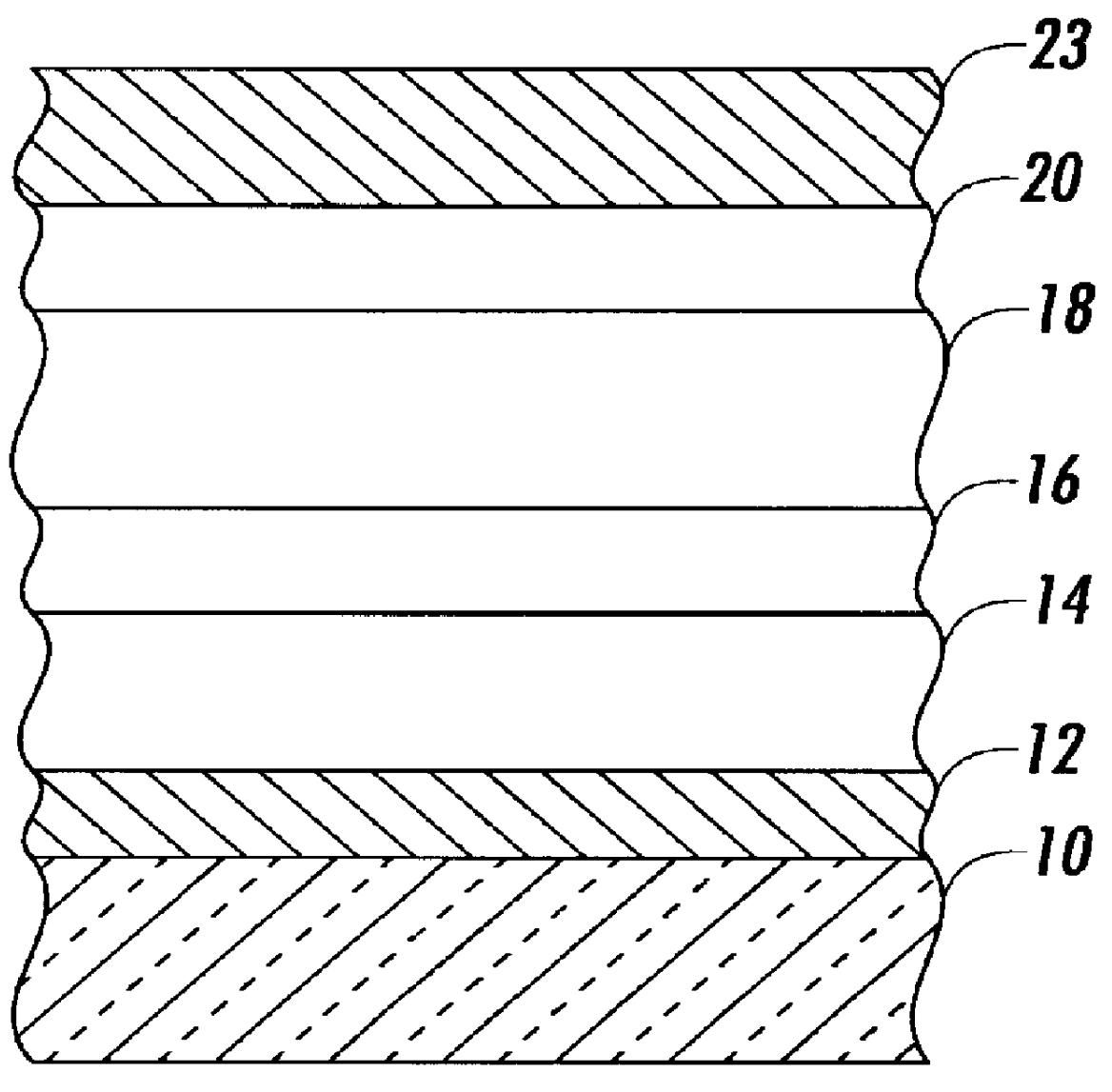
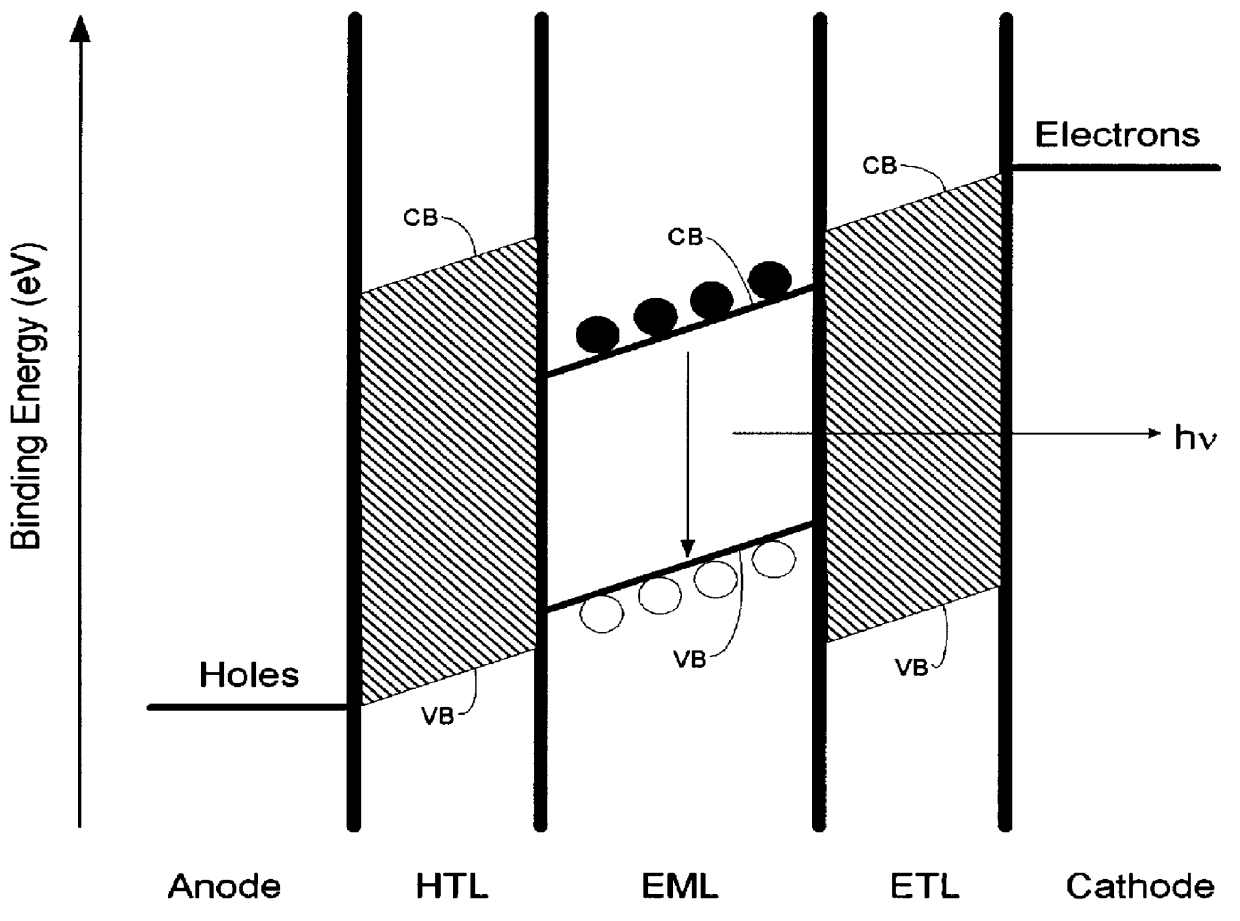
Organic electroluminescent device
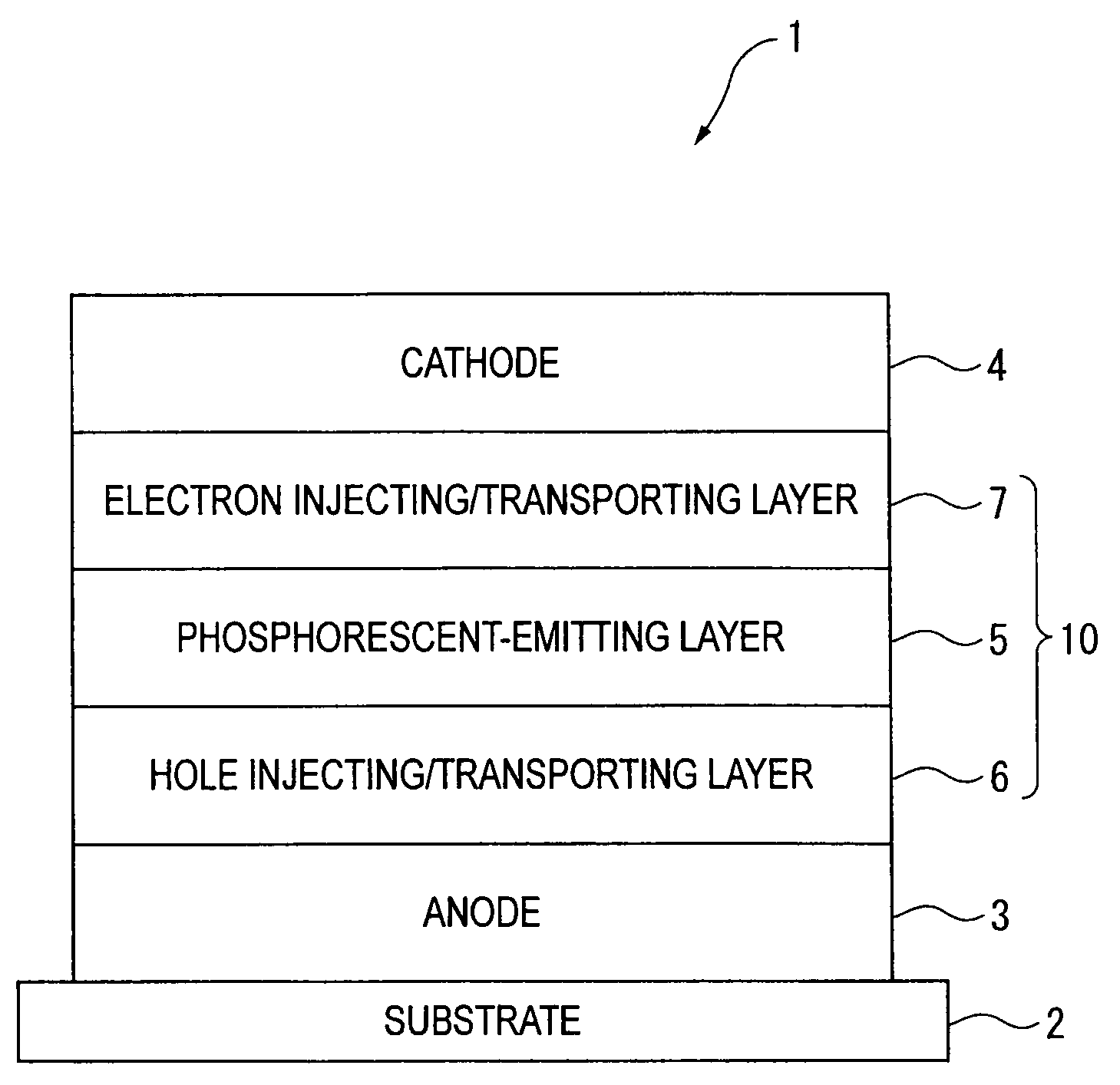
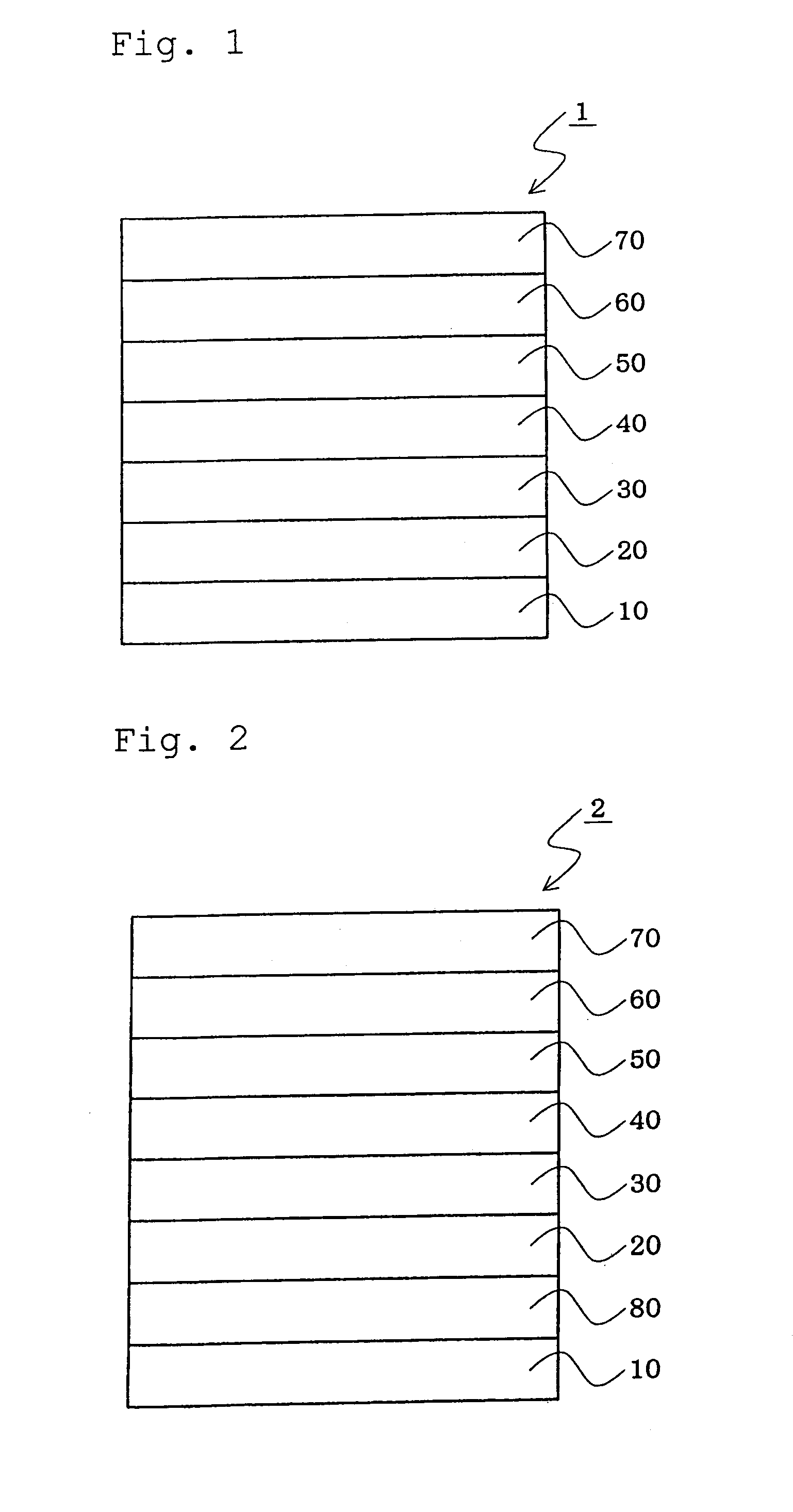
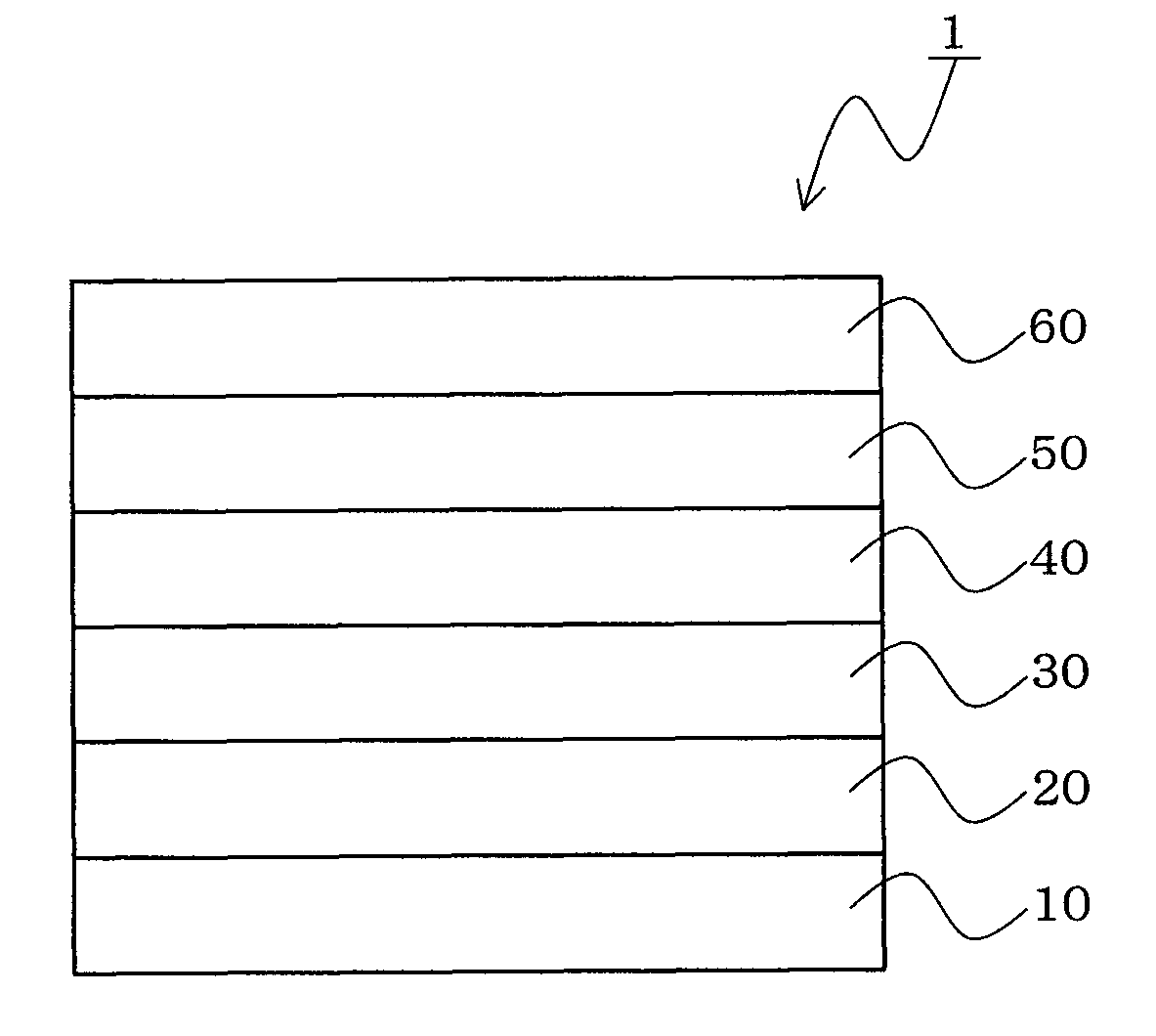
InactiveUS20070104977A1Improve efficiencyHigh color purityDischarge tube luminescnet screensLamp detailsDopantAryl
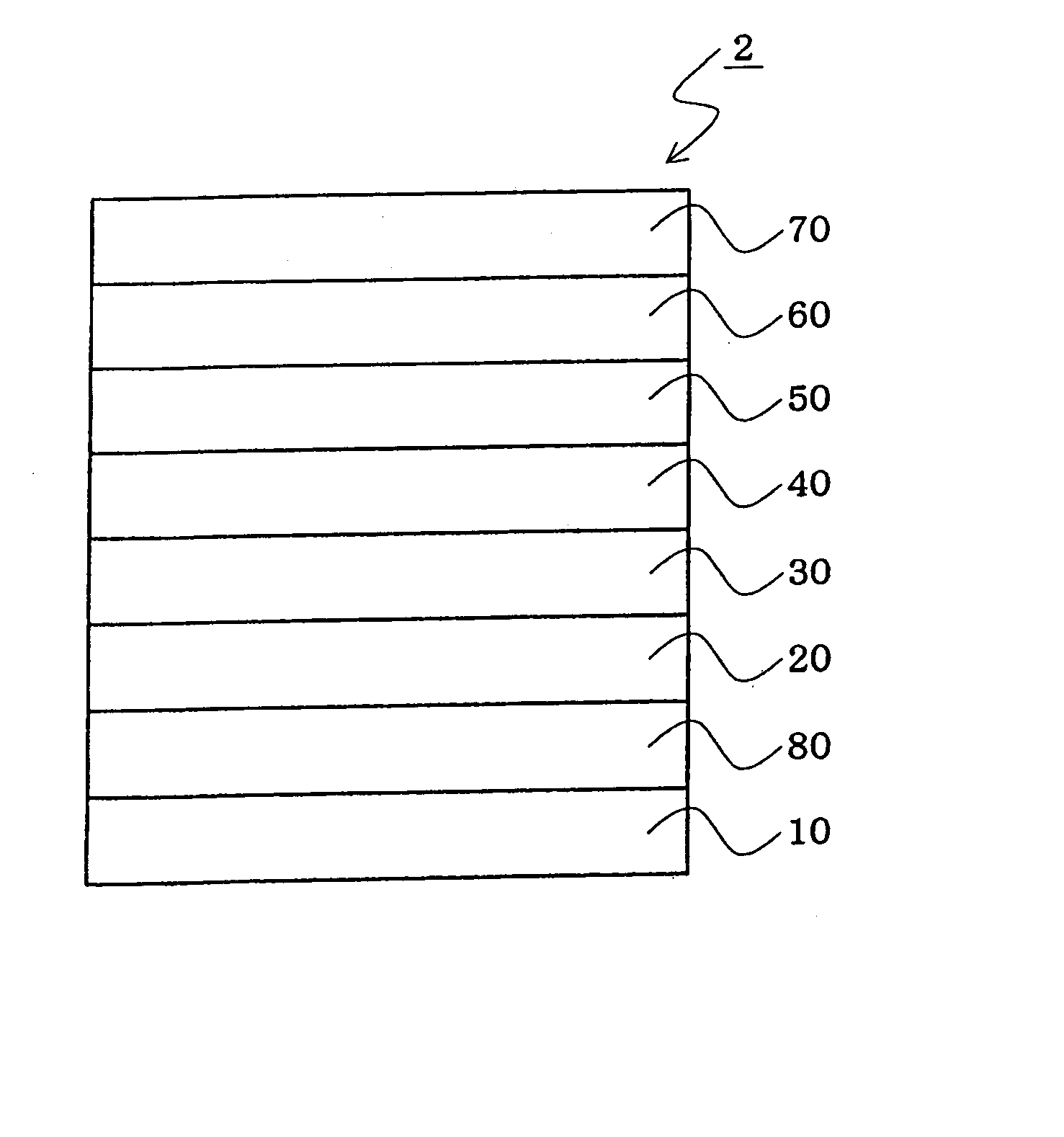
An organic electroluminescent device 1 comprising, an emitting layer (50) and an electron-transporting layer (60) between a cathode (80) and an anode (20), the electron-transporting layer (60) comprising a compound represented by formula (1), the emitting layer (50) comprising a host material which is a compound with an energy gap of 2.8 eV or less represented by formula (2) and a dopant which is an indenoperylene derivative, A-B (1) wherein A is an aromatic hydrocarbon group with three or more carbocycles and B is a substituted or unsubstituted heterocyclic group, X—(Y)n (2) wherein X is a condensed aromatic ring group with three or more carbocycles, Y is a group selected from substituted or unsubstituted aryl, substituted or unsubstituted diarylamino, substituted or unsubstituted arylalkyl and substituted or unsubstituted alkyl groups, and n is an integer of 1 to 6, provided that Ys may be the same or different when n is 2 or more.
Owner:IDEMITSU KOSAN CO LTD
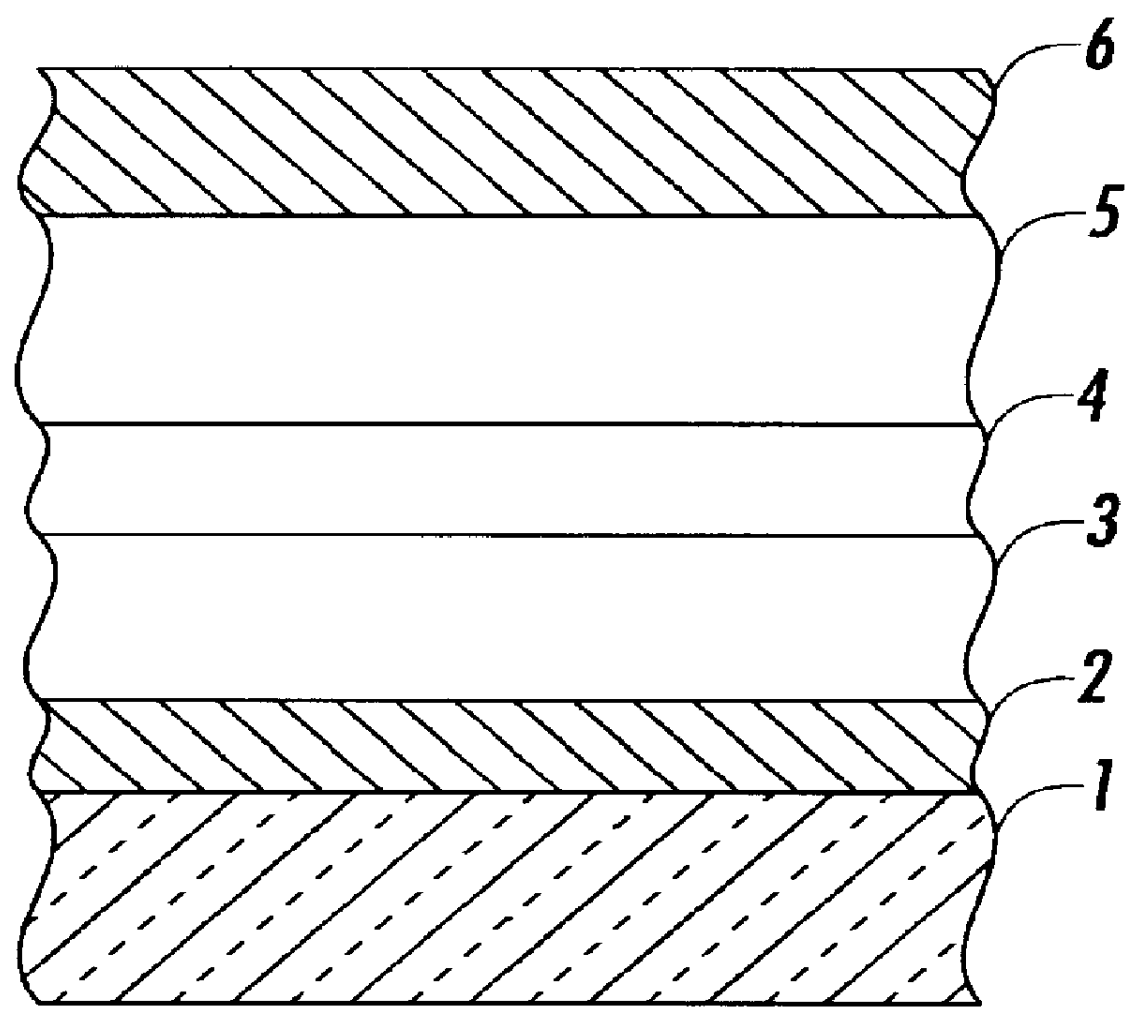
Light emitting element
InactiveUS20130105787A1Improve light emission efficiencySufficient durability lifeOrganic chemistrySolid-state devicesSilyleneAlkaline earth metal
Provided is an organic thin film light emitting element which has achieved all of improved luminous efficiency, improved driving voltage and improved durability life. Specifically provided is a light emitting element which comprises a hole transport layer and an electron transport layer between a positive electrode and a negative electrode and emits light by means of electrical energy. The light emitting element is characterized in that: the hole transport layer of the light emitting element contains a compound represented by general formula (1); the electron transport layer contains a donor compound; and the donor compound is an alkali metal, an inorganic salt containing an alkali metal, a complex of an alkali metal and an organic substance, an alkaline earth metal, an inorganic salt containing an alkaline earth metal, or a complex of an alkaline earth metal and an organic substance. (In the formula, R1-R20 each represents one group selected from the group consisting of hydrogen, deuterium, an alkyl group, a cycloalkyl group, an amino group, an aryl group, a heterocyclic group, a heteroaryl group, an alkenyl group, a cycloalkenyl group, an alkynyl group, analkoxy group, an alkylthio group, an arylether group, an arylthioether group, a halogen, a cyano group, a —P(═O)R24R25 group and a silyl group; R24 and R25 each represents an aryl group or a heteroaryl group; and these substituents may be further substituted, or adjacent two substituents may combine together to form a ring. Meanwhile, R21-R23 may be the same or different and each represents one group selected from the group consisting of an alkyl group, a cycloalkyl group, an aryl group and a heteroaryl group; and these substituents maybe further substituted.)
Owner:TORAY IND INC
Organoaminosilane Precursors and Methods for Depositing Films Comprising Same
Owner:VERSUM MATERIALS US LLC
Aromatic amine derivative and organic electroluminescence device using the same
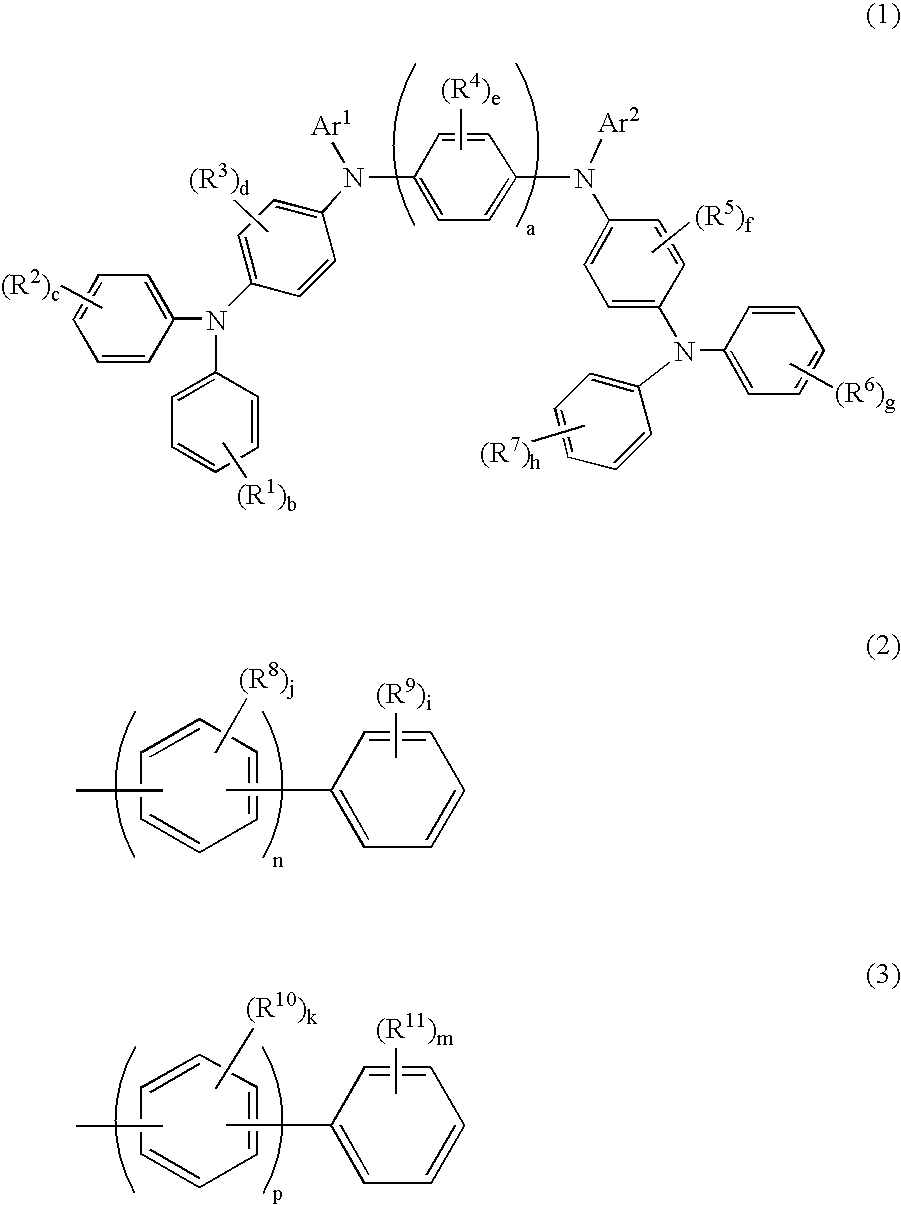
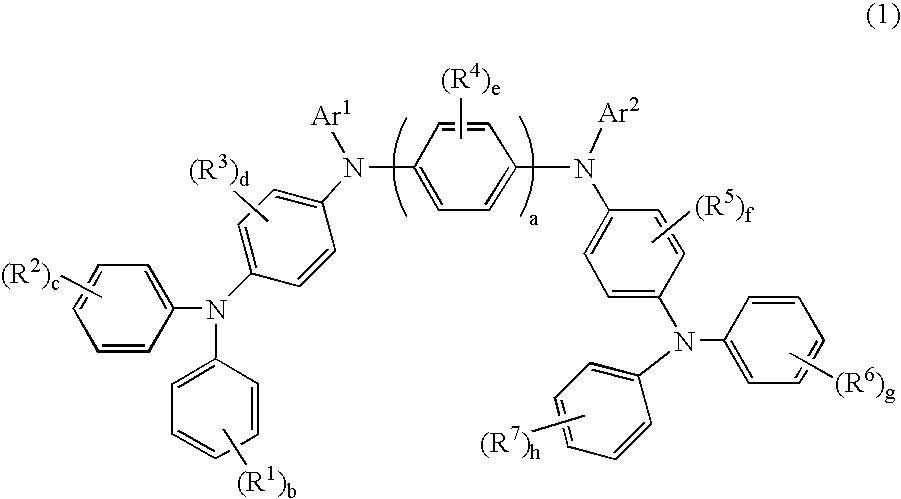
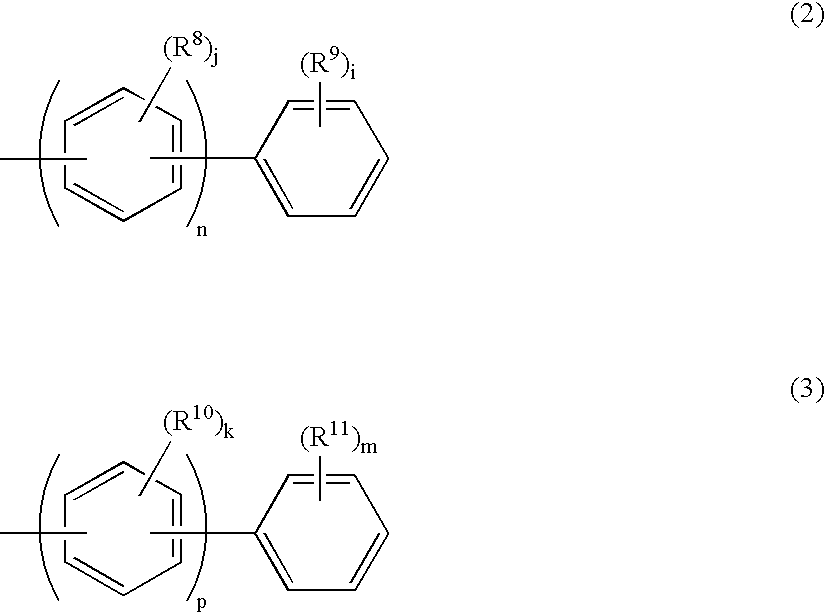
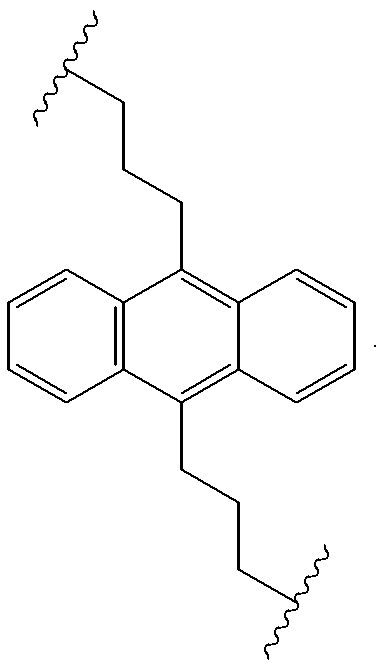
InactiveUS20080145707A1Small increase in driving voltageSolution to short lifeOrganic chemistryLayered productsArylHydrogen atom
The present invention provides a novel aromatic amine derivative enabling to obtain an organic electroluminescence device which is driven under a low voltage, exhibits small increase in the driving voltage after continuous driving for a long time and has a long life. The amine derivative is represented by the following general formula (1). In the formula, R1 to R7 each represent, for example, hydrogen atom or a substituted or unsubstituted aryl group having 5 to 50 nuclear carbon atoms; a represents an integer of 1 or greater; b, c, g and h each represent an integer of 1 to 5, and d, e and f each represent an integer of 1 to 4; and Ar1 and Ar2 represent a group represented by following general formulae (2) and (3), respectively, and the groups represented by Ar1 and Ar2 are not same with each other. R8 to R11 each represent, for example, hydrogen atom; and i and m each represent an integer of 1 to 5, j and k each represent an integer of 1 to 4, n and p each represent an integer of 0 or greater, and n≠p.
Owner:IDEMITSU KOSAN CO LTD
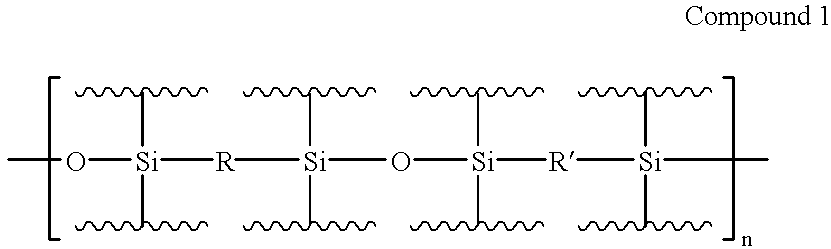
Organic electroluminescent materials and device made from such materials
Electroluminescent devices and materials including an electroluminescent organo-siloxane polymer. The main chain of the organo-siloxane polymer comprises an organic component that can be alkenyl, alkynyl, aralkyl, aryl, heteroaralkyl, and heteroaryl which can be substituted optionally with hydrogen, alkyl, aryl, heteroalkyl, heteroaralkyl, nitro, cyano, hydroxy, alkoxy, aryloxy, thio, alkylthio, arylthio, amino, halogen, dialkylamino, diarylamino, diaralkylamino, arylamino, alkylamino, arylalkylamino, carbonyloxy, carbonylalkoxy, carbonylalkyloxy, alkylcarbonyloxy, arylcarbonyloxy, alkoxylcarbonyloxy, sulfonyl, or sulfonyloxy. The organic component includes at least two covalent bonds coupling the organic component to the main chain of the organo-siloxane polymer. Such devices provide superior performance and mechanical stability compared with conventional organic electroluminescent materials and devices made from such materials.
Owner:ORGANIC DISPLAY TECH
Recovery of hydrophobicity of low-k and ultra low-k organosilicate films used as inter metal dielectrics
InactiveUS20050106762A1Low costHigh mechanical strengthSolid-state devicesSemiconductor/solid-state device manufacturingChemical treatmentSilylation
Often used to reduce the RC delay in integrated circuits are dielectric films of porous organosilicates which have a silica like backbone with alkyl or aryl groups (to add hydrophobicity to the materials and create free volume) attached directly to the Si atoms in the network. Si—R bonds rarely survive an exposure to plasmas or chemical treatments commonly used in processing; this is especially the case in materials with an open cell pore structure. When Si—R bonds are broken, the materials lose hydrophobicity, due to formation of hydrophilic silanols and low dielectric constant is compromised. A method by which the hydrophobicity of the materials is recovered using a novel class of silylation agents which may have the general formula (R2N)XSiR′Y where X and Y are integers from 1 to 3 and 3 to 1 respectively, and where R and R′ are selected from the group of hydrogen, alkyl, aryl, allyl and a vinyl moiety. Mechanical strength of porous organosilicates is also improved as a result of the silylation treatment.
Owner:GLOBALFOUNDRIES INC
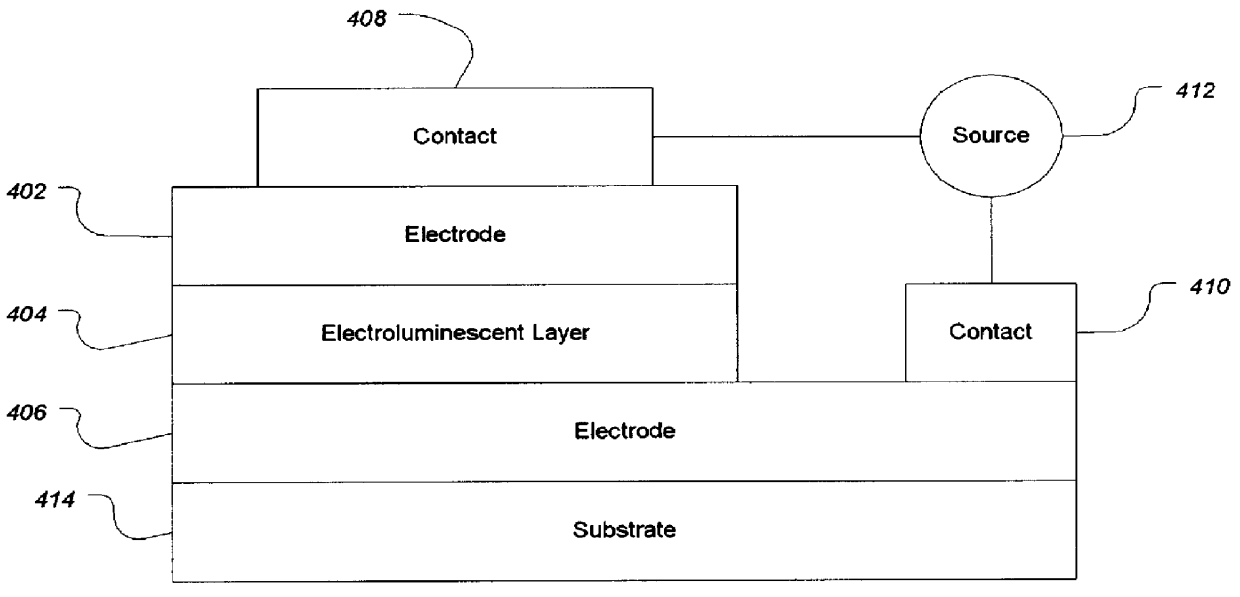
Electroluminescent (EL) devices
InactiveUS6057048ALow working voltageSpectrum spreadingOrganic chemistryDischarge tube luminescnet screensArylHalogen
An electroluminescent device comprised of an anode, a hole transporting layer, a light emitting layer, and a cathode, wherein said light emitting layer contains a component of the formula wherein Ar1, Ar2, Ar3, and Ar4 are each independently aryl or optionally aliphatic; R1 and R2 are independently selected from the group consisting of hydrogen, aliphatic, halogen, and cyano; L is a suitable linking group; and n is a number of from 0 to about 3.
Owner:LG DISPLAY CO LTD
Useful precursors for organic electroluminescent materials and devices made from such materials
InactiveUS6165383ADesirable efficiencyDesirable weightSilicon organic compoundsLayered productsArylAnthracene
Precursor materials useful for making organic electroluminescent devices such as anthracene derivatives having the structure: R40-R43 and R44-R47 are selected independently from the group consisting of hydrogen, alkyl, aryl, heteroalkyl, heteroaralkyl, nitro, cyano, hydroxy, alkoxy, aryloxy, thio, alkylthio, arylthio, amino, halogen, dialkylamino, diarylamino, diaralkylamino, arylamino, alkylamino, arylalkylamino, carbonyloxy, carbonylalkoxy, carbonylalkyloxy, alkylcarbonyloxy, arylcarbonyloxy, alkoxylcarbonyloxy, sulfonyl, sulfonyloxy. R48 and R49 are selected independently from the group consisting of trialkoxysilyl, dialkoxysilyl, trichlorosilyl, dichlorosilyl, heptachlorotrisiloxy, and pentachlorodisiloxy.
Owner:ORGANIC DISPLAY TECH
Modified polyethylene compositions
The present invention relates to a composition comprising more than 25 weight % (based on the weight of the composition) of one or more ethylene polymers having an Mw of 20,000 g / mole or more and at least 0.1 weight % of a liquid hydrocarbon modifier where the modifier has: 1) a viscosity index of 120 or more, and 2) an kinematic viscosity of 3 to 3000 cSt at 100° C., and 3) a pour point of −10° C. or less, and 4) a flash point of 200° C. or more; and wherein the modifier contains less than 5 weight % of functional groups selected from hydroxide, aryls, substituted aryls, halogens, alkoxys, carboxylates, esters, acrylates, oxygen, nitrogen, and carboxyl, based upon the weight of the modifier.
Owner:EXXONMOBIL CHEM PAT INC
Material for organic electroluminescent device, organic electroluminescent device, and organic electroluminescent display
ActiveUS8044390B2Improve heat resistanceDeposition stabilityOrganic chemistryElectroluminescent light sourcesHalogenHydrogen
A material for an organic electroluminescent device including an imine derivative represented by the following formula (Ia) or (Ib),wherein Y1 to Y4 are independently a carbon atom or a nitrogen atom; R1 to R4 are independently hydrogen, an alkyl group, an aryl group, a heterocycle, a halogen atom, a fluoroalkyl group or a cyano group; and R1 and R2, or R3 and R4 may be bonded together to form a ring.
Owner:IDEMITSU KOSAN CO LTD +1
High melting thermoplastic elastomeric alpha-olefin polymers (PRE/EPE effect) and catalysts therefor
InactiveUS6559262B1Activity of fluxional unbridged metallocene polymerization catalystsHigh molecular weightGroup 4/14 element organic compoundsMetallocenesElastomerEthylene Homopolymers
This invention relates generally to low ethylene insertions into I-olefin polymers and processes for production of such polymers using unbridged fluxional metallocenes, primarily substituted aryl indenyl metallocenes, and more particularly to use of unbridged, fluxional, cyclopentadienyl or indenyl metallocene catalyst systems in methods of production of high melting point I-olefin homo- and co-polymers, particularly elastomeric crystalline and amorphous block homo- and co-polymers of I-olefins. The activity of fluxional unbridged metallocene polymerization catalysts containing at least one 2-arylindene ligand is increased 10x or more by the addition of small (typically 0.1-10 wt. %) amounts of ethylene to the polymerization system, which increase is termed the Polymerization Rate-Enhancement effect (PRE), which is measured in terms of an Ethylene Enhancement Factor (EEF) as a dimensionless ratio in the range of from about 1.1 to about 10 or above. The amount of ethylene included in the reaction system can be selected and controlled to be so small as to result in essentially minimal (<2 mole %) incorporation of ethylene units into the resulting elastomeric polymer and the molecular weight may be increased. Amounts of ethylene to generate the PRE effect may be greater than 0.1 wt. % and preferably range up to about 2 wt. %. However, if a polymer with more ethylene is desired, additional ethylene may be incorporated into the polymerization feed, including up to 10 to about 50 mole % based on olefin units. A second important aspect of this invention is the ability to use a PRE activity-enhancing amount of ethylene in an olefin polymerization without substantially affecting the physical properties of the elastomer. In a third important aspect of this invention, I-olefin elastomers are produced through incorporation of ethylene using unbridged fluxional catalyst systems which may not otherwise produce acceptable elastomeric homopolymers. This effect is termed the EPE effect, for Elastomeric Property-Enhancing effect. The EPE amount of ethylene required to produce such elastomers typically overlaps the PRE activity-enhancing amount. Incorporation of up to about 5 mole % or more of ethylene typically will produce an elastomeric polymer using such catalyst systems. Typical useful amounts of incorporated ethylene include about 1 to 3 mole %. Preferred polymers of this invention retain sufficient crystallinity to provide a high melting point (by DSC) of about 80° C., preferably above 100° C., including in the range of from about 120° C. to about 140° C. and above. Novel flexible alpha-olefin homo and copolymers having elongation in excess of 600% and substantially no retained force are disclosed.
Owner:BP CORP NORTH AMERICA INC
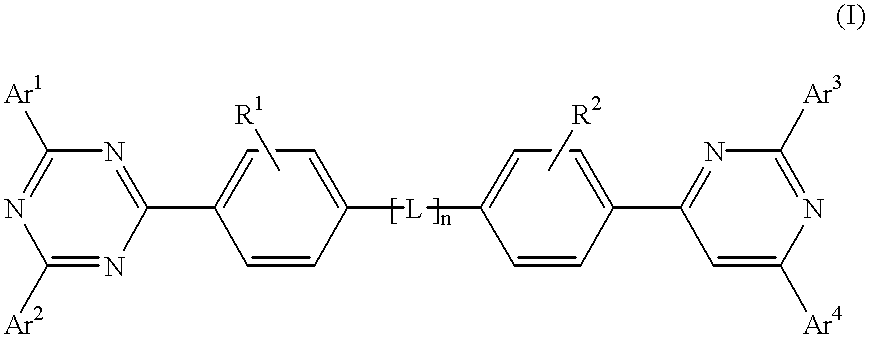
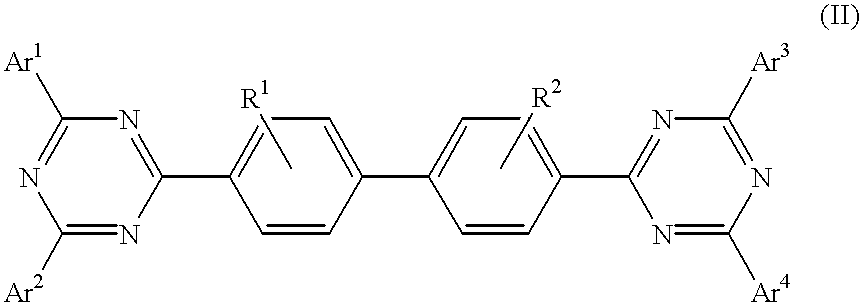
Triazine compositions
A triazine compound of the formulawherein Ar1, Ar2, Ar3, and Ar4 are each an aryl, aliphatic, or a mixture of aryl and aliphatic; R1 and R2 are selected from the group consisting of hydrogen, alkyl, aryl, alkoxy, halogen, and cyano; and L is L(n) wherein n is zero or 1, said L being a divalent group.
Owner:LG DISPLAY CO LTD
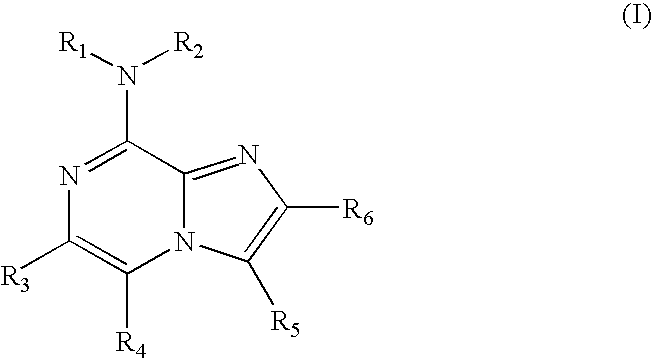
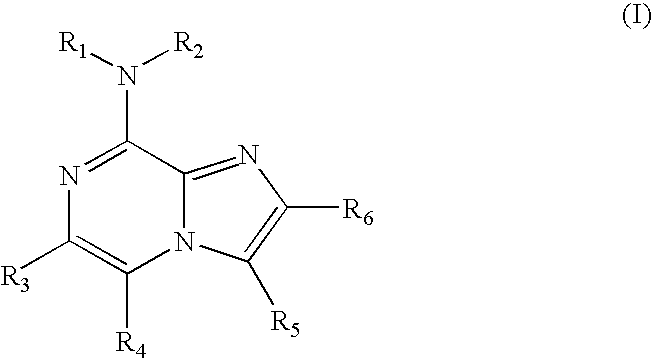
Use of 8-amino-aryl-substituted imidazopyrazines as kinase inhbitors
The present invention relates to 8-amino-aryl-substituted imidazopyrazines which modulate the activity of protein kinases ("PKs"). The compounds of this invention are therefore useful in treating disorders related to abnormal PK activity. Pharmaceutical compositions comprising these compounds, methods of treating diseases utilizing pharmaceutical compositions comprising these compounds and methods of preparing them are also disclosed.
Owner:SUGEN INC
Method for producing aromatic amino compound
InactiveUS7250532B2High yieldEasy to eliminateOrganic compound preparationAmino compound preparationArylCompound a
A method for producing aromatic amino compound (V):by synthesizing intermediate compound (IV):by the reaction of compound (I): H2N—R1 with a mixture of halogenated aryl compounds (II): Ar1—X and (III): Ar2—X in the presence of a noble metal catalyst, followed by eliminating the substituent R1 from the nitrogen atom in compound (IV) under an acidic condition or an alkaline condition or by addition of a reducing agent or an oxidizing agent. (R1: a substituent having 2 to 50 carbon atoms; Ar1 and Ar2: a substituted or unsubstituted hydrocarbon group or heterocyclic group having 6 to 50 carbon atoms and the same with or different from each other; and X: a halogen group). The aromatic amino compound useful as the charge transporting material can be produced efficiently at a great yield without using highly toxic raw materials.
Owner:IDEMITSU KOSAN CO LTD
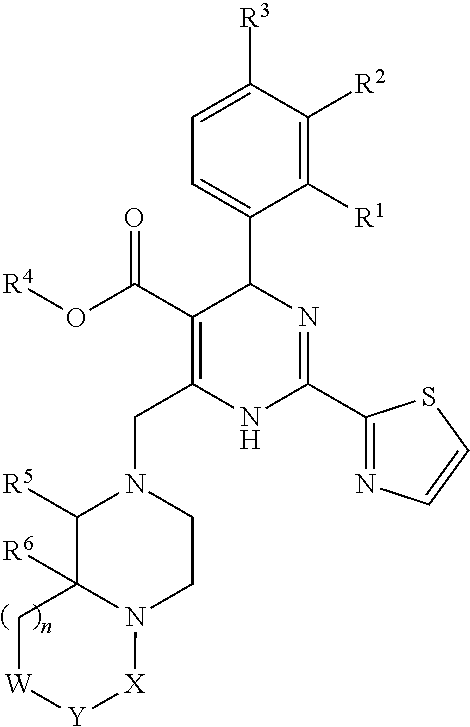
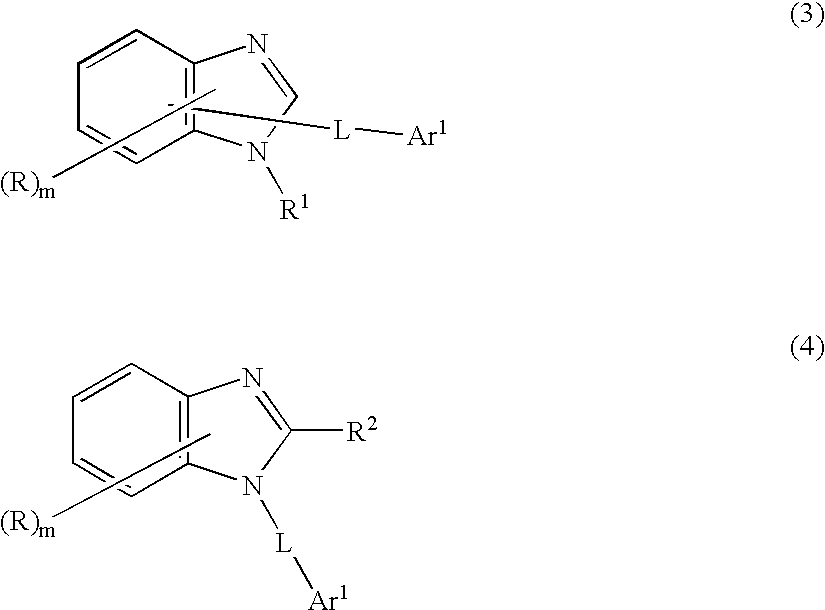
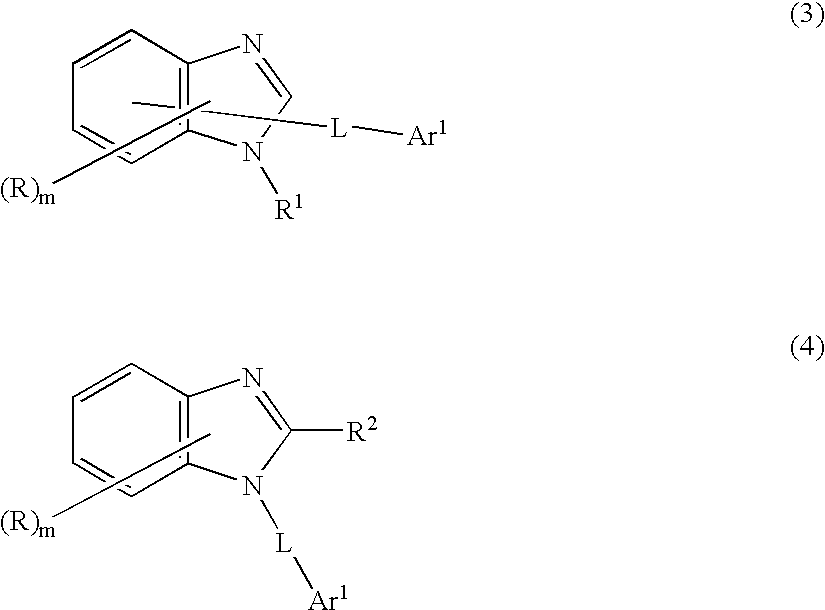
6-amino-1,4-dihydro-benzo[d][1,3] oxazin-2-ones and analogs useful as progesterone receptor modulators
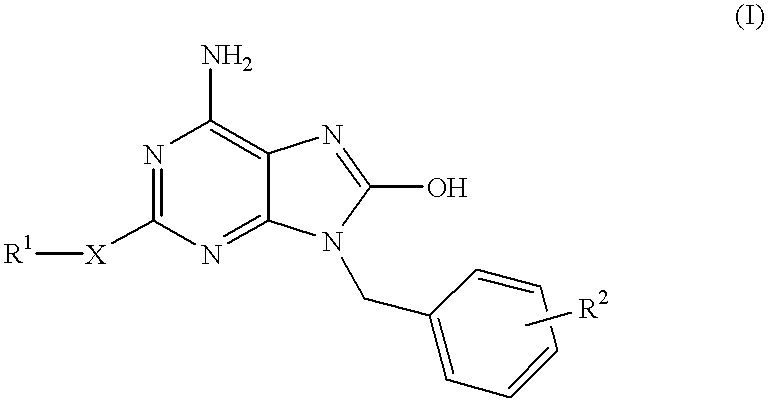
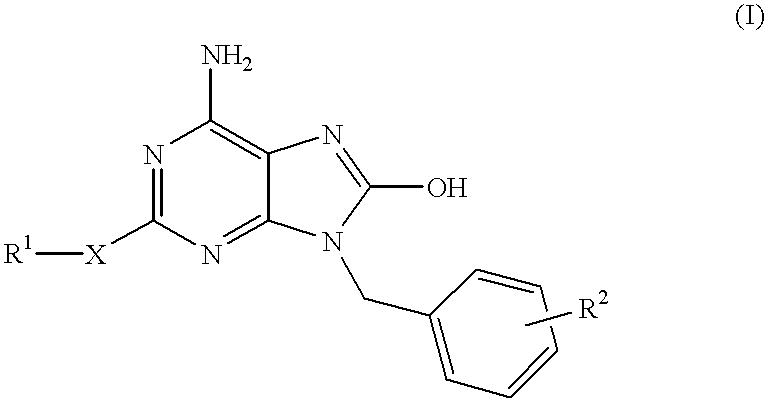
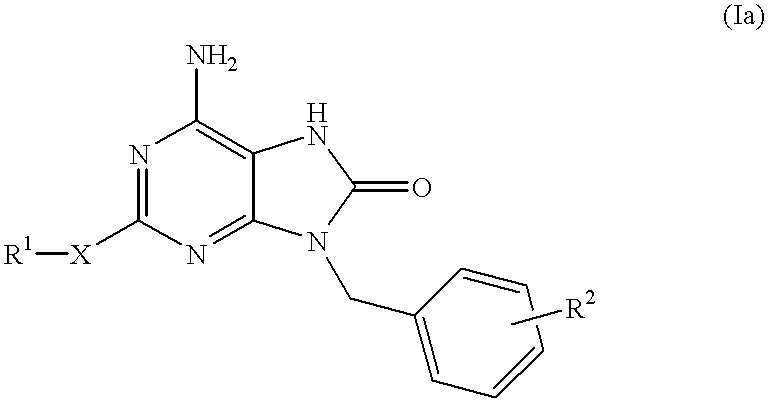
Compounds having the structure of formula I are provided. In formula I, R1 is H, OH, substituted or unsubstituted C1 to C3 alkyl, C1 to C3 perfluoroalkyl, or COR6; R6 is H, substituted or unsubstituted C1 to C4 alkyl, aryl, substituted or unsubstituted C1 to C4 alkoxy, substituted or unsubstituted C1 to C3 aminoalkyl; R2 and R3 are H, substituted or unsubstituted C1 to C6 alkyl, C1 to C6 perfluoroalkyl, substituted or unsubstituted C2 to C6 alkenyl, substituted or unsubstituted C2 to C6 alkynyl, substituted or unsubstituted C3 to C6 cycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heterocyclic; or R2 and R3 are fused to form spirocyclic rings; R4 is NHR7, OR7, NHSO2R7, or OSO2R7; Q is O, S, NR8, or CR9R10; or a pharmaceutically acceptable salt, ester, or prodrug thereof. Such compounds are useful as progesterone receptor modulators and for treating progesterone receptor related conditions.
Owner:WYETH LLC
Bioactive coating composition and methods
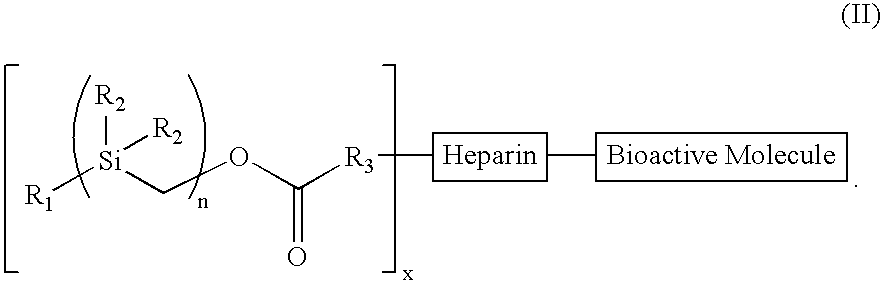
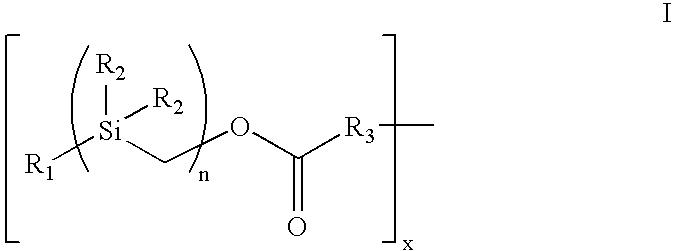
InactiveUS6921811B2Promotes desired biologicalPromotes therapeutic effectSuture equipmentsOrganic active ingredientsArylBody fluid
The present invention provides a bioactive coating composition, method and devices for bodily fluid-contacting surfaces. The coating comprises a complex of Formula II: wherein R1 is an C1-18alkyl or C6-32aryl group, each R2 is independently selected from the group consisting of C1-18alkyl and C6-32aryl, R3 is N or O, n is a number from 1 to 10, and x in a number from 1 to about 30, directly bound to a heparin-activity molecule via covalent bonding, with one or more bioactive molecules bound to the heparin-activity molecule. The bioactive molecule may be an adhesive molecule such as fibronectin, a growth factor such as basic fibroblast growth factor, or any other bioactive molecule that binds, by any mechanism, to a heparin-activity molecule
Owner:BIOSURFACE ENG TECH
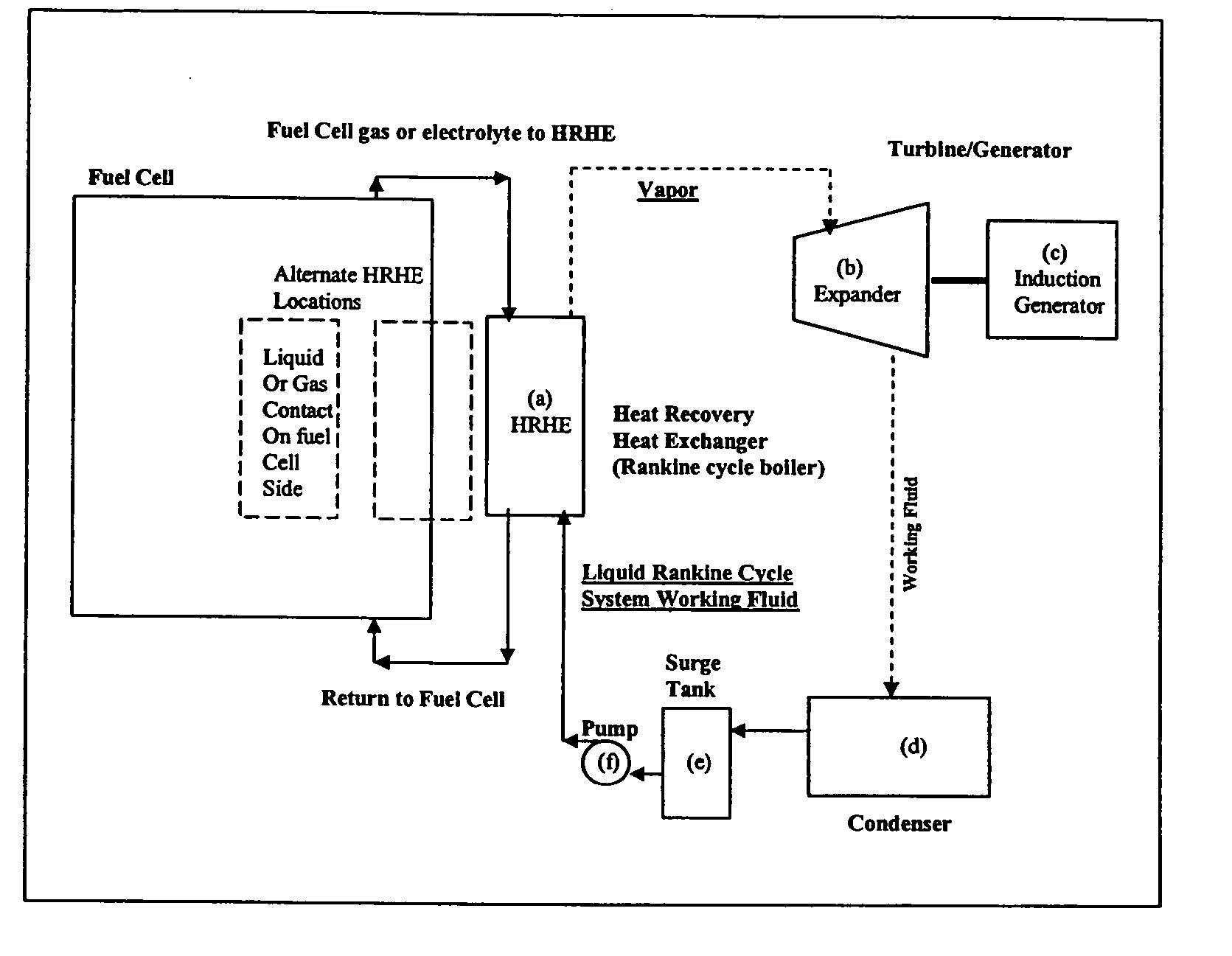
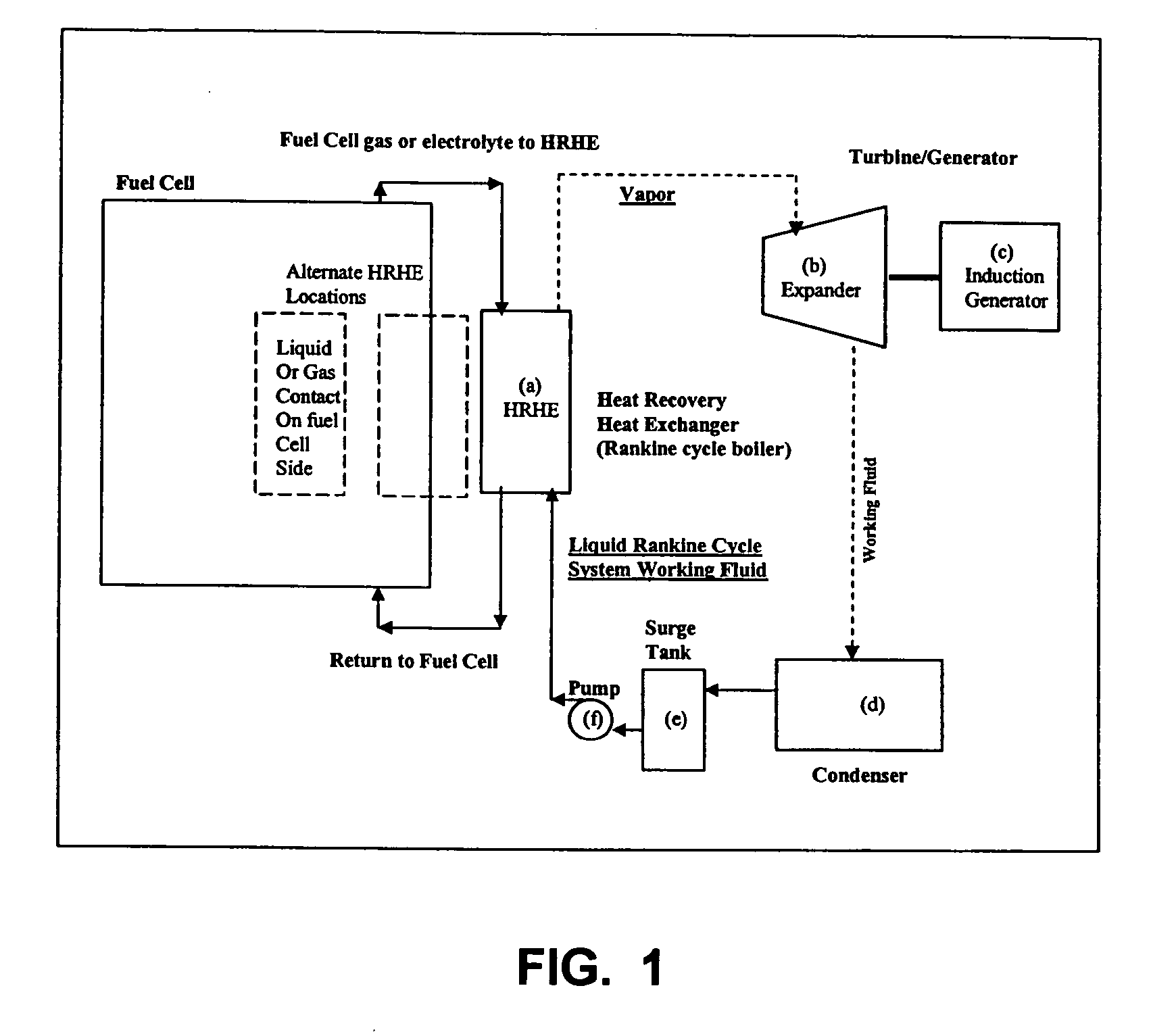
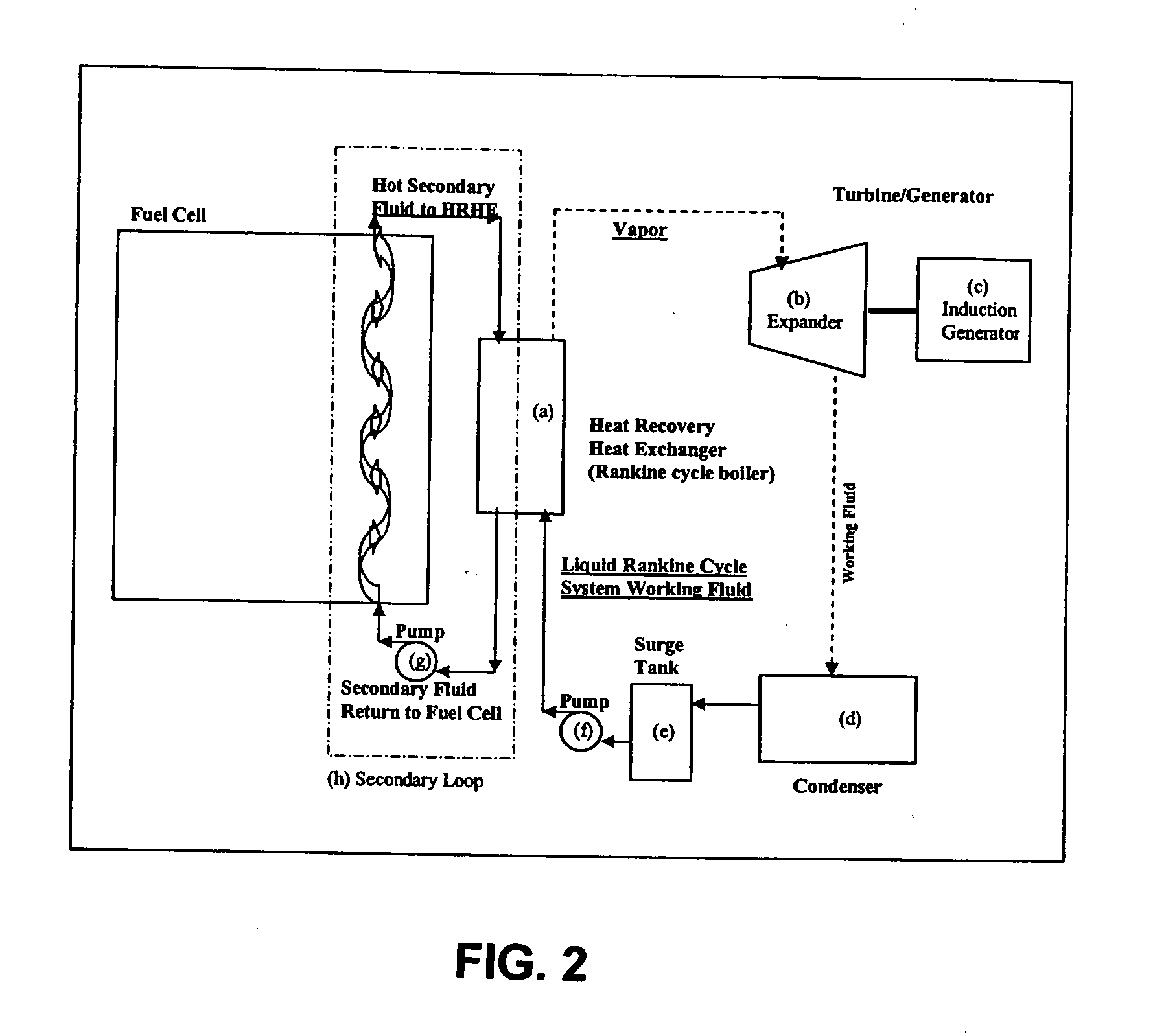
Working fluids for thermal energy conversion of waste heat from fuel cells using rankine cycle systems
A process for recovering waste heat which comprises: (a) passing a liquid phase working fluid through a heat exchanger in communication with a process which produces the waste heat; (b) removing a vapor phase working fluid from the heat exchanger; (c) passing the vapor phase working fluid to an expander, wherein the waste heat is converted into mechanical energy; and (d) passing the vapor phase working fluid from the expander to a condenser, wherein the vapor phase working fluid is condensed to the liquid phase working fluid. The preferred working fluid is an organic Rankine cycle system working fluid comprising compounds having the following general structure: where x, y, z, and m are each selected from the group consisting of: fluorine, hydrogen, Rf, and R, wherein R and Rf are each an alkyl, aryl, or alkylaryl of 1 to 6 carbon atoms, and wherein Rf is partially or fully fluorinated.
Owner:HONEYWELL INT INC
Novel 6-fused heteroaryldihydropyrimidines for the treatment and prophylaxis of hepatitis B virus infection
Owner:F HOFFMANN LA ROCHE INC
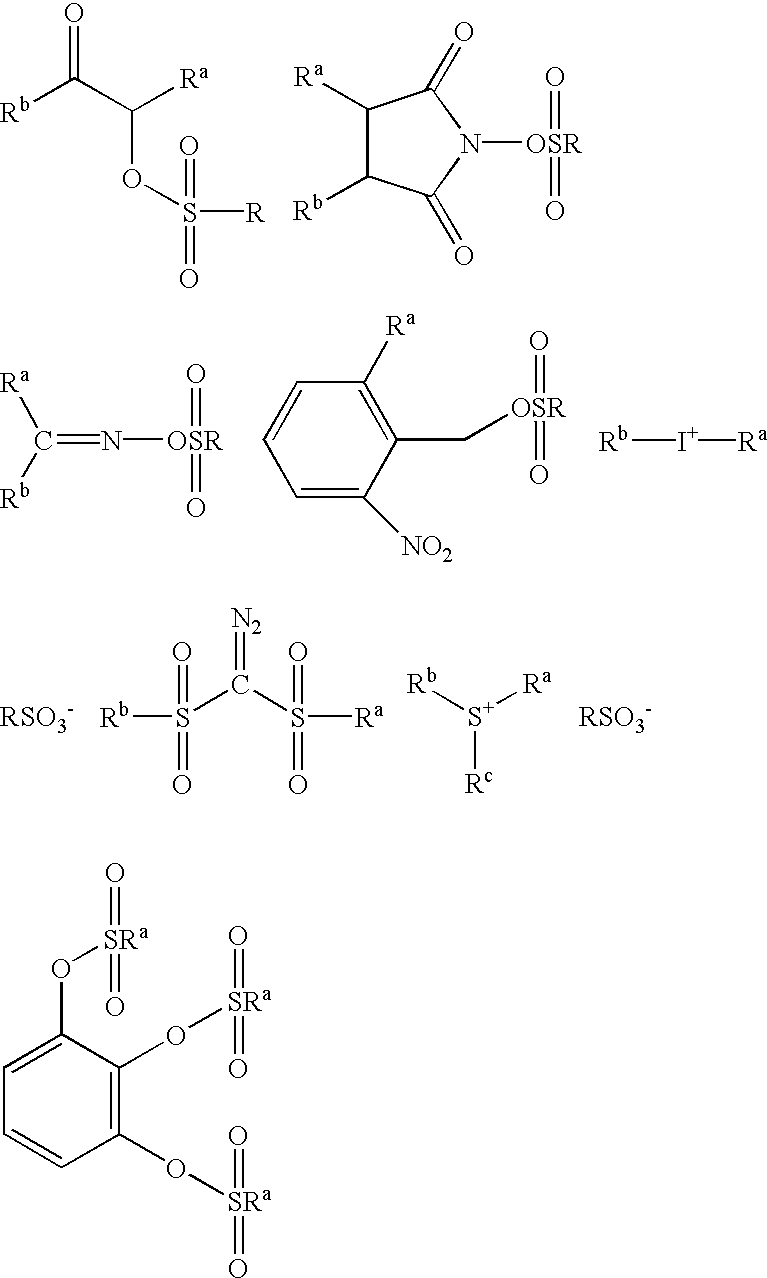
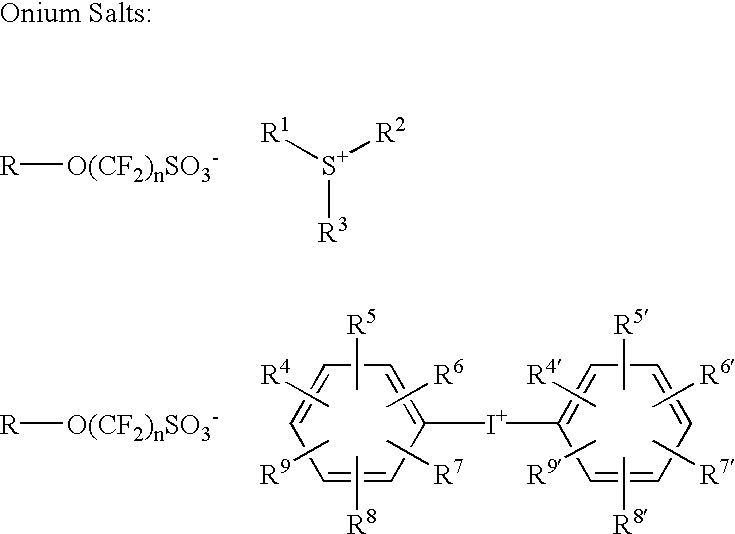
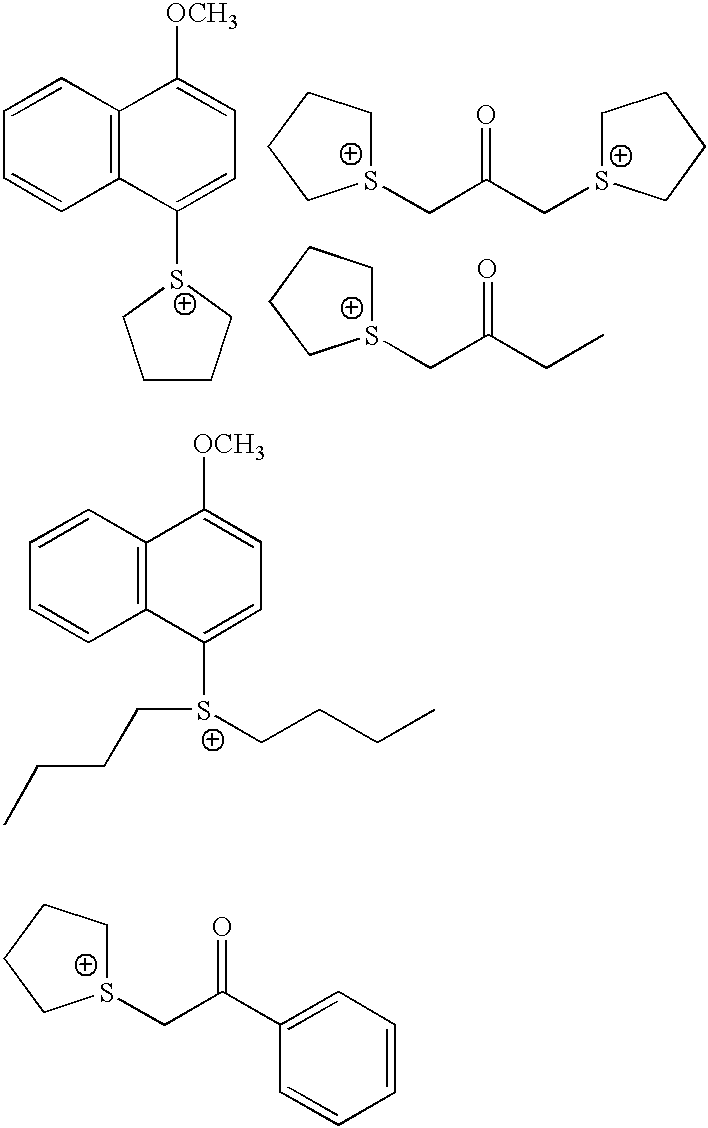
Photoacid generators for use in photoresist compositions
A photoacid compound having the following general structure:<paragraph lvl="0"><in-line-formula>R-O(CF2)nSO3X< / in-line-formula>wherein n is an integer between about 1 to 4; R is selected from the group consisting of: substituted or unsubstituted C1-C12 linear or branched alkyl or alkenyl, substituted or unsubstituted araalkyl, substituted or unsubstituted aryl, substituted or unsubstituted bicycloalkyl, substituted or unsubstituted tricycloalkyl, hydrogen, alkyl sulfonic acid, substituted or unsubstituted perfluoroalkyl, the general structure F((CF2)pO)m(CF2)q- wherein p is between about 1 to 4, m is between about 0 to 3 and q is between about 1 to 4, and substituted or unsubstituted partially fluorinated alkyl, halofluoroalkyl, perfluoroalkylsulfonic, or glycidyl; and X is selected from the group consisting of: organic cations and covalently bonded organic radicals.
Owner:FUJIFILM ELECTRONICS MATERIALS US
Polyester compound for preparing olefine polymerizing catalyst
Owner:CHINA PETROLEUM & CHEM CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
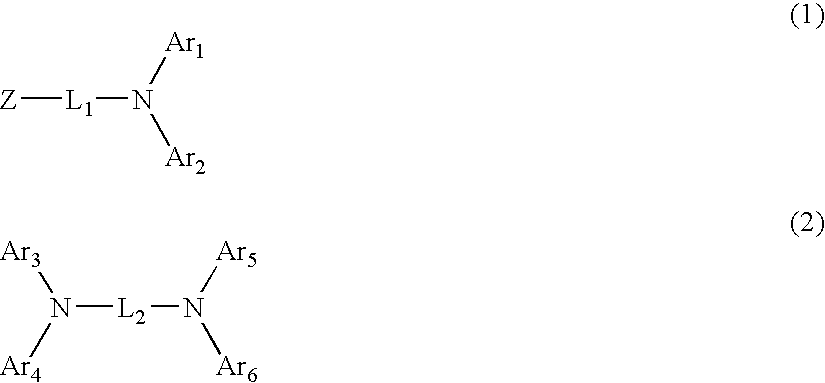








![6-amino-1,4-dihydro-benzo[d][1,3] oxazin-2-ones and analogs useful as progesterone receptor modulators 6-amino-1,4-dihydro-benzo[d][1,3] oxazin-2-ones and analogs useful as progesterone receptor modulators](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f527f94c-1c91-4445-8bcc-7ae4c7ef053d/US07247625-20070724-C00001.png)
![6-amino-1,4-dihydro-benzo[d][1,3] oxazin-2-ones and analogs useful as progesterone receptor modulators 6-amino-1,4-dihydro-benzo[d][1,3] oxazin-2-ones and analogs useful as progesterone receptor modulators](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f527f94c-1c91-4445-8bcc-7ae4c7ef053d/US07247625-20070724-C00002.png)
![6-amino-1,4-dihydro-benzo[d][1,3] oxazin-2-ones and analogs useful as progesterone receptor modulators 6-amino-1,4-dihydro-benzo[d][1,3] oxazin-2-ones and analogs useful as progesterone receptor modulators](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f527f94c-1c91-4445-8bcc-7ae4c7ef053d/US07247625-20070724-C00003.png)