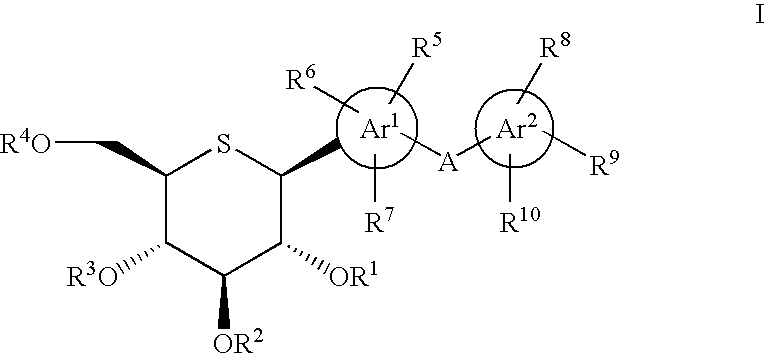
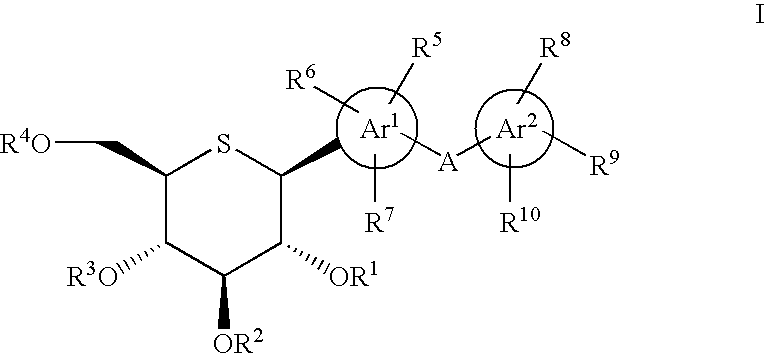
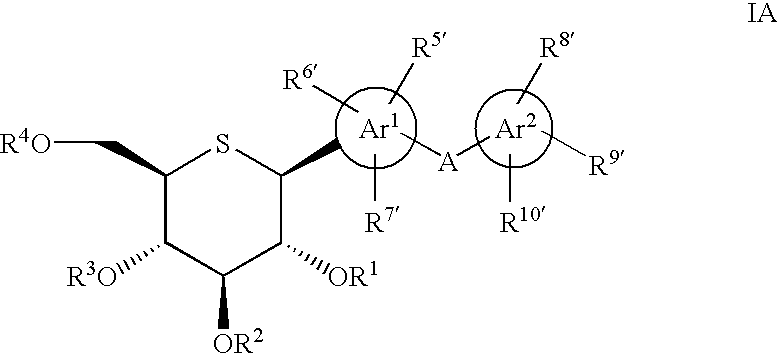
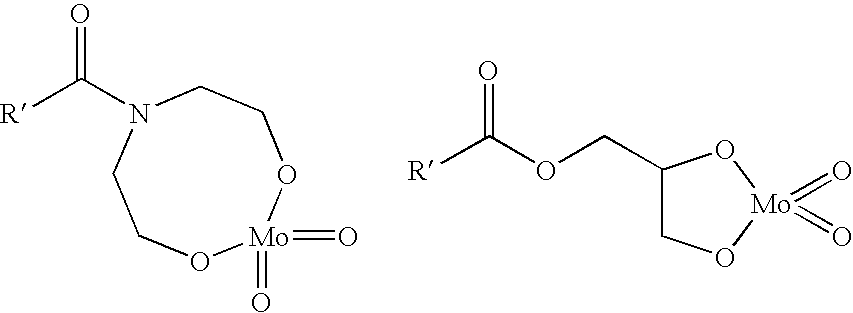
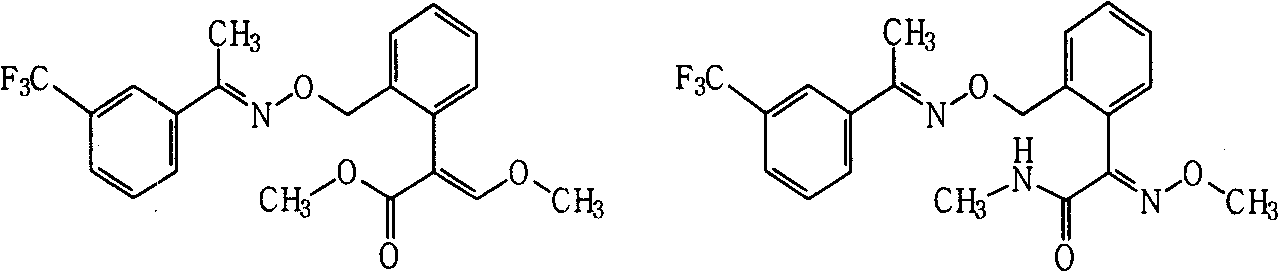
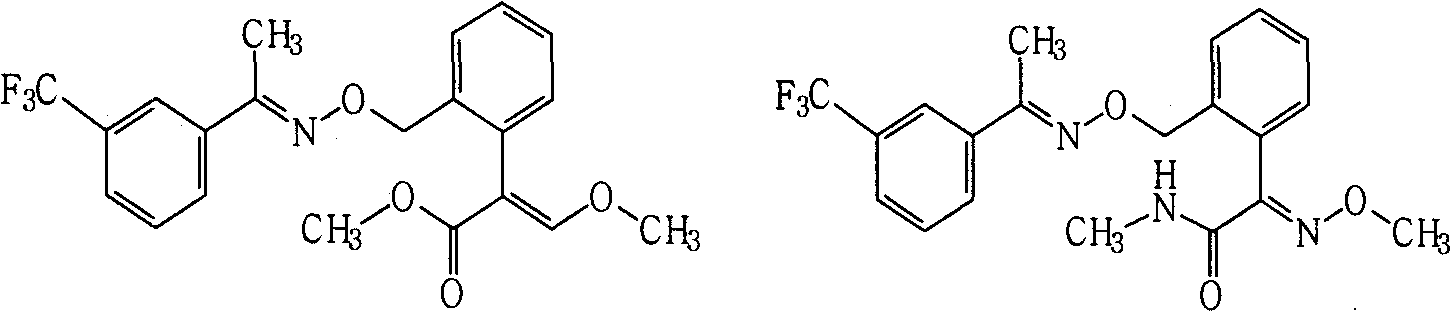
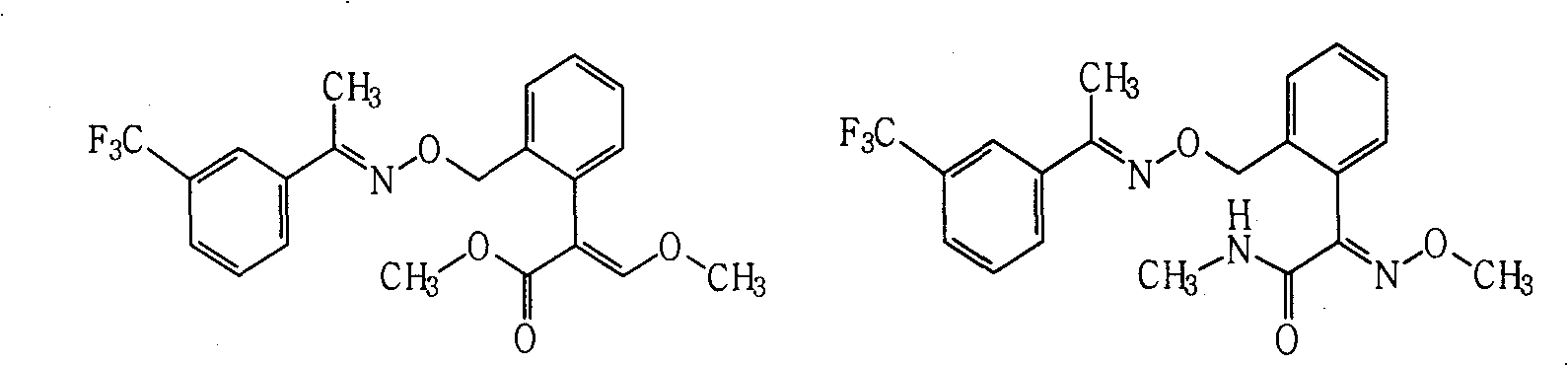
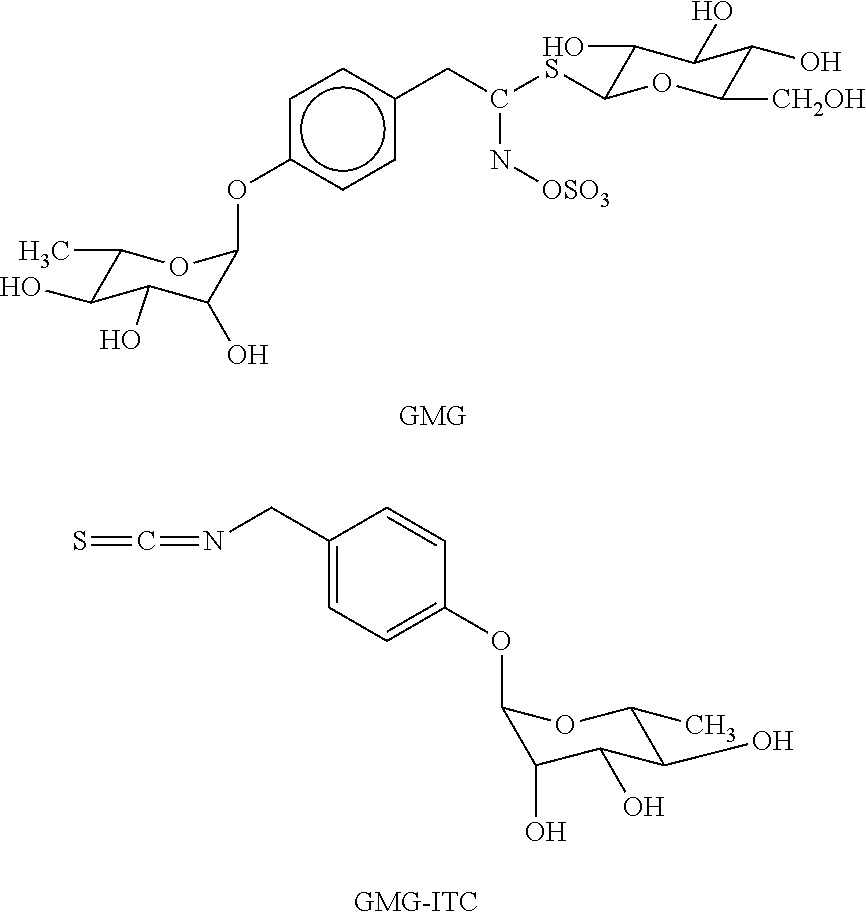
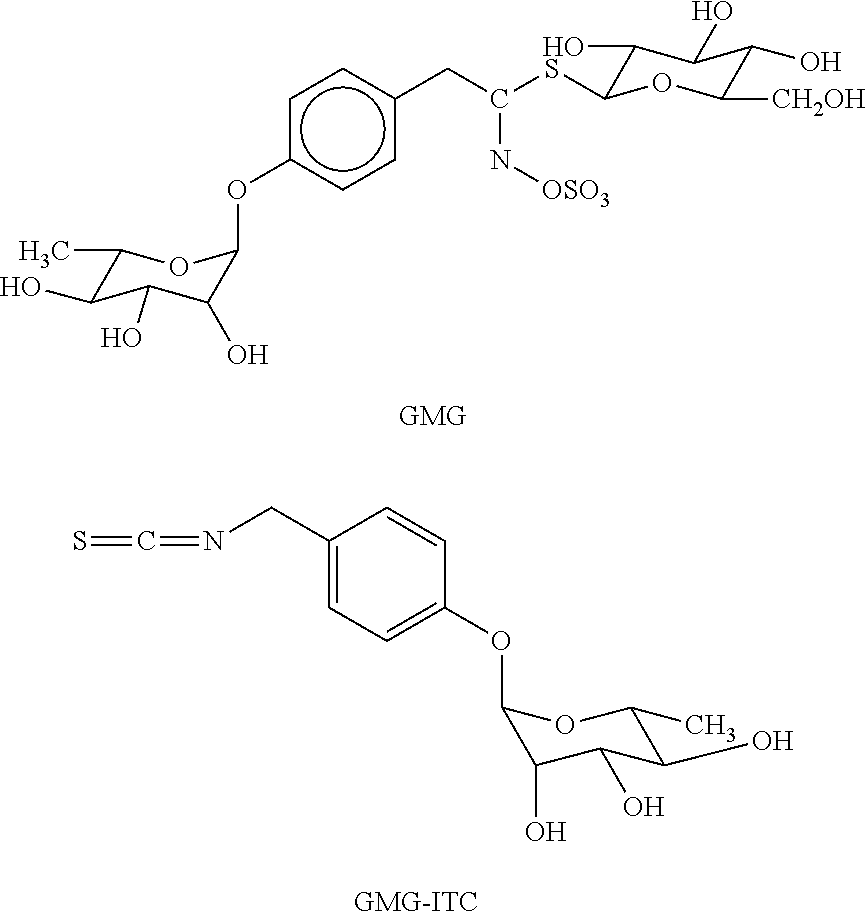
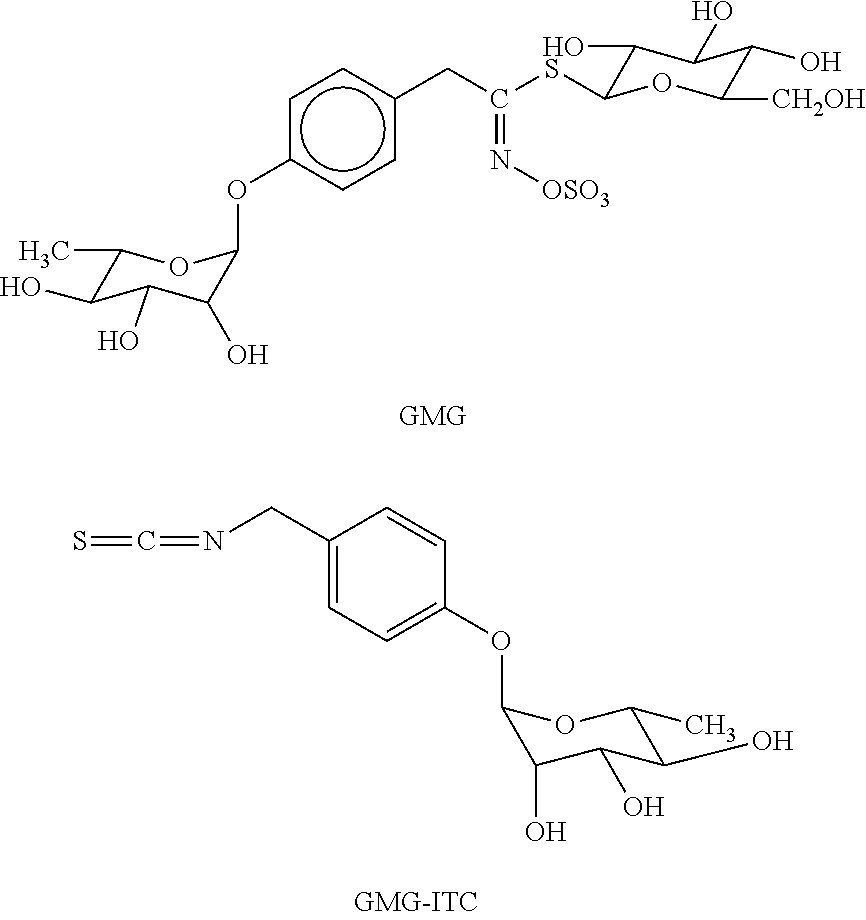
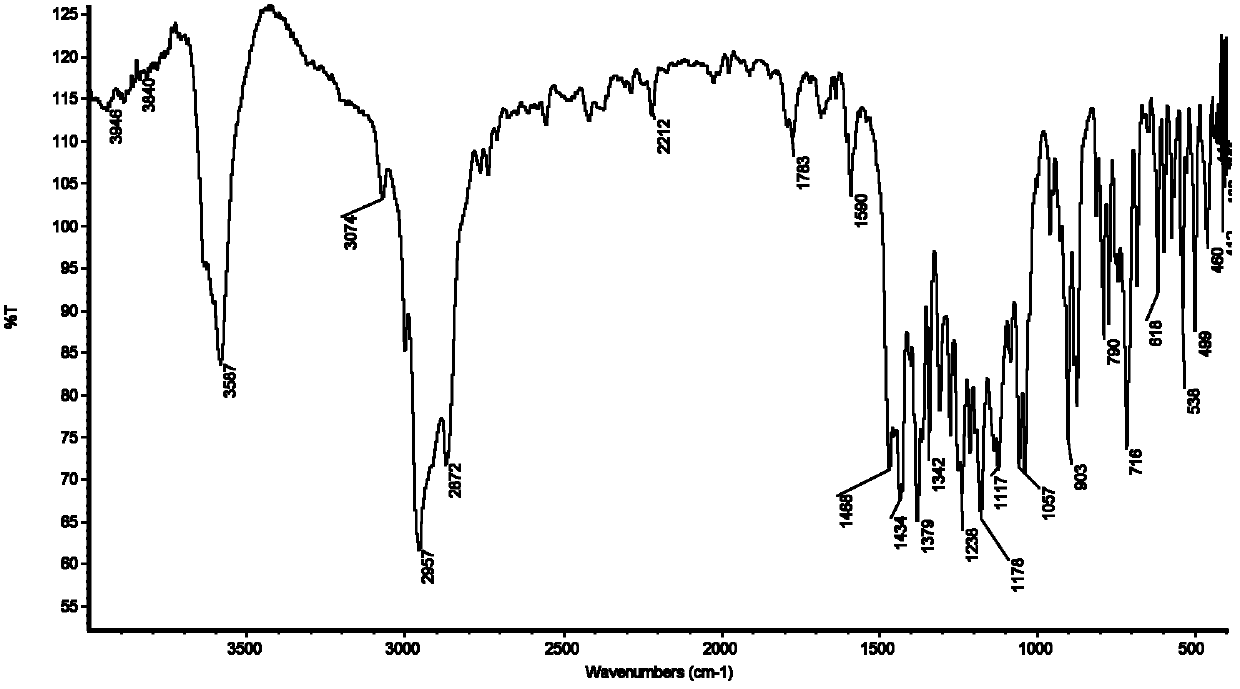
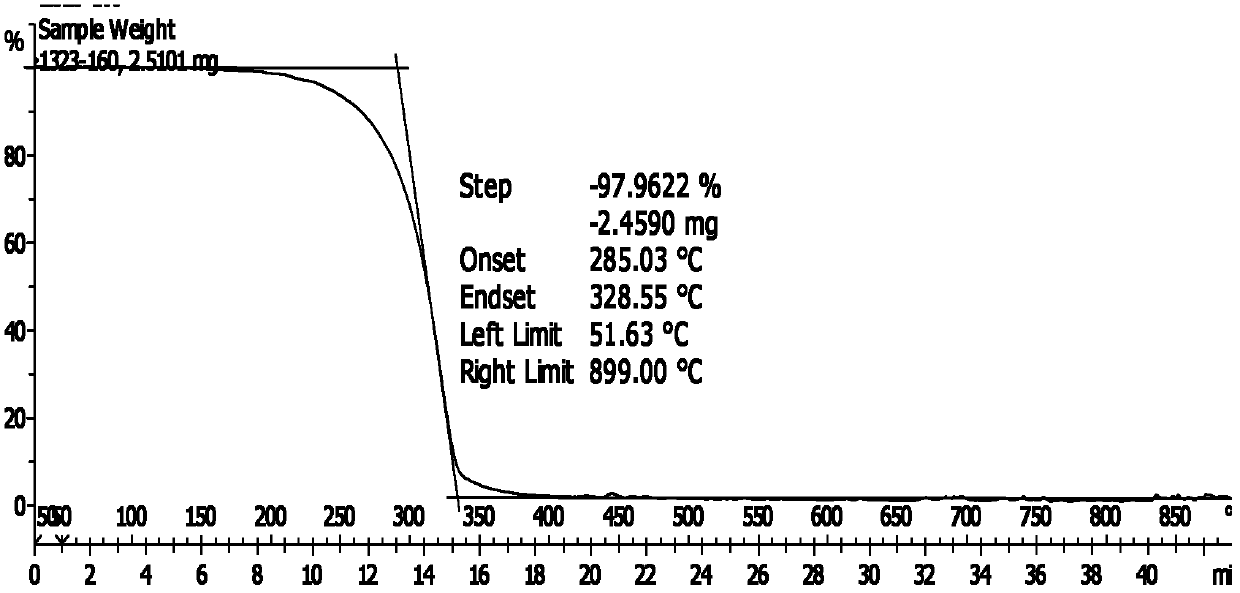
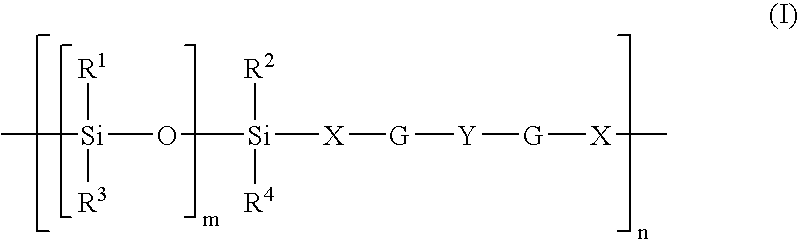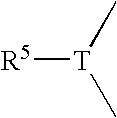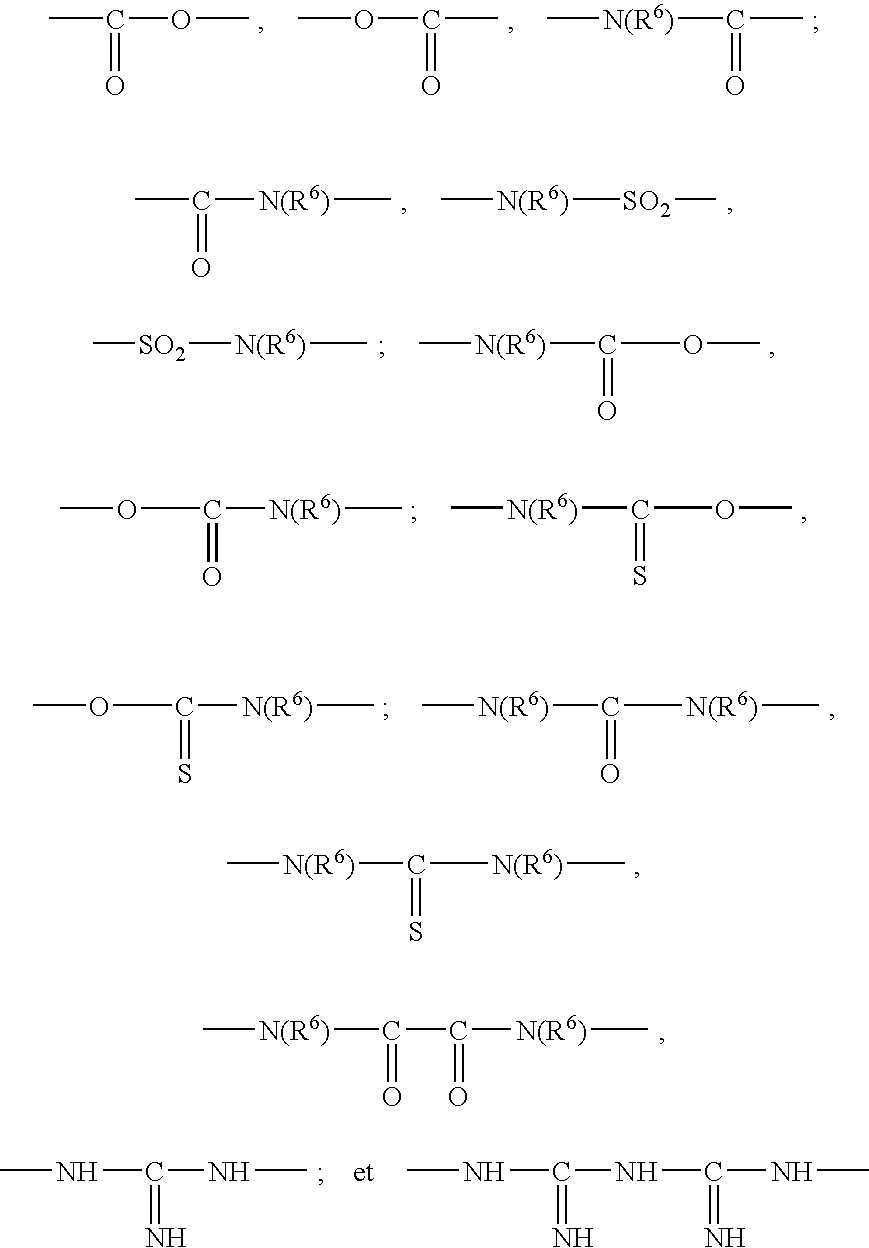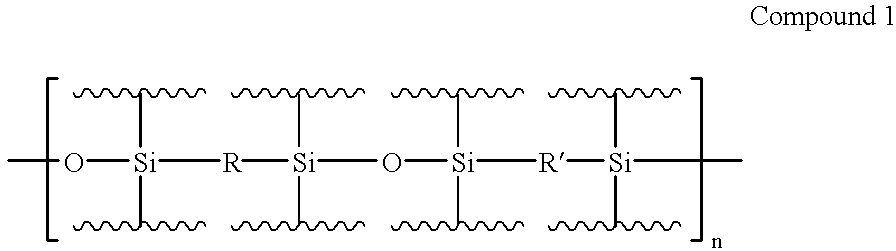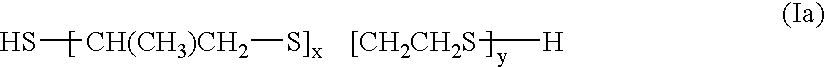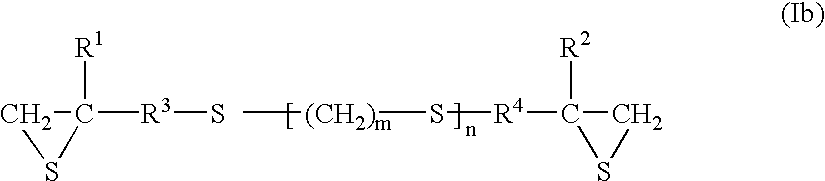Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3899 results about "Thio-" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
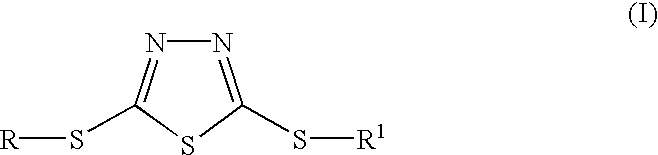
The prefix thio-, when applied to a chemical, such as an ion, means that an oxygen atom in the compound has been replaced by a sulfur atom. This term is often used in organic chemistry. For example, from the word ether, referring to an oxygen-containing compound having the general chemical structure R–O–R′, where R and R′ are organic functional groups and O is an oxygen atom, comes the word thioether, which refers to an analogous compound with the general structure R–S–R′, where S is a sulfur atom covalently bonded to two organic groups. A chemical reaction involving the replacement of oxygen to sulfur is called thionation or thiation.
Premium wear resistant lubricant
A premium synthetic lubricant having antiwear properties comprises a synthetic isoparaffinic hydrocarbon base stock and an effective amount of at least one antiwear additive. The antiwear additive is preferably at least one of a metal phosphate, a metal dialkyldithiophosphate, a metal dithiophosphate a metal thiocarbamate, a metal dithiocarbamate, an ethoxylated amine dialkyldithiophosphate and an ethoxylated amine dithiobenzoate. Metal dialkyldithiophosphates are preferred, particularly zincdialkyldithiophosphate (ZDDP). The base stock is derived from a waxy, Fischer-Tropsch synthesized hydrocarbon feed fraction comprising hydrocarbons having an initial boiling point in the range of about 650-750 DEG F., by a process which comprises hydroisomerizing the feed and dewaxing the isomerate. The lubricant may also contain hydrocarbonaceous and synthetic base stock material in admxture with the Fischer-Tropsch derived base stock.
Owner:EXXON RES & ENG CO
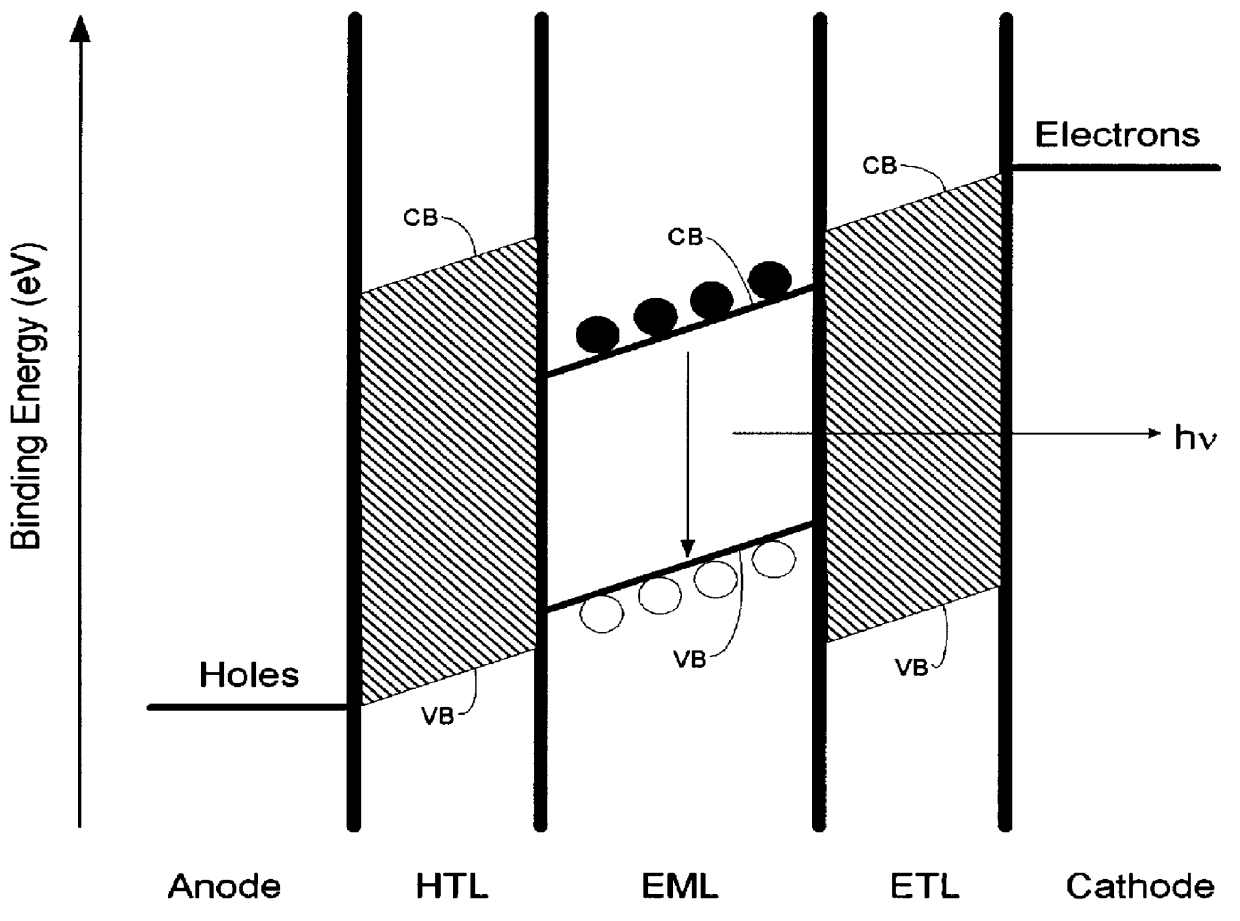
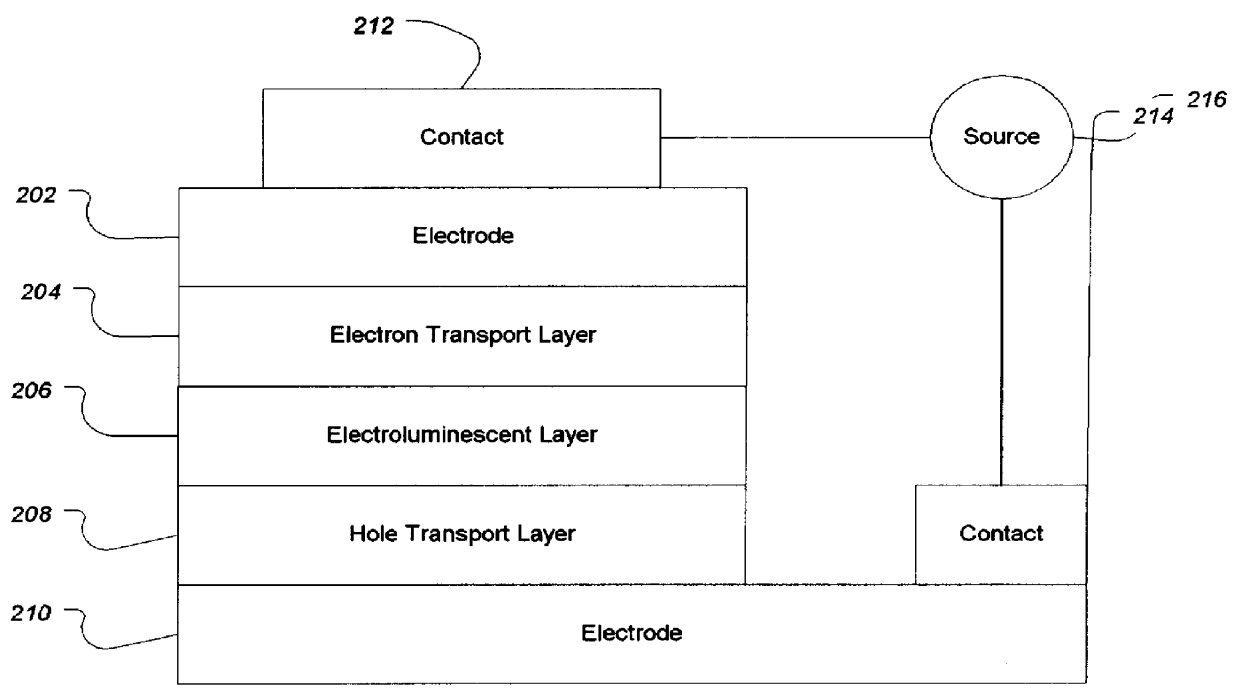
Organic electroluminescent materials and device made from such materials
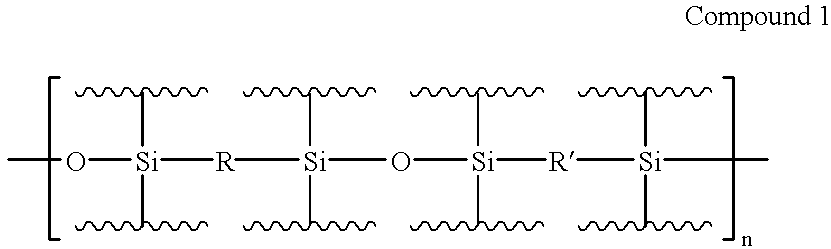
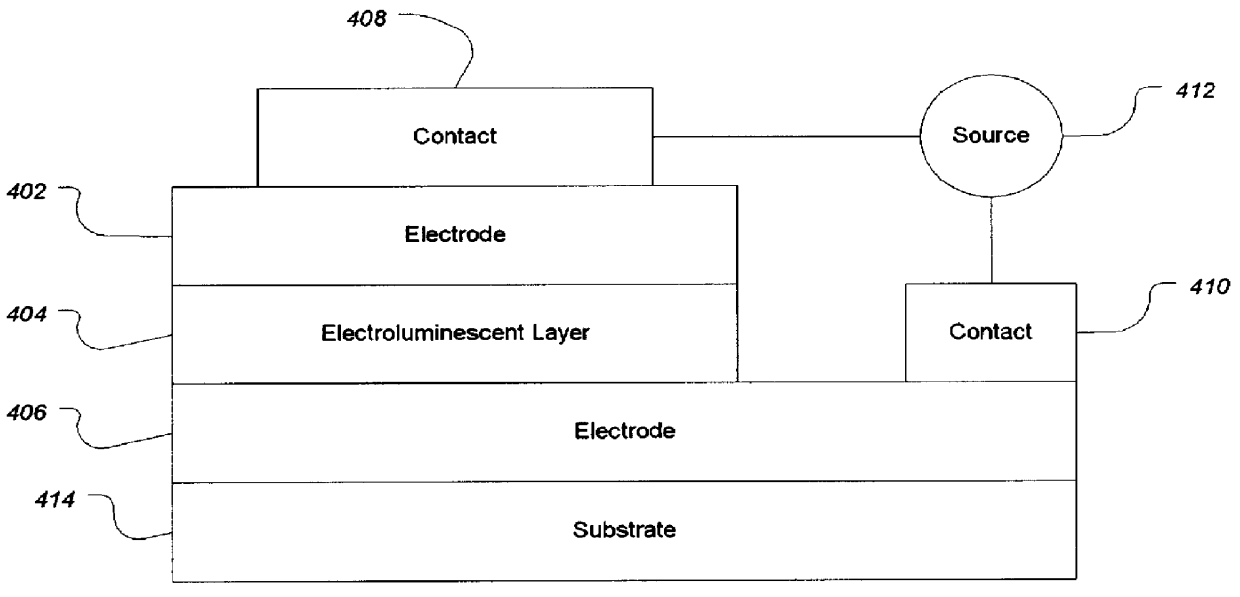
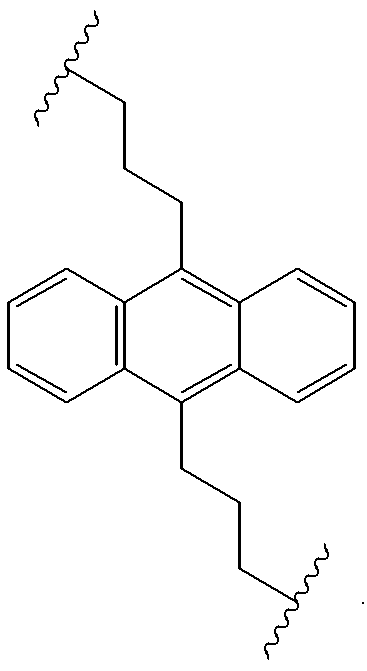
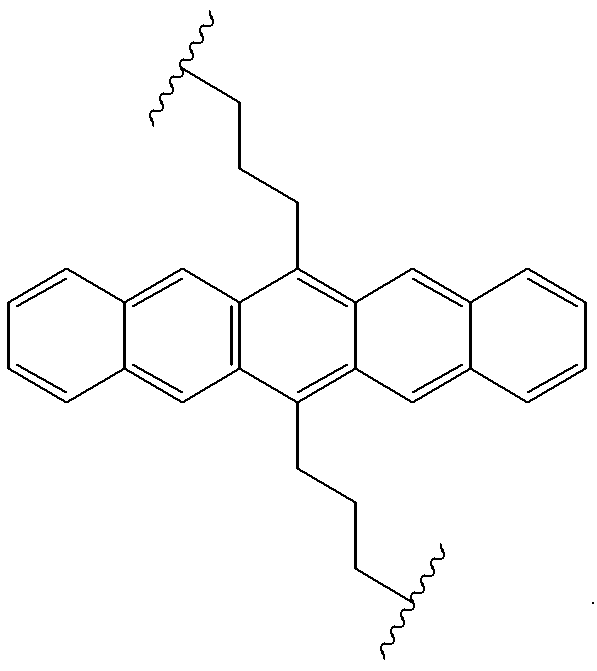
Electroluminescent devices and materials including an electroluminescent organo-siloxane polymer. The main chain of the organo-siloxane polymer comprises an organic component that can be alkenyl, alkynyl, aralkyl, aryl, heteroaralkyl, and heteroaryl which can be substituted optionally with hydrogen, alkyl, aryl, heteroalkyl, heteroaralkyl, nitro, cyano, hydroxy, alkoxy, aryloxy, thio, alkylthio, arylthio, amino, halogen, dialkylamino, diarylamino, diaralkylamino, arylamino, alkylamino, arylalkylamino, carbonyloxy, carbonylalkoxy, carbonylalkyloxy, alkylcarbonyloxy, arylcarbonyloxy, alkoxylcarbonyloxy, sulfonyl, or sulfonyloxy. The organic component includes at least two covalent bonds coupling the organic component to the main chain of the organo-siloxane polymer. Such devices provide superior performance and mechanical stability compared with conventional organic electroluminescent materials and devices made from such materials.
Owner:ORGANIC DISPLAY TECH
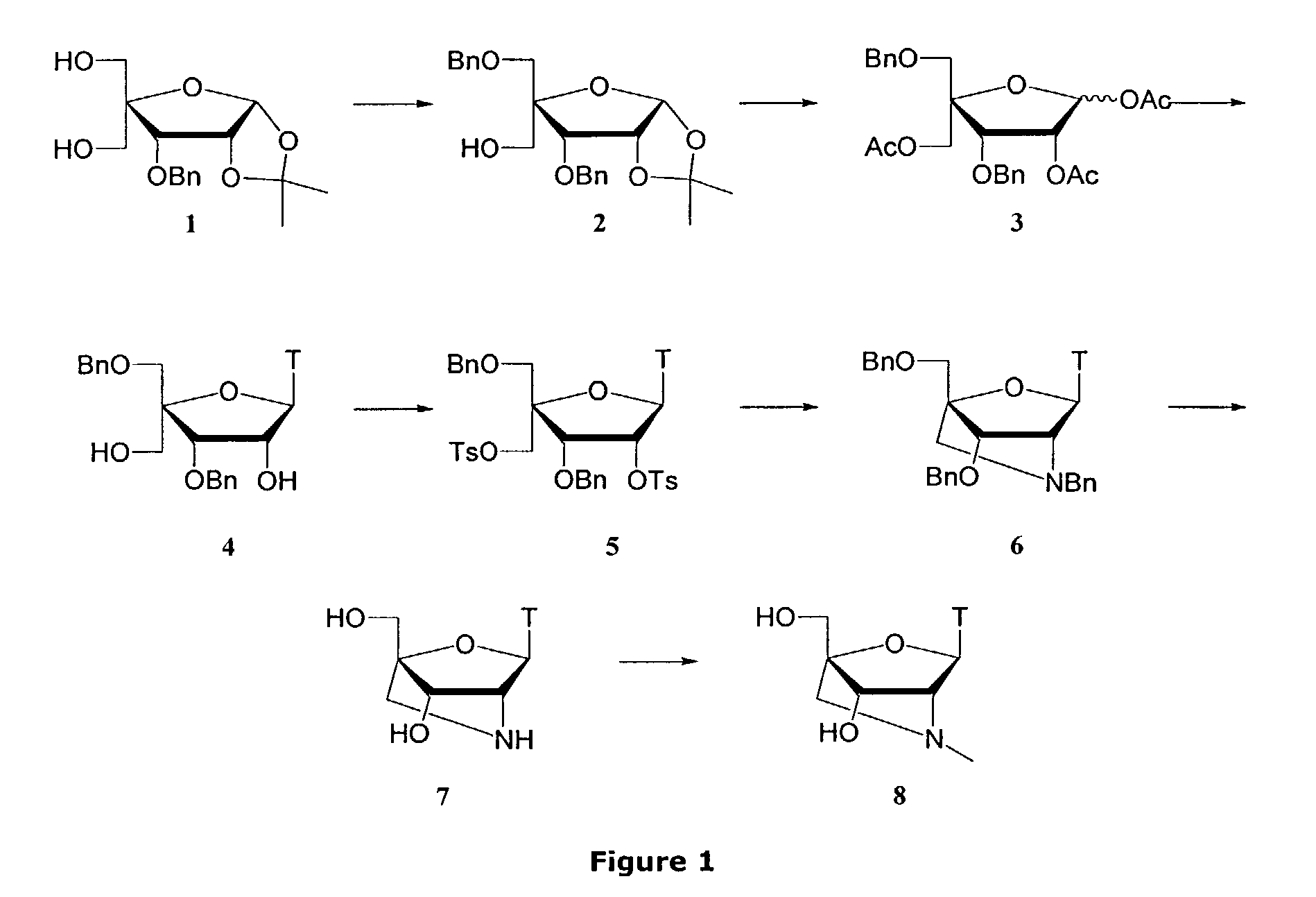
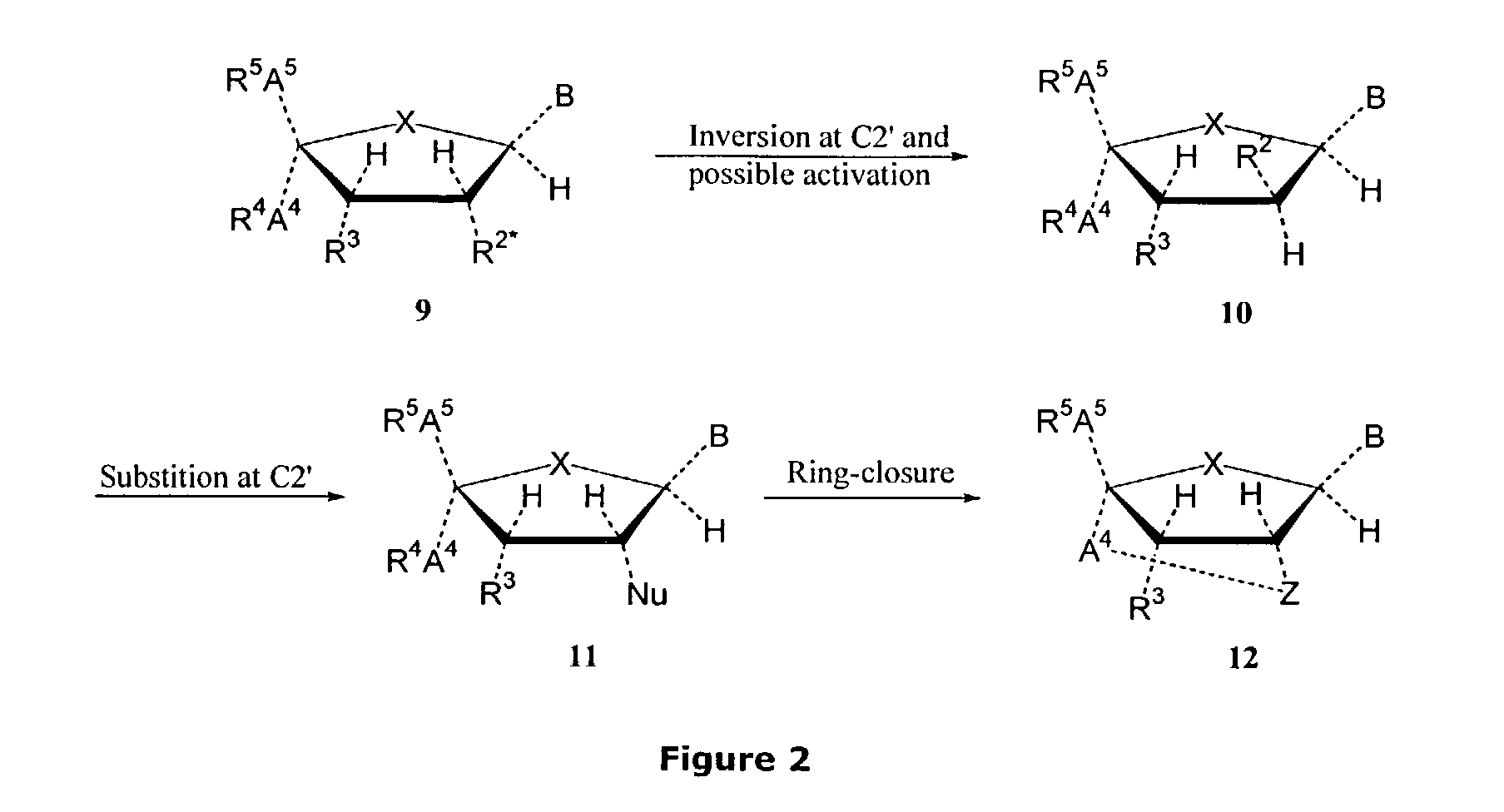
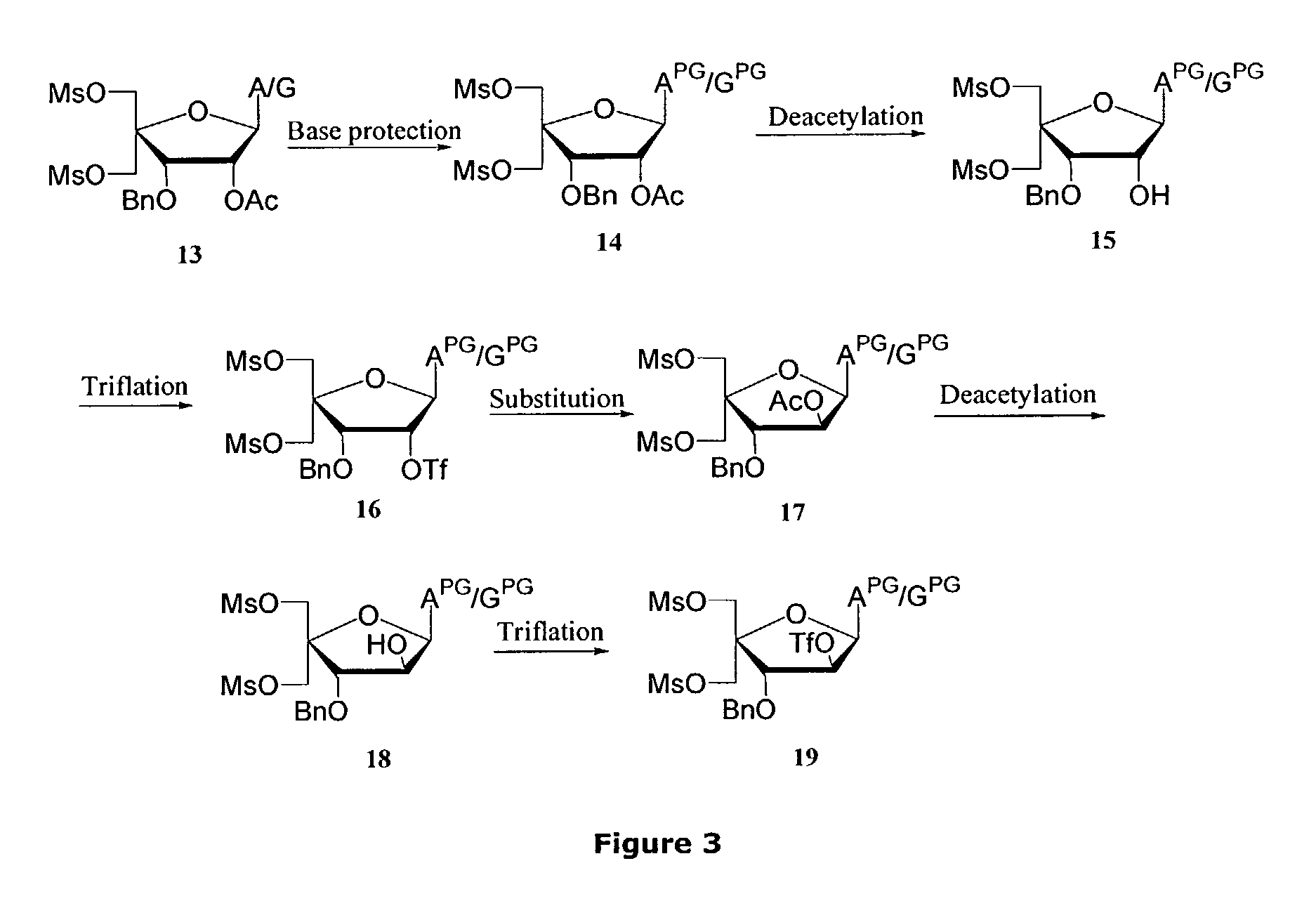
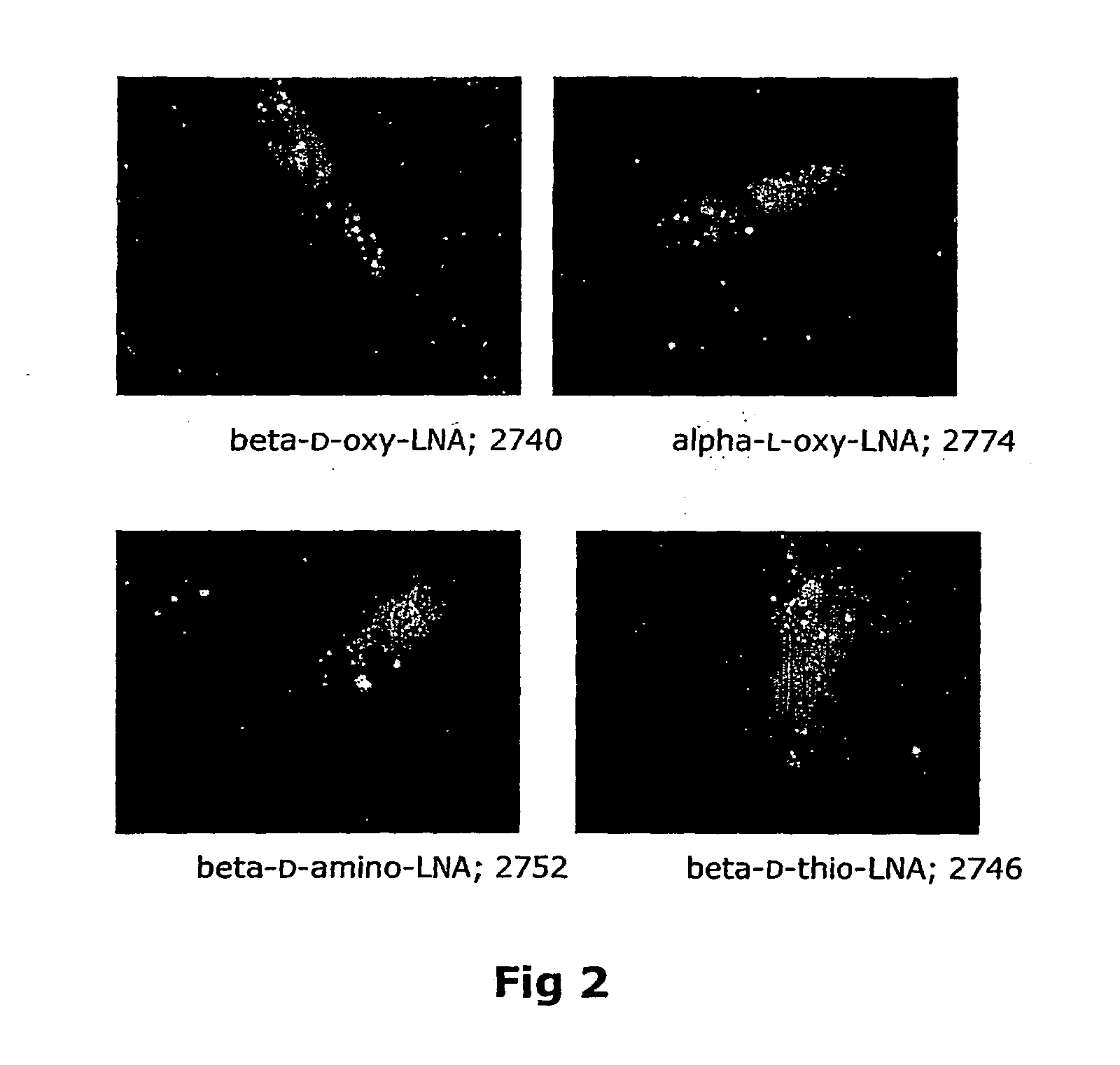
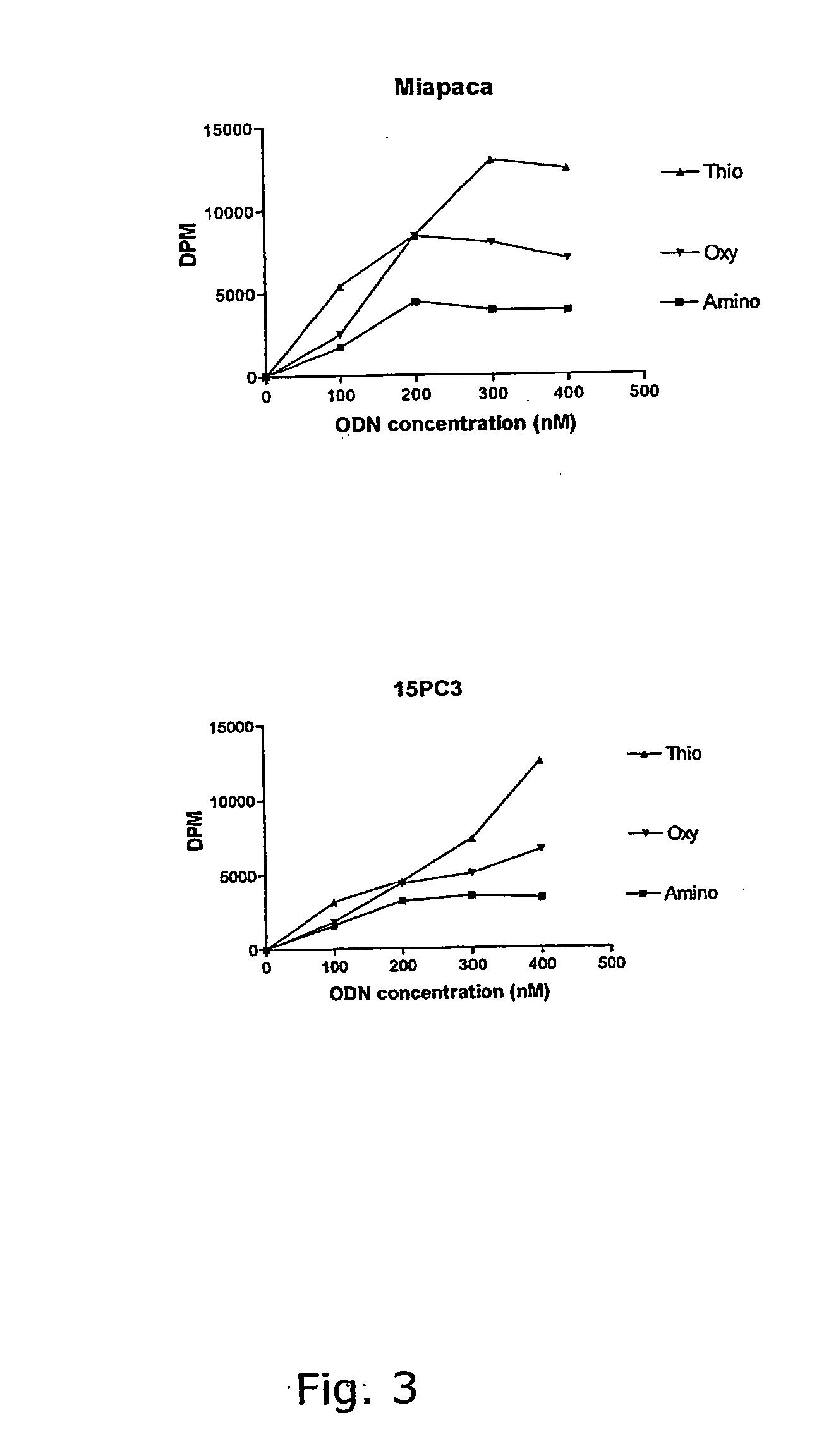
Synthesis of locked nucleic acid derivatives
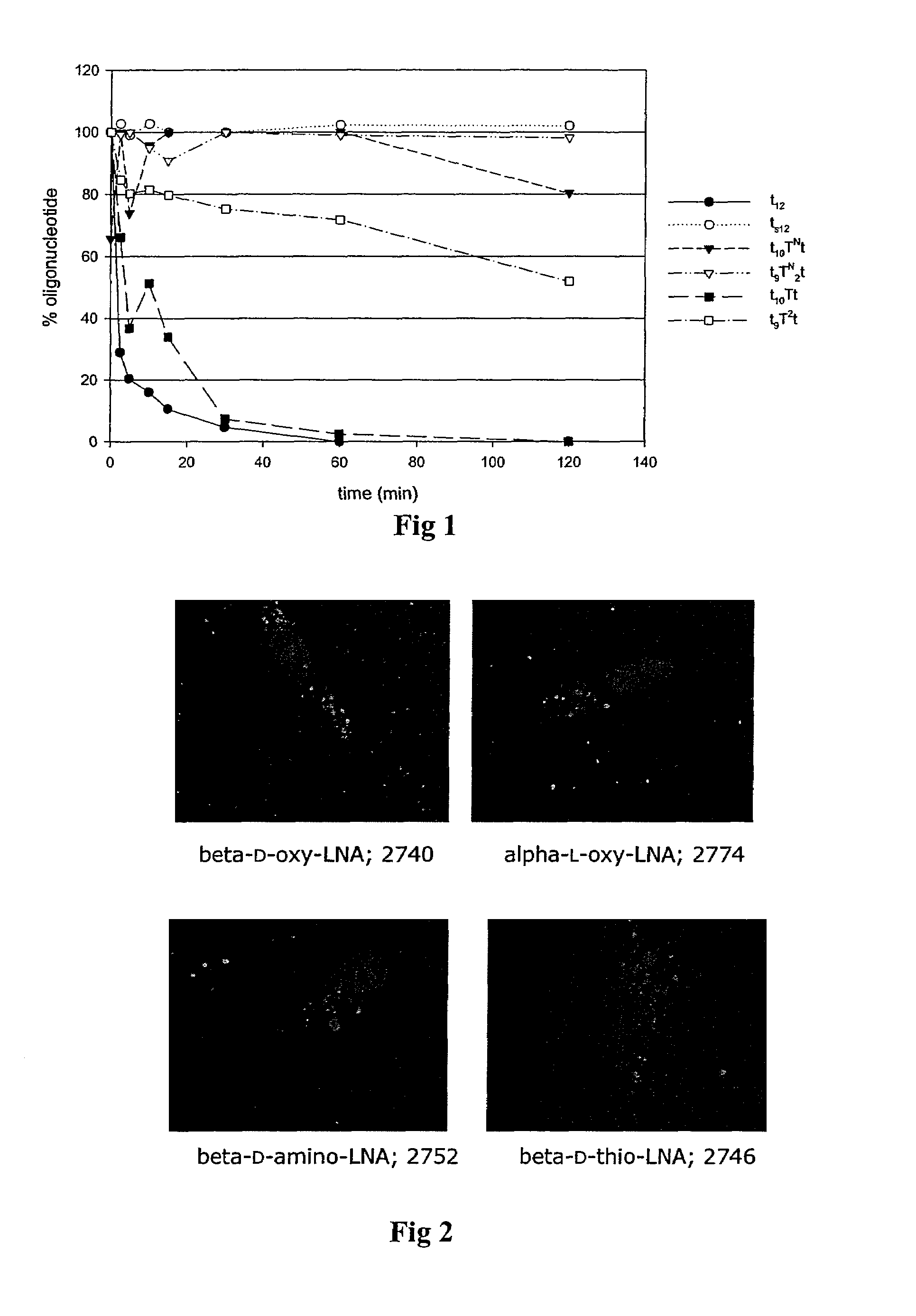
The invention relates to a novel strategy for the synthesis of Locked Nucleic Acid derivatives, such as α-L-oxy-LNA, amino-LNA, α-L-amino-LNA, thio-LNA, α-L-thio-LNA, seleno-LNA and methylene LNA, which provides scalable high yielding reactions utilizing intermediates that also can produce other LNA analogues such as oxy-LNA. Also, the compounds of the formula X are important intermediates that may be reacted with varieties of nucleophiles leading to a wide variety of LNA analogues.
Owner:SANTARIS PHARMA AS
Useful precursors for organic electroluminescent materials and devices made from such materials
InactiveUS6165383ADesirable efficiencyDesirable weightSilicon organic compoundsLayered productsArylAnthracene
Precursor materials useful for making organic electroluminescent devices such as anthracene derivatives having the structure: R40-R43 and R44-R47 are selected independently from the group consisting of hydrogen, alkyl, aryl, heteroalkyl, heteroaralkyl, nitro, cyano, hydroxy, alkoxy, aryloxy, thio, alkylthio, arylthio, amino, halogen, dialkylamino, diarylamino, diaralkylamino, arylamino, alkylamino, arylalkylamino, carbonyloxy, carbonylalkoxy, carbonylalkyloxy, alkylcarbonyloxy, arylcarbonyloxy, alkoxylcarbonyloxy, sulfonyl, sulfonyloxy. R48 and R49 are selected independently from the group consisting of trialkoxysilyl, dialkoxysilyl, trichlorosilyl, dichlorosilyl, heptachlorotrisiloxy, and pentachlorodisiloxy.
Owner:ORGANIC DISPLAY TECH
Oligonucleotides with alternating segments of locked and non-locked nucleotides
ActiveUS7687617B2Increased Design PossibilitiesEfficient substrateSugar derivativesActivity regulationNucleotideThio-
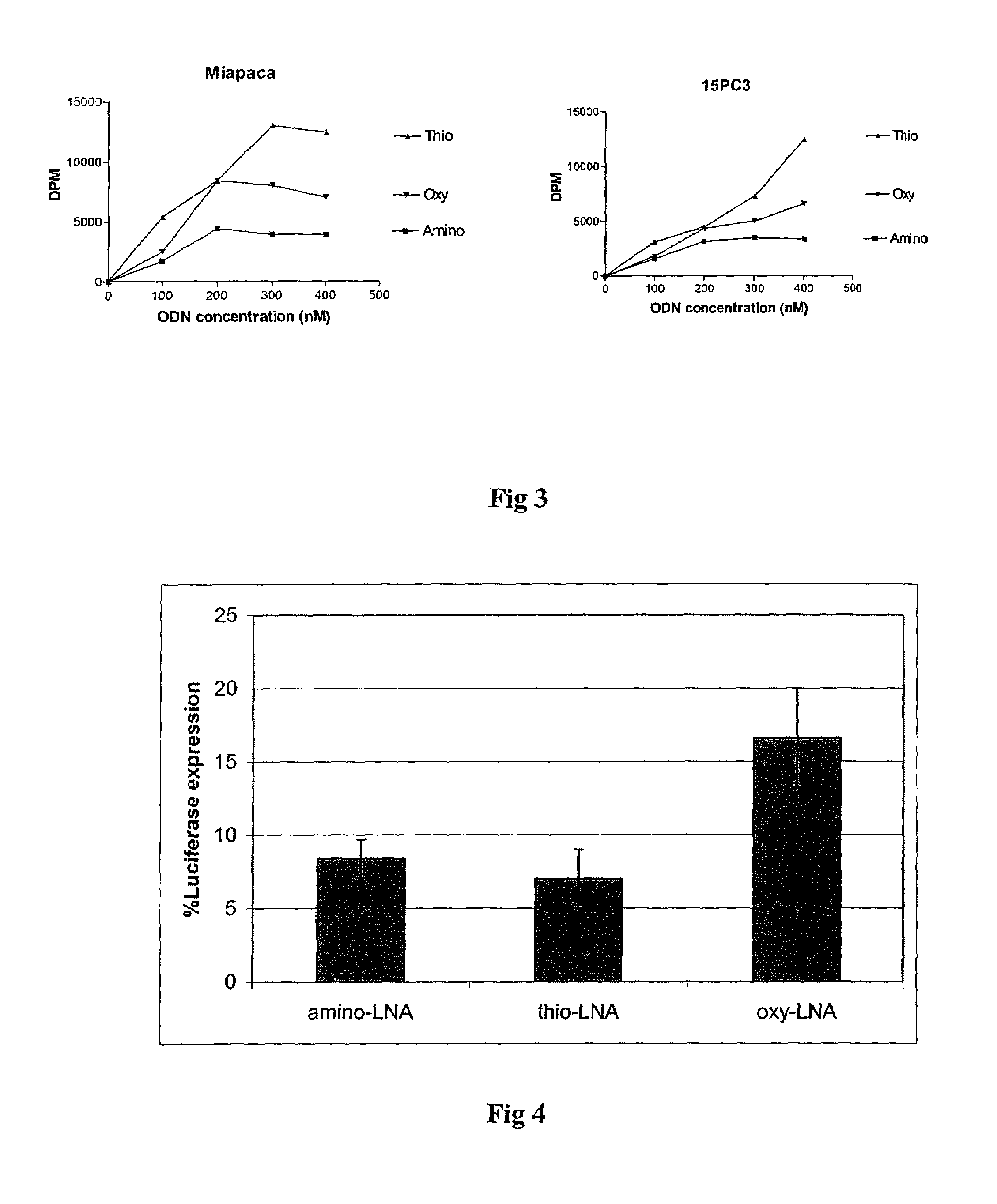
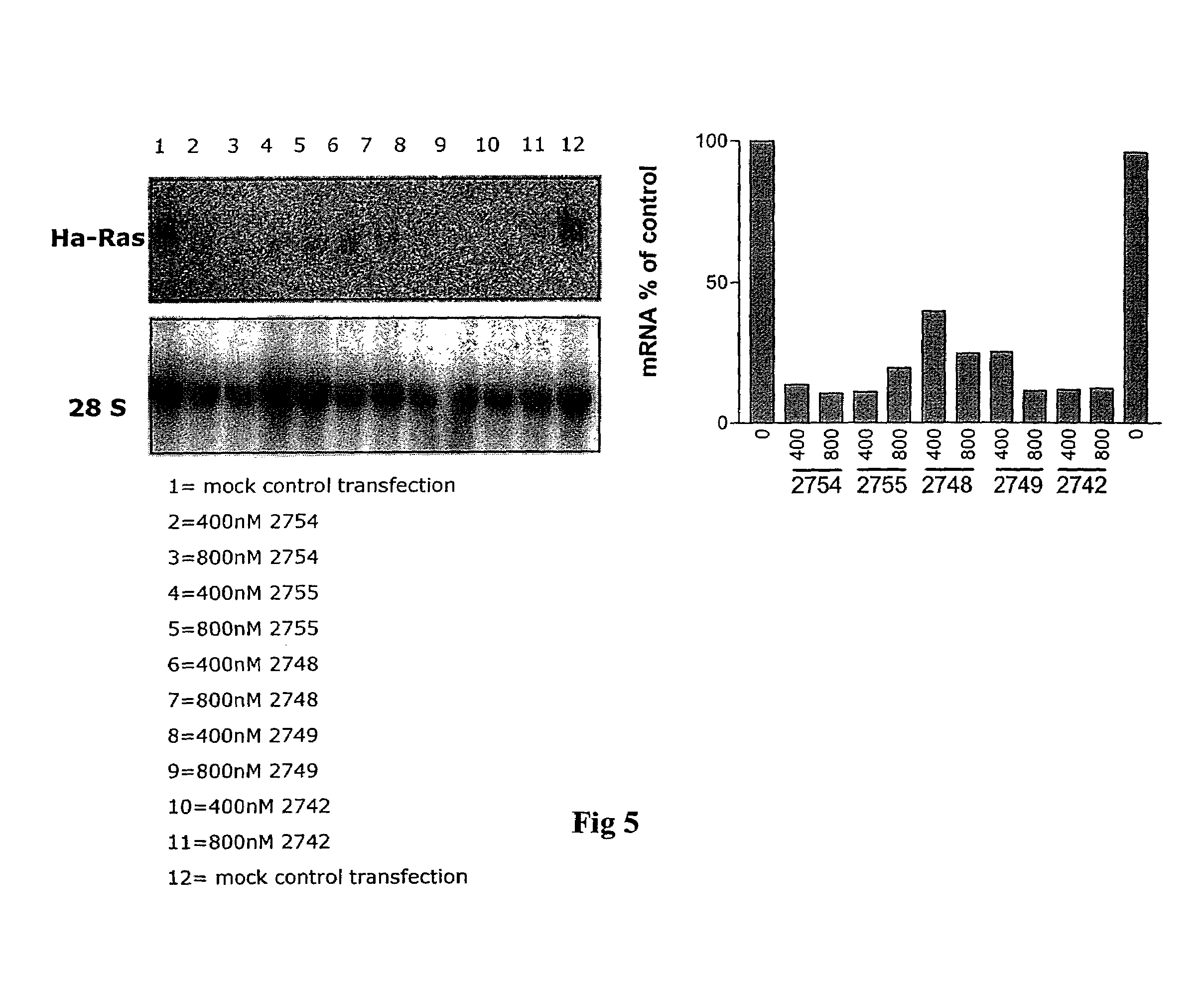
The present invention is directed to novel oligonucleotides with improved antisense properties. The novel oligonucleotides comprise at least one Locked Nucleic Acid (LNA) selected from beta-D-thio / amino-LNA or alpha-L-oxy / thio / amino-LNA. The oligonucleotides comprising LNA may also include DNA and / or RNA nucleotides. The present invention also provides a new class of pharmaceuticals which comprise antisense oligonucleotides and are useful in antisense therapy.
Owner:SANTARIS PHARMA AS
Microbial production of nuclease resistant DNA, RNA, and oligo mixtures
Owner:FRAYNE CONSULTANTS
Pharmaceutical co-crystal compositions
InactiveUS20070026078A1Improve solubilityLow hygroscopicityBiocidePowder deliveryThioketoneHydroxamic acid
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphonic acid, phosphinic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, sp2 amine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, s-heterocyclic ring, thiophene, n-heterocyclic ring, pyrrole, o-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES +2
Antisense design
ActiveUS20060128646A1Efficient deliveryAiding diffusionOrganic active ingredientsPeptide/protein ingredientsThio-Locked nucleic acid
A novel class of pharmaceuticals which comprises a Locked Nucleic Acid (LNA) which can be used in antisense therapy. These novel oligonucleotides have improved antisense properties. The novel oligonucleotides are composed of at least one LNA selected from beta-D-thio / amino-LNA or alpha-L-oxy / thio / amino-LNA. The oligonucleotides comprising LNA may also include DNA and / or RNA nucleotides.
Owner:ROCHE INNOVATION CENT COPENHAGEN
Cosmetic composition for care and/or treatment and/or makeup of the emulsion type structured with silicone polymers
InactiveUS20030235548A1Outstanding propertyIncrease flexibilityCosmetic preparationsHair cosmeticsThiocarbamatePolymer science
Cosmetic emulsion for care and / or makeup, comprising an aqueous phase and a liquid fatty phase dispersed one within the other, said liquid fatty phase comprising at least one silicone oil and being structured with at least one gelling polymer (homopolymer or copolymer) with a weight-average molecular mass ranging from 500 to 500,000, containing at least one moiety comprising: at least one polyorganosiloxane group, composed of 1 to 1000 organosiloxane units in the chain of the moiety or in the form of a graft, and at least two groups capable of establishing hydrogen interactions chosen from among the ester, amide, sulfonamide, carbamate, thiocarbamate, urea, thiourea, oxamido, guanidino, biguanidino groups, and combinations thereof, on condition that at least one of the groups is other than an ester group, the polymer being solid at room temperature and soluble in the liquid fatty phase at a temperature of 25 to 250° C., the aqueous phase, the liquid fatty phase and the gelling polymer forming a physiologically acceptable medium.
Owner:LOREAL SA
1-Thio-D-Glucitol Derivatives
The present invention provides a 1-thio-D-glucitol compound of the following formula, which shows the action of inhibiting the activity of SGLT2, a pharmaceutically acceptable salt of the compound, or a hydrate of the compound or the salt; and a pharmaceutical comprising such a compound as an active ingredient, especially, a pharmaceutical for preventing or treating diabetes, diabetes-related disease, or diabetic complication. The invention also provides a method for producing the 1-thio-D-glucitol compound and its intermediate.
Owner:TAISHO PHARMACEUTICAL CO LTD
Antioxidant additive compositions and lubricating compositions containing the same
InactiveUS6806241B2Low levelImprove corrosion inhibition and friction propertyOrganic chemistryLiquid carbonaceous fuelsAntioxidantDithiocarbamate
Disclosed are antioxidant compositions comprising of: (1) an organomolybdenum compound; (2) an alkylated diphenylamine; and (3) a sulfur compound selected from the group consisting of: (a) thiadiazole; (b) dithiocarbamate; and (c) mixtures of (a) and (b).
Owner:VANDERBILT CHEM LLC
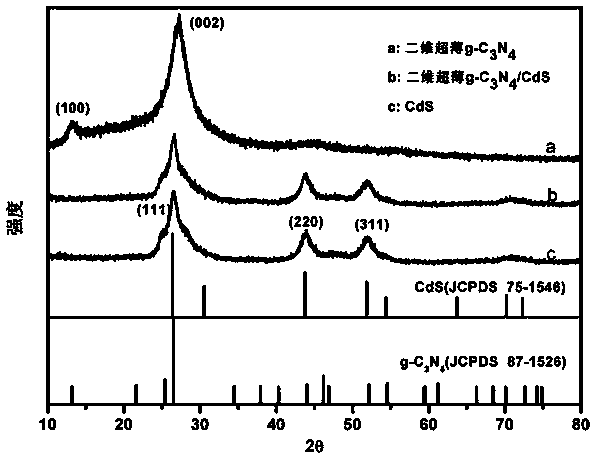
g-C3N4 nanosheet/CdS composite visible-light-driven photocatalyst
InactiveCN103785434AImprove separation efficiencyImprove photocatalytic efficiencyPhysical/chemical process catalystsWater/sewage treatment by irradiationPtru catalystThio-
The invention discloses a g-C3N4 nanosheet / CdS composite visible-light-driven photocatalyst and a preparing method and application thereof and belongs to the technical field of material preparation and photocatalysis. The catalyst is directly obtained with the solvothermal method by taking two-dimensional ultra-thin g-C3N4 nanosheets as the matrix, cadmium acetate dihydrate and thioacetamide as raw materials, and ethyl alcohol as the solvent. Compared with general block g-C3N4 / CdS, the two-dimensional ultra-thin g-C3N4 nanosheet / CdS composite photocatalyst prepared with the method has the advantages that the two substances are in tighter contact, the specific surface area is larger, photon-generated electron-hole can be better separated, and photocatalytic efficiency is higher. Under the shining of sunlight, the composite photocatalyst enables catalytic degradation of organic pollutants such as methyl orange in water to be achieved well. The preparing method is easy, raw materials are easy to obtain, visible light catalysis efficiency is high, and the application prospect is wide in the photocatalysis field.
Owner:FUZHOU UNIV
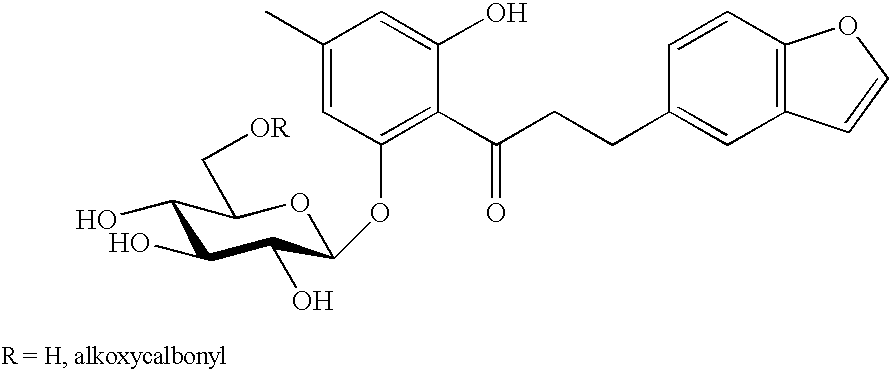
Aryl 5-thio-beta-d-glucopyranoside derivatives and remedies for diabetes containing the same
InactiveUS20050209309A1Accelerate excretion of urinaryIncrease excretionBiocideSenses disorderArylThio-
There is provided a 5-thio-β-D-glucopyranoside compound of the following formula, which has an inhibitory effect on SGLT2 activity, or a pharmaceutically acceptable salt thereof or a hydrate thereof. There is also provided a pharmaceutical preparation, particularly a prophylactic or therapeutic agent for diabetes, diabetes-related diseases or diabetic complications, which comprises such a compound as an active ingredient.
Owner:TAISHO PHARMACEUTICAL CO LTD
Heterocyclic compound and antitumor agent containing the same as active ingredient
The present invention relates to heterocyclic compounds represented by the formula I or pharmaceutically acceptable salts thereof and antitumor agents containing the heterocyclic compounds as effective components:wherein X represents nitrogen atom or CH; R1 represents CHnF3-n (wherein n is 1 or 2), hydroxy C1–C6 alkyl, NHR6 [wherein R6 represents hydrogen atom or COR (wherein R represents hydrogen atom, C1–C6 alkyl or C1–C6 alkoxy)]; R2 represents morpholino (which may be substituted with one to four C1–C6 alkyl), thiomorpholino, piperidino, pyrrolidinyl (which may be substituted with hydroxy C1–C6 alkyl), oxazolidinyl (which may be substituted with one or two C1–C6 alkyl) or tetrahydro-1,4-thiazin-1-oxo-4-yl; R3 and R4 each represent hydrogen atom or C1–C6 alkyl; and R5 represents hydrogen atom, amino or hydroxyl.
Owner:OHARA PHARMA
Pharmaceutical co-crystal compositions of drugs such as carbamazepine, celecoxib, olanzapine, itraconazole, topiramate, modafinil, 5-fluorouracil, hydrochlorothiazide, acetaminophen, aspirin, flurbiprofen, phenytoin and ibuprofen
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphinic acid, phosphonic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, imine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, S-heterocyclic ring, thiophene, N-heterocyclic ring, pyrrole, 0-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:UNIV OF SOUTH FLORIDA +3
Functionalized polymers and methods for their manufacture
A method for preparing a functionalized polymer, the method comprising the steps of (i) polymerizing monomer with a coordination catalyst to form a reactive polymer; and (ii) reacting the reactive polymer with a carboxylic or thiocarboxylic ester containing a silylated amino group.
Owner:BRIDGESTONE CORP
Ultraviolet light filter element
InactiveUS6872766B2Increased durabilityAvoid lightLiquid crystal compositionsOther chemical processesThio-Display device
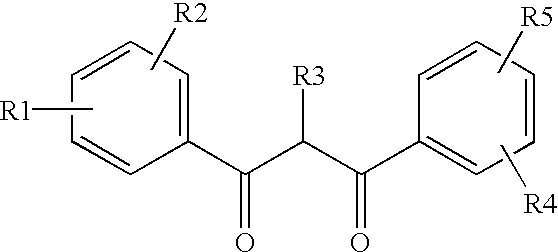
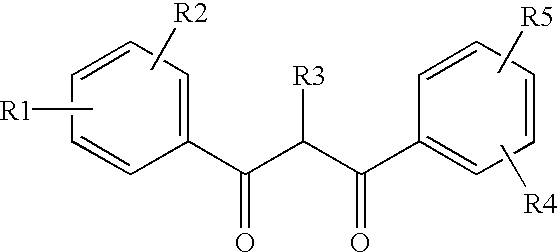
Ultraviolet light absorbing polymer film, coating, or molded article UV filter elements are described which comprise a polymer phase having molecularly dispersed therein a) a first ultraviolet absorbing dibenzoylmethane compound of formula (I) where R1 through R5 are each independently hydrogen, halogen, nitro, or hydroyxl, or further substituted or unsubstituted alkyl, alkenyl, aryl, alkoxy, acyloxy, ester, carboxyl, alkyl thio, aryl thio, alkyl amine, aryl amine, alkyl nitrile, aryl nitrile, arylsulfonyl, or 5-6 member heterocylce ring groups, and b) a second ultraviolet light absorbing compound which absorbs ultraviolet light at a wavelength for which the first compound is deficient at absorbing. In particular embodiments, the second ultraviolet light absorbing compound may comprise a hydroxyphenyl-s-triazine, hydroxyphenylbenzotriazole, formamidine, benzoxazinone, or benzophenone compound. In a specific embodiment of the invention, the above UV absorbing compounds are employed in cellulose acetate film for the fabrication of a protective film for polarizers for use in display applications.
Owner:EASTMAN KODAK CO
Process for synthesizing thiol terminated polymers
The present invention is directed to a process for the cleavage of one or more starting polymers having thiocarbonylthio groups of the formula —S—(C═S)— into derived polymers having thiol end groups of the formula —SH. The process includes contacting the aforedescribed starting polymers, with a reagent having the formula Xa Yb, wherein Xa is a nucleophilic group and Yb is an extracting group that results in the starting polymer and a byproduct, which is then separated from the derived polymer by conventional separation processes. The derived polymer is free from any odor or color that is sometimes associated with the starting polymer and it can be used in making optical lenses, such as contact lenses.
Owner:COMMONWEALTH SCI & IND RES ORG
Organic electroluminescent materials and devices made from such materials
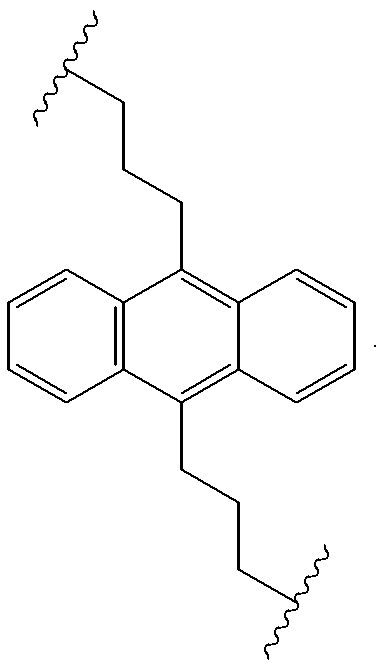
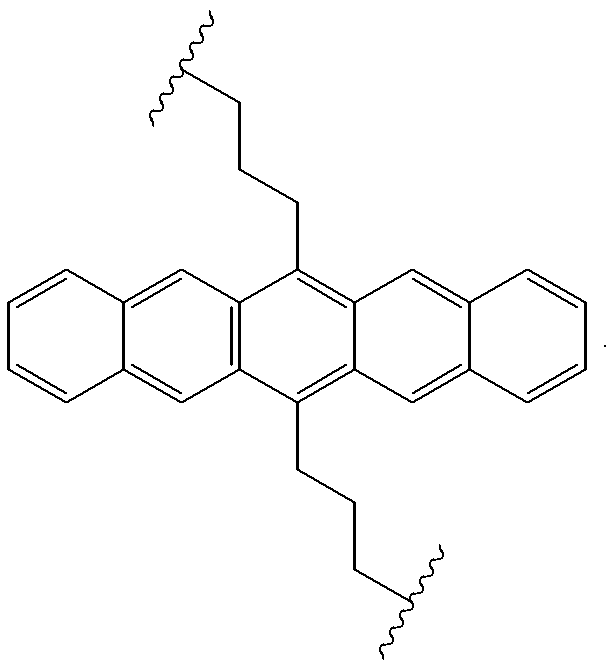
Organic electroluminescent materials having desirable efficiency, weight, and durability properties, as well as devices made from such materials. In one aspect, an organic electroluminescent material is electroluminescently conductively coupled directly with an anode and cathode such that the organic electroluminescent material emits light upon the application of a voltage across the anode and cathode. The organic electroluminescent material includes an organo-siloxane polymer having a main chain comprising anthracene and / or pentacene. The anthracene or pentacene can be substituted optionally with hydrogen, alkyl, aryl, heteroalkyl, heteroaralkyl, nitro, cyano, hydroxy, alkoxy, aryloxy, thio, alkylthio, arylthio, amino, halogen, dialkylamino, diarylamino, diaralkylamino, arylamino, alkylamino, arylalkylamino, carbonyloxy, carbonylalkoxy, carbonylalkyloxy, alkylcarbonyloxy, arylcarbonyloxy, alkoxylcarbonyloxy, sulfonyl, sulfonyloxy, alkyl bonded to the adjacent silicon atom of the organo-siloxane polymer, aryl bonded to the adjacent silicon atom of the organo-siloxane polymer, or the adjacent silicon atom of said organo-siloxane polymer.
Owner:ORGANIC DISPLAY TECH
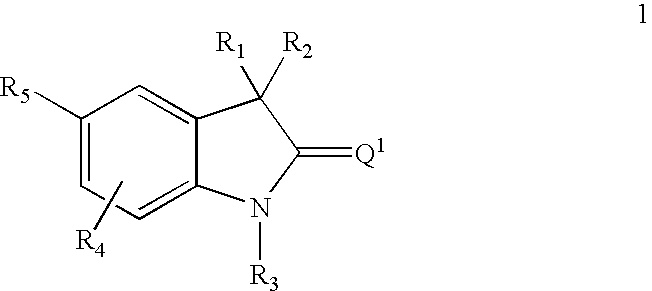
Thio-oxindole derivatives
This invention relates to methods of co-administering compounds of formula 1 which are agonists of the progesterone receptor which have the general structure: wherein: R1, R2, R3, R4, R5 and Q1 are as defined herein, or a pharmaceutically acceptable salt thereof, with estrogen, an estrone, or an estrogen receptor agonist for contraception, hormone replacement therapy, or treating progesterone-related carcinomas and adenocarcinomas.
Owner:WYETH LLC
Methods and pharmaceutical compositions useful for treating psoriasis
Pharmaceutical or cosmetic compositions including cruciferous plant extract or isothiocyanates such as 4-methyl-thio-butyl-isothiocyanate which are useful for treating psoriasis are provided.
Owner:S U L V E +1
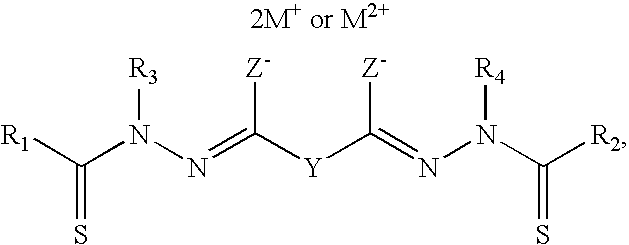
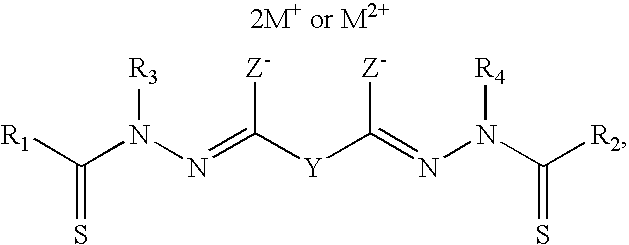
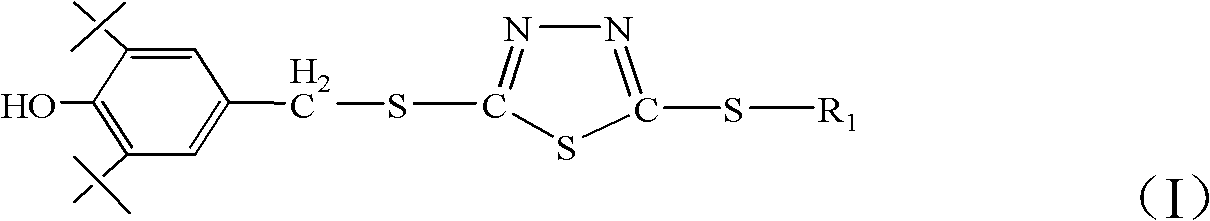
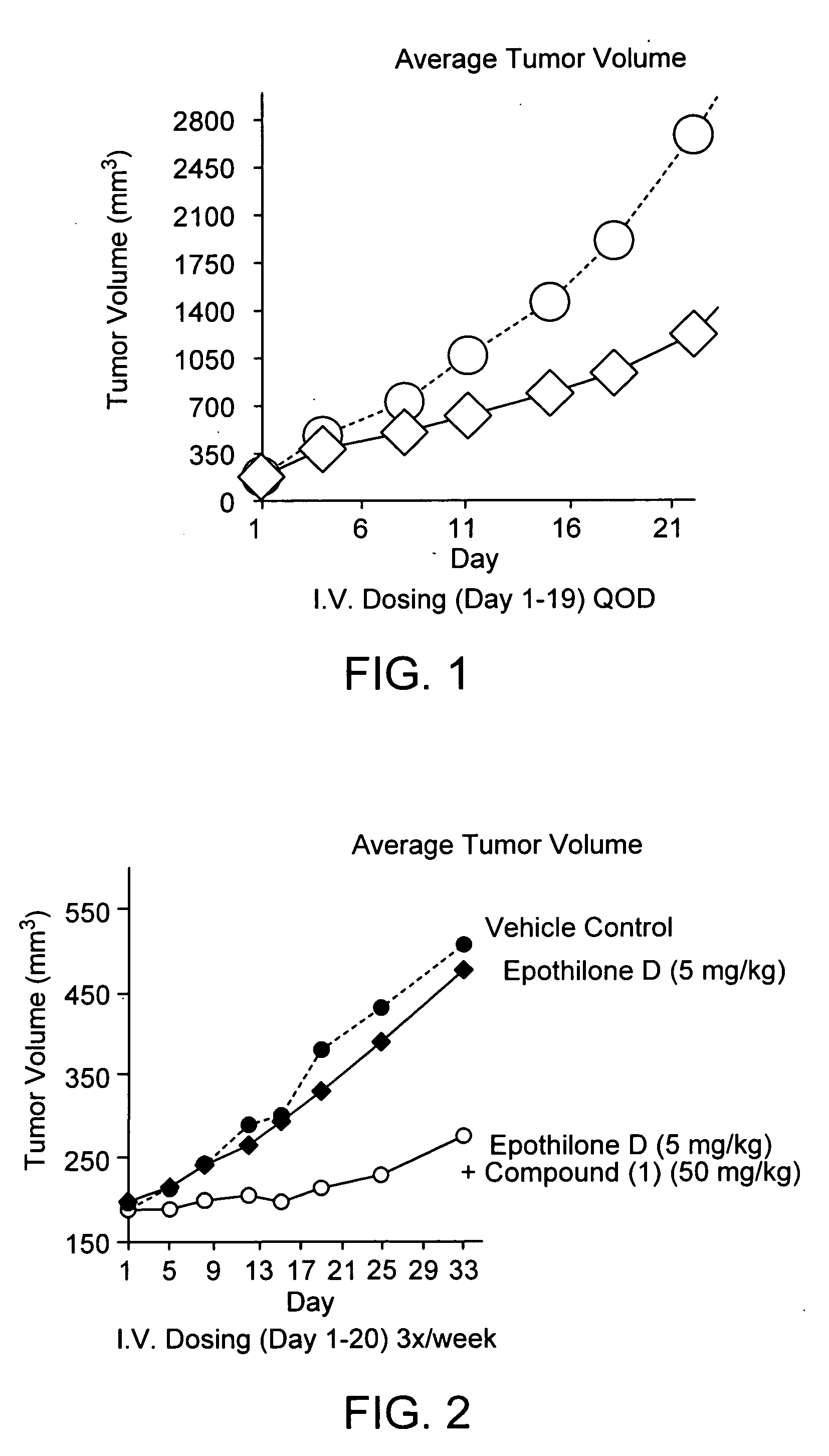
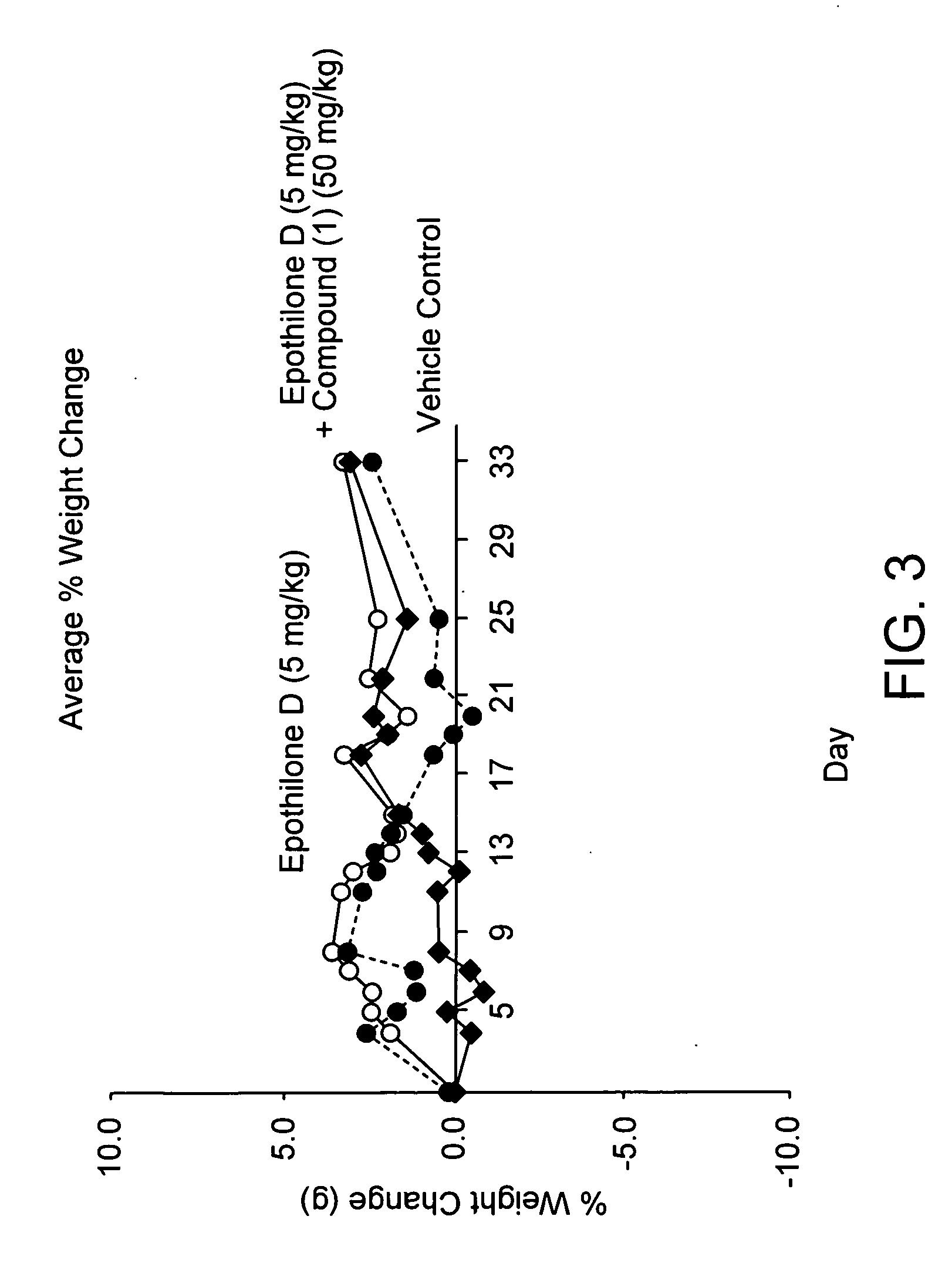
Bis(thio-hydrazide amide) formulation
InactiveUS20070088057A1Good water solubilityFast rebuildBiocideAmide active ingredientsThio-Compound (substance)
Disclosed herein are compositions comprising a compound represented by structural formula (I): 2 g of which is reconstitutable in 10 mL of a water in less than 10 minutes, and methods for preparing these compositions. Also disclosed are compositions comprising a compound represented by structural formula (I) and a pharmaceutically acceptable excipient, wherein the molar ratio of said compound to said excipient is from 1:20 to 1:1, and methods for preparing these compositions.
Owner:SYNTA PHARMA CORP
Fungicide composition
ActiveCN101637157AGood synergyImprove the effect of disease preventionBiocideFungicidesThiocarbamateCarbamate
The invention provides a fungicide composition containing an active component A and an active component B, and application thereof in preventing and controlling various agricultural fungal diseases. The active component A in the composition is one of two methoxyl acrylic ester compounds; and as shown above, the active component B of the compound A1 and the compound A2 is one of the following fungicide varieties: thiocarbamate compounds or salt thereof, aliphatic compounds or salt thereof, carbamate compounds or salt thereof, oxazole compounds or salt thereof, thiazole compounds or salt thereof, amide compounds or salt thereof, organophosphorus compounds or salt thereof, imidazole compounds or salt thereof, antibiotic compounds or salt thereof, pyridine compounds or salt thereof and triazole compounds or salt thereof.
Owner:SHENYANG SINOCHEM AGROCHEMICALS R&D CO LTD
Screen phenol-containing thiadiazole type antioxygen antiwear additive and preparation method thereof
InactiveCN103320199AAntioxidant is goodExcellent extreme pressureOrganic chemistryAdditivesAlkaneThio-
The invention discloses a screen phenol-containing thiadiazole type antioxygen antiwear additive and a preparation method thereof, wherein the chemical name of the additive is 2-(3,5-di-tert-butyl-4-hydroxy-benzyl)thio-5-alkylthio 1,3,4-thiadiazole. According to the preparation method, 2,5-dimercapto-1,3,4-thiadiazole is adopted as a raw material, the 2,5-dimercapto-1,3,4-thiadiazole and a halogenated alkane are subjected to a nucleophilic substitution reaction under a catalyst effect to generate monoalkyl thiadiazole, and the monoalkyl thiadiazole, formaldehyde and 2,6-di-tert-butylphenol are subjected to a condensation polymerization reaction under a catalyst effect to obtain 2-(3,5-di-tert-butyl-4-hydroxy-benzyl)thio-5-alkylthio 1,3,4-thiadiazole type lubricating oil multifunction additive, wherein the additive is characterized in that good oxidation resistance, extreme pressure property, wear resistance and corrosion resistance are provided for the lubricant with the additive, and the additive can be combined with other additives to provide good synergy effects, can be adopted to partly or completely replace ZDDP, and is applicable for internal combustion engine oils and industrial lubricating oils.
Owner:PETROCHINA CO LTD
Water tree resistant cable
InactiveUS6869995B2Reduce sweatingImprove propertiesBiocidePlastic/resin/waxes insulatorsPolyethylene glycolMethyl group
A composition comprising:(i) polyethylene, and, based on 100 parts by weight of component (i),(ii) about 0.3 to about 0.6 part by weight of 4,4′-thiobis(2-methyl-6-t-butylphenol); 4,4′-thiobis(2-t-butyl-5-methylphenol); 2,2′-thiobis(6-t-butyl-4-methylphenol); or a mixture of said compounds, and(iii) about 0.4 to about 1 part by weight of a polyethylene glycol having a molecular weight in the range of about 1000 to about 100,000.
Owner:UNION CARBIDE CHEM & PLASTICS TECH CORP
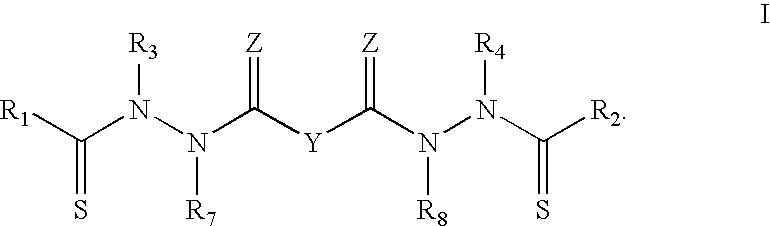
Bis(thio-hydrazide amides) for treatment of hyperplasia
InactiveUS20060142393A1Good treatment effectMinimize side effectsBiocideAmide active ingredientsThio-Percent Diameter Stenosis
Methods and medical devices for treating a proliferative disorder in a subject, e.g., restenosis in a blood vessel that has been implanted with a stent, employ a bis(thio-hydrazide amide) represented by Structural Formula I or a pharmaceutically acceptable salt or solvate thereof. Y is a covalent bond or an optionally substituted straight chained hydrocarbyl group, or, Y, taken together with both >C=Z groups to which it is bonded, is an optionally substituted aromatic group. R1-R4 are independently —H, an optionally substituted aliphatic group, an optionally substituted aryl group, or R1 and R3 taken together with the carbon and nitrogen atoms to which they are bonded, and / or R2 and R4 taken together with the carbon and nitrogen atoms to which they are bonded, form a non-aromatic heterocyclic ring optionally fused to an aromatic ring. R7-R8 are independently —H, an optionally substituted aliphatic group, or an optionally substituted aryl group. Z is O or S.
Owner:SYNTA PHARMA CORP
High index and high impact resistant polythiourethane/urea material, method of manufacturing same and its use in the optical field
A transparent, non elastomeric, high index, impact resistant polythiourethane / urea material comprising the reaction product of: a) at least one (alpha, omega)-diiso(thio)cyanate cycloaliphatic or aromatic prepolymer having a number average molecular weight ranging from 100 to 3000 gmol-1, and b) at least one primary diamine, in an equivalent molar ratio amine function / iso(thio)cyanate function from 0.5 to 2, preferably from 0.90 to 1.10, wherein, said prepolymer and diamine are free from disulfide (-S-S-) linkage and at least one of the prepolymer or the diamine contains one or more S atoms in its chain.
Owner:ESSILOR INT CIE GEN DOPTIQUE
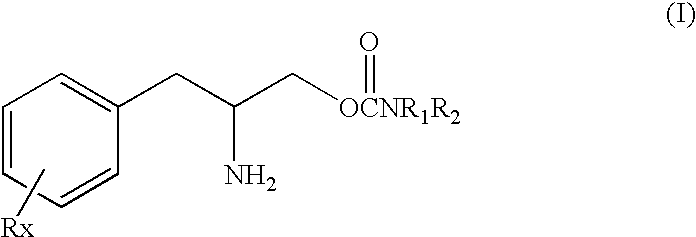
Treatment of sleep-wake disorders
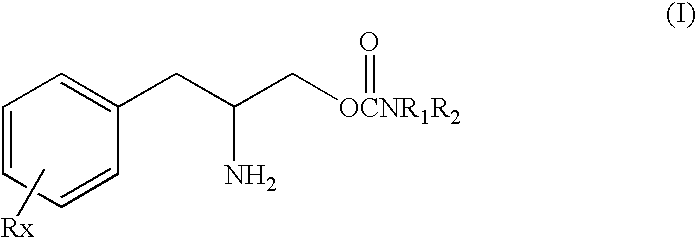
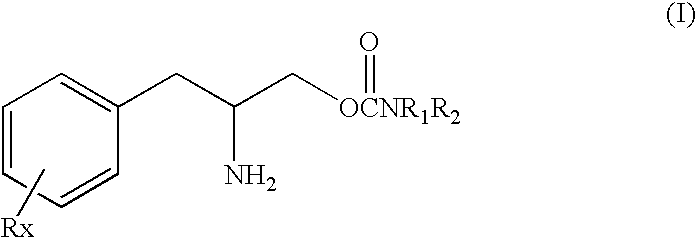
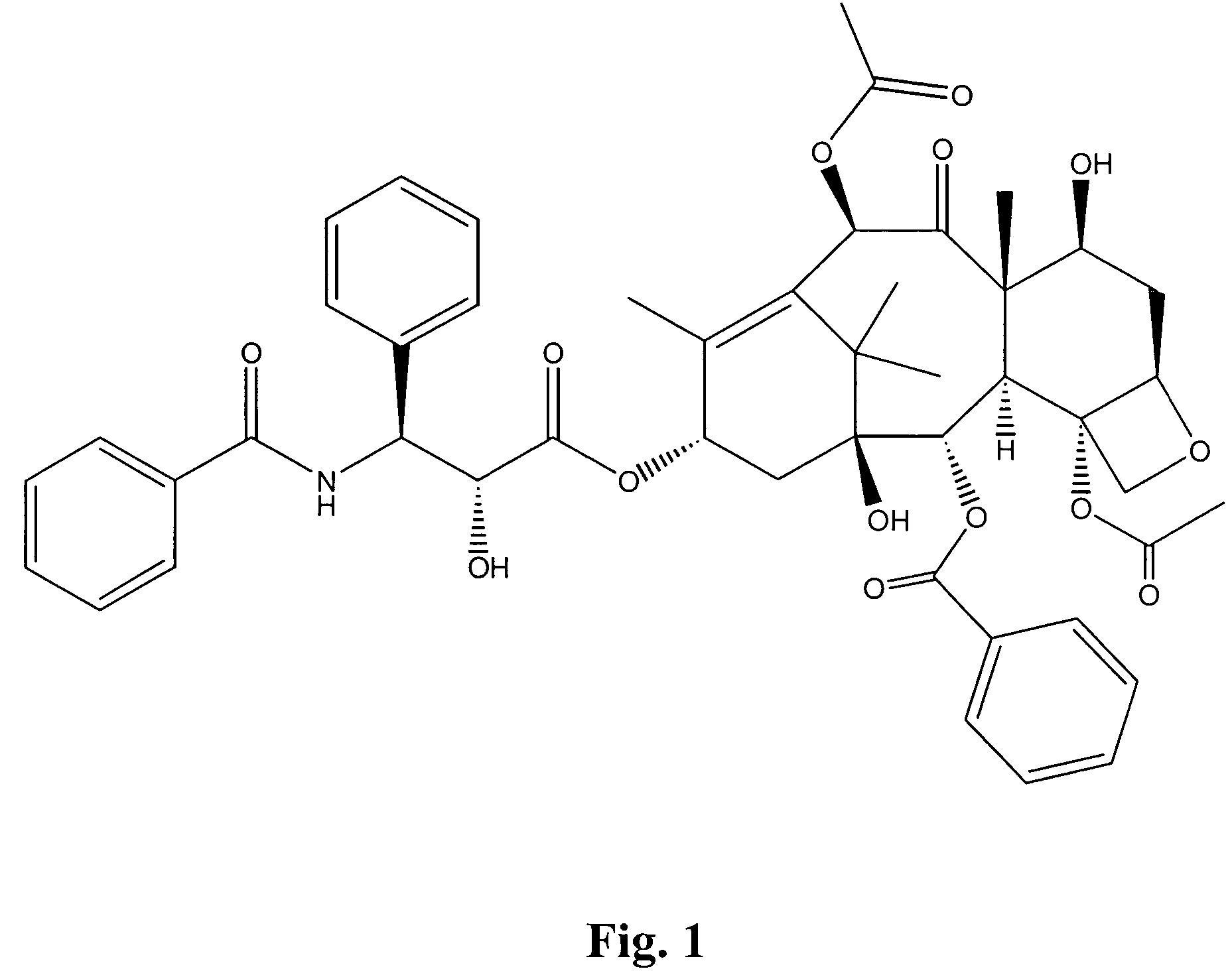
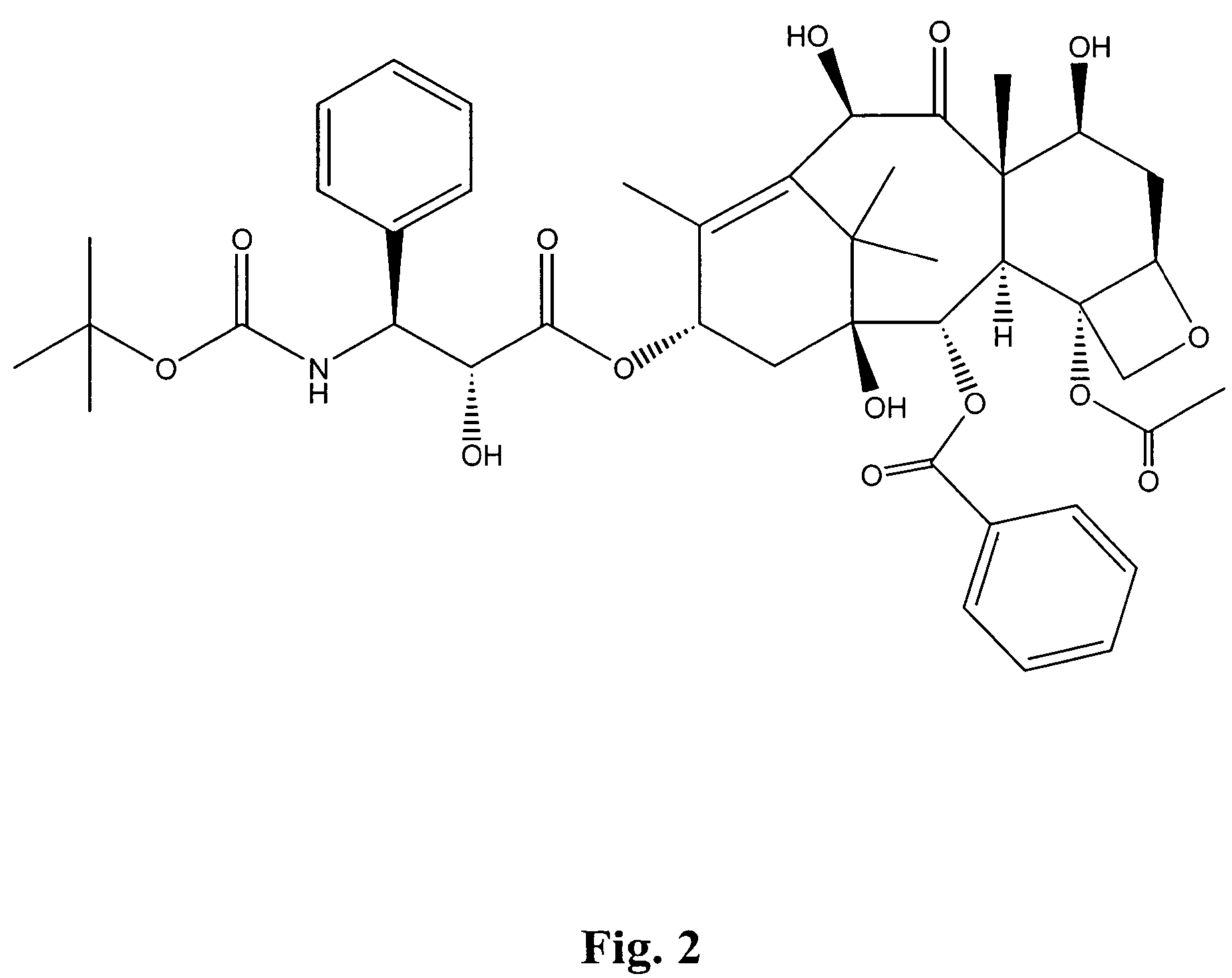
This invention is directed to a method of treating Excessive daytime Sleepiness (EDS) in a subject, comprising the step of administering a therapeutically effective amount of a compound of Formula (I): Formula (I) or a pharmaceutically acceptable salt or ester thereof wherein Rx is a member selected from the group consisting of hydrogen, lower alkyl of 1 to 8 carbon atoms, halogen selected from F, Cl, Br and I, alkoxy containing 1 to 3 carbon atoms, nitro, hydroxy, trifluoromethyl, and thioalkoxy containing 1 to 3 carbon atoms; x is an integer of 1 to 3, with the proviso that R may be the same or different when x is 2 or 3; R1 and R2 can be the same or different from each other and are independently selected from the group consisting of hydrogen, lower alkyl of 1 to 8 carbon atoms, aryl, arylalkyl, cycloalkyl of 3 to 7 carbon atoms; R1 and R2 can be joined to form a 5 to 7-membered heterocycle substituted with a member selected from the group consisting of hydrogen, alkyl, and aryl groups, wherein the cyclic compound can comprise 1 to 2 nitrogen atoms and 0 to 1 oxygen atom, wherein the nitrogen atoms are not directly connected with each other or with the ox en atom.
Owner:BIOPHARM
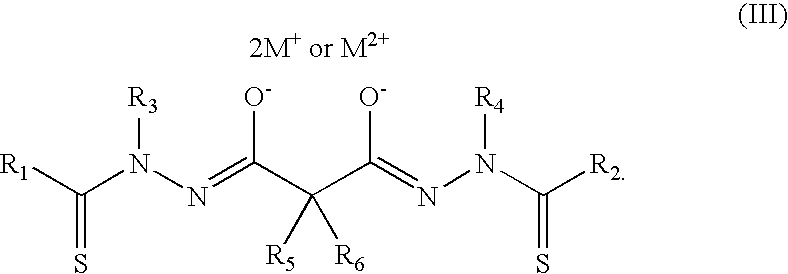
Bis(thio-hydrazide amide) salts for treatment of cancers
Disclosed are bis(thio-hydrazide amide) disalts, which are represented by Structural Formula (I):Y is a covalent bond or a substituted or unsubstituted straight chained hydrocarbyl group. R1-R4 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group, or R1 and R3 taken together with the carbon and nitrogen atoms to which they are bonded, and / or R2 and R4 taken together with the carbon and nitrogen atoms to which they are bonded, form a non-aromatic heterocyclic ring optionally fused to an aromatic ring. Z is —O or —S. M+ is a pharmaceutically acceptable monovalent cation and M2+ is a pharmaceutically acceptable divalent cation.Also, disclosed are pharmaceutical compositions comprising a bis(thio-hydrazide amide) disalt described above. Further disclosed are methods of treating a subject with cancer. The methods comprise the step of administering an effective amount of a bis(thio-hydrazide amide) disalt described above.
Owner:SYNTA PHARMA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com