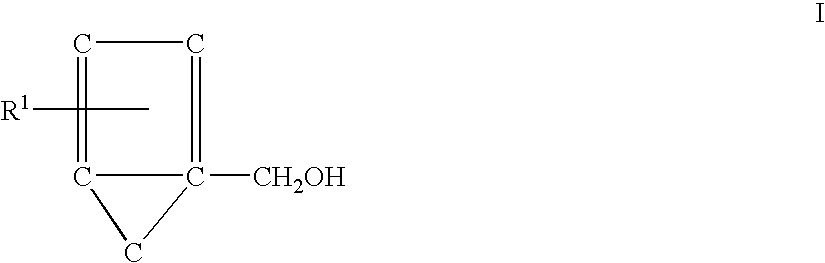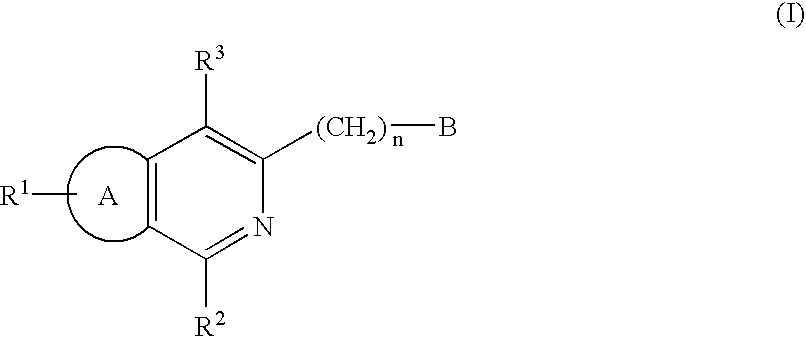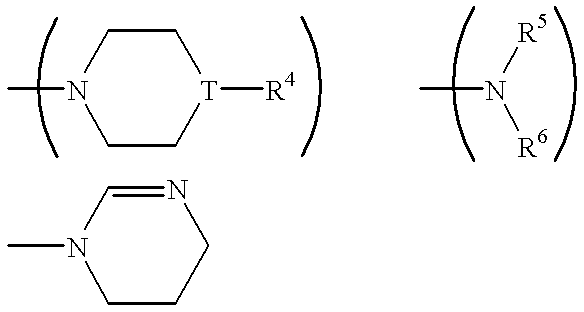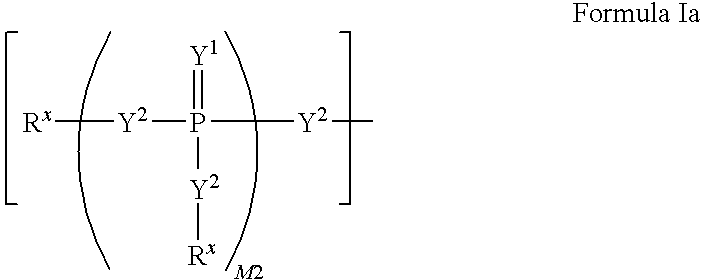Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
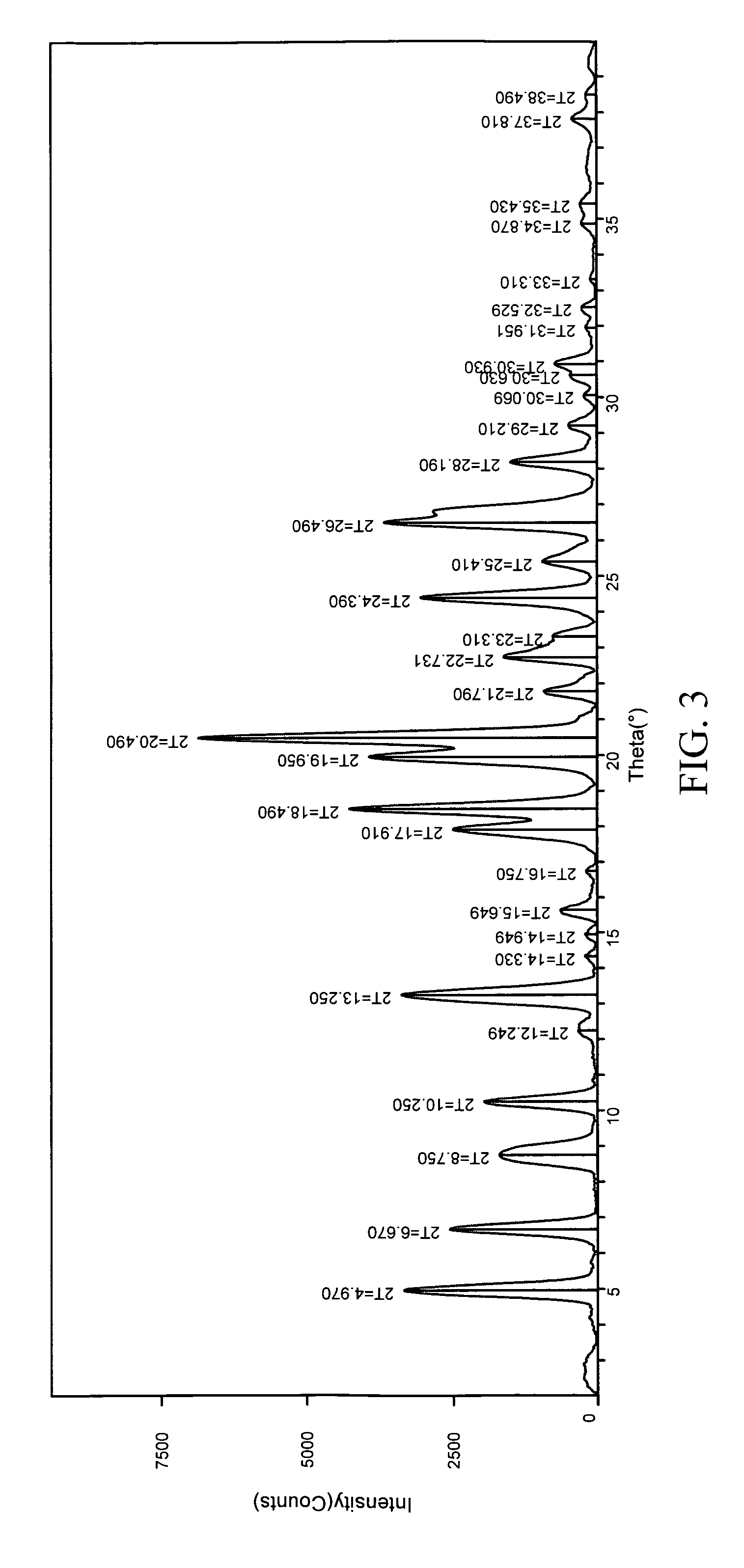
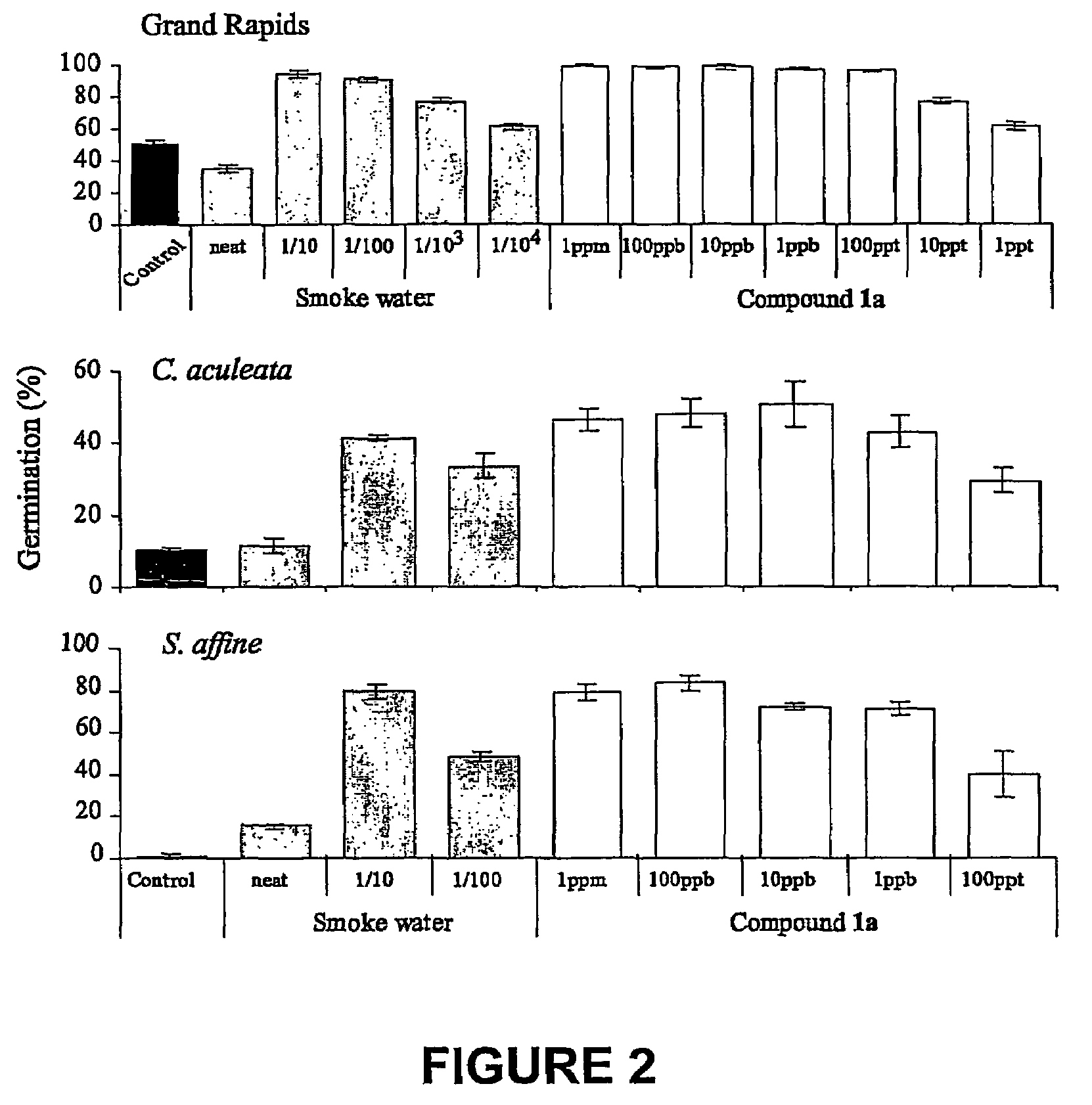
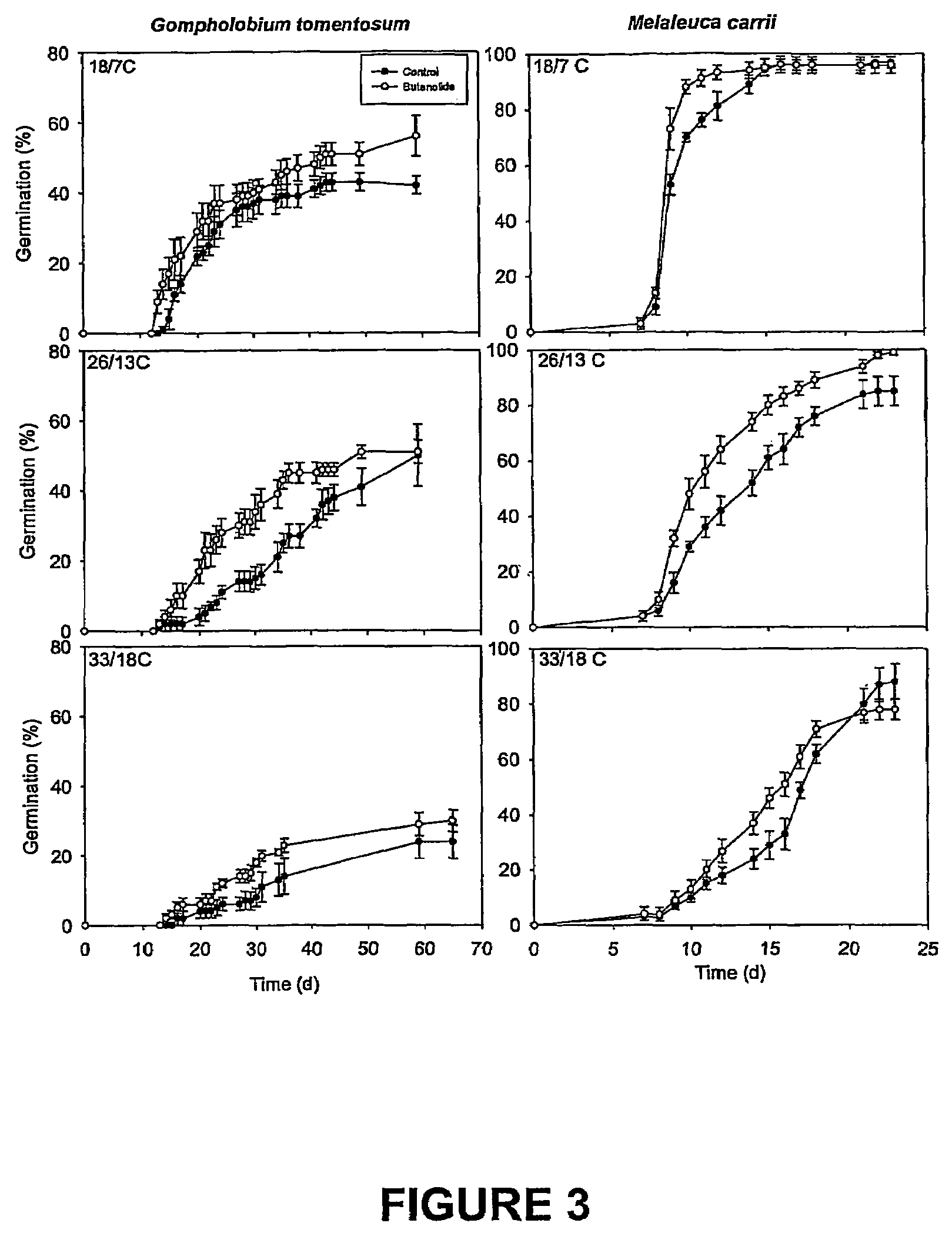
5154 results about "Furan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. Chemical compounds containing such rings are also referred to as furans. Furan is a colorless, flammable, highly volatile liquid with a boiling point close to room temperature. It is soluble in common organic solvents, including alcohol, ether, and acetone, and is slightly soluble in water. It is toxic and may be carcinogenic in humans. Furan is used as a starting point to other speciality chemicals.
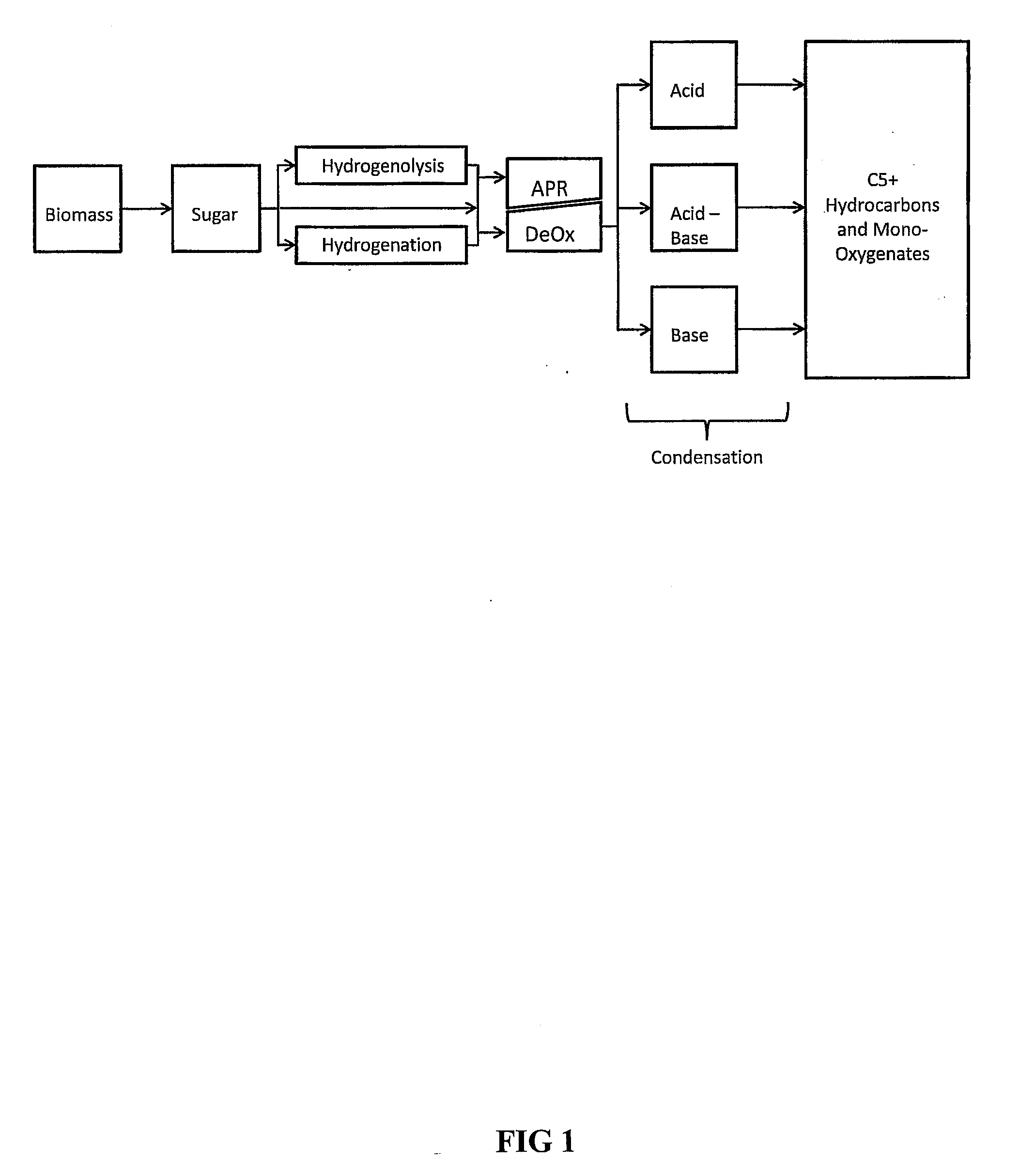
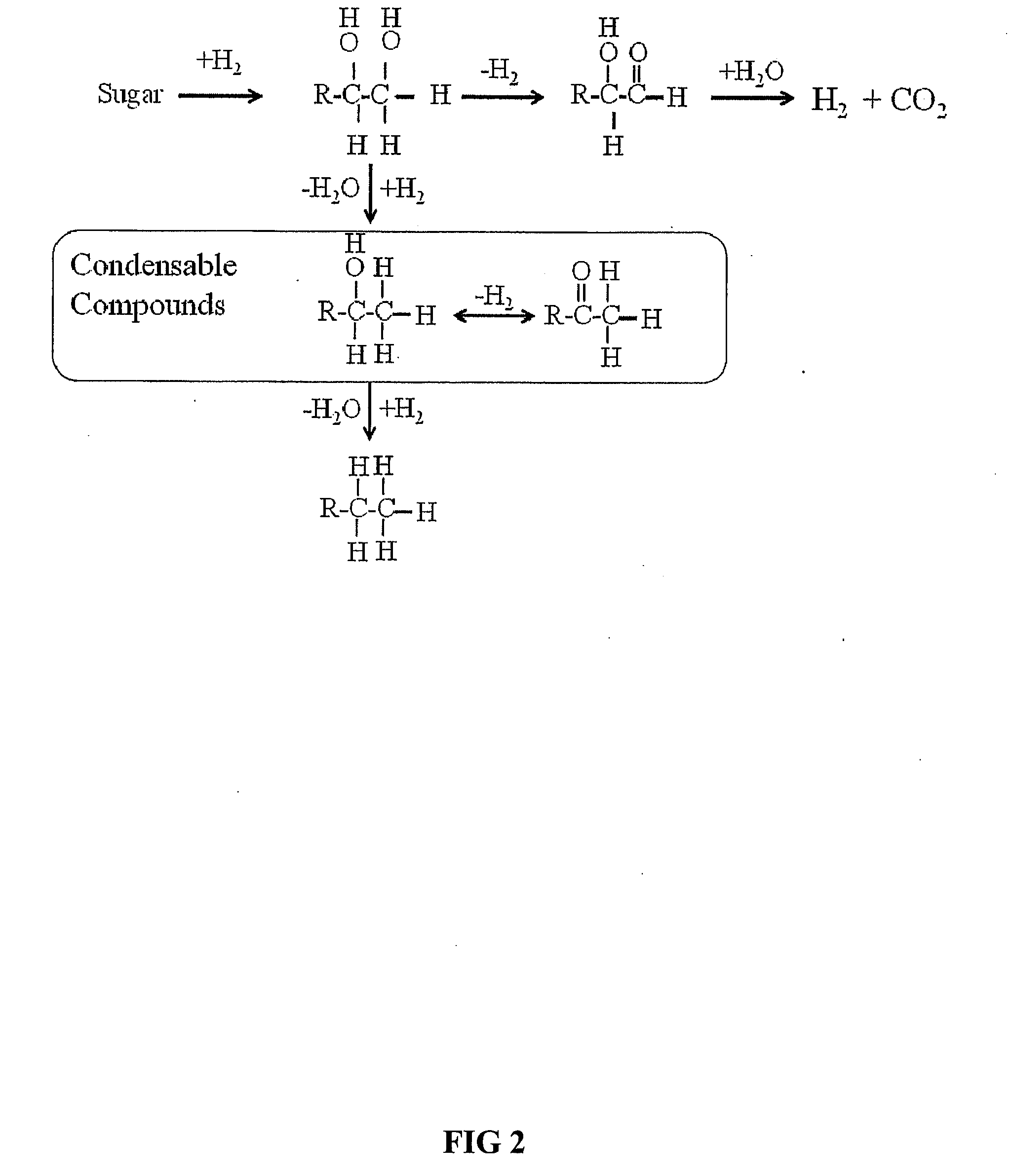
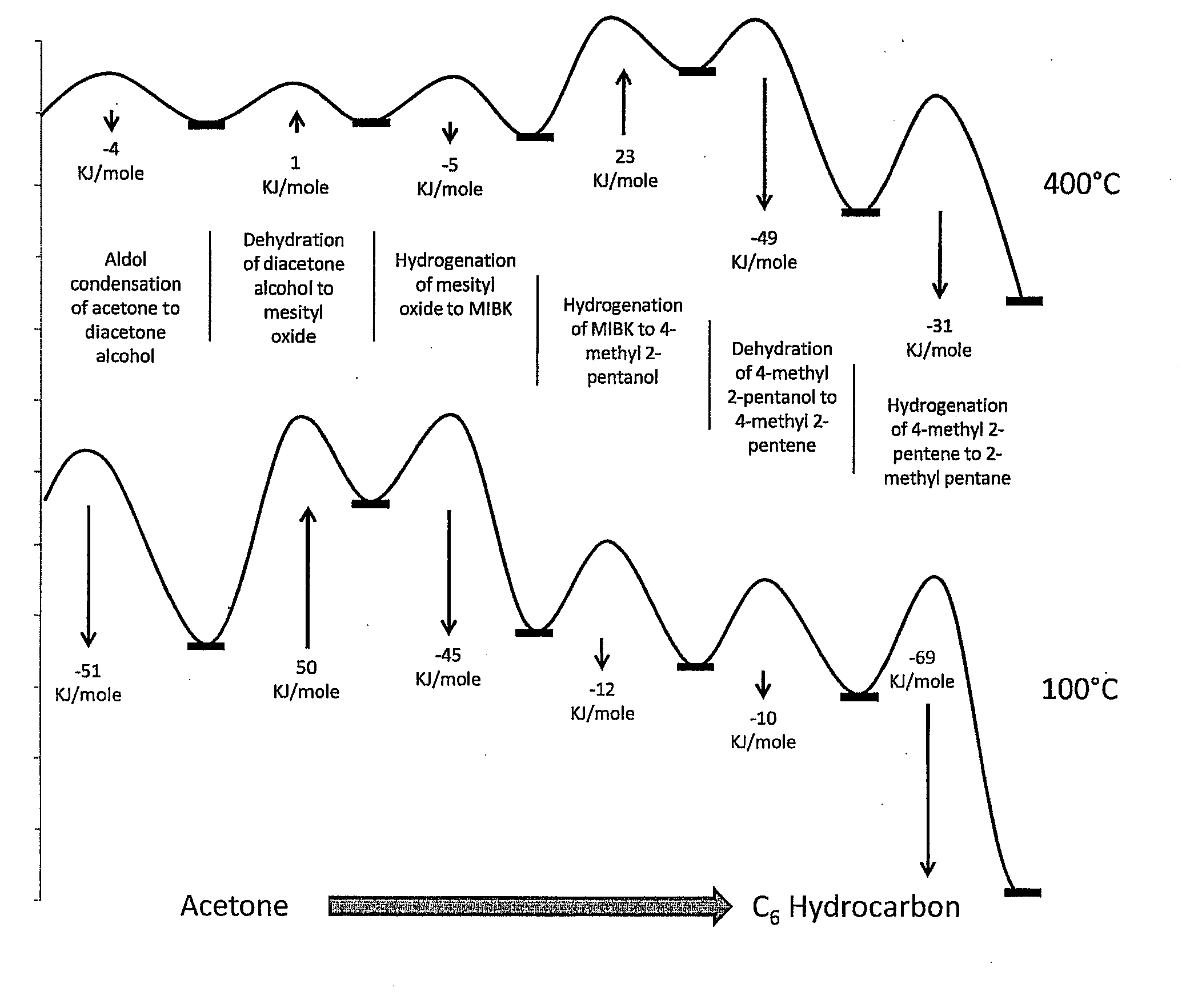
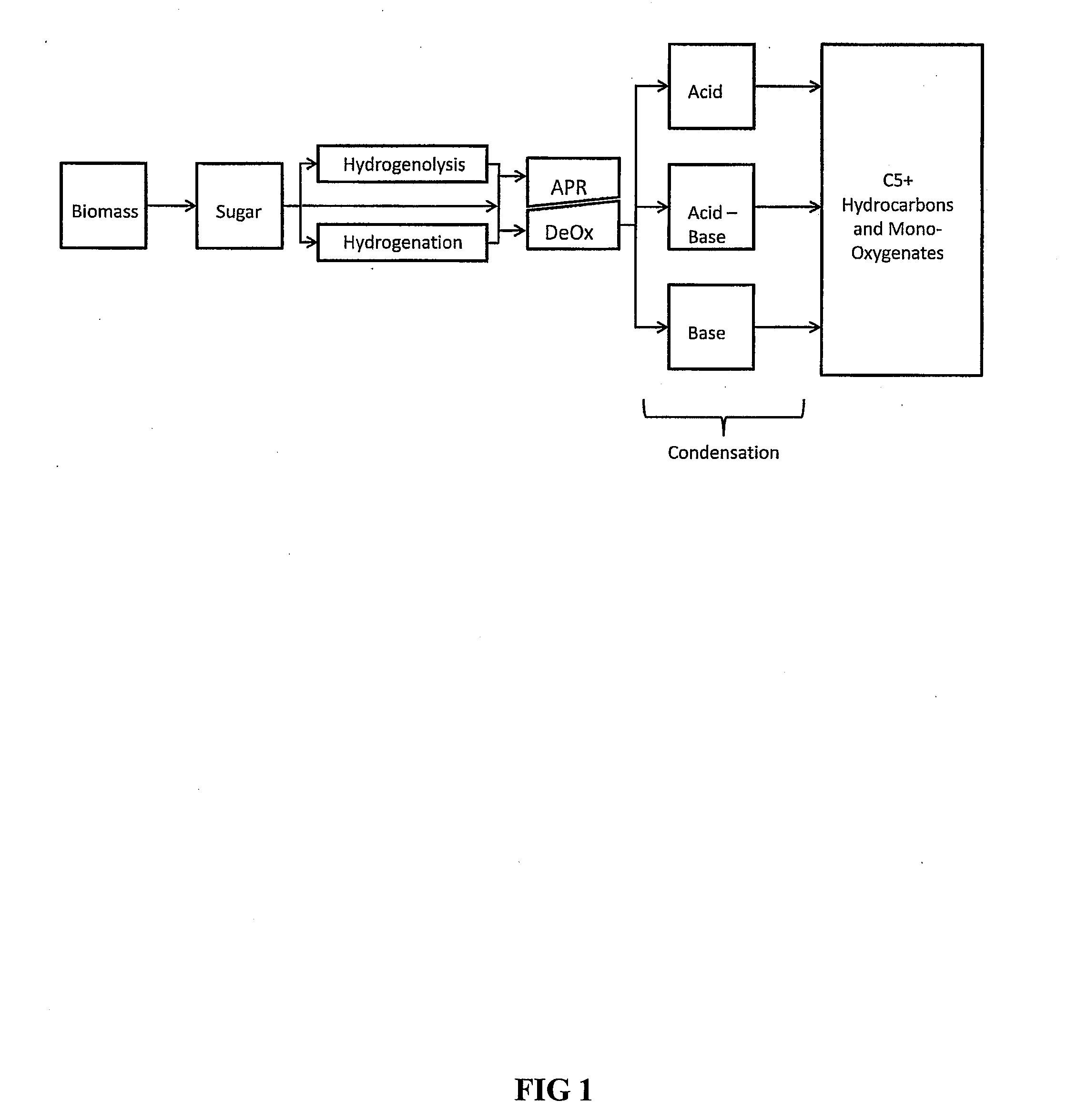
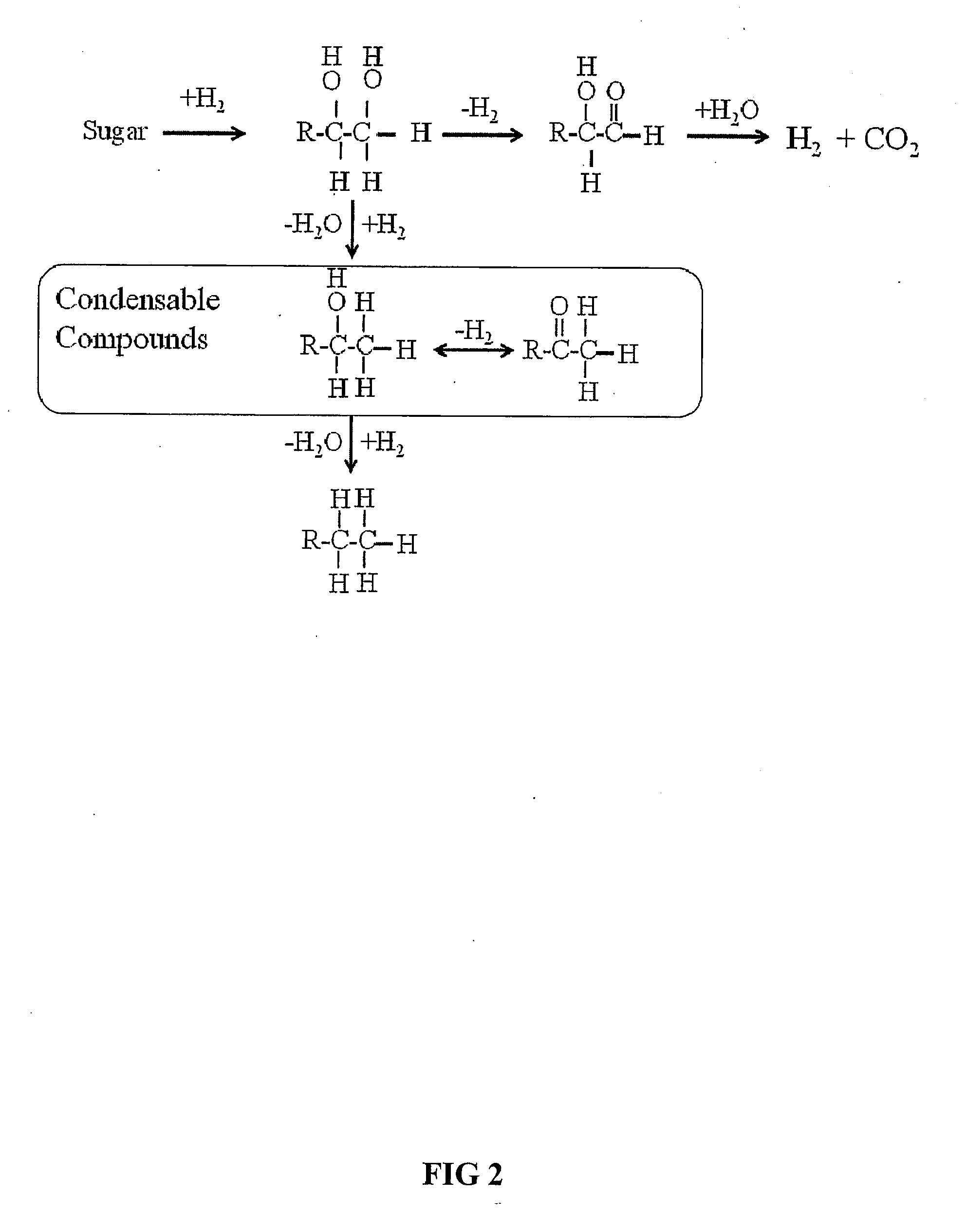
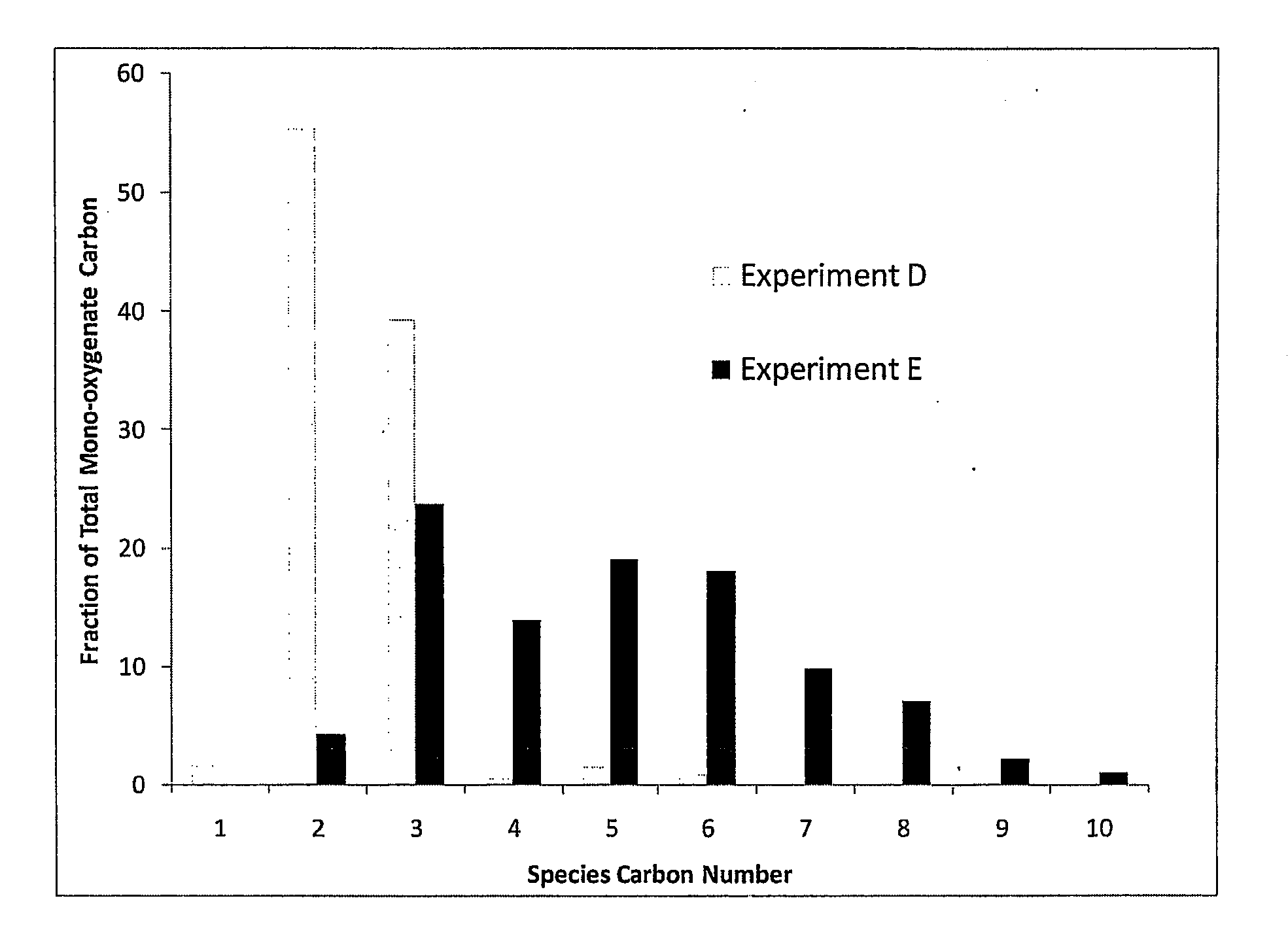
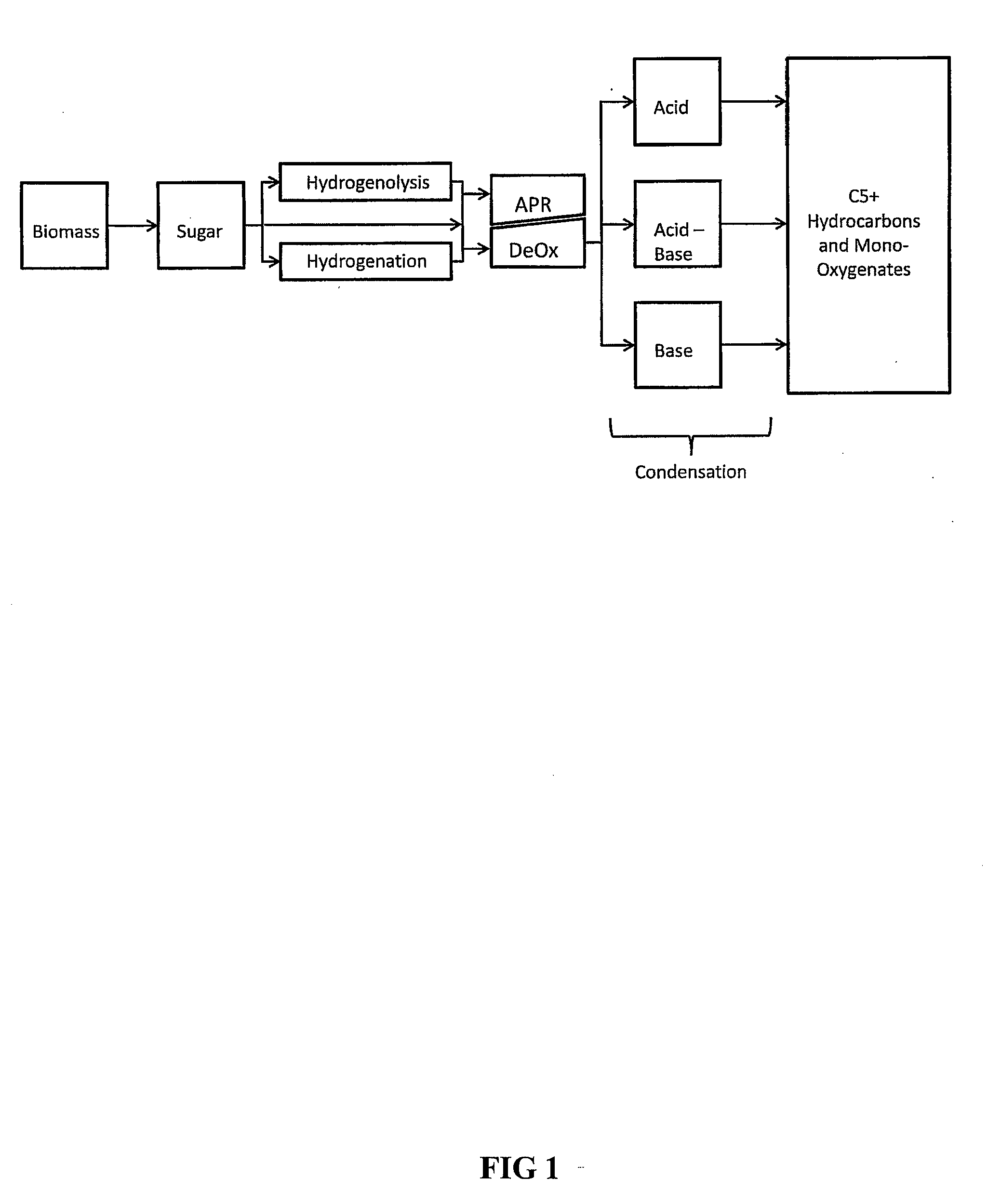
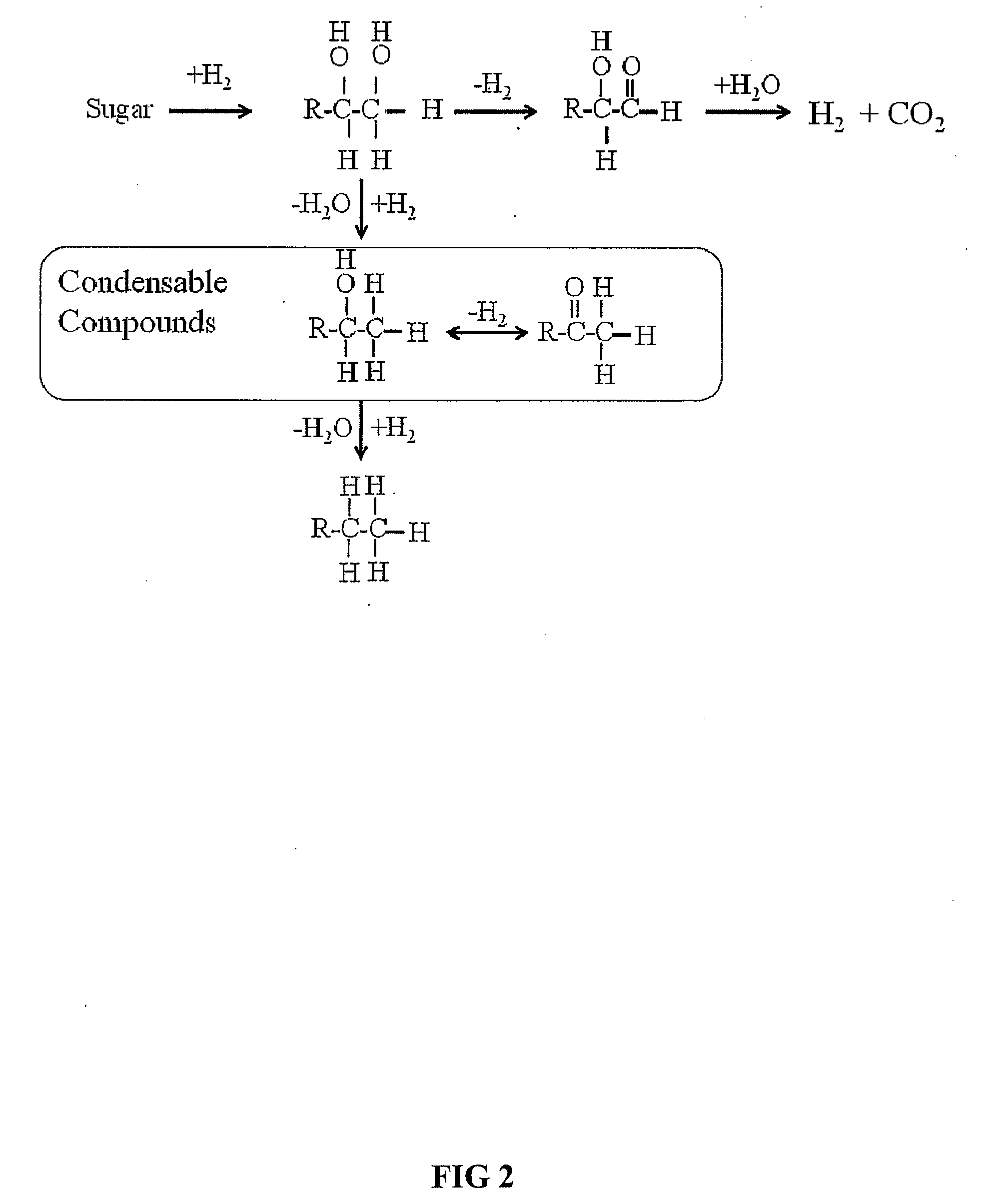
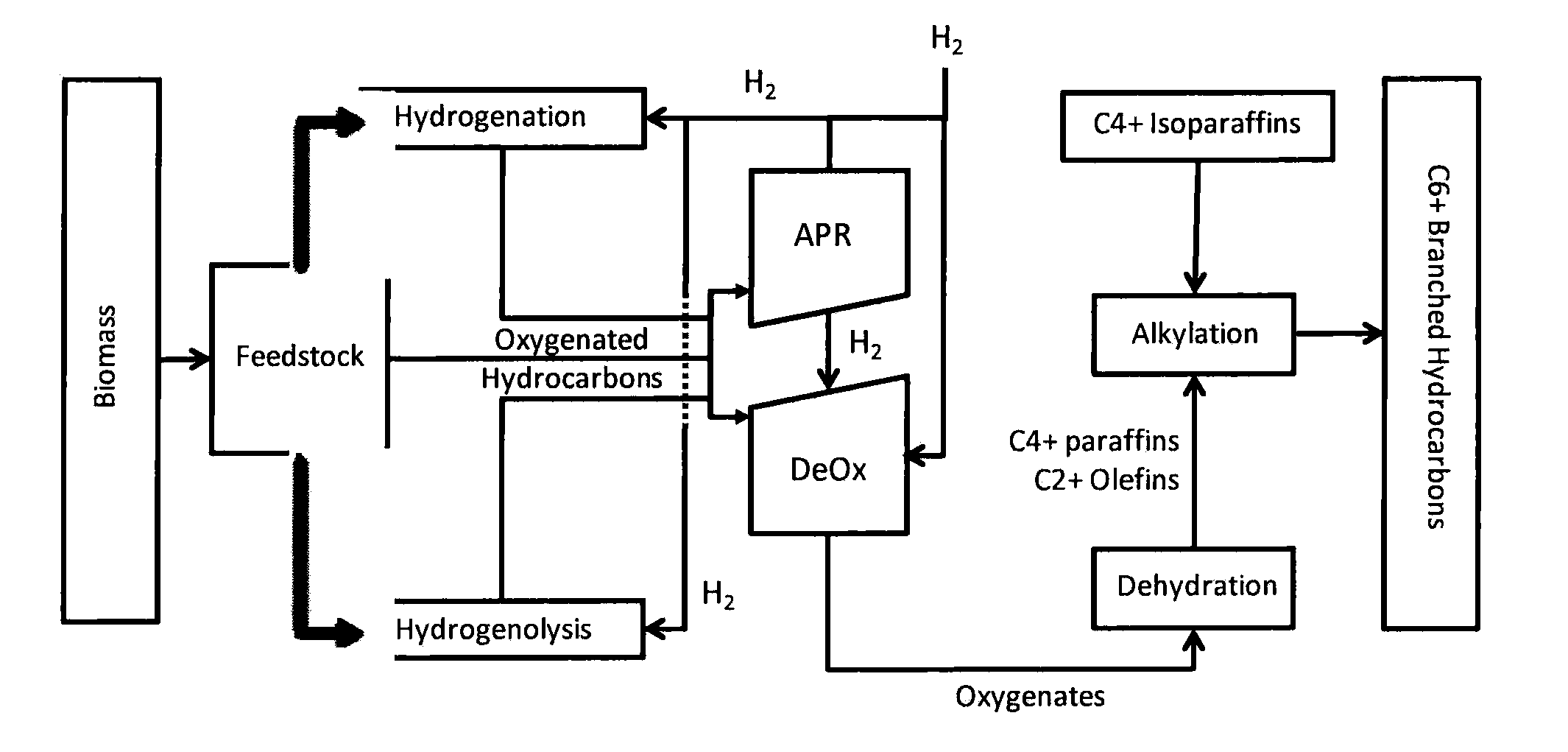
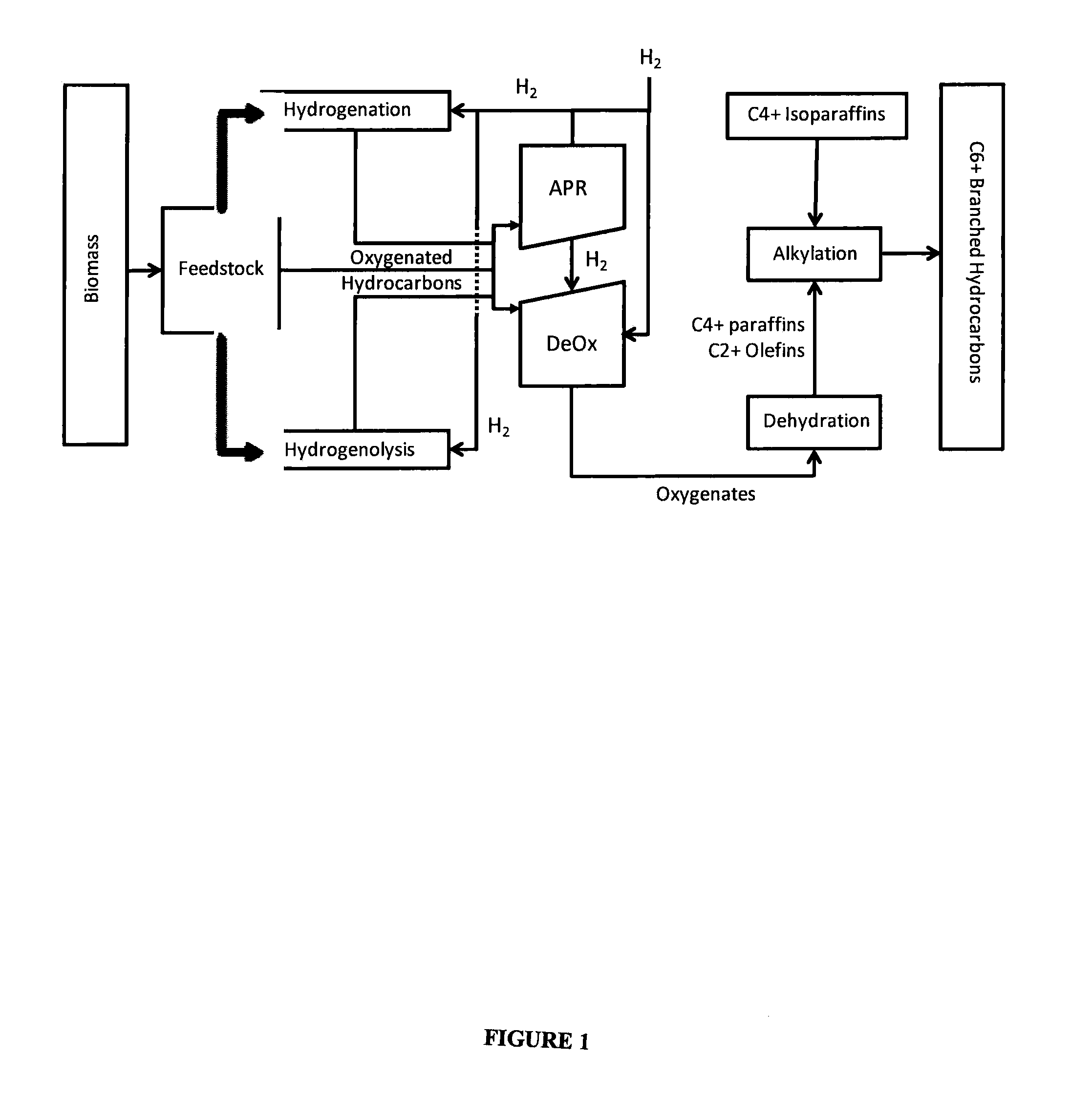
Synthesis of liquid fuels and chemicals from oxygenated hydrocarbons
ActiveUS20080216391A1Organic compound preparationHydrocarbon from oxygen organic compoundsFuranLiquid fuel
Processes and reactor systems are provided for the conversion of oxygenated hydrocarbons to hydrocarbons, ketones and alcohols useful as liquid fuels, such as gasoline, jet fuel or diesel fuel, and industrial chemicals. The process involves the conversion of mono-oxygenated hydrocarbons, such as alcohols, ketones, aldehydes, furans, carboxylic acids, diols, triols, and / or other polyols, to C4+ hydrocarbons, alcohols and / or ketones, by condensation. The oxygenated hydrocarbons may originate from any source, but are preferably derived from biomass.
Owner:VIRENT
Synthesis of liquid fuels and chemicals from oxygenated hydrocarbons
ActiveUS20080300435A1Oxygen-containing compound preparationLiquid hydrocarbon mixture productionFuranLiquid fuel
Processes and reactor systems are provided for the conversion of oxygenated hydrocarbons to hydrocarbons, ketones and alcohols useful as liquid fuels, such as gasoline, jet fuel or diesel fuel, and industrial chemicals. The process involves the conversion of mono-oxygenated hydrocarbons, such as alcohols, ketones, aldehydes, furans, carboxylic acids, diols, triols, and / or other polyols, to C4+ hydrocarbons, alcohols and / or ketones, by condensation. The oxygenated hydrocarbons may originate from any source, but are preferably derived from biomass.
Owner:VIRENT
Anti-viral nucleosides
4-Amino-1-((2R,3S,4S,5R)-5-azido-3,4-dihydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-1H-pyrimidin-2-one (I:R1=R2=R3=R4=H) and prodrugs thereof are hepatitis C(HCV) polymerase inhibitors. Also disclosed are compositions and methods for inhibiting HCV and treating HCV-mediated diseases, processes for making the compounds and synthetic intermediates employed in the process.
Owner:ROCHE PALO ALTO LLC
Synthesis of liquid fuels from biomass
ActiveUS20100076233A1Hydrocarbon by metathesis reactionLiquid hydrocarbon mixture productionFuranAlkane
Processes and reactor systems are provided for the conversion of oxygenated hydrocarbons to paraffins useful as liquid fuels. The process involves the conversion of water soluble oxygenated hydrocarbons to oxygenates, such as alcohols, furans, ketones, aldehydes, carboxylic acids, diols, triols, and / or other polyols, followed by the subsequent conversion of the oxygenates to paraffins by dehydration and alkylation. The oxygenated hydrocarbons may originate from any source, but are preferably derived from biomass.
Owner:VIRENT
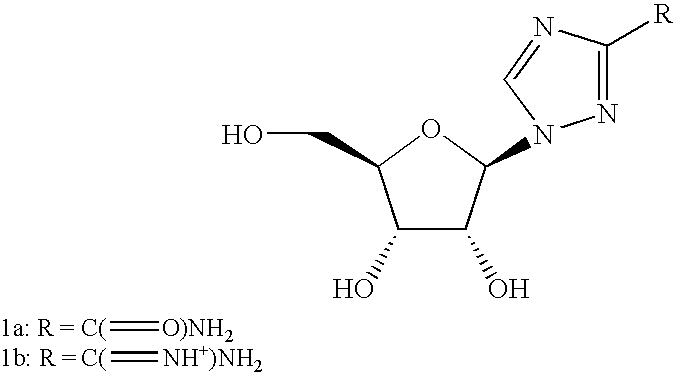
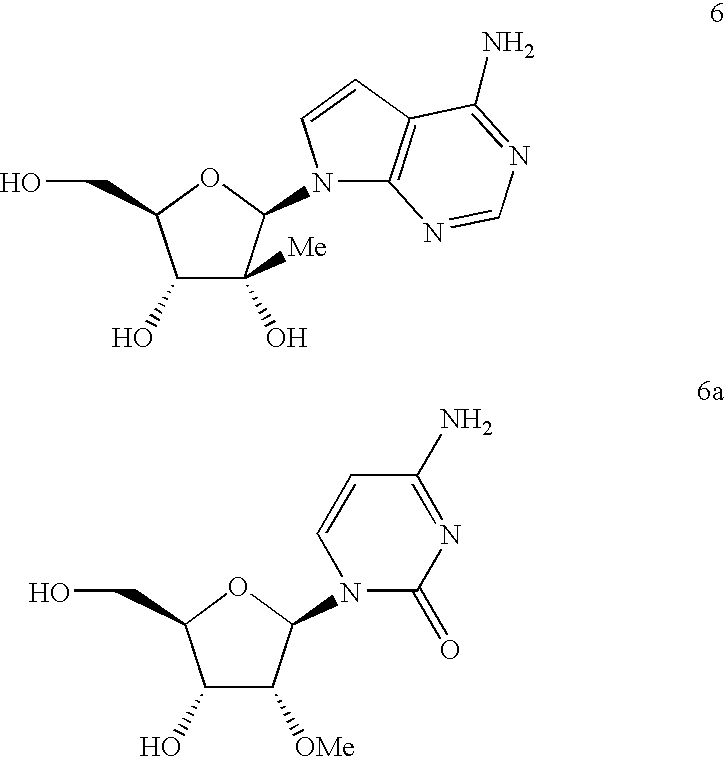
3-B-D-ribofuranosylthiazolo [4,5-d] pyridimine nucleosides and uses thereof
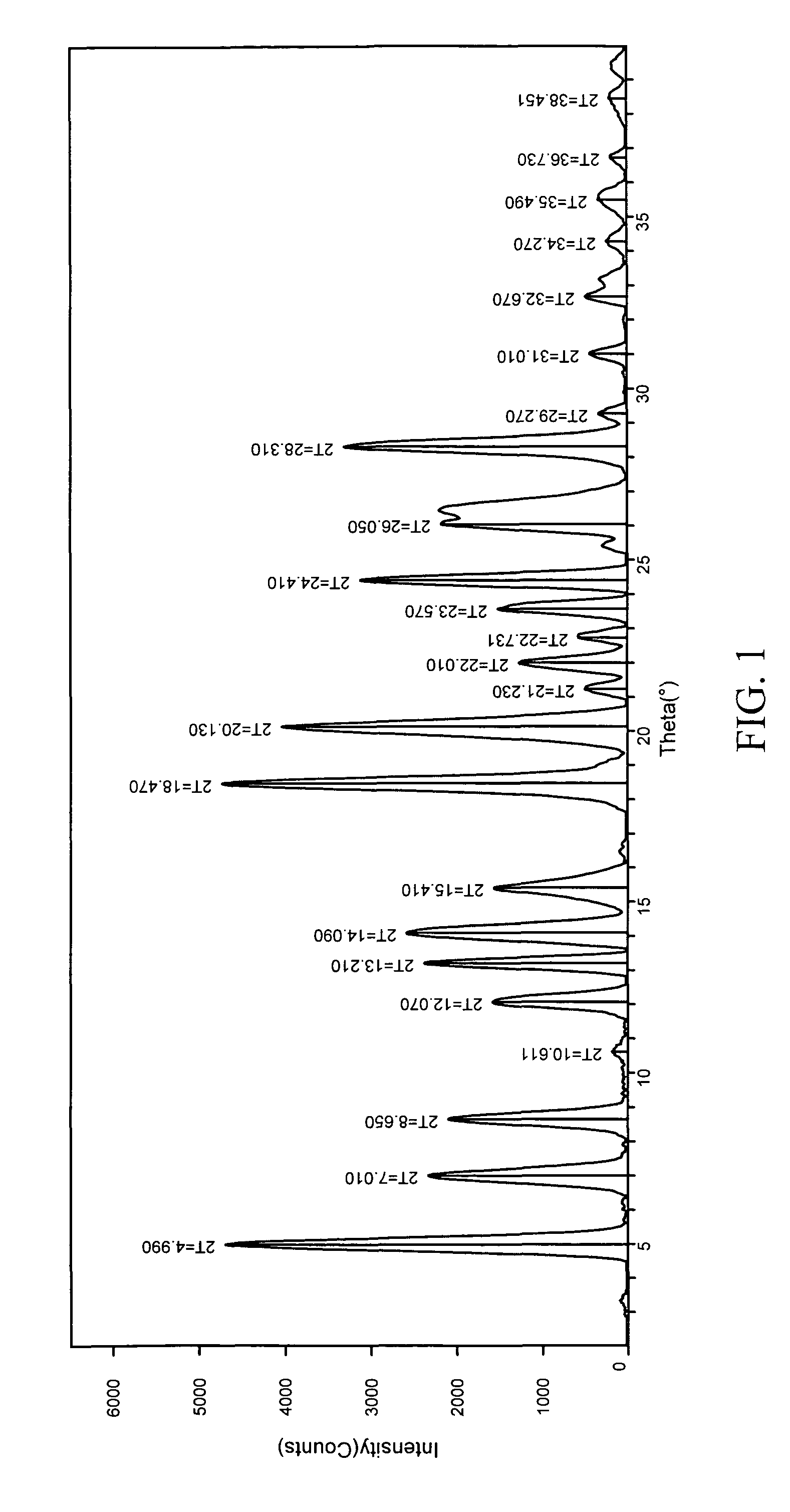
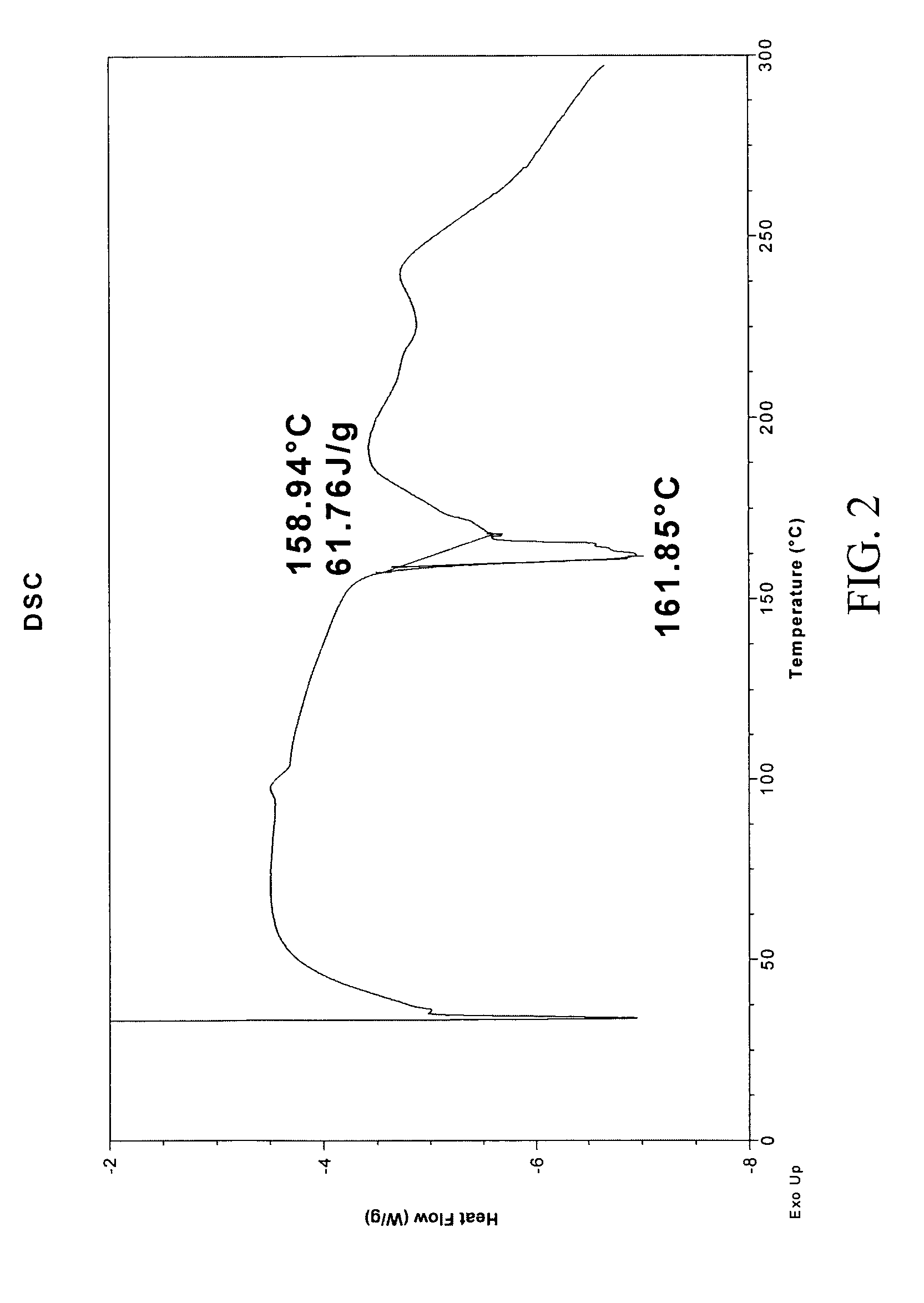
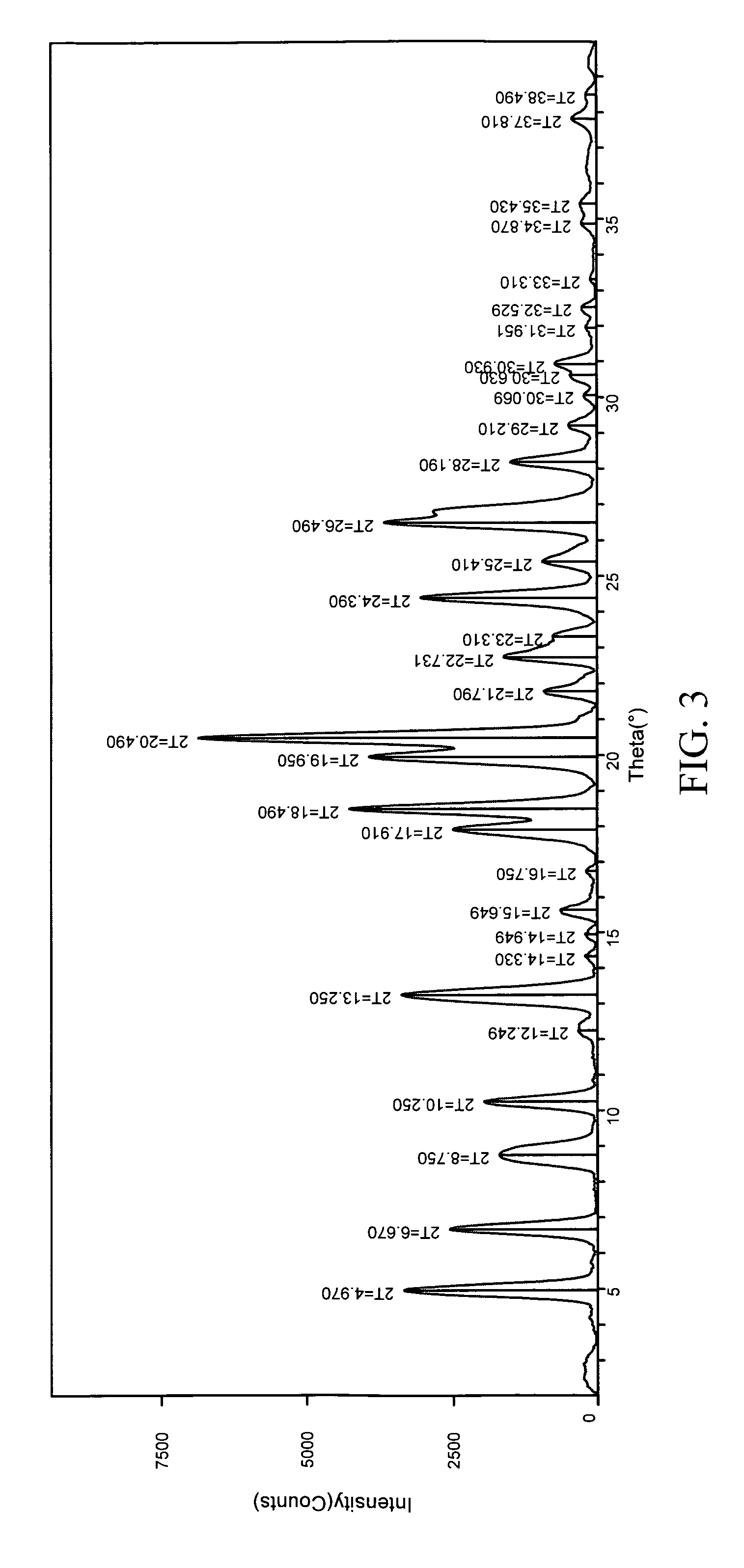
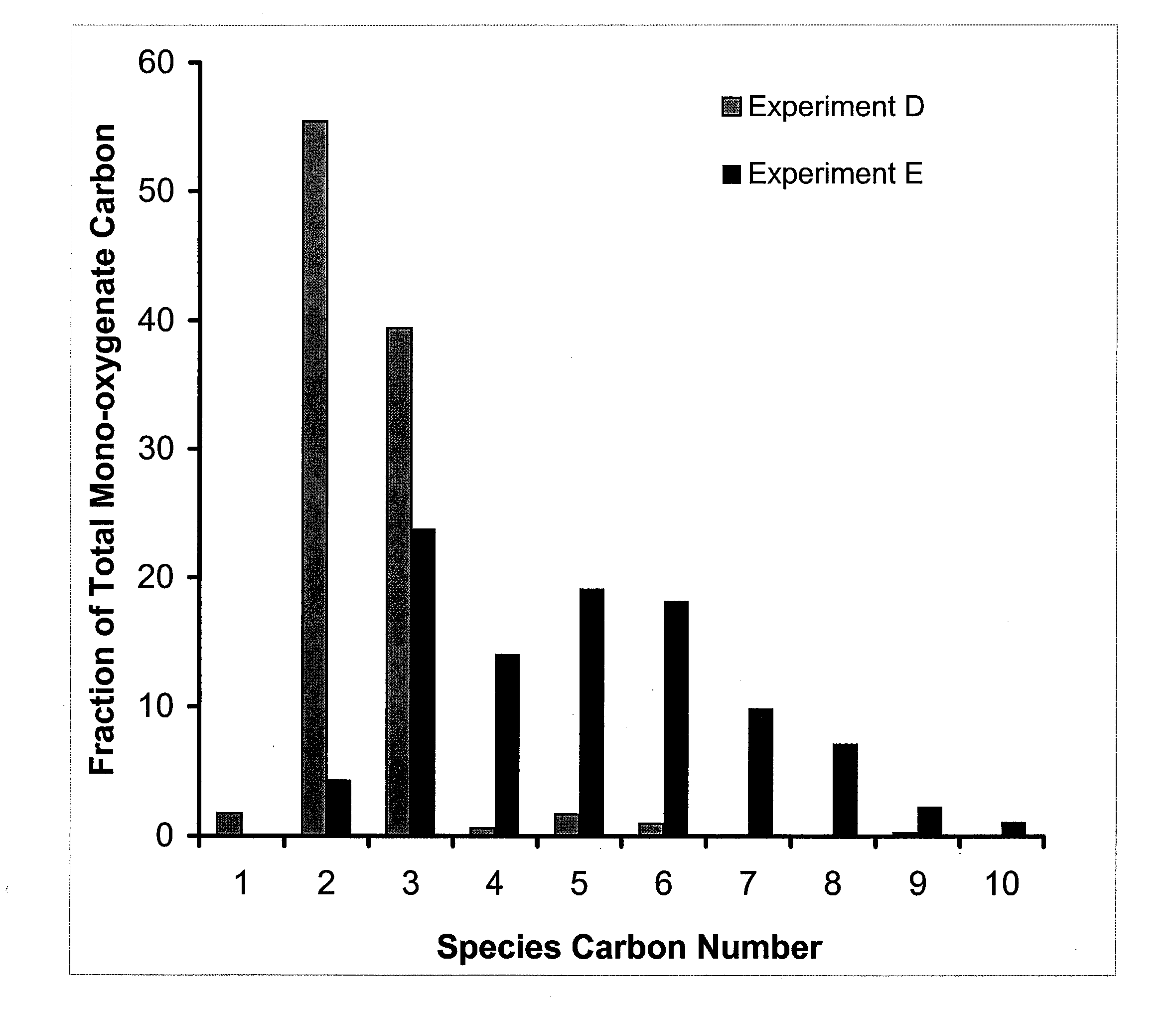
InactiveUS20050070556A1Improve oral availabilityMinimizing activationOrganic active ingredientsBiocideMedicineNucleoside X
Owner:ANDADYS PHARMA INC
Pharmaceutical co-crystal compositions
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphonic acid, phosphinic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, sp2 amine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, s-heterocyclic ring, thiophene, n-heterocyclic ring, pyrrole, o-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES +2
Synthesis of liqiud fuels and chemicals from oxygenated hydrocarbons
ActiveUS20080300434A1Oxygen-containing compound preparationHydrocarbon purification/separationFuranCarboxylic acid
Processes and reactor systems are provided for the conversion of oxygenated hydrocarbons to hydrocarbons, ketones and alcohols useful as liquid fuels, such as gasoline, jet fuel or diesel fuel, and industrial chemicals. The process involves the conversion of mono-oxygenated hydrocarbons, such as alcohols, ketones, aldehydes, furans, carboxylic acids, diols, triols, and / or other polyols, to C4+ hydrocarbons, alcohols and / or ketones, by condensation. The oxygenated hydrocarbons may originate from any source, but are preferably derived from biomass.
Owner:VIRENT
Particulate material having multiple curable coatings and methods for making and using same
InactiveUS7153575B2Retain it curabilityCurability potentialLiquid surface applicatorsSynthetic resin layered productsFuranPolymer science
The present invention relates to coated particulate matter wherein the particles are individually coated with a first set of one or more layers of a curable resin, for example, a combination of phenolic / furan resin or furan resin or phenolic-furan-formaldehyde terpolymer, on a proppant such as sand, and the first set of layers is coated with a second set of one or more layers of a curable resin, for example, a novolac resin with curative. Methods for making and using this coated product as a proppant, gravel pack and for sand control are also disclosed.
Owner:HEXION INC
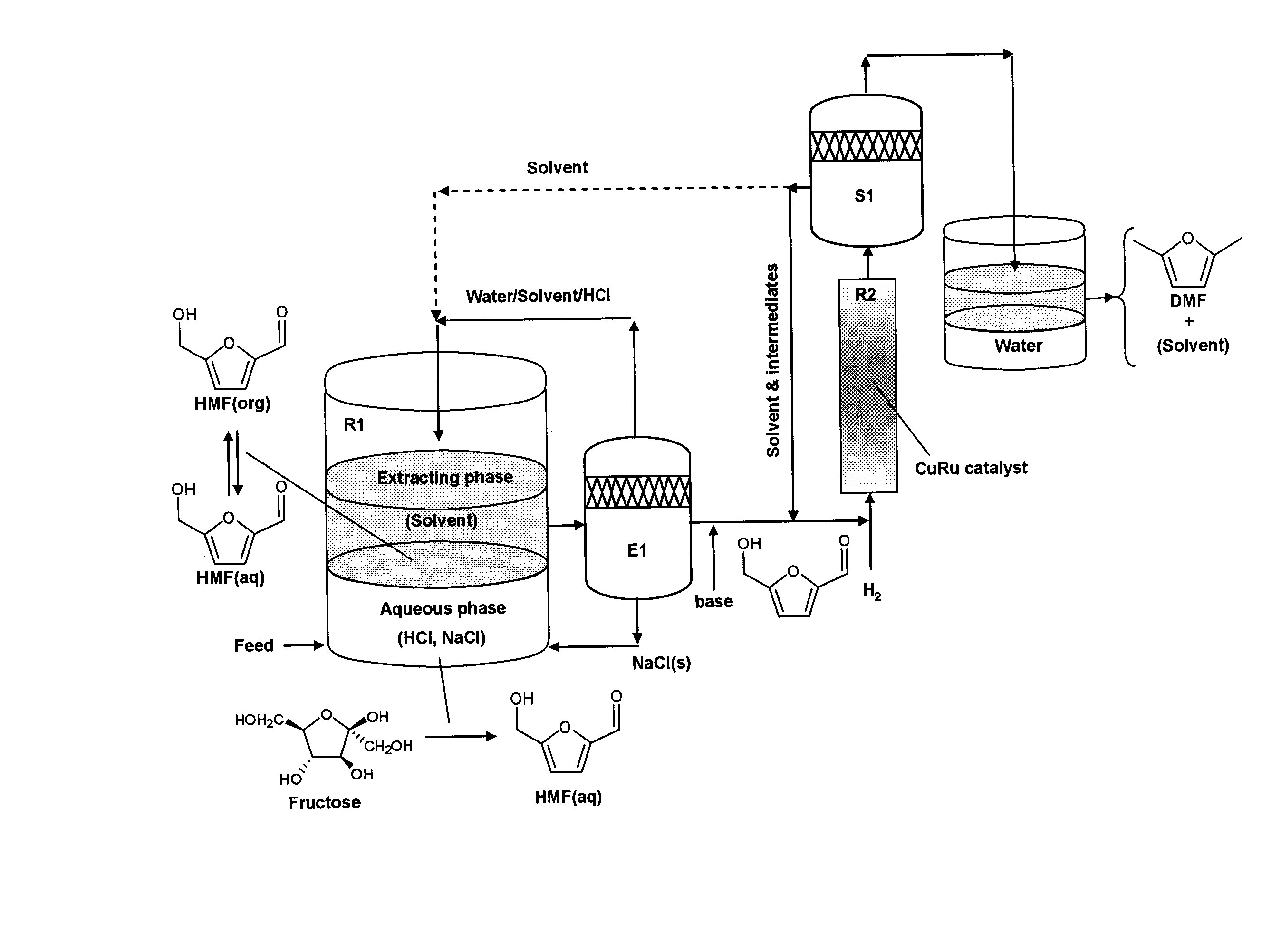
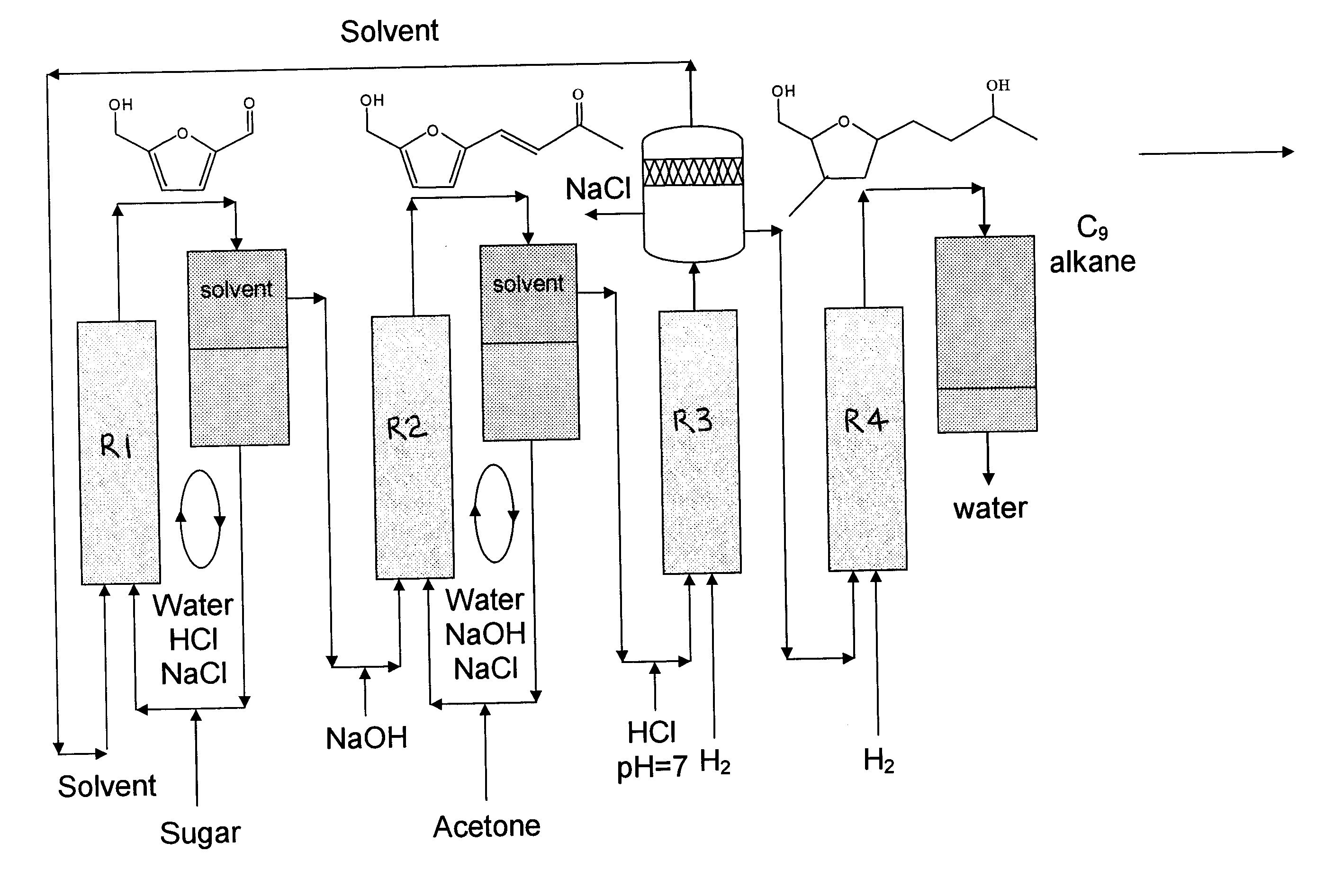
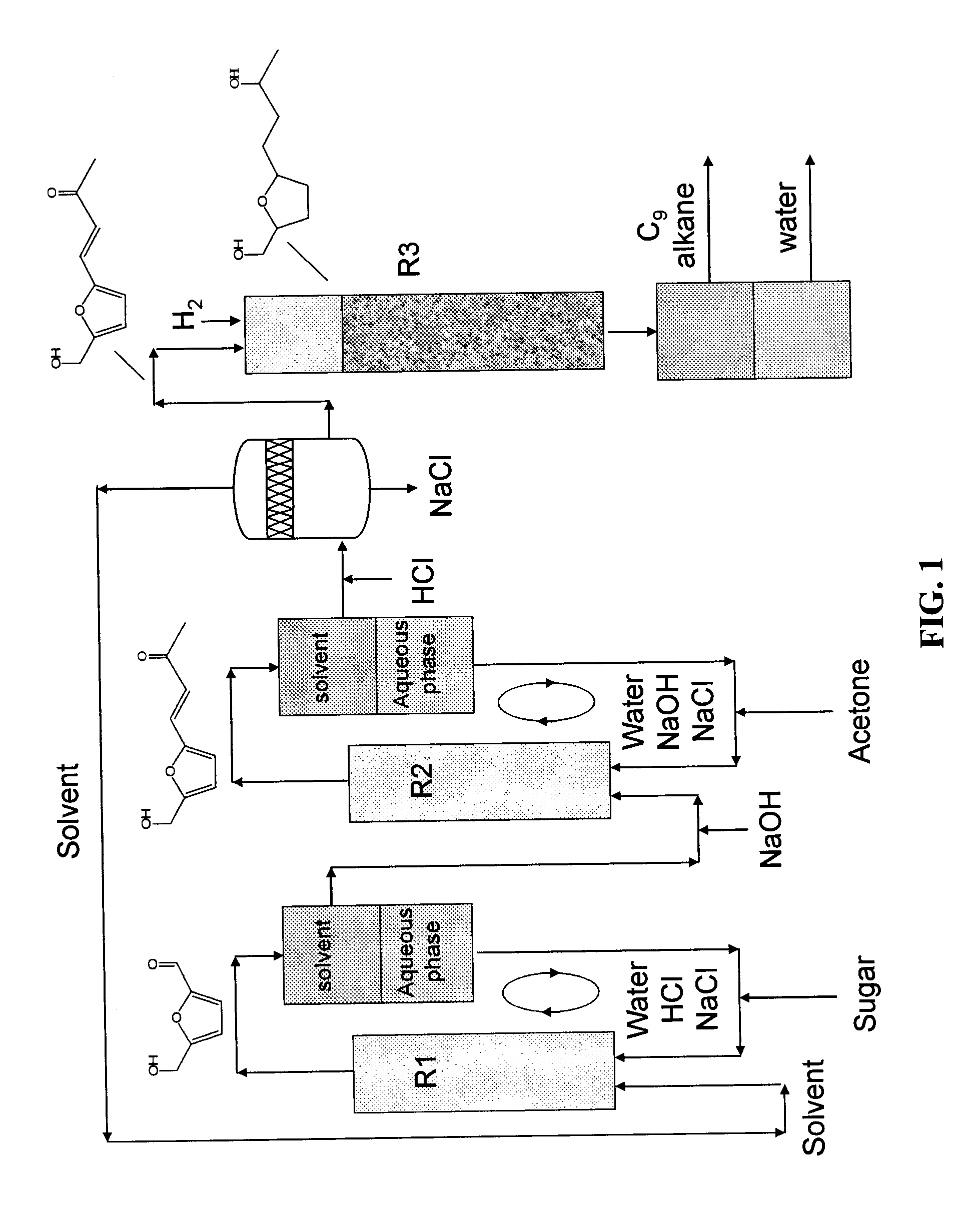
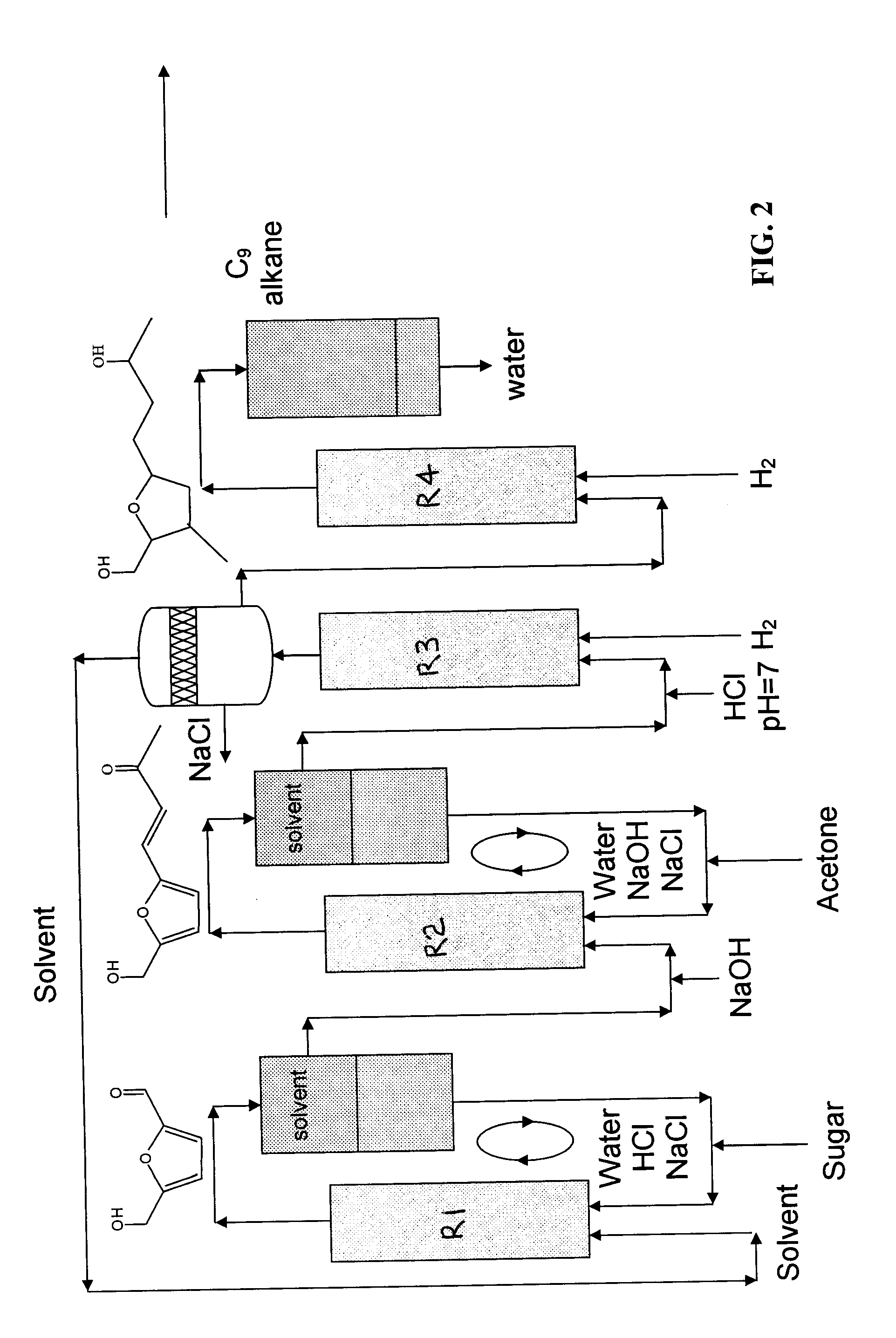
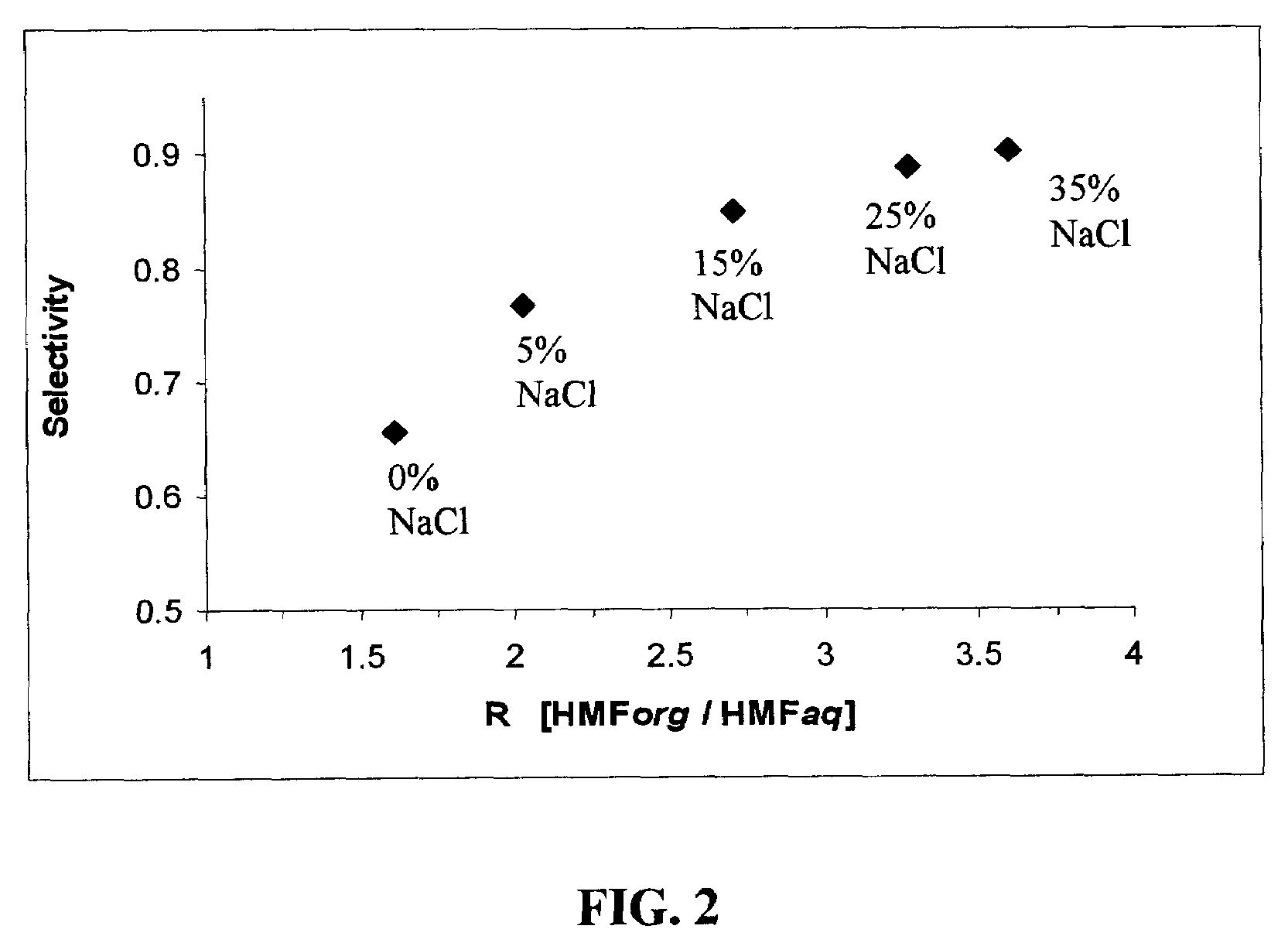
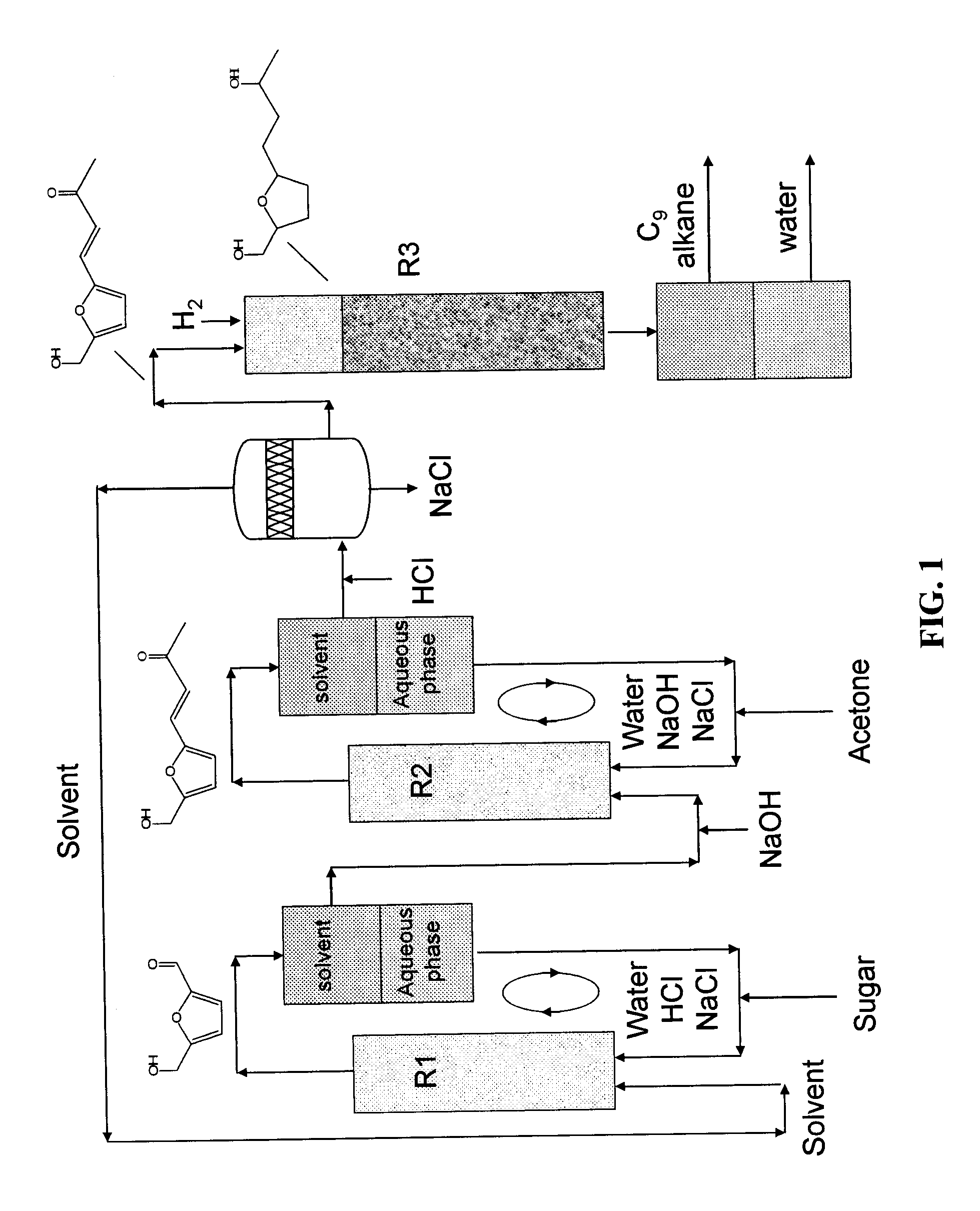
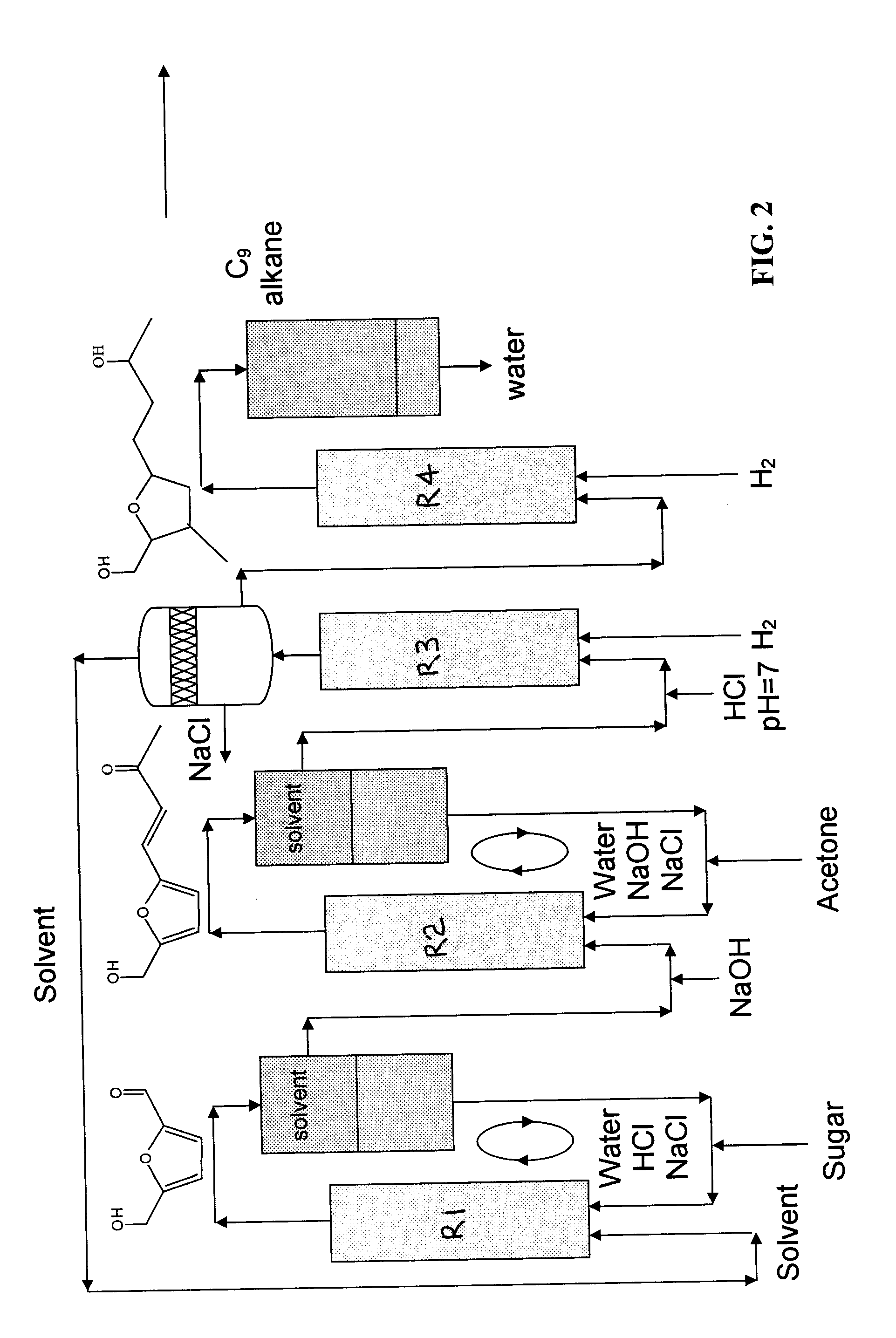
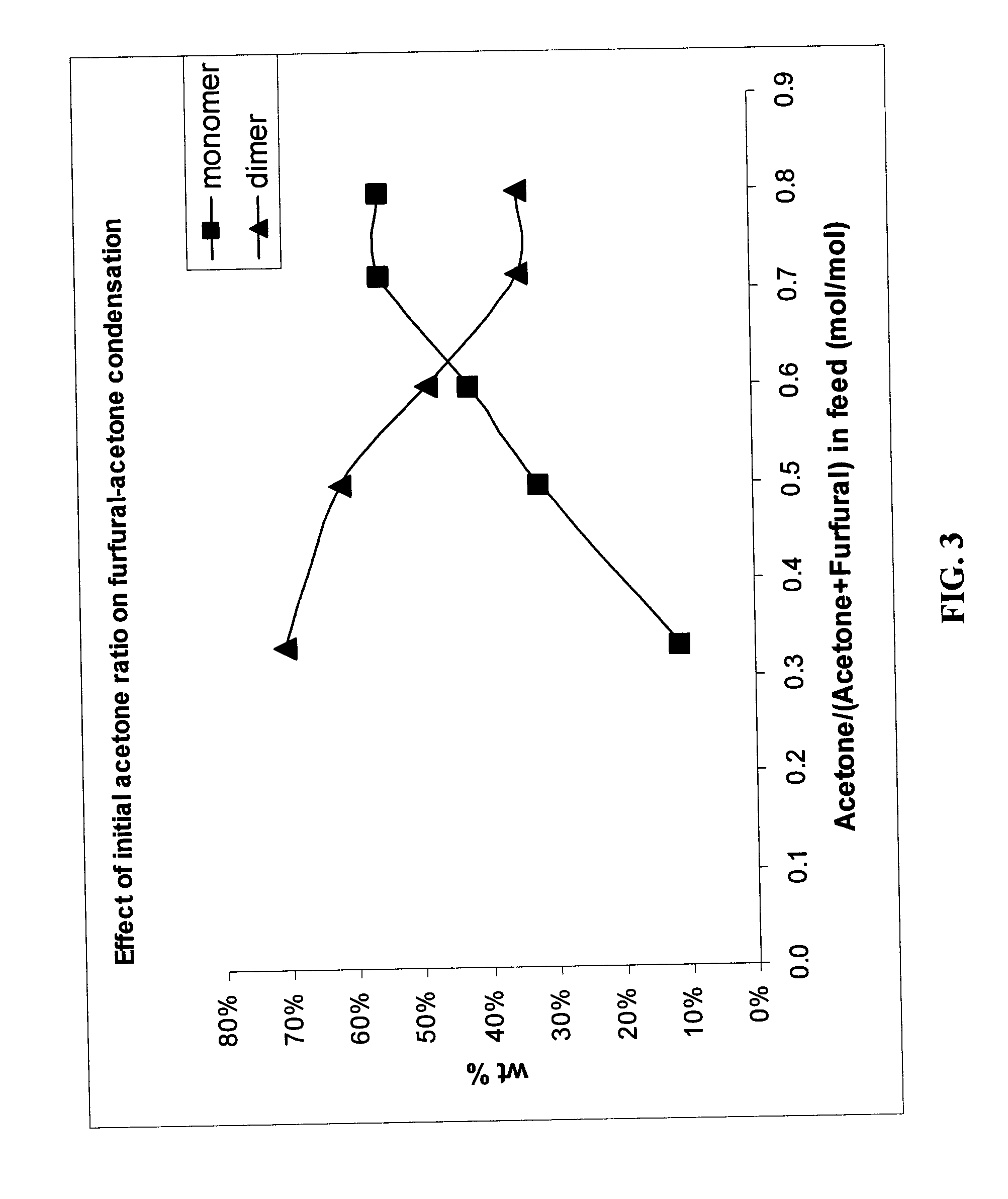
Catalytic process for producing furan derivatives in a biphasic reactor
Described is a catalytic process for converting sugars to furan derivatives (e.g. 5-hydroxymethylfurfural, furfural, dimethylfuran, etc.) using a biphasic reactor containing a reactive aqueous phase and an organic extracting phase. The process provides a cost-effective route for producing di-substituted furan derivatives. The furan derivatives are useful as value-added intermediates to produce polymers, as precursors to diesel fuel, and as fuel additives.
Owner:WISCONSIN ALUMNI RES FOUND
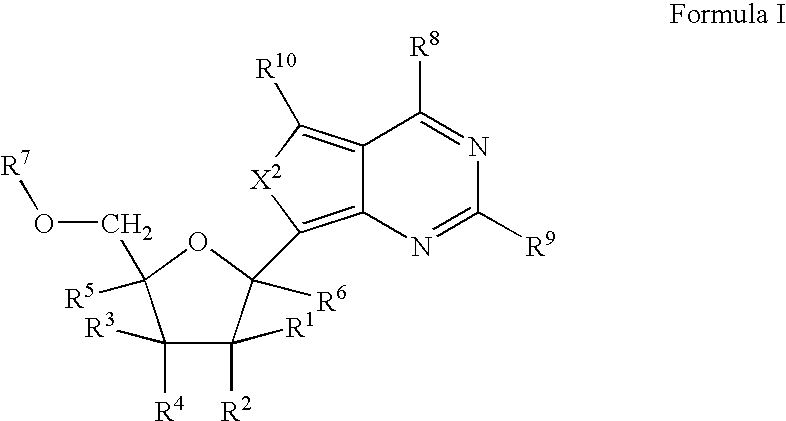
Tetracyclic cyclic GMP-specific phosphodiesterase inhibitors, process of preparation and use
A compound of formula (I) and salts and solvates thereof, in which: R0 represents hydrogen, halogen, or C1-6alkyl; R1 represents hydrogen, C1-6alkyl, C2-6alkenyl, C2-6alkynyl, haloC1-6alkyl, C3-8cycloalkyl, C3-8cycloalkylC1-3alkyl, arylC1-3alkyl, or heteroarylC1-3alkyl; R2 represents an optionally substituted monocyclic aromatic ring selected from benzene, thiophene, furan, and pyridine, or an optionally substituted bicyclic ring (a) attached to the rest of the molecule via one of the benzene ring carbon atoms, and wherein the fused ring (A) is a 5- or 6-membered ring which may be saturated or partially or fully unsaturated, and comprises carbon atoms and optionally one or two heteroatoms selected from oxygen, sulphur, and nitrogen; and R3 represents hydrogen or C1-3alkyl, or R1 and R3 together represent a 3- or 4-membered alkyl or alkenyl chain. A compound of formula (I) is a potent and selective inhibitor of cyclic guanosine 3',5'-monophosphate specific phosphodiesterase (cGMP specific PDE) having a utility in a variety of therapeutic areas where such inhibition is beneficial, including the treatment of cardiovascular disorders and erectile dysfunction.
Owner:ICOS CORP
Particulate material having multiple curable coatings and methods for making and using same
ActiveUS20030224165A1Retain it curabilityPotential bond strengthLiquid surface applicatorsSynthetic resin layered productsFuranParticulates
The present invention relates to coated particulate matter wherein the particles are individually coated with a first set of one or more layers of a curable resin, for example, a combination of phenolic / furan resin or furan resin or phenolic-furan-formaldehyde terpolymer, on a proppant such as sand, and the first set of layers is coated with a second set of one or more layers of a curable resin, for example, a novolac resin with curative. Methods for making and using this coated product as a proppant, gravel pack and for sand control are also disclosed.
Owner:HEXION INC
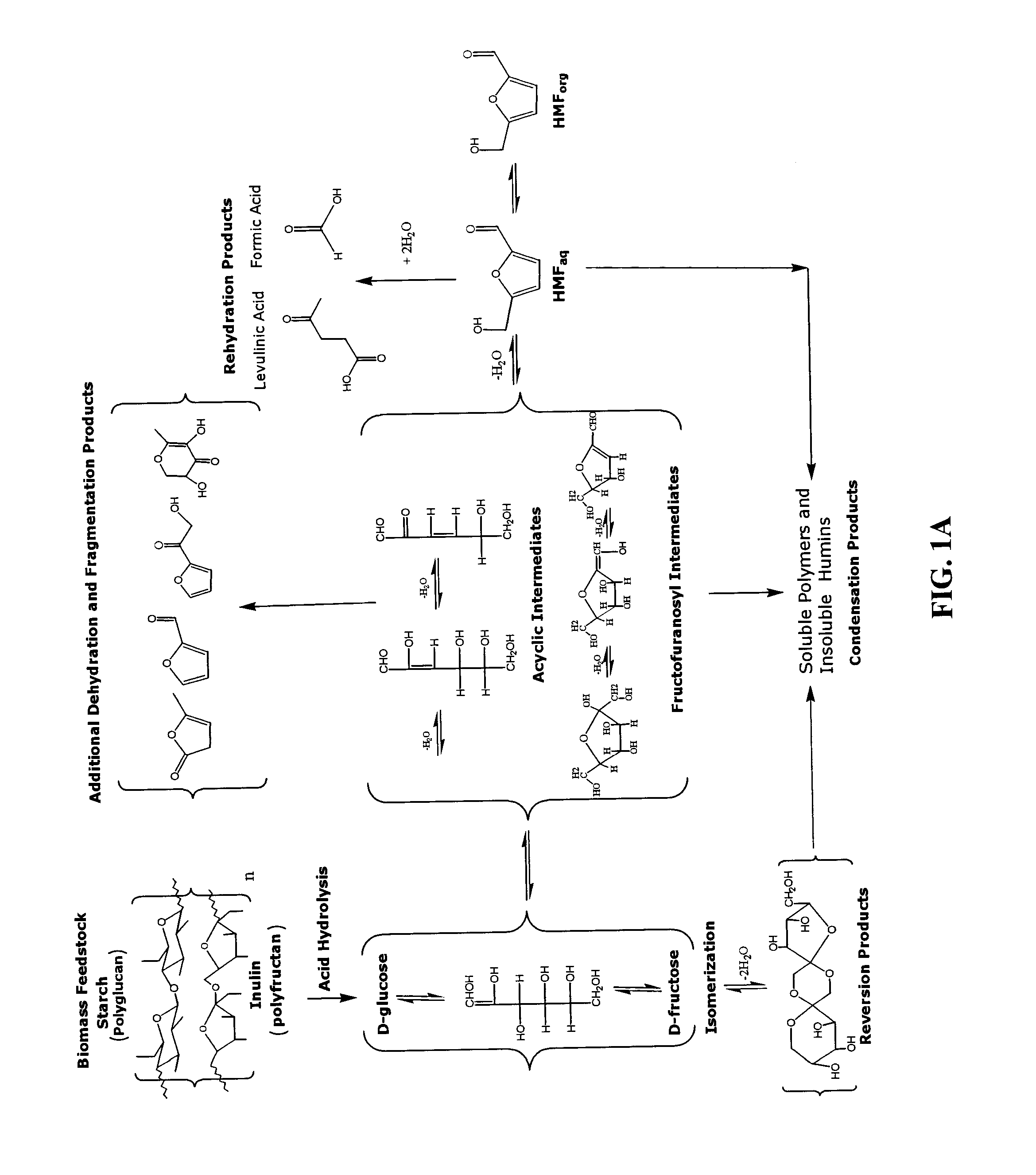
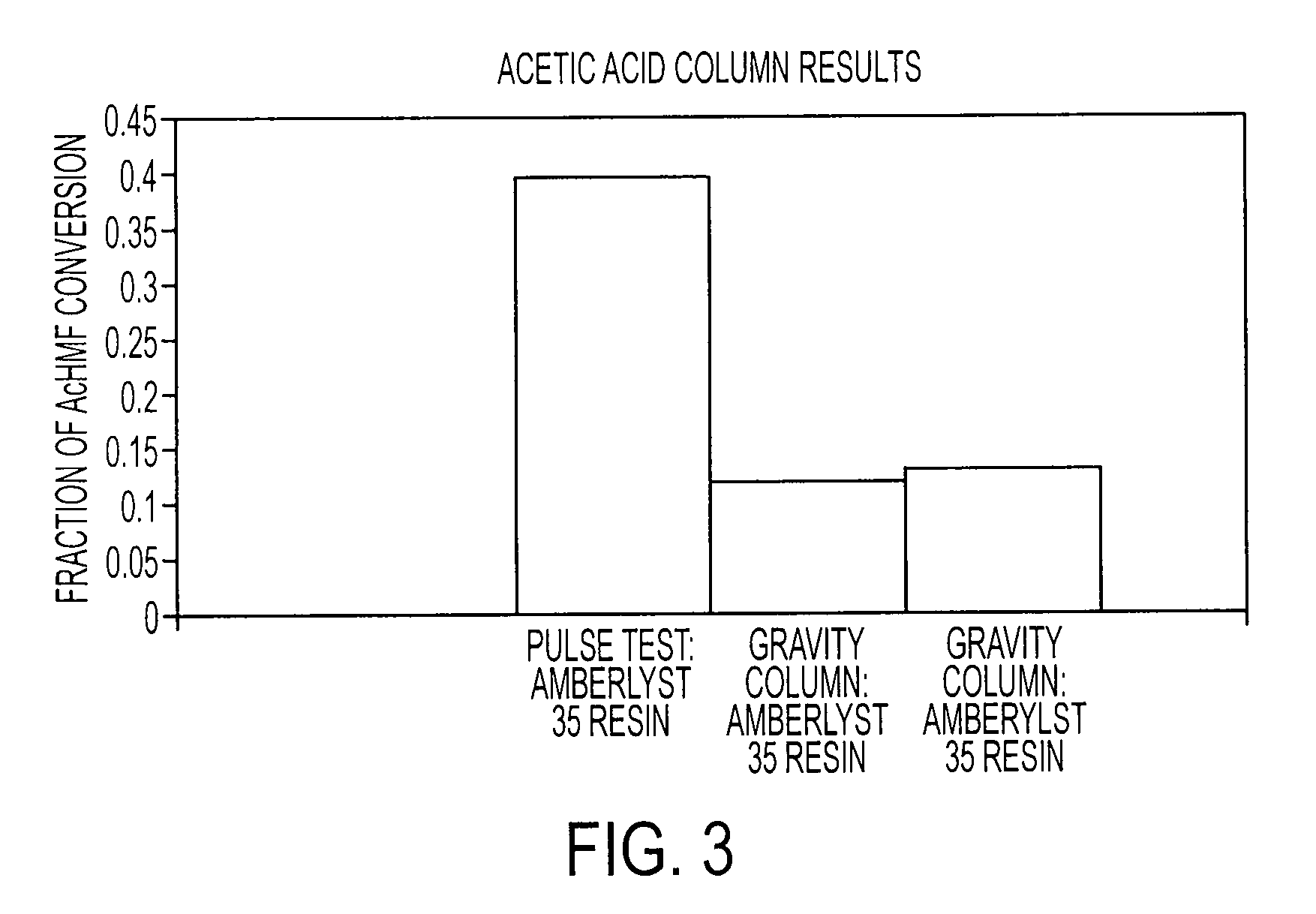
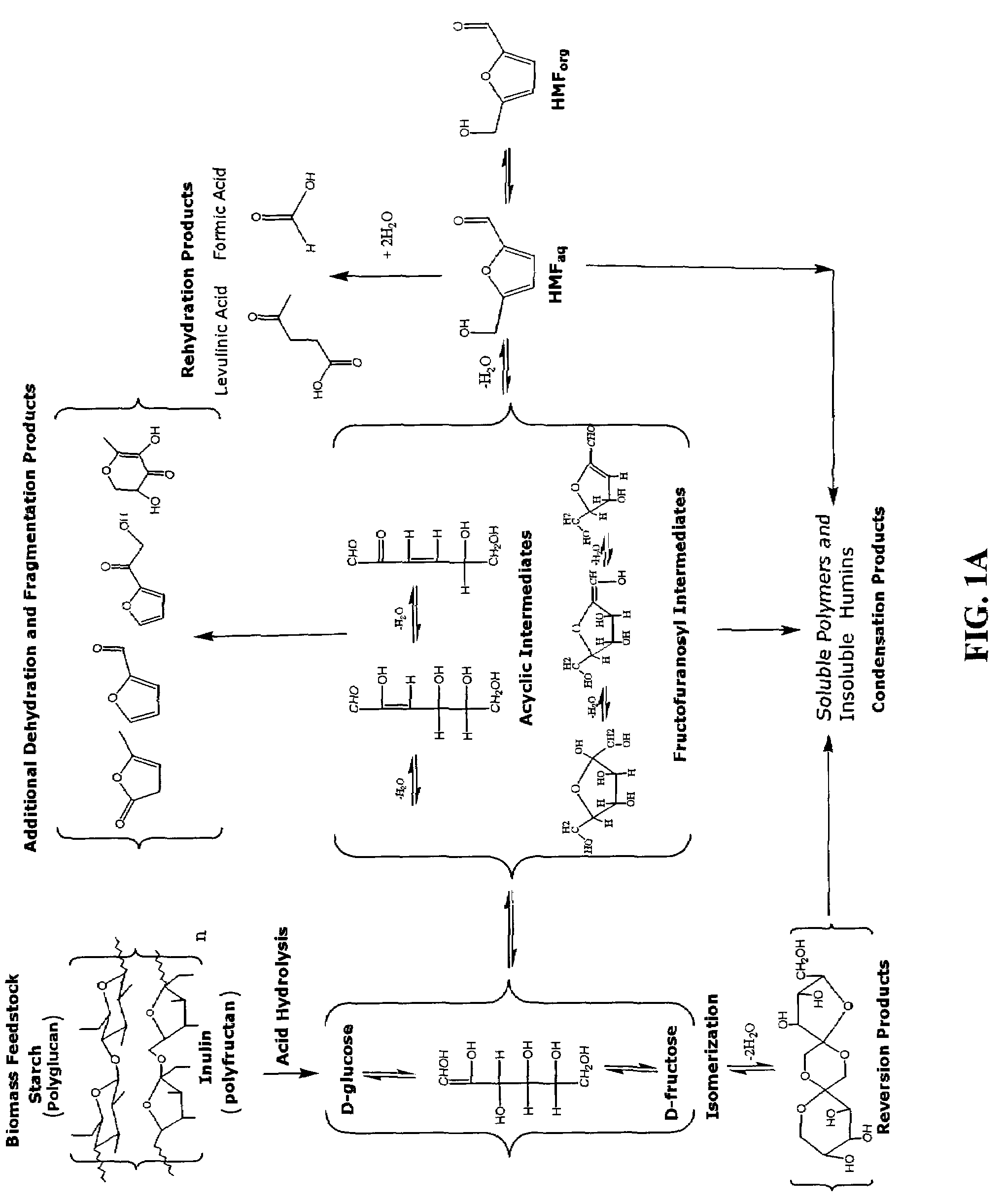
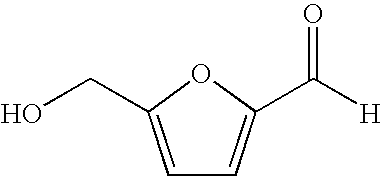
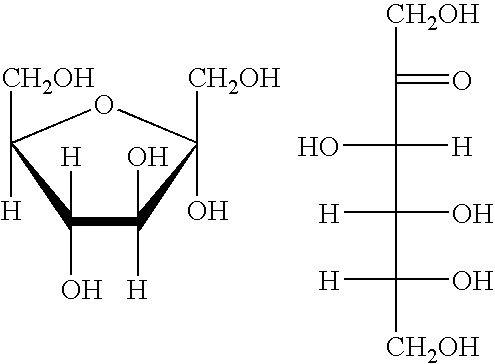
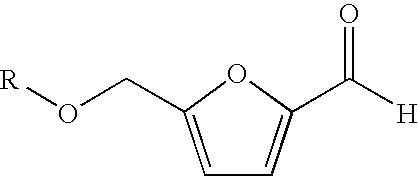
Conversion of carbohydrates to hydroxymethylfurfural (HMF) and derivatives
InactiveUS20090156841A1Increase conversion rateStable formOrganic compound preparationCarboxylic compound preparationMANGANESE ACETATEFuran
A method of producing substantially pure HMF, HMF esters and other derivatives from a carbohydrate source by contacting the carbohydrate source with a solid phase catalyst. A carbohydrate starting material is heated in a solvent in a column and continuously flowed through a solid phase catalyst in the presence of an organic acid, or heated with the organic acid and a solid catalyst in solution to form a HMF ester. Heating without organic acid forms HMF. The resulting product is purified by filtration to remove the unreacted starting materials and catalyst. The HMF ester or a mixture of HMF and HMF ester may then be oxidized to 2,5-furandicarboxylic acid (FDCA) by combining the HMF ester with an organic acid, cobalt acetate, manganese acetate and sodium bromide under pressure. Alternatively, the HMF ester may be reduced to form a furan or tetrahydrofuran diol.
Owner:ARCHER DANIELS MIDLAND CO
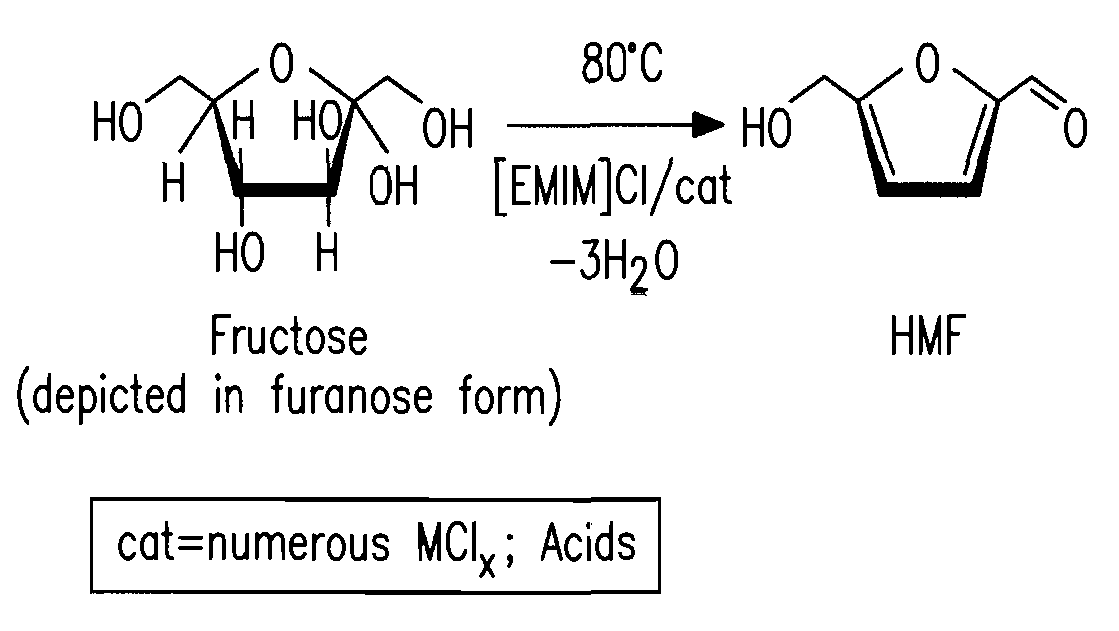
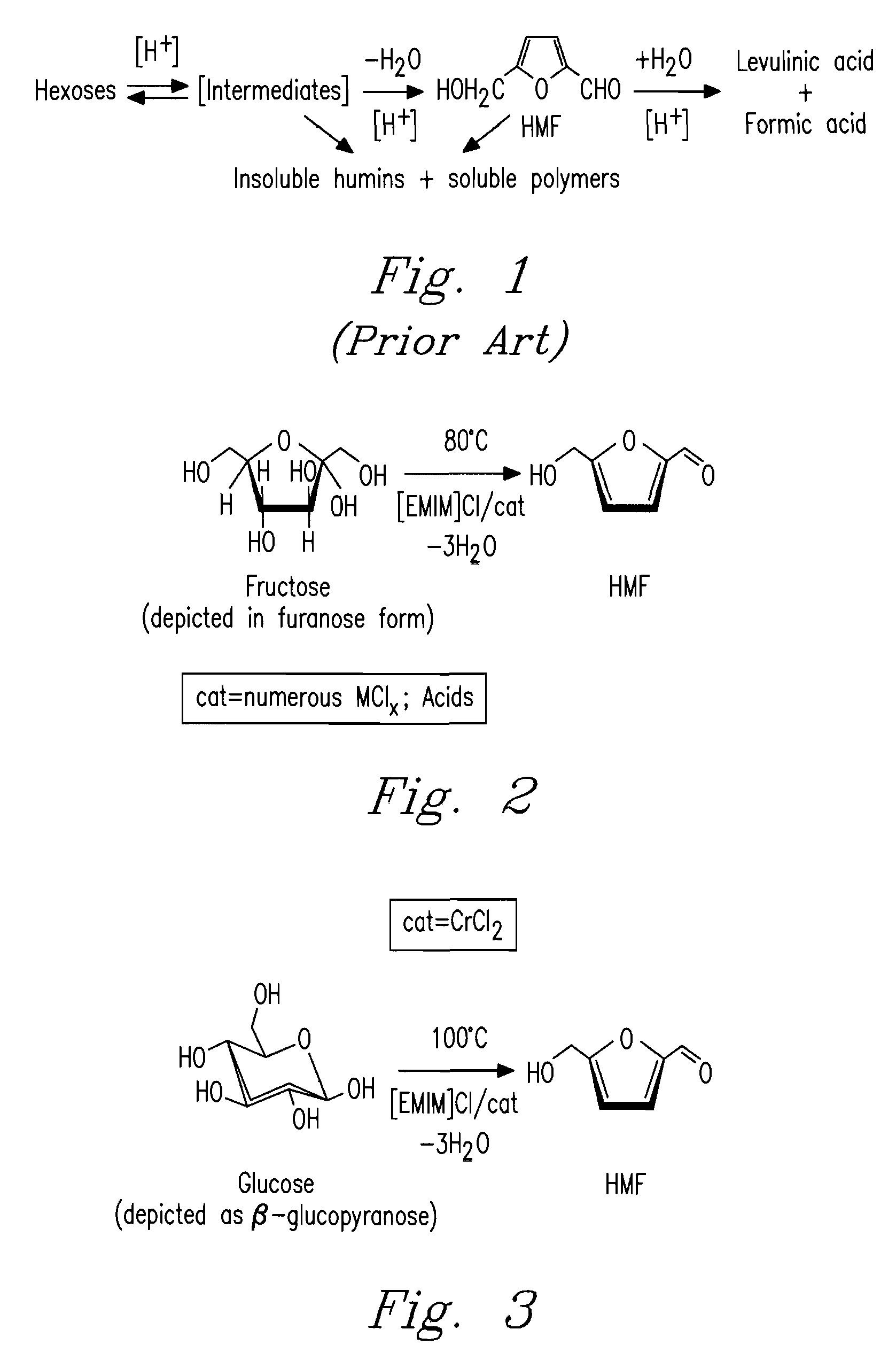
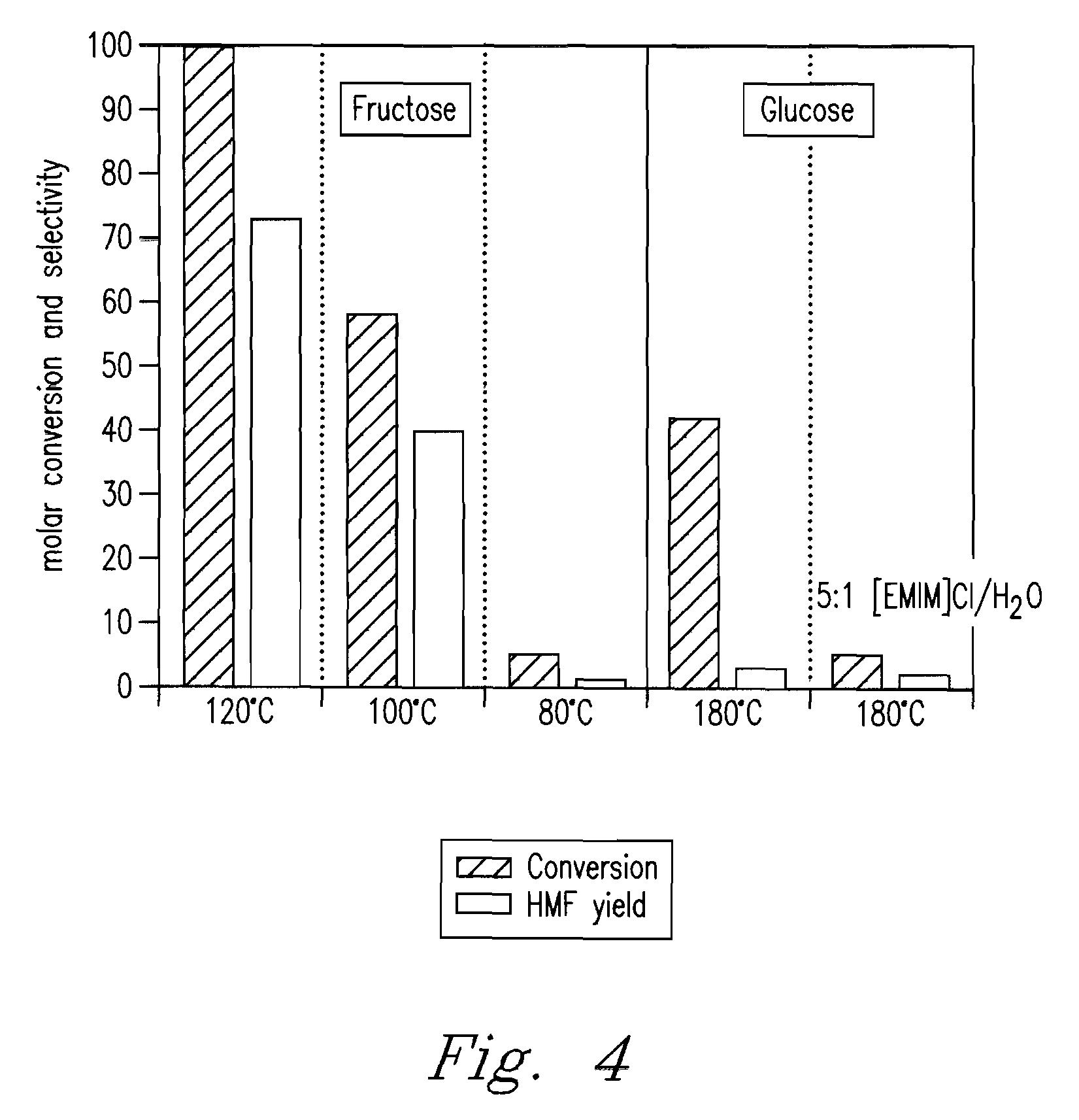
Methods for conversion of carbohydrates in ionic liquids to value-added chemicals
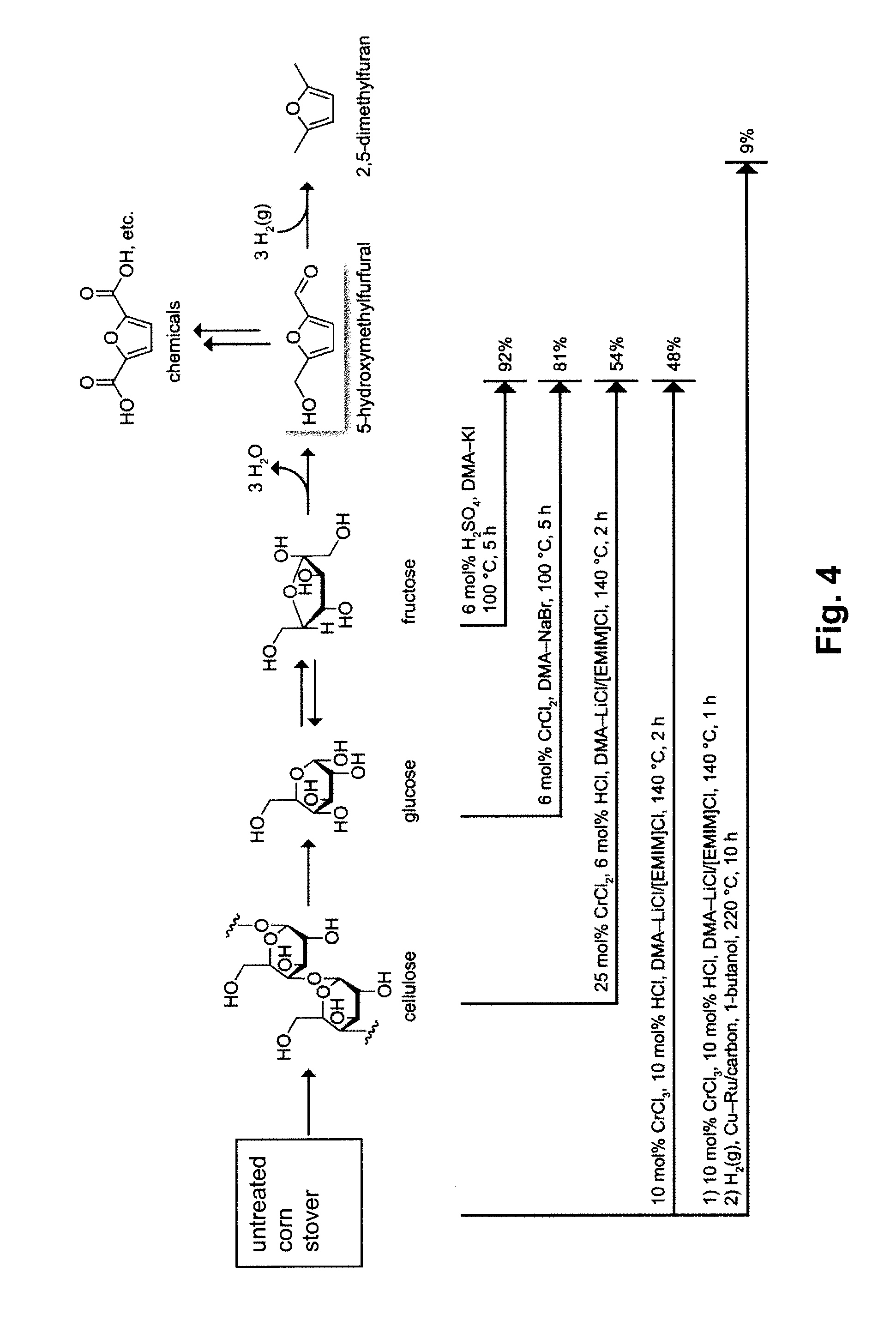
Methods are described for converting carbohydrates including, e.g., monosaccharides, disaccharides, and polysaccharides in ionic liquids to value-added chemicals including furans, useful as chemical intermediates and / or feedstocks. Fructose is converted to 5-hydroxylmethylfurfural (HMF) in the presence of metal halide and acid catalysts. Glucose is effectively converted to HMF in the presence of chromium chloride catalysts. Yields of up to about 70% are achieved with low levels of impurities such as levulinic acid.
Owner:BATTELLE MEMORIAL INST
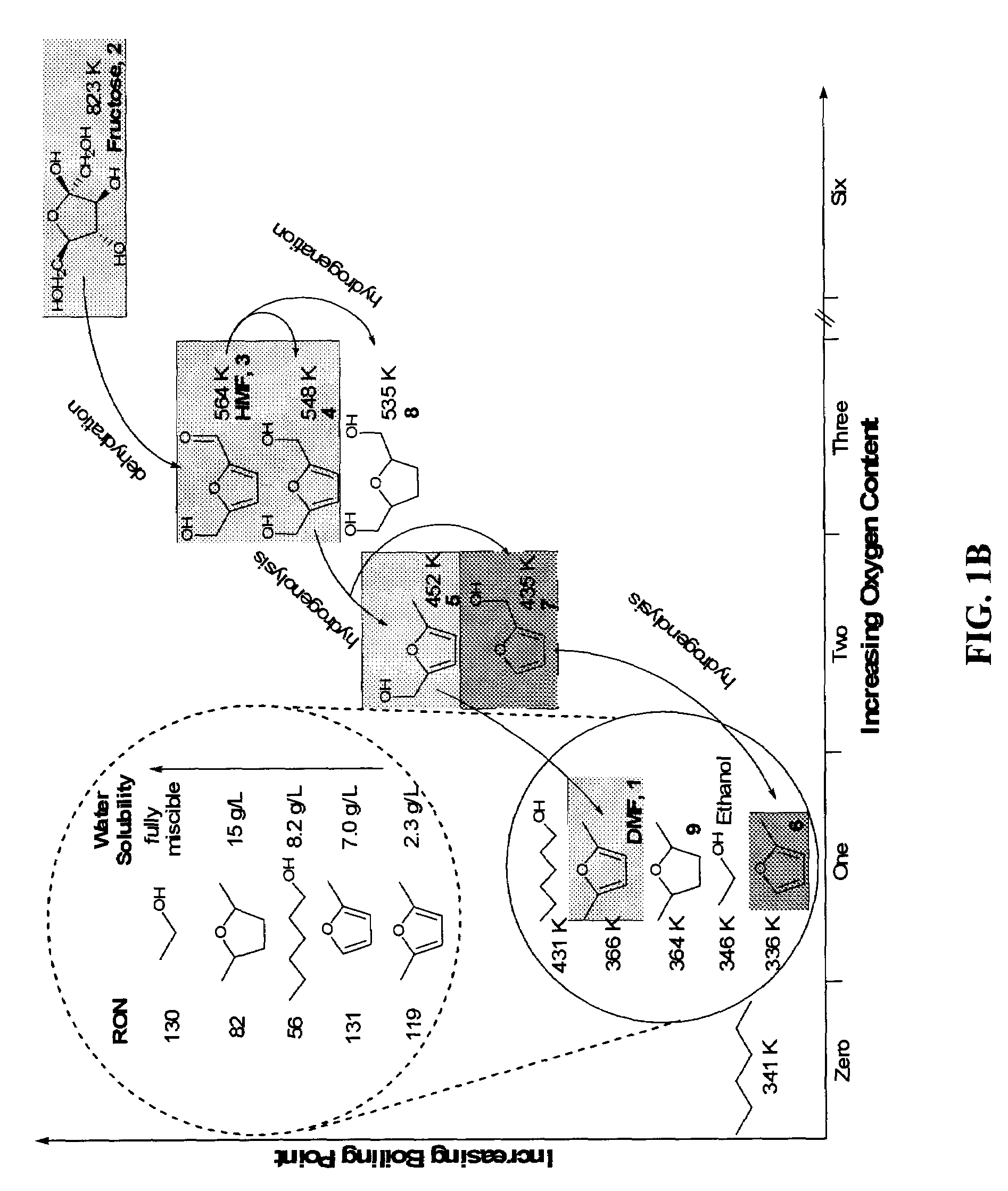
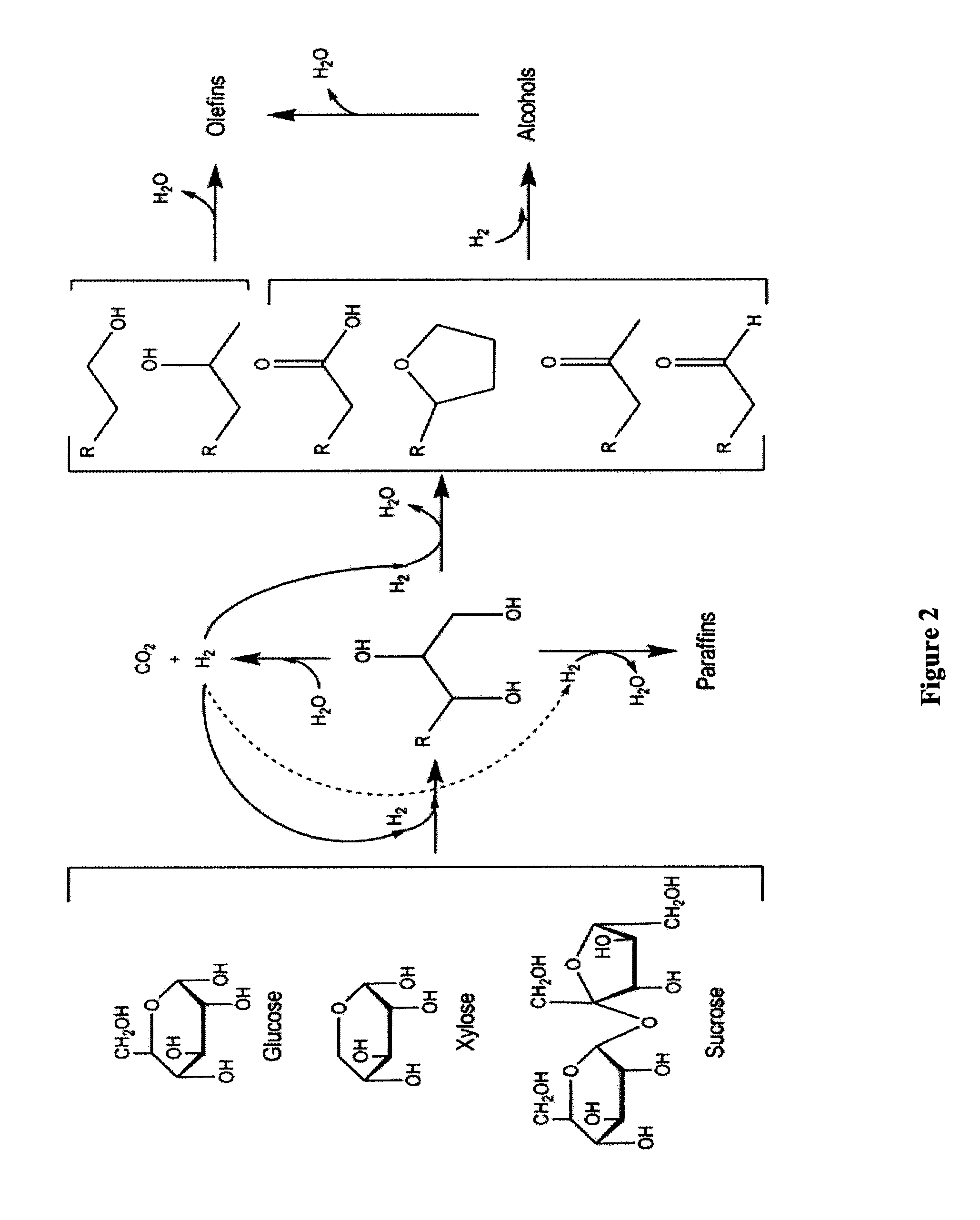
Production of liquid alkanes in the jet fuel range (c8-c15) from biomass-derived carbohydrates
Described is a method for making a composition comprising alkanes. The composition is suitable for use as a liquid transportation fuel in general, and jet fuel in particular. The method includes dehydrating a feedstock solution comprising a carbohydrate, in the presence of an acid catalyst, to yield at least one furan derivative compound, in a reaction vessel containing a biphasic reaction medium: an aqueous reaction solution and a substantially immiscible organic extraction solution. The furan derivative compound is then subjected to a self-aldol condensation reaction or a crossed-aldol condensation reaction with another carbonyl compound to yield a beta-hydroxy carbonyl compound and / or an alpha-beta unsaturated carbonyl compound. The beta-hydroxy carbonyl and / or alpha-beta unsaturated compounds are then hydrogenated to yield a saturated or partially saturated compound, followed by hydrodeoxygenation (e.g., dehydrating and hydrogenating) of the saturated or partially saturated compound to yield a composition of matter comprising alkanes.
Owner:WISCONSIN ALUMNI RES FOUND
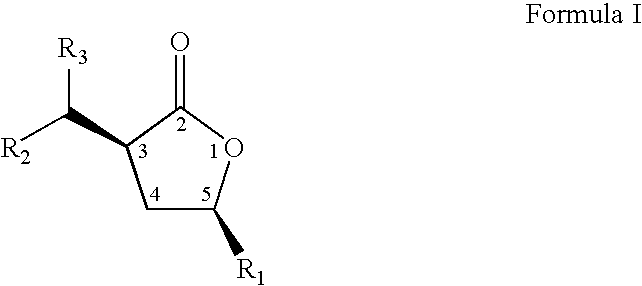
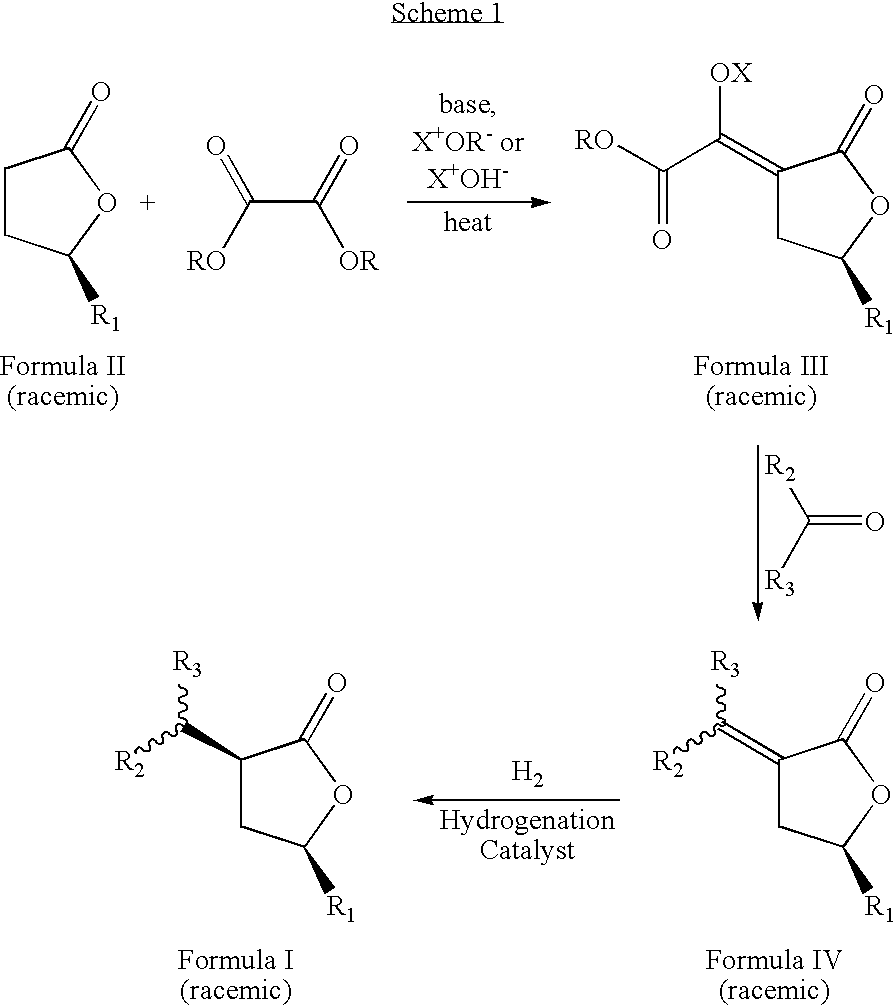
Cis-3,5-disubstituted-dihydro-furan-2-ones and the preparation and use thereof
The present invention relates to an improved process to prepare cis-3-dihydrocarbylmethano-5-hydrocarbyidihydro-furan-2-ones. The present invention also relates to novel compositions of matter comprising enantiomerically pure cis-3-dihydrocarbylmethano-5-hydrocarbyldihydro-furan-2-ones, being the (3S,5S), (3R,5R), (3S,5R), or (3R,5S) optically pure isomers, and a new, more cost efficient process to prepare said optically pure isomers.
Owner:EI DU PONT DE NEMOURS & CO
Pharmaceutical co-crystal compositions
InactiveUS20070026078A1Improve solubilityLow hygroscopicityBiocidePowder deliveryThioketoneHydroxamic acid
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphonic acid, phosphinic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, sp2 amine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, s-heterocyclic ring, thiophene, n-heterocyclic ring, pyrrole, o-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES +2
Vinylogous 4H-pyrones and their use in promoting plant growth
Owner:UNIV OF WESTERN AUSTRALIA
Catalytic process for producing furan derivatives in a biphasic reactor
Owner:WISCONSIN ALUMNI RES FOUND
Preparation method of aviation kerosene or diesel
The present invention relates to a novel synthetic route of a liquid chain hydrocarbon fuel totally independent of fossil energy based on a lignocellulose raw material to obtain a platform compound. The method includes three parts: 1) preparing oxygen-containing organic compounds with carbon chain length of 8-16 through the acid-catalyzed alkylation reaction by taking lignocelluloses-based carbonyl-containing platform compounds and furan platform compounds as raw materials on a novel solid catalyst; 2) effectively removing carbon-carbon double bonds and carbon-oxygen double bonds to prepare saturated oxygen-containing organic compounds by hydrogenation of the alkylated product; and 3) conducting hydrodeoxygenation for the hydrogenated alkylation product by using a metal-solid acid bifunctional catalyst to obtain the biomass aviation kerosene or high grade diesel fuel with the carbon chain length of 8-16 and having a high energy density and stability.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
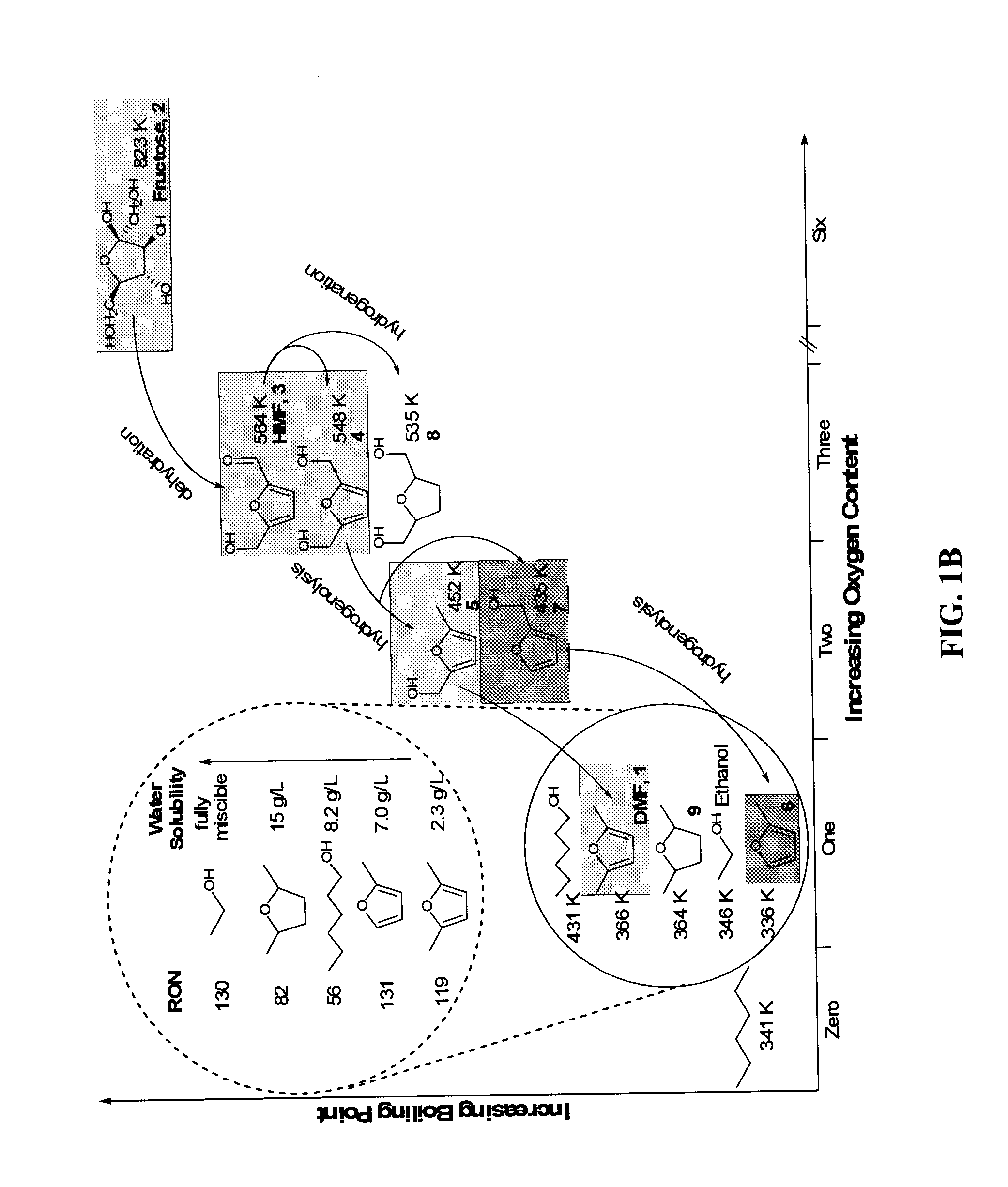
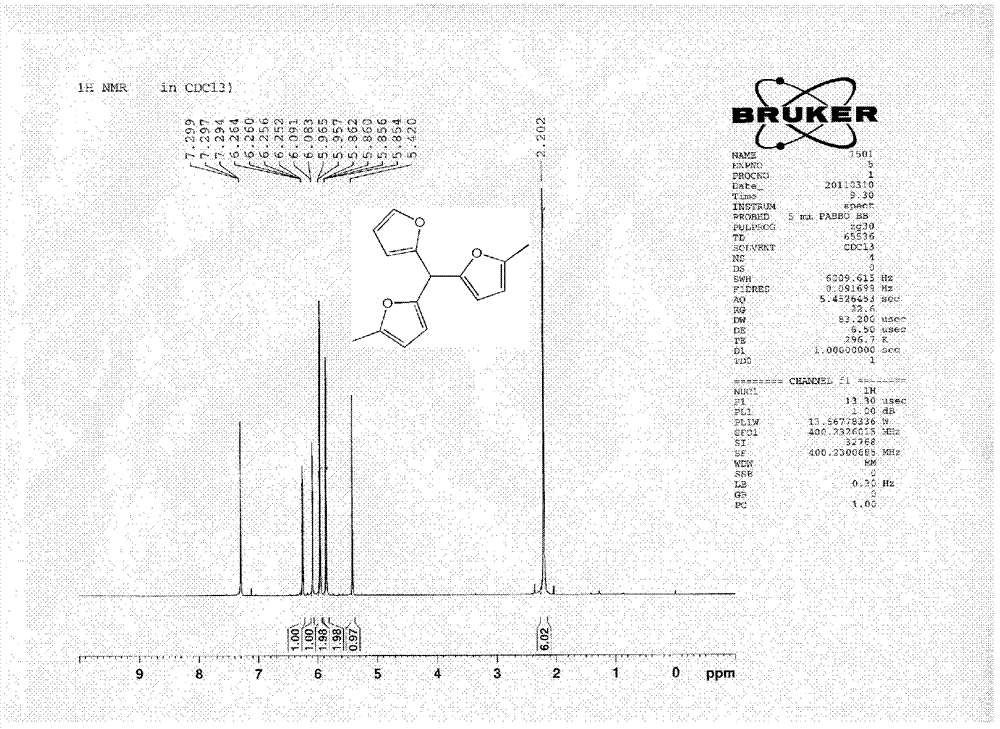
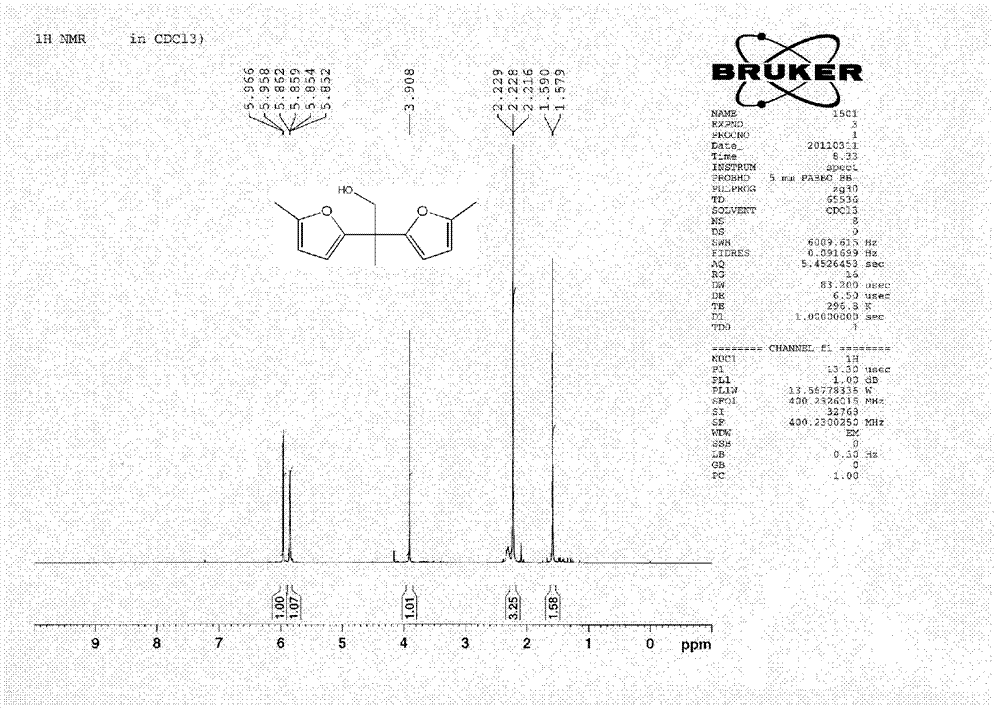
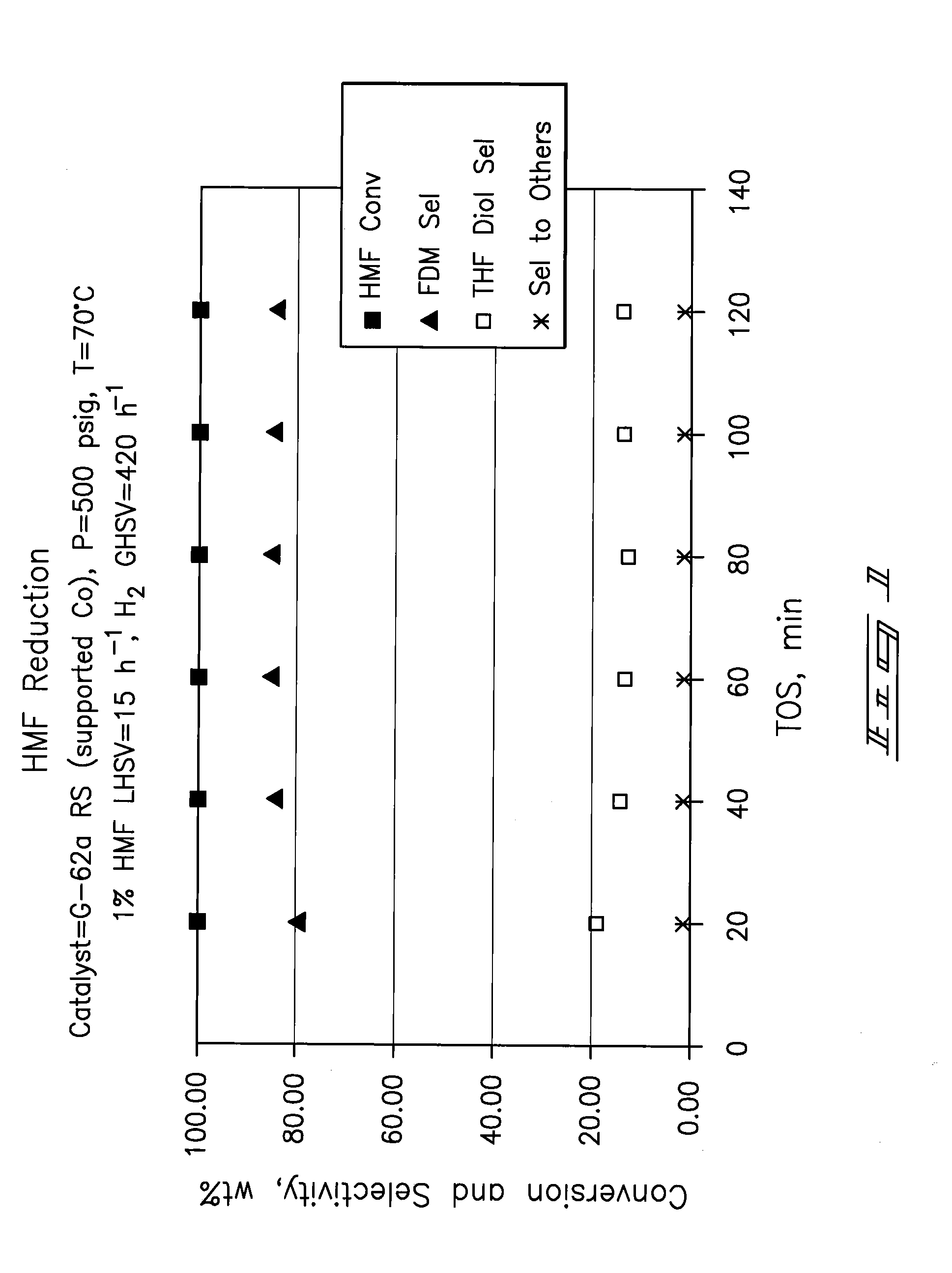
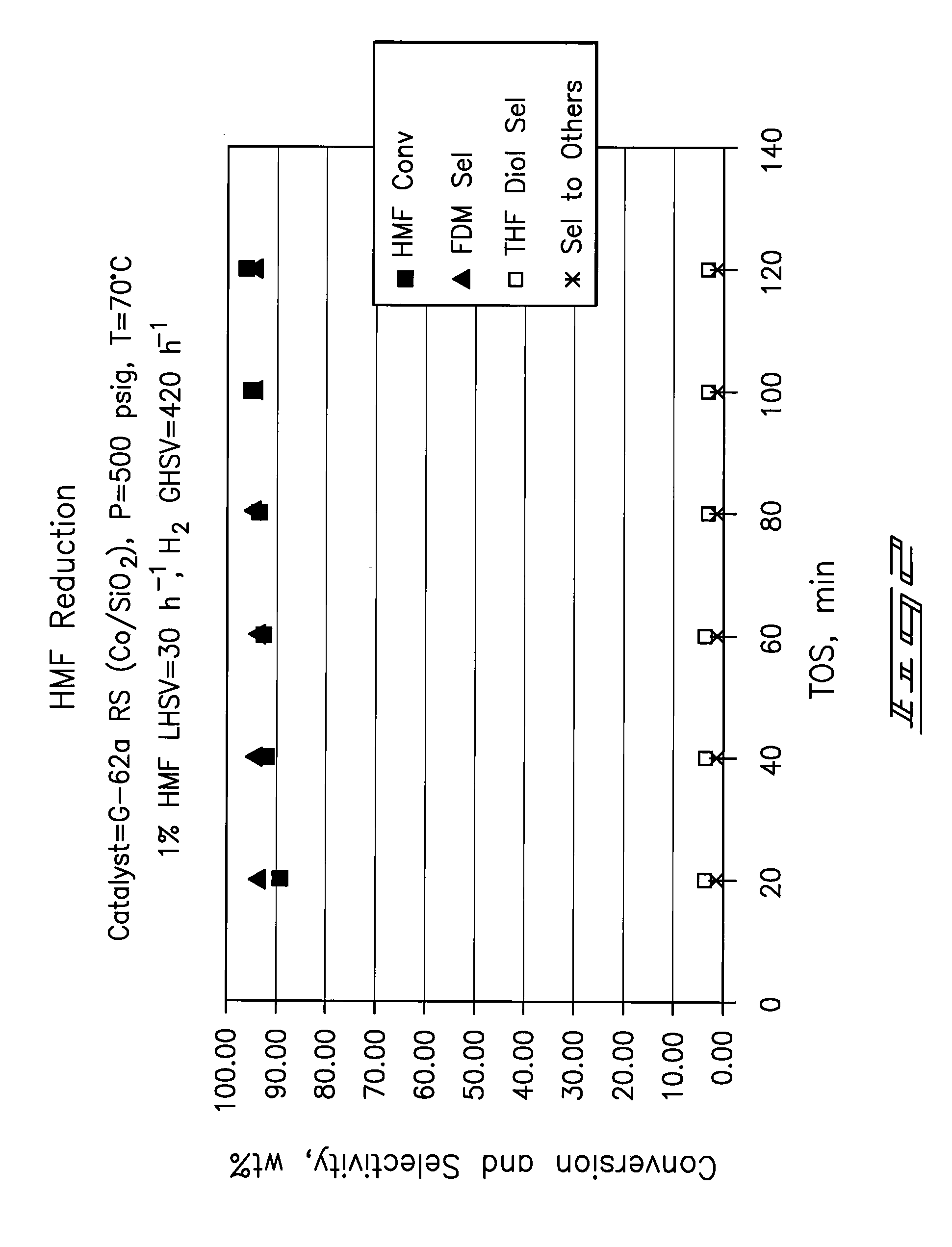
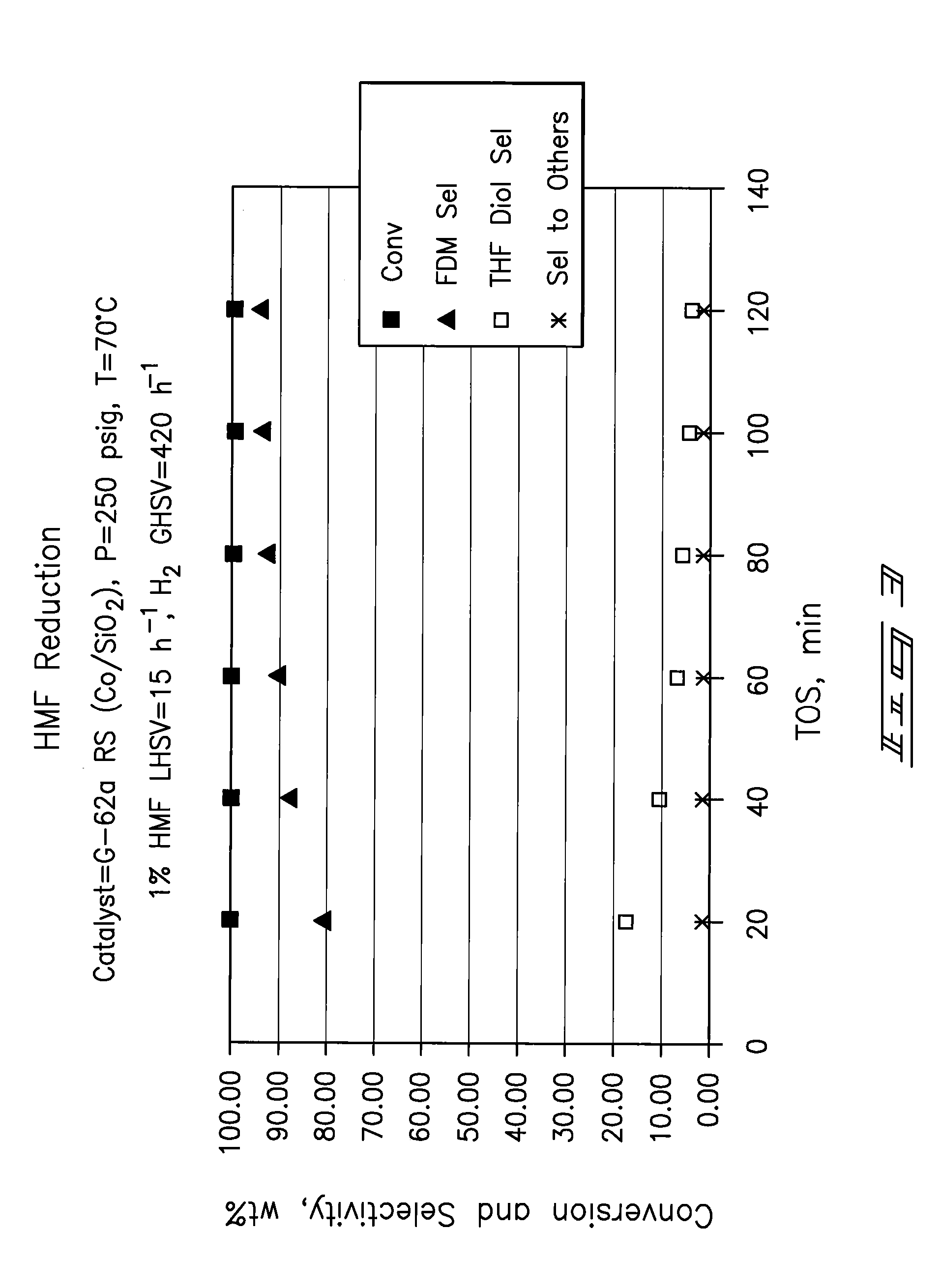
Hydroxymethylfurfural Reduction Methods and Methods of Producing Furandimethanol
A method of reducing hydroxymethylfurfural (HMF) where a starting material containing HMF in a solvent comprising water is provided. H2 is provided into the reactor and the starting material is contacted with a catalyst containing at least one metal selected from Ni, Co, Cu, Pd, Pt, Ru, Ir, Re and Rh, at a temperature of less than or equal to 250° C. A method of hydrogenating HMF includes providing an aqueous solution containing HMF and fructose. H2 and a hydrogenation catalyst are provided. The HMF is selectively hydrogenated relative to the fructose at a temperature at or above 30° C. A method of producing tetrahydrofuran dimethanol (THFDM) includes providing a continuous flow reactor having first and second catalysts and providing a feed comprising HMF into the reactor. The feed is contacted with the first catalyst to produce furan dimethanol (FDM) which is contacted with the second catalyst to produce THFDM.
Owner:BATTELLE MEMORIAL INST
Processes for the preparation and purification of hydroxymethylfuraldehyde and derivatives
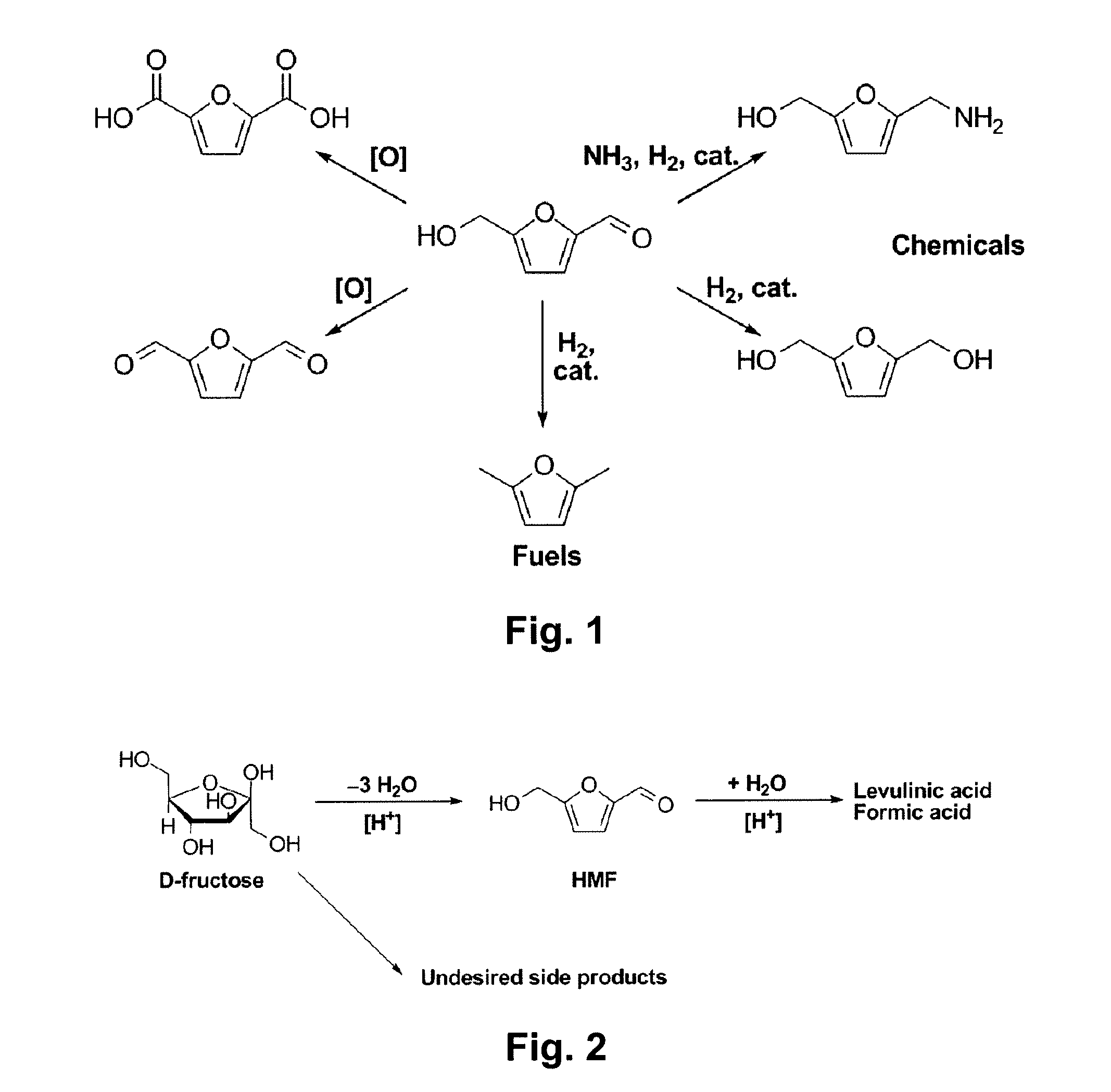
A method for utilizing an industrially convenient fructose source for a dehydration reaction converting a carbohydrate to a furan derivative is provided. Recovery methods also are provided. Embodiments of the methods improve upon the known methods of producing furan derivatives.
Owner:ARCHER DANIELS MIDLAND CO
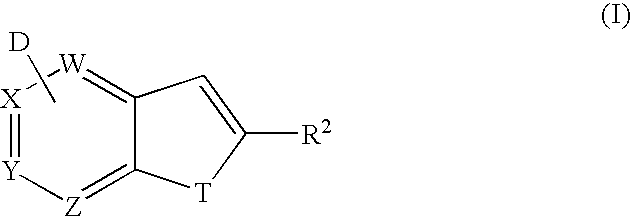
Method for synthesis of AZA-annelated pyrroles, thiophenes, and furans
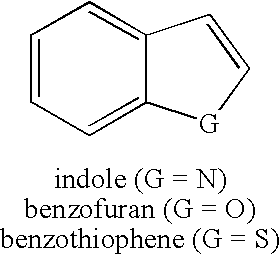
Methods of synthesis of intermediates that are useful as bioisosteres of the indole, benzofuran and benzothiophene scaffold are disclosed.
Owner:ADESIS
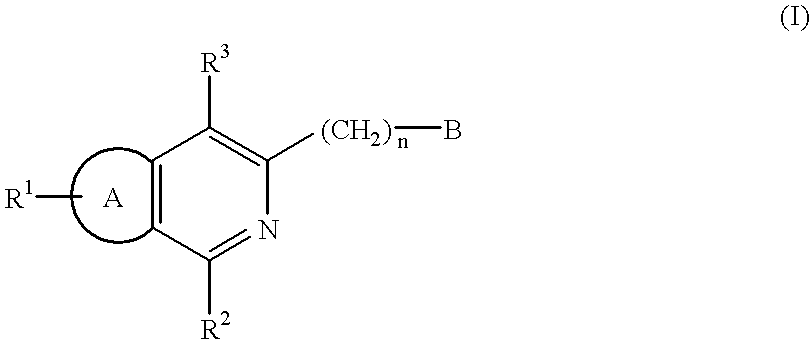
Certain 1,3-disubstituted isoquinoline derivatives
Condensed pyridine compounds represented by formula (I):wherein: R<1 >and R<3 >are, independently, hydrogen, halogen, lower alkyl, or lower alkoxy; R<2 >represents an amino substituent; ring A is a benzene ring, pyridine ring, thiophene ring, or furan ring; and B represents a substituent containing a ring structure. Also, pharmaceutically acceptable salt and hydrates thereof. These compounds are clinically useful medicaments having; serotonin antagonism, and in particular, for treating, ameliorating, or preventing spastic paralysis. They are also useful as central muscle relaxants for ameliorating myotonia.
Owner:EISIA R&D MANAGEMENT CO LTD
Carba-nucleoside analogs for antiviral treatment
Owner:GILEAD SCI INC
Human sweat malodor counteractant composition and process for using same
InactiveUS6379658B1Increase in level of perceived pleasantnessMalodor-reducing concentrationBiocideCosmetic preparationsFuranPropionate
Described is a process and compositions for counteracting human sweat malodor. The compositions containing at least 10 weight percent of either 3,7-dimethyl-2,6-nonadienenitrile or 3,7-dimethyl-2,6-octadienenitrile. Additional ingredients may be added including napthyl methyl ether, methyl beta-naphthyl ketone, benzyl acetone, methanoinden propionates, methyl ionone, tetramethylnaphtho furan, ethylene glycol cyclic ester of dodecanedioic acid, 1-cyclohexadecen-6-one, 1-cycliheptadecen- 10-one, and corn mint oil. These compositions are applied to either fabric or a defined surface area of the human epidermis.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
Production of liquid alkanes in the jet fuel range (C8-C15) from biomass-derived carbohydrates
ActiveUS7880049B2High selectivityHydrocarbon from oxygen organic compoundsLiquid hydrocarbon mixture productionAlkaneFuran
Described is a method for making a composition comprising alkanes. The composition is suitable for use as a liquid transportation fuel in general, and jet fuel in particular. The method includes dehydrating a feedstock solution comprising a carbohydrate, in the presence of an acid catalyst, to yield at least one furan derivative compound, in a reaction vessel containing a biphasic reaction medium: an aqueous reaction solution and a substantially immiscible organic extraction solution. The furan derivative compound is then subjected to a self-aldol condensation reaction or a crossed-aldol condensation reaction with another carbonyl compound to yield a beta-hydroxy carbonyl compound and / or an alpha-beta unsaturated carbonyl compound. The beta-hydroxy carbonyl and / or alpha-beta unsaturated compounds are then hydrogenated to yield a saturated or partially saturated compound, followed by hydrodeoxygenation (e.g., dehydrating and hydrogenating) of the saturated or partially saturated compound to yield a composition of matter comprising alkanes.
Owner:WISCONSIN ALUMNI RES FOUND
Lithium ion battery composite cathode material and preparation method
InactiveCN101604743APromote circulationImprove cycle performanceElectrode manufacturing processesActive material electrodesFuranEpoxy
The invention relates to a lithium ion battery composite cathode material and a preparation method. The cathode material comprises a hard carbon precursor material containing hetero atoms, and natural spherical graphite, wherein the hard carbon precursor material selects one or more than two of phenolic resin, polyfurfural, furan resin, polyvinyl alcohol, epoxide resin or polyacrylonitrile as raw materials, and accounts for 5 to 45 percent of the natural spherical graphite by mass; modifier of the hetero atoms is boron-containing modifier including boric acid and diboron trioxide, phosphor-containing modifier including phosphoric acid and phosphorus pentoxide, and nitrogen-containing modifier HNO3; and one of the modifiers accounts for 5 to 35 percent of the hard carbon precursor by mass. An analog battery formed by the cathode material which is prepared by the method has the capacity over 350 mAH / g and first coulomb efficiency up to 95.8 percent, and has good circulation performance. The preparation method has the advantages of low cost and simple process.
Owner:珠海华丽新能源科技有限公司
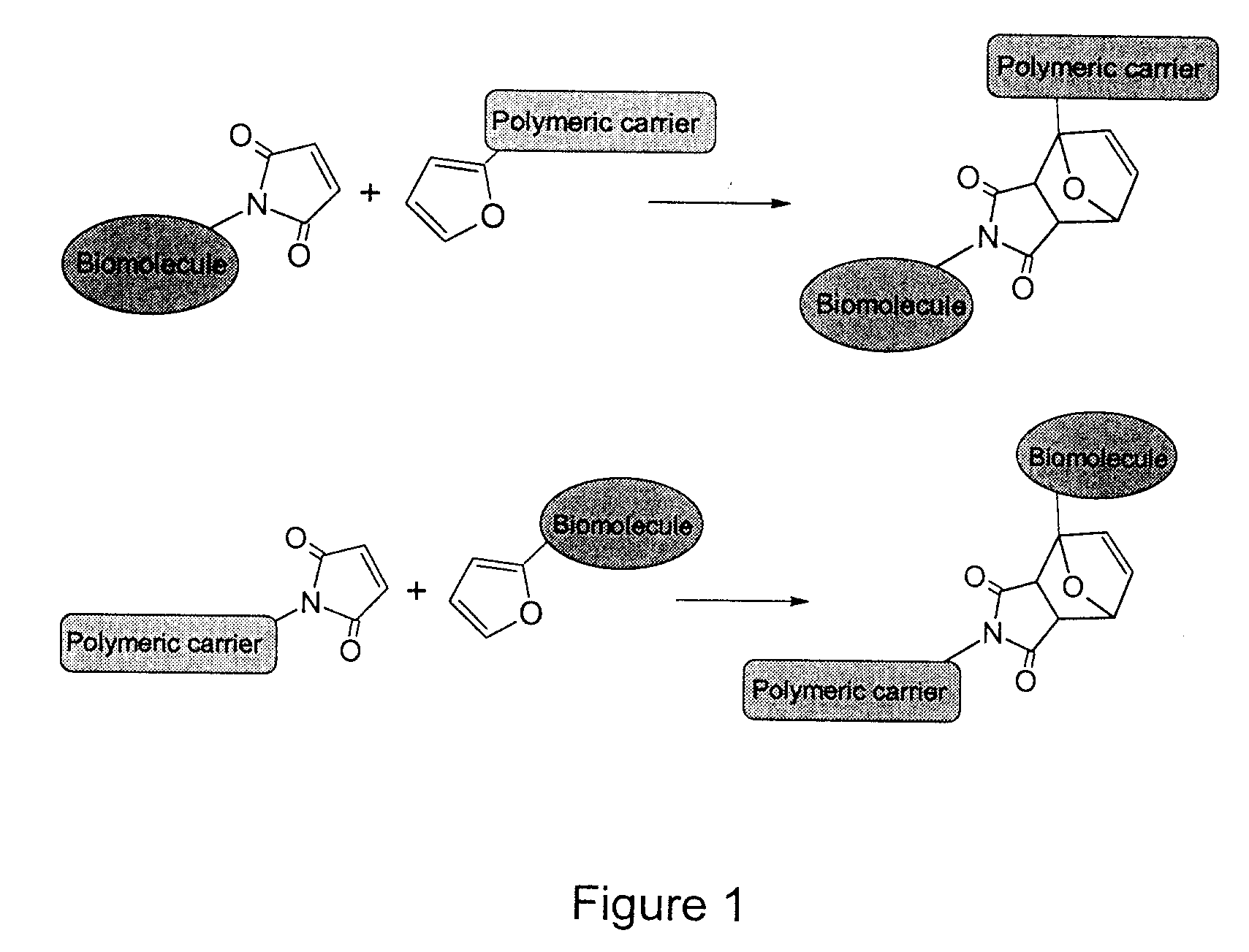
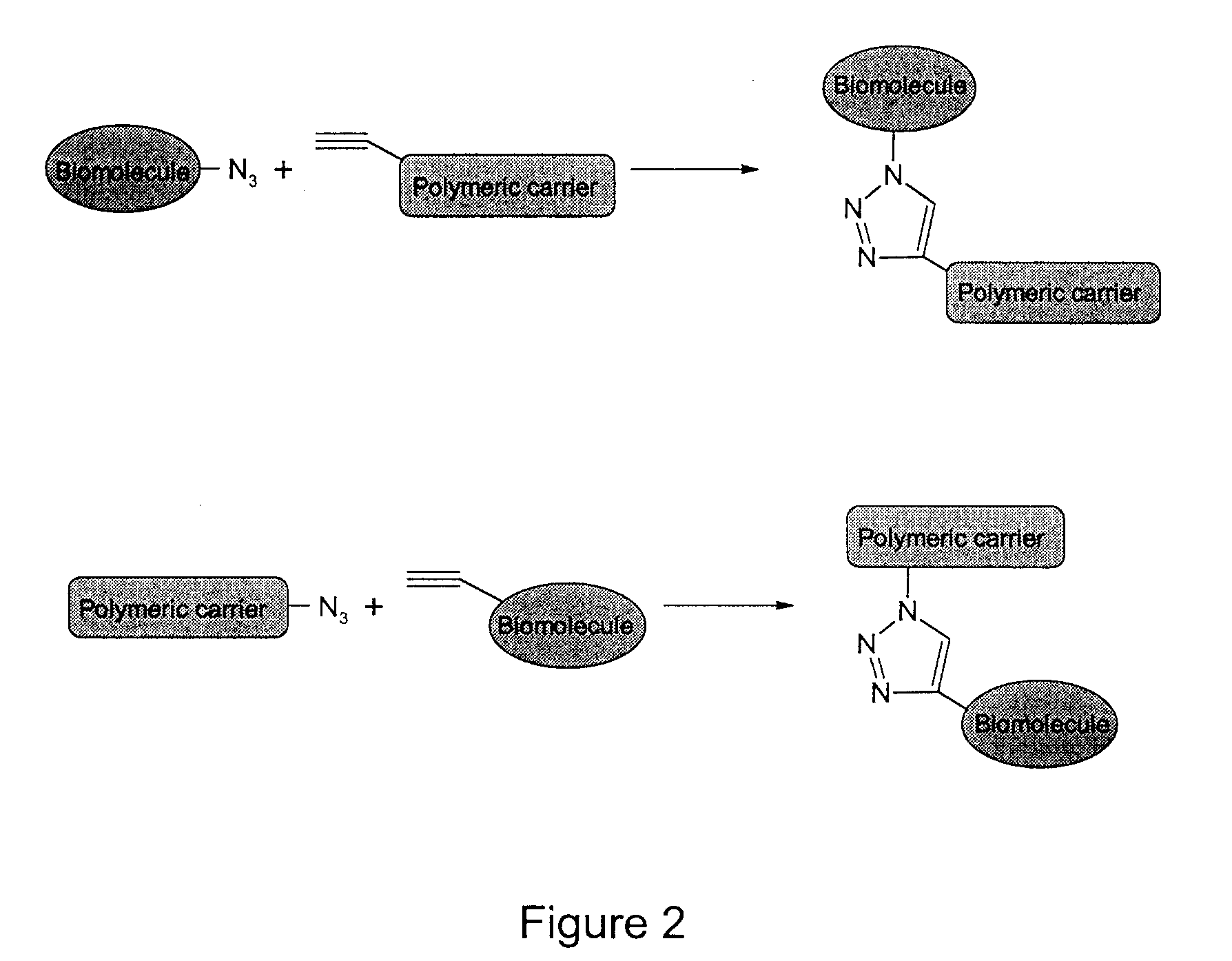
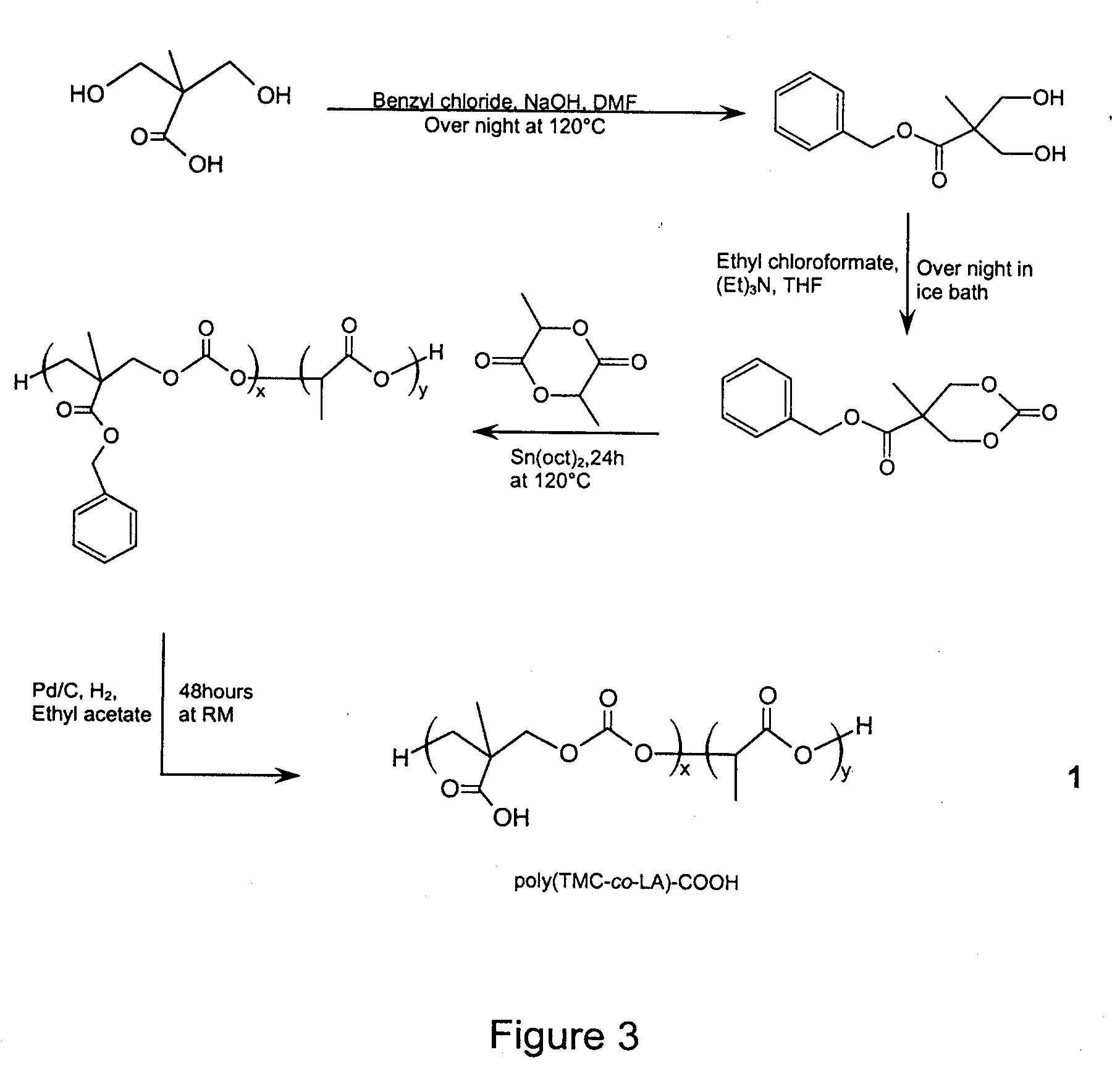
Method of Biomolecule Immobilization On Polymers Using Click-Type Chemistry
ActiveUS20090297609A1Improve efficiencyProcess environmental protectionBiocideOrganic active ingredientsFuranAlkyne
The present invention provides a method for the covalent immobilization of biomolecules on polymers for delivery of the biomolecules, which has the advantage of being simple, highly efficient, environmentally friendly and free of side products relative to traditional immobilization techniques. The invention provides a modified micro / nanoparticle system, which uses a functionalized polymer formed into micro or nanoparticles to bind a molecule to the particles using uses facile chemistry, the Diels-Alder cycloaddition between a diene and a dienophile with the polymer being functionalized with one of them and the molecule with the other, or the Huisgen 1,3-dipolar cycloaddition between a terminal alkyne and an azide to bind the molecule to the particle. The molecules and / or other therapeutic agents may be encapsulated within the polymer particles for intravenous therapeutic delivery. The invention also provides a novel synthetic biodegradable polymer, a furan / alkyne-functionalized poly(trimethylene carbonate) (PTMC)-based polymer, whose composition can be designed to meet the defined physical and chemical property requirements. In one example, the particle system self-aggregates from functionalized PTMC-based copolymers containing poly(ethylene glycol) (PEG) segments. The composition of the copolymers can be designed to meet various particle system requirements, including size, thermodynamic stability, surface PEG density, drug encapsulation capacity and biomolecule immobilization capacity.
Owner:SHOICHET MOLLY S +2
Method for preparation of aviation kerosene and diesel oil from biomass derivative
The invention relates to a new liquid chain hydrocarbon fuel synthetic route that acquires a platform chemical compound based on a lignocellulose raw material and is completely independent of fossil energy. The liquid fuel obtained by the method can be used as a substitute of aviation kerosene and diesel oil or as an additive for improving the cetane number and cold hardiness of fuels, thereby reducing the national dependence on imported petroleum in terms of liquid fuels. The method provided in the invention consists of two parts: 1) on a novel solid acid catalyst, an aldehyde group-containing compound (such as formaldehyde, acetaldehyde, propionaldehyde, and butyraldehyde, etc.) and a furan platform compound (such as furan, methyl furan, and hydroxylmethyl furan, etc.) undergo an acid catalyzed alkylation reaction to prepare an oxygen-containing organic compound with a carbon chain length of 8-16; and 2) hydrogenation and hydrodeoxygenation are conducted on an alkylation product to hydrogenate unsaturated bonds and remove the oxygen therein, thus preparing aviation kerosene or high grade diesel oil with a carbon chain length ranging from 8 to 16.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Chemical Transformation of Lignocellulosic Biomass into Fuels and Chemicals
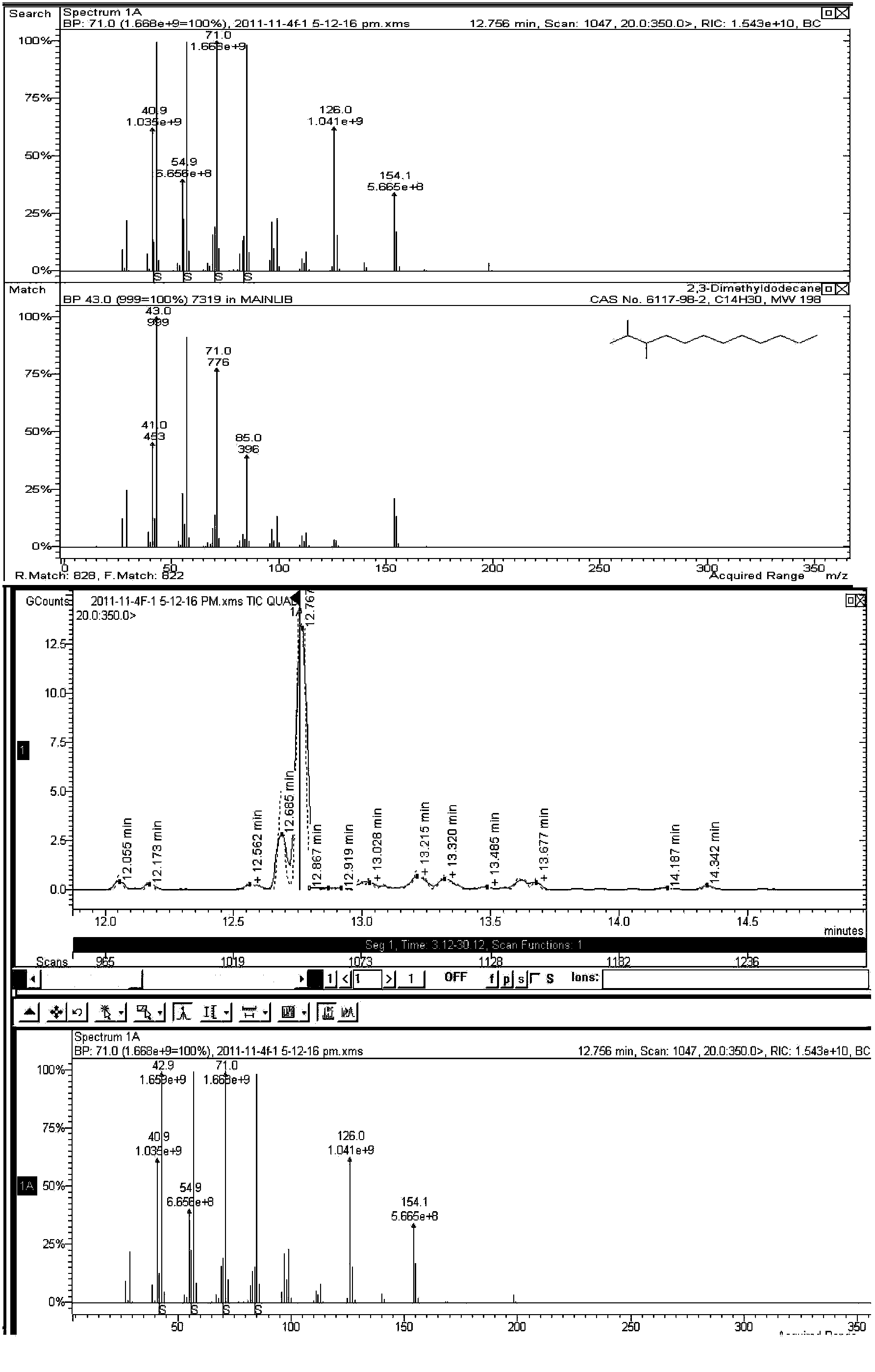
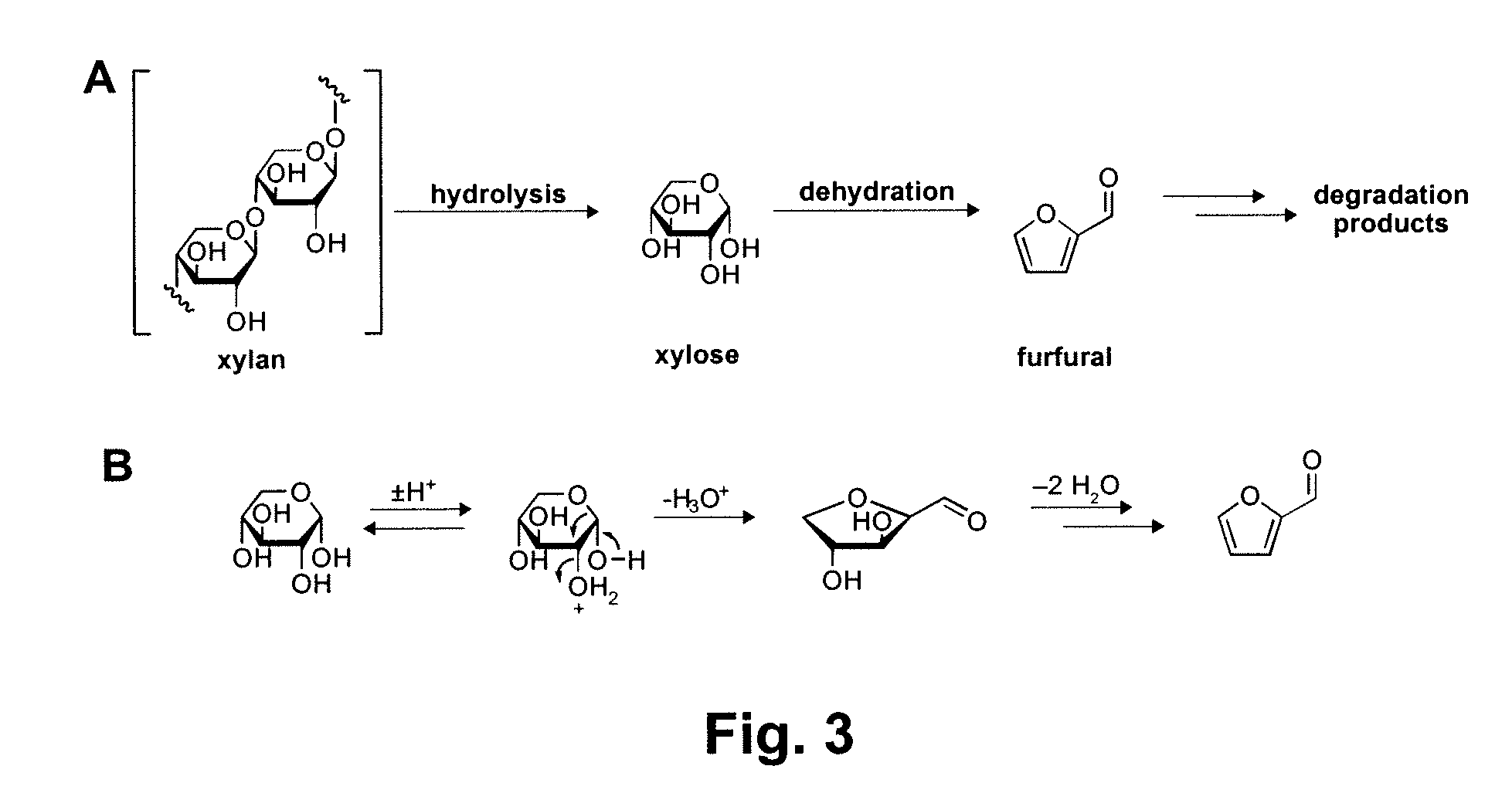
A method for converting a carbohydrate to a furan in a polar aprotic solvent in the presence of a chloride, bromide, or iodide salt or a mixture thereof and optionally in the presence of an acid catalyst, a metal halide catalyst and / or an ionic liquid (up to 40 wt %). The method can be employed in particular to produce furfural or 5-hydroxymethylfurfural.
Owner:WISCONSIN ALUMNI RES FOUND
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![3-B-D-ribofuranosylthiazolo [4,5-d] pyridimine nucleosides and uses thereof 3-B-D-ribofuranosylthiazolo [4,5-d] pyridimine nucleosides and uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6744ce42-a5ed-4f3e-a61b-f505358721e2/US20050070556A1-20050331-D00001.png)
![3-B-D-ribofuranosylthiazolo [4,5-d] pyridimine nucleosides and uses thereof 3-B-D-ribofuranosylthiazolo [4,5-d] pyridimine nucleosides and uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6744ce42-a5ed-4f3e-a61b-f505358721e2/US20050070556A1-20050331-D00002.png)
![3-B-D-ribofuranosylthiazolo [4,5-d] pyridimine nucleosides and uses thereof 3-B-D-ribofuranosylthiazolo [4,5-d] pyridimine nucleosides and uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6744ce42-a5ed-4f3e-a61b-f505358721e2/US20050070556A1-20050331-C00001.png)