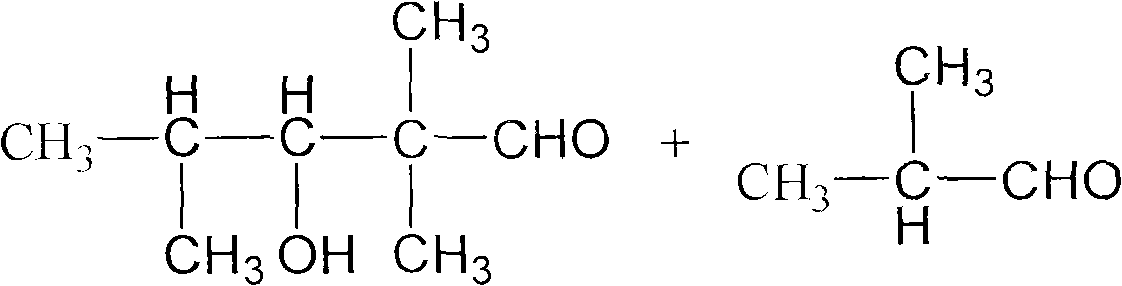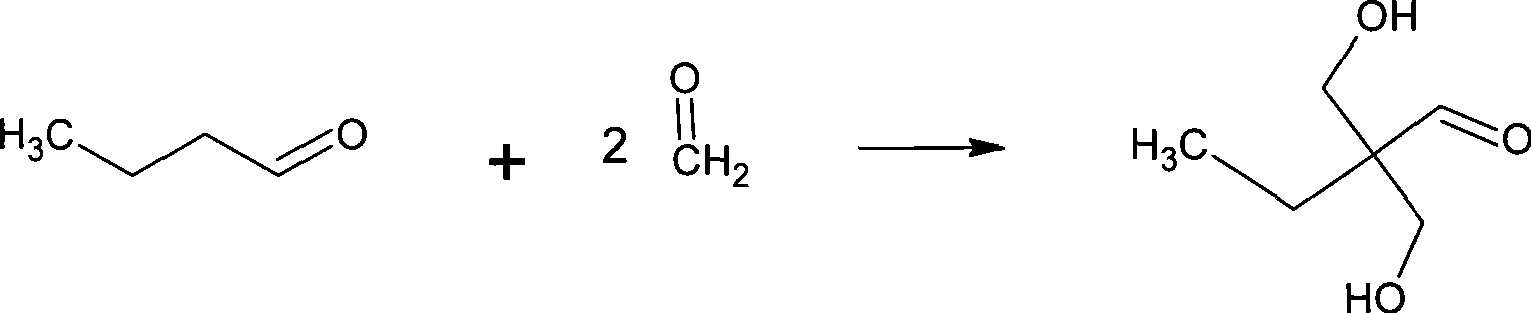Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
700 results about "Aldol condensation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
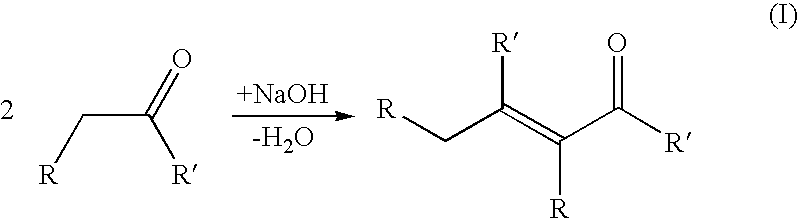
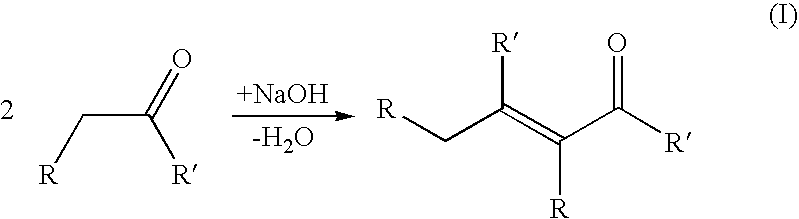
An aldol condensation is a condensation reaction in organic chemistry in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), followed by dehydration to give a conjugated enone.
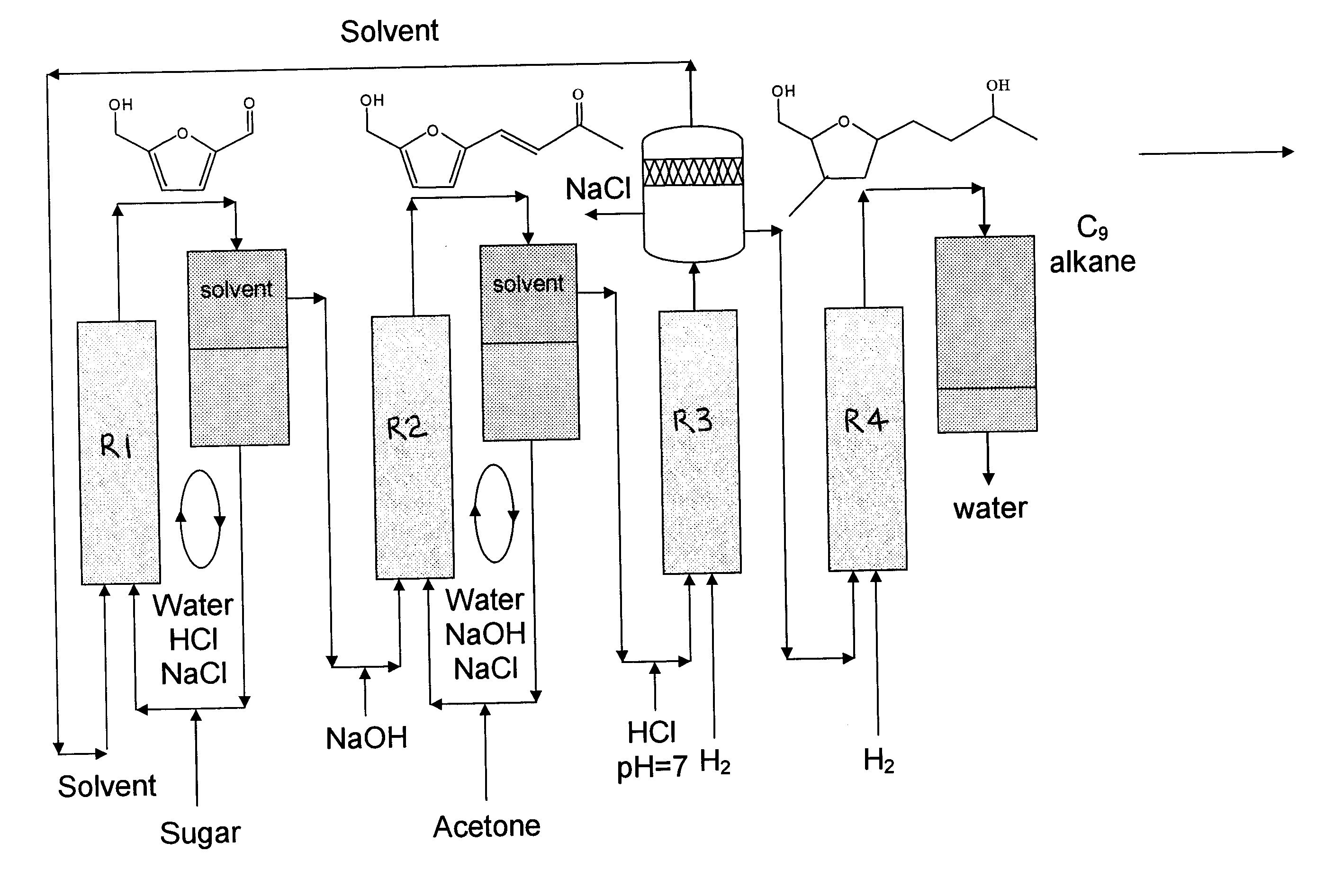
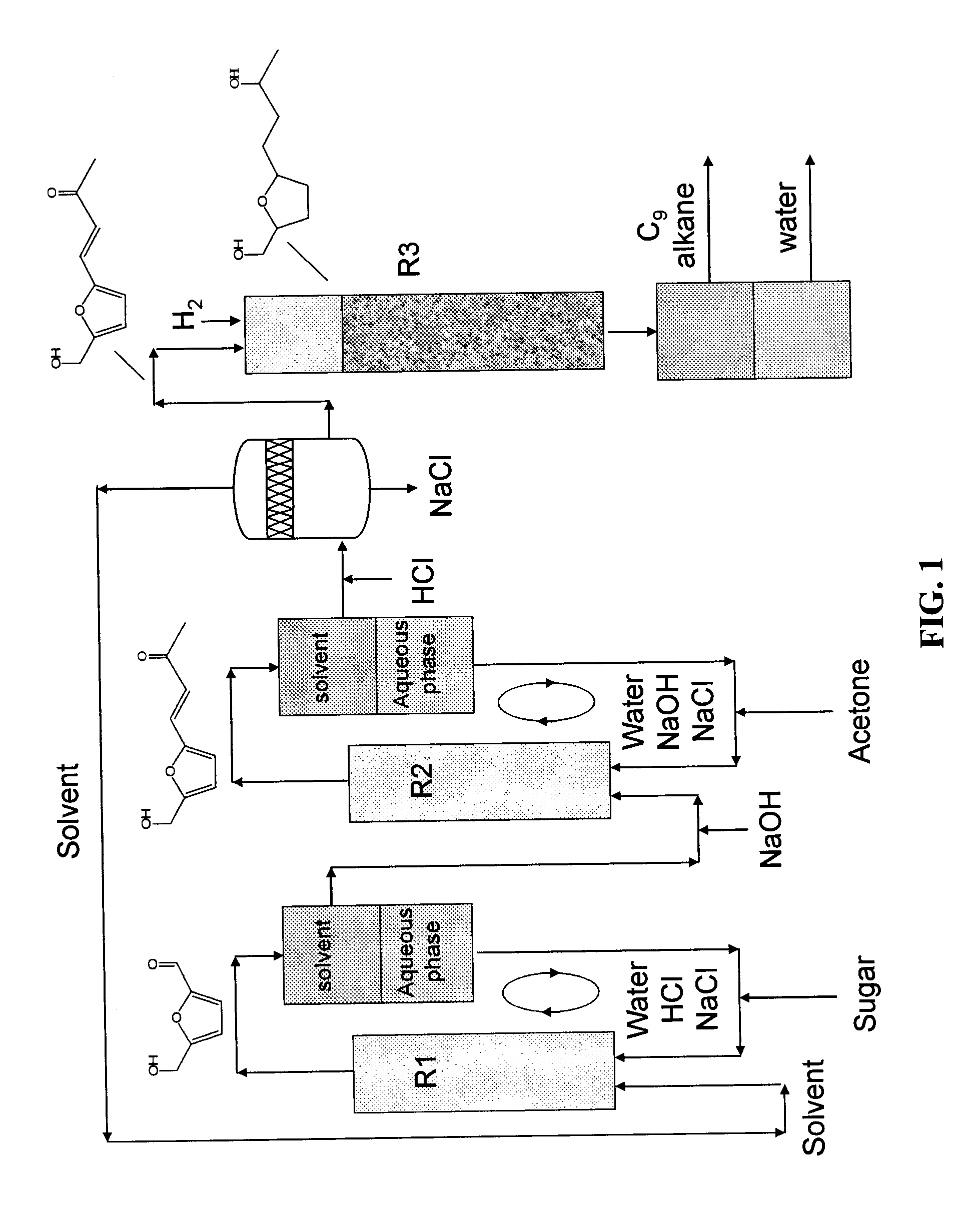
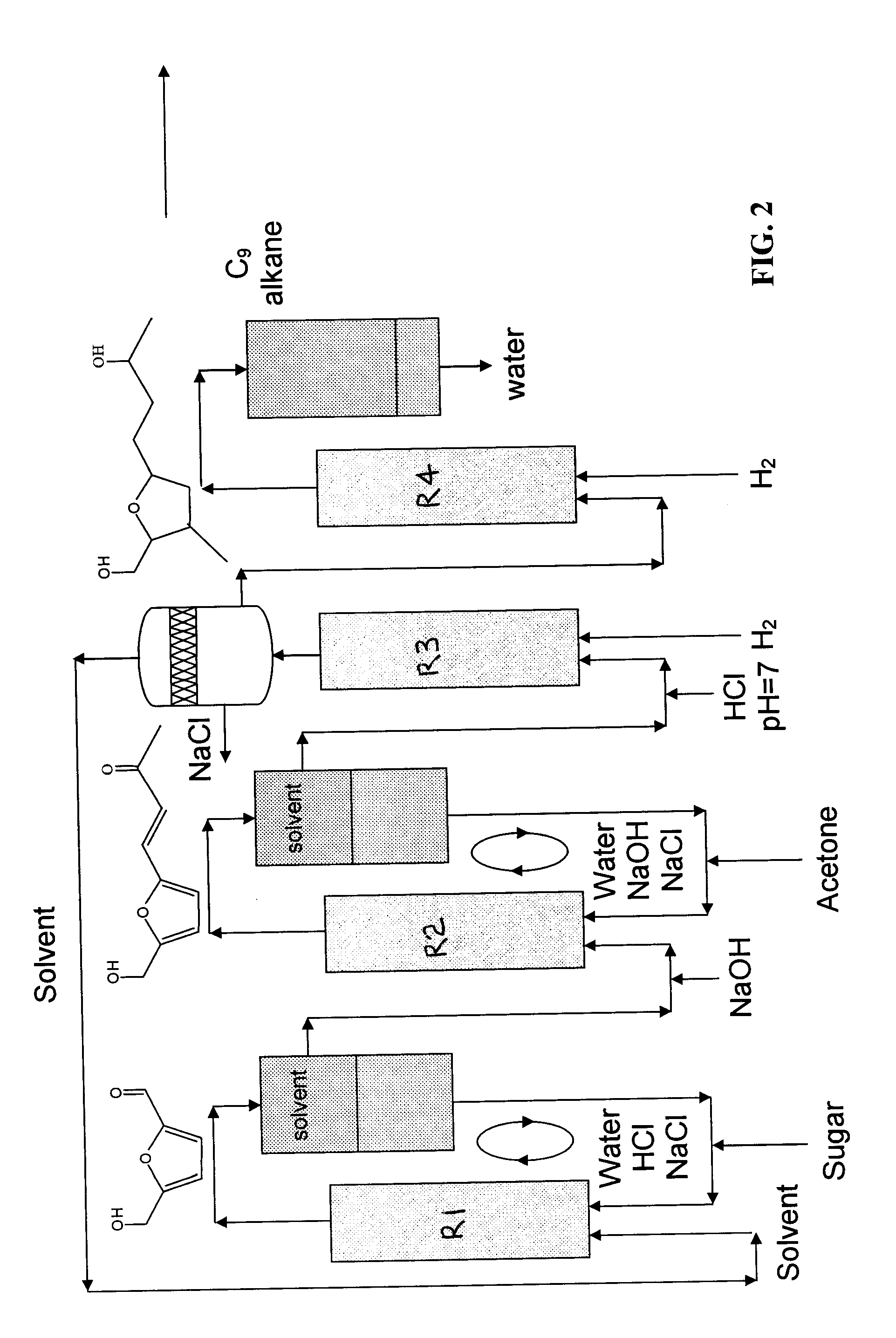
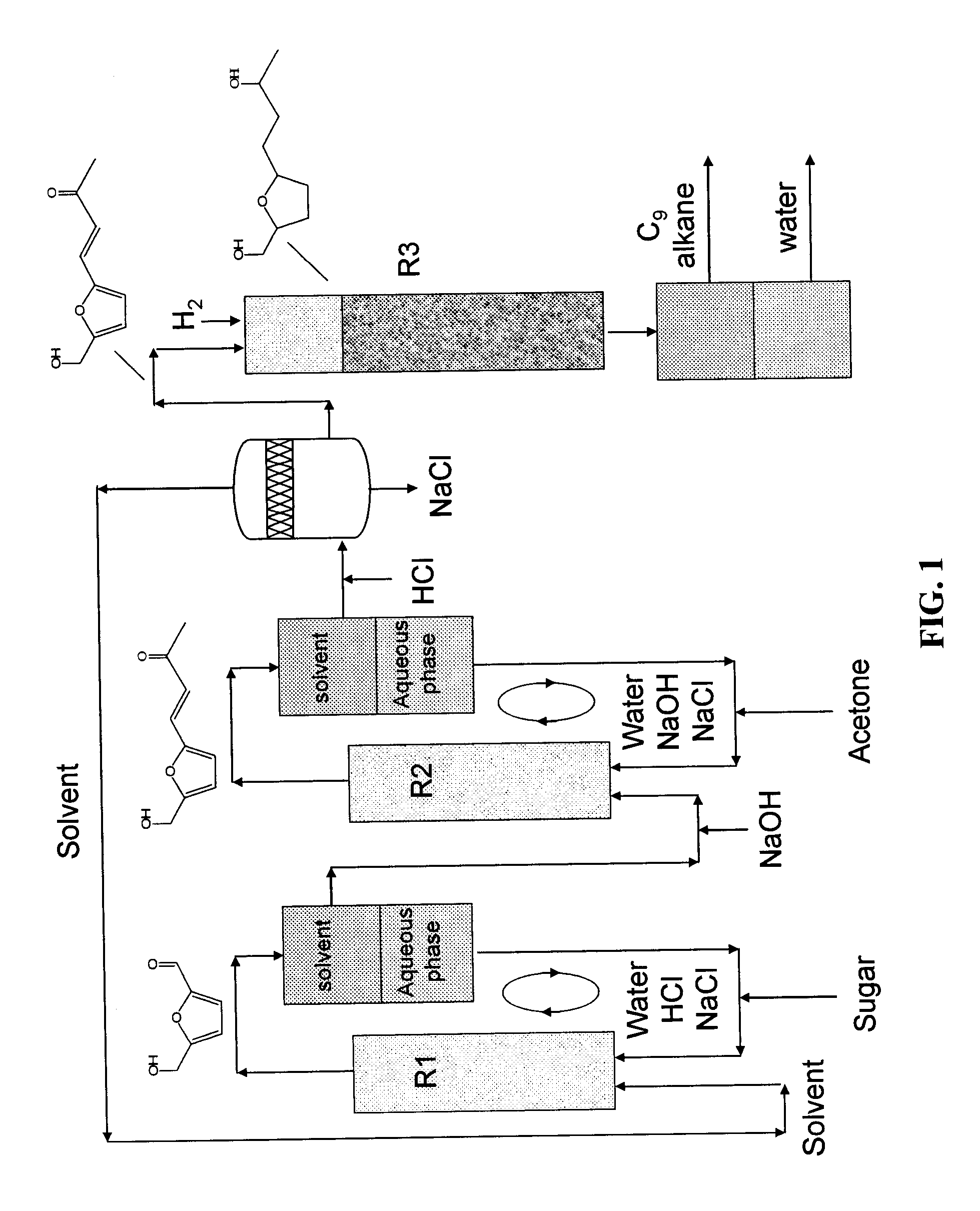
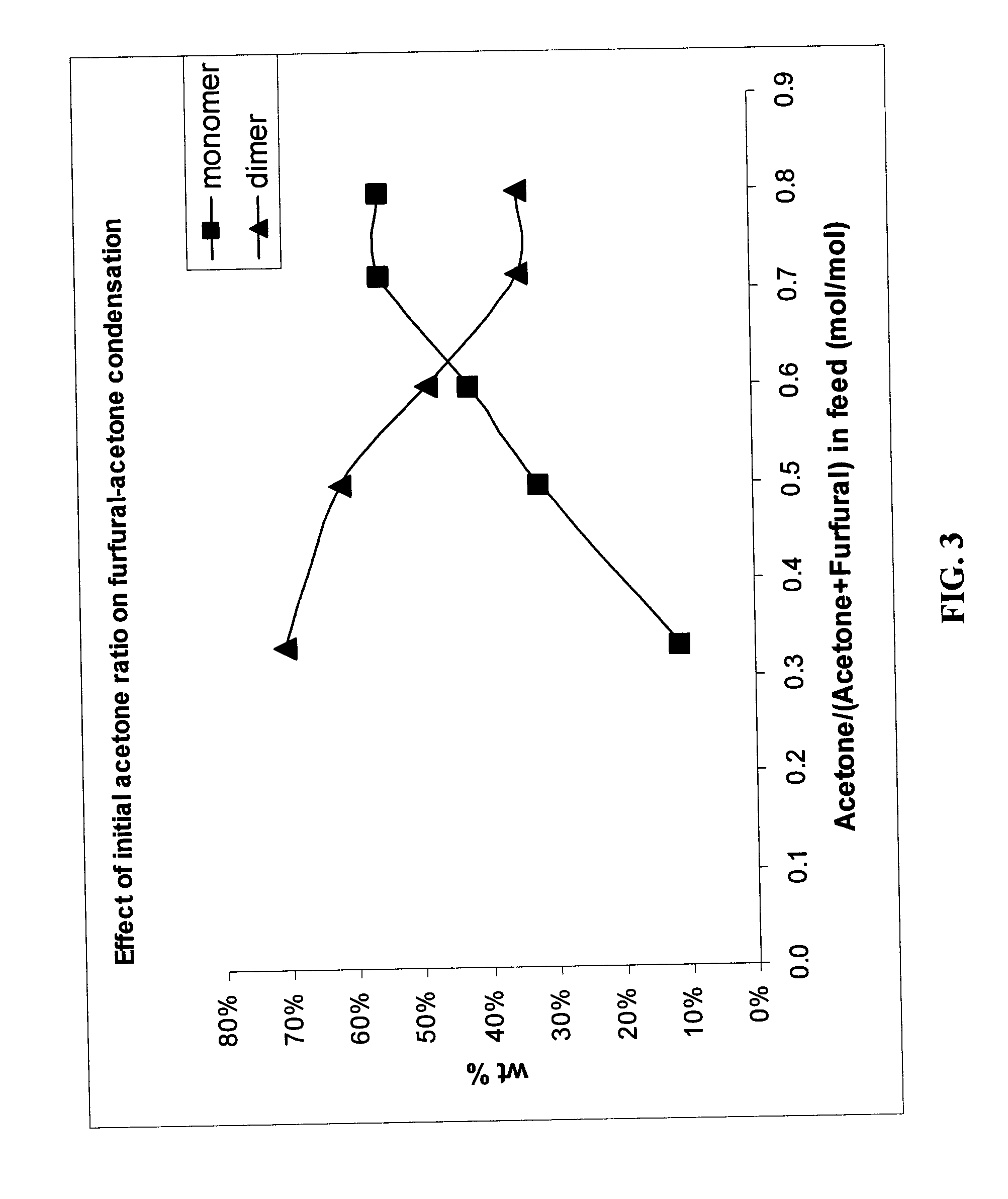
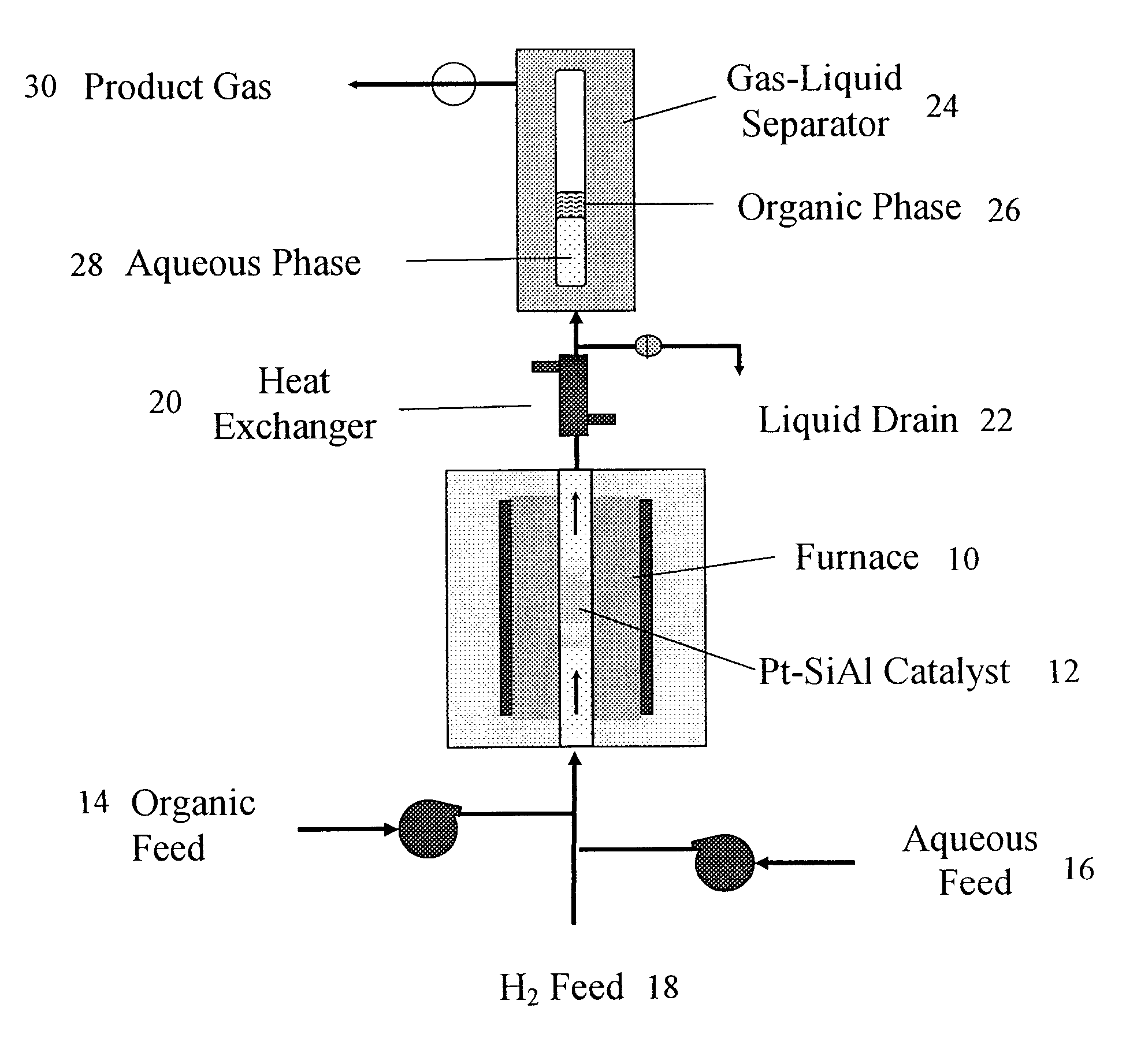
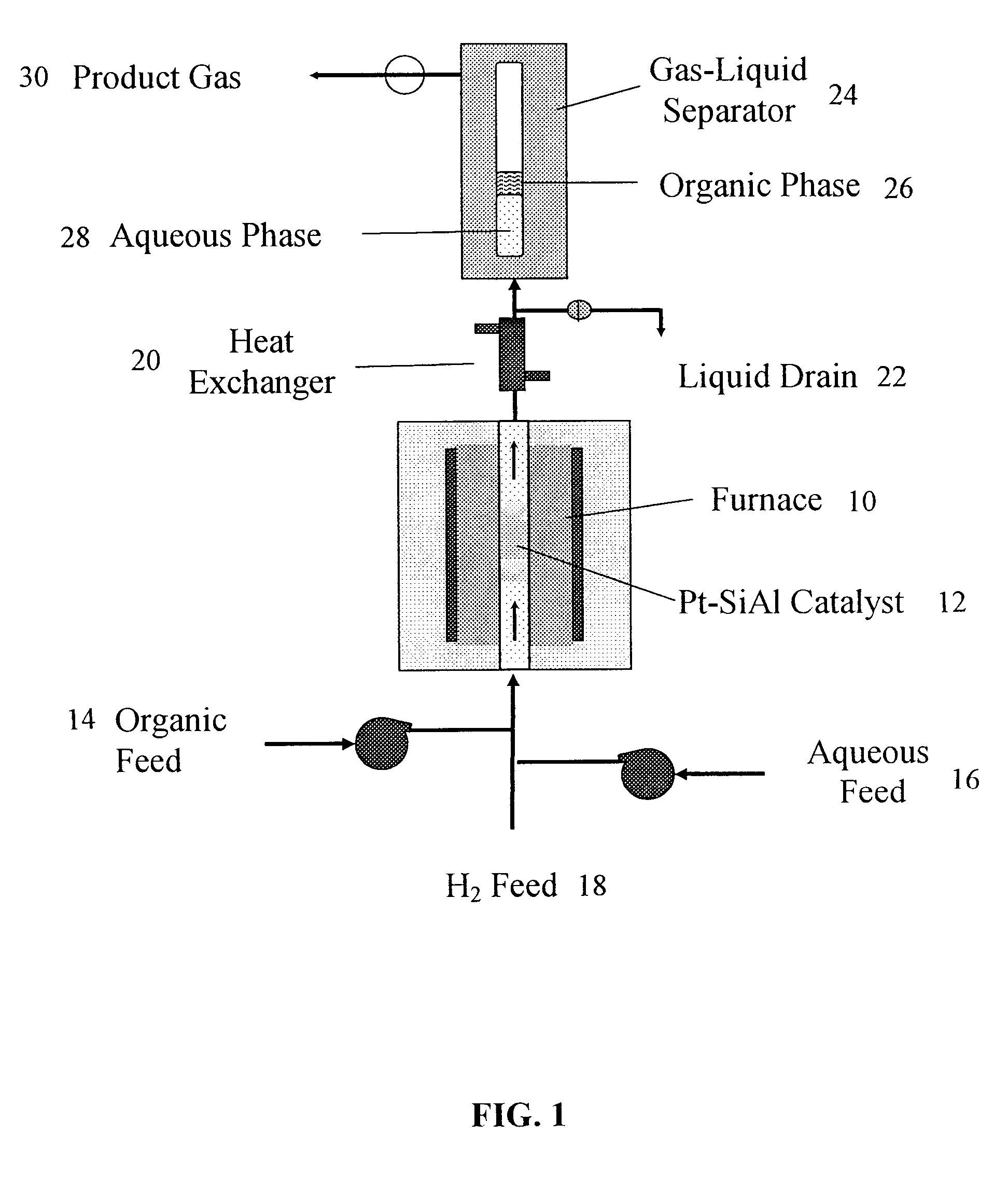
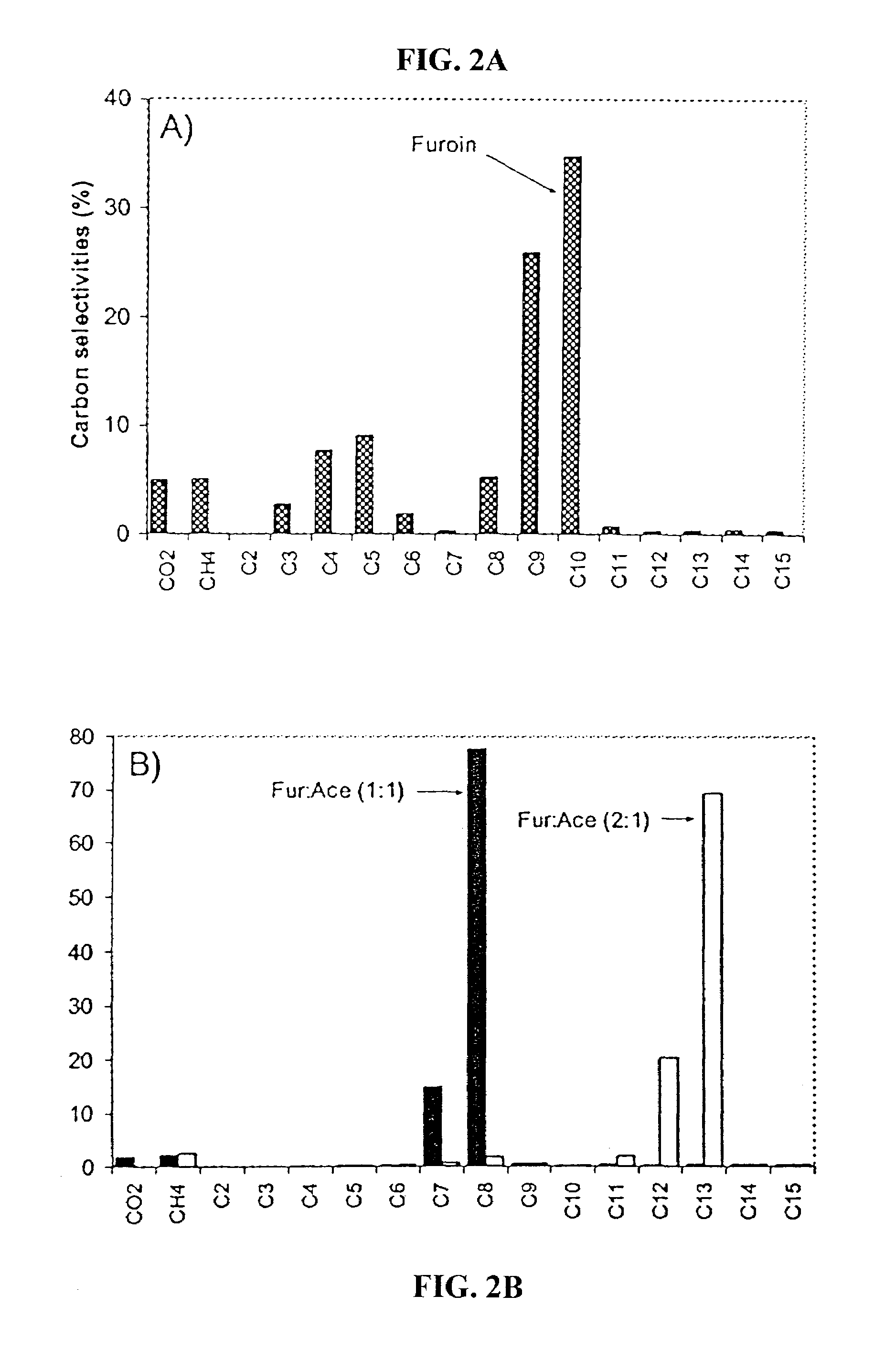
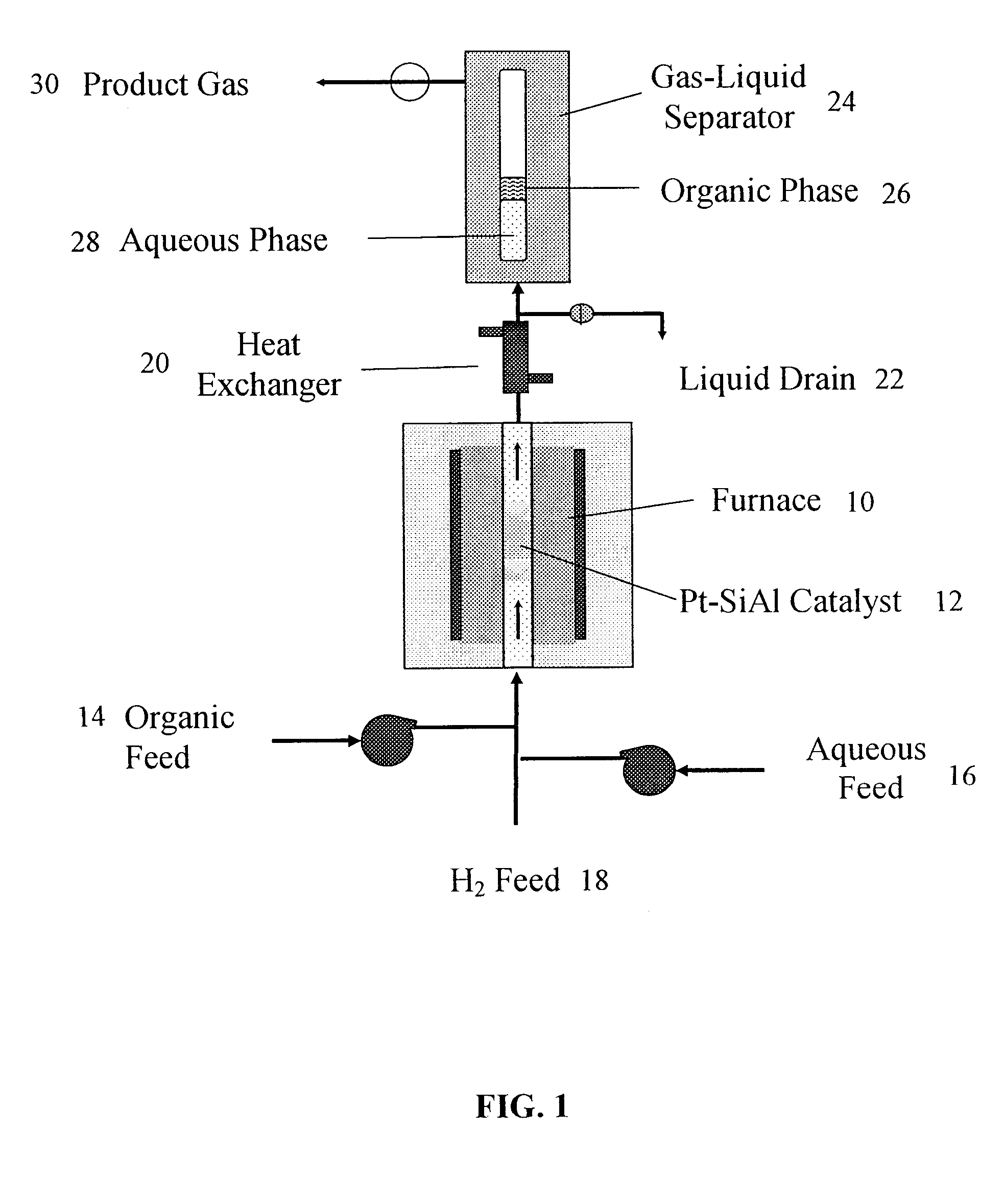
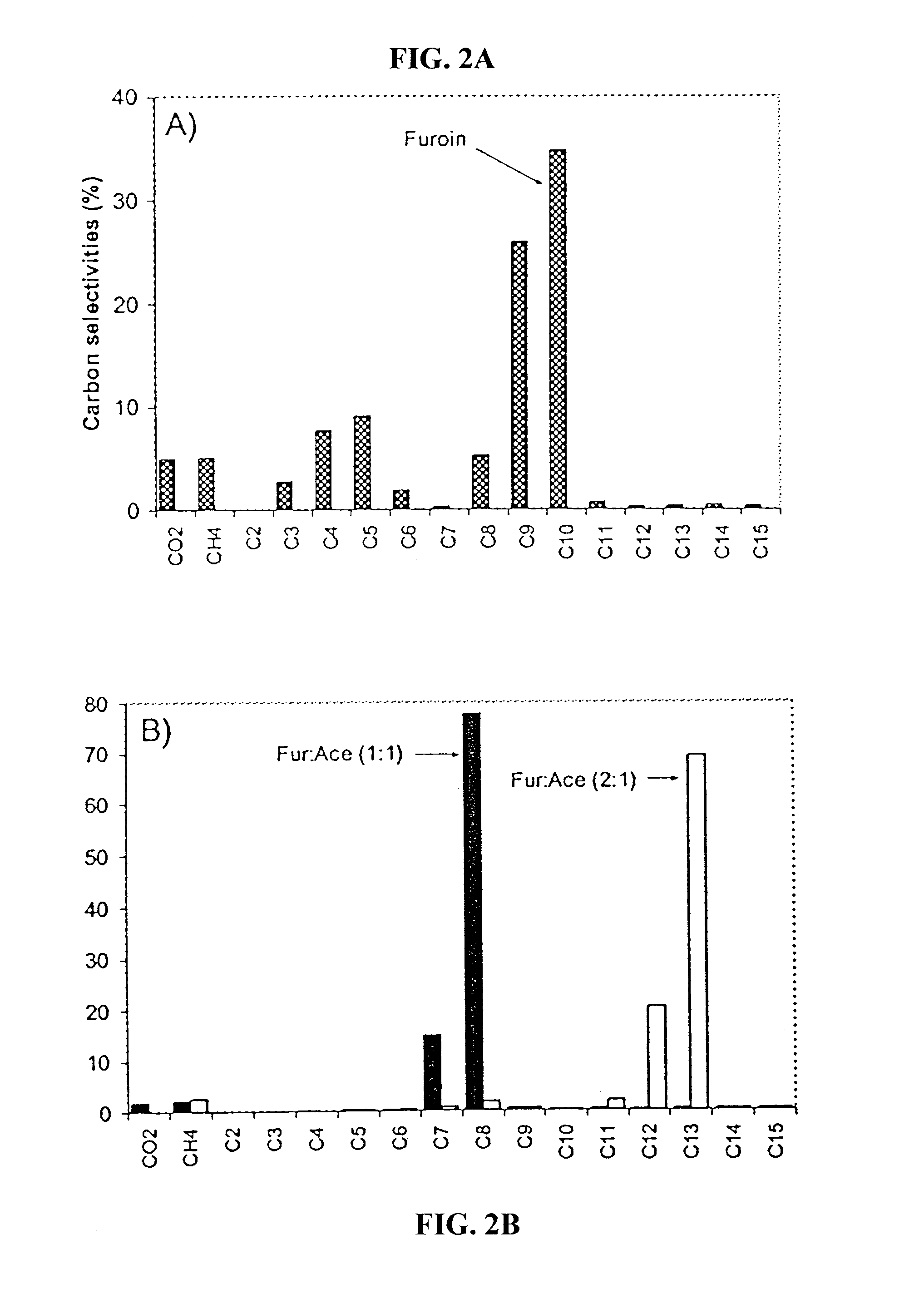
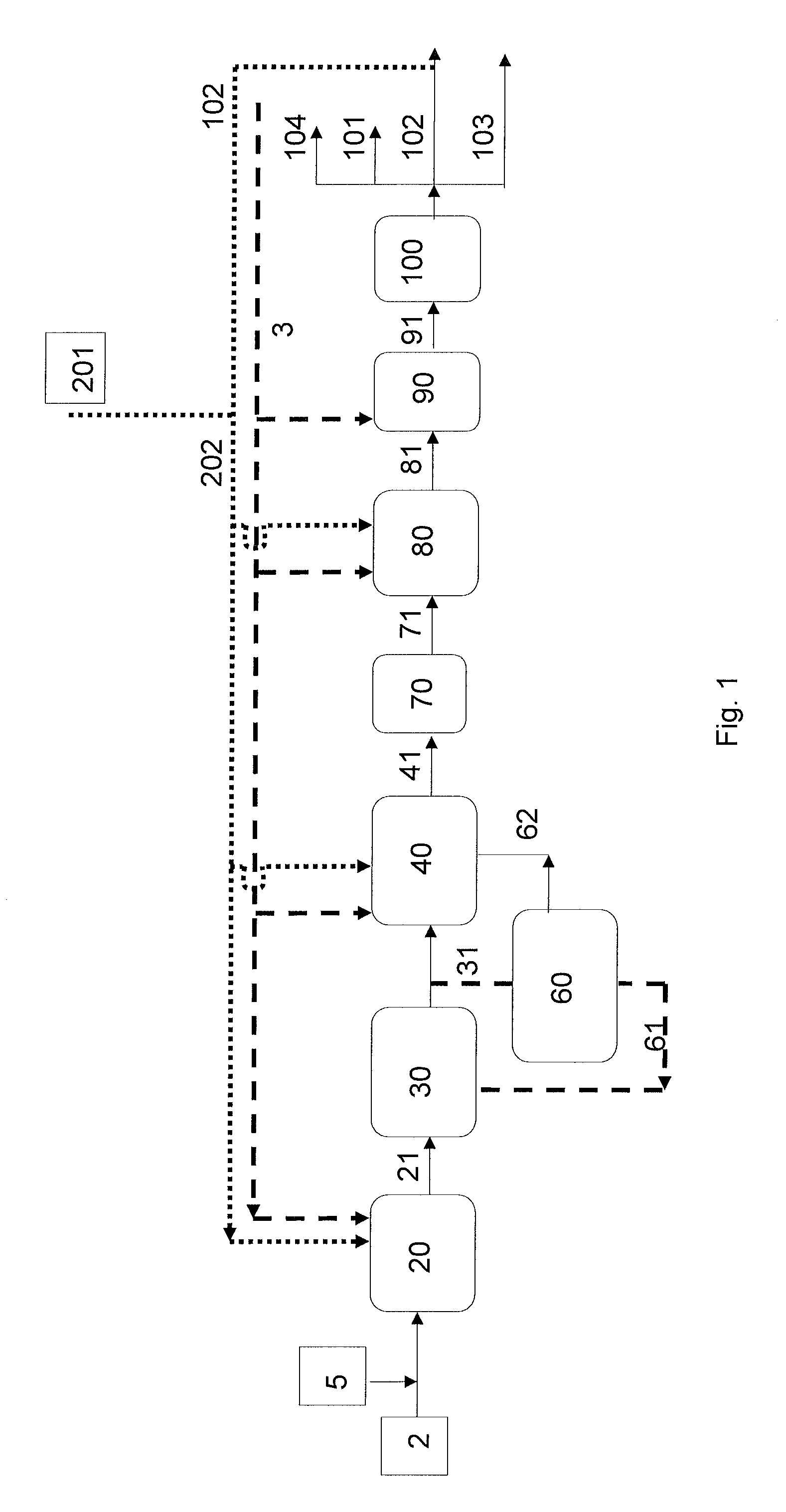
Production of liquid alkanes in the jet fuel range (c8-c15) from biomass-derived carbohydrates
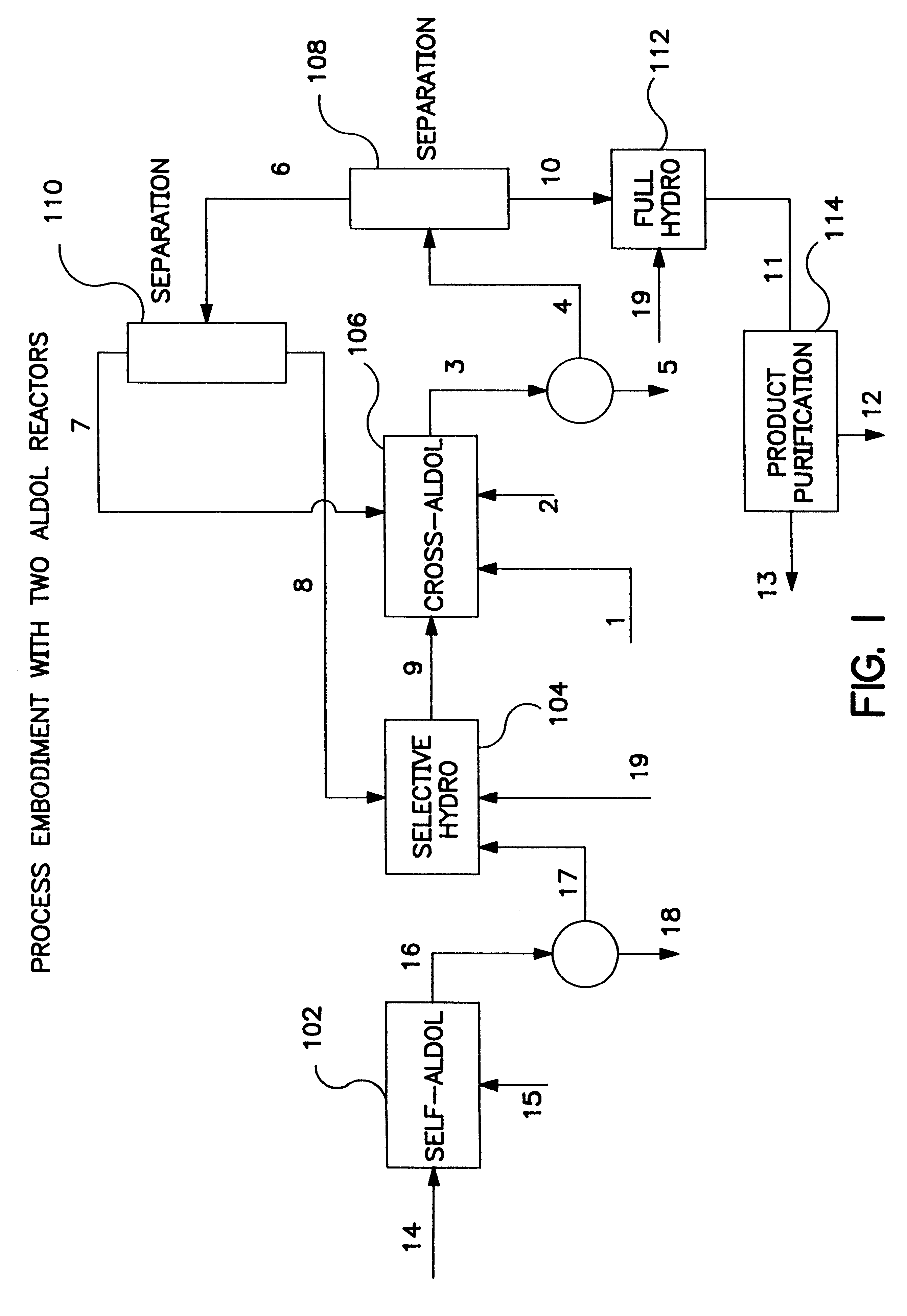
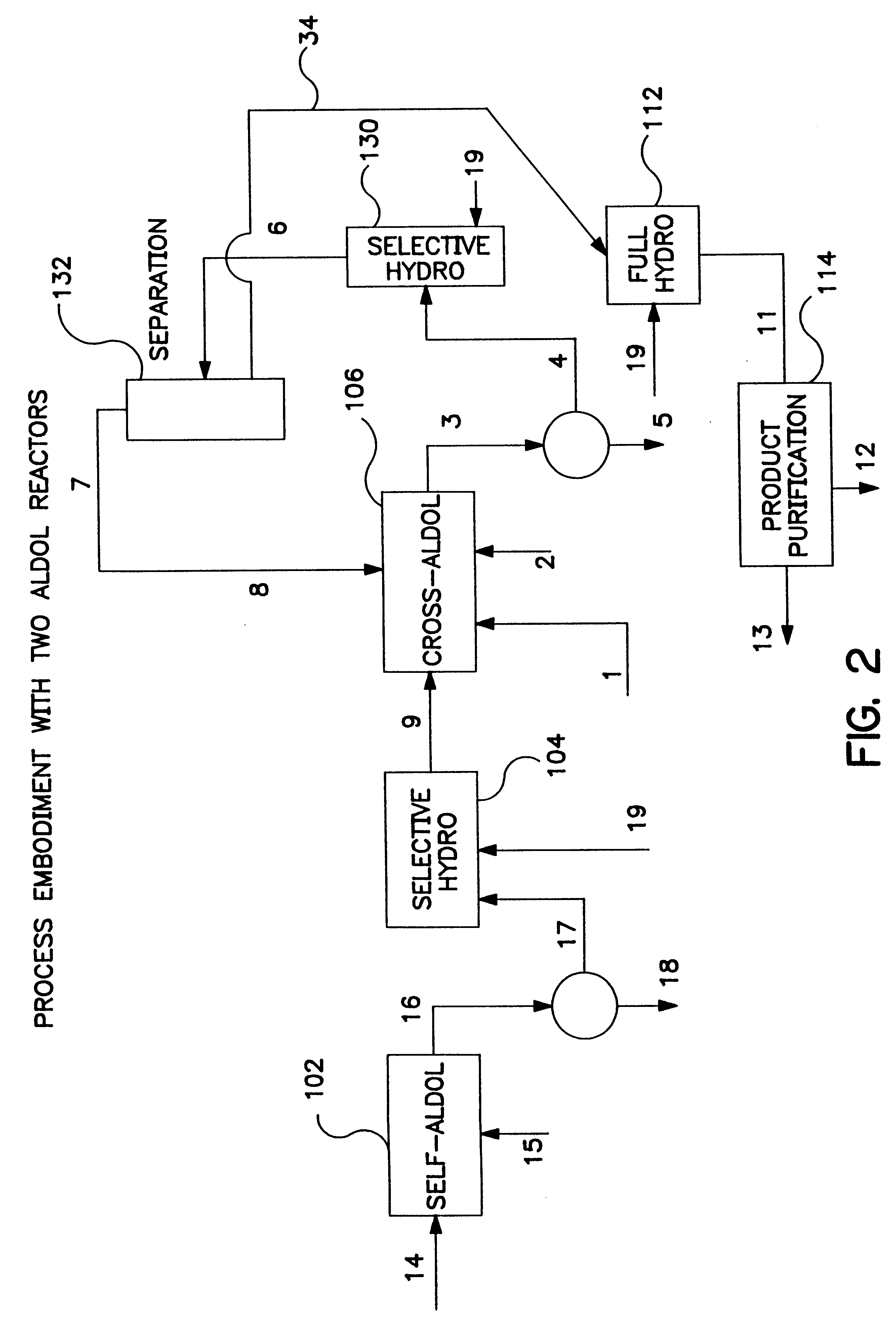
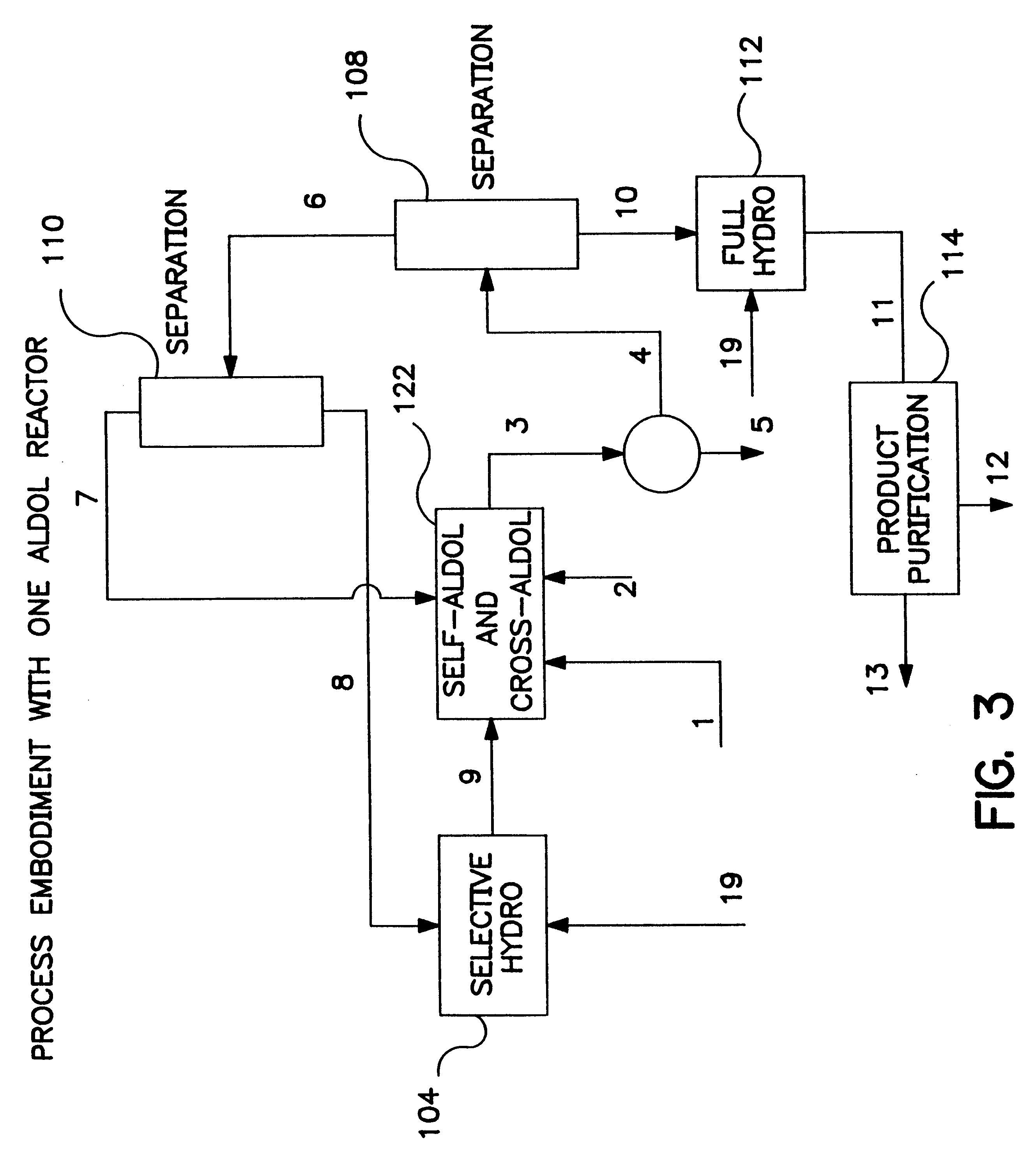
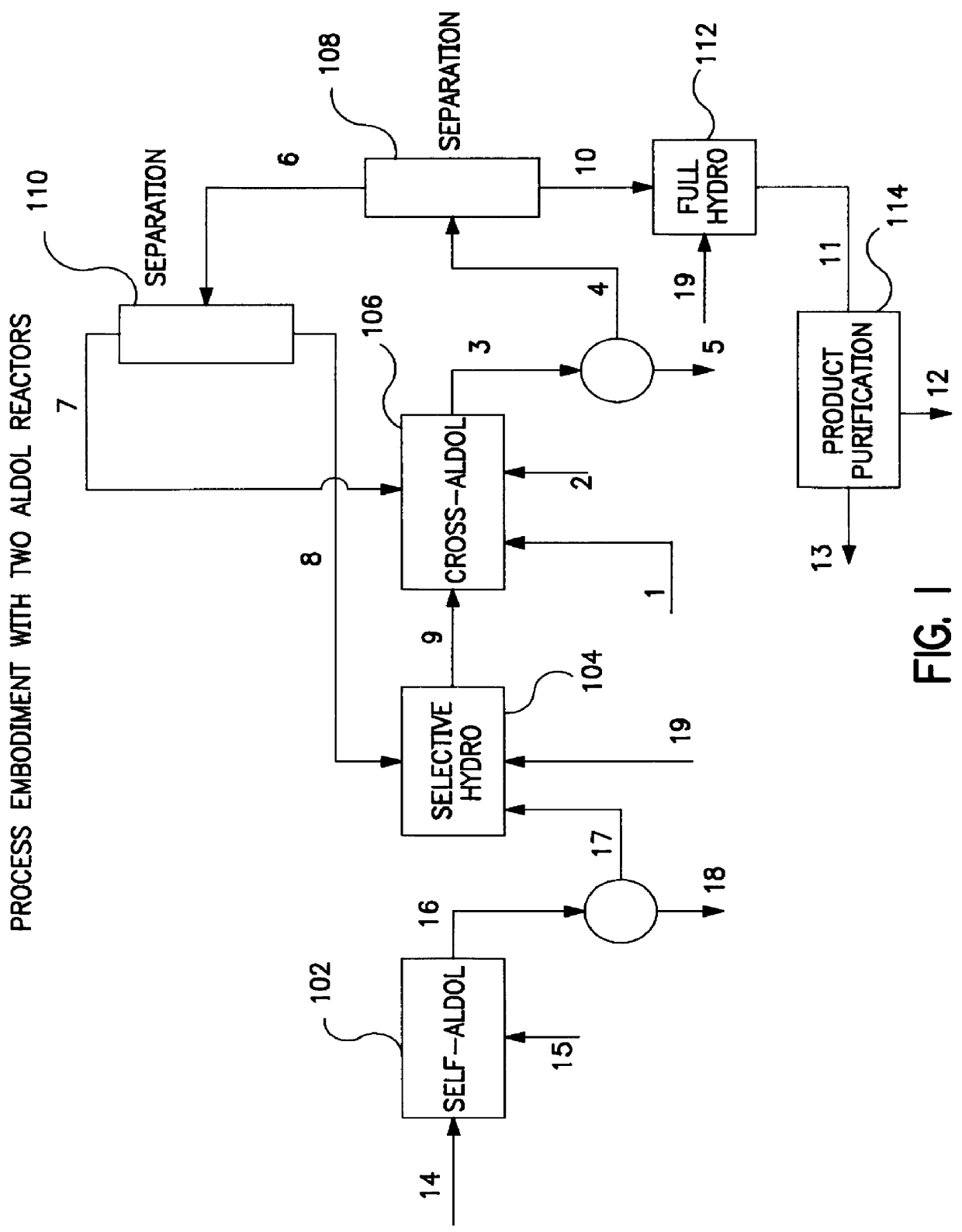
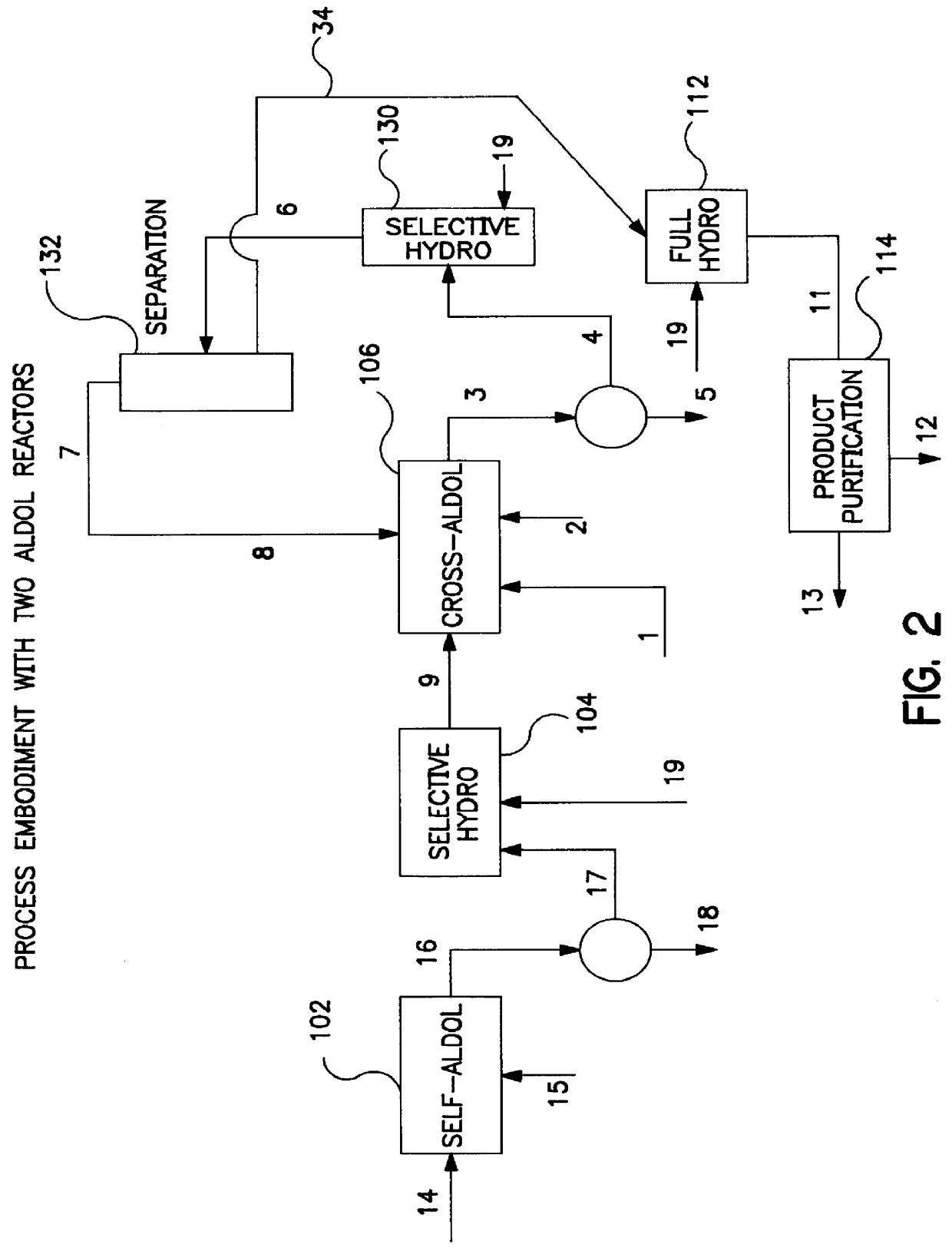
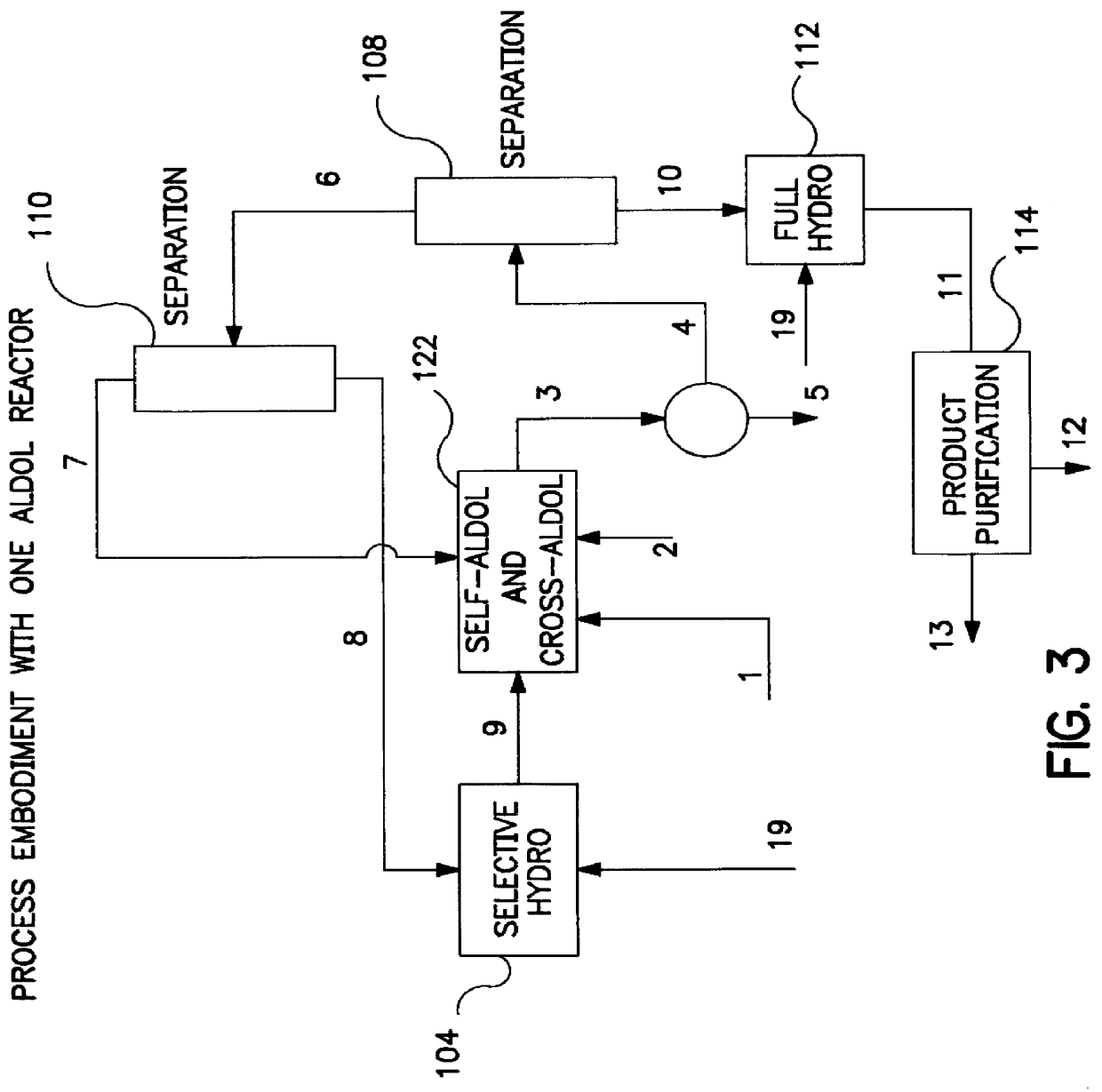
Described is a method for making a composition comprising alkanes. The composition is suitable for use as a liquid transportation fuel in general, and jet fuel in particular. The method includes dehydrating a feedstock solution comprising a carbohydrate, in the presence of an acid catalyst, to yield at least one furan derivative compound, in a reaction vessel containing a biphasic reaction medium: an aqueous reaction solution and a substantially immiscible organic extraction solution. The furan derivative compound is then subjected to a self-aldol condensation reaction or a crossed-aldol condensation reaction with another carbonyl compound to yield a beta-hydroxy carbonyl compound and / or an alpha-beta unsaturated carbonyl compound. The beta-hydroxy carbonyl and / or alpha-beta unsaturated compounds are then hydrogenated to yield a saturated or partially saturated compound, followed by hydrodeoxygenation (e.g., dehydrating and hydrogenating) of the saturated or partially saturated compound to yield a composition of matter comprising alkanes.
Owner:WISCONSIN ALUMNI RES FOUND
Process for producing a branched hydrocarbon component
InactiveUS20070135316A1Reduce carbon dioxide emissionsImprove stabilityOrganic compound preparationCatalytic naphtha reformingIsomerizationHydrodeoxygenation
The invention relates to a process for producing high-quality hydrocarbon base oil particularly of biological origin. The process of the invention comprises aldol condensation, hydrodeoxygenation, and isomerization steps. Aldehydes and / or ketones, preferably of biological origin are used as the feedstock.
Owner:NESTE OIL OY
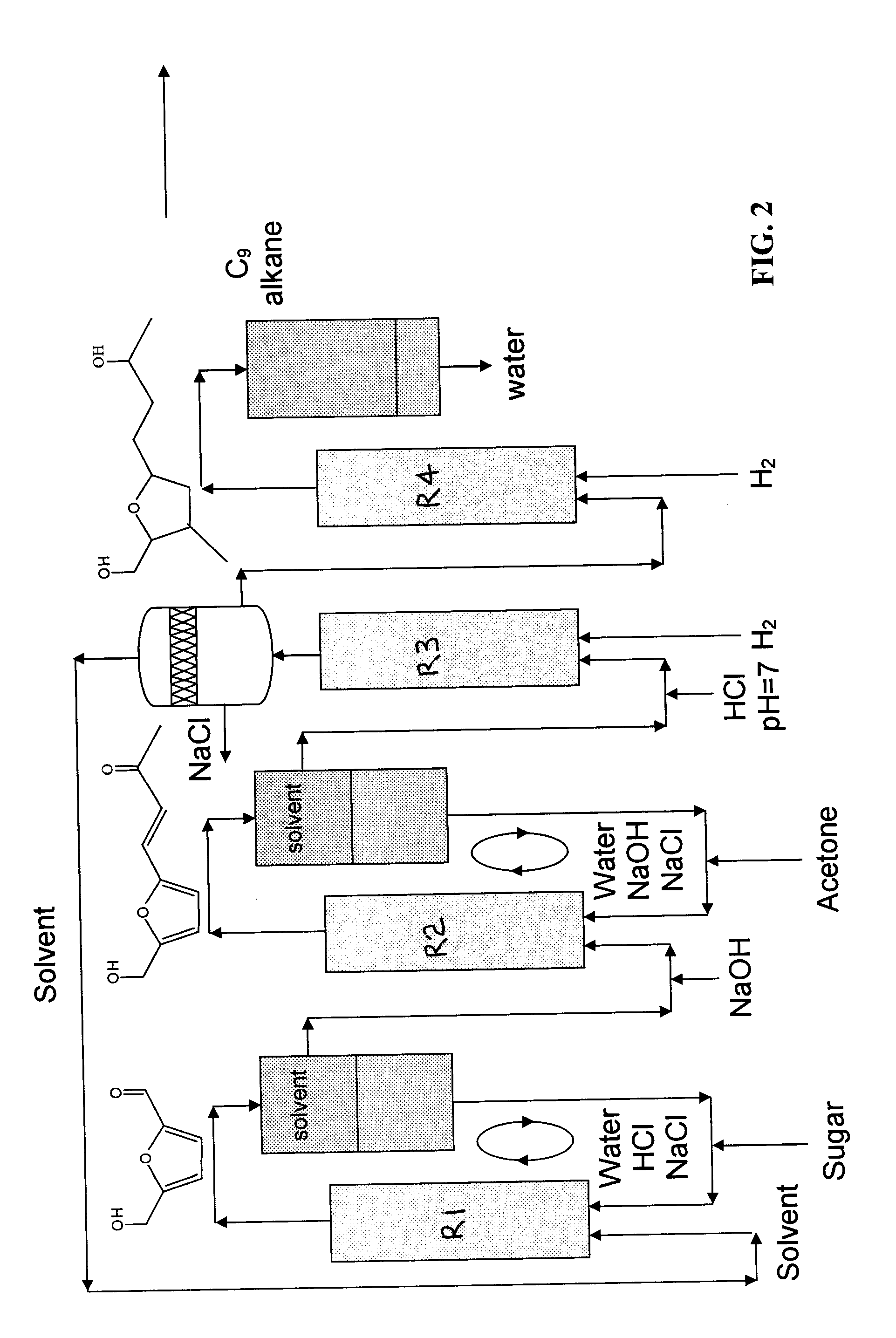
Production of liquid alkanes in the jet fuel range (C8-C15) from biomass-derived carbohydrates
ActiveUS7880049B2High selectivityHydrocarbon from oxygen organic compoundsLiquid hydrocarbon mixture productionAlkaneFuran
Described is a method for making a composition comprising alkanes. The composition is suitable for use as a liquid transportation fuel in general, and jet fuel in particular. The method includes dehydrating a feedstock solution comprising a carbohydrate, in the presence of an acid catalyst, to yield at least one furan derivative compound, in a reaction vessel containing a biphasic reaction medium: an aqueous reaction solution and a substantially immiscible organic extraction solution. The furan derivative compound is then subjected to a self-aldol condensation reaction or a crossed-aldol condensation reaction with another carbonyl compound to yield a beta-hydroxy carbonyl compound and / or an alpha-beta unsaturated carbonyl compound. The beta-hydroxy carbonyl and / or alpha-beta unsaturated compounds are then hydrogenated to yield a saturated or partially saturated compound, followed by hydrodeoxygenation (e.g., dehydrating and hydrogenating) of the saturated or partially saturated compound to yield a composition of matter comprising alkanes.
Owner:WISCONSIN ALUMNI RES FOUND
Method to make alkanes and saturated polyhydroxy compounds from carbonyl compounds
ActiveUS7671246B2Hydrocarbon by hydrogenationHydrocarbon purification/separationAldol condensationPolyhydroxy compound
Owner:WISCONSIN ALUMNI RES FOUND
Preparation method for aviation kerosene
The invention relates to a new synthesis route of a liquid branched paraffin fuel, the method adopts a lignocellulose based platform compound as a raw material and is completely independent of fossil energy. The liquid fuel obtained by the method can be used as an aviation kerosene (or diesel) substitute or as an additive to increase the cetane number and cold resistance of fuel. The method provided by the invention includes two steps of: 1) under the promotion of a base catalyst, subjecting a lignocellulose based furfural compound (including furfural, methylfurfural or 5 hydroxymethylfurfural) and branched chain keto (including methyl isobutyl ketone, and mesityl oxide, etc.) to aldol condensation reaction so as to synthesize an oxygen-containing organic compound with a carbon chain length of 9-16; and 2) conducting hydrodeoxygenation on the aldol condensation product generated in step1 to obtain biomass aviation kerosene branched hydrocarbon with a carbon chain length of 9-16, higher energy density, stability and low freezing point.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
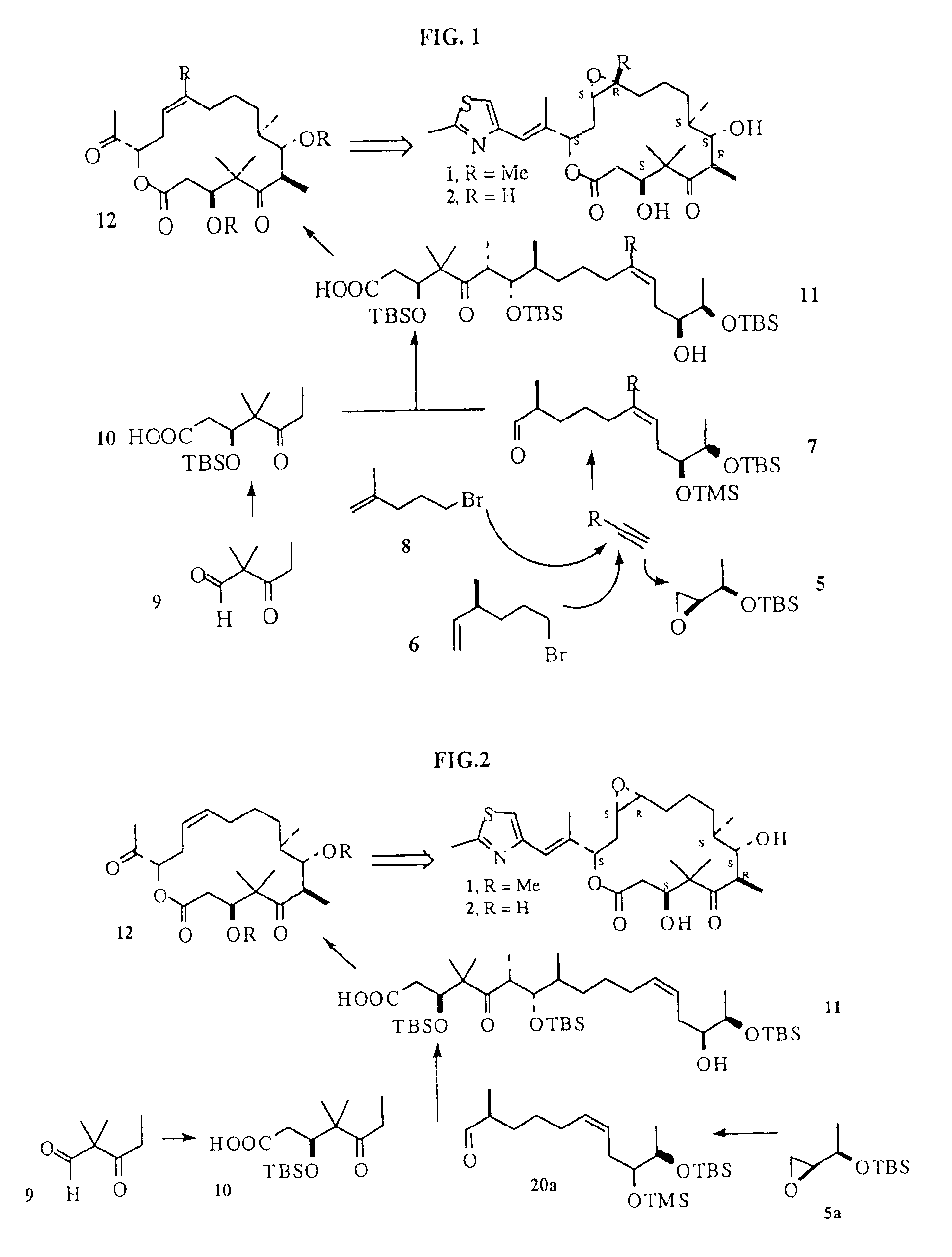
Synthesis of epothilones and related analogs
InactiveUS6989450B2Easy to understandOrganic active ingredientsGroup 4/14 element organic compoundsEpothiloneStereoisomerism
The present invention relates to methods for use in producing epothilones and analogs and derivatives thereof. A general method according to the present invention broadly comprises performing an aldol condensation of a first compound with a second compound thereby to form a third compound selected from the formulas: and stereoisomers thereof, and performing a macrolactonization of the third compound. The present invention also provides chemical compounds, and methods for producing such chemical compounds, that are useful in producing epothilones and analogs and derivatives thereof.
Owner:UNIVERSITY OF MISSISSIPPI
Cs-supported silica-based catalyst and preparation method and application thereof
InactiveCN103801280AImprove stabilityContinuous productionMolecular sieve catalystsOrganic compound preparationGas phaseMethyl acetate
The invention provides a Cs-supported silica-based catalyst. One of SBA-15, SiO2 or silicon dioxide prepared by a silica sol method is used as a carrier, the support is selected from one of Cs salt and a mixture of Cs and modified elements, the catalyst is prepared by a dipping method, the Cs content is 0.1wt%-10wt%, and the content of the modified elements is 0-2wt%. Meanwhile, the invention further provides a preparation method of the Cs-supported silica-based catalyst and application of the Cs-supported silica-based catalyst to the process of preparing methyl acrylate by catalyzing condensation reaction of methyl acetate and a methanal gas phase. The prepared catalyst is capable of realizing the aldol condensation of the methyl acetate and the methanal gas phase under proper conditions so as to prepare the methyl acrylate, wherein the activity of the Cs / SBA-15 catalyst is higher than that of other Cs-supported silica-based catalysts, and the selectivity on the methyl acrylate is good.
Owner:SHANGHAI HUAYI GRP CO
Stable, aqueous-phase, basic catalytsts and reactions catalyzed thereby
ActiveUS20080058563A1Hydrocarbon by hydrogenationHydrocarbon purification/separationHydrogenation reactionEther
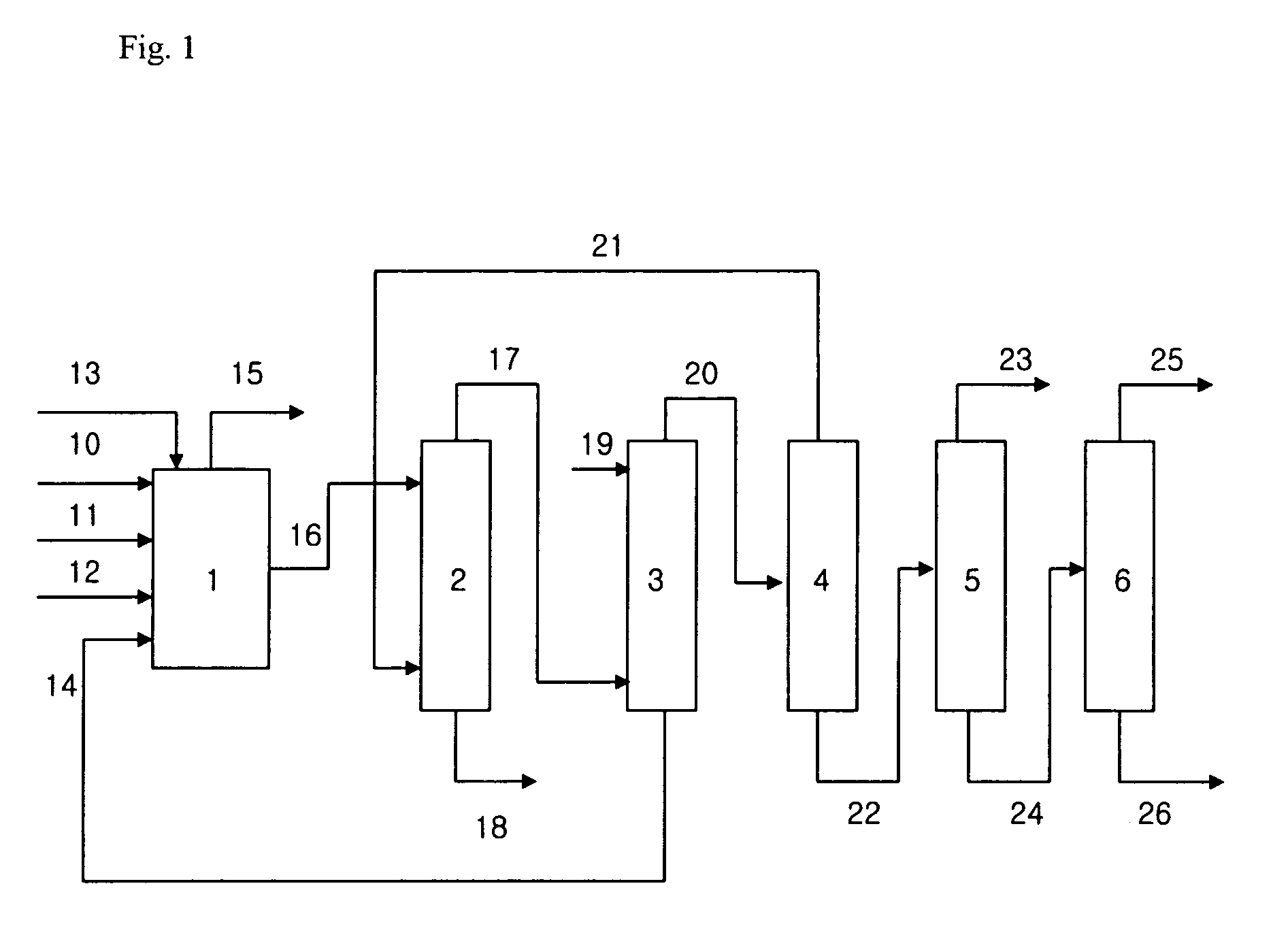
A catalytic process for converting biomass-derived carbohydrates to liquid alkanes, alkenes, and / or ethers is described. The process uses combinations of self- and crossed-aldol condensation reactions, dehydration reactions, and hydrogenation reactions, over specified metal-containing catalysts, to yield alkane, alkene, and ether products from carbohydrate reactants.
Owner:WISCONSIN ALUMNI RES FOUND
Method for preparing trimethylolproane
ActiveUS7253326B1Effective and economicalOrganic compound preparationOxygen compounds purification/separationAlcoholCannizzaro reaction
The present invention relates to a method for preparing trimethylolpropane (TMP) comprising the steps of: 1) synthesizing trimethylolpropane by using n-butyl aldehyde, an aqueous solution of formaldehyde and an aqueous solution of alkali metal hydroxide through aldol condensation reaction and Cannizzaro reaction; 2) extracting trimethylolpropane from a resultant mixture of the step 1) by contacting the resultant mixture with an alcohol having 6 to 10 carbons; 3) removing alkali metal ion from a resultant extract of the step 2) by contacting the resultant extract with water; and 4) distilling the alkali metal ion-removed extract obtained from the step 3). According to the present invention, a separate formaldehyde recovery process can be omitted, the extraction efficiency of TMP can be maximized with using a relatively small amount of extraction solvent, the separation and recovery processes for extraction solvent can be simplified since a mixture of solvents is not used for TMP extraction, and the yield of TMP can be maximized while the amount of generated waste water can be minimized, thereby producing TMP economically with good efficiency.
Owner:LG CHEM LTD
Method for preparing liquid alkane fuel by upgrading bio-oil in aqueous phase catalytic mode
ActiveCN101870881AHigh recovery rateReduce latent heat loss of vaporizationLiquid hydrocarbon mixture productionBio-feedstockAlkaneDesorption
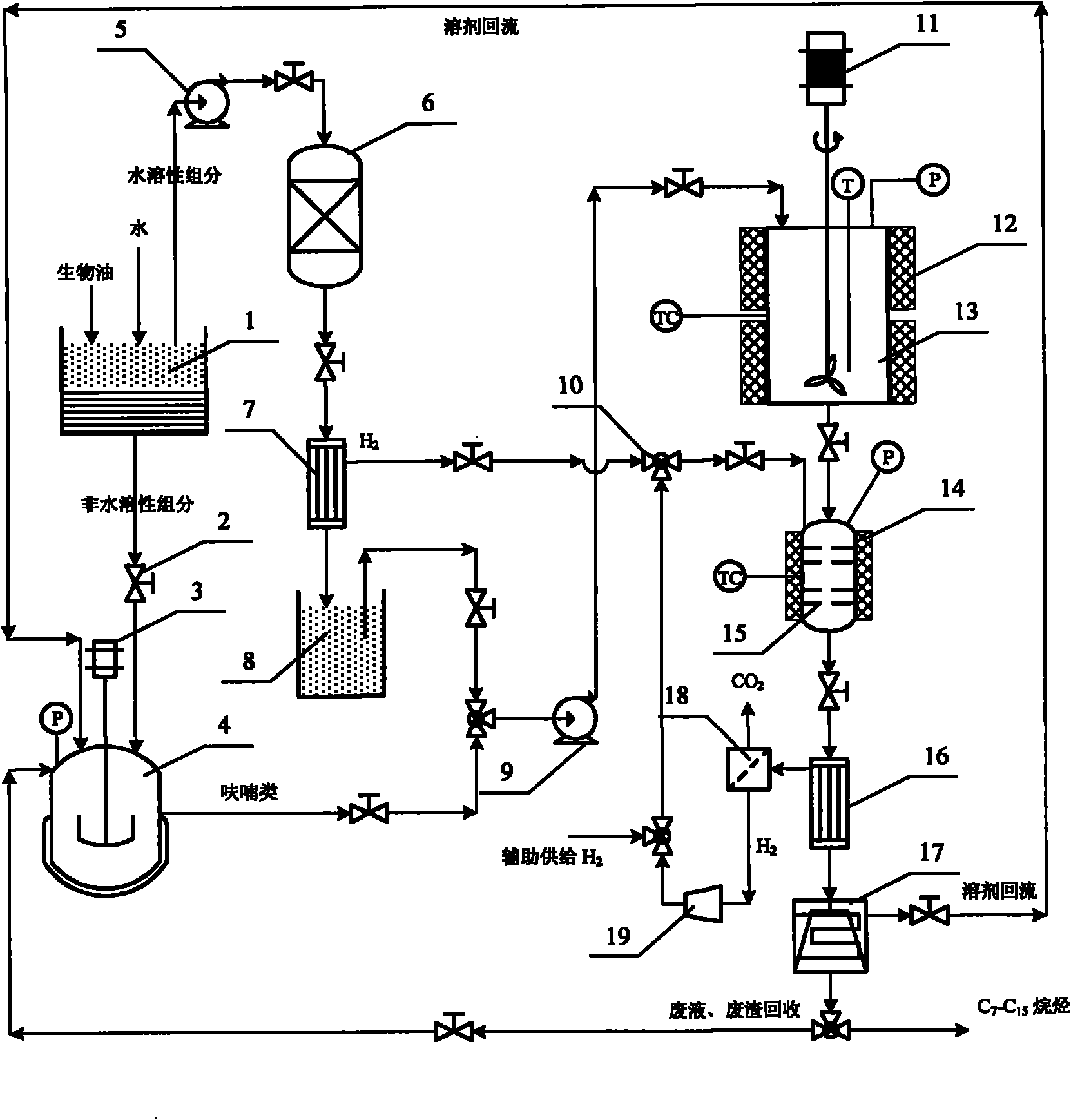
The invention provides a method for preparing liquid alkane fuel by upgrading bio-oil in an aqueous phase catalytic mode. The method comprises the following steps of: 1) pretreating the bio-oil to obtain a water-soluble component and a water-insoluble component; 2) performing a pressurized acid hydrolysis process on a furan compound of the water-insoluble component to prepare the furan compound; 3) performing a reforming and hydrogen production reaction on the water-soluble component; 4) mixing the furan compound with a liquid-phase product separated from the step 3) to perform an aldol condensation reaction to increase carbon chains; and 5) performing a hydrogenation and dehydration reaction on an aldol condensation product to obtain liquid linear alkane. The method has the advantages that: an upgrading process, which is performed in a double-phase system, can effectively contribute to the desorption of an intermediate product from the surface of a catalyst and the mass transfer of an upgraded product, and can reduce the risk of carbon deposition on the surface of the catalyst; the quality of oil obtained by the method is high; the energy density and the energy grade of the bio-oil are improved greatly; and the liquid alkane fuel can be obtained from all kinds of bio-oil so as to partially substitute for the conventional widely-used fossil gasoline and diesel oil fuel.
Owner:GUANGZHOU INST OF ENERGY CONVERSION - CHINESE ACAD OF SCI
Method of preparing long chain alkane for jet fuel by virtue of sugar platform compound
ActiveCN104650947AReduce usageAchieve phase separationLiquid hydrocarbon mixture productionBio-feedstockAlkaneIsomerization
The invention discloses a method of preparing long chain alkane for a jet fuel by virtue of a sugar platform compound. The method comprises the following steps: carrying out aldol condensation on the sugar platform compound in an aqueous solution under catalysis of alkali to generate a long chain oxygenated compound with ten to seventeen carbons; and then carrying out hydrogenation under the action of a metal catalyst, and finally carrying out hydrogenation, deoxidation, isomerization, cracking and cyclization under the action of the metal catalyst to generate a long chain n-alkane / isoparaffin with eight to fifteen carbons. According to the method disclosed by the invention, other organic solvents are prevented from being used, and in the condensation process, condensation products are separated from one another, a solid condensation product is directly separated from the aqueous solution, and the energy consumption is lowered; a solid product is dissolved by an alcohol solvent, the subsequent hydrogenation and deoxidation processes are carried out on the solid product, and the alcohol solvent can be provided by sugar / sugar alcohol hydrogenolysis; meanwhile, hydrogen needed by the technological process can be directly provided by the reforming process of a sugar alcohol solution, and the C component and the H component in a biological jet fuel and the solvent used in the process can be from biomass materials.
Owner:GUANGZHOU INST OF ENERGY CONVERSION - CHINESE ACAD OF SCI +1
Organic compounds and processes for their manufacture
Owner:EXXONMOBIL CHEM PAT INC
Process for producing a branched hydrocarbon base oil from a feedstock containing aldehyde and/or ketone
InactiveUS7850841B2Organic compound preparationCatalytic naphtha reformingIsomerizationHydrodeoxygenation
The invention relates to a process for producing high-quality hydrocarbon base oil particularly of biological origin. The process of the invention comprises aldol condensation, hydrodeoxygenation, and isomerization steps. Aldehydes and / or ketones, preferably of biological origin are used as the feedstock.
Owner:NESTE OIL OY
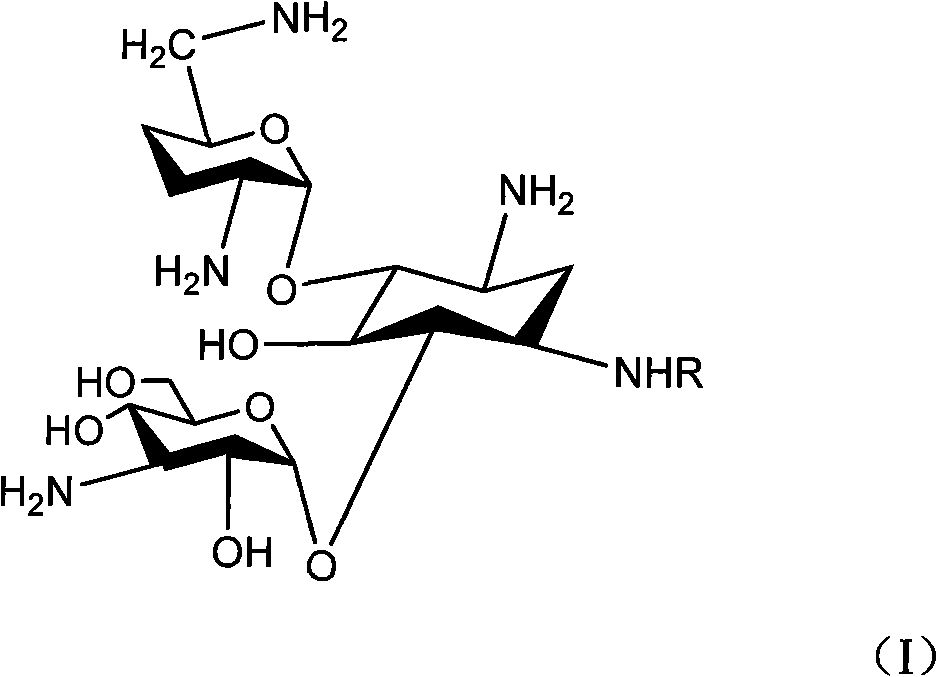
Method for synthesizing Arbekacin and intermediate dibekacin thereof
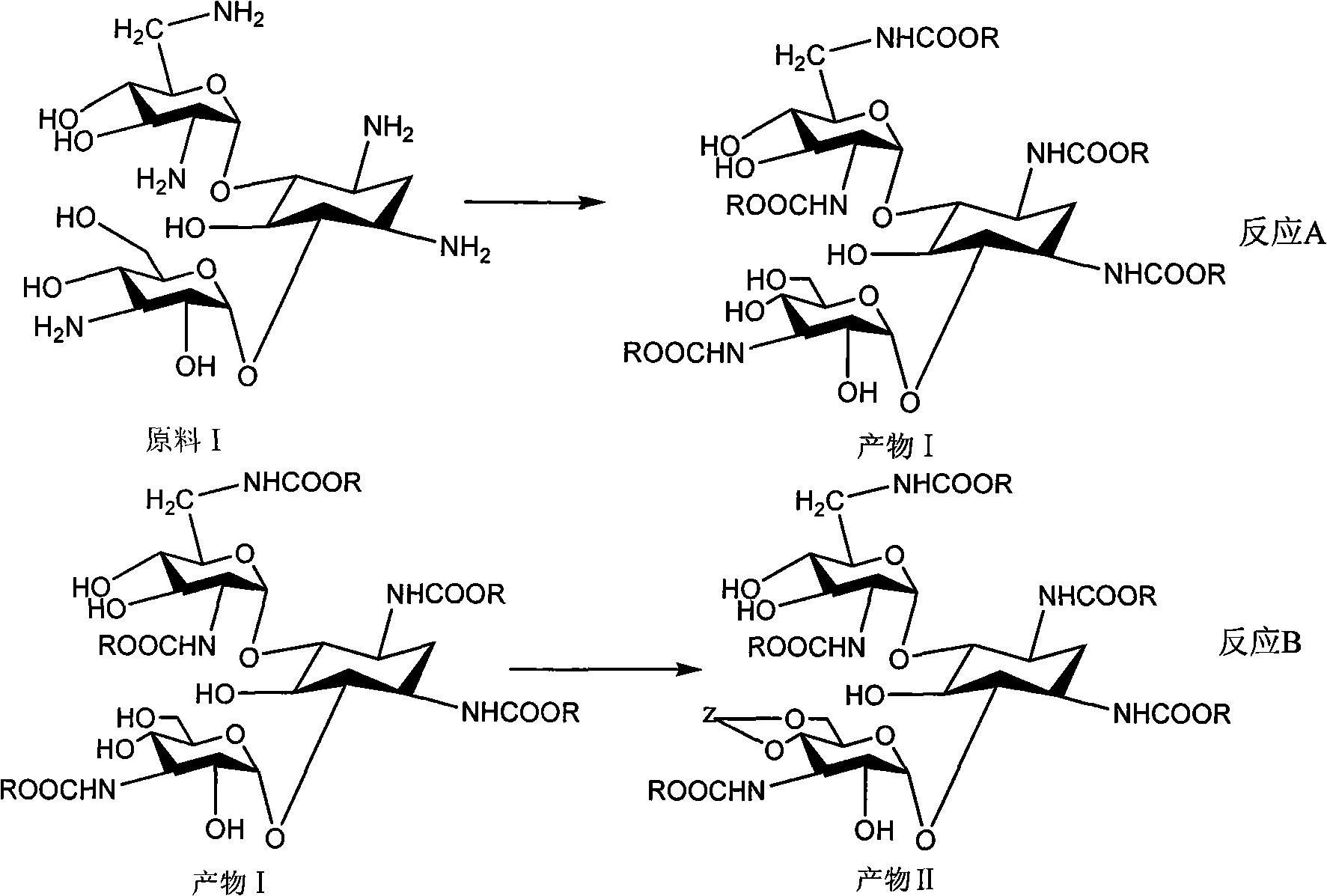
InactiveCN101575354AHigh yieldReduce generationSugar derivativesSugar derivatives preparationFluoroacetic acidSynthesis methods
The invention relates to an organic synthesis method, in particular to a method for synthesizing an Arbekacin and an intermediate dibekacin thereof. The method comprises the following steps of: takinga kanamycin B as initial raw material, carrying out the following processes of aldol condensation, sulfonylation, sodium iodide replacement and elimination to form double bond, de-protection under acidic condition, amino-electron reduction and final hydrogenation, thus obtaining the dibekacin; taking 3',4'-dideoxy -3',4'-didehydro-kanamycin B as raw material, using a di-tert-butyl dicarbonate toselectively protect the amidogen of 3, 2', 6', 3'' sites; subsequently using the synthesized active ester to protect the 1-site amidogen; subsequently using tri-fluoroacetic acid to remove BOC; and carrying out hydrazinolysis and catalysis and hydrogenation of platinum oxide, thus obtaining the Arbekacin. The synthesis method has the advantages of simple operation, high outcome yield, reducing thecost of raw material, optimizing the reaction route, lowering the requirements to the reaction conditions and being beneficial to industrial production.
Owner:BEIJING UNIV OF CHEM TECH +1
Ionic liquid precursors and their supported mesoporous materials, synthesis and applications
InactiveCN102276642AReduce usageReduce use costGroup 4/14 element organic compoundsOrganic compound preparationChemical reactionIonic conductance
The invention relates to an ionic liquid precursor and a mesoporous material for supporting the ionic liquid precursor, synthesis and application. A preparation method comprises the following steps of: 1) synthesizing the ionic liquid precursor; 2) synthesizing the mesoporous material for supporting the ionic liquid precursor; and 3) acidizing or alkalifying the synthesized mesoporous material for supporting the ionic liquid precursor according to the requirements so as to obtain a target product. The loading capacity of the mesoporous material for supporting the ionic liquid precursor is relatively adjusted, and a mesoporous material for supporting functionalized ionic liquid can be obtained by further acidizing or alkalifying the material according to the requirements. The amount, strength of acid and alkali, properties, variety and the like of ionic liquid supported on the material are adjustable, so that the material can meet the demand of application such as various acid and alkaline catalytic reactions, chemical adsorption and separation and the like. By the material, the using amount of the ionic liquid is greatly reduced, the better catalytic effect is achieved when the material is applied to aldol condensation reactions, and the recycling of a catalyst can be realized. The kind of material is expected to be used for catalyzing more chemical reactions, and may be applied in the fields of adsorption, separation, ionic conductance and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Organic compounds and processes for their manufacture
InactiveUS6090986AOrganic compound preparationPreparation by hydrogenationSimple Organic CompoundsAlcohol
PCT No. PCT / EP96 / 00267 Sec. 371 Date Oct. 27, 1997 Sec. 102(e) Date Oct. 27, 1997 PCT Filed Jan. 17, 1996 PCT Pub. No. WO96 / 22268 PCT Pub. Date Jul. 25, 1996Esters of branched C9 alcohols suitable as plasticizers are formed by esterification of a C9 alcohol produced by the aldol condensation from propanal and a C6 aldehyde and hydrogenation, the propanal optionally having been made from natural gas streams.
Owner:EXXON CHEM PAT INC
Aldol condensation reaction solid base catalyst and application
ActiveCN102019177AReduce usageReduce Disposal EmissionsOrganic compound preparationCarbonyl compound preparationAlkali metal oxideGas phase
The invention relates to an aldol condensation reaction solid base catalyst and application. A composite oxide is used as a carrier which carries at least one alkali metal oxide in the catalyst, the composite oxide is a titanium oxide-aluminum oxide composite oxide or a zirconium oxide-aluminum oxide composite oxide, the mass percentage of Ti or Zr in the composite oxide is 0.1 to 30 percent, thealkali metal oxide is calcium oxide or magnesium oxide, and the mass percentage of the alkali metal in the catalyst is 0.1 to 40 percent; a fixed bed reactor is adopted, aliphatic aldehyde is contacted with the catalyst under a liquid phase condition at the temperature of between 60 and 260 DEG C to generate unsaturated aldehyde by reaction, and the catalyst is solid, so alkali aqueous solution is avoided, treatment and discharge of organic waste water are reduced, and the catalyst has excellent catalytic performance for the aldol condensation reaction; and particularly, the catalyst has goodstability, the reaction temperature is low compared with gaseous phase reaction, and the reaction result is superior to the reaction result under the gaseous phase reaction condition.
Owner:PETROCHINA CO LTD
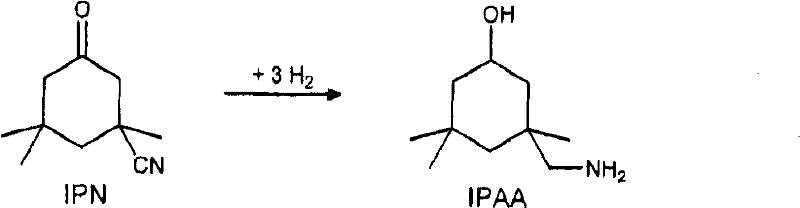
Process for preparing 3-aminomethyl-3,5,5-trimethylcyclohexylamine
InactiveCN102531916AIncrease concentrationOrganic compound preparationWater/sewage treatment by heatingIsophoroneAsymmetric hydrogenation
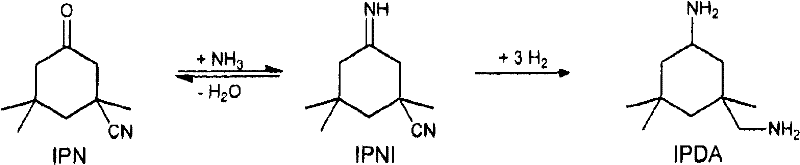
The invention relates to an improved process for preparing 3-aminomethyl-3,5,5-trimethylcyclohexylamine, referred to hereinafter as isophoronediamine or, in abbreviated form, IPDA, by: I. preparation of isophorone by catalyzed aldol condensations with acetone as reactant; II. Reaction of isophorone with HCN to form isophoronenitrile (IPN, 3-cyano-3,5,5-trimethylcyclohexanone); III. catalytic hydrogenation and / or catalytic reductive amination (also referred to as aminative hydrogenation) of 3-cyano-3,5,5-trimethylcyclohexanone, hereinafter called isophoronenitrile or, in abbreviated form, IPN, to give the isophoronediamine.
Owner:EVONIK DEGUSSA GMBH
Preparation method of neopentyl glycol
ActiveCN103449970AReduce saponification processing stepsHigh purityOrganic compound preparationHydroxy compound preparationPolymer sciencePtru catalyst
The invention provides a preparation method of neopentyl glycol, and belongs to the technical field of the preparation of alcohols. The preparation method includes the following steps: (1) regulating the pH value of a hydroxypivalaldehyde solution to 7.5-10; (2) obtaining a neopentyl glycol crude product by catalytic hydrogenation of hydroxypivalaldehyde treated by the step (1) under the effect of a hydrogenation catalyst at the temperature of 110-140 DEG C and with the pressure of 2.0-5.0 Mpa; and (3) purifying the neopentyl glycol crude product obtained in the step (2). The content of esters and other side products in the neopentyl glycol crude product prepared by the preparation method of the neopentylene glycol is extremely low, and the yield and purity of the neopentyl glycol are improved. In addition, a hydroxypivalaldehyde raw material system can be directly used for catalytic hydrogenation without any treatment, processes of aldol condensation and catalytic hydrogenation for preparation of the neopentyl glycol can be reduced, and the production efficiency can be improved.
Owner:BEIJING SJ ENVIRONMENTAL PROTECTION & NEW MATERIAL CO LTD +1
Method for synthesizing methyl acrylate by means of filling catalysts in sections
InactiveCN101575290AImprove reaction efficiencyImprove conversion rateComponent separationOrganic compound preparationFurnace temperatureMethyl acetate
The invention provides a method for synthesizing methyl acrylate by means of filling catalysts in sections; the method comprises the steps: methyl acetate and methylal are taken as raw materials for synthesizing methyl acrylate; the mole ratio of the methyl acetate to the methylal is 1:1 to 3:1; charging space velocity is 1 to 5h; the furnace temperature is fixed to be 350 DEG C to 420 DEG C; a reaction tube is divided into 9 sections; when two catalysts, i.e. phosphovanadate and Cs-Sb2O5 / SiO2, are filled in different sections from top to bottom, the phosphovanadate can be filled in the second section to the sixth section and the cesium or alkali metal catalyst can be filled in the second section to the ninth section; the conversion per pass of methylal is 30 percent to 50 percent; the selectivity of the methyl acrylate is 60 percent to 91 percent; and the yield per pass of the methyl acrylate is 40 percent to 50 percent. With the method for synthesizing the methyl acrylate by means of filling catalysts in sections, the reaction between the methyl acetate and the methylal can be further improved, the conversion rate of the methylal can be raised, and the yield of the target product, namely, the methyl acrylate, can be increased.
Owner:HARBIN INST OF TECH +1
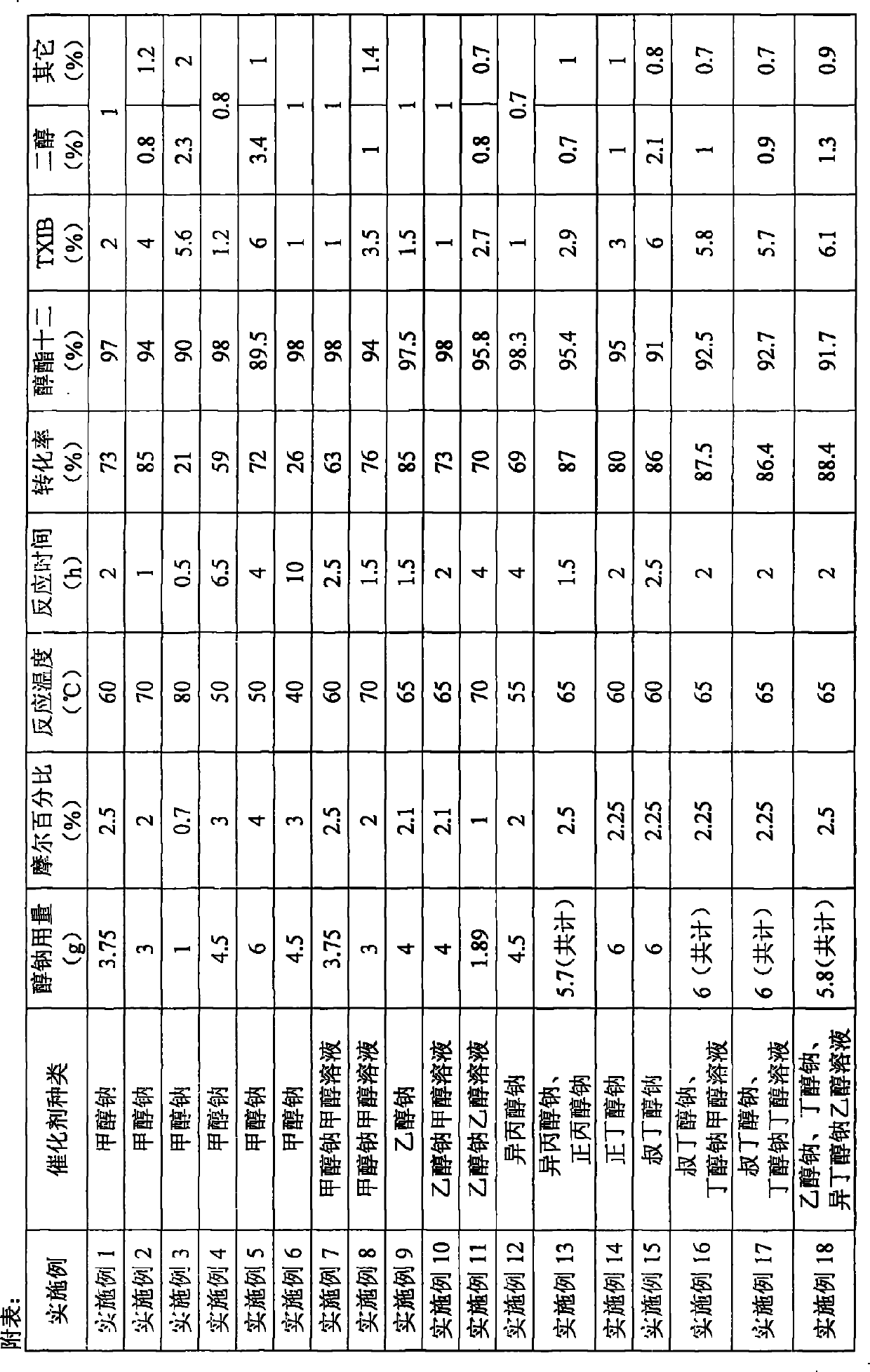
Method for preparing 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate
ActiveCN101948386AAvoid process problemsAvoid energy consumptionPreparation by aldehyde oxidation-reductionAlcoholOrganic synthesis
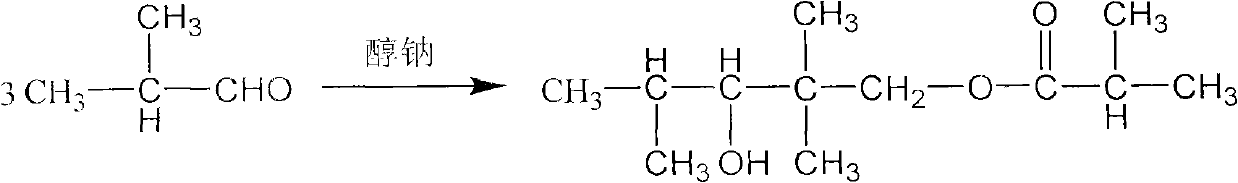
The invention discloses a method for preparing 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate, which relates to the technical field of organic synthesis. In the invention, isobutylaldehyde is used as a raw material, aldol condensation and Cannizzaro reactions are completed by a one-step reaction process in the presence of a catalyst, and the process adopts sodium alcoholate as the catalyst; and the isobutylaldehyde is used to form alcohol ester-12 in the presence of the sodium alcoholate catalyst, so the complex and high-energy consumption drawbacks of a two-step reaction process using an inorganic alkali as a catalyst are overcome, the drawbacks of toxic catalyst and high quality requirement on the isobutylaldehyde raw material of a one-step reaction process using Ba(OH)2 as a catalyst are overcome, and simple process, high single-pass yield, small catalyst dosage, low energy consumption, safety and environmental protection are realized.
Owner:YIXING HENGXING FINE CHEM
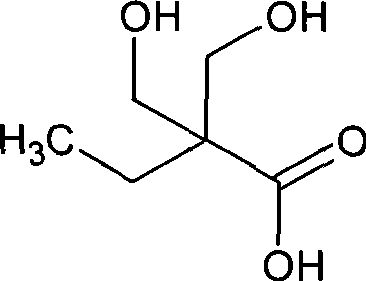
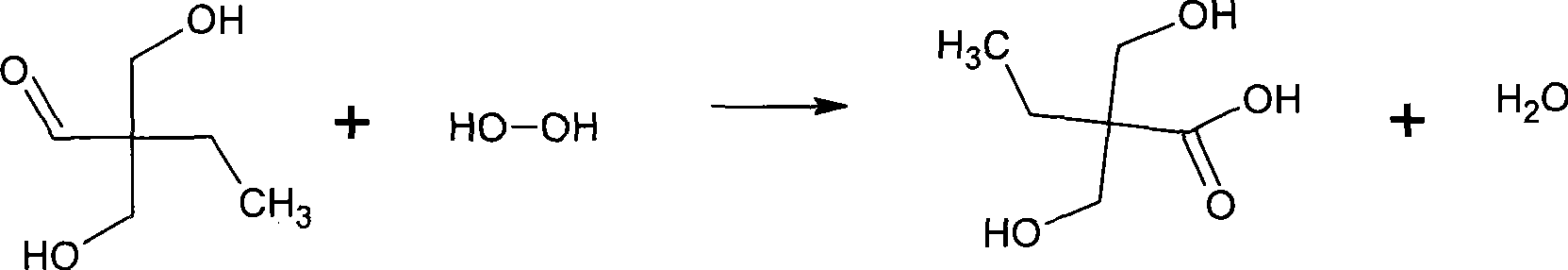
Method for synthesizing 2,2-bis(hydroxymenthyl)butyric acid
InactiveCN101381299AEasy to controlMild reaction conditionsCarboxylic preparation by oxidationOrganic synthesisOxygen
The invention belongs to the field of fine organic synthesis. The invention selects the mixture of NaCO3, Ba(OH)2, triethylamine and NaCO3, or the mixture of triethylamine and Ba(OH)2 as a catalyst, and makes use of butyraldehyde and formaldehyde to prepare 2, 2-hydroxymethyl butyric acid under the oxidation of hydrogen peroxide or oxygen through aldol condensation reaction. The invention prepares the 2, 2-hydroxymethyl butyric acid in the alkaline condition, thus the side reaction is less, the yield of the 2, 2-hydroxymethyl butyric acid is higher, and the industrialization of the invention is easy to realize.
Owner:QILU UNIV OF TECH
Preparation method of high-density liquid hydrocarbon fuel
InactiveCN106867565AThe synthetic route is simpleEfficient synthetic routeLiquid hydrocarbon mixture productionBio-feedstockCarbon numberAlkane
The invention relates to a preparation method of high-density liquid hydrocarbon fuel. The method totally comprises two parts: (1) on a first catalyst bed layer of a fixed bed continuous reactor, cyclopentanone (furfural water phase selective hydrogenation products) takes a self aldol condensation (and hydrogenation) reaction under the catalysis effect of acid / alkali catalysts or metal doping solid alkali to obtain oxygen-containing organic compounds with the carbon number being 15 and 10; (2) on a second catalyst bed layer of the fixed bed continuous reactor, the products generated on the first catalyst bed layer take a further hydrodeoxygenation reaction under the conditions of low temperature and no solvent under the catalysis effect of load metal A / X type bifunctional catalysts to obtain polycyclic hydrocarbon fuel with the carbon number being 15 and 10. The high-density liquid fuel (C15 tri-cycloalkane: 0.917 g / cm<3>; C10 bi-cycloalkane: 0.866g / cm<3>) has the advantages that the energy carried by an aircraft can be effectively improved; the engine oil consumption rate is reduced; the requirements of high flight speed, great load and long emitting range can be met.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for prevention of fouling in basic solution by inhibiting polymerization and solubilizing deposits using amino acids
InactiveUS6986839B2Prevent scalingRemoving polymeric depositThermal non-catalytic crackingDistillation corrosion inhibitionHydrolysisCaproic Acid
A method for inhibiting and dissolving the deposits formed on caustic or alkaline scrubbers used in scrubbing acidic gases such as carbon dioxide, hydrogen sulfide, which are formed during the pyrolytic cracking of naphtha, ethane, and propane. The cracking operations produce certain oxygenated compounds such as vinyl acetate or acetaldehyde, which undergo polymerization under alkaline condition. The vinyl acetate on hydrolysis releases acetaldehyde under alkaline conditions. Amino acids such as 6 amino caproic acid and lactams such as epsilon caprolactam not only prevent but also dissolve the polymers formed by aldol condensation.
Owner:DORF KETAL CHEM (I) PTE LTD
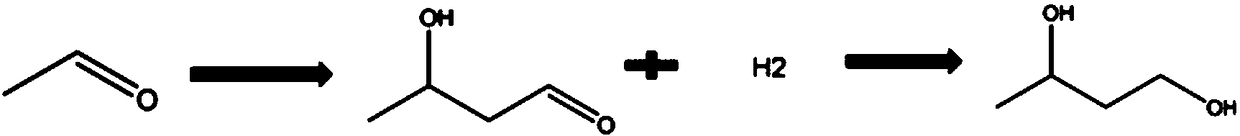
Preparation method of 1, 3-butylene glycol
ActiveCN109422624AControl PH valueTemperature controlOrganic compound preparationHydroxy compound preparationFixed bed1,3-Butanediol
The invention provides a preparation method of 1, 3-butylene glycol. The preparation method comprises following steps: A, acetaldehyde is introduced into a fixed bed reactor, under the effect of a supported type solid basic catalyst, aldol condensation reaction is carried out so as to obtain 3-hydroxybutyraldehyde; and B, 3-hydroxybutyraldehyde is subjected to continuous hydrogenation reaction inthe fixed bed reactor so as to obtain 1, 3-butylene glycol. According to the preparation method, the fixed bed reactor is adopted, at the same time, the supported type solid basic catalyst is adoptedto replace a conventional liquid alkali (such as sodium hydroxide) catalysts, and in the step of hydrogenation reduction, a supported nickel hydrogenation catalyst is adopted. The preparation method is capable of solving problems in the prior art product quality is poor, product yield is low, technology process is complex, and a large amount of waste water and waste residue is generated; aldol condensation quenching step is avoided; side reactions are reduced; relatively high reaction conversion rate and yield are achieved; no neutralizing or desalting process is needed in reaction process; and great improvement of traditional 1, 3-butylene glycol preparation technology is realized.
Owner:DONGYING HI TECH SPRING CHEM IND
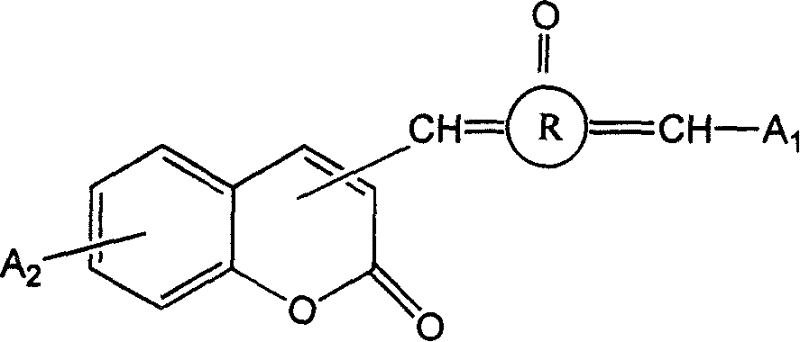
Coumarin dyestuff linked by naphthene ketones, method for synthesis and use thereof
The invention provides a coumarin dyestuff linked by naphthene ketones, method for synthesis and use, wherein the method employs the conventional hydroxyaldehyde condensation reaction and consists of, charging naphthene ketone, corresponding aldehydes with A1 groups into reactor, obtaining semi-naphthene ketone dyestuff with A1 groups, subjecting corresponding third position methyl substituted or fourth position methyl substituted coumarin having A2 group and selenious acid in another catalyst case to obtain coumarin aldehyde having A2 group, subjecting the A1 group semi-naphthene ketone dyestuff and A2 group coumarin aldehydes to reaction at the presence of mixed solvent of dimethylbenzene and alcohol, thus obtaining coumarin type dyestuffs coupled by naphthene ketones.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Catalyst for aldol condensation reaction and preparation method thereof
InactiveCN102600827AHigh activityEasy to prepareOrganic compound preparationCarbonyl compound preparationAlkaline earth metalRare earth
The invention relates to a catalyst for aldol condensation reaction and a preparation method of the catalyst. The catalyst is prepared by an immersion method, the active ingredient main catalyst is alkali metals or alkaline earth metals, and the cocatalyst is rare earth metals; and the catalyst is obtained by loading one or more of alkali metals or alkaline earth metals on a carrier, simultaneously loading one or more of rare earth metals and calcining at a certain temperature. The catalyst has the characteristics of simple preparation method, low price, high catalytic activity and long life.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Water resistant catalyst for aldol condensation as well as preparation method and application thereof
ActiveCN103551148AGood physical and mechanical propertiesIncrease resistance to poisoningOrganic compound preparationCarboxylic acid esters preparationPtru catalystPropanoic acid
The invention discloses a water resistant catalyst for aldol condensation. The water resistant catalyst comprises a main active ingredient, an active agent and a carrier, wherein the main active ingredient is one or more selected from oxides or salts of Cs; the active agent is one or more selected from oxides or salts of Sb, Nb, Ag, Al and Zr; the carrier comprises SiO2 and a carrier aid; the carrier aid is one or more selected from Al2O3, ZrO2, diatomite and kaolin; the SiO2 is hydrophobic nano SiO2; or (2) the catalyst is subjected to water-resistant treatment. The water resistant catalyst is used for aldol condensation, has the characteristics of high activity, high selectivity and long service life, is especially used for synthesizing methyl methacrylate through formaldehyde and methyl propionate. With calculation of methyl propionate, the selectivity of methyl methacrylate is 94 percent, and the single service life of the catalyst is over 400 hours. The catalyst is simple in preparation method and is suitable for industrial application.
Owner:HAO HUA CHENGDU TECH
Asymmetric synthesis for chiral huperzine A
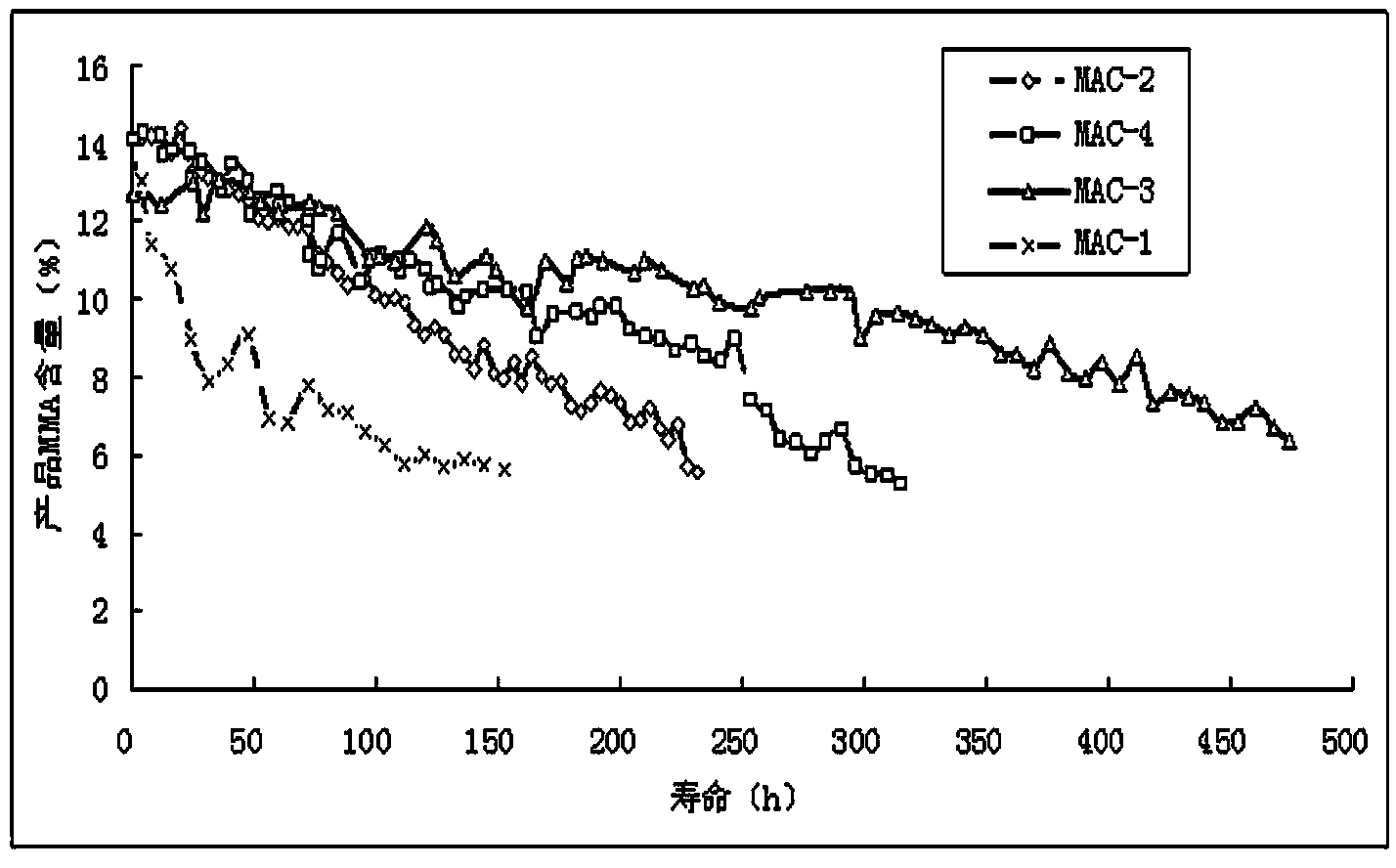
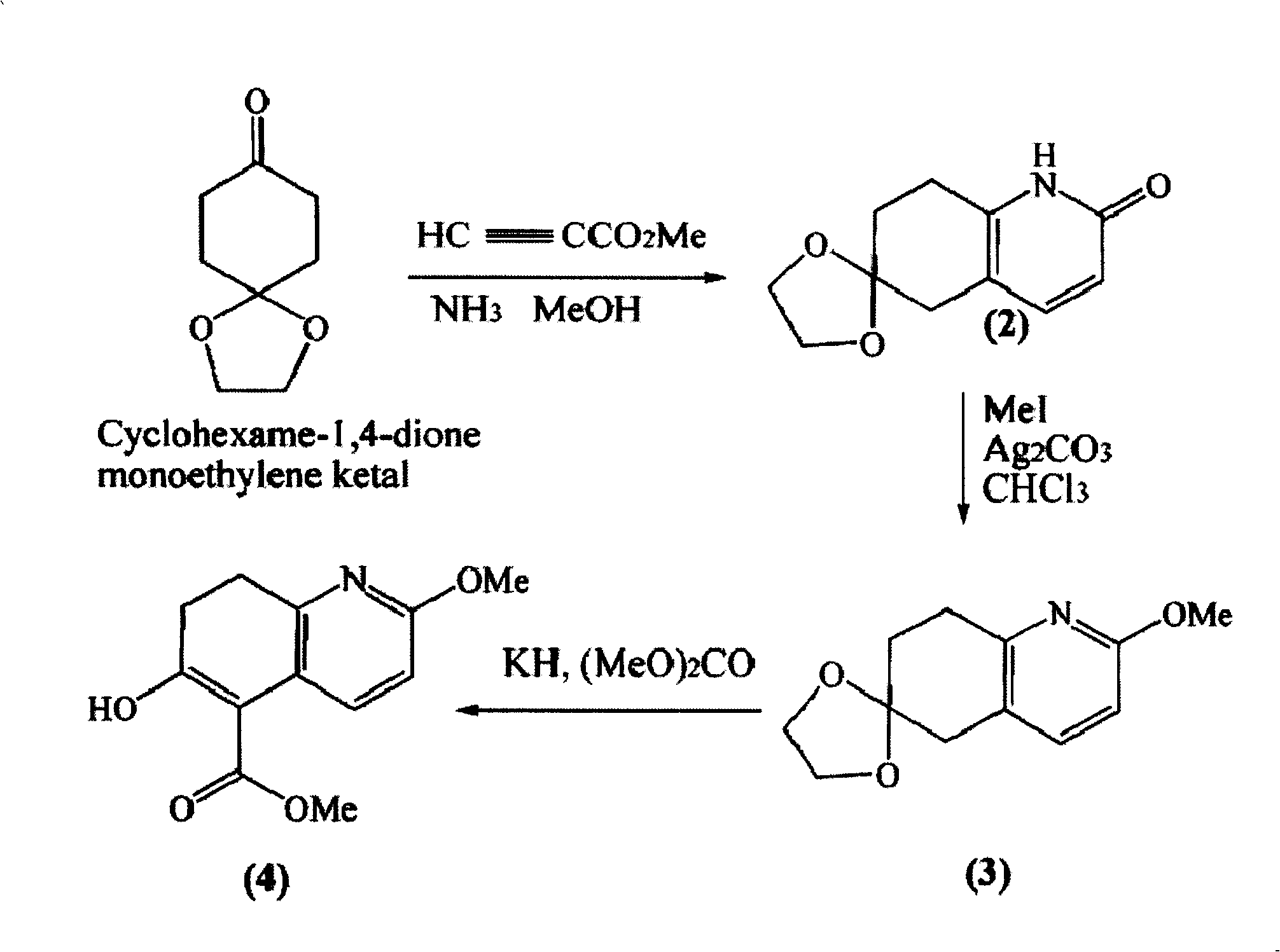
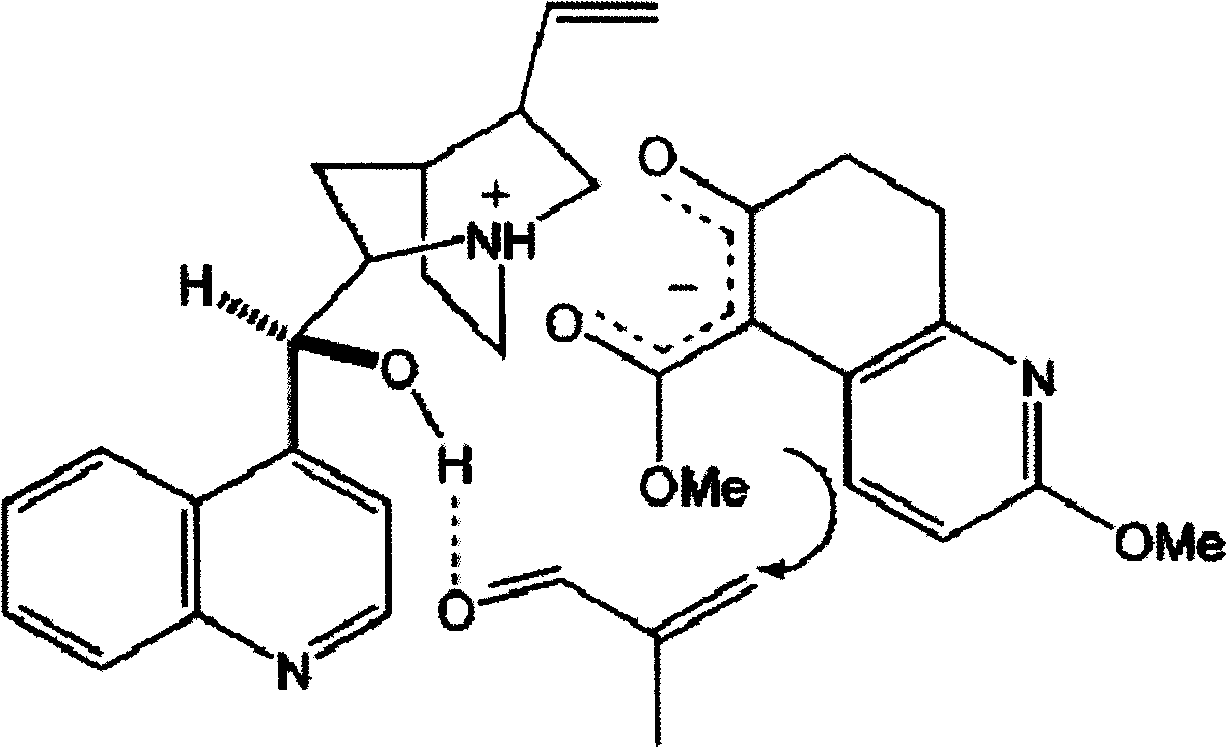
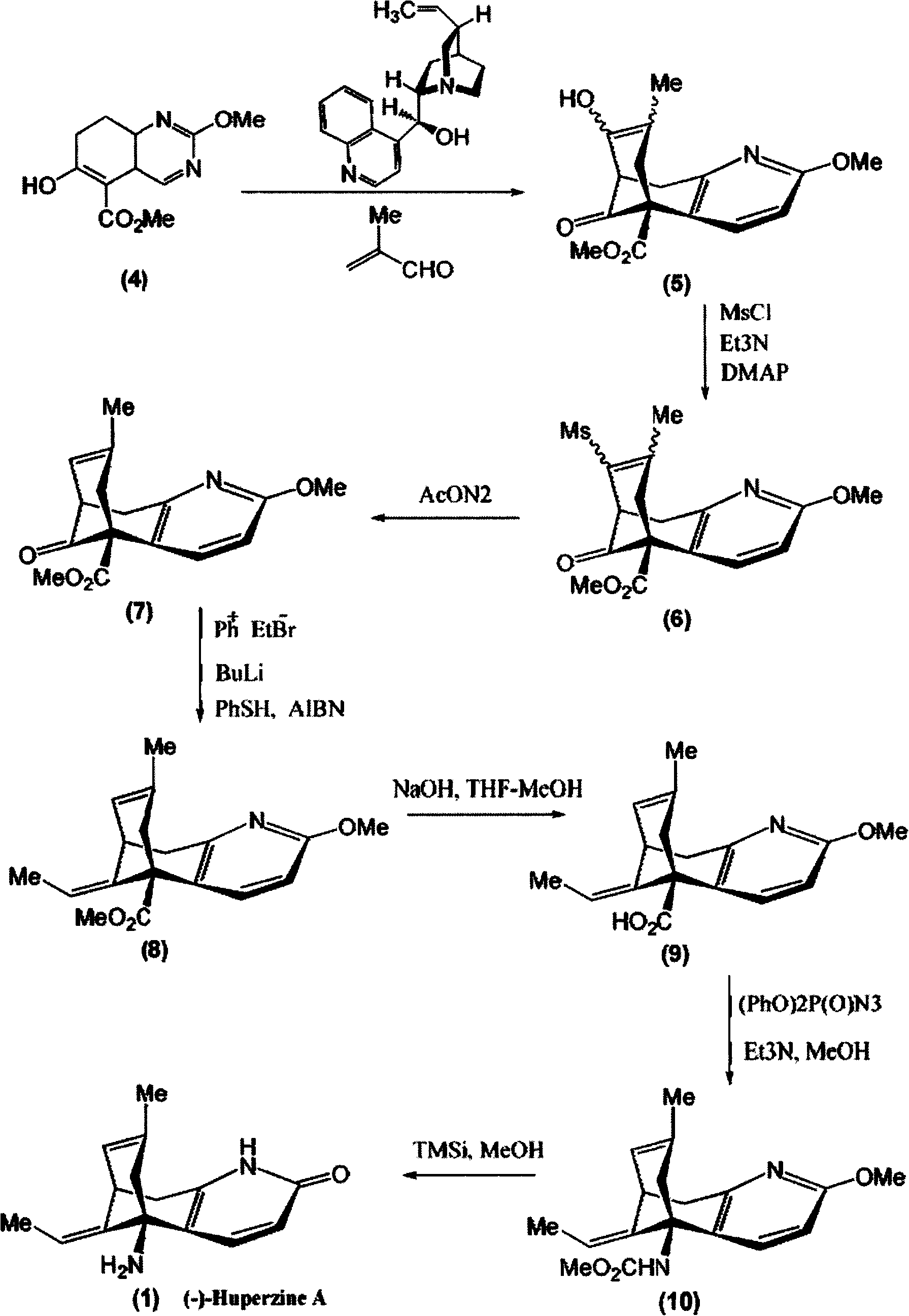
Disclosed is an asymmetric total-synthesis method for chiral huperzine a. The method takes 1,4-dihydro-spiro (4,5) -8 -decanone as starting material to get benzoate through hydroxymethylation and the treatment of benzoyl chloride and K2CO3; the benzoate is reacted with hydrogen sulfate O-methyl iso urea to get quinazoline; after the ketal is eliminated, Mander reagent is used for methyl esterification reaction so as to obtain beta-keto ester. Chiral ammonia, such as cinchona alkaloid, is used to promote the tandem asymmetric Michael addition / aldol condensation reaction of beta-keto esters and methyl acrolein. The compound carboxylate of diastereoisomer is reacted with MsCl, triethylamine and DMAP to get transformed. Through TMSI and MeOH processing, the protective group is removed so as to obtain optically pure-chiral huperzine a.
Owner:京山瑞生制药有限公司 +1
Process for the co-production of alcohols
InactiveUS20040138510A1Efficient processingPromote formationOrganic compound preparationPreparation by hydrogenationSalting outWater soluble
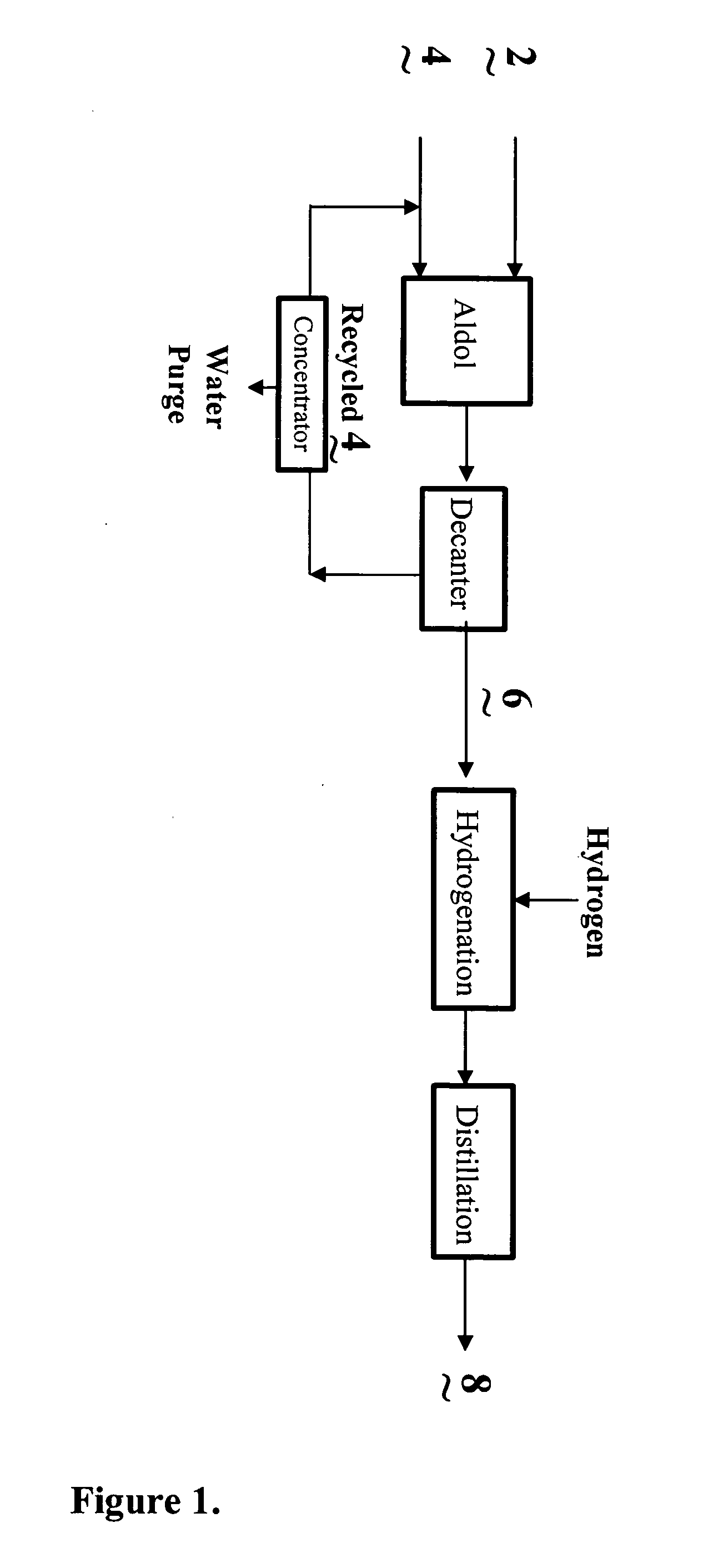
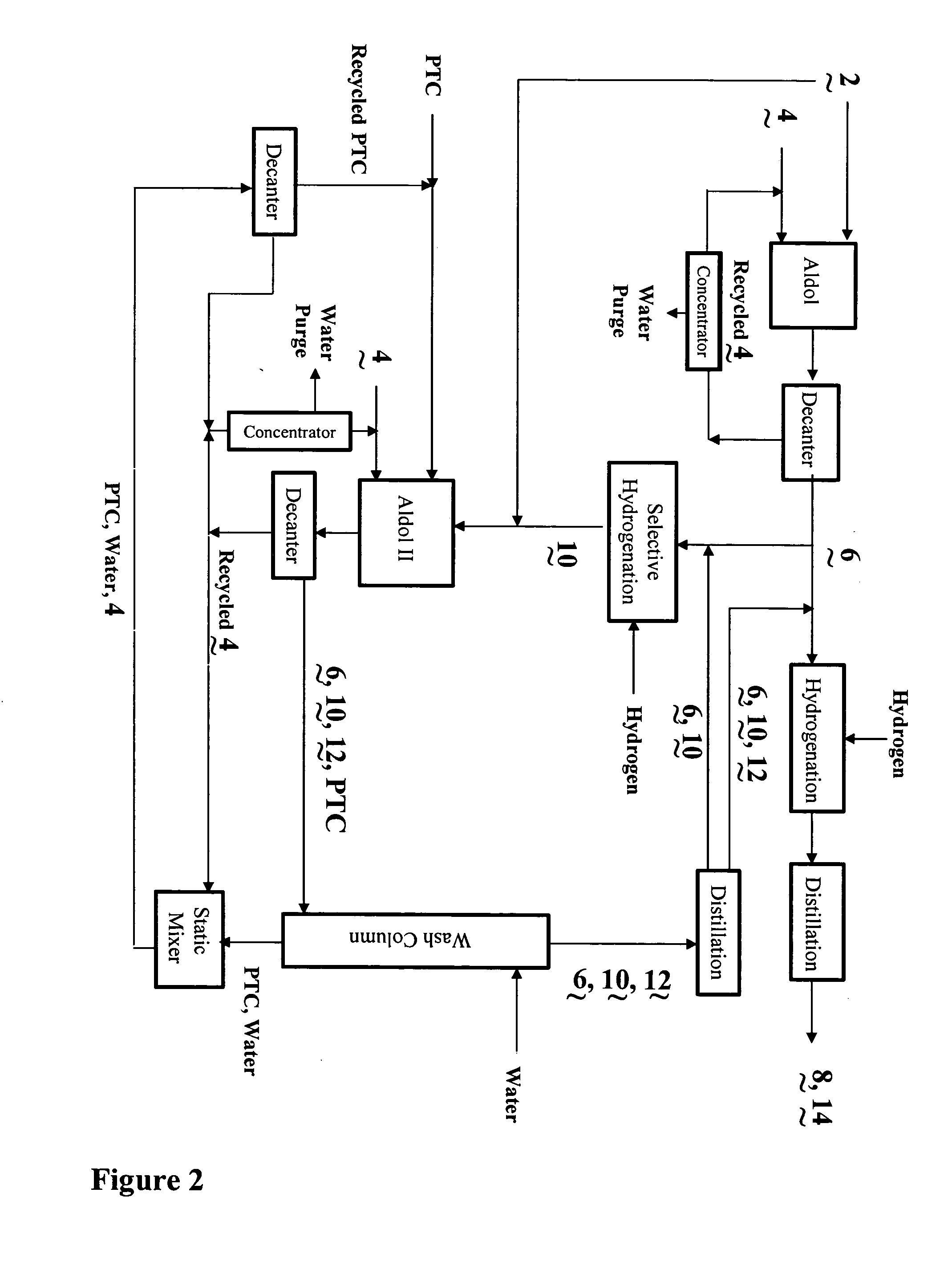
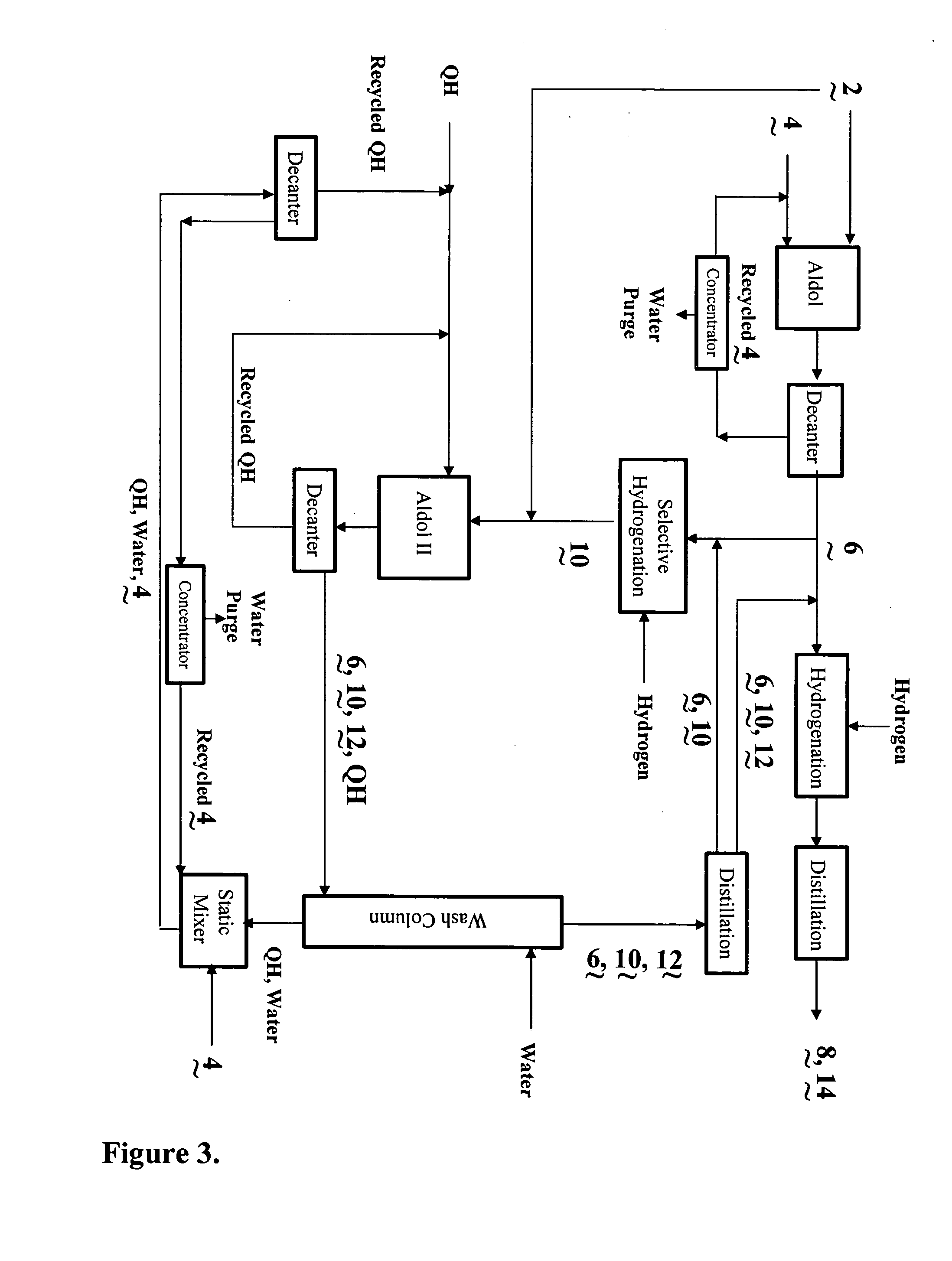
The present invention relates to the co-production of unsaturated aldehydes via a crossed-aldol condensation reaction catalyzed by recyclable water-soluble phase-transfer catalysts or the hydroxides thereof. The aldehydes are then hydrogenated to the desired alcohol products or saturated aldehyde feed stocks. Specifically, methods in which 2,4-diethyloctanol is co-produced with 2-ethylhexanol in batch and continuous processes are described. Recovery of the phase-transfer catalyst through water washing followed by "salting out" from the washings is also demonstrated.
Owner:BASF CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com