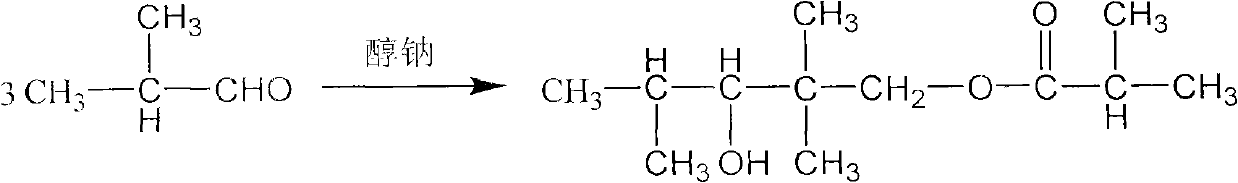
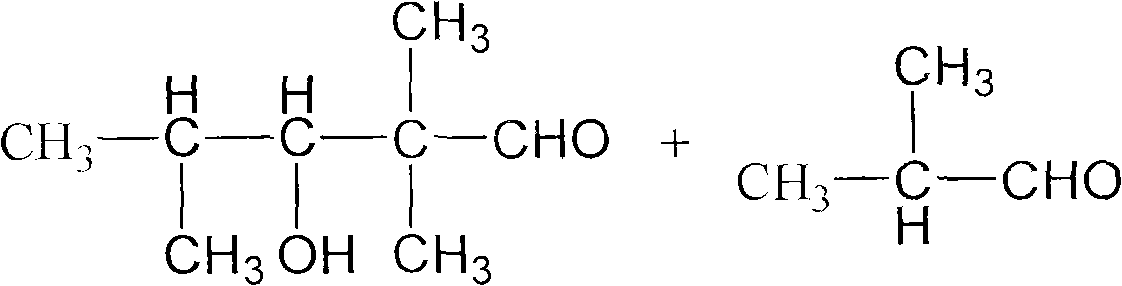Method for preparing 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate
A technology of pentanediol monoisobutyrate and trimethyl, applied in the field of preparation 2, can solve the problems of low isobutyraldehyde conversion rate target product selectivity, high quality requirements, no industrialization prospects, etc., and avoids the reaction process. Complicated, high yield per pass and less catalyst dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
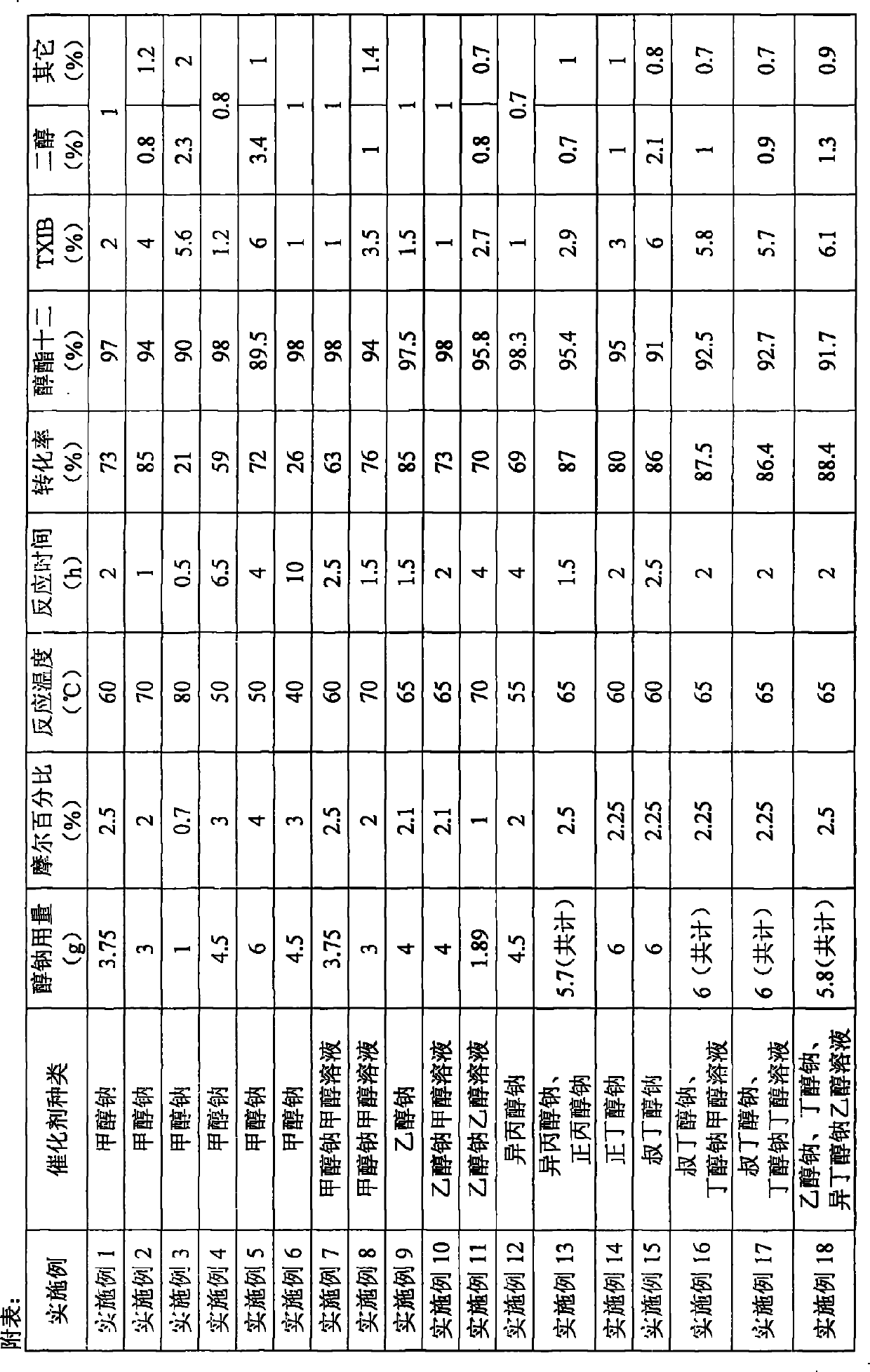
[0021] Embodiment 1, in the 500ml there-necked flask that is equipped with reflux condenser and stirring, add 200g isobutyraldehyde (2.778mol), catalyzer is dry solid sodium methylate powder 3.75g (0.0694mol), and sodium methylate is added intermittently, will The temperature of the reaction system was raised to 60° C., and the stirring reaction was continued for 2 h, and the reaction was carried out under normal pressure. Then it was cooled to room temperature, washed with water, and the reaction mixture was analyzed, and the conversion rate of isobutyraldehyde was 73%. Unreacted isobutyraldehyde was removed under reduced pressure, and the crude product was analyzed. The alcohol ester dodecane content was 97%, the by-product TXIB content was about 2%, and other components accounted for about 1%, of which 2,2,4-trimethyl -1,3-pentanediol content is very small. The crude product is then separated by vacuum distillation, and more than 99.5% of the finished product of alcohol es...
Embodiment 2
[0023] Example 2, the reaction device, isobutyraldehyde and its dosage are the same as in Example 1, the catalyst is 3 g (0.0556 mol) of sodium methoxide, the sodium methoxide is added intermittently, and the temperature of the reaction system is raised to 70° C., and the stirring reaction is continued for 1 h. Aftertreatment and product analysis are the same as in Example 1.
Embodiment 3
[0024] Example 3, the reaction device, isobutyraldehyde and its dosage are the same as in Example 1, the catalyst is 1 g (0.0185 mol) of sodium methoxide, sodium methoxide is added intermittently, and then the temperature of the reaction system is raised to 80° C., and the reaction is continued for 0.5 h with stirring. Aftertreatment and product analysis are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com