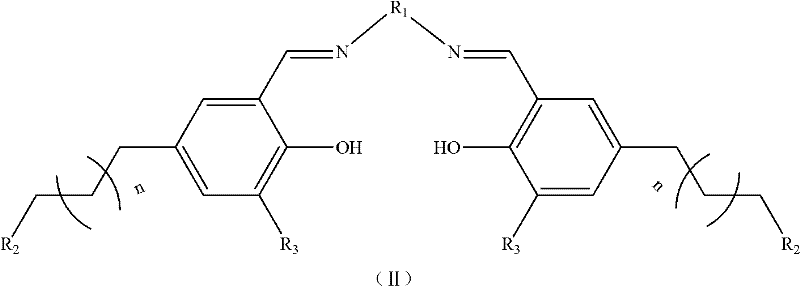
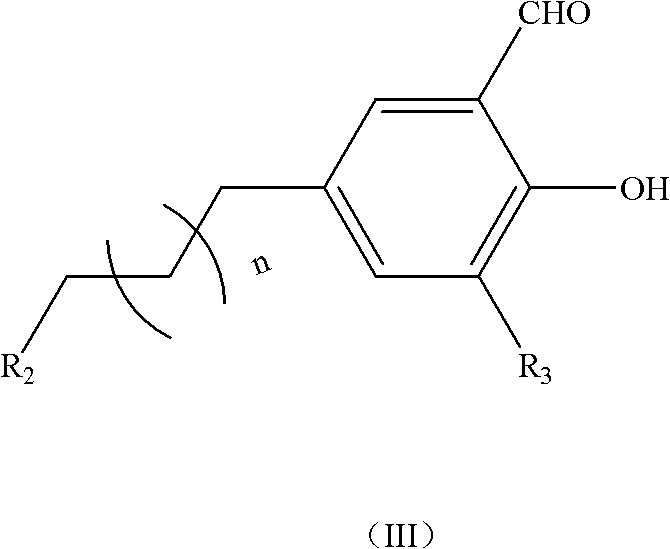
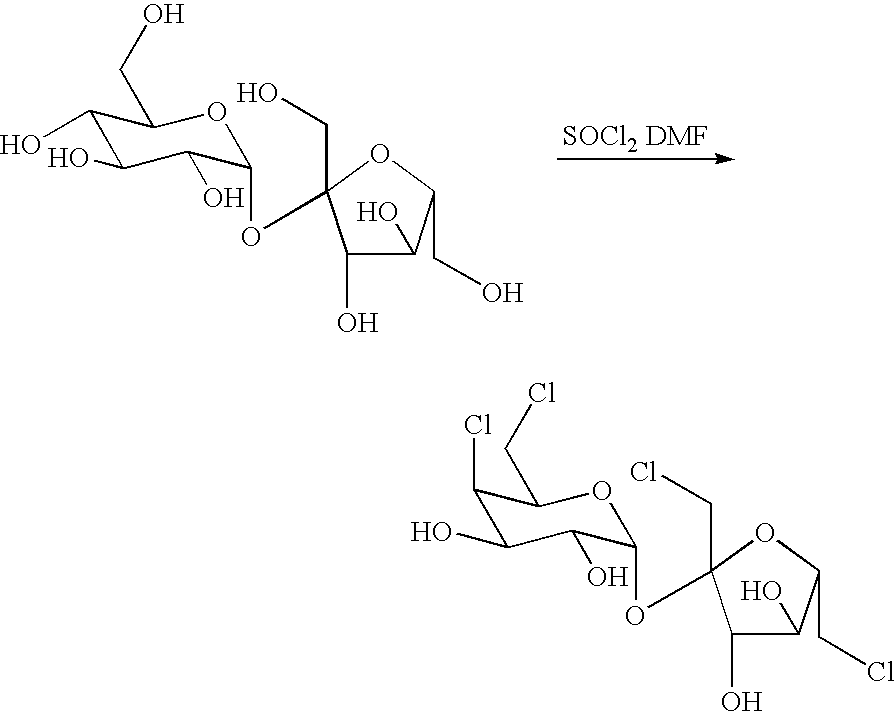
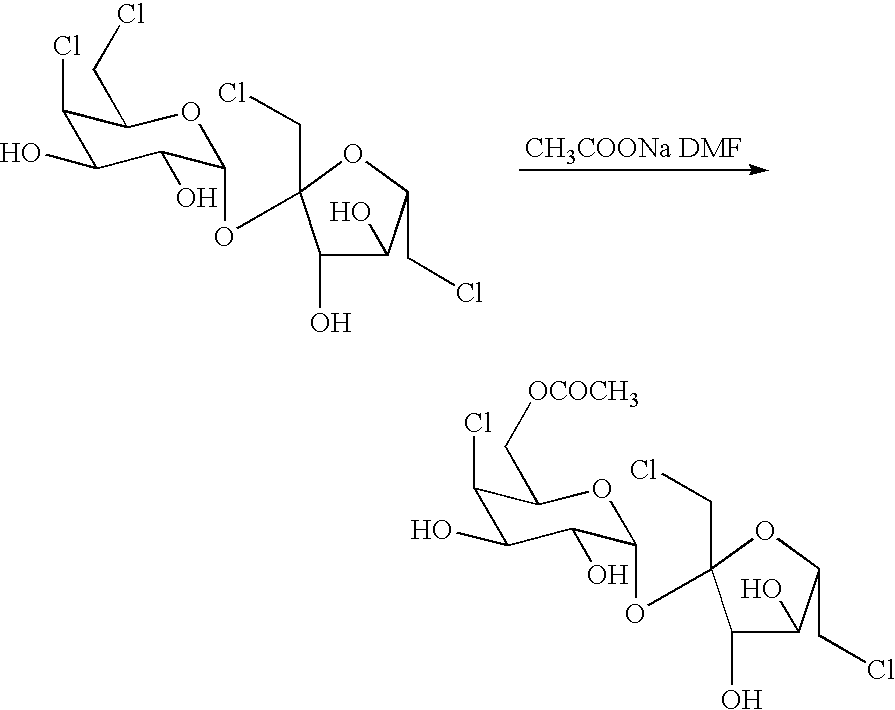
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1092 results about "Sodium methoxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sodium methoxide is a chemical compound with the formula CH₃ONa. This colorless solid, which is formed by the deprotonation of methanol, is a widely used reagent in industry and the laboratory. It is also a dangerously caustic base.
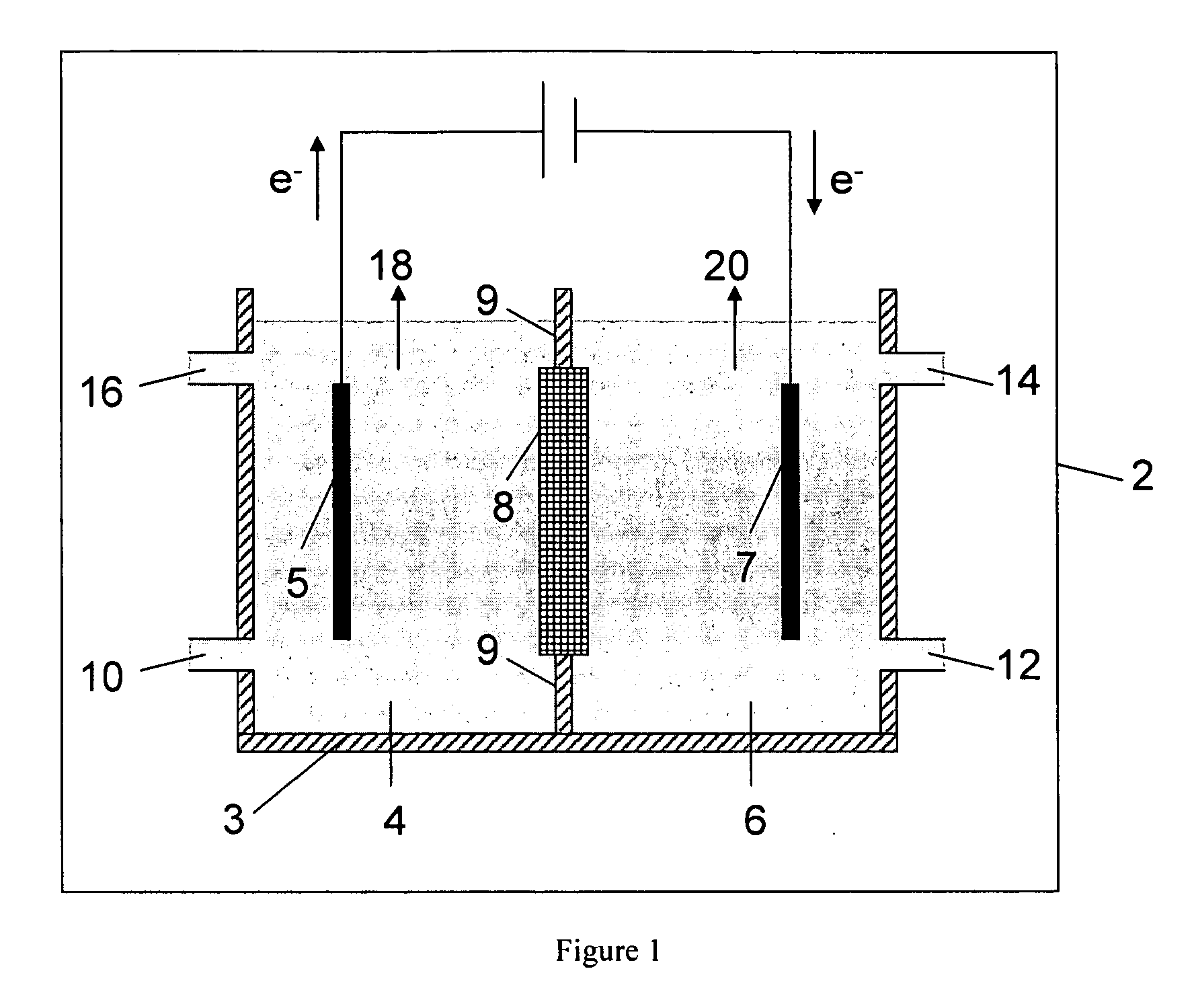
Electrolytic method to make alkali alcoholates using ceramic ion conducting solid membranes
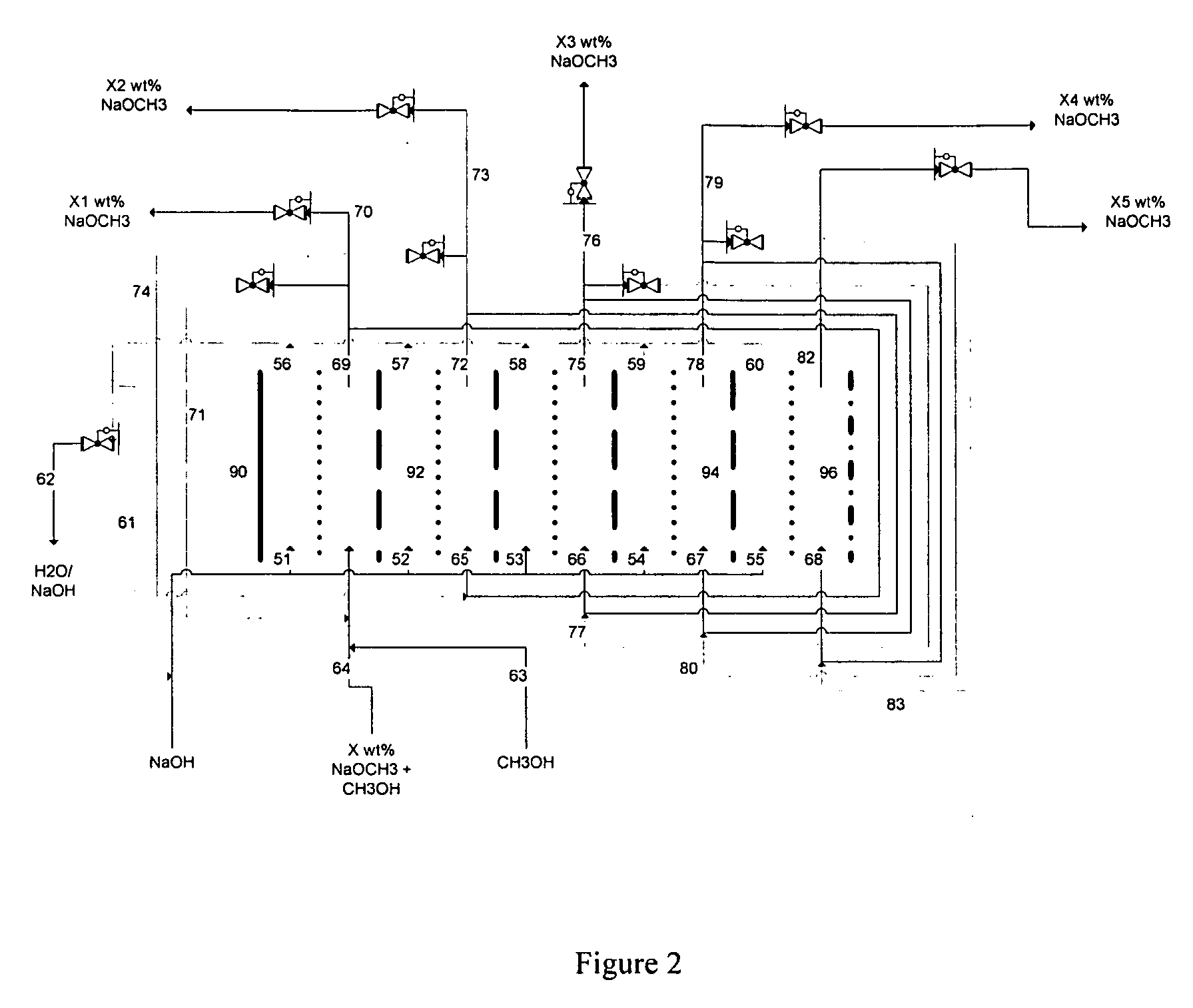
Disclosed are processes of making solutions of alkali alkoxides in their corresponding alcohols using an electrolytic process. In one embodiment, sodium methoxide in methanol is made from methanol and aqueous sodium hydroxide solution, where the aqueous sodium hydroxide solution is present in the anolyte compartment and a solution of sodium methoxide in methanol is present in the catholyte compartment, the two compartments are separated by a ceramic membrane that selectively transports sodium ions under the influence of an electric potential, and wherein the composition of the solution of sodium methoxide in methanol in the catholyte compartment of the electrolytic cell comprises between at least about 2% by weight sodium methoxide and at most about 20% by weight sodium methoxide.
Owner:ENLIGHTEN INNOVATIONS INC
Oleochemical Plasticizers with Thermal and Ultraviolet Radiation Stabilizing Activity for PVC Molding Resins and Process for Obtaining Thereof
The present invention is related with bioplasticizers or primary oleochemical plasticizers and the improved process for obtaining thereof. It refers primarily to epoxydized oleochemical plasticizers produced from vegetable oils, as substitute of traditional petrochemical plasticizers. The process starts with the epoxydized product of natural oils, such as sunflower, linseed, Jatropha curcas, soybean, etc., which are transesterified with an alcohol such as ethylic or methylic, in the presence of a catalyst such as sodium methoxide or sodium hydroxide in order to produce an alkylic esters mixture of the fatty acids that were present in the oil or oil mixture used as raw material in the epoxydized oil production. When the plasticizer obtained by the process already mentioned is used for the formulation of moldable poly(vinyl chloride), PVC, resins; the resulting plastic films get adequate hardness, static and dynamic thermal stability, and plasticizer extractability by solvents, such as n-hexane, gasoline and oil. Besides, when the PVC resin is formulated with a phthalic or terephthalic plasticizers mixture and the bioplasticizer, the bioplasticizer presents a full range solubility and or compatibility with the remainder of the resin compounds. The oxyrane chemical ring of the bioplasticizer is an excellent chemical neutralizer of the HCL that might be formed from the PVC, due to the action or interference of thermal or UV radiation.
Owner:RESINAS & MATERIALES
Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process
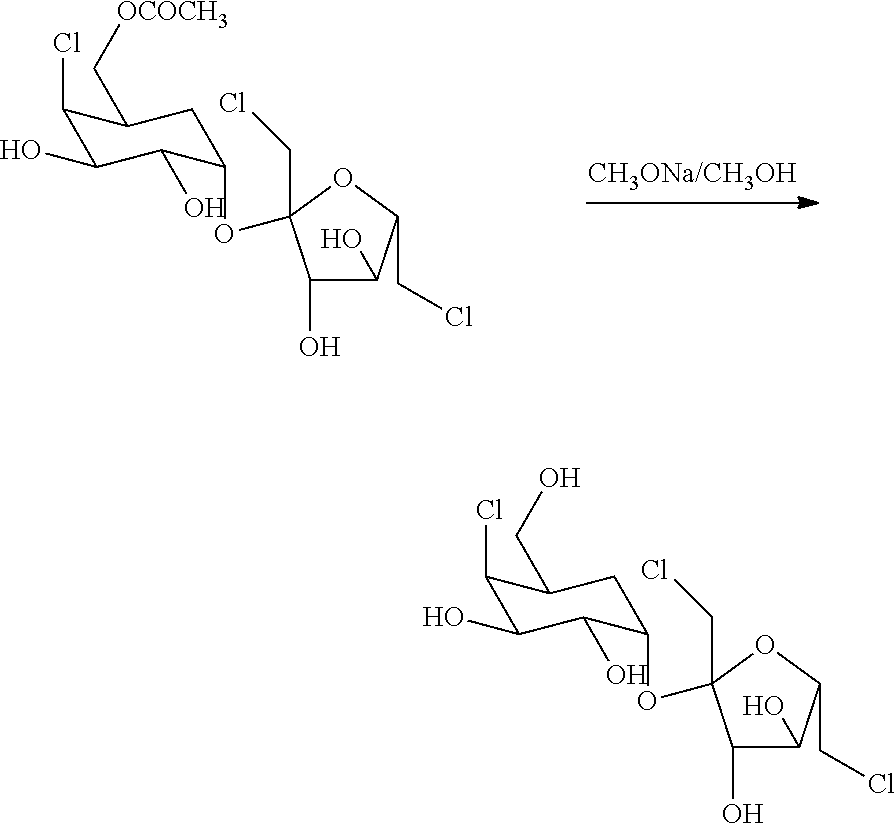
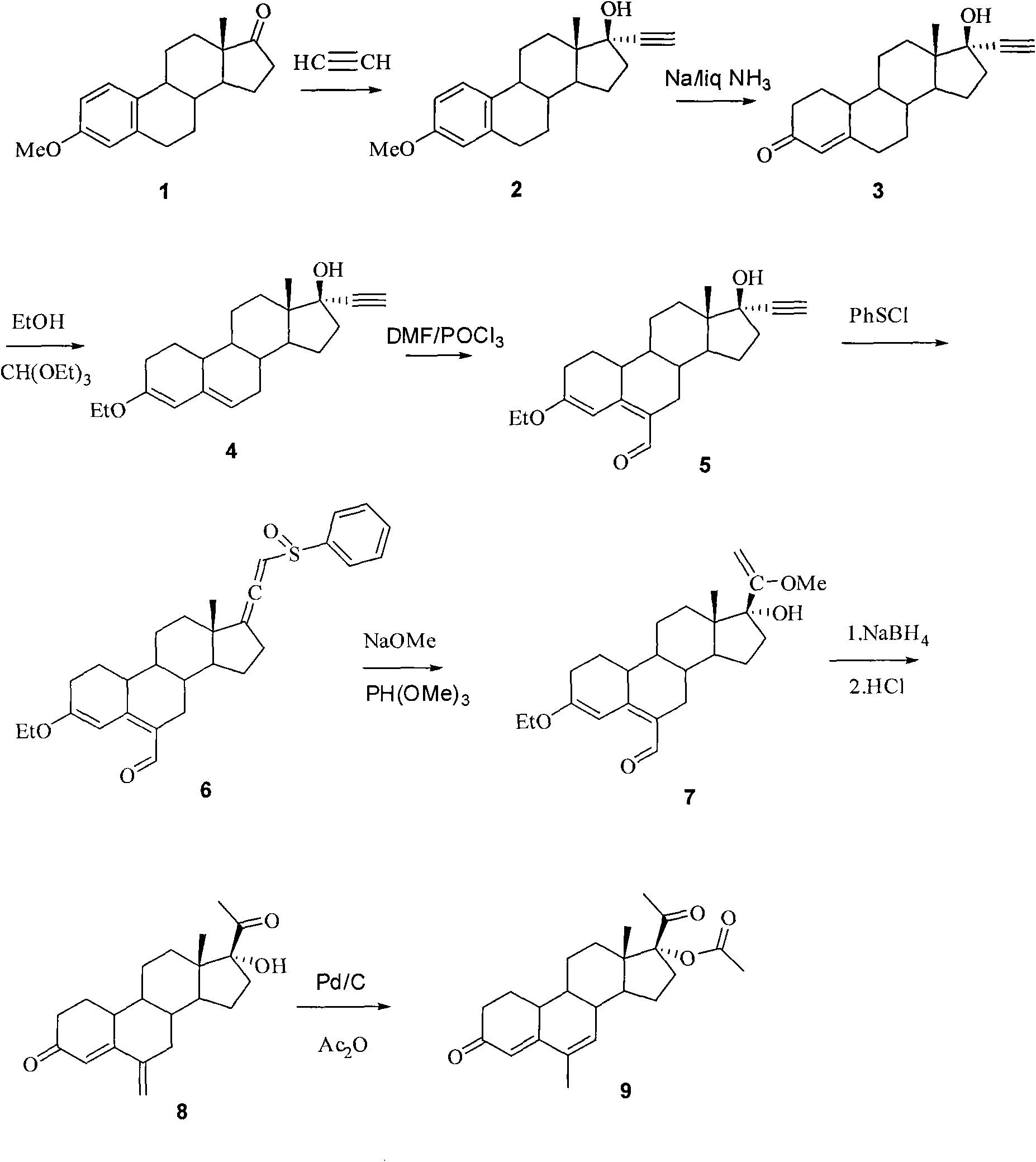
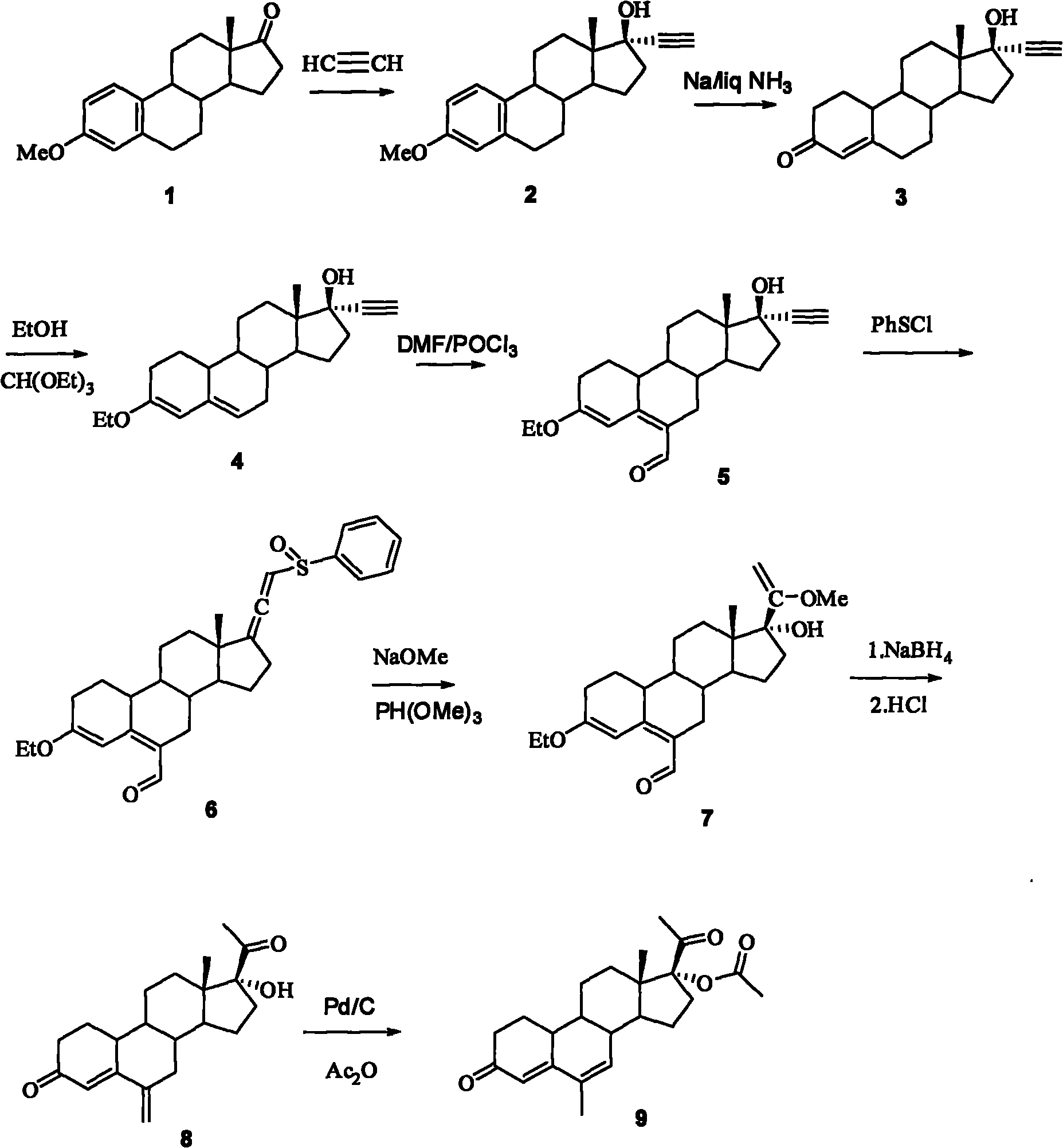
The present invention relates to a new industrial process for the synthesis of solvate- free 17a-acetoxy-11ss-[4-(N,N-dimethyl-amino)-phenyl]-19-norpregna-4,9-diene-3,20-dione [CDB -2914] of formula (I) which is a strong antiprogestogene and antiglucocorticoid agent. The invention also relates to compounds of formula (VII) and (VIII) used as intermediates in the process. The process according to the invention is the following: i) 3-(ethylene-dioxy)-estra-5(10),9(11)-diene-17-one of formula (X) is reacted with potassium acetilyde formed in situ in dry tetrahydrofuran by known method, ii) the obtained 3-(ethylene-dioxy)-17a-ethynyl-17ss-hydroxy-estra-5(10),9(11)-diene of formula (IX) is reacted with phenylsulfenyl chloride in dichloromethane in the presence of triethylamine and acetic acid, iii) the obtained isomeric mixture of 3-(ethylene-dioxy)-21-(phenyl-sulfinyl)-19-norpregna-5(10),9(11),17(20),20-tetraene of formula (VIII) is reacted first with sodium methoxide in methanol, then with trimethyl phosphite, iv) the obtained 3-(ethylene-dioxy)-17a-hydroxy-20-methoxy-19-norpregna-5(10),9(11),20-triene of formula (VII) is reacted with hydrogen chloride in methanol, then v) the obtained 3-(ethylene-dioxy)-17a-hydroxy-19-norpregna-5(10),9(11l); -diene-20- one of formula (VI) is reacted with ethylene glycol hi dichloromethane in the presence of trimethyl orthoformate and p-toluenesulfonic acid by known method, vi) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-19-norpregna- 5(10),9(11)-diene of formula (V) is reacted with hydrogen peroxide in a mixture of pyridine and dichloromethane in the presence of hexachloroacetone by known method, vii) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-5,10-epoxy-19-norpregn-9(11)-ene of formula (IV), containing approximately a 1:1 mixture of 5a,10a- and 5ss,10ss-epoxides, is isolated from the solution and reacted with a Grignard reagent obtained from 4-bromo-N,N-dimethyl-aniline in tetrahydrofuran.
Owner:RICHTER GEDEON NYRT
Trans free non-hydrogenated hard structural fat and non-hydrogenated hard palm oil fraction component
A trans free non hydrogenated high C-16 type palm fat suitable for the manufacture of trans free non hydrogenated hard structural fat that is being suitable for use in the manufacture of low SAFA (Saturated Fatty Acid) poly / mono unsaturated margarine and spreads and shortening and fat blends incorporating such hard Structural fat. The Structural fat is made from selectively fractionated non-hydrogenated high melting palm oil fraction with a C-16 fatty acid residue of at least 70%, which is subjected to chemical random interesterification using alkaline metal catalyst such as sodium methoxide / sodium methylate, with a dry fractionated non hydrogenated hard palm kernel stearin fraction. The structural fat that is produced has high yield ratios that can be economically and commercially incorporated in the oil blends for the manufacture of trans free margarine / spreads / shortening as well as other plastic W / O emulsions. Also described is a process for the manufacturing such structural fat as well as hard palm fraction including process for the manufacture of extra hard trans free structural fat by panning and pressing of above structural fat.
Owner:PREMIUM VEGETABLE OILS
Synthetic method of azoxystrobin and special intermediate for synthesis
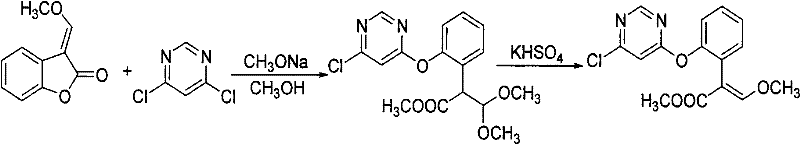
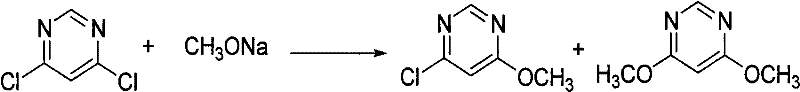
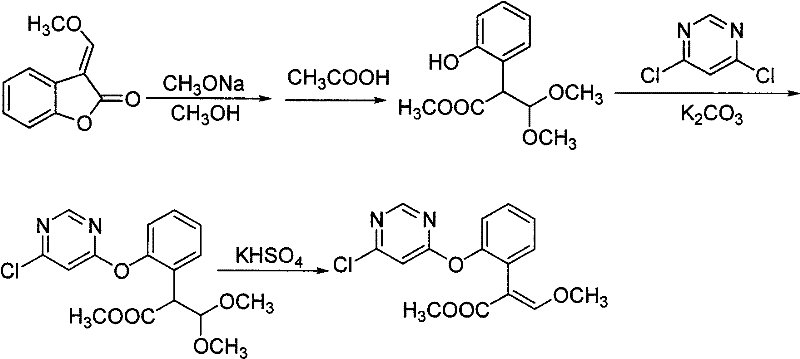
The invention relates to a synthetic process of a chemical substance, and particularly relates to a synthetic method for synthesizing (E)-2-[2-(6-chloro pyrimidine-4-yloxy)phenyl]-3-methoxy methacrylate and azoxystrobin; the method comprises the following steps: mixing a raw material of 3-(alpha-methoxy)methylene benzofuran-2-(3H)-ketone and potassium carbonate in a toluene solvent, cooling to 0-10 DEG C, adding sodium methoxide, reacting for 0.4-0.6 hours; adding 4,6-dichloropyrimidine and a catalyst of DABCO, reacting for 1-2 hours, filtering to remove inorganic salts, washing the filtrate with water, performing distillation to recover toluene; adding a catalyst of potassium bisulfate into the distillation residues of the above reaction, heating to 132-145 DEG C in a reduced-pressure condition for reaction; directly adding salicylonitrile to synthesize azoxystrobin or performing toluene dissolution, water washing, solvent recovery, recrystallization and filtration to obtain an intermediate. The production and synthesis of (E)-2-[2-(6-chloro pyrimidine-4-yloxy)phenyl]-3-methoxy methacrylate by the production process of the invention has high yield, and simple operations, and the used raw materials and processes are routine reagents and methods.
Owner:CHONGQING UNISPLENDOUR CHEM
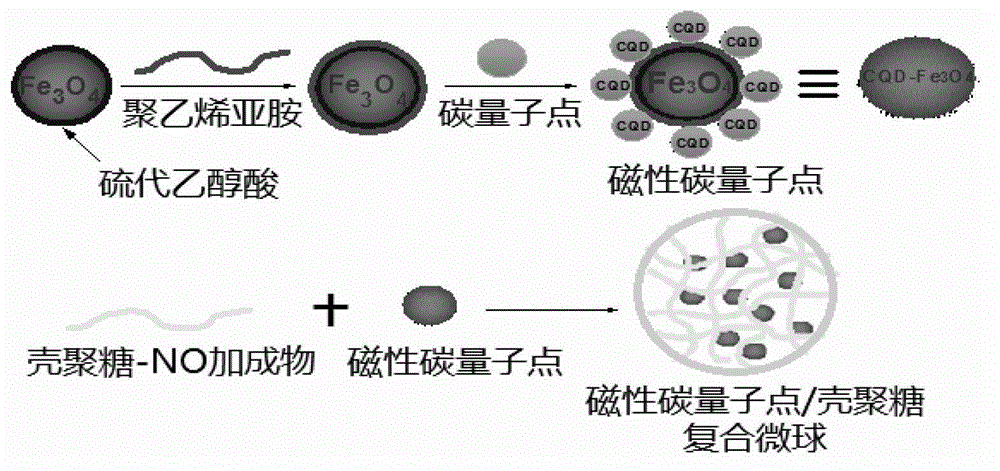
Method for preparing drug carrier based on magnetic carbon quantum dot/chitosan composite microsphere
InactiveCN102973948ALow costSimple methodInorganic active ingredientsInorganic non-active ingredientsSodium methoxideIron salts
The invention relates to a method for preparing a drug carrier based on a magnetic carbon quantum dot / chitosan composite microsphere. The method specifically comprises the following steps of: (1) carrying out a coprecipitation reaction on bivalent and trivalent iron salts in an alkaline aqueous solution so as to prepare nano magnetic ferroferric oxide; (2) carrying out microwave radiation reaction on a glucose and polyethylene glycol mixed solution to prepare carbon quantum dots, and forming magnetic carbon quantum dot composite particles through electrostatic adsorption; (3) reacting the chitosan which is dissolved in a mixed solution of sodium methoxide / absolute methanol with nitric oxide in a high-pressure kettle, and forming a chitosan-nitric oxide addition product; and (4) dropwise adding the magnetic carbon quantum dots into the addition product, and forming the magnetic carbon quantum dot / chitosan composite microsphere through electrostatic adsorption. Compared with the prior art, the method is simple, rapid, low in cost, and the prepared product can be developed into the drug carrier which integrates magnetic targeting, fluorescence imaging or tracing, nitric oxide in-situ release and fluorescence detection.
Owner:SHANGHAI JIAO TONG UNIV
Method for preparing folic acid
The invention relates to a preparation method of folic acid. The process steps are as follows: firstly, the intermediate product N-p-aminobenzoyl glutamic acid is prepared by using p-nitrobenzoyl chloride and sodium glutamate; Guanidine and sodium methoxide were used to prepare the intermediate product 6-hydroxy-2,4,5-triaminopyrimidine sulfate; finally, the above two intermediate products were used to prepare crude folic acid, which was purified to obtain pure folic acid. The beneficial effects of the invention are that the production cost is greatly reduced, the product efficiency is improved, and the pollution to the environment is reduced.
Owner:潘福星
Thickening agent synthesized by acrylic ester and preparation method thereof
InactiveCN101619543AGood thickening effectStrong electrolyte resistanceOrganic compound preparationDyeing processTextile printerSodium methoxide
The invention discloses a preparation method for synthesizing a thickening agent by acrylic ester. The thickening agent synthesized by acrylic ester comprises crylic acid, acrylamide, 25 percent of ammonia water, a functional monomer, EDTA, deionized water, tasteless kerosene, 3<#> white oil, diallyl phthalate, ammonium persulphate, Span 80 and isomeric hexadecanol polyethenoxy ether, wherein the functional monomer is prepared from alicyclic amine polyethenoxy ether, methyl methacrylate, nitroxide free radical pipradrol and sodium methoxide. The preparation method synthesizes a special weak cation type (methyl) acrylic ester functional monomer by adopting an ester exchange method, and the functional monomer and vinyl monomers of the acrylic acid, the acrylamide, and the like are adopted to synthesize a textile printing thickening agent by adopting inverse emulsion polymerization. The thickening agent has the characteristics of high thickening capacity, strong electrolyte resistant capacity and good water-retaining property.
Owner:成都德美精英化工有限公司
Method for synthesizing florfenicol
InactiveCN101265220AEasy marketEasy to operateAntibacterial agentsOrganic chemistrySodium methoxideSynthesis methods
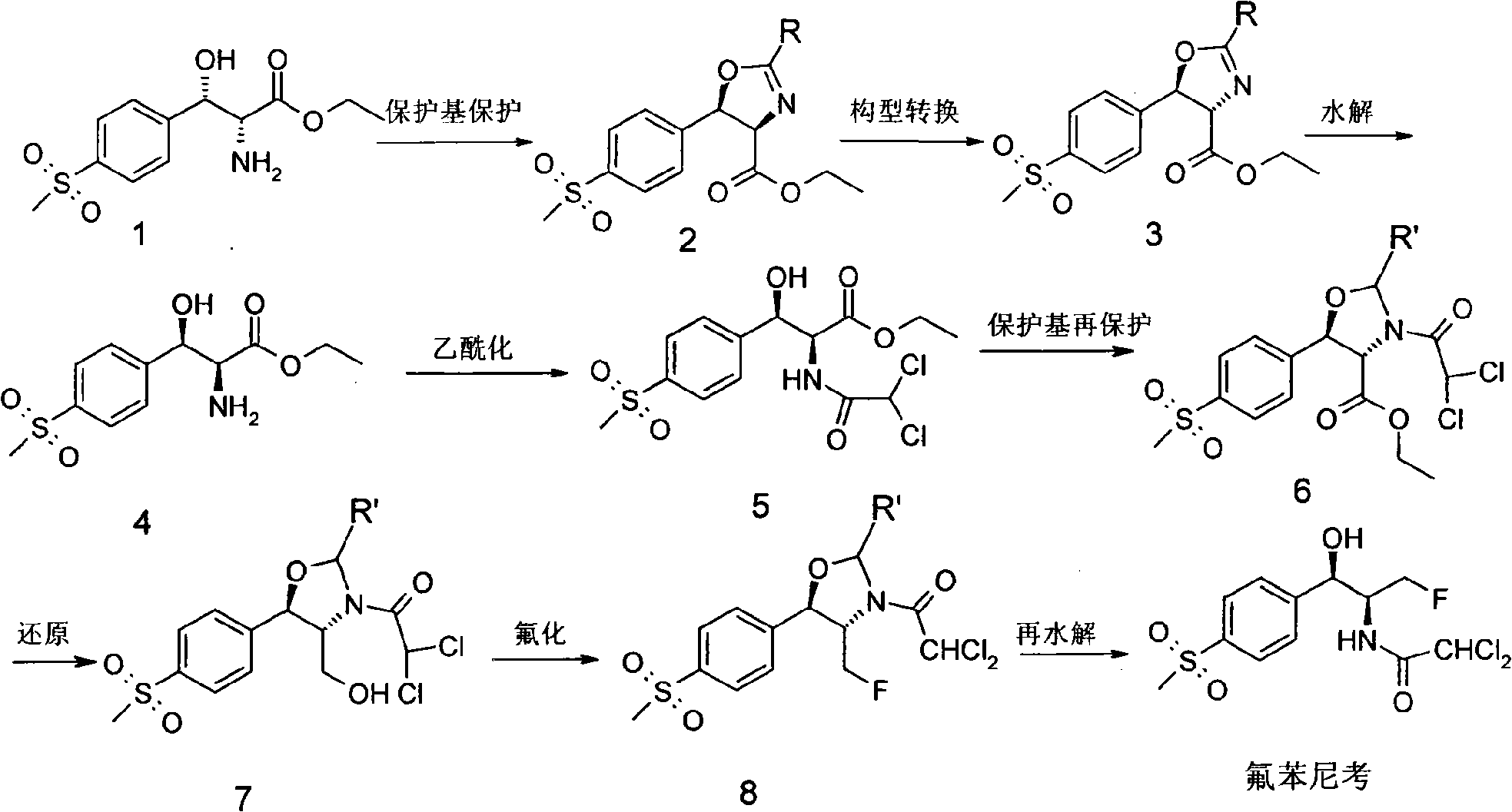
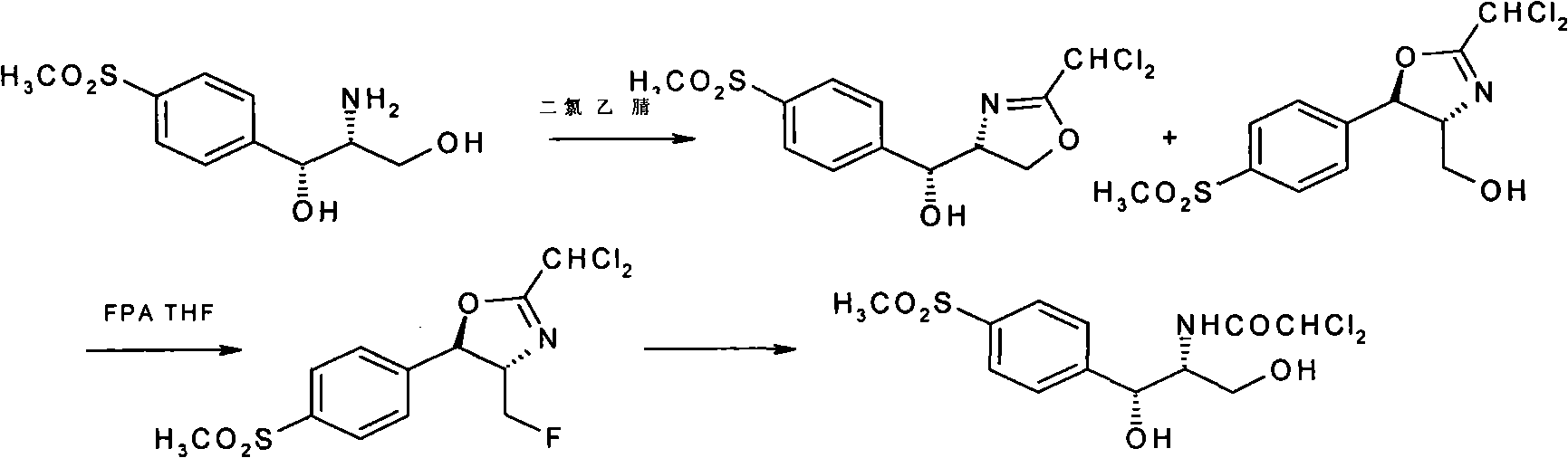
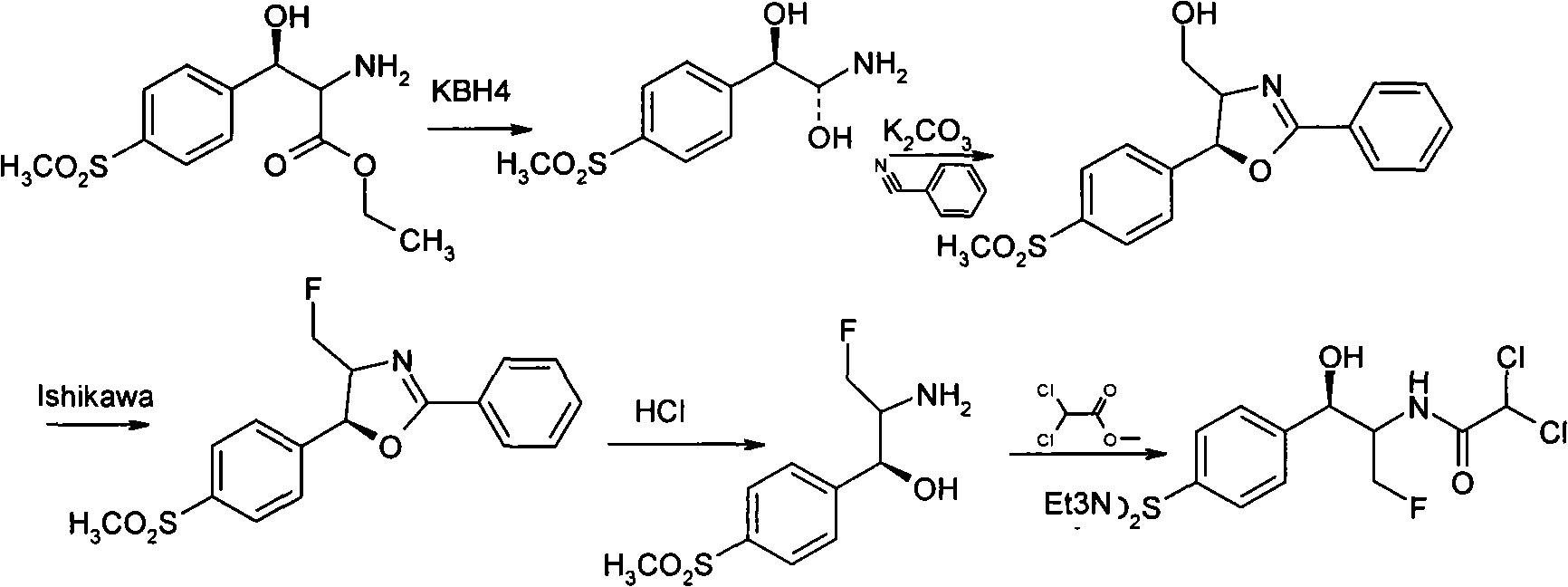
The invention discloses a synthesis method of synthesizing florfenicol. The synthesis method comprises the following steps: L-threo-(p-(methylsylfonyl) phenyl) serine ethyl ester which is used as raw material is processed by the protection by a protecting group, configurational transition, hydrolyzation, acetylation, re-protection, deoxidation, fluorination, and pre-hydrolyzation, so that the florfenicol is obtained, wherein, the protecting group R is one of benzoyl chloride, phthalic anhydride, cyanophenyl, and allyl compounds; the configurational transition is performed when sodium alcoholate or sodium methoxide exists. The raw material which is used in the method is a byproduct which is generated during the process of preparing thiamphenicol, is easy to get in the market, and is inexpensive; the technological operation is simple, the cost is low, the yield rate is high, and the synthesis method has industrialized value.
Owner:SHANGHAI RECORD PHARM CO LTD
Method for preparing Salen-metal complex
InactiveCN102212085ALow costHigh catalytic efficiencyOrganic-compounds/hydrides/coordination-complexes catalystsCobalt organic compoundsSodium methoxideFiltration
The invention provides a method for preparing a Salen-metal complex. The method comprises the following steps of: under the argon atmosphere, adding ligands(II), sodium methoxide, absolute methanol soluble ligands(II) and sodium methoxide into a reactor in sequence; stirring the mixture for 2 minutes, and adding 1mol / L cobalt acetate anhydrous / methanol solution dropwise, wherein the molar ratio of the ligands(II) to sodium methoxide to cobalt acetate is 1:1:1; and making the mixture reacted at the room temperature for 24 hours, adding anionic salt which has the same molar weight as the cobalt acetate, stirring the mixture for three days in the air, stopping the reaction, concentrating the mixture, performing dissolution and filtration by adding methylene chloride, drying the filtrate by anhydrous sodium sulfate overnight, filtering the mixture, concentrating under reduced pressure, and drying the mixture under the vacuum condition to obtain the Salen-metal complex. The Salen-metal complex prepared by the method has the advantages of simple method, low cost and high catalytic efficiency.
Owner:HEBEI UNIV OF TECH
Glass micro-bead filling modified mould nylon plate and preparation method thereof
InactiveCN101081927AImprove mechanical propertiesHigh impact strengthSodium methoxideToluene diisocyanate
The present invention relates to glass bead stuffing modified cast nylon plate and its production process, and belongs to the field of material science. The enhanced cast nylon plate is produced with the material comprising caprolactam as base component, hollow glass bead as the enhancing component, stearate as the dispersant, main antioxidant, auxiliary antioxidant, stabilizer, sodium methoxide as the catalyst and toluene diisocyanate as the co-catalyst in certain weight proportion, and through a molding and sintering process. The enhanced cast nylon plate has high comprehensive mechanical performance and physical performance.
Owner:JILIN UNIV
Process for the preparation of sucralose
InactiveUS7932380B2High purityLow costSugar derivativesSugar derivatives preparationSodium methoxideAcetic acid
A process for the preparation of a sucralose which comprises the steps of: reacting a sucrose with a chlorinating reagent in a non-proton type polar solvent to form chlorinated sucrose (4,6,1′,6′-tertchloro-4,6,1′,6′-tertdeoxylgalactosucrose); reacting the chlorinated sucrose and a carboxylate salt to form sucralose-6-acetate in a dissolvent; and finally de-acylating the sucralose-6-acetate in sodium methoxide / methanol system and then the desired product sucralose is thereby produced. The present invention is generally related to the industrial production of sucralose with the advantages of mild reaction conditions, high yield rate, and brief operation.
Owner:WANHE INT GROUP
Technological method for preparing epoxy fatty acid methyl ester plasticizer with waste vegetable oil
InactiveCN102344856ASimple processSimple processing methodFatty acid esterificationSodium methoxideEpoxy
The invention relates to a technological method for preparing an epoxy fatty acid methyl ester plasticizer with waste vegetable oil. The method comprises the steps of: subjecting impurity removed mixed swill-cooked dirty oil or catering hogwash oil and other waste vegetable oil to molecular distillation directly in a distillation column for removing fatty acid so as to convert the oil to neutral oil with an acid value of 0.8-1.2; then adding 1-3% sodium methoxide and 20-25% methanol, raising the temperature gradually to 66DEG C to 70DEG C under stirring for a reaction of 40-50min, leaving the oil to stand for 25-35min, and discharging crude glycerol, thus obtaining fatty acid methyl ester; then adding 2-4% formic acid, raising the temperature gradually to 45-55DEG C under stirring, starting to add 20-30% hydrogen peroxide dropwisely for a reaction of 6.5-7.5h, thus obtaining epoxy fatty acid methyl ester; then adding 15-25% sodium hydroxide liquid for neutralization so as to make the PH of the material ranging from 6.5 to 7.5, carrying out washing and distillation, thus obtaining the high-purity, non-toxic and colorless epoxy fatty acid methyl ester plasticizer. The preparation method of the invention has simple process and low cost, and can substitute o-benzene plasticizers to a greater degree.
Owner:肖连朝
Trans free non-hydrogenated hard structural fat and non-hydrogenated hard palm oil fraction component
Owner:PREMIUM VEGETABLE OILS
Method for synthesizing 6-methyl-17alpha-acetoxyl-19-norpregnane-4,6-diene-3,20-diketone
The invention relates to a method for synthesizing 6-methyl-17alpha-acetoxyl-19-norpregnane-4,6-diene-3,20-diketone. The method comprises the following steps: 1) reacting acetylene with estrone-3-methyl ether so as to obtain 17alpha-acetenyl estrone-3-methyl ether; 2) carrying out Birich reaction at a low temperature; 3) carrying out alkene etherification on the product obtained in the step 2); 4) carrying out a Vilsmeier reaction on the product obtained in the steps 3); 5) reacting the product obtained in the step 4) with benzene sulfenyl chloride; 6) reacting the product obtained in the step 5) with sodium methoxide and trimethyl phosphate; 7) reducing the formoxyl at the 6-position of the product obtained in the step 6); and 8) reacting the product obtained in the step 7) with Pd-C / cyclohexene, then reacting with acetic oxide so as to obtain the target product 6-methyl-17alpha-acetoxyl-19-norpregnane-4,6-diene-3,20-diketone. By using the method, the operation is simplified, and theproduction cost is reduced; and the reactions involved in the invention are simple to operate, and the yield is high.
Owner:黄云生 +1
Continuous reaction rectification process and rectification equipment for synthesizing isopropyl alcohol
ActiveCN102755759AIncrease conversion rate of exchange reactionReduce production energy consumptionOrganic compound preparationHydroxy compound preparationSodium methoxideTrans esterification
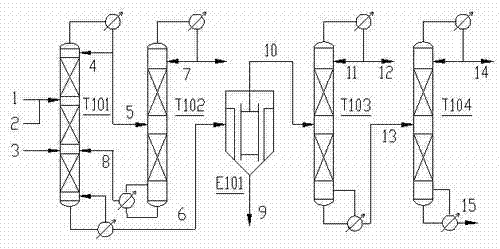
The invention provides a continuous reaction rectification process that isopropyl acetate and methyl alcohol are taken as raw materials to be synthesized into isopropyl alcohol through a trans-esterification method under the existence of basic catalysts such as sodium methylate. According to the invention, a reaction rectification technology is adopted, so that the isopropyl acetate and methyl alcohol are fed from the upper part and the lower part of a reaction section of a reaction rectifying tower respectively to be reacted and separated so as to realize continuous production; and products at the top and the bottom of the reaction rectifying tower are separated and purified by a methyl ester rectifying tower, a sodium methylate rectifying tower, an evaporator and a isopropyl alcohol refining tower, so that isopropyl alcohol is obtained, and raw material and catalyst which are not reacted are recycled at the same time. The continuous reaction rectification process for synthesizing isopropyl alcohol has the characteristics of continuity in operation, low energy consumption, high reaction conversion rate and isopropyl alcohol purity, stable quality, and the like.
Owner:FUZHOU UNIV
Methoxy group naphthyl fluorescence marked water treating agent and its preparing method
InactiveCN1781857ARaw materials are easy to getMild reaction conditionsScale removal and water softeningSodium methoxideFluorescence
The present invention discloses water treating agent containing methoxy naphthalene as fluorescent marker. The water treating agent is prepared through the first reaction of 4-chloro-1, 8-naphthalic anhydride, glacial acetic acid and 3-dimethylamino propylamine to obtain 4-chloro-N-3-dimethylamino propyl-naphthyl imide; the subsequent reaction with sodium methoxide to introduce methoxy group and obtain 4, 4-methoxyl-N-3-dimethylamino propyl-naphthyl imide, which is reacted with allyl chloride to obtain quaternary ammonium salt of 4-methoxyl-N-3-dimethylamino propyl-naphthyl imide allyl chloride as fluorescent monomer with fluorescent characteristic and double bond; and the final polymerization with phosphorus containing compound, acrylic acid and other monomer to obtain the multifunctional water treating agent with corrosion retarding, scale inhibiting, dispersing and fluorescent tracing functions.
Owner:NANJING UNIV OF SCI & TECH
Sugammadex preparation and purification method
InactiveUS20180016359A1Quality improvementSimple processNervous disorderSodium methoxidePurification methods
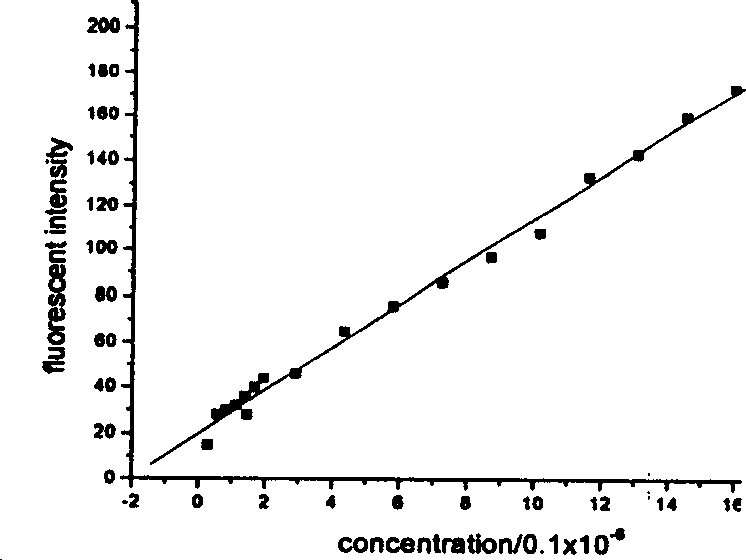
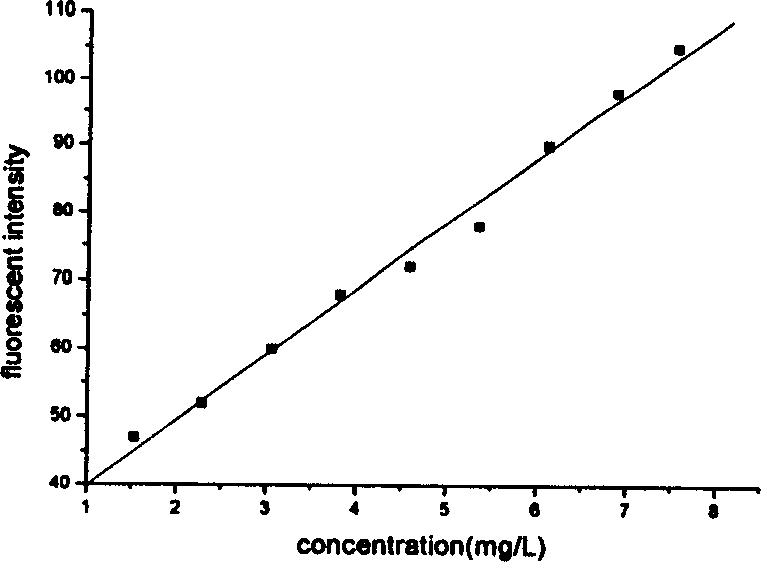
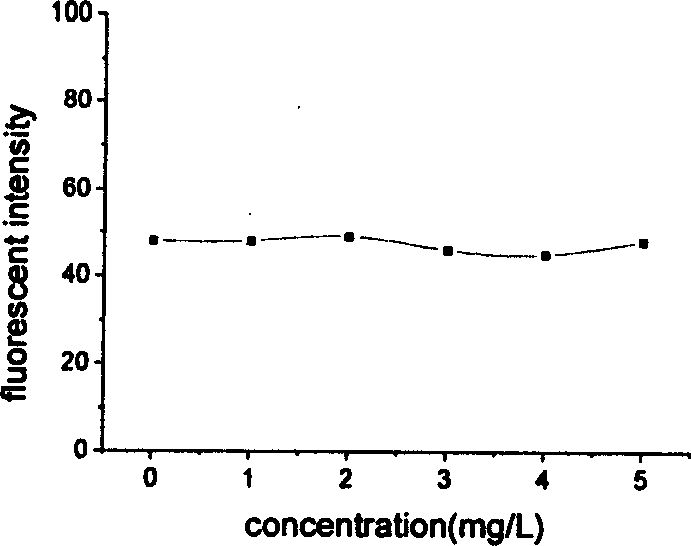
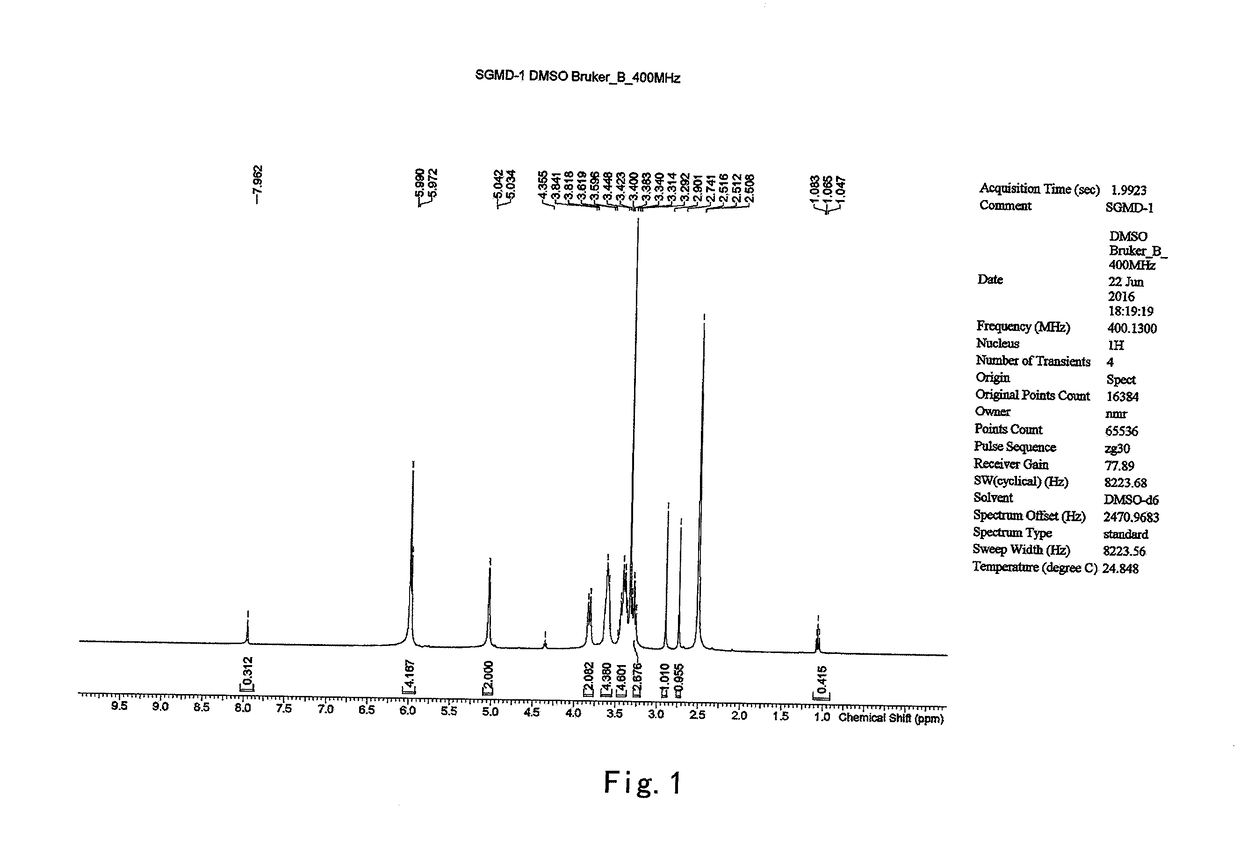
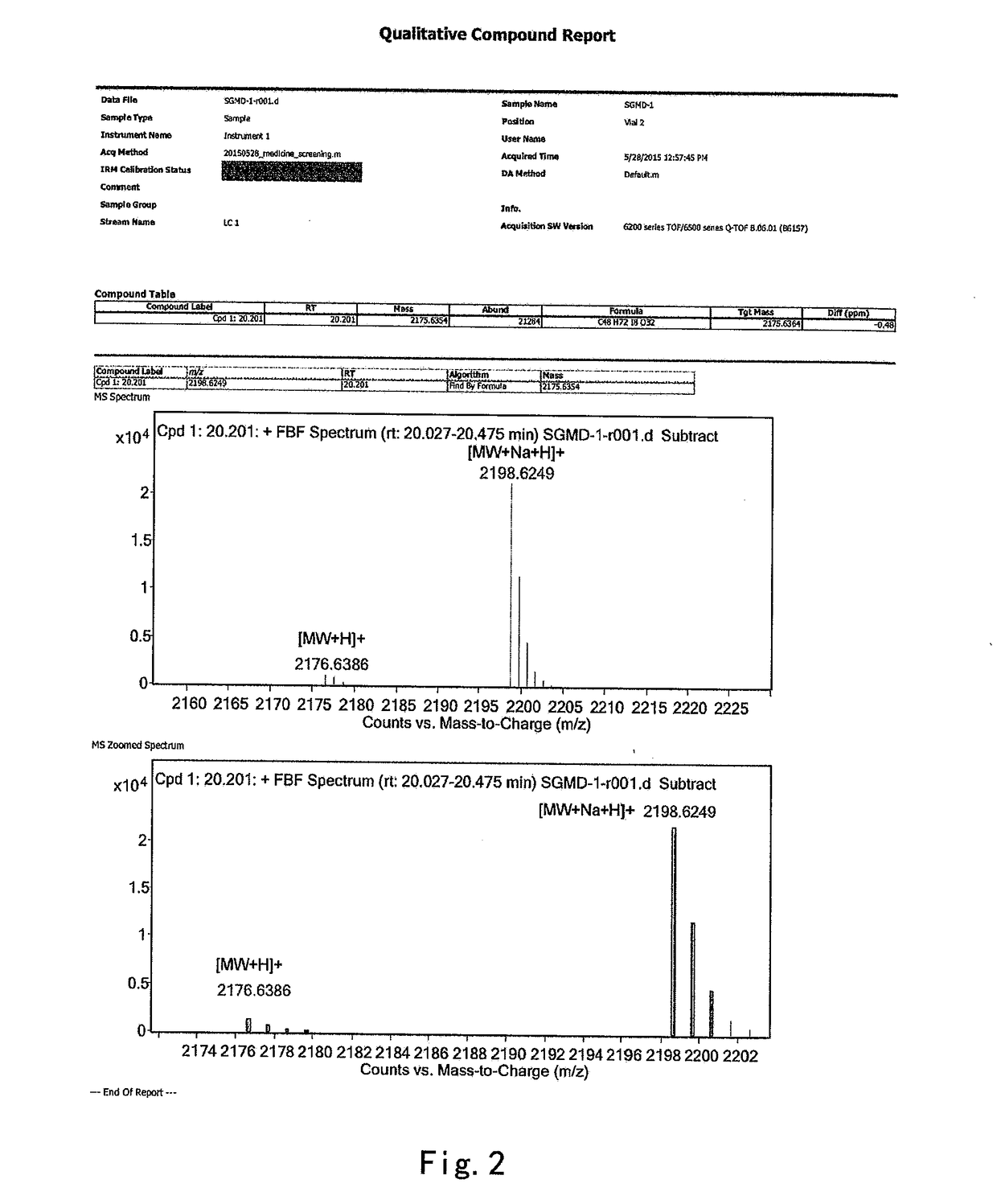
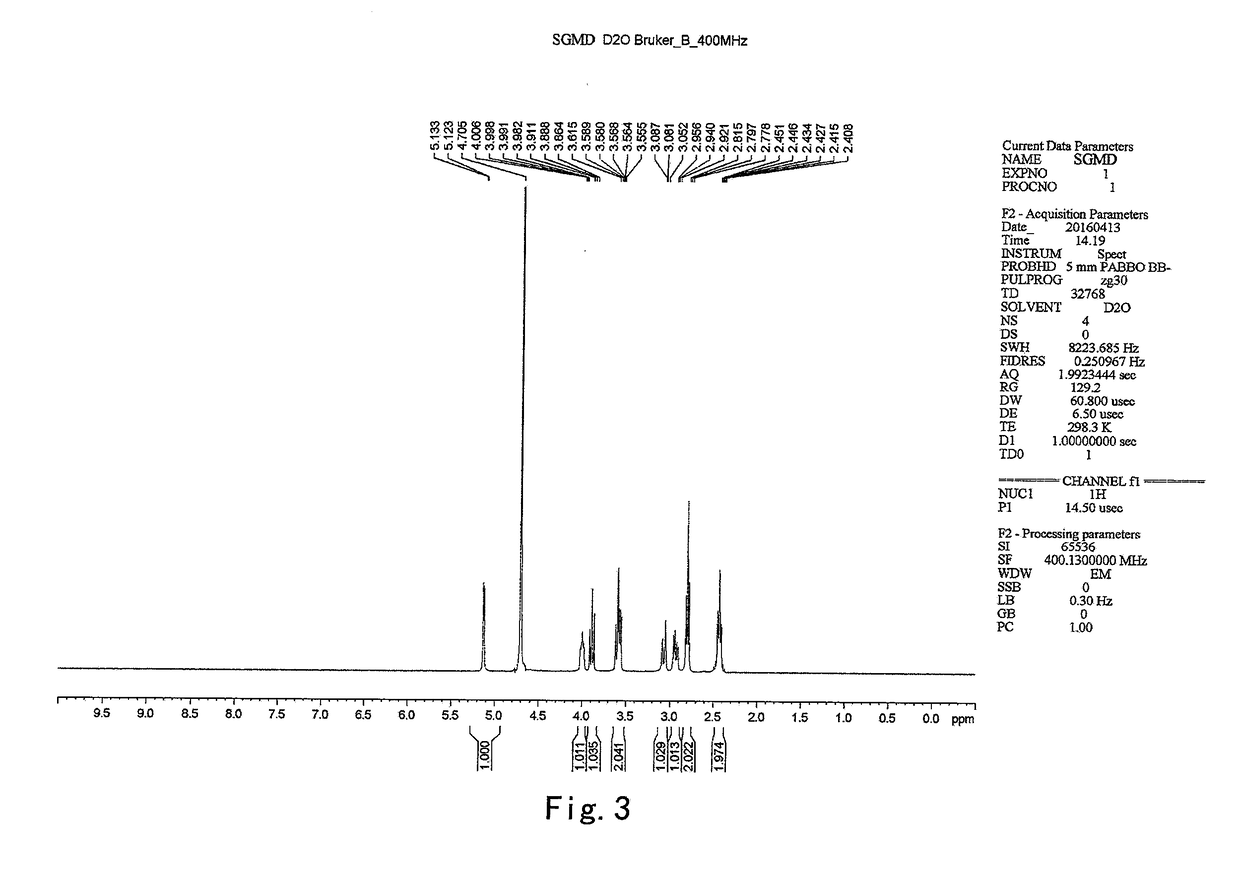
The present invention provides a process for the preparation of (6-per-deoxy-6-per-(2-carboxyethyl)thio-γ-cyclodextrin) sodium salt, comprising the steps of:reacting γ-cyclodextrin (SM1) with iodine in the presence of triphenylphosphine in an organic solvent to afford an intermediate, 6-per-deoxy-6-iodo-γ-cyclodextrin (abbreviated as SGMD-1);adding methanol solution of sodium methoxide into the reaction system followed by the addition of acetone without removal of the solvent under reduced pressure to obtain the crude SGMD-1 as a solid after filtration;purifying the crude SGMD-1 by recrystallization;reacting a obtained recrystallized intermediate (SGMD-1) with 3-mercaptopropionic acid in basic medium e.g., sodium hydride, to obtain the crude 6-per-deoxy-6-per-(2-carboxyethyl)thio-γ-cyclodextrin sodium salt (abbreviated as SGMD);purifying the crude SGMD by passing through adsorbents followed by recrystallization.
Owner:BEIJING CREATRON INST OF PHARMA RES CO LTD
Process for preparing biological diesel oil by using waste oil
InactiveCN1670128ASolve the idle problemImprove protectionBiofuelsLiquid hydrocarbon mixture productionOil and greaseSodium methoxide
The invention provides a method of using waste fat to producing organism diesel oil. Take the waste fat as material, pre-process by removing the mechanical impurity, phosphatide and sterol and put the waste fat in the reactor, then add methanol and sodium methoxide catalyst. The percentage of the added sodium methoxide of the total weight in waste fat is 0.7~1.3%; the methanol's percentage is 15%~35%. The reaction temperature is controlled ranging from 30~60 Deg. C, and reaction time is 40~90 minutes. After reacting, water scrubbing methyl estor layer and drying, get the said organism diesel oil. The reaction lasts 2-3 hours and the convert rate is higher than 95%. The value of cetane is lager than 50.
Owner:TIANJIN UNIV
Phosphine-based Marpropy multipolymer containing fluorescent base-group and its production
InactiveCN1939945ARaw materials are easy to getMild reaction conditionsSodium methoxideEthylenediamine
A phosphino-based mapropyl copolymer containing fluorescent group and its production are disclosed. The process is carried out by taking 4-bromine-1,8-naphthalic anhydride, glacial acetic acid and N, N-dimethyl-ethylenediamine as raw materials to obtain 4-bromine-N-(2-N,, N,-dimethylbenzidine-ethyl)naphthalimide, reacting it with sodium methoxide to induce methoxy-group to obtain 4-methoxy-N-(2-N,, N,- dimethylbenzidine-ethyl)naphthalimide, reacting it with allyl chloride to obtain fluorescent monomer 4-methoxy-N-(2-N,, N,- dimethylbenzidine-ethyl)naphthalimide allyl ammonia chloride with fluorescent characteristic and double-bond, polymerizing it with sub-sodium phosphate, maleic anhydride, acroleic acid and propenyl-sodium sulfate to obtain the final product. It has fluorescent tracting, corrosion-inhibting, scale-inhibiting and dispersion functions.
Owner:NANJING UNIV OF SCI & TECH
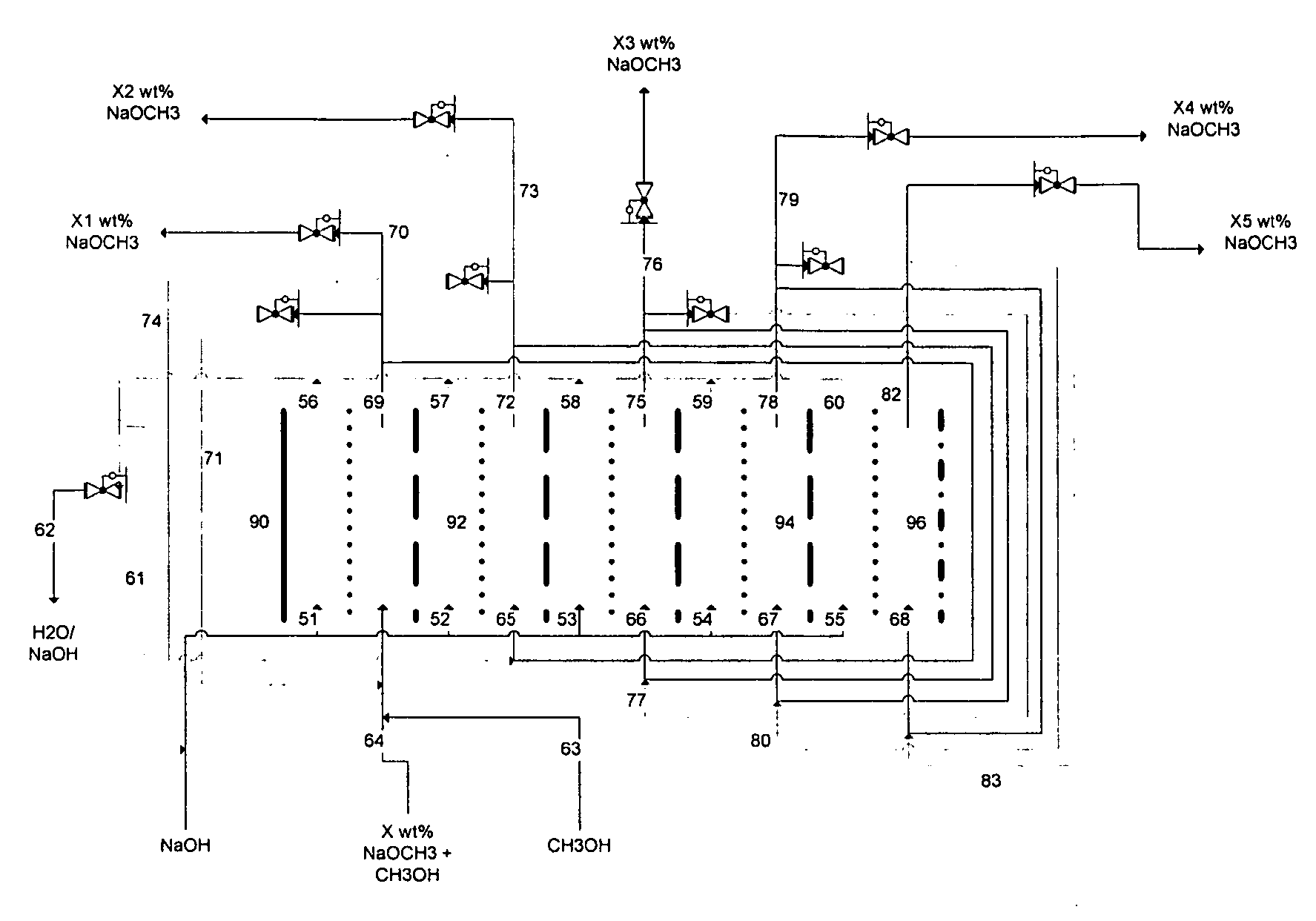
Technique of alkaline process for producing sodium methoxide/sodium ethylate
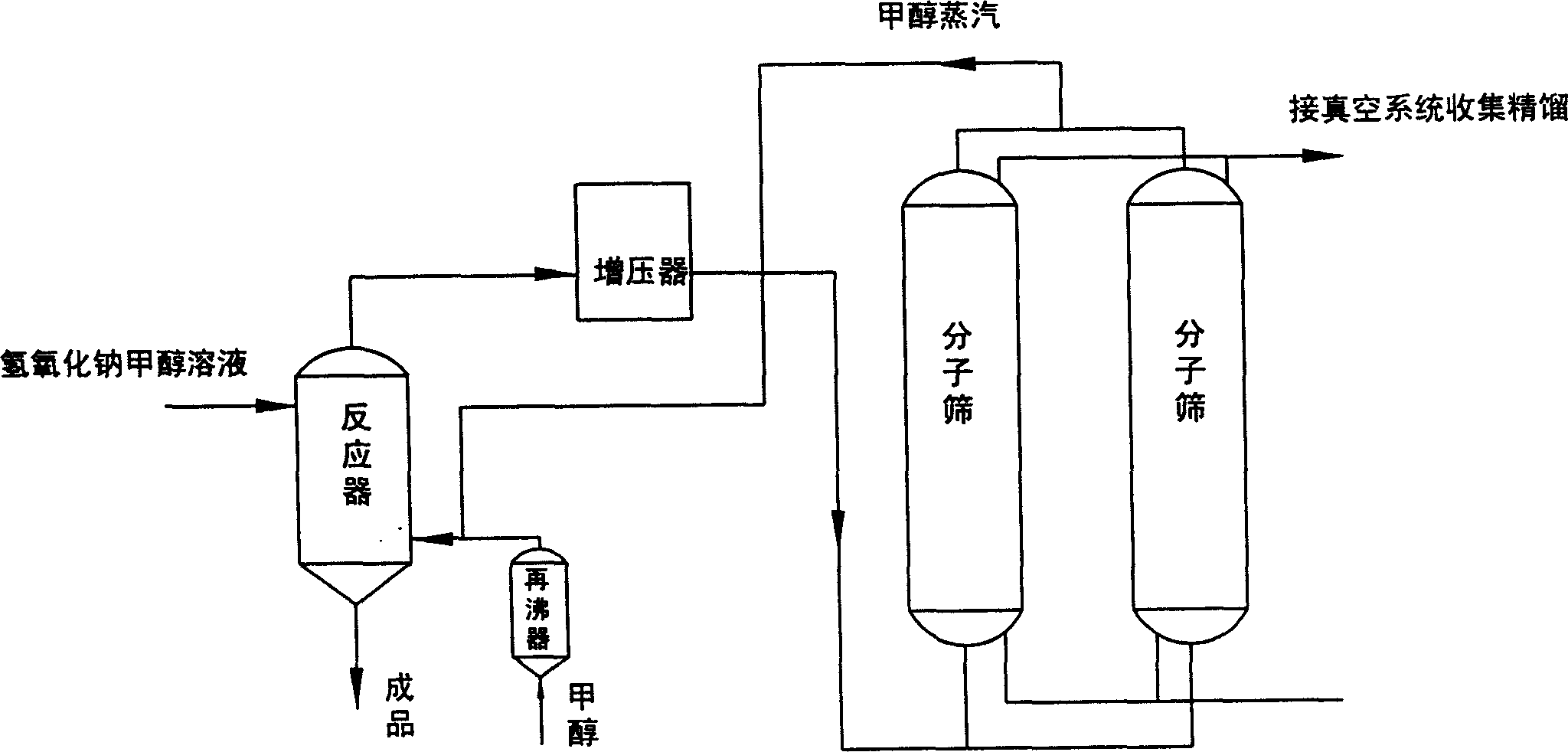
InactiveCN1626488AQuick responseContinuous responsePreparation of metal alcoholatesSodium methoxideMolecular sieve
A process for preparing sodium methoxde (or ethoxide) by alkali method includes reaction between excess methanol (or ethanol) and sodium hydroxide to obtain product, dewatering the vapour of methanol (or ethanol) by parallel two molecular sieves for alternative adsorption and desorption, and rectifying the solution of methanol (or ethanol) for reuse. Its advantage is no pollution.
Owner:于志波
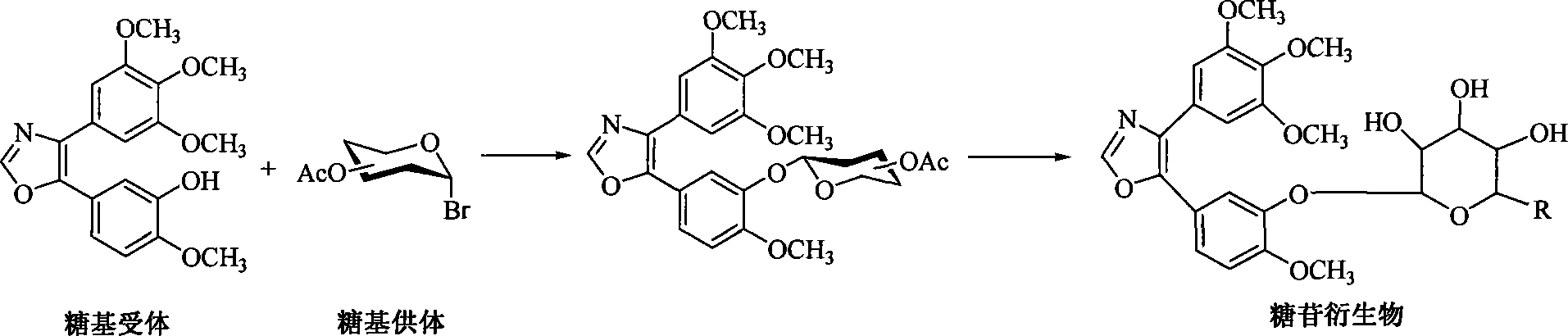
1,2-glycoside transderivative of oxazole compounds and preparation method thereof
InactiveCN101230079AImprove bioavailabilityImprove targeted tumor vasculatureOrganic active ingredientsSugar derivativesSolubilitySodium methoxide
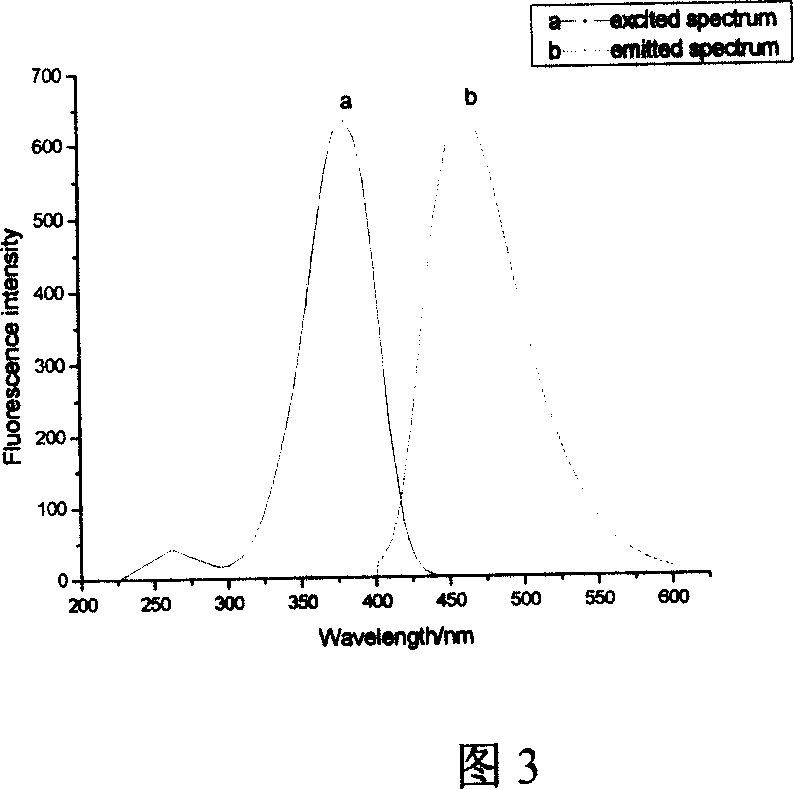
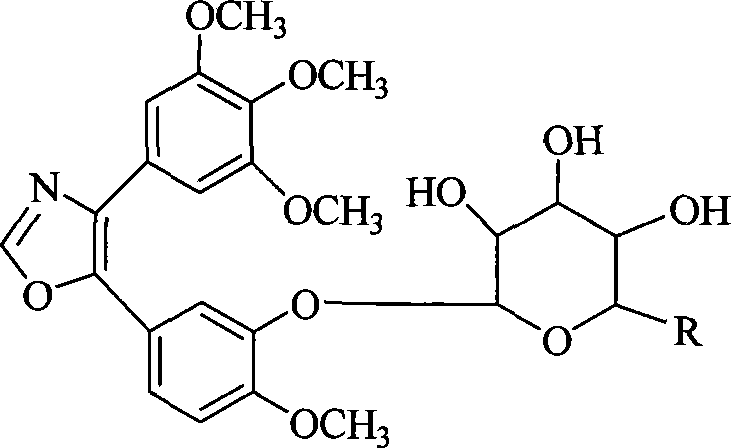
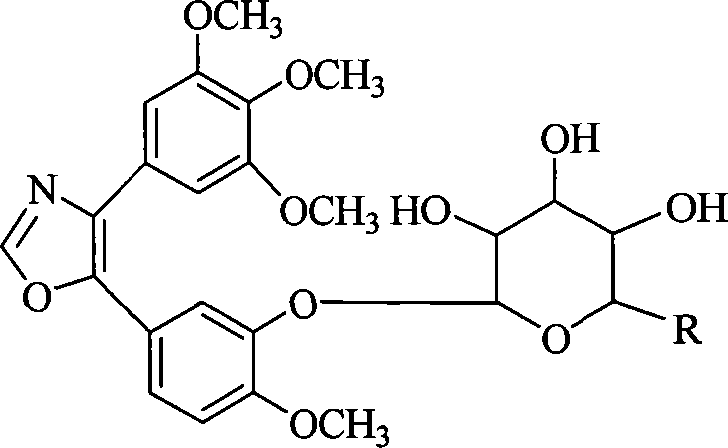
The invention discloses 1, 2-anti form glucoside derivative of oxazole compound. During the preparation, 4-(3, 4, 5-trimethoxy phenyl)-5-(3-hydroxyl-4-methoxy phenyl oxazole is used as the acceptor of glycosyl, bromo sugar of D-glucose, D-galactose, L-arabinose, D-xylose, L-fucose or lactose with full acetyl protection, or trichlorine imine ester of L-rhamnose or D-mannose with full acetyl protection is adopted as the donator of glycosyl, glycosylation is performed with the catalysis of alkali and tetrabutyl ammonium bromide or lewis acid, to obtain glucoside derivative containing acetyl protecting group; and then the acetyl protecting group is removed by utilizing methyl alcohol and sodium methoxide, to obtain 1,2 anti-form glucoside derivative of oxazole compound. The preparation method is simple, highly effective and universal, the route is short, the water-solubility of the product is good, the bioavailability is high, thereby the glucoside derivative can be applied as antineoplastic medicine inhibiting microtubule assembly and selectively targeting tumor blood vessels.
Owner:OCEAN UNIV OF CHINA
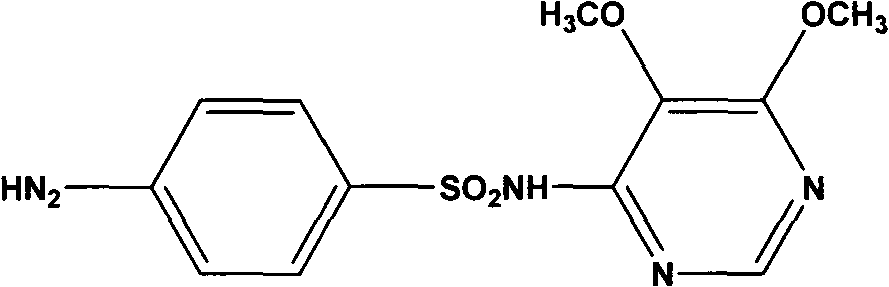
Preparation method of sulfadoxine
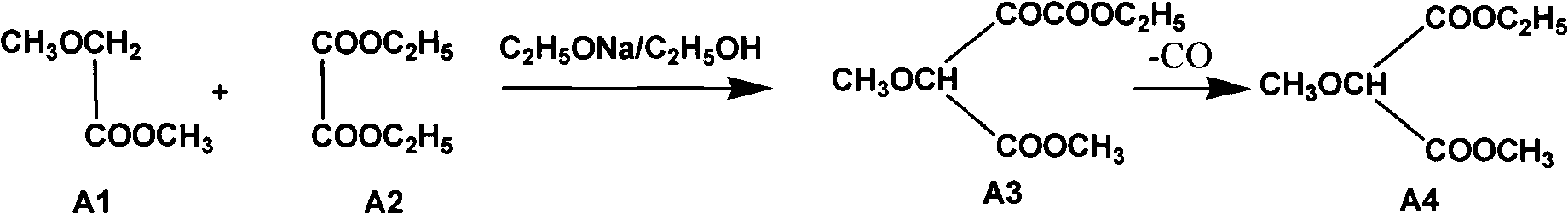
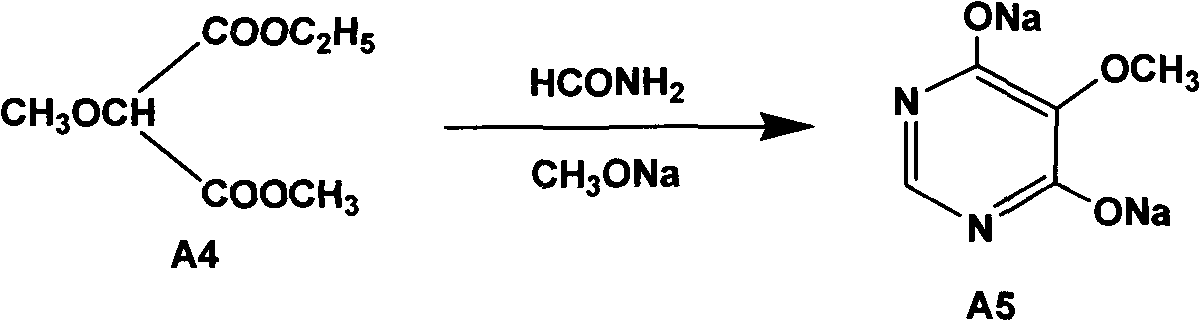
A preparation method of sulfadoxine belongs to the field of sulfanilamide antimicrobial drug preparation. Cyclization reaction comprises the following steps of: firstly pouring a sodium methoxide solution into a reactive pan, then successively adding methanamide and methyl ethyl methoxymalonate, keeping warm, recovering methanol, cooling for crystallization, drying by centrifugation, discharging,and drying to obtain 5-methoxy-4,6-disodium dihydroxypyrimidine; Chlorination reaction comprises the following steps of: firstly putting phosphorus oxychloride into a reaction vessel for heating, adding 5-methoxy-4,6-disodium dihydroxypyrimidine into the reaction vessel to react, decompressing and recovering phosphorus oxychloride until the material is dry, cooling, adding trichloro ethylene withuniformly stirring, putting into a hydrolysis pan for hydrolyzation, collecting a trichloro ethylene layer after standing and delaminating, followed by a neutralization reaction, controlling pH value, washing, removing a water layer, recovering trichloro ethylene, and releasing crystals to obtain 5-methoxy-4,6-dichloropyrimidine. The preparation method provided by the invention can be used to guarantee the product purity, prolong the service life of equipment, avoid the damage to the environment and human body, reduce emission, and save energy, and accords with foreign pharmacopoeia standard requirements.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
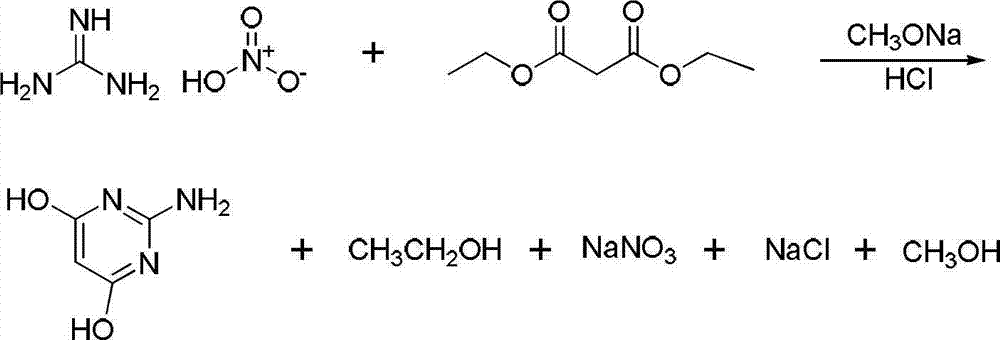
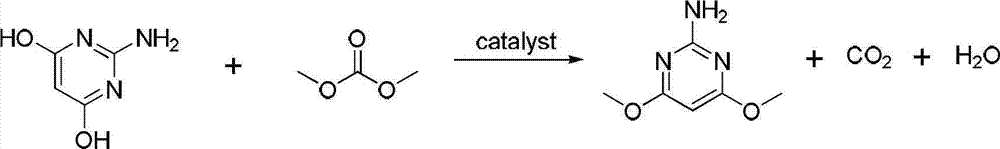
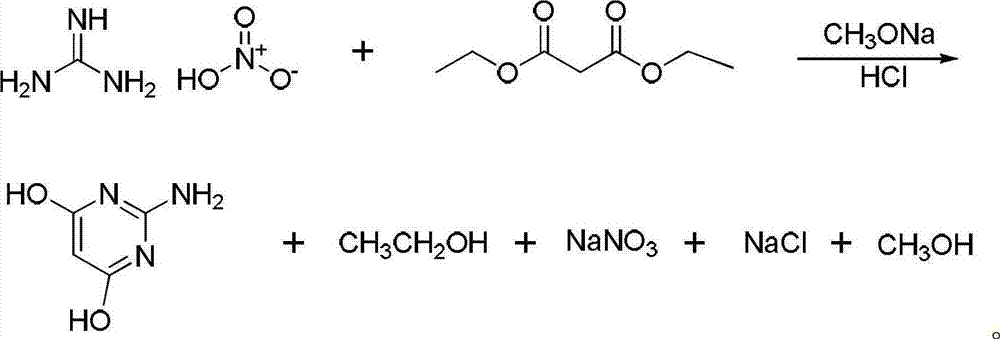
Method for synthesizing 2-amino-4,6-dimethoxypyrimidine
The invention relates to a method for synthesizing 2-amino-4,6-dimethoxypyrimidine serving as a fine chemical raw material. The method comprises the following steps of: performing cyclization and hydrolysis on guanidine nitrate, diethyl malonate, sodium methoxide, methanol and dimethyl carbonate serving as raw materials to generate 2-amino-4,6-dyhydroxypyrimidine, and performing methylation on the 2-amino-4,6-dyhydroxypyrimidine and dimethyl carbonate to generate the 2-amino-4,6-dimethoxypyrimidine. The raw materials are cheap; and the method is simple and convenient to operate, facilitates large-scale industrialized production, and has high application value. By using the dimethyl carbonate which is a green raw material as a methylation reagent, the reaction process is shortened, and generation of three wastes is greatly reduced; and because the route is shortened, the energy consumption is also reduced.
Owner:JIANGSU REPONT PESTICIDE FACTORY
Method for producing enriched boric-10 acid from trifluoride-anisole complex and application thereof
The invention relates to a method for producing enriched boric-10 acid from a trifluoride-anisole complex and application thereof. The method comprises the following steps: reacting a boron trifluoride-anisole complex with excessive sodium methoxide methanol solution, operating in an ice bath for 5-25 minutes, carrying out thermostatic water bath, reacting for 40-60 hours while keeping the reaction temperature within the range of 40-60 DEG C, stopping heating, and carrying out centrifugal stratification; fractionating the centrifugated supernatant: heating the mixture, starting to collect the fraction when the temperature rises to 50 DEG C, and stopping collecting the fraction when the temperature rises to 60 DEG C; and carrying out salting-out stratification on the collected fraction, mixing with deionized water, carrying out vacuum filtration on the mixture to obtain a solid, and drying to obtain the boric acid. The enriched boric-10 acid is used in the field of production of 10B-isotope-enriched downstream boric acid products, nuclear-grade boric acids and other enriched boric-10 acids by an anisole chemical exchange fractionation process. The production raw materials are from a closed system; and the invention has the impurity removal link, so the product purity is higher, thereby lowering the difficulty of subsequent boric acid production.
Owner:TIANJIN UNIV
Method for preparing lidocaine hydrochloride
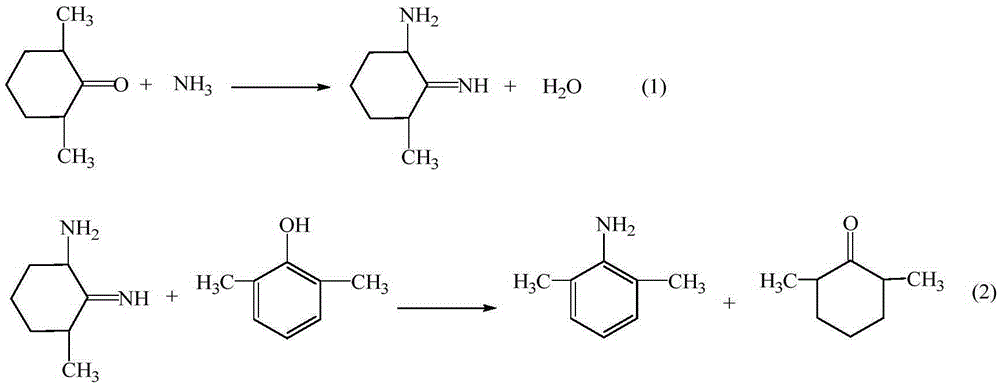
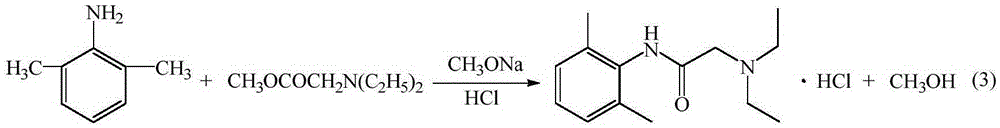
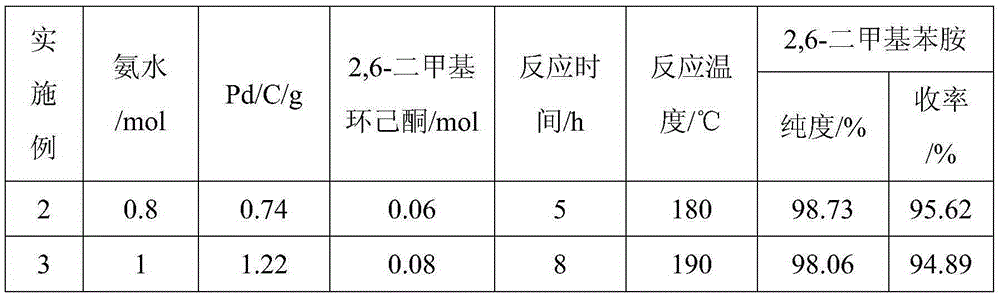
ActiveCN105294477AThe synthesis process is simpleHigh purityOrganic compound preparationCarboxylic acid amides preparationDimethylaniline N-oxideOrganic layer
The invention provides a method for preparing lidocaine hydrochloride, and belongs to the technical field of anesthetic synthesis. The method comprises the following steps: by taking 2,6-xylenol as a raw material, Pd / C as a main catalyst and 2,6-dimethylcyclohexanone as a promoter, performing liquid phase amination with ammonia water at high temperature, thereby obtaining a midbody 2,6-dimethylaniline; enabling sodium methylate, 2,6-dimethylaniline and N,N-lignocaine methyl acetate as raw materials to react at 90-95 DEGC, distilling while reaction is performed to remove methanol till no methanol can be evaporated out, continuously reacting for 30 minutes, cooling to the room temperature, adding dichloroethane, washing with water, and leaving to stand to layer, thereby obtaining an organic layer, namely, a lidocaine based dichloroethane solution; further adding hydrochloric acid into the lidocaine based dichloroethane solution, adjusting the pH value to be 3.5-4 by using hydrogen chloride, adding activated carbon to reflux for 20-40 minutes, filtering, concentrating the filtrate, cooling, crystallizing, and dying, thereby obtaining lidocaine hydrochloride. The lidocaine hydrochloride prepared by using the method is simple in synthesis process and high in product purity, that is, the purity can be greater than 99%, and the total yield is greater than 84%.
Owner:ZHEJIANG ESIGMA BIOTECH CO LTD
Preparation method of sulfadoxine
InactiveCN102304095AReduce usageQuality improvementOrganic chemistrySodium methoxideDimethylaniline N-oxide
The invention relates to a preparation method of sulfadoxine, which comprises the following steps: (1) reacting methyl methoxyacetate and excessive diethyl oxalate to generate 3-methoxy-2-oxo-methylethyl succinate, and decarbonylating to obtain 2-methoxy-methylethyl malonate; (2) reacting the 2-methoxy-methylethyl malonate and formamide to generate a cyclocompound; (3) carrying out chlorination reaction; (4) carrying out condensation reaction; and (5) carrying out methoxylation reaction. The purity of the 2-methoxy-methylethyl malonate obtained in the step (1) is strictly controlled, and the cyclocompound in the step (2) is controlled to exist in the form of anhydrous hydroxy sodium salt; in the step (3), no catalyst, including N,N-dimethylaniline, is used; and in the step (5), solid sodium hydroxide is substituted for sodium methoxide solution. The invention is easy to operate, has the advantage of high product quality, greatly lowers the production cost, and has obvious economic benefit and environmental benefit.
Owner:CHANGSHU NANHU INDAL CHEM
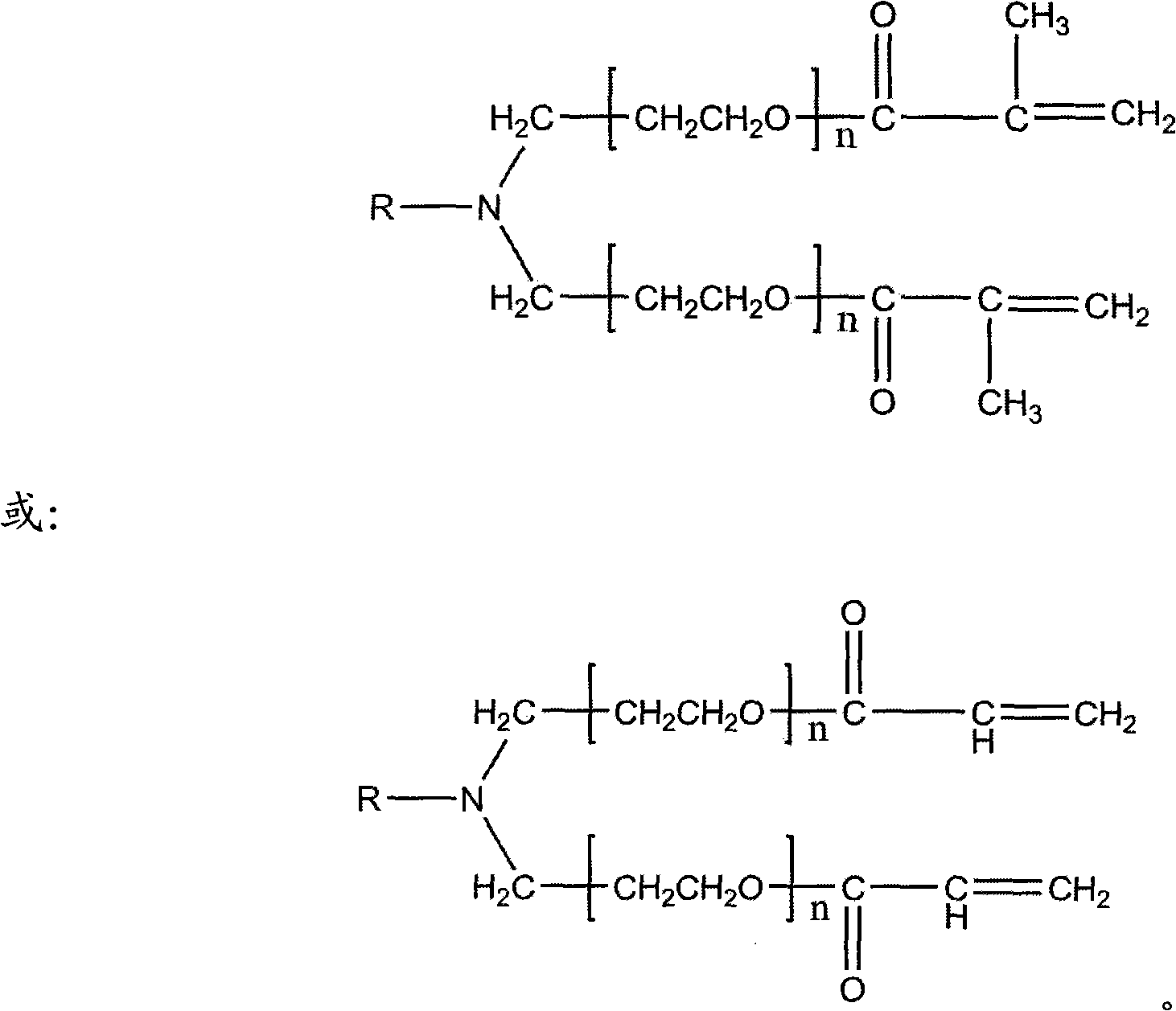
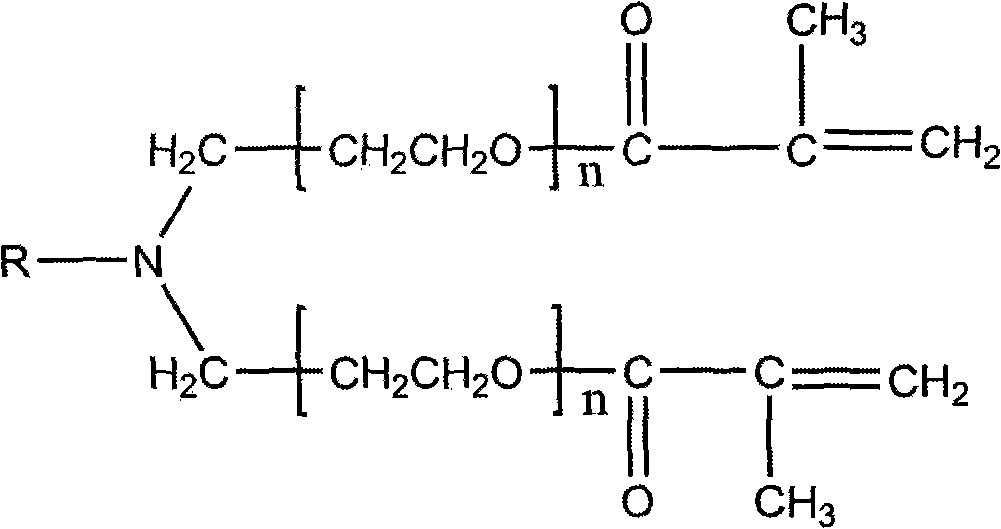
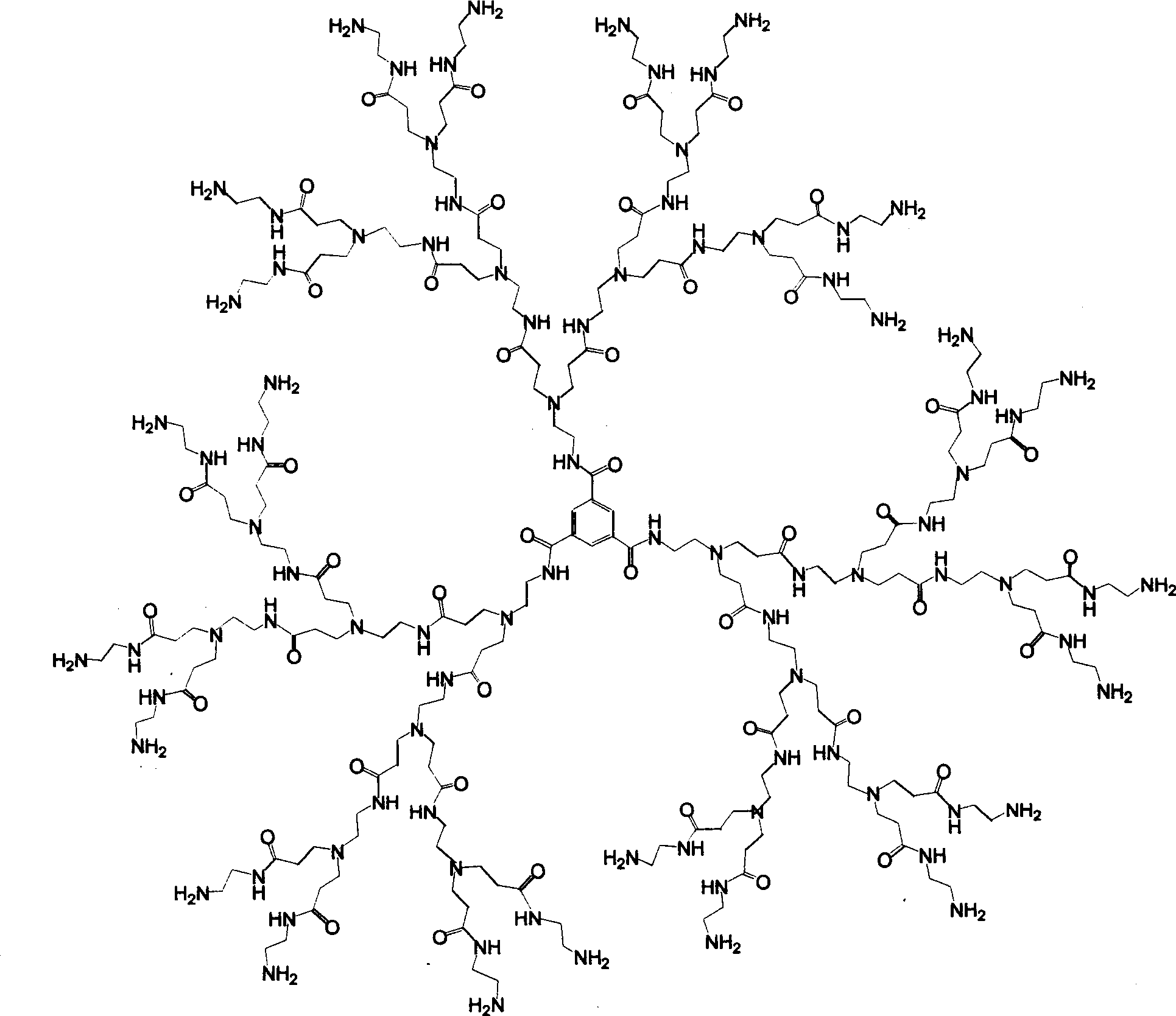
Tree-type high-molecular polyamide-amine compound and its preparing process and application
InactiveCN1379053ABiodegradableHigh efficiency and low toxicityVector-based foreign material introductionSodium methoxideMolecular materials
A tree-type polyamide-amine material series includes G1.5, G2.0, G2.5, G3.5...G8.0 three-type high-molecular materials. The above-mentioned half generation tree-type polyamid-amine material is prepared through Michael addition reaction of integer generation one on methyl acrylate under the catalysis of sodium methoxide. The above-mentioned integer generation tree-type polyamide-amine material is prepared through amidation reaction of half generation one on ethanediamine. Its advantages are biodegradability and low poison. It can be used as gene transfer carrier and release controlling carrierof medicine.
Owner:WUHAN UNIV
Preparation of 2,4-dichloro-5-methoxy pyrimidine
InactiveCN101486684ARaw materials are easy to getEasy to synthesizeOrganic chemistrySodium methoxideMethyl methoxyacetate
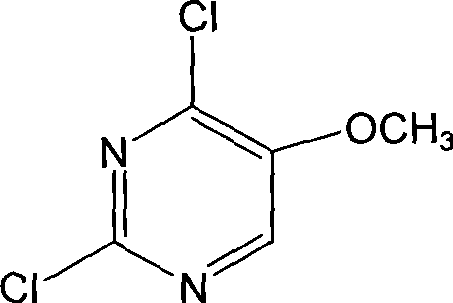
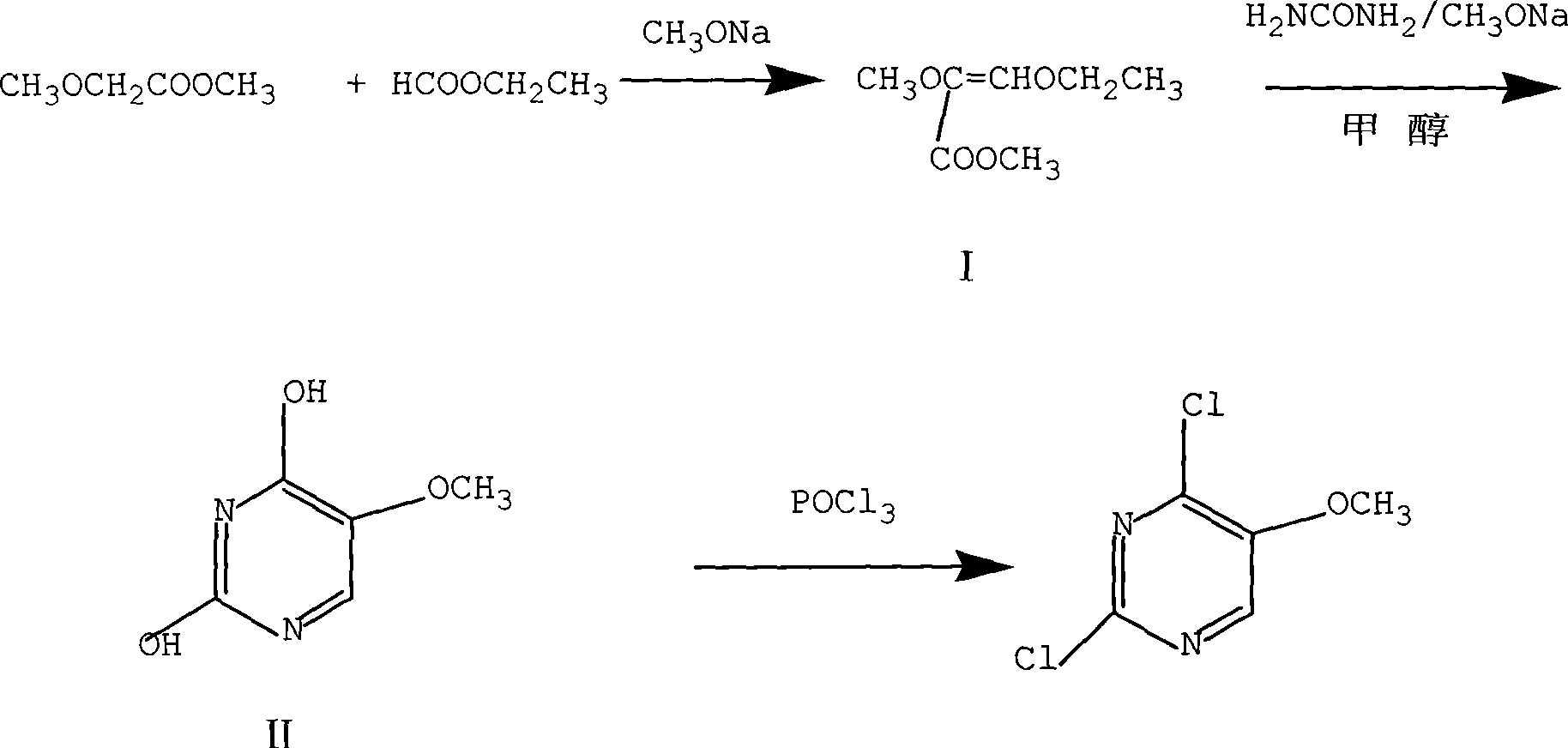
The invention discloses a preparation method of 2, 4-dichloro-5-methoxypyrimidine, pertaining to the technical field of pesticide intermediate preparation. The method includes steps as follows: 2, 4-dihydroxy-5-methoxypyrimidine is prepared, and ethyl formate and solid sodium methoxide are added into a reaction device and stirred. After the temperature is lowered, methyl methoxyacetate is added for carrying out a condensation reaction to obtain a compound I, and then methanol and carbamide are added into the compound I and a refluxing reaction is carried out. A compound II is obtained after condensation, dissolution with water, cooling, neutralization, filtration and drying; the 2, 4-dichloro-5-methoxypyrimidine is prepared, and a chlorinating agent and an acid-binding agent are added into the compound II; and then a temperature reaction, dilution and filtration are carried out in sequence to obtain a crude product of the 2, 4-dichloro-5-methoxypyrimidine. The crude product is refined to obtain a pure product of the 2, 4-dichloro-5-methoxypyrimidine. The method has the advantages of easy availability of all raw materials, convenient synthesis, not exacting technological conditions, overall yield up to 57 percent to 67 percent, purity over 99.6 percent and applicability to industrialized production.
Owner:JIANGSU HUAYI TECH
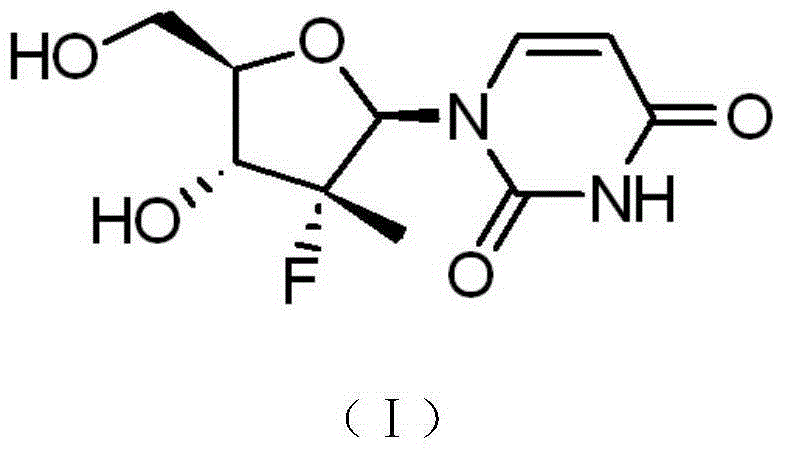
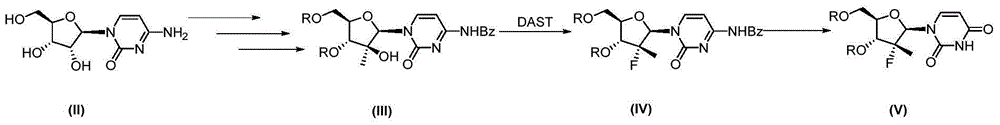
Synthesis method of intermediate compound of sofosbuvir
InactiveCN104987355AHigh yieldMild reaction conditionsSugar derivativesSugar derivatives preparationSodium methoxideSynthesis methods
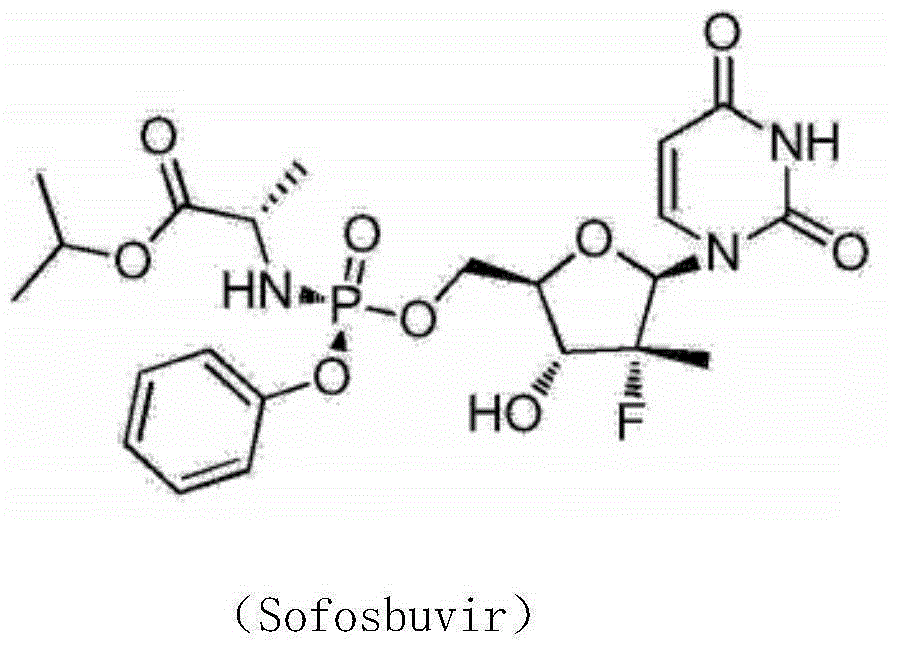
The invention provides a synthesis method of an intermediate compound of sofosbuvir as shown in formula (I) (see specification). The synthesis method of the intermediate compound of sofosbuvir, a synthetic route of which is as follows: (see specification) the synthesis method comprises the following steps: taking (3R, 4R, 5R)-3-fluoro-dihydro-4-hydroxl3-methyl furan-2(3H)-ketone as an initial raw material to react with pivaloyl chloride to generate an intermediate product (XII); reducing the intermediate product to obtain an intermediate product (XIII); enabling the intermediate product (XIII) to react with pivaloyl chloride to generate an intermediate product (XIV); generating an intermediate product (XV) by virtue of the reaction of the intermediate product (XIV) and a hydrogen bromide; performing silyl-hilbert-johnson reaction for the intermediate product (XV) and uracil to generate an intermediate product (XVI); enabling the intermediate product (XVI) to react with sodium methoxide to obtain a target product. The synthesis method of the intermediate compound (I) is moderate in reaction condition, simple in procedure, low in cost, environment-friendly and favorable for the industrialized production.
Owner:SHANGHAI TWISUN BIO PHARM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00221.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00231.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00232.PNG)