Method for synthesizing 2-amino-4,6-dimethoxypyrimidine
A technology of dimethoxypyrimidine and synthesis method is applied in the field of synthesis of fine chemical raw material 2-amino-4,6-dimethoxypyrimidine, and can solve the problem of large amount of waste water, waste acid, energy consumption and equipment loss, Complex process and other problems, to achieve the effect of high cost, reduce production cost, and simplify the process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
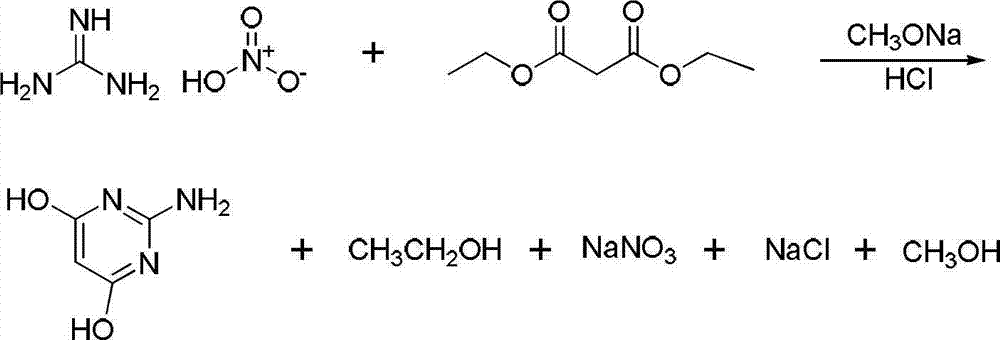
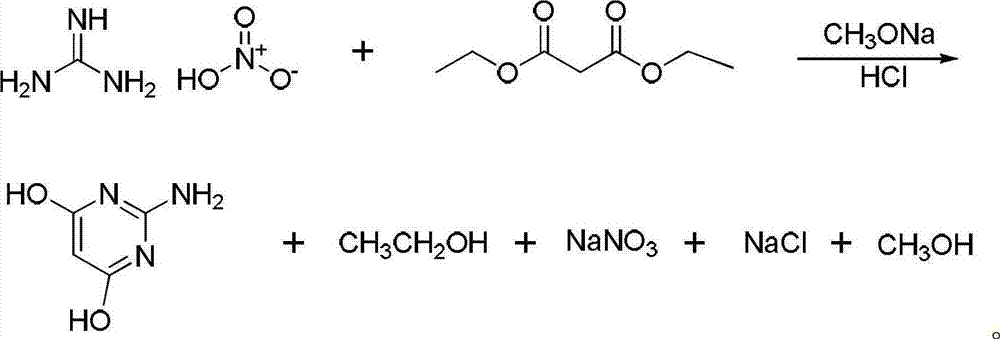
[0022] (1) Take 48.8g of guanidine nitrate and 64g of diethyl malonate in a 500ml four-necked bottle (the molar ratio of guanidine nitrate to diethyl malonate is 1:1), add 10g of anhydrous methanol and stir well. Another 216g of liquid sodium methoxide (the molar ratio of liquid sodium methoxide to guanidine nitrate is 1:3) was added dropwise to the mixture at 40°C, and the dropwise addition was completed in about 1 hour, then the temperature was raised to 68°C, and reacted in a reaction device with reflux 3.5h. Add a distillation device to recover methanol, leaving a white solid; then add water to the reaction flask to completely dissolve the white solid, filter to remove insoluble flocs, adjust the pH value to 5-6 with 0.1mol / L HCl solution, and filter with suction , washing with water, drying, grinding, and vacuum drying. 50.6 g of 2-amino-4,6-dihydroxypyrimidine was obtained, with a yield of 83%.
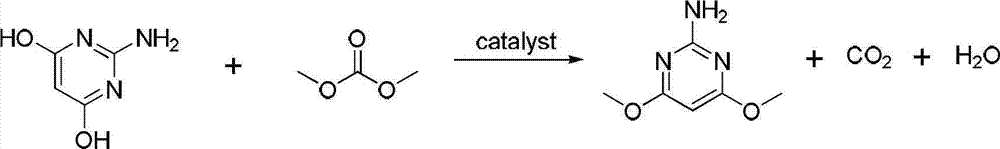
[0023] (2) Add 25.4g of 2-amino-4,6-dihydroxypyrimidine into a 150ml auto...
Embodiment 2
[0025] (1) Take 58.6g of guanidine nitrate and 64g of diethyl malonate in a 500ml four-necked bottle (the molar ratio of guanidine nitrate to diethyl malonate is 1.2:1), add 23.6g of anhydrous methanol and stir well . Another 216g of liquid sodium methoxide (the molar ratio of liquid sodium methoxide to guanidine nitrate is 1:3) was added dropwise to the mixture at 60°C, and the dropwise addition was completed in about 1 hour, then the temperature was raised to 68°C, and reacted in a reaction device with reflux 3.5h. Add a distillation device to recover methanol, leaving a white solid; then add water to the reaction flask to completely dissolve the white solid, filter to remove insoluble flocs, adjust the pH value to 5-6 with 0.1mol / L HCl solution, and filter with suction , washed several times with water, dried, ground, and vacuum-dried. 57.9 g of 2-amino-4,6-dihydroxypyrimidine was obtained, with a yield of 95%.
[0026] (2) Add 25.4g of 2-amino-4,6-dihydroxypyrimidine in...
Embodiment 3
[0028] (1) Take 73.2g of guanidine nitrate and 64g of diethyl malonate in a 500ml four-necked bottle (the molar ratio of guanidine nitrate to diethyl malonate is 1.5:1), add 50g of anhydrous methanol and stir well. Another 360g of liquid sodium methoxide (the molar ratio of liquid sodium methoxide to guanidine nitrate is 1:5) was added dropwise to the mixture at 60°C, and the dropwise addition was completed in about 1 hour, then the temperature was raised to 68°C and reacted in a reaction device with reflux . Add a distillation device to recover methanol, leaving a white solid; then add water to the reaction flask to completely dissolve the white solid, filter to remove insoluble flocs, adjust the pH value to 5-6 with 0.1mol / L HCl solution, and filter with suction , washed several times with water, dried, ground, and vacuum-dried. 58.7 g of 2-amino-4,6-dihydroxypyrimidine was obtained, with a yield of 96.3%.
[0029] (2) Add 25.4g of 2-amino-4,6-dihydroxypyrimidine into a 15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com