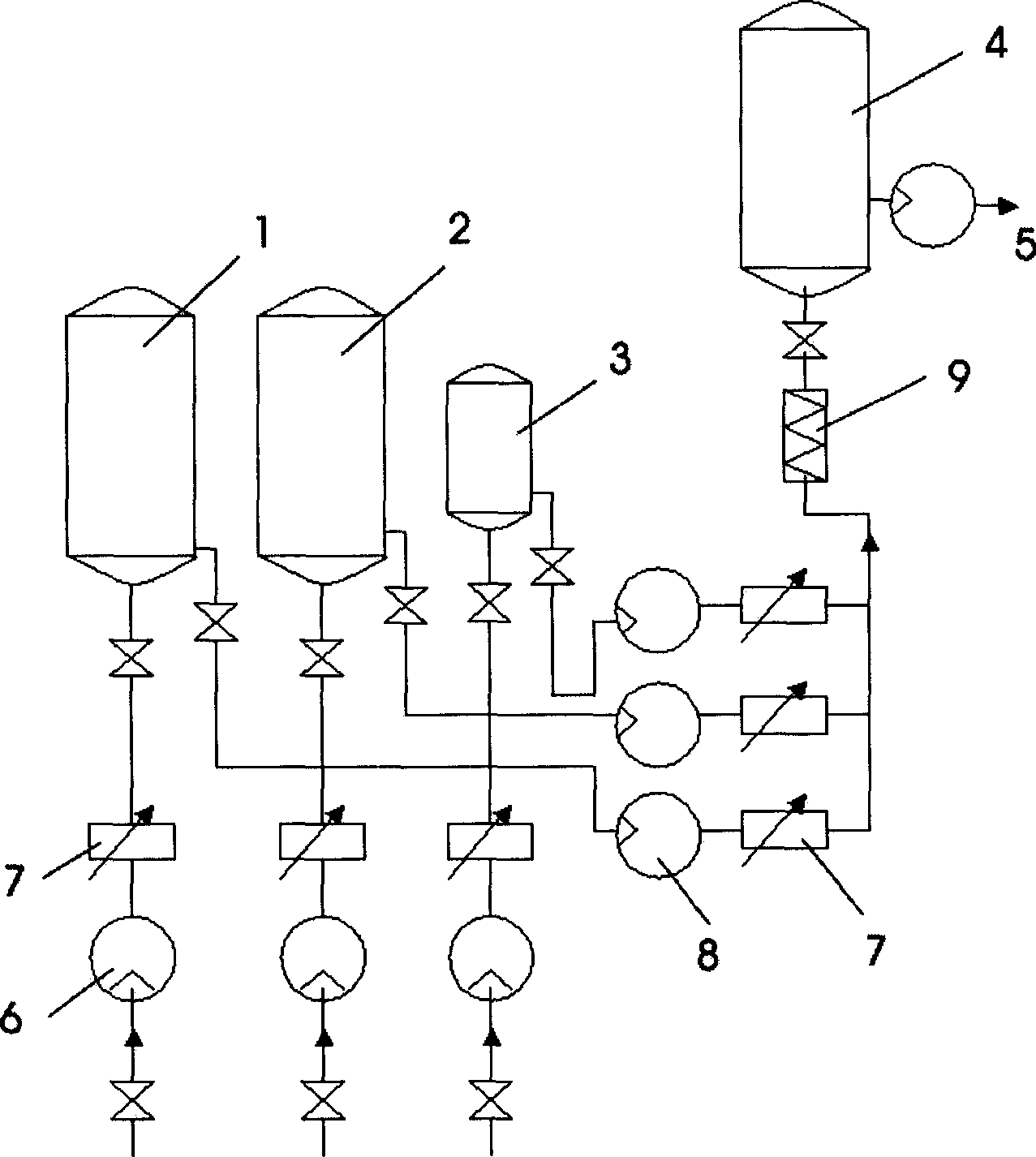
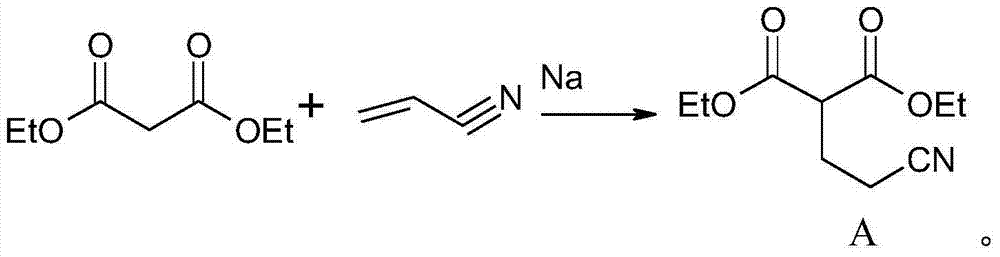
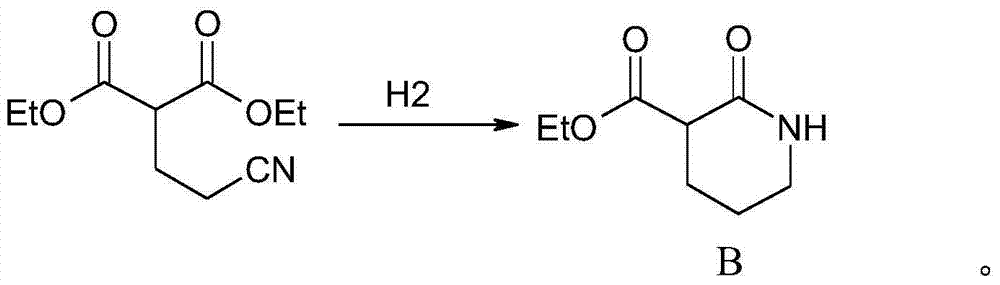
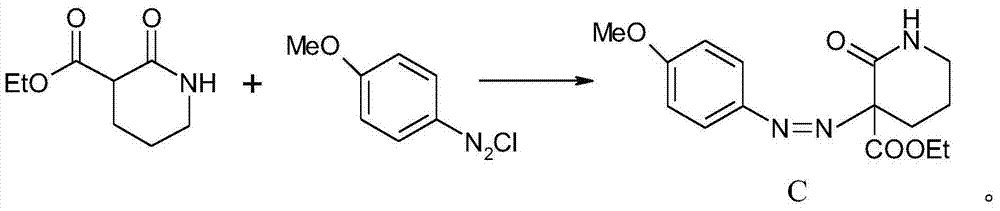
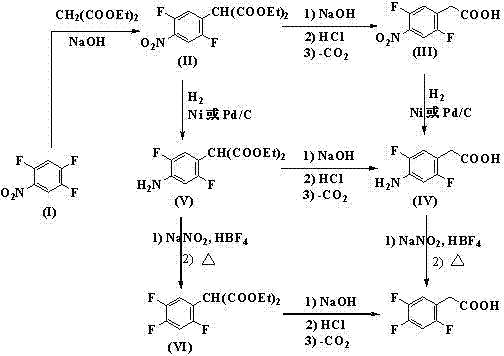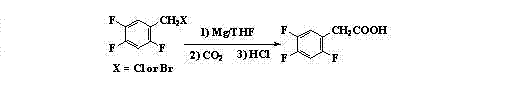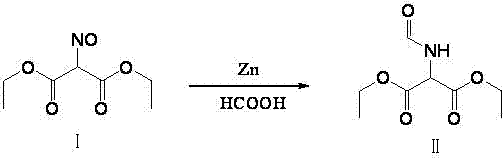Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
486 results about "Diethyl malonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
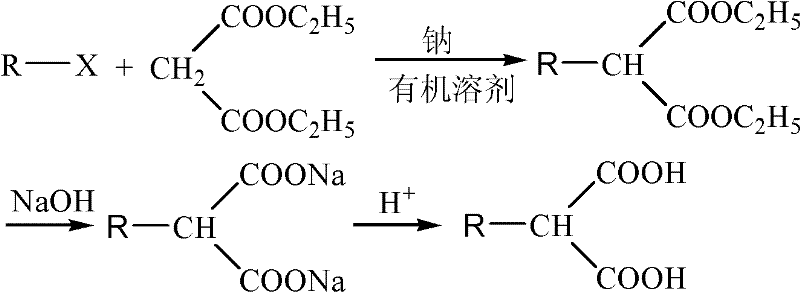
Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes. It is also used to synthesize other compounds such as barbiturates, artificial flavourings, vitamin B₁, and vitamin B₆.
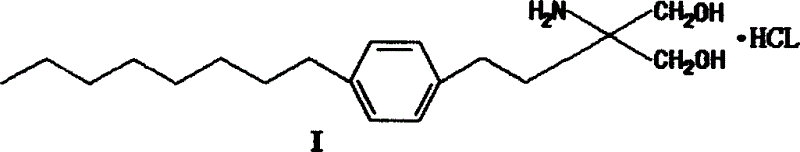
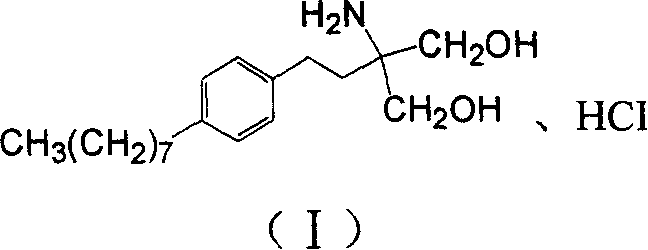
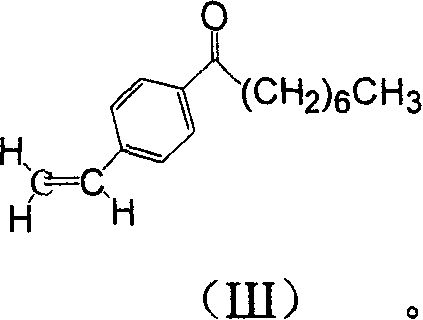
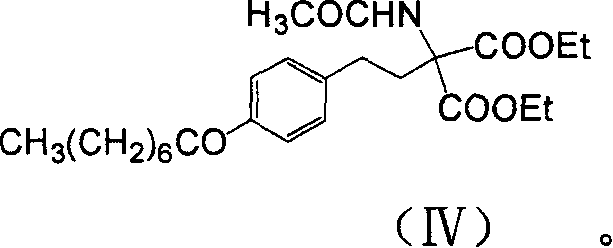
Method for preparing 2-para octylphenyl ehtyl-2-amino propanediol
InactiveCN1528738AEasy to manufactureEfficient manufacturingOrganic compound preparationAmino-hyroxy compound preparationSodium iodidePropanediol
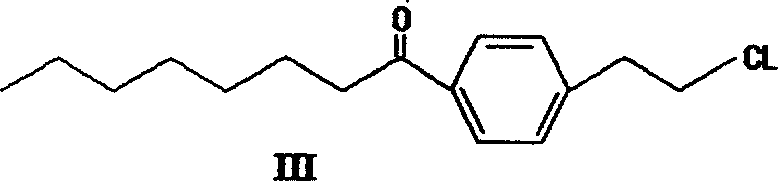
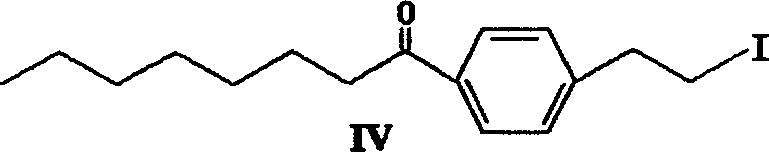
The invention provides a method to prepare a compound method, including the steps: ethylbenzene and capryl chloride make Friedel-Crafts reaction to generate p-capryl chloroethylbenzene; convert capryl chloroethylbenzene under the action of sodium iodide into p-capryl iodoethylbenzene; p-capryl iodoethylbenzene and acetylamino diethyl malonate condense under the action of alkali to generate 2-(p-capryl phenethyl)-2-acetylamino diethyl malonate; or p-capryl iodoethylbenzene makes elimination reaction under the action of alkali to generate p-capryl styrene, which together with acetylamino diethyl malonate condenses under the action of alkali into 2-(p-capryl phenethyl)-2- acetylamino diethyl malonate; a compound is reduced into 2-[4-(1-hydroxyoctyl) phenethyl-]2- acetylamino propylene alcohol; the other coumpound makes hydrogenolysis to obtain 2-(p-octyl phenethyl)-acetylamino propylene alcohol; make alkali hydrolyzation and then acidifies them into salt, so as to obtain it. It also provides a method to prepare intermediate in the above preparing course.
Owner:马启明
Method for preparing 2-P-octyl-phenenl-2-amino-propanediol hydrochloride
InactiveCN1814583AEasy to manufactureEfficient manufacturingOrganic compound preparationAmino-hyroxy compound preparationSilanesPropanediol
The invention offers 2-amphi capryl phenethyl-2-amino group propylene glycol hydrochloride preparation method. It includes the following steps: doing Friedel-Crafts reaction for styrene and capryl chloride to produce amphi capryl styrene; doing Michael addition reaction with kharophen diethyl malonate under alkali action to produce 2-amphi capryl phenethyl-2-kharophen diethyl malonate; reacting with triethyl silane to produce 2-amphi capryl phenethyl-2-kharophen diethyl malonate; reducing and hydrolyzing to produce 2-amphi capryl phenethyl-2-amino group propylene glycol; acidifying with hydrochloric acid to produce salt. It has the advantages of simple line, low cost, short period, little pollution, and high yield. It also offers the intermediate compound preparation method.
Owner:NANJING YOKO PHARMA GRP CO LTD
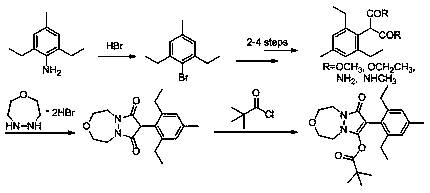
Synthesis method of pinoxaden
The invention discloses a synthesis method of pinoxaden, and belongs to the field of chemical engineering. The method comprises the following steps that 2,6-diethyl-4-methylaniline is subjected to sodium nitrite diazotization and thermal decomposition to generate 2,6-diethyl-4-methylbromobenzene; diethyl malonate is added into [1,4,5]-oxadiazepine dihydrobromide hydrate and an alkaline catalyst tosynthesize dihydro-1H-pyrazolo[1,2-d][1,4,5]-oxadiaza-7,9(2H, 8H)-diketone, the dihydro-1H-pyrazolo[1,2-d][1,4,5]-oxadiaza-7,9(2H, 8H)-diketone and 2,6-diethyl-4-bromomethyl benzene are subjected tocoupling reaction to obtain 8-(2,6-diethyl-4-methyl phenyl)tetrahydropyrazolo[1,2-d][1,4,5]xazepine-7, 9(2H, 8H)-diketone; the 8-(2,6-diethyl-4-methyl phenyl)tetrahydropyrazolo[1,2-d][1,4,5]xazepine-7, 9(2H, 8H)-diketone is reacted with pivaloyl chloride to synthesize the final product pinoxaden. According to the synthesis method of the pinoxaden, the synthesis steps are shortened, operation is simple and convenient, the cost is reduced, the economic efficiency is high, and environmental pollution is little.
Owner:兰州精细化工有限责任公司 +1
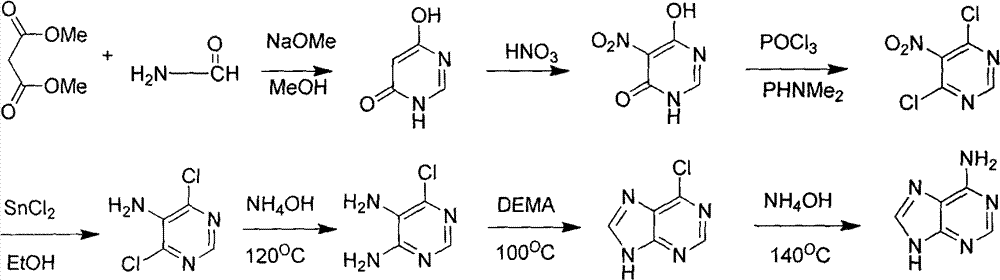
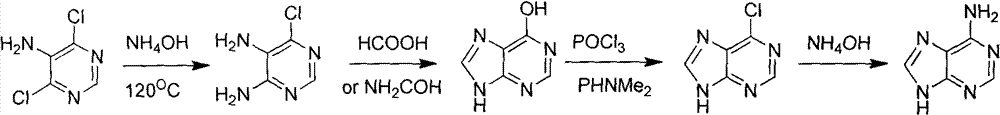
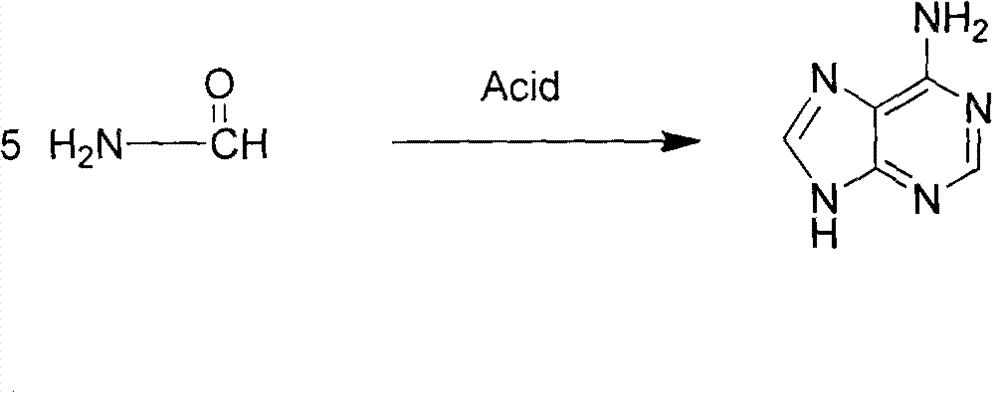
Novel chemical synthesis method for adenine
InactiveCN102887899AShort route stepsIncrease productivityOrganic chemistryChemical synthesisAcetic anhydride
The invention discloses a method for synthesizing adenine represented by a formula (I), wherein the method comprises the following steps of making formamide react with diethyl malonate in an ethanol solution of sodium ethoxide to obtain a raw material 4,6-dihydroxypyrimidine represented by a formula (VI); nitrifying the 4,6-dihydroxypyrimidine to obtain 4,6-dihydroxy-5-nitropyrimidine represented by a formula (V); carrying out chlorination reaction on the 4,6-dihydroxy-5-nitropyrimidine (V) to obtain 4,6-dichloro-5-nitropyrimidine represented by a formula (IV), carrying out aminolysis reaction on the 4,6-dichloro-5-nitropyrimidine (IV) and an saturated aminoethanol solution to obtain 4,6-diamino-5-nitropyrimidine represented by a formula (III); carrying out catalytic hydrogenation on the 4,6-diamino-5-nitropyrimidine (III), and reducing a nitro group to obtain 4,5,6-triaminopyrimidine represented by a formula (II); making the 4,5,6-triaminopyrimidine (II) react with ethyl orthoformate in acetic anhydride to obtain the adenine represented by the formula (I). The method provided by the invention has the advantages of cheap and easily-obtained raw materials, mild reaction conditions, single product, high total yield, low production cost and easiness in industrial production.
Owner:YANGZHOU UNIV
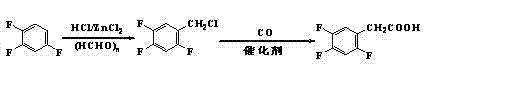
Preparation method 2,4,5-trifluorophenylacetic acid
ActiveCN103012111AFew synthetic stepsMild reaction conditionsOrganic compound preparationCarboxylic preparation by ozone oxidationPhenylacetic acidNitrobenzene
The invention discloses a preparation method 2,4,5-trifluorophenylacetic acid. The method is characterized by consisting of four reaction steps of: A, reaction of 2,4,5-trifluoronitrobenzene (I) and diethyl malonate which are condensed to prepare 2,5-difloro-4-nitrobenzophenone diethyl malonate; B, hydrolysis, acidification and decarboxylic reaction of dibasic ester; C, reduction reaction of nitryl; and D, diazotization fluoridation of amino. The four reaction steps can be sequentially carried out according to A, B, C and D, or A, C, B and D, or A, C, D and B. According to the preparation method provided by the invention, condensation of 2,4,5-trifluoronitrobenzene (I) and diethyl malonate is easy to realize by means of high substituting activity of nitryl p-fluorine, and the raw material 2,4,5-trifluoronitrobenzene (I) is low in cost and easy to obtain and can be easily prepared by nitration and fluorination of 2,4-dichlor fluorbenzene. Compared with the prior art, the preparation method provided by the invention has the characteristics of low-cost and easily obtained raw materials, mild reaction condition, high total yield, low production cost and the like, and is comparatively suitable for industrialized production.
Owner:江苏中丽新材料有限公司
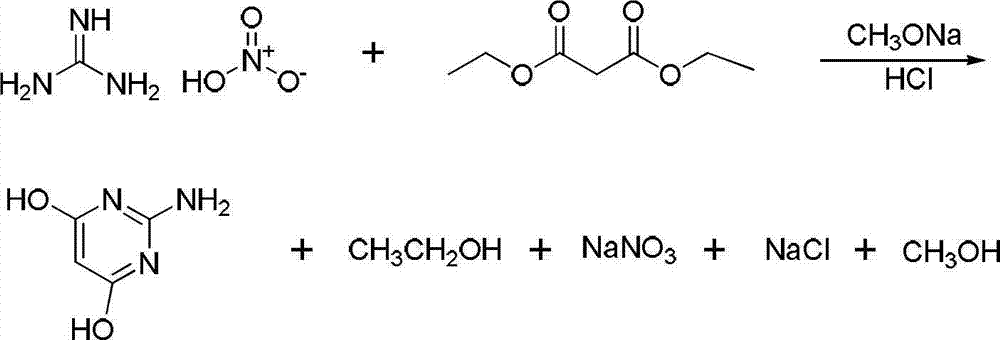
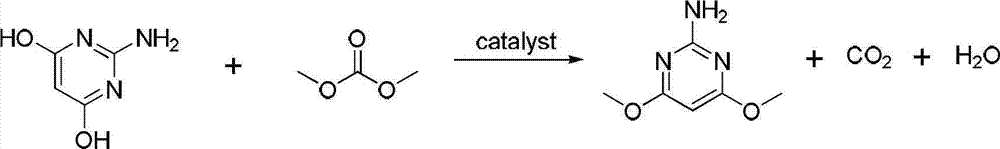
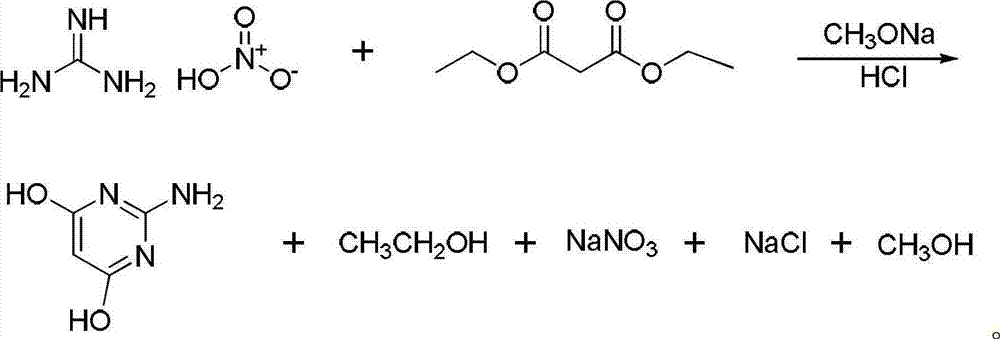
Method for synthesizing 2-amino-4,6-dimethoxypyrimidine
The invention relates to a method for synthesizing 2-amino-4,6-dimethoxypyrimidine serving as a fine chemical raw material. The method comprises the following steps of: performing cyclization and hydrolysis on guanidine nitrate, diethyl malonate, sodium methoxide, methanol and dimethyl carbonate serving as raw materials to generate 2-amino-4,6-dyhydroxypyrimidine, and performing methylation on the 2-amino-4,6-dyhydroxypyrimidine and dimethyl carbonate to generate the 2-amino-4,6-dimethoxypyrimidine. The raw materials are cheap; and the method is simple and convenient to operate, facilitates large-scale industrialized production, and has high application value. By using the dimethyl carbonate which is a green raw material as a methylation reagent, the reaction process is shortened, and generation of three wastes is greatly reduced; and because the route is shortened, the energy consumption is also reduced.
Owner:JIANGSU REPONT PESTICIDE FACTORY
Magnetic water-soluble fullerene, and preparation method and application thereof
InactiveCN103230604ALow toxicityLow costEnergy modified materialsInorganic non-active ingredientsSodium acetateSolubility
The invention relates to a magnetic water-soluble fullerene, and a preparation method and application thereof. The invention effectively solves the problems of low water solubility, low compatibility and poor targeting property of the fullerene. The method comprises the following steps: dissolving fullerene in toluene, adding sodium hydride and diethyl bromomalonate, stirring in a nitrogen protective atmosphere to remove sodium hydride and toluene, adding the dried diethyl-bromomalonate-substituted fullerene into the toluene, hydrolyzing until the toluene phase becomes colorless, adding concentrated hydrochloric acid, filtering to obtain a filter cake, removing insoluble substances and methanol, drying to obtain malonic-acid-substituted fullerene, dissolving in an ethylene glycol and di-acetal mixed solvent, adding sodium acetate and ferric iron salt to react, washing, drying to obtain ferroferric-oxide-carried fullerene, carrying out ultrasonic dispersion on the ferroferric-oxide-carried fullerene, amino water-soluble substance and EDC.HCl in phosphate, reacting in a dark place, carrying out vacuum filtration, and drying to obtain the magnetic water-soluble fullerene. The magnetic water-soluble fullerene has the advantages of favorable magnetic targeting propertt, strong water dispersity, low toxicity for organisms, high physical and chemical stability, good quality and low cost.
Owner:ZHENGZHOU UNIV
Fire retardant for air-conditioning cabinet
The invention relates to a fire retardant for an air-conditioning cabinet and belongs to the technical field of flame retardant articles. The fire retardant is prepared from water, 2,2-dimethylsuccinic acid, 3-methyl-2-butylene-1-farnesyl acetate, (2E, 6E)-3,7,11-trimethyl-2,6,10-dodecatrien-1-ol, ethylene glycol monosalicylate and diethyl methylmalonate. The fire retardant for an air-conditioning cabinet can effectively realize flame retardation effects of the air-conditioning cabinet by interaction of chemical ingredients. The fire retardant has stable chemical components, does not decompose at a high temperature, attaches to the inner and outer wall of the air-conditioning cabinet, does not influence normal air-conditioning use, effectively prevents circuit flame spread, prevents air-conditioning fire hazard and blast, has excellent flame retardation effects and can be widely popularized in the fields of air-conditioning production and marketing.
Owner:安徽斯迈特新材料股份有限公司
High content environment-friendly methanol gasoline
InactiveCN1884448ATo achieve a large proportion of accessionIncrease motivationLiquid carbonaceous fuelsKetoneTrimethylolpropane
The invention relates the methanol petrol, comprising 61-80 parts methanol, 10-29 parts base oil, 0.2-0.3 parts ethane, 0.2-0.3 parts skellysolve B, 3-5 parts methyl tert-butyl ether, 0.5-0.8 parts ethanol, 0.2-0.3 part 2, 2- dimethylbutane, 0.3-0.5 part tert-butanol, 0.2-0.3 part normal propyl alcohol, 0.1-0.3 part 2- ethyl (-1-) ethanol, 0.3-0.5 part ethylene glycol, 0.1-0.2 part 1, 3- dihydroxybutane, 0.2-0.5 part neopentyl glycol, 0.1-0.2 part 1, 6- dihydroxy hexane, 0.3-0.8 part trimethylolpropane, 0.2-0.8 part pentaerythrite, 0.6-0.8 part diisopropyl ether, 0.2-0.3 part ethyldioxy acetic acid ethyl ester, 0.2-0.4 part isoproyl nitrite, 0.2-0.5 part dimethyl ketone, 0.1-0.2 part diethyl malonate, 0.2-0.4 part diphenyl carbonate, 0.02-0.05 part 102TB corrosion inhibitor, and 0.02-0.05 part 107PT anti-swelling agent. The invention has the advantages of high methanol content, environment protection, and good earthquake resistance and dynamic.
Owner:顾杏泉
Preparation technology of water-solubility fluorescent carbon quantum dot
The invention discloses a preparation technology of a water-solubility fluorescent carbon quantum dot. The preparation technology comprises the following steps of: adding glucose to a dried florence flask containing diethyl malonate according to a proportion of 5g / 20 milliliter, cooling to a room temperature after refluxing at 140-160 DEG C, collecting brown precipitates, and carrying out vacuum drying for 2 hours at 100-120 DEG C; and dissolving the dried precipitates in a 10ml of distilled water, carrying out high-speed centrifugal seperation (1200r / m) to remove a trace amount of precipitates, and cooling and drying the centrifuged solvent, thus obtaining a target fluorescent carbon point. The preparation technology is simple in the entire flow operation, low in cost and high in feasibility, can be used for macro-production of the water-solubility fluorescent carbon quantum dot and meets industrialization application; the particle size of the product is uniform and is mainly in the range of 2-4 nanometers; the fluorescence quantum efficiency is high and can reach 24%; added ions do not need to be removed by dialysis during after treatment as no positive ions are added in the preparation process; and the water-solubility fluorescent carbon quantum dot has good water-solubility and can be widely applied in practical application.
Owner:MINNAN NORMAL UNIV
Oil-resistant anti-corrosion waterborne paint and preparation method thereof
InactiveCN105199570AImprove anti-corrosion performanceImprove heat resistanceAnti-corrosive paintsPolyester coatingsIsooctyl acrylateCalcite
The invention discloses oil-resistant anti-corrosion waterborne paint which is prepared from alkyd resin, phenol formaldehyde epoxy resin, m-xylene phenolic resin, butyl etherified amino resin, hydroxyl bromopropanoic acid resin, phthalic acid dibutyl ester, hydroxyethyl acrylate, 2-ethylhexyl acrylate, diethyl malonate, methyl methacrylate, aluminum triphosphate, zinc phosphate, anhydrous ethanol, aluminum oxide, chlorinated paraffin, methyl cellulose, talcum powder, organic bentonite, silicon alloy powder, calcite, zinc stearate, titanium dioxide, kaoline, calcium carbonate, silica powder, dibutyltin dilaurate, magnesium stearate, thickeners, diluent, defoaming agents, coupling agents, propylene glycol monomethyl ether and deionized water. The invention further discloses a preparation method of the oil-resistant anti-corrosion waterborne paint. The obtained waterborne paint is good oil resistance and corrosion resistance and high in adhesion and hardness.
Owner:ANHUI KAILIN ADVANCED MATERIAL CO LTD
Synthesis method of melatonin
The invention belongs to the field of chemical synthesis of medicines and specifically relates to a synthesis method of melatonin. The method comprises the following steps: performing addition, ammonolysis of ester, coupling, rearrangement, hydrolysis of amide, decarboxylation, acylation and the like from diethyl malonate and acrylonitrile to obtain melatonin. According to the synthesis method provided by the invention, the starting raw materials are easy to obtain, the reaction in each step is relatively mild, a used solvent is small in amount and can be recovered and applied mechanically, an intermediate can directly enter the next-step reaction without refining and drying, and thus the synthesis method is suitable for industrial production.
Owner:湖北金赛药业有限公司
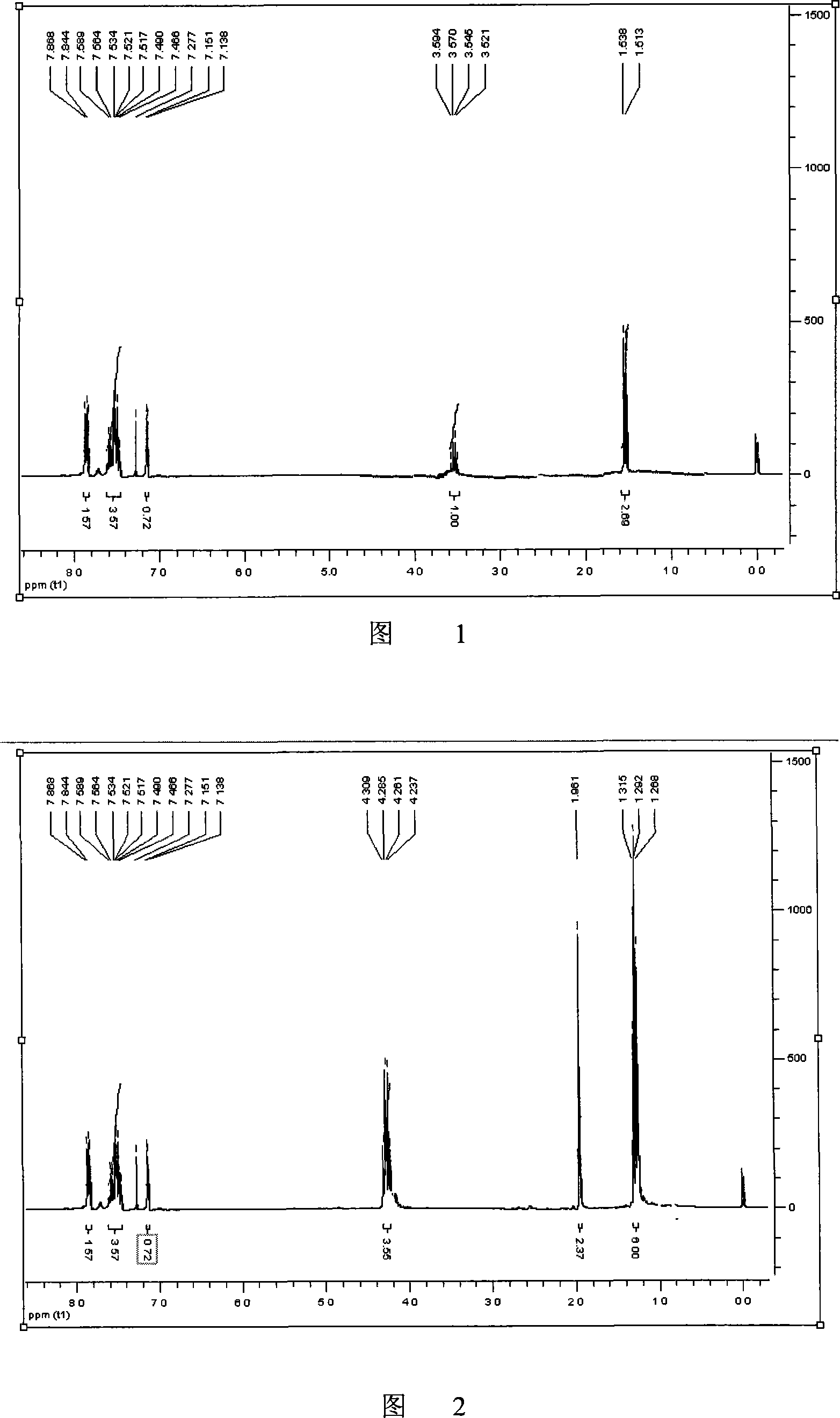
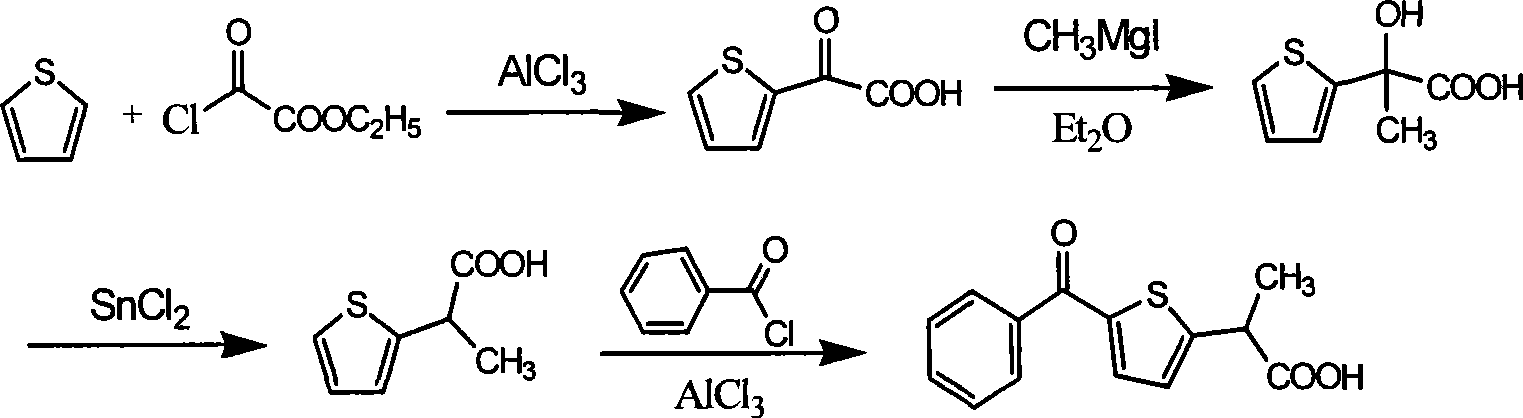
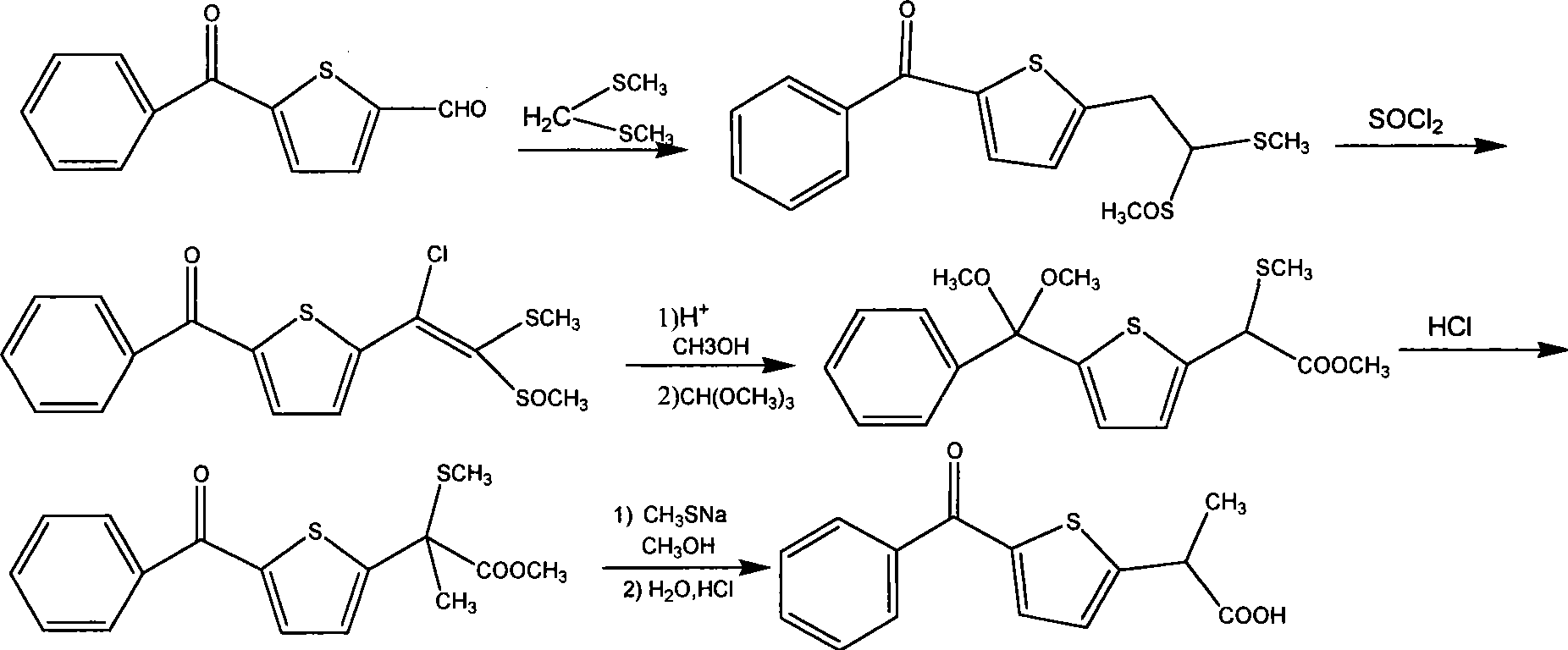
Method for synthesizing non-steroidal antiphlogiston tiaprofenic acid
The invention relates to tiaprofenic acid, that is, five-benzoyl-alpha-methyl-two-thiophene acetic acid, which is the ramification of the thiophene propionate and a new typed anti-inflaminatoroy and pain easing medicine. The invention is characterized in that two-thiophene benzophenone is obtained from the thiophene benzoylate by the benzene; the latter reacts with MDA-diethyl and generates the important intermediates five-benzoyl-alpha-methyl-two thiophene diethyl malonate under the action of the metal complex and the radiation of the power ultrasonic; the tiaprofenic acid is obtained after the saponification, acidization and decarboxylic reaction of the compound. The invention has the advantages of high yield up to seventy one percent calculated according to the thiophene, low cost of material, convenient and safe process, good product quality, and applicability to the industrial production.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
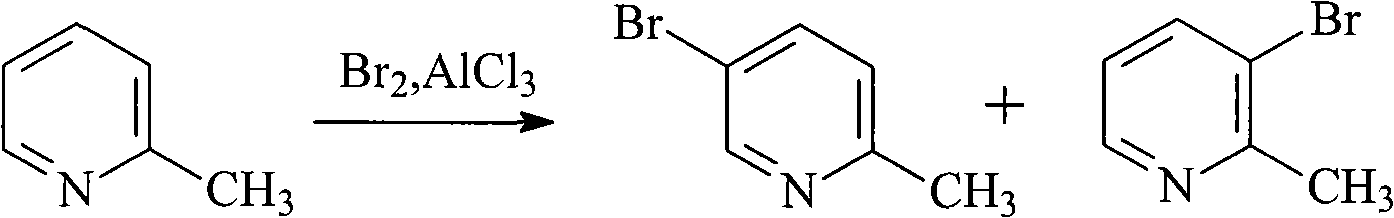
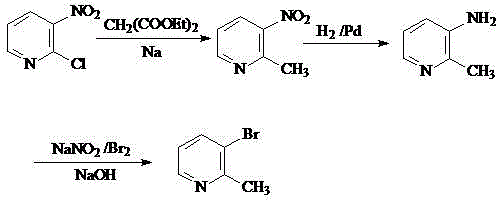
Method for preparing 5-bromo-2-methylpyridine
InactiveCN101560183AHigh yieldLow priceOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsCatalytic effectSodium nitrite
The invention discloses a method for preparing intermediate 5-bromo-2-methylpyridine. In the prior art, the dosage of aluminium trichloride is large; the catalytic effect is poor; the by-products are more; the yield of products is low; and the obtained products are difficult to separate. The method comprises the following steps: reacting diethyl malonate with alkali metal to generate salts, dripping 5-nitryl-2-chloropyridine into the salts for condensation reaction, and subsequently performing decarboxylation on the obtained product under acidic condition to obtain 5-nitryl-2-methylpyridine; performing hydrogenation reduction on the 5-nitryl-2-methylpyridine under the catalysis of Pd / C catalyst to obtain 5-amido-2-methylpyridine; and reacting the 5-amido-2-methylpyridine with acid to generate salts, dripping bromine, dripping a sodium nitrite water solution, and obtaining the 5-bromo-2-methylpyridine. The method has mild reaction conditions, easy operation, simple post-treatment, good catalytic effect, high yield of each step and high yield of final products, and is particularly suitable for industrialized production.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Preparation method of 2-methyl-3-bromopyridine
The invention belongs to the field of organic synthesis and particularly relates to a preparation method of 2-methyl-3-bromopyridine. The preparation method includes the following steps that 1, diethyl malonate reacts with alkali metal to generate salt, then a methylbenzene solution of 2-chlorine-3-nitropyridine is added dropwise to be conducted a condensation reaction, and the 2-methyl-3-nitropyridine is obtained through decarboxylation under an acidic condition; 2, under the catalysis of Pd / C, methanol serves as a solvent, a hydrogenation reduction and suction filtration are conducted on the 2-methyl-3-nitropyridine, filtered liquid is condensed, and 2-methyl-3-aminopyridine is obtained; 3, the 2-methyl-3-aminopyridine reacts with acid to generate salt, the cooling is conducted to enable the temperature to be in -10 DEG C-0 DEG C, bromine is added dropwise, after the addition, then a sodium nitrite solution is added dropwise, after addition, pH of the solution is adjusted to be alkaline, then extracting, drying and concentration are conducted, and the 2-methyl-3-bromopyridine is obtained. The preparation method of the 2-methyl-3-bromopyridine has the advantages that the reaction condition is moderate, the operation is easy, the post-processing is simple, enlarged production is easy, and the preparation method is very suitable for industrial production; the catalysis effect is good, and the reaction yield ratio is high; the price of the raw material is low, and the production cost is low.
Owner:洪帅金
Preparation method and application of topramezone
PendingCN111440160AAvoid smelly problemsHigh yieldBiocideOrganic chemistryHydroxylamine HydrochloridePotassium carbonate
The invention discloses a preparation method and application of topramezone, and the preparation method comprises the following steps: taking 2-methylbenzaldehyde, a bromination reagent, a catalyst, hydroxylamine hydrochloride, an alkali, ethylene gas, a sulfonylation reagent and a preset solvent as reaction raw materials, and preparing 3-[3-bromo-methyl-6-(methylsulfonyl) phenyl]-4, 5-dihydroisoxazole through a first reaction process; taking diethyl malonate, triethyl orthoformate, nickel sulfate, monobasic saturated carboxylic acid, methylhydrazine, a hydrocarbon solvent, an ethanol solutionand hydrochloric acid as reaction raw materials, and carrying out a second reaction process to prepare 1-methyl-5-hydroxypyrazole; and taking the 3-[3-bromo-methyl-6-(methylsulfonyl) phenyl]-4, 5 dihydroisoxazole,-1-methyl-5-hydroxypyrazole, triethylamine, potassium carbonate, palladium chloride, triphenylphosphine, 1, 4-dioxane, water, a saturated NaHCO3 solution and a hydrochloric acid solutionas reaction raw materials, and carrying out a third reaction process to prepare the topramezone. The problems that a sulfur-containing intermediate can emit odor and the raw materials are difficult to obtain in the existing process are solved.
Owner:黑龙江省绥化农垦晨环生物制剂有限责任公司
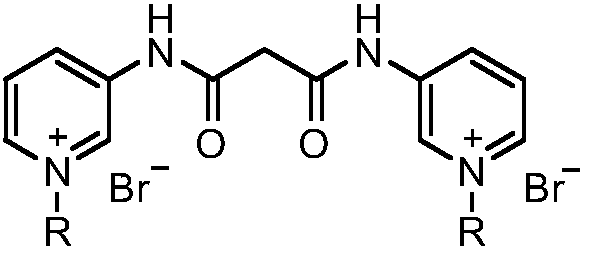
Pyridine bis-quaternary ammonium salt surfactant and preparation method and application thereof
PendingCN109096183ARaw materials are easy to getEasy to makeBiocideOrganic chemistryEscherichia coliAmpicillin
The invention discloses a pyridine bis-quaternary ammonium salt surfactant and a preparation method and an application thereof. The preparation method of the pyridine bis-quaternary ammonium salt surfactant includes utilizing 3-aminopyridine as a starting raw material to react with diethyl malonate to obtain N,N'-di(3-pyridyl) malonamide and then reacting with brominated alkane under a solvent-free condition to obtain the pyridine bis-quaternary ammonium salt surfactant. The pyridine bis-quaternary ammonium salt surfactant not only has good surface activity with the critical micelle concentration as low as 1.69*10^5 mol L-1, but also has excellent antibacterial property with the minimum semi-inhibitory concentration of escherichia coli as 2.699-6.538Mug / mL, and the antibacterial effect isbetter than that of ampicillin.
Owner:SHANXI UNIV
Non-ferrous metal oxide ore chelate collector and preparation method thereof
InactiveCN102527523APromote chelationWide variety of sourcesPreparation from carboxylic acid saltsFlotationMalonateCarbonate
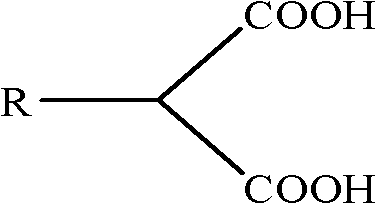
The invention provides a non-ferrous metal oxide ore chelate collector and a preparation method thereof. The invention is characterized in that the chelate collector is alkyl malonic acid; and the preparation method of the chelate collector is completed in three steps of reaction: 1, solid-liquid phase transfer catalytic hydrocarbylation: directly performing a solid-liquid phase reaction for 0.5 to 4.0 hours at the temperature of 60-200 DEG C by using a phase transfer catalyst, anhydrous carbonate, halogenated hydrocarbon and diethyl malonate as raw materials to prepare alkyl diethyl malonate; 2, basic hydrolysis: hydrolyzing the alkyl diethyl malonate into alkyl sodium malonate by adopting a NaOH solution condition; and 3, acidification: transforming the alkyl sodium malonate into alkyl malonic acid in the acidic condition with the pH of 0.5-6.5, cooling, crystallizing and separating a product, and filtering to obtain the product. The chelate collector has the characteristics of wide raw material sources, low production cost, simple preparation process, stable property and high collection capability and selectivity.
Owner:SHENYANG RES INST OF NONFERROUS METALS +1
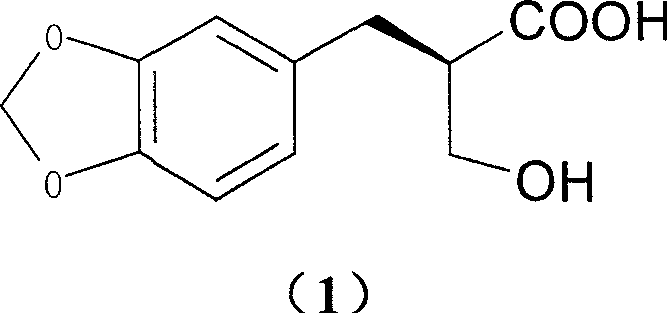
Compound 2-methylol-3-substituted phenyl propionic acid with optical activity and its resolving process
The present invention provides optically active compound 2-methylol-3-(3, 4- methylene dioxy) phenyl propionic acid and the resolution process for preparing the compound. By using substituted benzyl diethyl malonate as initial material and through basic hydrolysis, reduction and acidification, 2-methylol-3-substituted phenyl propionic acid is prepared, which is further resolved to obtain its optical isomer. The present invention has cheap material and other reagent, less wastes produced, simple operation, high yield and low cost, and is suitable for industrial production.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD +1
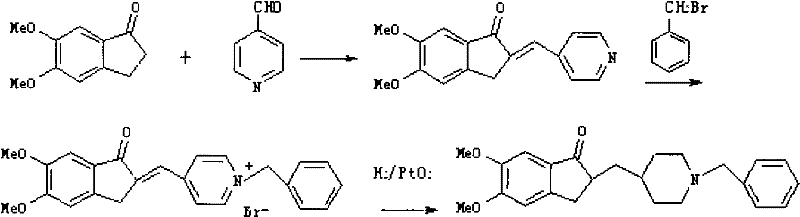
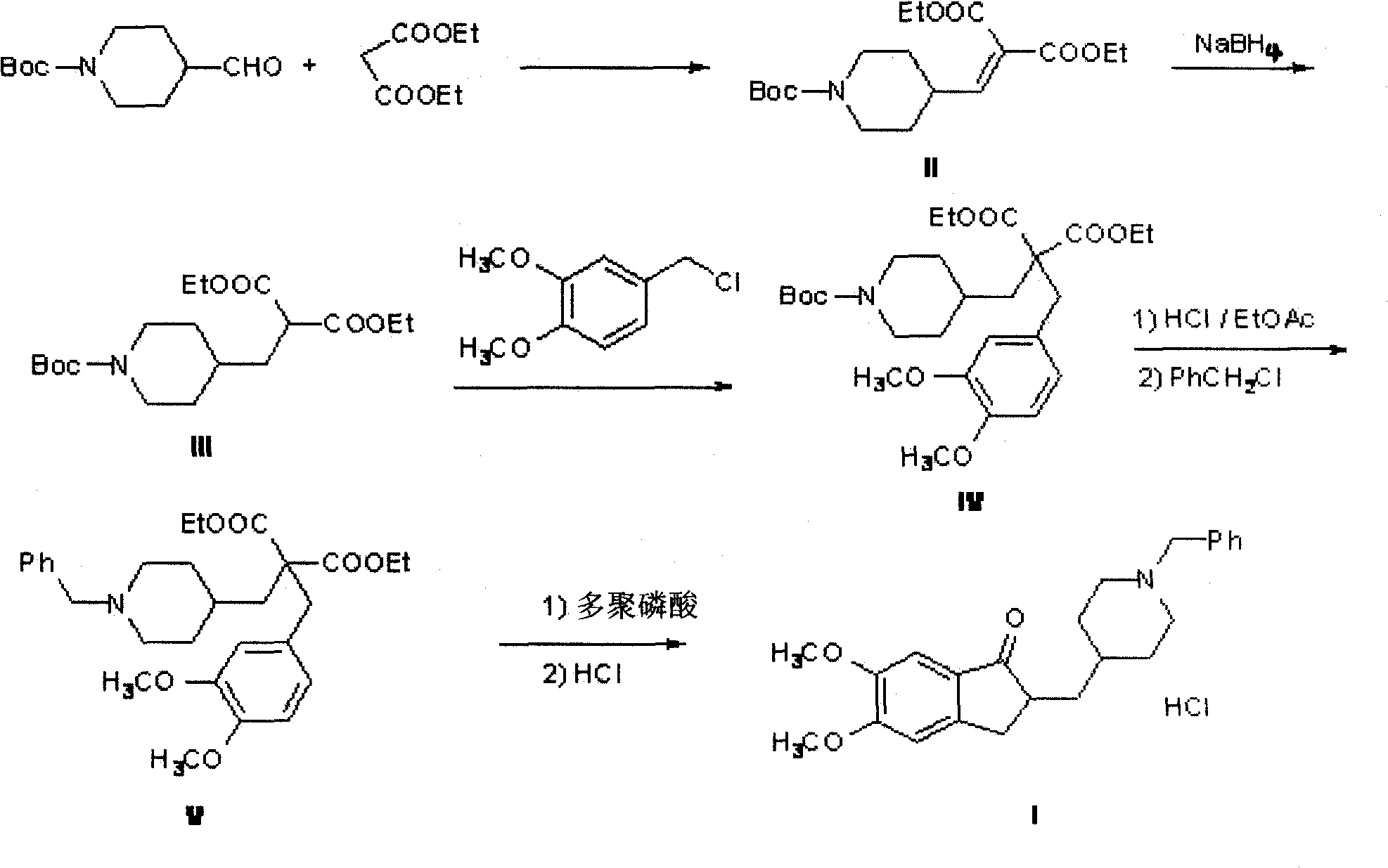
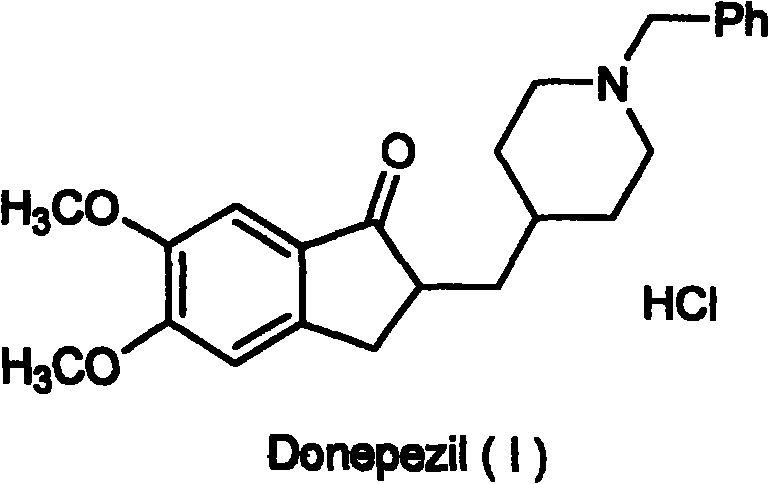
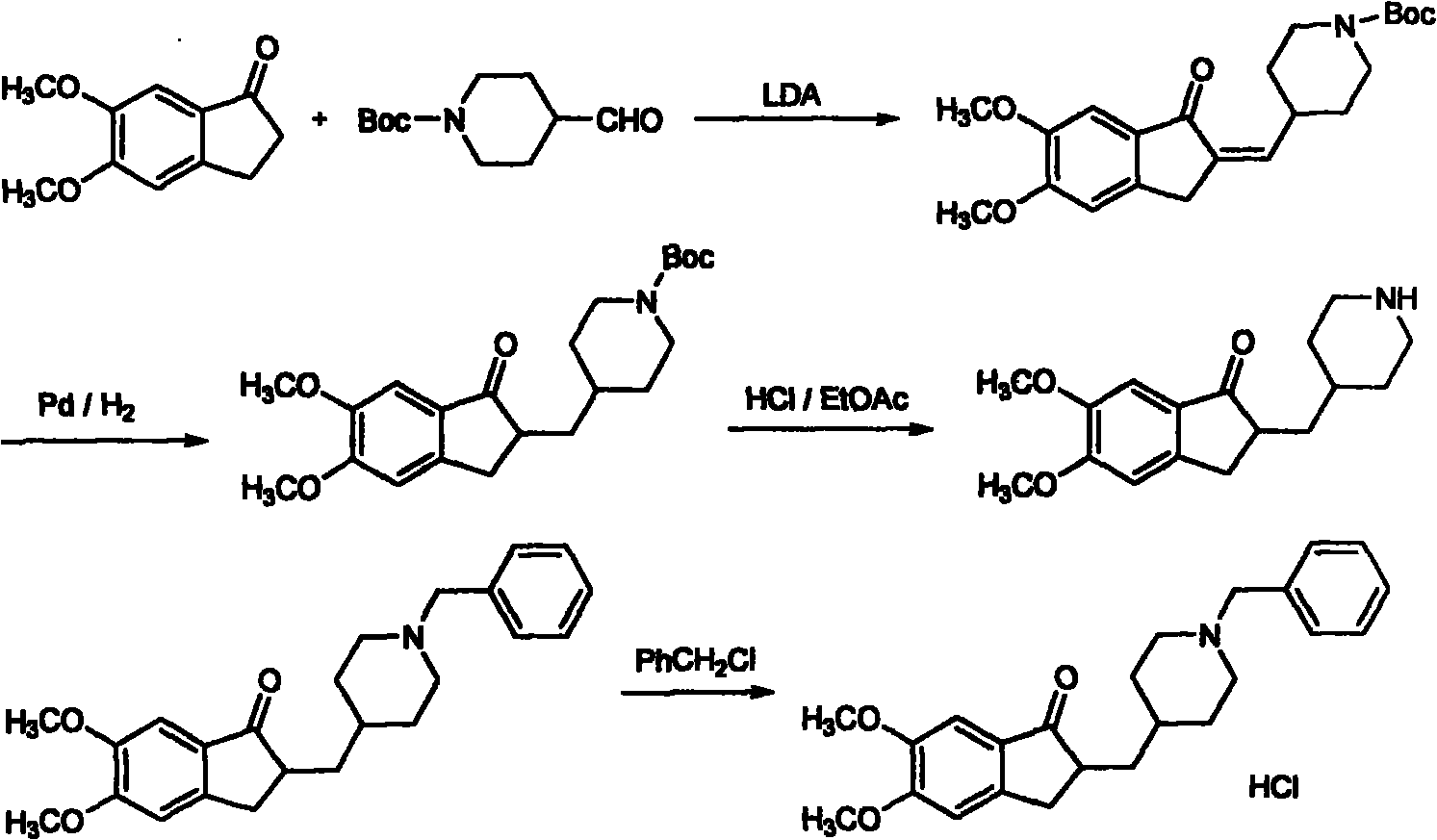
Synthesizing technology of donepezil hydrochloride
The invention relates to the synthesis of donepezil hydrochloride. According to a published patent CN100436416 which relates to a synthesizing technology of donepezil hydrochloride, diethyl malonate is adopted as an initial material, and 5 steps of condensation, reduction, substitution, ring-closing, and decarboxylation are adopted, such that donepezil hydrochloride is obtained. The steps are complicated, and the total yield is not high. The invention aims at providing a donepezil hydrochloride synthesizing technology employing 5,6-dimethoxy-2-(4-pyridyl)methylene-indan-1-one as an initial material. The technical scheme of the invention comprises steps that: the raw material is processed through hydrogenation, cooling, and filtration; a solvent glacial acetic acid is removed by reduced-pressure distillation; the obtained solution is processed through neutralization and extraction; a filtrate is condensed, and is dissolved in dichloromethane; the mixture is stirred, and triethylamine and benzyl chloride are dropped into the solution; the solution is cooled, the filtrate is condensed, and the obtained material is dissolved in methanol; a methanol solution of hydrogen chloride is dropped into the solution, such that a salt is formed; and the solution is cooled, crystallized, filtered, and dried, such that donepezil hydrochloride is obtained. The synthesizing technology provided by the invention is advantaged in short synthetic route and improved total yield. With the technology, the donepezil hydrochloride content is higher than 99%. The technology is suitable for industrialized productions.
Owner:陕西方舟制药有限公司
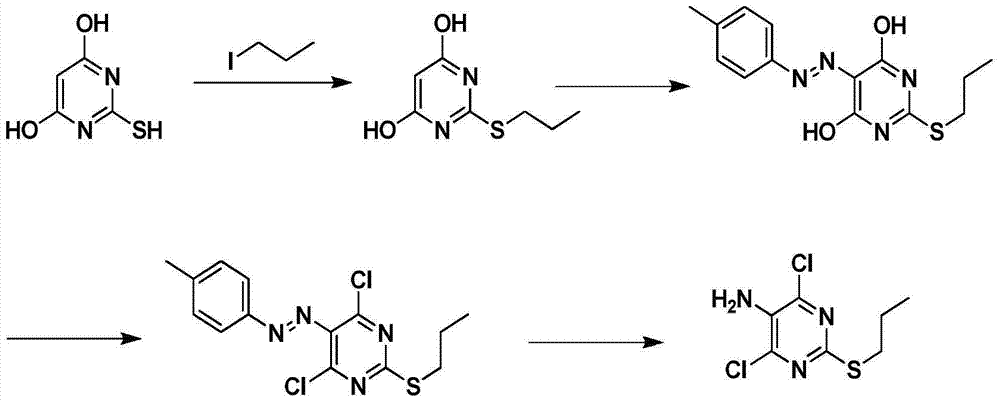
Preparation method of 2-propylthio-4,6-dichloro-5-aminopyrimidine
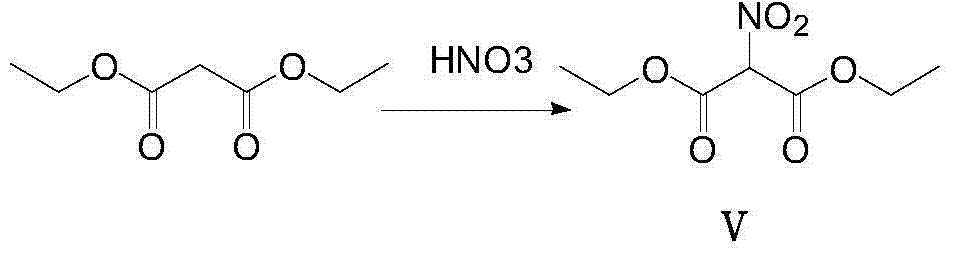
InactiveCN103923020AHigh nitrification yieldEasy post-processingOrganic chemistryPalladium on carbonDimethylaniline N-oxide
The invention provides a preparation method of 2-propylthio-4,6-dichloro-5-aminopyrimidine, which comprises the following steps: reacting diethyl malonate and fuming nitric acid at 0-15 DEG C, carrying out acid washing and alkali washing to obtain a compound V, dissolving the compound V in ethanol, adding sodium ethylate and thiocarbamide, and reacting to obtain a compound IV; reacting the compound IV with halogenated n-butane in an alkaline solution to obtain a compound III; chlorinating hydroxy group of the compound III in phosphorus oxychloride containing N,N- dimethylaniline to produce a compound II; and finally, reducing the compound II by using hydrogen in alcohol under the catalytic action of palladium on carbon to obtain the 2-propylthio-4,6-dichloro-5-aminopyrimidine. The method optimizes the technique, lowers the production cost, and enhances the reaction yield.
Owner:CHAMBROAD CHEM IND RES INST CO LTD
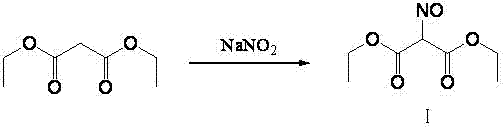
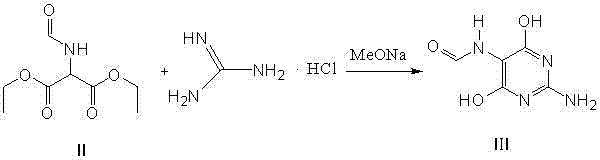
Method for preparing 2-amino-4,6-dichloro-5-formamido pyrimidine
The invention relates to a method for preparing 2-amino-4,6-dichloro-5-formamido pyrimidine. The method comprises the following steps of: performing nitrosation on malonic acid diethyl ester and acetic acid serving as raw materials and sodium nitrite at first; then, performing reduction and formylation with formic acid in the presence of zinc powder to form formyl amino malonic acid diethyl ester; finally, performing condensation and cyclization with guanidine hydrochloride to produce 2-amino-4,6-dichloro-5-formamido pyrimidine; performing chlorination by using quaternary ammonium salt as a catalyst; fractionally performing hydrolysis under an alkali action to obtain a product. The method has easily-available raw materials, and is short in reaction time, simple in aftertreatment and high in hydrolysis selectivity; the cost is obviously reduced; the total yield is up to 74 percent and the purity of the product is up to 99.0 percent.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
Carboxylate type anionic Gemini surfactant and preparation method thereof
ActiveCN106540630ALow critical micelle concentrationEnhance aggregation abilityOrganic compound preparationTransportation and packaging1-bromodecaneCarboxylic salt
Belonging to the technical field of surfactant science and application, the invention discloses a carboxylate type anionic Gemini surfactant and a preparation method thereof. The synthesis of the surfactant includes: taking diethyl malonate and 1-bromodecane as the starting raw materials to synthesize a first step product 2-decyl diethyl malonate, carrying out bilateral substitution reaction, saponification, acidification, deacidification and acid-base neutralization reaction on the 2-decyl diethyl malonate and dibromoalkane in order so as to obtain the final carboxylate type anionic Gemini surfactant. The surfactant has very low cmc, thus indicating very high surface activity. The surfactant with the above structure is conducive to enriching the related knowledge of Gemini surfactant self-assembly, and the unique properties of the carboxylate type anionic Gemini surfactant are also beneficial to application in industrial production.
Owner:东营万福能源科技有限公司
Preparation of medical intermediate AMD by electro-reduction
InactiveCN101100759AReduce dosageReduce generationElectrolysis componentsElectrolytic organic productionSodium acetateAcetic acid
Process for preparing AMD as medical intermediate from diethyl malonate is carried out by: preparing nitroso-diethyl malonate from diethyl malonate and sodium nitrite, using sodium acetate and sodium sulfate and acetic acid as electrolyte, electrically reducing to prepare amino-diethyl malonate in frame bath with membranes, and acidating the reduced products to obtain AMD. It is simple and moderate, and has no pollution and high purity.
Owner:安徽省恒锐新技术开发有限责任公司
Water-based paint
InactiveCN104277687AImprove uniformityHigh mechanical strengthPolyester coatingsIsooctyl acrylateWater based
The invention discloses a water-based paint. The paint comprises alkyd resin, hydroxypropyl bromic acid resin, 2-hydroxyethyl acrylate, 2-ethylhexyl acrylate, diethyl malonate, methyl methacrylate, acrylic acid, talcum powder, calcite, titanium white, dibutyltin dilaurate, 10% silicon oil, magnesium stearate, N,N-dimethylethanolamine, organobentonite, lecithin, diluter, assistant, propylene glycol monomethyl ether and deionized water. The paint has the advantages of uniformly dispersed raw materials in the paint preparation process, favorable uniformity, high mechanical strength, favorable impact resistance, weather resistance, low energy consumption, favorable adhesive force, favorable scratch and abrasion resistance, low cost and no environment pollution.
Owner:QINGDAO JIASHANG CREATIVE CULTURE
Method for preparing minodronic acid intermediate
ActiveCN102250090ARaw materials are easy to getRaw materials are cheap and easy to getOrganic chemistryAcetic acidPyridine
The invention relates to the field of medicine synthesis, in particular to a method for preparing a minodronic acid intermediate 2-(imidazo[1,2-a]pyridin-3-yl)acetic acid (I). The method is characterized by comprising the following steps of: performing condensation on 3-bromoimidazo[1,2-a]pyridine (II) and diethyl malonate to obtain 2-(imidazo[1,2-a]pyridin-3-yl)diethyl malonate (III); and hydrolyzing and performing decarboxylation to obtain the 2-(imidazo[1,2-a]pyridin-3-yl)acetic acid (I). The method has the advantages of cheap and readily available raw materials, a few reaction steps, simple operation, high product quality, low cost, suitability for industrial production and the like.
Owner:广东宏远集团药业有限公司
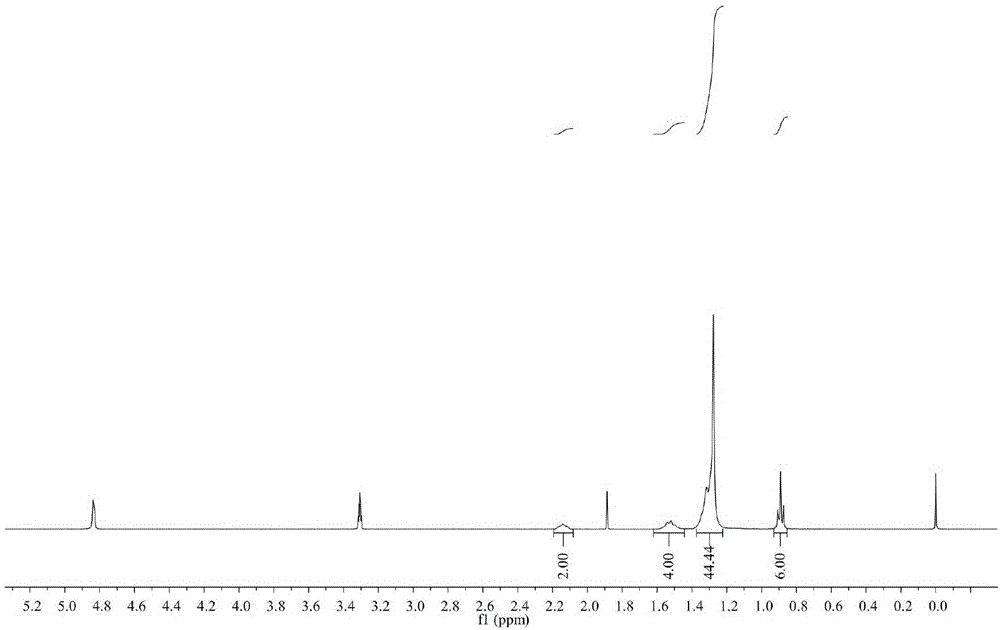
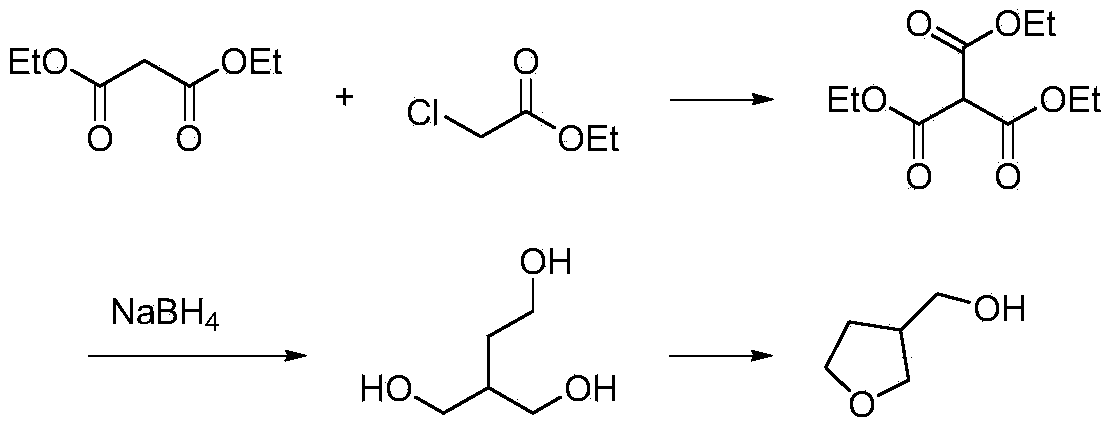
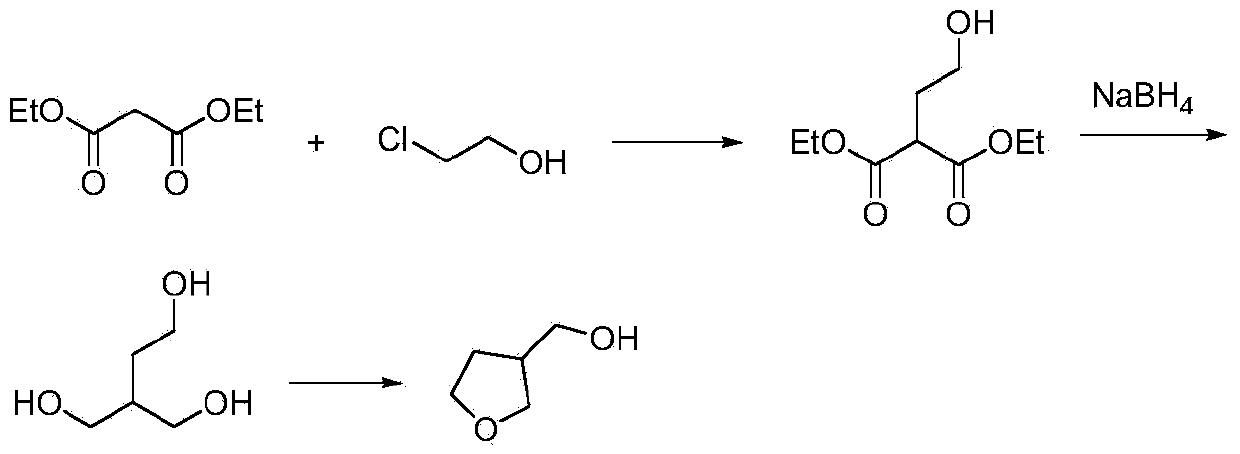
Synthetic method for 3-hydroxymethyl tetrahydrofuran
The invention discloses a synthetic method for 3-hydroxylmethal tetrahydrofuran. The synthetic method comprises the following steps: with 2-chloroethanol and diethyl malonate as raw materials, reacting under the action of alkaline in the presence of an organic solvent or in absence of a solvent to obtain an intermediate 2-ethoxy-diethyl succinate; then, reducing the intermediate 2-ethoxy-diethyl succinate by virtue of metal borohydride to obtain 2-hydroxymethyl-1,4-butanediol; and finally, producing 3-hydroxylmethal tetrahydrofuran under the action of a dehydrating agent. According to the synthetic method, the usage amount of the reducing agent metal borohydride is remarkably lowered, and yield of a byproduct sodium metaborate is reduced at the same time. The invention provides the synthetic method for the drug intermediate 3-hydroxylmethal tetrahydrofuran.
Owner:XIAN MODERN CHEM RES INST
Method for synthesizing famciclovir
InactiveCN101550137AFew reaction stepsRaw materials are cheap and easy to getOrganic chemistryAntiviralsEthyl groupAcetic oxide
The invention relates to a method for synthesizing famciclovir, which includes preparing sodium salt of diethyl malonate at the acting of sodium ethylate by taking diethyl malonate as raw charge and ethyl hydrate as dissolvent, preparing bromo ethyl group diethyl malonate by carrying out nucleophilic substitution reaction with 1, 2-dibromoethane and sodium salt of diethyl malonate, preparing bromo ethyl group propanediol by deoxidizing bromo ethyl group diethyl malonate at the acting of sodium borohydride, preparing 2-acetyl oxygen radicel methyl radicel -4-bromo butyl acetic ester by carrying out reaction with bromo ethyl group propanediol and acylating agent acetic oxide, preparing 2-(2-acetyl oxygen radicel-4-bromo butyl acetic ester)-6-chloropurine by carrying out condensation with 2-acetyl oxygen radicel methyl radical-4-bromo butyl acetic ester and 2-amidocyanogen-6-chloropurine, and then preparing famciclovir by carrying out dechlorination with 2-(2-acetyl oxygen radicel methyl radical -4-bromo butyl acetic ester)-6-chloropurine. This method is suitable for the industrial production with short production line, high yield and low cost.
Owner:彭洋
Preparation method for asymmetric synthesis of pregabalin
ActiveCN103833562AHigh yieldFew stepsOrganic compound preparationAmino-carboxyl compound preparationPtru catalystHydrolysis
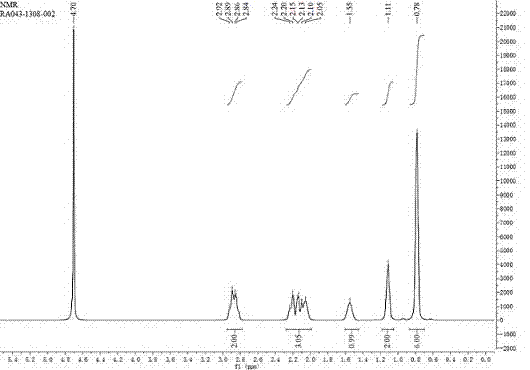
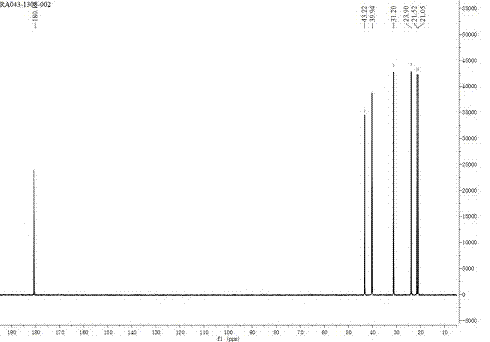
The invention provides a novel method for chiral synthesis of pregabalin. The novel method comprises the following steps that 1, diethyl malonate and 3-methyl butyraldehyde undergo a Knoevenagel reaction to produce an intermediate 2-(3-methylbutenyl)-diethyl malonate, 2, in a nitromethane solution, 2-(3-methylbutenyl)-diethyl malonate and nitromethane undergo an asymmetric addition reaction in the presence of a chiral catalyst to produce an intermediate (S)-2-(3-methyl-2-nitromethyl)diethyl n-butylmalonate, 3, under the acidic conditions, (S)-2-(3-methyl-2-nitromethyl)diethyl n-butylmalonate simultaneously undergoes hydrolysis, decarboxylation and reduction reactions, and pregabalin or its corresponding isomer is separated by alkalization, 4, the pregabalin or its corresponding isomer is subjected to acidity adjustment, decoloration and filtration, the filtrate is subjected to basicity adjustment and crystallization, and the crystals are dried to form the refined pregabalin, and 5, the refined pregabalin is subjected to 1HNMR, 13CNMR, MS and IR identification.
Owner:RAFFLES PHAMRMATECH CO LTD
Novel donepezil synthesis process
InactiveCN100436416CLow priceSynthetic operation is simpleOrganic chemistryDonepezilCombinatorial chemistry
The invention discloses a novel process for synthesizing Donepezil by using diethyl malonate as the starting raw material, which comprises condensation, reduction, substitution, cycling reaction and decarboxylation.
Owner:PKUCARE PHARMA R&D CENT +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com