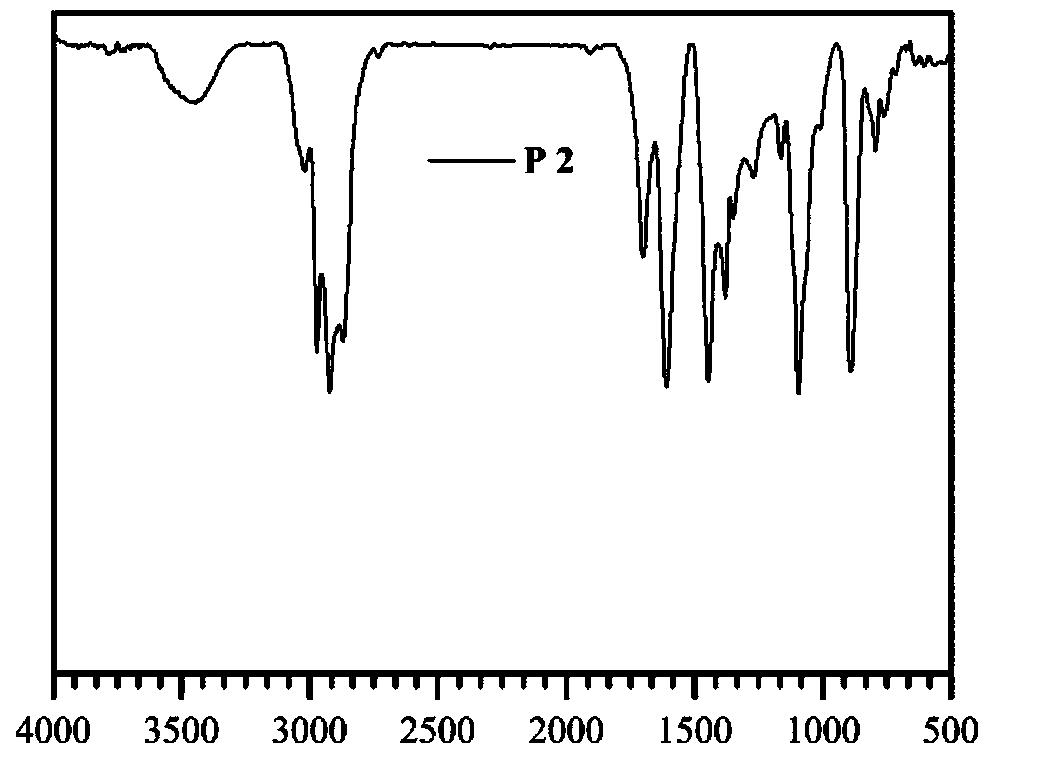
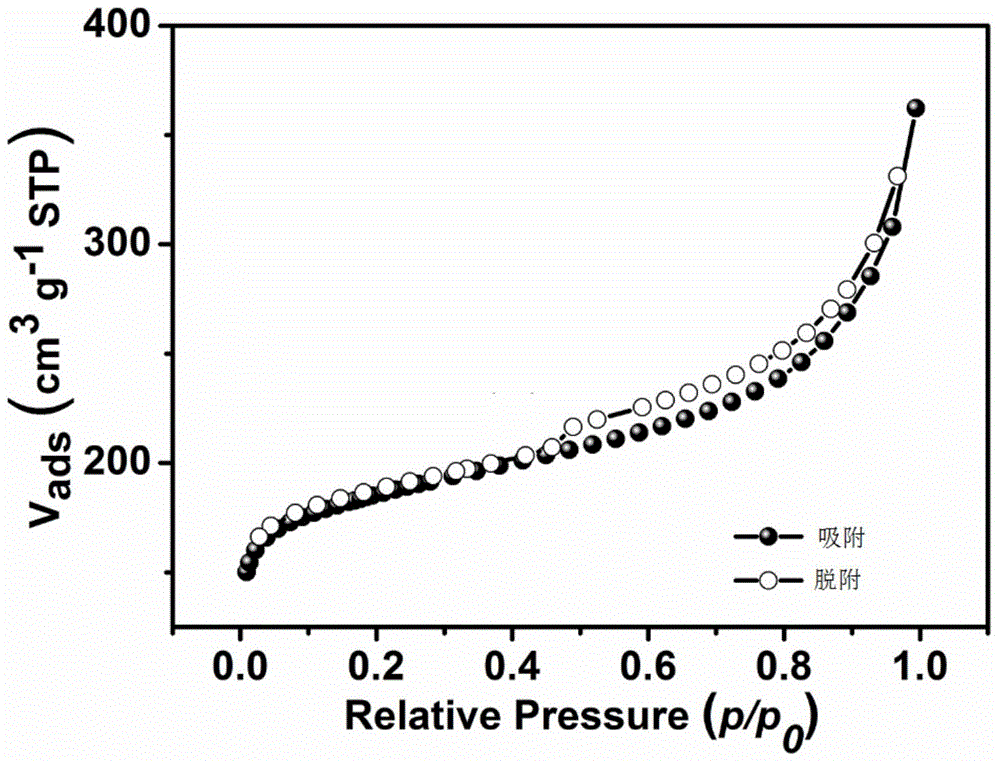
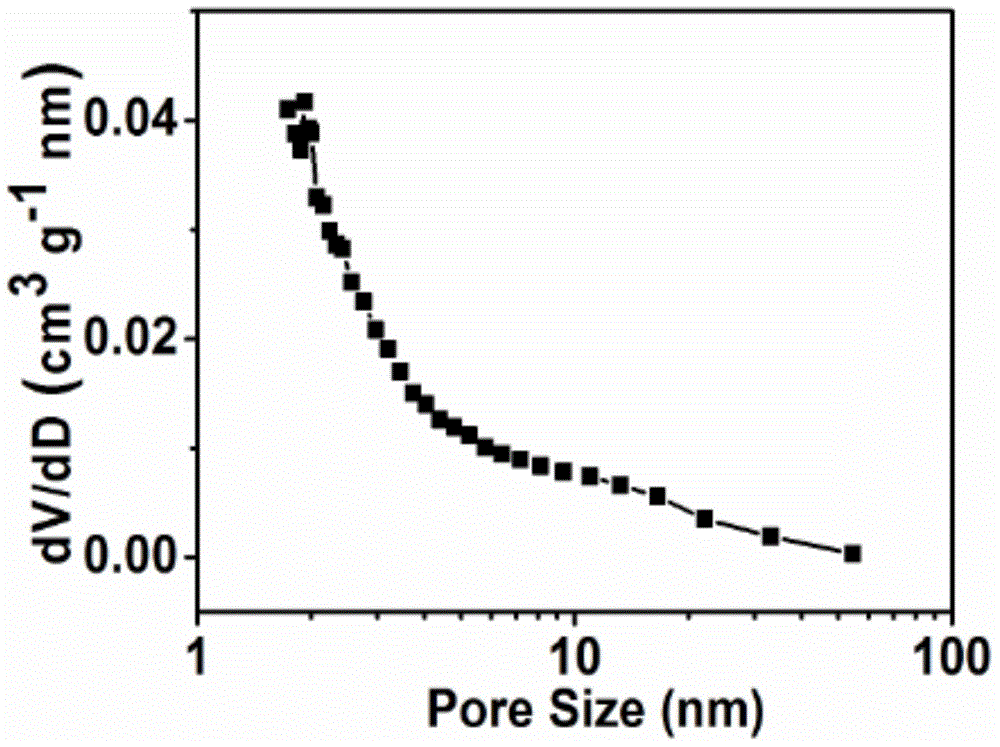
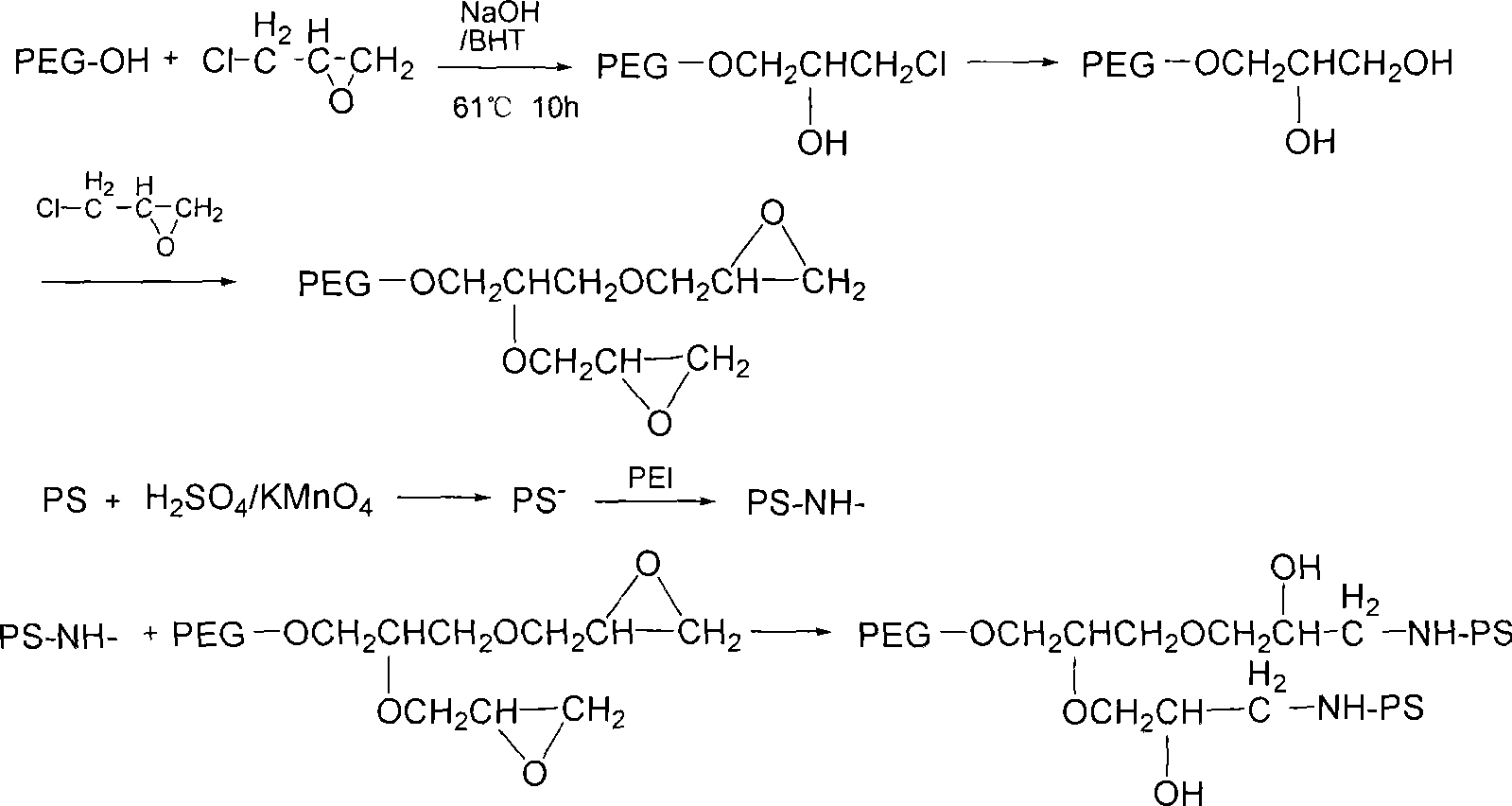
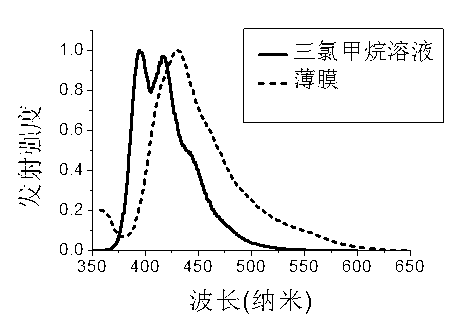
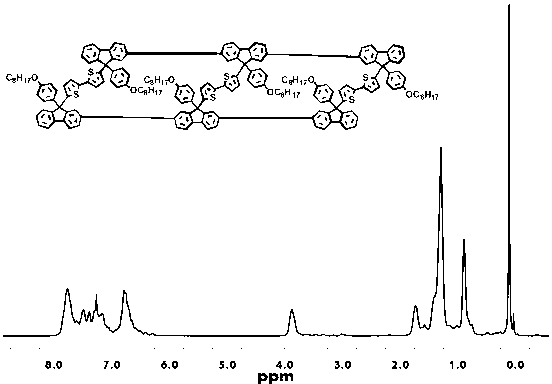
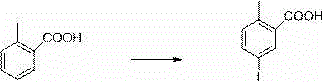
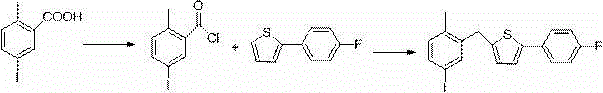
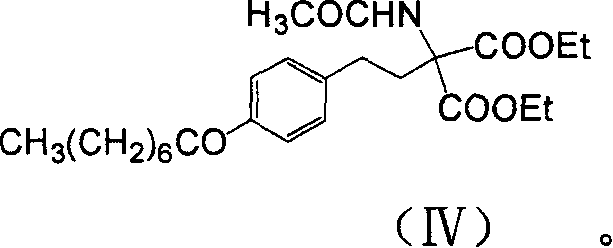
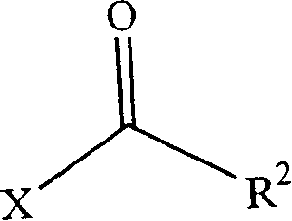
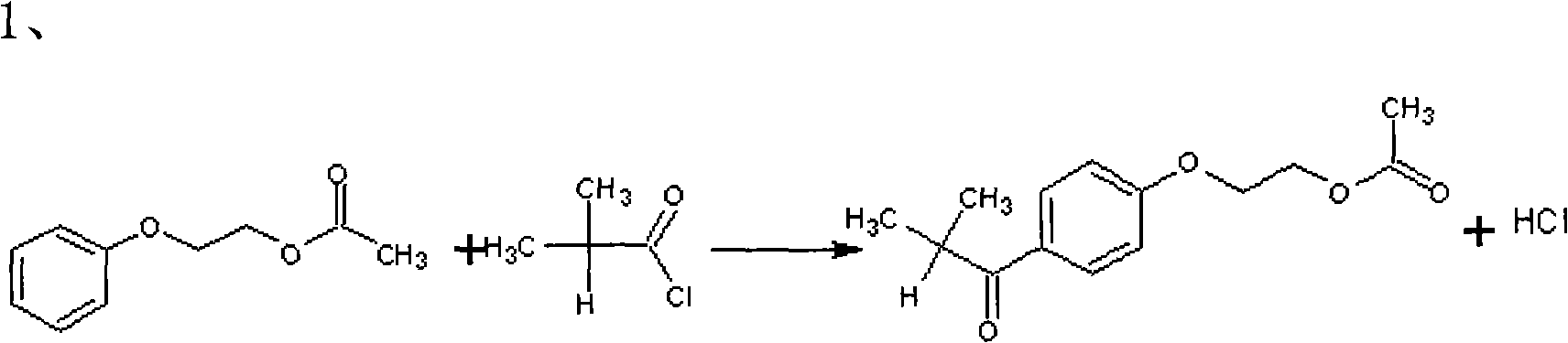
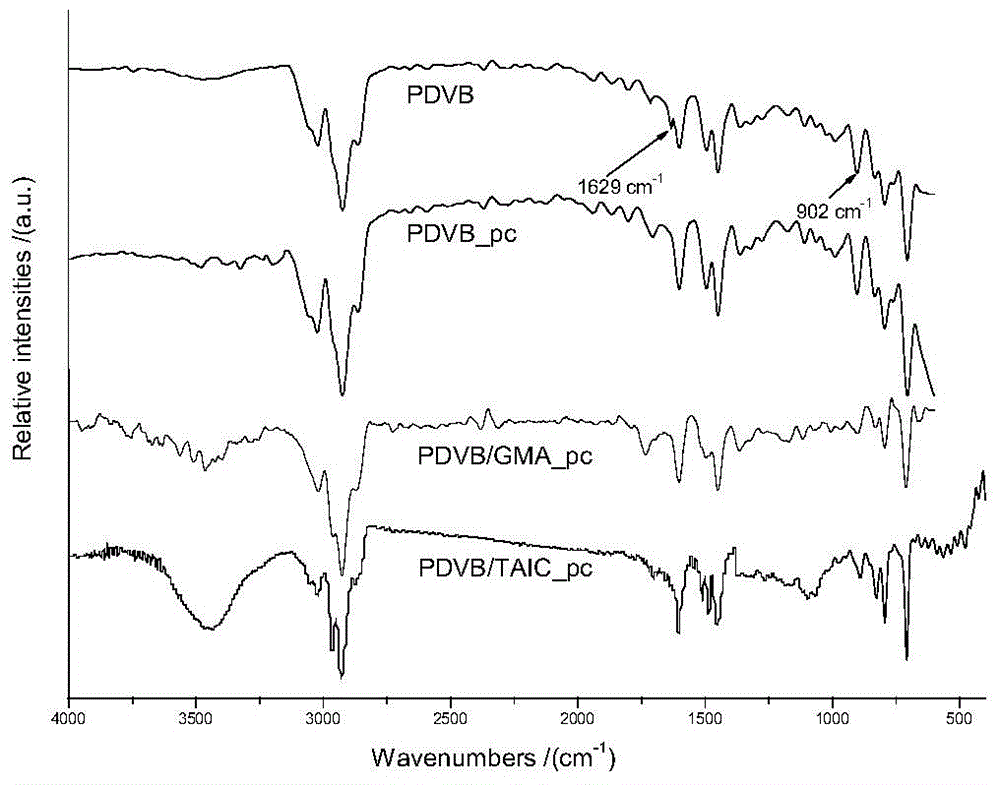
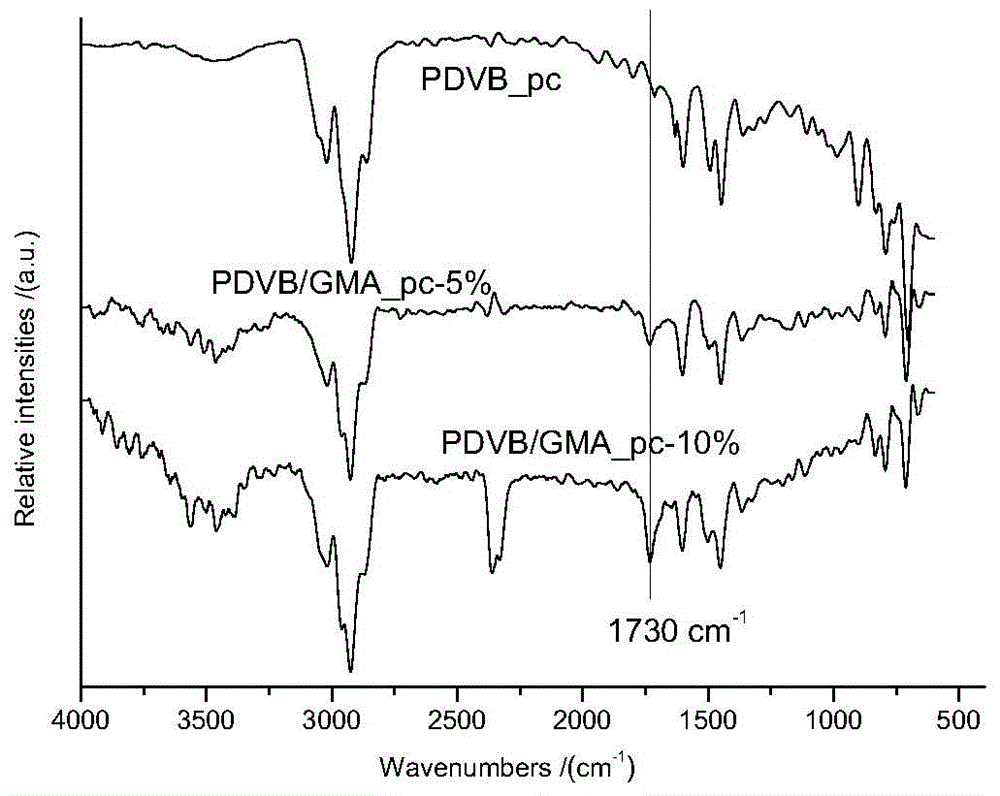
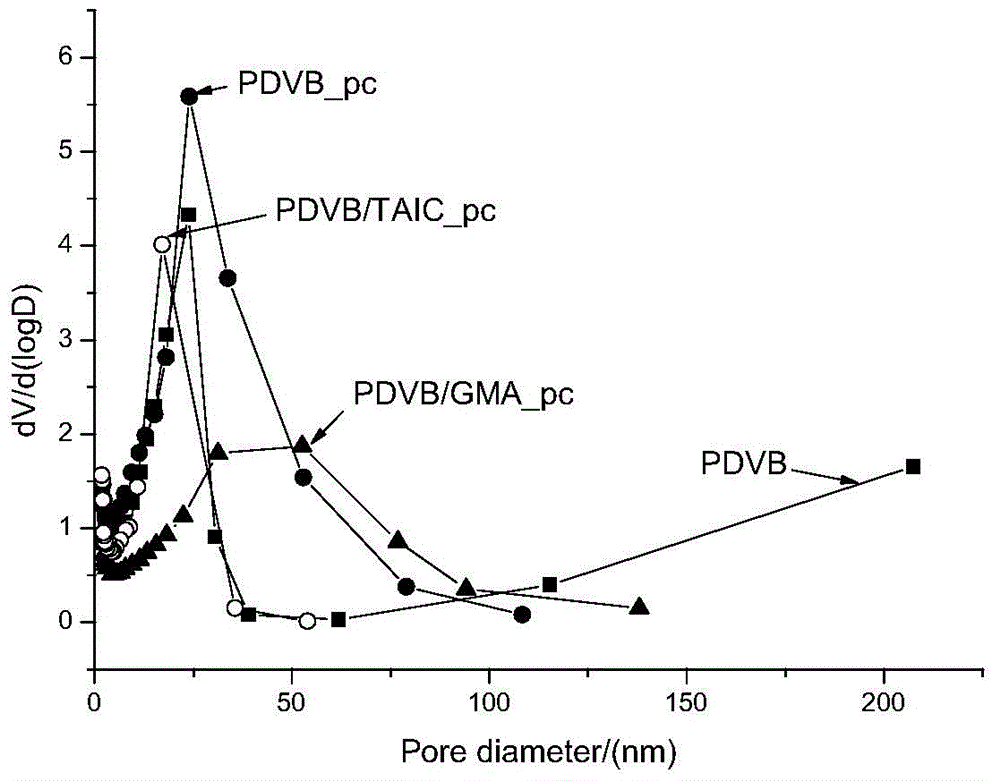
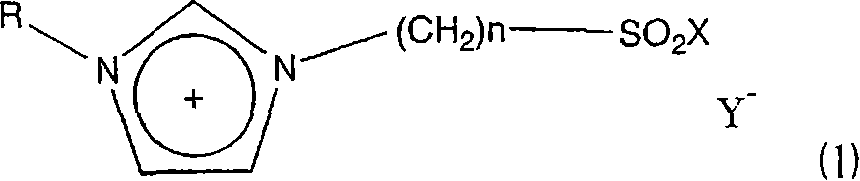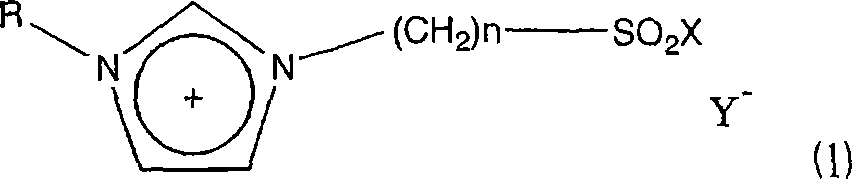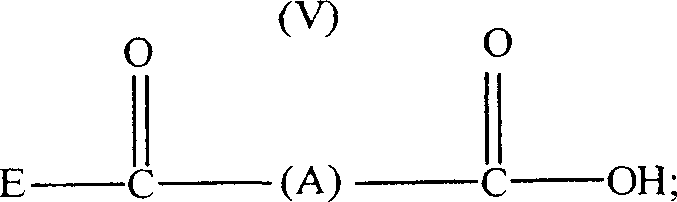Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
530 results about "Friedel–Crafts reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.
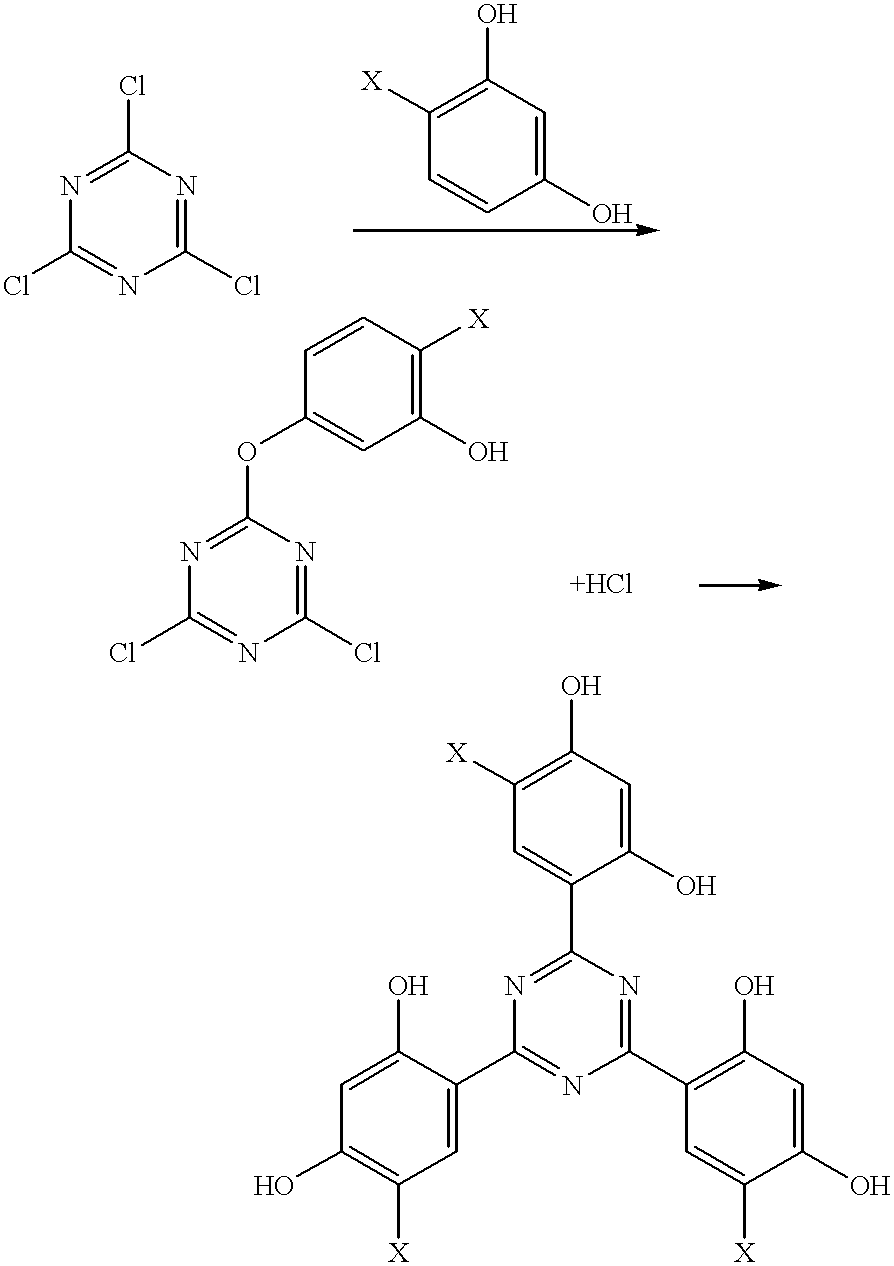
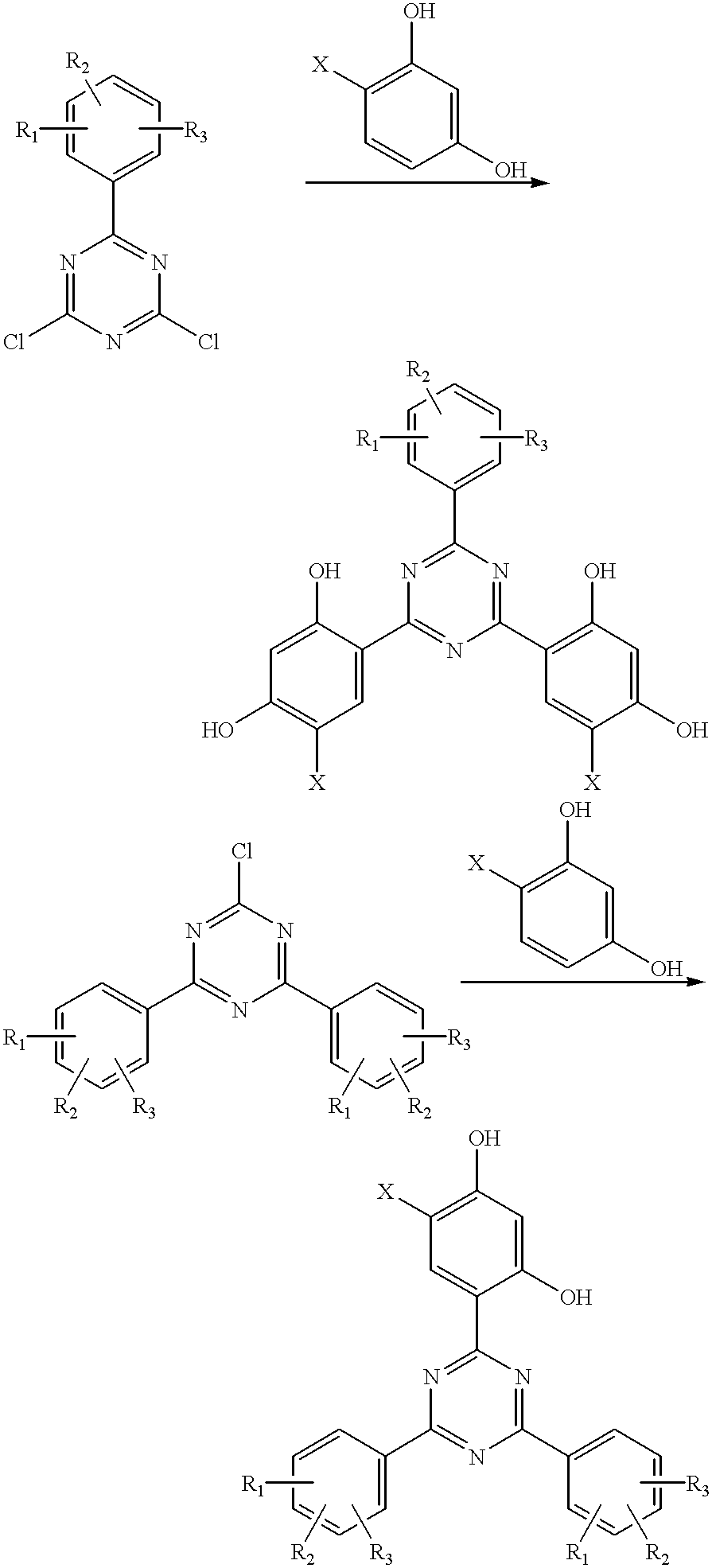
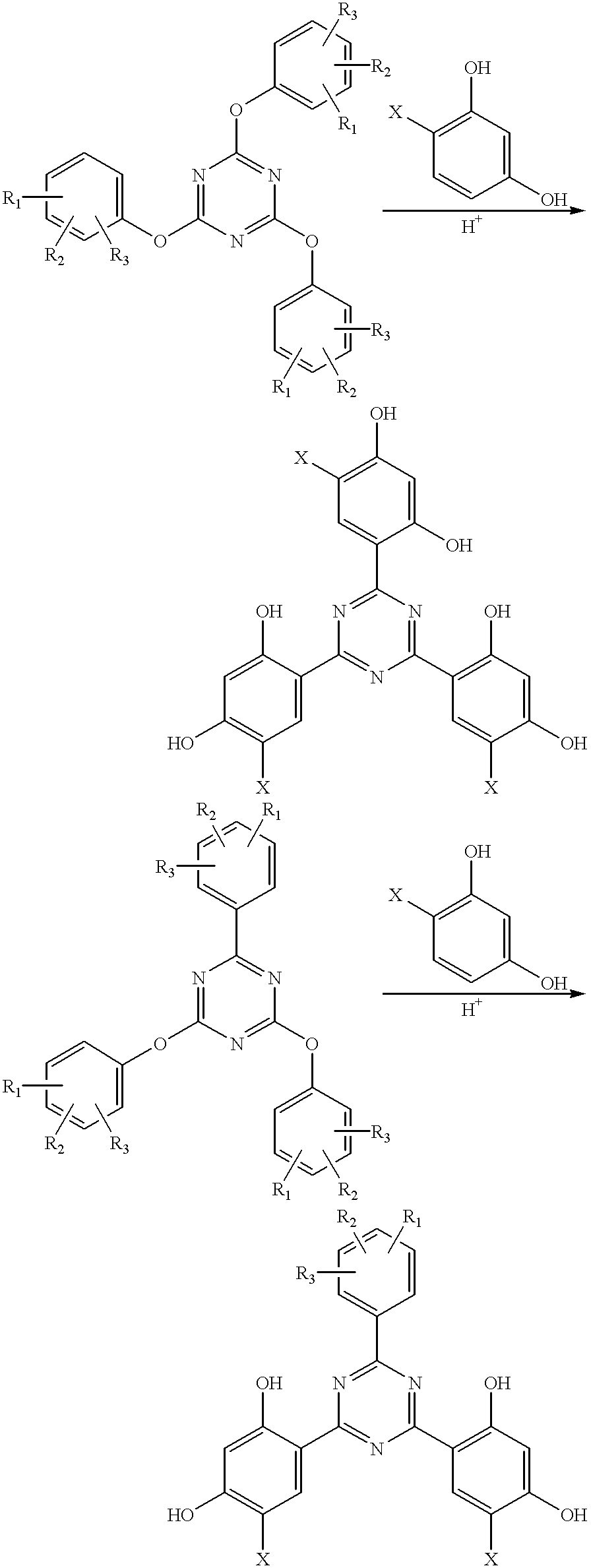
Methods for the preparation of tris-aryl-o-hydroxyphenyl-s-triazines
InactiveUS6242598B1Reduce in quantityPoor leaving groupOrganic chemistryLewis acid catalysisResorcinol
A process for preparing 2-(2,4-dihydroxyphenyl)-4.6-diaryl-s-triazines in three steps starting with cyanuric chloride is described. Step 1 involves the nucleophilic (basic) displacement of one chlorine atom with a phenolic moiety. Step 2 involves a Friedel-Crafts reaction using a Lewis acid catalyst (preferably aluminum chloride) to replace the remaining two chlorine atoms with aryl groups such as xylyl. Finally, step 3 involves replacing the phenolic moiety with resorcinol using either a Lewis acid or protic acid catalyst or combinations thereof. Some additional processes only peripherally related to the three-step process outlined above are also described for the preparation of various s-triazine compounds. The s-triazines prepared are useful as UV absorbers for the stabilization of organic substrates against the adverse effects of actinic light.
Owner:CIBA SPECIALTY CHEM CORP
Process for preparing 4,4'-dichlorodiphenylsulfone employing sulfoxide oxidation method
InactiveCN104557626AMeet the synthesis requirementsFully contactedOrganic chemistryOrganic compound preparationSolventHydrogen peroxide
The invention relates to a process for preparing 4,4'-dichlorodiphenylsulfone, in particular to a process for preparing 4,4'-dichlorodiphenylsulfone employing a sulfoxide oxidation method. The process comprises the following steps: carrying out friedel-crafts reaction with chlorobenzene and thionyl chloride under the action of a catalyst to obtain the 4,4'-dichlorodiphenylsulfone; dissolving the obtained 4,4'-dichlorodiphenylsulfone with a solvent; adding hydrogen peroxide and carrying out oxidation reaction to obtain a 4,4'-dichlorodiphenylsulfone crude product; carrying out secondary oxidation refining on the obtained 4,4'-dichlorodiphenylsulfone crude product; and finally preparing high-purity 4,4'-dichlorodiphenylsulfone. The purity of the 4,4'-dichlorodiphenylsulfone purified by the refining process is greater than 99.5%; the refining yield is greater than 98.5%; and the process completely meets the synthesis requirements of high-performance polymers.
Owner:SHANDONG KAISHENG NEW MATERIALS
Preparation method of improved 4,4-dichlorodiphenylsulfone
InactiveCN102351756AReduce manufacturing costImprove product qualityOrganic chemistryOrganic compound preparationDichloropropaneOrganic solvent
The invention discloses a preparation method of improved 4,4-dichlorodiphenylsulfone. The method comprises steps that: (1) Al2Cl3 is adopted as a catalyst; chlorobenzene, thionyl chloride are adopted as reaction materials; the materials are subject to a Friedel-Crafts reaction; a mother liquor obtained after the reaction is hydrolyzed; the hydrolyzed mother liquor is refluxed for 45 to 60min under a temperature of 95 to 100 DEG C; after refluxing, the mother liquor is cooled to below 20 DEG C, such that the liquor is divided into an organic phase and a water phase; the organic phase is processed through reduced-pressure distillation and centrifugal washing until the organic phase turns neutral, and the organic phase is preserved as a product M for later use, wherein a main component in the product M is 4,4-dichlorodiphenylsulfone; (2) the product M and an oxidizing agent of hydrogen peroxide are adopted as raw materials, and 1,2-dichloropropane is adopted as an organic solvent; an oxidizing reaction is carried out upon the main component 4,4-dichlorodiphenylsulfone in the product M under the effect of a composite catalyst, such that a 4,4-dichlorodiphenylsulfone crude product is synthesized, wherein the composite catalyst is phosphotungstic acid or silicotungstic acid loaded on active carbon. Therefore, the method provided by the invention has advantages of high yield and good product quality. With the method, wastewater can be circulated and reused.
Owner:WUJIANG BEISHE SHENGYUAN TEXTILE PROD AUXILIARIES PLANT
New preparation method of 4,4-dichlorodiphenyl sulfone
InactiveCN102351758AReduce manufacturing costShort reaction timeOrganic chemistryOrganic compound preparationAcetic acidChlorobenzene
The invention discloses a new preparation method of 4,4-dichlorodiphenyl sulfone. The method comprises the following steps: 1) through using AlCl3 as a catalyst, and chlorobenzene and thionyl chloride as reaction raw materials, performing a Friedel-Crafts reaction at 25-35 DEG C, then hydrolyzing the mother liquor obtained through the Friedel-Crafts reaction, refluxing at 95-100 DEG C for 45-60 minutes, cooling the obtained mother liquor to less than 20 DEG C after refluxing to obtain an organic phase and an aqueous phase; 2) performing reduced pressure distillation on the organic phase, centrifuging, washing the obtained product to be neutral and be used as a product A for later use, wherein the main component of the product A is 4,4-dichlorodiphenyl sulfoxide; and 3) through using the product A and the oxidant hydrogen peroxide as raw materials and acetic acid as satalytic solvent, carrying out an oxidation reaction on the main component 4,4-dichlorodiphenyl sulfoxide of the product A to obtain crude 4,4-dichlorodiphenyl sulfone. Therefore, the existing synthesis method of 4,4-dichlorodiphenyl sulfone is changed, the production cost of 4,4-dichlorodiphenyl sulfone is low, the product quality is good and the reaction time is short.
Owner:WUJIANG BEISHE SHENGYUAN TEXTILE PROD AUXILIARIES PLANT
Ionic liquid and method of reaction using the same
InactiveCN1852898ASuppress generationCatalystsHydrocarbon preparation catalystsBeckmann rearrangementAlkyl transfer
A novel acidic ionic liquid which is useful as a catalyst for alkylation, nitration, Beckmann rearrangement, etc. and is stable to air and water. It is an ionic liquid represented by the following formula (1): (1) wherein X represents halogeno or hydroxy; Y<-> represents CF3SO3<->, BF4<->, PF6<->, CH3COO<->, CF3COO<->, (CF3SO2)2N<->, (CF3SO2)3C<->, F<->, Cl<->, Br<->, or I<->; n is an integer of 2 to 16; and R represents methyl, allyl, or vinyl. This ionic liquid not only functions as a BrDnsted acid or a Lewis acid but is a liquid insoluble in many organic solvents. The liquid is hence useful as a catalyst or solvent for Friedel-Crafts reaction, nitration, and Beckmann rearrangement. It can be easily separated from the reaction mixture and reused.
Owner:SUMITOMO CHEM CO LTD +1
Method for preparing functionalized graphene and composite material of functionalized graphene
InactiveCN102862976AGood dispersionImprove interface performanceMaterial nanotechnologyCarbon compoundsEpoxyOrganic solvent
The invention discloses a method for preparing functionalized graphene and a composite material of the functionalized graphene. The method for preparing the functionalized graphene comprises the following steps of: performing Friedel-Crafts reaction on natural graphite which is taken as a raw material to obtain modified graphite, extracting, purifying, and uniformly dispersing in an organic solvent with ultrasonic to form stable graphene suspension liquid. The method for preparing the composite material comprises the following steps of: adding epoxy resin into the graphene suspension liquid, stirring for dissolving, uniformly mixing with ultrasonic, distilling under reduced pressure to remove the organic solvent to obtain a graphene / epoxy resin composite; and adding an epoxy resin curing agent, an accelerant and micrometer silver sheets sequentially, and heating for curing to obtain the composite material of a graphene polymer, namely a graphene / epoxy resin conducting composite material. The edge functionalized graphene has a high interaction between functional groups at the edge and a polymer matrix, so that the dispersion of the graphene in the polymer matrix can be promoted, the agglomeration degree can be reduced, and the interface performance of the composite material can be enhanced.
Owner:SOUTH CHINA UNIV OF TECH
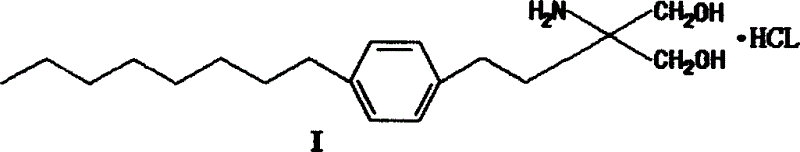
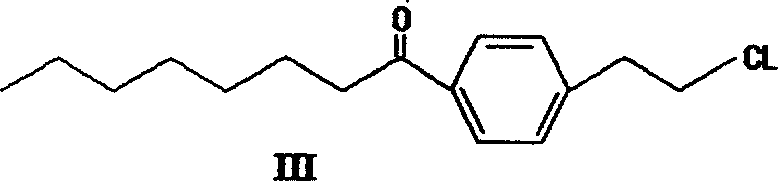
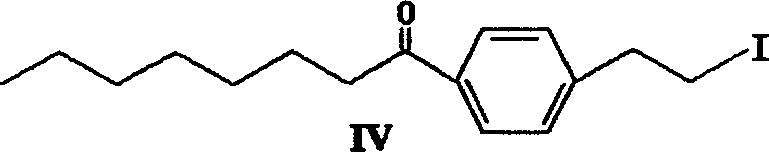
Method for preparing 2-para octylphenyl ehtyl-2-amino propanediol
InactiveCN1528738AEasy to manufactureEfficient manufacturingOrganic compound preparationAmino-hyroxy compound preparationSodium iodidePropanediol
The invention provides a method to prepare a compound method, including the steps: ethylbenzene and capryl chloride make Friedel-Crafts reaction to generate p-capryl chloroethylbenzene; convert capryl chloroethylbenzene under the action of sodium iodide into p-capryl iodoethylbenzene; p-capryl iodoethylbenzene and acetylamino diethyl malonate condense under the action of alkali to generate 2-(p-capryl phenethyl)-2-acetylamino diethyl malonate; or p-capryl iodoethylbenzene makes elimination reaction under the action of alkali to generate p-capryl styrene, which together with acetylamino diethyl malonate condenses under the action of alkali into 2-(p-capryl phenethyl)-2- acetylamino diethyl malonate; a compound is reduced into 2-[4-(1-hydroxyoctyl) phenethyl-]2- acetylamino propylene alcohol; the other coumpound makes hydrogenolysis to obtain 2-(p-octyl phenethyl)-acetylamino propylene alcohol; make alkali hydrolyzation and then acidifies them into salt, so as to obtain it. It also provides a method to prepare intermediate in the above preparing course.
Owner:马启明
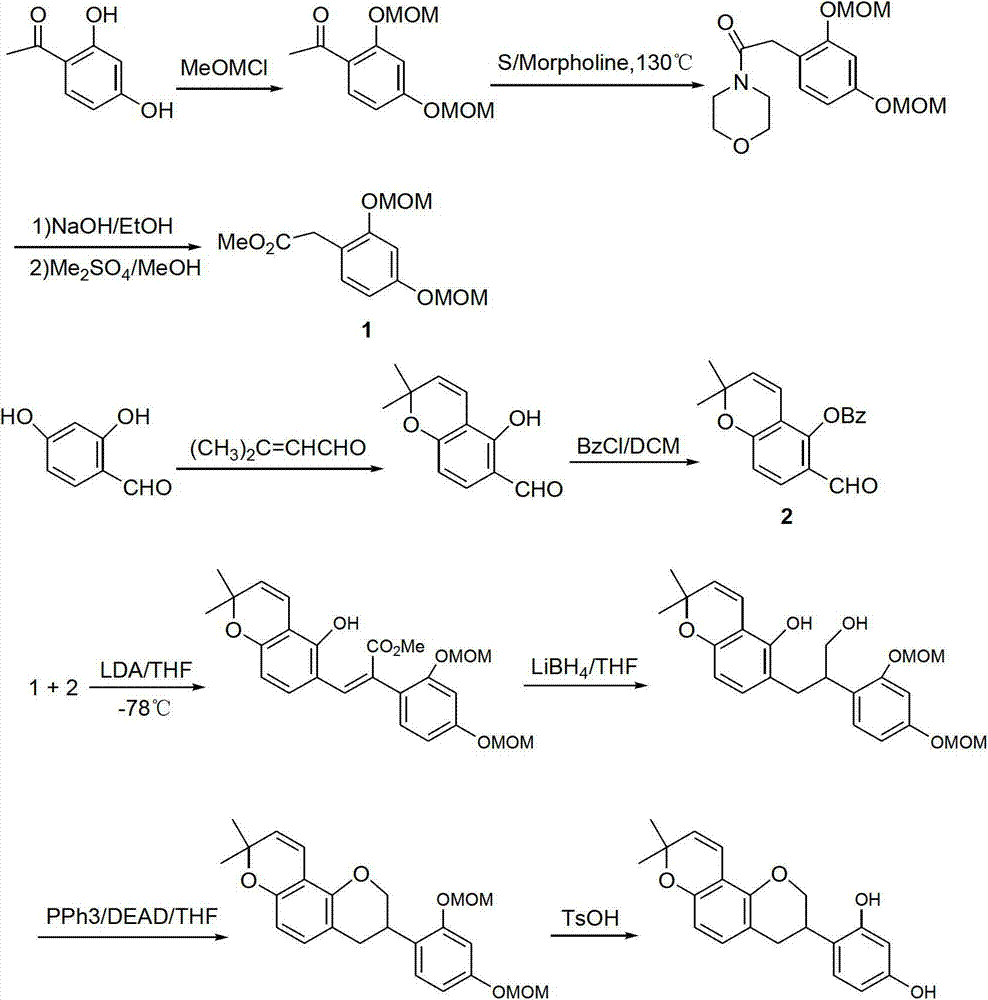
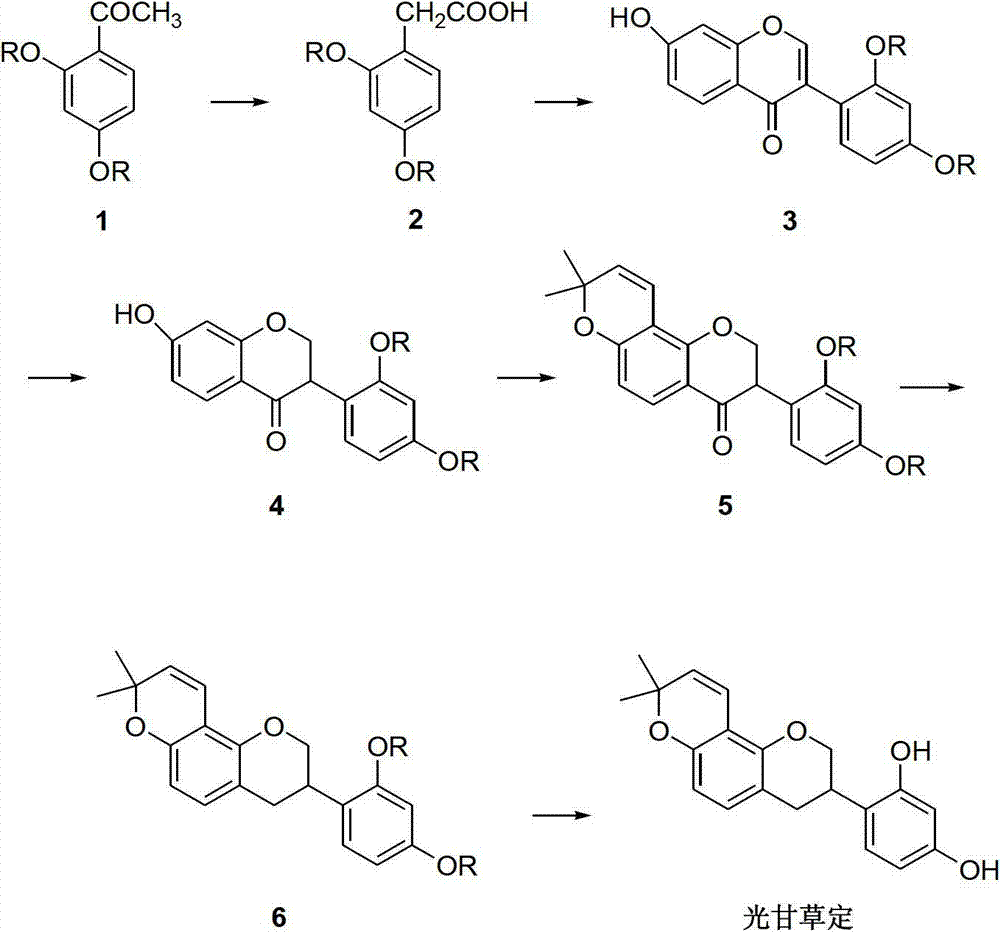
Method for synthesizing glabridin
The invention relates to a method for synthesizing glabridin. The method comprises the following steps: using acetophenone protected by phenol hydroxy as a raw material, carrying out a Willgerodt-Kindler reaction to obtain aryl phenylacetic acid, and carrying out a Friedel-Crafts reaction to obtain an isoflavones compound; causing the isoflavones compound to carry out Pd / C catalytic hydrogenation to obtain a isoflavanone compound; and causing the isoflavanone compound to carrying out a crclizationreaction, a conyl reduction reaction and a removing phenolic hydroxyl group protection group reaction to obtain the glabridin. The operation and the serparation of the steps of the method are simple, the yield is higher, the used reagents are common reagents, are cheap and are easily obtained, the path is shorter, and the tptal yield is not lower than 20 percent.
Owner:山东济清科技服务有限公司
Preparation method, material and application of porous polymer
The invention discloses a preparation method, a material and an application of a porous polymer. The preparation method comprises the following steps: (1) evenly dispersing the raw material (aromatic compounds, mixtures of the aromatic compounds, polymers of the aromatic compounds and / or mixtures of the polymers) into a crosslinking agent and solvent, so as to obtain a raw material mixed solution, wherein the crosslinking agent solvent is one or more of alkane dihalides; (2) adding a catalyst, carrying out Friedel-Craft reaction, and carrying out super-crosslinking, so as to obtain a crude product; and (3) washing and extracting a filter cake which is obtained after the coarse product is filtered, removing a catalyst, and drying, so as to obtain a porous polymer. The preparation method disclosed by the invention is simple in process, uses easily available raw materials, the prepared porous polymer is adjustable in pore size and specific surface area, has low cost and good adaptability, and can be used as a catalyst carrier, a separation membrane, a gas storage material or an ion adsorbing material, especially, can be used as materials for storing hydrogen and capturing carbon dioxide or methane storage materials.
Owner:武汉华科中英纳米科技有限公司
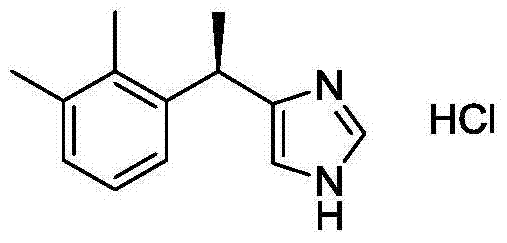
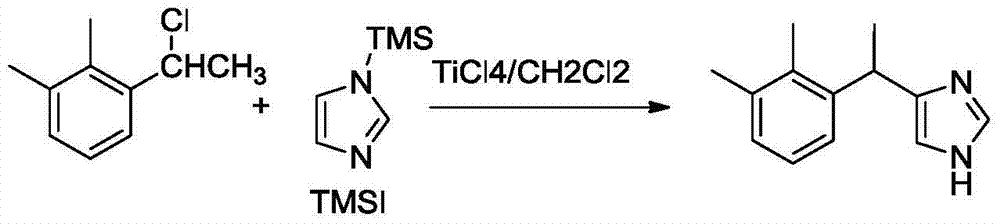
New method for preparing dexmedetomidine hydrochloride
The invention provides a new method for preparing dexmedetomidine hydrochloride. According to the method, Lewis acid is used as a catalyst, and racemized medetomidine is prepared by a Friedel-Crafts reaction; and the racemized medetomidine is resolved by (+)-di-p-toluoyl-tartaric acid [(+)-DDTA] to obtain dexmedetomidine. The dexmedetomidine is salified in hydrochloric acid to obtain dexmedetomidine hydrochloride.
Owner:北京华禧联合科技发展有限公司
Pyridine diimine iron olefin polymerizing catalyst, as well as preparation method and application thereof
InactiveCN101412771AExtended service lifeHigh catalytic activityIron organic compoundsBiological activationStructural formula
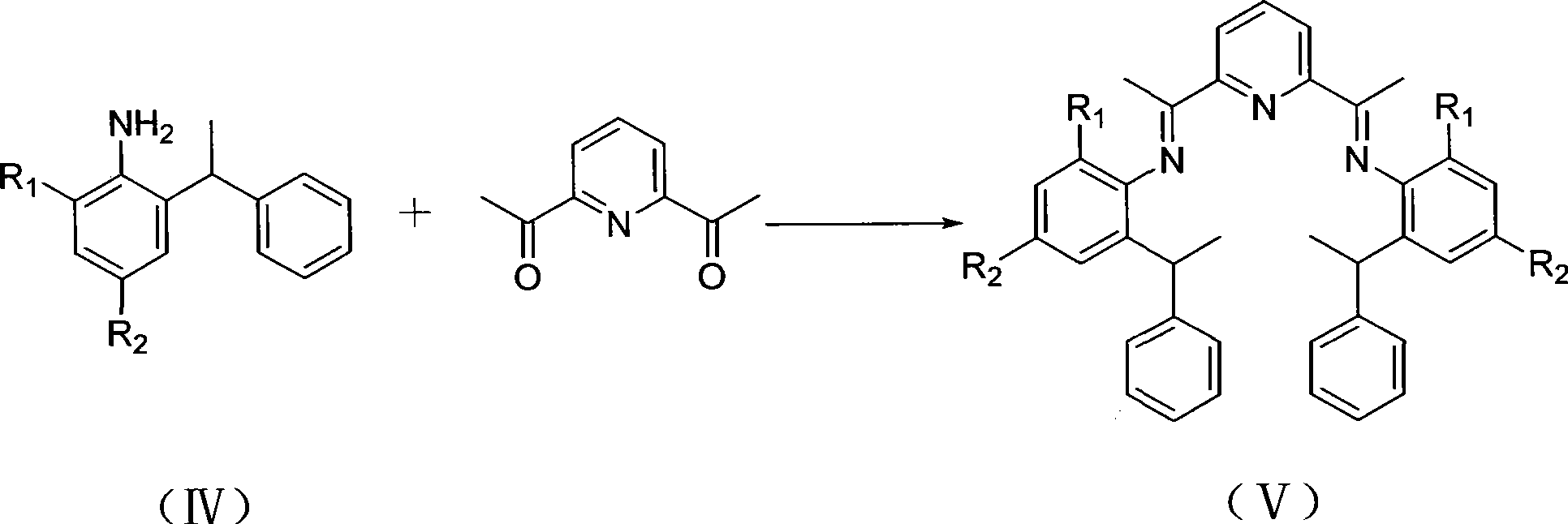
The invention discloses a bis (imino) pyridine iron olefin polymerization catalyst and a preparation method and application thereof. The catalyst is a novel bis(imino)pyridine iron complex with the asymmetric substitution of position 2 and 6 of an aromatic ring; and the structural formula of the catalyst is shown in formula (I), wherein X is halogen, R1 is alkyl, and R2 is the alkyl or oxyl. The catalyst can be obtained through a Friedel-Crafts reaction, a ketoamine condensation reaction and a coordination reaction. The preparation method is simple, and raw materials are cheap. The catalyst can catalyze the polymerization of ethylene under the activation of MAO to prepare polyethylene with high molecular weight and wide distribution.
Owner:SUN YAT SEN UNIV
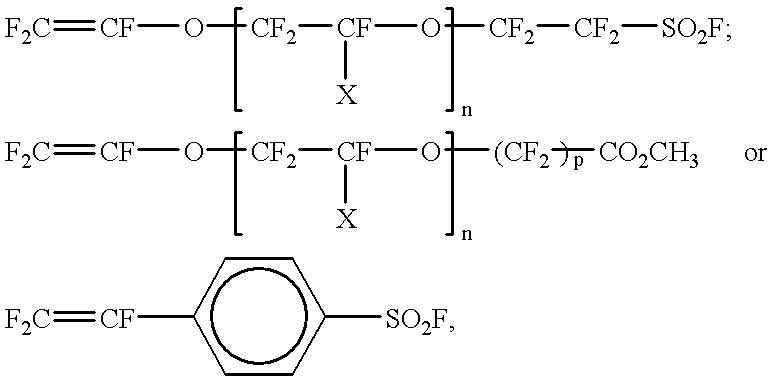
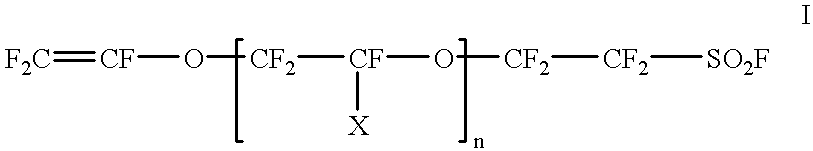
Cross-linked sulphonated polymers and method for preparing same
InactiveUS20020002240A1Improve mechanical propertiesFine surfaceSemi-permeable membranesOrganic diaphragmsCross-linkElectrolysis
The present invention is concerned with cross-linked sulfonated polymers, eventually perfluorinated, and their preparation process. When molded in the form of membranes, the polymers are useful in electrochemical cells, in a chlorine-sodium electrolysis process, as separator in an electrochemical preparation or organic and inorganic compounds, as separators between an aqueous phase and an organic phase, or as catalyst for Diels-Alder additions, Friedel-Craft reactions, aldol concentrations, cationic polymerisation, esterification, and acetal formation.
Owner:MICHOT CHRISTOPHE +1
Nitrogen and sulfur co-doped carbon-loaded non-noble metal type oxygen reduction catalyst and preparation method thereof
InactiveCN104998673AImprove stabilityExcellent resistance to methanolPhysical/chemical process catalystsCell electrodesDistillationVacuum drying
The invention discloses a nitrogen and sulfur co-doped carbon-loaded non-noble metal type oxygen reduction catalyst and a preparation method thereof and discloses a M-N-S-C oxygen reduction catalyst and a preparation method thereof. The raw materials of the catalyst comprise copolymer P (TPT+Tp) of tripyrrole-[1,3,5]-triazine (TPT) and thiophene (Tp) and non-noble metal salt. The preparation method of the catalyst includes the following steps that firstly, a Friedel-Crafts reaction is adopted for synthesizing the copolymer P (TPT+Tp) of the tripyrrole-[1,3,5]-triazine (TPT) and the thiophene (Tp); secondly, the P (TPT+Tp) and the non-noble metal salt are added into ethanol, mixtures are placed in an ultrasonic dispersing instrument, a whole system is evenly dispersed due to ultrasound, and then the ethanol is dried by distillation and placed in a vacuum drying box to be dried for 4 h at the temperature of 80 DEG C; thirdly, thermal treatment is performed for the first time and nitrogen doped materials are obtained; fourthly, the nitrogen doped materials are completely washed through dilute acid; fifthly, thermal treatment is performed for the second time, and then the M-N-C oxygen reduction catalyst can be obtained.
Owner:XIANGTAN UNIV
Surface hydrophilic modification of polystyrene material and product
ActiveCN101440168AIncreased hydrophilicityReduced non-specific adsorption capacityComponent separationChromatographic separationMicrosphere
The invention provides a method for preparing surface hydrophilic modified polystyrene materials, and a product thereof, and the method is characterized by grafting and coupling the surface of polystyrene with a layer of hydrophilic macromolecular polyvinyl alcohol (PVA) through a stable chemical bond, particularly performing hydrophilic modification on the surfaces of super-macroporous polystyrene microspheres. The method comprises the following concrete steps: (1) benzene ring of polystyrene is subjected to halogen acetylation or halogen methylation in an organic solvent under the action of acidic catalysts through Friedel-Crafts reaction, and is added to active radical halogen acetyl or halogen methyl; and (2) under alkaline conditions and the action of phase transfer catalyst, Williamson etherification is utilized to couple the hydrophilic PVA to the surface of polystyrene through ether linkage. The method has the advantages of simple operation and mild conditions. A hydrophilic coating is stable, not easy to fall off and rich in hydroxyl. The method has great application potential in biotechnology, particularly the chromatographic separation field.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Nitrogen-doped carbon-supported non-noble metal (M-N-C) oxygen reduction catalyst and preparation method thereof
InactiveCN105170168AImprove stabilityExcellent resistance to methanolPhysical/chemical process catalystsCell electrodesUltrasonic dispersionPyrrole
The invention discloses an M-N-C oxygen reduction catalyst and a preparation method thereof, wherein the raw materials of the catalyst comprise a copolymer (P(TPT+Py)) of tripyrrole-[1,3,5]-triazine (TPT) and pyrrole (Py) and a non-noble metal salt. The preparation method comprises: (1) using a Friedel-Crafts reaction to synthesize a copolymer (P(TPT+Py)) of tripyrrole-[1,3,5]-triazine (TPT) and pyrrole (Py); (2) adding (P(TPT+Py)) and a non-noble metal salt to ethanol, placing into an ultrasonic dispersion device, carrying out an ultrasonic treatment to make the whole system be uniformly dispersed, evaporating the ethanol, placing into a vacuum oven, and drying for 4 h at a temperature of 80 DEG C; (3) carrying out a first heat treatment to obtain a nitrogen-doped carbon material; (4) completely washing the obtained nitrogen-doped carbon material with a diluted acid; and (5) carrying out a second heat treatment so as to obtain the M-N-C oxygen reduction catalyst.
Owner:XIANGTAN UNIV
Functionalized elastomer nanocomposite
InactiveUS20050277723A1Improve air tightnessWithout separate inflatable insertsWith separate inflatable insertsElastomerGeneral purpose
An embodiment of the present invention is a nanocomposite comprising a clay and an elastomer comprising at least C2 to C10 olefin derived units; wherein the elastomer also comprises functionalized monomer units pendant to the elastomer. Desirable embodiments of the elastomer include poly(isobutylene-co-p-alkylstyrene) elastomers and poly(isobutylene-co-isoprene) elastomers, which are functionalized via Friedel-Crafts reaction with a Lewis acid and a functionalizing agent such as acid anhydrides and / or acylhalides. The clay is exfoliated in one embodiment by the addition of exfoliating agents such as alkyl amines and silanes to the clay. The composition can include secondary rubbers such as general purpose rubbers, and curatives, fillers, and the like. The nanocomposites of the invention have improved air barrier properties such as are useful for tire innerliners and innertubes.
Owner:EXXONMOBIL CHEM PAT INC
Fluorenyl organic framework material, preparation and application method thereof
ActiveCN103232473ATest thermal stabilitySave raw materialsOrganic chemistrySolid-state devicesSolubilityOrganic field-effect transistor
The invention relates to a fluorenyl organic framework material, a preparation and application method thereof. A material which is provided with a framework structure shaped as a Chinese character ri and constructed by the method disclosed by the invention is taken as a new generation organic semiconductor to be applied to an organic electronic device. The structure of the material is shown in the specification. The material has the characteristics that (1) monomers are synthesized via a Grignard reaction and a Friedel-Crafts reaction, the raw material is inexpensive and the synthesis method is simple; (2) the material has high heat stability and glass transition temperature; (3) the material is good in flexibility and high in solubility; and (4) due to the accumulation effect of diaryl fluorine, the material is good in electrooptic activity. The fluorenyl organic framework material can be applied to the organic electronic field of film devices such as organic light emitting display, organic light storage, organic photovoltaic cells, organic field effect transistors and organic laser, and the like. Electroluminescent devices prepared by using the material disclosed by the invention show satisfactory results in luminance, luminous efficiency and voltage resistance stability.
Owner:NANJING UNIV OF POSTS & TELECOMM
New synthesis process of canagliflozin
The present invention discloses a new synthesis process of canagliflozin. The new synthesis process comprises: adopting 2-methyl benzoic acid as a starting raw material, and adopting a self-made catalyst, iodic acid and iodine to carry out a reaction to produce an intermediate 1, or adopting 2-methyl benzoic acid as a starting raw material, and adding liquid bromine under effects of a metal reagent and a catalyst to synthesize an intermediate 2; optionally selecting the intermediate 1 or 2 to carry out an acylation reaction with thionyl chloride, and then carrying out a Friedel-Crafts reaction to produce an intermediate 3; adopting ALPHA-D-glucose as a raw material, carrying out a reaction with pivaloyl chloride to protect all hydroxyl, and carrying out a reaction with zinc bromide and bromotrimethylsilane to produce an intermediate 4; linking the intermediate 3 and the intermediate 4 to produce an intermediate 5; and finally under an acid condition, removing the pivaloyl to produce the target compound. The new synthesis process has characteristics of high yield, mild condition, safety, reliability, cheap and easily available raw material, and easy production cost control, and is suitable for industrial production.
Owner:HAIMEN RUIYI MEDICAL TECH
Method for preparing mangiferin aglycone
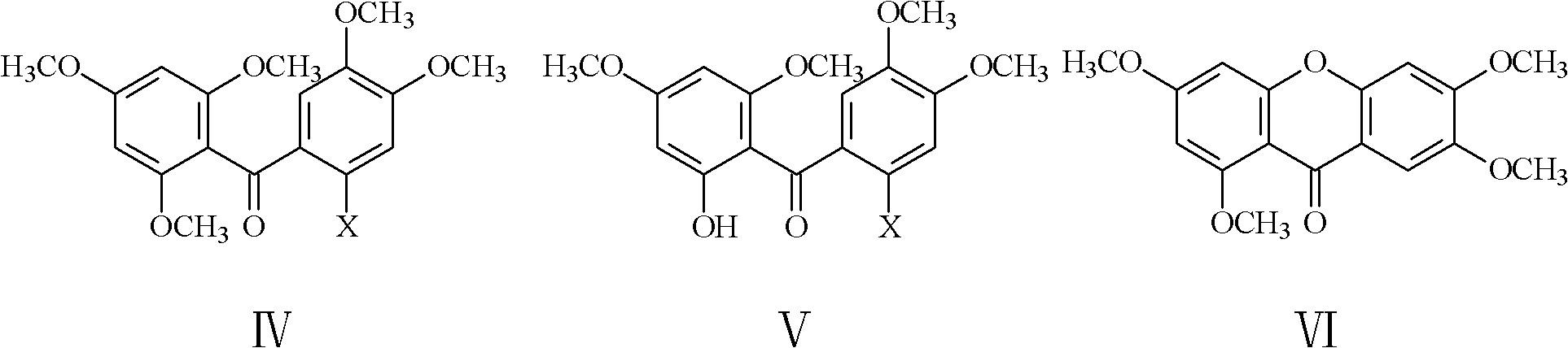
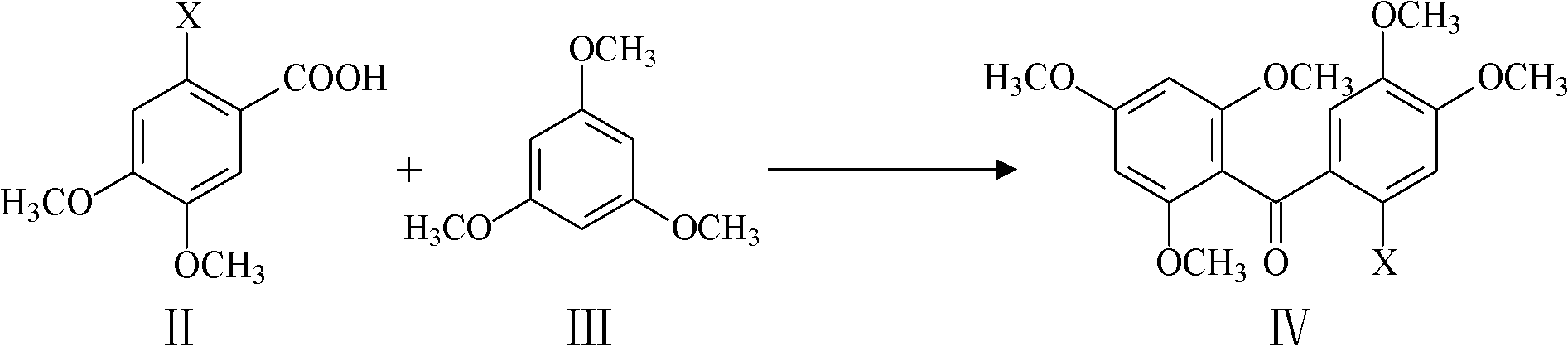
The invention relates to the field of medicinal chemistry, and discloses a method for preparing mangiferin aglycone. The method comprises that: step 1, 2-halogenate-4,5-dimethoxy benzoic acid and 1,3,5-trimethoxy benzene are subjected to Friedel-Crafts reaction, so a compound which has a structure in a formula IV is generated; step 2, the compound in the formula IV is selectively demethylated, and a compound which has the structure in the formula V is generated; step 3, the compound in the formula V is cyclized, and a compound which has the structure in the formula VI is generated; and step 4, the compound in the formula VI is completely demethylated, and mangiferin aglycone is generated. The method for preparing mangiferin aglycone improves the synthetic yield of the mangiferin aglycone, has the characteristics of cheap and easily-obtained raw materials, less synthetic steps, convenience in operation, easiness in control and high product purity, and can be widely applied in industrial production.
Owner:KPC PHARM INC +1
Method for preparing 2-P-octyl-phenenl-2-amino-propanediol hydrochloride
InactiveCN1814583AEasy to manufactureEfficient manufacturingOrganic compound preparationAmino-hyroxy compound preparationSilanesPropanediol
The invention offers 2-amphi capryl phenethyl-2-amino group propylene glycol hydrochloride preparation method. It includes the following steps: doing Friedel-Crafts reaction for styrene and capryl chloride to produce amphi capryl styrene; doing Michael addition reaction with kharophen diethyl malonate under alkali action to produce 2-amphi capryl phenethyl-2-kharophen diethyl malonate; reacting with triethyl silane to produce 2-amphi capryl phenethyl-2-kharophen diethyl malonate; reducing and hydrolyzing to produce 2-amphi capryl phenethyl-2-amino group propylene glycol; acidifying with hydrochloric acid to produce salt. It has the advantages of simple line, low cost, short period, little pollution, and high yield. It also offers the intermediate compound preparation method.
Owner:NANJING YOKO PHARMA GRP CO LTD
Functionalized elastomer nanocomposite
InactiveCN1665871AWithout separate inflatable insertsWith separate inflatable insertsElastomerSilanes
An embodiment of the present invention is a nanocomposite comprising a clay and an elastomer comprising at least C2 to C10 olefin derived units; wherein the elastomer also comprises functionalized monomer units pendant to the elastomer. Desirable embodiments of the elastomer include poly(isobutylene-co-p-alkylstyrene) elastomers and poly(isobutylene-co-isoprene) elastomers, which are functionalized via Friedel-Crafts reaction with a Lewis acid and a functionalizing agent such as acid anhydrides and / or acylhalides. The clay is exfoliated in one embodiment by the addition of exfoliating agents such as alkyl amines and silanes to the clay. The composition can include secondary rubbers such as general purpose rubbers, and curatives, fillers, and the like. The nanocomposites of the invention have improved air barrier properties such as are useful for tire innerliners and innertubes.
Owner:EXXONMOBIL CHEM PAT INC
Preparation method of 2-hydroxyl-1-{4-(2-hydroxyethyl) phenyl}-2-methyl-1-acetone
InactiveCN101811951AShorten the timeSimple processOrganic compound preparationCarbonyl compound preparationN dimethylformamidePhenyl Ethers
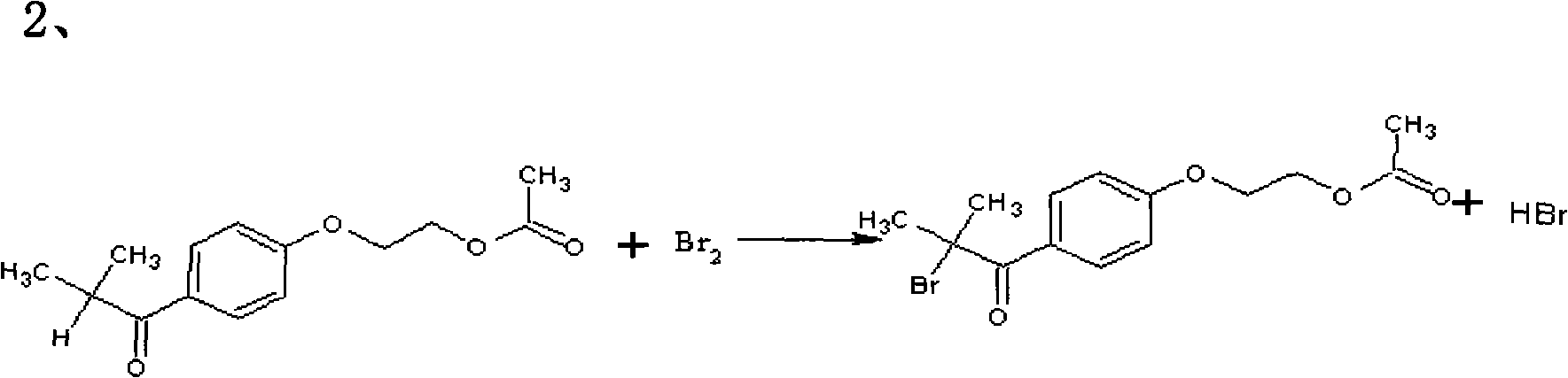
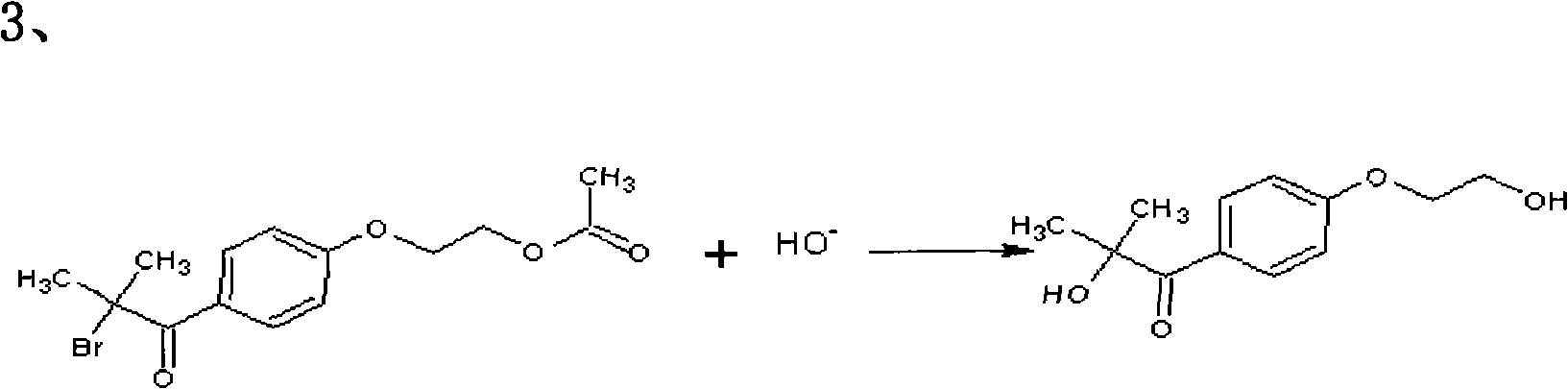
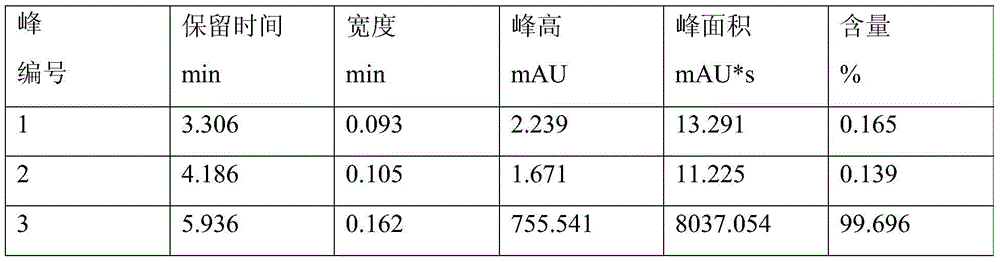
The invention relates to a preparation method of 2-hydroxyl-1-{4-(2-hydroxyethyl) phenyl}-2-methyl-1-acetone, which comprises the following steps that: ethylene glycol phenyl ether acetate serves as the raw material and carries out friedel-crafts reaction with isobutyl chloride with the molar ratio of 1 to 1.2 times of equivalent under the catalysis of lewis acid, the reaction temperature is -5 to 5DEG C, and the reaction time is 3 to 6h; bromination is carried out under the catalysis of N, N-dimethylformamide or iodine; at room temperature, phase transfer catalyst is used for carrying out catalysis and hydrolysis to a brominated product; and finally the product is prepared through purification and crystallization. The preparation method of 2-hydroxyl-1-{4-(2-hydroxyethyl) phenyl}-2-methyl-1-acetone adopts dichloromethane or dichloroethane as the solvent during the bromination process, does not use acetic acid any more, carries out reaction under the catalysis of DMF or I2, so that the reaction time is greatly shortened. The phase transfer catalyst is introduced during the hydrolysis process, so that the intermediate brominated product directly has reaction with alkali in water, thereby preventing using a large amount of ethanol as the solvent, simplifying the process, reducing the production cost and improving the yield.
Owner:GANSU JINDUN CHEM
Synthetic process of 4,4'-dichlorodiphenyl sulfone
ActiveCN104402780BLow costPost-processing is simpleOrganic chemistryOrganic compound preparationChlorobenzeneOrganic synthesis
The invention belongs to the field of organic synthesis, and in particular relates to a synthesis process of 4, 4'-dichlorodiphenyl sulfone. The synthesis process comprises the following steps: firstly, performing a Friedel-Crafts reaction on sulfoxide chloride and chlorobenzene under the action of a catalyst; cooling and hydrolyzing -after the reaction; heating and dissolving ; cooling, separating out crystals and filtering so as to obtain 4, 4'-dichlorodiphenyl sulfoxide; dissolving 4, 4'-dichlorodiphenyl sulfoxide in glacial acetic acid, adding hydrogen peroxide for an oxidizing reaction; lowering the temperature and cooling after the reaction is completed, and filtering so as to obtain 4, 4'-dichlorodiphenyl sulfone. Chlorobenzene is used both as a reaction material and a solvent, and other solvent impurities are not mixed in chlorobenzene, so that after-treatment is easier when the cost is saved, and the product is high in purity. The synthesis process has the advantages of simple process, low cost and few impurities. The obtained product has high purity and has the content of above 99.2% and the yield of above 90%, so that the product is good in economic benefit and is suitable for industrial production.
Owner:SHANDONG KAISHENG NEW MATERIALS
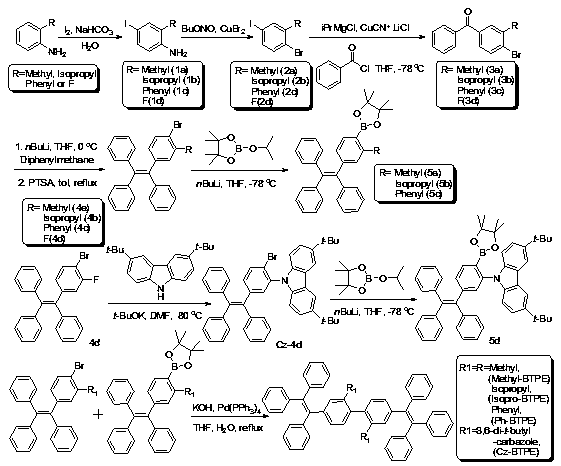
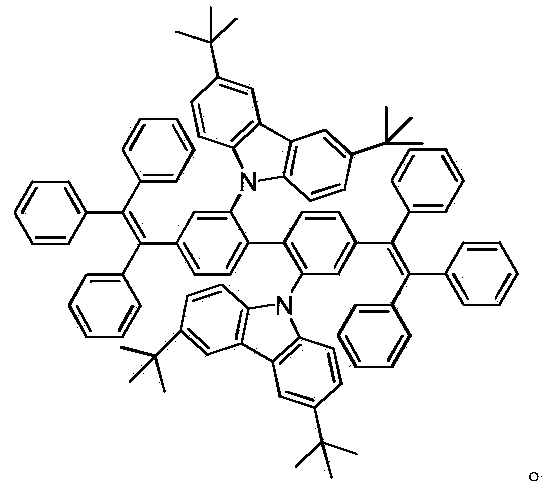
Compound containing tetraphenyl ethylene unit, preparation method and applications thereof
InactiveCN103396285AReduce the degree of conjugationIncrease brightnessSolid-state devicesSemiconductor/solid-state device manufacturingQuantum yieldAniline
Owner:WUHAN UNIV
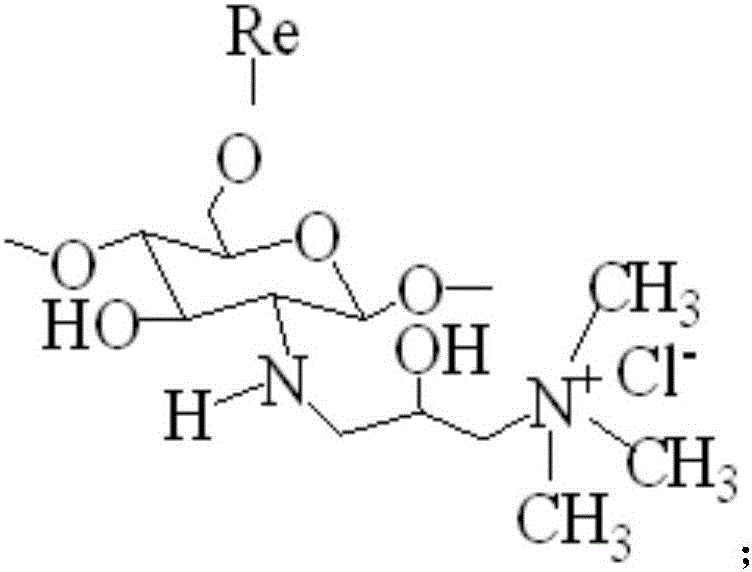
Reactive chitosan quaternary ammonium salt, and preparation method and application thereof
The invention relates to a reactive chitosan quaternary ammonium salt, and a preparation method and application thereof. The preparation method comprises the following steps: carrying out Friedel-Crafts reaction on chitosan and 2,3-epoxy propyl-trimethyl ammonium chloride to generate N-2-hydroxypropyltrimethyl ammonium chloride chitosan, and carrying out substitution reaction on the N-2-hydroxypropyltrimethyl ammonium chloride chitosan and a compound to generate the reactive chitosan quaternary ammonium salt. The reactive chitosan quaternary ammonium salt is an aid for reactive dye cotton fabric salt-free dyeing, is soluble in water, and has the advantages of no toxic or side effect and favorable environment friendliness. When being used for reactive dye salt-free dyeing of cotton fabrics, the reactive chitosan quaternary ammonium salt has favorable dyeing effects, reduces the environmental pollution of salts in wastewater, and greatly lowers the dye printing production cost. When the reactive chitosan quaternary ammonium salt is used in the dyeing process to obtain the cotton fabric, the dye-uptake rate of the reactive dye is 70-85%. The fixation rate is 47-55%, the dry fastness to rubbing is greater than Grade 4, and the wet fastness to rubbing is greater than Grade 3.
Owner:WUYI UNIV
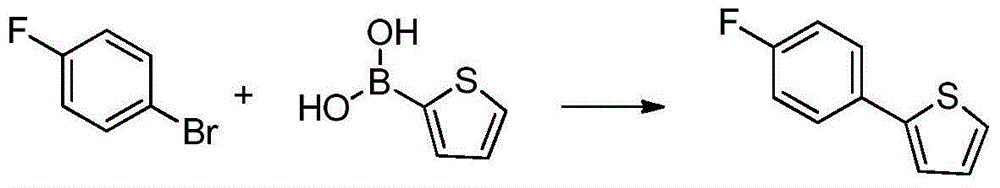
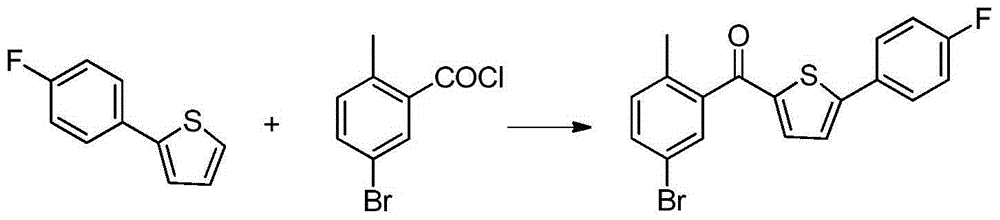
Preparation method of 2-(5-bromo-2-methylbenzyl)-5-(4-fluorophenyl)thiophene
InactiveCN104892566AEasy to purifySimple and fast operationOrganic chemistryBromobenzoic AcidsKetone
The invention relates to a preparation method of 2-(5-bromo-2-methylbenzyl)-5-(4-fluorophenyl)thiophene, which comprises the following steps: carrying out coupling reaction on 2-bromothiophene and p-bromofluorobenzene to obtain 2-(4-fluorophenyl)thiophene; carrying out Friedel-Craft reaction on the 2-(4-fluorophenyl)thiophene and 2-methyl-5-bromobenzoic acid to obtain 5-bromo-2-methylphenyl-2-(4-fluorophenyl)thienyl ketone; and finally, carrying out reduction reaction on the 5-bromo-2-methylphenyl-2-(4-fluorophenyl)thienyl ketone to obtain the 2-(5-bromo-2-methylbenzyl)-5-(4-fluorophenyl)thiophene. By using the cheap and accessible p-bromofluorobenzene and 2-methyl-5-bromobenzoic acid as the raw materials for synthesizing the canagliflozin intermediate, the product is easy for purification, and the method is simple to operate and has the advantages of low cost and environment friendliness.
Owner:SHANGHAI INST OF TECH
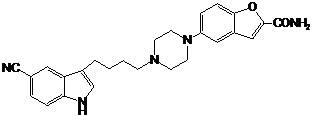
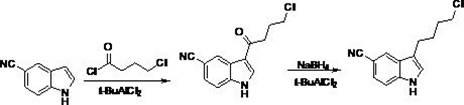
Preparation method of vilazodone
The invention provides a preparation method of vilazodone, which comprises the following steps: reacting 5-cyanoindole, which is used as the initial raw material, with substituted phenylsulfonyl chloride under alkaline conditions, carrying out Friedel-Crafts reaction under the catalytic action of Lewis acid, reducing the product, and carrying out substitution reaction with 5-(1-piperazino)-benzofuryl-2-formamide to obtain the vilazodone. The invention also provides three intermediate compounds related to the vilazodone preparation method. The preparation method provided by the invention has the advantages of low cost, high yield and simple after-treatment, and is easy to operate and convenient for industrial production; and all the reagents are conventional reagents.
Owner:上海泛凯生物医药科技有限公司 +1
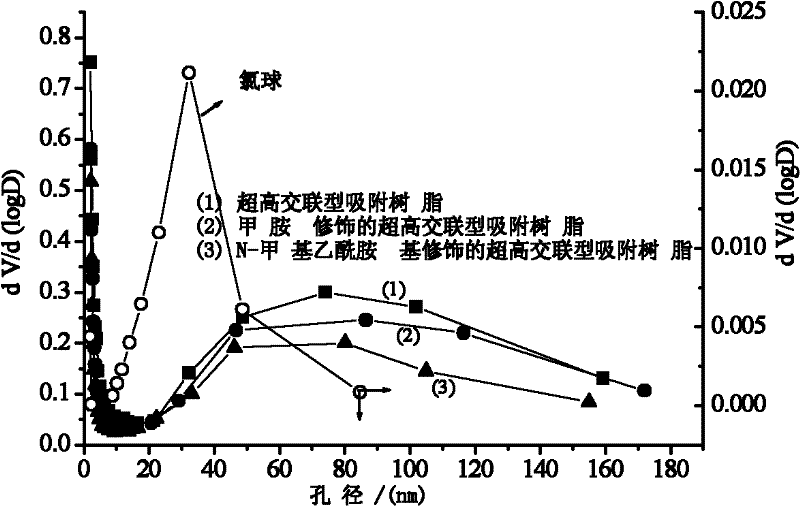
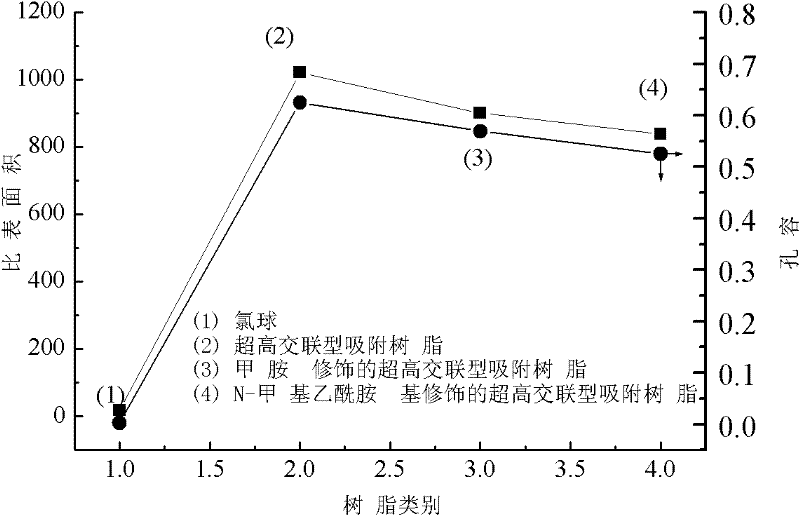
Preparation method of ultra-high crosslinking type adsorptive resin modified by N-methyl acetamido
InactiveCN102350316AEffective control of pore structurePolarity can be effectively adjustedOther chemical processesAlkali metal oxides/hydroxidesOrganic acidN-methylacetamide
The invention relates to a preparation method of ultra-high crosslinking type adsorptive resin modified by N-methyl acetamido. Lewis acid is added into a chlorine ball swollen in a solvent to be as a catalyst, and Friedel-Crafts reaction is carried out at 80 to 100 DEG C so as to obtain the ultra-high crosslinking type adsorptive resin; and the obtained resin is further aminated and acetylated soas to prepare the ultra-high crosslinking type adsorptive resin modified by the N-methyl acetamido. According to the preparation method of the ultra-high crosslinking type adsorptive resin modified by the N-methyl acetamido, the surface of the ultra-high crosslinking type adsorptive resin is loaded with the N-methyl acetamido, which can obviously enhance the adsorption capacity of the resin to weak polar and polar substances (such as phenol and salicylic acid). The ultra-high crosslinking type adsorptive resin modified by the N-methyl acetamido has an extensive application prospect in the fields of the treatment, the recycle and the like of phenols and organic acid wastewater.
Owner:CENT SOUTH UNIV
Acylation reactions of aromatic substrates
InactiveUS6630606B2Increase ratingsHigh selectivityCarbonyl compound preparation by condensationHydrocarbon by hydrocarbon and non-hydrocarbon condensationAlkyl transferProduct formation
Method of performing alkylation or acylation reactions of aromatic substrates under supercritical or near-critical reaction conditions. In particular, a method of performing Friedel-Crafts alkylation or acylation reactions is disclosed under those conditions. Friedel-Crafts reactions may be effected using a heterogeneous catalyst in a continuous flow reactor containing a super-critical or near-critical reaction medium. Selectivity of product formation can be achieved by varying one or more of the temperature, pressure, catalyst, flow rates and also by varying the ratios of aromatic substrate to acylating or alkylating agent.
Owner:THOMAS SWAN & CO LTD +1
Preparation method and application of polar group-modified pendent double bond post-crosslinked polydivinylbenzene resin
InactiveCN103910823ALarge specific surface areaGood selective adsorptionOther chemical processesWater/sewage treatment by sorptionDouble bondOrganic matter
The invention discloses a preparation method and an application of polar group-modified pendent double bond post-crosslinked polydivinylbenzene resin. The preparation method comprises: firstly performing a polymerization reaction of divinylbenzene monomers, polar monomers, and a pore forming agent to obtain a polar group-modified pendent double bond post-crosslinked polydivinylbenzene resin precursor; performing further crosslinking through a Friedel-Crafts reaction to obtain the polar group-modified pendent double bond post-crosslinked polydivinylbenzene resin. The preparation method is simple, and low in cost, and the prepared post-crosslinked resin has a high specific surface area and a special pore structure; the post-crosslinked resin can be used for adsorbing and removing organic matter in waste water, has the effect of selective adsorption, and especially has obvious selective adsorption effect on Congo red and / or rhodamine B.
Owner:CENT SOUTH UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com