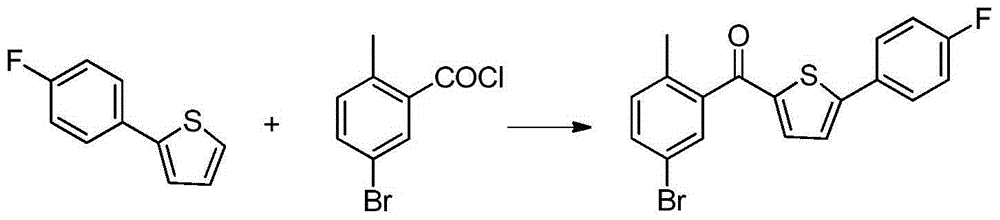
Preparation method of 2-(5-bromo-2-methylbenzyl)-5-(4-fluorophenyl)thiophene
A technology of methyl benzyl and methyl phenyl, applied in the field of medicinal chemistry, can solve the problems of high cost, low yield and complex process of the preparation method, and achieve the effects of easy purification, environmental friendliness and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
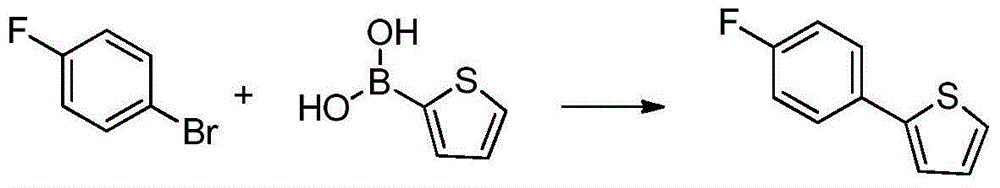
[0039] Example 1 Preparation of 2-(4-fluorophenyl)thiophene III:
[0040]
[0041] Grignard reagent preparation: N 2 Under protection, magnesium chips (11.7g, 480mmol), THF (15.0mL) were added to a 500mL three-necked flask equipped with a thermometer, a reflux condenser, and a constant pressure dropping funnel, 2 drops of p-fluorobromobenzene, and 2 capsules of After iodine is heated to initiate the reaction, dissolve p-fluorobromobenzene (70.0g, 400mmol) in THF (250mL), then add the THF solution of p-fluorobromobenzene dropwise under heating and reflux, keep warm and reflux for 2h after dropping, and detect the conversion of raw materials by GC completely.
[0042] Coupling reaction: N 2Under protection, 2-bromothiophene (52.2g, 320mmol) was added to a 1000mL three-necked flask equipped with a thermometer, a reflux condenser, and a constant pressure dropping funnel, and bis(acetylacetonate)palladium (23.4mg, 0.0320mmol) was added under stirring. , THF (200mL), heated to...
Embodiment 2
[0043] Example 2 Preparation of 2-(4-fluorophenyl)thiophene III:
[0044]
[0045] Grignard reagent preparation: N 2 Under protection, magnesium chips (11.7g, 480mmol), THF (15.0mL) were added to a 500mL three-neck flask equipped with a thermometer, a reflux condenser, and a constant pressure dropping funnel, 2 drops of p-fluorobromobenzene, and 2 capsules of After iodine is heated to initiate the reaction, dissolve p-fluorobromobenzene (70.0g, 400mmol) in THF (250mL), then add the THF solution of p-fluorobromobenzene dropwise under heating and reflux, keep warm and reflux for 2h after dropping, and detect the conversion of raw materials by GC completely.
[0046] Coupling reaction: N 2 Under protection, 2-bromothiophene (52.2g, 320mmol) was added to a 1000mL three-neck flask equipped with a thermometer, a reflux condenser, and a constant pressure dropping funnel, and PdCl was added under stirring. 2 (dppf) (23.4mg, 0.0320mmol), THF (200mL), heated up to 50°C, then added...
Embodiment 3
[0047] Example 3 Preparation of 2-(4-fluorophenyl)thiophene III:
[0048]
[0049] Grignard reagent preparation: N 2 Under protection, magnesium chips (11.7g, 480mmol), THF (15.0mL) were added to a 500mL three-neck flask equipped with a thermometer, a reflux condenser, and a constant pressure dropping funnel, 2 drops of p-fluorobromobenzene, and 2 capsules of After iodine is heated to initiate the reaction, dissolve p-fluorobromobenzene (70.0g, 400mmol) in THF (250mL), then add the THF solution of p-fluorobromobenzene dropwise under heating and reflux, keep warm and reflux for 2h after dropping, and detect the conversion of raw materials by GC completely.
[0050] Coupling reaction: N 2 Under protection, 2-bromothiophene (52.2g, 320mmol) was added to a 1000mL three-neck flask equipped with a thermometer, a reflux condenser, and a constant pressure dropping funnel, and PdCl was added under stirring. 2 (dppf) (23.4mg, 0.0320mmol), THF (200mL), heated up to 50°C, then added...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com