Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1918 results about "Pyran" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In chemistry, pyran, or oxine, is a six-membered heterocyclic, non-aromatic ring, consisting of five carbon atoms and one oxygen atom and containing two double bonds. The molecular formula is C₅H₆O. There are two isomers of pyran that differ by the location of the double bonds. In 2H-pyran, the saturated carbon is at position 2, whereas, in 4H-pyran, the saturated carbon is at position 4.
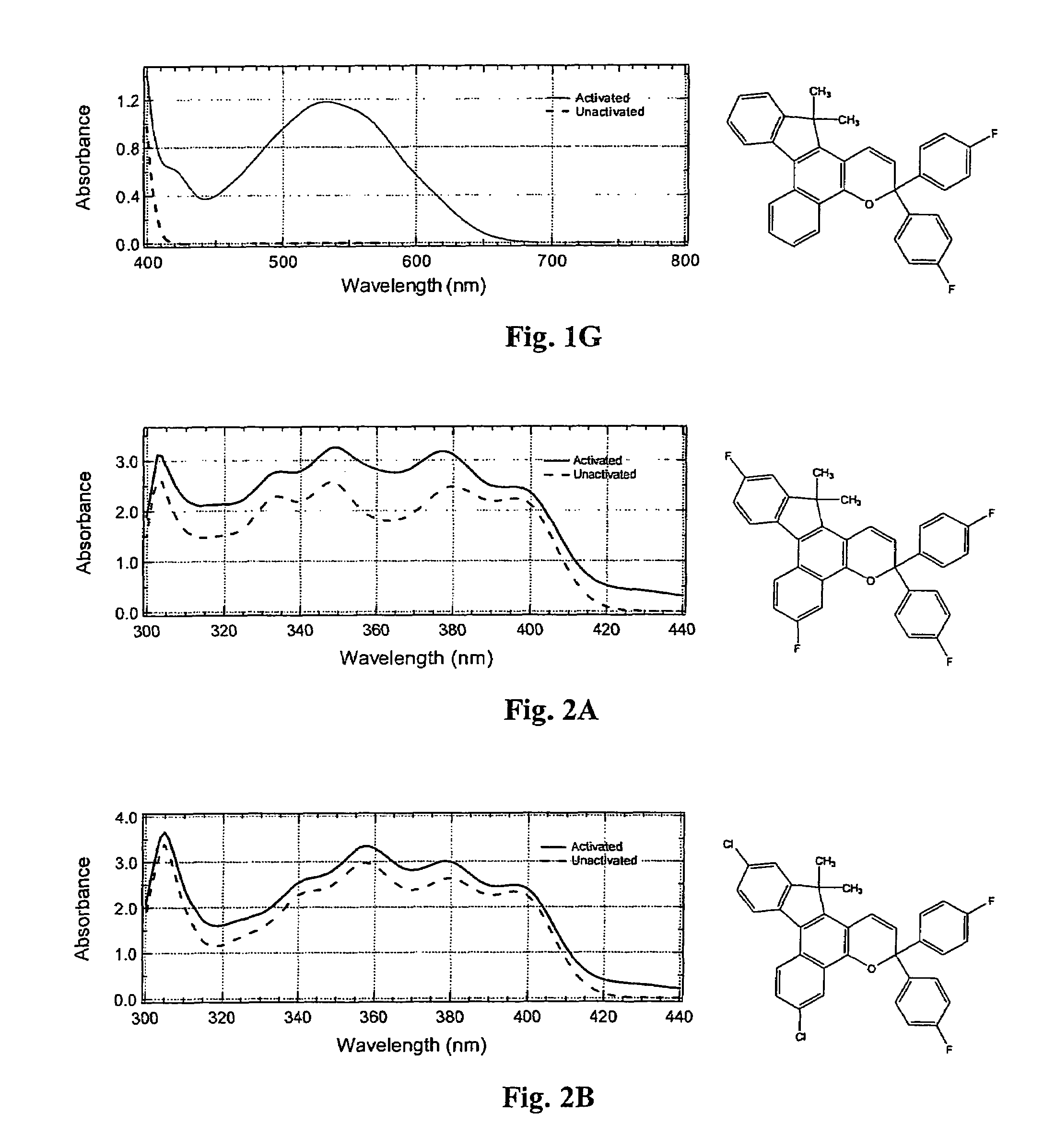
Photochromic compounds
Various non-limiting embodiments disclosed herein relate generally to photochromic compounds, which may be thermally reversible or non-thermally reversible, and articles made therefrom. Other non-limiting embodiments relate to photochromic-dichroic compounds, which may be thermally reversible or non-thermally reversible, and articles made therefrom. For example, one non-limiting embodiment provides a thermally reversible, photochromic compound adapted to have at least a first state and a second state, wherein the thermally reversible, photochromic compound has an average absorption ratio greater than 2.3 in at least one state as determined according to CELL METHOD. Another non-limiting embodiment provides a photochromic compound comprising: (a) at least one photochromic group chosen from a pyran, an oxazine, and a fulgide; and (b) at least one lengthening agent L attached to the at least one photochromic group and represented by the formula —[S1]c-[Q1-[S2]d]d′-[Q2-[S3]e]e′-[Q3-[S4]f]f′—S5—P, which is described herein.
Owner:TRANSITIONS OPTICAL INC
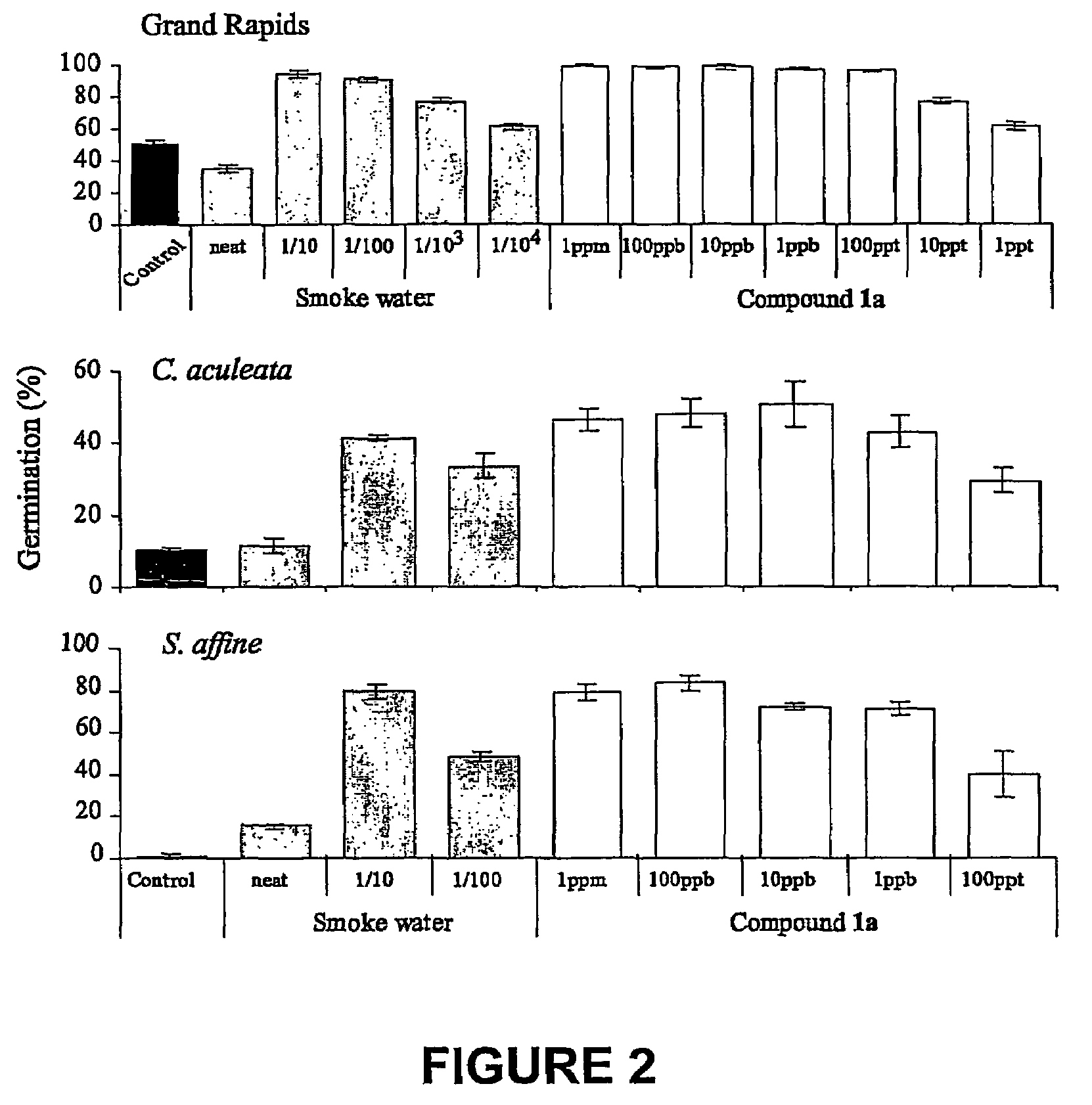
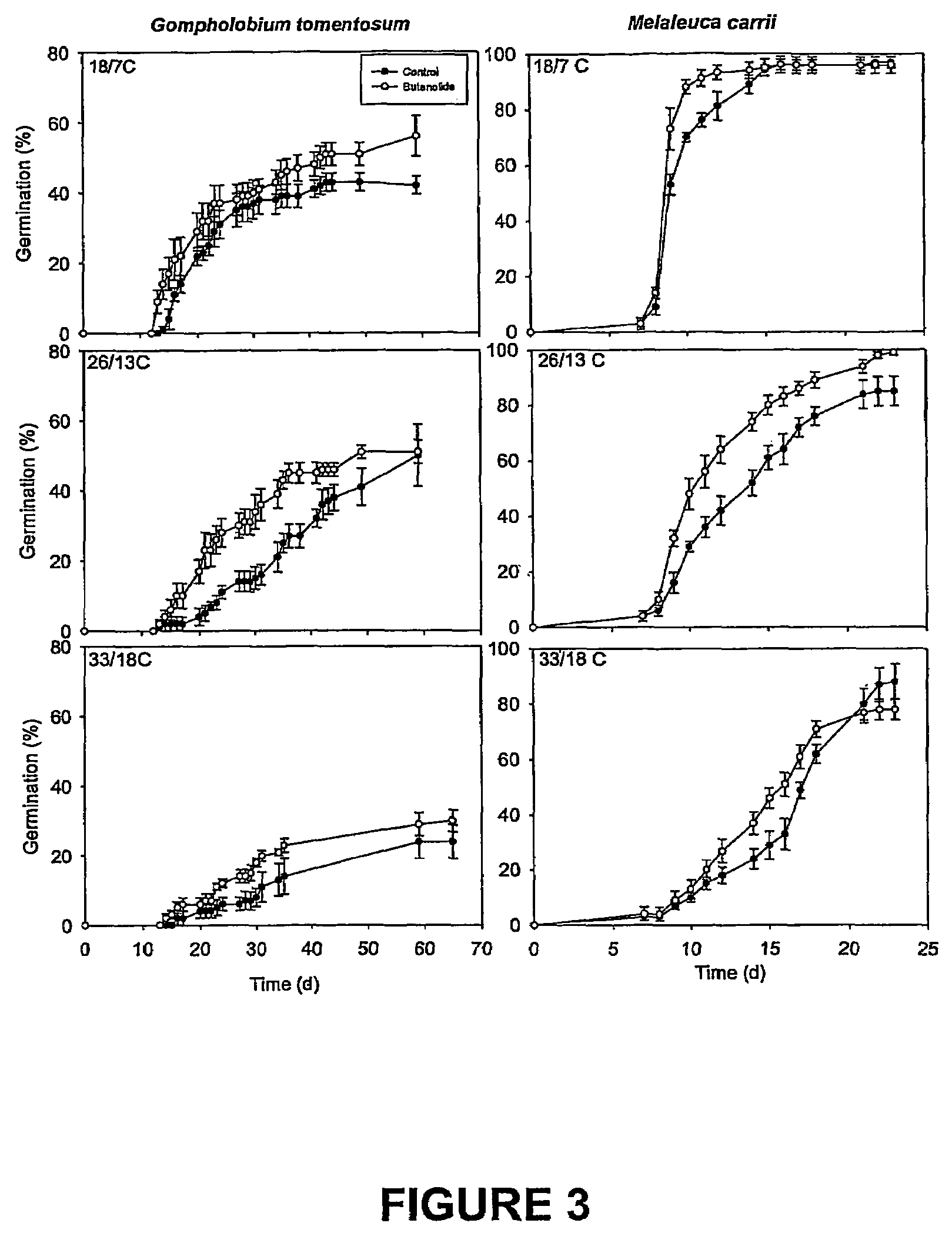
Vinylogous 4H-pyrones and their use in promoting plant growth
Owner:UNIV OF WESTERN AUSTRALIA
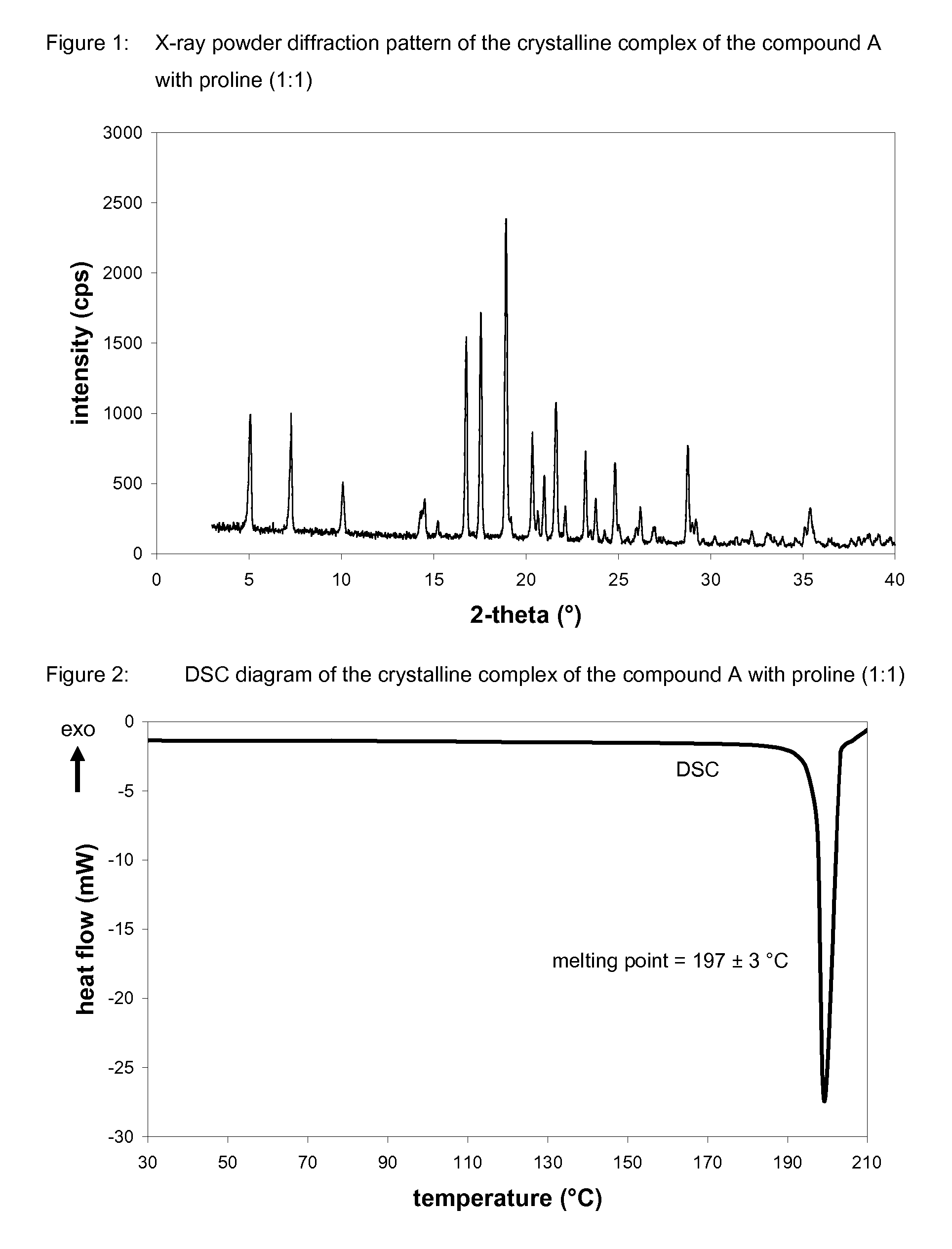
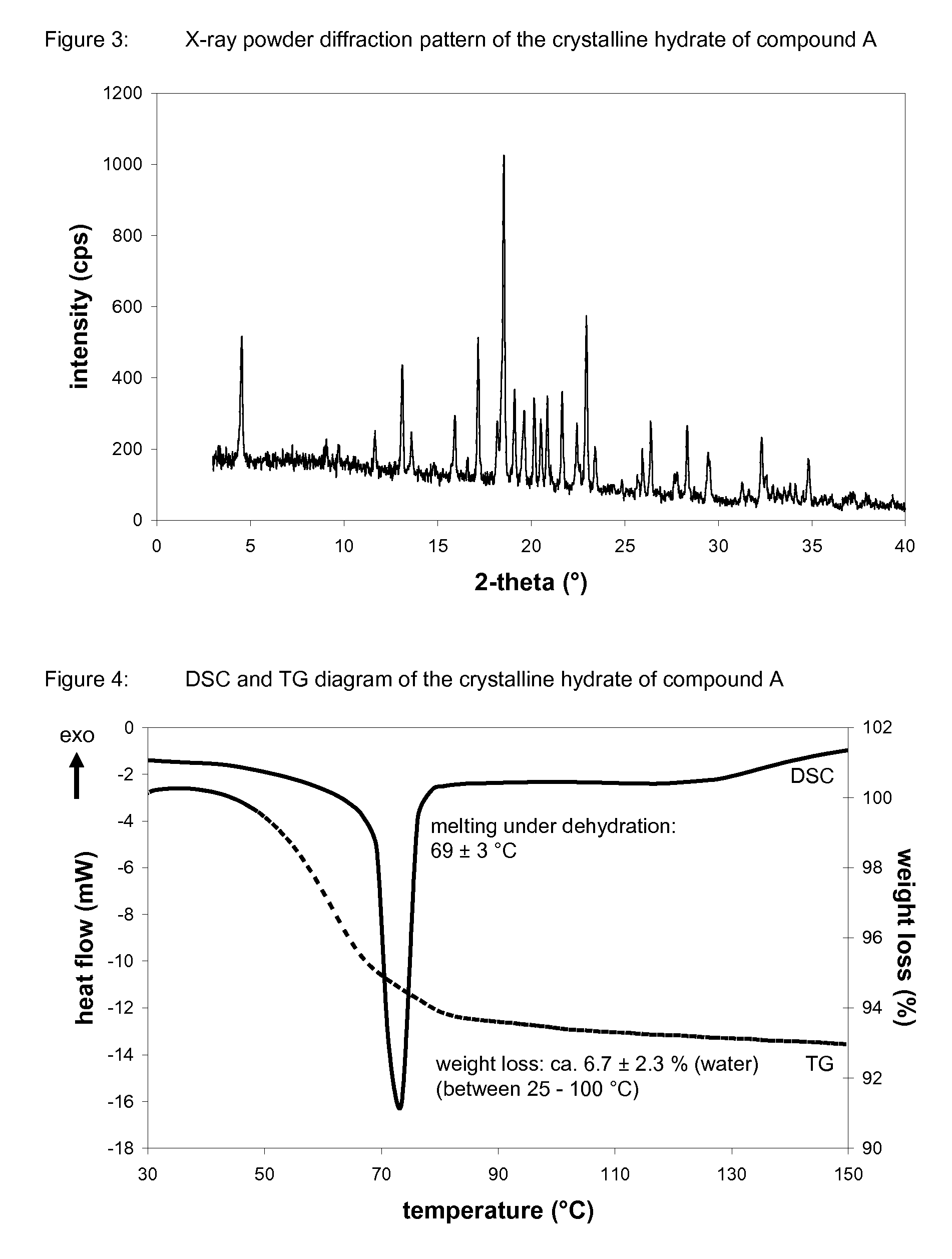
Crystalline forms of 1-chloro-4-(beta-D-glucopyranos-1-yl)-2-[4-ethynyl-benzyl)-benzene, methods for its preparation and the use thereof for preparing medicaments
The invention relates to a crystalline hydrate of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-(4-ethynyl-benzyl)-benzene and to crystalline complexes between 1-chloro-4-(β-D-glucopyranos-1-yl)-2-(4-ethynyl-benzyl)-benzene and a natural amino acid, to methods for the preparation thereof, as well as to uses thereof for preparing medicaments.
Owner:BOEHRINGER INGELHEIM INT GMBH
Propiophenone derivatives and process for preparing the same
InactiveUS6048842AHigh activityGood hypoglycemic activityAntibacterial agentsBiocideD-GlucopyranoseHydroxy group
A propiophenone derivative of the formula (I): wherein OX is a hydroxy group which may optionally be protected, Y is a lower alkyl group, and Z is a beta -D-glucopyranosyl group wherein one or more hydroxy groups may optionally be protected, or a pharmaceutically acceptable salt thereof. Said compounds have excellent hypoglycemic activity so that they are useful in the prophylaxis or treatment of diabetes.
Owner:MITSUBISHI TANABE PHARMA CORP
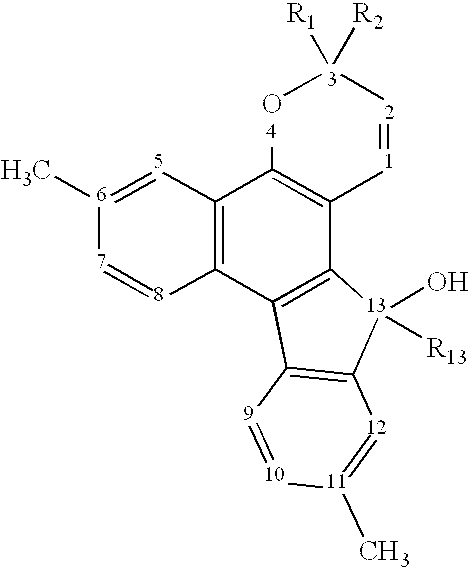
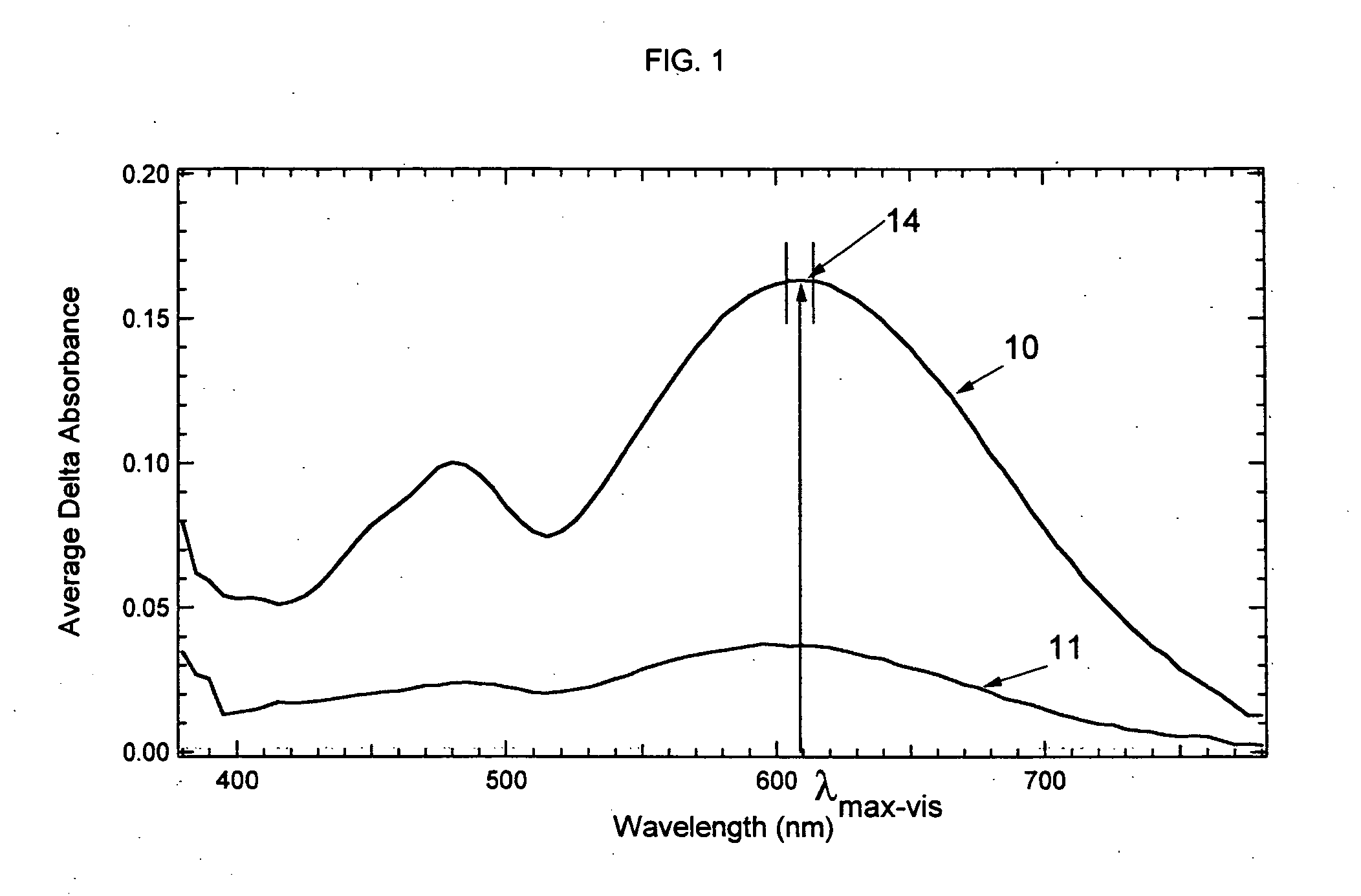
Photochromic materials demonstrating improved fade rates
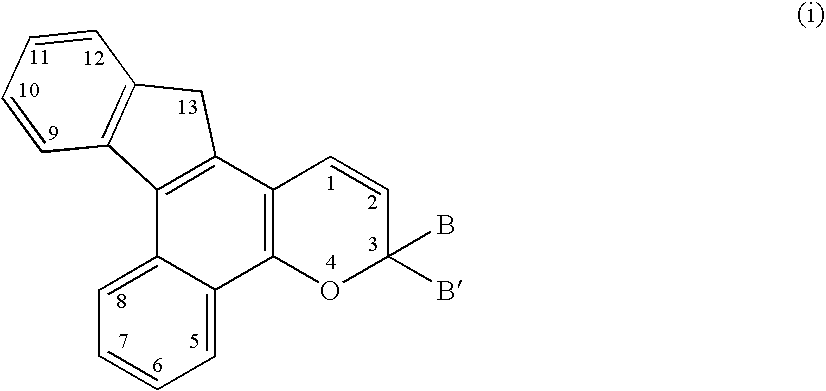
Various photochromic materials are provided that are essentially free of polymerizable unsaturated groups, and comprise: a) an indeno[2′,3′:3,4]naphtho[1,2-b]pyran; and b) an electron-withdrawing, non-conjugating group bonded at the 11-position of the indeno[2′,3′:3,4]naphtho[1,2-b]pyran. Alternative embodiments include various substituents at other positions of the indeno[2′,3′:3,4]naphtho[1,2-b]pyran. Also provided are photochromic articles including a substrate and one of the above photochromic materials, in contact with at least a portion of the substrate.
Owner:TRANSITIONS OPTICAL INC +1
Diaryl-2H-naphthopyrans
InactiveUS6036890AHigh yieldOrganic chemistrySpiro-condensed pyran compound compositionsRoom temperatureEye lens
Photochromic diaryl-2H-naphthopyran structures, which dye photochromically well at room temperature, lighten up quickly when incorporated into ophthalmic lenses, possess longevity, and due to their spacial structures, have a reduced tendency of the molecules to migrate in plastics.
Owner:OPTISCHE WERKE G RODENSTOCK
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
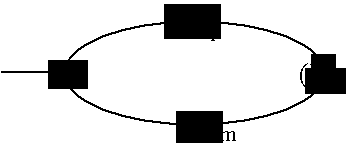
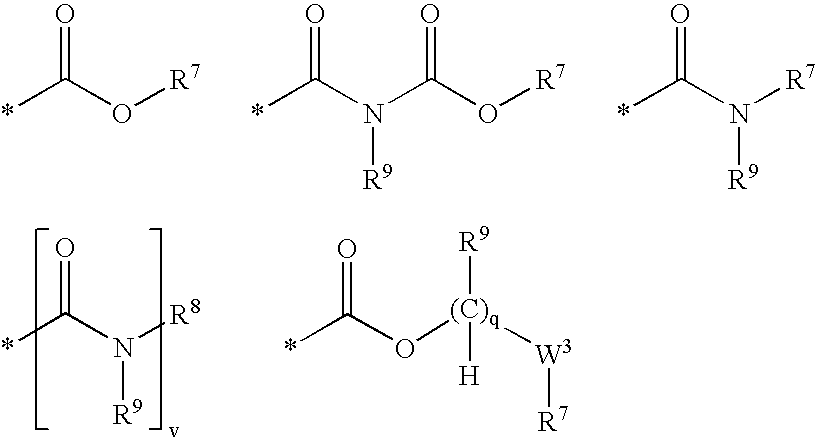
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments
The invention relates to crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, to a method for the preparation thereof, as well as to the use thereof for preparing medicaments.
Owner:BOEHRINGER INGELHEIM INT GMBH
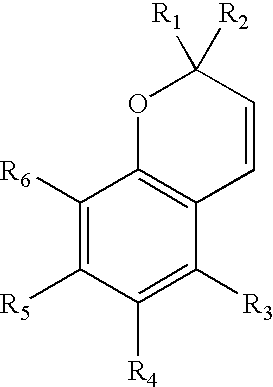
Photochromic naphthopyran compounds: compositions and articles containing those naphthopyran compounds
InactiveUS7008568B2High sensitivity to solar radiationEliminate needOrganic chemistryDiffusing elementsCompound aSulfur
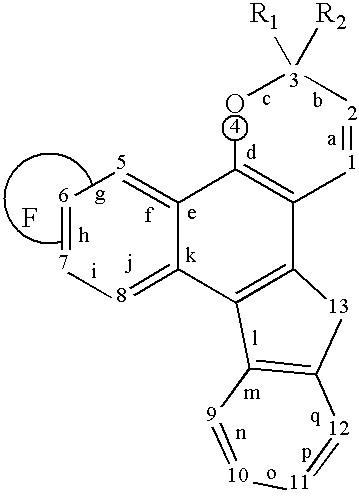
A photochromic naphthopyran displays good color distribution when the naphthopyran has a central nucleus of the formula: wherein F is a 5-member, 6-member, or 7-member heterocyclic ring group having only one heteroatom, the heteroatom selected from the group consisting of oxygen, sulfur, and nitrogen, the 2,3 or 3,2 positions of the heterocyclic ring fused to the g, h, or i side;R1 and R2 are the atoms or groups providing photochromic properties to the naphthopyran.
Owner:HOYA OPTICAL LABS OF AMERICA INC
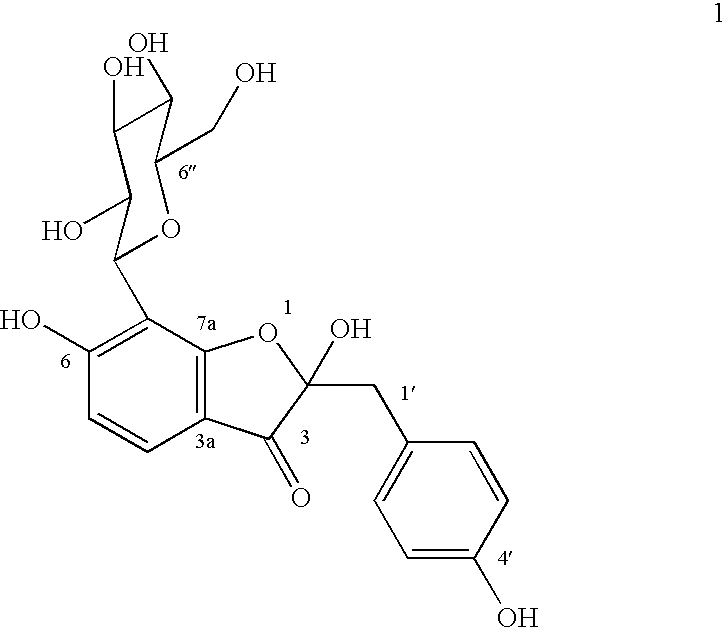
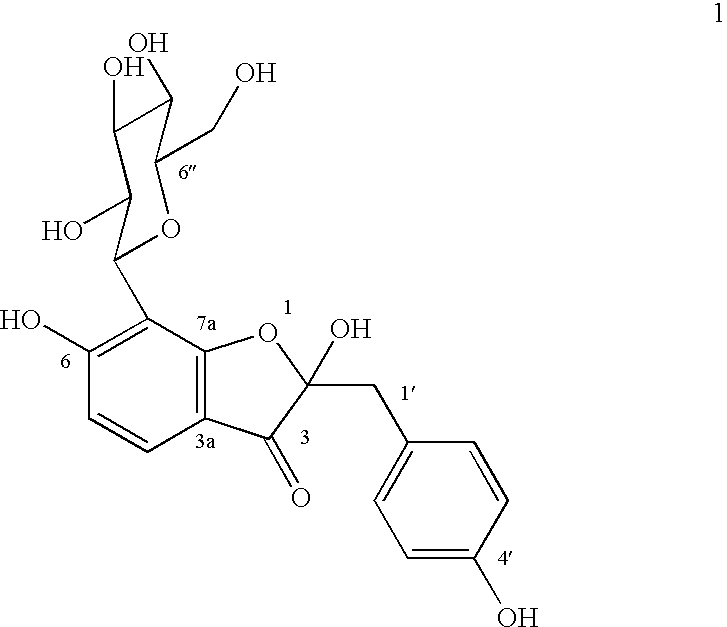
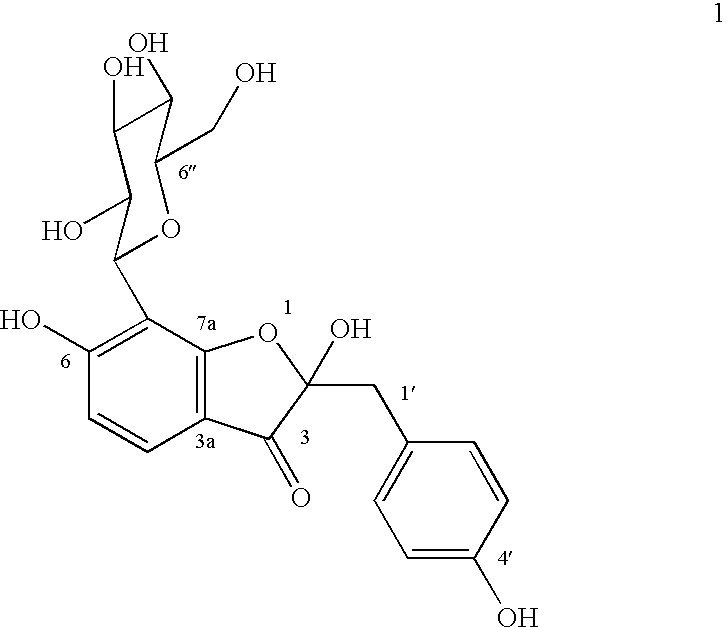
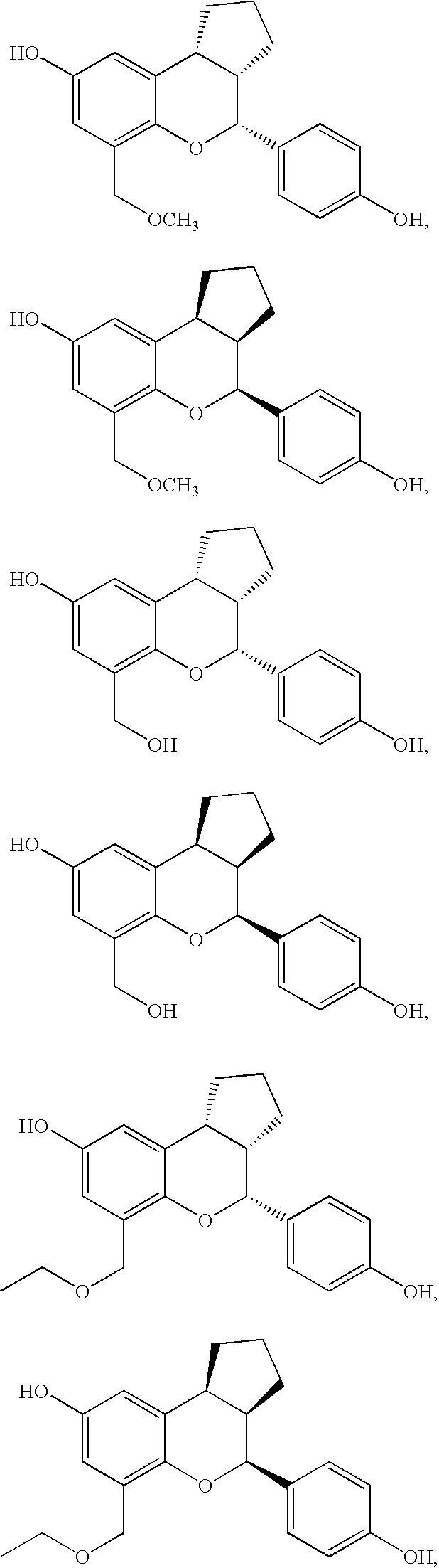
Glucopyranoside and process of isolation thereof from pterocarpus marsupium pharmaceutical composition containing the same and use thereof
A novel glucopyranoside, 2,6-dihydroxy-2-(P-hydroxybenzyl)-3(2H) benzofuran-7-C-beta-D-glucopyranoside of the formula 1isolated from Pterocarpus marsupium and to a process for the isolation thereof is disclosed. The invention also relates to a pharmaceutical composition containing 2,6-dihydroxy-2-(P-hydroxybenzyl)-3(2H)benzofuran-7-C-beta-D-glucopyranoside and to method for the treatment of diabetes using said compound.
Owner:COUNCIL OF SCI & IND RES
Salts and crystalline forms of an apoptosis-inducing agent
Salts and crystalline forms of 4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-({3-nitro-4-[(tetrahydro-2H-pyran-4-ylmethyl)amino]phenyl}-sulfonyl)-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide are suitable active pharmaceutical ingredients for pharmaceutical compositions useful in treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBVIE INC
Tissue degeneration protection
InactiveUS20100099640A1Improve solubilityHigh dissolution rateBiocideSugar derivativesPharmaceutical medicinePharmacology
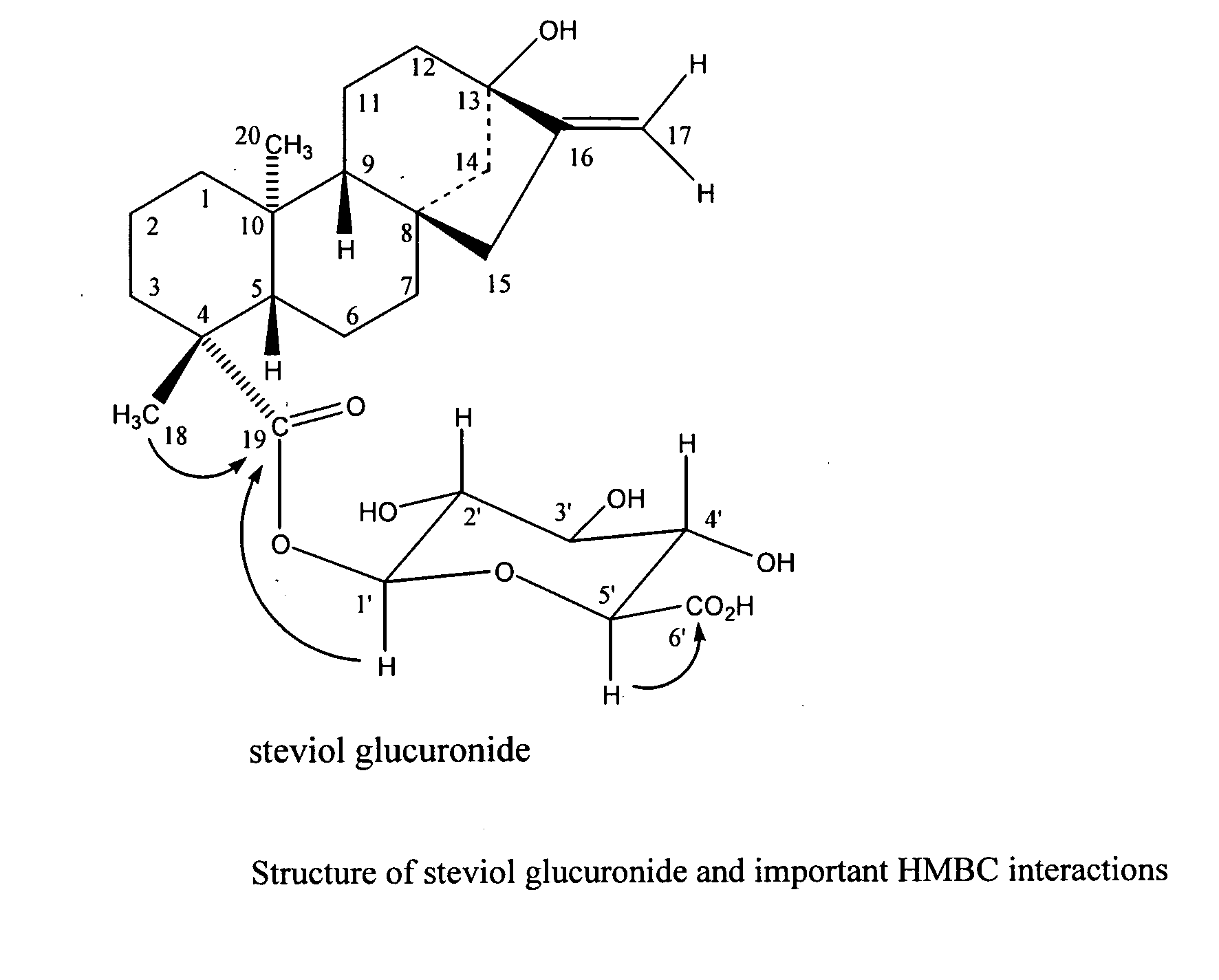
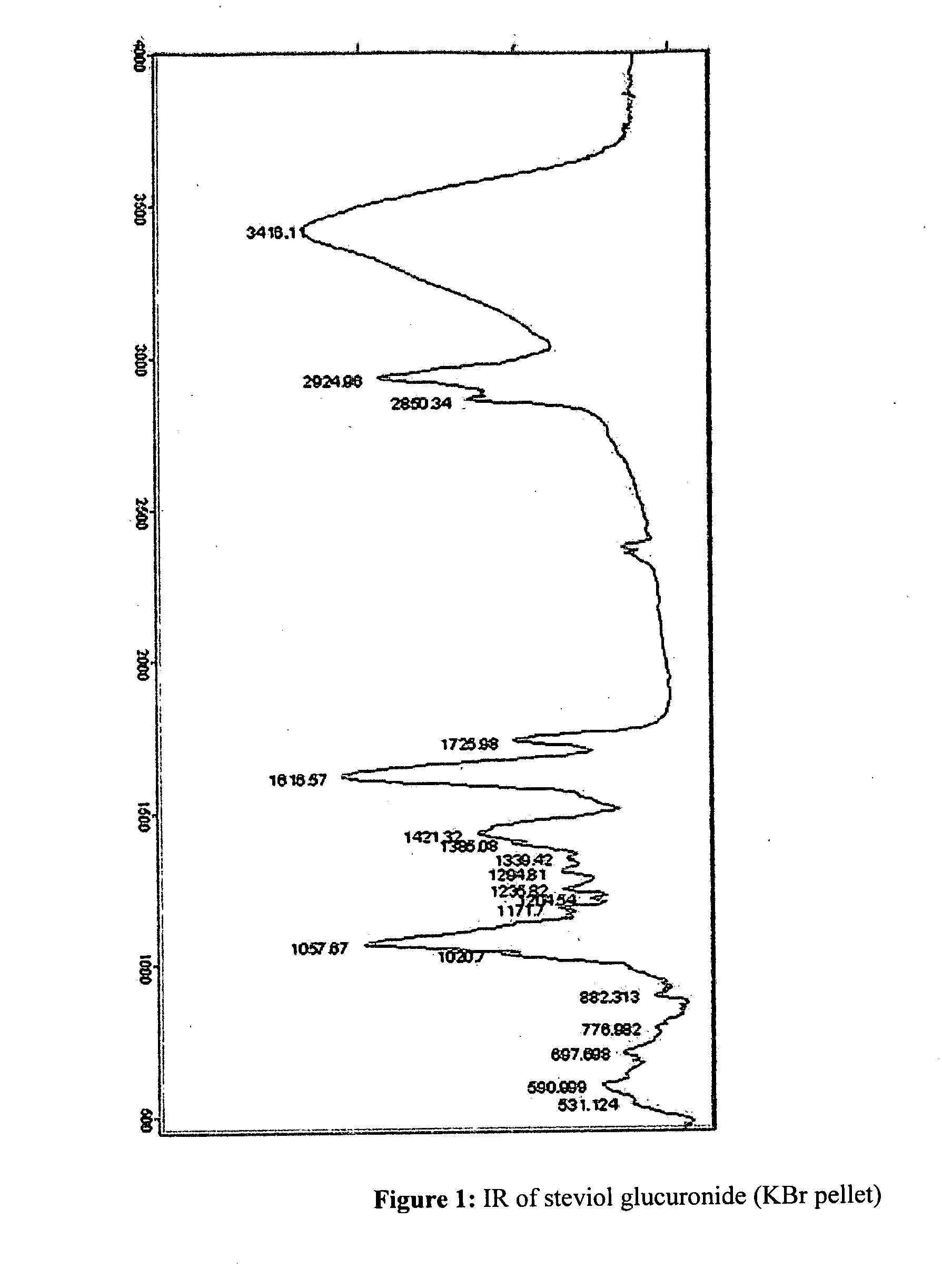
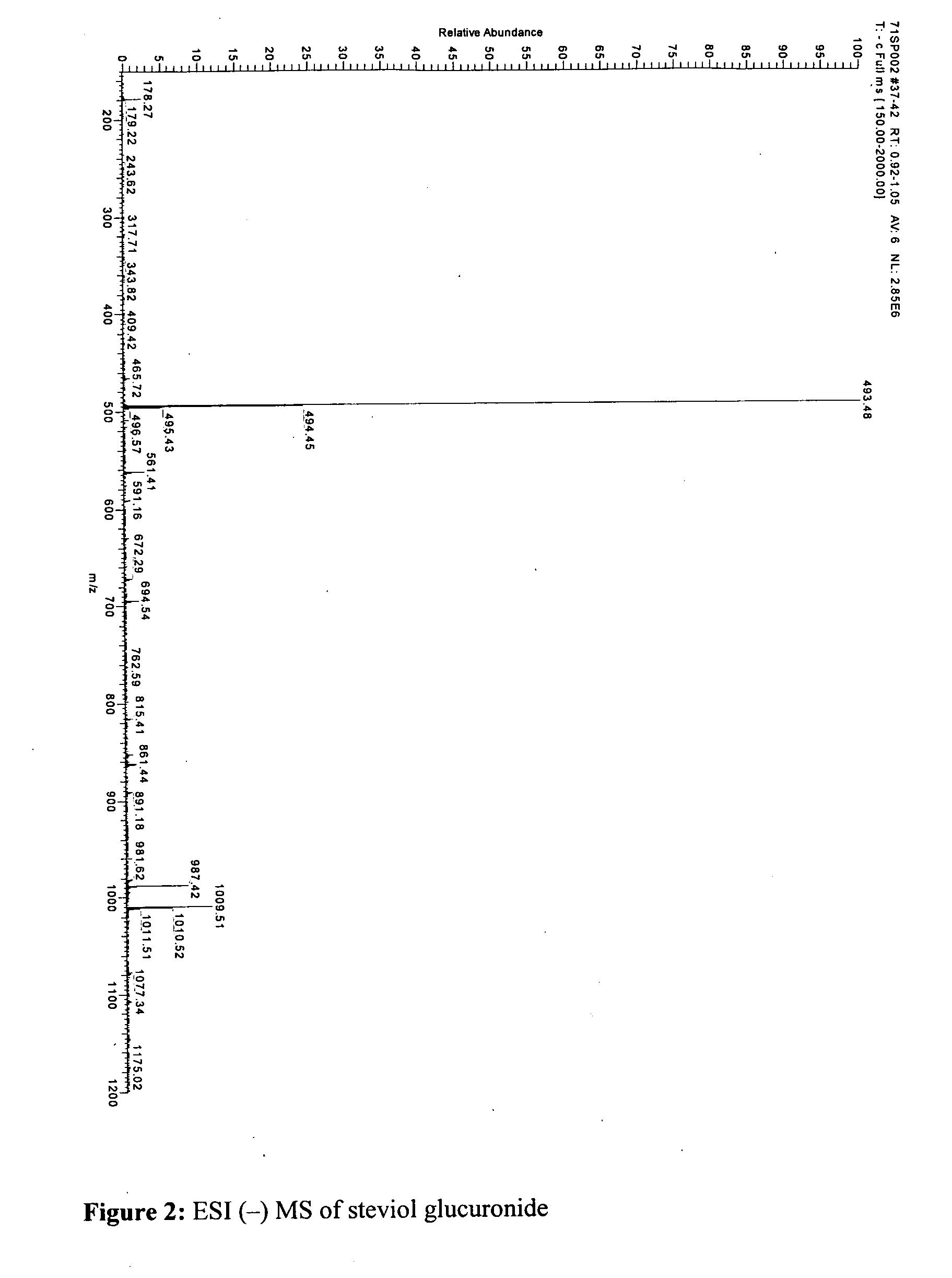
The present invention provides isolated or essentially pure diterpenoic tetrahydropyran, such as steviol-19-glucuronide, steviol, stevioside and rebaudioside processes for obtaining the same and methods for obtaining stable pharmaceutically acceptable salts of the same for use of such compounds or compositions in a treatment of cardiovascular disorders or vascular disease or for the manufacture of medicaments to treat a condition of a cardiovascular disorder or vascular disease.
Owner:GEUNS JOANNES +1
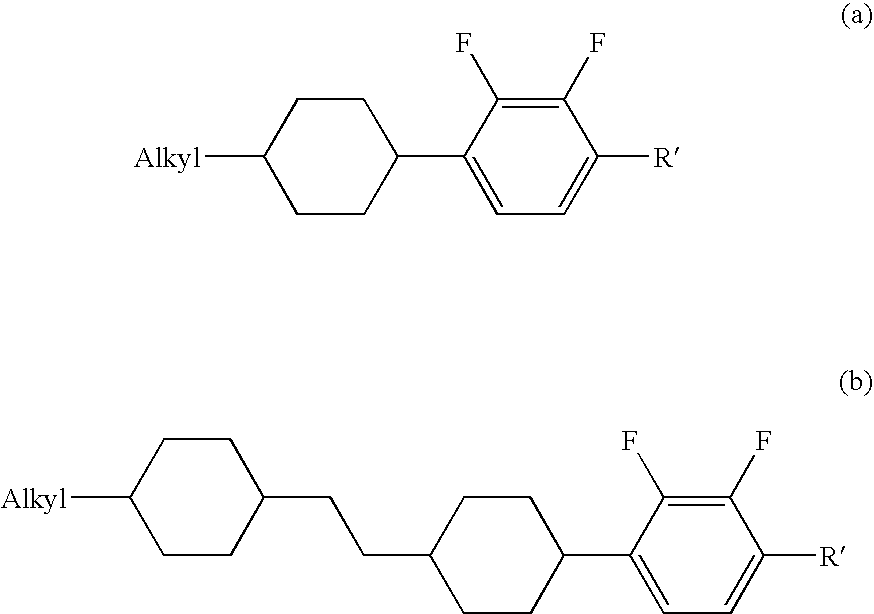
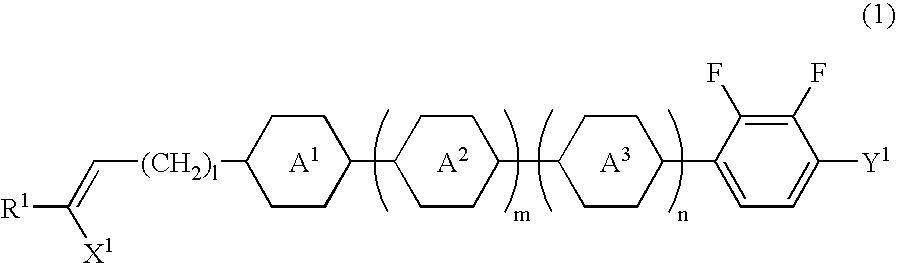
Alkenyl compound having a negative delta epsilon value, liquid crystal composition, and liquid crystal display device
InactiveUS20040065866A1Low viscosityImprove solubilityLiquid crystal compositionsSilicon organic compoundsLiquid crystallineDithiane
Owner:JNC CORP
Photochromic compounds and compositions
Described herein are compounds generally comprising an indeno[2′,3′:3,4]naptho[1,2-b]pyran structure. Such compounds may be useful for their photochromic properties, and be used in certain photochromic compositions. Such compositions may further comprise other photochromic compositions and / or materials. Additionally, such compounds and / or compositions may be suitable for preparing certain photochromic articles. Also described herein are methods for preparing certain photochromic compounds, compositions, and articles.
Owner:TRANSITIONS OPTICAL INC
17-hydroxy c27 steroid compound and its synthesis and use
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Photochromic indeno-fused naphthopyrans
Owner:TRANSITIONS OPTICAL INC
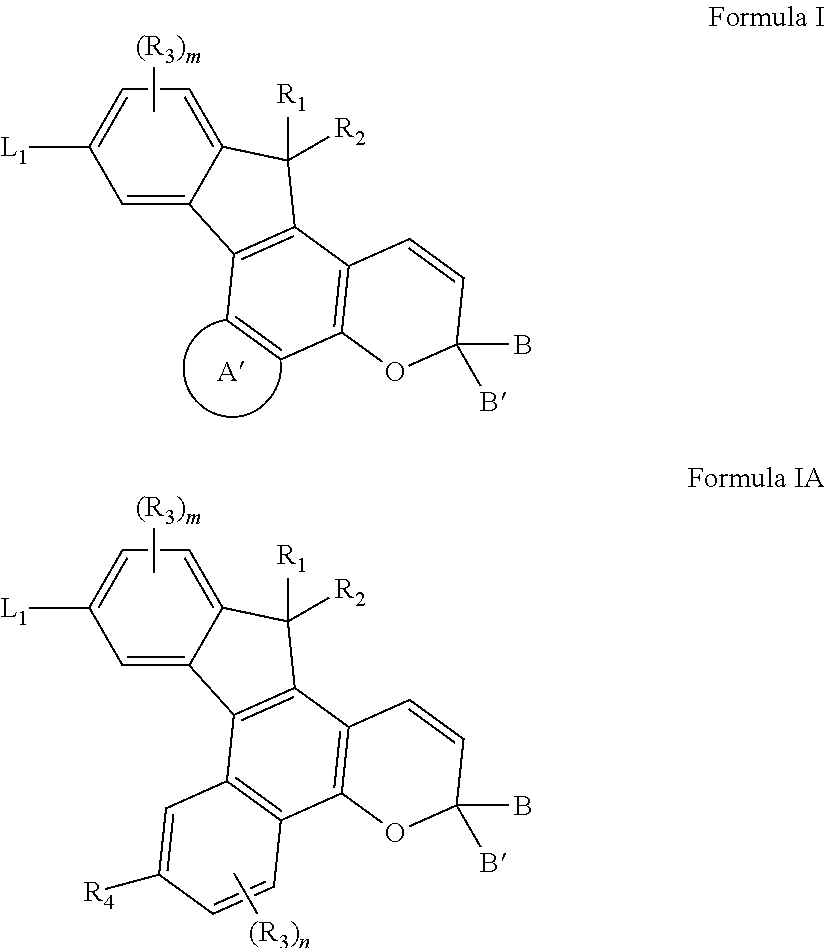
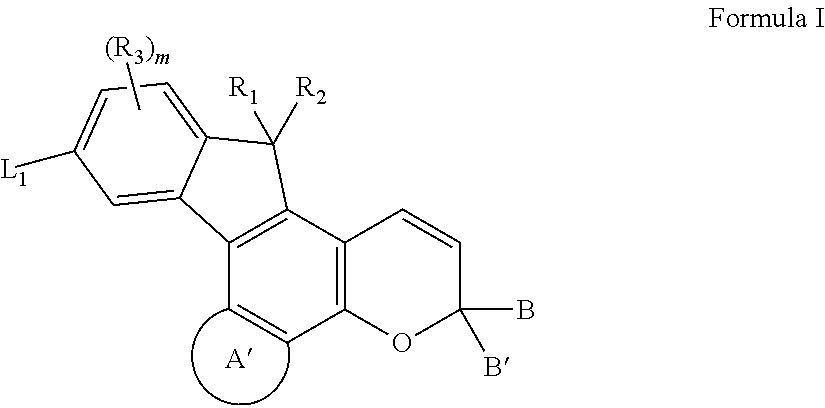
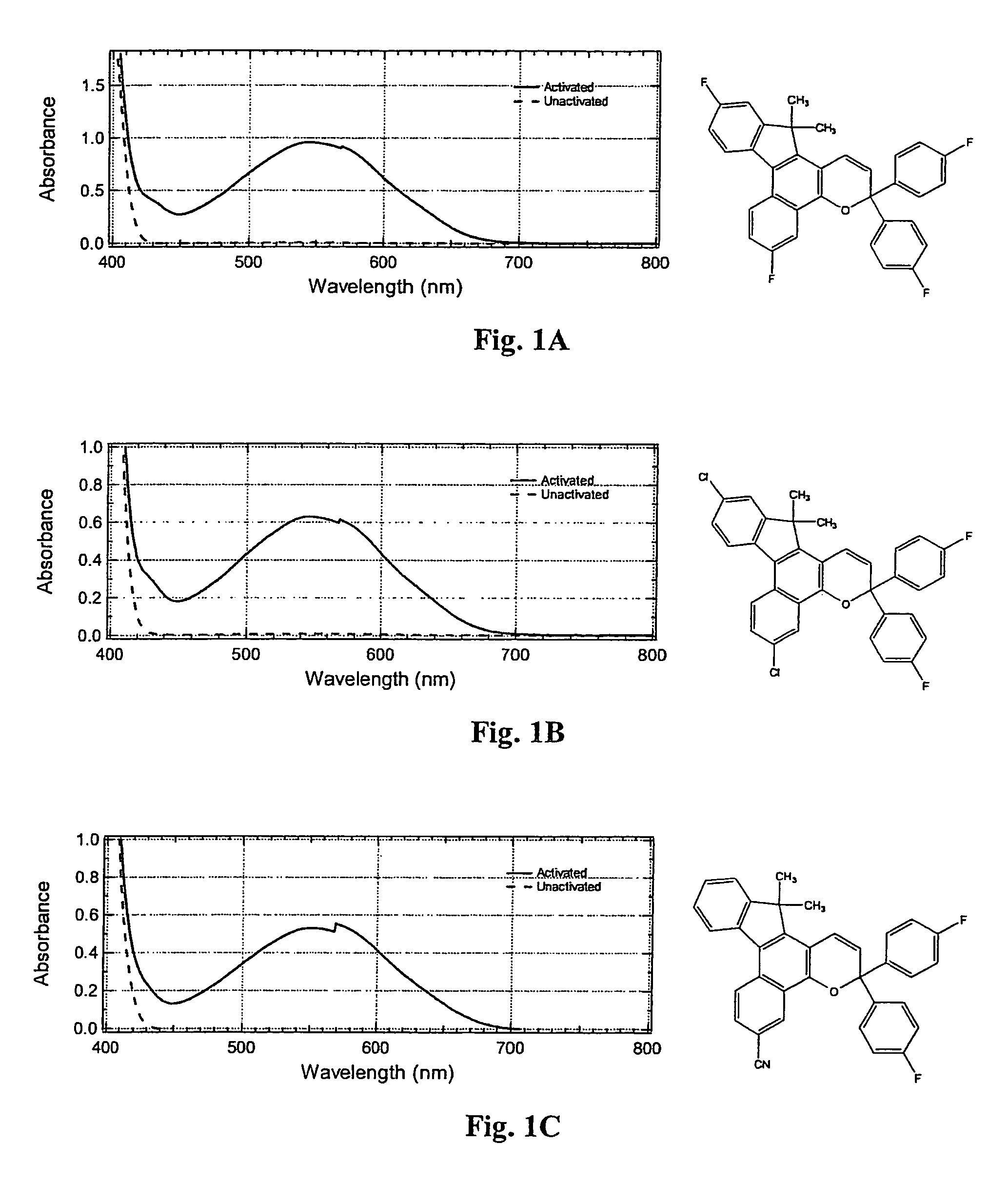
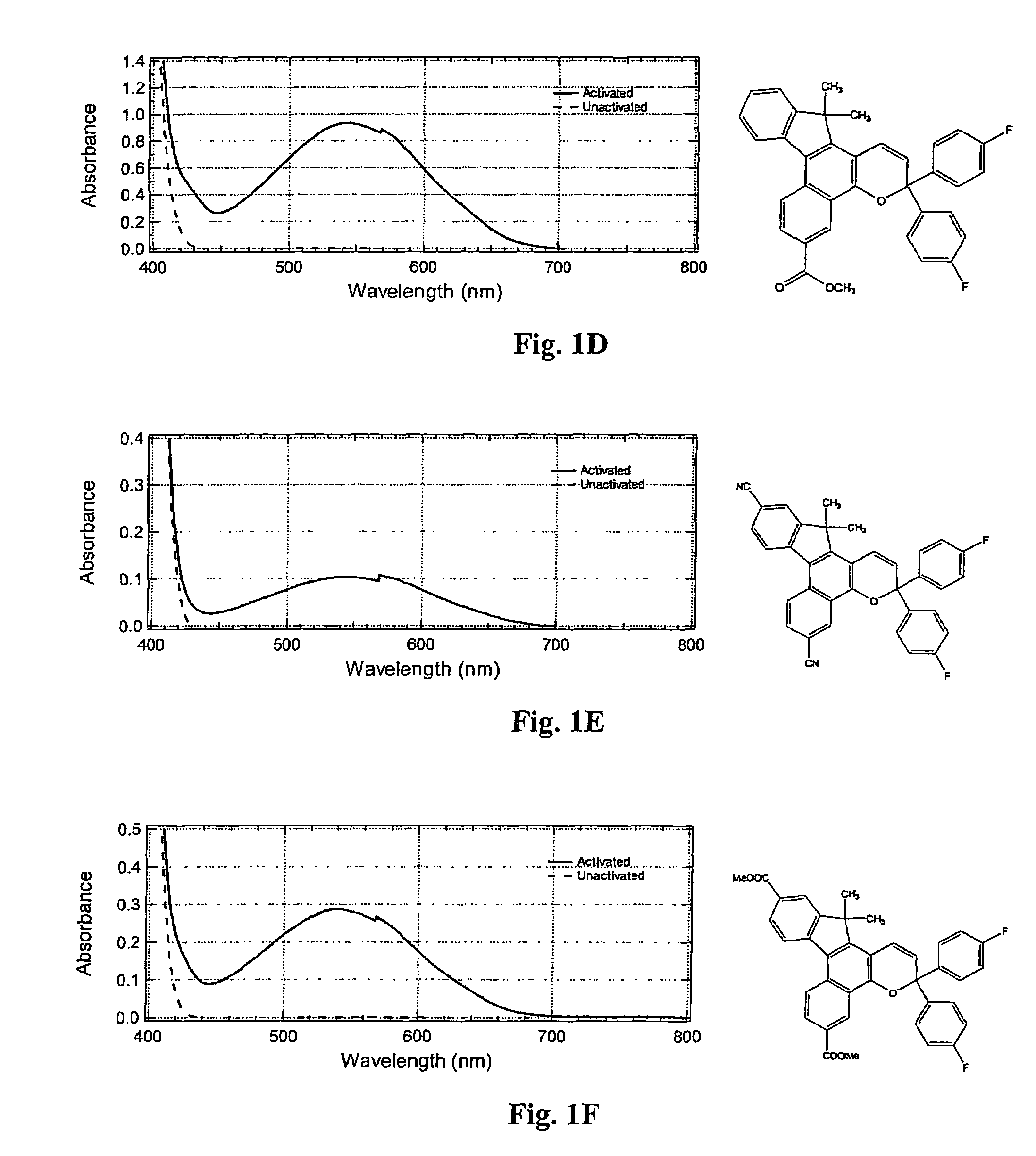
Photochromic materials having electron-withdrawing substituents
Photochromic materials comprising indeno-fused naphthopyrans having a first electron-withdrawing substituent and, in certain non-limiting embodiments, a second electron-withdrawing substituent are disclosed. The photochromic materials according to the various embodiments may display faster fade rates, bathochromic shift, and higher performance ratings compared to comparable indeno-fused naphthopyrans without the electron-withdrawing substituents. Photochromic compositions and articles, such as optical elements, incorporating the photochromic materials are also disclosed.
Owner:TRANSITIONS OPTICAL INC
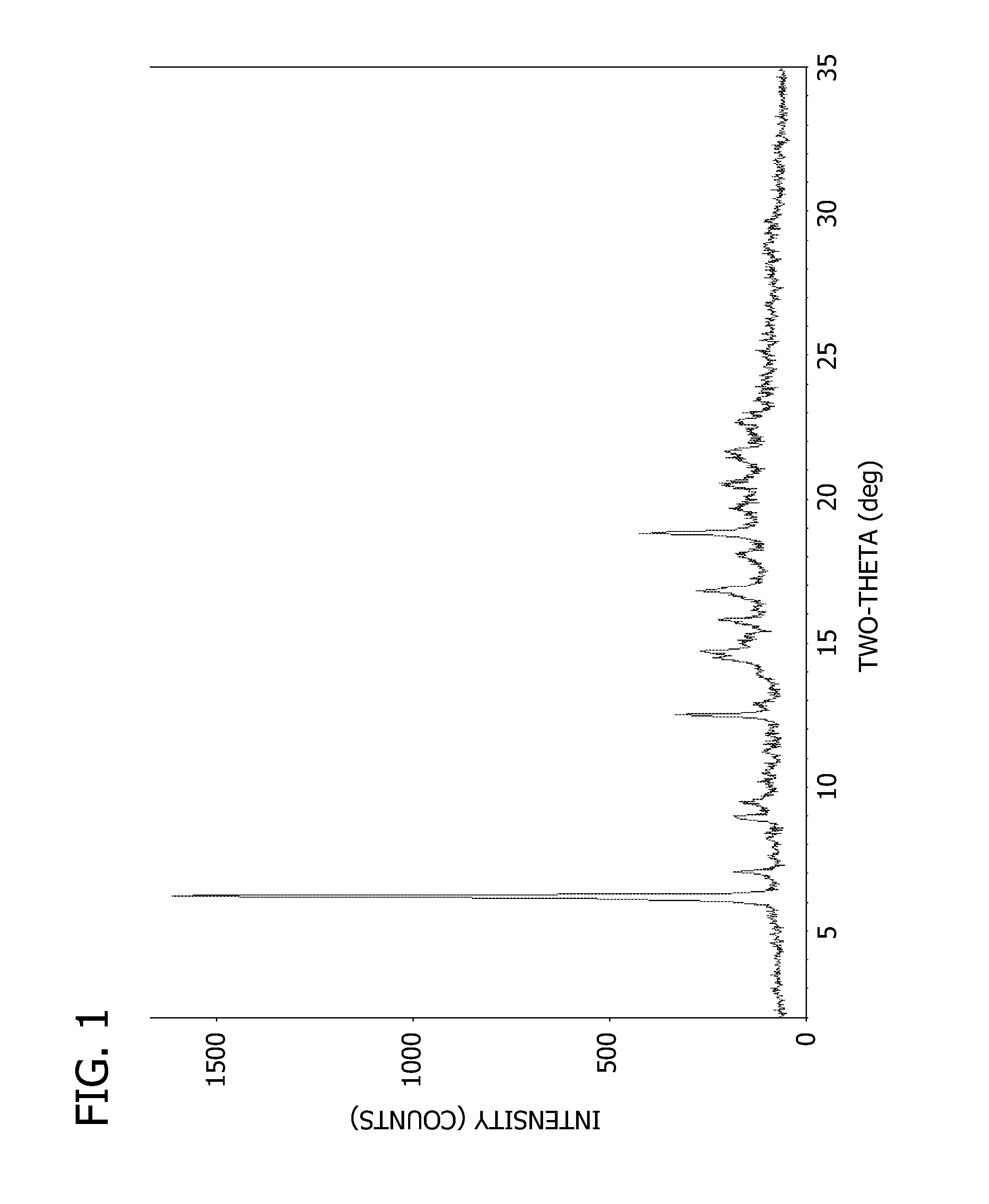
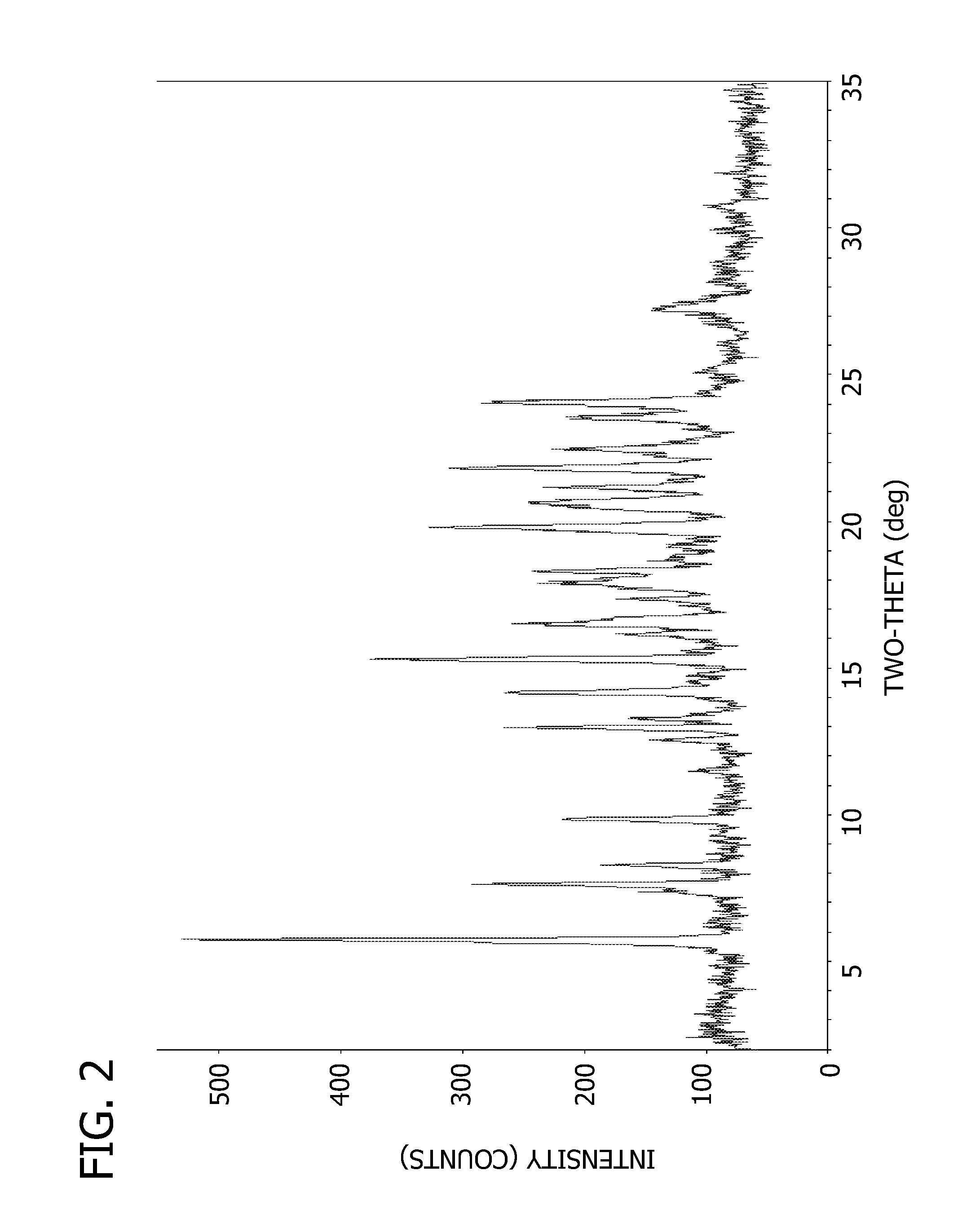
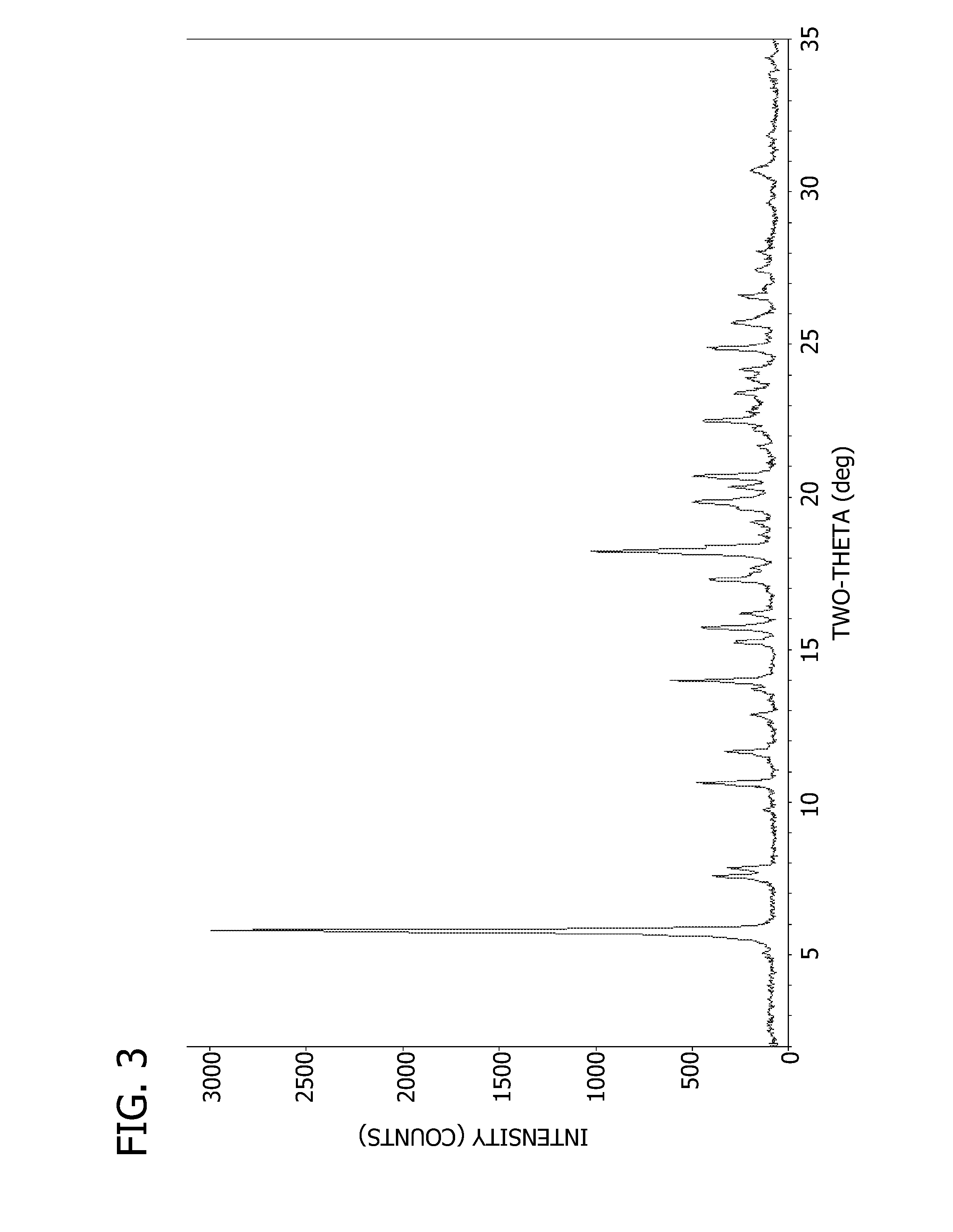
Pharmaceutical Dosage Forms Comprising 6'-Fluoro-(N-Methyl- or N,N-Dimethyl-)-4-Phenyl-4',9'-Dihydro-3'H-Spiro[Cyclohexane-1,1'-Pyrano[3,4,b]Indol]-4-Amine
ActiveUS20150111939A1Extension of timeModifies the physical and chemical propertiesBiocideNervous disorderPhenyl groupPyran
A pharmaceutical dosage form for administration twice daily, once daily or less frequently, which contains 6′-fluoro-(N-methyl- or N,N-dimethyl)-4-phenyl-4′,9′-dihydro-3′H-spiro[cyclohexane-1,1′-pyrano[3,4,b]indol]-4-amine or a physiologically acceptable salt thereof.
Owner:PARK THERAPEUTICS INC
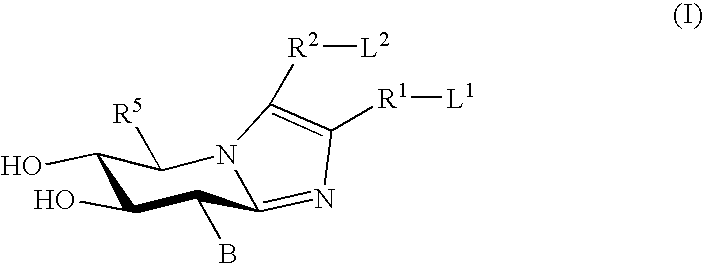
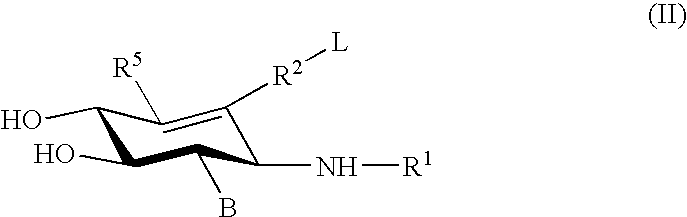
Glucoimidazole and polyhydroxycyclohexenyl amine derivatives to treat gaucher disease
InactiveUS20050137223A1Stabilize GCaseEffective and stableBiocideNervous disorderPyranoseActive site
Owner:AMICUS THERAPEUTICS INC
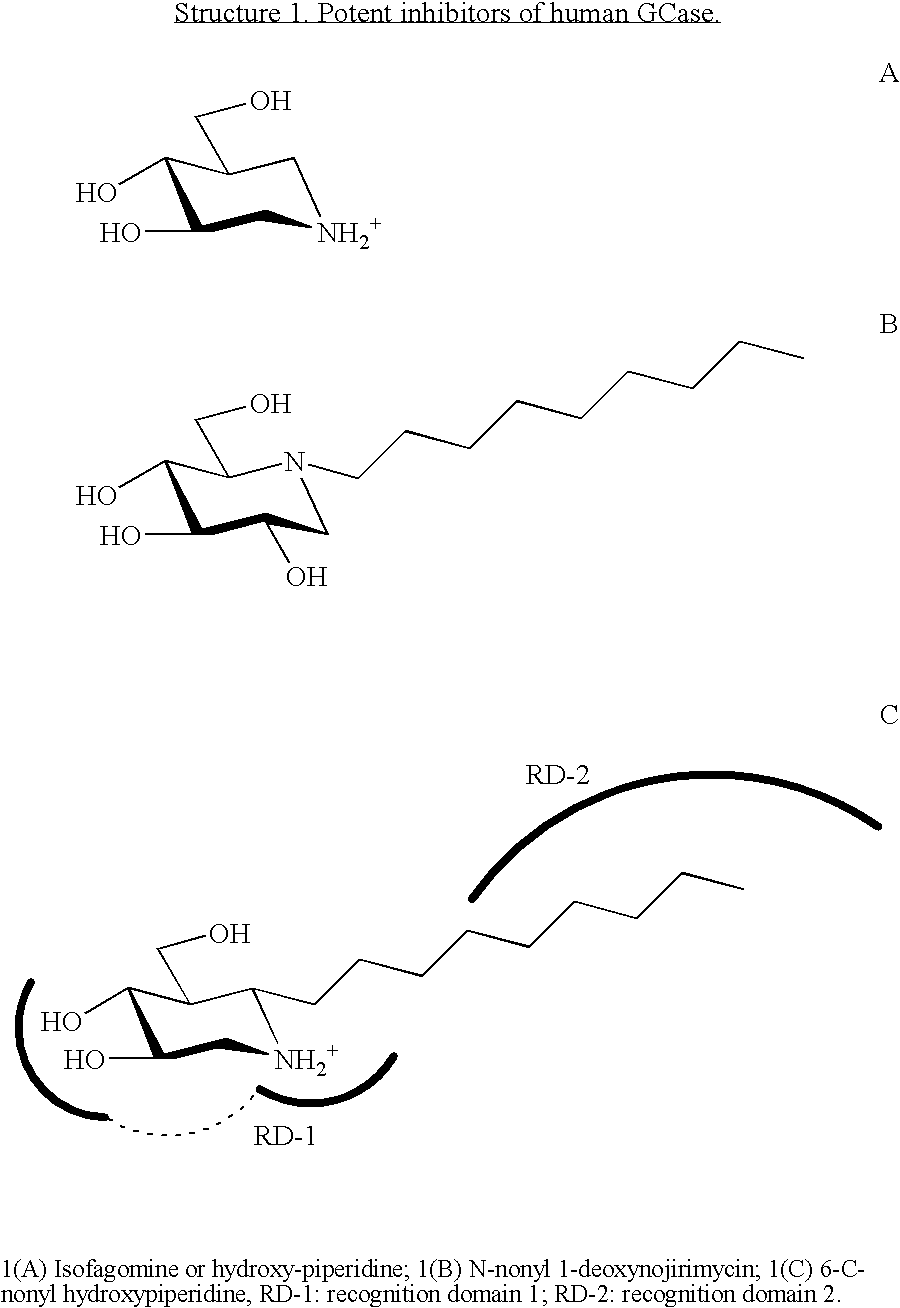
Hydroxy piperidine derivatives to treat gaucher disease
The present invention provides novel hydroxy piperidine (HP) derivatives having (i) a positive charge at the position corresponding to the anomeric position of a pyranose ring; (ii) a short, flexible linker emanating from the corresponding position of the ring oxygen in a pyranose; and (iii) a lipophilic moiety connected to the linker and pharmaceutically acceptable salts thereof. The linker can be absent if the lipophilic moiety corresponds to a hydrocarbon chain with a linear length of 6 or more carbons. The present invention further provides a method for treating individuals having Gaucher disease by administering the novel HP derivative as “active-site specific chaperones” for the mutant glucocerebrosidase associated with the disease.
Owner:AMICUS THERAPEUTICS INC
Salts and crystalline forms of an apoptosis-inducing agent
Salts and crystalline forms of 4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-({3-nitro-4-[(tetrahydro-2H-pyran-4-ylmethyl)amino]phenyl}-sulfonyl)-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide are suitable active pharmaceutical ingredients for pharmaceutical compositions useful in treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBVIE INC
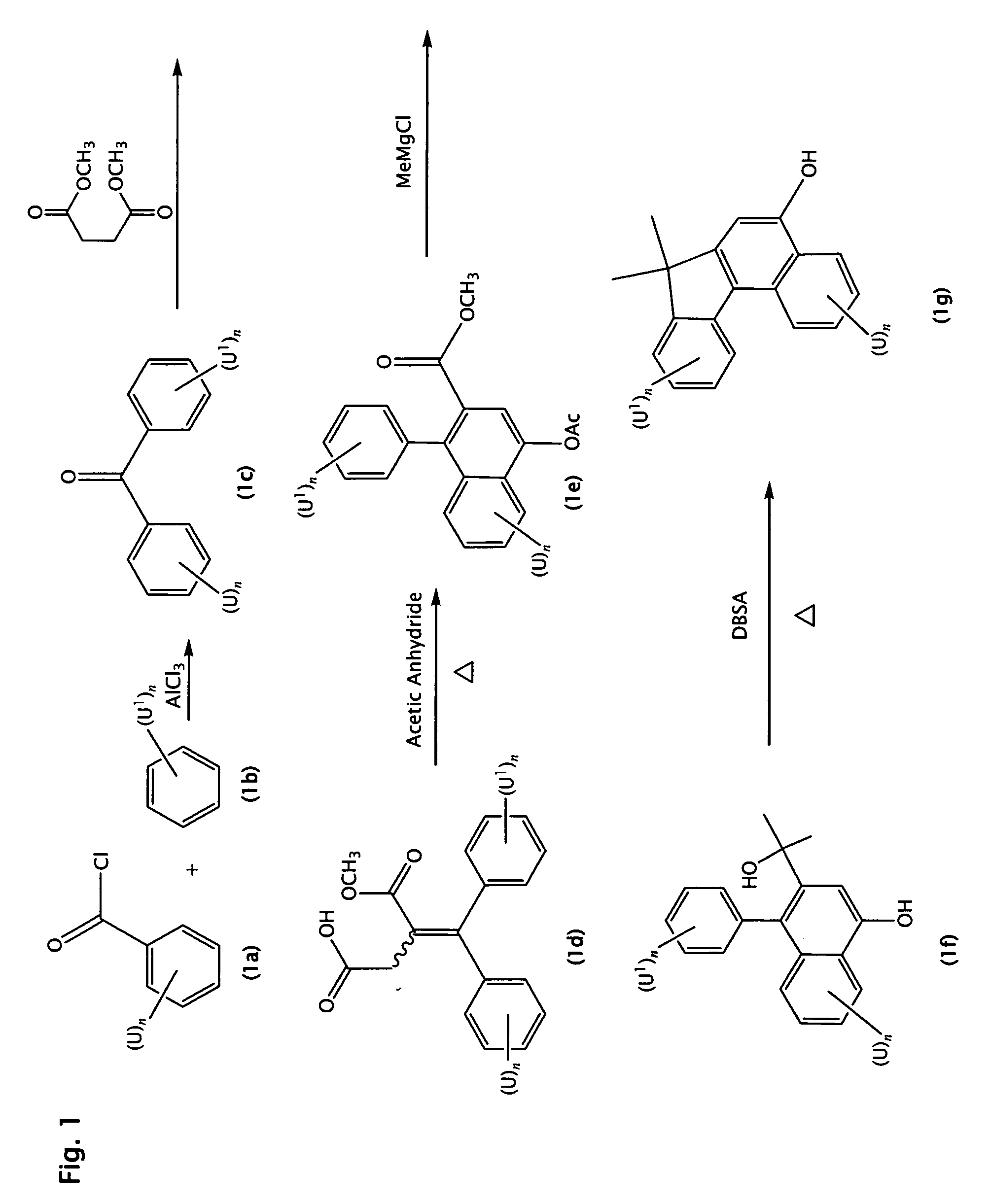
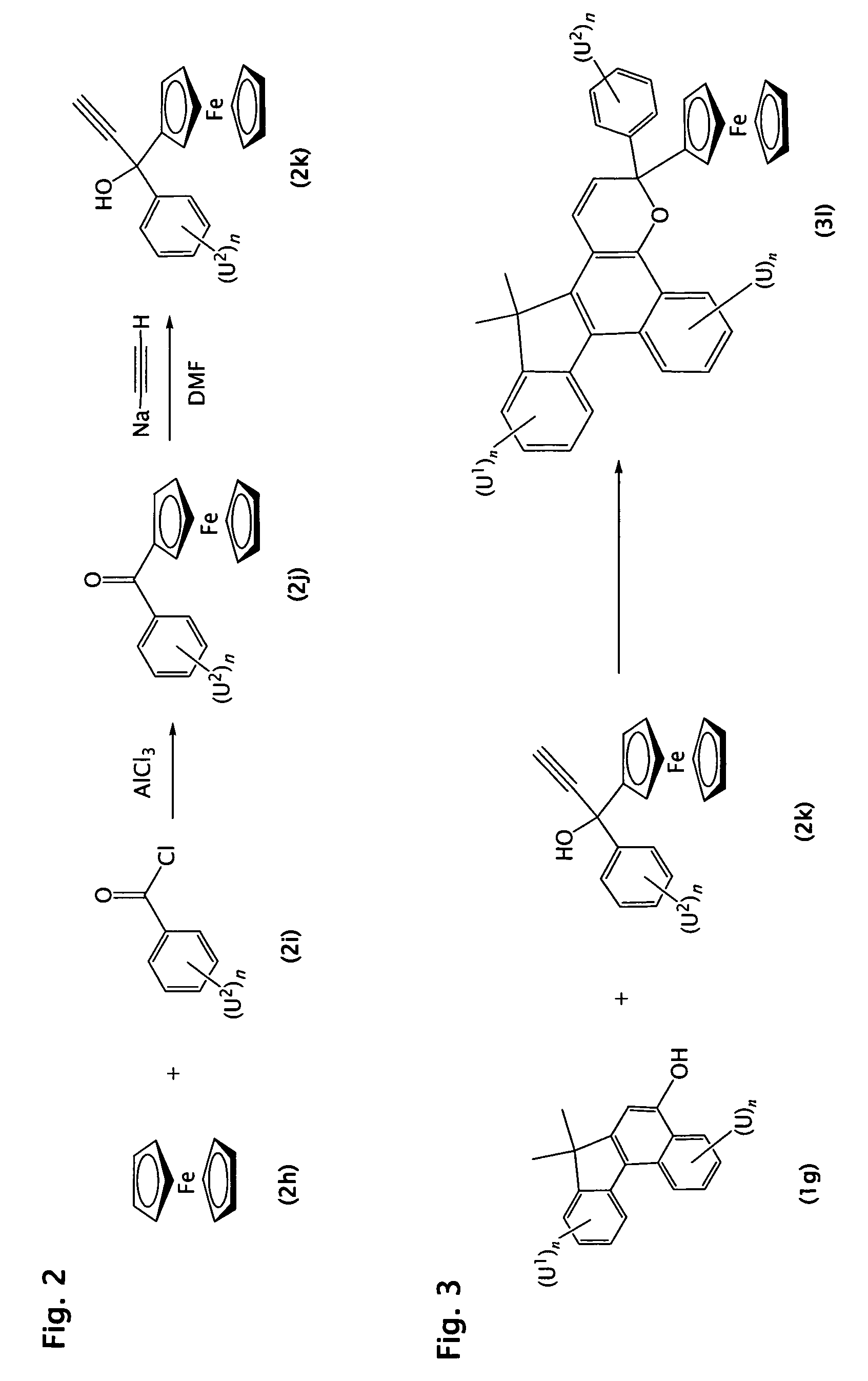
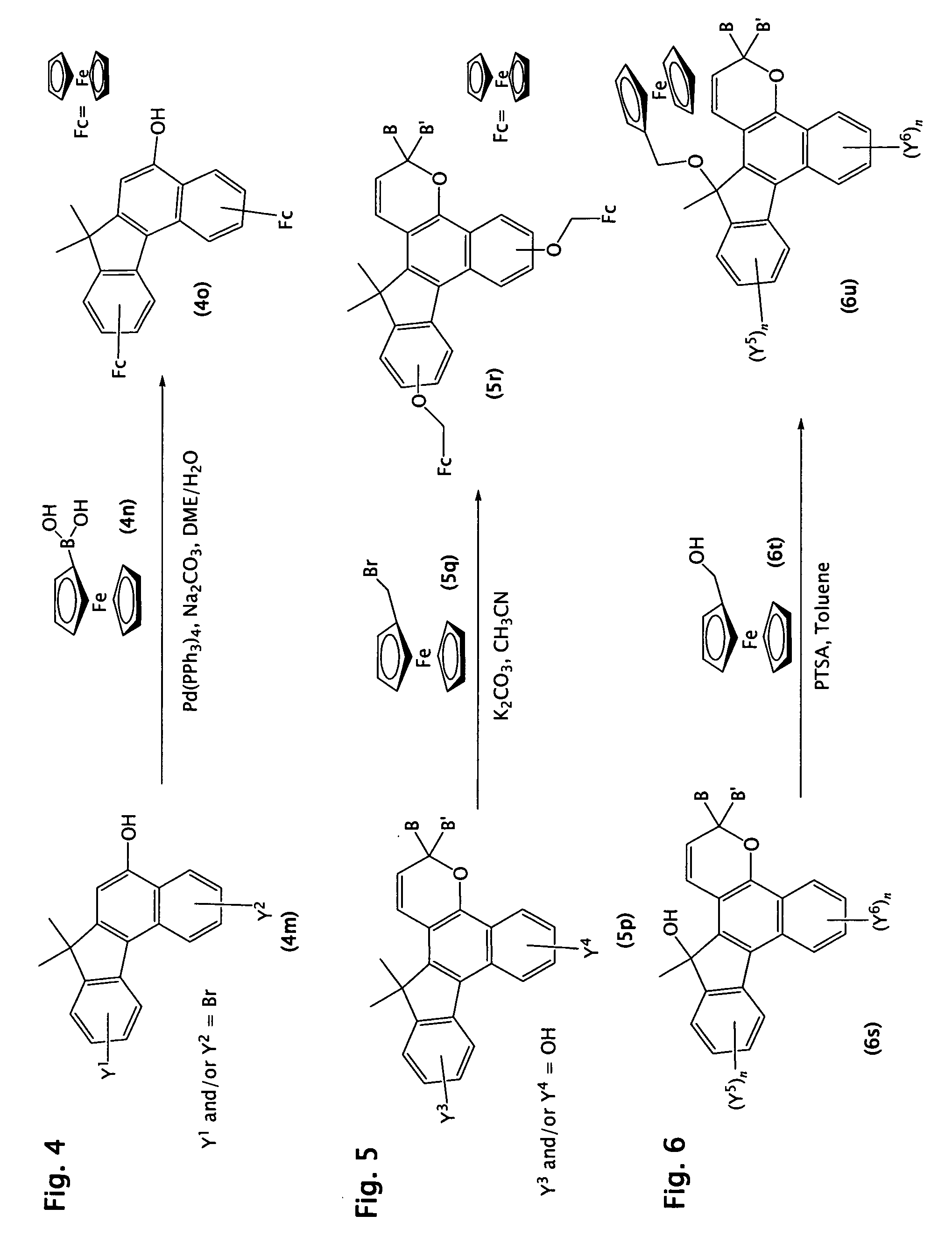
Photochromic materials comprising metallocenyl groups
Various non-limiting embodiments of the present invention relate to photochromic materials having a metallocenyl group. More particularly, various non-limiting embodiments disclosed herein provide photochromic materials including an indeno-fused naphthopyran, such as an indeno[2′,3′:3,4]naphtho[1,2-b]pyran, and a metallocenyl group bonded to at least one available position on the indeno-fused naphthopyran. Other non-limiting embodiments disclosed herein provide photochromic composition and photochromic articles, such as but not limited to ophthalmic lens, which include the disclosed photochromic materials and method of making the same.
Owner:TRANSITIONS OPTICAL INC
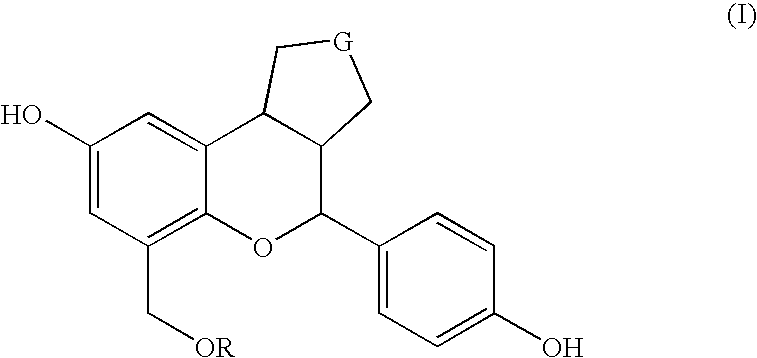
Substituted benzopyrans as selective estrogen receptor-beta agonists
The present invention relates to substituted benzopyran derivatives, stereoisomers, and pharmaceutical acceptable salts thereof and processes for the preparation of the same. The compounds of the present invention are useful as Estrogen Receptor ? agonists. Such agonists are useful for the treating Estrogen Receptor ? mediated diseases such as prostate cancer or BPH.
Owner:ELI LILLY & CO
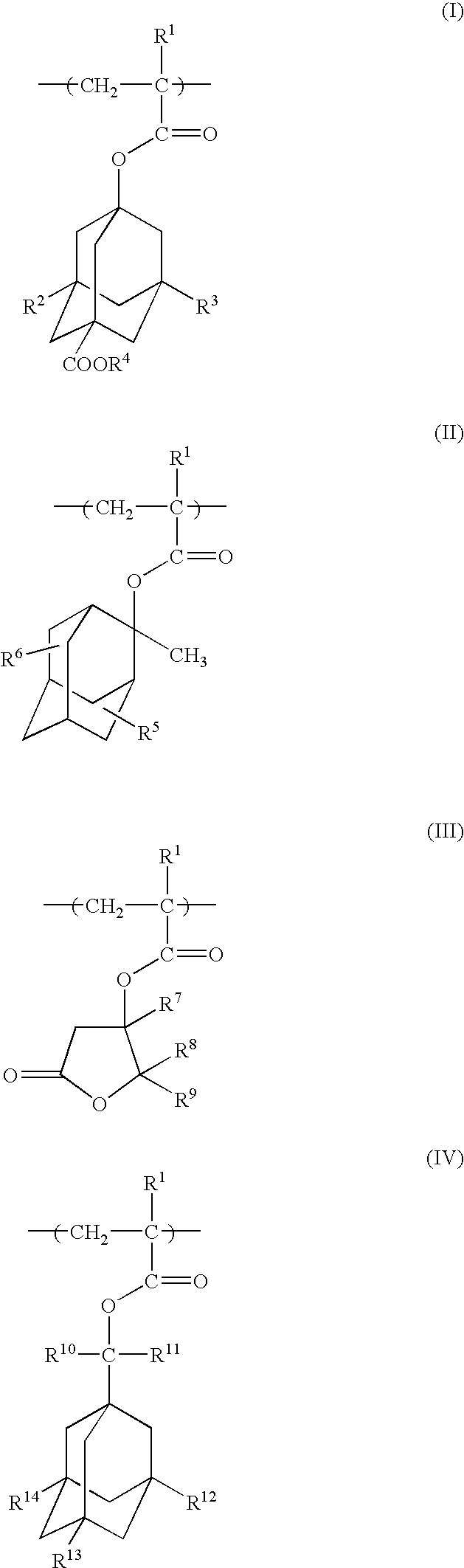
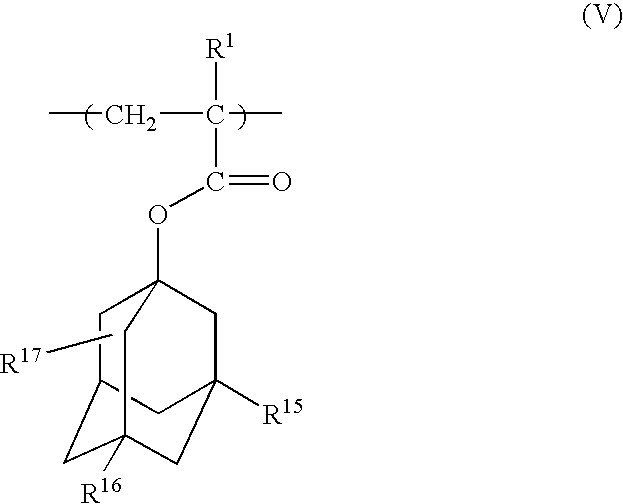
Photoresist polymeric compound and photoresist resin composition
InactiveUS6692889B1Improve corrosion resistanceSatisfactory transparencyPhotosensitive materialsRadiation applicationsSolubilityHydrogen atom
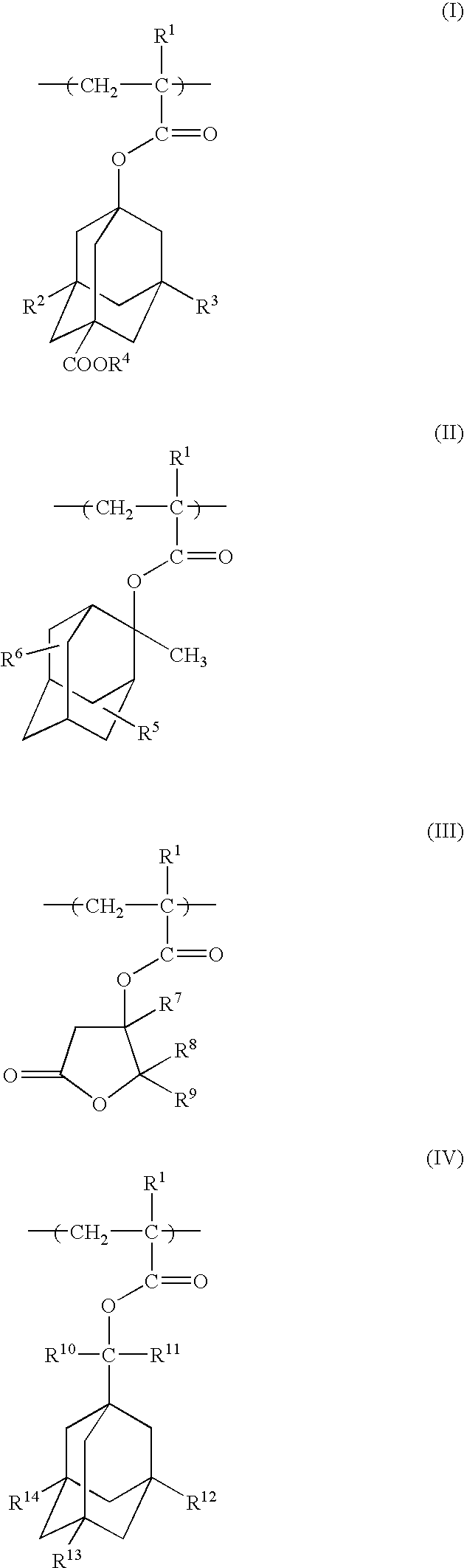
A polymeric compound of the invention includes at least one monomer unit selected from the following formulae (I), (II), (III) and (IV):(wherein R<1 >is a hydrogen atom or a methyl group, R<2 >and R<3 >are each a hydrogen atom, a hydroxyl group or a -COOR<4 >group, where R<4 >is, e.g., a t-butyl group or a 2-tetrahydropyranyl group; R<5 >and R<6 >are each a hydrogen atom, a hydroxyl group or an oxo group; R<7>, R<8 >and R<9 >are each a hydrogen atom or a methyl group; R<10 >and R<11 >are each a hydrocarbon group having 1 to 8 carbon atoms; R<12>, R<13 >and R<14 >are each a hydrogen atom, a hydroxyl group or a methyl group, where if all of R<12 >to R<14 >are each a hydrogen atom or a hydroxyl group, R<10 >and R<11 >are not coincidentally methyl groups) [wherein, when the compound includes, for example, a monomer unit of Formula (III), the compound further includes at least one monomer unit selected from among, for example, a monomer unit represented by the following Formula (V):(wherein R<15 >and R<16 >are each a hydrogen atom, a hydroxyl group or a carboxyl group; R<17 >is a hydroxyl group, an oxo group or a carboxyl group; and R<1 >has the same meaning as defined above)]. This polymeric compound has high etching resistance, as well as satisfactory transparency, alkali-solubility and adhesion, and is therefore useful as a photoresist resin.
Owner:DAICEL CHEM IND LTD
Pyranoindazoles and their use for the treatment of glaucoma
InactiveUS6696476B2Good chemical stabilityOrganic active ingredientsBiocideElevated intraocular pressureTreatment glaucoma
Pyranoindazoles are disclosed. Also disclosed are methods for the lowering and controlling of normal or elevated intraocular pressure as well as a method for the treatment of glaucoma using compositions containing one or more of the compounds of the present invention.
Owner:NOVARTIS AG
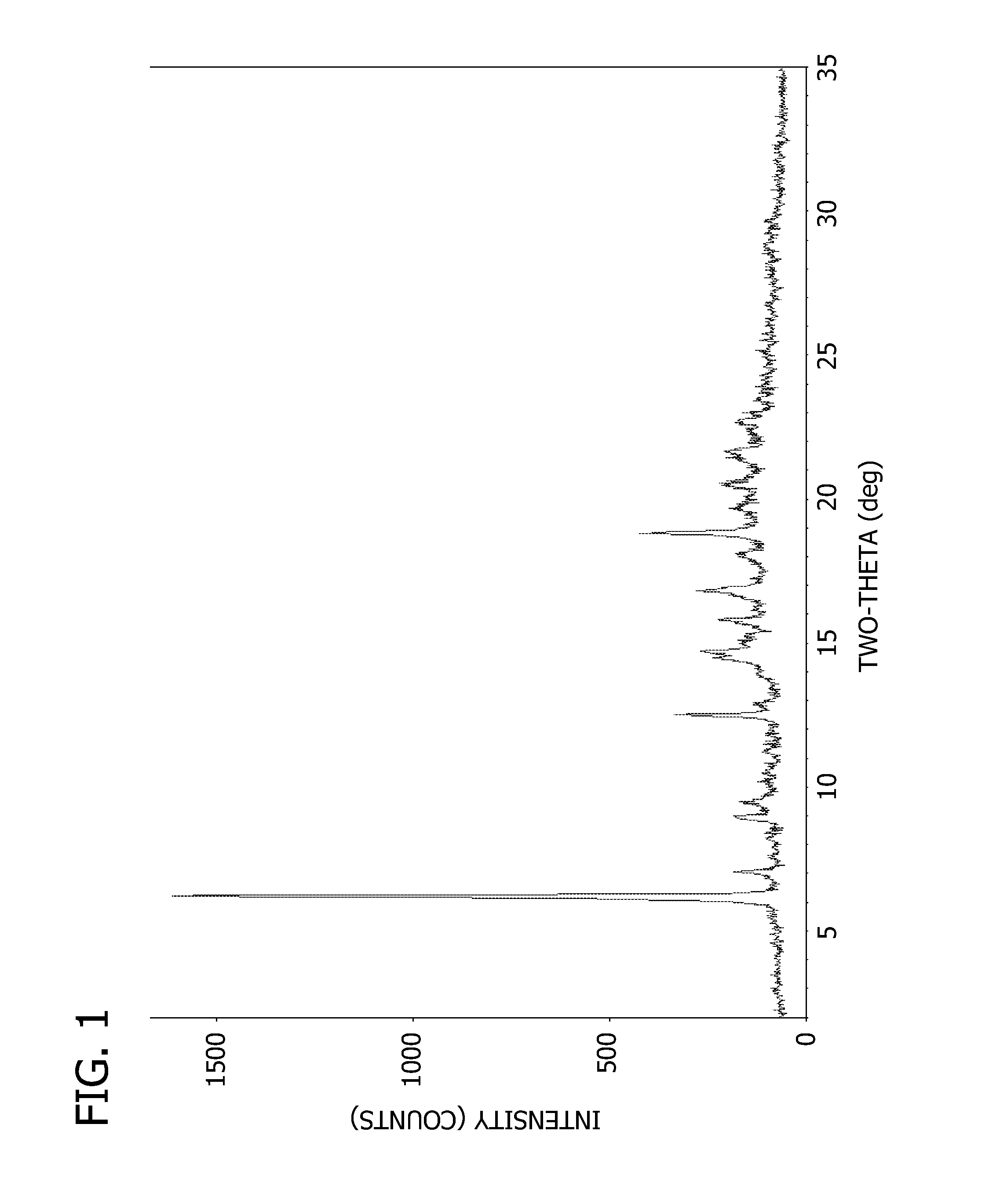
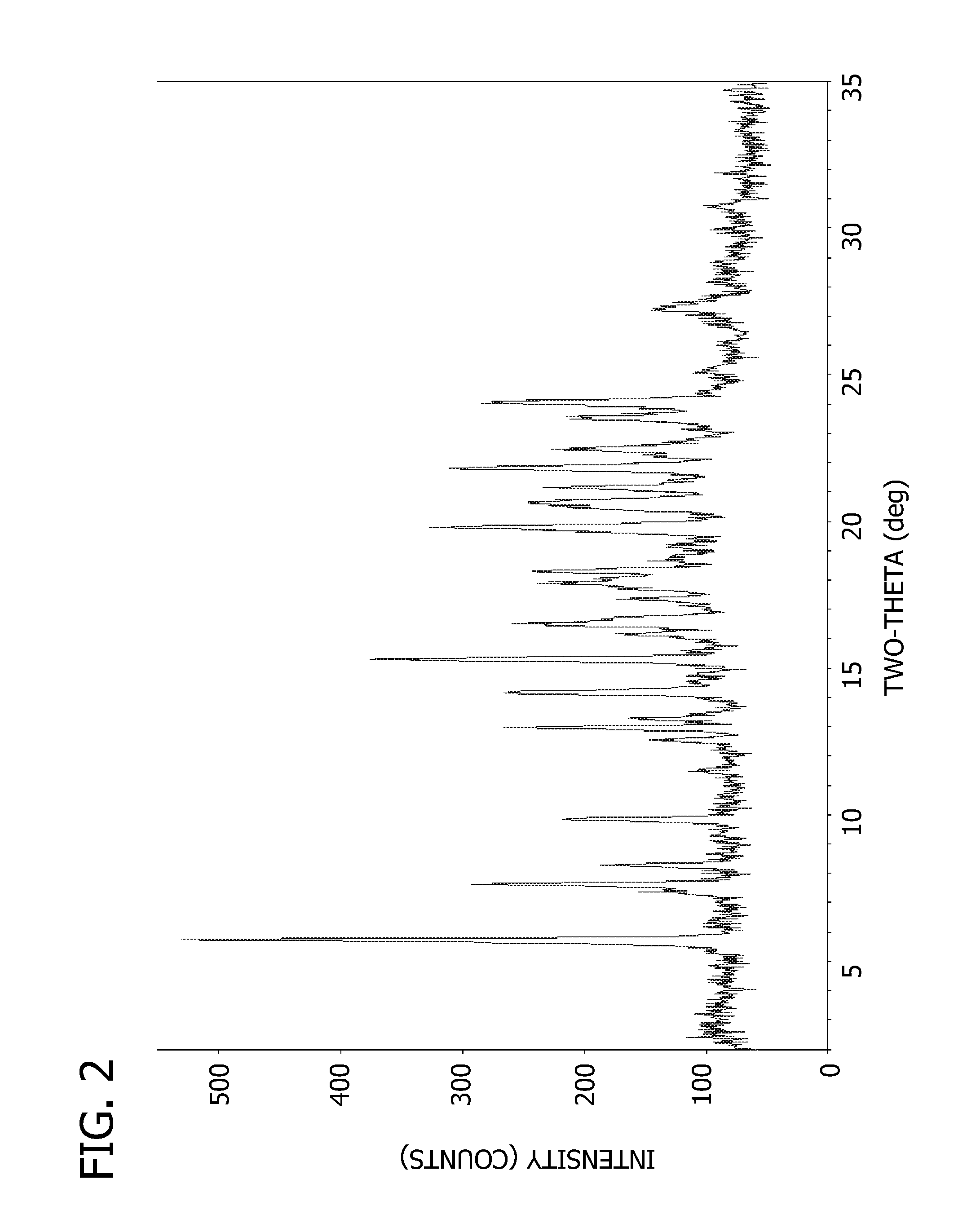
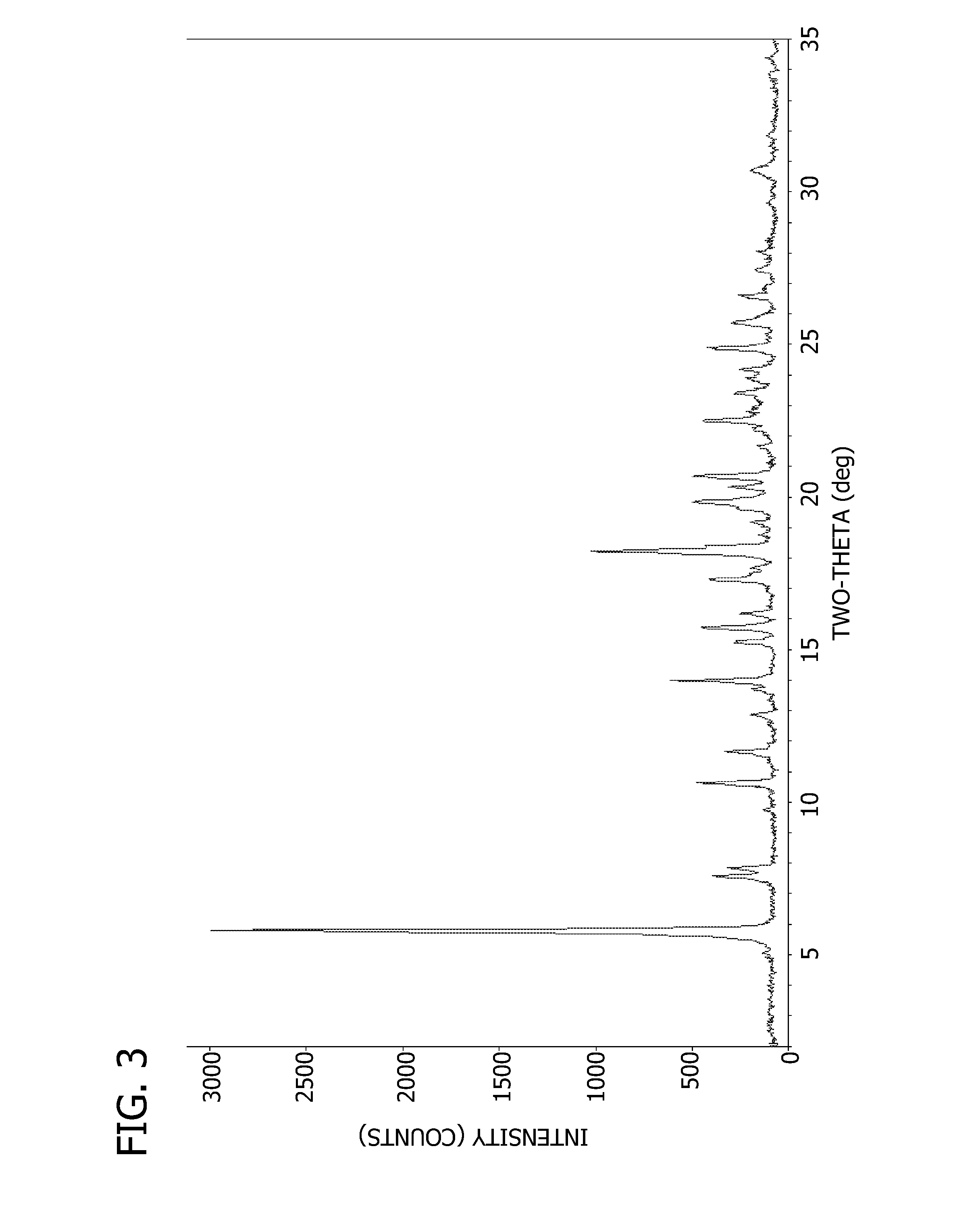
Crystalline form of 4-(beta-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments
The invention relates to a crystalline form of 4-(β-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, to a method for the preparation thereof, as well as to the use thereof for preparing medicaments.
Owner:BOEHRINGER INGELHEIM INT GMBH
Novel benzopyran derivatives as potassium channel openers
The present invention is directed to novel benzopyran derivatives, pharmaceutical compositions containing them and their use in the treatment of disorders related to potassium channel.
Owner:JANSSEN PHARMA NV
Pharmaceutical compositions comprising aryl-substituted acyclic enediyne compounds
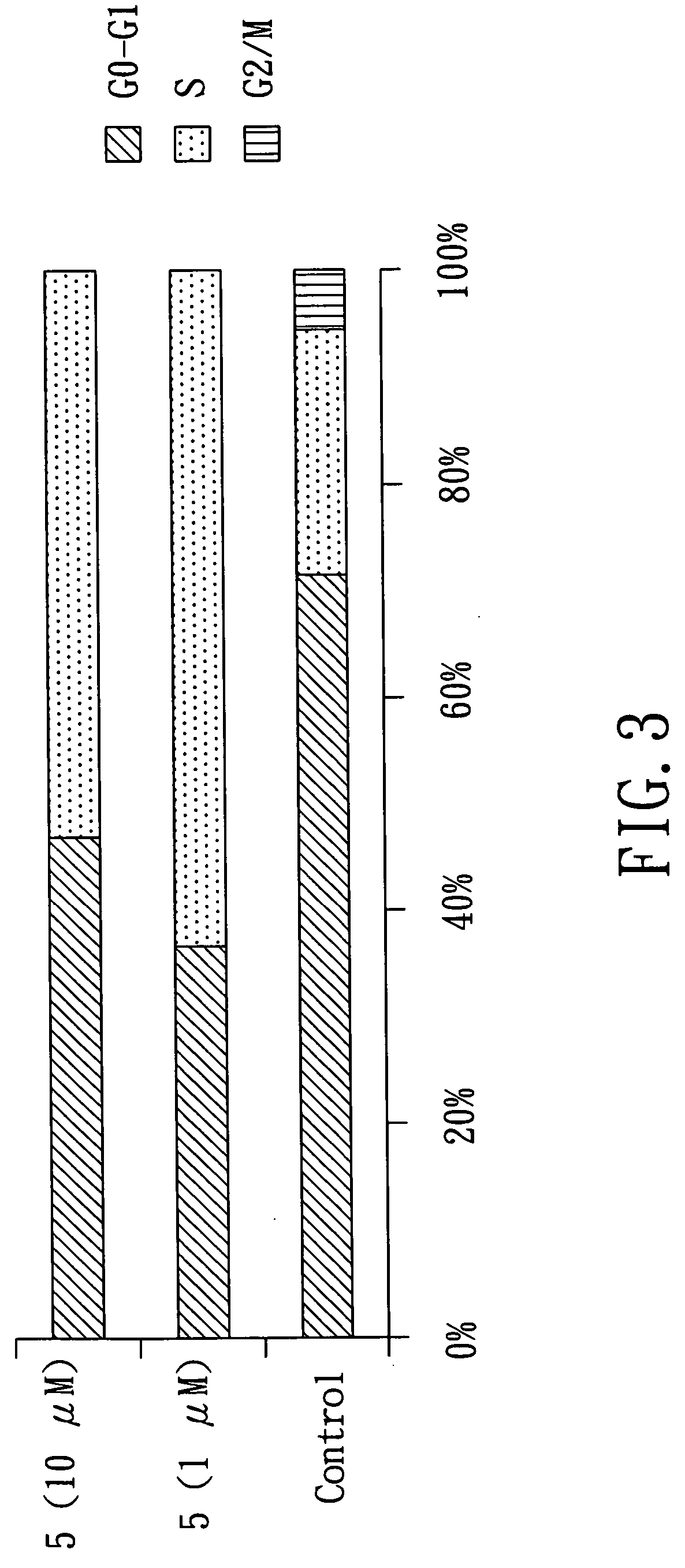
A pharmaceutical compositions comprises a compound of formula (I): or a pharmaceutically acceptable salt thereof: wherein R1═R2 ═H; or R1 and R2 together form a moiety represented by the formula R3 represents a substituted or unsubstituted alkyl having 4-30 carbon atoms, or a substituted or unsubstituted aryl group having 3-30 carbon atoms; and R4 represents a substituted or unsubstituted aryl group having 3-30 carbon atoms; with the proviso that R3 is not butyl, pentyl, tetrahydropyranyloxymethyl, tetrahydropyranyloxypropyl or phenyl when R1═R2═H and R4 is o-cyanophenyl,; and with the proviso that R3 is not butyl when R1═R2═H and R4 is phenyl. The pharmaceutical composition may be used to treat a subject afflicted with a tumor / cancer by inhibiting topoisomerase I activities or blocking the S phase or G2 / M phase of the tumor / cancer cells.
Owner:KAOHSIUNG MEDICAL UNIVERSITY
Phenanthrene and imidazole-coumarin double-fluorescent group ratio fluorescent molecular probe for iron ion detection and synthesis and use methods thereof
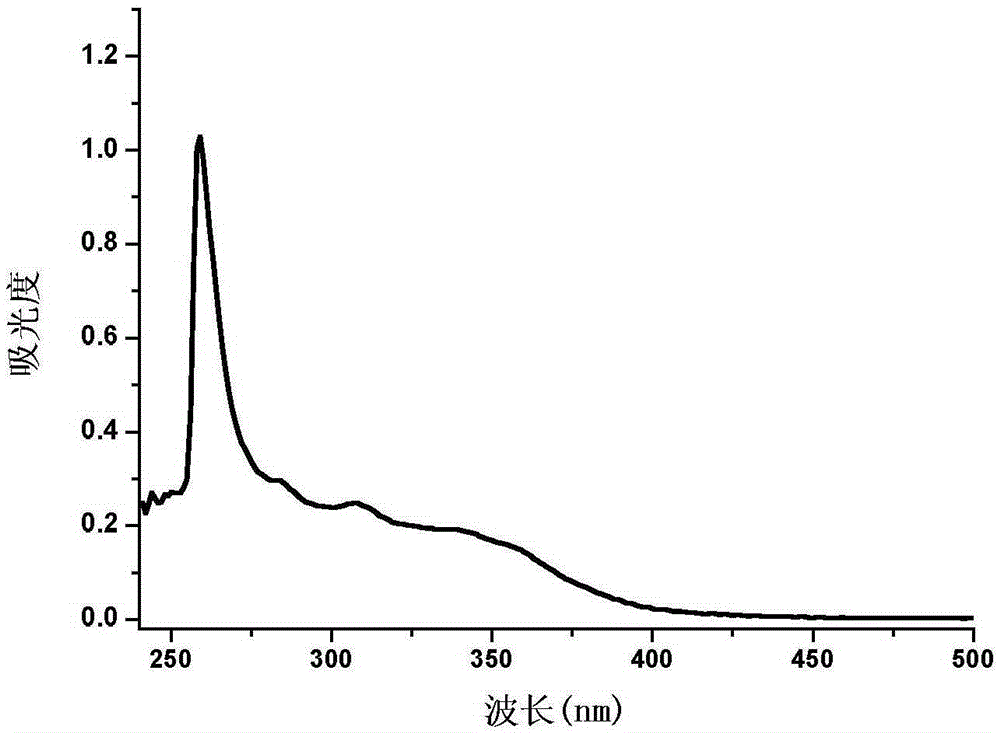
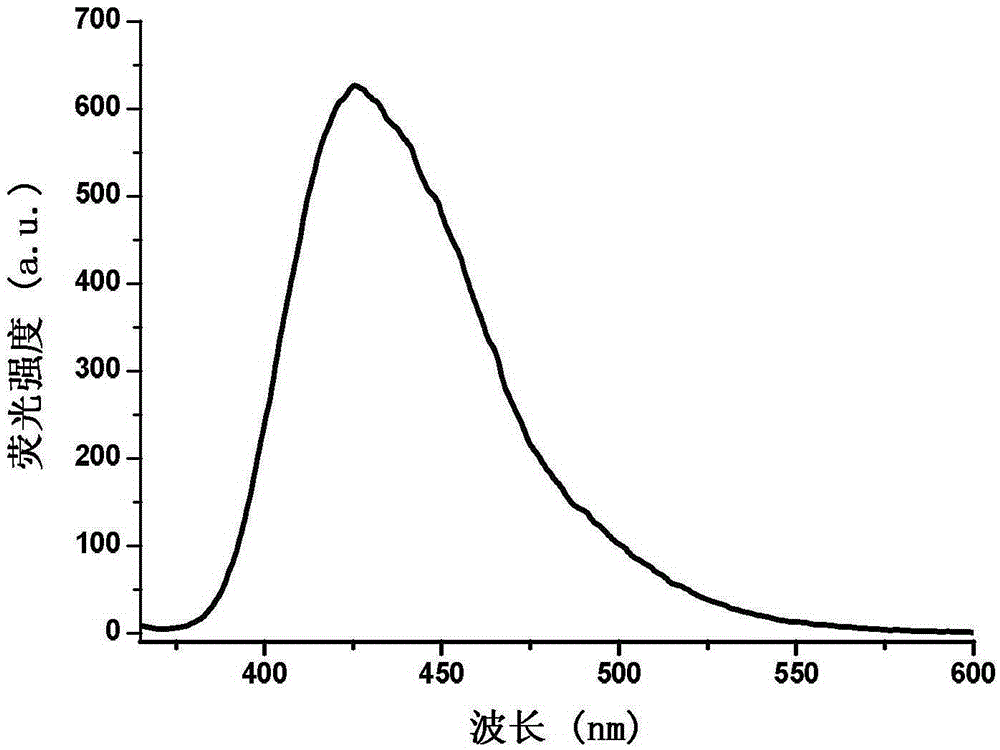
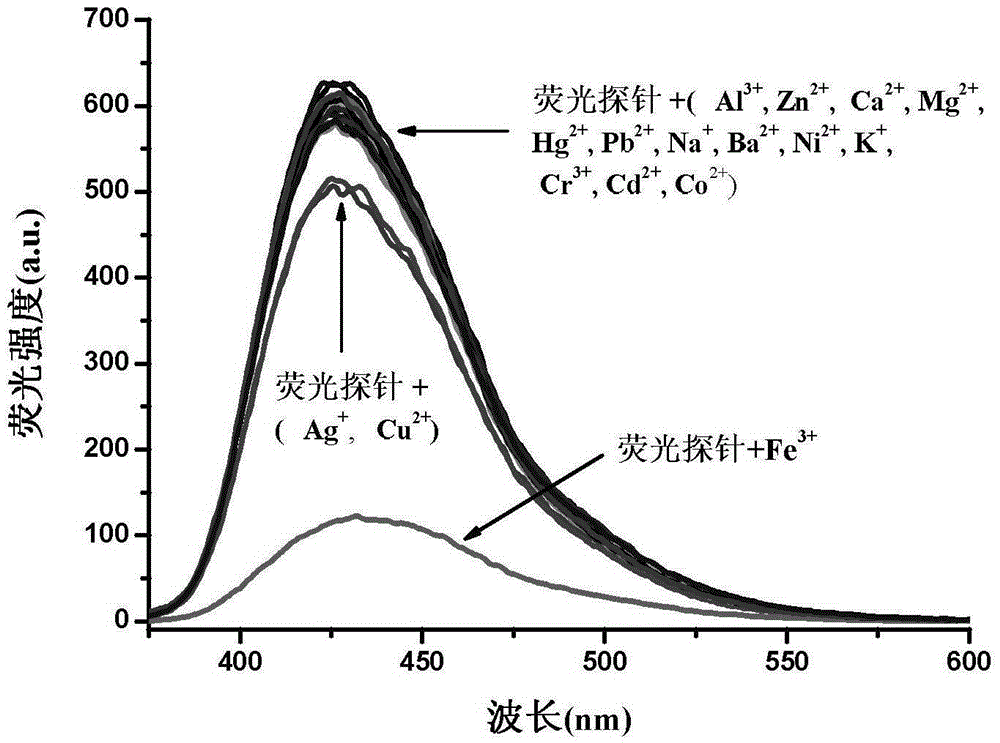
InactiveCN105255481AHigh fluorescence intensityExcellent fluorescence performanceOrganic chemistryFluorescence/phosphorescenceKetonePhenyl group
The invention provides a phenanthrene and imidazole-coumarin double-fluorescent group ratio fluorescent molecular probe for iron ion detection and synthesis and use methods thereof, relates to fluorescent molecular probes and synthesis and application thereof and aims to solve the problem that an existing Fe<3+> fluorescent probe is prone to being interfered by pH, concentration and other metal ions. The fluorescent molecular probe is 4-methyl-7-hydroxide radical-8-[2-(1- phenyl group-1H-phenanthrene and [9, 10-d] imidazole-2-)benzene ammonia methylene]-2H-pyran-2-ketone. The phenanthrene and imidazole-coumarin double-fluorescent group ratio fluorescent molecular probe is formed by conducting condensation on 1-N-phenyl group-2-(2-aminophenyl)-1H-phenanthrene and [9, 10-d] imidazole and 4-methyl-7-hydroxide radical-8- formyl group coumarin, and the yield is 75-85%. The fluorescent molecular probe is dissolved in mixed liquid of N, N- dimethylformamide and an HEPES buffering solution, existence of iron ions is judged through the absorbance value or fluorescence intensity change before and after adding of test samples, and the fluorescent molecular probe can be used for detection of Fe<3+> pollution in water.
Owner:QIQIHAR UNIVERSITY
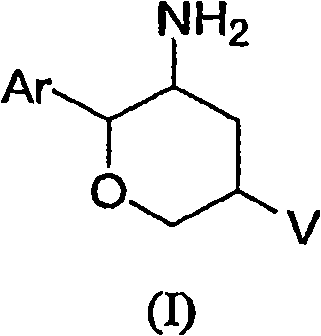
Aminotetrahydropyrans as dipeptidyl peptidase-IV inhibitors for the treatment or prevention of diabetes
The present invention is directed to novel substituted aminotetrahydropyrans of structural formula (I) which are inhibitors of the dipeptidyl peptidase-IV enzyme and which are useful in the treatment or prevention of diseases in which the dipeptidyl peptidase-IV enzyme is involved, such as diabetes and particularly Type 2 diabetes. The invention is also directed to pharmaceutical compositions comprising these compounds and the use of these compounds and compositions in the prevention or treatment of such diseases in which the dipeptidyl peptidase-IV enzyme is involved.
Owner:SCHERING AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





![Crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/713cb55c-505a-47ae-a0dd-2350f315dca1/US07723309-20100525-D00001.png)
![Crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/713cb55c-505a-47ae-a0dd-2350f315dca1/US07723309-20100525-D00002.png)
![Crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/713cb55c-505a-47ae-a0dd-2350f315dca1/US07723309-20100525-C00001.png)


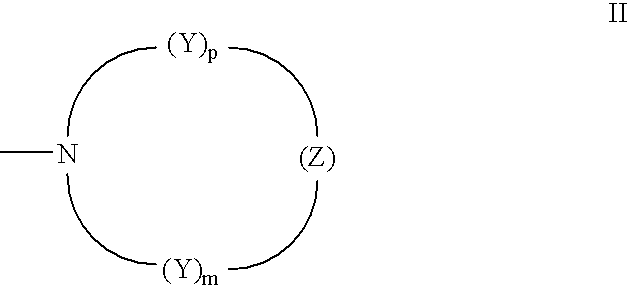
![Pharmaceutical Dosage Forms Comprising 6'-Fluoro-(N-Methyl- or N,N-Dimethyl-)-4-Phenyl-4',9'-Dihydro-3'H-Spiro[Cyclohexane-1,1'-Pyrano[3,4,b]Indol]-4-Amine Pharmaceutical Dosage Forms Comprising 6'-Fluoro-(N-Methyl- or N,N-Dimethyl-)-4-Phenyl-4',9'-Dihydro-3'H-Spiro[Cyclohexane-1,1'-Pyrano[3,4,b]Indol]-4-Amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/677d5899-3318-439e-beec-6e8f16d3f270/US20150111939A1-20150423-D00001.PNG)
![Pharmaceutical Dosage Forms Comprising 6'-Fluoro-(N-Methyl- or N,N-Dimethyl-)-4-Phenyl-4',9'-Dihydro-3'H-Spiro[Cyclohexane-1,1'-Pyrano[3,4,b]Indol]-4-Amine Pharmaceutical Dosage Forms Comprising 6'-Fluoro-(N-Methyl- or N,N-Dimethyl-)-4-Phenyl-4',9'-Dihydro-3'H-Spiro[Cyclohexane-1,1'-Pyrano[3,4,b]Indol]-4-Amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/677d5899-3318-439e-beec-6e8f16d3f270/US20150111939A1-20150423-D00002.PNG)
![Pharmaceutical Dosage Forms Comprising 6'-Fluoro-(N-Methyl- or N,N-Dimethyl-)-4-Phenyl-4',9'-Dihydro-3'H-Spiro[Cyclohexane-1,1'-Pyrano[3,4,b]Indol]-4-Amine Pharmaceutical Dosage Forms Comprising 6'-Fluoro-(N-Methyl- or N,N-Dimethyl-)-4-Phenyl-4',9'-Dihydro-3'H-Spiro[Cyclohexane-1,1'-Pyrano[3,4,b]Indol]-4-Amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/677d5899-3318-439e-beec-6e8f16d3f270/US20150111939A1-20150423-D00003.PNG)

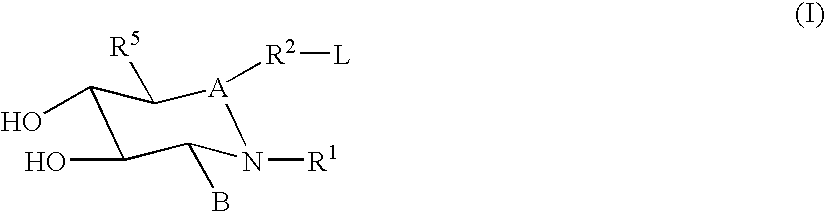




![Crystalline form of 4-(beta-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline form of 4-(beta-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9af34e3e-cad4-4b67-b618-8a019eb52d2e/US08283326-20121009-D00001.png)
![Crystalline form of 4-(beta-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline form of 4-(beta-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9af34e3e-cad4-4b67-b618-8a019eb52d2e/US08283326-20121009-C00001.png)
![Crystalline form of 4-(beta-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline form of 4-(beta-D-glucopyranos-1-yl)-1-methyl-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9af34e3e-cad4-4b67-b618-8a019eb52d2e/US08283326-20121009-C00002.png)