Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
7356results about How to "Reduce release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
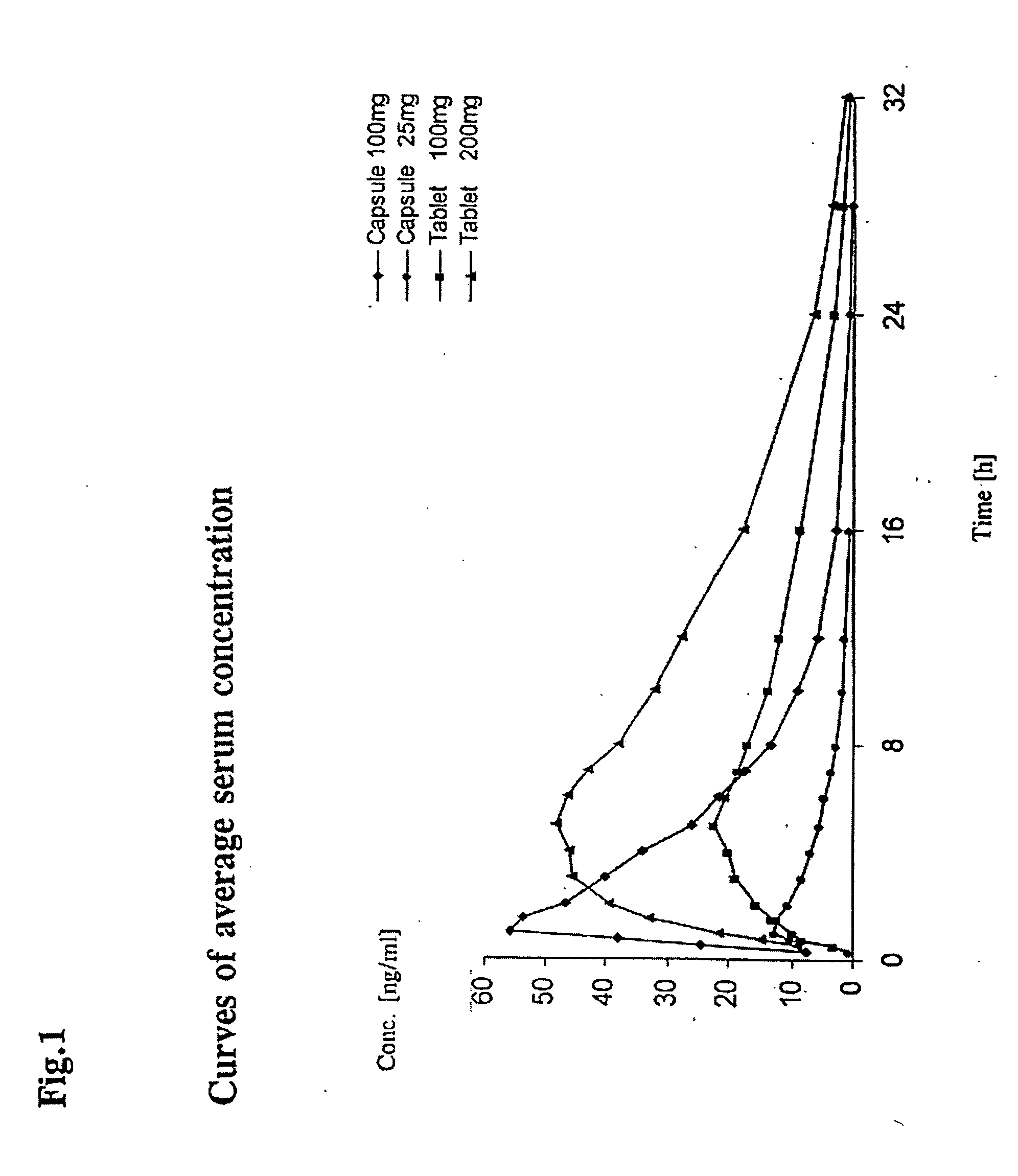
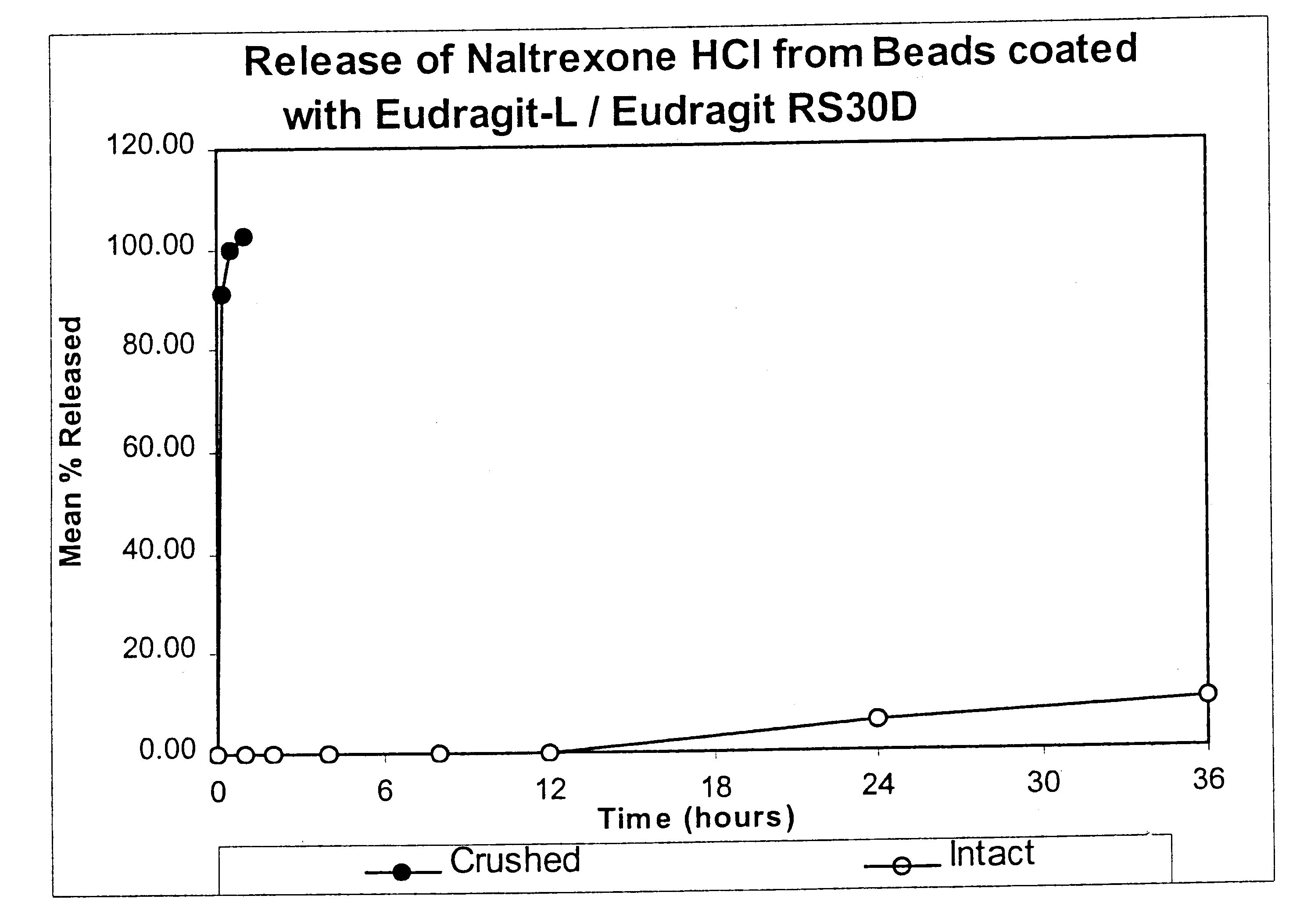
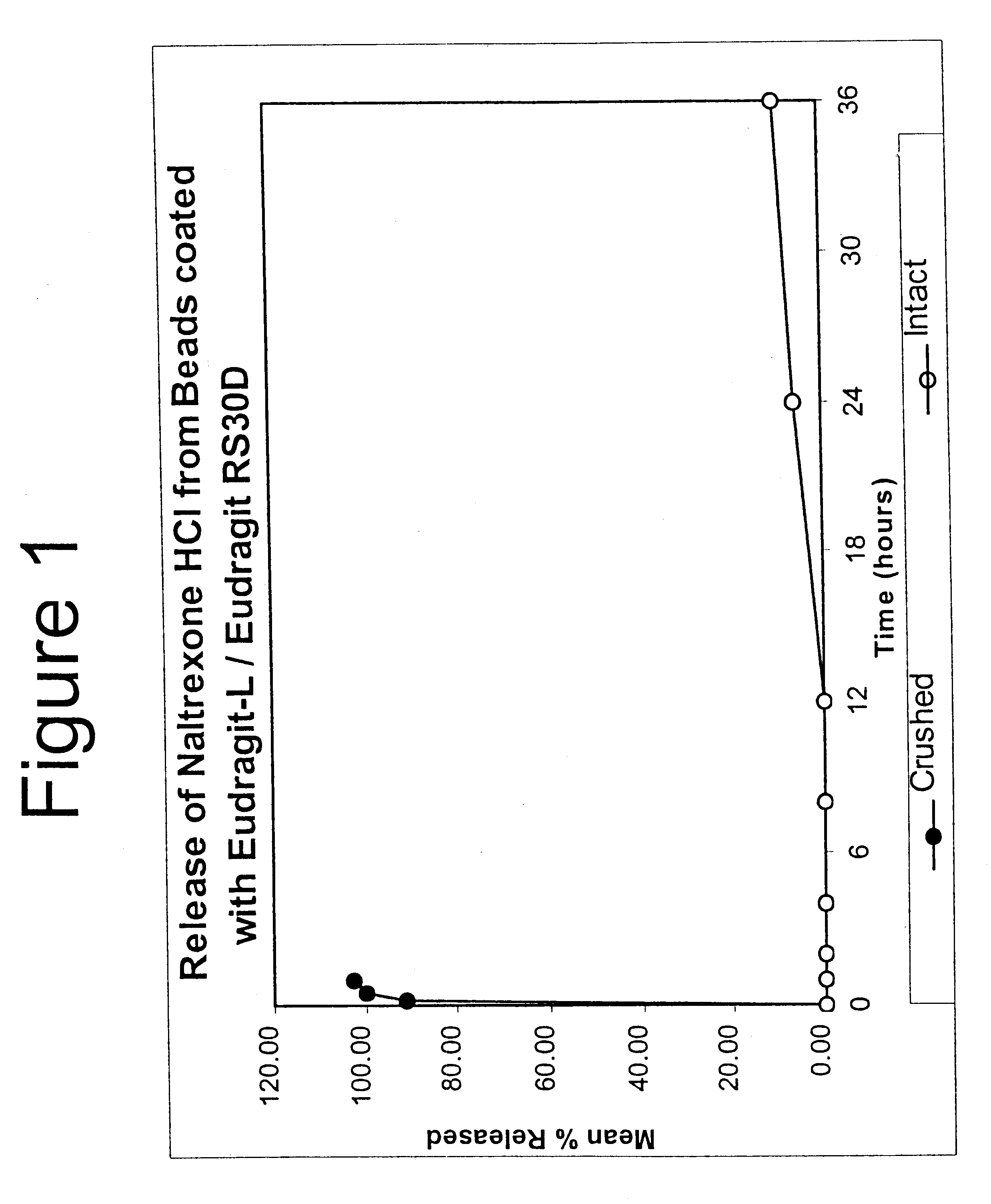
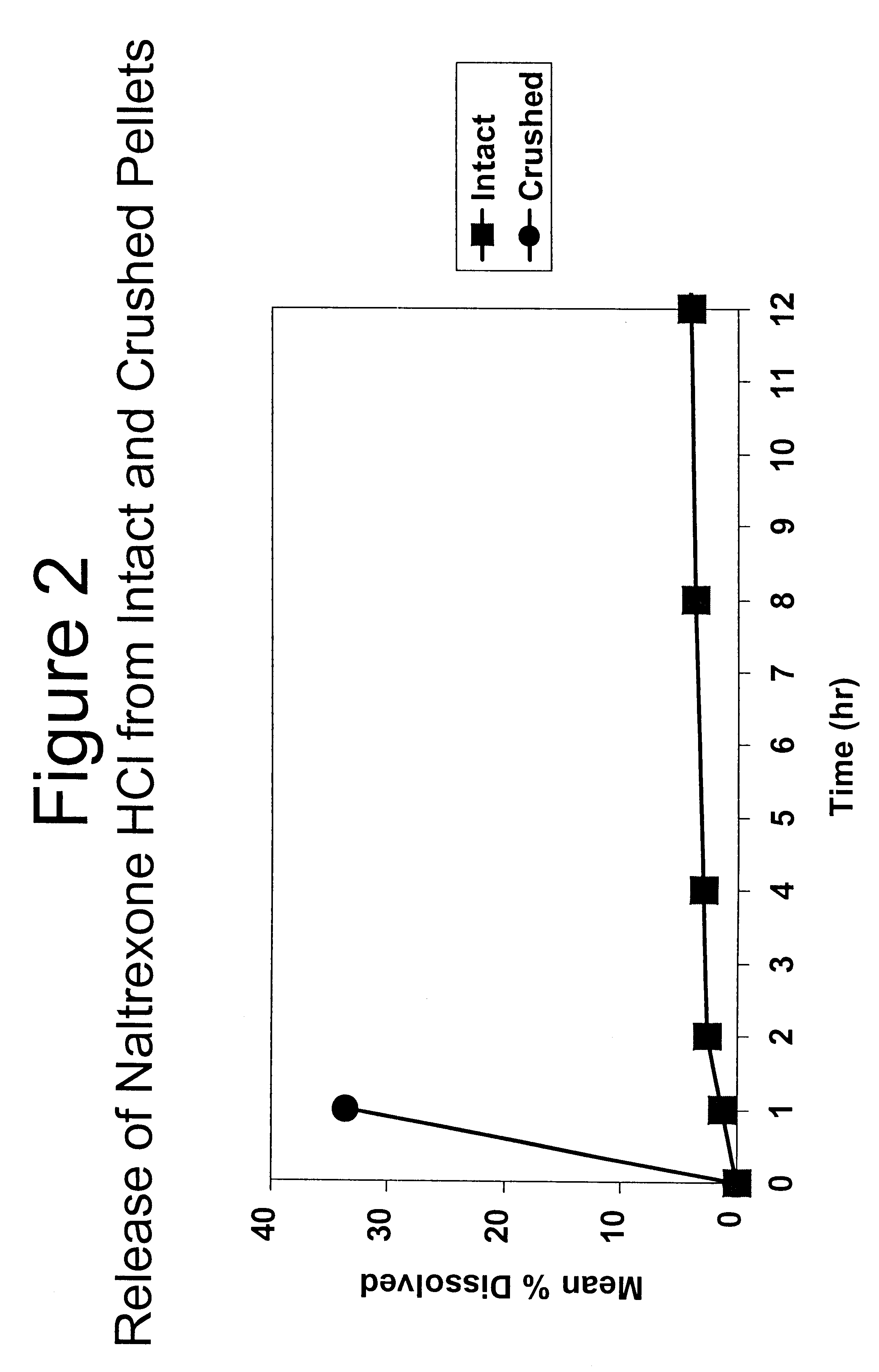
Tamper-resistant oral opioid agonist formulations
InactiveUS6696088B2Lower potentialReduce releasePowder deliveryNervous disorderOpioid AgonistOpioid antagonist
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
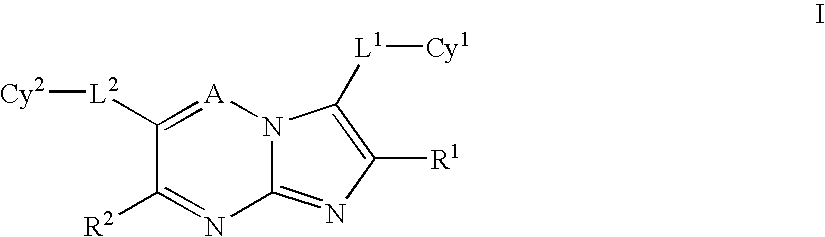
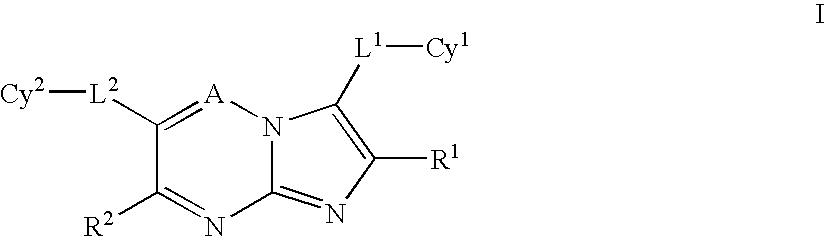
Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors
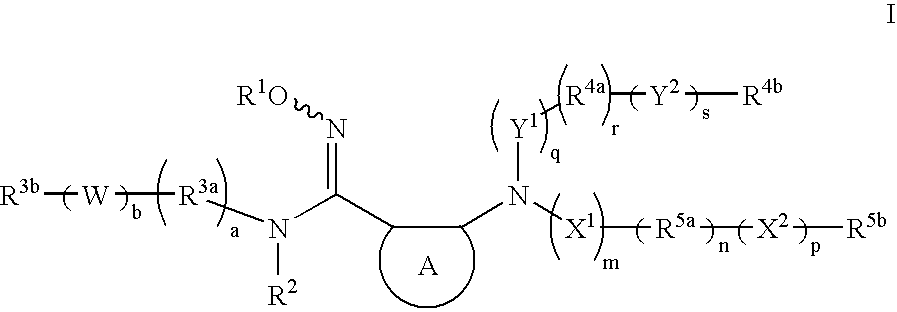
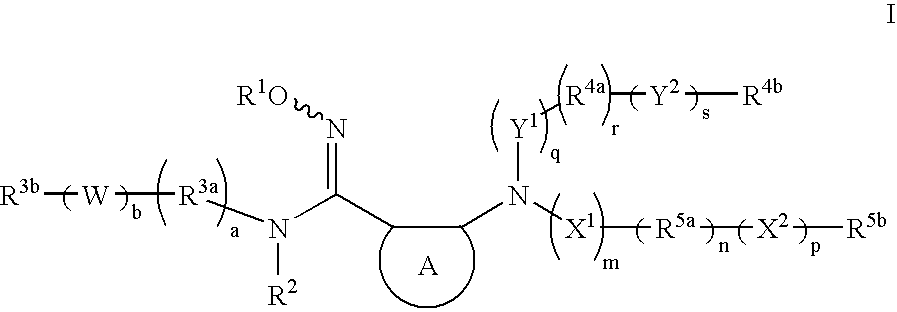
The present invention provides heteroaryl substituted pyrrolo[2,3-b]pyridines and heteroaryl substituted pyrrolo[2,3-b]pyrimidines that modulate the activity of Janus kinases and are useful in the treatment of diseases related to activity of Janus kinases including, for example, immune-related diseases, skin disorders, myeloid proliferative disorders, cancer, and other diseases.
Owner:INCYTE HLDG & INCYTE
Electro-optical device and electronic device
InactiveUS6689492B1Reduce heatReduce releaseDischarge tube luminescnet screensSemiconductor/solid-state device detailsDisplay deviceEngineering
An object of the present invention is to provide an EL display device, which has a high operating performance and reliability. A third passivation film 45 is disposed under an EL element 203 which comprises a pixel electrode (anode) 46, and EL layer 47 and a cathode 48, to make a structure in which heat generated by the EL element 203 is radiated. Further, the third passivation film 45 prevents alkali metals within the EL element 203 from diffusing into the TFTs side, and prevents moisture and oxygen of the TFTs side from penetrating into the EL element 203. More preferably, heat radiating effect is given to a fourth passivation film 50 to make the EL element 203 to be enclosed by heat radiating layers.
Owner:SEMICON ENERGY LAB CO LTD
Tobacco and/or tobacco substitute composition for use as a snuff in the oral cavity
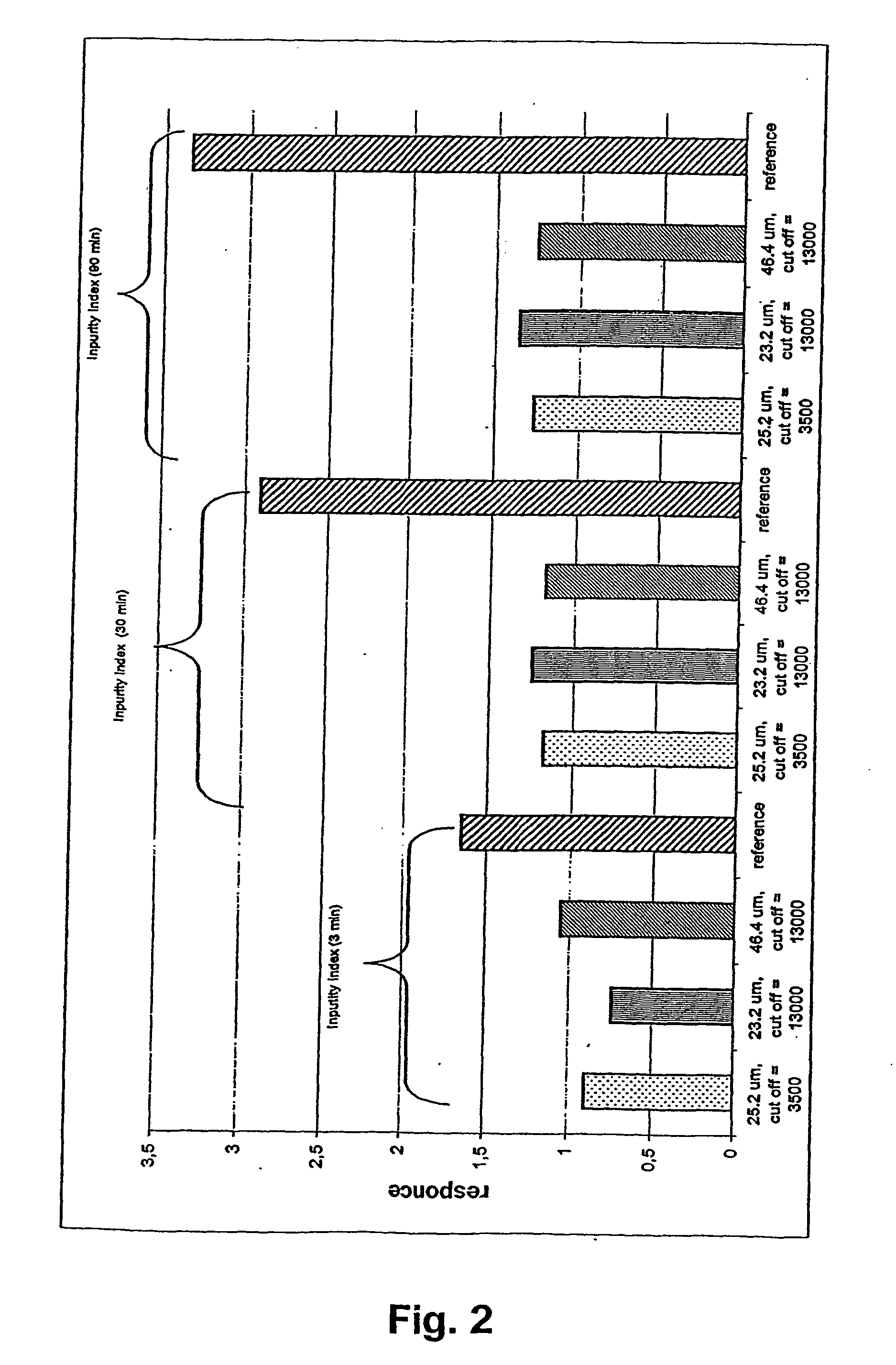
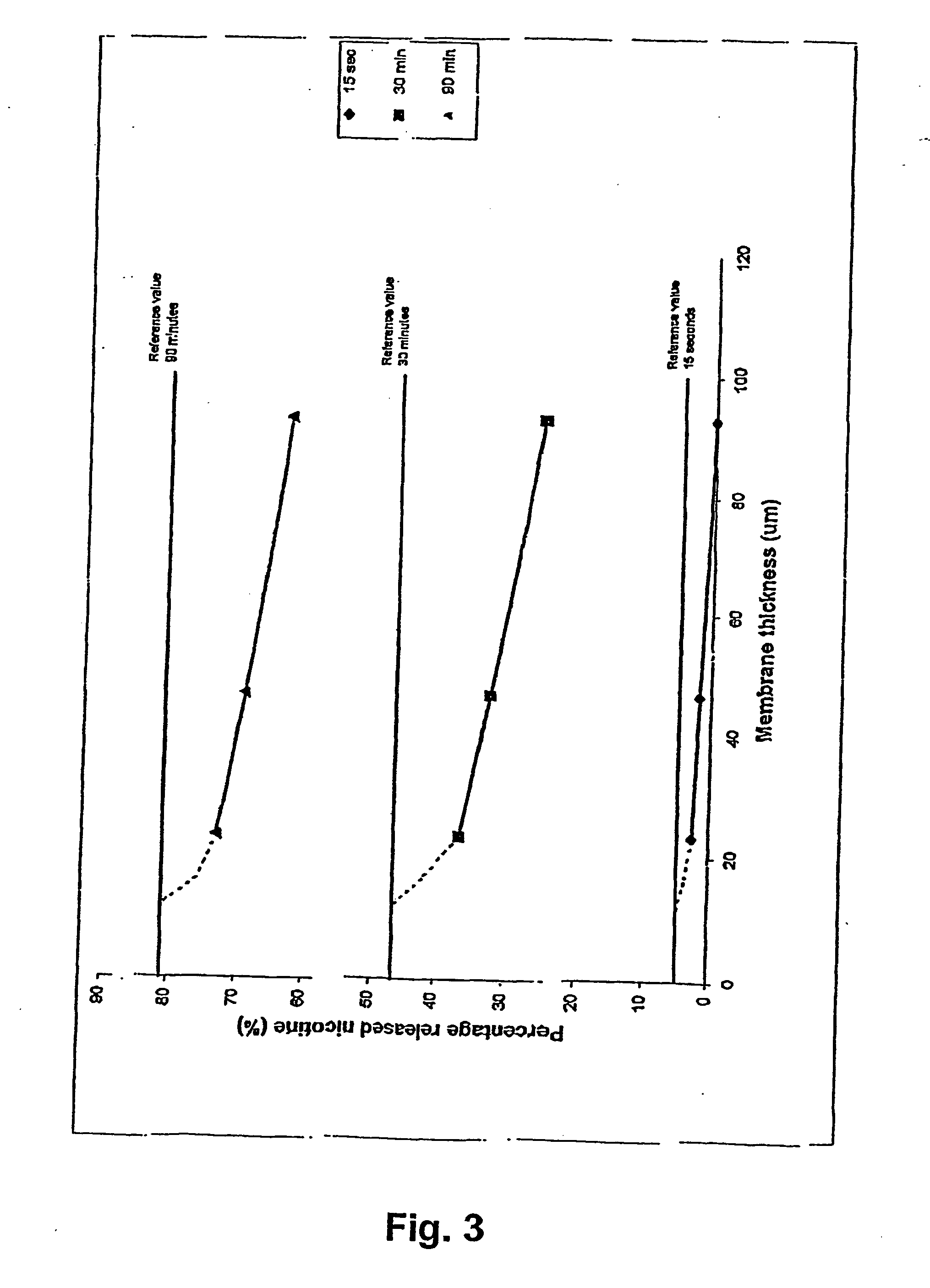
InactiveUS20050061339A1Reduce releaseAvoid spreadingTobacco treatmentSide effectEnvironmental health
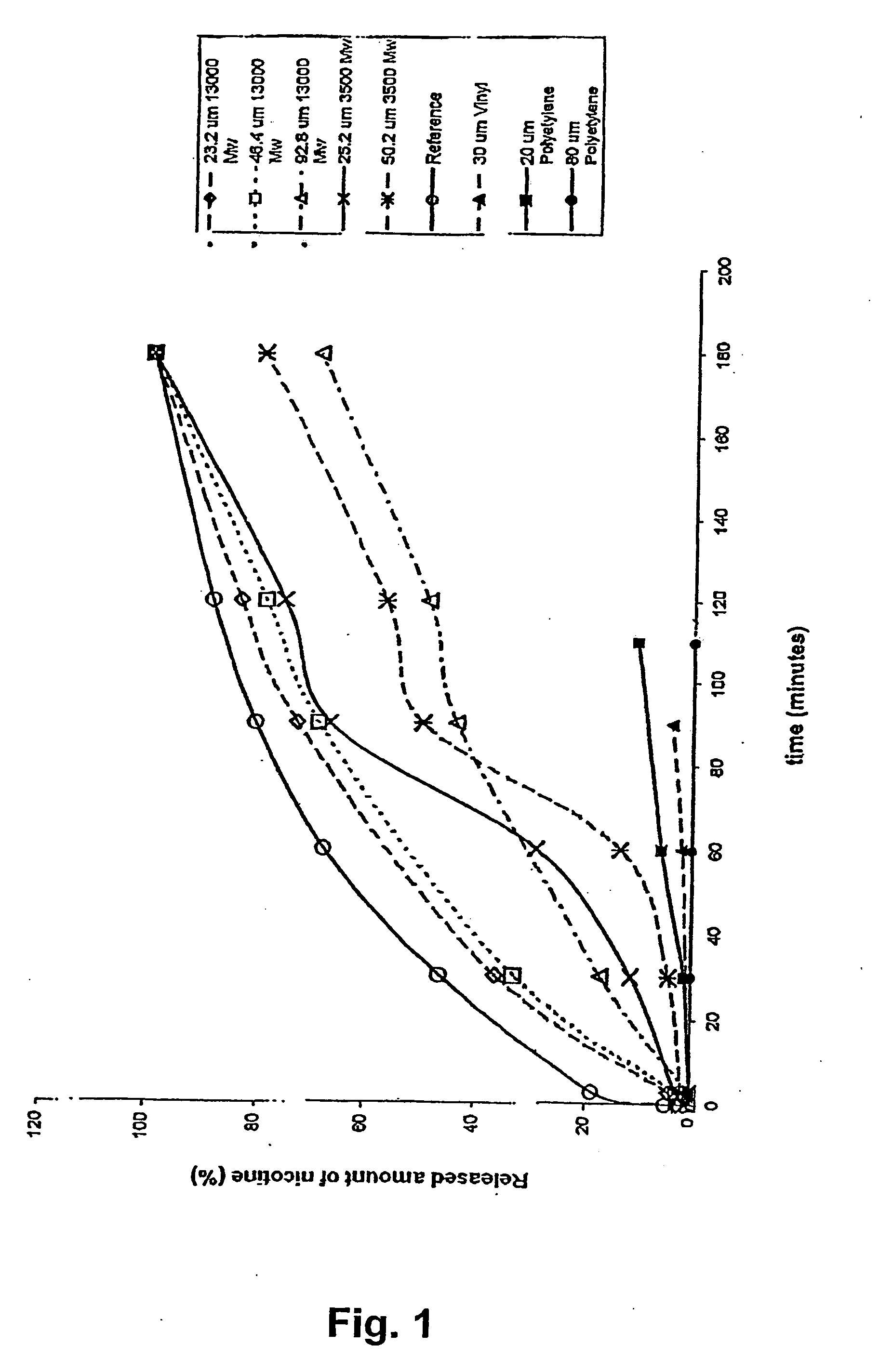
A novel composition for the use as snuff in the oral cavity, the composition comprising tobacco and / or a tobacco substitute encapsulated in a membrane material comprising one or more membranes at least one of which being water permeable and water-insoluble. A novel composition enables a selective release of e.g. nicotine while it at the same time reduces the release of substances, which normally lead to unwanted side effects. The novel compositions may be used as a healthier alternative to snuff and other tobacco products such as, e.g., cigarettes, cigars and pipe. Methods for giving up smoking, reducing nicotine craving, reducing side effects normally related to smoking and snuffing of tobacco as welt a method for the preparation of a composition according to the invention.
Owner:GALENICA
Apparatus and Method for Ocular Treatment
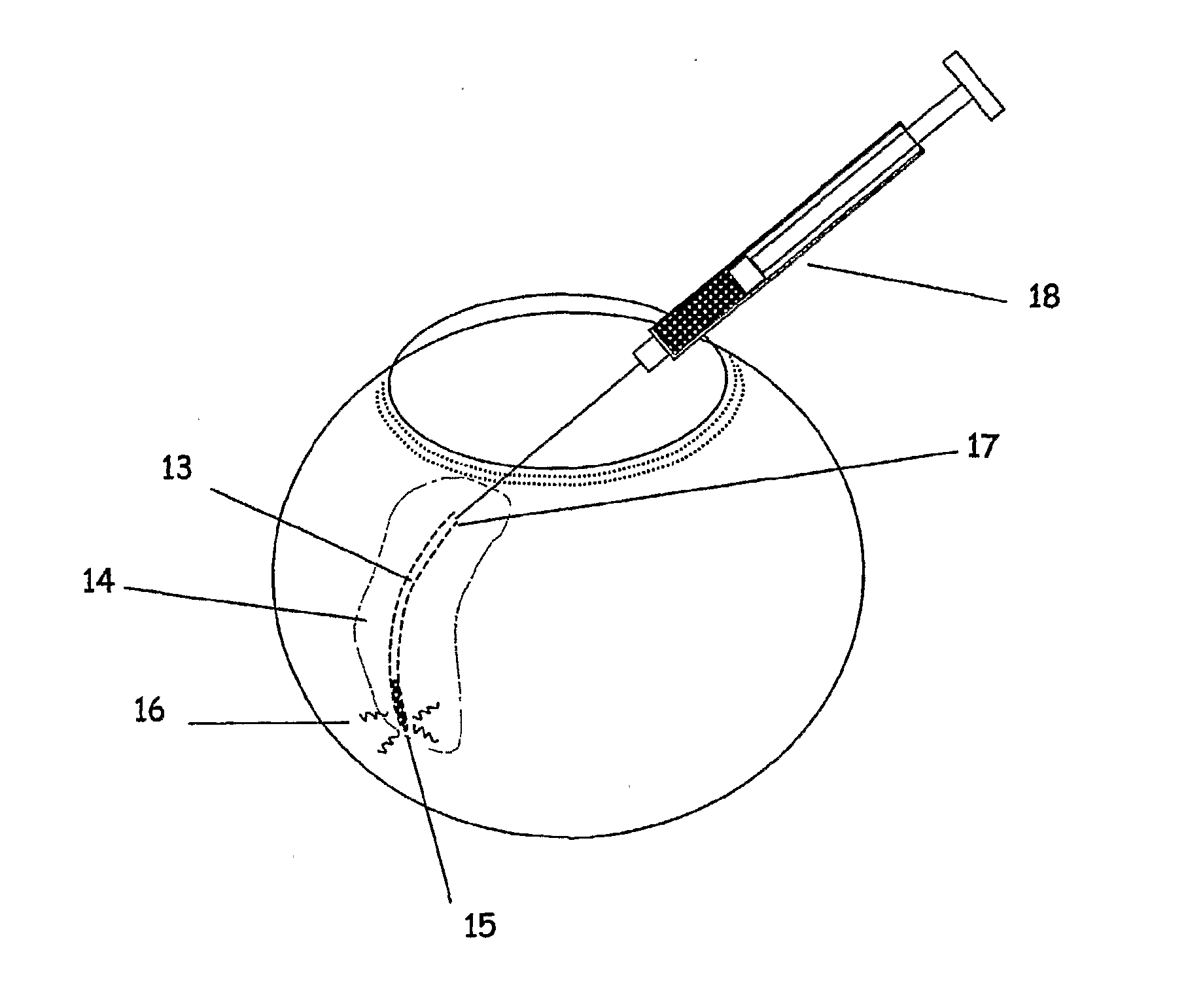
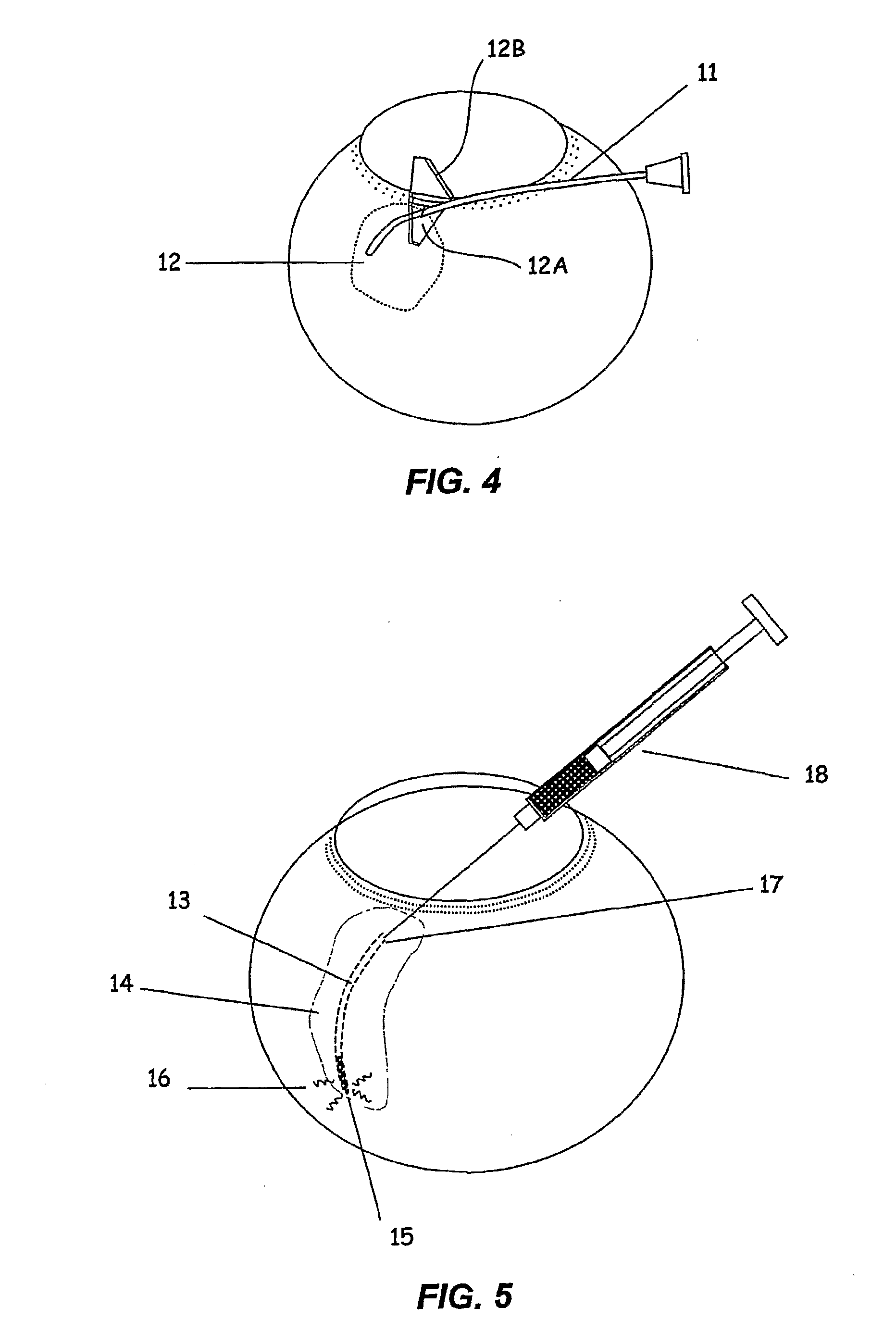
InactiveUS20080058704A1Reduce releaseFacilitate tissue targetingUltrasonic/sonic/infrasonic diagnosticsLaser surgeryLess invasive surgerySuprachoroidal space
The invention provides tools, materials and related methods to surgically access the suprachoroidal space of an eye for the purpose of performing minimally invasive surgery or to deliver drugs to the eye. The invention provides a flexible microcannula device (11, 13) that may be placed into the suprachoroidal space (12, 14) through a small incision (12A) of the overlying tissues, maneuvered into the appropriate region of the space, and then activated to treat tissues adjacent to the distal tip of the device.
Owner:ISCI INTERVENTIONAL CORP
Coating for implantable devices and a method of forming the same
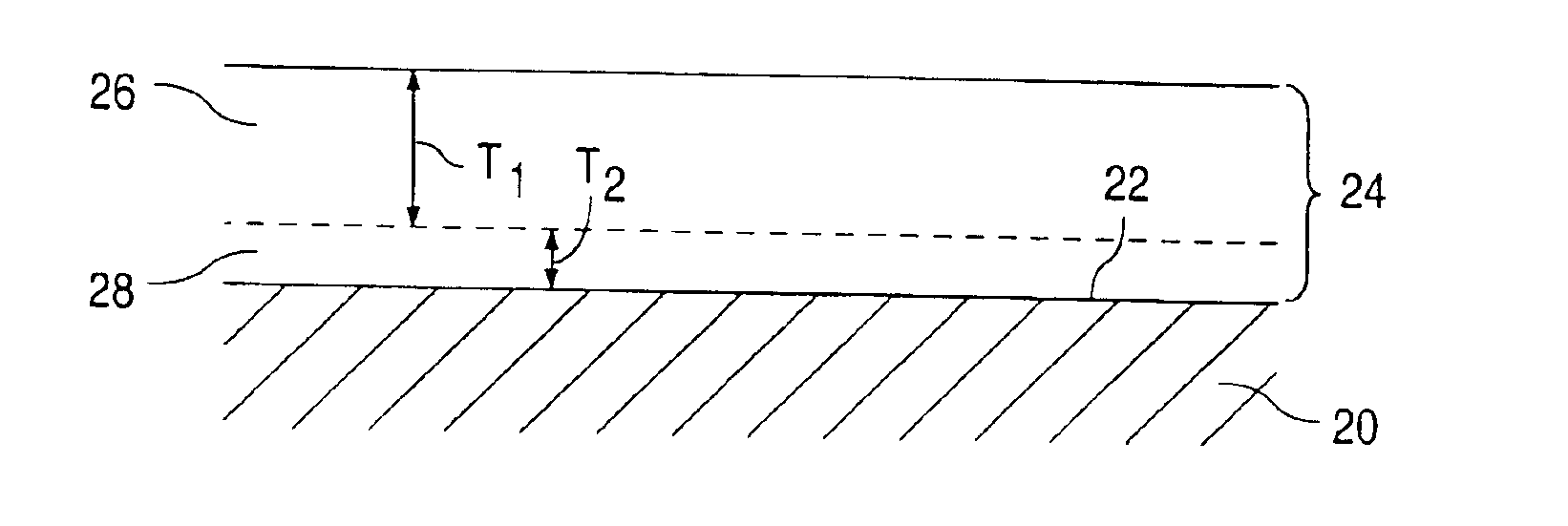
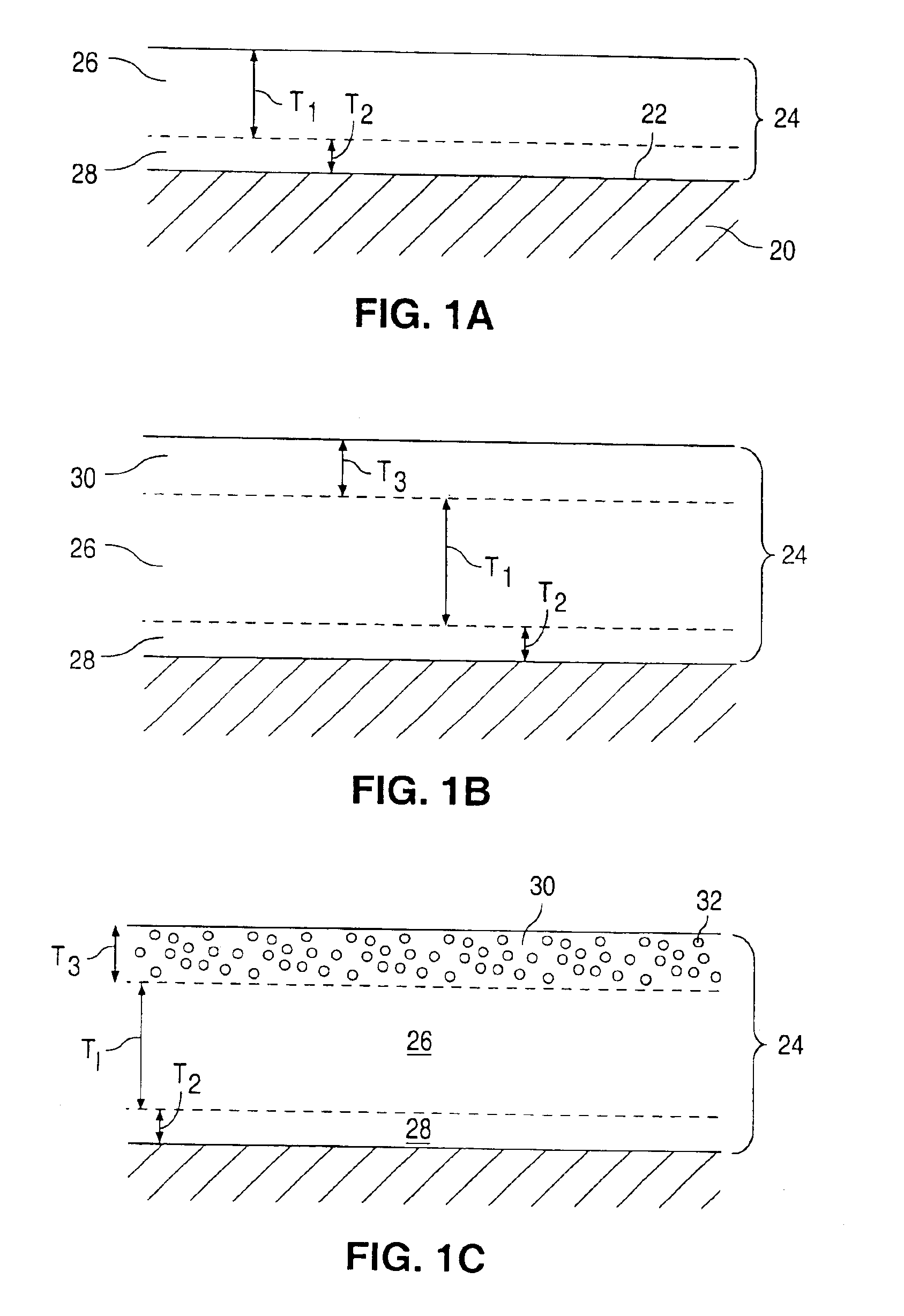
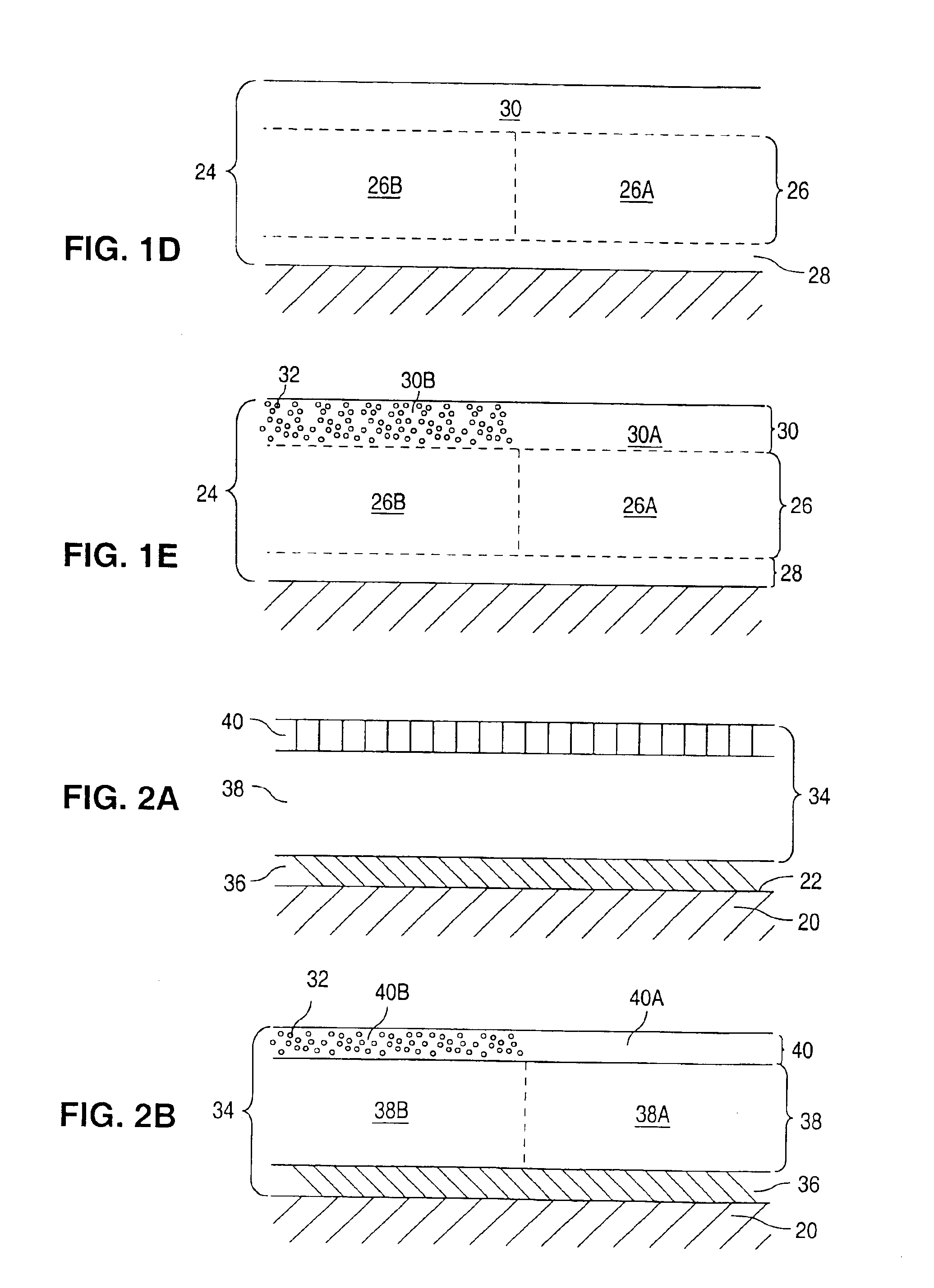
InactiveUS6908624B2Reduce releaseReduce probabilitySurgeryPharmaceutical delivery mechanismProsthesisActive ingredient
Coatings for implantable devices or endoluminal prosthesis, such as stents, are provided, including a method of forming the coatings. The coatings can be used for the delivery of an active ingredient or a combination of active ingredients.
Owner:ABBOTT CARDIOVASCULAR
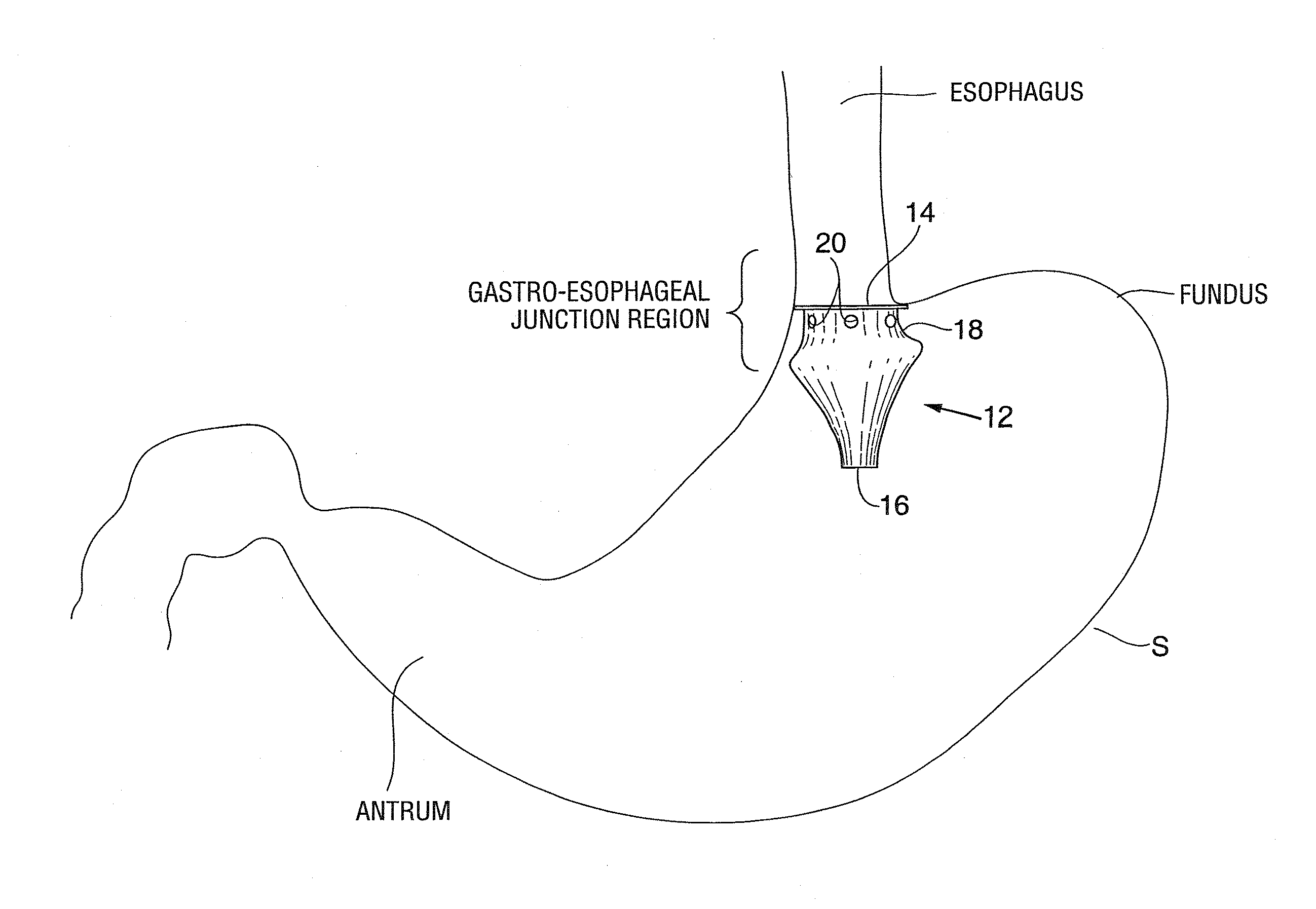
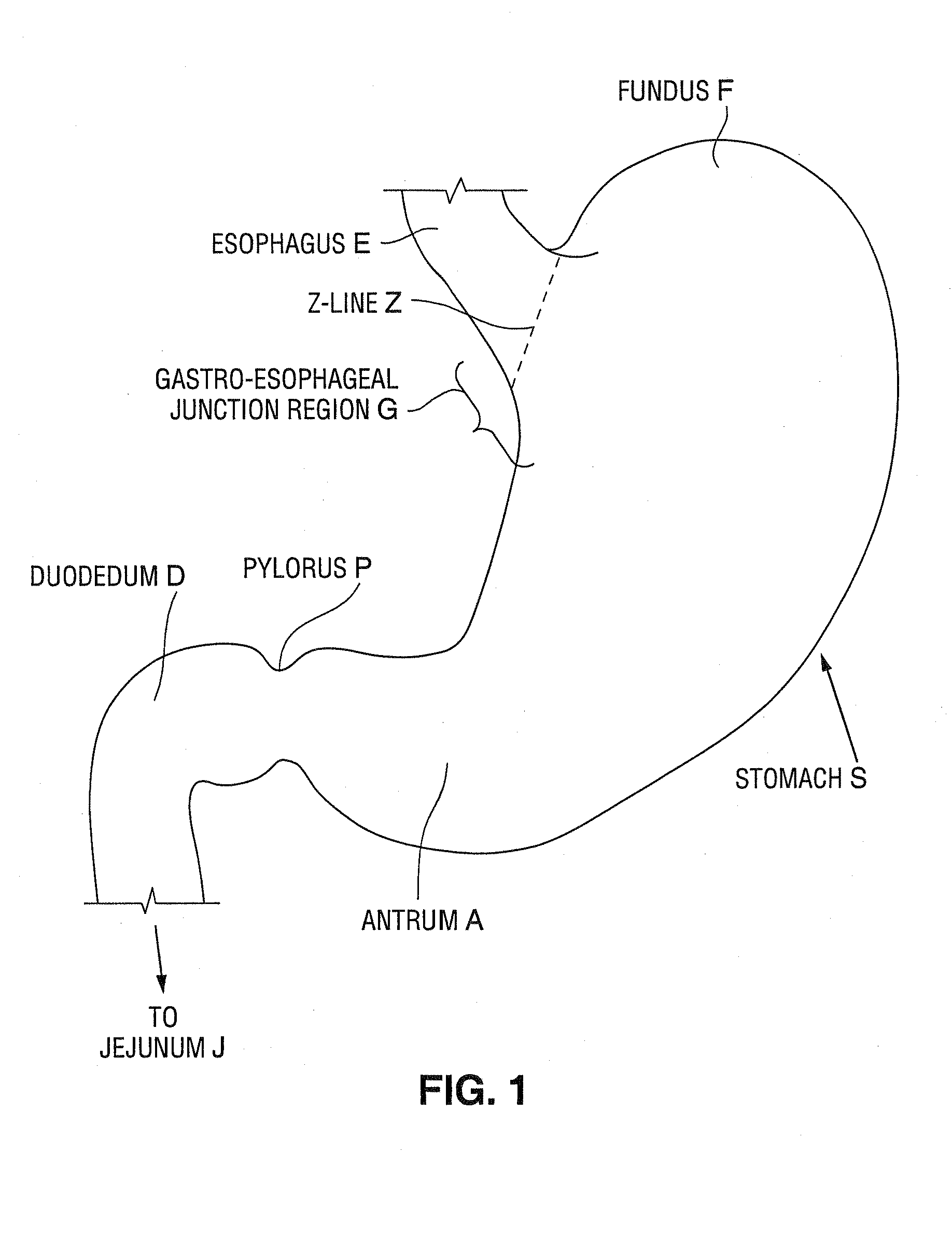
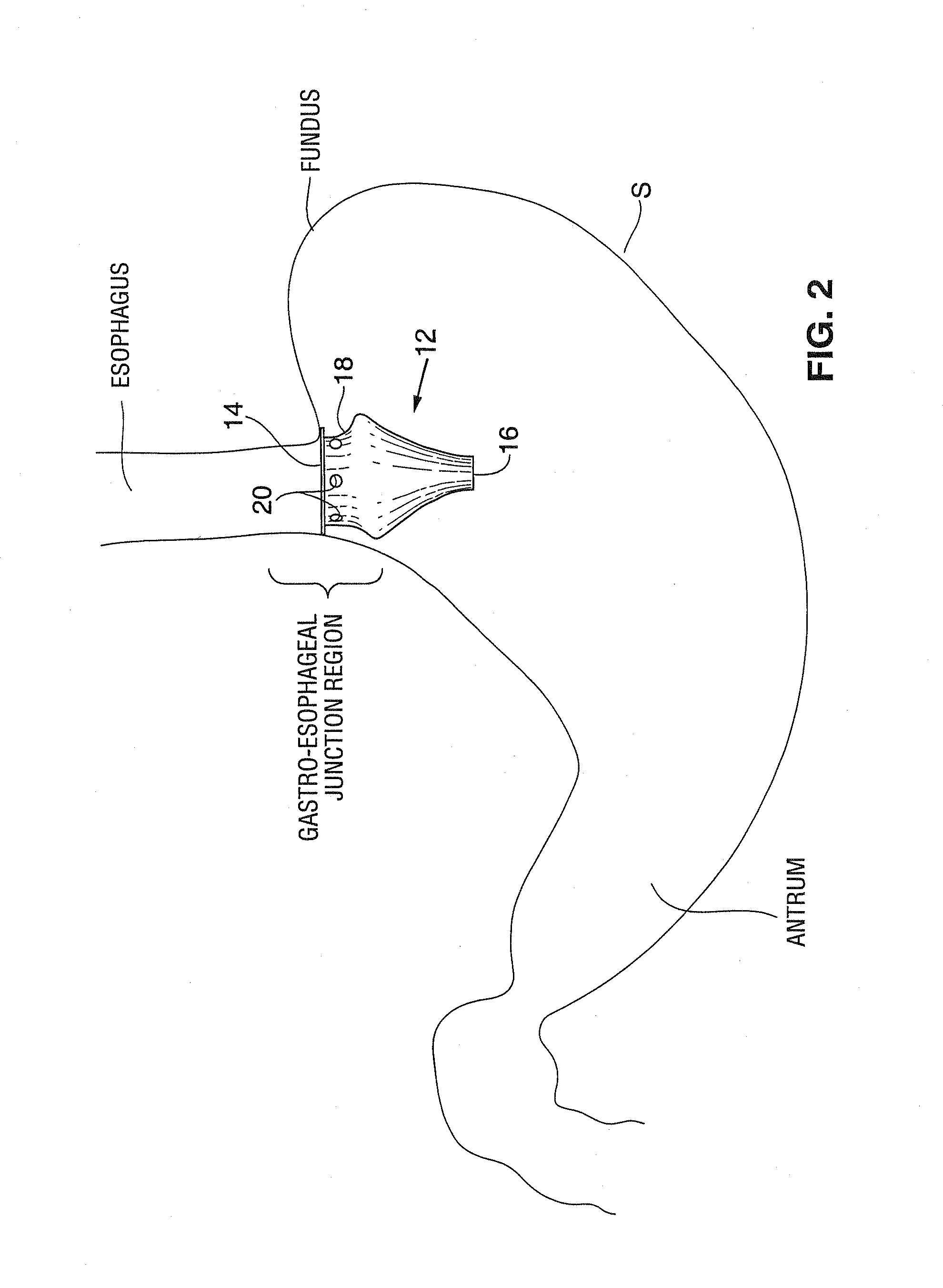
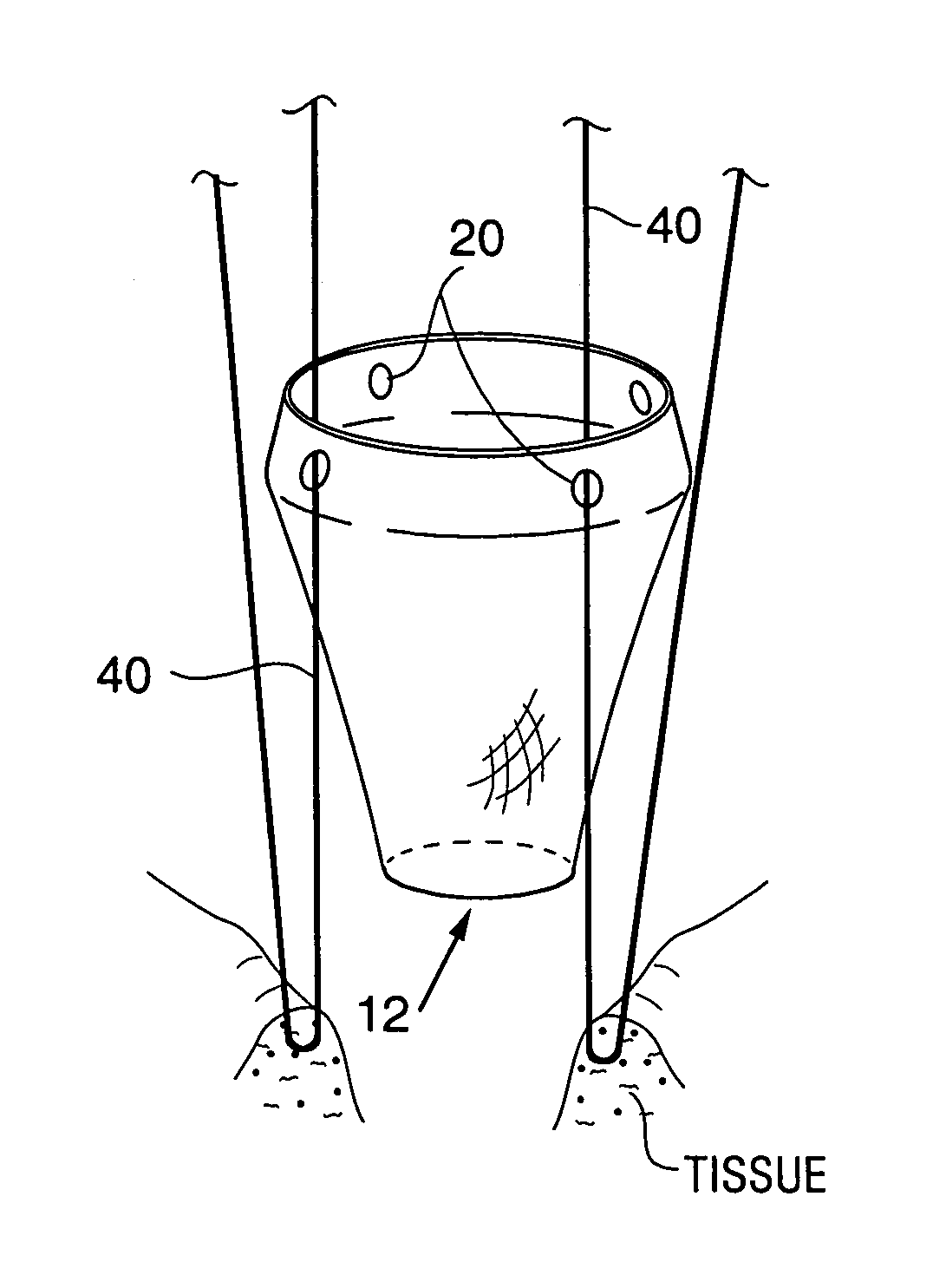
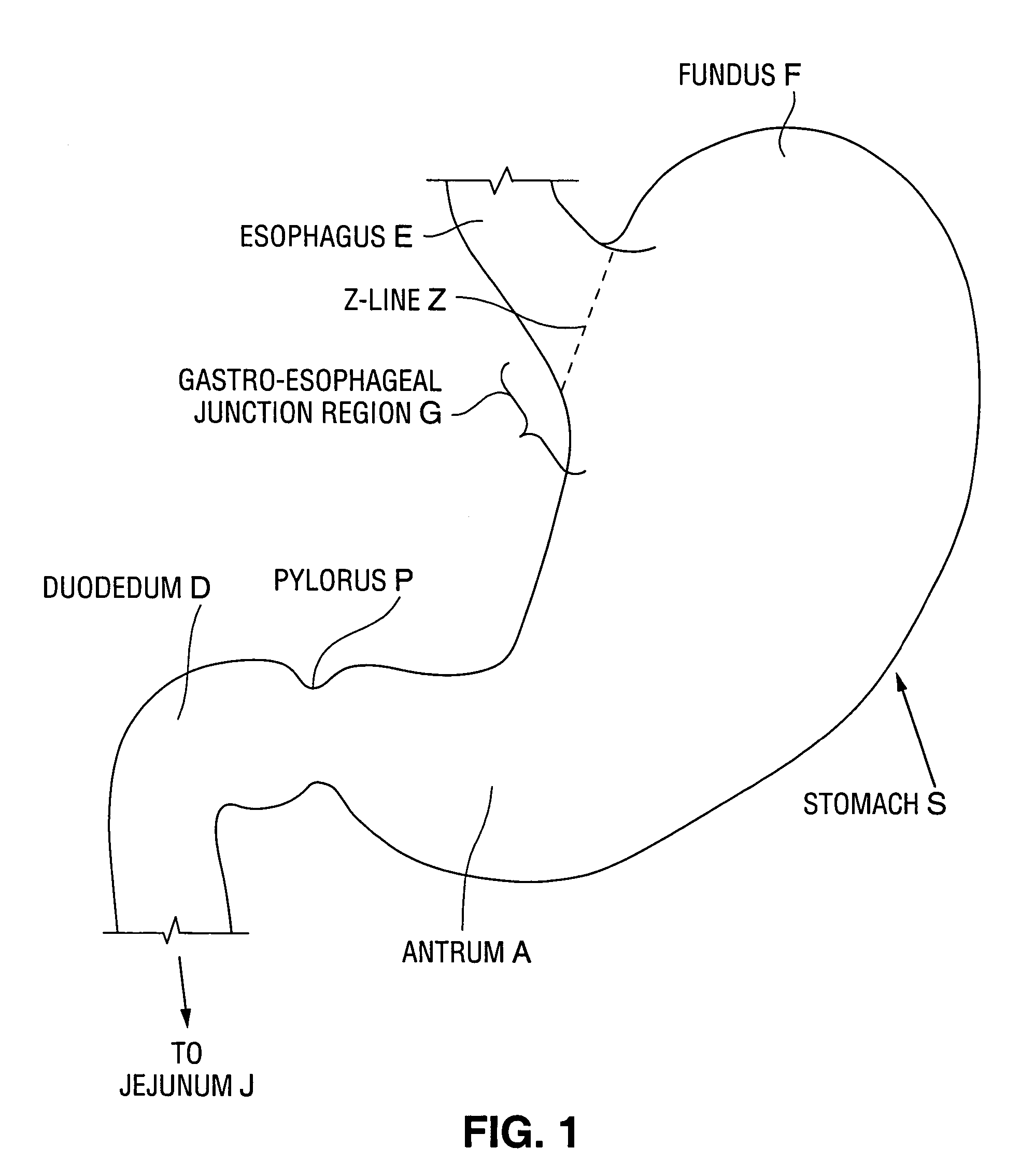
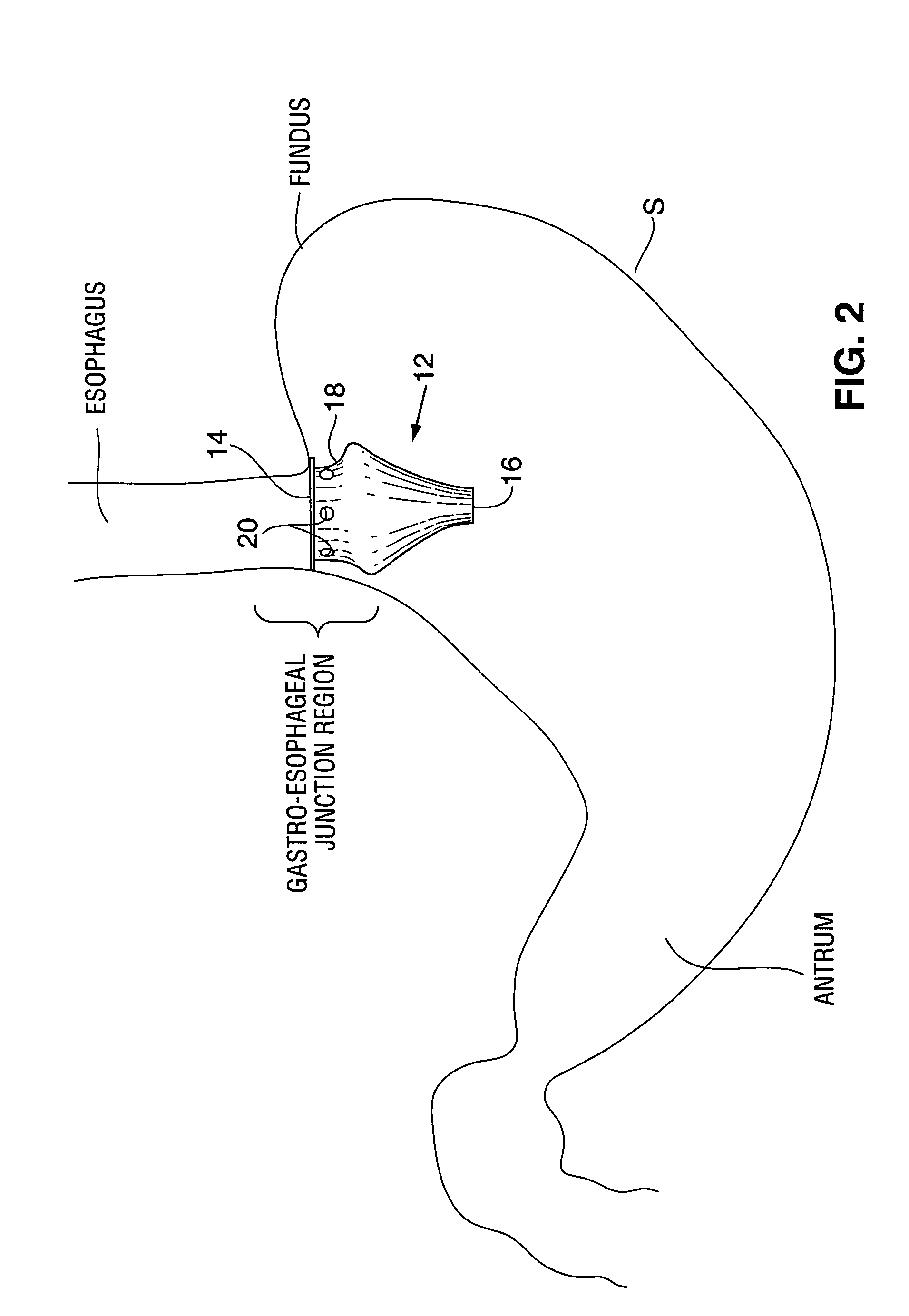
Satiation devices and methods
A device for inducing weight loss in a patient includes a tubular prosthesis positionable at the gastro-esophageal junction region, preferably below the z-line. In a method for inducing weight loss, the prosthesis is placed such that an opening at its proximal end receives masticated food from the esophagus, and such that the masticated food passes through the pouch and into the stomach via an opening in its distal end.
Owner:BOSTON SCI SCIMED INC
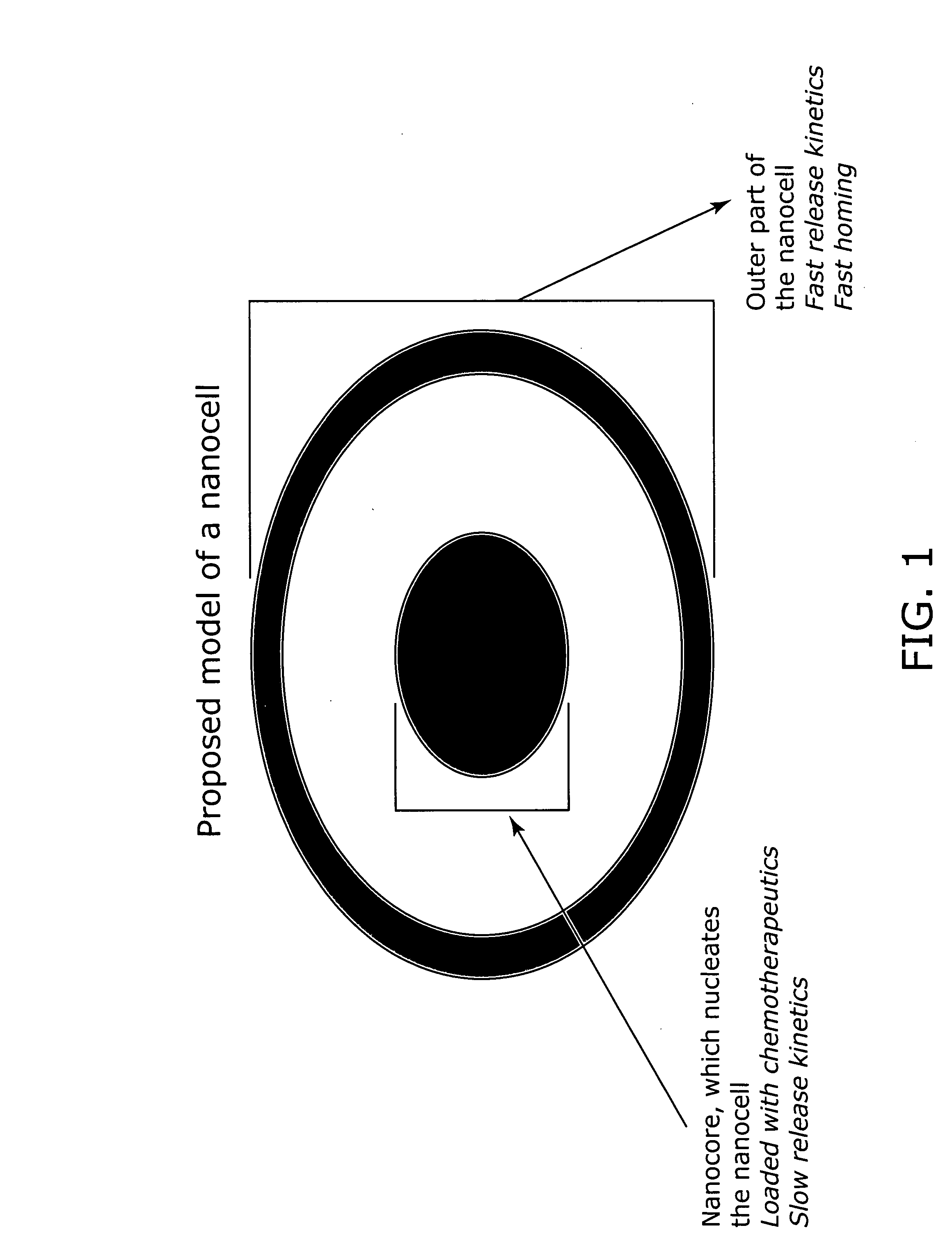
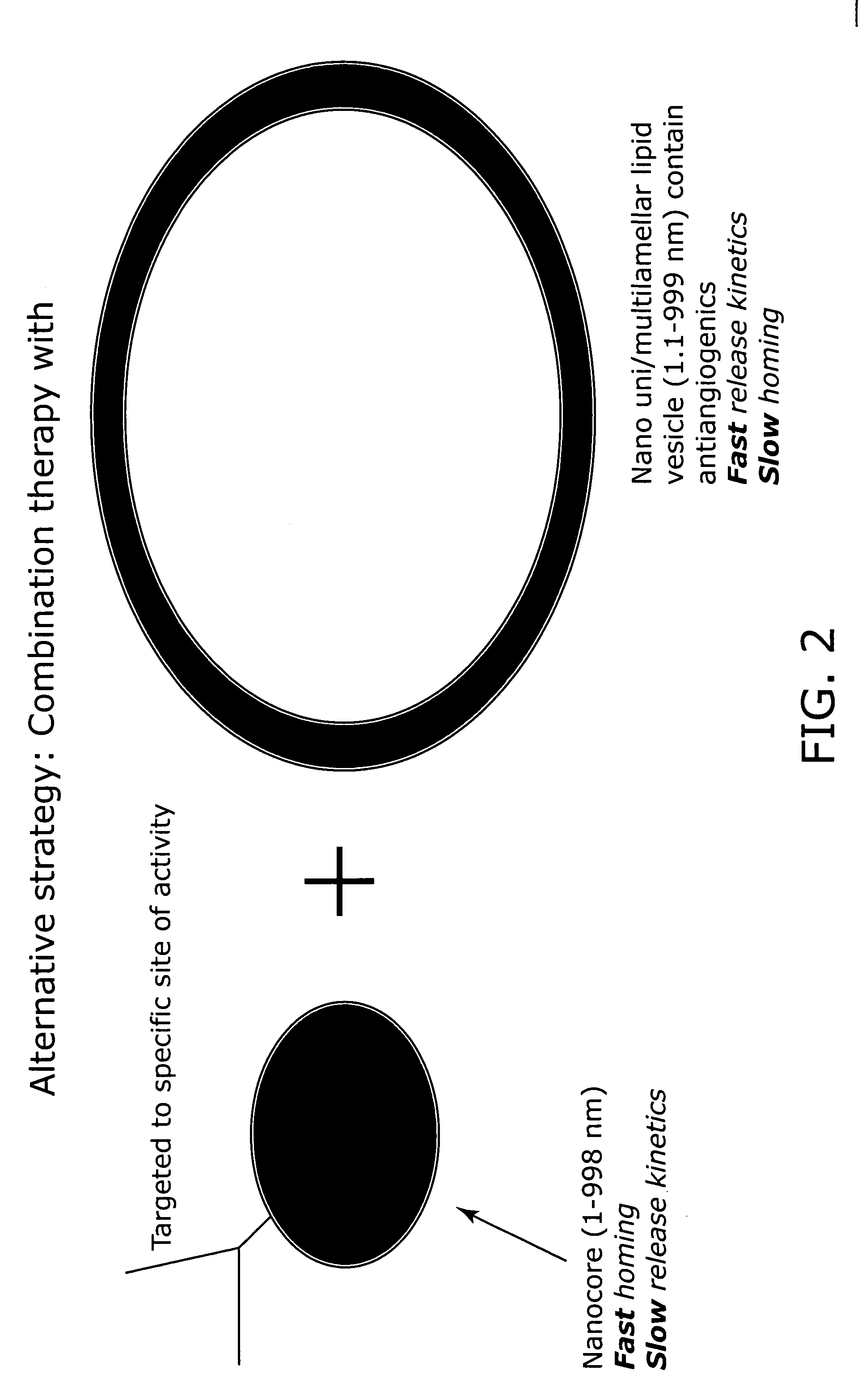
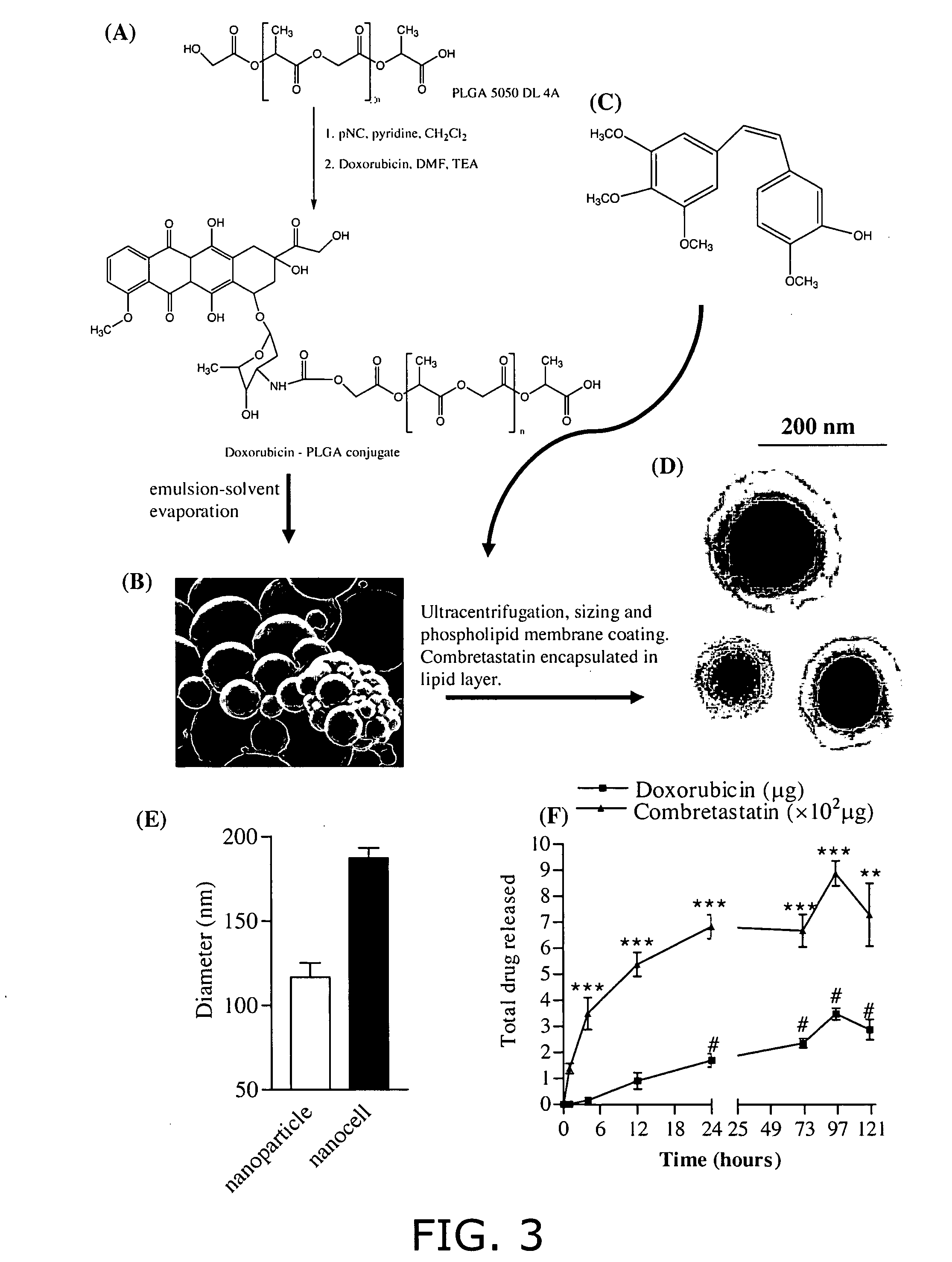
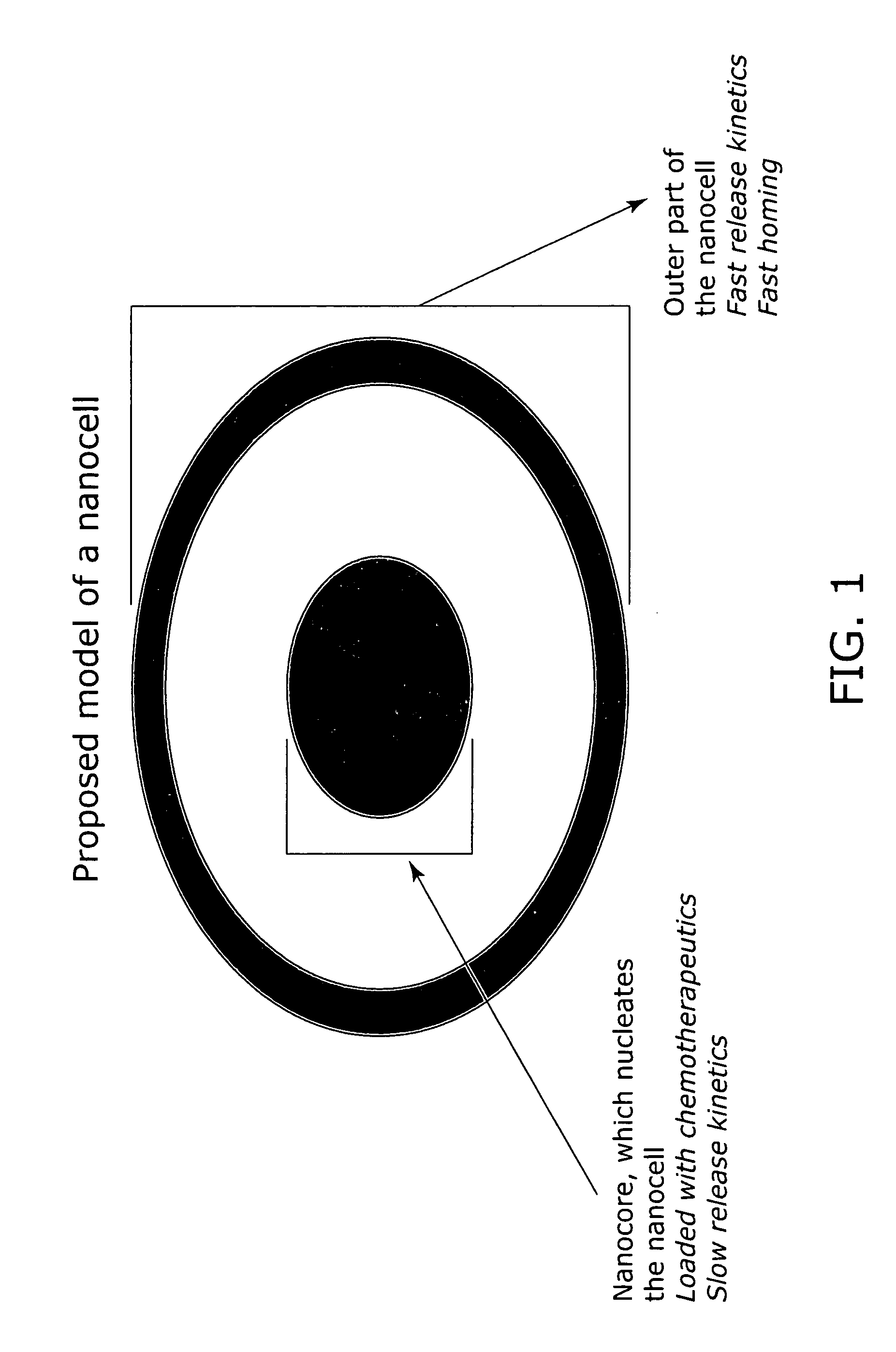
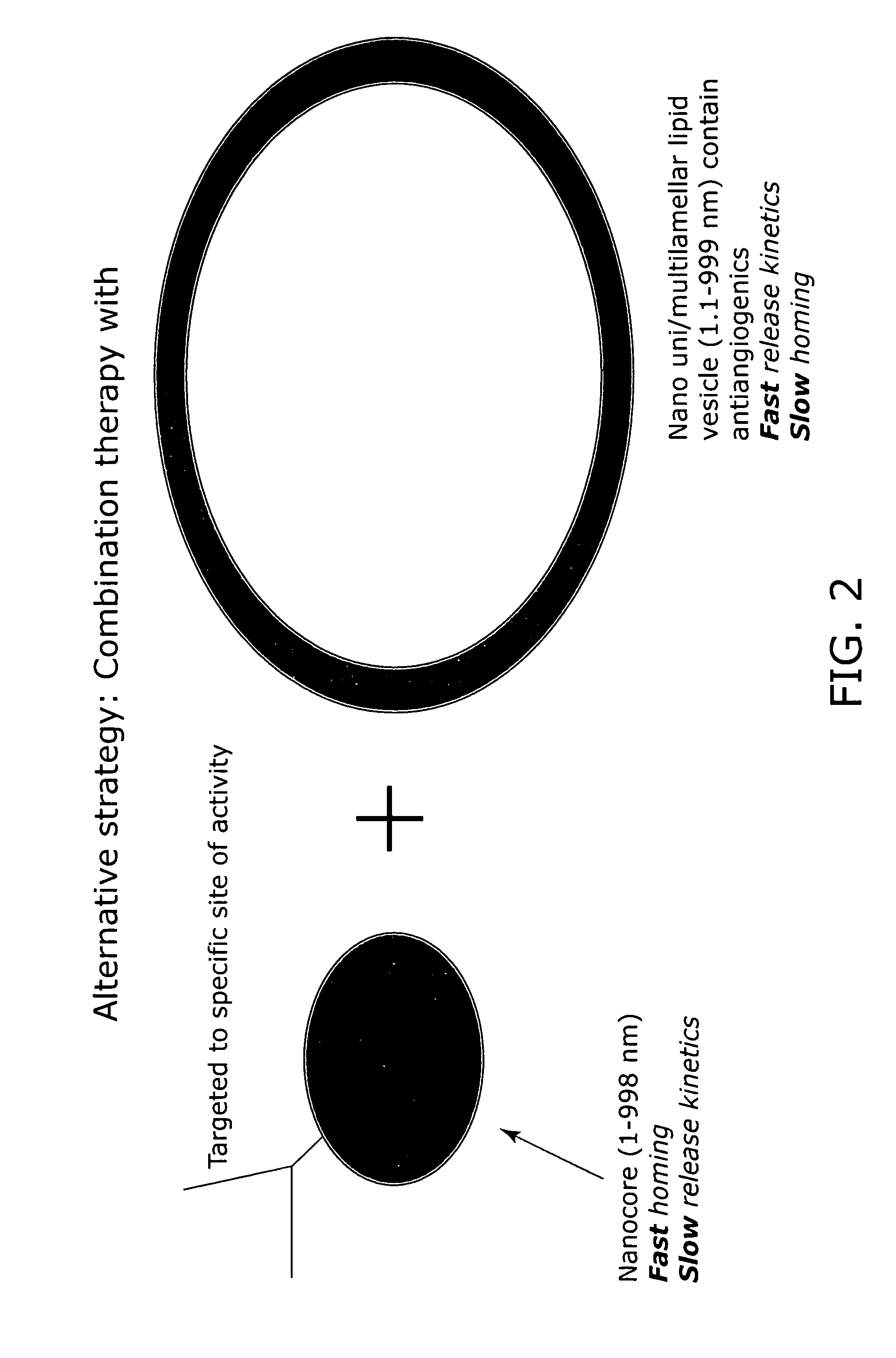
Nanocell drug delivery system
InactiveUS20050266067A1Avoid flowIncreased toxicityAntibacterial agentsOrganic active ingredientsLipid formationAntigen
Nanocells allow the sequential delivery of two different therapeutic agents with different modes of action or different pharmacokinetics. A nanocell is formed by encapsulating a nanocore with a first agent inside a lipid vesicle containing a second agent. The agent in the outer lipid compartment is released first and may exert its effect before the agent in the nanocore is released. The nanocell delivery system may be formulated in pharmaceutical composition for delivery to patients suffering from diseases such as cancer, inflammatory diseases such as asthma, autoimmune diseases such as rheumatoid arthritis, infectious diseases, and neurological diseases such as epilepsy. In treating cancer, a traditional antineoplastic agent is contained in the outer lipid vesicle of the nanocell, and an antiangiogenic agent is loaded into the nanocore. This arrangement allows the antineoplastic agent to be released first and delivered to the tumor before the tumor's blood supply is cut off by the antianiogenic agent.
Owner:MASSACHUSETTS INST OF TECH
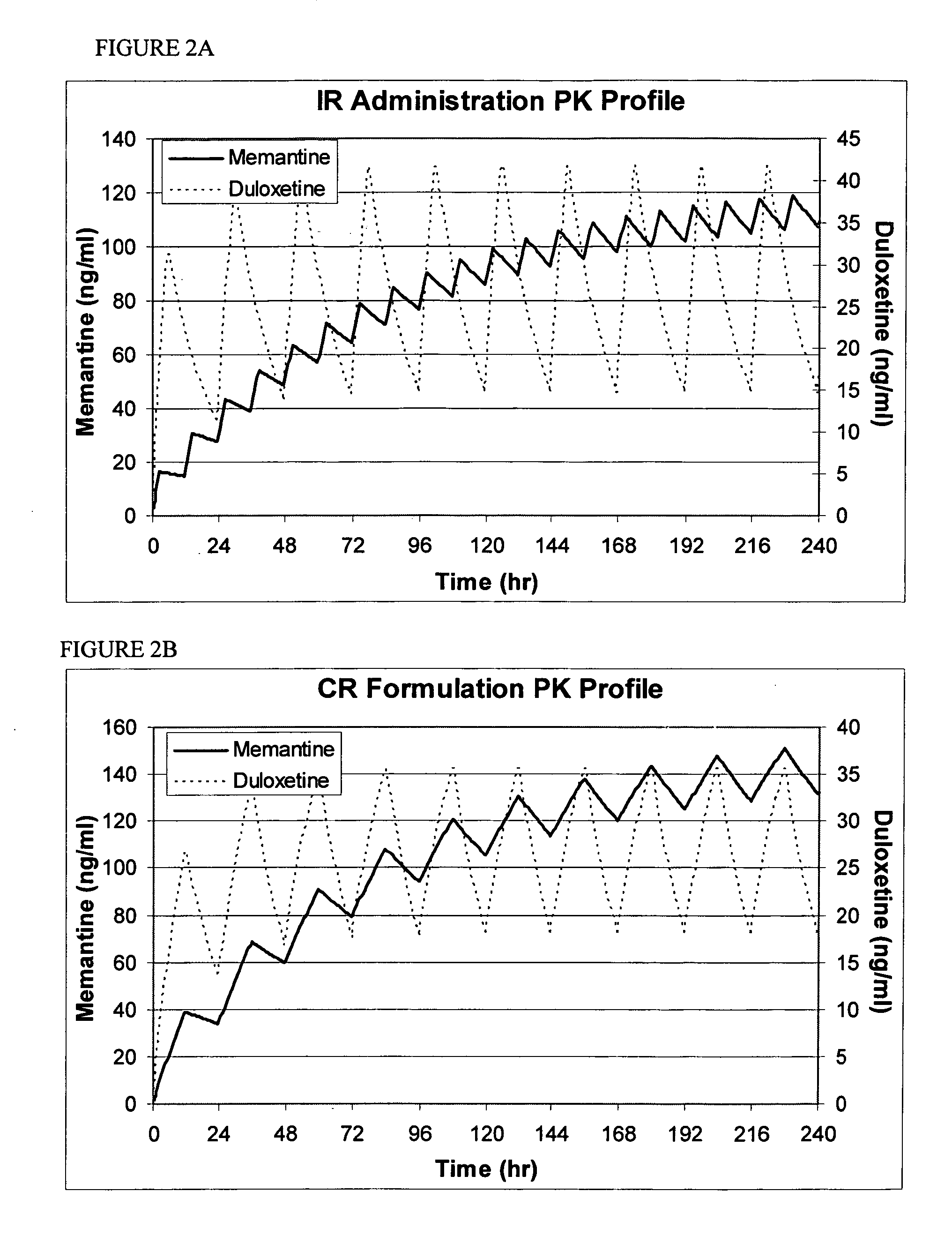
Methods and compositions for the treatment of psychiatric conditions
InactiveUS20050209218A1Low variabilityMaximizes therapeutic benefitBiocideNervous disorderDiseasePsychogenic disease
Owner:ADAMAS PHARMA INC
Methods and devices for providing acoustic hemostasis
InactiveUS6083159AEasy to aimReduce releaseChiropractic devicesEye exercisersInternal bleedingRadiology
Methods and apparatus for the remote coagulation of blood using high-intensity focused ultrasound (HIFU) are provided. A remote hemostasis method comprises identifying a site of internal bleeding and focusing therapeutic ultrasound energy on the site, the energy being focused through an intervening tissue. An apparatus for producing remote hemostasis comprises a focused therapeutic ultrasound radiating surface and a sensor for identifying a site of internal bleeding, with a registration means coupled to the radiating surface and the sensor to bring a focal target and the bleeding site into alignment. The sensor generally comprises a Doppler imaging display. Hemostasis enhancing agents may be introduced to the site for actuation by the ultrasound energy.
Owner:THS INT
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
ActiveUS7399488B2Good treatment effectSmall dosePowder deliveryNervous disorderAdditive ingredientWater insoluble
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, a drug is modified to increase its lipophilicity. In preferred embodiments the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble, but enzymatically degradable by enzymes present in the human gastrointestinal tract. The abuse-deterrent composition retards the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
Inorganic/organic nano composite antibacterial agent and its fabric product application
The invention relates to an inorganic / organic nano-composite antibacterial and application of fiber product thereof. 0.01-20 proportions of inorganic antibacterial, 0.01-35 proportions of organic antibacterial, 0.0001-0.4 proportion of inorganic / organic antibacterial compatibility finishing agent, 0.05-40 proportions of macromolecule bond, 0.0001-0.4 proportion of stabilizer that prevents color change of the inorganic antibacterial, 0.1-30 proportions of high efficiency emulsifying agent and 100 proportions of distilled water are added in sequence by weight proportion, wetted, dispersed, ground, dispersed at high-speed, stirred, etc., so as to acquire the nano-composite antibacterial. The antibacterial can process the fiber product by a dip-dye method, a roll-dye method, a spray method, etc. Dresses, socks and socks, blankets, beddings, gowns, patient wears, masks, medicinal gauzes, air filtering nettings, etc., made from the inorganic / organic nano-composite antibacterial of the invention are characterized by high antibacterial efficiency, rapid antibacterial function, long lasting antibacterial performance, laundering durability, etc., and the inorganic / organic nano-composite antibacterial of the invention is applicable to life, medical care, etc., and has the functions of preventing the occurrence and transmission of diseases, protecting the health of people and improving life quality.
Owner:BEIJING CHAMGO NANO TECH
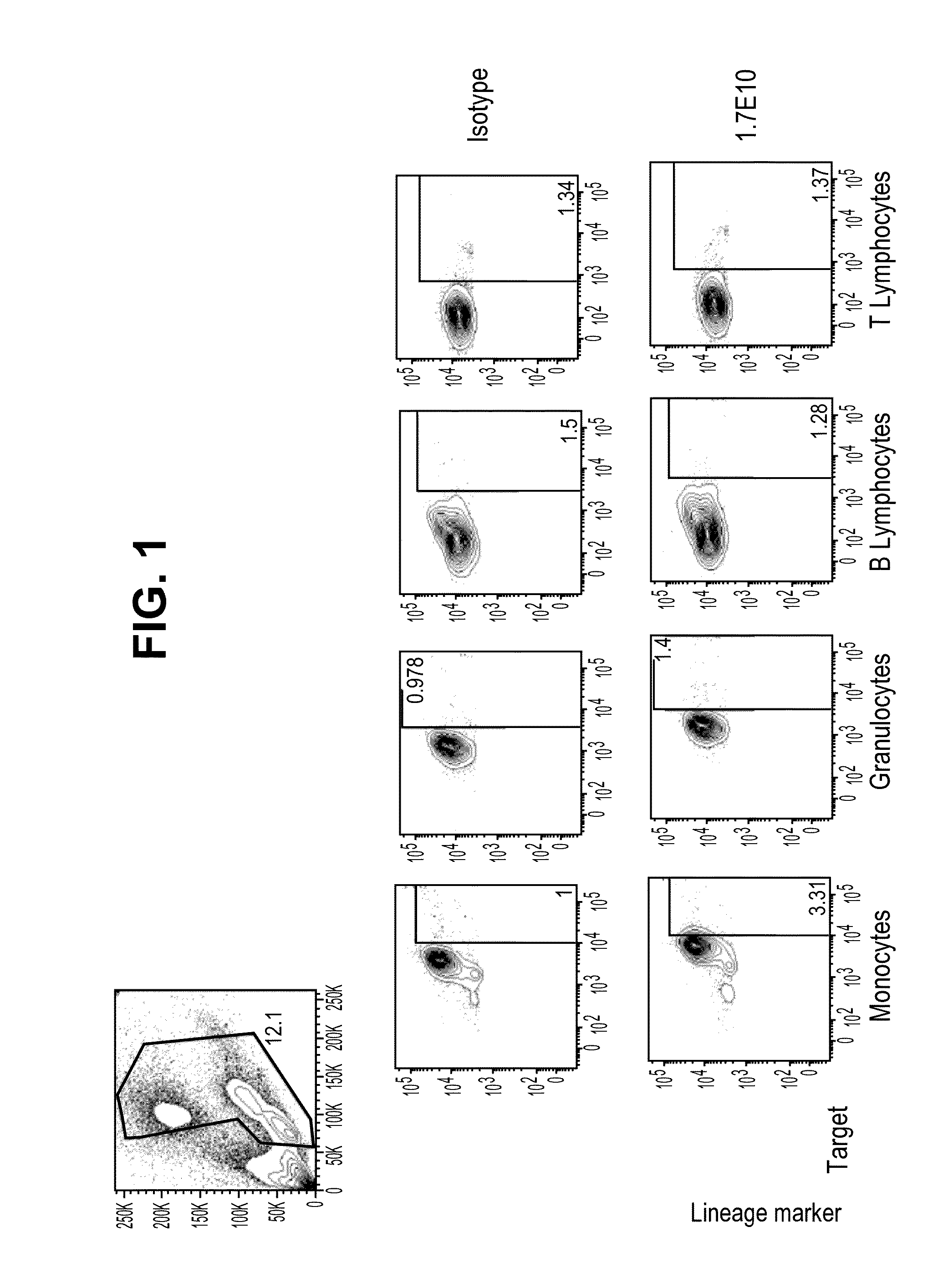
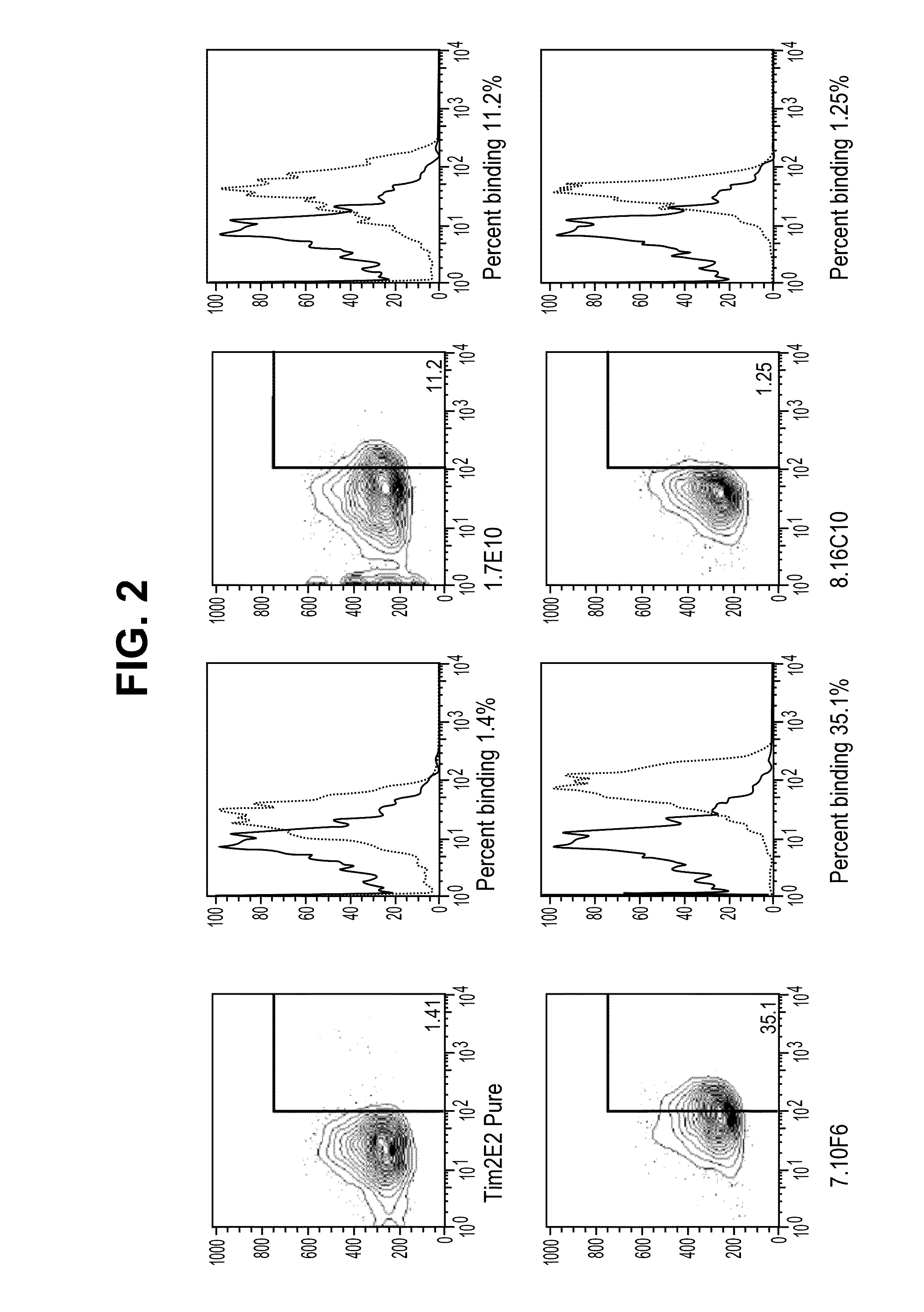
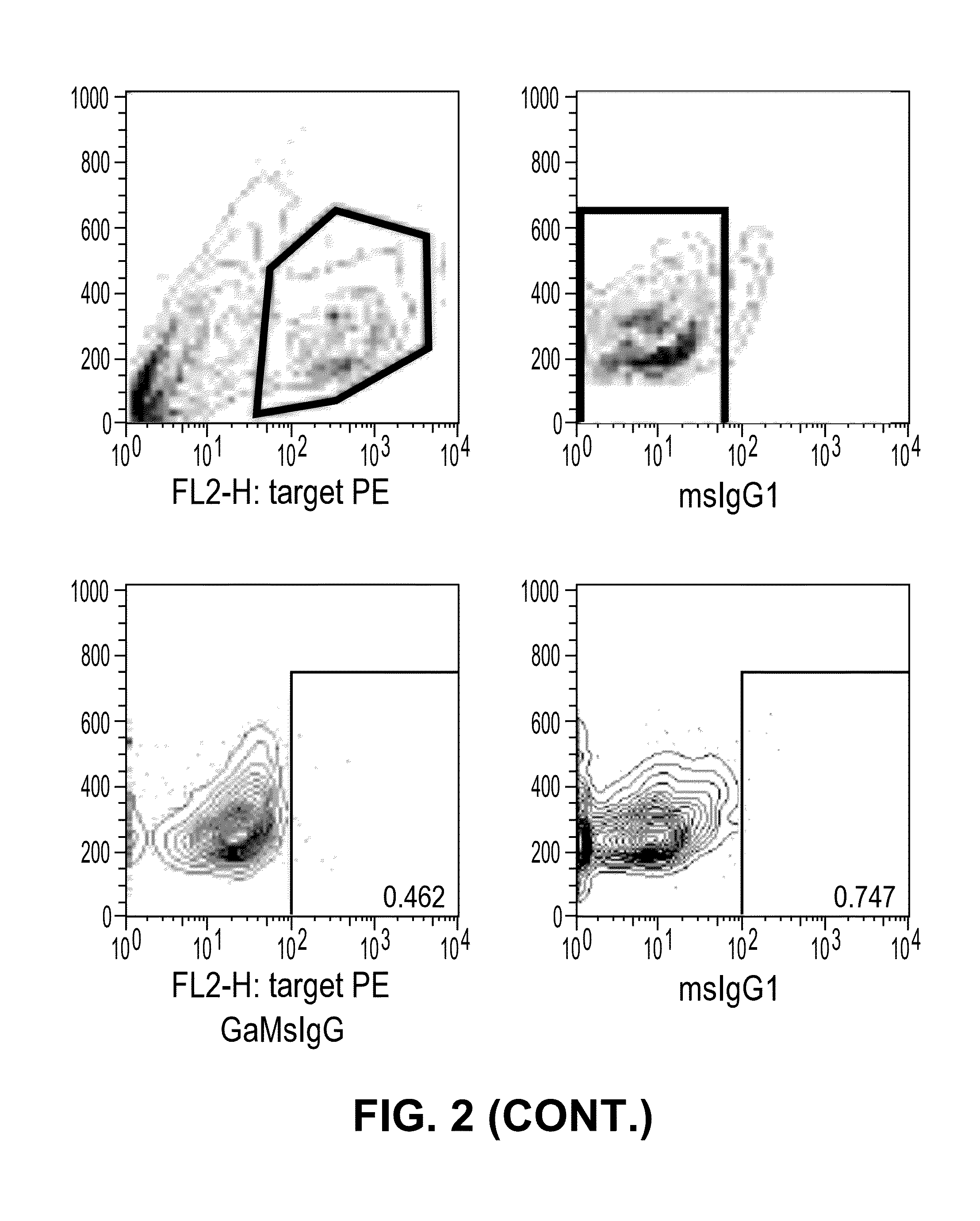
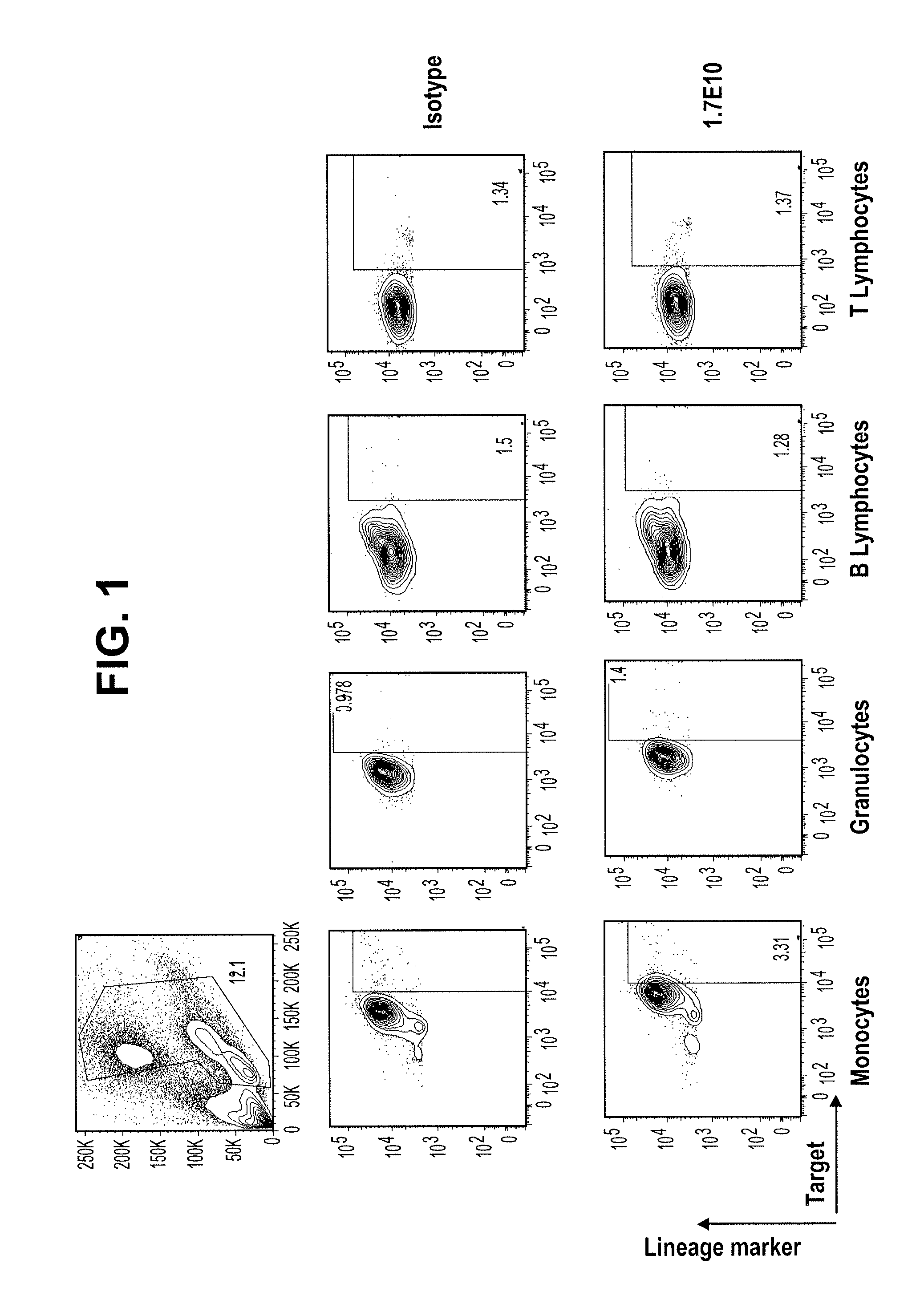
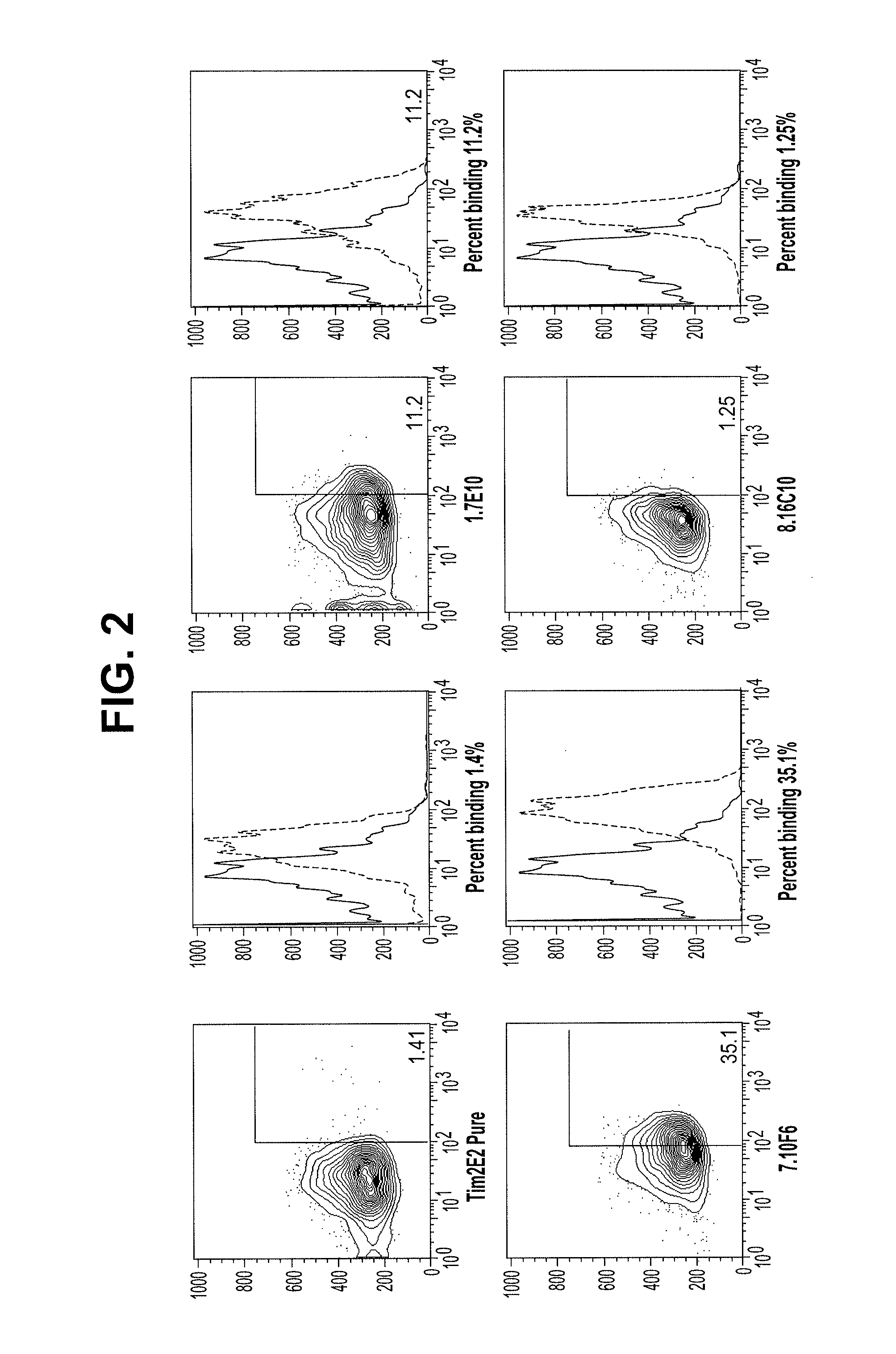
Antibodies that specifically bind to TIM3
ActiveUS8841418B2Promote growthReduce inflammationHeavy metal active ingredientsPeptide/protein ingredientsCancer cellAntibody
Provided herein are antibodies specific for TIM3 that can be used to detect cancer cells, in particular, cancer stem cells. The antibodies can also be used in therapeutic compositions for treating cancer and reducing inflammation.
Owner:ONK THERAPEUTICS LTD
Nanocell drug delivery system
InactiveUS20070053845A1Lose efficacyIncreased toxicityPowder deliveryPeptide/protein ingredientsNervous systemAsthma
Nanocells allow the sequential delivery of two different therapeutic agents with different modes of action or different pharmacokinetics. A nanocell is formed by encapsulating a nanocore with a first agent inside a lipid vesicle containing a second agent. The agent in the outer lipid compartment is released first and may exert its effect before the agent in the nanocore is released. The nanocell delivery system may be formulated in pharmaceutical composition for delivery to patients suffering from diseases such as cancer, inflammatory diseases such as asthma, autoimmune diseases such as rheumatoid arthritis, infectious diseases, and neurological diseases such as epilepsy. In treating cancer, a traditional antineoplastic agent is contained in the outer lipid vesicle of the nanocell, and an antiangiogenic agent is loaded into the nanocore. This arrangement allows the antineoplastic agent to be released first and delivered to the tumor before the tumor's blood supply is cut off by the antianiogenic agent.
Owner:MASSACHUSETTS INST OF TECH
HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINES AND PYRROLO[2,3-b]PYRIMIDINES AS JANUS KINASE INHIBITORS
The present invention provides heteroaryl substituted pyrrolo[2,3-b]pyridines and heteroaryl substituted pyrrolo[2,3-b]pyrimidines that modulate the activity of Janus kinases and are useful in the treatment of diseases related to activity of Janus kinases including, for example, immune-related diseases, skin disorders, myeloid proliferative disorders, cancer, and other diseases.
Owner:INCYTE HLDG & INCYTE
Methods of synthesis and use of chemospheres
InactiveUS20110104052A1Prevents immunoclearanceExtended half-lifePowder deliveryBiocideDiagnostic agentComputed tomography
The present invention provides, in general, compositions comprising a hydrogel and an agent, for example a therapeutic agent or an imaging agent, for locoregional delivery. In certain preferred embodiments of the invention, the hydrogel compositions are detectable by Magnetic Responance and CT Scan and are used for locoregional delivery of therapeutic agents, for example chemotherapeutic agents. The invention also features polymer matrix compositions comprising nanoparticles that can be loaded after polymerization with bioactive agents, for example a diagnostic agent or therapeutic agent.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
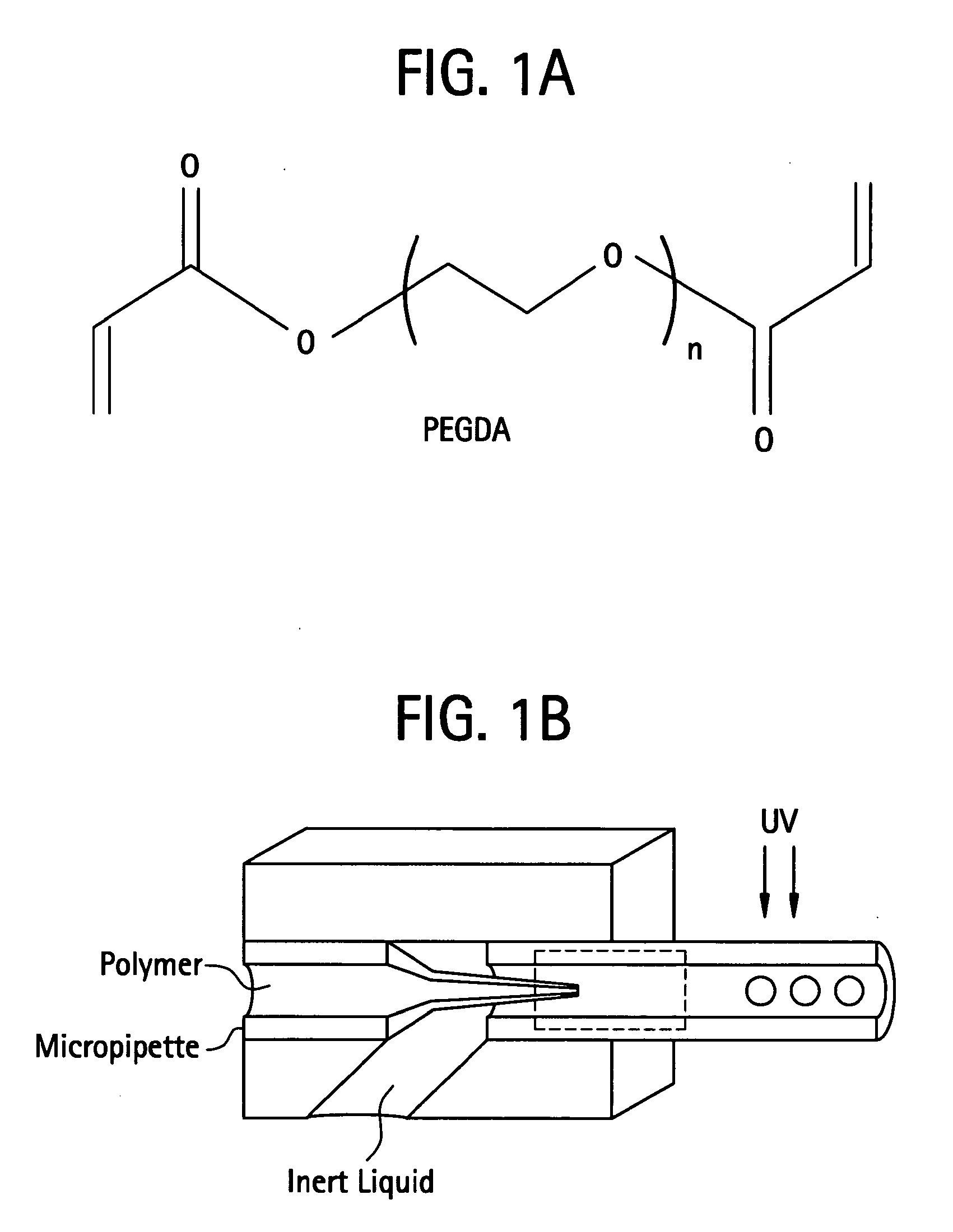
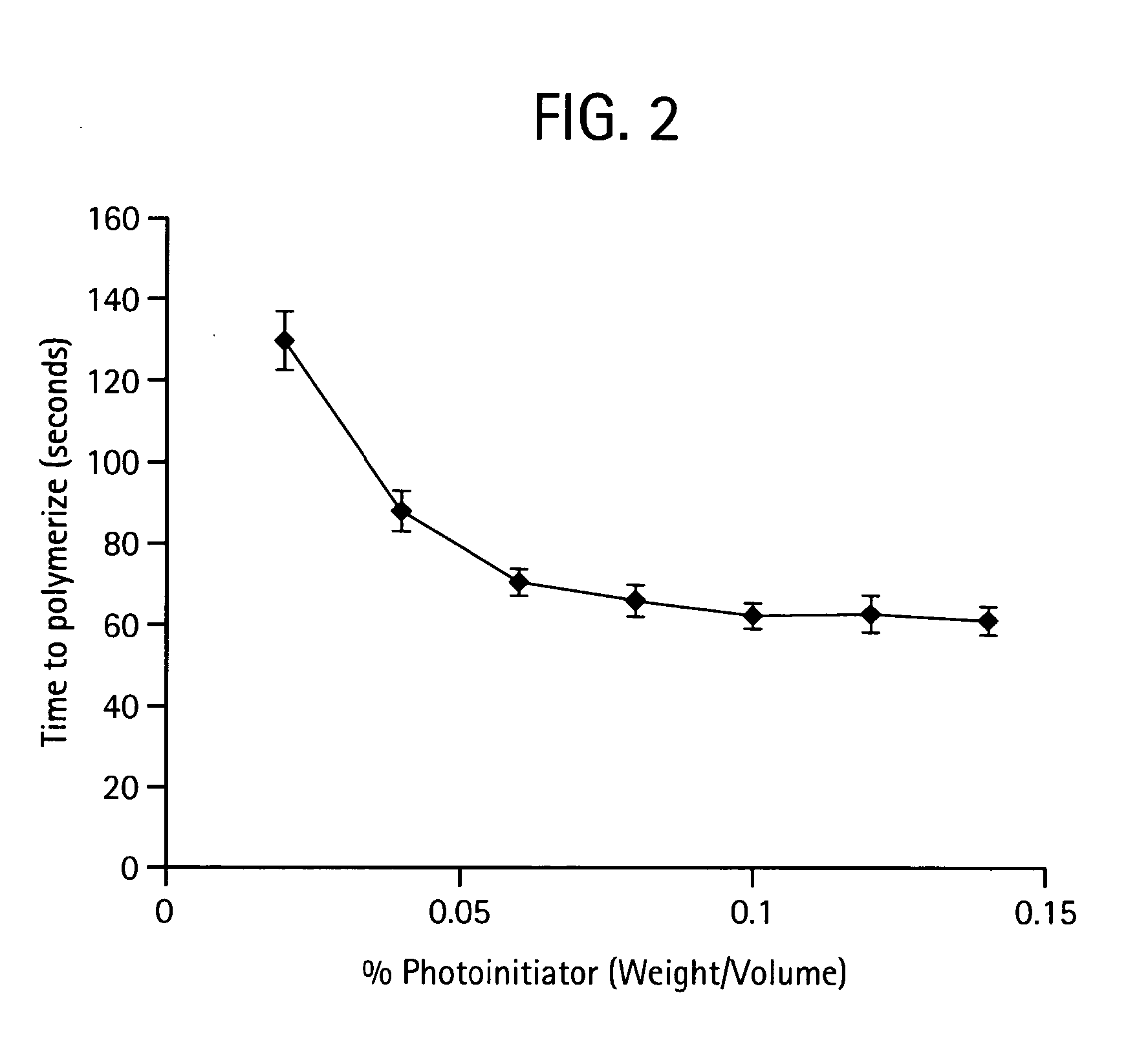
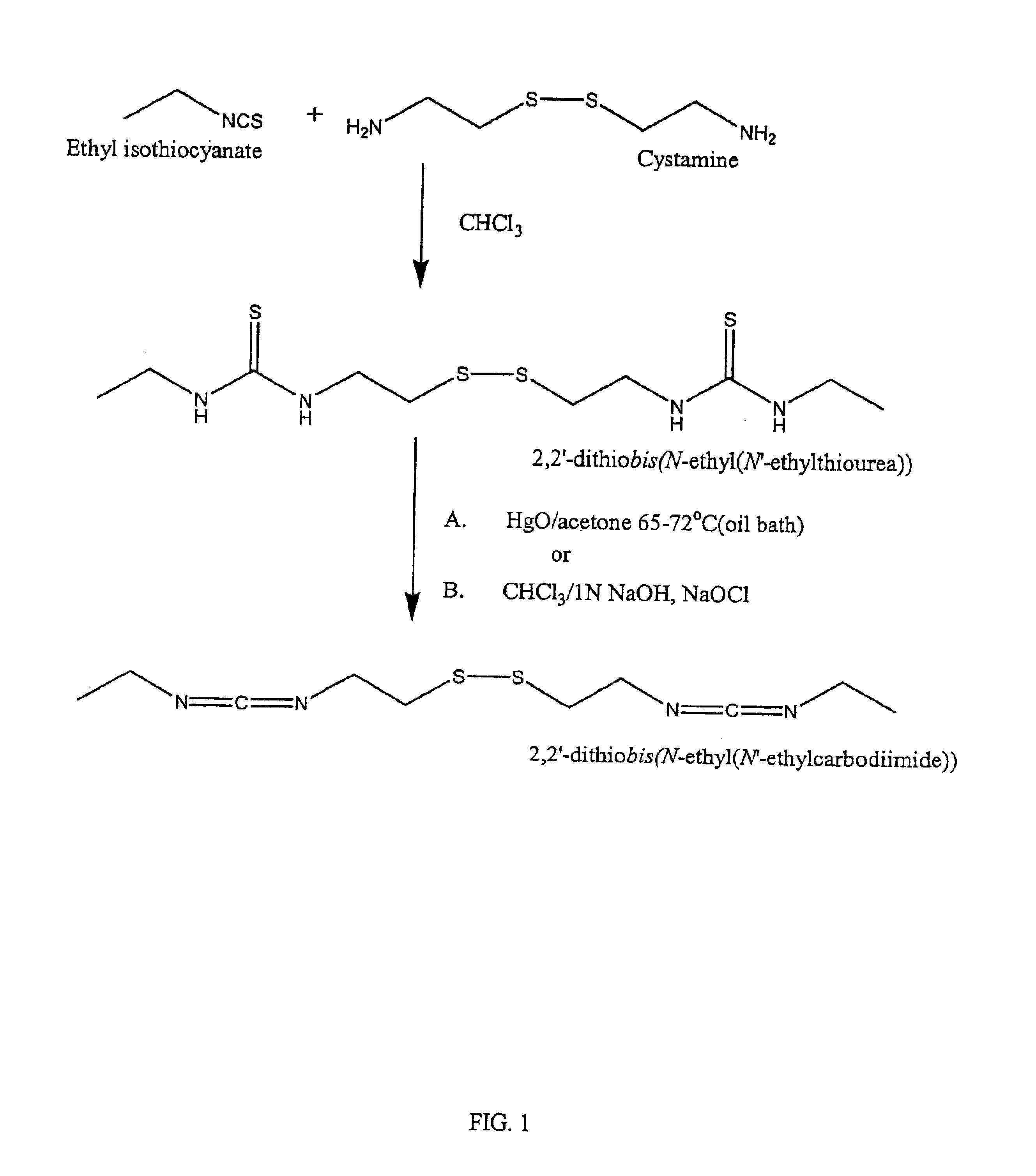
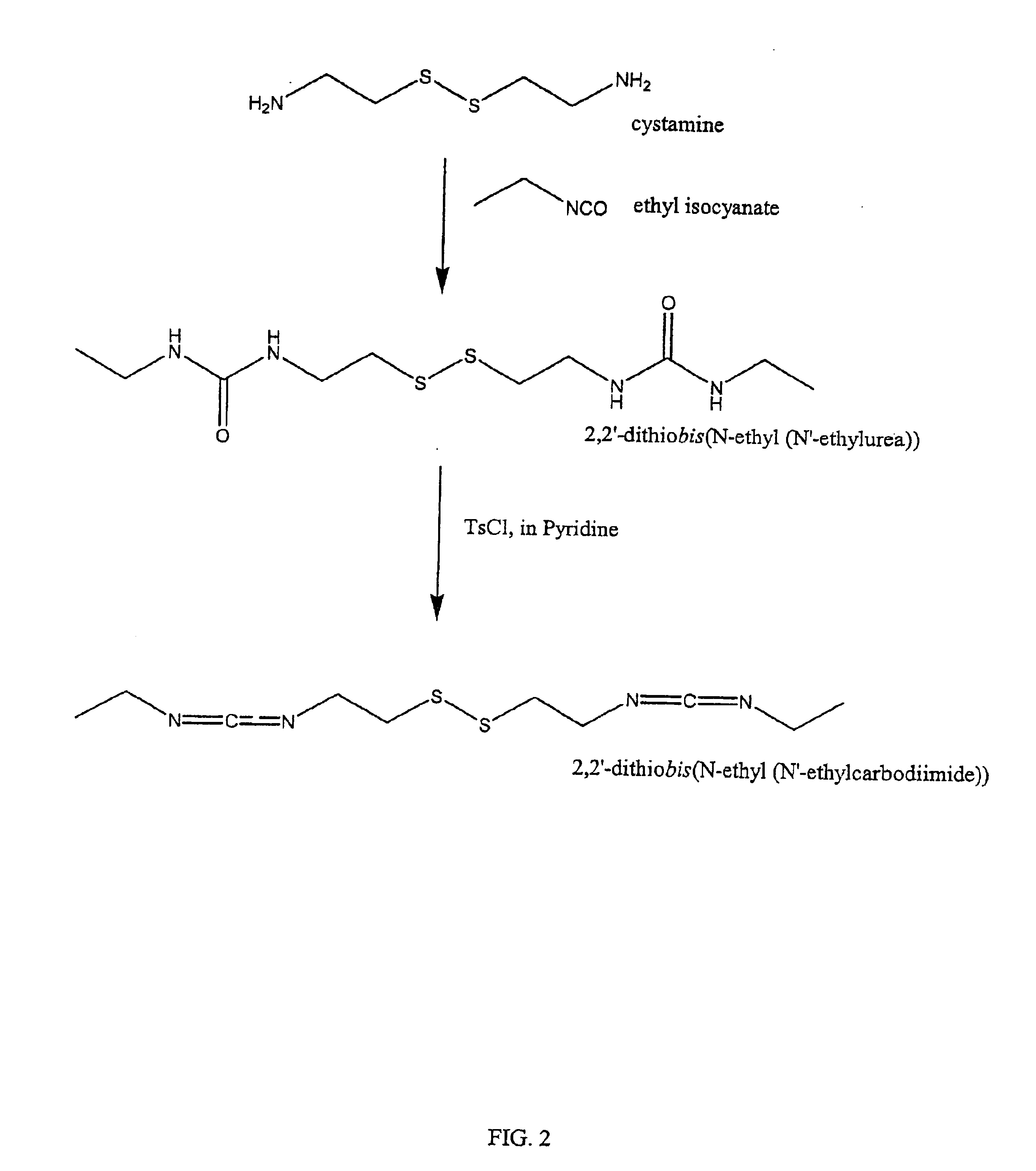
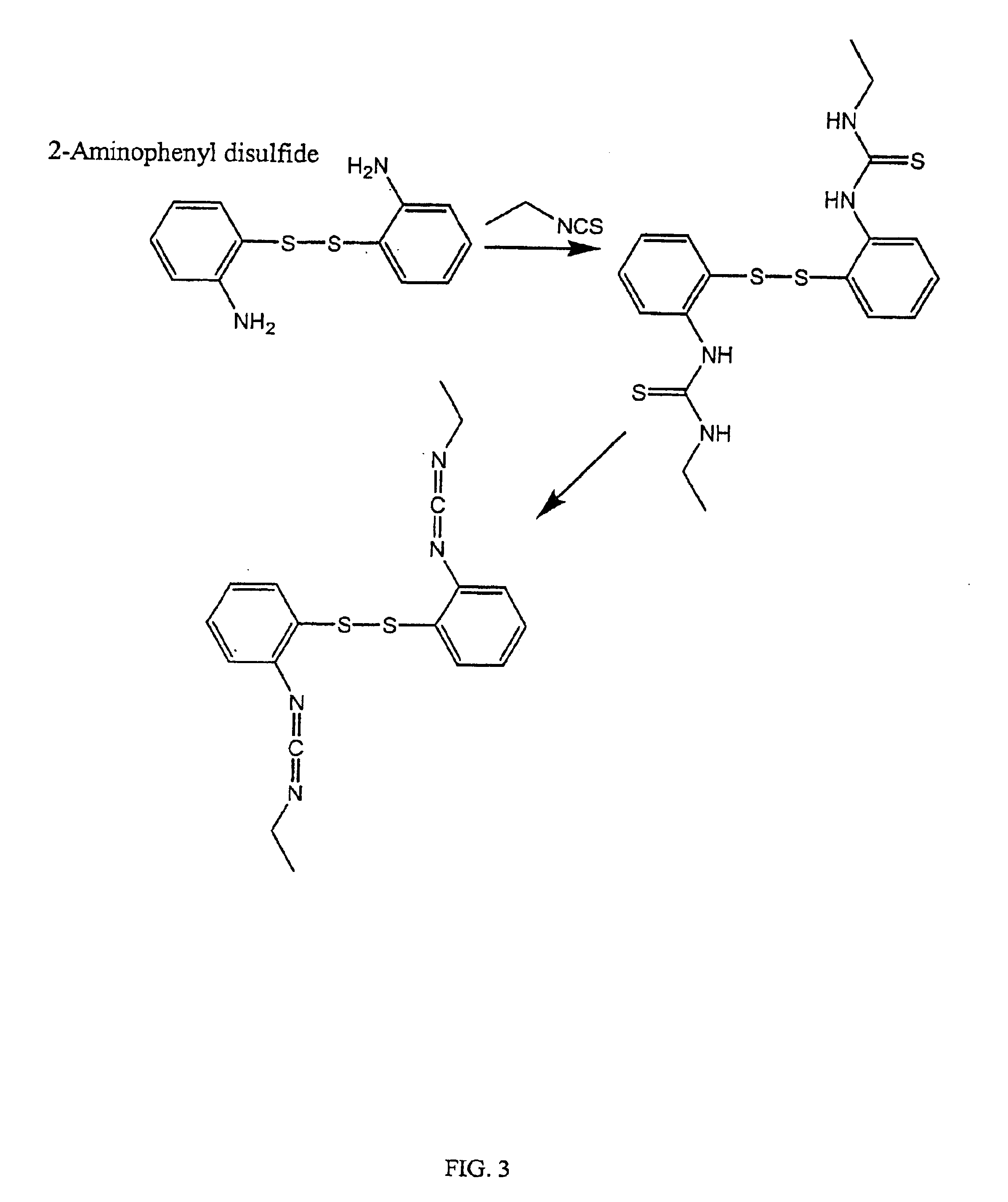
Thiol-modified hyaluronan
InactiveUS6884788B2High activityControl moreBiocideOrganic active ingredientsUrea derivativesCross-link
The present invention relates to biscarbodiimides, thiourea derivatives, urea derivatives, and cross-linked hyaluronan derivatives having at least one intramolecular disulfide bond, and methods of preparation thereof. The invention also includes thiolated hyaluronan derivatives and salts thereof having at least one pendant thiol group or a modified pendant thiol group, and methods of preparation thereof. An example of a modified pendant thiol group is a sulfhydryl group linked to a small molecule such as a bioactive agent, for example a drug or pharmaceutically active moiety. A hyaluronan derivative having a sulfhydryl group linked to a pharmaceutically active moiety is useful as a sustained or controlled release drug delivery vehicle. Compositions containing the hyaluronan derivatives of the invention can reversibly viscosify in vivo or in vitro, in response to mild changes in condition, and are thus useful in ophthalmic surgery and in tissue engineering.
Owner:ANIKA THERAPEUTICS INC
Delivery of therapeutic capable agents
The present invention provides improved stents and other prostheses for delivering substances to vascular and other luminal and intracorporeal environments. In particular, the present invention provides for therapeutic capable agent eluting stents with minimized undesirable loss of the therapeutic capable agent during expansion of the stent.
Owner:ALTAI MEDICAL TECH
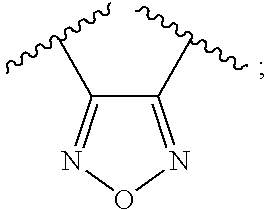
1,2,5-oxadiazoles as inhibitors of indoleamine 2,3-dioxygenase
ActiveUS20100015178A1Prevent immunosuppressionUseful in preparationBiocideSenses disorderDiseaseDioxygenase
The present invention is directed to 1,2,5-oxadiazole derivatives, and compositions of the same, which are inhibitors of indoleamine 2,3-dioxygenase and are useful in the treatment of cancer and other disorders, and to the processes and intermediates for making such 1,2,5-oxadiazole derivatives.
Owner:INCYTE
Antimicrobial mesoporous silica nanoparticles
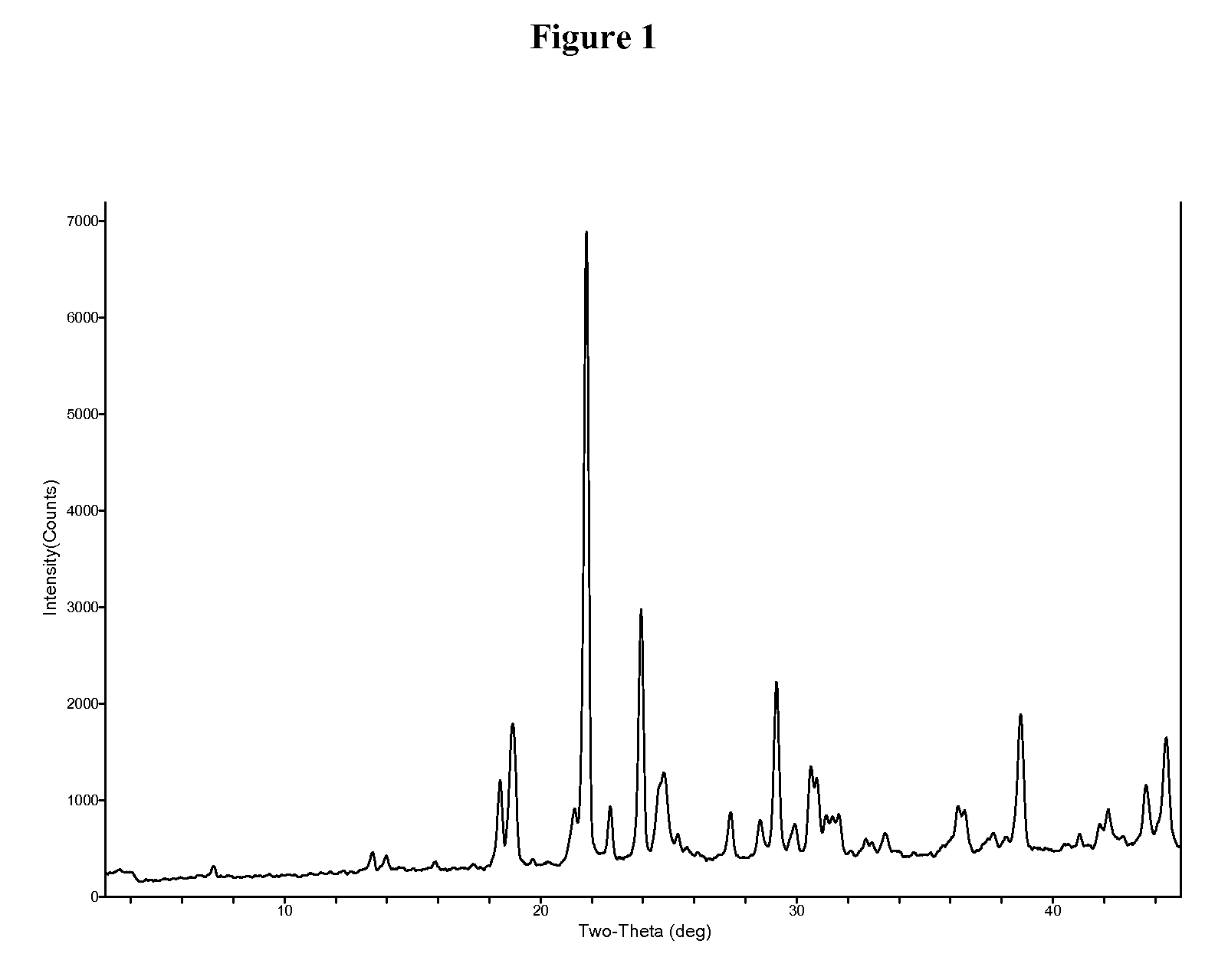
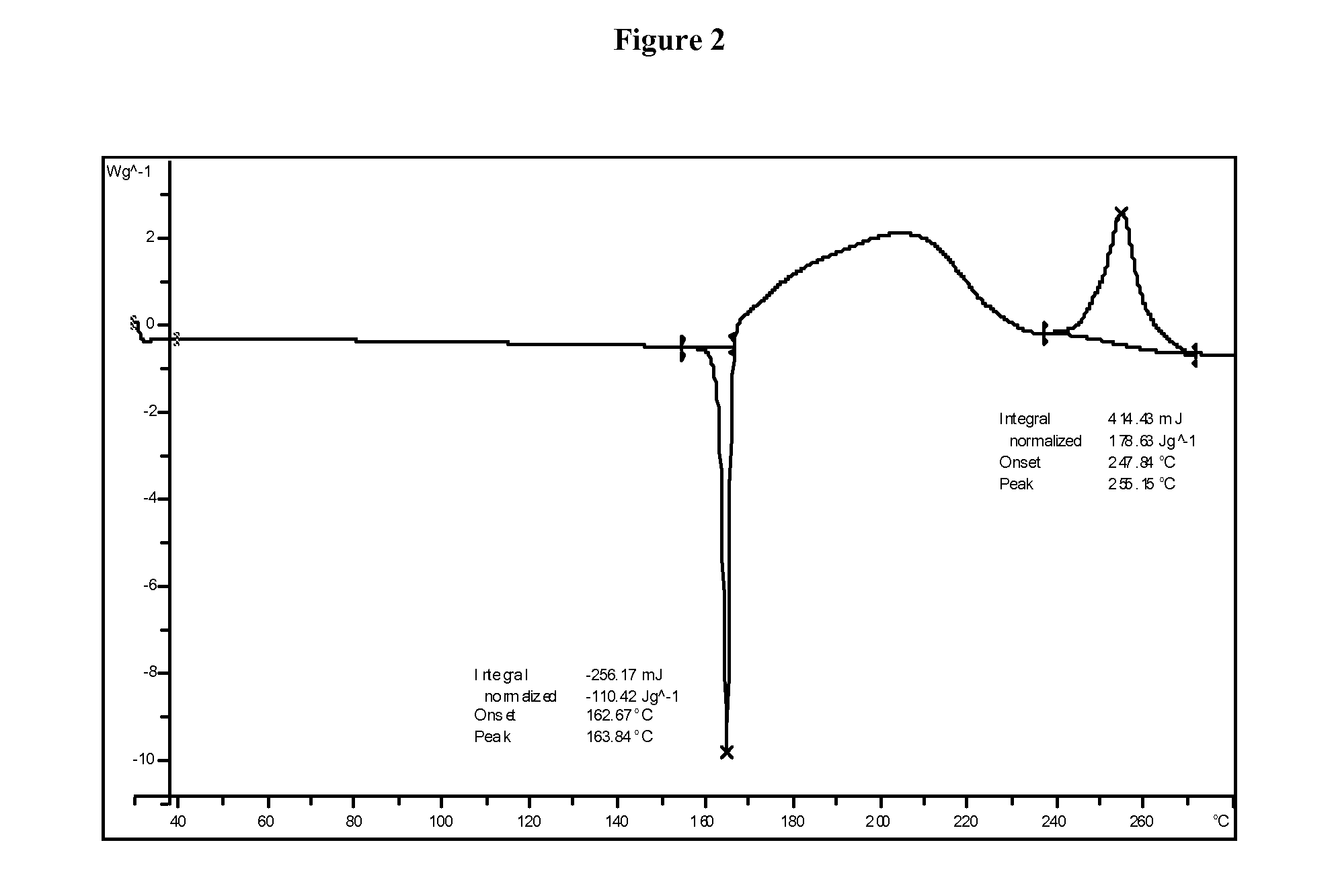
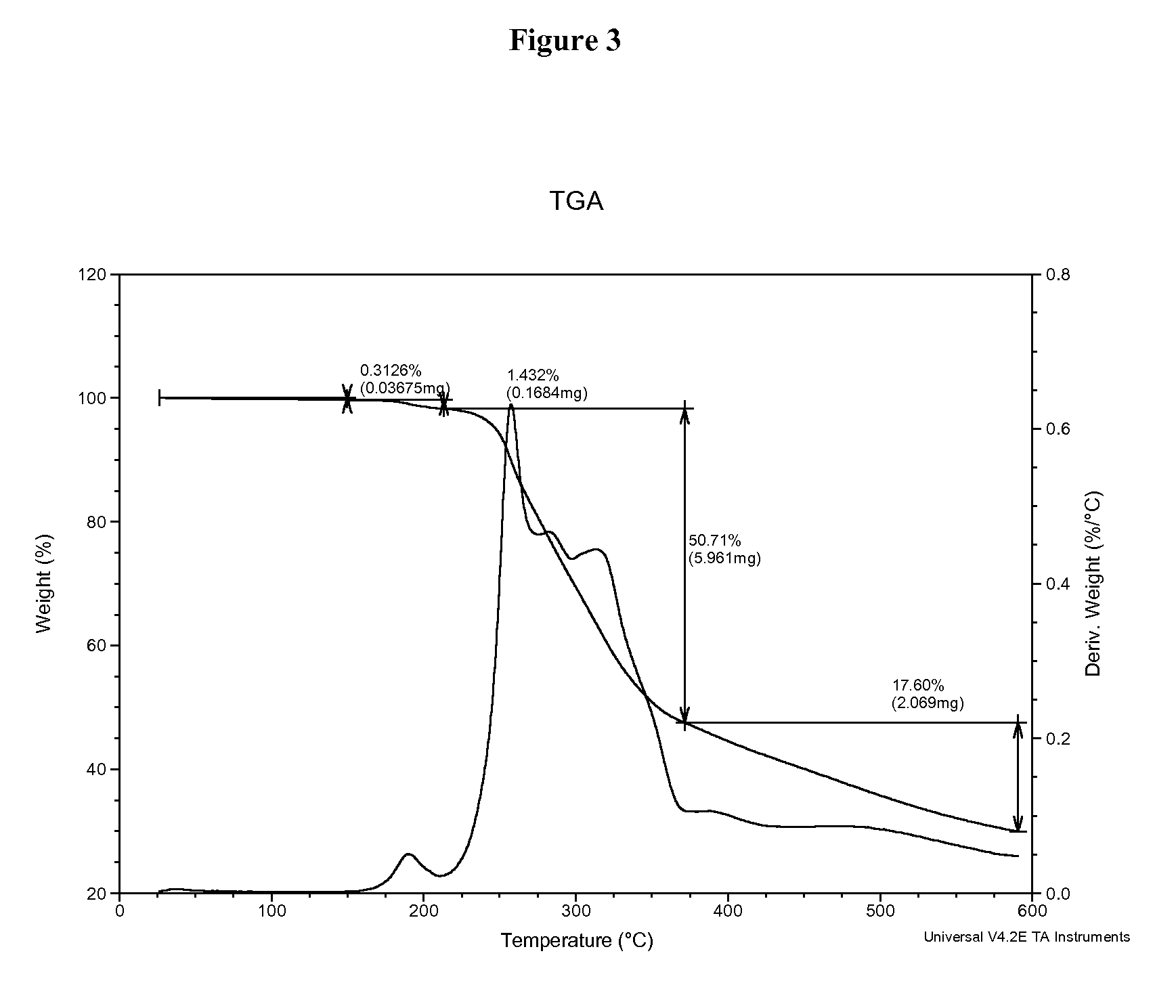
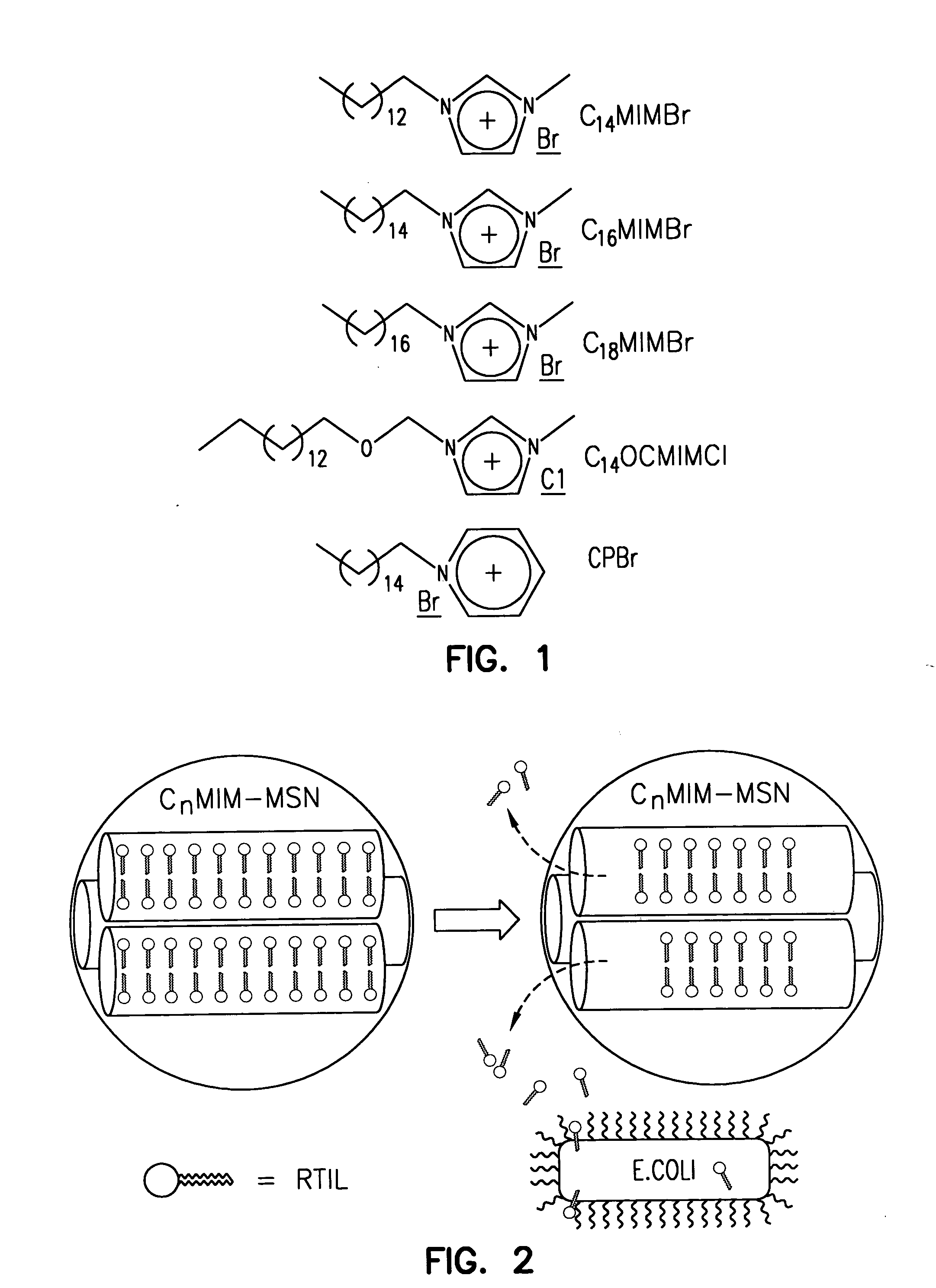
InactiveUS20060018966A1Reduce productionSlow diffusion ratePowder deliveryBiocideMesoporous silicaSilicon dioxide
Methods for preparing a series of mesoporous silicates, such as room-temperature ionic liquid (RTIL)-templated mesoporous silicate particles, with various particle morphologies are provided. Methods for preparing silicate particles with antimicrobial agents within the MSN pores is also provided. The particles can be used as controlled-release nanodevices to deliver antimicrobial agents.
Owner:IOWA STATE UNIV RES FOUND
Modulators of indoleamine 2,3-dioxygenase and methods of using the same
The present invention is directed to modulators of indoleamine 2,3-dioxygenase (IDO), as well as compositions and pharmaceutical methods thereof.
Owner:INCYTE
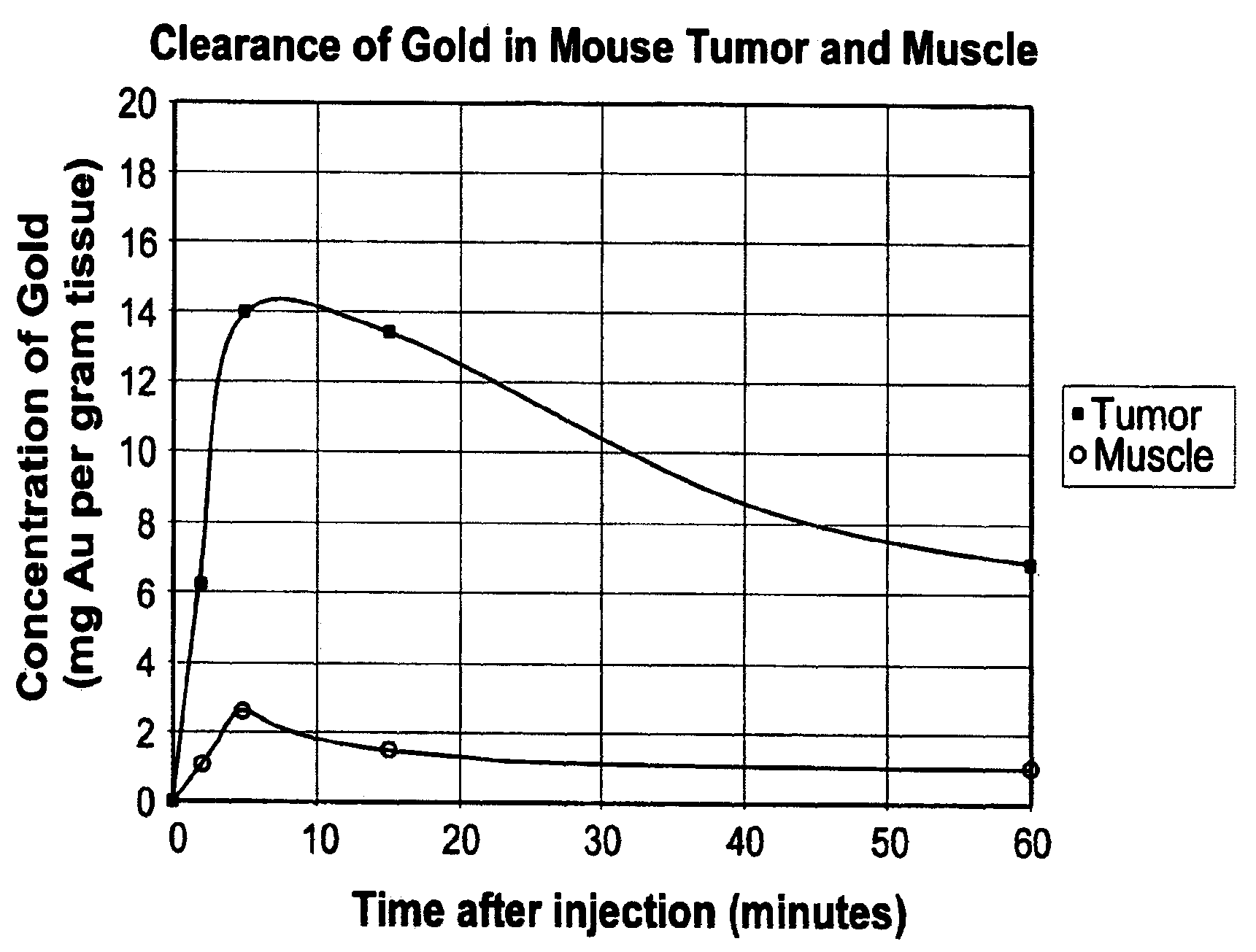
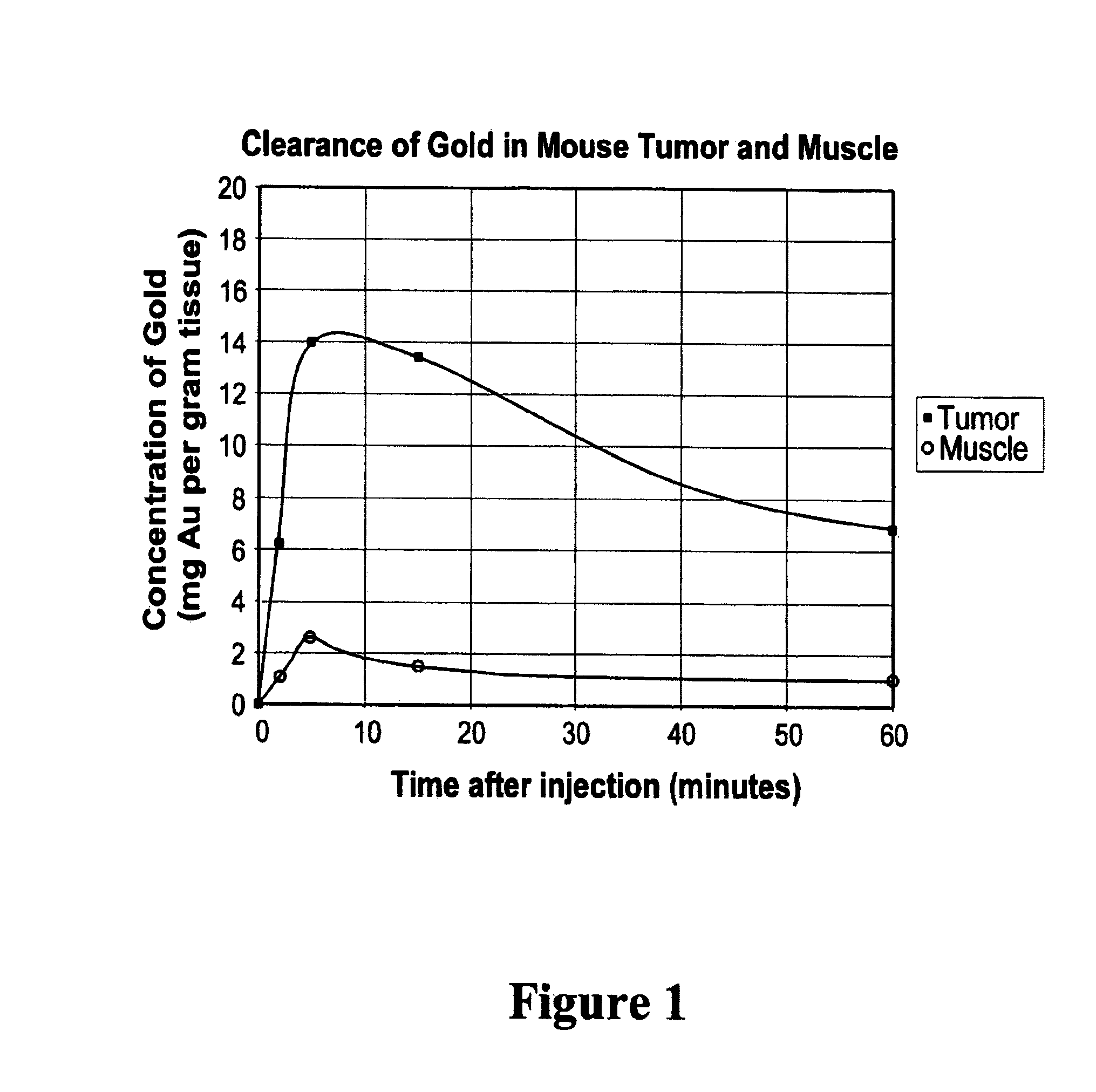
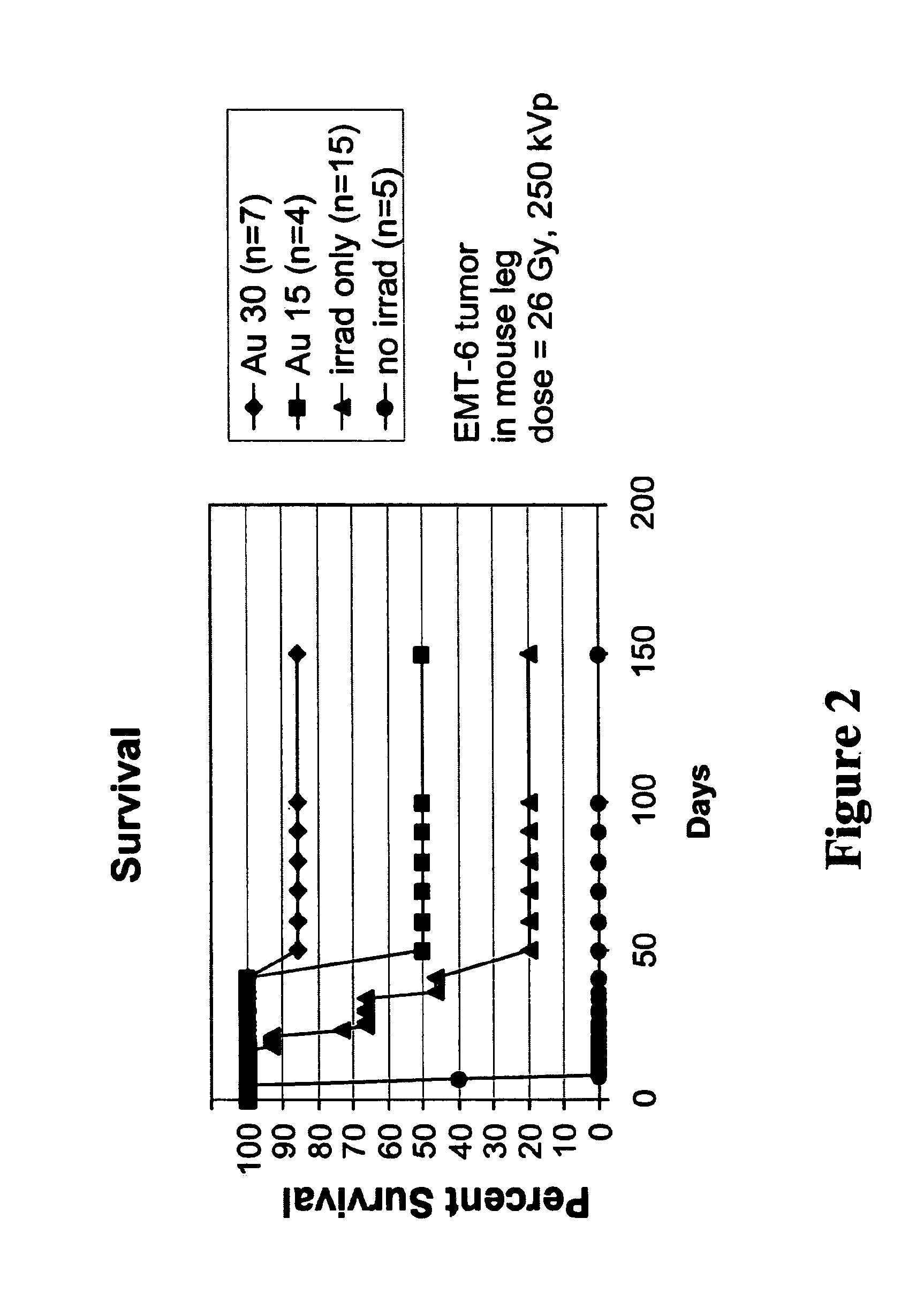
Methods of enhancing radiation effects with metal nanoparticles
Owner:NANOPROBES
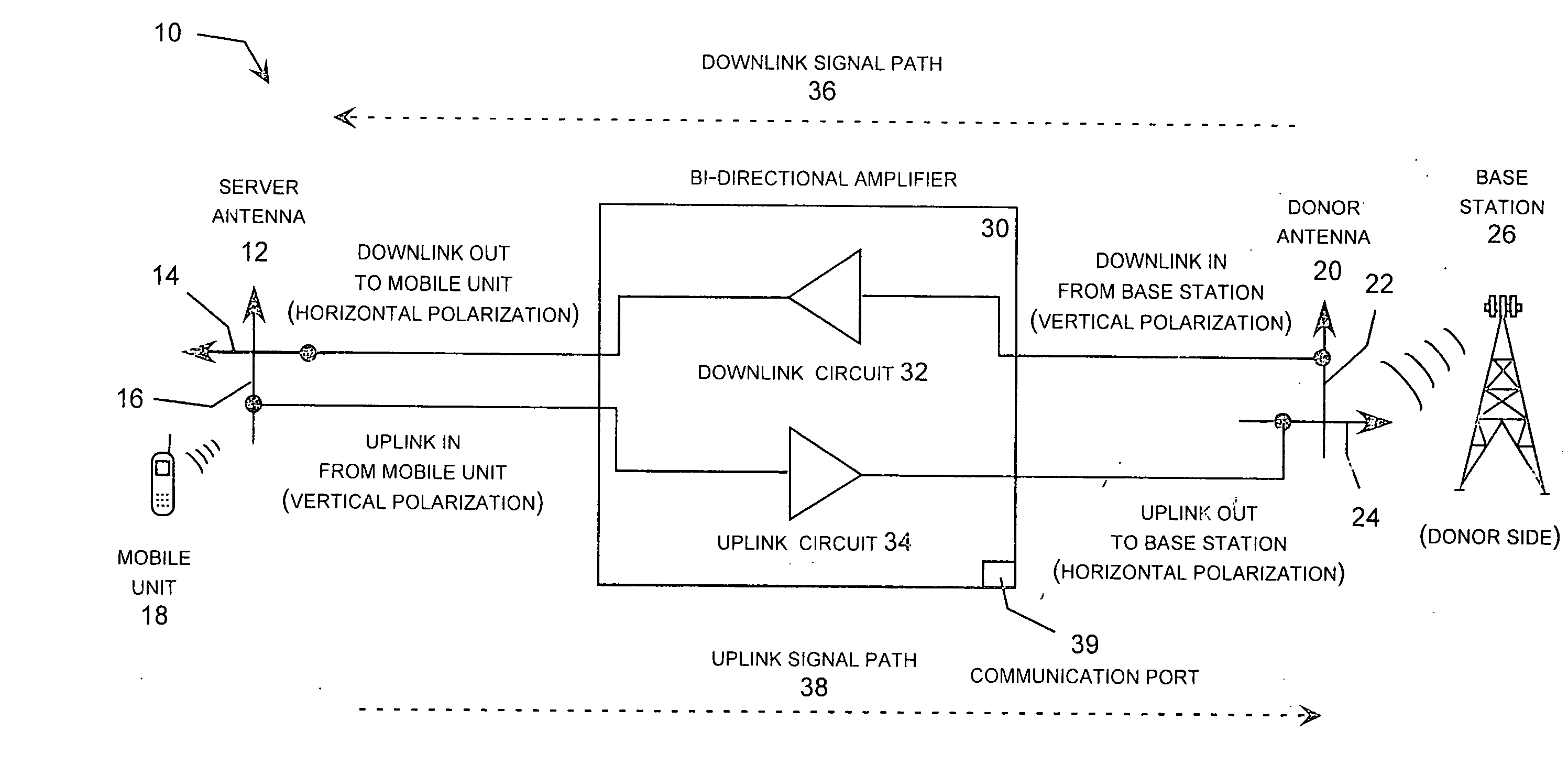
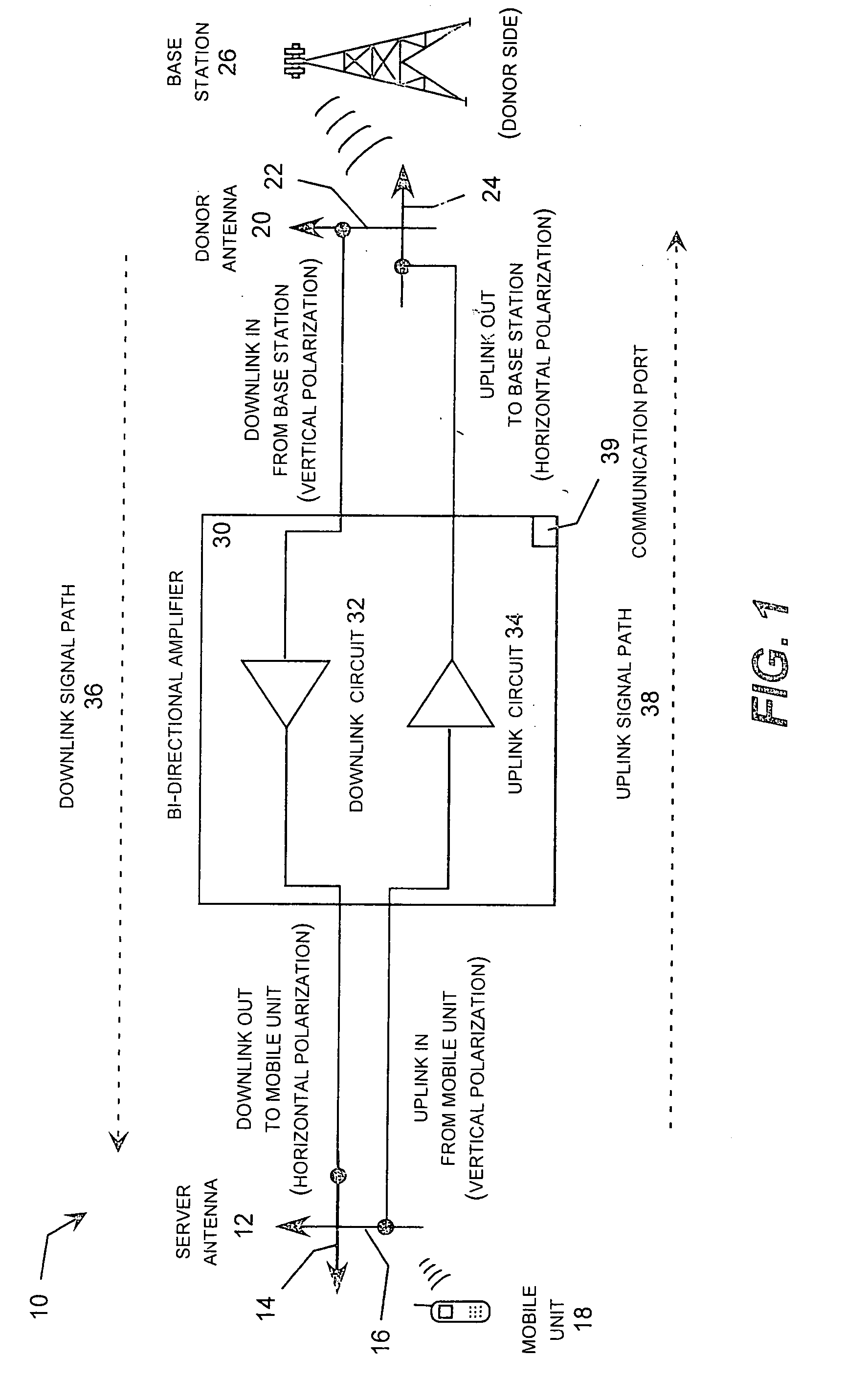
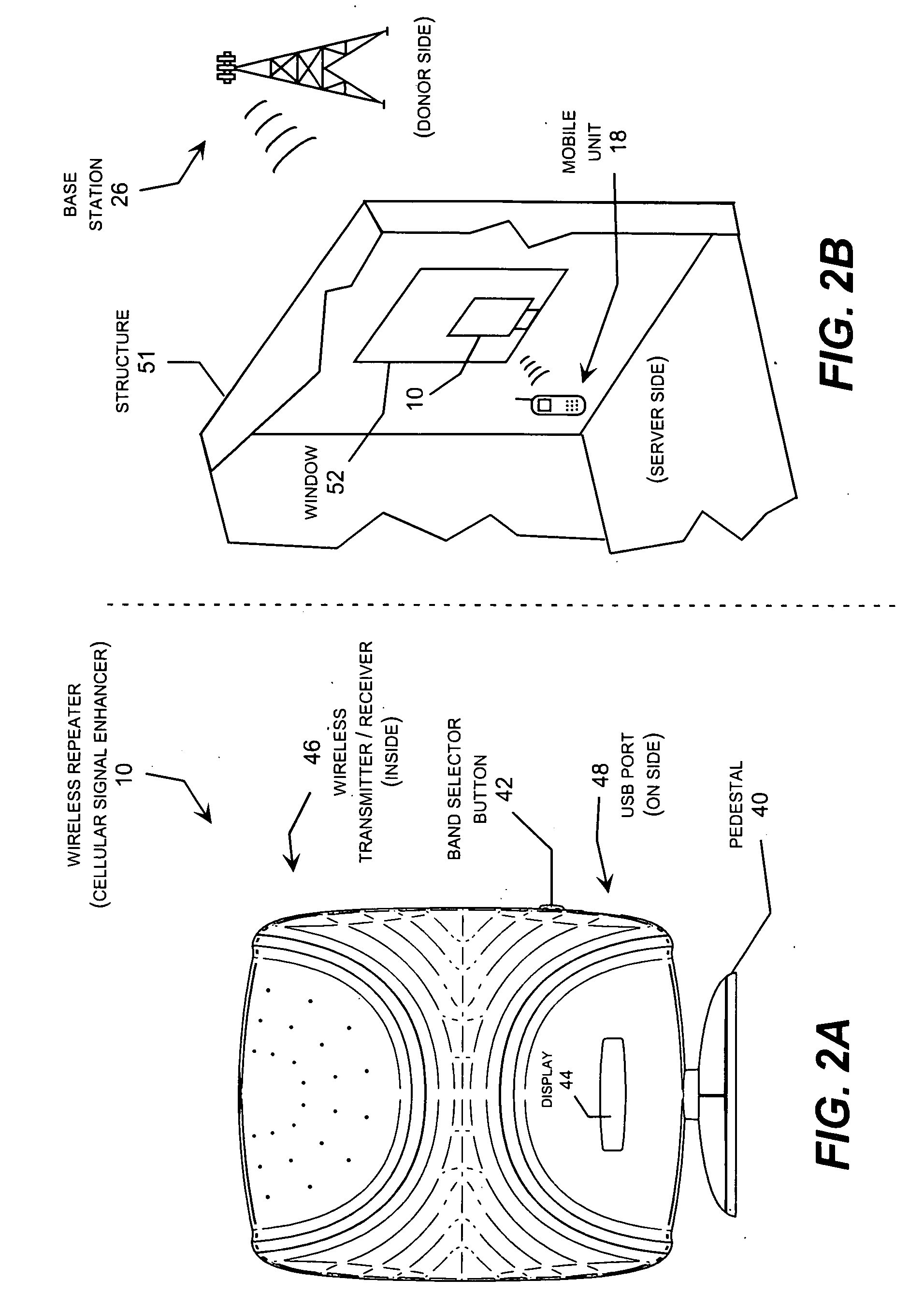
Wireless repeater with feedback suppression features
InactiveUS20060205343A1Improve server-donor feedback suppressionSmall and portableTransmission systemsActive radio relay systemsElectrical conductorWireless repeater
A wireless repeater with features that improve the server-donor antenna isolation including multi-purpose server and donor mounting plates that each carry an antenna feed circuit board and associated antenna elements mounted in a back-to-back arrangement with the duplex repeater electronics board sandwiched between the server and donor mounting plates. To improve the server-donor antenna isolation, one or both mounting plates may include corner, side tabs, which may be asymmetric, and raised walls around one or both mounting plates. In addition, the radomes covering the donor antenna and / or the server antennas may carry one or more parasitic conductors, such as a two parasitic strips arranged in parallel lines or for parasitic strips arranged in a square configuration.
Owner:ANDREW CORP
Controlled dose drug delivery system
ActiveUS20070264323A1Meet needsReduce the amount requiredOrganic active ingredientsBiocideImmediate releaseDose delivery
A multiple pulsed dose drug delivery system for pharmaceutically active amphetamine salts, comprising a pharmaceutically active amphetamine salt covered with an immediate-release coating and a pharmaceutically active amphetamine salt covered with an enteric coating wherein the immediate release coating and the enteric coating provide for multiple pulsed dose delivery of the pharmaceutically active amphetamine salt. The product can be composed of either one or a number of beads in a dosage form, including either capsule, tablet, or sachet method for administering the beads.
Owner:TAKEDA PHARMA CO LTD
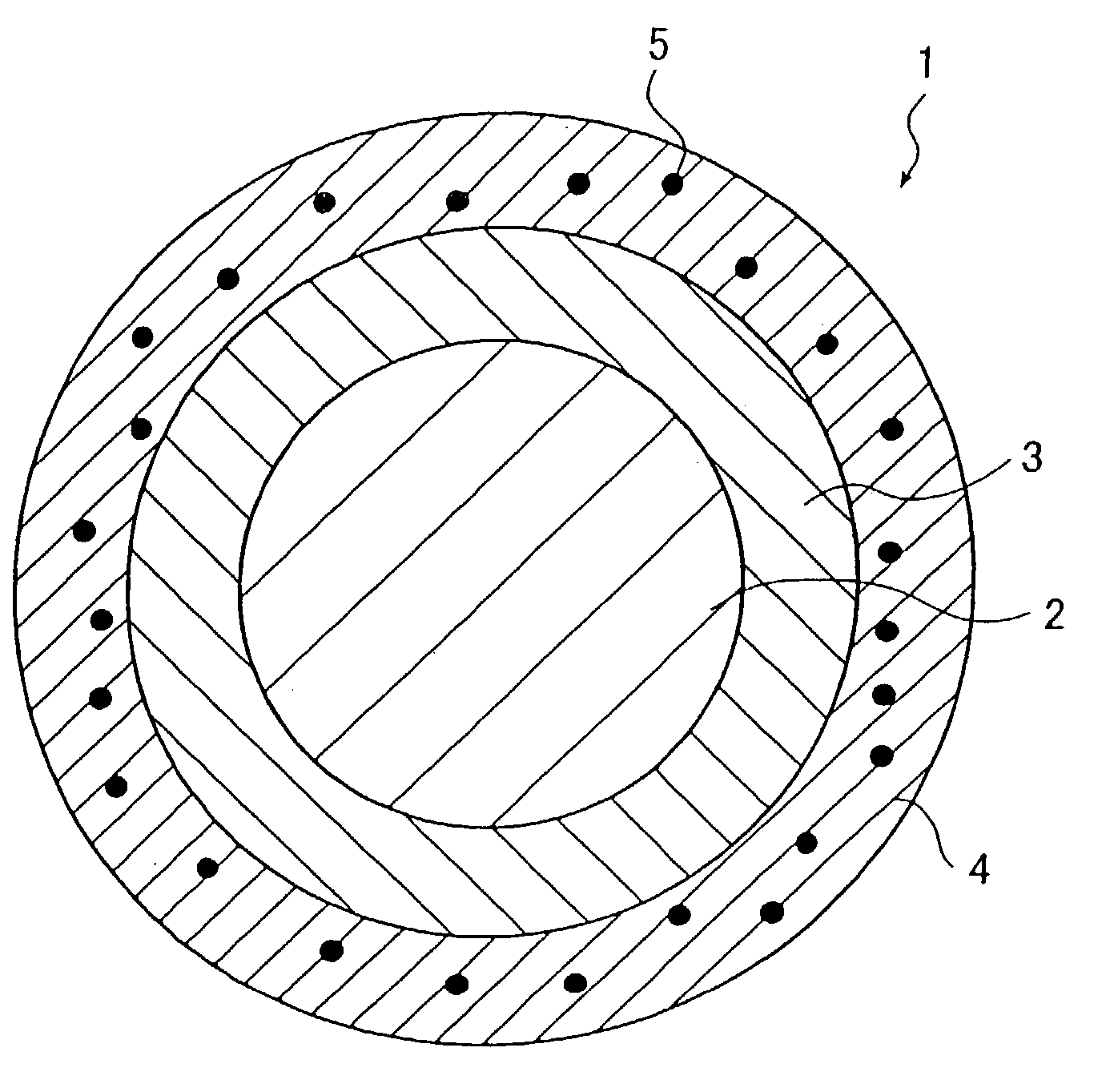
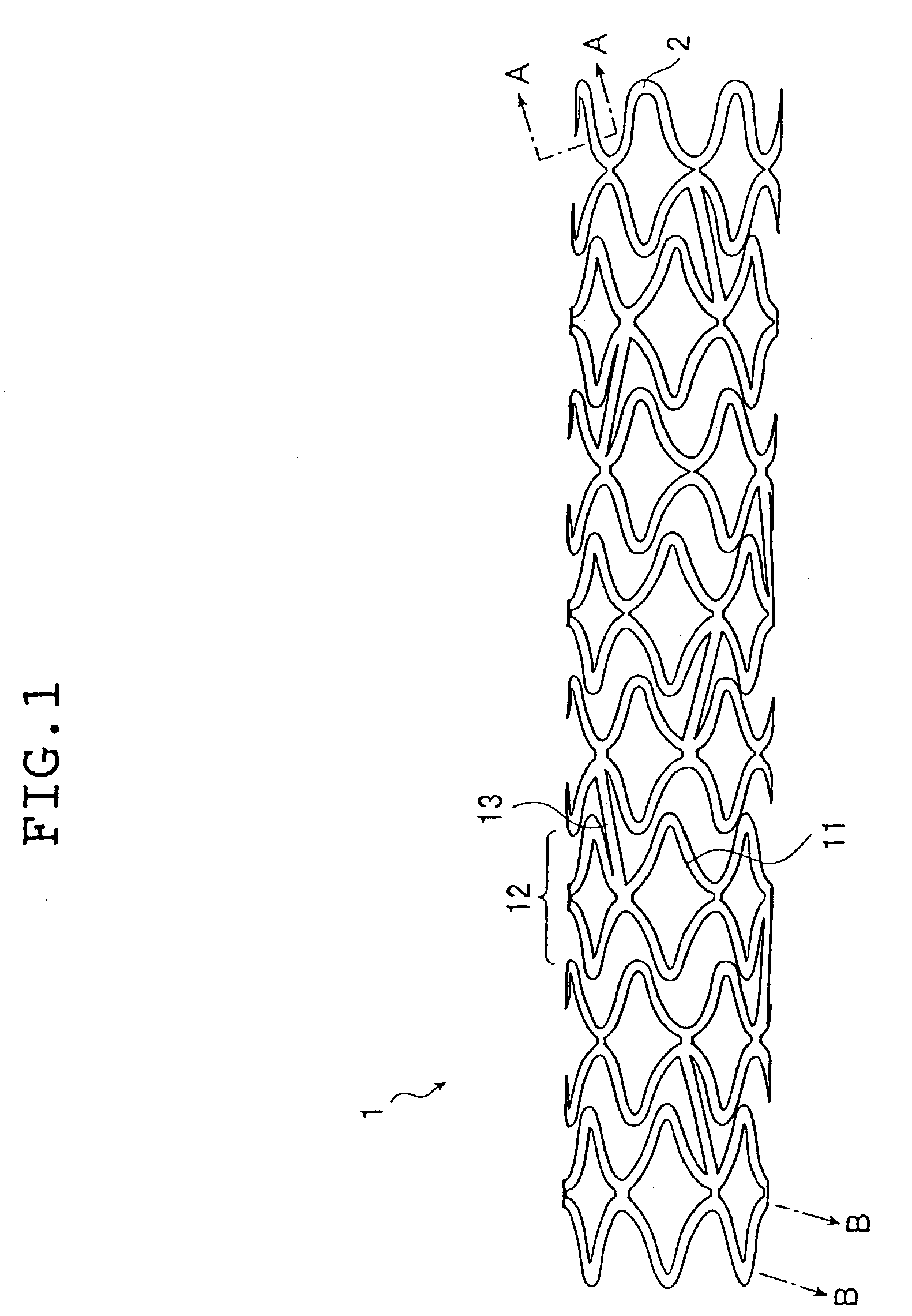
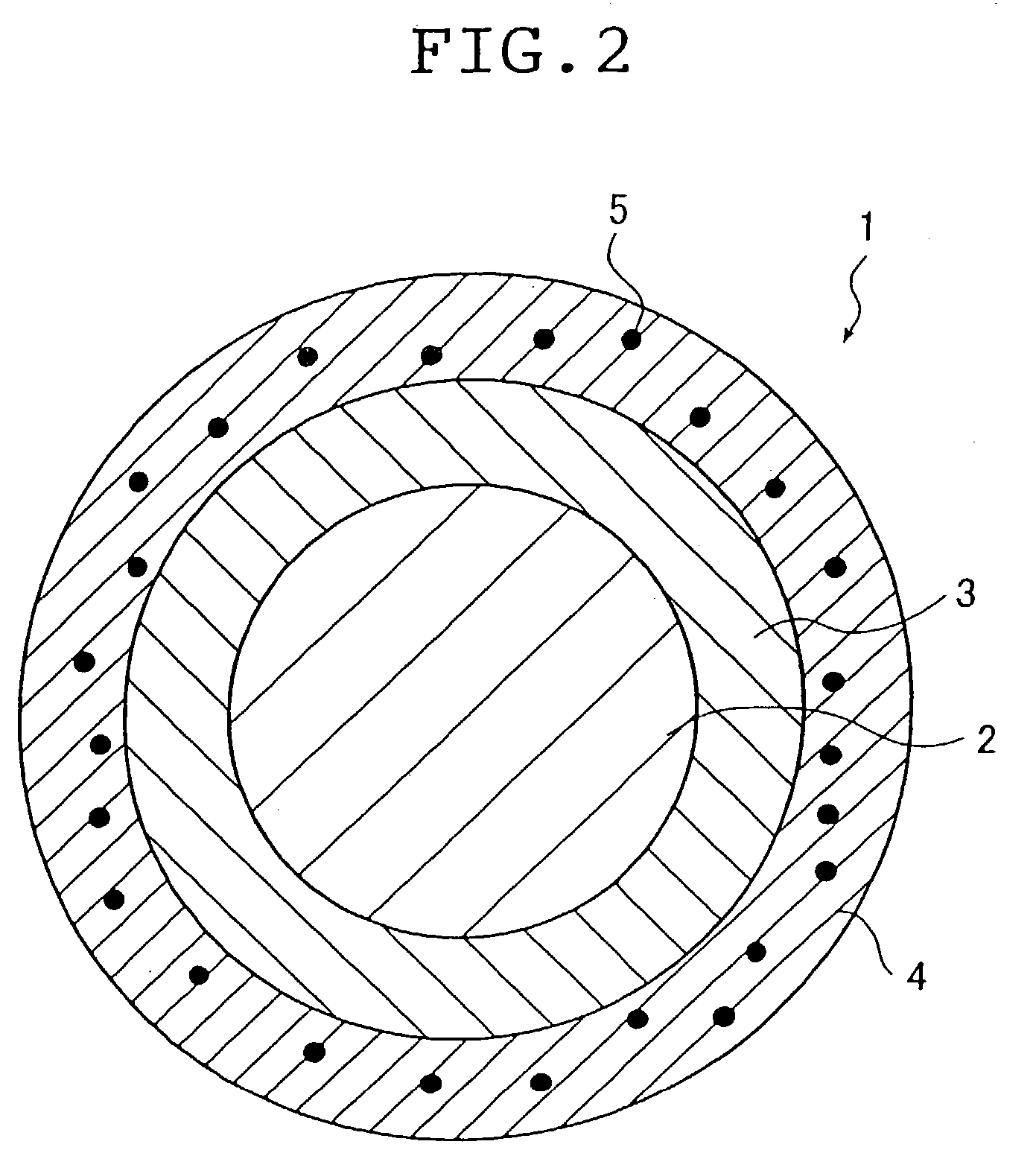
Stent
A stent capable of stably loading a biologically / physiologically active substance, without causing its decomposition or deterioration, and permitting it to release itself slowly over a long period of time without being rapidly released in a short period, after being implanted in a lesion. The stent (1) comprises a cylindrical stent main body (2) having an opening on each end and extending in axial direction between the two openings, and a sustained release coating formed on the surface of said stent main body from which a biologically / physiologically active substance is released, and wherein said sustained release coating is composed of a layer of a biologically / physiologically active substance (3) which covers the surface of said stent main body, and a polymer layer (4) which covers said layer of a biologically / physiologically active substance (3) on said layer of a biologically / physiologically active substance (3), and said layer of a biologically / physiologically active substance (3) contains at least one kind of fat-soluble biologically / physiologically active substance, and said polymer layer (4) contains a polymer miscible with said biologically / physiologically active substance and fine particles (5) equal to or smaller than 5 mum in particle diameter dispersed in the polymer.
Owner:TERUMO KK
Delayed release pharmaceutical composition containing 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol
InactiveUS20050058706A1Good repeatabilityGood treatment effectOrganic active ingredientsBiocideHydrophobic polymerBULK ACTIVE INGREDIENT
A pharmaceutical formulation for delayed release of the active ingredient 3-(3-dimethylamino-1-ethyl-2-methylpropyl)phenol or a pharmaceutically acceptable salt thereof in a matrix containing between 1 and 80 wt. % of at least one pharmaceutically acceptable hydrophilic or hydrophobic polymer as a matrix forming agent and exhibiting in vivo the following release rate: 3 to 35% by weight (based on 100% by weight active ingredient) 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 0.5 hours; 5 to 50% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 1 hour; 10 to 75% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 2 hours; 15 to 82% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 3 hours; 30 to 97% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 6 hours; more than 50% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 12 hours; more than 70% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 18 hours, and more than 80% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 24 hours.
Owner:GRUNENTHAL GMBH
Antibodies that specifically bind to tim3
ActiveUS20130022623A1Promote growthReduce inflammationHeavy metal active ingredientsPeptide/protein ingredientsCancer cellAntibody
Provided herein are antibodies specific for TIM3 that can be used to detect cancer cells, in particular, cancer stem cells. The antibodies can also be used in therapeutic compositions for treating cancer and reducing inflammation.
Owner:ONK THERAPEUTICS LTD
Chronotherapeutic dosage forms and methods of treatment using chronotherapy
InactiveUS20030082230A1Blood pressure controlReduce morbidityOrganic active ingredientsInorganic non-active ingredientsActive agentPharmaceutical formulation
A chronotherapeutic pharmaceutical formulation comprising a core containing an active agent (e.g., a drug) and a delayed release compression coating comprising a natural or synthetic gum applied onto the surface of the core.
Owner:PENWEST PHARMA CO
Satiation devices and methods
A device for inducing weight loss in a patient includes a tubular prosthesis positionable at the gastro-esophageal junction region, preferably below the z-line. In a method for inducing weight loss, the prosthesis is placed such that an opening at its proximal end receives masticated food from the esophagus, and such that the masticated food passes through the pouch and into the stomach via an opening in its distal end.
Owner:BAROSENSE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/78e0b760-3b4b-4848-b642-2e00beb09f2a/US20070135461A1-20070614-C00001.png)
![Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/78e0b760-3b4b-4848-b642-2e00beb09f2a/US20070135461A1-20070614-C00002.png)
![Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/78e0b760-3b4b-4848-b642-2e00beb09f2a/US20070135461A1-20070614-C00003.png)
![HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINES AND PYRROLO[2,3-b]PYRIMIDINES AS JANUS KINASE INHIBITORS HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINES AND PYRROLO[2,3-b]PYRIMIDINES AS JANUS KINASE INHIBITORS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9ca91ab9-beda-49e3-8a59-55336dec4de6/US20090181959A1-20090716-C00001.png)
![HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINES AND PYRROLO[2,3-b]PYRIMIDINES AS JANUS KINASE INHIBITORS HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINES AND PYRROLO[2,3-b]PYRIMIDINES AS JANUS KINASE INHIBITORS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9ca91ab9-beda-49e3-8a59-55336dec4de6/US20090181959A1-20090716-C00002.png)
![HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINES AND PYRROLO[2,3-b]PYRIMIDINES AS JANUS KINASE INHIBITORS HETEROARYL SUBSTITUTED PYRROLO[2,3-b]PYRIDINES AND PYRROLO[2,3-b]PYRIMIDINES AS JANUS KINASE INHIBITORS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9ca91ab9-beda-49e3-8a59-55336dec4de6/US20090181959A1-20090716-C00003.png)