Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
122 results about "Gastro-" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
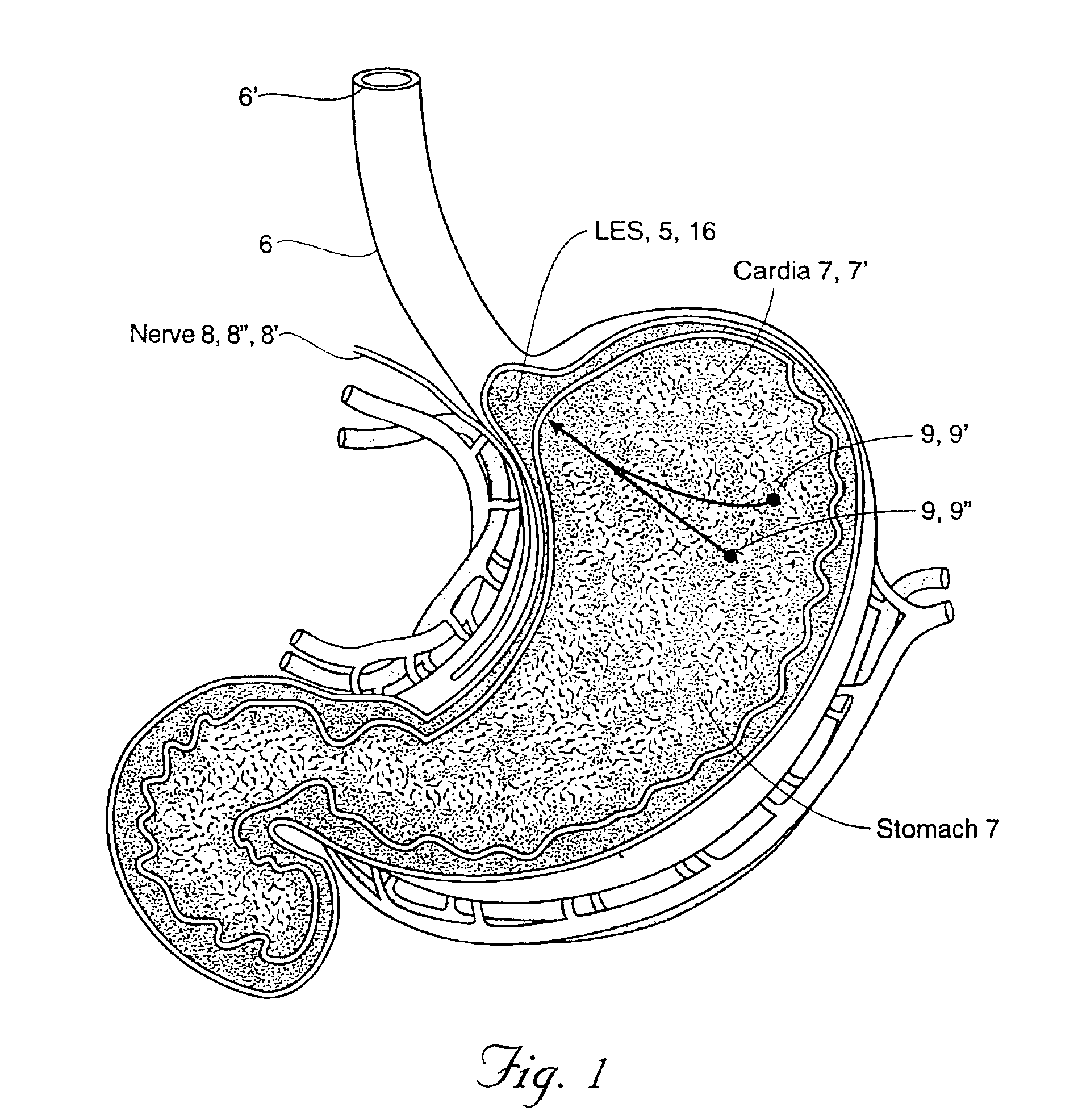
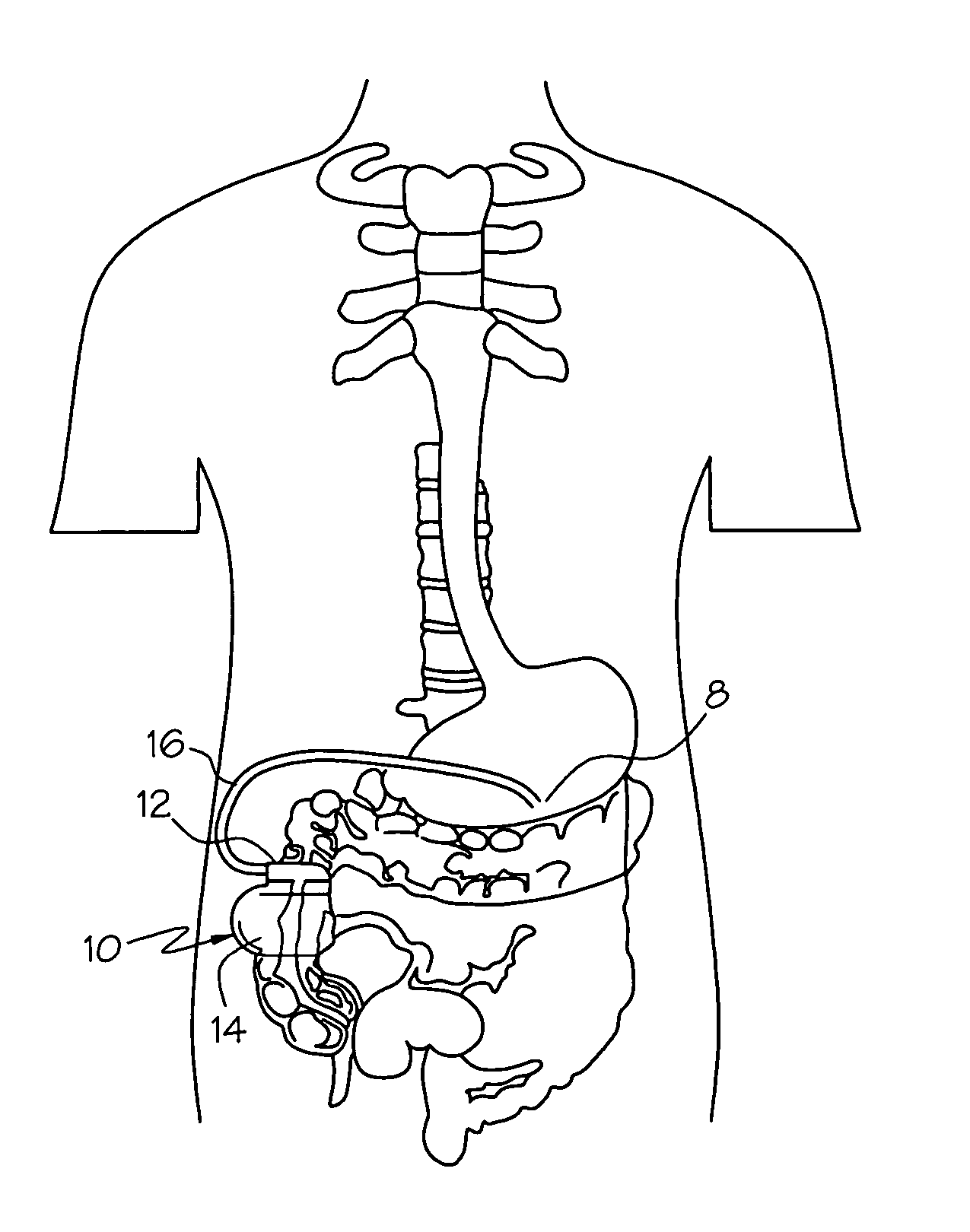
Gastro- is a common English-language prefix derived from the ancient Greek γαστήρ gastēr ("stomach").
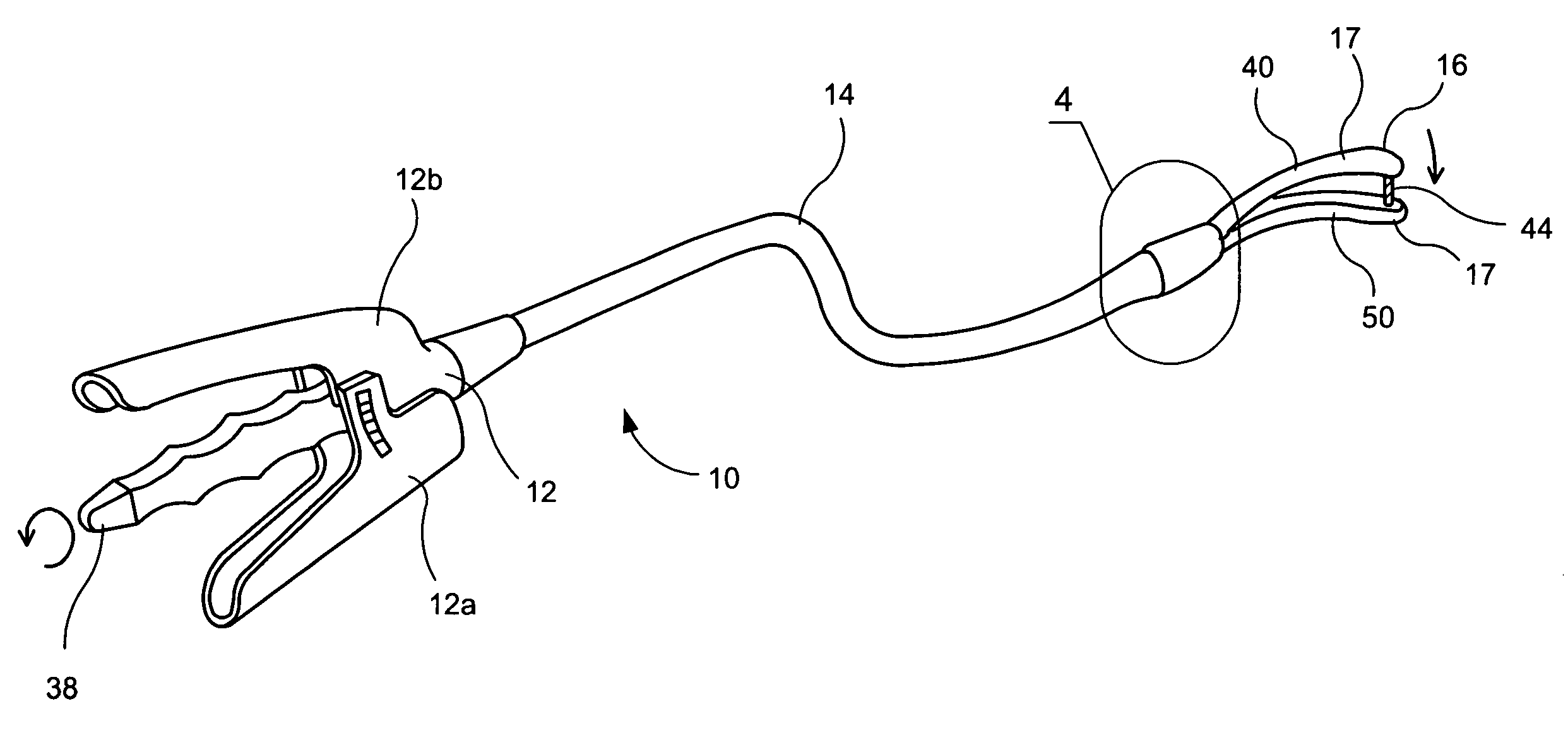
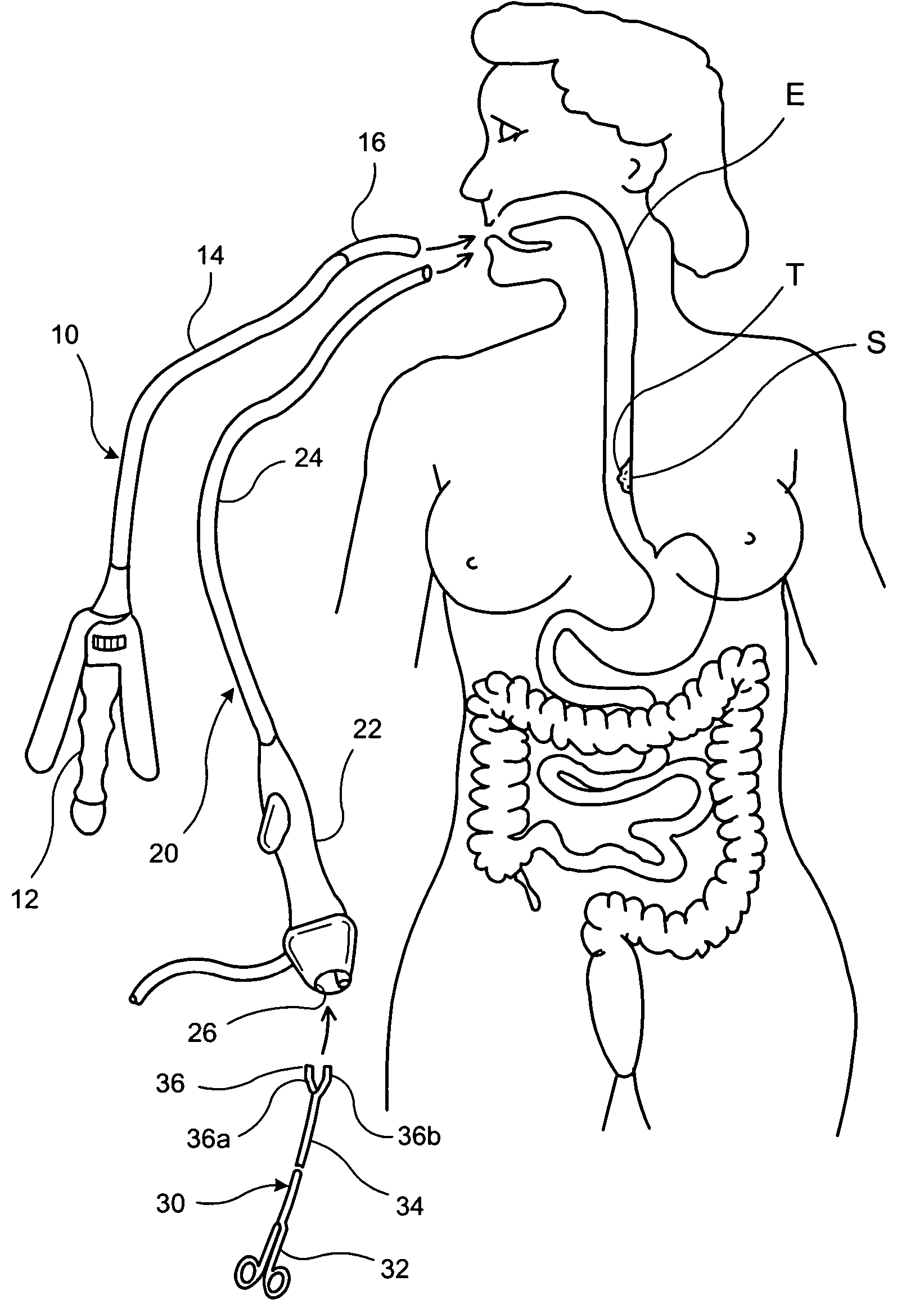
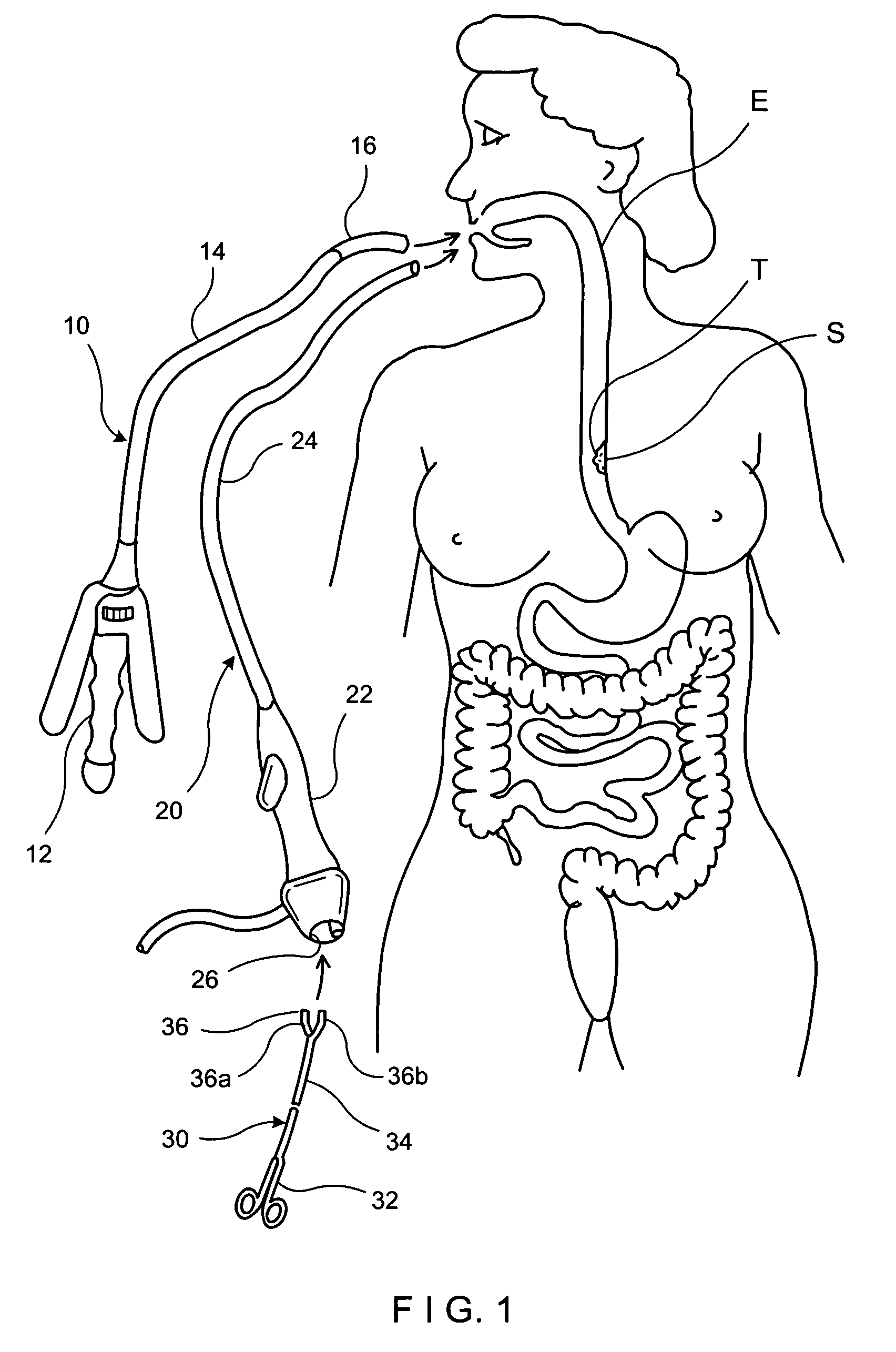
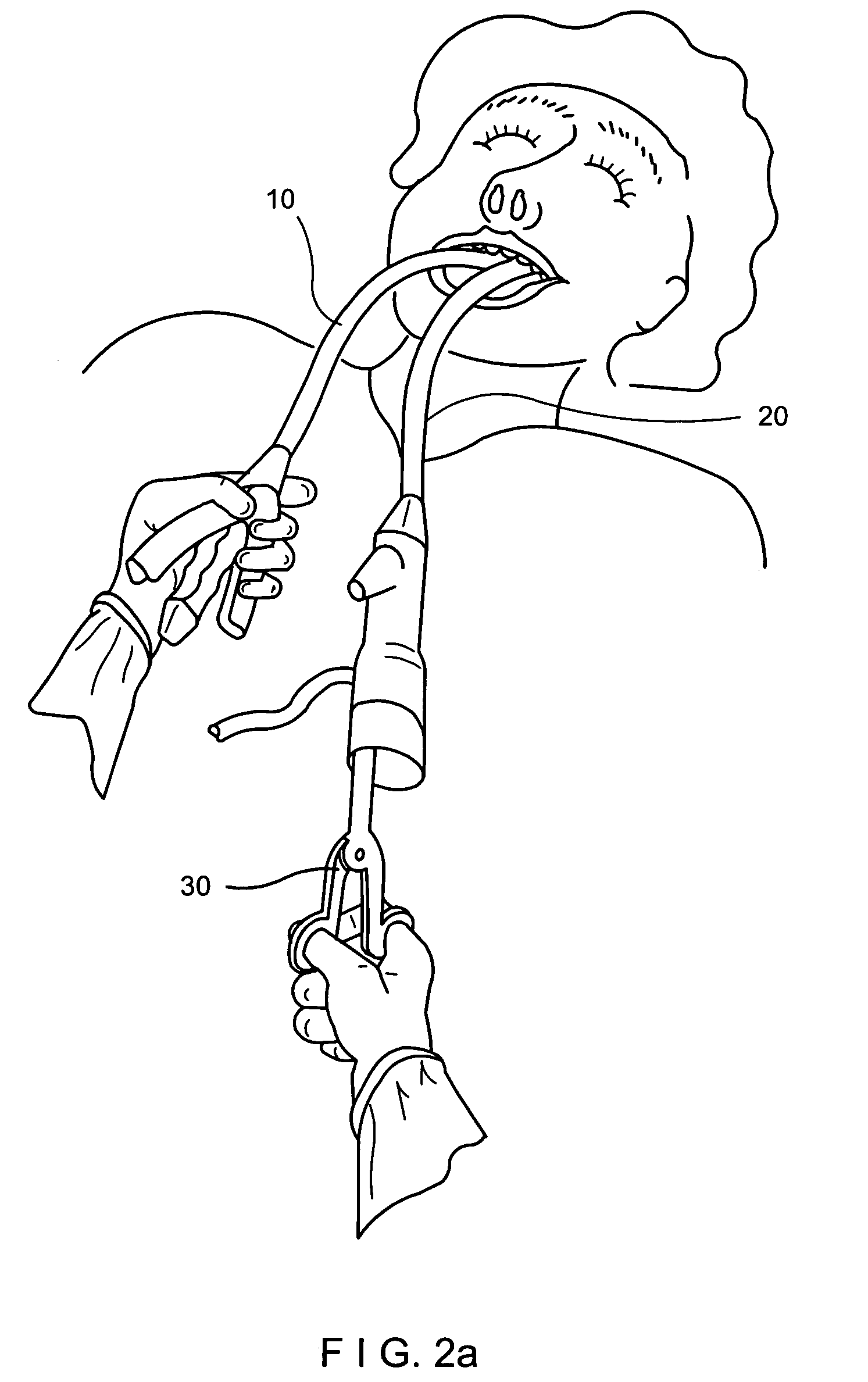
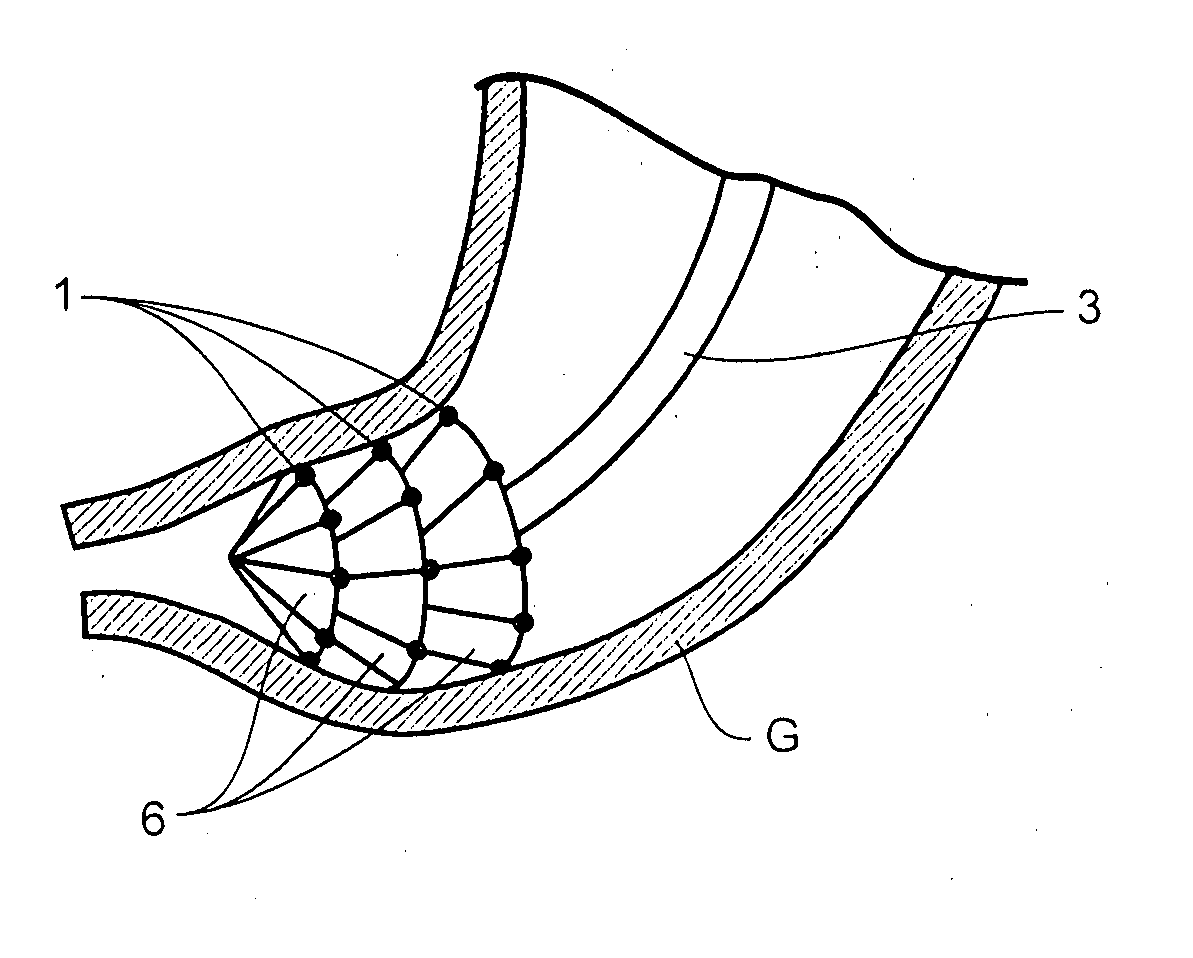
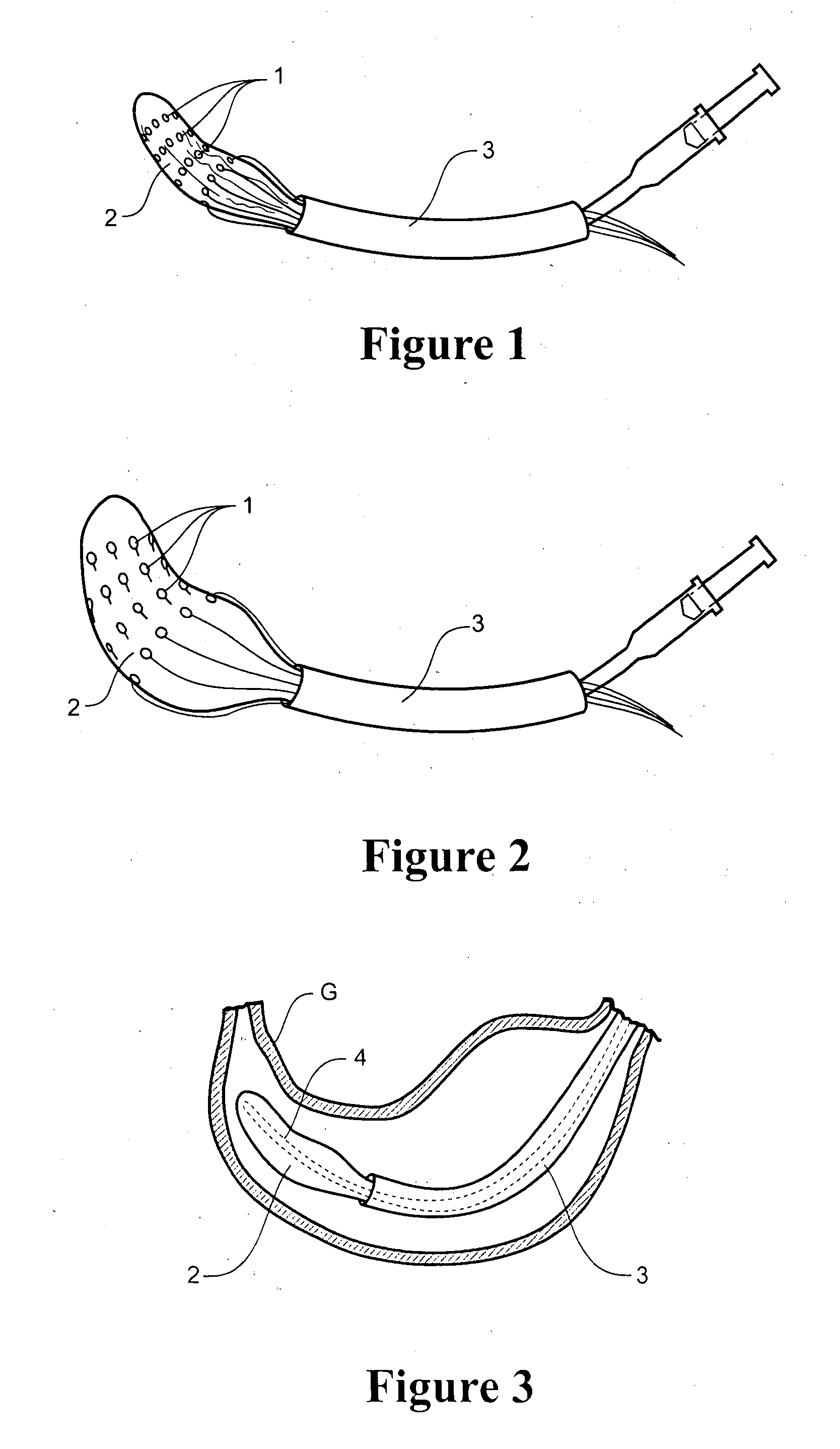
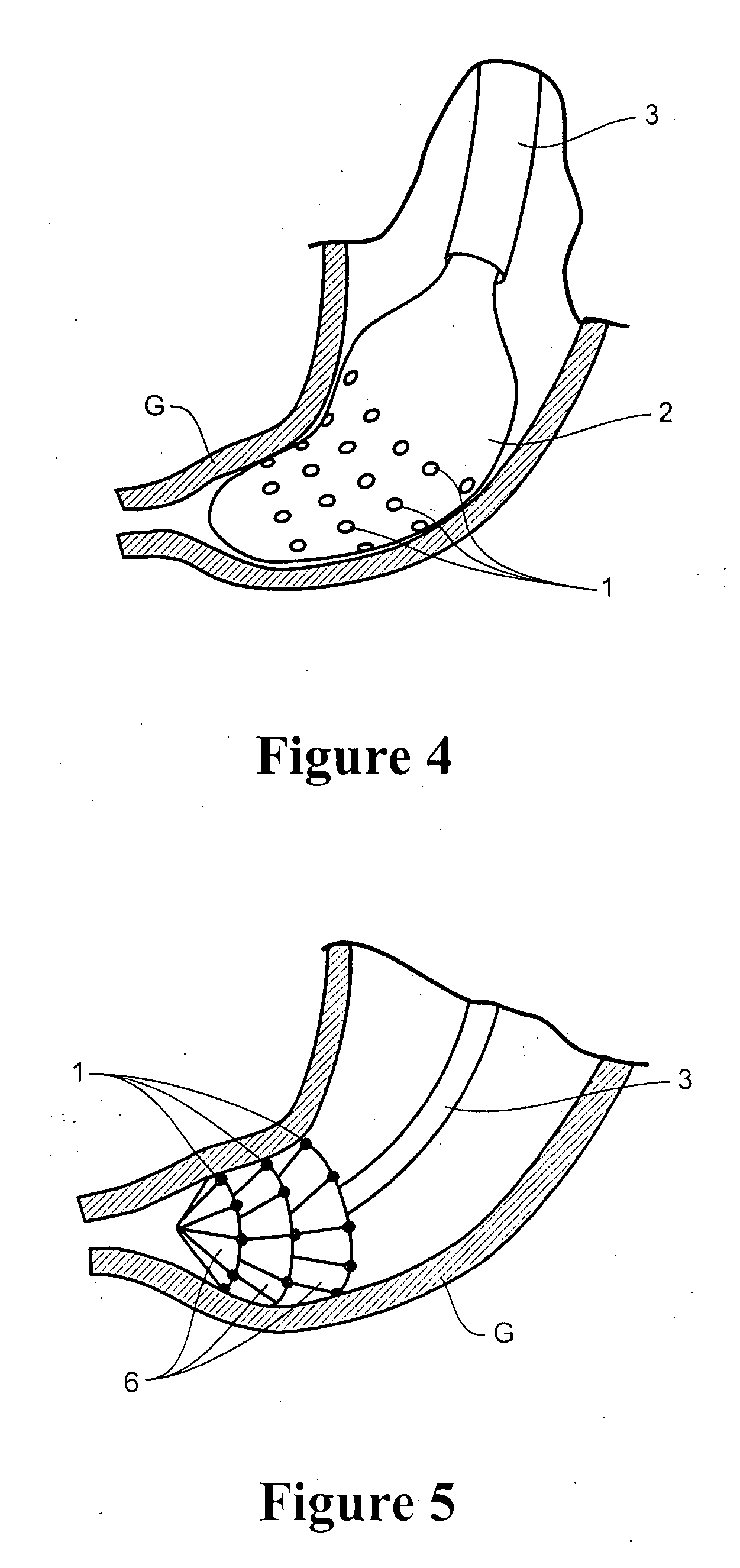
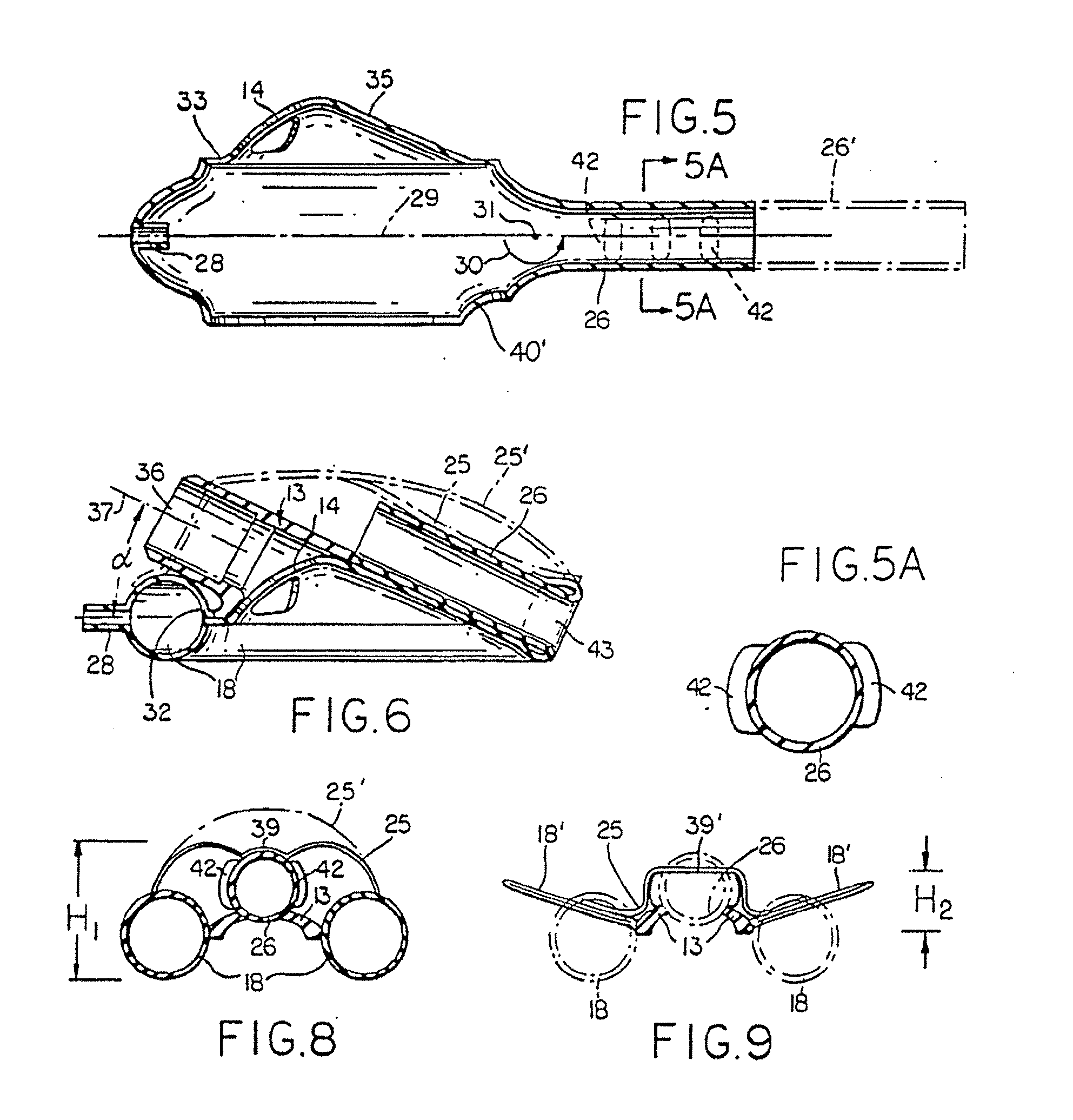
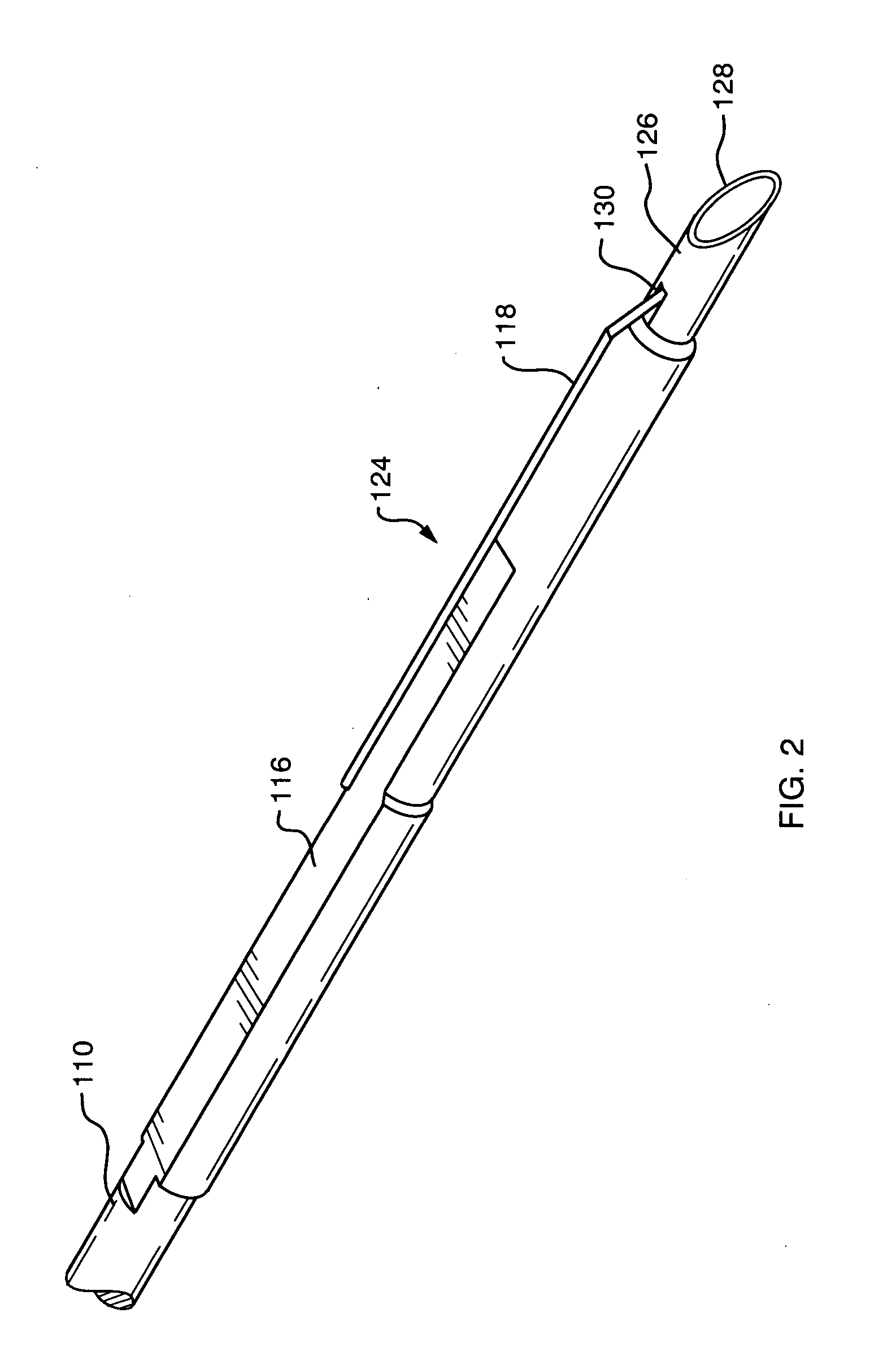
Apparatus and method for resectioning gastro-esophageal tissue
A system for stapling tissue comprises a flexible endoscope and an operative head including a pair of opposed, curved tissue clamping jaws sized to pass through an esophagus, the jaws being moveable with respect to one another between an open tissue receiving configuration and a closed tissue clamping configuration, a first one of the curved jaws including a stapling mechanism and a second one of the jaws including a staple forming anvil surface, the stapling mechanism including staple slots through which staples are fired arranged in a row extending from a proximal end of the first jaw to a distal end thereof in combination with a control handle which, when the operative head is in an operative position within one of a patient's stomach and esophagus, remains outside the patient, the control handle including a first actuator for moving the jaws relative to one another and a second actuator for operating the stapling mechanism.
Owner:REX MEDICAL LP
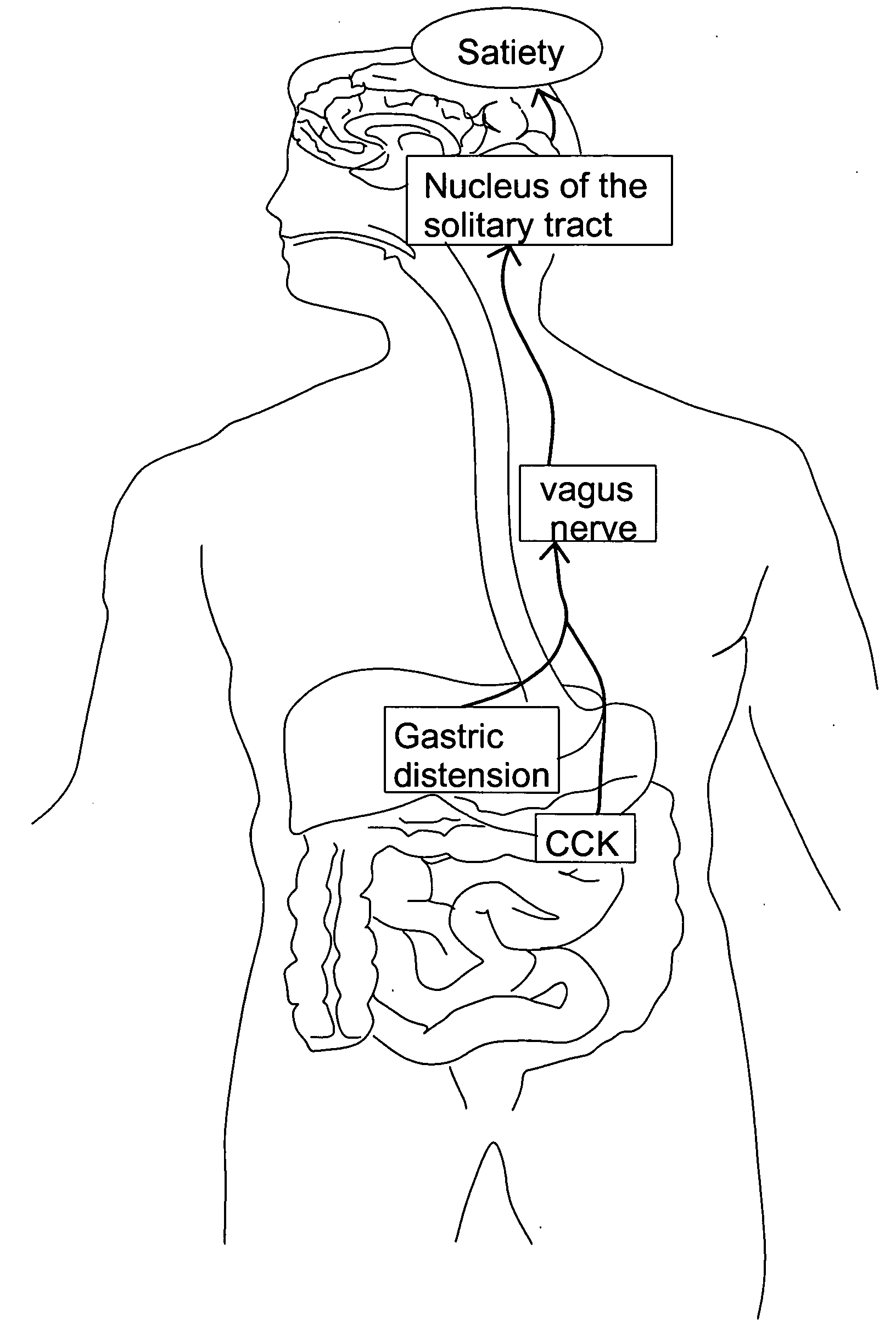
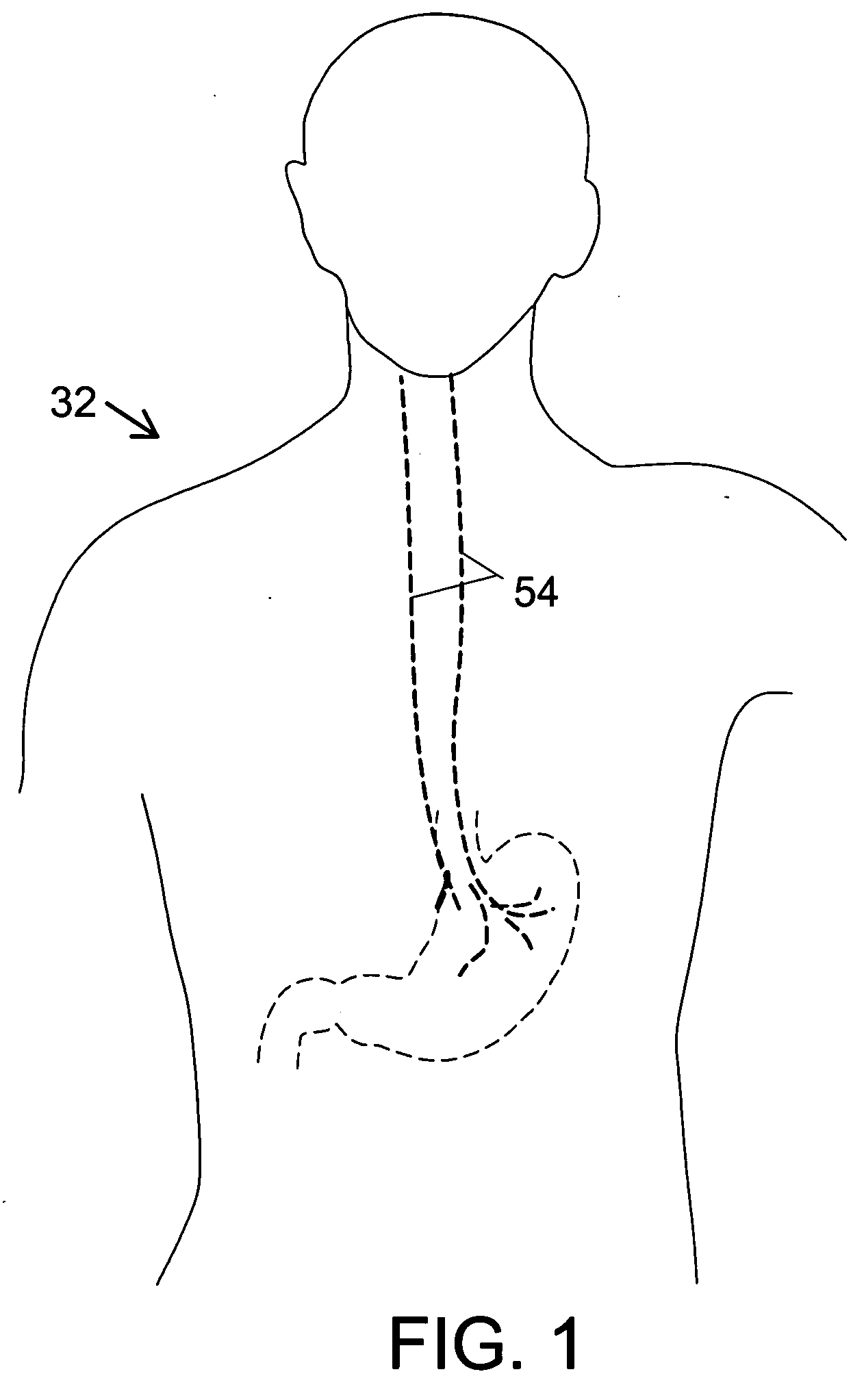
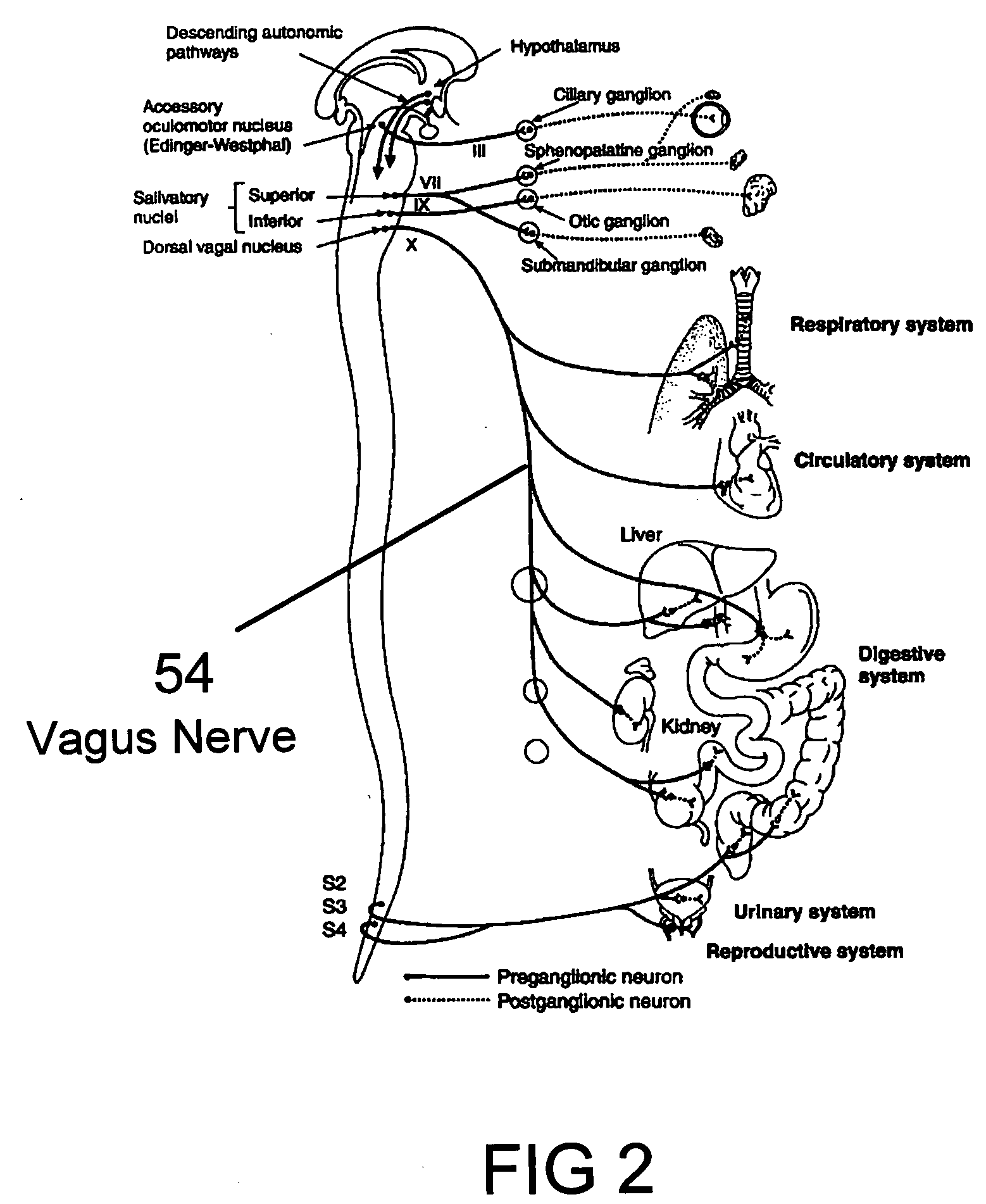
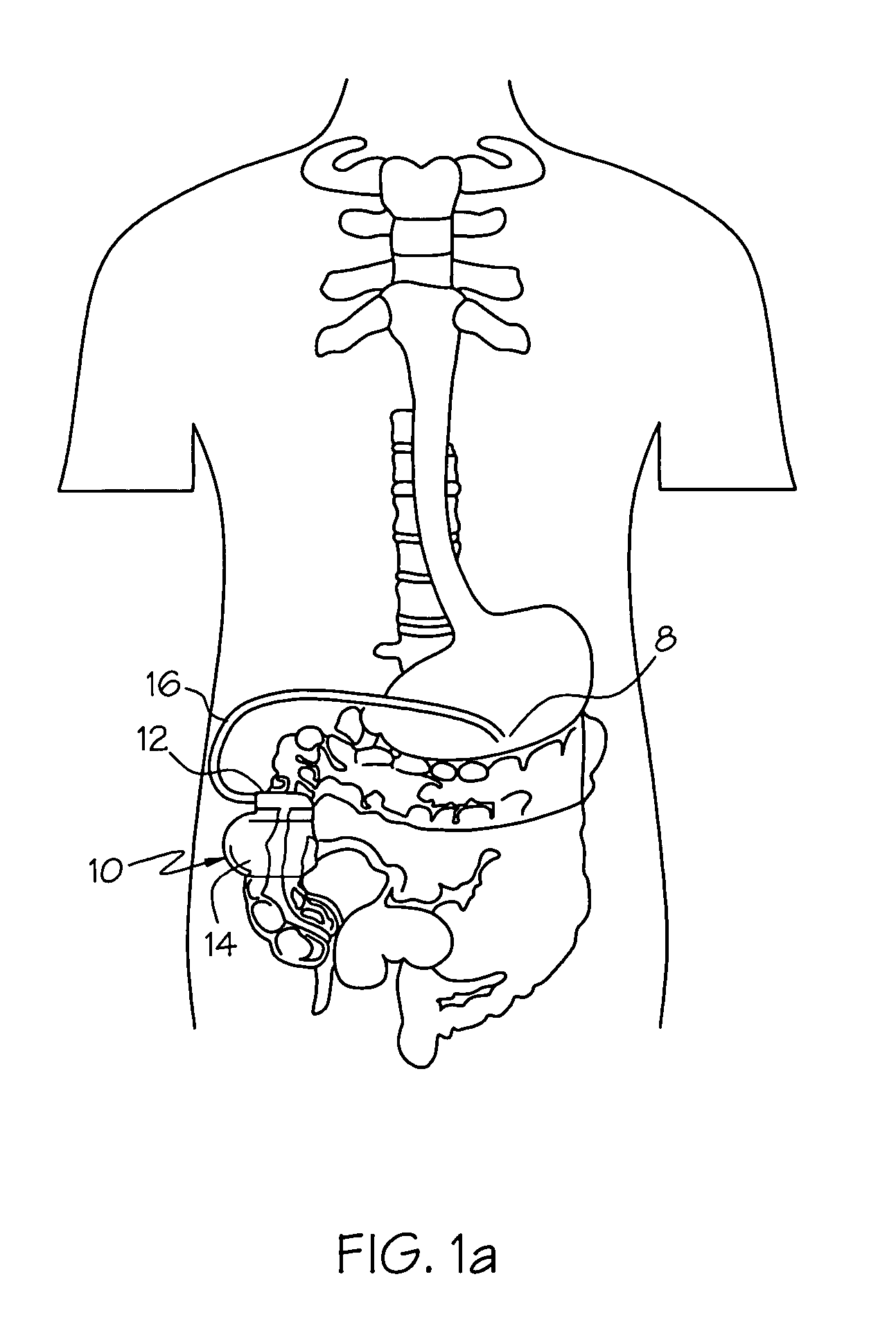
Method and system for vagal blocking and/or vagal stimulation to provide therapy for obesity and other gastrointestinal disorders
InactiveUS20050137644A1Eliminates repeated surgeryOvercomes shortcomingElectrotherapyArtificial respirationDiseaseDisease irritable bowel
Method and system to provide therapy for obesity and gastrointestinal disorders such as FGIDs, gastroparesis, gastro-esophageal reflex disease (GERD), pancreatitis and ileus comprises vagal blocking and / or vagal stimulation. Vagal blocking may be in the afferent or efferent direction, and may be with or without stimulation pulses. Blocking may be provided by one of a number of different electrical blocking techniques. Electrical signals may be provided with an external stimulator in conjunction with an implanted stimulus-receiver, or an implanted stimulus-receiver comprising a high value capacitor for temporary power source. In one embodiment, the external stimulator may comprise an optional telemetry unit. The addition of the telemetry unit to the external stimulator provides the ability to remotely interrogate and change stimulation programs over a wide area network, as well as other networking capabilities,
Owner:NEURO & CARDIAC TECH
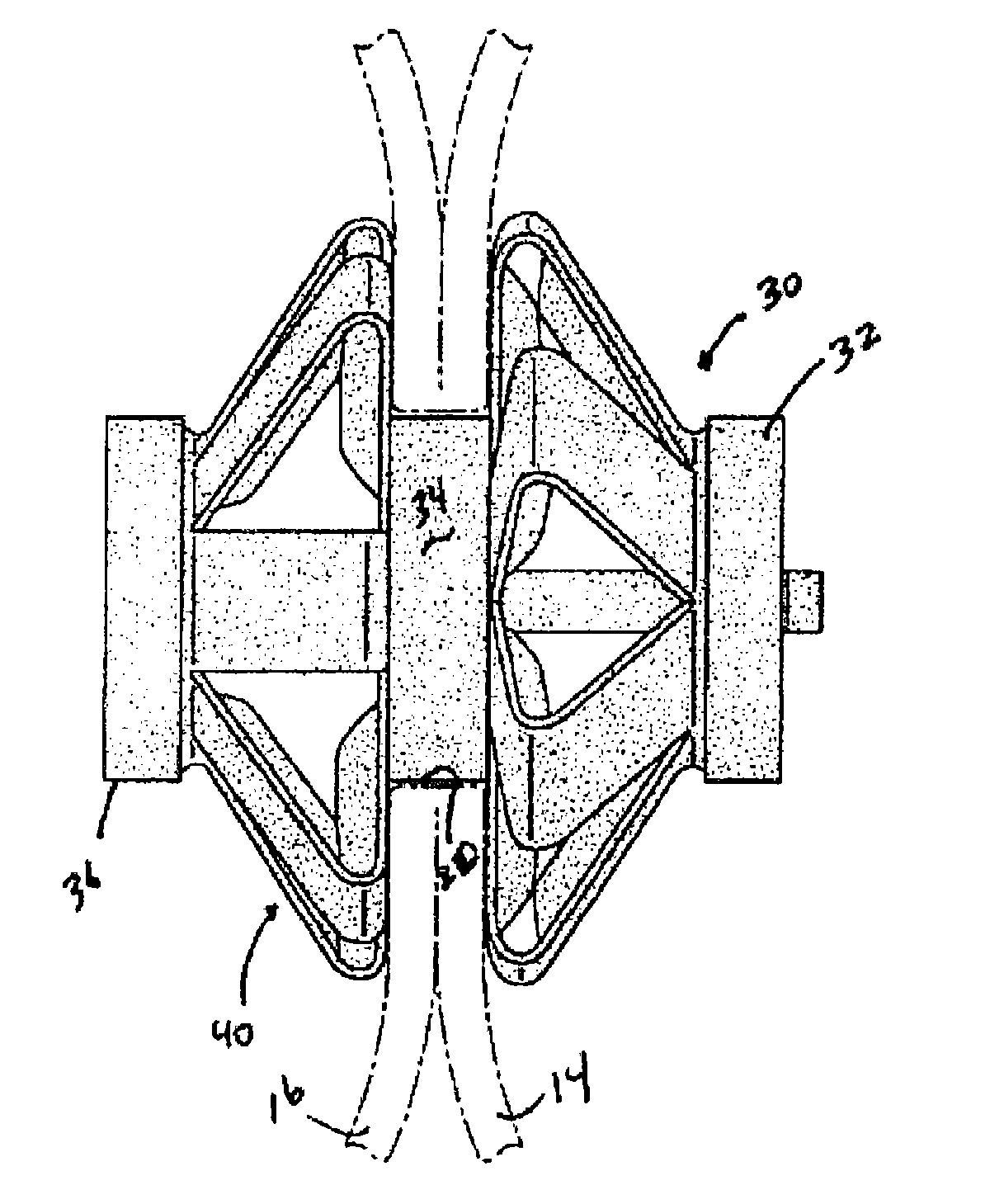
Gastro-occlusive device
A gastro-occlusive device, comprising a balloon disposable in a stomach cavity of a patient, and inflatable therein to occlude a portion of the volume of the stomach cavity, a gas flow tube coupled at a distal end thereof with the balloon and extending outwardly through a stomach wall for coupling with a gas source for selectively inflating the balloon, to occlude at least a portion of the volume of the stomach cavity. The gastro-occlusive device may be employed in combination with a feeding tube unit, a drain unit or other ancillary apparatus, and is useful for treatment of morbid obesity and various eating disorders.
Owner:POLYZEN INC
Method to treat gastric reflux via the detection and ablation of gastro-esophageal nerves and receptors
Owner:MEDERI RF LLC
Method to treat gastric reflux via the detection and ablation of gastro-esophageal nerves and receptors
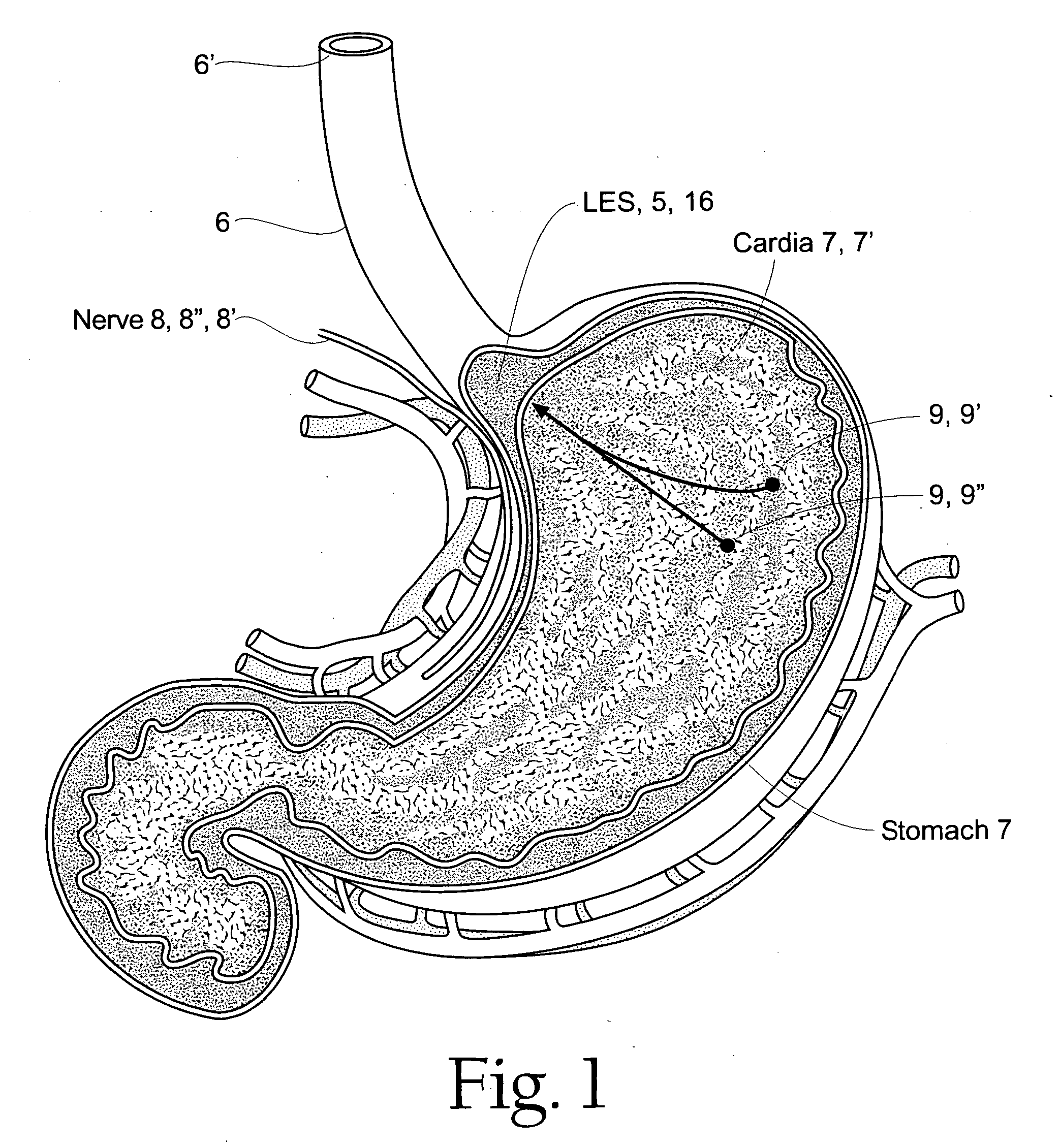
A method of treating a sphincter provides a sphincter electropotential mapping device with at least one of a mapping electrode or a treatment electrode. The sphincter electropotential mapping device is introduced into at least a portion of the sphincter, the lower esophageal sphincter, stomach, the cardia or the fundus. Bioelectric activity causing a relaxation of the sphincter is detected and energy is delivered from either the mapping electrode or the treatment electrode to treat the bioelectric activity. A method of treating a sphincter provides a sphincter electropotential mapping device with at least one of a mapping electrode or a treatment electrode. The sphincter electropotential mapping device is introduced into at least a portion of the sphincter, the lower esophageal sphincter, stomach, the cardia or the fundus. Bioelectric activity causing a relaxation of the sphincter is detected and energy is delivered from either the mapping electrode or the treatment electrode to treat the bioelectric activity.
Owner:MEDERI RF LLC
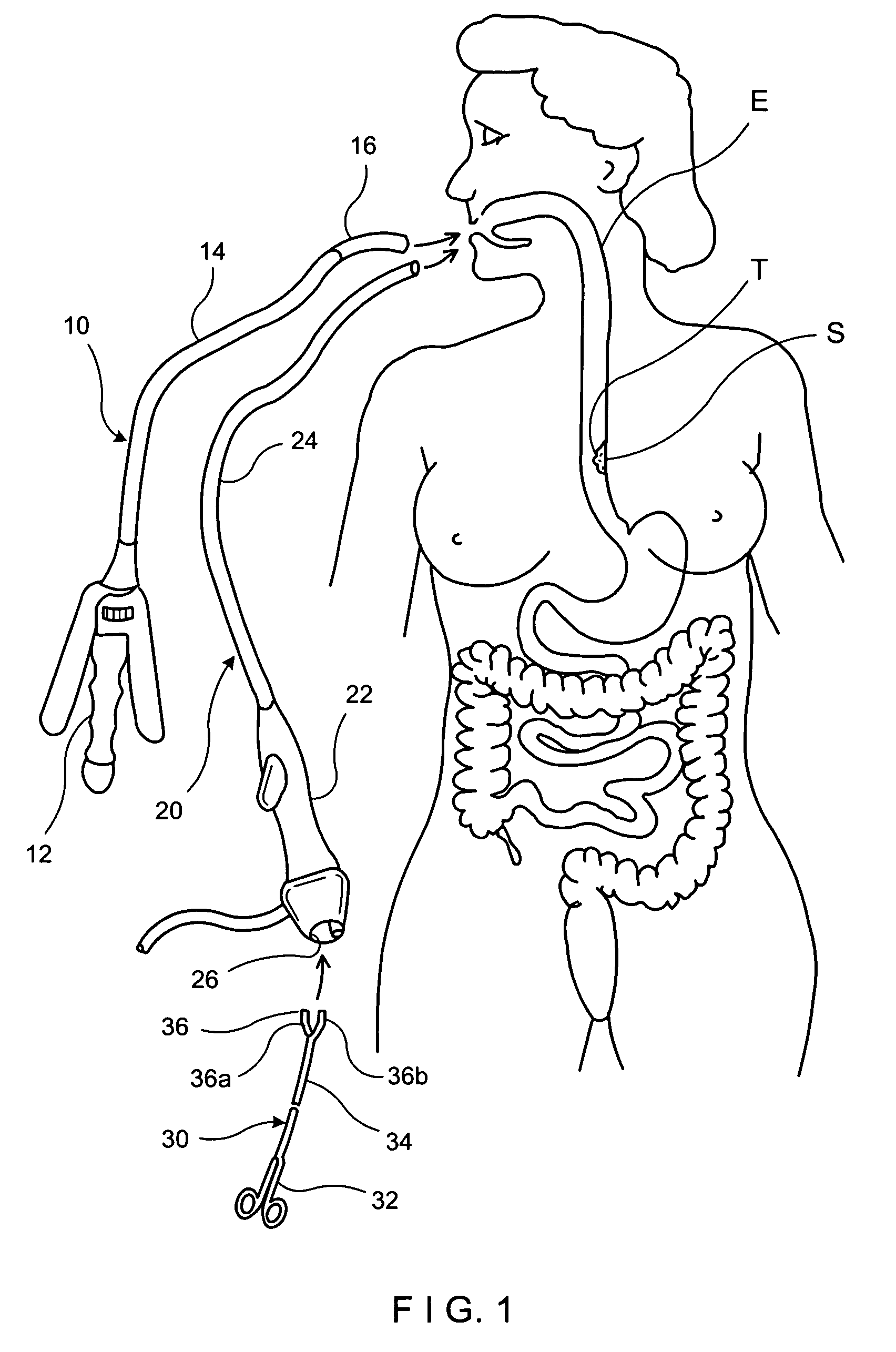
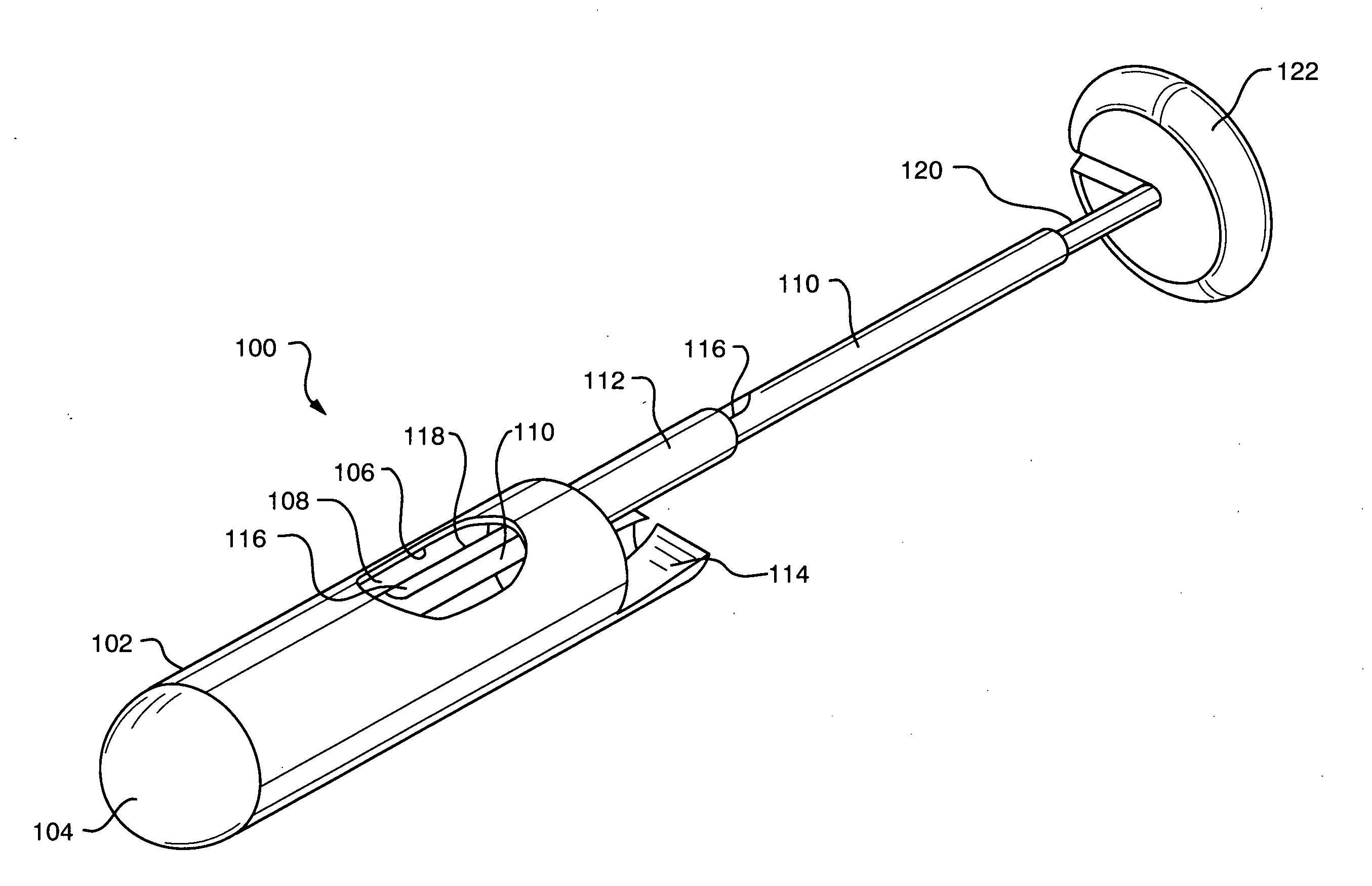
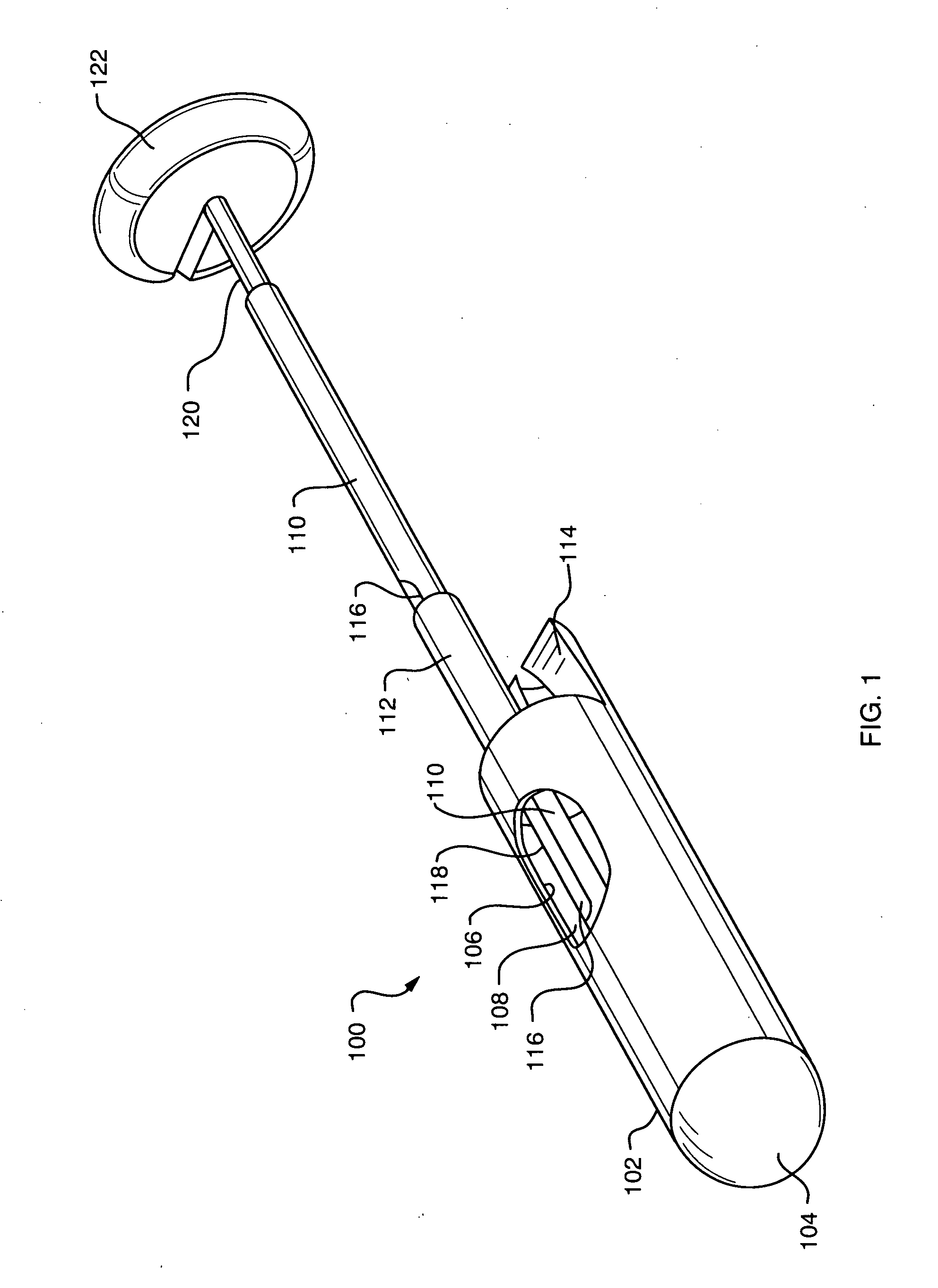
Tissue capturing and suturing device and method
InactiveUS7220266B2Easy to produceEliminate needSuture equipmentsDiagnosticsGastro-esophageal reflux diseaseEndoscopic Procedure
A combination tissue apposition and suture capturing device (100) for performing endoscopic procedures typically in the gastro-esophageal tract. The device (100) is particularly adapted for forming multiple plications used in a gastroplasty procedure devised to cure or ameliorate gastro-esophageal reflux disease. The device includes a tissue sewing capsule (102) attached to the distal end of an endoscope having a needle (120) that is deposited in a capsule (102) distal tip cavity following the suturing of a tissue fold and retrieved to enable the suturing of a subsequent tissue fold without the need for multiple intubations. A suture clip delivery device (200) is also disclosed that is adapted to fit within the capsule (102) to enable suture (118) capture without the need for multiple intubations. The combination device eliminates the need for an overtube and maximizes the speed efficiency of the gastroplasty procedure. A method for using the combination device is also disclosed.
Owner:CR BARD INC
Apparatus and method for resectioning gastro-esophageal tissue
A system for stapling tissue comprises a flexible endoscope and an operative head including a pair of opposed, curved tissue clamping jaws sized to pass through an esophagus, the jaws being moveable with respect to one another between an open tissue receiving configuration and a closed tissue clamping configuration, a first one of the curved jaws including a stapling mechanism and a second one of the jaws including a staple forming anvil surface, the stapling mechanism including staple slots through which staples are fired arranged in a row extending from a proximal end of the first jaw to a distal end thereof in combination with a control handle which, when the operative head is in an operative position within one of a patient's stomach and esophagus, remains outside the patient, the control handle including a first actuator for moving the jaws relative to one another and a second actuator for operating the stapling mechanism.
Owner:REX MEDICAL LP
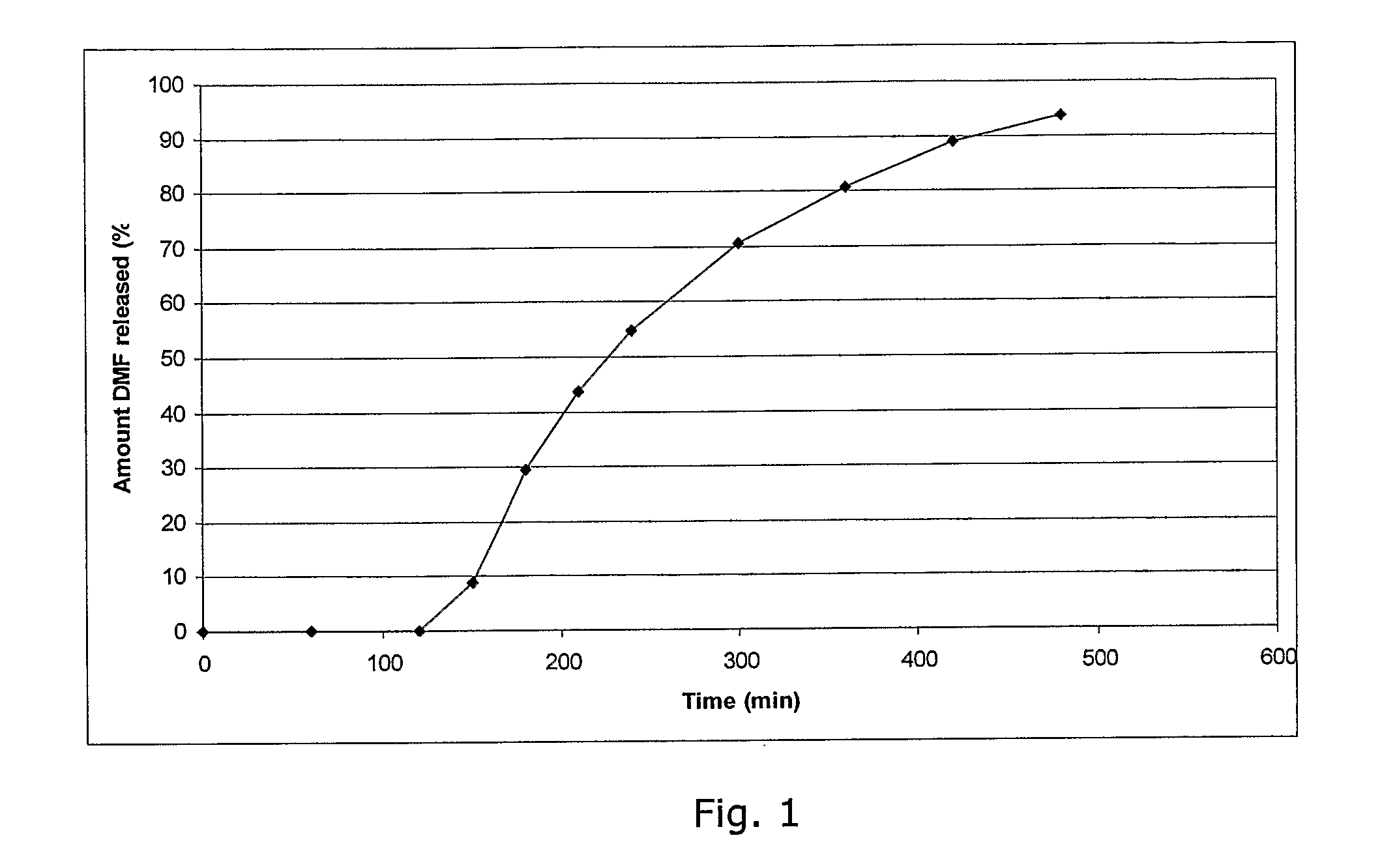
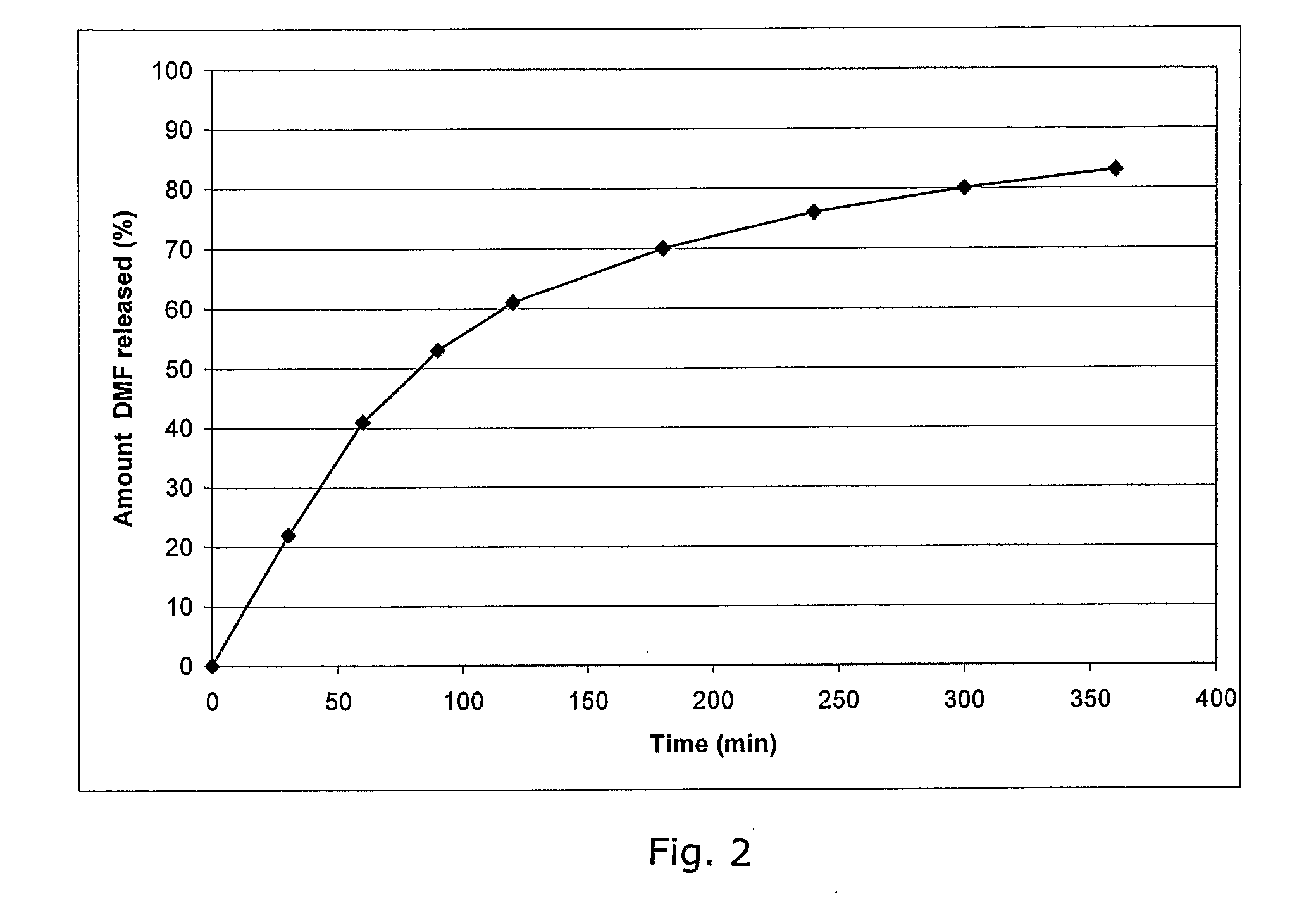
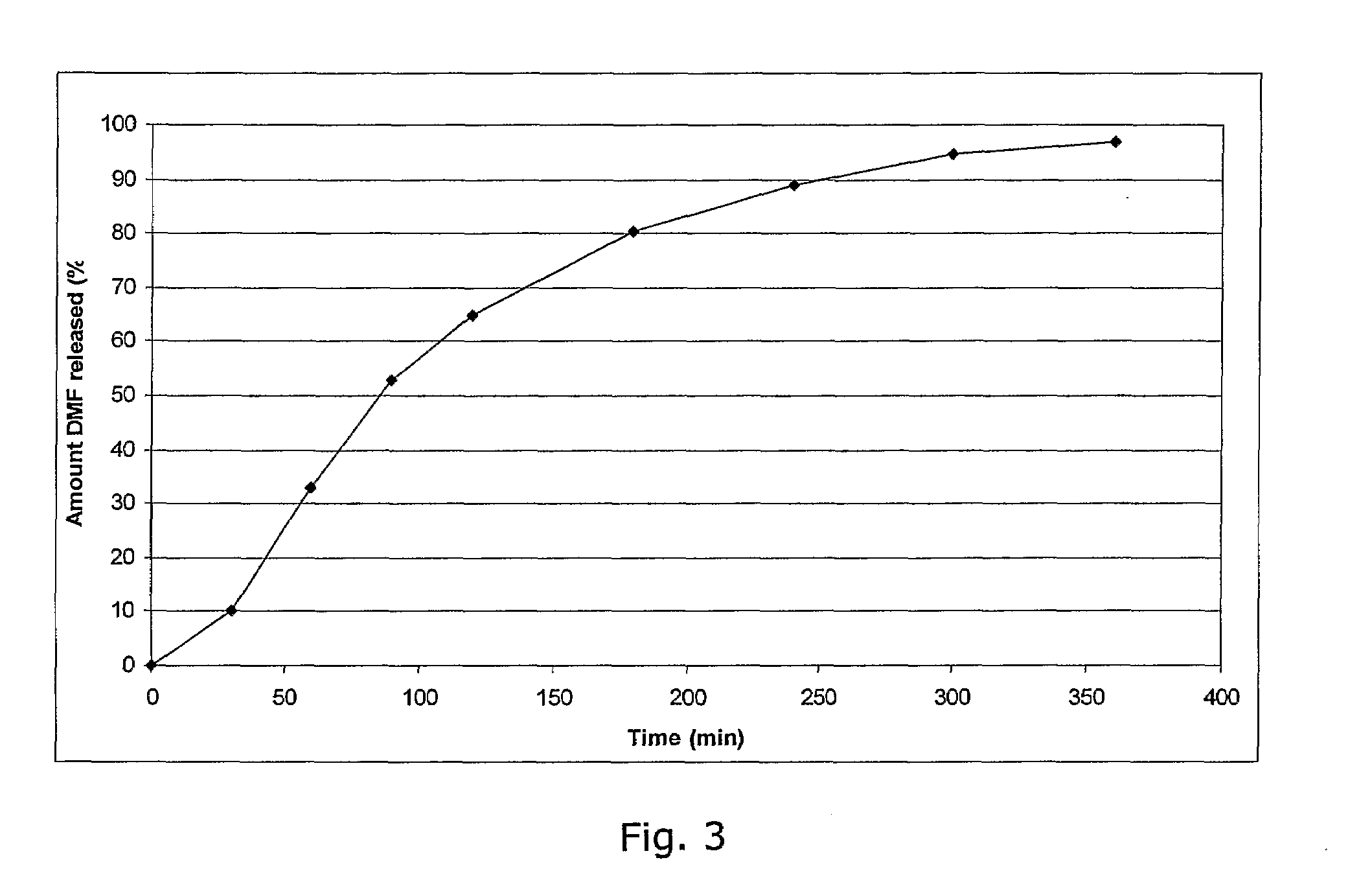
Controlled release pharmaceutical compositions comprising a fumaric acid ester
The present invention relates to controlled release pharmaceutical compositions comprising fumaric acid ester(s) as active substance(s). The compositions are suitable for use in the treatment of e.g. psoriasis or other hyperproliferative, inflammatory or autoimmune disorders and are designated to release the fumaric acid ester in a controlled manner so that local high concentrations of the active substance within the gastrointestinal tract upon oral administration can be avoided and, thereby, enabling a reduction in gastro-intestinal related side-effects.
Owner:BIOGEN SWISS MFG GMBH
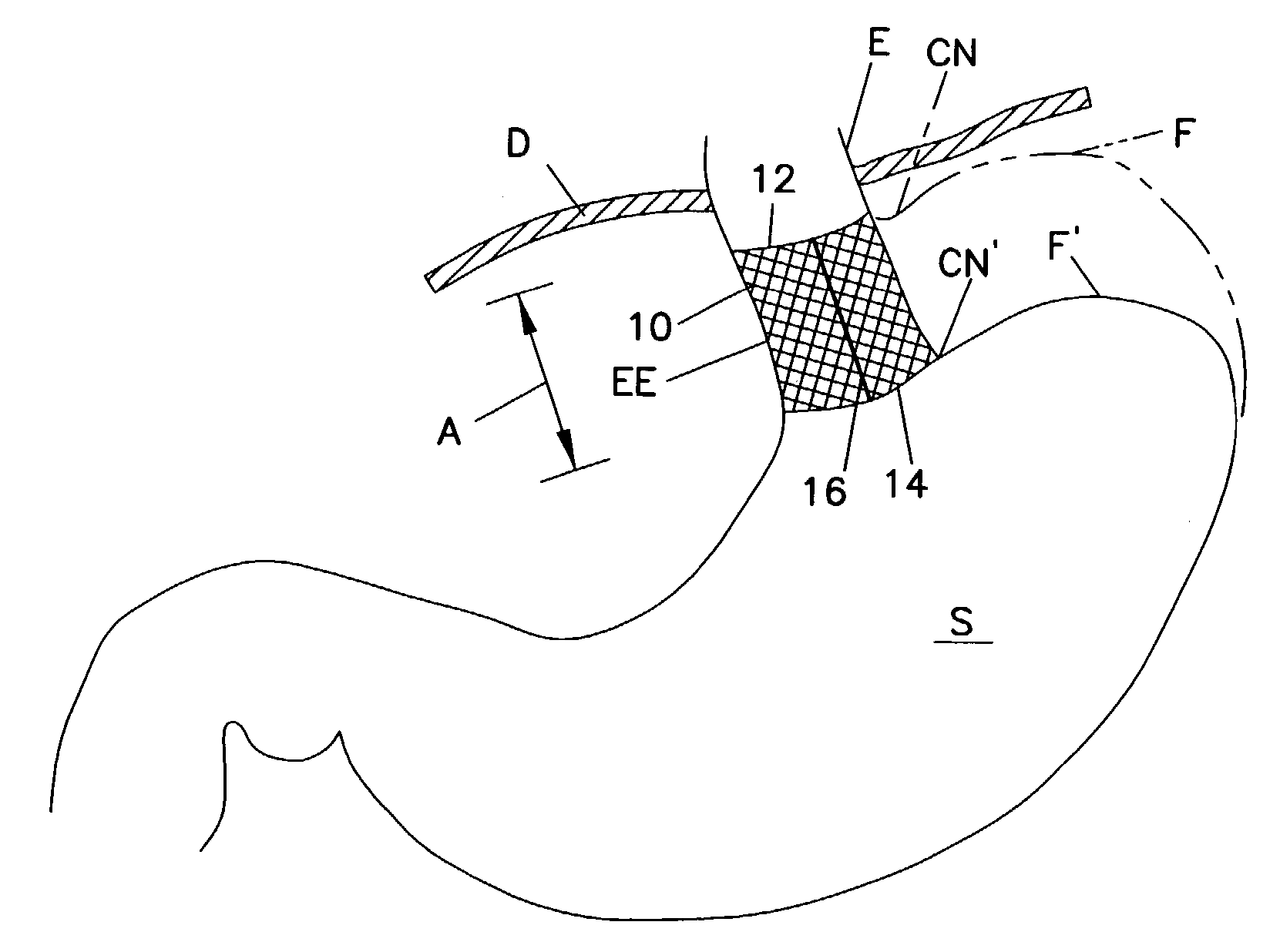
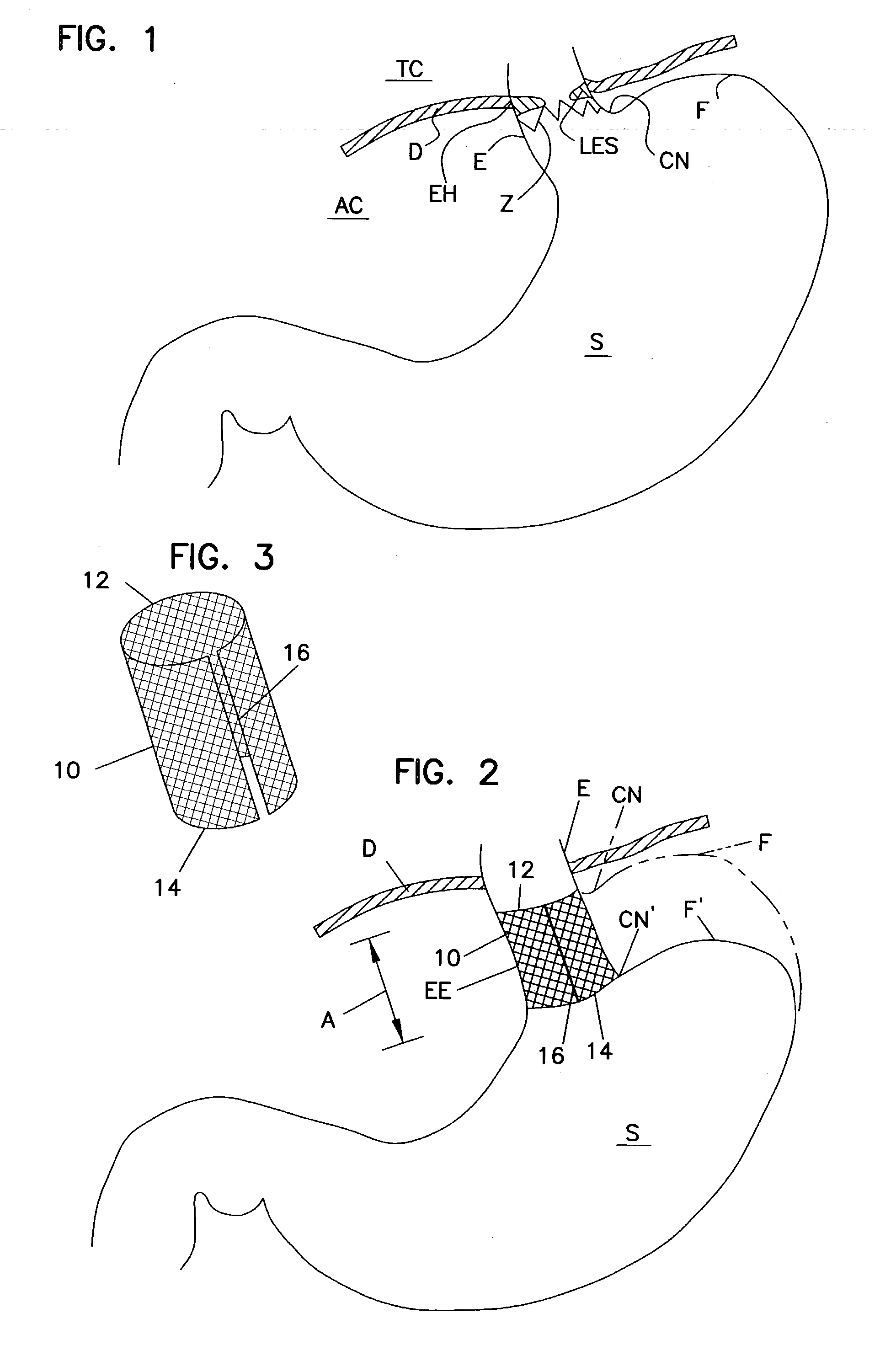
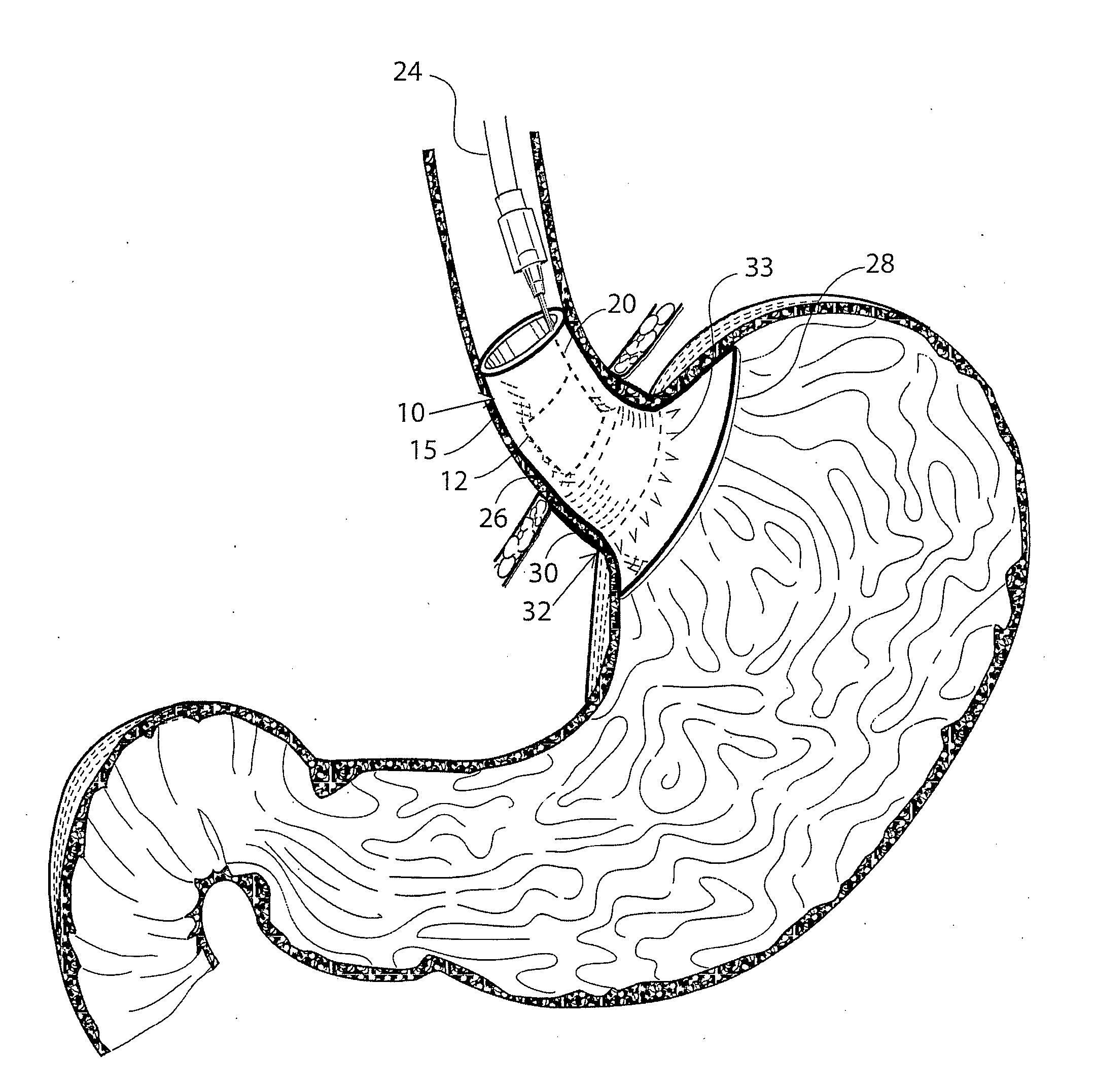
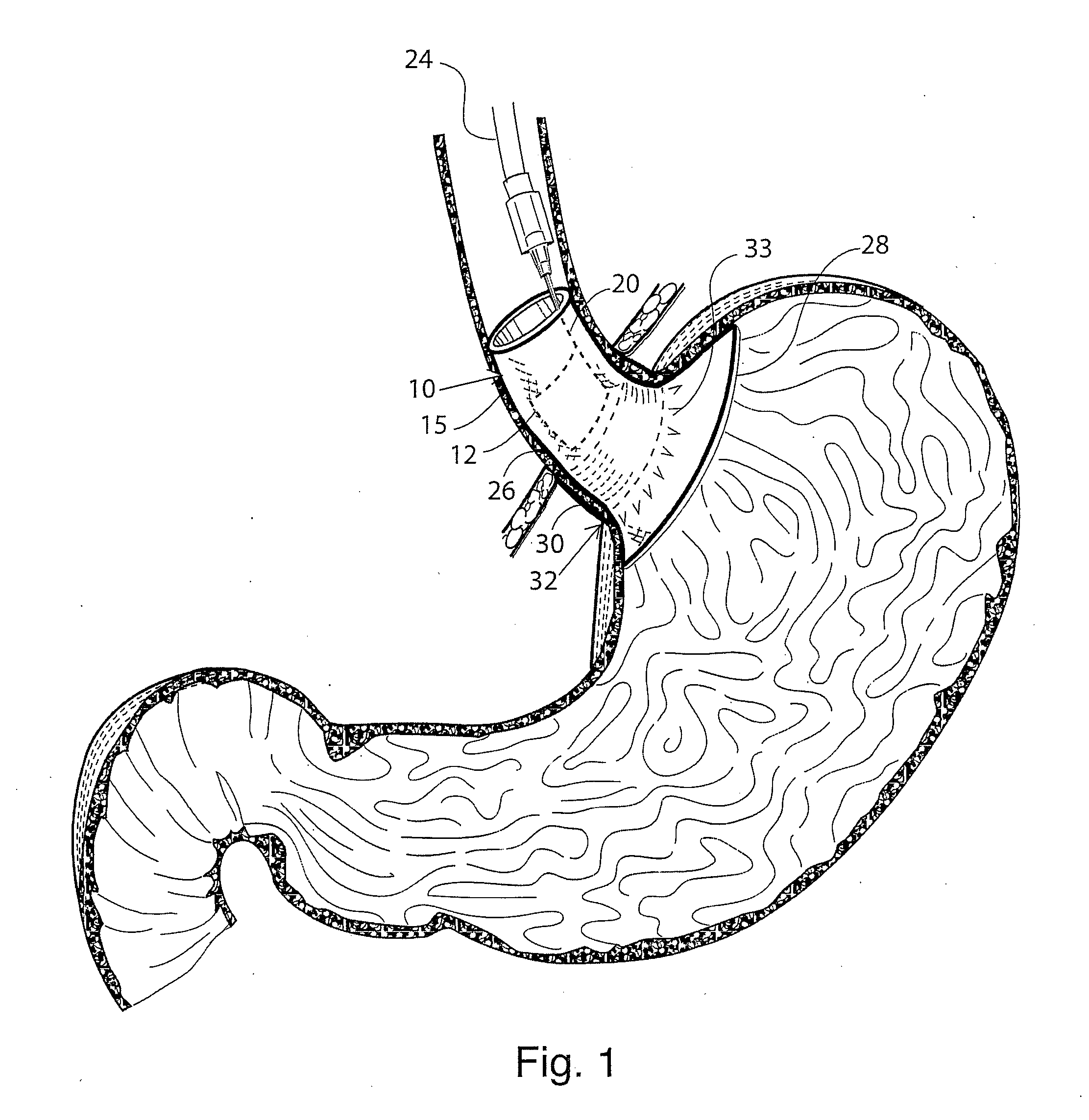
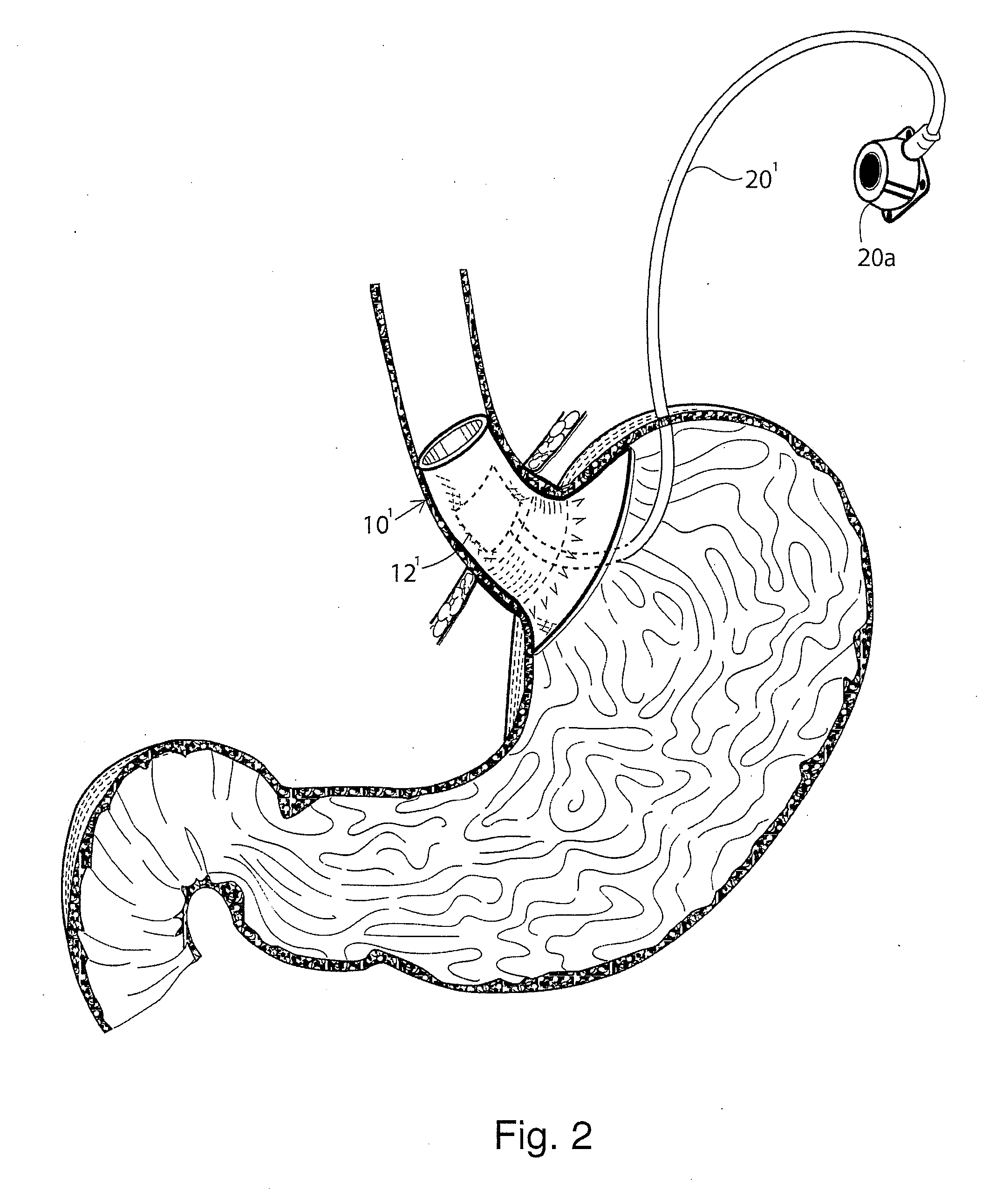
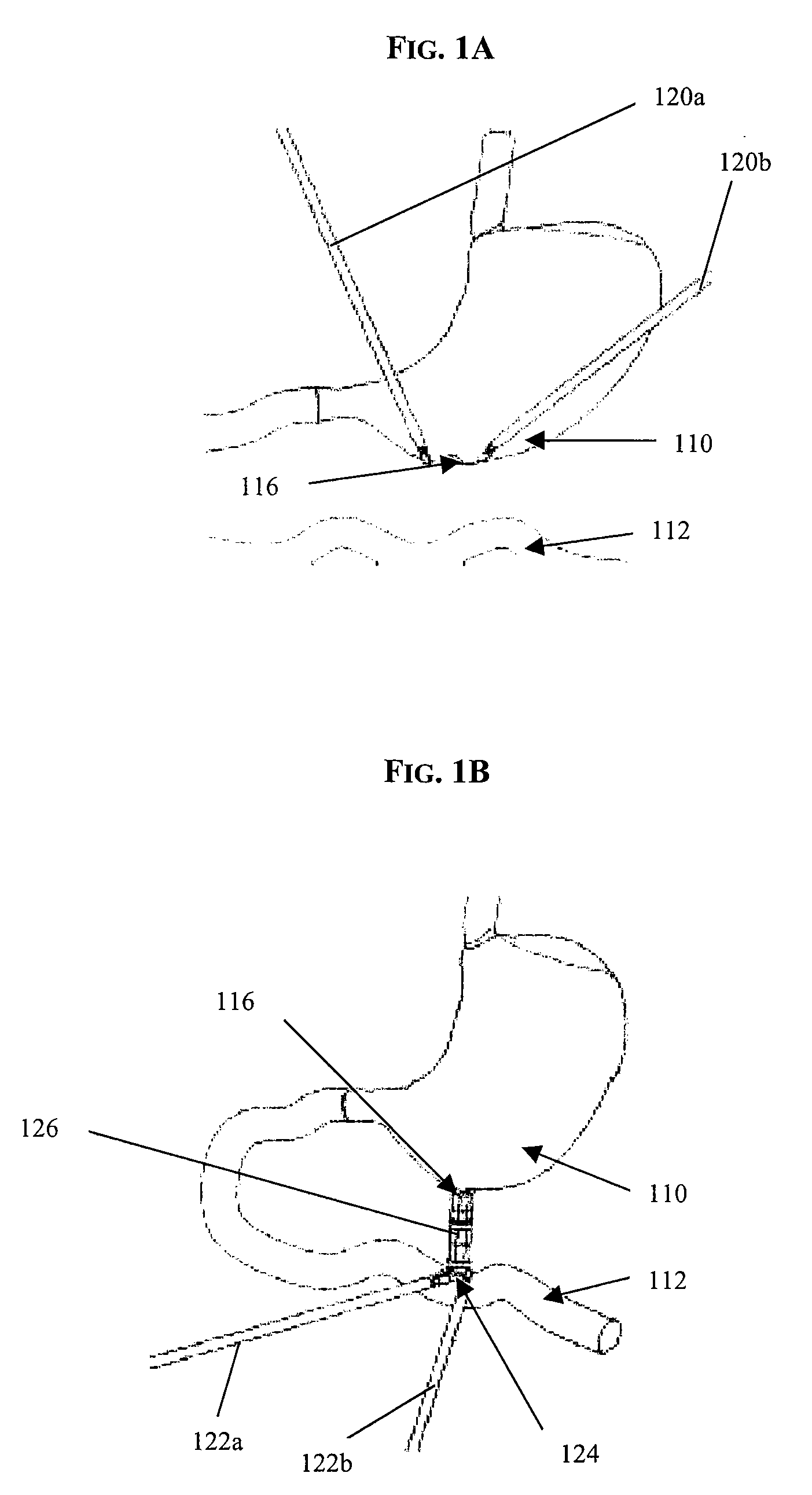
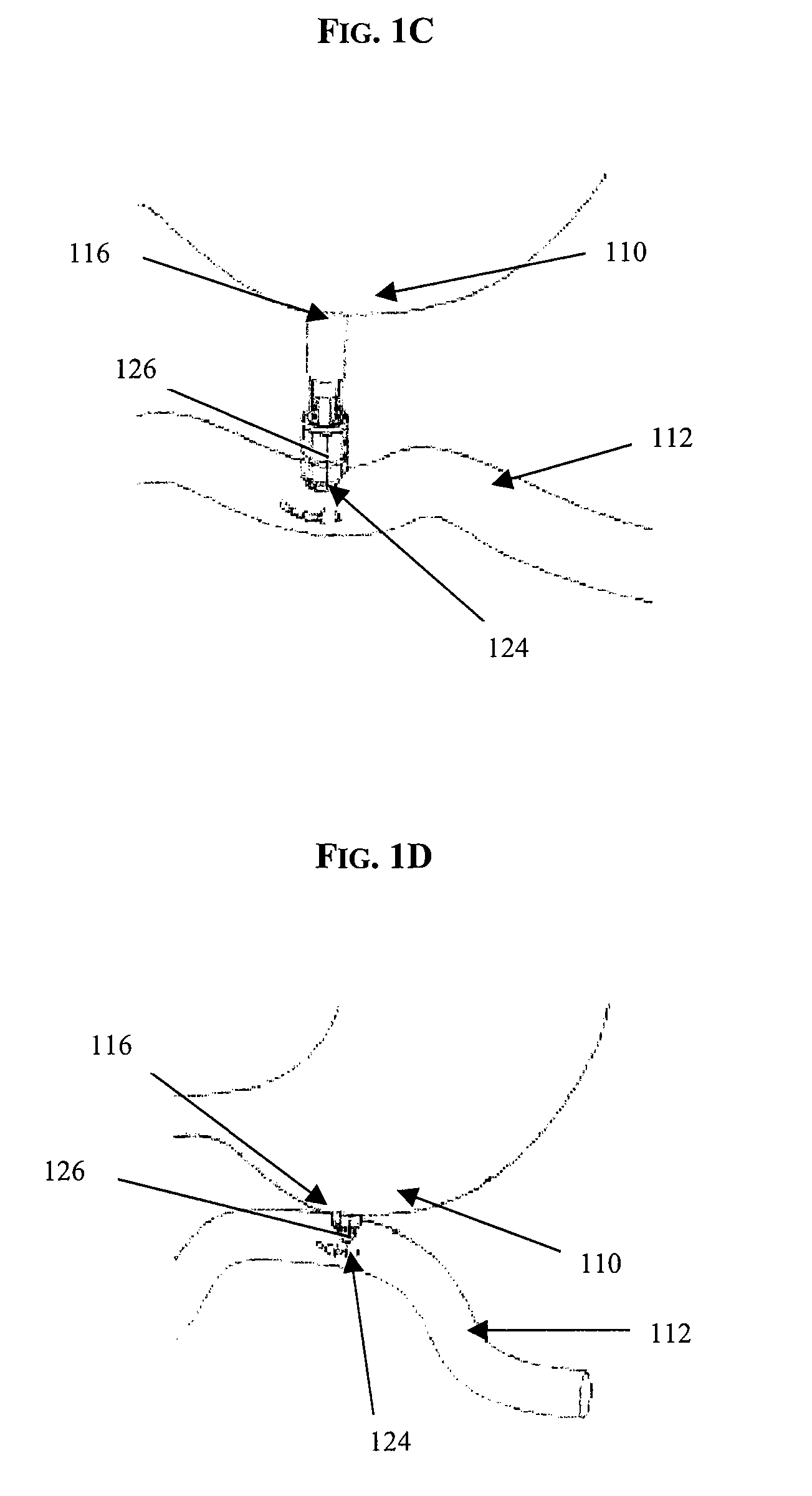
Gastro-esophageal reflux disease (GERD) treatment method and apparatus
The method of the invention includes accessing a juncture of an esophagus and a stomach of the patient on a distal side of a diaphragm. The esophagus and a fundus of the stomach intersect at a cardiac notch located at an original cardiac notch position. A reducing element is placed at the junction with the reducing element selected to reposition the cardiac notch to a position more distal to a lower esophageal sphincter of the patient.
Owner:RESHAPE LIFESCIENCES INC
Compositions and methods for characterizing and restoring gastrointestinal, skin, and nasal microbiota
ActiveUS20100074872A1Growth inhibitionFacilitate calorie uptakeBiocideMetabolism disorderBacteroidesDisease
The present invention relates to characterizing changes in mammalian bacterial gastrointestinal, cutaneous and nasal microbiota associated with antibiotic treatment and various disease conditions (such as asthma, allergy, obesity, metabolic syndrome, gastrointestinal reflux disease (GERD), eosinophilic esophagitis, gastro-esophageal junction adenocarcinomas (GEJAC), infections due to bacteria that are resistant to antibiotics, including Methicillin-resistant Staphylococcus aureus (MRSA), Clostridium difficile, vancomycin-resistant enterococci, etc.) and related diagnostic and therapeutic methods. Therapeutic methods of the invention involve the use of live bacterial inoculants that are capable of restoring healthy mammalian bacterial gastrointestinal, skin, and nasal microbiota.
Owner:NEW YORK UNIV
System and method for mapping gastro-intestinal electrical activity
InactiveUS20130035576A12D-image generationSurgical instrument detailsSmooth muscleBiological activation
A gastro-electrical activity mapping system and comprises a catheter insertable through a natural orifice into the gastro-intestinal (GI) tract and comprising an array of electrodes for contacting an interior surface of a section of the GI tract to detect electrical potentials at multiple electrodes, and a signal analysis and mapping system arranged to receive and process electrical signals from multiple electrodes of the array and spatially map GI smooth muscle electrical activity as an activation time map, a velocity map, or an amplitude map, which may be in the form of contour plots and may be mapped on an anatomical computer model of at least the section of the GI tract and may be animated. A GI mapping method and catheter are also claimed.
Owner:AUCKLAND UNISERVICES LTD
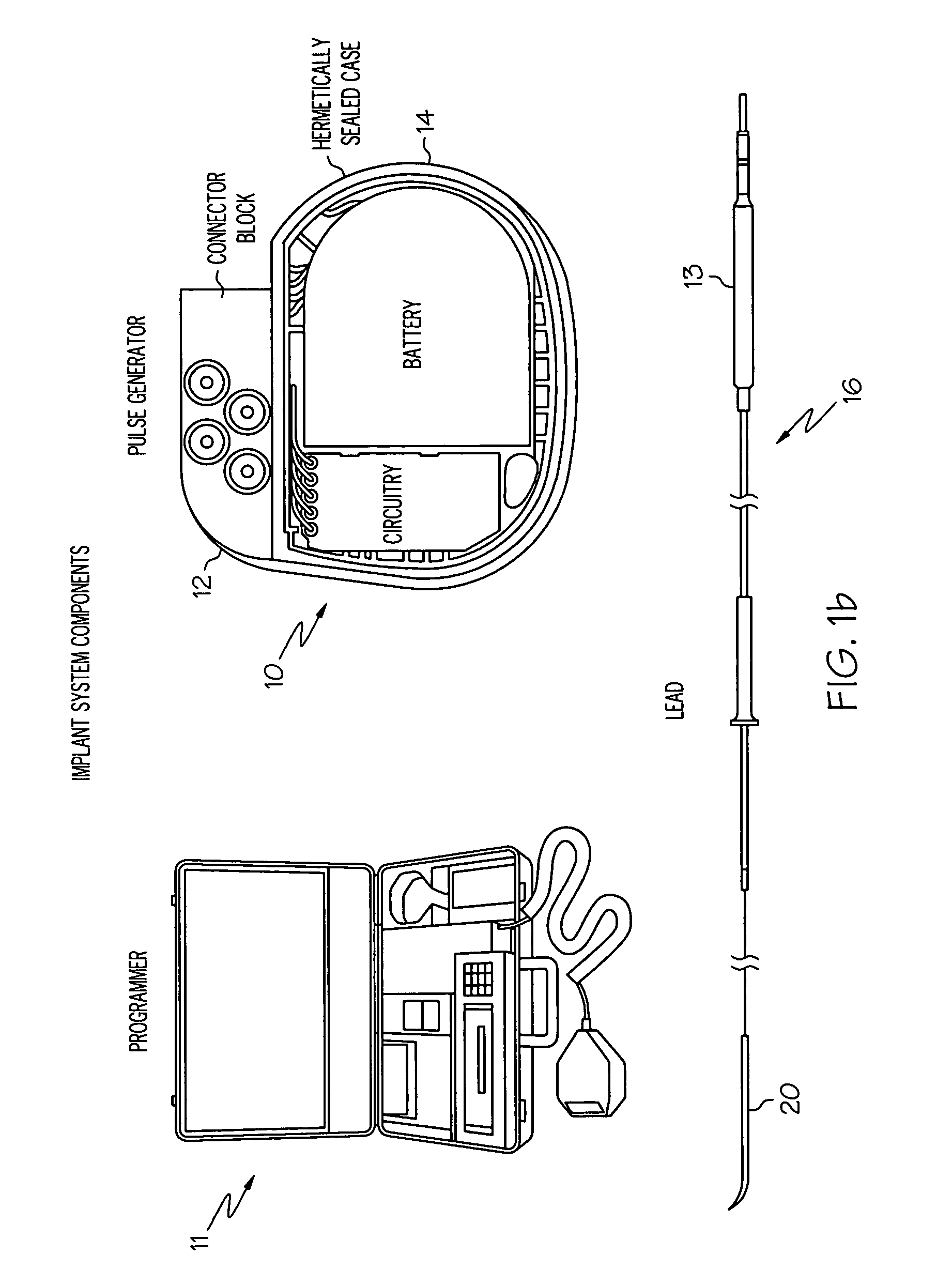
Gastro-electric stimulation for increasing the acidity of gastric secretions or increasing the amounts thereof
InactiveUS7742818B2Low costTherapy is simpleInternal electrodesExternal electrodesMedicineGastric secretions
A gastro-electric stimulation system includes an INS for producing an electrical stimulation signal, at least one medical electrical lead, and at least two electrical contacts. The medical electrical lead has a proximal end and a distal end, the proximal end being connected to the INS and the distal end being adapted for placement in or near a patient's stomach or appropriate nerve or nerve portion. The electrodes are disposed near the distal end of the medical electrical lead, and the electrodes are electrically connected through the medical electrical lead to the INS to receive the electrical stimulation signal and convey such signal to the selected electrode implant position. The electrical stimulation signal is provided in an amount and manner adapted to decrease the pH of the gastric acid in the patient's stomach and / or to increase the amount of gastric acid produced thereby.
Owner:MEDTRONIC INC
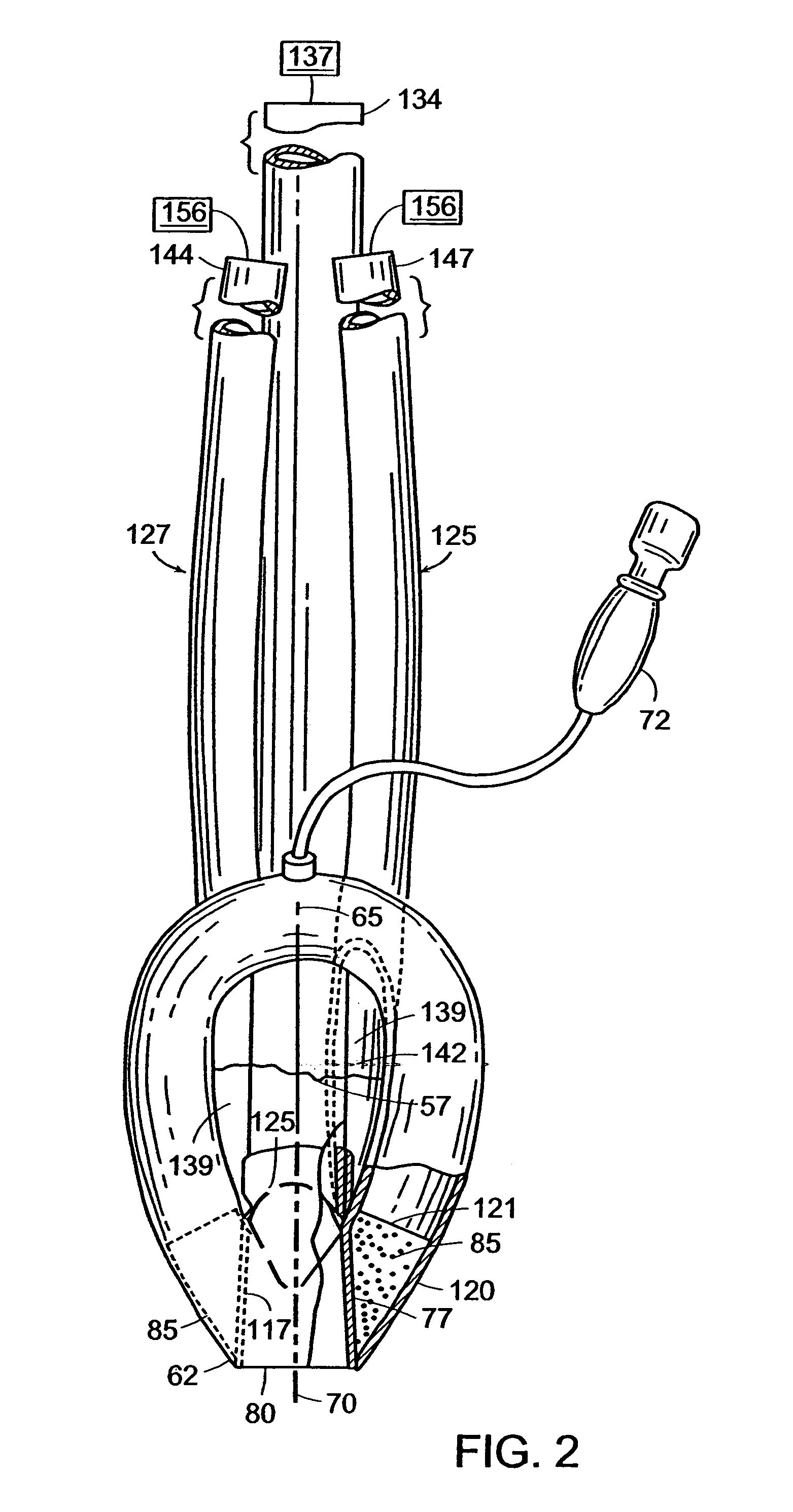
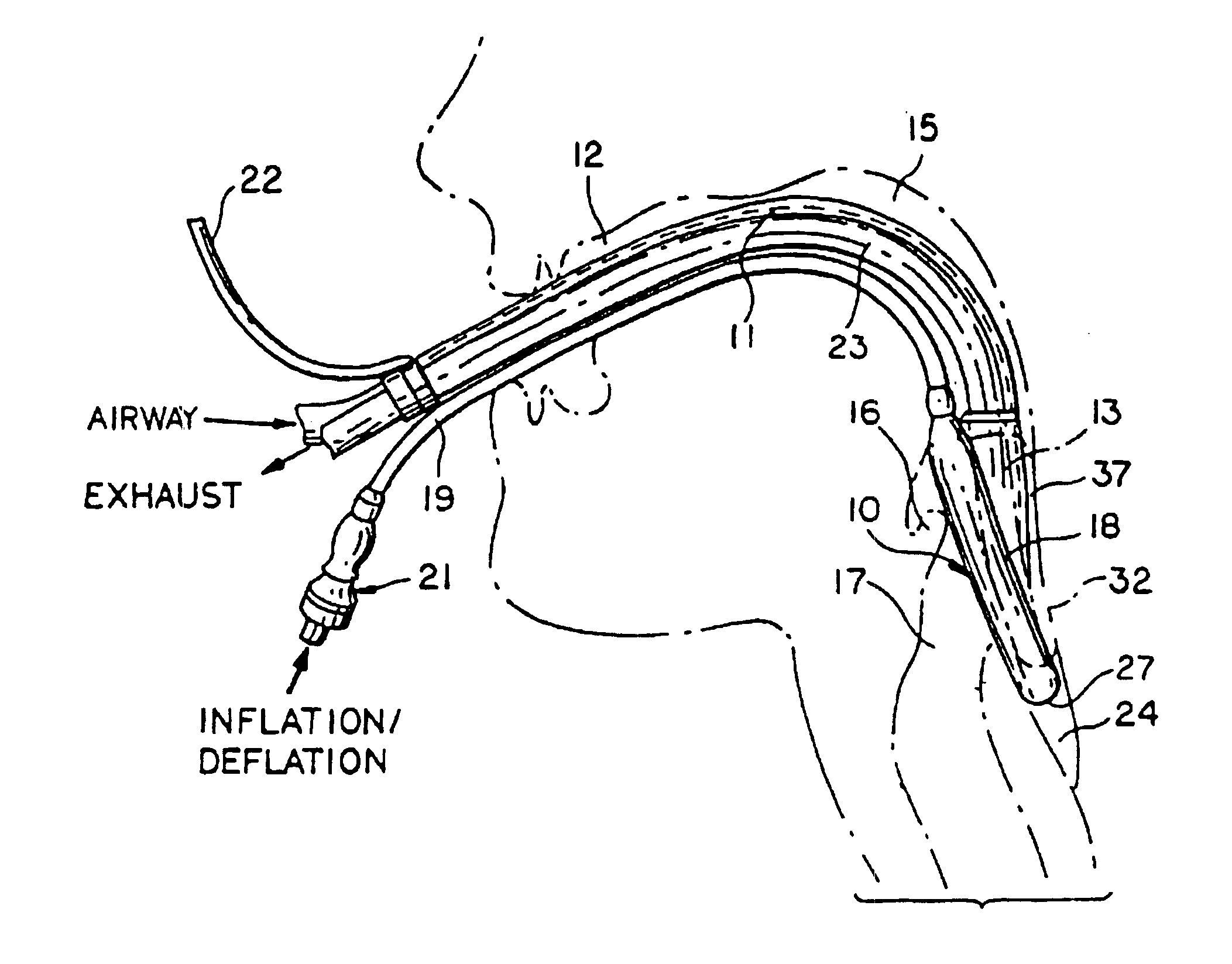
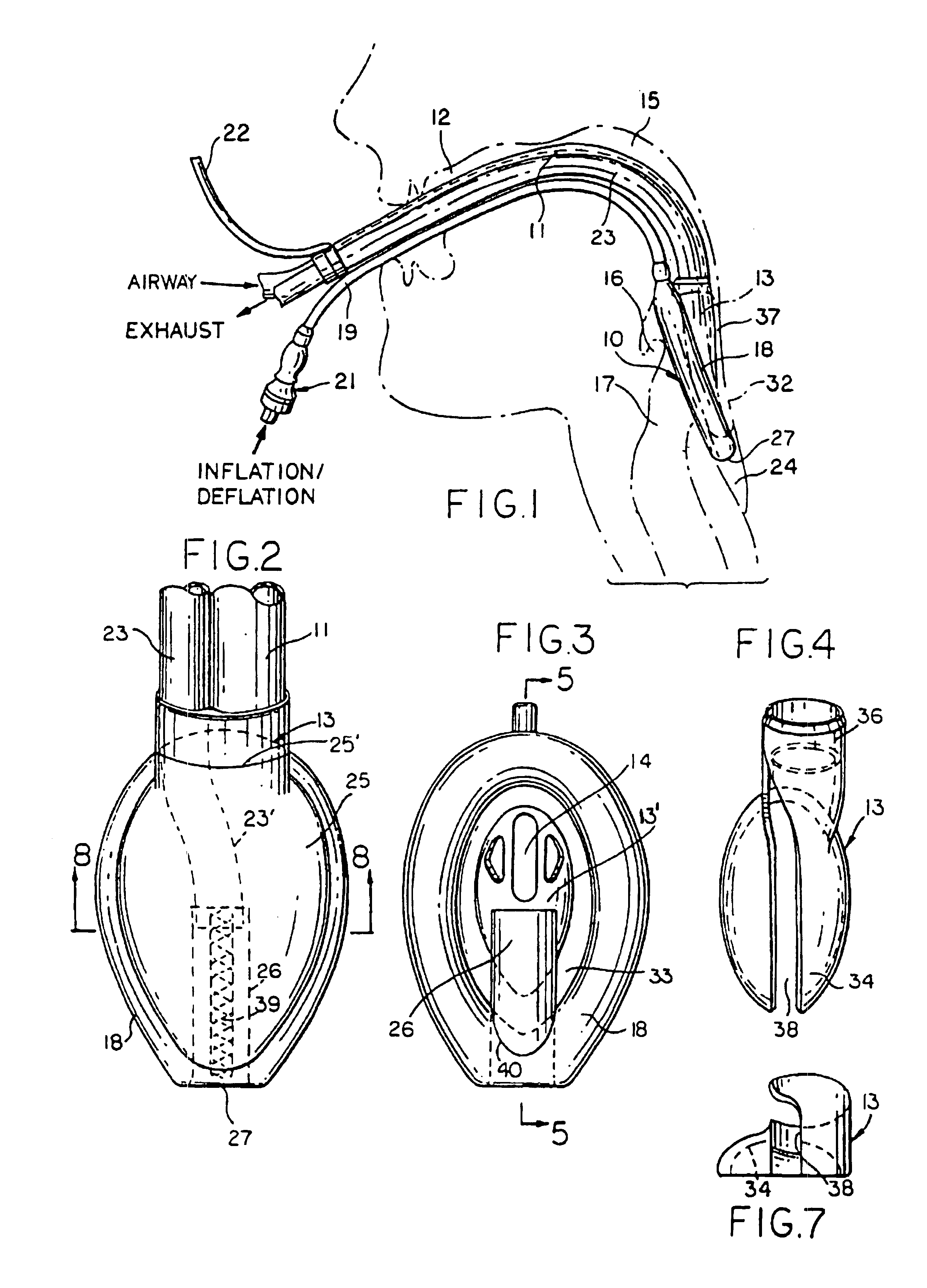
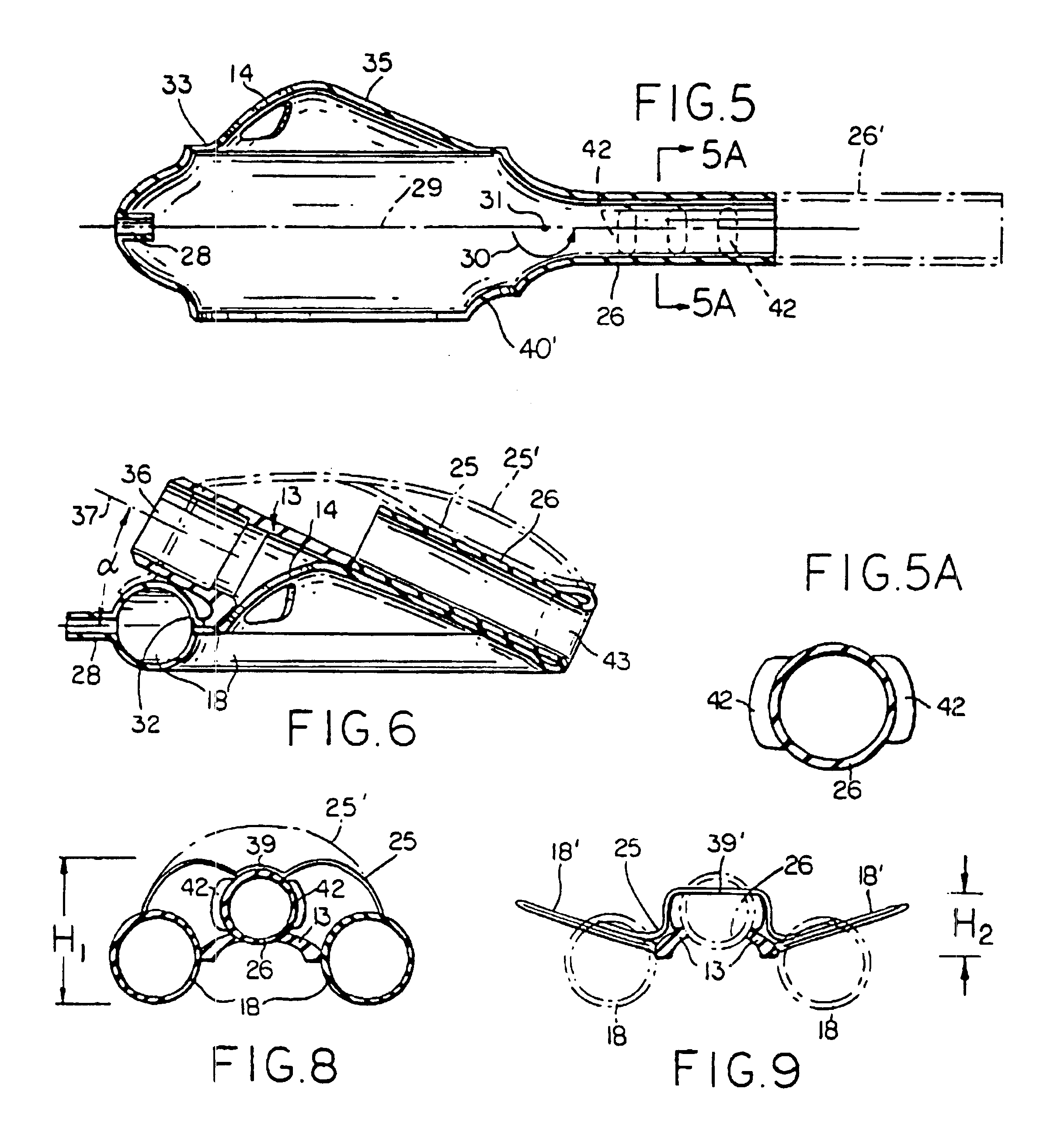
Laryngeal mask with large-bore gastric drainage
An artificial airway device for use in unconscious patients comprises a laryngo-pharyngeal mask including an expandable masking ring. The expandable mask sealingly surrounds the laryngeal inlet when expanded to obstruct communication between the laryngeal inlet and oesophagus. One or more airway tubes connected to the mask provide for fluid flow to a portion of the mask facing the laryngeal inlet when said mask sealingly surrounds the laryngeal inlet. A gastro-tube connected to the mask provides a fluid flow-path to the mask when the mask sealingly surrounds the laryngeal inlet. The distal end of the gastro tube passes through the masking ring at its narrower distal region where, when installed in a patient, it abuts against the oesophagus; and the distal portion of the gastro-tube flattens when the mask is deflated to facilitate smooth passage behind the larynx during insertion through the mouth and throat of the patient.
Owner:TELEFLEX LIFE SCI PTE LTD
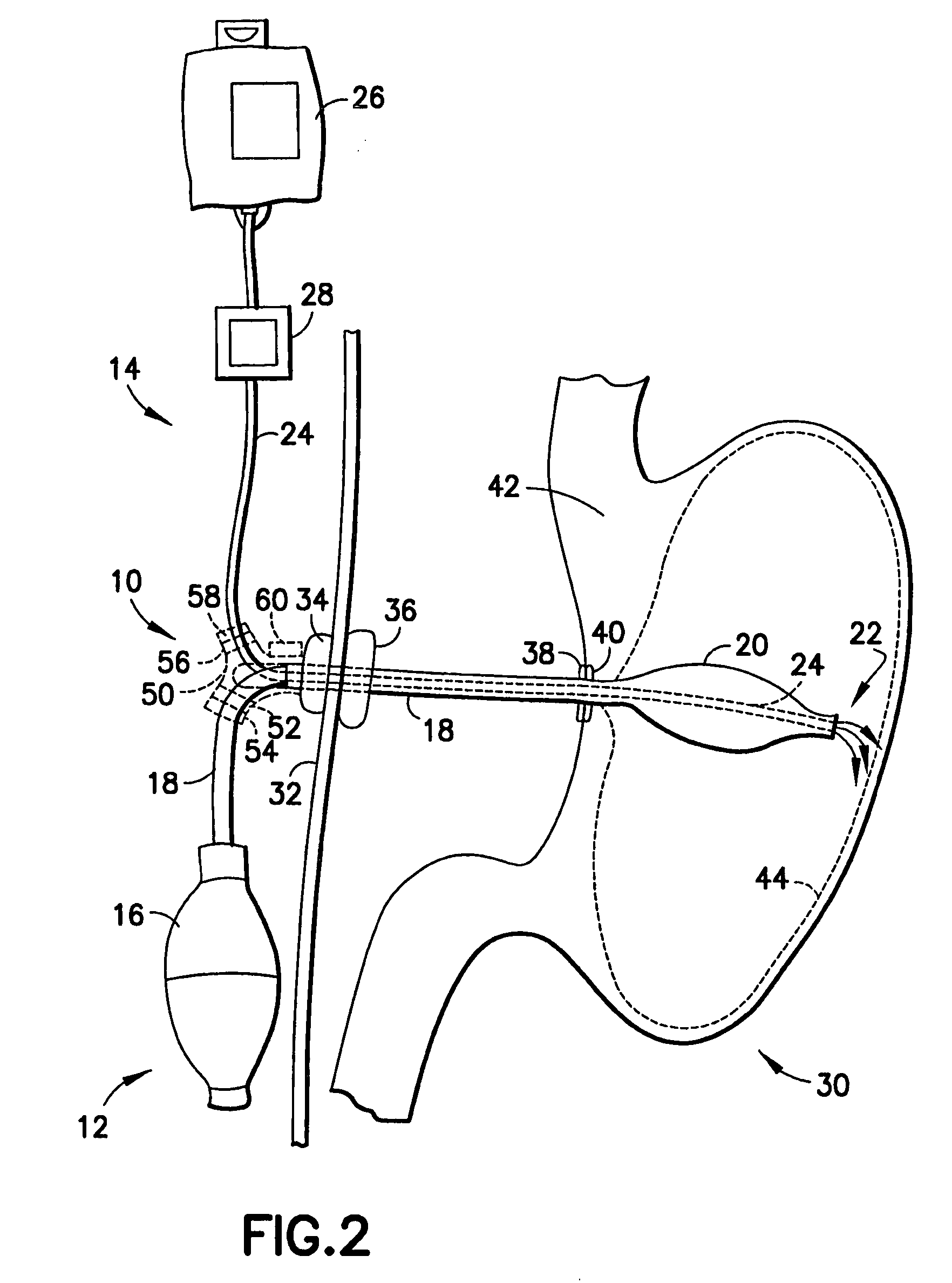
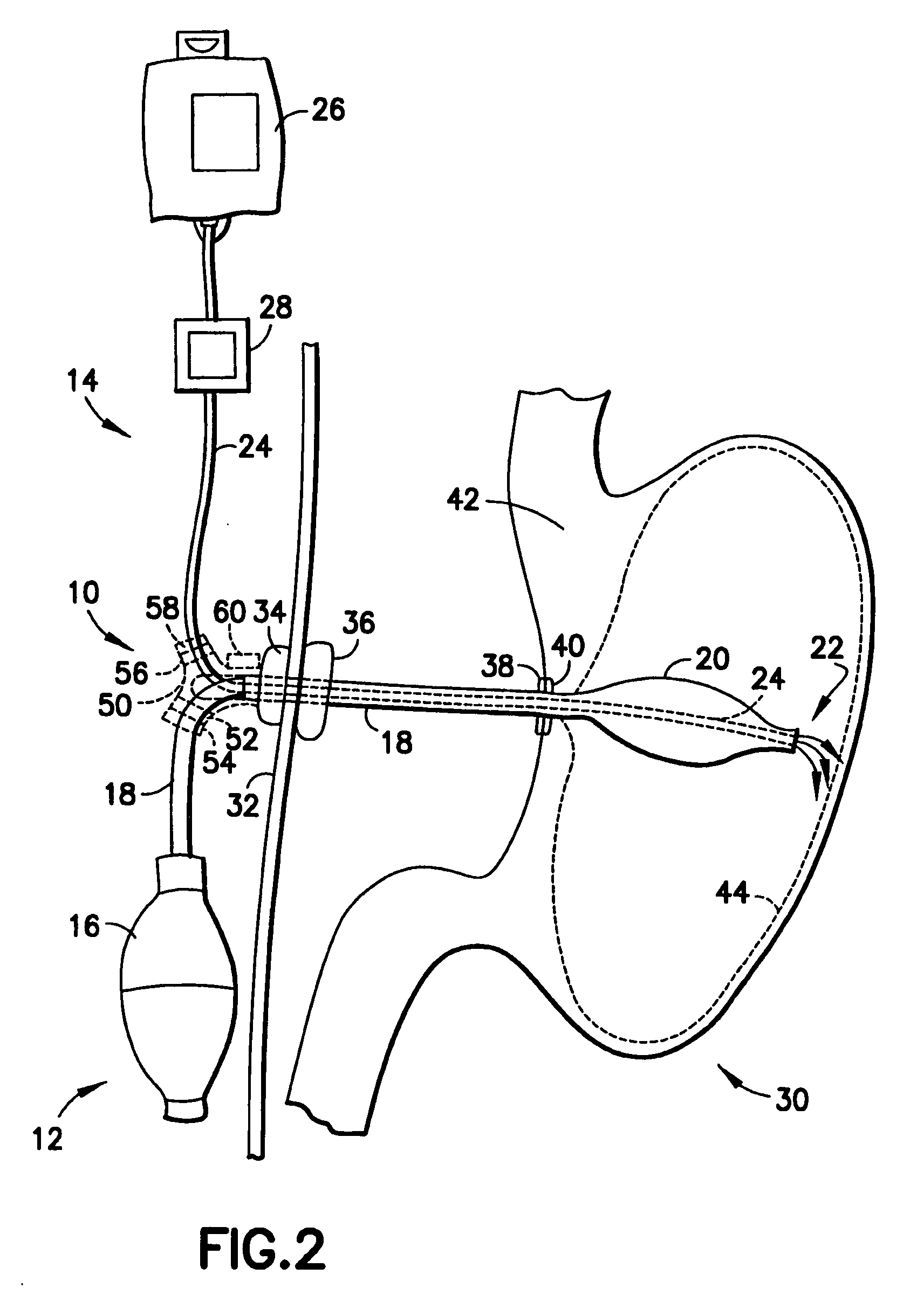
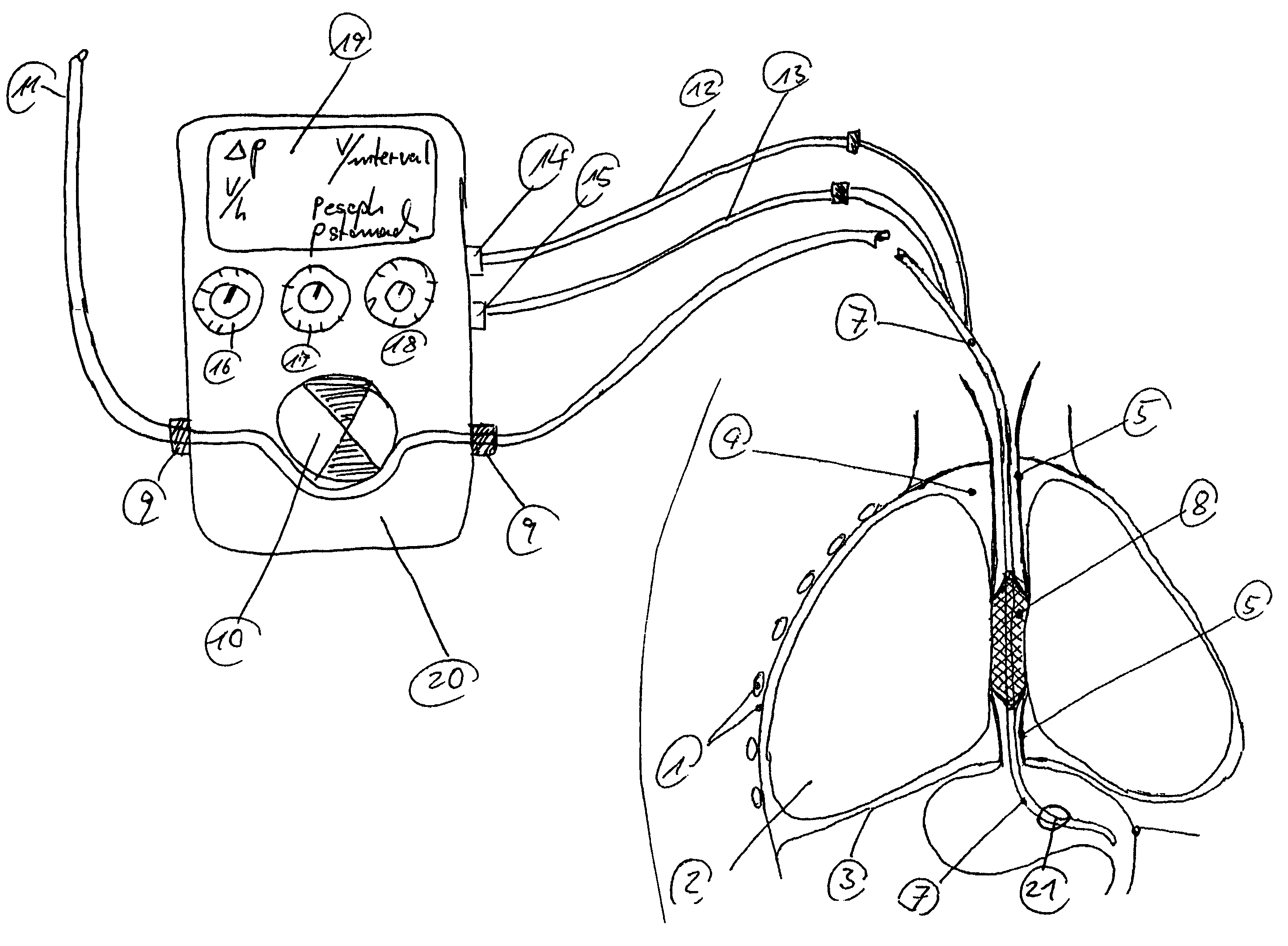
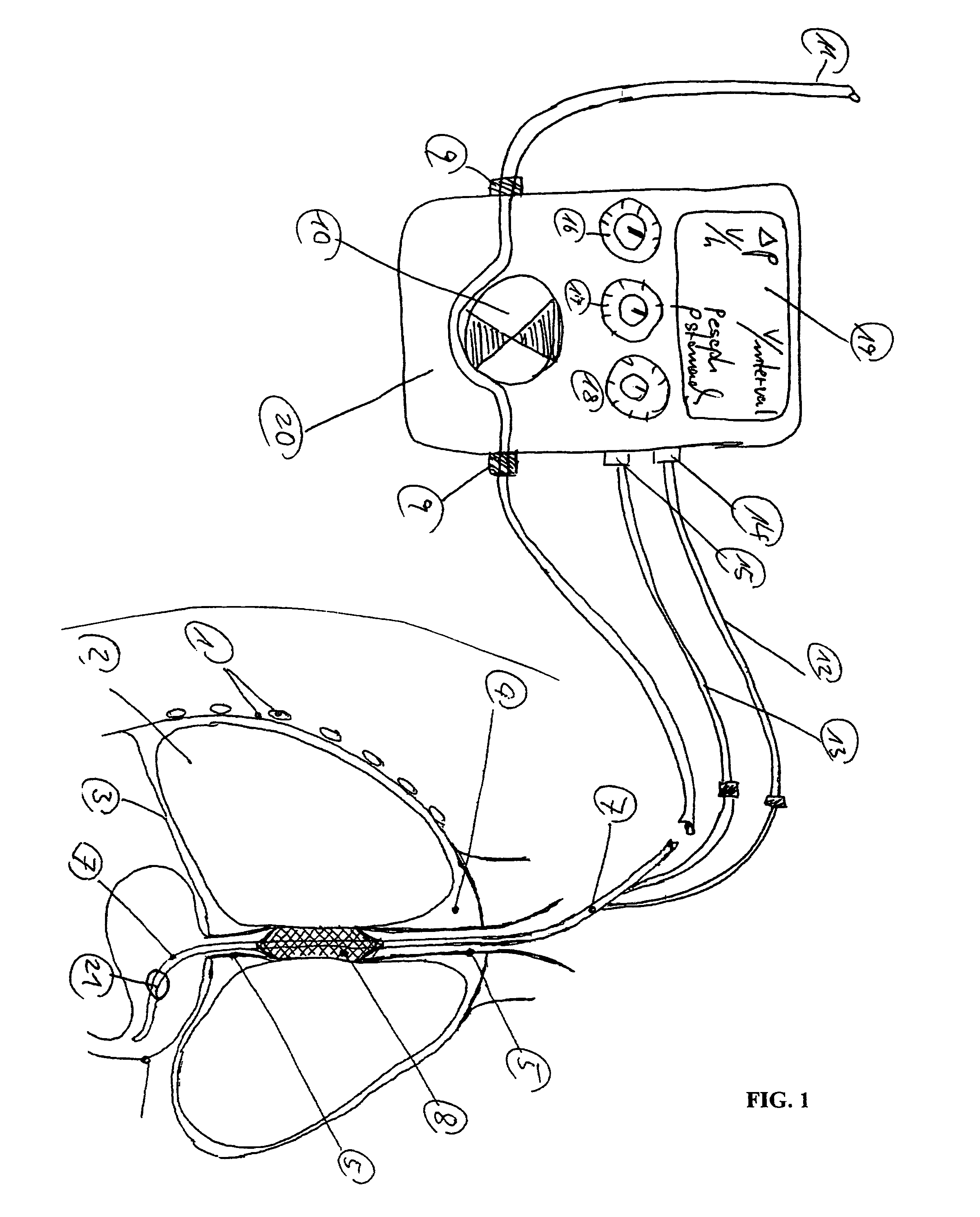
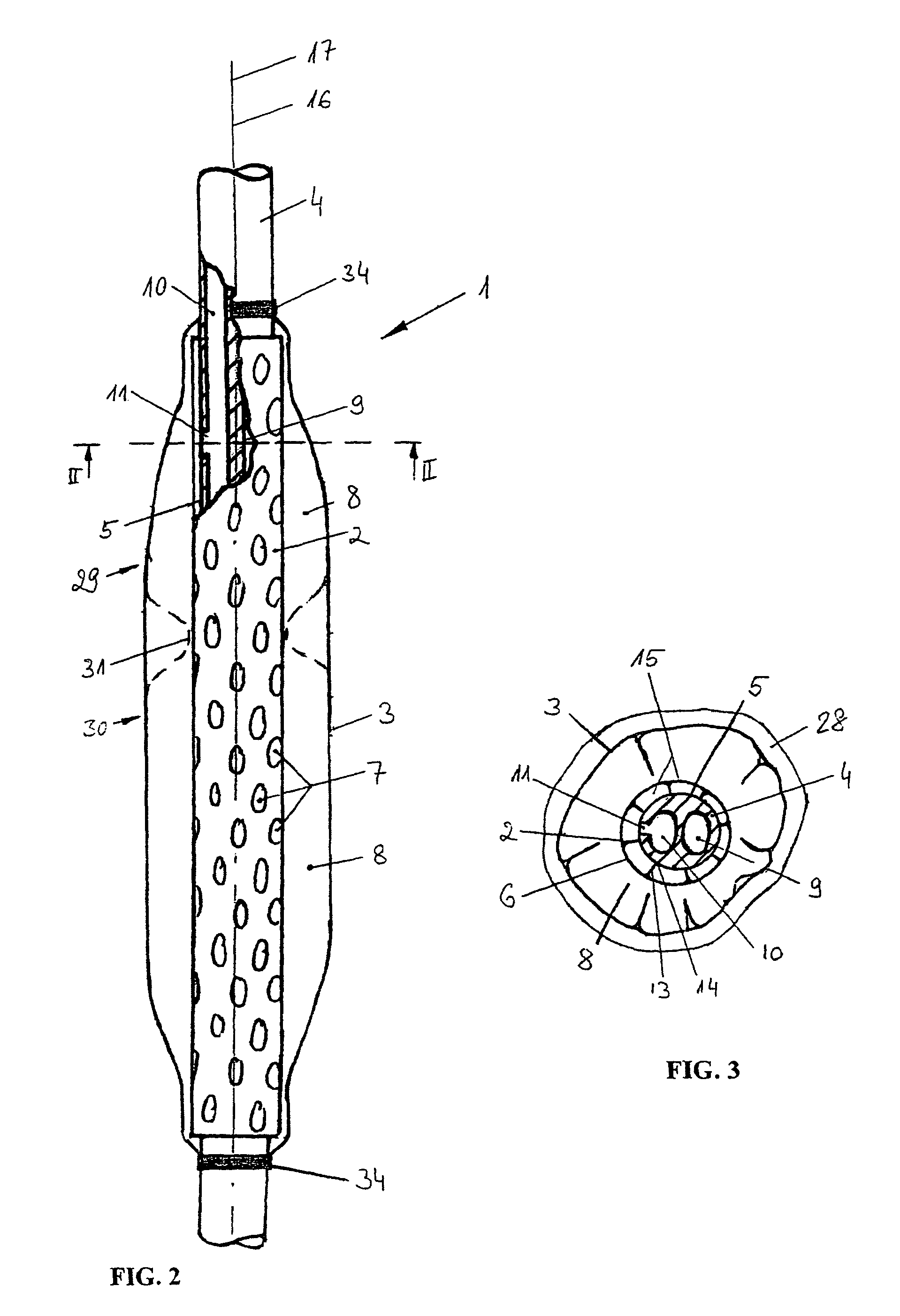
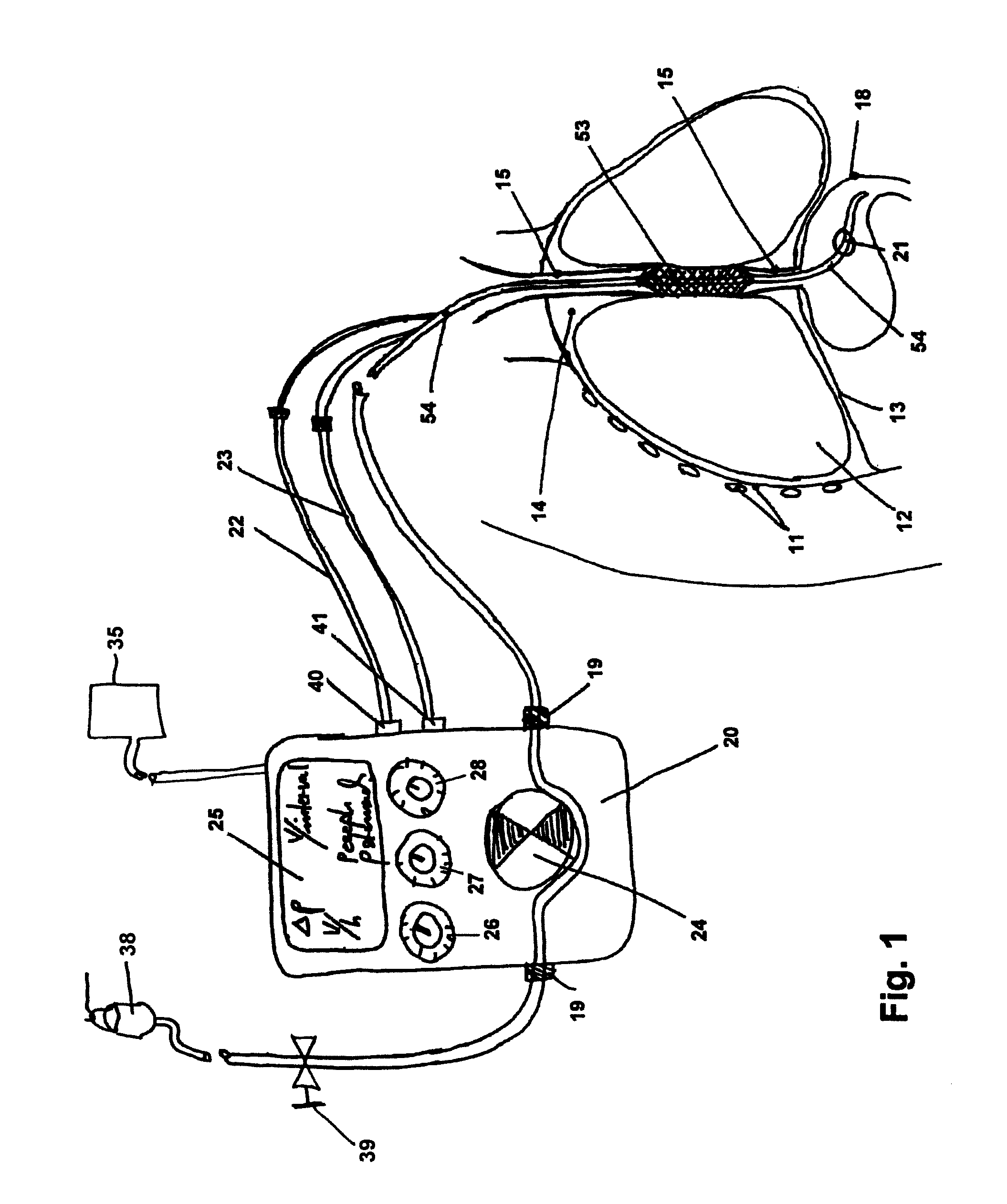
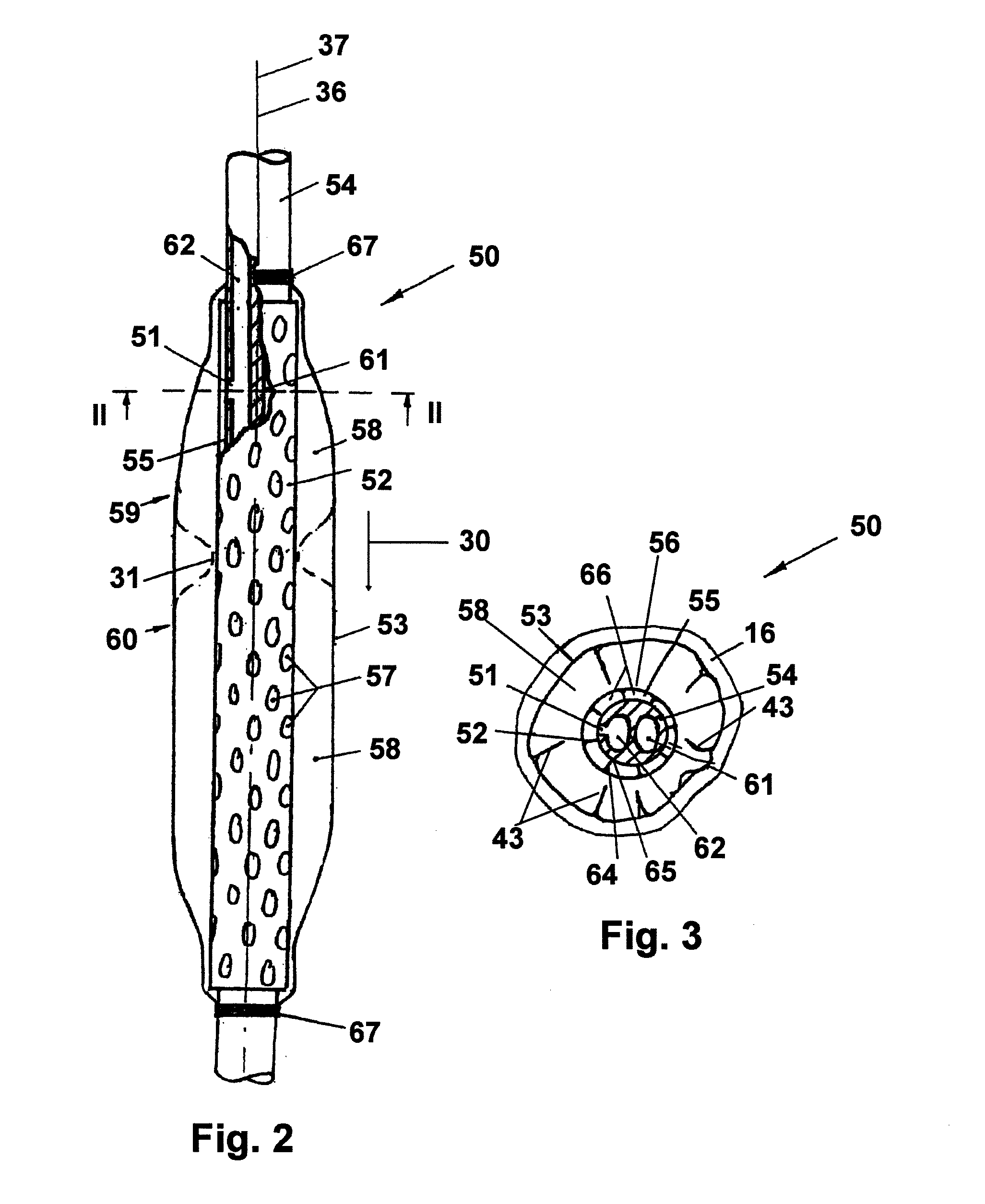
Gastro-esophageal reflux control system and pump
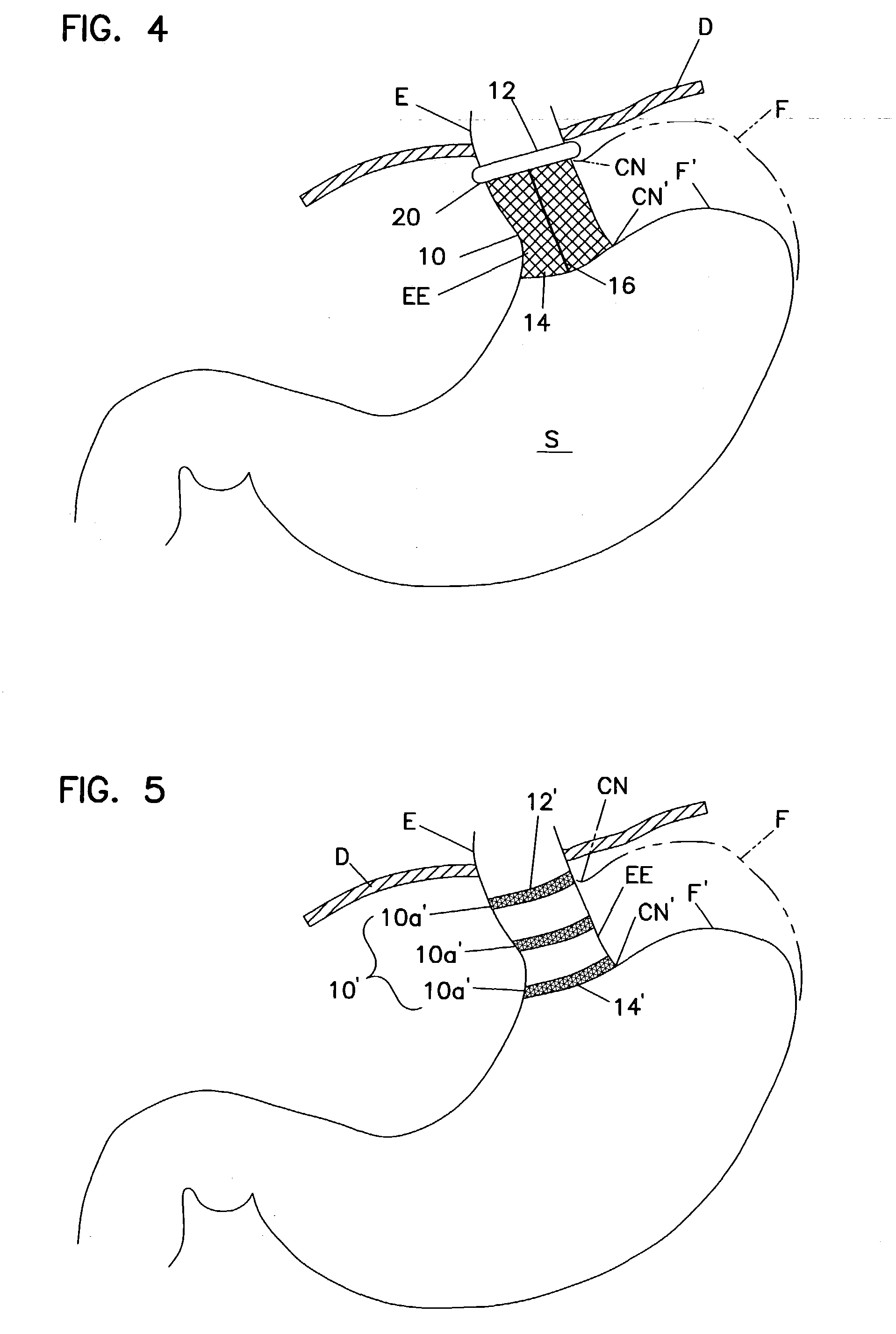
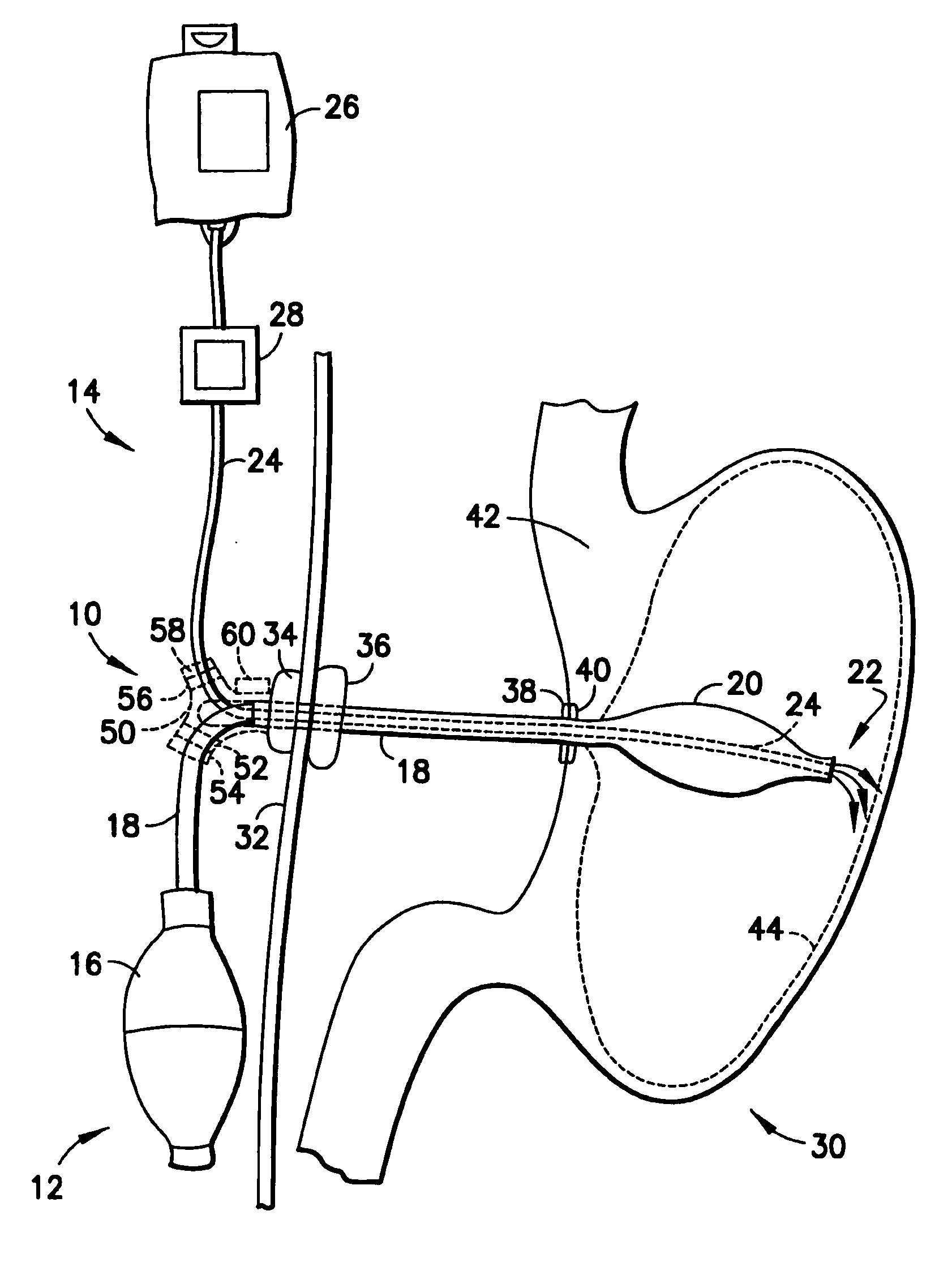
An enteral feeding unit or system that minimizes the occurrence of gastro-esophogeal-pharynegal reflux during feeding is described. The enteral feeding unit or device includes an automatable feeding pump with a feedback sensor for sensing a relative pressure in a patient's stomach and esophagus, and a regulator system for controlling or monitoring feeding rate to said patient as a function of said relative gastro-esophageal pressure. The system includes a stomach probe that has a fluid-tight closure of the esophagus. The stomach probe, according to the invention, is characterized by a tampon-bladder for watertight closure of the esophagus, in which the tampon-bladder forms from flexible and / or elastic material at least a closed inner cavity for the reception of a fluid medium, through a means (11) of establishing a prescribed pressure for the medium in the tampon-bladder (16) by an inner lumen forming the actual stomach probe, from which an outer hose-like lumen (18) extending to the tampon-bladder (16) is so arranged that between the outer lumen (18) and the inner lumen (17) a channel is formed connected to the inner cavity of the tampon-bladder (16) arranged on the outer lumen (18) by a number of openings (20), whereby the inner cavity of the tampon-bladder (16) is connected via the canal formed between the inner and outer lumina (17, 18) with the means of production of pressure in the tampon-bladder, that is, with a suitably graded reservoir or equalizing vessel (11) for the liquid medium situated above the tampon-bladder and outside the patient.
Owner:AVENT INC
Surgical instruments for treating gastro-esphageal reflux
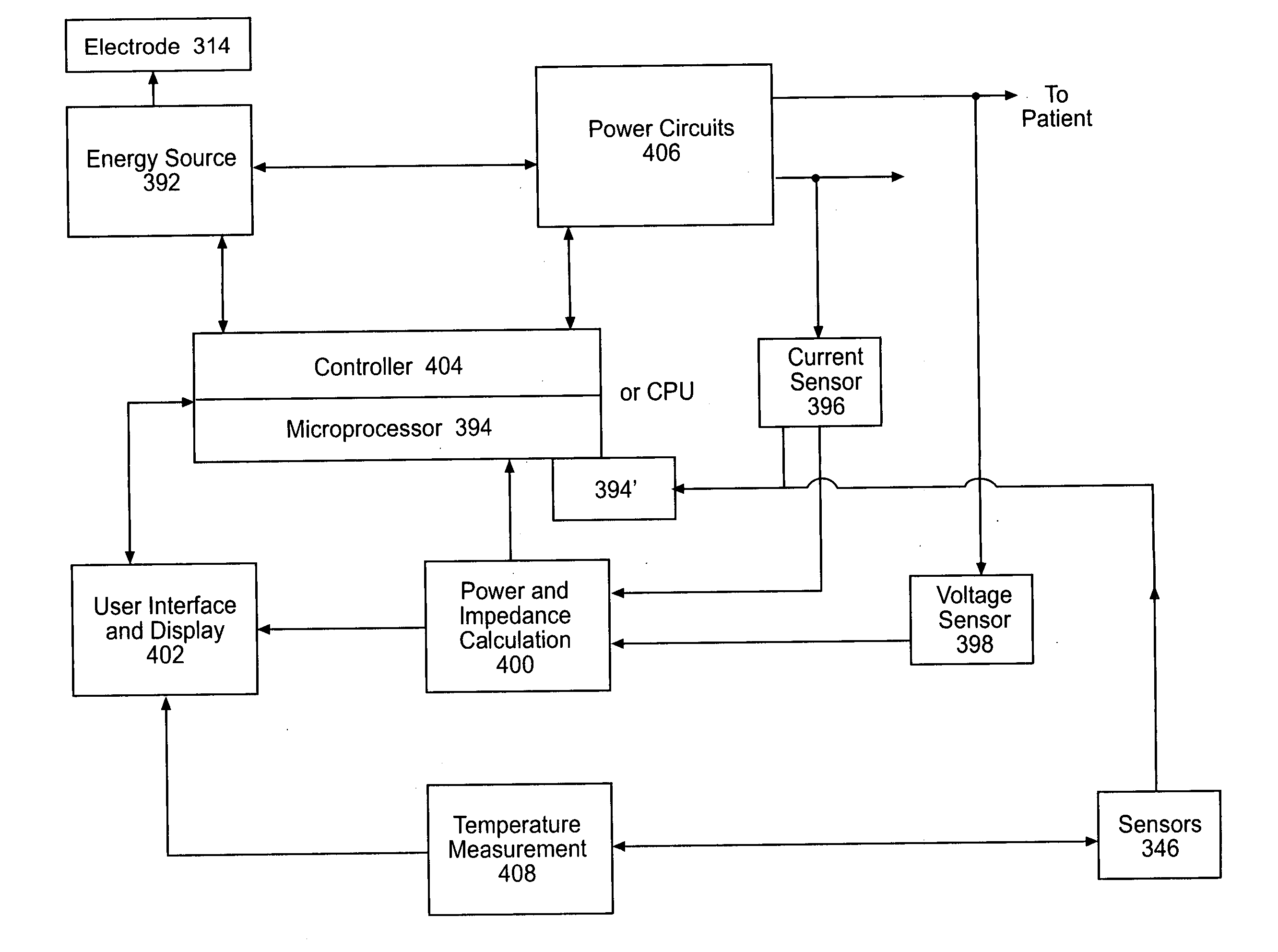
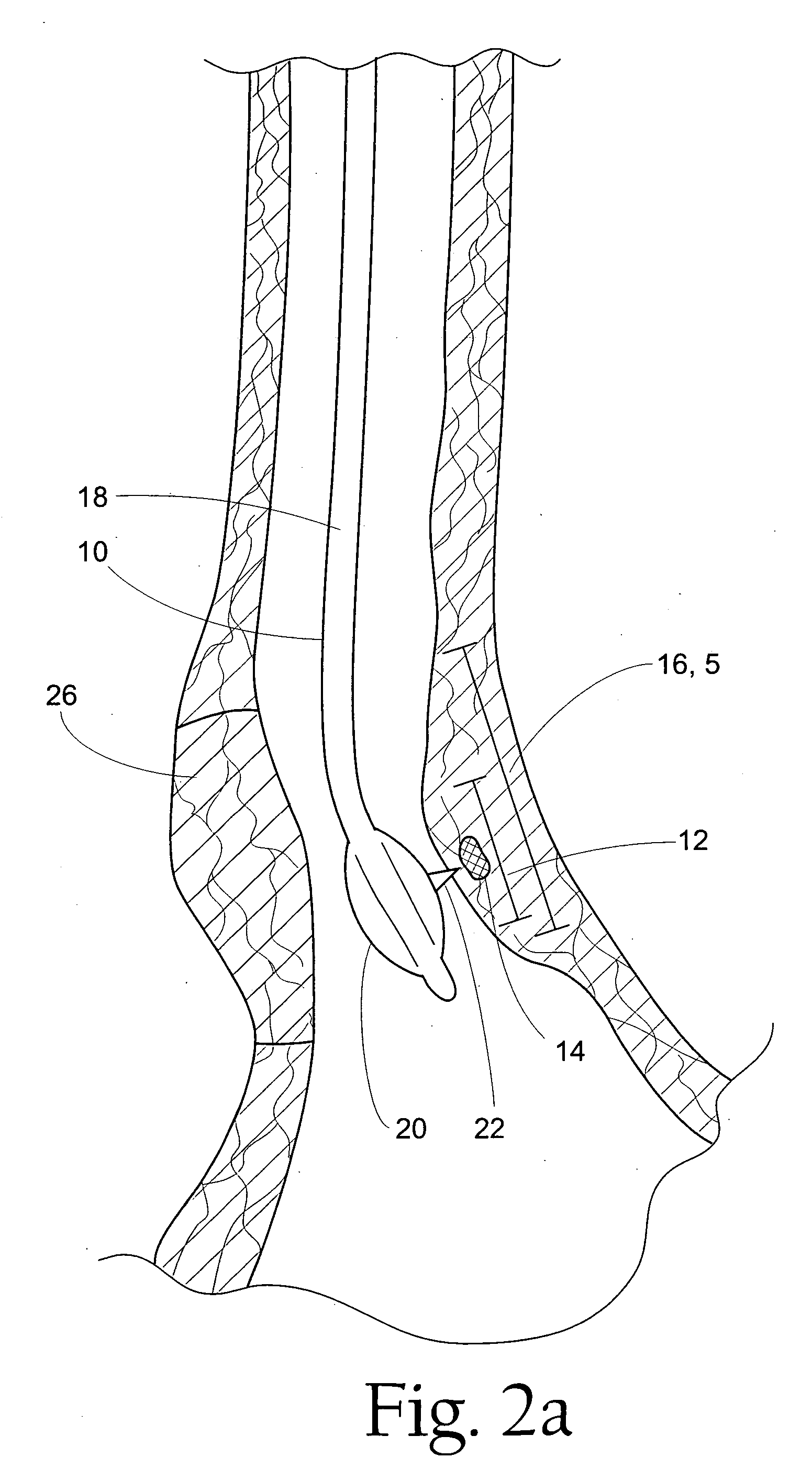
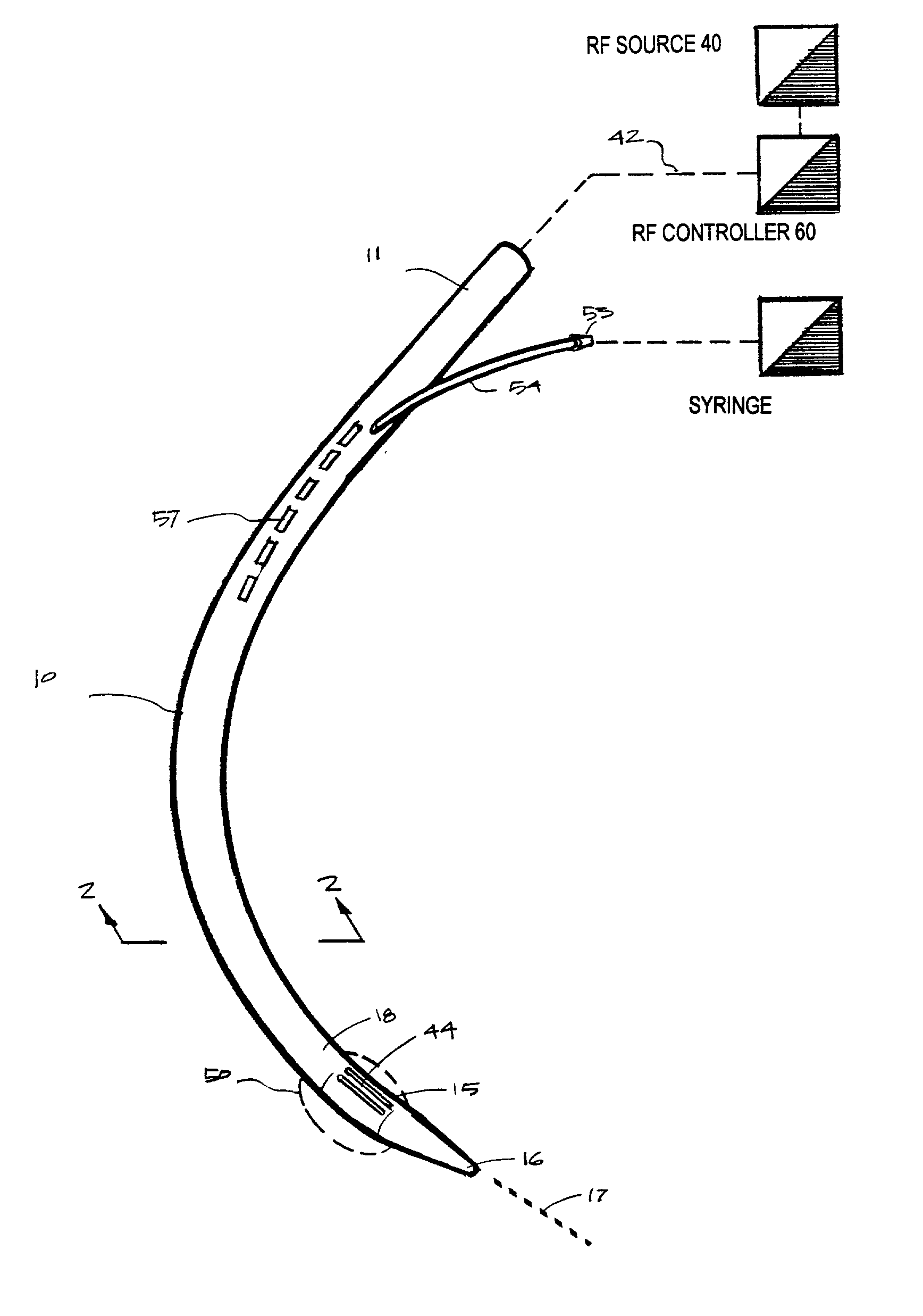
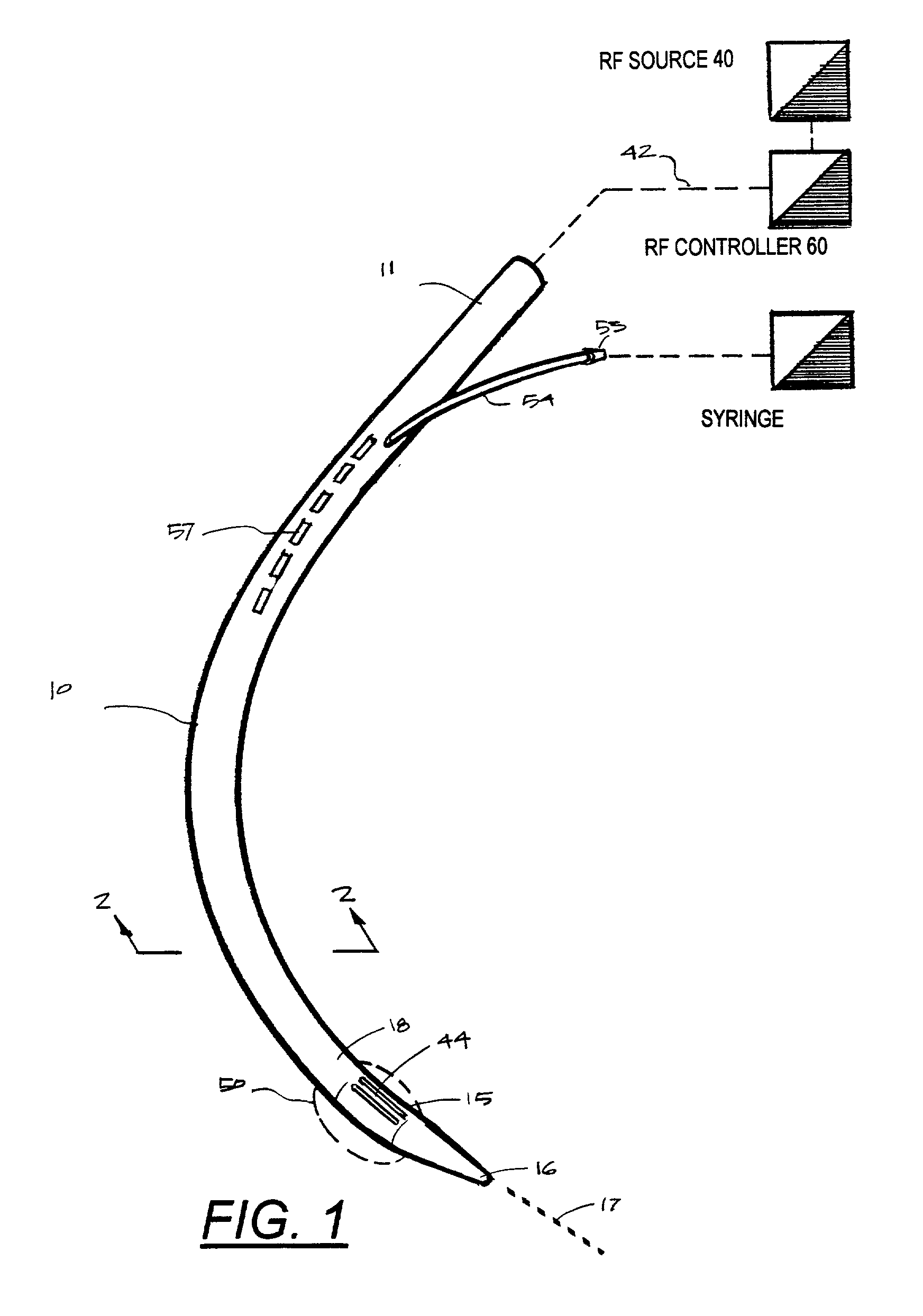
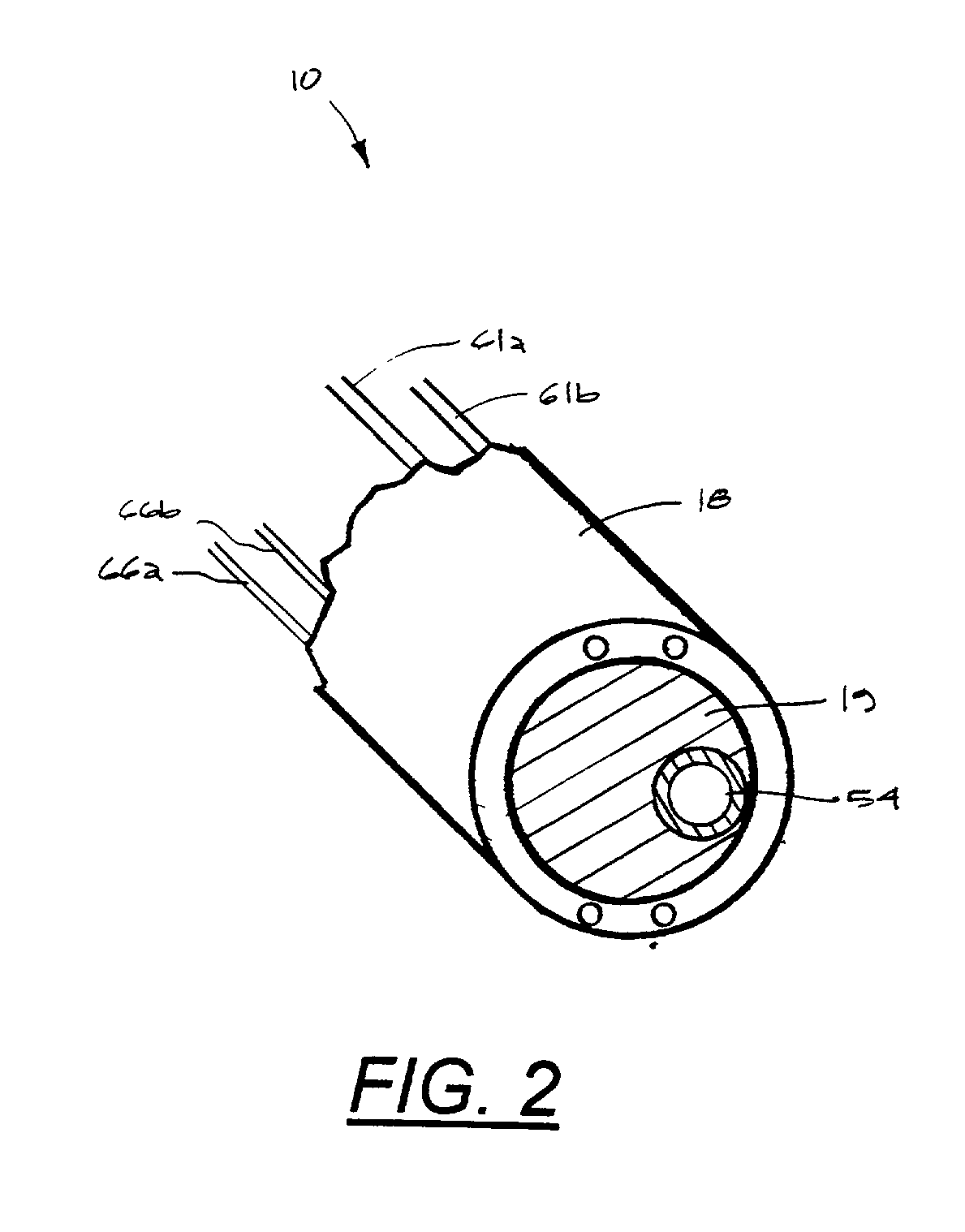
Instruments for thermally-mediated treatment of a patient's lower esophageal sphincter (LES) to induce an injury healing response to thereby populate the extracellular compartment of walls of the LES with collagen matrices to altere the biomechanics of the LES to provide an increased intra-esophageal pressure for preventing acid reflux. A preferred embodiment is a bougie-type device for trans-esophageal introduction that carries conductive electrodes for delivering Rf energy to walls of the LES (i) to induce the injury healing response or (ii) to "model" collagenous tissues of the LES by shrinking collagen fibers therein. Typically, an Rf source is connected to at least one conductive electrode that may be operated in a mono-polar or bi-polar fashion. A sensor array of individual sensors is provided in the working end. A computer controller is provided, which together with feedback circuitry, is capable of full process monitoring and control of: (i) power delivery; (ii) parameters of a selected therapeutic cycle, (iii) mono-polar or bi-polar energy delivery, and (iv) multiplexing of current flow between various paired electrodes. The controller can determine when the treatment is completed based on time, temperature, tissue impedance or any combination thereof.
Owner:MEDERI RF LLC
Gastro-occlusive device
A gastro-occlusive device, comprising a balloon disposable in a stomach cavity of a patient, and inflatable therein to occlude a portion of the volume of the stomach cavity, a gas flow tube coupled at a distal end thereof with the balloon and extending outwardly through a stomach wall for coupling with a gas source for selectively inflating the balloon, to occlude at least a portion of the volume of the stomach cavity. The gastro-occlusive device may be employed in combination with a feeding tube unit, a drain unit or other ancillary apparatus, and is useful for treatment of morbid obesity and various eating disorders.
Owner:POLYZEN INC
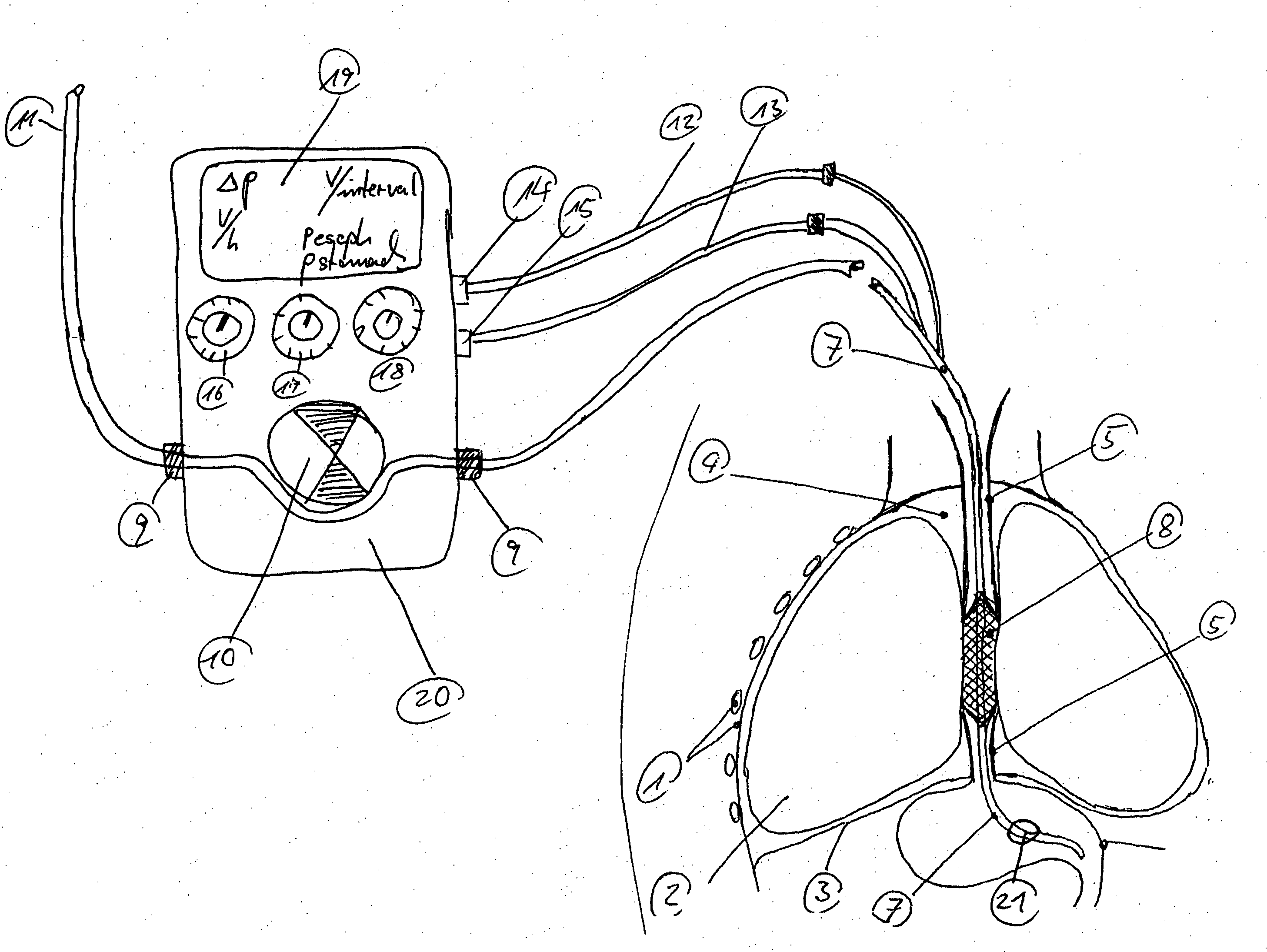
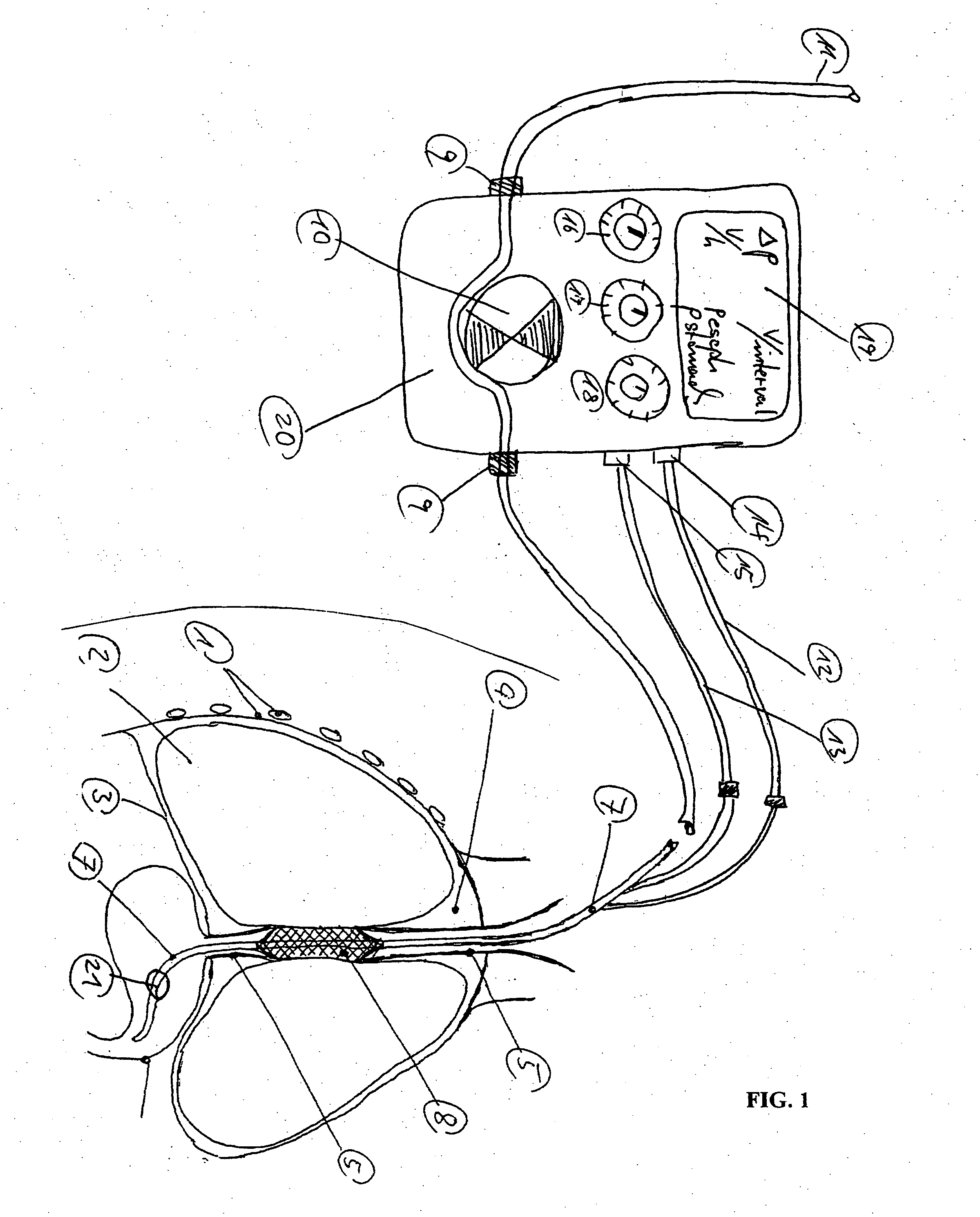
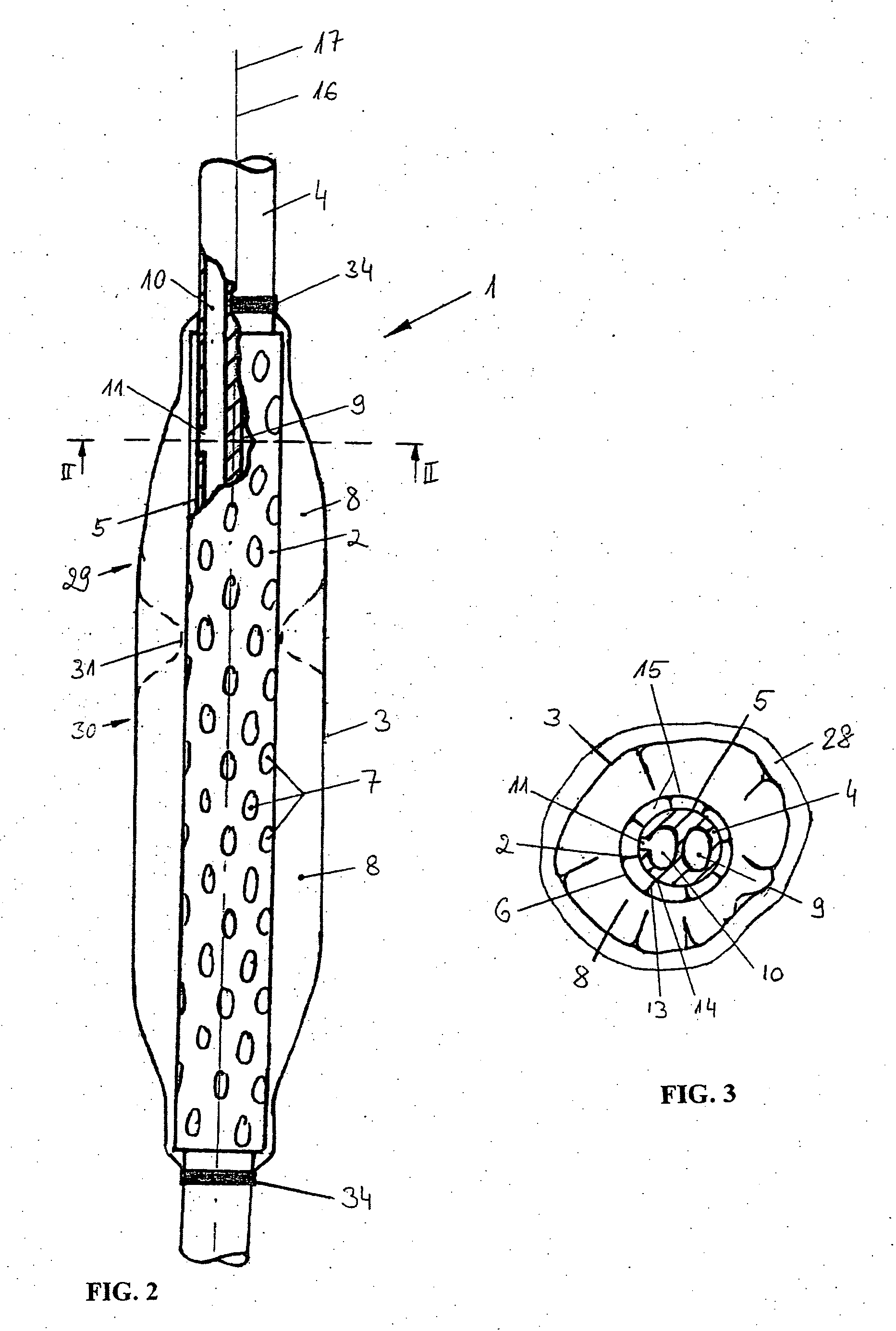
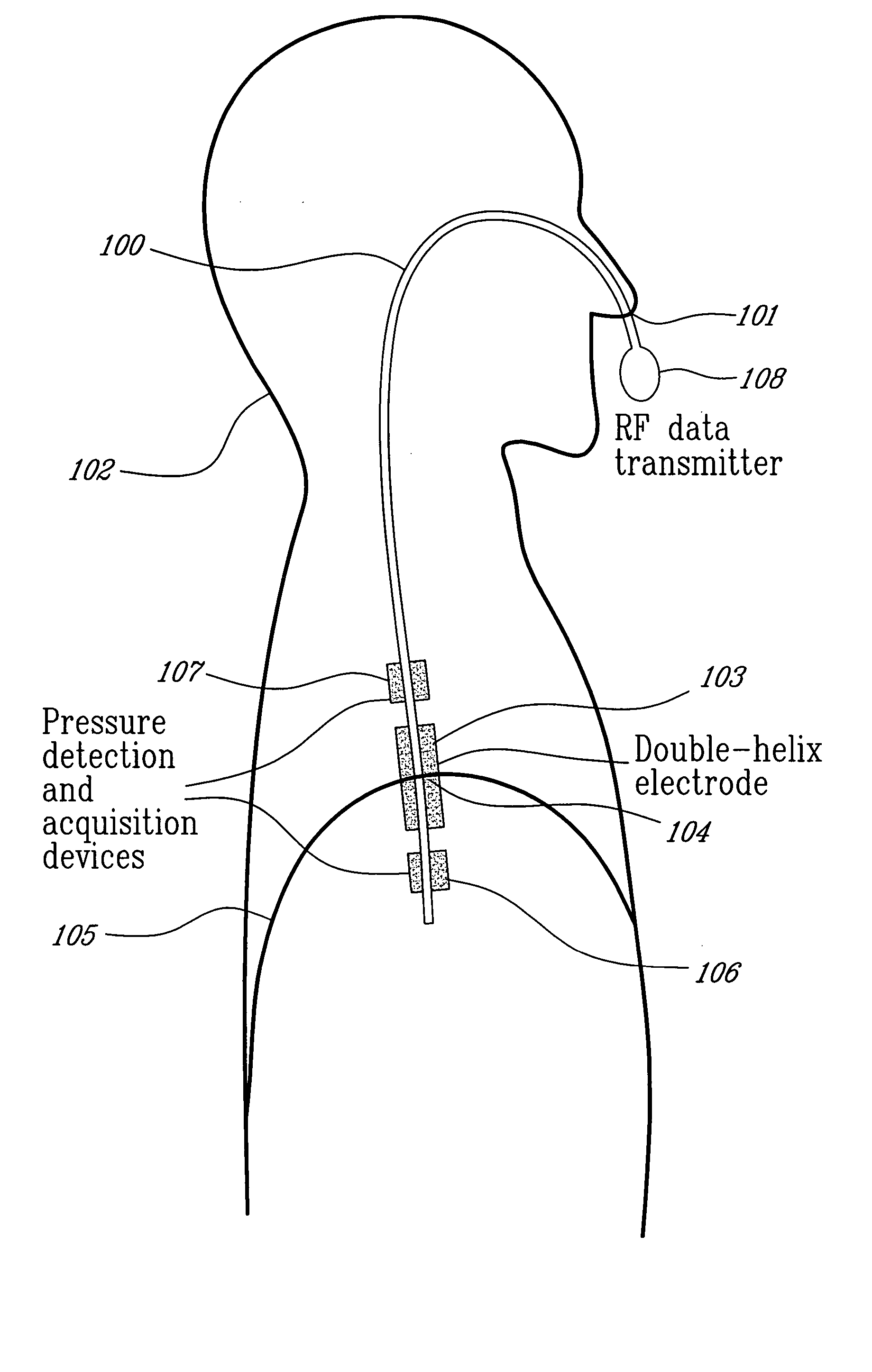
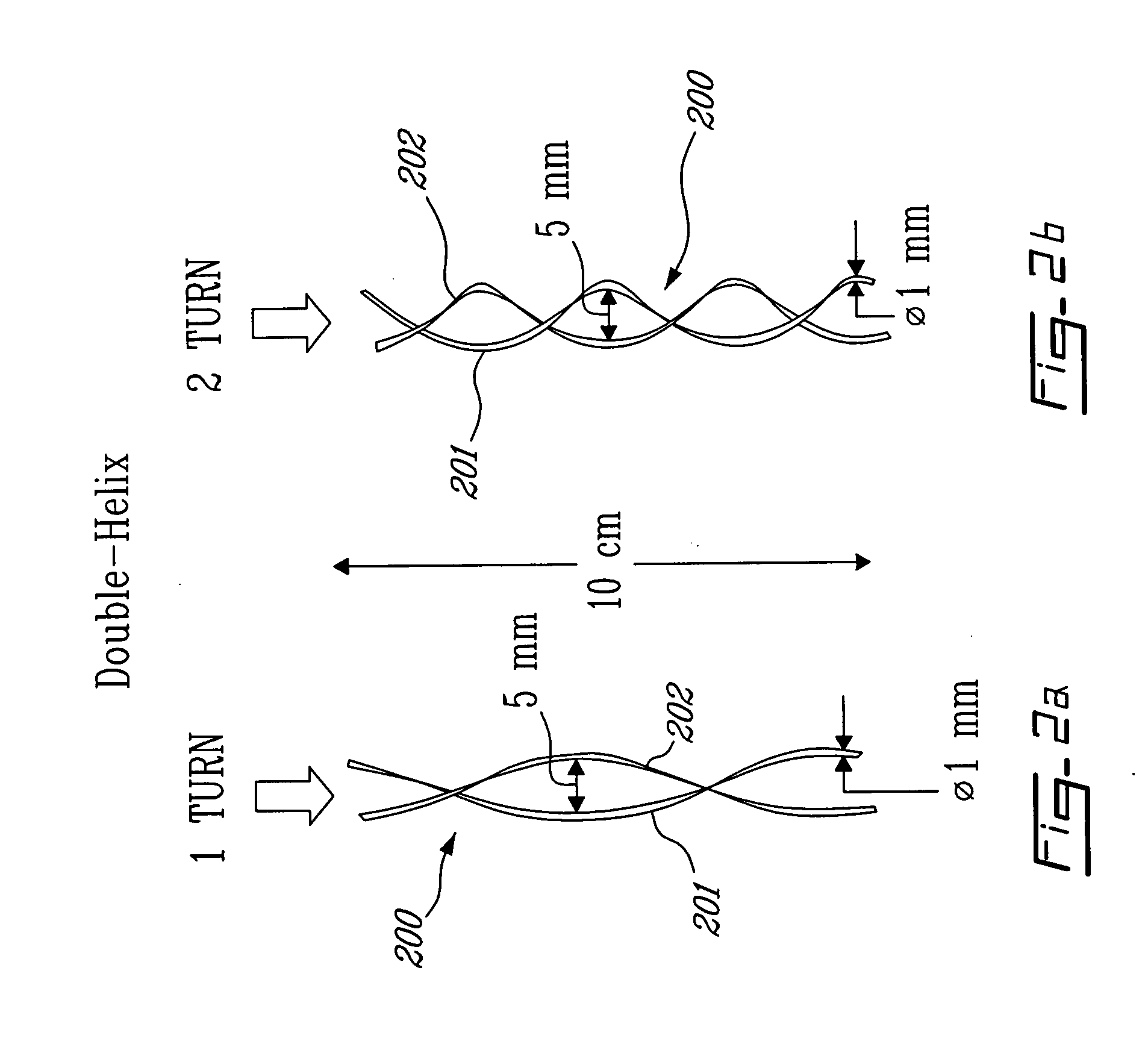
Catheter for transdiaphragmatic pressure and diaphragm electromyogram recording using helicoidal electrodes
A first aspect of the present invention is concerned with a double-helix electrode structure for sensing electrical activity of a patient's diaphragm, comprising first and second helical electrodes disposed in a double-helix arrangement for being positioned in the gastro-esophageal sphincter of the patient's diaphragm in view of sensing electrical activity of the patient's diaphragm. According to a second aspect, the present invention provides a pressure detection and acquisition device comprising a semiconductor substrate, a pressure sensor implemented on the semiconductor substrate and producing, when subjected to an external pressure, a pressure representative signal, and a signal acquisition and transmission circuit integrated to the semiconductor substrate, connected to the pressure sensor, and supplied with the pressure representative signal. Other aspects of the present invention relate to an EMGdi signal and pressure acquisition catheter.
Owner:SAWAN MOHAMAD +3
Gastro-esophageal reflux control system and pump
An enteral feeding unit or system that minimizes the occurrence of gastro-esophogeal-pharynegal reflux during feeding is described. The enteral feeding unit or device includes an automatable feeding pump with a feedback sensor for sensing a relative pressure in a patient's stomach and esophagus, and a regulator system for controlling or monitoring feeding rate to said patient as a function of said relative gastro-esophageal pressure. The system includes a stomach probe that has a fluid-tight closure of the esophagus. The stomach probe, according to the invention, is characterized by a tampon-bladder for watertight closure of the esophagus, in which the tampon-bladder forms from flexible and / or elastic material at least a closed inner cavity for the reception of a fluid medium, through a means (11) of establishing a prescribed pressure for the medium in the tampon-bladder (16) by an inner lumen forming the actual stomach probe, from which an outer hose-like lumen (18) extending to the tampon-bladder (16) is so arranged that between the outer lumen (18) and the inner lumen (17) a channel is formed connected to the inner cavity of the tampon-bladder (16) arranged on the outer lumen (18) by a number of openings (20), whereby the inner cavity of the tampon-bladder (16) is connected via the canal formed between the inner and outer lumina (17, 18) with the means of production of pressure in the tampon-bladder, that is, with a suitably graded reservoir or equalizing vessel (11) for the liquid medium situated above the tampon-bladder and outside the patient.
Owner:AVENT INC
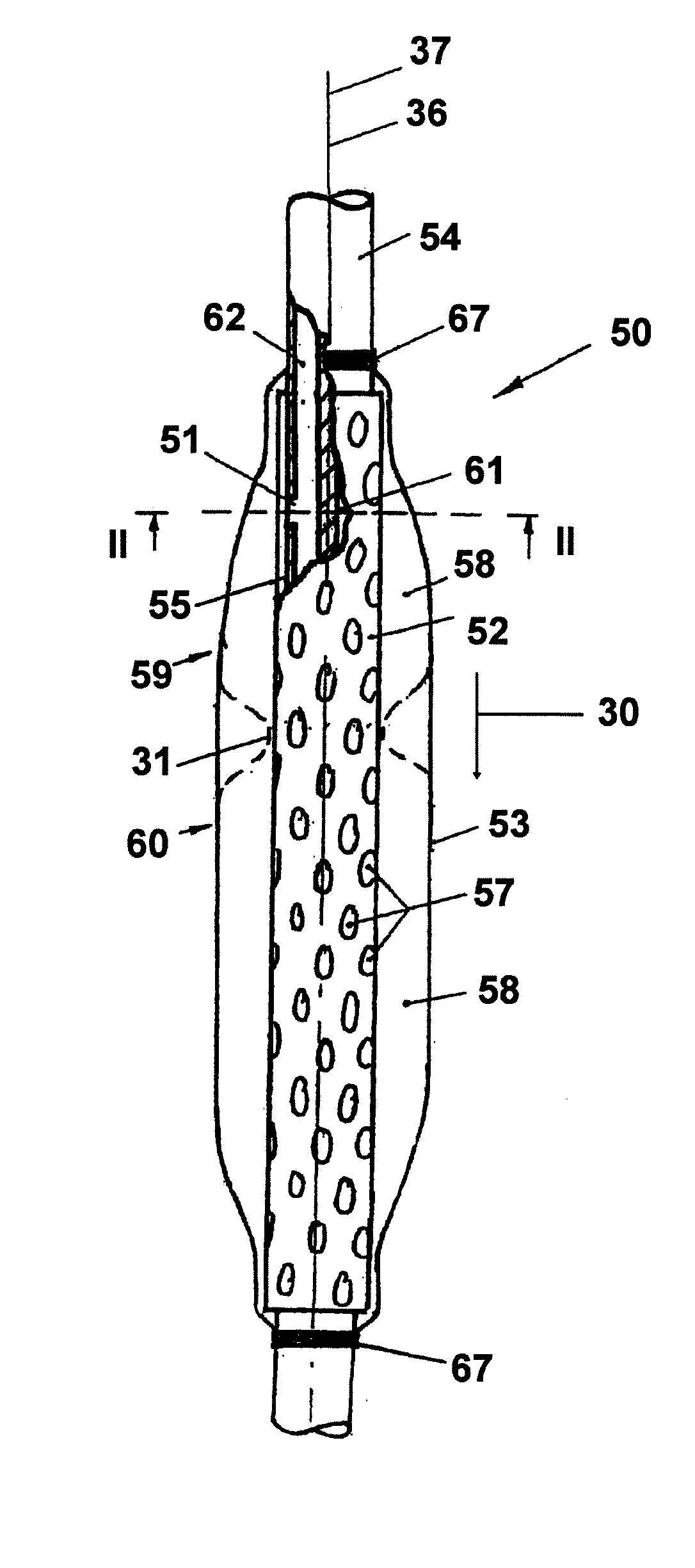
Gastro-esophageal reflux control system and pump
An enteral feeding unit that reduces the occurrence of gastro-esophogeal-pharynegal reflux during feeding includes an automatable feeding pump with a feedback sensor for sensing a relative pressure in a patient's stomach and esophagus, and a regulator system for controlling and monitoring feeding rate to the patient as a function of the relative gastro-esophageal pressure. The system includes a stomach probe that provides a fluid-tight closure of the esophagus. The stomach probe includes a tampon-bladder for watertight closure of the esophagus, in which the tampon-bladder is formed of flexible and / or elastic material. At least an inner cavity of the bladder is provided for the reception of a fluid medium. A prescribed pressure for the medium in the tampon-bladder (53) is maintained by an inner lumen forming the stomach probe, from which an outer hose-like lumen (62) extending to the tampon-bladder (53) is so arranged that between the outer lumen (62) and the inner lumen (61) a channel is formed connected to the inner cavity of the tampon-bladder (53) arranged on the outer lumen (62) by a number of openings (57). The inner cavity (58) of the tampon-bladder (53) is connected via a canal formed between the inner and outer lumina (62) with a suitably graded reservoir or equalizing vessel for the liquid medium situated above the tampon-bladder and outside the patient.
Owner:AVENT INC
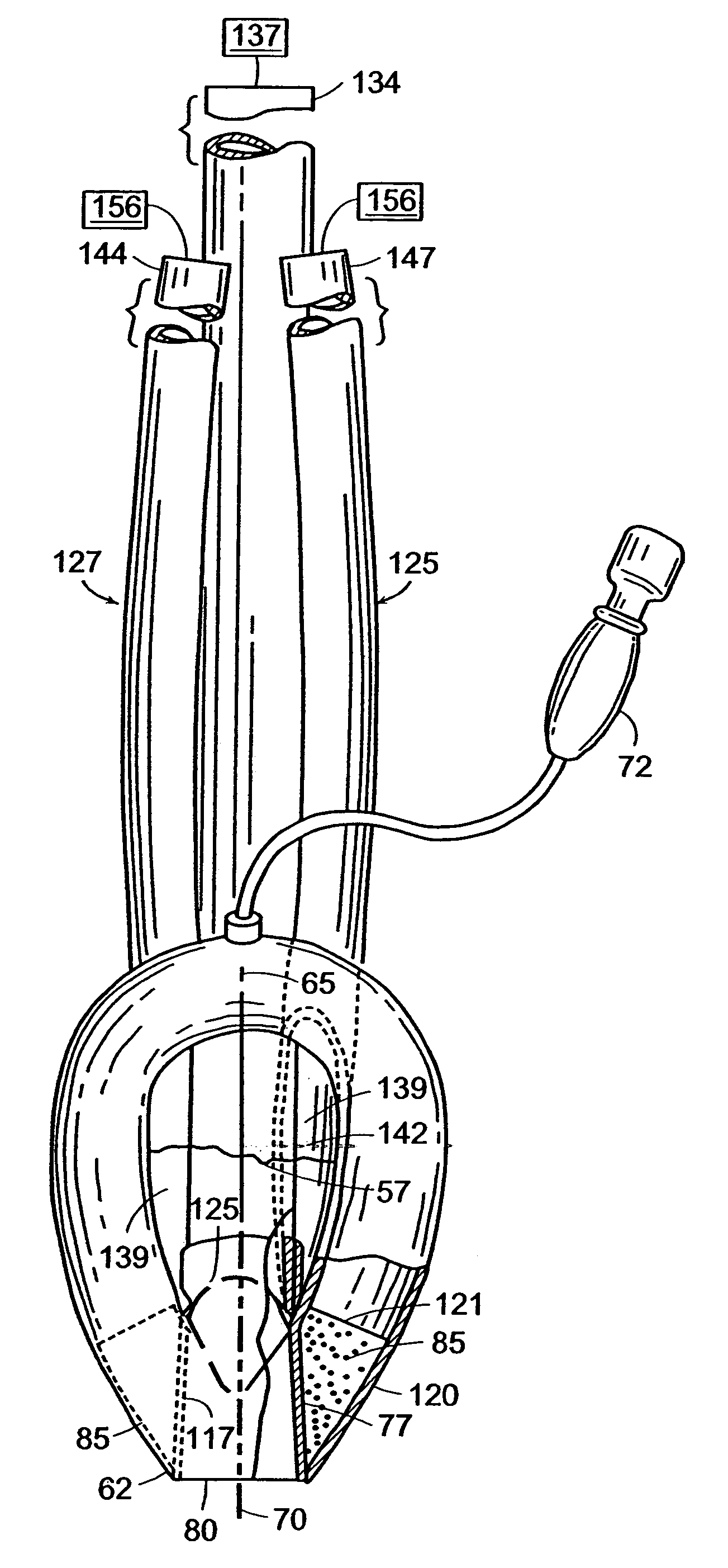
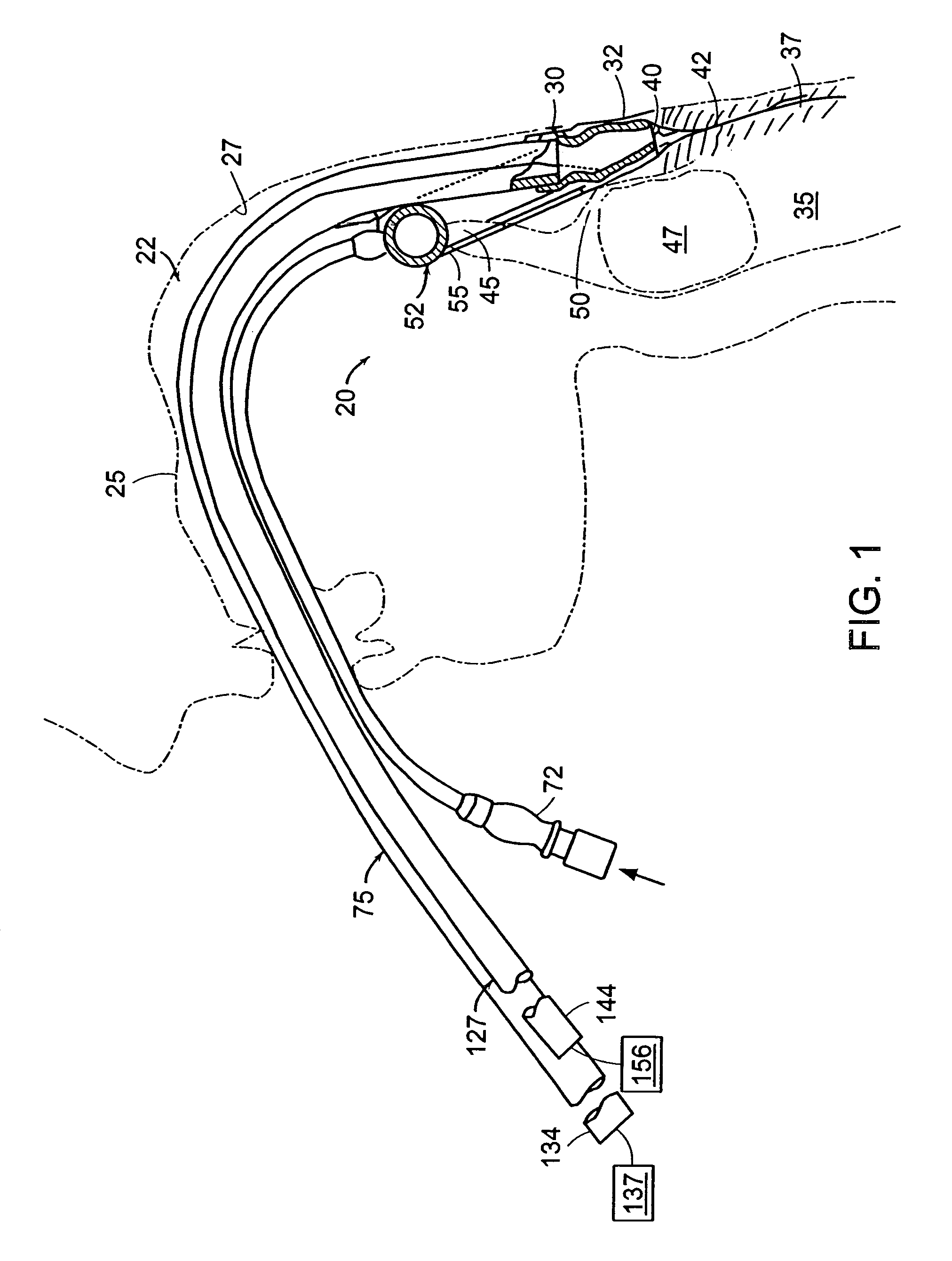
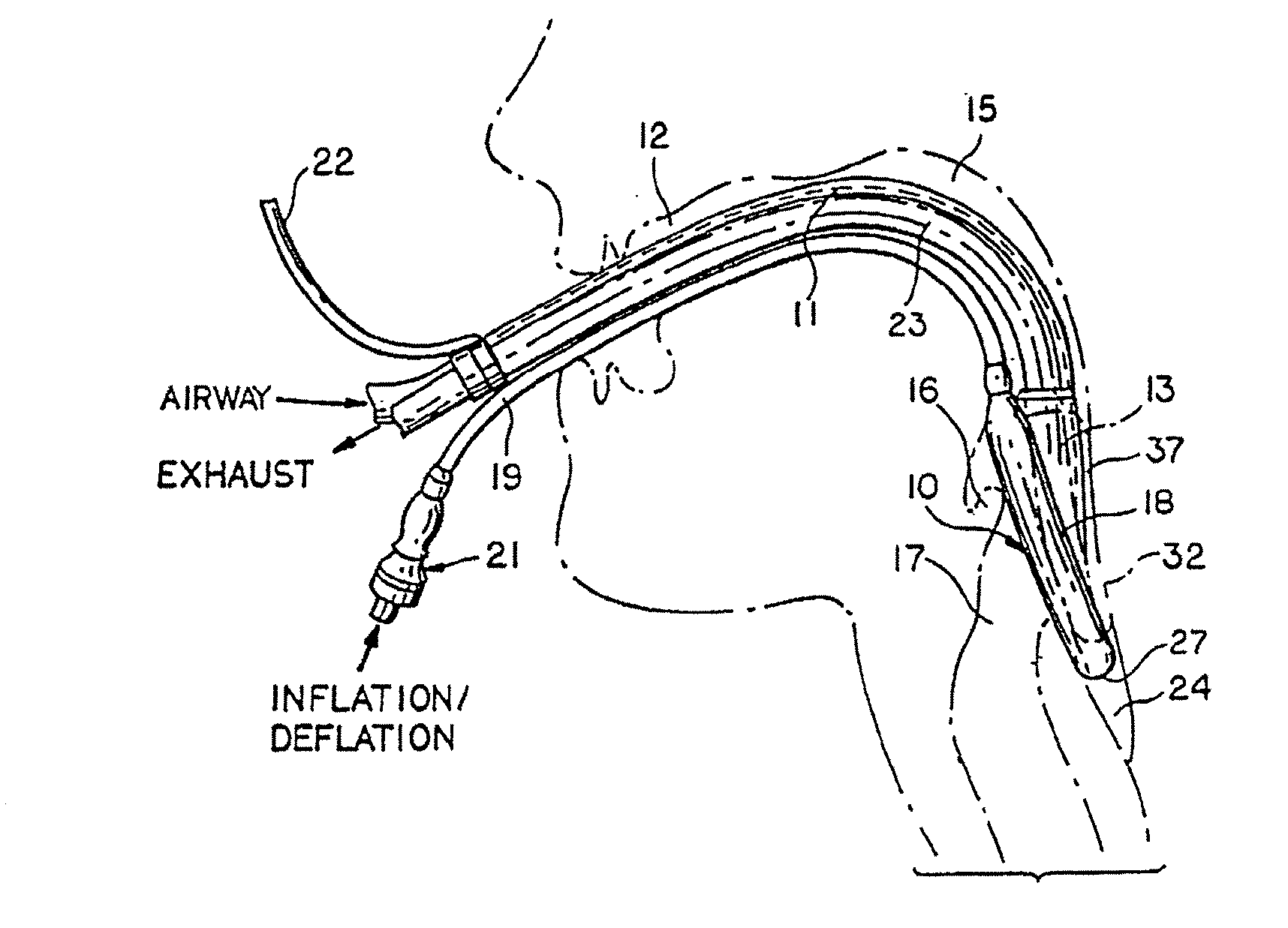
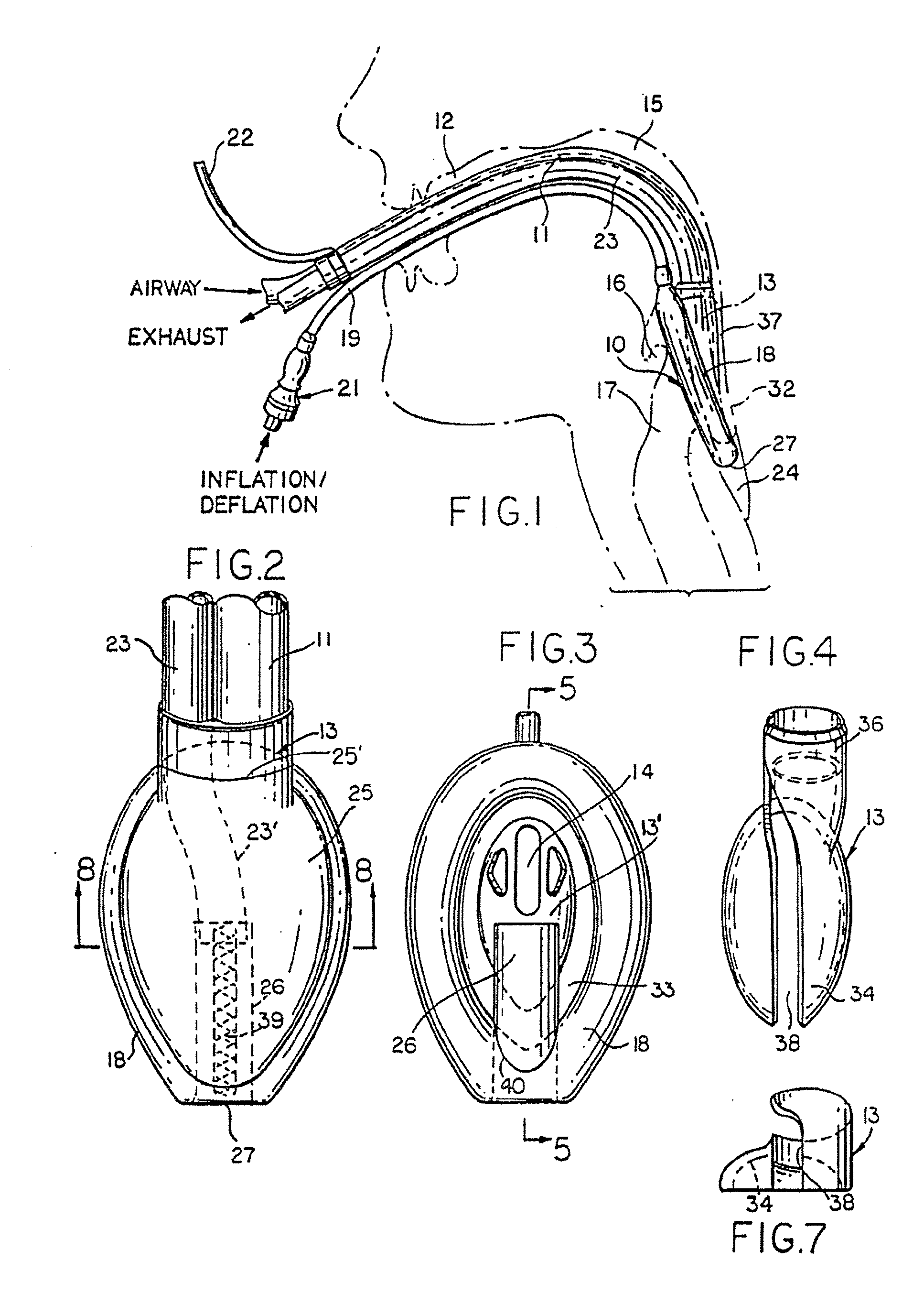
Gastro-Laryngeal Mask
A gastro-laryngeal mask features softly compliant construction of the distal half of the mask, wherein the mask is of generally elliptical configuration, with an inflatable peripheral cuff to seal and support the mask around the laryngeal inlet. A back cushion is inflatable to engage the back wall of the pharynx and thus to forwardly load the peripheral-cuff seal to the laryngeal inlet. An evacuation tube for external removal of a possible gastric discharge completes an evacuation or discharge passage contained within the mask and opening through the distal end of the peripheral cuff. Special provision is made for assuring integrity of the discharge passage within the flexible distal half of the mask, i.e., assuring against collapse of the distal-end half of the softly compliant evacuation tube in the distal region of the mask, such that inflation of the mask does not compromise viability of the evacuation tube by compressing softly compliant material of the evacuation tube during periods of mask inflation. The special provision also favors such collapse of the mask when deflated as to provide a leading flexible edge for piloting a safe and correct advancing insertional advance of the deflated mask in the patient's throat, in avoidance of epiglottis interference and to the point of locating engagement in the upper sphincter of the oesophagus.
Owner:THE LARYNGEAL MASK +1
Medical agent delivery system and method
InactiveUS20080015523A1Medical devicesDiagnostic recording/measuringBiomedical engineeringDelivery system
A medical agent delivery system and method of dispensing a medical agent include providing a medical agent dispensing member and supporting said medical agent dispensing member at the gastro-esophageal region of the patient.
Owner:SENTINEL GRP LLC
Method for Hybrid Gastro-Jejunostomy
ActiveUS20060217748A1Promote formationEasy to insertSurgical veterinaryWound clampsBiomedical engineeringJejunostomy
Disclosed herein are methods for joining one piece to tissue to another piece of tissue. In one embodiment, the method can include inserting an applier device having an actuation portion into a first body lumen through a natural body orifice, forming a first opening in a first piece of tissue within the first lumen and a second opening in a second piece of tissue defining a portion of a second lumen adjacent to the first piece of tissue, and inserting the applier device through the first and second openings such that the actuation portion is between the first and second piece of tissue. The method can further include deploying a fastener into the first and second pieces of tissue through the actuation portion of the applier device, thereby joining the first and second pieces of tissue to form an anastomosis between the first and second lumens.
Owner:ETHICON ENDO SURGERY INC
Gastro-resistant and ethanol-resistant controlled-release formulations comprising hydromorphone
The invention provides a controlled-release composition comprising hydromorphone or a pharmaceutically acceptable form thereof in association with a gastro-resistant and ethanol-resistant compound and method of use thereof.
Owner:SPECTOR DONALD
Gastro-laryngeal mask
A gastro-laryngeal mask features softly compliant construction of the distal half of the mask, wherein the mask is of generally elliptical configuration, with an inflatable peripheral cuff to seal and support the mask around the laryngeal inlet. A back cushion is inflatable to engage the back wall of the pharynx and thus to forwardly load the peripheral-cuff seal to the laryngeal inlet. An evacuation tube for external removal of a possible gastric discharge completes an evacuation or discharge passage contained within the mask and opening through the distal end of the peripheral cuff. Special provision is made for assuring integrity of the discharge passage within the flexible distal half of the mask, i.e., assuring against collapse of the distal-end half of the softly compliant evacuation tube in the distal region of the mask, such that inflation of the mask does not compromise viability of the evacuation tube by compressing softly compliant material of the evacuation tube during periods of mask inflation. The special provision also favors such collapse of the mask when deflated as to provide a leading flexible edge for piloting a safe and correct advancing insertional advance of the deflated mask in the patient's throat, in avoidance of epiglottis interference and to the point of locating engagement in the upper sphincter of the oesophagus.
Owner:THE LARYNGEAL MASK +1
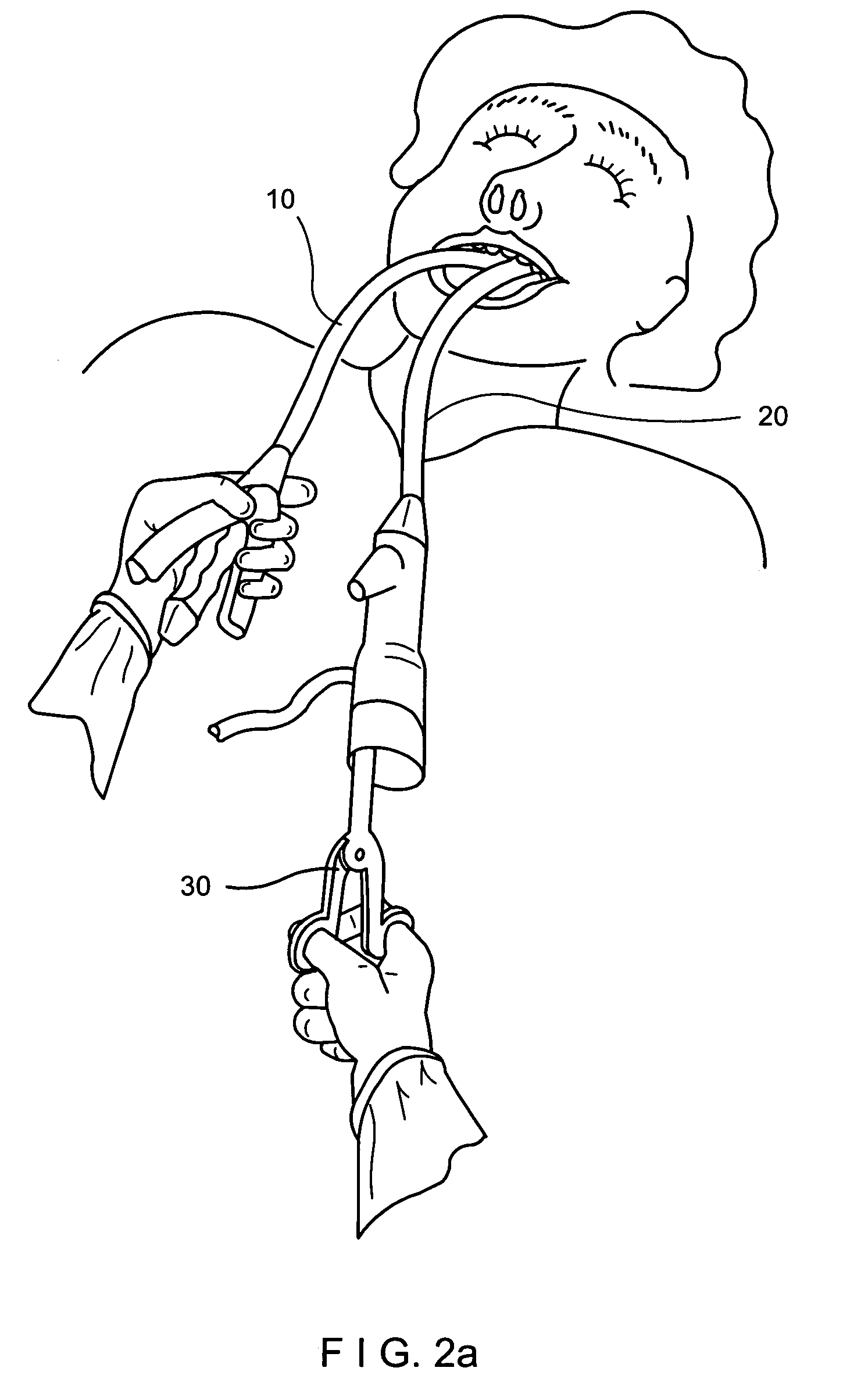
Tissue capturing and suturing device and method
ActiveUS20070219566A1Easy to produceEliminate needSuture equipmentsSurgical needlesGastro-esophageal reflux diseaseEndoscopic Procedure
A combination tissue apposition and suture capturing device (100) for performing endoscopic procedures typically in the gastro-esophageal tract. The device (100) is particularly adapted for forming multiple plications used in a gastroplasty procedure devised to cure or ameliorate gastro-esophageal reflux disease. The device include a tissue sewing capsule (102) attached to the distal end of an endoscope having a needle (120) that is deposited in a capsule (102) distal tip cavity following the suturing of a tissue fold and retrieved to enable the suturing of a subsequent tissue fold without the need for multiple intubations. A suture clip delivery device (200) is also disclosed that is adapted to fit within the capsule to enable suture capture without the need for multiple intubations. The combination device eliminates the need for an overtube and maximizes the speed efficiency of the gastroplasty procedure. A method for using the combination device is also disclosed.
Owner:CR BARD INC
Compositions for treatment of gastro-esophageal reflux disorders
The present invention provides compositions for treatment of gastro-esophageal reflux disorders. The compositions include at least two of the following: (i) one or more digestive enzymes; (ii) one or more probiotics; and (iii) stevia. Also provided are processes for preparing the compositions useful for treatment of gastro-esophageal reflux disorders, and methods of treating subjects against gastro-esophageal reflux disorders, which include administering to a subject a therapeutically effective amount of the compositions of the present invention.
Owner:EISENSTEIN JEREMY B
Methods of treating gastro-esophogeal reflux disease using (-) norcisapride in combination with proton pump inhibitors or H2 receptor antagonists
InactiveUS6548518B2Prevent and alleviate symptomReducing and avoiding adverse effectBiocideDigestive systemNorcisapride5-HT3 receptor
Owner:SEPACOR INC
Controlled release pharmaceutical compositions comprising a fumaric acid ester
The present invention relates to controlled release pharmaceutical compositions comprising fumaric acid ester(s) as active substance(s). The compositions are suitable for use in the treatment of e.g. psoriasis or other hyperproliferative, inflammatory or autoimmune disorders and are designated to release the fumaric acid ester in a controlled manner so that local high concentrations of the active substance within the gastrointestinal tract upon oral administration can be avoided and, thereby, enabling a reduction in gastro-intestinal related side-effects.
Owner:前进药物股份公司
Compositions for prevention and treatement of symptoms of gastrointestinal distress
InactiveUS20070020249A1Preventing gastro-intestinal distressReduce severityBiocidePeptide/protein ingredientsStress conditionsAlcohol sugars
A composition for the treatment or prevention of gastro-intestinal distress associates with the consumption of food products containing sugar alcohols includes at least one enzyme, at least one probiotic organism and simethicone. The composition may be consumed immediately prior to but not more than 20 minutes prior to consuming a sugar alcohol-containing food product in order to reduce the severity of at least one gastro-intestinal stress condition associated with sugar alcohol consumption by at least about 50%.
Owner:DOWNS BERNARD WILLIAM
Control of growth and repair of gastro-intestinal tissues by gastrokines and inhibitors
A novel group of gastrokines called Gastric Antrum Mucosal Protein is characterized. A member of the group is designated AMP-18. AMP-18 genomic DNA, cDNA and the AMP-18 protein are sequenced for human, mouse and pig. The AMP-18 protein and active peptides derived from it are cellular growth factors. Surprisingly, peptides capable of inhibiting the effects of the complete protein, are also derived from the AMP-18 protein. Cytoprotection and control of mammalian gastro-intestinal tissue growth and repair (restitution) is facilitated by the use of the proteins, making the proteins candidates for therapies in inflammatory bowel disease, mucositis, and gastric ulcers.
Owner:CHICAGO UNIV OF THE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com