Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
5386 results about "Controlled release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
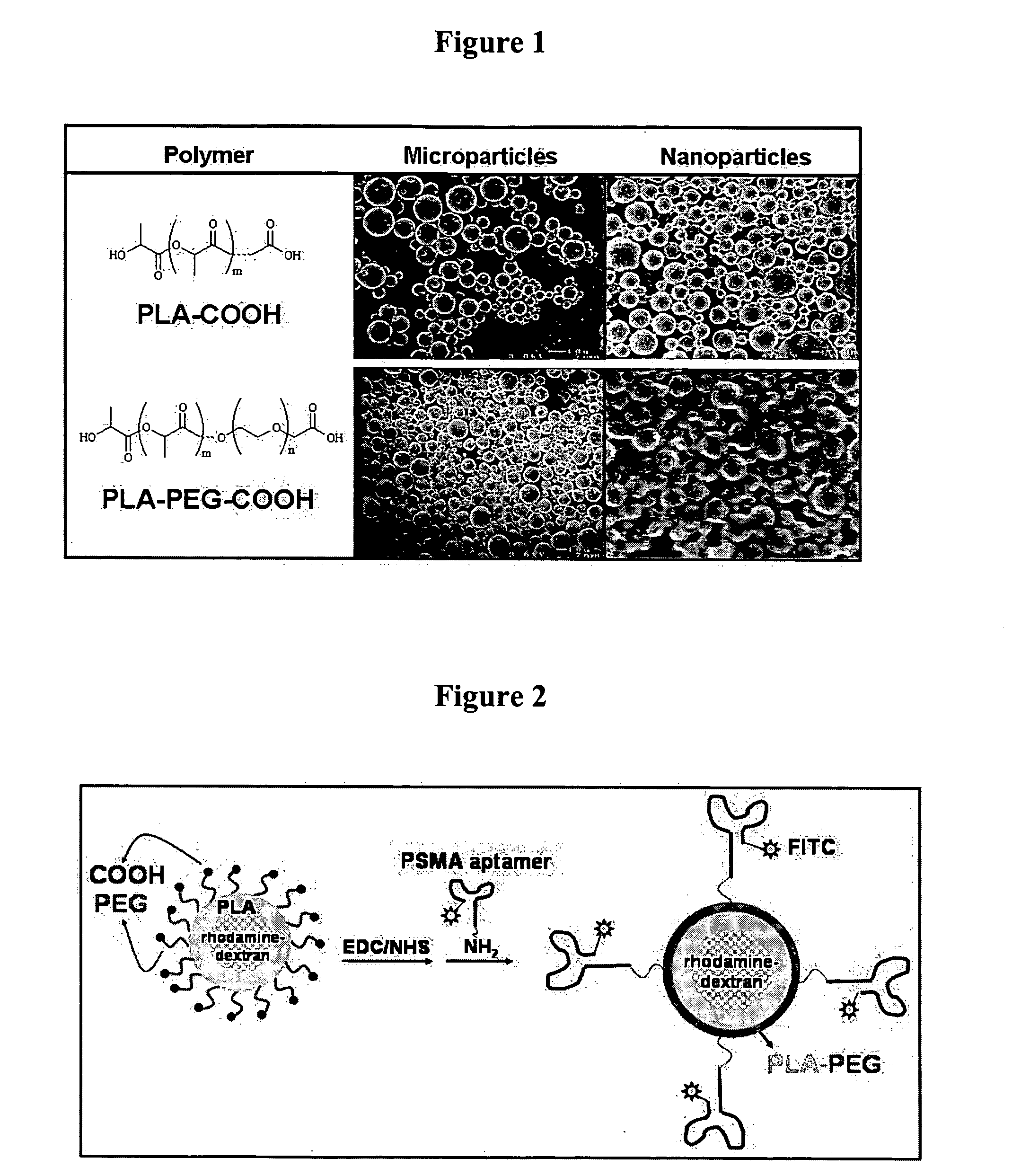
Multiparticulate modified release composition
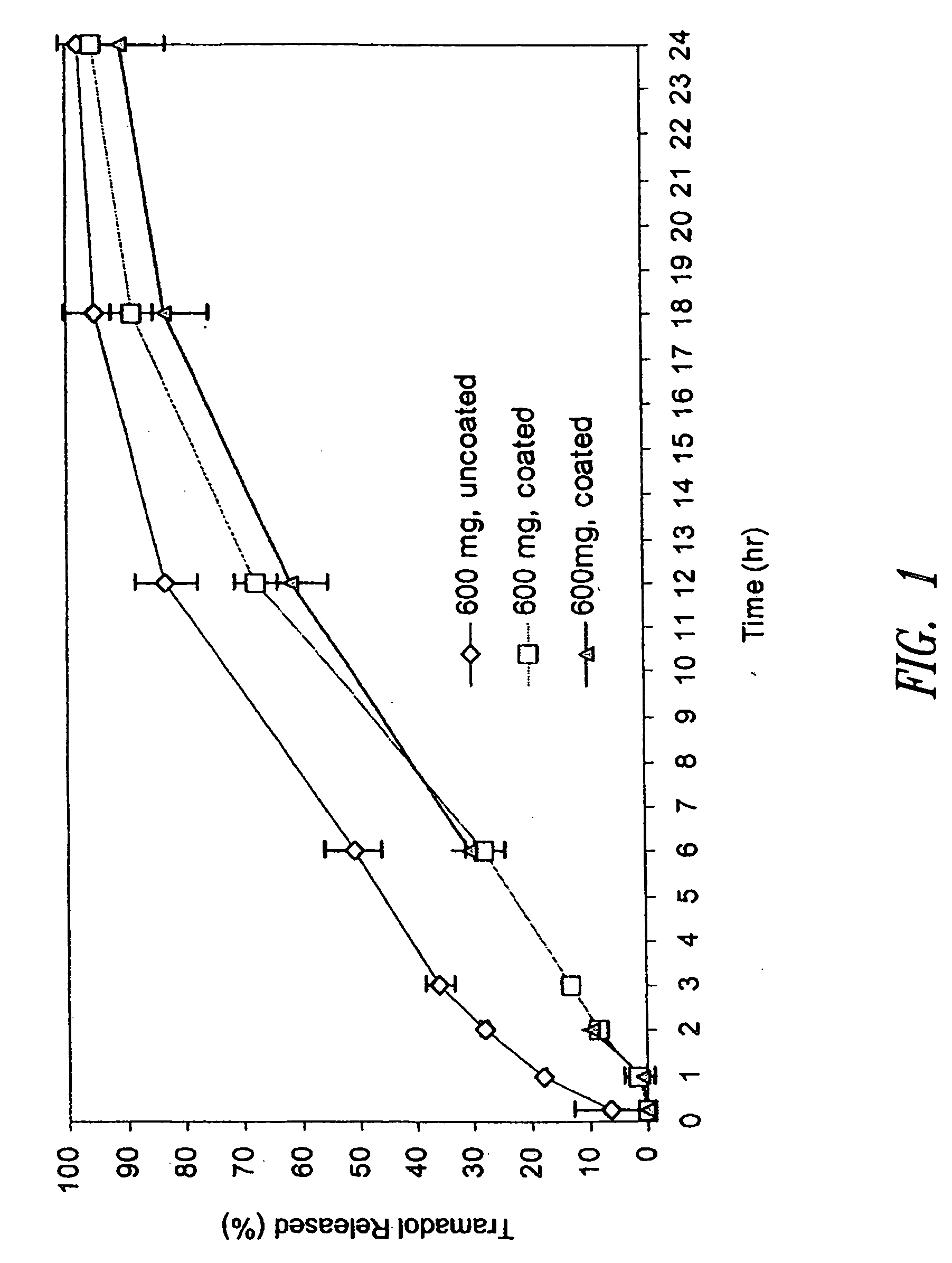
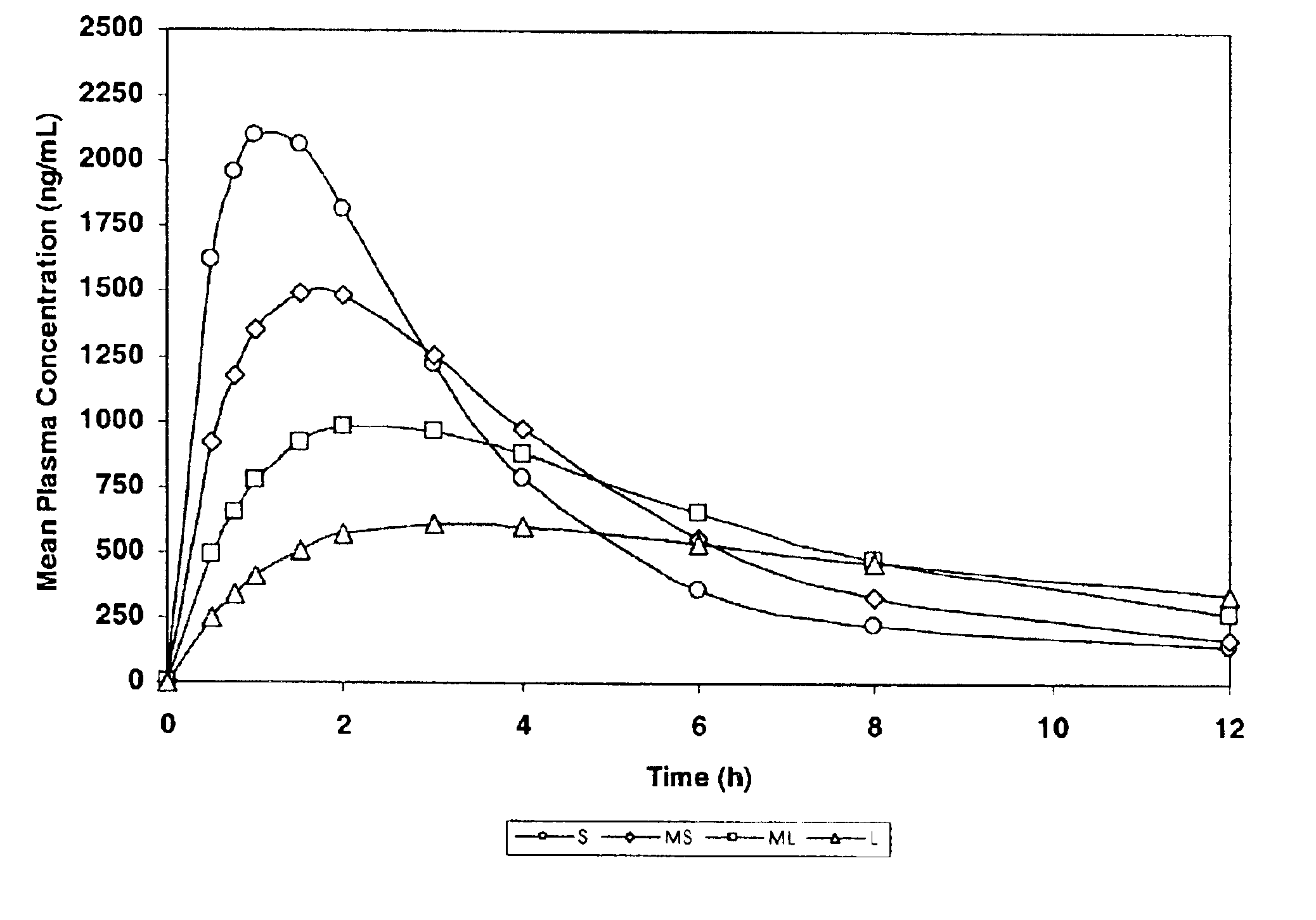
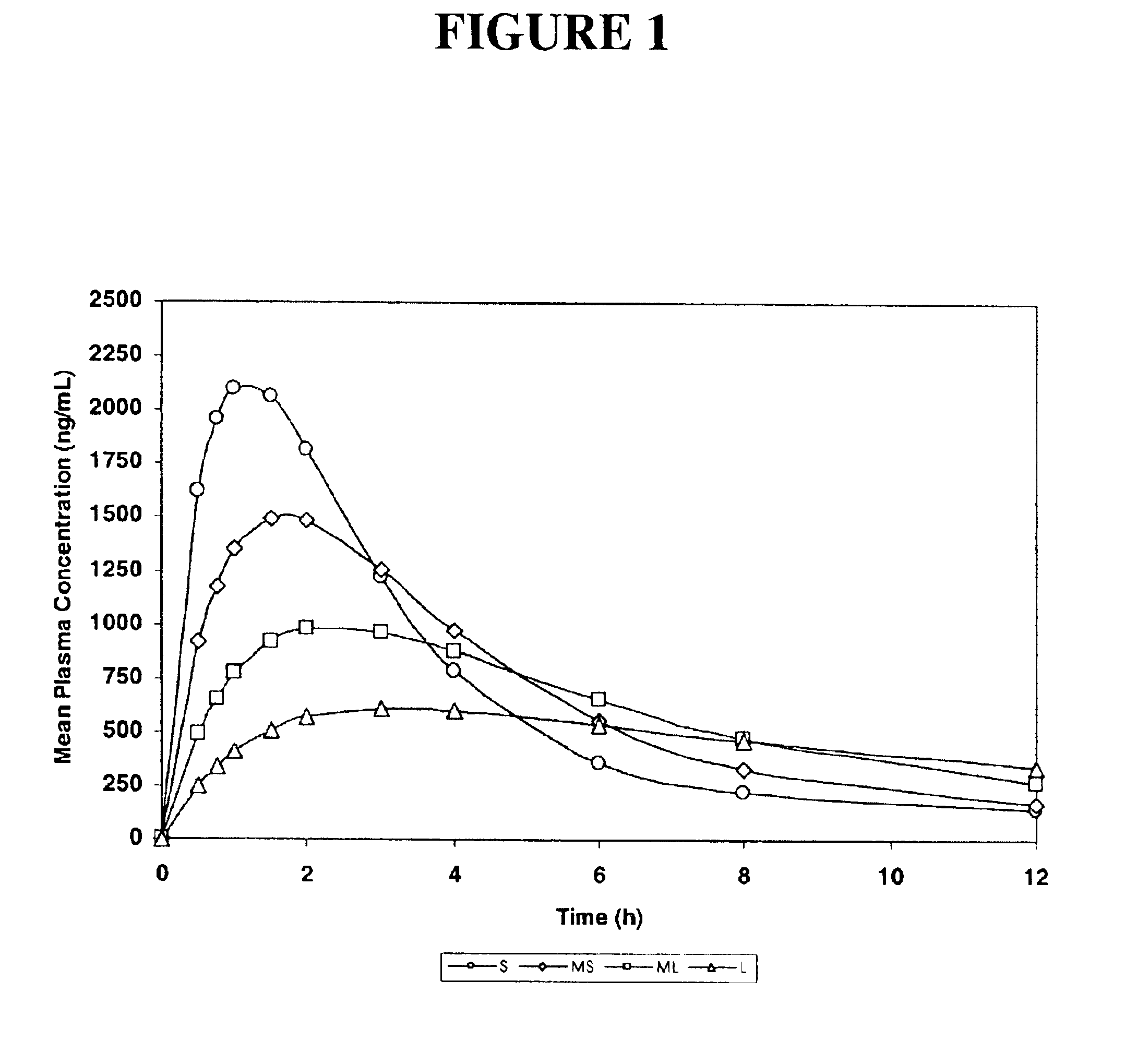
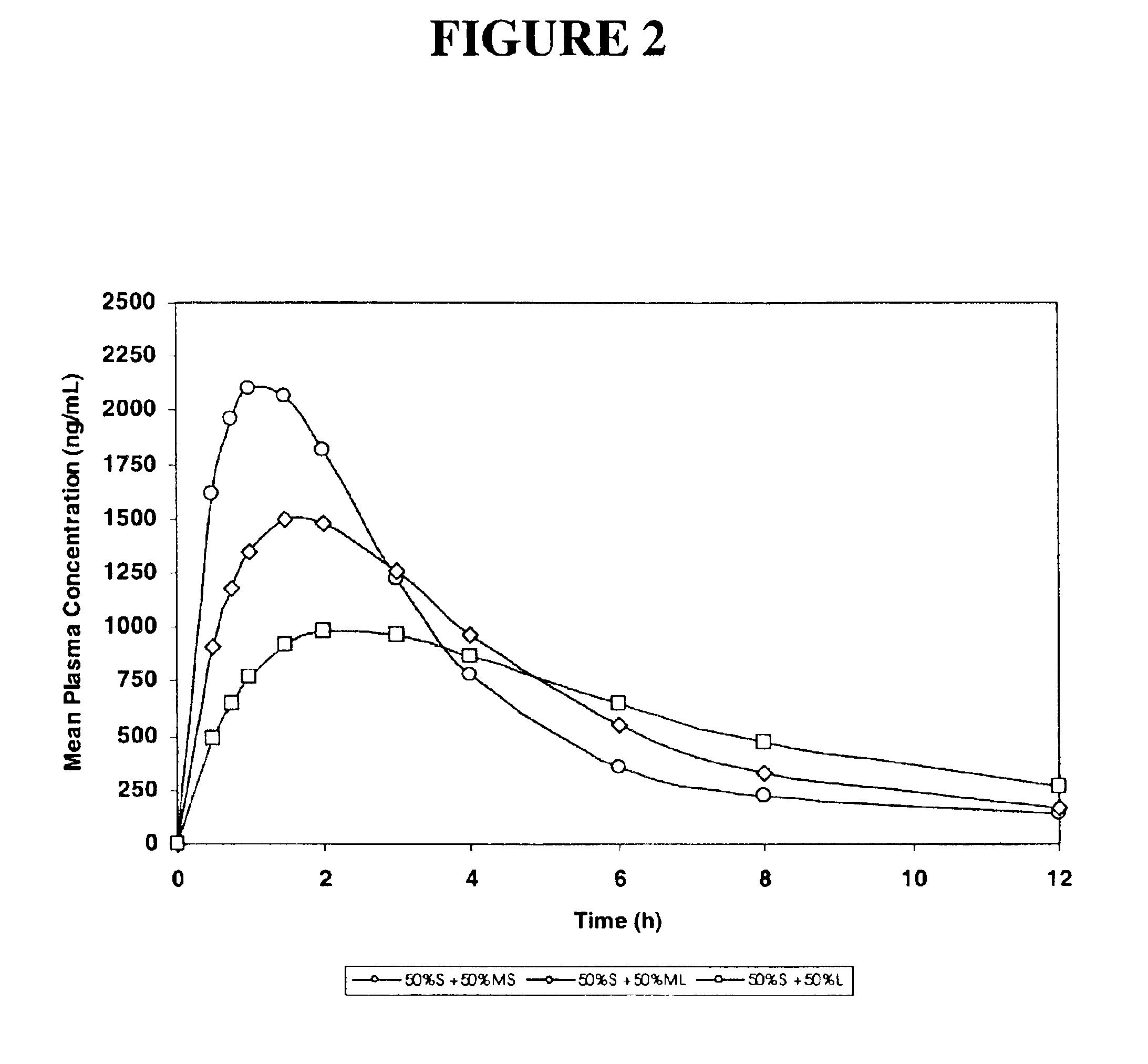
The invention relates to a multiparticulate modified release composition that in operation delivers an active ingredient in a pulsed or bimodal manner. The multiparticulate modified release composition comprises an immediate release component and a modified release component; the immediate release component comprising a first population of active ingredient containing particles and the modified release component compnsimg a second population of active ingredient containing particles coated with a controlled release coating; wherein the combination of the immediate release and modified release components in operation deliver the active ingredient in a pulsed or a bimodal manner. The invention also relates to a solid oral dosage form containing such a multiparticulate modified release composition. The plasma profile achieved by the multiparticulate modified release composition is advantageous in reducing patient tolerance to the active ingredient and in increasing patient compliance by reducing dosage frequency.
Owner:ALKERMES PHARMA IRELAND LTD +1
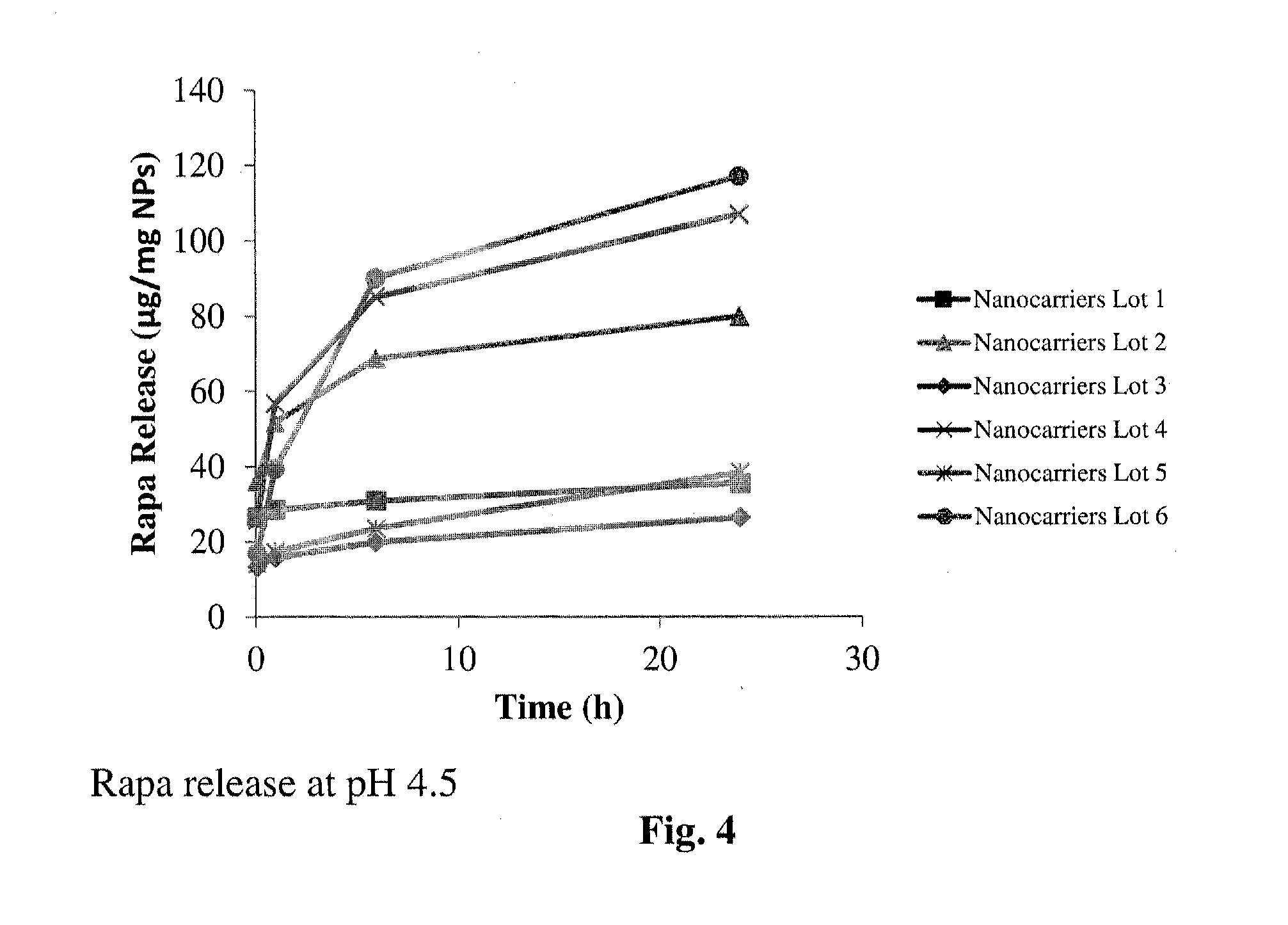
Controlled release of immunosuppressants from synthetic nanocarriers
InactiveUS20120301498A1Reduce in quantityReduce percentagePowder deliveryOrganic active ingredientsControlled releaseAntigen
Disclosed are synthetic nanocarrier compositions that provide controlled release of immunosuppressants as well as related methods. The synthetic nanocarrier compositions may also include antigen in some embodiments.
Owner:SELECTA BIOSCI
Drug releasing coatings for medical devices
ActiveUS20080118544A1Avoid dependenceAvoid disadvantagesOrganic active ingredientsBiocideControlled releaseDrug release
The invention relates to a medical device for delivering a therapeutic agent to a tissue. The medical device has a layer overlying the exterior surface of the medical device. The layer contains a therapeutic agent and an additive. The additive has a hydrophilic part and a hydrophobic part and the therapeutic agent is not enclosed in micelles or encapsulated in particles or controlled release carriers.
Owner:LUTONIX INC
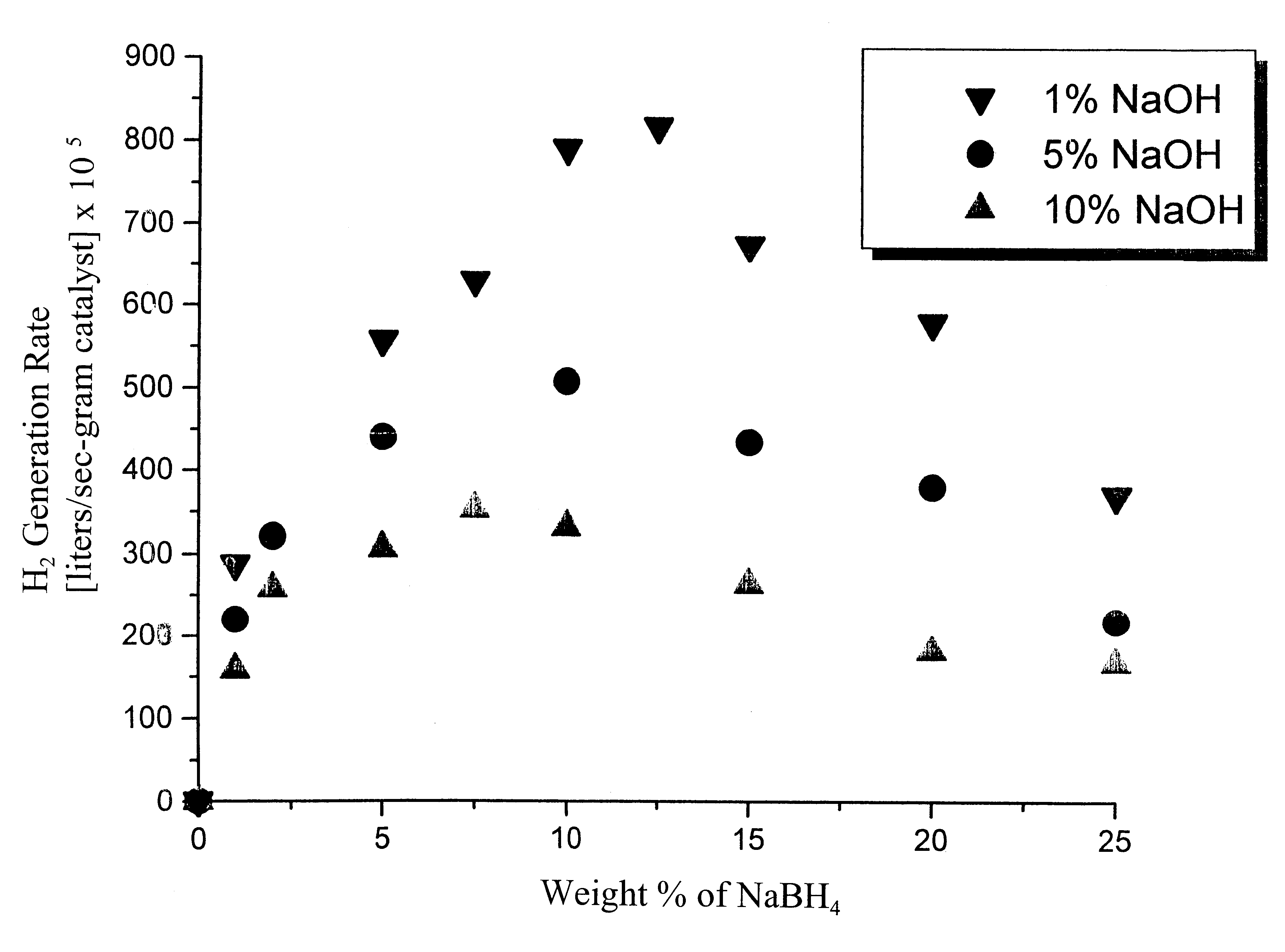
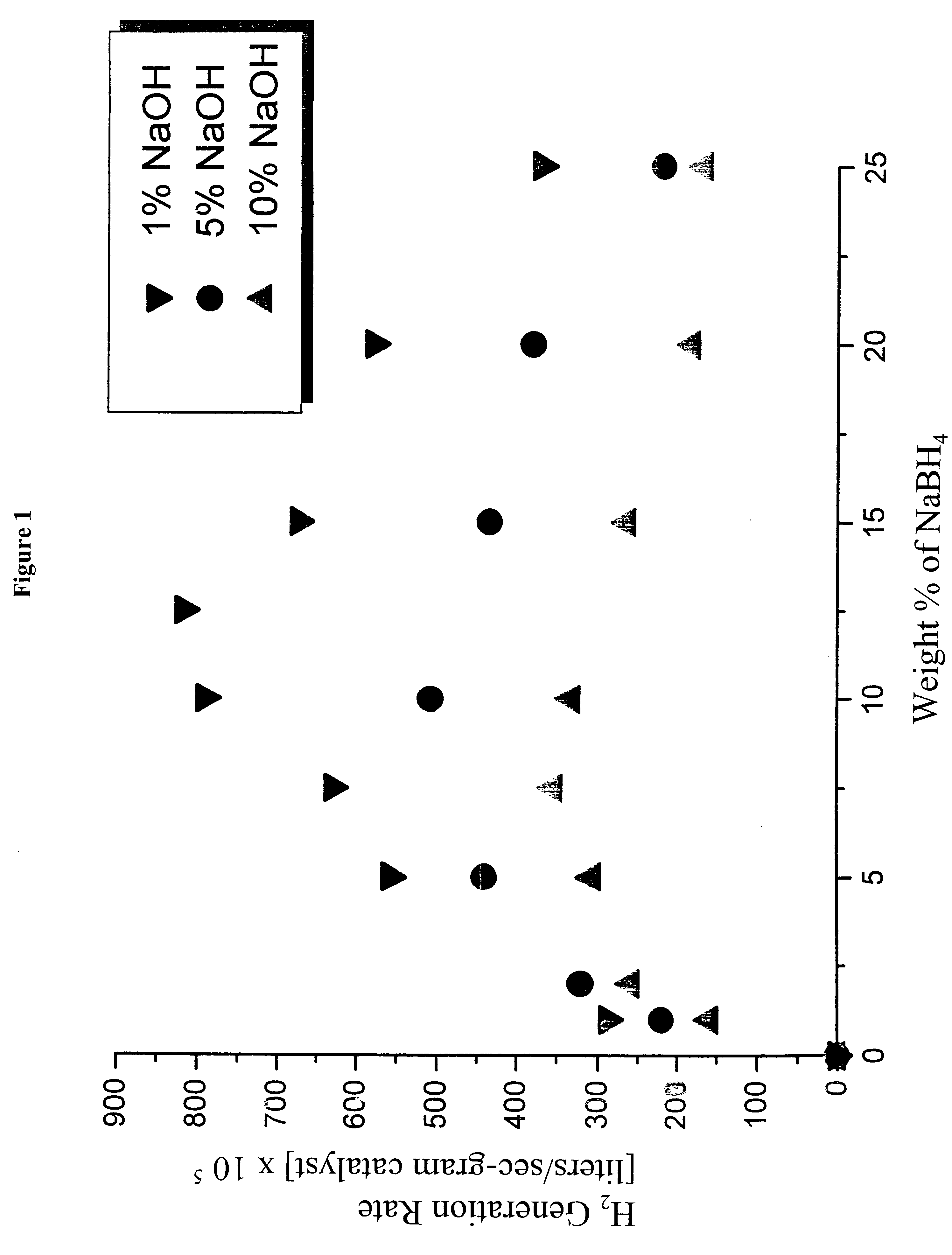
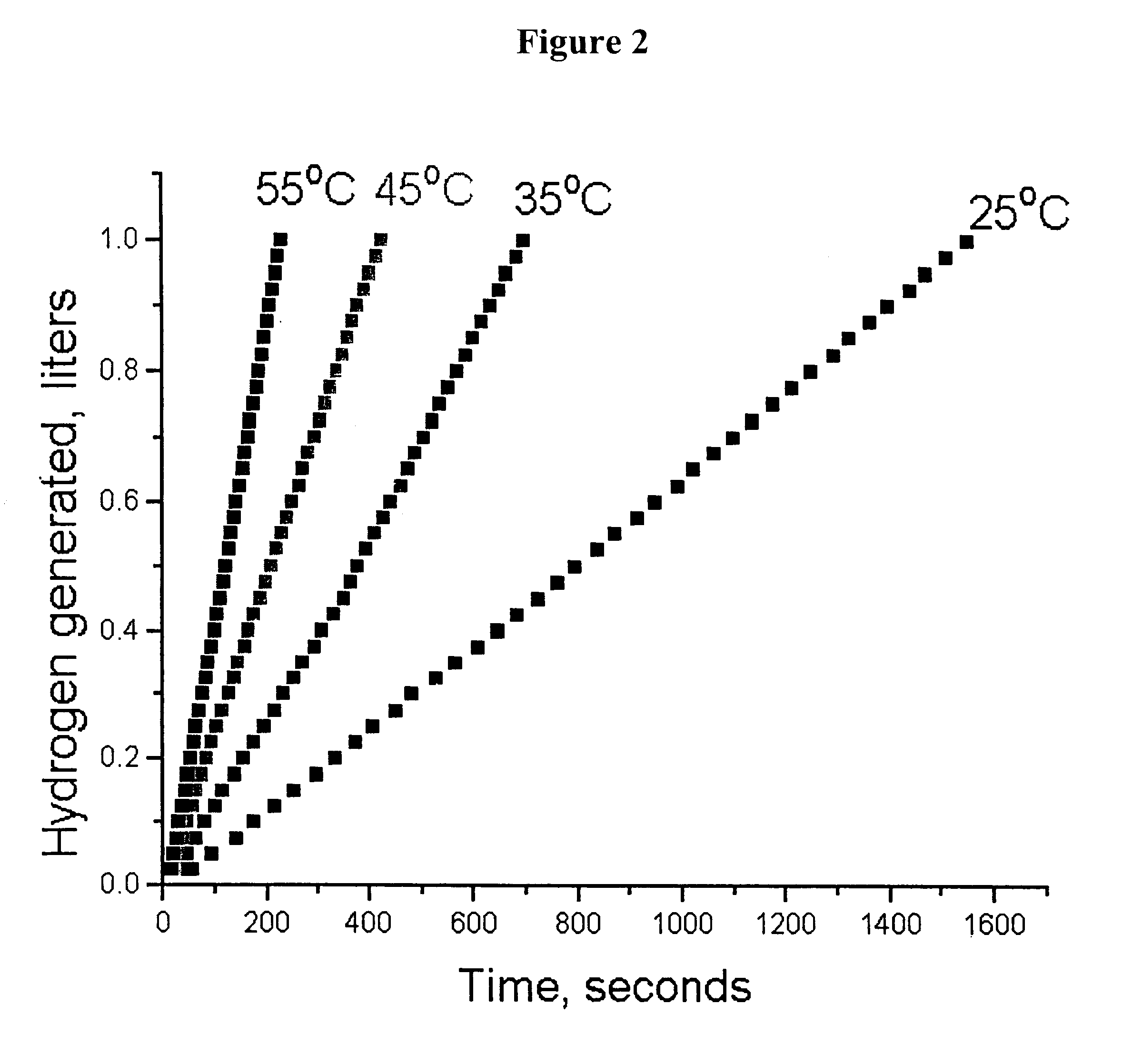
System for hydrogen generation
InactiveUS6534033B1Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationControlled releaseBorohydride
The present invention relates to a composition and method for storage and controlled release of hydrogen. In particular, the present invention relates to the use of borohydride based solutions as a hydrogen storage source and a catalyst system to release hydrogen therefrom.
Owner:SILICON VALLEY BANK +1
Once-a-day, oral, controlled-release, oxycodone dosage forms
Oxycodone formulations are provided which produce substantially flat in vivo steady state plasma profiles. Tolerance levels associated with such profiles and tolerance levels associated with biphasic profiles are shown not to be statistically different. The substantially flat in vivo steady state plasma profiles are produced by dosage forms having substantially zero order in vitro release profiles. Such release profiles produce low single dose in vivo Cmax levels which can reduce the probability of adverse side effects.
Owner:ALZA CORP
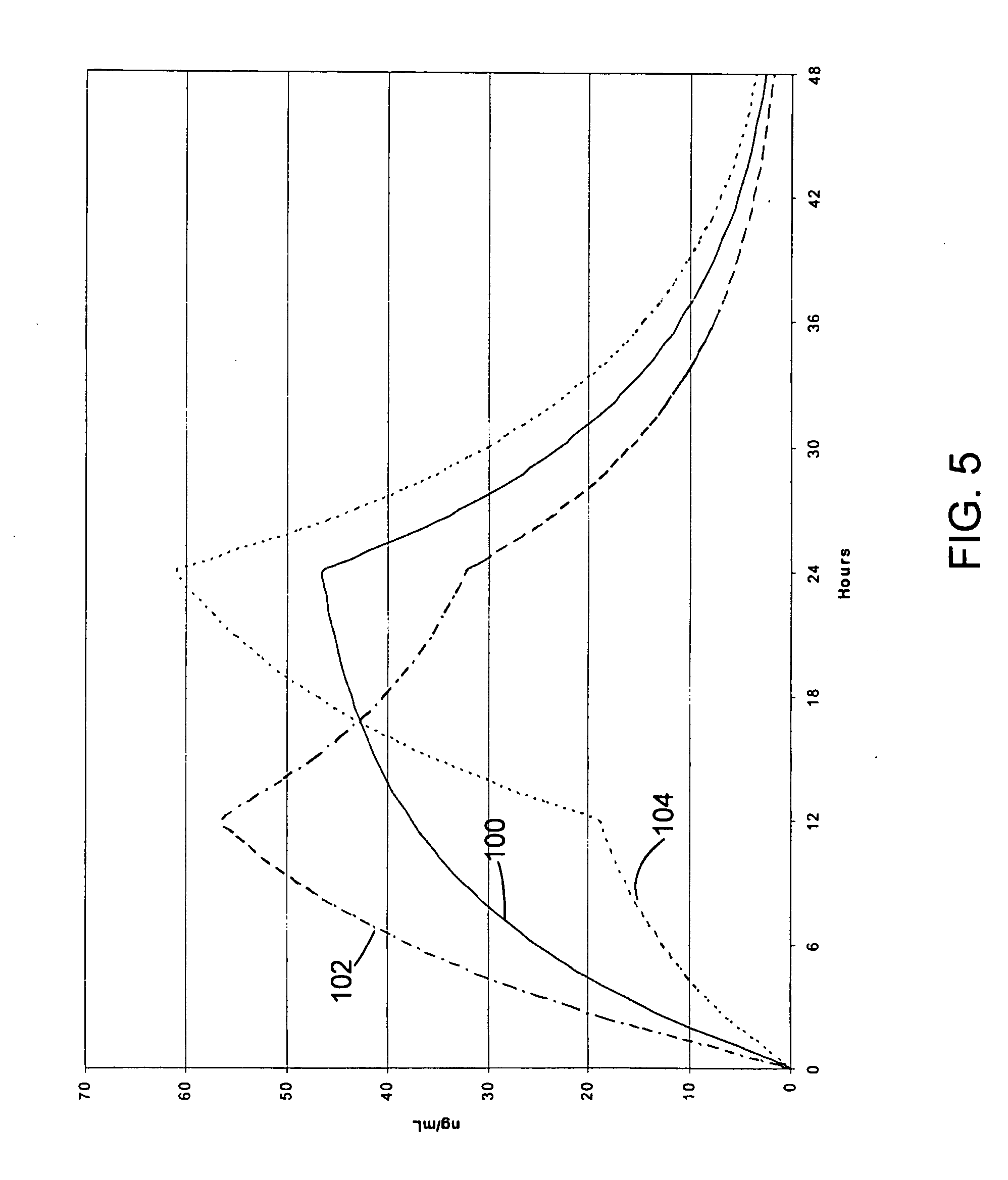
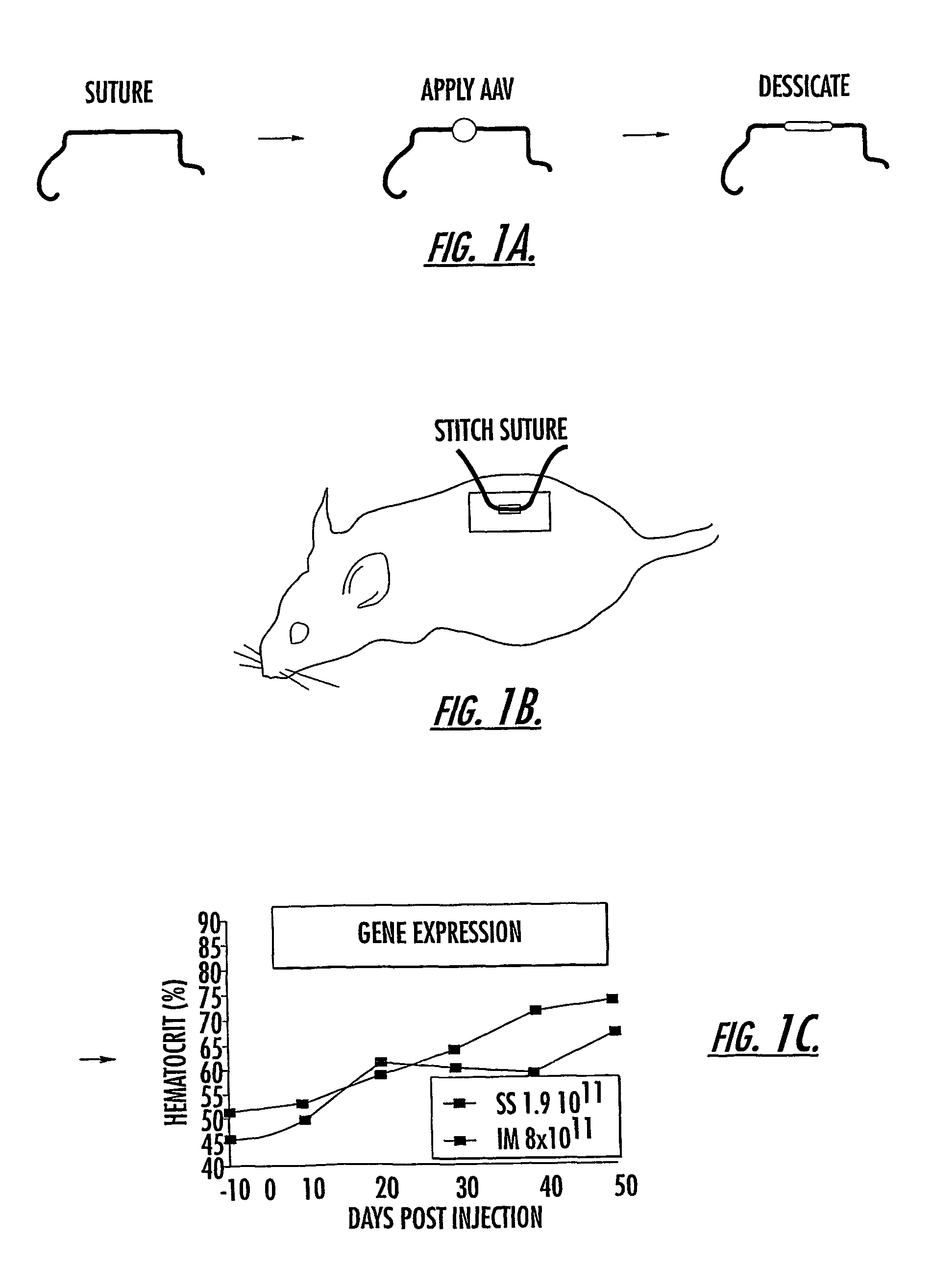
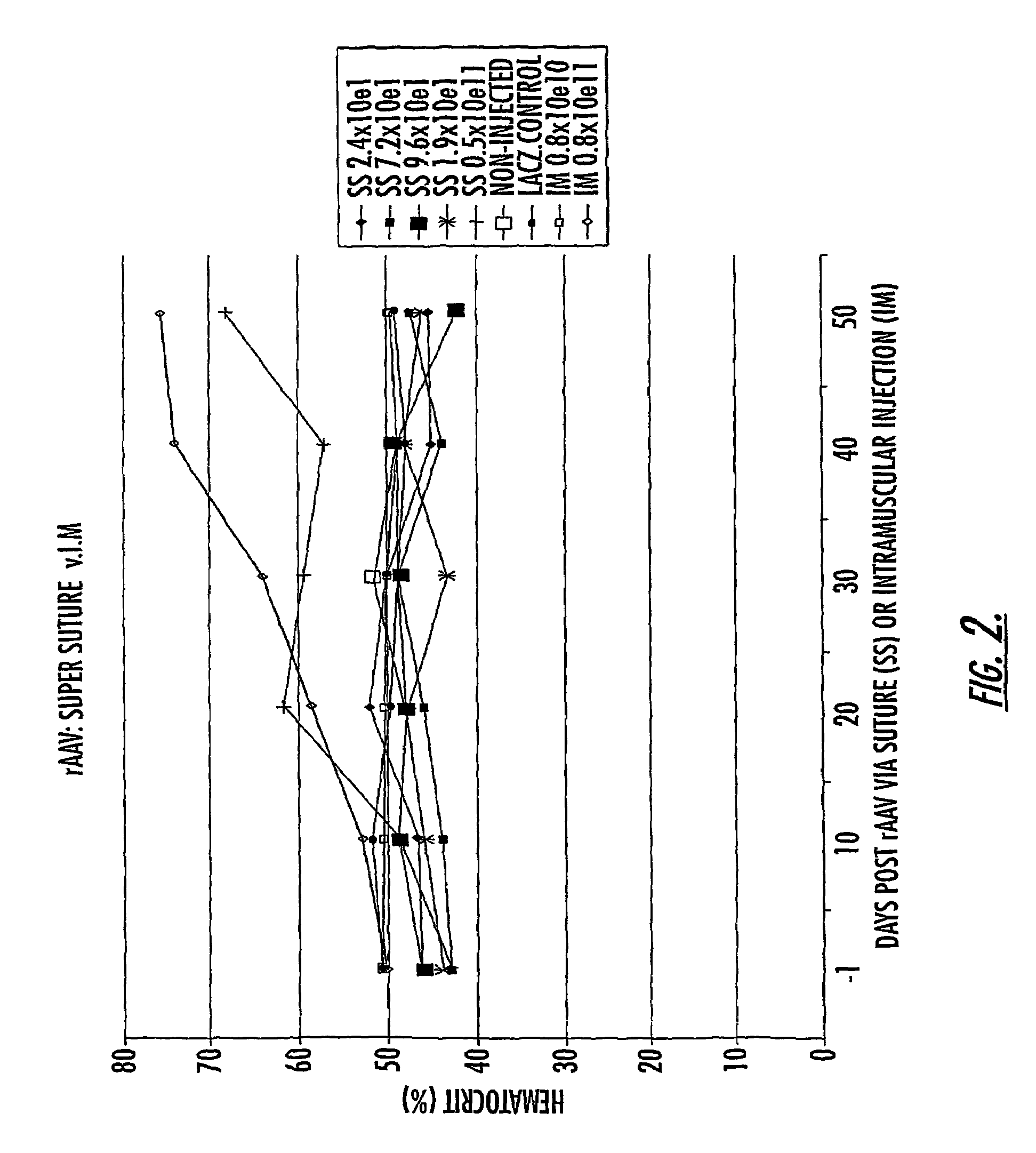
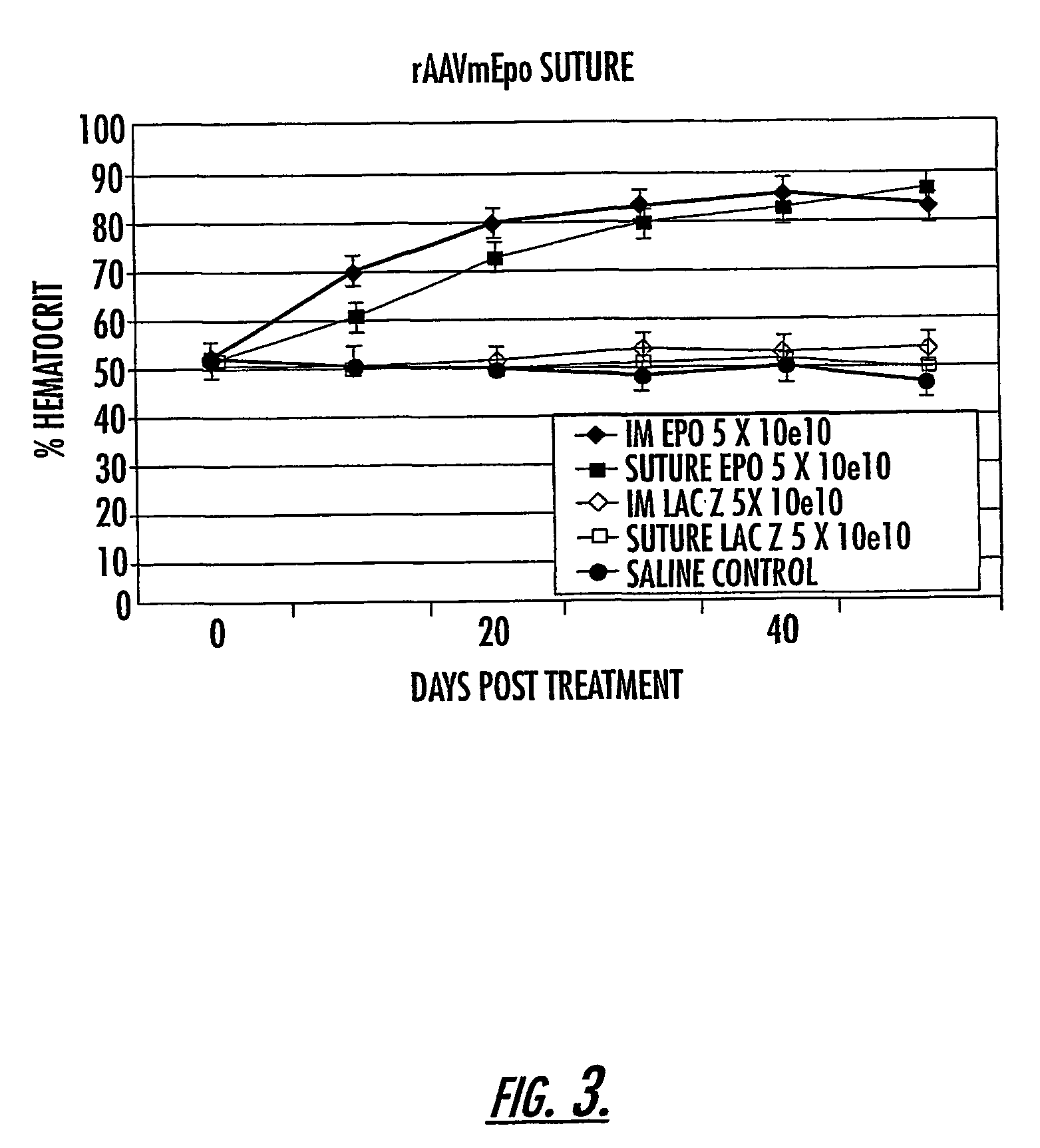
Methods and compounds for controlled release of recombinant parvovirus vectors
InactiveUS7201898B2Prevent relapseImproved pulmonary mechanicsSuture equipmentsAntibacterial agentsControlled releaseSupport matrix
The invention uses recombinant parvoviruses, and particularly recombinant adeno-associated virus (rAAV) to deliver genes and DNA sequences for gene therapy following manipulation of the therapeutic virus for packaging and transport. The invention delivers therapeutic viral vectors via rAAV affixed to support matrixes (i.e., sutures, surgically implantable materials, grafts, and the like).
Owner:NORTH CAROLINA AT CHAPEL HILL THE UNIV OF
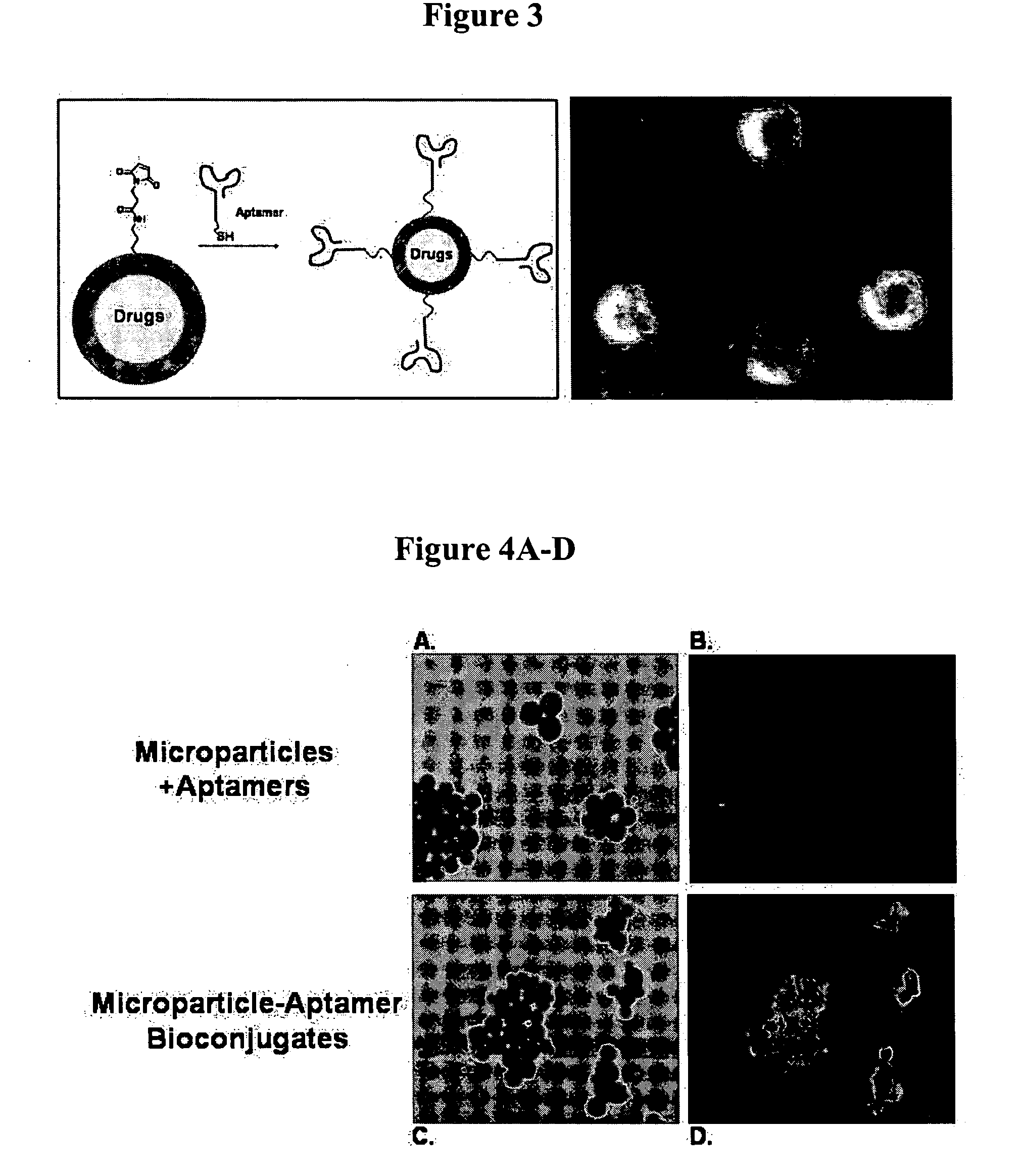
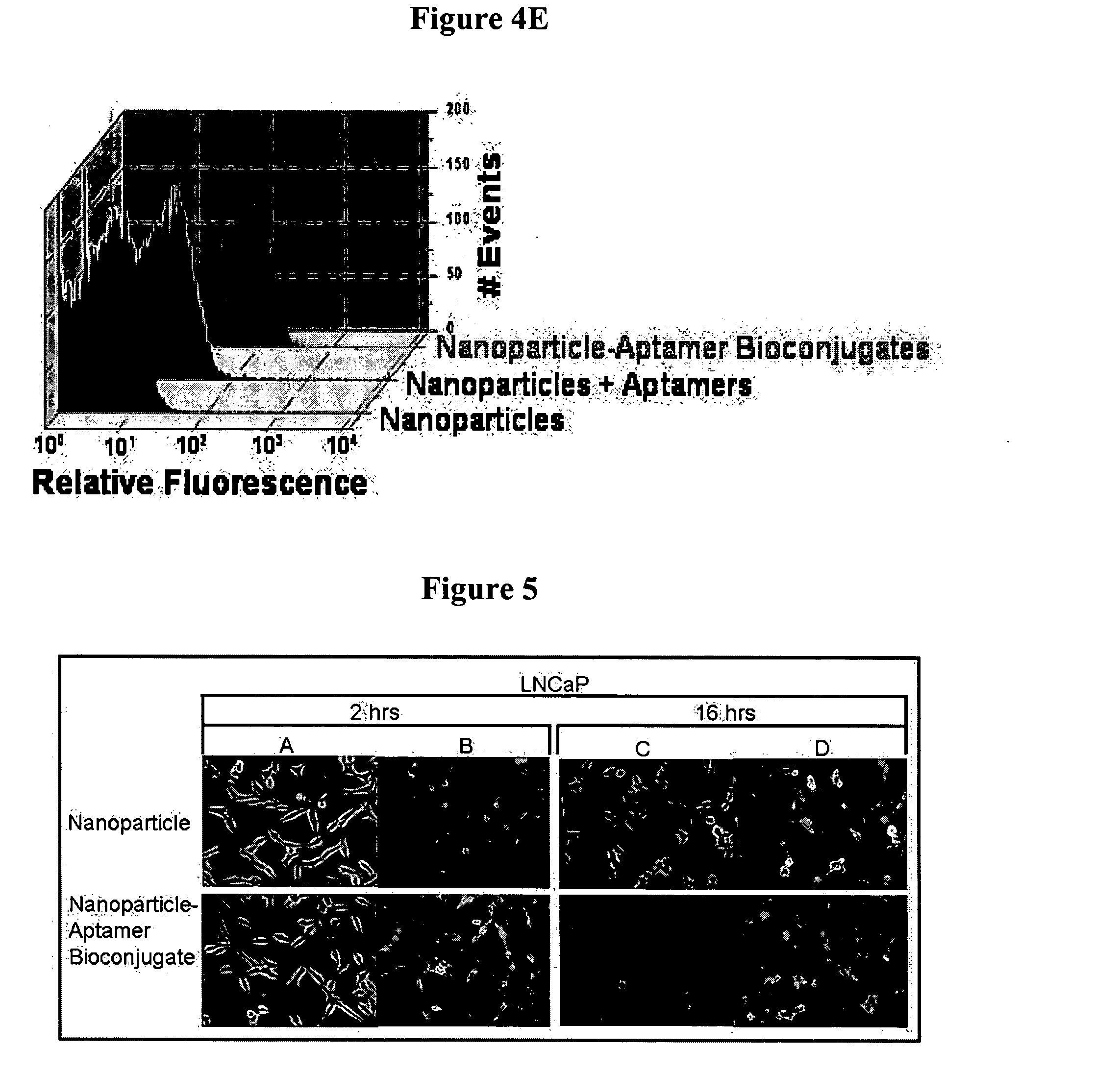
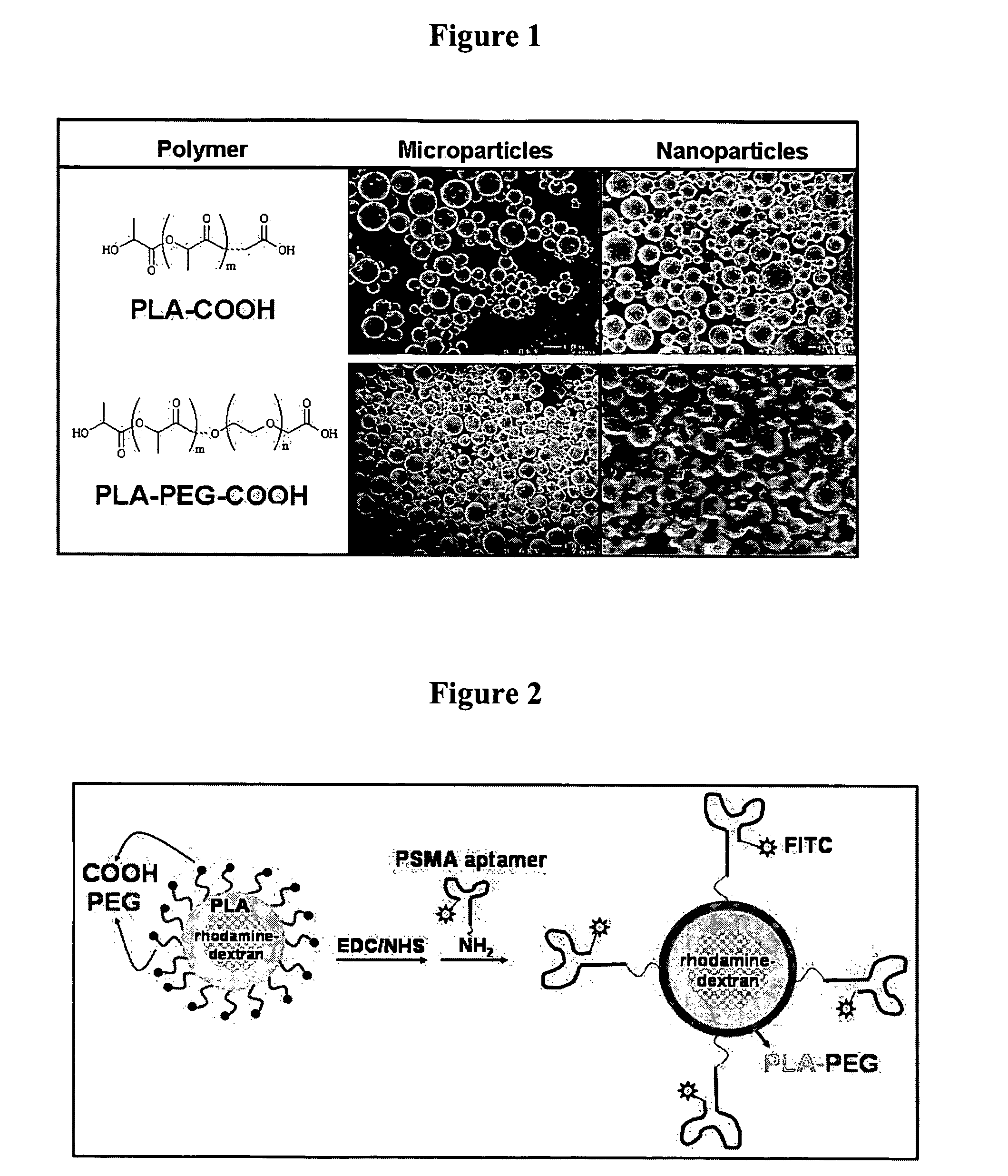
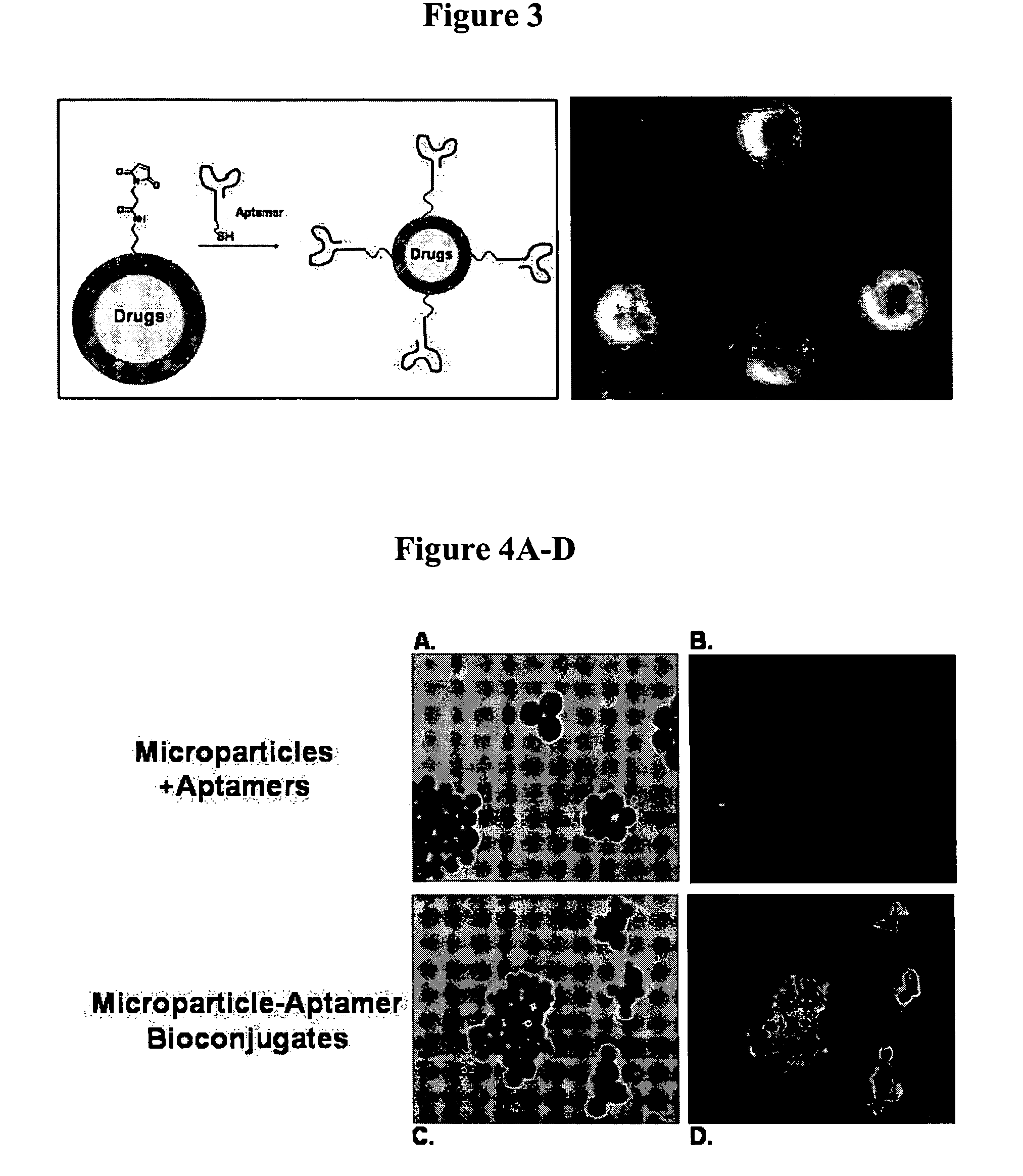
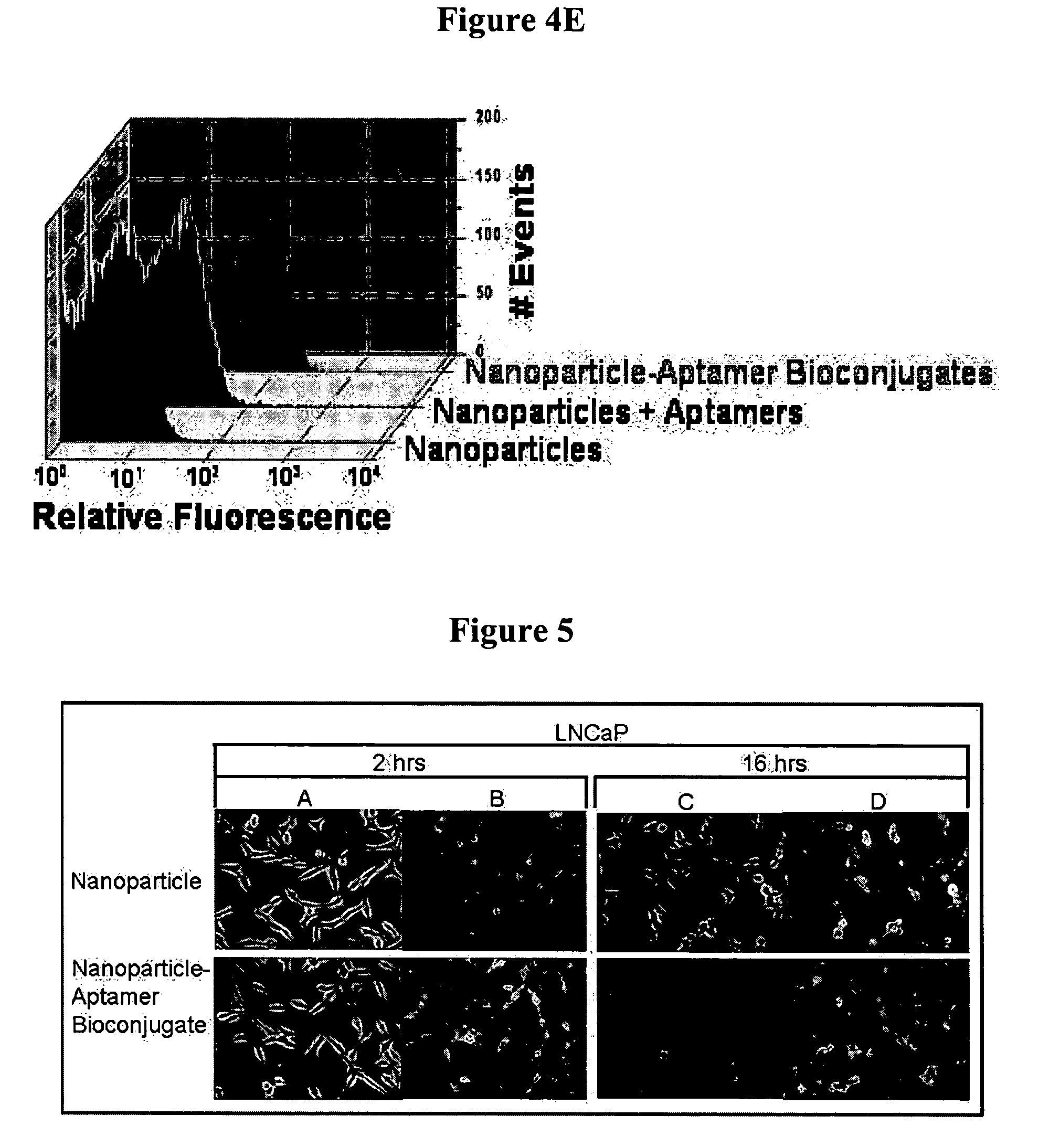
Targeted delivery of controlled release polymer systems
ActiveUS20050037075A1Strong specificityHigh affinityPowder deliveryPeptide/protein ingredientsControlled releaseCell Surface Antigens
The present invention relates to a conjugate that includes a nucleic acid ligand bound to a controlled release polymer system, a pharmaceutical composition that contains the conjugate, and methods of treatment using the conjugate. The controlled release polymer system includes an agent such as a therapeutic, diagnostic, prognostic, or prophylactic agent. The nucleic acid ligand that is bound to the controlled release polymer system, binds selectively to a target, such as a cell surface antigen, and thereby delivers the controlled release polymer system to the target.
Owner:MASSACHUSETTS INST OF TECH
Drug eluting implantable medical device
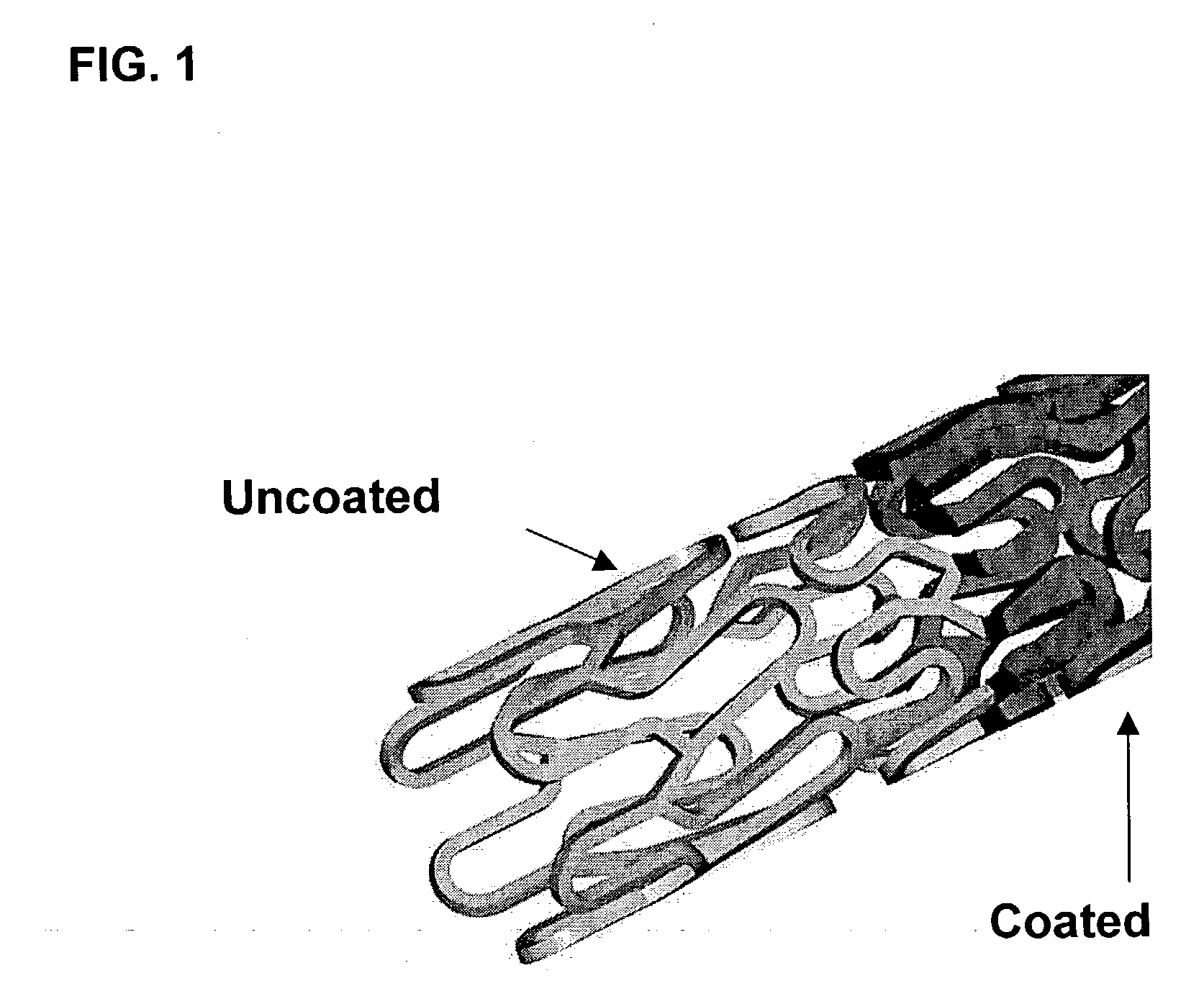
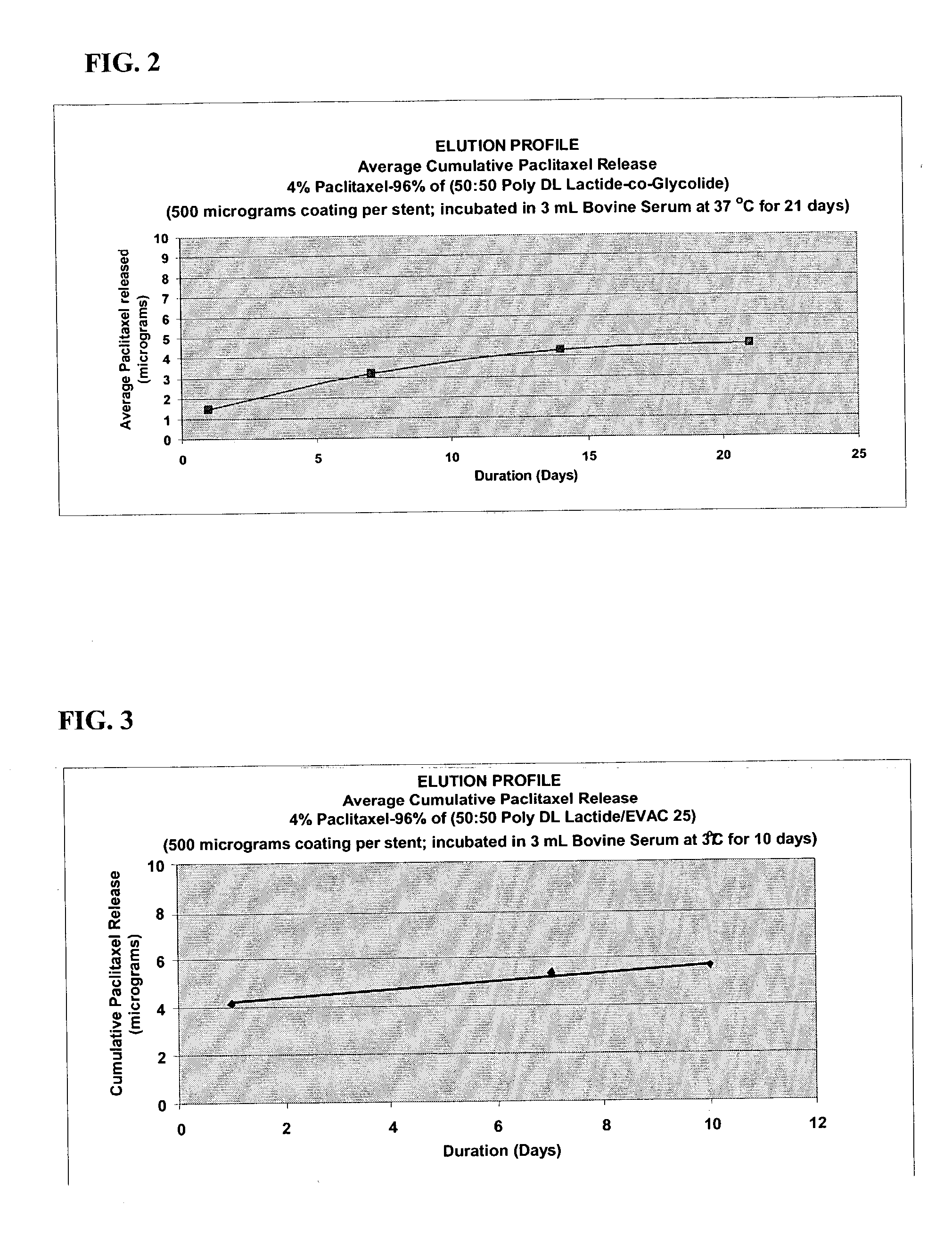
A drug eluting medical device is provided for implanting into vessels or luminal structures within the body of a patient. The coated medical device, such as a stent, vascular, or synthetic graft comprises a coating consisting of a controlled-release matrix of a bioabsorbable, biocompatible, bioerodible, biodegradable, nontoxic material, such as a Poly(DL-Lactide-co-Glycolide) polymer, and at least one pharmaceutical substance, or bioactive agent incorporated within the matrix or layered within layers of matrix. In particular, the drug eluting medical device when implanted into a patient, delivers the drugs or bioactive agents within the matrix to adjacent tissues in a controlled and desired rate depending on the drug and site of implantation.
Owner:ORBUSNEICH MEDICAL PTE LTD
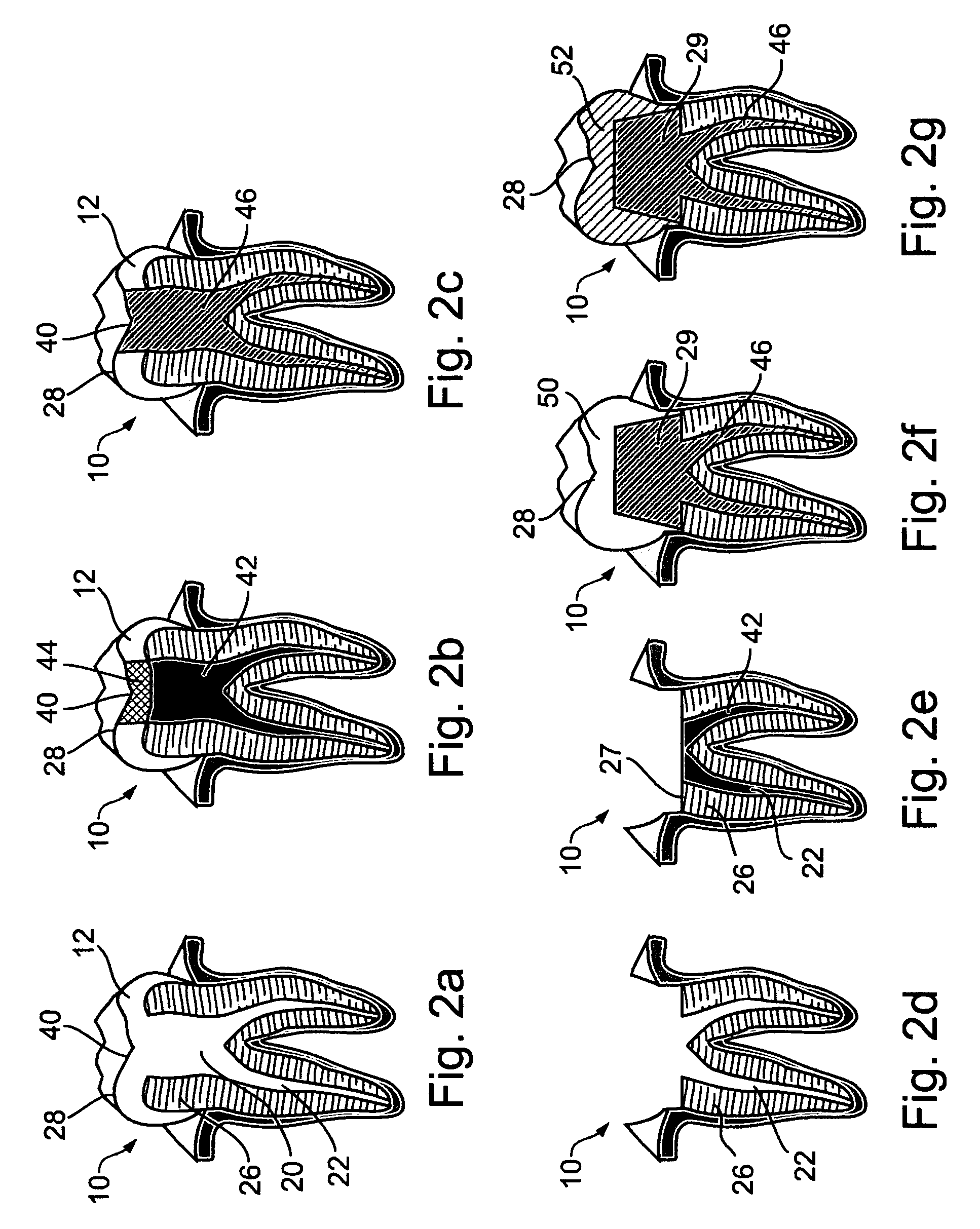
Oral devices and methods for controlled drug release
Drug dosage forms, which are housed in oral devices, and methods for controlled drug release are provided. The oral devices are permanently or removably inserted in the oral cavity and refilled or replaced as needed. The controlled drug release may be passive, based on the dosage form, or electronically controlled, for a high-precision, intelligent, drug delivery. Additionally, the controlled release may be any one of the following: release in accordance with a preprogrammed schedule, release at a controlled rate, delayed release, pulsatile release, chronotherapeutic release, closed-loop release, responsive to a sensor's input, release on demand from a personal extracorporeal system, release in accordance with a schedule specified by a personal extracorporeal system, release on demand from a monitoring center, via a personal extracorporeal system, and release in accordance with a schedule specified by a monitoring center, via a personal extracorporeal system. Drug absorption in the oral cavity may be assisted by an electrotransport mechanism. The oral devices require refilling or replacement at relatively long intervals of weeks or months, maintain a desired dosage level in the oral cavity, hence in the gastrointestinal tract, for extended periods, address situations of narrow drug therapeutic indices, and by being automatic, ensure adherence to a prescribed medication regimen.
Owner:WOLFAF ANDY +1
Ocular delivery of polymeric delivery formulations
InactiveUS20060210604A1Large doseEliminate lossSenses disorderPharmaceutical delivery mechanismControlled releaseMetabolite
The present invention provides a flowable composition suitable for use as a controlled release implant. The flowable composition can be administered into the ocular region of a mammal. The composition includes: (a) a biodegradable, biocompatible thermoplastic polymer that is at least substantially insoluble in aqueous medium, water or body fluid; (b) a biological agent, a metabolite thereof, a biological agently acceptable salt thereof, or a prodrug thereof; and (c) a biocompatible organic liquid, at standard temperature and pressure, in which the thermoplastic polymer is soluble. The present invention also provides methods of medical treatment that include administering the flowable composition into the ocular region of a mammal.
Owner:QLT USA INC
Controlled release bioactive agent delivery device
InactiveUS20050019371A1Minimize damageInterference minimizationOrganic active ingredientsSenses disorderControlled releaseMeth-
Owner:SURMODICS INC
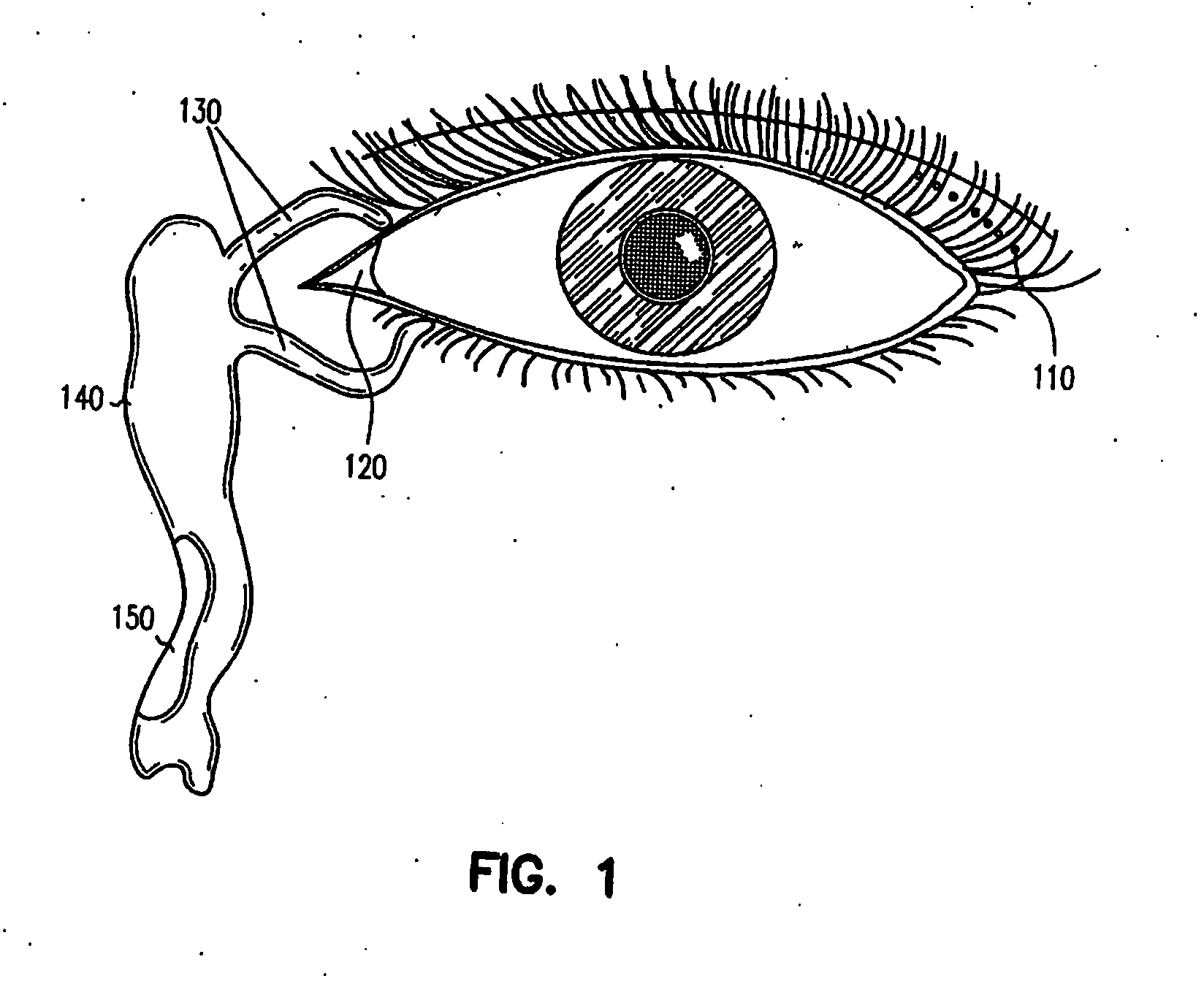
Flexible microchip devices for ophthalmic and other applications
InactiveUS6976982B2Small sizeVariable shapeEye surgerySolid-state devicesControlled releaseBiomedical engineering
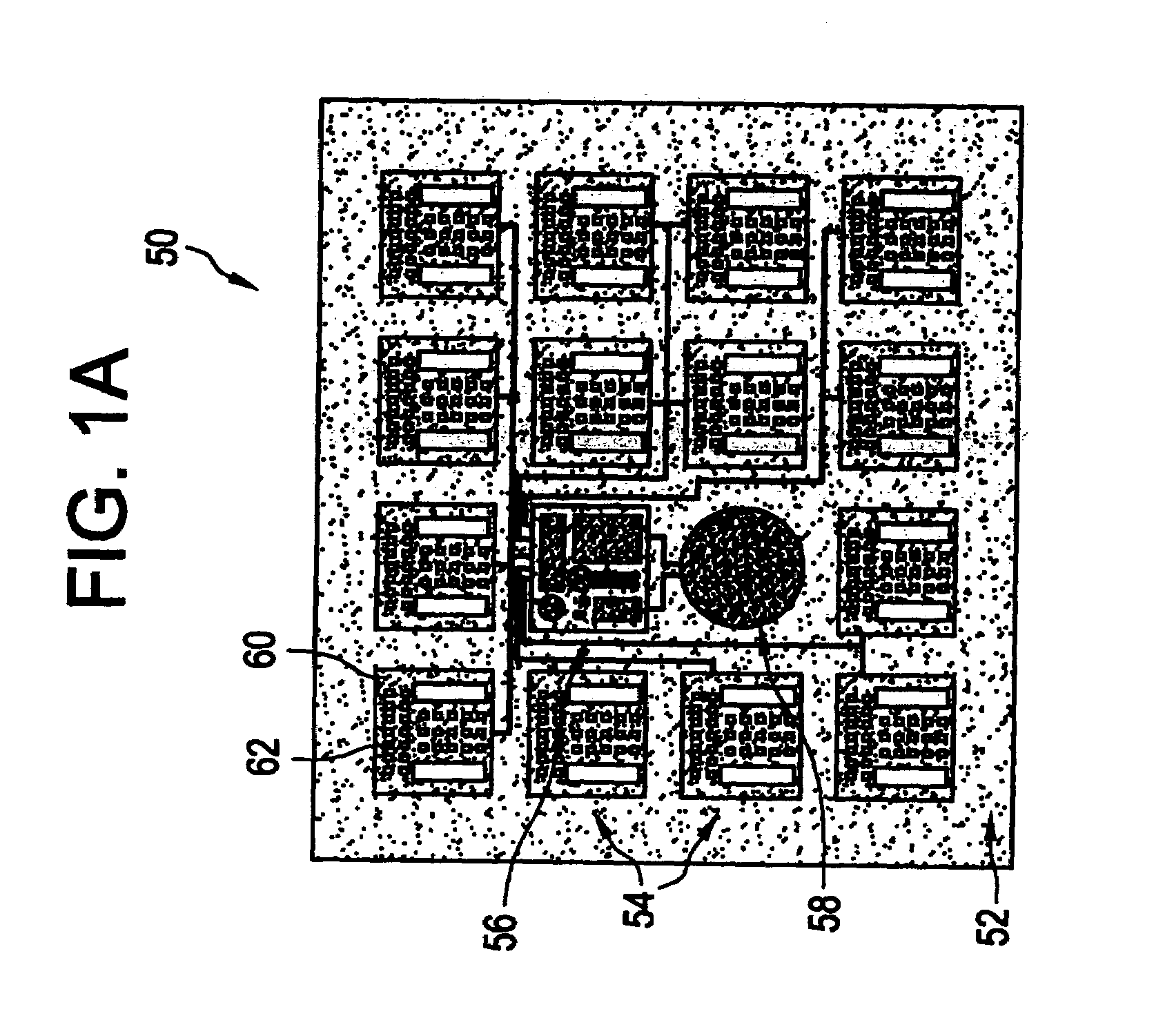
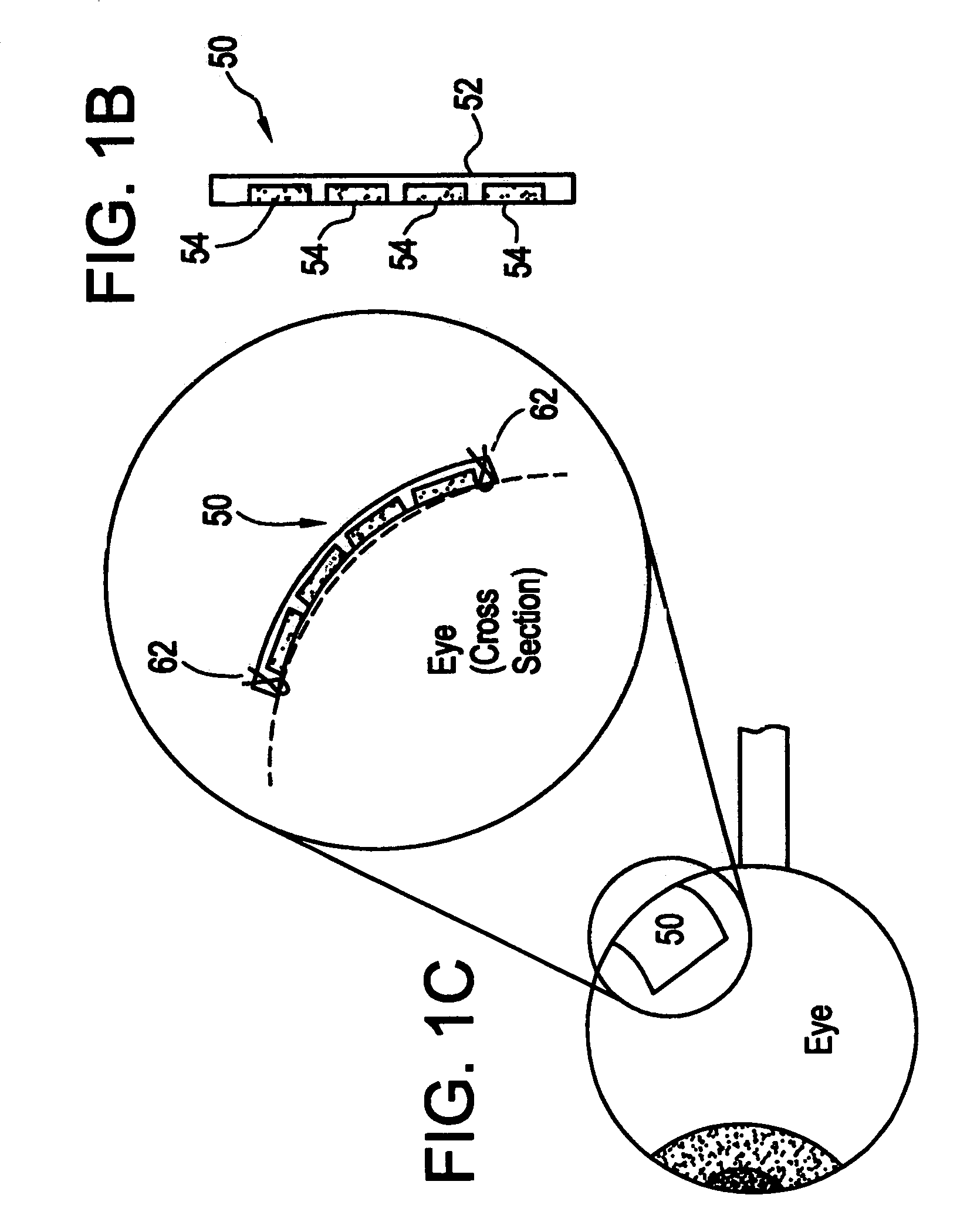
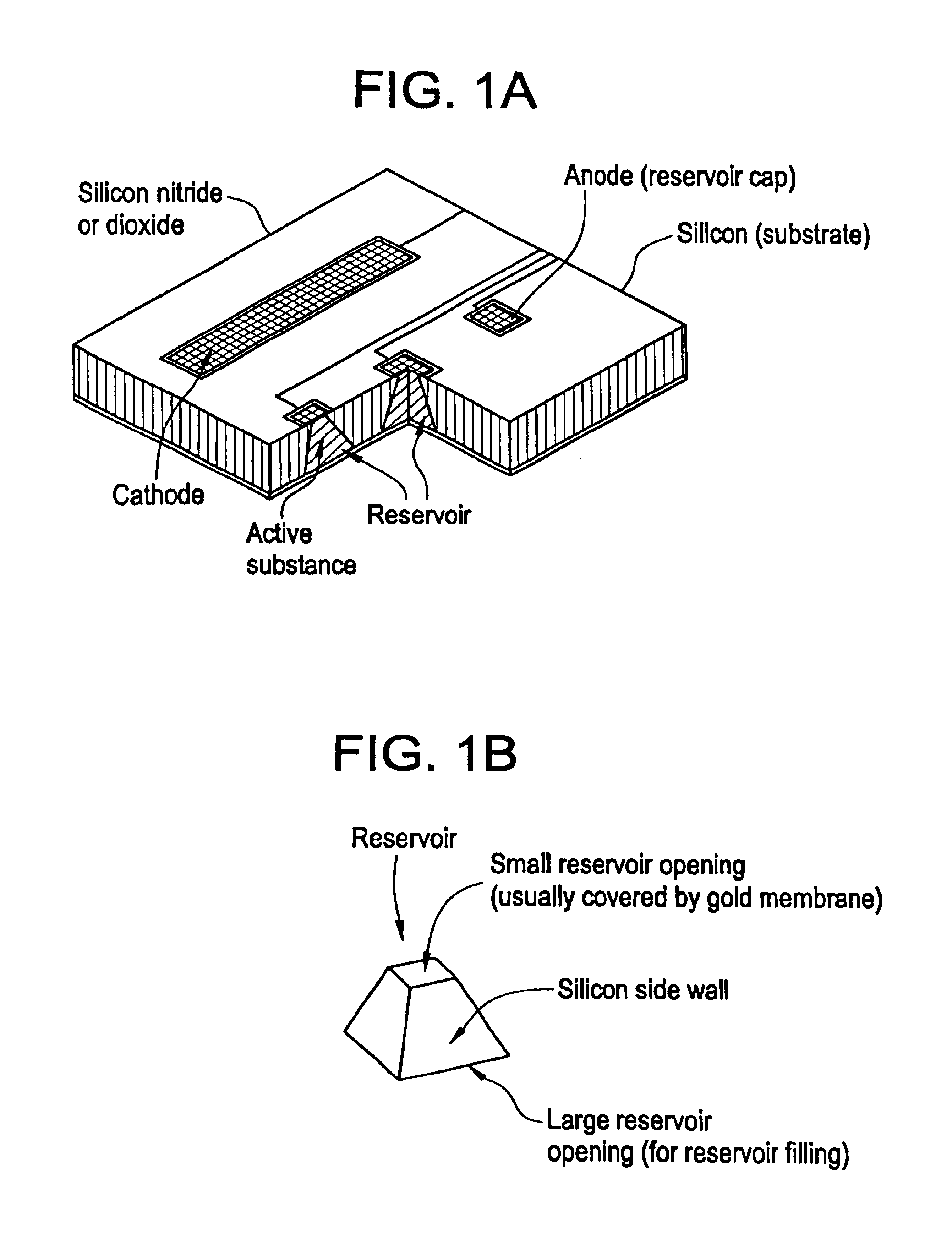
Microchip device arrays that can conform to a curved surface are provided for the controlled release or exposure of reservoir contents. The arrays comprise two or more microchip device elements, each of which includes a plurality of reservoirs that contain molecules for controlled release or components for selective exposure, and a means for flexibly connecting the device elements. The reservoirs can contain one or more drugs and / or one or more secondary devices, such as a sensor or a component thereof. Preferably, the microchip devices contain and controllably release therapeutic, prophylactic, and diagnostic molecules to and into the eye of a patient in need thereof.
Owner:MICROCHIPS BIOTECH INC
Abuse-resistant pharmaceutical compositions
An abuse-resistant controlled release pharmaceutical composition comprising a pharmaceutically effective amount of discrete particles of an active capable of abuse, wherein surfaces of said particles are wetted with a water insoluble coating material, and preferably wherein said composition comprises a matrix, in which said particles are distributed, and which renders the abuse-capable compound within the matrix difficult to separate from the matrix; and a method for the preparation of a controlled release pharmaceutical composition having a reduced potential for abuse, comprising applying a pressure force to a mixture comprising a water insoluble material, and particles of a pharmaceutically active compound capable of inducing in a subject a reaction that is physiologically or psychologically detrimental if administered in an immediate release dosage form, thereby resulting in surface coated particles, and incorporating said surface coated particles into a pharmaceutical composition
Owner:ELAN PHRMA INT LTD
Sustained release pharmaceutical compositions for highly water soluble drugs
ActiveUS20070020335A1Reduce spreadReduce erosionPowder deliveryOrganic active ingredientsControlled releaseActive agent
The present invention provides pharmaceutical compositions for controlled release of pharmaceutically active agents, especially those with a high water solubility, high dose, and / or short half-life. In addition, the present application provides methods for preparing and using such pharmaceutical compositions.
Owner:FARNAM +1
Microchip devices with improved reservoir opening
InactiveUS6875208B2Improve uniformityImprove reliabilityMedical devicesMicromachined deliveryControlled releaseCurrent distribution
Microchip devices and methods of manufacture thereof are provided to increase the uniformity and reliability of active exposure and release of microchip reservoir contents. In one embodiment, the microchip device for the controlled release or exposure of molecules or secondary devices comprises: (1) a substrate having a plurality of reservoirs; (2) reservoir contents comprising molecules, a secondary device, or both, located in the reservoirs; (3) reservoir caps positioned on the reservoirs over the reservoir contents; (4) electrical activation means for disintegrating the reservoir cap to initiate exposure or release of the reservoir contents in selected reservoirs; and (5) a current distribution means, a stress induction means, or both, operably engaged with or integrated into the reservoir cap, to enhance reservoir cap disintegration.
Owner:MASSACHUSETTS INST OF TECH
Compositions having a combination of immediate release and controlled release characteristics
InactiveUS6908626B2Rapid in vivo dissolutionSlower in vivo dissolutionPowder deliveryAerosol deliveryControlled releaseNanoparticle
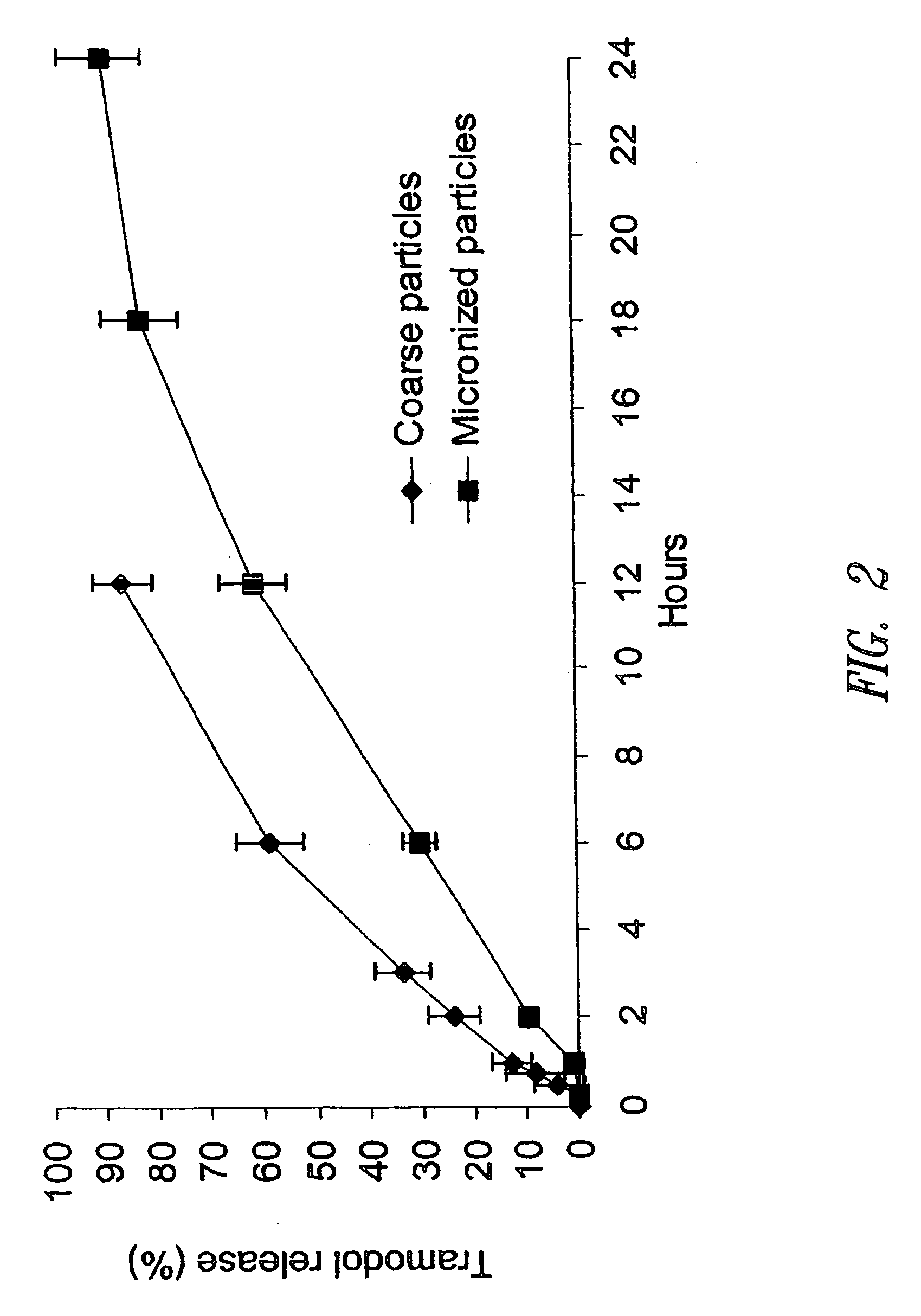
Disclosed are compositions exhibiting a combination of immediate release and controlled release characteristics. The compositions comprise at least one poorly soluble active ingredient having a nanoparticulate particle size, at least one surface stabilizer adsorbed onto the surface of the nanoparticulate active agent particles, and at least one active ingredient having a microparticulate particle size.
Owner:BAUDAX BIO INC +1
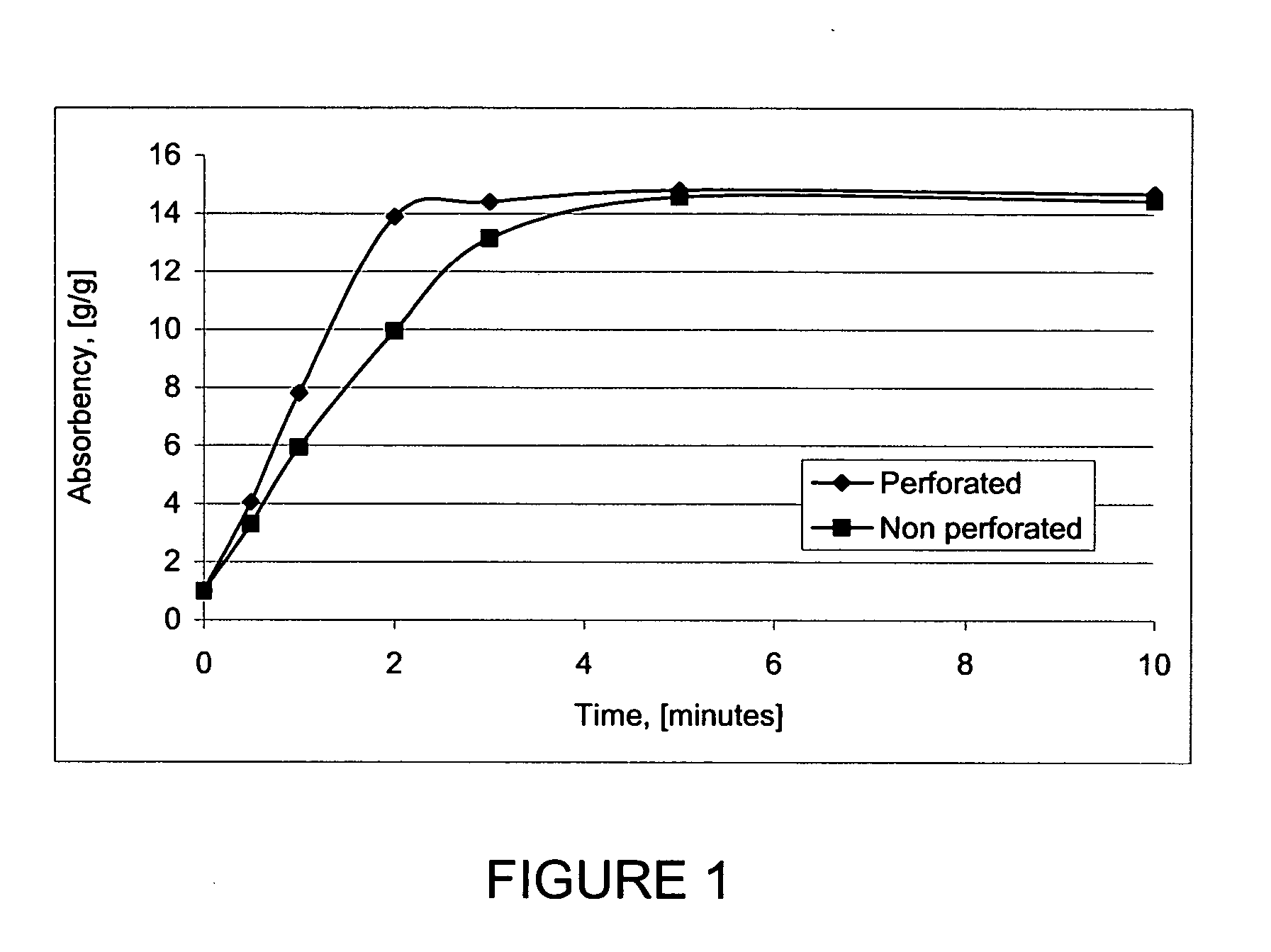
Gelled biopolymer based foam
InactiveUS20050137272A1Improve water absorptionWet strengthCosmetic preparationsToilet preparationsPersonal careCross-link
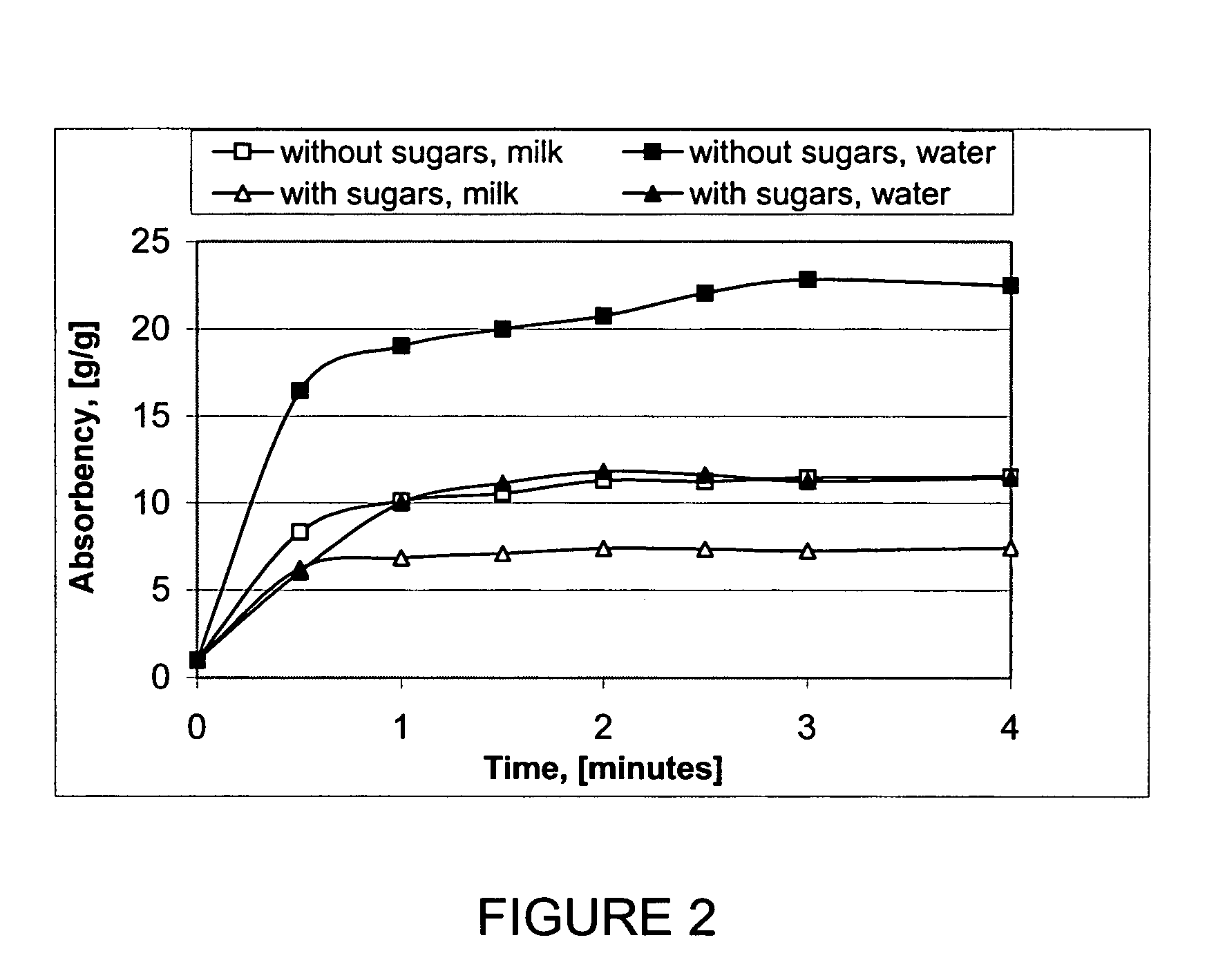
Gelled biopolymer based foams are disclosed. The gelled foams comprise a cross-linked biopolymer, preferably alginate; optionally, a foaming agent such as hydroxy propyl methyl cellulose; and a plasticizer, preferably glycerin sorbitol, or a mixture thereof, that forms the predominant portion of the gelled foam. The foams are soft and pliable and have high absorbency. They are used as wound dressing materials, controlled release delivery systems, cell culture, barrier media for preventing tissue adherence, and bioabsorbable implants. They also have various personal care applications, especially in oral hygiene, and can be used in food applications.
Owner:FMC BIOPOLYMER AS
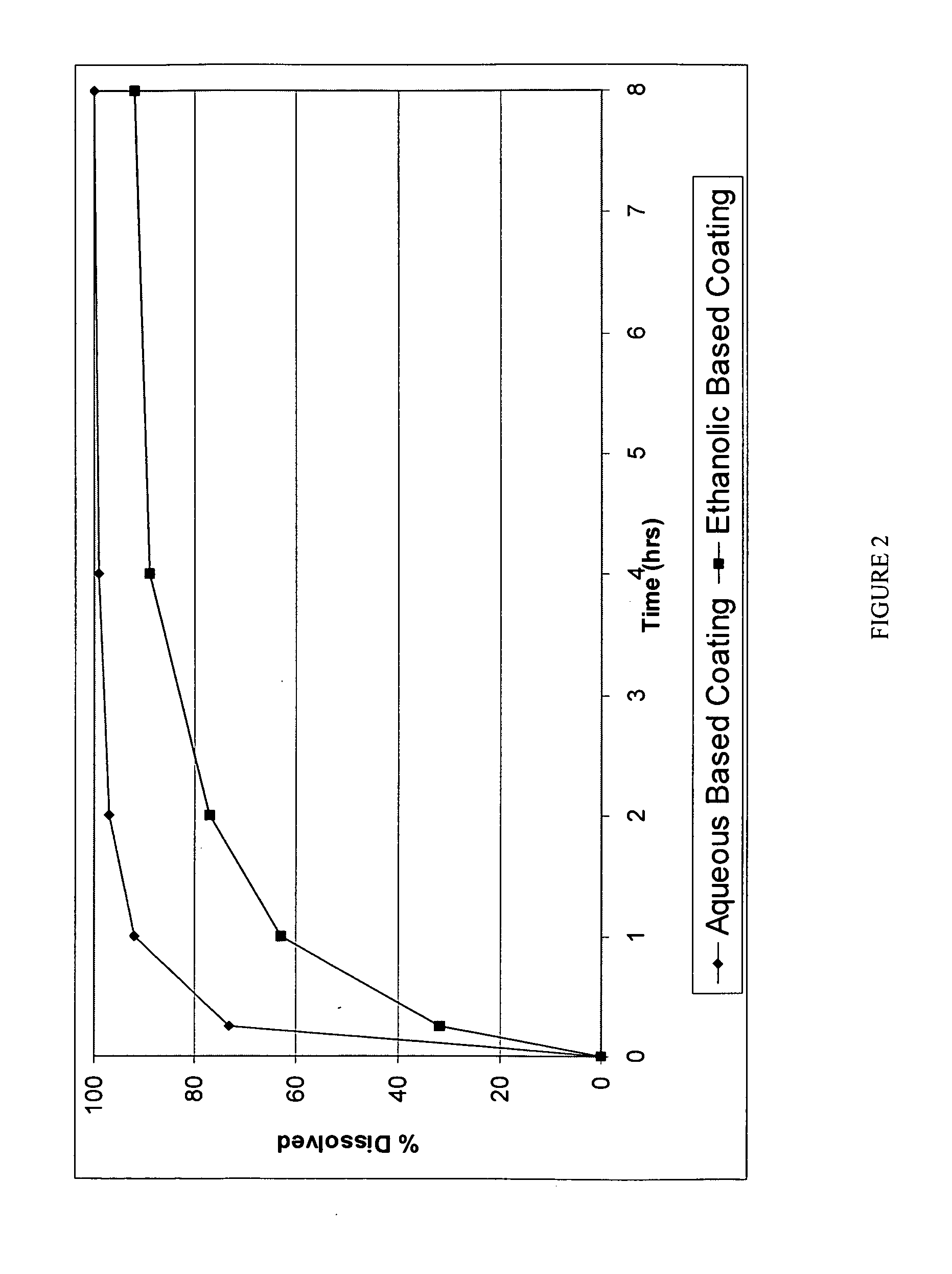
Morphine controlled release system
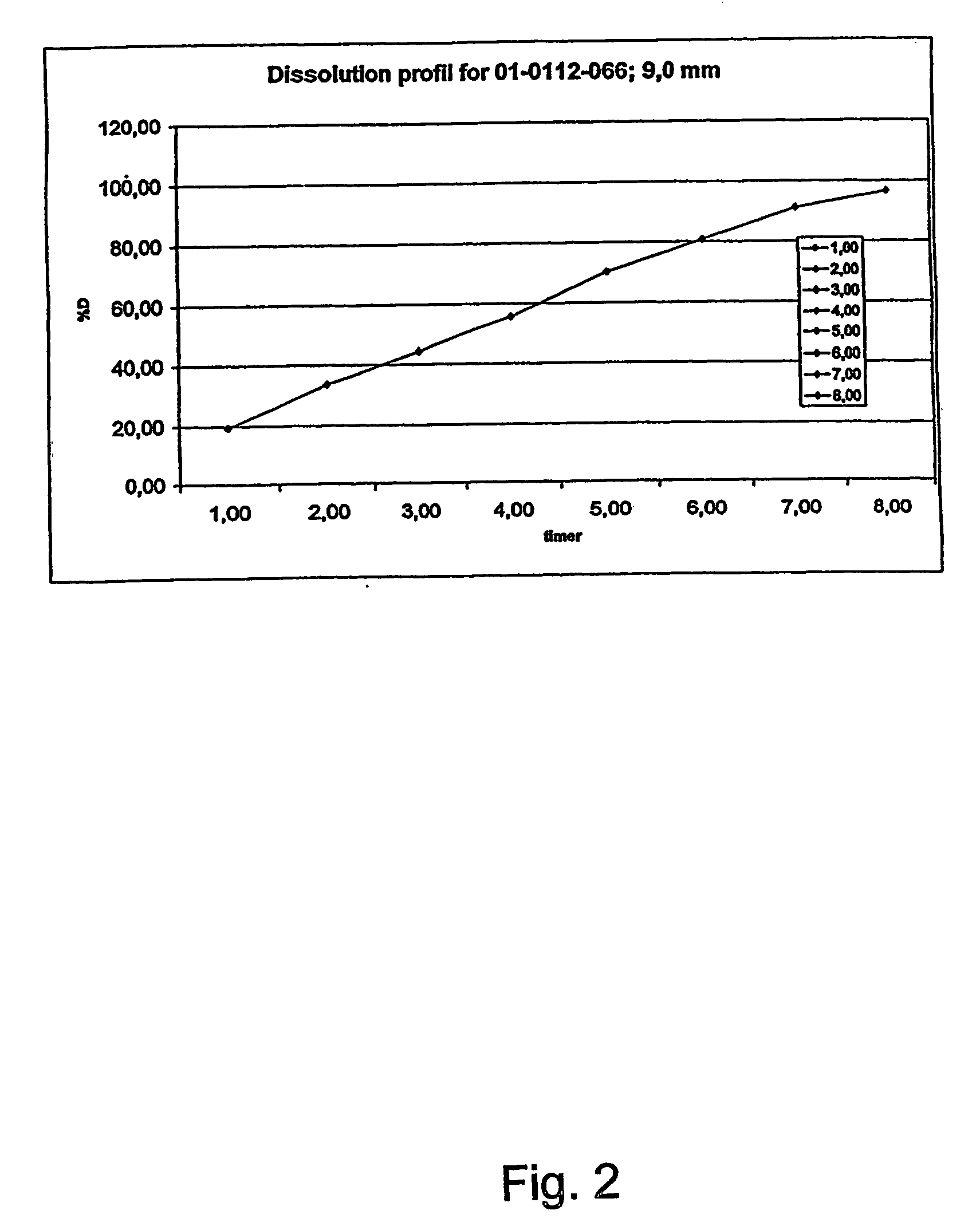
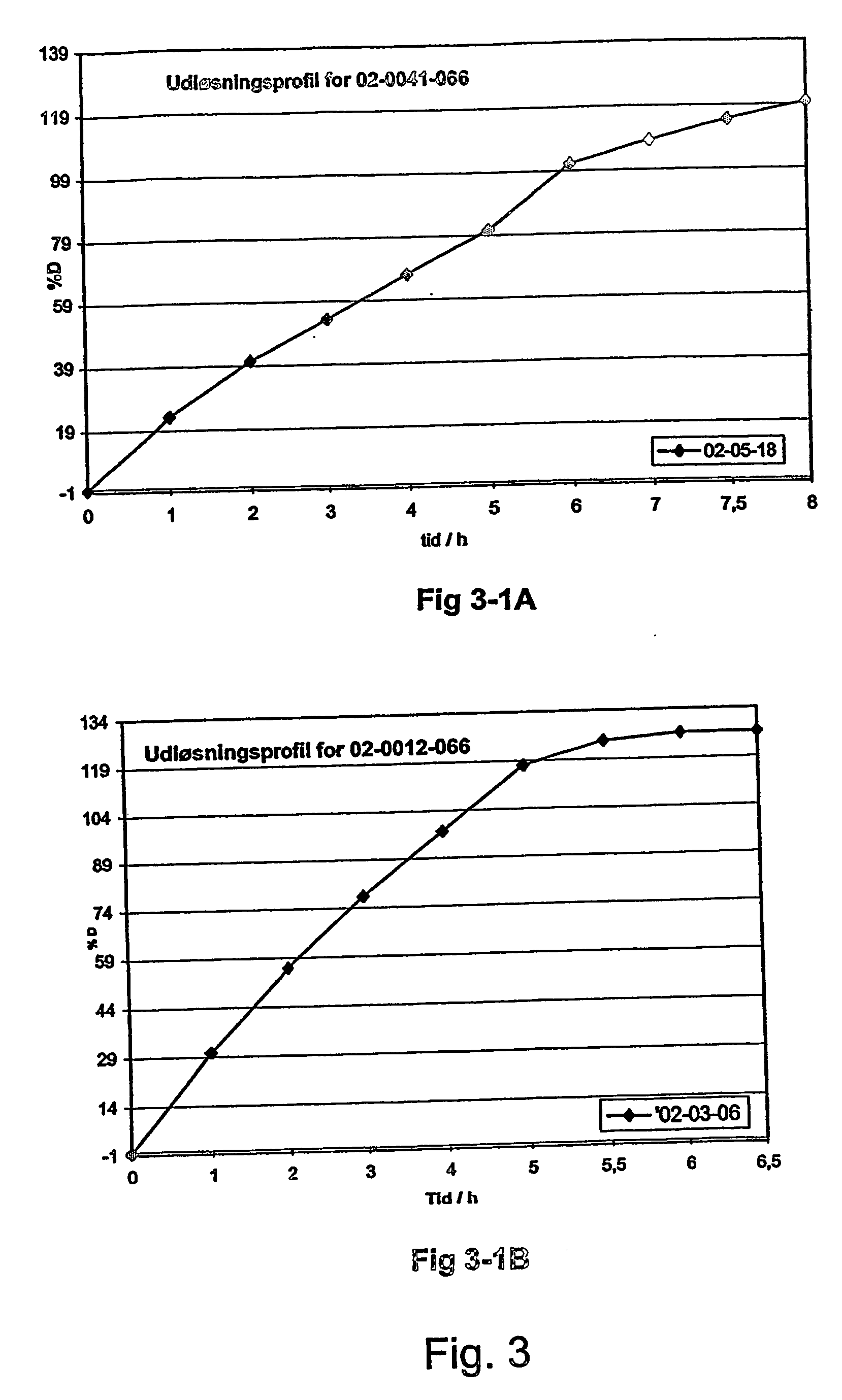
InactiveUS20070003617A1Low administration frequencyAffecting extent of drug bioavailabilityBiocideNervous disorderMorphineDissolution
A composition for controlled release of an opioid from a pharmaceutical composition, the method comprises controlling the release of at least one opioid into an aqueous medium by erosion of at least one surface of a pharmaceutical composition comprising I) a matrix composition comprising a) polymer or a mixture of polymers, b) an opioid and, optionally, c) one or more pharmaceutically acceptable excipients, and (i) a coating. The matrix composition has a conus-like shape so the surface area exposed to the aqueous medium increases at least during initial erosion of the matrix composition, and the dissolution of the opioid-when tested in a Dissolution Test as described herein with or without application of sinkers-results in a zero order release of at least 80% of the opioid contained in the composition. Such compositions are especially suitable for controlled release of an opioid to obtain a delayed pead concentration and a prolonged therapeutically effective plasma concentration upon oral administration. Once or twice daily administration is possible. The matrix typically comprises PEO and the active substance is typically an opioid such as morphine or a glucuronide thereof.
Owner:EGALET LTD
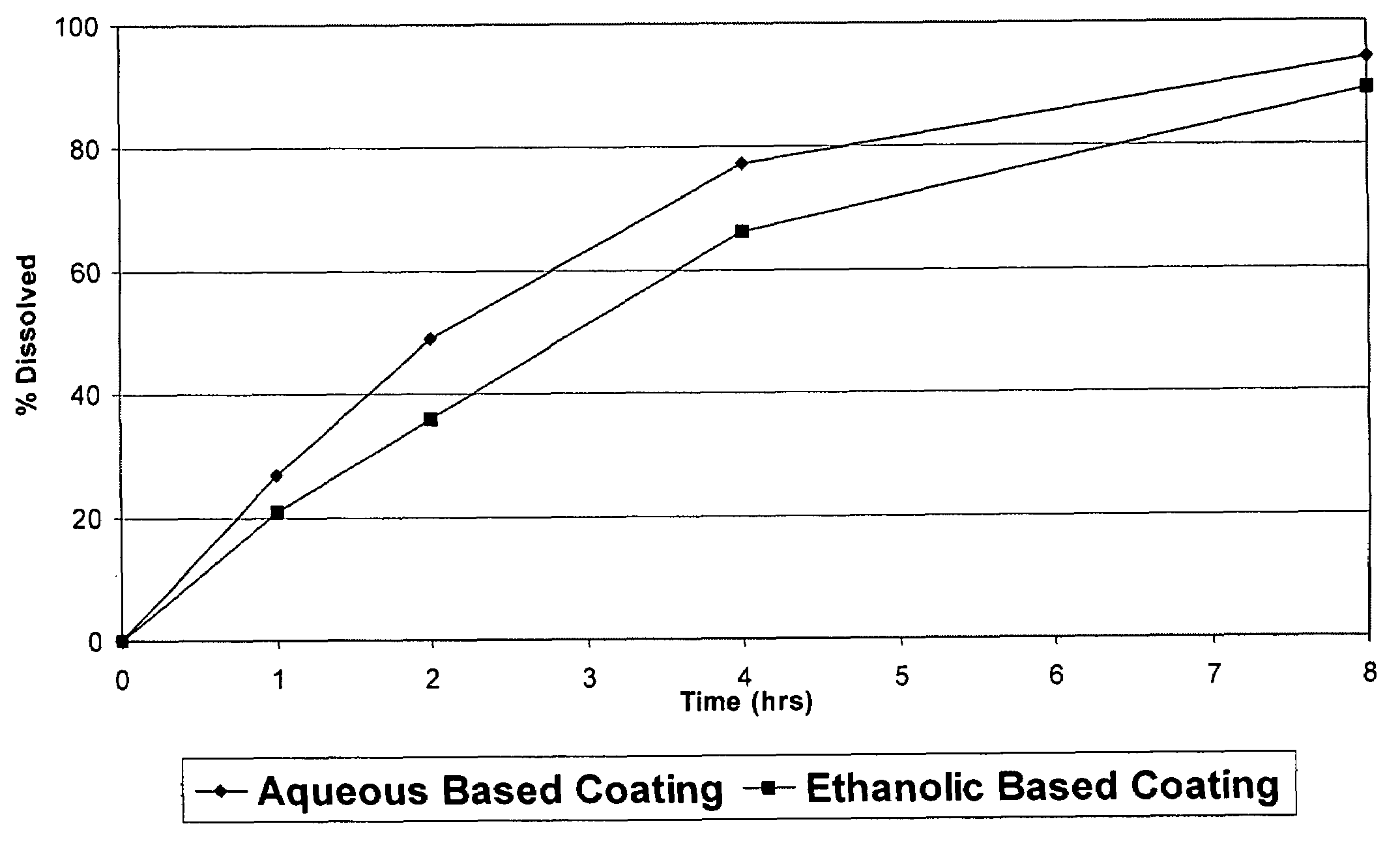
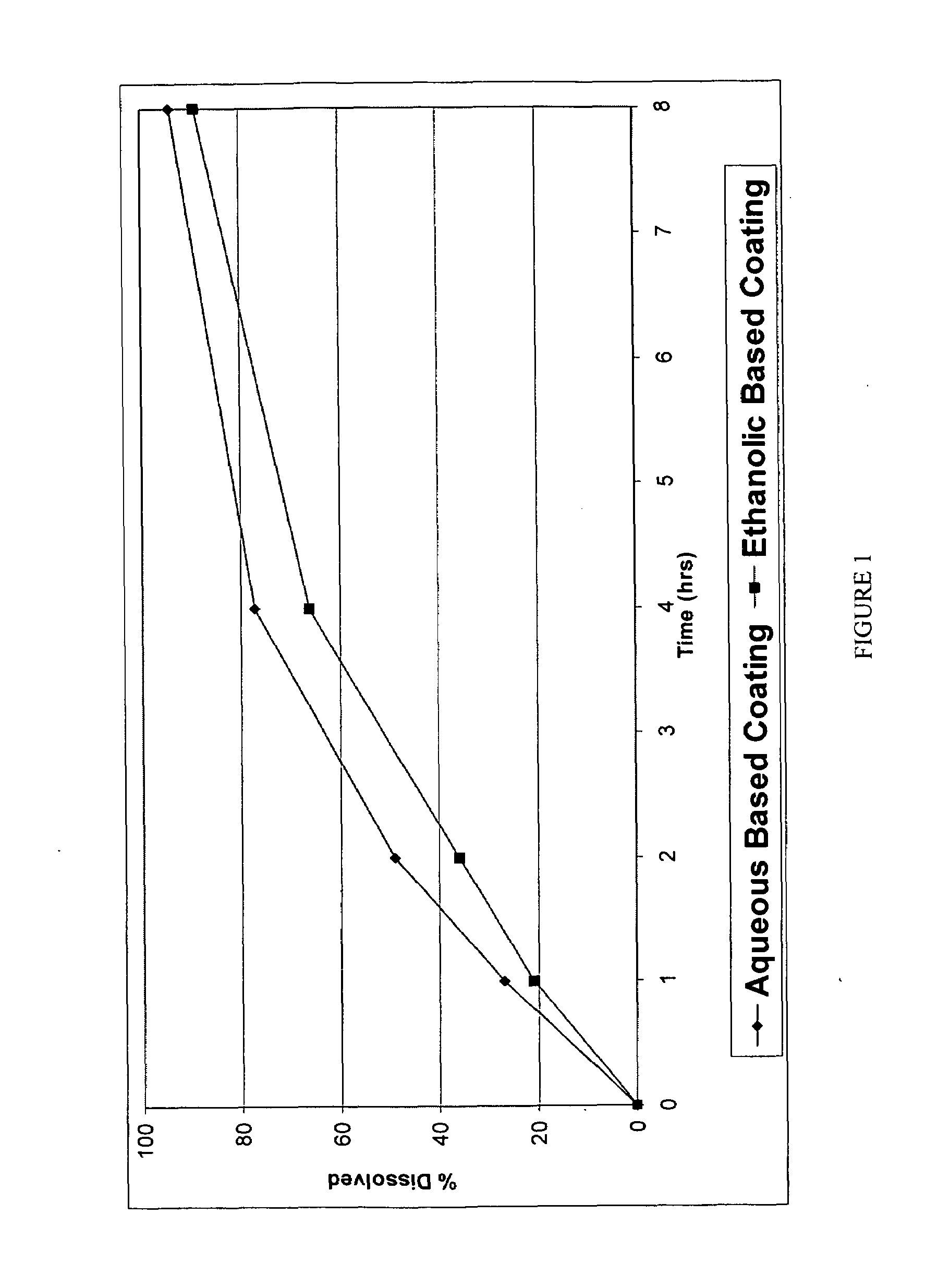
Pharmaceutical tablet, completely coated, for controlled release of active principles that present problems of bio-availability linked to gastro-intestinal absorption
Described herein is a particular type of pharmaceutical tablet, for oral use, which is formed by one or more layers, and is specifically designed for controlled release of active principles that present problems of bio-availability linked to absorption in the gastro-intestinal tract, and in particular active principles that present an erratic and unpredictable absorption linked to the presence or absence of food at the level of the stomach and / or of the first portion of the small intestine, the said pharmaceutical form being characterized in that it is completely coated with one or more films of a biocompatible and biodegradable polymeric material.
Owner:JAGOTEC AG
Powder formulation disintegrating system and method for dry powder inhalers
A disperser for dry powders which can be used with different dose systems, dose weights ranging from 2 to 25 mg and different types of powder formulation. In one embodiment, the disperser acts both as a de-agglomeration (disintegration; aerosolization) means and as an air classifier for especially adhesive mixtures. Only fine drug particles are emitted whereas the larger agglomerates and carrier crystals are retained by the disperser. Another embodiment enables time controlled release of carrier crystals in these mixtures. Yet another embodiment has optimized performance with spherical pellets, containing no carrier crystals. Other possible embodiments of the invention make it possible to control the total inhaler resistance and the powder deposition in the upper respiratory tract by means of the addition of a so-called sheath flow of clean air. Modifications also enable carrier retainment in the mouthpiece and elimination of the tangential flow component of the discharge cloud.
Owner:ASTRAZENECA AB
Stent for controlled release of drug
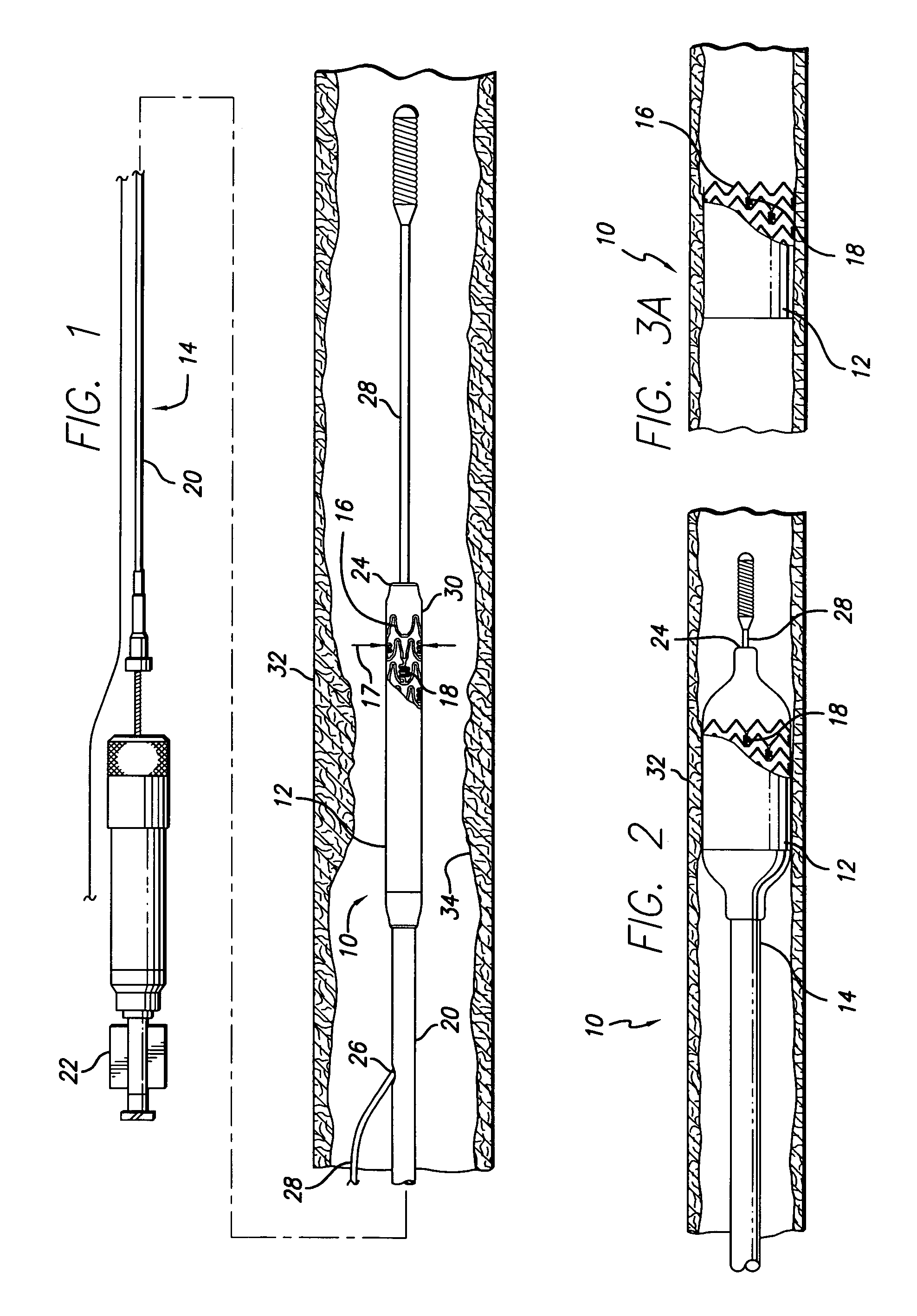
Devices for the controlled release of one or more drugs into a patient are provided. The devices include an implantable stent, at least two reservoirs in the stent, and a release system contained in each of the at least two reservoirs, wherein the release system may have one or more drugs for release at a controlled rate. Reservoir caps optionally are provided to control the time at which release of the one or more drugs is initiated.
Owner:BOSTON SCI SCIMED INC
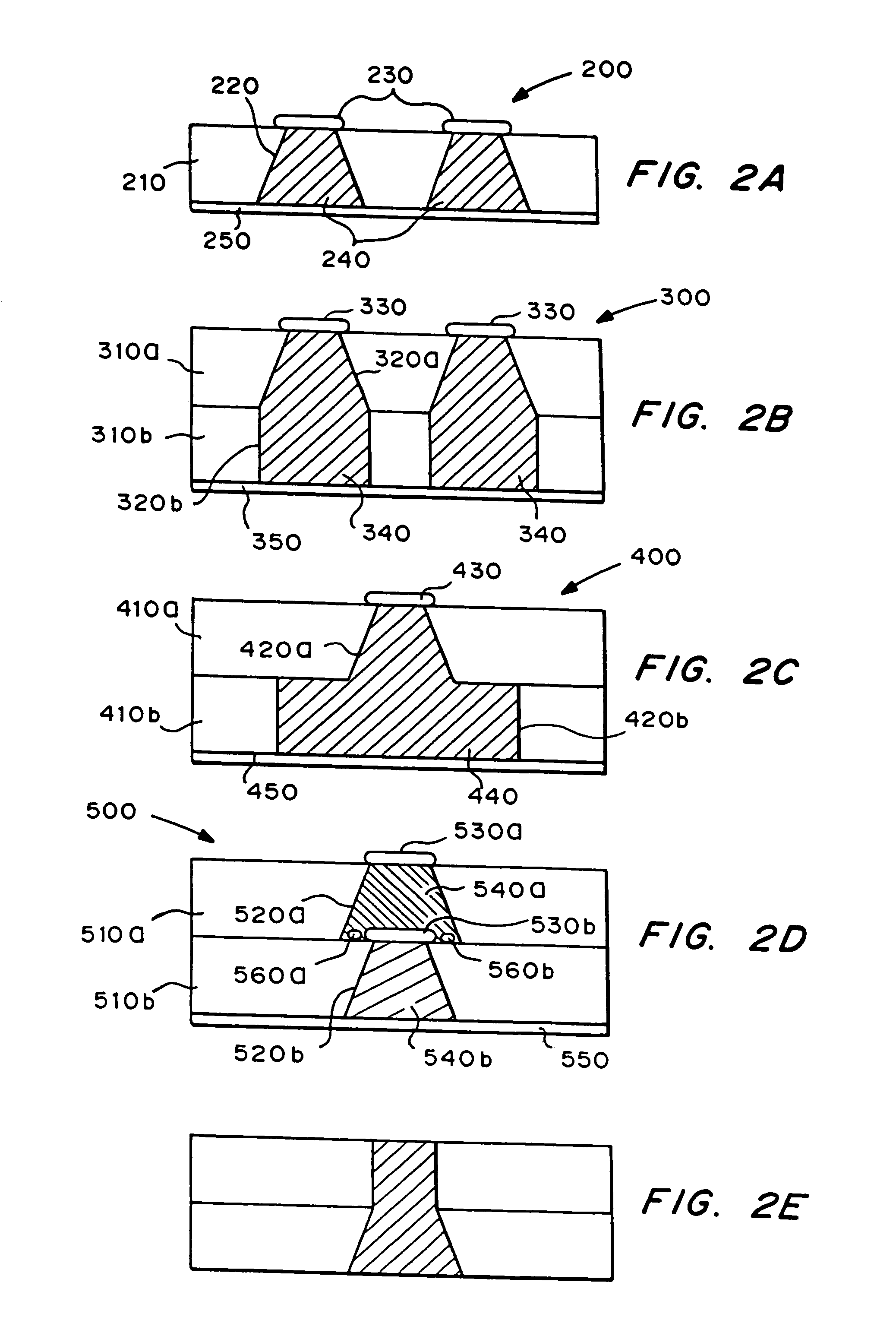
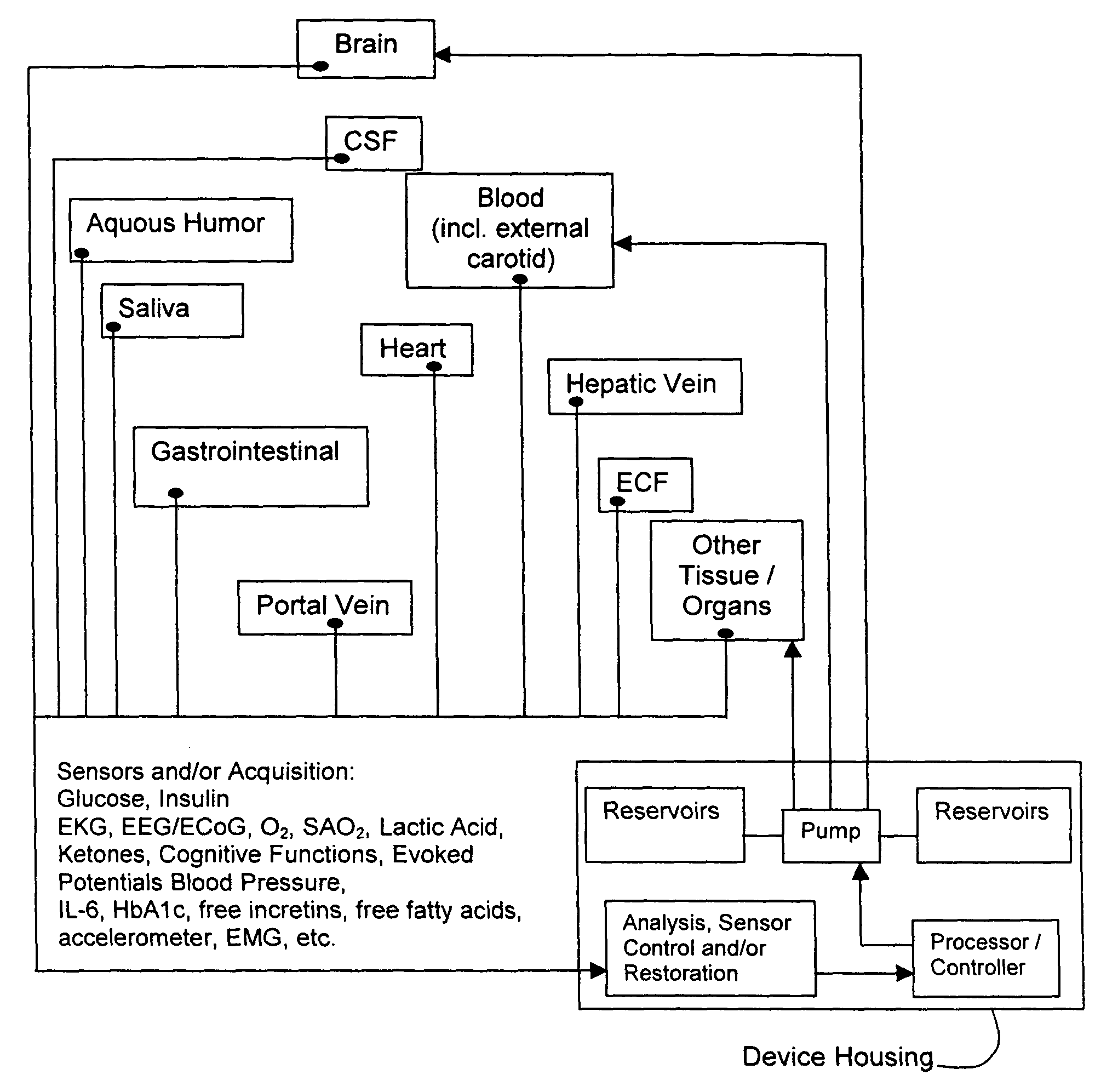
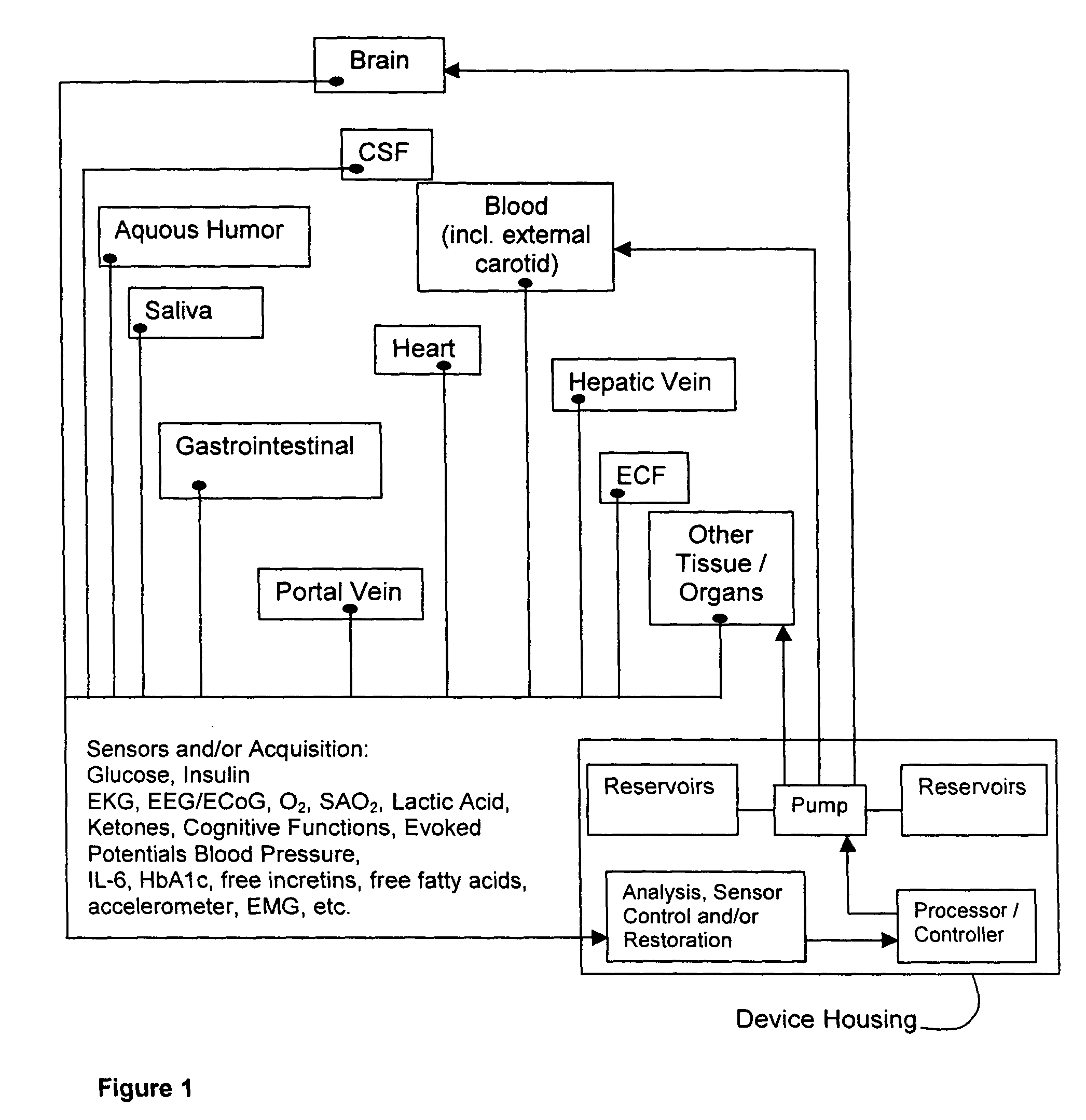
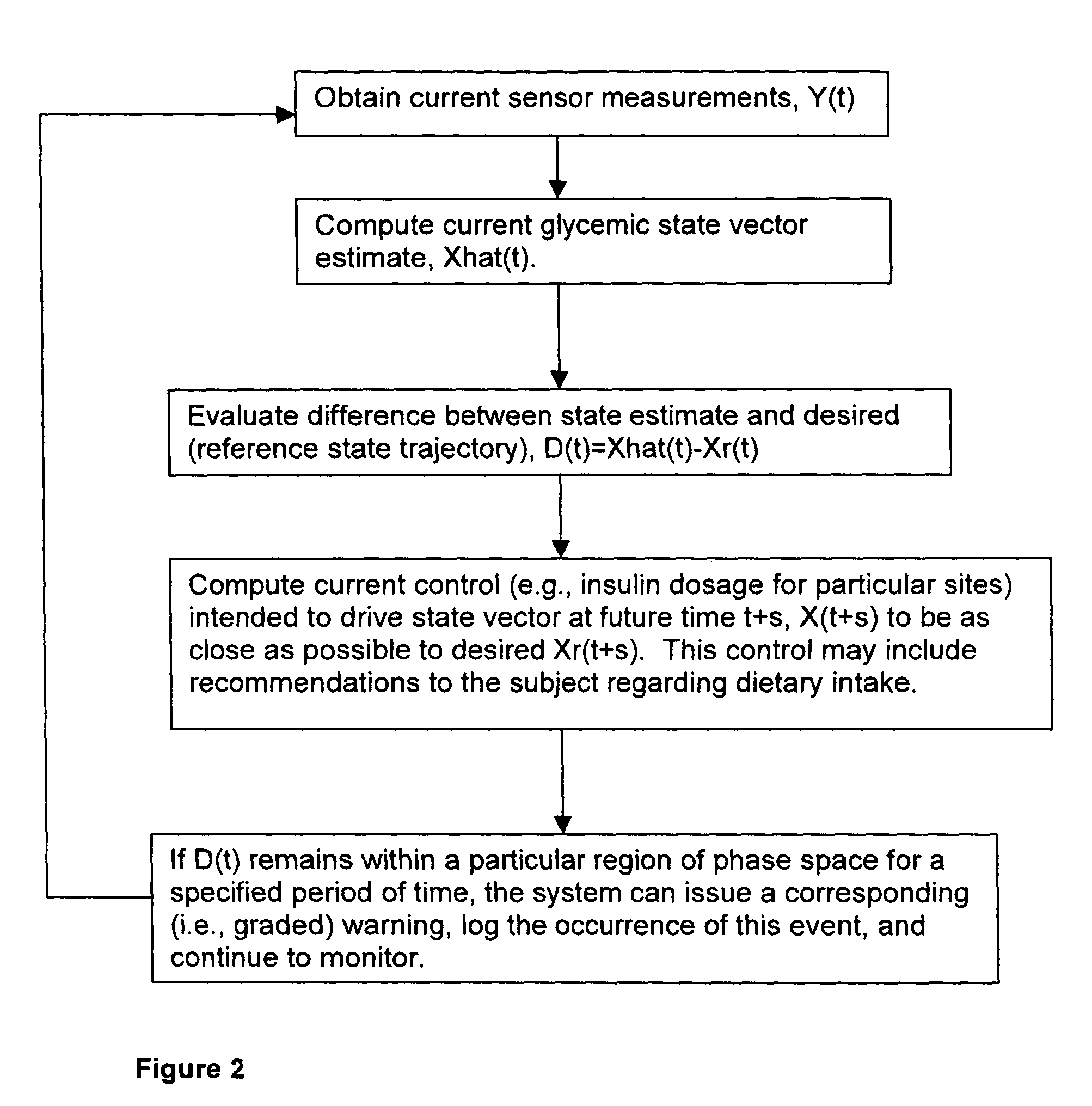
Method and system for implantable glucose monitoring and control of a glycemic state of a subject
ActiveUS7727147B1Preventing and correcting occurrenceImprove performanceMedical devicesDiagnostic recording/measuringTelecommunications linkTransfer mechanism
A method and system for monitoring and / or controlling a glycemic state of a subject, comprising a housing device having one or more chambers, extendable and retractable sensors, extendable and retractable catheters, insulin reservoir, neuroprotective agent reservoir, release mechanism for releasing insulin and neuroprotective agent into the subject, and a control mechanism with a processor for receiving and analyzing outputs from the sensors and for controlling the release mechanism, a clock mechanism for providing logging and / or circadian information to the processor, an internal analysis chamber, a byproduct storage chamber, a sampling mechanism, a transfer mechanism, a self-sealing membrane, a calibration chamber, a replacement sensor chamber, a prevention mechanism to prevent deposition of unwanted substances on a sensor or catheter, a removal mechanism to remove unwanted substances from a sensor or catheter, a warning mechanism, a real-time clock and non-volatile memory to store and log information processed by the processor, a failure detection mechanism, and wired and / or wireless communication linkages both internally and externally.
Owner:FLINT HILLS SCI L L C
Drug-eluting stent cover and method of use
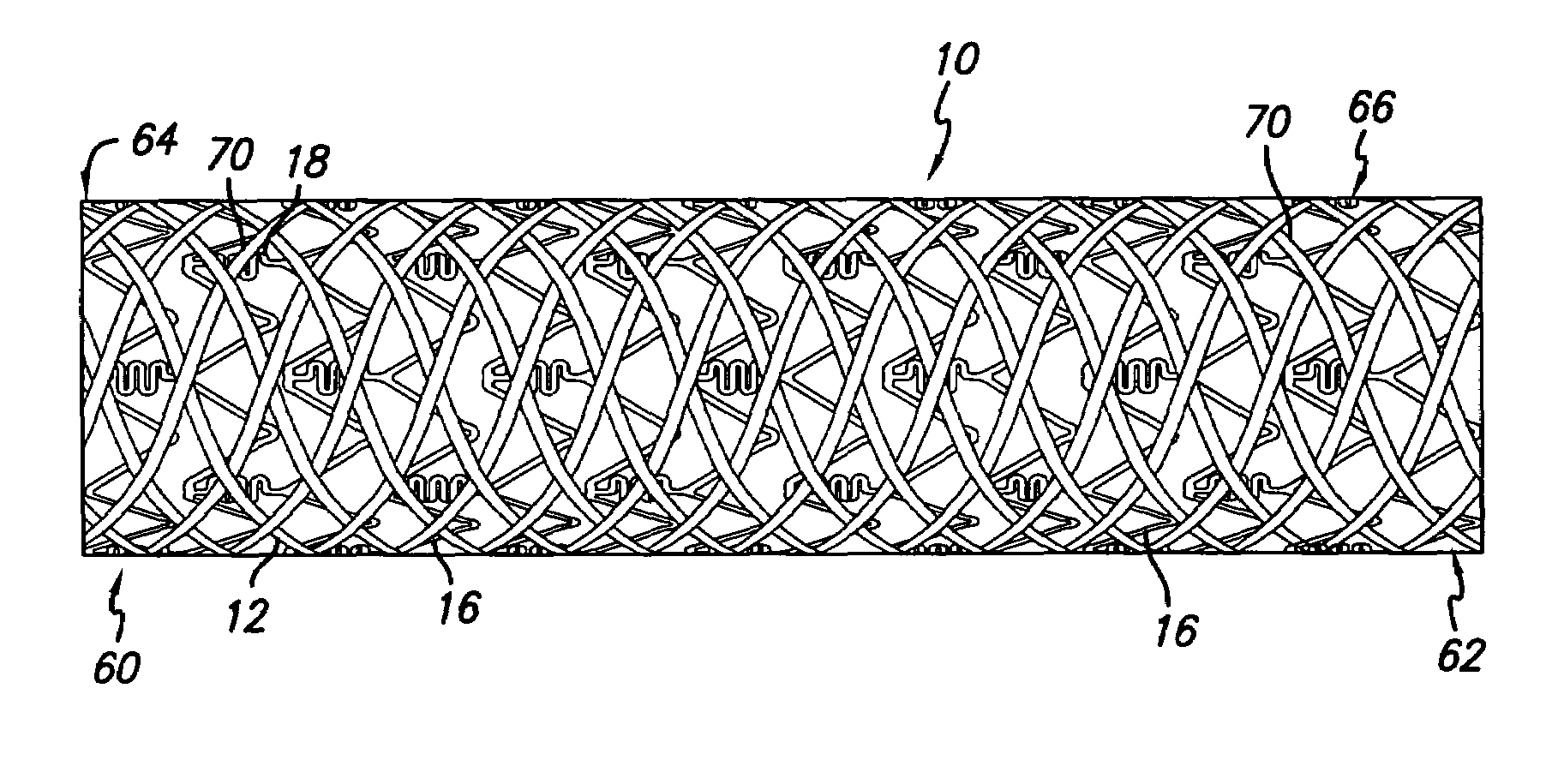
An intravascular stent includes an eluting sheath fabricated from a mesh for controlled release of therapeutic drugs and for delivery of the therapeutic drugs in localized drug therapy in a blood vessel. The eluting sheath is attached to at least a portion of an outside surface area of the stent structure and is fabricated from a mesh designed to neck down in response to a radially outward directed force resulting in the uniform expansion of the stent. The eluting sheath can be loaded with at least one therapeutic drug for the release thereof at a treatment site to facilitate repair of a damaged vessel. The stent has a high degree of flexibility in the longitudinal direction, yet has adequate vessel wall coverage and radial strength sufficient to hold open an artery or other body lumen.
Owner:ABBOTT CARDIOVASCULAR
Implantable elastomeric depot compositions and uses thereof
InactiveUS20050079202A1Reduce dosing frequencyPatient compliance is goodProsthesisDrageesControlled releaseElastomer
Methods and compositions for systemically or locally administering a beneficial agent to a subject are described, and include, for example, implantable elastomeric depot compositions that can be injected into a desired location and which can provide controlled release of a beneficial agent over a prolonged duration of time. The compositions include a biocompatible, elastomeric polymer, a biocompatible solvent having low water miscibility that forms an elastomeric viscous gel with the polymer and limits water uptake by the implant, and a beneficial agent.
Owner:DURECT CORP
Pharmaceutical dosage form
ActiveUS20090202634A1Improved controlled releaseEasy to adjustBiocideOrganic active ingredientsControlled releaseBreaking strength
The invention relates to a pharmaceutical dosage form, preferably with controlled release of a pharmacologically active compound (A) contained therein, the pharmaceutical dosage form very preferably being tamper-resistant and most preferably having a breaking strength B1 of at least 500 N in direction of extension E1 and having a breaking strength B2 of less than 500 N in direction of extension E2.
Owner:GRUNENTHAL GMBH
Plasticized bioerodible controlled delivery system
InactiveUS6372245B1Easy to useExtend posting timeSolution deliveryDrug compositionsControlled releaseSufficient time
A controlled release medicament delivery system comprises a plasticized bioerodible polymer, such as a polyorthoester. Medicament desirably is entrapped in the plasticized polymer. The resulting delivery system is able to release the medicament in a controlled and sustained manner. The formulation is particularly advantageous for use as a once-a-day eyedrop. During preparation, the polymer may be heated to an elevated temperature for a sufficient time to substantially reduce its molecular weight.
Owner:INSITE VISION
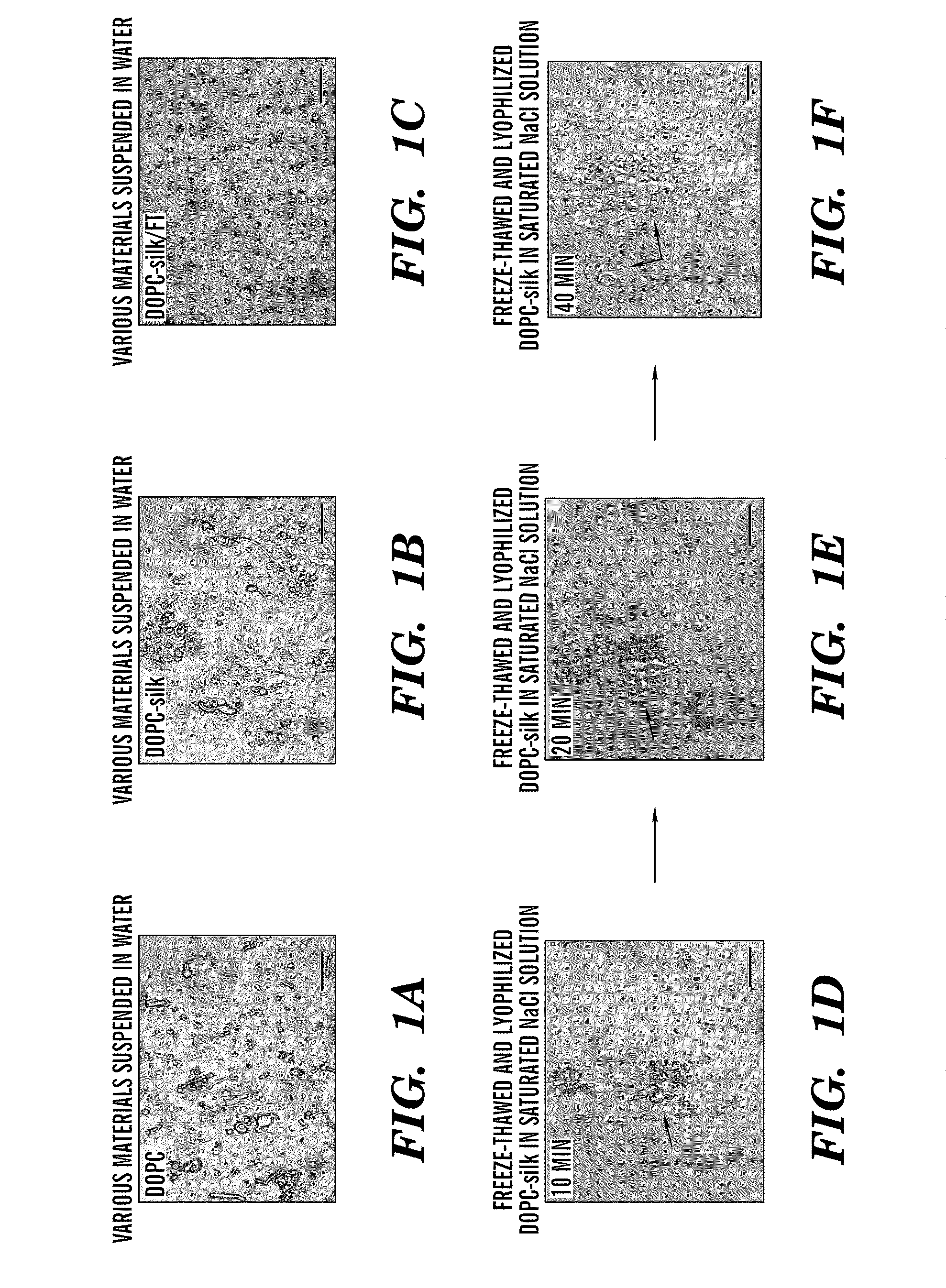
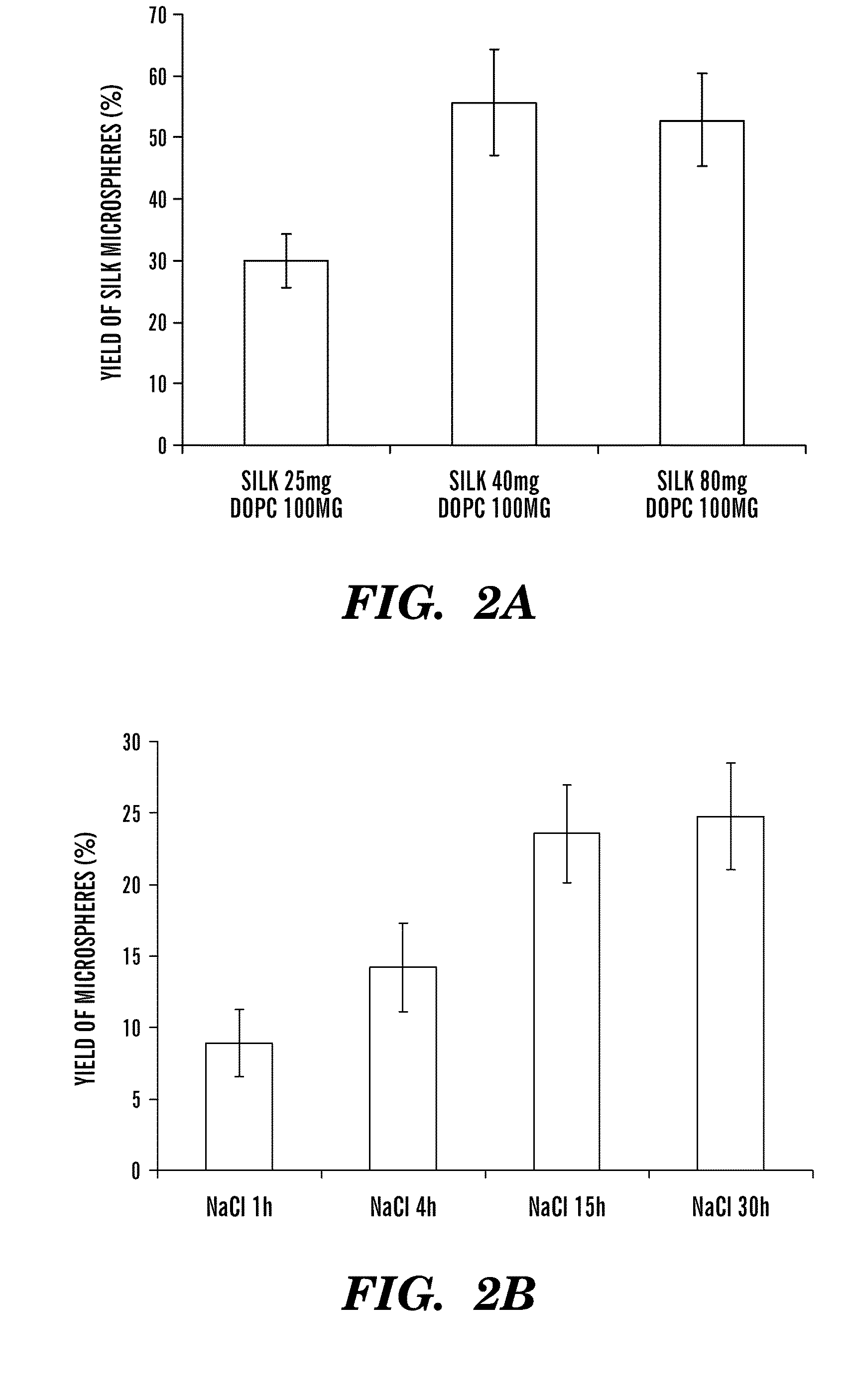
Silk microspheres for encapsulation and controlled release
A method was developed to prepare silk fibroin microspheres using lipid vesicles as templates to efficiently load therapeutic agents in active form for controlled release. The lipids are subsequently removed through the use of a dehydration agent, such as methanol or sodium chloride, resulting in β-sheet structure dominant silk microsphere structures having about 2 μm in diameter. The therapeutic agent can be entrapped in the silk microspheres and used in pharmaceutical formulations for controlled-release treatments.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Abuse resistant drug formulation
A pharmaceutical composition may include a coated particulate which may include at least one active pharmaceutical ingredient, particularly one susceptible to abuse by an individual. The coated particles may include a fat / wax and have improved controlled release and / or crush resistance. Method of making these coated particulate and dosage forms therewith are also described.
Owner:CIMA LABS
Photopolymerizable biodegradable hydrogels as tissue contacting materials and controlled-release carriers
InactiveUS6306922B1Fast gelationRapid polymerizationImmobilised enzymesPowder deliveryThermal energyUltraviolet lights
Hydrogels of polymerized and crosslinked macromers comprising hydrophilic oligomers having biodegradable monomeric or oligomeric extensions, which biodegradable extensions are terminated on free ends with end cap monomers or oligomers capable of polymerization and cross linking are described. The hydrophilic core itself may be degradable, thus combining the core and extension functions. Macromers are polymerized using free radical initiators under the influence of long wavelength ultraviolet light, visible light excitation or thermal energy. Biodegradation occurs at the linkages within the extension oligomers and results in fragments which are non-toxic and easily removed from the body. Preferred applications for the hydrogels include prevention of adhesion formation after surgical procedures, controlled release of drugs and other bioactive species, temporary protection or separation of tissue surfaces, adhering of sealing tissues together, and preventing the attachment of cells to tissue surfaces.
Owner:BOARD OF REGENTS
Controlled release polymer nanoparticle containing bound nucleic acid ligand for targeting
The present invention relates to a conjugate that includes a nucleic acid ligand bound to a controlled release polymer system, a pharmaceutical composition that contains the conjugate, and methods of treatment using the conjugate. The controlled release polymer system includes an agent such as a therapeutic, diagnostic, prognostic, or prophylactic agent. The nucleic acid ligand that is bound to the controlled release polymer system, binds selectively to a target, such as a cell surface antigen, and thereby delivers the controlled release polymer system to the target.
Owner:MASSACHUSETTS INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com