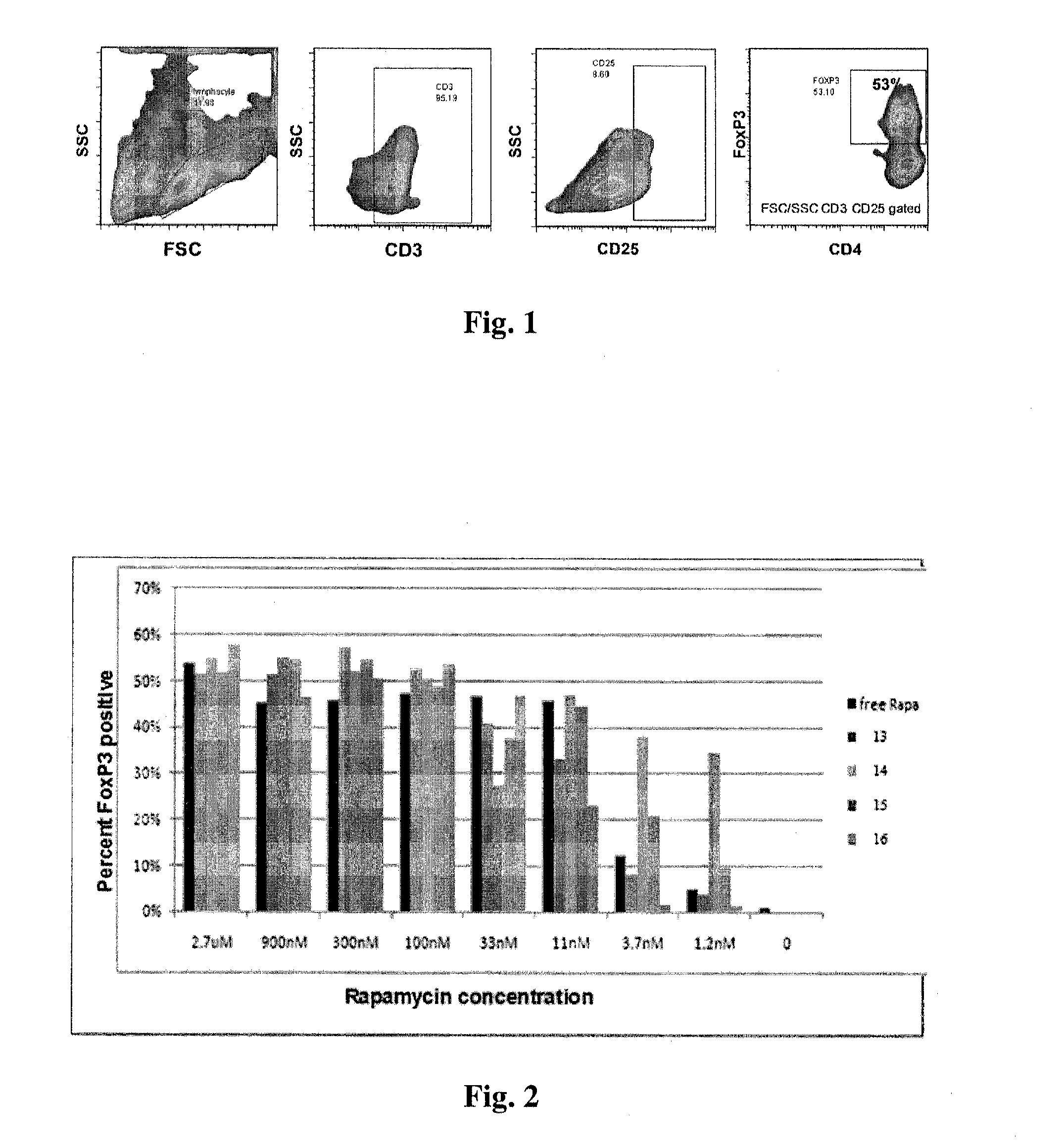
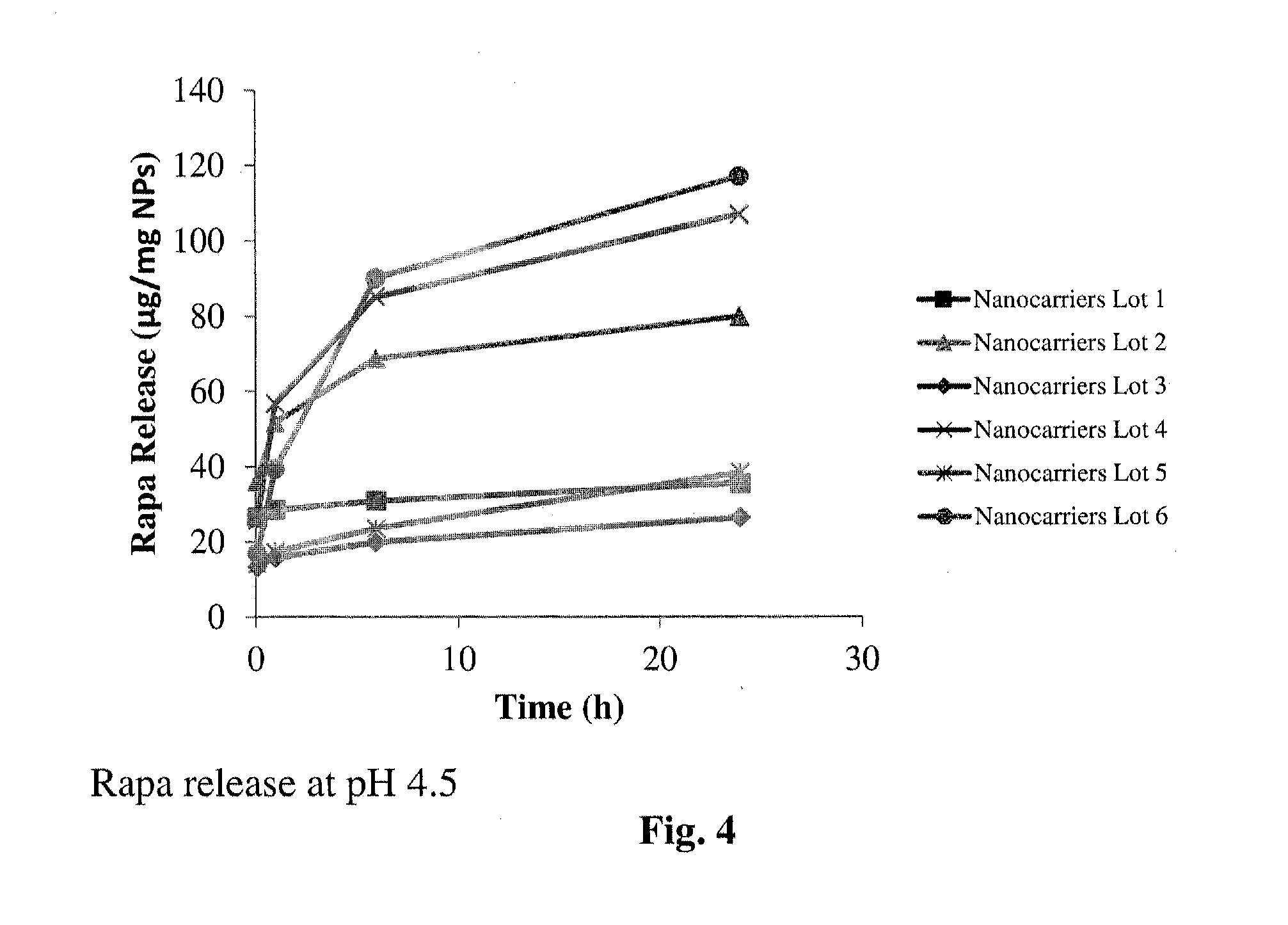
Controlled release of immunosuppressants from synthetic nanocarriers
a technology of synthetic nanocarriers and immunosuppressants, which is applied in the direction of liposome delivery, peptide/protein ingredients, powder delivery, etc., can solve the problems of insufficient information regarding the optimal release of agents from delivery vehicles, the risk of severe side effects, tumors, etc., and achieve the effect of reducing the undesired immune respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
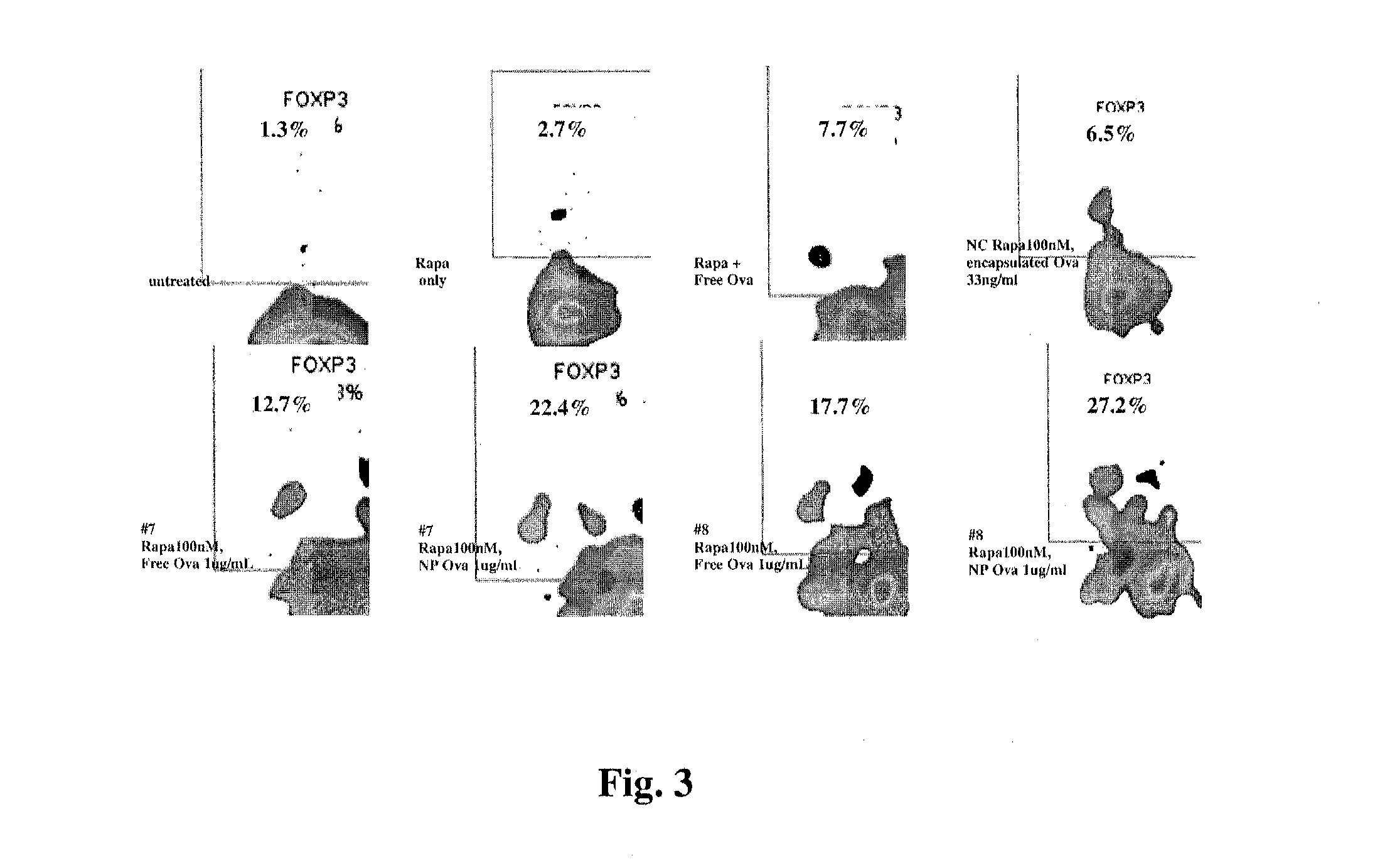
Image
Examples
example 1
Mesoporous Silica Nanoparticles with Coupled Ibuprofen (Prophetic)
[0244]Mesoporous SiO2 nanoparticle cores are created through a sol-gel process. Hexadecyltrimethyl-ammonium bromide (CTAB) (0.5 g) is dissolved in deionized water (500 mL), and then 2 M aqueous NaOH solution (3.5 mL) is added to the CTAB solution. The solution is stirred for 30 min, and then Tetraethoxysilane (TEOS) (2.5 mL) is added to the solution. The resulting gel is stirred for 3 h at a temperature of 80° C. The white precipitate which forms is captured by filtration, followed by washing with deionized water and drying at room temperature. The remaining surfactant is then extracted from the particles by suspension in an ethanolic solution of HCl overnight. The particles are washed with ethanol, centrifuged, and redispersed under ultrasonication. This wash procedure is repeated two additional times.
[0245]The SiO2 nanoparticles are then functionalized with amino groups using (3-aminopropyl)-triethoxysilane (APTMS)....
example 2
Liposomes Containing Cyclosporine A (Prophetic)
[0248]The liposomes are formed using thin film hydration. 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) (32 μmol), cholesterol (32 μmol), and cyclosporin A (6.4 μmol) are dissolved in pure chloroform (3 mL). This lipid solution is added to a 50 mL round-bottom flask, and the solvent is evaporated on a rotary evaporator at a temperature of 60° C. The flask is then flushed with nitrogen gas to remove remaining solvent. Phosphate buffered saline (2 mL) and five glass beads are added to the flask, and the lipid film is hydrated by shaking at 60° C. for 1 h to form a suspension. The suspension is transferred to a small pressure tube and sonicated at 60° C. for four cycles of 30s pulses with a 30 s delay between each pulse. The suspension is then left undisturbed at room temperature for 2 h to allow for complete hydration. The liposomes are washed by centrifugation followed by resuspension in fresh phosphate buffered saline.
example 3
Polymeric Nanocarrier Containing Polymer-Rapamycin Conjugate
(Prophetic)
[0249]Preparation of PLGA-Rapamycin Conjugate
[0250]PLGA polymer with acid end group (7525 DLG1A, acid number 0.46 mmol / g, Lakeshore Biomaterials; 5 g, 2.3 mmol, 1.0 eq) is dissolved in 30 mL of dichloromethane (DCM). N,N-Dicyclohexylcarbodimide (1.2 eq, 2.8 mmol, 0.57 g) is added followed by rapamycin (1.0 eq, 2.3 mmol, 2.1 g) and 4-dimethylaminopyridine (DMAP) (2.0 eq, 4.6 mmol, 0.56 g). The mixture is stirred at rt for 2 days. The mixture is then filtered to remove insoluble dicyclohexylurea. The filtrate is concentrated to ca. 10 mL in volume and added to 100 mL of isopropyl alcohol (IPA) to precipitate out the PLGA-rapamycin conjugate. The IPA layer is removed and the polymer is then washed with 50 mL of IPA and 50 mL of methyl t-butyl ether (MTBE). The polymer is then dried under vacuum at 35 C for 2 days to give PLGA-rapamycin as a white solid (ca. 6.5 g).
[0251]Preparation of nanocarrier containing PLGA-rap...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wt % | aaaaa | aaaaa |
| weight average molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com