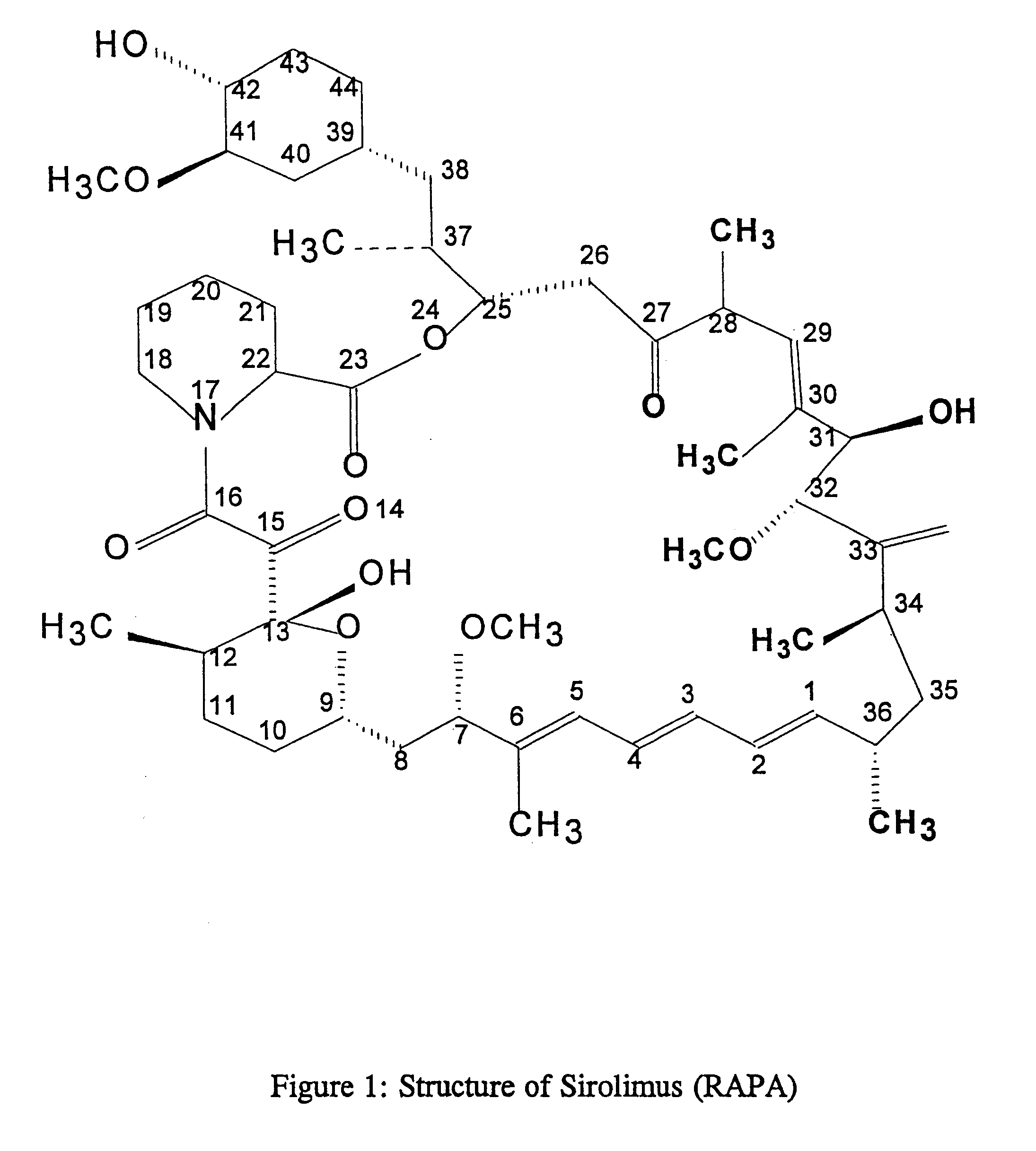
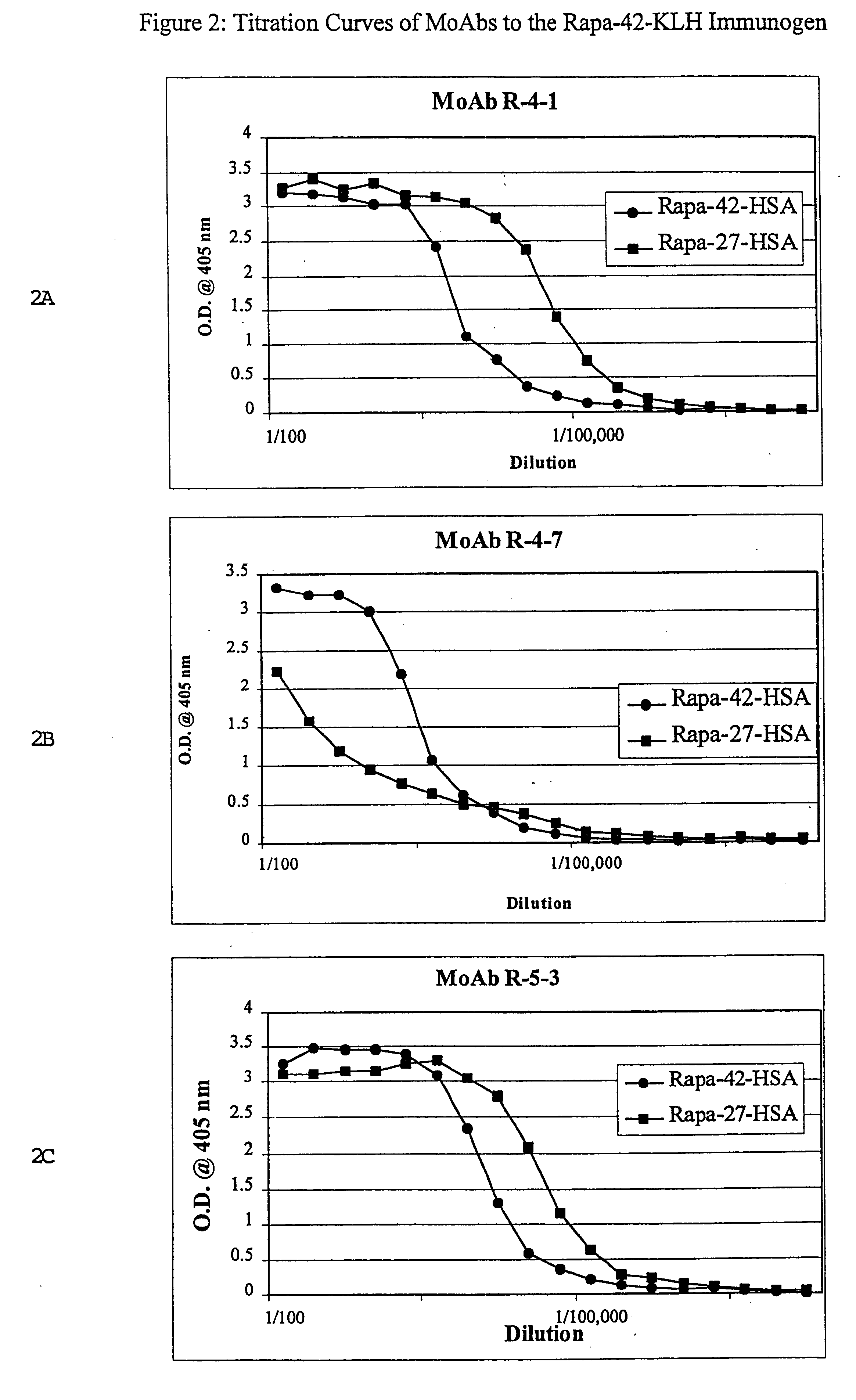
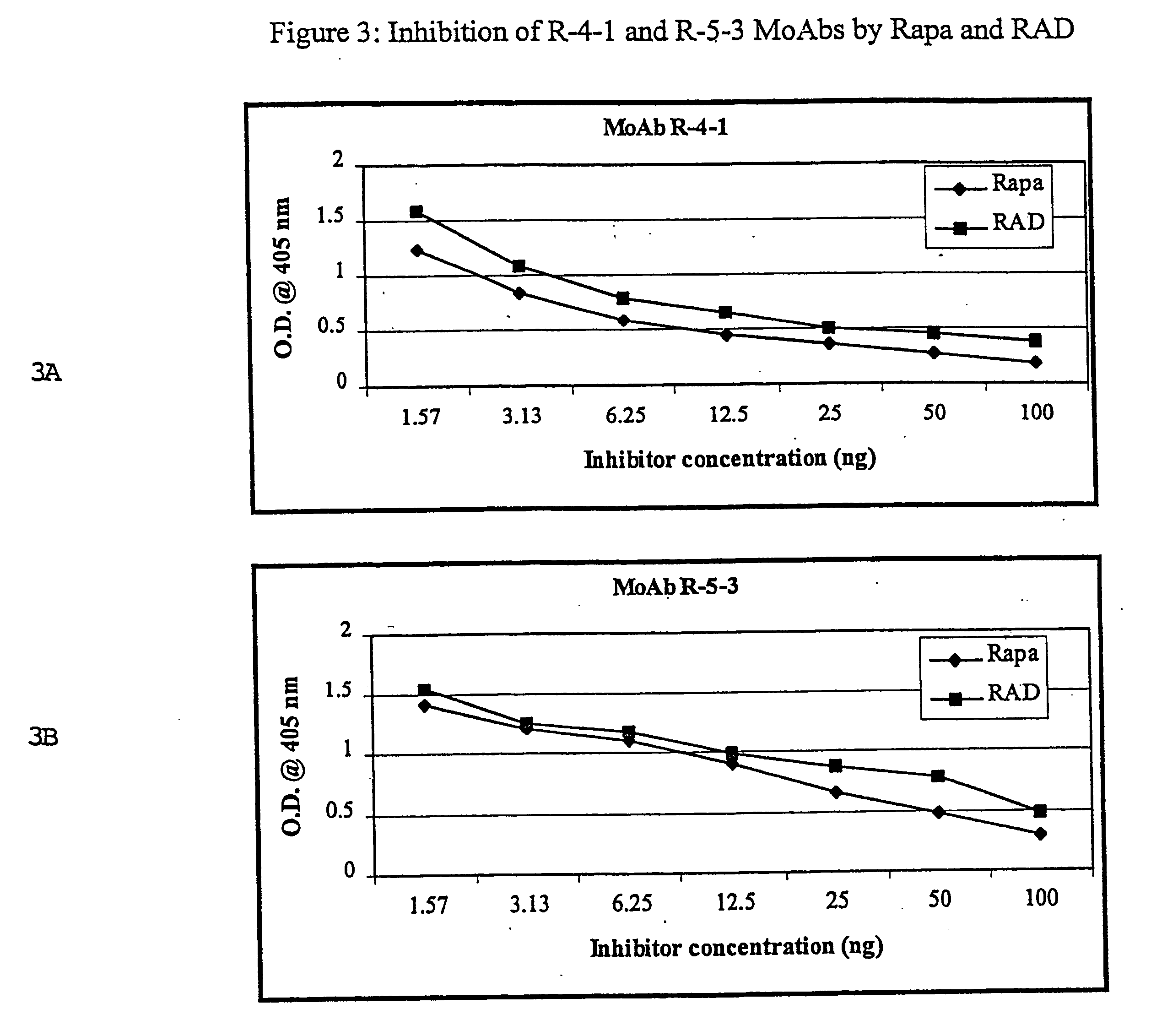
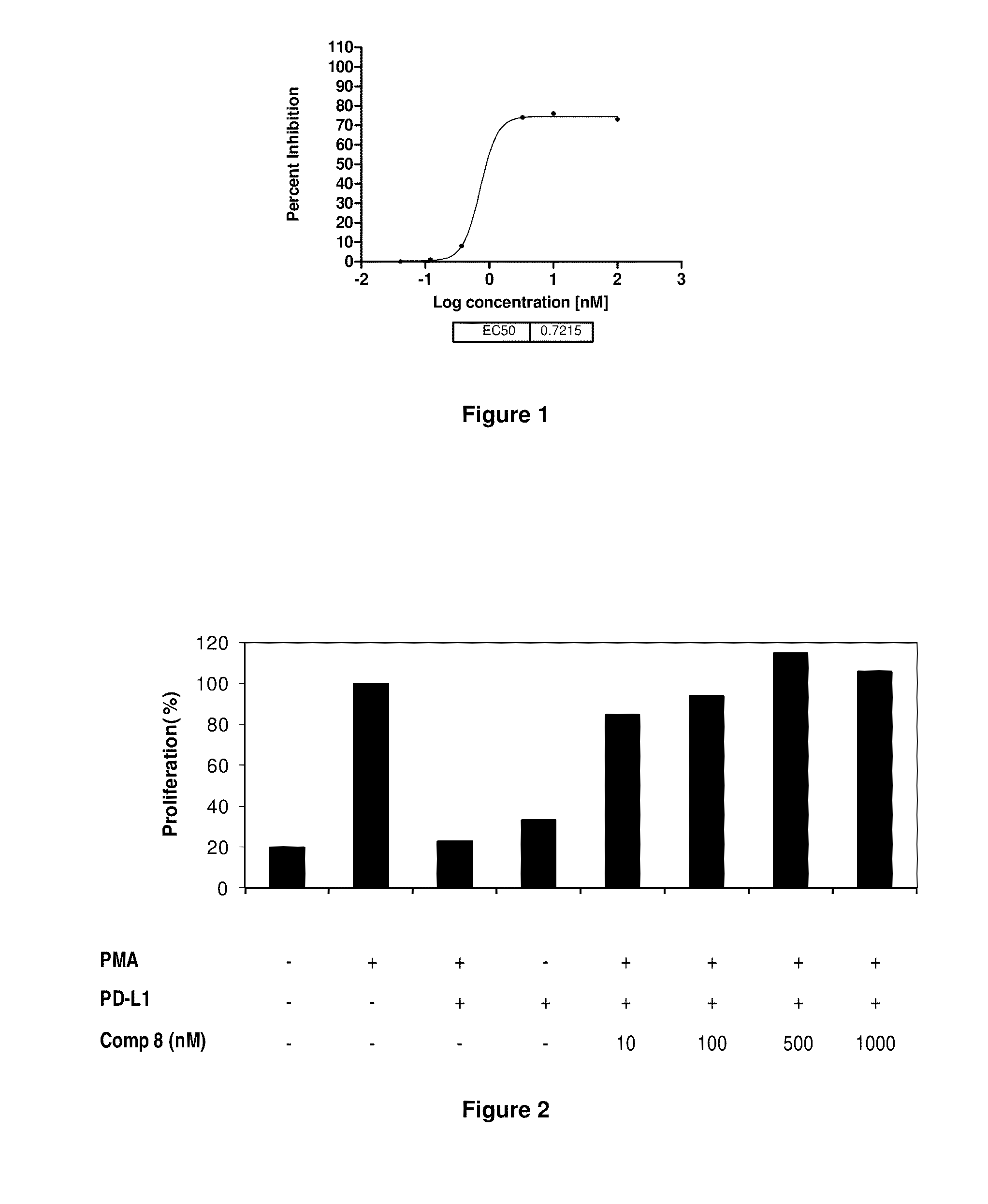
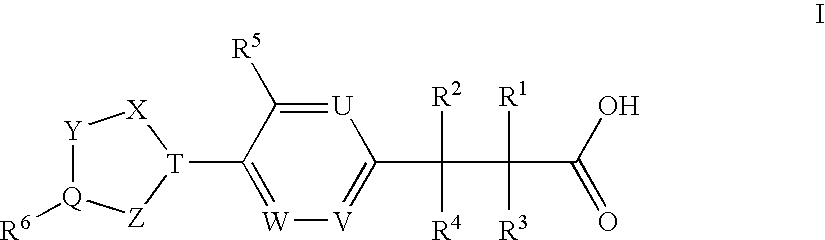
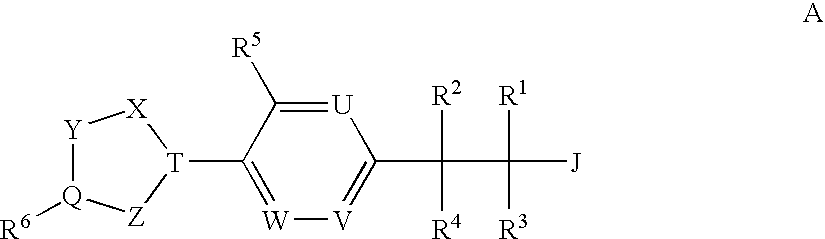
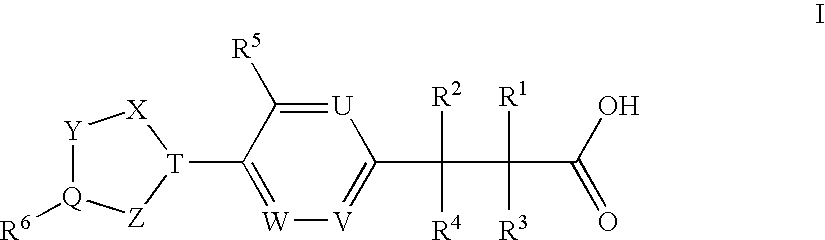
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1593 results about "Immunosuppression" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunosuppression is a reduction of the activation or efficacy of the immune system. Some portions of the immune system itself have immunosuppressive effects on other parts of the immune system, and immunosuppression may occur as an adverse reaction to treatment of other conditions.
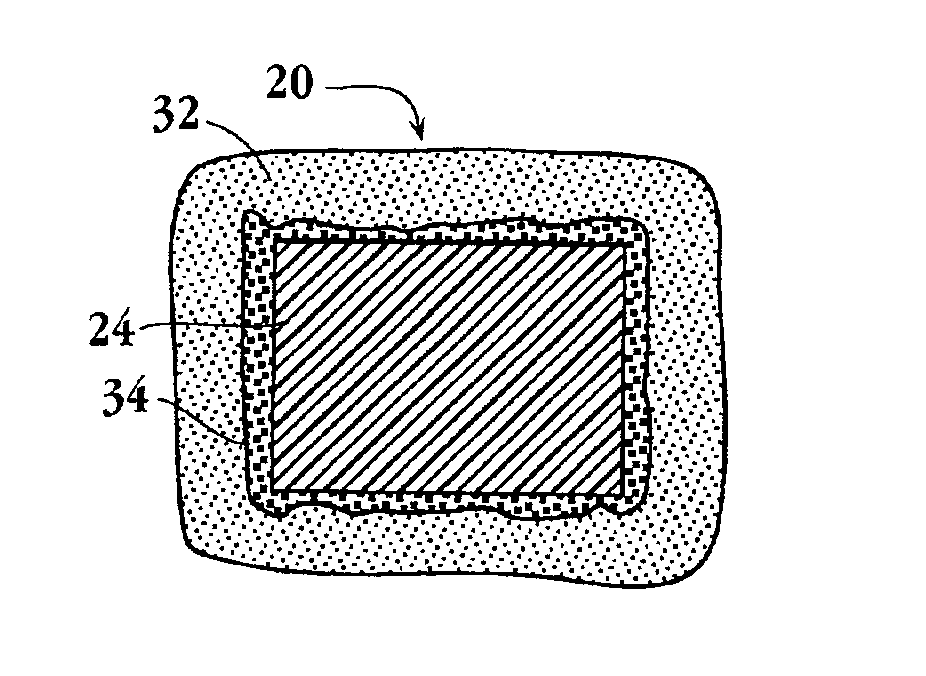
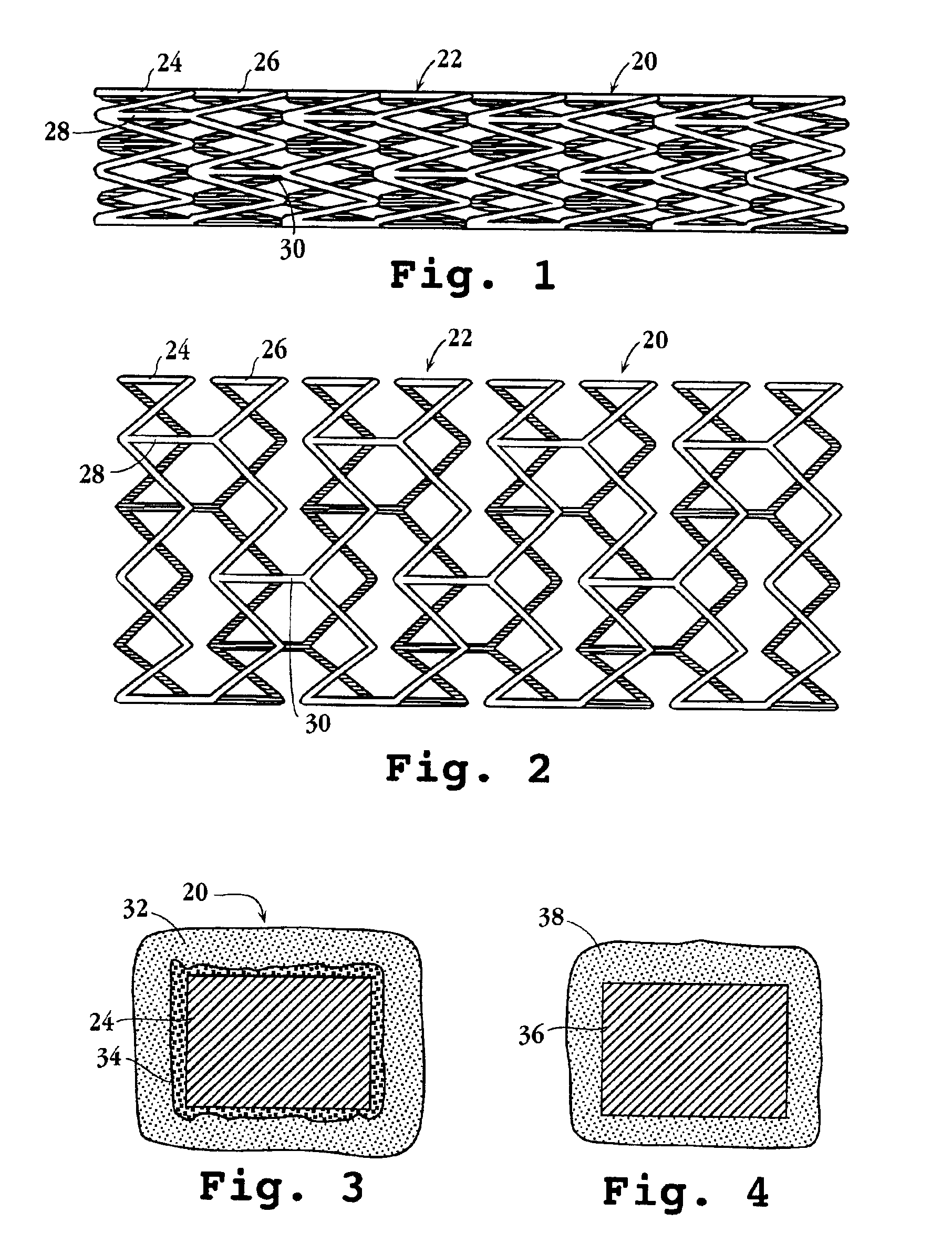
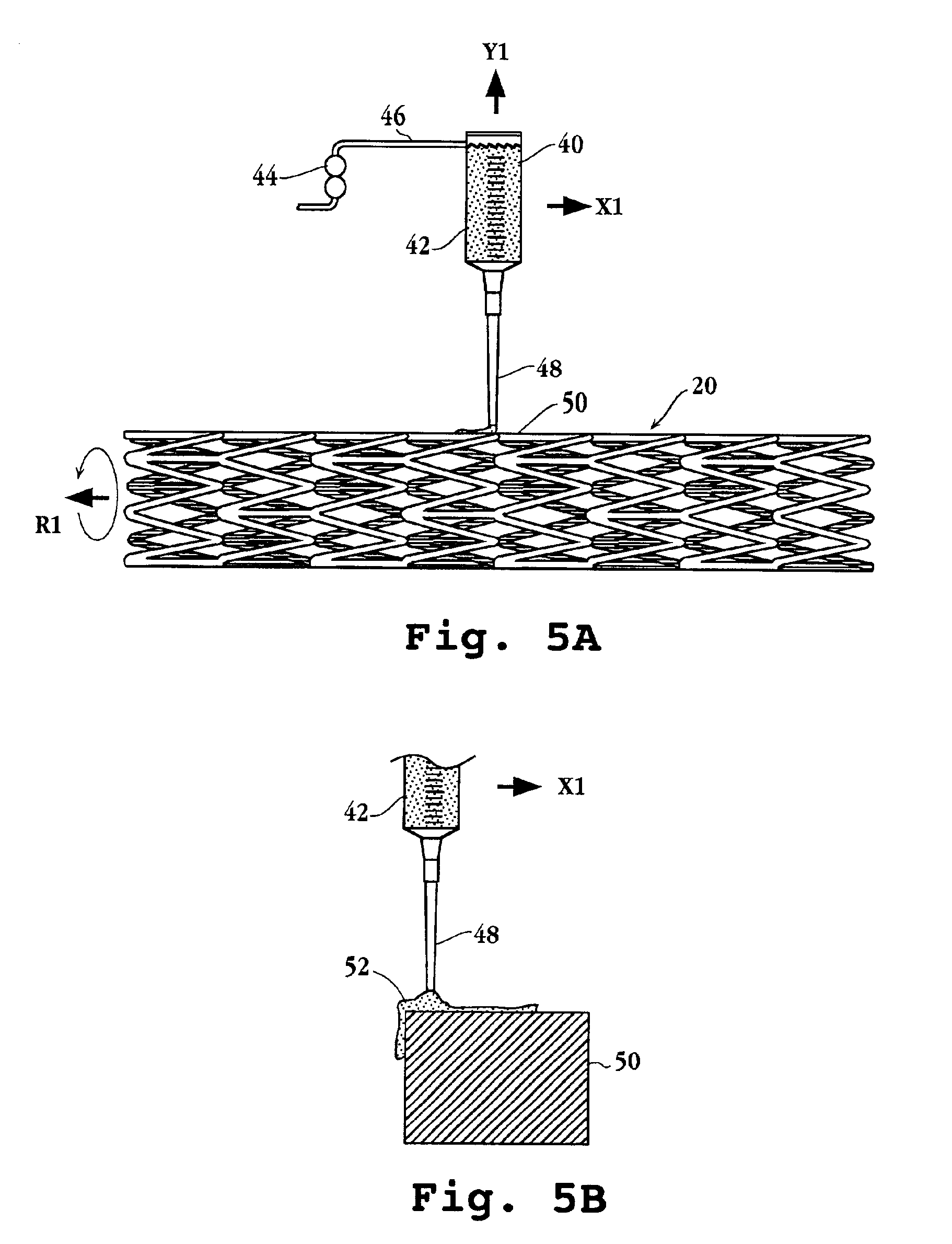
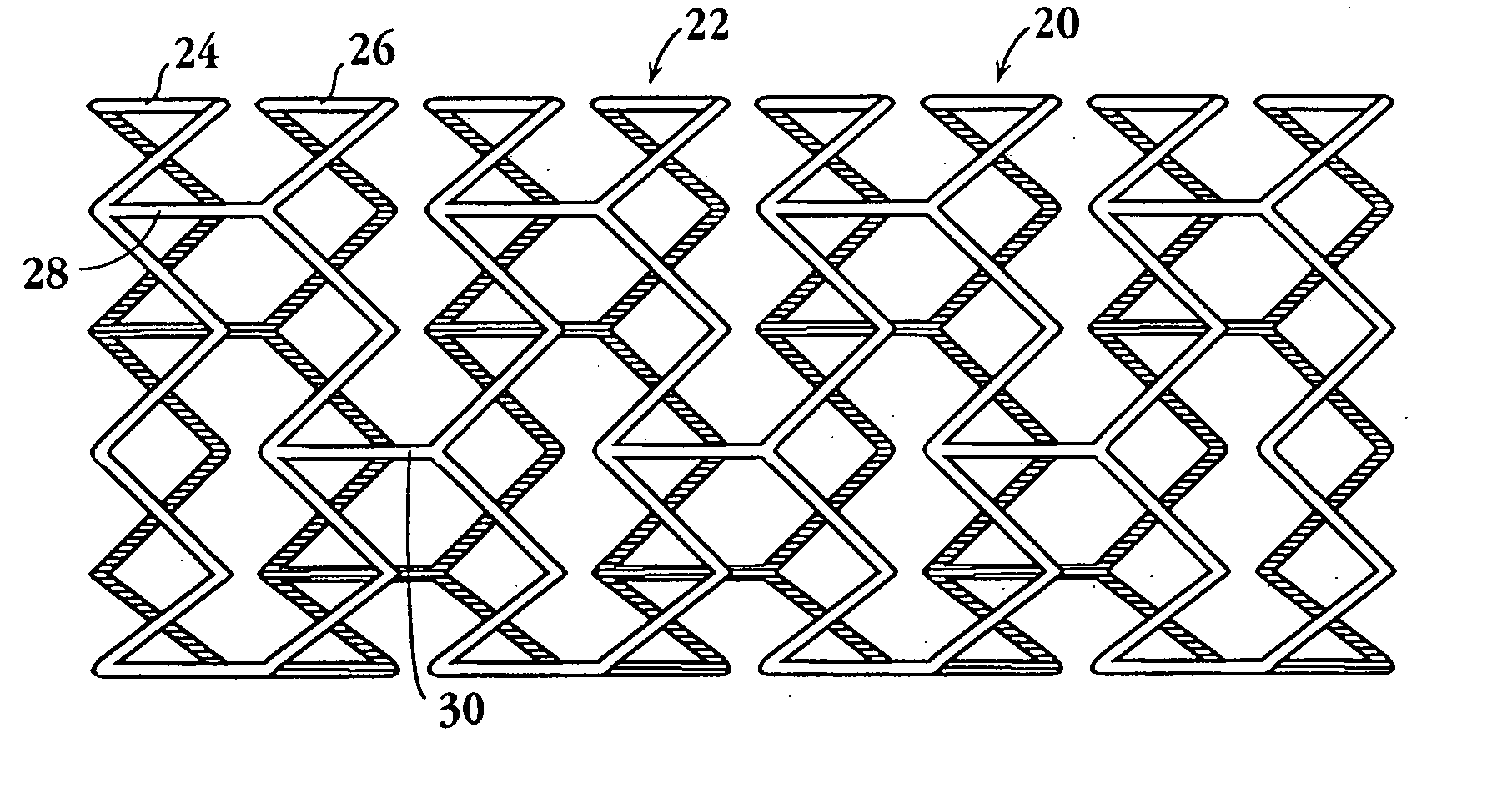
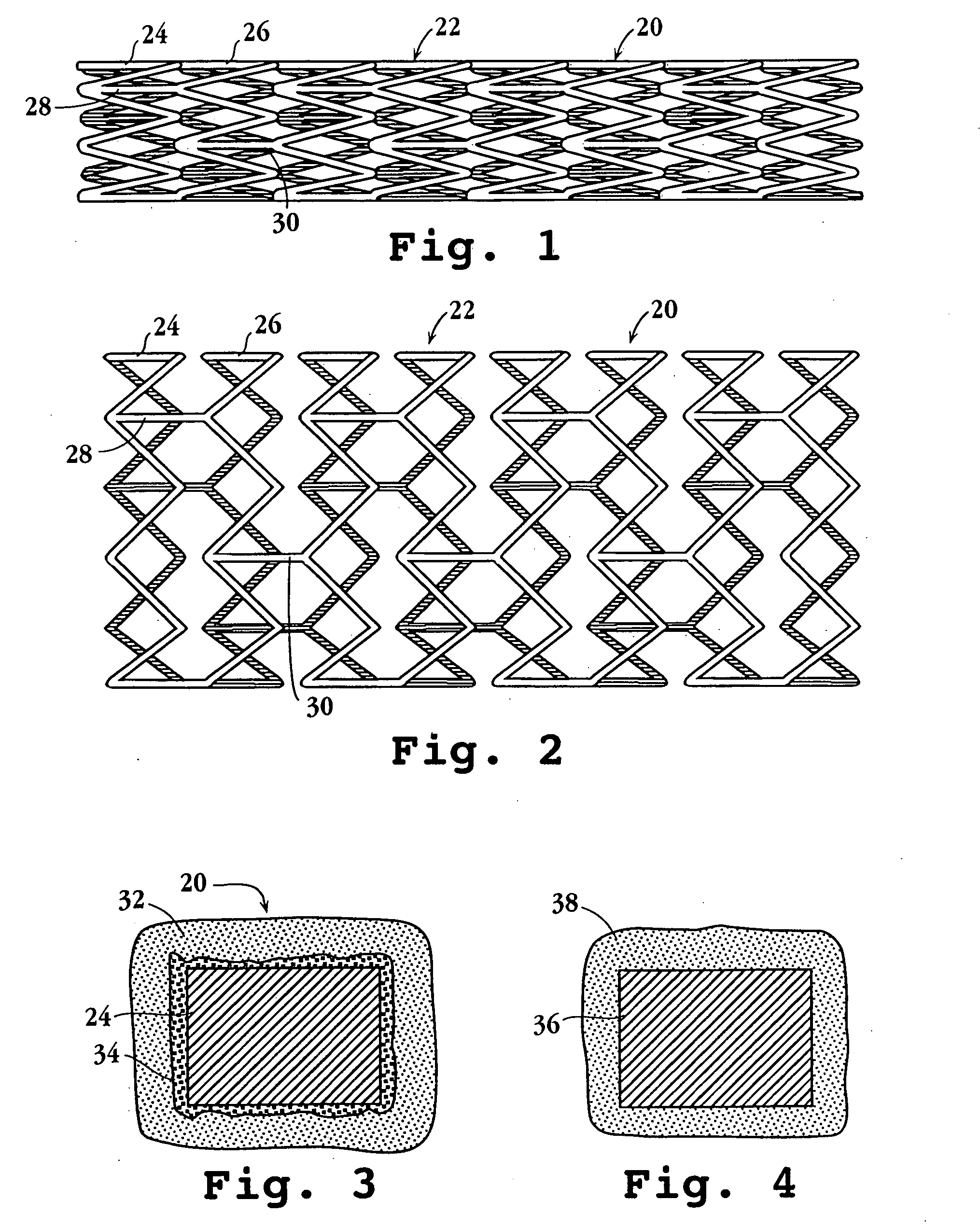
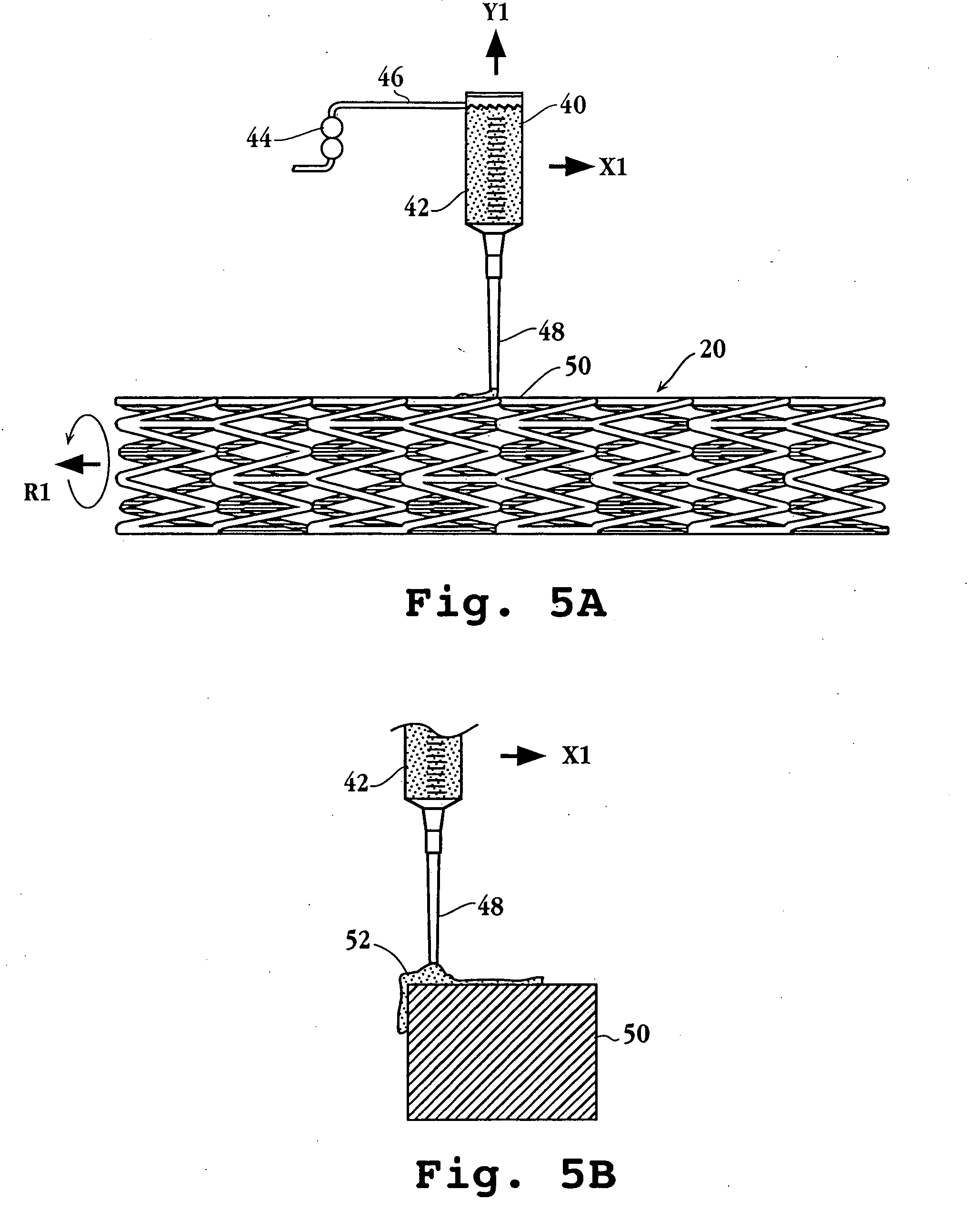
Drug-delivery endovascular stent and method for treating restenosis
InactiveUS6939376B2Efficient releaseOrganic active ingredientsOrganic chemistryRestenosisPoly dl lactide
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-dl-lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
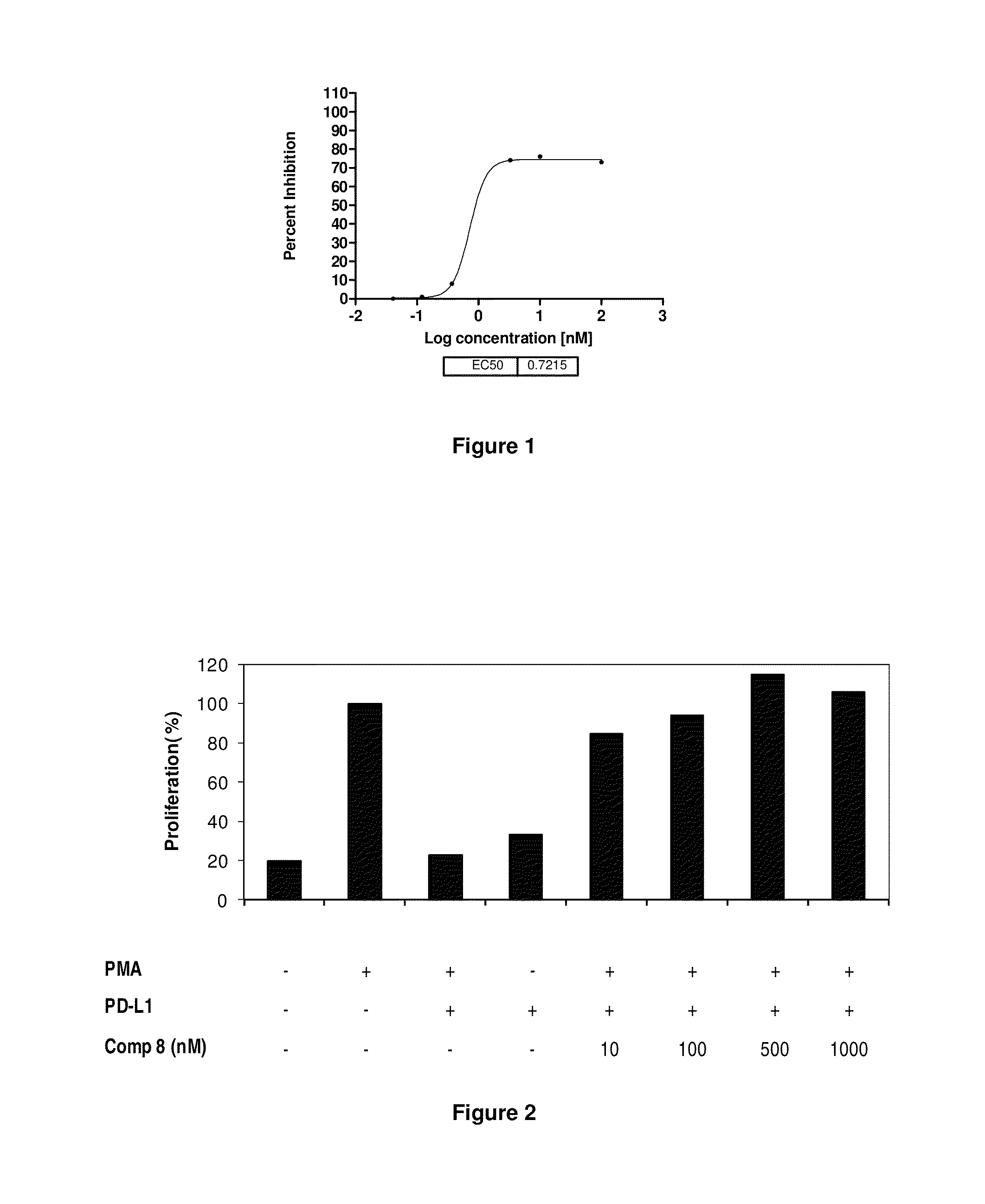
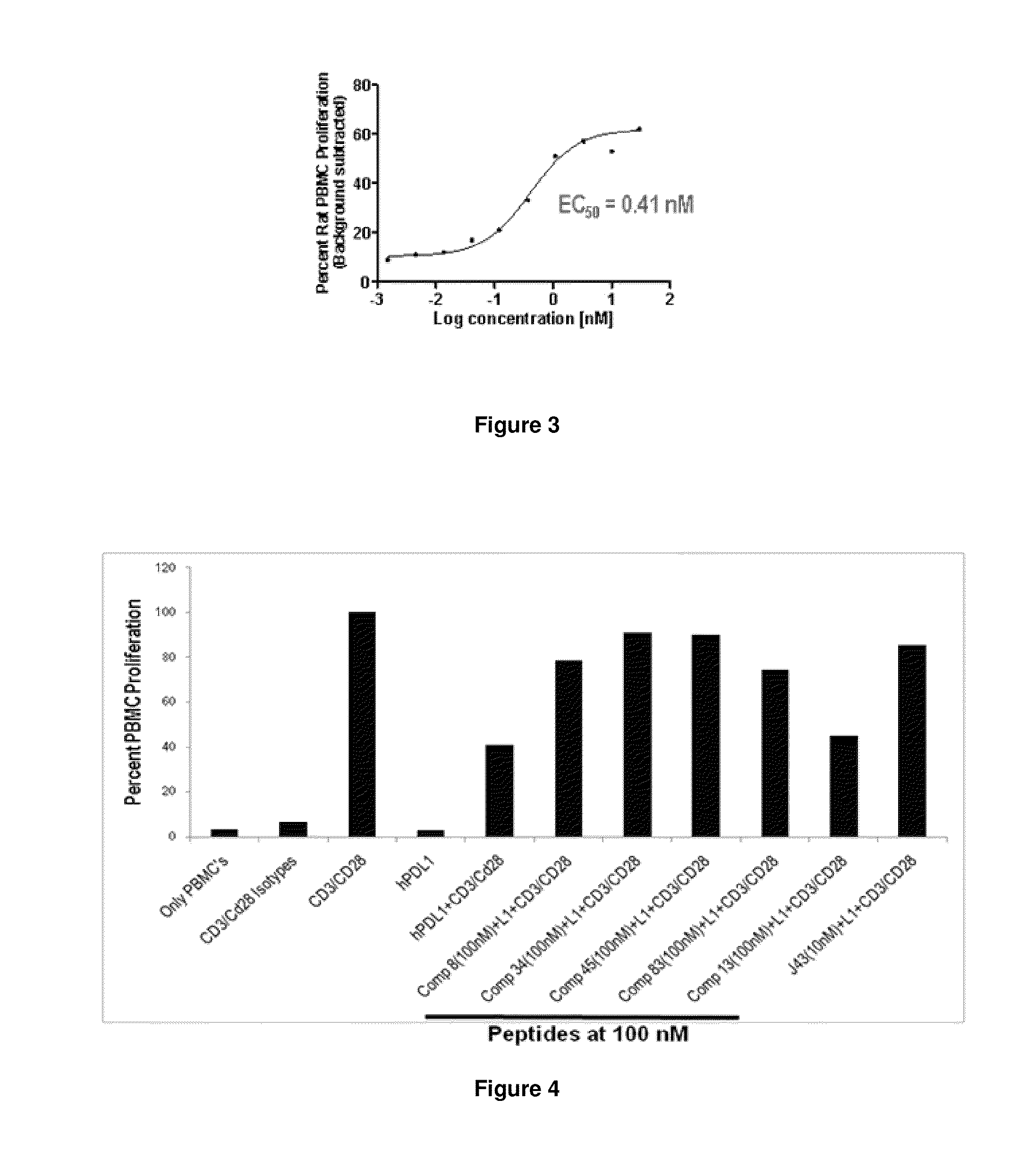
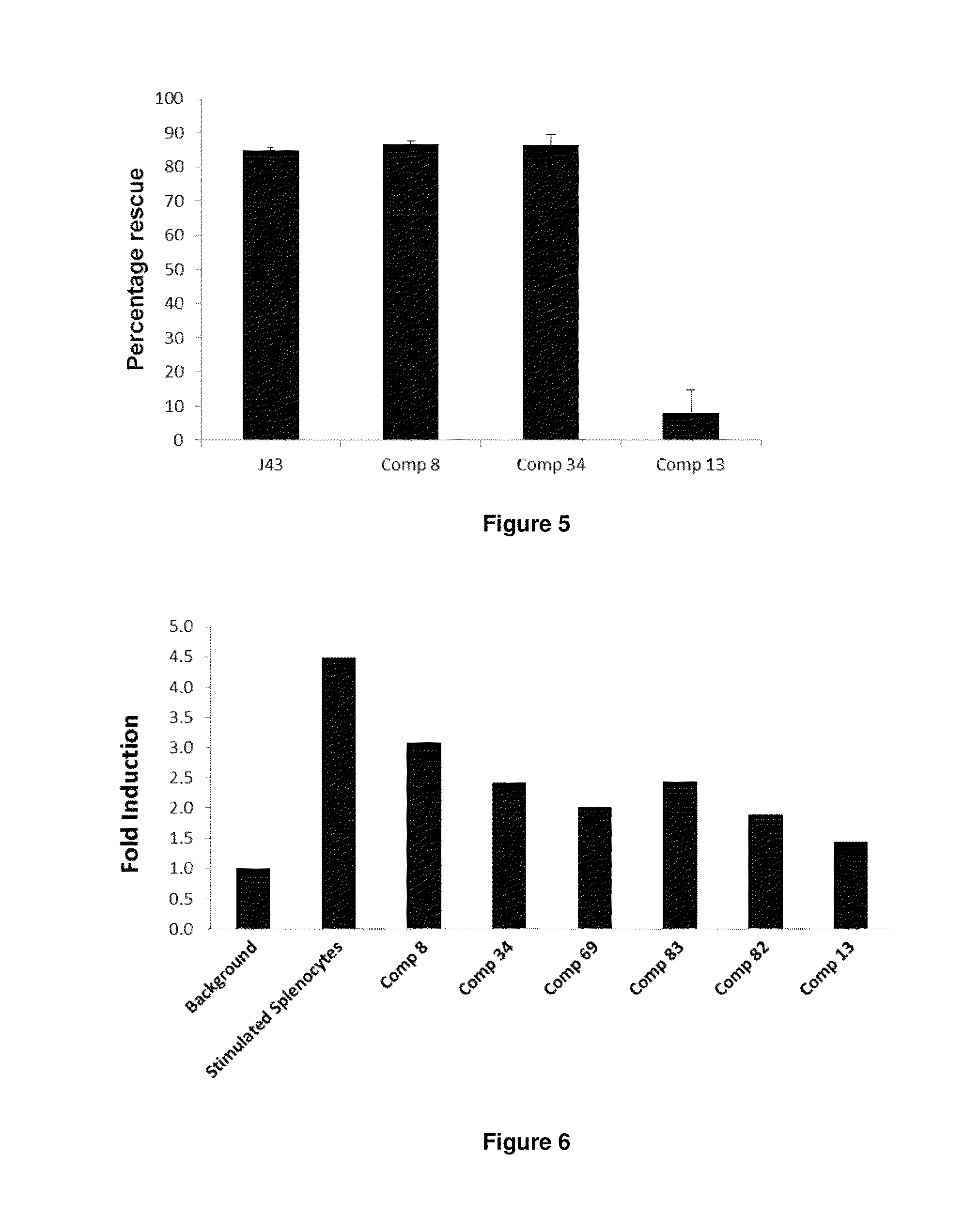
Immunosuppression modulating compounds
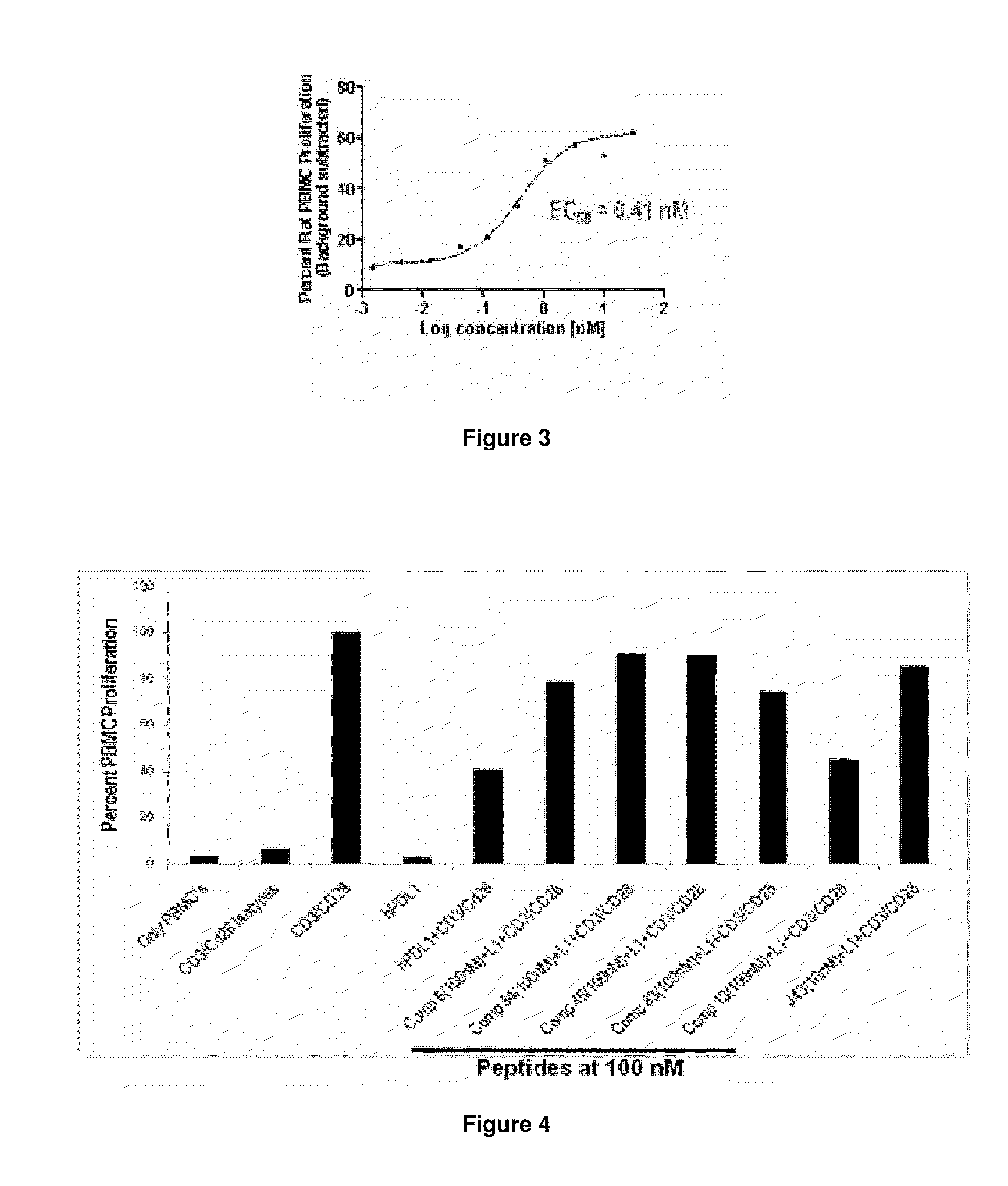
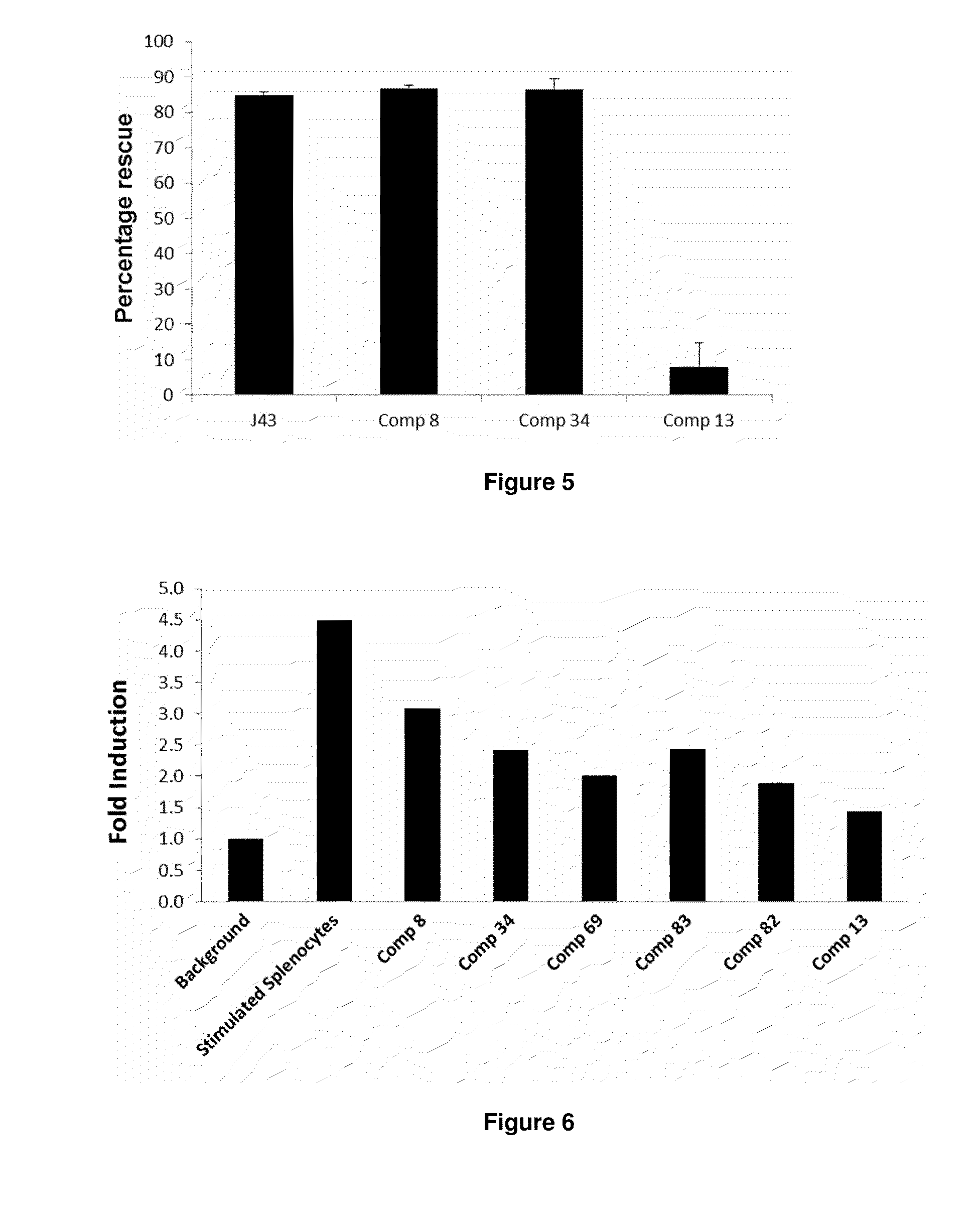
The present invention provides immunosuppression compounds capable of inhibiting the programmed cell death 1 (PD1) signalling pathway. The present invention further provides peptide based compositions for treatment of cancer or treatment of infections via immunopotentiation caused by inhibition of immunosuppressive signaling induced by PD-1, PD-L1, or PD-L2 and therapies using them, immunopotentiative substrates included as the active ingredient. Further, the invention provides an application of the compositions containing the peptide moieties for preventive and / or therapeutic agents for cancer, cancer metastasis, immunodeficiency, an infectious disease or the like and an application of peptide moieties as a testing or diagnostic agent or a research agent for such a disease.
Owner:AURIGENE DISCOVERY TECH
Drug-delivery endovascular stent and method of forming the same
ActiveUS20050038505A1Prevent restenosisEfficient releaseOrganic active ingredientsOrganic chemistryPolymer substrateInsertion stent
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-d / -lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
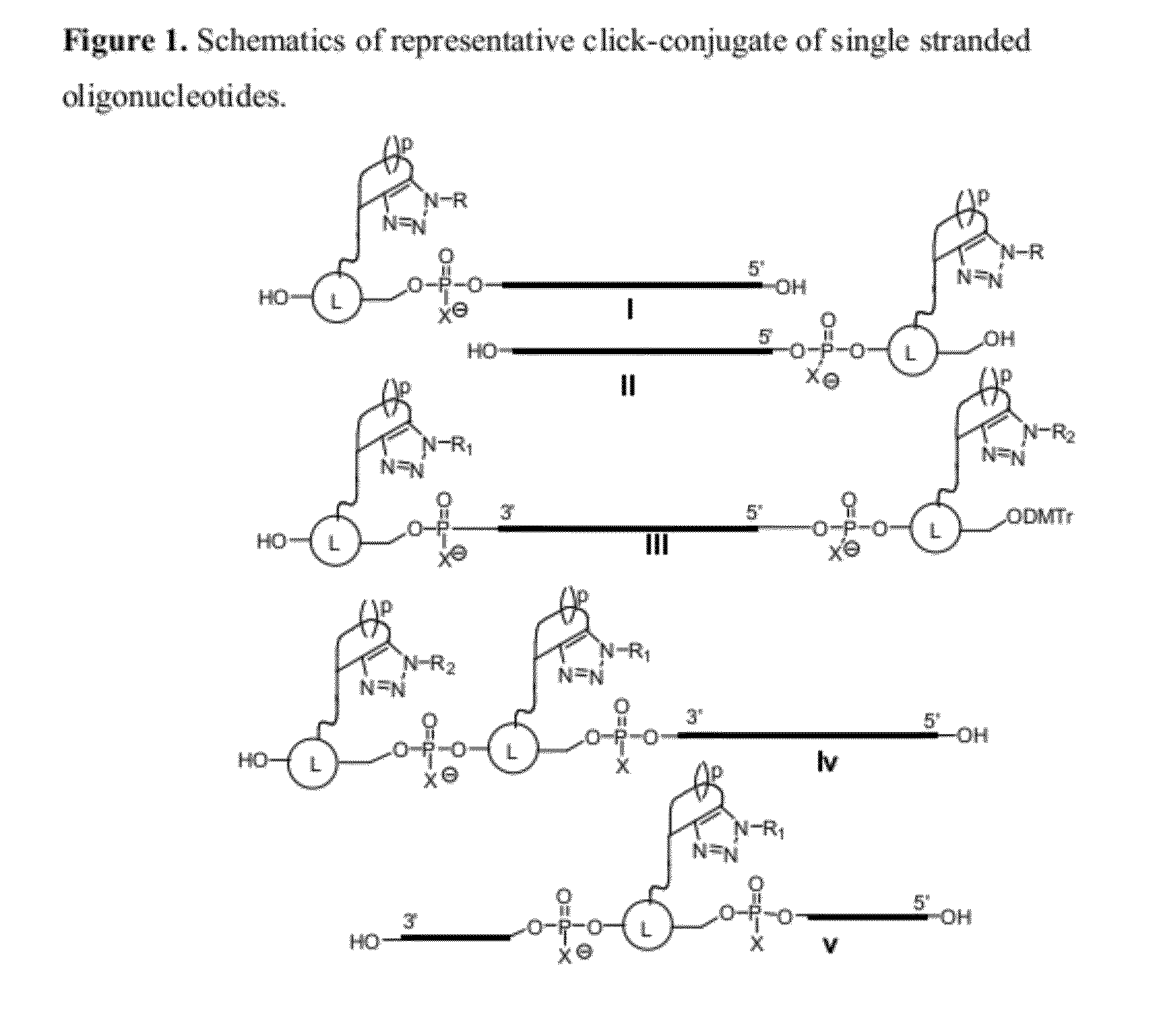
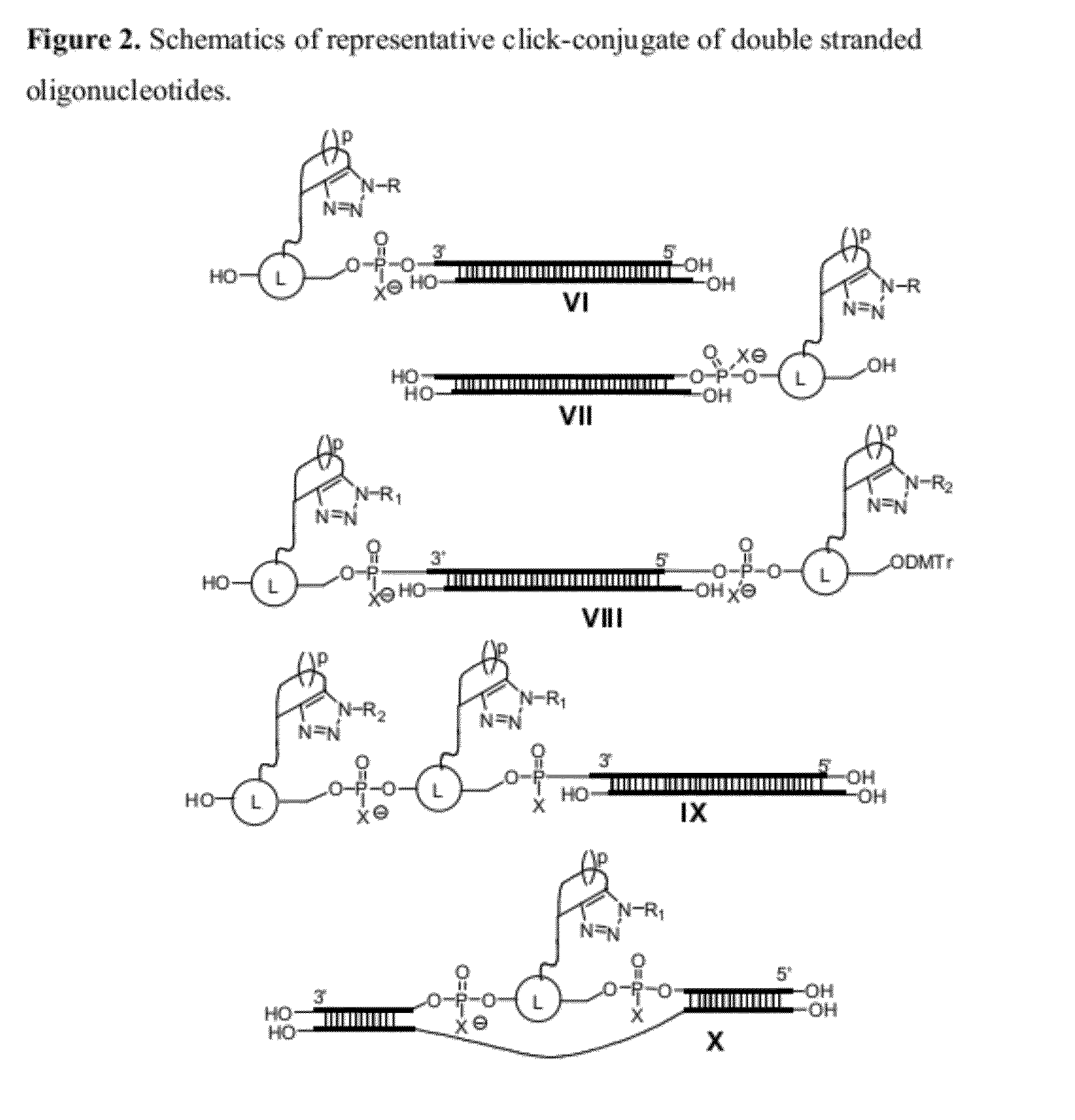
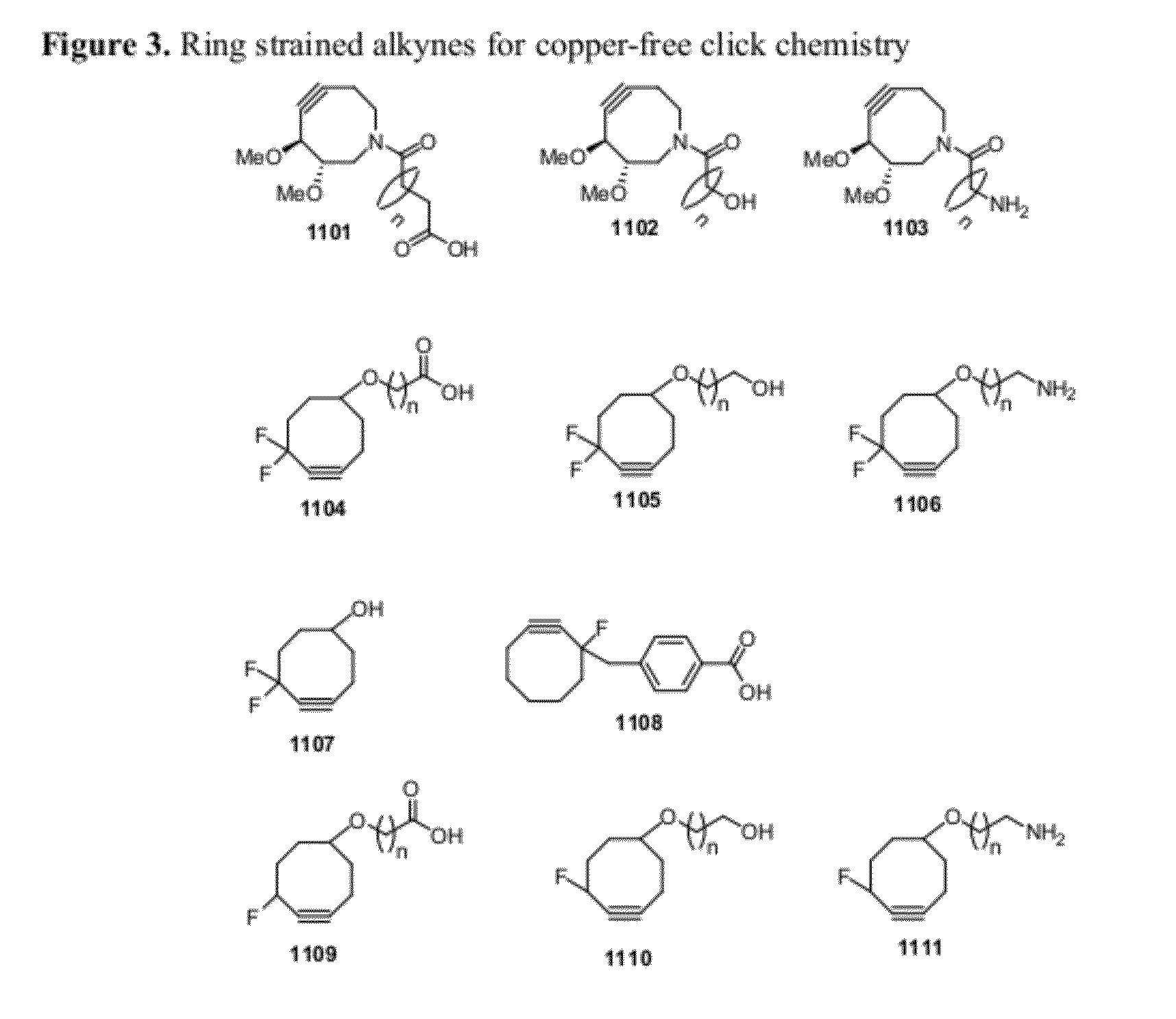
Chemical modifications of monomers and oligonucleotides with cycloaddition
The invention features compounds of formula I or II:In one embodiment, the invention relates compounds and processes for conjugating ligand to oligonucleotide. The invention further relates to methods for treating various disorders and diseases such as viral infections, bacterial infections, parasitic infections, cancers, allergies, autoimmune diseases, immunodeficiencies and immunosuppression.
Owner:ALNYLAM PHARMA INC
Injectable compositions of nanoparticulate immunosuppressive compounds
InactiveUS20060210638A1Improve complianceImprove efficacyPowder deliveryBiocideDepressantCompound (substance)
The invention is directed to an injectable nanoparticulate immunosuppressant composition for the formation of a subcutaneous or intramuscular depot. The invention is also directed to an injectable composition of nanoparticulate tacrolimus and / or sirolimus which eliminates the need to use polyoxyl 60 hydrogenated castor oil (HCO-60) and / or polysorbate 80 as a solubilizer. This invention further discloses a method of making an injectable nanoparticulate tacrolimus and / or sirolimus composition and is also directed to methods of treatment using the injectable nanoparticulate formulations comprising tacrolimus, sirolimus, or combination thereof for a subcutaneous or intramuscular depot for the prophylaxis of organ rejection and for the treatment of psoriasis or other immune diseases
Owner:ELAN PHRMA INT LTD
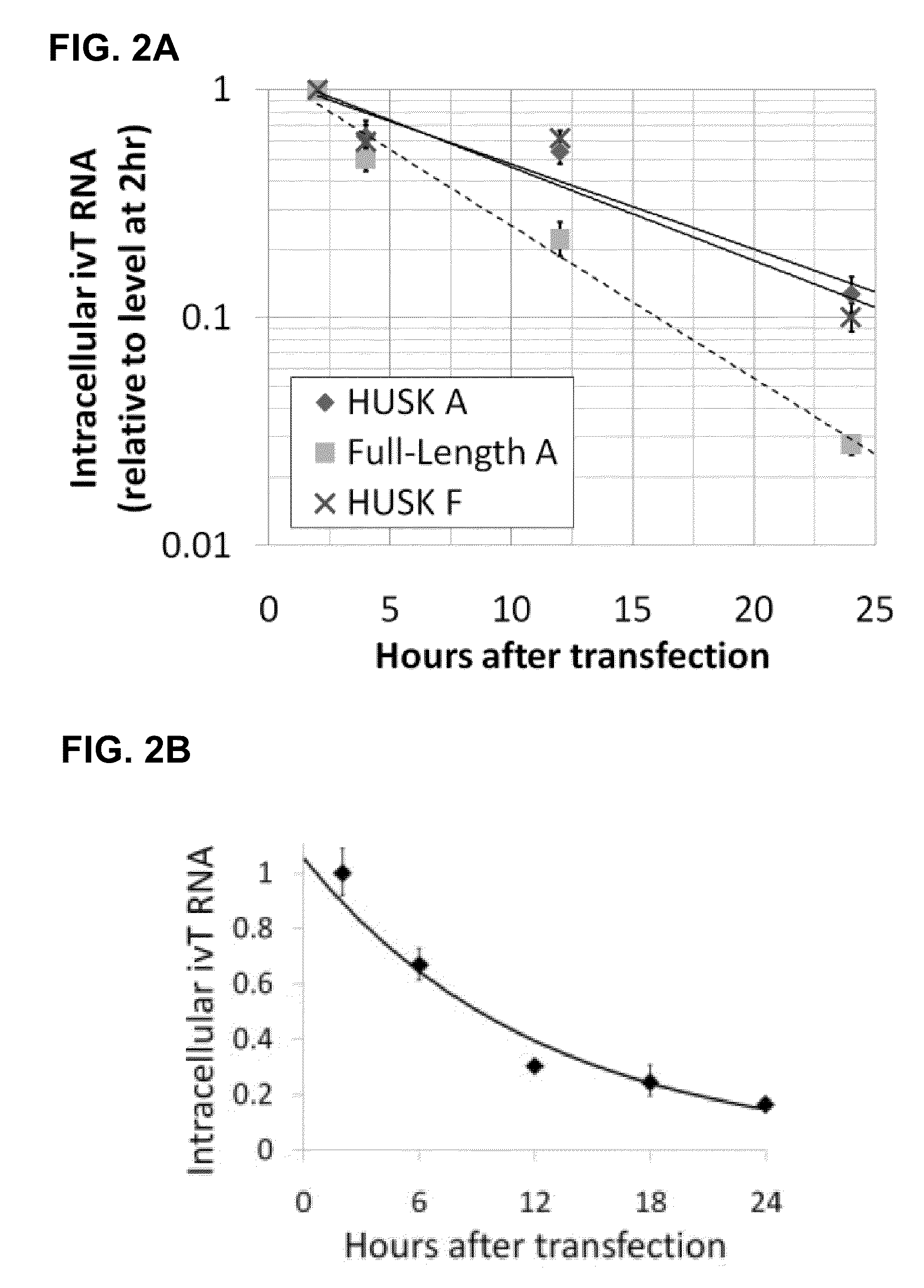
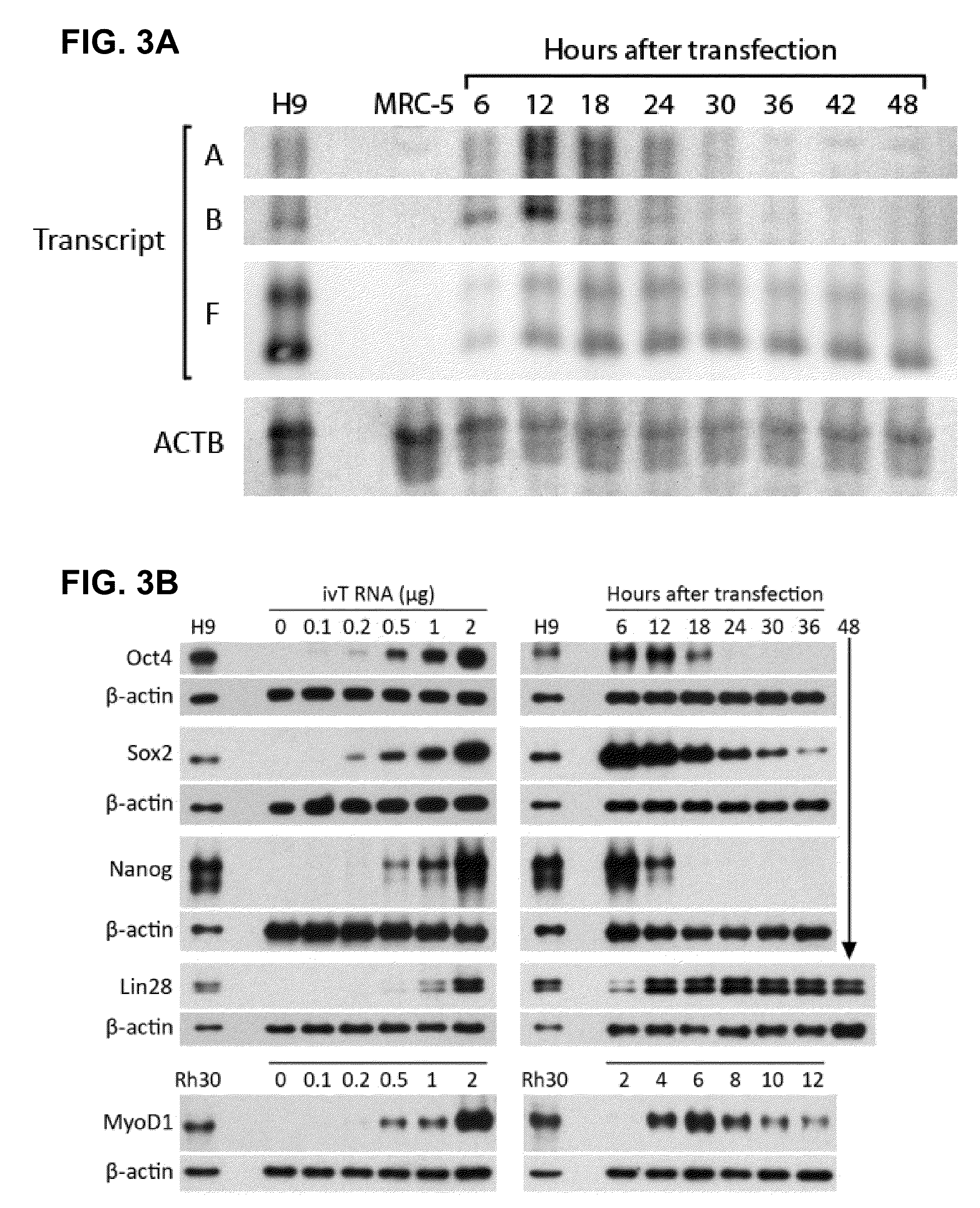
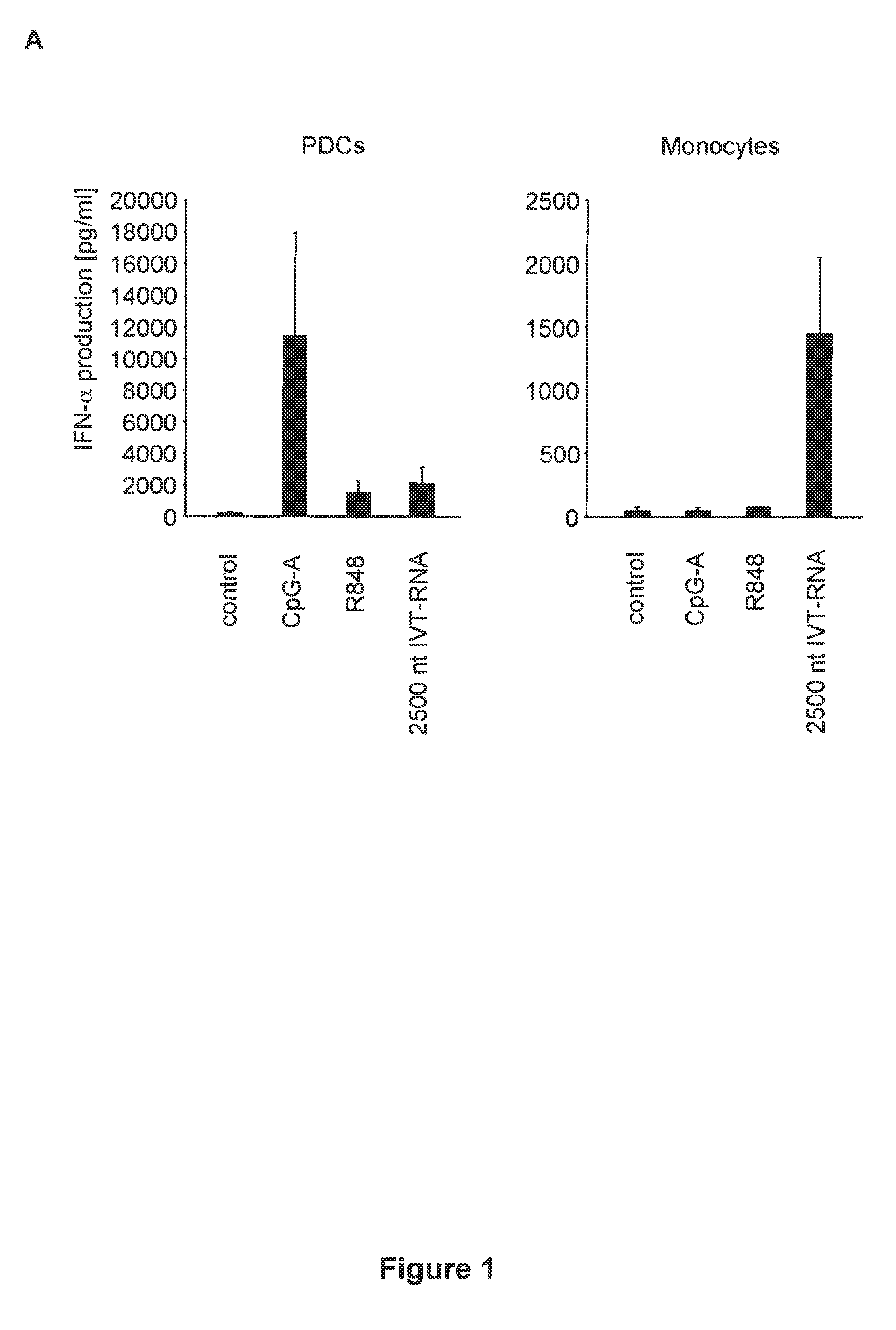
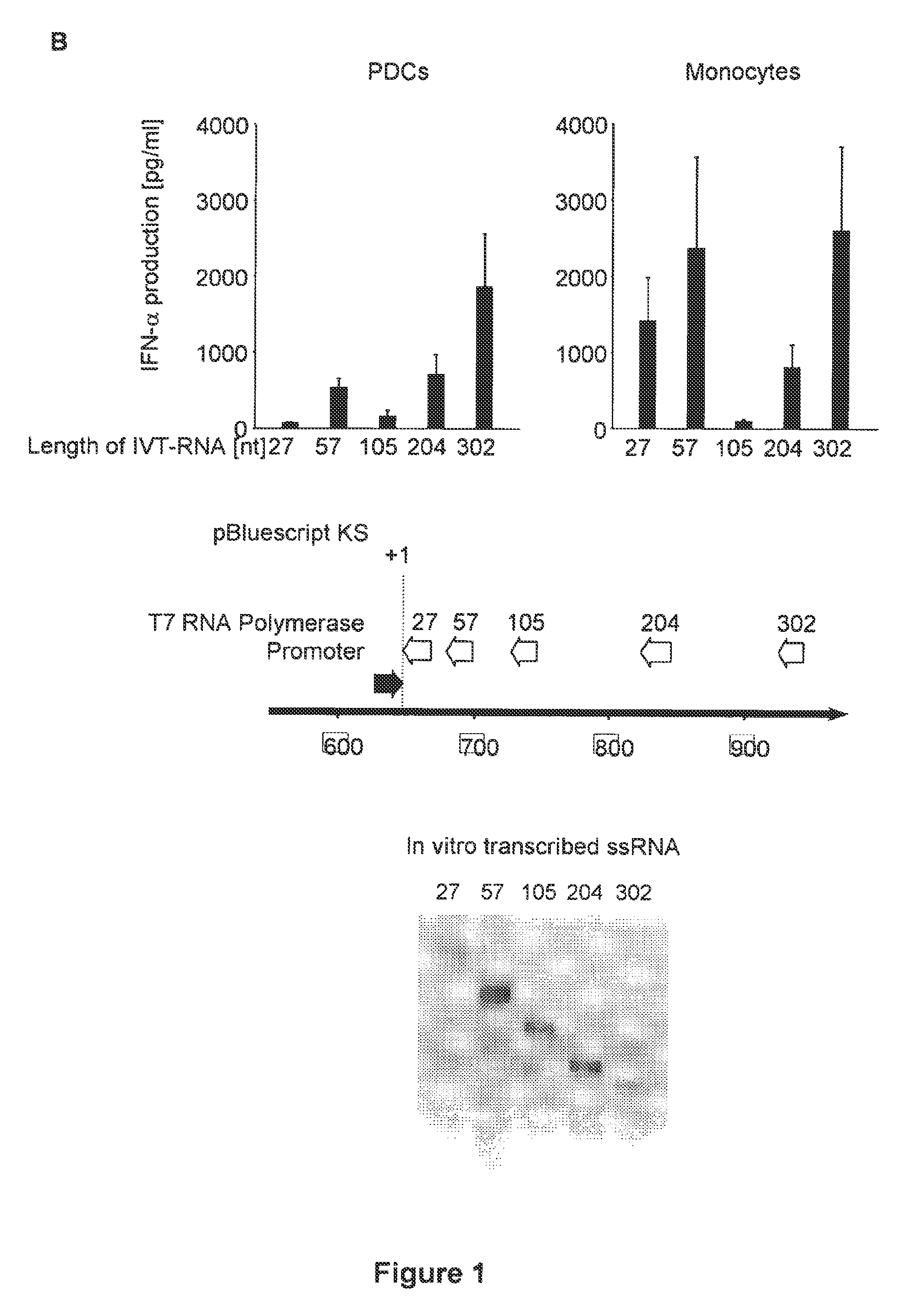
Innate immune suppression enables repeated delivery of long RNA molecules
InactiveUS20100273220A1Suppressing innate immune responseReduce expressionSugar derivativesArtificial cell constructsENCODETransient transfection
The present invention relates in part to methods for suppressing the innate immune response of a cell to transfection with an exogenous nucleic acid, to methods for increasing expression of a protein encoded by an exogenous nucleic acid by repeated delivery of the exogenous nucleic acid to a cell, and to methods of changing the phenotype of a cell by differentiating, transdifferentiating or dedifferentiating cells by repeatedly delivering one or more nucleic acids that encode defined proteins. A method is provided for extended transient transfection by repeated delivery of an in vitro-transcribed RNA (“ivT-RNA”) to a cell to achieve a high and sustained level of expression of a protein encoded by an ivT-RNA transcripts.
Owner:MASSACHUSETTS INST OF TECH
Immunomodulatory oligonucleotides
Oligonucleotides containing unthylated CpG dinucleotides and therapeutic utilities based on their ability to stimulate an immune response in a subject are disclosed. Also disclosed are therapies for treating diseases associated with immune system activation that are initiated by unthylated CpG dinucleotides in a subject comprising administering to the subject oligonucleotides that do not contain unmethylated CpG sequences (i.e. methylated CpG sequences or no CpG sequence) to outcompete unmethylated CpG nucleic acids for binding. Further disclosed are methylated CpG containing dinucleotides for use antisense therapies or as in vivo hybridization probes, and immunoinhibitory oligonucleotides for use as antiviral therapeutics.
Owner:IOWA RES FOUND UNIV OF +3
Immunomodulatory oligonucleotides
InactiveUS20050004062A1Improve responseIncrease the number ofOrganic active ingredientsSugar derivativesHybridization probeIn vivo
Oligonucleotides containing unthylated CpG dinucleotides and therapeutic utilities based on their ability to stimulate an immune response in a subject are disclosed. Also disclosed are therapies for treating diseases associated with immune system activation that are initiated by unthylated CpG dinucleotides in a subject comprising administering to the subject oligonucleotides that do not contain unmethylated CpG sequences (i.e. methylated CpG sequences or no CpG sequence) to outcompete unmethylated CpG nucleic acids for binding. Further disclosed are methylated CpG containing dinucleotides for use antisense therapies or as in vivo hybridization probes, and immunoinhibitory oligonucleotides for use as antiviral therapeutics.
Owner:COLEY PHARM GRP INC +1
Antagonizing interleukin-21 receptor activity
InactiveUS20060039902A1Reduce riskSufficient amountCompounds screening/testingCompound screeningWhite blood cellFibrosis
Methods and compositions for inhibiting interleukin-21 (IL-21) / IL-21 receptor (MU-1) activity using antagonists of IL-21 or IL-21 receptor (“IL-21R” or “MU-1”), are disclosed. IL-21 / IL-21R antagonists can be used to induce immune suppression in vivo, e.g., for treating, ameliorating or preventing autoimmune or inflammatory disorders, including, e.g., inflammatory bowel disease (IBD), rheumatoid arthritis (RA), transplant / graft rejection, psoriasis, asthma, fibrosis, and systemic lupus erythematosus (SLE).
Owner:WYETH LLC
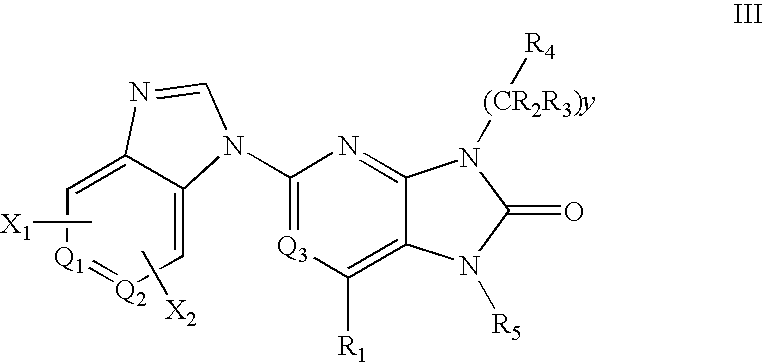
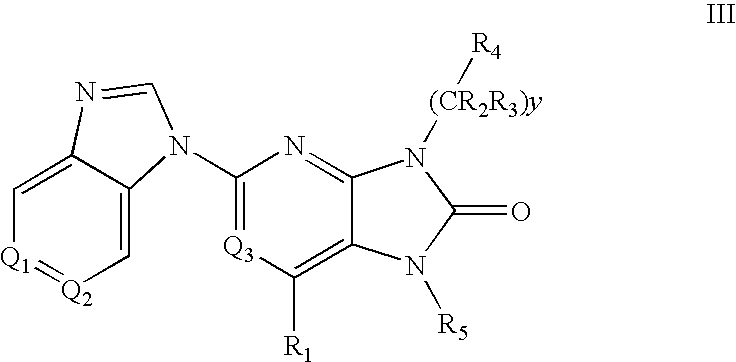
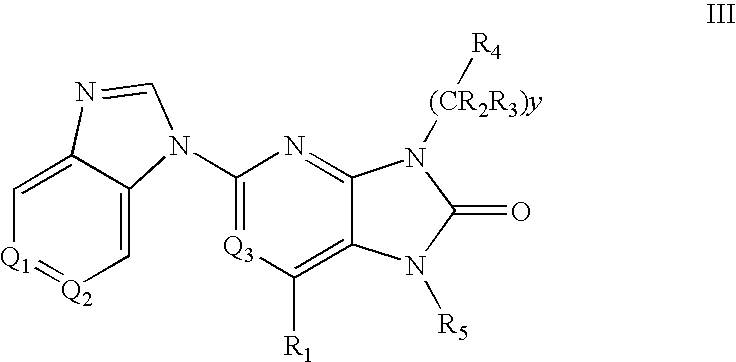
7-Substituted Purine Derivatives for Immunosuppression
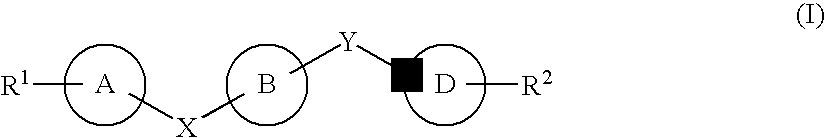
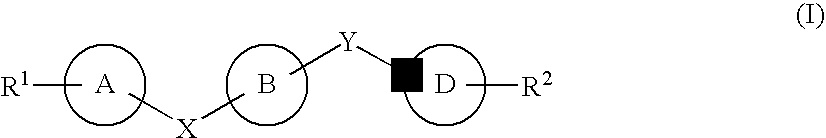
The present invention provides novel purinone and related derivatives useful for the prevention and treatment of autoimmune diseases, inflammatory disease, mast cell mediated disease and transplant rejection. The compounds are of the general formula III:
Owner:WYETH LLC
Treatment of B-cell associated diseases
InactiveUS6846476B2Enhanced killing and depletionReduce the possibilityRadioactive preparation carriersAntibody ingredientsDiseaseAutoimmune responses
Treatment of B-cell associated diseases including autoimmune and B-cell malignancies such as leukemias, lymphomas, using the combination of an anti-CD20 antibody, preferably RITUXAN® and a radiolabeled anti-CD22 antibody, preferably an 90Y labeled humanized anti-CD22 antibody, is described. These therapeutic regimens provide for enhanced depletion of B cells, and therefore reduce the risk in B cell malignancy treatment of relapse associated with RITUXAN® and, moreover, provide for prolonged immunosuppression of B-cell immune responses, especially in the context of autoimmune diseases and transplant.
Owner:BIOGEN INC
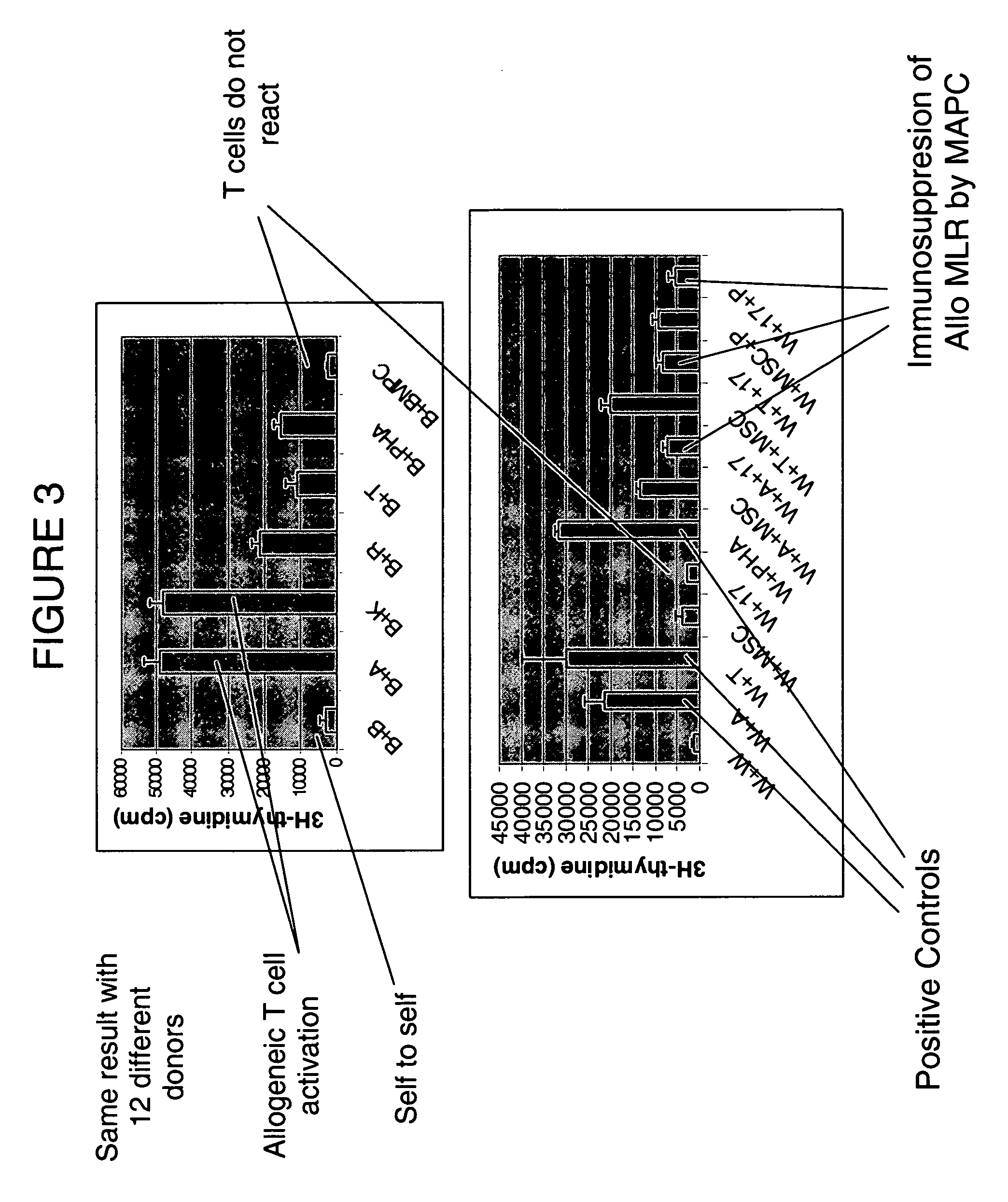
Immunomodulatory properties of multipotent adult progenitor cells and uses thereof
Isolated cells are described that are not embryonic stem cells, not embryonic germ cells, and not germ cells. The cells can differentiate into at least one cell type of each of at least two of the endodermal, ectodermal, and mesodermal lineages. The cells do not provoke a harmful immune response. The cells can modulate immune responses. As an example, the cells can suppress an immune response in a host engendered by allogeneic cells, tissues, and organs. Methods are described for using the cells, by themselves or adjunctively, to treat subjects. For instance, the cells can be used adjunctively for immunosuppression in transplant therapy. Methods for obtaining the cells and compositions for using them also are described.
Owner:ABT HOLDING COMPANY +1
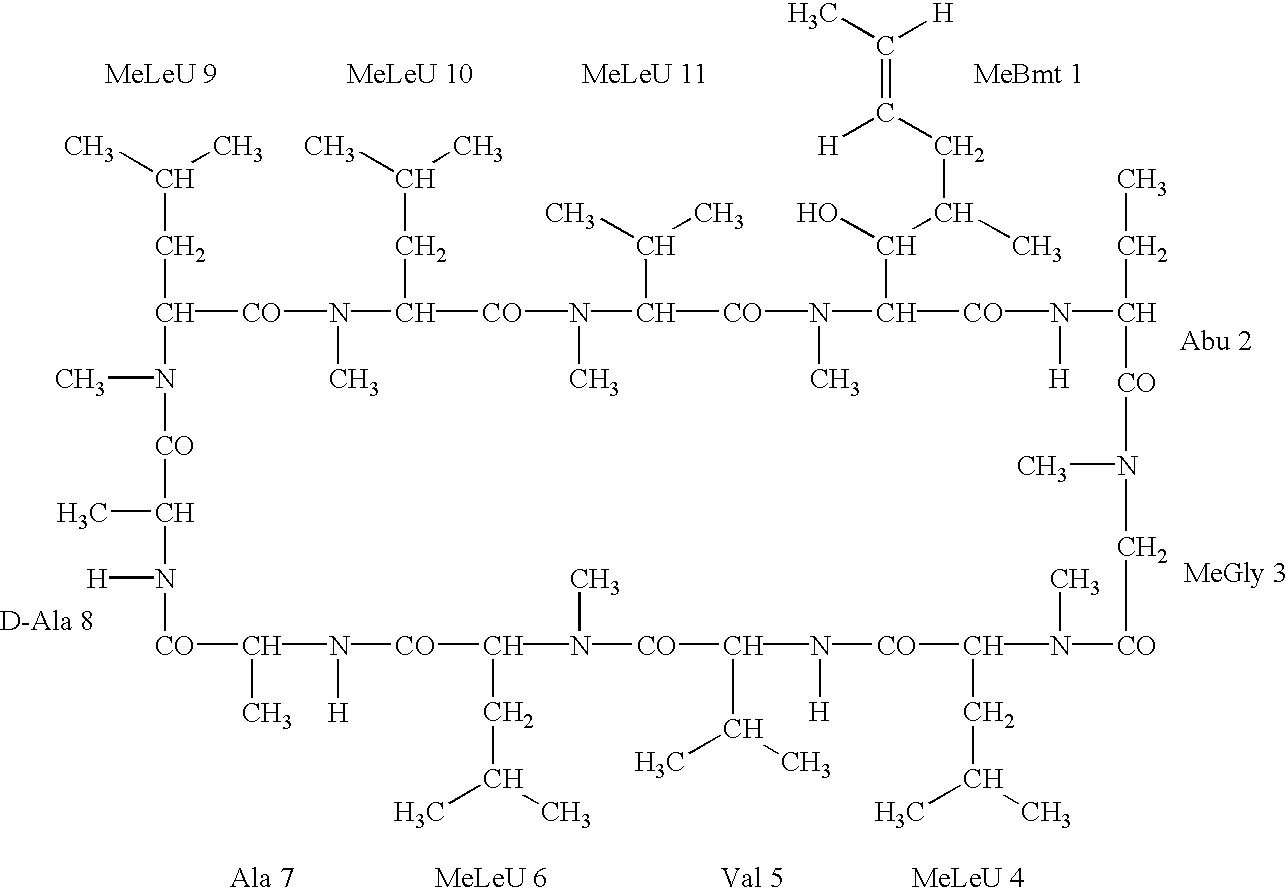
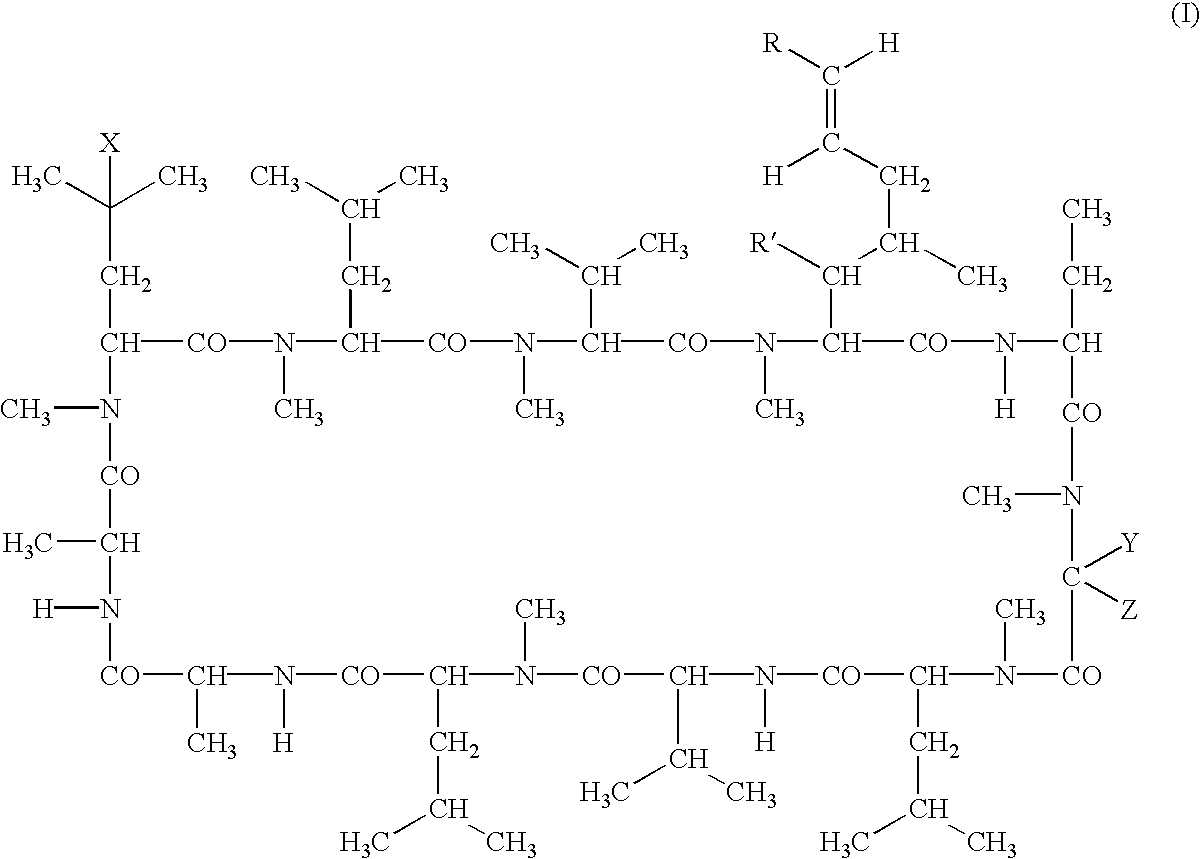
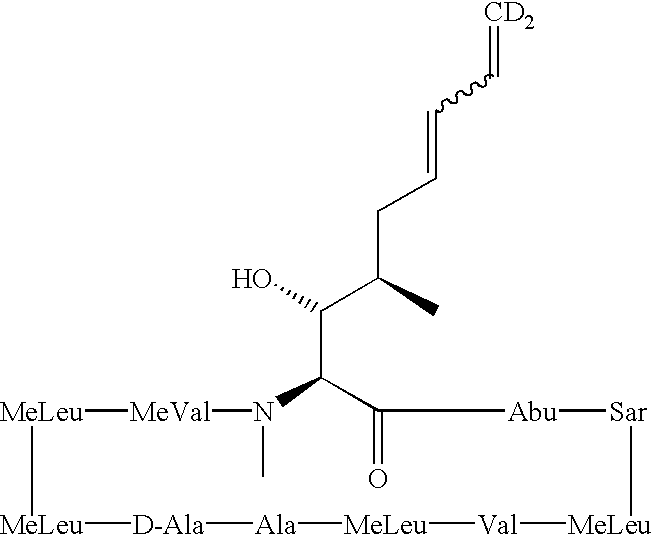
Deuterated cyclosporine analogs and their use as immunomodulating agents
InactiveUS6605593B1Improve practicalityAltered physicochemical and pharmacokinetic propertyNervous disorderAntipyreticCyclosporinsImmunomodulating Agent
Cyclosporine derivatives are disclosed which possess enhanced efficacy and reduced toxicity over naturally occurring and other presently known cyclosporins and cyclosporine derivatives. The cyclosporine derivatives of the present invention are produced by chemical and isotopic substitution of the cyclosporine A (CsA) molecule by: (1) Chemical substitution and optionally deuterium substitution of amino acid 1; and (2) deuterium substitution at key sites of metabolism of the cyclosporine A molecule such as amino acids 1, 4, 9. Also disclosed are methods of producing the cyclosporine derivatives and method of producing immunosuppression with reduced toxicity with the disclosed cyclosporine derivatives.
Owner:AURINIA PHARMA
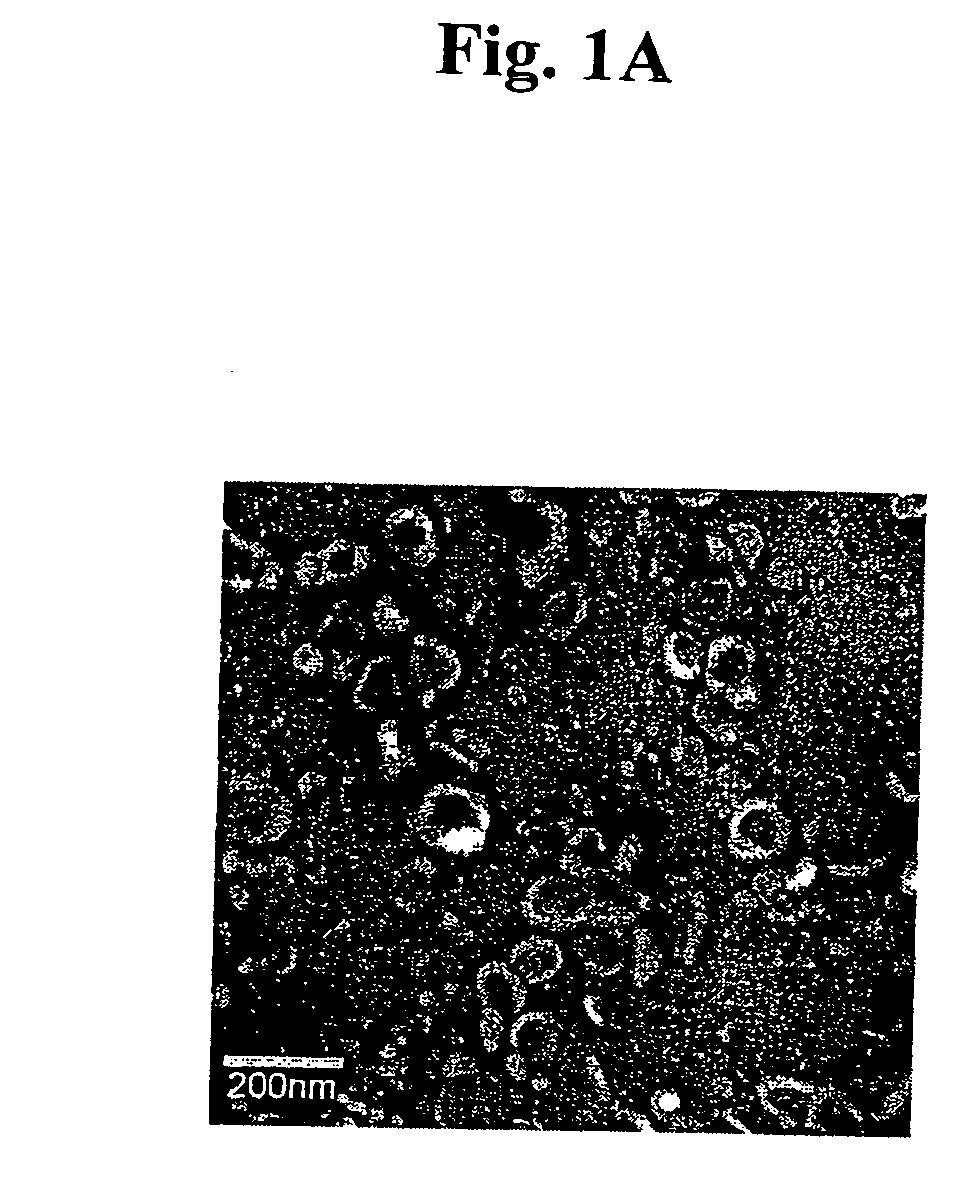
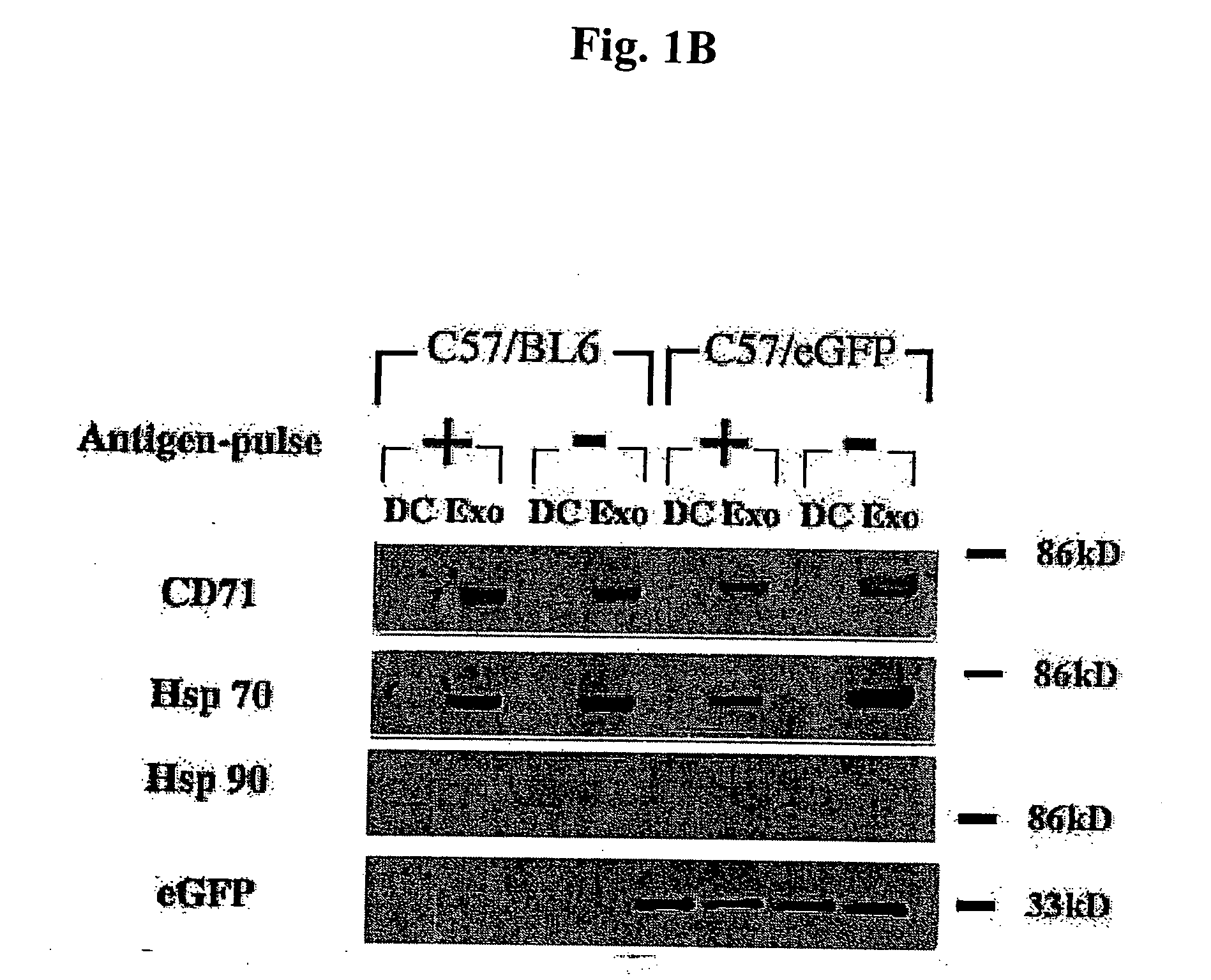
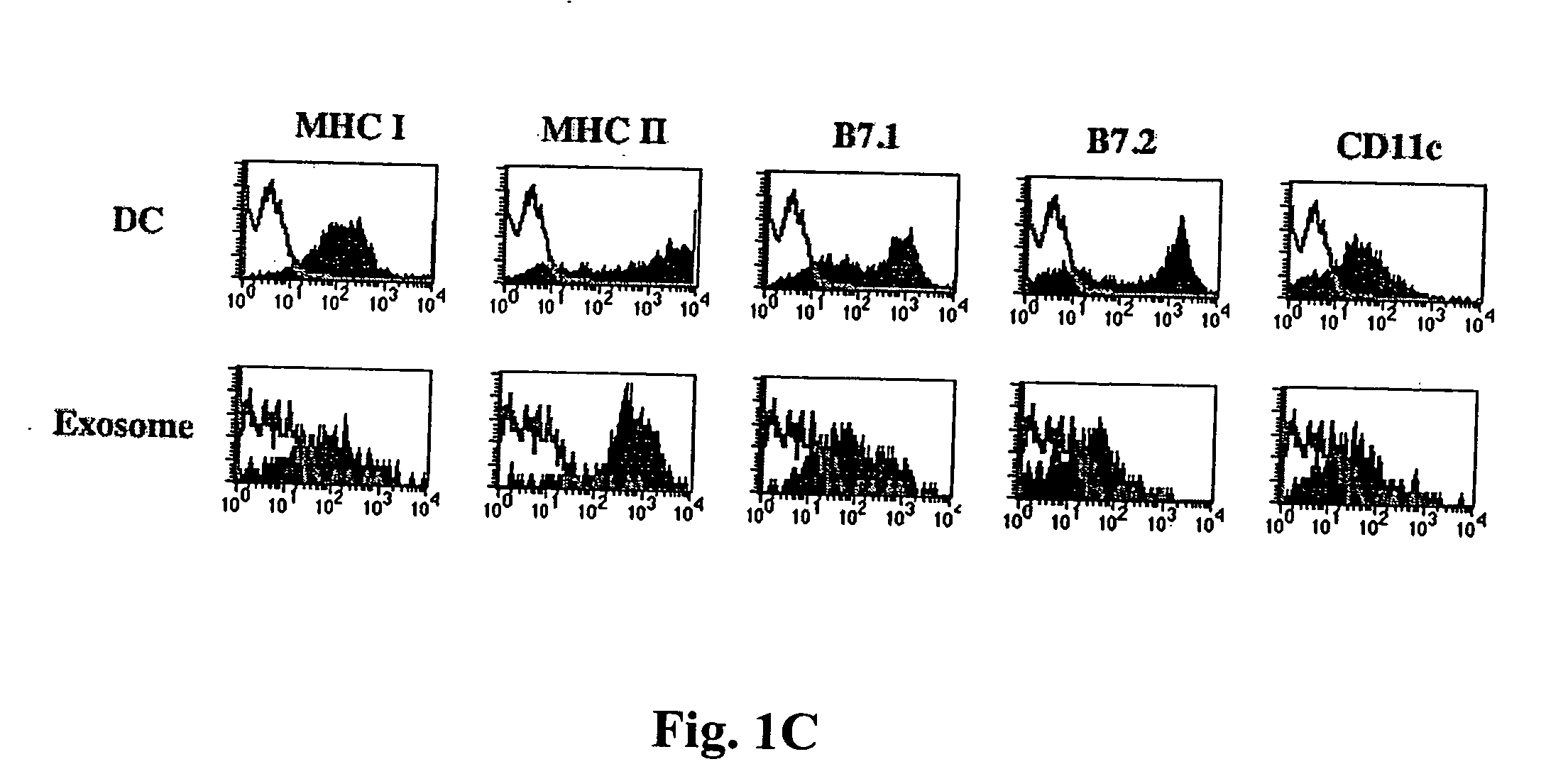
Immunosuppressive exosomes
InactiveUS20060116321A1Suppress undesirable immune responseStimulate immune responseNervous disorderPeptide/protein ingredientsCytokine SuppressionDendritic cell
The present invention relates to methods and compositions for use in mediating an immunosuppressive reaction. The compositions of the invention comprise exosomes having immunosuppressive activity. Such exosomes may be derived from a variety of different cell types, including antigen presenting cells such as dendritic cells and macrophages. Prior to isolation of exosomes, the cells may be genetically engineered to express molecules capable of enhancing the immunosuppressive activity of said exosomes and / or may be exposed to one or more agents, such as cytokines or cytokine inhibitors, which are also capable of enhancing the immunosuppressive activity of exosomes. The present invention also relates to the use of such exosomes for the treatment of diseases and disorders associated with undesirable activation of the immune system. The present invention also includes exosomes isolated directly from serum that have been shown to be immunosuppressive.
Owner:PITTSBURGH UNIV OF THE +1
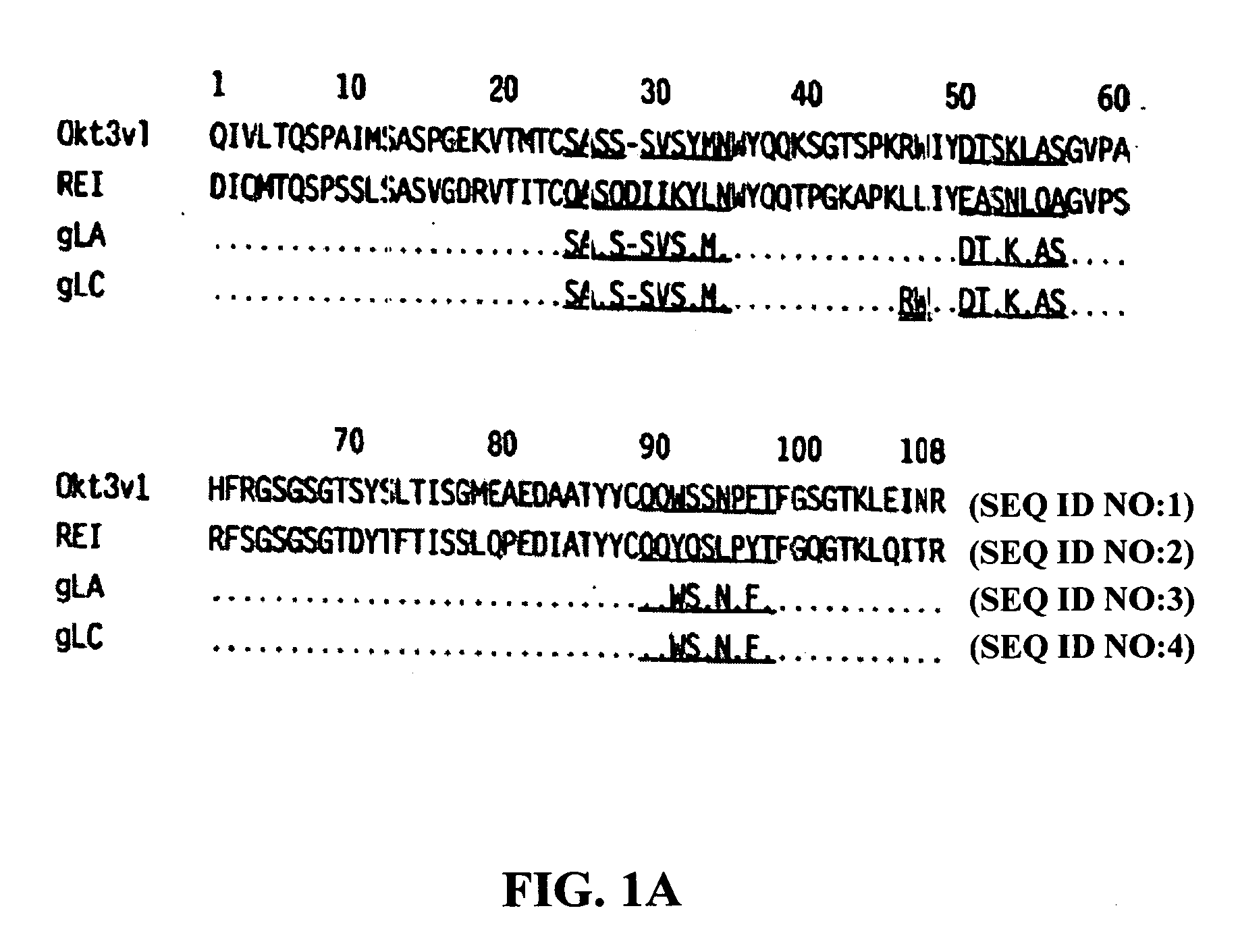
Methods for the treatment of autoimmune disorders using immunosuppressive monoclonal antibodies with reduced toxicity
ActiveUS20070077246A1Treating and preventing and ameliorating symptomSlow and reduce damagePeptide/protein ingredientsAntipyreticAutoimmune conditionAutoimmune disease
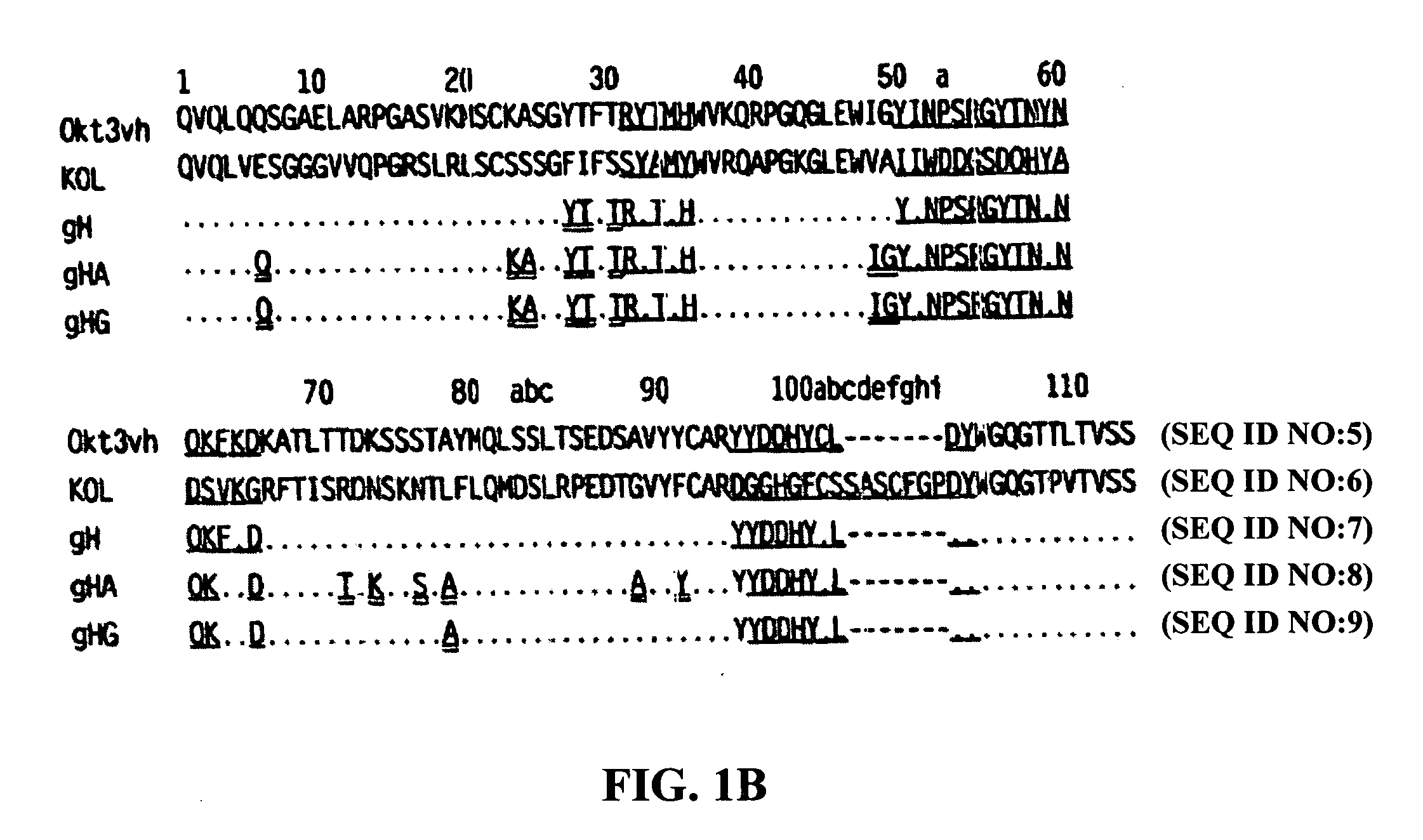
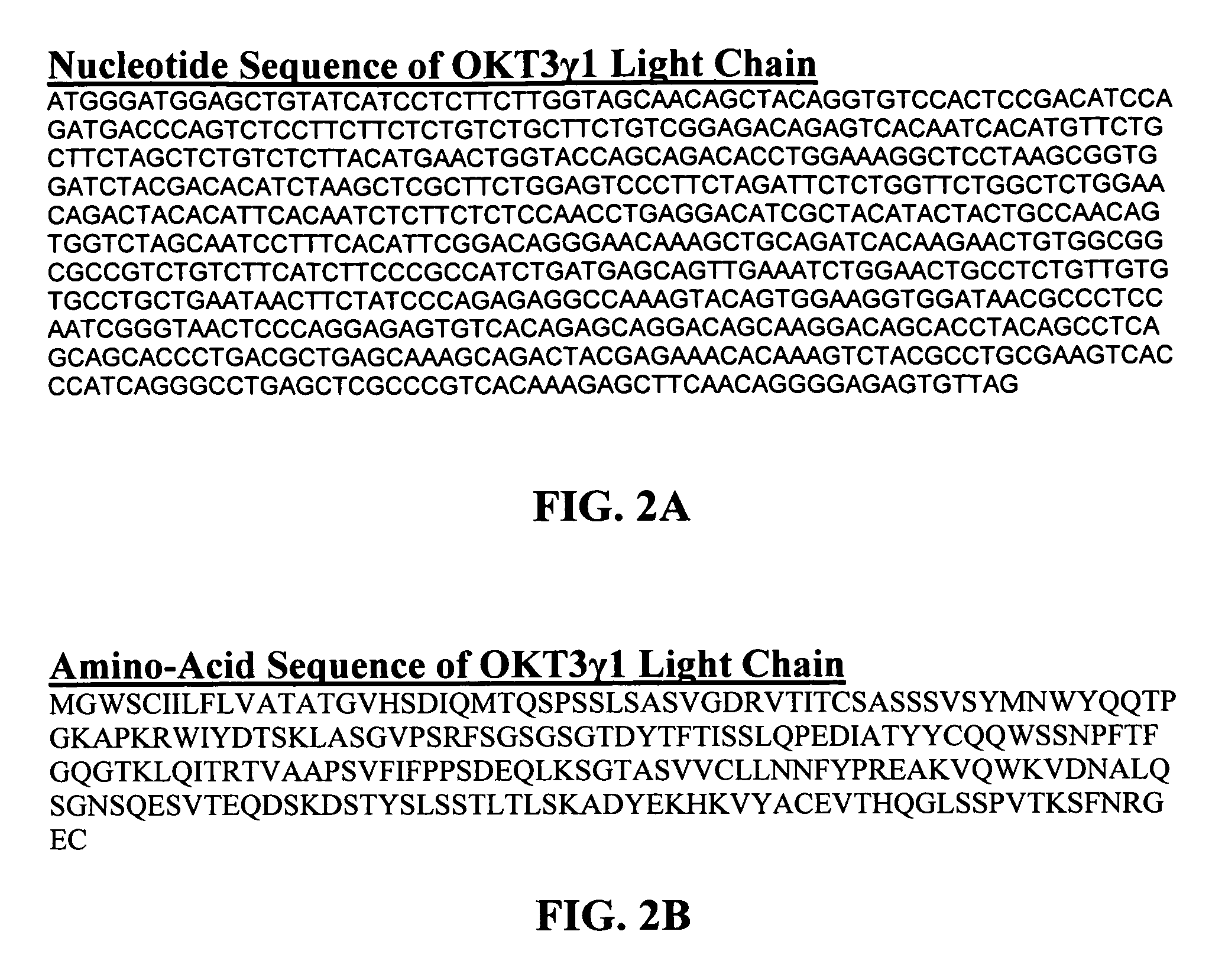
The present invention provides methods of treating, preventing or ameliorating the symptoms of T cell-mediated immunological diseases, particularly autoimmune diseases, through the use of anti-CD3 antibodies. In particular, the methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the invention provides for modification of the anti-CD3 antibodies such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-CD3 antibodies.
Owner:PROVENTION BIO INC
Nitrogenated heterocyclic derivative , and pharmaceutical agent comprising the derivative as active ingredient
InactiveUS20090131403A1Prevention and/or treatmentEasy to useBiocideSenses disorderAcquired immunodeficiencyAutoimmune condition
The compound represented by formula (I), a salt thereof, an N-oxide thereof, a solvate thereof, or a prodrug thereof specifically binds CCR5, so it is useful for preventing and / or treating CCR5-related diseases, for example, various inflammatory diseases (asthma, nephritis, nephropathy, hepatitis, arthritis, rheumatoid arthritis, rhinitis, conjunctivitis, ulcerative colitis, etc.), immunological diseases (autoimmune diseases, rejection in organ transplantation, immunosuppression, psoriasis, multiple sclerosis, etc.), infectious diseases (infection with human immunodeficiency virus, acquired immunodeficiency syndrome, etc.), allergic diseases (atopic dermatitis, urticaria, allergic bronchopulmonary aspergillosis, allergic eosinophilic gastroenteritis, etc.), ischemic reperfusion injury, acute respiratory distress syndrome, shock accompanying bacterial infection diabetes cancer metastasis and so on.Wherein all symbols in formula are as defined in the specification
Owner:ONO PHARMA CO LTD
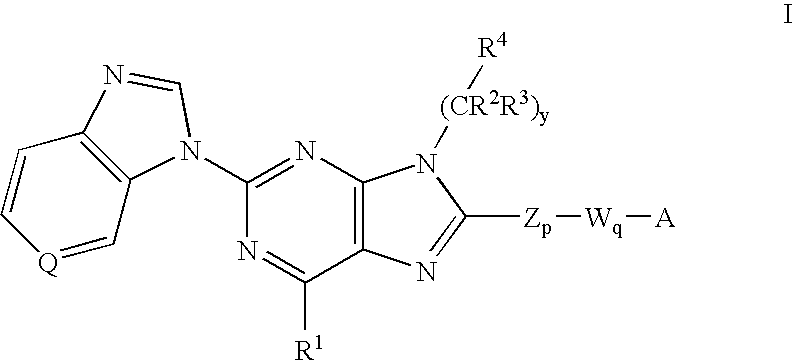
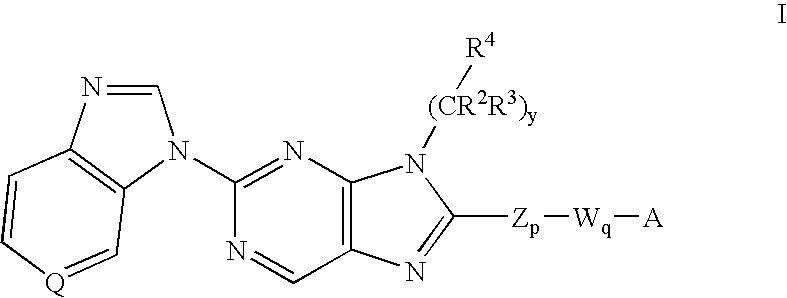
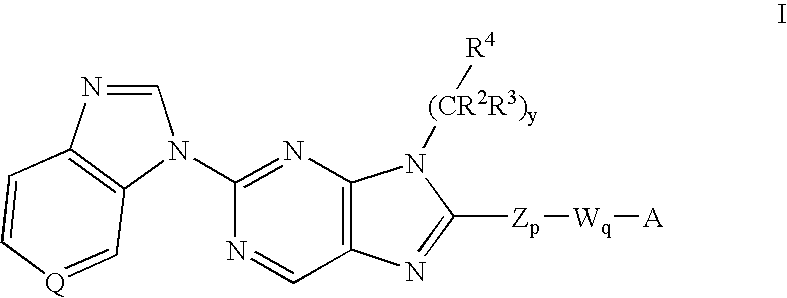
8-substituted 2-(benzimidazolyl)purine derivatives for immunosuppression
The present invention provides novel purines useful for the prevention and treatment of autoimmune diseases, inflammatory disease, mast cell mediated disease and transplant rejection. The compounds are of the general formula I:
Owner:WYETH LLC
Compositions and Methods for Targeted Immunomodulatory Antibodies and Fusion Proteins
ActiveUS20130039911A1Function increaseOrganic active ingredientsPeptide/protein ingredientsTumor targetAntitumor immunity
The present invention is based on the seminal discovery that targeted immunomodulatory antobodies and fusion proteins can counter act or reverse immune tolerance of cancer cells. Cancer cells are able to escape elimination by chemotherapeutic agents or tumor-targeted antobodies via specific immunosuppressive mechanisms in the tumor microenvironment and such ability of cancer cells is recognized as immune tolerance. Such immuno-suppressive mechanisms include immunosuppressive cytokines (for example, Transforming growth factor beta (TGF-β)) and regulatory T cells and / or immunosuppressive myeloid dendritic cells (DCs). By conteracting tumor-induced immune tolerance, the present invention provides effective compositions and methods for cancer treatment, optional in combination with another existing cancer treatment. The present invention provides strategies to counteract tumor-induced immune tolerance and enhance the antitumor efficacy of chemotherapy by activating and leveraging T cell-mediated adaptive antitumor immunity against resistant or disseminated cancer cells.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method for production of antibodies to specific sites of rapamycin
InactiveUS6709873B1Immunoglobulins against fungi/algae/lichensBiological testingPolyclonal antibodiesDivinyl sulfone
Owner:ISODIAGNOSTIKA
Immunosuppression modulating compounds
InactiveUS20110318373A1Reduce the binding forceAntibacterial agentsBiocideDiseaseSignalling pathways
The present invention provides immunosuppression compounds capable of inhibiting the programmed cell death 1 (PD1) signalling pathway. The present invention further provides peptide based compositions for treatment of cancer or treatment of infections via immunopotentiation caused by inhibition of immunosuppressive signaling induced by PD-1, PD-L1, or PD-L2 and therapies using them, immunopotentiative substrates included as the active ingredient. Further, the invention provides an application of the compositions containing the peptide moieties for preventive and / or therapeutic agents for cancer, cancer metastasis, immunodeficiency, an infectious disease or the like and an application of peptide moieties as a testing or diagnostic agent or a research agent for such a disease.
Owner:AURIGENE DISCOVERY TECH
Treatment of B-cell associated diseases such as malignancies and autoimmune diseases using a cold anti-CD20 antibody/radiolabeled anti-CD22 antibody combination
InactiveUS20050112060A1Enhanced killing and depletionPrevent and inhibit relapseRadioactive preparation carriersAntibody ingredientsAutoimmune conditionRegimen
Owner:BIOGEN INC
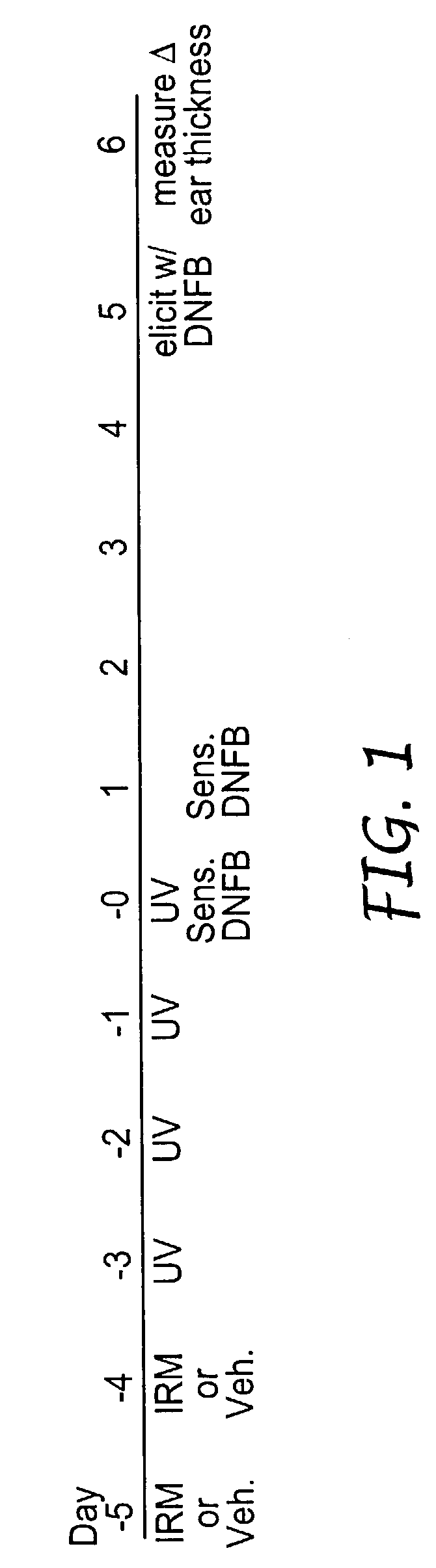
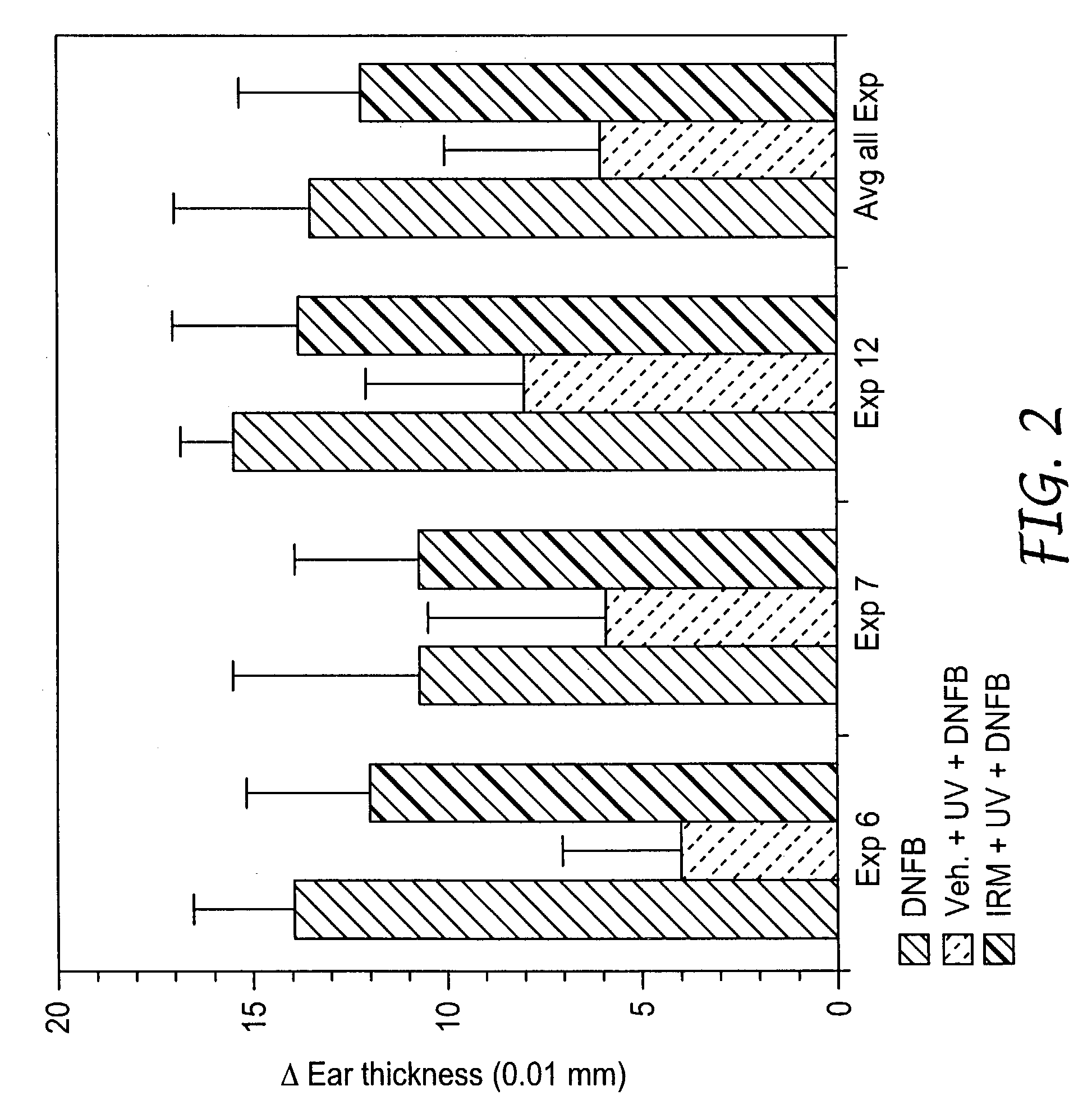
Method of reducing and treating UVB-induced immunosuppression
InactiveUS7030129B2Reducing UV-induced immunosuppressionPrevent immunosuppressionCosmetic preparationsBiocideAgonistImmunosuppression
Methods of preventing and / or treating UV-induced immunosuppression by administration of immune response modifier compounds are disclosed herein. Suitable immune response modifier compounds include agonists of one or more TLRs.
Owner:3M INNOVATIVE PROPERTIES CO
Compositions and methods for targeted immunomodulatory antibodies and fusion proteins
ActiveUS8993524B2Function increaseOrganic active ingredientsPeptide/protein ingredientsTumor targetAntitumor immunity
The present invention is based on the seminal discovery that targeted immunomodulatory antibodies and fusion proteins can counter act or reverse immune tolerance of cancer cells. Cancer cells are able to escape elimination by chemotherapeutic agents or tumor-targeted antibodies via specific immunosuppressive mechanisms in the tumor microenvironment and such ability of cancer cells is recognized as immune tolerance. Such immune suppressive mechanisms include immunosuppressive cytokines (for example, Transforming growth factor beta (TGF-β) and regulatory T cells and / or immunosuppressive myeloid dendritic cells (DCs). By counteracting tumor-induced immune tolerance, the present invention provides effective compositions and methods for cancer treatment, optional in combination with another existing cancer treatment. The present invention provides strategies to counteract tumor-induced immune tolerance and enhance the antitumor efficacy of chemotherapy by activating and leveraging T cell-mediated adaptive antitumor immunity against resistant or disseminated cancer cells.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Drug-delivery endovascular stent and method of forming the same
ActiveUS7727275B2Efficient releaseOrganic active ingredientsOrganic chemistryPolymer substratePercent Diameter Stenosis
Owner:BIOSENSORS INT GROUP
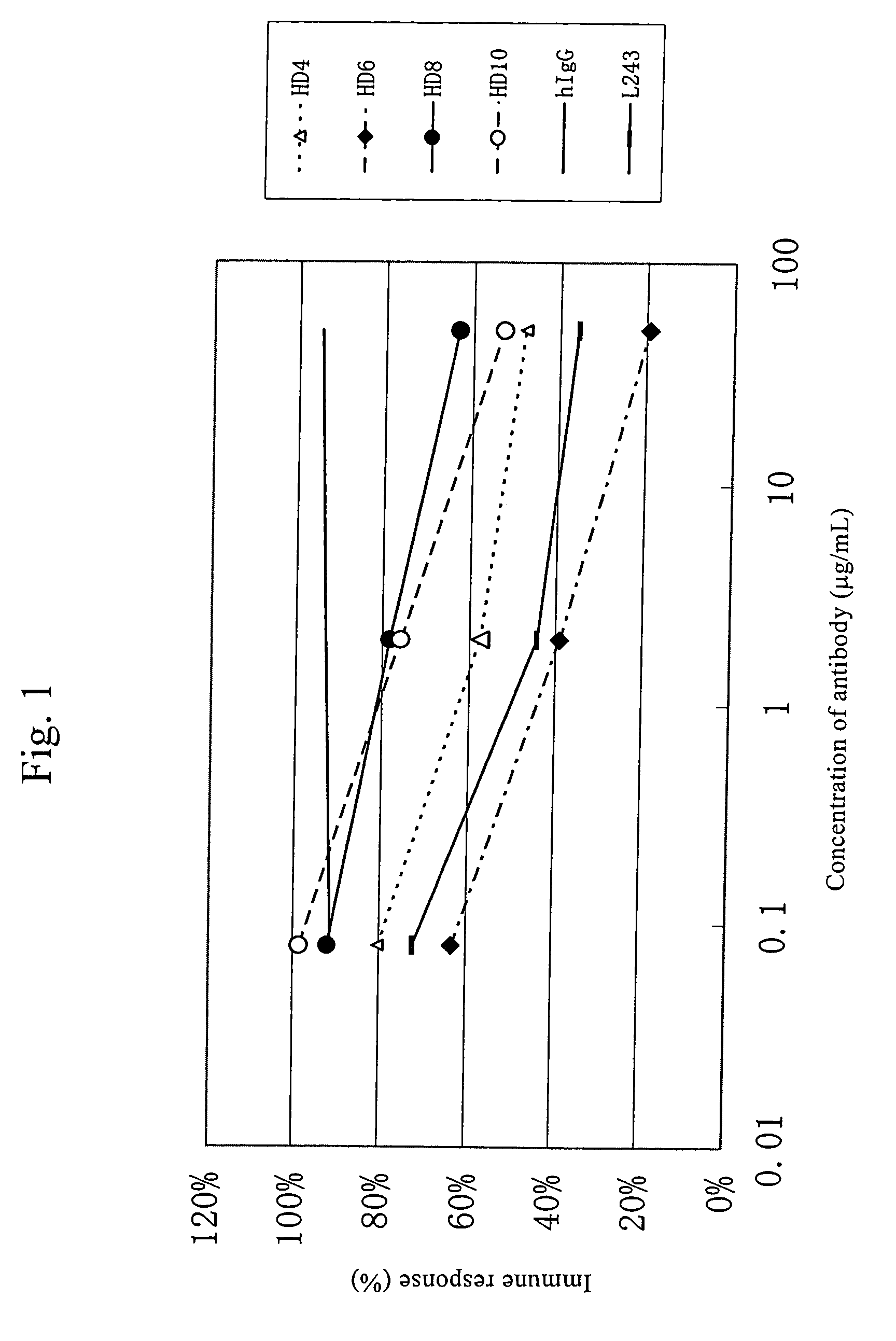
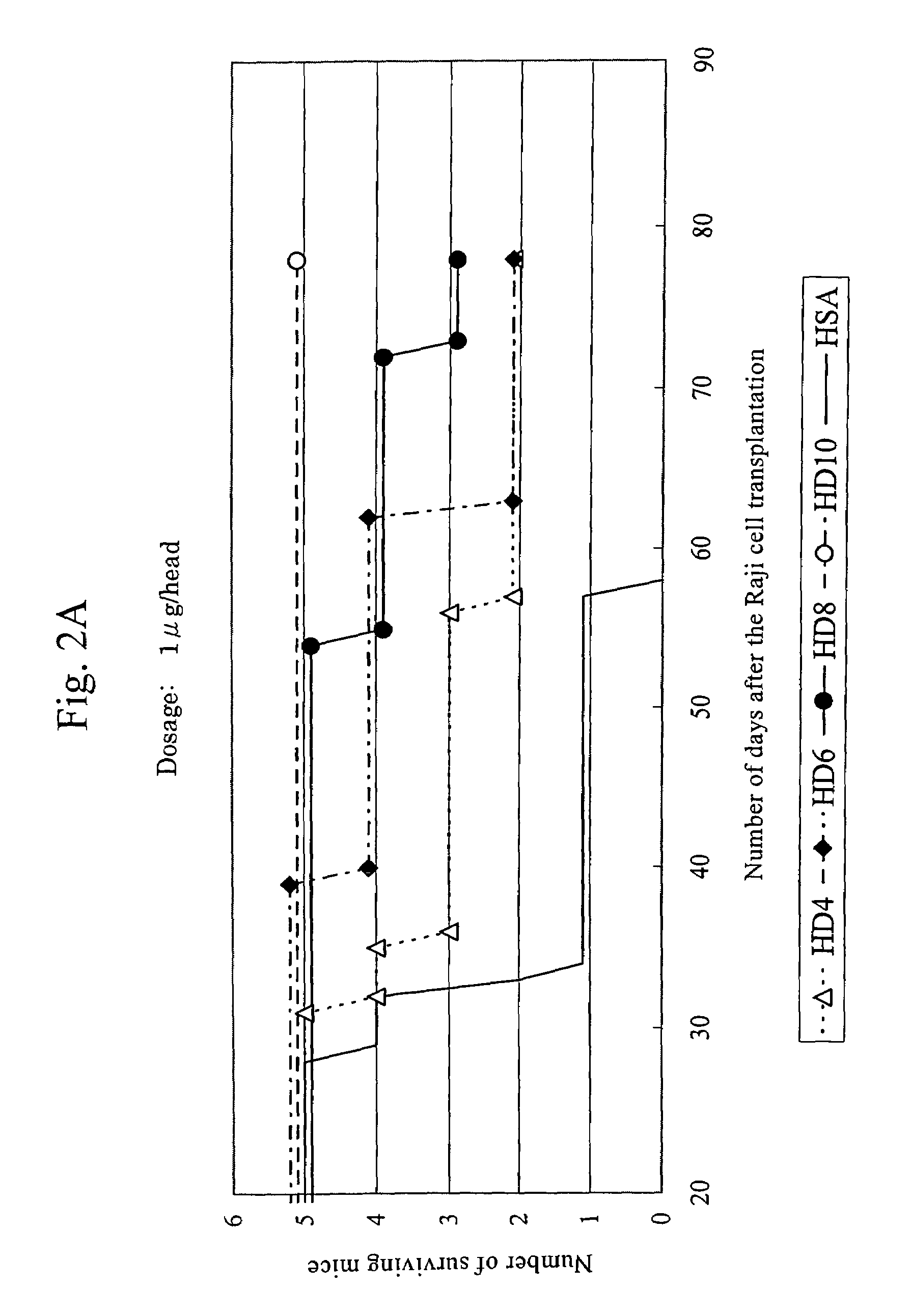
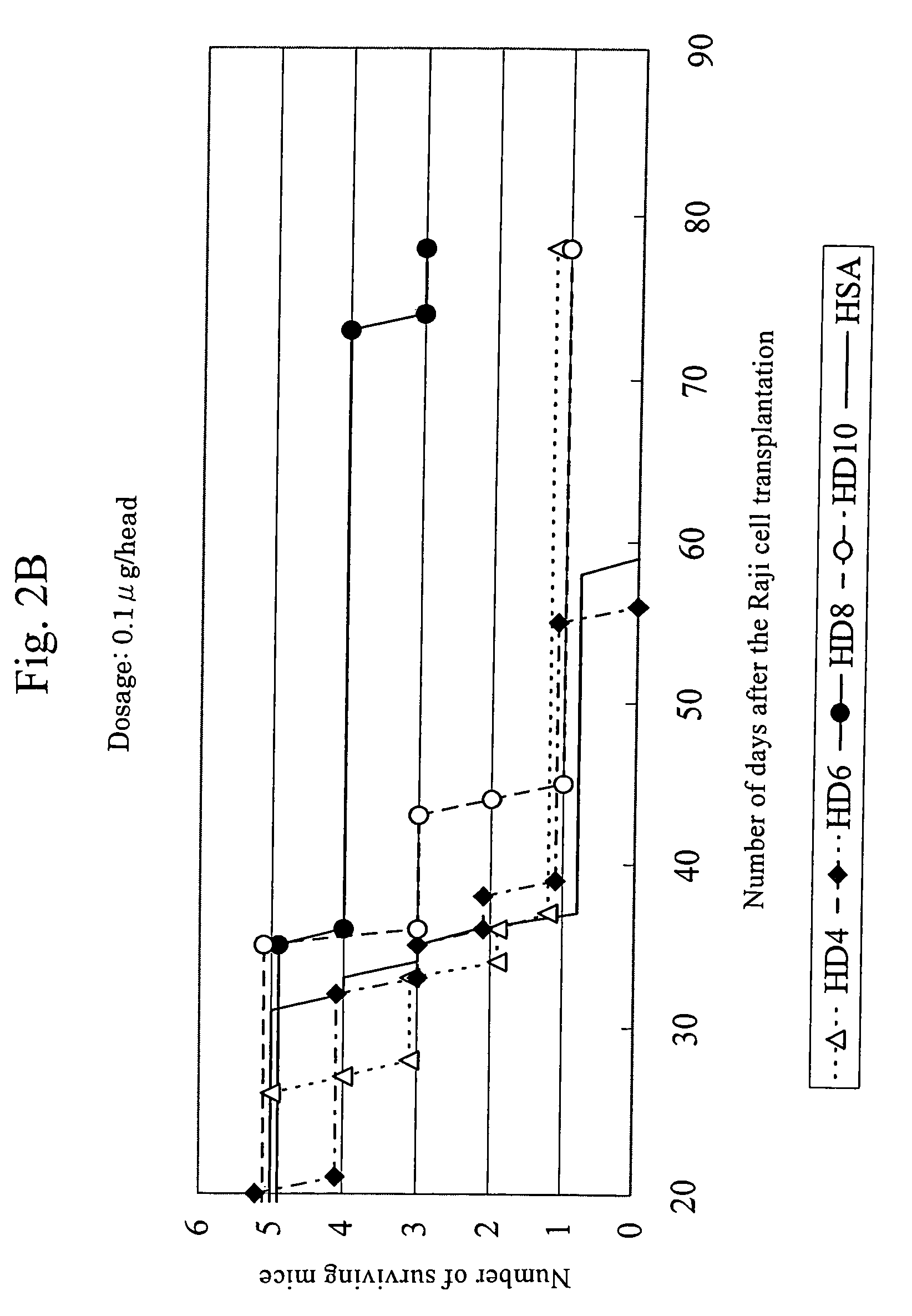
Anti-HLA-DR antibody
InactiveUS7262278B2Suppress lowering survival ratioSurvival ratioFungiBacteriaCancer cellMonoclonal antibody
This invention provides an anti-HLA-DR monoclonal antibody. This invention relates to an antibody binding to HLA-DR or a functional fragment thereof having (a) life-extending effects in nonhuman animals bearing HLA-DR-expressing cancer cells and (b) activity of suppressing immune responses lower than that of L243, or an antibody binding to HLA-DR or a functional fragment thereof exhibiting immunosuppressive activity equivalent to or higher than that of the mouse anti-HLA-DR monoclonal antibody L243 (ATCC HB-55).
Owner:KIRIN BREWERY CO LTD
Tolerogenic populations of dendritic cells
InactiveUS20100080816A1Snake antigen ingredientsArtificial cell constructsAutoimmune responsesCMKLR1
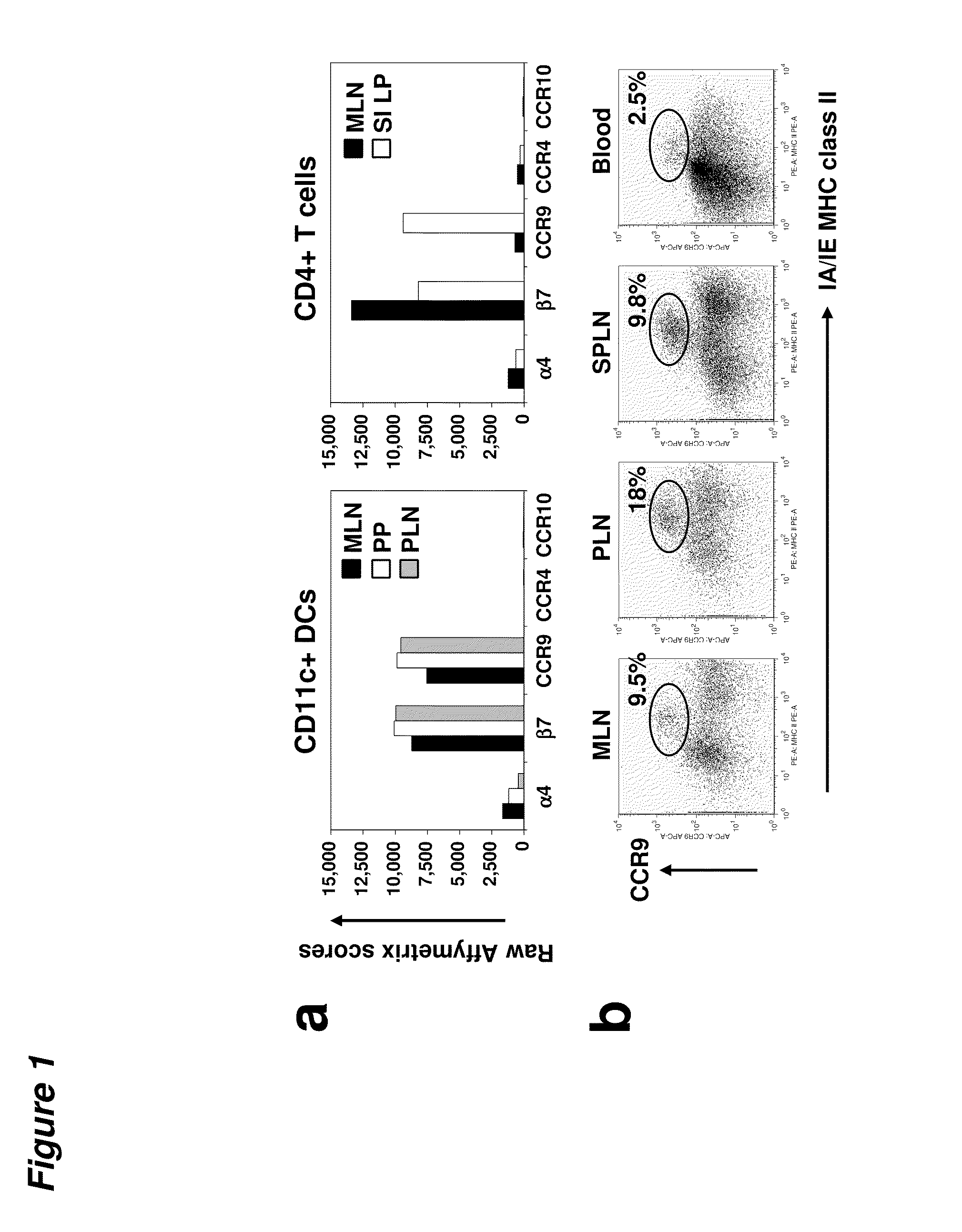
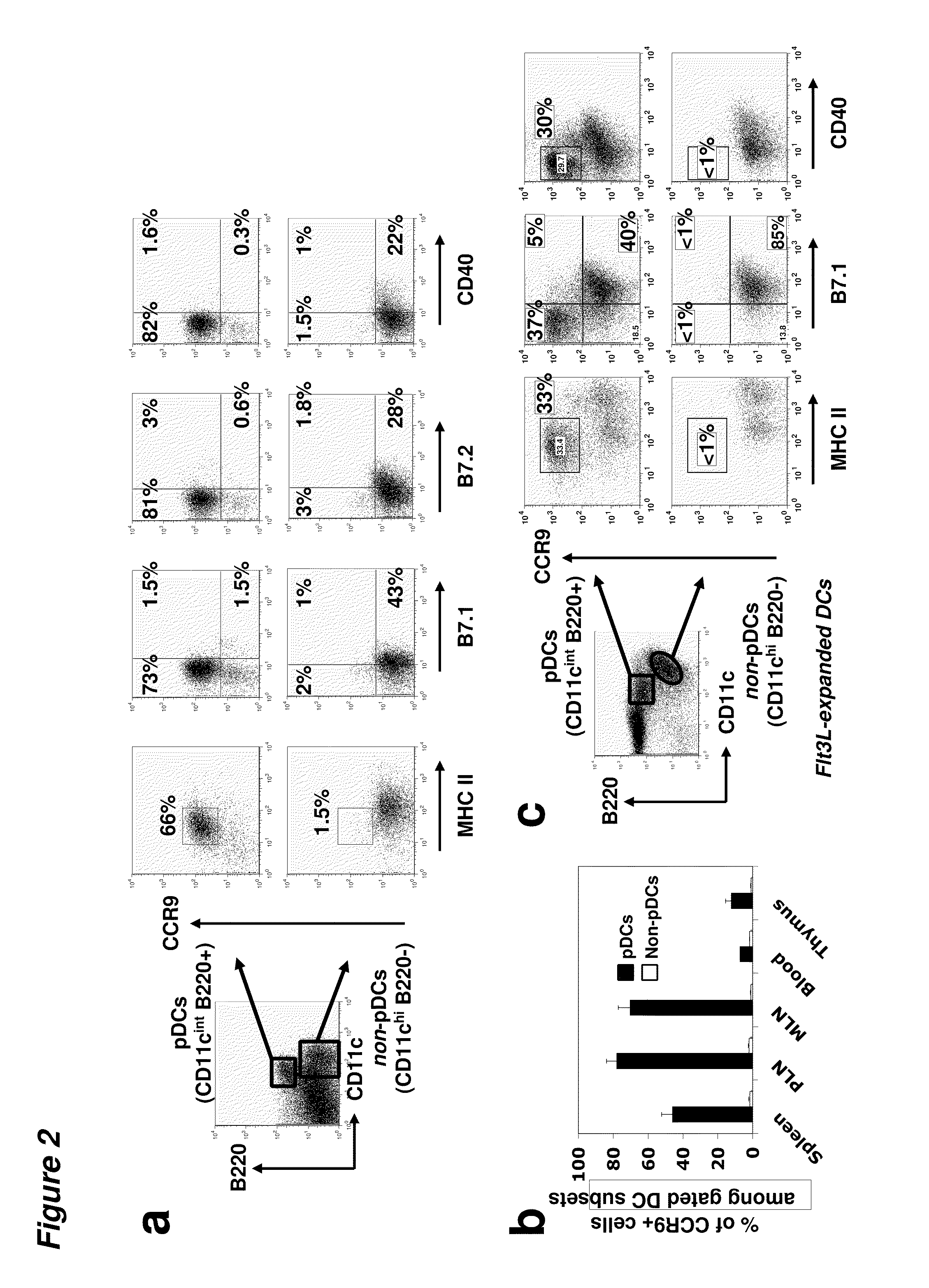
Tolerogenic populations of dendritic cells are provided, where the dendritic cells are characterized by expression of select tissue-specific homing receptors including the chemokine receptors CCR9; or CMKLR1; or the integrin CD103. The dendritic cells may be conventional / myeloid or plasmacytoid dendritic cells. The cells may be isolated from lymphoid tissue, from blood, or from in vitro culture, e.g. bone marrow culture, etc. Methods are provided for their identification, isolation and targeting in immunotherapeutic interventions in suppressing inflammatory disorders including autoimmunity, transplantation responses and allergic diseases. In some embodiments dendritic cell populations are fixed to render them immunosuppressive, thus allowing the cells to be typed and banked for future use.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Structure and use of 5' phosphate oligonucleotides
Oligonucleotides bearing free, uncapped 5′ phosphate group(s) are recognized by RIG-I, leading to the induction of type I IFN, IL-18 and IL-1β production. Bacterial RNA also induces type I IFN production. 5′ phosphate oligonucleotides and bacterial RNA can be used for inducing an anti-viral response or an anti-bacterial response, in particular, type I IFN and / or IL-18 and / or IL-1β production, in vitro and in vivo and for treating various disorders and diseases such as viral infections, bacterial infections, parasitic infections, tumors, allergies, autoimmune diseases, immunodeficiencies and immunosuppression. Single-stranded 5′ triphosphate RNA can be used for inducing an anti-viral response, an anti-bacterial response, or an anti-tumor response, in particular, type I IFN and / or IL-18 and / or IL-1β production, in a target cell-specific manner.
Owner:UNIVERSITY OF BONN
Antigen specific immunosuppression by dendritic cell therapy
InactiveUS20080311140A1Enhance protein expressionHigh expressionBiocideGenetic material ingredientsDiseaseAutoimmune disease
Owner:BAYLOR COLLEGE OF MEDICINE
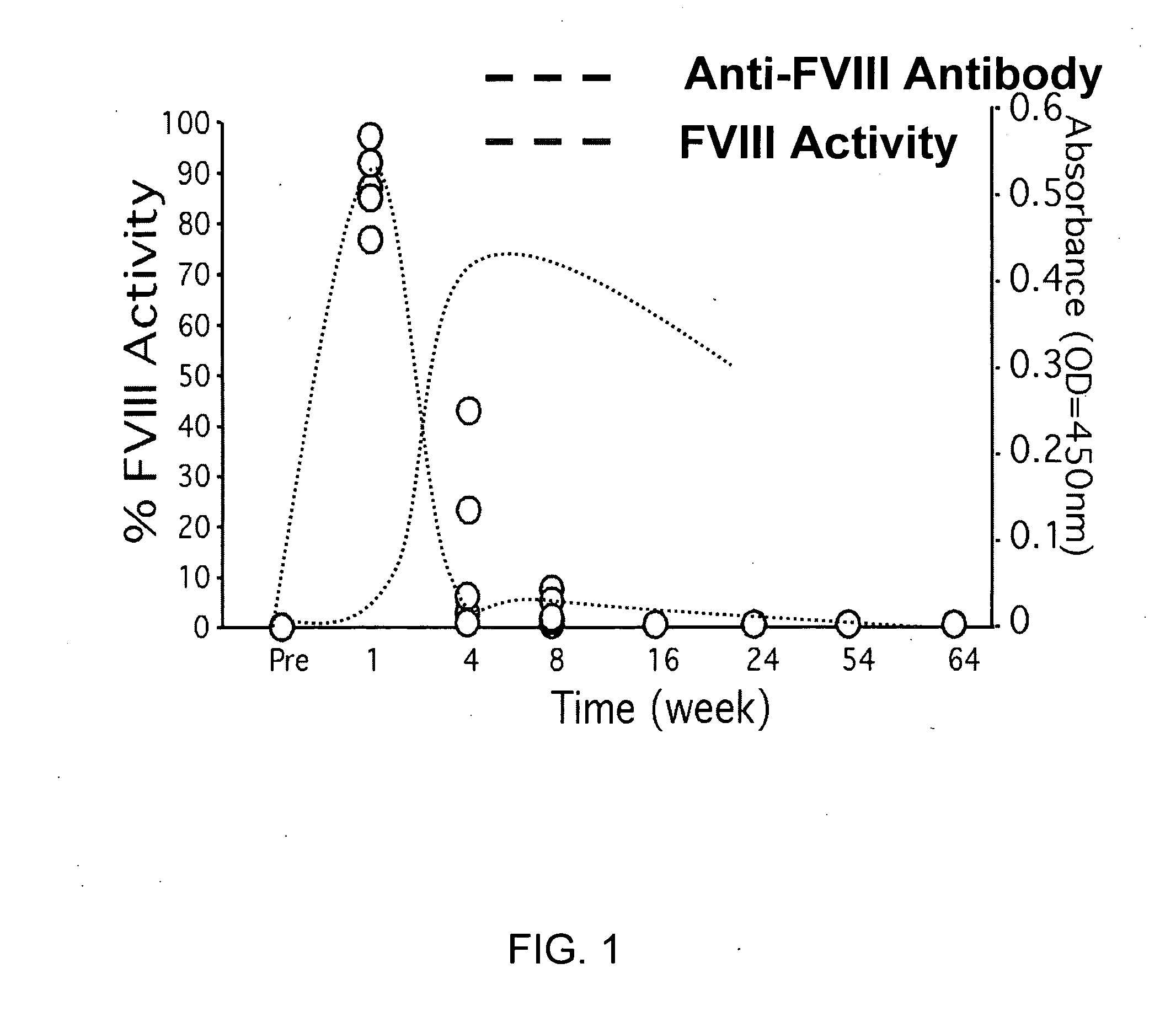
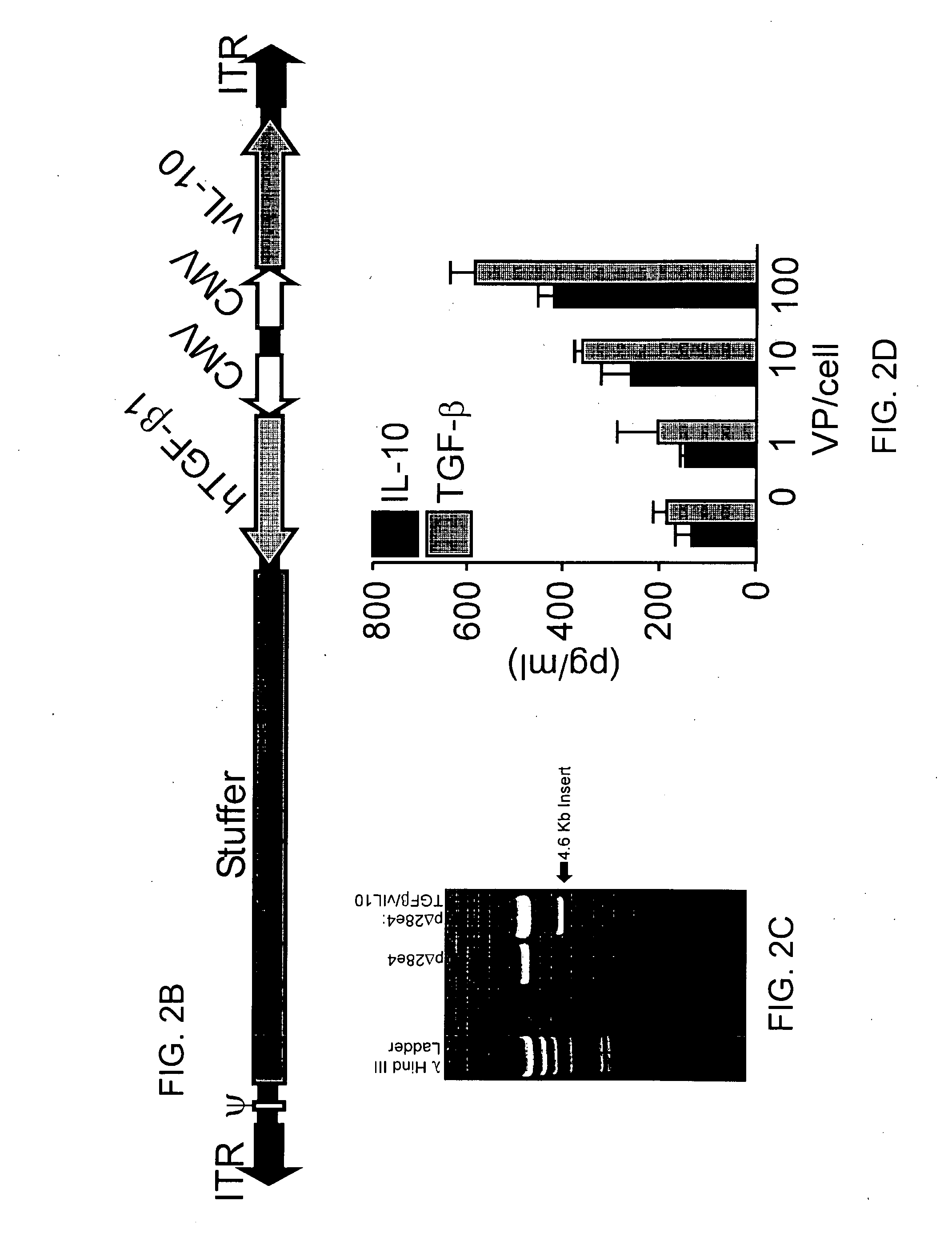
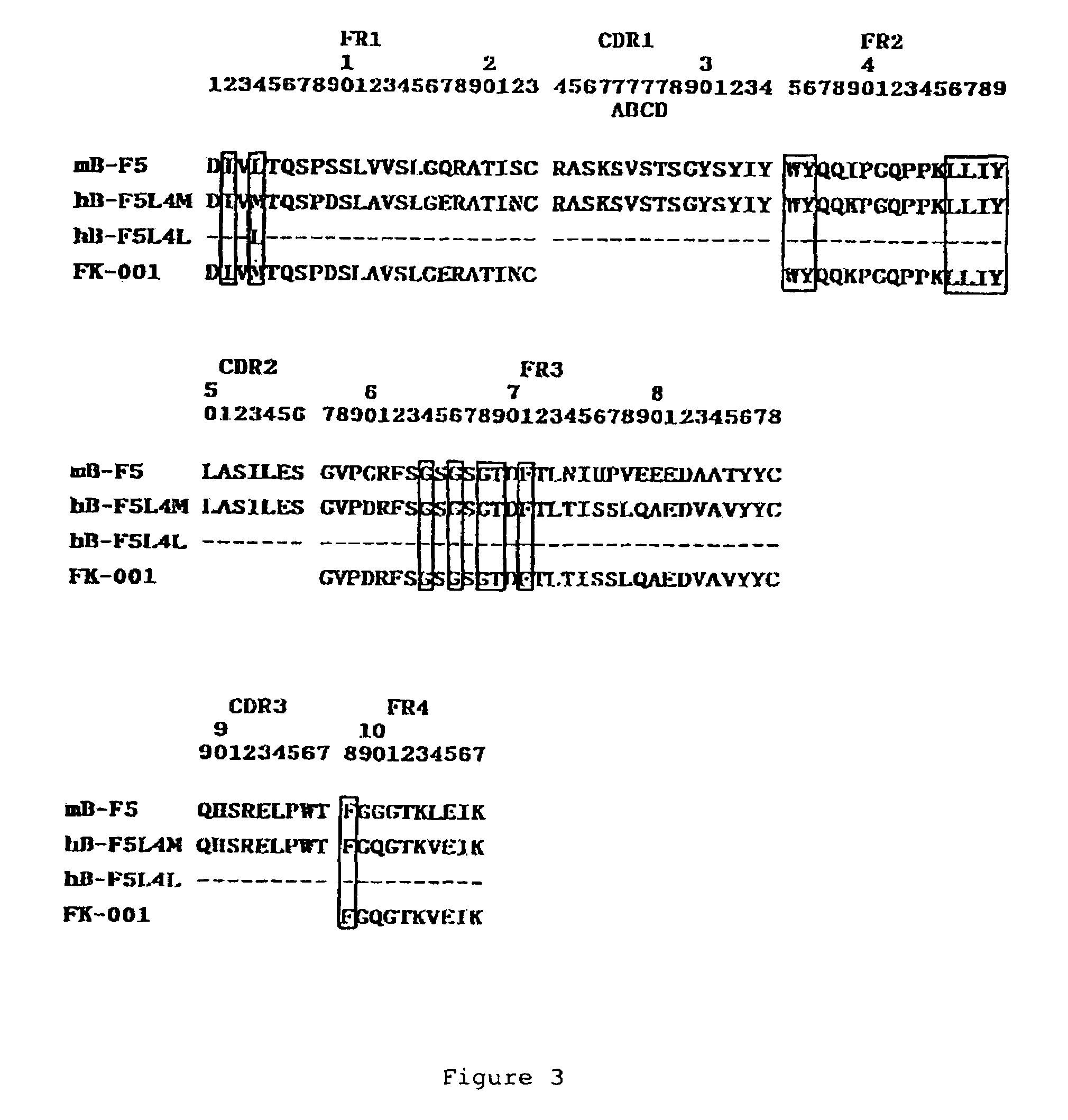
Humanized anti-CD4 antibody with immunosuppressive properties
A humanized antibody derived from mouse monoclonal anti-CD4 antibody B-F5 is able to activate CD25+CD4+ regulatory T cells and is useful for preparing immunosuppressive compositions.
Owner:OGEN GMBH
(3,4-Disubstituted)Propanoic Carboxylates as Sip (Edg) Receptor Agonists
The present invention encompasses compounds of Formula I:as well as the pharmaceutically acceptable salts thereof. The compounds are S1P1 / Edg1 receptor agonists and thus have immunosuppressive, anti-inflammatory and hemostatic activities by modulating leukocyte trafficking, sequestering lymphocytes in secondary lymphoid tissues, and enhancing vascular integrity. The invention is also directed to pharmaceutical compositions containing such compounds and methods of treatment or prevention.
Owner:MERCK SHARP & DOHME CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com