Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
397 results about "Tacrolimus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
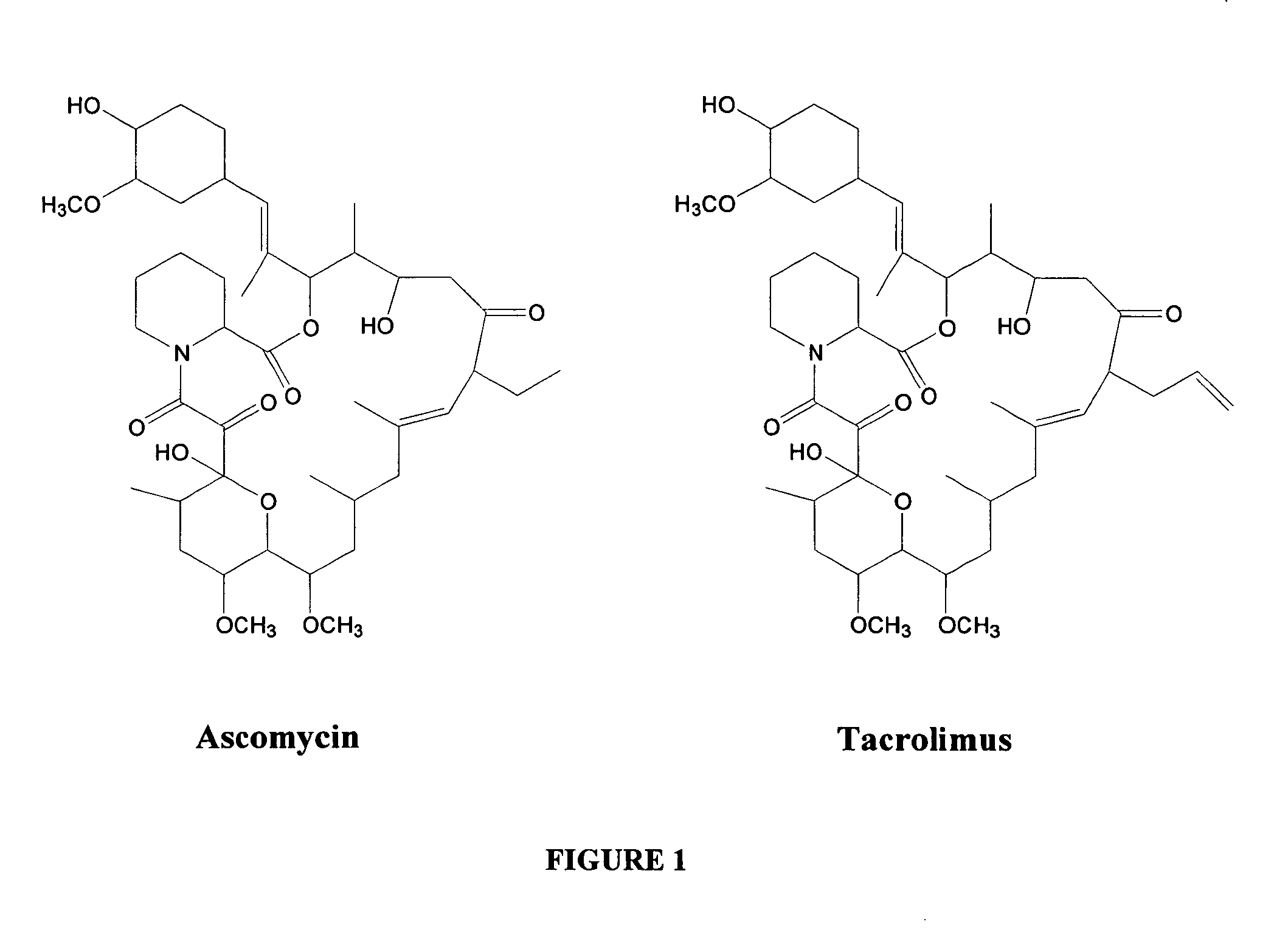
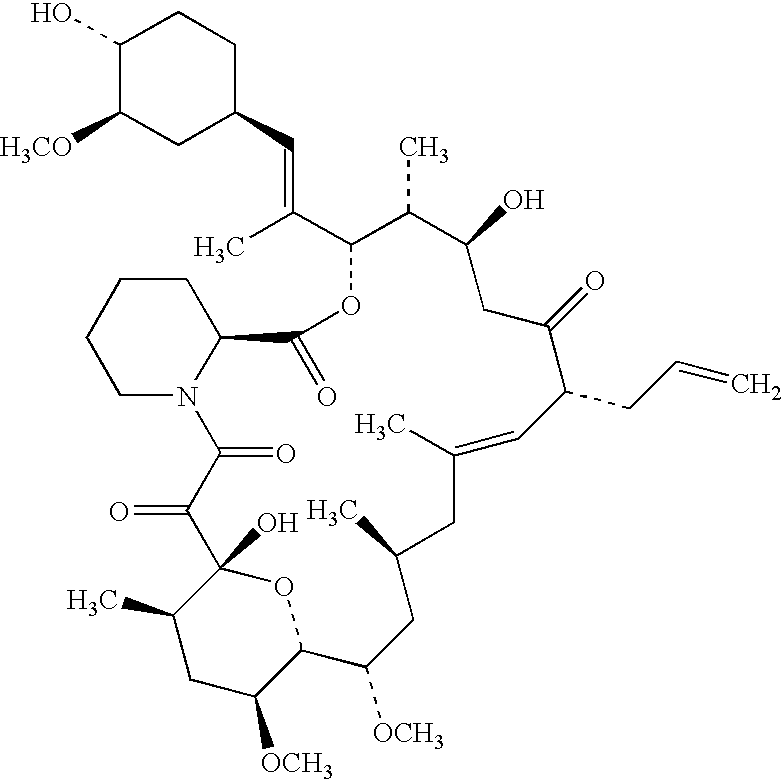
A macrolide isolated from Streptomyces tsukubaensis. Tacrolimus binds to the FKBP-12 protein and forms a complex with calcium-dependent proteins, thereby inhibiting calcineurin phosphatase activity and resulting in decreased cytokine production. This agent exhibits potent immunosuppressive activity in vivo and prevents the activation of T-lymphocytes in response to antigenic or mitogenic stimulation. Tacrolimus possesses similar immunosuppressive properties to cyclosporine, but is more potent.
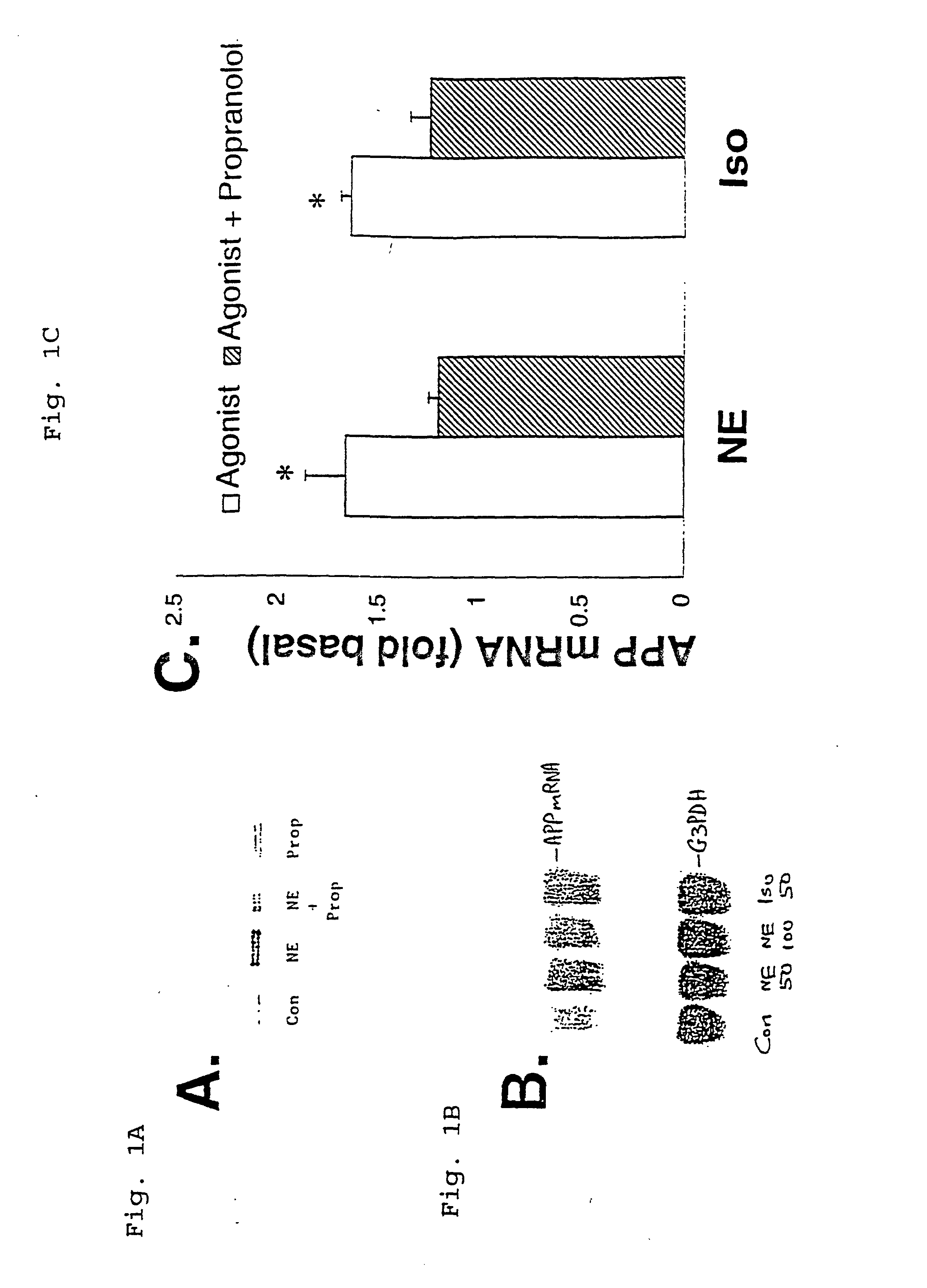
Composition and methods for treatment of neurological disorders and neurodegenerative diseases
InactiveUS6187756B1Increased formationPromote activationBiocideElcosanoid active ingredientsDiseaseGlial fibrillary acidic protein
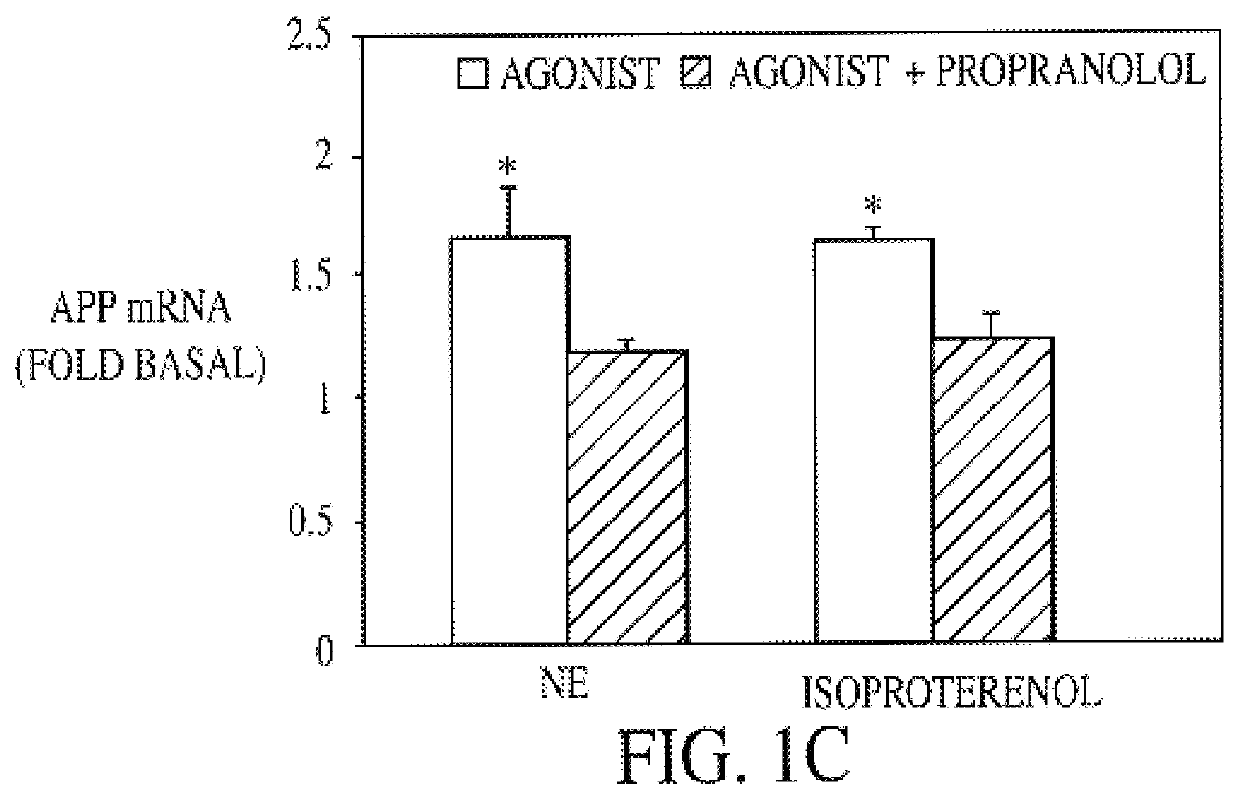
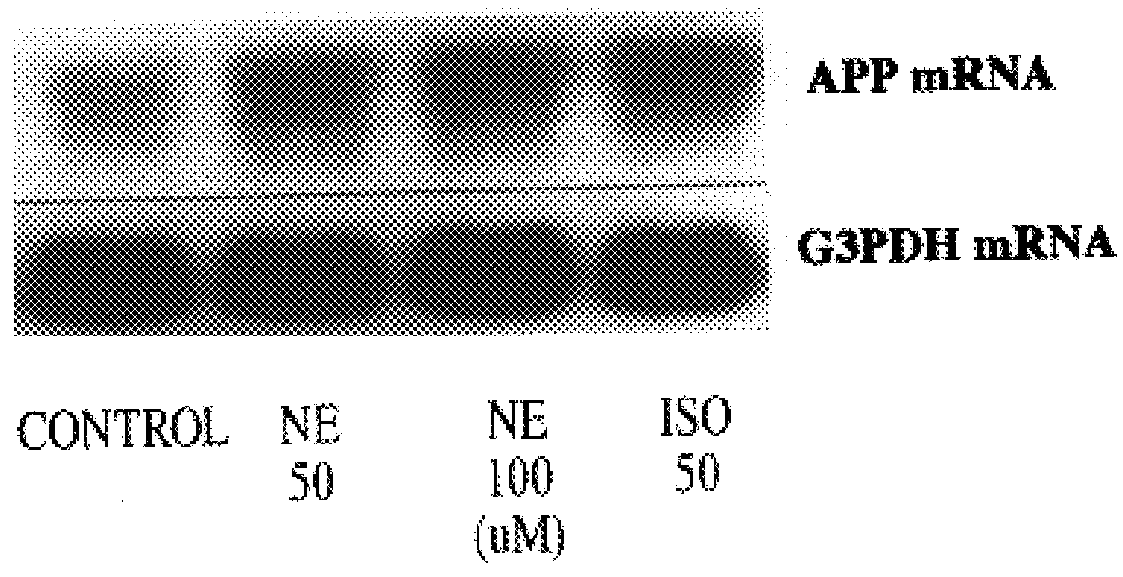
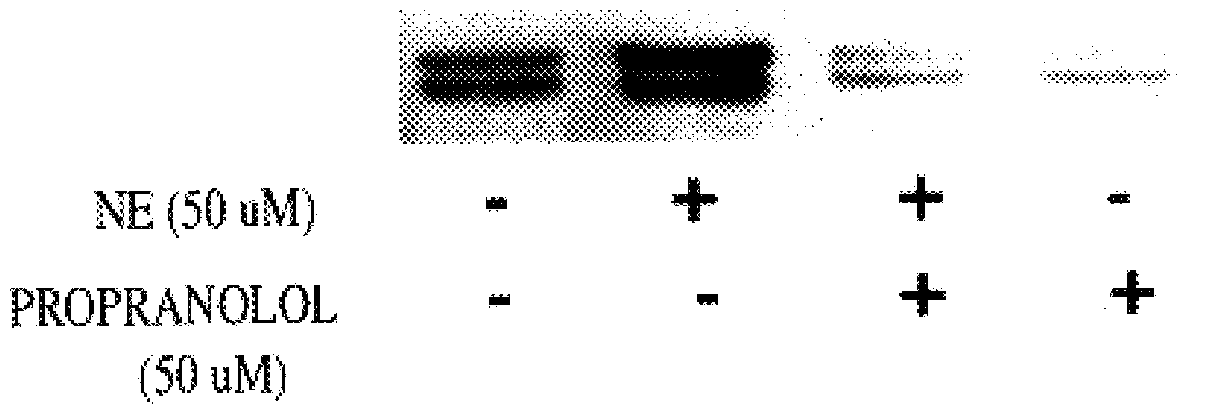
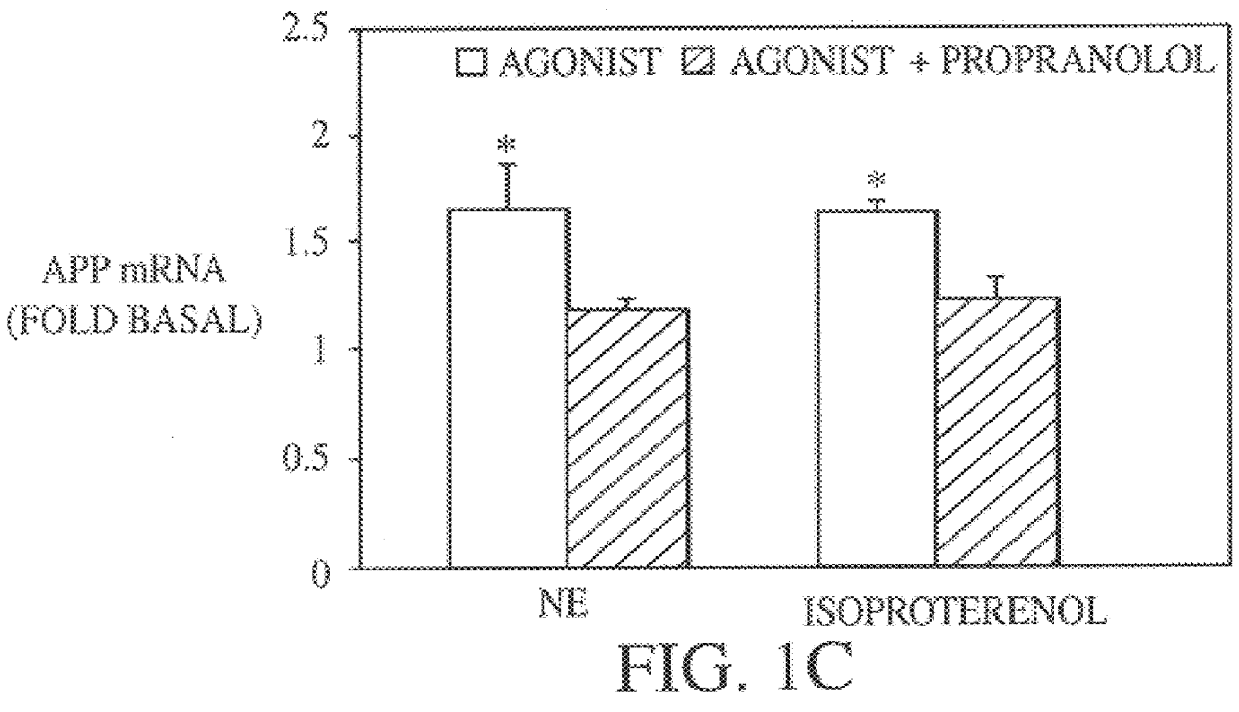
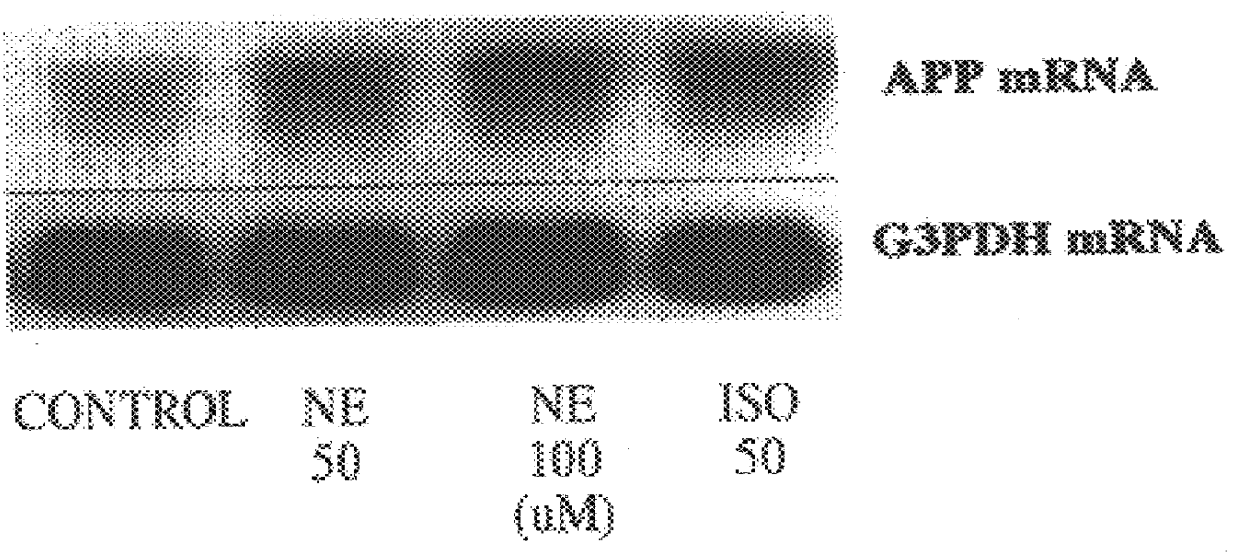
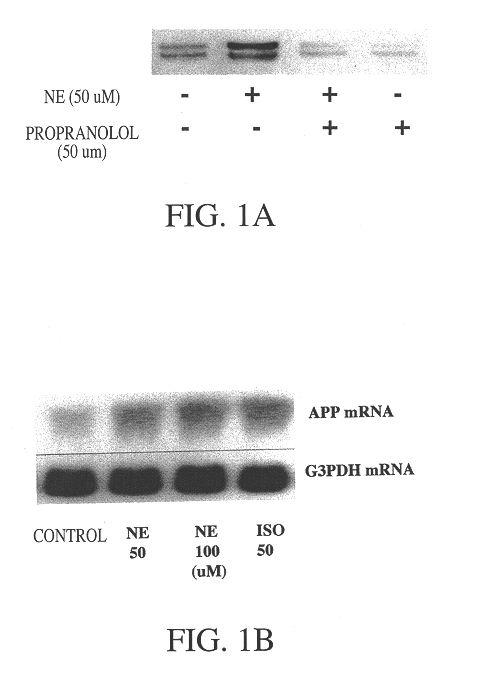
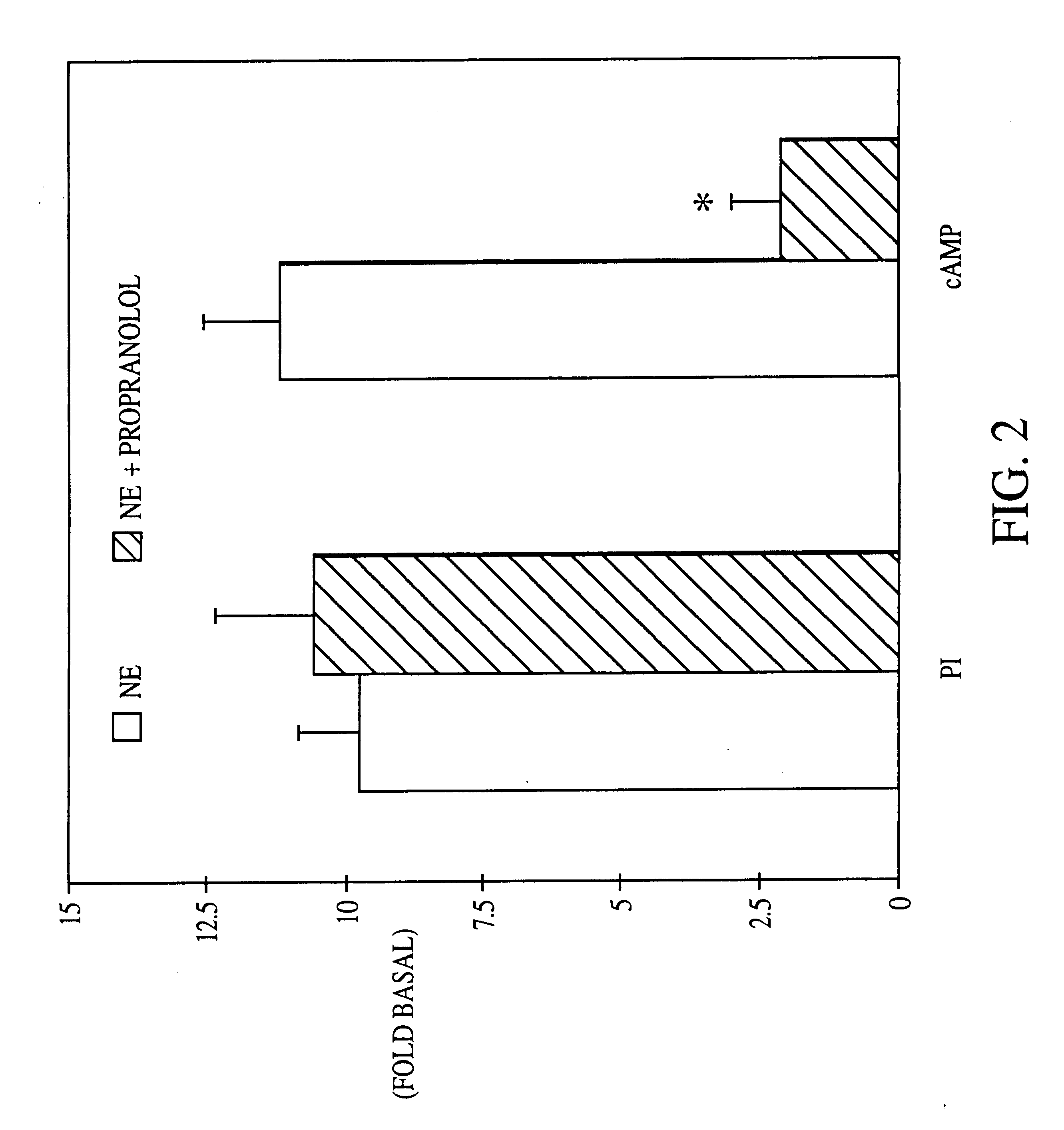
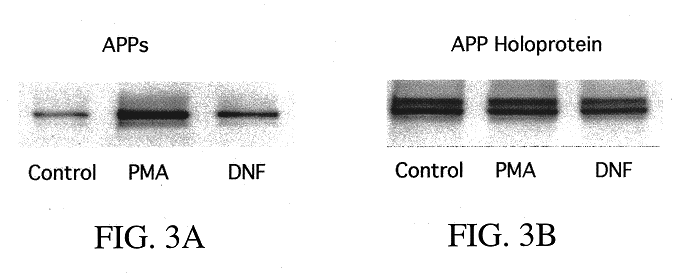
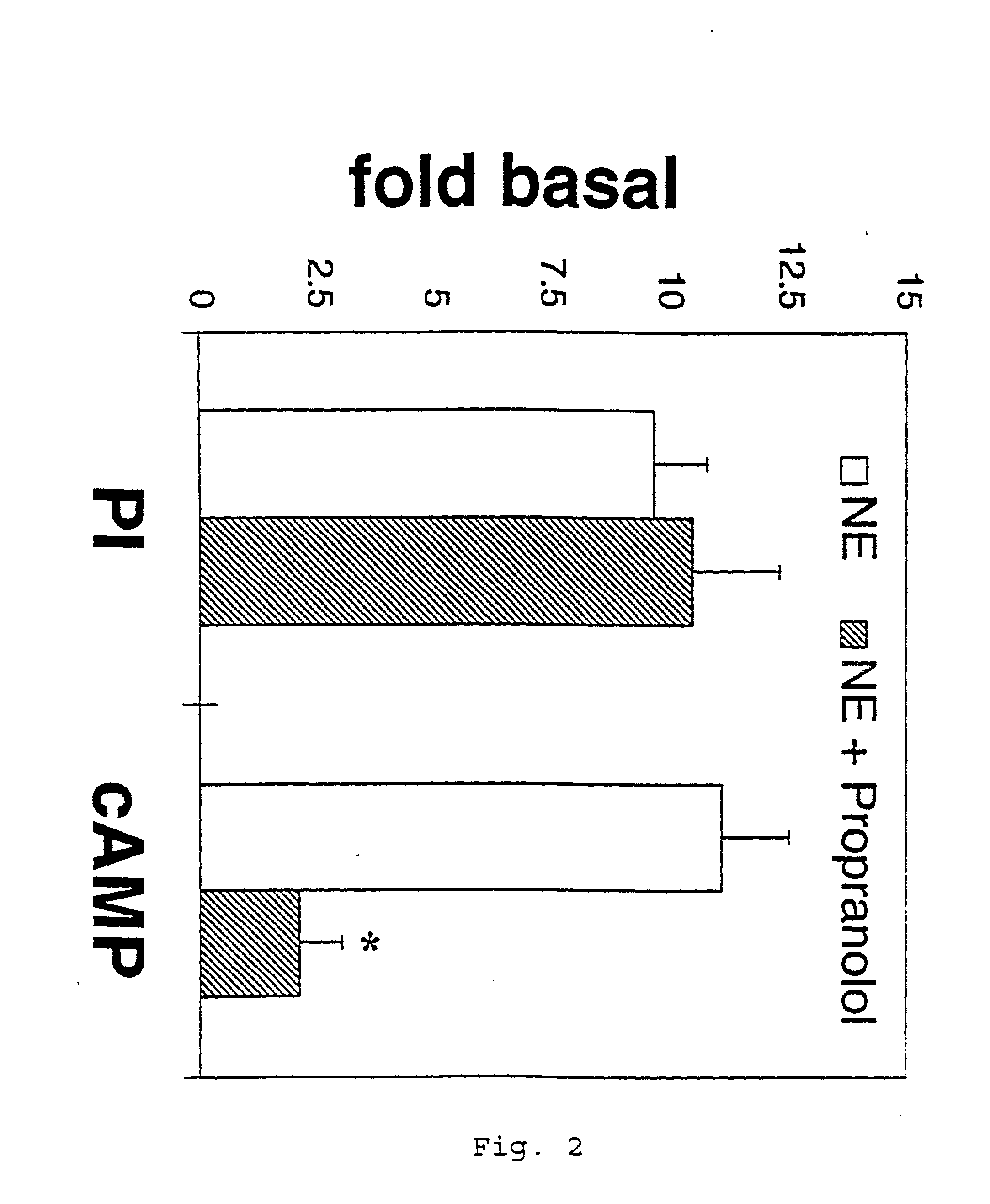
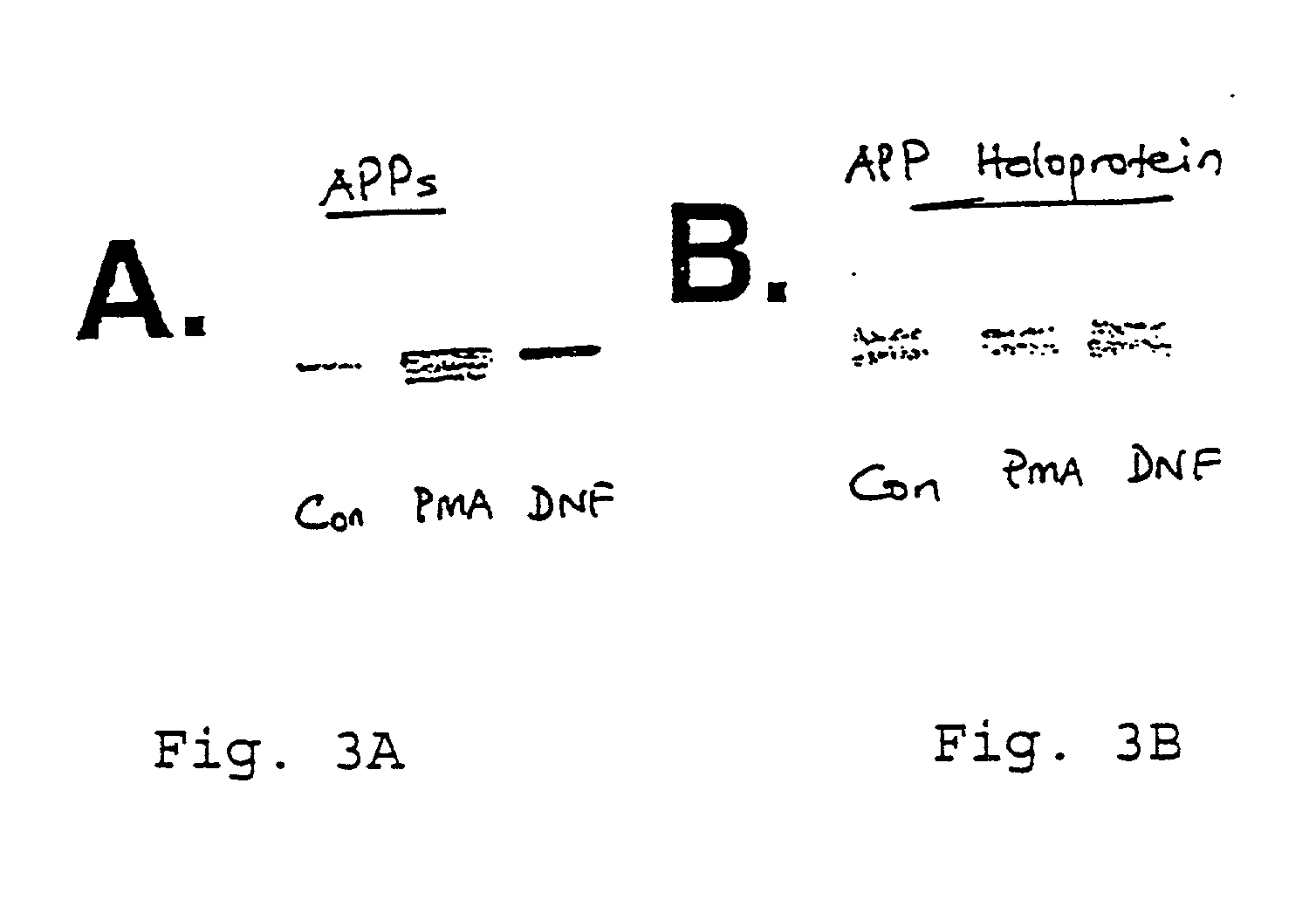
It has been discovered that the stimulation of beta-adrenergic receptors, which activate cAMP formation, give rise to increased APP and GFAP synthesis in astrocytes. Hence, the in vitro or in vivo exposure of neuronal cells to certain compositions comprising beta-adrenergic receptor ligands or agonists, including, e.g., norepinephrine, isoproterenol and the like, increases APP mRNA transcription and consequent APP overproduction. These increases are blocked by beta-adrenergic receptor antagonists, such as propranolol. The in vitro or in vivo treatment of these cells with 8Br-cAMP, prostaglandin E2 (PG E2), forskolin, and nicotine ditartrate also increased APP synthesis, including an increase in mRNA and holoprotein levels, as well as an increase in the expression of glial fibrillary acidic protein (GFAP). Compositions and methods are disclosed of regulating APP overexpression and mediating reactive astrogliosis through cAMP signaling or the activation of beta-adrenergic receptors. It has further been found that the increase in APP synthesis caused by 8Br-cAMP, PG E2, forskolin, or nicotine ditartrate is inhibited by immunosuppressants or anti-inflammatory agents, such as cyclosporin A, and FK-506 (tacrolimus), as well as ion-channel modulators, including ion chelating agents such as EGTA, or calcium / calmodulin kinase inhibitors, such as KN93. The present invention has broad implications in the alleviation, treatment, or prevention of neurological disorders and neurodegenerative diseases, including Alzheimer's Disease.
Owner:MASSACHUSETTS INST OF TECH
Injectable compositions of nanoparticulate immunosuppressive compounds
InactiveUS20060210638A1Improve complianceImprove efficacyPowder deliveryBiocideDepressantCompound (substance)
The invention is directed to an injectable nanoparticulate immunosuppressant composition for the formation of a subcutaneous or intramuscular depot. The invention is also directed to an injectable composition of nanoparticulate tacrolimus and / or sirolimus which eliminates the need to use polyoxyl 60 hydrogenated castor oil (HCO-60) and / or polysorbate 80 as a solubilizer. This invention further discloses a method of making an injectable nanoparticulate tacrolimus and / or sirolimus composition and is also directed to methods of treatment using the injectable nanoparticulate formulations comprising tacrolimus, sirolimus, or combination thereof for a subcutaneous or intramuscular depot for the prophylaxis of organ rejection and for the treatment of psoriasis or other immune diseases
Owner:ELAN PHRMA INT LTD
Compositions and methods for treatment of neurological disorders and neurodegenerative diseases
InactiveUS6043224AIncreased formationPromote activationBiocideElcosanoid active ingredientsGlial fibrillary acidic proteinDisease
It has been discovered that the stimulation of beta -adrenergic receptors, which activate cAMP formation, give rise to increased APP and GFAP synthesis in astrocytes. Hence, the in vitro or in vivo exposure of neuronal cells to certain compositions comprising beta -adrenergic receptor ligands or agonists, including, e.g., norepinephrine, isoproterenol and the like, increases APP mRNA transcription and consequent APP overproduction. These increases are blocked by beta -adrenergic receptor antagonists, such as propranolol. The in vitro or in vivo treatment of these cells with 8Br-cAMP, prostaglandin E2 (PG E2), forskolin, and nicotine ditartrate also increased APP synthesis, including an increase in mRNA and holoprotein levels, as well as an increase in the expression of glial fibrillary acidic protein (GFAP). Compositions and methods are disclosed of regulating APP overexpression and mediating reactive astrogliosis through cAMP signaling or the activation of beta -adrenergic receptors. It has further been found that the increase in APP synthesis caused by 8Br-cAMP, PG E2, forskolin, or nicotine ditartrate is inhibited by immunosuppressants or anti-inflammatory agents, such as cyclosporin A, and FK-506 (tacrolimus), as well as ion-channel modulators, including ion chelating agents such as EGTA, or calcium / calmodulin kinase inhibitors, such as KN93. The present invention has broad implications in the alleviation, treatment, or prevention of neurological disorders and neurodegenerative diseases, including Alzheimer's Disease.
Owner:MASSACHUSETTS INST OF TECH
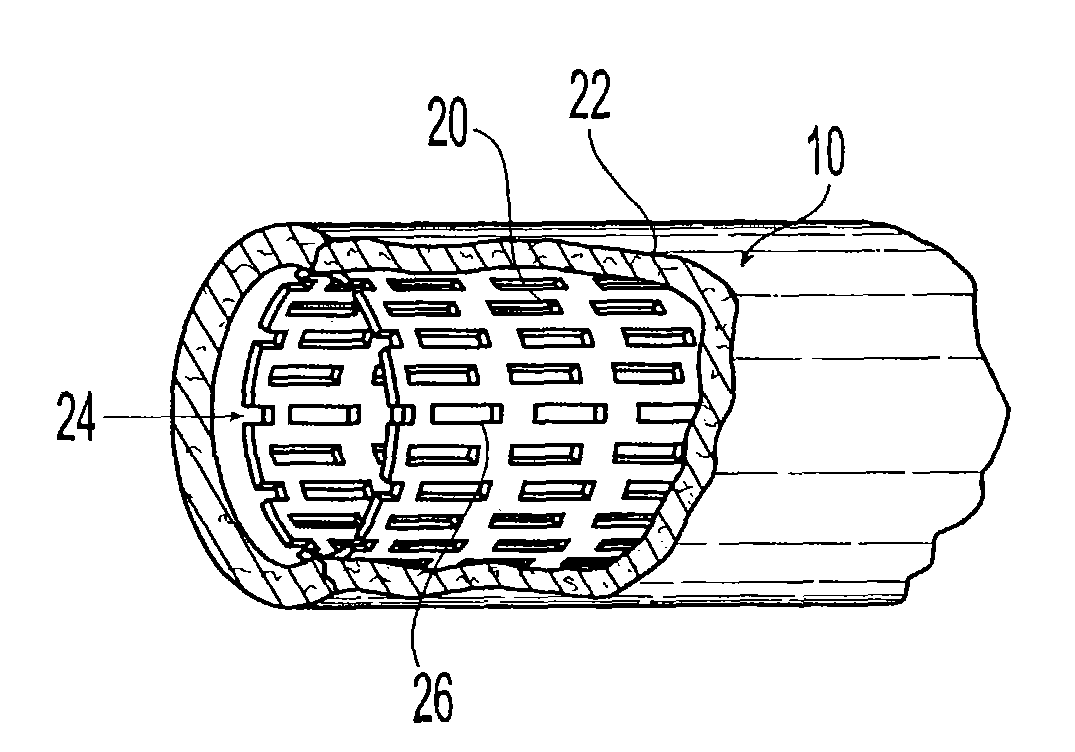
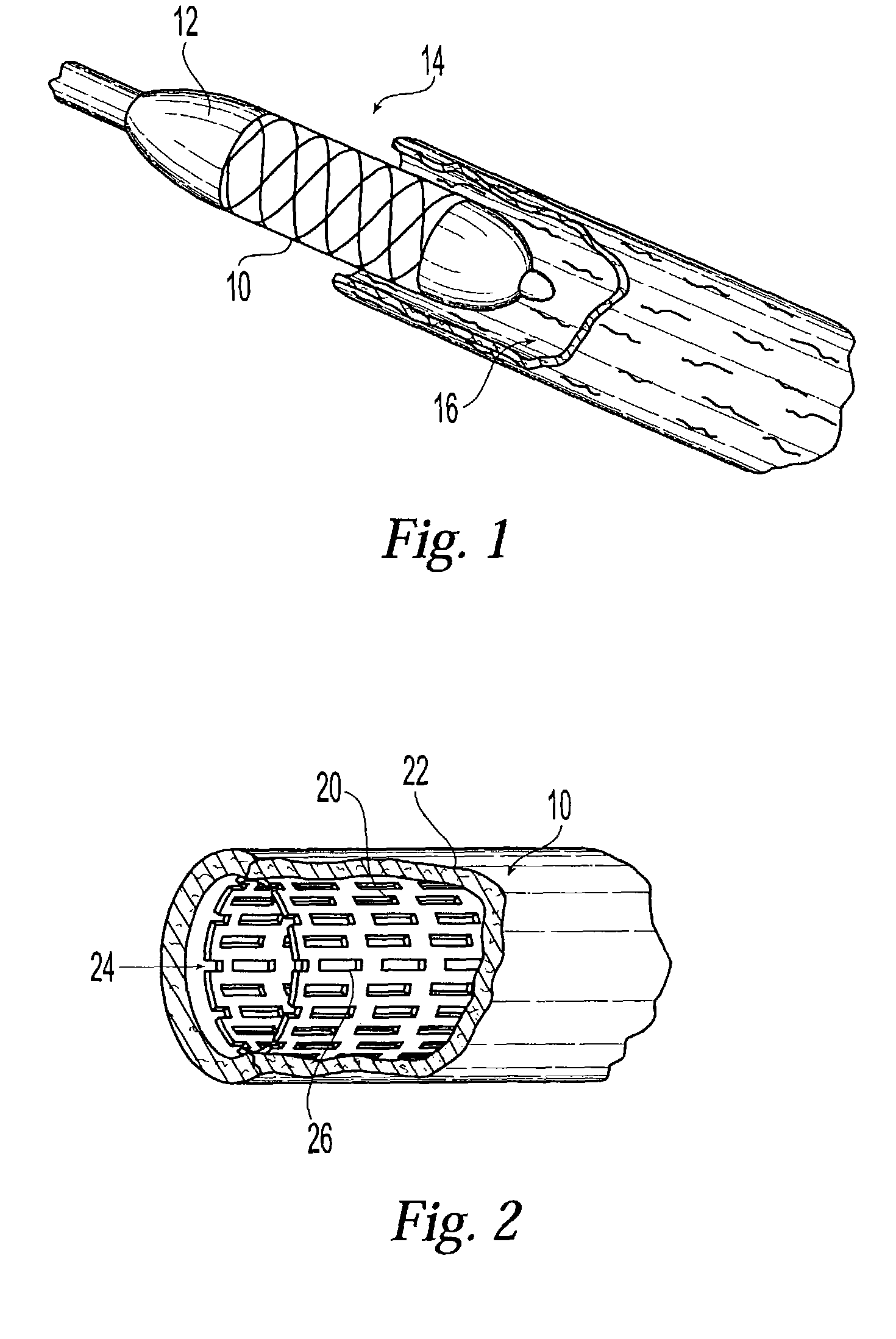
Drug eluting coatings for medical implants
ActiveUS20040037886A1Minimizing restenosisMinimizing thrombosisSuture equipmentsBiocideEverolimusCyclosporins
Drug eluting coating compositions are composed of at least one therapeutic agent dispersed in modified, biologically active binders. The therapeutic agents included in the coating composition are paclitaxel, sirolimus, tacrolimus, everolimus, actinomycin-D, dexamethasone, mycophenolic acid, cyclosporins, estradiol, and derivatives and analogs thereof. These therapeutic agents are applied to the surface of the medical device by a modified, biologically active binders. By using these biologically active binders, the therapeutic agents can be applied to at least one surface of a medical implant without using inert polymer carriers.
Owner:BIOVENTION INC
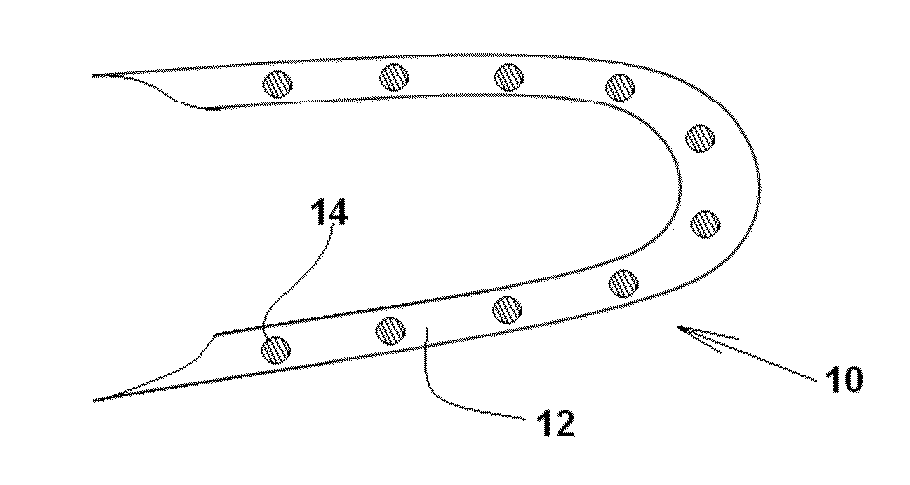
Stent device and method
Owner:BOSTON SCI SCIMED INC
Nanoparticulate tacrolimus formulations
InactiveUS20060159766A1Dissolve fastImprove complianceMaterial nanotechnologyBiocideMedicineNanoparticle
The present invention is directed to nanoparticulate tacrolimus compositions. The composition comprising tacrolimus particles having an effective average particle size of less than about 2000 nm and at least one surface stabilizer.
Owner:ELAN PHRMA INT LTD
Compositions and methods for treatment of neurological disorders and neurodegenerative diseases
InactiveUS6469055B2Increased formationPromote activationBiocideNervous disorderGlial fibrillary acidic proteinDisease
It has been discovered that the stimulation of beta-adrenergic receptors, which activate cAMP formation, give rise to increased APP and GFAP synthesis in astrocytes. Hence, the in vitro or in vivo exposure of neuronal cells to certain compositions comprising beta-adrenergic receptor ligands or agonists, including, e.g., norepinephrine, isoproterenol and the like, increases APP mRNA transcription and consequent APP overproduction. These increases are blocked by beta-adrenergic receptor antagonists, such as propranolol. The in vitro or in vivo treatment of these cells with 8Br-cAMP, prostaglandin E2 (PG E2), forskolin, and nicotine ditartrate also increased APP synthesis, including an increase in mRNA and holoprotein levels, as well as an increase in the expression of glial fibrillary acidic protein (GFAP). Compositions and methods are disclosed of regulating APP overexpression and mediating reactive astrogliosis through cAMP signaling or the activation of beta-adrenergic receptors. It has further been found that the increase in APP synthesis caused by 8Br-cAMP, PG E2, or forskolin is inhibited by immunosuppressants, immunophilin ligands, or anti-inflammatory agents, such as cyclosporin A, and FK-506 (tacrolimus), as well as ion-channel modulators, including ion chelating agents such as EGTA, or calcium / calmodulin kinase inhibitors, such as KN93. The present invention has broad implications in the alleviation, treatment, or prevention of neurological disorders and neurodegenerative diseases, including Alzheimer's Disease.
Owner:MASSACHUSETTS INST OF TECH
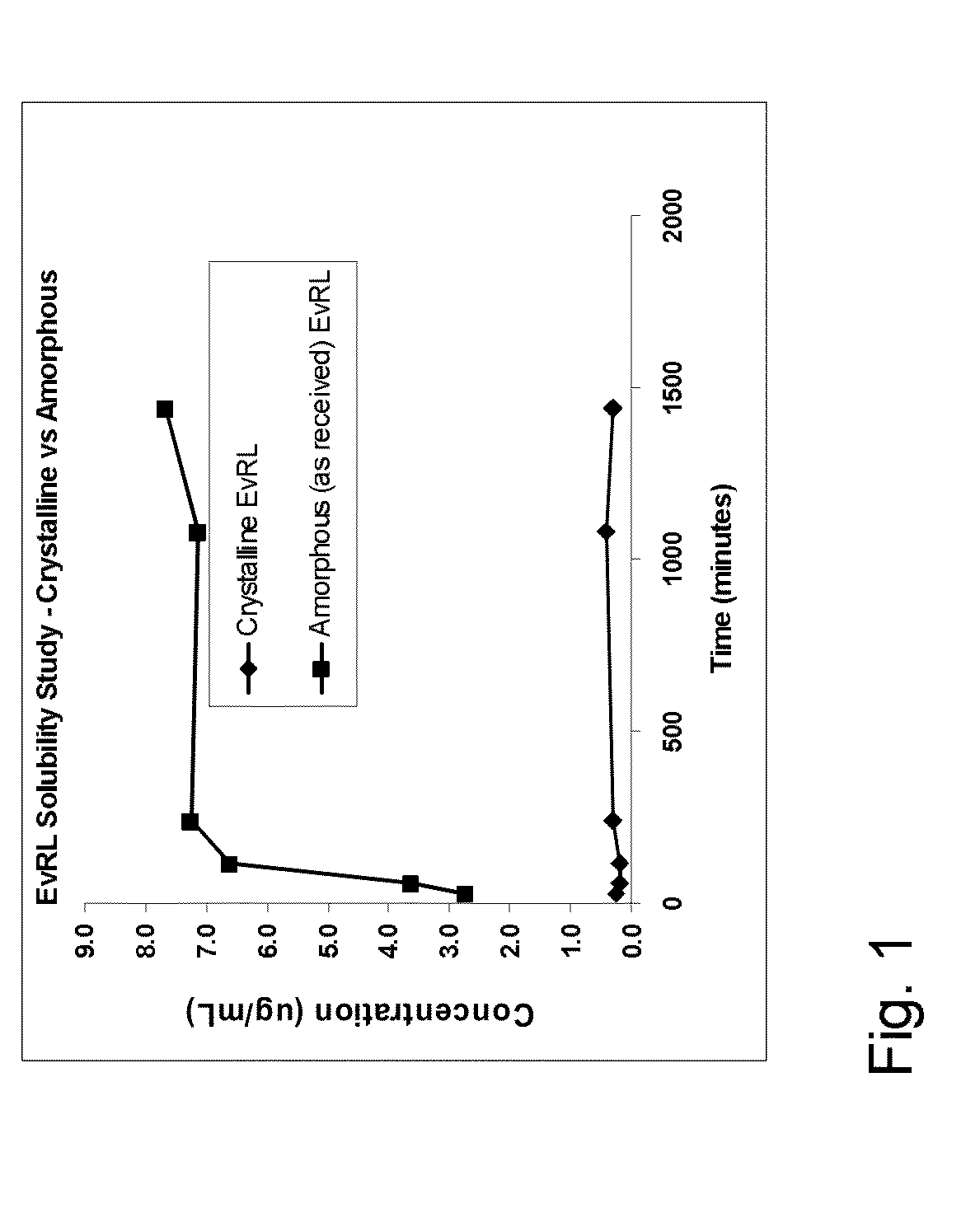
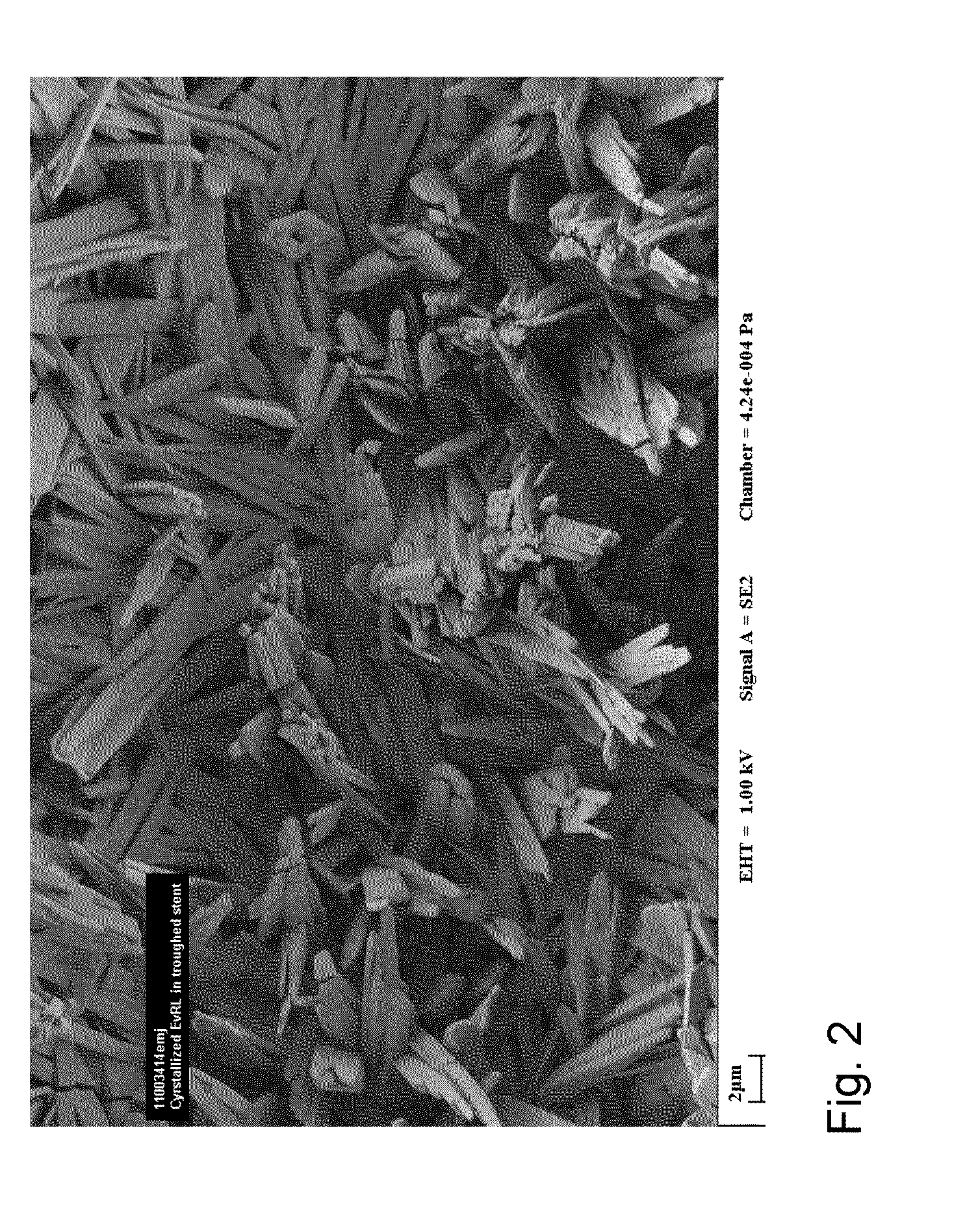
Medical Device with Crystalline Drug Coating
A medical device having a polymer-free outer surface layer comprising a crystalline drug selected from the group consisting of everolimus, tacrolimus, sirolimus, zotarolimus, biolimus, and rapamycin. The device may be produced by a method comprising the steps of providing a medical device; applying a solution of the drug to said portion of the outer surface to form a coating of amorphous drug; and vapor annealing the drug with a solvent vapor to form crystalline drug; wherein a seed layer of a crystalline form of said drug having a maximum particle size of about 10 μm or less is applied to at least said portion of the outer surface of the device before or after applying the drug solution, but before vapor annealing the amorphous coating.
Owner:BOSTON SCI SCIMED INC
Ocular solutions
InactiveUS7083803B2Reduce inflammationReduce bacterial growthBiocideSenses disorderDiseaseEverolimus
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease.
Owner:PEYMAN GHOLAM A DR
Ocular solutions
InactiveUS20050063996A1Reduce turbidityReduce inflammationAntibacterial agentsBiocideEverolimusCell migration
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions.
Owner:PEYMAN GHOLAM A DR
Compositions and methods for treatment of neurological disorders and neurodegenerative diseases
InactiveUS20020052407A1Prevent APP over-expressionInhibit overexpressionBiocideNervous disorderGlial fibrillary acidic proteinDisease
It has been discovered that the stimulation of beta-adrenergic receptors, which activate cAMP formation, give rise to increased APP and GFAP synthesis in astrocytes. Hence, the in vitro or in vivo exposure of neuronal cells to certain compositions comprising beta-adrenergic receptor ligands or agonists, including, e.g., norepinephrine, isoproterenol and the like, increases APP mRNA transcription and consequent APP overproduction. These increases are blocked by beta-adrenergic receptor antagonists, such as propranolol. The in vitro or in vivo treatment of these cells with 8Br-cAMP, prostaglandin E2 (PG E2), forskolin, and nicotine ditartrate also increased APP synthesis, including an increase in mRNA and holoprotein levels, as well as an increase in the expression of glial fibrillary acidic protein (GFAP). Compositions and methods are disclosed of regulating APP overexpression and mediating reactive astrogliosis through cAMP signaling or the activation of beta-adrenergic receptors. It has further been found that the increase in APP synthesis caused by 8Br-cAMP, PG E2, or forskolin is inhibited by immunosuppressants, immunophilin ligands, or anti-inflammatory agents, such as cyclosporin A, and FK-506 (tacrolimus), as well as ion-channel modulators, including ion chelating agents such as EGTA, or calcium / calmodulin kinase inhibitors, such as KN93. The present invention has broad implications in the alleviation, treatment, or prevention of neurological disorders and neurodegenerative diseases, including Alzheimer's Disease.
Owner:MASSACHUSETTS INST OF TECH
Ocular solutions
InactiveUS20060228394A1Reduce inflammationReduce bacterial growthAntibacterial agentsBiocideEverolimusOcular structure
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease. The agent(s) may be formulated with polymers or other components for extended or slow release to provide a substantially constant concentration over the course of treatment.
Owner:MINU
Ocular solutions
InactiveUS7087237B2Reduce inflammationReduce bacterial growthAntibacterial agentsBiocideEverolimusMacrolide resistance
Containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions.
Owner:PEYMAN GHOLAM A DR
Combination pharmaceutical compositions
InactiveUS20100215737A1Slow changeGood for healthPowder deliveryBiocideControlled releaseImmediate release
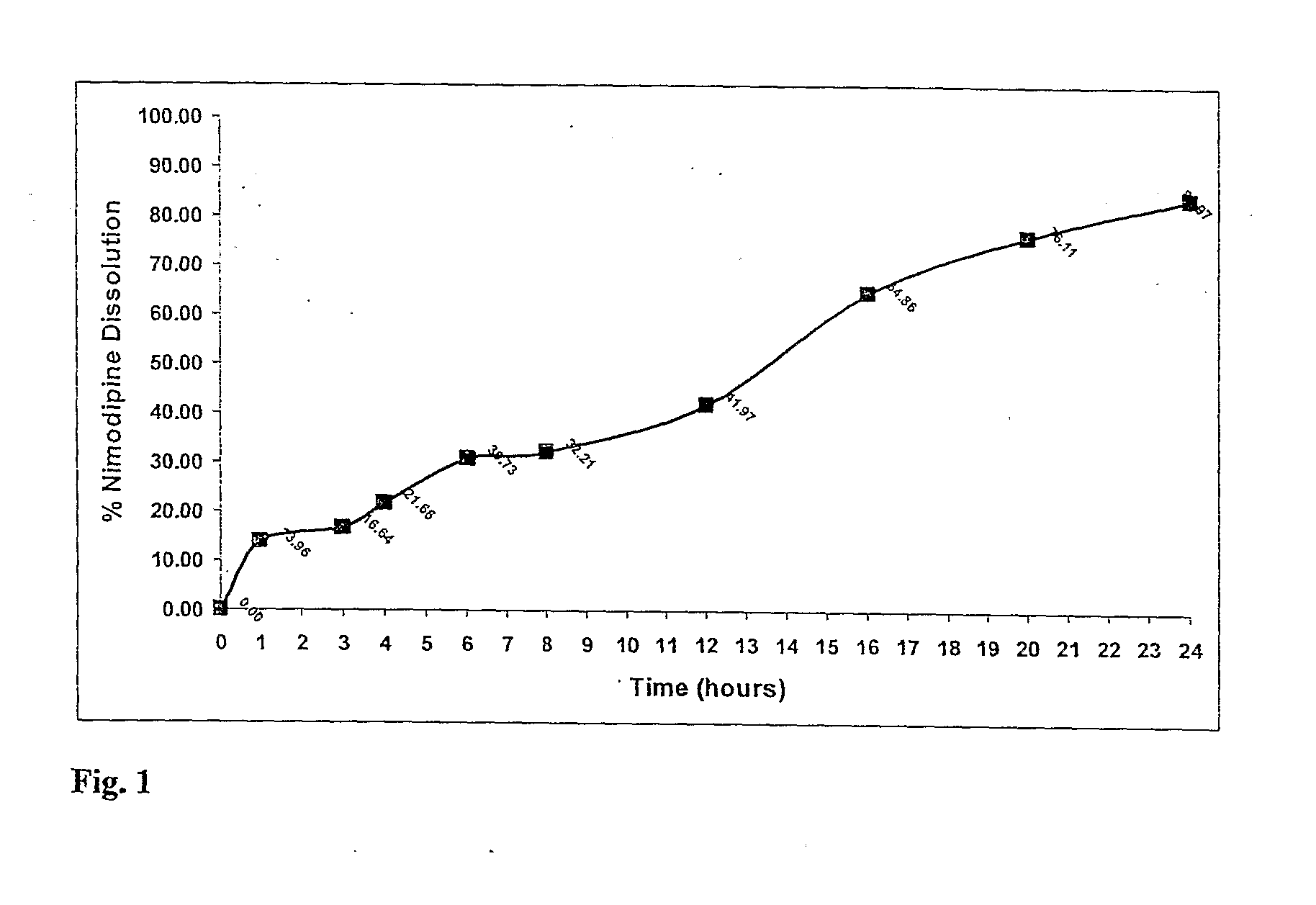
A modified release dosage product (5) comprises a plurality of minicapsules or minispheres (1, 2) containing nimodipine, and a plurality of minicapsules or minispheres (3), (4) containing tacrolimus. There are uncoated minicapsules or minispheres (1) encapsulating micronized nimodipine for immediate release and a controlled release polymer coated minicapsule or minisphere (2) encapsulating micronized nimodipine for delayed, sustained, controlled or targeted release. There are uncoated seamless minicapsules (3), the core of which comprises tacrolimus lipid-based formulation for immediate release and a controlled release polymer coated seamless minicapsule (4), the core of which comprises tacrolimus lipid-based formulation for delayed, sustained, controlled release or targeted release. The final dosage form may be a hard gelatin capsule (5).
Owner:COULTER IVAN
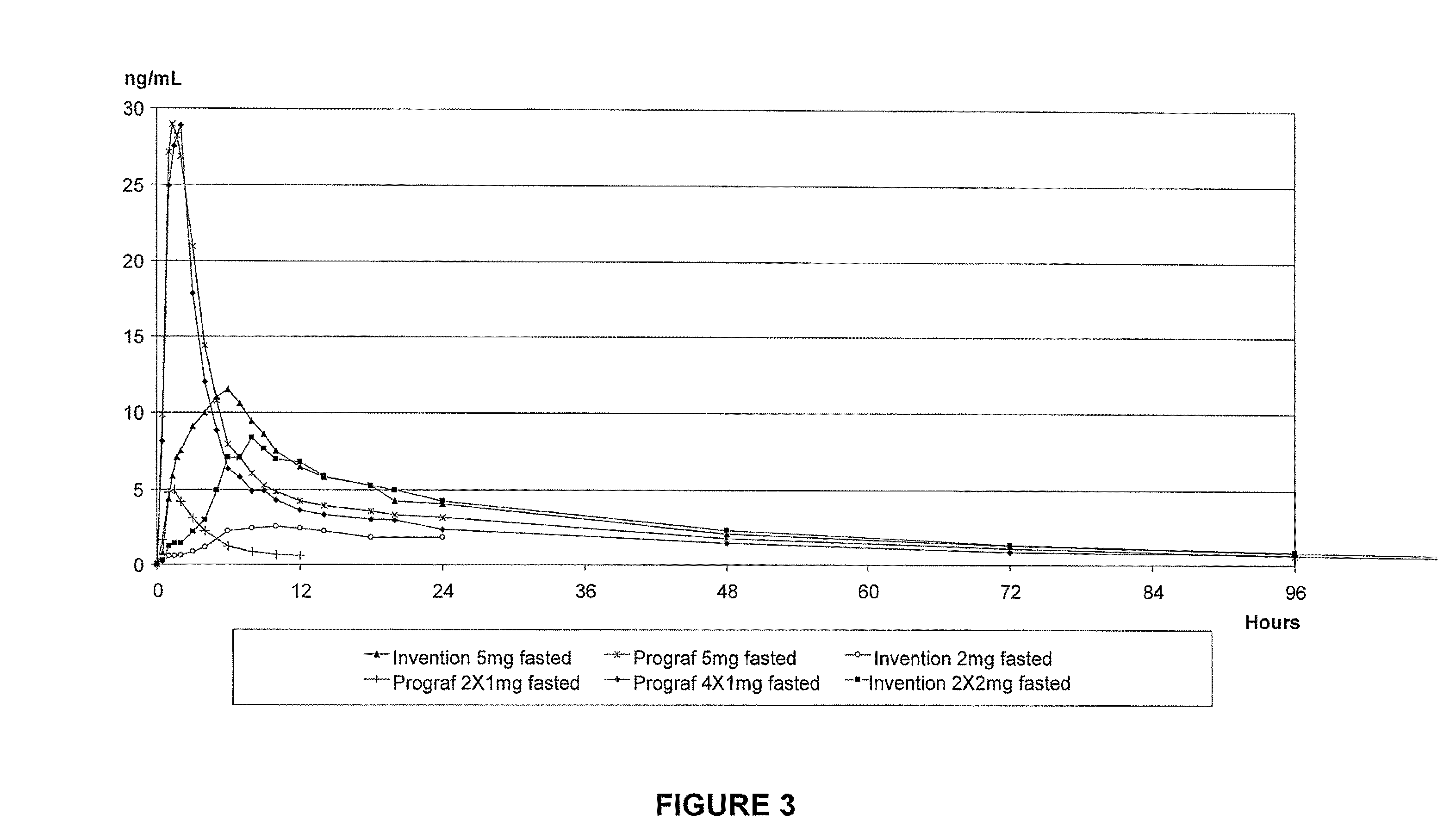
Tacrolimus for improved treatment of transplant patients
ActiveUS20100105717A1Improve bioavailabilityReduce riskBiocideOrganic chemistryTherapeutic effectIn vivo
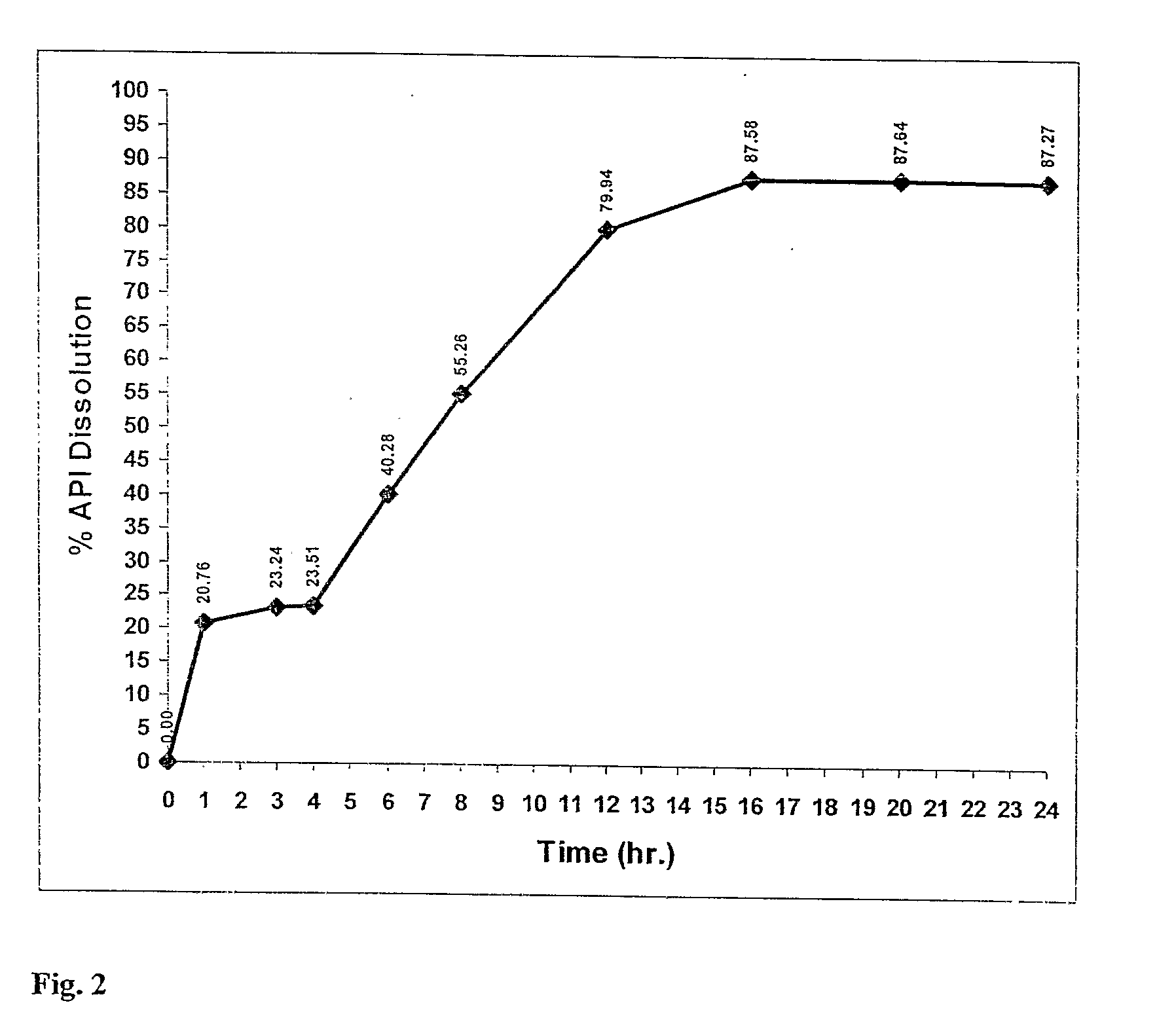
An extended release oral dosage form comprising as active substance tacrolimus or a pharmaceutically active analogue thereof for a once daily immunosuppressive treatment of a patient in need thereof, preferable a kidney or liver transplant patient. The dosage form releases the active substance over an extended period of time. It also provides improved pharmacokinetic parameters due to an extended and constant in vivo release including substantial decreased peak concentrations, despite increased bioavailability, substantial extended times for maximal concentration, and higher minimal concentrations when compared with conventional immediate release dosage forms and a recent modified release tacrolimus dosage form.
Owner:VELOXIS PHARM INC
Aqueous Systems For The Preparation Of Lipid Based Pharmaceutical Compounds; Compositions, Methods, And Uses Thereof
InactiveUS20100068251A1Simple and rapid and to produceSimple and rapid inspectionBiocidePowder deliveryDiseaseOrganic solvent
The present invention relates to a methods of preparing active compounds complexed with lipids using aqueous systems that are free of organic solvents, and methods of using the complexes, e.g., in treating a disease in a subject. In some embodiments, the present invention comprises a composition comprising a complex comprising at least one active compound, e.g., a polyene antibiotic, an immunosuppressant agent such as tacrolimus or a taxane or taxane derivative, and one or more lipids. In some embodiments, the present invention provides a method comprising preparing a composition comprising a lipid complex comprising at least one active compound and at least one lipid and administering the composition to a subject. In certain embodiments the subject is a mammal. In certain preferred embodiments, the subject is human.
Owner:JINA PHARMA
Intravenous pacemaker electrode
InactiveUS7643885B2Low change over timeGuaranteed long-term use effectElectrotherapyEverolimusTransvenous pacemakers
Owner:SIEMENS AG
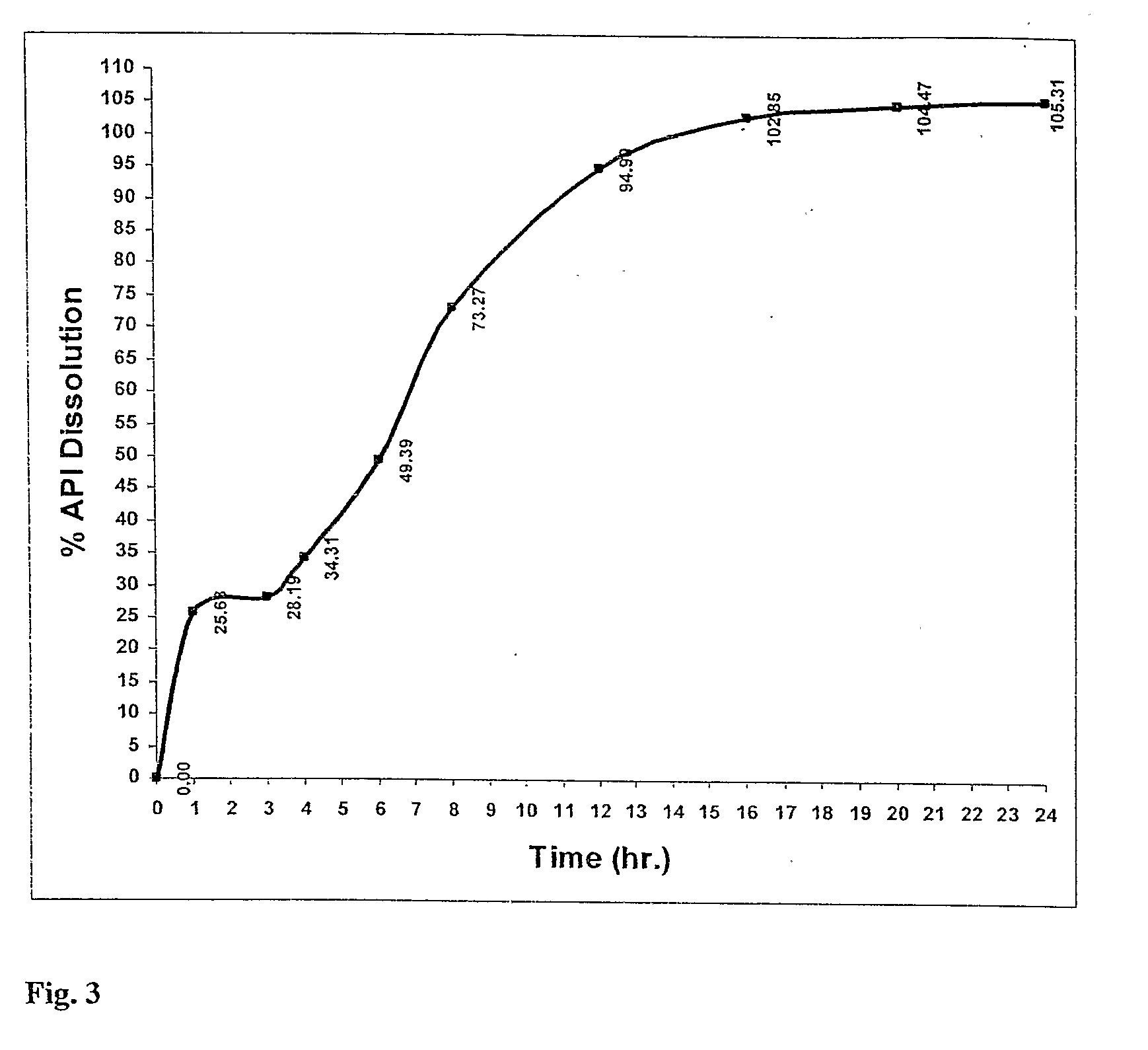
Solid dispersion of tacrolimus
InactiveUS20060177500A1High dissolution rateImprove bioavailabilityPowder deliveryOrganic active ingredientsDrug releaseBioavailability
The present invention relates to the carrier of the solid dispersion of tacrolimus, which is prepared by using the solid surfactant having a property of HLB value higher than or equal to about 7. The surfactants carry out a function of a carrier and a function of a dissolution enhancer, simultaneously. As a result, the dissolution rate of tacrolimus is improved, and the oral absorbability and the bioavailability may be increased due to rapid drug release.
Owner:CHONG KUN DANG PHARMA CORP
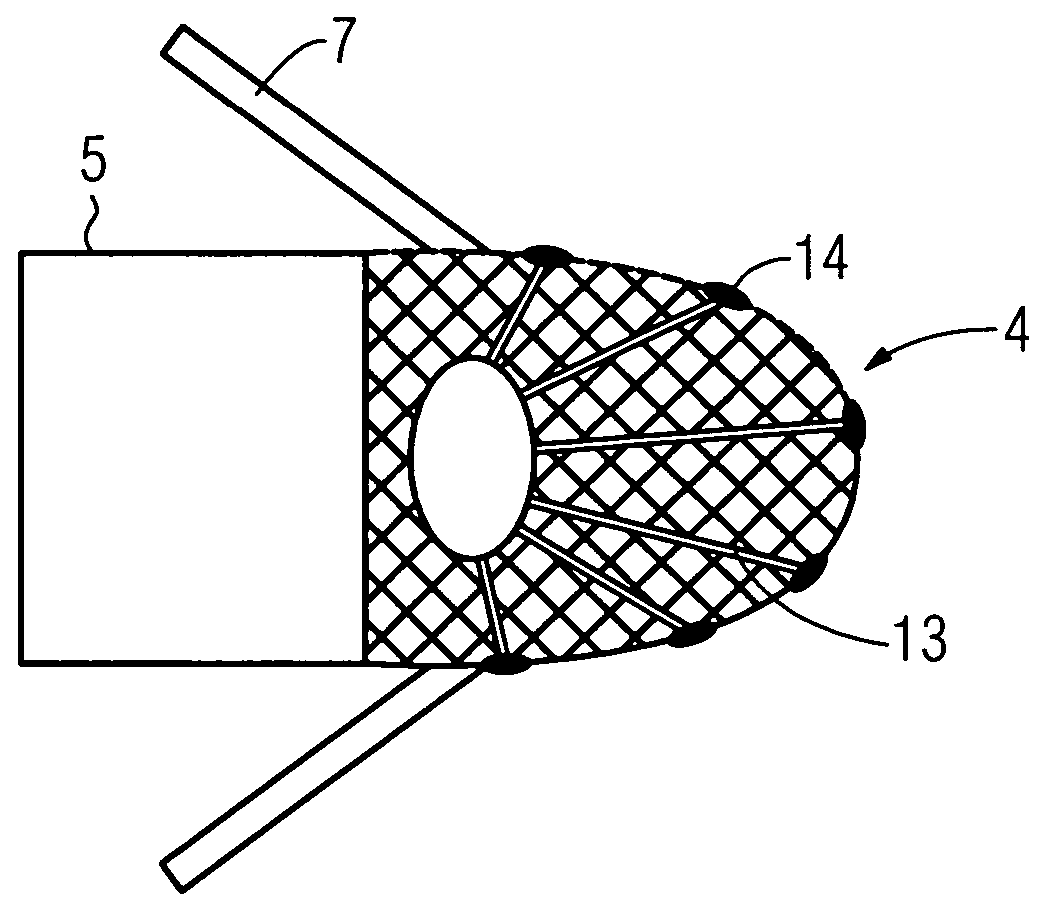
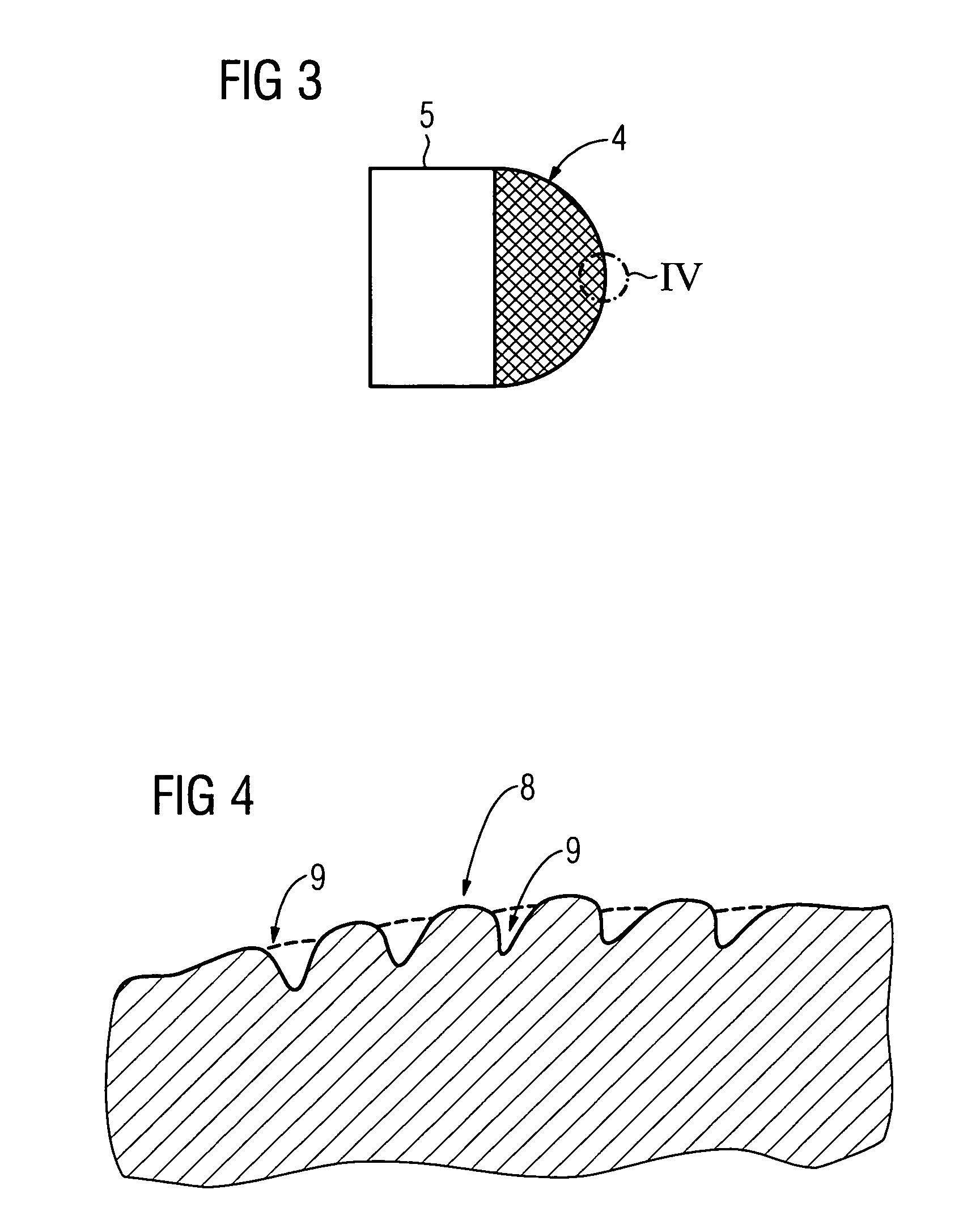
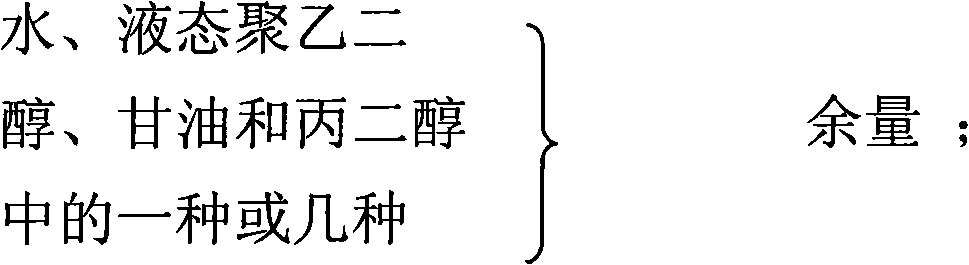
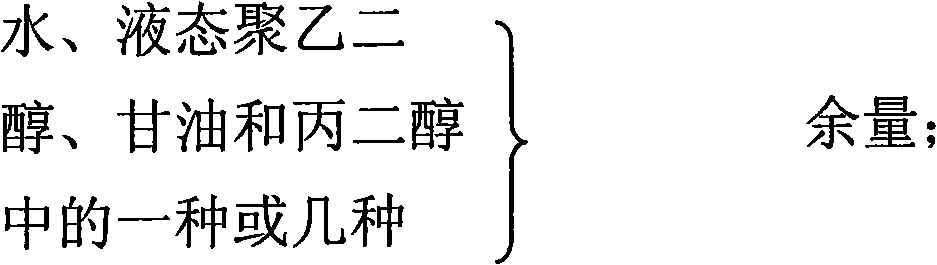
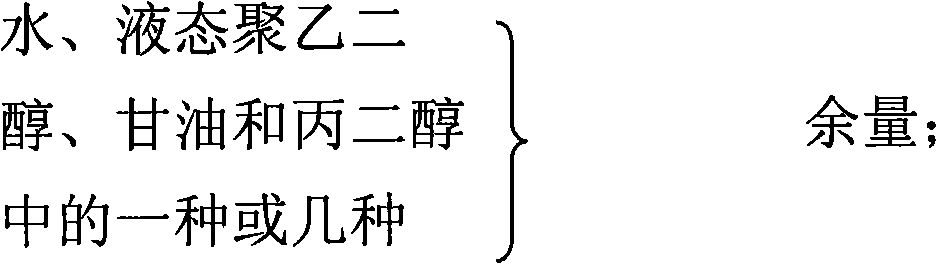
Gel composition containing tacrolimu and its preparation method and medicinal application
InactiveCN101288643AEasy to cleanNo bad smellOrganic active ingredientsPharmaceutical delivery mechanismDiseaseAdditive ingredient
The invention relates to a gel composition containing tacrolimus, which contains the tacrolimus and ingredients of a matrix, wherein, the ingredients of the matrix contain one or more of liquid polyethylene glycol, glycerin and propylene glycol, the content of the tacrolimus in the gel is 0.01 percent to 0.5 percent, and the weight ratio of the tacrolimus to one or more of the liquid polyethylene glycol, the glycerin and the propylene glycol is 1: (50 to 3000). The invention further relates to a preparation method of the gel composition of the tacrolimus and the application of the gel composition in the preparation of drugs for the treatment of atopic dermatitis, vitiligo, psoriasis, hormone-dependent dermatitis, intractable neurodermatitis, lupus erythematosus, alopecia areata and other diseases.
Owner:杨喜鸿
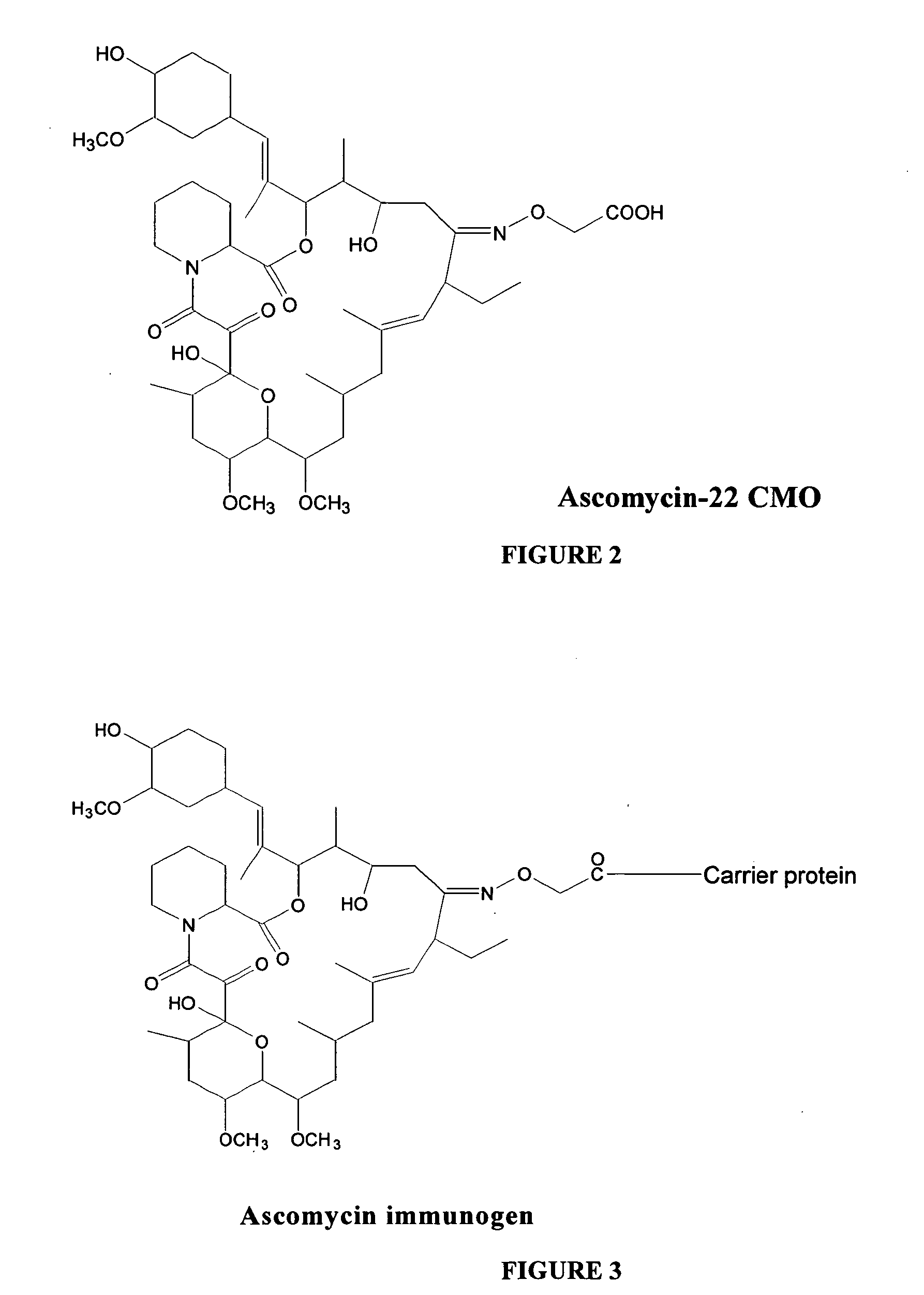
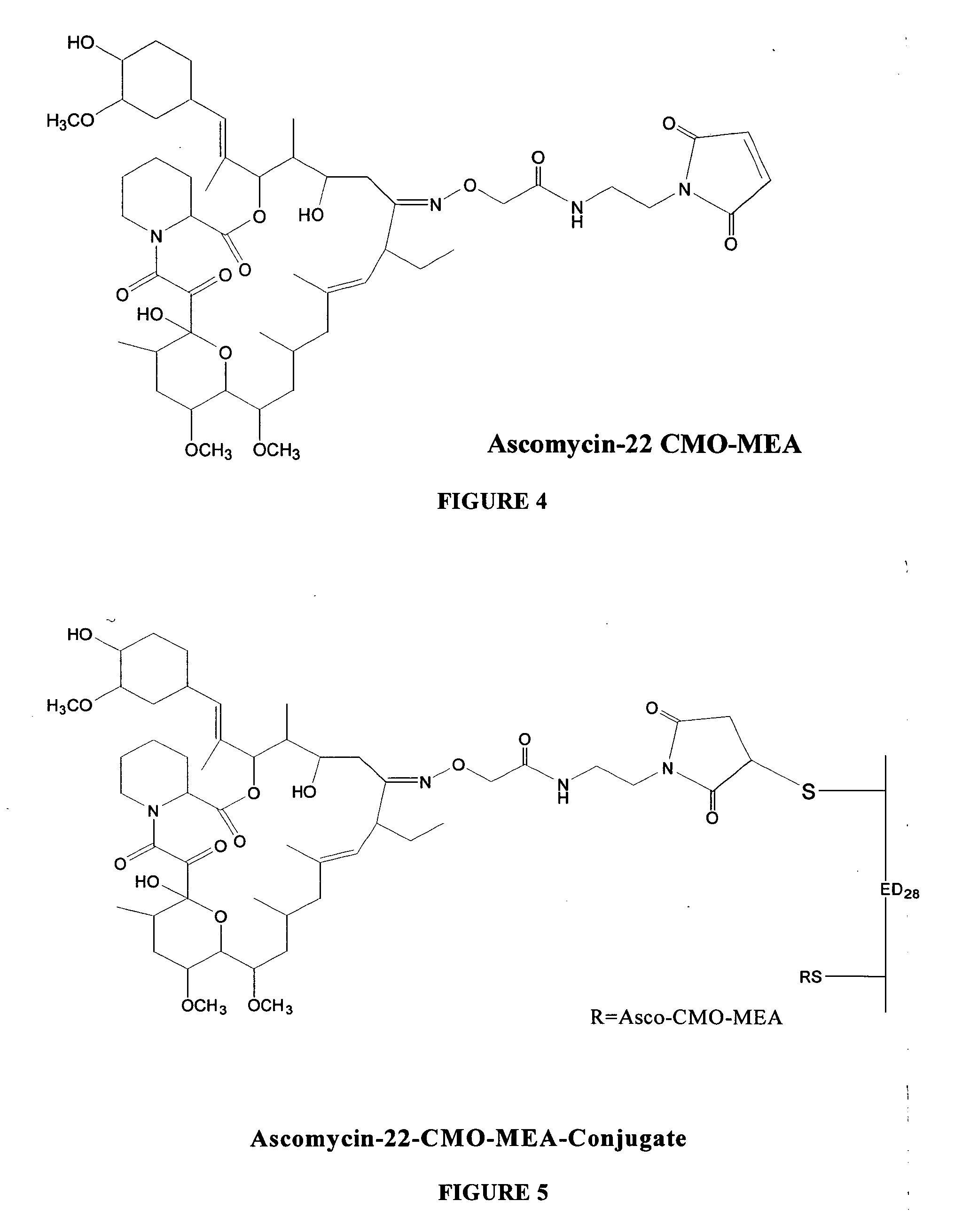
Hapten, immunogens and derivatives of ascomycin useful for preparation of antibodies and immunoassays
The invention teaches derivatives of ascomycin and methods of preparing immunogens and other conjugates useful in immunoassays for quantitatively measuring concentrations of tacrolimus in patient specimens. Antibodies produced from the disclosed immunogens capable of binding to tacrolimus with cross-reactivity of no more than 5% with each of 15-O-demethyl tacrolimus, 31-O-demethyl tacrolimus, and 13,31-O-didemethyl tacrolimus, less than 40% with 13-O-demethyl tacrolimus, and less than 1% with cyclosporin, rapamycin, mycophenolic acid, prednisone, hydrocortisol, and prednisolone are described. Further, immunoassays for measuring the concentration of tacrolimus using such antibodies are taught.
Owner:MICROGENICS CORP
Differential hemolysis of a whole blood sample
ActiveUS20090253210A1Ion-exchange process apparatusParticle separator tubesChromatographic separationImmunosuppressive drug
The invention relates to a method for differentially hemolyzing whole blood. It discloses method for detecting an analyte in a liquid sample known or suspected to comprise red blood cells and suspected or known to comprise eukaryotic cells, the method comprising the steps of processing said liquid sample with a membrane solubilizing agent under conditions appropriate to lyse cell membranes of red blood cells and at the same time not to cause precipitation of sample constituents, subjecting the processed sample to a chromatographic separation, and detecting the analyte. The differential hemolysis of red blood cells is of advantage in a method of detecting an analyte in a liquid sample that may comprise both erythrocytes as well as nucleated cells. The differential solubilization of red blood cells can be easily combined with an online detection methodology, like LC-MS, and is advantageous in the detection of many analytes, e.g. in the detection of folate or of immunosuppressive drugs, like tacrolimus or sirolimus.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Drug eluting coatings for medical implants
InactiveUS7438925B2Control releaseMinimize any pathologies associatedSuture equipmentsStentsEverolimusCyclosporins
Drug eluting coating compositions are composed of at least one therapeutic agent dispersed in modified, biologically active binders. The therapeutic agents included in the coating composition are paclitaxel, sirolimus, tacrolimus, everolimus, actinomycin-D, dexamethasone, mycophenolic acid, cyclosporins, estradiol, and derivatives and analogs thereof. These therapeutic agents are applied to the surface of the medical device by a modified, biologically active binders. By using these biologically active binders, the therapeutic agents can be applied to at least one surface of a medical implant without using inert polymer carriers.
Owner:BIOVENTION INC
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
Nanoparticulate tacrolimus formulations
InactiveUS20080152720A1Dissolve fastImprove compliancePowder deliveryOrganic active ingredientsNanoparticleSolid particle
The present invention is directed to pharmaceutical nanoparticulate compositions of an immunosuppressive agent. The pharmaceutical compositions comprise solid particles of an immunosuppressive agent having an effective average particle size of less than about 2000 nm and one or more surface stabilizers associated with the surface of the immunosuppressive agent particles.
Owner:ELAN PHRMA INT LTD
Ocular solutions
InactiveUS20050063997A1Reduce turbidityReduce inflammationBiocideSenses disorderEverolimusOcular structure
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease. The agent(s) may be formulated with polymers or other components for extended or slow release to provide a substantially constant concentration over the course of treatment.
Owner:PEYMAN GHOLAM A DR
Solid dispersions comprising tacrolimus
ActiveUS7994214B2Improve bioavailabilityLow water solubilityAntibacterial agentsBiocideBioavailabilityPharmaceutical Substances
Owner:VELOXIS PHARM INC
Solid dispersions comprising tacrolimus
ActiveUS20100008984A1Improve bioavailabilityLow water solubilityAntibacterial agentsBiocideSolid solutionBioavailability
A pharmaceutical composition comprising tacrolimus (FK-506) dissolved and / or dispersed in a hydrophilic or water-miscible vehicle to form a solid dispersion or solid solution at ambient temperature have improved bioavailability.
Owner:VELOXIS PHARM INC
Methods to measure immunosuppressive tacrolimus, sirolimus, and cyclosporin a complexes in a blood sample
The present invention provides methods, diagnostic assays, and diagnostic kits based on said methods, to determine levels of immunosuppressive complexes containing immunosuppressive drugs tacrolimus, sirolimus and cyclosporine A separately and in combination, formed in the blood of a drug-treated patient or in a patient candidate to immunosuppressive drug therapy. These methods, assays and kits are especially useful when using automated systems.
Owner:ABBOTT LAB INC
Injectable compositions of nanoparticulate immunosuppressive compounds
The present invention relates to an injectable nanoparticulate immunosuppressant composition for the formation of a subcutaneous or intramuscular depot. The invention is also directed to an injectable composition of nanoparticulate tacrolimus and / or sirolimus which eliminates the need to use polyoxyl 60 hydrogenated castor oil (HCO-60) and / or polysorbate 80 as a solubilizer. This invention further discloses a method of making an injectable nanoparticulate tacrolimus and / or sirolimus composition and is also directed to methods of treatment using the injectable nanoparticulate formulations comprising tacrolimus, sirolimus, or combination thereof for a subcutaneous or intramuscular depot for the prophylaxis of organ rejection and for the treatment of psoriasis or other immune diseases.
Owner:ELAN PHRMA INT LTD
Tacrolimus sustained-release preparation and preparation method thereof
InactiveCN101664394AOvercome the problems of poor water solubility and narrow therapeutic windowImprove securityOrganic active ingredientsGranular deliverySolubilityCyclodextrin
The invention provides a tacrolimus sustained-release preparation and a preparation method thereof. The invention adopts a solid dispersion technology, or cyclodextrin inclusion technology or a solubilizing method for micronizing drugs and then adding one or more types of surfactants and the like, and obviously improves solubility thereof so as to improve the bioavailability thereof, then adds oneor more types of skeleton materials and other auxiliary materials so as to prepare a skeleton type sustained-release preparation, or adopts a sustained-release material to carry out coating so as toprepare a diaphragm-controlled type or osmotic pump type sustained-release preparation. The tacrolimus sustained-release preparation has better solubility and dissolution rate, high bioavailability and sustained-controlled-release effect, thus maintaining stable blood and drug concentration, reducing the occurrence rate of adverse reaction, and improving clinical drug safety; in addition, the invention has easy obtaining for materials, simple and feasible preparation technique, high yield and low cost, can realize large-scale industrialized production and has obviously economical benefit.
Owner:宋洪涛
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com