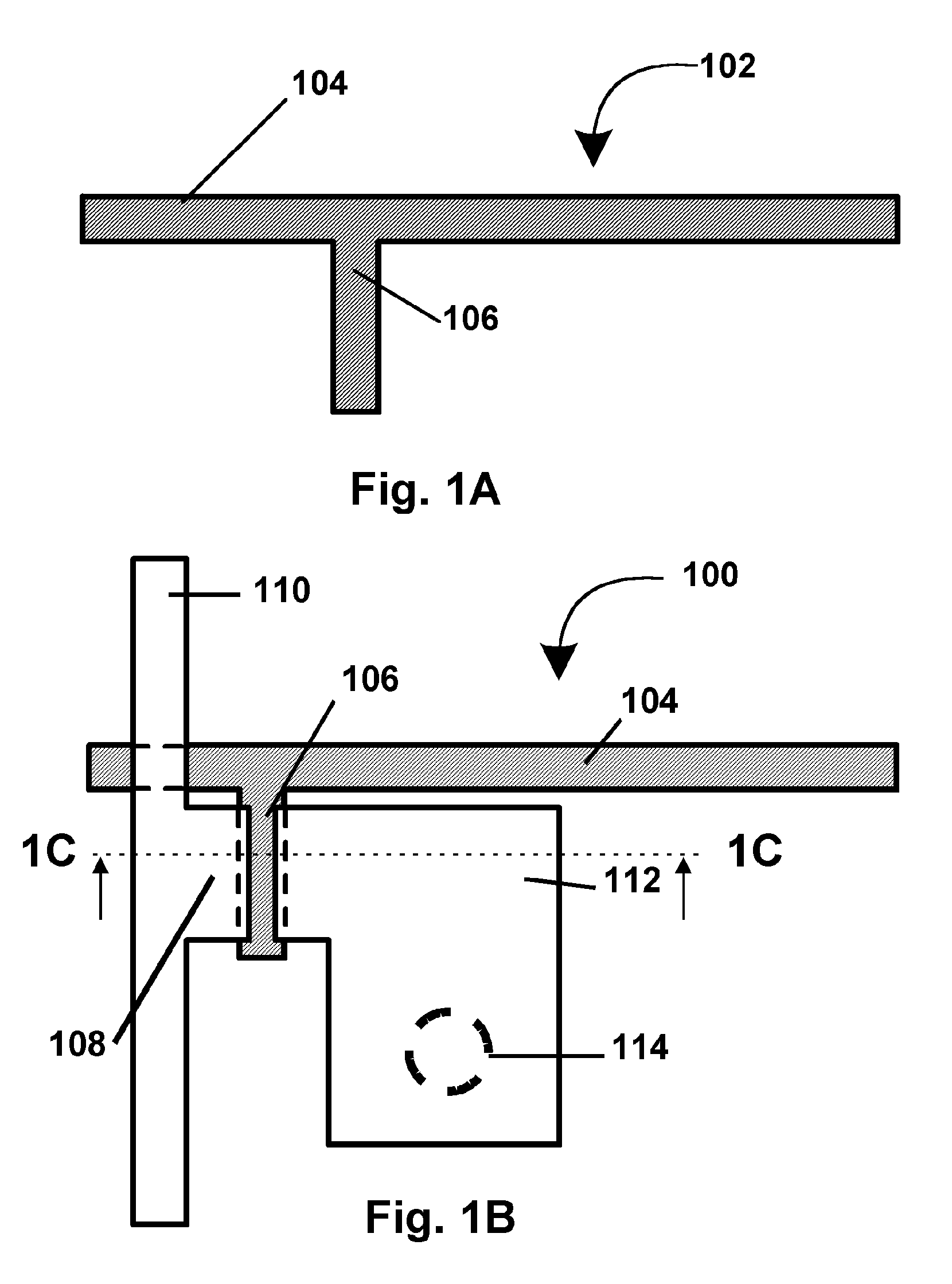
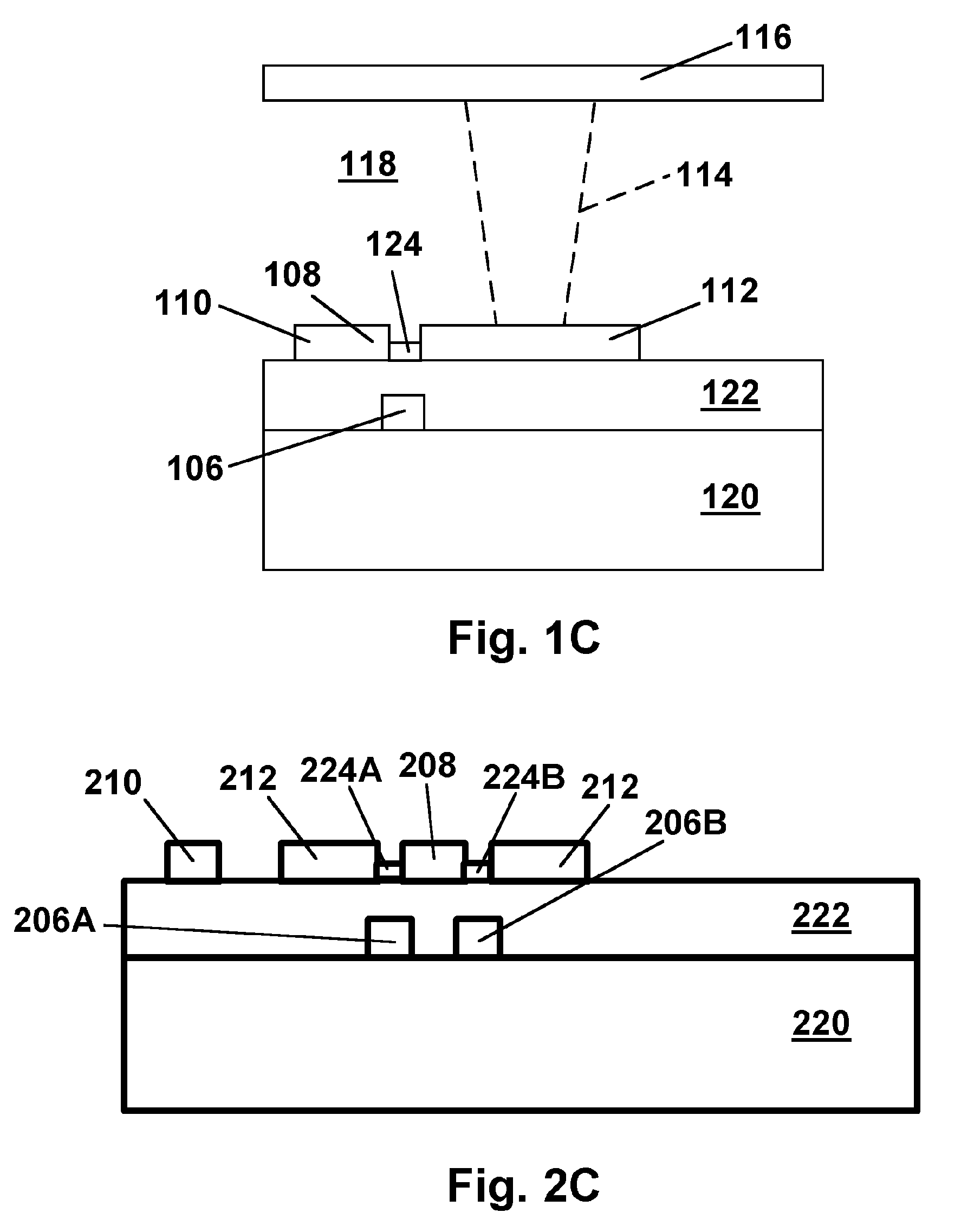
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1810results about How to "Slow change" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
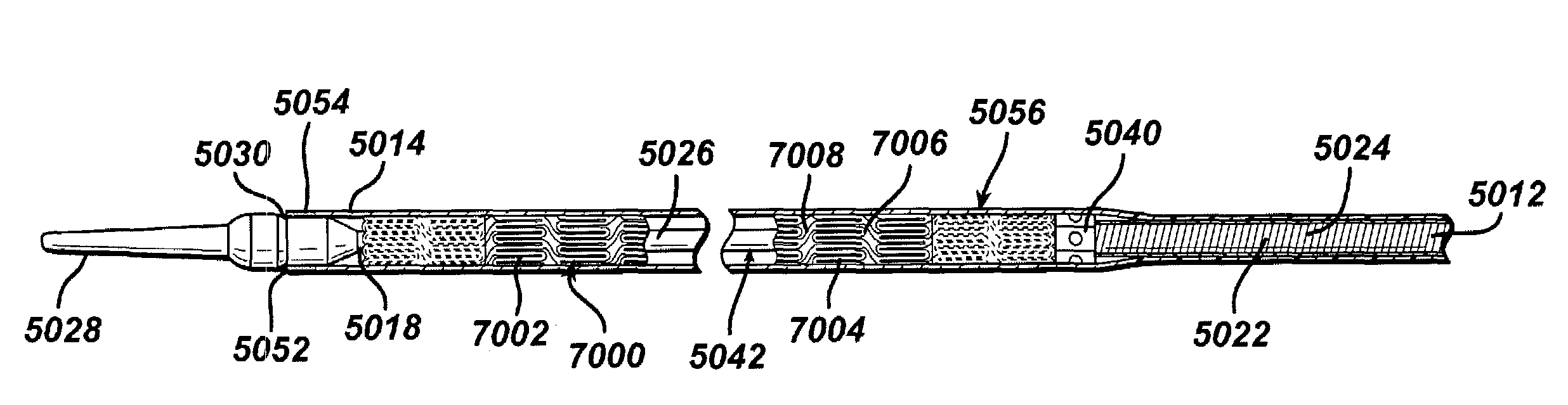
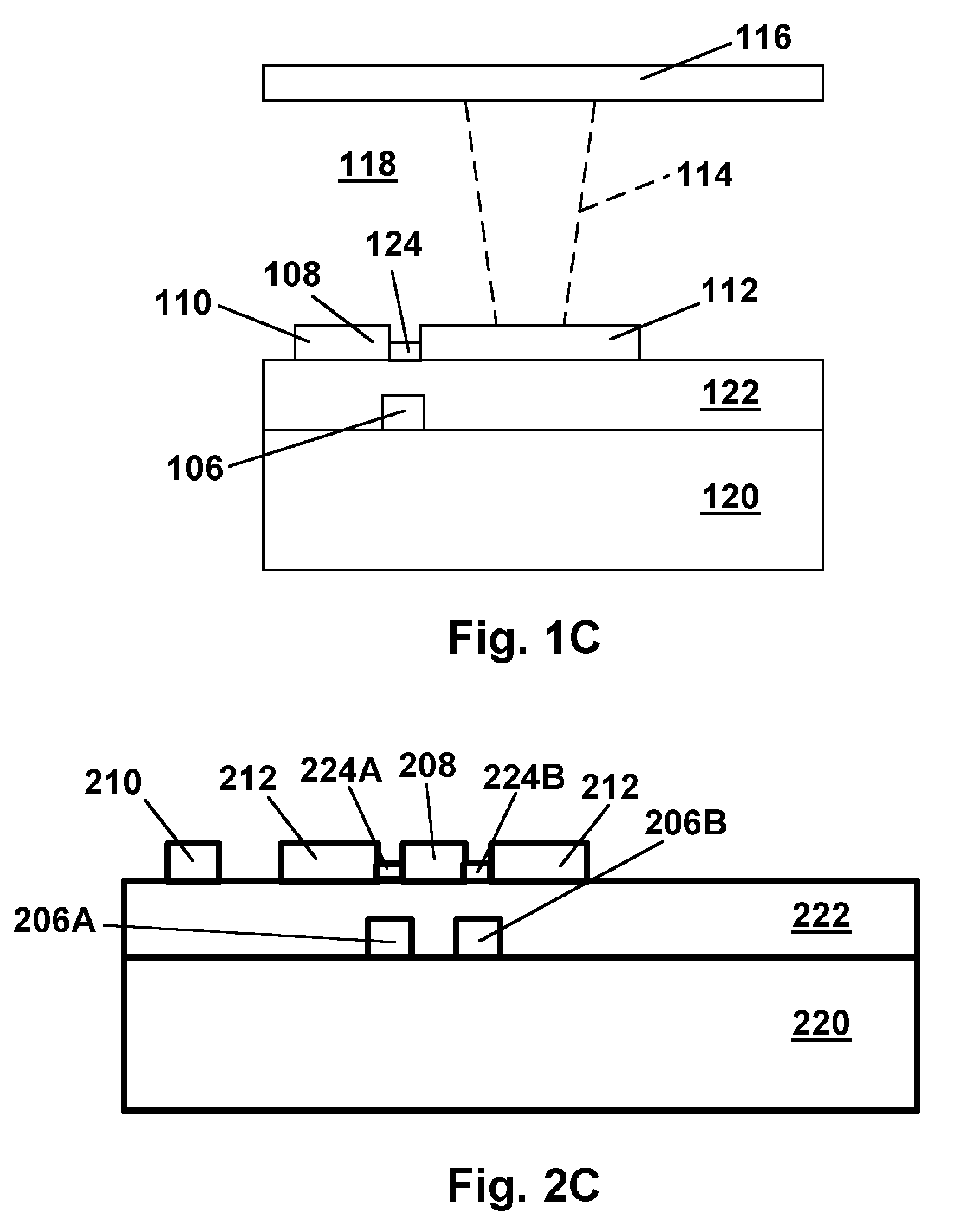
Modified delivery device for coated medical devices
InactiveUS7527632B2Minimize potential risk of damageReduce frictionStentsEar treatmentBiological bodyMedical device
Medical devices, and in particular implantable medical devices, including self-expanding stents, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
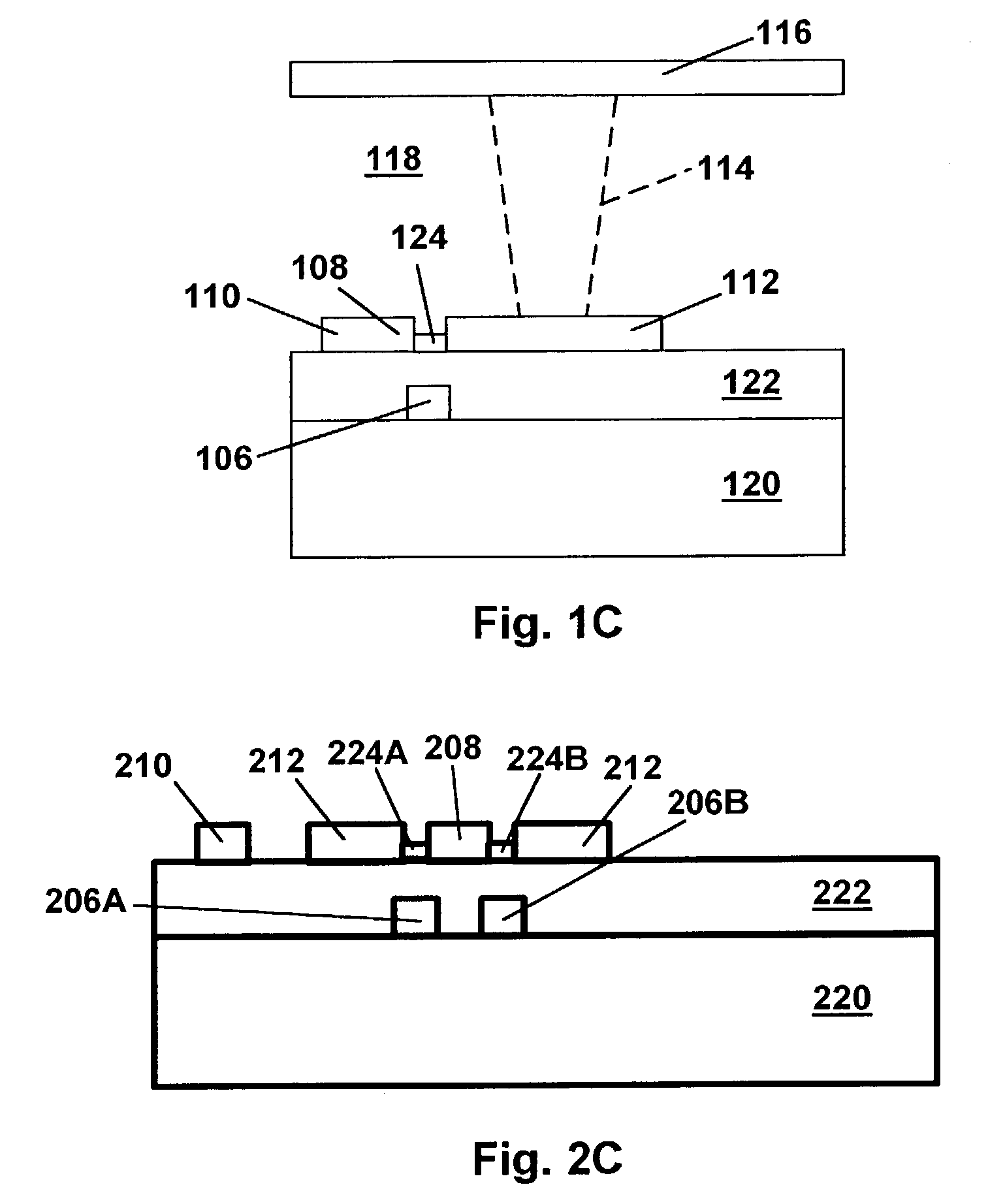
Backplanes for display applications, and components for use therein
ActiveUS7116318B2Reduce manufacturing costThe process steps are simpleTransistorSolid-state devicesCapacitanceCoupling
A display pixel unit provides reduced capacitative coupling between a pixel electrode and a source line. The unit includes a transistor, the pixel electrode, and the source line. The source line includes an extension that provides a source for the transistor. A patterned conductive portion is disposed adjacent to the source line.
Owner:E INK CORPORATION
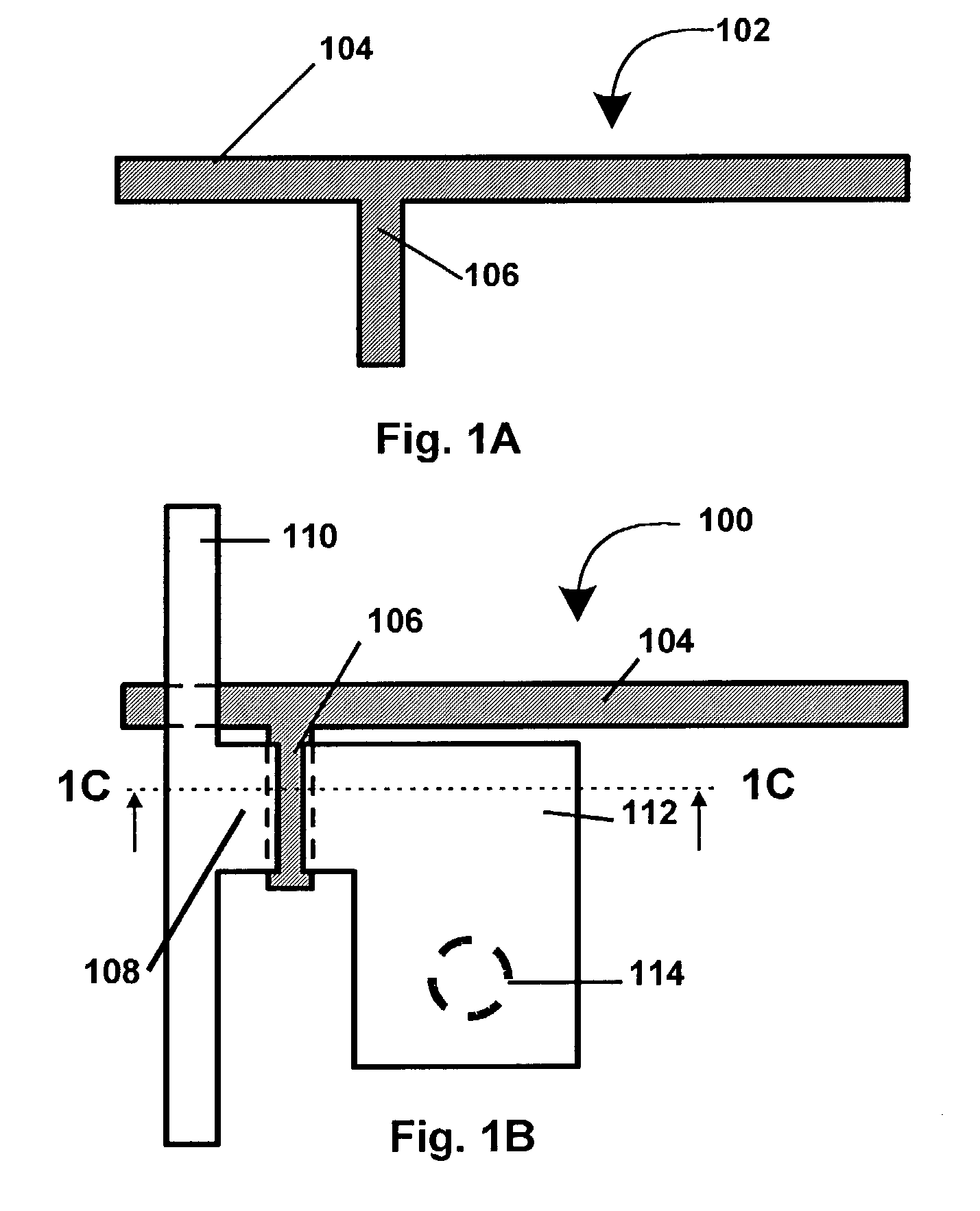
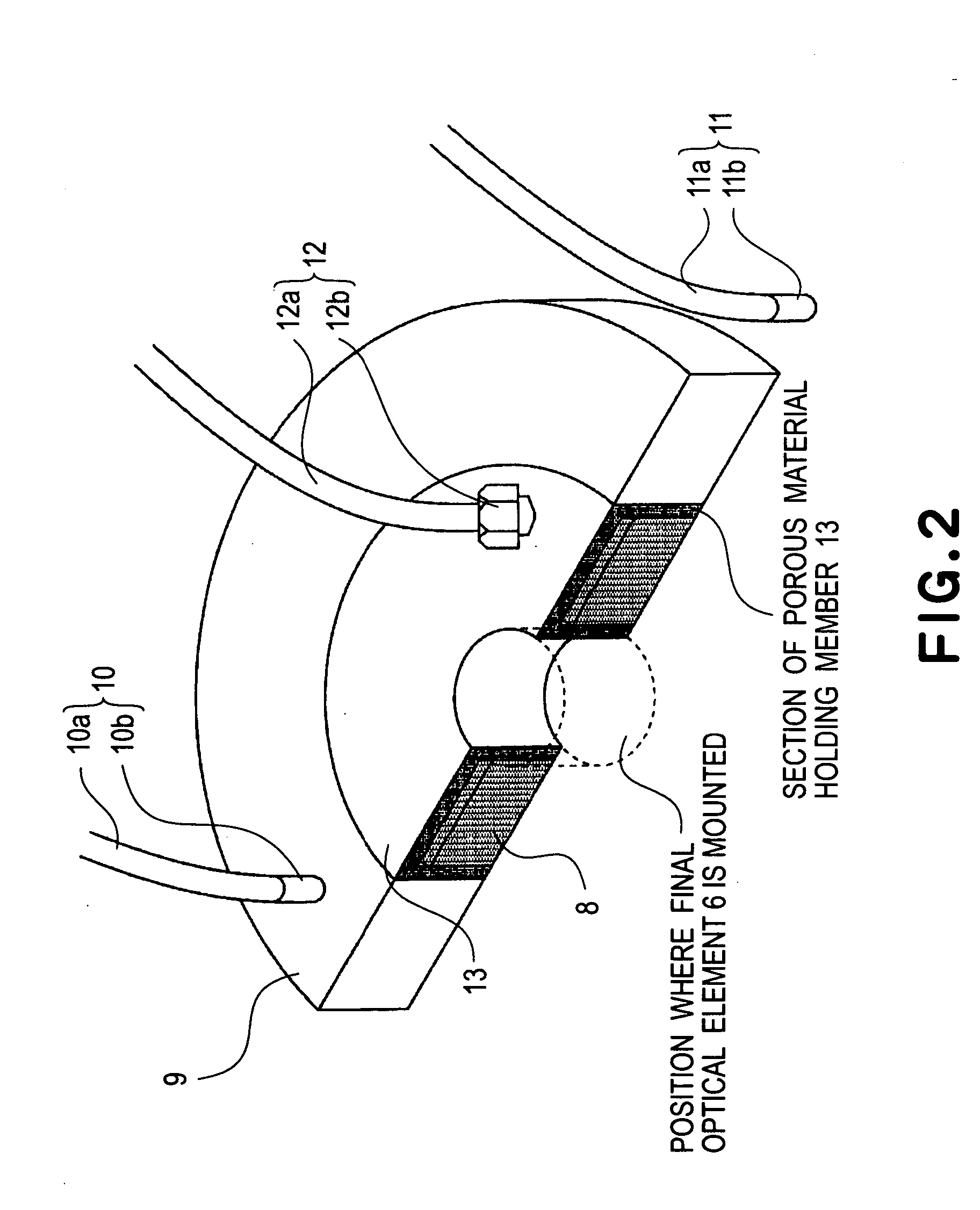
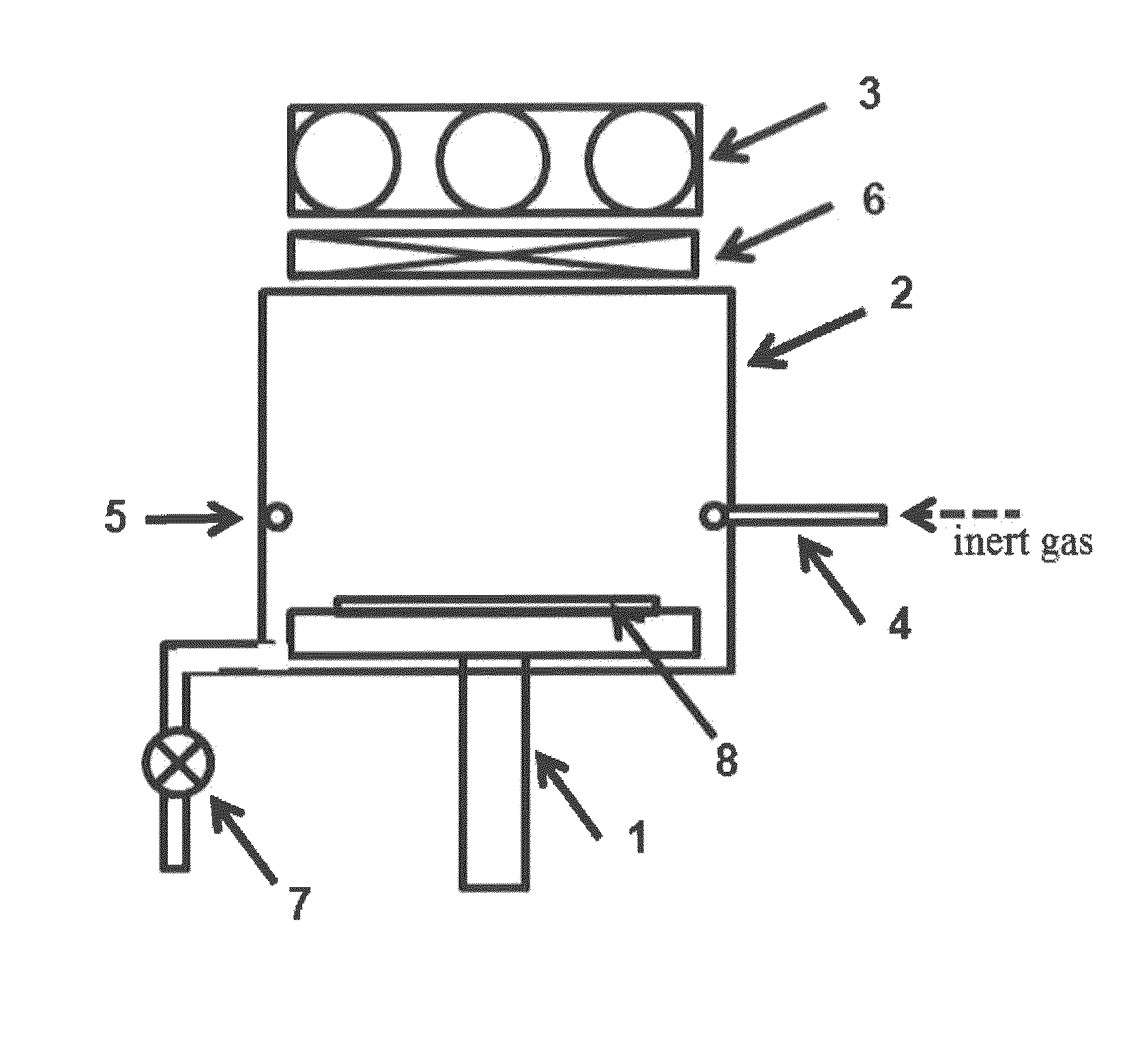
Liquid immersion type exposure apparatus
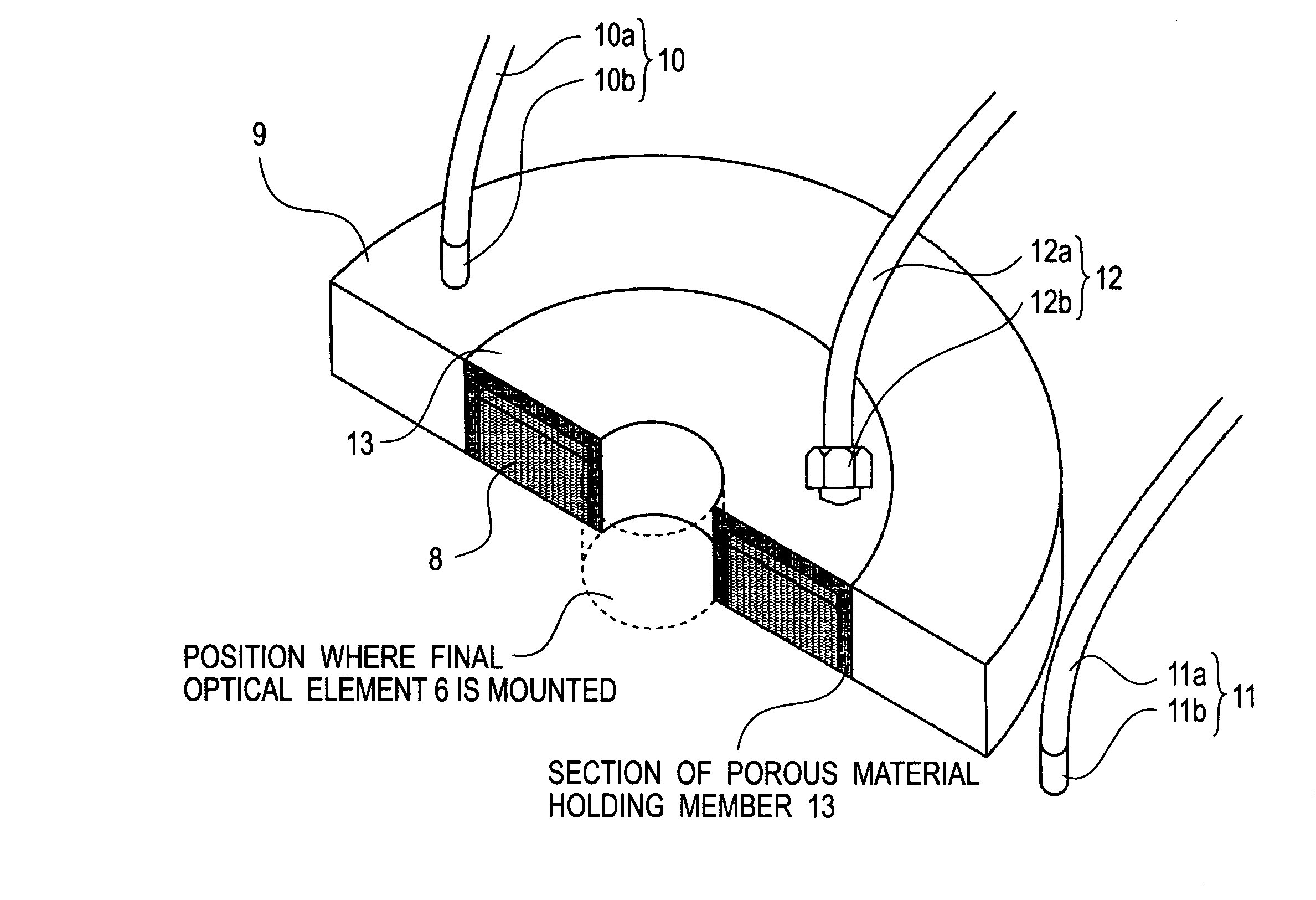
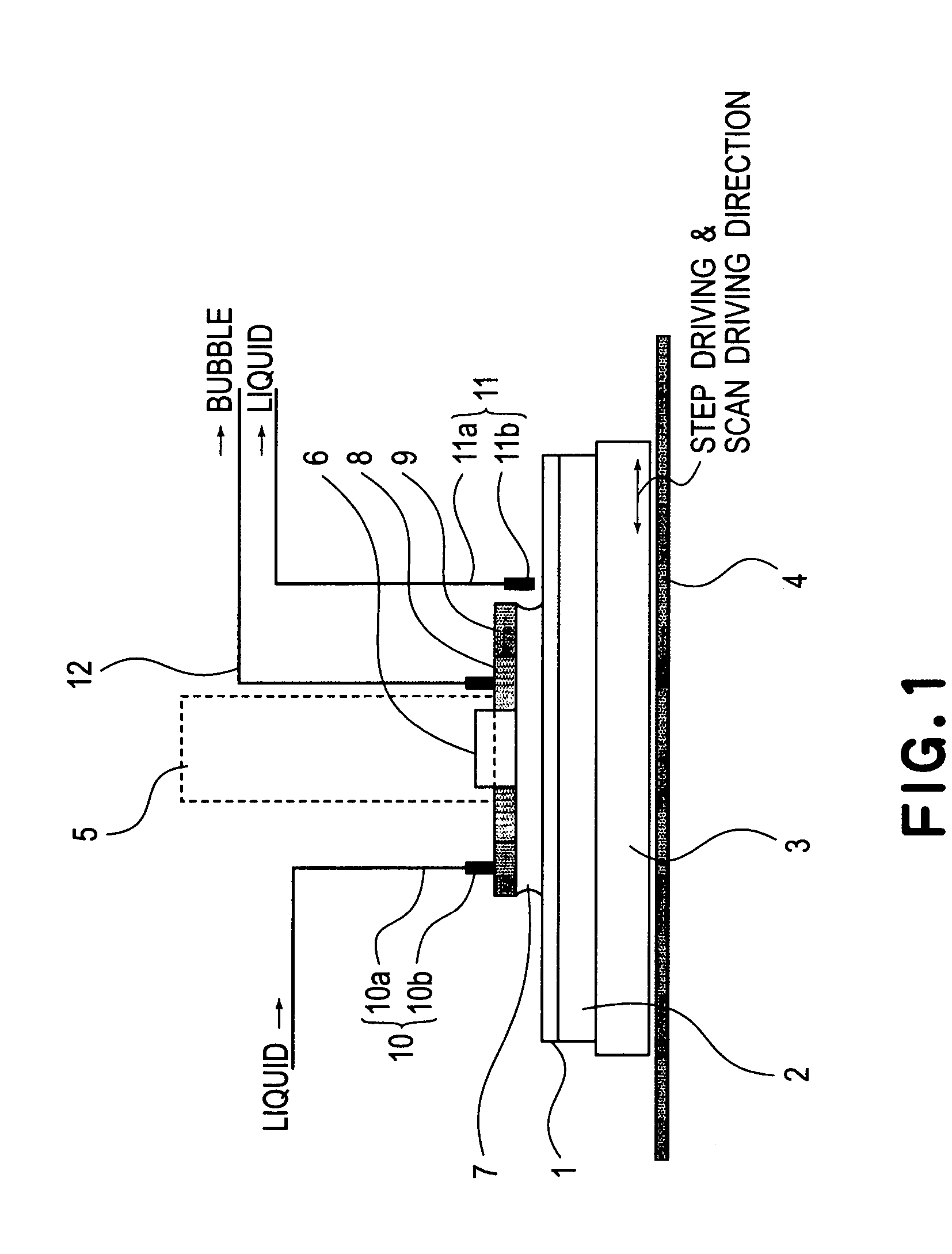
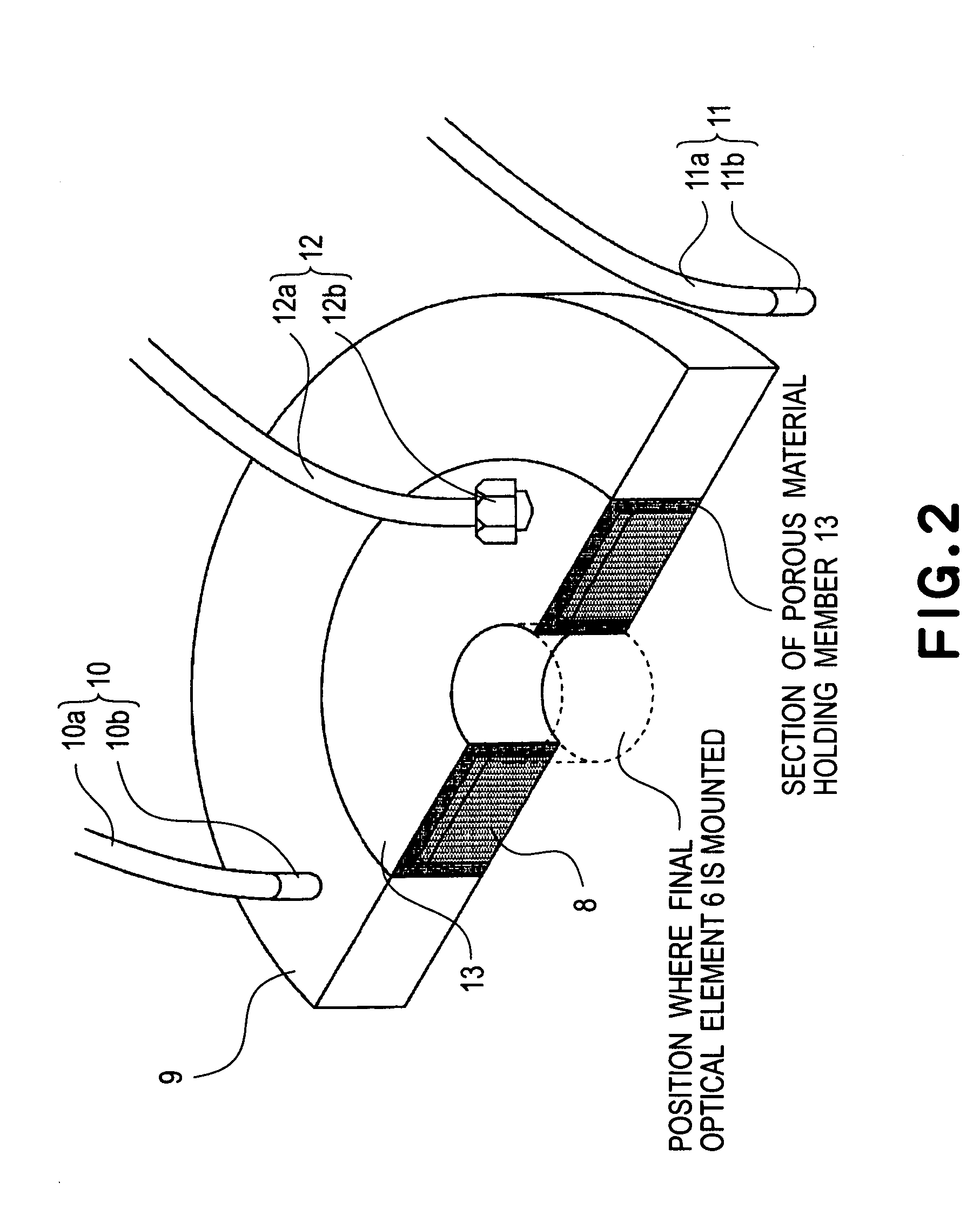
InactiveUS20050233081A1High resolutionLow refractive indexSemiconductor/solid-state device manufacturingPhotomechanical exposure apparatusLiquid mediumCollection system
Disclosed is an exposure apparatus which includes a projection optical system for projecting a pattern of a reticle onto a substrate, wherein the substrate is exposed through a liquid medium kept at least in a portion between the substrate and an optical element of the projection optical system which optical element is nearest to the substrate, a supplying system for supplying a liquid medium, a collecting system for collecting a liquid medium, and an exhausting system for removing a bubble in the liquid medium through a bubble removing material having such property that it passes a gas but it does not pass a liquid.
Owner:CANON KK
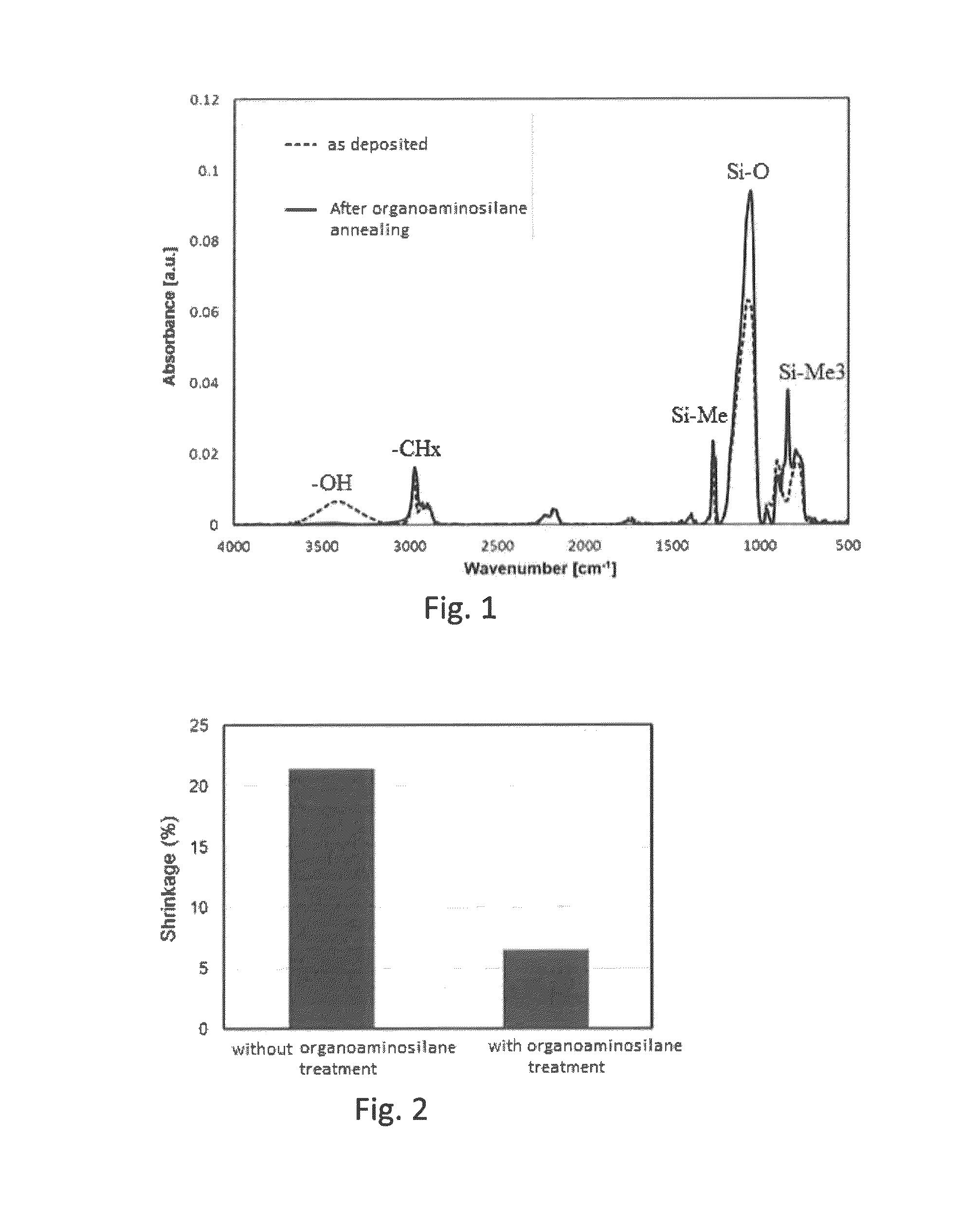
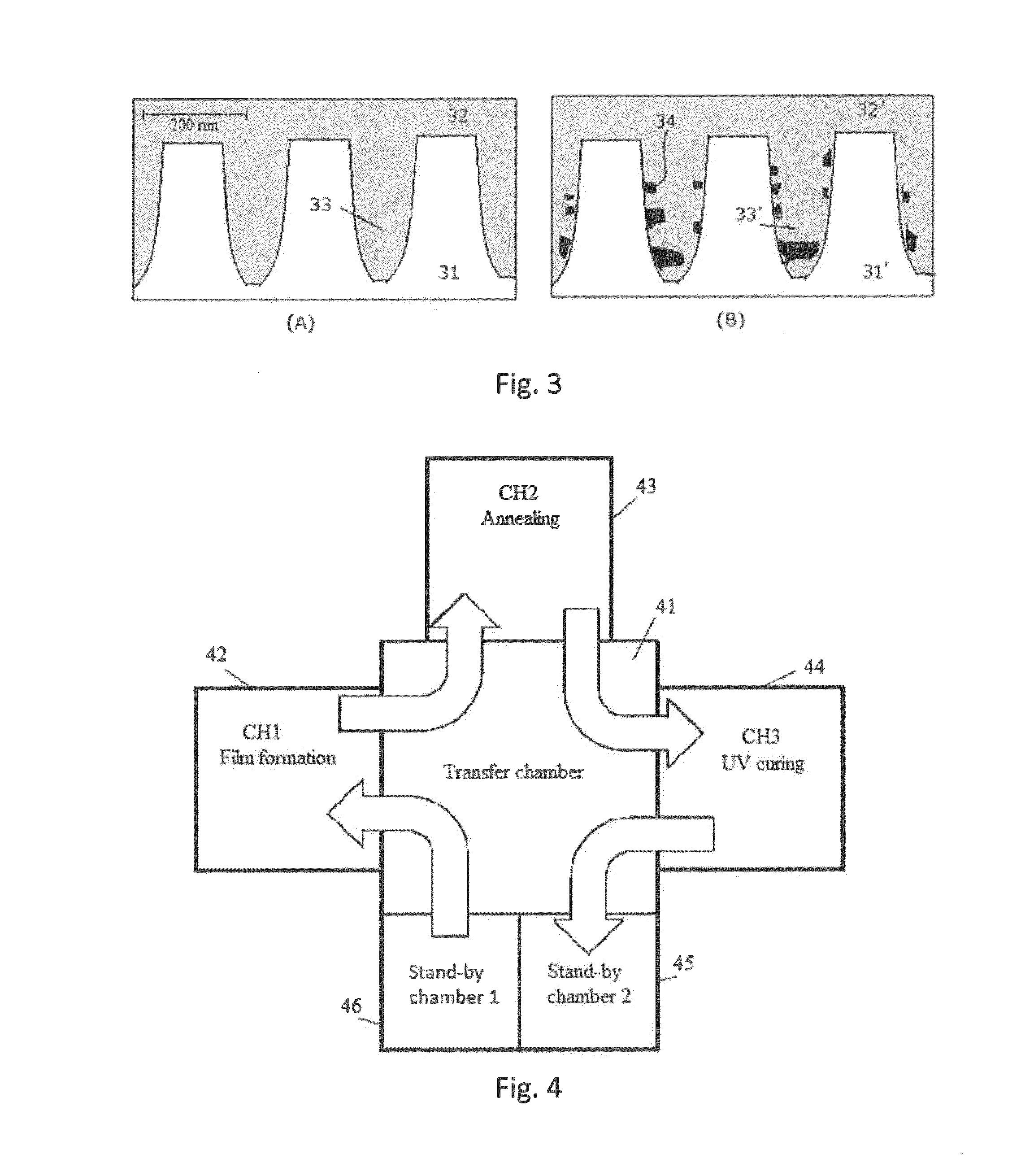
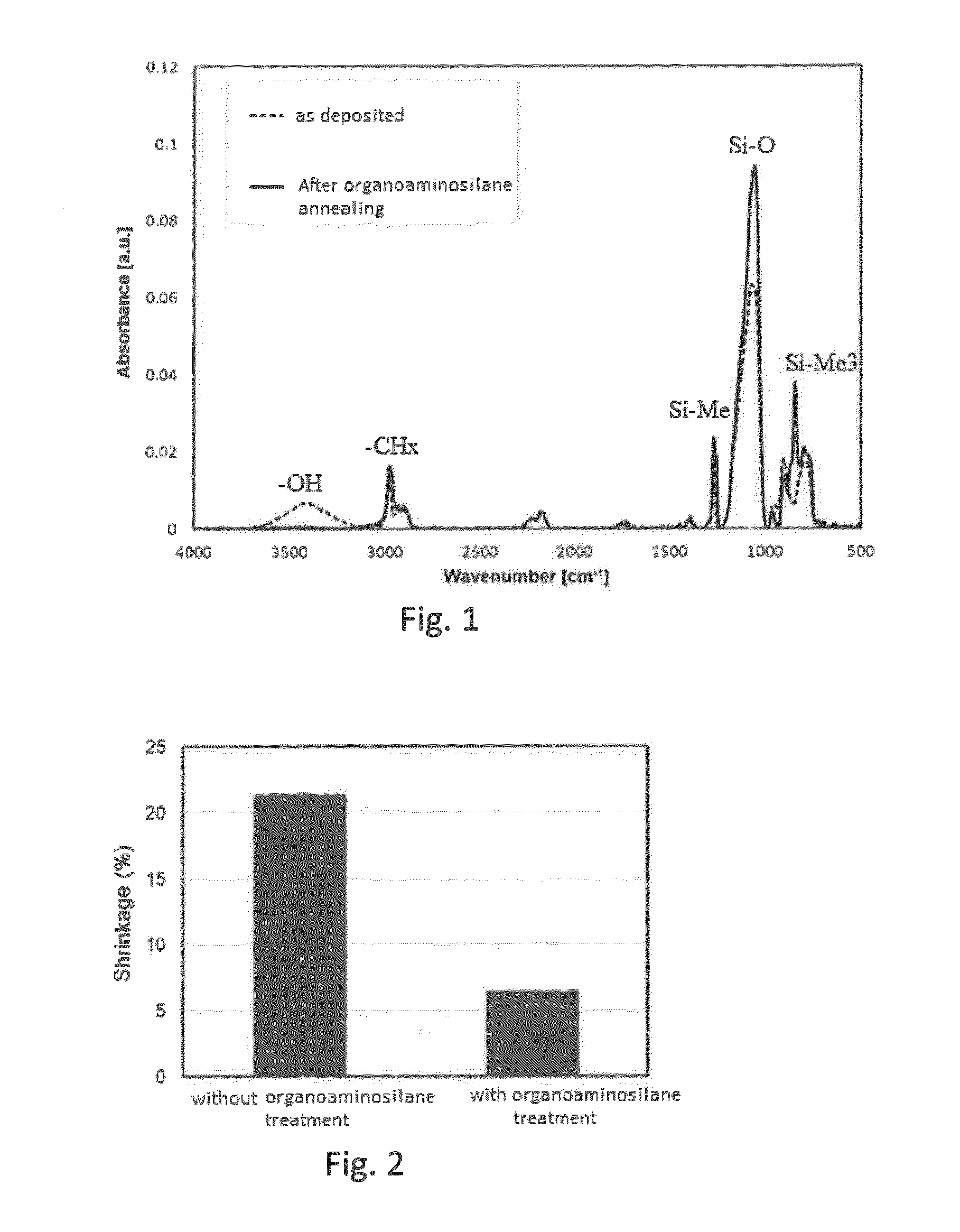
Method for forming SiOCH film using organoaminosilane annealing
ActiveUS9190263B2Improve liquidityReduce the amount requiredSemiconductor/solid-state device manufacturingPolymer scienceSilanes
Owner:ASM IP HLDG BV
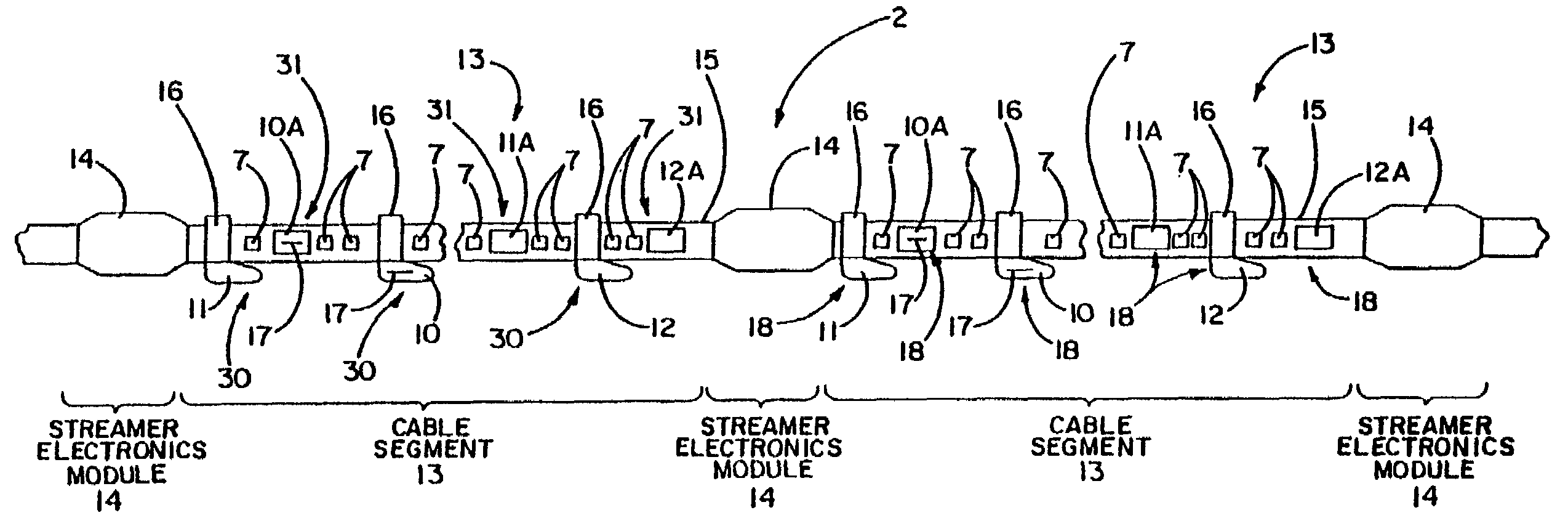
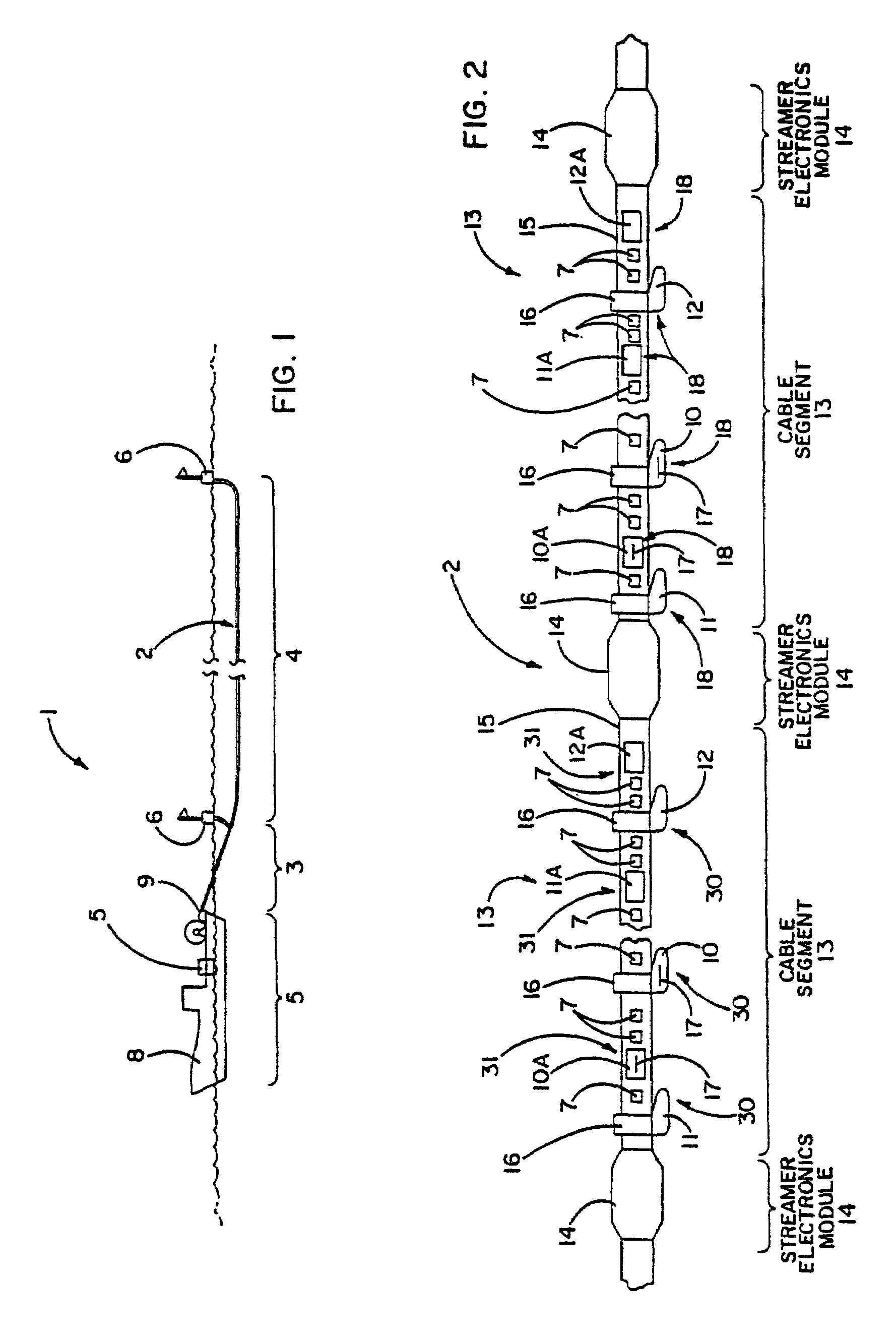
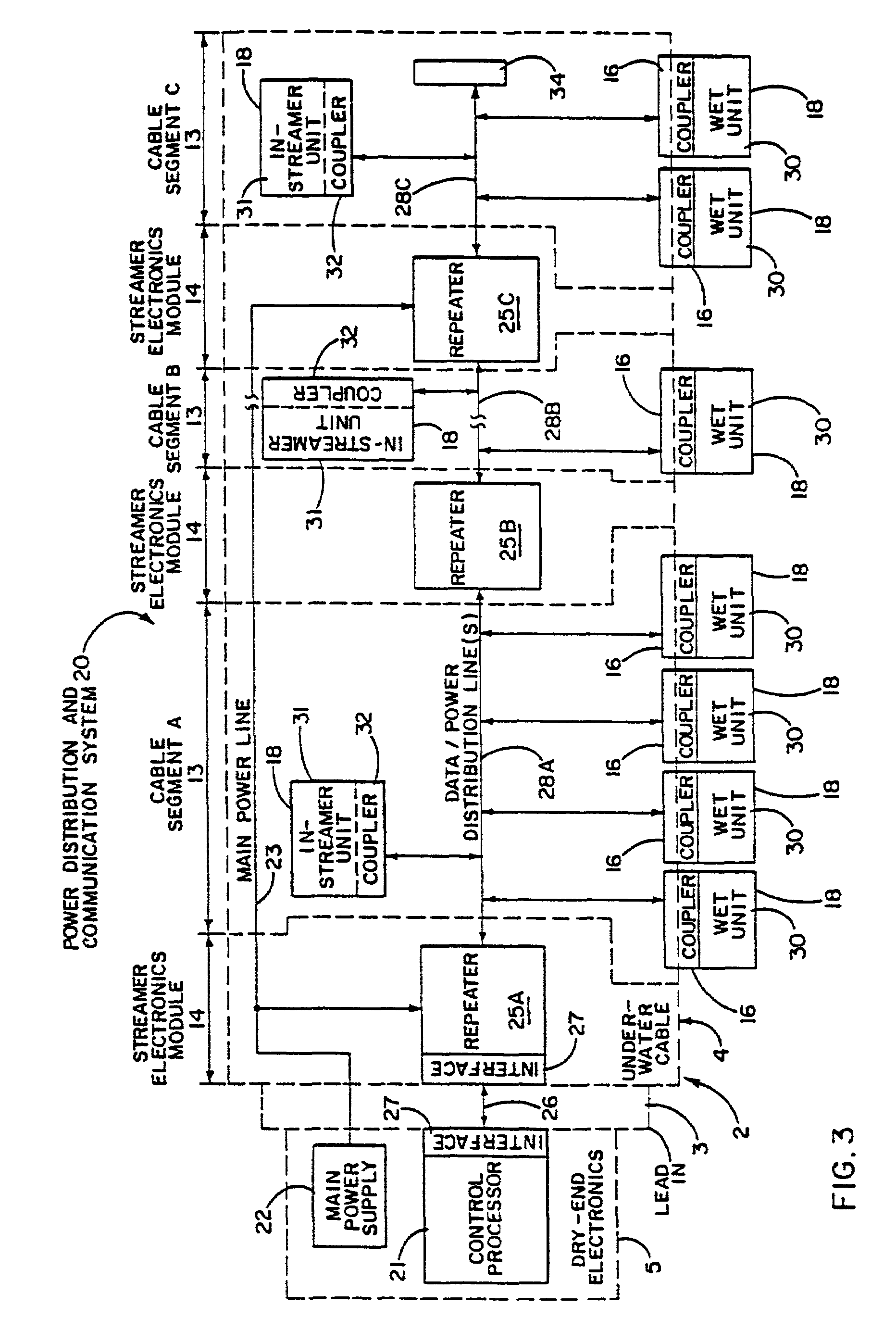
Electrical power distribution and communication system for an underwater cable
ActiveUS7176589B2Improve reliabilityImprove operationBus-bar/wiring layoutsInsulated cablesCommunications systemElectrical devices
An underwater cable arrangement includes systems and method for distributing and / or transferring power and / or data to internal devices and external devices disposed along an underwater cable. Under water coupling systems and underwater electrical devices may be used in the distribution and / or transfer of the power and / or data.
Owner:INPUT OUTPUT INC
Method for Forming SiOCH Film Using Organoaminosilane Annealing
ActiveUS20150056821A1Improve liquidityReduce the amount requiredSemiconductor/solid-state device manufacturingPolymer scienceSilanes
Owner:ASM IP HLDG BV
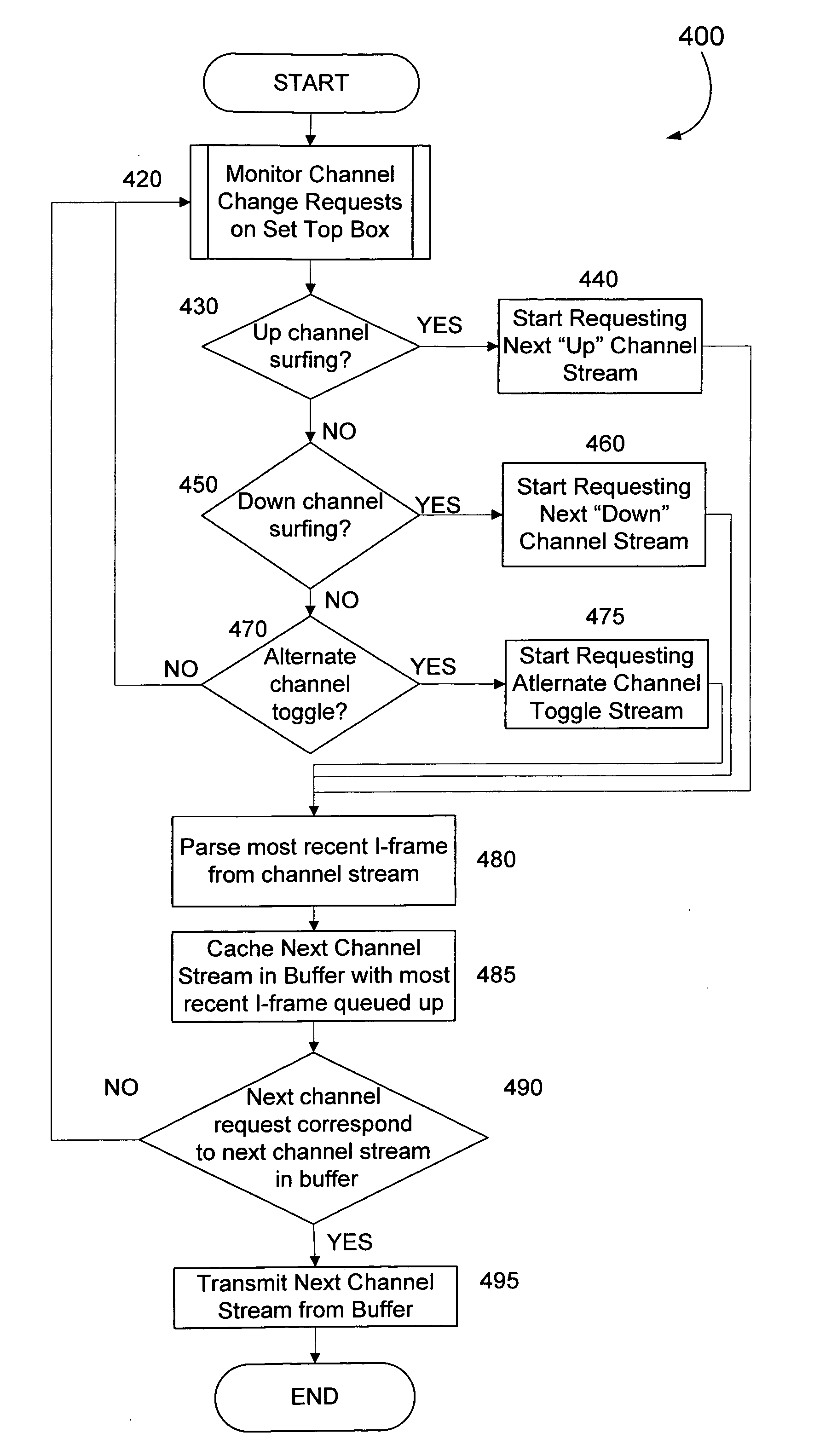
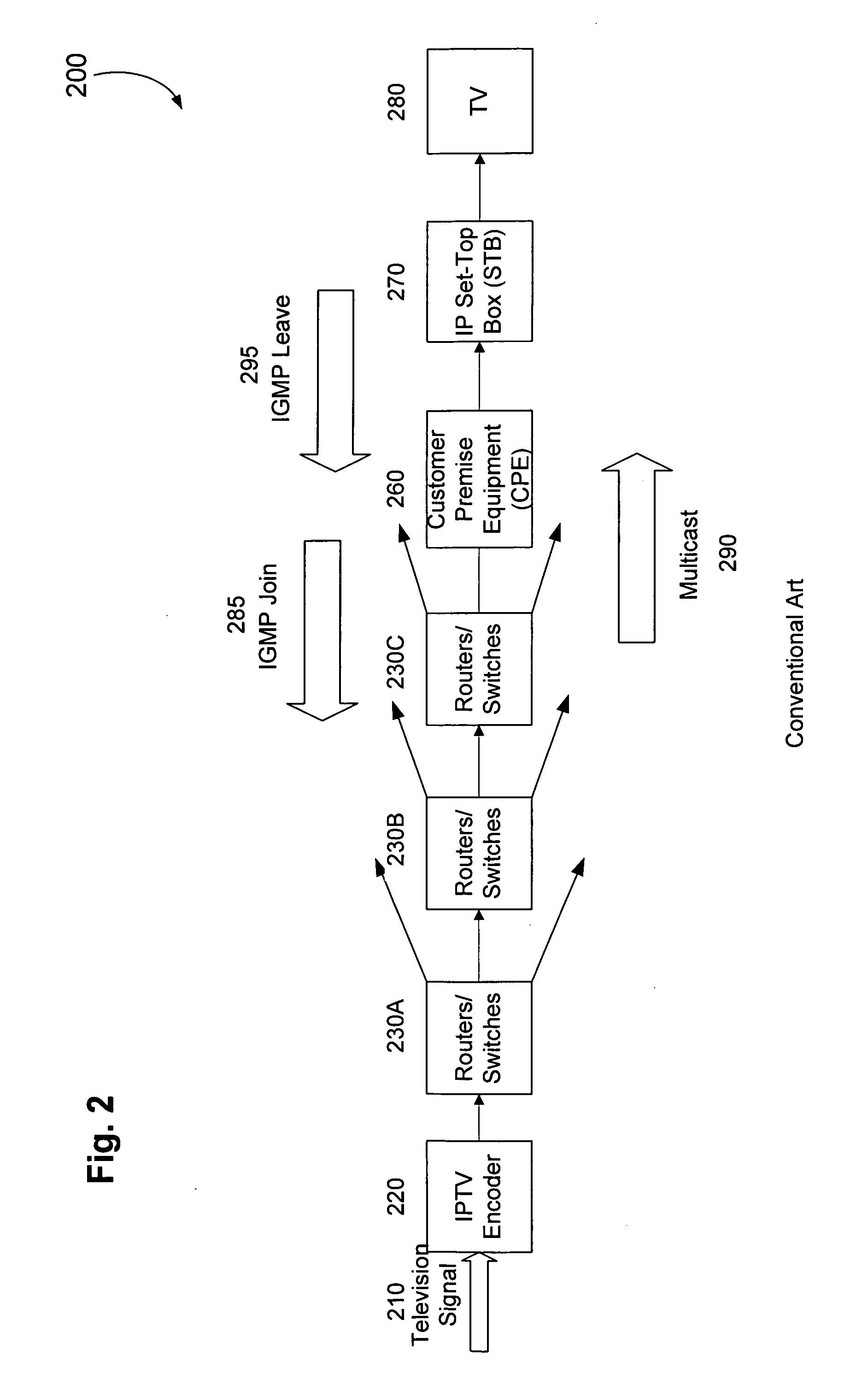
Minimizing channel change time for IP video
InactiveUS20060075428A1Reduce delaysSlow changeTelevision system detailsColor television detailsDigital videoSet top box
Subscribers to Internet Protocol TV services usually complain about one key characteristic—the additional delay digital video introduces when subscribers change channels, especially when subscribers “channel surf.” The problem is traced to at least three sources of delay in a convention Internet Protocol video deployment system. The channel changing delay can be minimized by caching video packets for the most likely next channel in a buffer in anticipation of a television subscriber changing channels and / or by having an adaptable buffer length in the set top box.
Owner:ENABLENCE USA FTTX NETWORKS
Radio communication apparatus and current reducing method
InactiveUS20120306705A1Slow changeAvoid changeAntenna supports/mountingsSubstation equipmentElectricityElectrical conductor
Owner:LENOVO INNOVATIONS LTD HONG KONG
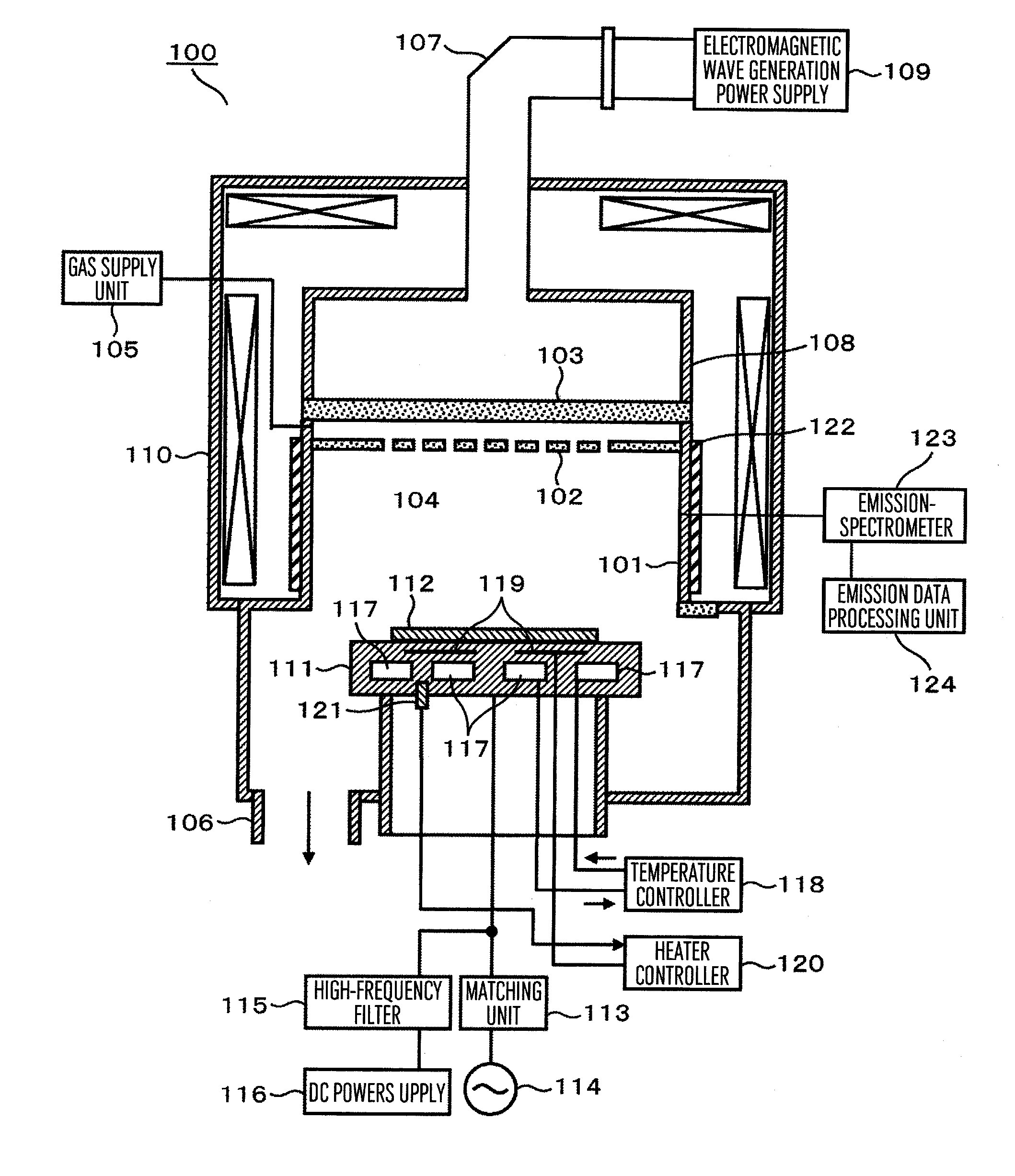
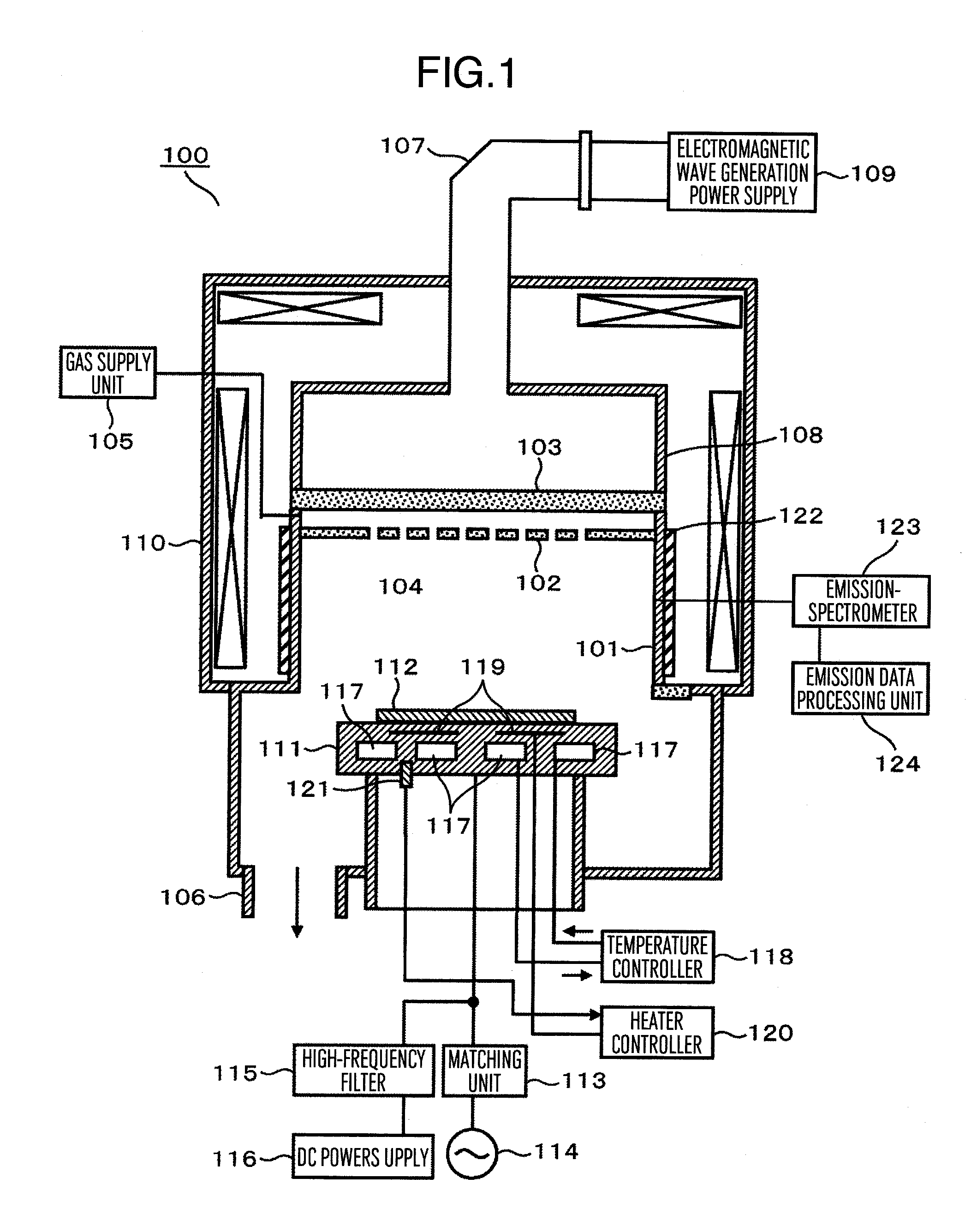
Plasma processing apparatus and plasma processing method
ActiveUS20110226734A1Reduce changeImprove efficiencyElectric discharge tubesDecorative surface effectsResistFilm structure
In a plasma processing apparatus comprising a processing chamber arranged in a vacuum chamber, a sample stage arranged under the processing chamber and having its top surface on which a wafer to be processed is mounted, a vacuum decompression unit for evacuating the interior of the processing chamber to reduce the pressure therein, and introduction holes arranged above said sample stage to admit process gas into the processing chamber, the wafer having its top surface mounted with a film structure and the film structure being etched by using plasma formed by using the process gas, the film structure is constituted by having a resist film or a mask film, a poly-silicon film and an insulation film laminated in this order from top to bottom on a substrate and before the wafer is mounted on the sample stage and the poly-silicon film underlying the mask film is etched, plasma is formed inside the processing chamber to cover the surface of members inside the processing chamber with a coating film containing a component of Si.
Owner:HITACHI HIGH-TECH CORP
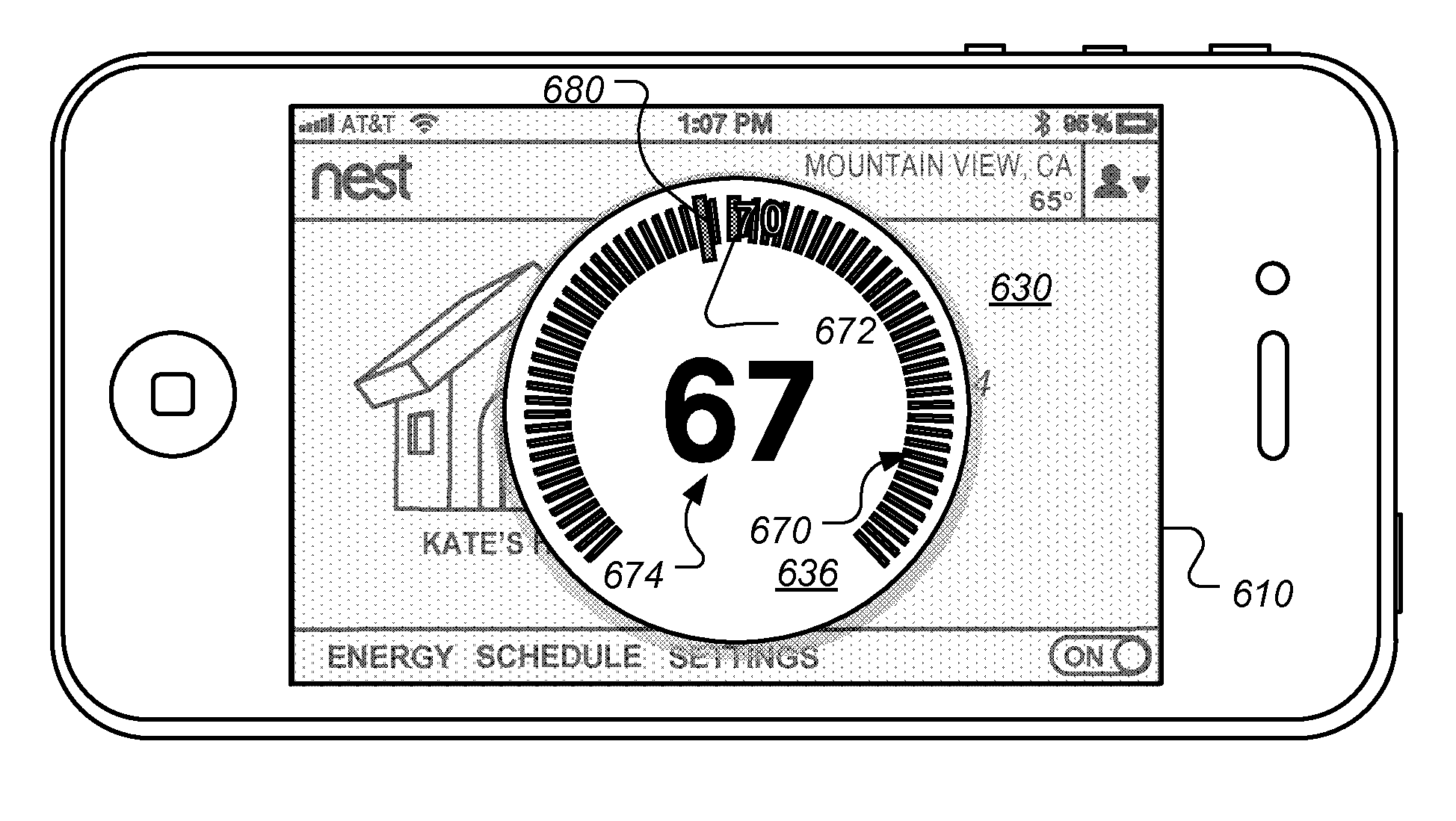
Touchscreen device user interface for remote control of a thermostat
ActiveUS20140319232A1Reduce riskImprove user experienceTemperature control without auxillary powerMechanical apparatusControl signalDisplay device
Systems and methods are described for interactively and graphically interfacing with a user on an HVAC system controlled by a thermostat. The user interface is implemented on a touch screen display on a remote wirelessly connected device such as smartphone or a tablet PC. The interface displays a screen that mimics the display on the thermostat including allowing one or more input methods that are analogous to input methods used on the thermostat. Touch screen gestures such as touch and drag, touch and hold and tapping are used in an intuitive way. The user experience is enhanced by allowing large-scale changes while reducing the risk of sudden unintended changes. The control signals are judiciously tailored to protect the HVAC equipment from unwarranted over-controlling, reduce unnecessary network traffic, and prevent the waste of energy.
Owner:GOOGLE LLC
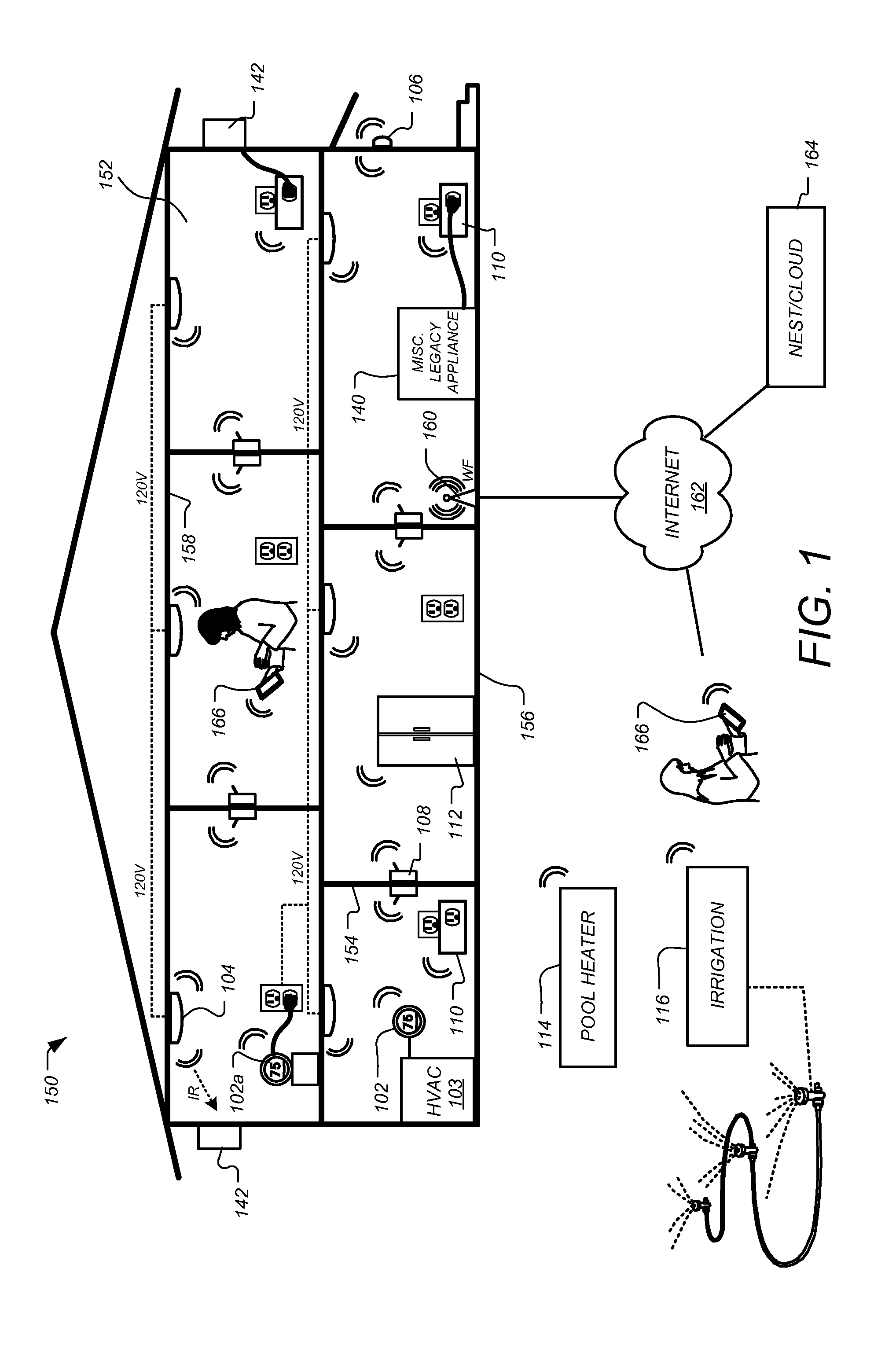
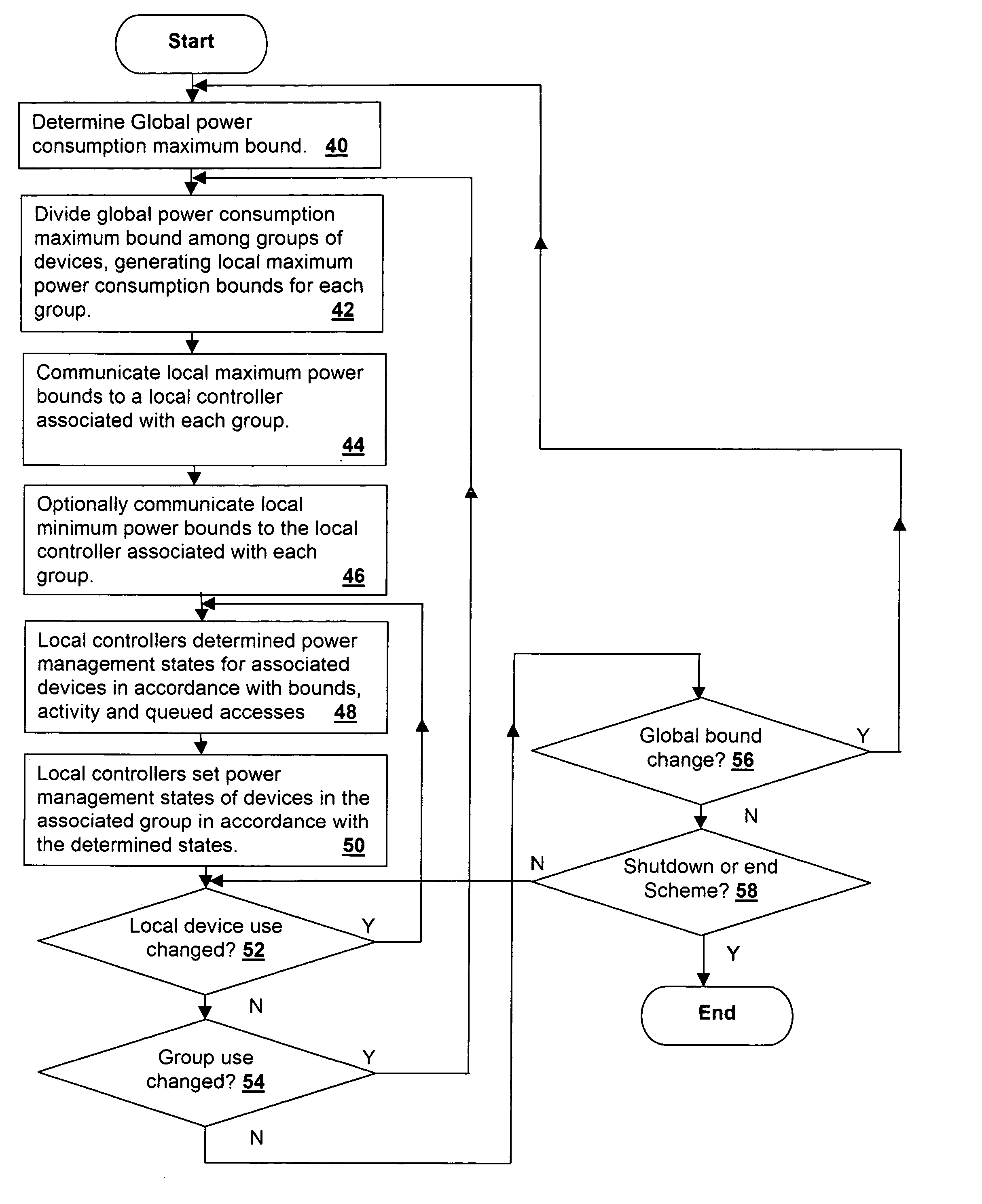
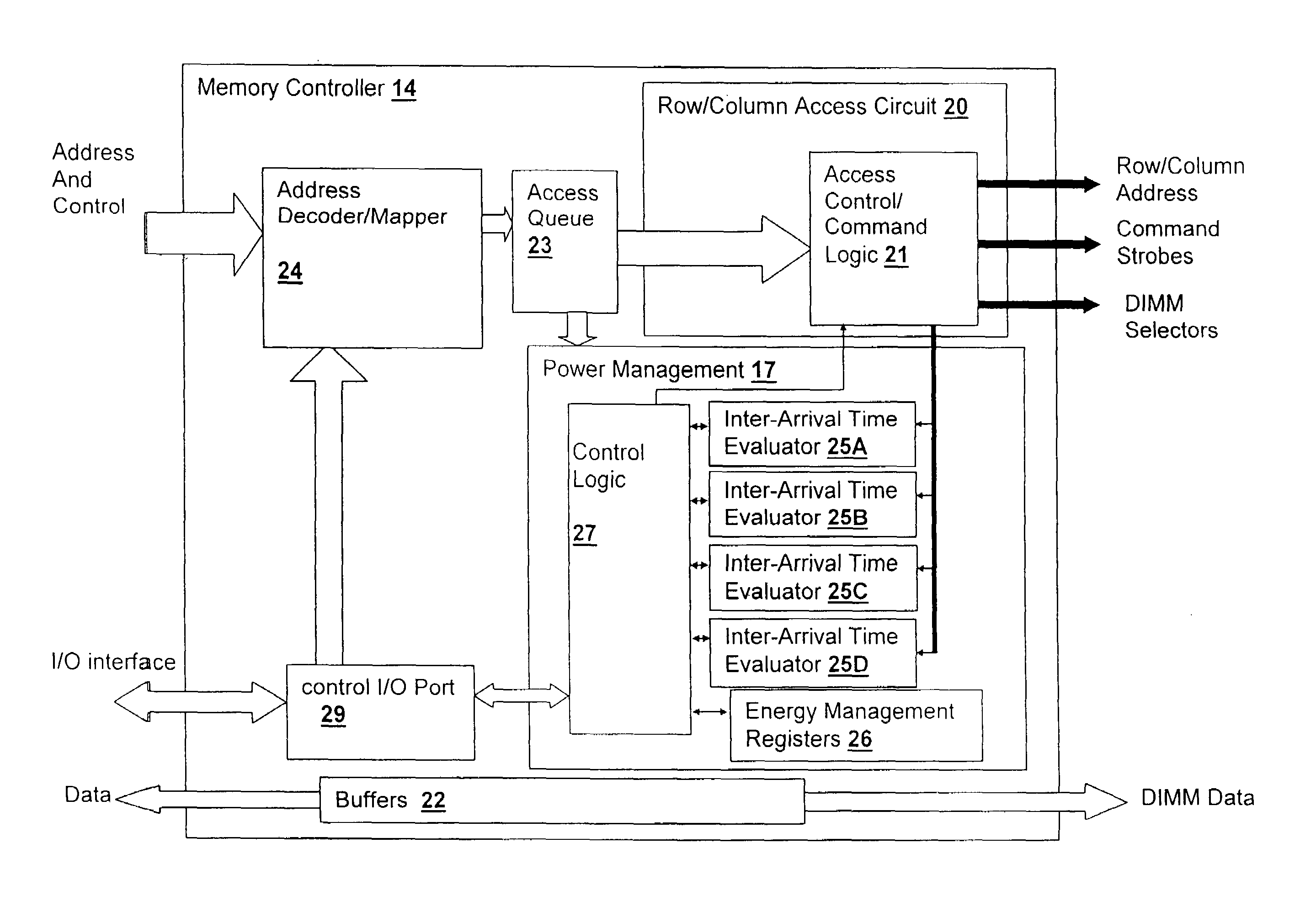
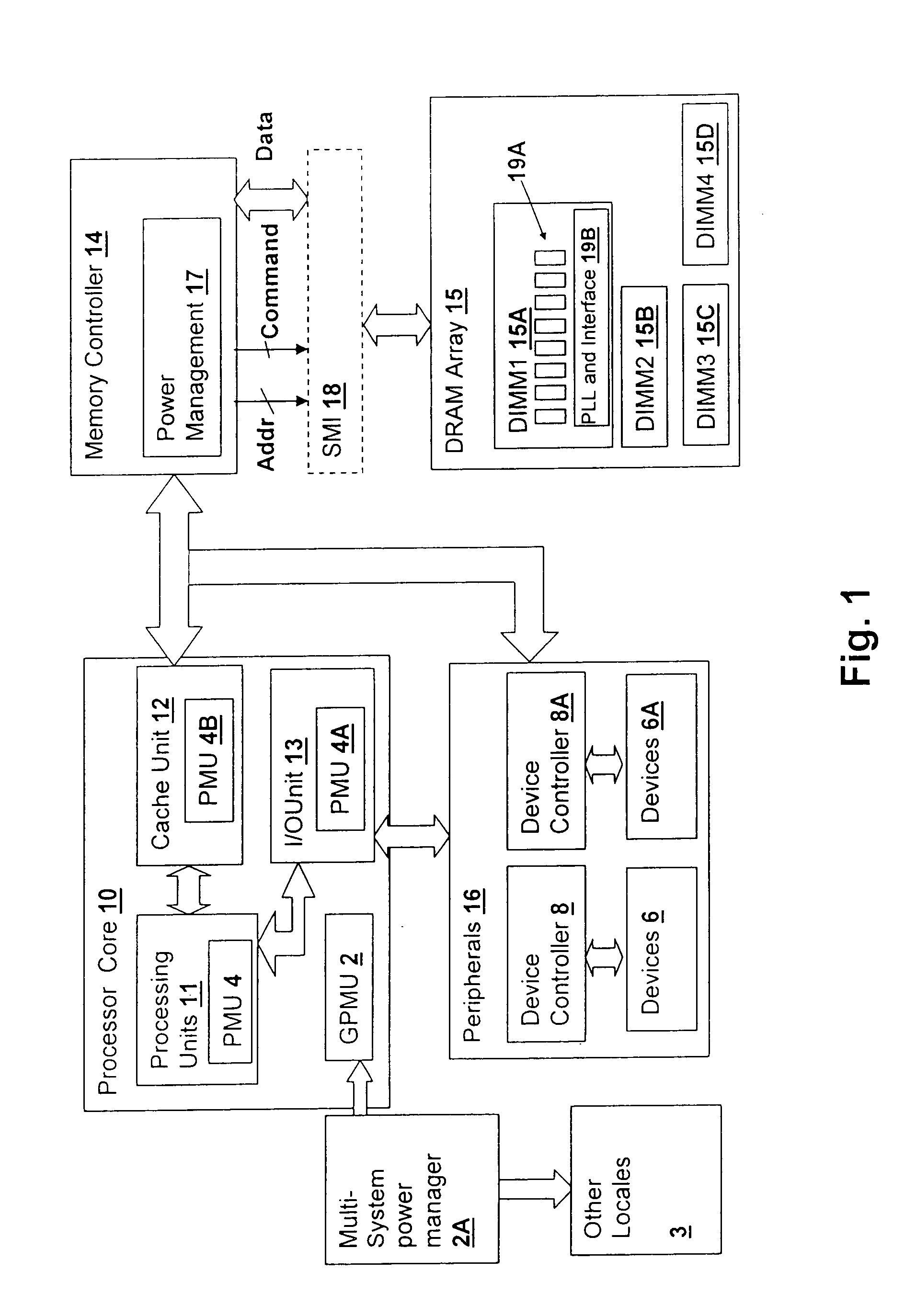
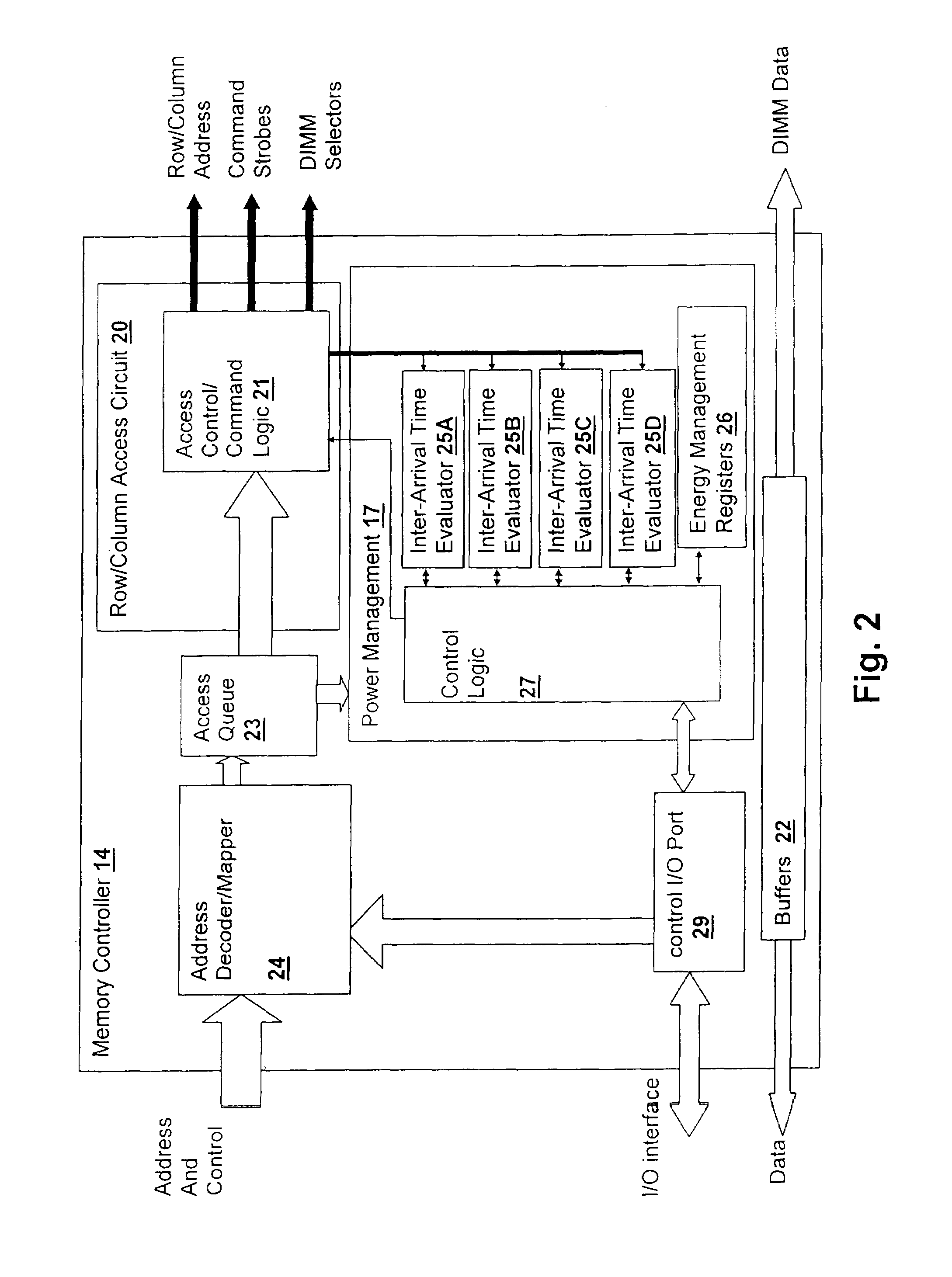
Method and system for power management including local bounding of device group power consumption
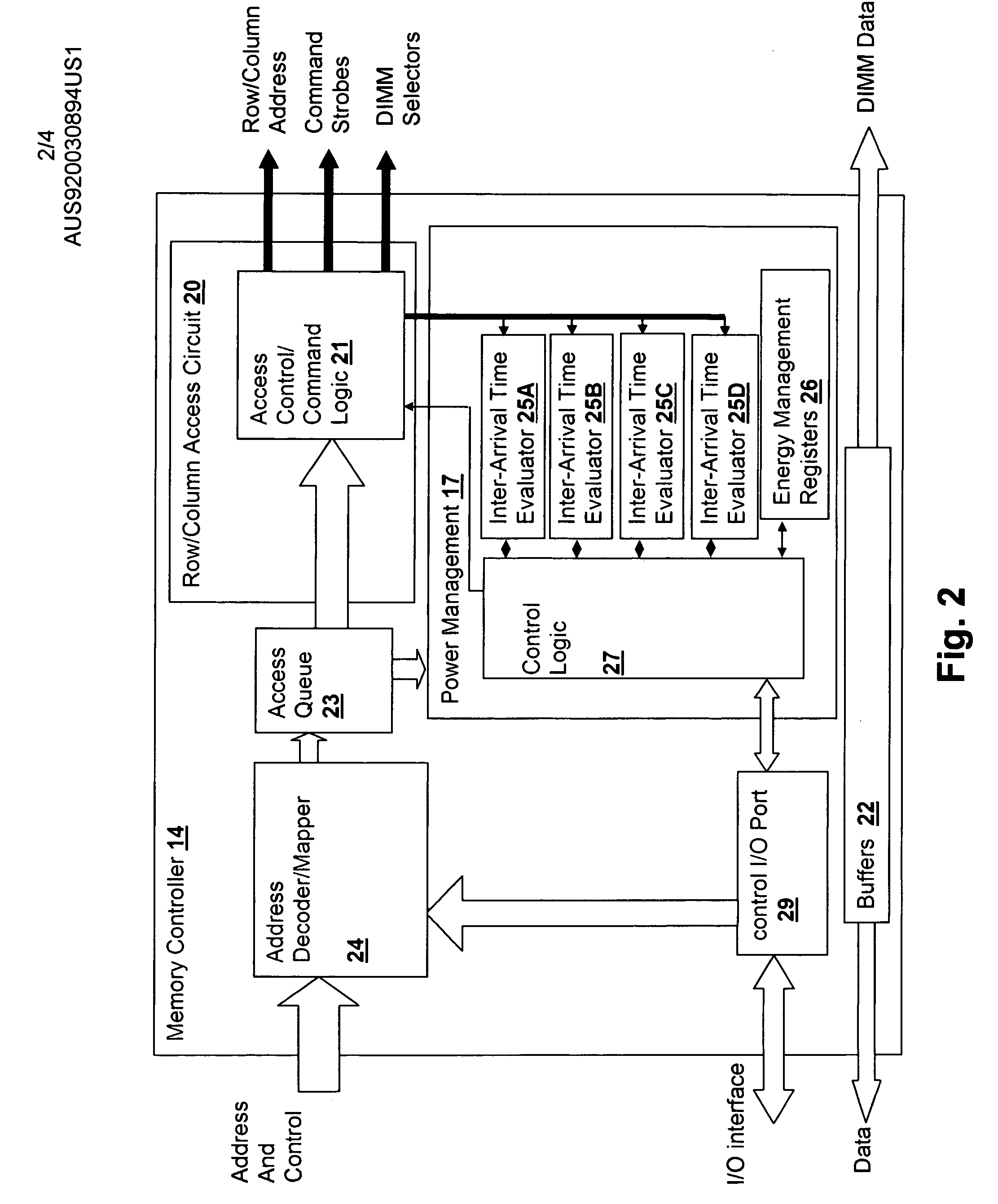
ActiveUS20050125703A1Inhibit currentSlow changeEnergy efficient ICTVolume/mass flow measurementMemory controllerGlobal system
A method and system for power management including local bounding of device group power consumption provides the responsiveness of local power control while meeting global system power consumption and power dissipation limits. At the system level, a global power bound is determined and divided among groups of devices in the system so that local bounds are determined that meet the global system bound. The local bounds are communicated to device controllers associated with each group of devices and the device controllers control the power management states of the associated devices in the group to meet the local bound. Thus, by action of all of the device controllers, the global bound is met. The controllers may be memory controllers and the devices memory modules, or the devices may be other devices within a processing system having associated local controllers. Alternatively or in concert, the devices may be entire processing systems and the associated controller a power management controller for associated processing systems, whereby multiple processing locales may be power-managed consistent with a global power consumption budget.
Owner:HUAWEI TECH CO LTD
System and process for controlling the coding bit rate of streaming media data employing a limited number of supported coding bit rates
InactiveUS20060165166A1Maximize qualityStable and high qualityPicture reproducers using cathode ray tubesPicture reproducers with optical-mechanical scanningStreaming dataNatural variation
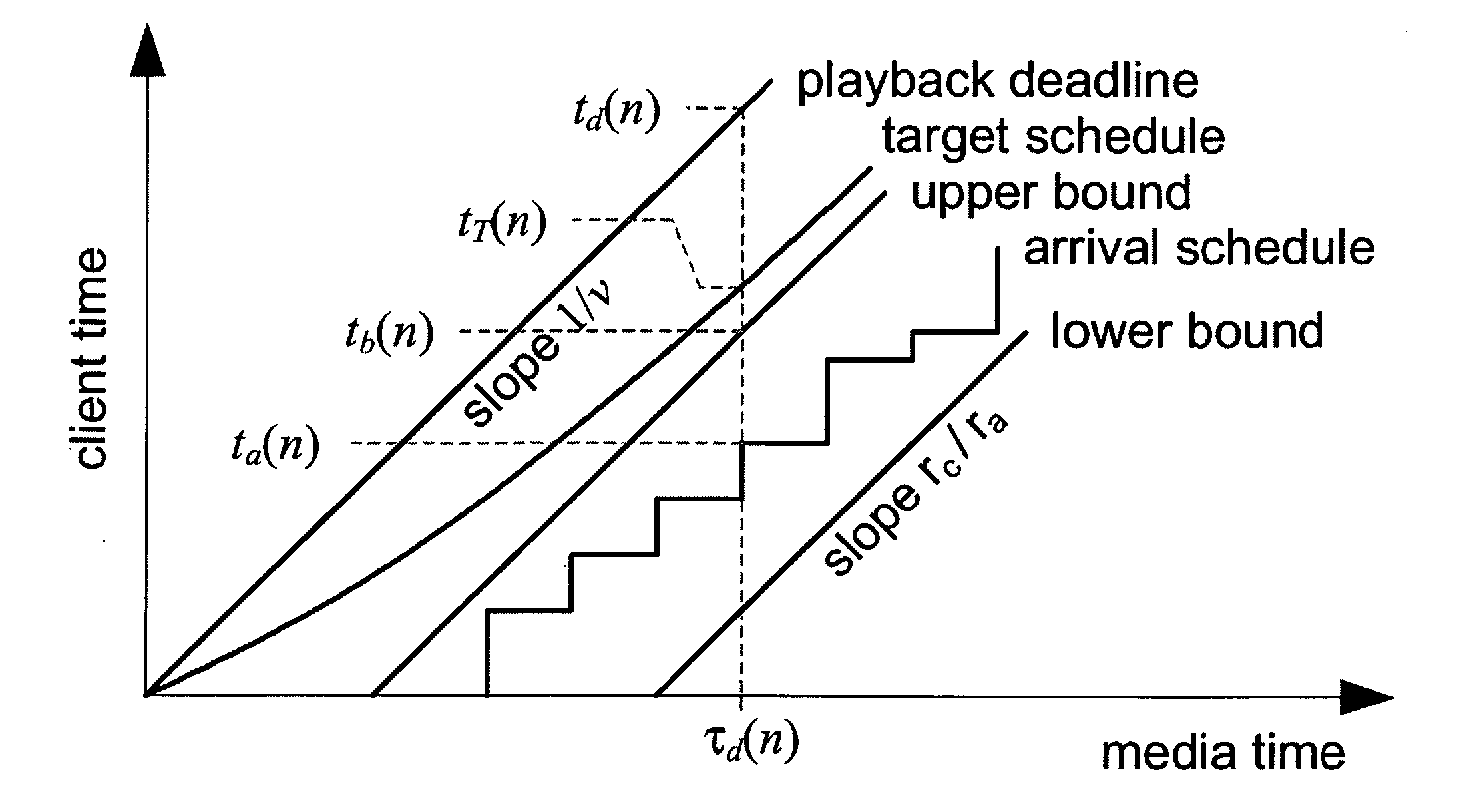
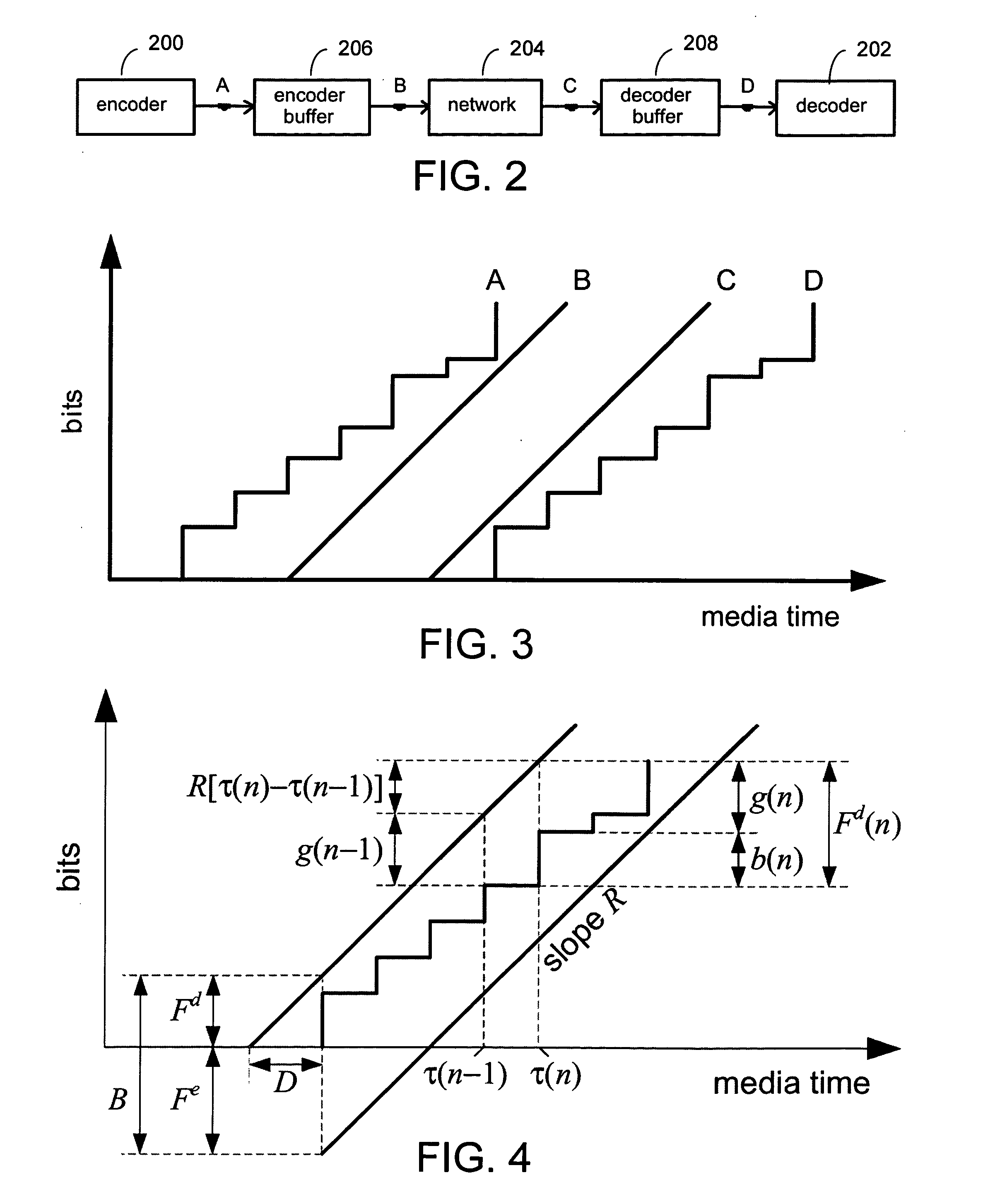
A system and process for controlling the coding bit rate of streaming media data is presented where a server streams data that exhibits one of a number of coding bit rates supported by the server. Initially, the server chooses the coding bit rate. However, after this startup period, the client provides coding bit rate requests. The server transmits the streaming media data at the most appropriate supported coding bit rate closest to the rate requested. The coding bit rates requested are those estimated to provide a high quality playback of the streaming data while still keeping a decoder buffer of the client filled to a desired level. A leaky bucket model is incorporated so that the changes in buffer duration due to natural variation in the instantaneous coding bit rate are not mistaken for changes in buffer duration due to network congestion.
Owner:MICROSOFT TECH LICENSING LLC
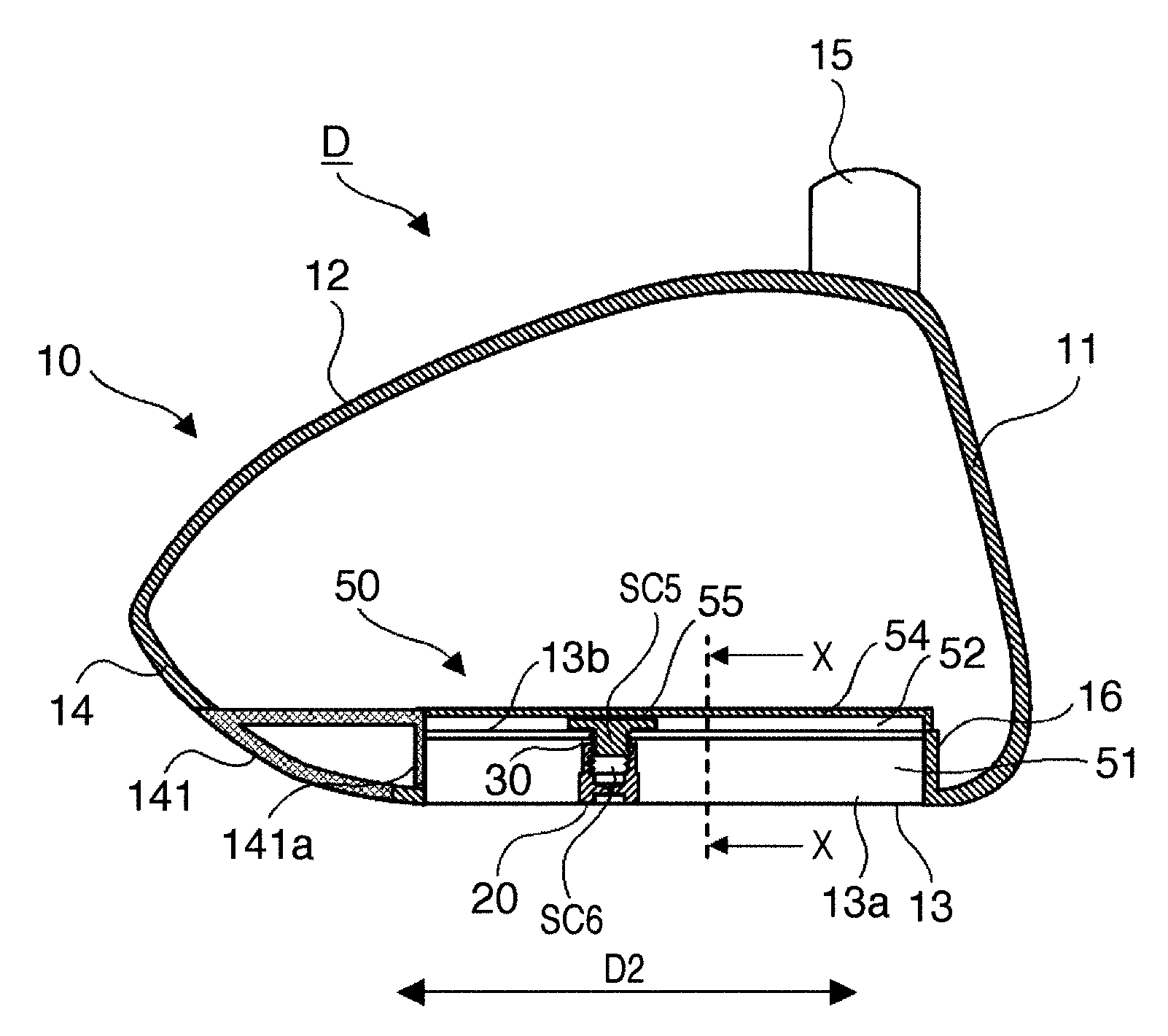
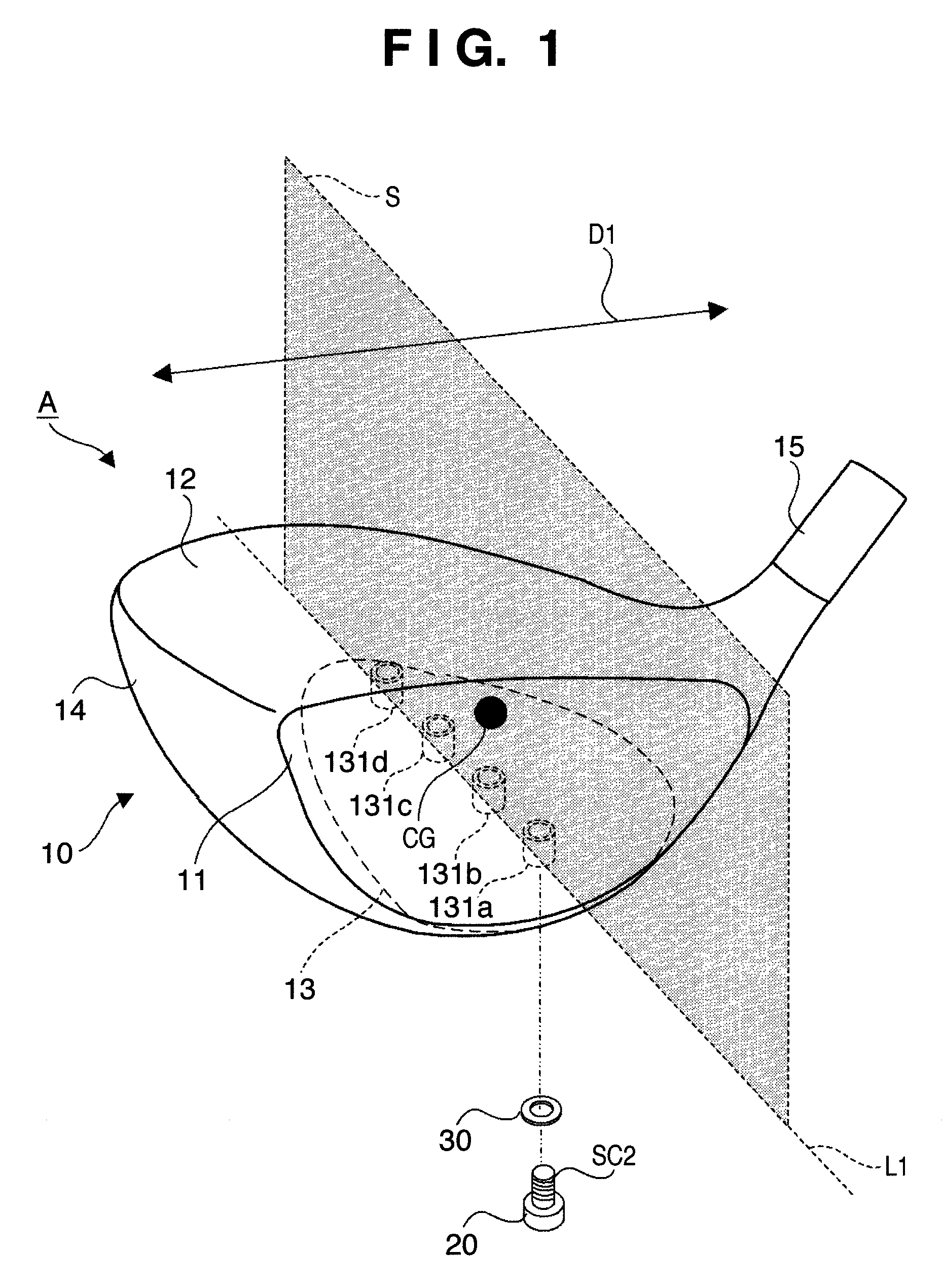
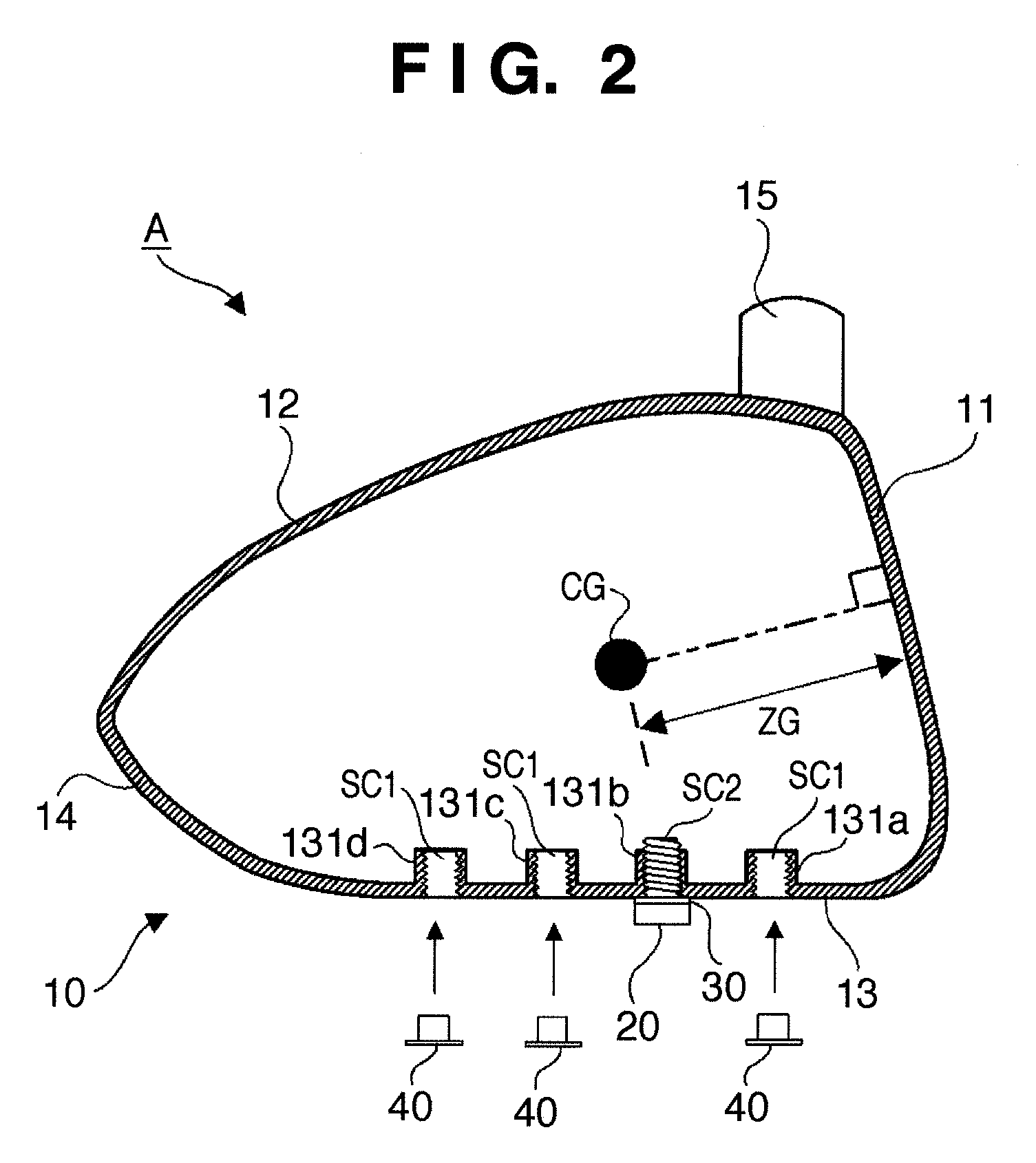
Golf club head
ActiveUS8192303B2Change in lateral directionality of flightSlow changeGolf clubsRacket sportsEngineeringGolf Ball
A golf club head of this invention includes a head body, a weight member attached to the head body, and a fixing unit to fix the weight member at any one of a plurality of attachment positions of the head body. The plurality of attachment positions are located on a straight line included in a plane. The plane includes the center-of-gravity position of the head body without the weight member attached thereto and is perpendicular to the toe-and-heel direction of the head body.
Owner:BRIDGESTONE SPORTS
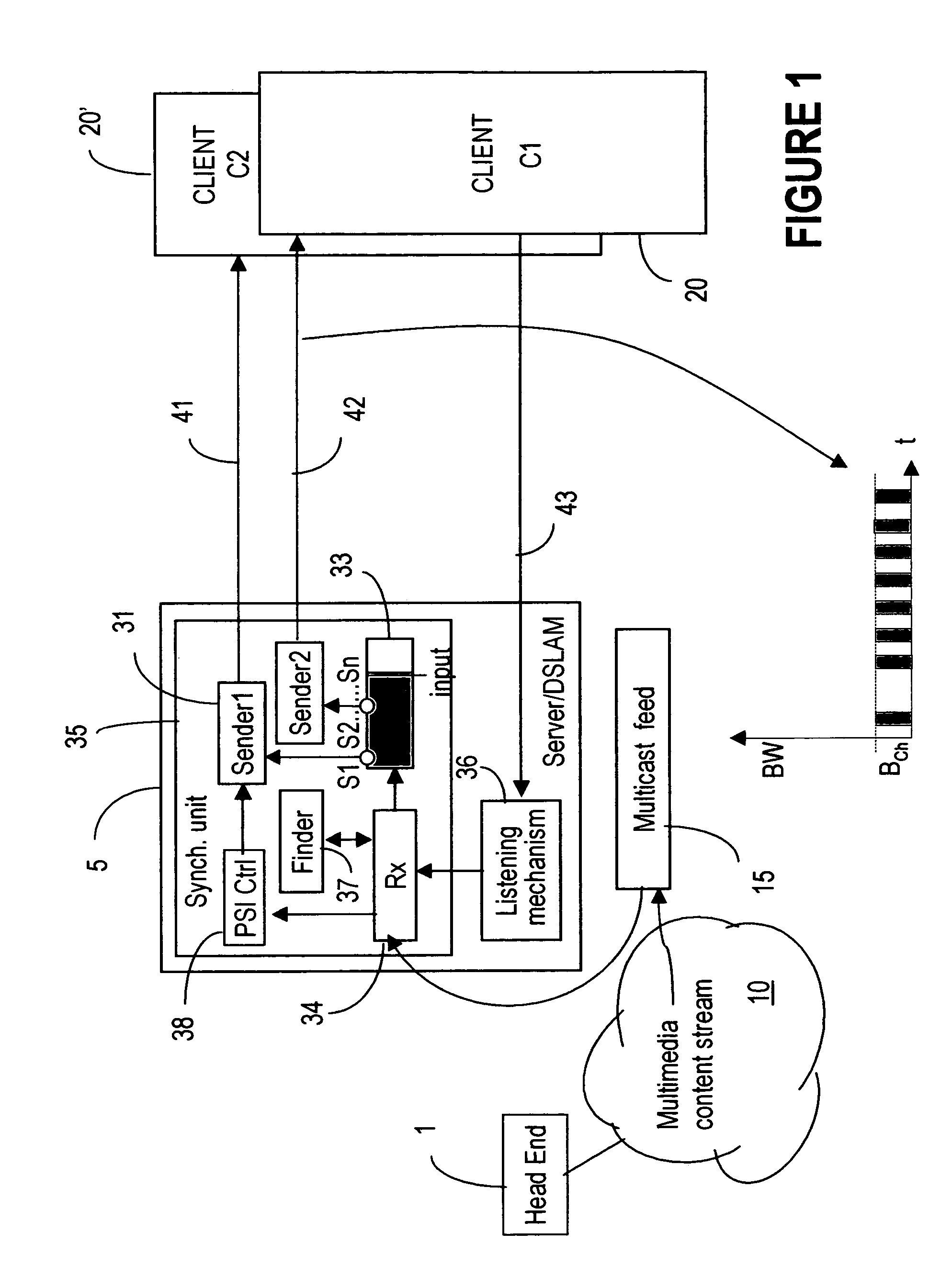
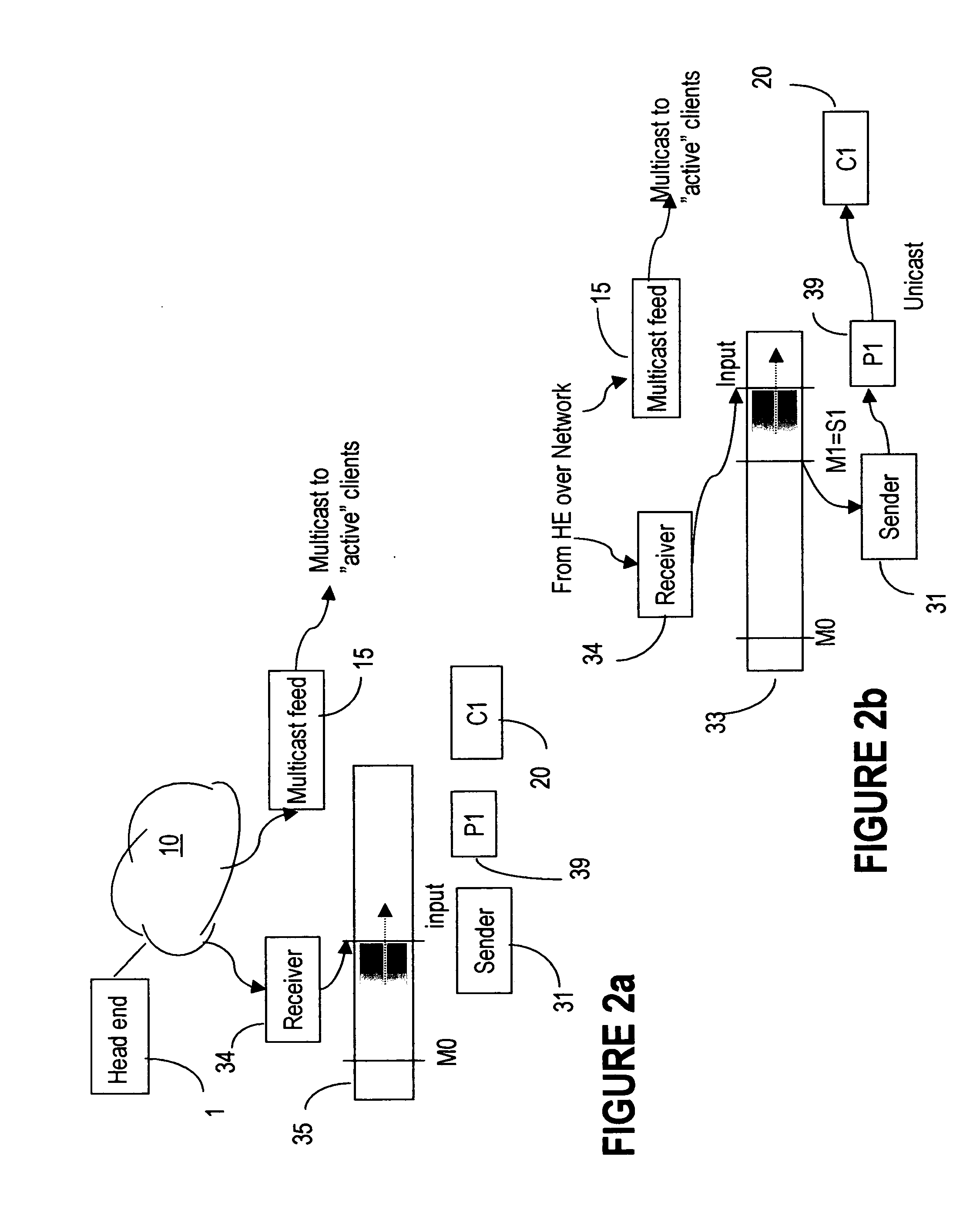
Milestone synchronization in broadcast multimedia streams
ActiveUS20060242240A1Reduce channel change delayQuick displayTelevision system detailsPicture reproducers using cathode ray tubesMultimedia streamsMilestone
A server at the edge of a broadband network distributes multimedia content streams to clients, while ensuring that the first data delivered to each client is key data (milestones) needed for correctly decoding the stream content. This is obtained by buffering the packets in the incoming stream and transmitting the packets from the buffer in an outgoing stream, starting with the most recent milestone placed in the buffer before a request to join the respective incoming stream is received. As the writing to and reading from the buffer are performed at different rates, the incoming and outgoing streams are eventually synchronized, at which point the client may be switched to receive the incoming stream directly.
Owner:WSOU INVESTMENTS LLC
Backplanes for display applications, and components for use therein
InactiveUS7605799B2Slow changeLow cost manufacturingTransistorStatic indicating devicesCapacitanceDisplay device
A thin-film transistor includes a gate electrode having first and second gate electrode edges on opposed sides, and a drain electrode having a first edge that overlaps the first gate electrode edge, and a second edge that overlaps the second gate electrode edge. A diode array is fabricated by successive deposition of a conductive layer, a doped semiconductor layer and an undoped semiconductor layer adjacent to the substrate. A display pixel unit provides reduced capacitative coupling between a pixel electrode and a source line. The source line includes an extension that provides a source for the transistor. A patterned conductive portion is disposed adjacent to the source line. Another display pixel unit provides reduced pixel electrode voltage shifts using a source line and a balance line.
Owner:E INK CORPORATION
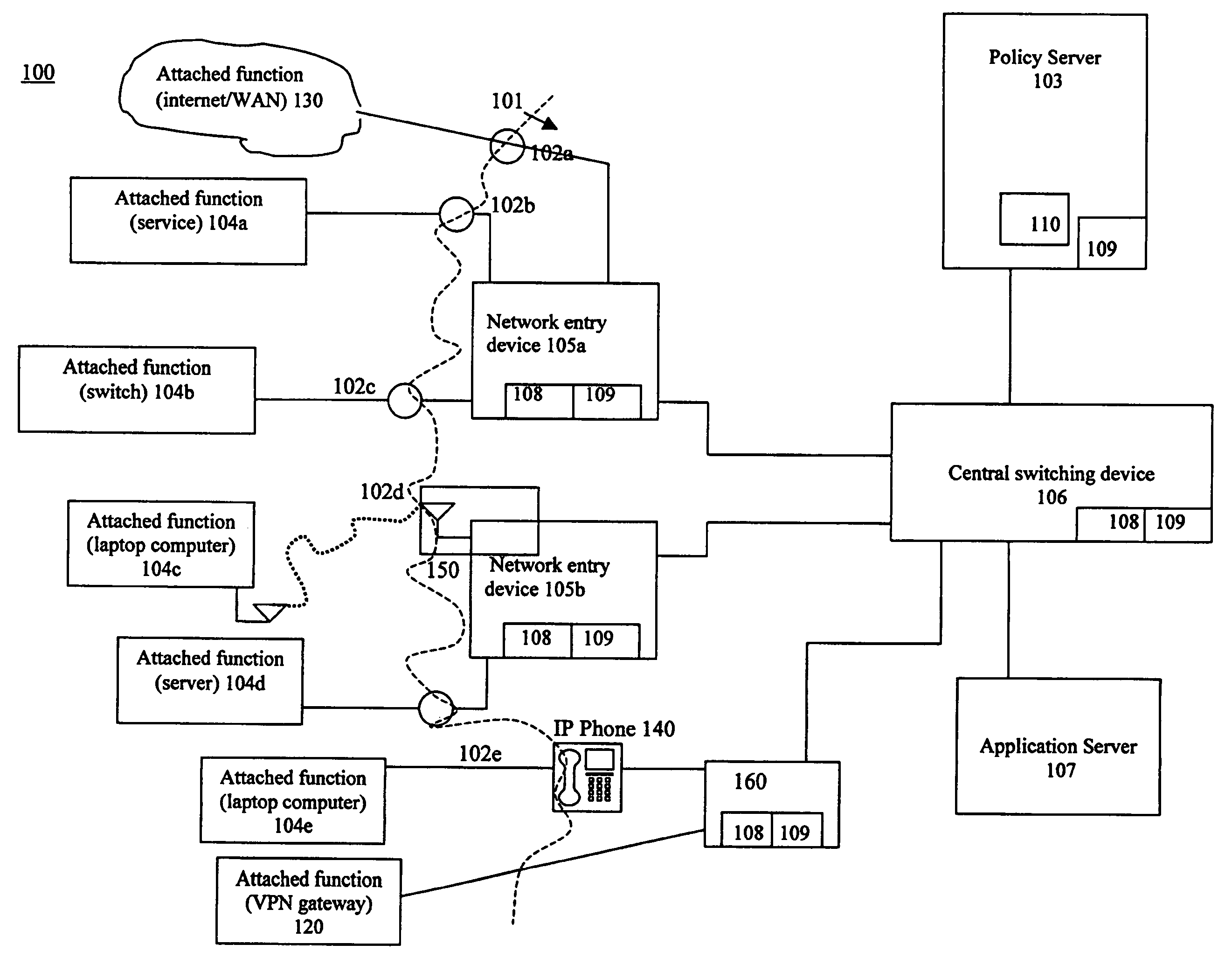
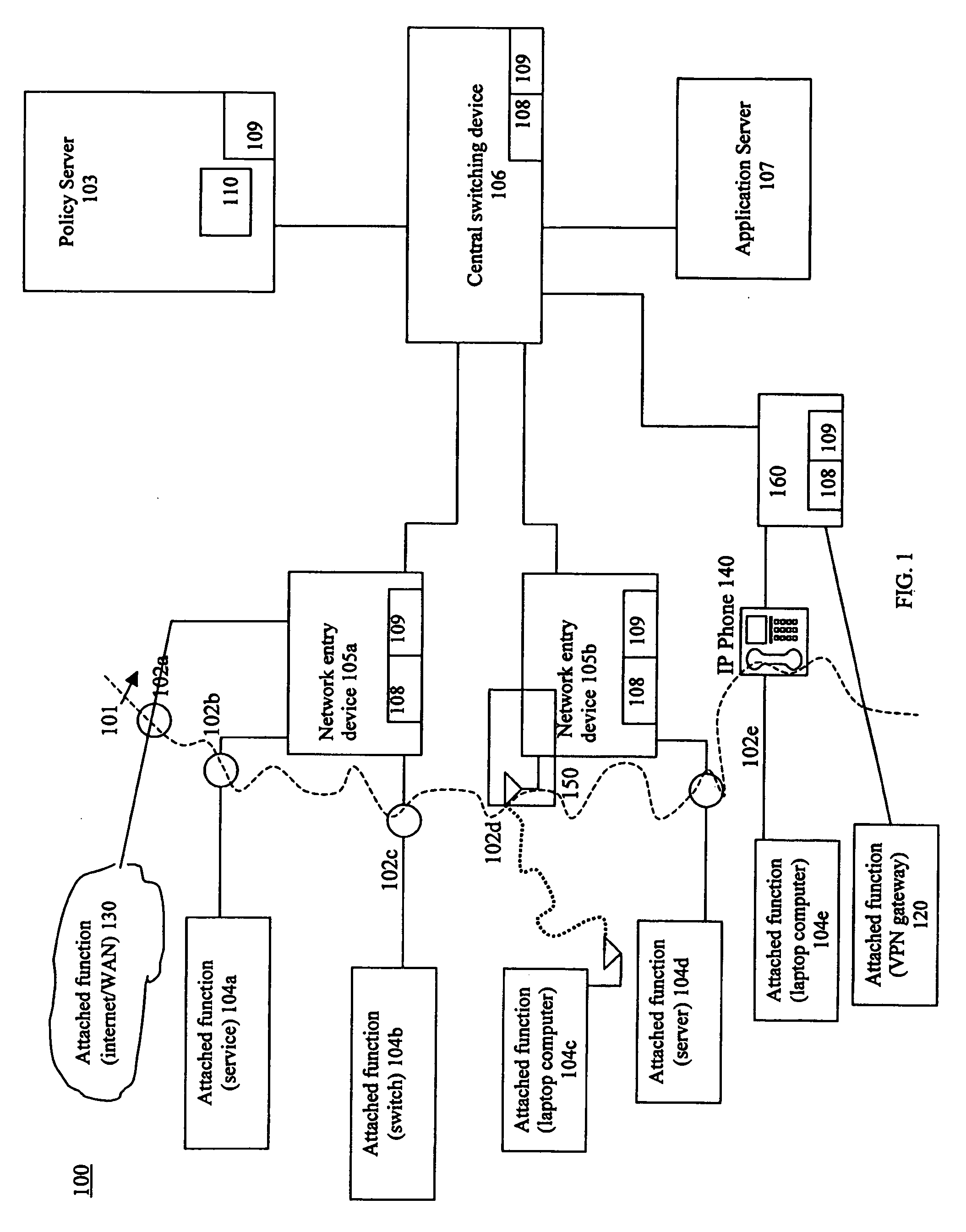
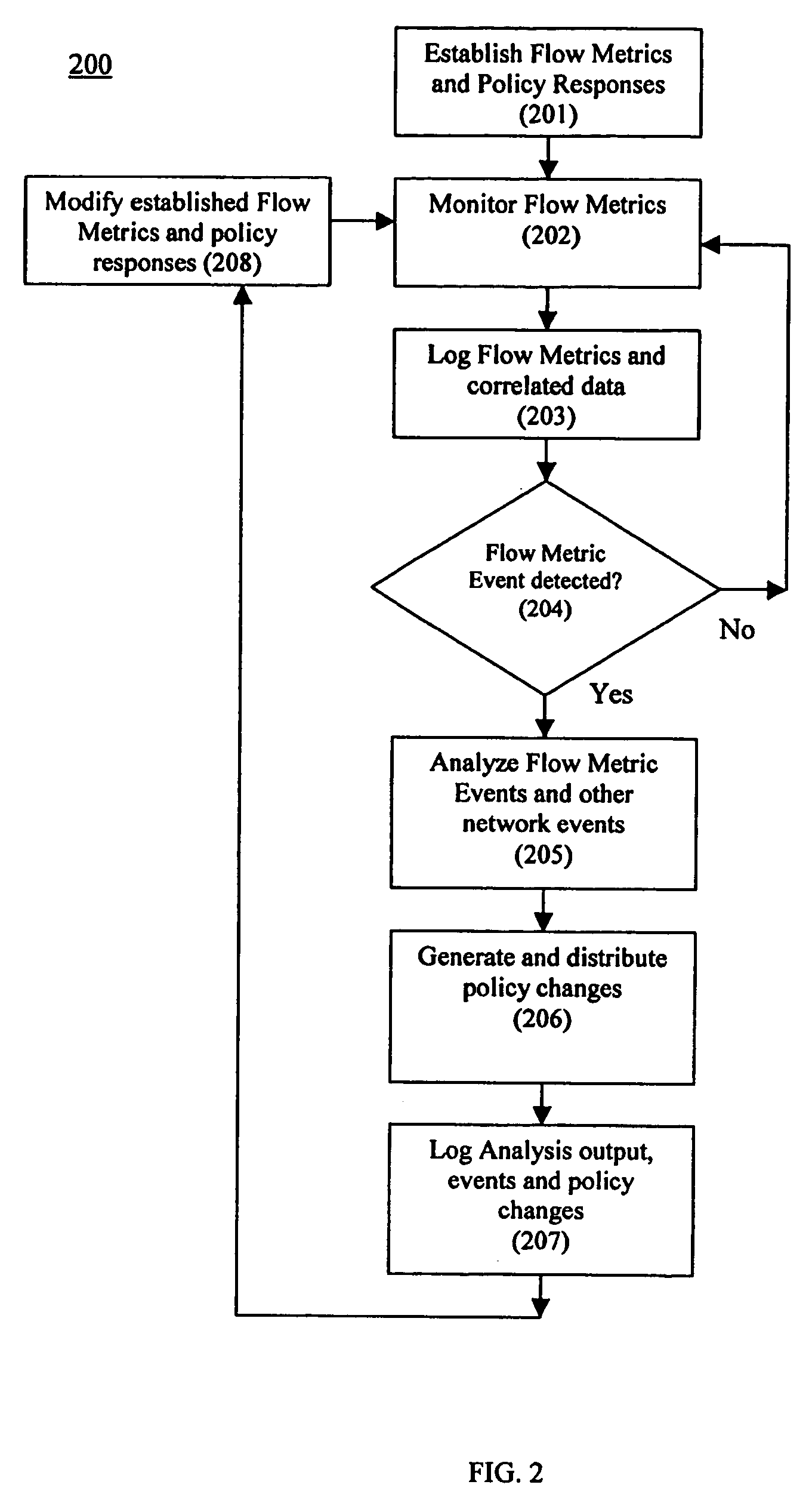
Using flow metric events to control network operation
InactiveUS20060075093A1Minimize impactSlow changeDigital computer detailsTransmissionNetworked systemTraffic flow
A system and method to monitor, detect, analyze and respond to, triggering conditions associated with packet and signal flows in a network system including attached functions and a network infrastructure. The system includes a detection function, an analysis function, and a response function. The detection function includes a monitoring sub-function, a flow definition sub-function, and a monitor counter sub-function. The flow definition sub-function defines the types of activities associated with the traffic flow that may indicate a triggering condition requiring analysis and potentially a response. The monitor sub-function observes traffic flows. The monitor counter sub-function counts the defined types of activities occurring in the device. The analysis function analyzes the event from the monitored flows, flow counters, status and other network information and determines whether a response is required. The response function initiates a response to a perceived event or attack based on the events detected in the flow metrics and other data. The response function further includes a sub-function for activating changes throughout the network system based on receiving and sending event notifications. Responses generated by the response function include dynamic policy changes.
Owner:ENTERASYS NETWORKS
Liquid immersion type exposure apparatus
InactiveUS7053983B2High resolutionSlow changeSemiconductor/solid-state device manufacturingPhotomechanical exposure apparatusLiquid mediumEngineering
Disclosed is an exposure apparatus which includes a projection optical system for projecting a pattern of a reticle onto a substrate, wherein the substrate is exposed through a liquid medium kept at least in a portion between the substrate and an optical element of the projection optical system which optical element is nearest to the substrate, a supplying system for supplying a liquid medium, a collecting system for collecting a liquid medium, and an exhausting system for removing a bubble in the liquid medium through a bubble removing material having such property that it passes a gas but it does not pass a liquid.
Owner:CANON KK
Communication control apparatus and method
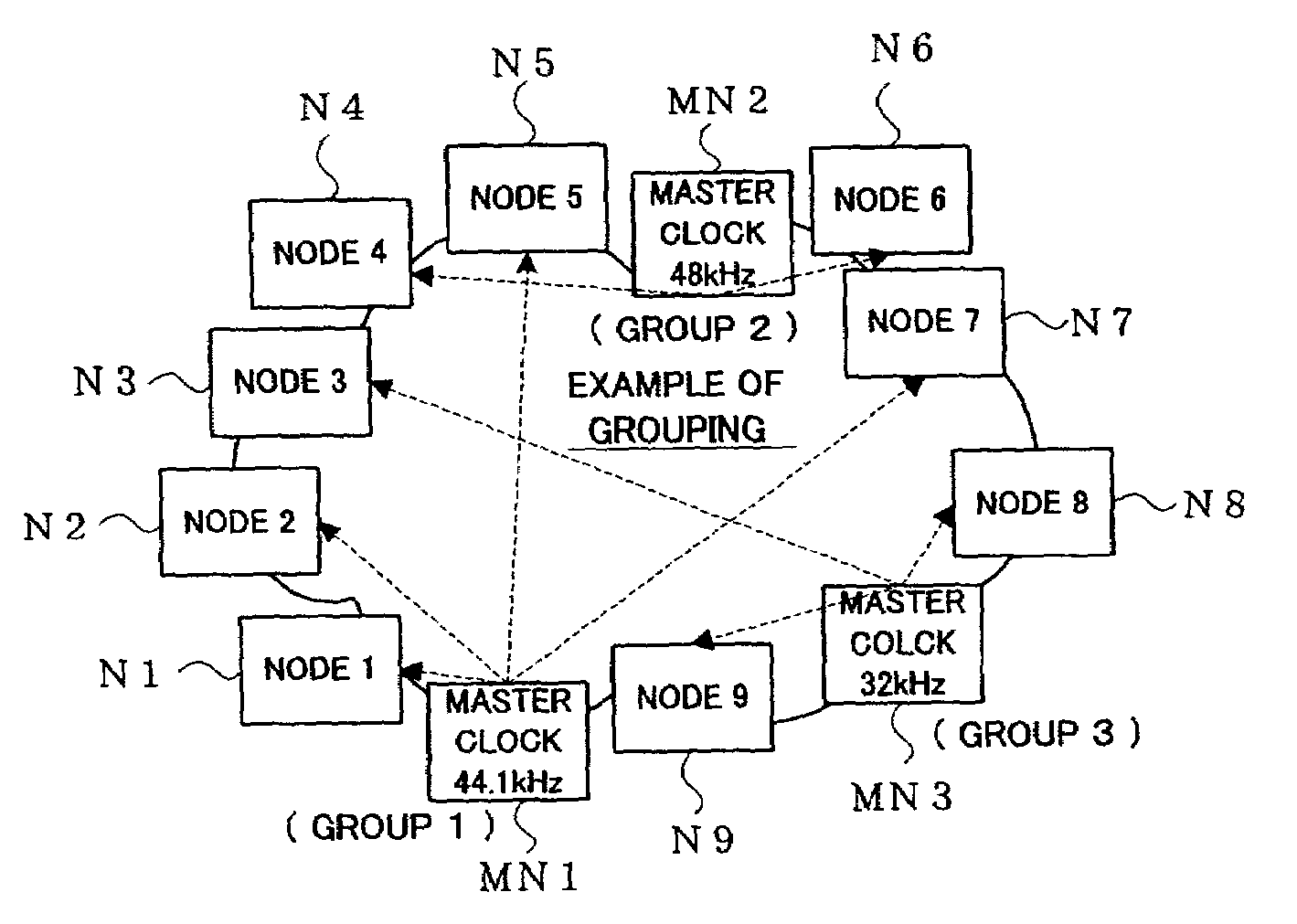
ActiveUS7158488B2Easy to identifyEasy to set upElectrophonic musical instrumentsLaser detailsNetwork packetExecution control
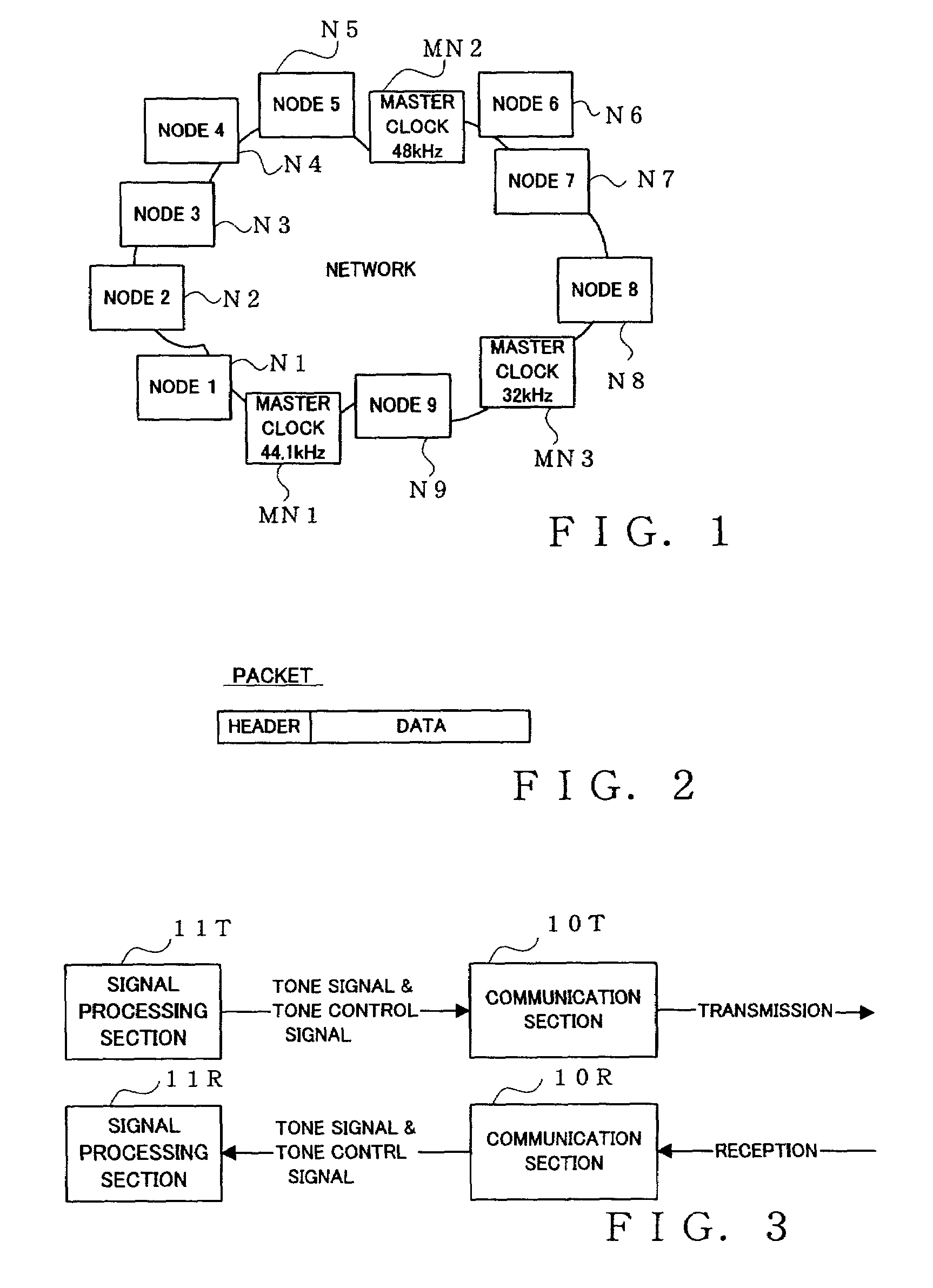
One or more nodes on a communication network are selected and set as one node group. In each of the nodes, group identification information representing the node group to which the node belongs is stored in advance. The group identification information is attached to the header of a data packet to be transmitted, so that nodes constituting a node group that should commonly receive data can be readily identified by comparing the group identification information imparted to the transmitted data packet and the group identification information stored in each receiving node. Given node can choose to become a node of a particular function, such as a clock master node, in which case control is performed to delete, from the node group, a node having so far played the role of the node of the particular function in such a manner that there exists only one node of the particular function per node group.
Owner:YAMAHA CORP
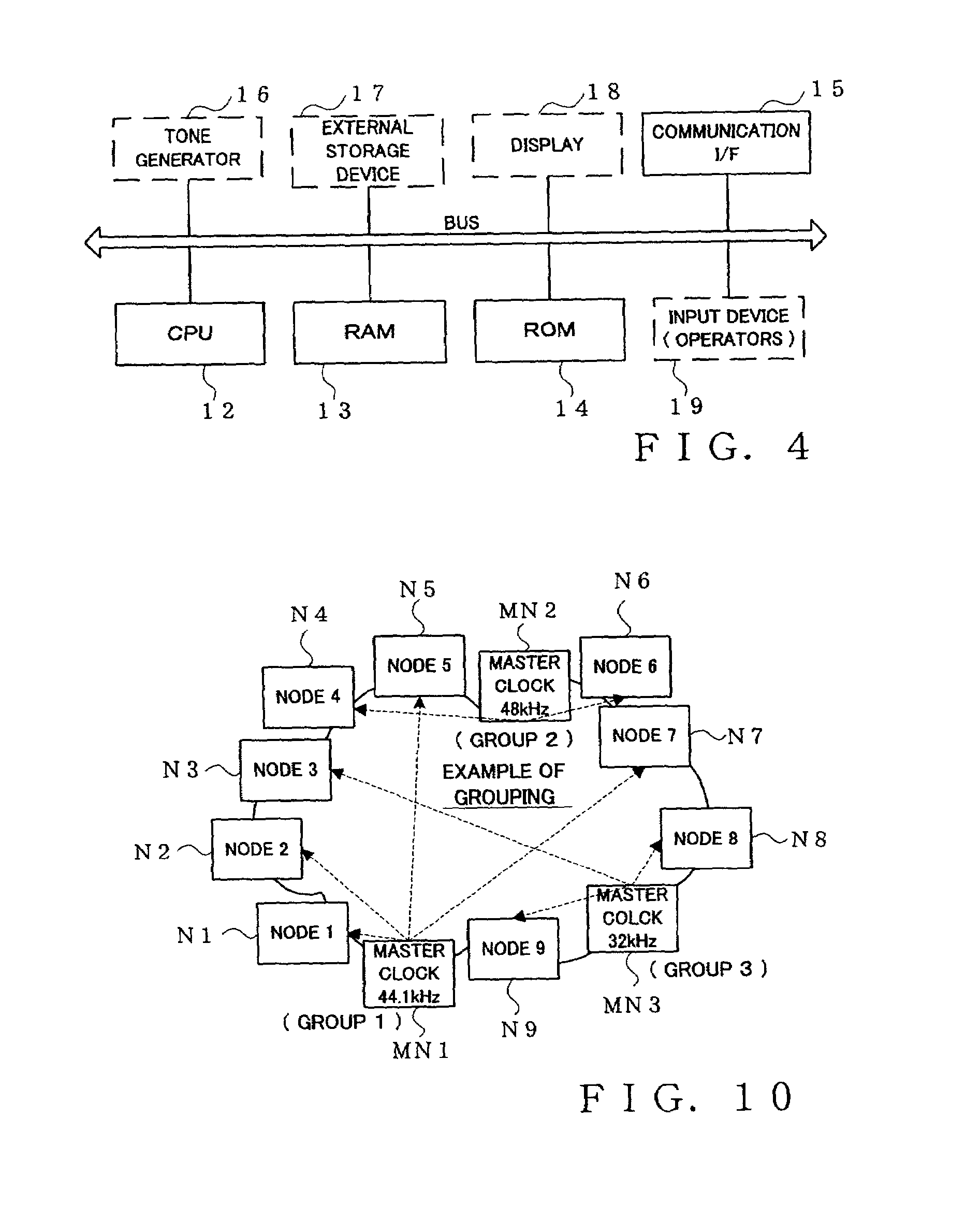
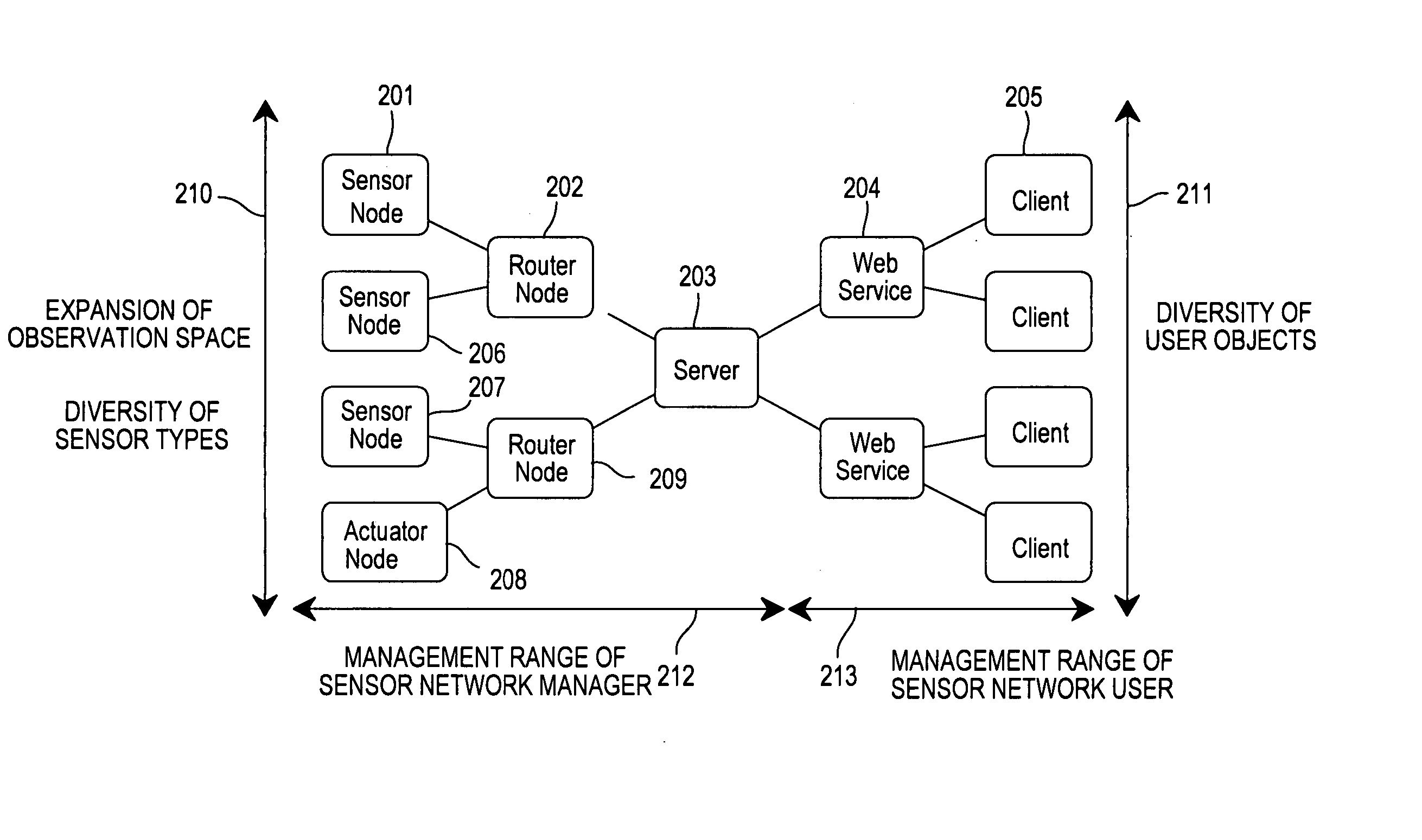
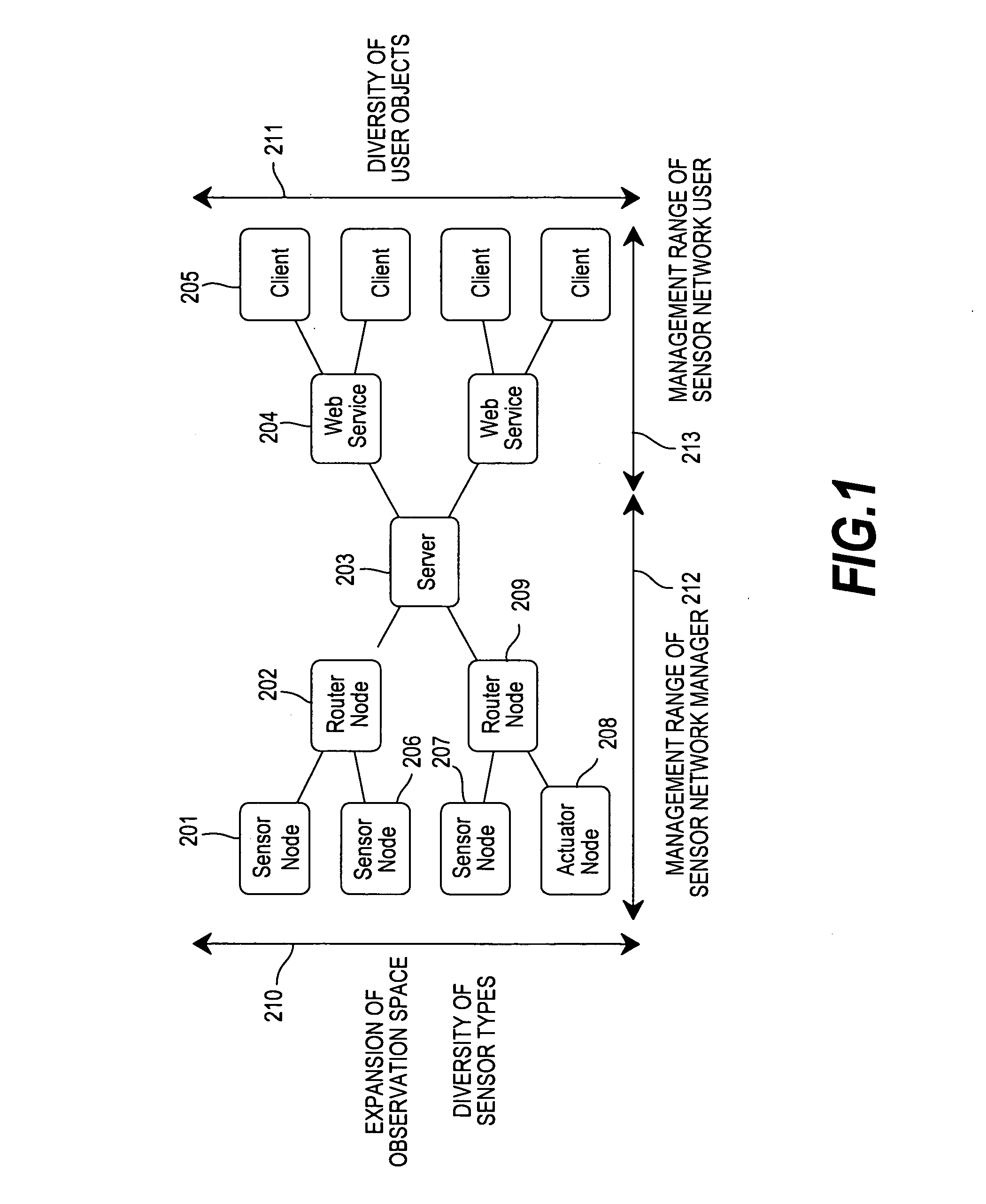
Sensor network system, method for data processing of a sensor network system
InactiveUS20060282498A1Reduce in quantityReduce load concentrationNetwork topologiesMultiple digital computer combinationsSensor nodeClient-side
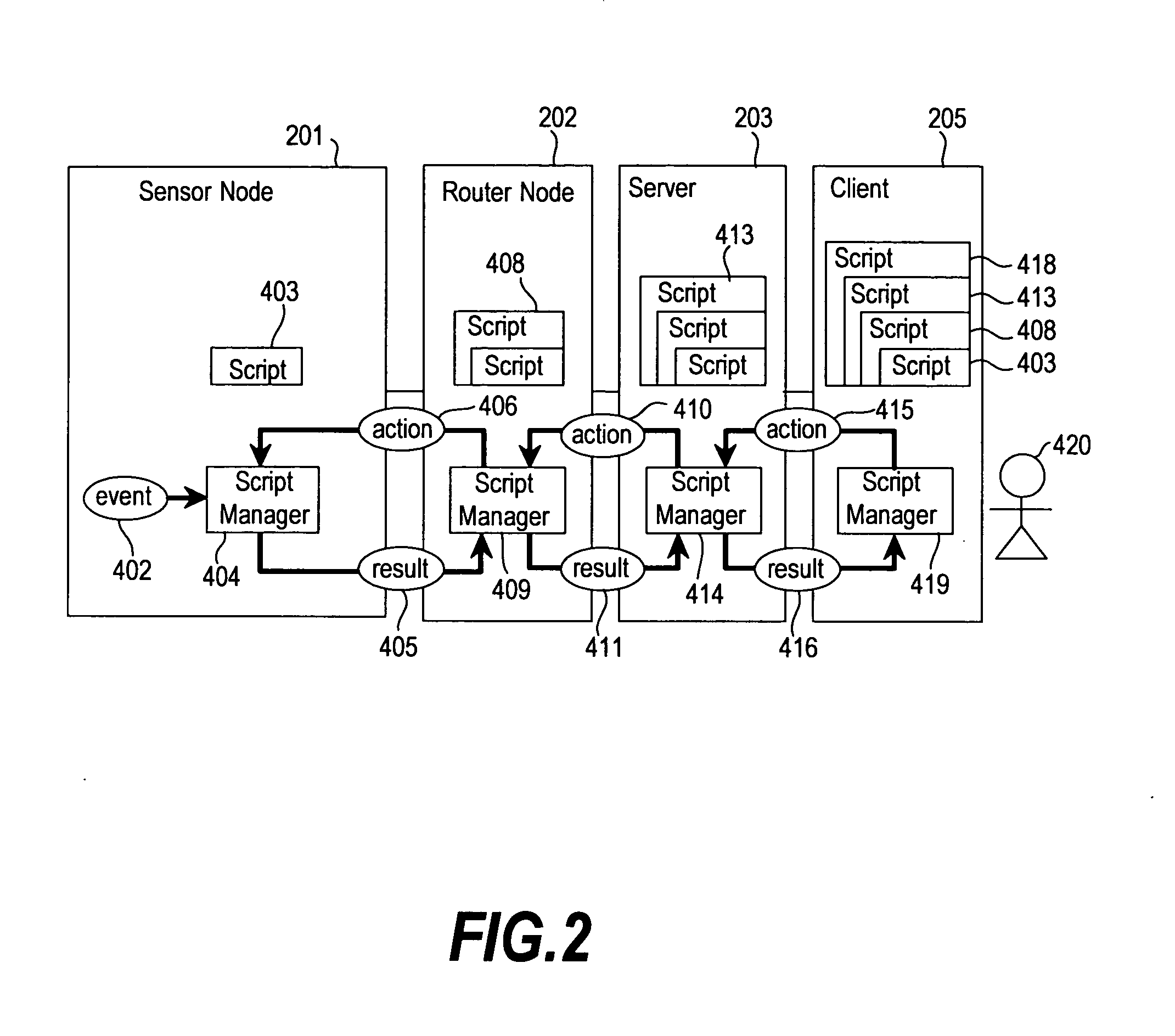
An operation flow of each node is dynamically changed according to a user request or a situation. A client (205) includes: a step (415) of extracting a script for a lower node from scripts in which processing is preset for a plurality of nodes to distribute the script extracted to the lower node; a step of executing processing for a self node from the script; a step of causing the lower node to receive the script distributed to execute processing for the node; and a step 410 of causing the lower node to extract a script for a node lower than the self node from the scripts, and to distribute the extracted script to the lower node if the script for the lower node is present. Based on a script executed by each node, an event from a lowest sensor node is sent through an intermediate node to the client 205.
Owner:HITACHI LTD
Buffer solution for electroporation and a method comprising the use of the same
InactiveUS20050064596A1High transfection efficiencyReduce cell deathPeptide/protein ingredientsGenetic material ingredientsElectroporationIon
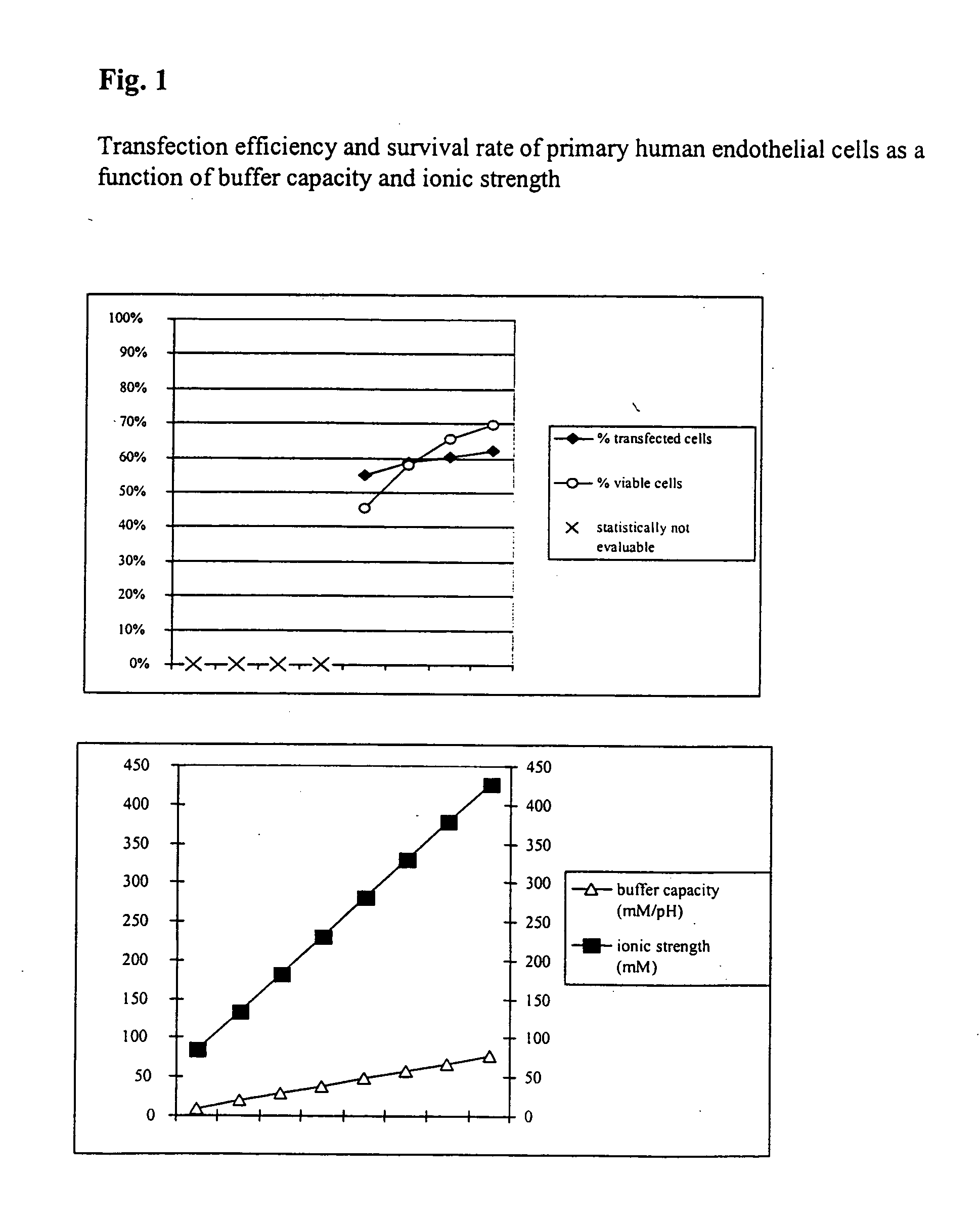
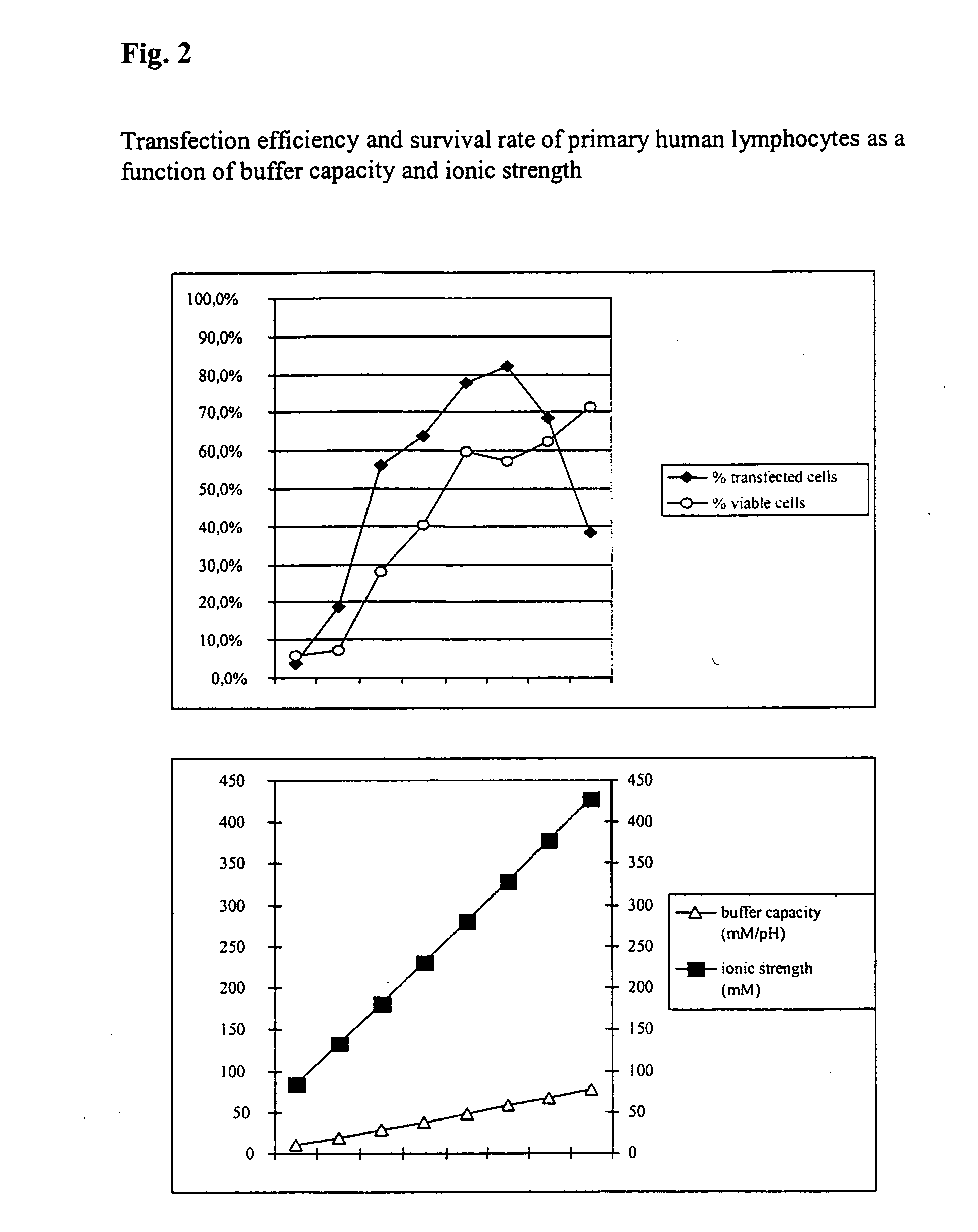
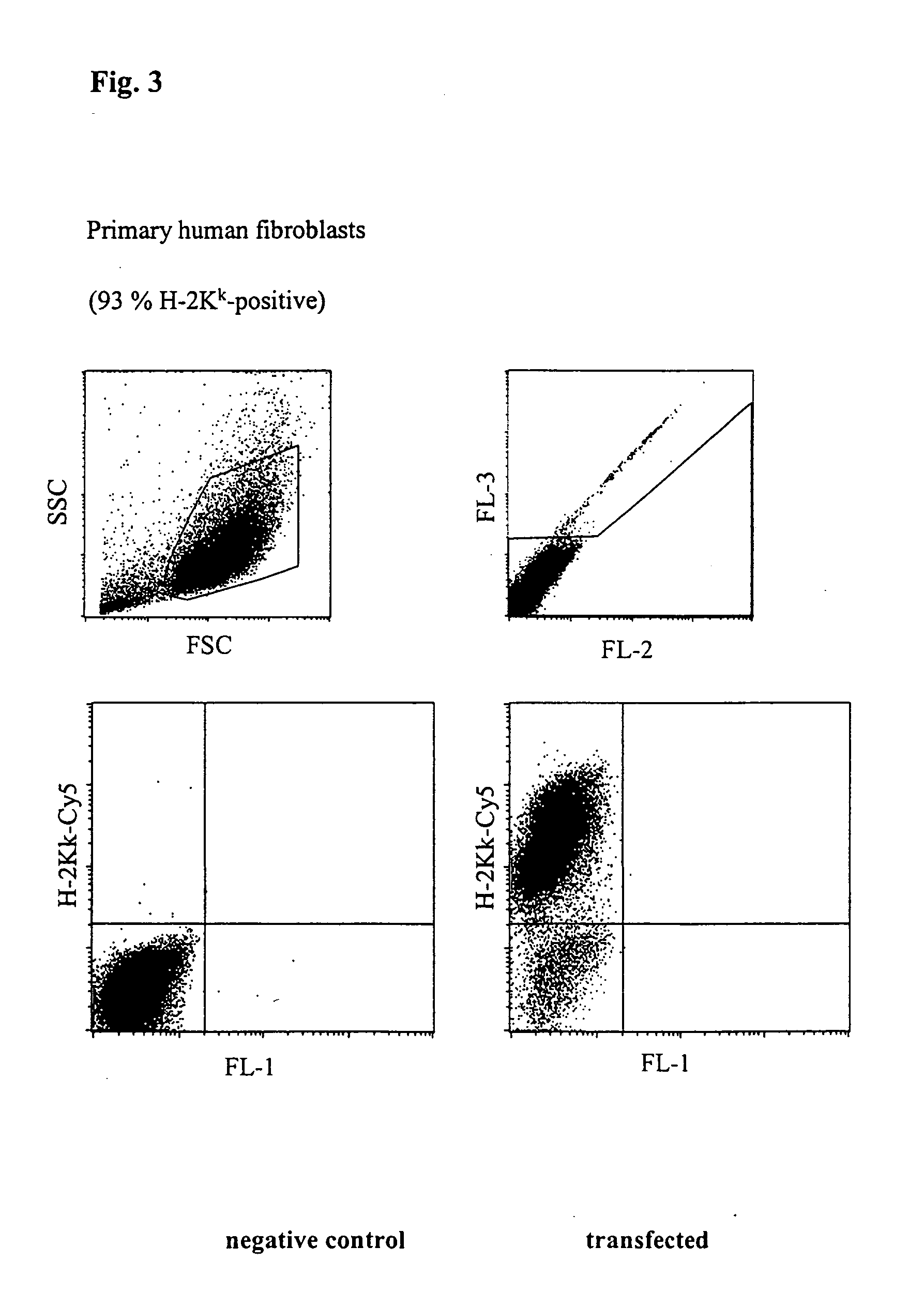
The invention relates to a buffer solution for suspending animal or human cells and for dissolving biologically active molecules in order to introduce said biologically active molecules into the cells using an electric current and to a method for introducing biologically active molecules into animal or human cells using an electric current and a buffer solution. The inventive buffer solution has a buffering capacity of at least 20 mmol*I−1*pH−1 and an ionic strength of at least 200 mmol*I−1 during a change to the pH value from pH 7 to pH 8 and at a temperature of 25° C. The use of a buffer solution of this type in the corresponding method allows biologically active molecules to be introduced into animal and human cells with a high degree of transfection efficiency and at the same time a low cell mortality. Different cell types, in particular dormant and actively dividing cells of low activity, can be successfully transfected in said buffer solution.
Owner:LONZA COLOGNE
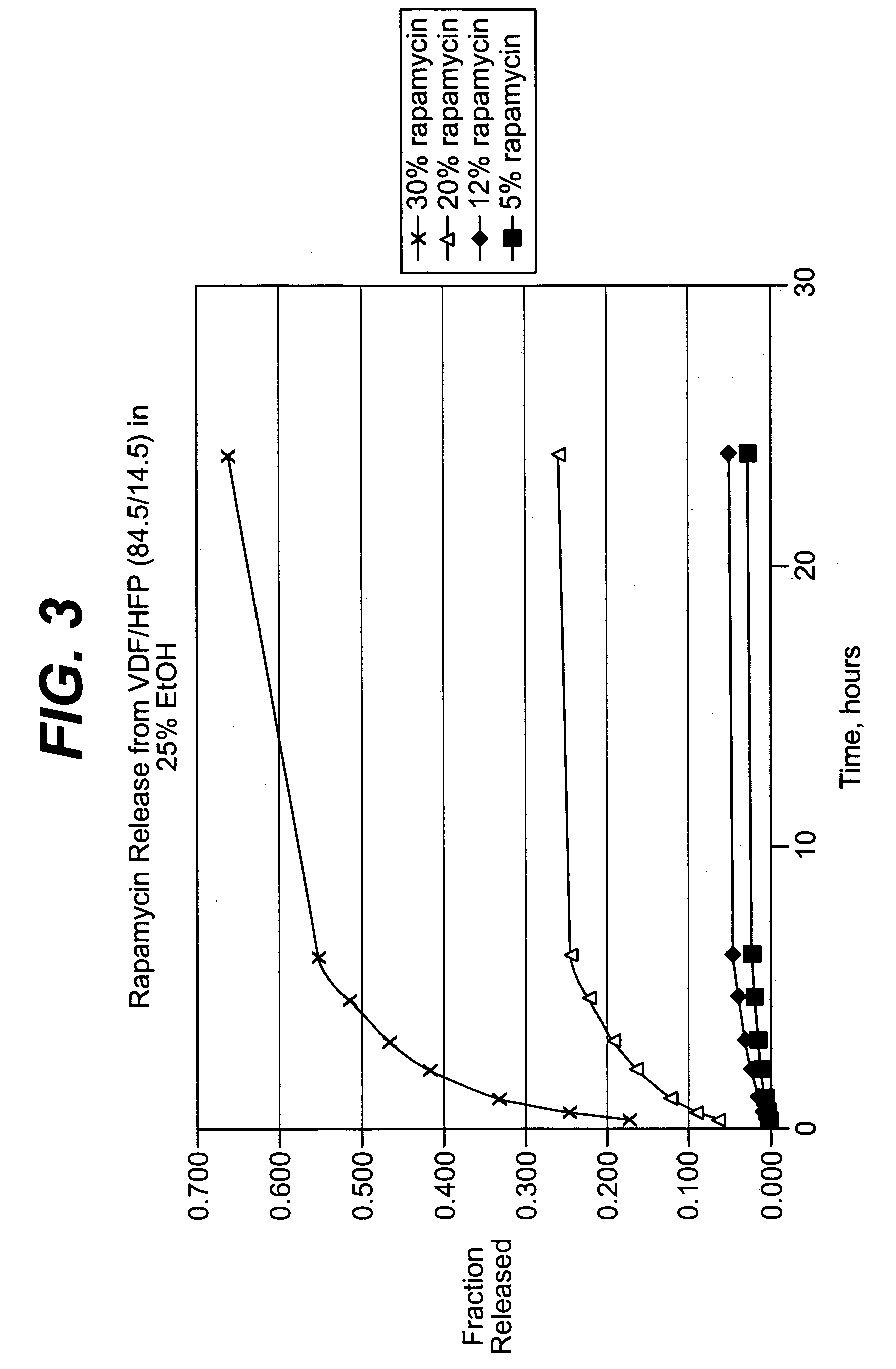
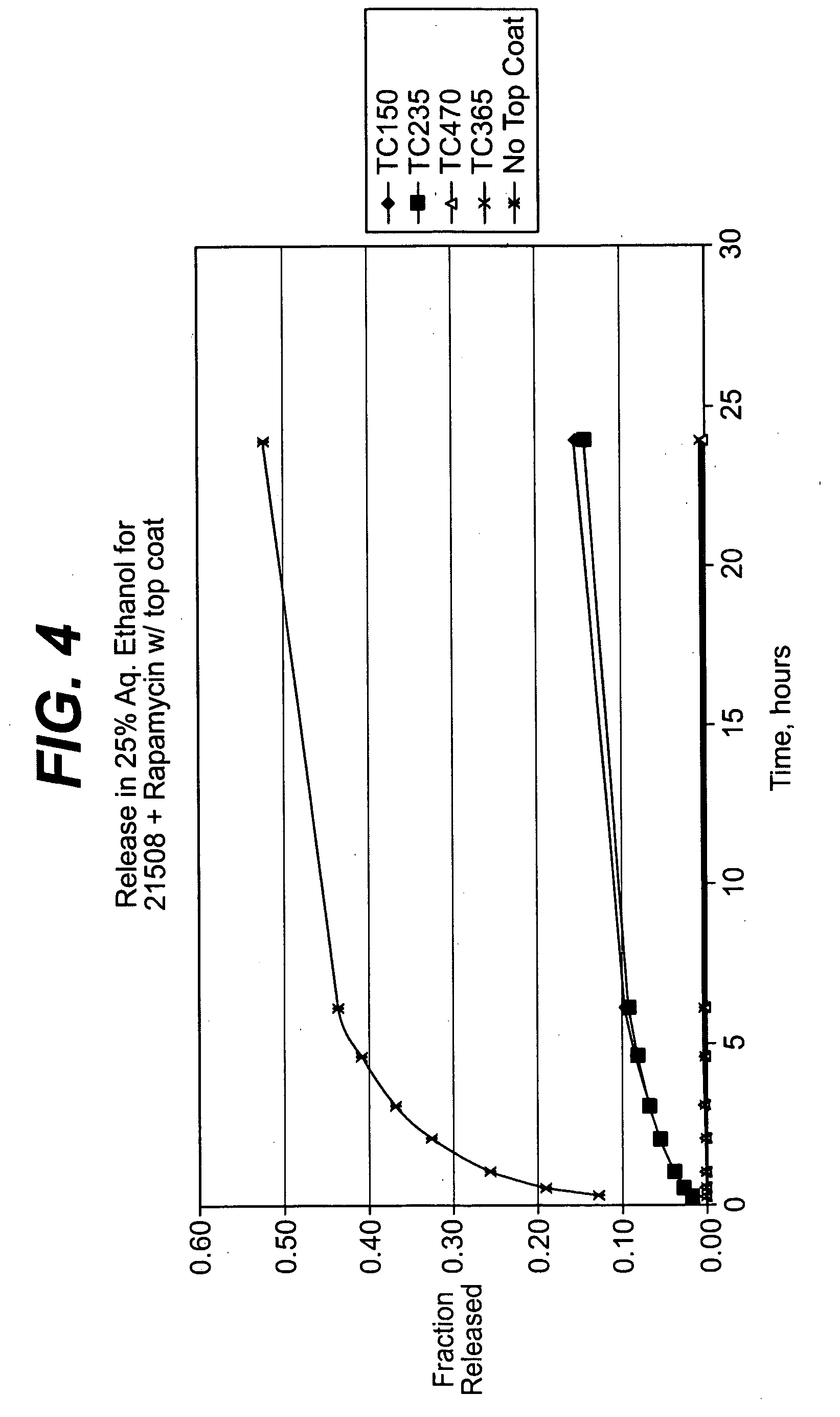
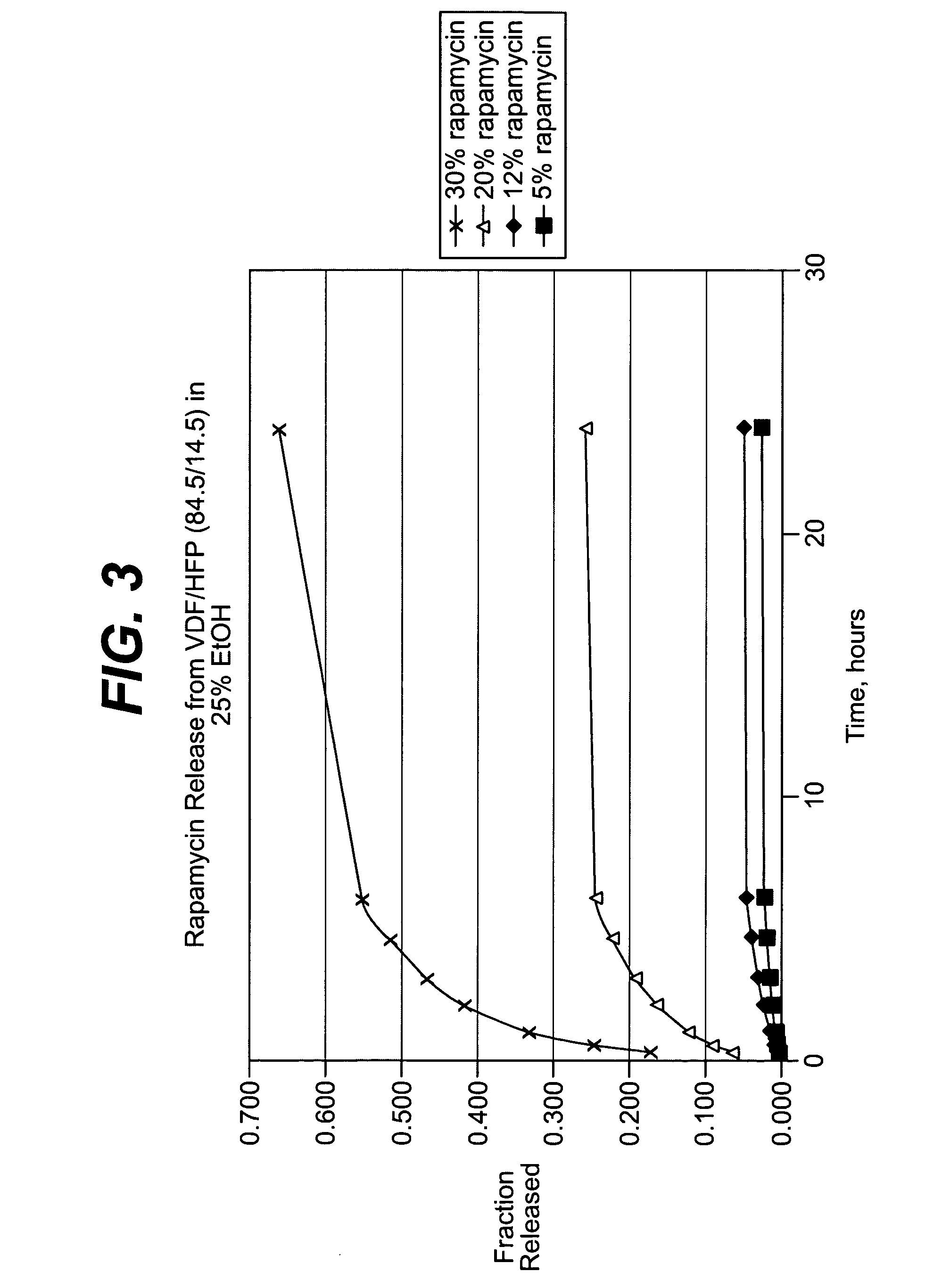
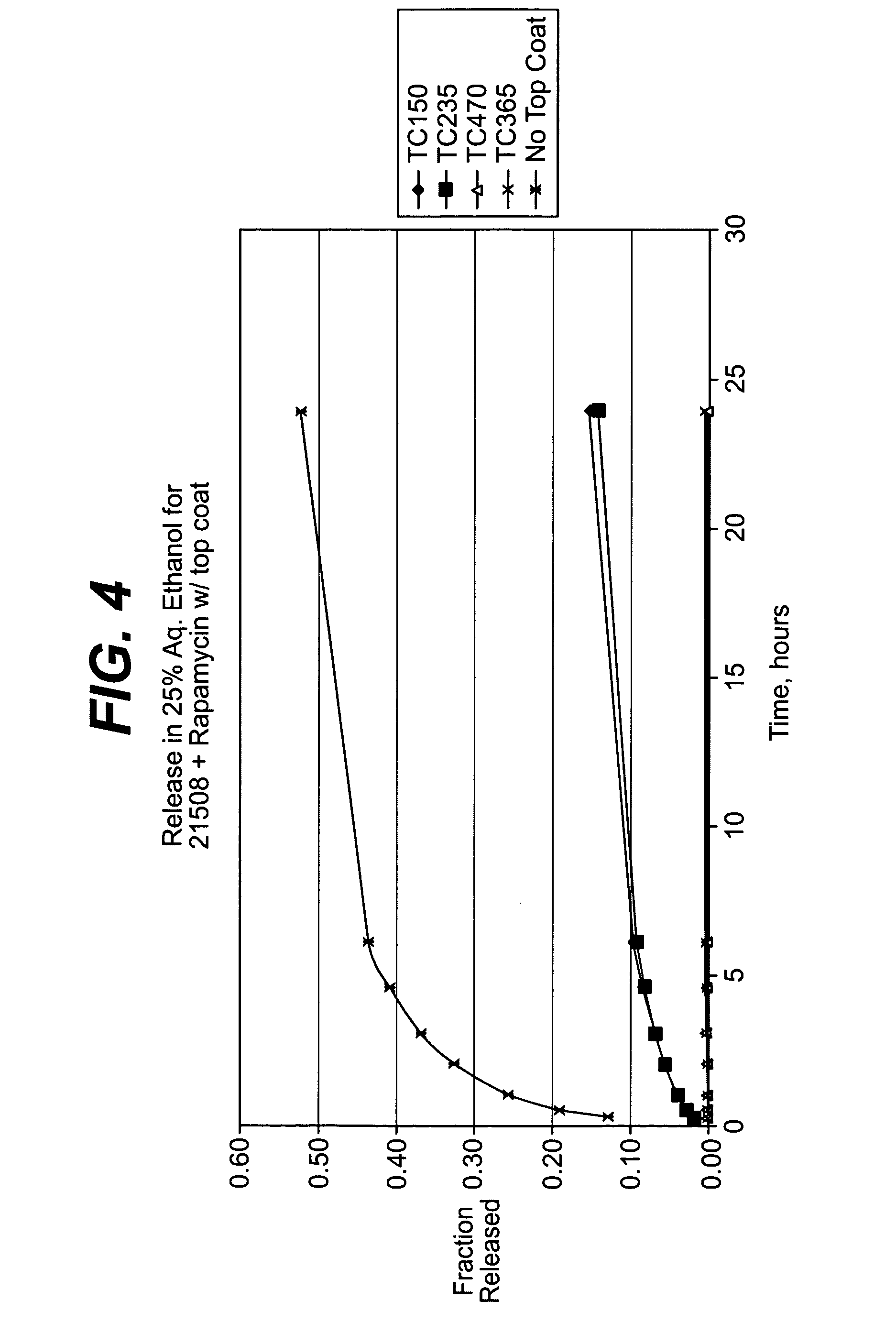
Solution formulations of sirolimus and its analogs for CAD treatment
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Biosensor
ActiveUS20050158850A1Suppresses nonspecific adsorptionSuppressing decreaseBioreactor/fermenter combinationsBiological substance pretreatmentsHydrophobic polymerBiosensor
It is an object of the present invention to provide a biosensor, which is not significantly affected by the baseline fluctuation and suppresses nonspecific adsorption. The present invention provides a biosensor, which comprises a metal surface or metal film coated with a hydrophobic polymer, and has two or more types of different surfaces in a region coated with a hydrophobic polymer.
Owner:FUJIFILM CORP +1
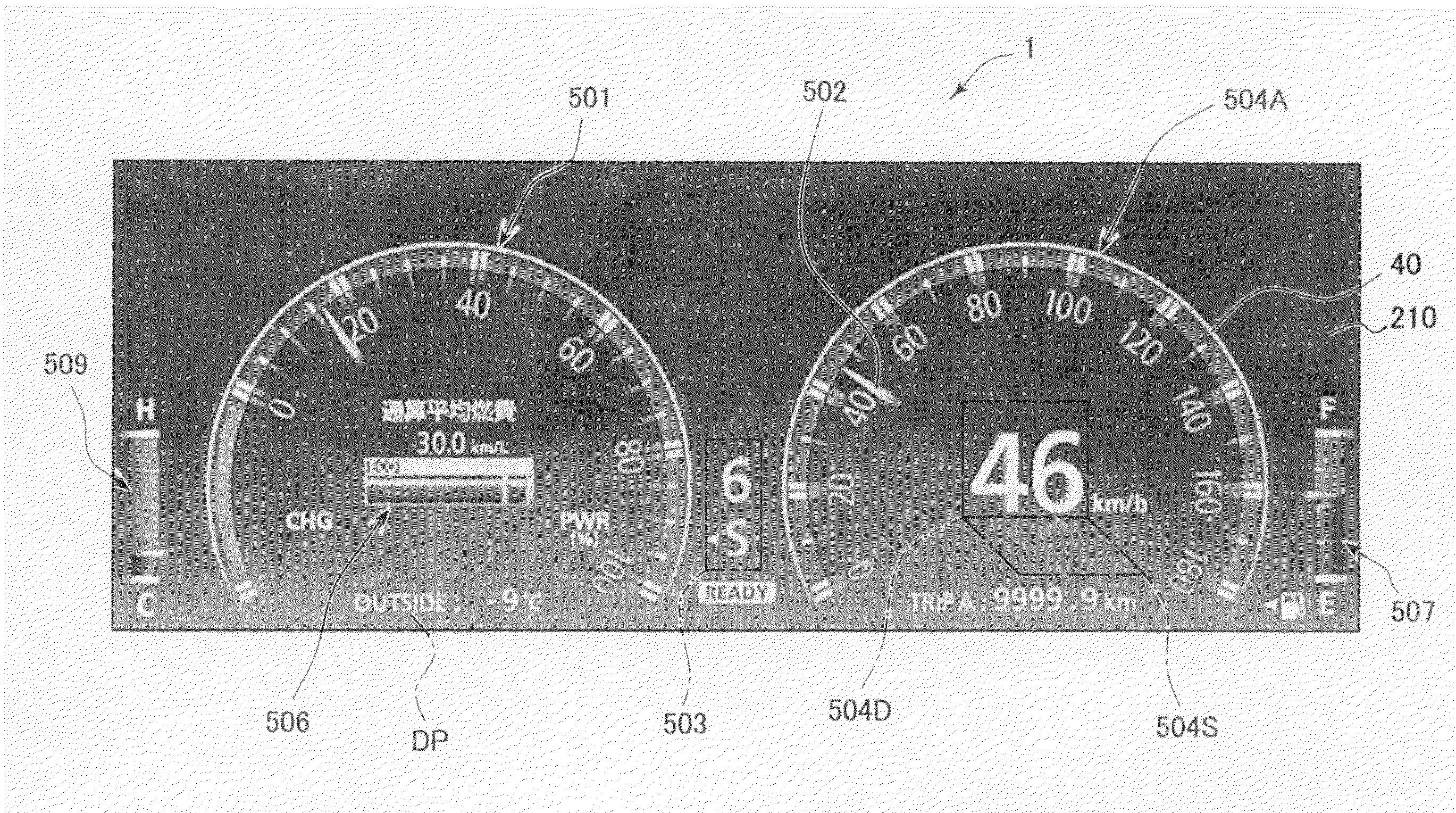
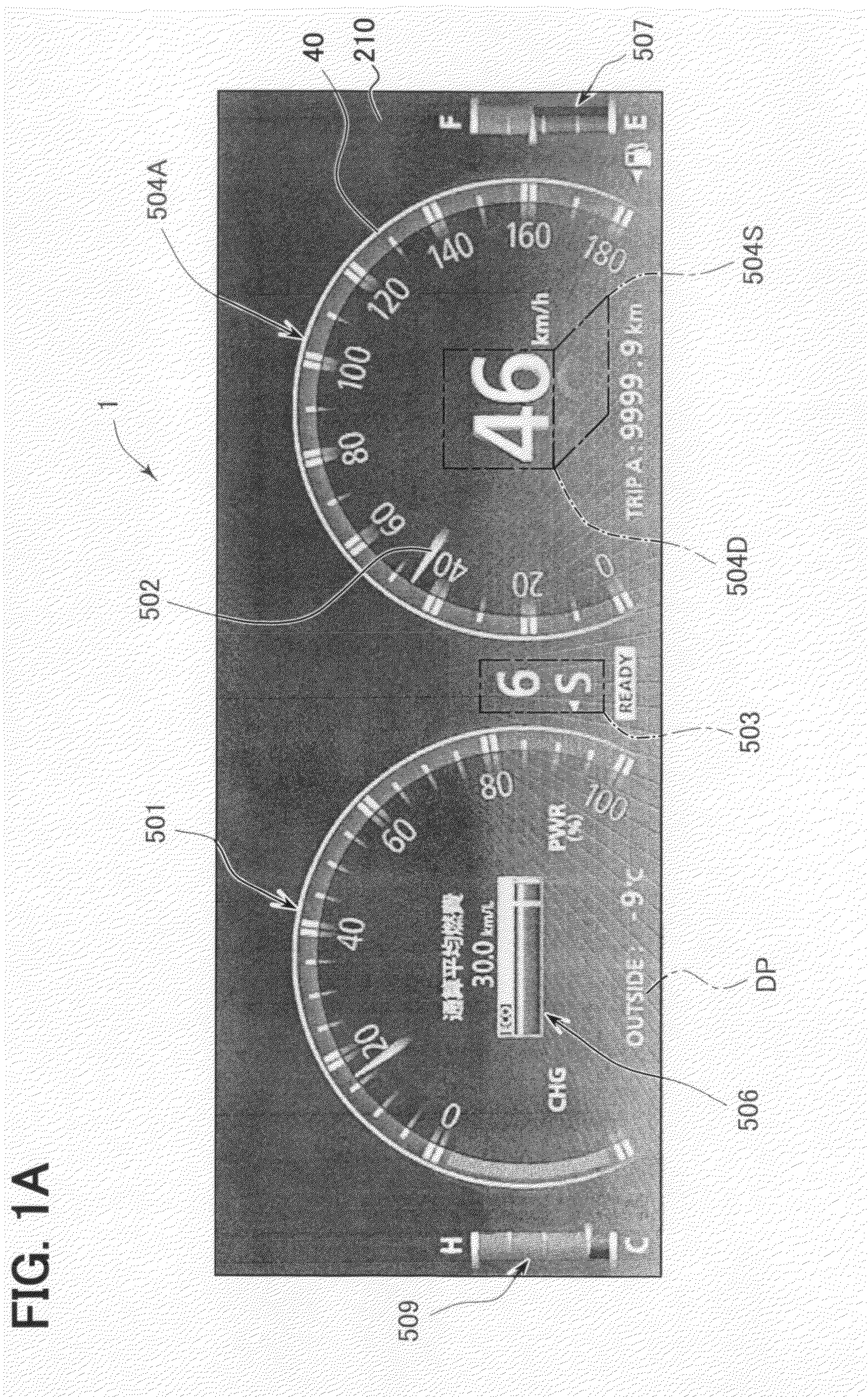
Vehicular meter unit and display device
ActiveUS20080309475A1Easy to useDisplay is limitedInstrument arrangements/adaptationsProjectorsAnimationDisplay device
In a first display mode, a common image function component is displayed to occupy at least a part of a display region of a second specific image function component. In a second display mode, the common image function component is continuously displayed outside the second specific image function component to avoid the overlap therebetween (i.e., such that an exclusive positional relationship is made therebetween). When the display mode is switched, a final transition of the display mode to a display state in the second display mode is made while or after a moving image showing a transition process, in which the common image function component is moved from a display position in the first display mode to a display position in the second display mode, is displayed as a mode transition animation.
Owner:DENSO CORP
System and process for controlling the coding bit rate of streaming media data employing a linear quadratic control technique and leaky bucket model
InactiveUS20060143678A1Maximize qualitySmoothness of the average coding bit rate over consecutiveTwo-way working systemsDigital video signal modificationModel controlClient buffer
A system and process for controlling the coding bit rate of streaming media data is presented. This coding bit rate control involves dynamically adjusting the coding bit rate to control client buffer duration to prevent the buffer from underflowing, while keeping the average coding bit rate close to the average transmission bit rate of the network (an thus maximizing the quality of the data playback). Using the theory of optimal linear quadratic control, the client buffer duration is kept as close as possible to a target level while still keeping the coding bit rate (and hence the quality) as constant as possible. In addition, a leaky bucket model is incorporated into the control loop so that the changes in buffer duration due to natural variation in the instantaneous coding bit rate are not mistaken for changes in buffer duration due to network congestion.
Owner:MICROSOFT TECH LICENSING LLC
Injectable formulations of taxanes for cad treatment
InactiveUS20050272806A1Minimize potential risk of damageReduce frictionBiocideOrganic active ingredientsAntioxidantBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices. Liquid formulations, including solutions and suspensions of the various drugs, agents and / or compounds, may be locally or regionally delivered. In each of these instances, antioxidants are utilized to prolong product integrity.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
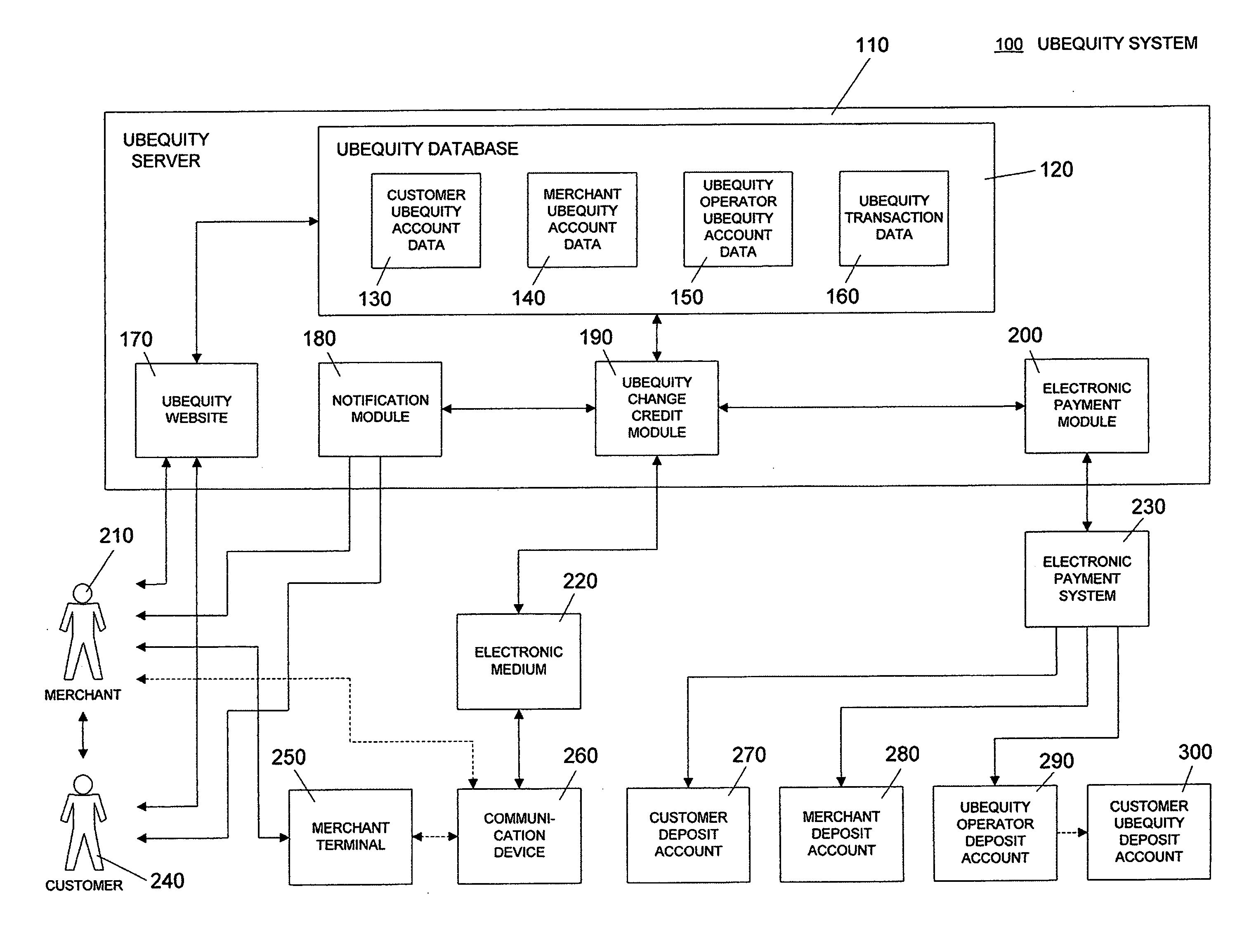
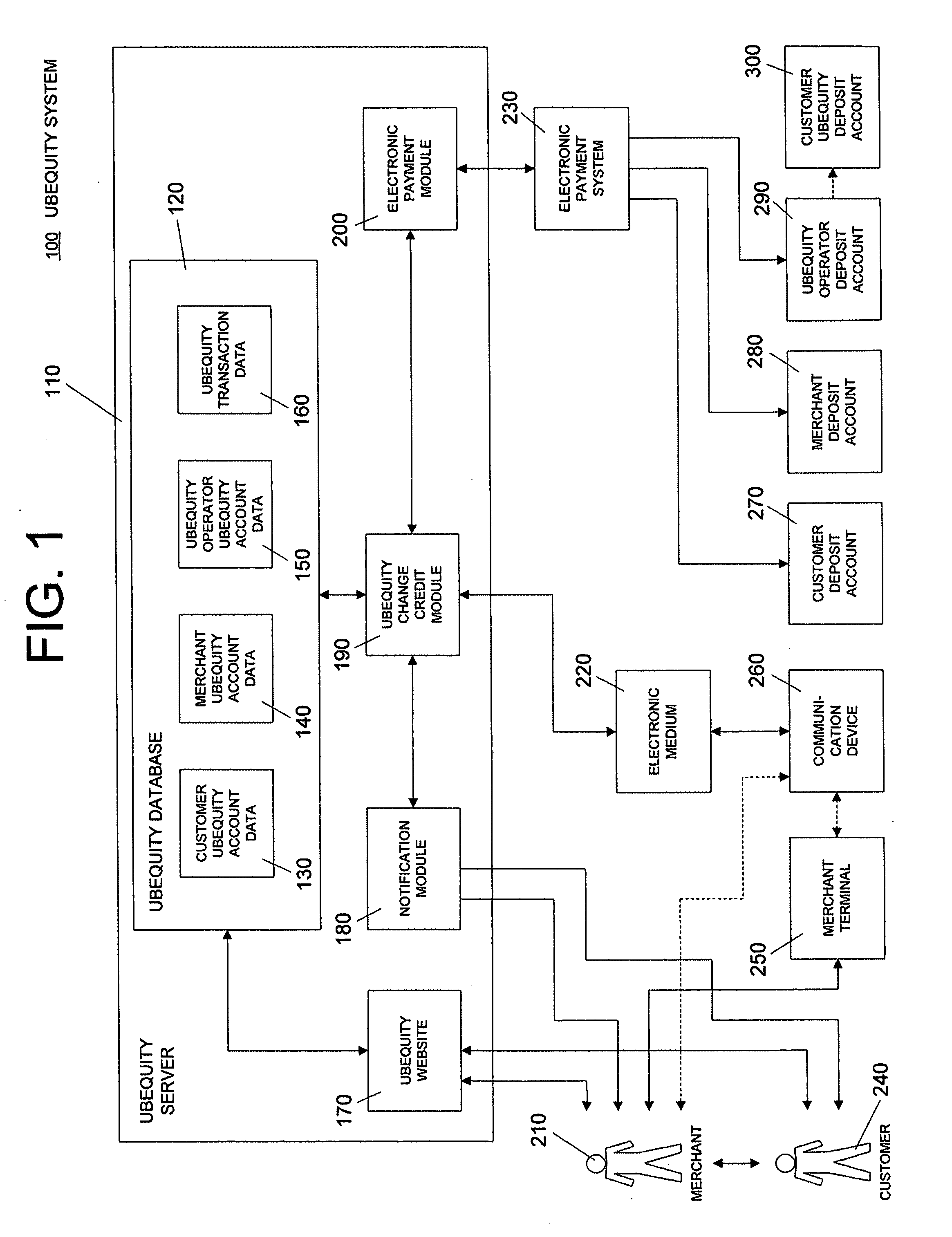
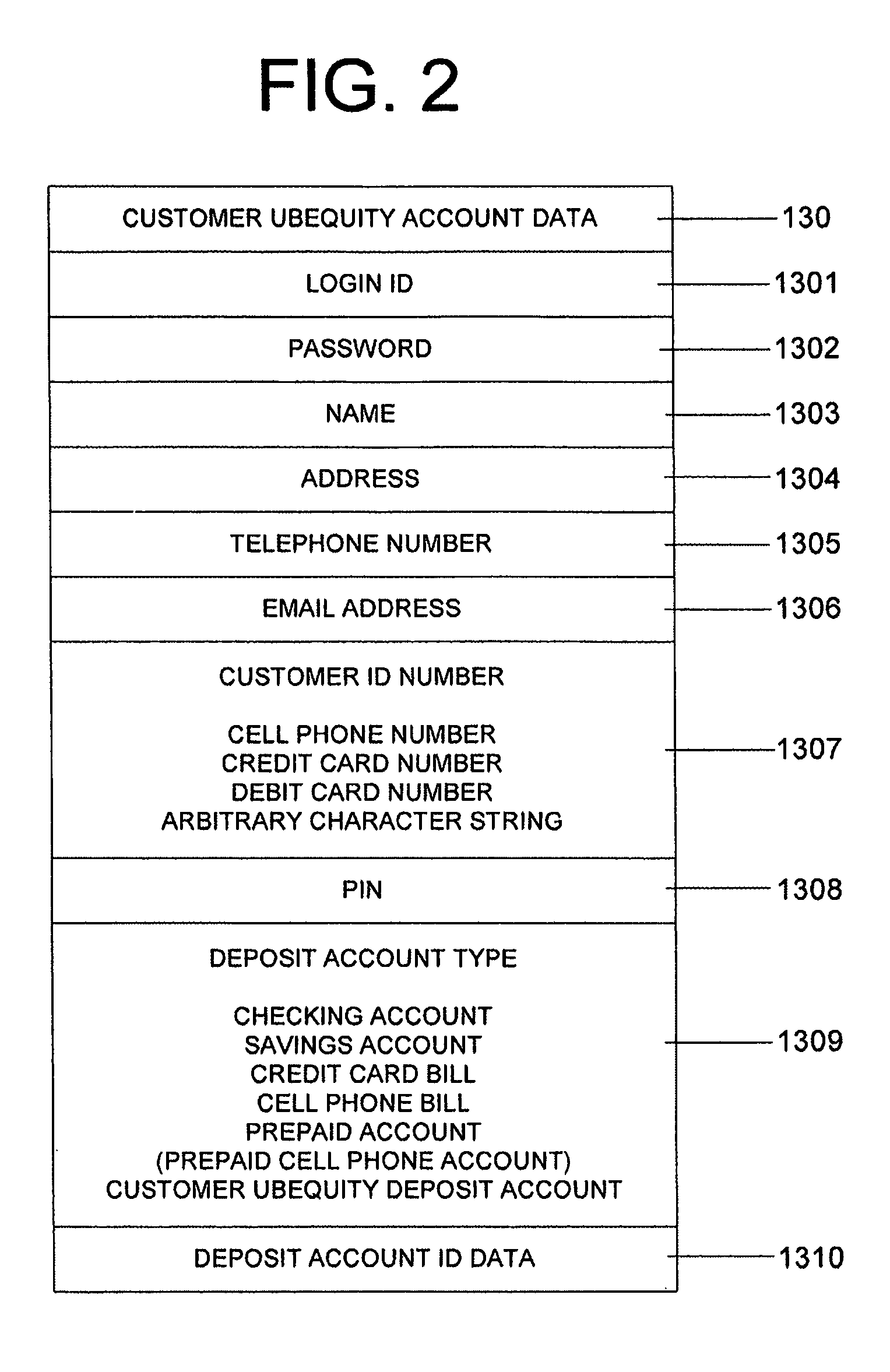
System and method of reducing or eliminating change in cash transaction by crediting at least part of change to buyer's account over electronic medium
InactiveUS20070156579A1Efficient reductionEliminate effectiveFinancePayment architectureDatabaseCommunication device
A method of reducing or eliminating change in a cash transaction includes receiving a cash payment for an article or a service from a buyer; and crediting at least part of change from the cash payment due the buyer to an account of the buyer over an electronic medium. A system of reducing or eliminating change in a cash transaction includes a merchant terminal that processes a cash payment received from a buyer for an article or a service; a communication device that sends a message over an electronic medium specifying that at least part of change from the cash payment is to be credited to an account of the buyer; and a change credit apparatus that credits the at least part of the change to the buyer's account.
Owner:UBEQUITY
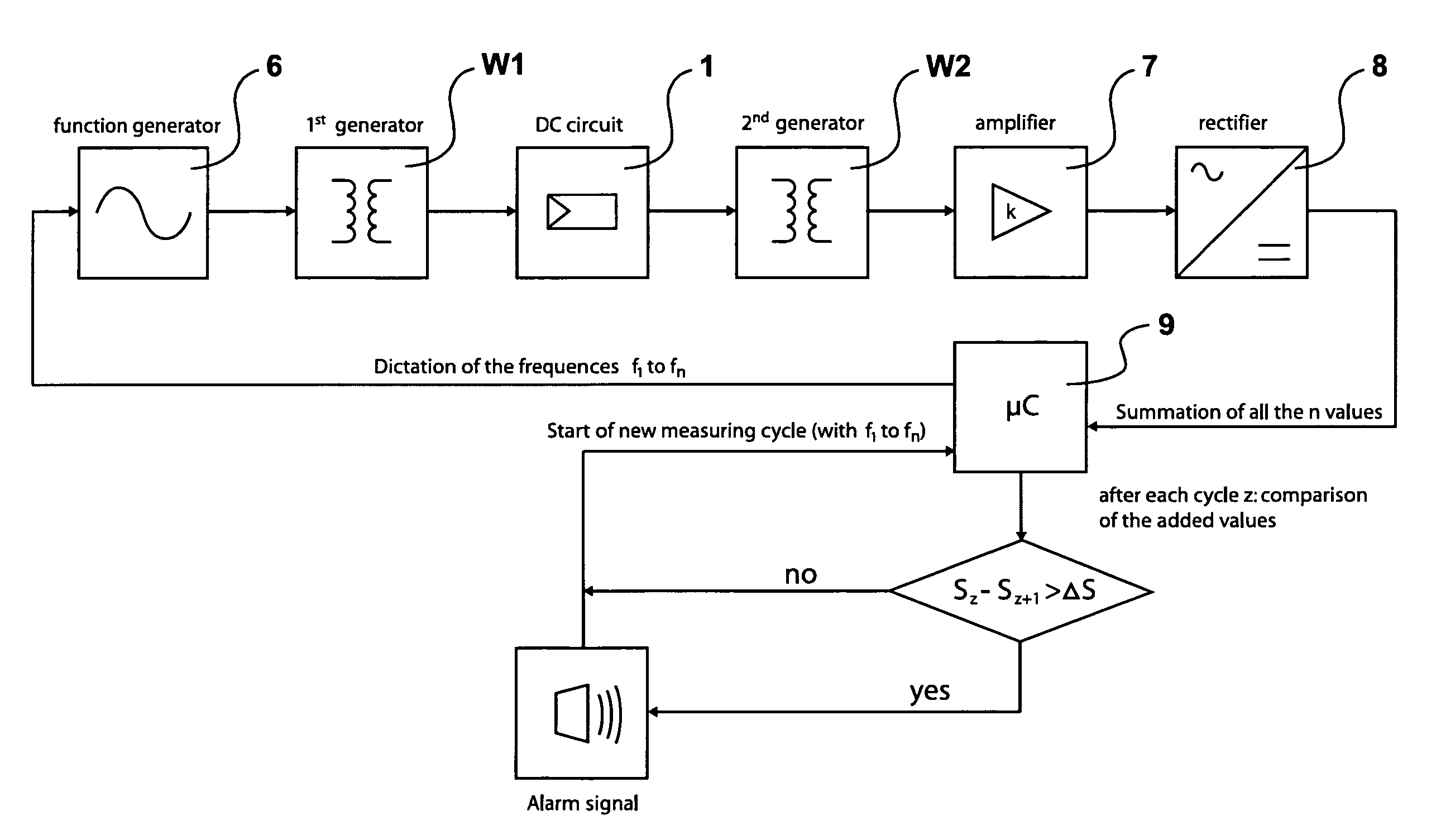
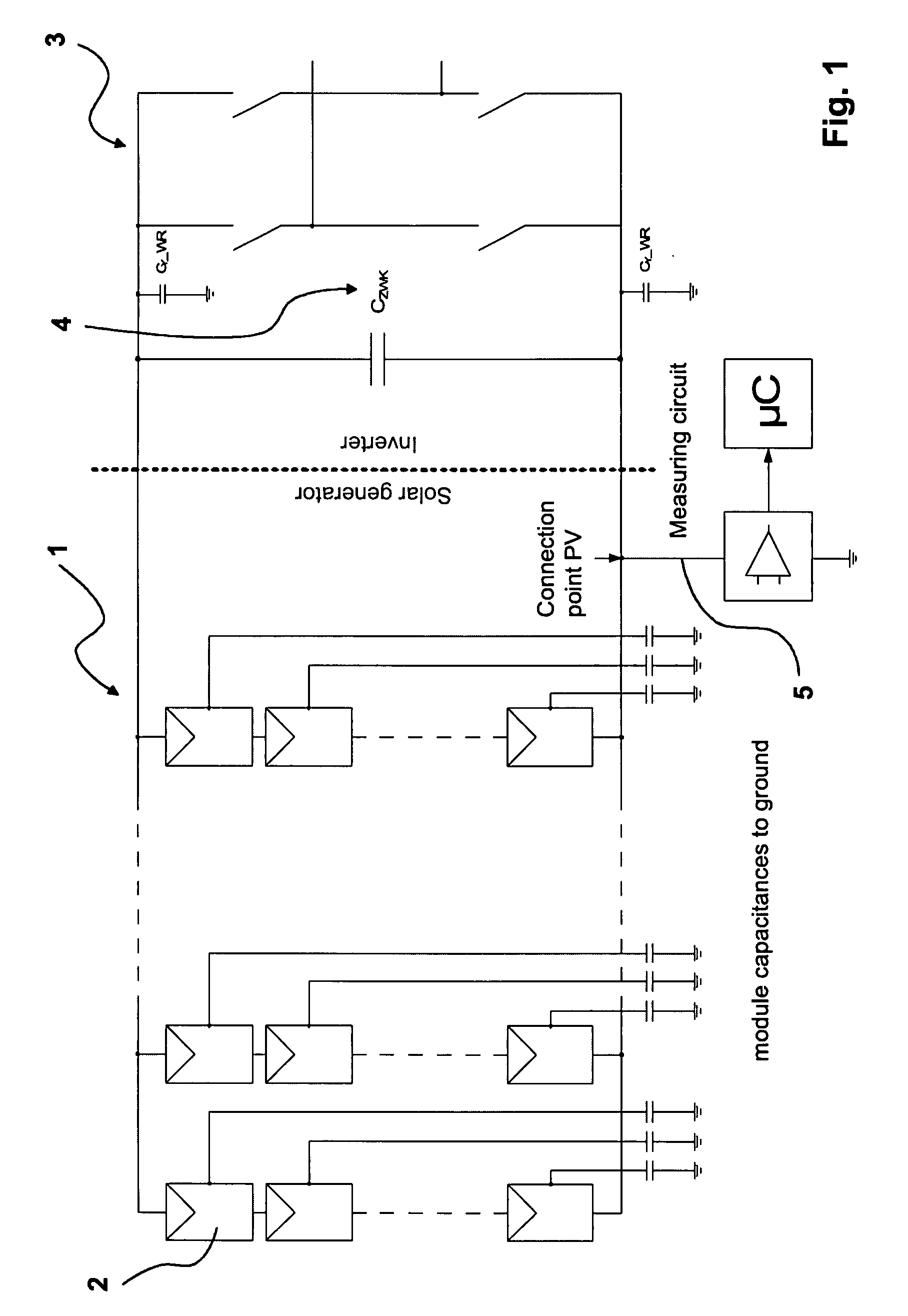
Method of monitoring a photovoltaic generator
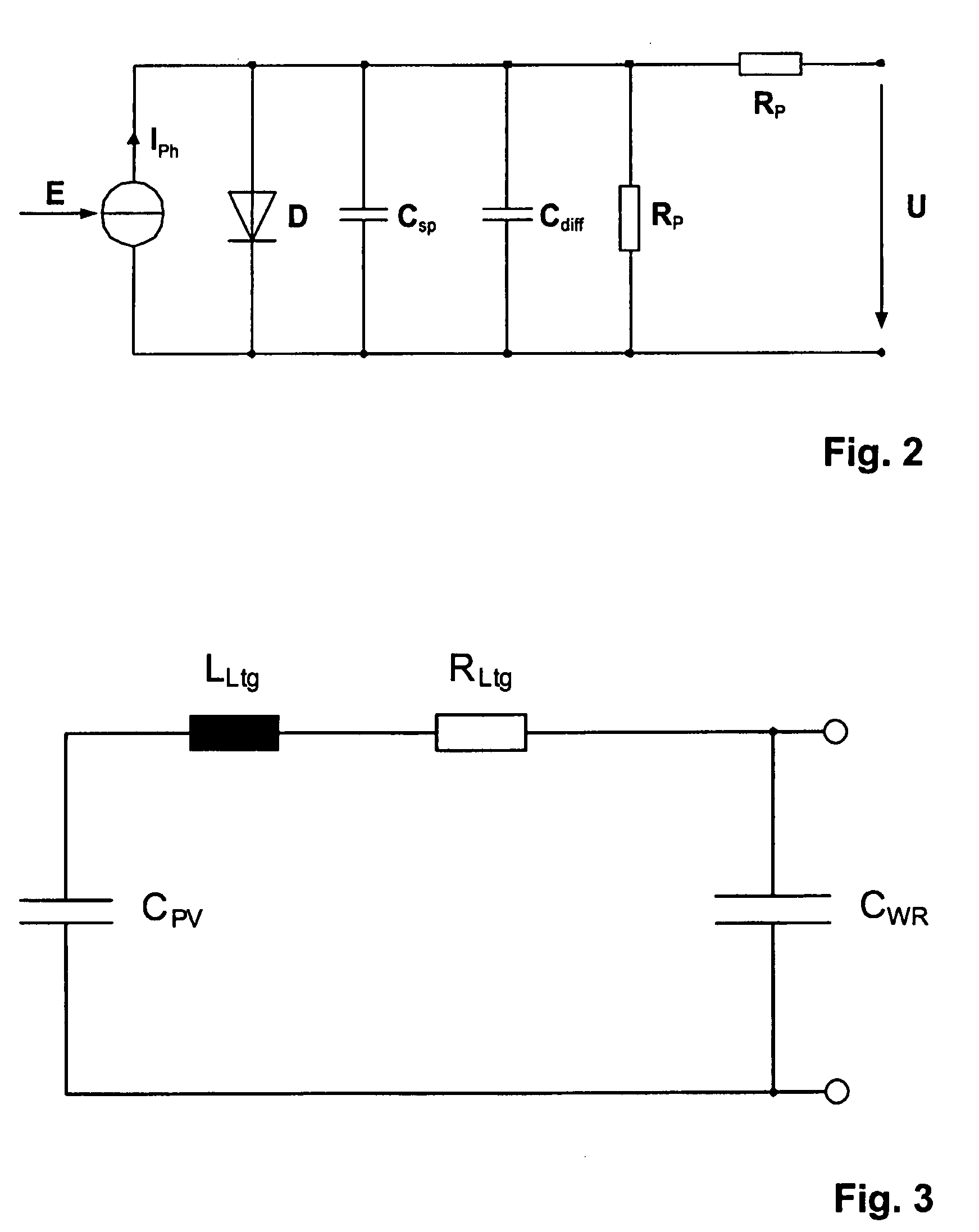
ActiveUS7812592B2Cost advantageTheft of goodPhotovoltaic monitoringPV power plantsFrequency spectrumElectrical battery
The subject matter of the present invention is a method for monitoring a photovoltaic generator (1) for generating current with a number of solar cells connected between two external connections by repeated feeding of a current with a frequency spectrum into the generator current circuit, detecting thereby a respective frequency response in the frequency spectrum with the supplied current as the input variable and an electric variable of the generator as the output variable, and detecting a change in the frequency response for monitoring the photovoltaic generator (1) in the event of a change during repeated feeding.
Owner:SMA SOLAR TECH AG
Method and system for power management including local bounding of device group power consumption
ActiveUS7155623B2Inhibit currentSlow changeEnergy efficient ICTVolume/mass flow measurementMemory controllerGlobal system
A method and system for power management including local bounding of device group power consumption provides the responsiveness of local power control while meeting global system power consumption and power dissipation limits. At the system level, a global power bound is determined and divided among groups of devices in the system so that local bounds are determined that meet the global system bound. The local bounds are communicated to device controllers associated with each group of devices and the device controllers control the power management states of the associated devices in the group to meet the local bound. Thus, by action of all of the device controllers, the global bound is met. The controllers may be memory controllers and the devices memory modules, or the devices may be other devices within a processing system having associated local controllers. Alternatively or in concert, the devices may be entire processing systems and the associated controller a power management controller for associated processing systems, whereby multiple processing locales may be power-managed consistent with a global power consumption budget.
Owner:HUAWEI TECH CO LTD
Liquid Container, Liquid Consuming Apparatus, Liquid Supply System and Liquid Container Unit
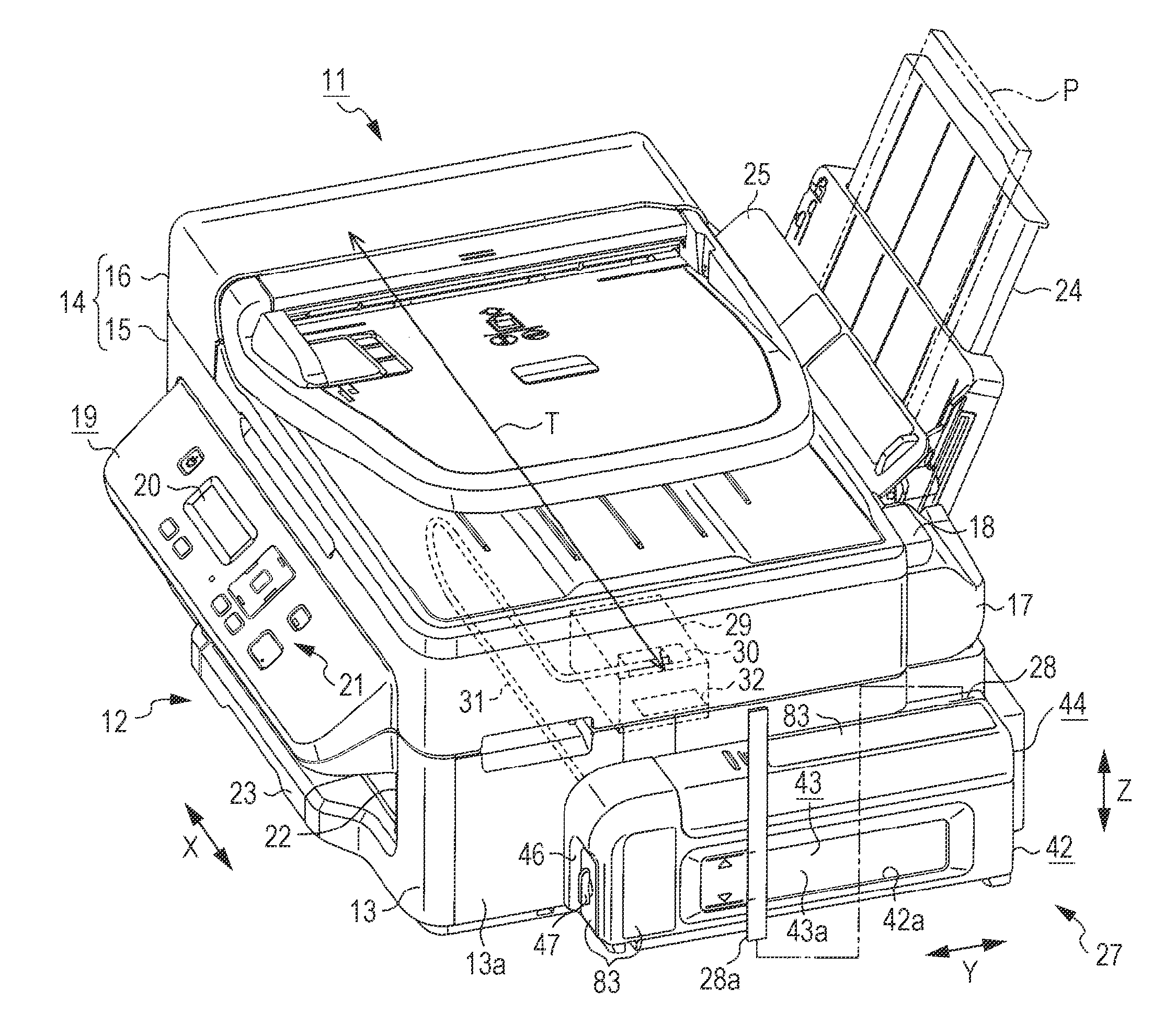
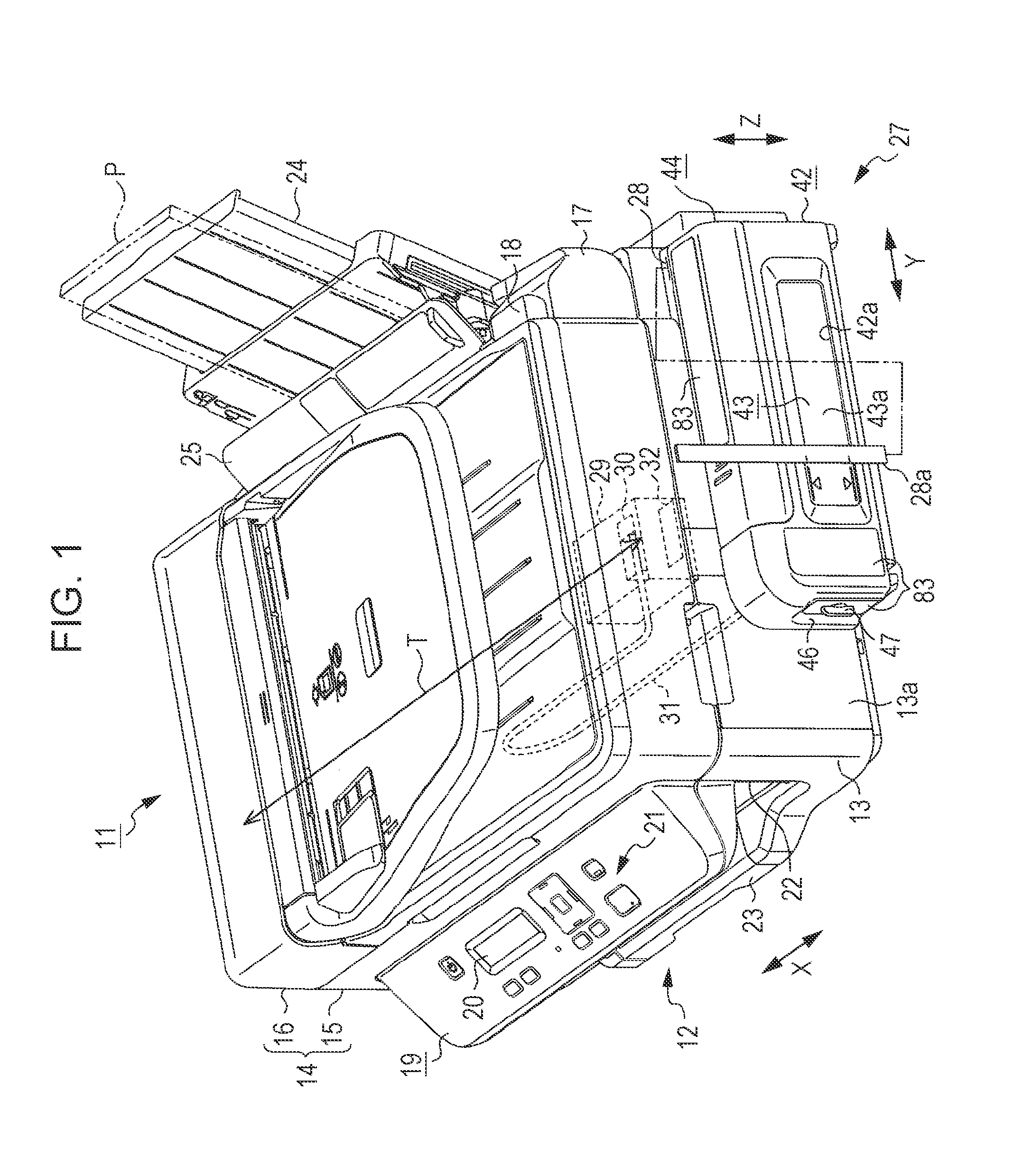
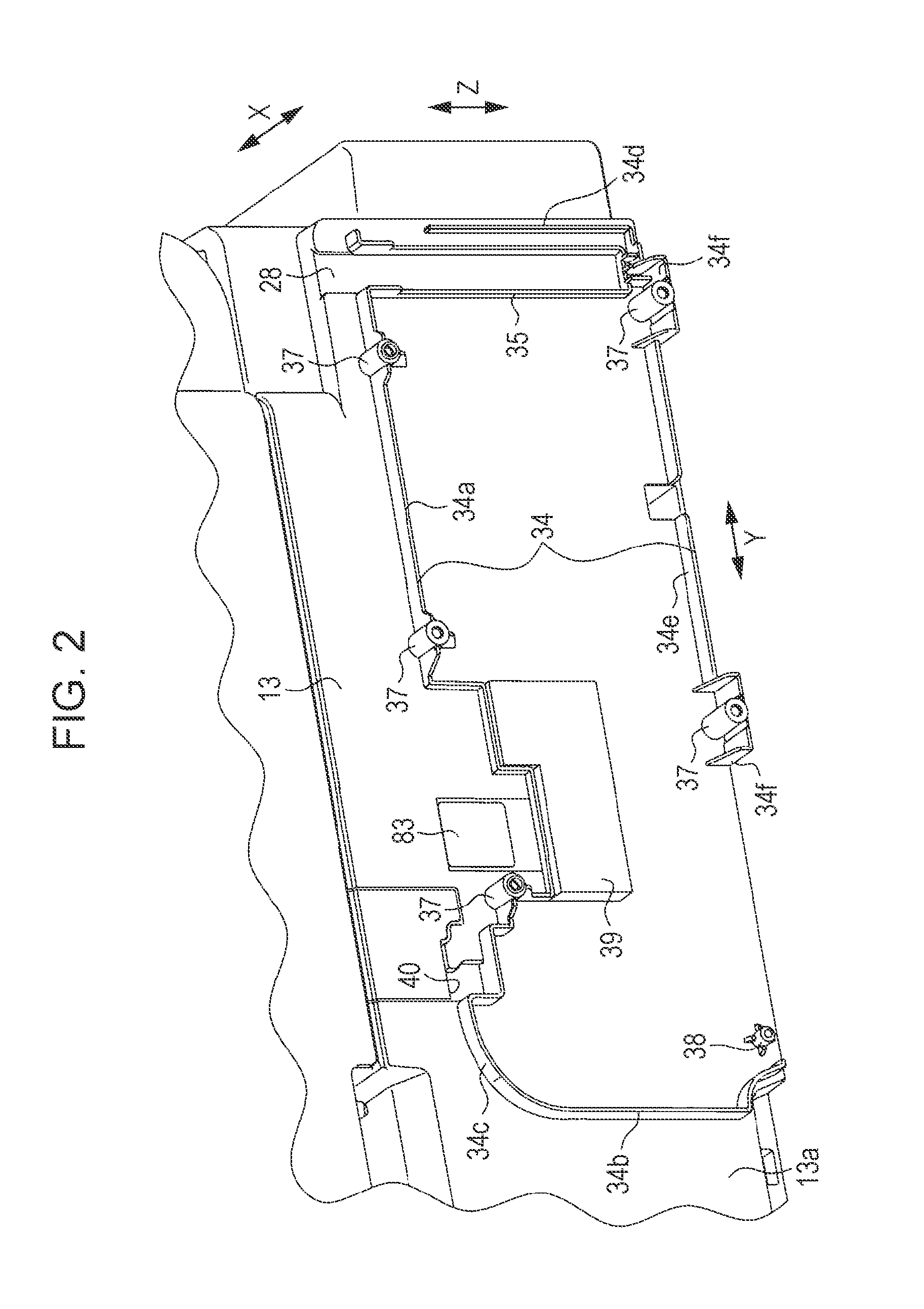
InactiveUS20140043408A1Reduce the possibilityImprove portabilityPrintingOther printing apparatusInjection portEngineering
A liquid container includes an ink chamber containing an ink to be supplied via a tube to a liquid ejecting head consuming the ink; an outlet port from which the ink contained in the ink chamber flows to the tube side; an injection port through which the ink can be injected into the ink chamber; and an air intake port taking air into the ink chamber from a further vertically upper position than a liquid level of the ink when the ink is contained in the ink chamber. If the ink equal to 5% of containing capacity containable in the ink chamber flows from the outlet port, the liquid container has an area where a fluctuation range of the liquid level of the ink inside the ink chamber becomes 5% or less of the cubic root of the containing capacity.
Owner:SEIKO EPSON CORP
Backplanes for display applications, and components for use therein
InactiveUS20070035532A1Slow changeLow cost manufacturingTransistorSolid-state devicesCapacitanceCoupling
Owner:E INK CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com