Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2535results about How to "Stay flexible" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
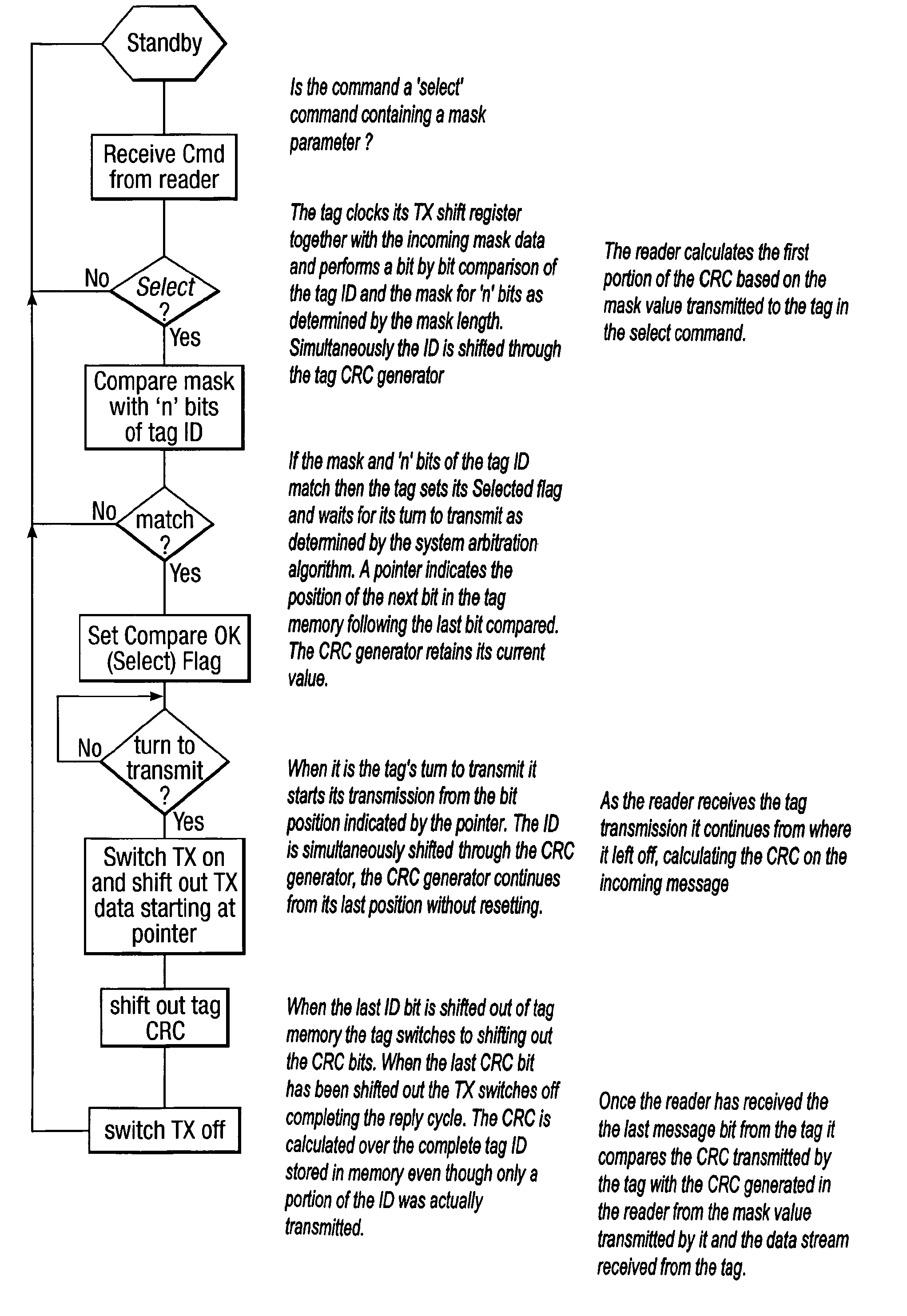
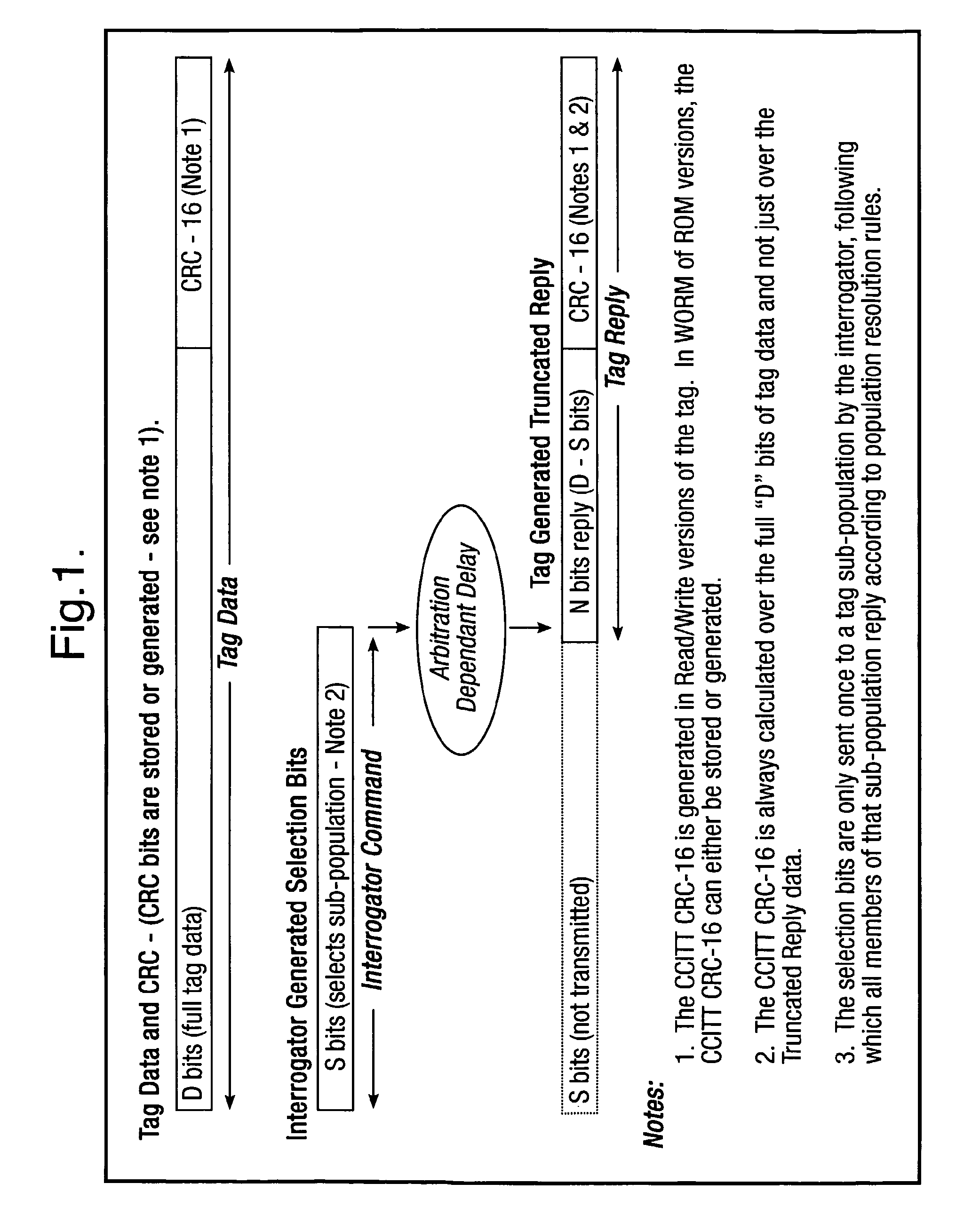
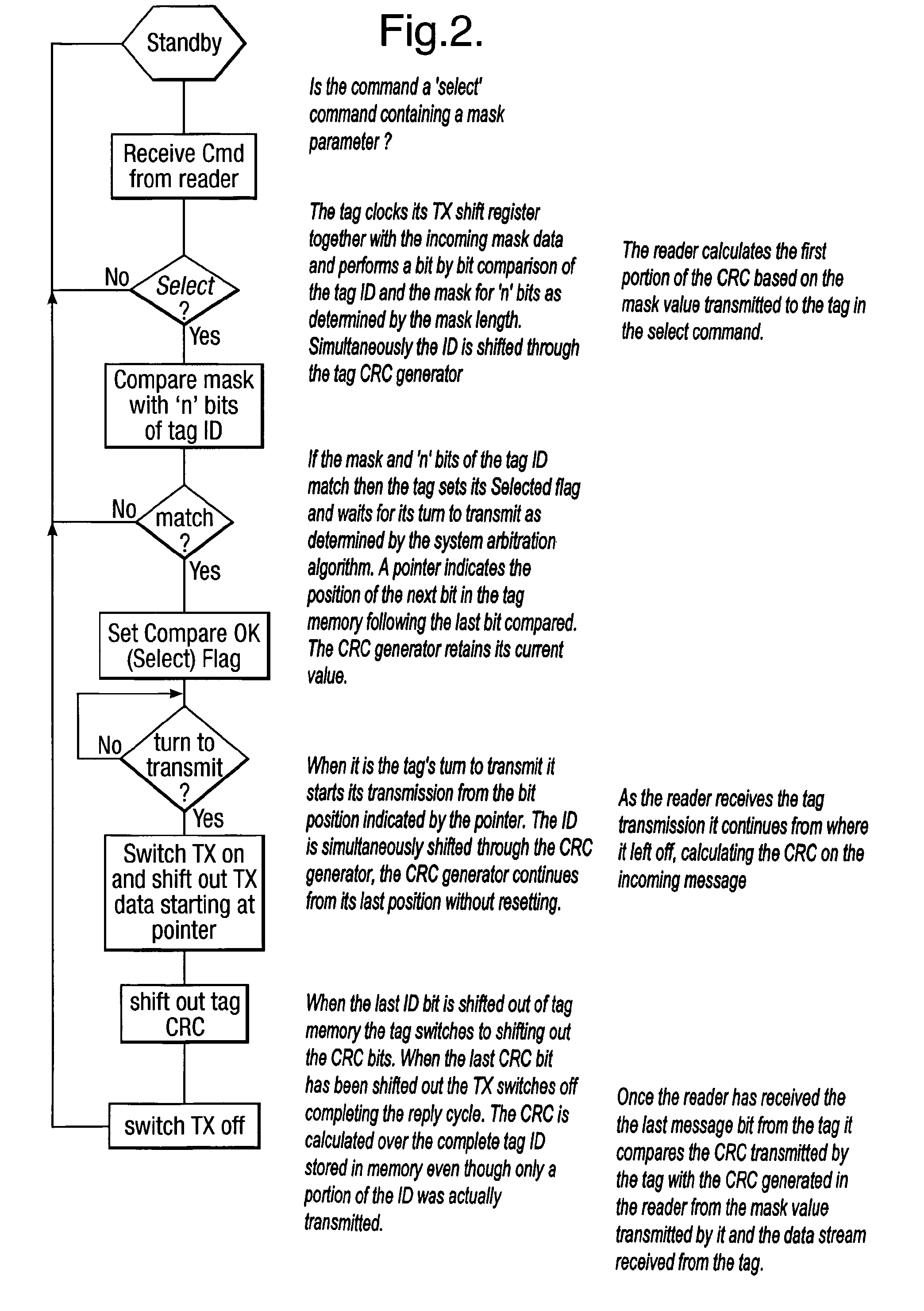
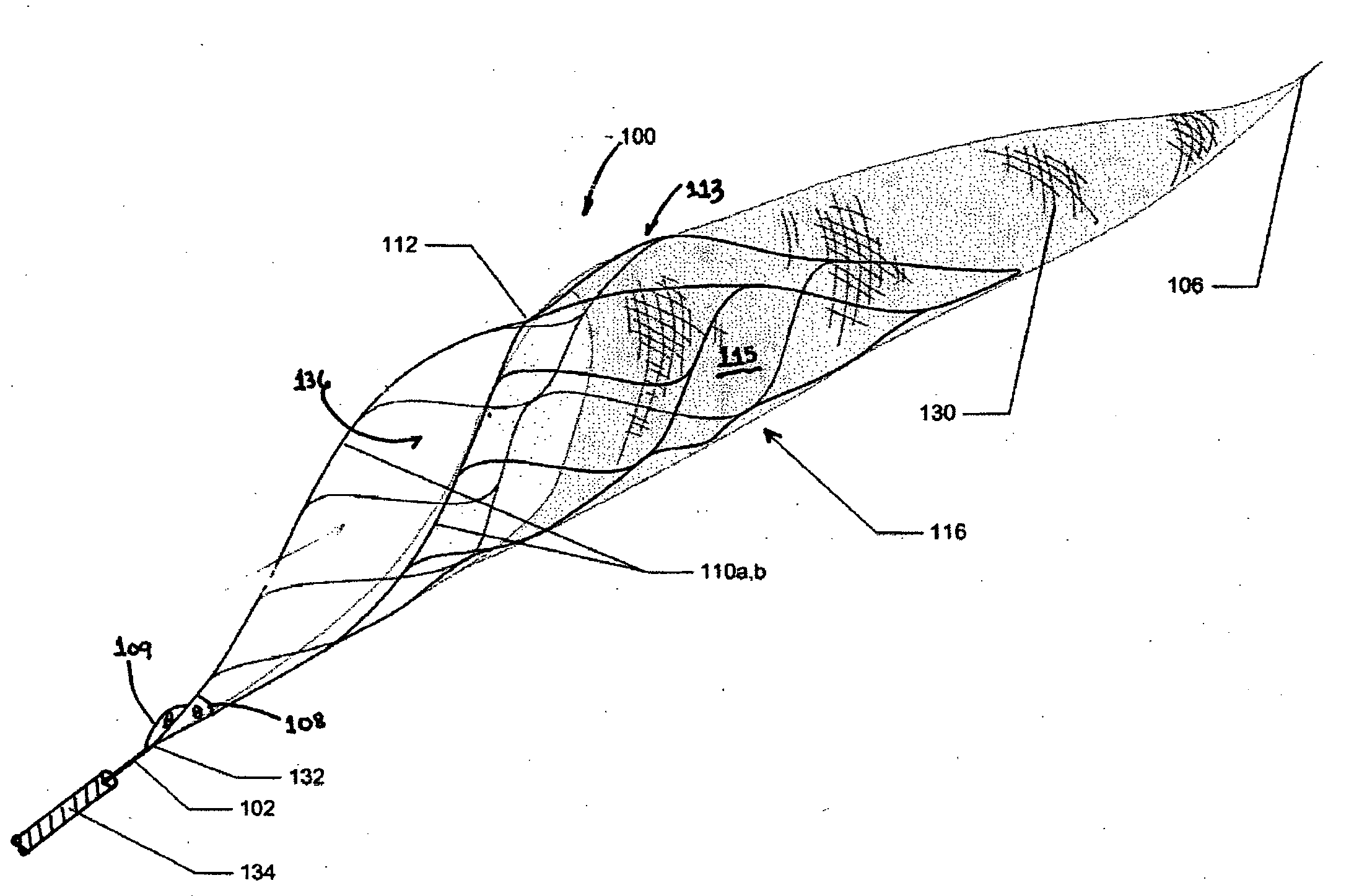
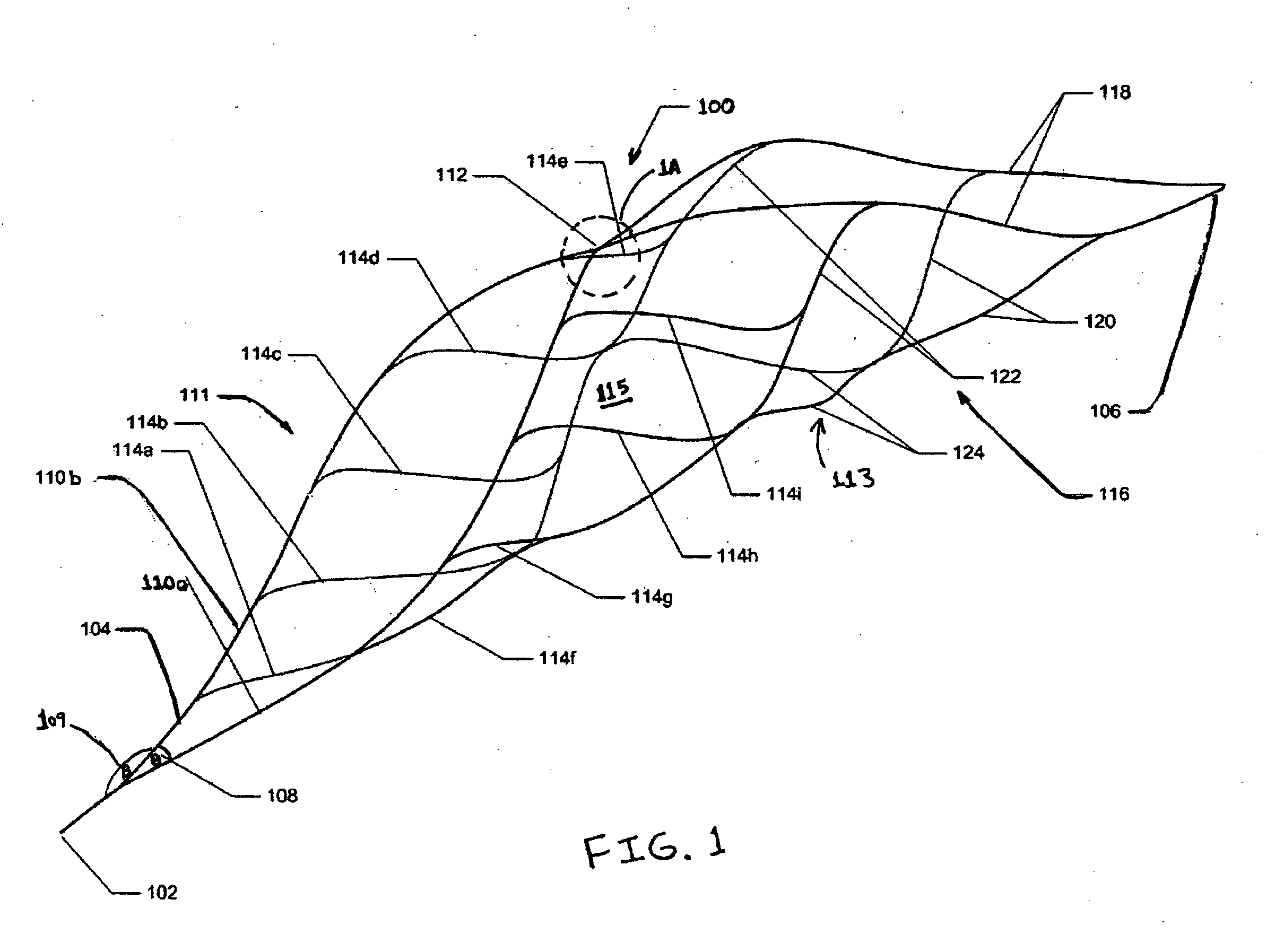
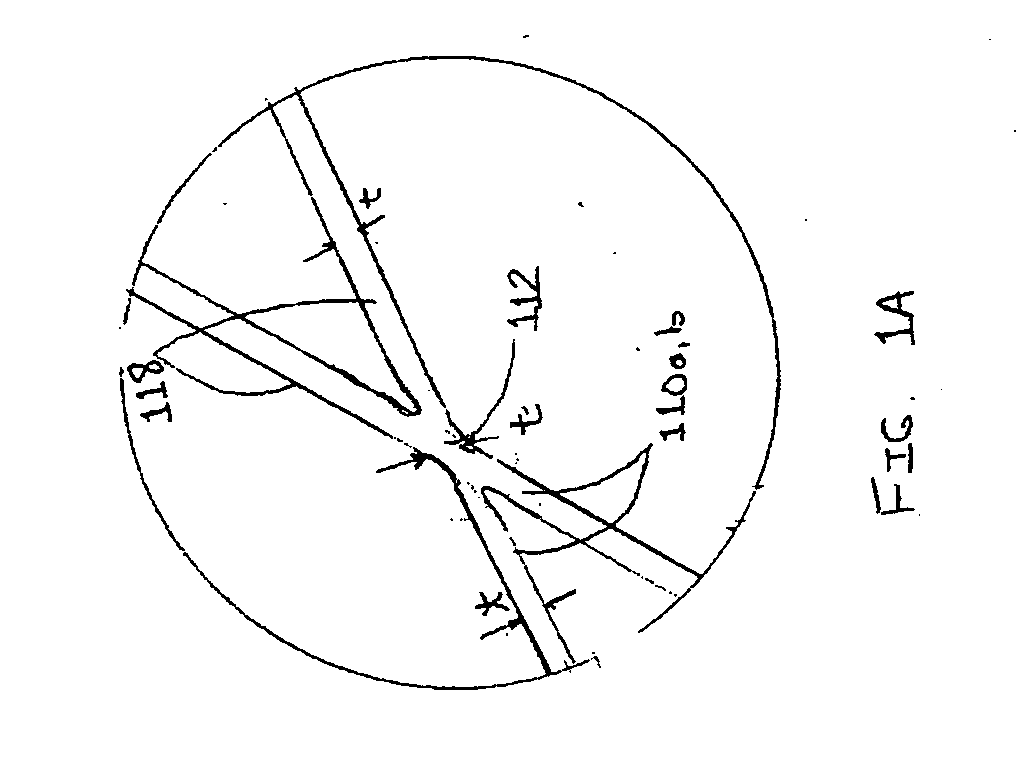
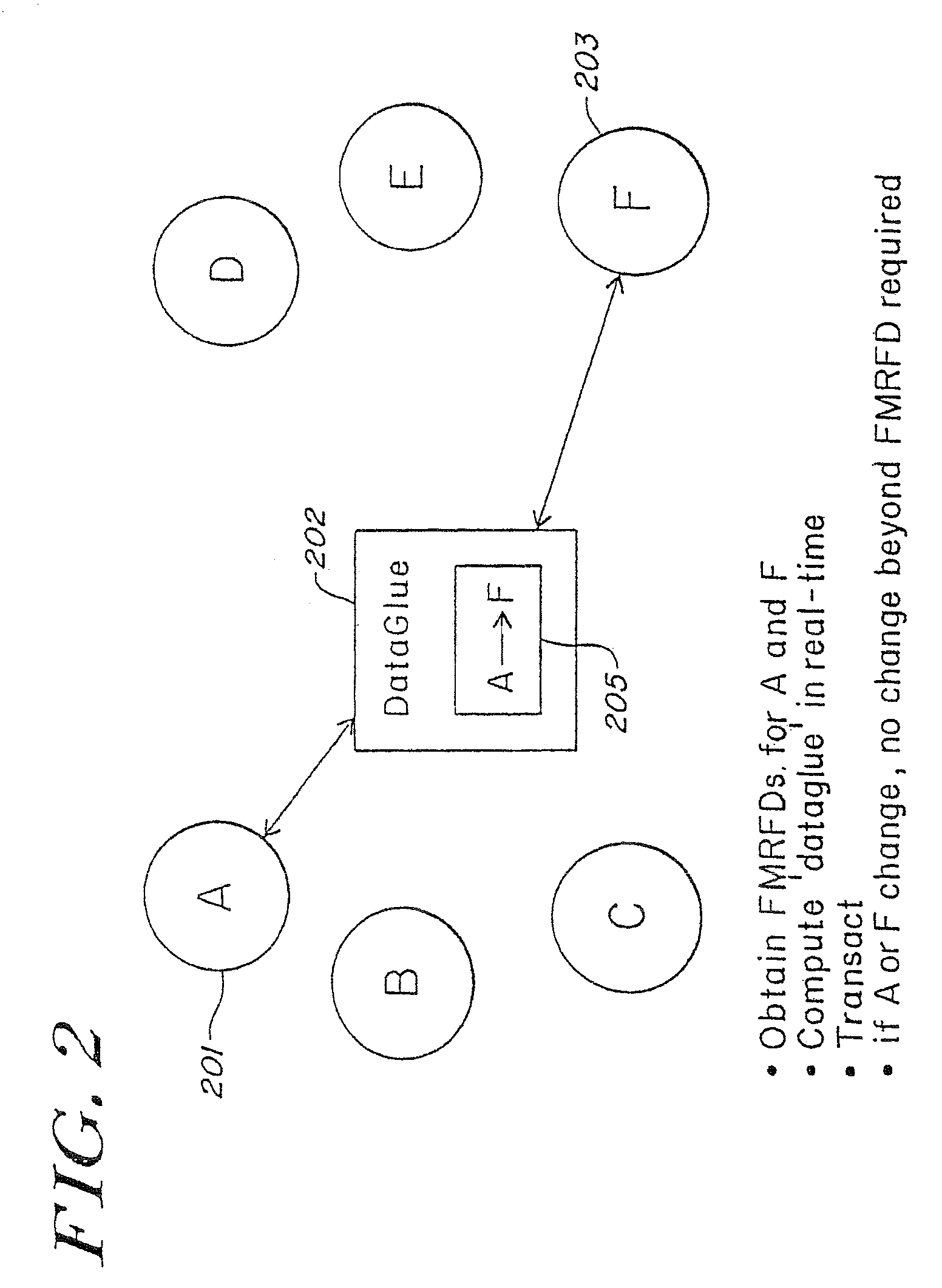
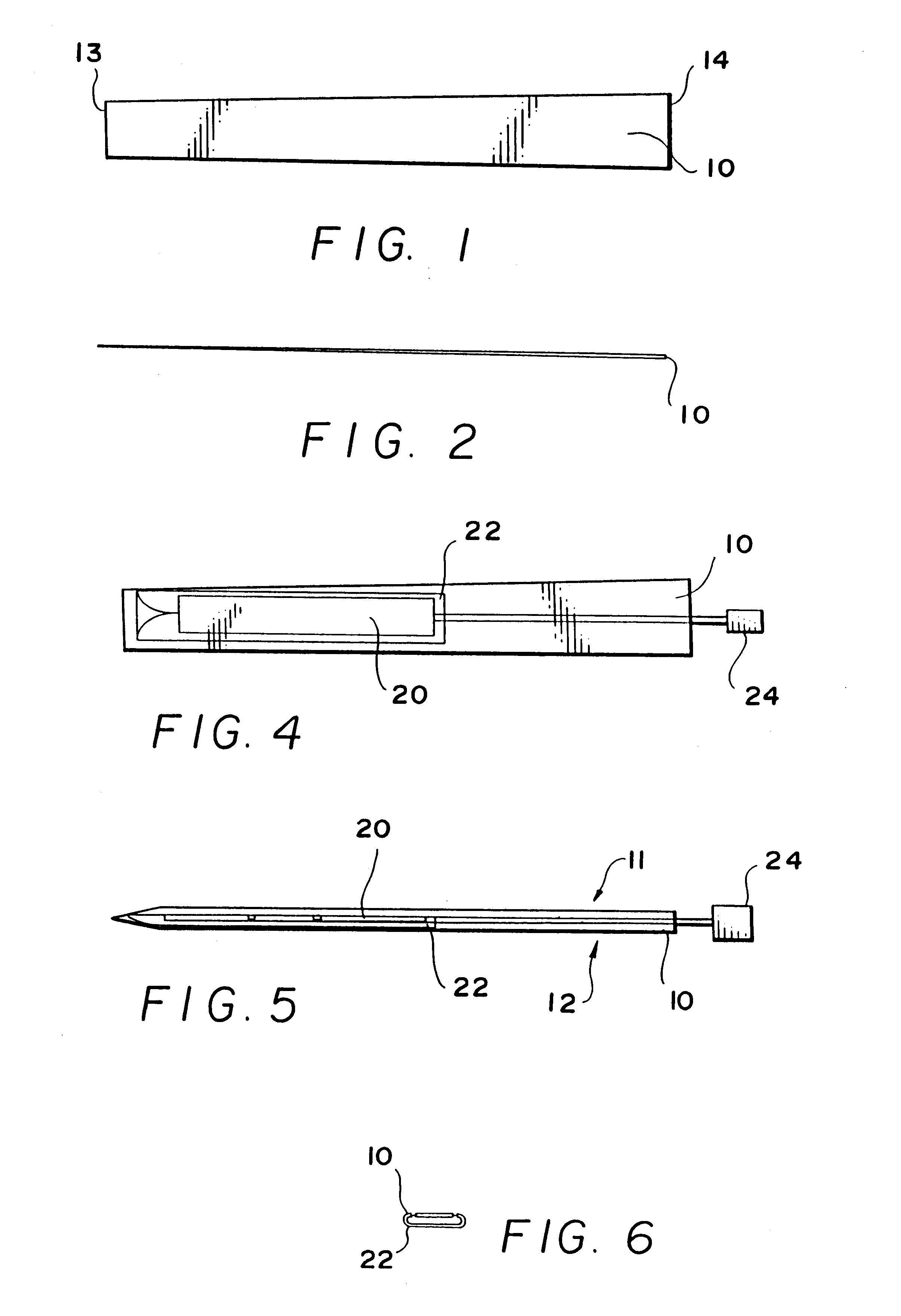
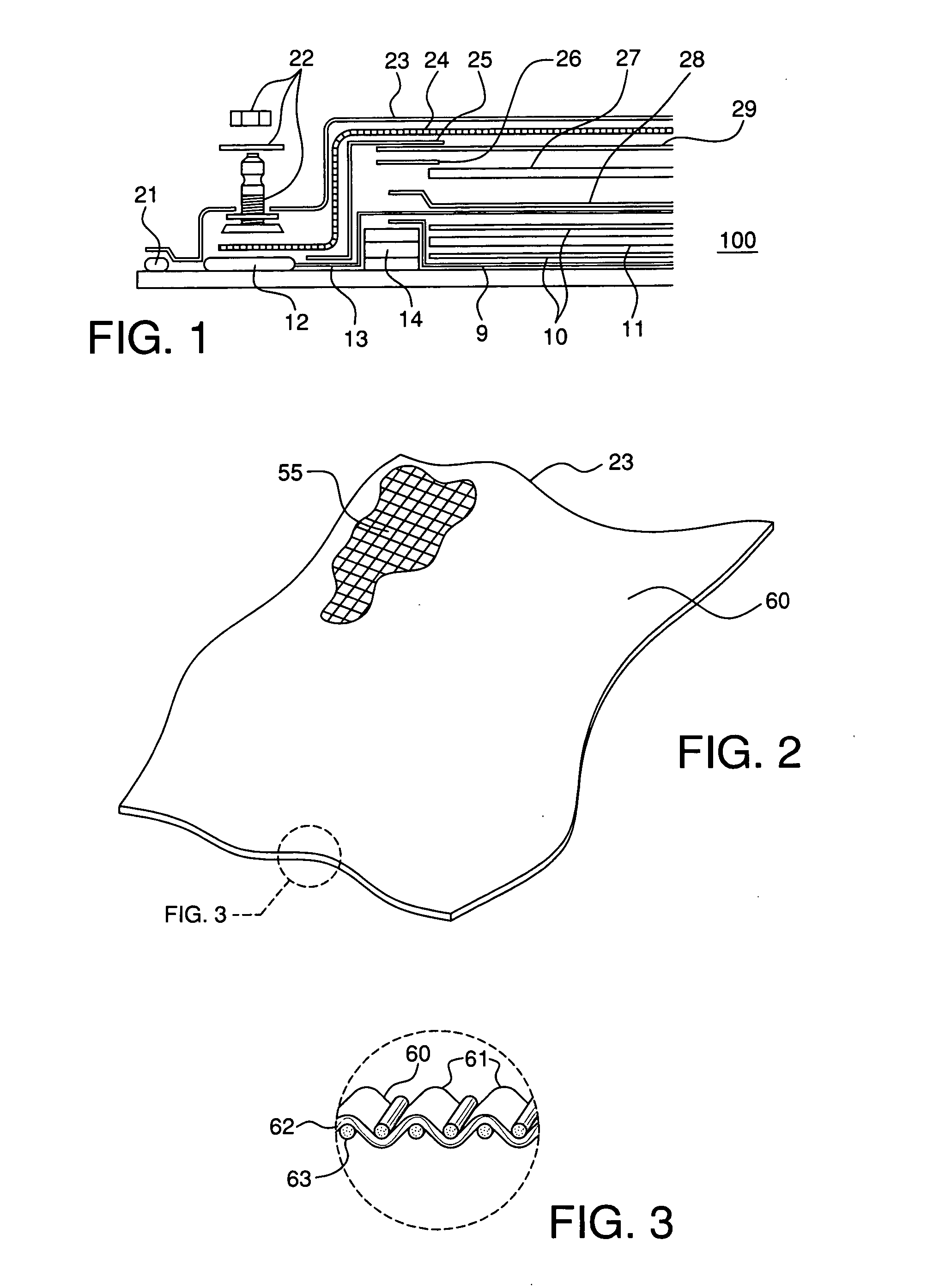
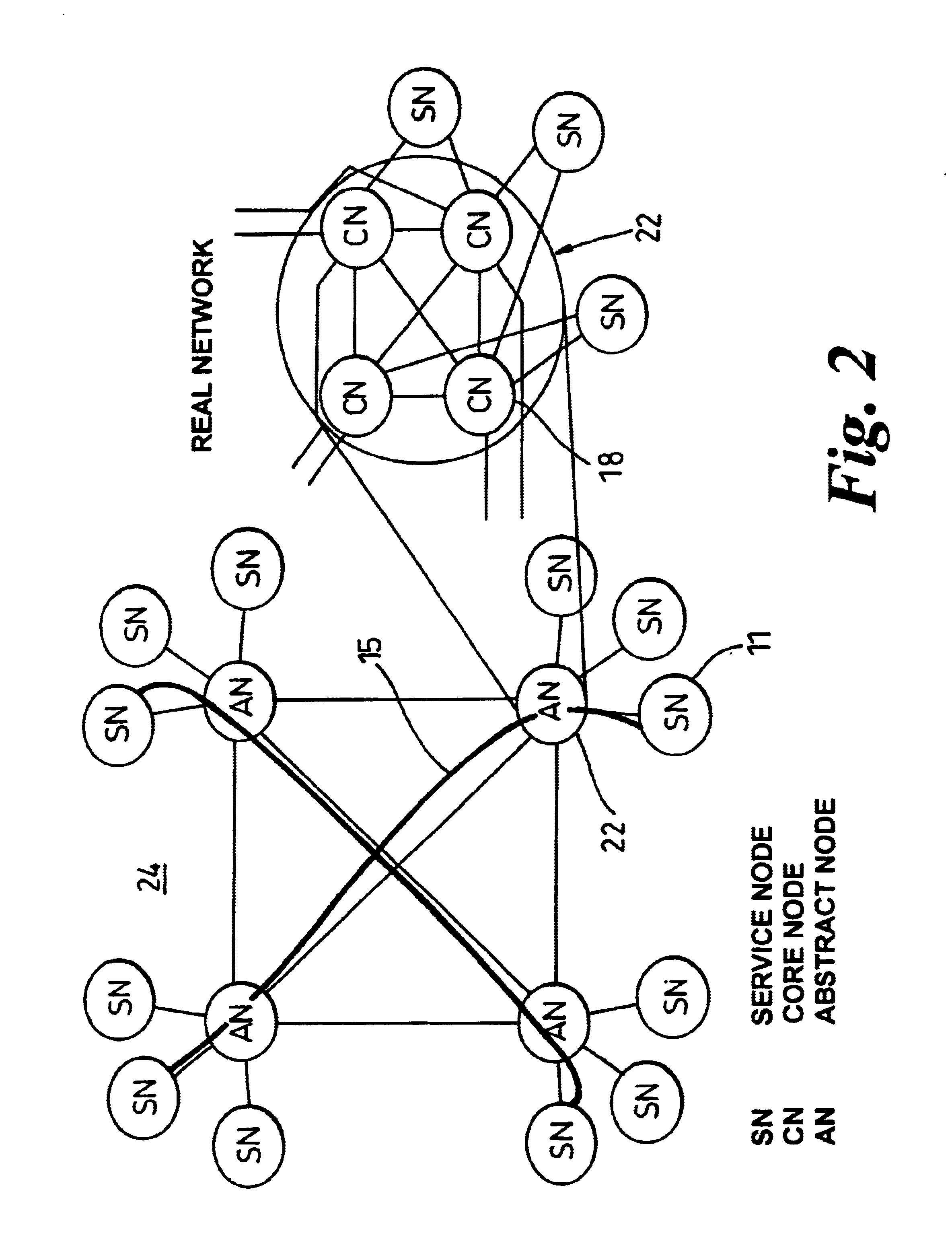
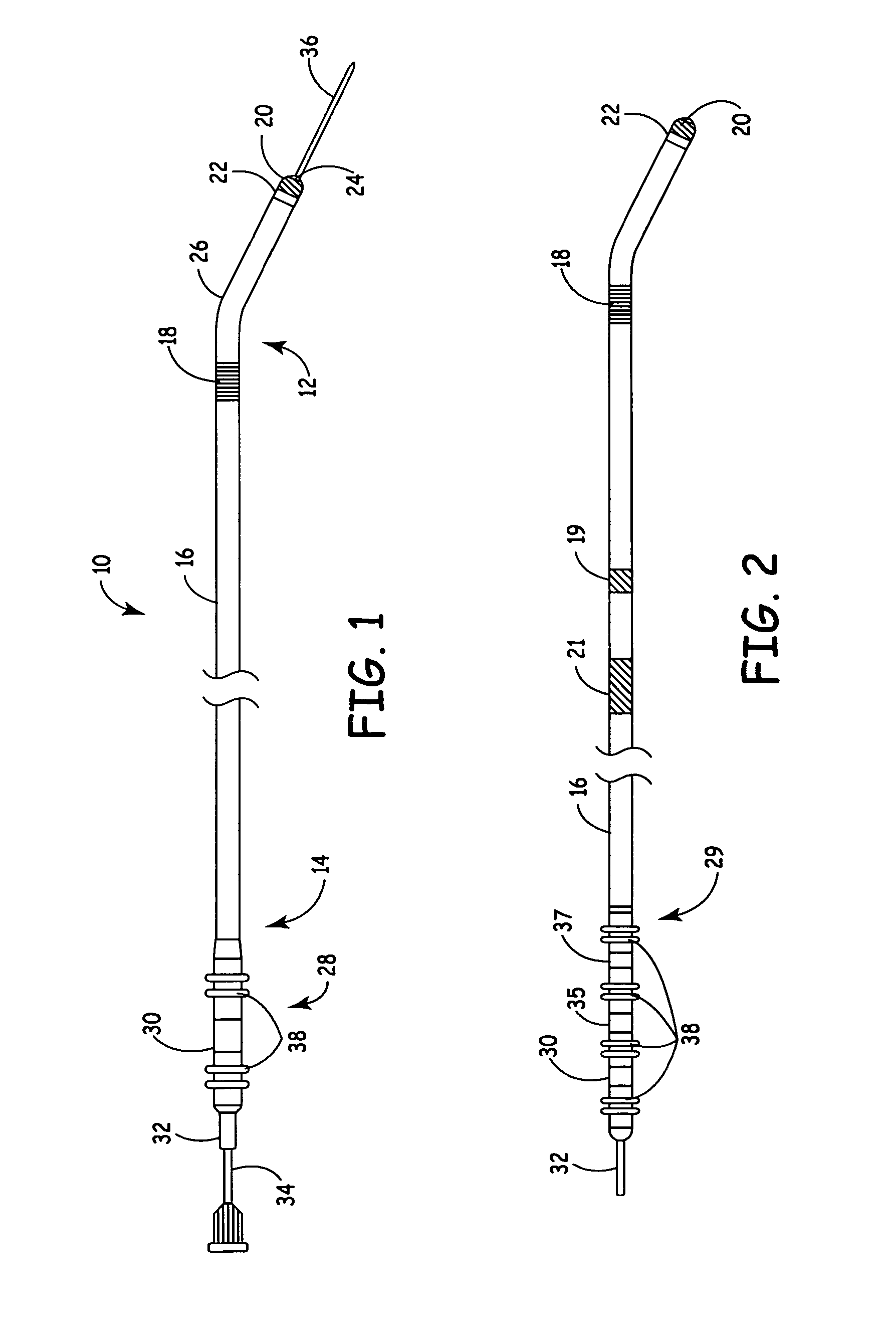
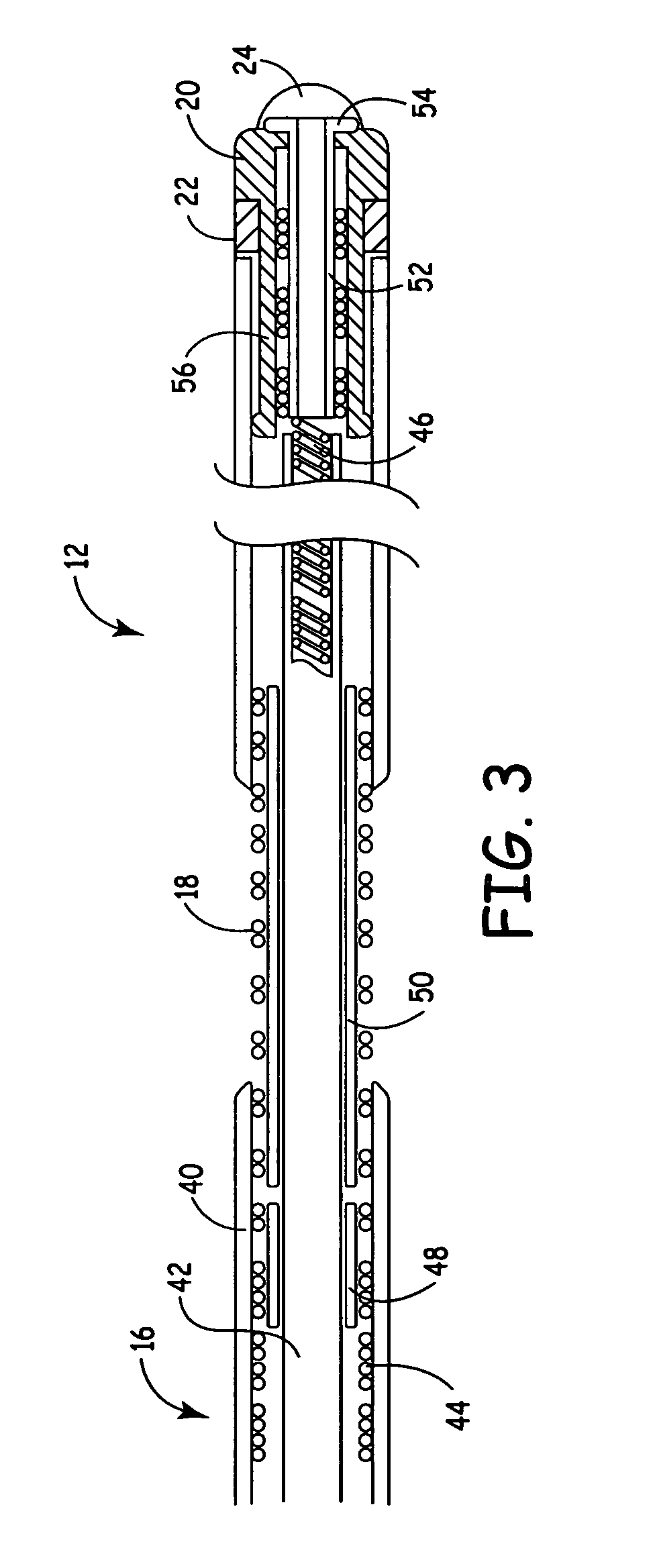
Method and system for calculating and verifying the integrity of data in a data transmission system
ActiveUS7987405B2Not having capability to accuratelyStay flexibleError preventionMemory record carrier reading problemsComputer hardwareData integrity
A method is described of calculating and verifying the integrity of data in a data communication system. The system comprises a base station and one or more remote stations, such as in an RFID system. The method includes transmitting a select instruction from the base station to the one or more remote stations, the select instruction containing a data field which matches a portion of an identity or other data field in one or more of the remote stations; transmitting from a selected remote station or stations a truncated reply containing identity data or other data of the remote station but omitting the portion transmitted by the base station; calculating in the base station a check sum or CRC from the data field originally sent and the truncated reply data received and comparing the calculated check sum or CRC with the check sum or CRC sent by the remote station.
Owner:ZEBRA TECH CORP
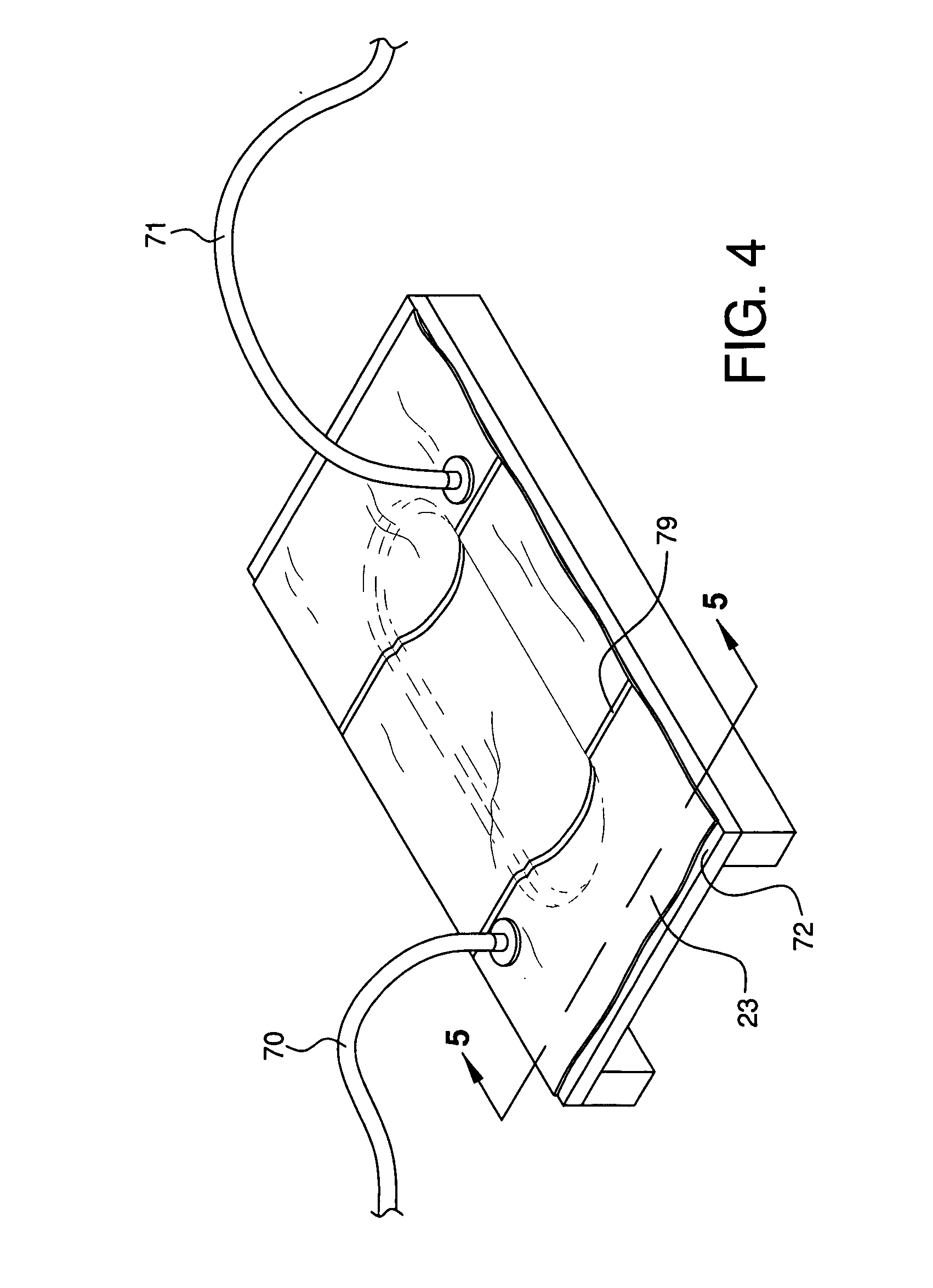
Modified delivery device for coated medical devices
InactiveUS7527632B2Minimize potential risk of damageReduce frictionStentsEar treatmentBiological bodyMedical device
Medical devices, and in particular implantable medical devices, including self-expanding stents, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
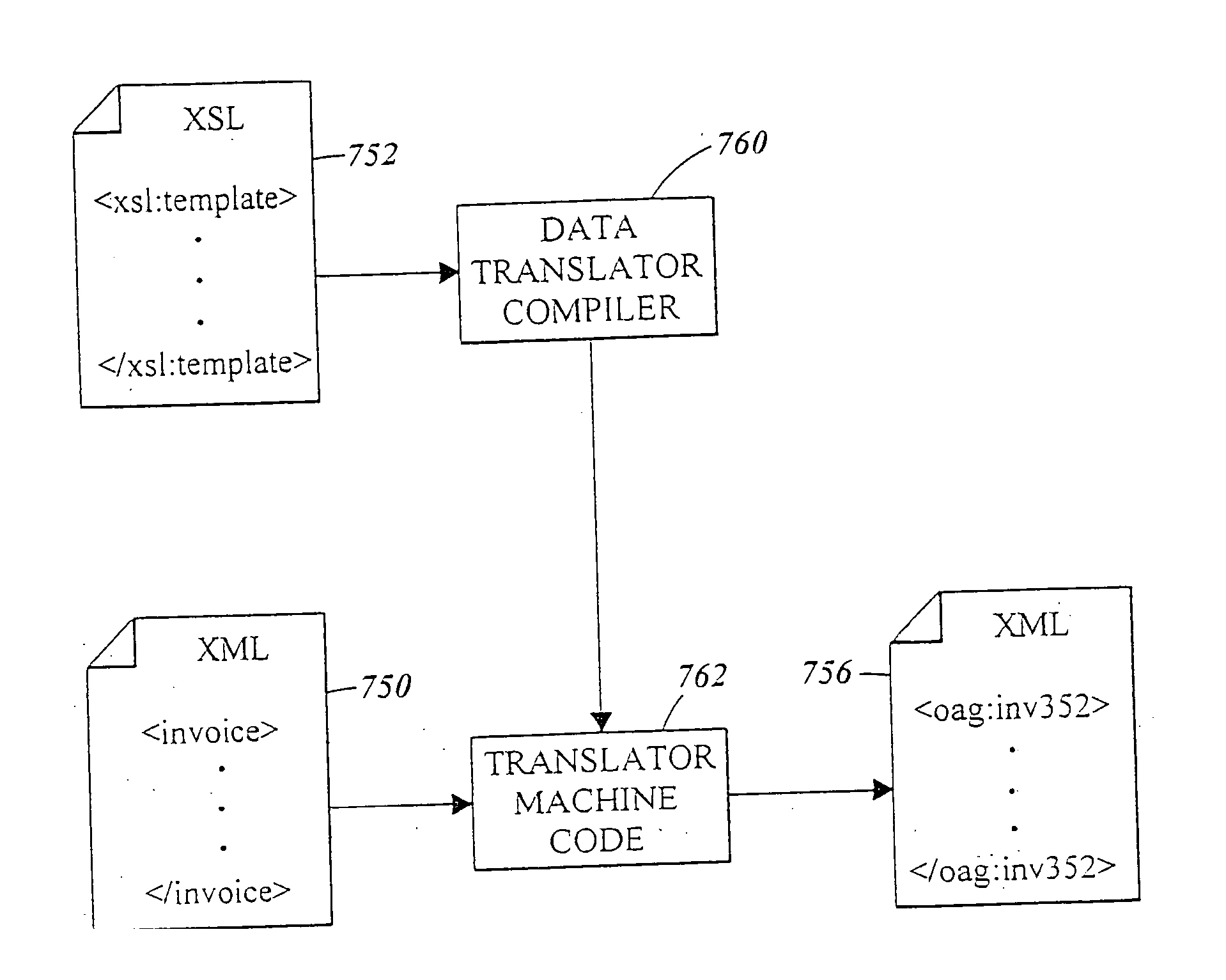
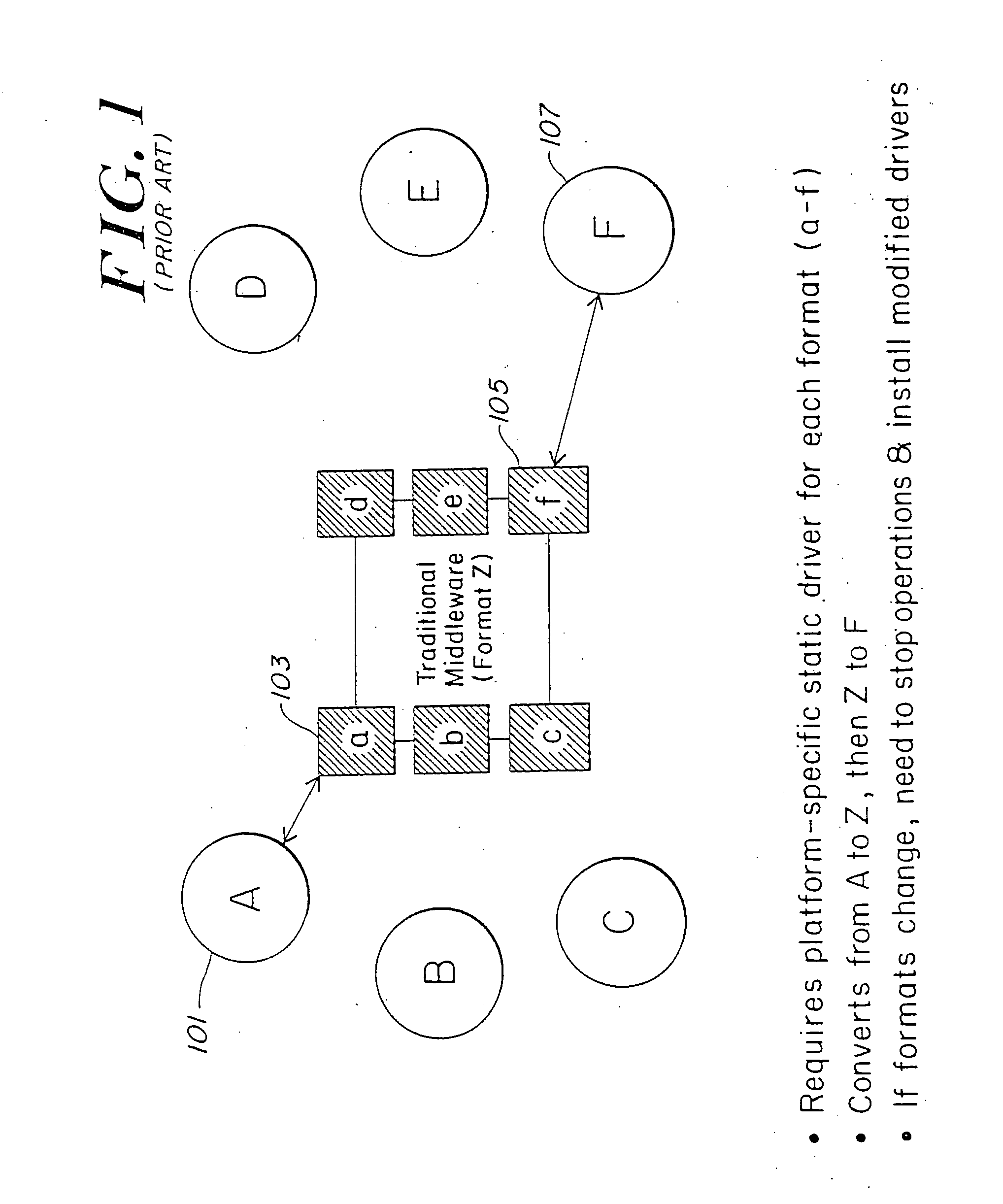
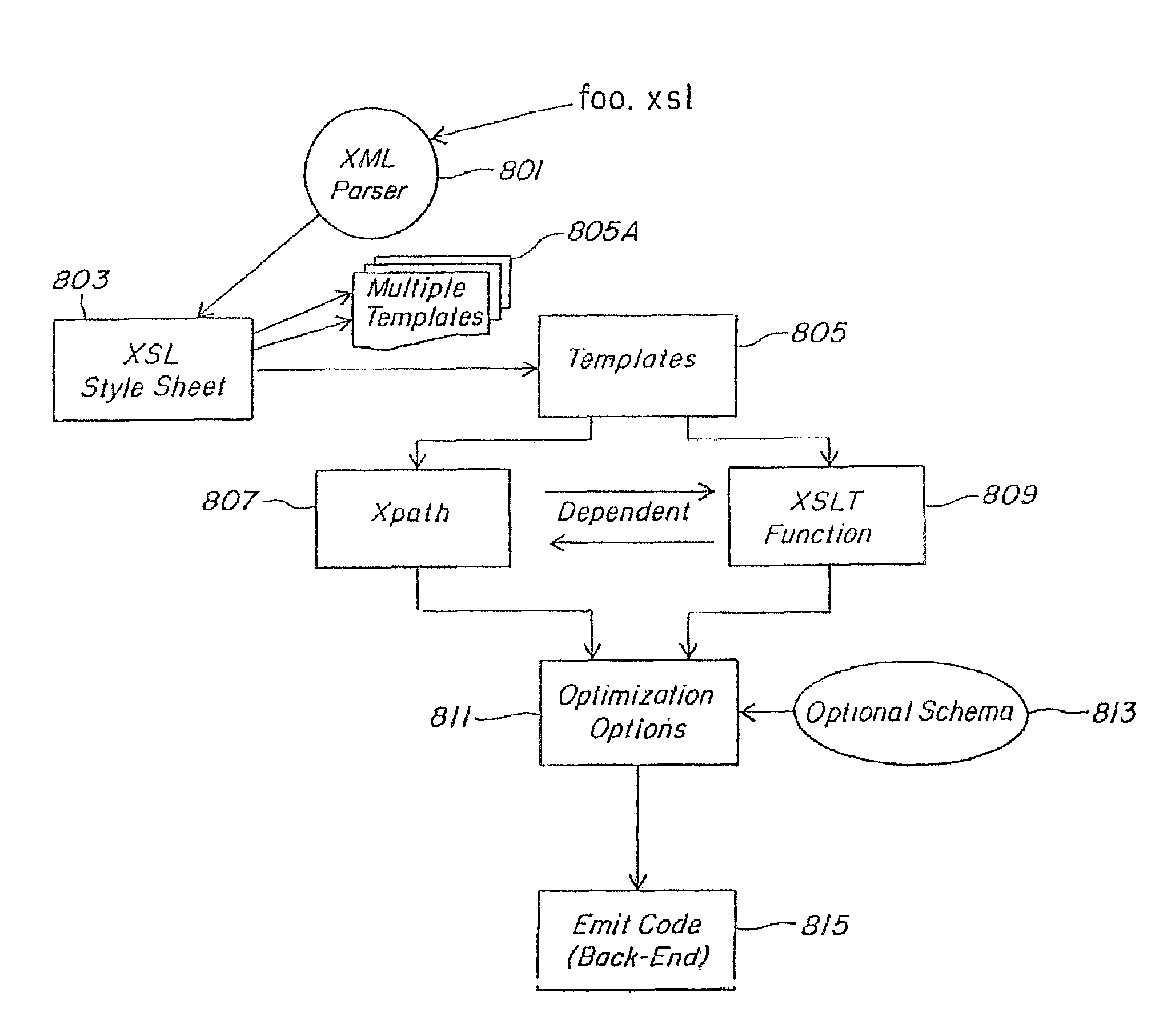
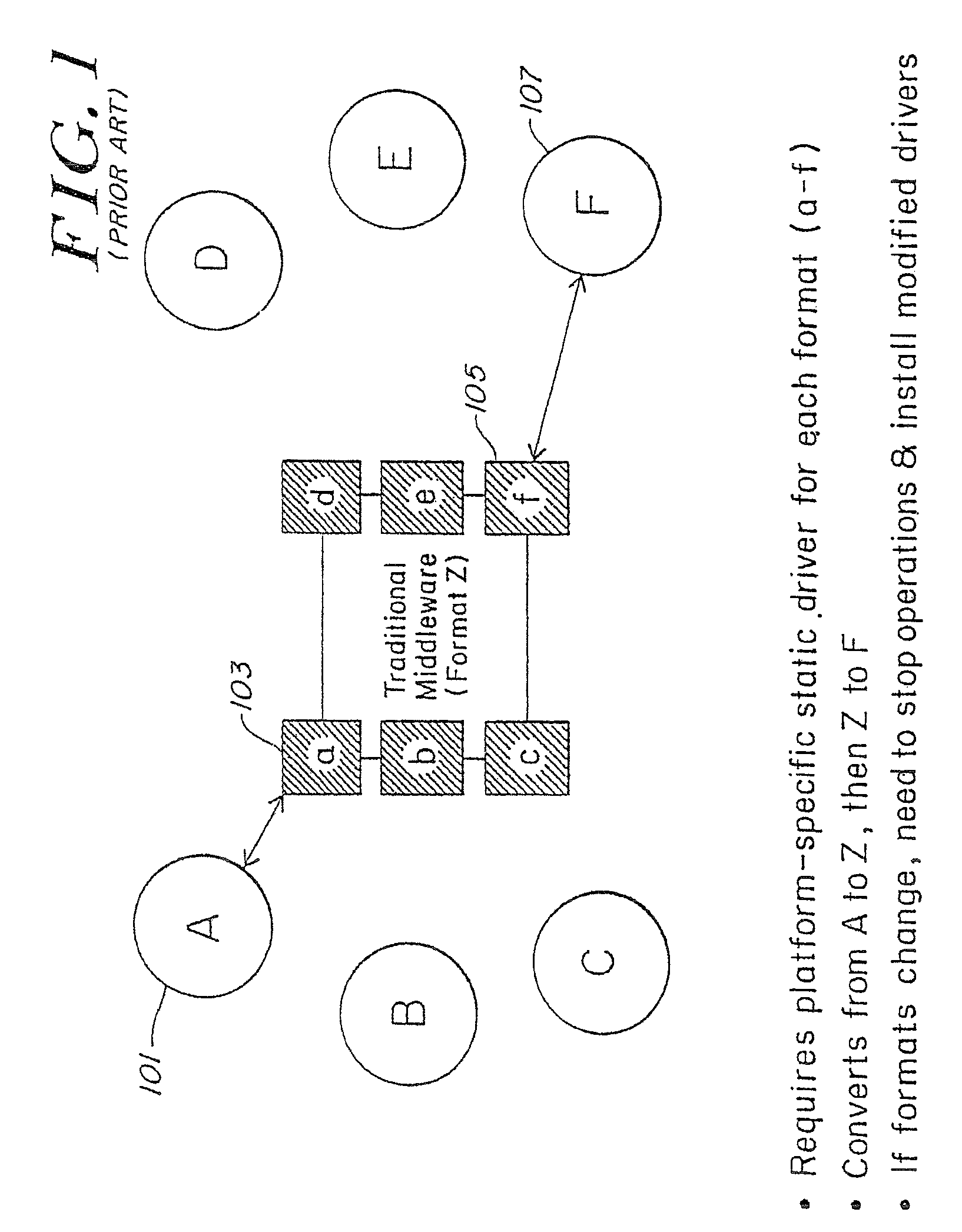
Method and apparatus of streaming data transformation using code generator and translator
InactiveUS20050273772A1Promote generationStay flexibleNatural language data processingMultiple digital computer combinationsStreaming dataObject code
A high level transformation method and apparatus for converting data formats in the context of network applications, among other places. A flexible transformation mechanism is provided that facilitates generation of translation machine code. A translator is dynamically generated by a translator compiler engine. When fed an input stream, the translator generates an output stream by executing the native object code generated on the fly by the translator compiler engine. In addition, the translator may be configured to perform a bi-directional translation between the two streams as well as translation between two distinct protocol sequences. Further a translator may working in streaming mode, to facilitate streaming processing of documents.
Owner:IBM CORP
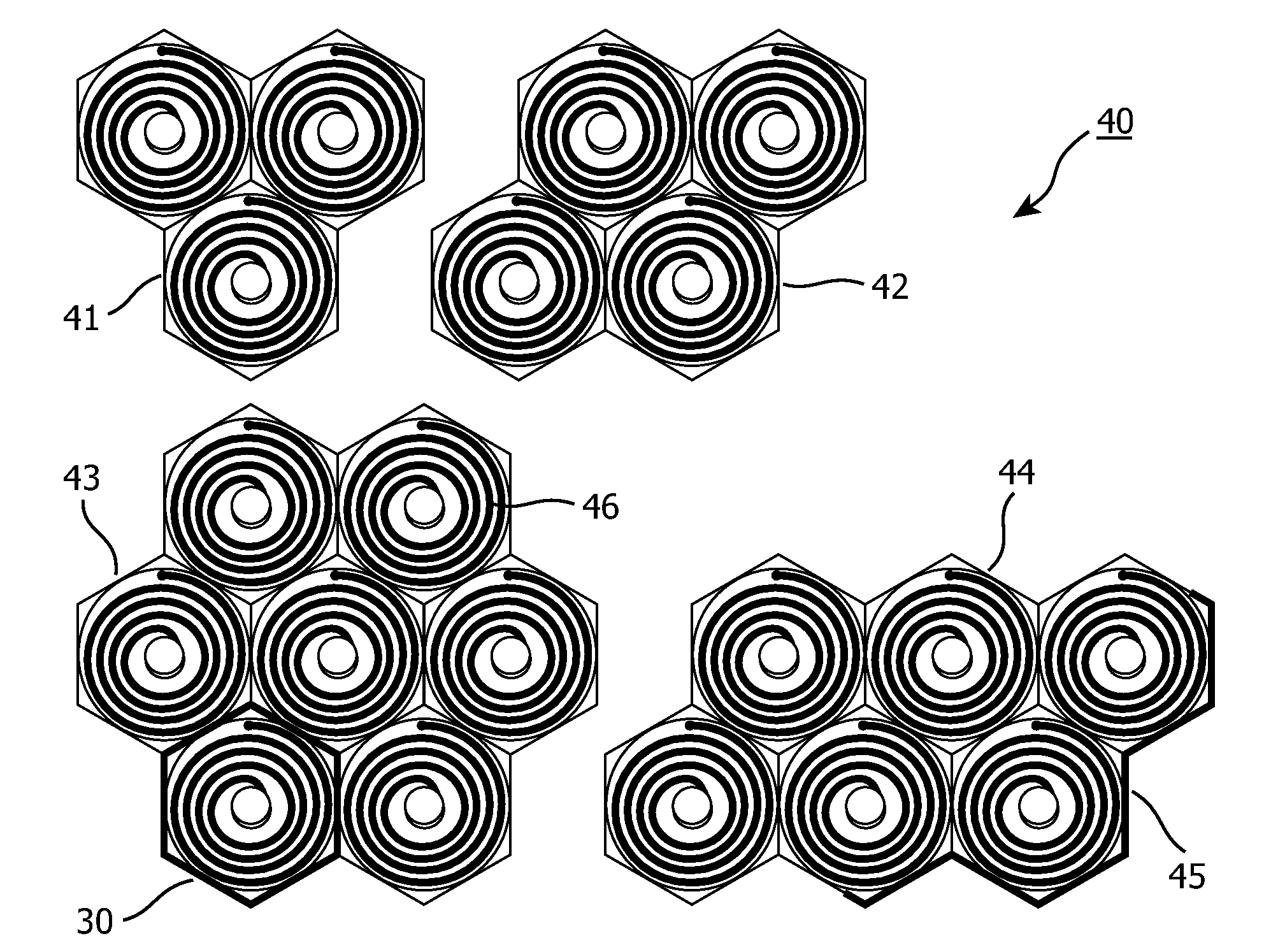
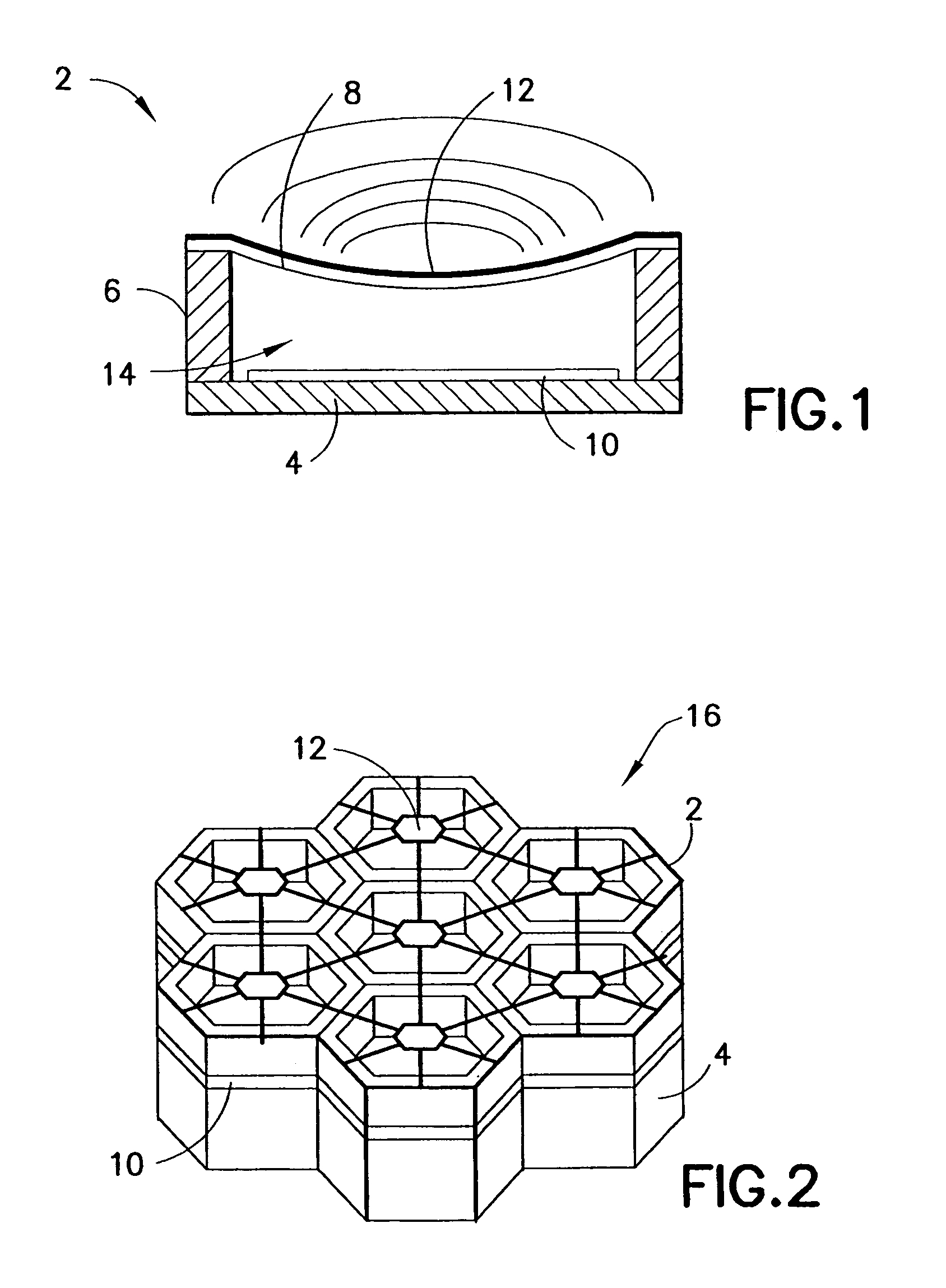
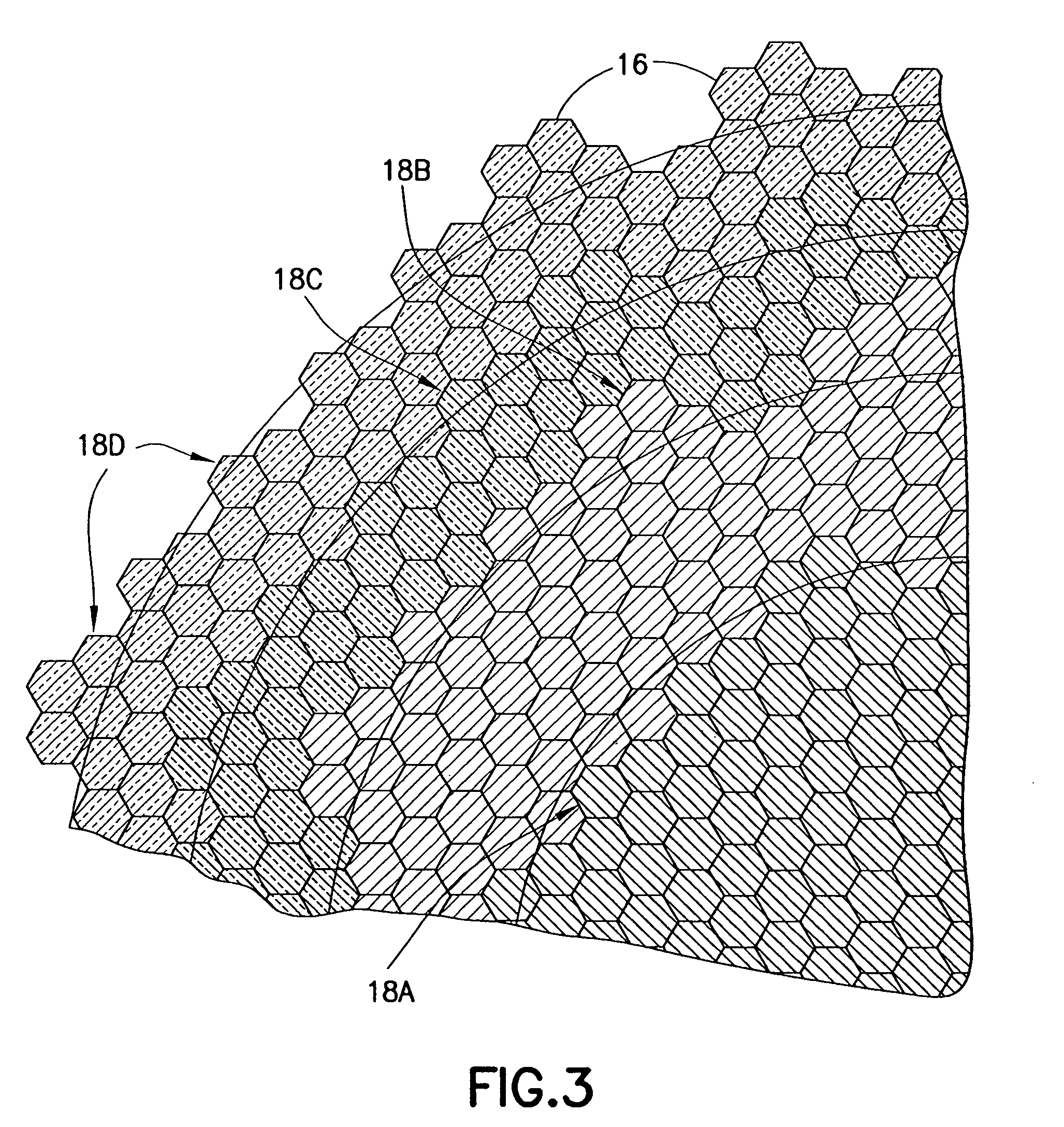
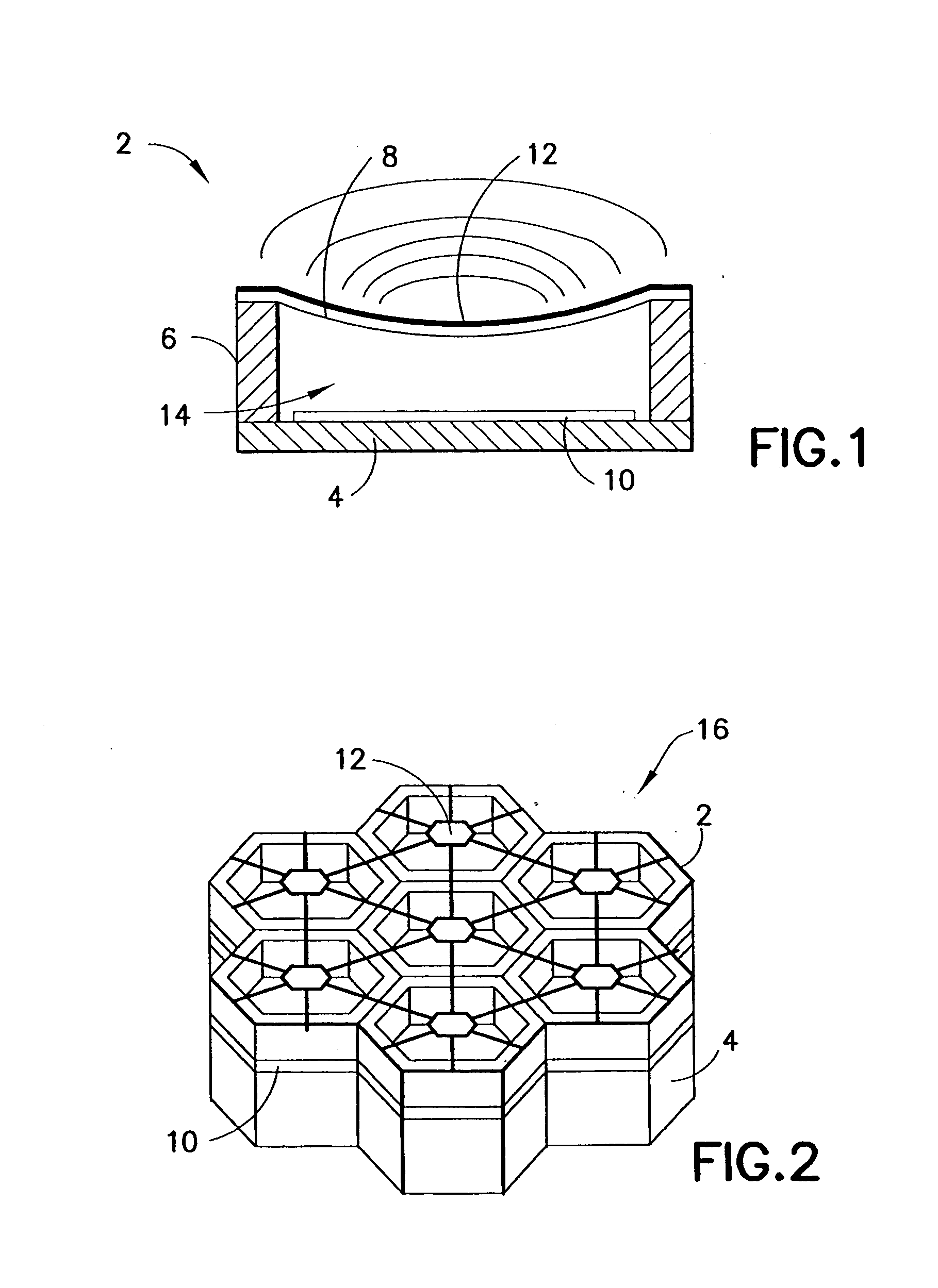
Transmitter module for use in a modular power transmitting system
ActiveUS20130069444A1Improve power stabilityImprove mechanical stabilityTransformersCoupling device detailsElectric power transmissionTransmitter coil
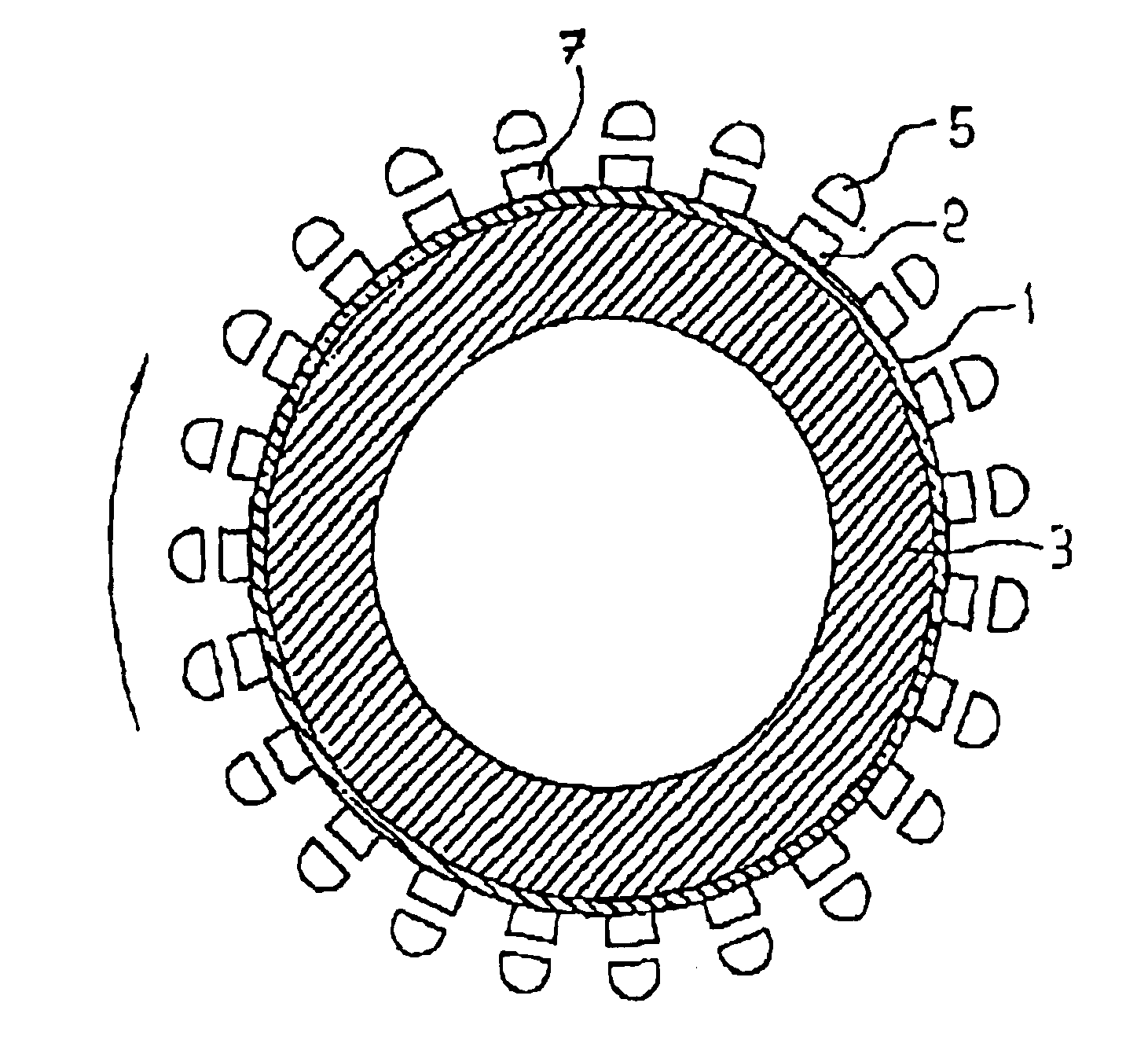
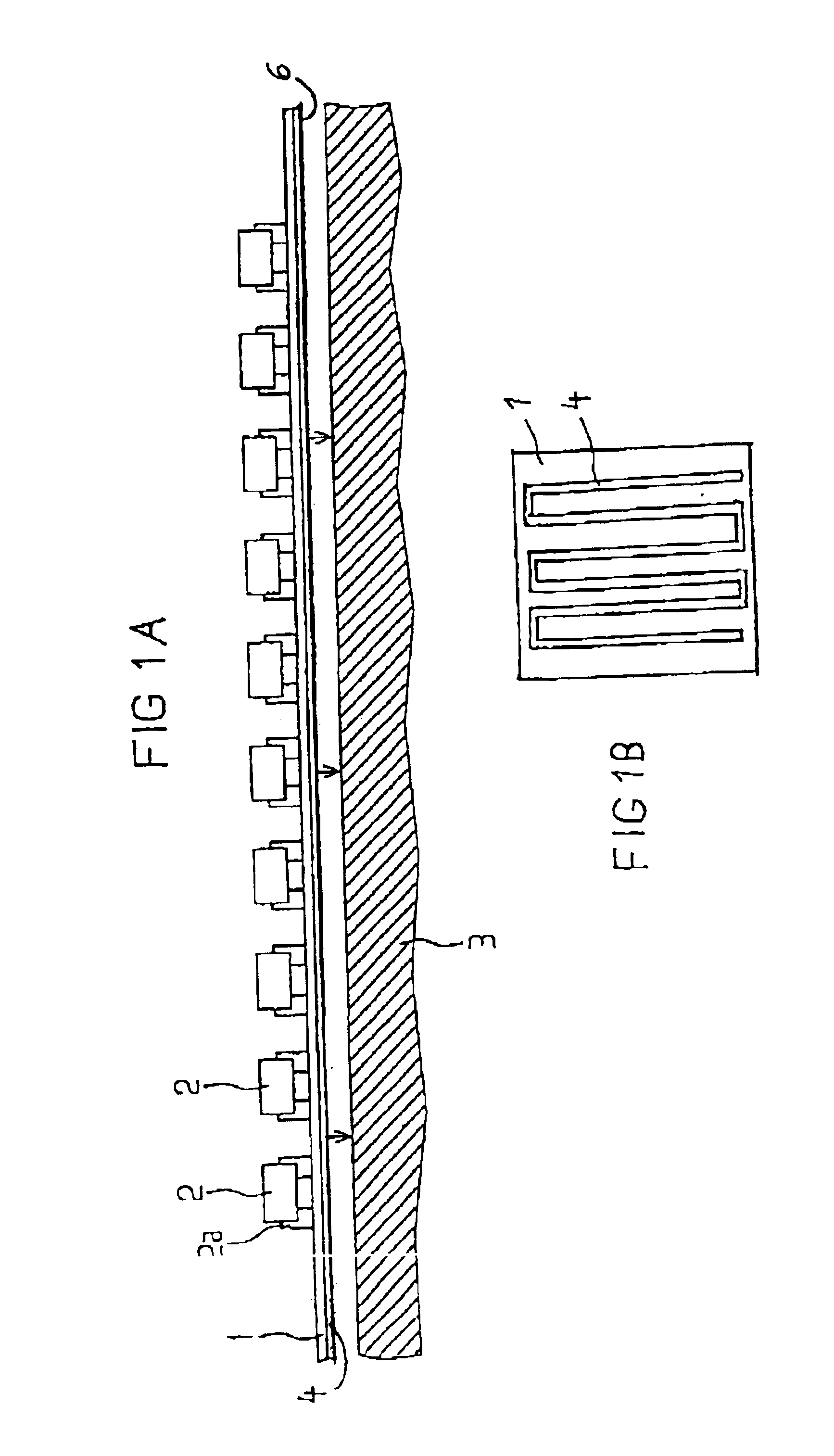
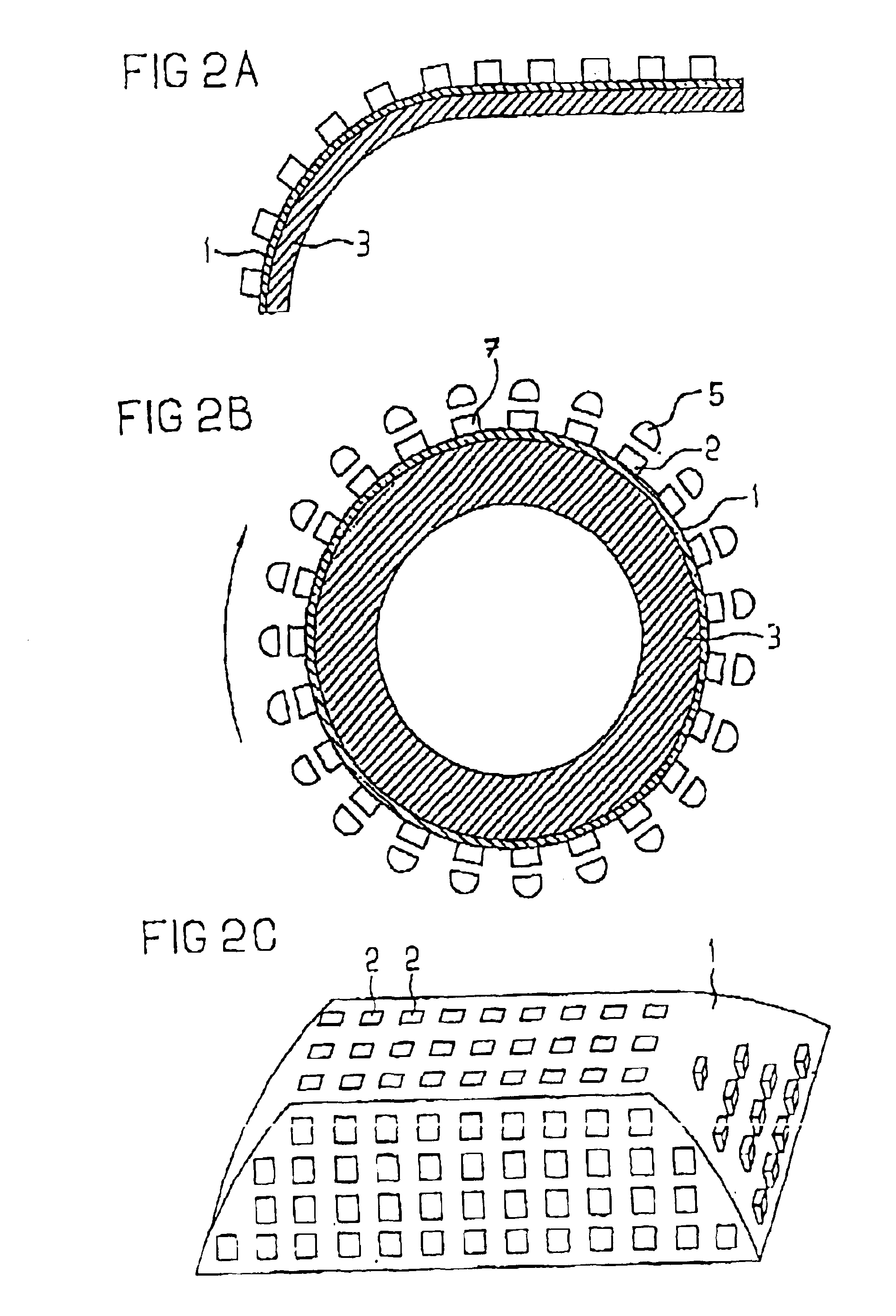
A modular power transmitting system comprises multiple transmitter modules being connected together for transmitting power inductively to a receiver. The transmitter module is connected with other transmitter modules for transmitting power inductively to the receiver, wherein the transmitter module (40) comprises at least one transmitter cell (30), each transmitter cell having one transmitter coil (33) by which the transmitter cell transmitting power to the receiver, the transmitter module having an outer periphery (45) being shaped so as to fit to neighboring transmitter modules for forming an power transmitting surface, the at least one transmitter cell being arranged such that the power transmitting surface is constituted by an uninterrupted pattern of adjacent transmitter coils extending in said surface, and interconnection units (110,111) for connecting with neighboring transmitter modules for sharing a power supply.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
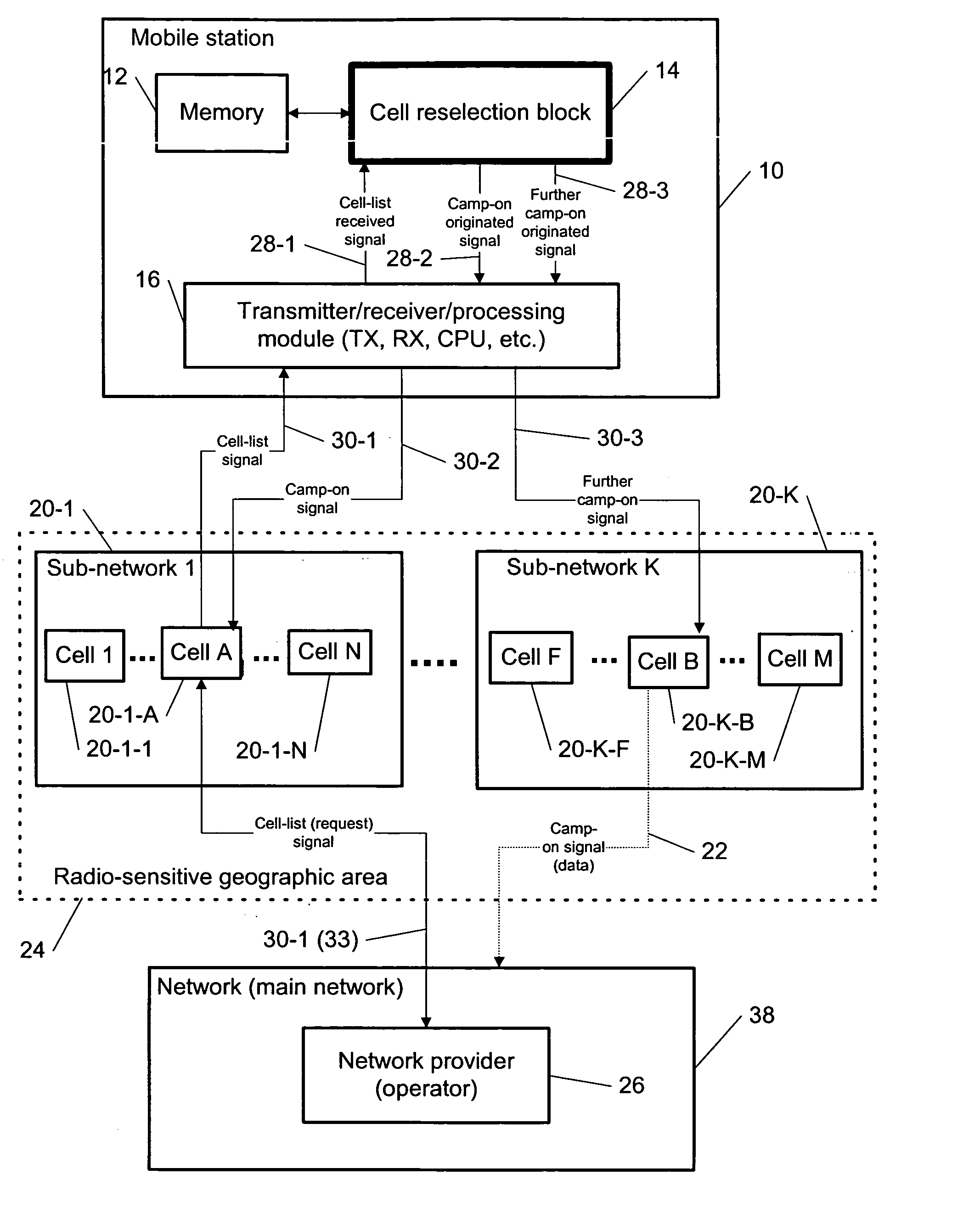
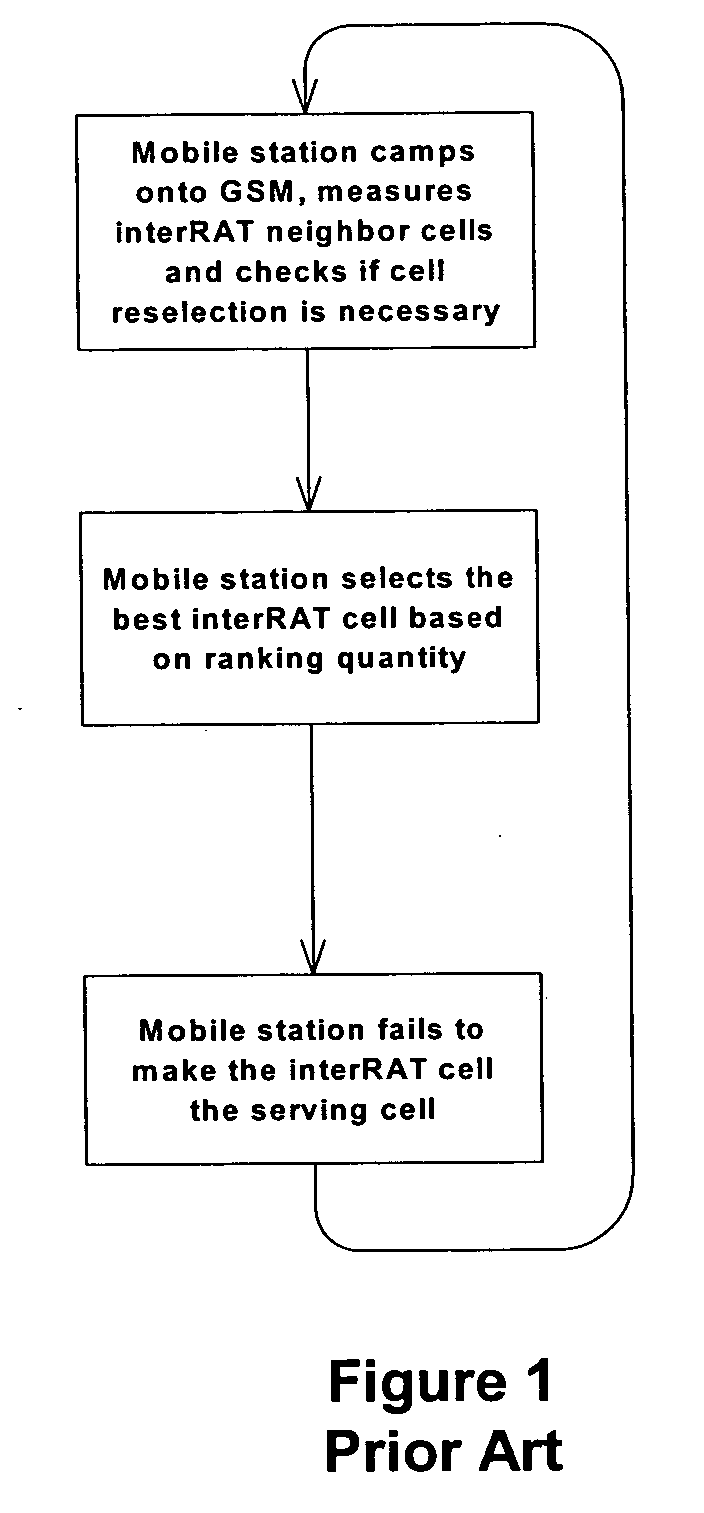
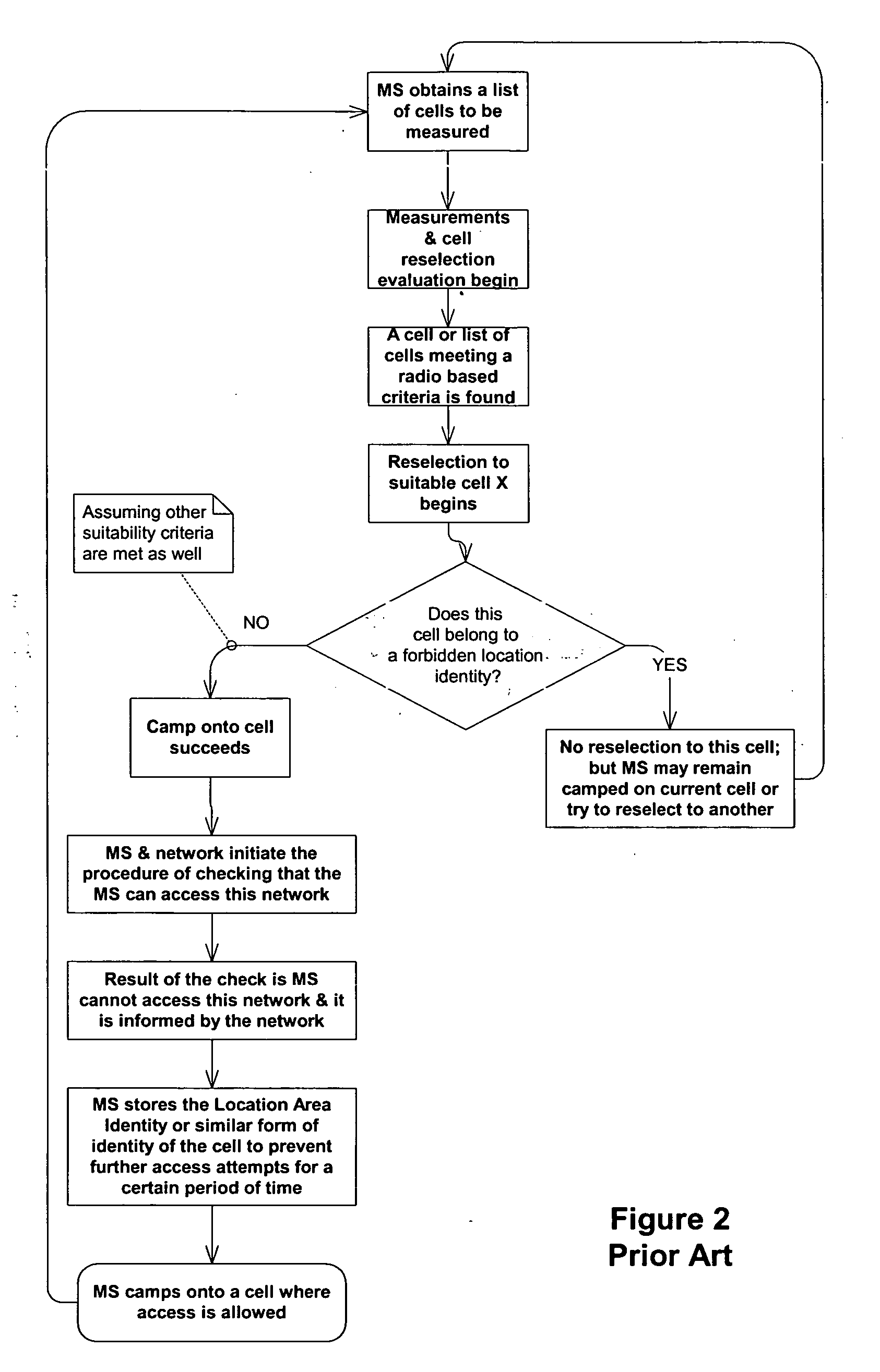
Cell reselection for improving network interconnection
InactiveUS20060084443A1Improving network interconnectionImprove interoperabilityAssess restrictionRadio/inductive link selection arrangementsCell selectionInterconnection
This invention describes a new methodology for a cell reselection by a mobile station (MS) for improving network interconnection and interoperability in a limited mobile access environment. The invention is applicable to any kind of networks and their interconnections. The invention describes how the MS can better recover from failed intersystem cell reselection attempts so that there are fewer subsequent failed attempts using two major improvements. First, the MS takes into consideration during cell reselection evaluation and candidate-cell selection, whether the MS had previously been unsuccessful in reselecting the considered cell. This means treating neighbor cells to which the MS had failed reselection before with a lower priority in subsequent cell reselection evaluations. Second, the MS is allowed to stop monitoring and thus, to stop evaluating cells if it was earlier found that the access to those cells is forbidden.
Owner:RPX CORP
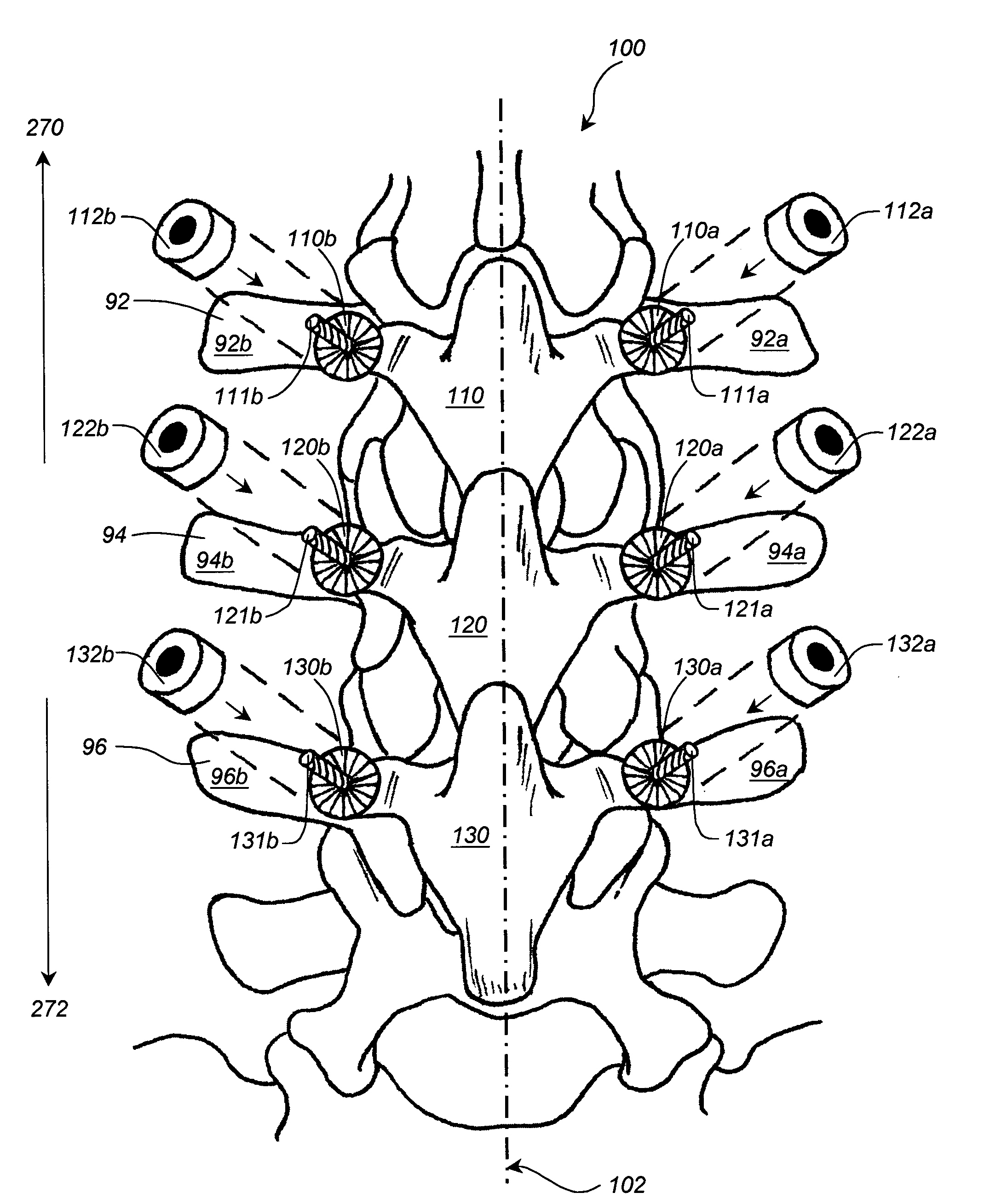
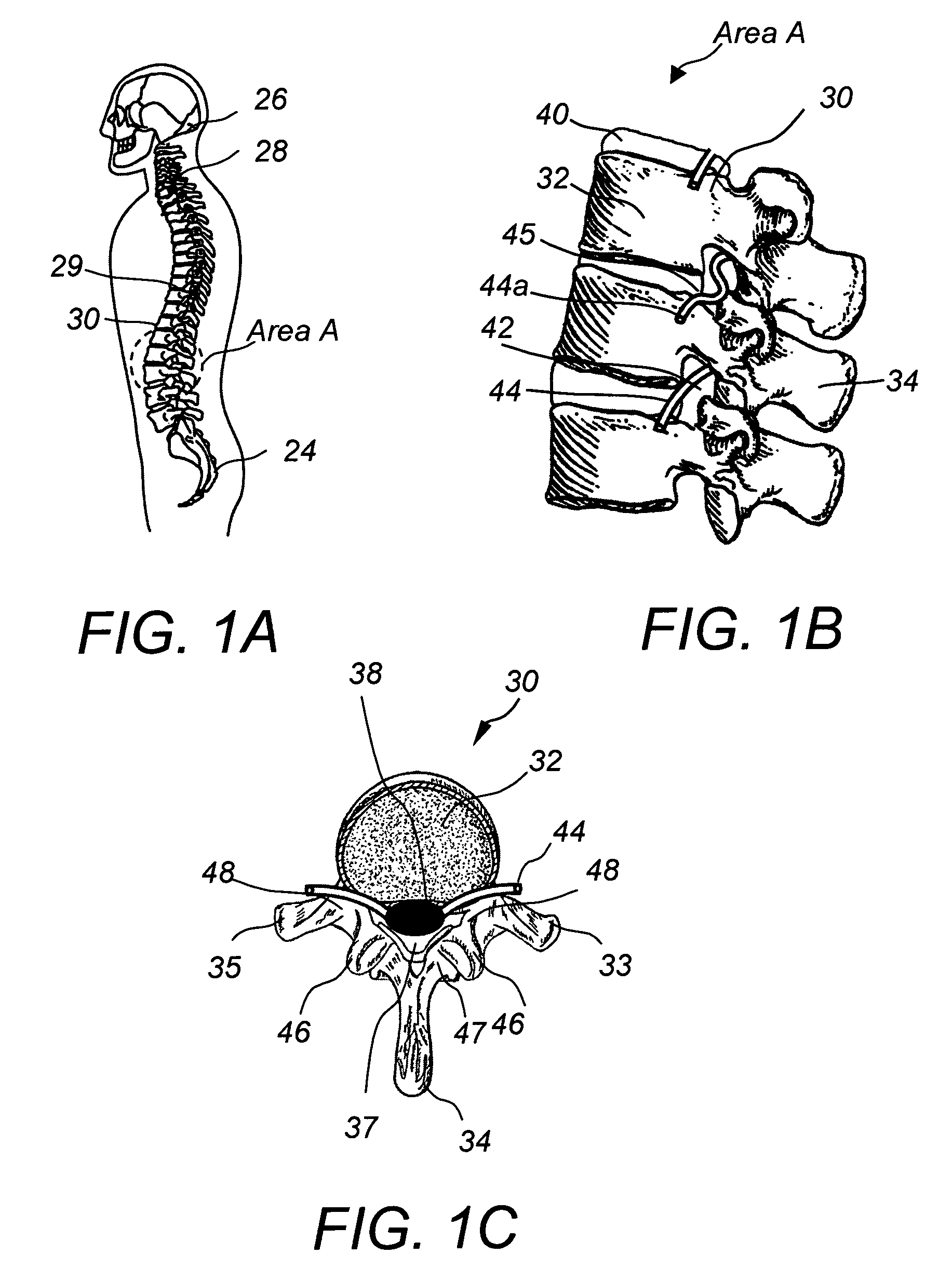
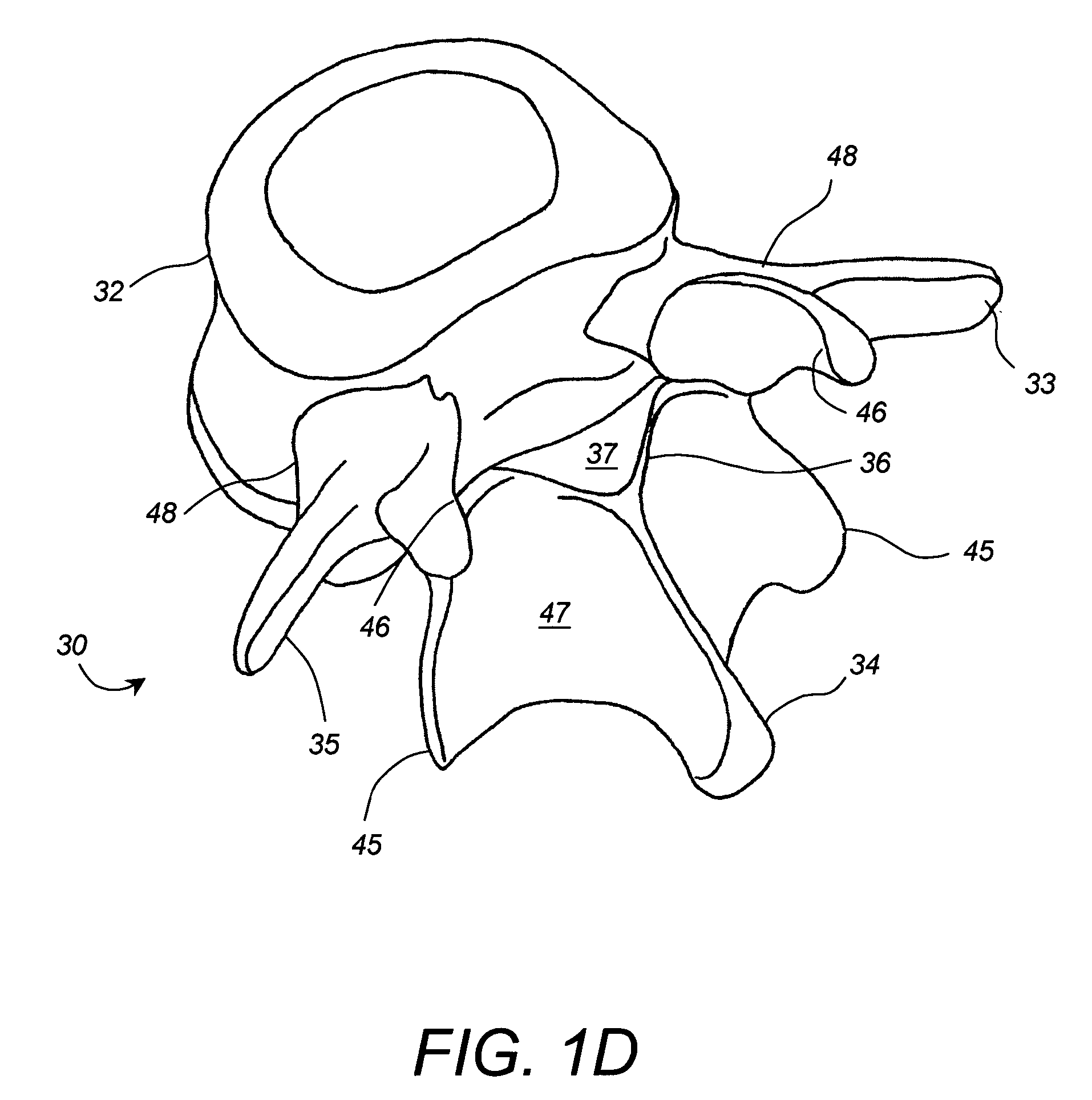
Apparatus and method for connecting spinal vertebrae
InactiveUS7282064B2Process stabilityRetain spinal flexibilityInternal osteosythesisBone implantMiddle linePlastic surgery
An orthopedic implantable device articulately connecting a first spinal vertebra to an adjacent second spinal vertebra includes a pair of first components adapted to be attached to locations left and right of a midline of the first vertebra, respectively; and a pair of second components adapted to be attached to locations left and right of a midline of the second vertebra, respectively. Each of the first components includes a body and a male articulation member attached to the first component body and each of the second components includes a body and a female articulation member attached to the second component body. The first components are articulately connected to the second components by engaging the male articulation members to the female articulation members, thereby articulately connecting the first vertebra to said second vertebra along lines left and right of the midlines, respectively.
Owner:LESFACETS LLC
Touch Screen Shield
ActiveUS20110279383A1Promote formationStay flexibleLamination ancillary operationsProtective equipmentEngineeringTouchscreen
A shield that is attachable to a touch sensitive screen is disclosed. The shield may be attached to the touch sensitive screen only at its outer peripheral portion. An air gap is enclosed between the shield and the touch sensitive screen to form a planar air bearing. The shield preferably does not touch the active area of the touch sensitive screen when the user is not touching the shield but only viewing the touch sensitive screen through the shield. This mitigates unwanted optical artifacts such as trapped air bubbles, Newton rings and chromatic interference while maintaining the sensitivity of the touch sensitive screen.
Owner:RACING OPTICS
Light-emitting diode arrangement
InactiveUS6848819B1Technological manufacturing outlayImprove cooling effectPoint-like light sourceLighting support devicesLed arrayEngineering
An LED array surface-mounted on a circuit board and applied to a cooling member, such that any generated heat is optimally eliminated. The cooling member can be in any desired shape so that motor vehicle lights, such as blinkers, can be adapted to the outside contour of the vehicle. For a rotating light, the circuit board can be applied around a cooling member fashioned as a hollow cylindrical member which is adapted to rotate.
Owner:OSRAM OPTO SEMICONDUCTORS GMBH
Methods of treatment using a bariatric sleeve
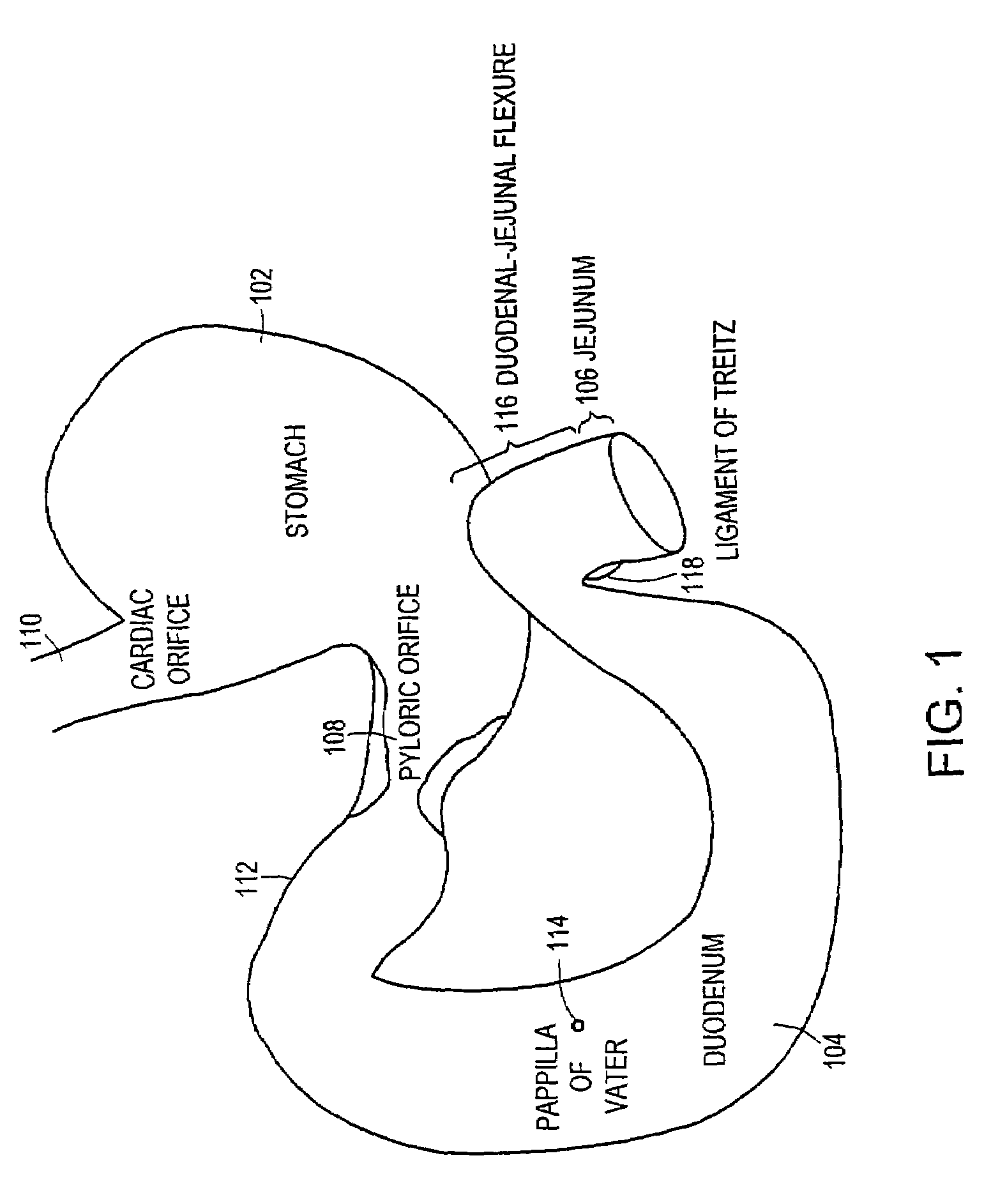
ActiveUS7695446B2Promote healingControlled absorptionSuture equipmentsStentsDiseaseIntestinal structure
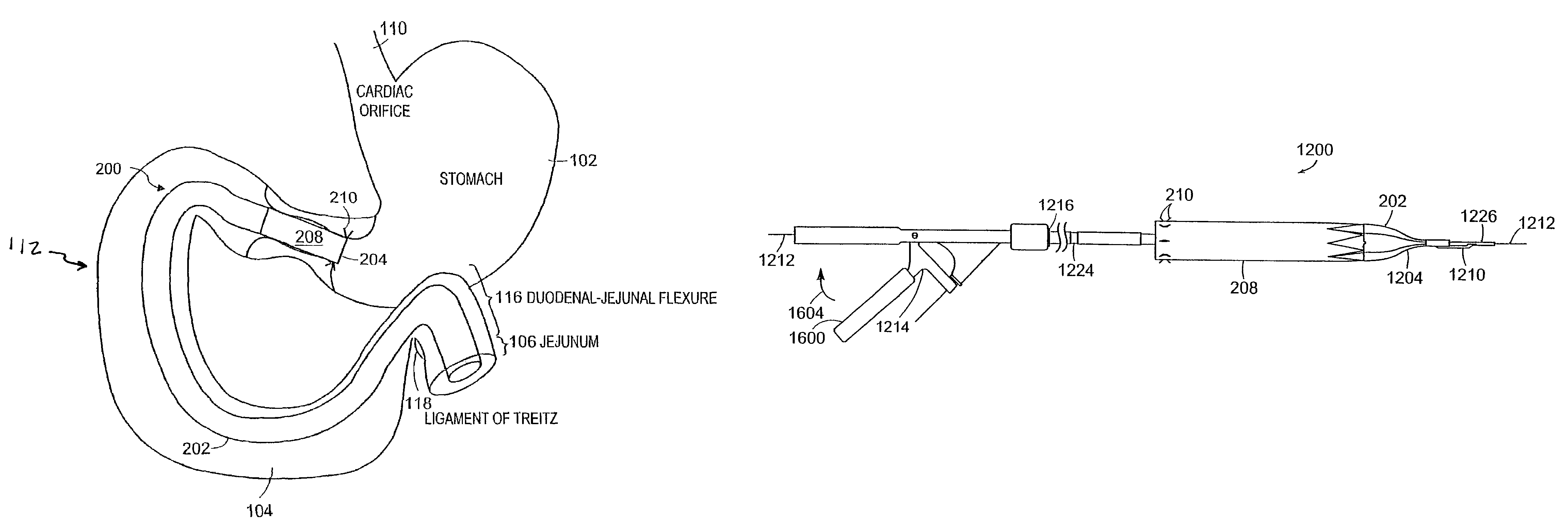
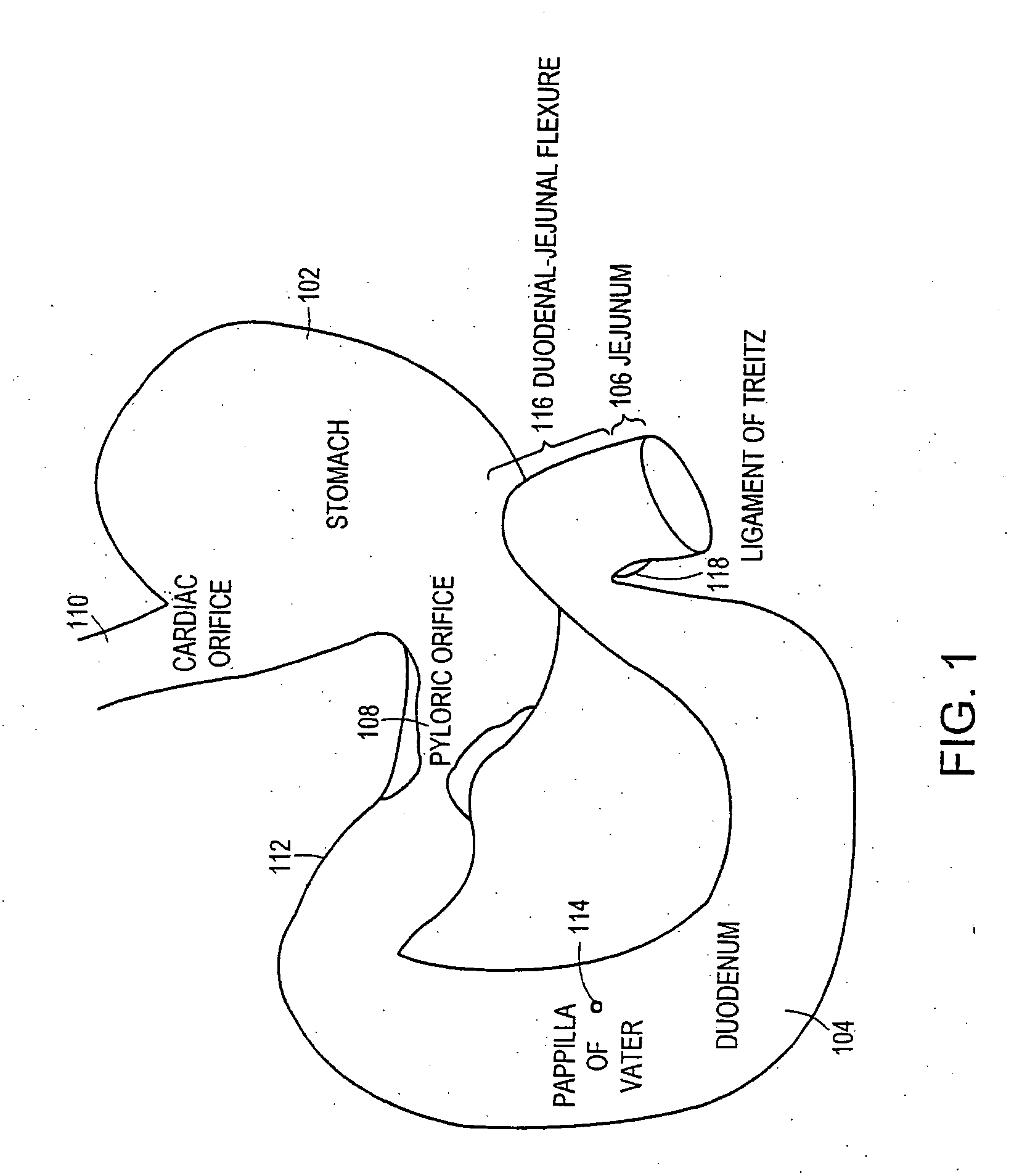
Methods of treatment using a gastrointestinal implant device removably anchored within an animal's gastrointestinal tract. For example, the implant device includes a collapsible anchor for anchoring the device coupled to a proximal end of a flexible sleeve. The implant device can be anchored within the stomach, within the pyloric orifice, and / or distal to the pylorus and extended into the duodenum. All partially-digested food, or chyme, exiting the stomach is funneled through the device. Methods of treatment include treating obesity by one or more of: limiting the absorption of nutrients within the duodenum; delaying the mixing of chyme with digestive enzymes; alter hormonal triggers; and providing negative feedback. Alternatively or in addition, the desired result includes treating a diseases, such as diabetes, or temporarily shielding a portion of the intestine to promote healing within the intestine.
Owner:GI DYNAMICS INC
Intracardiac device for restoring the functional elasticity of the cardiac structures, holding tool for the intracardiac device, and method for implantation of the intracardiac device in the heart
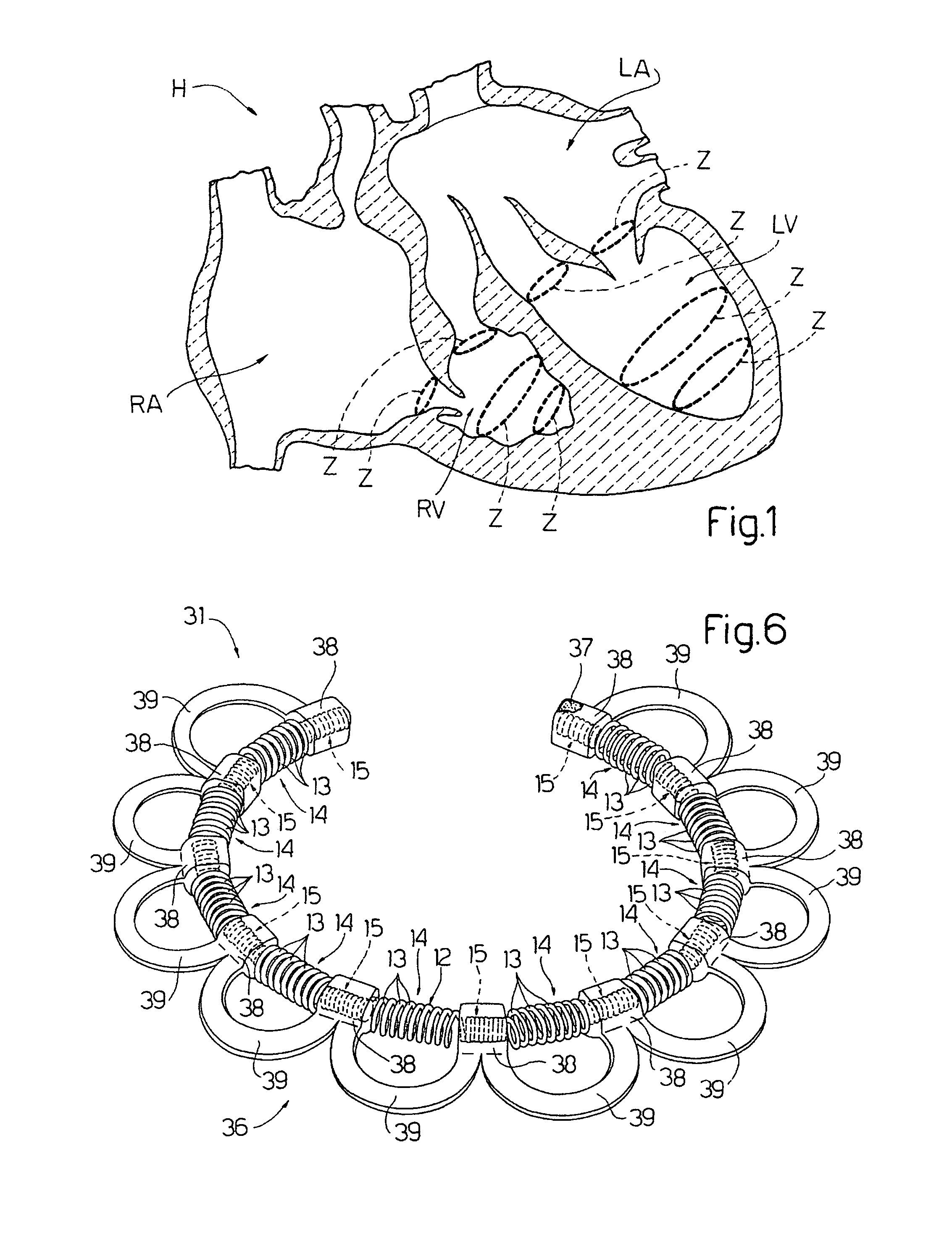
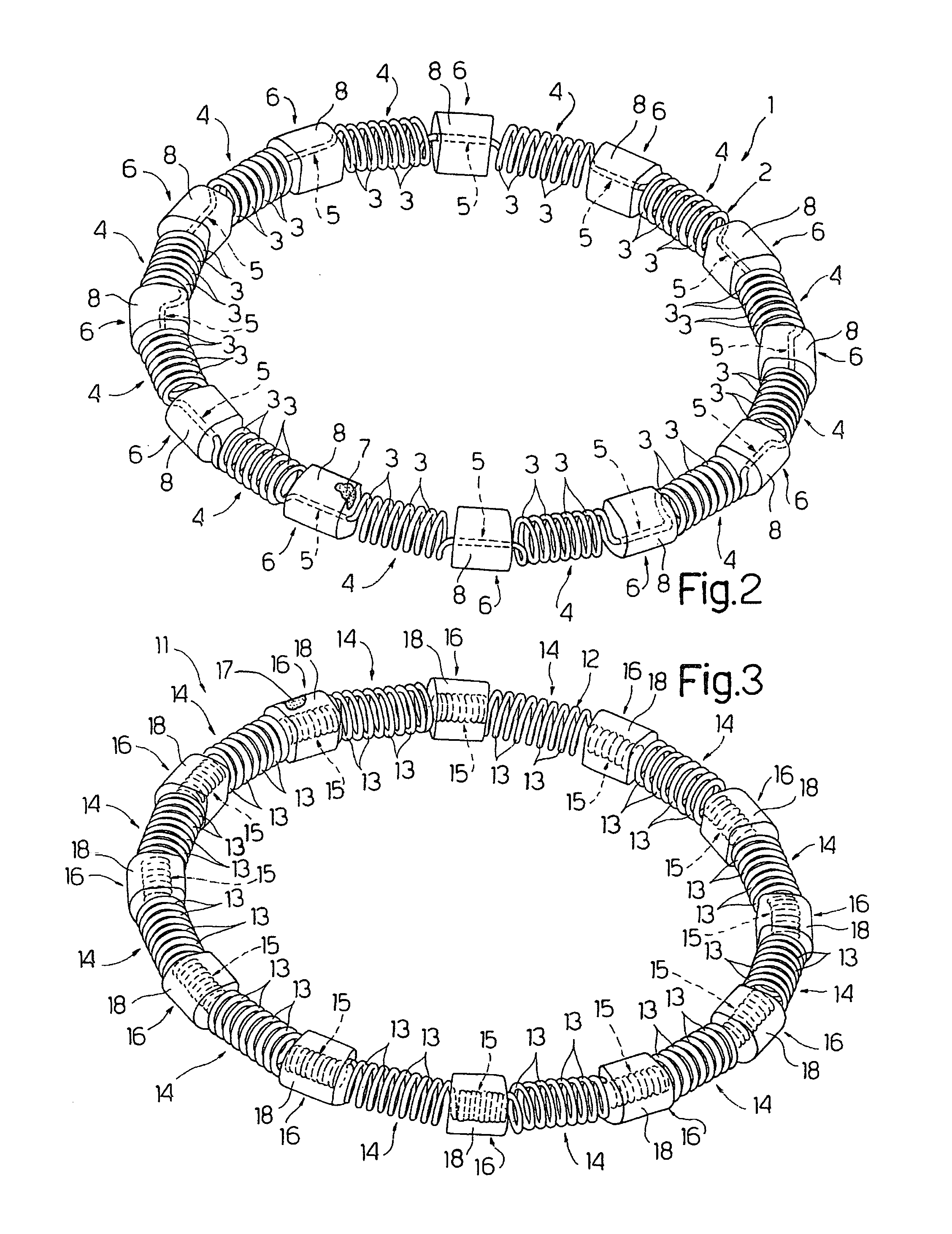
ActiveUS20100030014A1Minimize tissutal reactionReduce thrombogenic capacityAnnuloplasty ringsBandagesCardiac cyclePlasma viscosity
An intracardiac device for restoring the functional elasticity of the cardiac structures, in particular for the treatment of cardiomyopathies and or valvulopathies, by storing energy from the cardiac structures and ceding energy to the cardiac structures during the cardiac cycle, has an elongated shape, is at least partially wound in coils and is attachable to a cardiac structure; the coils are selected in material, number and dimension so as to allow an elastic elongation of the intracardiac device higher than 10% of the rest length of the intracardiac device and are exposed, in use, to the blood flow.
Owner:CUBE
Vascular thrombectomby apparatus and method of use
InactiveUS20070288054A1Modulus of elasticityStay flexibleSurgeryDilatorsMembrane configurationBlood vessel
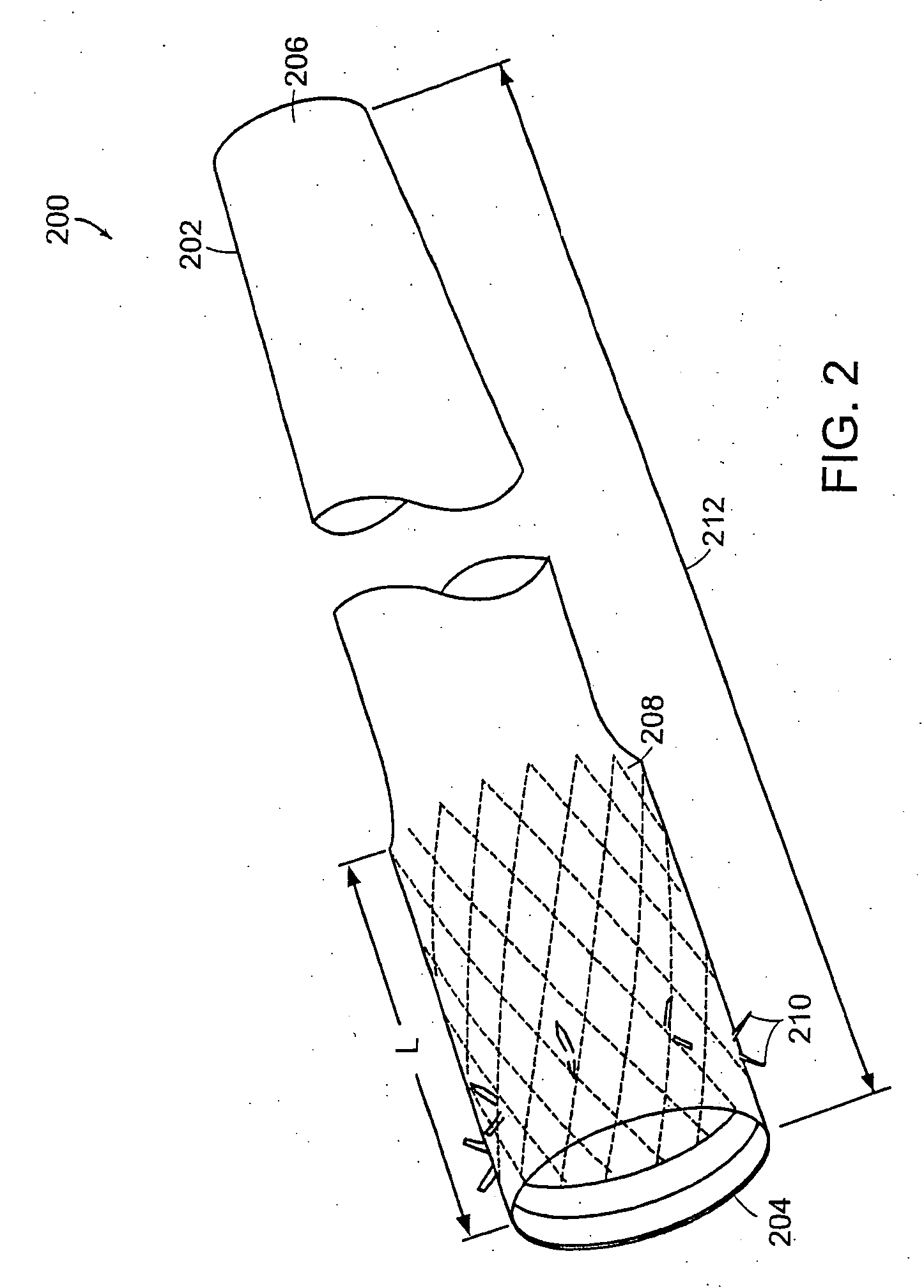
An apparatus is provided for removing matter from within a conduit. The apparatus generally comprises a separation edge attached to a wire at its proximal end, a frame attached to the distal end of the separation edge, and a membrane attached to the wire and disposed over the frame enclosing its interior. The membrane generally comprises a net constructed from a vaporized metal deposited on a mandrel. Alternatively, the membrane may comprise a braided tube constructed from wire. The apparatus may be employed as a filter or as a means for actively dislodging matter from the wall of a conduit. When employed as a filter, the apparatus is positioned downstream of the matter where it ensures that matter does not escape downstream as it is being removed. When employed to actively remover matter from a conduit, the apparatus is positioned downstream of the matter. The apparatus is then pulled proximally whereby it engages the matter and dislodges it from the wall of the conduit
Owner:CORDIS CORP
Methods of treatment using a bariatric sleeve
Methods of treatment using a gastrointestinal implant device removably anchored within an animal's gastrointestinal tract. For example, the implant device includes a collapsible anchor for anchoring the device coupled to a proximal end of a flexible sleeve. The implant device can be anchored within the stomach, within the pyloric orifice, and / or distal to the pylorus and extended into the duodenum. All partially-digested food, or chyme, exiting the stomach is funneled through the device. Methods of treatment include treating obesity by one or more of: limiting the absorption of nutrients within the duodenum; delaying the mixing of chyme with digestive enzymes; alter hormonal triggers; and providing negative feedback. Alternatively or in addition, the desired result includes treating a diseases, such as diabetes, or temporarily shielding a portion of the intestine to promote healing within the intestine.
Owner:GI DYNAMICS
Method and apparatus of streaming data transformation using code generator and translator
InactiveUS7590644B2Promote generationStay flexibleDigital data processing detailsNatural language data processingStreaming dataObject code
A high level transformation method and apparatus for converting data formats in the context of network applications, among other places. A flexible transformation mechanism is provided that facilitates generation of translation machine code. A translator is dynamically generated by a translator compiler engine. When fed an input stream, the translator generates an output stream by executing the native object code generated on the fly by the translator compiler engine. In addition, the translator may be configured to perform a bi-directional translation between the two streams as well as translation between two distinct protocol sequences. Further a translator may working in streaming mode, to facilitate streaming processing of documents.
Owner:INT BUSINESS MASCH CORP
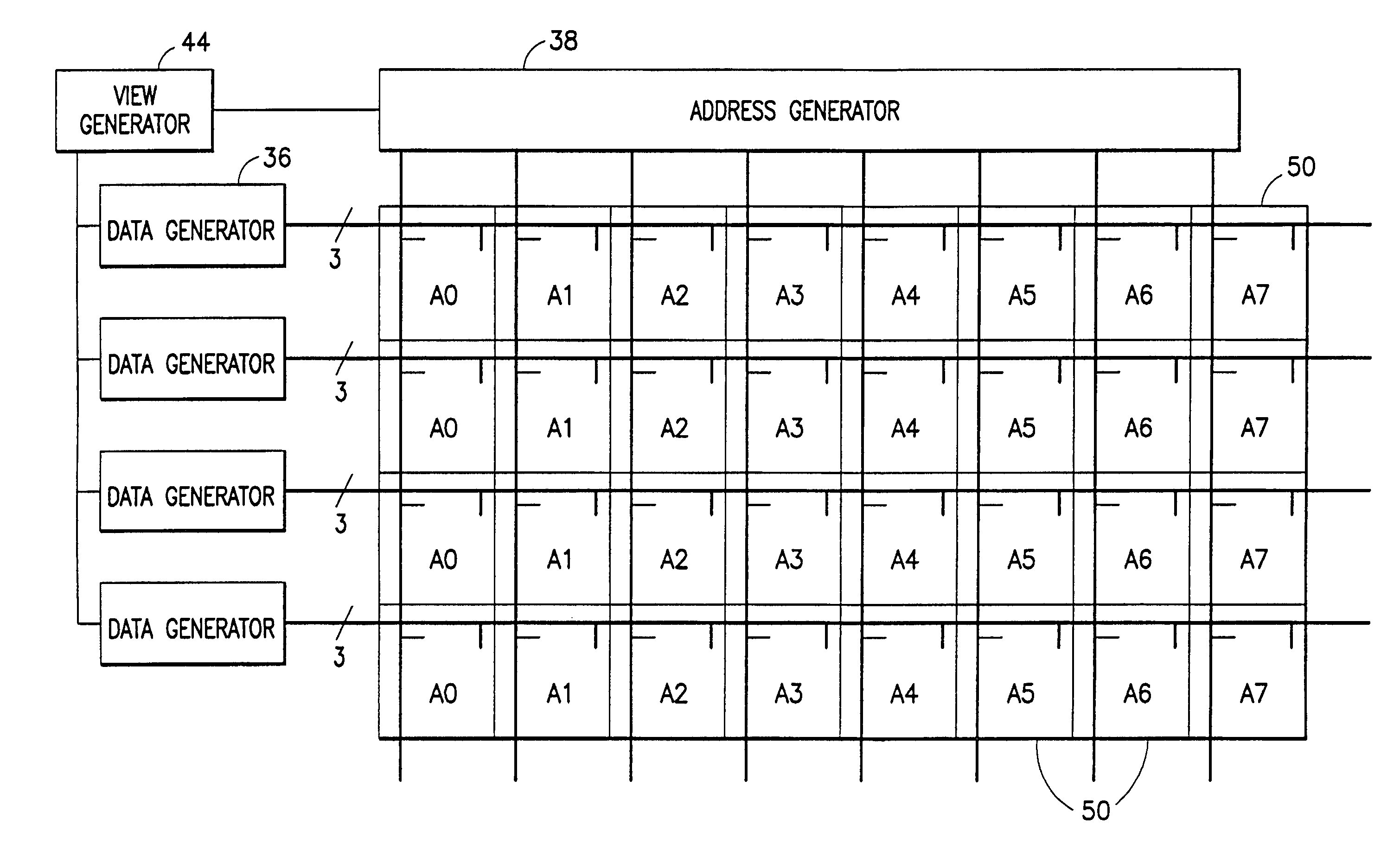
Method and apparatus for controlling scanning of mosaic sensor array
InactiveUS7313053B2Quick configurationMinimize powerUltrasonic/sonic/infrasonic diagnosticsWave based measurement systemsComputer hardwareSensor array
A scanning architecture that makes it possible to update only those ultrasonic transducer subelements of a mosaic transducer array that change from view to view. The configuration of the switch matrix is fully programmable. The switch matrix includes access switches that connect subelements to bus lines and matrix switches that connect subelements to subelements. Each subelement has a unit switch cell associated therewith, each unit switch cell comprising at least one access switch, at least one matrix switch, and addressing and control logic. Optionally, each unit switch cell also includes latches for storing the future switch states of the switches to be programmed. The switches themselves have memory for storing their current switch states.
Owner:GENERAL ELECTRIC CO
System for performing coupled finite analysis
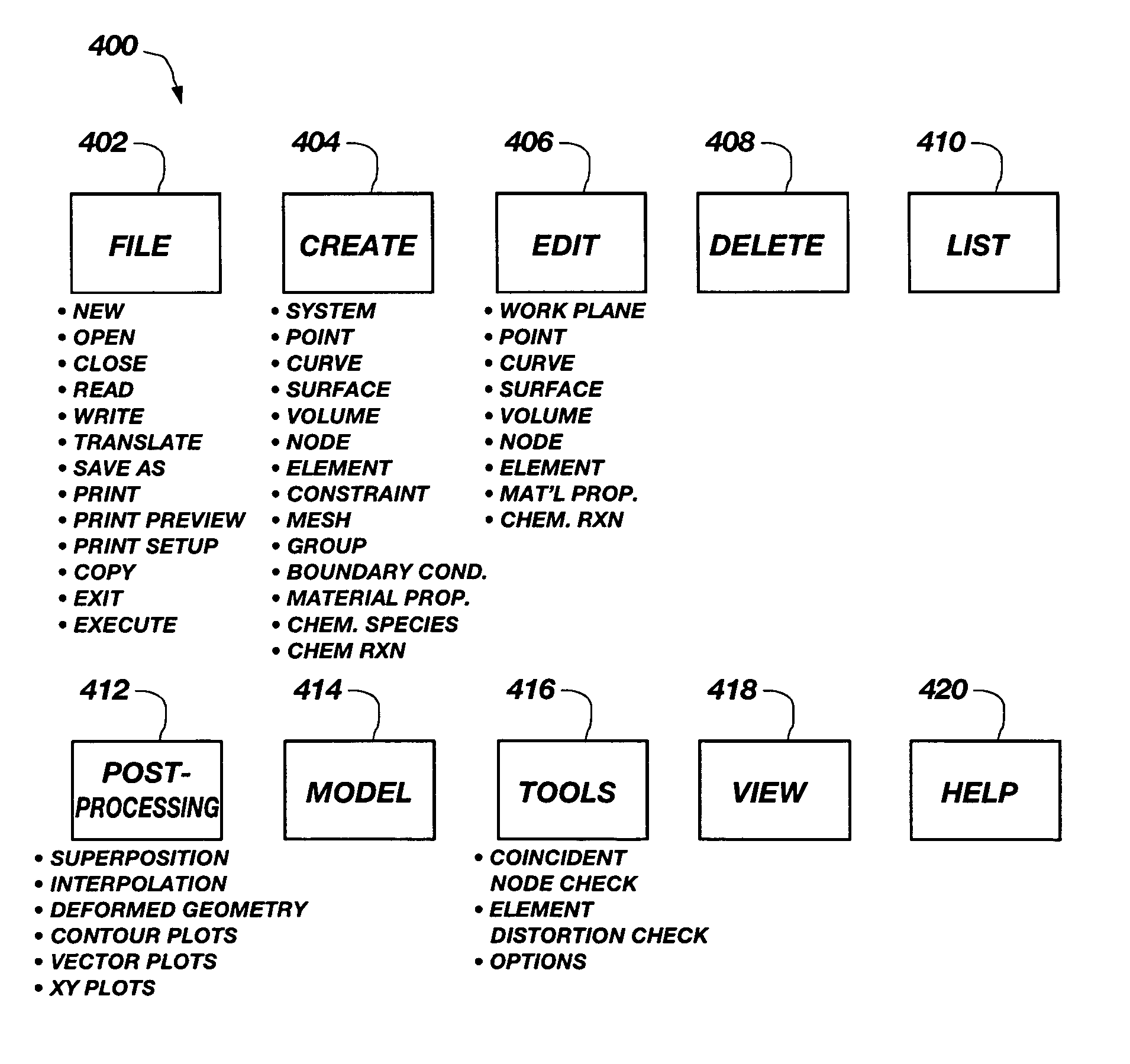
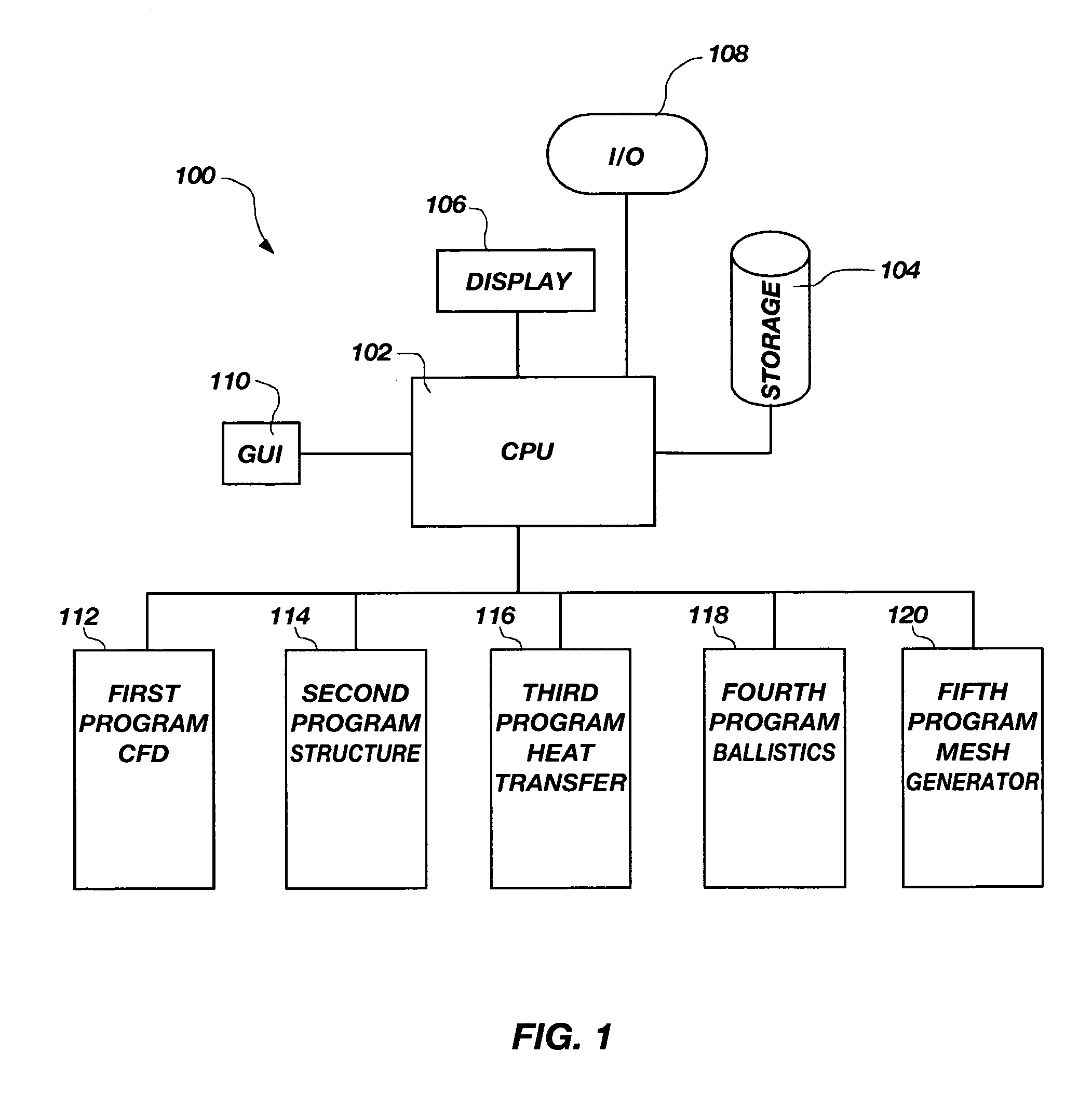
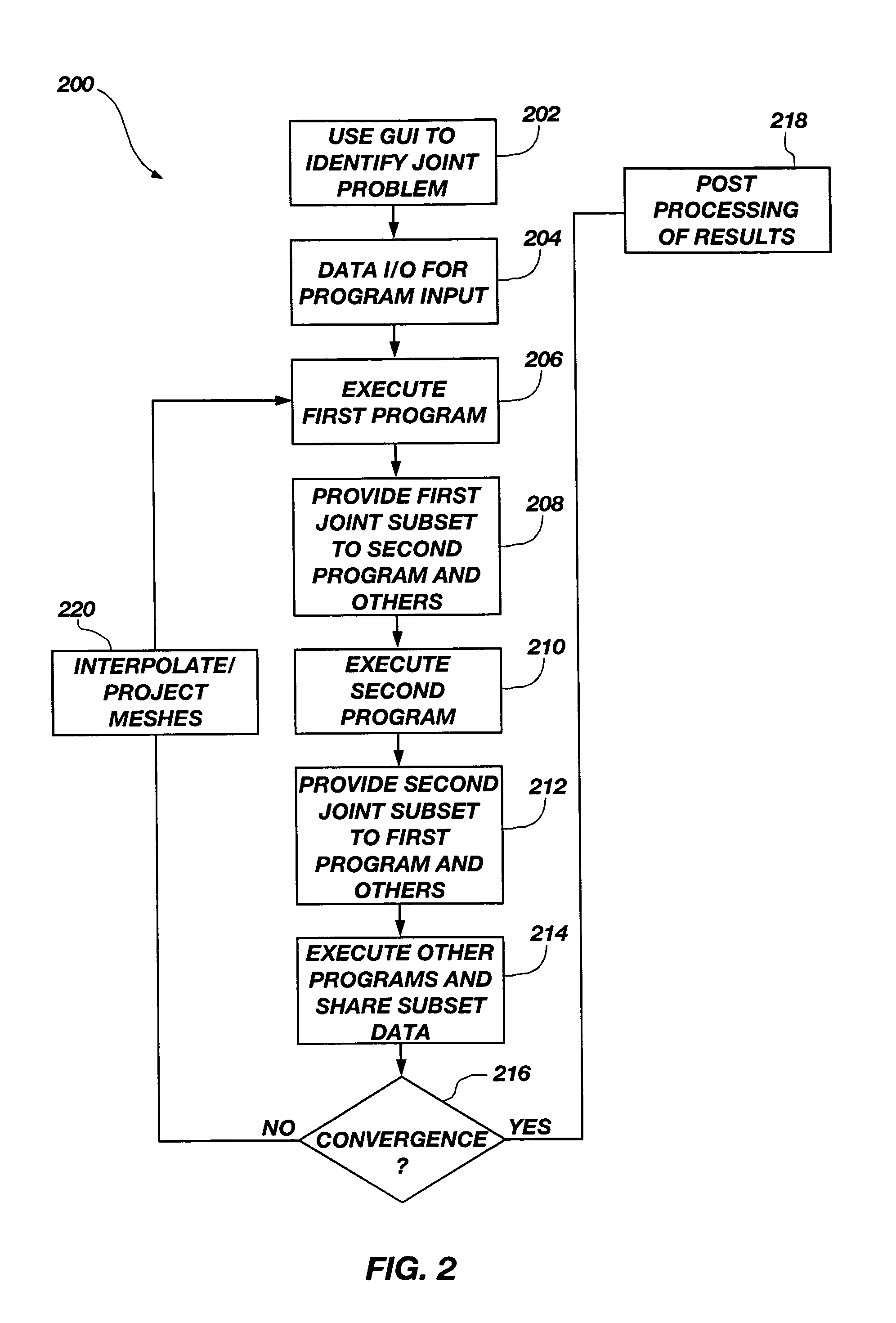
ActiveUS7127380B1Stay flexibleAccelerated settlementComputation using non-denominational number representationDesign optimisation/simulationComputational scienceGraphics
A graphical user interface, together with a comparable scripting interface, couples a plurality of finite element, finite volume, or finite difference analytical programs and permits iterative convergence of multiple programs through one set of predefined commands. The user is permitted to select the joint problem for solution by choosing program selections. Data linkages that couple the program are predefined by an expert system administrator to permit less skilled modelers access to a comprehensive and multifaceted solution that would not be possible for the less skilled modelers to complete absent the graphical user interface.
Owner:NORTHROP GRUMMAN SYST CORP
Disposable pulse oximeter assembly and protective cover therefor
InactiveUS6253098B1Improve qualityImprove readingRespiratorsMedical devicesPolypropyleneOff the shelf
This invention is a protective covering to protect off-the-shelf disposable pulse oximeter sensors from bodily or surgical fluids. The protective covering will envelop and encase the inserted pulse oximeter sensor up to a point on the connection cable extending from the pulse oximeter sensor. The protective covering is a polypropylene, rubber, or similar material, which preferably is tapered from the large width at the entrance to the narrower width at the blind end. The protective covering is bilaminar in nature to contain a substantially rectangular pulse oximeter.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
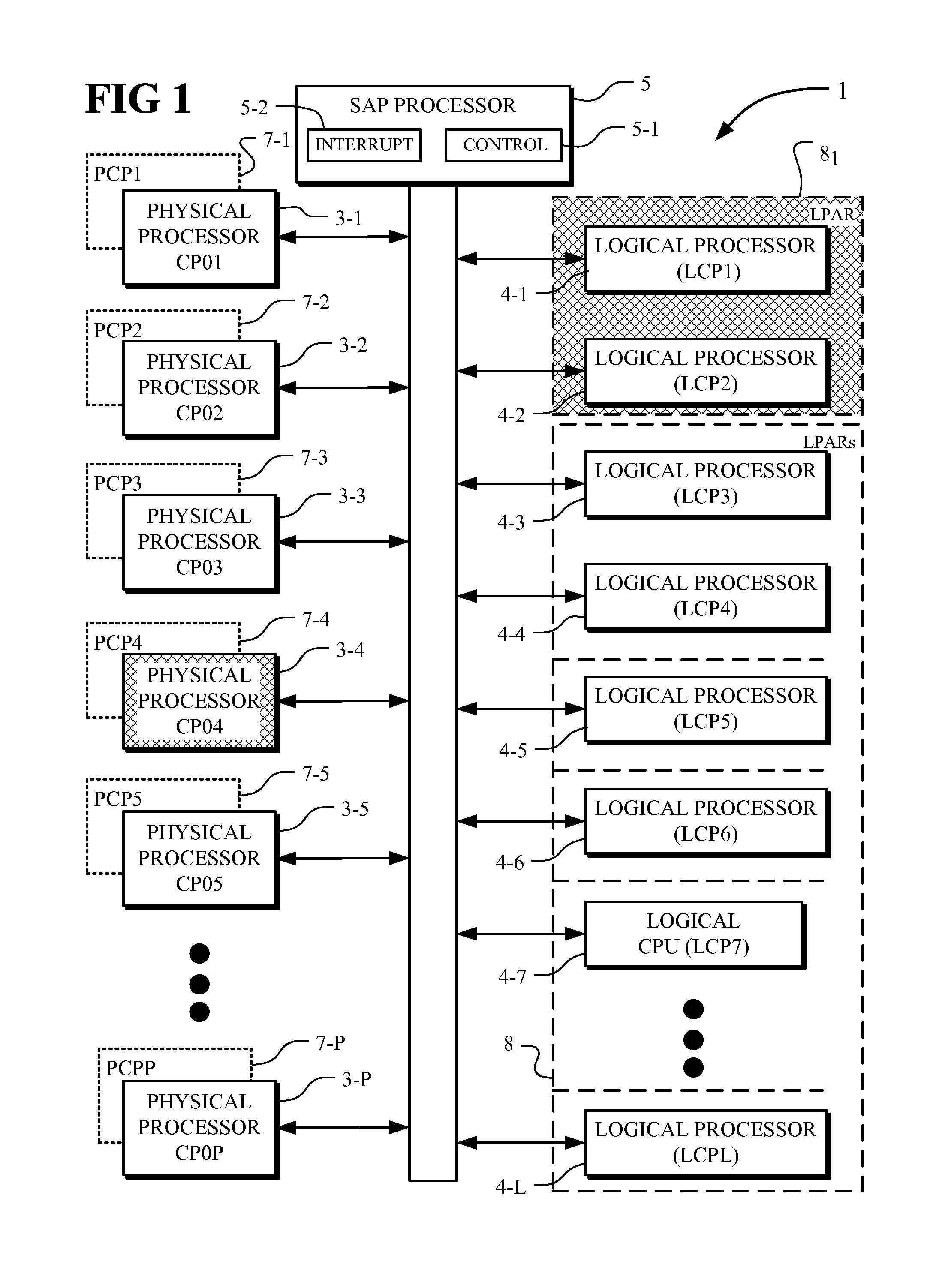
Processor exclusivity in a partitioned system
ActiveUS20090013153A1Eliminating all formMeet changing requirementsSoftware simulation/interpretation/emulationMemory systemsVirtualizationComputerized system
A computer system including a plurality of physical processors (CPs) having physical processor performances (PCPs), a plurality of logical processors (LCPs), a plurality of logical partitions (LPARs) where each partition includes one or more of the logical processors (LCPs), and a system assist processor having a control element. The control element controls the virtualization of the physical processors (CPs), the logical partitions (LPARs) and the logical processors (LCPs) and allocates the physical processor performances (PCPs) to the logical partitions (LPARs). The control element operates to exclusively bind logical processors (LCPs) to the physical processors (CPs). For a logical processor (LCP) exclusively bound to a physical processor (CP), the logical processor (LCP) has exclusive use of the underlying physical processor (CP) and no other logical processor (LCP) can be dispatched on the underlying physical processor (CP) even if the underlying physical processor (CP) is otherwise available.
Owner:IBM CORP
Reusable vacuum bag and methods of its use
InactiveUS20050086916A1Eliminate disposableEliminate expenseDispersed particle filtrationConfectioneryFiberShell molding
Reusable vacuum bags are provided which include a fabric layer containing reinforcement fibers and a release surface disposed on at least the first side of the fabric layer. The vacuum bag is capable of withstanding multiple mold cycles of the vacuum of less than ambient pressure without significant leakage. In addition, the described vacuum bag can be used in resin transfer molding and standard bagging operations with commercial benefit.
Owner:SAINT GOBAIN BRUNSWICK TECH +1
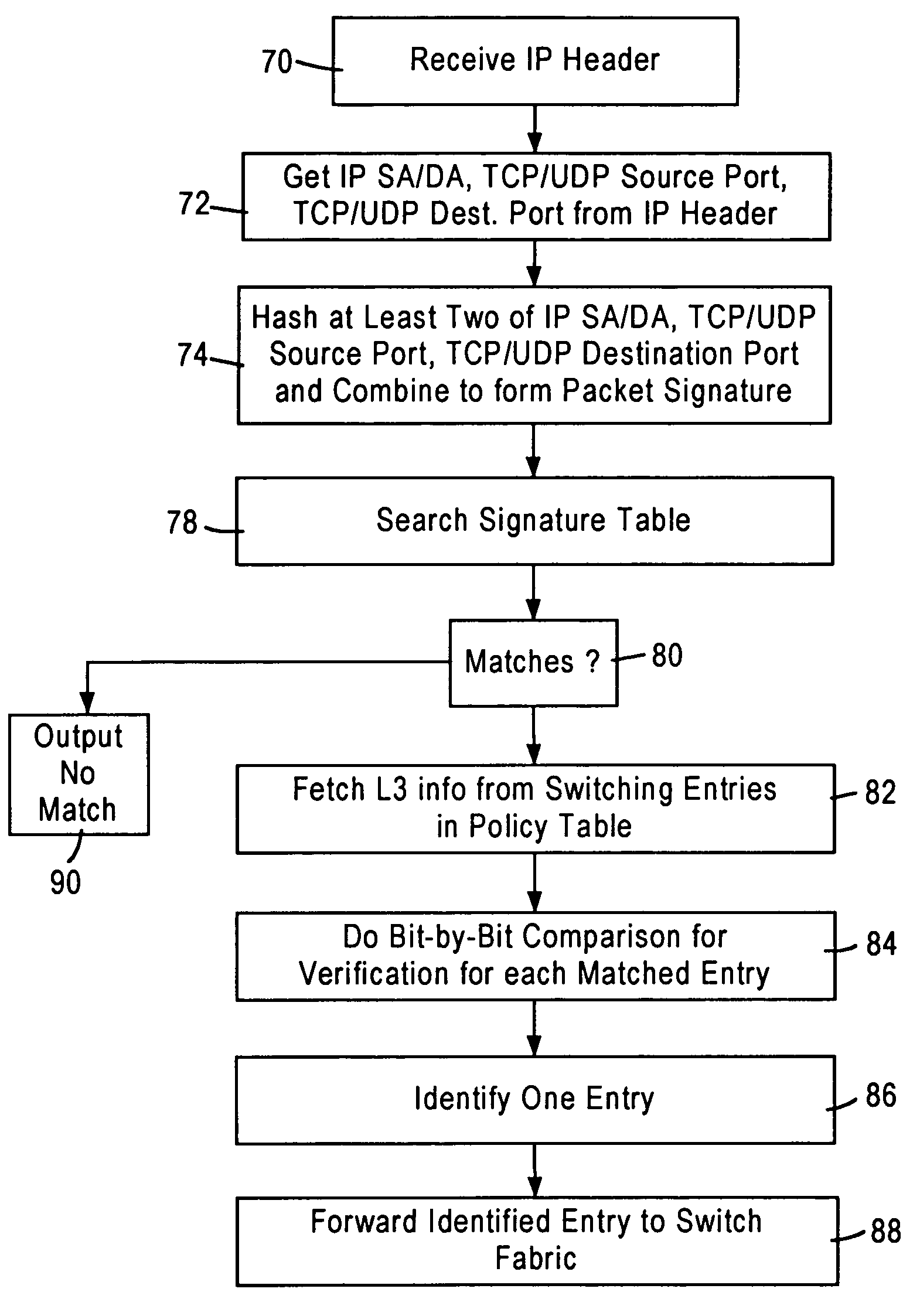
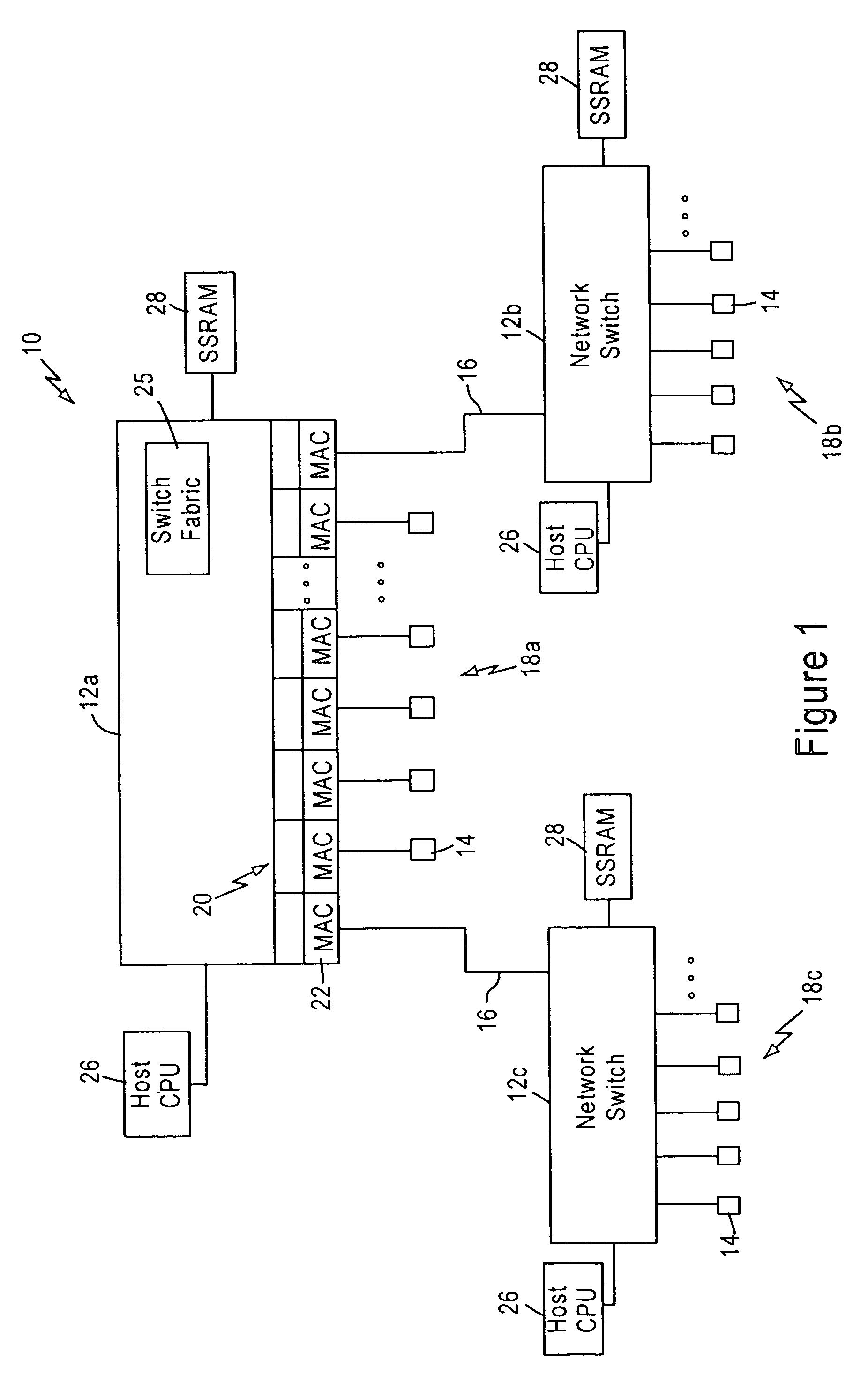
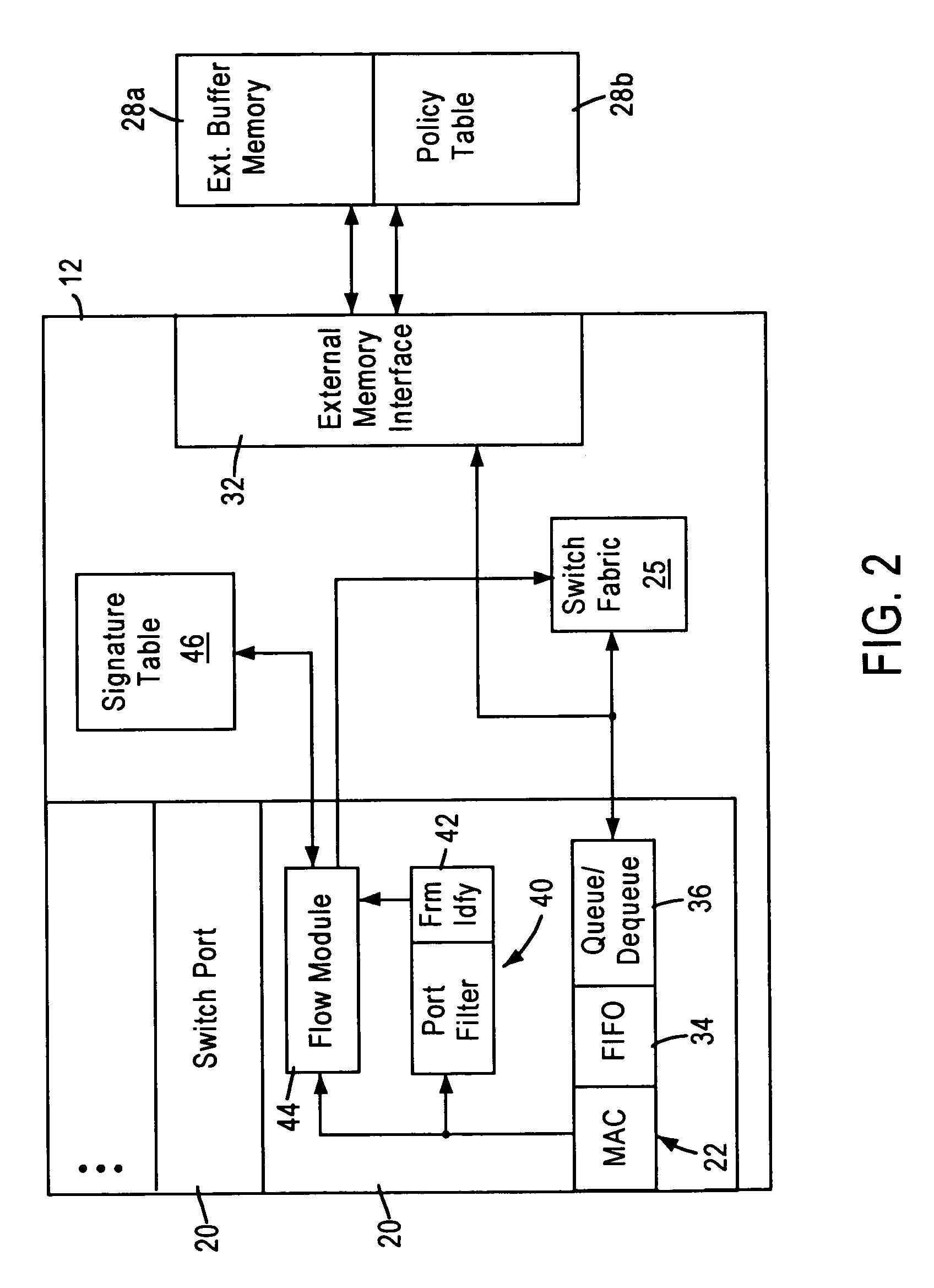
Arrangement for searching packet policies using multi-key hash searches in a network switch
InactiveUS6950434B1Increase speedStay flexibleData switching by path configurationHash functionNetwork packet
A network switch, configured for performing layer 2 and layer 3 switching in an Ethernet (IEEE 802.3) network without blocking of incoming data packets, includes network switch ports, each including a flow module configured for generating a packet signature based on layer 3 information within a received data packet. The flow module generates first and second hash keys according to a prescribed hashing function upon obtaining first and second portions of layer 3 information. The flow module combines the first and second hash keys to form the packet signature, and searches an on-chip signature table that indexes addresses of layer 3 switching entries by entry signatures, where the entry signatures are generated using the same prescribed hashing function on the first and second layer 3 portions of the layer 3 switching entries.
Owner:GLOBALFOUNDRIES INC
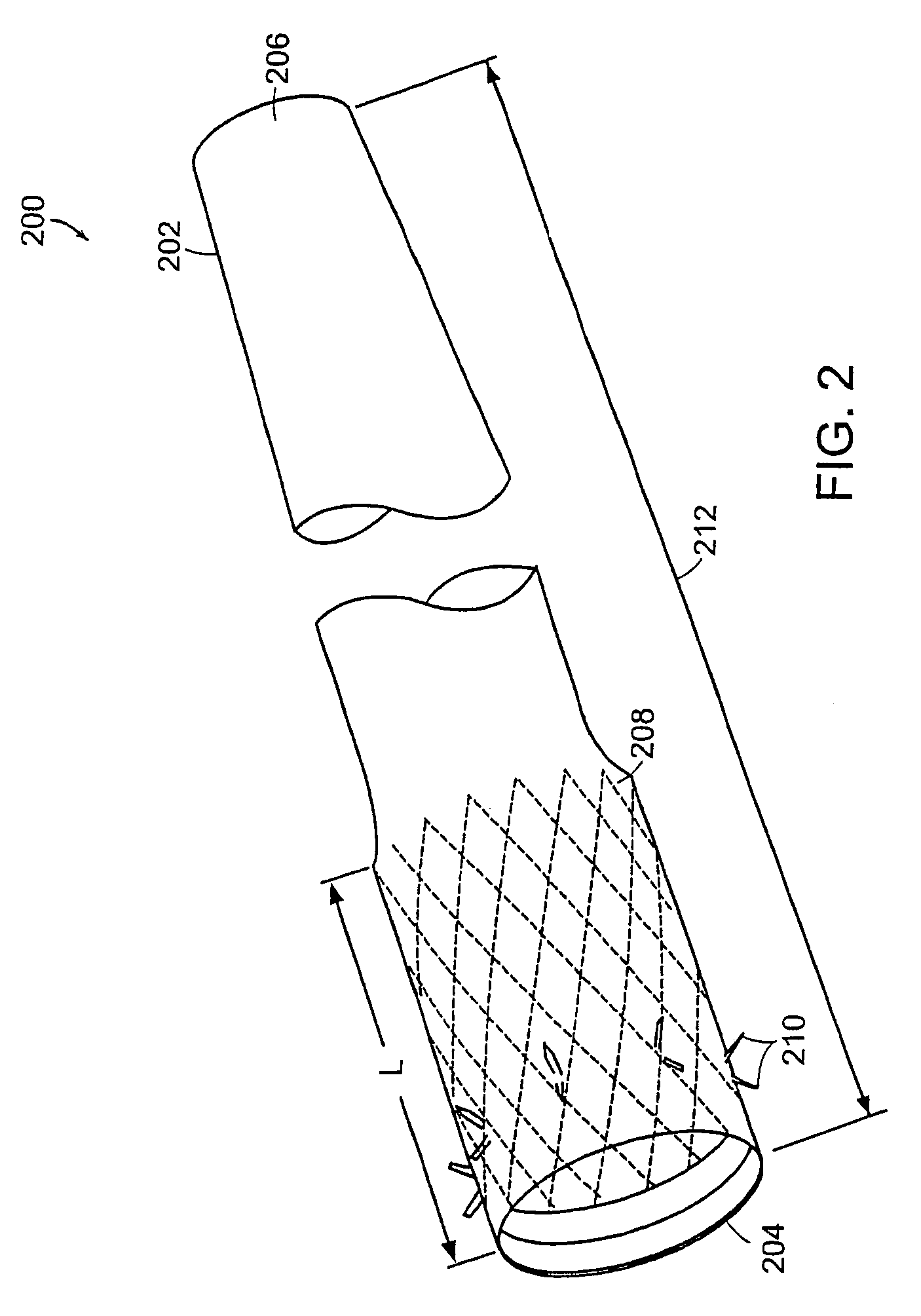
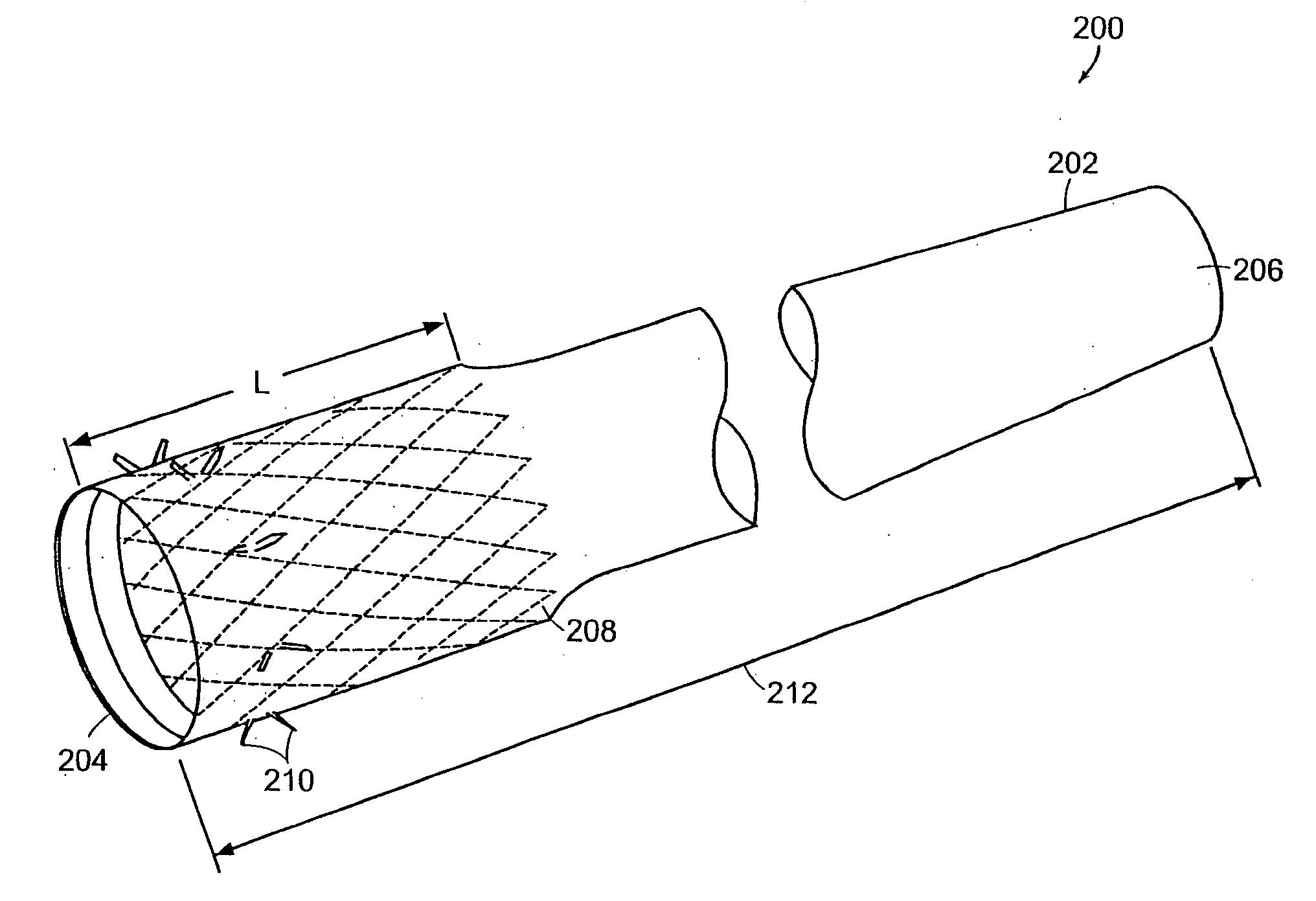
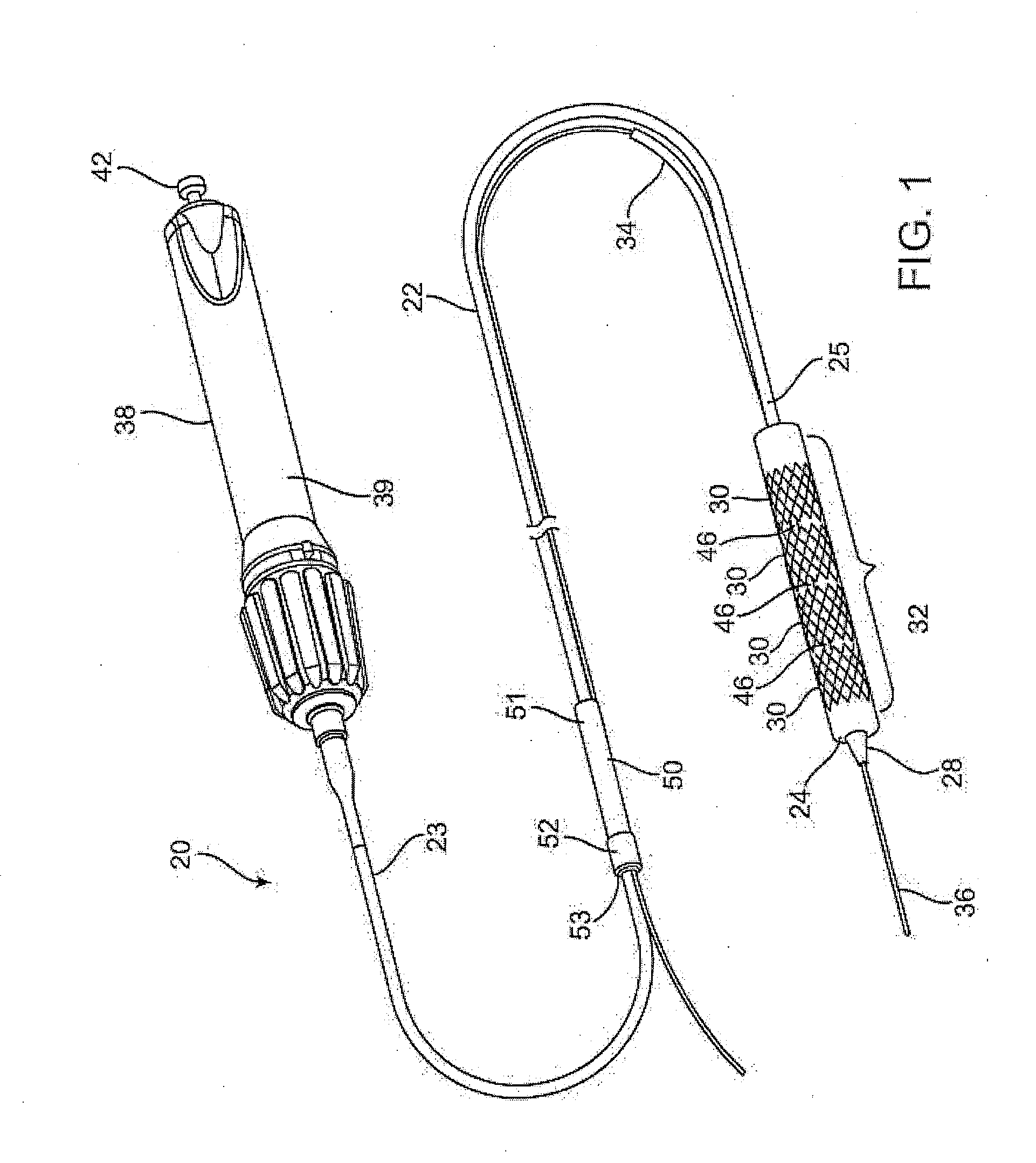
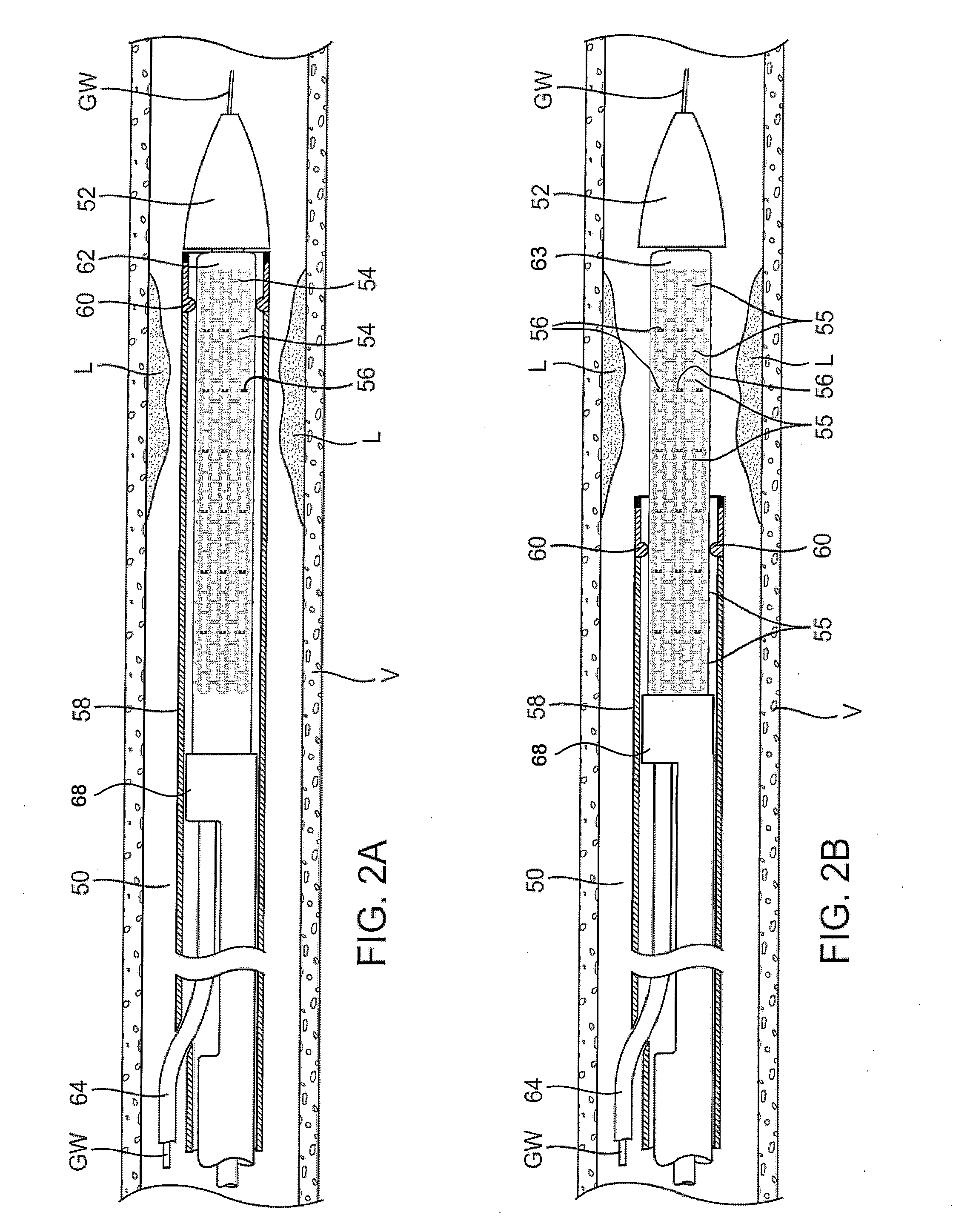
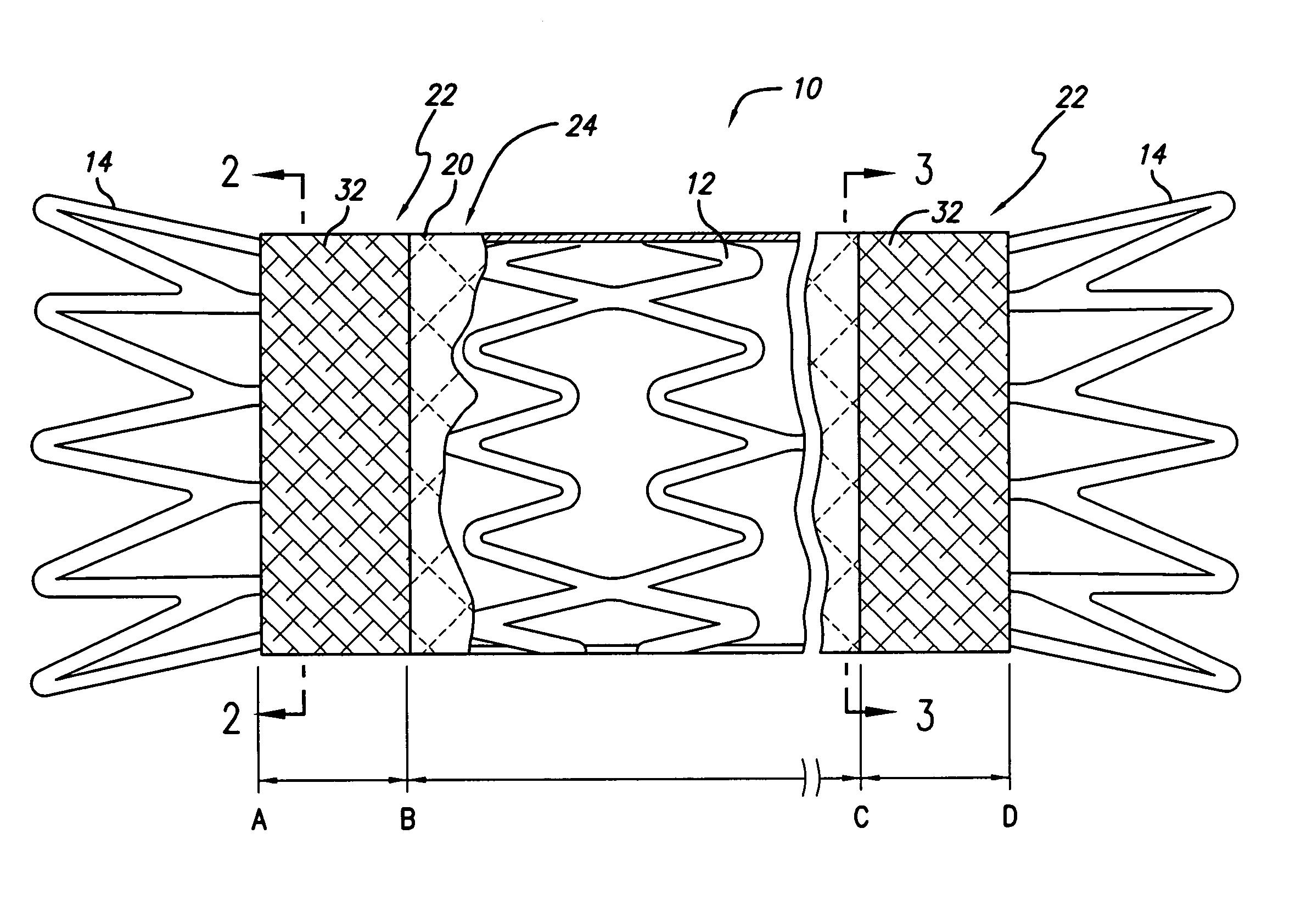
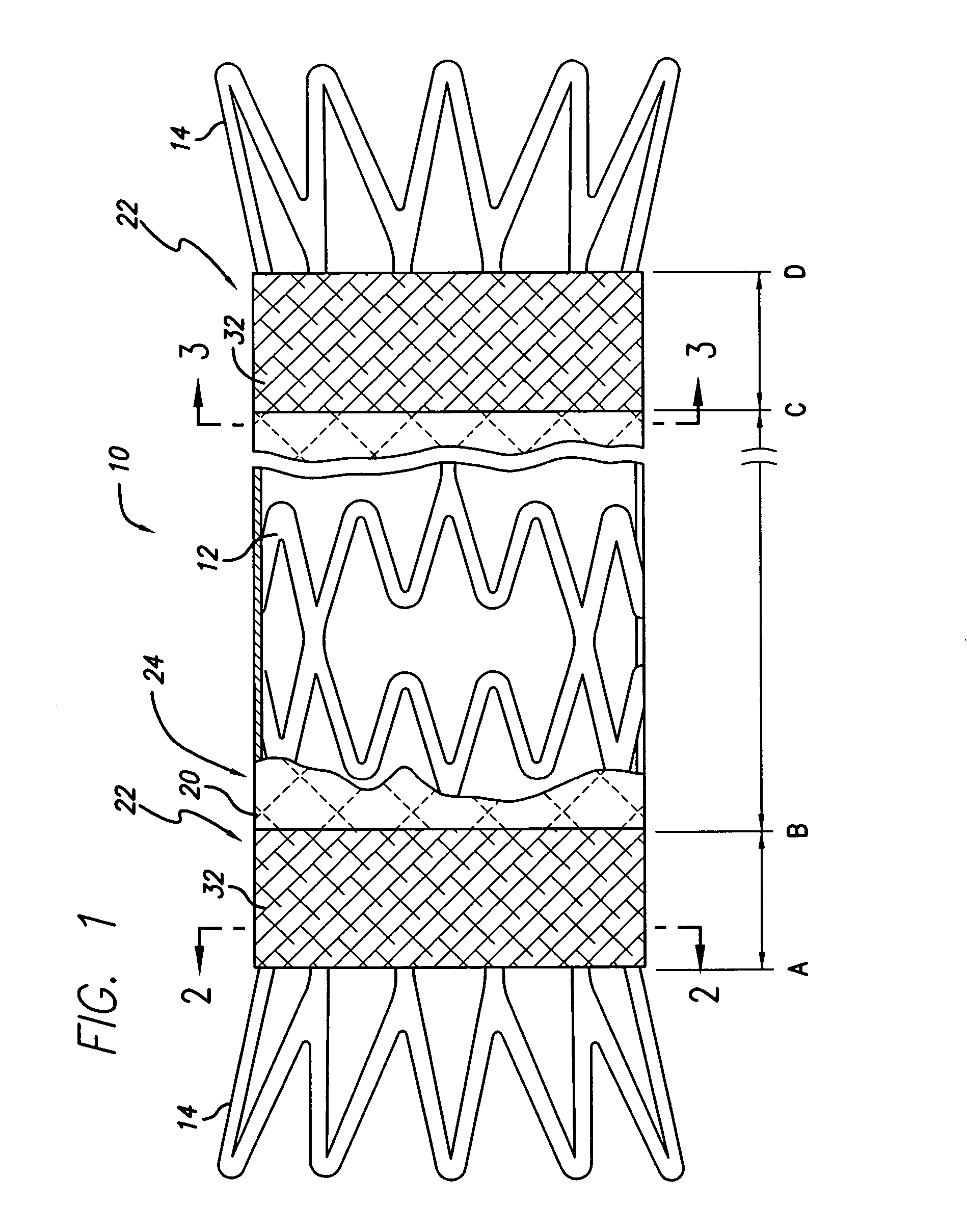
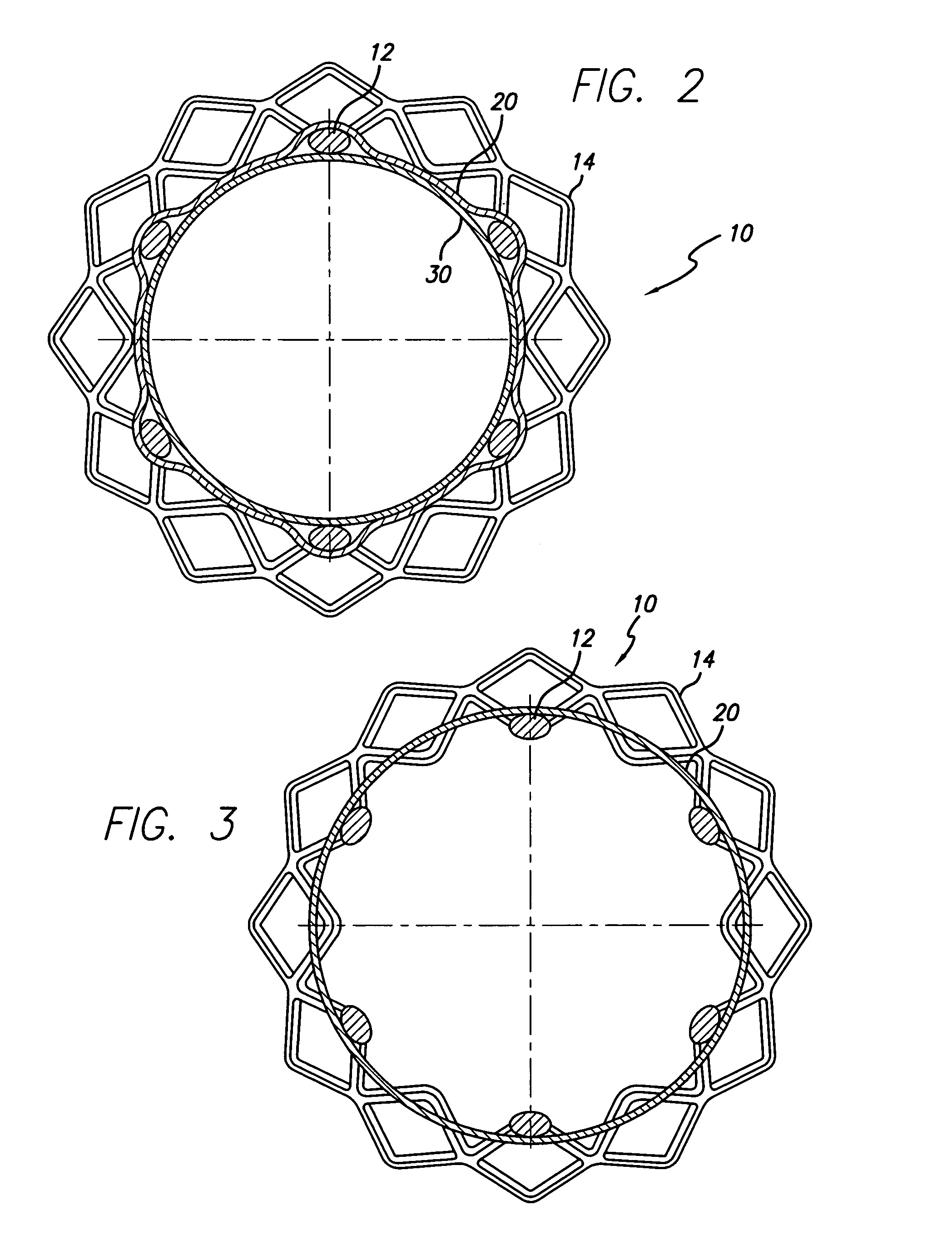
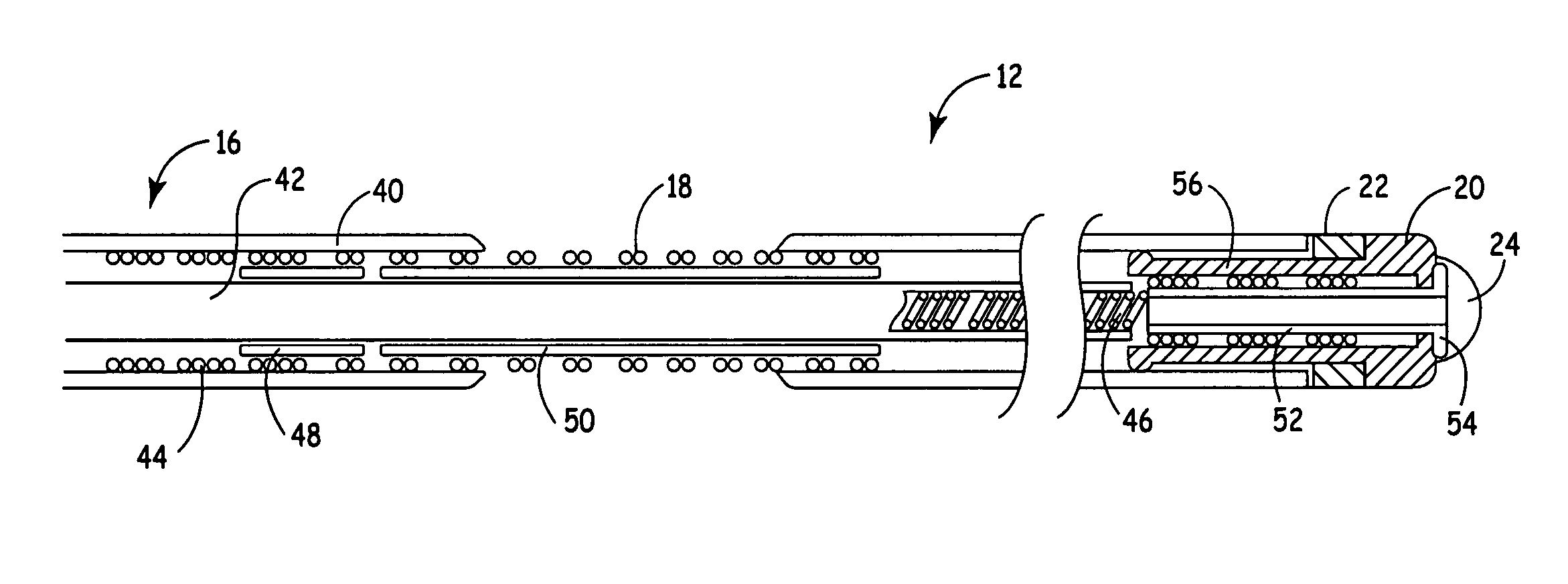
Apparatus and methods for interlocking stent segments
Apparatus and methods for interlocking stent segments which provide for a secure engagement between the expanded stent segments are described herein. Stent segments which are able to slide freely relative to one another along the deployment catheter prior to expansion may be secured to one another when expanded and / or deployed into the vessel. Securement upon expansion of the stent segments may be accomplished, in part, by utilizing one or more coupling mechanisms between adjacent stent segments which securely interlock the segments to one another by taking advantage of the changing geometry of the stents during expansion.
Owner:XTENT INC
Covered stent with encapsulated ends
InactiveUS7083640B2Increase flexibilityFlexibility of stent is retainedStentsSurgeryInsertion stentCovered stent
A flexible covered stent having a stent covered on a first surface by a first layer of biocompatible material and on a second surface by both a second and third layer of biocompatible material, the first and second layers and the first and third layers of biocompatible material being bonded to one another through openings in a wall in the stent. The first layer of biocompatible material is longer than both the second and third layers of biocompatible material such that at least a portion of the second surface of the stent is not covered by either second or third layer, imparting flexibility to the stent.
Owner:CR BARD INC
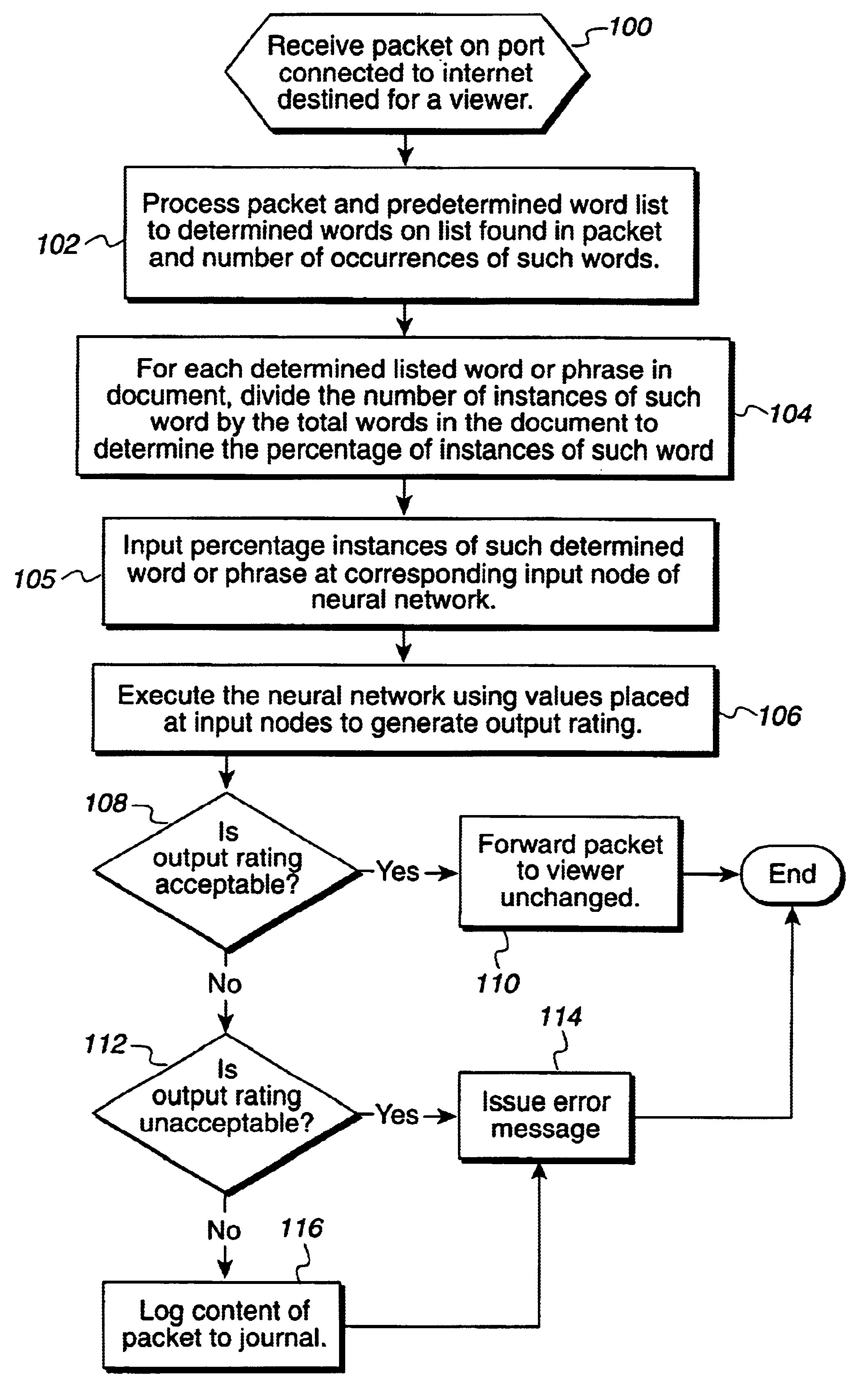
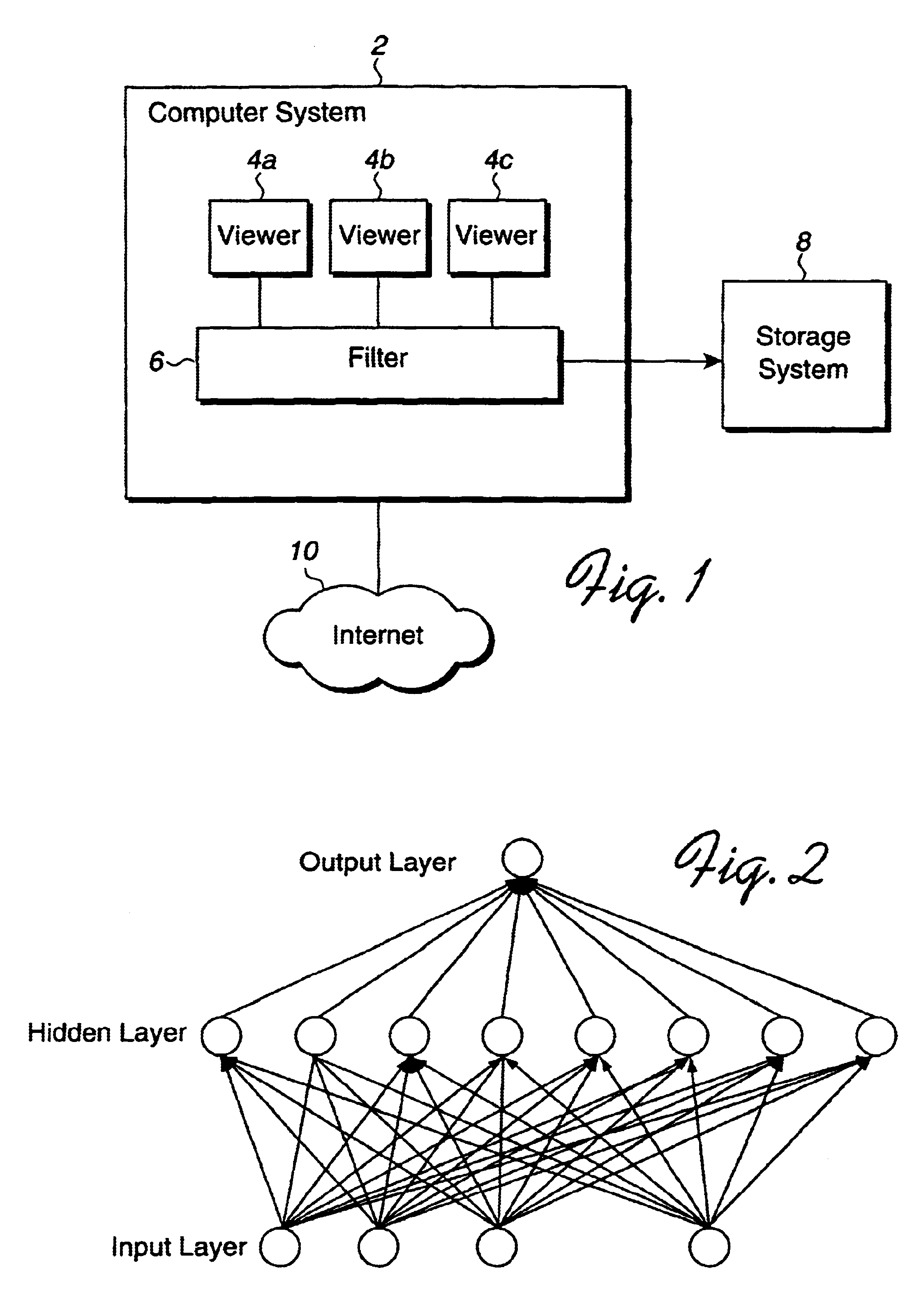
Method, system, and program for filtering content using neural networks
InactiveUS6633855B1Low costStay flexibleDigital data information retrievalDigital computer detailsProgramming languageData objects
Disclosed is a method, system, and program for filtering a data object for content deemed unacceptable by a user. A data object requested by a viewer program is received. The data object is processed to determine predefined language statements. Information on the determined language statements is inputted into a neural network to produce an output value. A determination is then made as to whether the output value indicates that the data object is unacceptable. Viewer program access to the data object is inhibited upon determining that the data object is unacceptable.
Owner:IBM CORP
Method and apparatus for controlling scanning of mosaic sensor array
InactiveUS20050057284A1Quick configurationMinimize powerUltrasonic/sonic/infrasonic diagnosticsElectronic switchingSensor arrayUltrasonic sensor
A scanning architecture that makes it possible to update only those ultrasonic transducer subelements of a mosaic transducer array that change from view to view. The configuration of the switch matrix is fully programmable. The switch matrix includes access switches that connect subelements to bus lines and matrix switches that connect subelements to subelements. Each subelement has a unit switch cell associated therewith, each unit switch cell comprising at least one access switch, at least one matrix switch, and addressing and control logic. Optionally, each unit switch cell also includes latches for storing the future switch states of the switches to be programmed. The switches themselves have memory for storing their current switch states.
Owner:GENERAL ELECTRIC CO
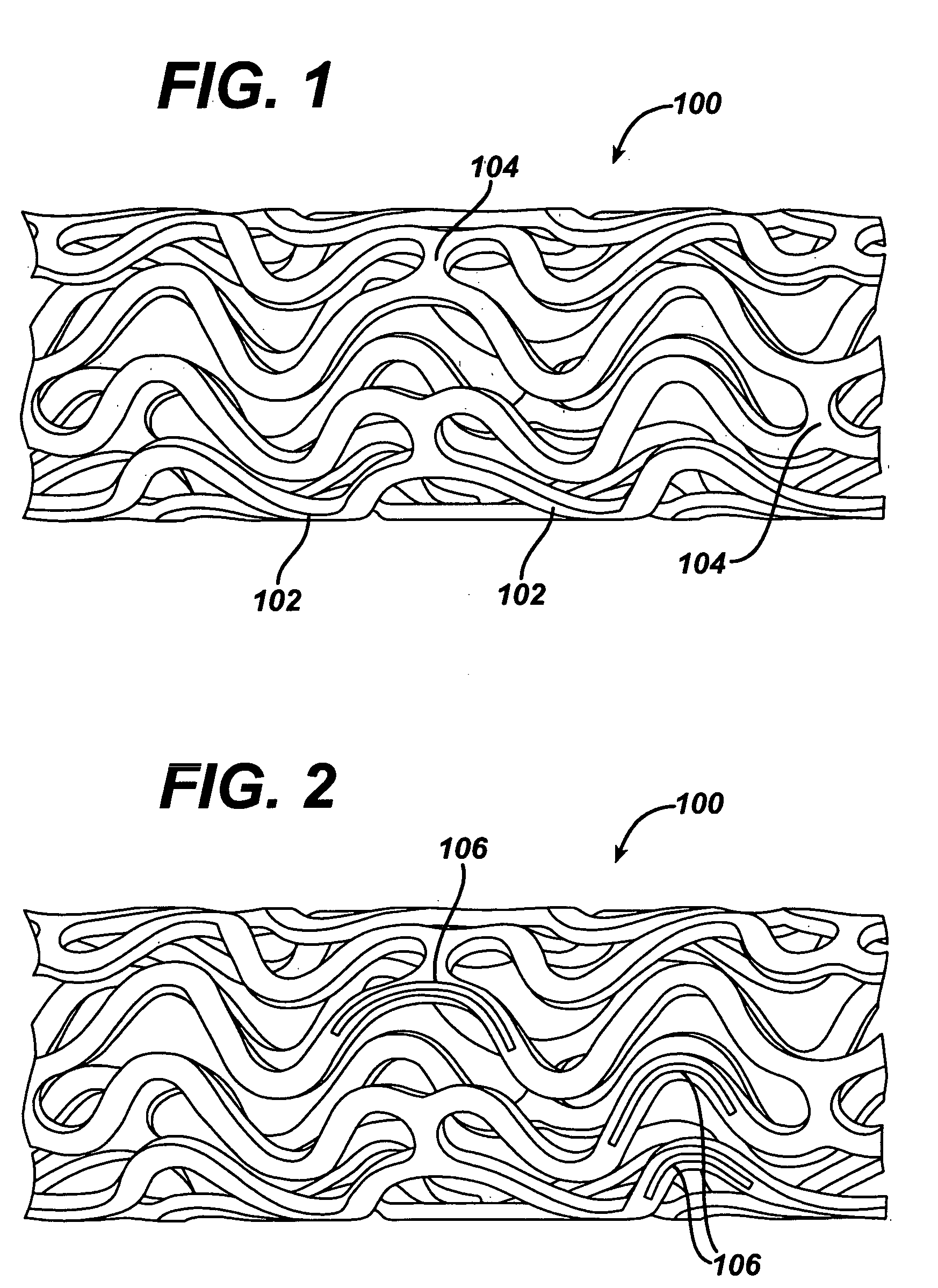
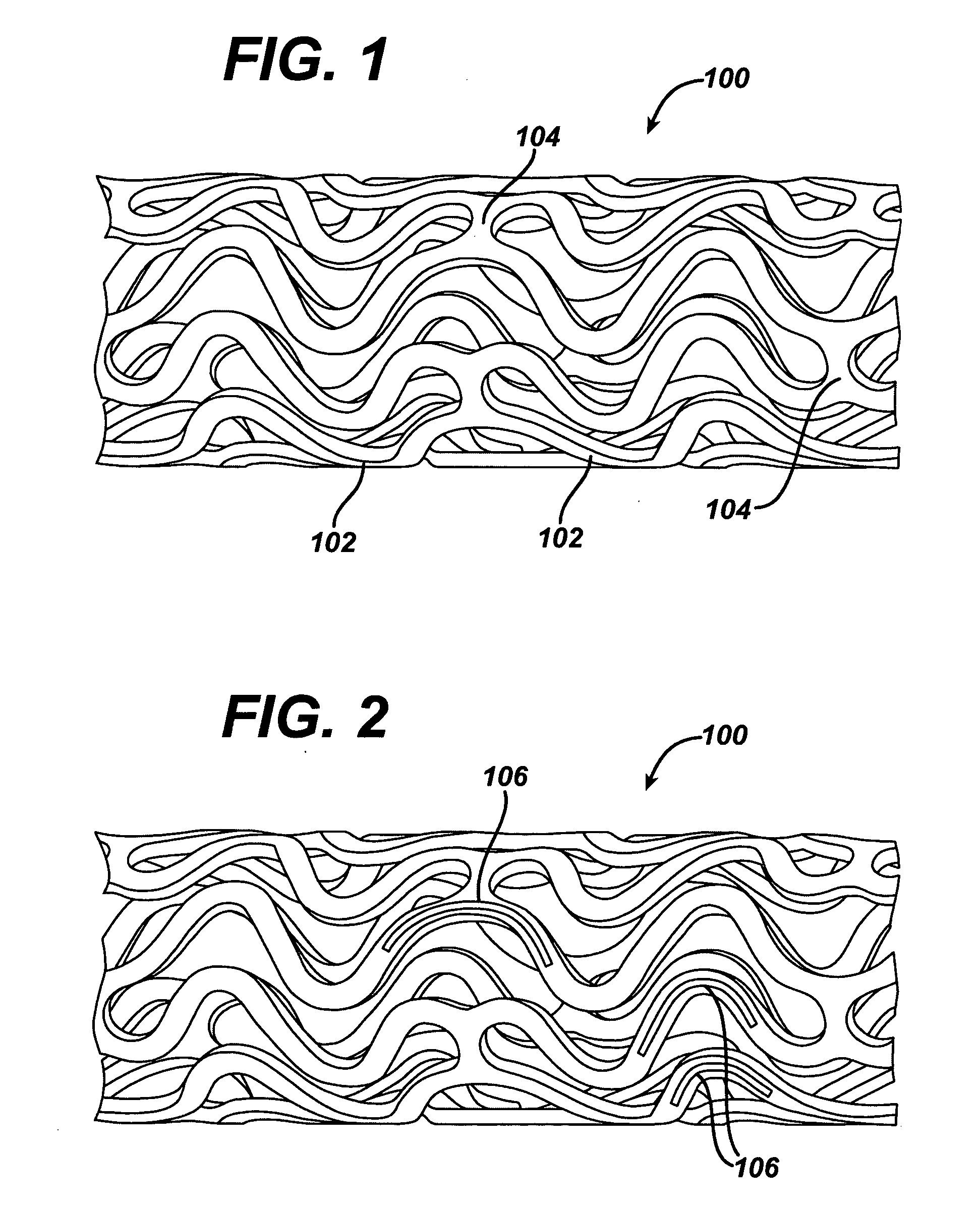
Polymeric stent and method of manufacture
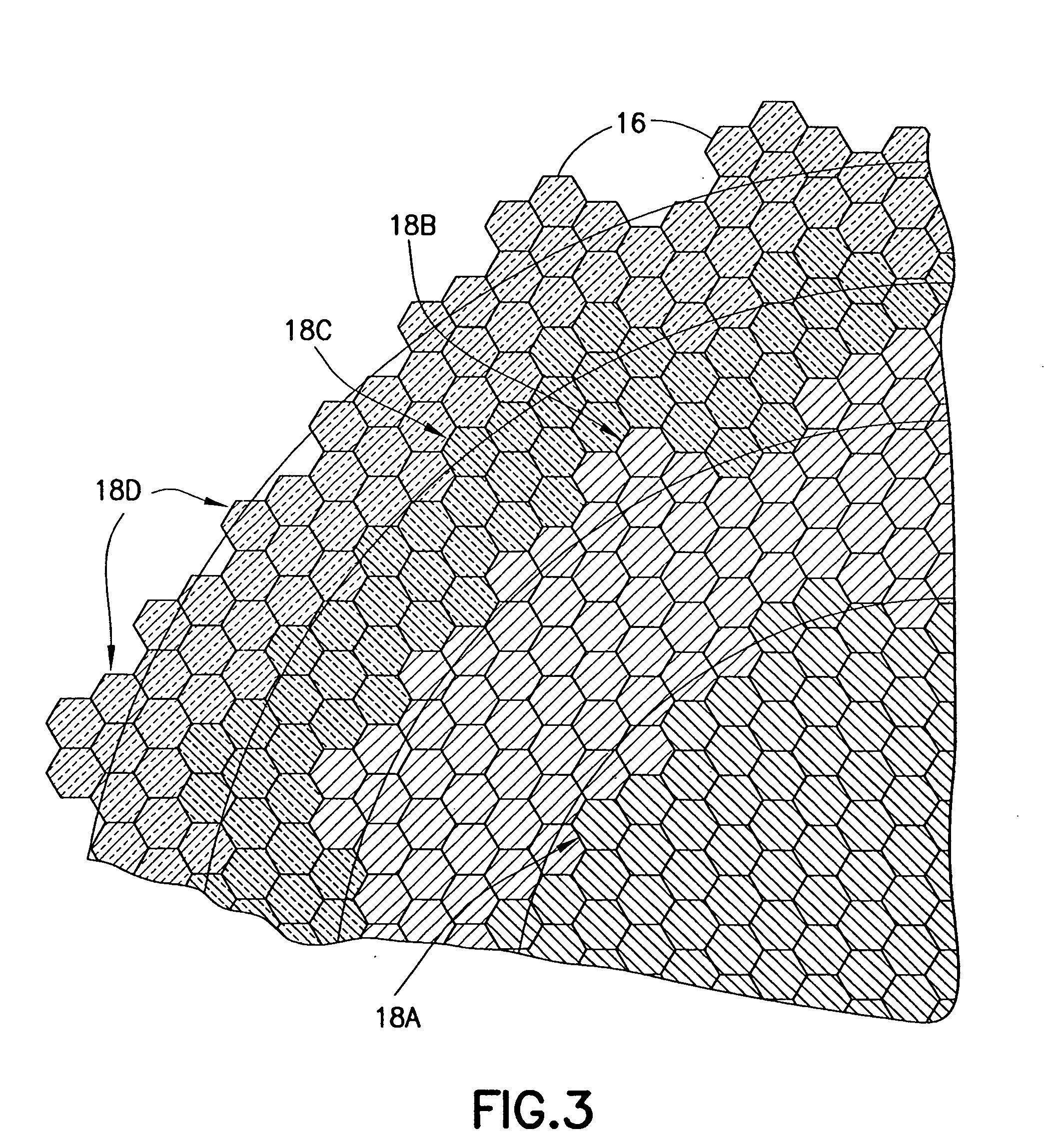
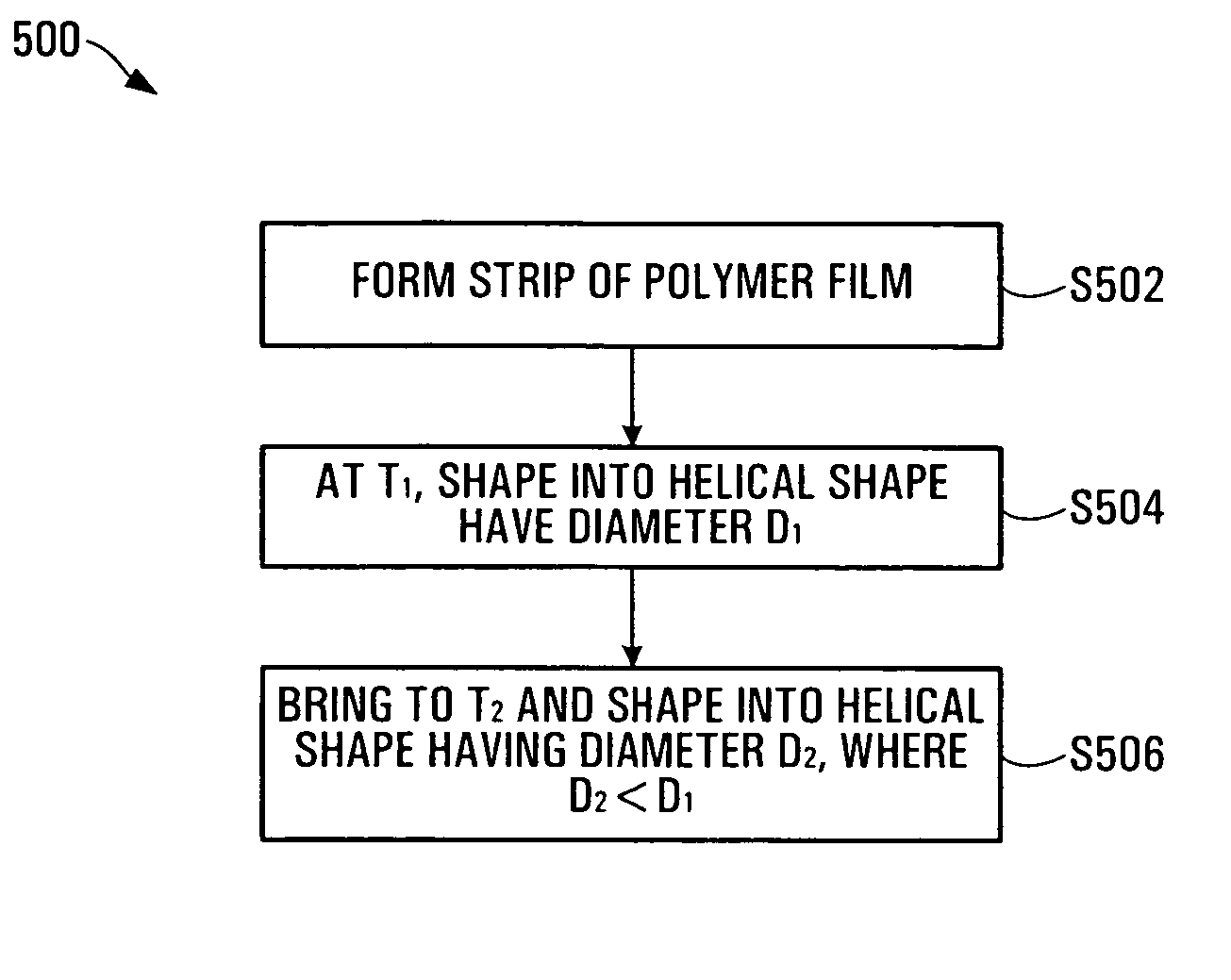
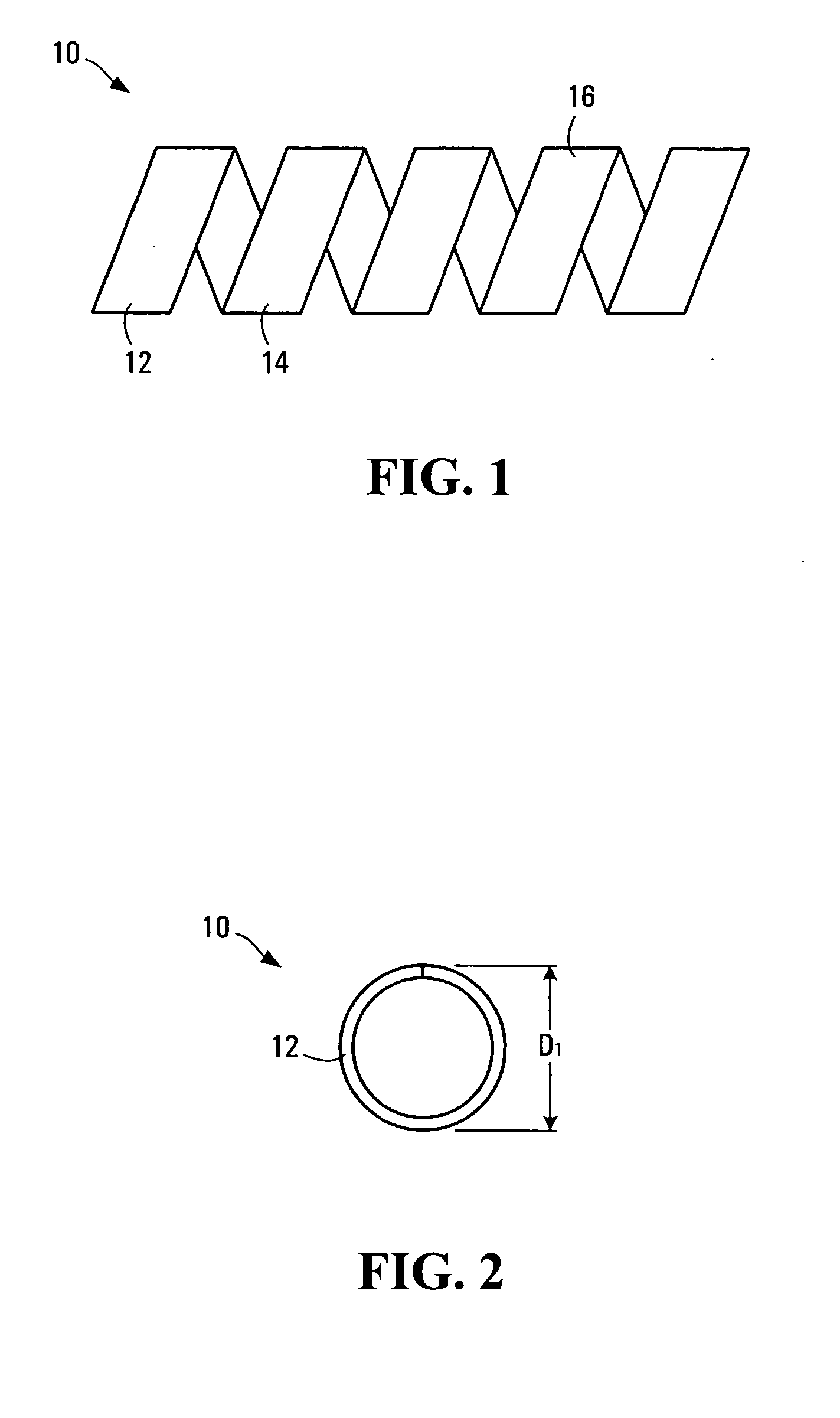
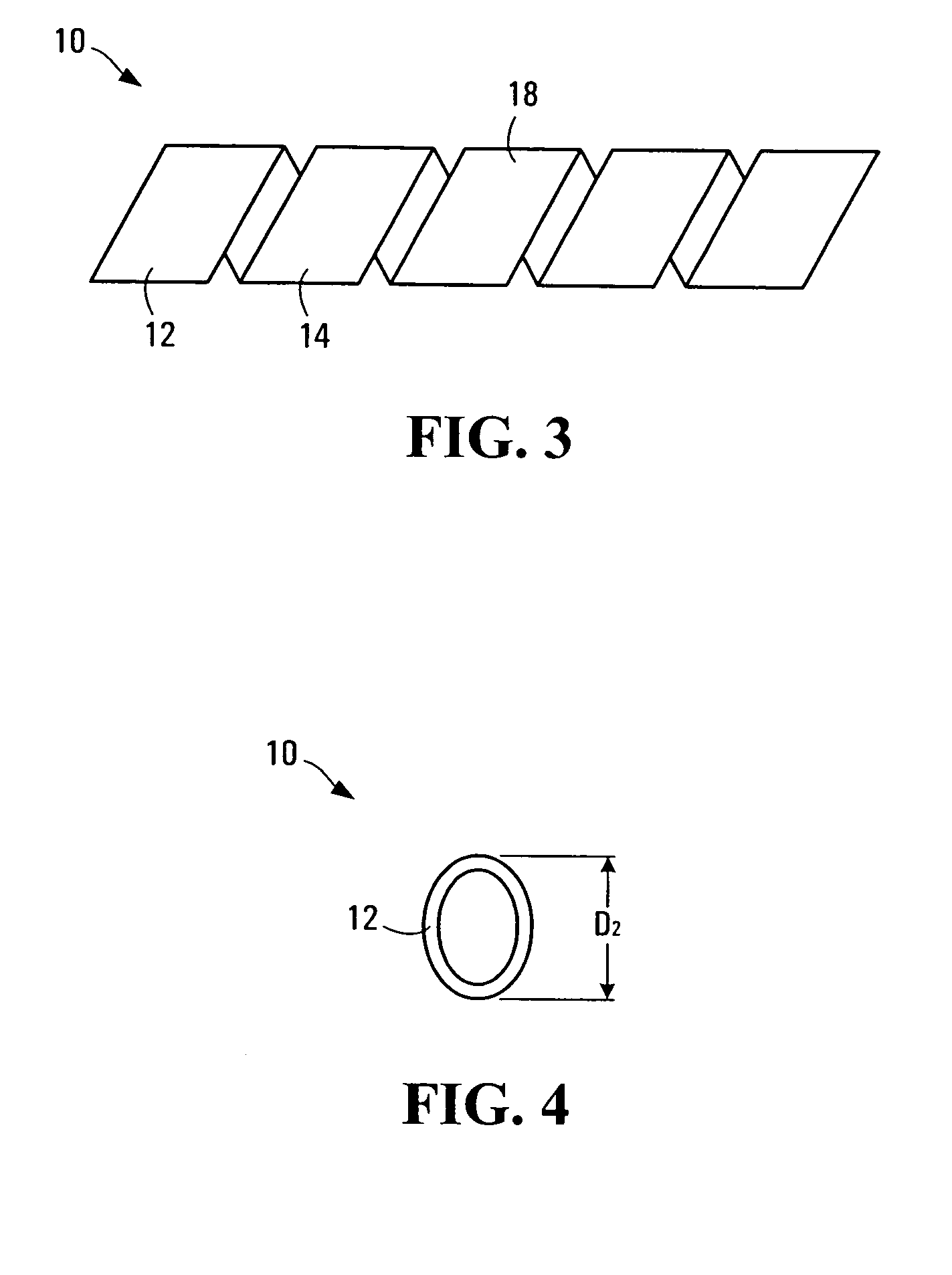
InactiveUS20050021131A1Stay flexiblePowerfulStentsHollow filament manufactureInsertion stentBiomedical engineering
A stent formed of polymeric material, useful for the expansion of a lumen and the delivery of one or more therapeutic agents in situ is disclosed. The stent may be multi-layered, and may change shape at a state transition temperature governed by the materials forming the layers. Methods of use and manufacture are also disclosed.
Owner:NANYANG TECH UNIV
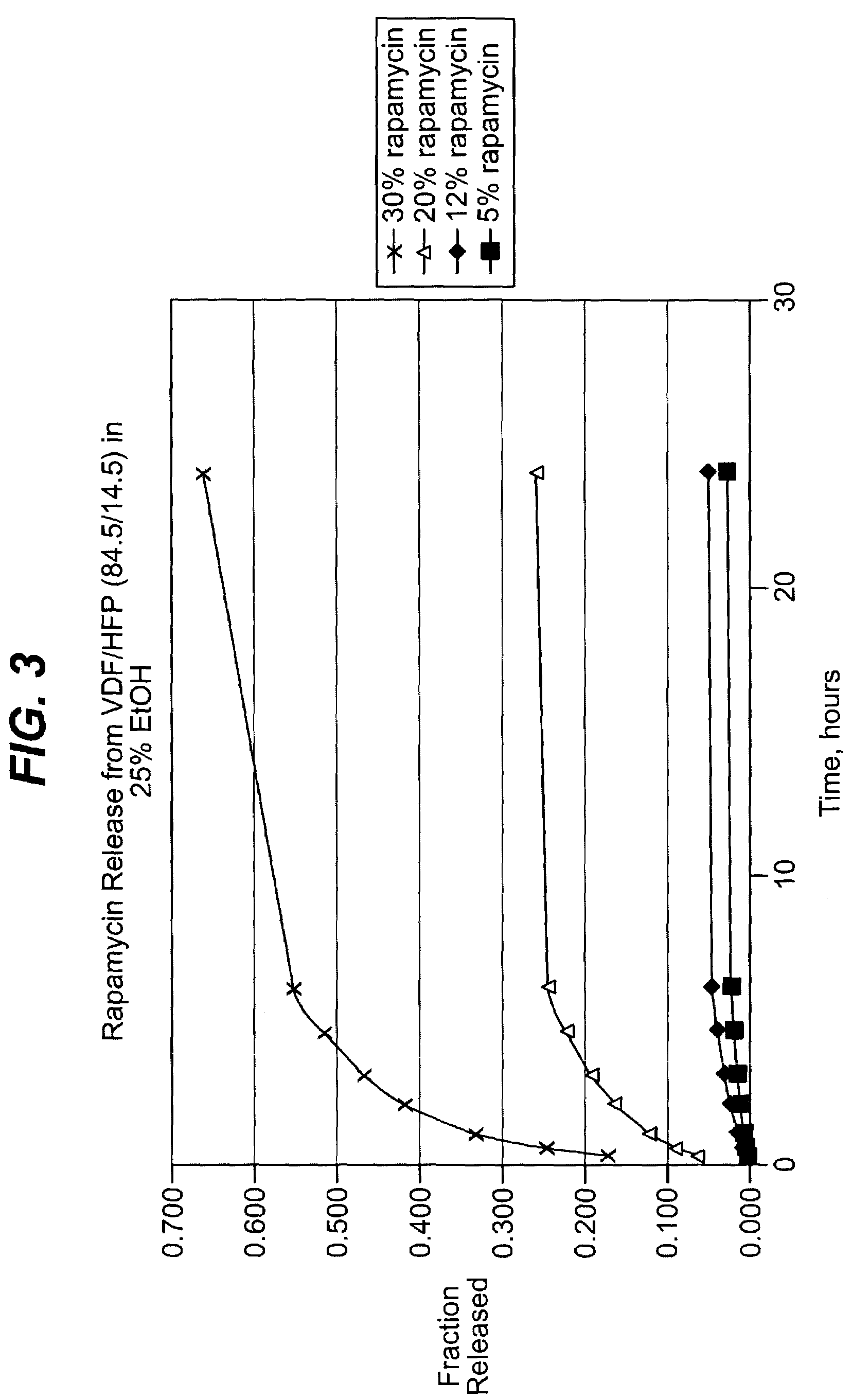
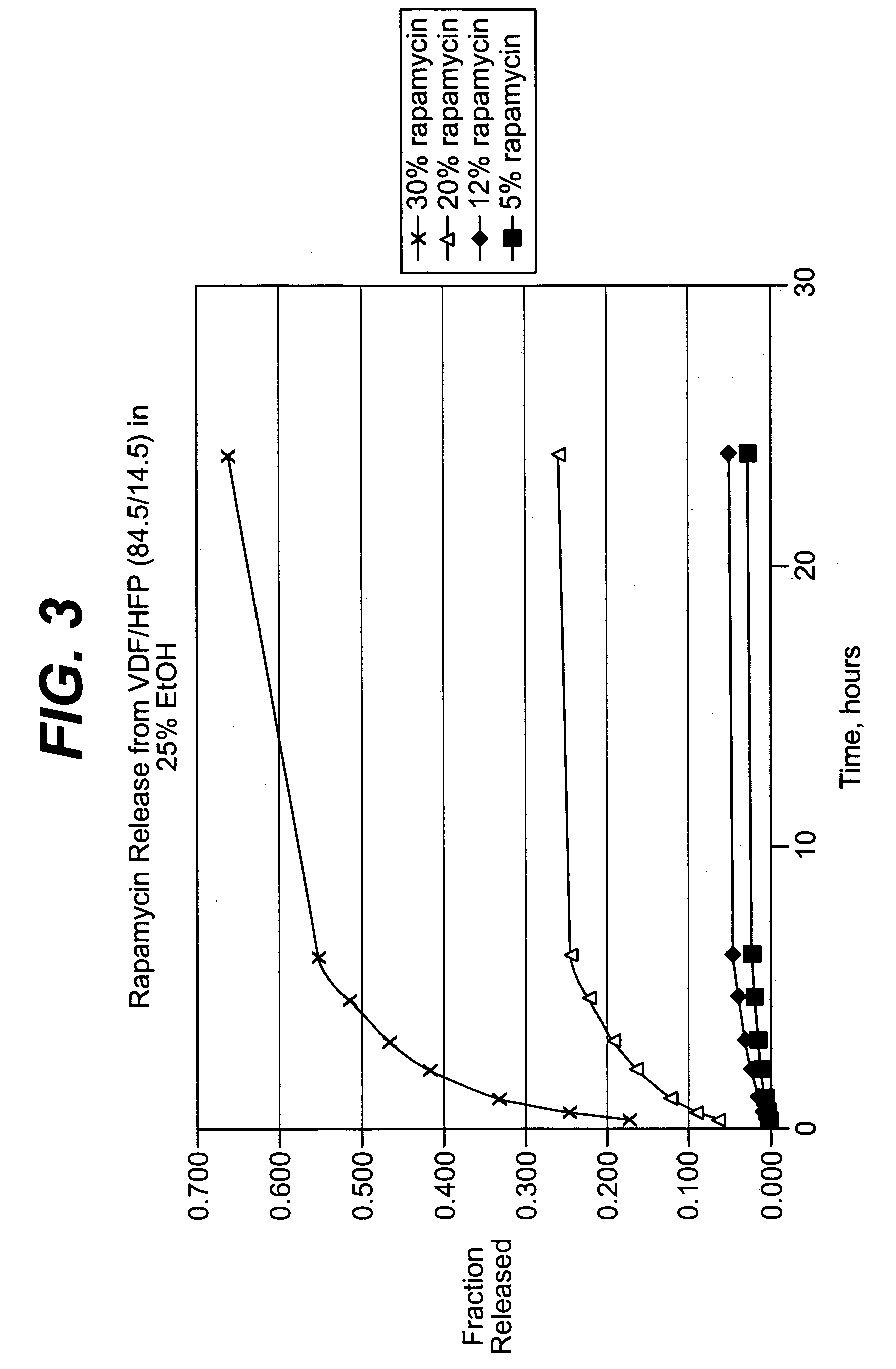
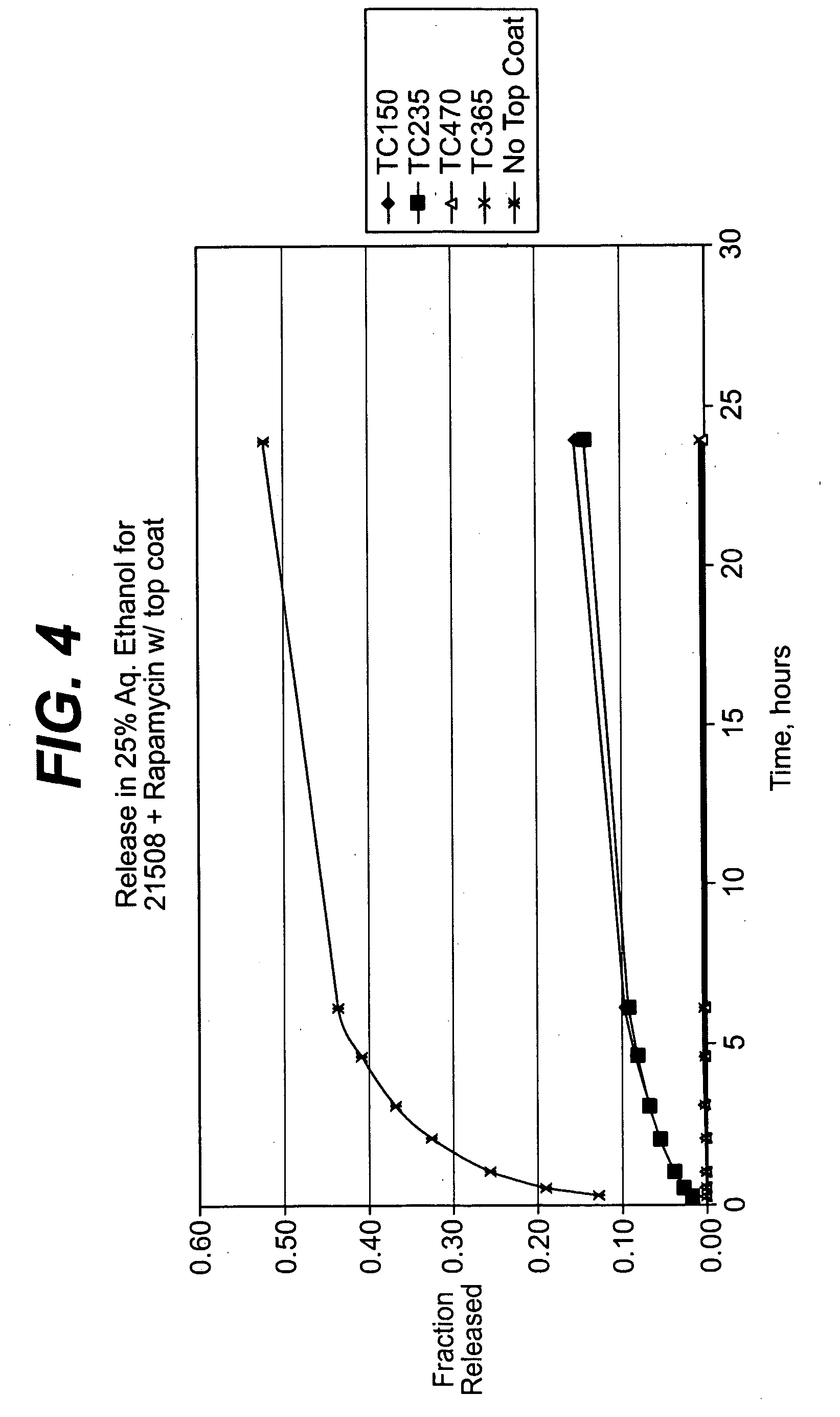
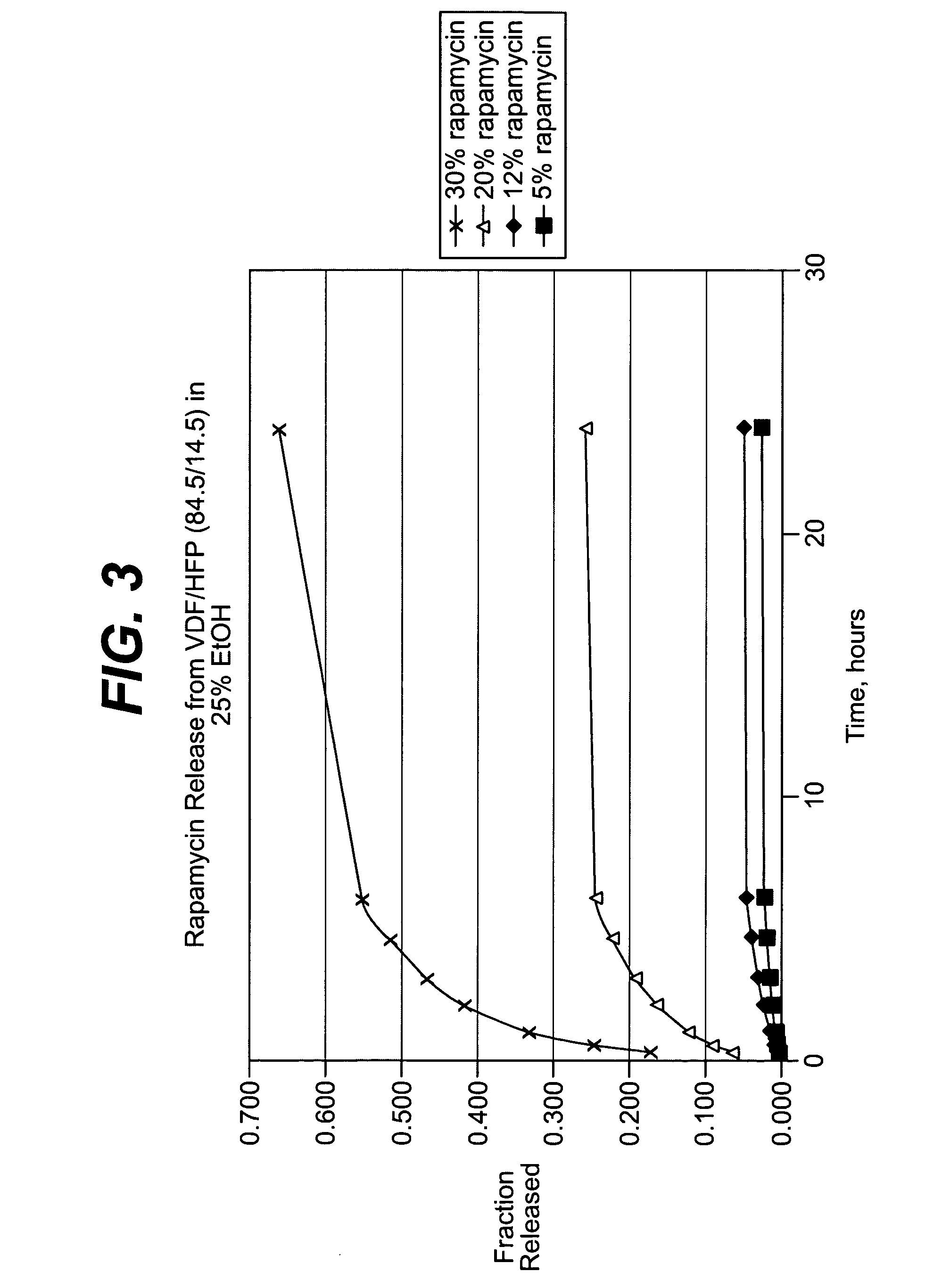
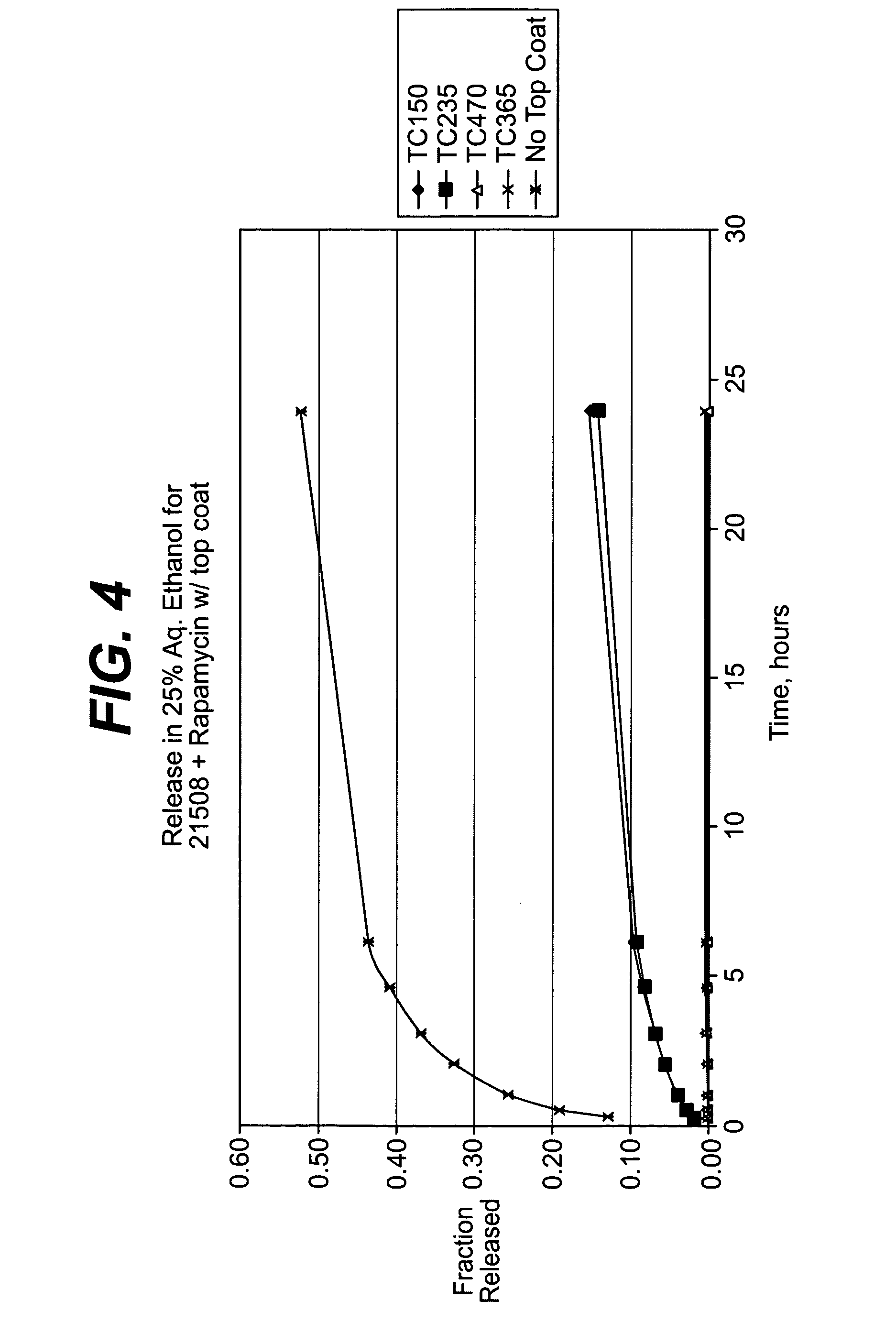
Solution formulations of sirolimus and its analogs for CAD treatment
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Semiconductor device and method of manufacturing the same
InactiveUS7456091B2Speed up preparationReduce loadSemiconductor/solid-state device detailsSolid-state devicesBreaking strengthUltimate tensile strength
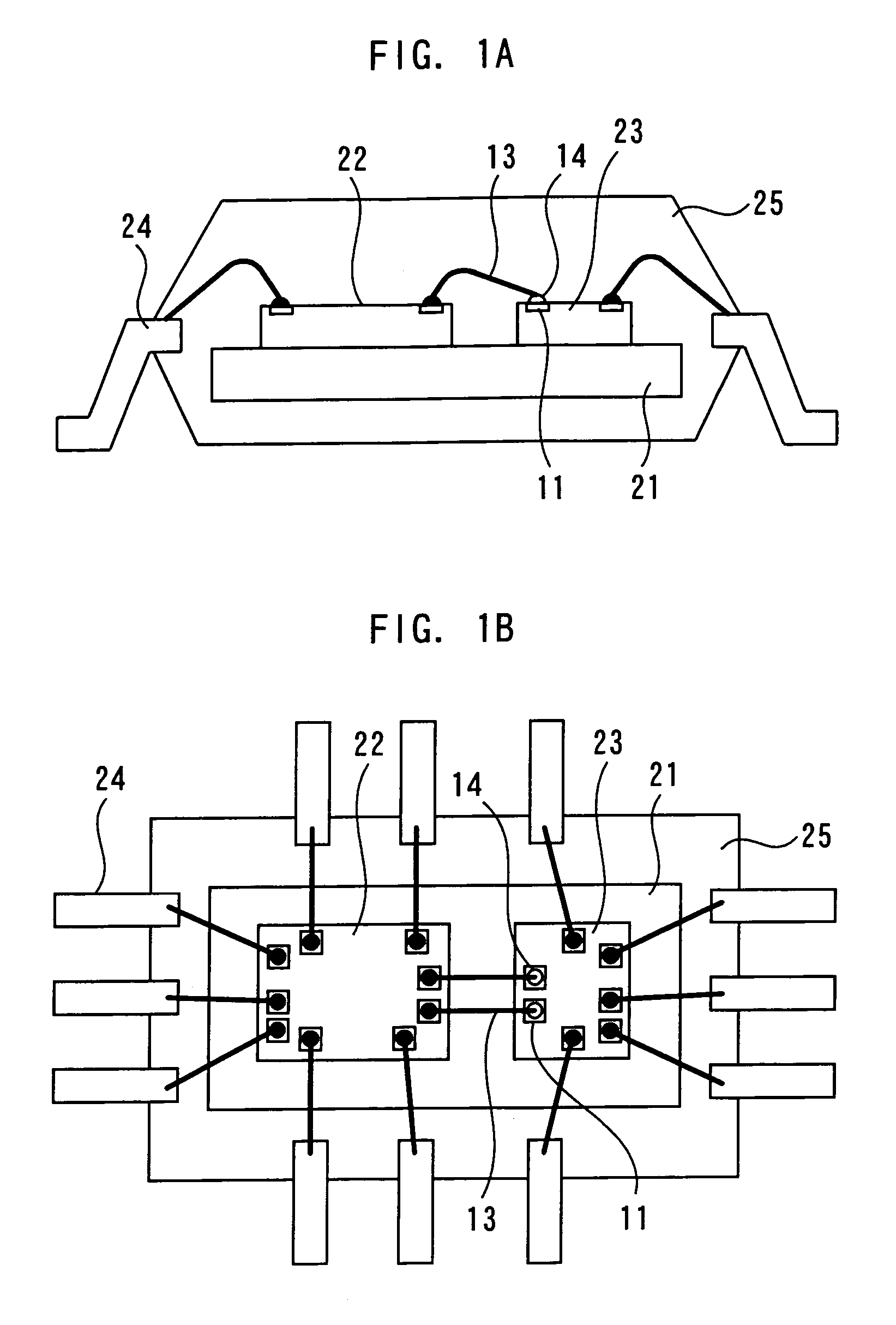
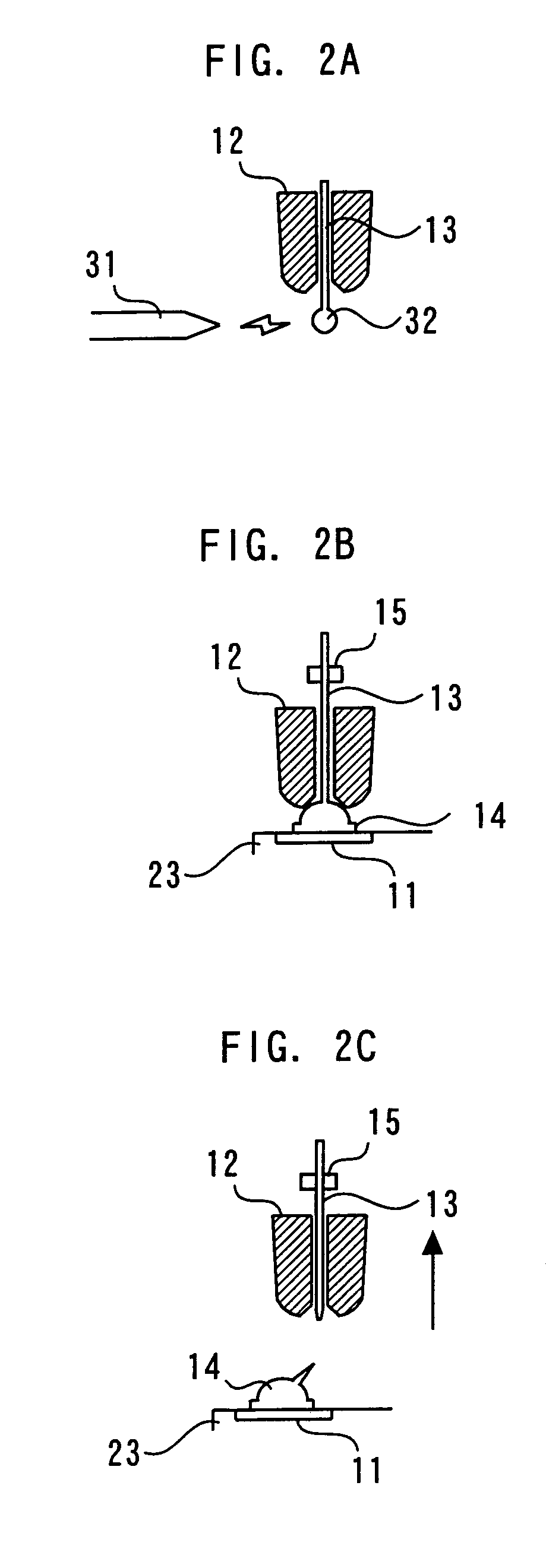
A semiconductor device of the present invention includes a chip which has a pad; a bump electrode formed on the pad; and a wire whose stitch bonding is made on the bump electrode. The wire satisfies a condition: (modulus-of-elasticity / breaking strength per unit area) ≧400.
Owner:RENESAS ELECTRONICS CORP
Communications network
InactiveUS6886043B1Guaranteed service qualityMinimise control of networkMultiple digital computer combinationsNetworks interconnectionQuality of serviceCombined use
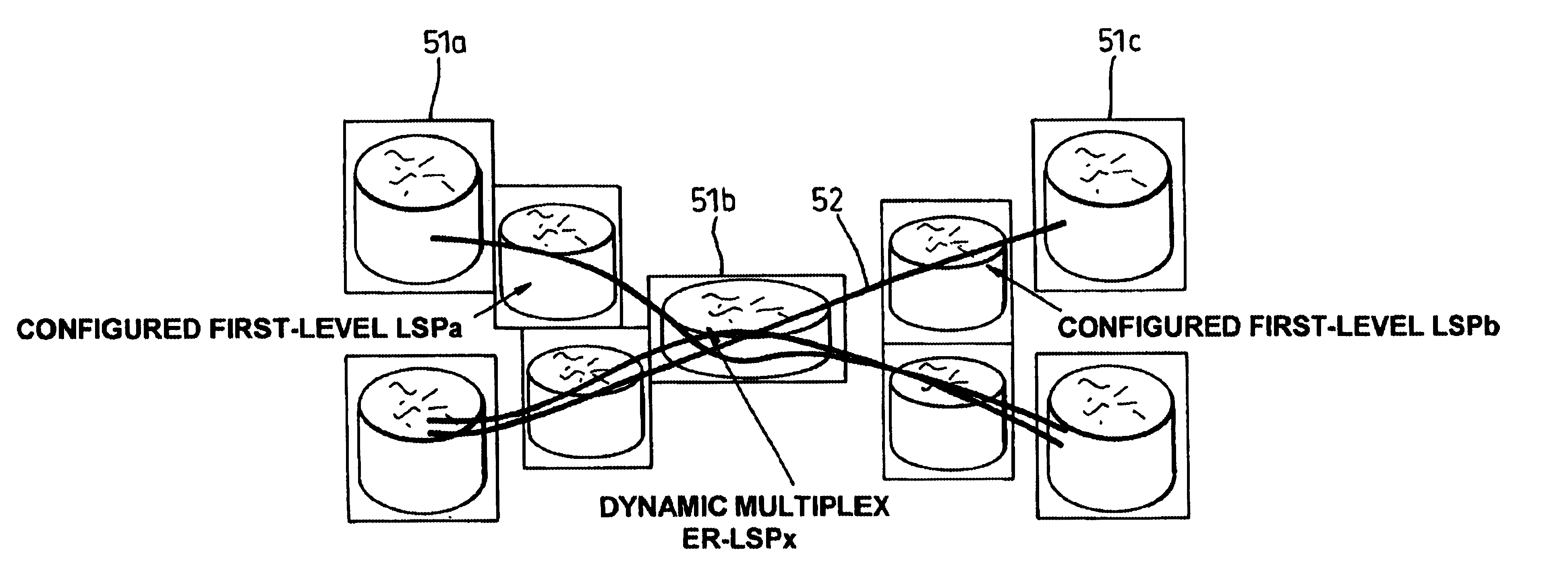
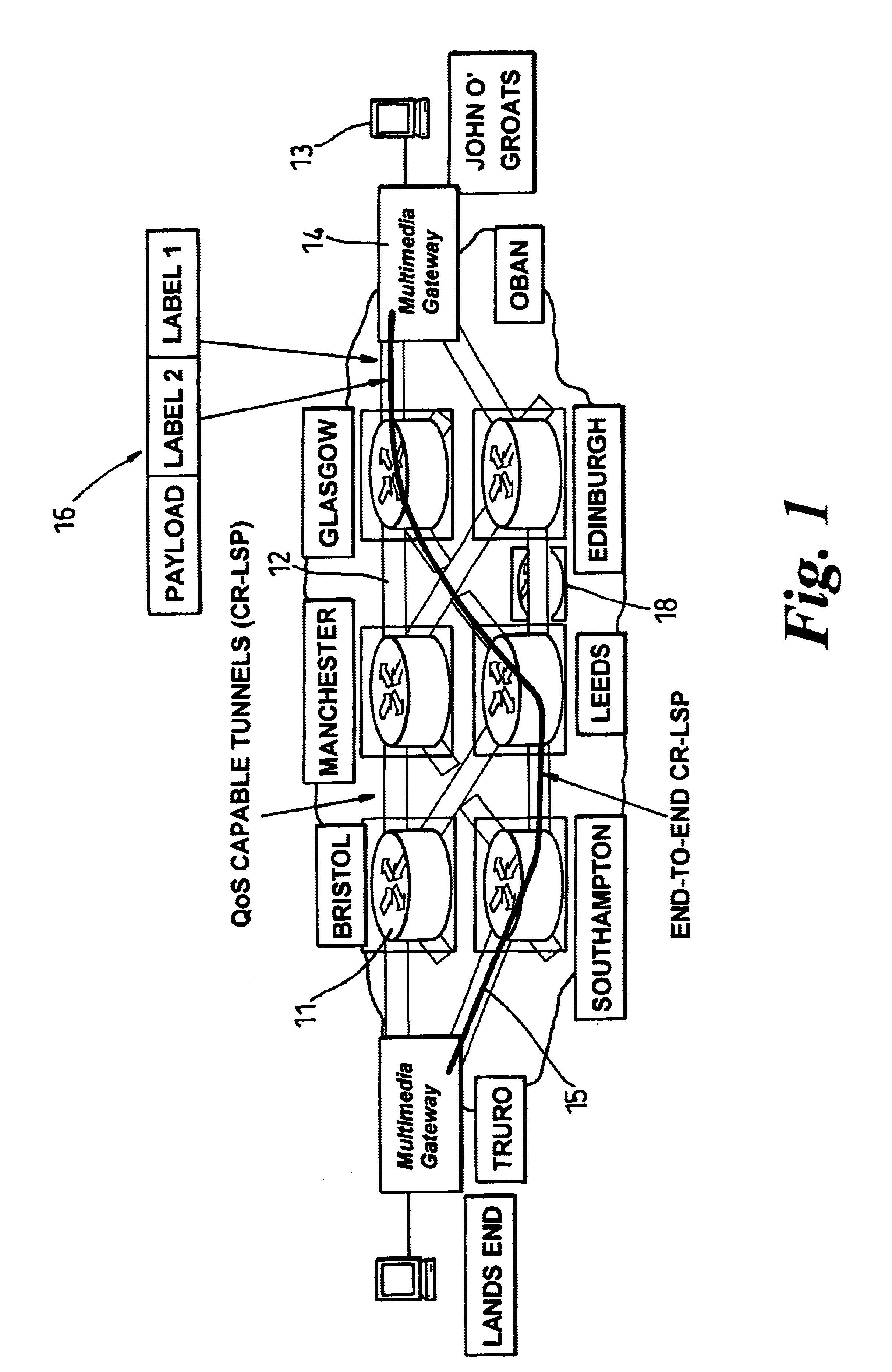
In a label switch communications network, a third level of label is employed in conjunction with a dynamic multiplex constraint-based routed label switched path (CR-LSP) in order to achieve implicit switching at nodes within the network. Implicit switching occurs when a switching function takes place at a node without the need for any control function being required at that node. A three-layer label stack provided at the edge of the network achieves end-to-end connection oriented behaviour with guaranteed Quality of Service. The use of the three-layer label stack concentrates the control of the network to the two edges of the network and a single central switching stage of the network.
Owner:GENBAND US LLC
Touch screen shield
ActiveUS20120188743A1Reduce scratchesPromote formationMechanical working/deformationMagnetic/electric field screeningEngineeringTouchscreen
A shield that is attachable to a touch sensitive screen is disclosed. The shield may be attached to the touch sensitive screen only at its outer peripheral portion. An air gap is enclosed between the shield and the touch sensitive screen to form a planar air bearing. The shield preferably does not touch the active area of the touch sensitive screen when the user is not touching the shield but only viewing the touch sensitive screen through the shield. This mitigates unwanted optical artifacts such as trapped air bubbles, Newton rings and chromatic interference while maintaining the sensitivity of the touch sensitive screen.
Owner:RACING OPTICS
Injectable formulations of taxanes for cad treatment
InactiveUS20050272806A1Minimize potential risk of damageReduce frictionBiocideOrganic active ingredientsAntioxidantBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices. Liquid formulations, including solutions and suspensions of the various drugs, agents and / or compounds, may be locally or regionally delivered. In each of these instances, antioxidants are utilized to prolong product integrity.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
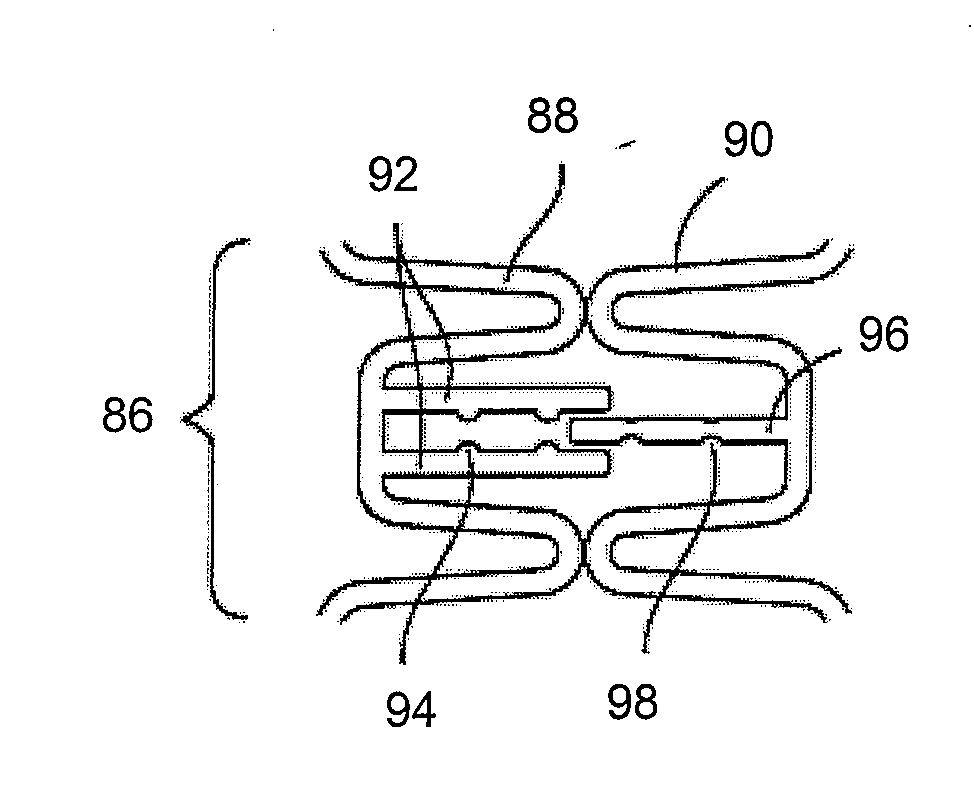
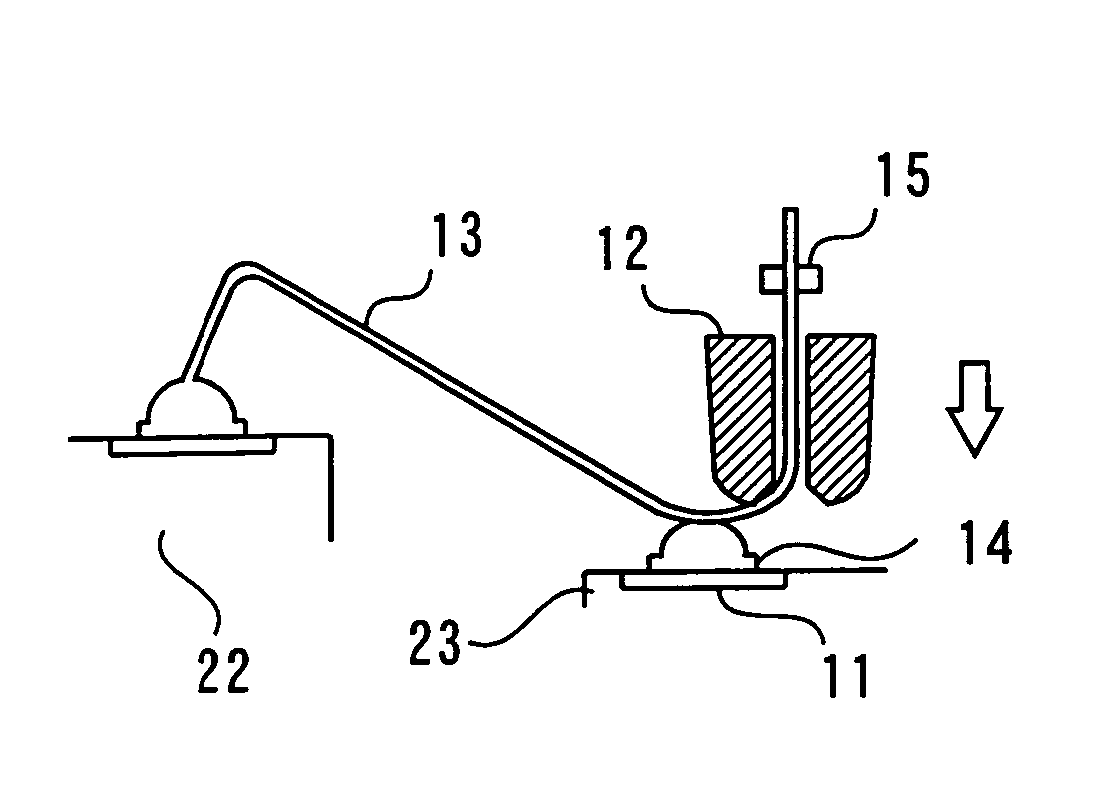
Cardiac vein lead with flexible anode and method for forming same
ActiveUS7031777B2Avoid contaminationMaintaining flexibilityTransvascular endocardial electrodesExternal electrodesCardiac VeinLeft Ventricles
A bipolar cardiac vein lead and method of assembly is provided wherein the lead includes a flexible coil anode electrode such that the lead may be advanced through a tortuous pathway. The coil electrode is coupled to a conductor using a method of assembly that minimizes or eliminates rigid components, maintaining flexibility of the distal lead end. Multi-polar cardiac vein leads may include multiple flexible coil electrodes to achieve pacing and / or sensing in the left atrium and the left ventricle or at multiple left heart sites.
Owner:MEDTRONIC INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com