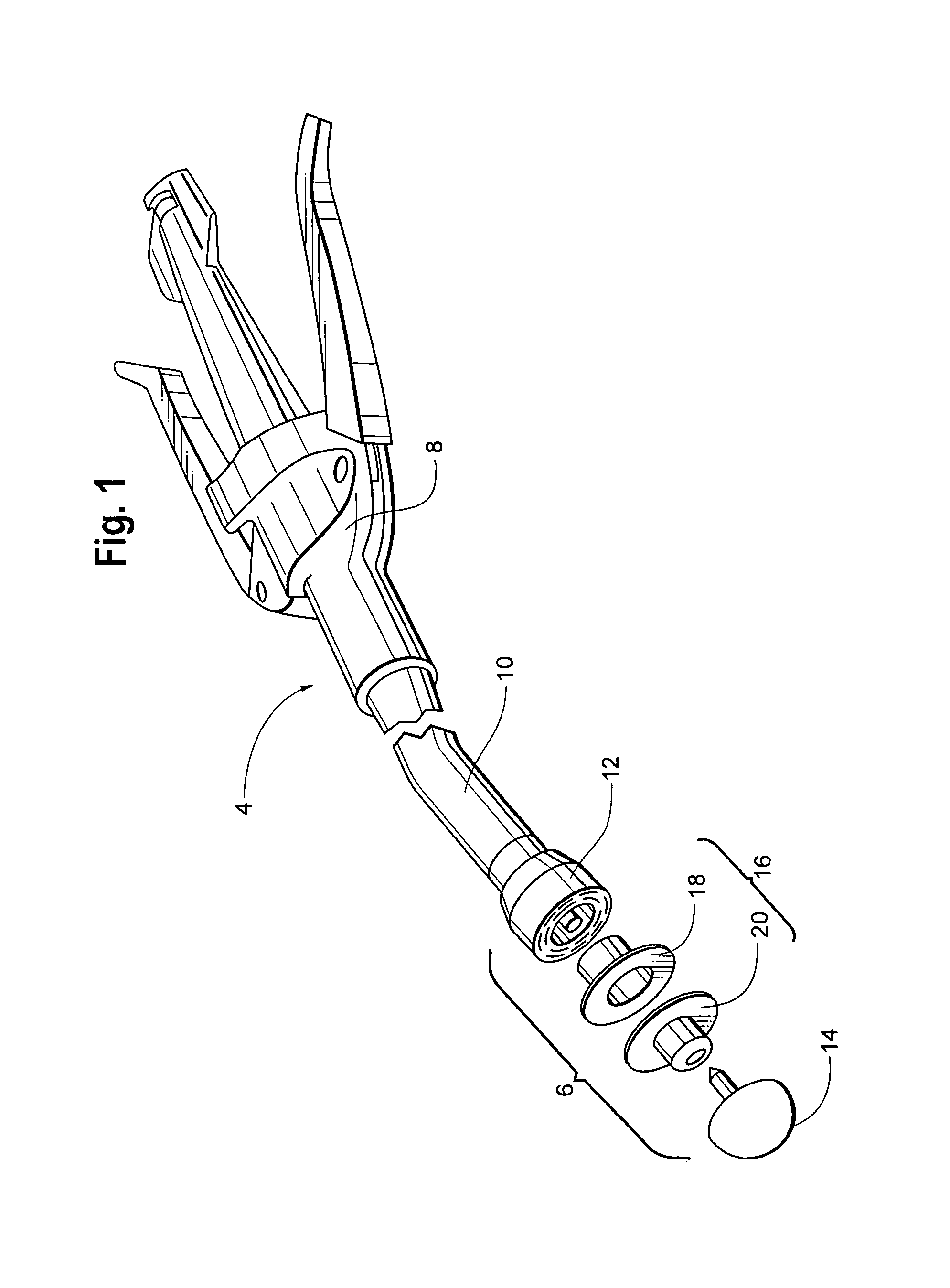
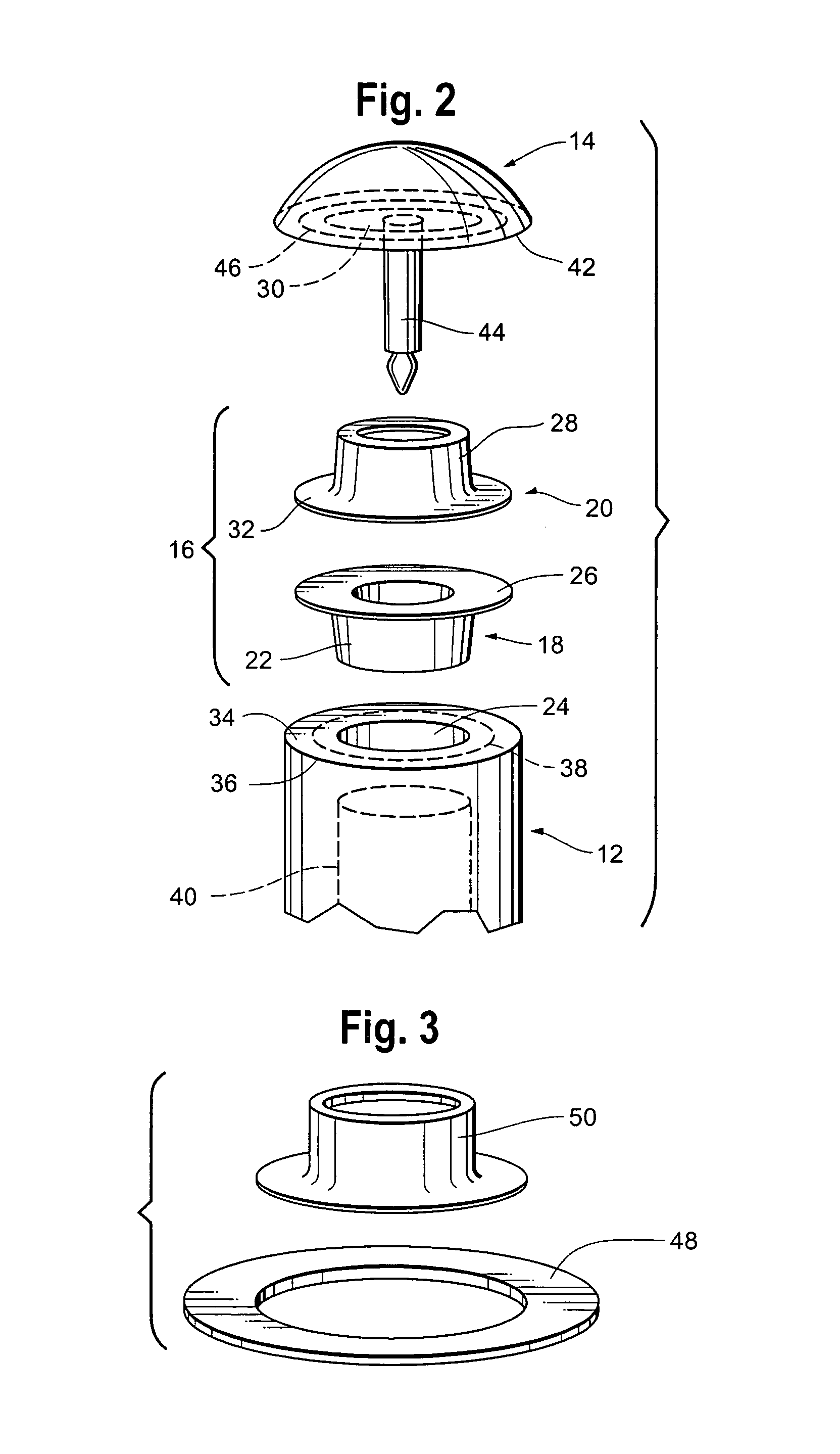
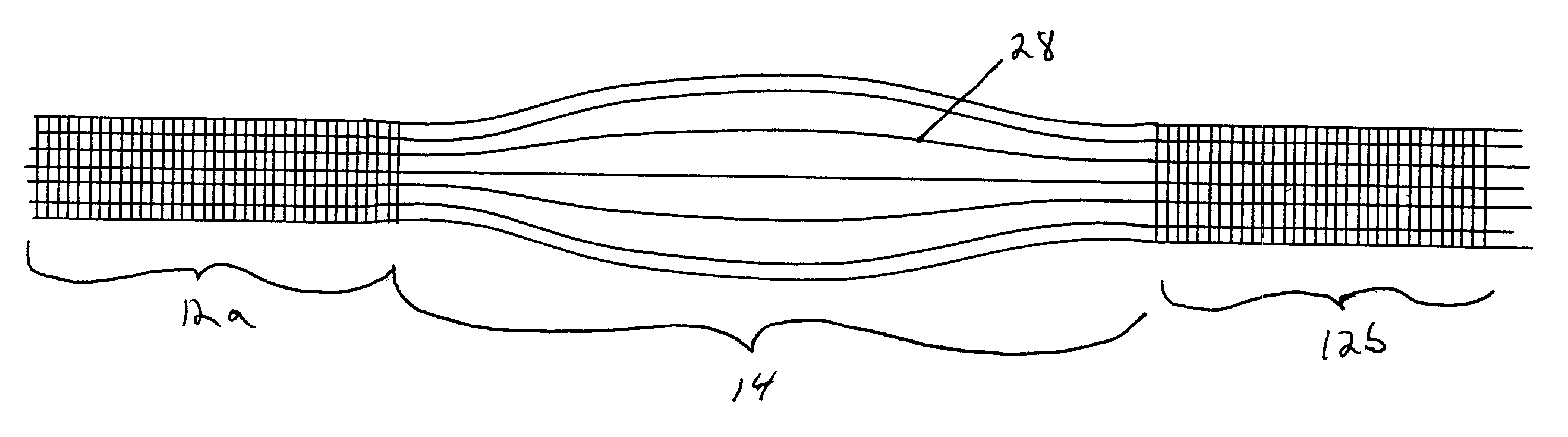
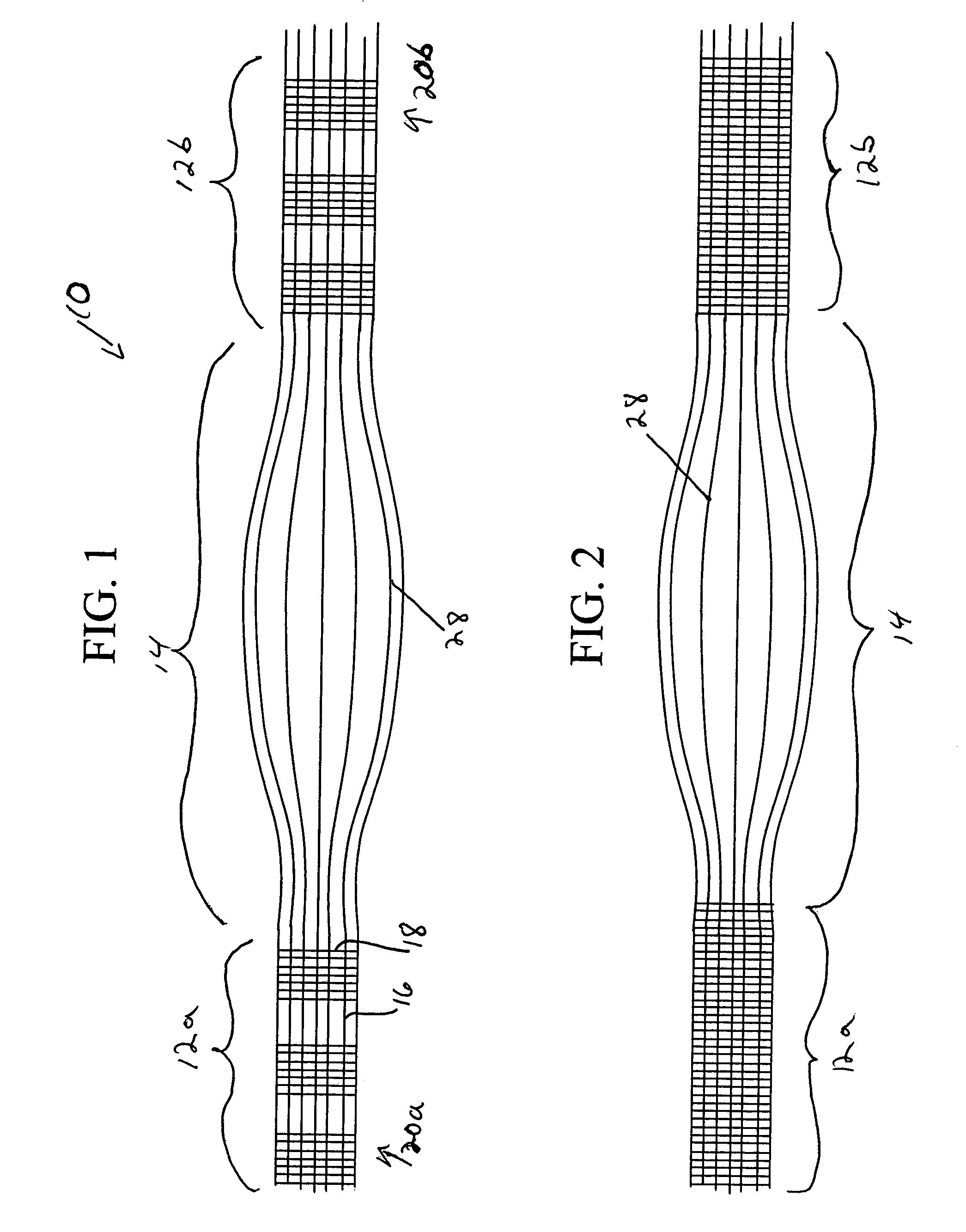
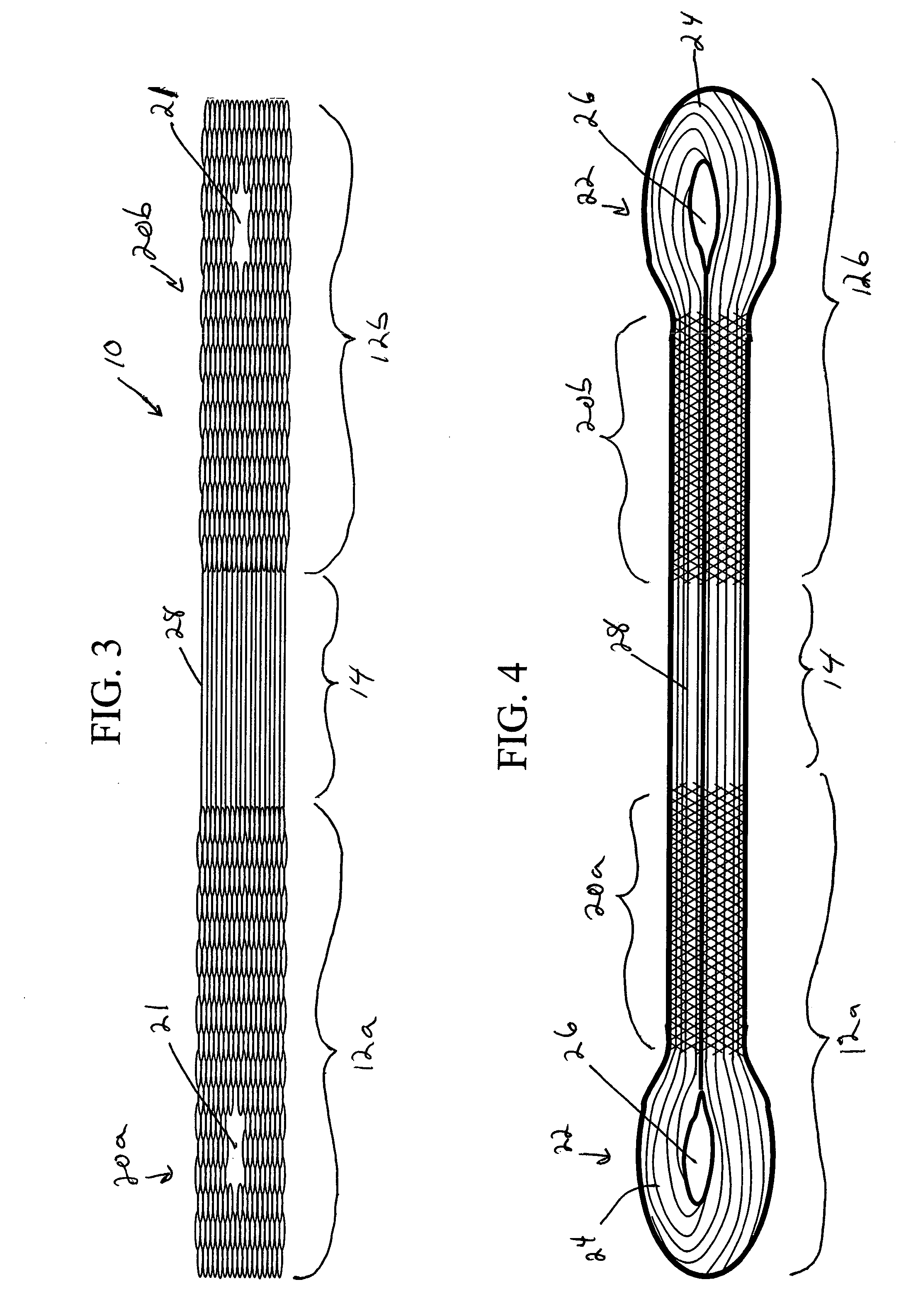
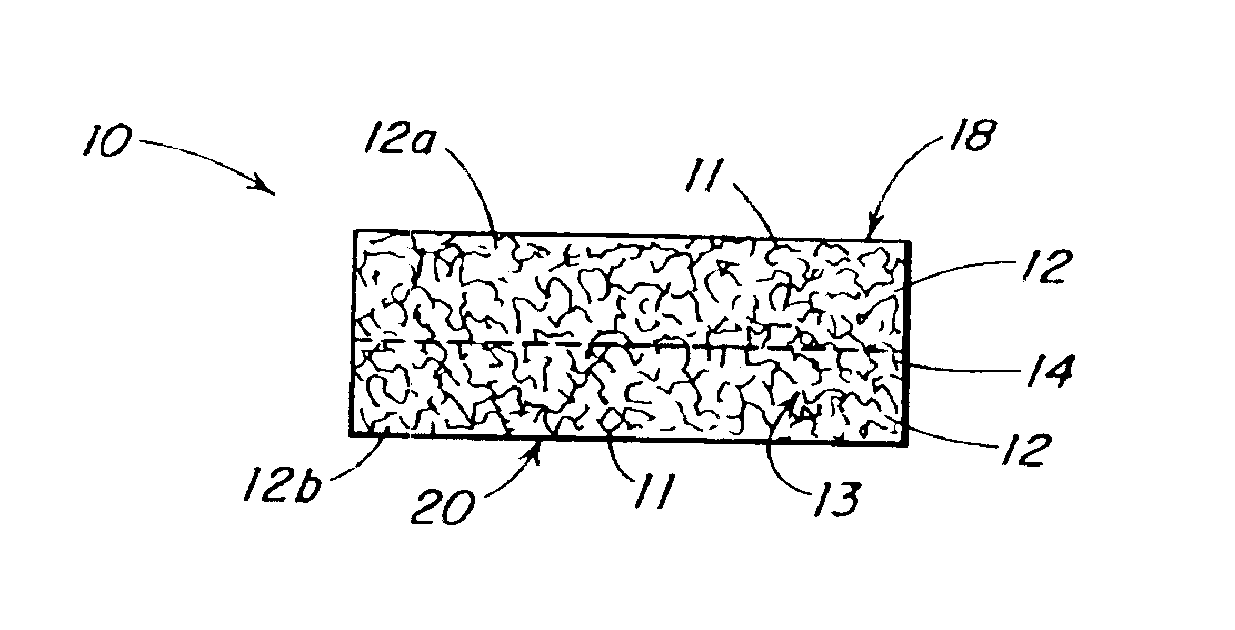
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
23319 results about "Stent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
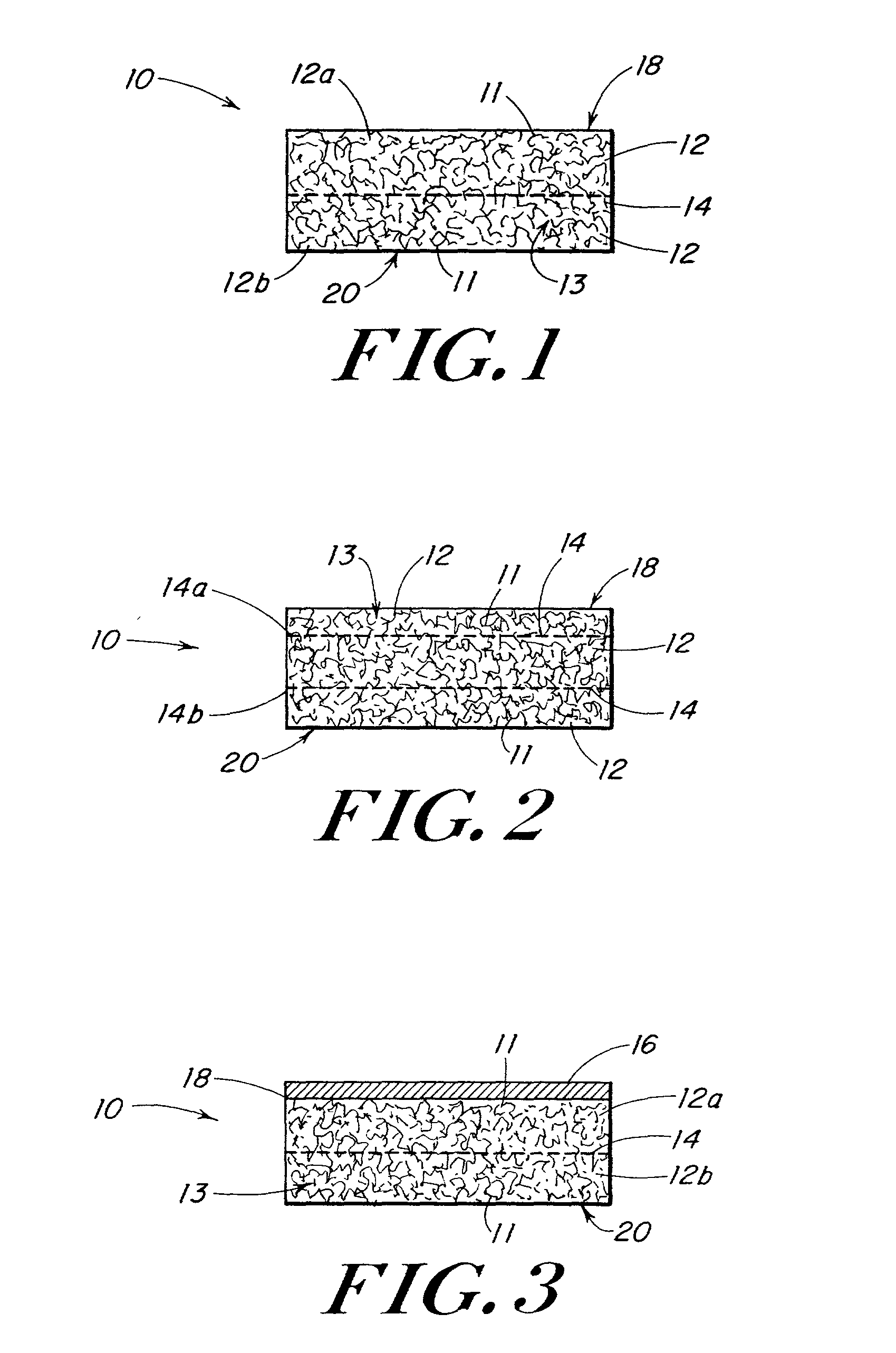
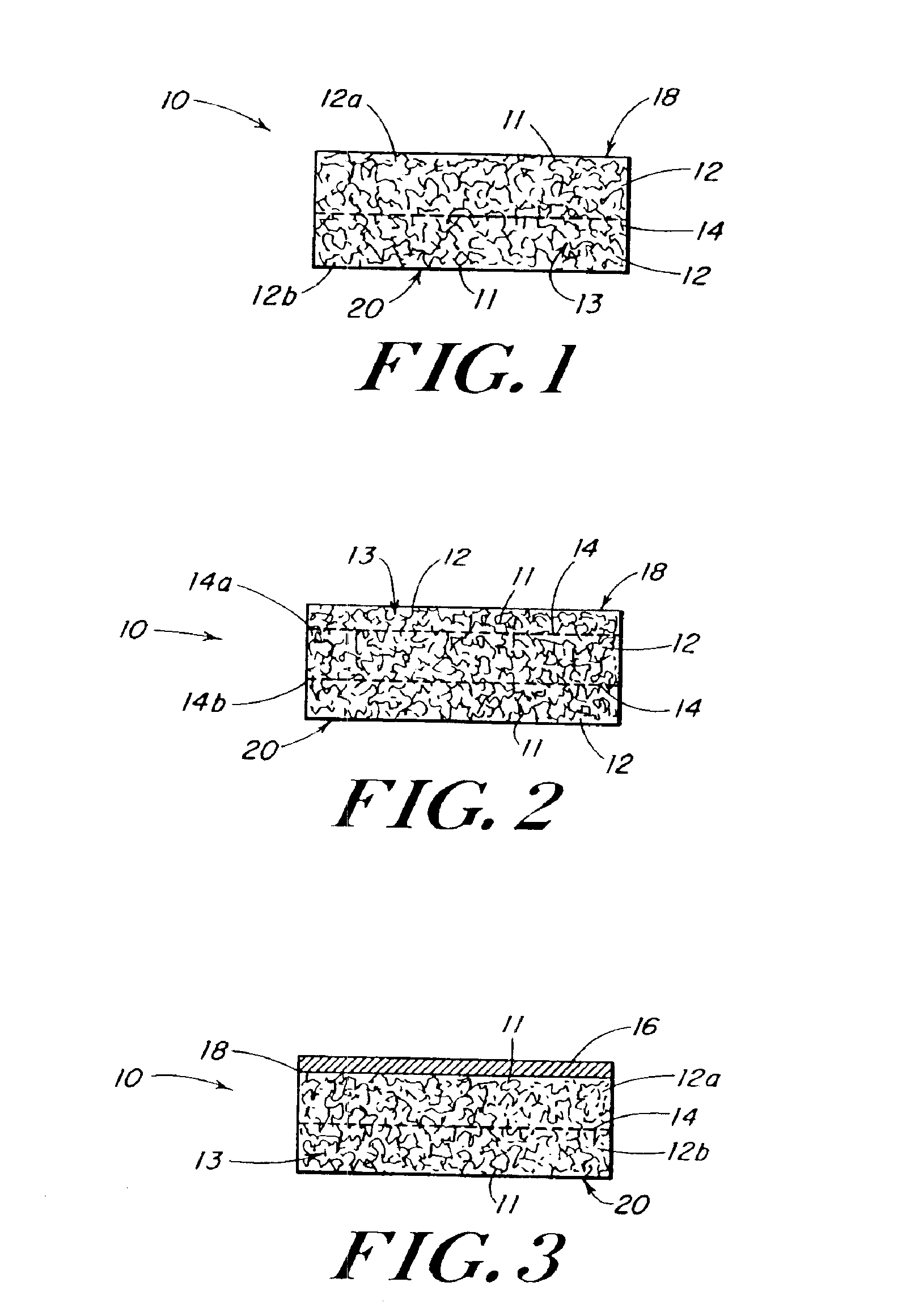
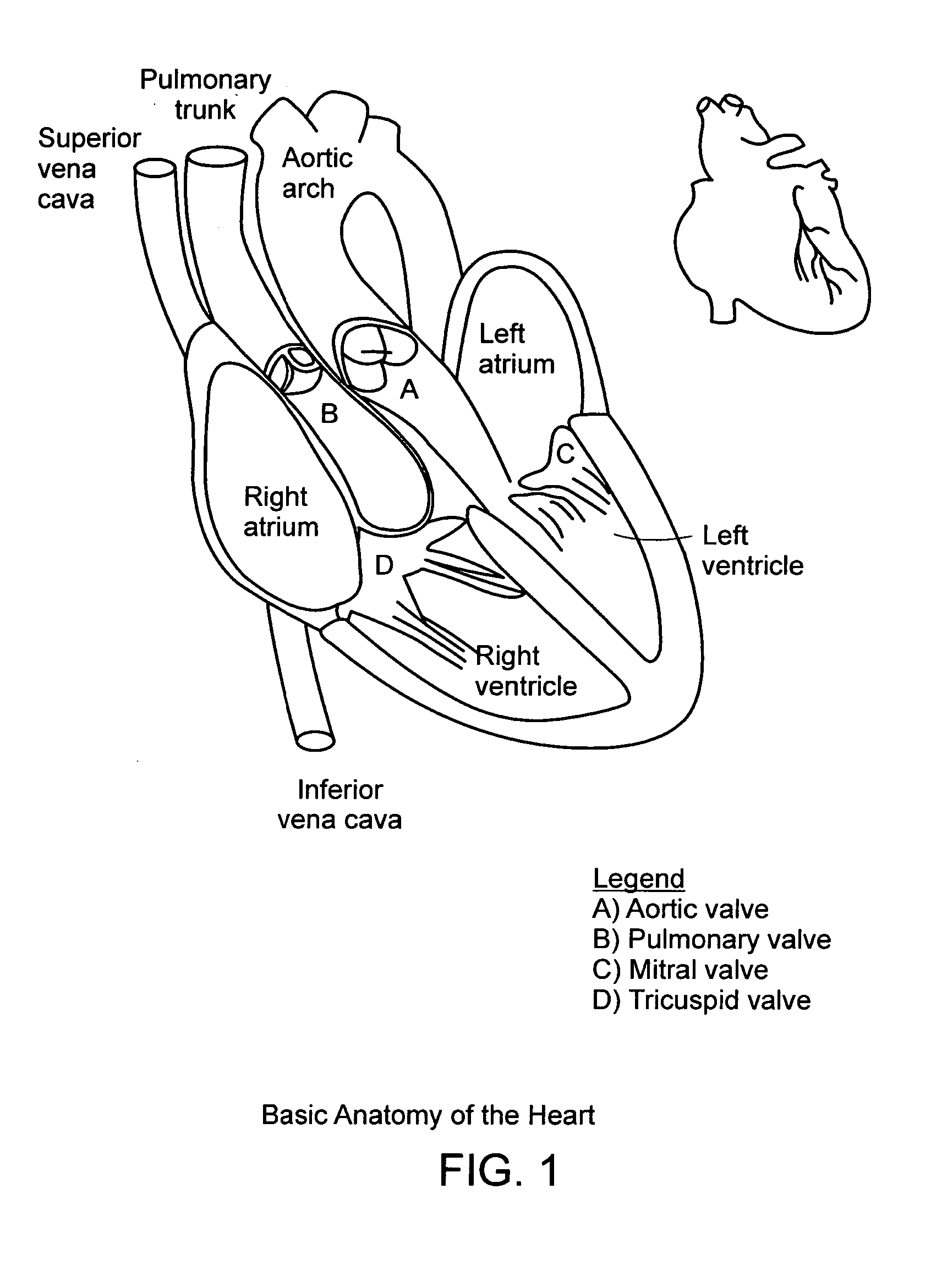
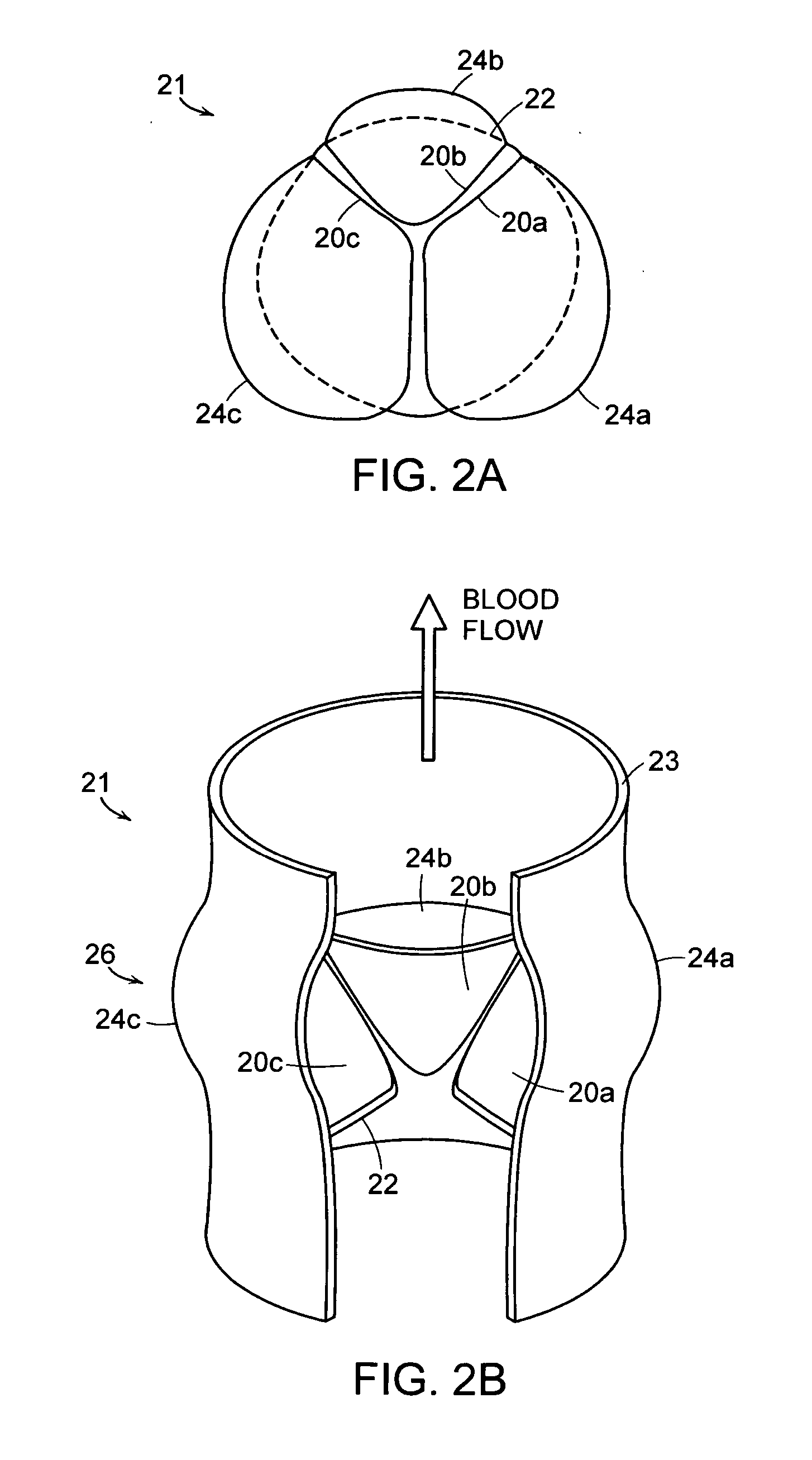
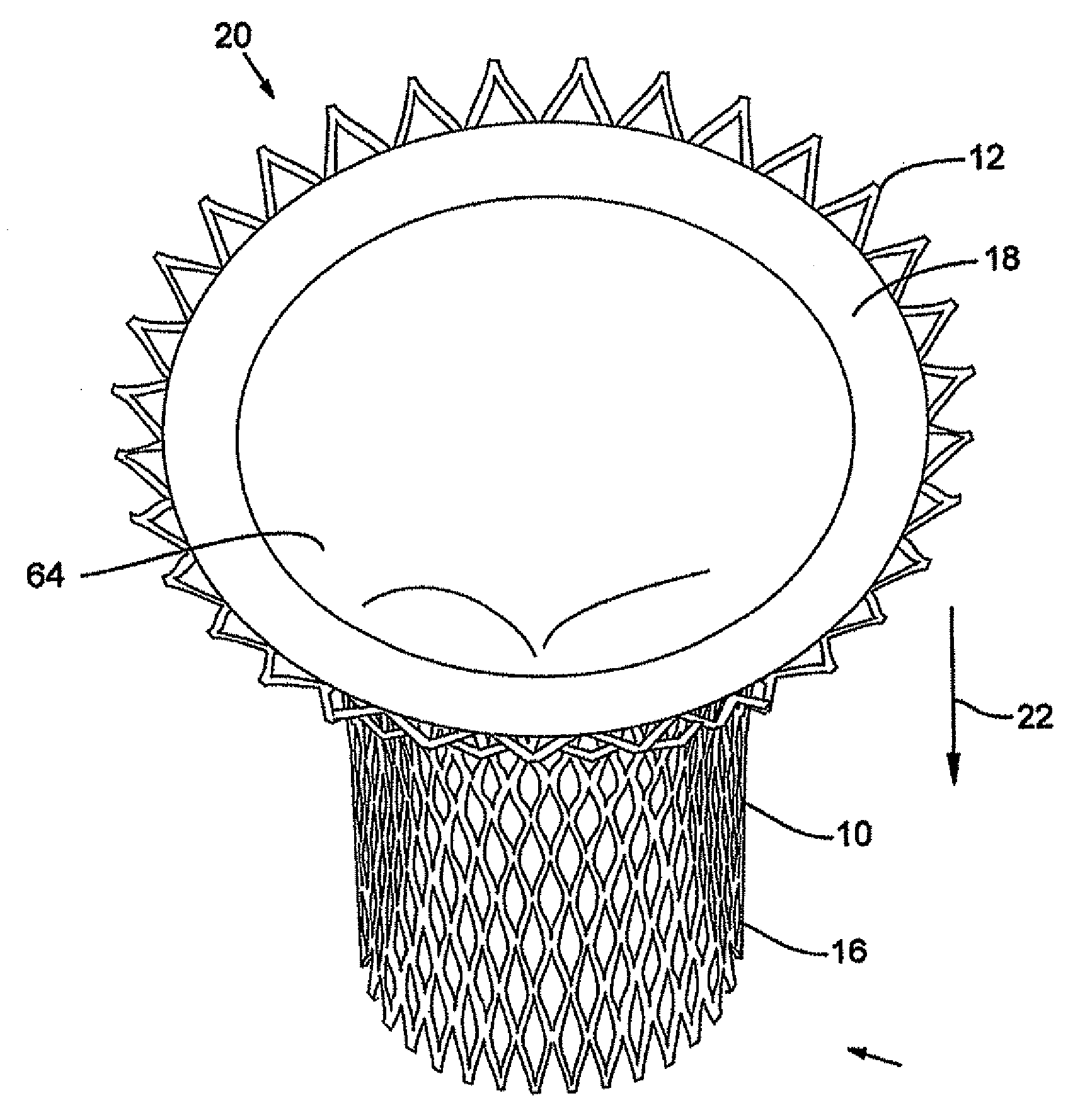
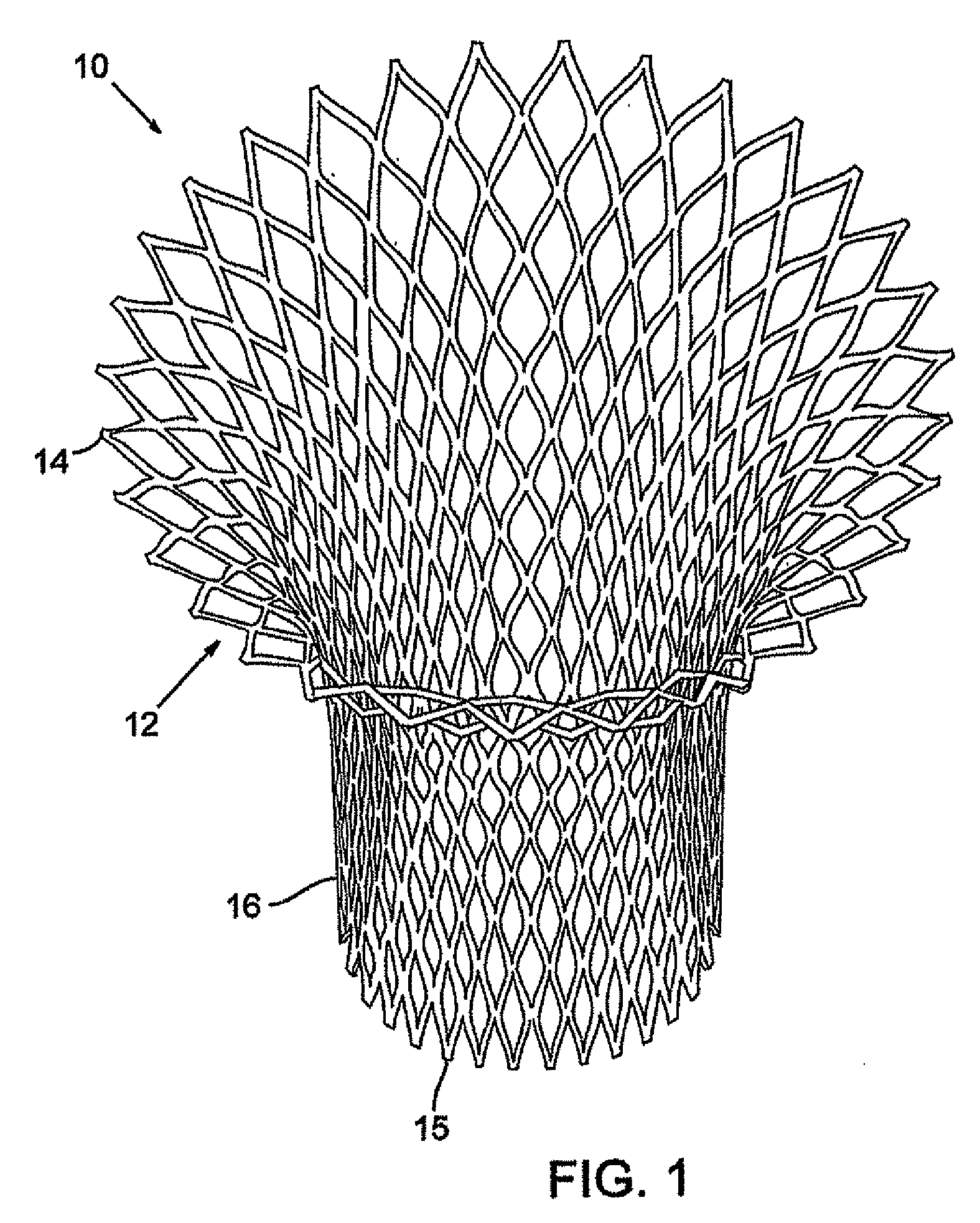
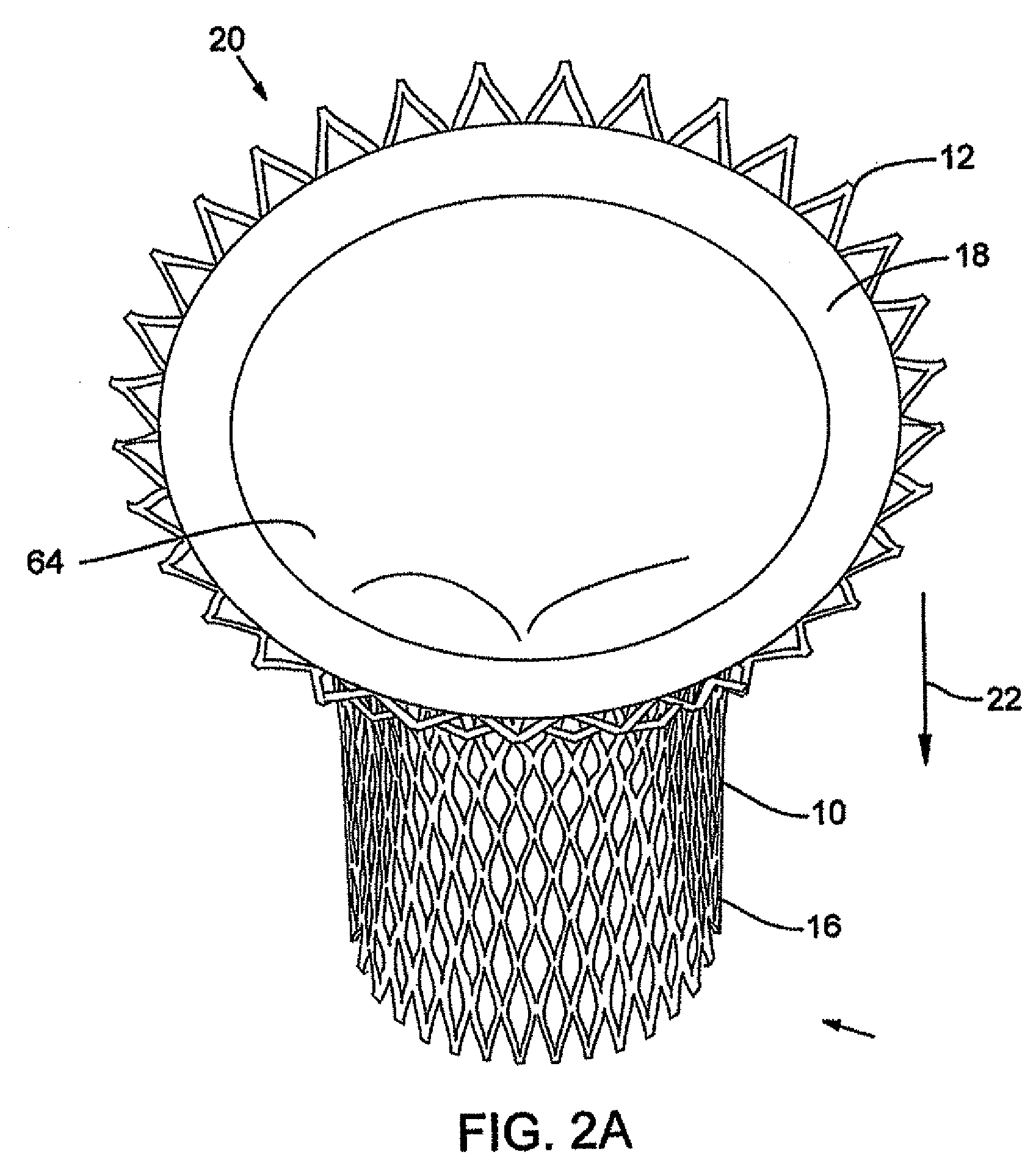
In medicine, a stent is a metal or plastic tube inserted into the lumen of an anatomic vessel or duct to keep the passageway open, and stenting is the placement of a stent. There is a wide variety of stents used for different purposes, from expandable coronary, vascular and biliary stents, to simple plastic stents used to allow the flow of urine between kidney and bladder. "Stent" is also used as a verb to describe the placement of such a device, particularly when a disease such as atherosclerosis has pathologically narrowed a structure such as an artery.
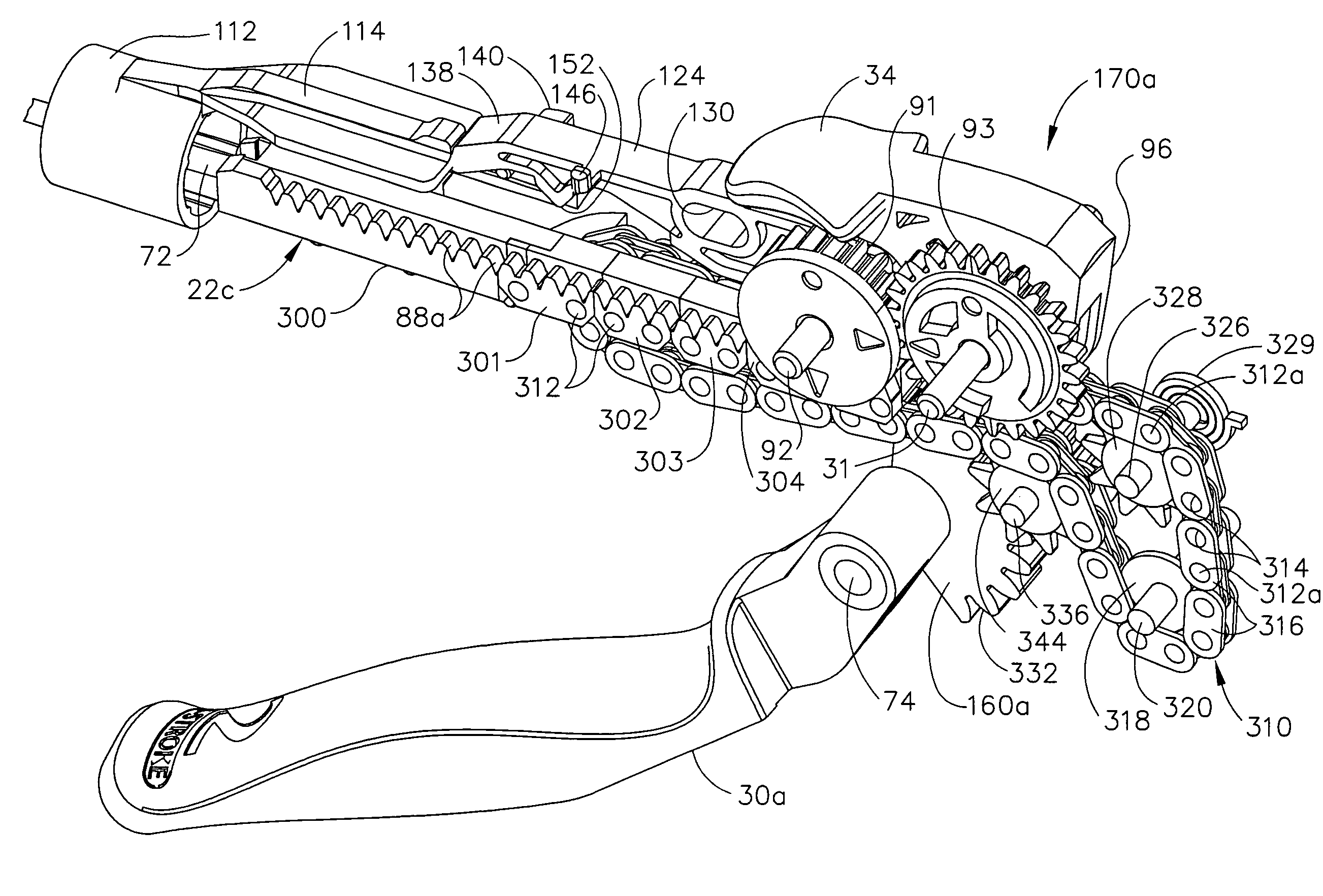
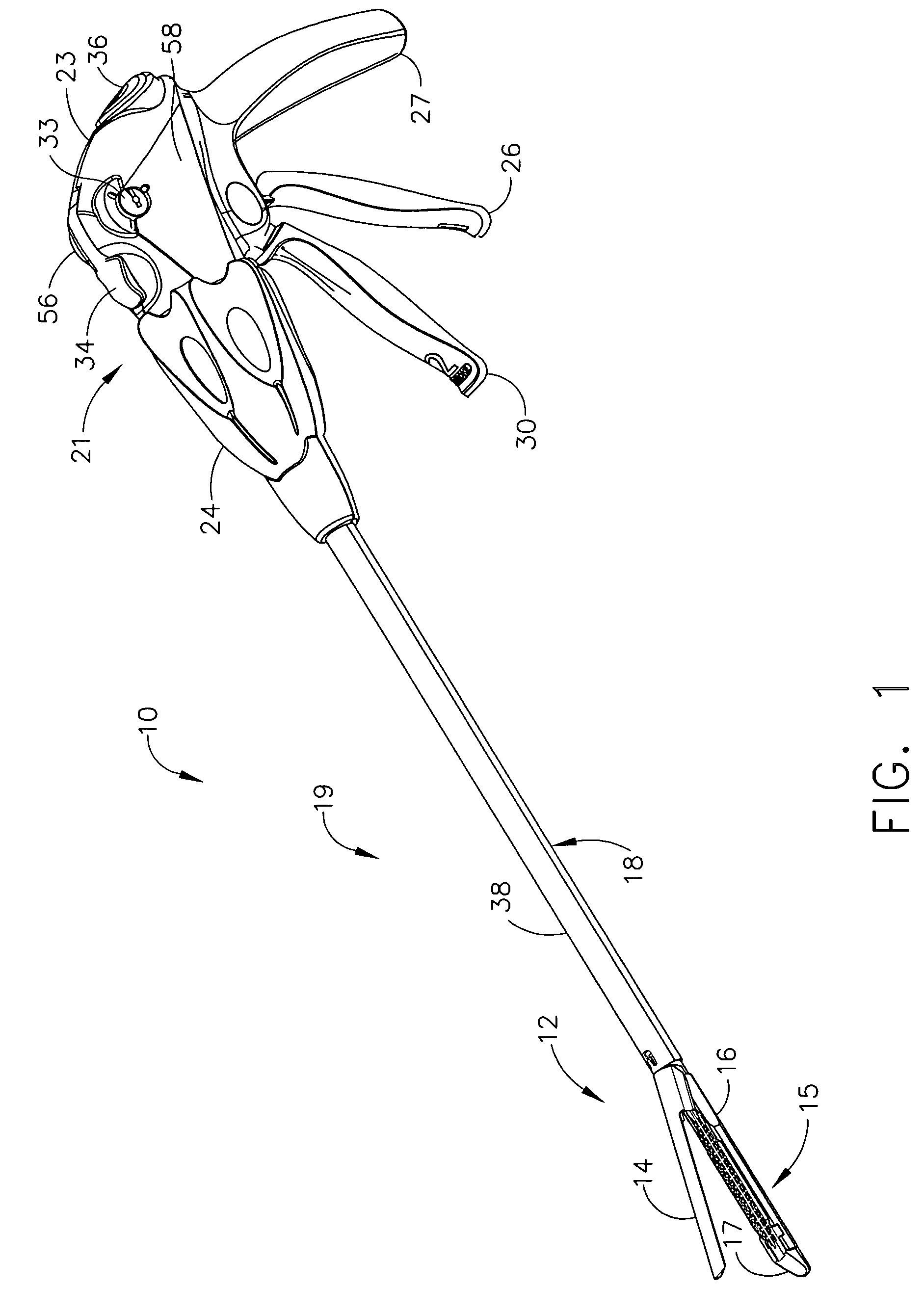
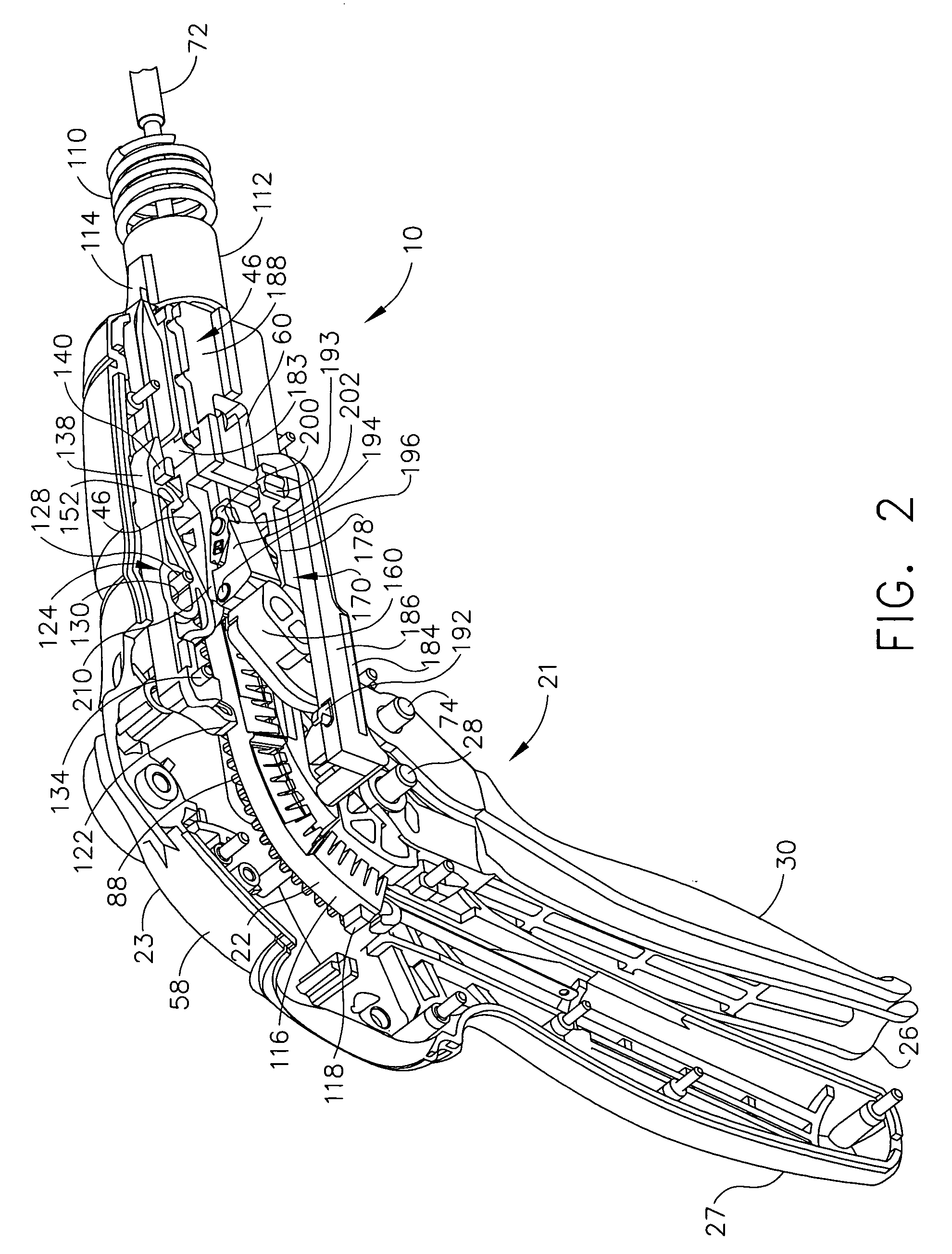
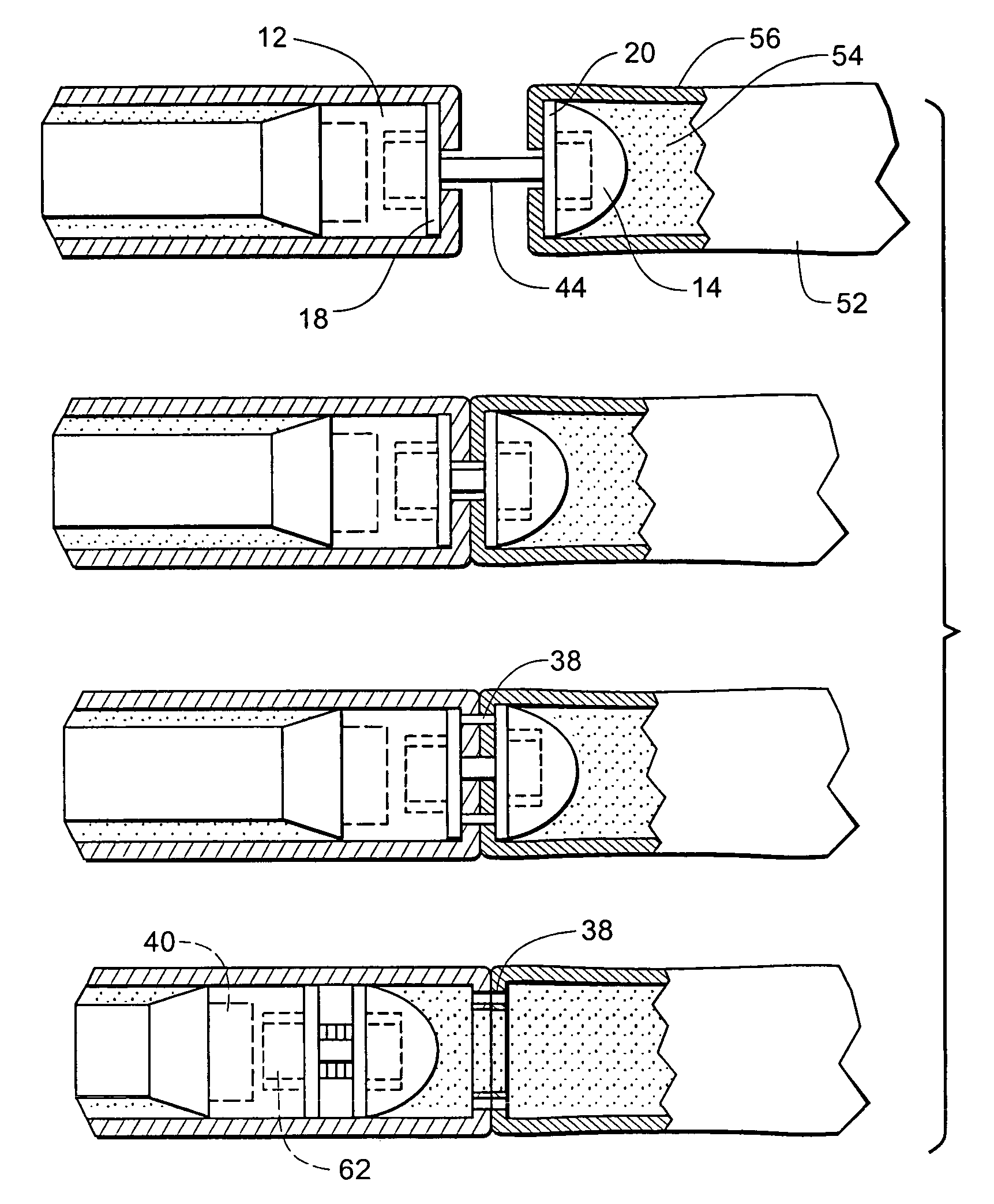
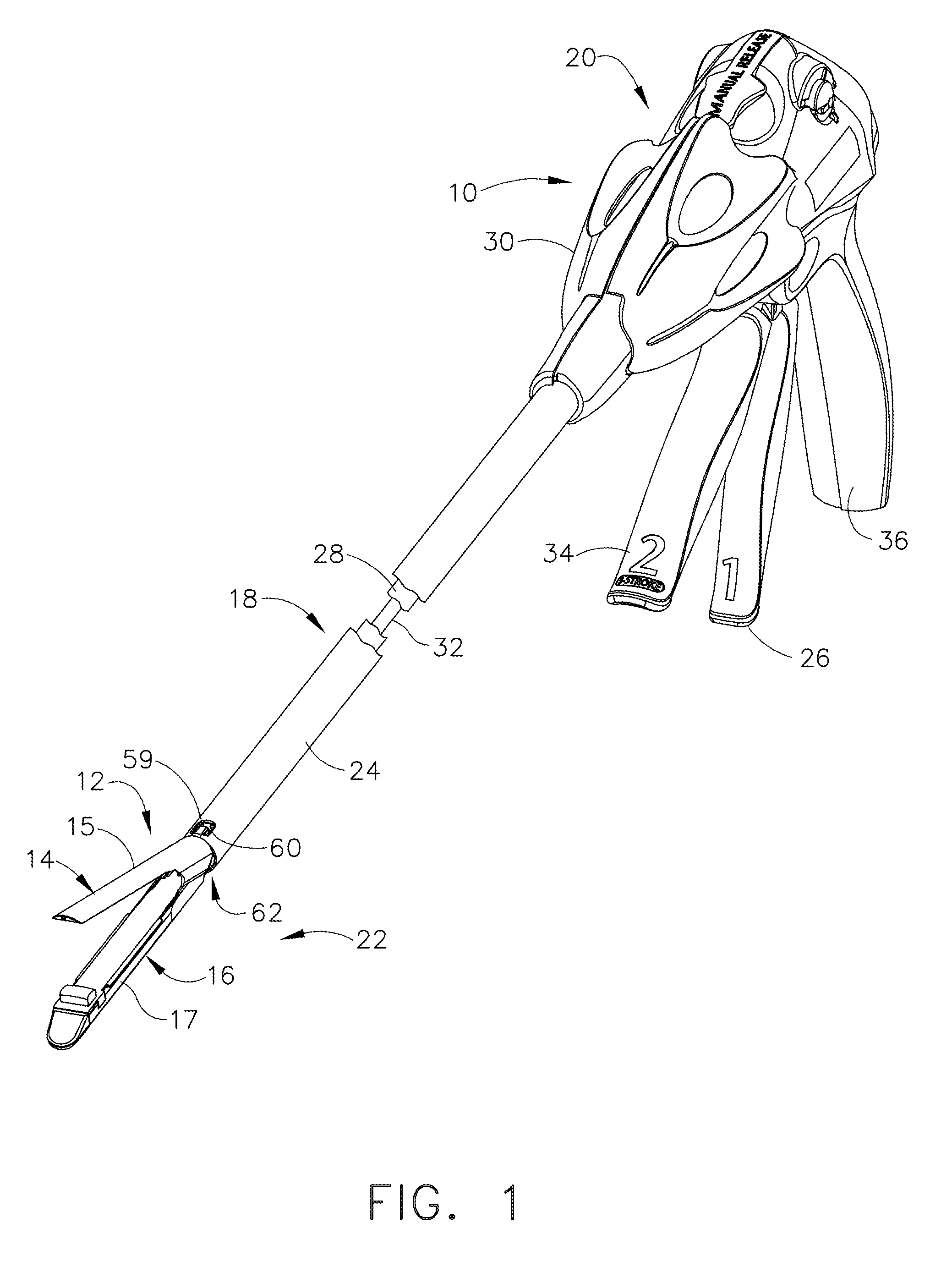
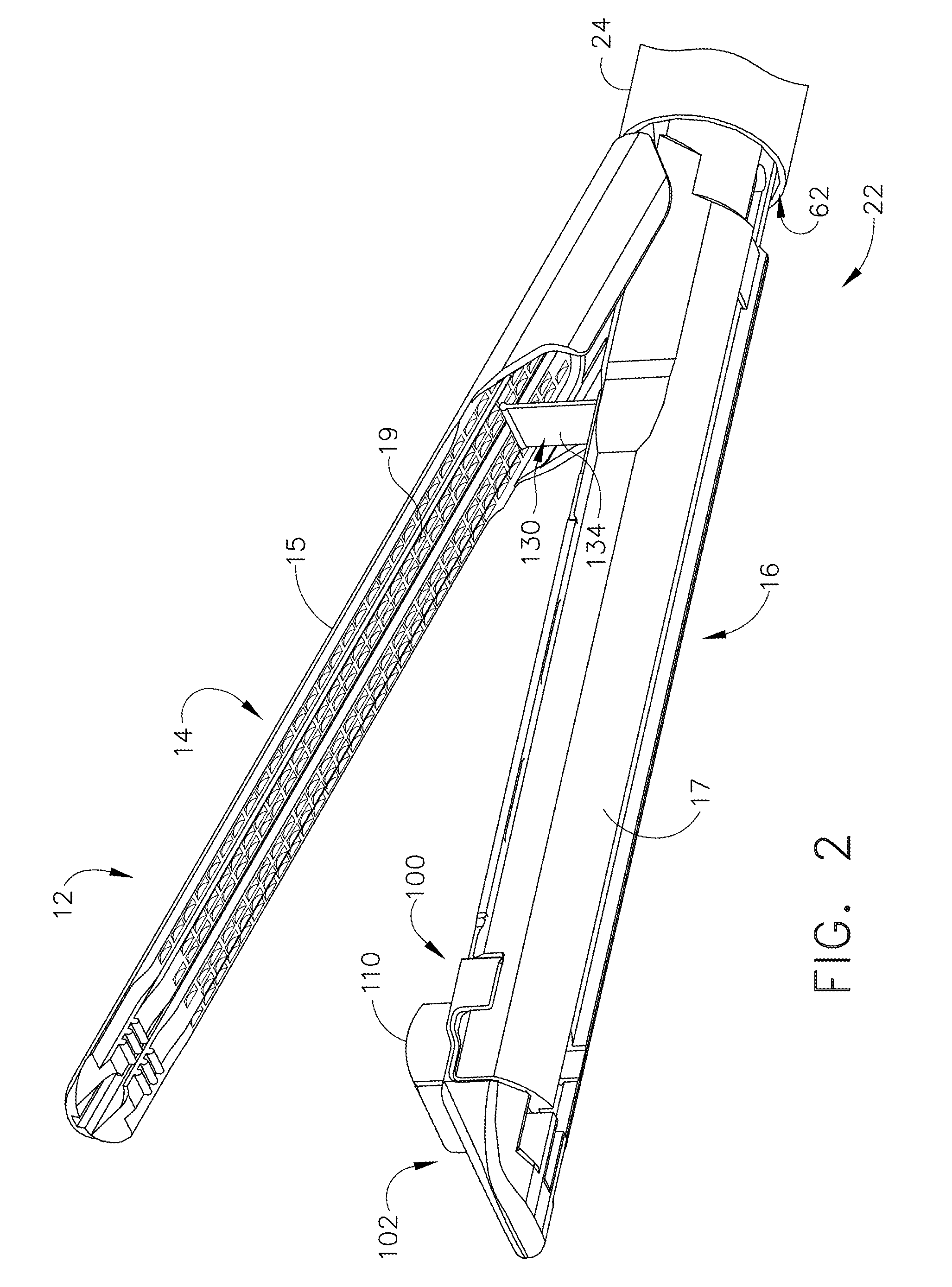
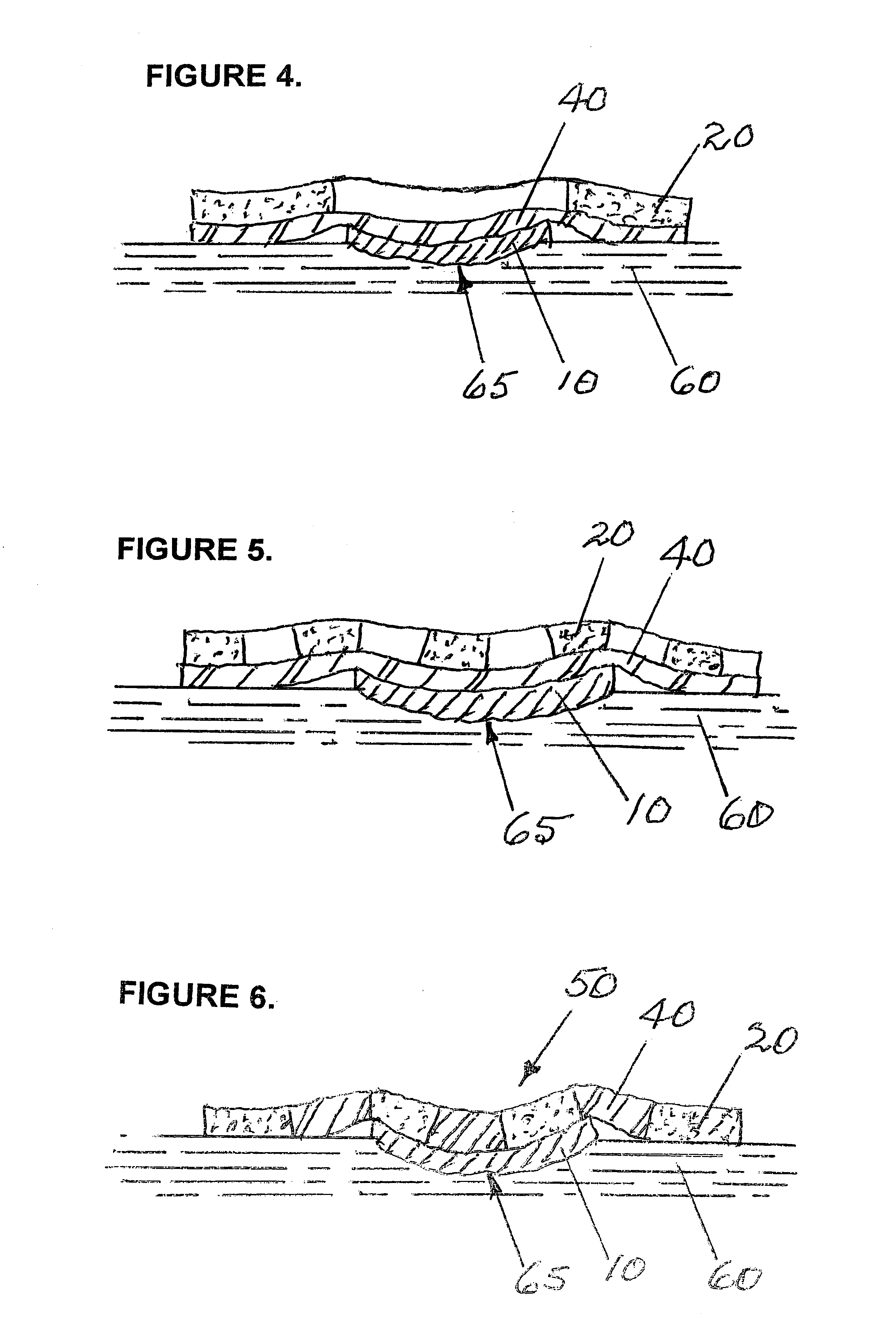
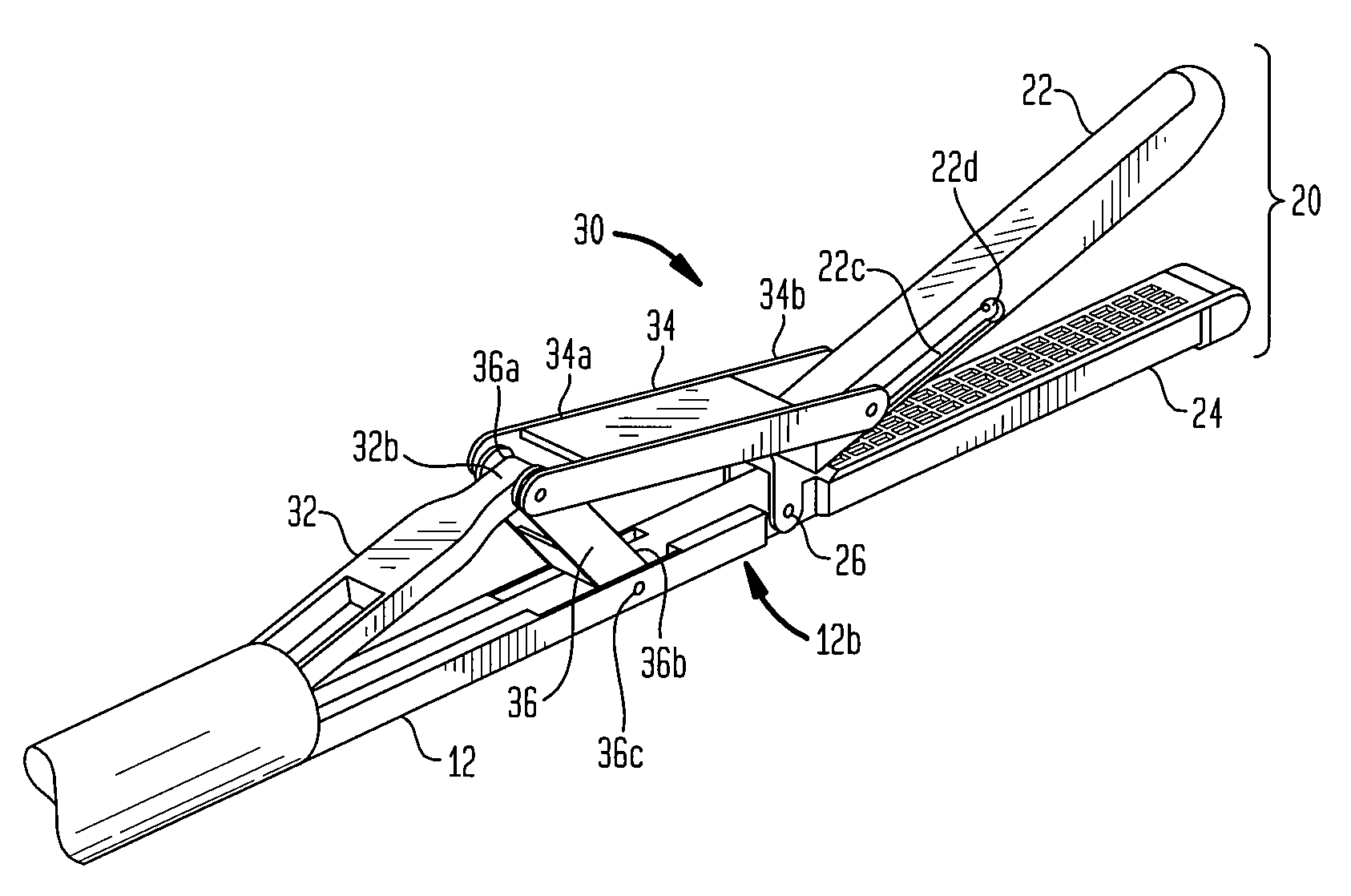
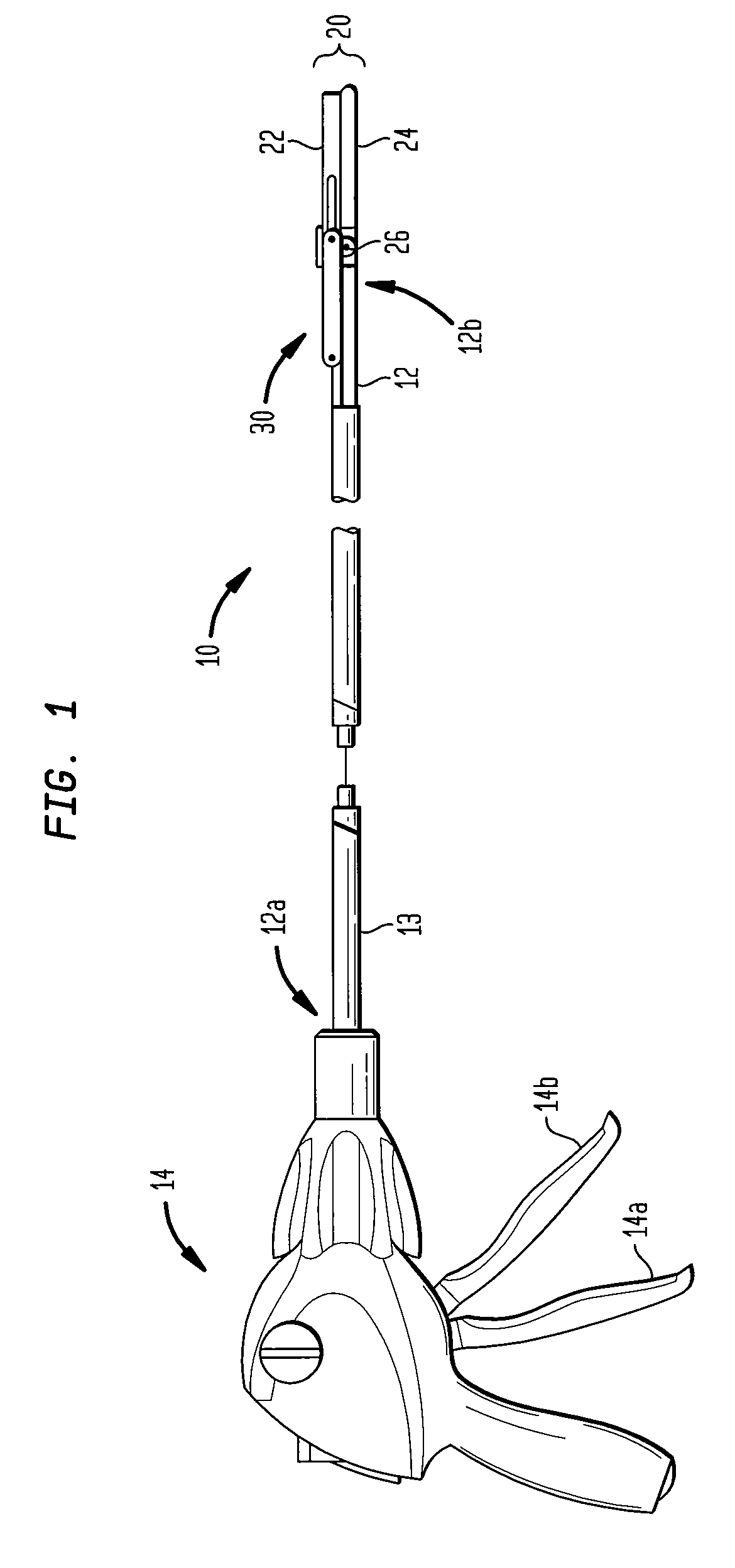
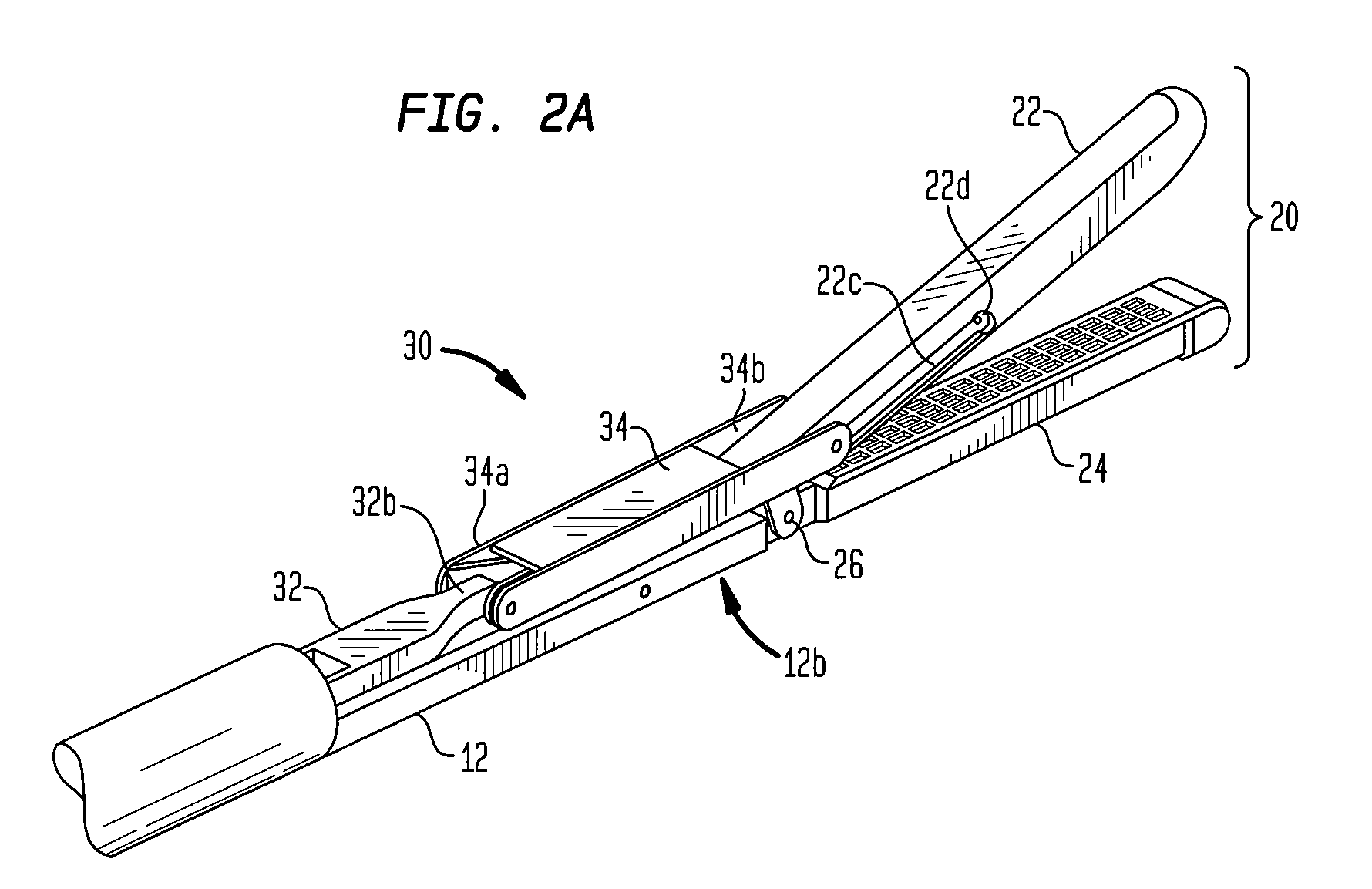
Surgical stapling instrument incorporating a multi-stroke firing mechanism with a flexible rack
InactiveUS7303108B2Shorten the lengthSuture equipmentsStapling toolsSurgical stapleReciprocating motion
Owner:CILAG GMBH INT
Circular stapler buttress combination
A combination medical device comprising a circular stapler instrument and one or more portions of preformed buttress material adapted to be stably positioned upon the staple cartridge and / or anvil components of the stapler prior or at the time of use. Positioned buttress material(s) are delivered to a tissue site where the circular stapler is actuated to connect previously severed tissue portions. The buttress material is retained and provides an improved seal between the joined tissue sections. The buttress material is made up of two regions, one of which serves primarily to secure the buttress material to the stapler prior to actuation, and one of which serves primarily to form the improved seal. The former region is severed and discarded upon activation of the circular stapler to form an anastomoses. Methods of use and preparation of the buttress material are also described.
Owner:SYNOVIS LIFE TECH
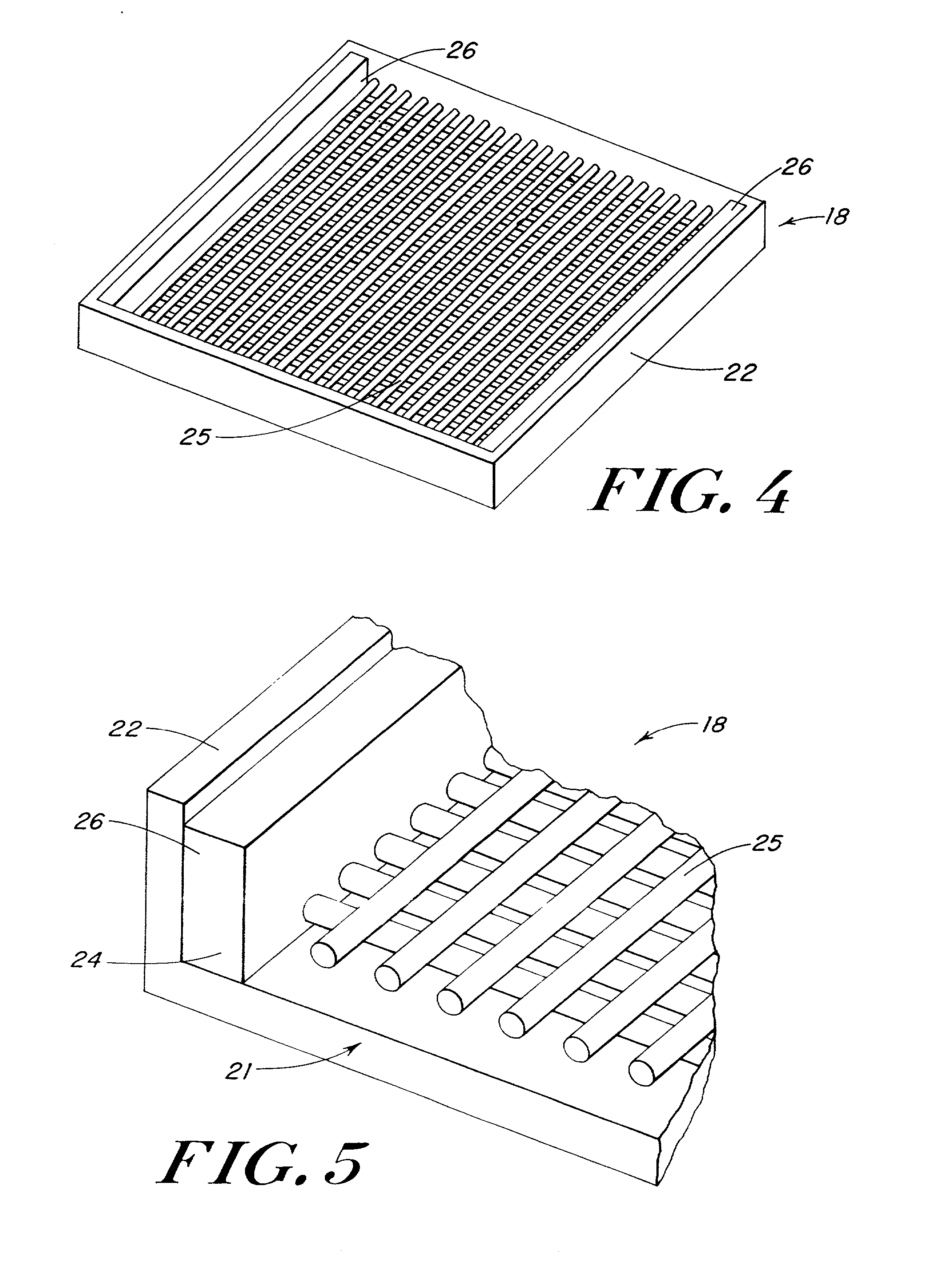
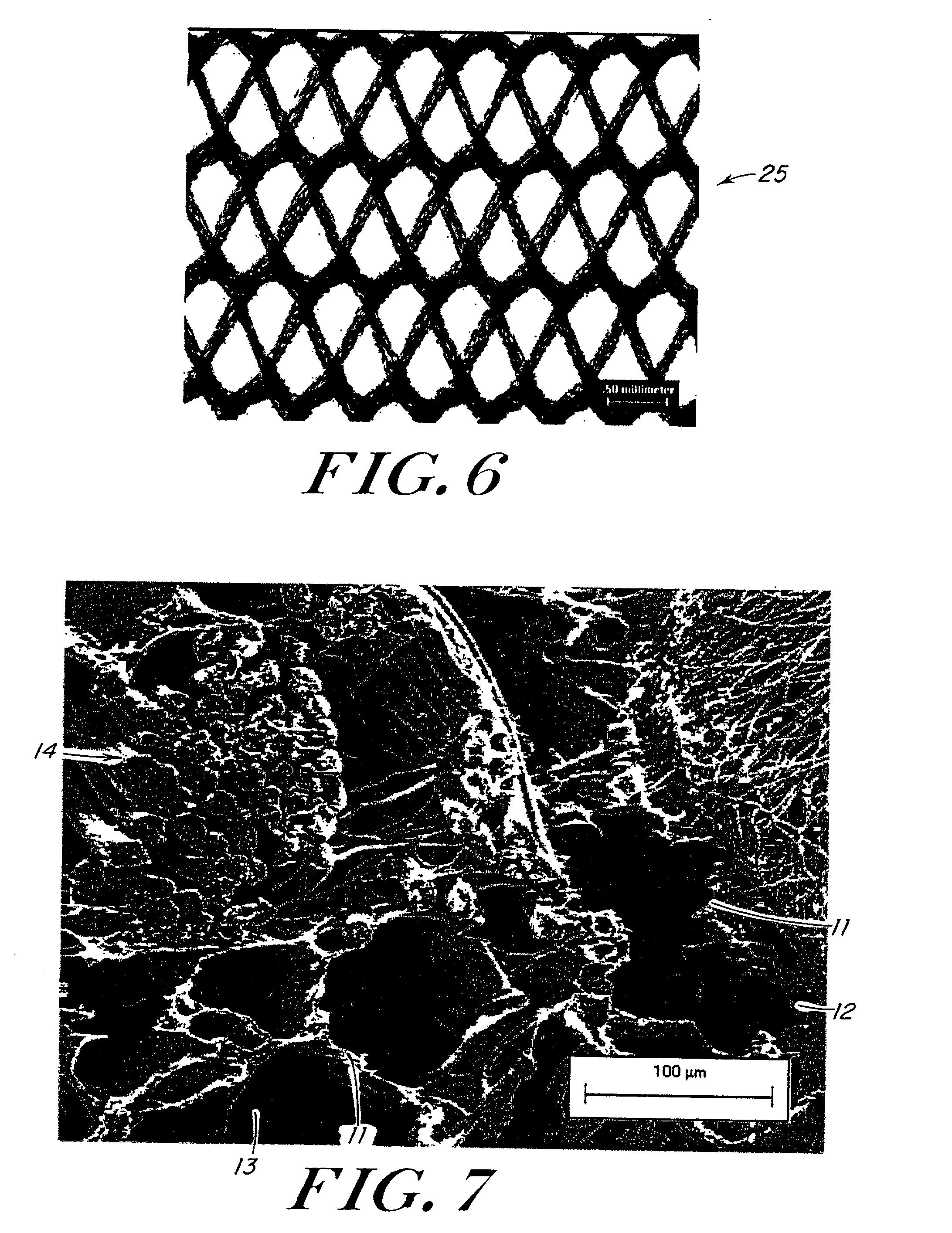
Porous tissue scaffoldings for the repair of regeneration of tissue
InactiveUS6333029B1Promote growthPromote regenerationPeptide/protein ingredientsAntipyreticRepair tissueOpen cell
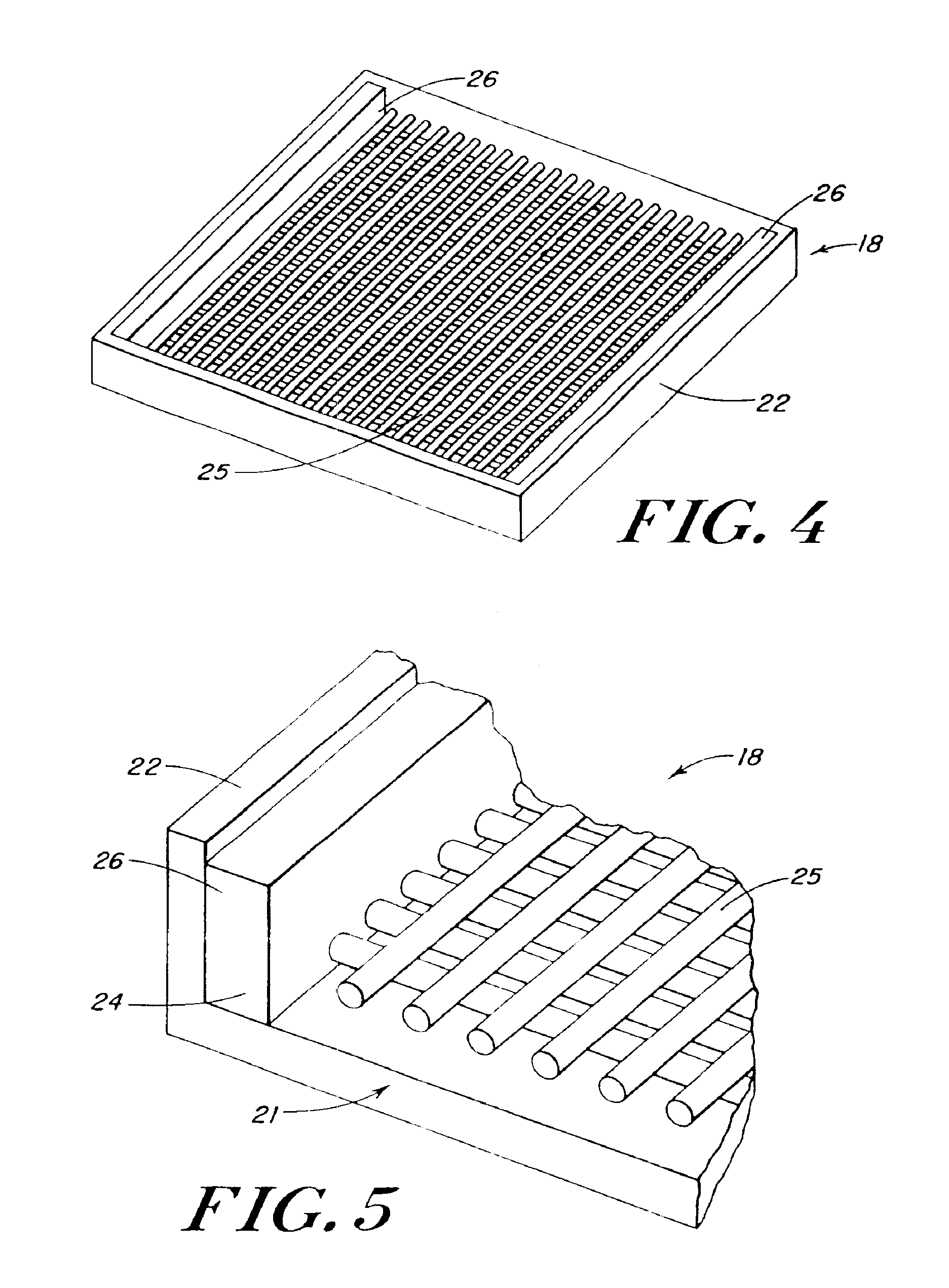
The present patent describes a three-dimensional inter-connected open cell porous foams that have a gradient in composition and / or microstructure through one or more directions. These foams can be made from a blend of absorbable and biocompatible polymers that are formed into foams having a compositional gradient transitioning from predominately one polymeric material to predominately a second polymeric material. These gradient foams are particularly well suited to tissue engineering applications and can be designed to mimic tissue transition or interface zones.
Owner:ETHICON ENDO SURGERY INC
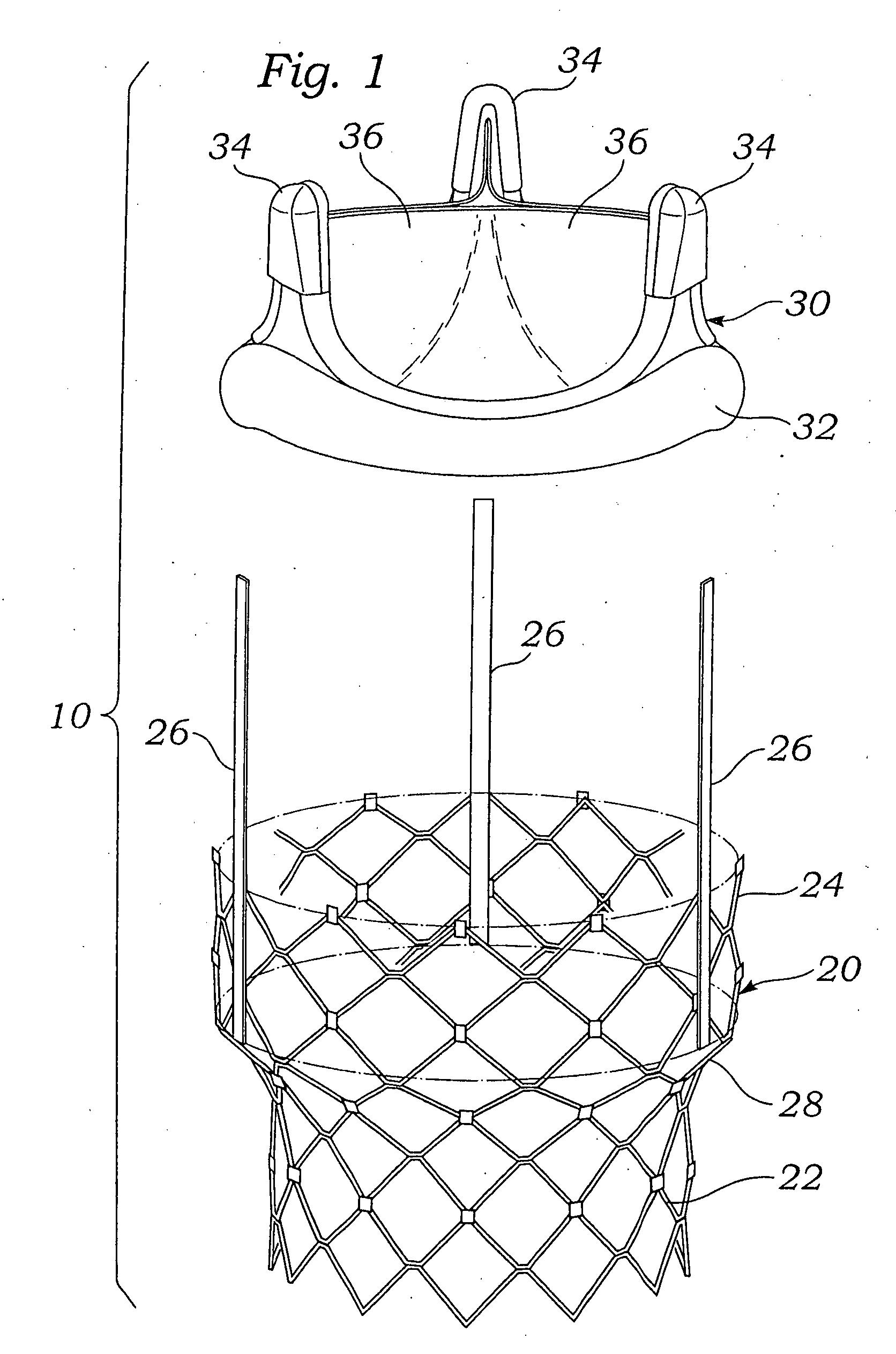
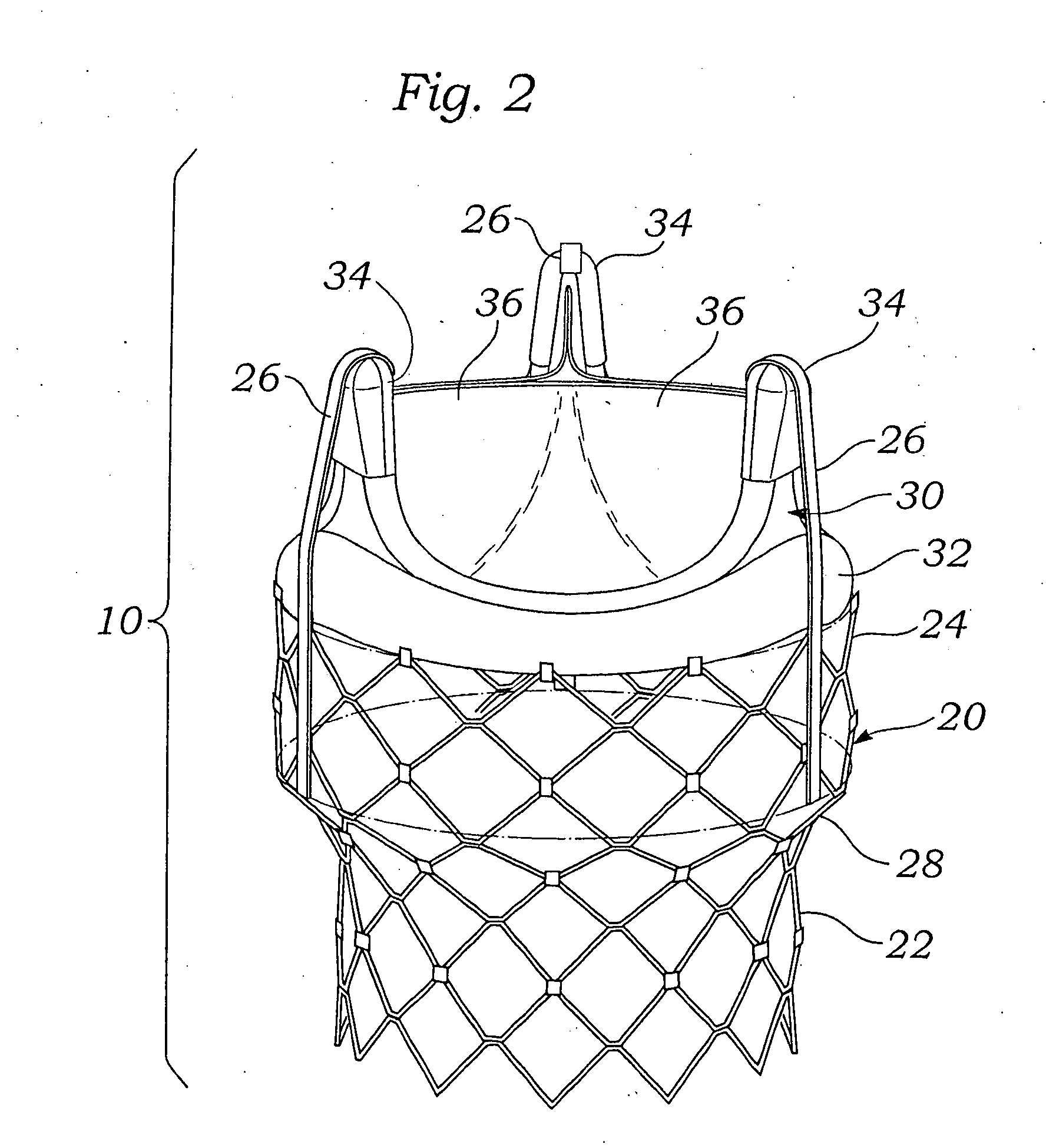
Implantable prosthetic valve
InactiveUS6893460B2Prevent blood flowImprove sealingStentsBalloon catheterGuide tubeBiomedical engineering
A valve prosthesis device is disclosed suitable for implantation in body ducts. The device comprises support stent, comprised of a deployable construction adapted to be initially crimped in a narrow configuration suitable for catheterization through the body duct to a target location and adapted to be deployed by exerting substantially radial forces from within by means of a deployment device to a deployed state in the target location, the support stent provided with a plurality of longitudinally rigid support beams of fixed length; valve assembly comprising a flexible conduit having an inlet end and an outlet, made of pliant material attached to the support beams providing collapsible slack portions of the conduit at the outlet. When flow is allowed to pass through the valve prosthesis device from the inlet to the outlet the valve assembly is kept in an open position, whereas a reverse flow is prevented as the collapsible slack portions of the valve assembly collapse inwardly providing blockage to the reverse flow.
Owner:EDWARDS LIFESCI PVT
Medical devices and applications of polyhydroxyalkanoate polymers
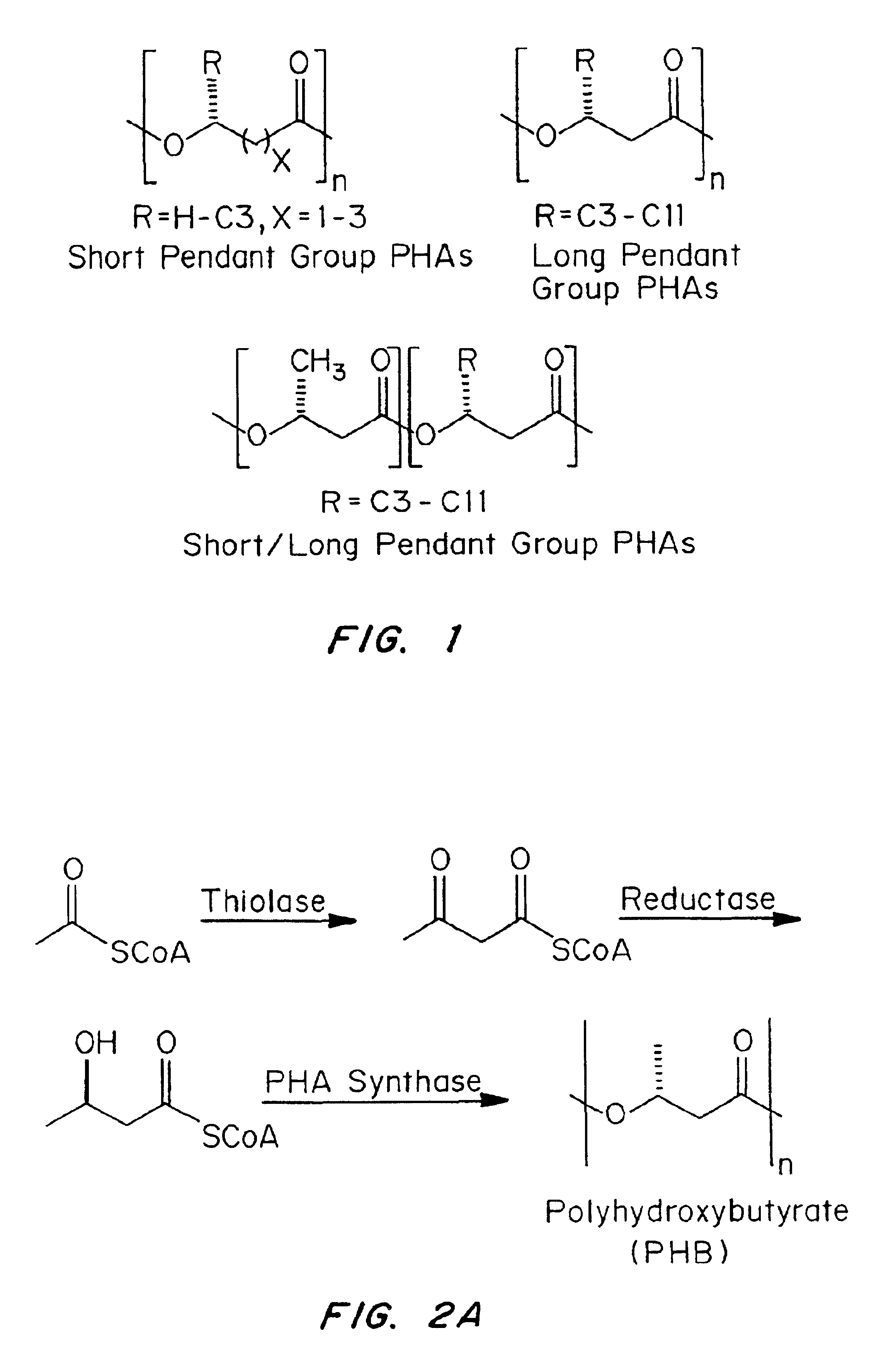
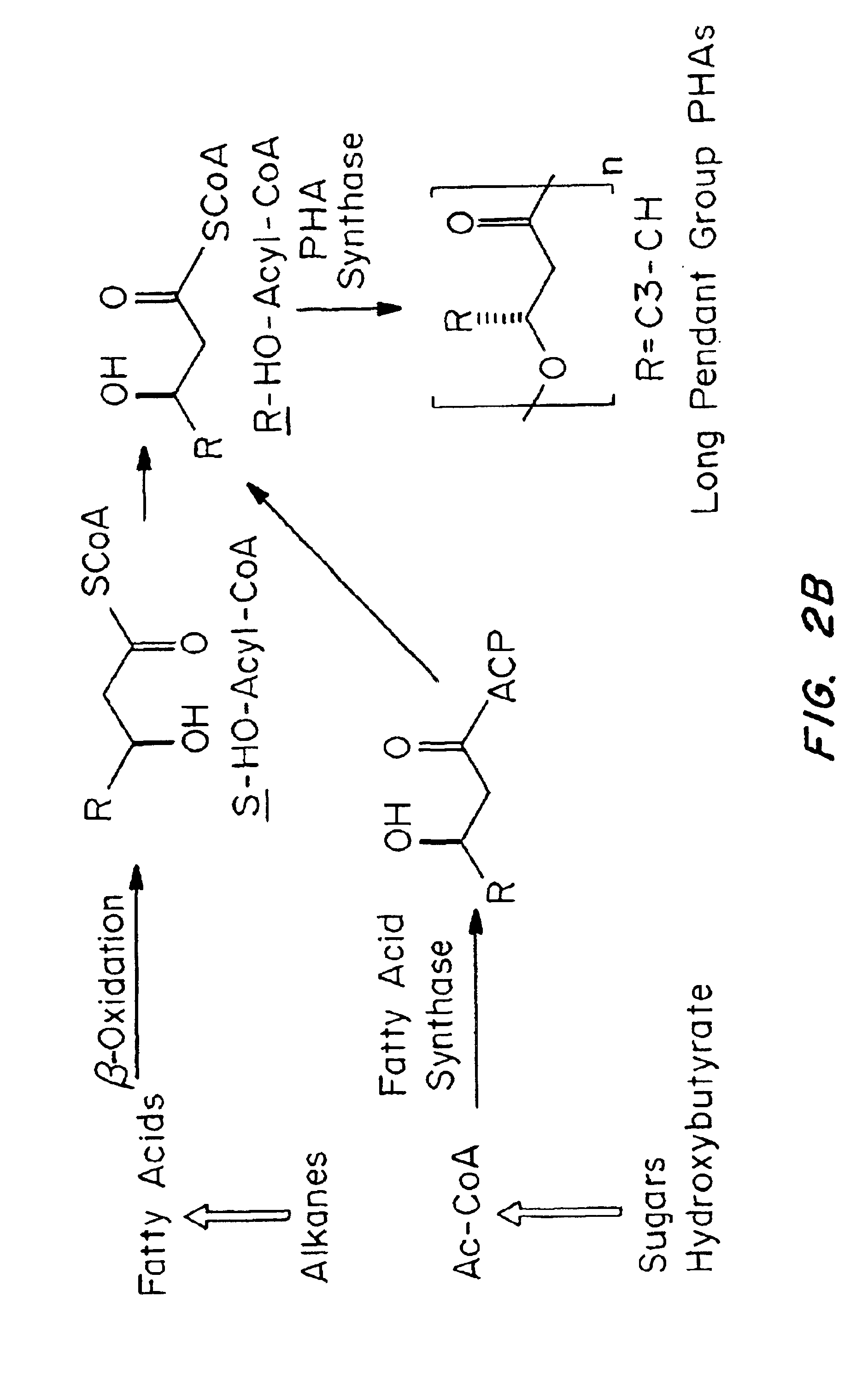
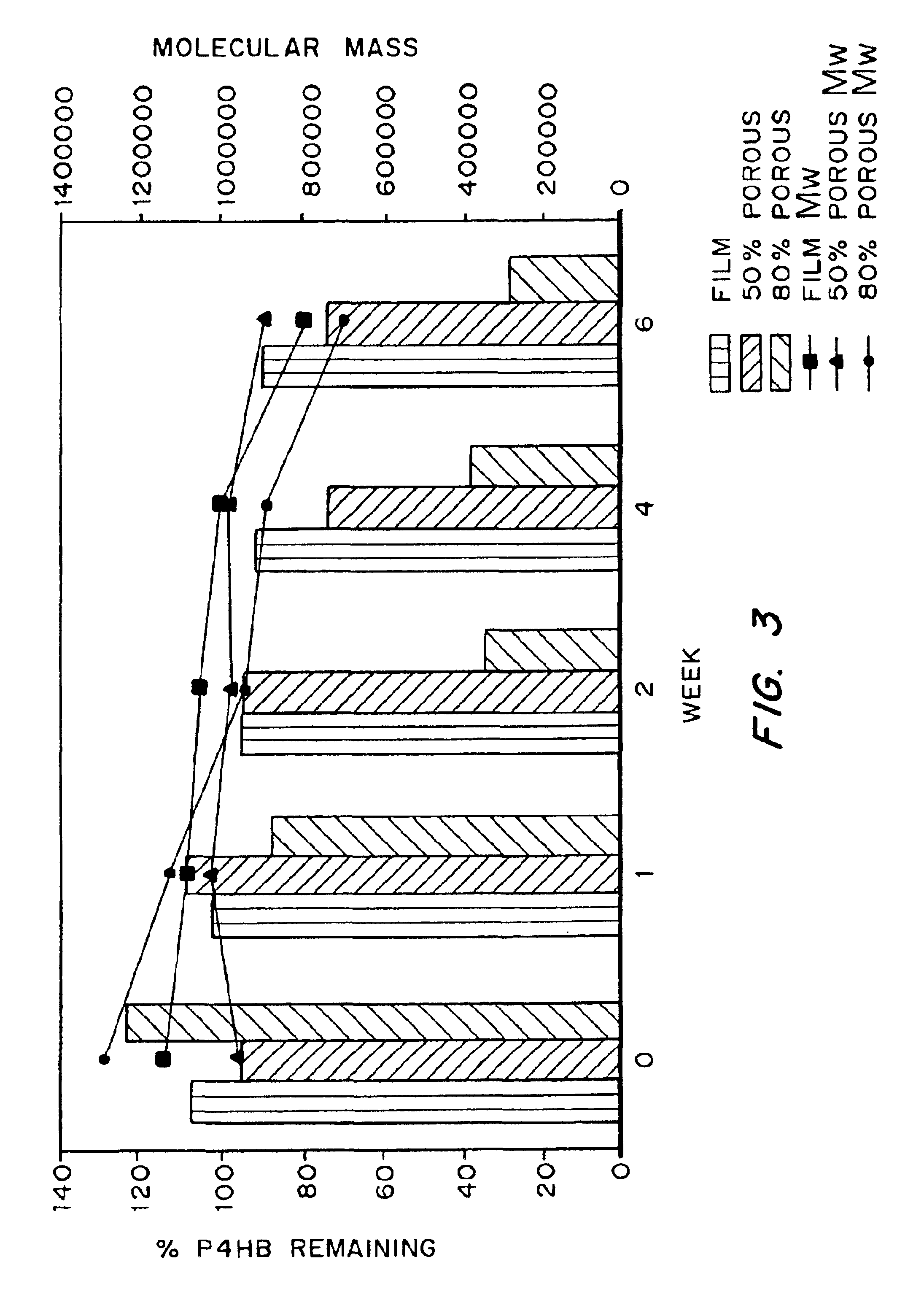
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
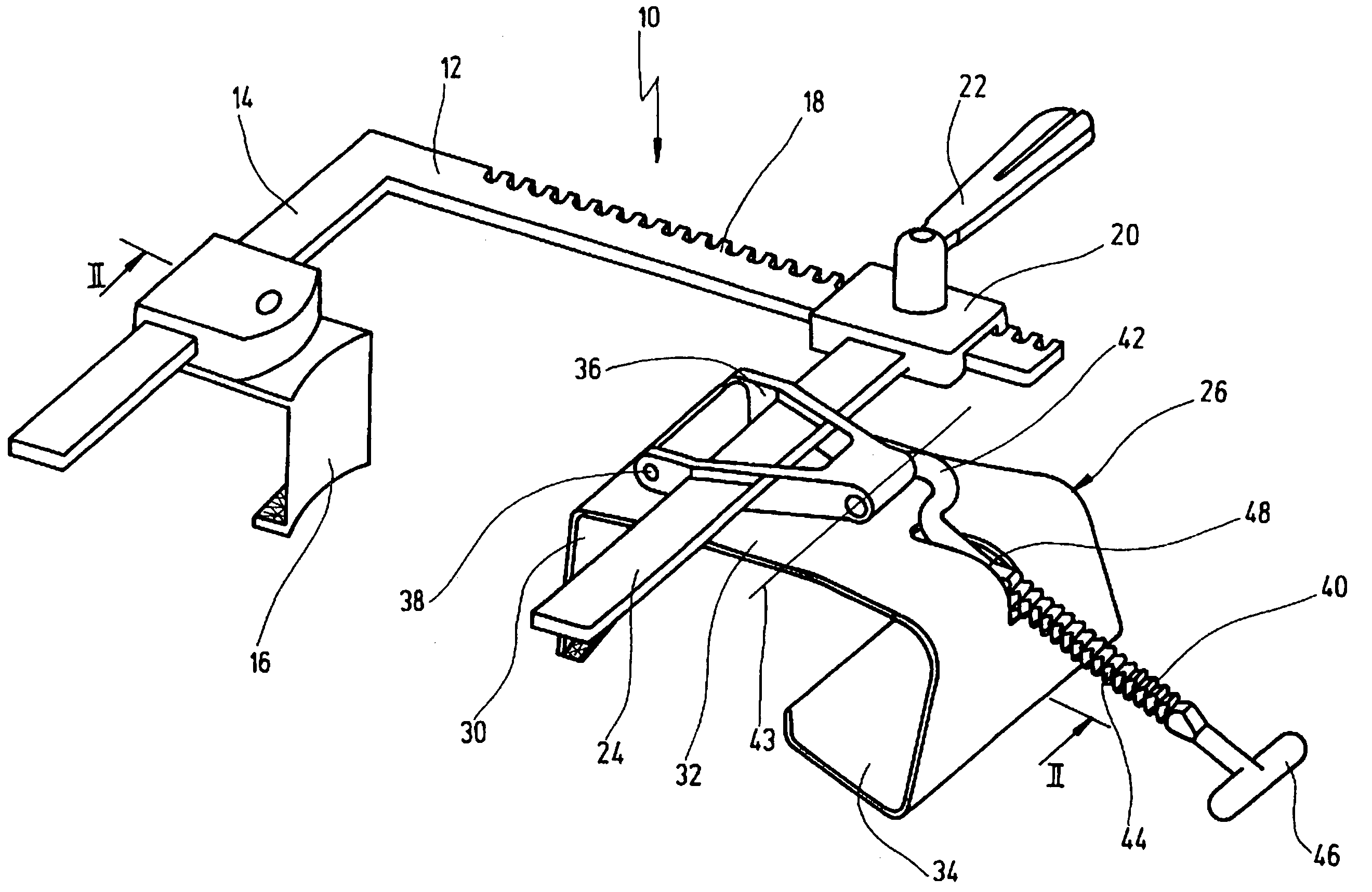
Laparoscopic tissue thickness and clamp load measuring devices
A surgical instrument having opposed jaws that can be selectively moved between open and closed positions. Various embodiments include components for measuring the thickness of tissue clamped between the opposed jaws. Some embodiments are configured to ascertain the amount of compressive force that is being applied to the tissue while the thickness of the tissue is being determined. The tissue thickness data is displayed on the instrument itself and / or on a display that is remote from the instrument. The various embodiments may comprise different types of surgical instruments such as surgical staplers and graspers. A jaw arrangement with jaws shaped to define a cradle that corresponds to a cross-sectional shape of an object is also disclosed. The components that generate the thickness data may be electrically or mechanically actuated.
Owner:CILAG GMBH INT
Bioabsorbable wound dressing
ActiveUS7041868B2Promote wound healingEasy to useAdhesive dressingsNon-surgical orthopedic devicesCell adhesionAdhesion process
A wound dressing includes a first layer located adjacent the wound and which comprises a material that is bioabsorbable, porous and adapted for serving as a scaffold for cell attachment and proliferation; and a second layer which is in contact with the first layer and which comprises an absorbent, gel forming material adapted for serving as a barrier to cell adhesion and penetration. A method of treating a wound with the dressing is also disclosed.
Owner:AVENT INC
Surgical stapler with an end effector support
A surgical stapler is provided having a cartridge arm for holding staples, an anvil pivotally connected thereto to deform fired staples from the cartridge arm, and a support for substantially preventing anvil deflection during staple firing to help insure proper staple formation. Some supports allow pivoting between the anvil and cartridge arm when in a first position, but prevent or hinder the pivoting action when in a second position. Supports can also be adapted to contact the anvil at a distal position relative to the pivot point between the anvil and cartridge arm. Exemplary structures of supports embodied as one or more links or as a sleeve are described. Methods of use are also discussed.
Owner:ETHICON ENDO SURGERY INC
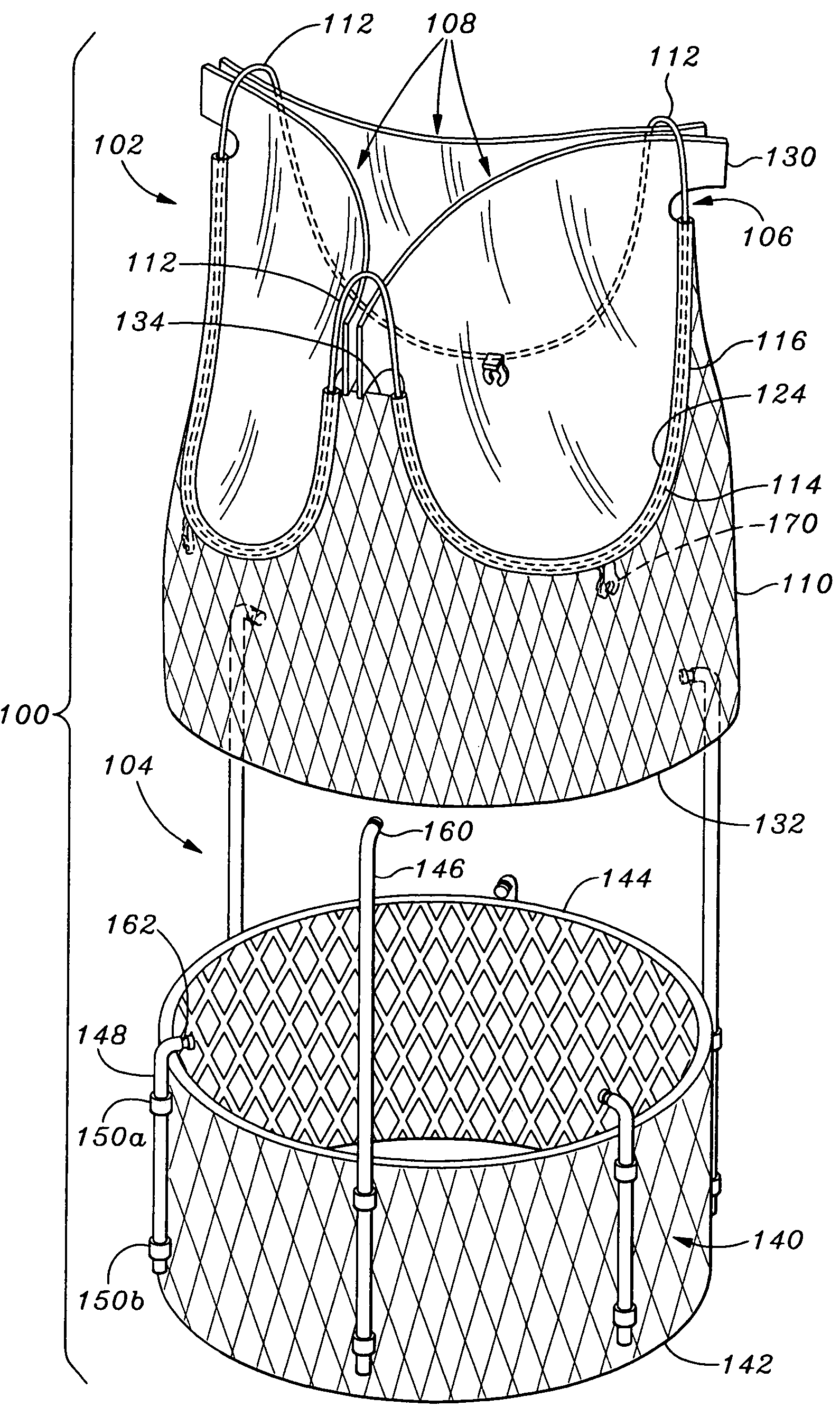
Use of reinforced foam implants with enhanced integrity for soft tissue repair and regeneration
A biocompatible tissue repair stimulating implant or "scaffold" device is used to repair tissue injuries, particularly injuries to ligaments, tendons, and nerves. Such implants are especially useful in methods that involve surgical procedures to repair injuries to ligament, tendon, and nerve tissue in the hand and foot. The repair procedures may be conducted with implants that contain a biological component that assists in healing or tissue repair.
Owner:DEPUY MITEK INC
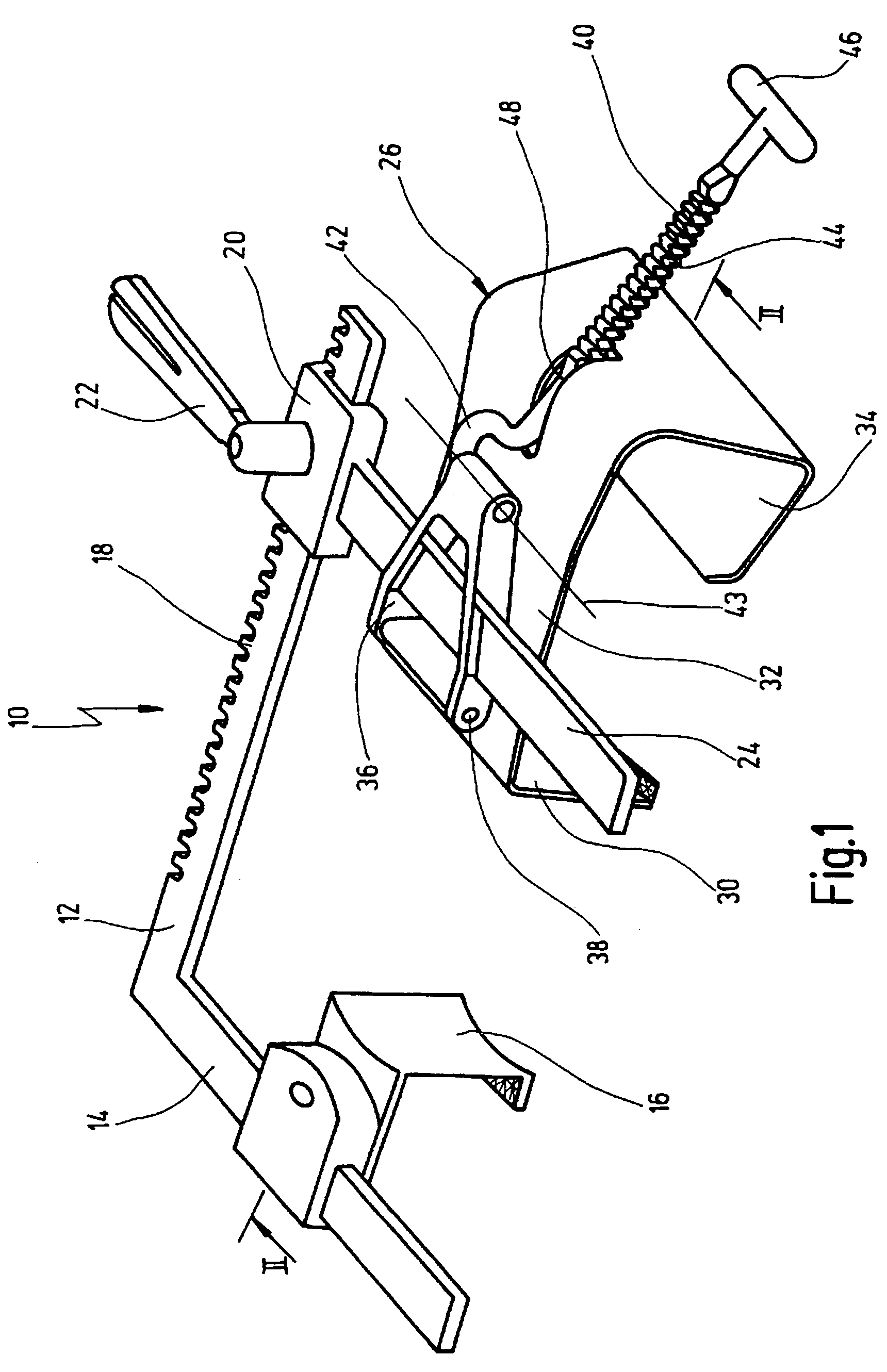
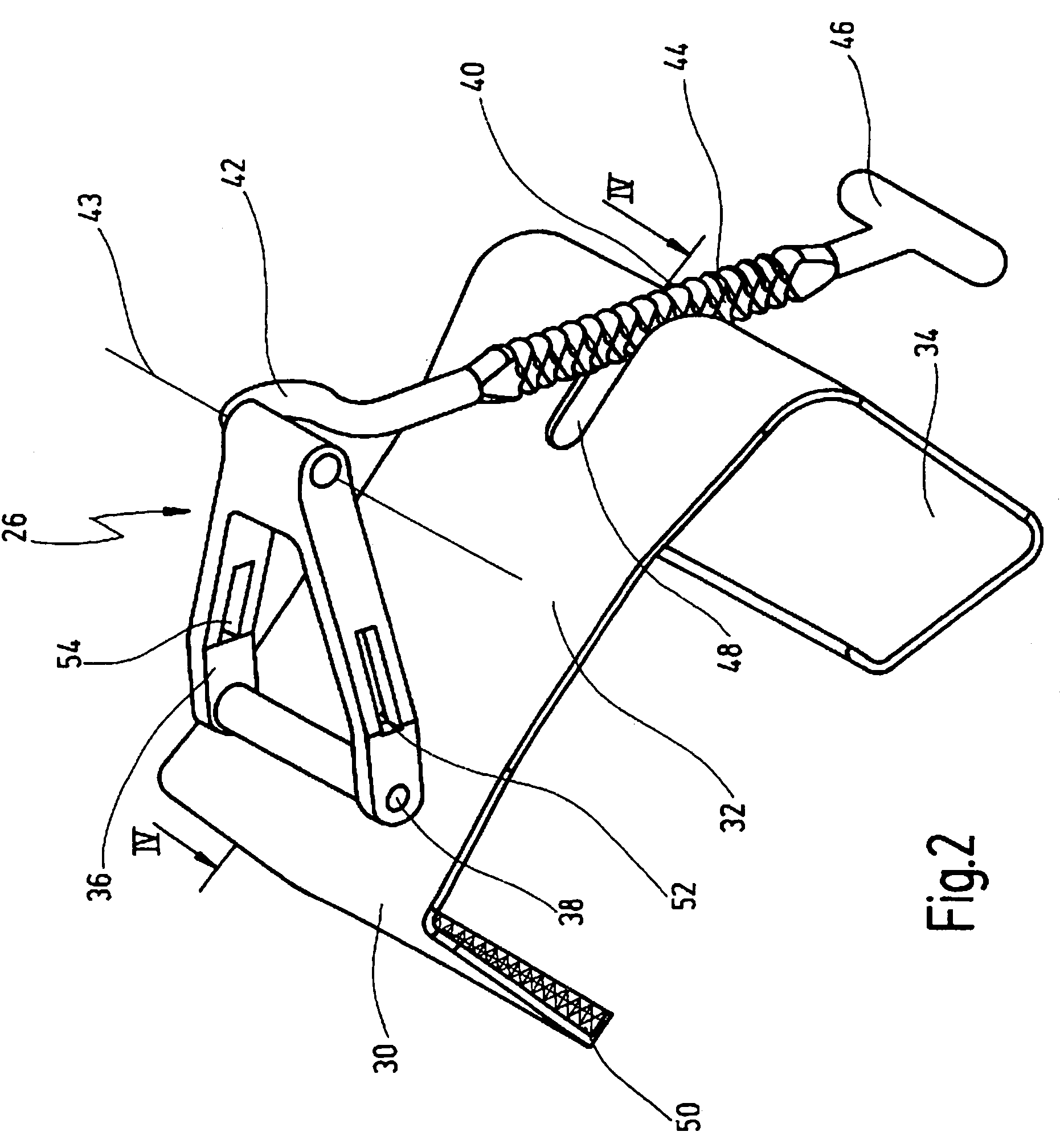
Retractor for performing heart and thorax surgeries
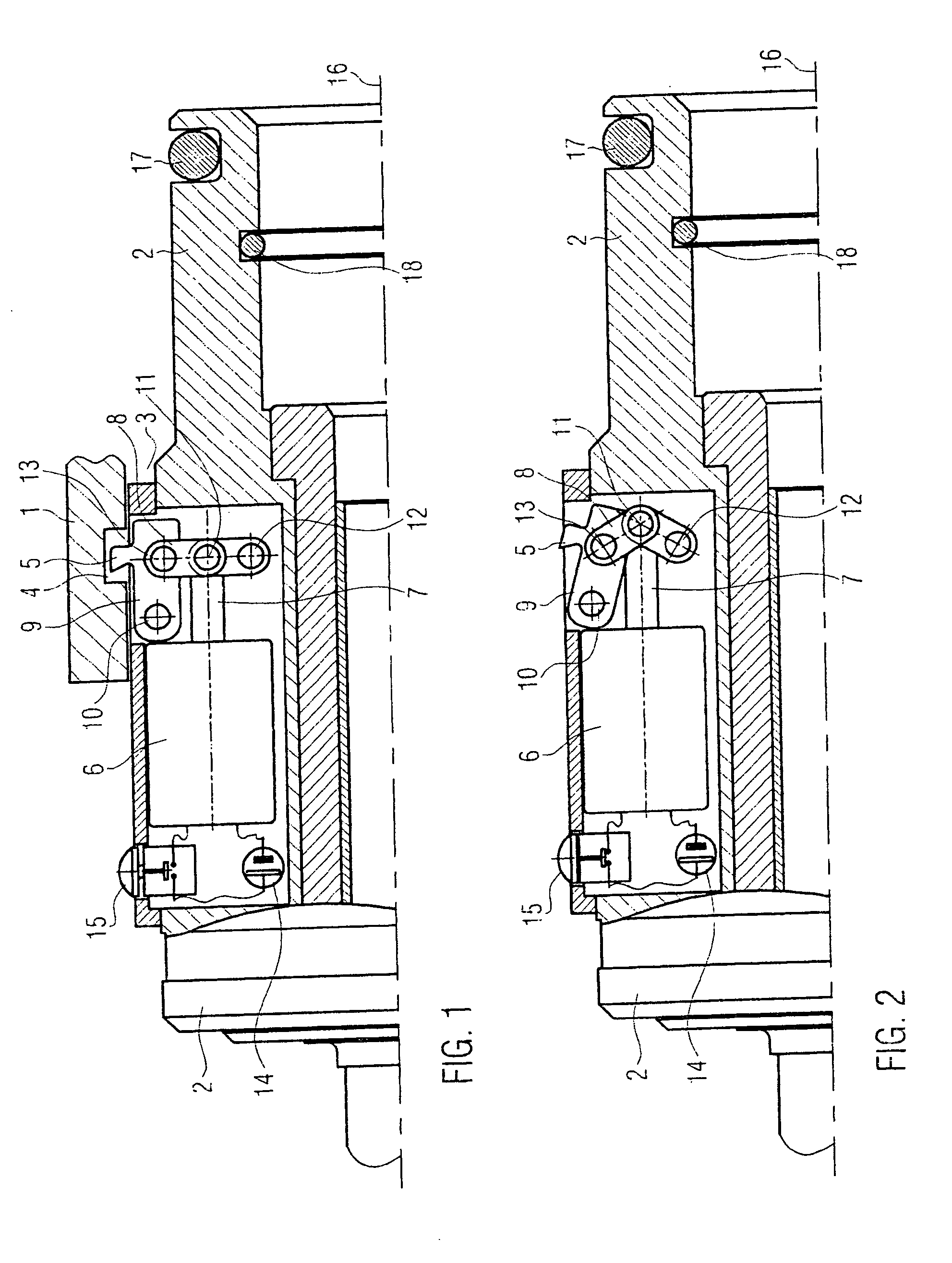
A retractor has a rail, a first holding arm protruding from this rail at an angle at a second holding arm extending approximately parallel to the first holding arm. A distance between the two arms being modifiable. At least one functional element is mounted on one of these holding arms, which functional element has a section extending transversely to the holding arms and a swivel bracket protruding from this transverse section. The functional element being pivotable about a pin extending roughly parallel to the arms via the swivel bracket, a swiveled position can be set by grasping the function element with a hand. A locking mechanism serves for locking the functional element in a swiveled position.
Owner:KARL STORZ GMBH & CO KG
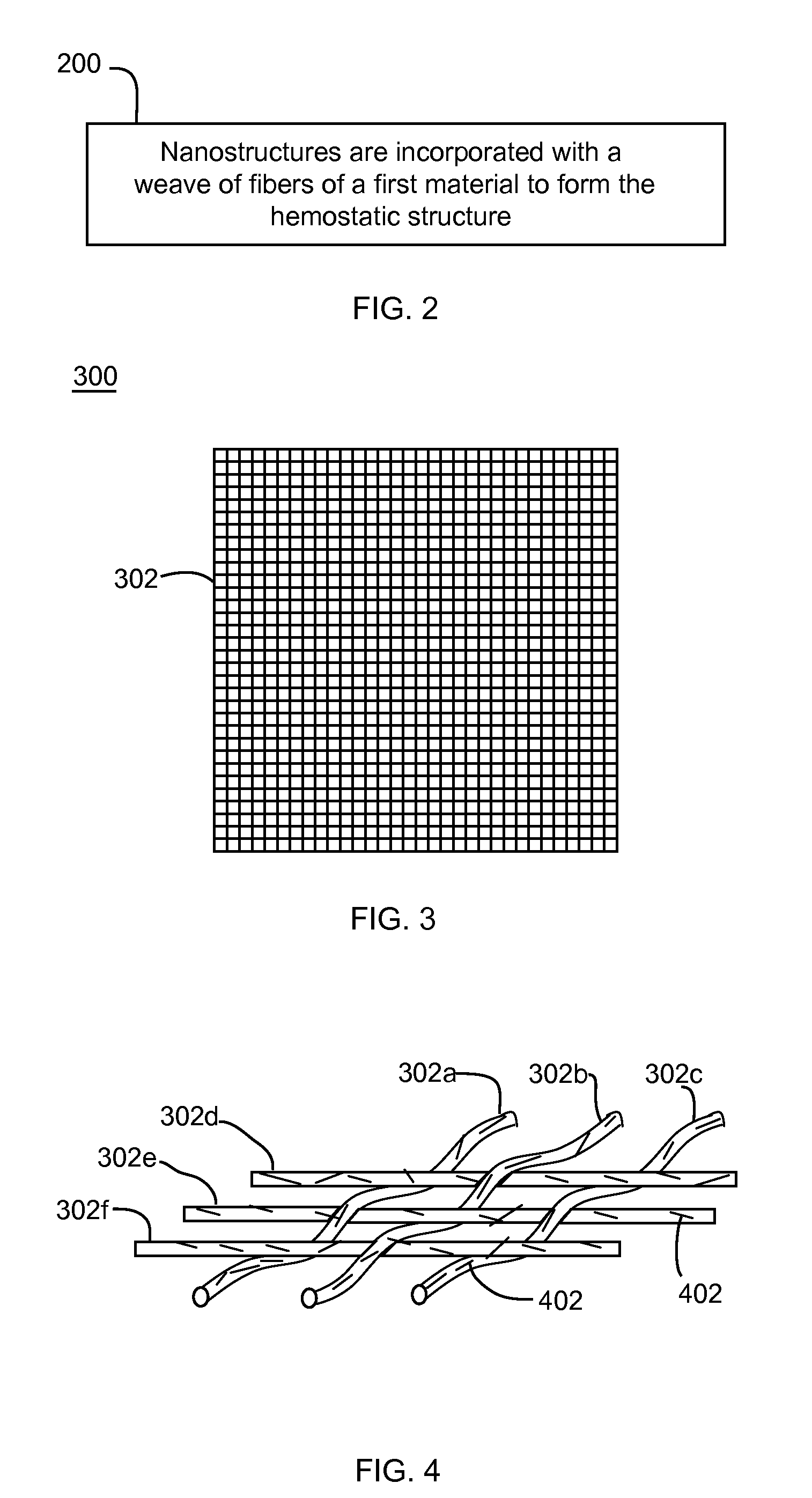
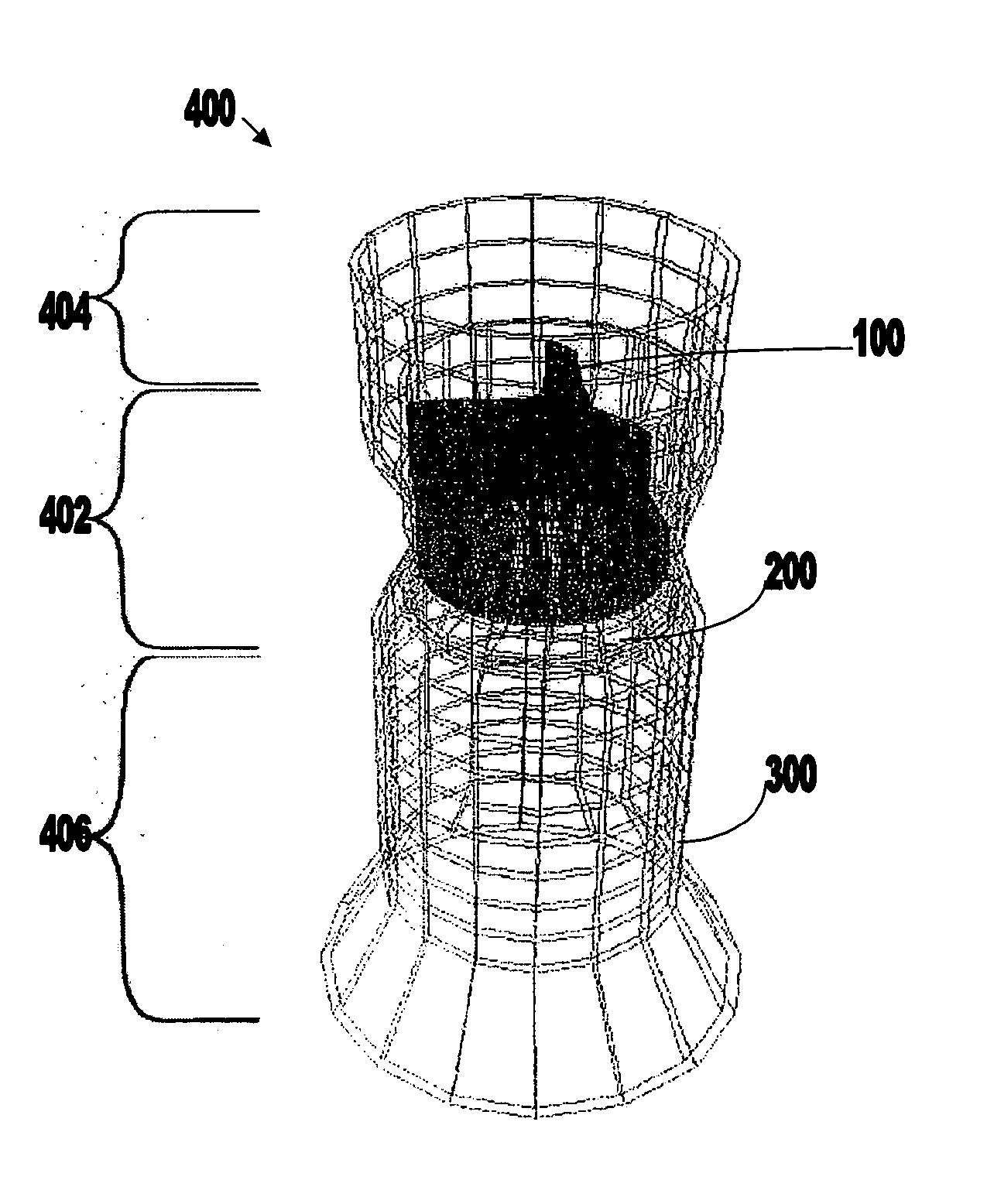
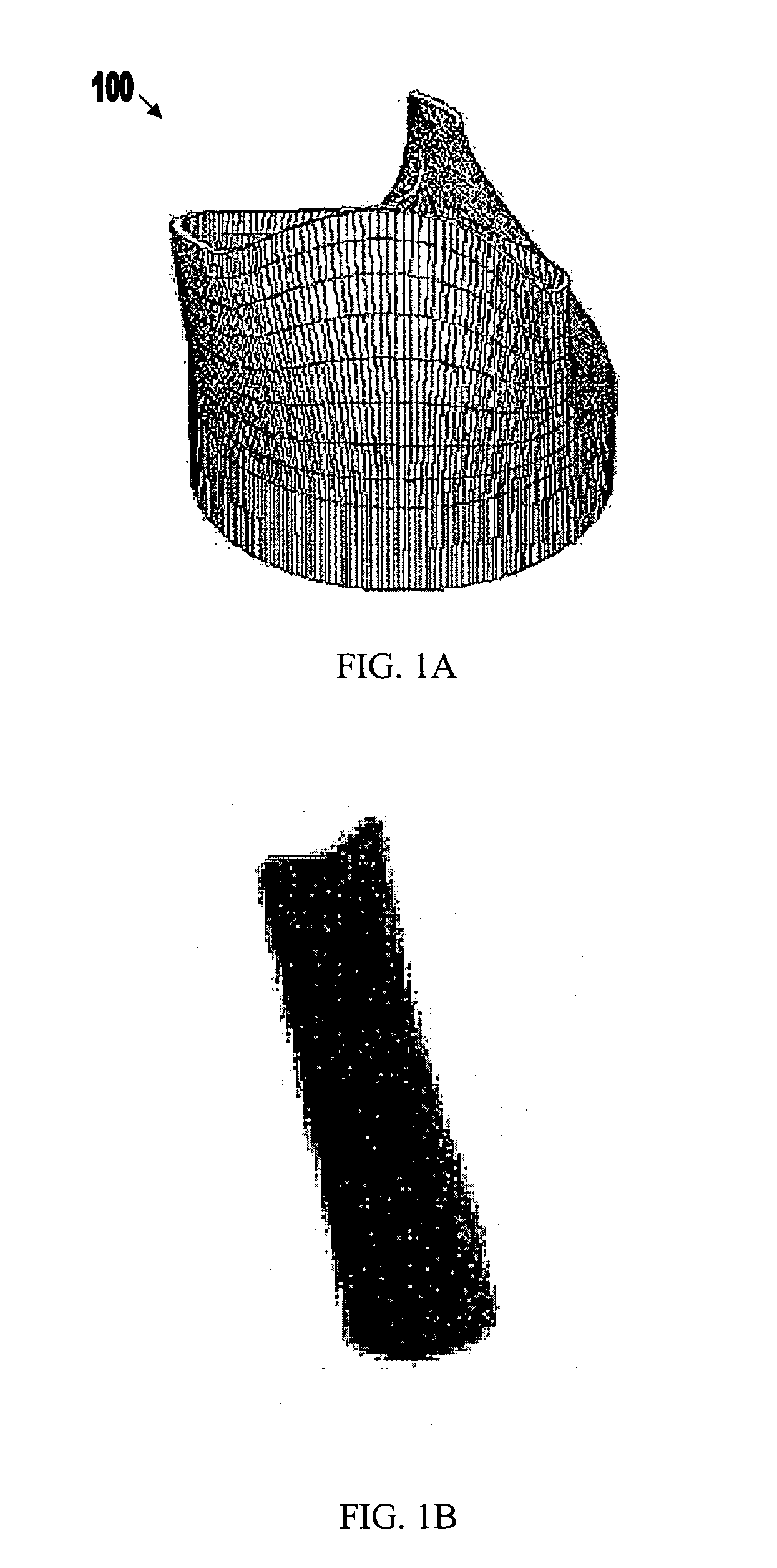
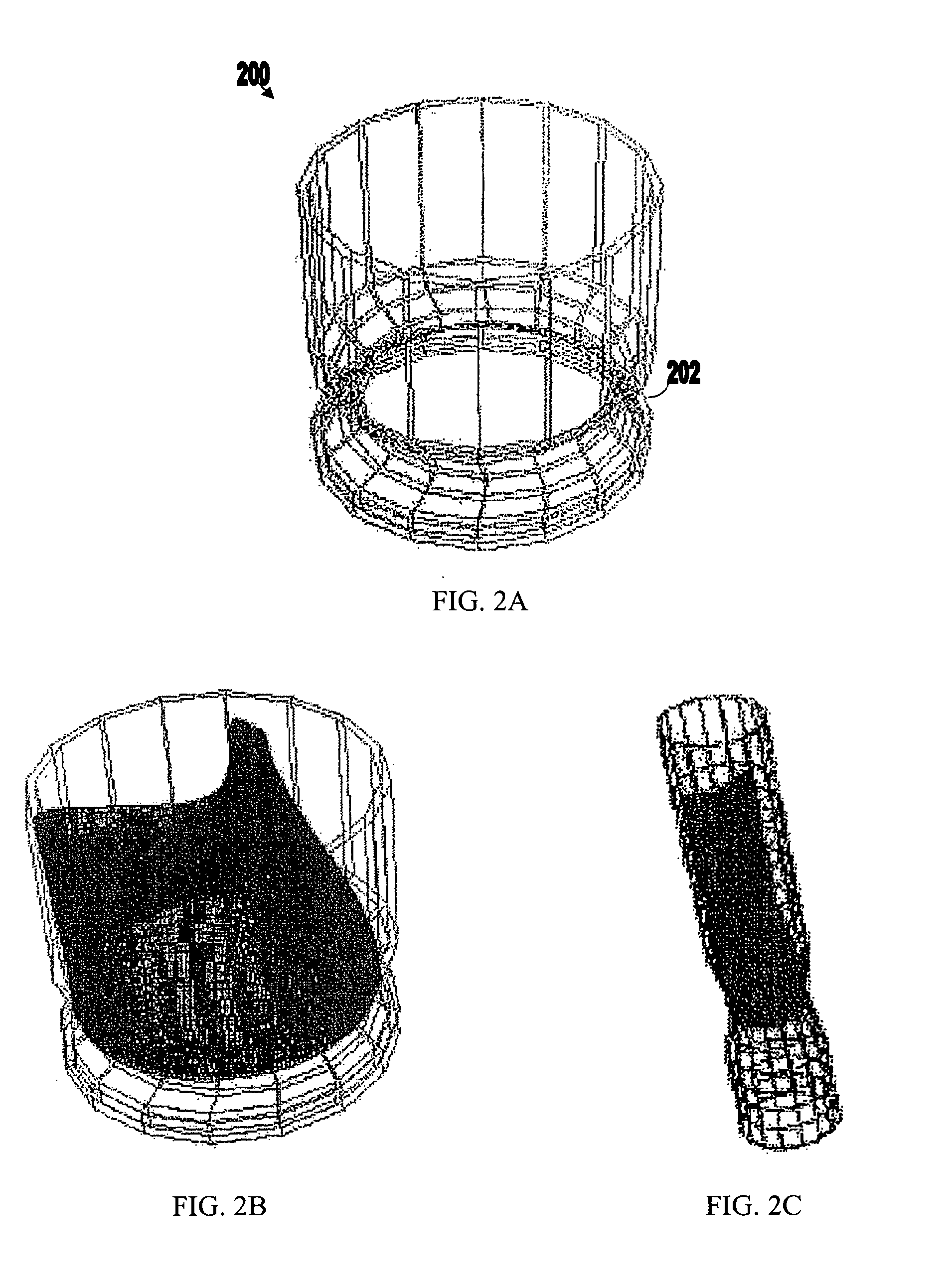
Nanostructure-enhanced platelet binding and hemostatic structures
InactiveUS8319002B2Enhancing overall rate and strengthInduce platelet binding and efficient hemostasisBiocideSurgical adhesivesPlateletNanofiber
Methods, systems, and apparatuses for nanomaterial-enhanced platelet binding and hemostatic medical devices are provided. Hemostatic materials and structures are provided that induce platelet binding, including platelet binding and the coagulation of blood at a wound / opening caused by trauma, a surgical procedure, ulceration, or other cause. Example embodiments include platelet binding devices, hemostatic bandages, hemostatic plugs, and hemostatic formulations. The hemostatic materials and structures may incorporate nanostructures and / or further hemostatic elements such as polymers, silicon nanofibers, silicon dioxide nanofibers, and / or glass beads into a highly absorbent, gelling scaffold. The hemostatic materials and structures may be resorbable.
Owner:NANOSYS INC
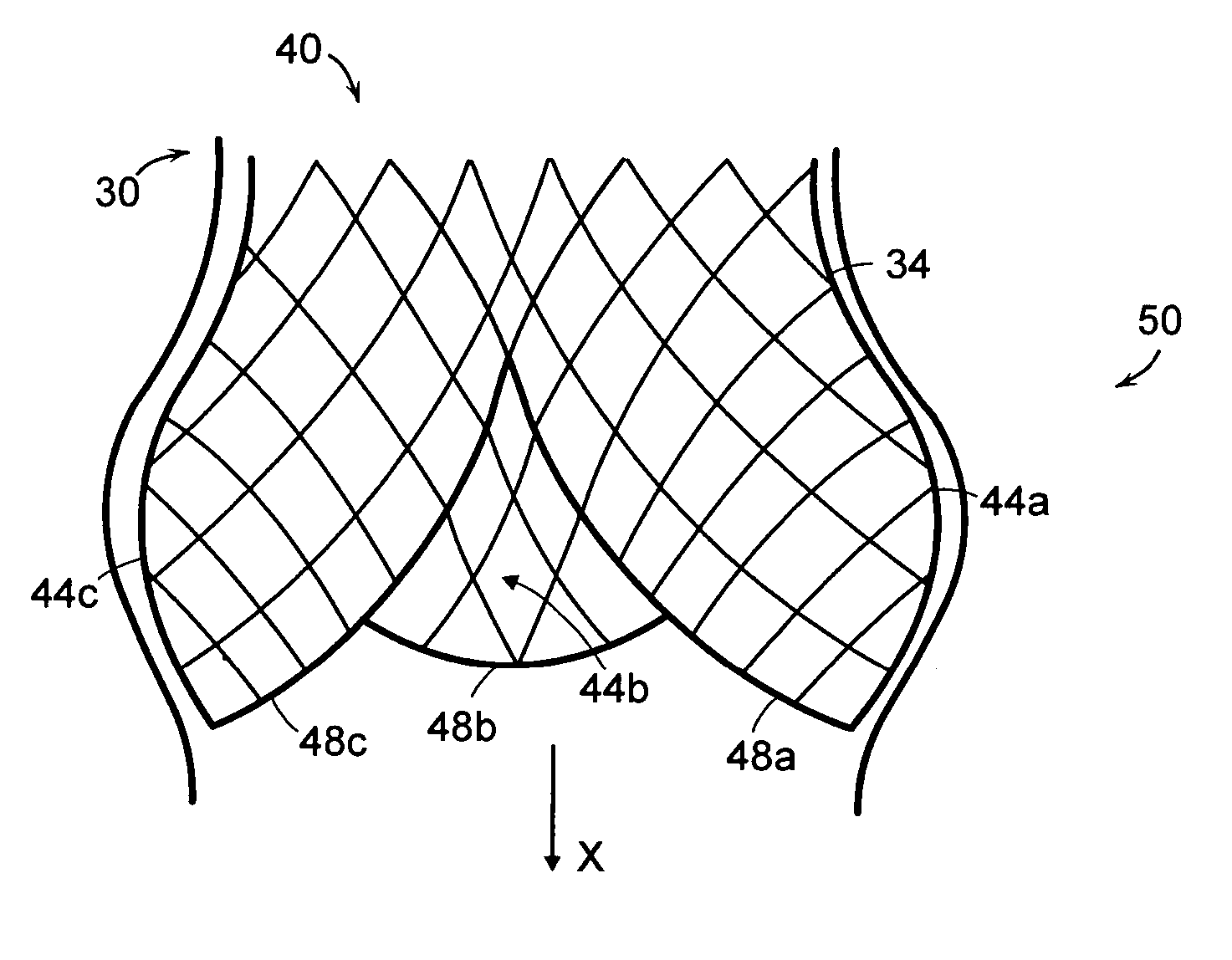
Stent-valves for valve replacement and associated methods and systems for surgery
InactiveUS20070213813A1Simple methodReduce riskStentsBalloon catheterLess invasive surgeryInsertion stent
Stent-valves (e.g., single-stent-valves and double-stent-valves), associated methods and systems for their delivery via minimally-invasive surgery, and guide-wire compatible closure devices for sealing access orifices are provided.
Owner:SYMETIS
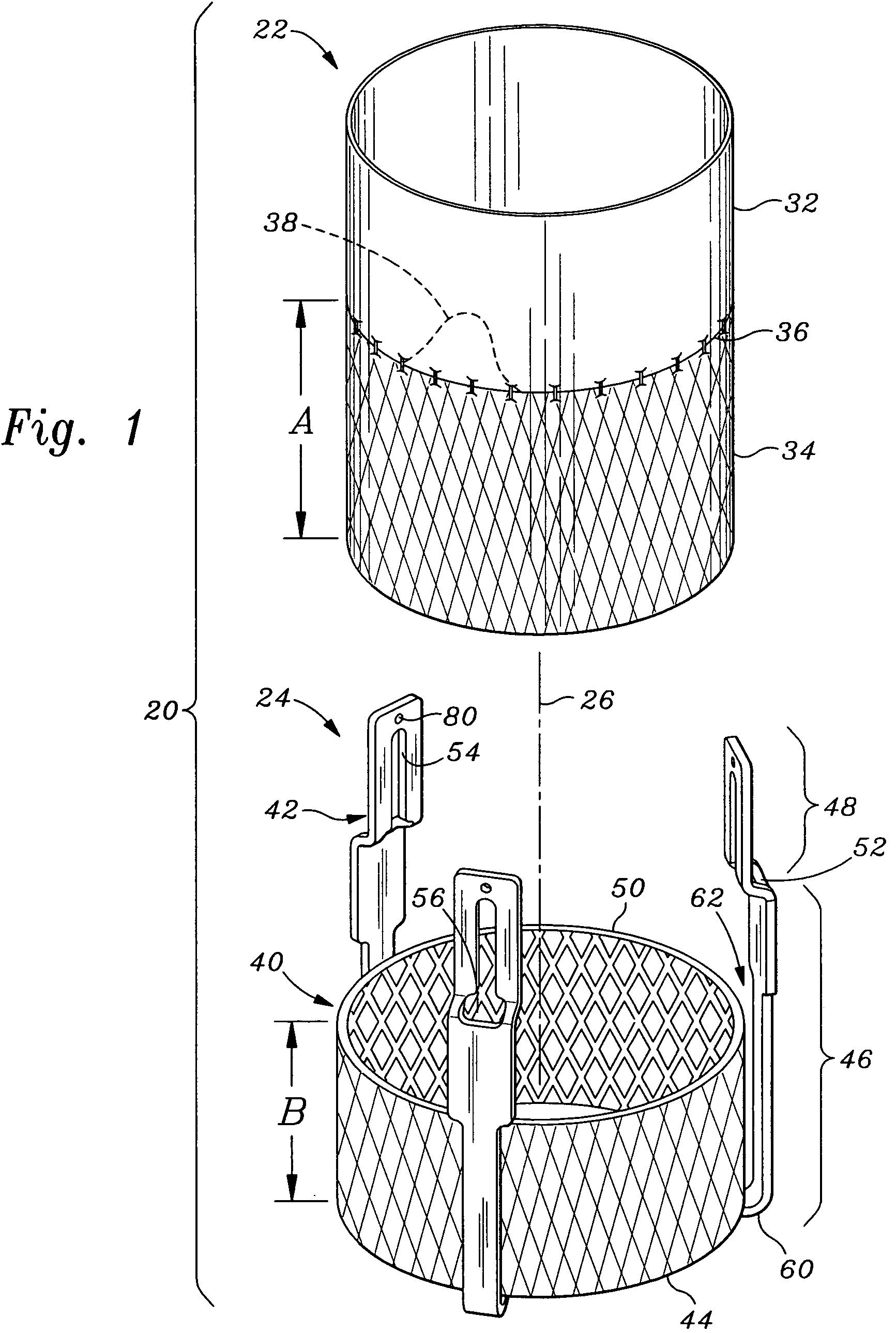
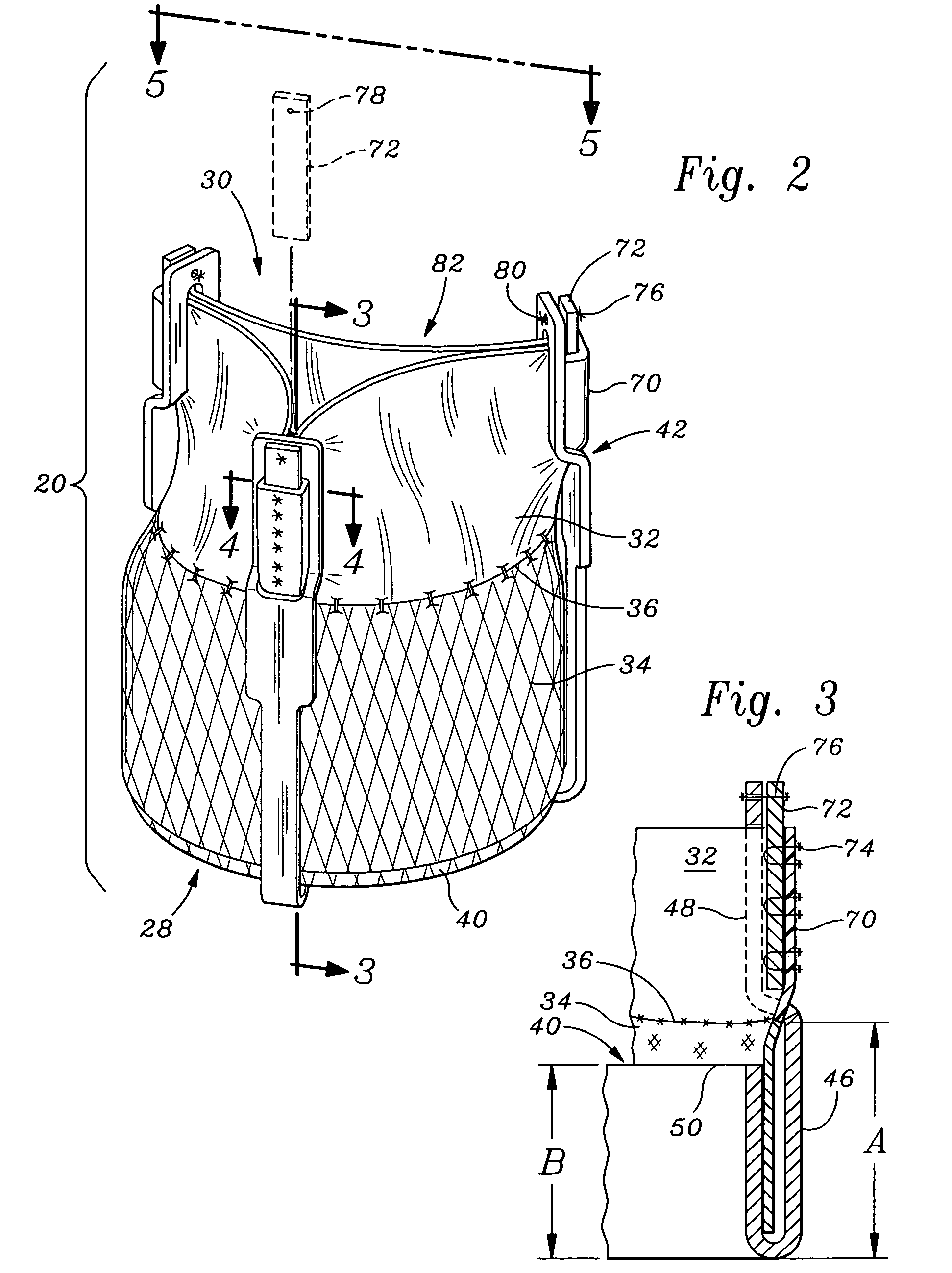
Scaffold for connective tissue repair
A connective tissue scaffold including opposed first and second anchoring segments formed from a plurality of bioresorbable polymeric fibers oriented in a direction substantially parallel to a longitudinal axis of the scaffold and a plurality of bioresorbable polymeric fibers oriented in a direction substantially transverse to a longitudinal axis of the scaffold. A central segment joins the first and second anchoring segments and includes a plurality of bioresorbable polymeric fibers oriented in a direction substantially parallel to the longitudinal axis of the scaffold. The scaffold can also a tissue particle and / or biological component.
Owner:DEPUY SYNTHES PROD INC
Reinforced foam implants with enhanced integrity for soft tissue repair and regeneration
InactiveUS6852330B2Sufficient structural integritySufficient propertyPowder deliveryOrganic active ingredientsTissue repairSoft tissue repair
A biocompatible tissue repair stimulating implant or “scaffold” device, and methods for making and using such a device, are provided. The implant includes one or more layers of a bioabsorbable polymeric foam having pores with an open cell pore structure. A reinforcement component is also present within the implant to contribute enhanced mechanical and handling properties. The implant houses a biological component that may be released to tissue adjacent the location in which the implant is implanted to faciliate and / or expedite the healing of tissue. This biological component resides primarily within the foam component of the implant, being incorporated within pores formed within the foam.
Owner:DEPUY SYNTHES PROD INC
Transcatheter delivery of a replacement heart valve
A replacement heart valve apparatus. The heart valve apparatus includes a stent and a valve frame having a substantially cylindrical body defining a lumen. The valve frame includes a plurality of curved wire pairs attached to the substantially cylindrical body. Each curved wire pair includes an inner curved wire and an outer curved wire. The wire frame further having a plurality of leaflets. Each leaflet is attached to a respective inner curved wire and extends over a respective outer curved wire, so as to position the body of the leaflet within the lumen of the valve frame.
Owner:CHILDRENS MEDICAL CENT CORP
Attachment of absorbable tissue scaffolds to fixation devices
The present invention relates to tissue scaffold implant devices useful in the repair and / or regeneration of diseased and / or damaged musculoskeletal tissue and that include a tissue scaffold component fixedly attached to a scaffold fixation component via at least one of sutures, fabrics, fibers, threads, elastomeric bands, reinforcing elements and interlocking protrusions for engaging and maintaining the scaffold component fixedly attached to the fixation component.
Owner:ETHICON INC
Ergonomic and semi-automatic manipulator, and applications to instruments for minimally invasive surgery
Owner:DEXTERITE SURGICAL
Hybrid biologic-synthetic bioabsorbable scaffolds
ActiveUS8366787B2Increase surface areaGood mechanical integritySuture equipmentsBone implantBioabsorbable scaffoldCell-Extracellular Matrix
A bioprosthetic device is provided for soft tissue attachment, reinforcement, and or reconstruction. The device comprises a naturally occurring extracellular matrix portion and a three-dimensional synthetic portion. In illustrated embodiments, the naturally occurring extracellular matrix portion comprises layers of small intestine submucosa, and the three-dimensional synthetic portion comprises a foam or a three-dimensional mesh, textile, or felt.
Owner:DEPUY SYNTHES PROD INC
Scaffolds with viable tissue
ActiveUS20050177249A1Promote cell growthProsthesisTissue regenerationTissue defectBiomedical engineering
A composite implant is provided for repairing a tissue defect in a patient. In one embodiment, the implant is a porous tissue scaffold having at least one pocket formed therein and adapted to contain a viable tissue. The tissue scaffold can have a variety of configurations, and in one embodiment it includes top and bottom portions that can be at least partially mated to one another, and in an exemplary embodiment that are heated sealed to one another around a perimeter thereof to form an enclosed pocket therebetween. The pocket is preferably sealed with a viable tissue disposed therein. In another embodiment, the tissue scaffold is substantially wedge-shaped and the pocket comprises a hollow interior formed in the tissue scaffold, and / or at least one lumen extending into the tissue scaffold. The tissue scaffold can also optionally include at least one surface feature formed thereof to promote blood vessel formation.
Owner:DEPUY SYNTHES PROD INC
Kit enabling a prosthetic valve to be placed in a body enabling a prosthetic valve to be put into place in a duct in the body
InactiveUS20050043790A1Destruction damageRisk of destructionHeart valvesBlood vesselsProsthetic valveInsertion stent
The present invention is an assembly comprising a prosthetic valve to be implanted; a radially expandable stent comprising at least one zone intended to be expanded to allow the stent, in the expanded state, to bear against the wall of the body duct to be fitted with the valve, this bearing making it possible to immobilize this stent with respect to this wall; and means for mounting the valve with respect to the stent, making it possible to connect the valve to the stent in such a way that the placement of the stent allows the valve to be mounted in the body duct, and expansion means such as a balloon catheter being provided to trigger expansion of the stent at the implantation site. According to the invention, the valve and the stent are designed in such a way that, at the moment when the stent is expanded, the valve is situated outside the zone or zones of the stent that are subjected to said expansion means. The invention thus consists in separating the valve and said zone or zones to be expanded, so that the expansion of the stent can be effected with an expansion force suitable for perfect anchoring of this stent in the wall of the body duct to be fitted with the valve, and without any risk of destruction or damage of the valve.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
Device and method for replacing mitral valve
InactiveUS20090276040A1Prevent movementRelieve pressureSuture equipmentsStentsNative tissueProsthesis
A prosthetic mitral valve assembly and method of inserting the same is disclosed. In certain disclosed embodiments, the prosthetic mitral valve assembly has a flared upper end and a tapered portion to fit the contours of the native mitral valve. The prosthetic mitral valve assembly can include a stent or outer support frame with a valve mounted therein, The assembly can be adapted to expand radially outwardly and into contact with the native tissue to create a pressure fit. One embodiment of a method includes positioning the mitral valve assembly below the annulus such that the annulus itself can restrict the assembly from moving in an upward direction towards the left atrium. The mitral valve assembly is also positioned so that the leaflets of the mitral valve hold the assembly to prevent downward movement of the assembly towards the left ventricle.
Owner:EDWARDS LIFESCIENCES CORP
Surgical coupling device
InactiveUS20070179477A1Simple structureSimple and safe operationDiagnosticsSpannersCouplingEngineering
A surgical coupling device detachably connects a tool holder to a surgical instrument, wherein the tool holder is provided with a recess into which a coupling portion of the instrument can be inserted detachably, wherein a locking recess is provided in an inner wall of the recess, with which a locking member can be engaged detachably, the locking member being supported at the instrument, wherein the locking member is selectively movable into a locking position and a release position by a servo drive.
Owner:GEBR BRASSELER GMBH & CO KG
System and method for implanting a two-part prosthetic heart valve
Expandable heart valves for minimally invasive valve replacement surgeries are disclosed. In a first embodiment, an expandable pre-assembled heart valve includes a plastically-expandable annular base having plurality of upstanding commissure posts. A tubular flexible member including a prosthetic section and a fabric section is provided, with the prosthetic section being connected to the commissure posts and defining leaflets therebetween, and the fabric section being attached to the annular base. In a second embodiment, an expandable heart valve includes an annular tissue-engaging base and a subassembly having an elastic wireform and a plurality of leaflets connected thereto. The annular base and subassembly are separately stored and connected just prior to delivery to the host annulus. Preferably, the leaflet subassembly is stored in its relaxed configuration to avoid deformation of the leaflets. The expandable heart valves maybe implanted using a balloon catheter. Preferably, the leaflets of the heart valves are secured to the commissure regions of the expandable stents using a clamping arrangement to reduce stress.
Owner:EDWARDS LIFESCIENCES CORP
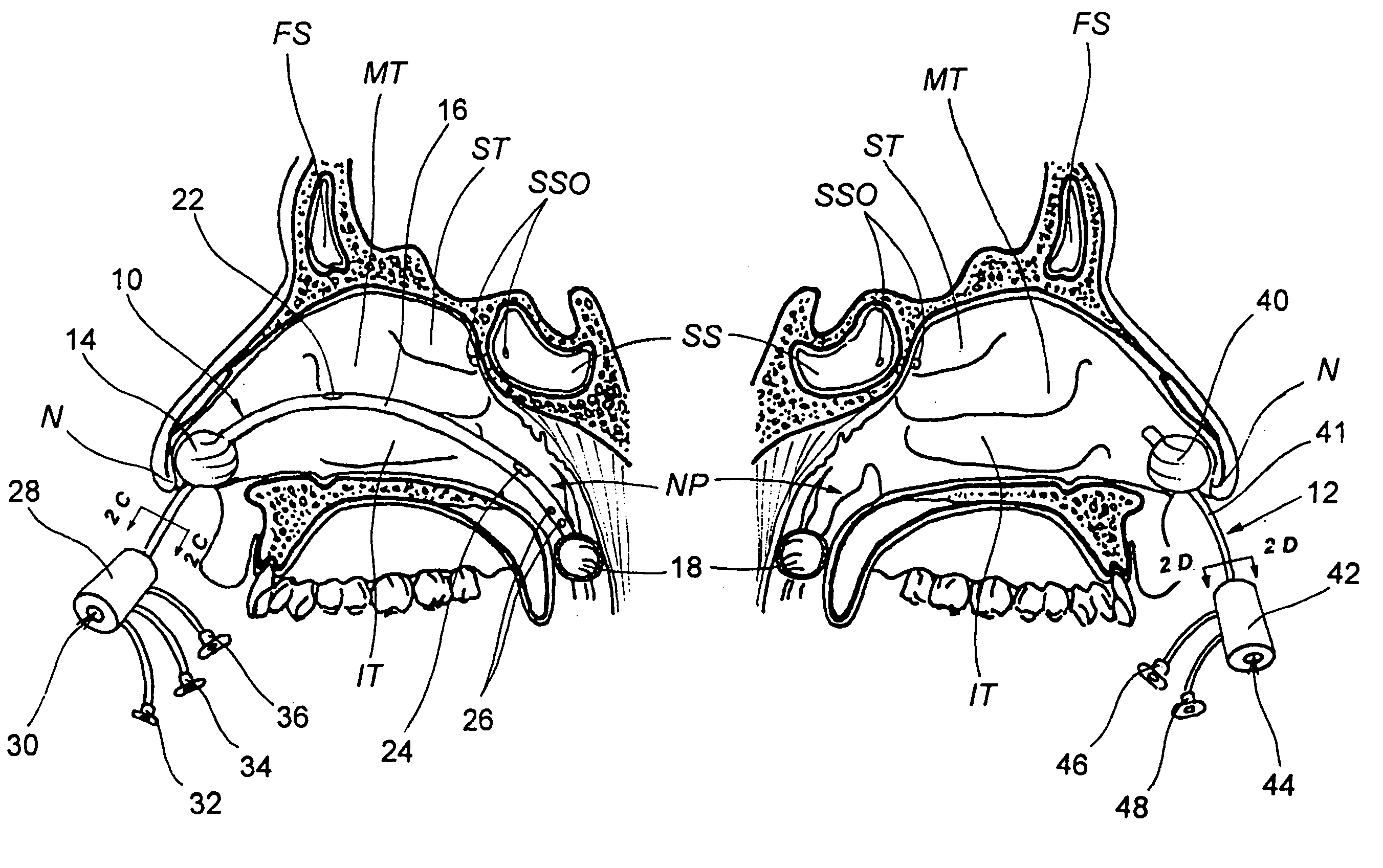
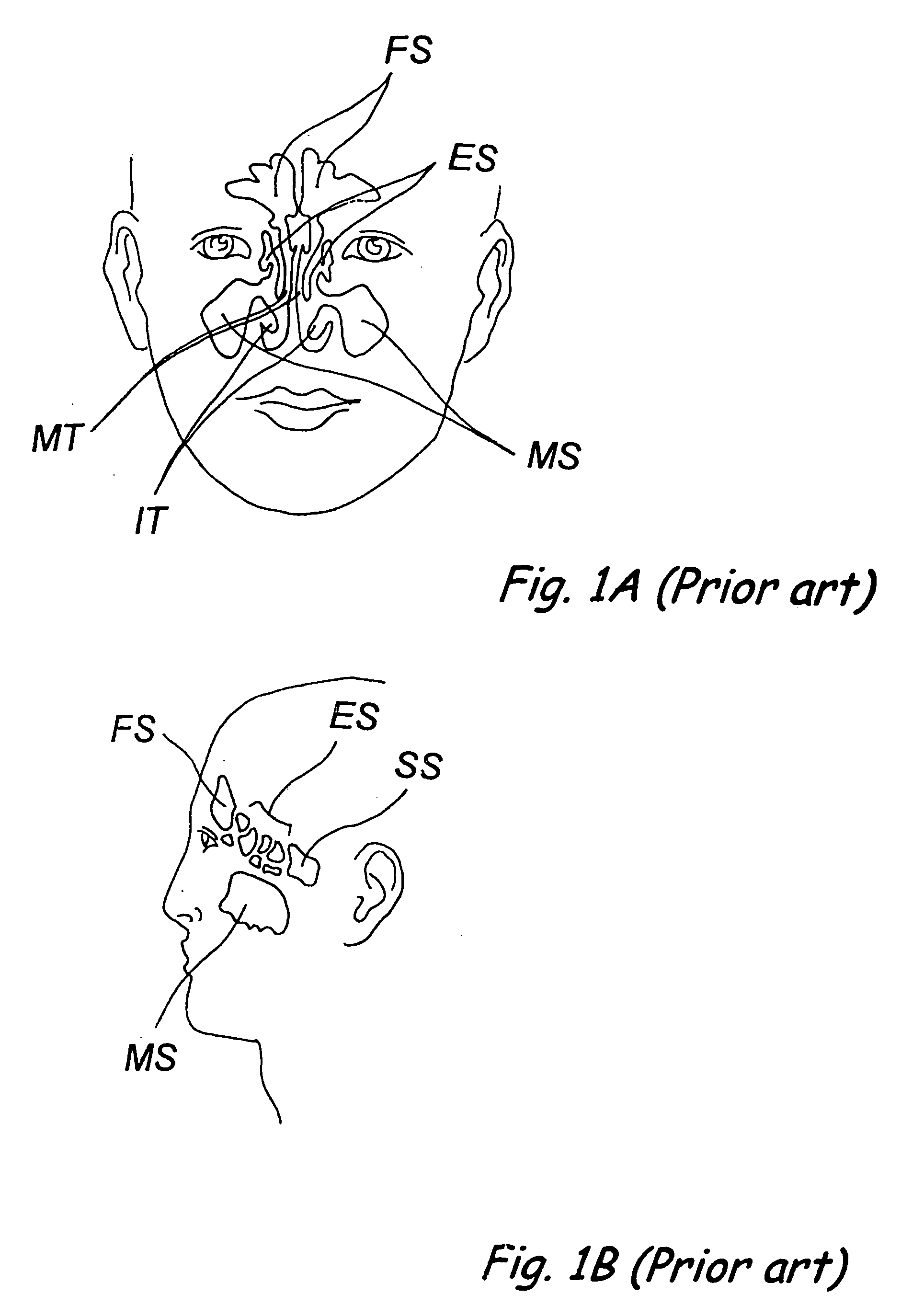
Apparatus and methods for dilating and modifying ostia of paranasal sinuses and other intranasal or paranasal structures
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat.
Owner:ACCLARENT INC
Methods for rapid deployment of prosthetic heart valves
ActiveUS20060287717A1Quickly and easily replacingUse minimizedStentsAnnuloplasty ringsInsertion stentCoupling
A two-stage or component-based valve prosthesis that can be quickly and easily implanted during a surgical procedure is provided. The prosthetic valve comprises a support structure that is deployed at a treatment site. The prosthetic valve further comprises a valve member configured to be quickly connected to the support structure. The support structure may take the form of a stent that is expanded at the site of a native valve. If desired, the native leaflets may remain and the stent may be used to hold the native valve open. In this case, the stent may be balloon expandable and configured to resist the powerful recoil force of the native leaflets. The support structure is provided with a coupling means for attachment to the valve member, thereby fixing the position of the valve member in the body. The valve member may be a non-expandable type, or may be expandable from a compressed state to an expanded state. The system is particularly suited for rapid deployment of heart valves in a conventional open-heart surgical environment.
Owner:EDWARDS LIFESCIENCES CORP
Devices, systems and methods for diagnosing and treating sinusitus and other disorders of the ears, nose and/or throat
Sinusitis, enlarged nasal turbinates, tumors, infections, hearing disorders, allergic conditions, facial fractures and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies, endoscopic studies and transillumination studies. Access and occluder devices may be used to establish fluid tight seals in the anterior or posterior nasal cavities / nasopharynx and to facilitate insertion of working devices (e.g., scopes, guidewires, catheters, tissue cutting or remodeling devices, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting devices, substance delivery implants, etc.
Owner:ACCLARENT INC
Porous Substrates for Implantation
InactiveUS20100137990A1Easy to integrateGood biocompatibilitySuture equipmentsDental implantsPorous substrateAnimal body
A porous substrate or implant for implantation into a human or animal body constructed from a structural material and having one or more regions which when implanted are subjected to a relatively lower mechanical loading. The region(s) are constructed with lesser mechanical strength by having a lesser amount of structural material in said region(s) relative to other regions. This is achieved by controlling pore volume fraction in the regions. A spacer is adapted to define an open-cell pore network by taking a model of the required porous structure, and creating the spacer to represent the required porous structure using three-dimensional modelling. Material to form the substrate about the spacer in infiltrated the scaffold structure formed.
Owner:NATIONAL UNIVERSITY OF IRELAND
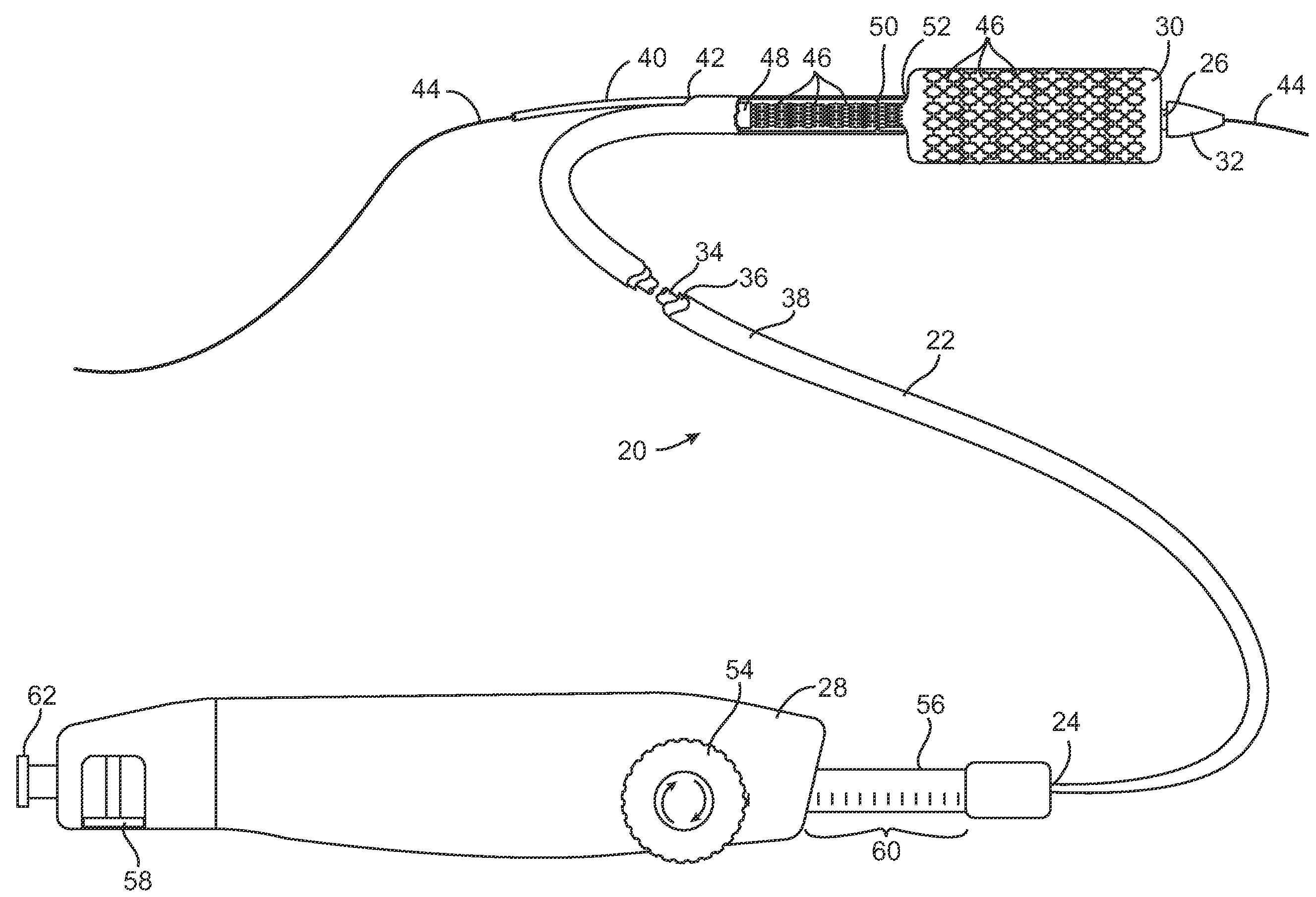
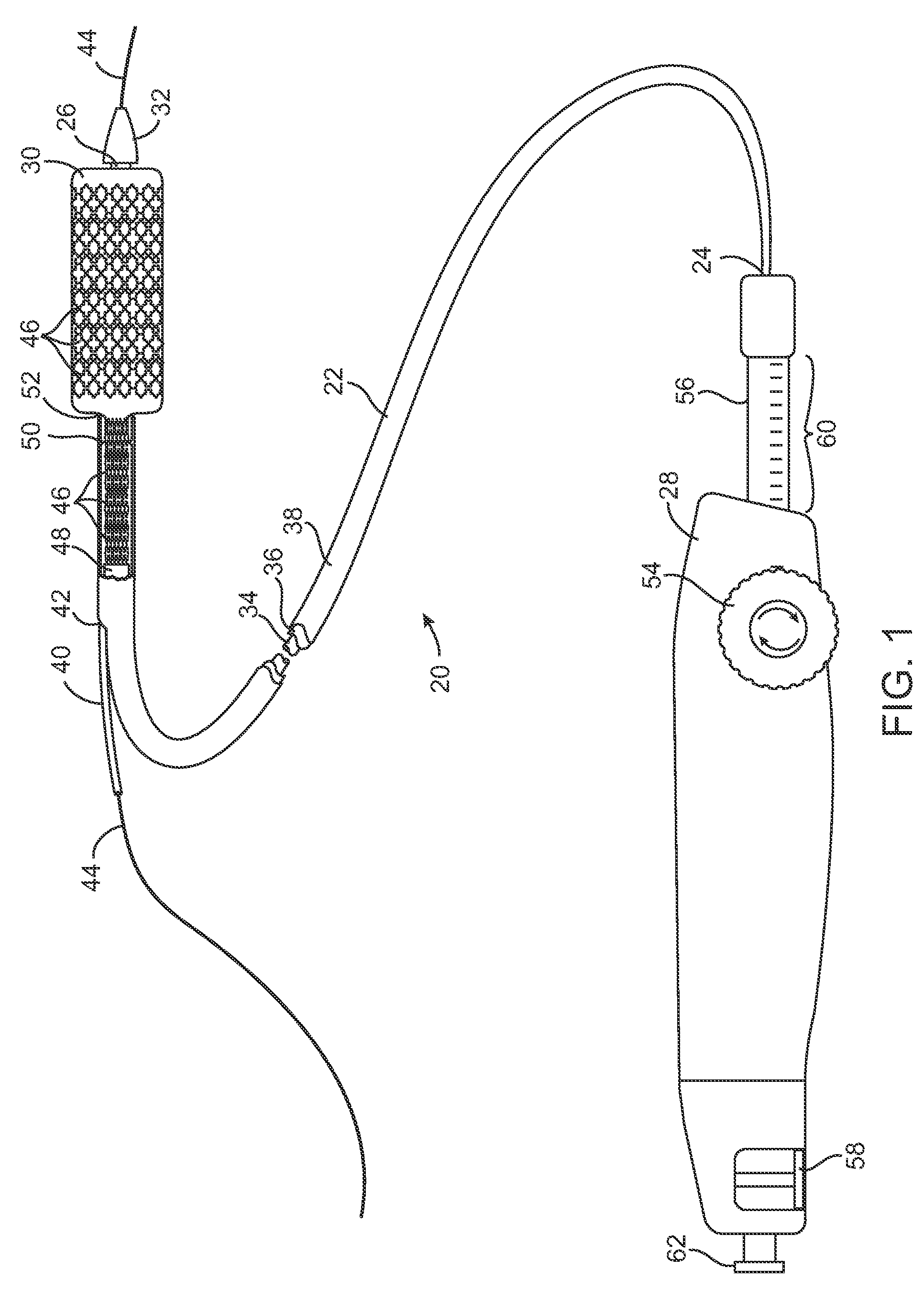
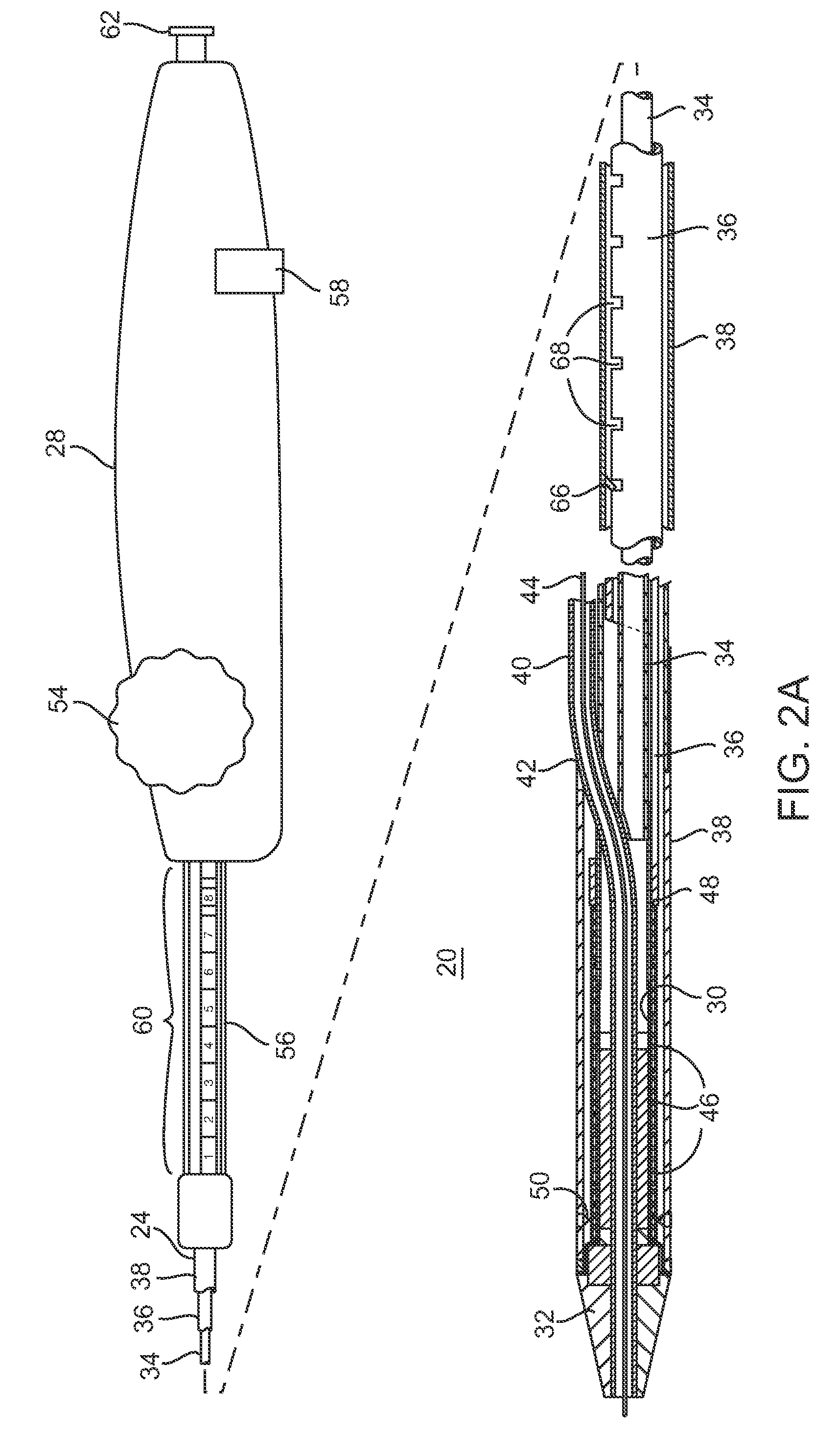
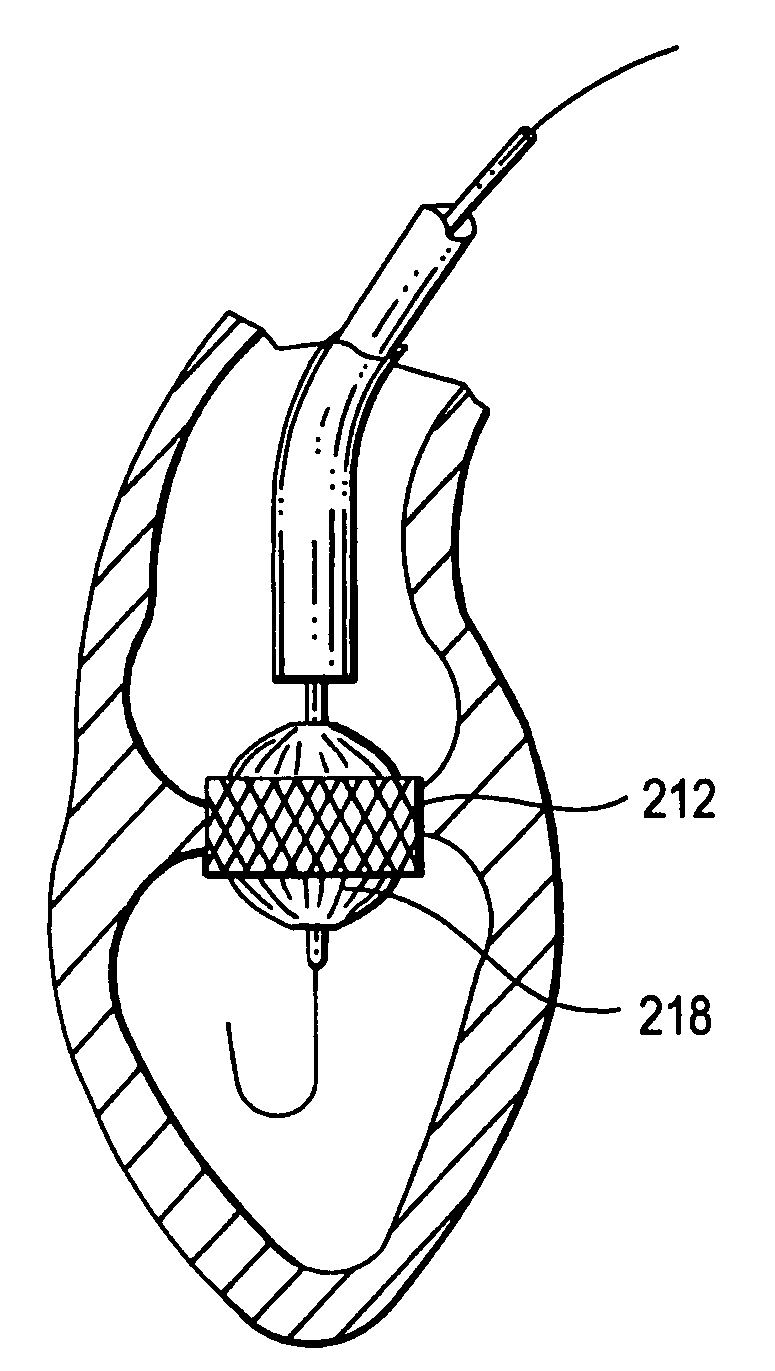
Devices and methods for controlling and indicating the length of an interventional element
Devices and methods are provided for controlling and indicating the deployed length of an interventional element on an interventional catheter. The interventional element may be a stent or series of stents, a balloon, or any other interventional element for which length control is necessary or desirable. Devices for controlling the length of the interventional element include gear driven actuators, motors, and other mechanisms. Devices for indicating length of an interventional element to the user include sensors, detents, visual displays and other mechanisms providing visual, audible, and tangible indications of length to the user. The control and indication devices preferably work in tandem to enable highly precise adjustment of interventional element length.
Owner:JW MEDICAL SYSTEMS LTD
Device and method for assisting in the implantation of a prosthetic valve
ActiveUS20060004439A1Increase thrustReduce the overall diameterBalloon catheterHeart valvesBalloon dilatation catheterProsthetic valve
A system for percutaneously introducing a prosthetic valve into a patient's vasculature comprises a balloon dilatation catheter, a prosthetic valve mounted coaxial to the dilatation balloon, and a pusher member comprising a longitudinally extending tubular member encompassing the shaft of the catheter. The distal end of the pusher member preferably corresponds to the proximal end of the stent component of the prosthetic valve. The pusher member provides enhanced longitudinal pushability for facilitating advancement of the prosthetic valve to a treatment site. The system is well-suited for advancing a prosthetic valve or other medical device through an introducer sheath having a relatively small inner diameter. The introducer sheath may be formed with a tapered proximal end portion for receiving the prosthetic valve and for reducing a diameter of the prosthetic valve during advancement therethrough.
Owner:EDWARDS LIFESCI PVT
Rolled minimally-invasive heart valves and methods of manufacture
Expandable heart valves for minimally invasive valve replacement surgeries are disclosed. The valves are rolled into a first, contracted configuration for minimally invasive delivery using a catheter, and then unrolled or unfurled at the implantation site. One- and two-piece stents may be used in conjunction with a plurality of flexible leaflet-forming membranes. The stents may include an annulus section, a sinus section with the membranes attached over sinus apertures, and an outflow section. Lockout tabs and making slots secure the stents in their expanded shapes. Alignment structure ensures concentric unfurling of the stent. Anchoring elements at the stent edges or in the stent body secure the valve within the annulus. A method of manufacture includes shape setting the sheet-like stent to ensure an outward bias during deployment. The stent may also include dear tracks for engagement with a gear mechanism for deployment. The stent is desirably made of a superelastic material such as Nitinol and may have areas removed or thinned to reduce the bending stresses when rolled into its small spiral for catheter delivery.
Owner:EDWARDS LIFESCIENCES CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com