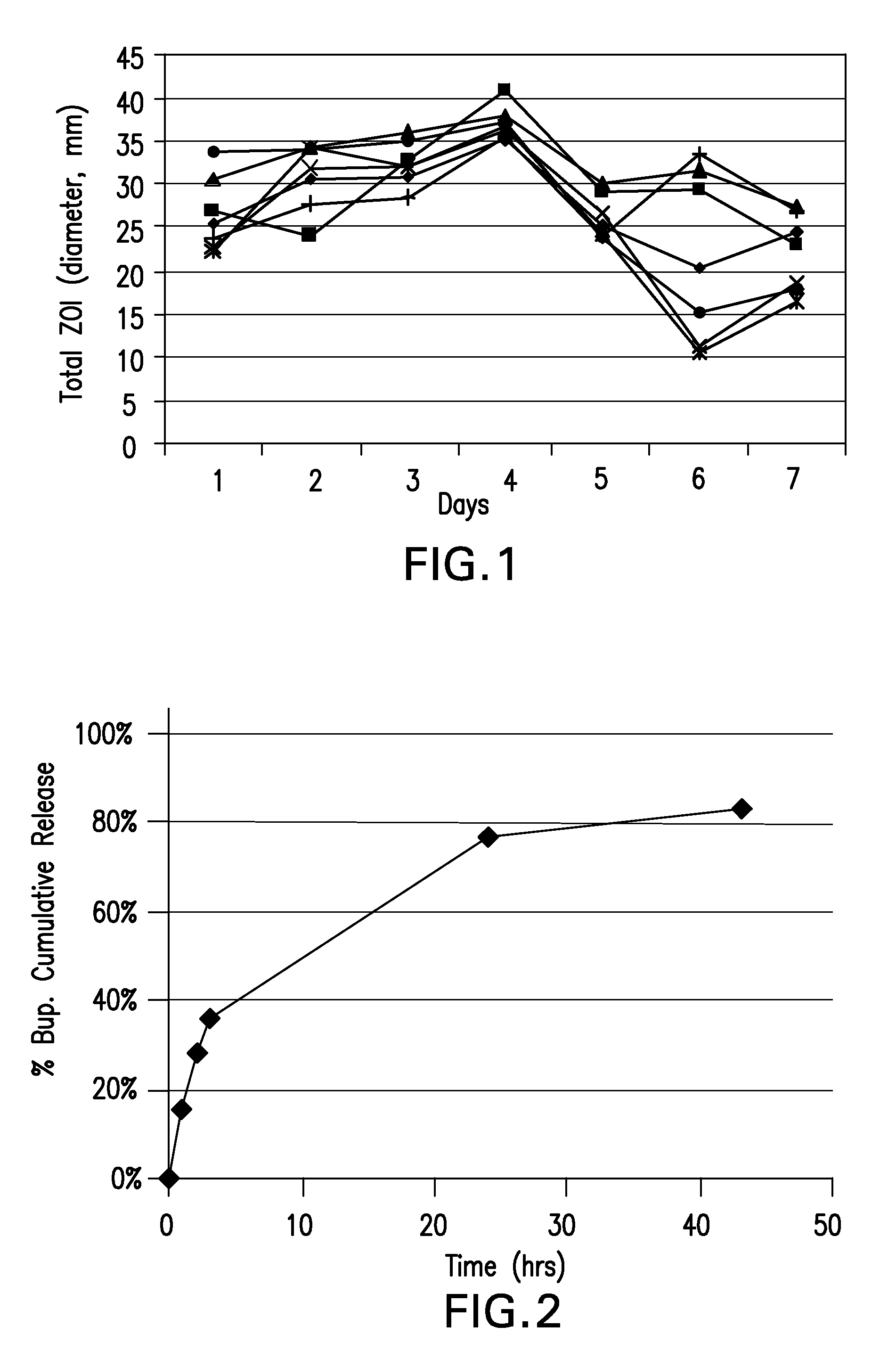
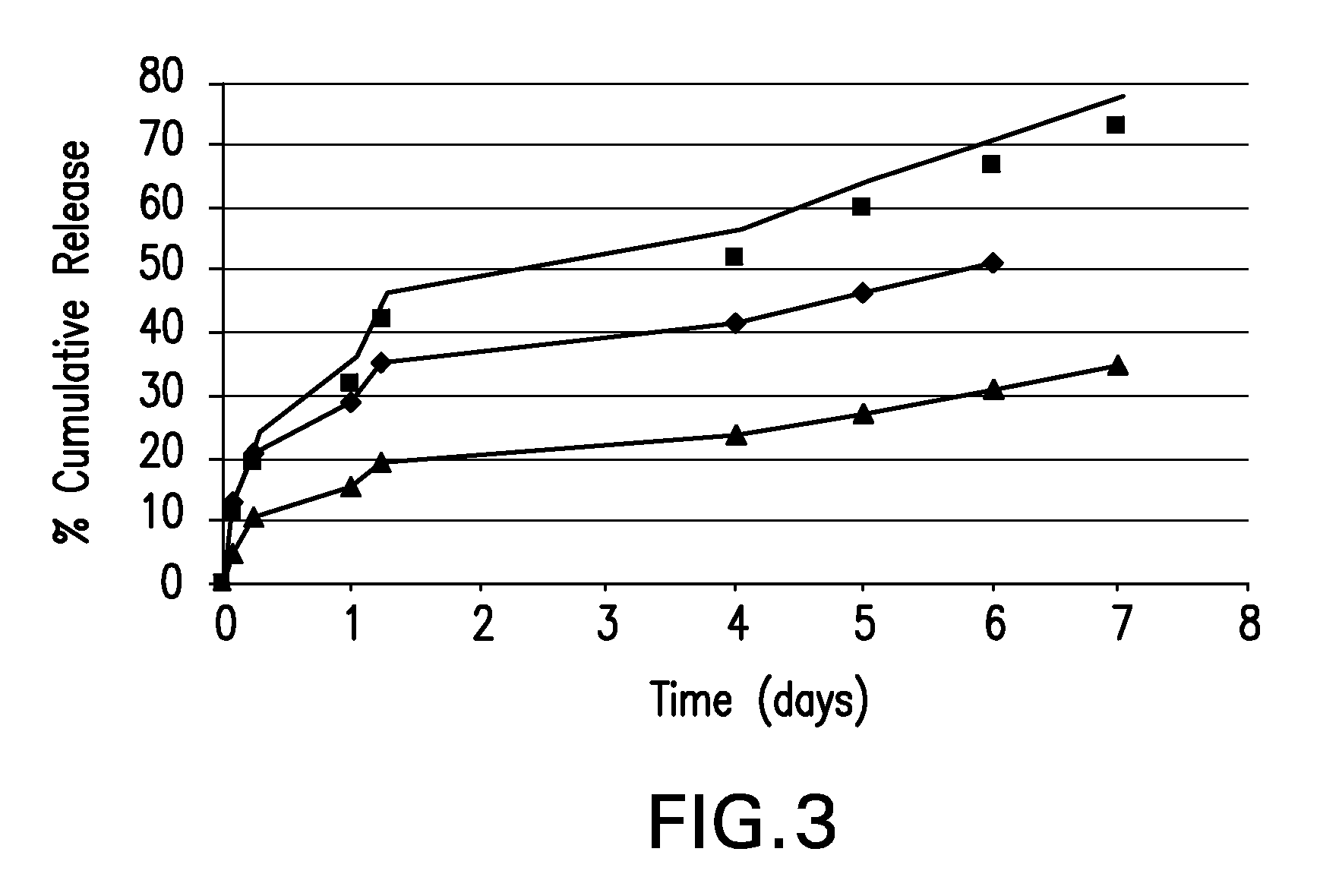
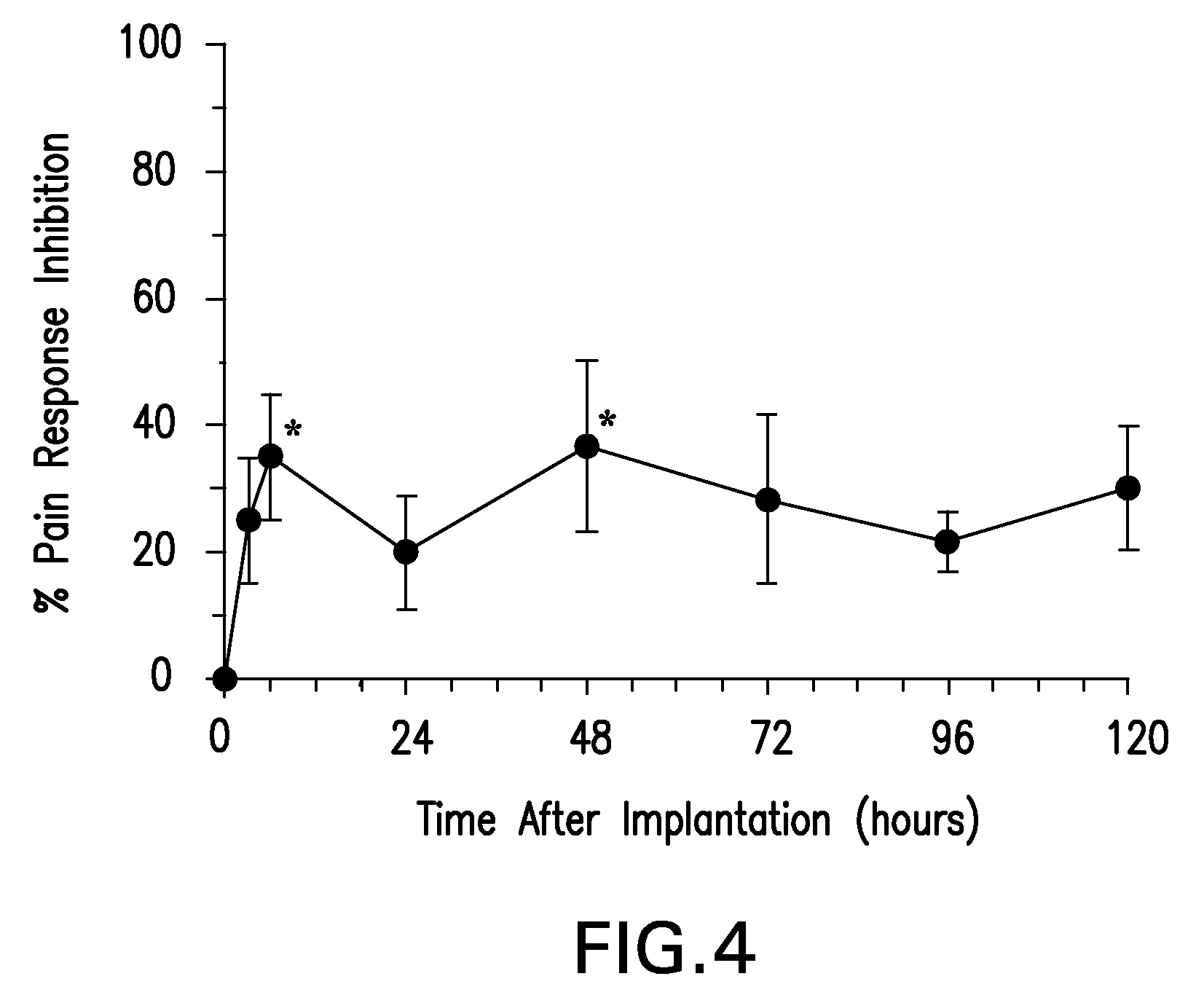
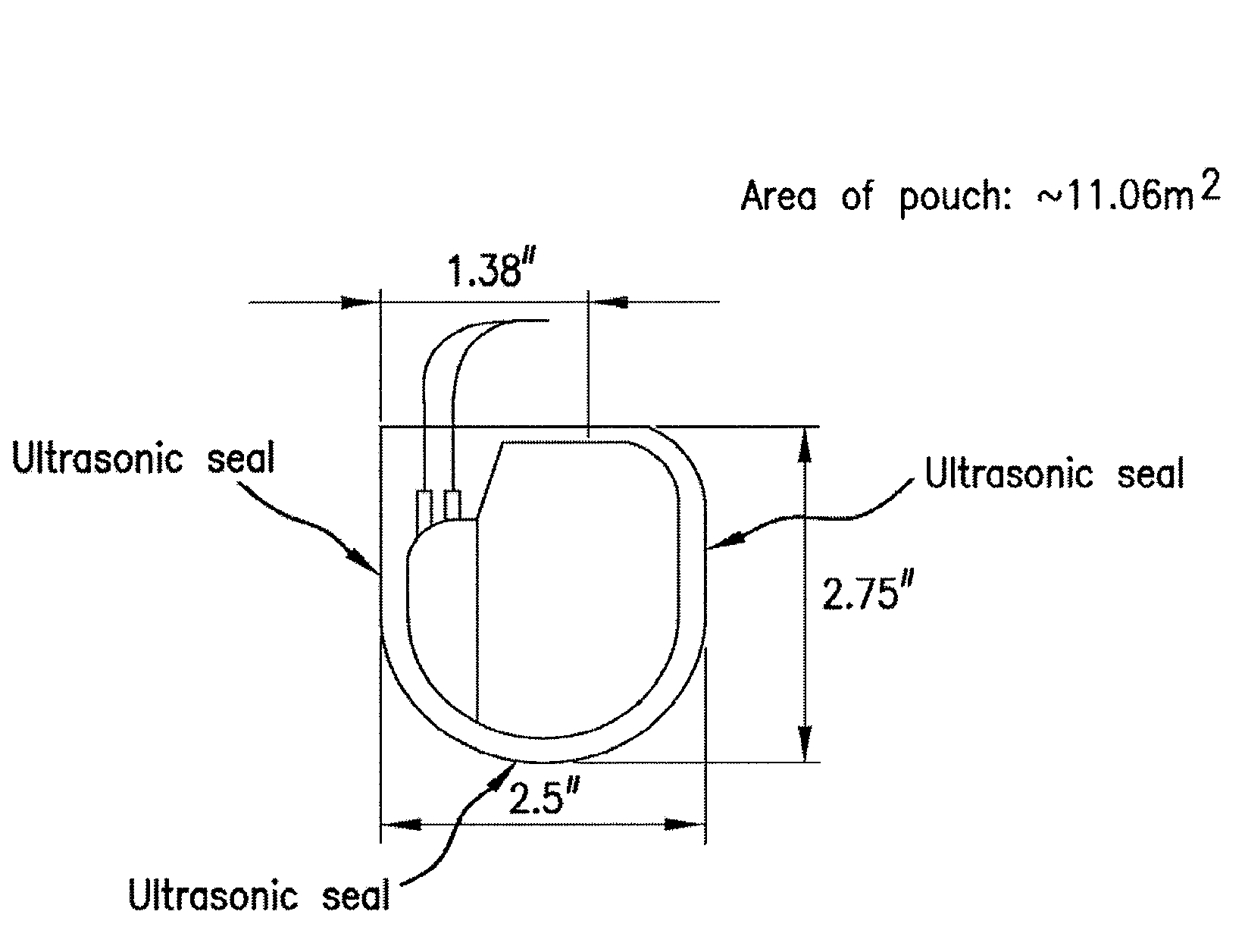
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
146 results about "Surgical mesh" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
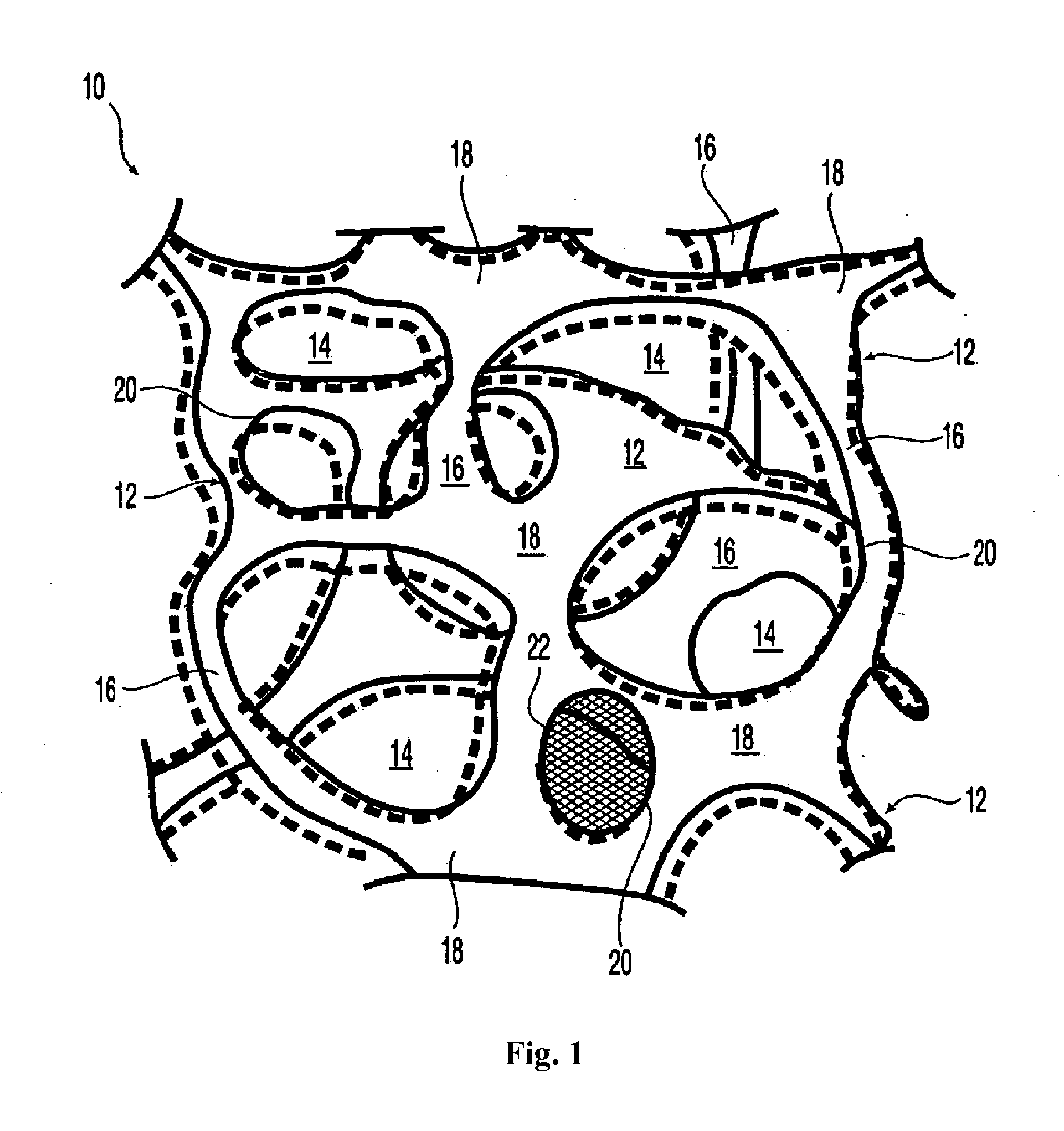
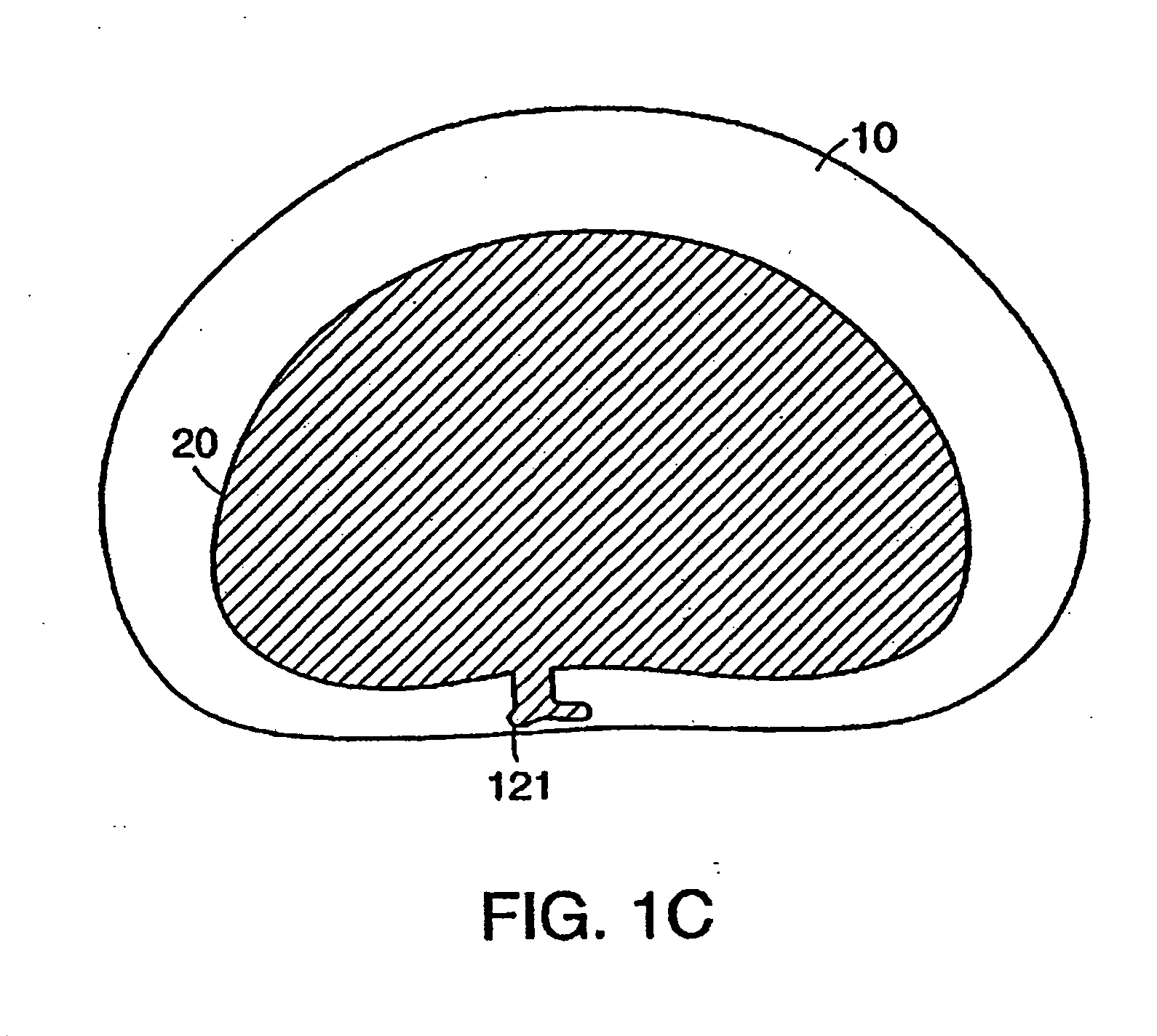
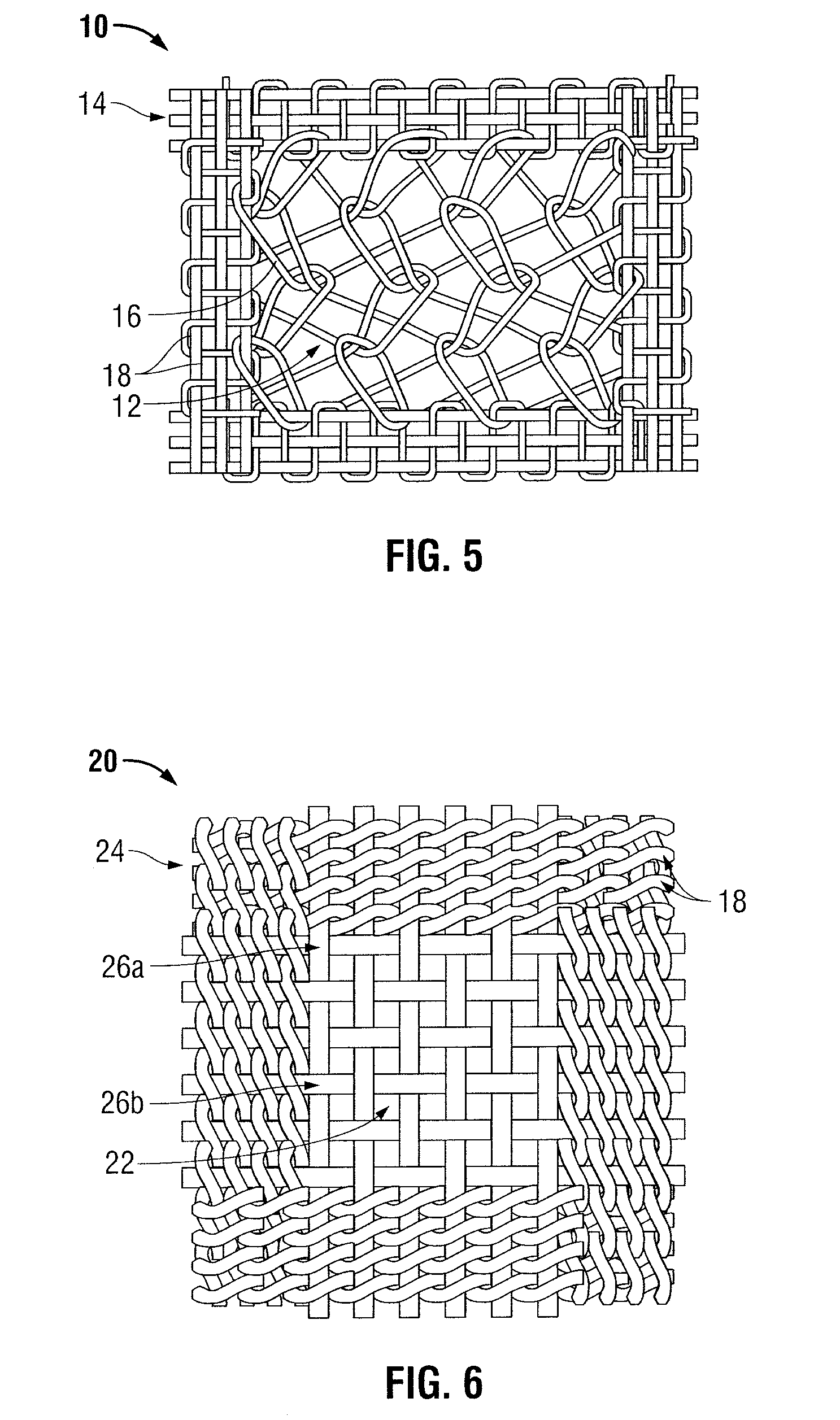
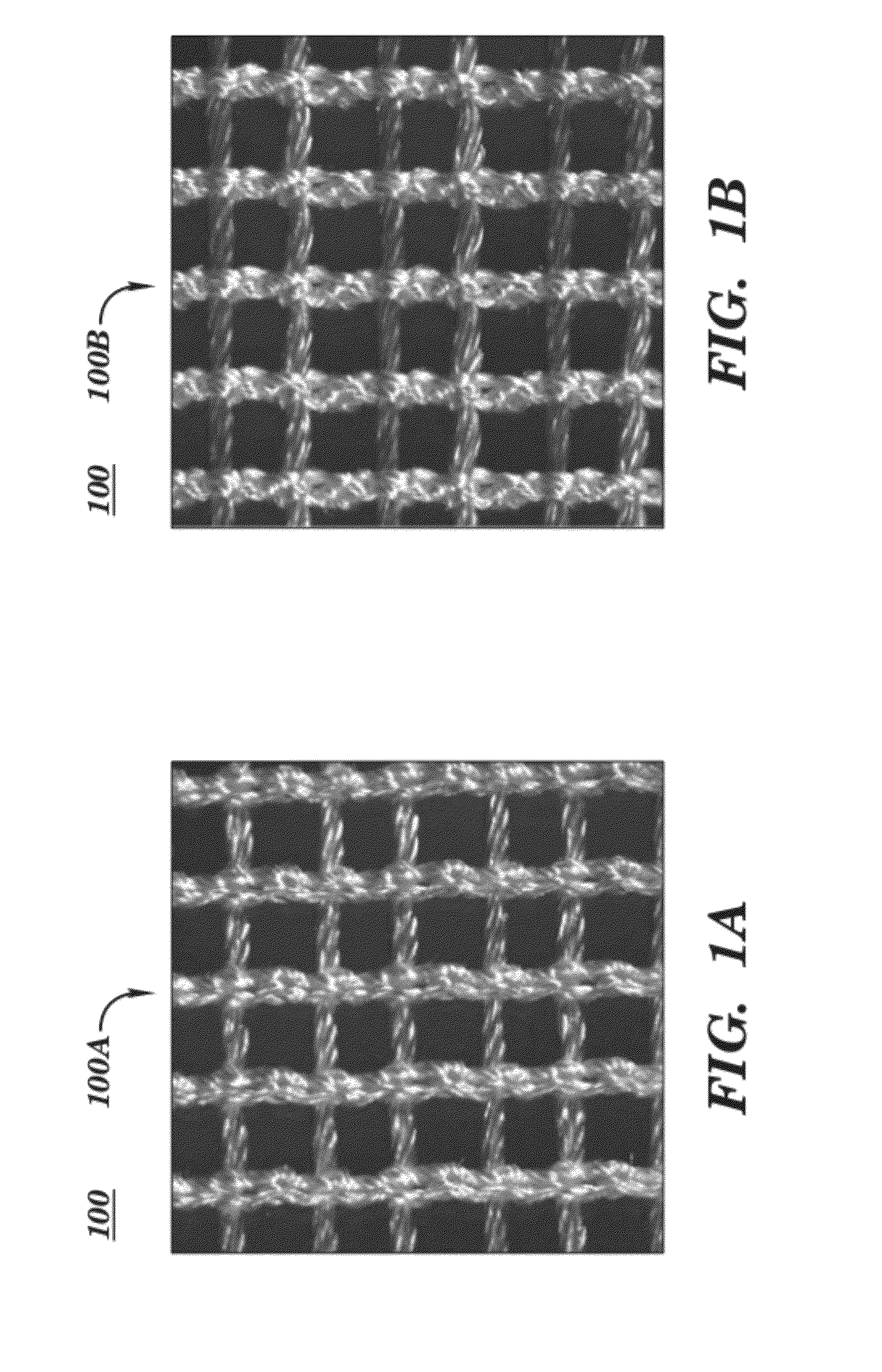
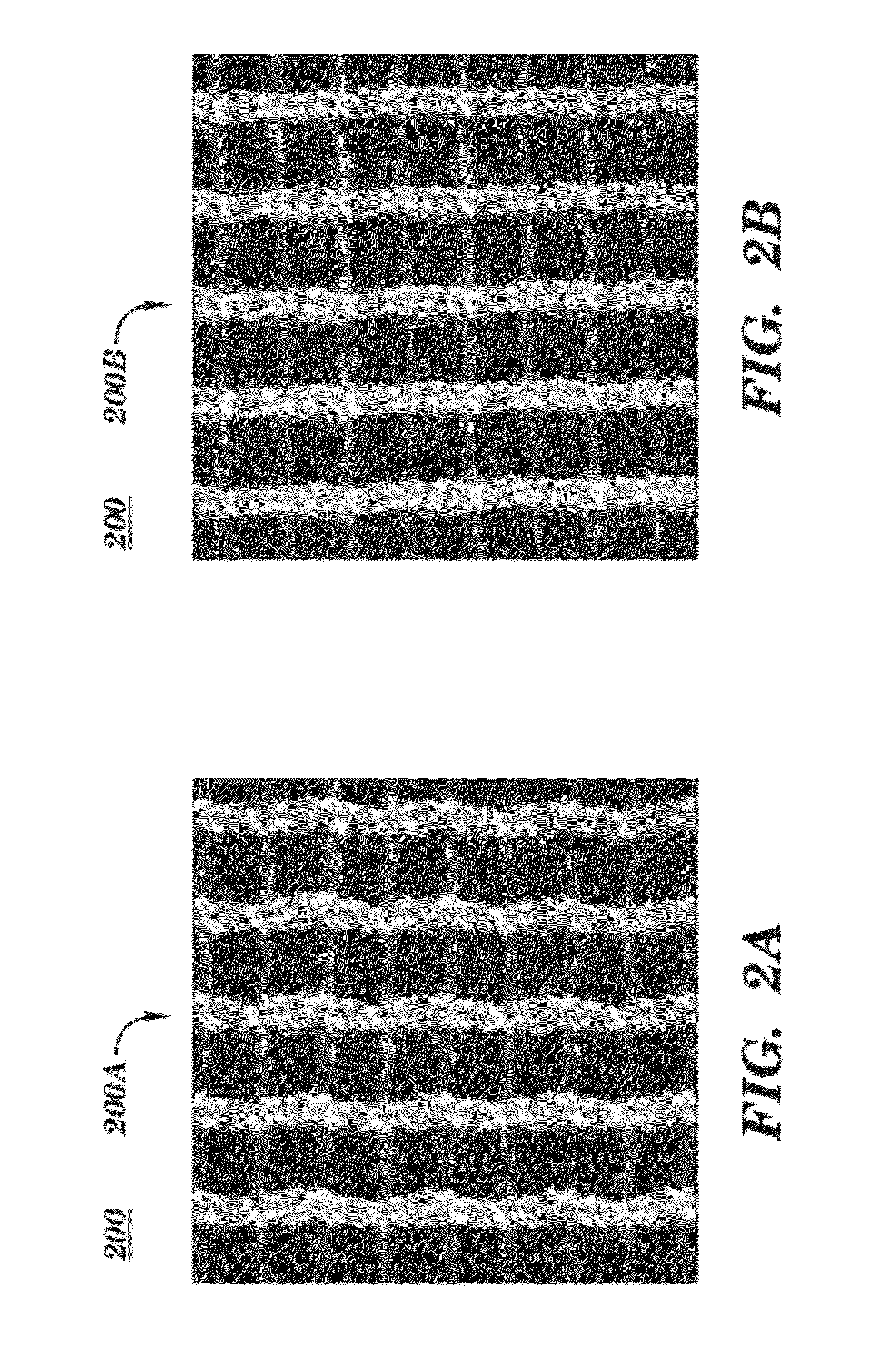
Surgical mesh is a loosely woven sheet which is used as either a permanent or temporary support for organs and other tissues during surgery. Surgical mesh is created from both inorganic and biological materials and is used in a variety of surgeries. Though hernia repair surgery is the most common application, it can also be used for reconstructive work, such as in pelvic organ prolapse.
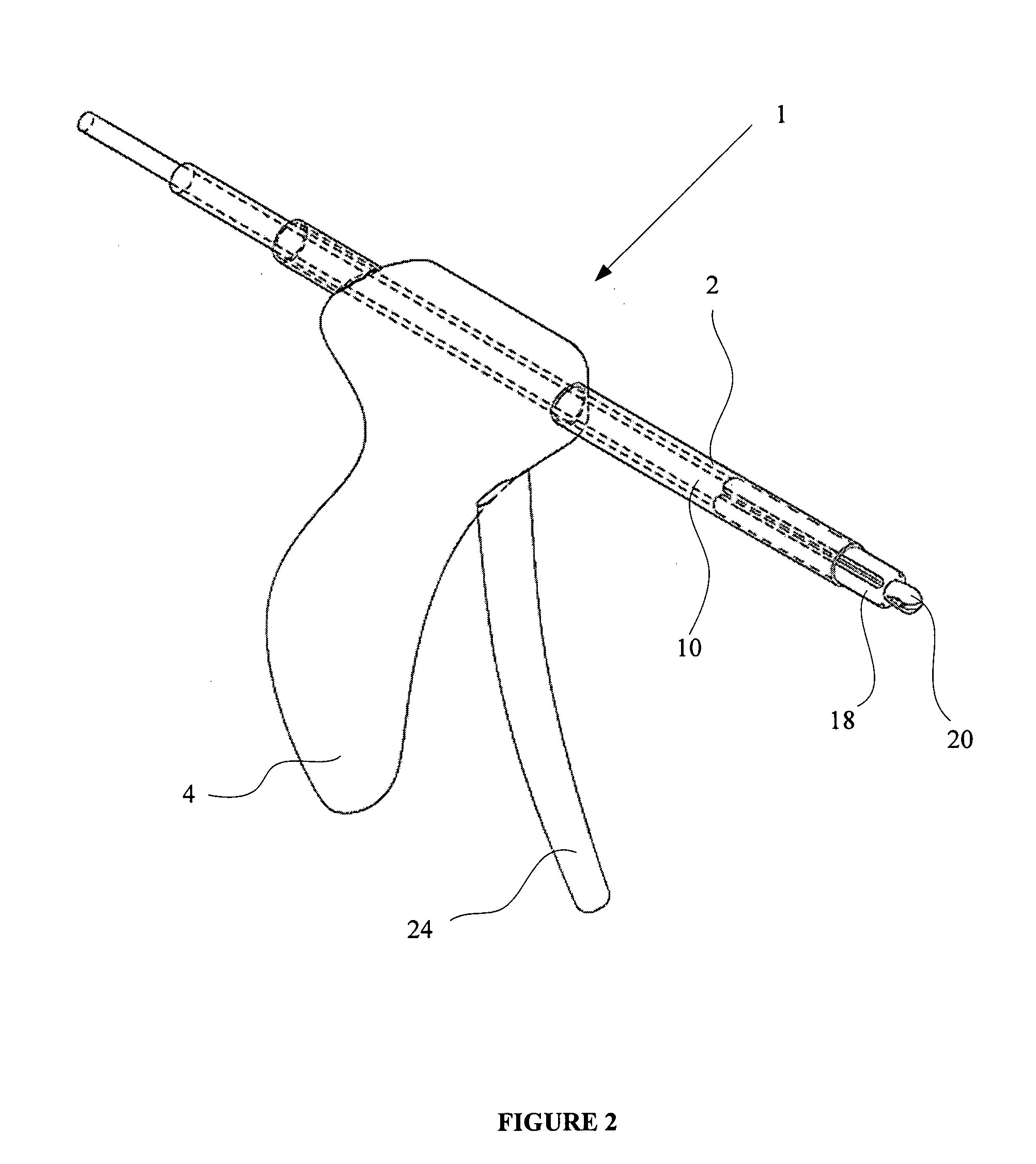
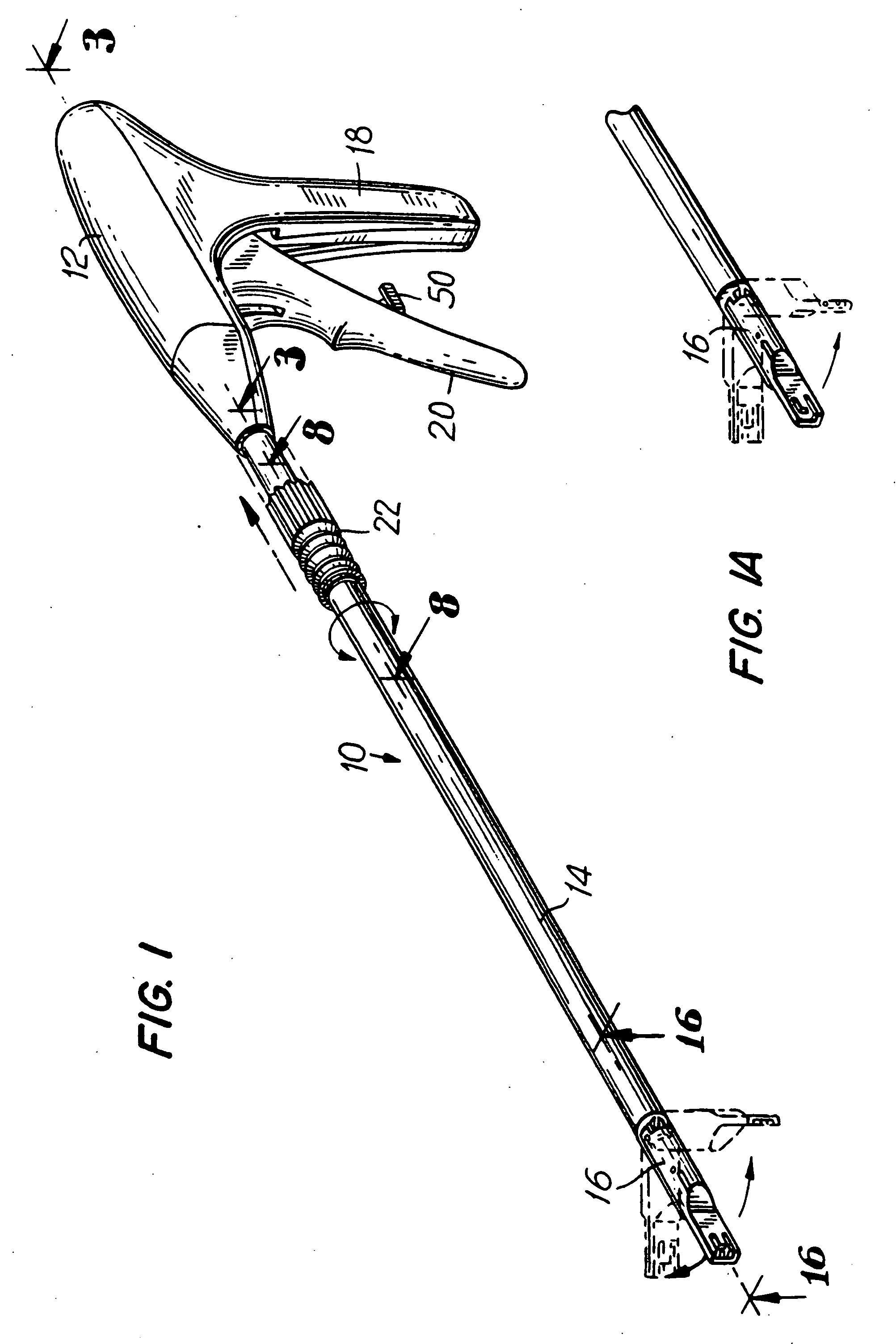
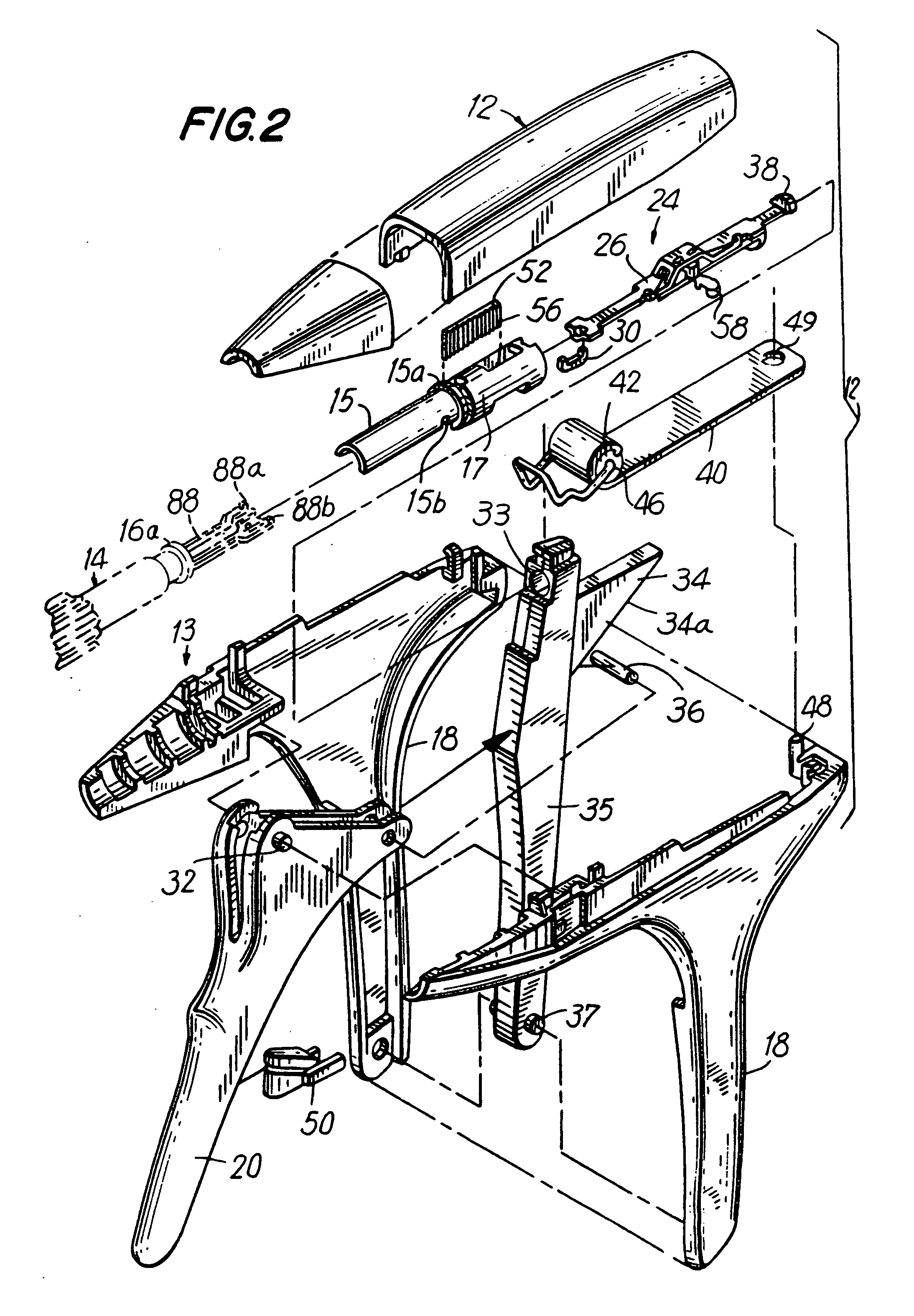
Apparatus for applying surgical fastners to body tissue
InactiveUS7624903B2Increase awarenessAvoid deformationSuture equipmentsStapling toolsSurgical stapleSupporting system
Owner:COVIDIEN LP
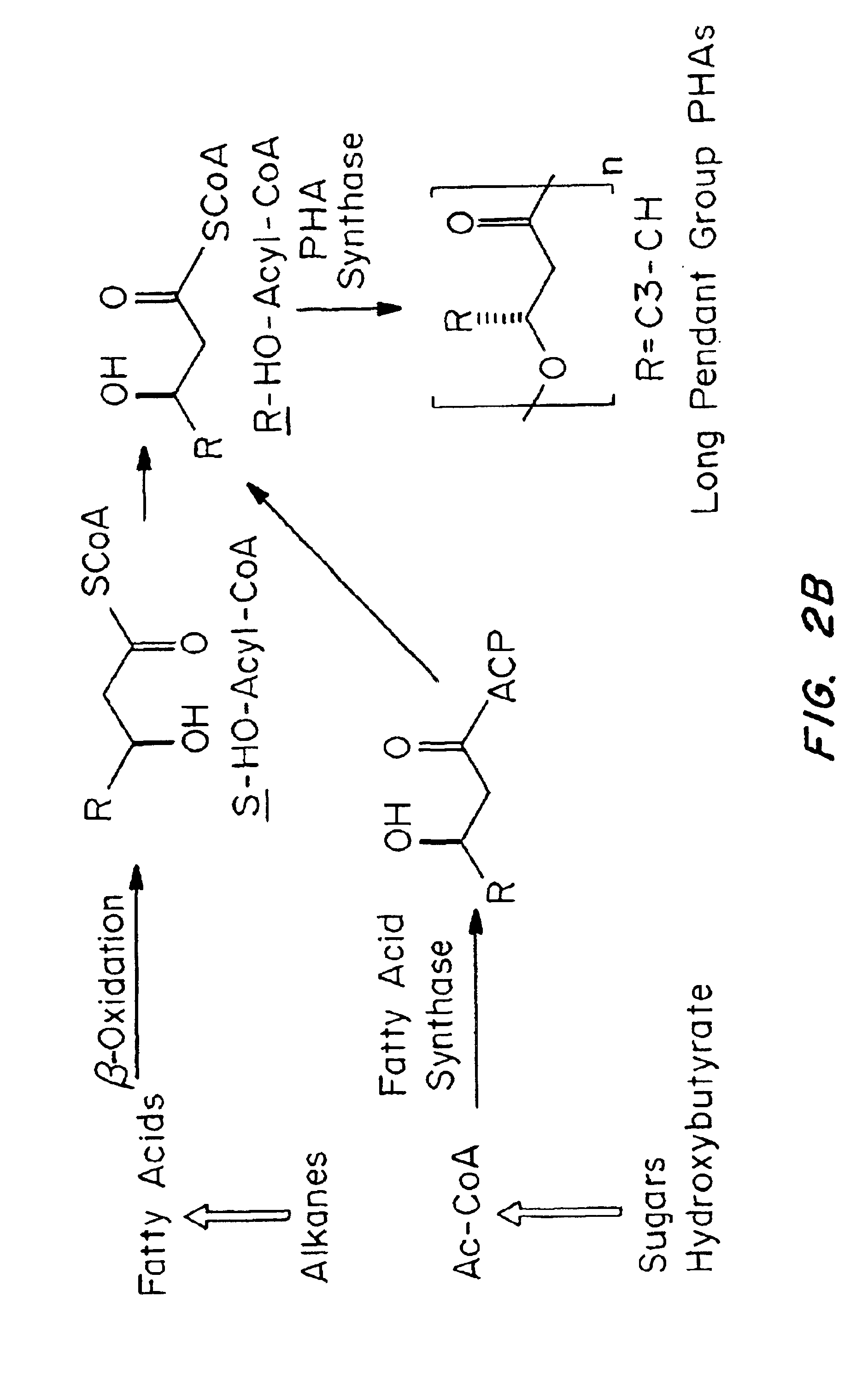
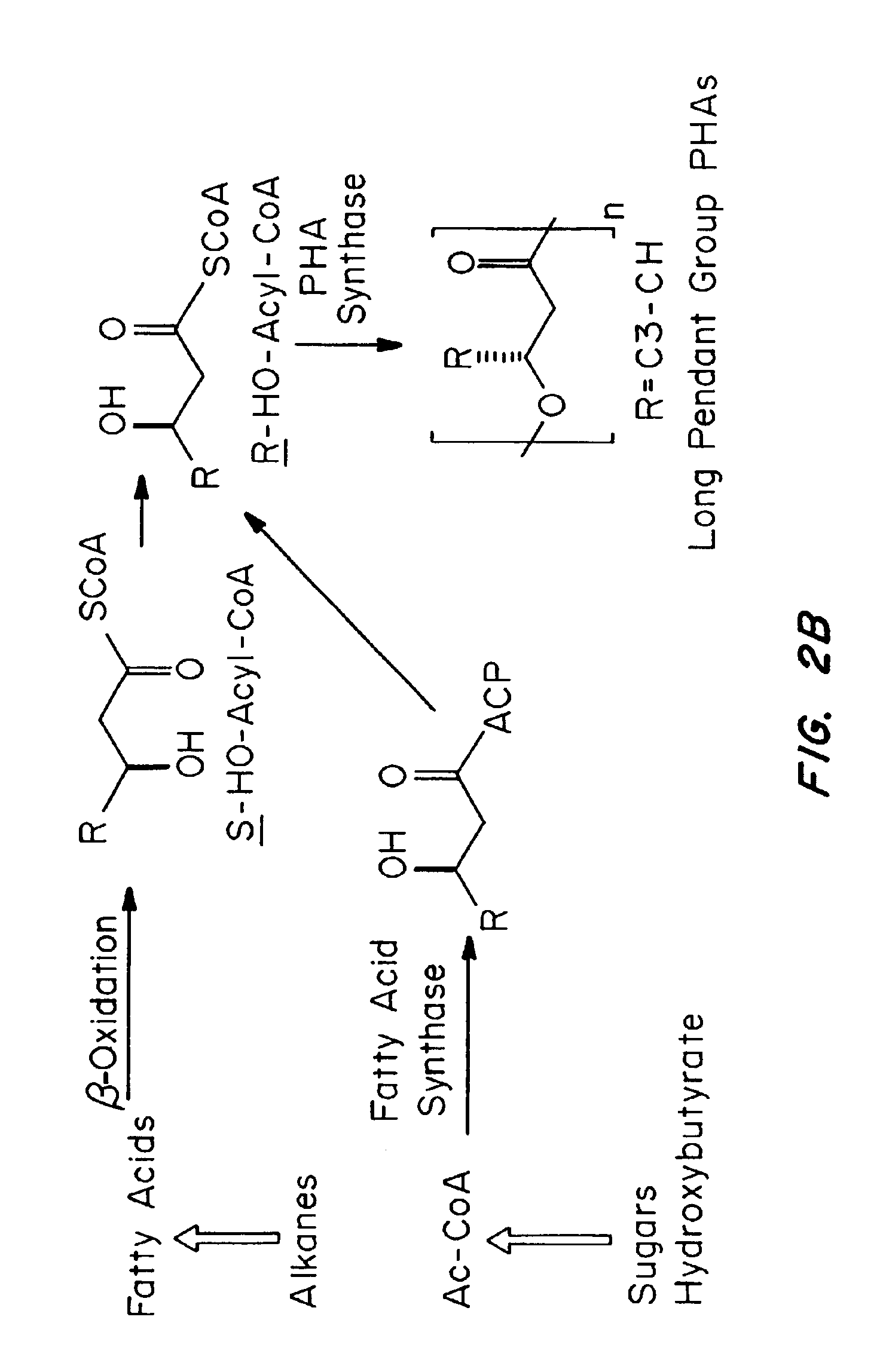
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
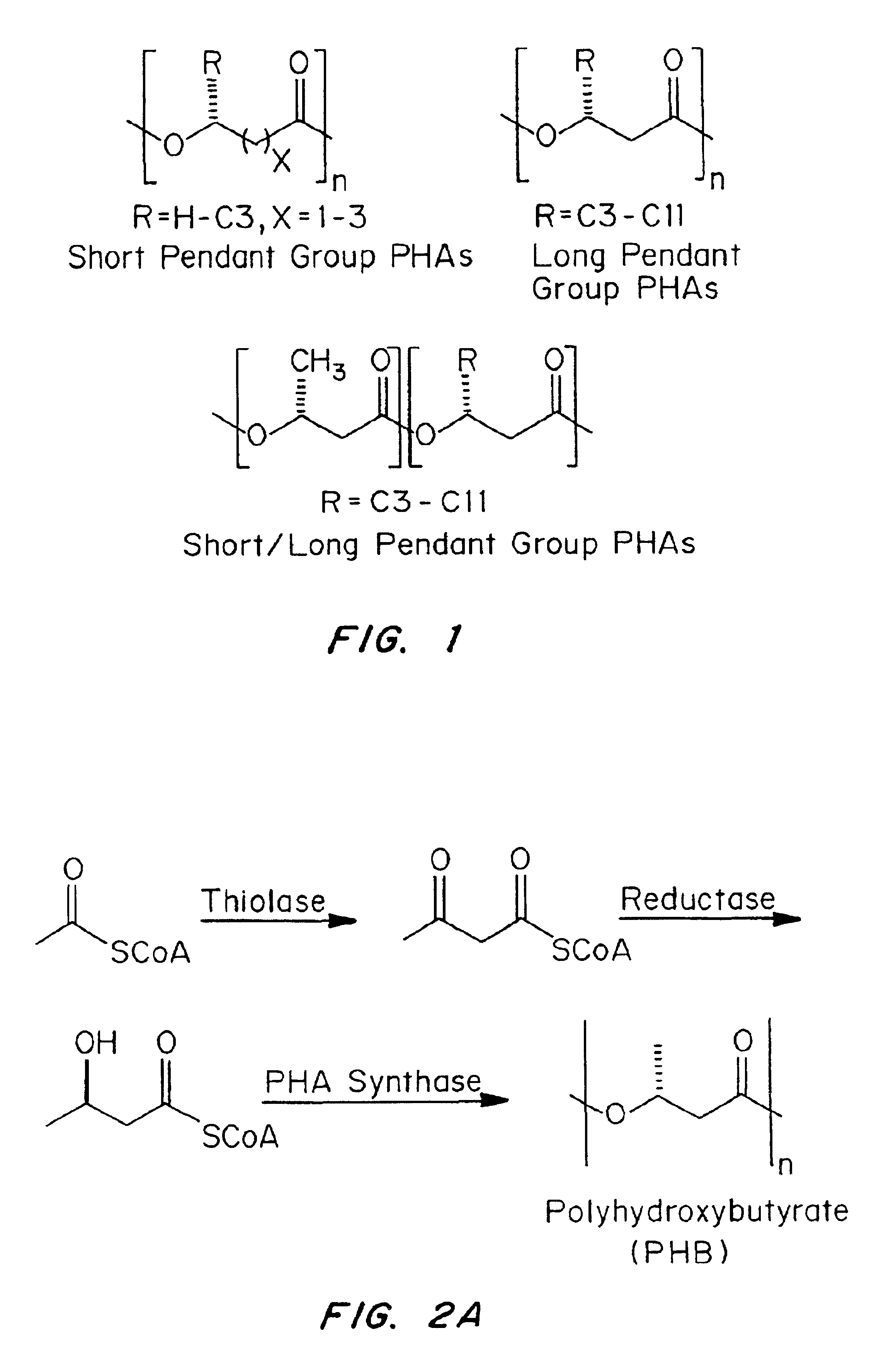
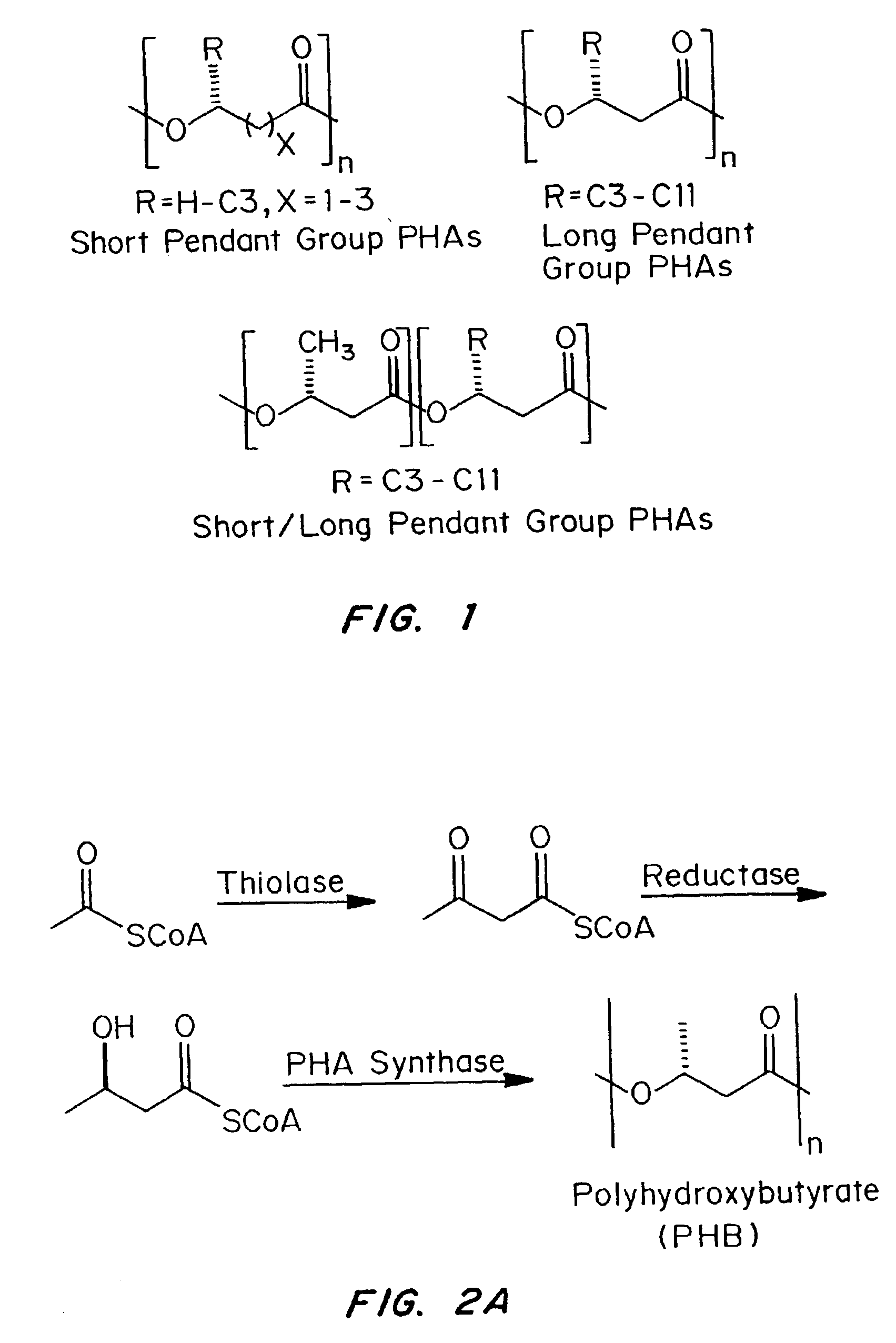
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
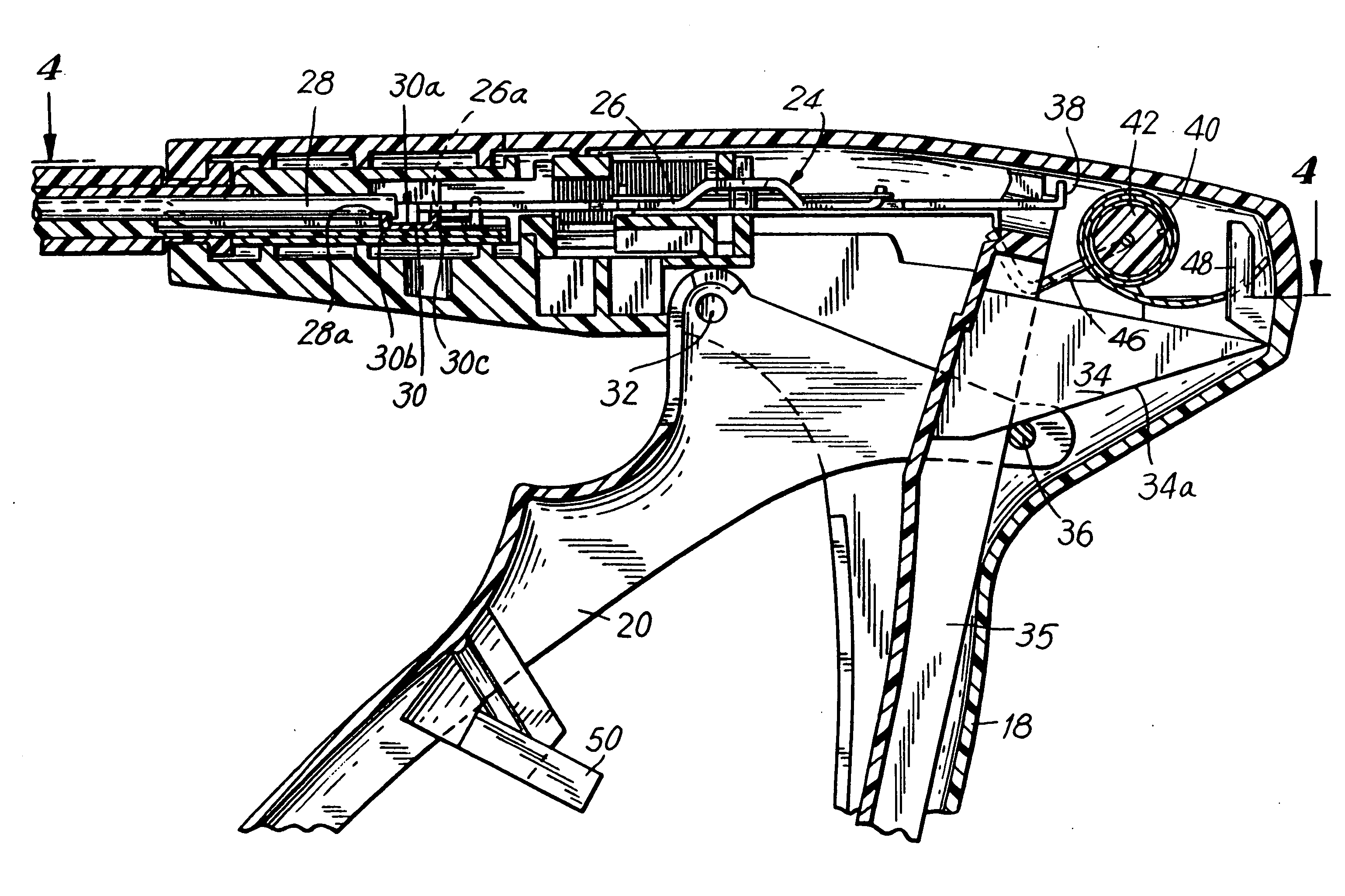
Apparatus and method for applying surgical staples to attach an object to body tissue
InactiveUS20020117534A1Avoid deformationSuture equipmentsStapling toolsSupporting systemSurgical staple
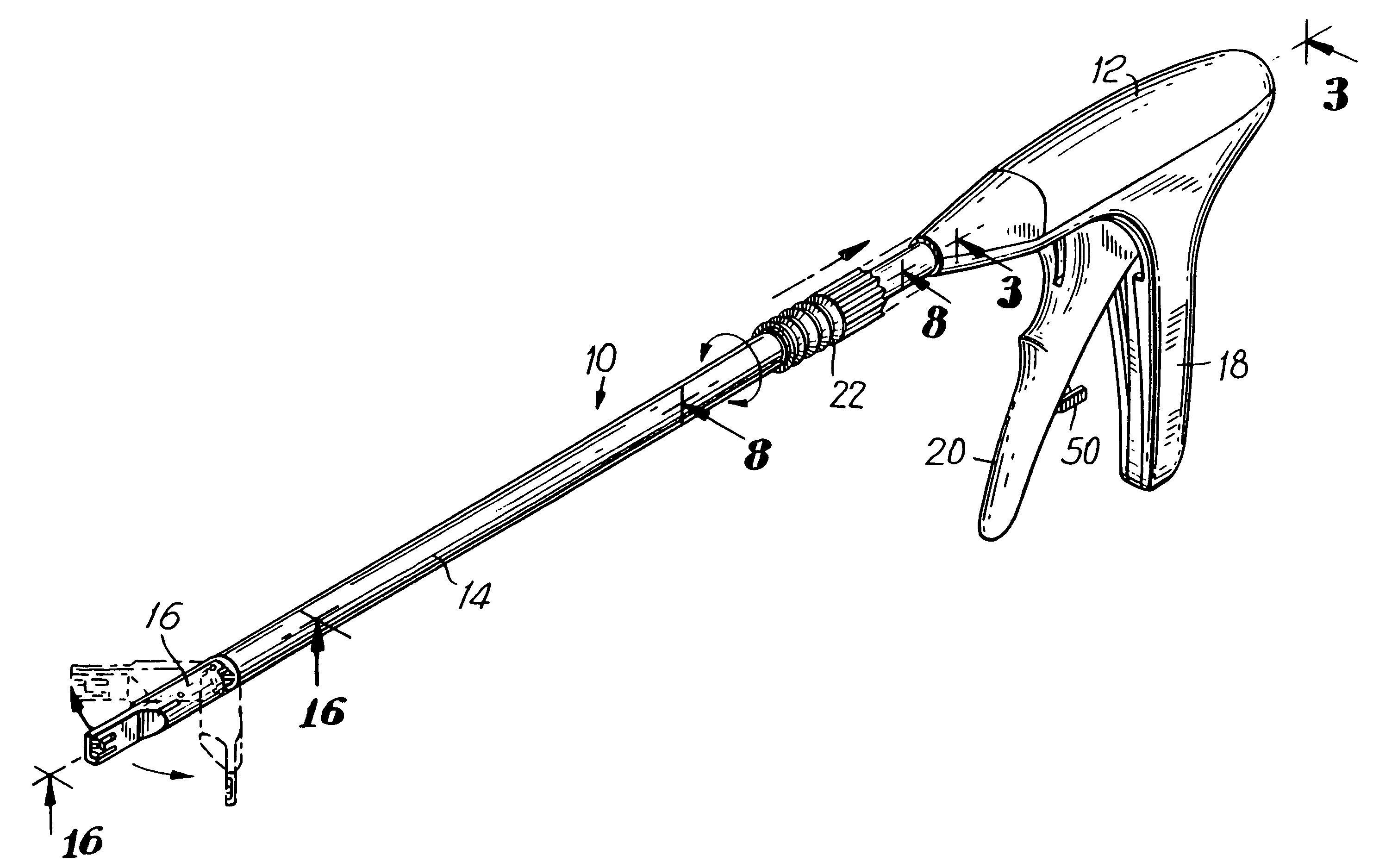
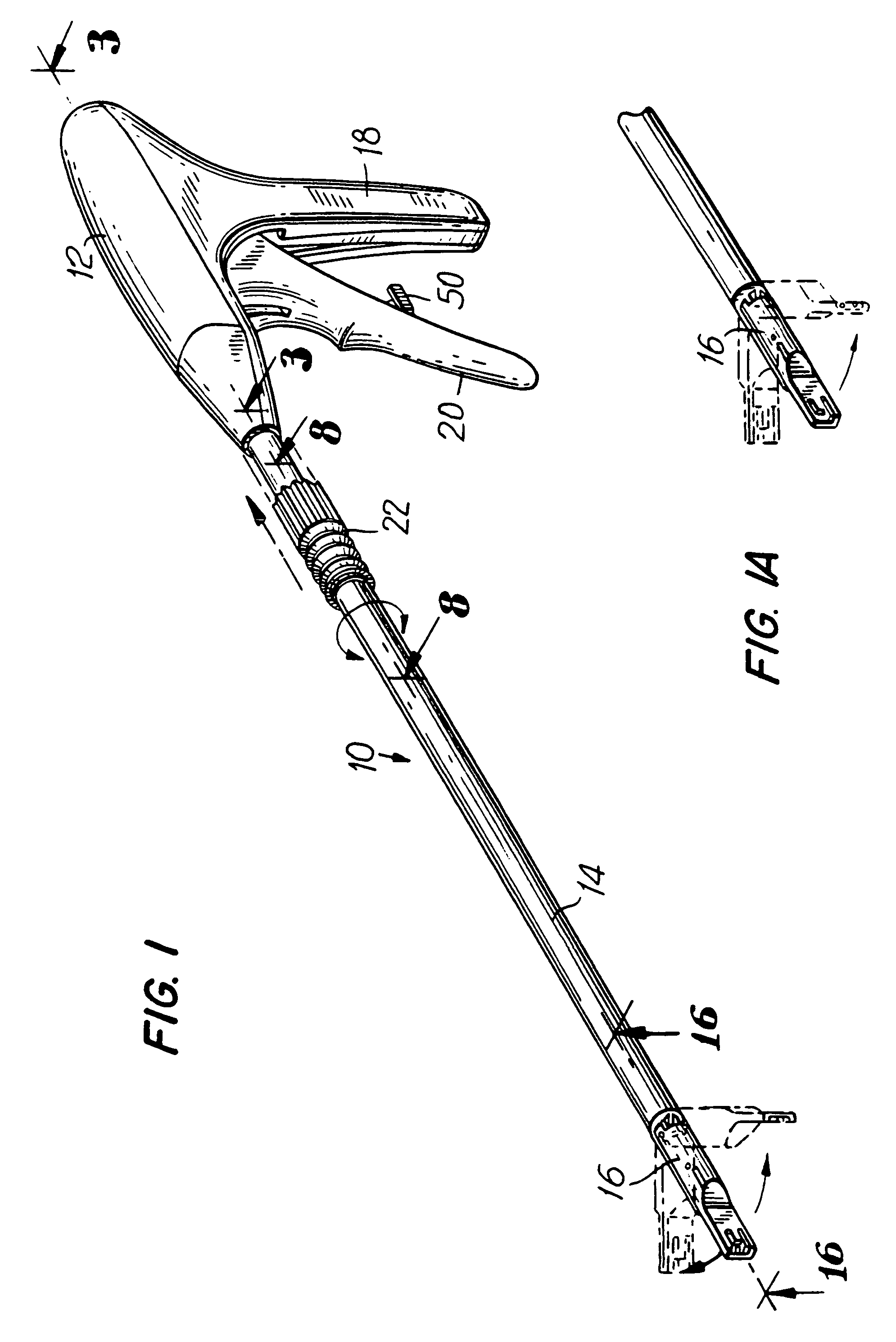
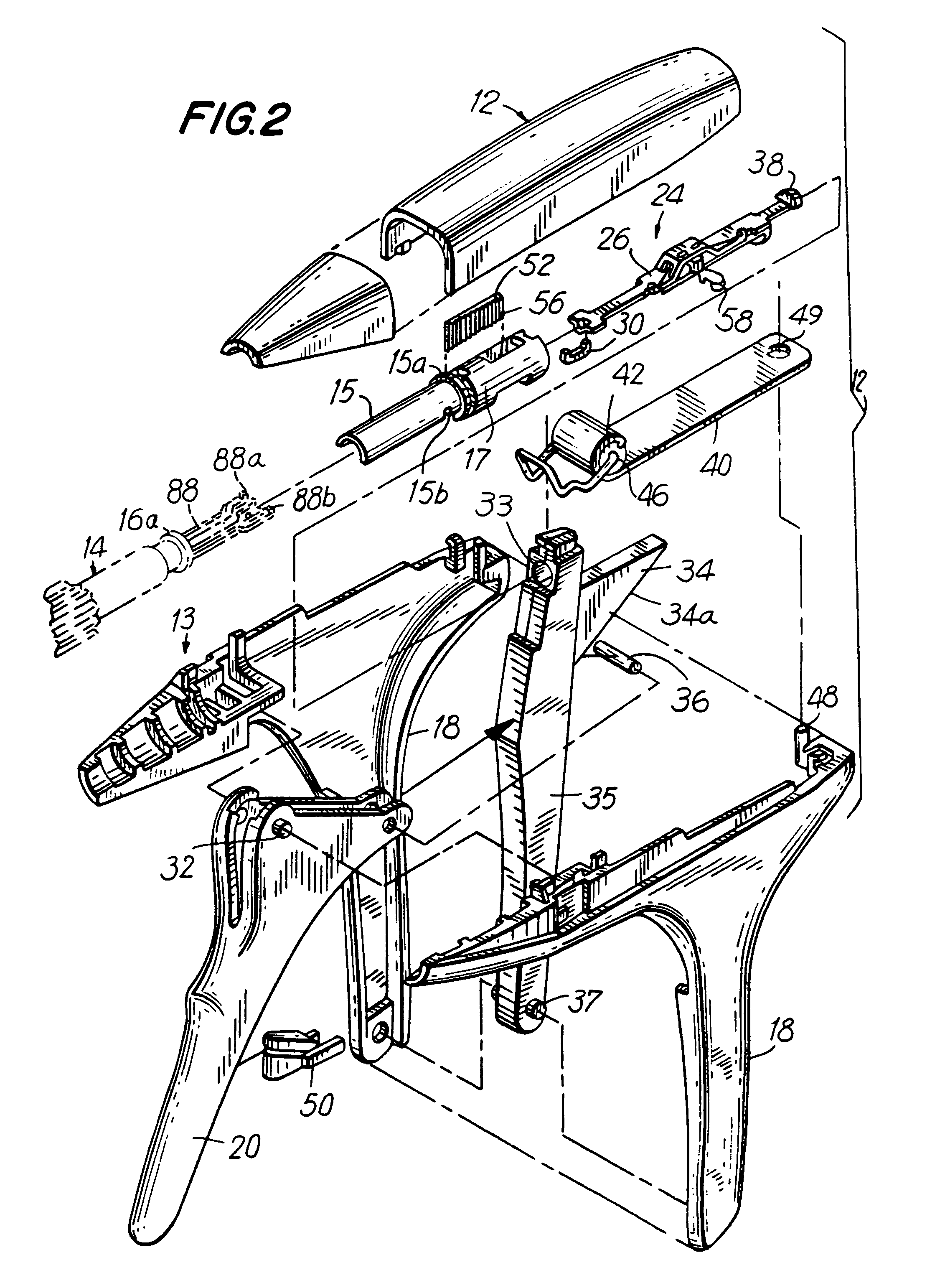
An apparatus is disclosed for endoscopic application of surgical staples adapted to attach surgical mesh to body tissue in laparoscopic hernia surgery. The apparatus includes a frame, and a generally elongated endoscopic section connected to the frame and extending distally therefrom. A staple storage cartridge is removably supported on a pivotal support system at the distal end portion of the endoscopic section with each staple being configured and adapted to attach the mesh to the body tissue. An elongated pusher system formed of several assembled components and extending from the frame to the endoscopic section is provided for individually advancing at least one staple at a time distally for positioning adjacent the surgical mesh and the body tissue. The pusher system also includes a trigger system to actuate the pusher. The trigger system is provided with perceptible tactile sensing means to indicate when the legs of the staple being advanced are exposed so as to be visible to the user for positioning and orientation purposes. Anvil means provides for individually closing each staple to encompass at least a portion of the surgical mesh and to penetrate the body tissue in a manner to attach the portion of the mesh to the body tissue. Projecting distally of the cartridge support system is a pair of legs which are dimensioned and configured to engage the staple during closure to prevent unwanted roll or deformation outside of the plane of the staple.
Owner:TYCO HEALTHCARE GRP LP
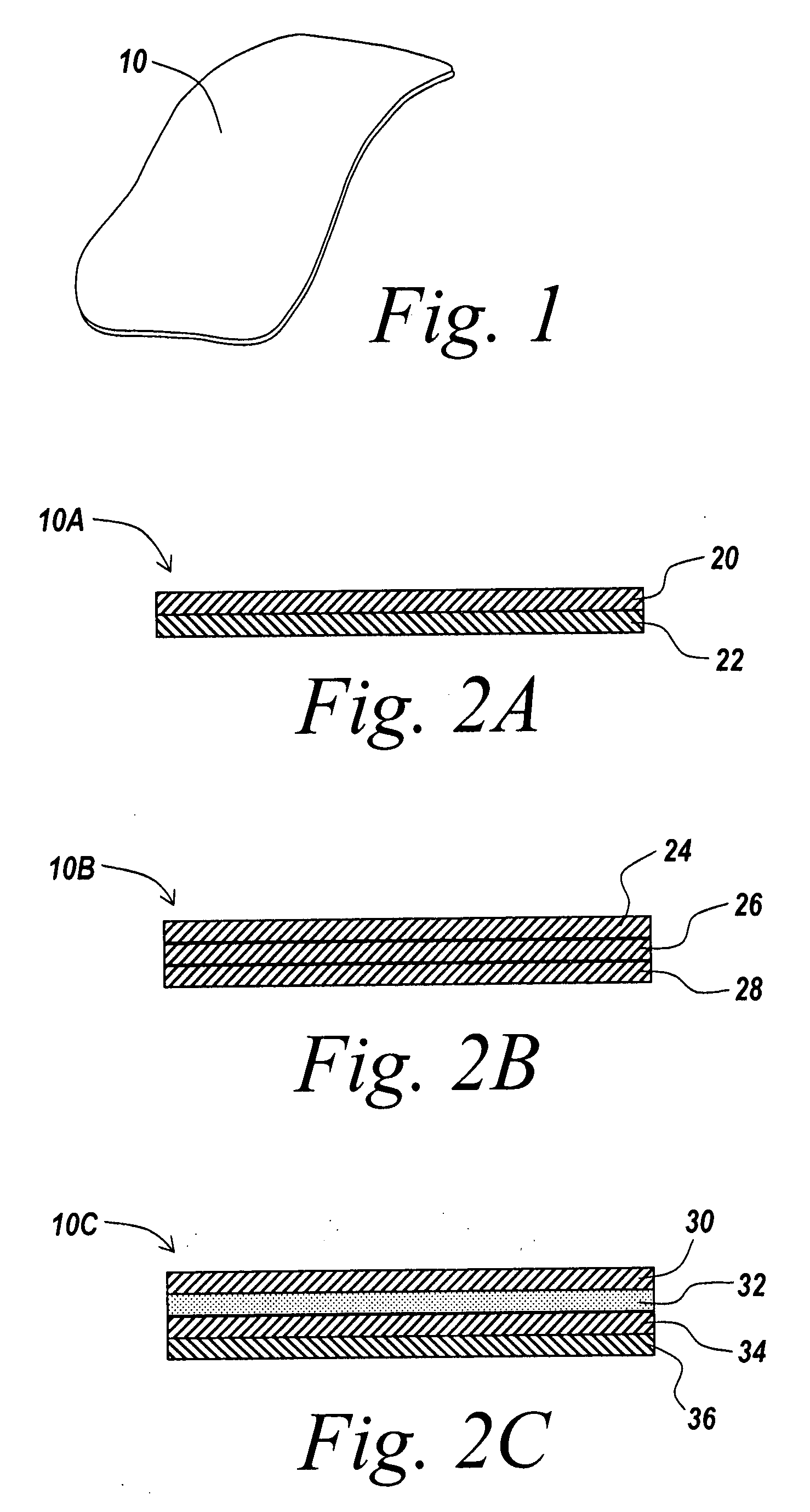
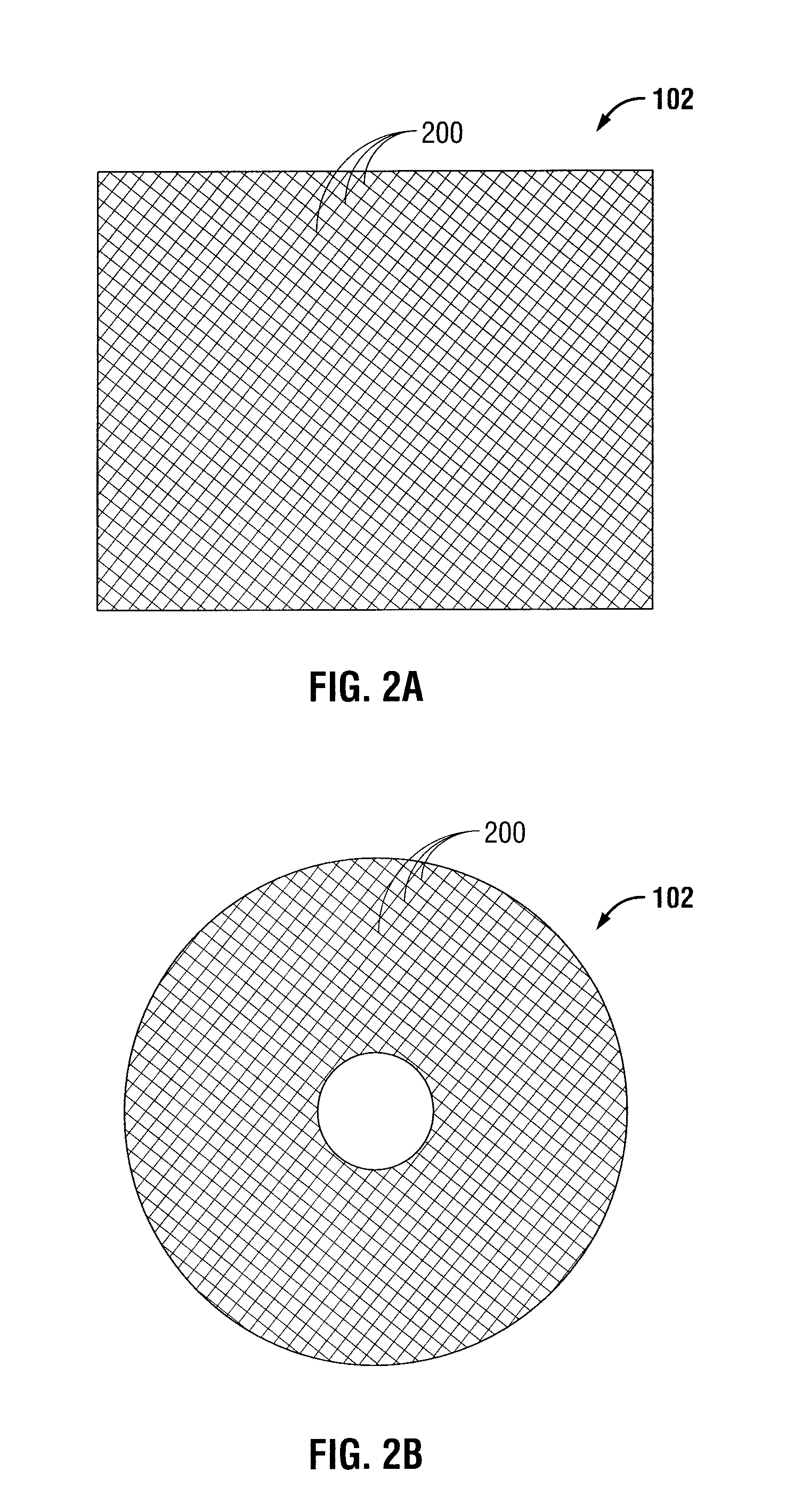
Surgical meshes
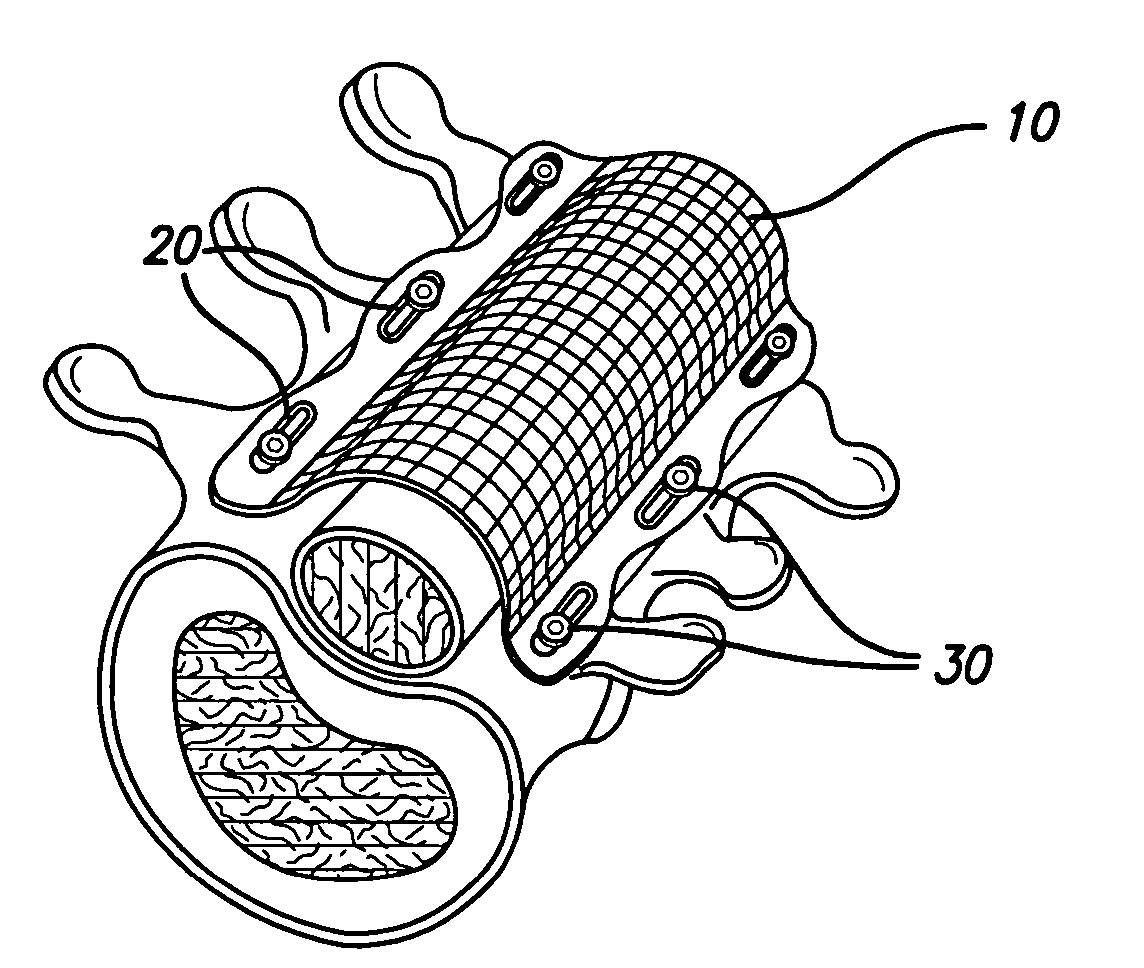
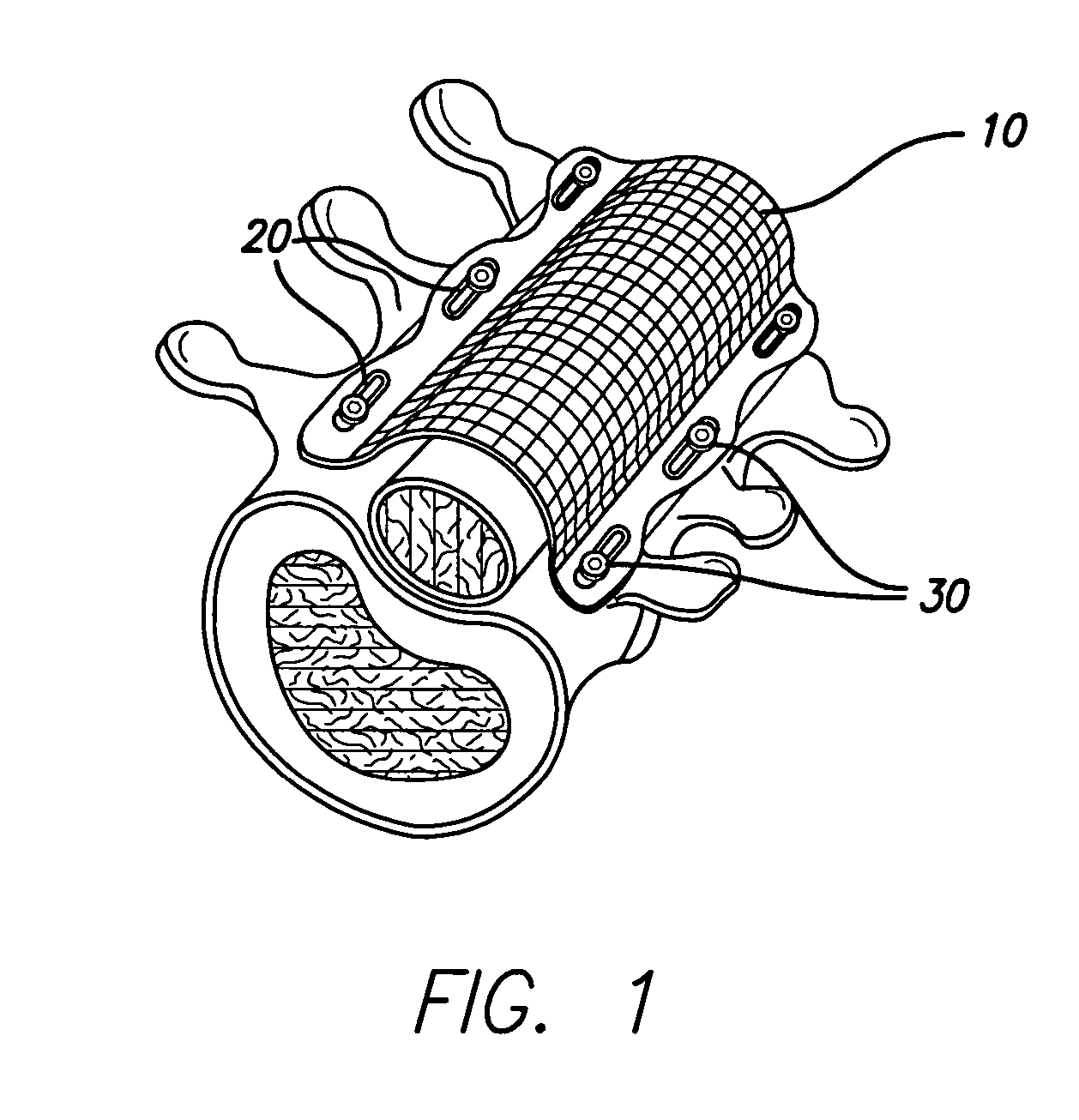
An implant includes a central portion composed of at least one yarn interconnected to form openings therein and a peripheral portion extending around the central portion. The peripheral portion includes at least one high strength yarn.
Owner:TYCO HEALTHCARE GRP LP
Implantable devices having swellable grip members
The present disclosure relates to implantable medical devices including swellable tissue gripping elements and methods of forming such devices. The implantable medical device may comprise a biocompatible substrate having a surface comprising at least one swellable grip member. The implantable medical device may take on the form of a surgical mesh, patch, buttress, or pledget and the swellable member may comprise spikes and / or spiked naps. The implantable medical device may also contain a bioactive agent.
Owner:TYCO HEALTHCARE GRP LP
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6867247B2Reduce probabilityHigh porositySuture equipmentsStentsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
Medical instruments and methods for using the same
InactiveUS20070185506A1Quickly and easily releasingAttachment of the sheet to the surface quicker and easierDisinfectionProsthesisHuman bodyHernia surgical mesh
A medical instrument used to position a surgical mesh sheet to a surface of the human body. The instrument contains a mechanism for holding the mesh sheet against the tissue surface in more than two locations during the attaching process, making attachment of the sheet to the surface quicker and easier. The instrument also contains a mechanism for grasping and then quickly and easily releasing the mesh sheet from the instrument once it is in place. The invention can also be used to hold the mesh in position without the use of the mechanism that grips the mesh.
Owner:JACKSON KELLY
Barrier layer with underlying medical device and one or more reinforcing support structures
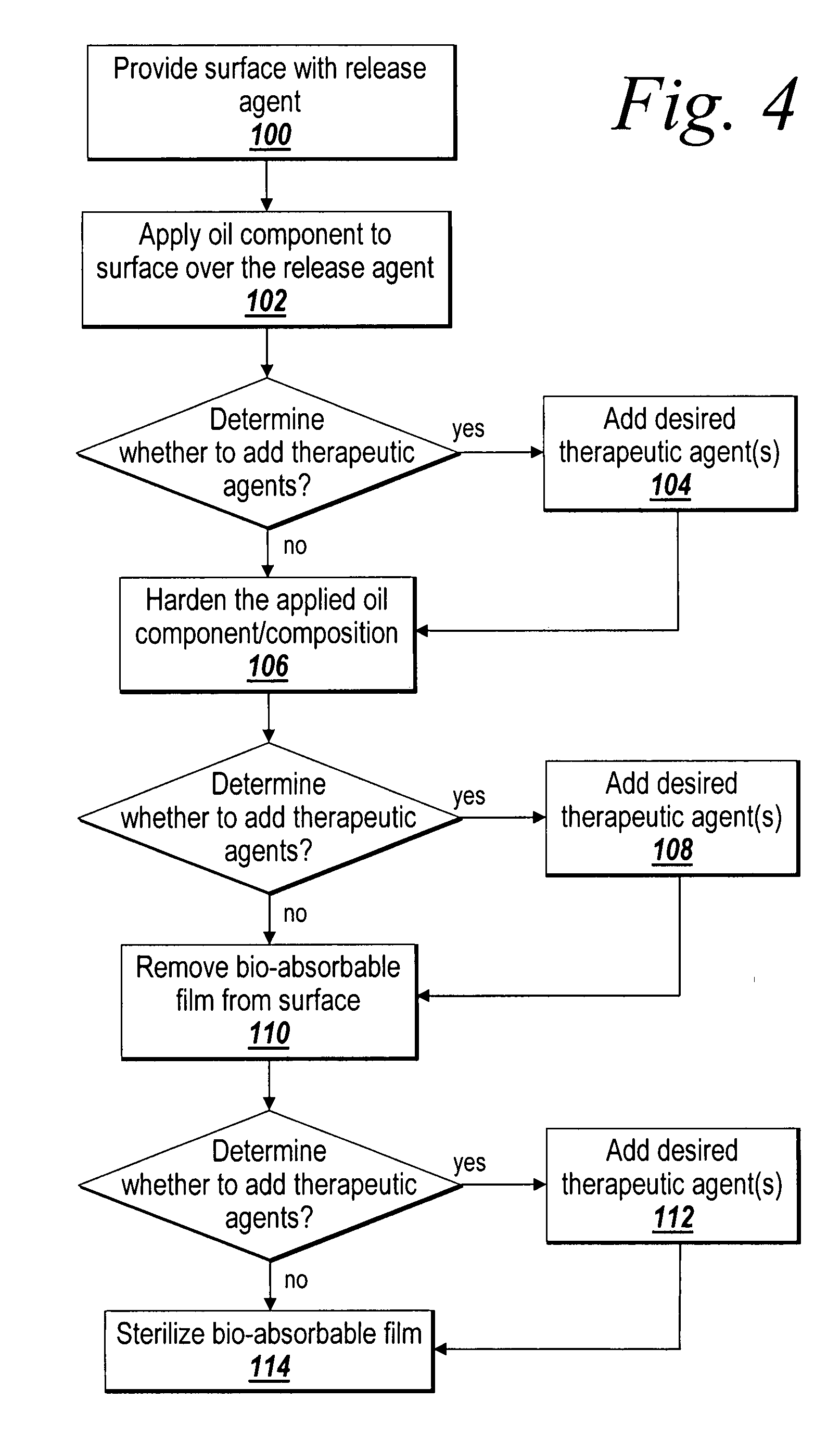
A barrier layer device is formed of an underlying biocompatible structure having a barrier layer coating that can exhibit anti-inflammatory properties, non-inflammatory properties, and / or adhesion-limiting properties, as well as generate a modulated healing effect on injured tissue. As implemented herein, the barrier layer is a non-polymeric cross-linked gel derived at least in part from a fatty acid compound, and may include a therapeutic agent. The underlying structure can be in the form of a surgical mesh. The barrier device is further provided with anchoring reinforcements to aid with the fastening of the barrier device for implantation purposes and reinforcing truss sections or portions that prohibit or substantially reduce the occurrence of excessive stretching and tearing. The barrier device is implantable in a patient for short term or long term applications, and can include controlled release of the therapeutic agent.
Owner:ATRIUM MEDICAL
Resilient intervertebral disc implant
InactiveUS20050240269A1Resist fatigueStress resistantInternal osteosythesisCannulasIntervertebral discBiomedical engineering
Resilient surgical meshes that, in some aspects, can be compressed or otherwise configured, for minimally invasive delivery in the intervertebral discs are provided. According to one or more embodiments, the surgical mesh can be robust, fatigue resistant, stable and capable of withstanding the dynamic environment generic to intervertebral discs.
Owner:INTRINSIC THERAPEUTICS
Temporarily Stiffened Mesh Prostheses
ActiveUS20070198040A1Low experience requirementReduce implantationPretreated surfacesAdhesive dressingsFiberSide effect
The present invention relates to medical prostheses and methods of manufacturing those devices. In particular, the prostheses are temporarily stiffened meshes with particular coatings to provide initial stiffness and thereby permit easier surgical handling for treatment or reconstruction of soft tissue defects. Preferred embodiments include surgical meshes coated with one or more biodegradable polymers that can act as a stiffening agent by coating the filaments or fibers of the mesh to temporarily immobilize the contact points of those filaments or fibers and / or by increasing the stiffness of the mesh by at least 1.1 times its original stiffness. The devices of the invention can also provide relief from various post-operative complications associated with their implantation, insertion or surgical use. By including biologically active agents and / or drugs in the coating, the devices provide prophylaxis for and can alleviate side effects or complications associated with the surgery or use of prostheses in general.
Owner:MEDTRONIC INC
Ventral Hernia Repair With Barbed Suture
The present disclosure is directed to a method and system for the repair of ventral hernias. The method includes the steps of providing a needle; providing a barbed suture having a distal end attached to said needle; providing a surgical mesh; rolling said surgical mesh, said barbed suture, and said needle to form a rolled mesh having said needle oriented substantially parallel to a longitudinal axis of said rolled mesh; transferring said rolled mesh into a body cavity via a laparoscopic device; unrolling and laying said surgical mesh under a ventral hernia in an abdominal wall; threading said needle and barbed suture through said surgical mesh and said abdominal wall; and trimming said barbed suture.
Owner:TYCO HEALTHCARE GRP LP
Apparatus for applying surgical fasteners to body tissue
InactiveUS20090105535A1Increase awarenessAvoid deformationSuture equipmentsStapling toolsSurgical stapleSupporting system
An apparatus is disclosed for endoscopic application of surgical staples adapted to attach surgical mesh to body tissue in laparoscopic hernia surgery. The apparatus includes a frame, and a generally elongated endoscopic section connected to the frame and extending distally therefrom. A staple storage cartridge is removably supported on a pivotal support system at the distal end portion of the endoscopic section with each staple being configured and adapted to attach the mesh to the body tissue. An elongated pusher system formed of several assembled components and extending from the frame to the endoscopic section is provided for individually advancing at least one staple at a time distally for positioning adjacent the surgical mesh and the body tissue. The pusher system also includes a trigger system to actuate the pusher. The trigger system is provided with perceptible tactile sensing means to indicate when the legs of the staple being advanced are exposed so as to be visible to the user for positioning and orientation purposes. Anvil means provides for individually closing each staple to encompass at least a portion of the surgical mesh and to penetrate the body tissue in a manner to attach the portion of the mesh to the body tissue. Projecting distally of the cartridge support system is a pair of legs which are dimensioned and configured to engage the staple during closure to prevent unwanted roll or deformation outside of the plane of the staple.
Owner:TYCO HEALTHCARE GRP LP
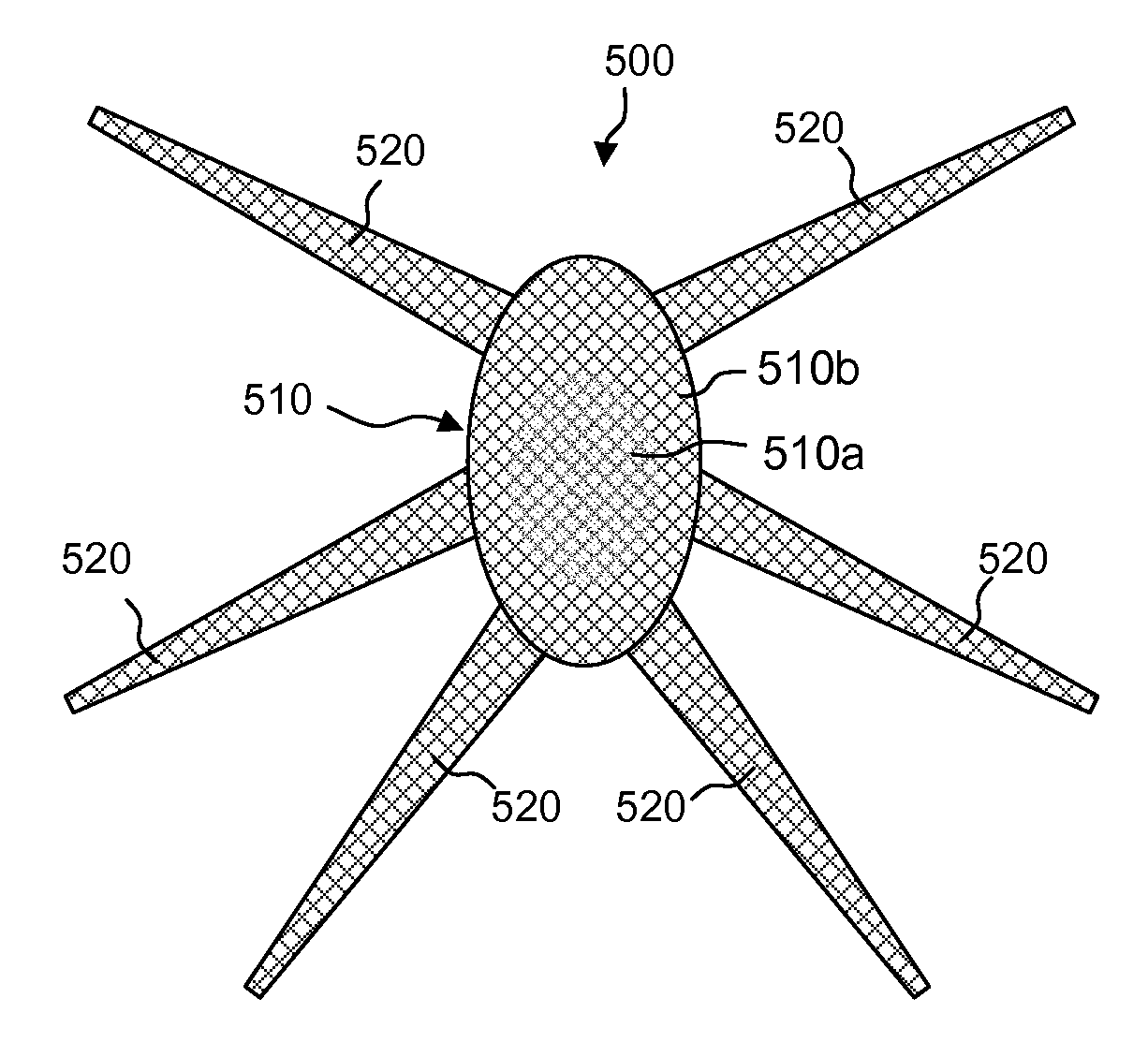
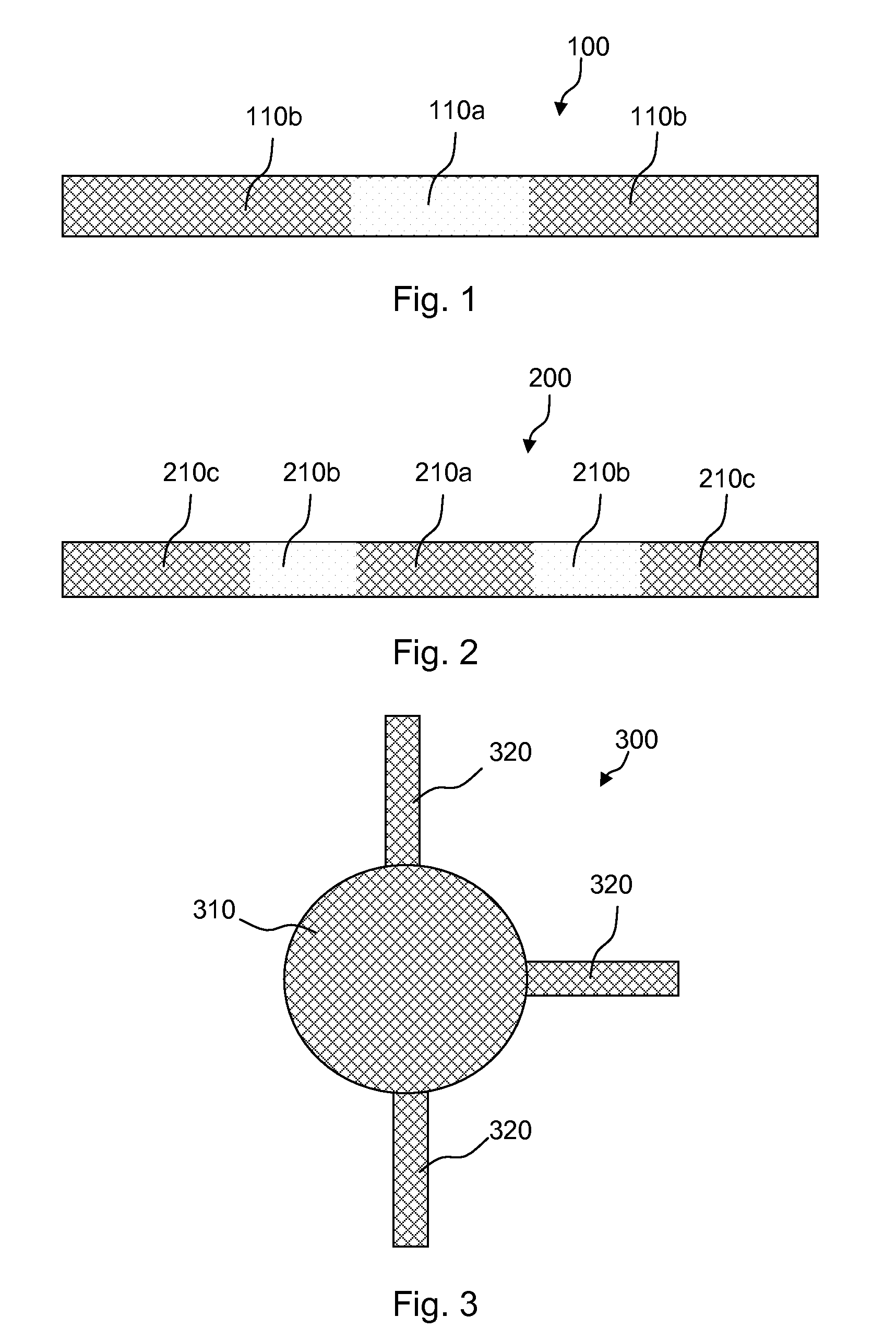
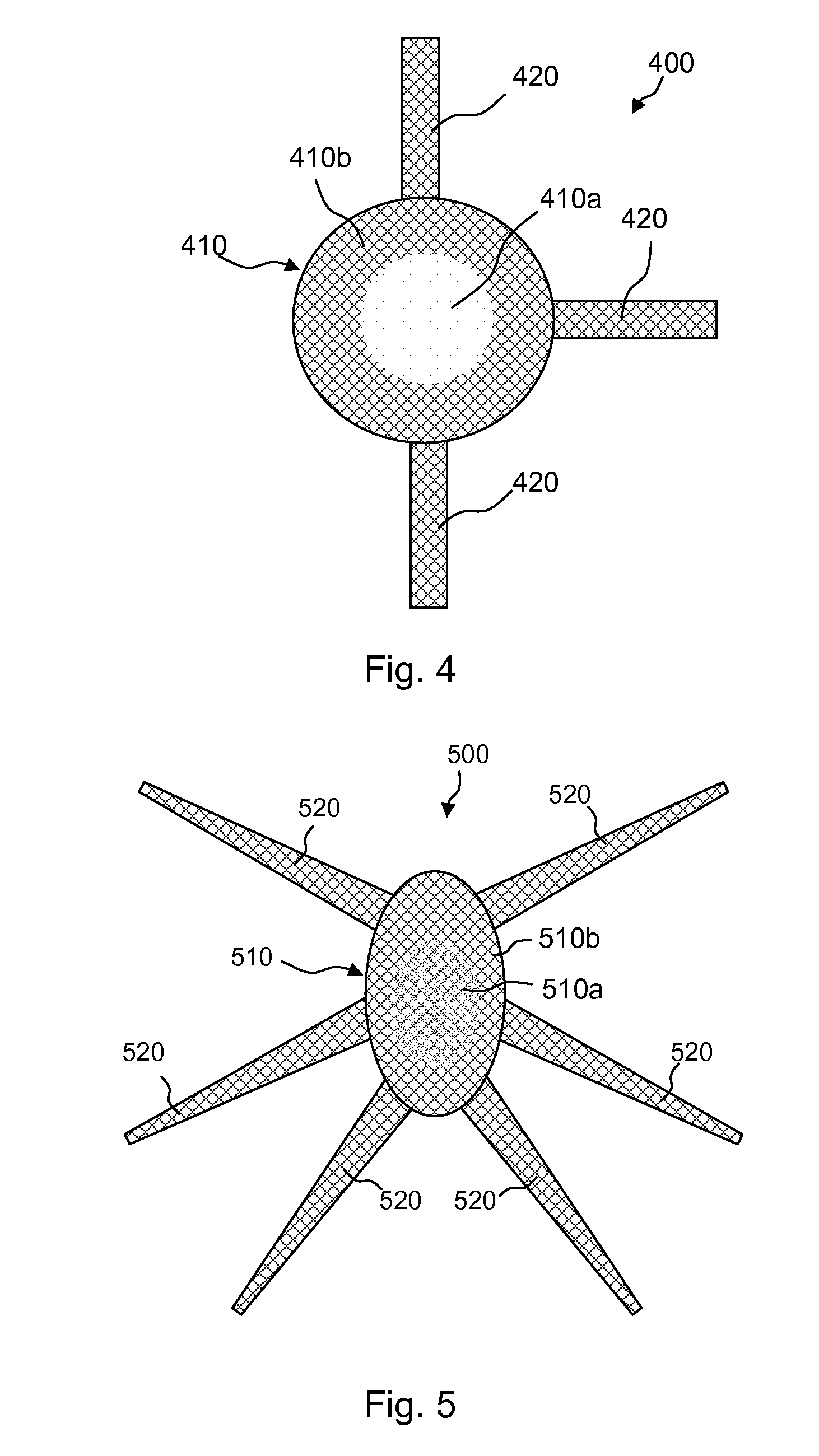
Meshes of variable construction
According to one aspect, the present invention provides a substantially two-dimensional surgical mesh comprising a base material, a first area having a first characteristic and a second area having a second characteristic that differs from the first characteristic. The surgical mesh may further comprise a third area having a third characteristic that may be the same as or different from the first and second characteristics, and so on.
Owner:BOSTON SCI SCIMED INC
Composite mesh devices and methods for soft tissue repair
InactiveUS20100318108A1Reduce adhesionDifferent and simple configurationCovering/liningsWarp knittingComposite meshSoft tissue repair
A composite implantable device for promoting tissue ingrowth therein comprising a biodurable reticulated elastomeric matrix having a three-dimensional porous structure having a continueous network of interconnected and intercommunicating open pores and a support structure is disclosed. The support structure may be a polymeric surgical mesh comprising a plurality of intersecting one-dimensional reinforcement elements, wherein said mesh is affixed to a face of said first matrix. Methods of making and using the implantable device are also provided.
Owner:BIOMERIX CORP
Stabilized intervertebral disc barrier
InactiveUS20050234557A1Resist fatigueStress resistantInternal osteosythesisCannulasIntervertebral discBiomedical engineering
Presented are new resilient sheet-like surgical meshes that may be compressed for minimally invasive delivery in the intervertebral discs. According to one or more embodiments, the surgical mesh can be robust, fatigue resistant, stable and capable of withstanding the dynamic environment generic to intervertebral discs.
Owner:INTRINSIC THERAPEUTICS
Absorbable fastener and applying apparatus
InactiveUS8034076B2Minimize formationReducing fastener-associated long-term discomfort to the patientSuture equipmentsStaplesAbsorbent materialBody tissue
A surgical fastener apparatus, for securing a surgical mesh material to body tissue including a pair of anchors each having a retaining structure formed on an outer surface thereof; and a suture tether interconnecting the pair of anchors to one another. The pair of anchors have a substantially cylindrical body having a conically tapered distal end and a planar proximal end. The retaining structure includes a series of semi-circular angled projections having a planar proximal surface and a tapered distal end, wherein a center of each of the angled projections is spaced a distance from a longitudinal central axis of the body portion. The surgical fastener is made from a bioabsorbable material which reabsorbs into said body tissue at an appropriate rate, such as for example, polyglycolic acid and polylactic acid.
Owner:COVIDIEN LP
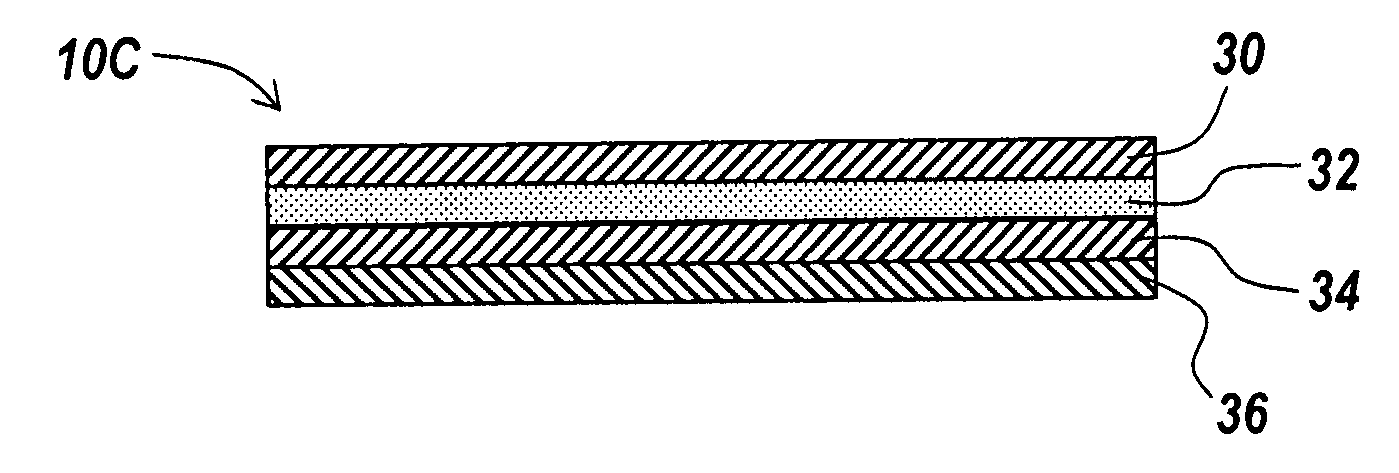
Coated surgical mesh
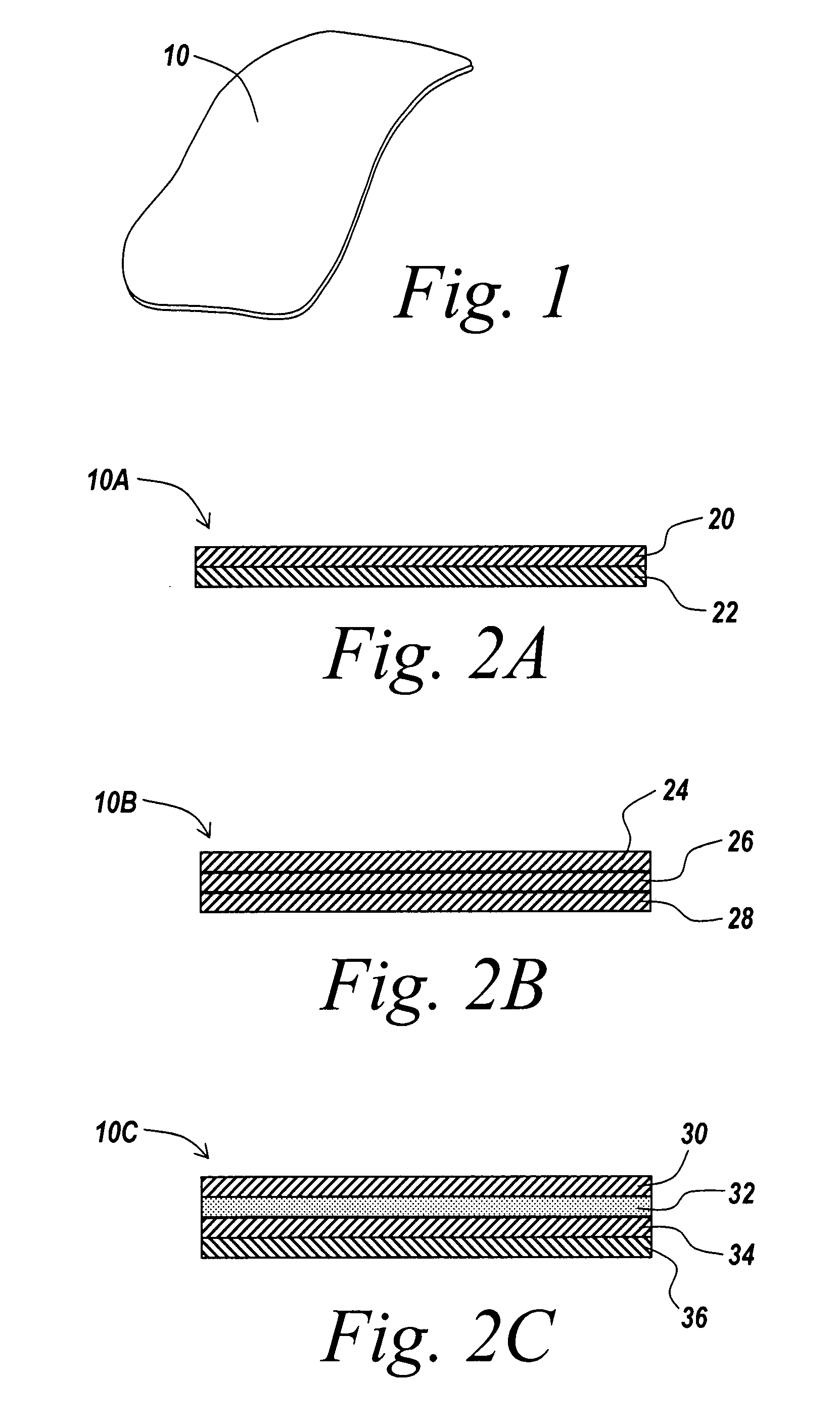
A surgical mesh is formed of a biocompatible mesh structure with a coating that provides anti-inflammatory, non-inflammatory, and anti-adhesion functionality for a implantation in a patient. The coating is generally formed of a fish oil, can include vitamin E, and may be at least partially cured. In addition, the coating can include a therapeutic agent component, such as a drug or other therapeutic agent.
Owner:ATRIUM MEDICAL
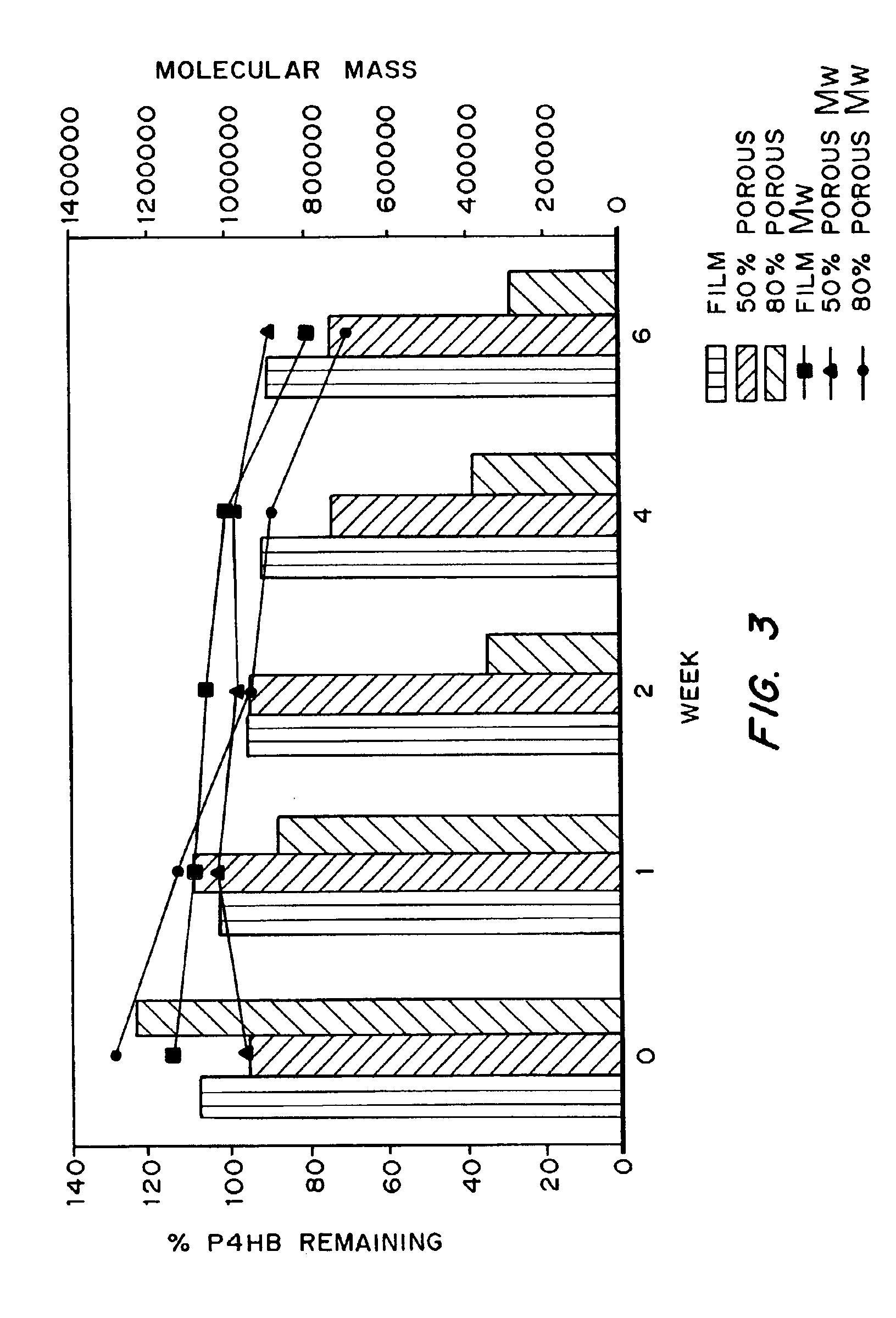
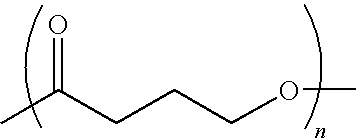
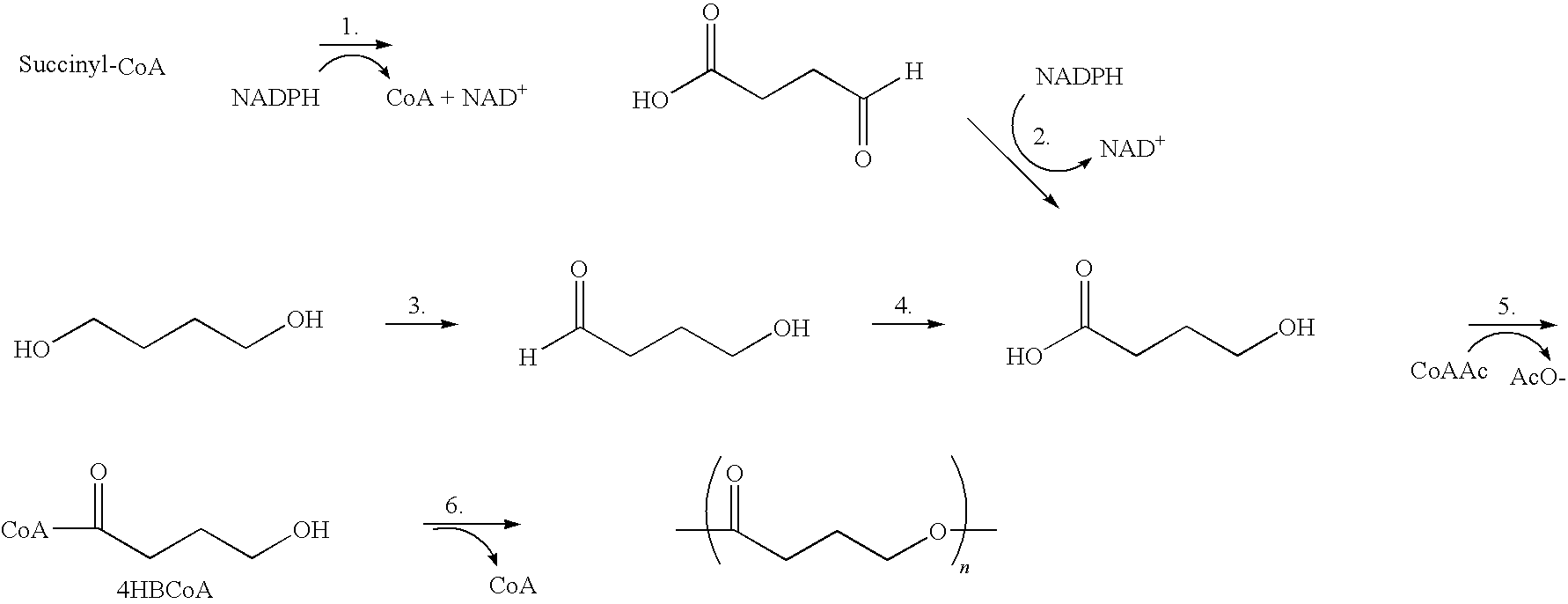
Polyhydroxyalkanoate medical textiles and fibers
ActiveUS8034270B2Easy to operateProlonged strength retentionSurgerySynthetic resin layered productsPolyesterFiber
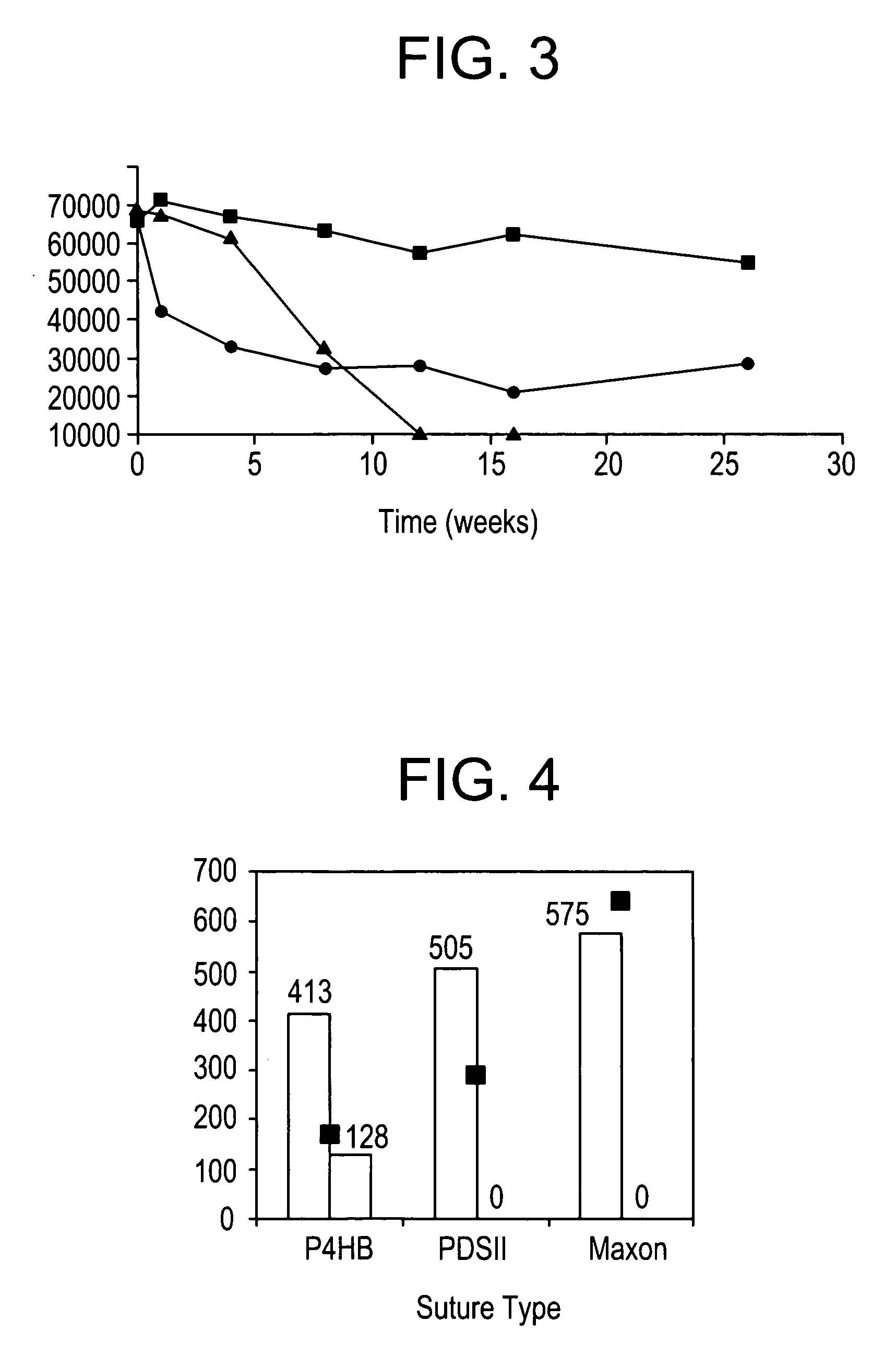
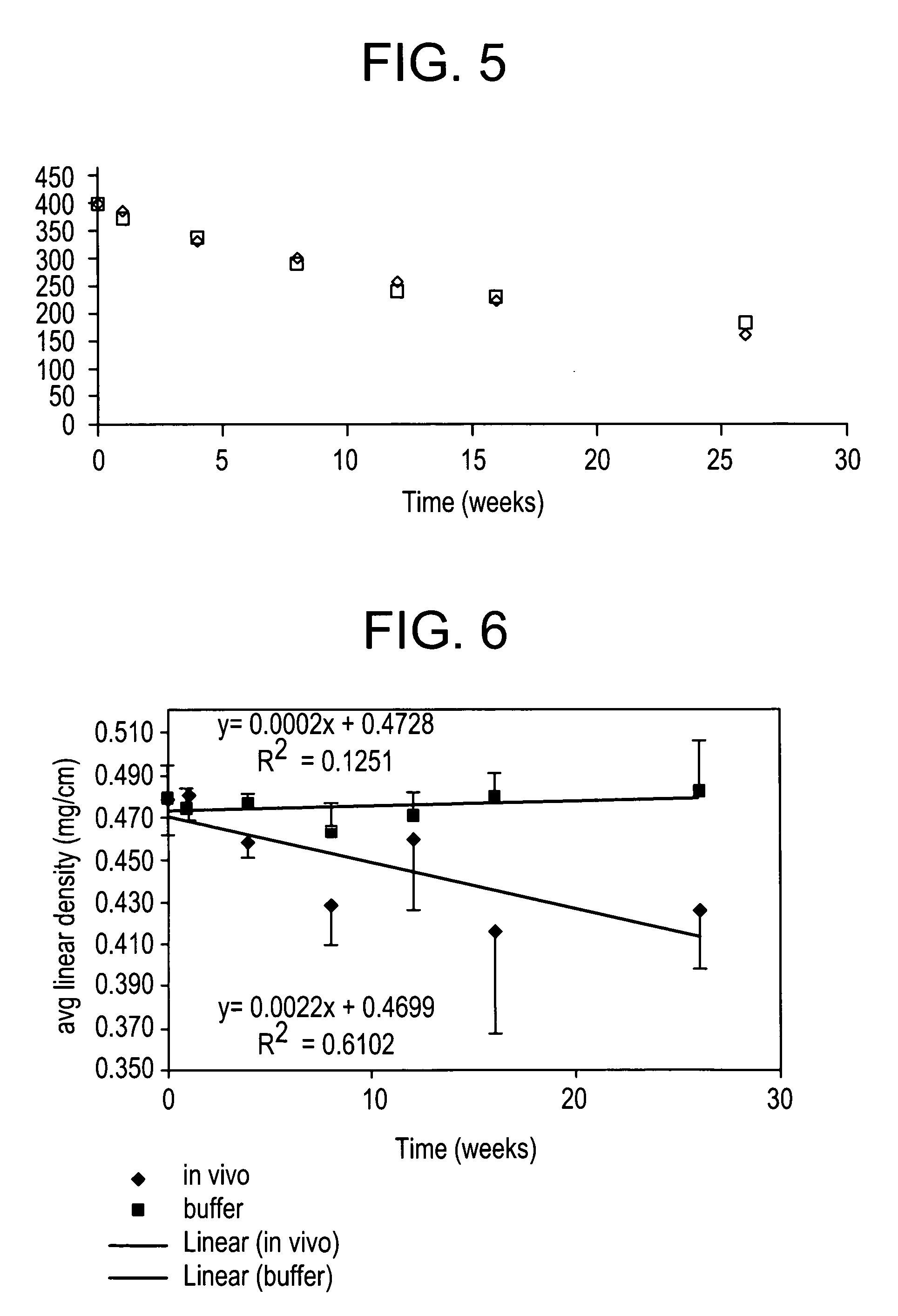
Absorbable polyester fibers, braids, and surgical meshes with prolonged strength retention have been developed. These devices are preferably derived from biocompatible copolymers or homopolymers of 4-hydroxybutyrate. These devices provide a wider range of in vivo strength retention properties than are currently available, and could offer additional benefits such as anti-adhesion properties, reduced risks of infection or other post-operative problems resulting from absorption and eventual elimination of the device, and competitive cost. The devices may also be particularly suitable for use in pediatric populations where their absorption should not hinder growth, and provide in all patient populations wound healing with long-term mechanical stability. The devices may additionally be combined with autologous, allogenic and / or xenogenic tissues to provide implants with improved mechanical, biological and handling properties.
Owner:TEPHA INC
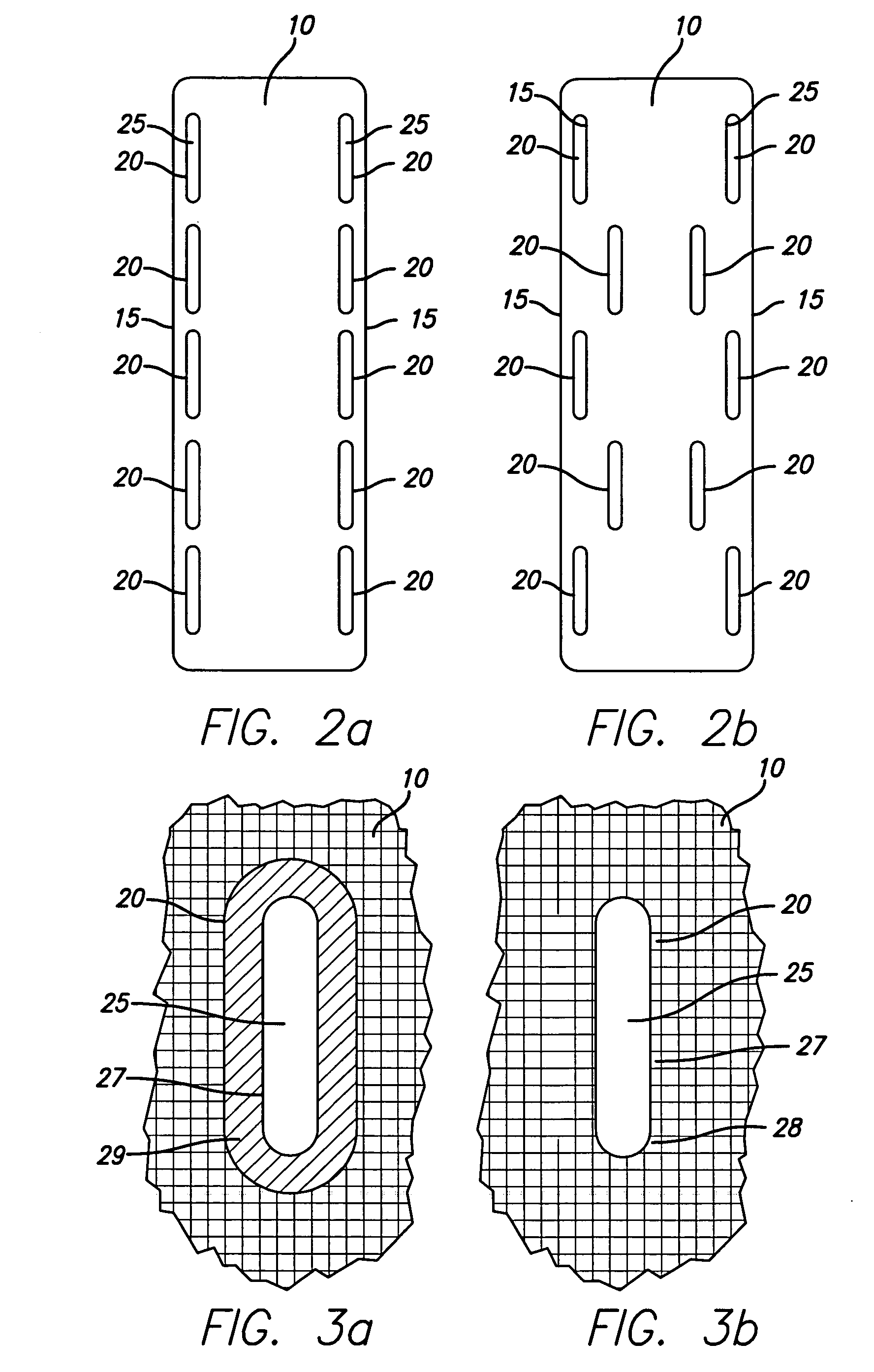
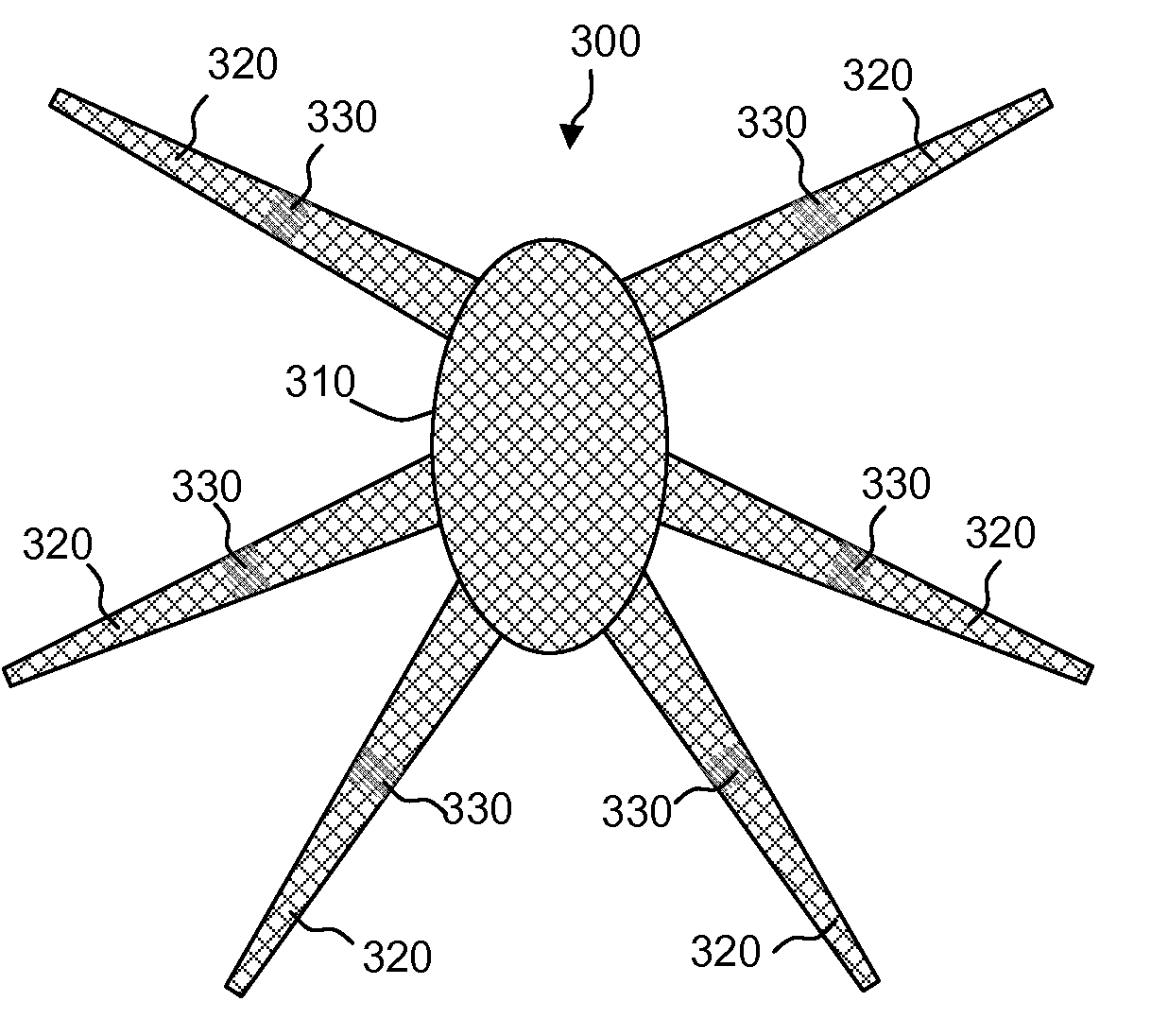
Bone anchored surgical mesh
InactiveUS20060264948A1Inhibition of attachmentAvoid developmentInternal osteosythesisJoint implantsDura materSurgical department
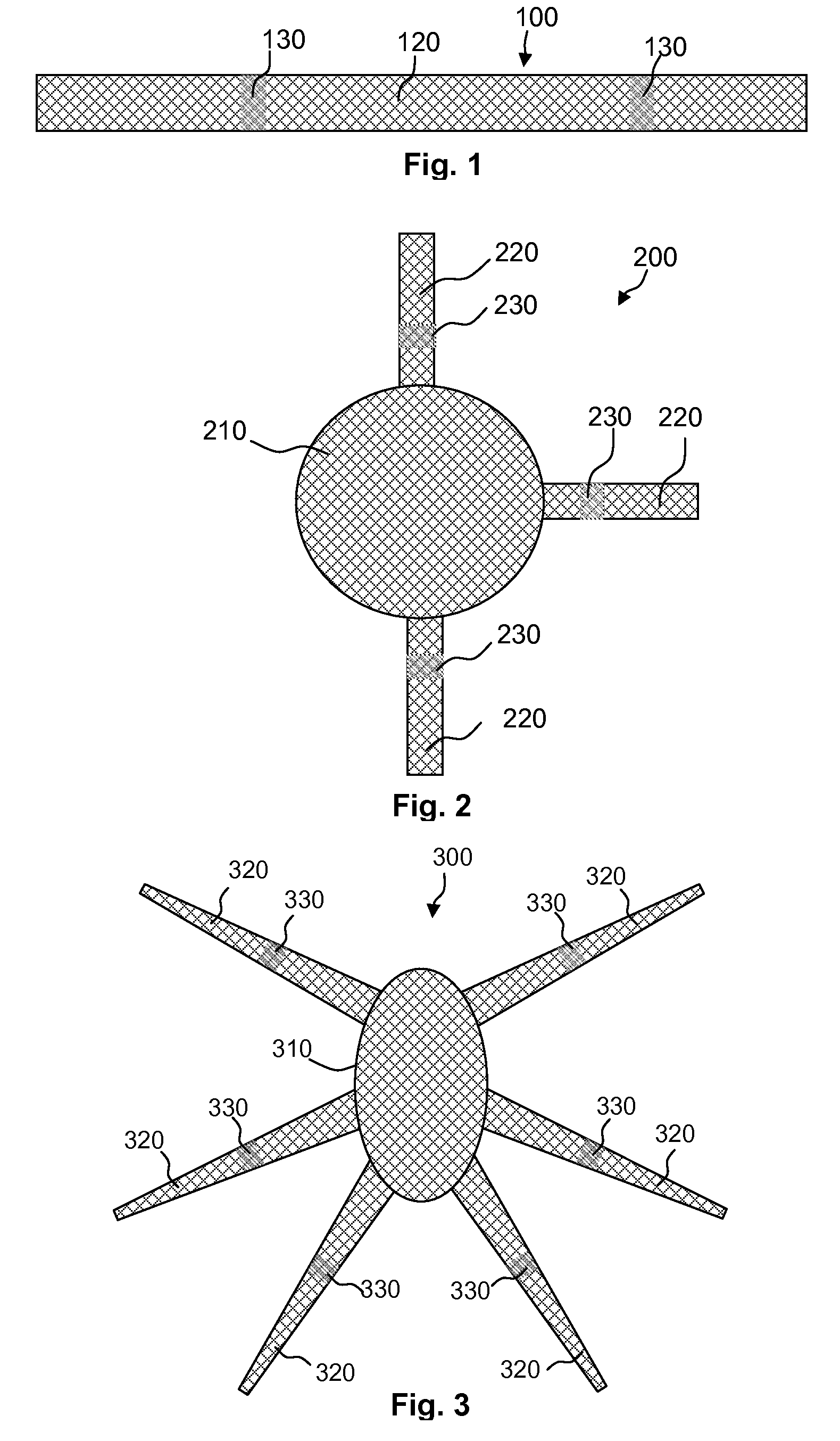
A bone-anchored surgical mesh has slot-like anchoring members that allow for the variable placement of screws and other bone fasteners. This permits the surgeon discretion in the placement of bone fasteners used to attach the mesh to the patient's bone. The elongate openings of the anchoring members allow for a sliding motion between the bone fasteners and the anchoring members, and facilitates positioning and articulation of the mesh. The anchoring members may include bushings to aid the sliding motion of the anchoring member on the bone fastener. In one embodiment, the mesh consists of shorter modular strips that overlap each other such that a single bone fastener is passed through two overlapping anchoring members to lock the two modular mesh strips together. Additional modular mesh strips can be added on at either end, as desired, to provide the desired length of dural coverage.
Owner:ONIKE TECH
Surgical instrument, surgical mesh and surgical retraction means of the instrument, and surgical method using the instrument
InactiveUS20140088368A1Easily and efficiently drawing backMinimize damageSuture equipmentsSurgical needlesBody cavity useSurgical department
A surgical instrument, a surgical mesh and a surgical retraction means of the instrument, and a surgical method using the instrument, in which the surgical retraction means is connected to the mesh and to a body tissue at opposite ends, and so the tissue retraction force can be distributed to several holding parts that fasten the mesh to the inner surface of a body cavity, thereby efficiently drawing back the body tissue in various directions. The surgical instrument includes a mesh fastened in an open state to the inner surface of the body cavity using a fastening means; and a surgical retraction means selectively and removably connected at a first end to a point of the mesh and connected at a second end to a part of the internal tissue that is required to be drawn back, so that the surgical retraction means can efficiently draw back the internal tissue.
Owner:PARK KWANG TAI
Tissue separating device with reinforced support for anchoring mechanisms
ActiveUS20080113001A1Promoting tissue in-growthLimit and reduce degree of adhesion formationSurgeryAbsorbent padsCross-linkControl release
A barrier layer device is formed of an underlying biocompatible structure having a barrier layer coating that can exhibit anti-inflammatory properties, non-inflammatory properties, and / or adhesion-limiting properties, as well as generate a modulated healing effect on injured tissue. As implemented herein, the barrier layer is a non-polymeric cross-linked gel derived at least in part from a fatty acid compound, and may include a therapeutic agent. The underlying structure can be in the form of a surgical mesh. The barrier device is further provided with reinforced sections or portions to aid with the fastening of the barrier device for implantation purposes and prohibits or substantially reduces the occurrence of excessive stretching and tearing. The barrier device is implantable in a patient for short term or long term applications, and can include controlled release of the therapeutic agent.
Owner:ATRIUM MEDICAL
Stabilized intervertebral disc barrier
InactiveUS7553329B2Resist fatigueStress resistantInternal osteosythesisCannulasIntervertebral discHernia surgical mesh
Presented are new resilient sheet-like surgical meshes that may be compressed for minimally invasive delivery in the intervertebral discs. According to one or more embodiments, the surgical mesh can be robust, fatigue resistant, stable and capable of withstanding the dynamic environment generic to intervertebral discs.
Owner:INTRINSIC THERAPEUTICS
Mesh Pouches for Implantable Medical Devices
ActiveUS20080132922A1Reduces and prevents implantReduces and prevents and surgery-related complicationElectrotherapyDiagnosticsFiberSide effect
Biodegradable polymer-coated surgical meshes formed into pouches are described for use with cardiac rhythm management devices (CRMs) and other implantable medical devices. Such meshes are formed into a receptacle, e.g., a pouch or other covering, capable of encasing, surrounding and / or holding the cardiac rhythm management device or other implantable medical device for the purpose of securing it in position, inhibiting or reducing bacterial growth, providing pain relief and / or inhibiting scarring or fibrosis on or around the CRM or other implantable medical device. Preferred embodiments include surgical mesh pouches coated with one or more biodegradable polymers that can act as a stiffening agent by coating the filaments or fibers of the mesh to temporarily immobilize the contact points of those filaments or fibers and / or by increasing the stiffness of the mesh by at least 1.1 times its original stiffness. The pouches of the invention can also provide relief from various post-operative complications associated with their implantation, insertion or surgical use, and, optionally, include one or more drugs in the polymer matrix of the coating to provide prophylactic effects and / or alleviate side effects or complications associated with the surgery or implantation of the CRM or other implantable medical device.
Owner:MEDTRONIC INC
Preventing biofilm formation on implantable medical devices
ActiveUS20100168808A1Reduces and prevents implantReduces and prevents and surgery-related complicationMammary implantsDiagnosticsBiofilmMedical device
Biodegradable polymer-coated surgical meshes formed into pouches are described for use with cardiac rhythm management devices (CRMs) and other implantable medical devices. Such meshes are formed into a receptacle, e.g., a pouch or other covering, capable of encasing, surrounding and / or holding the cardiac rhythm management device or other implantable medical device and preventing or retarding the formation of a biofilm.
Owner:MEDTRONIC INC
Surgical meshes
An implant includes a central portion composed of at least one yarn interconnected to form openings therein and a peripheral portion extending around the central portion. The peripheral portion includes at least one high strength yarn.
Owner:TYCO HEALTHCARE GRP LP
Method of making a polyhydroxyalkanoate filament
ActiveUS7641825B2Improve handlingImprove methodSuture equipmentsMovable spraying apparatusPolyesterFiber
Absorbable polyester fibers, braids, and surgical meshes with improved handling properties have been developed. These devices are preferably derived from biocompatible copolymers or homopolymers of 4-hydroxybutyrate. These devices provide a wider range of in vivo strength retention properties than are currently available and have a decreased tendency to curl, in the preferred embodiment, due to the inclusion of relaxation and annealing steps following methods are characterized by the following physical properties: (i) elongation to break from about 17% to about 85% (ii) Young's modulus of less than 350,000 psi, (iii) knot to straight ratio (knot strength / tensile strength) of 55-80% or (iv) load at break from 1100 to 4200 grams.
Owner:TEPHA INC
Surgical meshes with radiopaque coatings
ActiveUS20090281558A1Anti-incontinence devicesPharmaceutical containersImaging techniqueBiomedical engineering
According to an aspect of the present invention, implantable medical articles are provided, which comprise a surgical mesh that is at least partially covered with a coating that comprises a radiopaque material such as a metal or a metallic compound. The radiopaque material is present in the coating in an amount such that the coated portions of the mesh are visible using radiographic imaging techniques. Other aspects of the invention pertain to methods of making and using such medical articles.
Owner:BOSTON SCI SCIMED INC
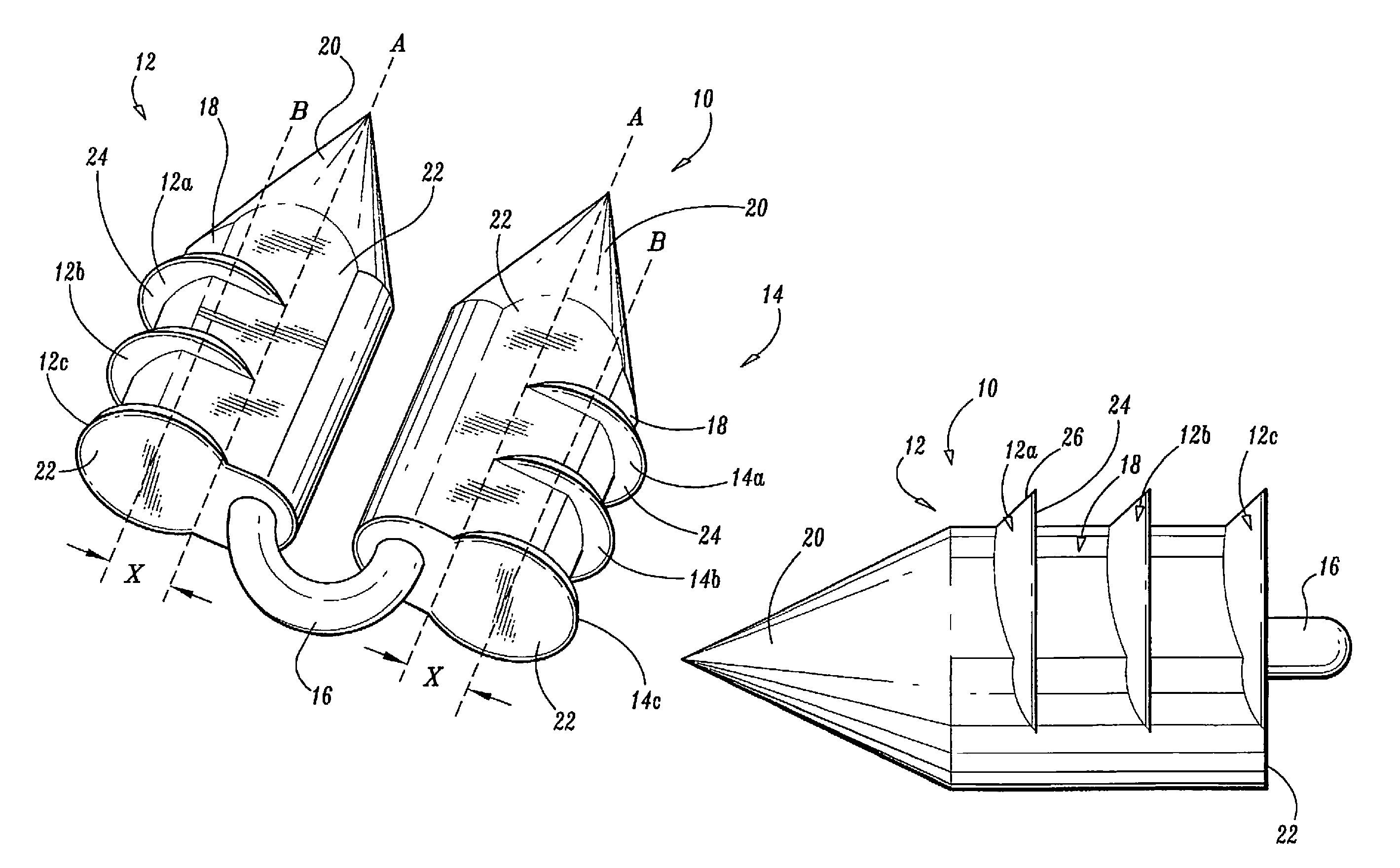
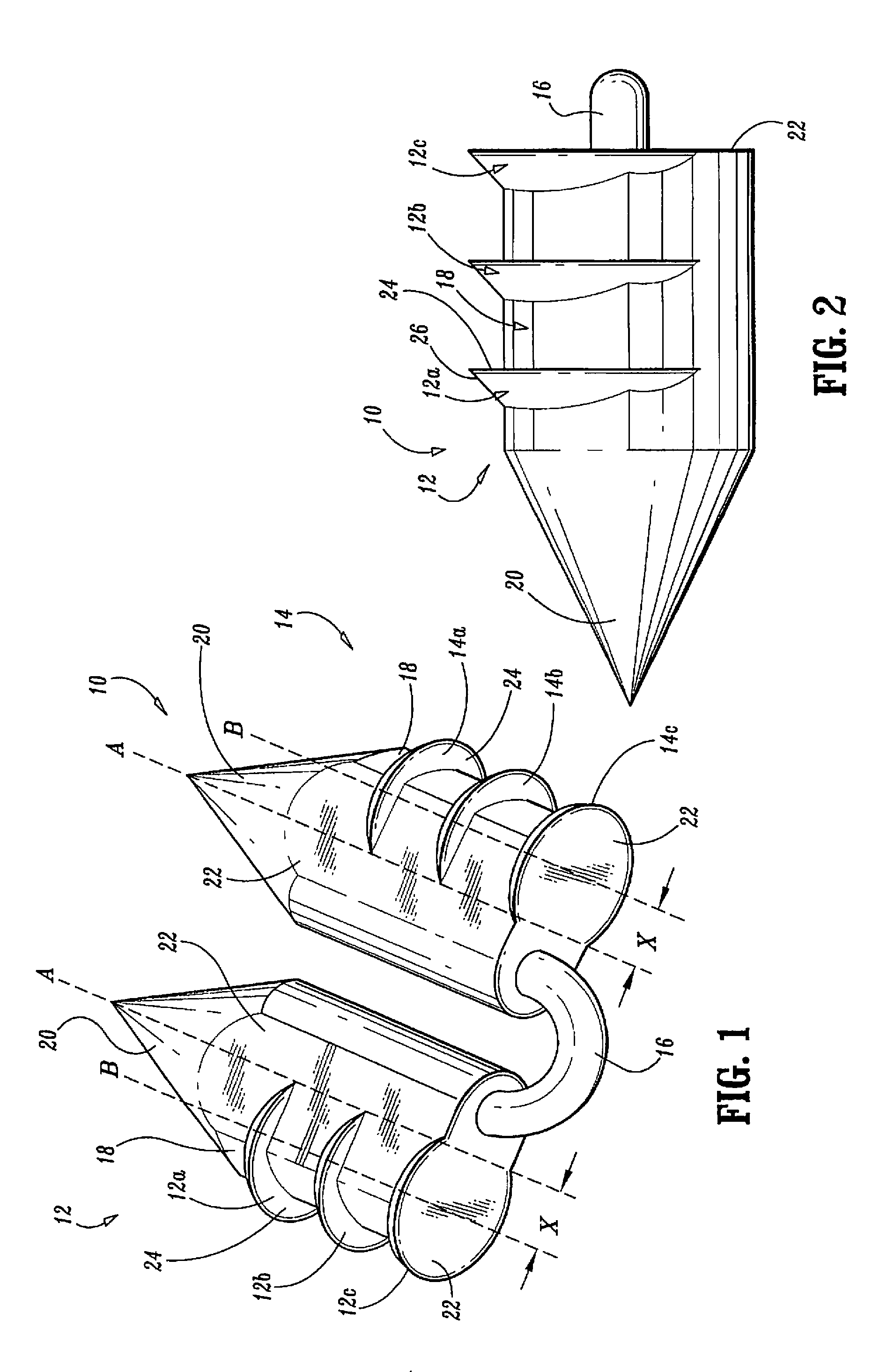
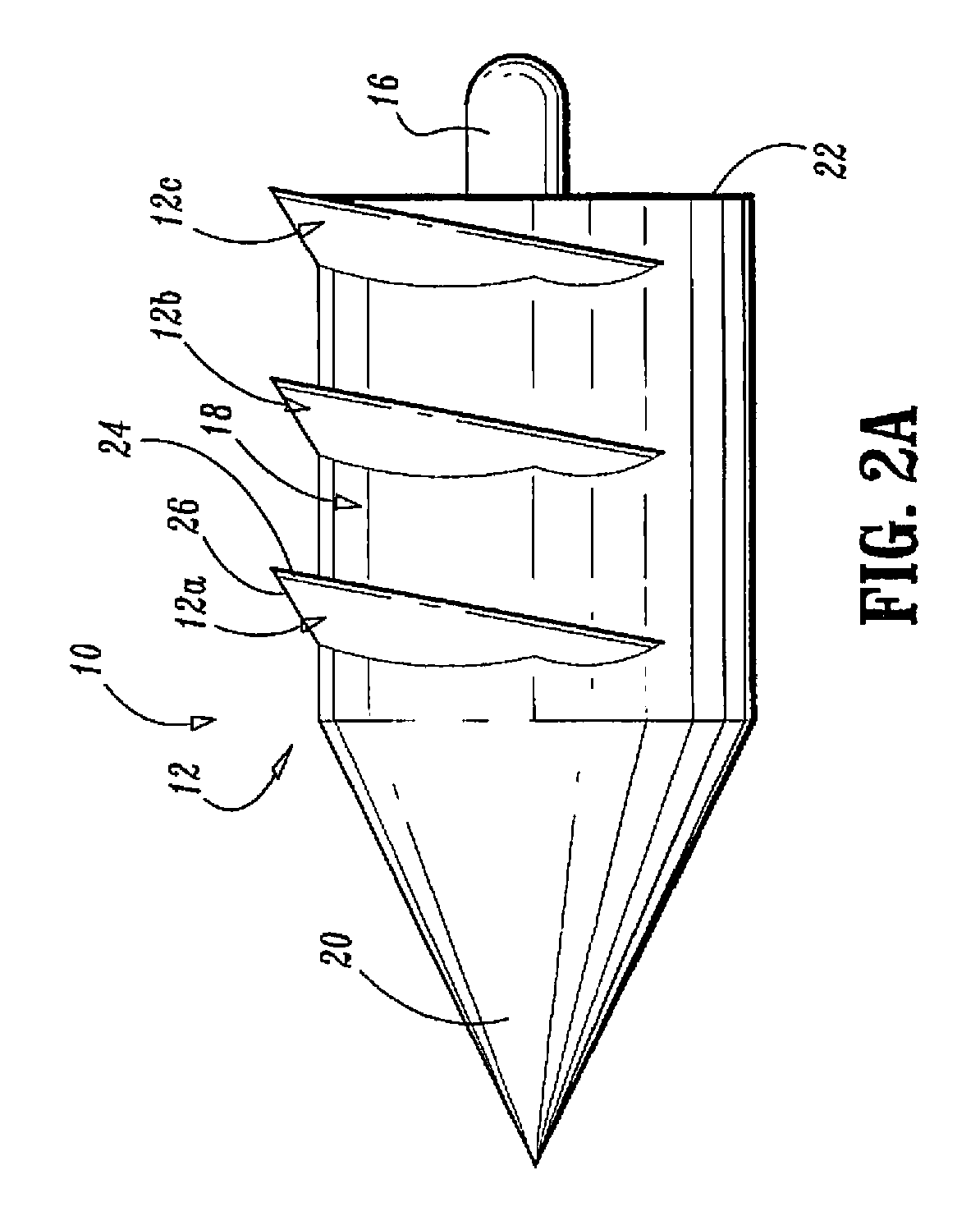
Surgical fasteners
Surgical fasteners, including tacks are disclosed for fastening a surgical mesh to underlying tissue during surgical procedures to repair body tissue, such as in hernia repair. Such tacks can include a head, a elongated body portion having a distal end and a proximal end, and an anchoring element on an outer surface of the body portion for inhibiting the removal of the tack from the mesh and body tissue.
Owner:TYCO HEALTHCARE GRP LP
Mechanically Attached Medical Device Coatings
The present invention relates to nanofibrous coatings on medical devices such a surgical mesh or stent, wherein the coating is mechanically attached to the device. The principal mechanism for attaching the coating is through causing the fibers to permeate and entangle with the substrate.
Owner:THE UNIVERSITY OF AKRON
Implantable silk prosthetic device and uses thereof
InactiveUS20120150204A1Minimizes tissue erosionMinimizes fistulaMammary implantsWeft knittingYarnProsthesis
A method of using a biocompatible surgical silk mesh prosthetic device in body aesthetics and body contouring, the surgical mesh employing a knit pattern that substantially prevents unraveling and preserves the stability of the mesh device, especially when the mesh device is cut. An example prosthetic device employs a knitted mesh including at least two yarns laid in a knit direction and engaging each other to define a plurality of nodes. The at least two yarns include a first yarn and a second yarn extending between and forming loops about two nodes. The second yarn has a higher tension at the two nodes than the first yarn. the second yarn substantially prevents the first yarn from moving at the two nodes and substantially prevents the knitted mesh from unraveling at the nodes.
Owner:ALLERGAN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com