Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
345 results about "Tissue ingrowth" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
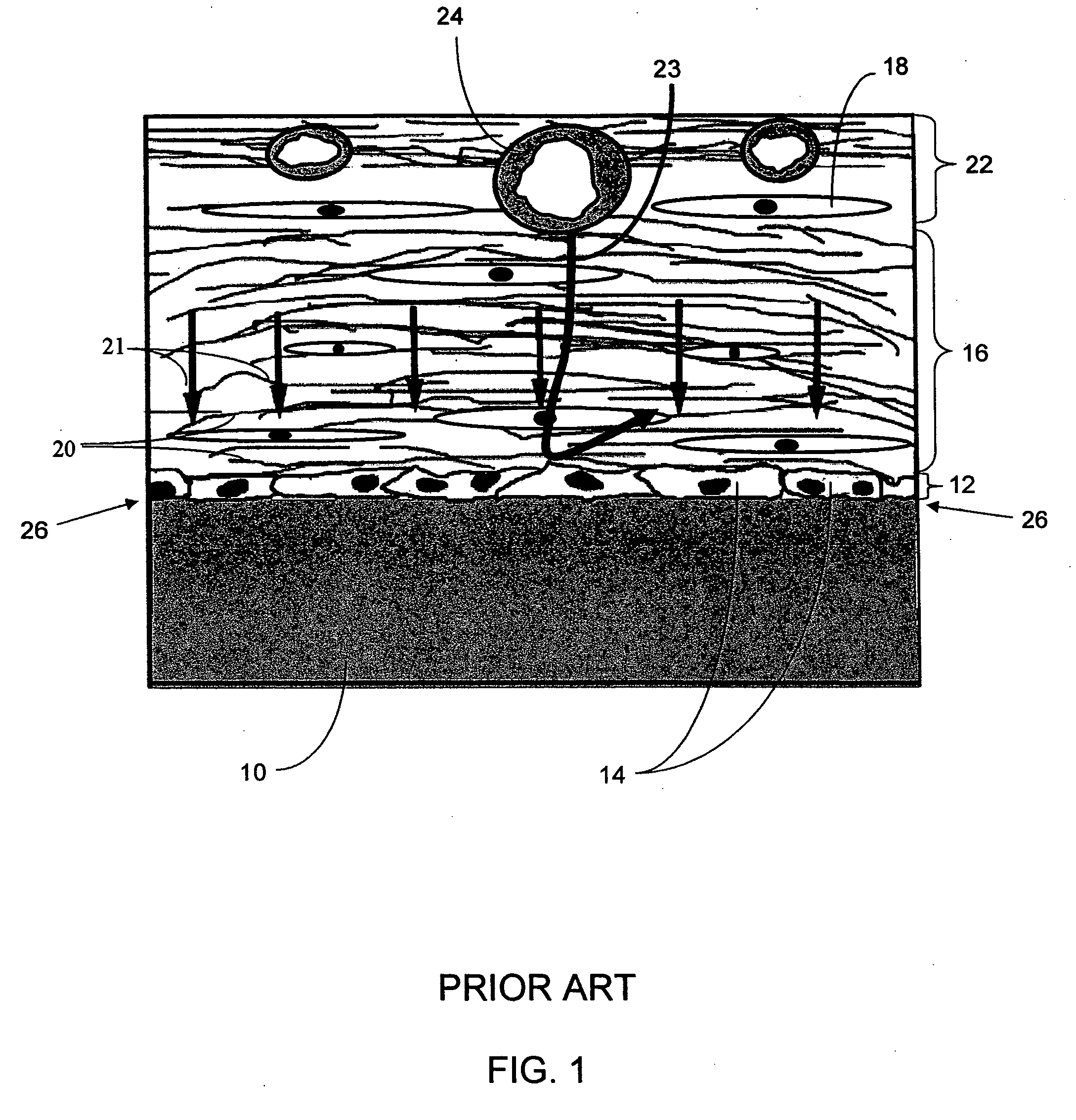
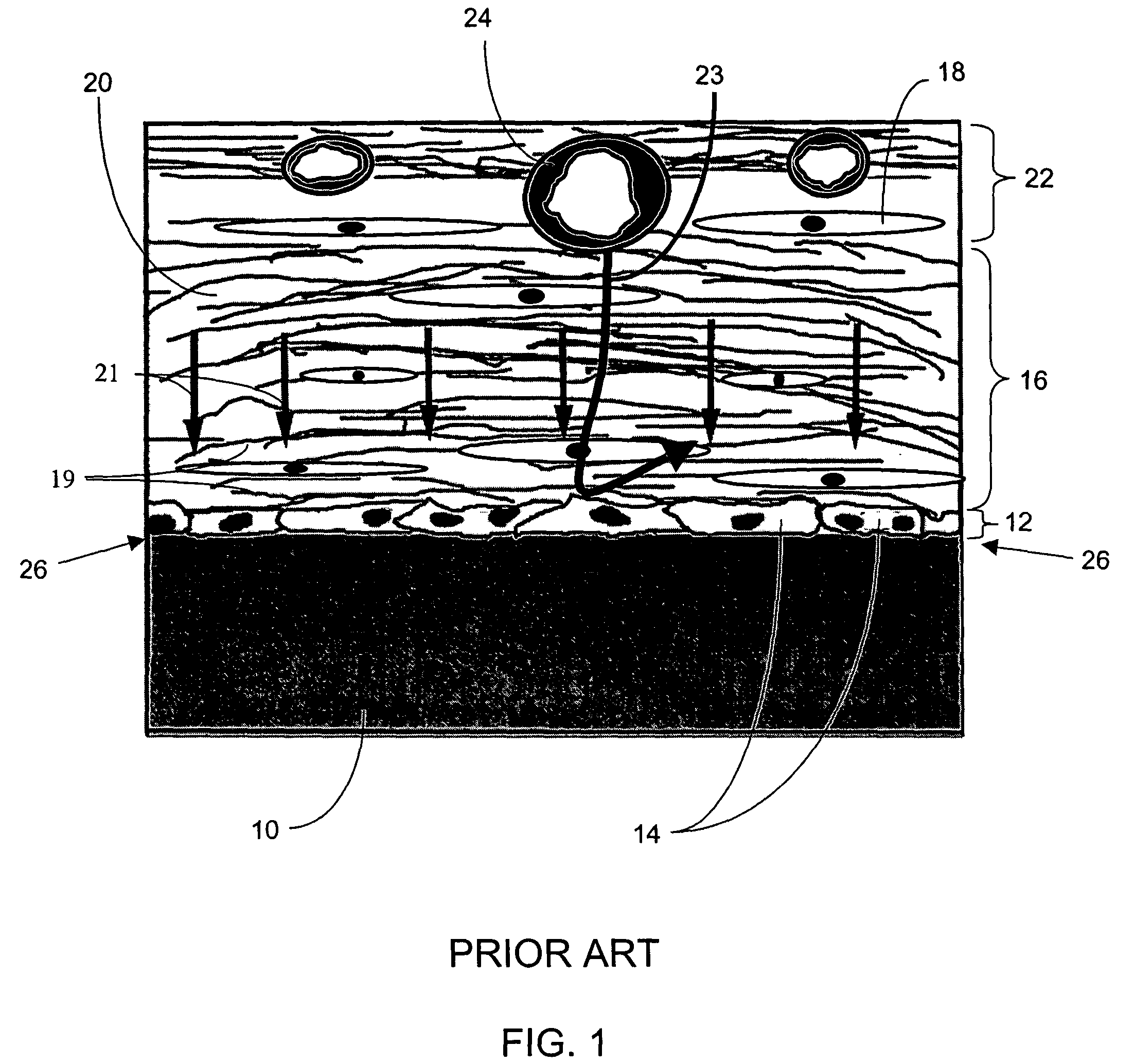
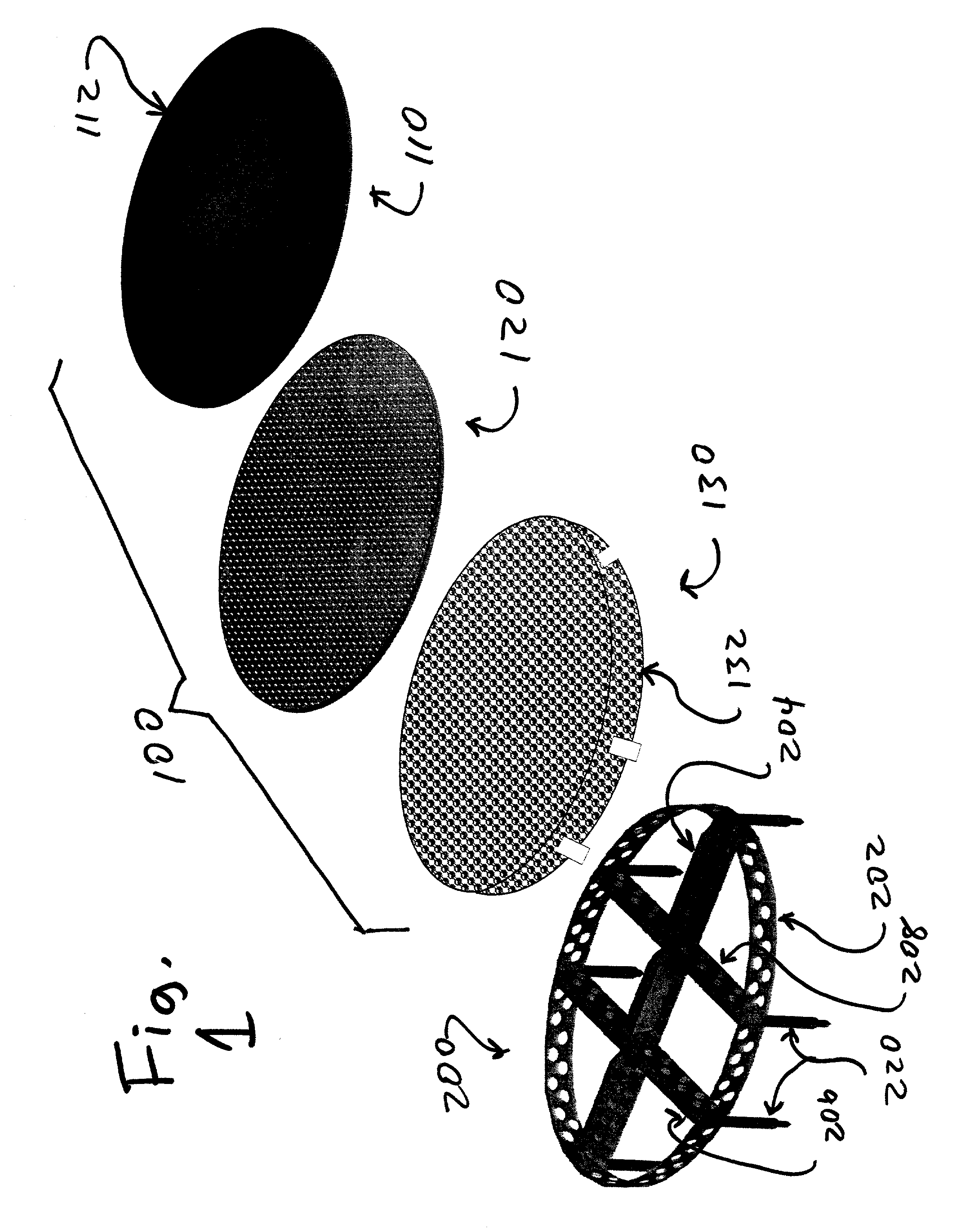
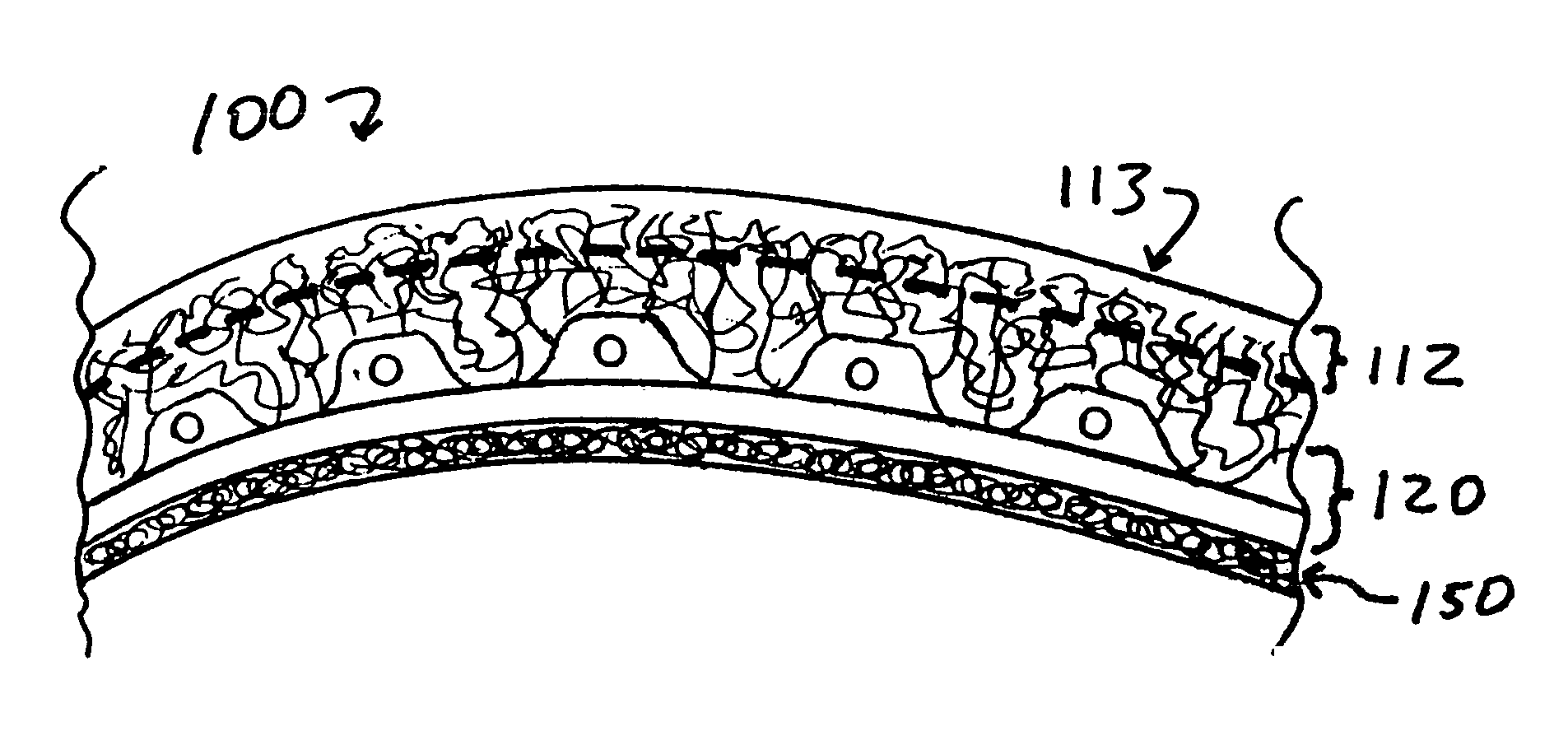
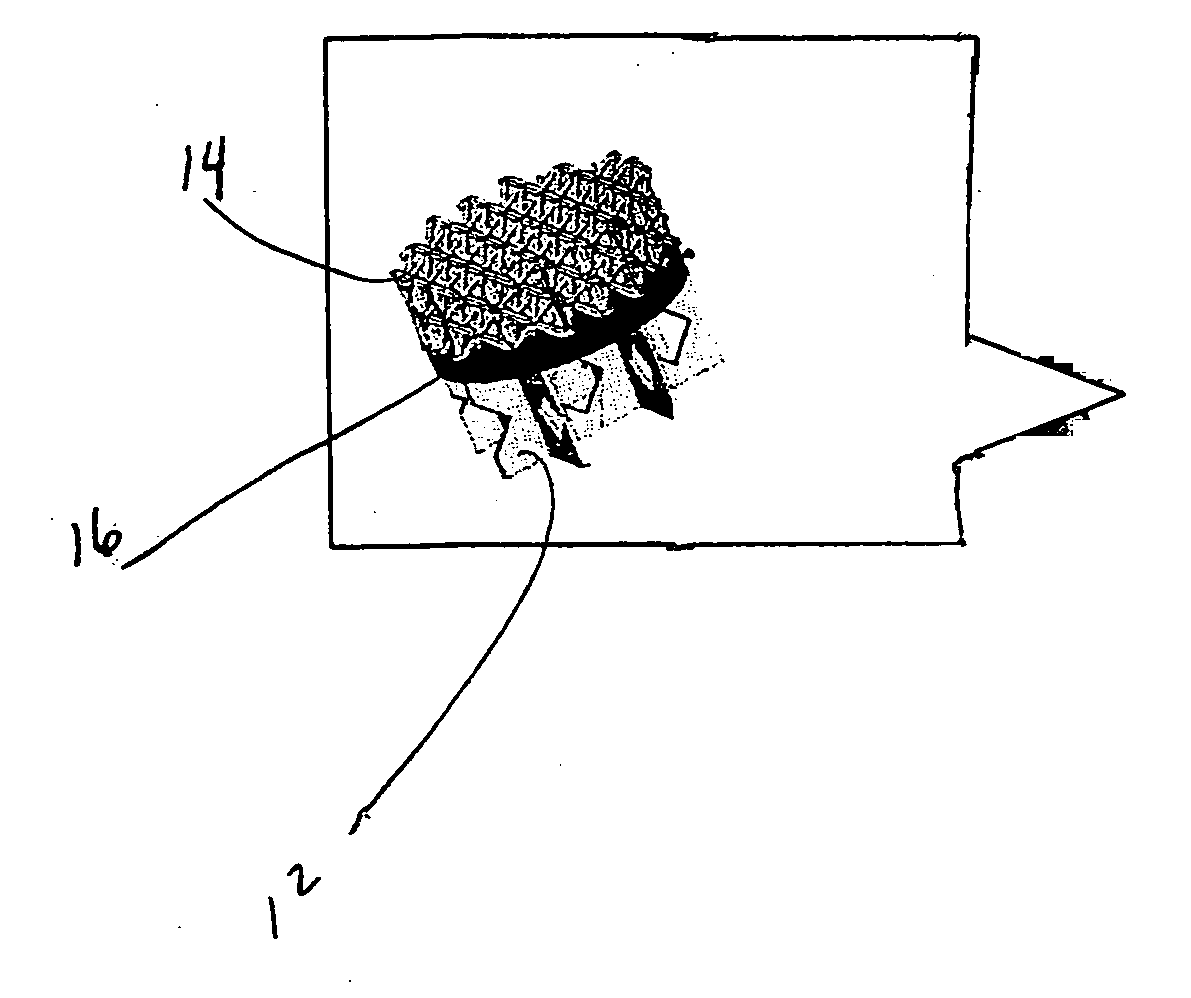
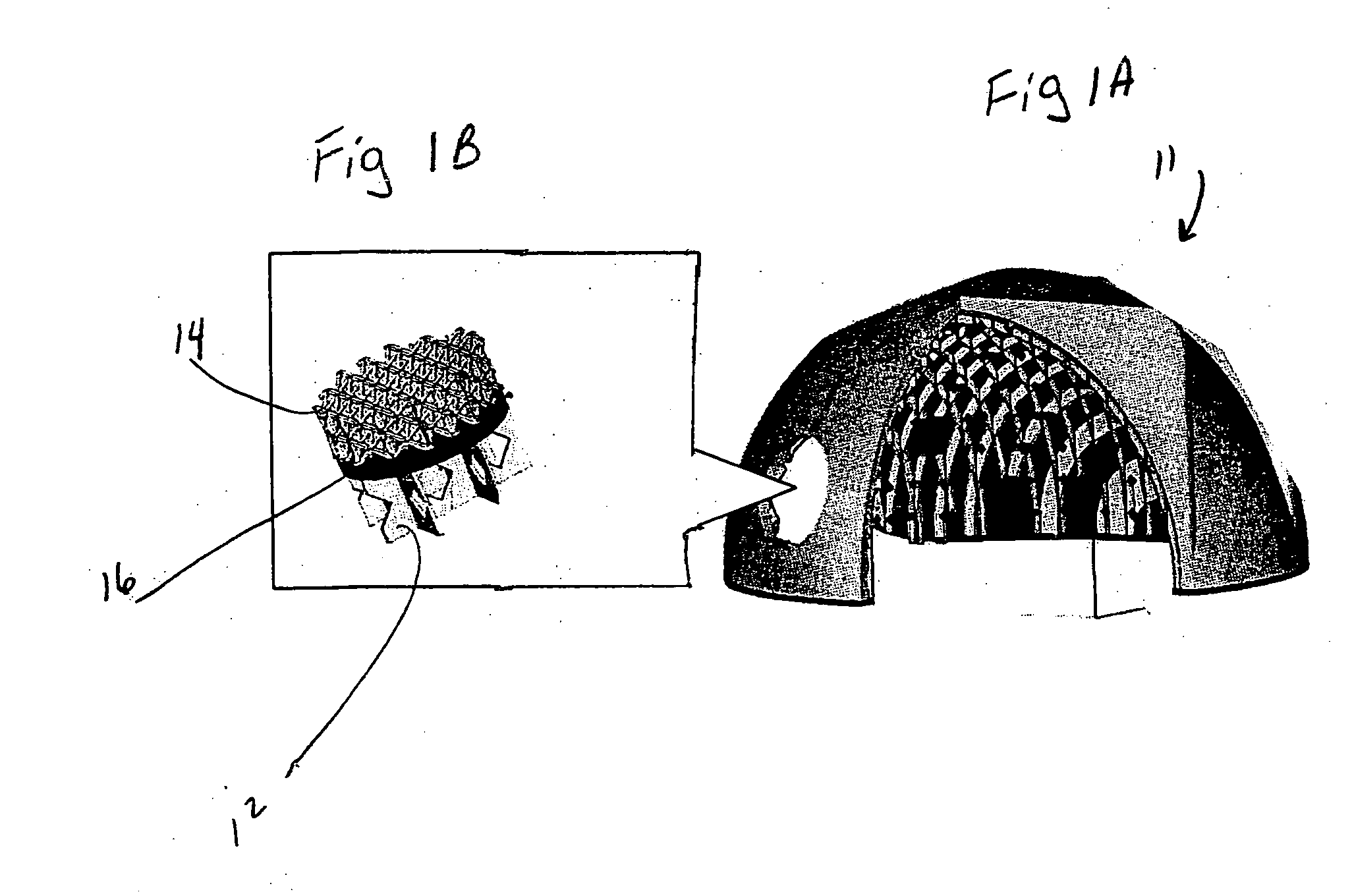
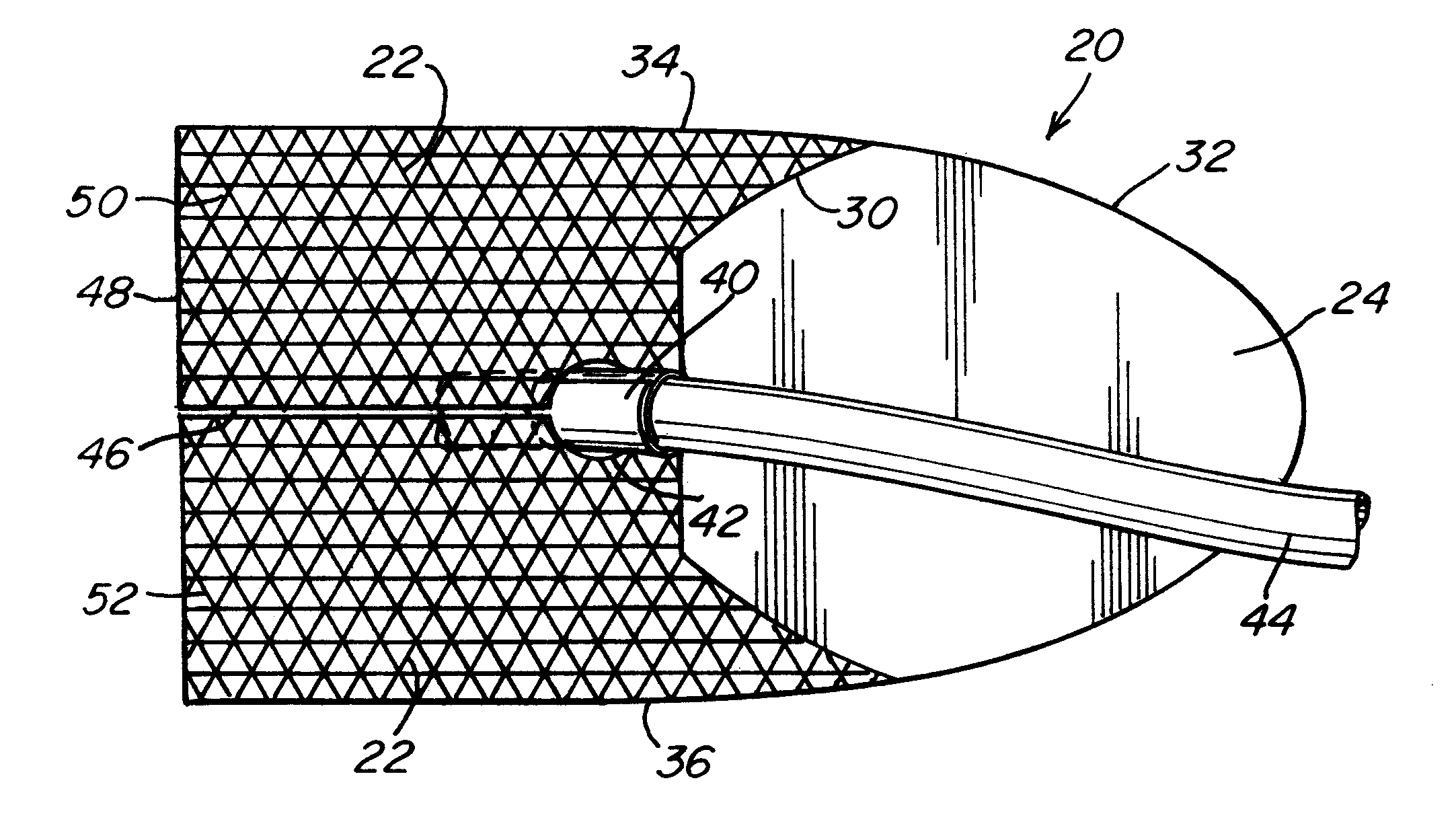
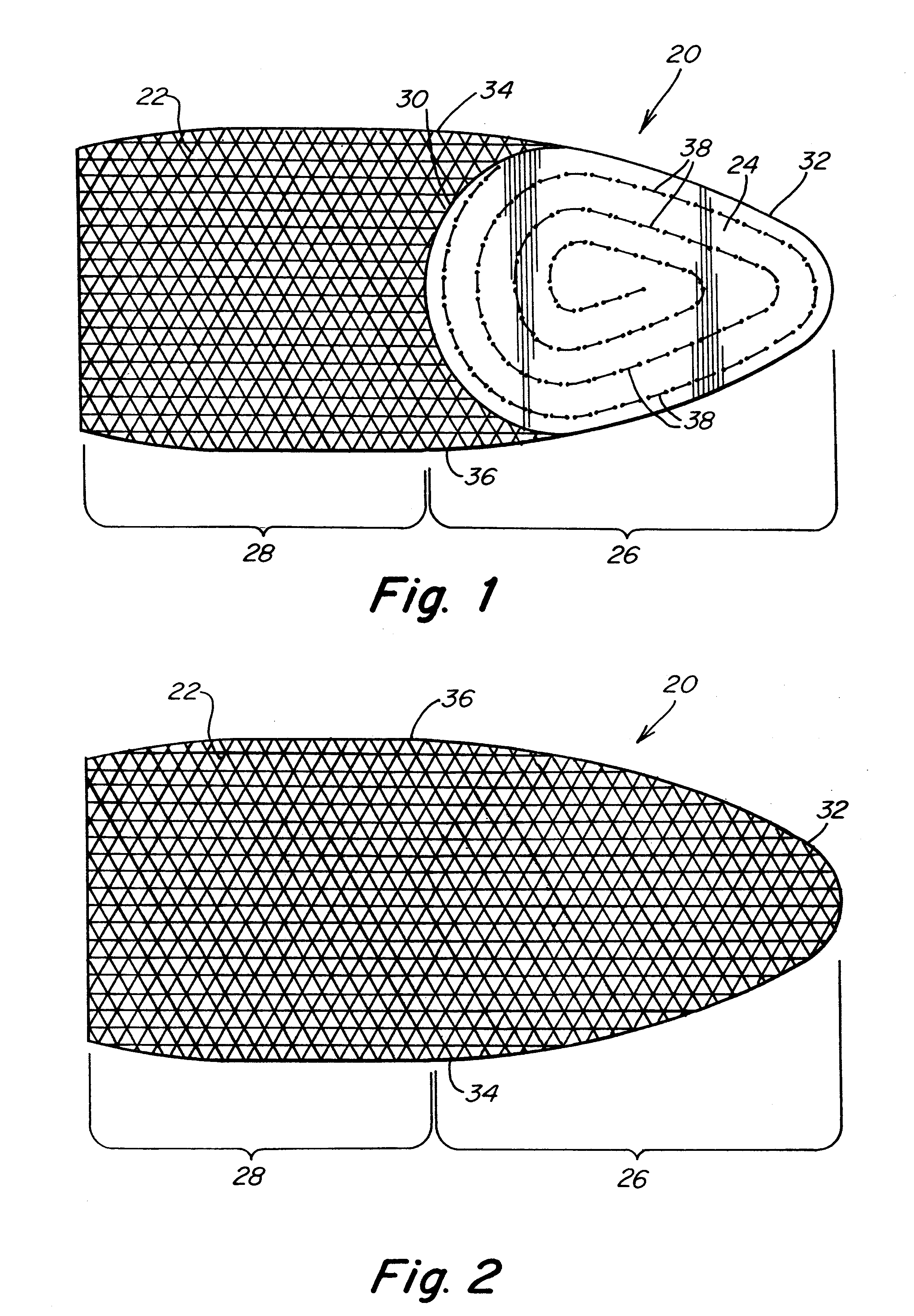
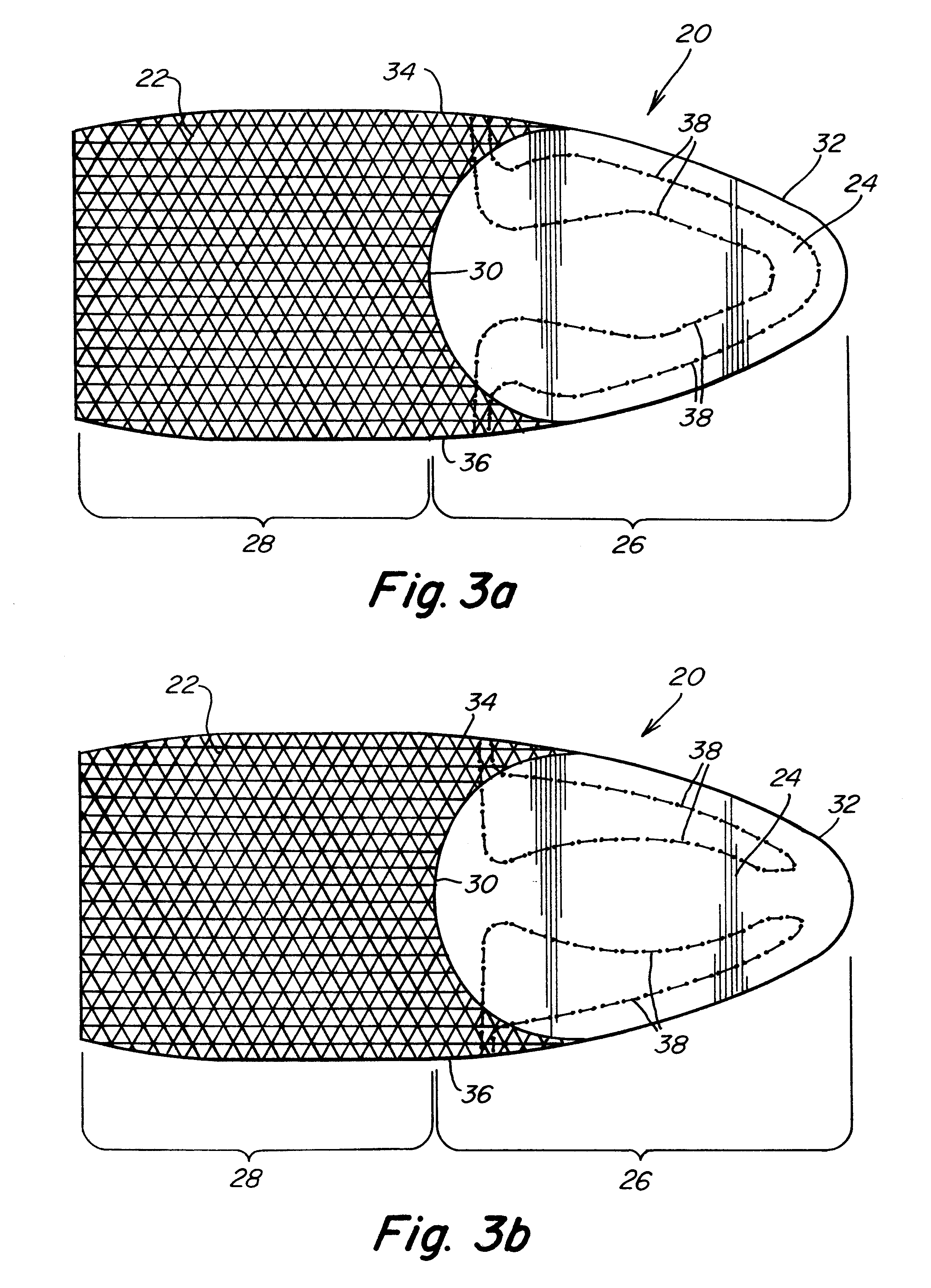
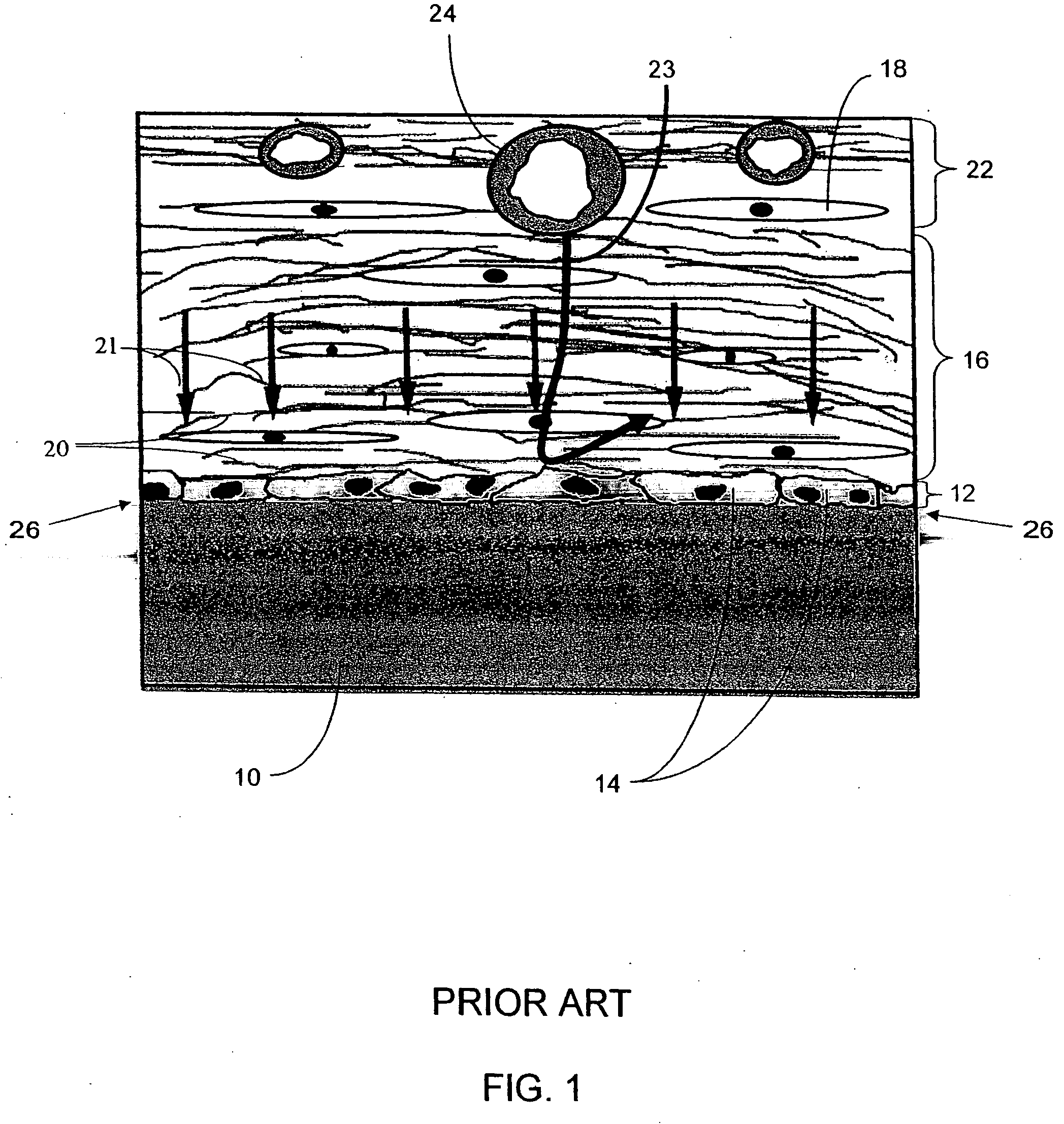
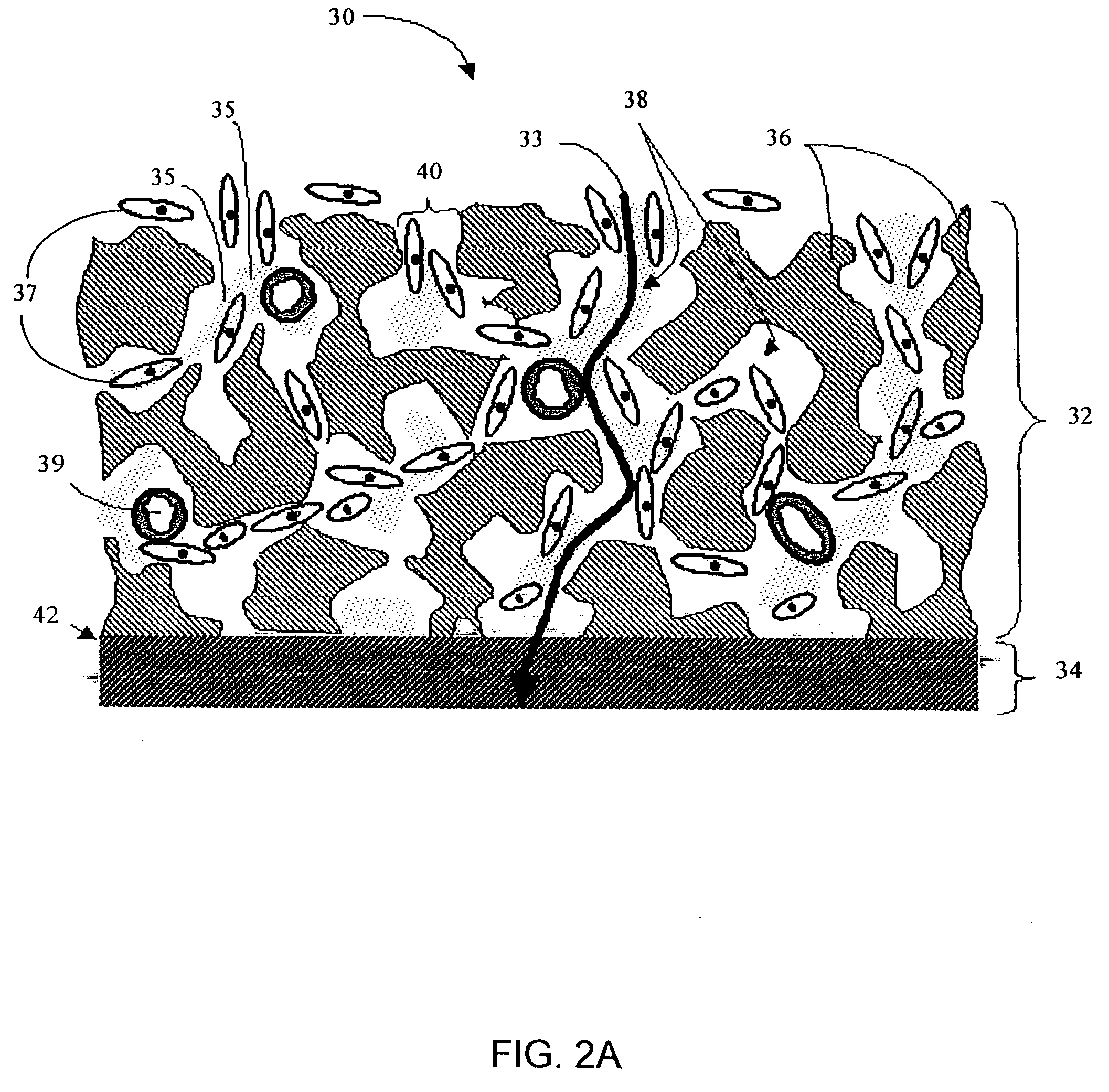
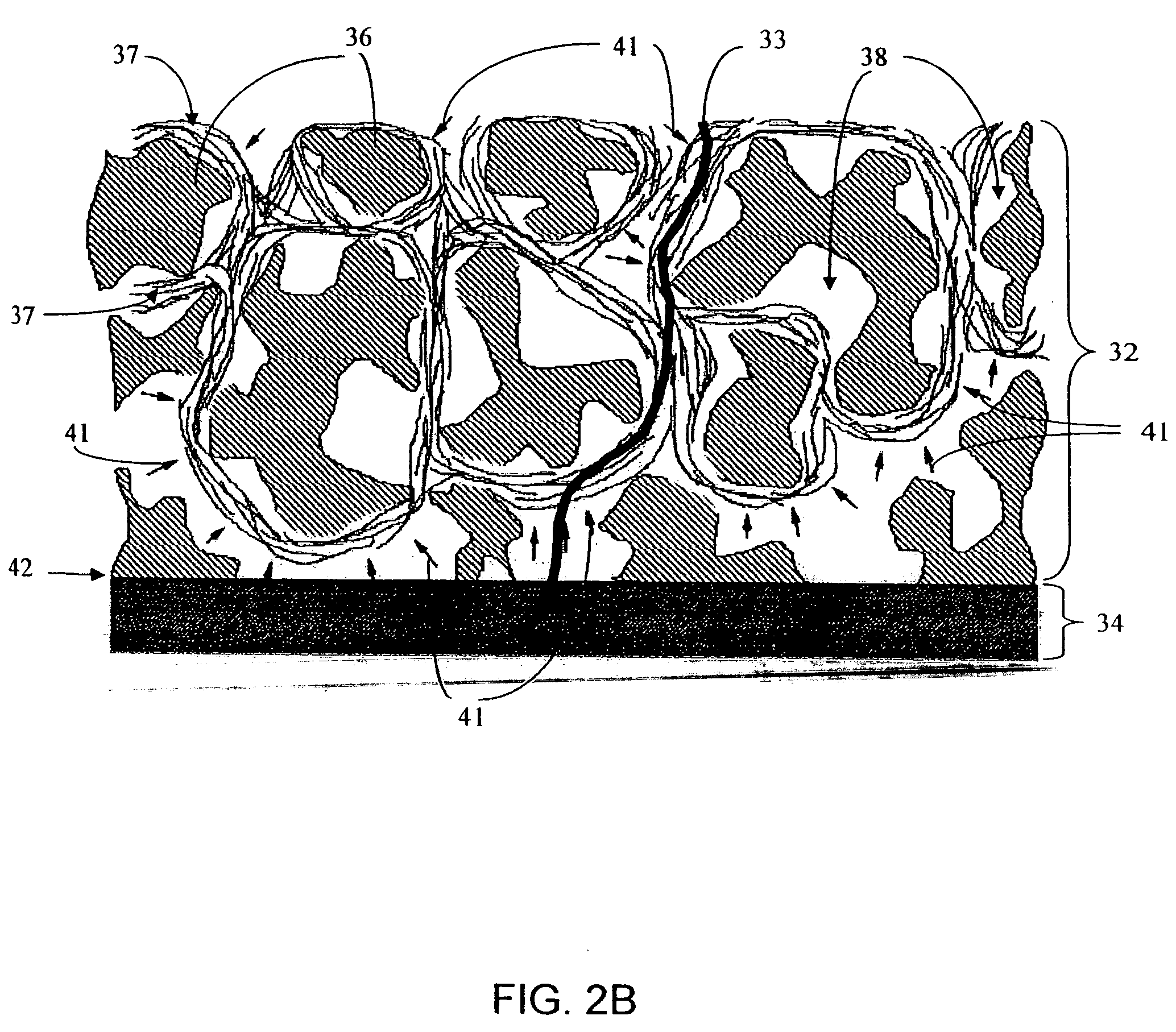
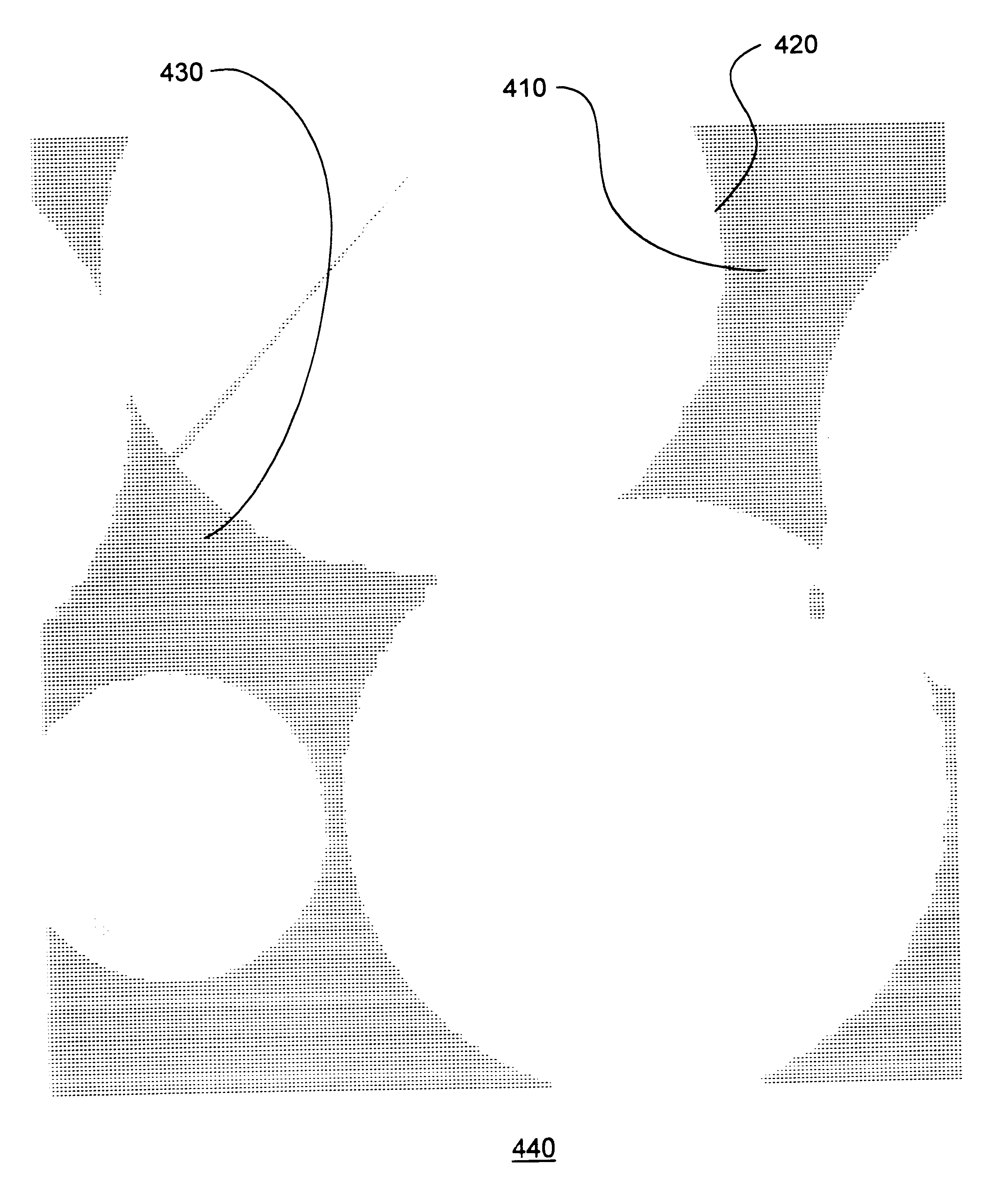
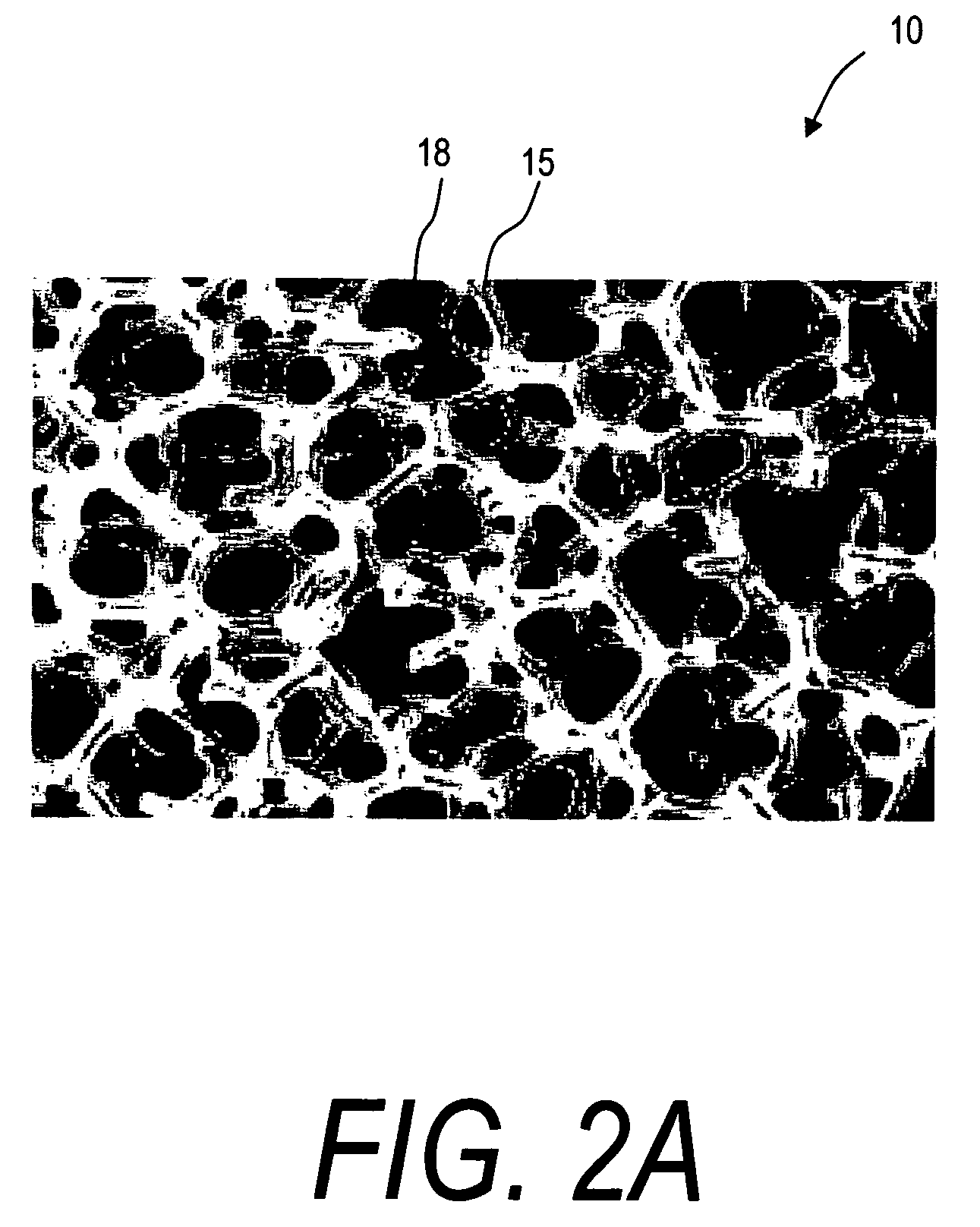
Biointerface membranes incorporating bioactive agents
InactiveUS20050031689A1Improve performancePowder deliveryAdditive manufacturing apparatusBiointerfaceActive agent
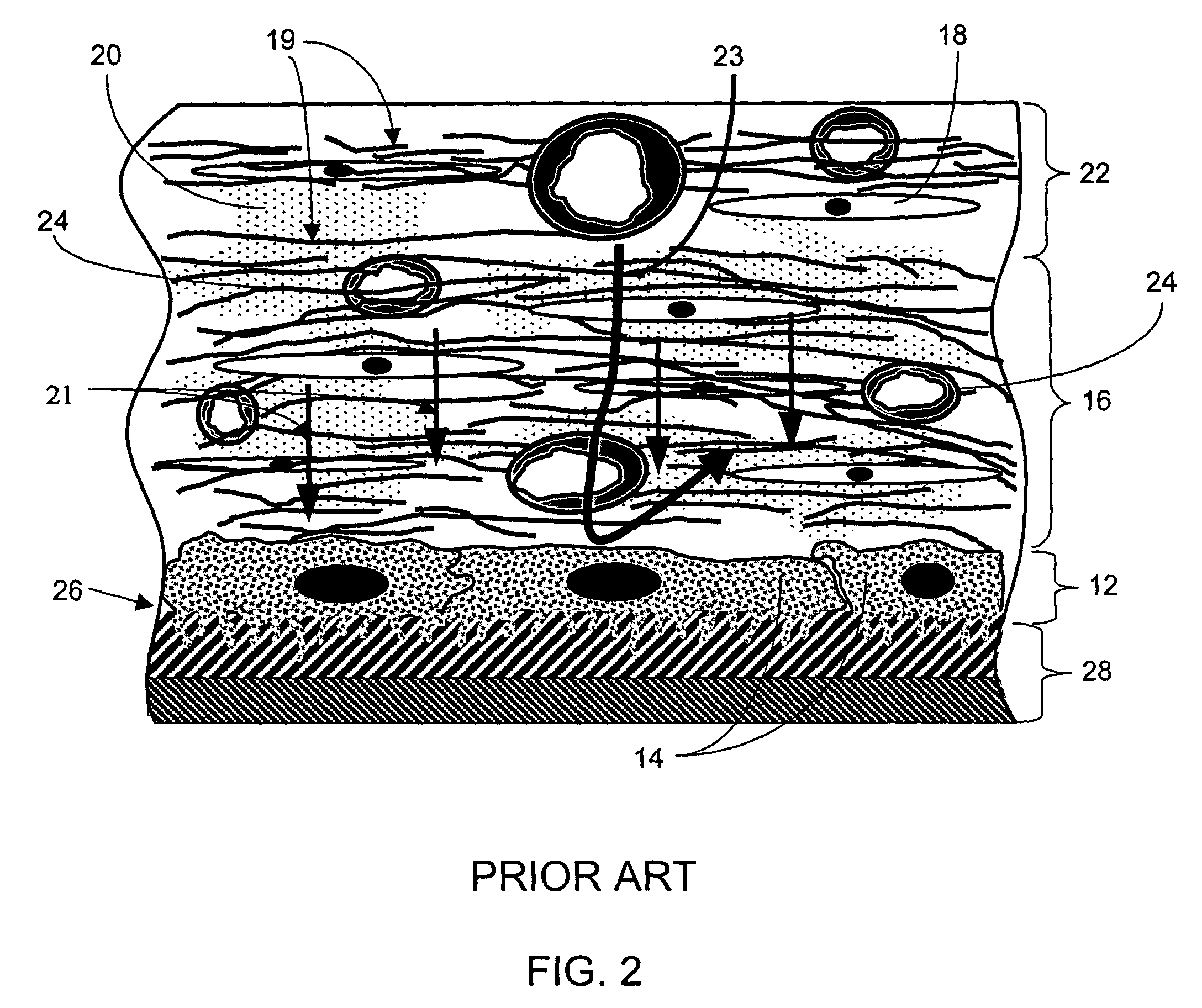
A biointerface membrane for an implantable device including a nonresorbable solid portion with a plurality of interconnected cavities therein adapted to support tissue ingrowth in vivo, and a bioactive agent incorporated into the biointerface membrane and adapted to modify the tissue response is provided. The bioactive agents can be chosen to induce vascularization and / or prevent barrier cell layer formation in vivo, and are advantageous when used with implantable devices wherein solutes are transported across the device-tissue interface.
Owner:DEXCOM
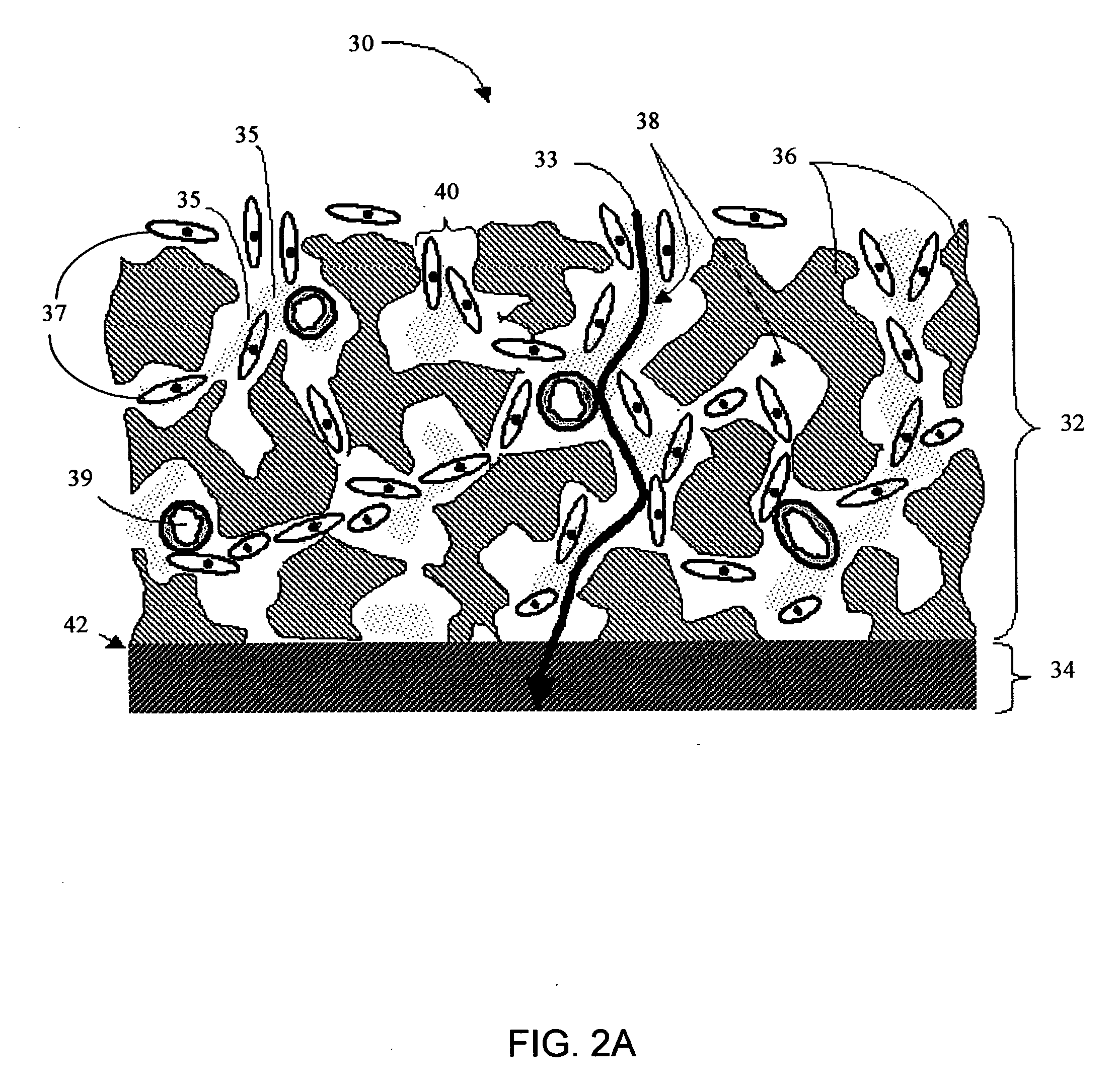
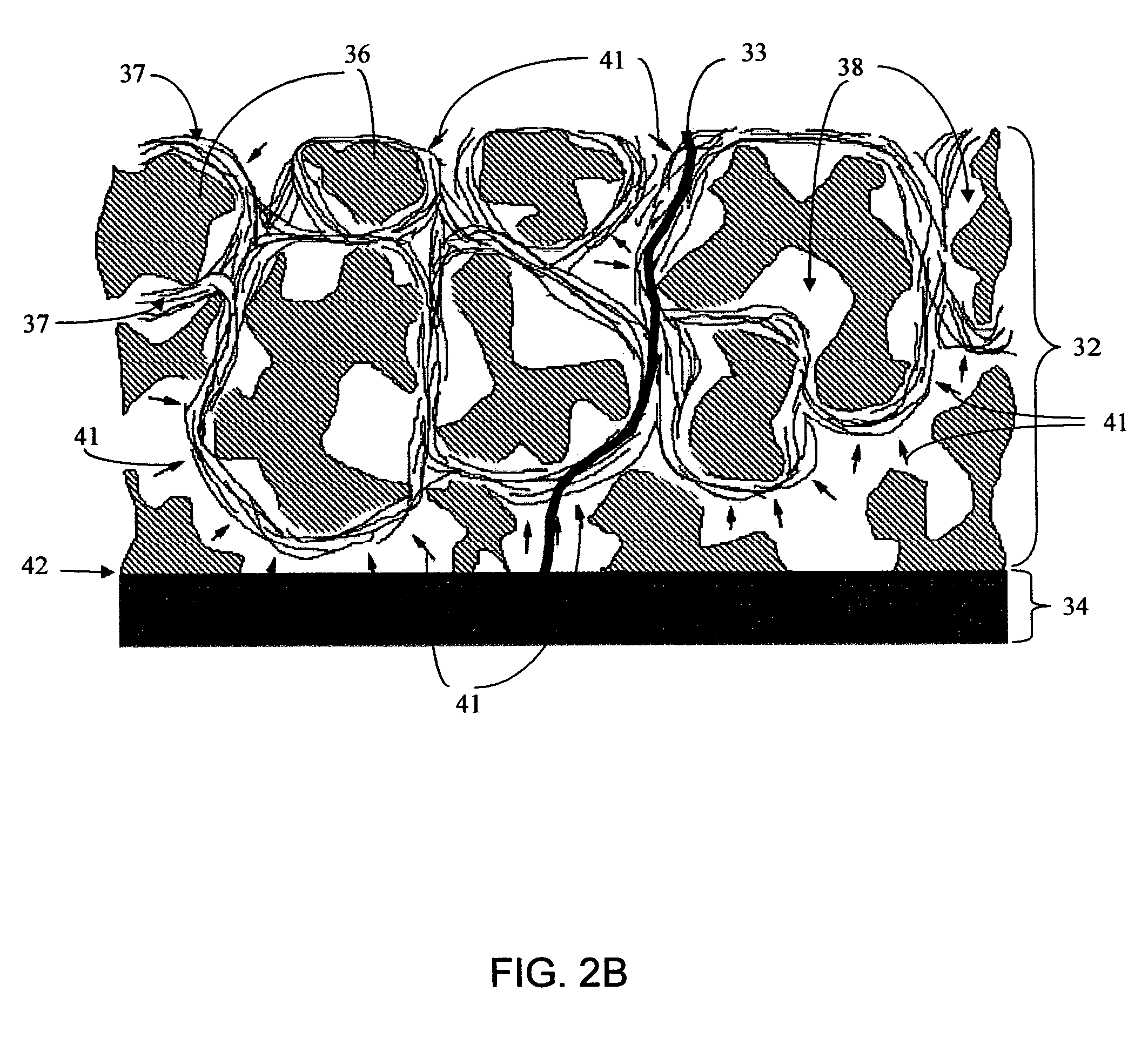
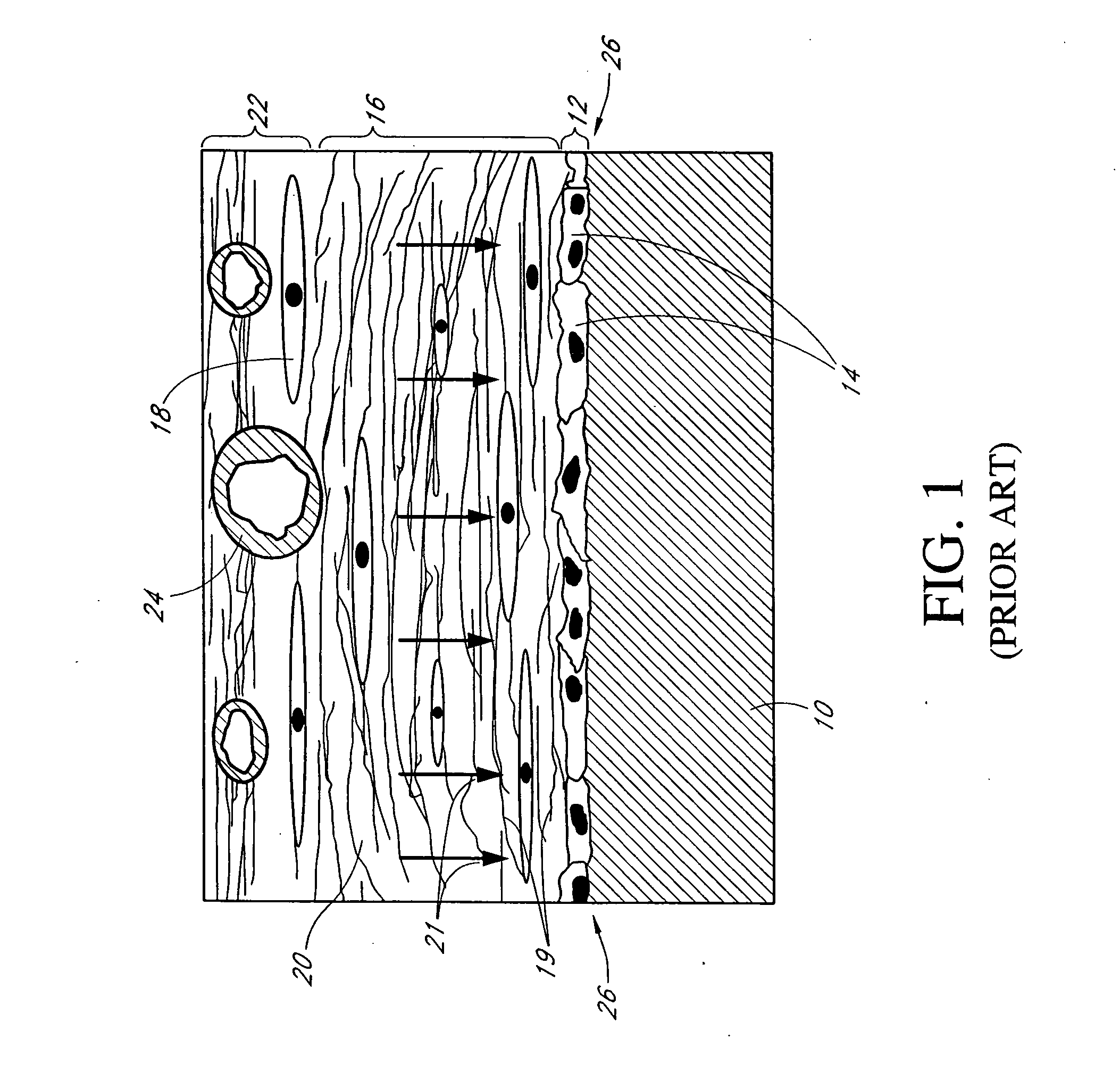
Porous membranes for use with implantable devices
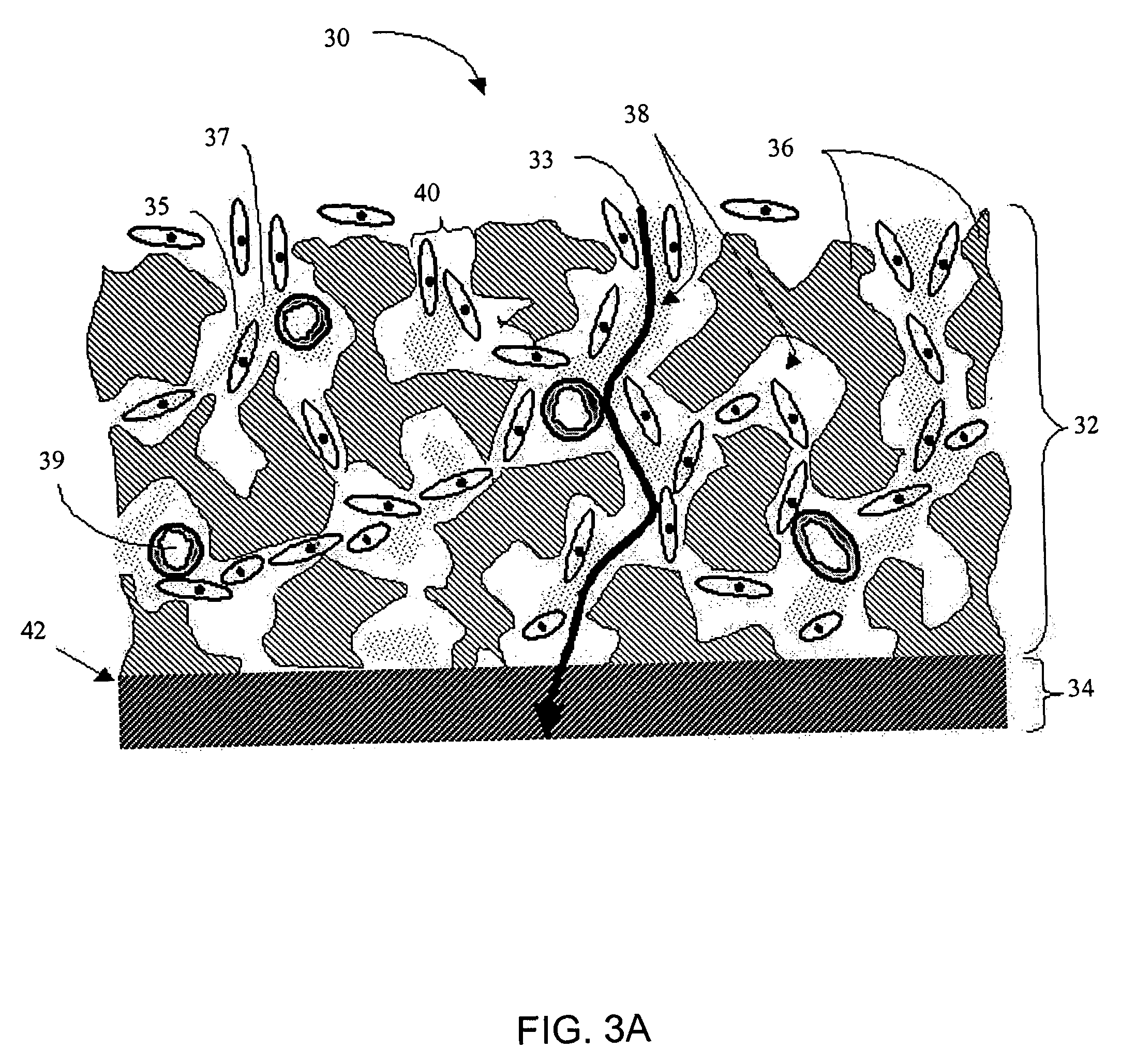
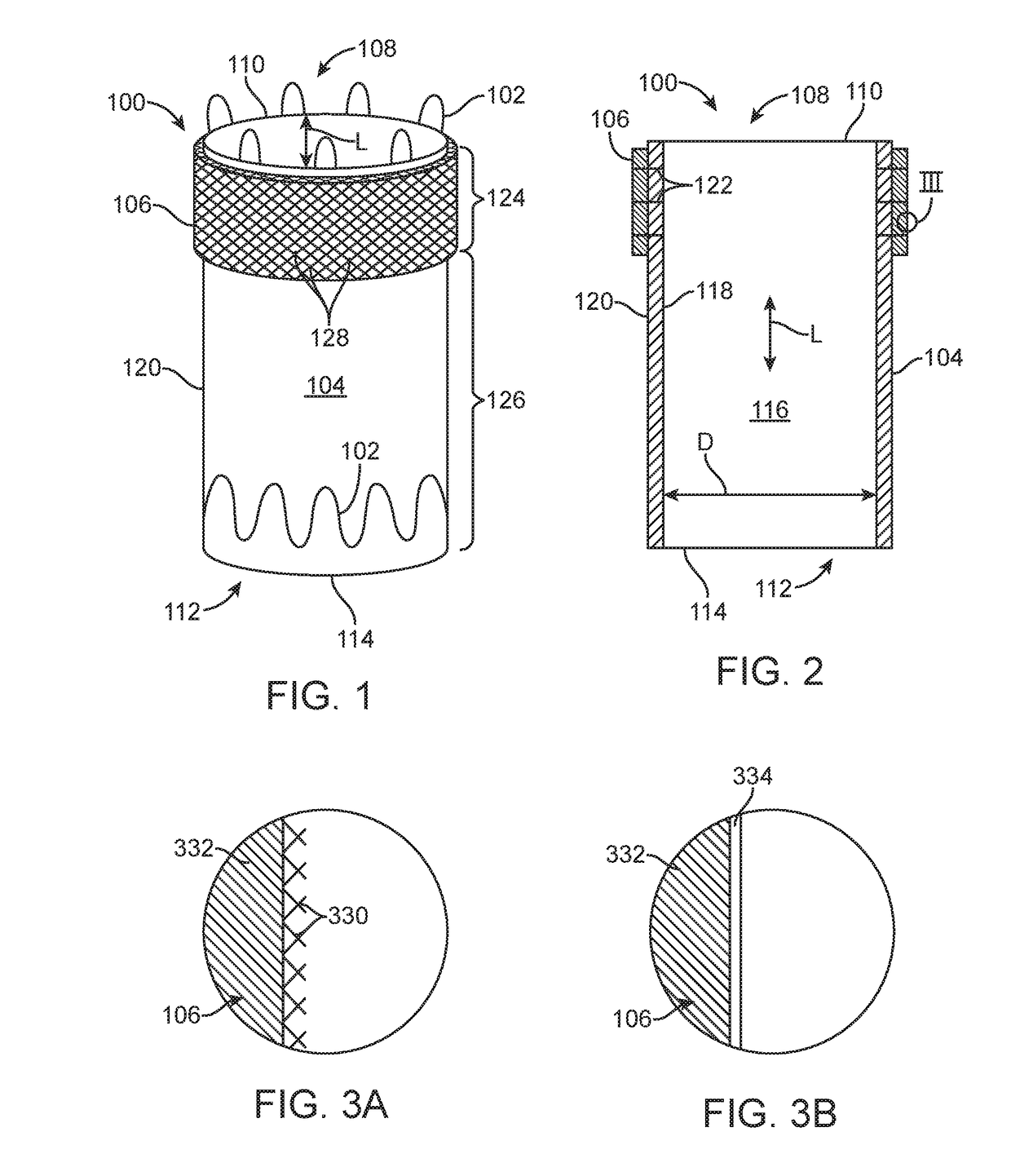
A membrane for implantation in soft tissue comprising a first domain that supports tissue ingrowth, disrupts contractile forces typically found in a foreign body response, encourages vascularity, and interferes with barrier cell layer formation, and a second domain that is resistant to cellular attachment, is impermeable to cells and cell processes, and allows the passage of analytes. The membrane allows for long-term analyte transport in vivo and is suitable for use as a biointerface for implantable analyte sensors, cell transplantation devices, drug delivery devices, and / or electrical signal delivering or measuring devices. The membrane architecture, including cavity size, depth, and interconnectivity, provide long-term robust functionality of the membrane in vivo.
Owner:DEXCOM INC
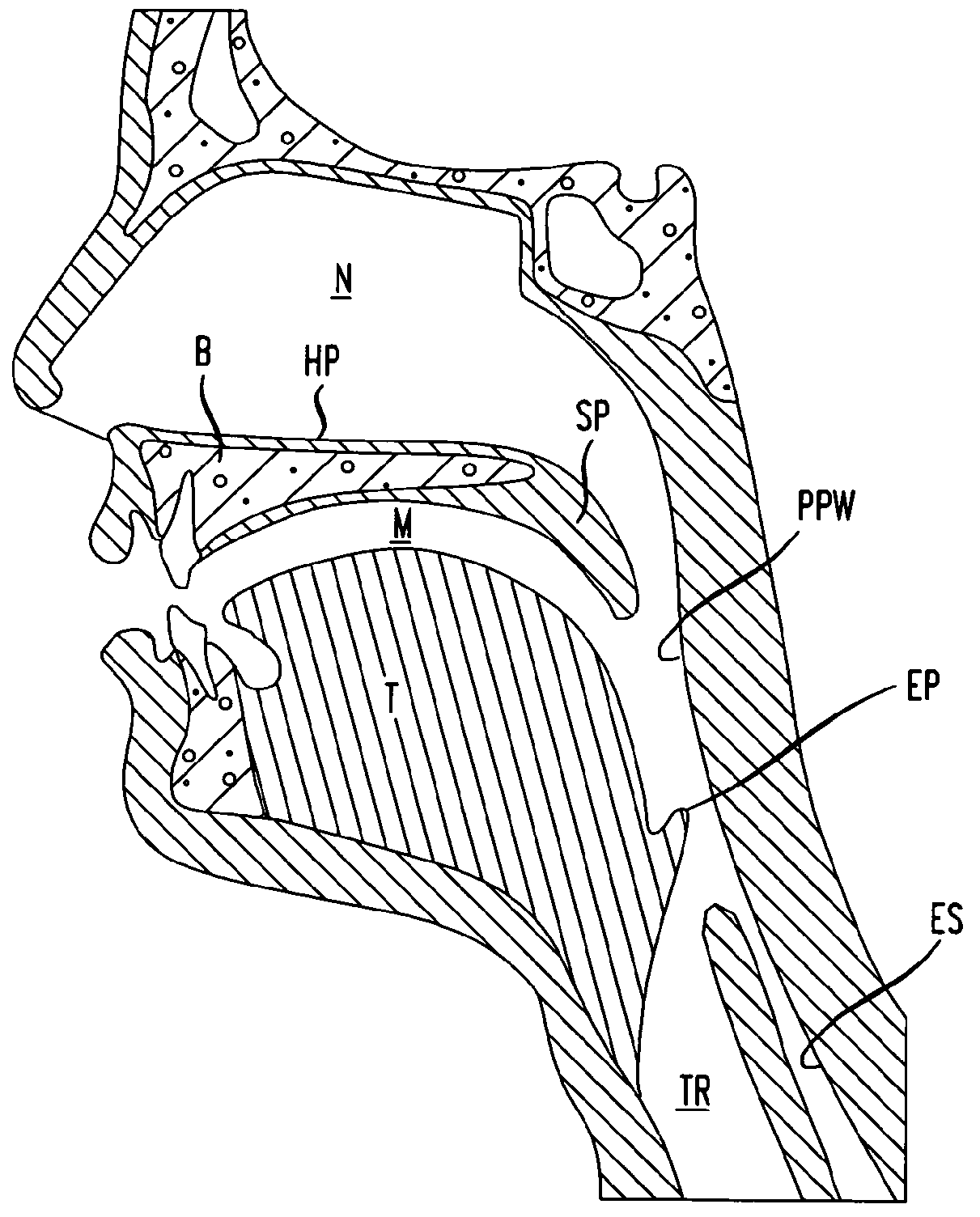
Methods and devices for treatment of obstructive sleep apnea
ActiveUS8413661B2Avoid blockingAlters the geometry of the airwayElectrotherapyDiagnosticsHand heldNeck of pancreas
Owner:ETHICON INC
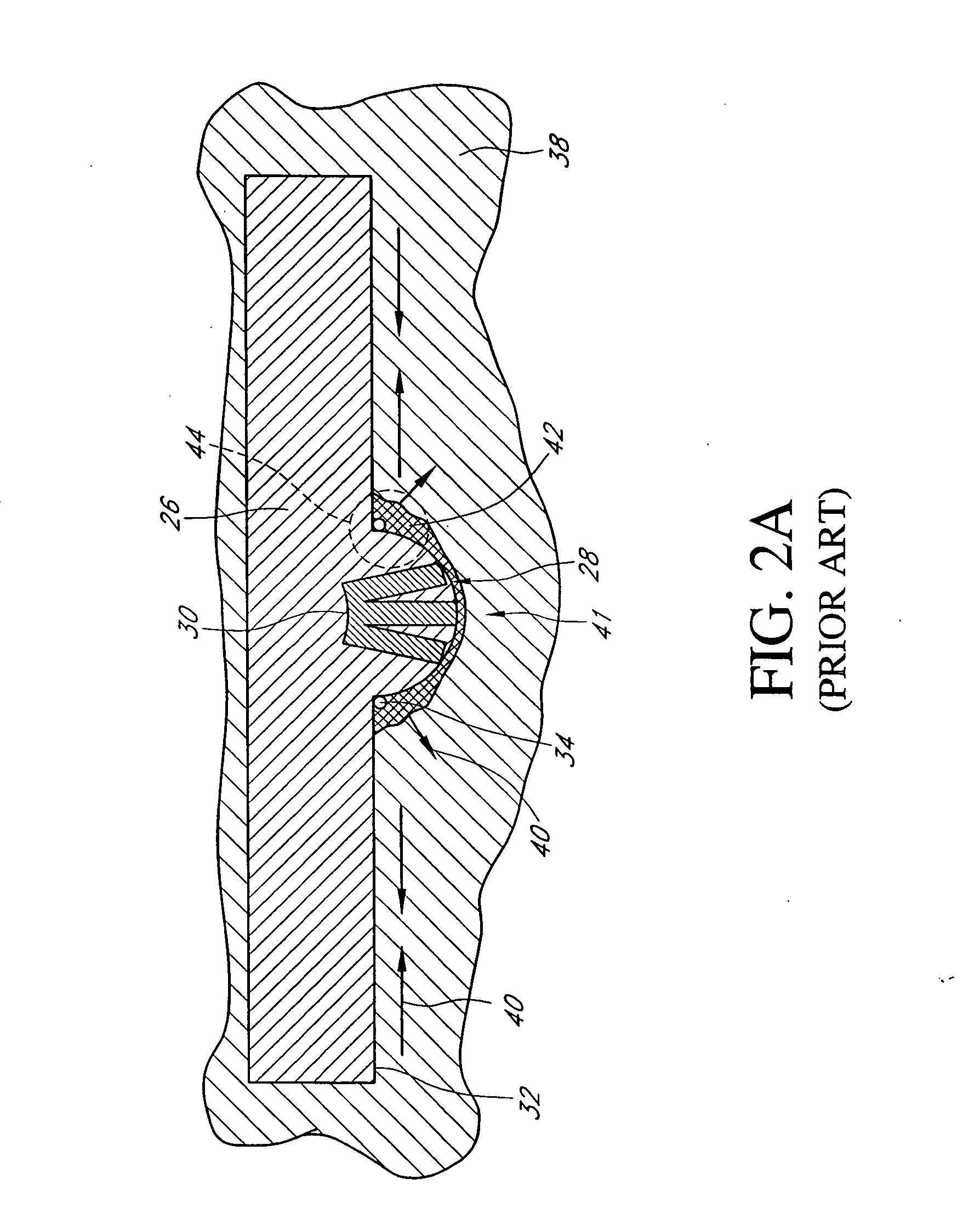
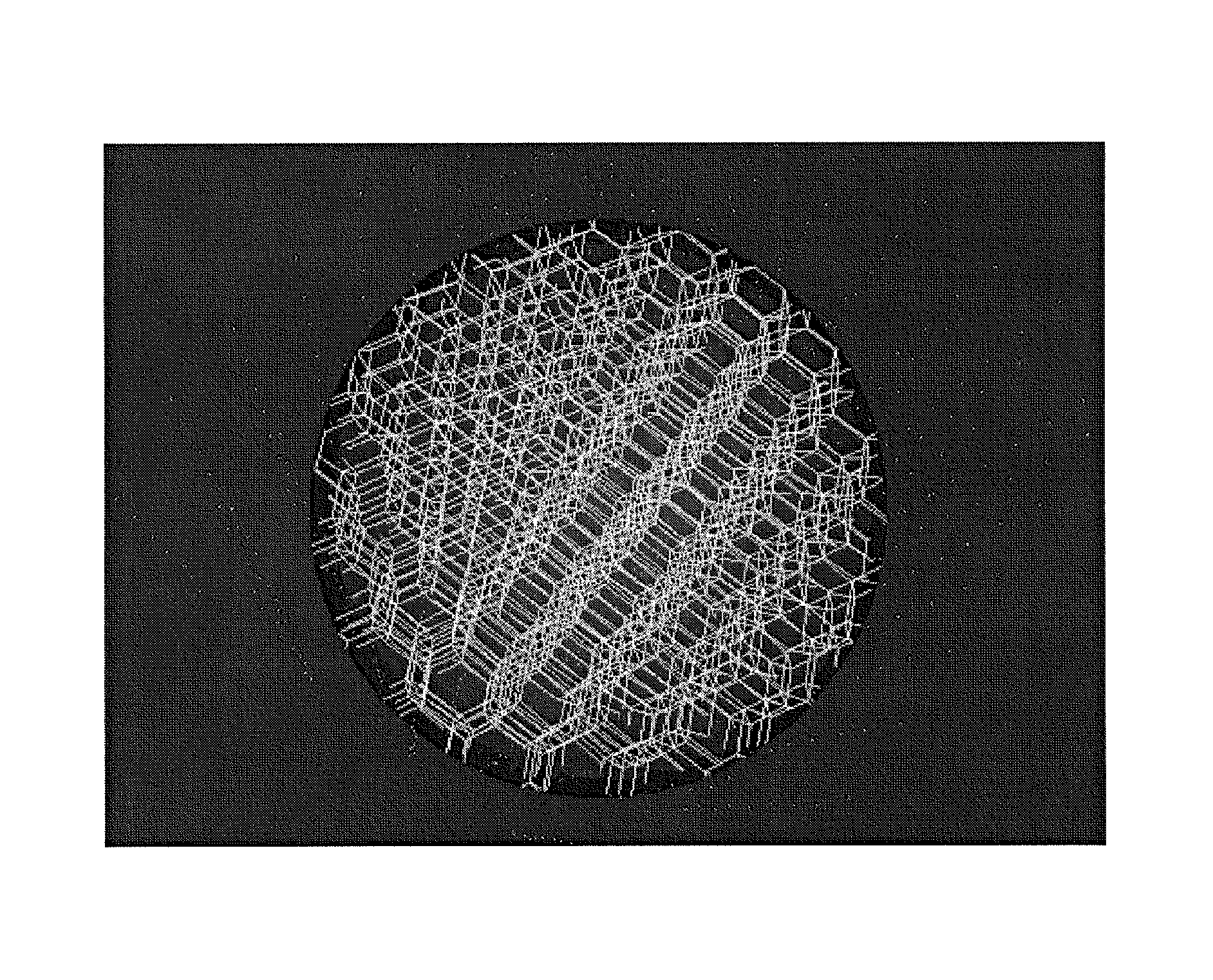
Laser-produced porous structure
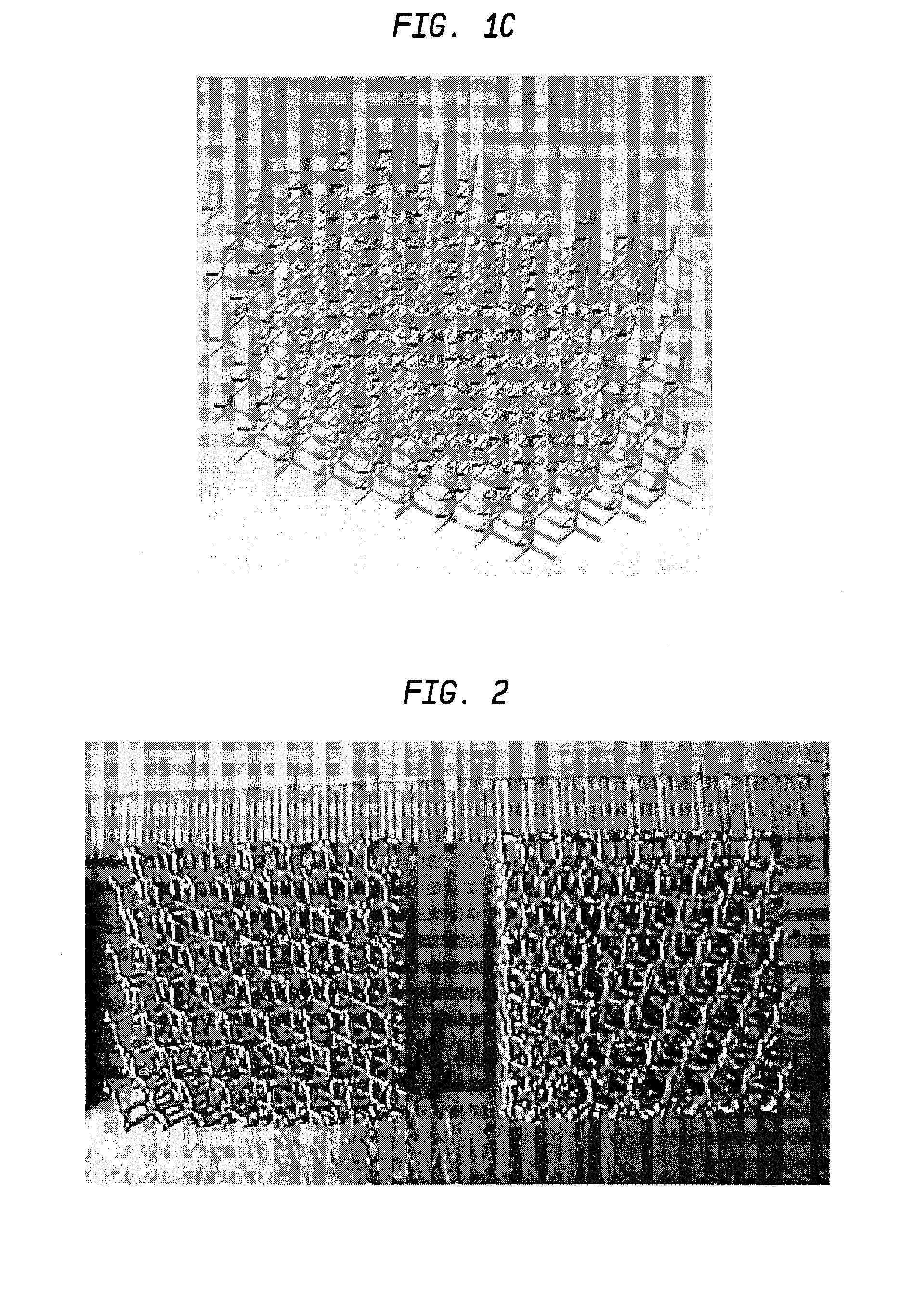
The present invention disclosed a method of producing a three-dimensional porous tissue in-growth structure. The method includes the steps of depositing a first layer of metal powder and scanning the first layer of metal powder with a laser beam to form a portion of a plurality of predetermined unit cells. Depositing at least one additional layer of metal powder onto a previous layer and repeating the step of scanning a laser beam for at least one of the additional layers in order to continuing forming the predetermined unit cells. The method further includes continuing the depositing and scanning steps to form a medical implant.
Owner:UNIV OF LIVERPOOL +1
Cartilage repair implant with soft bearing surface and flexible anchoring device
InactiveUS9050192B2Strong and more permanent fixationSoft and bendableJoint implantsHip jointsCartilage repairSurgical implant
A surgical implant for replacing hyaline cartilage in a knee or other articulating synovial joint has an anchoring side on one side of the implant adapted for fixing the implant to one of the bones in the joint, and a bearing surface on the opposite side of the implant for lubricious rubbing and sliding contact with another bone in the joint. The anchoring side can be configured with an irregular surface for tissue ingrowth. The bearing side can include hydrogel. The implant can be rolled up from an original shape and surgically inserted by arthroscopic means, and opens into its original shape when released inside the joint.
Owner:FORMAE
Implants for replacing cartilage, with negatively-charged hydrogel surfaces and flexible matrix reinforcement
ActiveUS9314339B2Strong and durableStrong and secure anchoringFinger jointsWrist jointsFiberChemical agent
A permanent non-resorbable implant allows surgical replacement of cartilage in articulating joints, using a hydrogel material (such as a synthetic polyacrylonitrile polymer) reinforced by a flexible fibrous matrix. Articulating hydrogel surface(s) are chemically treated to provide a negative electrical charge that emulates the negative charge of natural cartilage, and also can be treated with halogenating, cross-linking, or other chemical agents for greater strength. For meniscal-type implants, the reinforcing matrix can extend out from the peripheral rim of the hydrogel, to allow secure anchoring to soft tissue such as a joint capsule. For bone-anchored implants, a porous anchoring layer enables tissue ingrowth, and a non-planer perforated layer can provide a supportive interface between the hard anchoring material and the softer hydrogel material.
Owner:FORMAE
Method of forming an implantable device
ActiveUS9352071B2Greater and beneficial tissue ingrowthLow melting pointWeft knittingMedical devicesImplanted devicePliability
Owner:ETHICON INC
Laser-produced porous surface
ActiveUS20070142914A1Promote bone ingrowthGood stiffness characteristicsAdditive manufacturing apparatusMolten spray coatingLight beamMetal powder
A method of forming an implant having a porous tissue ingrowth structure and a bearing support structure. The method includes depositing a first layer of a metal powder onto a substrate, scanning a laser beam over the powder so as to sinter the metal powder at predetermined locations, depositing at least one layer of the metal powder onto the first layer and repeating the scanning of the laser beam.
Owner:UNIV OF LIVERPOOL +1
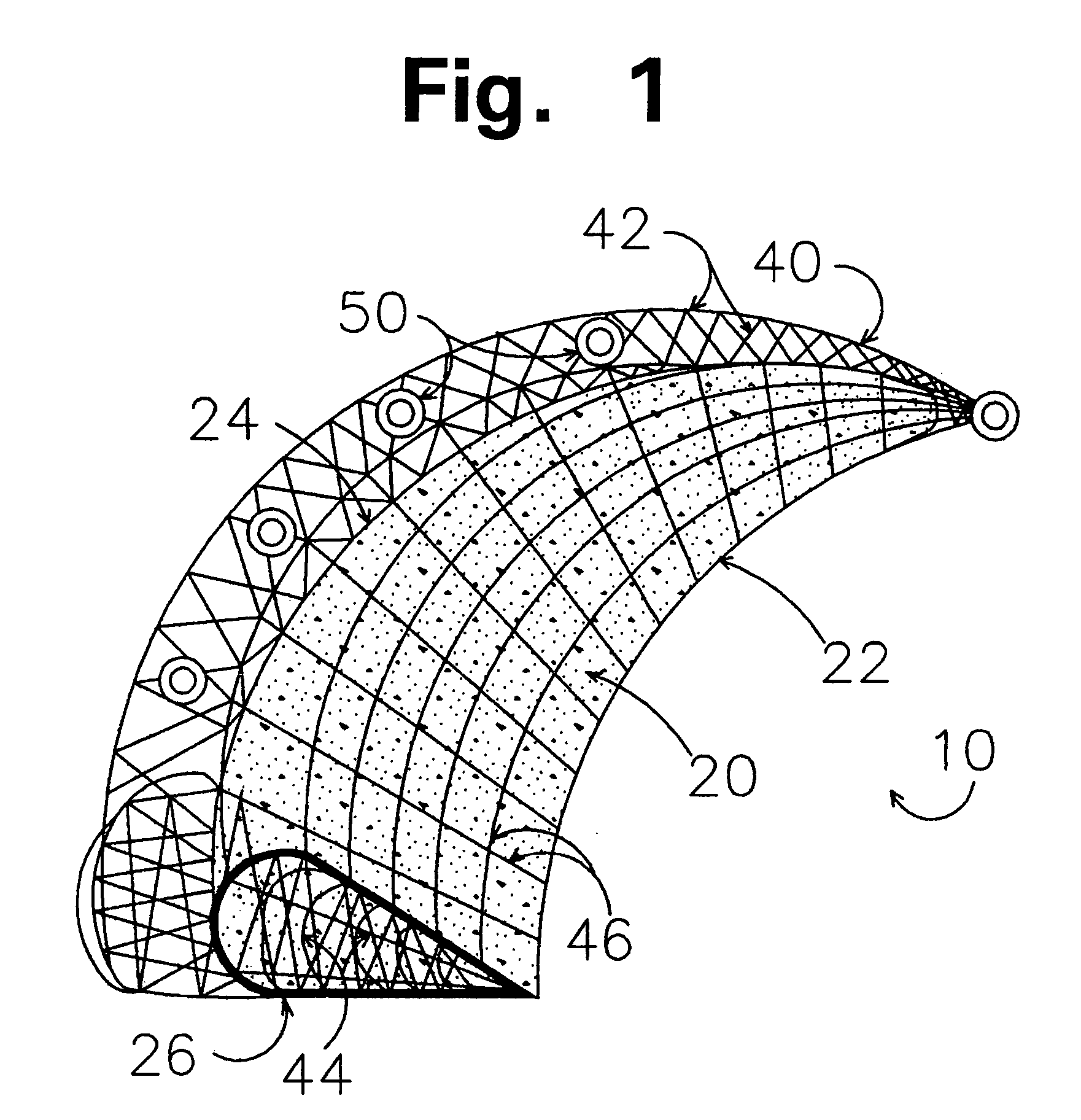
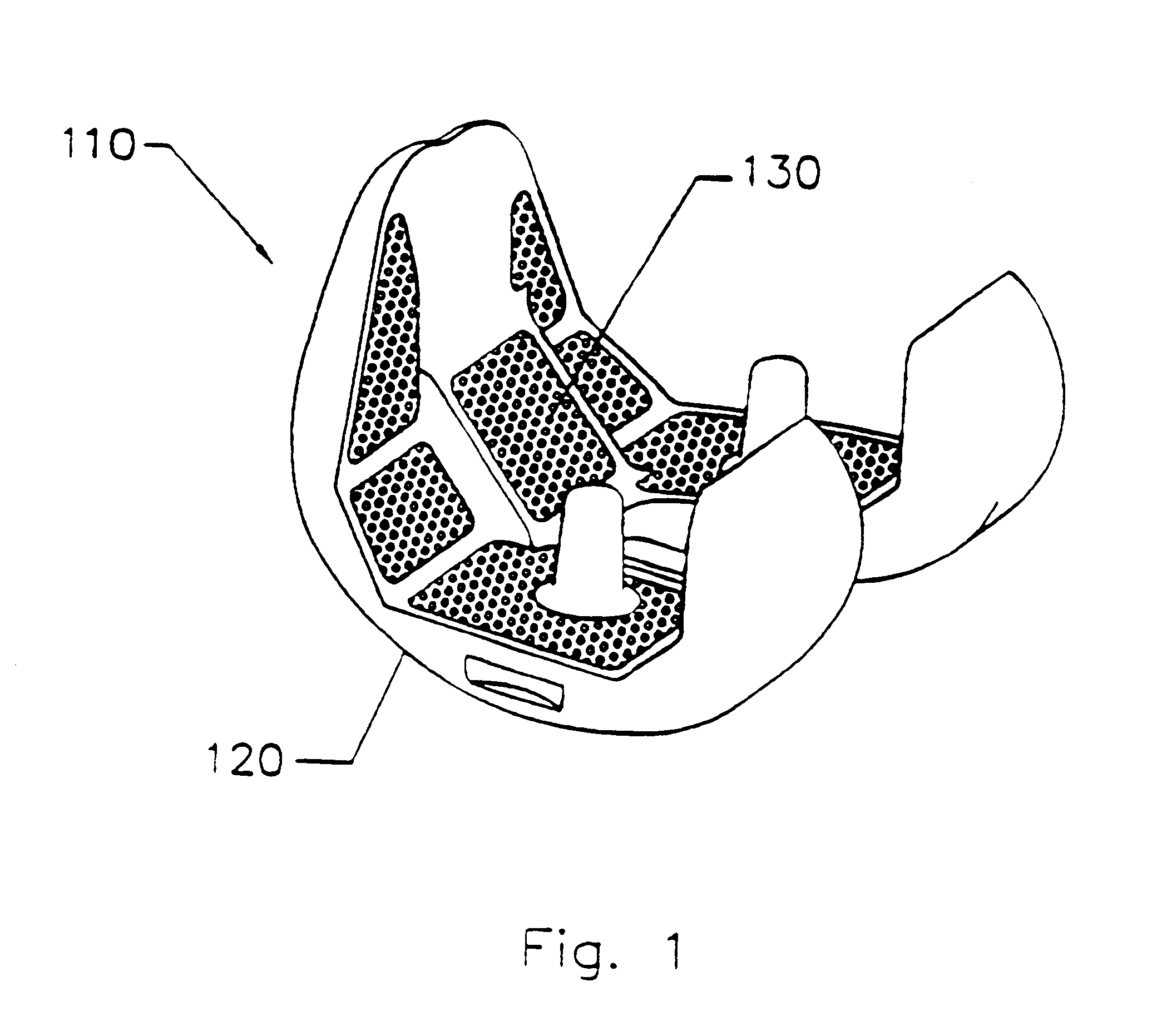
Prosthetic repair fabric
InactiveUS6258124B1Promoting enhanced tissue ingrowthControl incidenceDiagnosticsLigamentsProsthesisSpermatic cord
A prosthetic repair fabric and method for repairing an inguinal hernia in the inguinal canal. The prosthesis including a layer of mesh fabric that is susceptible to the formations of adhesions with sensitive tissue and organs, and a barrier layer that inhibits the formation of adhesions with sensitive tissue and organs. The mesh fabric including a medial section and a lateral section that are configured to be positioned adjacent the medial corner and the lateral end of the inguinal canal, respectively, when the prosthesis is placed in the inguinal canal to repair the defect. The barrier layer is positioned on the mesh fabric to inhibit the formation of adhesions between the spermatic cord and the mesh fabric. At least a portion of the lateral section of the mesh fabric is free of the barrier layer on both of its sides to promote enhanced tissue ingrowth therein. The barrier layer may include at least one flap that is to be folded through the mesh fabric to isolate the spermatic cord from internal edges of the fabric when the spermatic cord is routed through the prothesis.
Owner:CR BARD INC
Biointerface membranes incorporating bioactive agents
InactiveUS20060198864A1Improve performanceAdditive manufacturing apparatusSurgeryBiointerfaceActive agent
A biointerface membrane for an implantable device including a nonresorbable solid portion with a plurality of interconnected cavities therein adapted to support tissue ingrowth in vivo, and a bioactive agent incorporated into the biointerface membrane and adapted to modify the tissue response is provided. The bioactive agents can be chosen to induce vascularization and / or prevent barrier cell layer formation in vivo, and are advantageous when used with implantable devices wherein solutes are transported across the device-tissue interface.
Owner:DEXCOM INC
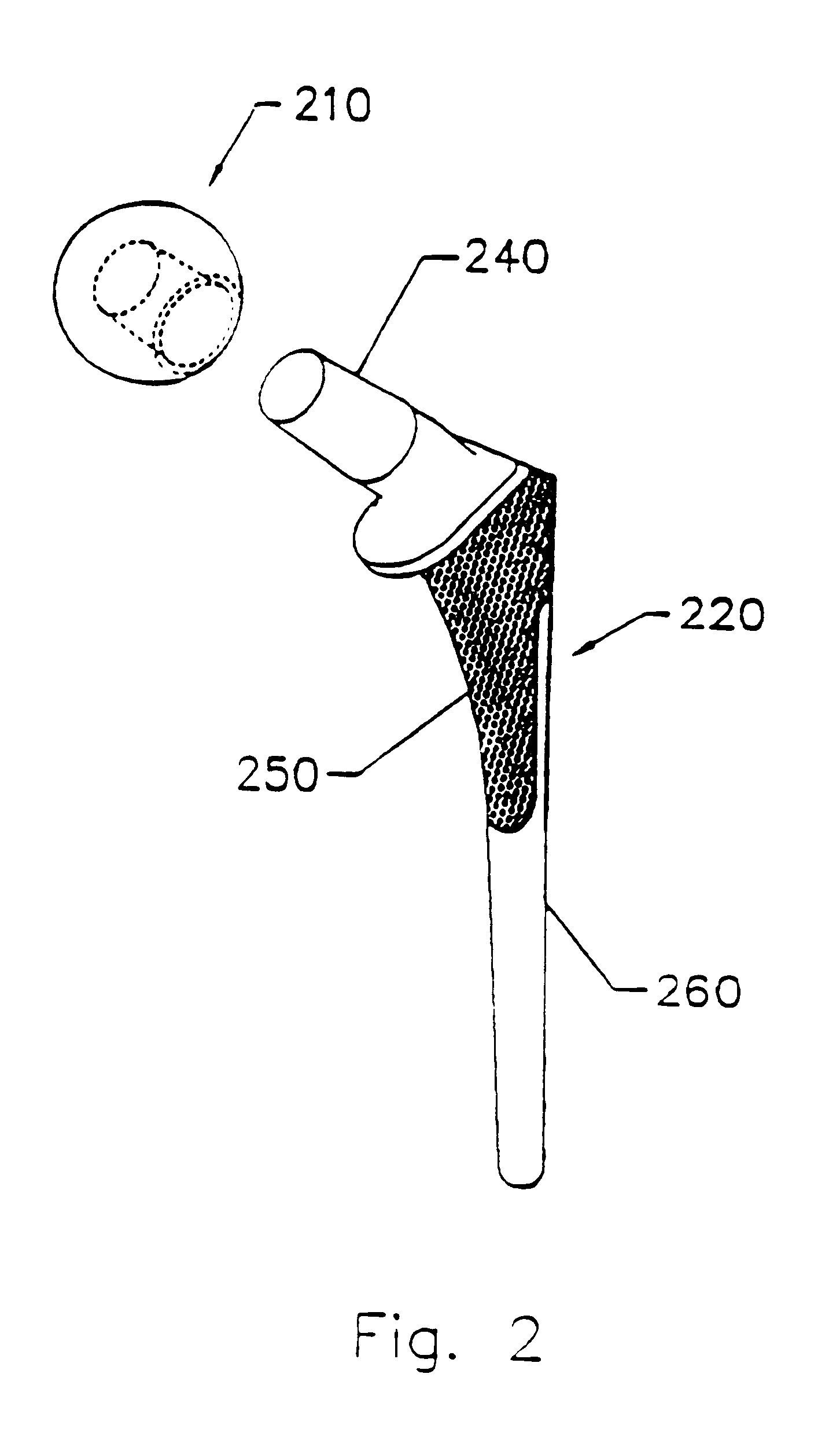
Optimized sensor geometry for an implantable glucose sensor
InactiveUS20060200022A1Precise processAccurate measurementDiagnostic recording/measuringSensorsGlucose sensorsAnalyte
An implantable sensor for use in measuring a concentration of an analyte such as glucose in a bodily fluid, including a body with a sensing region adapted for transport of analytes between the sensor and the bodily fluid, wherein the sensing region is located on a curved portion of the body such that when a foreign body capsule forms around the sensor, a contractile force is exerted by the foreign body capsule toward the sensing region. The body is partially or entirely curved, partially or entirely covered with an anchoring material for supporting tissue ingrowth, and designed for subcutaneous tissue implantation. The geometric design, including curvature, shape, and other factors minimize chronic inflammatory response at the sensing region and contribute to improved performance of the sensor in vivo.
Owner:DEXCOM
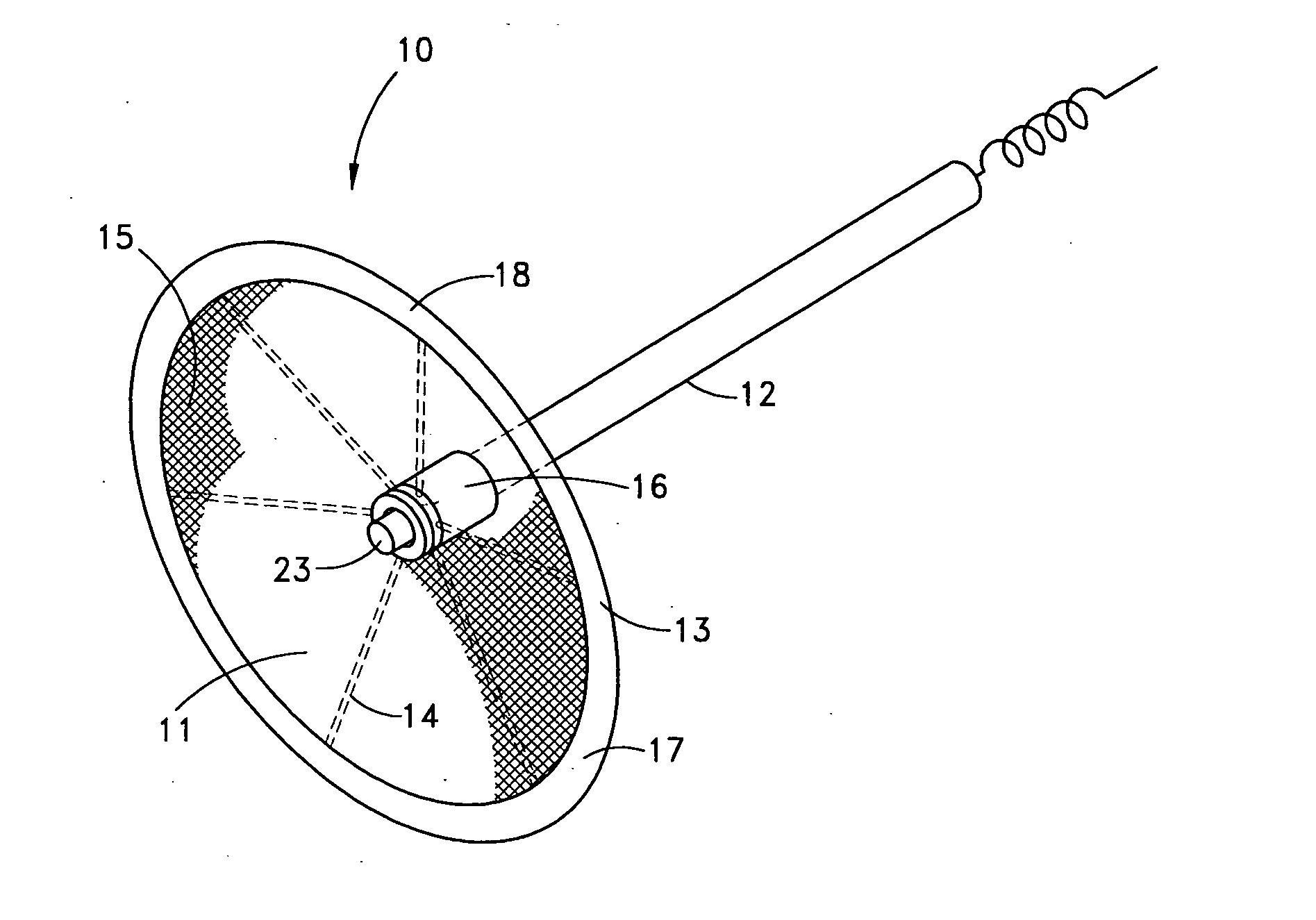
Methods and devices for occluding body lumens and/or enhancing tissue ingrowth
InactiveUS20060009798A1Enhance ingrowthTissue ingrowth is further enhancedStentsFallopian occludersMesh gridEpithelial tissue
The present invention provides devices, methods and systems for the occlusion of various lumens in a body of a patient including devices and methods for enhancing tissue ingrowth, particularly endothelial tissue growth within an occlusive device. The system includes an occlusive device and a delivery device for placing the occlusive device in a body lumen. The occlusive device is generally a tubular member with a mesh member disposed thereon. The occlusive device is configured to be radially expandable along a longitudinal axis of the tubular member and implantable with a delivery catheter such that the occlusive device is in a collapsed state when positioned in the delivery catheter and in an expanded state when positioned in a lumen of a patient. The mesh member of the occlusive device is configured to promote epithelial tissue ingrowth.
Owner:BAYER ESSURE
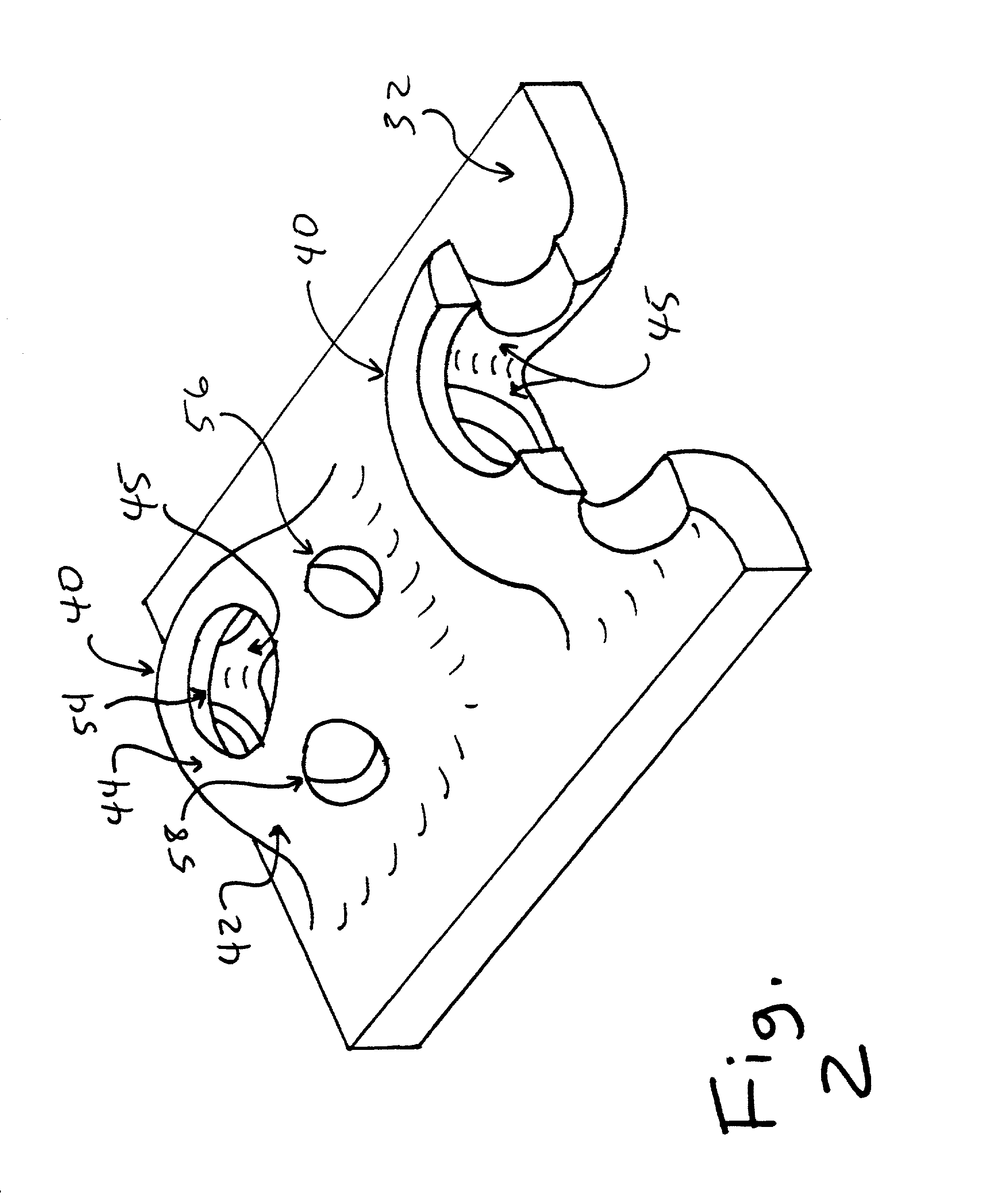
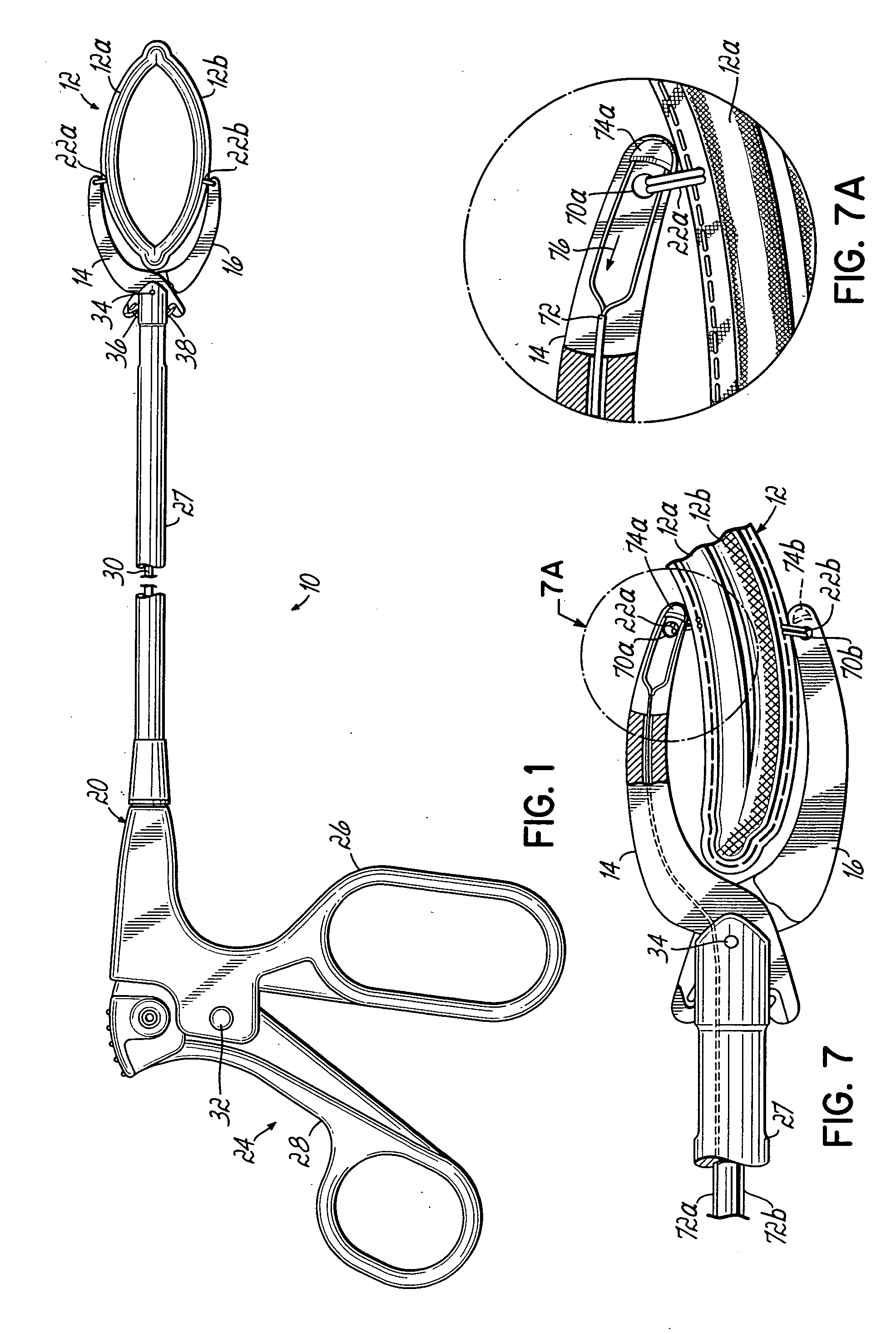
Coaptation enhancement implant, system, and method
ActiveUS8845717B2Safe and effective and operation of valveImprove sealingSuture equipmentsHeart valvesBody shapeCatheter
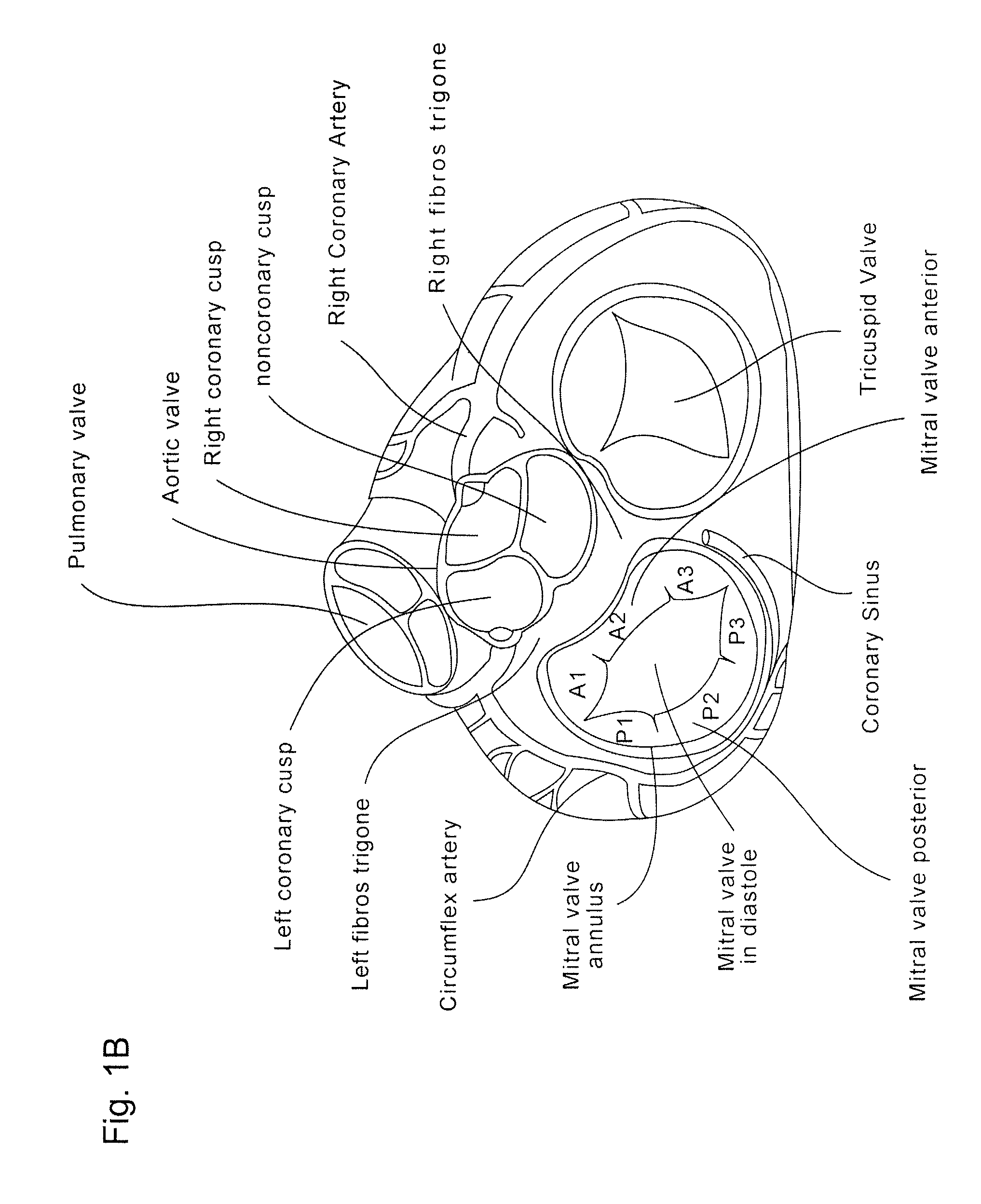
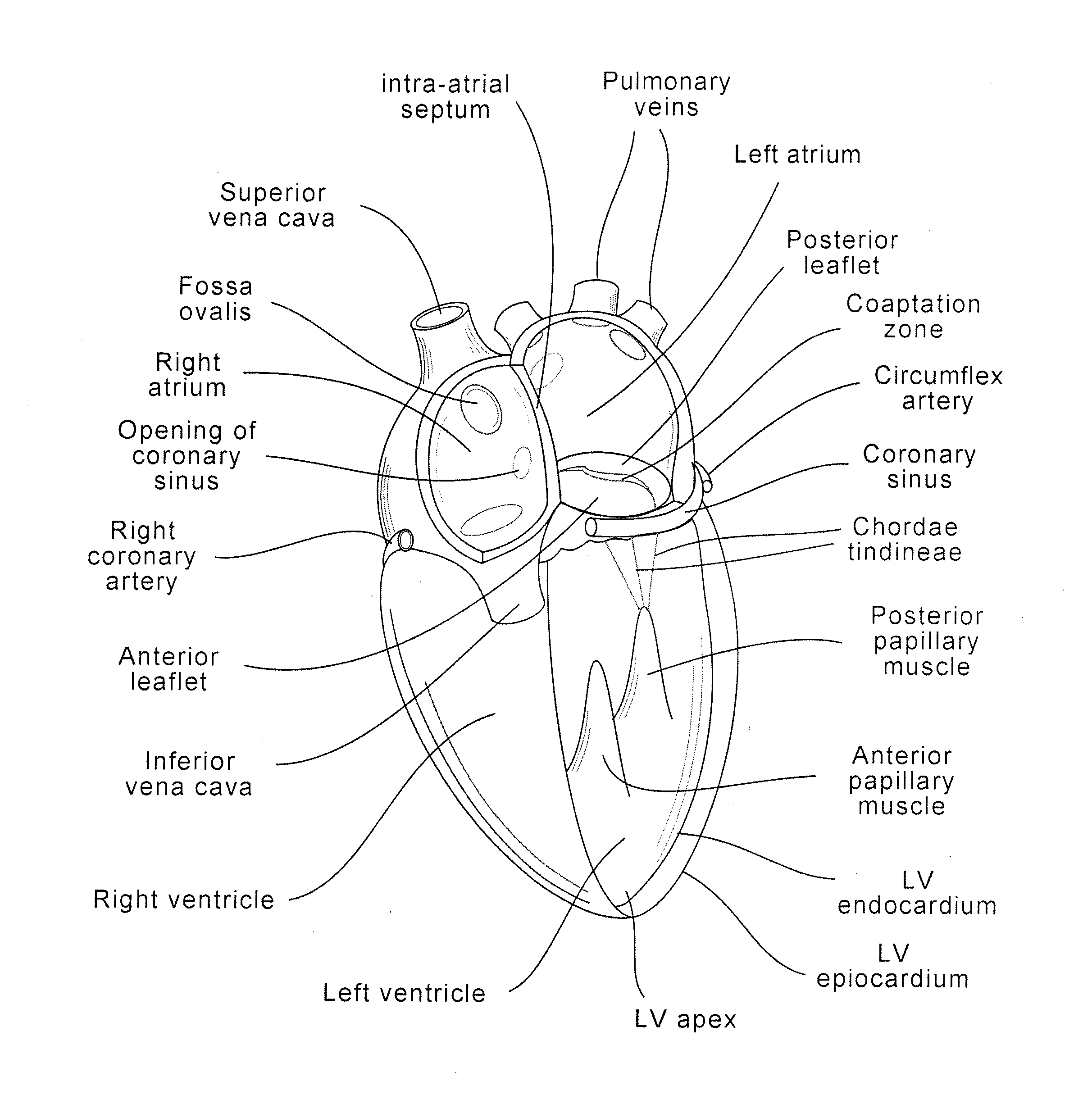
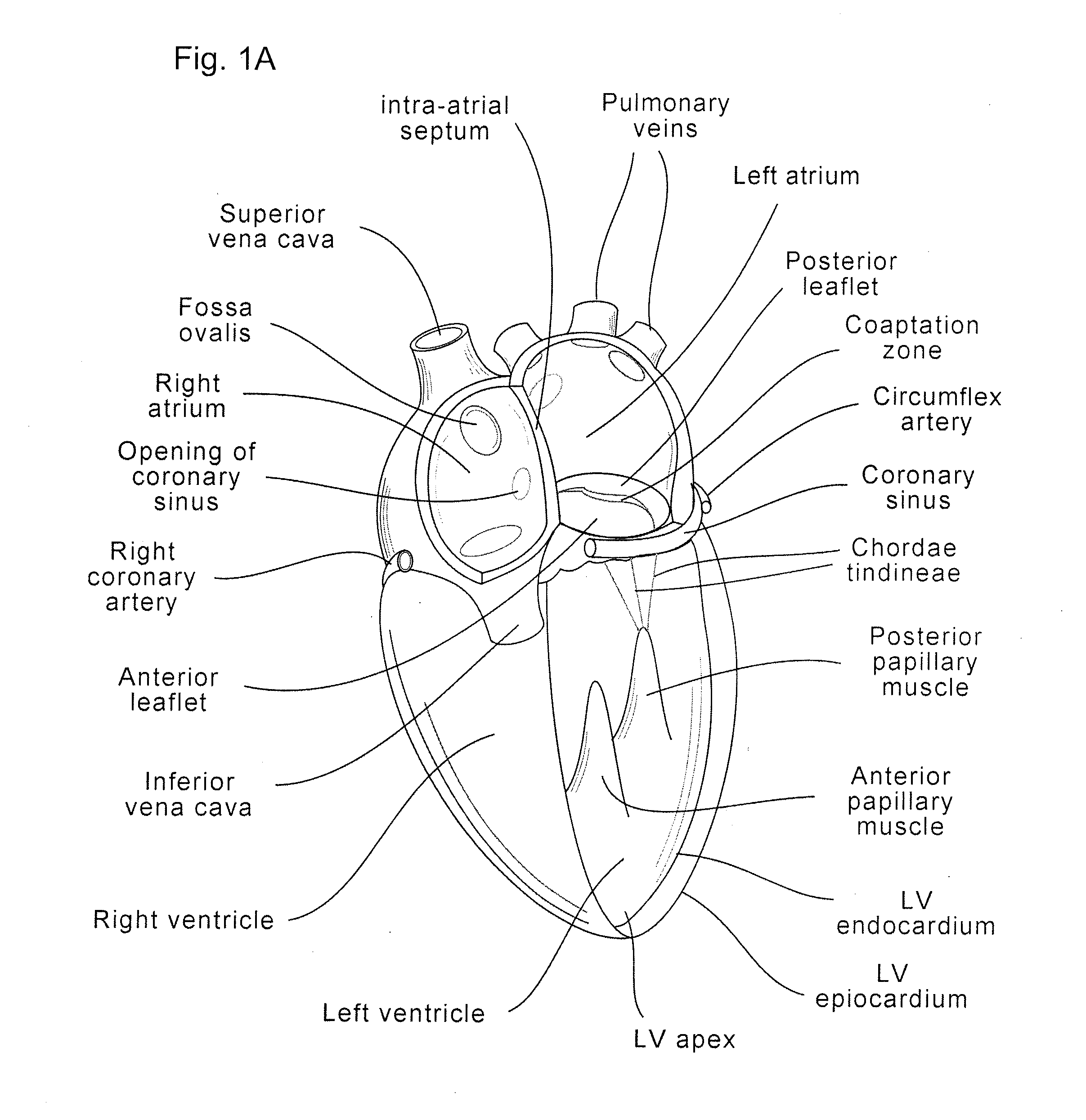
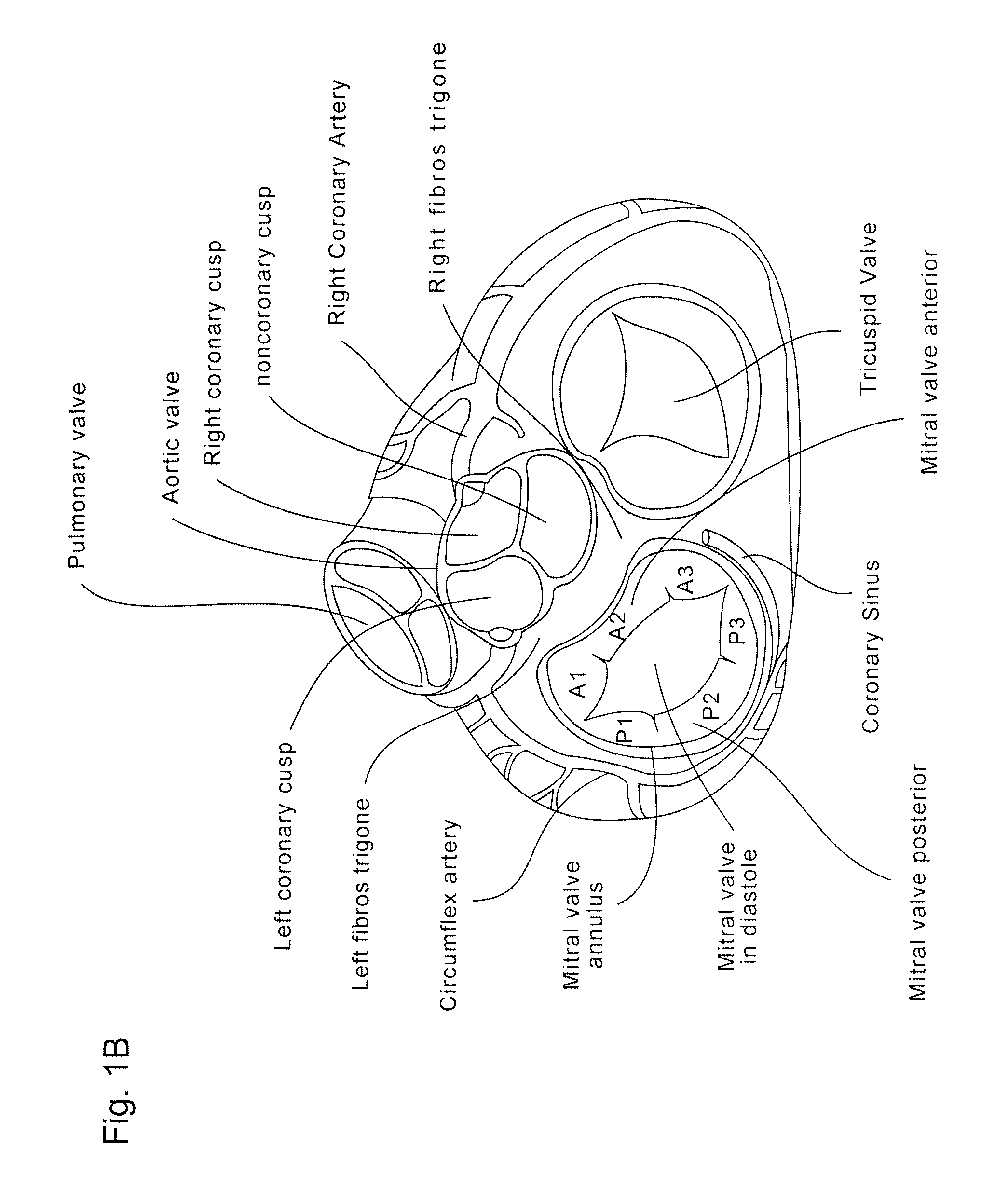
Implants, implant systems, and methods for treatment of mitral valve regurgitation and other valve diseases generally include a coaptation assist body which remains within the blood flow path as the leaflets of the valve move, the valve bodies often being relatively thin, elongate (along the blood flow path), and / or conformable structures which extend laterally from commissure to commissure, allowing the native leaflets to engage and seal against the large, opposed surfaces on either side of the valve body during the heart cycle phase when the ventricle contracts to empty that chamber of blood, and allows blood to pass around the valve body so that blood flows from the atrium to the ventricle during the filling phase of the heart cycle. Separate deployment of independent anchors near each of the commissures may facilitate positioning and support of an exemplary triangular valve body, with a third anchor being deployed in the ventricle. An outer surface of the valve body may accommodate tissue ingrowth or endothelialization, while a fluid-absorbing matrix can swell after introduction into the heart. The valve body shape may be selected after an anchor has been deployed, and catheter-based deployment systems may have a desirable low profile.
Owner:POLARES MEDICAL INC
Stent-within-stent arrangements
A variety of stent arrangements are described in which multiple stents expand and coordinate to block the spaces between the struts of the outer stent to create a tubular stent not prone to tissue in-growth. One or more stents are selectively positioned within an outer stent such that the struts of the one or more stents at least partially fill the openings of the outer stent. Alternatively, the one or more stents may be permanently affixed to the outer stent to produce a stent arrangement in which the openings between the struts of the outer stent are blocked by the struts of the one or more stents.
Owner:WILSONCOOK MEDICAL
Apparatus and methods for occluding a hollow anatomical structure
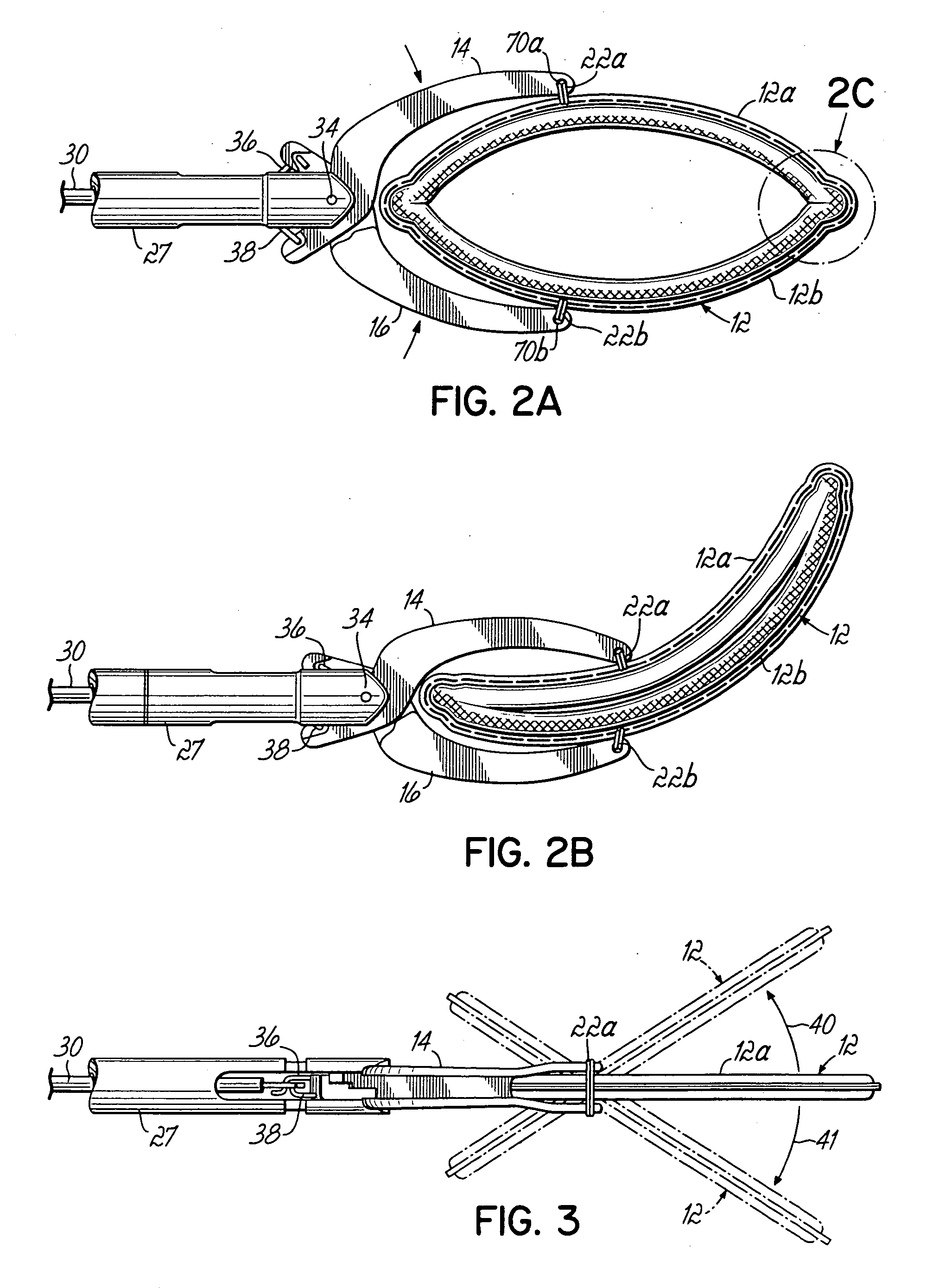
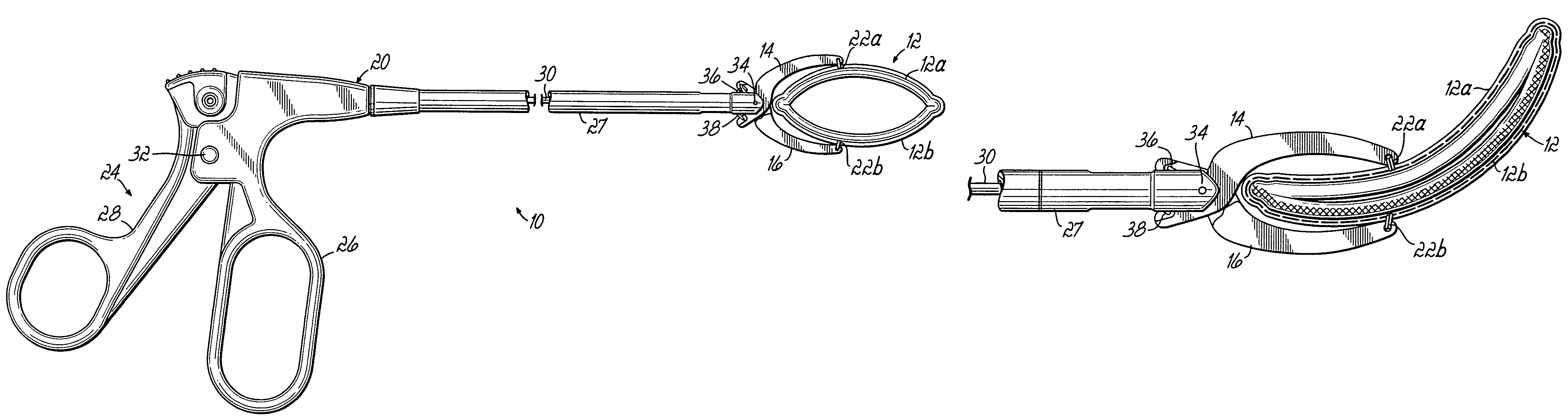
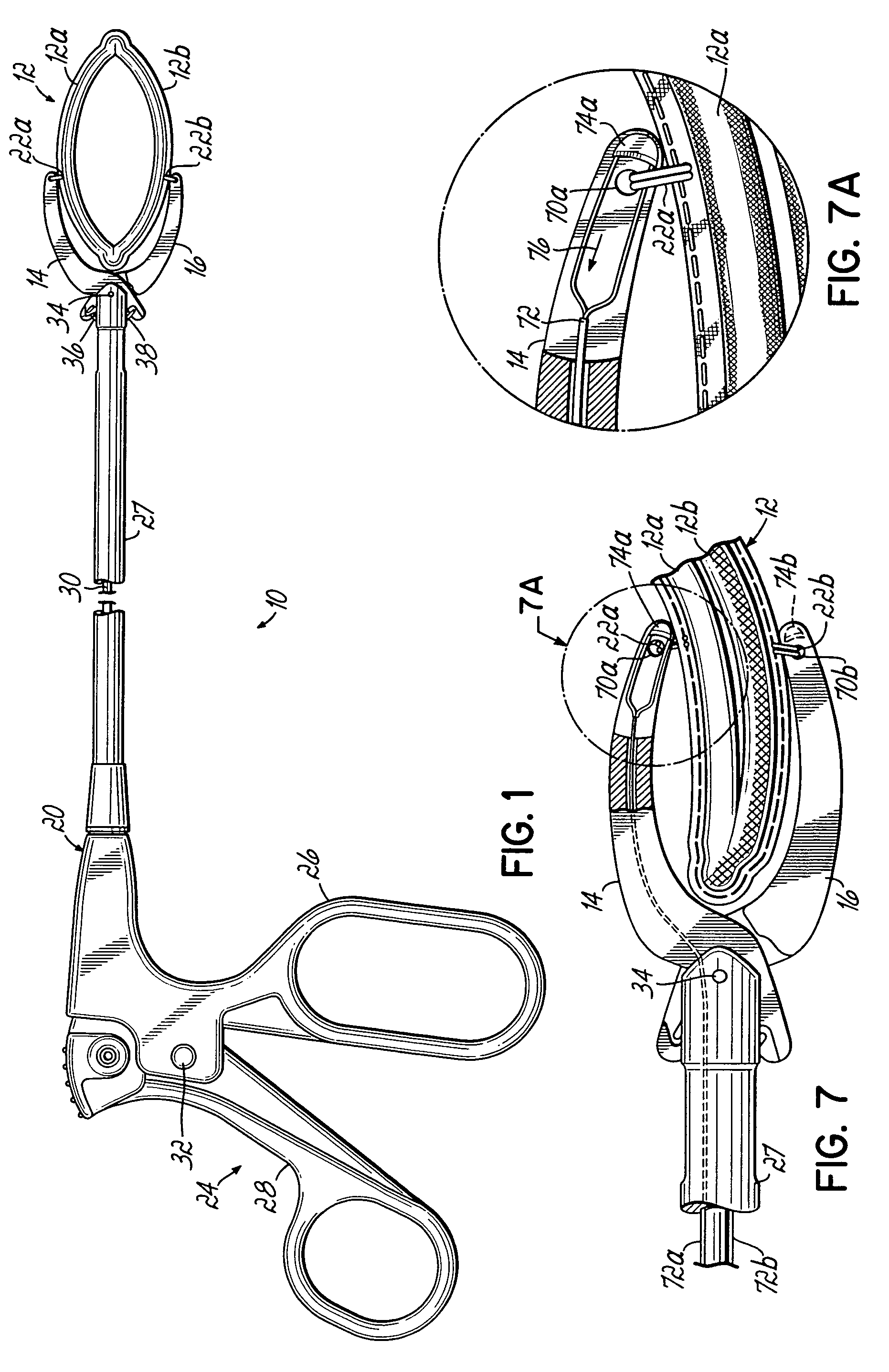
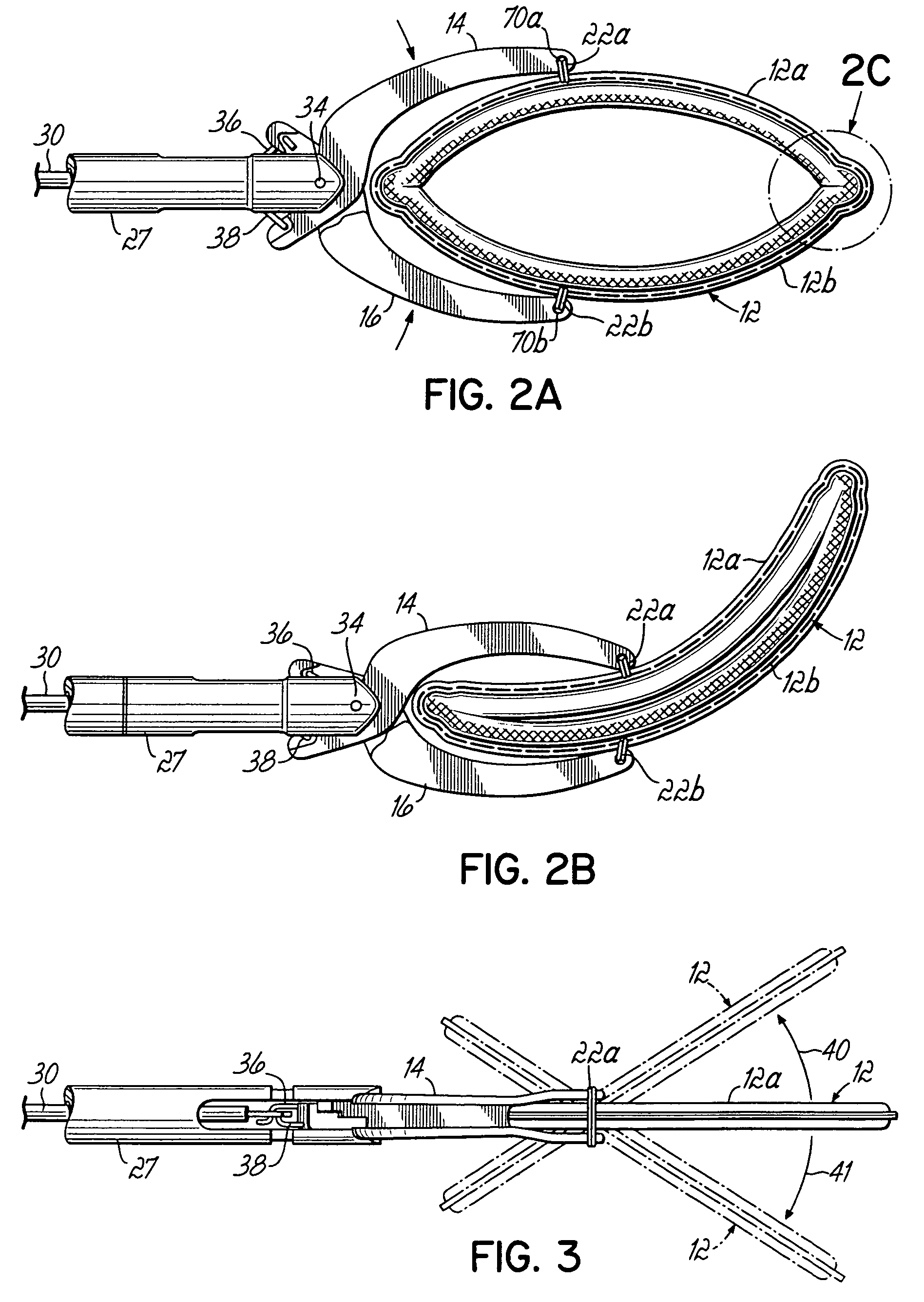
ActiveUS20050277959A1Positively attachingPromote resultsSurgical forcepsWound clampsAnatomical structuresMinimally invasive procedures
A device for occluding a hollow anatomical structure includes a clamp having at least first and second clamping portions adapted to be placed on opposite sides of the anatomical structure. At least one of the first and second clamping portions is movable toward the other from an open position to a clamping or closed position to occlude the anatomical structure. The clamp has an annular shape configured to surround the hollow anatomical structure in the open position and a flattened shape in the clamping position configured to occlude the hollow interior of the anatomical structure. The clamp is preferably covered with fabric to promote tissue ingrowth. A clamp delivery and actuation device is provided for allowing the clamp to be applied in either an open surgical procedure or a minimally invasive procedure.
Owner:ATRICURE
Apparatus and methods for occluding a hollow anatomical structure
ActiveUS7645285B2More forcePositively attachingSurgical forcepsWound clampsAnatomical structuresMinimally invasive procedures
A device for occluding a hollow anatomical structure includes a clamp having at least first and second clamping portions adapted to be placed on opposite sides of the anatomical structure. At least one of the first and second clamping portions is movable toward the other from an open position to a clamping or closed position to occlude the anatomical structure. The clamp has an annular shape configured to surround the hollow anatomical structure in the open position and a flattened shape in the clamping position configured to occlude the hollow interior of the anatomical structure. The clamp is preferably covered with fabric to promote tissue ingrowth. A clamp delivery and actuation device is provided for allowing the clamp to be applied in either an open surgical procedure or a minimally invasive procedure.
Owner:ATRICURE
Implant with composite coating
InactiveUS6261322B1High strengthCost effectiveImpression capsBone implantBiocompatible coatingBiocompatibility Testing
Systems and methods are described for implants with composite coatings to promote tissue in-growth and / or on-growth. An implant includes: a substrate; a structured surface formed on at least a portion of the substrate; and a biocompatible coating deposited on at least a fraction of the structured surface. The systems and methods provide advantages in that the implant has good biocompatibility while the biocompatible coating has good strength.
Owner:SHALBY ADVANCED TECH INC
Method for left atrial appendage occlusion
InactiveUS20050004652A1Resisting compressionPrevent rotationStentsBalloon catheterLeft atrial appendage occlusionAppendage
Disclosed is an occlusion device for use in a body lumen such as the left atrial appendage. The occlusion device includes an occlusion member and may also include a stabilizing member. The stabilizing member inhibits compression of the left atrial appendage, facilitating tissue in-growth onto the occlusion member. Methods are also disclosed.
Owner:BOSTON SCI SCIMED INC
Laser-produced porous structure
The present invention disclosed a method of producing a three-dimensional porous tissue in-growth structure. The method includes the steps of depositing a first layer of metal powder and scanning the first layer of metal powder with a laser beam to form a portion of a plurality of predetermined unit cells. Depositing at least one additional layer of metal powder onto a previous layer and repeating the step of scanning a laser beam for at least one of the additional layers in order to continuing forming the predetermined unit cells. The method further includes continuing the depositing and scanning steps to form a medical implant.
Owner:UNIV OF LIVERPOOL +1
Coaptation enhancement implant, system, and method
ActiveUS20120197388A1Mal-coaptation is mitigatedImprove valve functionSuture equipmentsBone implantBody shapeAbdominal cavity
Implants, implant systems, and methods for treatment of mitral valve regurgitation and other valve diseases generally include a coaptation assist body which remains within the blood flow path as the leaflets of the valve move, the valve bodies often being relatively thin, elongate (along the blood flow path), and / or conformable structures which extend laterally from commissure to commissure, allowing the native leaflets to engage and seal against the large, opposed surfaces on either side of the valve body during the heart cycle phase when the ventricle contracts to empty that chamber of blood, and allows blood to pass around the valve body so that blood flows from the atrium to the ventricle during the filling phase of the heart cycle. Separate deployment of independent anchors near each of the commissures may facilitate positioning and support of an exemplary triangular valve body, with a third anchor being deployed in the ventricle. An outer surface of the valve body may accommodate tissue ingrowth or endothelialization, while a fluid-absorbing matrix can swell after introduction into the heart. The valve body shape may be selected after an anchor has been deployed, and catheter-based deployment systems may have a desirable low profile.
Owner:POLARES MEDICAL INC
Implants and methods for treating bone
This invention relates to biomedical implants for filling, supporting or treating bone. In one embodiment, the implant comprises an electrospun polymer scaffold that is thereafter plated with a metal to provide a selected high modulus. Such an implant can be fabricated with a selected porosity for tissue ingrowth. The implant can be further provided with a varied modulus along the length of the implant body for inducing bending of the implant for packing in a bone. In another embodiment, the implant is fabricated in an elongated configuration for introducing into bone to treat a vertebral fracture. In another embodiment, the implant can be configured with helical threads for helically driving the implant into a bone.
Owner:DFINE INC
Implants and methods for treating bone
ActiveUS20060089715A1Eliminate needInternal osteosythesisSpinal implantsVertebra compression fractureBiodegradable magnesium
An orthopedic implant comprising a deformable, expandable implant body configured for treating abnormalities in bones, such as compression fractures of vertebra, necrosis of femurs and the like. An exemplary implant body comprises a small cross-section threaded element that is introduced into a bone region and thereafter is expanded into a larger cross-section, monolithic assembly to provide a bone support. In one embodiment, the implant body is at least partly fabricated of a magnesium alloy that is biodegradable to allow for later tissue ingrowth.
Owner:DFINE INC
Optimized sensor geometry for an implantable glucose sensor
InactiveUS20060211921A1Accurate measurementDiagnostic recording/measuringSensorsGlucose sensorsChronic inflammatory response
An implantable sensor for use in measuring a concentration of an analyte such as glucose in a bodily fluid, including a body with a sensing region adapted for transport of analytes between the sensor and the bodily fluid, wherein the sensing region is located on a curved portion of the body such that when a foreign body capsule forms around the sensor, a contractile force is exerted by the foreign body capsule toward the sensing region. The body is partially or entirely curved, partially or entirely covered with an anchoring material for supporting tissue ingrowth, and designed for subcutaneous tissue implantation. The geometric design, including curvature, shape, and other factors minimize chronic inflammatory response at the sensing region and contribute to improved performance of the sensor in vivo.
Owner:DEXCOM
Laser-produced porous structure
The present invention disclosed a method of producing a three-dimensional porous tissue in-growth structure. The method includes the steps of depositing a first layer of metal powder and scanning the first layer of metal powder with a laser beam to form a portion of a plurality of predetermined unit cells. Depositing at least one additional layer of metal powder onto a previous layer and repeating the step of scanning a laser beam for at least one of the additional layers in order to continuing forming the predetermined unit cells. The method further includes continuing the depositing and scanning steps to form a medical implant.
Owner:UNIV OF LIVERPOOL +1
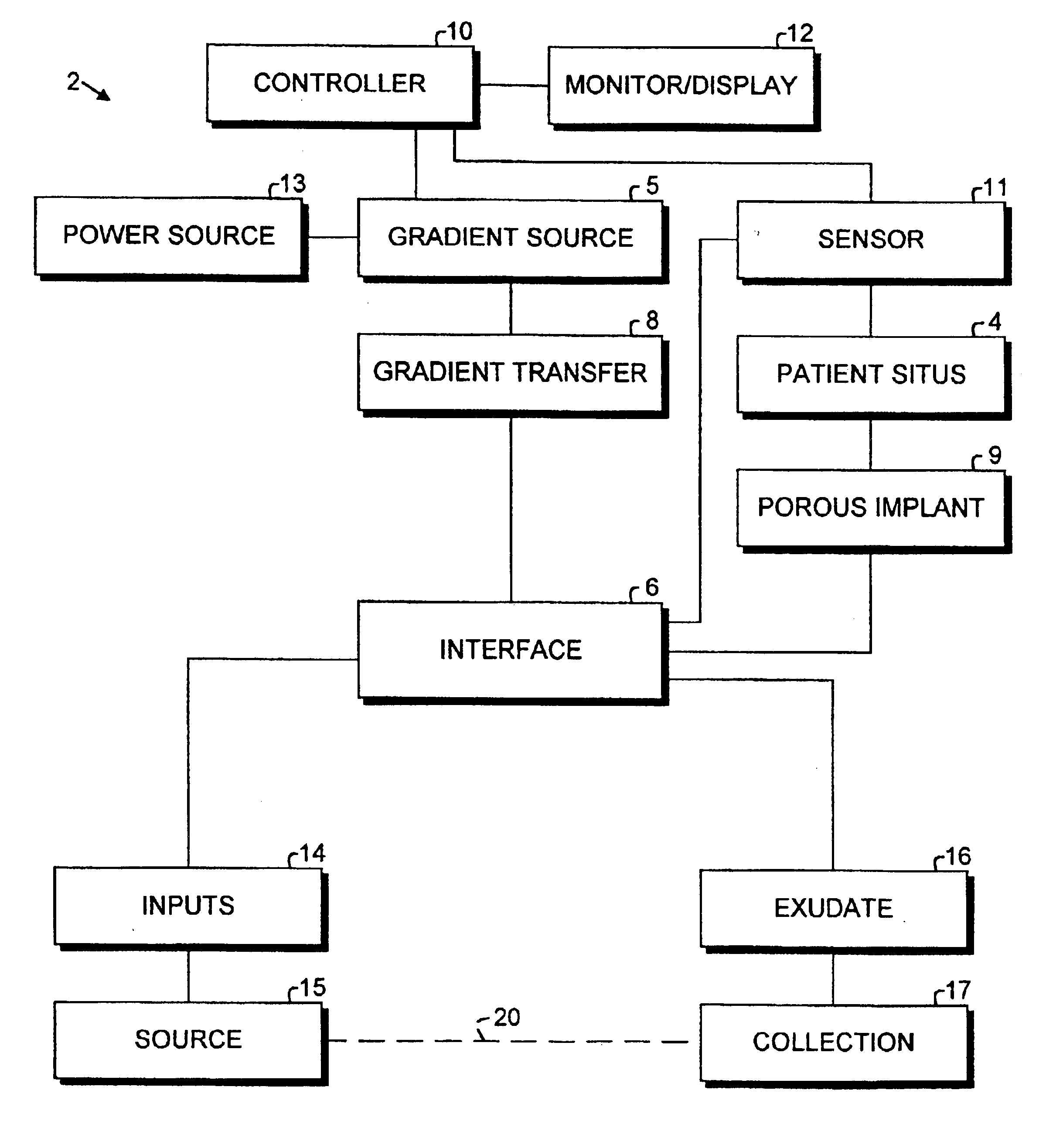
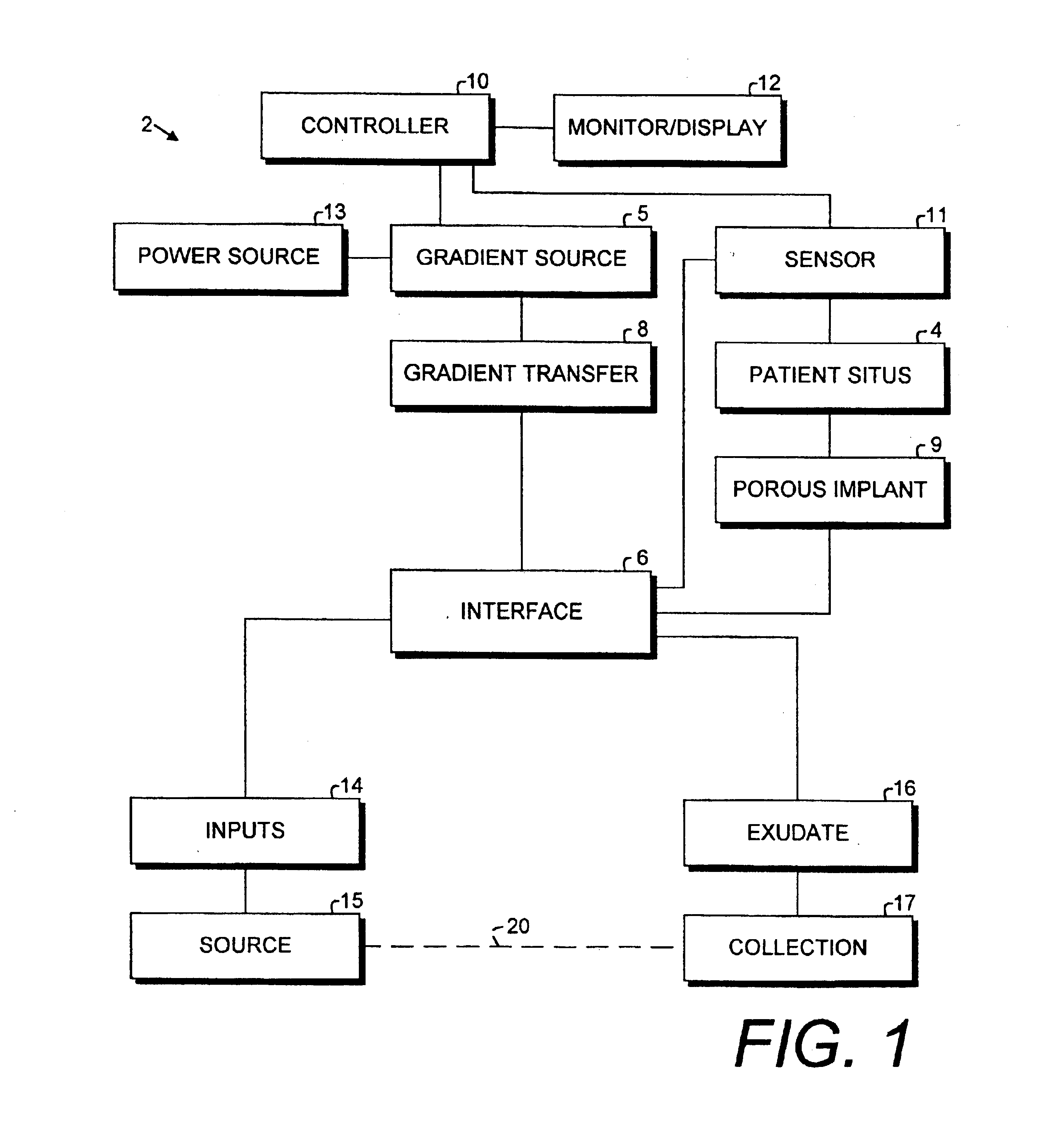
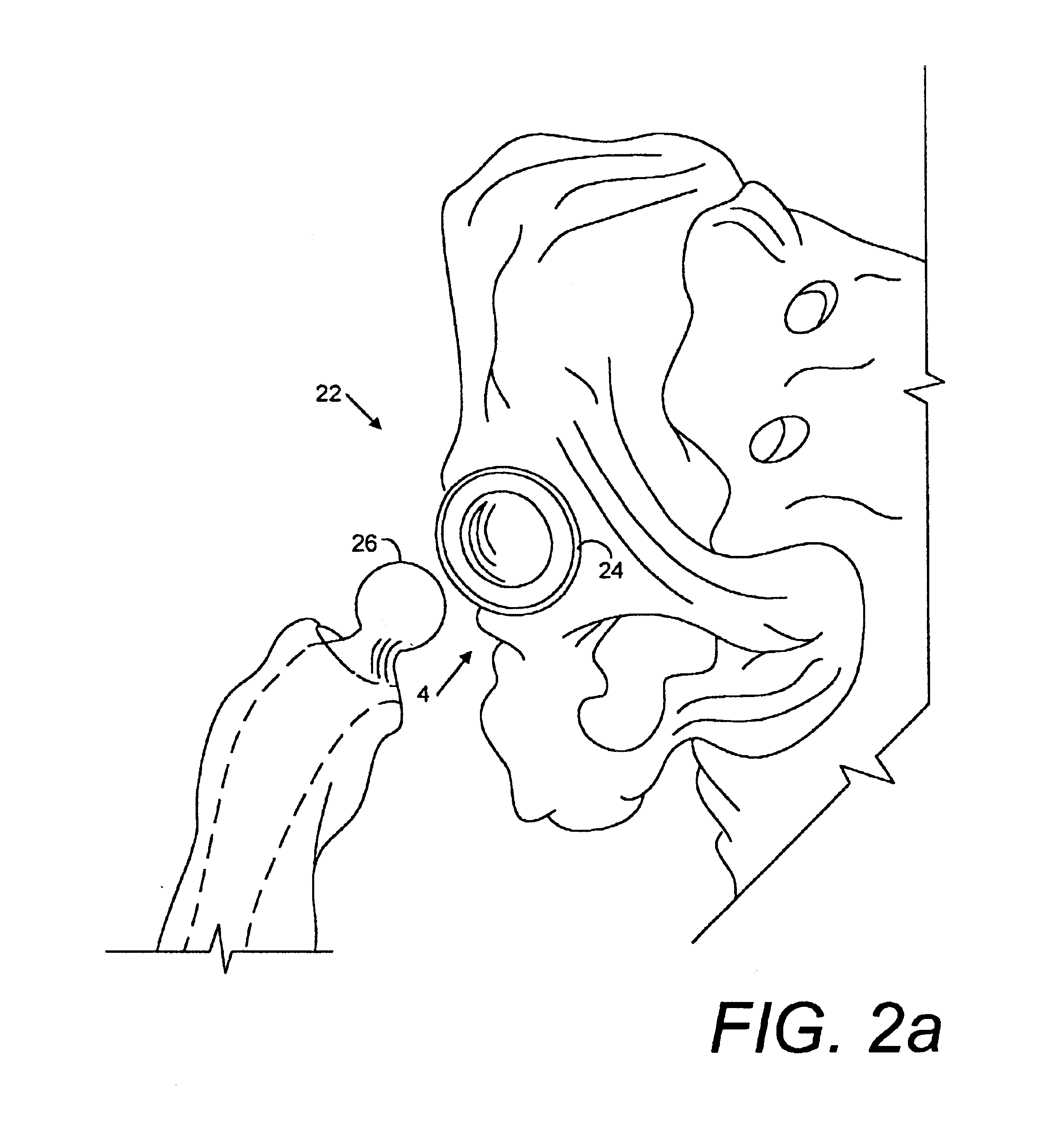
Porous implant system and treatment method
A porous implant system includes a gradient source adapted for transferring a gradient to an interface connected to an implant at a patient situs. The gradient source is controlled by a programmable controller. The implant is bonded to the patient by tissue ingrowth, which is facilitated by the gradient formed across the porous portion of the implant. A treatment method and includes the steps of providing a porous implant, connecting same to a gradient source through an interface, forming a gradient across the implant and controlling the operation of the gradient source according to a predetermined and preprogrammed treatment protocol.
Owner:BUBB STEPHEN K
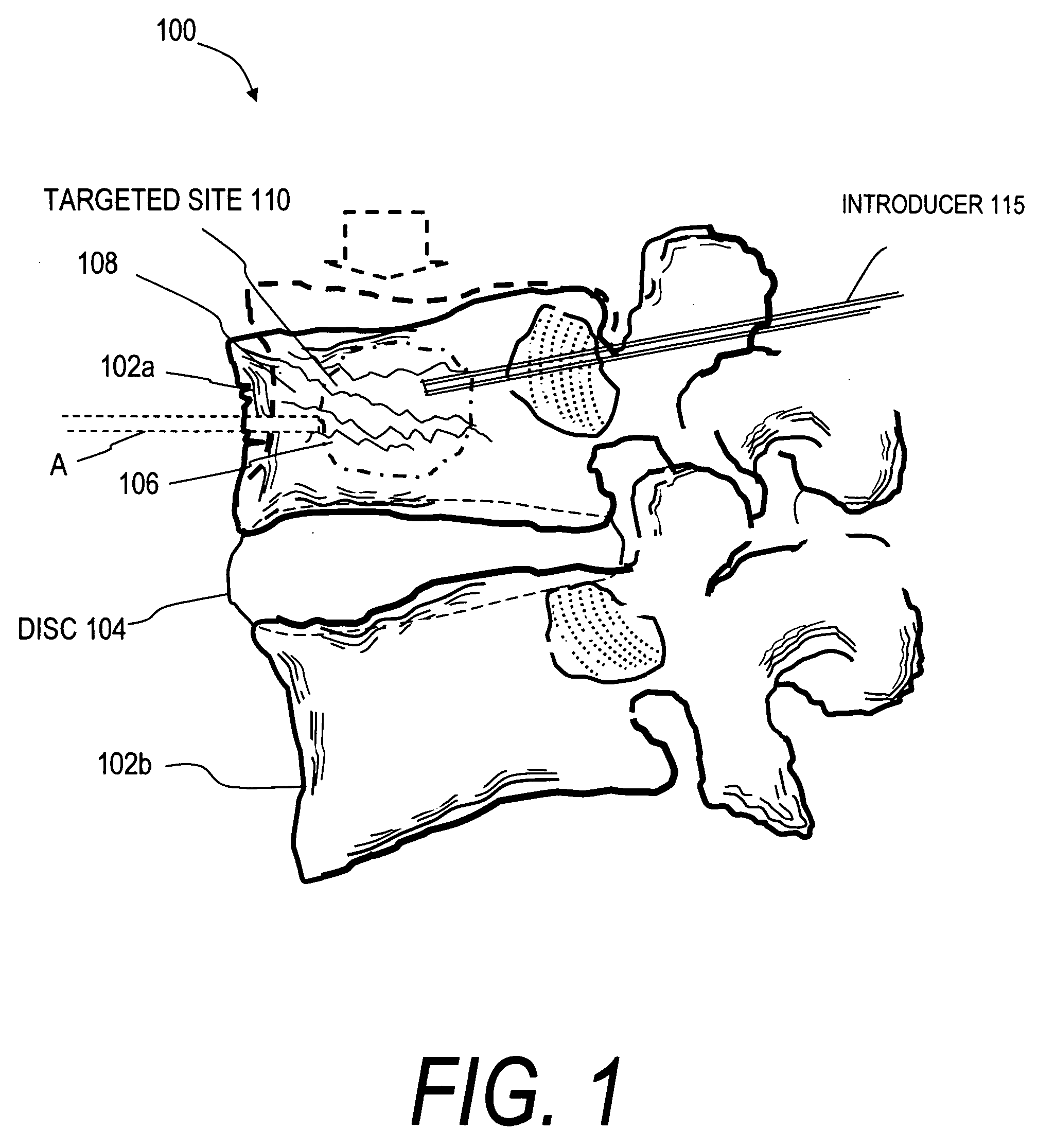
Interbody fusion device and method for restoration of normal spinal anatomy
InactiveUS7238186B2Maintain patency and stabilityRapid and stable arthrodesisInternal osteosythesisBone implantSpinal columnPorous tantalum
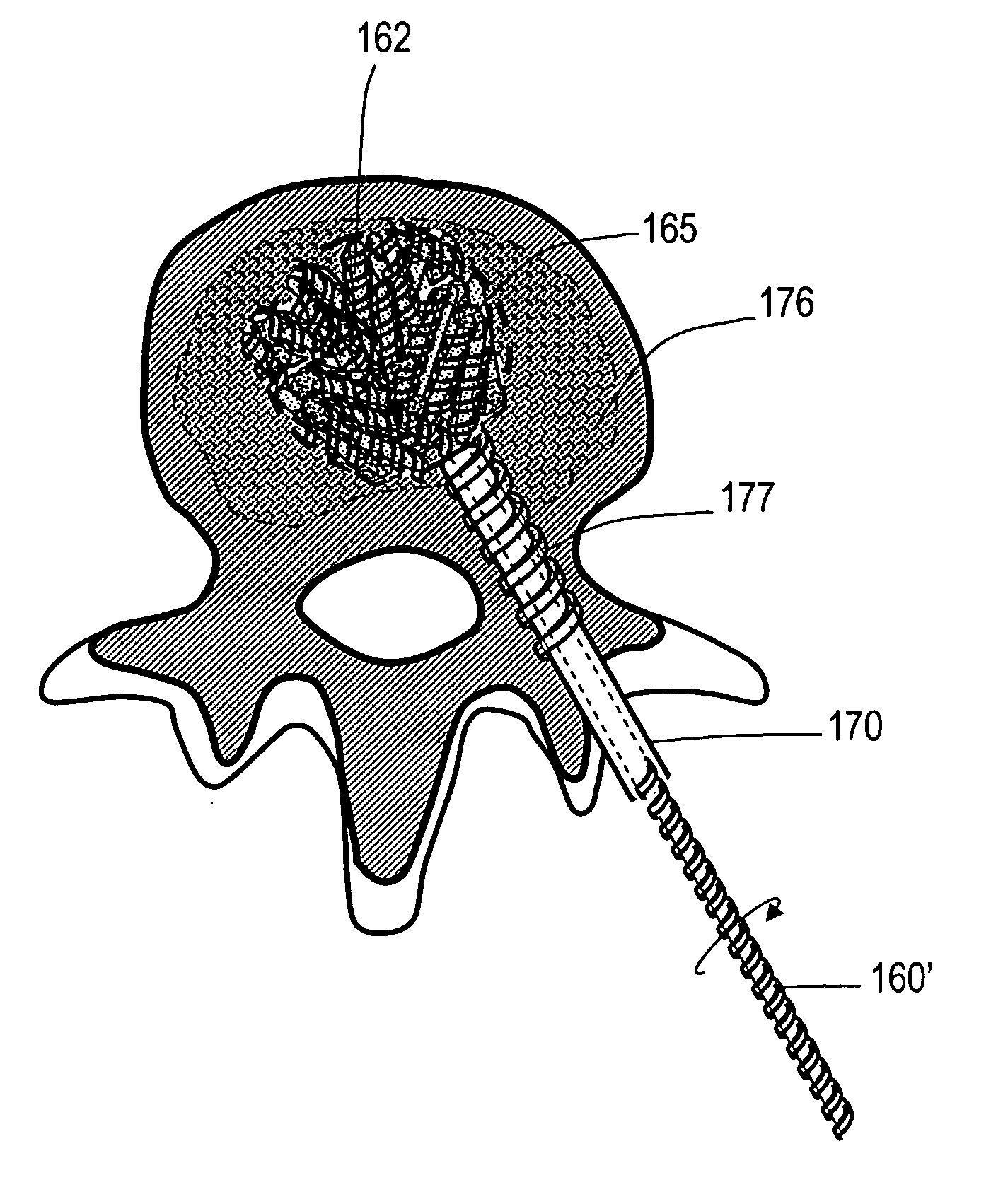
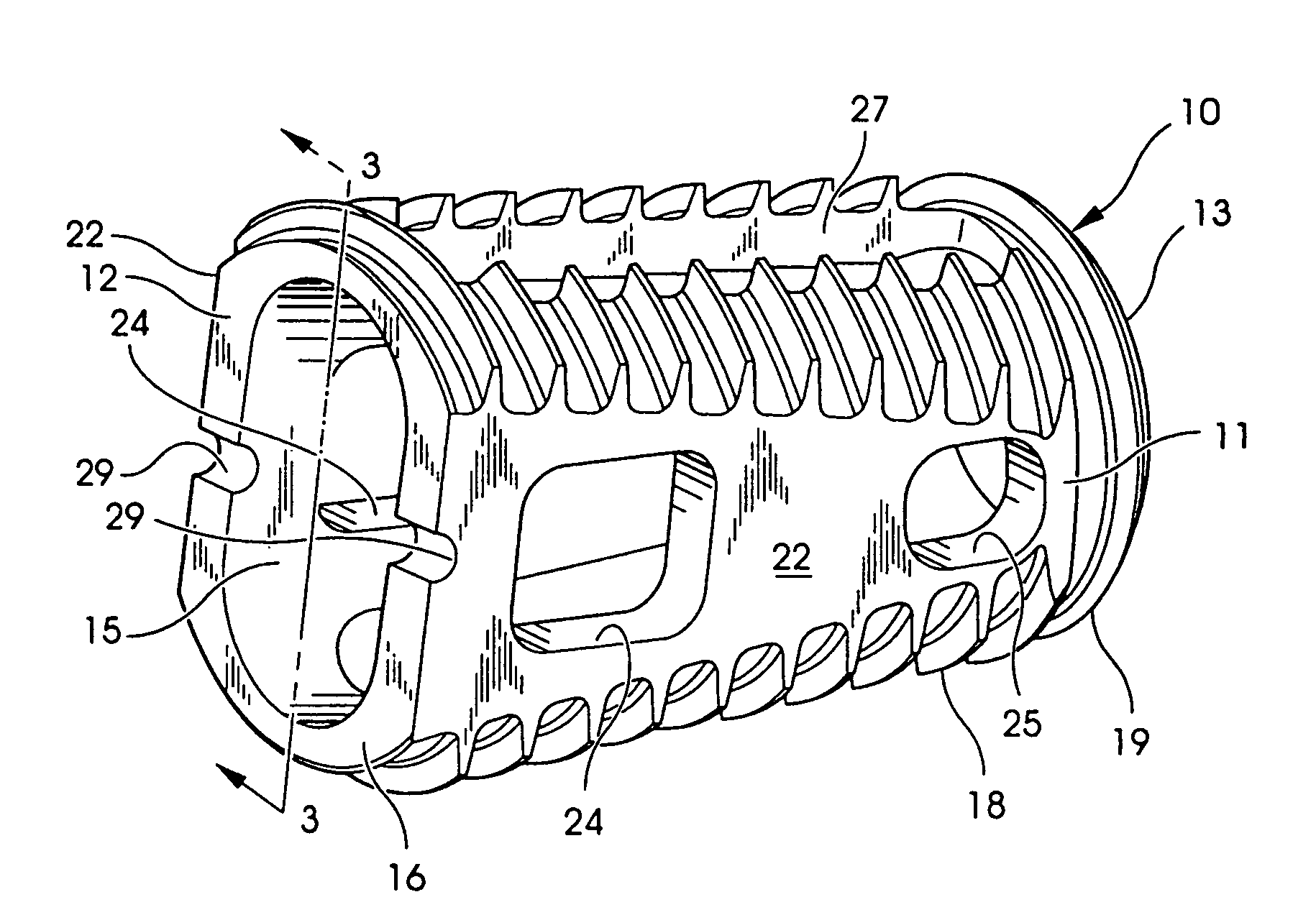
An interbody fusion device in one embodiment includes a tapered body defining a hollow interior for receiving bone graft or bone substitute material. The body defines exterior threads which are interrupted over portions of the outer surface of the device. The fusion device defines truncated side walls so that on end view the body takes on a cylindrical form. The side walls are provided with vascularization openings, and the body wall device includes opposite bone ingrowth slots extending through the interrupted thread portion of the body. In another embodiment, the tapered body is solid and formed of a porous biocompatible material having sufficient structural integrity to maintain the intradiscal space and normal curvature. The material is preferably a porous tantalum having fully interconnected pores to facilitate complete bone tissue ingrowth into the implant. An implant driver is provided which engages the truncated side walls to complete the cylindrical form of the implant at the root diameter of the interrupted threads, to thereby facilitate threaded insertion of the implant to the intra-discal space between adjacent vertebrae. Methods for posterior and anterior insertion of the fusion device are also disclosed.
Owner:WARSAW ORTHOPEDIC INC
Methods and devices for conduit occlusion
The present invention comprises systems, methods and devices for the delivery of compositions for occluding or of means for opening conduits. The implantable occlusive material may be delivered pre-formed or in situ cured and, may be a resorbable material that supports tissue ingrowth that eventually replaces the material leaving little or no original material in place. The delivery system is positioned to allow for placement of the occlusive material into the body conduit. Use of delivery systems, methods and devices for re-opening an occluded body conduit are also included.
Owner:FEMASYS INC
Devices for maintaining surgically created openings
InactiveUS20050137518A1Simple processImprove scalabilityStentsBronchiCatheterObstructive Pulmonary Diseases
Devices and methods are directed to improving the gaseous exchange in a lung of an individual having, for instance, chronic obstructive pulmonary disease. More particularly, conduits may be deployed in the lung to maintain collateral openings (or channels) surgically created through airway walls. This tends to facilitate both the exchange of oxygen ultimately into the blood and decompress hyper-inflated lungs. The conduit includes a radially expandable center section having a first end, a second end, and a passageway extending from the first end to the second end. A control segment may be associated with the conduit to limit the degree of radial expansion. The conduit further includes a plurality of deflectable members extending from the ends of the center section. A tissue barrier may coaxially surround the conduit such that tissue ingrowth is prevented. The conduits may also include hold-down members and bioactive coatings that serve to prevent ejection of the conduit as well as prevent narrowing of the passageway due to tissue ingrowth.
Owner:BRONCUS TECH
Stent graft with external scaffolding and method
ActiveUS20170231749A1Promote tissue ingrowthEasy to integrateStentsBlood vesselsTissue integrationStent grafting
A scaffolded stent-graft includes a graft material comprising an inner surface and an outer surface. The inner surface defines a lumen within the graft material. The scaffolded stent-graft further includes a scaffold comprising a mesh coupled to the graft material at the outer surface. The scaffold is configured to promote tissue ingrowth therein. In this manner, the scaffold enhances tissue integration into the scaffolded stent-graft. The tissue integration enhances biological fixation of the scaffolded stent-graft in vessels minimizing the possibility of endoleaks and migration.
Owner:MEDTRONIC VASCULAR INC
Bodily lumen closure apparatus and method
ActiveUS20050155608A1Improve sealingPrevent leakageStentsFallopian occludersCell-Extracellular MatrixSource material
An absorbable and expandable closure member used to occlude or exclude a body lumen or cavity, such as a blood vessel, fallopian tube, duct, aneurysmal sac, etc., comprising a closure member comprising one of more sheets of a biomaterial that are rolled, stacked, or folded to form a multilayer construct of a generally cylindrical configuration for deployment through a delivery system, either as a singularly or part of a multiplicity of closure members. The biomaterial is derived from a source material, such as small intestinal submucosa or another remodelable material (e.g., an extracellular matrix) having properties for stimulating ingrowth of adjacent tissue into the biomaterial deployed within the bodily lumen. The closure member is deployed to the bodily lumen from a delivery sheath, cartridge, and / or over a inner guiding member, such as a wire guide or catheter.
Owner:OREGON HEALTH & SCI UNIV +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com