Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2113 results about "Inflammatory response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inflammatory response: A fundamental type of response by the body to disease and injury, a response characterized by the classical signs of "dolor, calor, rubor, and tumor " -- pain, heat (localized warmth), redness, and swelling. Innumerable insults (a mosquito bite, a splinter, a virus infection,...
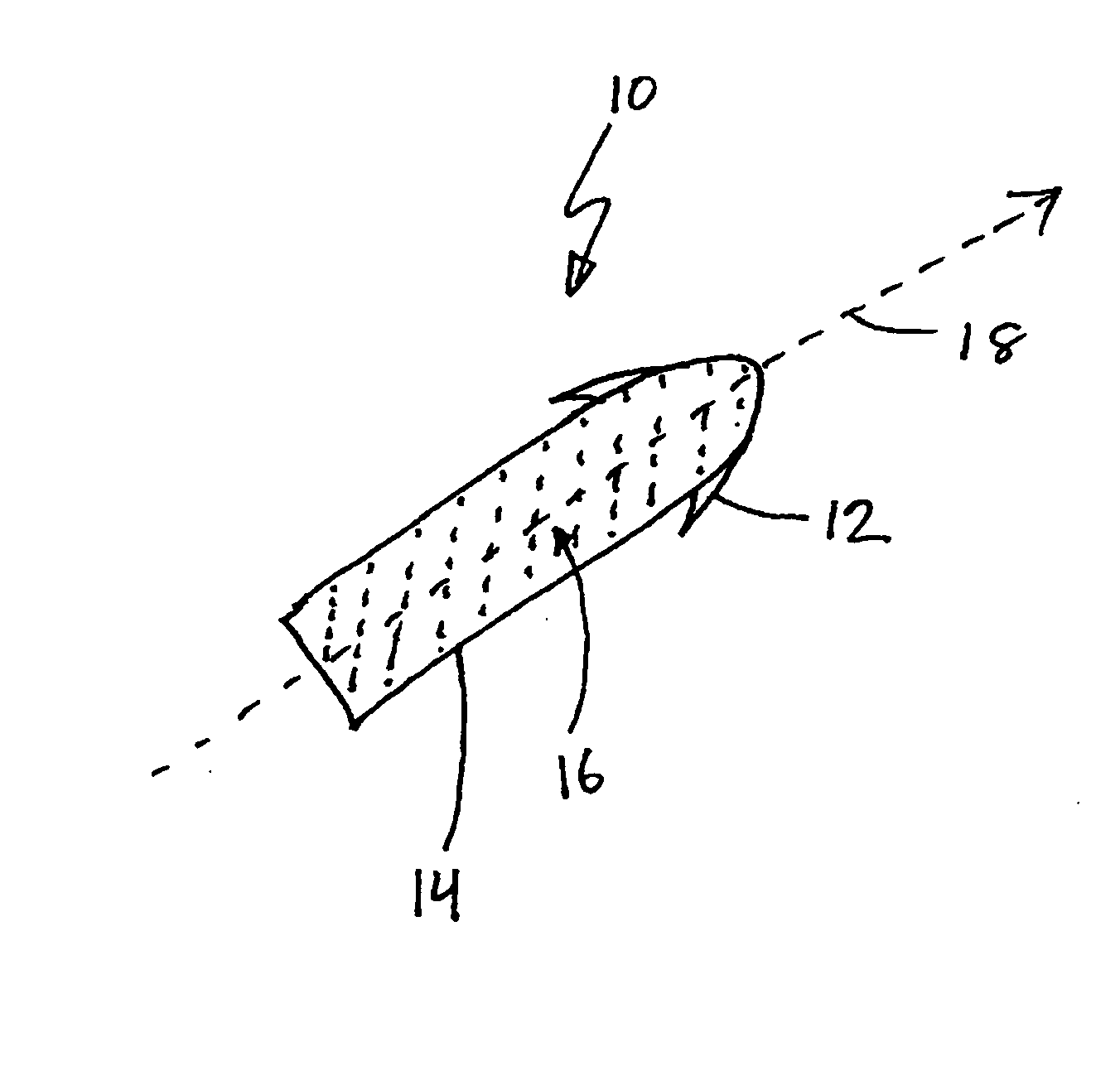
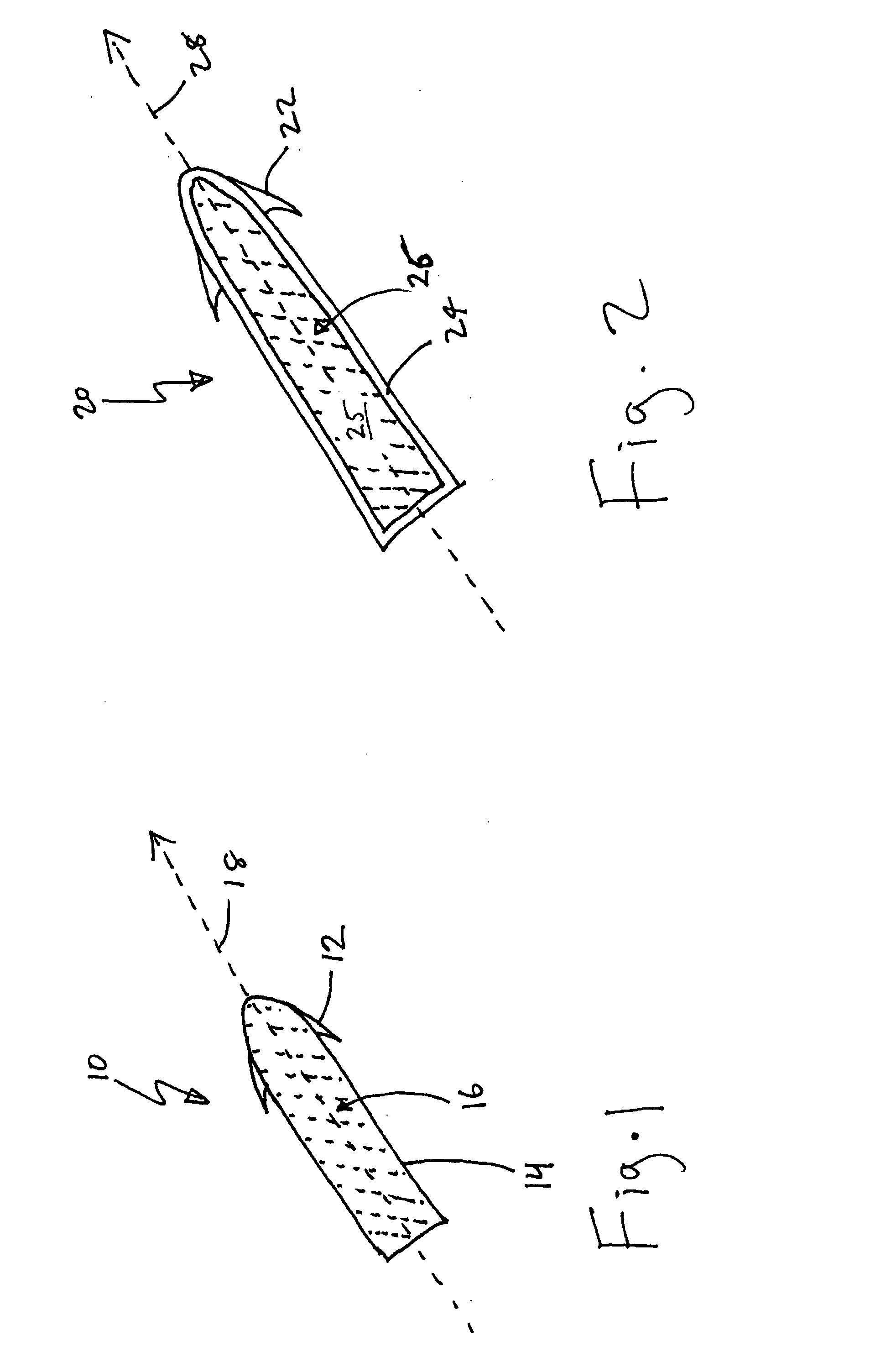
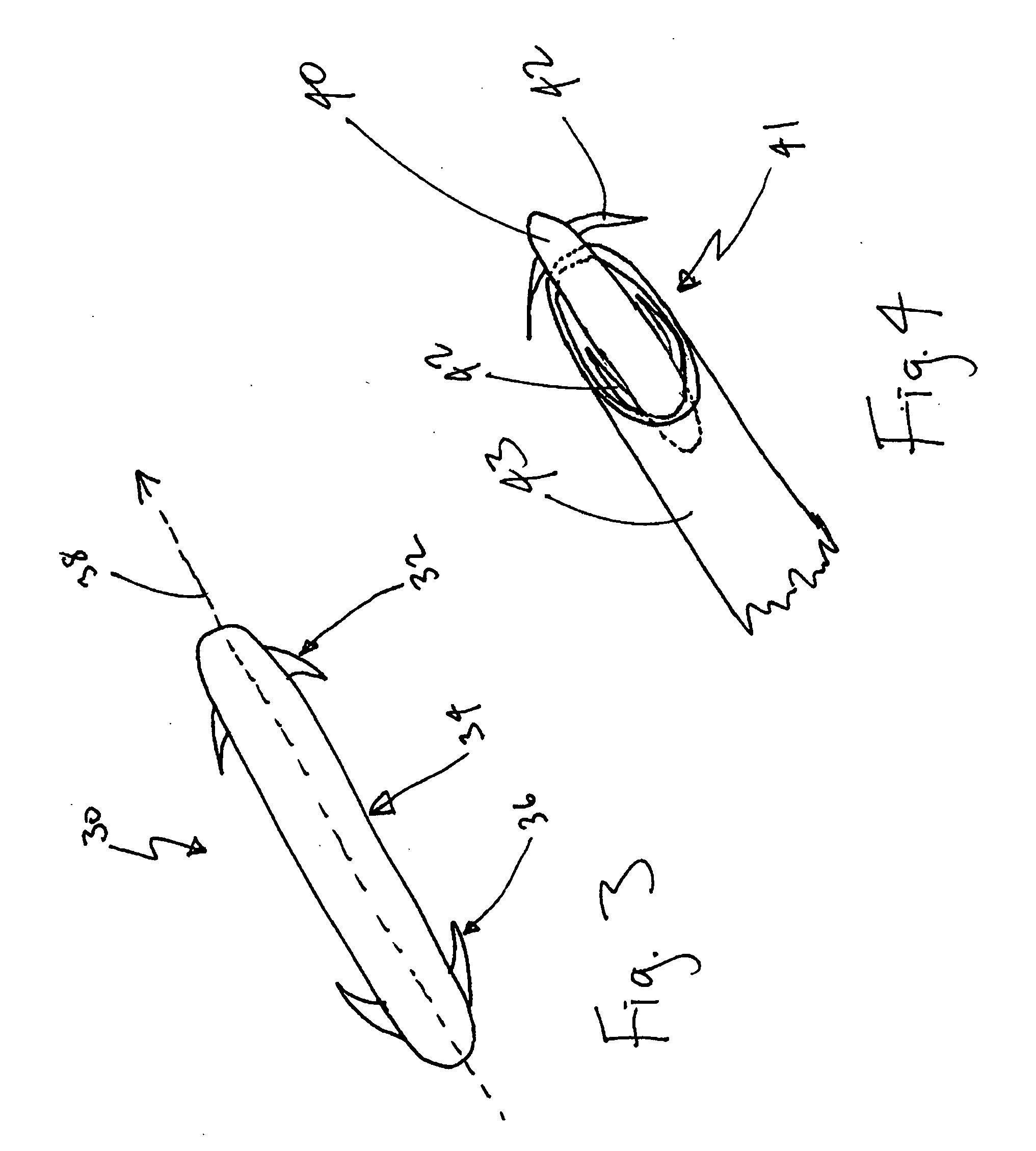
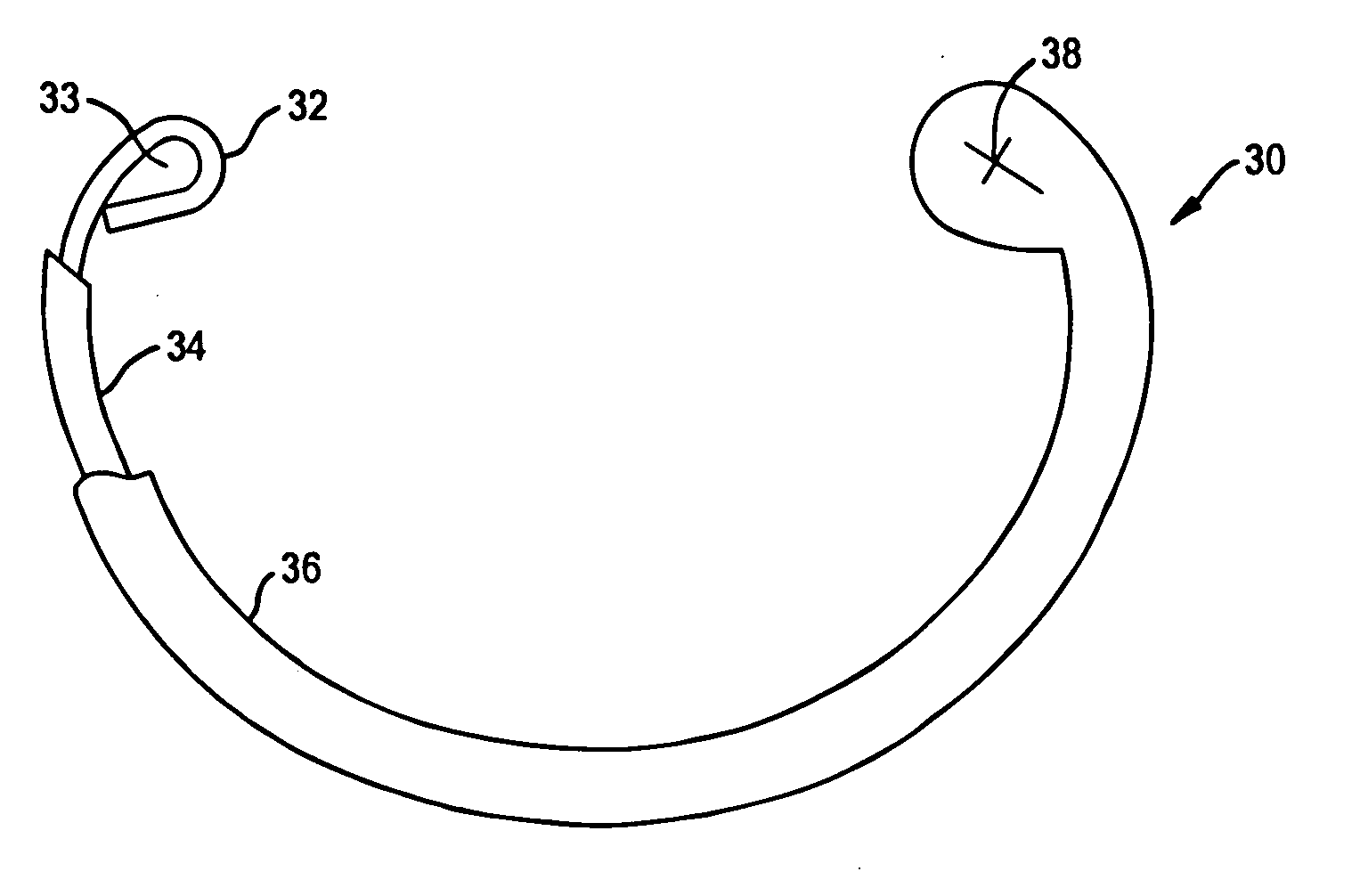
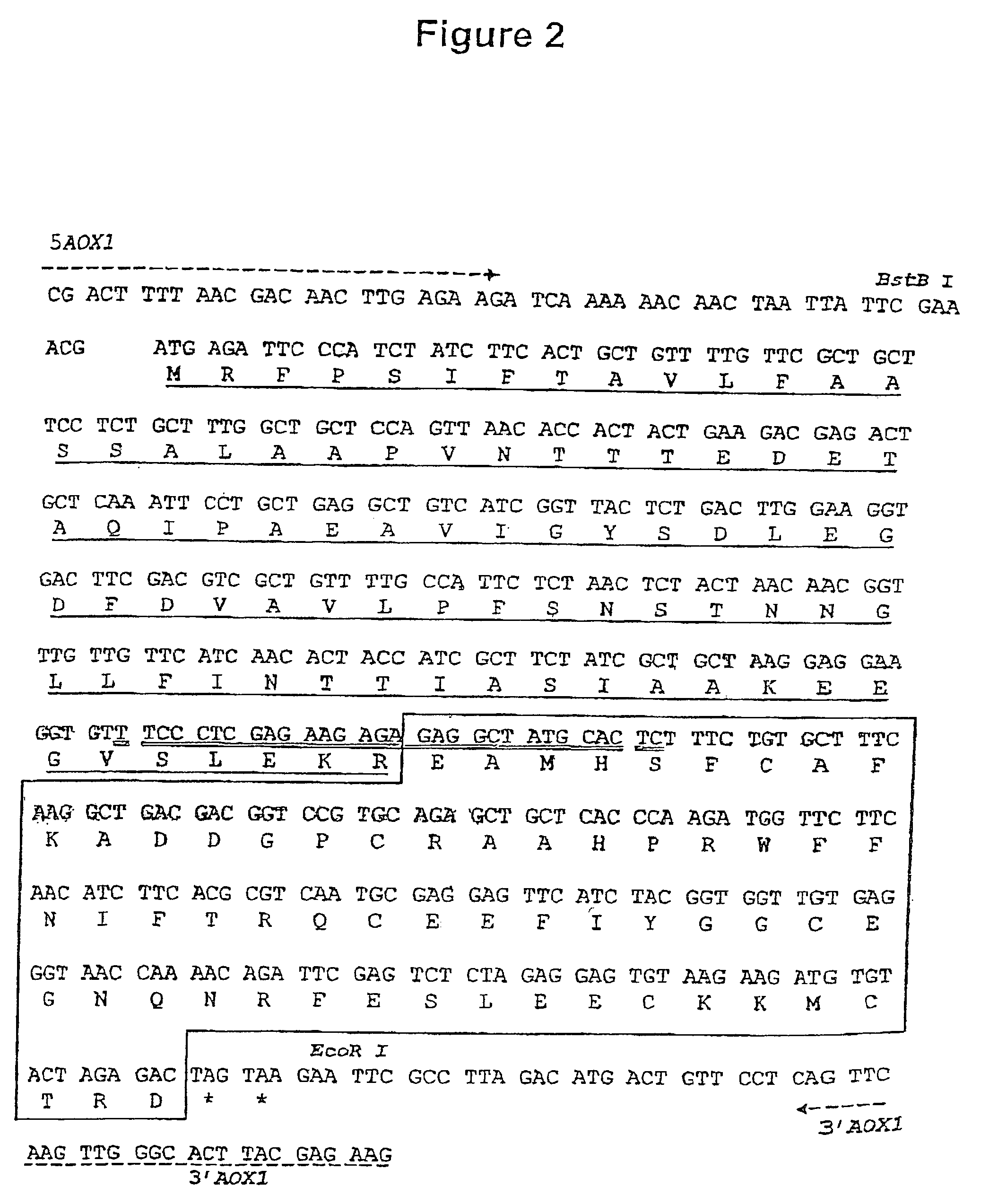
Drug depot implant designs
ActiveUS7727954B2Uniform drug distributionMinimal disruptionPowder deliveryPeptide/protein ingredientsSkeletal injuryChronic pain
Owner:WARSAW ORTHOPEDIC INC
Negative pressure treatment system with heating and cooling provision
InactiveUS7144390B1Prevent vacuum leakageReduce inflammationSurgical needlesPlastersWound siteSurgery
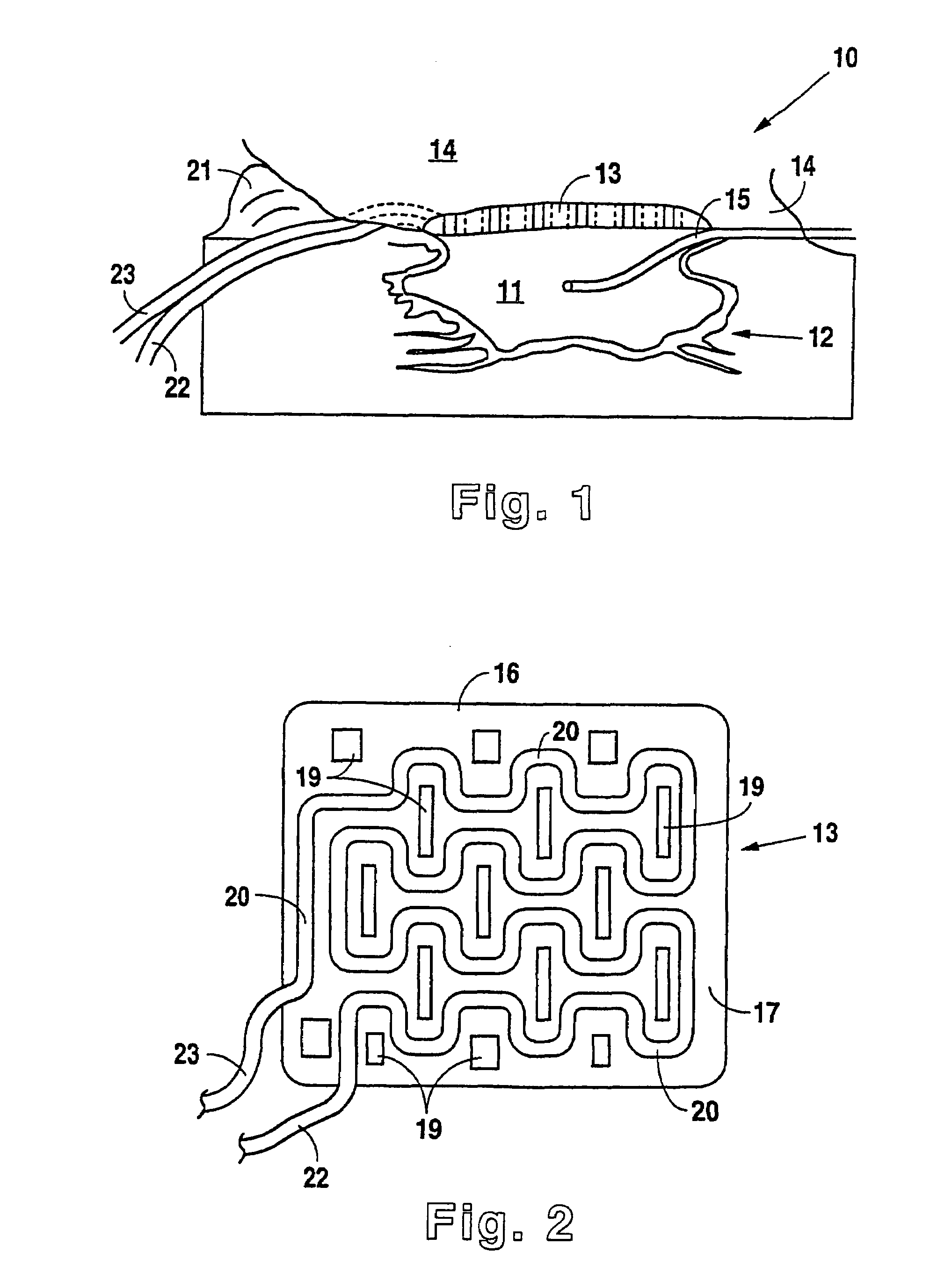
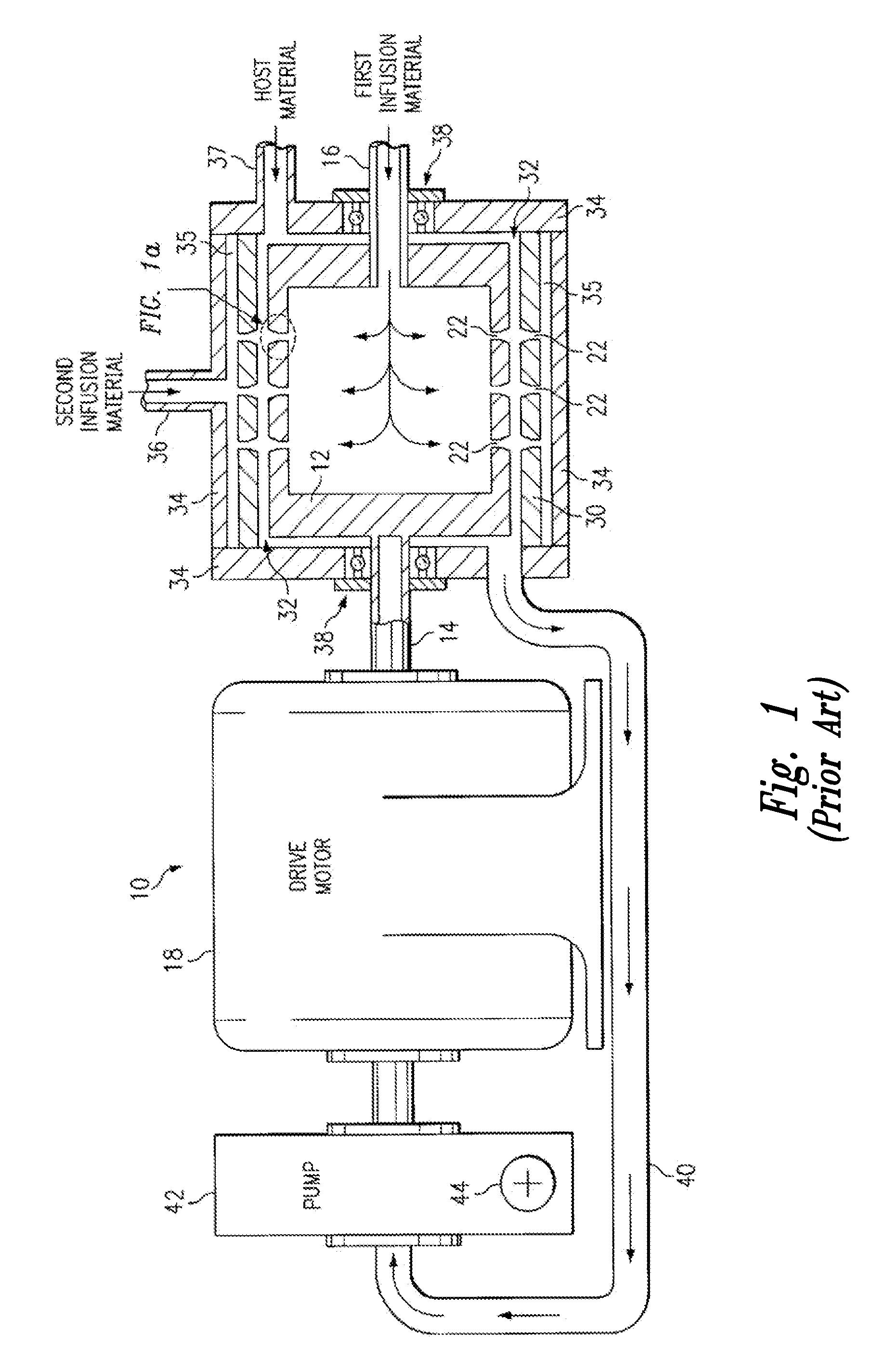
A method, and apparatus (10) for the controlled acceleration, and / or retardation of the body's inflammatory response generally comprises a foam pad (11) for insertion substantially into a wound site, a heating, a cooling pad (13) for application over the wound site (12), a wound drape (14) or sealing enclosure of the foam pad (11), the heating, and cooling pad (13) at wound site (12). The foam pad (11) is placed in fluid communication with a vacuum source for promotion of the controlled acceleration or retardation of the body's inflammatory response. The heating, and cooling provision controls the local metabolic function as part of the inflammatory response.
Owner:KCI LICENSING INC
Drug depot implant designs and methods of implantation
ActiveUS20070243225A1Uniform drug distributionMinimal disruptionPowder deliveryPeptide/protein ingredientsSkeletal injurySacroiliac joint
The present invention relates to novel drug depot implant designs for optimal delivery of therapeutic agents to subjects. The invention provides a method for alleviating pain associated with neuromuscular or skeletal injury or inflammation by targeted delivery of one or more therapeutic agents to inhibit the inflammatory response which ultimately causes acute or chronic pain. Controlled and directed delivery can be provided by drug depot implants, comprising therapeutic agents, specifically designed to deliver the therapeutic agent to the desired location by facilitating their implantation, minimizing their migration from the desired tissue location, and without disrupting normal joint and soft tissue movement.
Owner:WARSAW ORTHOPEDIC INC
Drug depot implant designs and methods of implantation
ActiveUS20070243228A1Uniform drug distributionMinimal disruptionBiocidePeptide/protein ingredientsSkeletal injurySacroiliac joint
The present invention relates to novel drug depot implant designs for optimal delivery of therapeutic agents to subjects. The invention provides a method for alleviating pain associated with neuromuscular or skeletal injury or inflammation by targeted delivery of one or more therapeutic agents to inhibit the inflammatory response which ultimately causes acute or chronic pain. Controlled and directed delivery can be provided by drug depot implants, comprising therapeutic agents, specifically designed to deliver the therapeutic agent to the desired location by facilitating their implantation, minimizing their migration from the desired tissue location, and without disrupting normal joint and soft tissue movement.
Owner:WARSAW ORTHOPEDIC INC
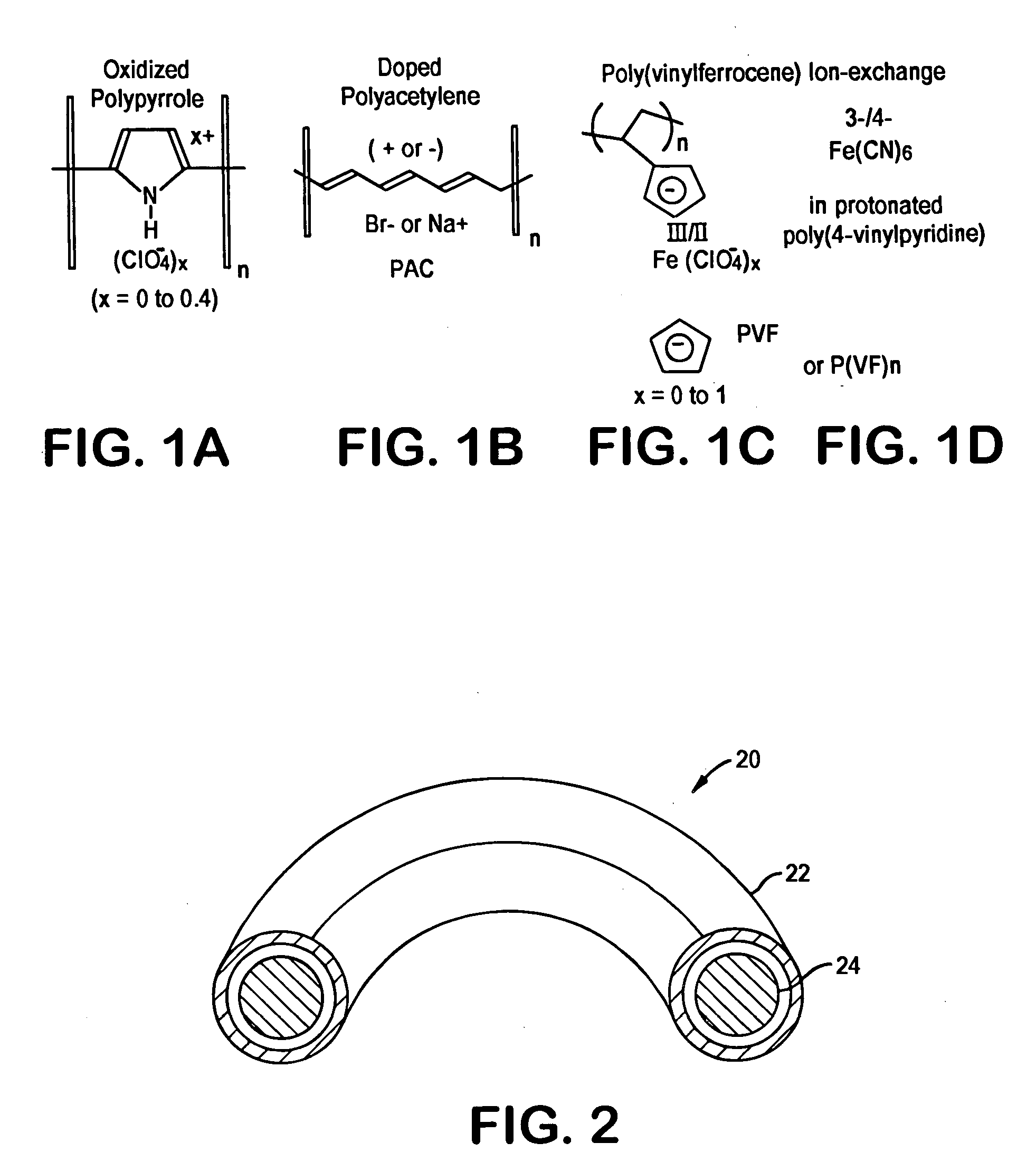
Implantable heart valve prosthetic devices having intrinsically conductive polymers
A heart valve sewing prosthesis including an intrinsically conductive polymer. The invention includes annuloplasty rings and bands, and sewing rings or cuffs for prosthetic heart valves. Some annuloplasty rings and sewing rings include fabric that is coated with an intrinsically conductive polymer. The coating can be formed over individual filaments or fibers, or on the fabric surface as a surface layer. One intrinsically conductive polymer is polypyrrole. The intrinsically conductive polymer can be doped to facilitate the intrinsic conductivity. Some devices have a polypyrrole surface layer doped with dialkyl-napthalene sulfonate. The intrinsically conductive polymer can be deposited on a fabric using in-situ polymerization of monomeric or oligomeric species, together with a dopant. Animal studies using implanted annuloplasty rings having an intrinsically conductive polymer coating have demonstrated a substantial reduction in pannus formation and inflammatory response.
Owner:MEDTRONIC INC
Silver-containing, sol/gel derived bioglass compositions
Silver-containing, sol-gel derived bioactive glass compositions and methods of preparation and use thereof are disclosed. The compositions can be in the form of particles, fibers and / or coatings, among other possible forms, and can be used, for example, for treating wounds, improving the success of skin grafts, reducing the inflammatory response and providing anti-bacterial treatments to a patient in need thereof. Anti-bacterial properties can be imparted to implanted materials, such as prosthetic implants, sutures, stents, screws, plates, tubes, and the like, by incorporating the compositions into or onto the implanted materials. The compositions can also be used to prepare devices used for in vitro and ex vivo cell culture.
Owner:IMPERIAL INNOVATIONS LTD
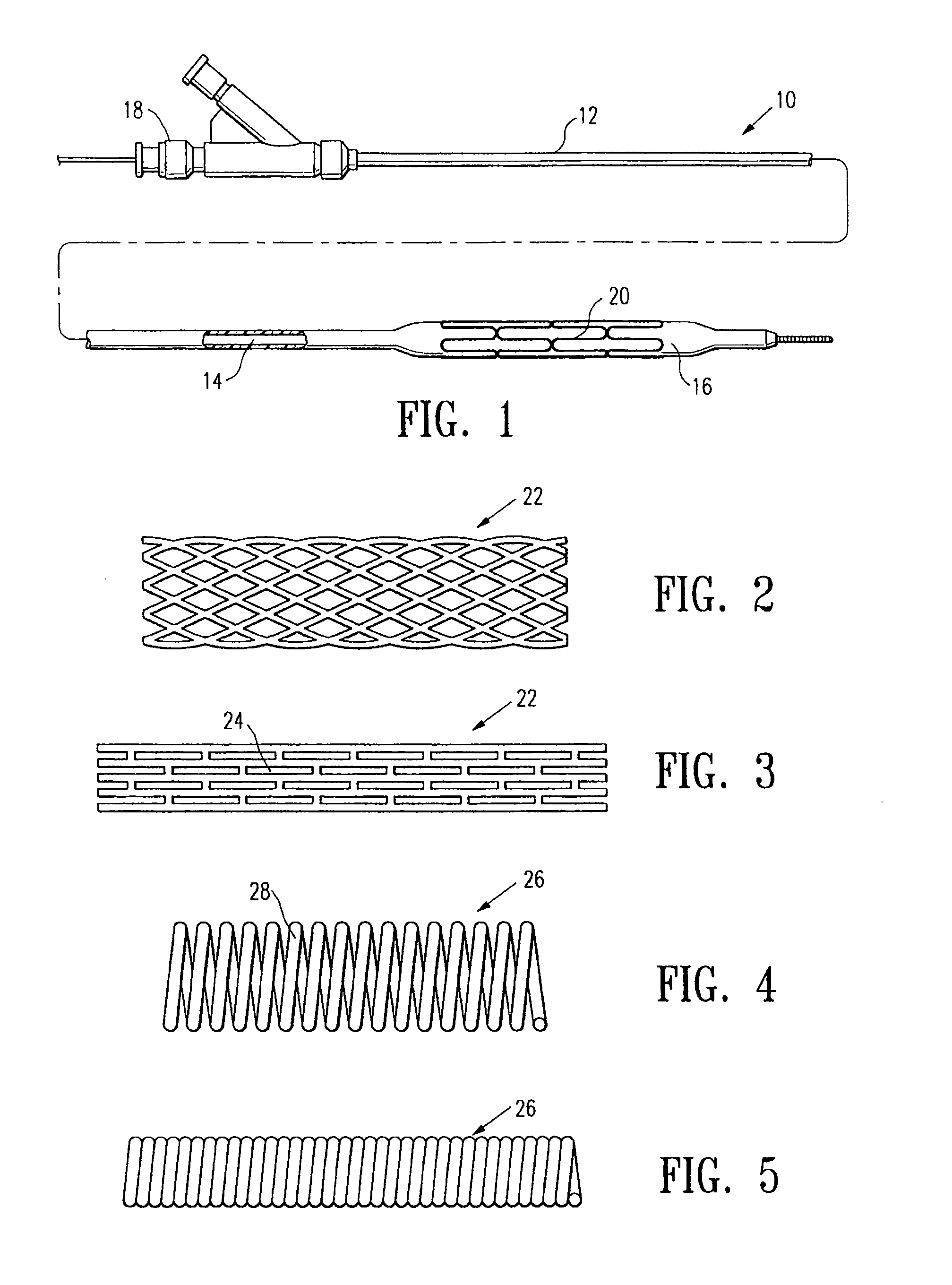
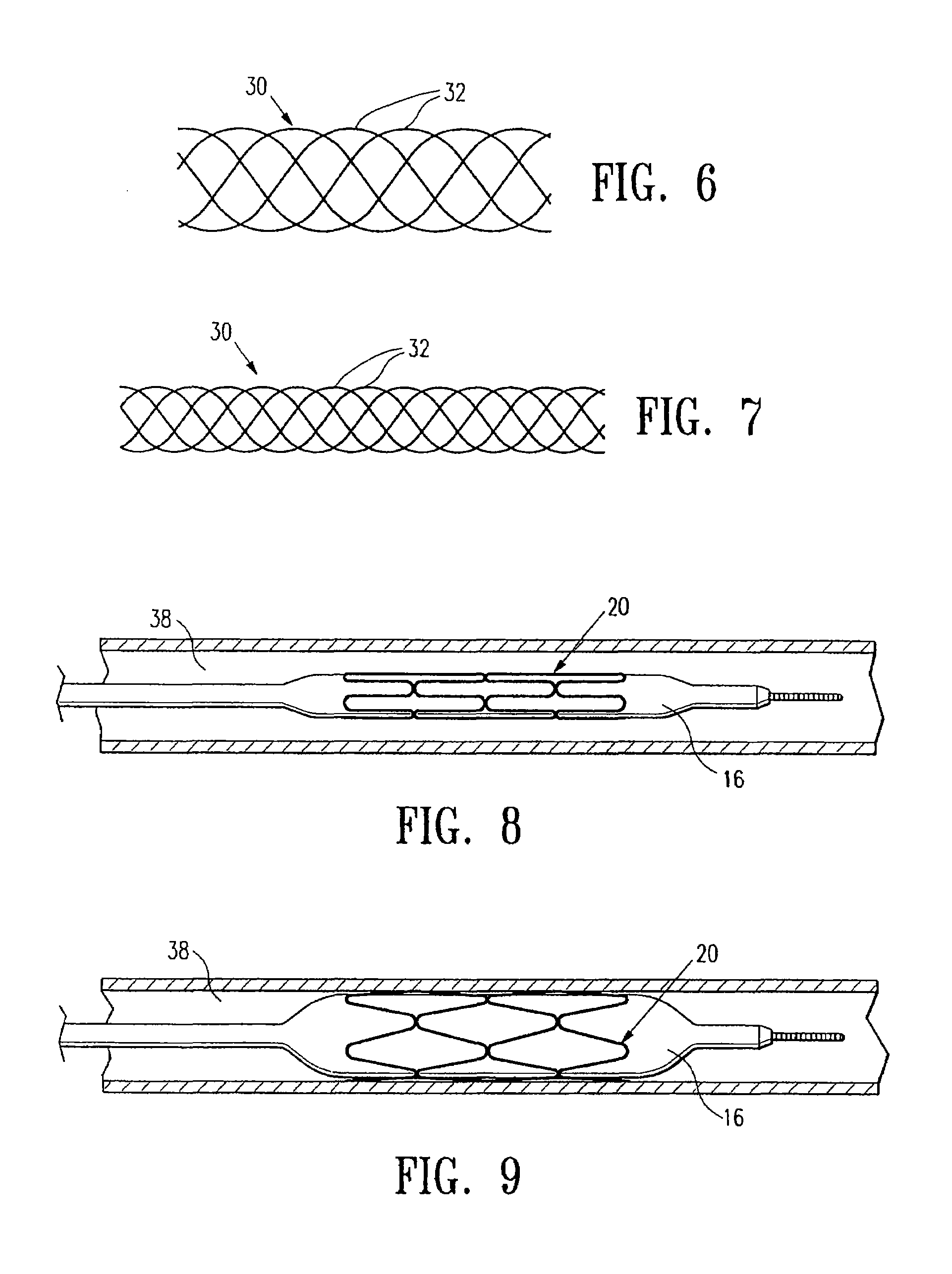
Biodegradable polymer coils for intraluminal implants
An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location of a separable coil comprised at least in part of at least one biocompatible and absorbable polymer or protein and growth factors. Typically a catheter associated with the separable coil is used to dispose the coil into a selected body lumen. The biocompatible and absorbable polymer or protein is thrombogenic. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and absorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides. The biocompatible and absorbable protein is at least one protein selected from the group consisting of collagen, fibrinogen, fibronectin, vitronectin, laminin, and gelatin. In one embodiment the coil is composed of the biocompatible and absorbable polymer or protein with a radio-opaque material is disposed thereon. Alternatively, the coil is composed of a radio-opaque material, and the biocompatible and absorbable polymer or protein is disposed thereon. This apparatus may be positioned within intracranial aneurysms or any aneurysm in the body as well as within other body cavities.
Owner:RGT UNIV OF CALIFORNIA
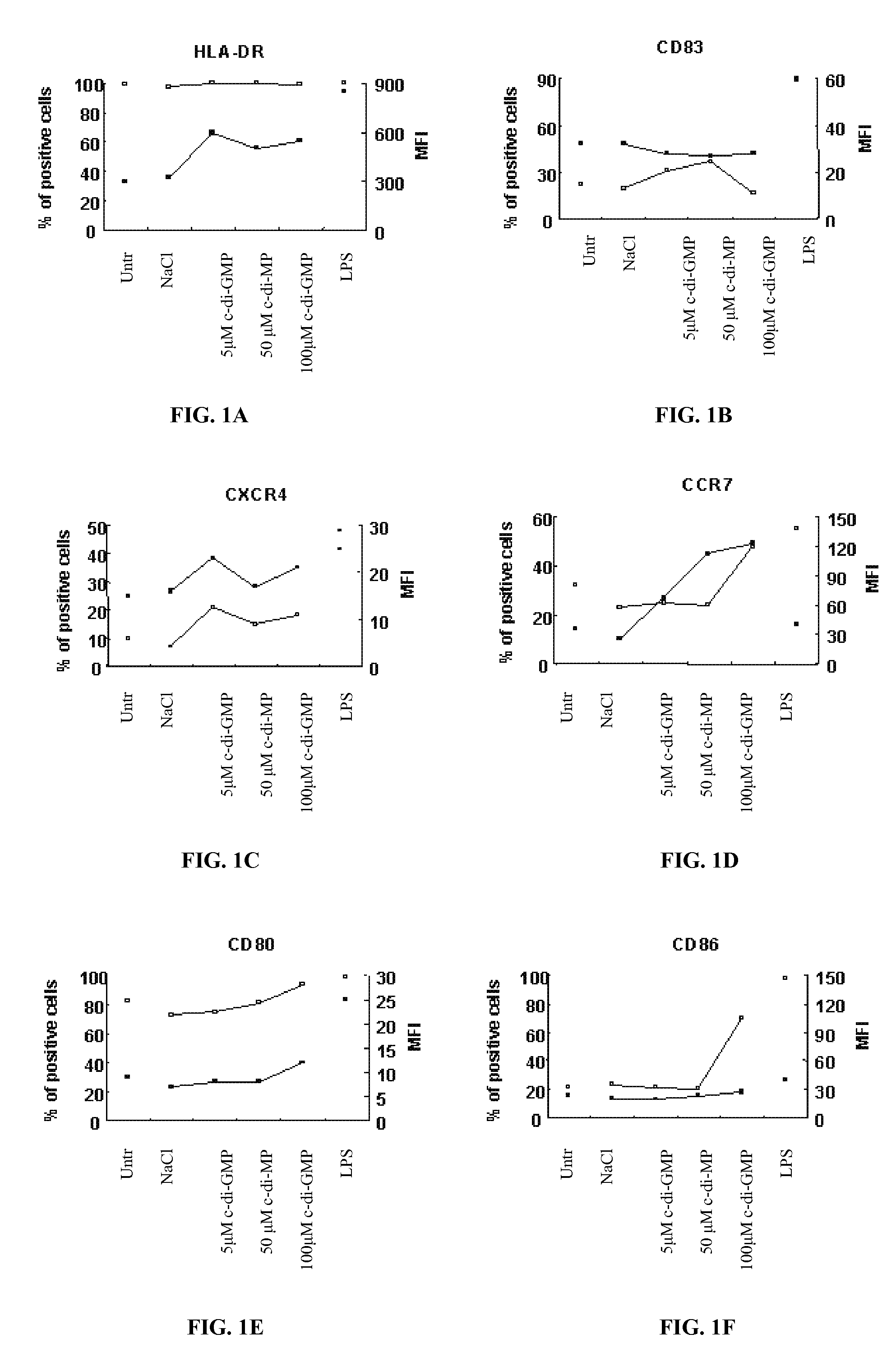
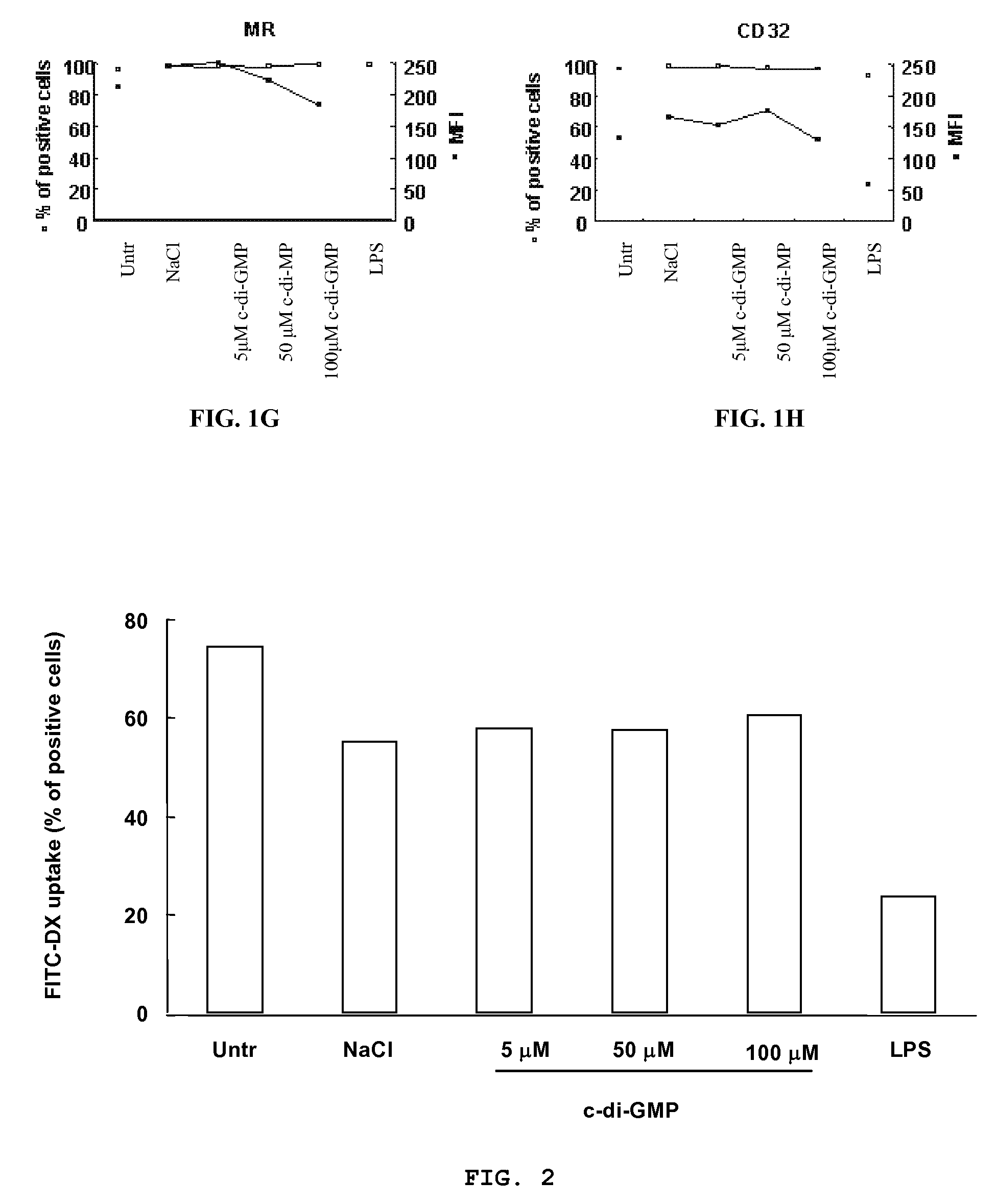
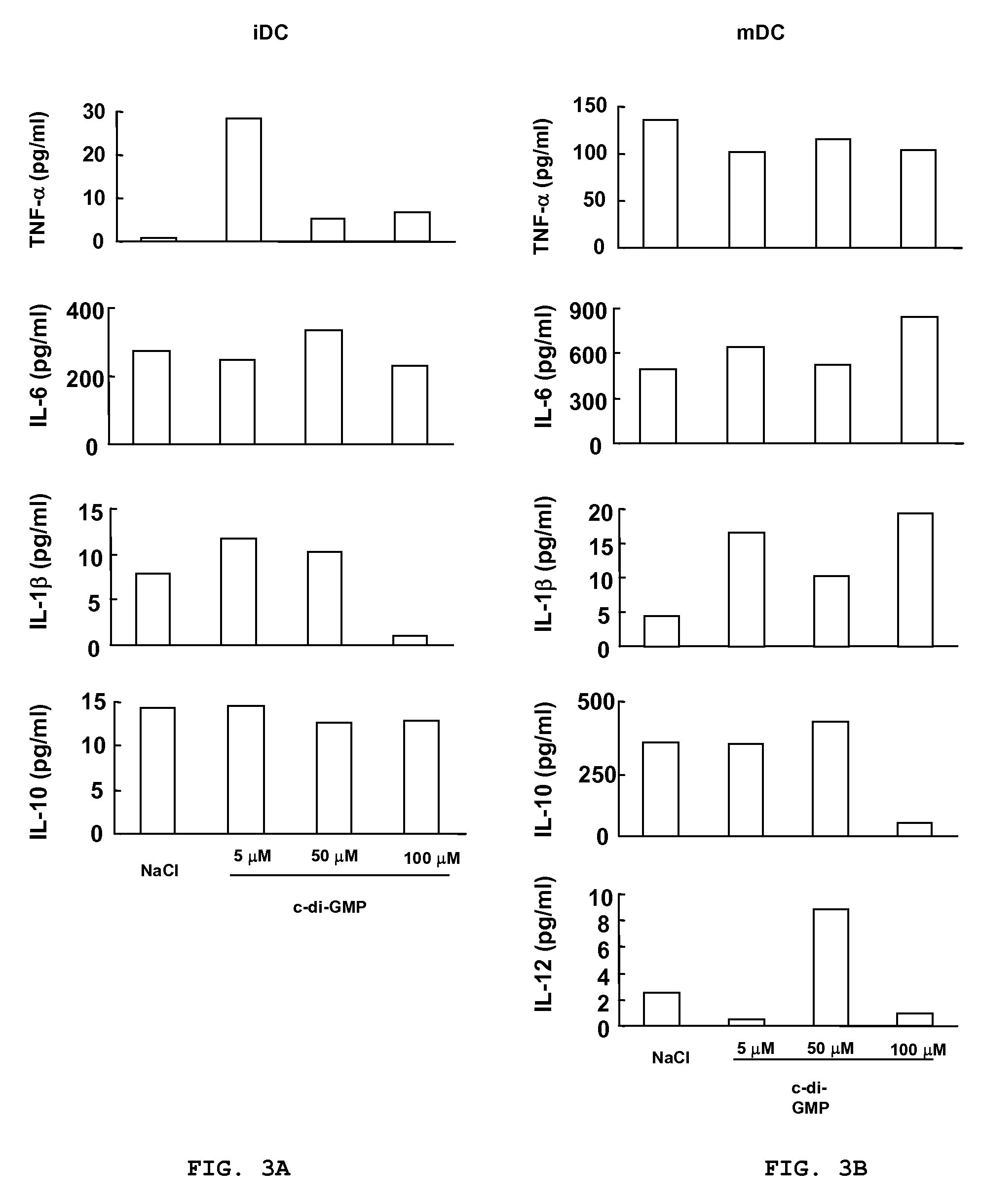
Method for stimulating the immune, inflammatory or neuroprotective response
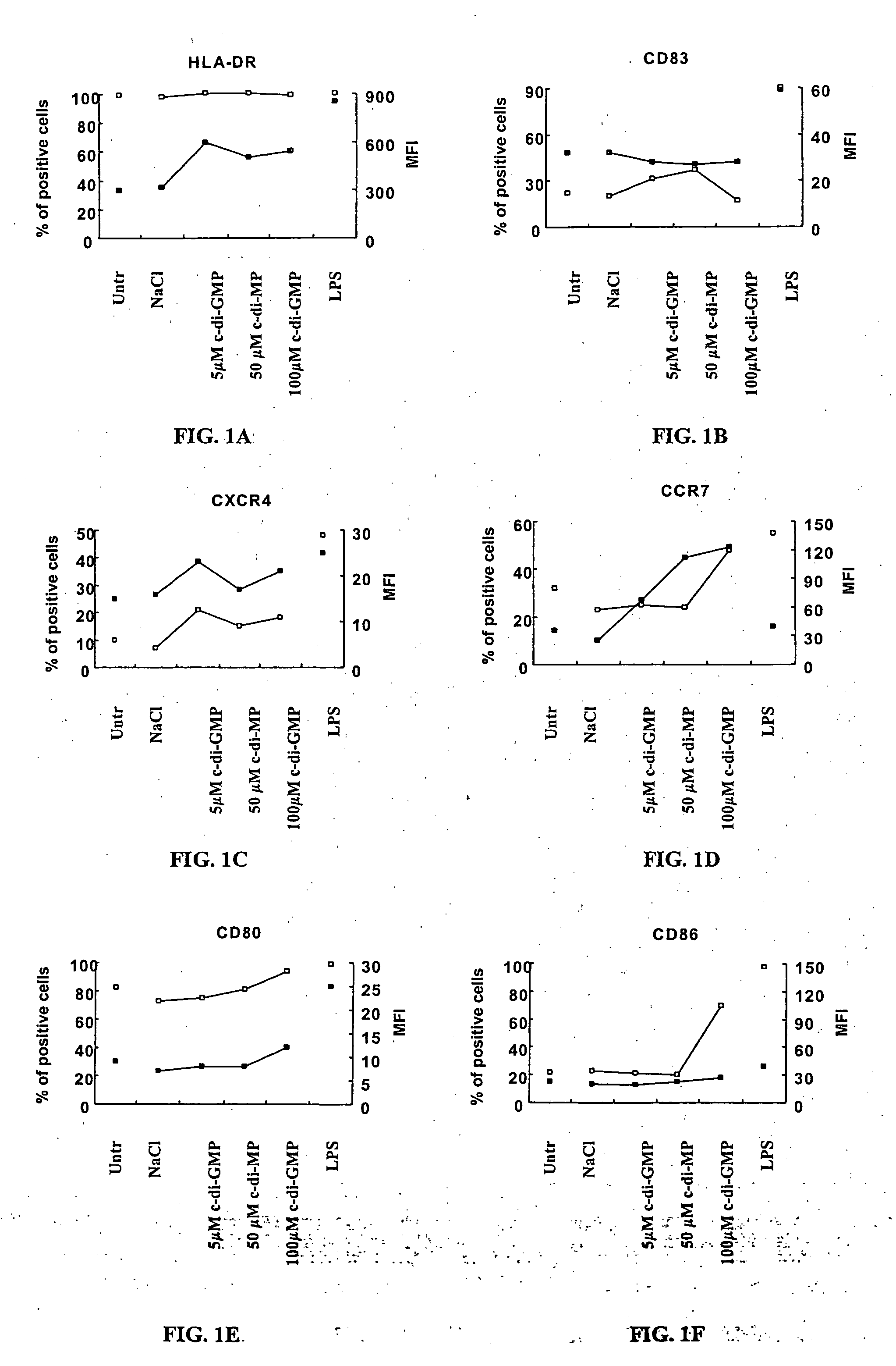
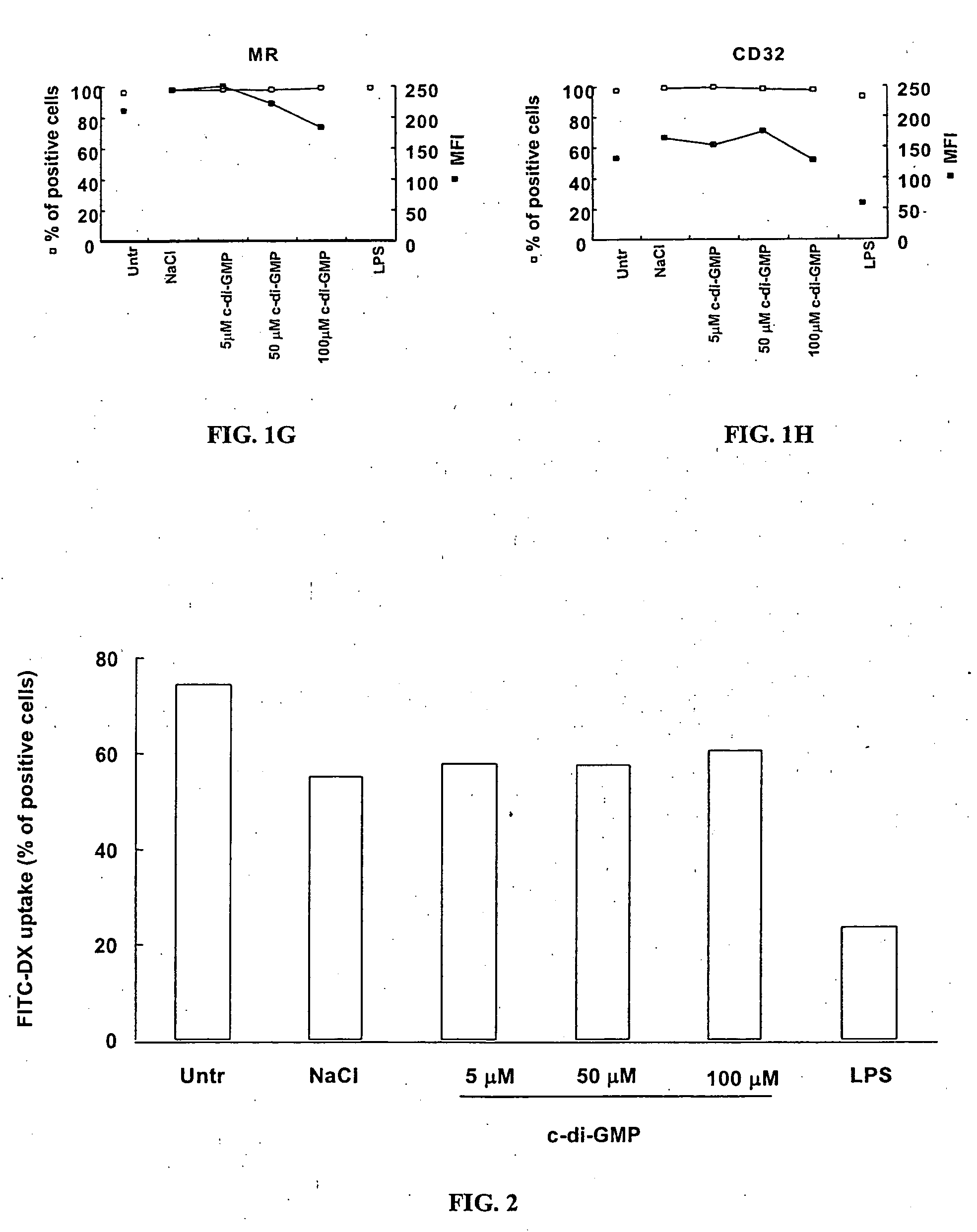
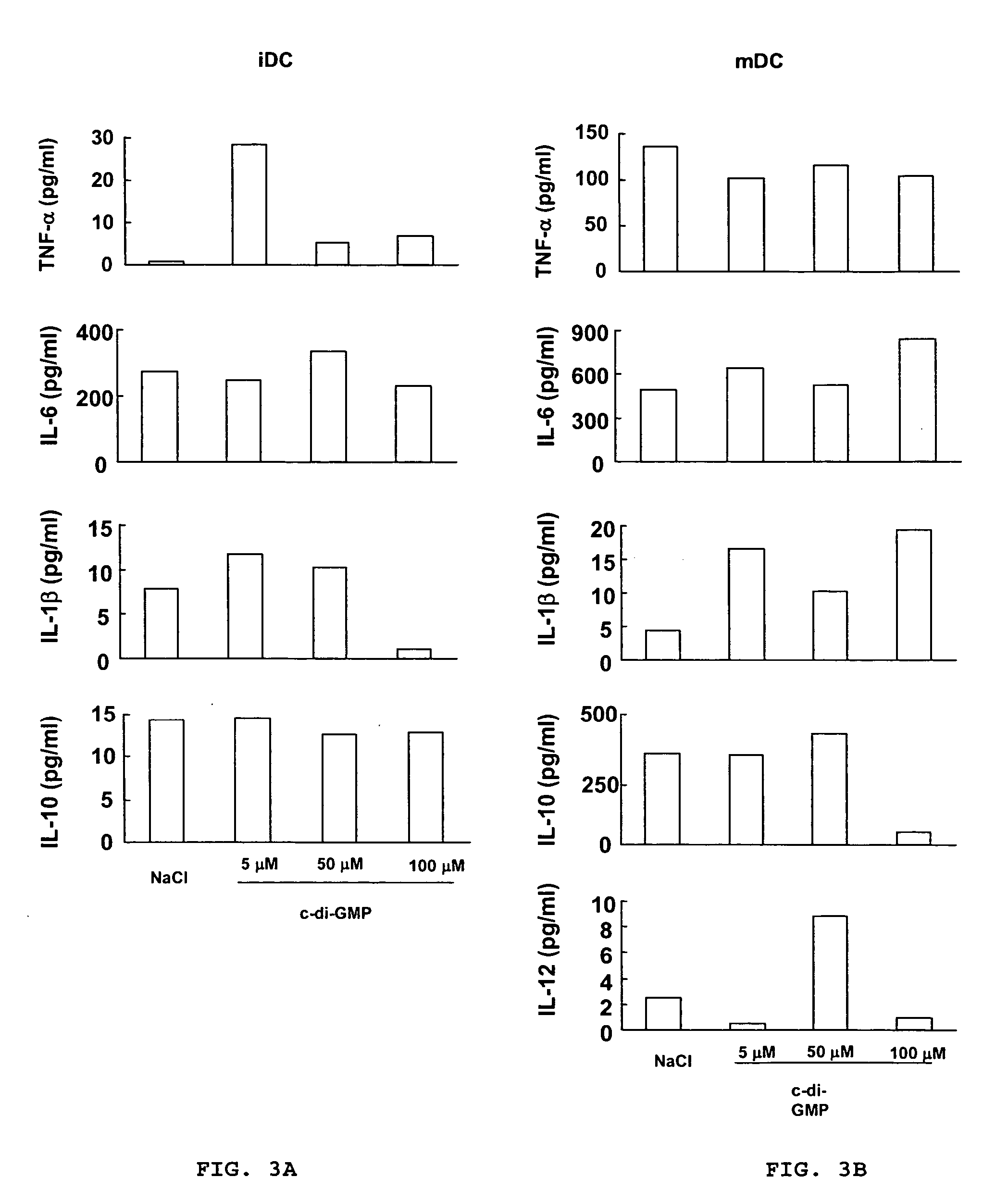
Cycic di-GMP, or a cyclic dinucleotide analogue thereof that has the same effect as cyclic di-GMP, stimulates or enhances immune or inflammatory response in a patient or enhances the immune response to a vaccine by serving as an adjuvant. Cyclic di-GMP, or a cyclic dinucleotide analogue thereof, also has neuroprotective properties for use as a neuroprotective agent to inhibit, treat, or ameliorate the effects of injuries, diseases,
Owner:KARAGEN PHARMA INC +1
Bioprosthetic tissue preparation with synthetic hydrogels
ActiveUS20050119736A1Reduce calcificationReduce stiffnessSuture equipmentsHeart valvesCross-linkIn situ polymerization
Methods for treating xenogenic tissue for implantation into a human body including in-situ polymerization of a hydrogel polymer in tissue, and tissue treated according to those methods, where the polymerization takes place in tissue that has not been fixed with glutaraldehyde. The polymerization may only fill the tissue, bind the polymer to the tissue, or cross-link the tissue through the polymer, depending on the embodiment. One method includes free radical polymerization of a first vinylic compound, and can include cross-linking through use of a second compound having at least two vinyl groups. Another method utilizes nucleophilic addition polymerization of two compounds, one of which can include PEG and can further include hydrolytically degradable regions. In one embodiment, applicants believe the in-situ polymerization inhibits calcification, and that the polymerization of tissue un-fixed by glutaraldehyde allows for improved penetration of the polymer. The methods find one use in the treatment of porcine heart valve tissue, intended to extend the useful life of the valves by inhibiting calcification. The incorporation of degradable hydrogel regions may initially fill the tissue and reduce any initial inflammatory response, but allow for later infiltration by cells to remodel the tissue.
Owner:MEDTRONIC INC
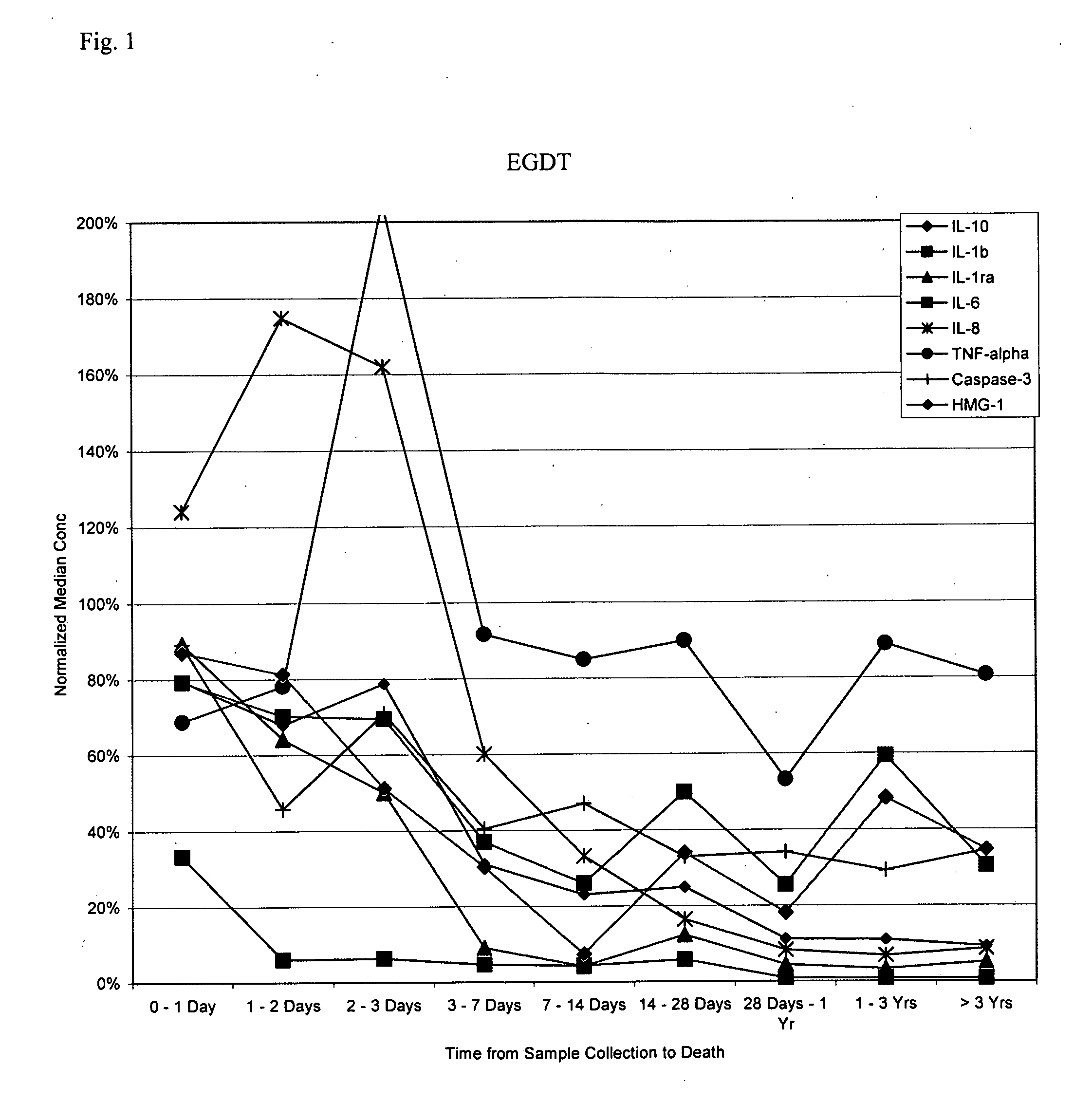
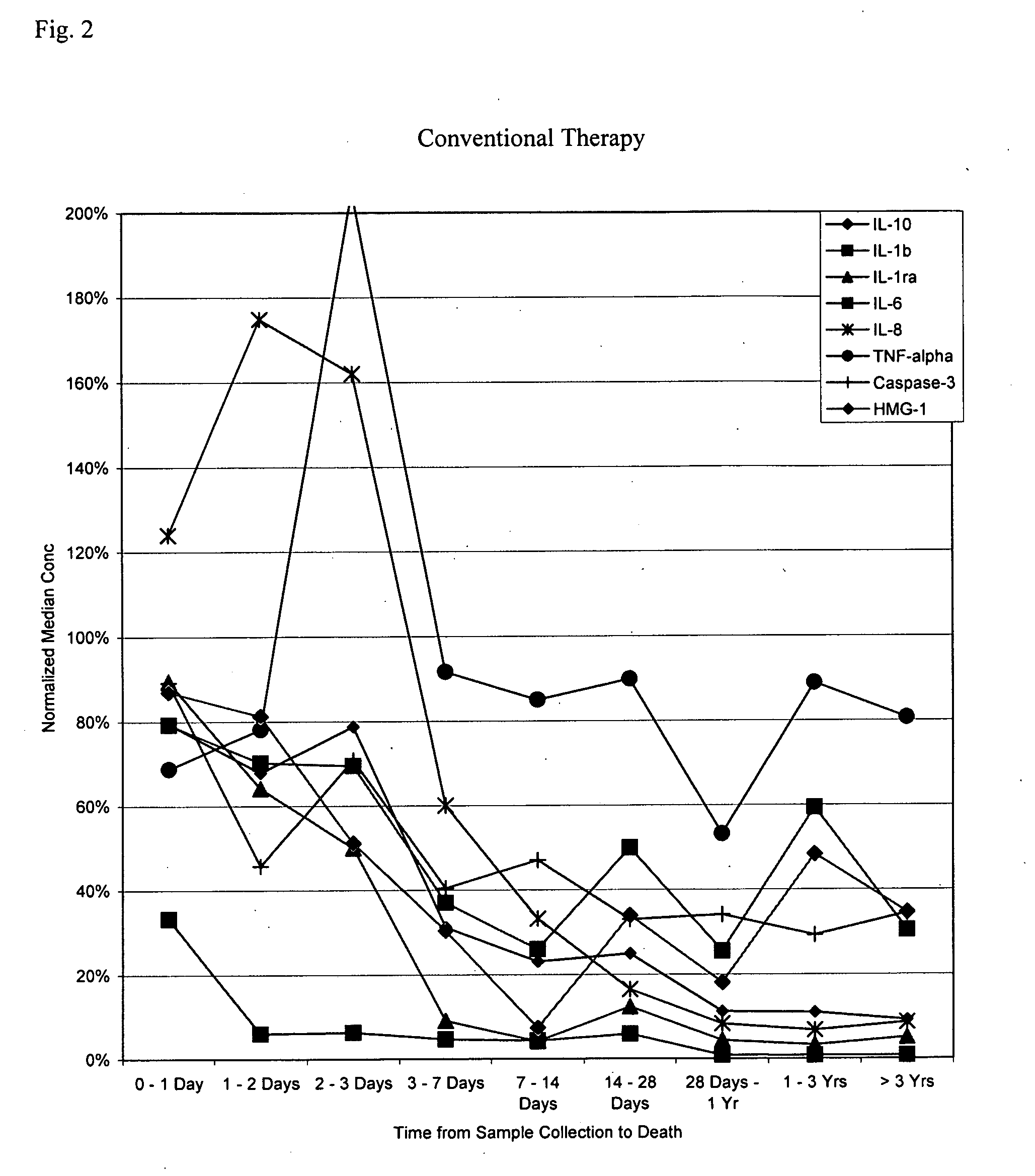
Methods and compositions for determining treatment regimens in systemic inflammatory response syndromes
InactiveUS20050148029A1Improve discriminationMicrobiological testing/measurementDisease diagnosisMedicineWhole body
The present invention relates to methods and compositions for symptom-based differential diagnosis, prognosis, and determination of treatment regimens in subjects. In particular, the invention relates to methods and compositions selected to rule in or out SIRS, or for differentiating sepsis, severe sepsis, septic shock and / or MODS from each other and / or from non-infectious SIRS.
Owner:BIOSITE INC
Method of biochemical treatment of persistent pain
InactiveUS20050152905A1Reduce releaseAvoid exposureBiocidePeptide/protein ingredientsInterleukin 6Interleukin-1beta
This invention relates to a method for the biochemical treatment of persistent pain disorders by inhibiting the biochemical mediators of inflammation in a subject comprising administering to said subject any one of several combinations of components that are inhibitors of biochemical mediators of inflammation. Said process for biochemical treatment of persistent pain disorders is based on Sota Omoigui's Law, which states: ‘The origin of all pain is inflammation and the inflammatory response’. Sota Omoigui's Law of Pain unifies all pain syndromes as sharing a common origin of inflammation and the inflammatory response. The various biochemical mediators of inflammation are present in differing amounts in all pain syndromes and are responsible for the pain experience. Classification and treatment of pain syndromes should depend on the complex inflammatory profile. A variety of mediators are generated by tissue injury and inflammation. These include substances produced by damaged tissue, substances of vascular origin as well as substances released by nerve fibers themselves, sympathetic fibers and various immune cells. Biochemical mediators of inflammation that are targeted for inhibition include but are not limited to: prostaglandin, nitric oxide, tumor necrosis factor alpha, interleukin 1-alpha, interleukin 1-beta, interleukin-4, Interleukin-6 and interleukin-8, histamine and serotonin, substance P, Matrix Metallo-Proteinase, calcitonin gene-related peptide, vasoactive intestinal peptide as well as the potent inflammatory mediator peptide proteins neurokinin A, bradykinin, kallidin and T-kinin.
Owner:OMOIGUI OSEMWOTA SOTA
Compositions and methods for treating insulin resistance and diabetes mellitus
Provided are electrokinetically-altered fluids (gas-enriched electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures in an amount sufficient to provide modulation of at least one of cellular membrane potential and cellular membrane conductivity, and therapeutic compositions and methods for use in treating diabetes and diabetes-associated conditions or disorders (e.g., insulin resistance), or symptoms thereof. Provided are electrokinetically-altered ioinic aqueous fluids optionally in combination with other therapeutic agents. Particular aspects provide for regulating or modulating intracellular signal transduction associated with said inflammatory responses by modulation of at least one of cellular membranes, membrane potential, membrane proteins such as membrane receptors, including but not limited to G-Protein Coupled Receptors (GPCR), and intercellular junctions (e.g., tight junctions, gap junctions, zona adherins and desmasomes). Other embodiments include particular routes of administration or formulations for the electrokinetically-altered fluids (e.g., electrokinetically-altered gas-enriched fluids and solutions) and therapeutic compositions.
Owner:REVALESIO CORP
Controlled and directed local delivery of anti-inflammatory compositions
InactiveUS20060046961A1Relieve painLimiting bone lossOrganic active ingredientsPeptide/protein ingredientsSkeletal injuryControl release
The invention provides a method for alleviating pain associated with neuromuscular or skeletal injury or inflammation by controlled and directed delivery of one or more biological response modifiers to inhibit the inflammatory response which ultimately causes acute or chronic pain. Controlled and directed delivery can be provided by implantable or infusion pumps, implantable controlled release devices, or by sustained release compositions comprising biological response modifiers.
Owner:SDGI HLDG
Methods of wound care and treatment
InactiveUS20100021464A1Reduce scarsImprove the level ofBiocideSenses disorderCell membraneWound care
Provided are electrokinetically-altered fluids (e.g., gas-enriched electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures in an amount sufficient to provide modulation of at least one of cellular membrane potential and cellular membrane conductivity, and therapeutic compositions and methods for use in treating a wound to a surface tissue or a symptom thereof. The electrokinetically-altered fluids or therapeutic compositions and methods include electrokinetically-altered ionic aqueous fluids optionally in combination with other therapeutic agents. Particular aspects provide for regulating or modulating intracellular signal transduction associated with said inflammatory responses by modulation of at least one of cellular membranes, membrane potential, membrane proteins such as membrane receptors, including but not limited to G-Protein Coupled Receptors (GPCR), and intercellular junctions (e.g., tight junctions, gap junctions, zona adherins and desmasomes). Other embodiments include particular routes of administration or formulations for the electrokinetically-altered fluids (e.g., electrokinetically-altered gas-enriched fluids and solutions) and therapeutic compositions.
Owner:REVALESIO CORP
Contraceptive system and method of use
InactiveUS7073504B2Obstruct passageImprove protectionMale contraceptivesFallopian occludersGynecologyBalloon catheter
A device and method of using the device for contraception or sterilization and particularly for reversible contraception by occluding a reproductive lumen to prevent the passage of reproductive cells through the lumen for a desired period of time until the patient wishes to become fertile again and then be reopened. The occluding member preferably comprises a tubular framework formed from a shape memory material configured to be implanted in a reproductive lumen. The occluding member is implanted within a body lumen, secured to the wall of the reproductive lumen and then collapsed to collapse the wall and occlude the lumen. Alternatively, the occluding member may be collapsed upon a solid plug. The closure of the reproductive lumen may be reversed by introducing a balloon catheter and by a series of inflations of the balloon reexpanding the collapsed occluding member or by removing the plug. The occluding member and the plug may be configured to facilitate endothelialization, to provoke an inflammatory responses or to deliver a drug.
Owner:BAYER HEALTHCARE LLC
Modified starch material of biocompatible hemostasis
InactiveUS20090062233A1Promoting tissue healingGood effectPowder deliveryBiocideAfter treatmentWound surface
A modified starch material for biocompatible hemostasis, biocompatible adhesion prevention, tissue healing promotion, absorbable surgical wound sealing and tissue bonding, when applied as a biocompatible modified starch to the tissue of animals. The modified starch material produces hemostasis, reduces bleeding of the wound, extravasation of blood and tissue exudation, preserves the wound surface or the wound in relative wetness or dryness, inhibits the growth of bacteria and inflammatory response, minimizes tissue inflammation, and relieves patient pain. Any excess modified starch not involved in hemostatic activity is readily dissolved and rinsed away through saline irrigation during operation. After treatment of surgical wounds, combat wounds, trauma and emergency wounds, the modified starch hemostatic material is rapidly absorbed by the body without the complications associated with gauze and bandage removal.
Owner:JI XIN
Biocompatible hemostatic, antiblocking, healing-promoting and surgical wound-closing modified starch material
InactiveCN101485897AMaterials that promote healingPromote repairOrganic active ingredientsSurgical adhesivesTissue fluidBandage
The invention relates to a modified starch material for biocompatible hemostasis, biocompatible anti-adhesion, tissue healing promotion, absorbable surgery sealing and tissue adhesion. The invention applies biocompatible modified starch to animal tissues, wherein the modified starch material has the molecular weight of more than 15,000 daltons, and the particle diameter of between 1 and 1,000 mu m. The modified starch hemostatic material has stypticity, reduces hemorrhage, blood oozing and tissue fluid oozing of wounds, maintains relative moistening or drying of wound surfaces or the wounds, inhibits bacterium growth and inflammation reaction, and contributes to locally diminish inflammation of the wounds and relieve pain of patients. Moreover, the modified starch material can wash local parts after operation is over and remove redundant modified starch which does not participate in hemostasis, and can easily remove haemostatic under the condition of debridement treatment after self-help and first-aid treatment of war wounds; and hemostatic materials with a small amount of modified starch can be absorbed by the body, so that the pain of tearing of gauzes and bandages on people is avoided.
Owner:BEIJING UNIVERSAL LIKANG TECH CO LTD
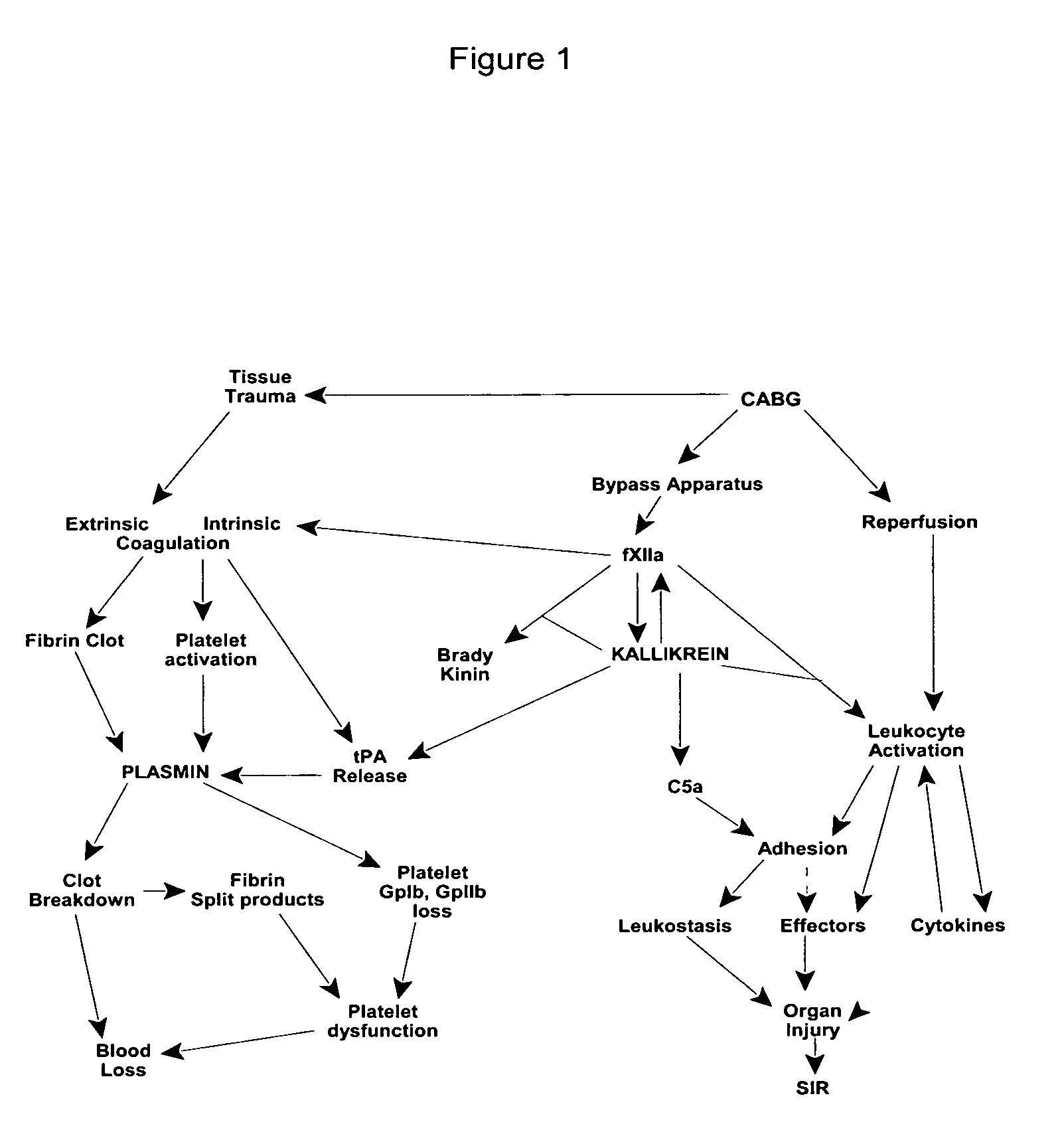
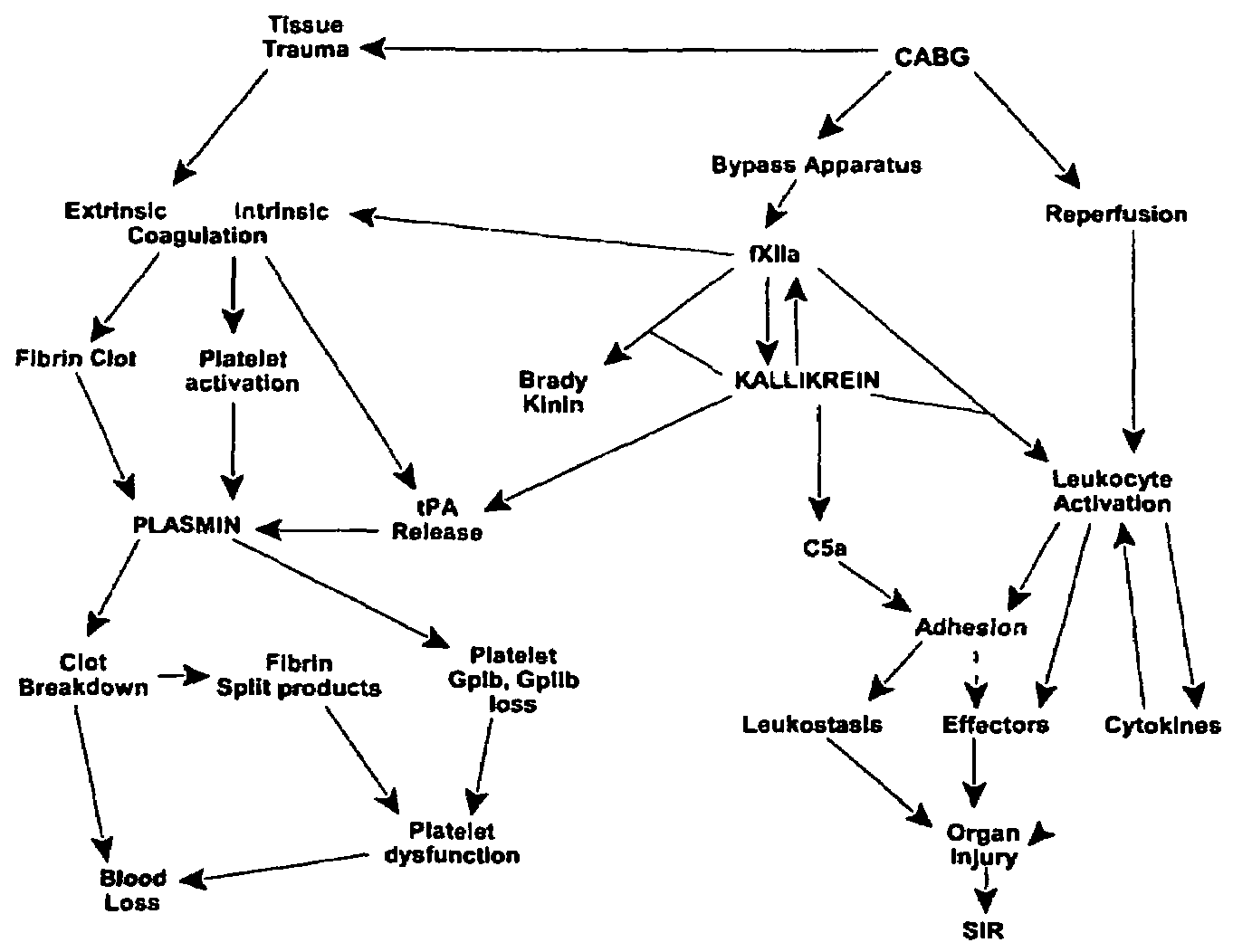
Prevention and reduction of blood loss
InactiveUS7064107B2Preventing and reducing onsetReduce and preventNervous disorderHydrolasesWhole bodySurgical department
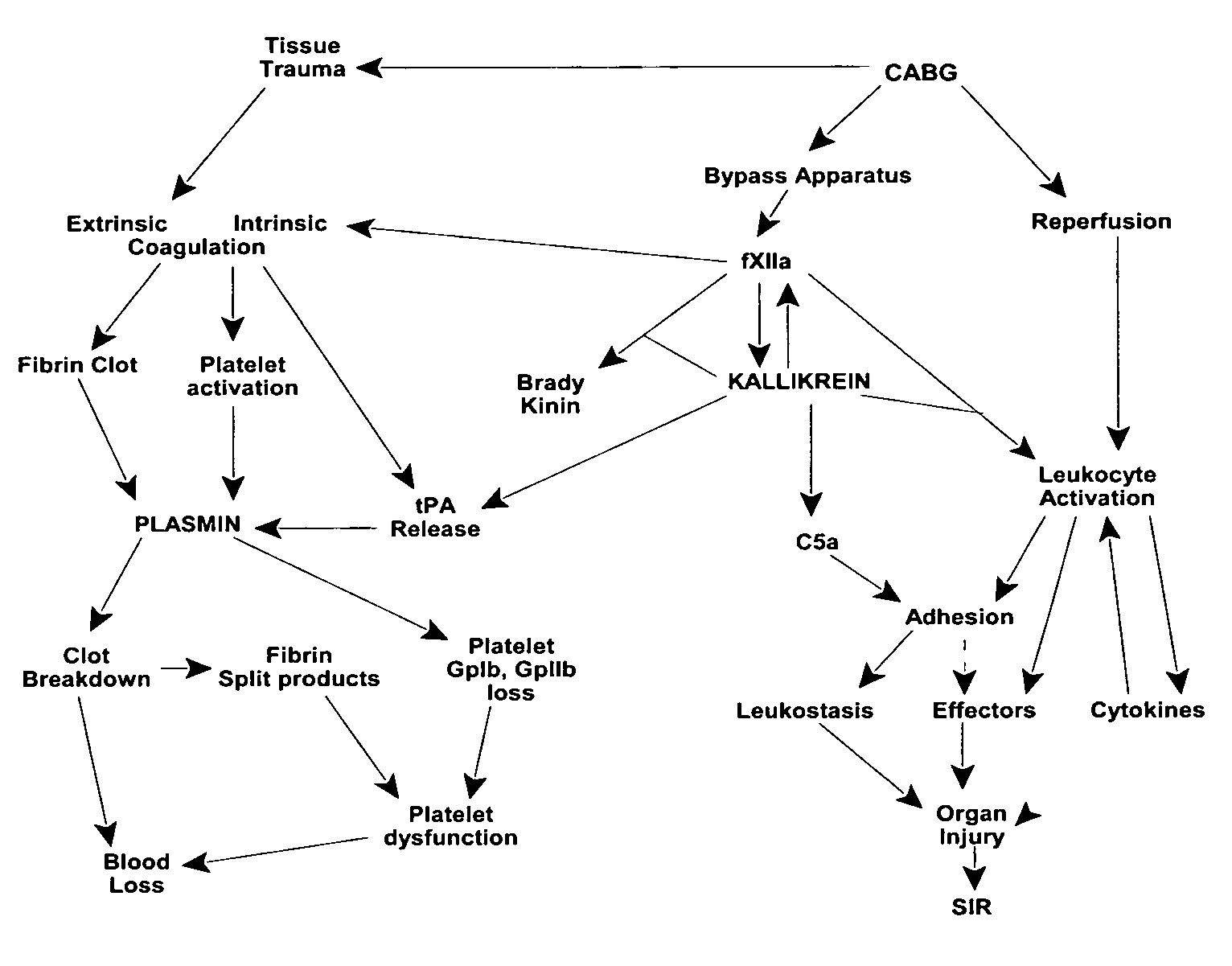
Methods are described for preventing or reducing ischemia and / or systemic inflammatory response in a patient such as perioperative blood loss and / or systemic inflammatory response in a patient subjected to cardiothoracic surgery, e.g., coronary artery bypass grafting and other surgical procedures, especially when such procedures involve extra-corporeal circulation, such as cardiopulmonary bypass.
Owner:TAKEDA PHARMA CO LTD
Barrier layer
Owner:ATRIUM MEDICAL
Prevention and reduction of blood loss
Methods are described for preventing or reducing ischemia and / or systemic inflammatory response in a patient such as perioperative blood loss and / or systemic inflammatory response in a patient subjected to cardiothoracic surgery, e.g. coronary artery bypass grafting and other surgical procedures, especially when such procedures involve extra-corporeal circulation, such as cardiopulmonary bypass.
Owner:TAKEDA PHARMA CO LTD
Methods and compositions for diagnosis and /or prognosis in systemic inflammatory response syndromes
InactiveUS20070092911A1Improve discriminationDisease diagnosisBiological testingWhole bodyDifferential diagnosis
The present invention relates to methods and compositions for symptom-based differential diagnosis, prognosis, and determination of treatment regimens in subjects. In particular, the invention relates to methods and compositions selected to rule in or out SIRS, or for differentiating sepsis, severe sepsis, septic shock and / or MODS from each other and / or from non-infectious SIRS.
Owner:BIOSITE INC
Processes for removing cells and cell debris from tissue and tissue constructs used in transplantation and tissue reconstruction
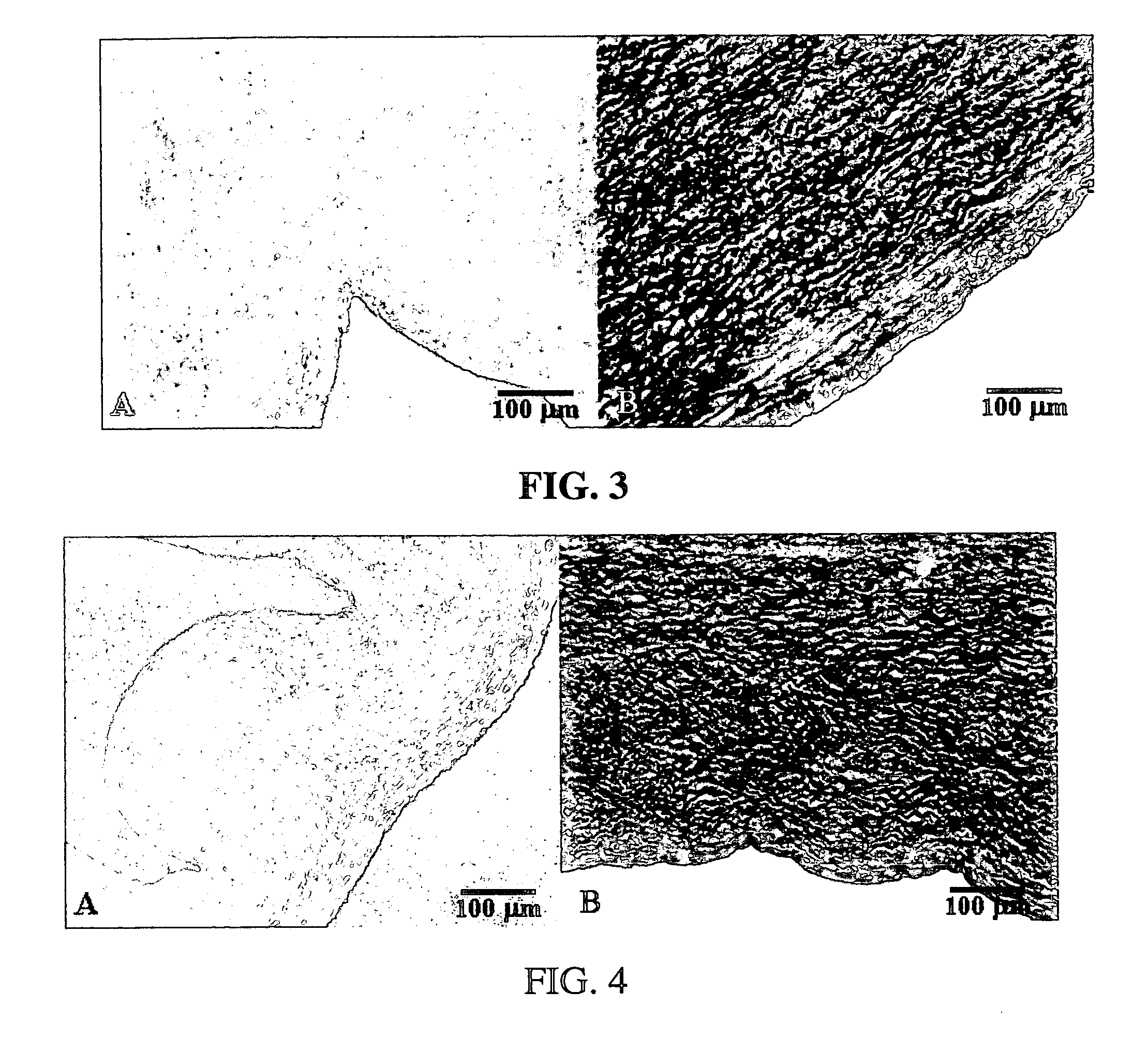
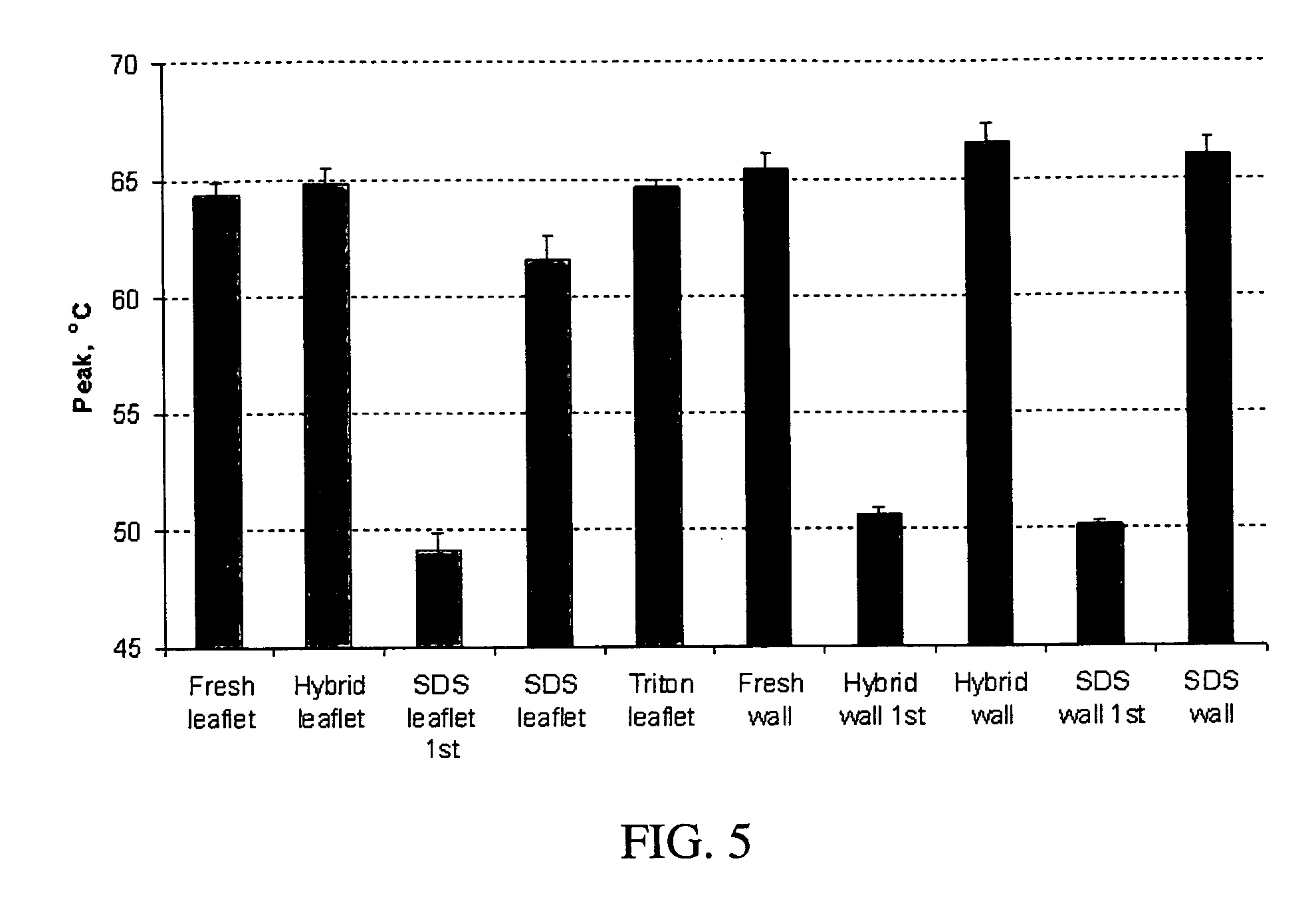
InactiveUS20070123700A1Improve inflammatory responseImprove responseHeart valvesDead animal preservationPresent methodFresh Tissue
Methods for decellularizing mammalian tissue for use in transplantation and tissue engineering. The invention includes methods for simultaneous application of an ionic detergent and a nonionic detergent for a long time period, which may exceed five days. One method utilizes SDS as the ionic detergent and Triton-X 100 as the nonionic detergent. A long rinse step follows, which may also exceed five days in length. This long duration, simultaneous extraction with two detergents produced tissue showing stress-strain curves and DSC data similar to that of fresh, unprocessed tissue. The processed tissue is largely devoid of cells, has the underlying structure essentially intact, and also shows a significantly improved inflammatory response relative to fresh tissue, even without glutaraldehyde fixation. Significantly reduced in situ calcification has also been demonstrated relative to glutaraldehyde fixed tissue. Applicants believe the ionic and non-ionic detergents may act synergistically to bind protein to the ionic detergent and may remove an ionic detergent-protein complex from the tissue using the non-ionic detergent. The present methods find one exemplary use in decellularizing porcine heart valve leaflet and wall tissue for use in transplantation.
Owner:UEDA YUICHIRO +1
Novel class of therapeutic protein based molecules
ActiveUS20050112751A1Prevent and inhibit adhesion and functionInhibitory responseAntibacterial agentsSenses disorderTherapeutic proteinViral infection
The present invention provides new compositions and methods for preventing and treating pathogen infection. In particular, the present invention provides compounds having an anchoring domain that anchors the compound to the surface of a target cell, and a therapeutic domain that can act extracellularly to prevent infection of a target cell by a pathogen, such as a virus. The present invention also comprises therapeutic compositions having sialidase activity, including protein-based compounds having sialidase catalytic domains. Compounds of the invention can be used for treating or preventing pathogen infection, and for treating and reducing allergic and inflammatory responses. The invention also provides compositions and methods for enhancing transduction of target cells by recombinant viruses. Such compositions and methods can be used in gene therapy.
Owner:ANSUN BIOPHARMA
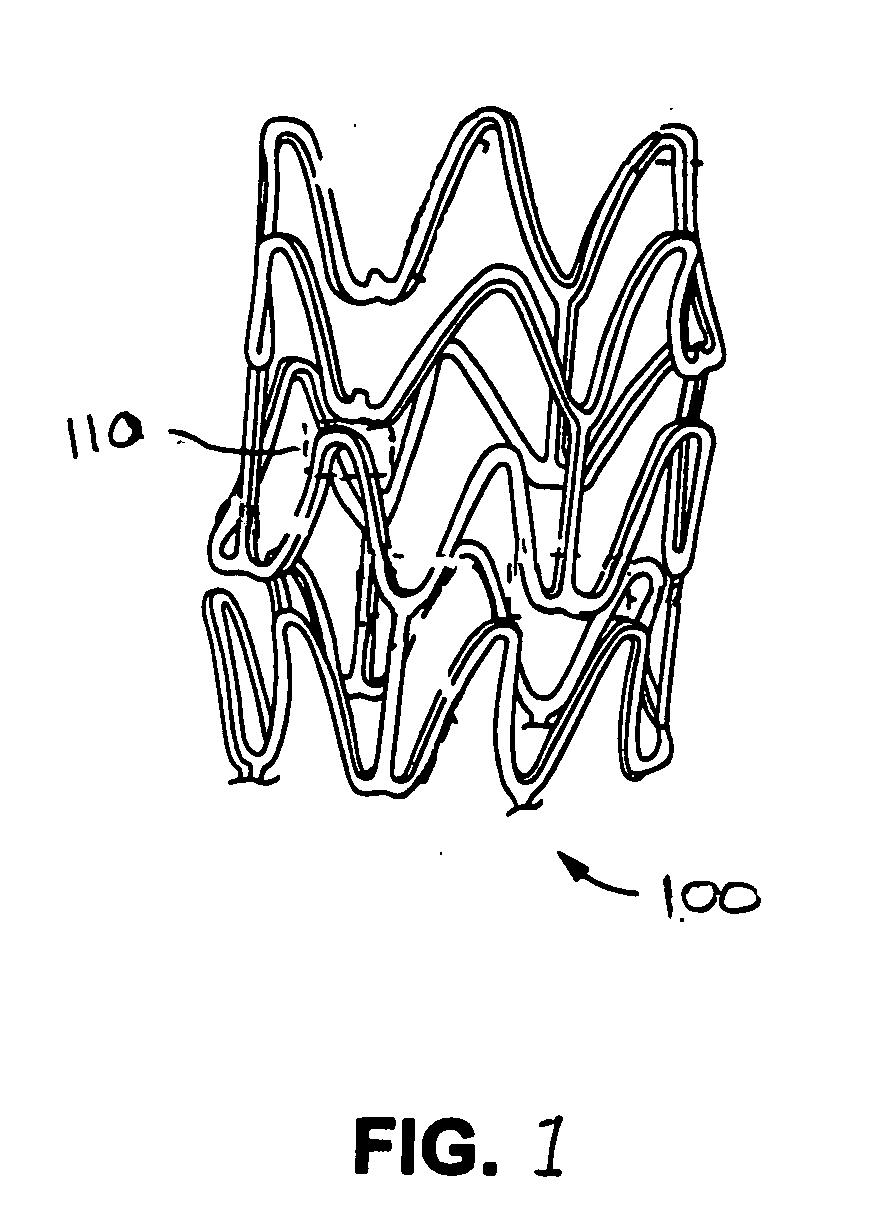
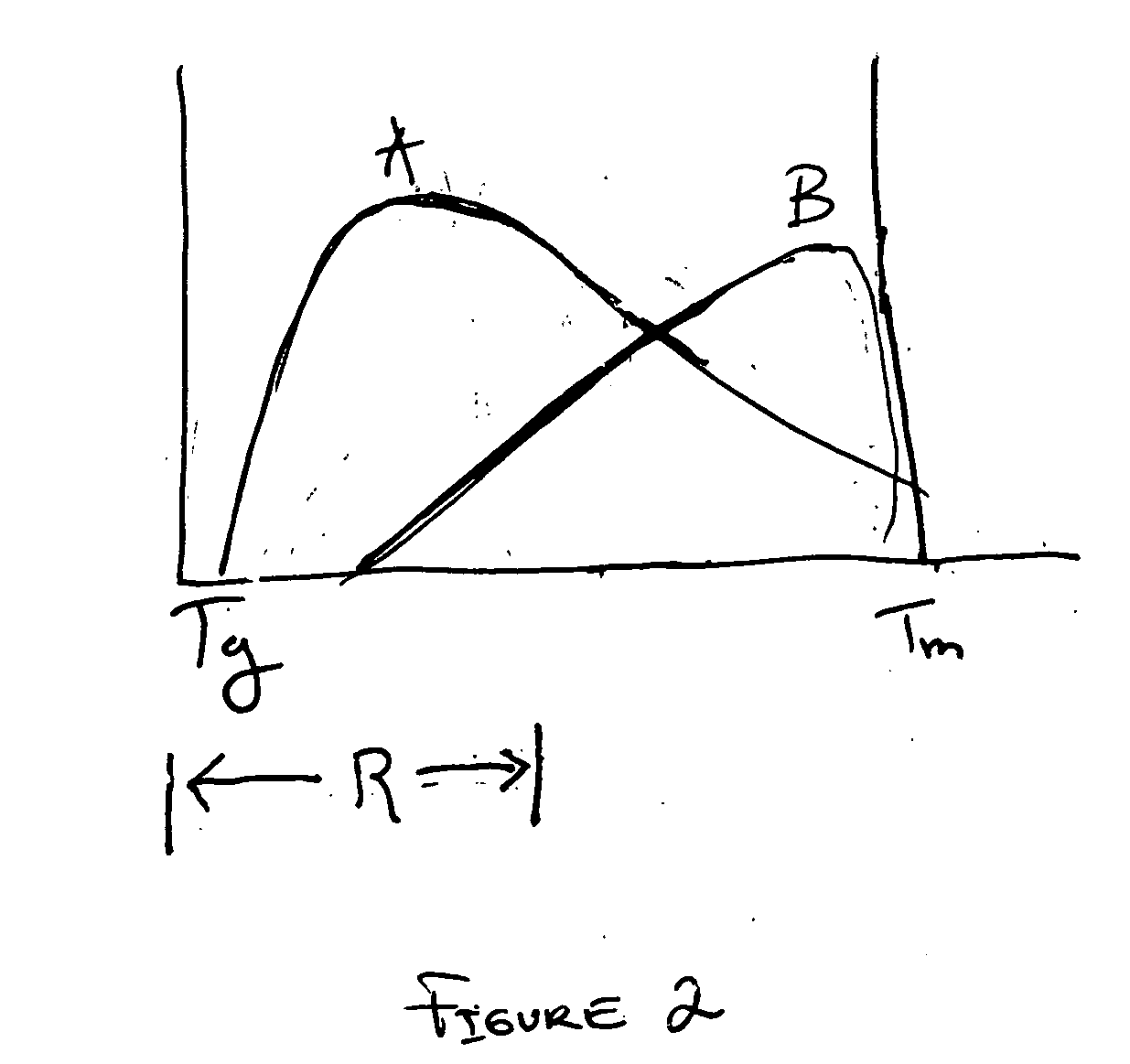
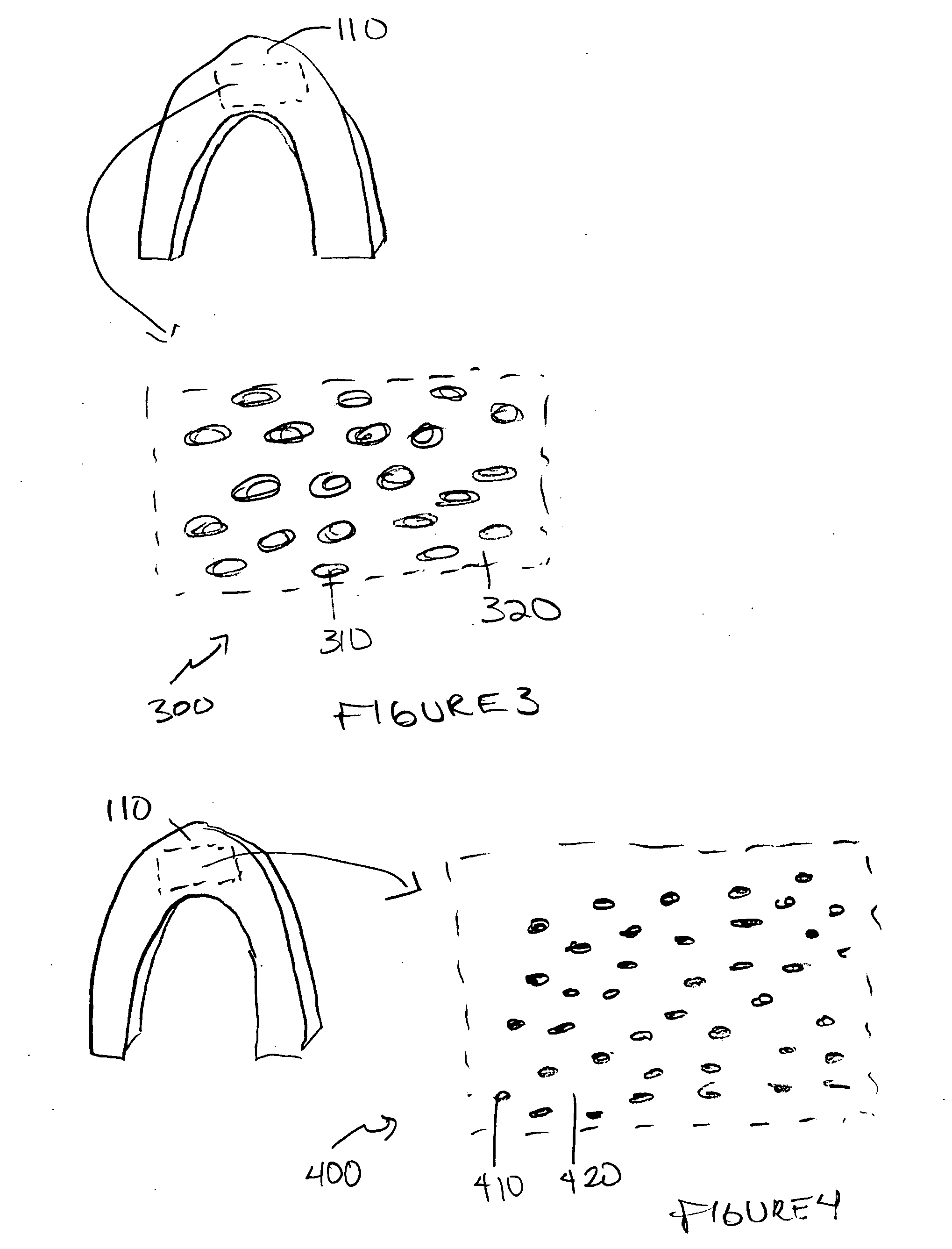
Method of fabricating an implantable medical device to reduce chance of late inflammatory response
The invention provides a method for fabricating an implantable medical device to increase biocompatibility of the device, the method comprising: heat setting a polymer construct, wherein the polymer construct is at a temperature range of from about Tg to about 0.6(Tm−Tg)+Tg such that the set polymer construct comprises a crystalline structure having crystals at a size less than about 2 microns; and fabricating an implantable medical device from the heat set polymer construct.
Owner:ABBOTT CARDIOVASCULAR
Compositions and methods for treating inflammation
Provided are electrokinetically-altered fluids (gas-enriched (e.g., oxygen-enriched) electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures in an amount sufficient to provide, upon contact with a cell, modulation of at least one of cellular membrane potential and cellular membrane conductivity, and therapeutic compositions and methods for using same in treating inflammation or at least one symptom thereof. The electrokinetically-altered fluid compositions and methods include electrokinetically-altered ionic aqueous fluids optionally in combination with other therapeutic agents. Particular aspects provide for regulating or modulating intracellular signal transduction associated with inflammatory responses by modulation of at least one of cellular membranes, membrane potential, membrane proteins such as membrane receptors, including but not limited to G-Protein Coupled Receptors (GPCR), and intercellular junctions (tight junctions, gap junctions, zona adherins and desmasomes). Other embodiments include particular routes of administration or formulations for the electrokinetically-generated fluids (electrokinetically-generated gas-enriched fluids and solutions) and therapeutic compositions.
Owner:REVALESIO CORP
Methods of wound care and treatment
InactiveUS20110081384A1Reduce scarsImprove the level ofAntibacterial agentsPowder deliveryCell membraneWound care
Provided are electrokinetically-altered fluids (e.g., gas-enriched electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures in an amount sufficient to provide modulation of at least one of cellular membrane potential and cellular membrane conductivity, and therapeutic compositions and methods for use in treating a wound to a surface tissue or a symptom thereof. The electrokinetically-altered fluids or therapeutic compositions and methods include electrokinetically-altered ionic aqueous fluids optionally in combination with other therapeutic agents. Particular aspects provide for regulating or modulating intracellular signal transduction associated with said inflammatory responses by modulation of at least one of cellular membranes, membrane potential, membrane proteins such as membrane receptors, including but not limited to G-Protein Coupled Receptors (GPCR), and intercellular junctions (e.g., tight junctions, gap junctions, zona adherins and desmasomes). Other embodiments include particular routes of administration or formulations for the electrokinetically-altered fluids (e.g., electrokinetically-altered gas-enriched fluids and solutions) and therapeutic compositions.
Owner:REVALESIO CORP
Methods and compositions for improved non-viral gene therapy
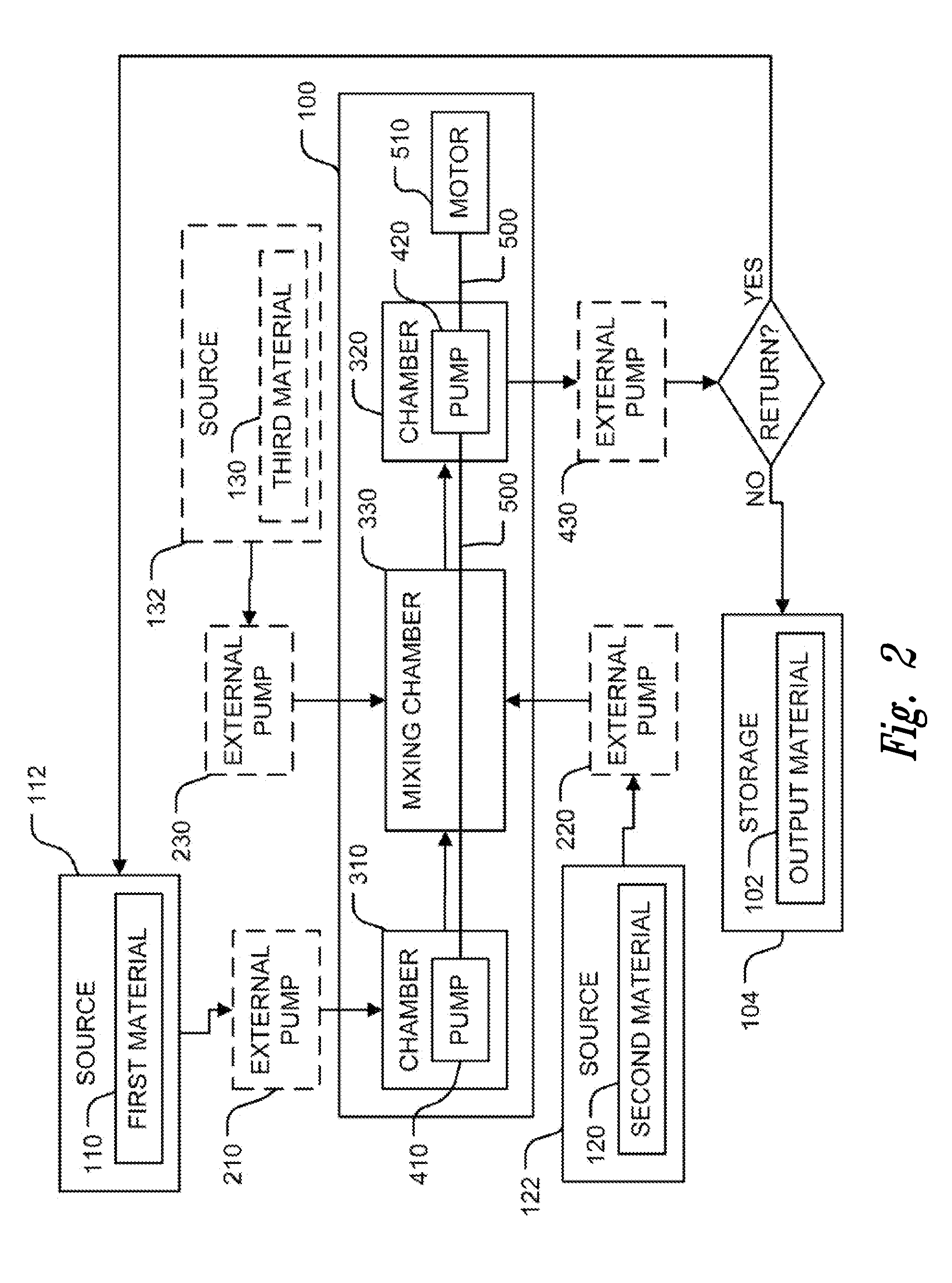
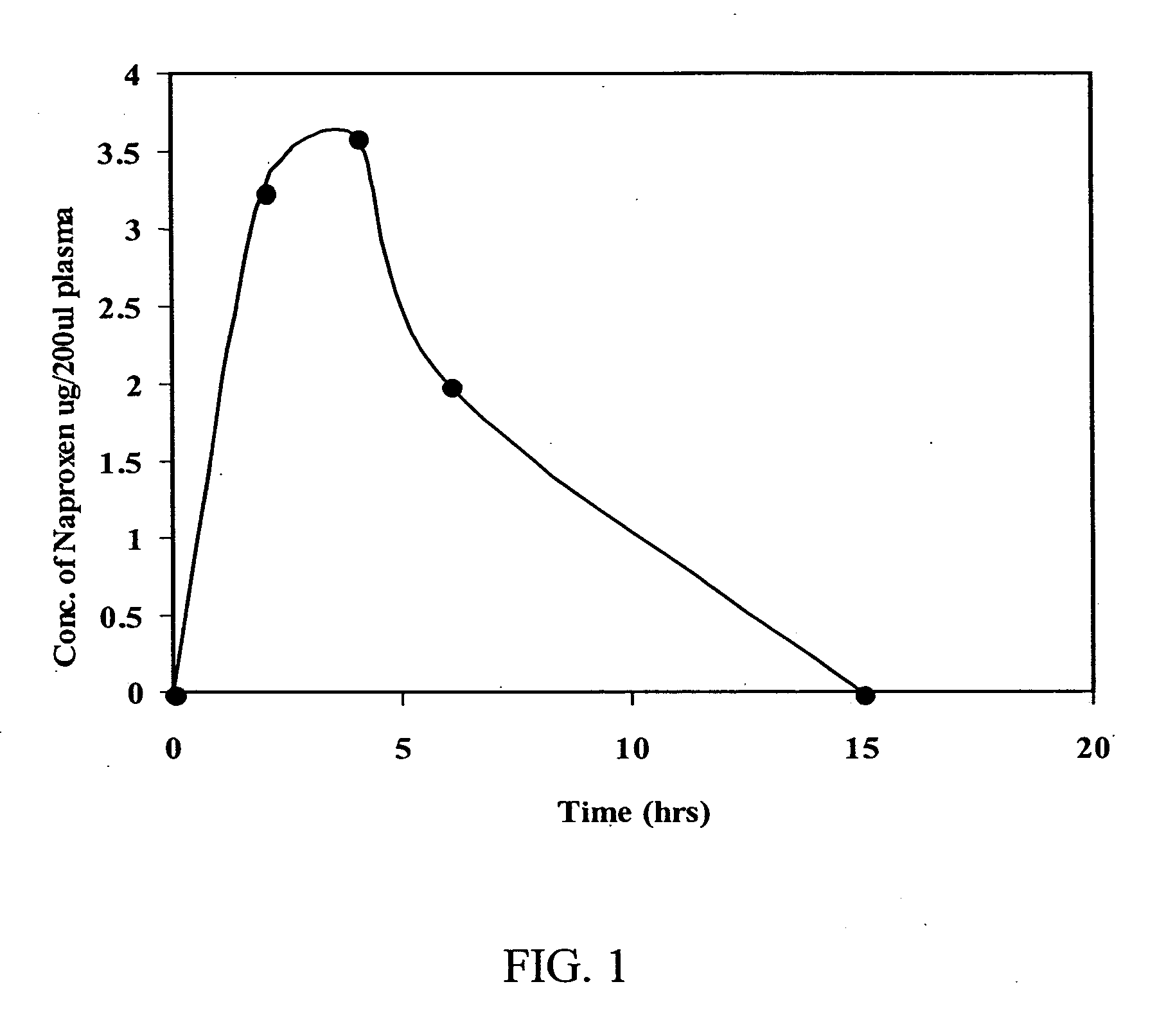
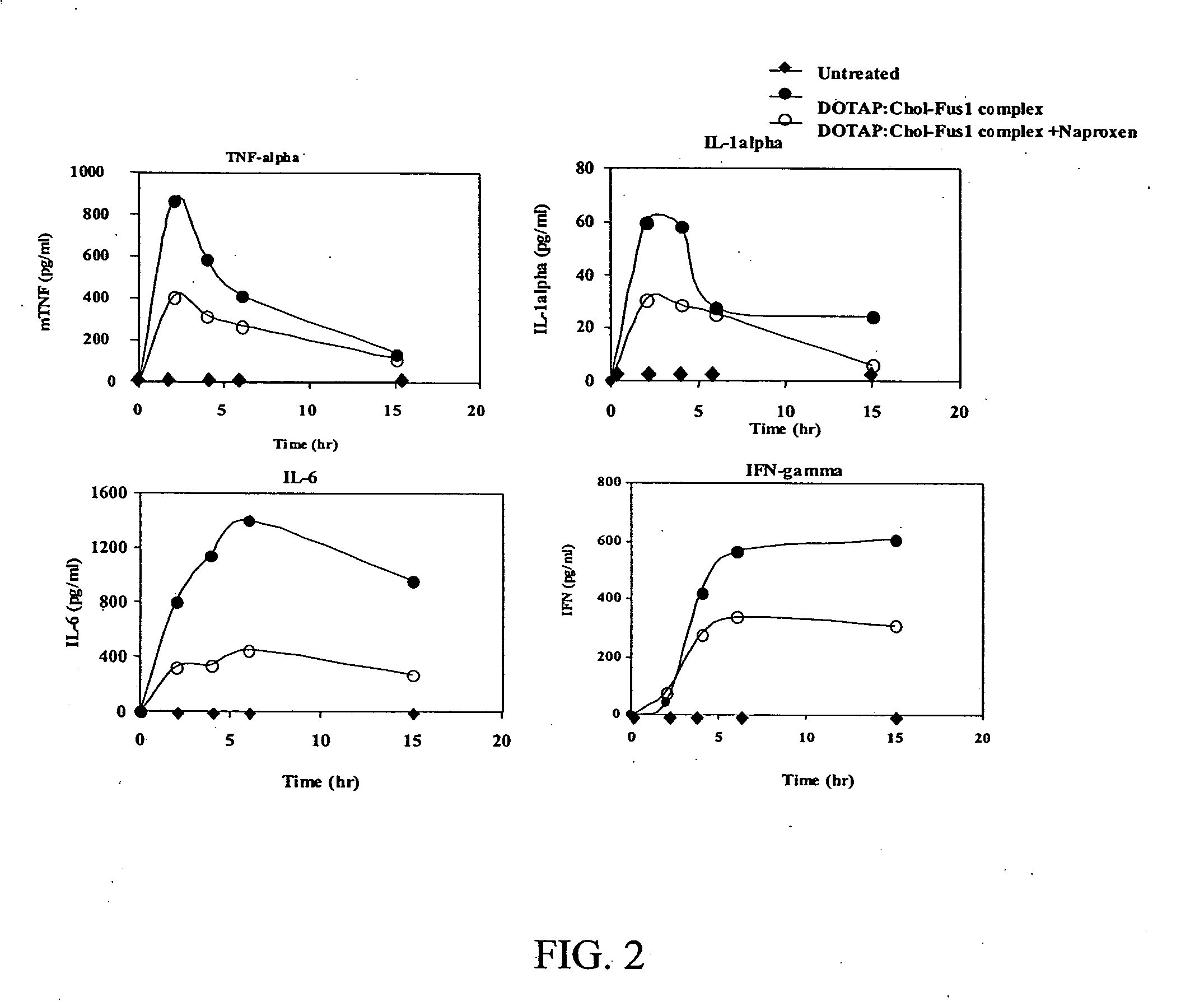
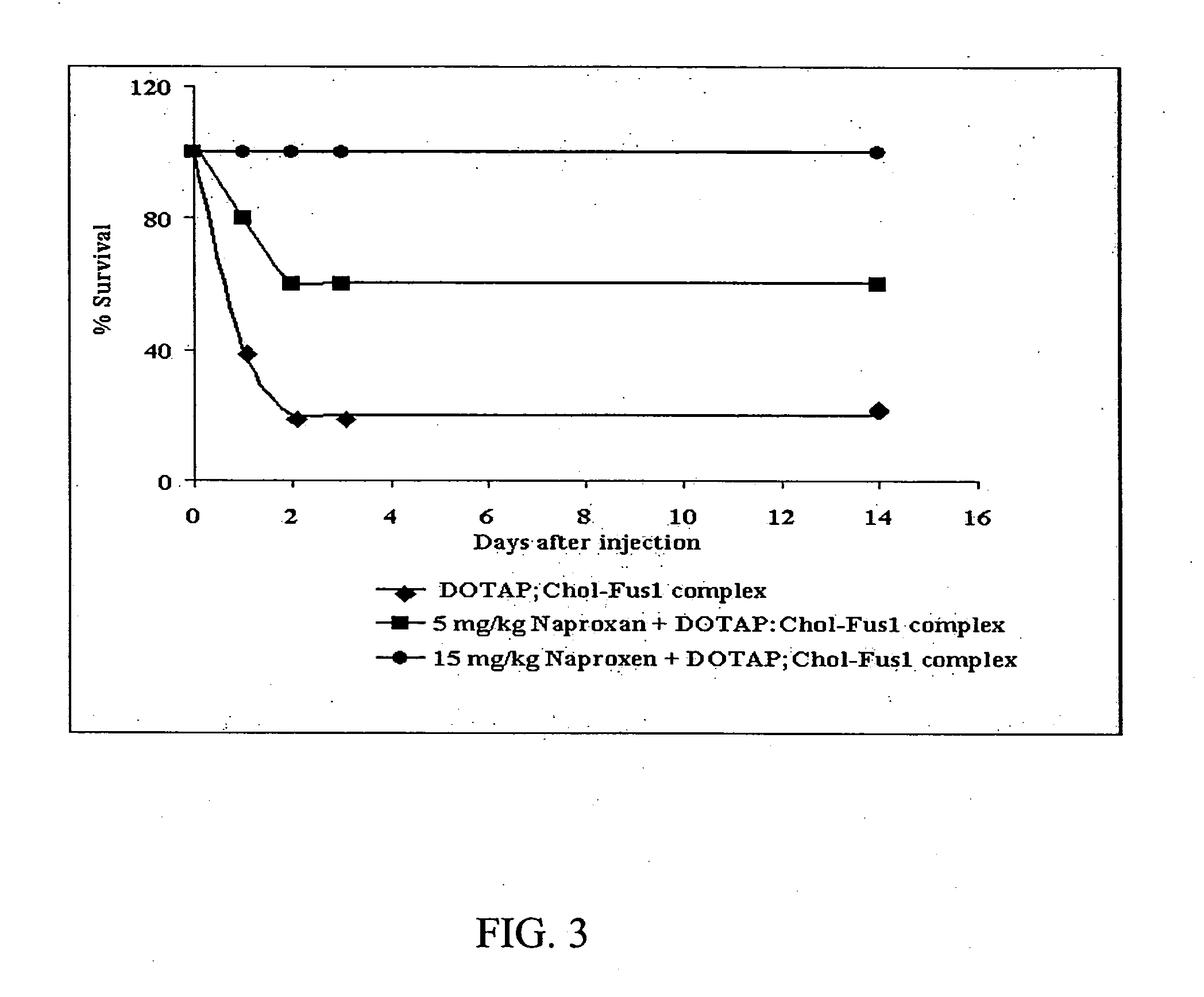
Methods to prevent or reduce inflammation secondary to administration of a lipid-nucleic acid complex in a subject, that include administering to the subject a non-steroidal anti-inflammatory agent, a salicylate, an anti-rheumatic agent, an antihistamine, or an immunsuppressive agent with the lipid-nucleic acid complex are disclosed. Also disclosed are methods of screening for inhibitors of the inflammatory response associated with administration of a lipid-nucleic acid complex to a subject, including providing a candidate substance suspected of preventing or inhibiting the inflammation associated with administration of a lipid-nucleic acid complex to the subject. Also disclosed are compositions that include a lipid, a nucleic acid, and a non-steroidal anti-inflammatory agent, a salicylate, an anti-rheumatic agent, an antihistamine, or an immunosuppressive agent.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Treatment of inflammatory conditions
InactiveUS20070021357A1Reduction and elimination and inhibition of functionTreat painBiocideSenses disorderOxidative stressSugar
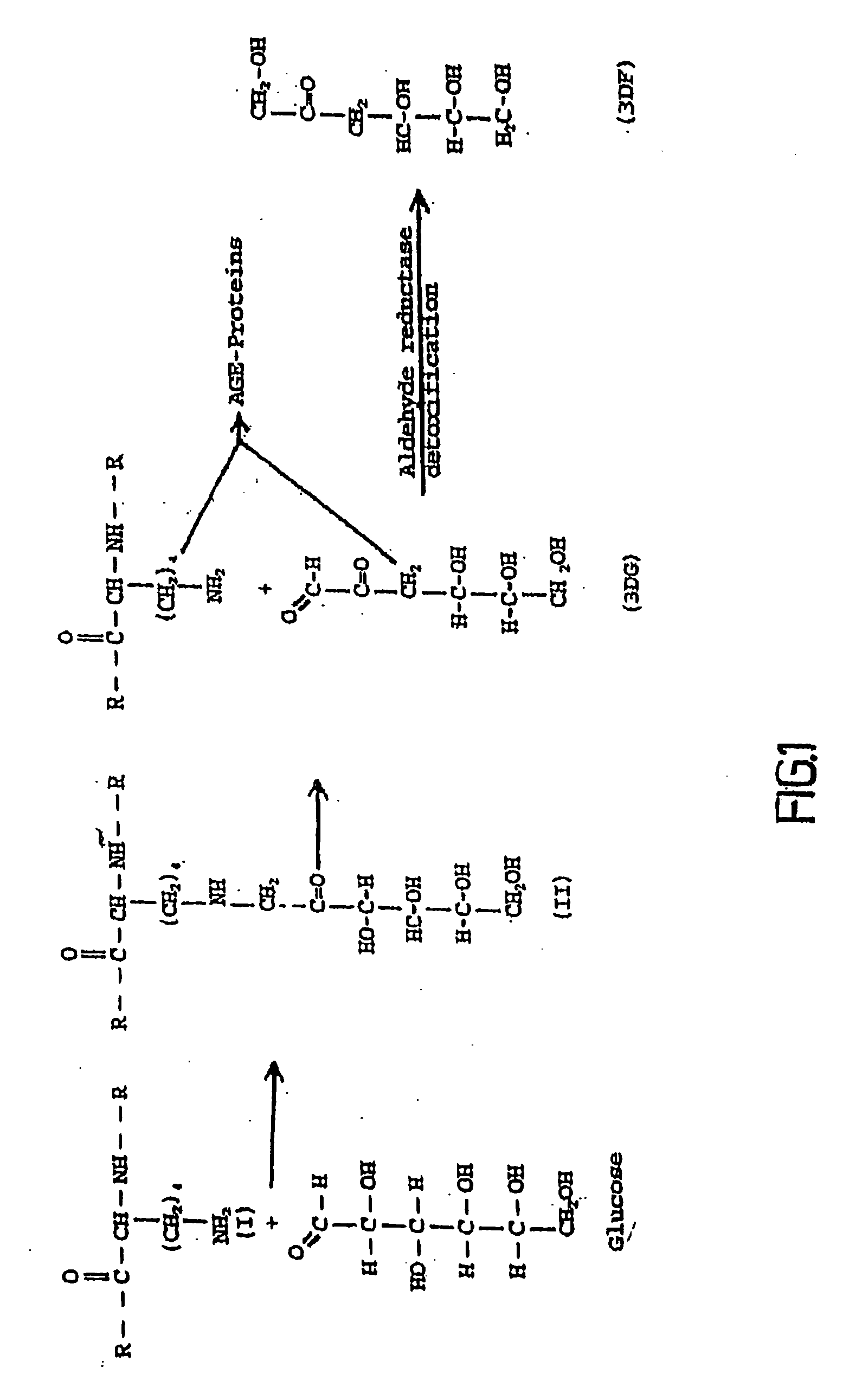
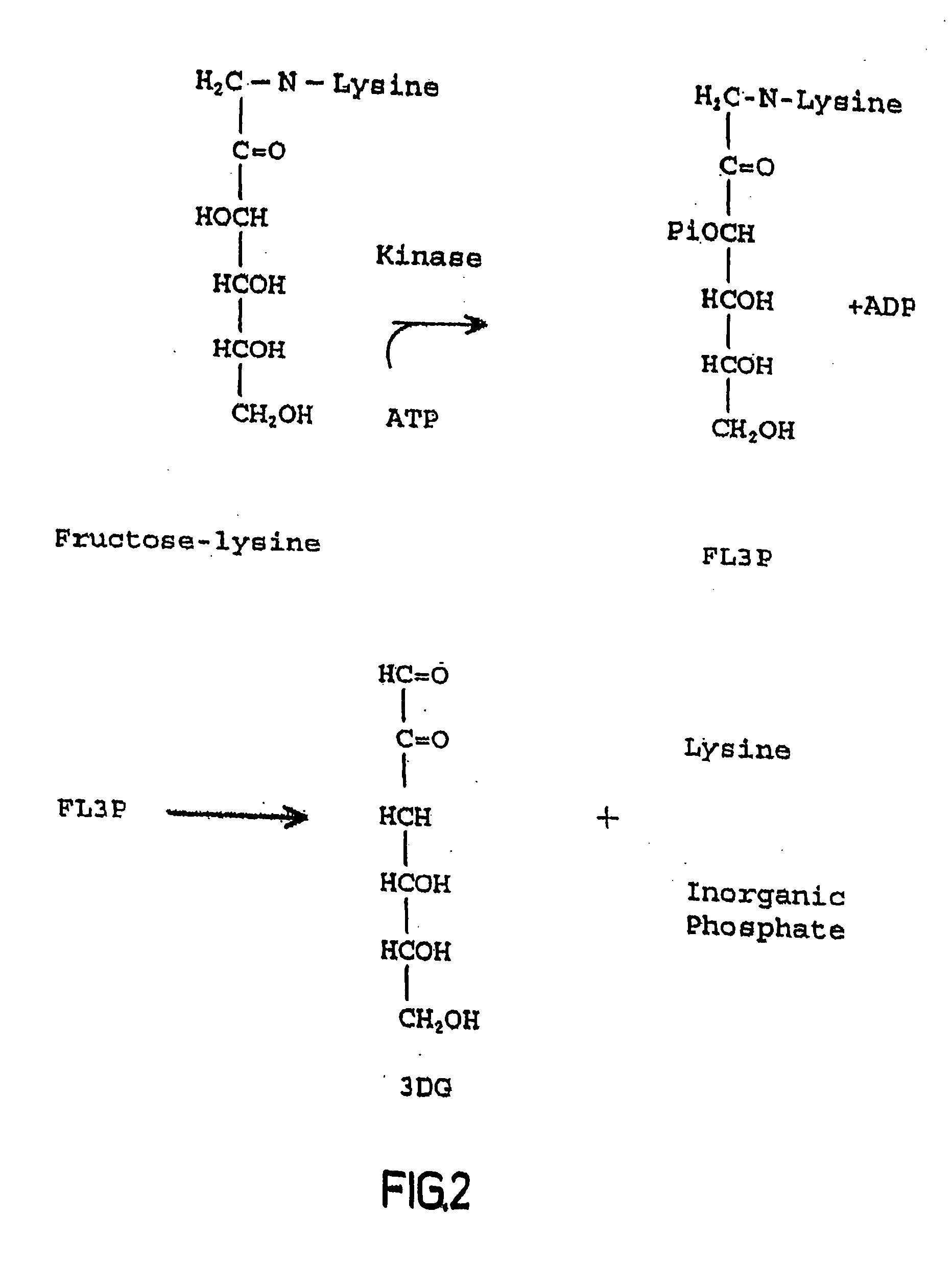
The invention relates to methods of inhibiting production and function of 3-deoxyglucosone and other alpha-dicarbonyl sugars in skin thereby treating or prevention various diseases, disorders or conditions. Additionally, the invention relates to treatment of various diseases, disorders or conditions associated with or mediated by oxidative stress since 3DG induces ROS and AGEs, which are associated with the inflammatory response caused by oxidative stress.
Owner:DYNAMIS THERAPEUTICS
Method for stimulating the immune, inflammatory or neuroprotective response
Owner:KARAGEN PHARMA INC
Methods of treating inflammation using antibodies to M-CSF
InactiveUS7108852B2Inhibiting and minimizing capabilityInhibiting and minimizing accumulationSnake antigen ingredientsImmunoglobulins against cytokines/lymphokines/interferonsAutoimmune responsesAutoimmune disease
A hematopoetic factor called “colony stimulating factor” (CSF) is capable of synergizing the attracting capabilities of chemokines and of inducing the accumulation and / or activation in vitro and in vivo of key components of inflammatory responses. Various types of agents that inhibit or otherwise hinder the production, release or activity of CSF could be used therapeutically in the treatment of ischemia and other inflammatory diseases, such as autoimmune disease, and various chronic inflammatory diseases such as rheumatoid arthritis and psoriasis.
Owner:WARNER-LAMBERT CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com