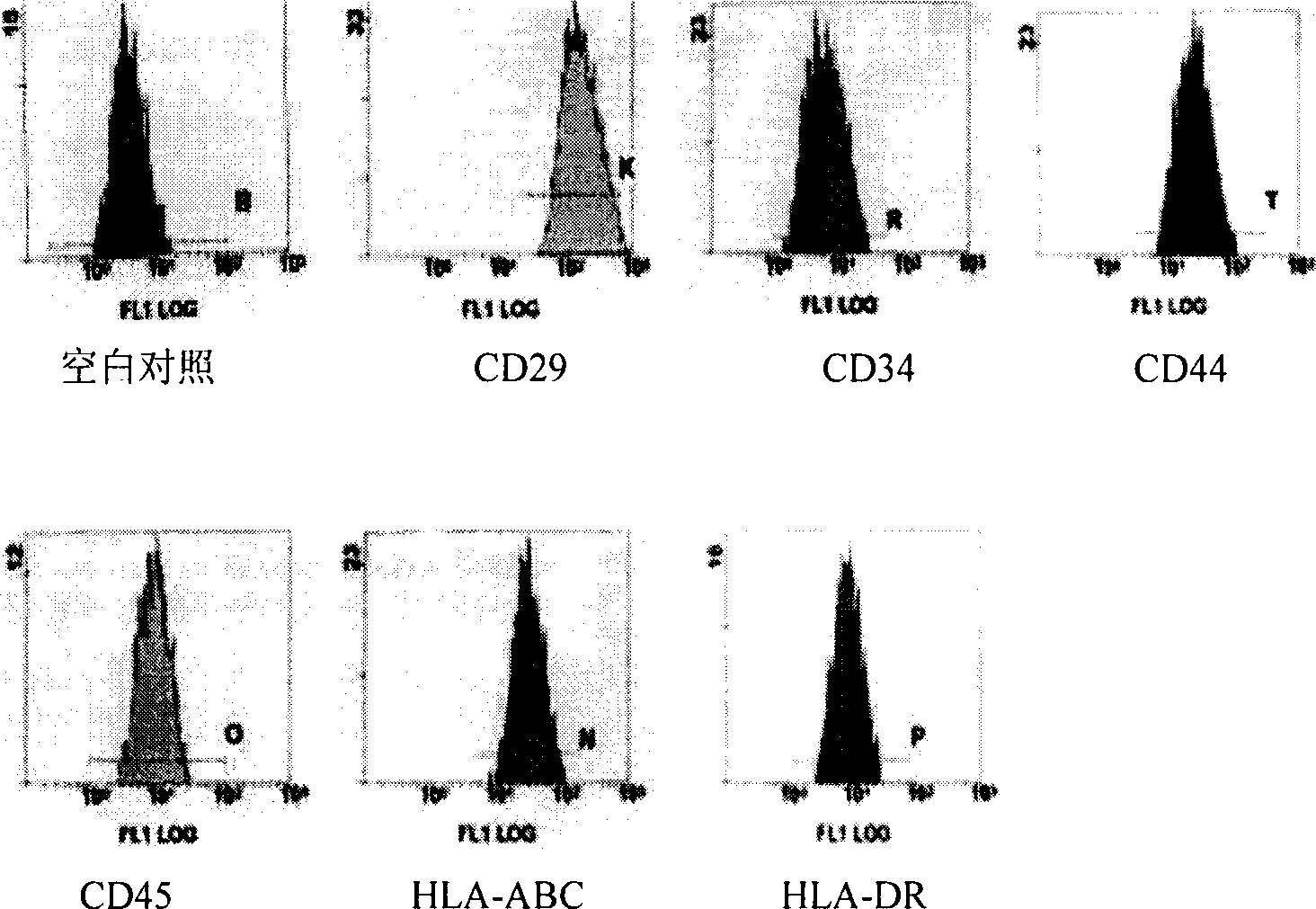
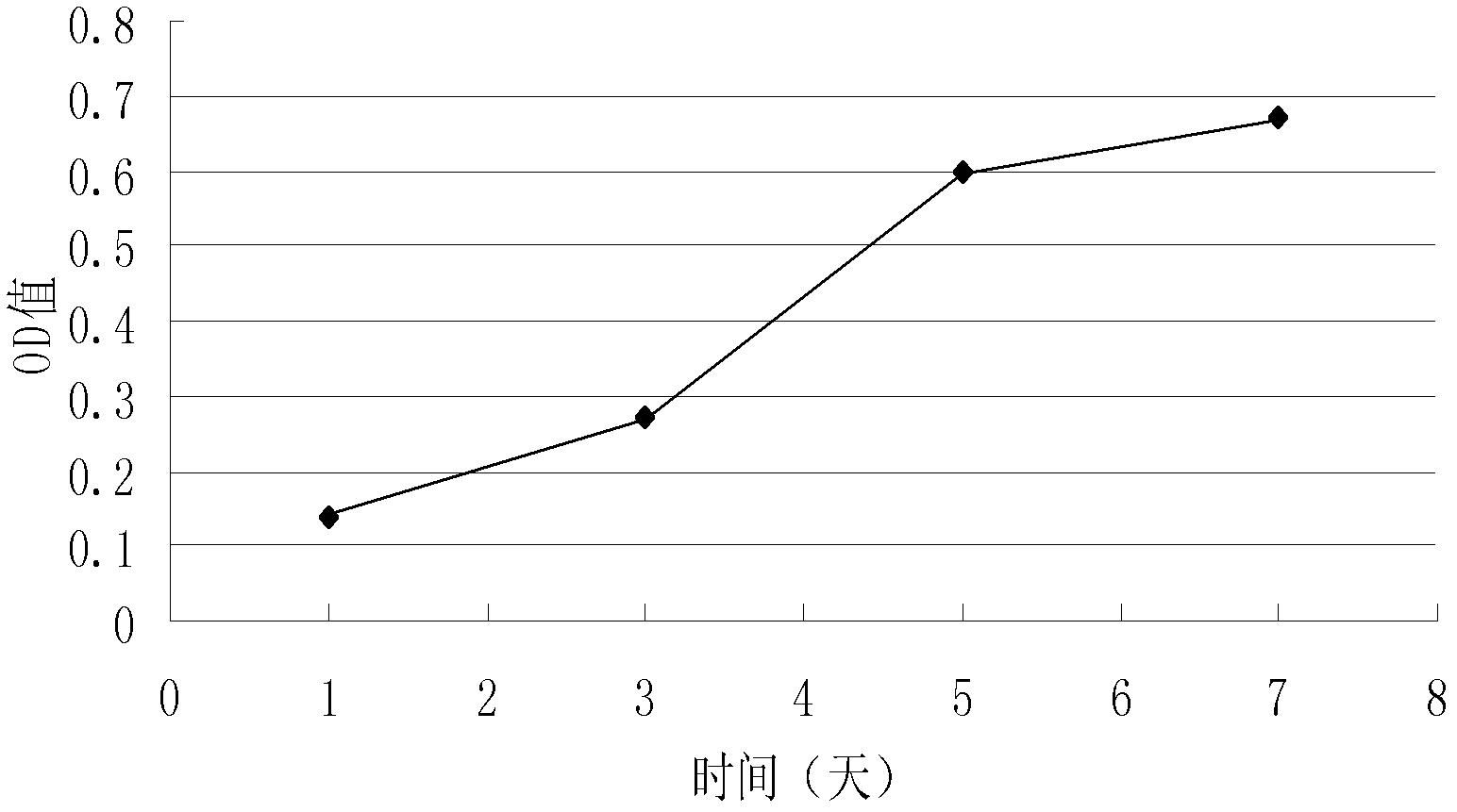
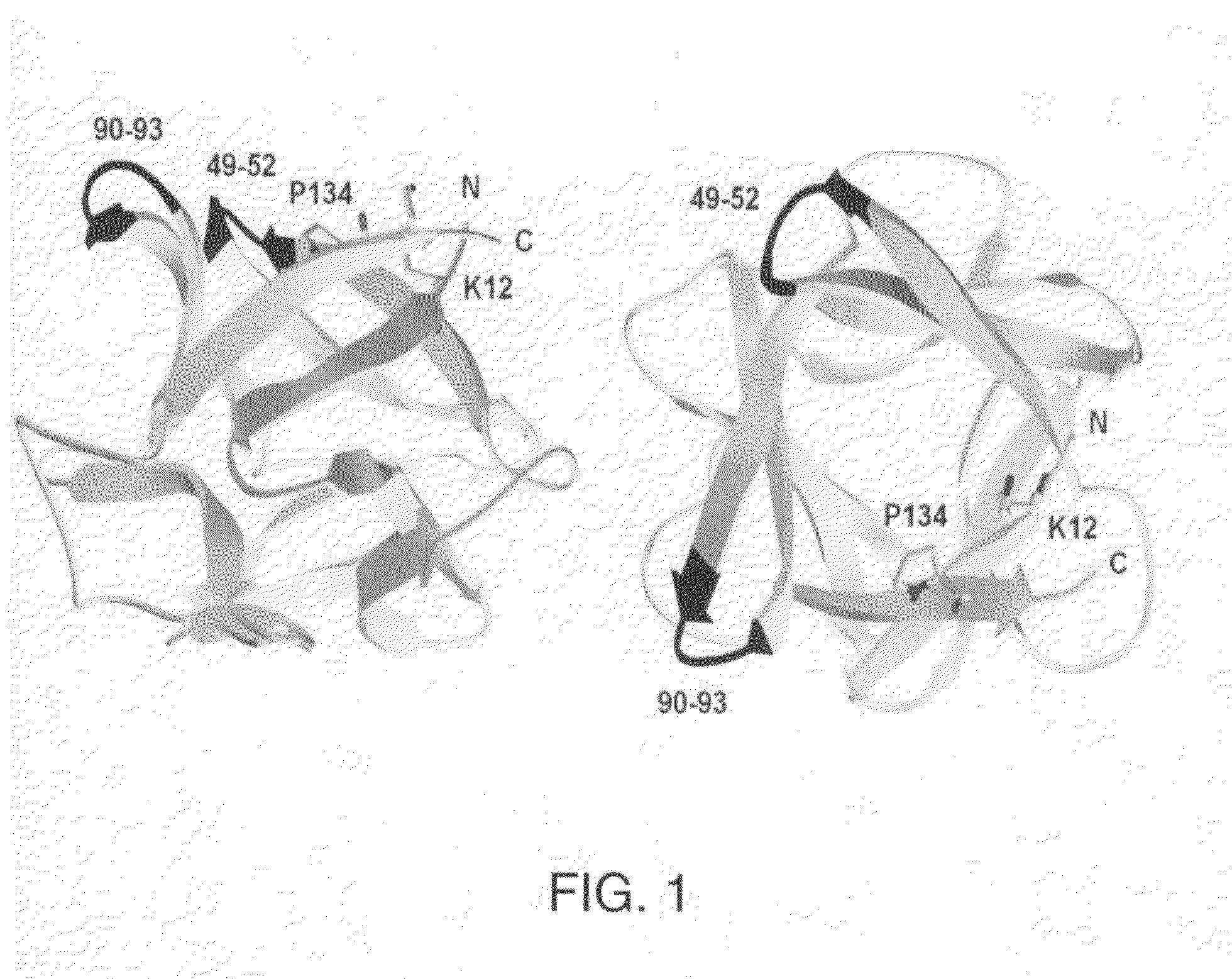
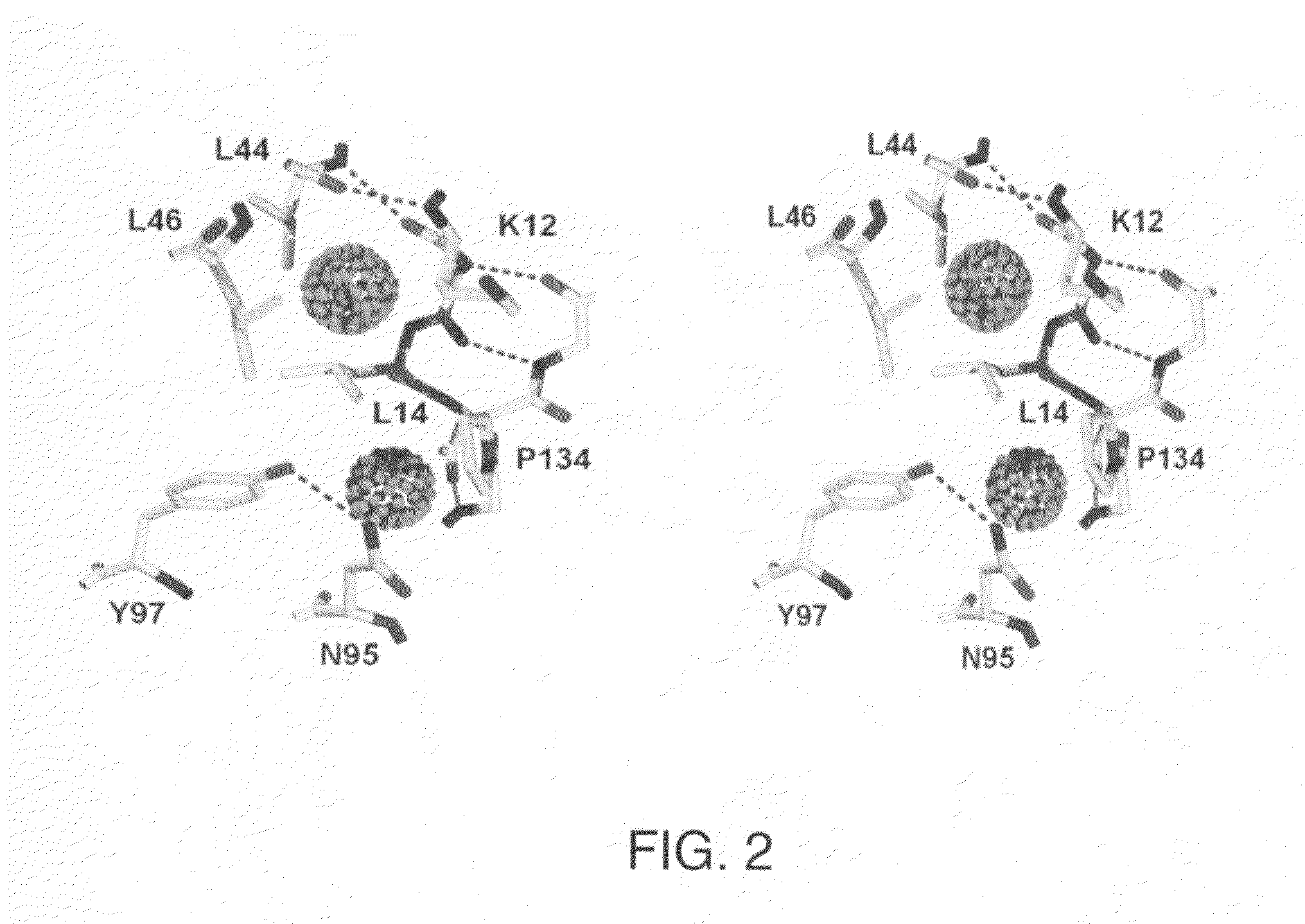
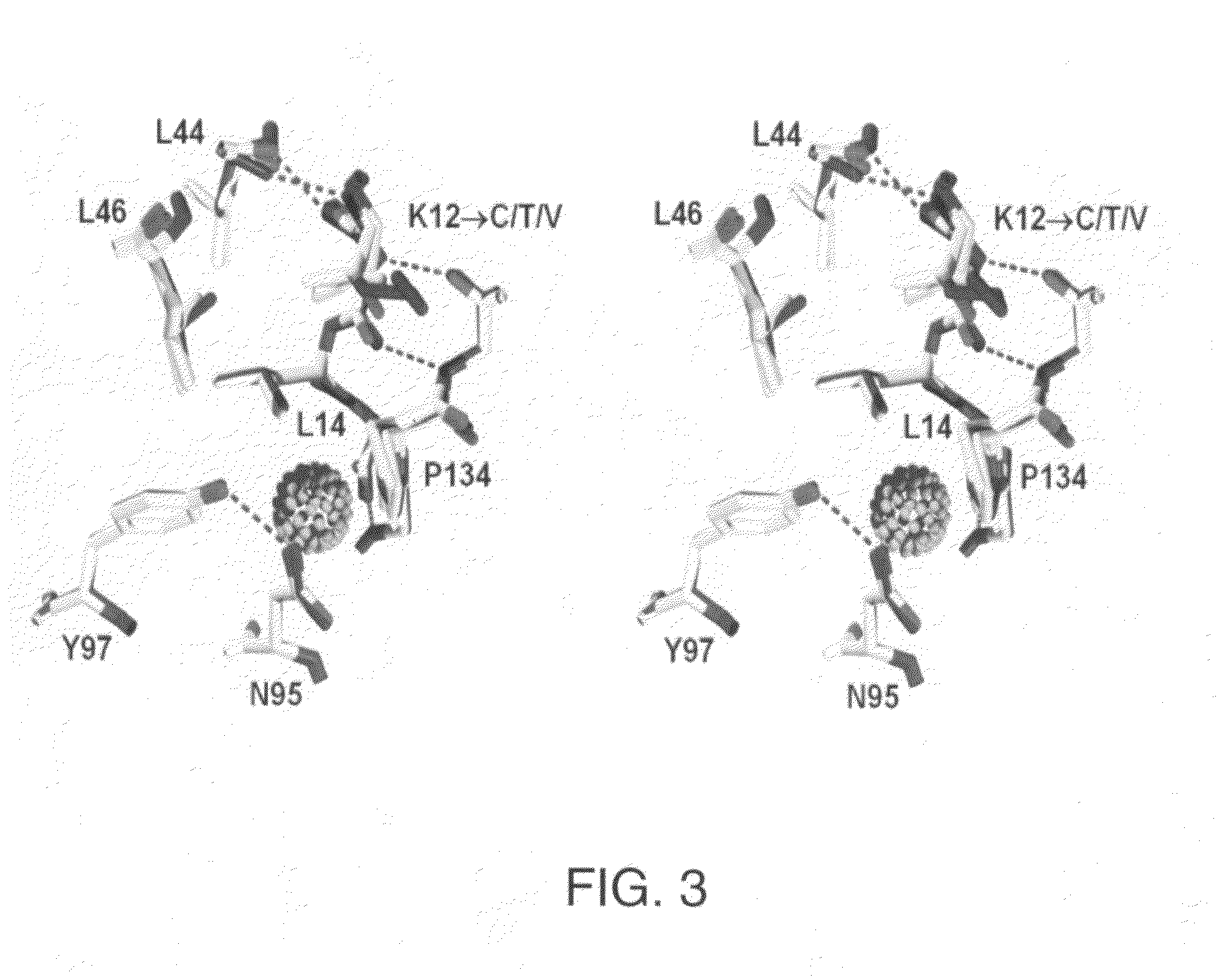
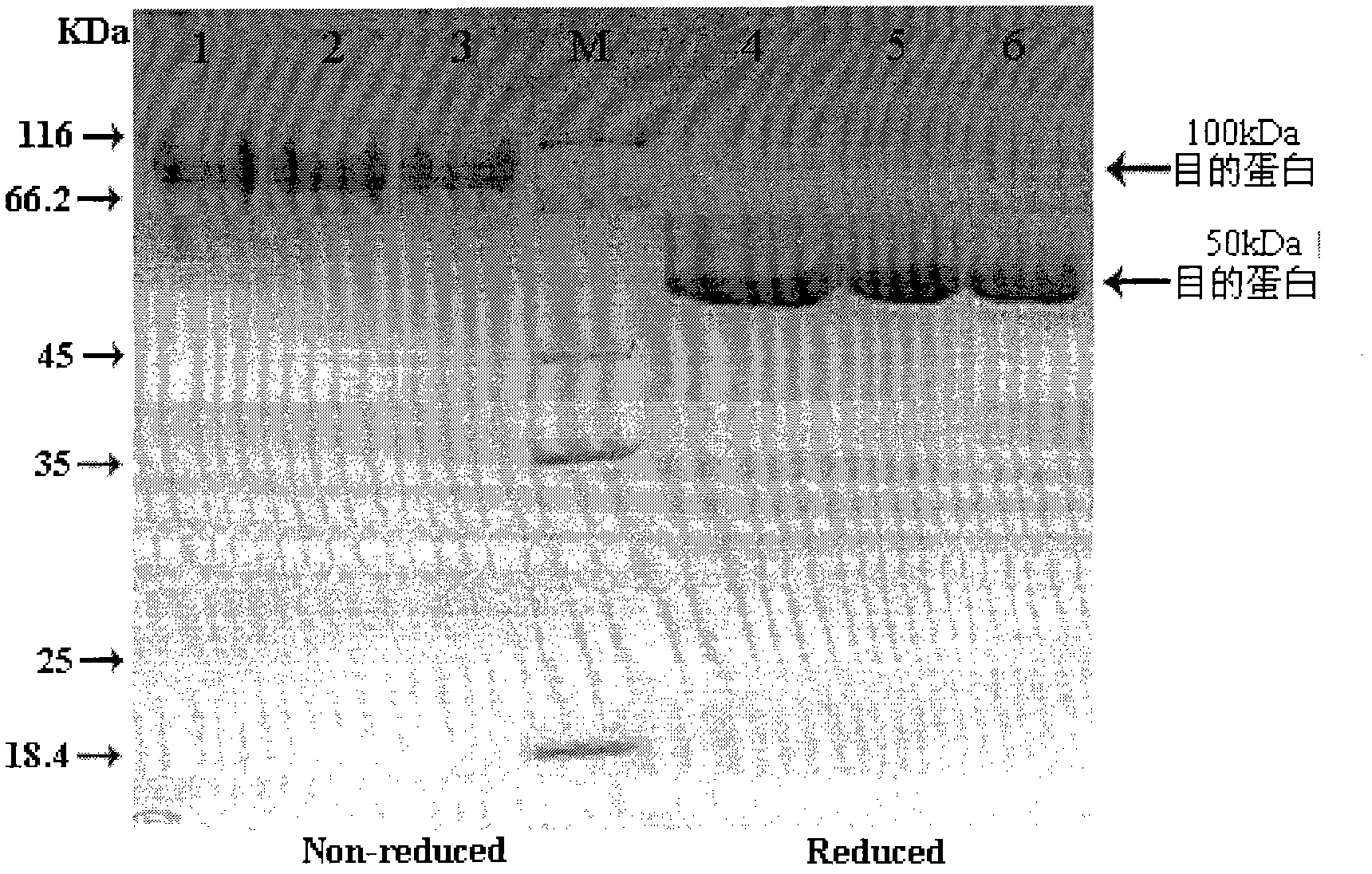
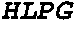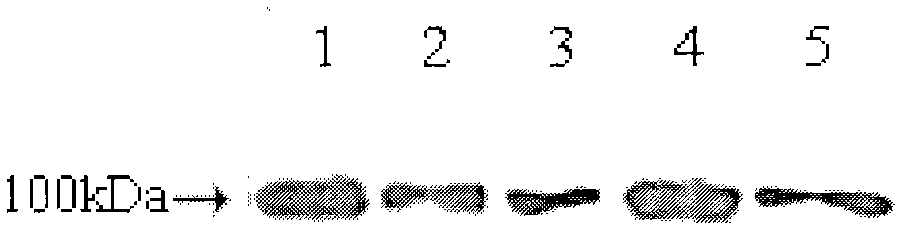Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
689 results about "Basic fibroblast growth factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
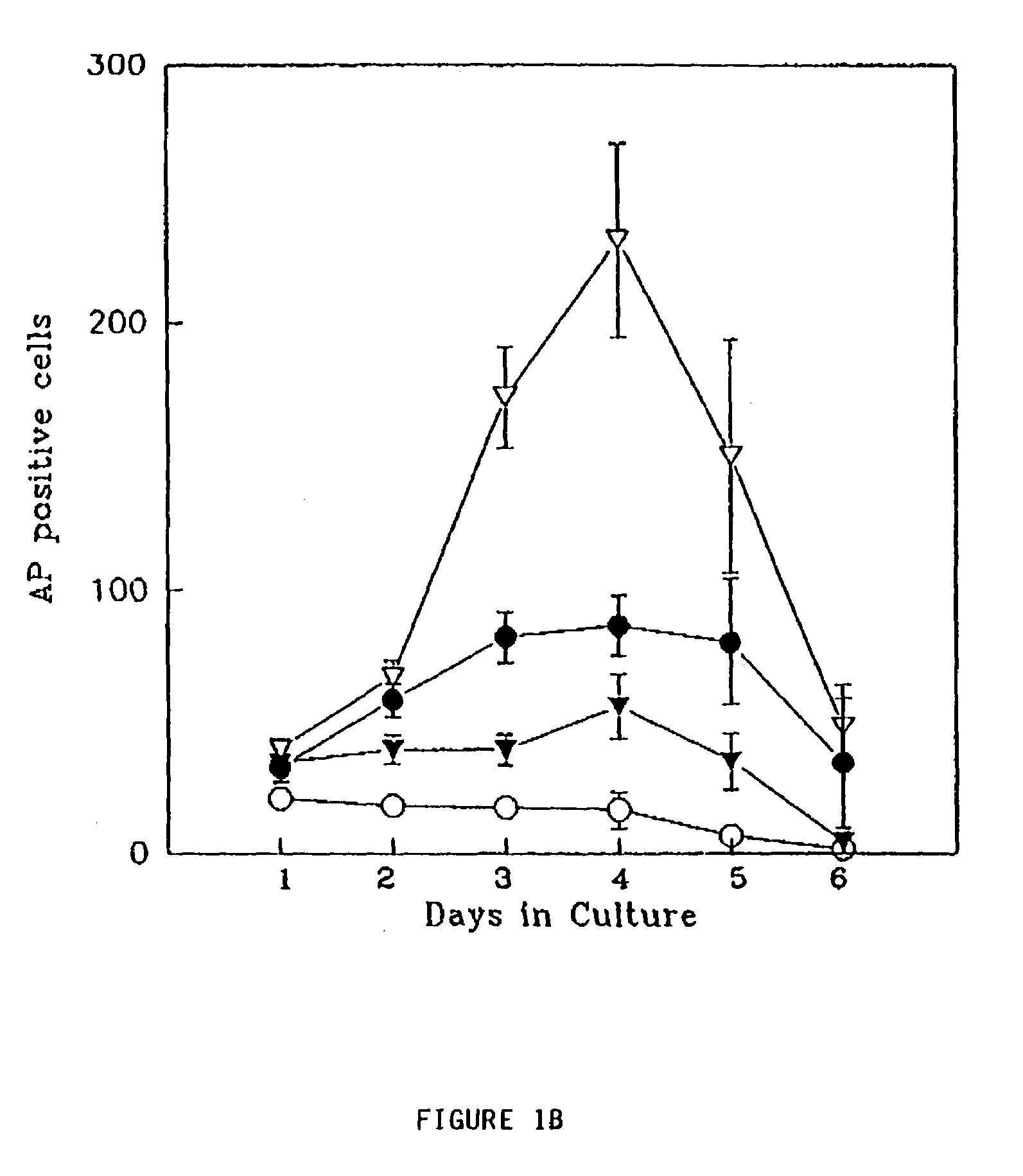
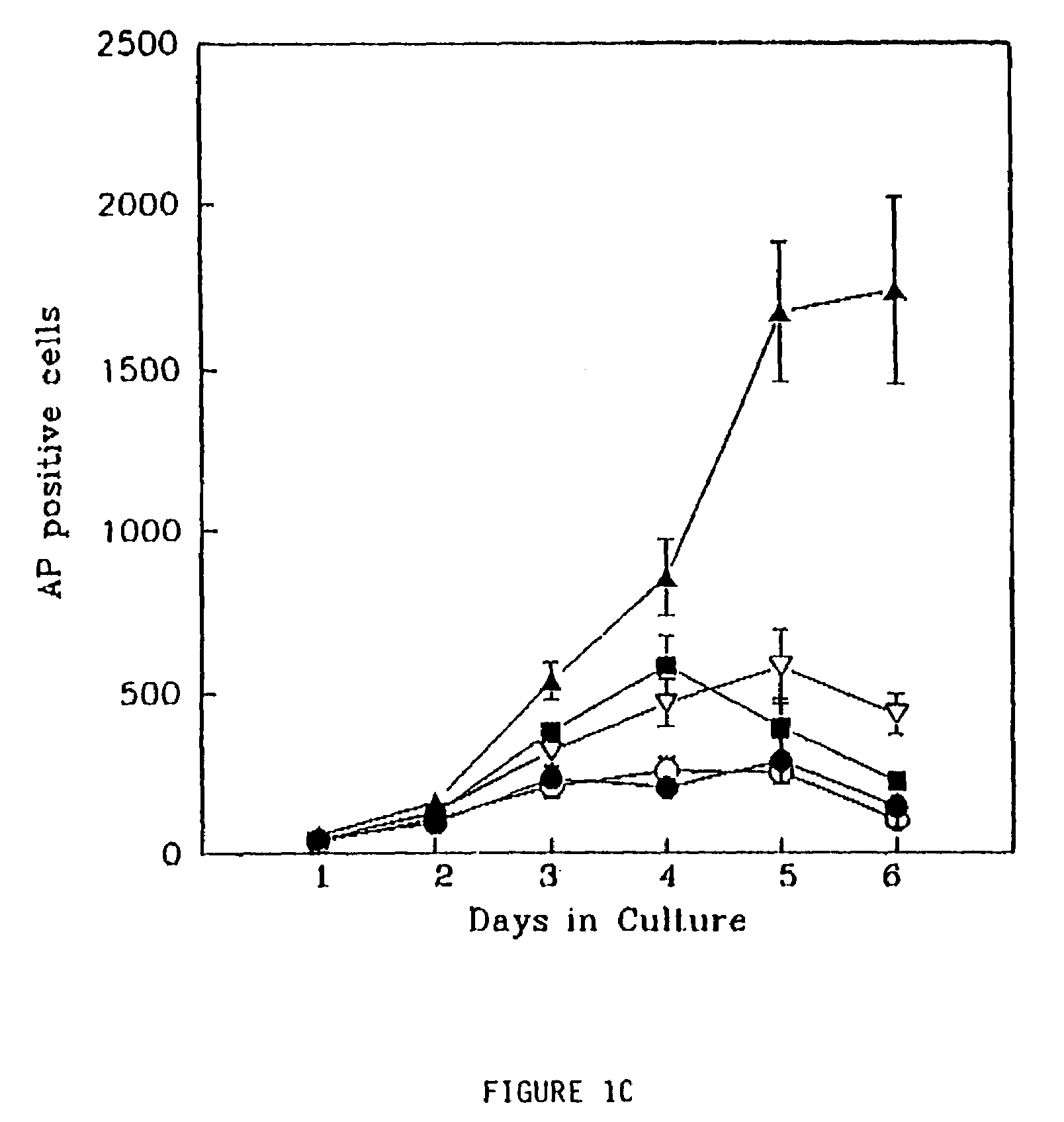
FGF2, also known as basic fibroblast growth factor (bFGF) and FGF-β, is a growth factor and signaling protein encoded by the FGF2 gene. It is synthesized primarily as a 155 amino acid polypeptide, resulting in an 18 kDa protein. However, there are four alternate start codons which provide N-terminal extensions of 41, 46, 55, or 133 amino acids, resulting in proteins of 22 kDa (196 aa total), 22.5 kDa (201 aa total), 24 kDa (210 aa total) and 34 kDa (288 aa total), respectively. Generally, the 155 aa/18 kDa low molecular weight (LMW) form is considered cytoplasmic and can be secreted from the cell, whereas the high molecular weight (HMW) forms are directed to the cell's nucleus.
Defined media for stem cell culture
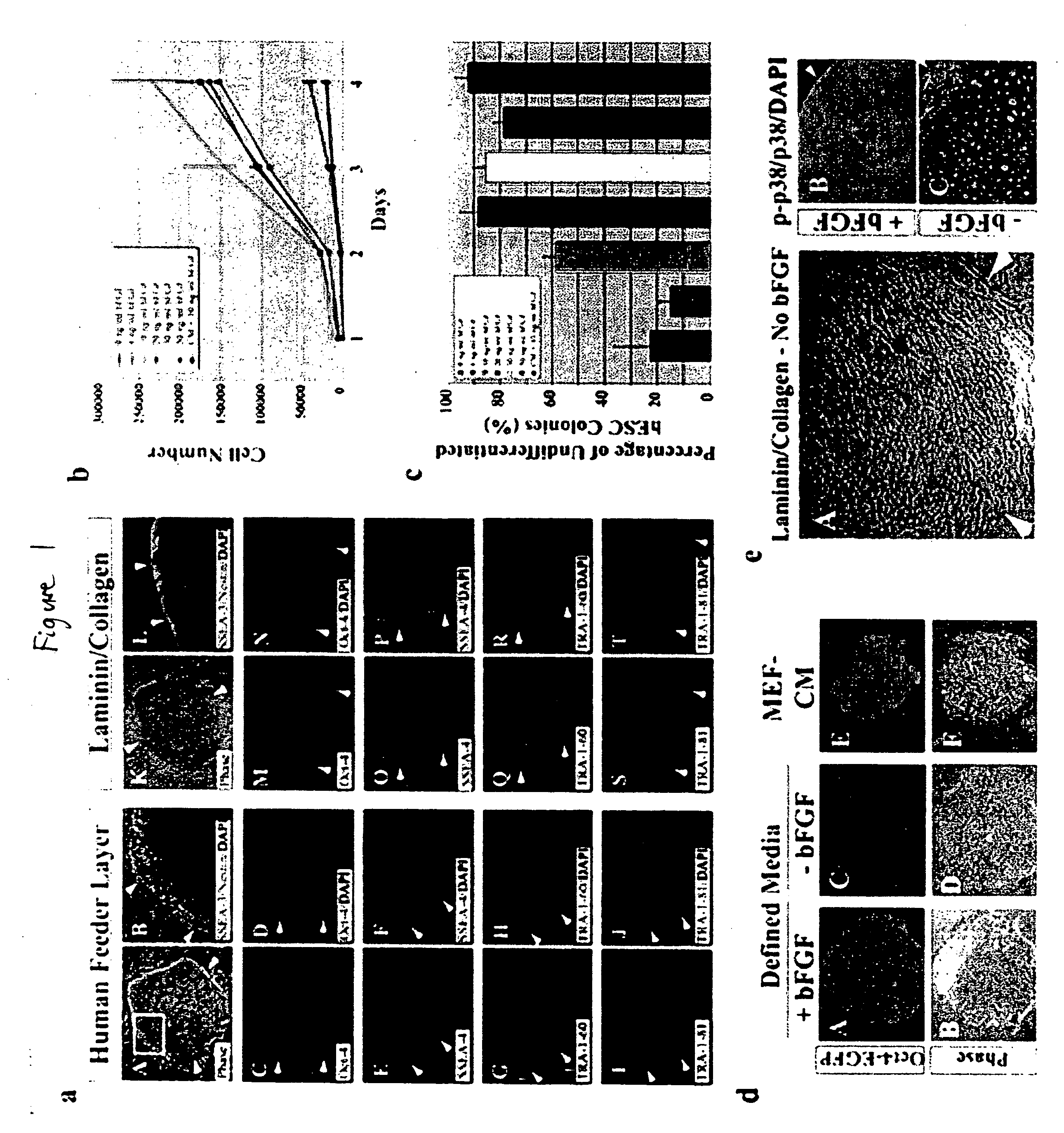
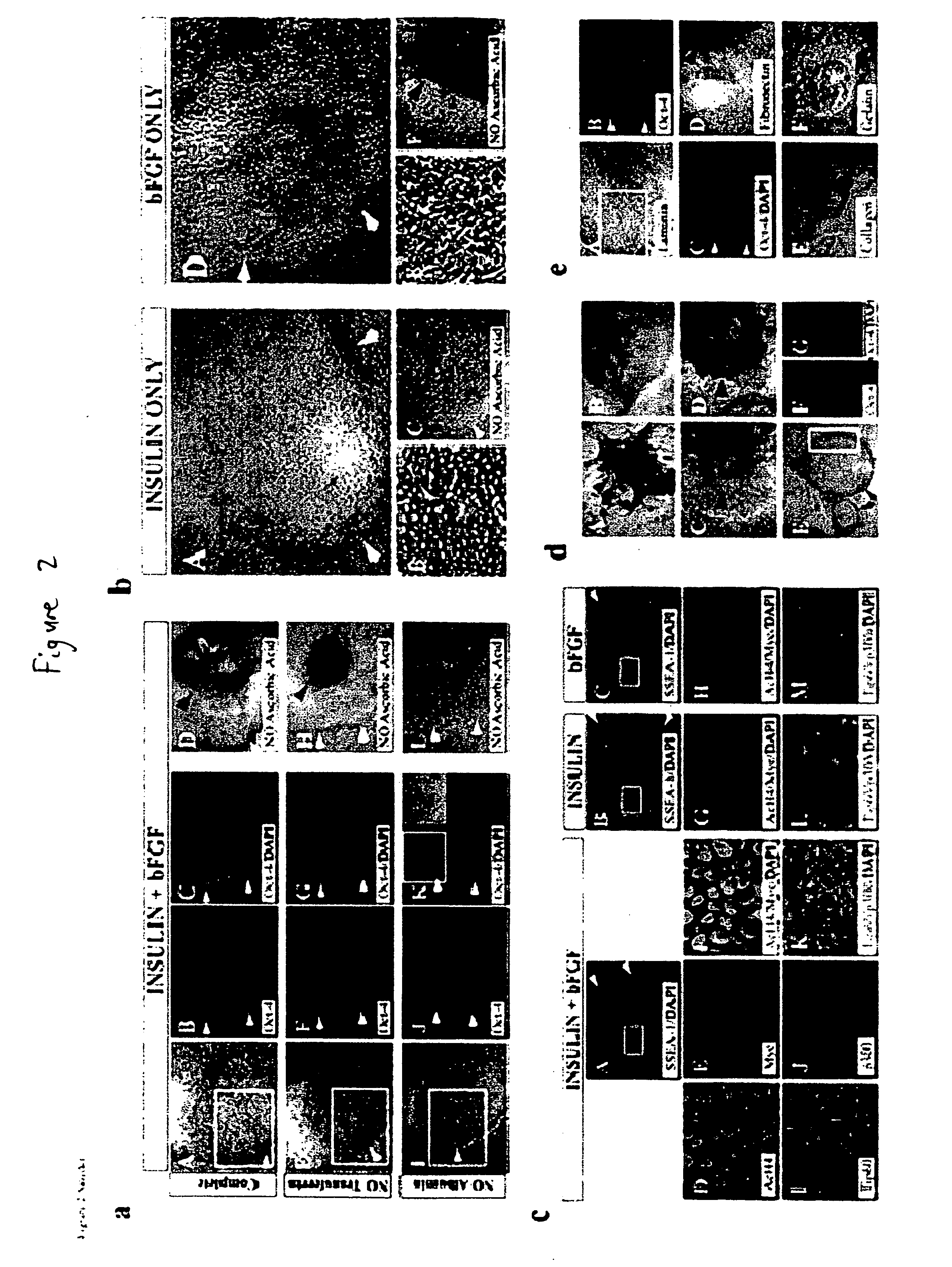
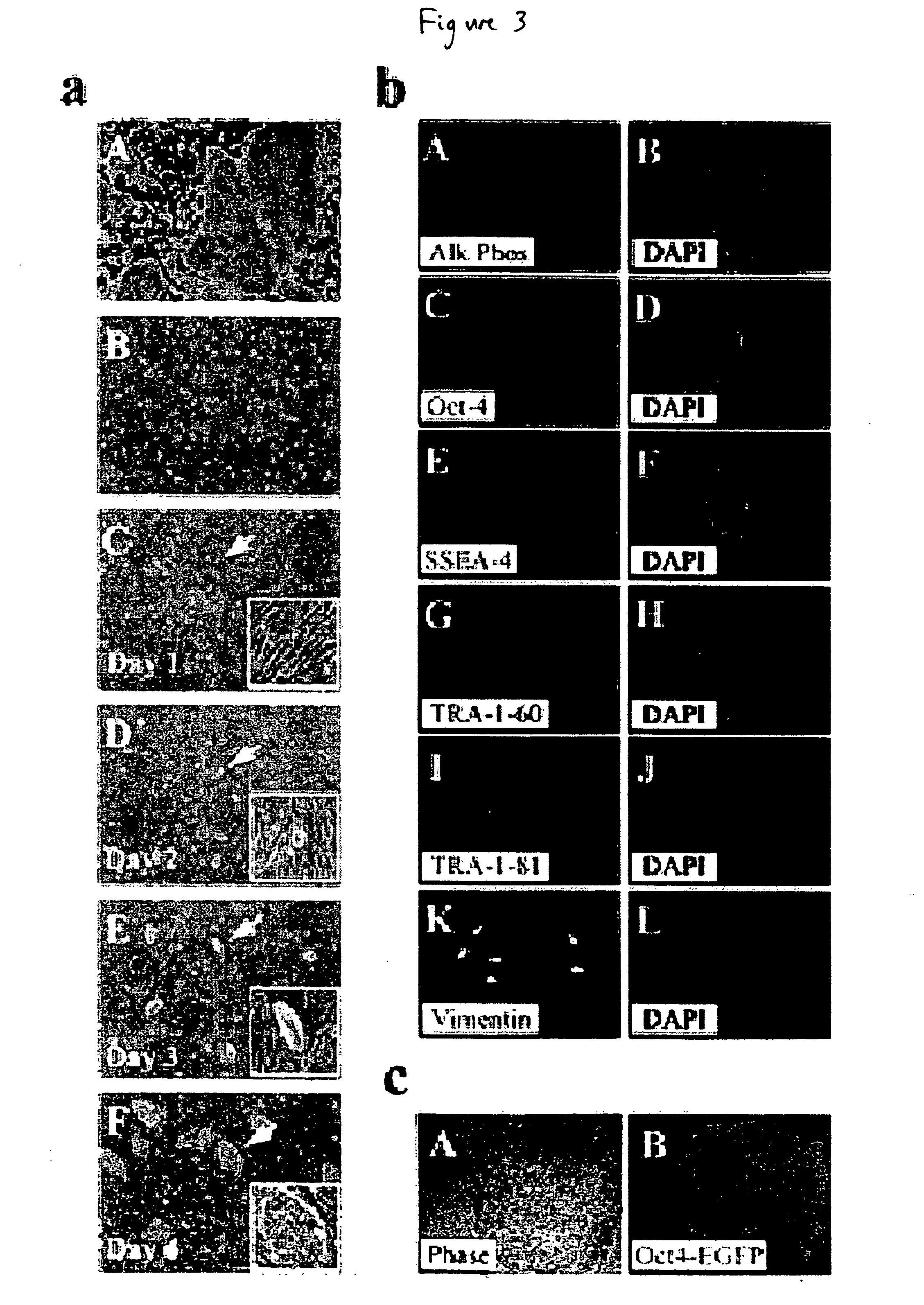
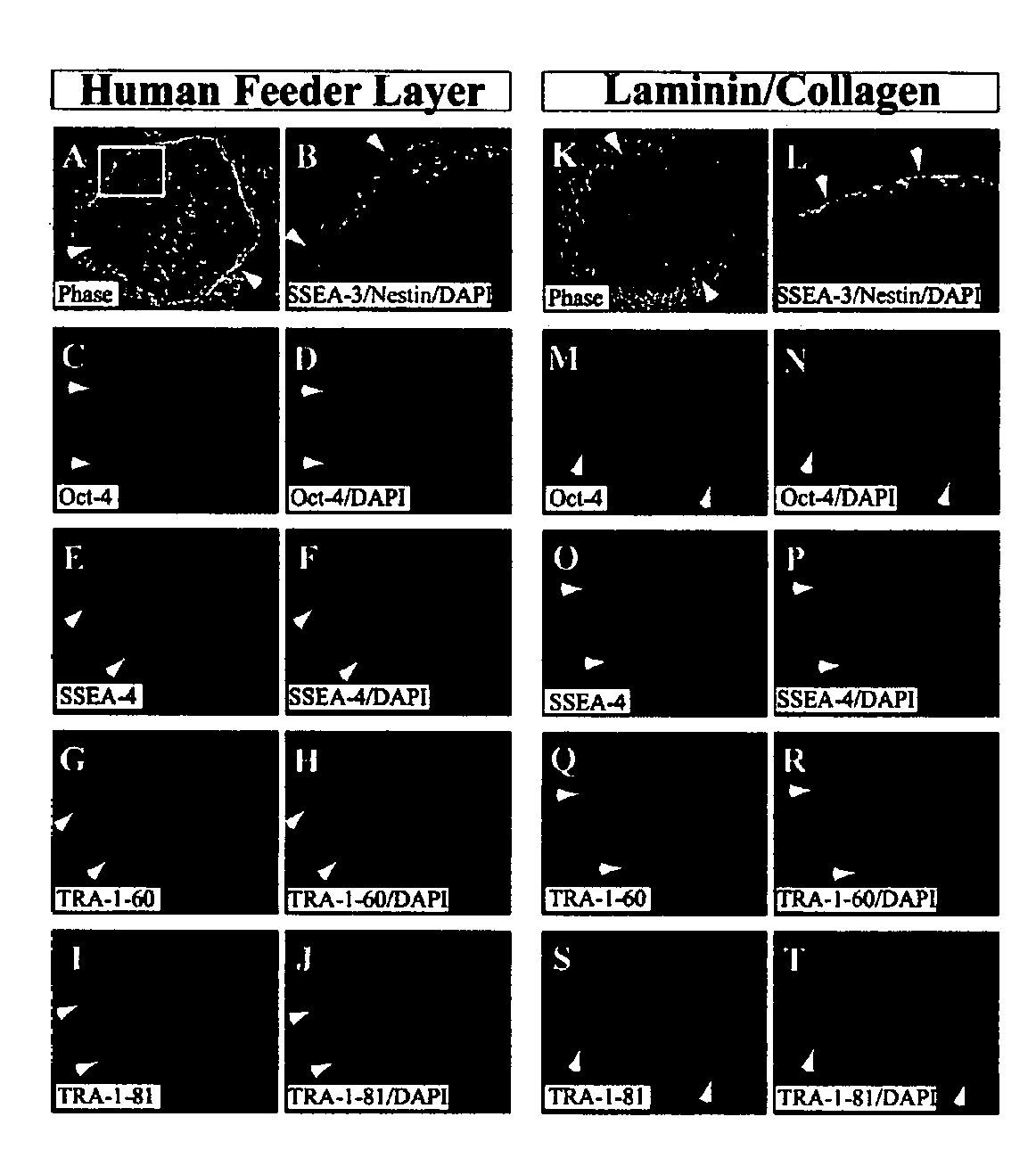
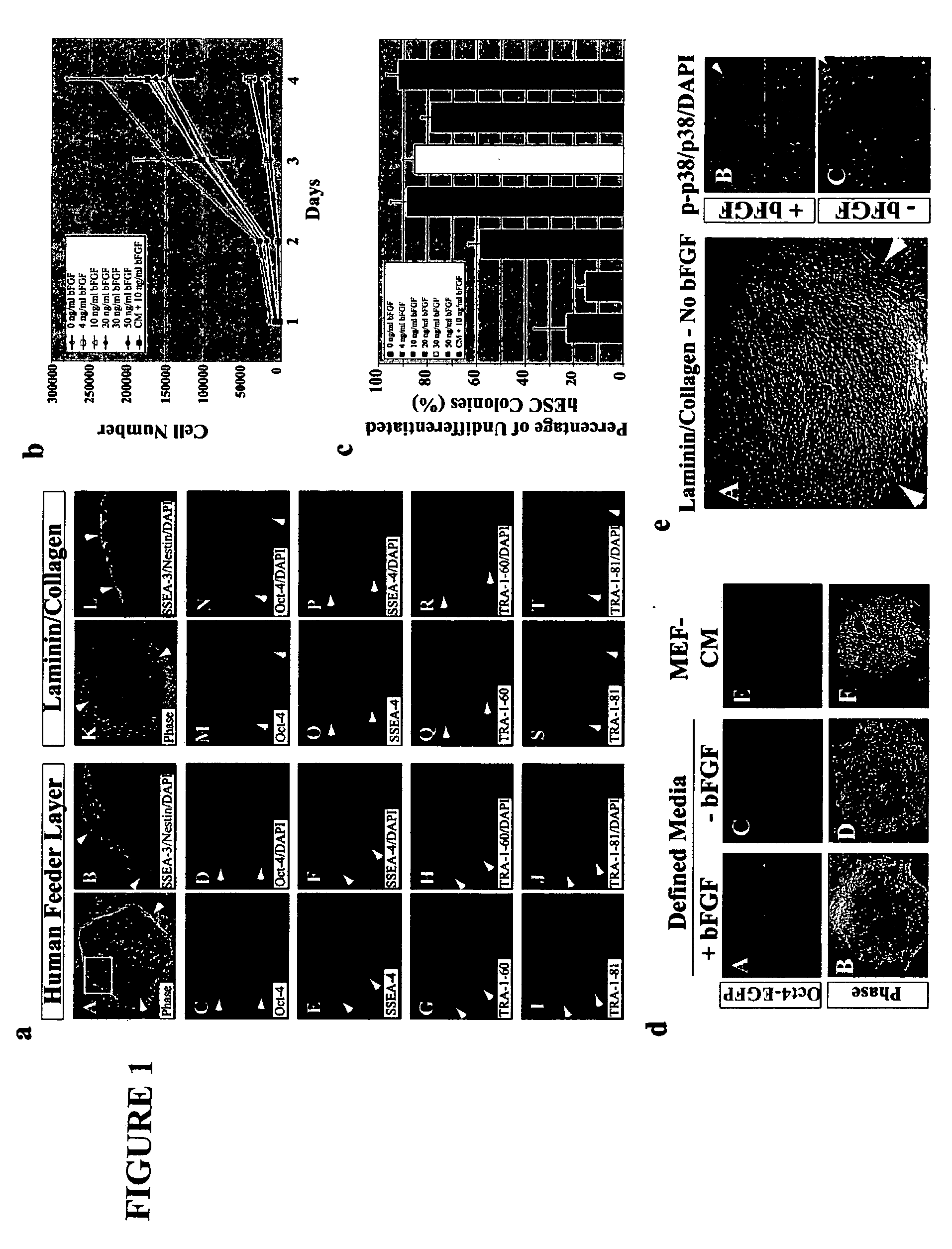
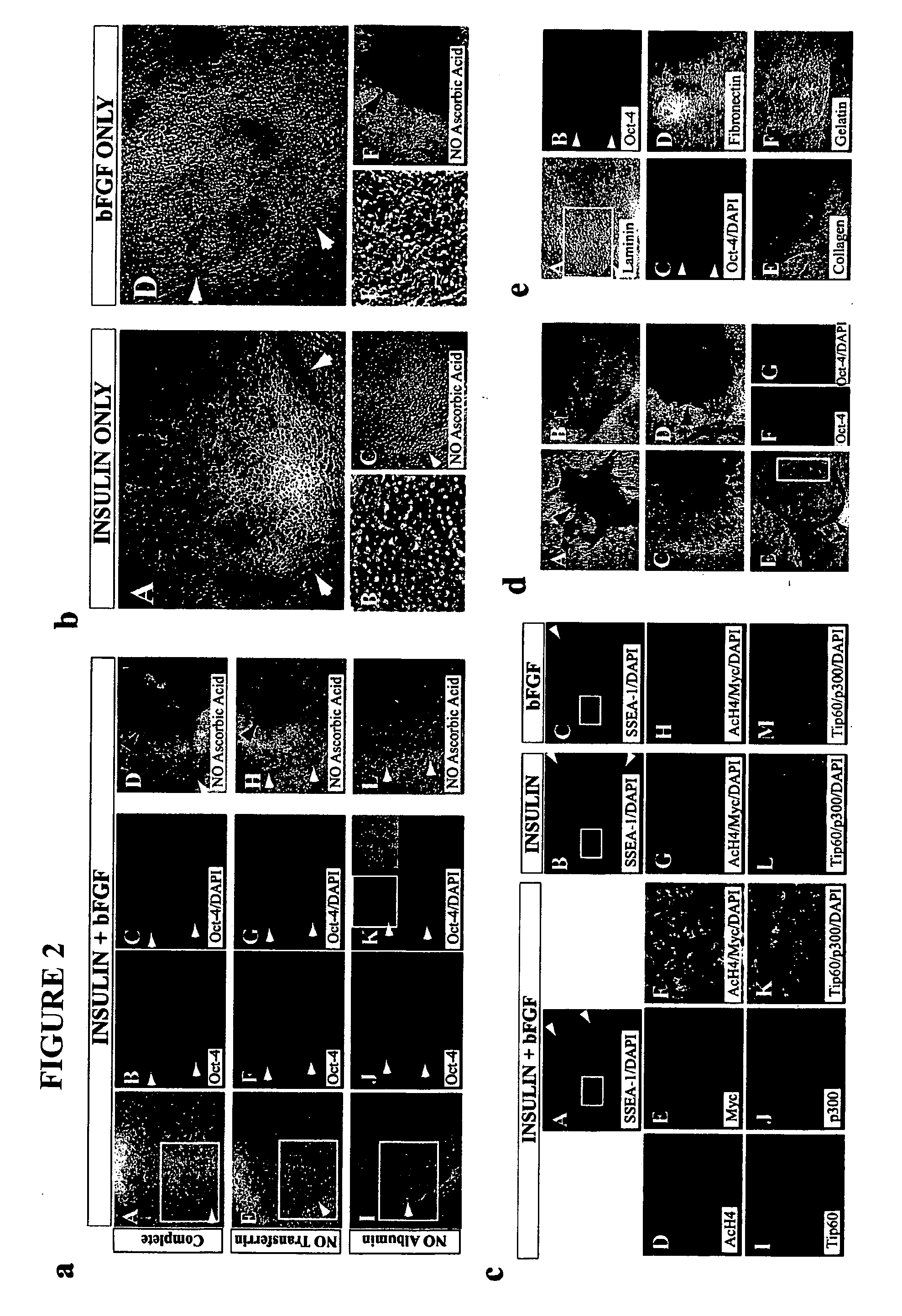
Stem cells, including mammalian, and particularly primate primordial stem cells (pPSCs) such as human embryonic stem cells (hESCs), hold great promise for restoring cell, tissue, and organ function. However, cultivation of stem cells, particularly undifferentiated hESCs, in serum-free, feeder-free, and conditioned-medium-free conditions remains crucial for large-scale, uniform production of pluripotent cells for cell-based therapies, as well as for controlling conditions for efficiently directing their lineage-specific differentiation. This instant invention is based on the discovery of the formulation of minimal essential components necessary for maintaining the long-term growth of pPSCs, particularly undifferentiated hESCs. Basic fibroblast growth factor (bFGF), insulin, ascorbic acid, and laminin were identified to be both sufficient and necessary for maintaining hESCs in a healthy self-renewing undifferentiated state capable of both prolonged propagation and then directed differentiation. Having discerned these minimal molecular requirements, conditions that would permit the substitution of poorly-characterized and unspecified biological additives and substrates were derived and optimized with entirely defined constituents, providing a “biologics”-free (i.e., animal-, feeder-, serum-, and conditioned-medium-free) system for the efficient long-term cultivation of pPSCs, particularly pluripotent hESCs. Such culture systems allow the derivation and large-scale production of stem cells such as pPSCs, particularly pluripotent hESCs, in optimal yet well-defined biologics-free culture conditions from which they can be efficiently directed towards a lineage-specific differentiated fate in vitro, and thus are important, for instance, in connection with clinical applications based on stem cell therapy and in drug discovery processes.
Owner:THE BURNHAM INST
Negative pressure wound therapy system with provision for introduction of an agent
InactiveUS7534240B1Promote wound healingRapid reepithelializationPeptide/protein ingredientsSurgical needlesWound siteGrafting
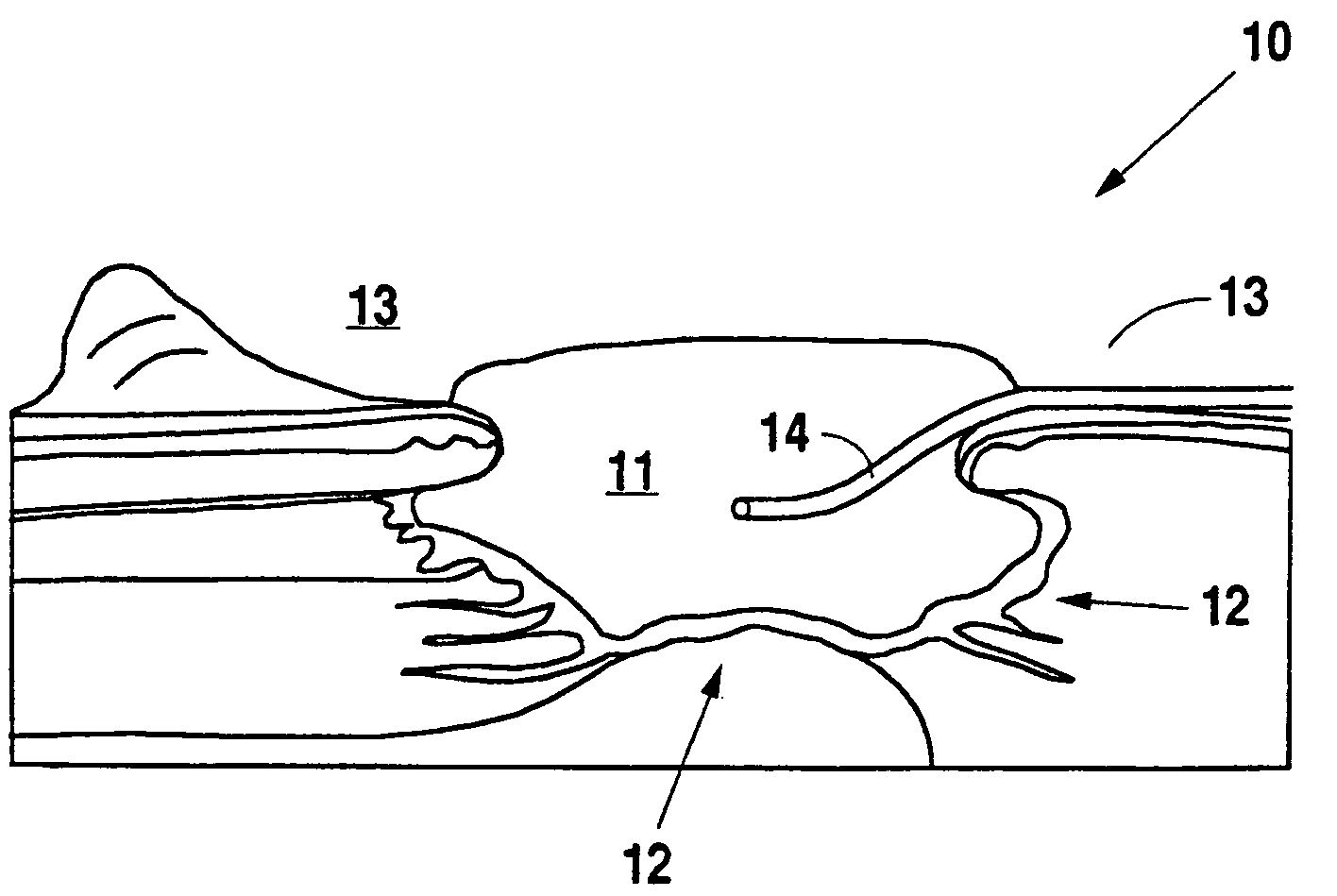
A method and apparatus for the introduction to a wound under negative pressure therapy of a wound healing agent, generally comprises a foam pad (11) for insertion substantially into a wound site (12), and a wound drape (13) for sealing enclosure of the foam pad at the wound site. The foam pad is placed in fluid communication with a vacuum source for promotion of fluid drainage. Additionally, the foam pad is predisposed, through grafting or other techniques known to those of ordinary skill in the art, with basic fibroblast growth factor (bFGF), anti-microbial or other factors, also known to those of ordinary skill in the art, for the promotion of increased wound healing.
Owner:KCI LICENSING INC
Defined media for pluripotent stem cell culture
Stem cells, including mammalian, and particularly primate primordial stem cells (pPSCs) such as human embryonic stem cells (hESCs), hold great promise for restoring cell, tissue, and organ function. However, cultivation of stem cells, particularly undifferentiated hESCs, in serum-free, feeder-free, and conditioned-medium-free conditions remains crucial for large-scale, uniform production of pluripotent cells for cell-based therapies, as well as for controlling conditions for efficiently directing their lineage-specific differentiation. This instant invention is based on the discovery of the formulation of minimal essential components necessary for maintaining the long-term growth of pPSCs, particularly undifferentiated hESCs. Basic fibroblast growth factor (bFGF), insulin, ascorbic acid, and laminin were identified to be both sufficient and necessary for maintaining hESCs in a healthy self-renewing undifferentiated state capable of both prolonged propagation and then directed differentiation. Having discerned these minimal molecular requirements, conditions that would permit the substitution of poorly-characterized and unspecified biological additives and substrates were derived and optimized with entirely defined constituents, providing a “biologics”-free (i.e., animal-, feeder-, serum-, and conditioned-medium-free) system for the efficient long-term cultivation of pPSCs, particularly pluripotent hESCs. Such culture systems allow the derivation and large-scale production of stem cells such as pPSCs, particularly pluripotent hESCs, in optimal yet well-defined biologics-free culture conditions from which they can be efficiently directed towards a lineage-specific differentiated fate in vitro, and thus are important, for instance, in connection with clinical applications based on stem cell therapy and in drug discovery processes.
Owner:THE BURNHAM INST
Bioactive coating composition and methods
InactiveUS6921811B2Promotes desired biologicalPromotes therapeutic effectSuture equipmentsOrganic active ingredientsArylBody fluid
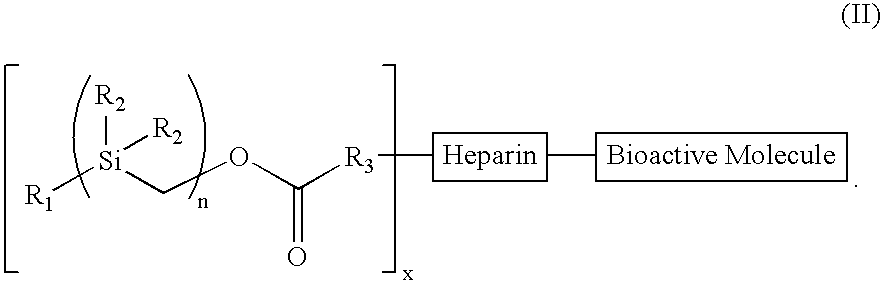
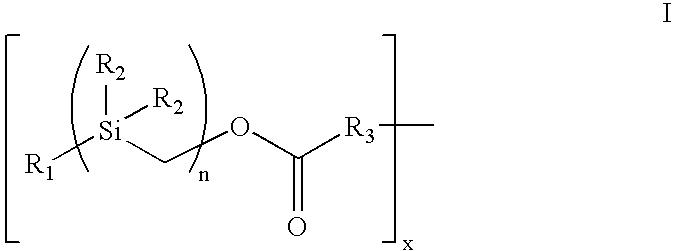
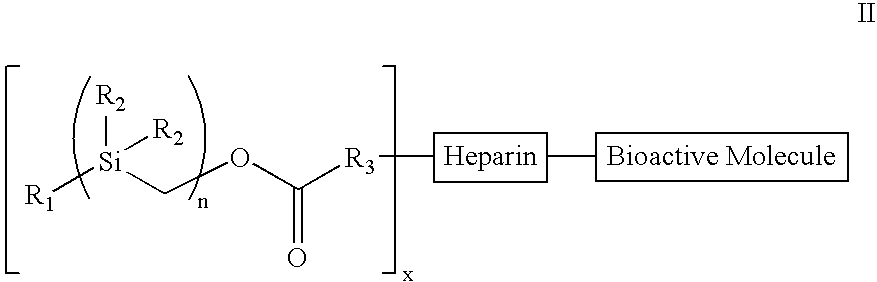
The present invention provides a bioactive coating composition, method and devices for bodily fluid-contacting surfaces. The coating comprises a complex of Formula II: wherein R1 is an C1-18alkyl or C6-32aryl group, each R2 is independently selected from the group consisting of C1-18alkyl and C6-32aryl, R3 is N or O, n is a number from 1 to 10, and x in a number from 1 to about 30, directly bound to a heparin-activity molecule via covalent bonding, with one or more bioactive molecules bound to the heparin-activity molecule. The bioactive molecule may be an adhesive molecule such as fibronectin, a growth factor such as basic fibroblast growth factor, or any other bioactive molecule that binds, by any mechanism, to a heparin-activity molecule
Owner:BIOSURFACE ENG TECH
Pluripotential embryonic stem cells and methods of making same
The present invention provides a non-mouse, including human, pluripotential embryonic stem cell which can:(a) be maintained on feeder layers for at least 20 passages; and(b) give rise to embryoid bodies and multiple differentiated cell phenotypes in monolayer culture.The invention further provides a method of making a pluripotential embryonic stem cell comprising culturing germ cells and germ cell progenitors in a composition comprising a growth enhancing amount of basic fibroblast growth factor, leukemia inhibitory factor, membrane associated steel factor, and soluble steel factor to primordial germ cells under cell growth conditions, thereby making a pluripotential embryonic stem cell.Also provided are compositions useful to produce the pluripotent embryonic stem cells and methods of screening associated with the method of making the embryonic stem cell.
Owner:VANDERBILT UNIV
Biodegradable polymer coils for intraluminal implants
An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location of a separable coil comprised at least in part of at least one biocompatible and absorbable polymer or protein and growth factors. Typically a catheter associated with the separable coil is used to dispose the coil into a selected body lumen. The biocompatible and absorbable polymer or protein is thrombogenic. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and absorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides. The biocompatible and absorbable protein is at least one protein selected from the group consisting of collagen, fibrinogen, fibronectin, vitronectin, laminin, and gelatin. In one embodiment the coil is composed of the biocompatible and absorbable polymer or protein with a radio-opaque material is disposed thereon. Alternatively, the coil is composed of a radio-opaque material, and the biocompatible and absorbable polymer or protein is disposed thereon. This apparatus may be positioned within intracranial aneurysms or any aneurysm in the body as well as within other body cavities.
Owner:RGT UNIV OF CALIFORNIA
Muteins of fibroblast growth factor 21
The present invention relates to novel muteins of human fibroblast growth factor 21 with improved pharmaceutical properties. Both protein and the respective encoding nucleic acid species are disclosed. The invention also embodies vectors and host cells for the propagation of said nucleic acid sequences and the production of said muteins. Also disclosed are methods for treating type 2 diabetes, obesity, metabolic syndrome, and in reducing the mortality and morbidity of critically ill patients.
Owner:ELI LILLY & CO
Muteins of fibroblast growth factor 21
ActiveUS7622445B2Reduce sensitivityReduced O-glycosylationPeptide/protein ingredientsMetabolism disorderMutated proteinNucleic acid sequence
The present invention relates to novel muteins of human fibroblast growth factor-21 with reduced susceptibility for proteolytic degradation when expressed in yeast. Both protein and the respective encoding nucleic acid species are disclosed. The invention also embodies vectors and host cells for the propagation of said nucleic acid sequences and the production of said muteins. Also disclosed are methods for treating type 2 diabetes, obesity, or metabolic syndrome.
Owner:ELI LILLY & CO
Muteins of fibroblast growth factor 21
The present invention relates to novel muteins of human fibroblast growth factor 21 with reduced capacity of O-glycosylation when expressed in yeast compared to wild-type human FGF-21. Both protein and the respective encoding nucleic acid species are disclosed. The invention also embodies vectors and host cells for the propagation of said nucleic acid sequences and the production of said muteins. Also disclosed are methods for treating type 2 diabetes, obesity, or metabolic syndrome.
Owner:ELI LILLY & CO
Muscle-derived cells (MDCs) for treating muscle- or bone-related injury or dysfunction
The present invention provides muscle-derived cells, preferably myoblasts and muscle-derived stem cells, genetically engineered to contain and express one or more heterologous genes or functional segments of such genes, for delivery of the encoded gene products at or near sites of musculoskeletal, bone, ligament, meniscus, cartilage or genitourinary disease, injury, defect, or dysfunction. Ex vivo myoblast mediated gene delivery of human inducible nitric oxide synthase, and the resulting production of nitric oxide at and around the site of injury, are particularly provided by the invention as a treatment for lower genitourinary tract dysfunctions. Ex vivo gene transfer for the musculoskeletal system includes genes encoding acidic fibroblast growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor, platelet derived growth factor, transforming growth factor-β, transforming growth factor-α, nerve growth factor and interleukin-1 receptor antagonist protein (IRAP), bone morphogenetic protein (BMPs), cartilage derived morphogenetic protein (CDMPs), vascular endothelial growth factor (VEGF), and sonic hedgehog proteins.
Owner:UNIVERSITY OF PITTSBURGH
Muteins of fibroblast growth factor 21
InactiveUS7655627B2Reduced deamidationReduce capacityPeptide/protein ingredientsMetabolism disorderADAMTS ProteinsWild type
The present invention relates to novel muteins of human fibroblast growth factor 21 with reduced deamidation compared to wild-type human FGF-21. Both protein and the respective encoding nucleic acid species are disclosed. The invention also embodies vectors and host cells for the propagation of said nucleic acid sequences and the production of said muteins. Also disclosed are methods for treating type 2 diabetes, obesity, or metabolic syndrome.
Owner:ELI LILLY & CO
Fibroblast growth factor-21-Fc fusion proteins
ActiveUS9006400B2Easy to controlImproved profilePeptide/protein ingredientsAntibody mimetics/scaffoldsProtein CFibroblast growth factor receptor 1
The invention relates to the identification of fusion proteins comprising polypeptide and protein variants of fibroblast growth factor 21 (FGF21) with improved pharmaceutical properties. Also disclosed are methods for treating FGF21-associated disorders, including metabolic conditions.
Owner:NOVARTIS AG
Method for in vitro amplifying, and in 3D solid culturing nerve stem
InactiveCN101092606AUniform penetrationIncrease the cultivation areaNervous system cellsCuticleCell growth
This invention relates to a method for amplifying neural stem cells in vitro by 3-dimensional culture. The method comprises: selecting microcarrier with 3-dimensional environment, pre-treating, coating the microcarrier with 40-60 ng / mL alkaline fibroblast growth factor, 40-60 ng / mL epidermal growth factor, and B27 DMEM / F12 neural stem cell serum-free culture medium, adding 1X105-1X106 neural stem cells into the culture bottle, taking out the microcarrier grown with neural stem cells, removing the microcarrier, and rinsing cells to obtain neural stem cells. The porous microcarrier can enlarge the culture area. The alkaline fibroblast growth factor and epidermal growth factor can promote cell multiple fission and improve cell microenvironment, which is advantageous for multiple fission of neural stem cells.
Owner:CHINA JAPAN FRIENDSHIP HOSPITAL
Bioabsorbable polymeric implants and a method of using the same to create occlusions
InactiveUS20020040239A1Peptide/protein ingredientsPharmaceutical containersPoly-L-lactideVascular compartment
A new embolic agent, bioabsorbable polymeric material (BPM) is incorporated to a Guglielmi detachable coil (GDC) to improve long-term anatomic results in the endovascular treatment of intracranial aneurysms. The embolic agent, comprised at least in part of at least one biocompatible and bioabsorbable polymer and growth factors, is carried by hybrid bioactive coils and is used to accelerate histopathologic transformation of unorganized clot into fibrous connective tissue in experimental aneurysms. An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location. Thrombogenicity of the biocompatible and bioabsorbable polymer is controlled by the composition of the polymer. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and bioabsorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid / poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides.
Owner:RGT UNIV OF CALIFORNIA
Method for differentiating mesenchymal stem cells into neural cells
A method for differentiating mesenchymal stem cells of bone marrow into neural cells comprises culturing the mesenchymal stem cells in a medium containing epidermal growth factor(EGF), basic fibroblast growth factor(bFGF) and hepatocyte growth factor(HGF), and the neural cells produced thereby can be employed for the treatment of a neural disease.
Owner:PHARMICELL +1
Chimeric fibroblast growth factor 23 proteins and methods of use
ActiveUS20140243260A1Peptide/protein ingredientsAntibody mimetics/scaffoldsChimera ProteinFibroblast growth factor 23
The present invention relates to an isolated chimeric protein. The isolated chimeric protein includes an N-terminus coupled to a C-terminus, where the N-terminus includes an N-terminal portion from a fibroblast growth factor (“FGF”) 23 molecule and the C-terminus includes a C-terminal portion from an FGF19 molecule. The present invention also relates to a pharmaceutical composition including an isolated chimeric protein and a pharmaceutically acceptable carrier. The isolated chimeric protein includes an N-terminus coupled to a C-terminus, where the N-terminus includes an N-terminal portion from a fibroblast growth factor (“FGF”) 23 molecule and the C-terminus includes a C-terminal portion from an FGF19 molecule, and a pharmaceutically-acceptable carrier. Yet another aspect of the present invention relates to a method for treating a subject suffering from a disorder. This method includes selecting a subject suffering from the disorder and administering to the subject a therapeutically effective amount of a chimeric protein according to the present invention.
Owner:NEW YORK UNIV
Fibroblast growth factor-like polypeptides
The present invention provides novel Fibroblast Growth Factor-like (FGF-like) polypeptides and nucleic acid molecules encoding the same. The invention also provides vectors, host cells, antibodies and methods for producing FGF-like polypeptides. Also provided for are methods for the diagnosis and treatment of diseases associated with FGF-like polypeptides.
Owner:AMGEN INC
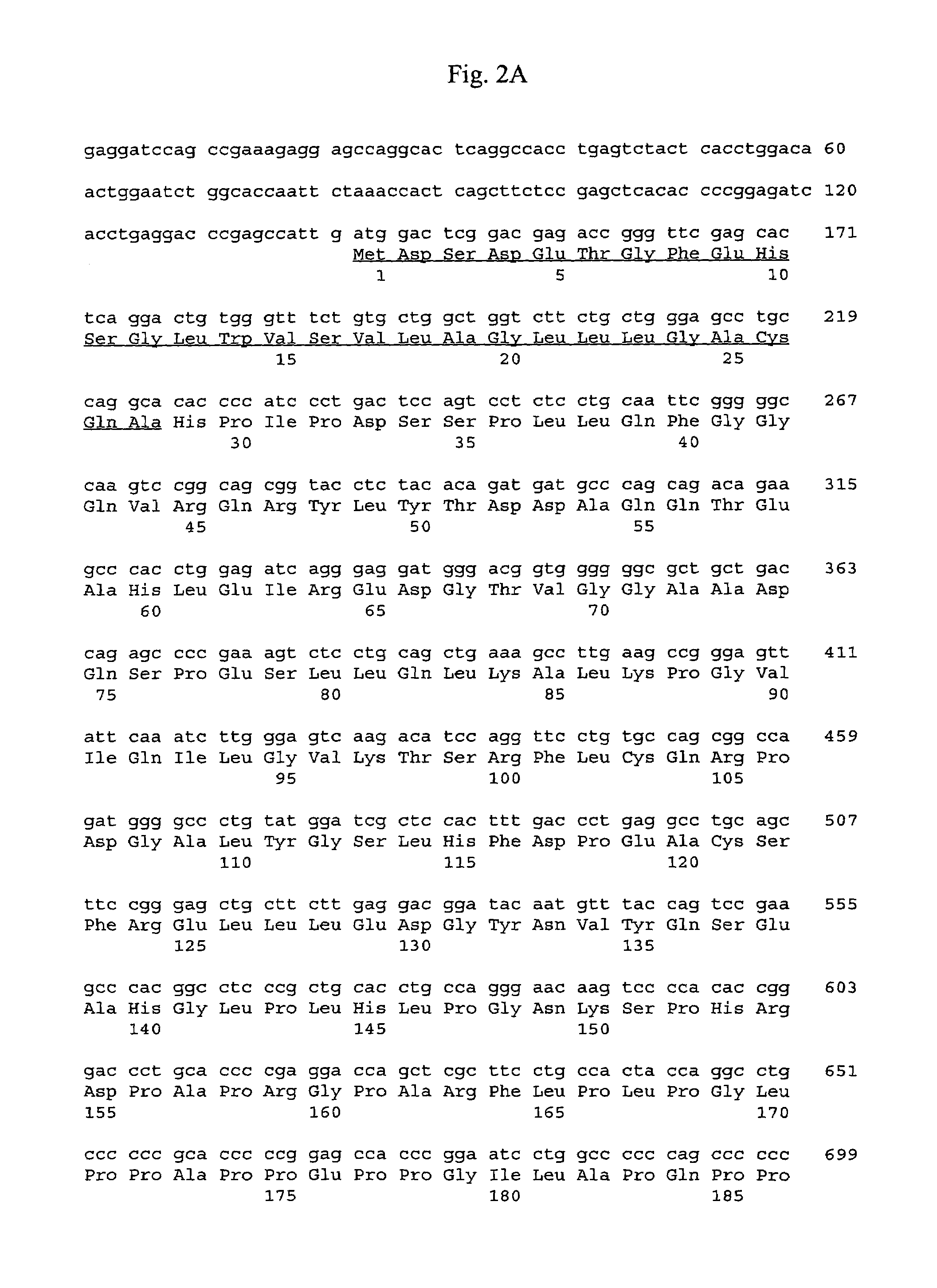
Clinical-grade human mesenchymal stem cell serum-free complete medium
ActiveCN103243071APromote growthLow toxicitySkeletal/connective tissue cellsInsulin-like growth factorCuticle
The invention relates to a human mesenchymal stem cell culture medium. According to the culture medium, the basal culture medium comprises the following components based on the final concentration: 1-2g / L of human serum albumin, 5-10mg / L of transferring, 2-8mg / L of fibronectin, 1-4mg / L of laminin, 50g / L of Fe(NO3)3.9H2O, 417g / L of FeSO4.7H2O, 1-3mu g / L of estradiol, 2-5mu g / L of testosterone, 1-3mu g / L of progesterone, 39.25-117.74 mu g / L of dexamethasone, 5-10mg / L of insulin, 376.36mg / L of riboflavin, 80.96-242.87mg / L of coenzyme A, 4.41-6.17mg / L of butanediamine, 1-2mg / L of taurine, 0.61-1.85mg / L of aminoethanol, 8.81-26.42mg / L of pyruvic acid, 3.78-7.56mu g / L of sodium selenate, 292.3-584.6mg / L of L-glutamine, 2-8mu g / L of vascular endothelial growth factor, 4-10mu g / L of epidermal growth factor, 4-10mu g / L of basic fibroblast growth factor, 1-5mu g / L of leukaemia inhibitory factor, 1-5mu g / L of insulin-like growth factor-I and 2-8mu g / L of stem cell factor. The culture medium does not contain the animal serum, the potential animal endogenous endotoxin or virus of the animal serum is eliminated, and the culture medium is conveniently applied to clinics.
Owner:QINGDAO RESTORE BIOTECHNOLOGY CO LTD
Complete medium with low serum concentration for cultivating mesenchymal stem cells and method for cultivating mesenchymal stem cells using same
The invention discloses a complete medium with low serum concentration for cultivating mesenchymal stem cells and a method for cultivating the mesenchymal stem cells using same. The complete medium comprises a cell basic medium, fetal calf serum with final concentration of 1-100 mul / ml, an epidermal growth factor with final concentration of 1-100 ng / ml, and a basic fibroblast growth factor with final concentration of 1-100 ng / ml. The complete medium with low serum concentration successfully reaches equal or even better function of promoting cell proliferation than a culture reagent with high serum concentration. The cultured cells have the typical biological characteristics of mesenchymal stem cells, and can also express an omnipotent mark of the embryonic stem cell and high express the idiosyncratic mark of the neuron under the condition of in vitro inducement. And the difference between the cell batches is little, the cost is low and the security is good. Compared with the prior cultivating method, the method has advantages of simple operation, low probability of pollution and high success ratio of cultivating cells.
Owner:INST OF HEMATOLOGY & BLOOD HOSPITAL CHINESE ACAD OF MEDICAL SCI +1
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in mammals using carprofen and derivatives
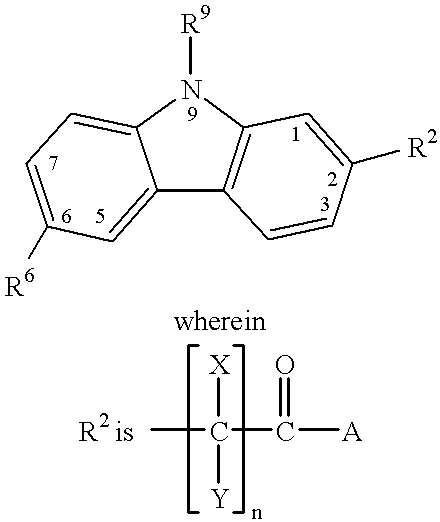
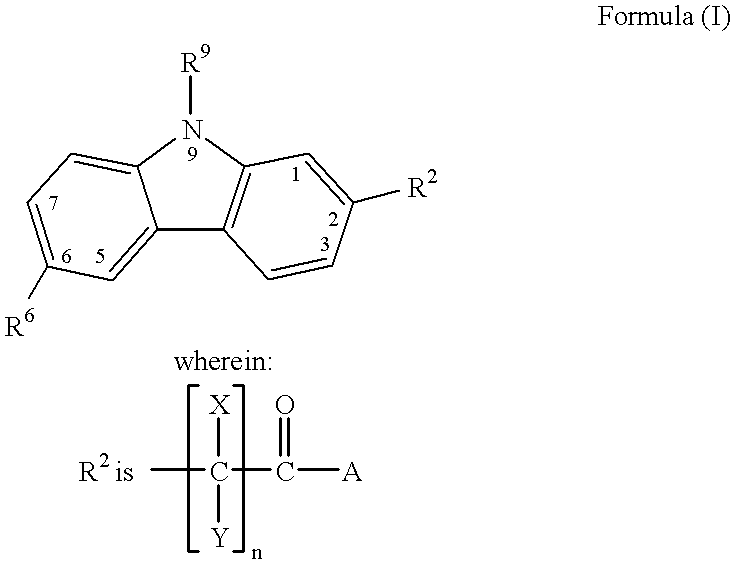
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in the affected joint of a mammal is accomplished by administering a chondroprotective compound of Formula (I):where A is hydroxy, (C1-C4)alkoxy, amino, hydroxy-amino, mono-(C1-C2)alkylamino, di-(C1-C2)alkylamino; X and Y are independently H or (C1-C2)alkyl; and n is 1 or 2; R6 is halogen, (C1-C3)alkyl, trifluoromethyl, or nitro; R9 is H; (C1-C2)alkyl; phenyl or phenyl-(C1-C2)alkyl, where phenyl is optionally mono-substituted by fluoro or chloro; -C(=O)-R, where R is (C1-C2)alkyl or phenyl, optionally mono-substituted by fluoro or chloro; or -C(=O)-O-R', where R1 is (C1-C2)alkyl.This treatment ameliorates, diminishes, actively treats, reverses or prevents any injury, damage or loss of articular cartilage or subchondral bone subsequent to said early stage of said degeneration. Whether or not a mammal needs such treatment is determined by whether or not it exhibits a statistically significant deviation from normal standard values in synovial fluid or membrane from the affected joint, with respect to at least five of the following substances: increased interleukin-1 beta (IL-1beta); increased tumor necrosis factor alpha (TNFalpha); increased ratio of IL-1beta to IL-1 receptor antagonist protein (IRAP); increased expression of p55 TNF receptors (p55 TNF-R); increased interleukin-6 (IL-6); increased leukemia inhibitory factor (LIF); decreased insulin-like growth factor-1 (IGF-1); decreased transforming growth factor beta (TGFbeta); decreased platelet-derived growth factor (PDGF); decreased basic fibroblast growth factor (b-FGF); increased keratan sulfate; increased stromelysin; increased ratio of stromelysin to tissue inhibitor of metalloproteases (TIMP); increased osteocalcin; increased alkaline phosphatase; increased cAMP responsive to hormone challenge; increased urokinase plasminogen activator (uPA); increased cartilage oligomeric matrix protein; and increased collagenase.
Owner:PFIZER INC +1
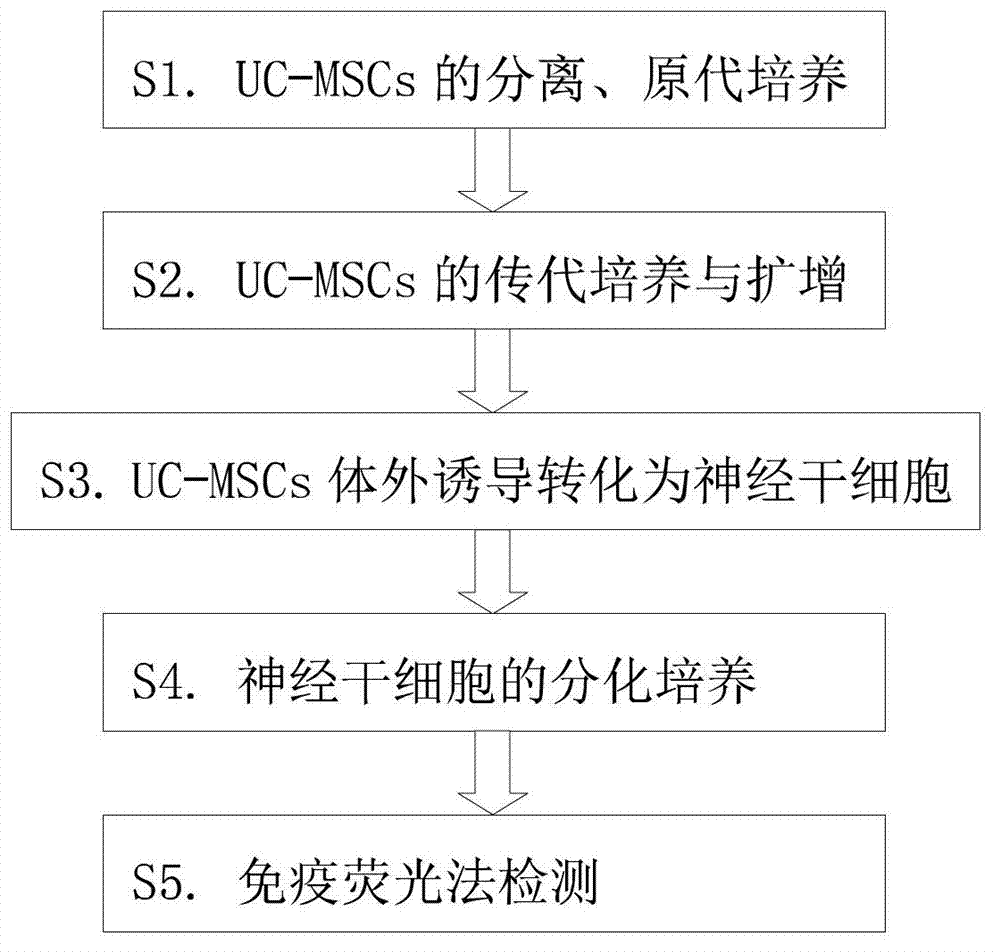
Inducing method for differentiating umbilical cord mesenchymal stem cells into neural stem cells
InactiveCN103031275AVigorousNervous system cellsSkeletal/connective tissue cellsGerm layerMesenchymal stem cell
The invention provides an inducing method for differentiating umbilical cord mesenchymal stem cells (UC-MSCs) into neural stem cells. The inducing method comprises the following steps: carrying out separation and primary culture of UC-MSCs, subculture and amplification of UC-MSCs, induction in vitro of UC-MSC to be transformed into neural stem cells, and differentiation culture of neural stem cells and detection by an immumofluorescence method. The inducing method provided by the invention uses all-transretinoic acid combined with alkaline fibroblast growth factors (bFGF) and epidermal growth factor (EGF) to induce the UC-MSCs to be transformed into neural stem cells, wherein the UC-MSCs still have stronger activity after repeated passage by division growth. The transretinoic acid is combined with cytokines to induce the UC-MSCs to be converted into the neural stem cells, so that the mesenchymal cells are differentiated into non mesenchymal cells cross germinal layer, and the mesenchymal cells probability become more ideal seed cells in the future clinical application.
Owner:陆华
Serum-free culture medium for mesenchymal stem cells
ActiveCN102433302AGood growthFast growthSkeletal/connective tissue cellsINSULIN HUMANPancreatic hormone
The invention discloses a serum-free culture medium for mesenchymal stem cells. The serum-free culture medium for the mesenchymal stem cells comprises the following ingredients: fibronectin at the final concentration of 25mu g / ml, basic fibroblast growth factors at the final concentration of 10ng / ml, human epidermal growth factors at the final concentration of 15ng / ml, recombinant human insulin at the final concentration of 1mg / ml, human transferrin at the final concentration of 0.55mg / ml, human blood albumin in a volume ratio of 5 percent, sodium selenite at the final concentration of 0.67mug / ml, L-carnitine at the final concentration of 5mM and resveratrol at the final concentration of 30mu M. When the mesenchymal stem cells are cultured by the serum-free culture medium for the mesenchymal stem cells, animal-derived serum is not contained, so infection risks can be controlled; the L-carnitine and the resveratrol which are added into the serum-free culture medium for the mesenchymalstem cells can effectively improve the growth state of the mesenchymal stem cells; and the growth speed of the mesenchymal stem cells is remarkably improved, and the biological characteristics of themesenchymal stem cells are kept unchanged.
Owner:CHENGDU QINGKE BIOTECH
Serum-free medium for umbilical cord mesenchymal stem cells
InactiveCN105420182AOvercoming pollutionOvercoming Cell Expansion NumbersSkeletal/connective tissue cellsPancreatic hormoneStem cell culture
The invention provides a serum-free medium for umbilical cord mesenchymal stem cells and belongs to the technical field of cell culture. The serum-free medium for umbilical cord mesenchymal stem cells comprises a basal culture medium body and added ingredients, wherein the basal culture medium body is a DMEM culture medium; the added ingredients include a basic fibroblast growth factor hFGF, a epidermal growth factor hEGF, insulin hI, a leukaemia inhibitory factor hLIF and astragalus polysaccharide. The umbilical cord mesenchymal stem cells are subjected to subculture and amplification in the medium, and the surfaces of the cultured cells are marked and analyzed. The serum-free medium for umbilical cord mesenchymal stem cells overcomes the defect of exogenous pollution of serum and solves the problem of contradiction between the cell expansion number and cost reduction. The medium is free of serum, thereby preventing influence of animal-derived serum ingredients on cell culture. The medium can be used for studying the differentiation and proliferation adjusting mechanism of the umbilical cord mesenchymal stem cells.
Owner:SHANDONG JINGYUAN BIOTECH CO LTD
Dual function proteins for treating metabolic disorders
ActiveUS20130129724A1Easy to controlImproved profilePeptide/protein ingredientsAntibody mimetics/scaffoldsFibre cellMetabolic profile
The present invention relates to the identification of new proteins comprising fibroblast growth factor 21 (FGF21) and other metabolic regulators, including variants thereof, known to improve metabolic profiles in subjects to whom they are administered. Also disclosed are methods for treating FGF21-associated disorders, GLP-1-associated disorders, and Exendin-4-associated disorders, including metabolic conditions.
Owner:NOVARTIS AG
Remodeling and glycopegylation of fibroblast growth factor (FGF)
ActiveUS20120172300A1Cost effectiveImprove pharmacokineticsSenses disorderNervous disorderFibre cellMutant
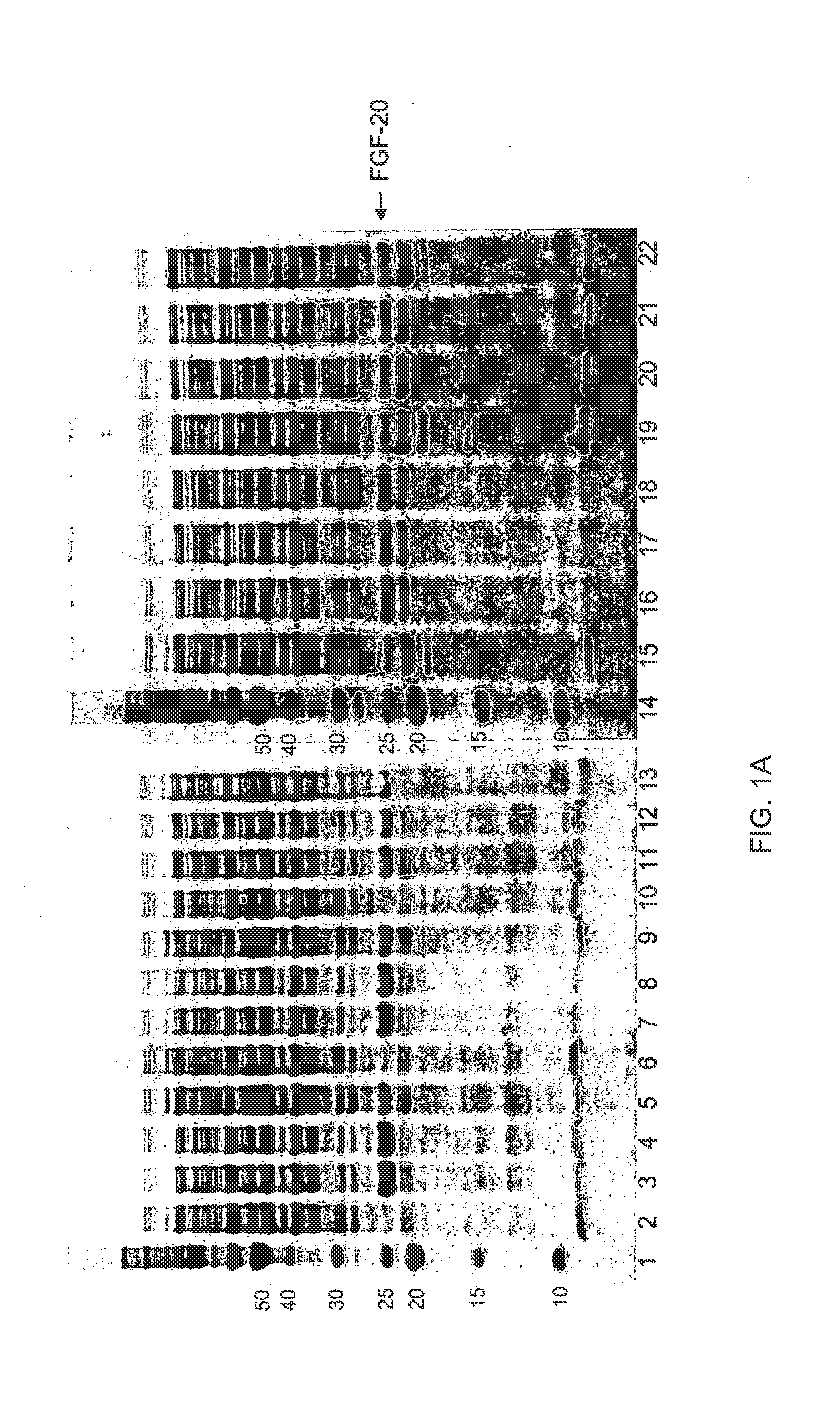
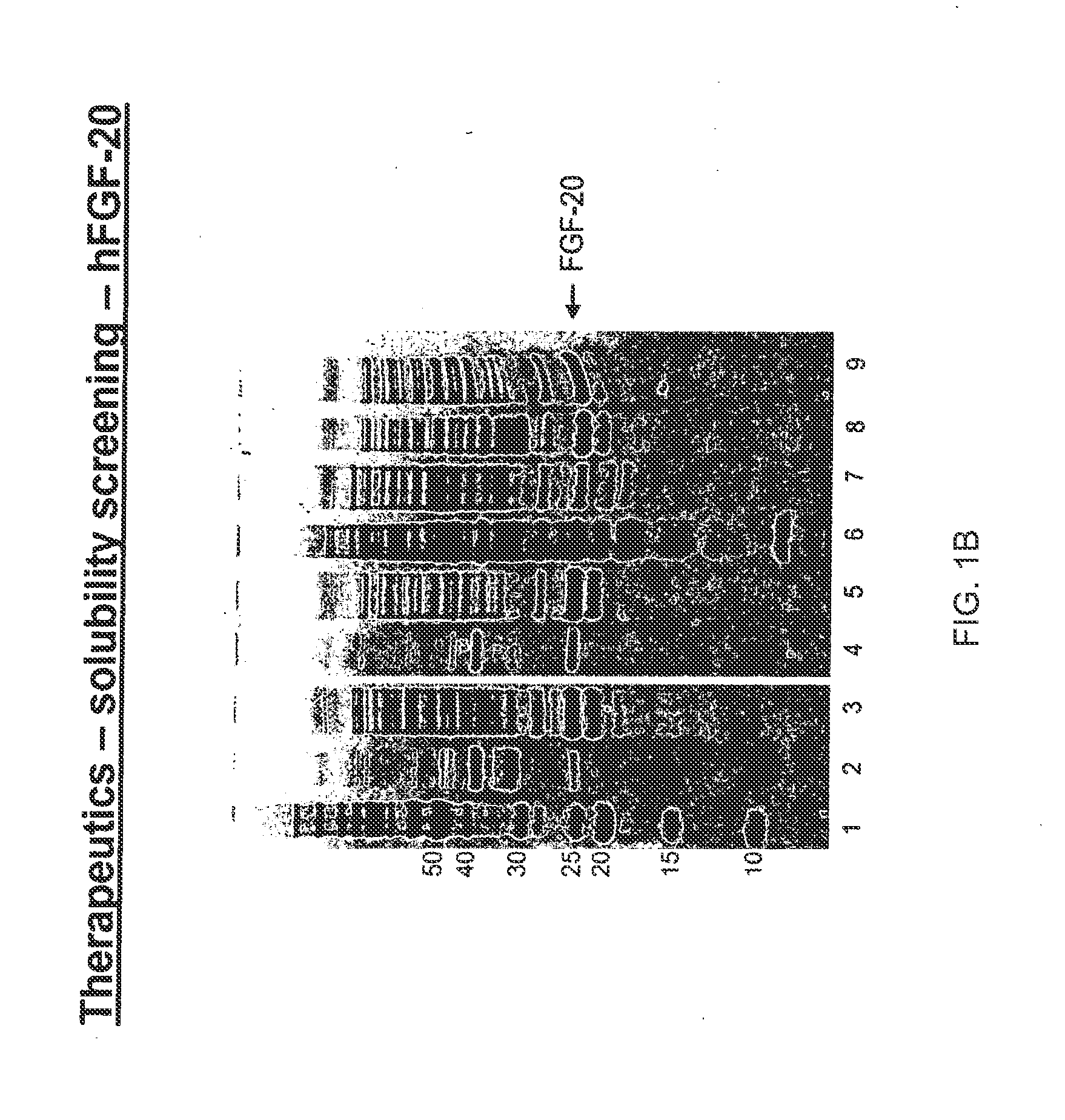
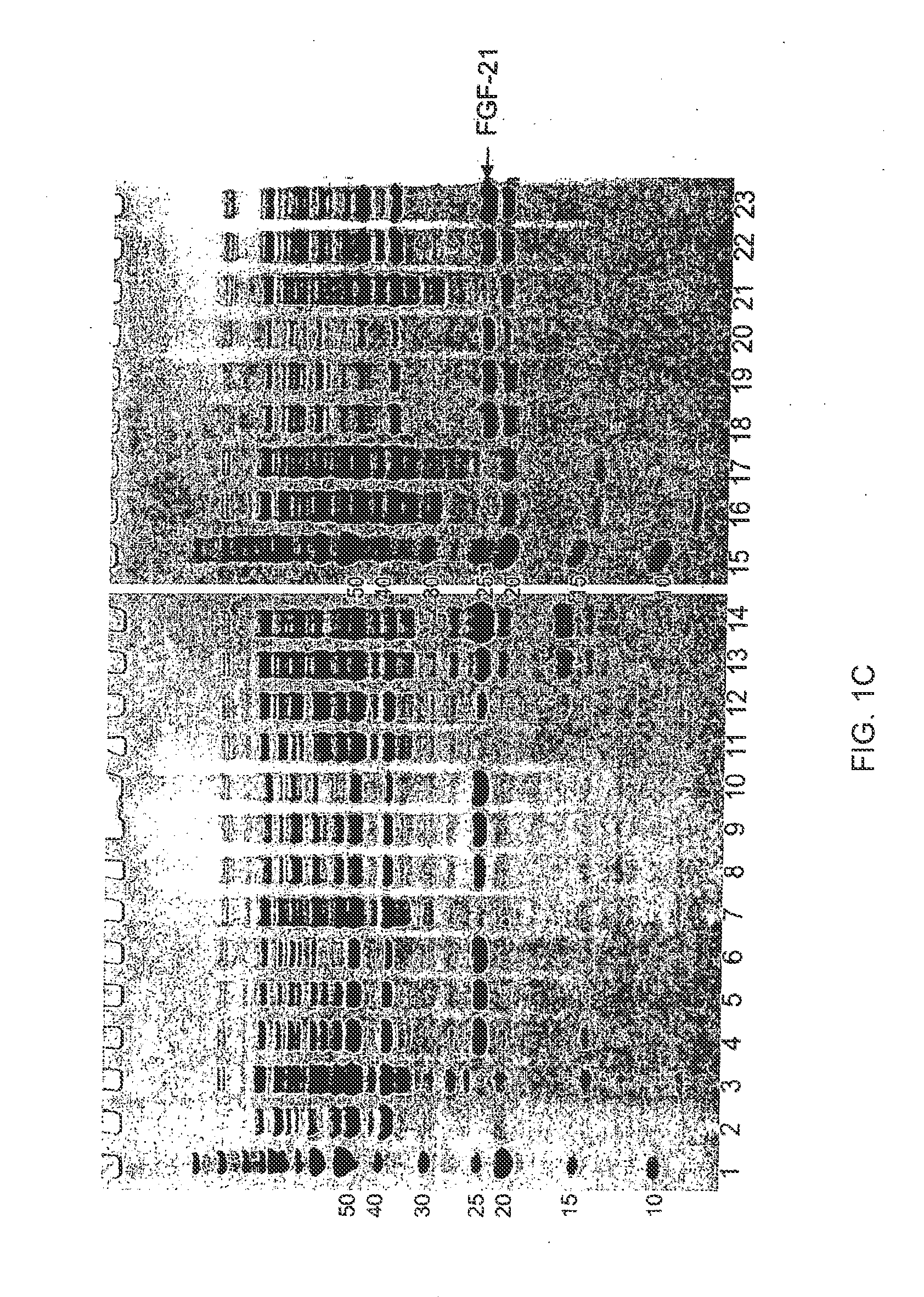
The present invention relates to mutants of Fibroblast Growth Factor (FGF), particularly FGF-20 and FGF-21, which contain newly introduced N-linked or O-linked glycosylation site(s). The polynucleotide coding sequences for the mutants, expression cassettes comprising the coding sequences, cells expressing the mutants, and methods for producing the mutants are also disclosed. Further disclosed are pharmaceutical compositions comprising the mutants and method for using the mutants.
Owner:89BIO LTD
Separating and culturing process of human amnion mesenchyme stem cell and its medical composition
ActiveCN1810959AWide variety of sourcesUnrestricted by ethicsMammal material medical ingredientsSkeletal/connective tissue cellsSingle cell suspensionCulture mediums
The present invention is separating and culturing process of human amnion mesenchyme stem cell and its medical composition. The separating and culturing process includes digesting human amnion successively with trypsin, collagenase and deoxyribonuclease, and filtering to prepare single cell suspension; culturing in DMEM / F12 culture medium with VDMEM and VF12 in the equal ratio and containing ox embryo blood serum in 10-20 vol% and basic fibroblast growth factor of ultimate concentration 10-20 ng / ml inside a culture box at 37 deg.c, saturated humidity and CO2 in 5 vol%; and replacing liquid and culture passage to proliferate and purify human amnion mesenchyme stem cell. The process has wide material source no ethnic limitation and wide application foreground. The medical composition may be used in various kinds of treatment.
Owner:SHENZHEN BEIKE BIOTECH +4
Culture medium containing human umbilical cord mesenchymal stem cell exudates and preparation method and applications thereof
The invention relates to a culture medium containing human umbilical cord mesenchymal stem cell exudates and a preparation method and applications thereof. An umbilical cord mesenchymal stem cell finite cell line is established to identify biological characteristics of the umbilical cord mesenchymal stem cell, and the preparation method for the human umbilical cord mesenchymal stem cell exudates is established to verify that the human umbilical cord mesenchymal stem cell (HUCMSC) exudates can promote cell regeneration, improve cell function and suppress cell apoptosis in vitro. A detection of enzyme-linked immunosorbent assay (ELISA) shows that human umbilical cord mesenchymal stem cell exudates contain abundant cell factors which can promote cell proliferation and suppress cell apoptosis, such as hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF).
Owner:斯坦姆生物科技江苏有限公司
Mutant polypeptides of fibroblast growth factor 1
ActiveUS7595296B1Good effectFacilitated DiffusionPeptide/protein ingredientsDepsipeptidesWild typeX-ray
The β-trefoil protein human fibroblast growth factor-1 (FGF-1) is made up of a six-stranded anti-parallel β-barrel closed off on one end by three β-hairpins, thus exhibiting a three-fold axis of structural symmetry. The N- and C-termini β-strands hydrogen bond to each other and are postulated from both NMR and X-ray structure data to represent a structurally-weakened region of the β-barrel. Val mutations within the N- and C-termini β-strands are shown to stabilize the structure and to increase van der Waals contacts by filling local cavities present within this region. Mutations that increase van der Waals contacts between both the N- and C-termini β-strands are generally associated with significant reductions in the unfolding kinetics, and also increase the cooperativity of unfolding. Surprisingly, several mutant polypeptides herein disclosed greatly exceed the wild-type polypeptide in ability to stimulate human fibroblasts to proliferate.
Owner:FLORIDA STATE UNIV RES FOUND INC
Fibroblast growth factor 21 mutations
InactiveUS9023791B2Good biological propertiesPeptide/protein ingredientsAntibody mimetics/scaffoldsBiologyFGF21
The present invention provides novel polypeptide and protein variants of fibroblast growth factor 21 (FGF21) and pharmaceutical compositions comprising FGF21 polypeptide and protein variants.
Owner:NOVARTIS AG
Preparation and application of human fibroblast growth factor 21 fusion protein and mutant of human fibroblast growth factor 21 fusion protein
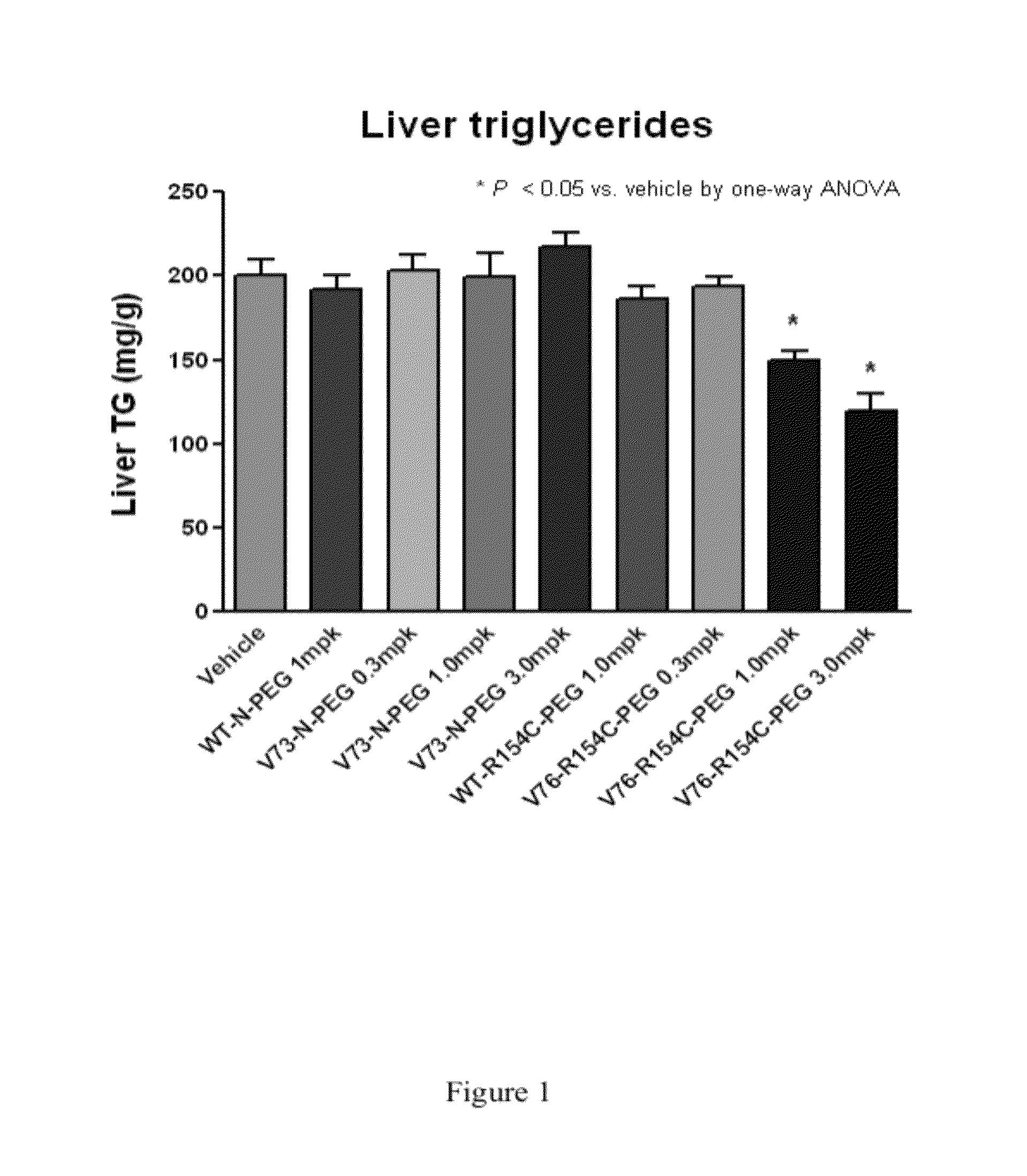
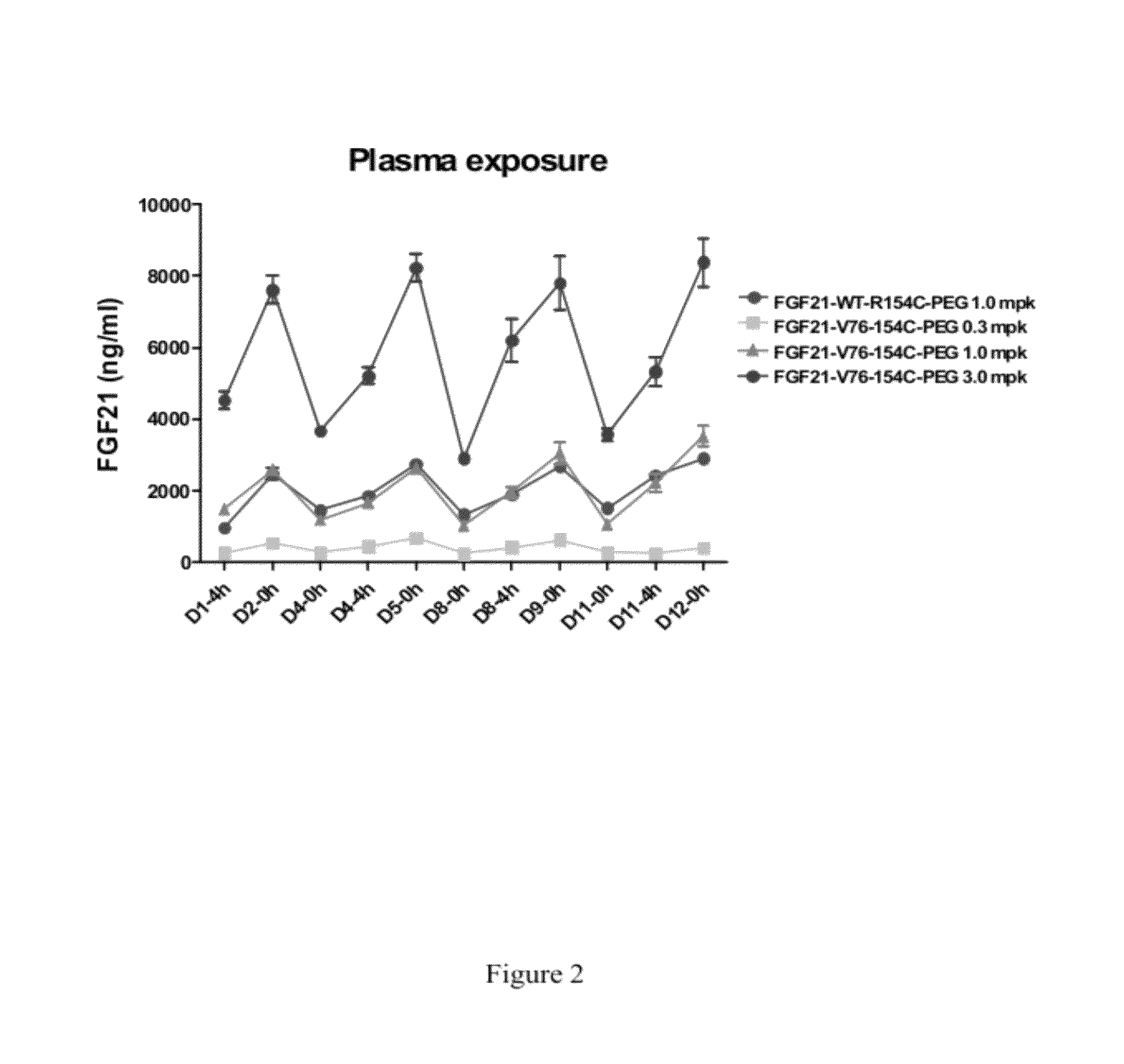
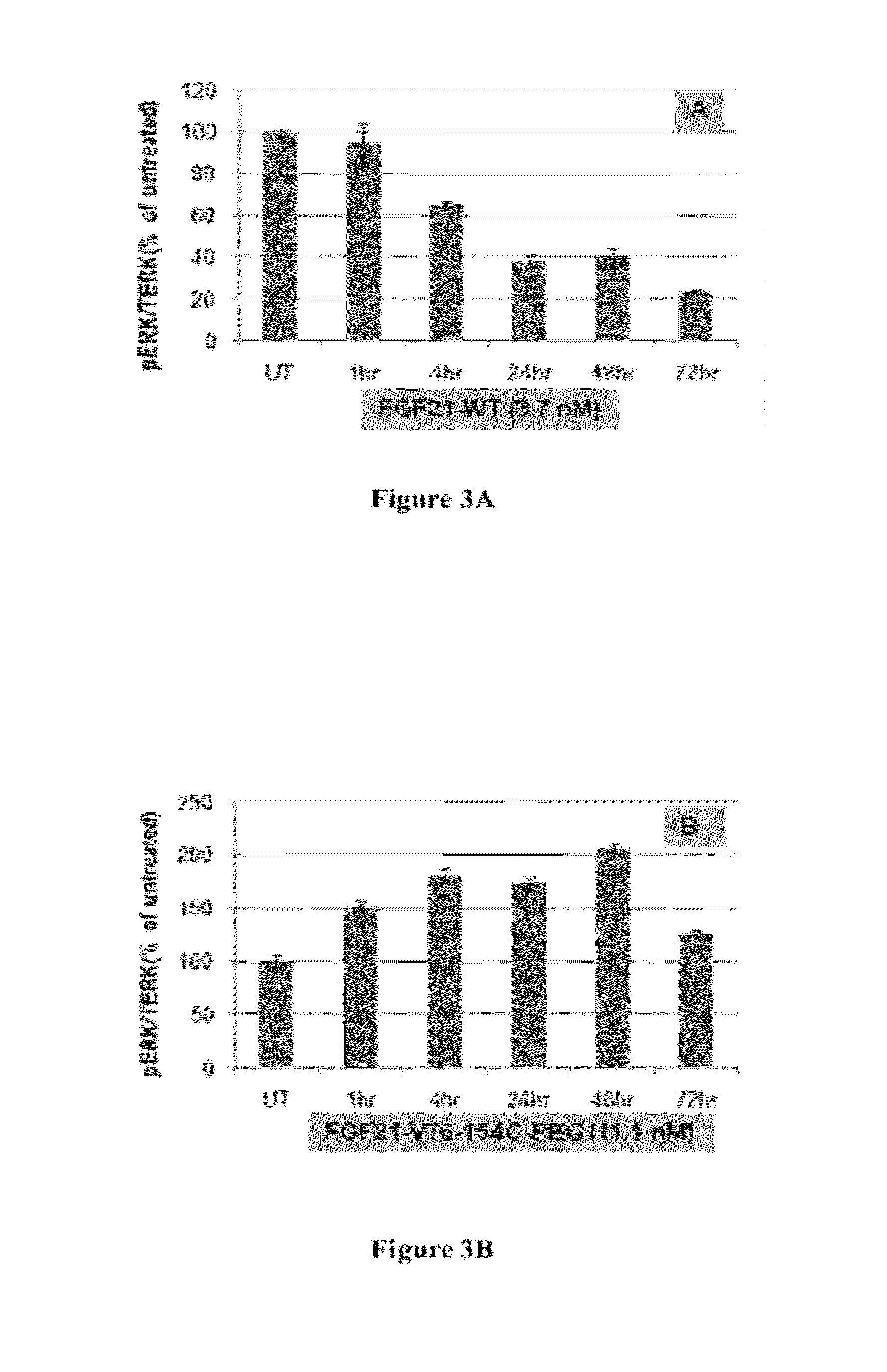
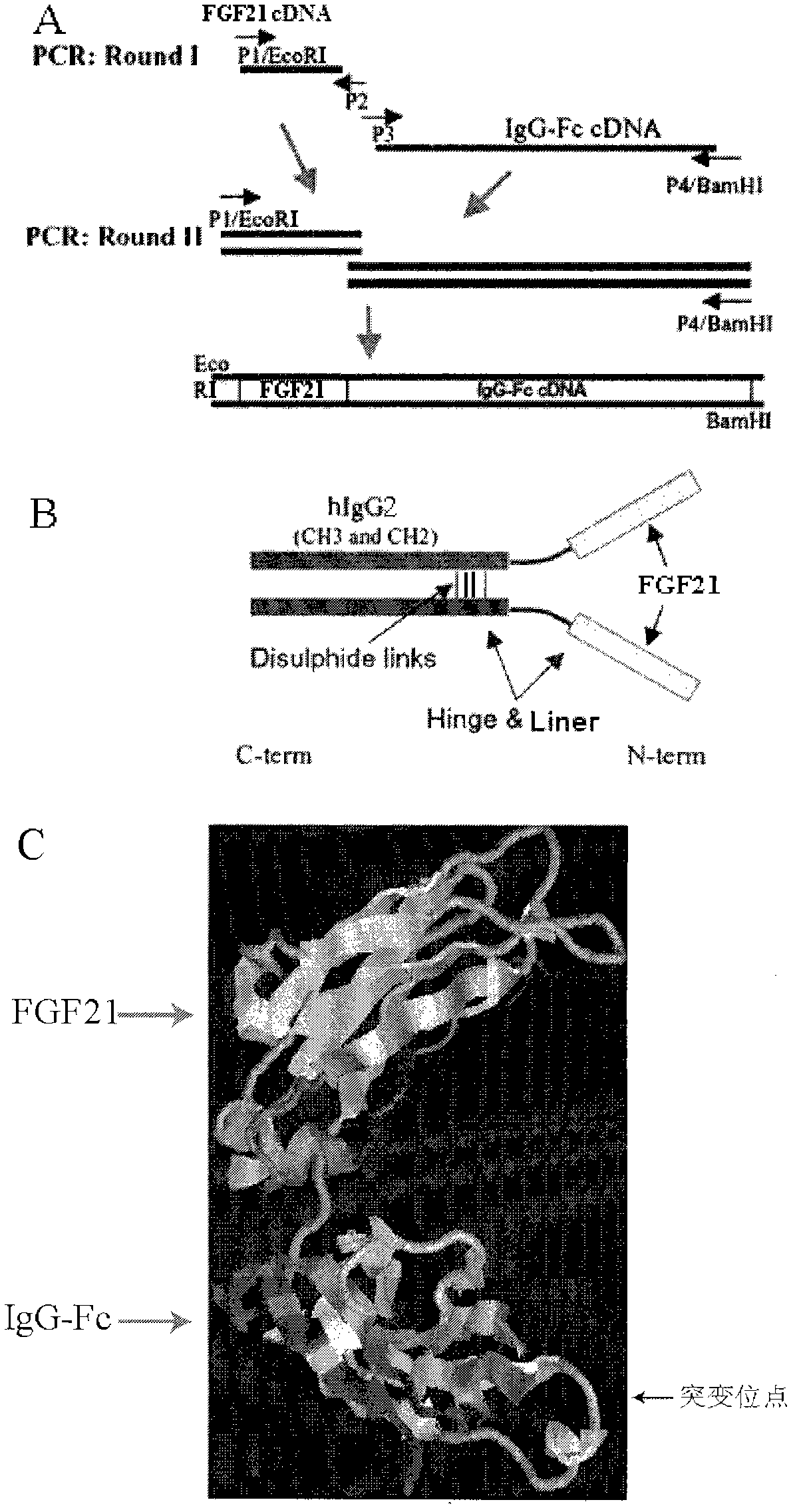
InactiveCN102558358AGood effectStrong penetrating powerPeptide/protein ingredientsMetabolism disorderHalf-lifeHinge region
The invention provides a fusion protein used for detecting and treating human I-type diabetes mellitus and human II-type diabetes mellitus and obesity as well as a preparation and an application of the fusion protein. The fusion protein contains a human FGF21 or a mutant thereof and a human IgG2-Fc (hinge region -CH2-CH3) or a human IgG2-Fc mutant ( T250Q / M428L and T307Q / N434A), can improve the living condition of the obesity patients, reduce the blood sugar level of diabetics, effectively reduce aggregation and degradation of the FGF21 protein, and meanwhile prolong in vivo half-life period of the FGF21, and especially through the fusion of the FGF21 and the mutant and the IgG-Fc (T250Q / M428L and T307Q / N434A) mutant, the affinity with FcRn is enhanced, and in vivo half-life period of the FGF21 is further prolonged.
Owner:北京康明百奥科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com