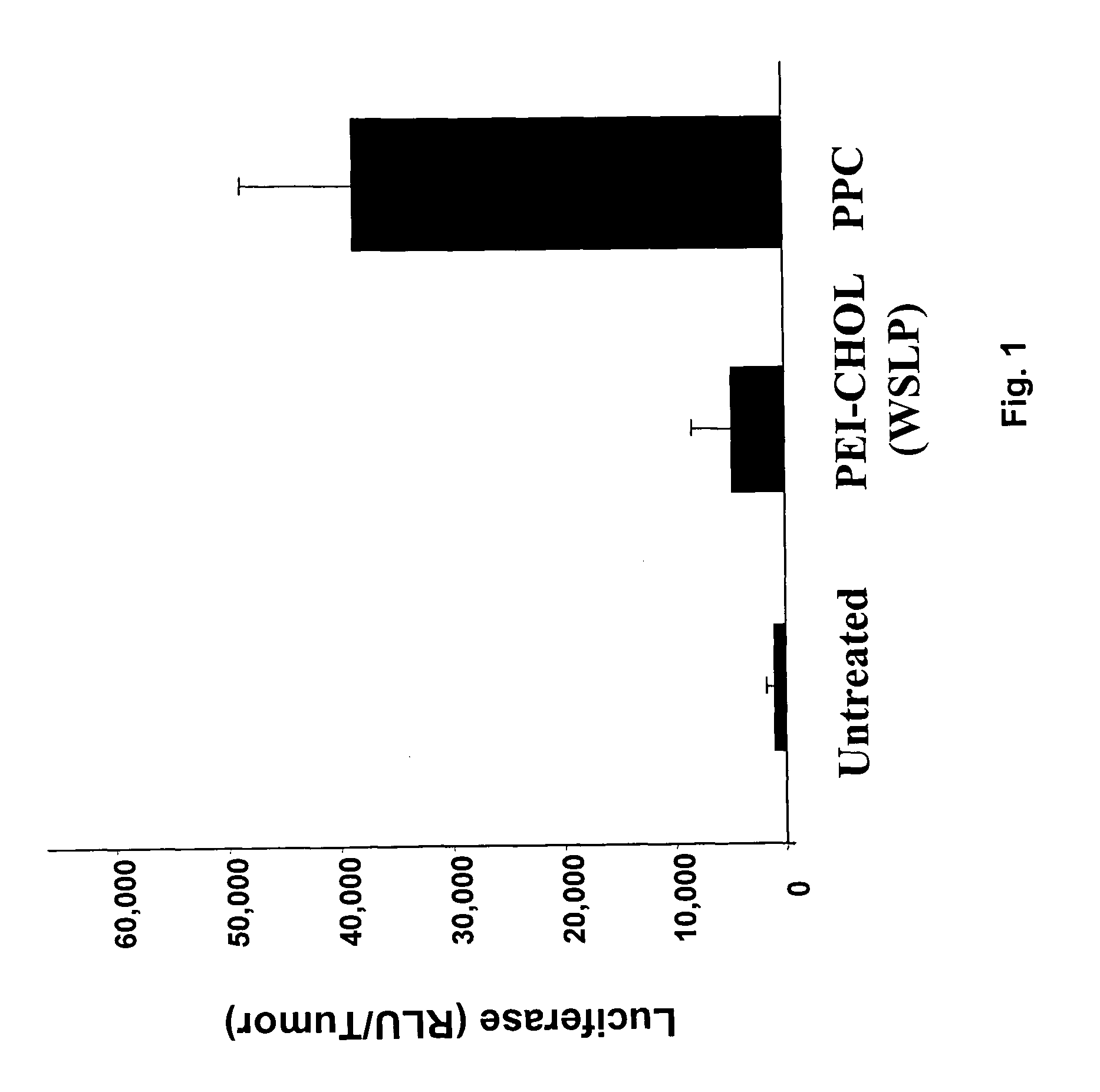
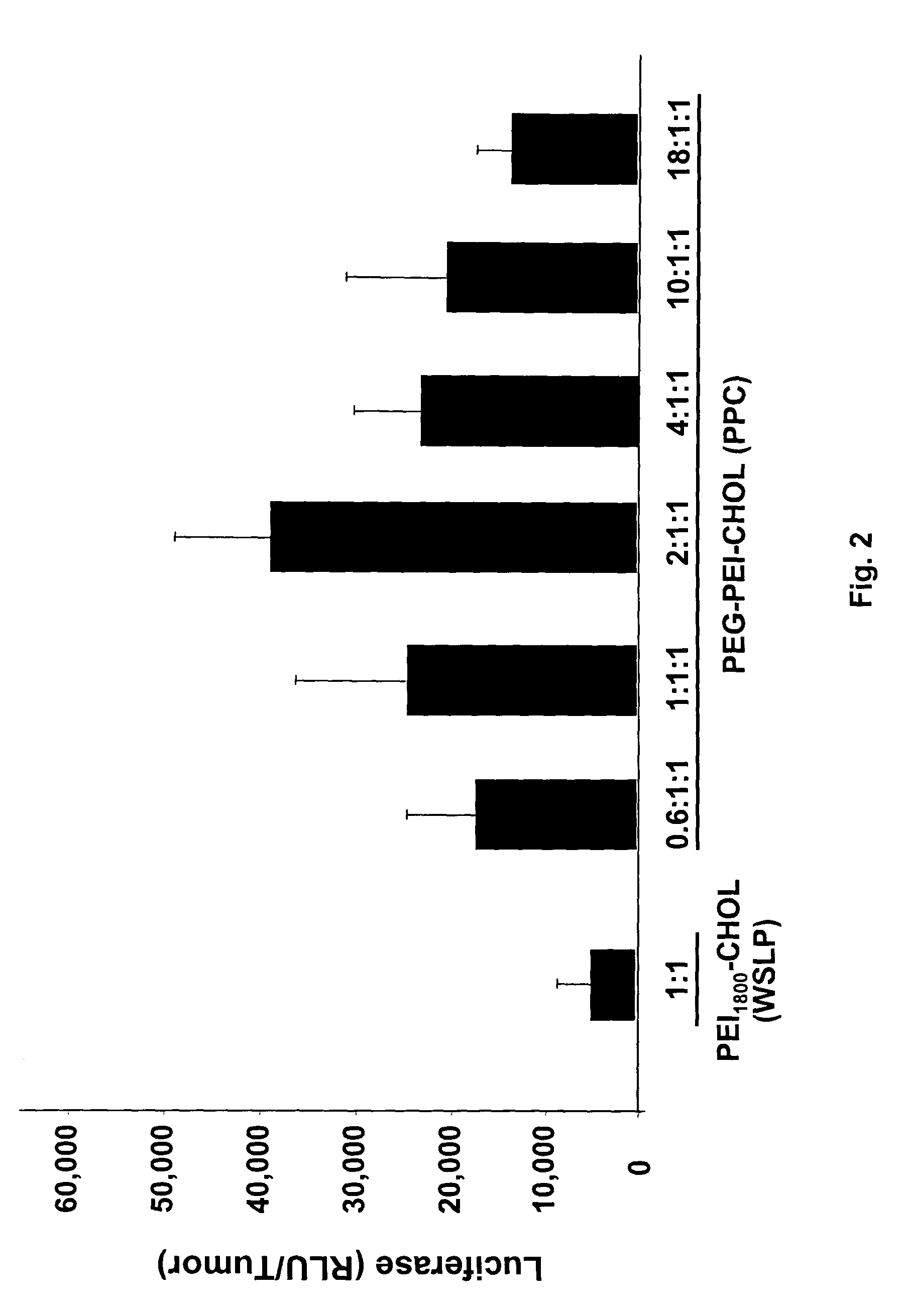
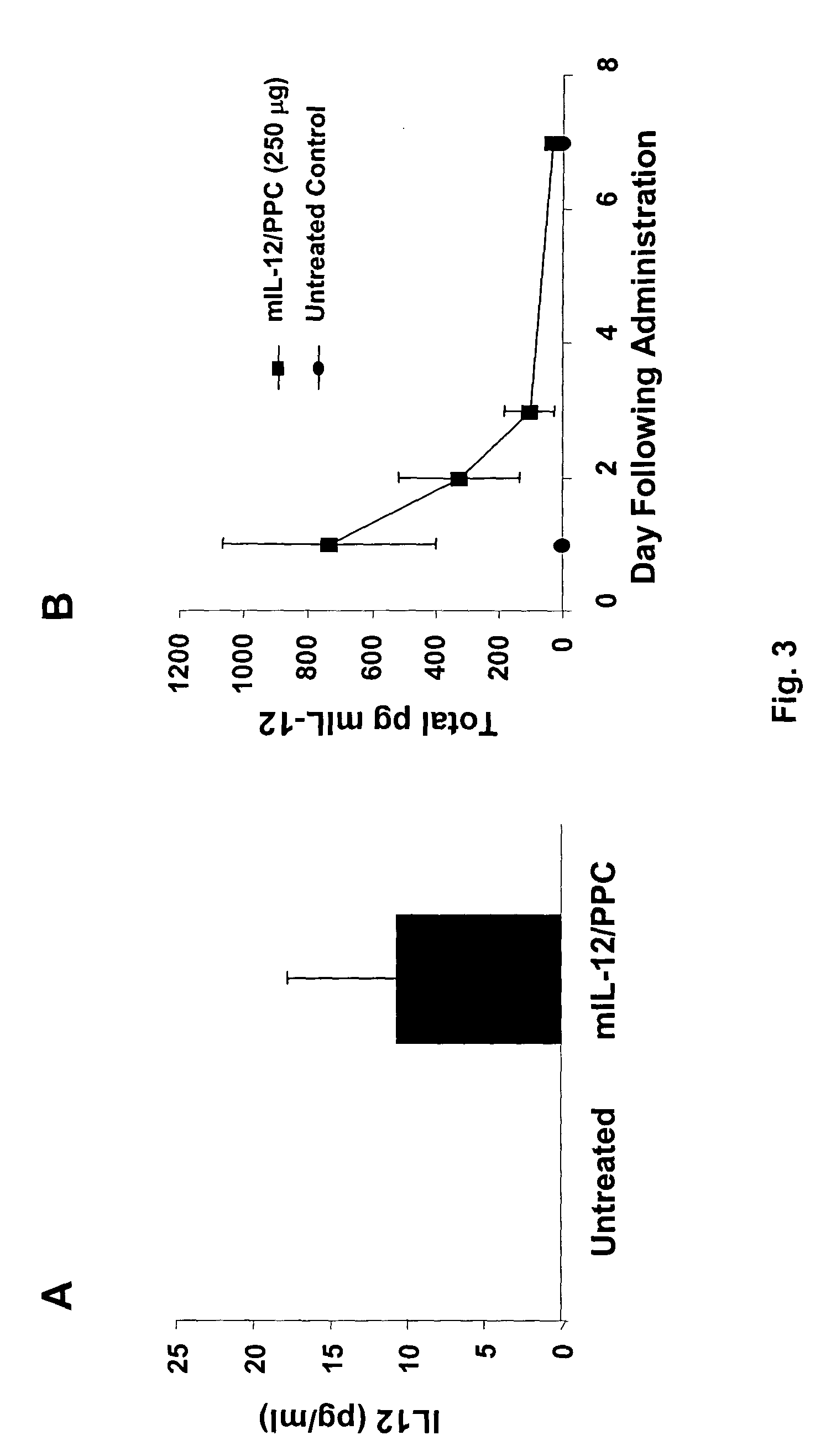
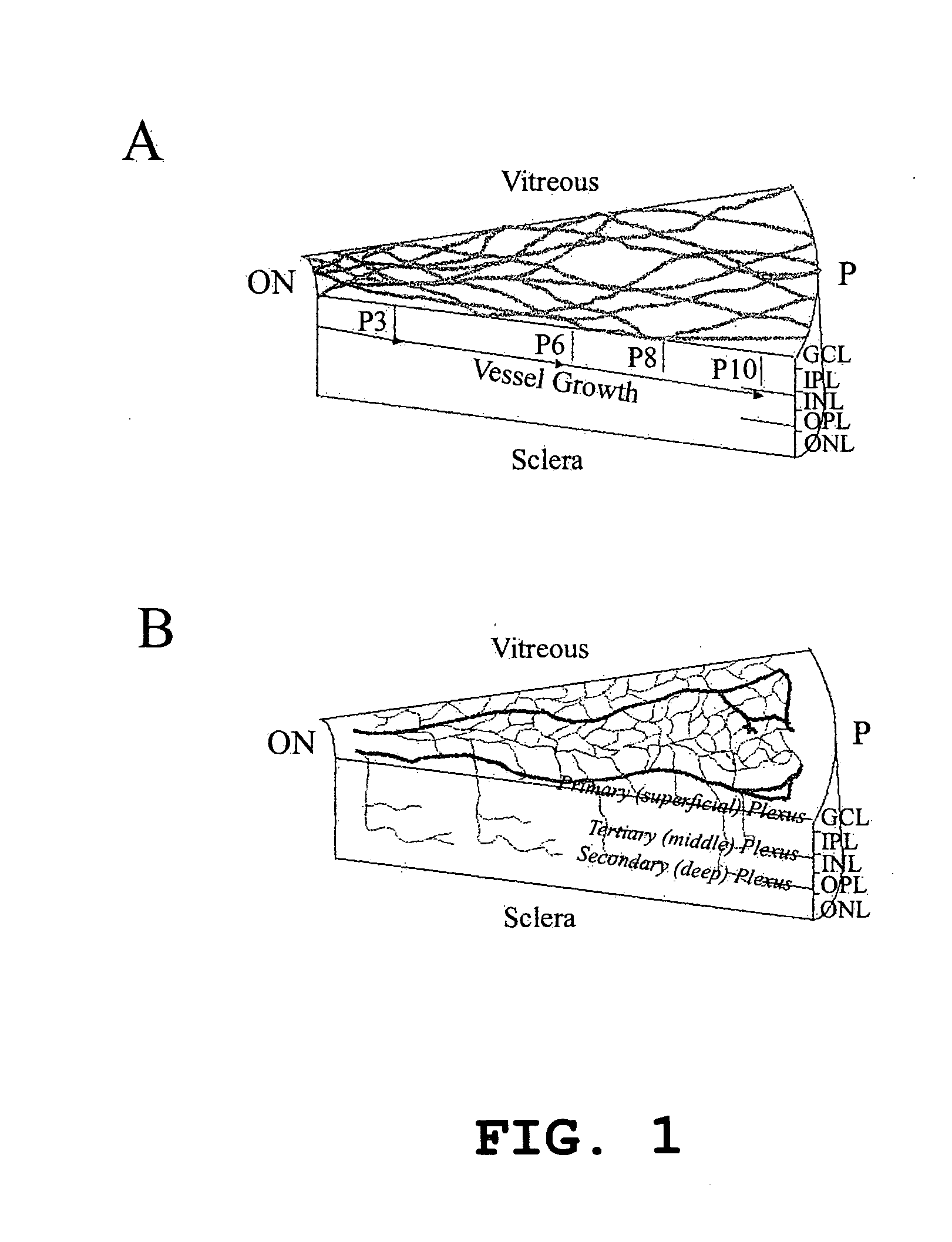
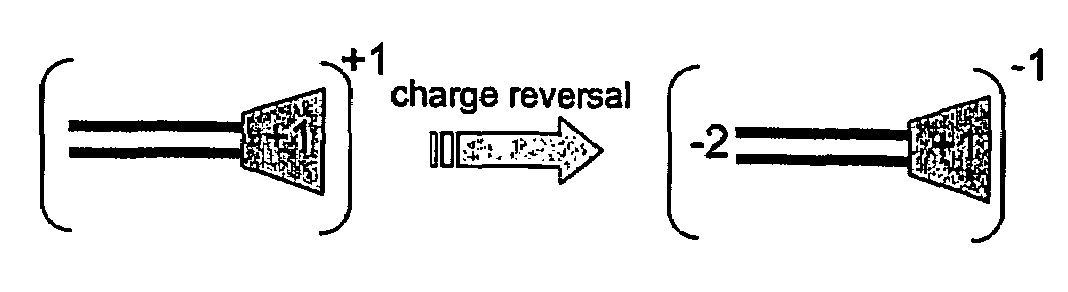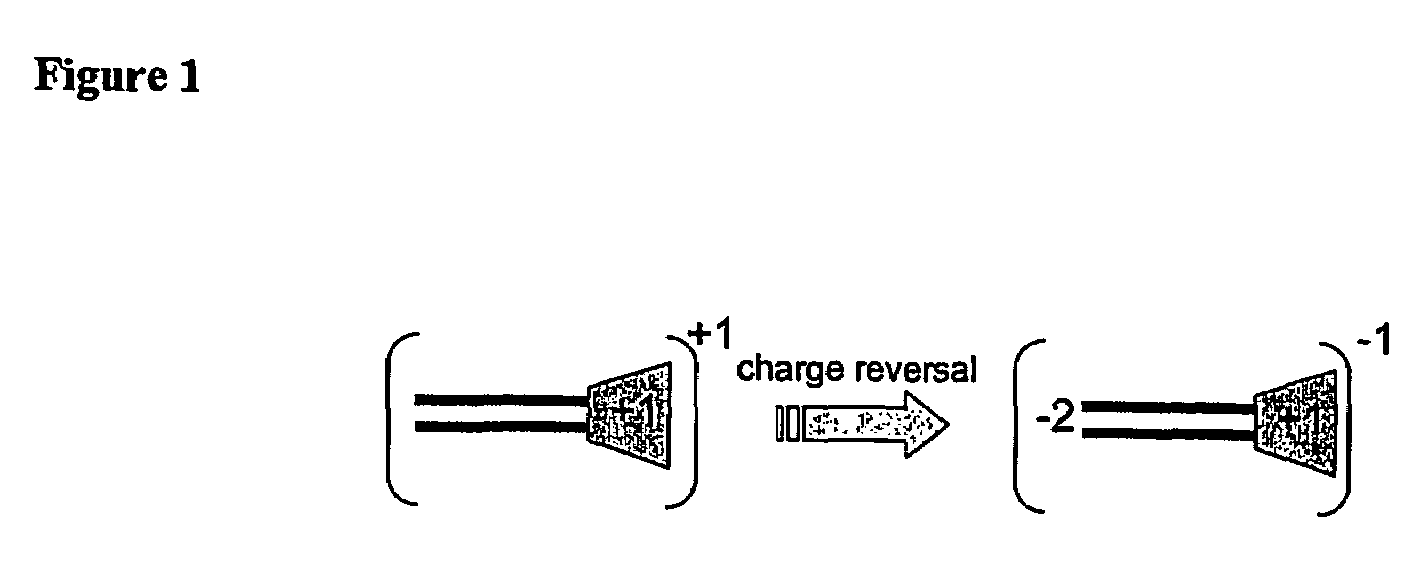Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
794 results about "Gene delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gene delivery is the process of introducing foreign genetic material, such as DNA or RNA, into host cells. Genetic material must reach the nucleus of the host cell to induce gene expression. Successful gene delivery requires the foreign genetic material to remain stable within the host cell and can either integrate into the genome or replicate independently of it. This requires foreign DNA to be synthesized as part of a vector, which is designed to enter the desired host cell and deliver the transgene to that cell's genome. Vectors utilized as the method for gene delivery can be divided into two categories, recombinant viruses and synthetic vectors (viral and non-viral).
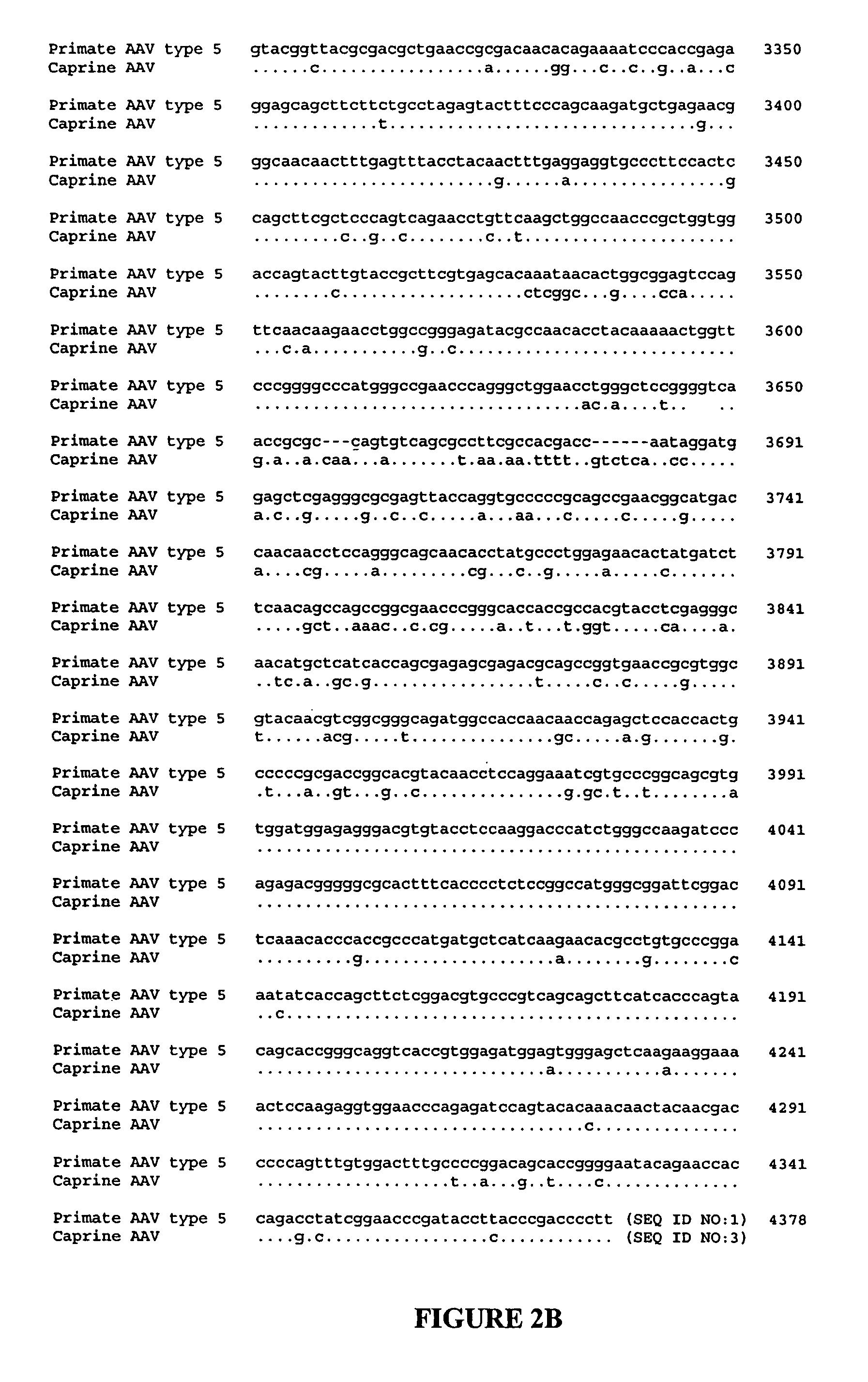
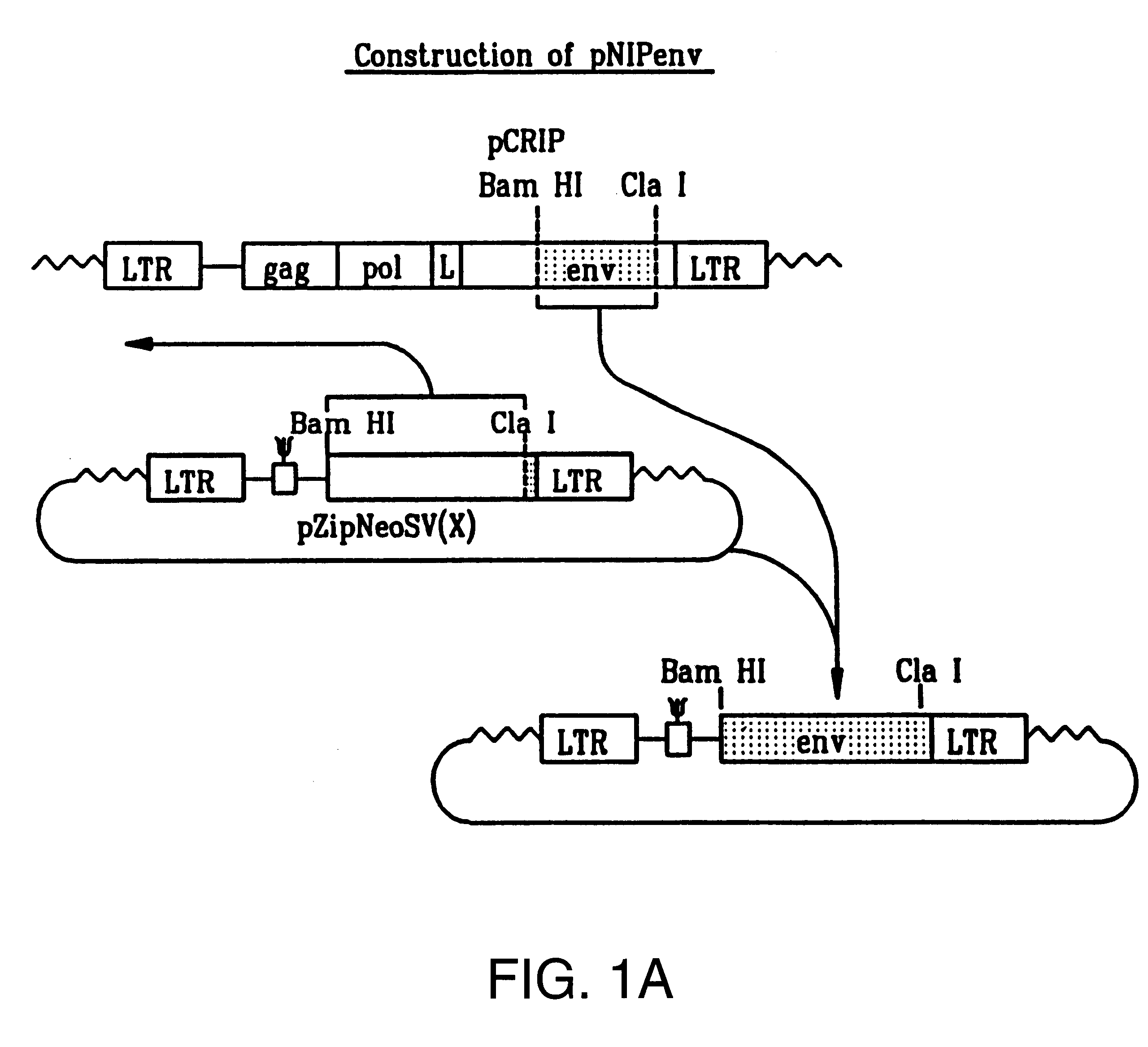
Methods for generating high titer helper-free preparations of released recombinant AAV vectors
InactiveUS6989264B2Genetic therapy composition manufactureGroup 5/15 element organic compoundsGene deliveryHeterologous
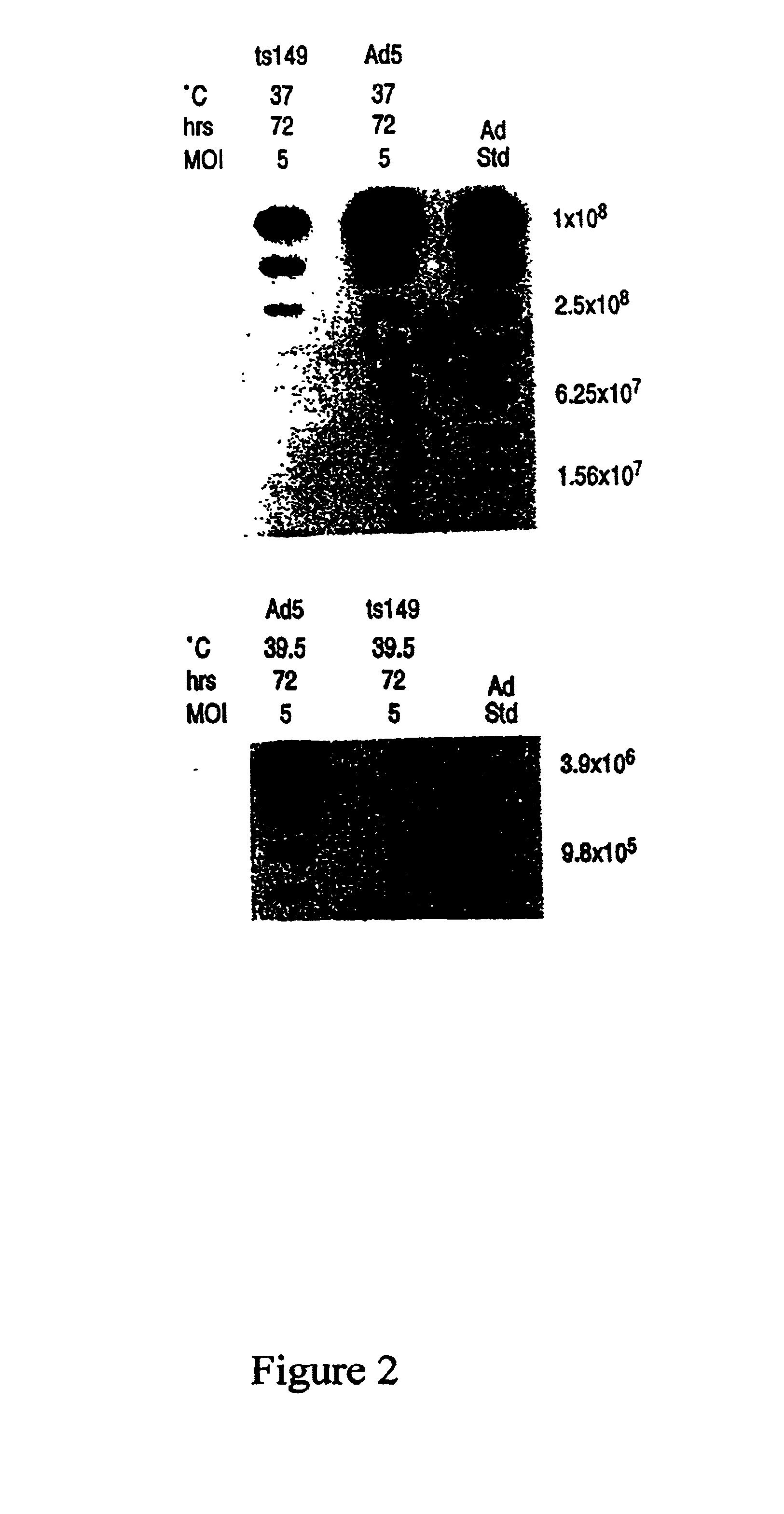
This invention provides methods and compositions for producing high titer, substantially purified preparations of recombinant adeno-associated virus (AAV) that can be used as vectors for gene delivery. At the onset of vector production, AAV producer cells of this invention typically comprise one or more AAV packaging genes, an AAV vector comprising a heterologous (i.e. non-AAV) transgene of interest, and a helper virus such as an adenovirus. The AAV vector preparations produced are generally replication incompetent but are capable of mediating delivery of a transgene of interest (such as a therapeutic gene) to any of a wide variety of tissues and cells. The AAV vector preparations produced according to this invention are also substantially free of helper virus as well as helper viral and cellular proteins and other contaminants. The invention described herein provides methods of producing rAAV particles by culturing producer cells under conditions, such as temperature and pH, that promote release of virus. Also provided is a quantitative, high-throughput assay useful in the assessment of viral infectivity and replication, as well as in the screening of agent that affect viral infectivity and / or replication.
Owner:TARGETED GENETICS CORPORTION
Rapid Diffusion of Large Polymeric Nanoparticles in the Mammalian Brain
ActiveUS20130183244A1Reduce deliveryHigher drug payloadPowder deliveryNervous disorderGene deliveryHydrophilic coating
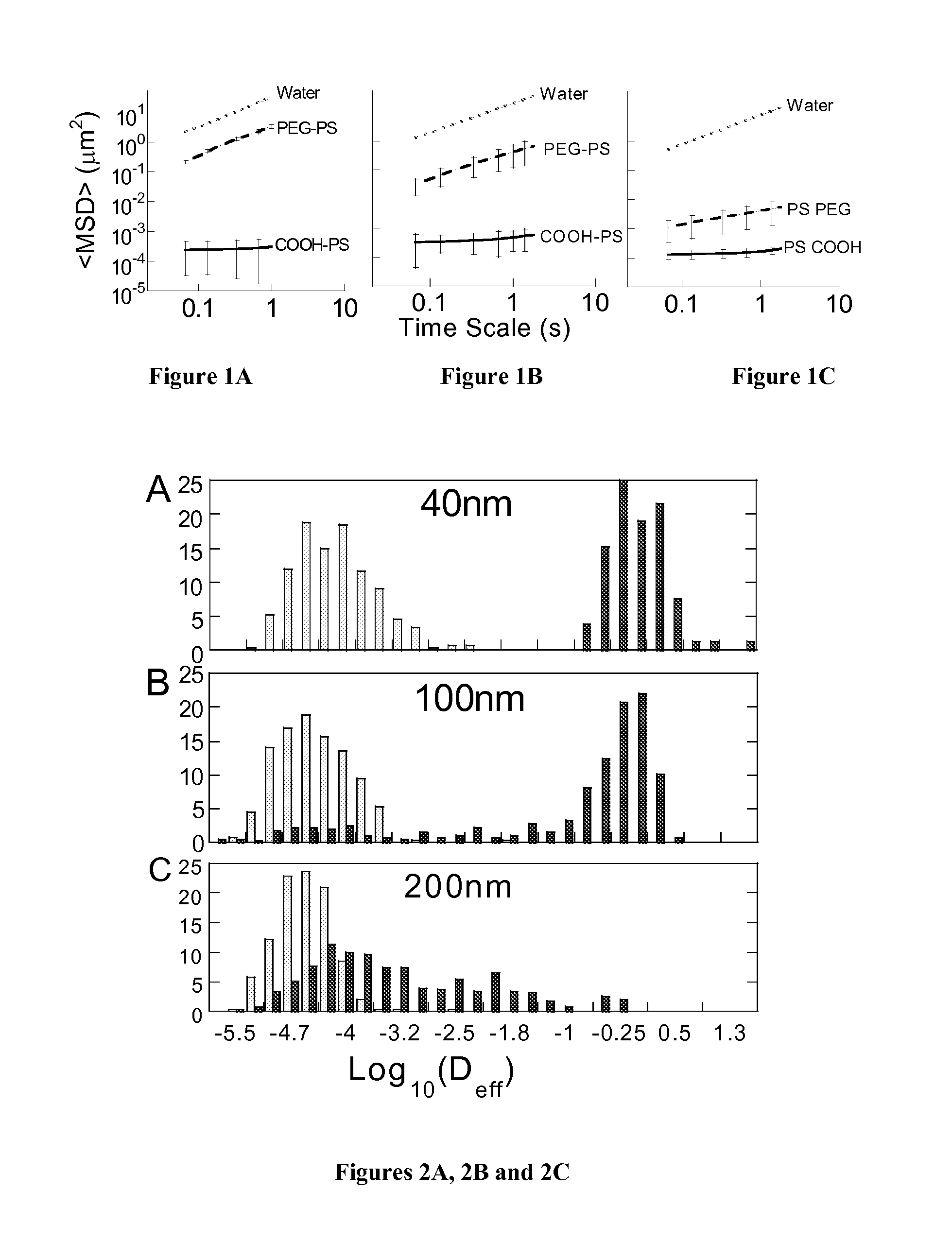
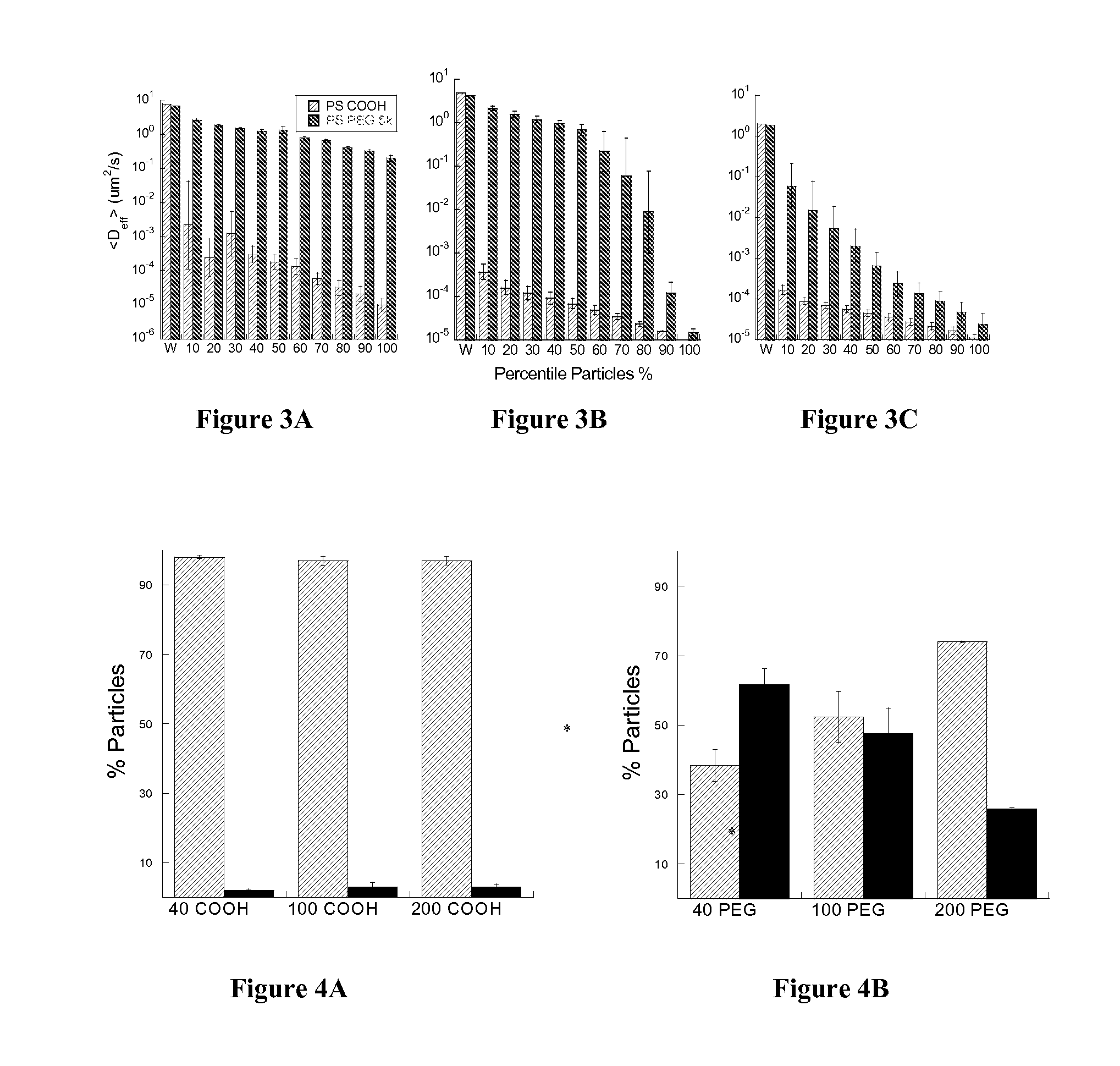
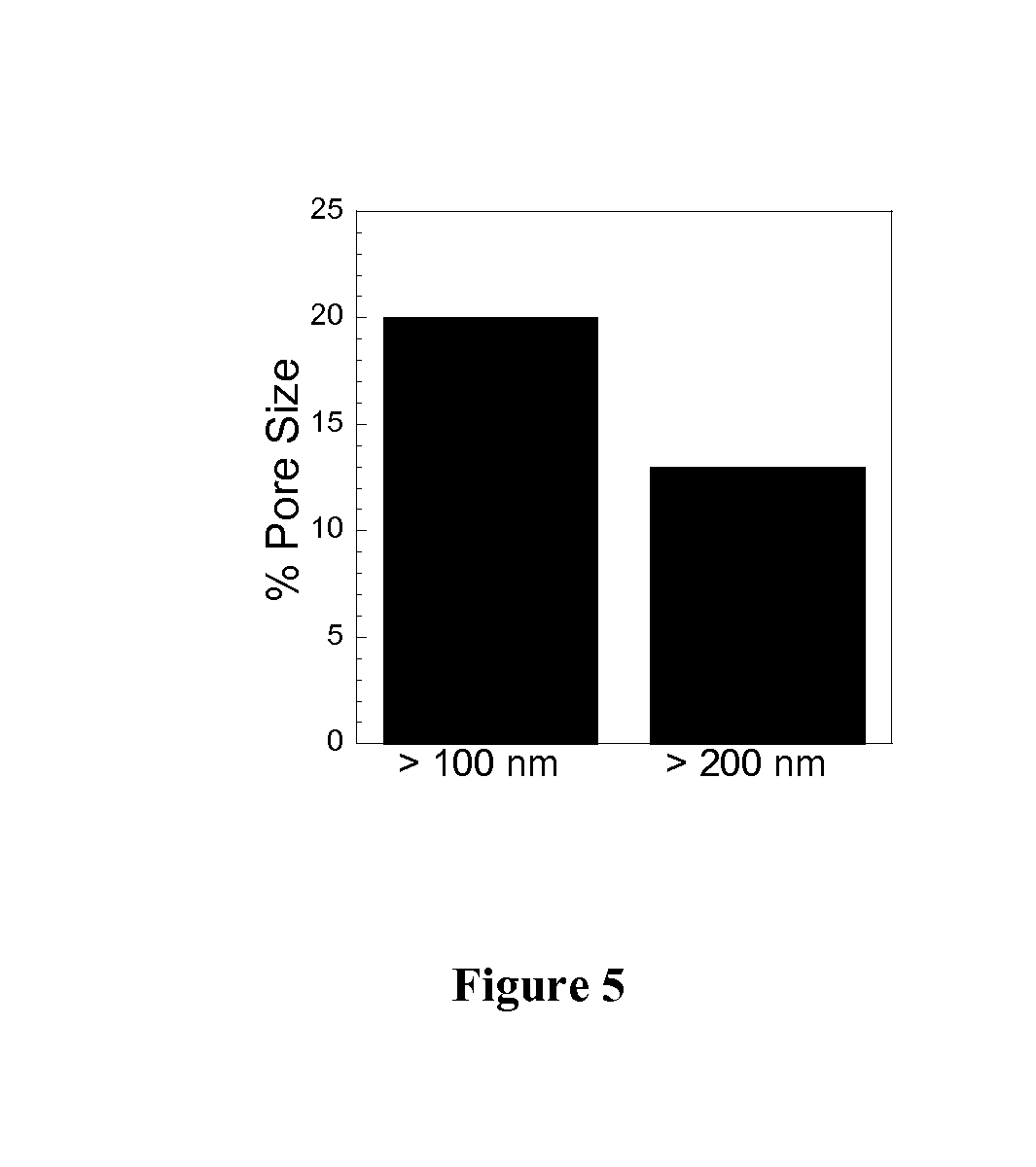
Non-adhesive particles as large as 110 nm can diffuse rapidly in the brain ECS, if coated with hydrophilic coatings such as PEG coatings and preferably having neutral surface charge. The ability to achieve brain penetration with larger particles will significantly improve drug and gene delivery within the CNS since larger particles offer higher drug payload, improved drug loading efficiency, and significantly longer drug release durations.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Antigen delivery system and method of production
The present invention concerns polymer particle vaccine delivery systems in which a water insoluble protein antigen, e.g. a lipidated HpaA protein, is incorporated with particles comprising a polymer matrix. The present invention also concerns a method for incorporating such a water insoluble protein antigen with a polymer matrix in order to produce a polymer particle vaccine delivery system. In addition, the invention also provides a vaccine composition comprising the polymer particle delivery system. The vaccine can be used to treat and / or reduce the risk of for example Helicobacter infection.
Owner:ASTRAZENECA AB
Magnetically guidable carriers and methods for the targeted magnetic delivery of substances in the body
InactiveUS7189198B2Facilitated releaseLess hydrophobicPowder deliveryElectrotherapyMagnetic gradientGene delivery
A method of delivering a substance to targeted tissue comprising the steps of: delivering a plurality of magnetically responsive particles which are carrying the substance and have a hydrophobic coating into the patient's vasculature upstream of the targeted tissue; and applying a magnetic gradient in the vicinity of the targeted tissue to draw the magnetically responsive particles against the wall of the patient's vasculature in the vicinity of the targeted tissue, to allow the substance on the magnetic particles to migrate through the wall of the patent's vasculature to targeted tissue.
Owner:STEREOTAXIS
Transient Transfection with RNA
ActiveUS20080260706A1Lymphocyte transfectabilitySimilar efficiencyBiocideGenetic material ingredientsGene deliveryDNA construct
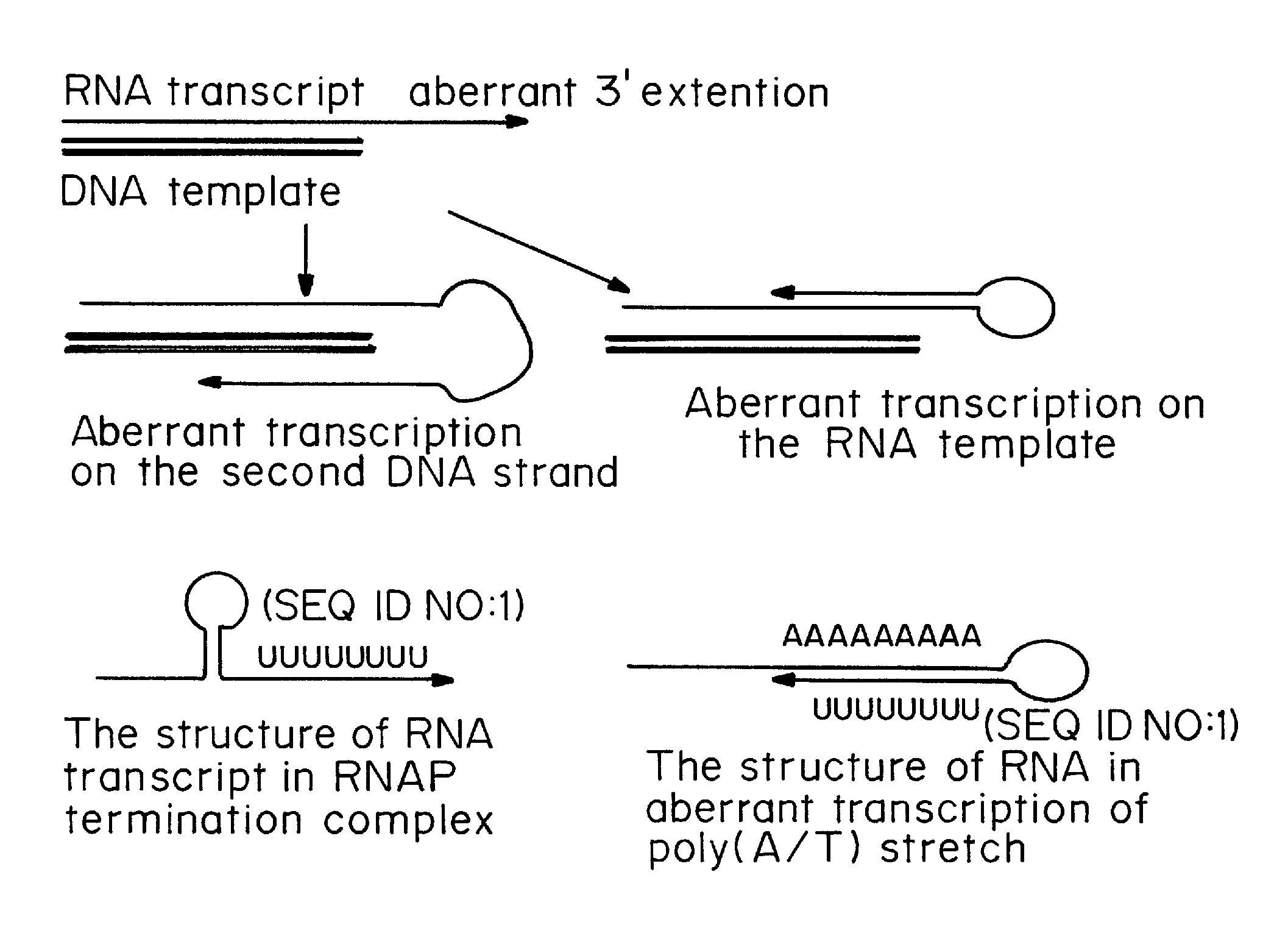
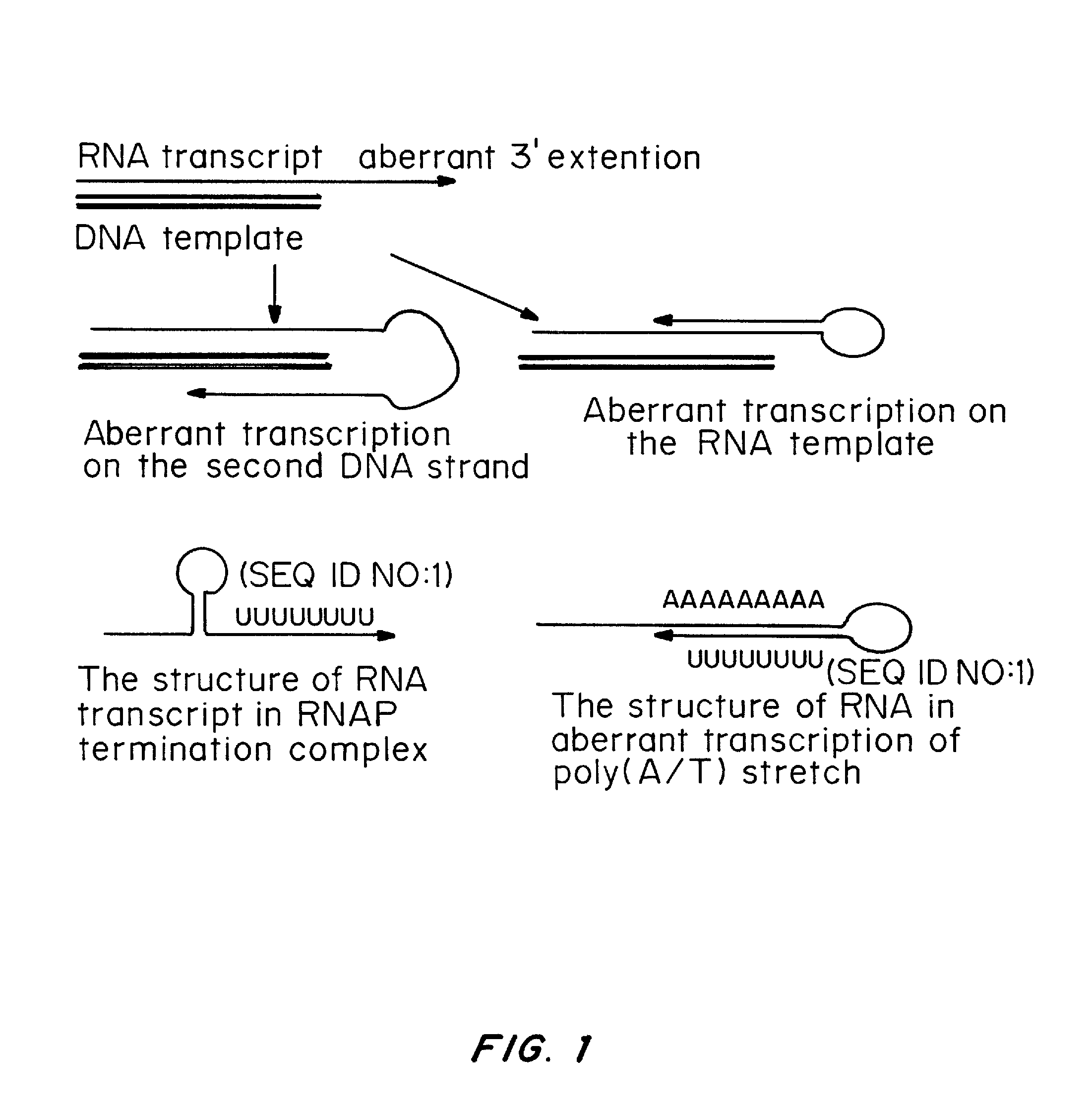
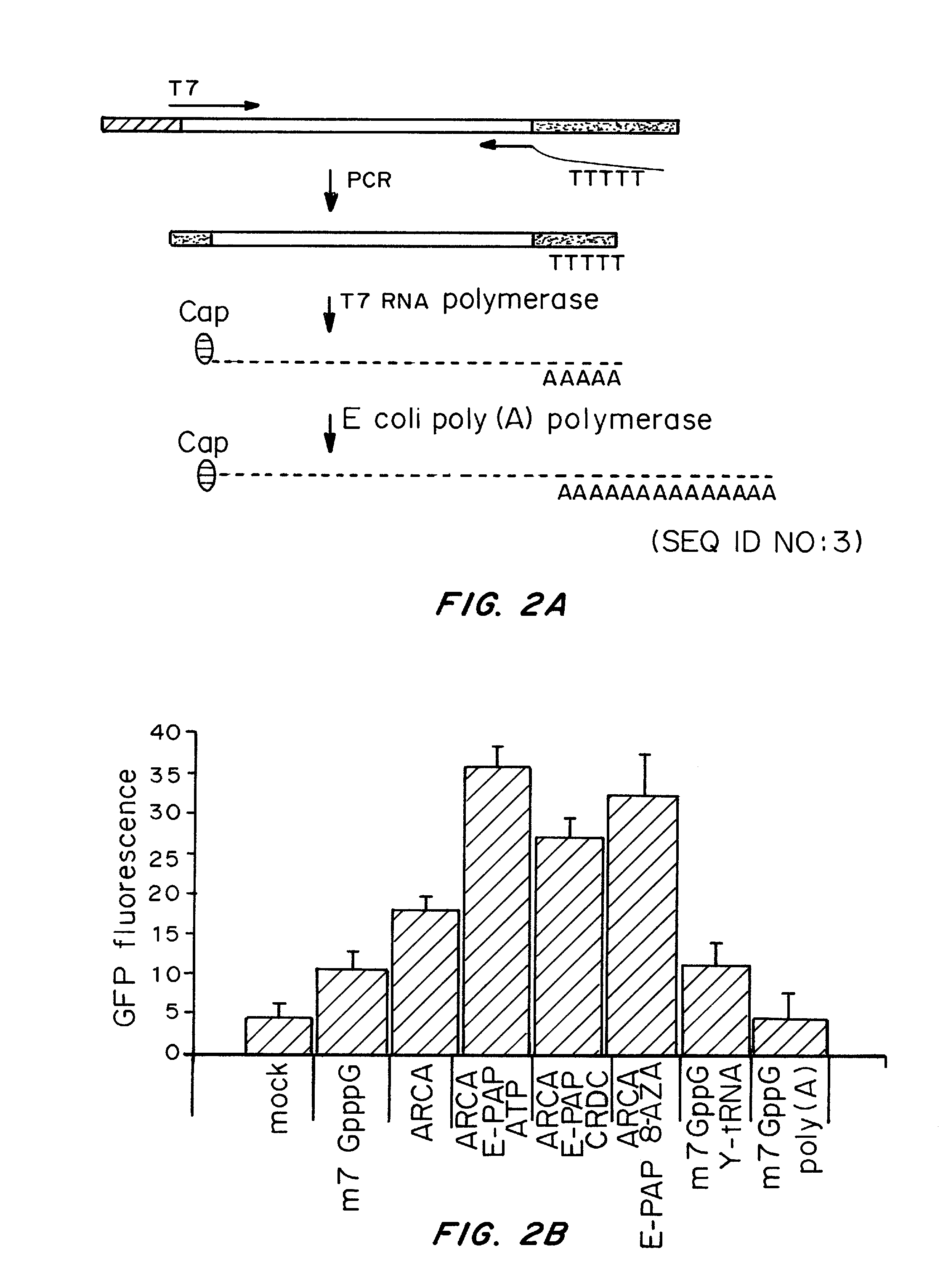
A method of mRNA production for use in transfection is provided, that involves in vitro transcription of PCR generated templates with specially designed primers, followed by polyA addition, to produce a construct containing 3′ and 5′ untranslated sequence (“UTR”), a 5′ cap and / or Internal Ribosome Entry Site (IRES), the gene to be expressed, and a polyA tail, typically 50-2000 bases in length. This RNA can efficiently transfect different kinds of cells. This approach results in increased efficiency (fidelity and productivity) of mRNA synthesis and is less time consuming because it does not require cloning, and also consequently eliminates the unwanted errors and effects related to RNA made on DNA templates obtained with cloning techniques. The results of transfection of RNAs demonstrate that RNA transfection can be very effective in cells that are exceedingly difficult to transfect efficiently with DNA constructs. Further, the levels of gene expression following mRNA transfection are consistent from cell to cell in an experiment and these levels can be controlled over a wide range simply by changing the amount of mRNA that is transfected, and without obvious cytotoxic effects due to the levels of RNA per se. Due to high efficiency the cells can be simultaneously transfected with multiple genetic constructs. The method can be used to deliver genes into cells not- or only poorly transfectable for DNA, in vitro and in vivo.
Owner:YALE UNIV
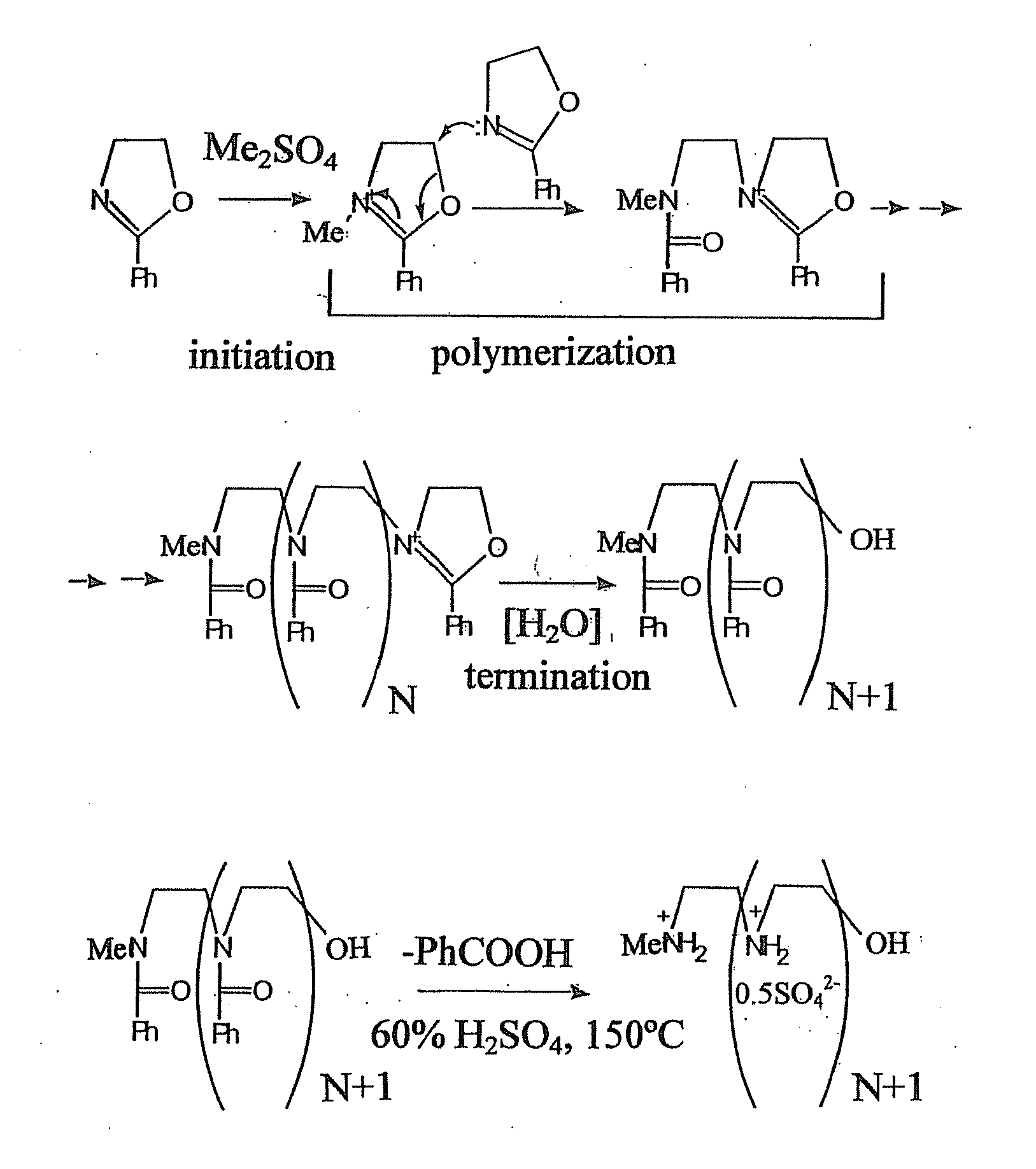
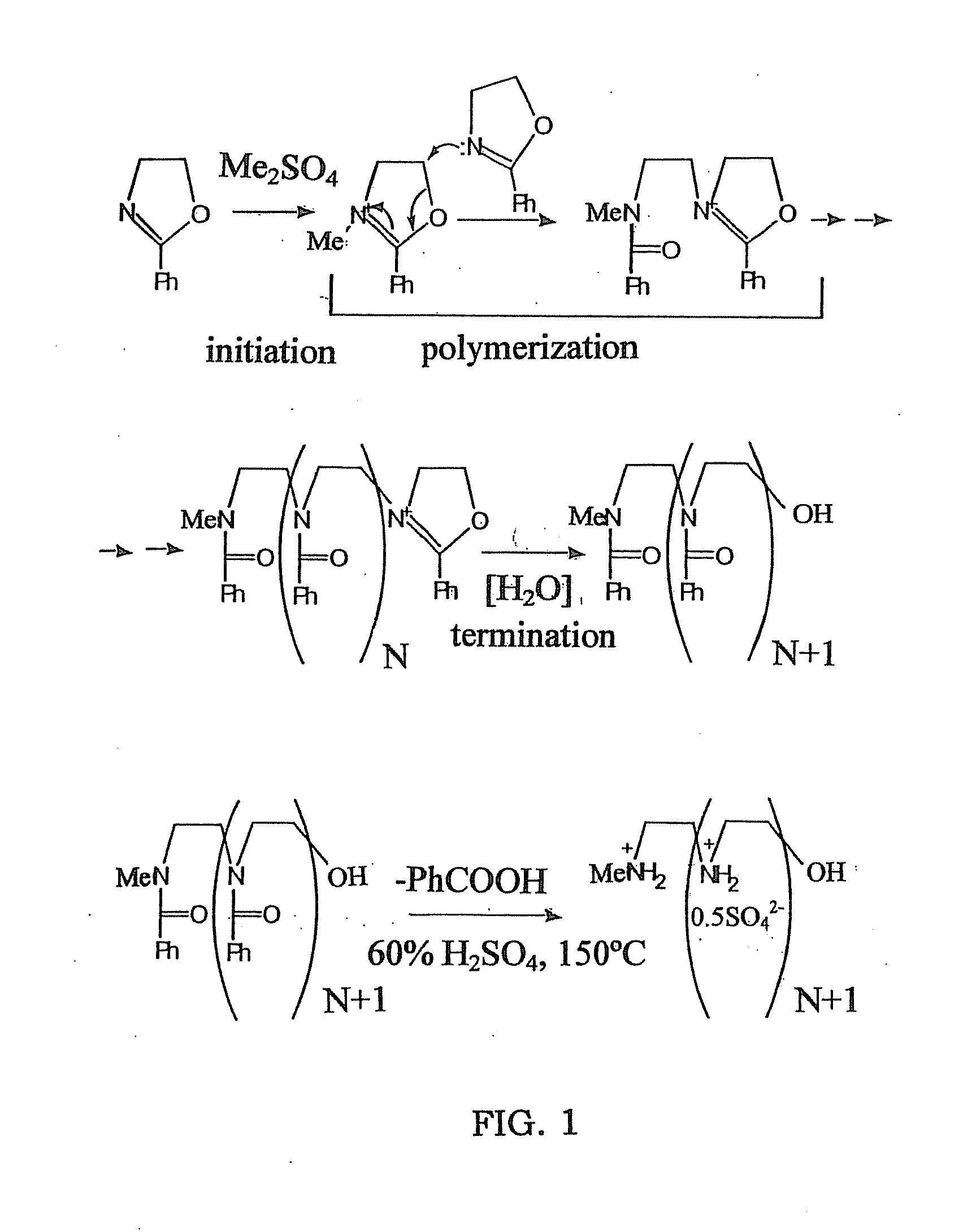
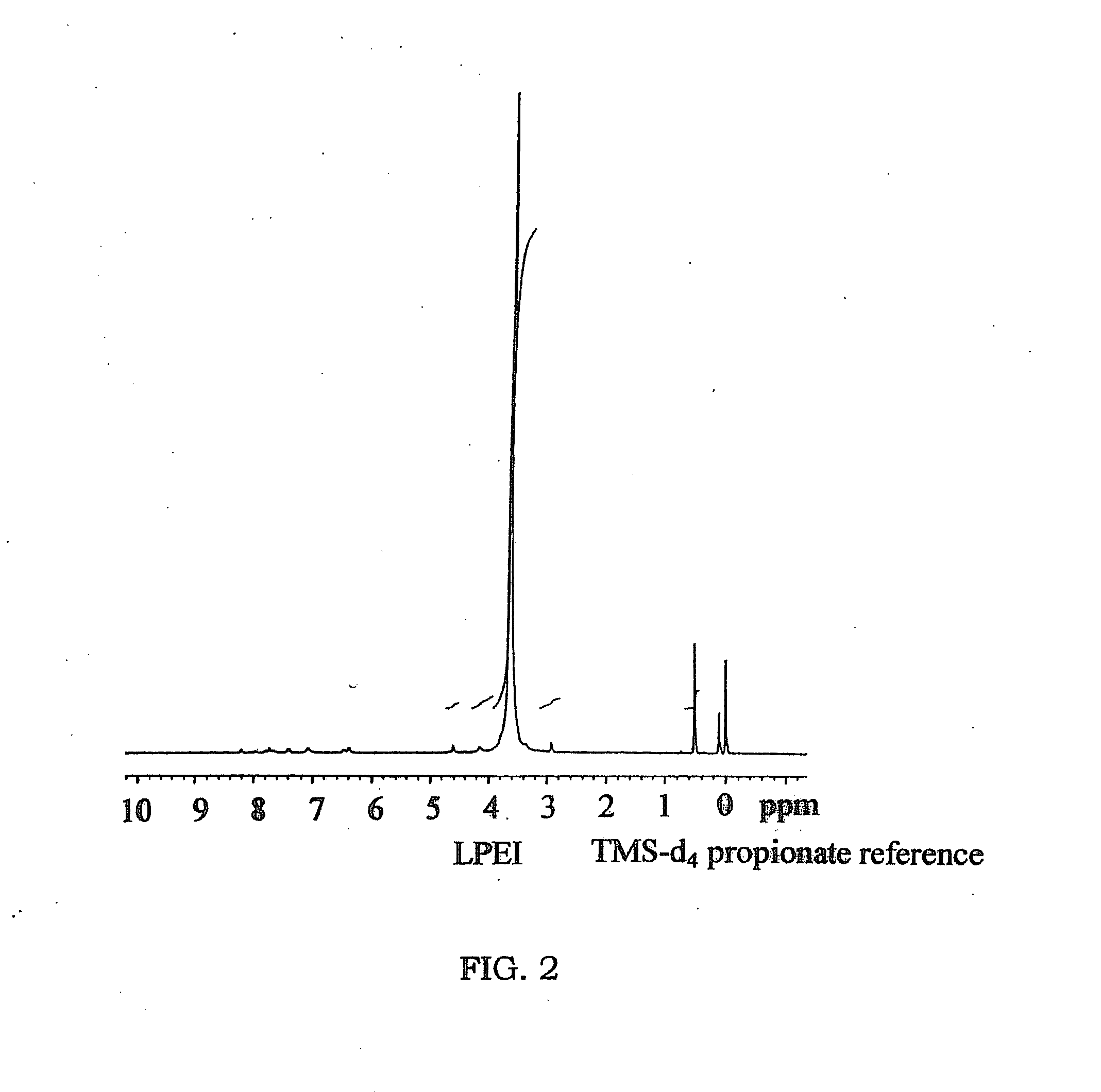
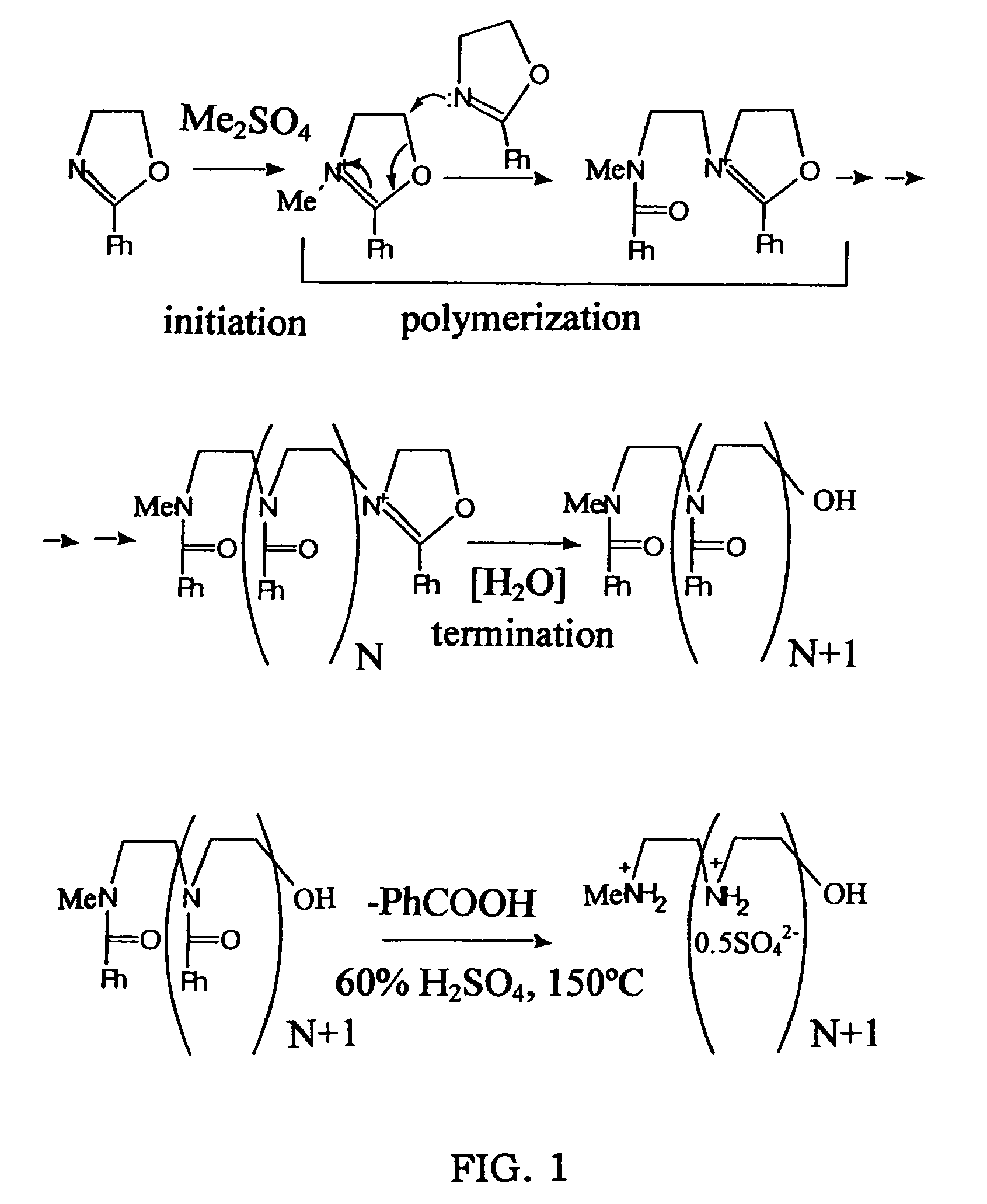
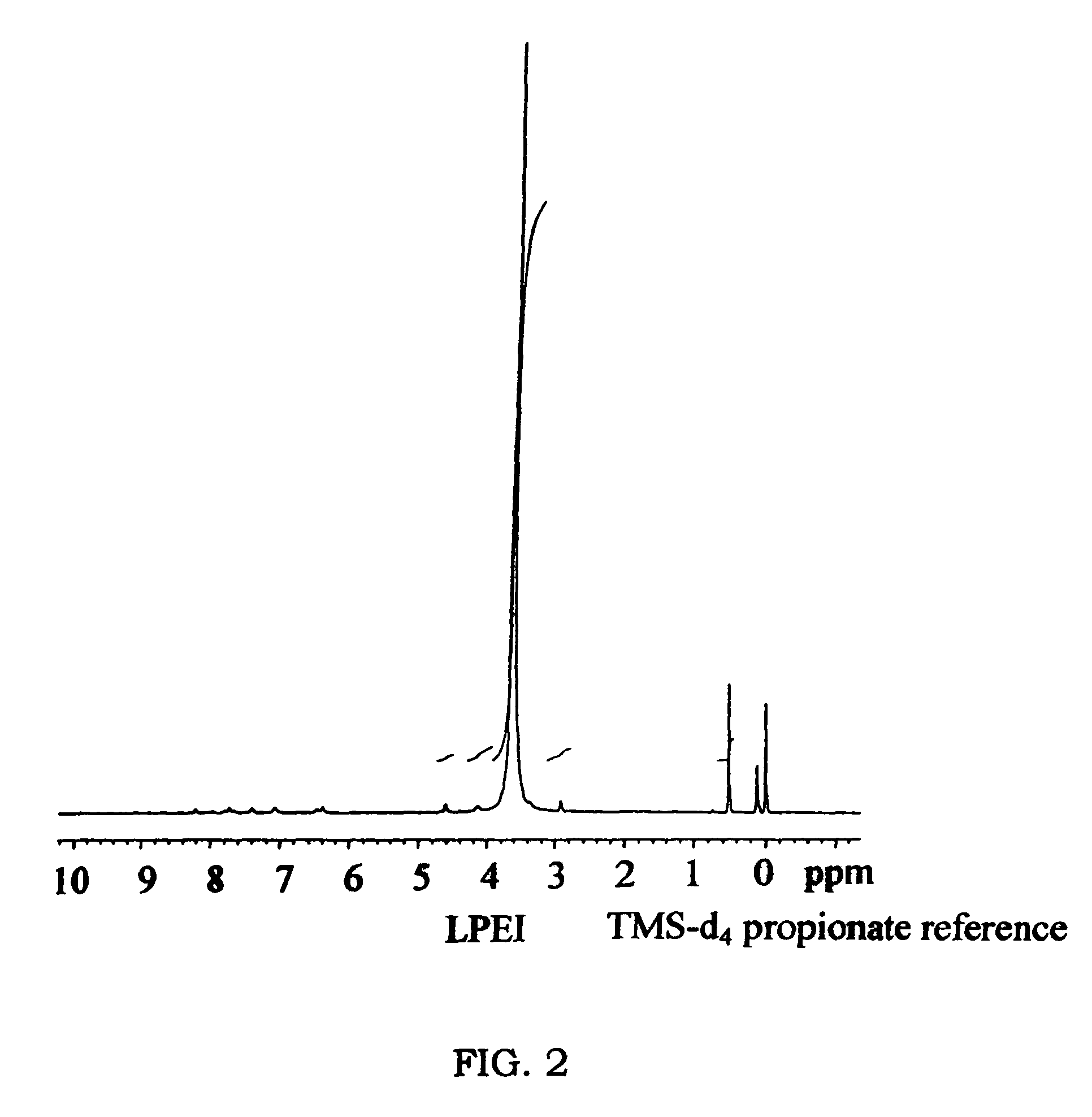
Biodegradable Cross-Linked Cationic Multi-block Copolymers for Gene Delivery and Methods of Making Thereof
ActiveUS20120009145A1Easy to controlGuaranteed effective sizeCosmetic preparationsOrganic active ingredientsCross-linkGene delivery
A biodegradable cross-linked cationic multi-block copolymer of linear polyethylenimine (LPEI) wherein the LPEI blocks are linked together by hydrophilic linkers with a biodegradable disulfide bond and methods of making thereof. The biodegradable cross-linked cationic multi-block copolymer may also contain pendant functional moieties which are preferably receptor ligands, membrane permeating agents, endosomolytic agents, nuclear localization sequences, pH sensitive endosomolytic peptides, chromogenic or fluorescent dyes.
Owner:CLSN LAB
Biodegradable cross-linked cationic multi-block copolymers for gene delivery and methods of making thereof
ActiveUS8057821B2Easy to controlGuaranteed effective sizePowder deliveryGenetic material ingredientsCross-linkGene delivery
A biodegradable cross-linked cationic multi-block copolymer of linear polyethylenimine (LPEI) wherein the LPEI blocks are linked together by hydrophilic linkers with a biodegradable disulfide bond and methods of making thereof. The biodegradable cross-linked cationic multi-block copolymer may also contain pendant functional moieties which are preferably receptor ligands, membrane permeating agents, endosomolytic agents, nuclear localization sequences, pH sensitive endosomolytic peptides, chromogenic or fluorescent dyes.
Owner:CLSN LAB
AAV vectors for gene delivery to the lung
ActiveUS7427396B2Efficient transductionDecreased immunoreactivityPowder deliveryBiocideGene deliveryVirosome
Owner:AVIGEN
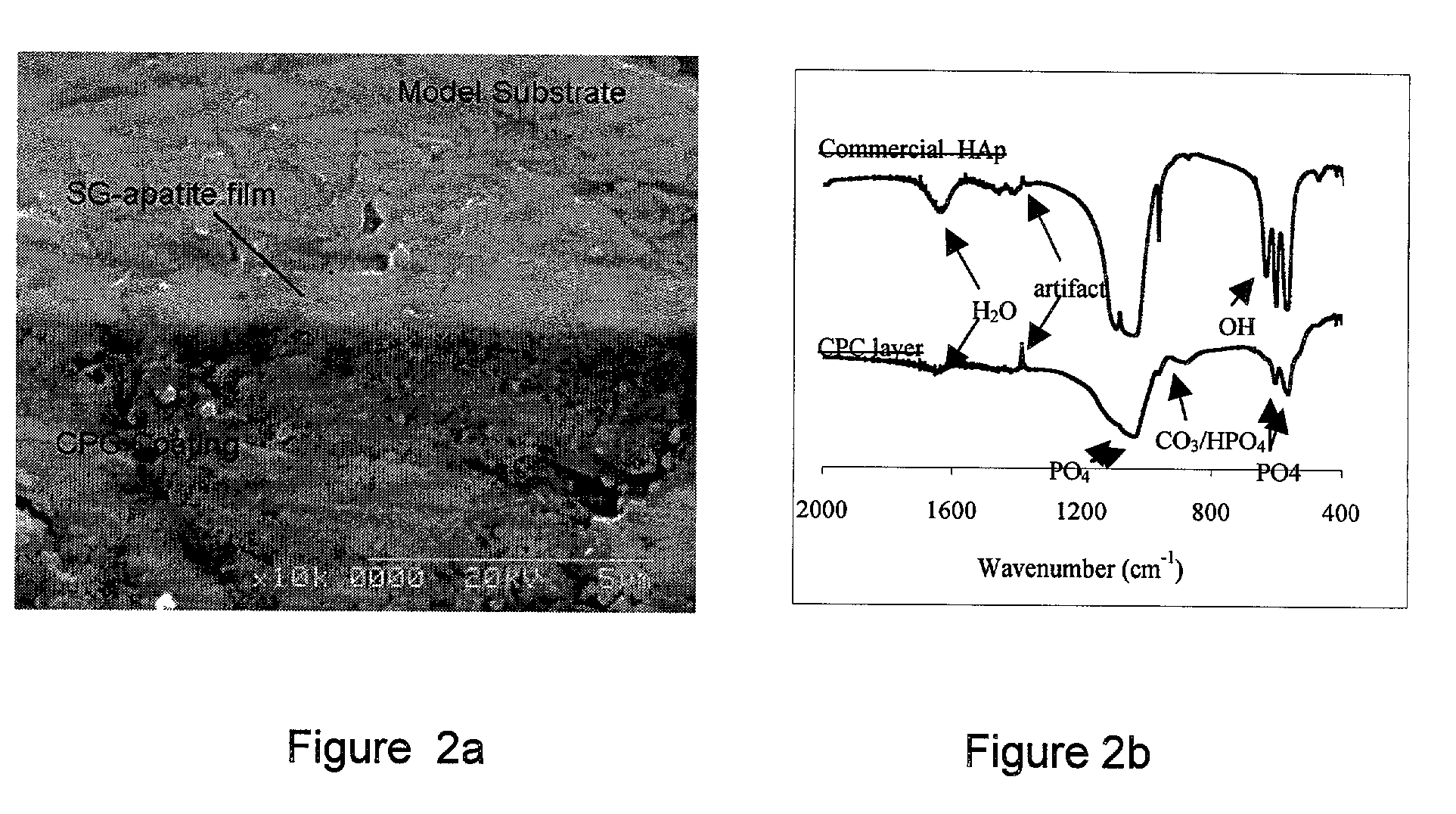
Biofunctional hydroxyapatite coatings and microspheres for in-situ drug encapsulation
InactiveUS20020155144A1Improve relationshipImprove interface strengthPowder deliveryOrganic active ingredientsGene deliverySide effect
Owner:THE UNIV OF BRITISH COLUMBIA
Methods of treating Parkinson's disease using recombinant adeno-associated virus virions
ActiveUS7588757B2Reduce deliveryIncrease in fine motor taskingBiocidePeptide/protein ingredientsGene deliveryDisease
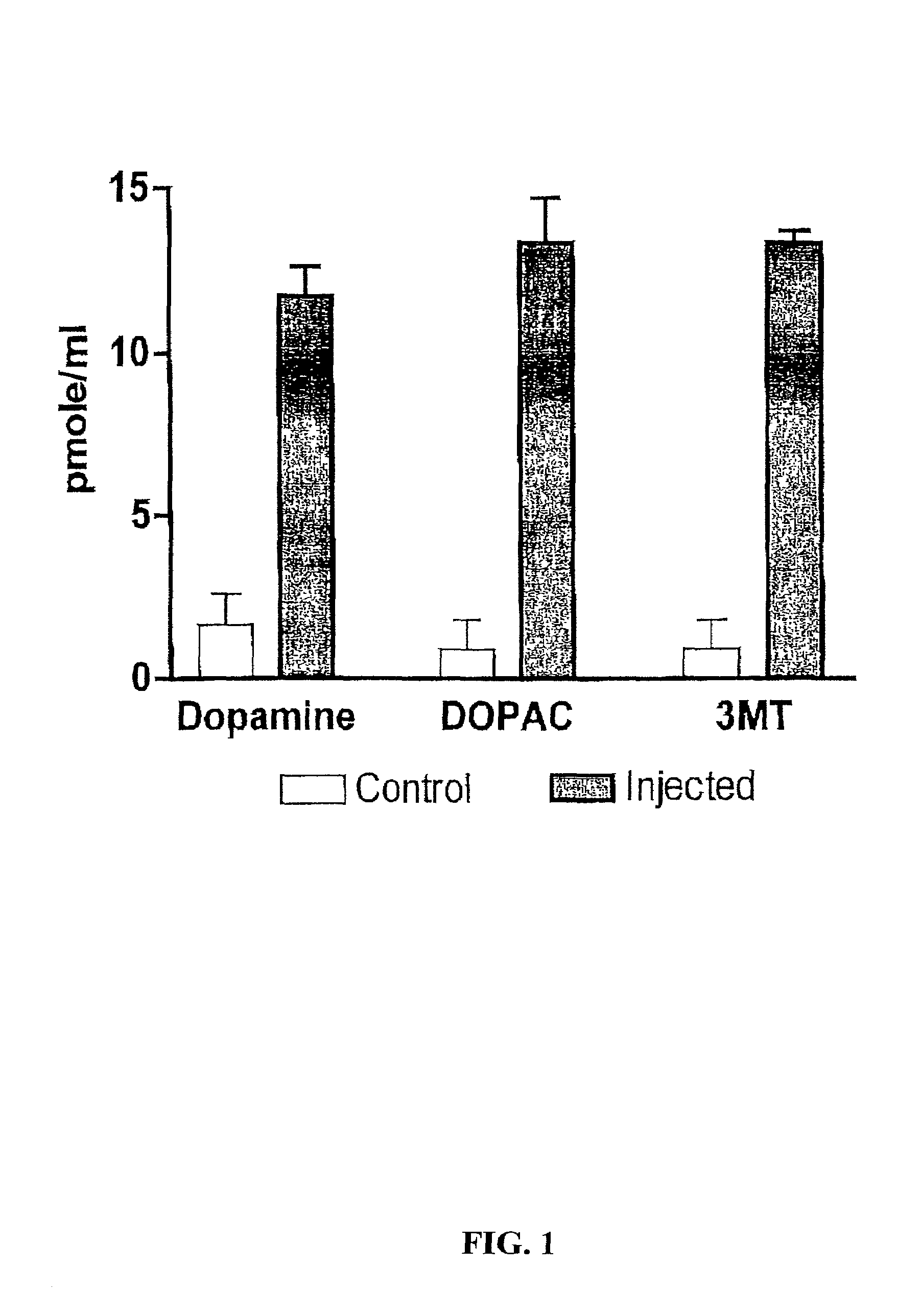
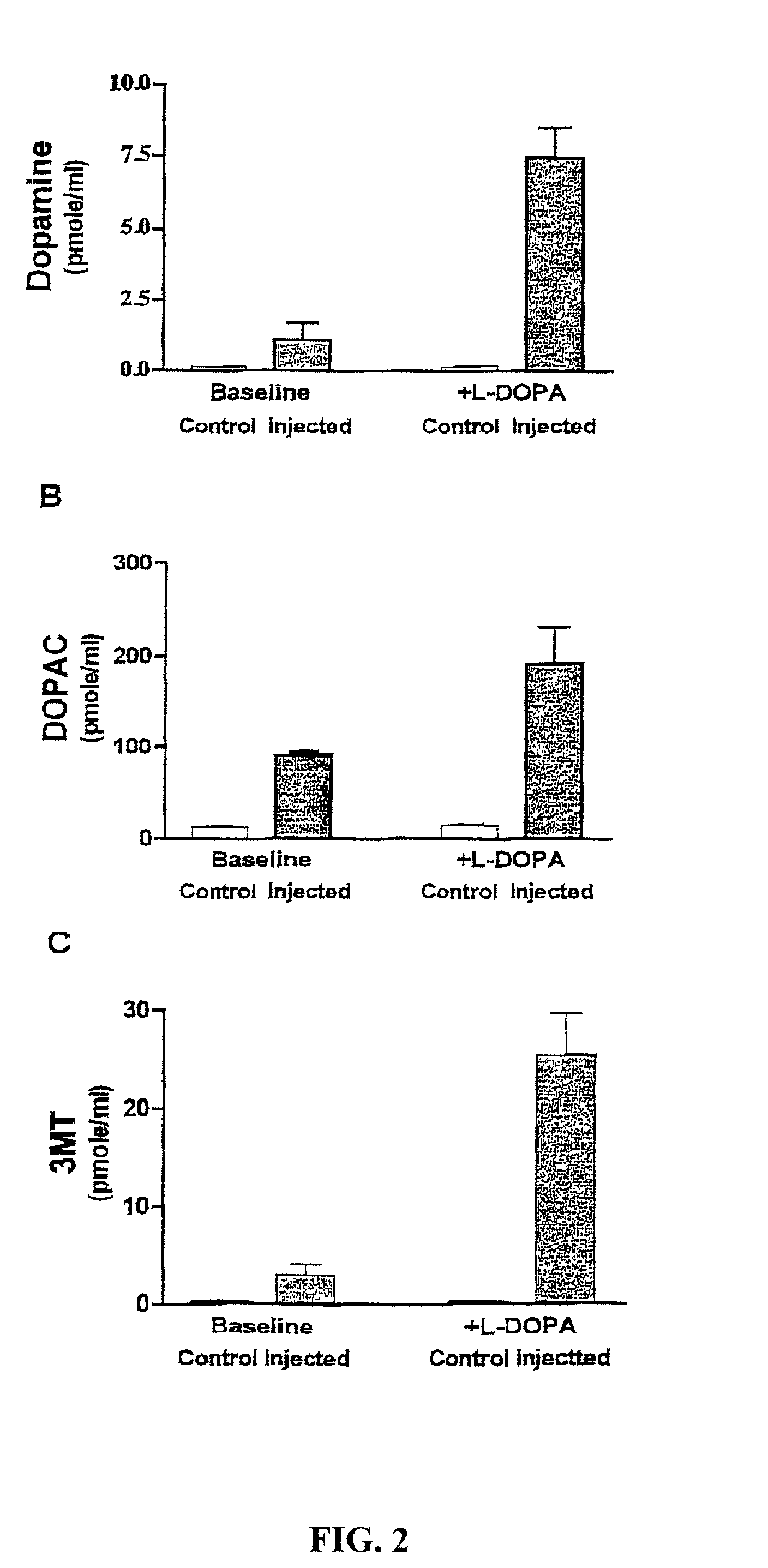
Methods for treating Parkinson's disease (PD) are provided. Recombinant adeno-associated virus (rAAV) virions are used to deliver genes encoding dopamine-synthesizing enzymes to the central nervous system of a primate. Once delivered, the genes are expressed, which then results in dopamine synthesis and amelioration in the clinical signs and symptoms of PD. The methods of the present invention can be used to deliver the three central dopamine synthesizing enzymes: tyrosine hydroxylase, aromatic L-amino acid decarboxylase, and guanosine triphosphate cyclohydrolase I thereby enhancing dopamine biosynthesis and providing for enhanced therapeutic efficacy.
Owner:GENZYME CORP
Modified Poloxamers for Gene Expression and Associated Methods
InactiveUS20100004313A1Inhibit expressionReduce deliveryGenetic material ingredientsPharmaceutical delivery mechanismGene deliverySolid tissue
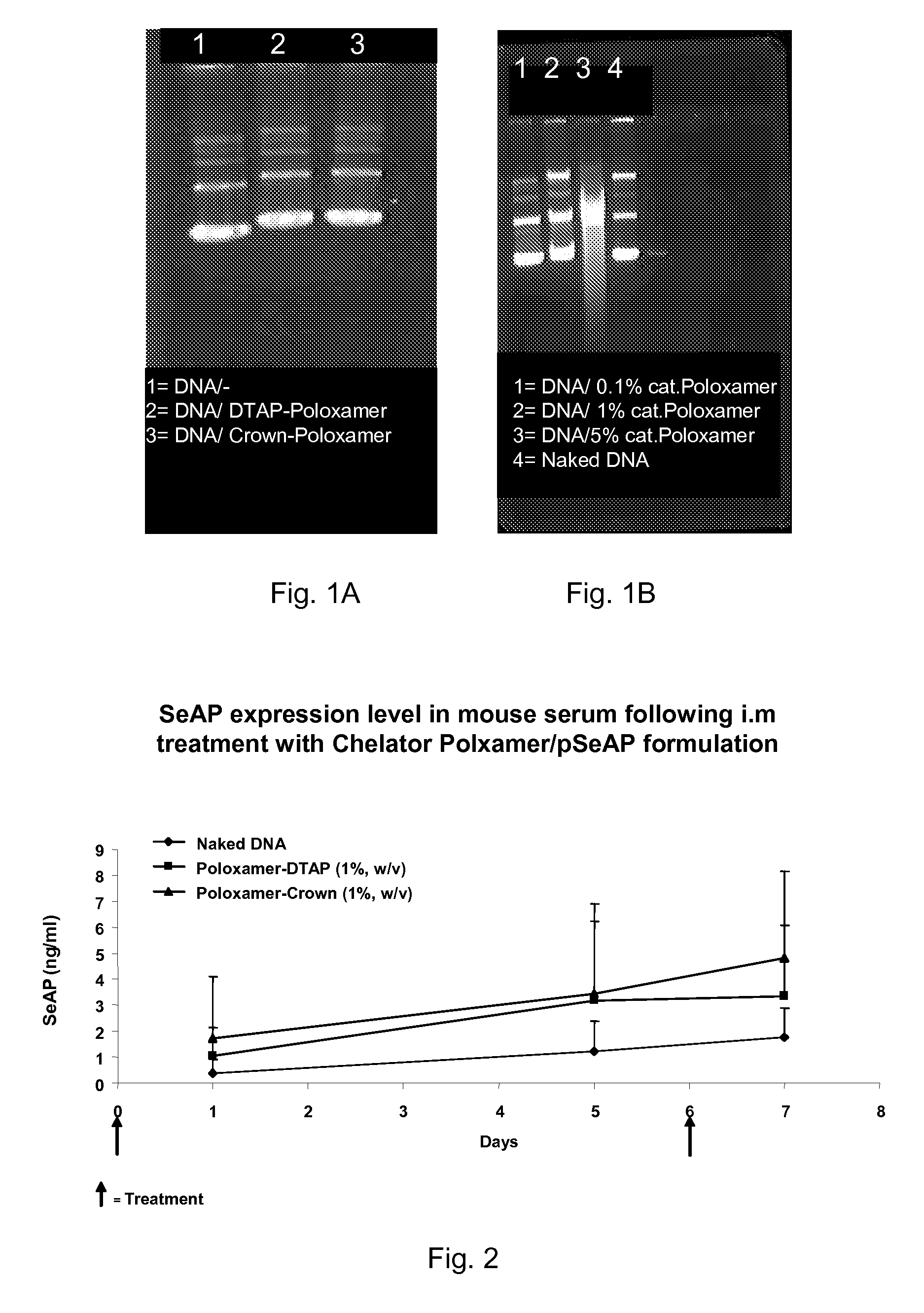
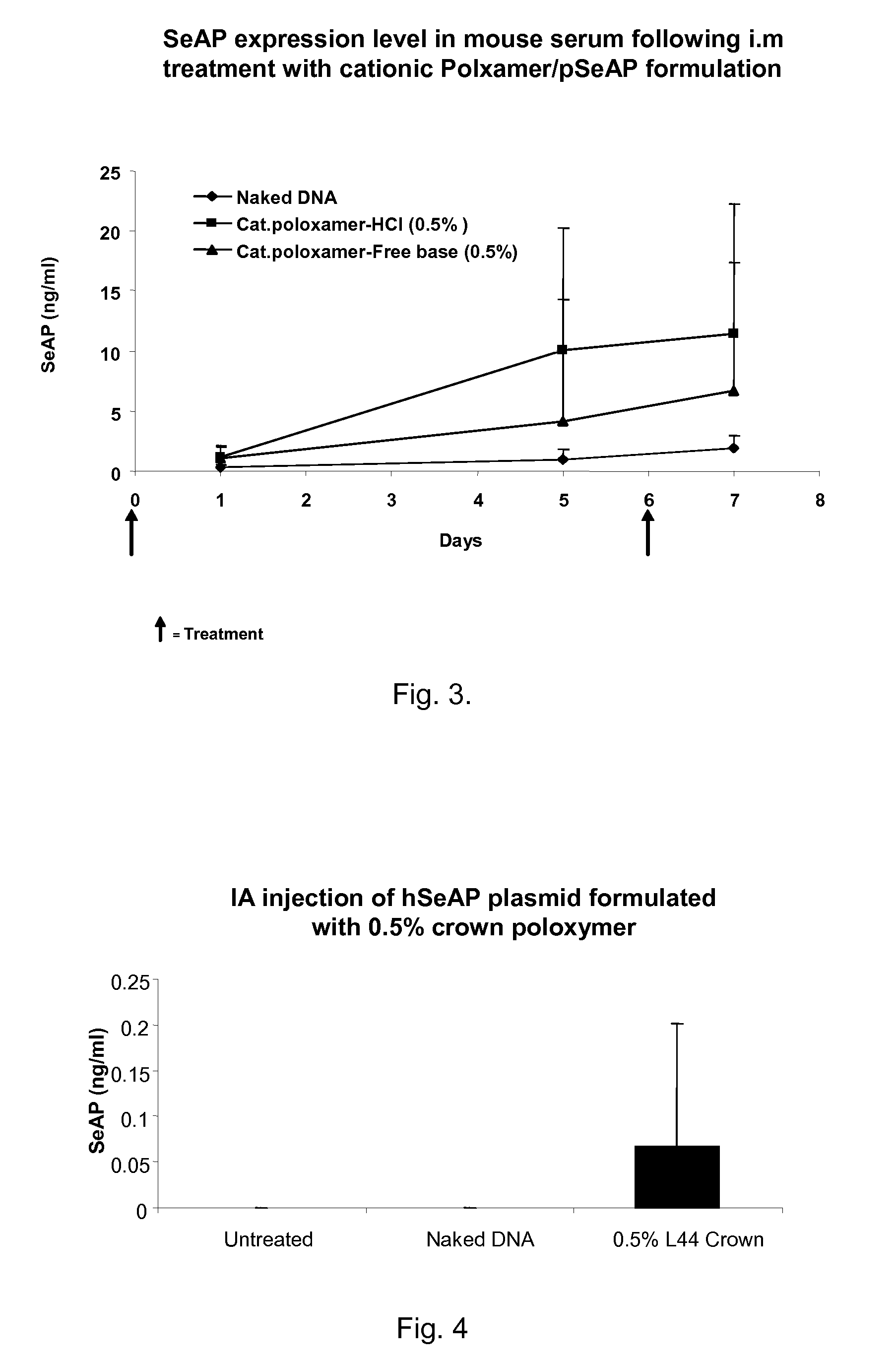
Nucleotide delivery polymers, compositions, and associated methods for the enhancement of gene delivery and expression in solid tissues are provided. In one aspect, for example, a nucleotide delivery polymer may include a poloxamer backbone having a metal chelator covalently coupled to at least one terminal end of the poloxamer backbone. In another aspect, the nucleotide expression polymer has a metal chelator coupled to at least two terminal ends of the poloxamer backbone.
Owner:CLSN LAB
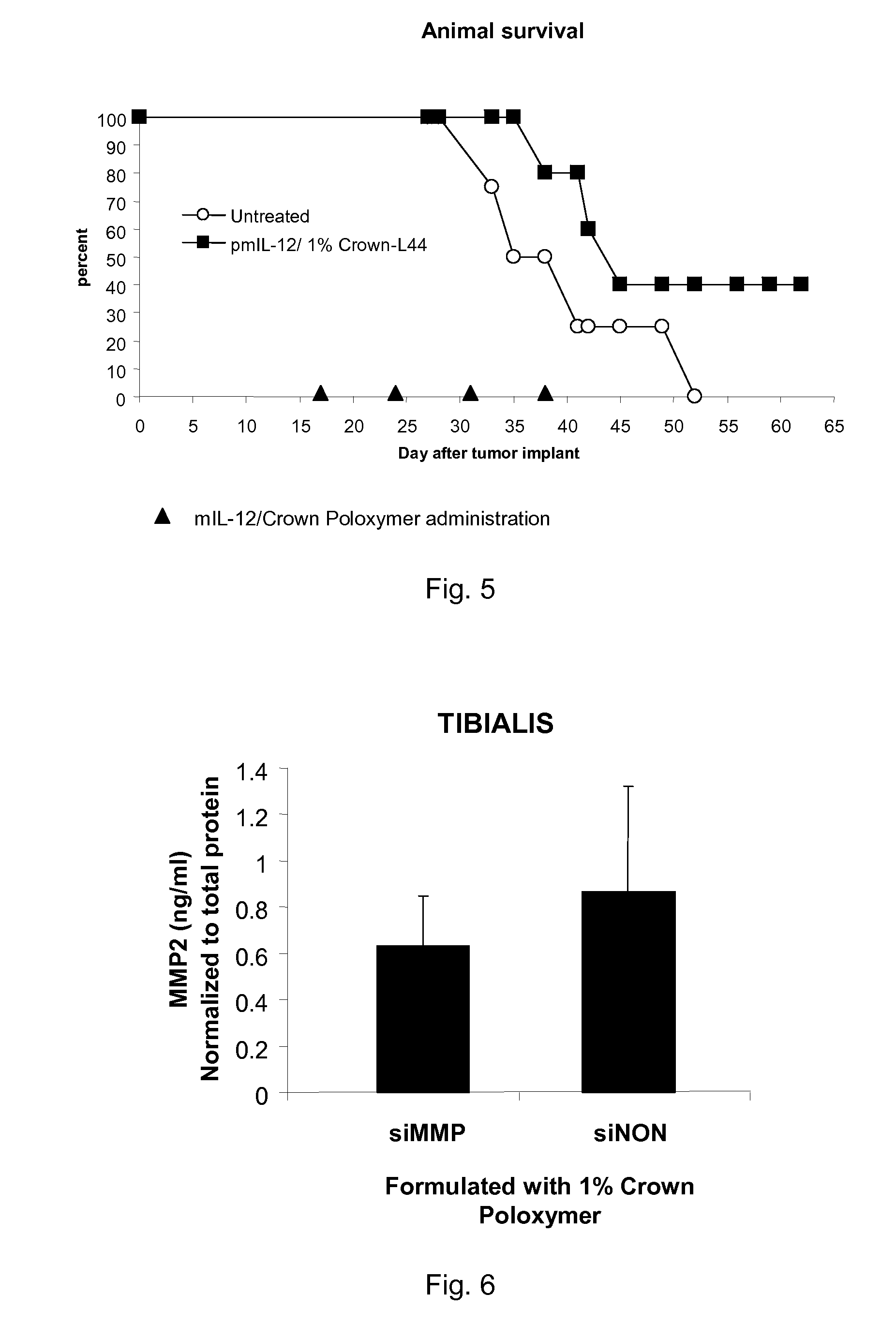
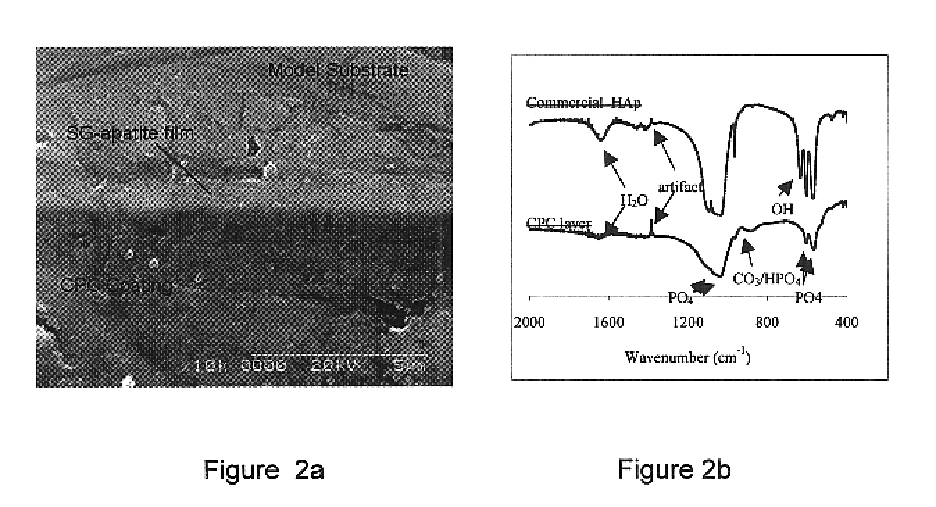
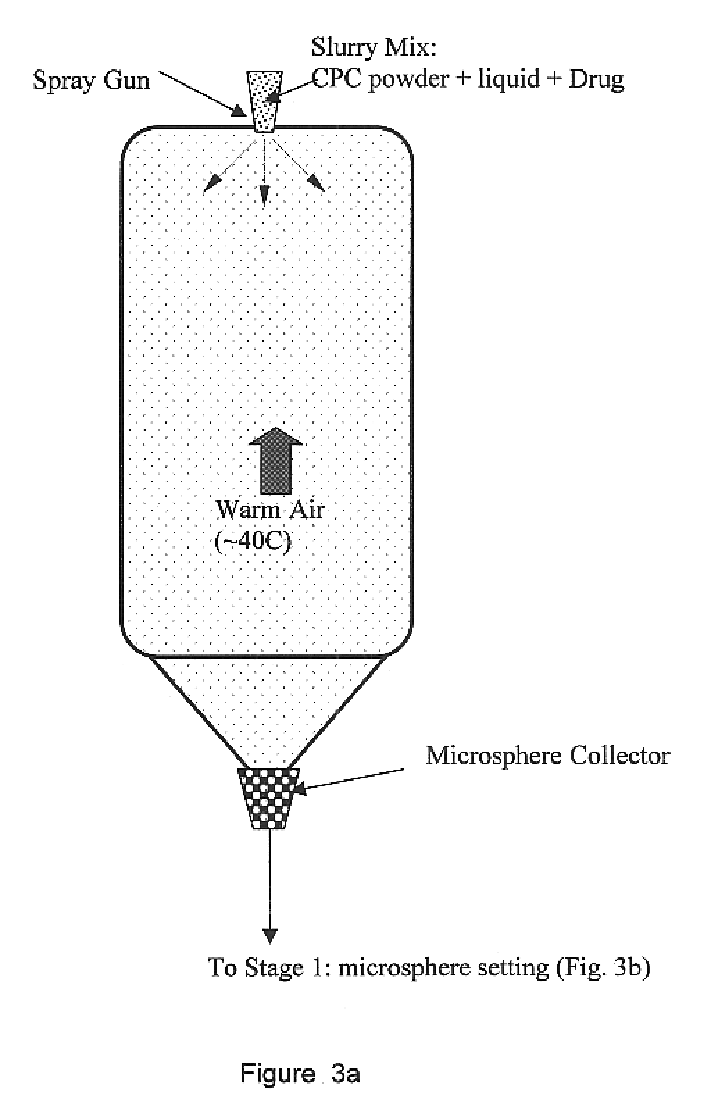
Biofunctional hydroxyapatite coatings and microspheres for in-situ drug encapsulation
InactiveUS6730324B2High strengthEasy to makePowder deliveryOrganic active ingredientsGene deliverySide effect
This invention relates to novel room-temperature process for obtaining calcium phosphate, in particular hydroxyapatite, coatings and microspheres that encapsulate drugs, proteins, genes, DNA for therapeutical use. The coatings and microspheres are designed to perform a defined biological function related to drug delivery, such as gene therapy through gene delivery. A novel method for encapsulation, and subsequent controlled release of therapeutically active agents from such biofunctional coatings and microspheres is disclosed. Such coatings and microspheres are useful for side-effects free, long-term, targeted, controlled release and delivery of drugs, proteins, DNA, and other therapeutic agents.
Owner:THE UNIV OF BRITISH COLUMBIA
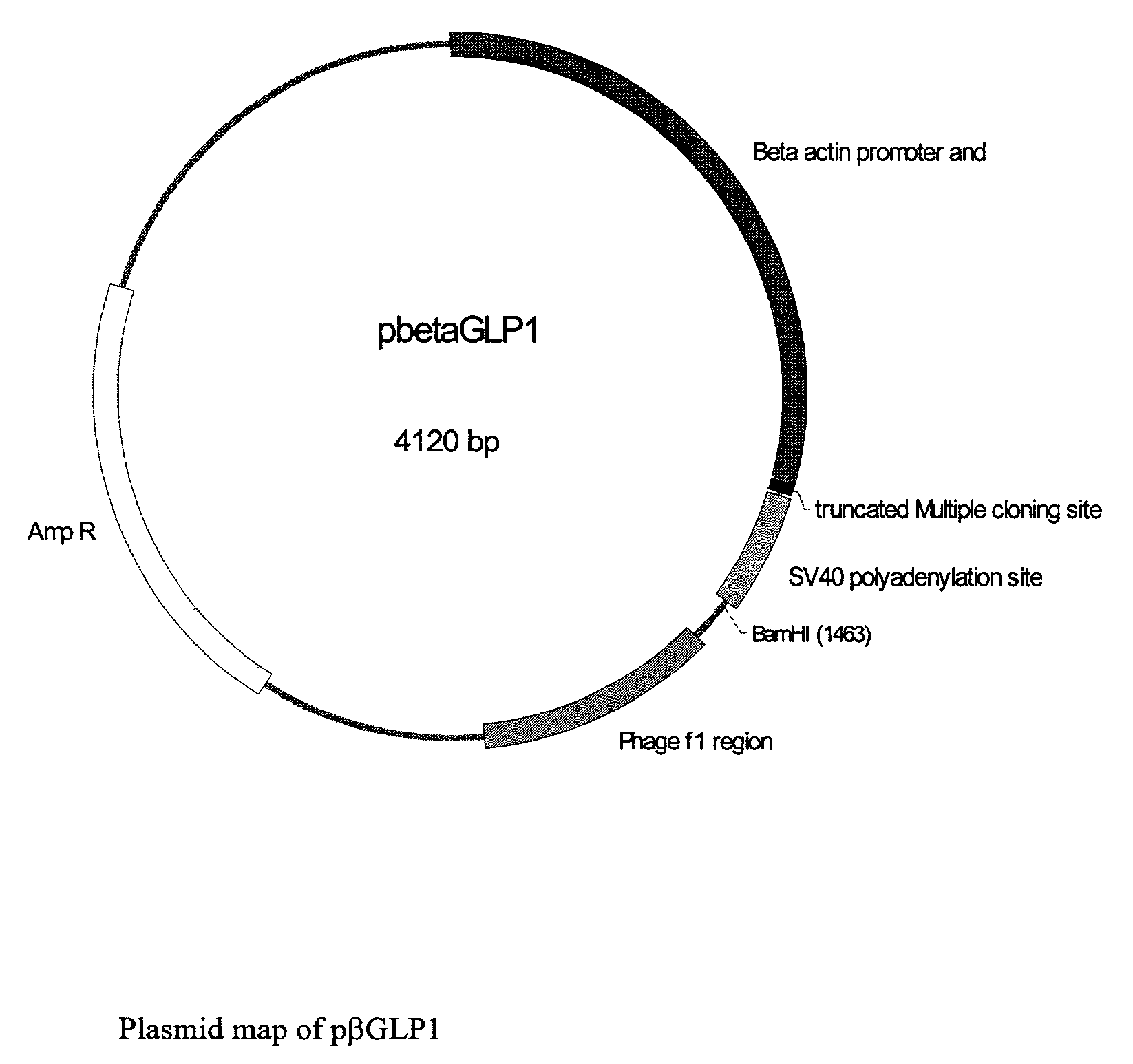
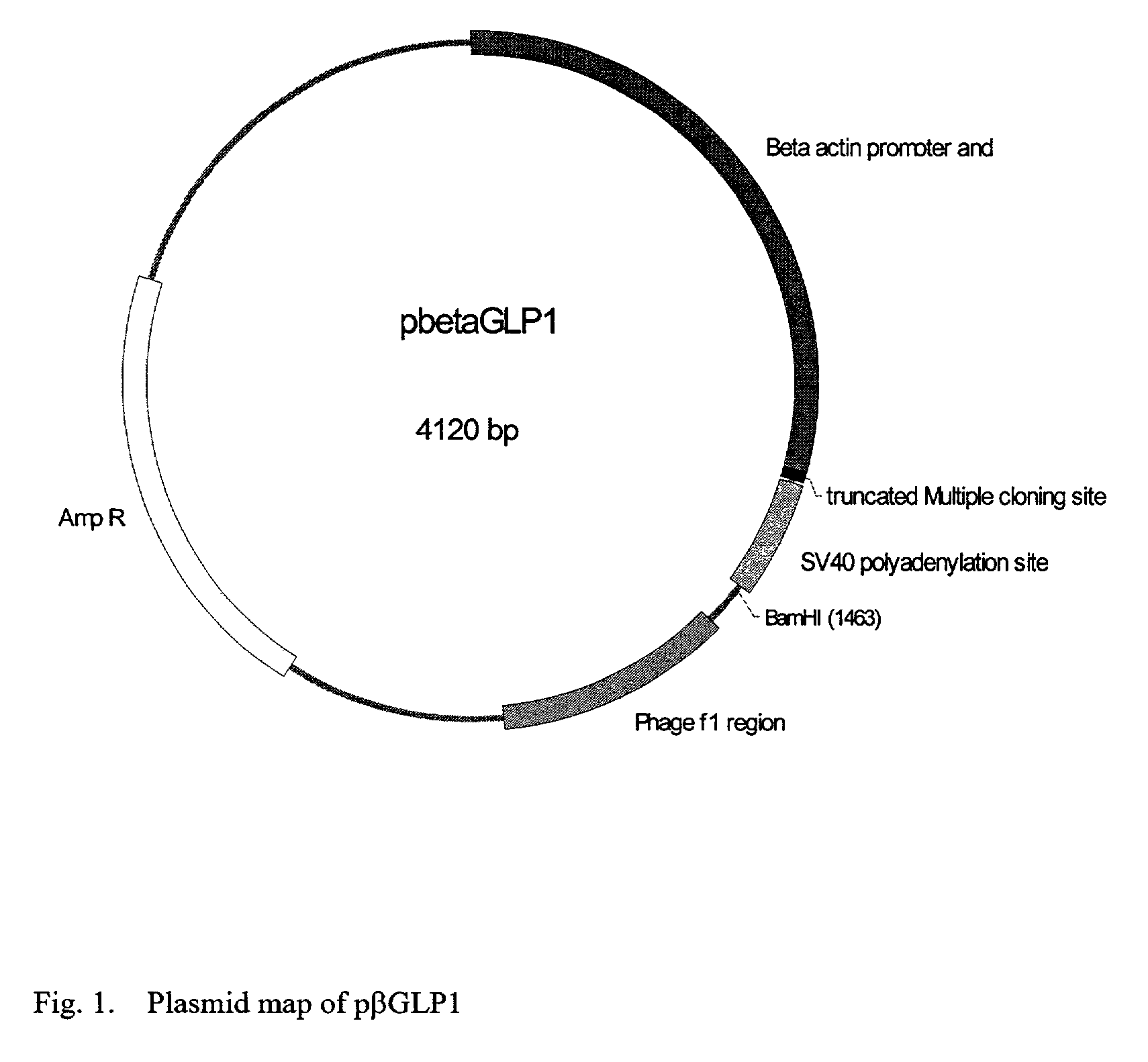
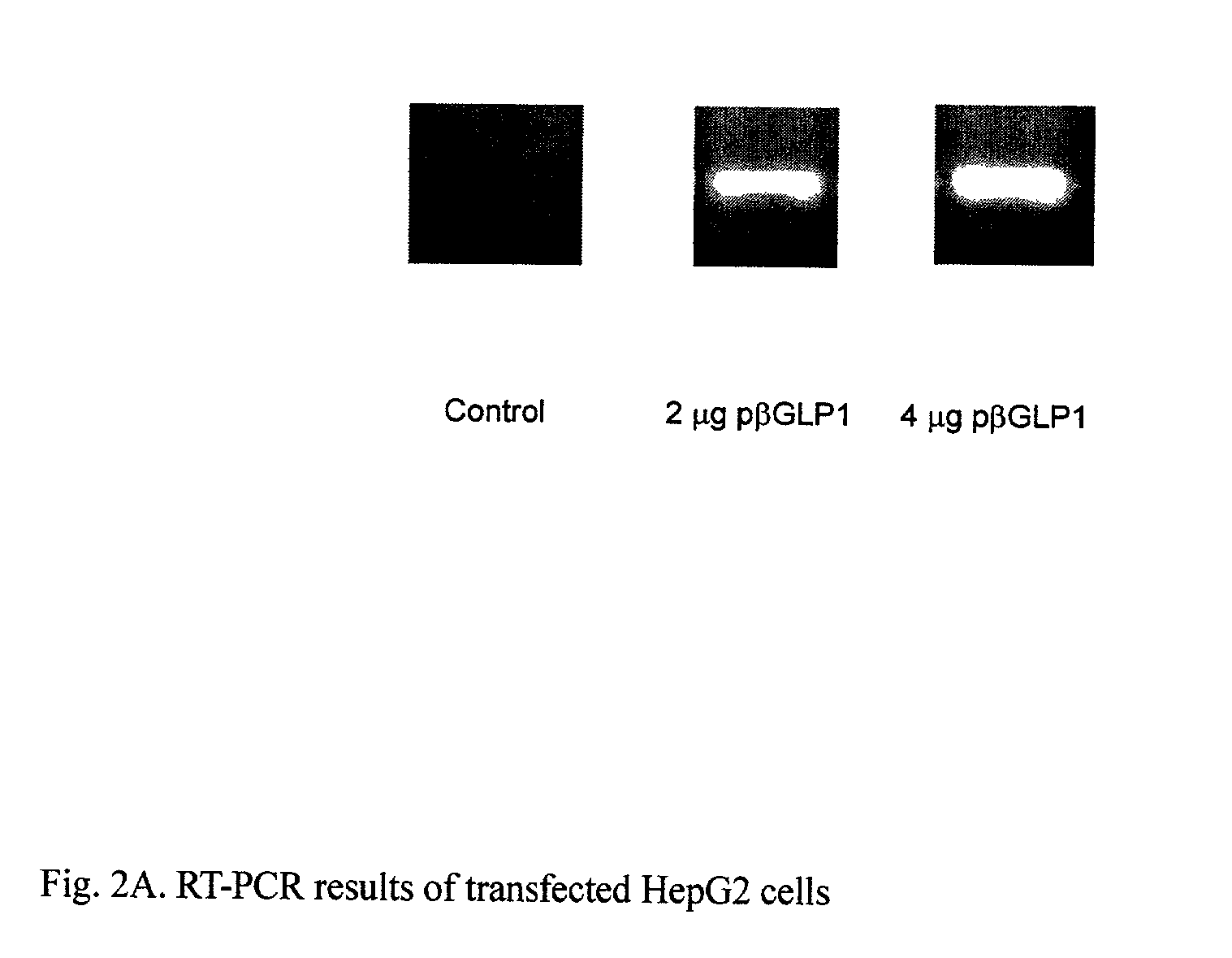
GLP-1 gene delivery for the treatment of type 2 diabetes
ActiveUS7374930B2Efficient transfectionEasy to controlSugar derivativesPeptide/protein ingredientsGene deliveryGlucose polymers
This patent discloses compositions and methods of use thereof to normalize the blood glucose levels of patients with type 2 diabetes. It relates particularly to a plasmid comprising a chicken β actin promoter and enhancer; a modified GLP-1 (7-37) cDNA (pβGLP1), carrying a furin cleavage site, which is constructed and delivered into a cell for the expression of active GLP-1.
Owner:CLSN LAB
Use of recombinant gene delivery vectors for treating or preventing diseases of the eye
Gene delivery vectors, such as, for example, recombinant adeno-associated viral vectors, and methods of using such vectors are provided for use in treating or preventing diseases of the eye.
Owner:CHIRON CORP +1
Combination of immuno gene therapy and chemotherapy for treatment of cancer and hyperproliferative diseases
ActiveUS7964571B2Inhibiting growth and metastasisImprove survivalHeavy metal active ingredientsPeptide/protein ingredientsGene deliveryWhole body
Owner:CLSN LAB
Hematopoietic stem cells and methods of treatment of neovascular eye diseases therewith
InactiveUS20050063961A1Stably incorporated into neovasculature of the eyePromote repairBiocideSenses disorderDiseaseProgenitor
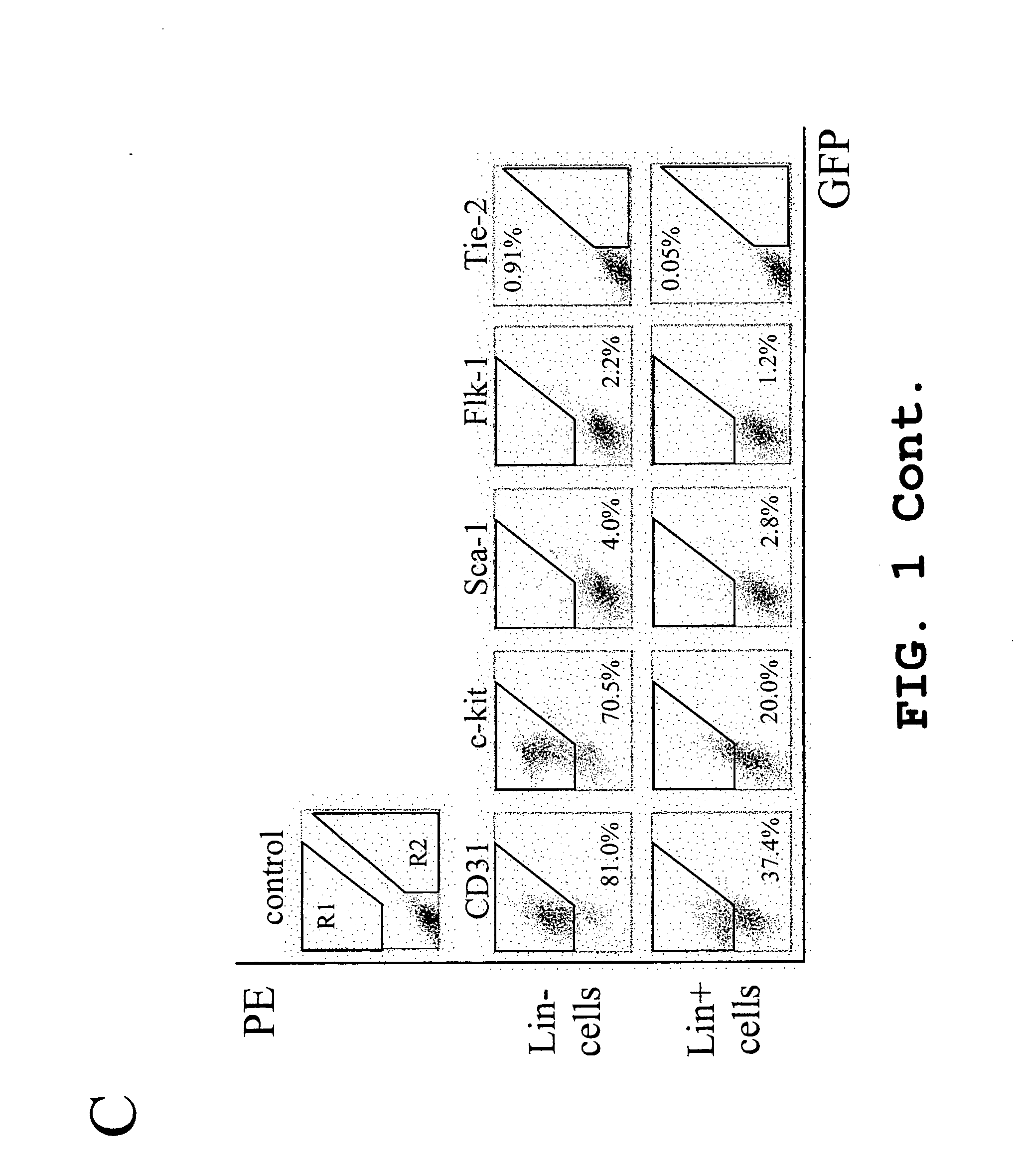
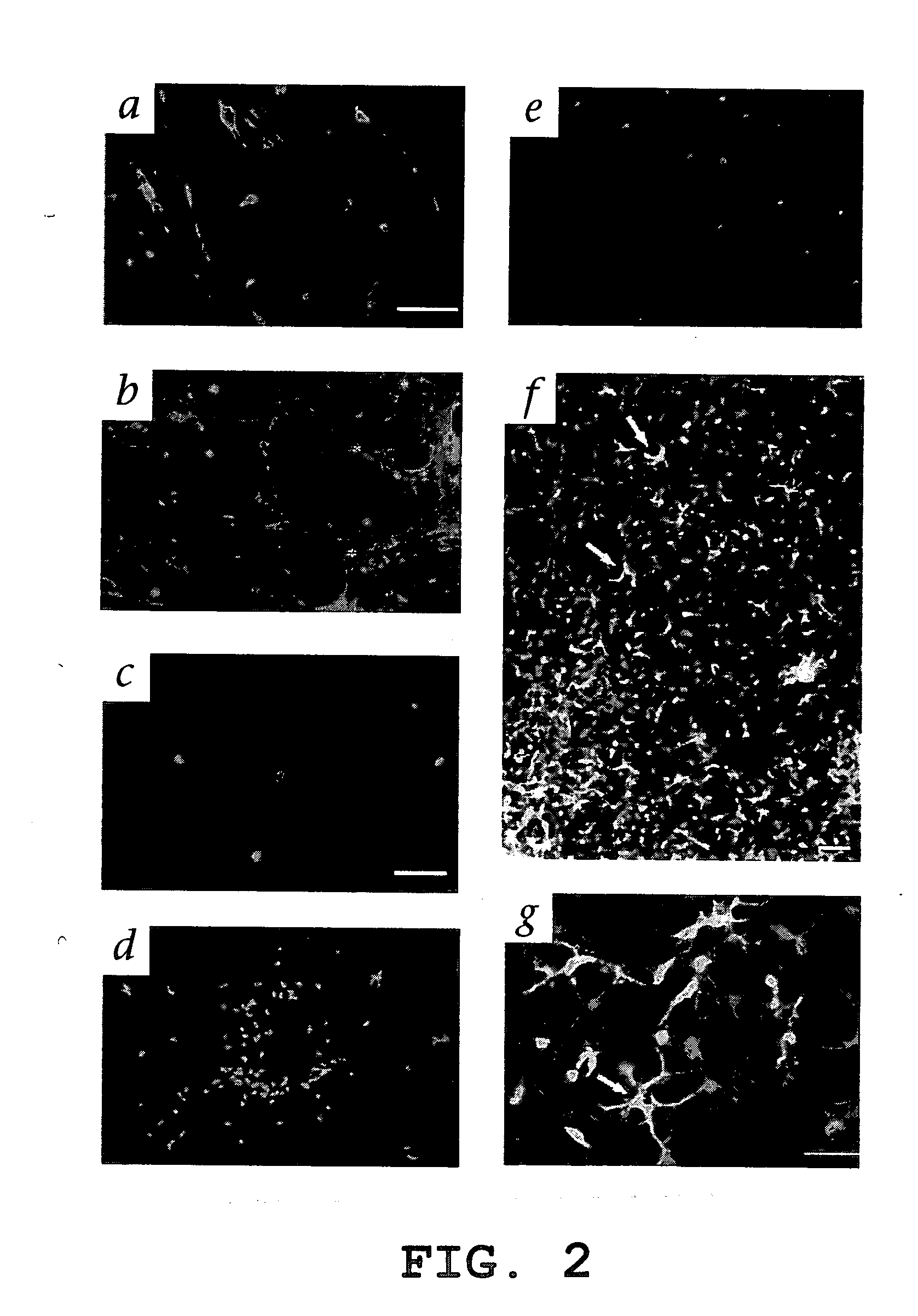
Isolated, mammalian, adult bone marrow-derived, lineage negative hematopoietic stem cell populations (Lin− HSCs) contain endothelial progenitor cells (EPCs) capable of rescuing retinal blood vessels and neuronal networks in the eye. Preferably at least about 20% of the cells in the isolated Lin− HSCs express the cell surface antigen CD31. The isolated Lin− HSC populations are useful for treatment of ocular vascular diseases. In a preferred embodiment, the Lin− HSCs are isolated by extracting bone marrow from an adult mammal; separating a plurality of monocytes from the bone marrow; labeling the monocytes with biotin-conjugated lineage panel antibodies to one or more lineage surface antigens; removing of monocytes that are positive for the lineage surface antigens from the plurality of monocytes, and recovering a Lin− HSC population containing EPCs. Isolated Lin− HSCs that have been transfected with therapeutically useful genes are also provided, and are useful for delivering genes to the eye for cell-based gene therapy. Methods of preparing isolated stem cell populations of the invention, and methods of treating ocular diseases and injury are also described.
Owner:THE SCRIPPS RES INST
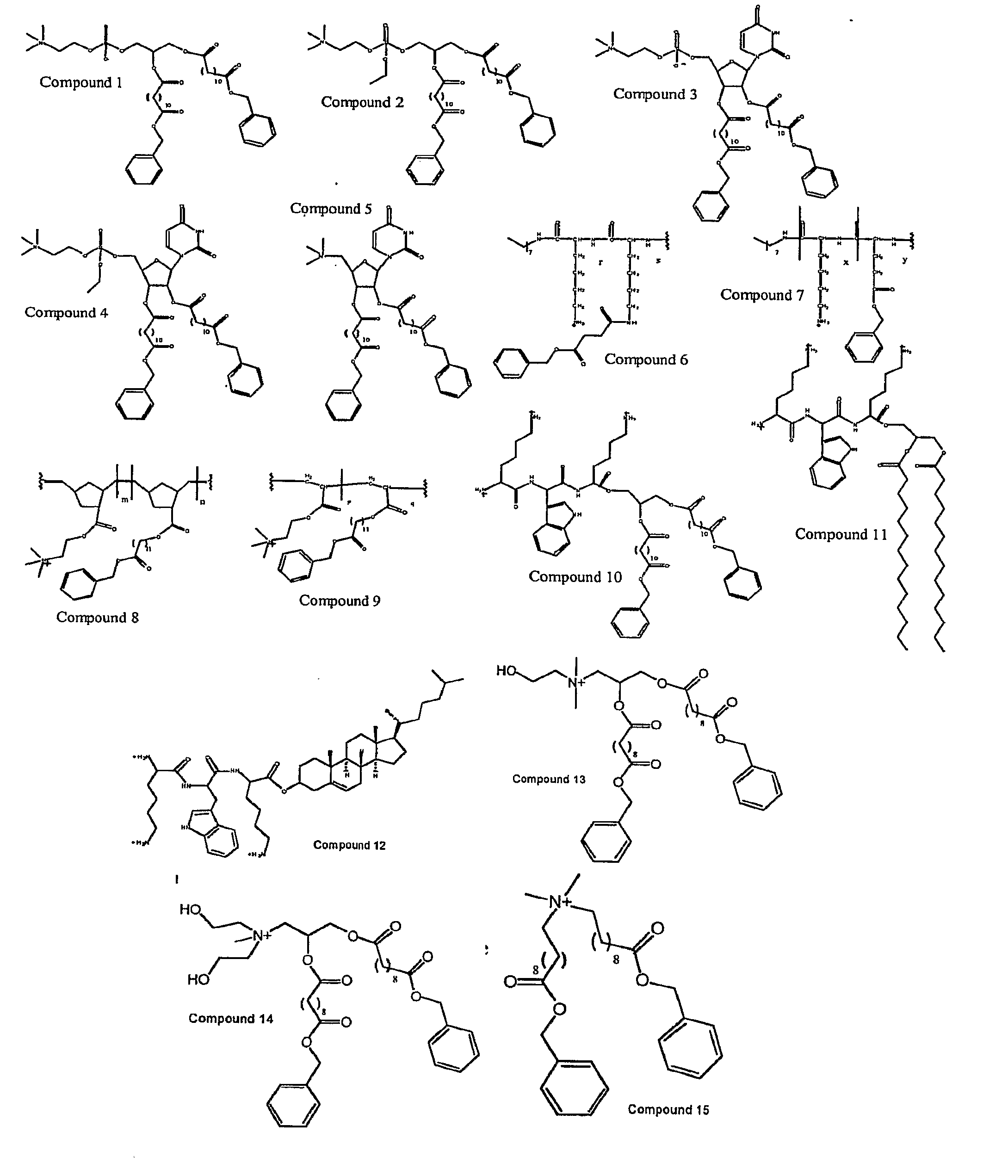
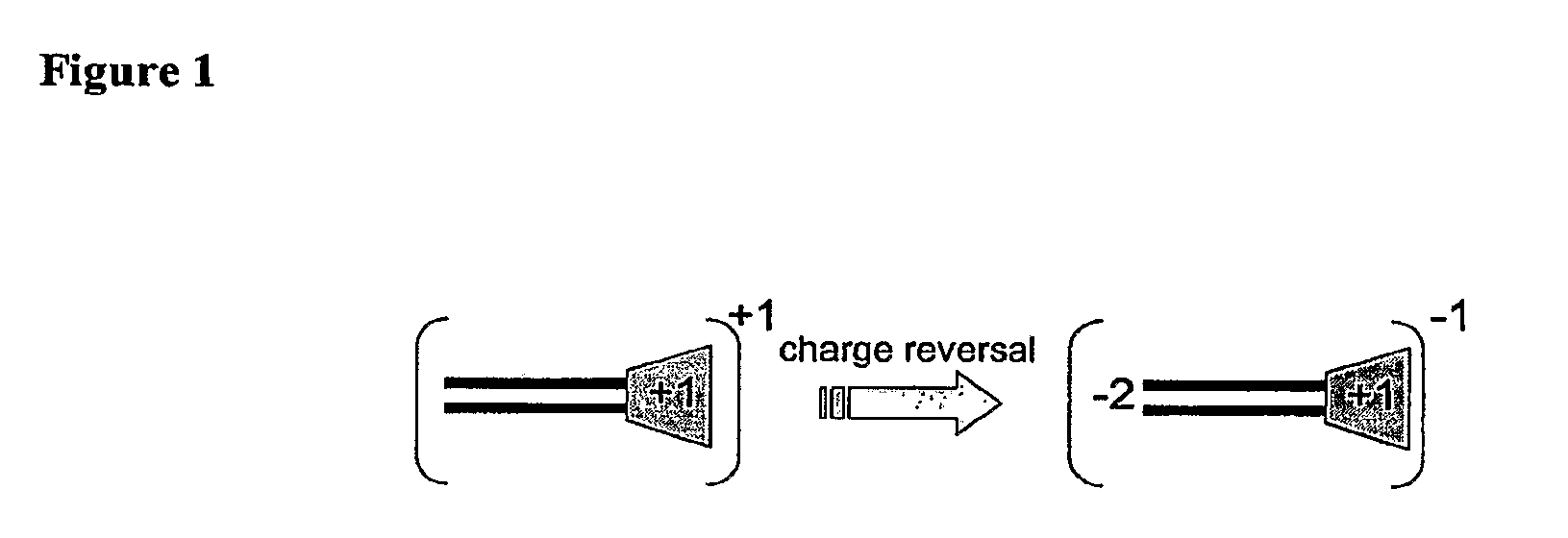
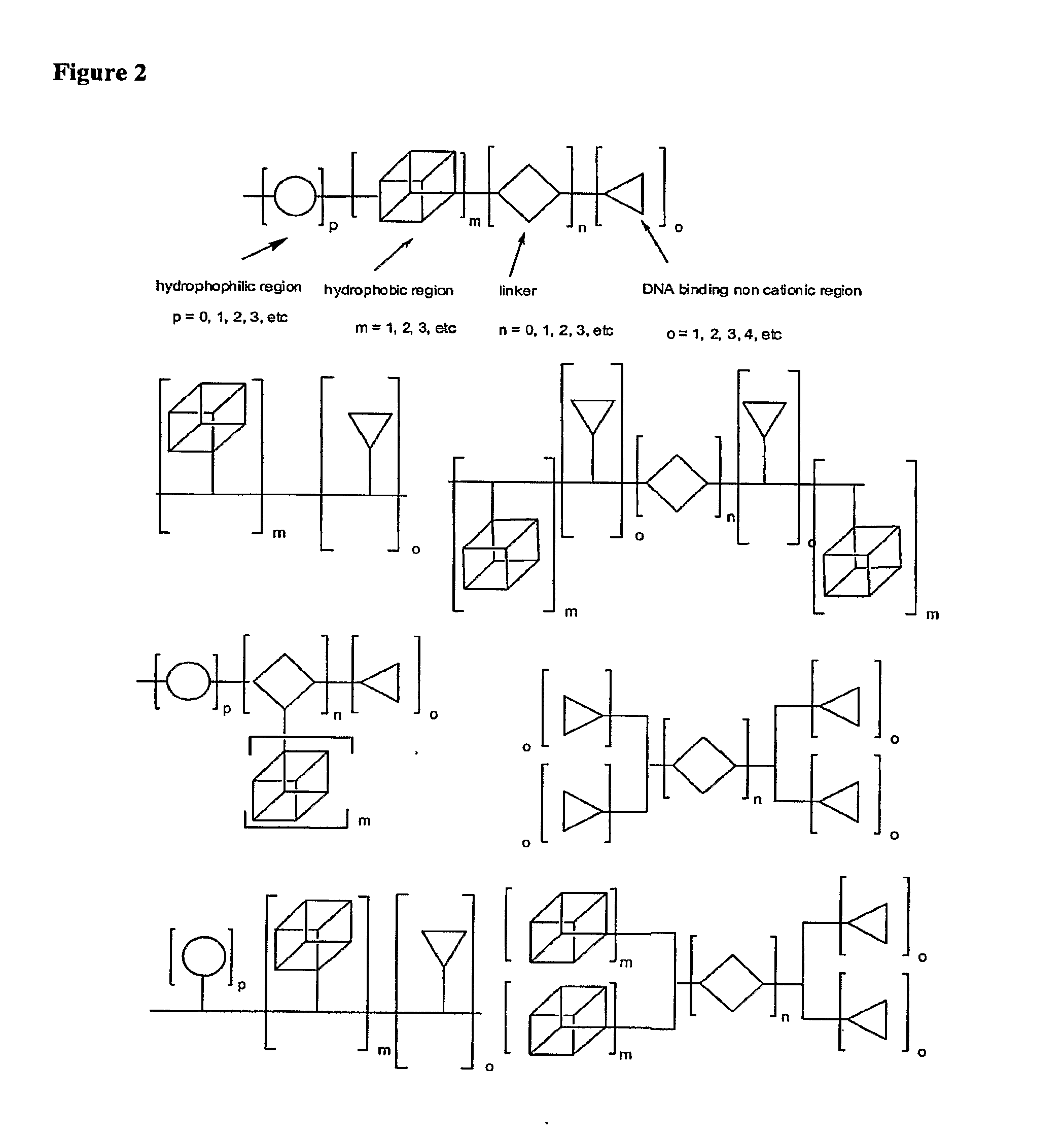
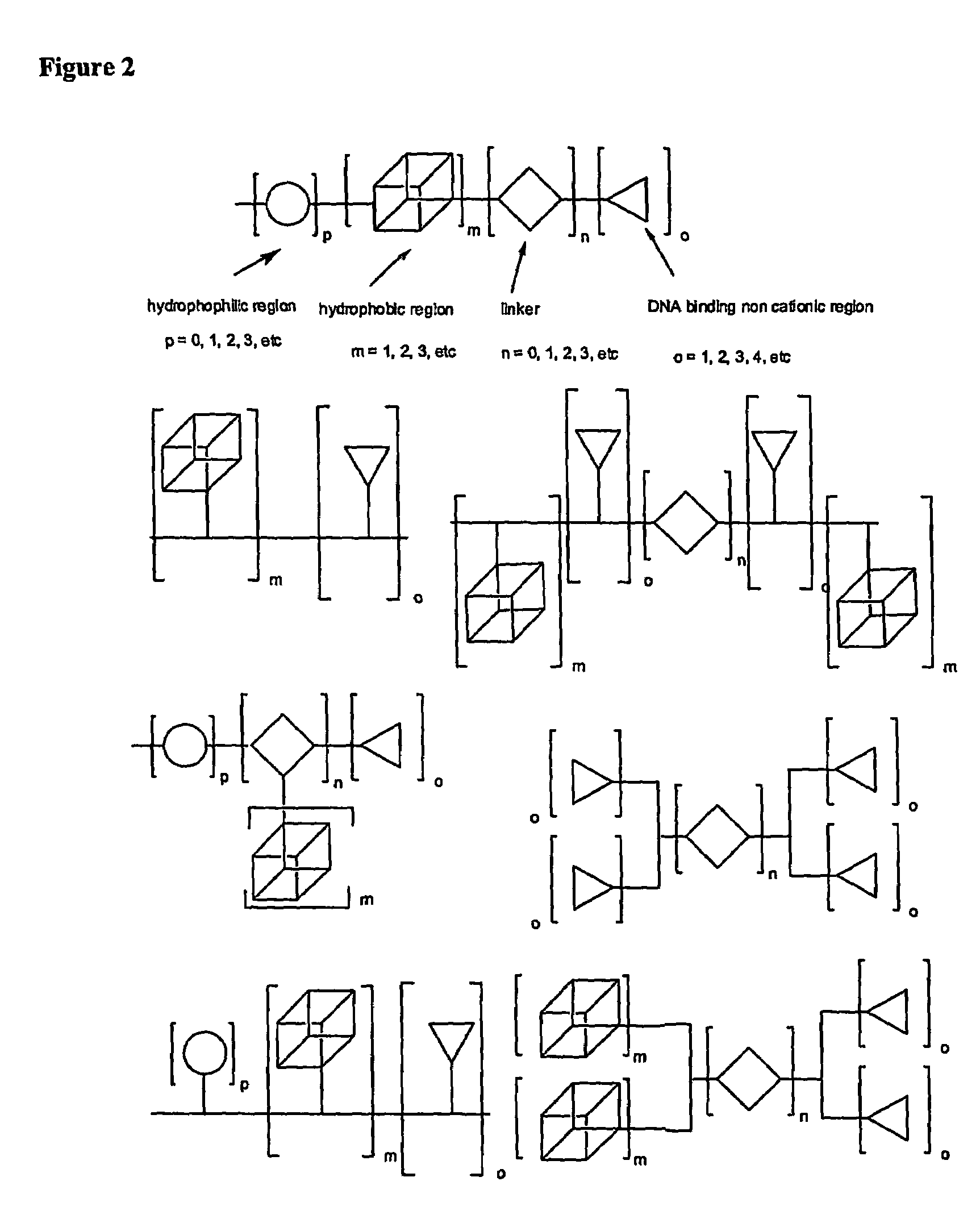
Molecules for Gene Delivery and Gene Therapy, and Methods of Use Thereof
InactiveUS20090221684A1Organic active ingredientsPeptide/protein ingredientsGene deliveryGenetic Materials
One aspect of the present invention relates to a synthetic non-viral vector composition for gene therapy. Another aspect of the invention relates to the use of the composition for in vitro, ex vivo and / or in vivo transfer of genetic material. The invention also encompasses a pharmaceutical composition (useful for delivery of nucleic acids to a cell), containing a non-cationic amphiphilic molecule or macro-molecule; or a cationic amphiphilic molecule or macromolecule that transforms from a cationic entity to an anionic, neutral, or zwitterionic entity upon a chemical, photochemical, or biological reaction. Another aspect of the invention relates to multicationic compounds that are composed of three or more amino acids. The present invention also relates to the use of the pharmaceutical composition for delivery of nucleic acids to a cell. Moreover, the invention encompasses the non-viral vector compositions tethered to a surface. The surface-tethered compositions are useful for the delivery of nucleic acids to cells in contact with the surface. An additional embodiment of the invention relates to a hydrogel comprising a composition of the invention, and methods of using same for the delivery of genetic material to a cell.
Owner:TRUSTEES OF BOSTON UNIV
Biodegradable mixed polymeric micelles for gene delivery
InactiveUS6210717B1Kind be easilySimple contentOrganic active ingredientsNanotechGene deliveryPolyester
A biodegradable, mixed polymeric micelle used to deliver a selected nucleic acid into a targeted host cell contains an amphiphilic polyester-polycation copolymer and an amphiphilic polyester-sugar copolymer. The polyester-polycation copolymer forms an electrostatic interaction with polyanionic nucleic acids, and the polyester-sugar copolymer directs the micelle-nucleic acid complex to cells in vivo. Additional copolymers with similar properties may also be included. The composition improves delivery efficiency by providing a particulate gene carrier for which particle size and charge density are easily controlled by multivariate means. Various kinds of ligands and other functional compounds may be also be introduced using the composition. The composition may be used in a method for transforming a targeted host cell with a selected nucleic acid.
Owner:SAMYANG BIOPHARMLS CORP
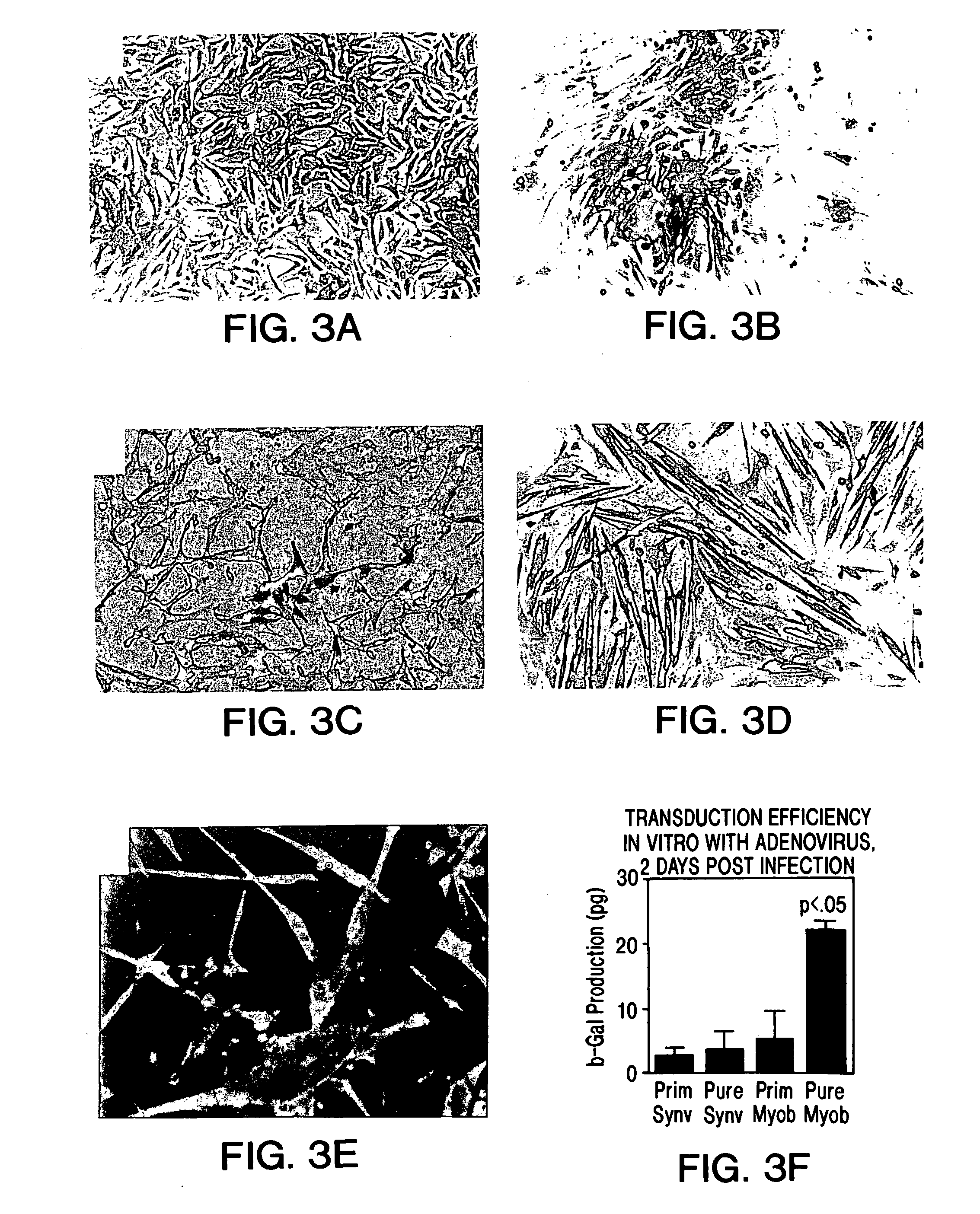
Muscle-derived cells (MDCs) for treating muscle- or bone-related injury or dysfunction
The present invention provides muscle-derived cells, preferably myoblasts and muscle-derived stem cells, genetically engineered to contain and express one or more heterologous genes or functional segments of such genes, for delivery of the encoded gene products at or near sites of musculoskeletal, bone, ligament, meniscus, cartilage or genitourinary disease, injury, defect, or dysfunction. Ex vivo myoblast mediated gene delivery of human inducible nitric oxide synthase, and the resulting production of nitric oxide at and around the site of injury, are particularly provided by the invention as a treatment for lower genitourinary tract dysfunctions. Ex vivo gene transfer for the musculoskeletal system includes genes encoding acidic fibroblast growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor, platelet derived growth factor, transforming growth factor-β, transforming growth factor-α, nerve growth factor and interleukin-1 receptor antagonist protein (IRAP), bone morphogenetic protein (BMPs), cartilage derived morphogenetic protein (CDMPs), vascular endothelial growth factor (VEGF), and sonic hedgehog proteins.
Owner:UNIVERSITY OF PITTSBURGH
Mesoporous silica nanoparticle-mediated delivery of DNA into arabidopsis root
InactiveUS20130185823A1MicroorganismsOther foreign material introduction processesGerm layerGene delivery
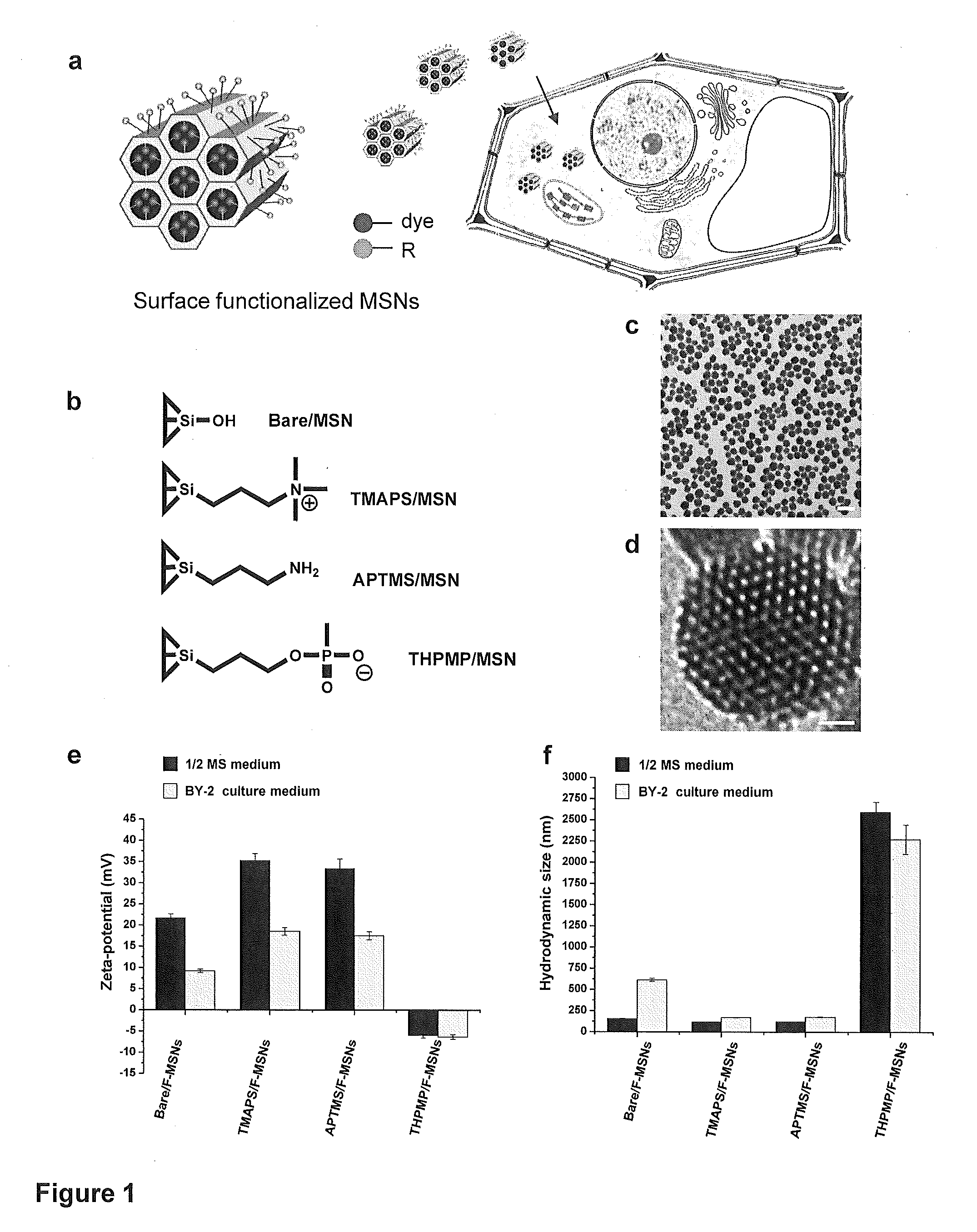
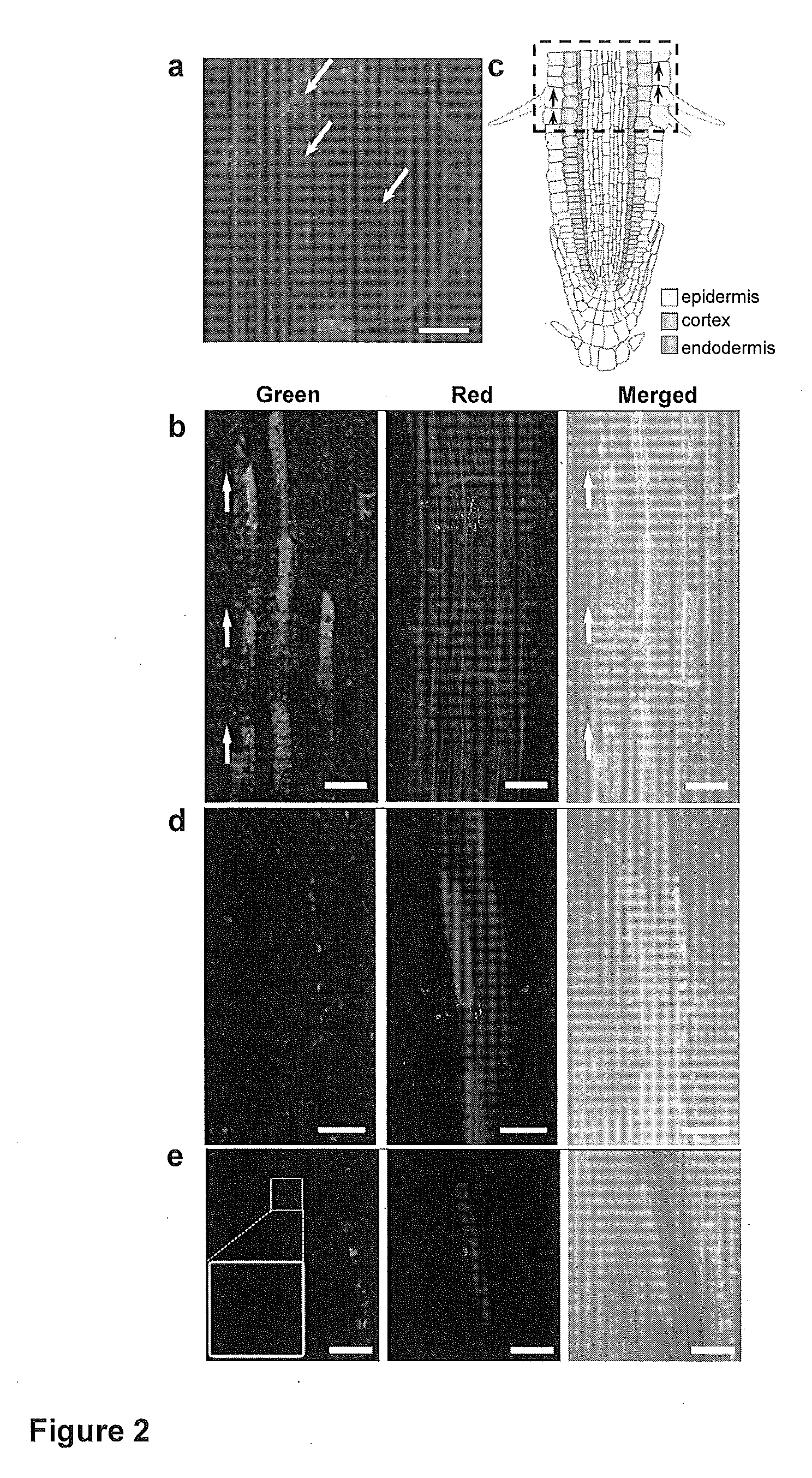
Transient gene expression is a powerful tool for plant genomics studies. Recently, the use of nanomaterials has drawn great interest. Delivery with mesoporous silica nanoparticles (MSNs) has many advantages. We used surface-functionalized MSNs to deliver and express foreign DNA in Arabidopsis thaliana root cells without the aid of particle bombardment. Gene expression was detected in the epidermis layer and in the more inner cortex and endodermis root tissues. This method is superior to the conventional gene-gun method to deliver DNA, which delivers the gene to the epidermis layer only. Less DNA is needed for the MSN method. Our system is the first use of nanoparticles to deliver DNA to plants with good efficiency and without external aids. MSNs, with multifunctionality and the capability of cargo delivery to plant cells as we demonstrated, provide a versatile system for biomolecule delivery, organelle targeting, and even agriculture, such as improved nutrient uptake.
Owner:ACAD SINIC +1
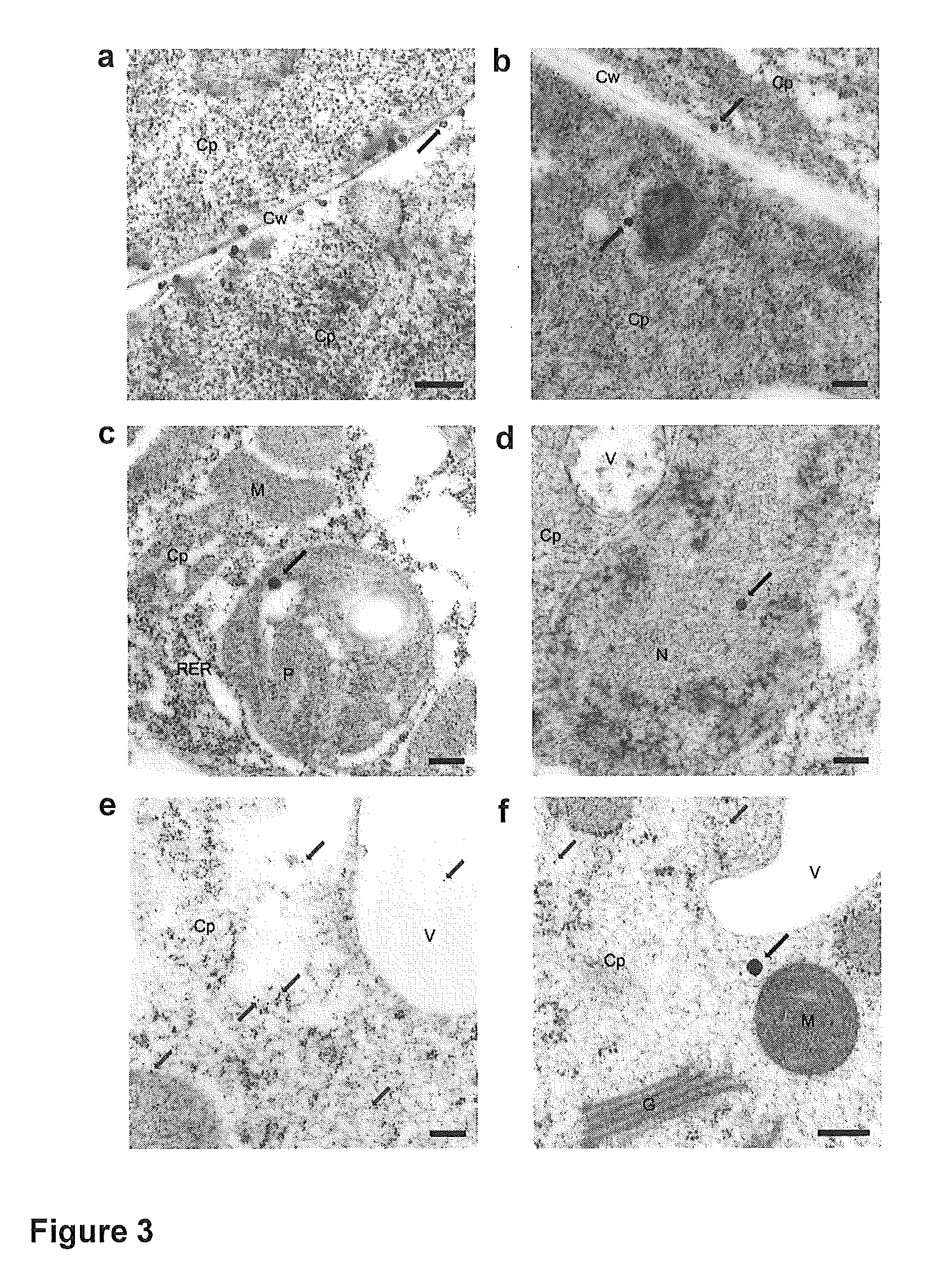
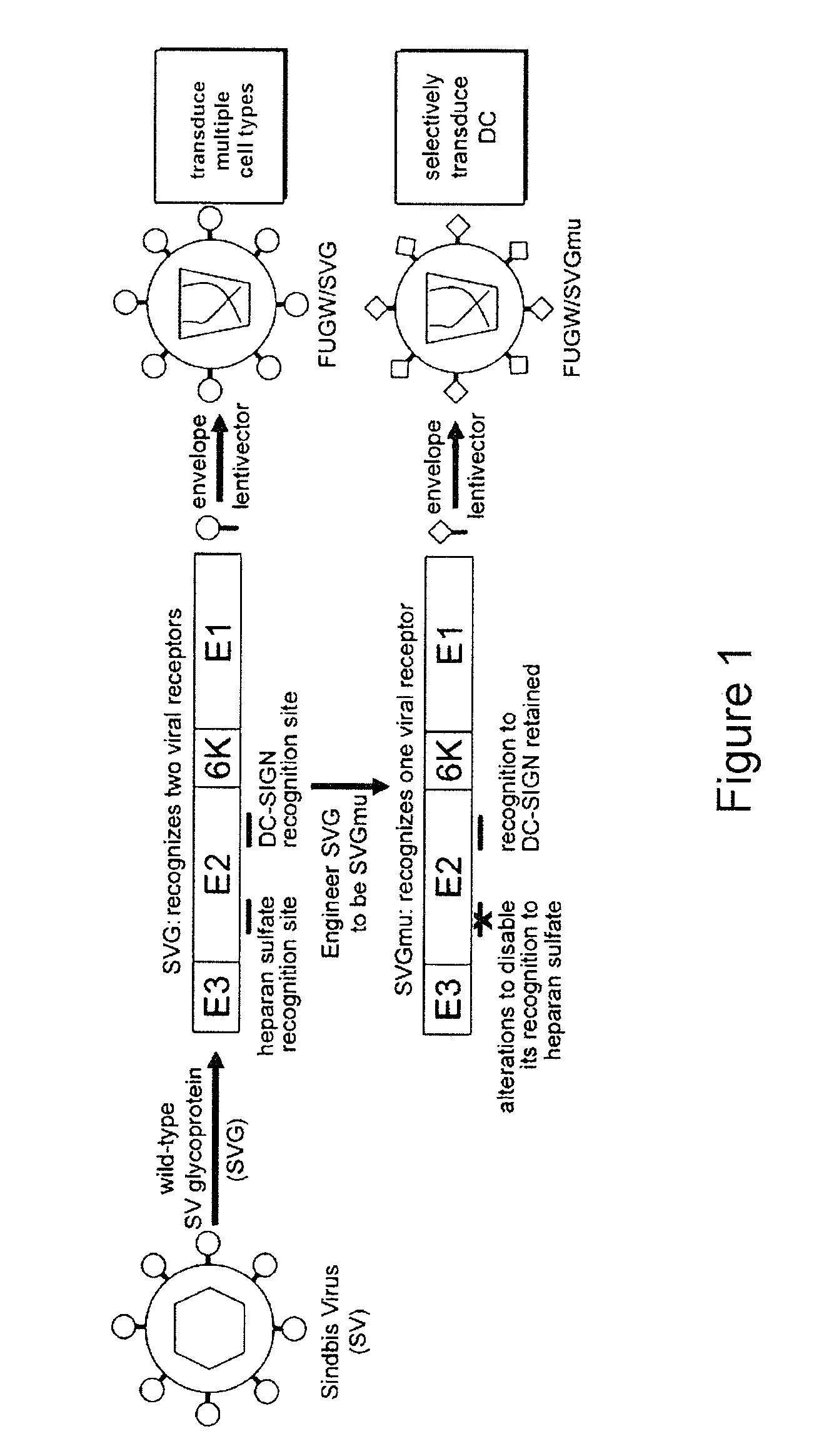
Targeted gene delivery for dendritic cell vaccination
Methods and compositions are provided for delivery of a polynucleotide encoding a gene of interest, typically an antigen, to a dendritic cell (DC). The virus envelope comprises a DC-SIGN specific targeting molecule. The methods and related compositions can be used to treat patients suffering from a wide range of conditions, including infection, such as HIV / AIDS, and various types of cancers.
Owner:CALIFORNIA INST OF TECH
Method of targeted gene delivery using viral vectors
Methods and compositions are provided for delivering a polynucleotide encoding a gene of interest to a target cell using a virus. The virus envelope comprises a cell-specific binding determinant that recognizes and binds to a component on the target cell surface, leading to endocytosis of the virus. A separate fusogenic molecule is also present on the envelope and facilitates delivery of the polynucleotide across the membrane and into the cytosol of the target cell. The methods and related compositions can be used for treating patients having suffering from a wide range of conditions, including infection, such as HIV; cancers, such as non-Hodgkin's lymphoma and breast cancer; and hematological disorders, such as severe combined immunodeficiency.
Owner:CALIFORNIA INST OF TECH
Methods and compositions for efficient gene transfer using transcomplementary vectors
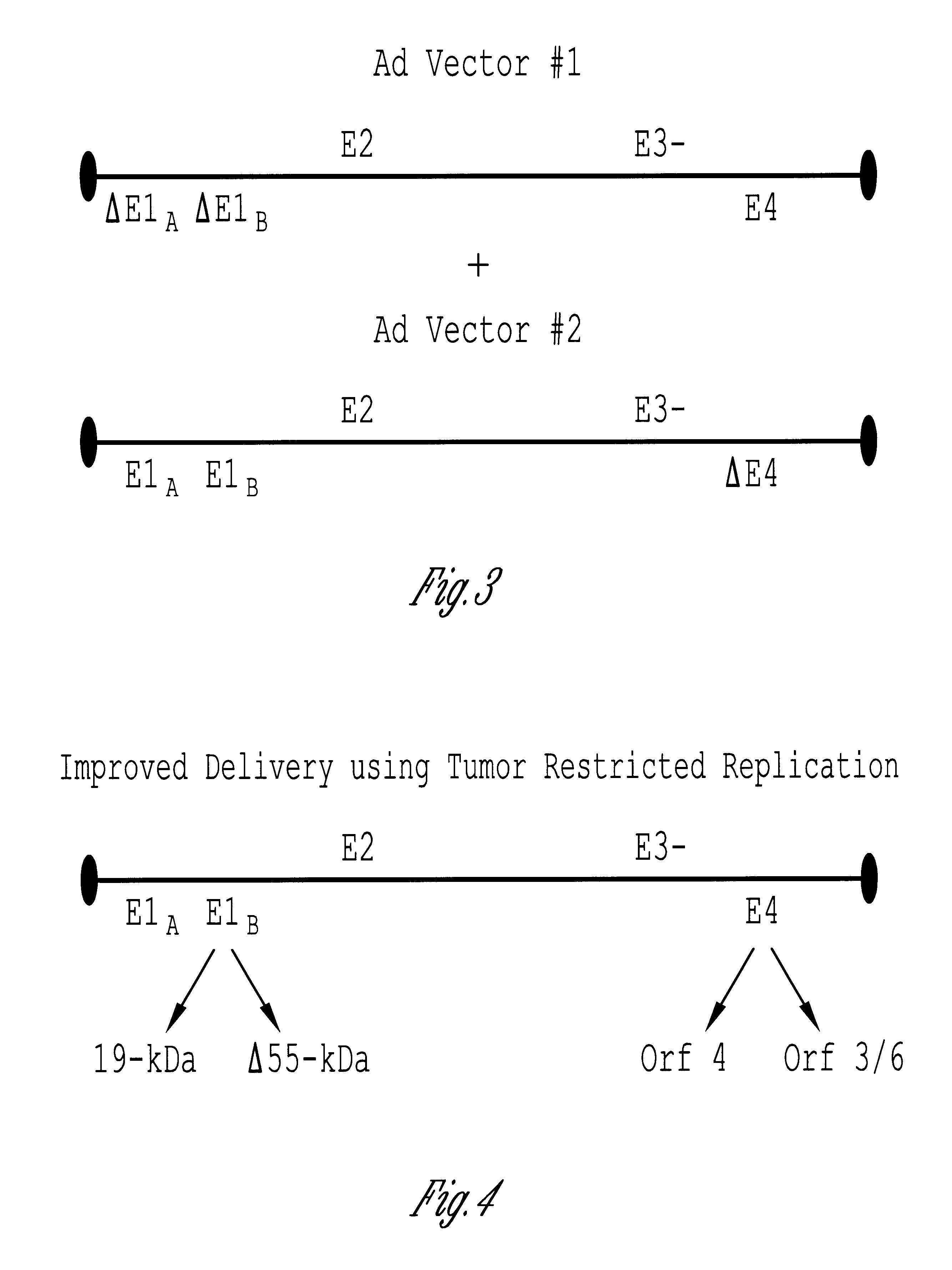
The invention includes a viral vector method and composition comprising transcomplementary replication incompetent viral vectors, preferably adenoviral vectors, which are cotransformed to a recipient cell. The two vectors complement each other and thus allow viral replication, in a synergistic combination which enhances both gene delivery and gene expression of genetic sequences contained within the vector.
Owner:HUMAN GENE THERAPY RES INST
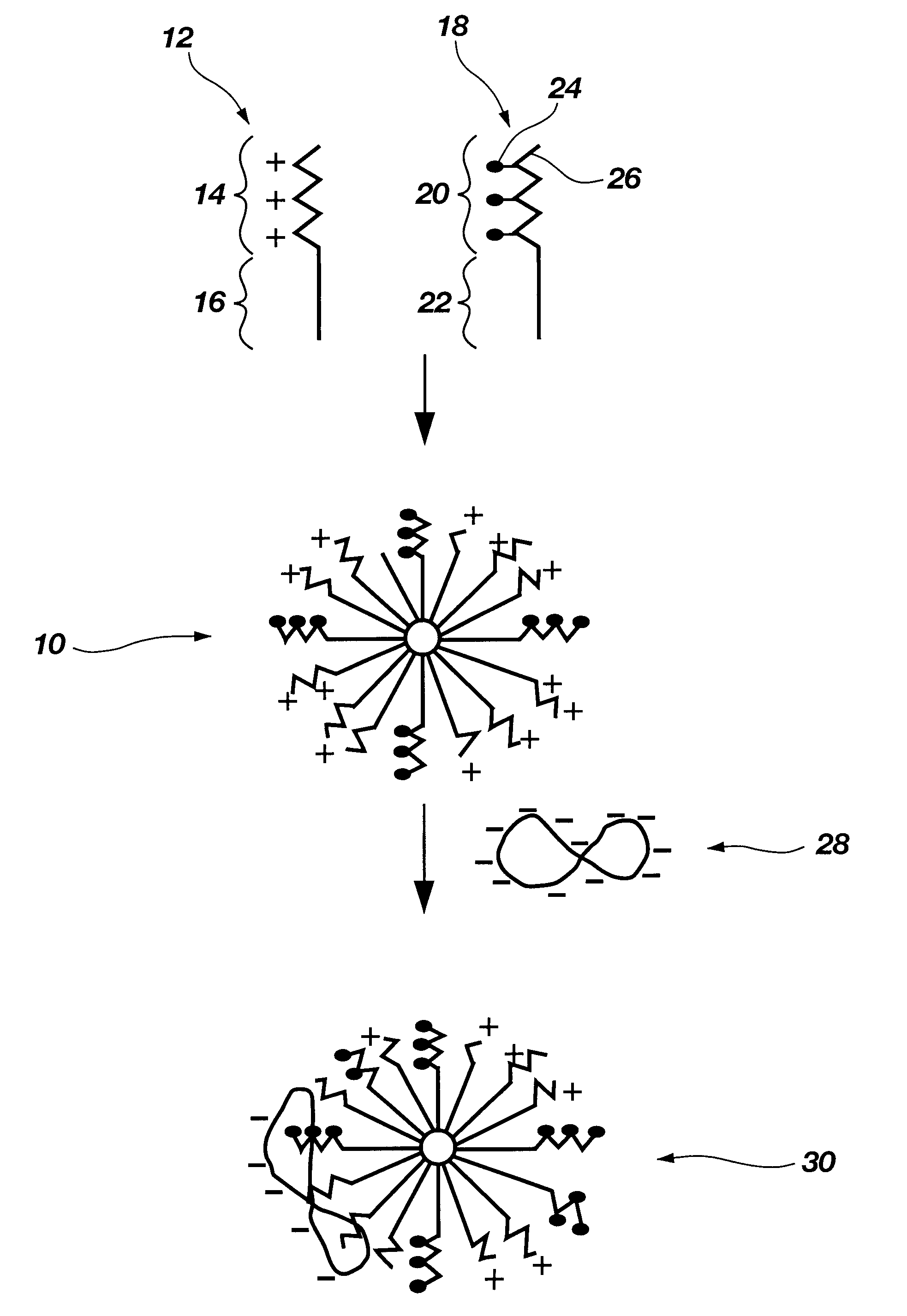
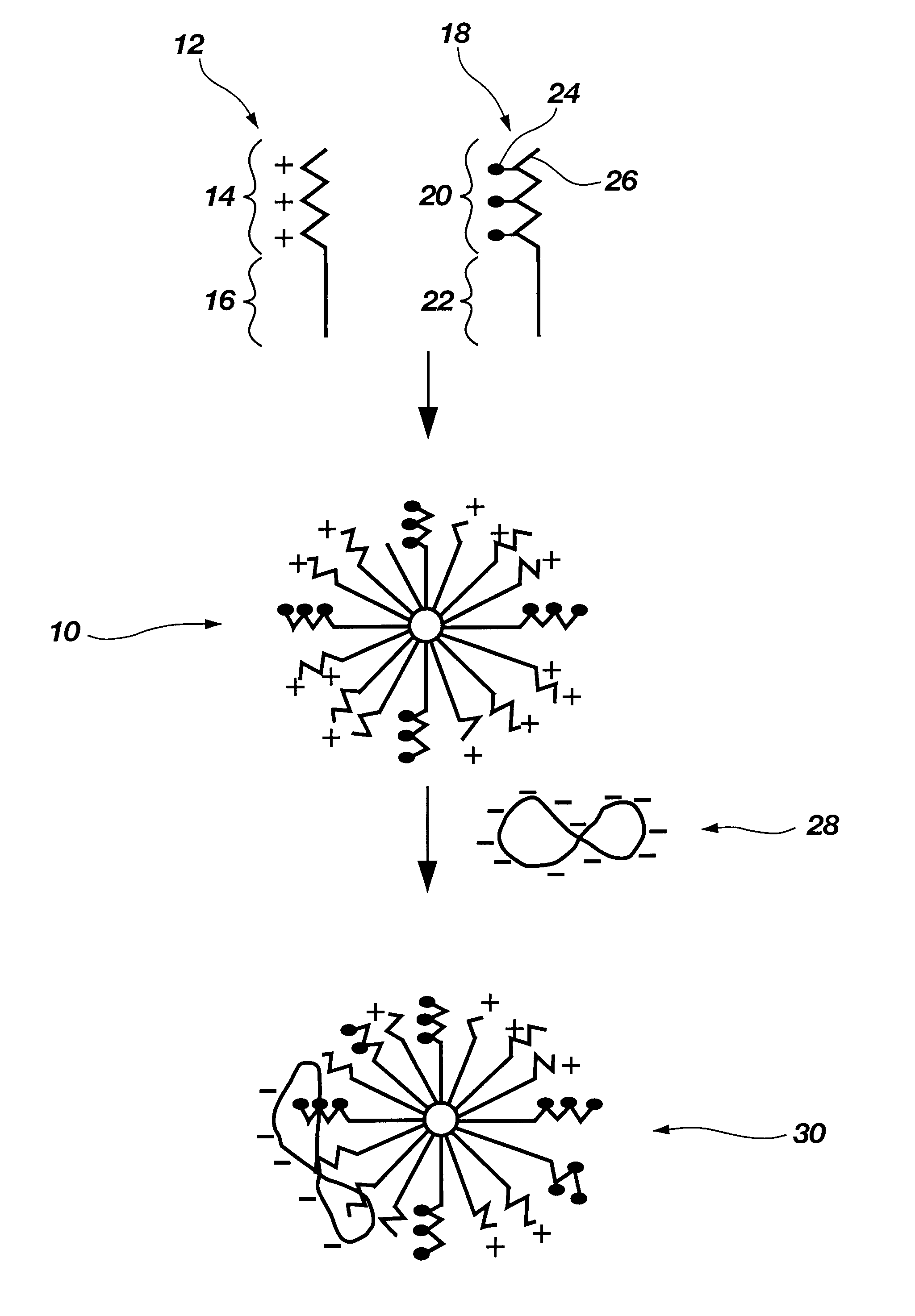
Functional synthetic molecules and macromolecules for gene delivery
The present invention describes a synthetic non-viral vector composition for gene therapy and the use of such compositions for in vitro, ex vivo and / or in vivo transfer of genetic material. The invention proposes a pharmaceutical composition containing 1) a non-cationic amphiphilic molecule or macromolecule and its use for delivery of nucleic acids or 2) a cationic amphiphilic molecule or macromolecule that transforms from a cationic entity to an anionic, neutral, or zwitterionic entity by a chemical, photochemical, or biological reaction and its use for delivery of nucleic acids. Moreover this invention describes the use of these non-viral vector compositions in conjunction with a surface to mediate the delivery of nucleic acids. An additional embodiment is the formation of a hydrogel with these compositions and the use of this hydrogel for the delivery of genetic material. A further embodiment of this invention is the use of a change in ionic strength for the delivery of genetic material.
Owner:FIFTH BASE
Gene delivery system and methods of use
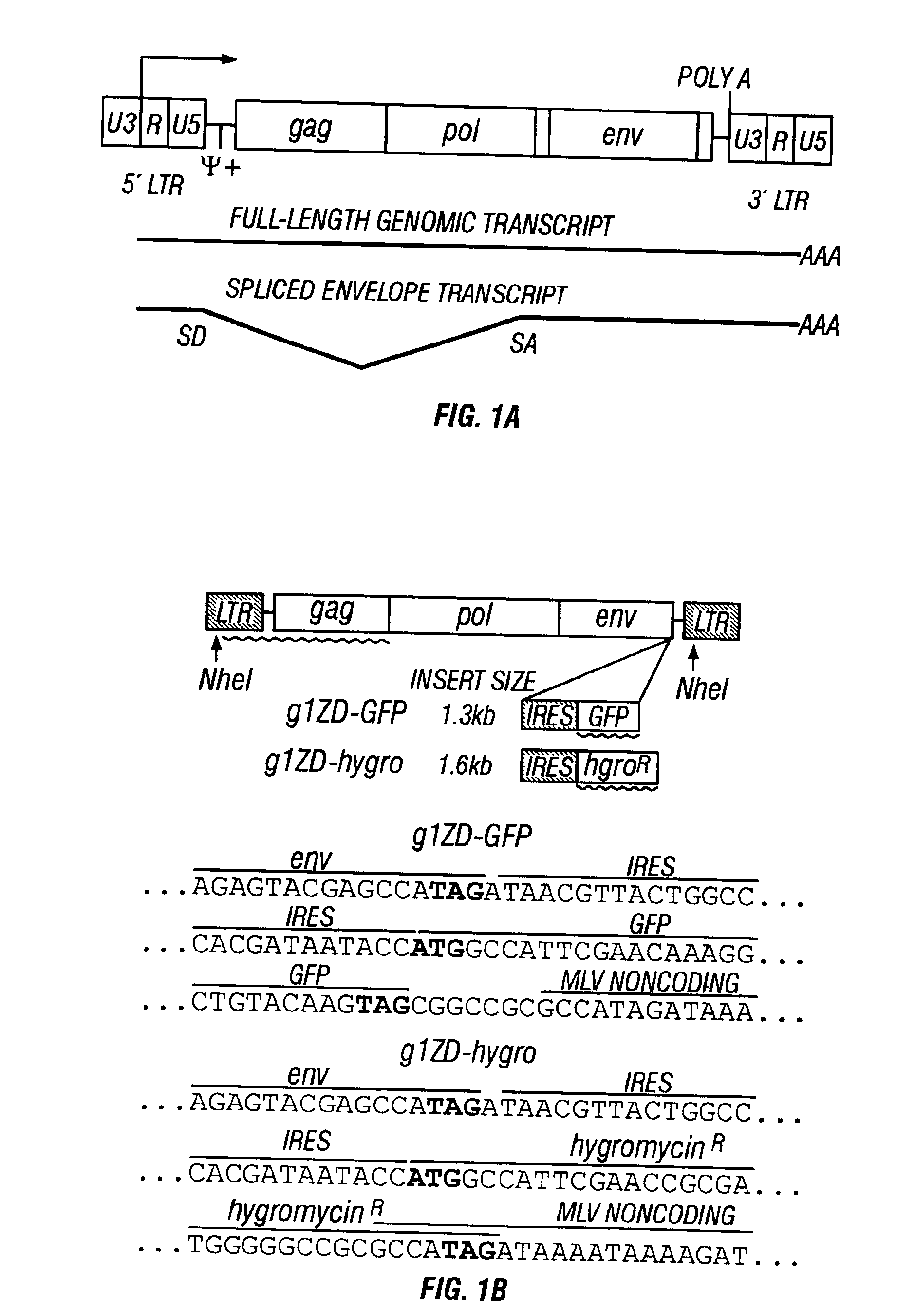
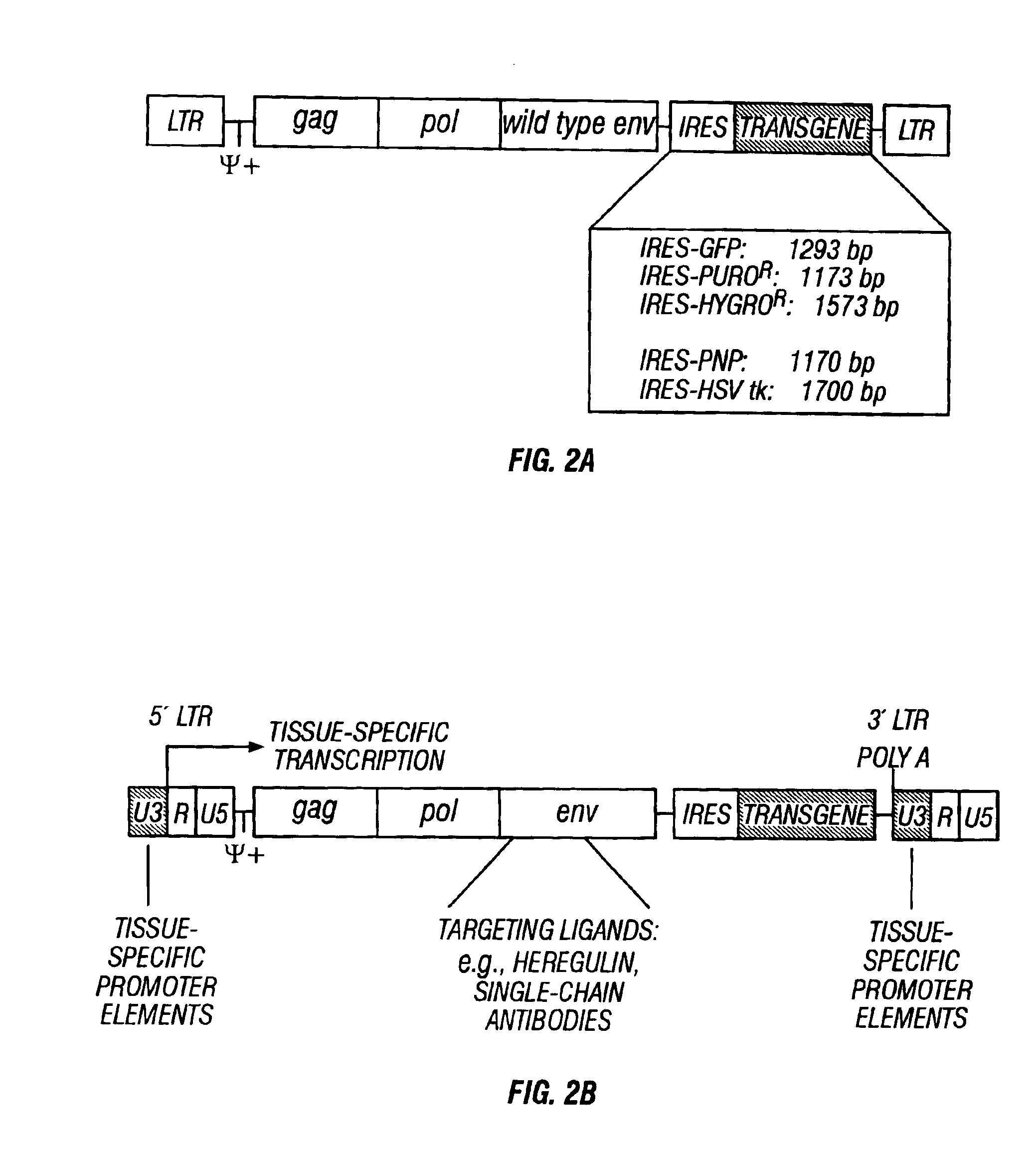
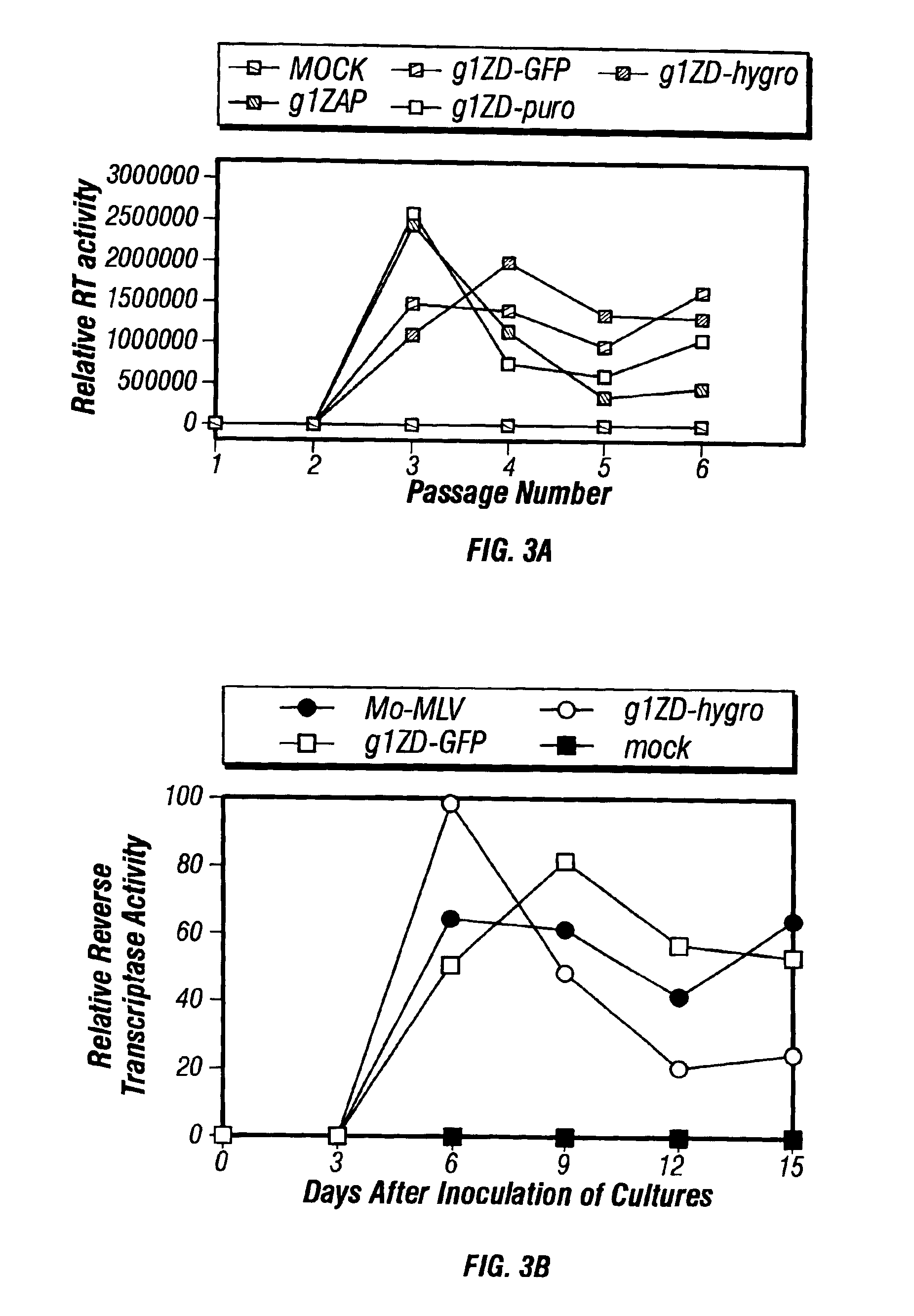
InactiveUS6899871B2Reduce and eliminate native pathogenic potentialEfficient transductionBiocideVectorsGene deliveryHeterologous
A recombinant replication competent retrovirus for gene deliver and gene therapy is provided. The recombinant retrovirus has a heterologous nucleic acid sequence, a sequence encoding a cell- or tissue-specific ligand or a sequence for transcriptional targeting, or a combination of both a cell- or tissue-specific ligand and a cell- or tissue-specific transcriptional targeting sequence.
Owner:UNIV OF SOUTHERN CALIFORNIA
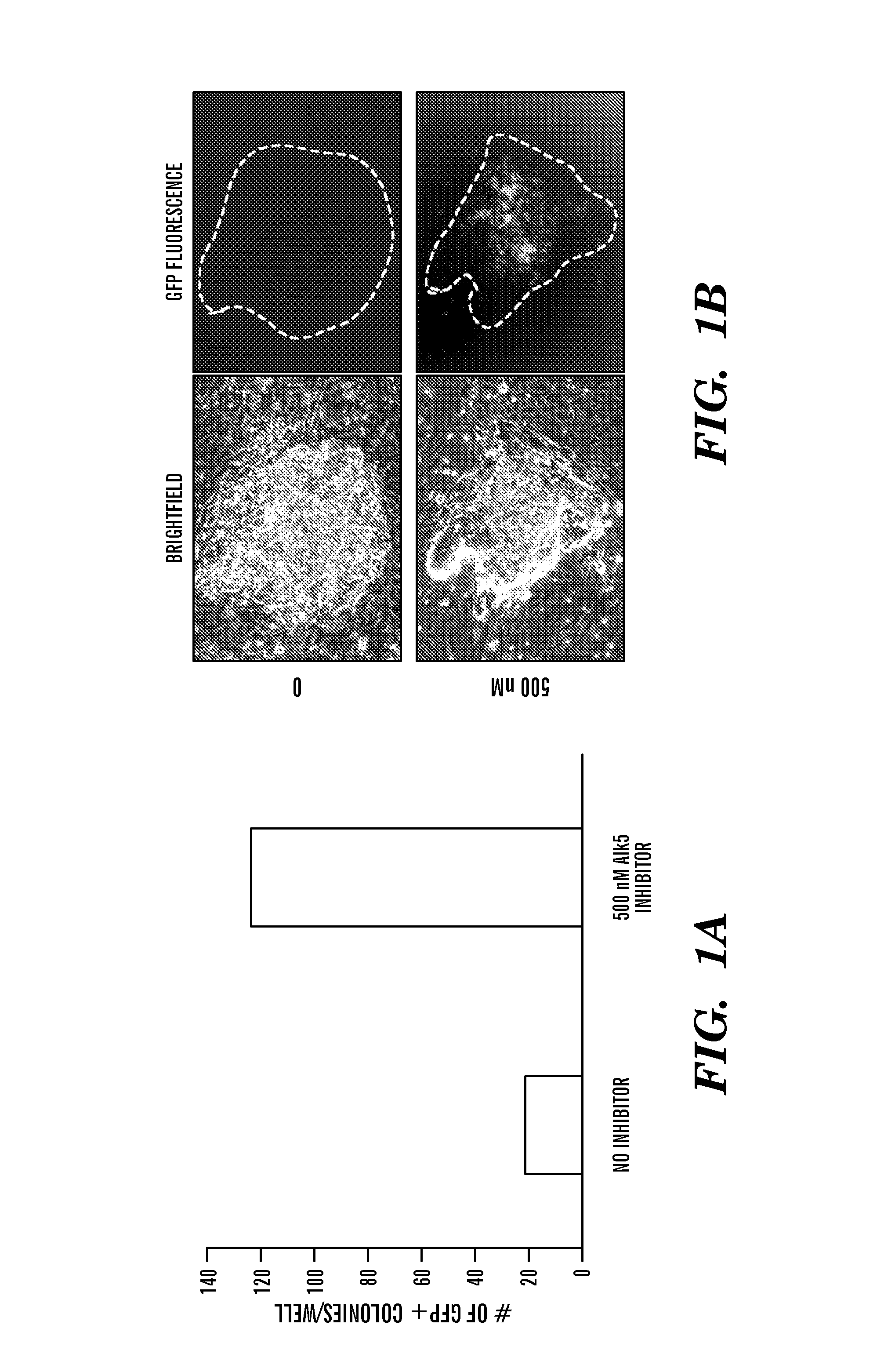
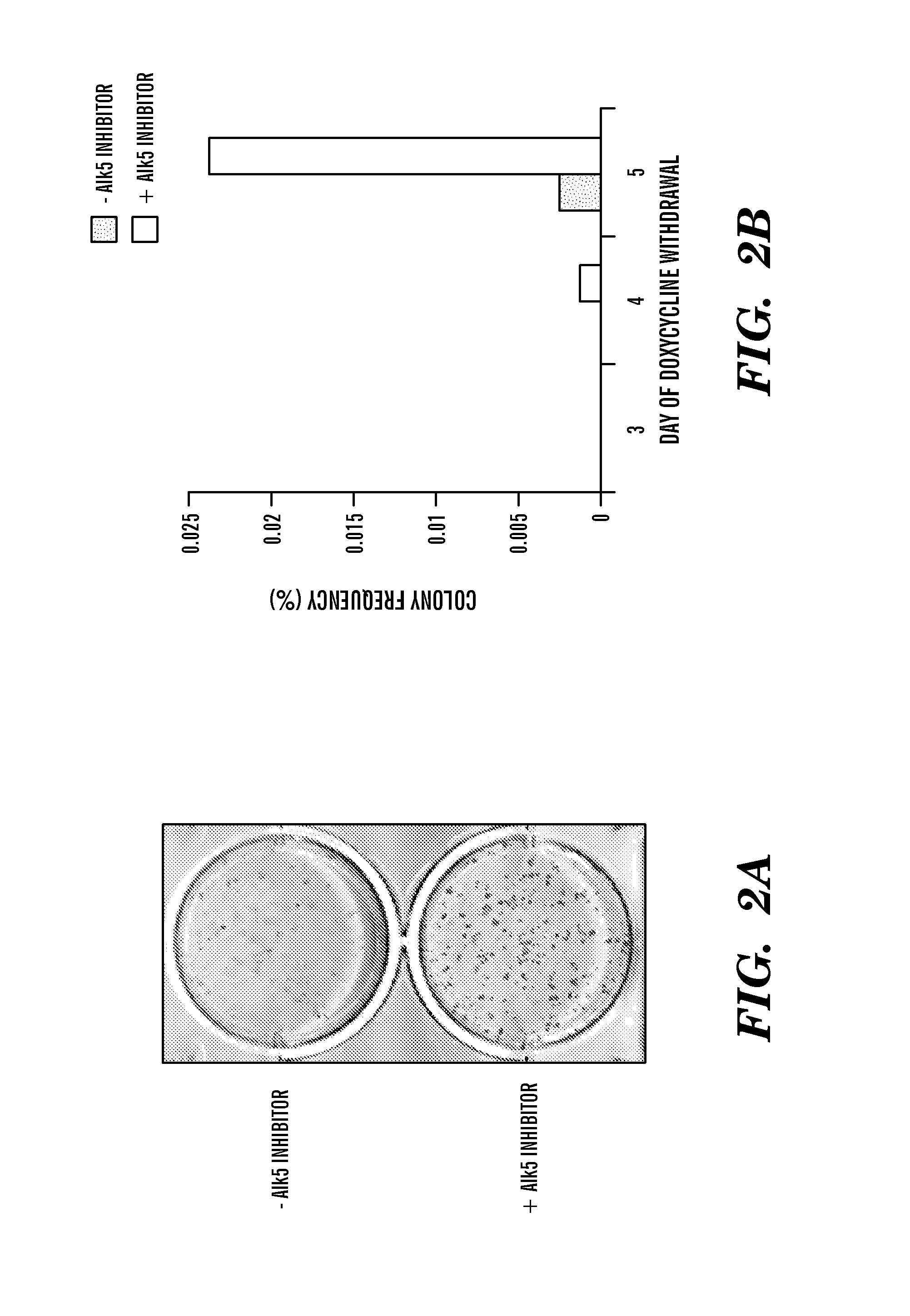
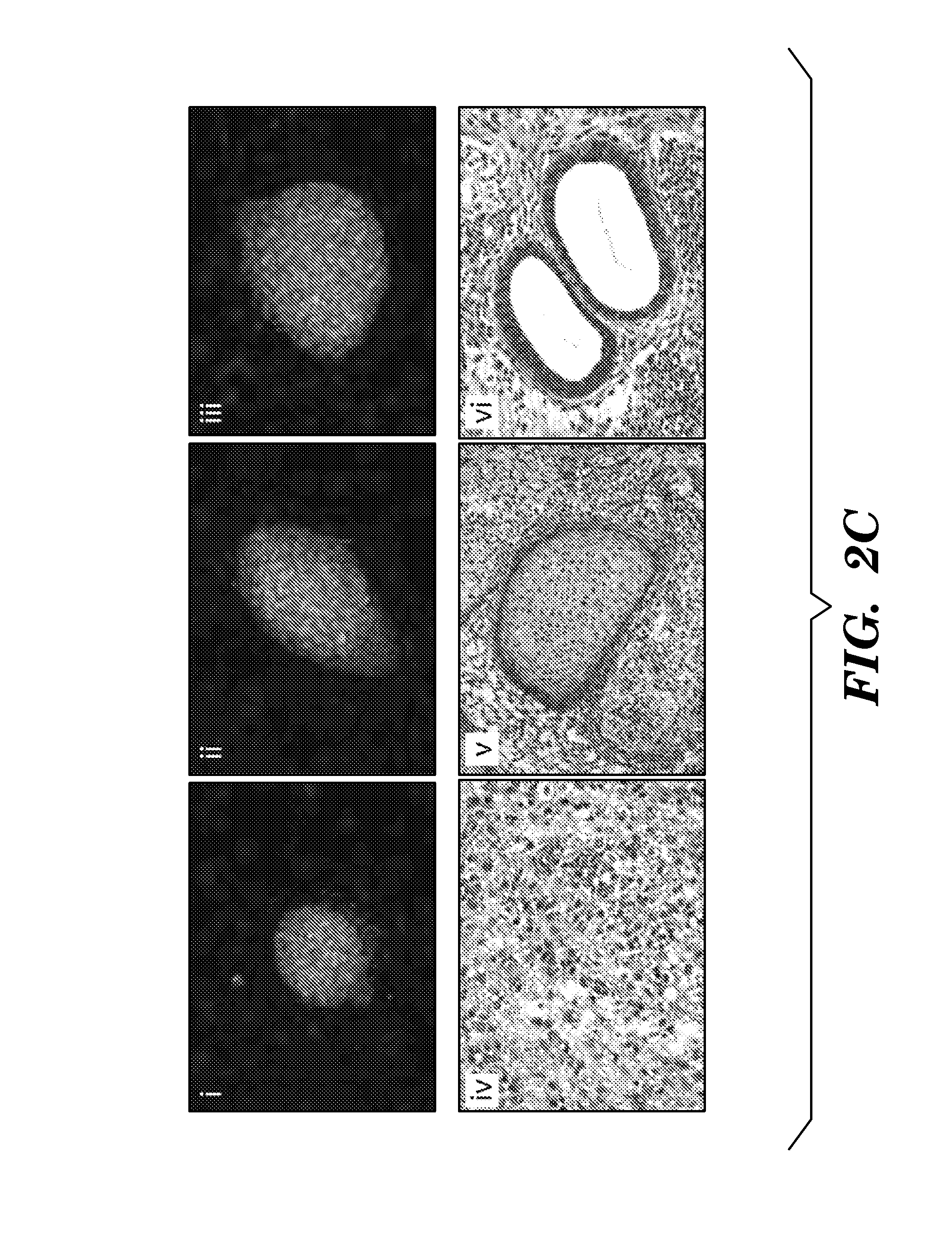
TGF-beta receptor inhibitors to enhance direct reprogramming
ActiveUS8298825B1Improve efficiencyIncrease ratingsGenetically modified cellsSkeletal/connective tissue cellsHuman Induced Pluripotent Stem CellsGene delivery
In general, iPS cells are produced by delivery of stem cell-associated genes into adult somatic cells (e.g., fibroblasts). Described herein are methods for enhancing the efficiency and rate of induced pluripotent stem cell production by treating somatic cells with a transforming growth factor-beta receptor (TGFβR) inhibitor. Also described herein are iPS cell compositions made according to the methods described herein and iPS cell compositions comprising an iPS cell in an admixture with a TGFβR inhibitor. Further described herein are kits for producing iPS cells using a TGFβR inhibitor.
Owner:THE GENERAL HOSPITAL CORP
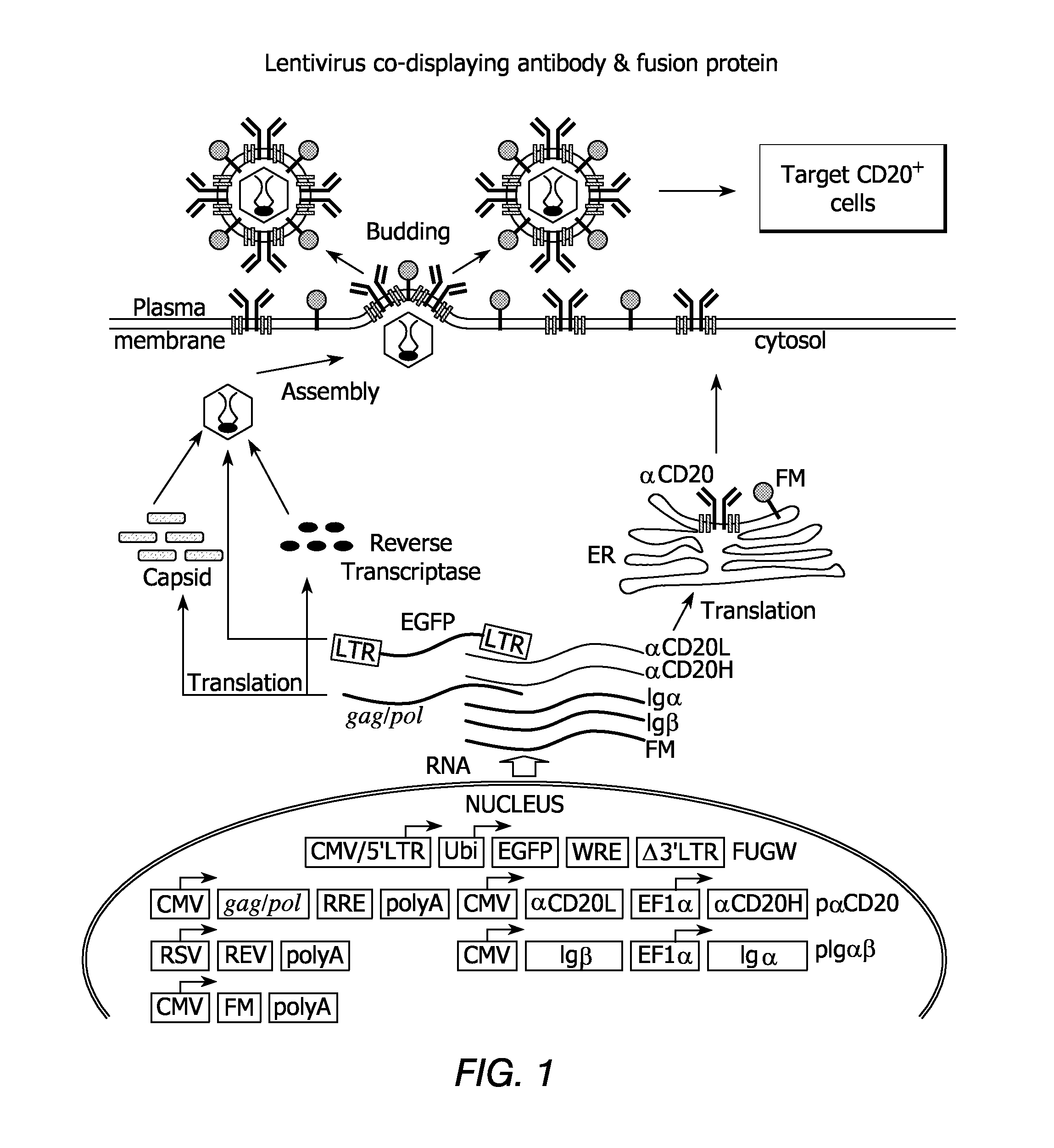
Recombinant viruses displaying a nonviral polypeptide on their external surface
InactiveUS6297004B1Genetic material ingredientsImmunological disordersGene deliveryAntibody fragments
We have made retrovirus particles displaying a functional antibody fragment. We fused the gene encoding an antibody fragment directed against a hapten with that encoding the viral envelope protein (Pr80env) of the ecotropic Moloney murine leukemia virus. The fusion gene was co-expressed in ecotropic retroviral packaging cells with a retroviral plasmid carrying the neomycin phosphotransferase gene (neo), and retroviral particles with specific hapten biding activities were recovered. Furthermore the hapten-binding particles were able to transfer the neo gene and the antibody-envelope fusion gene to mouse fibroblasts. In principle, the display of antibody fragments on the surface of recombinant retroviral particles could be used to target virus to cells for gene delivery, or to retain the virus in target tissues, or for the construction of libraries of viral display packages.
Owner:BIOFOCUS DICOVERY
Magnetically-driven biodegradable gene delivery nanoparticles formulated with surface-attached polycationic complex
InactiveUS20060057211A1Reduce deliveryImprove protectionBiocideHeavy metal active ingredientsPolyelectrolyteGene delivery
A particle including a matrix-forming agent and a polyelectrolyte-amphiphilic agent adduct wherein the polyelectrolyte-amphiphilic agent adduct is in physical communication with the matrix-forming agent. The particle further includes a coated magnetic field-responsive agent and a biomaterial. Methods of making the particle are provided. Also provided are methods of delivery of the biomaterial to a target cell or a target tissue including administering the particle having the matrix-forming agent, polyelectrolyte-amphiphilic agent adduct, the coated magnetic field-responsive agent and the biomaterial; providing a magnetic device associated with the target cell or the target tissue; applying a magnetic force to the particle; and guiding the particle toward the magnetic device by the magnetic force.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Vaccination by topical application of recombinant vectors
InactiveUS20030045492A1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseGenetic material ingredientsGene deliveryVaccination
The present invention relates to techniques of skin-targeted non-invasive gene delivery to elicit immune responses and uses thereof. The invention further relates to methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal following topical application of vectors, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal as well as such a method further including disposing the vector in and / or on the delivery device. The vector can be gram negative bacteria, preferably Salmonella and most preferably Salmonella typhimurium.
Owner:UAB RES FOUND
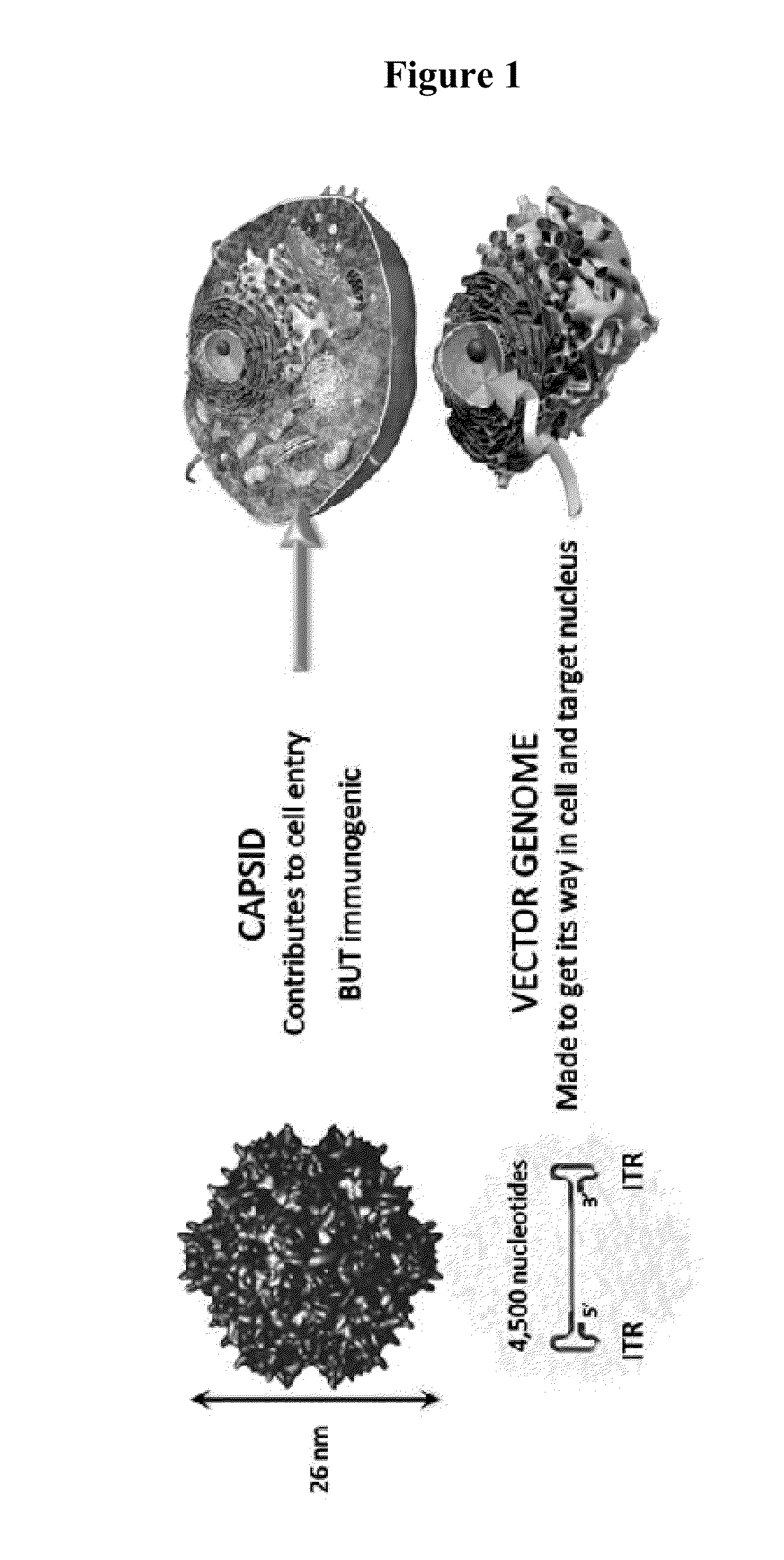
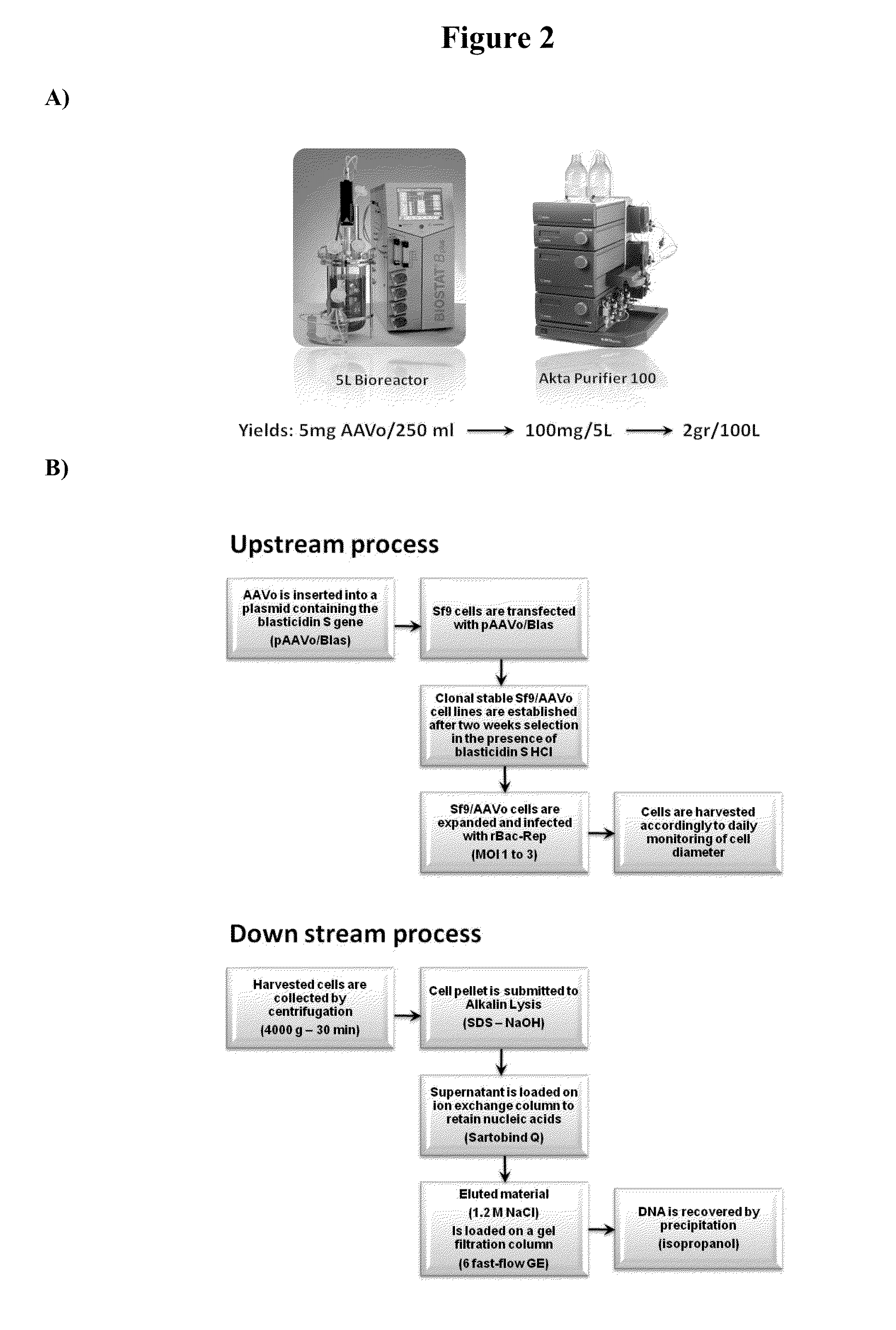
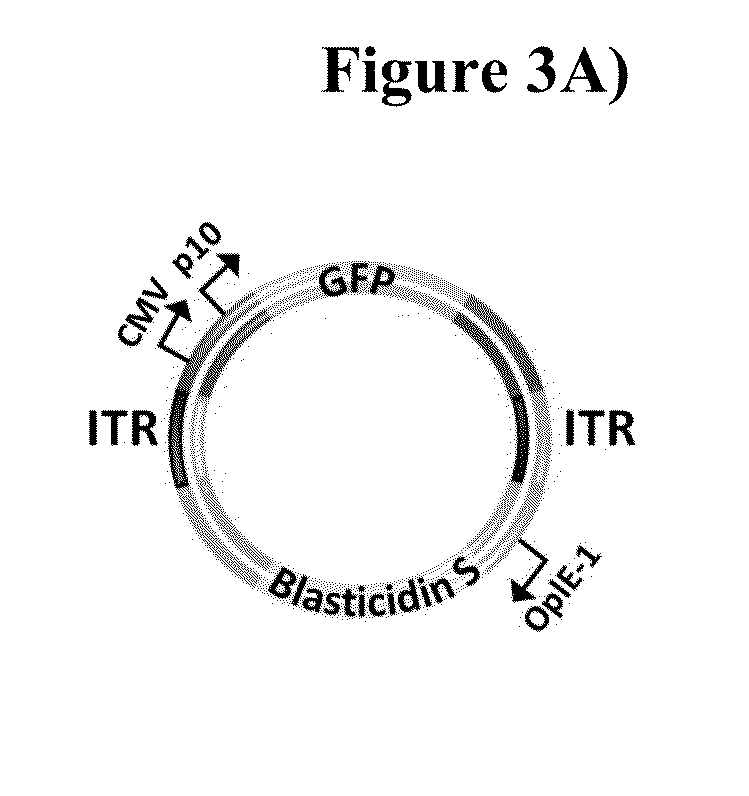
Capsid-free aav vectors, compositions, and methods for vector production and gene delivery
ActiveUS20140107186A1High-efficiency transductionEfficient and convenient methodNervous disorderGenetic material ingredientsGene deliveryTherapeutic intent
An isolated linear nucleic acid molecule comprising in this order: a first adeno-associated virus (AAV) inverted terminal repeat (ITR), a nucleotide sequence of interest and a second AAV ITR, wherein said nucleic acid molecule is devoid of AAV capsid protein coding sequences. The said nucleic acid molecule can be applied to a host at repetition without eliciting an immune response. Methods for producing and purifying this nucleic acid molecule, and use of the same for therapeutic purposes are also provided.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +4
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com