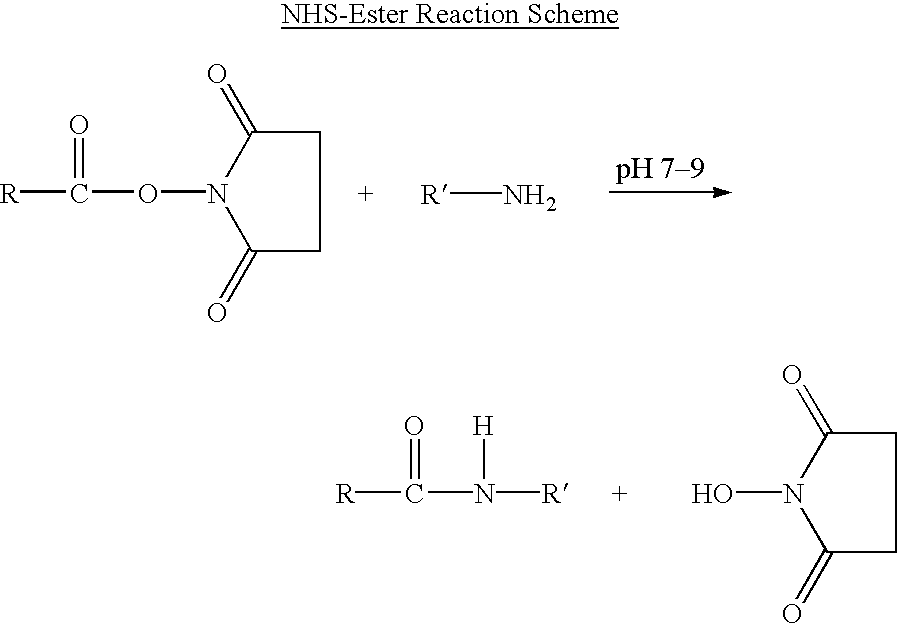
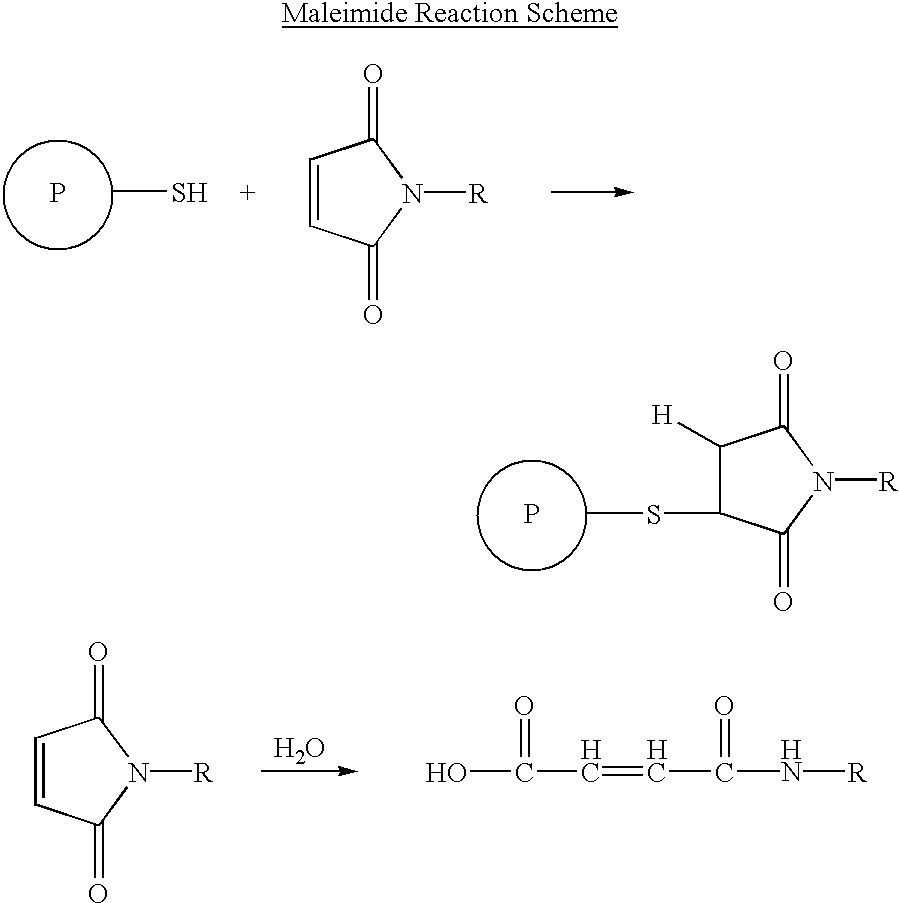
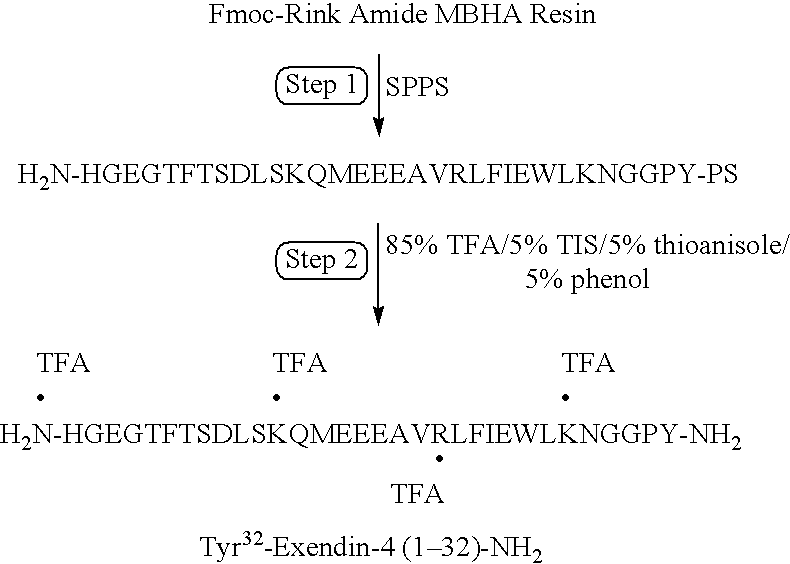
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2121 results about "Ex vivo" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ex vivo (Latin: "out of the living") means that which takes place outside an organism. In science, ex vivo refers to experimentation or measurements done in or on tissue from an organism in an external environment with minimal alteration of natural conditions. Ex vivo conditions allow experimentation on an organism's cells or tissues under more controlled conditions than is possible in in vivo experiments (in the intact organism), at the expense of altering the "natural" environment.
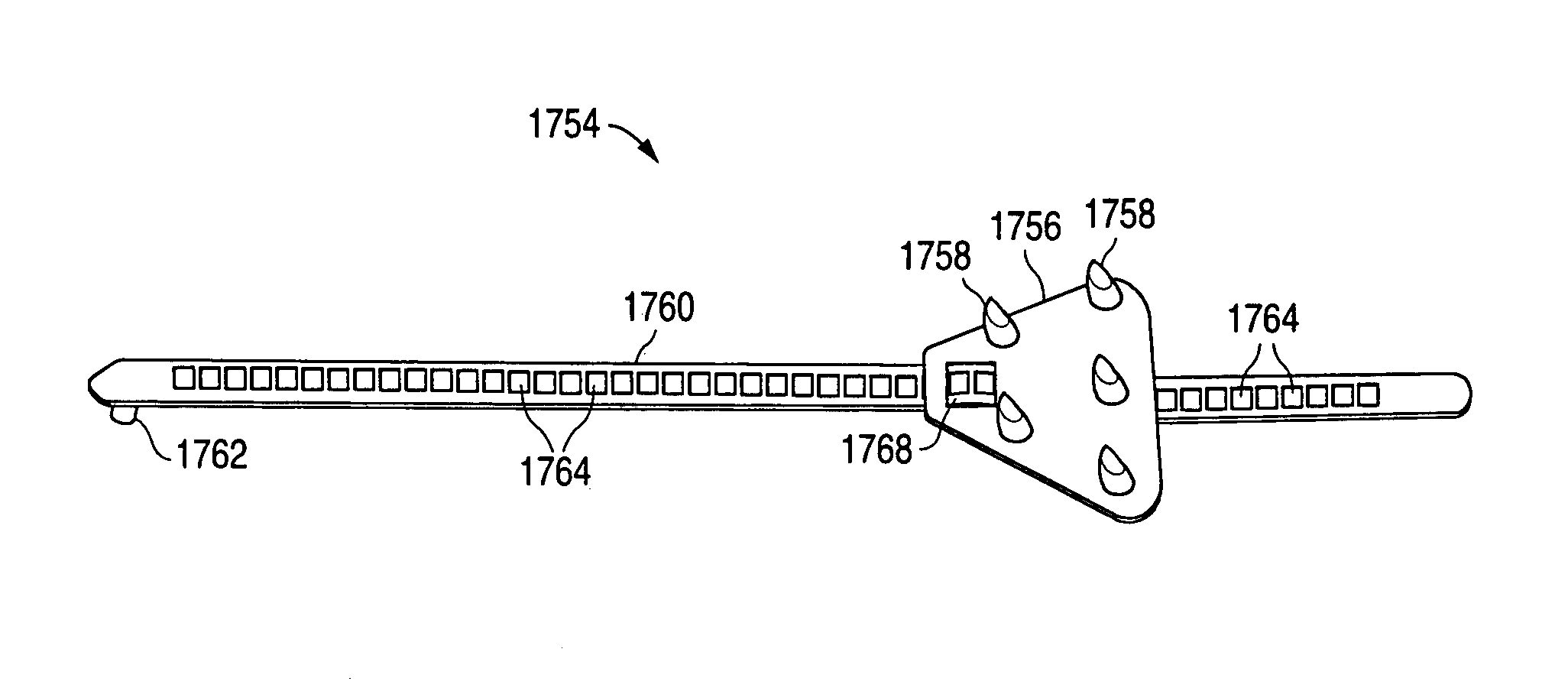
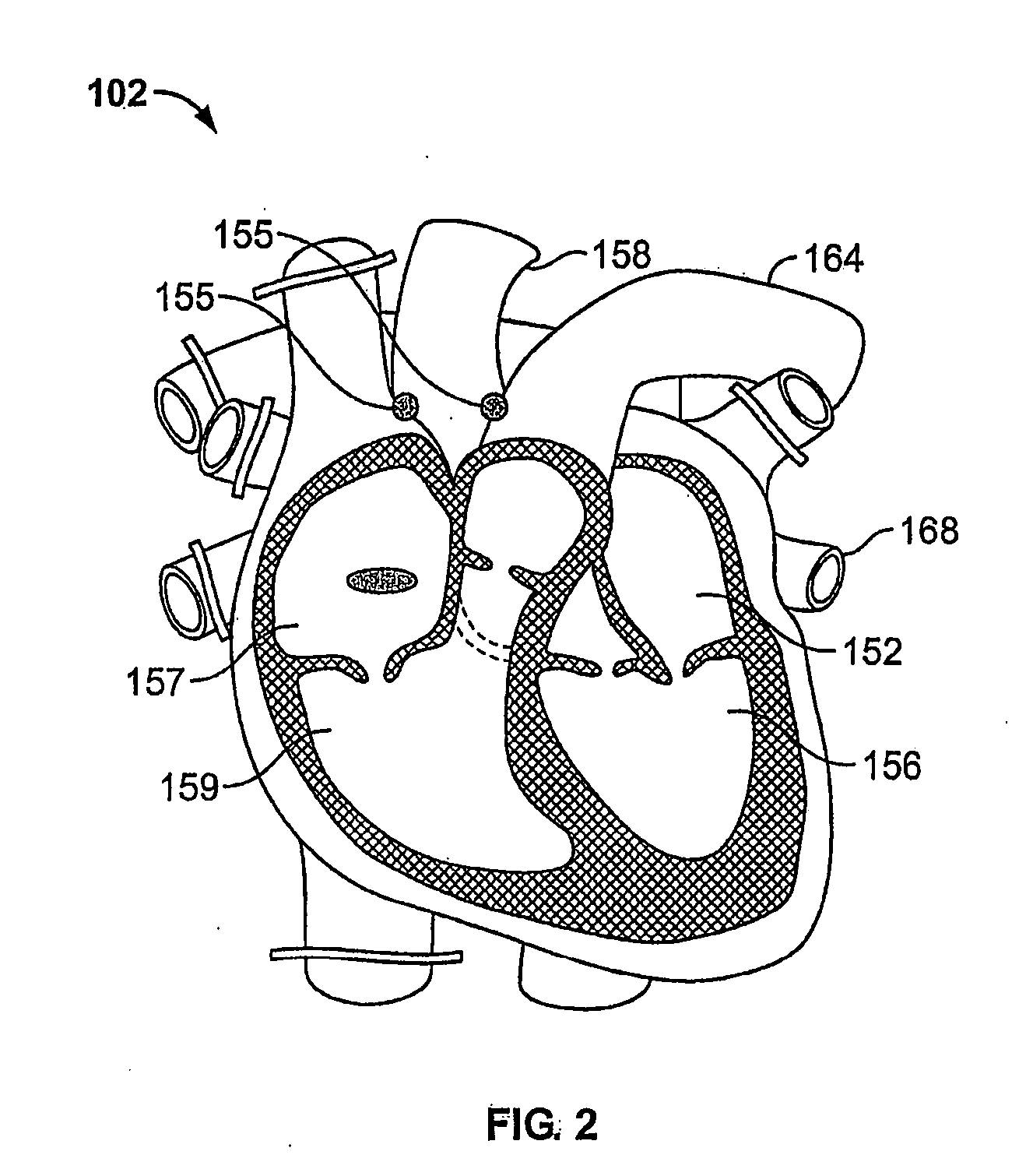
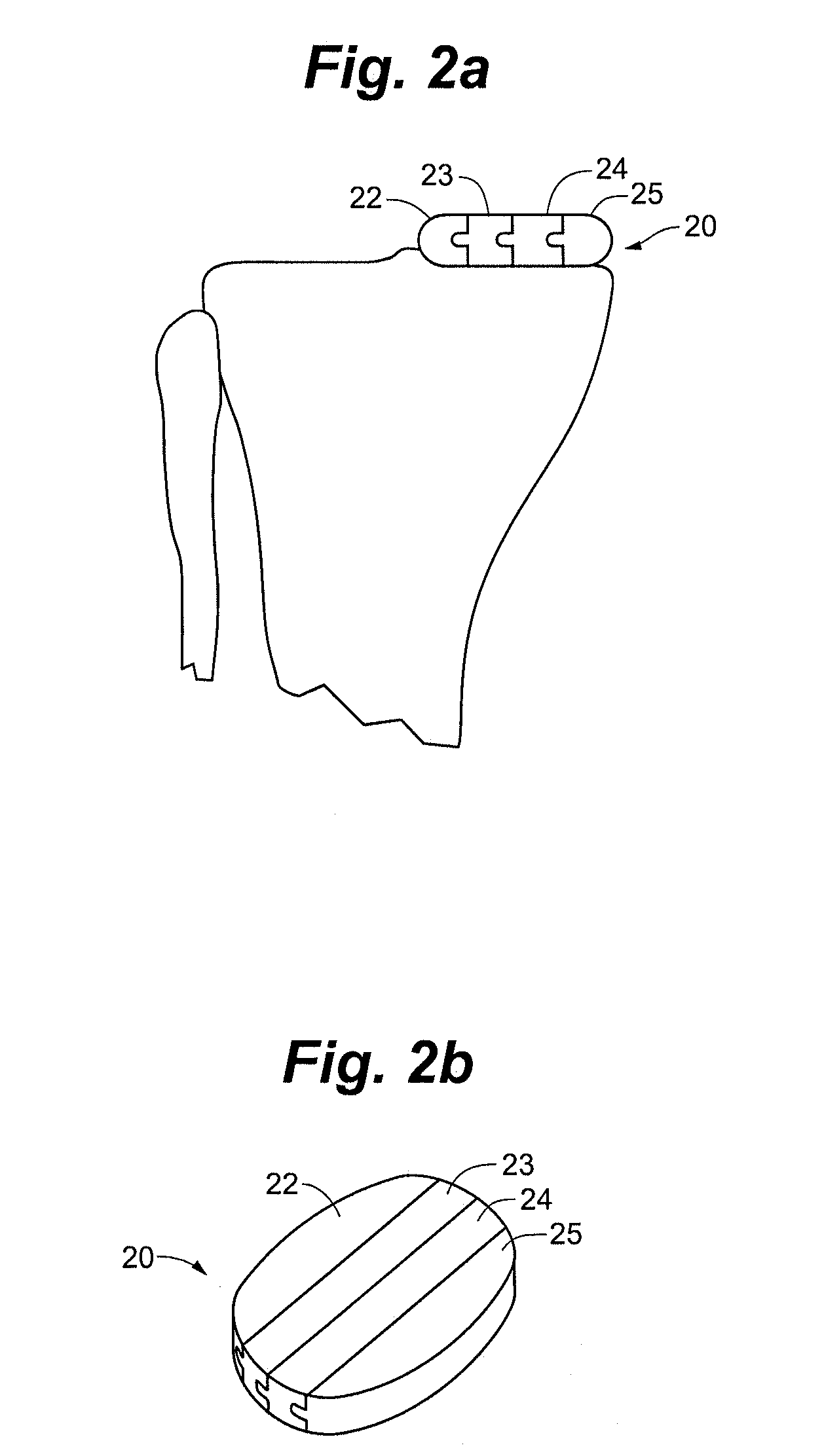
Remotely anchored tissue fixation device
InactiveUS7172615B2Degree of approximation necessaryVariable flexibilitySuture equipmentsCosmetic implantsWound healingBiomedical engineering
A tissue approximation device and processes for using the device, particularly in the mid-face region, are provided. The device is an implantable, biodegradable construct that has attachment points emanating from at least one supportive backing. The device also has a connecting member or leash which extends between the backing and an anchor which is attached to bone or soft tissue. Attachment to soft tissue is accomplished by a second backing having attachment points emanating from the backing and attachment to bone is accomplished by a post. The connecting member allows for repeated adjustments in length between the anchor and the backing in vivo or ex vivo until the desired amount of tissue approximation is achieved. The device improves the mechanical phase of wound healing and evenly distributes tension over the contact area between the device and tissue.
Owner:OXFORD FINANCE +2
Method and system for mammalian joint resurfacing
A method and system for the creation or modification of the wear surface of orthopedic joints, involving the preparation and use of one or more partially or fully preformed and procured components, adapted for insertion and placement into the body and at the joint site. In a preferred embodiment, component(s) can be partially cured and generally formed ex vivo and further and further formed in vivo at the joint site to enhance conformance and improve long term performance. In another embodiment, a preformed balloon or composite material can be inserted into the joint site and filled with a flowable biomaterial in situ to conform to the joint site. In yet another embodiment, the preformed component(s) can be fully cured and formed ex vivo and optionally further fitted and secured at the joint site. Preformed components can be sufficiently pliant to permit insertion through a minimally invasive portal, yet resilient enough to substantially assume, or tend towards, the desired form in vivo with additional forming there as needed.
Owner:ADVANCED BIO SURFACE
Systems and methods for ex vivo organ care
The invention, in various embodiments, provides systems, methods and solutions using an organ ex vivo.
Owner:TRANSMEDICS
Long lasting synthetic glucagon-like peptide {GLP-1}
Modified insulinotropic peptides are disclosed. The modified insulinotropic peptides are capable of forming a peptidase stabilized insulinotropic peptide. The modified insulinotropic peptides are capable of forming covalent bonds with one or more blood components to form a conjugate. The conjugates may be formed in vivo or ex vivo. The modified peptides are administered to treat humans with diabetes and other related diseases.
Owner:CONJUCHEM
Silver-containing, sol/gel derived bioglass compositions
Silver-containing, sol-gel derived bioactive glass compositions and methods of preparation and use thereof are disclosed. The compositions can be in the form of particles, fibers and / or coatings, among other possible forms, and can be used, for example, for treating wounds, improving the success of skin grafts, reducing the inflammatory response and providing anti-bacterial treatments to a patient in need thereof. Anti-bacterial properties can be imparted to implanted materials, such as prosthetic implants, sutures, stents, screws, plates, tubes, and the like, by incorporating the compositions into or onto the implanted materials. The compositions can also be used to prepare devices used for in vitro and ex vivo cell culture.
Owner:IMPERIAL INNOVATIONS LTD
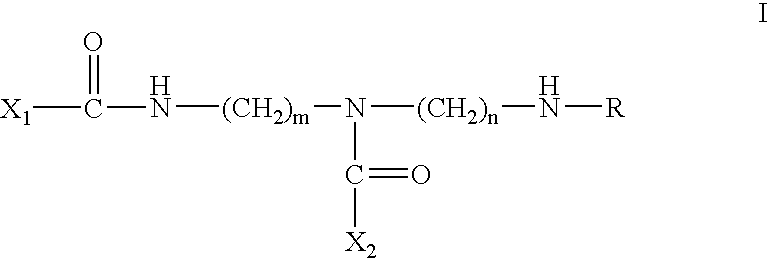
Inhibitors of serine proteases, particularly HCV NS3-NS4A proteases
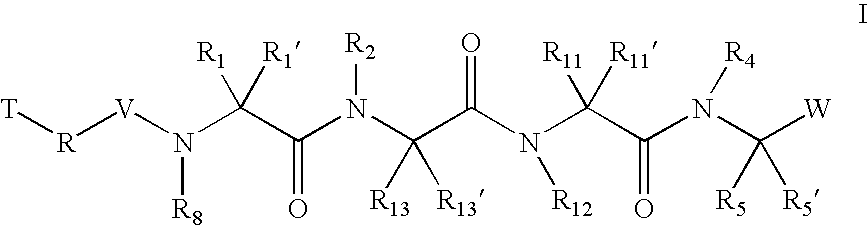
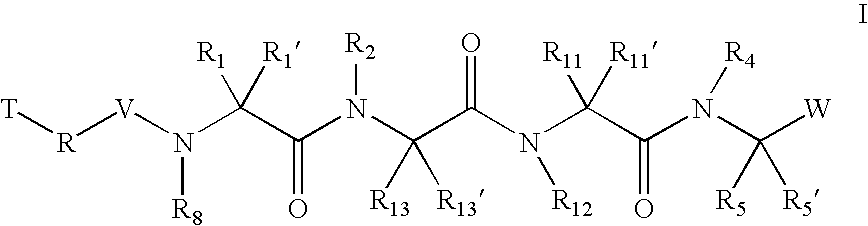
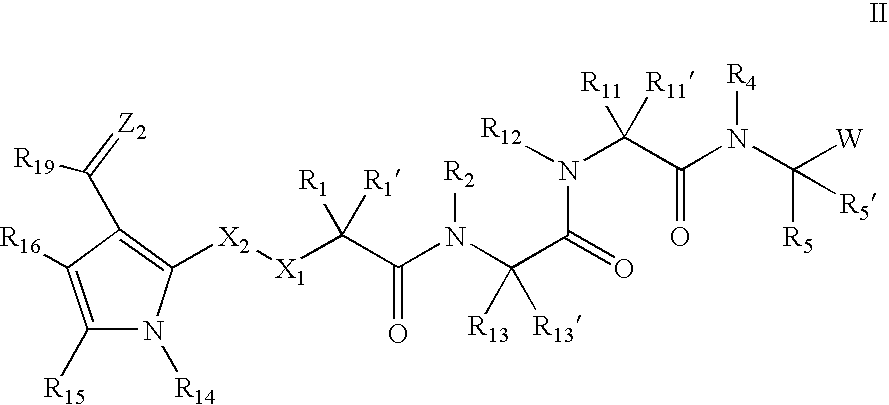
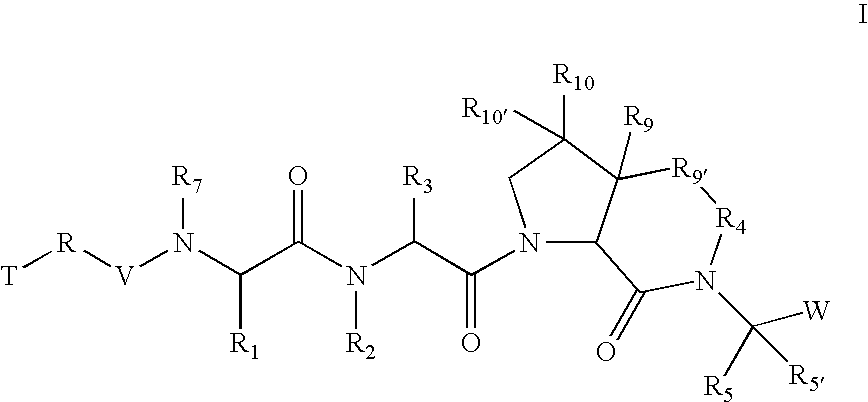
The present invention relates to compounds of formula I:or a pharmaceutically acceptable salt or mixtures thereof that inhibit serine protease activity, particularly the activity of hepatitis C virus NS3–NS4A protease. As such, they act by interfering with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The invention further relates to compositions comprising these compounds either for ex vivo use or for administration to a patient suffering from HCV infection and to processes for preparing the compounds. The invention also relates to methods of treating an HCV infection in a patient by administering a composition comprising a compound of this invention. The invention further relates to processes for preparing these compounds.
Owner:VERTEX PHARMA INC
Autogenic living scaffolds and living tissue matrices: methods and uses thereof
ActiveUS20050226856A1Preventing host rejectionThicker and strongBiocideSkin implantsTransdifferentiationOrganism
A 3-dimensional structure comprising suitable cells (or entities) and the ECM (or matrix) that has been completely produced and arranged by these cells (or entities) that promotes the differentiation, dedifferentiation and / or transdifferentiation of cells and / or formation of tissue in vitro and in vivo, while at the same time promoting cell growth, proliferation, migration, acquisition of in vivo-like morphology, or combinations thereof, and that 1. provides structural and / or nutritional support to cells, tissue, organs, or combinations thereof, termed an “Autogenic Living Scaffold” (ALS); or 2. is capable of being transformed into a more complex tissue (or matrix) or a completely different type of tissue (or matrix), termed a “Living Tissue Matrix” (LTM). Autogenic means it is self-produced. The living cells that produce the LTM or ALS, or are added to Autogenic Living Scaffolds, may be genetically engineered or otherwise modified. The matrix component of the ALS or LTM provides a structural framework for cells that guide their direction of growth, enables them to be correctly spaced, prevents overcrowding, enables cells to communicate between each other, transmit subtle biological signals, receive signals from their environment, form bonds and contacts that are required for proper functioning of all cells within a unit such as a tissue, or combinations thereof. The ALS or LTM may thus provide proper or supporting mechanical and chemical environments, signals, or stimuli to other cells, to the cells that produce the ALS, to surrounding tissue at an implantation site, to a wound, for in vitro and ex vivo generation and regeneration of cells, tissue and organs, or combinations thereof. They may also provide other cells with nutrients, growth factors, and / or other necessary or useful components. They may also take in or serve as buffers for certain substances in the environment, and have also some potential at adapting to new environments.
Owner:GENESIS TECH LTD
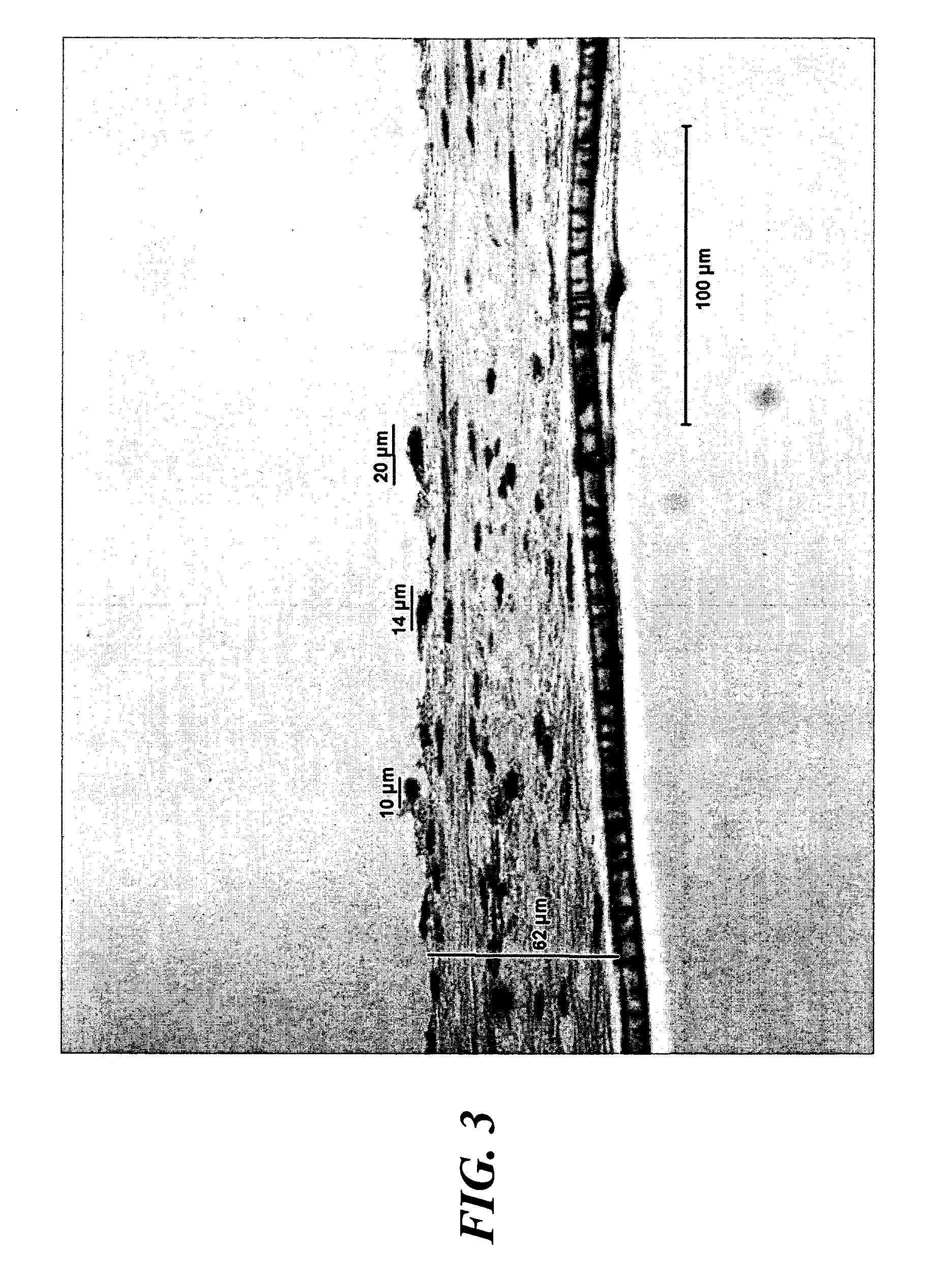
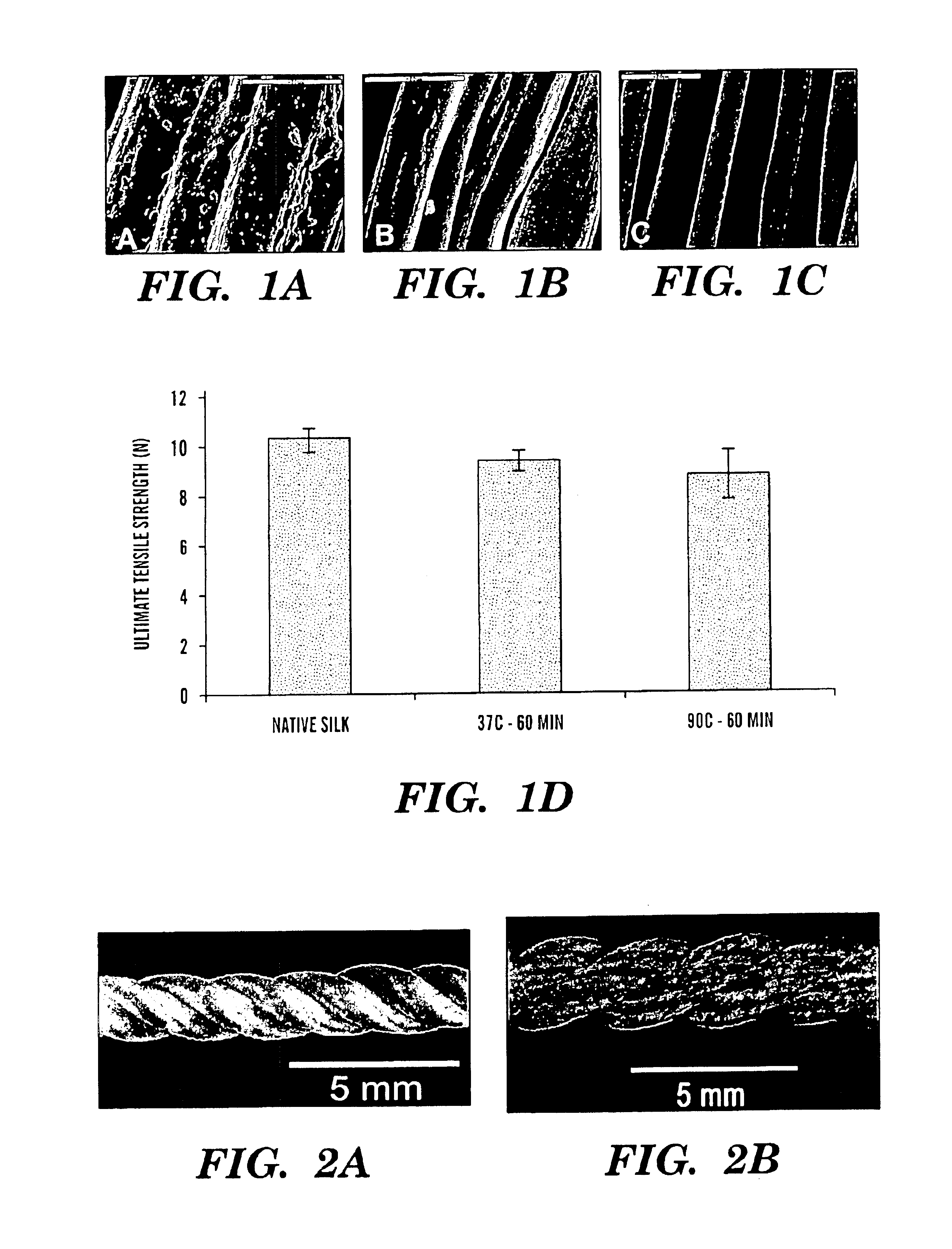
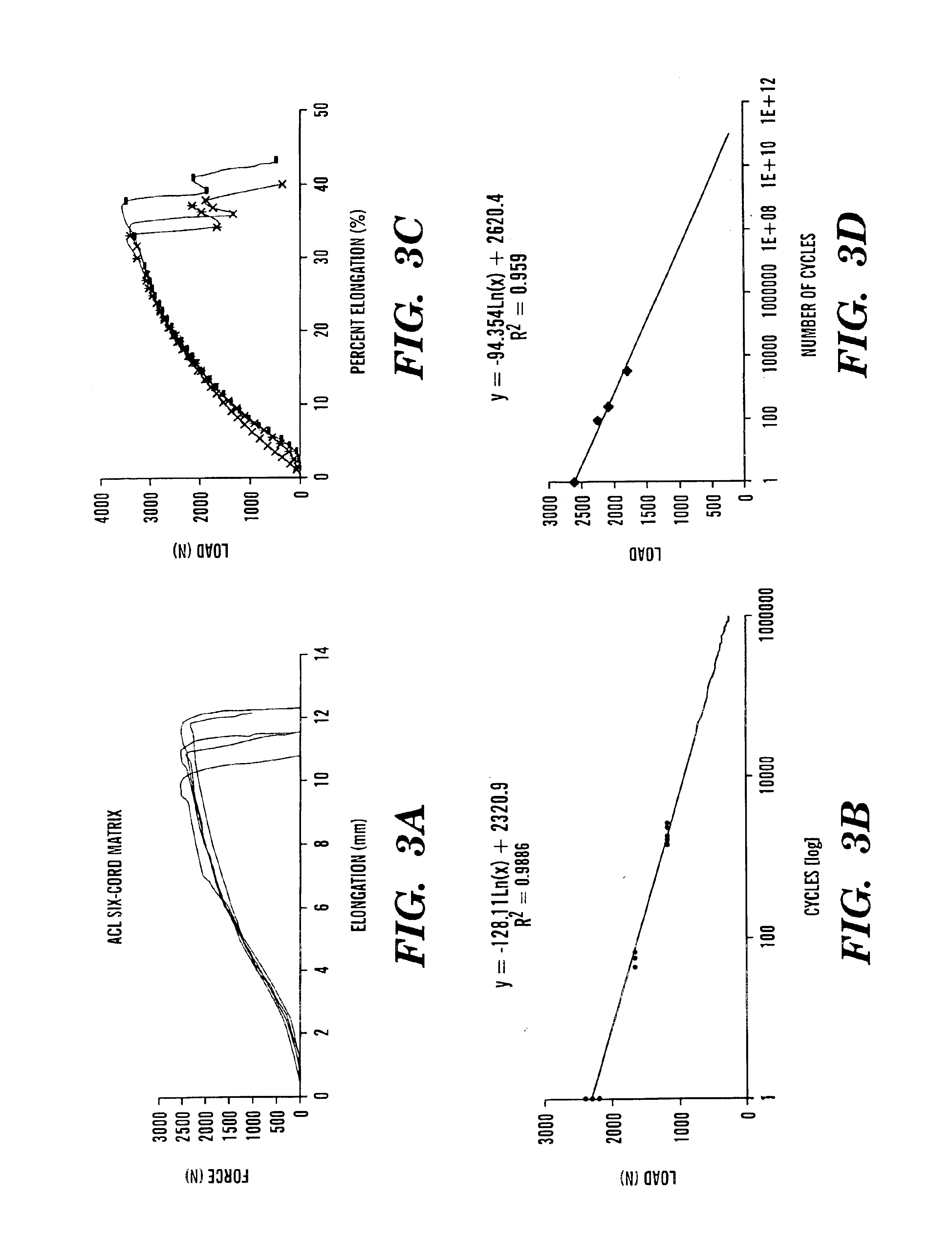
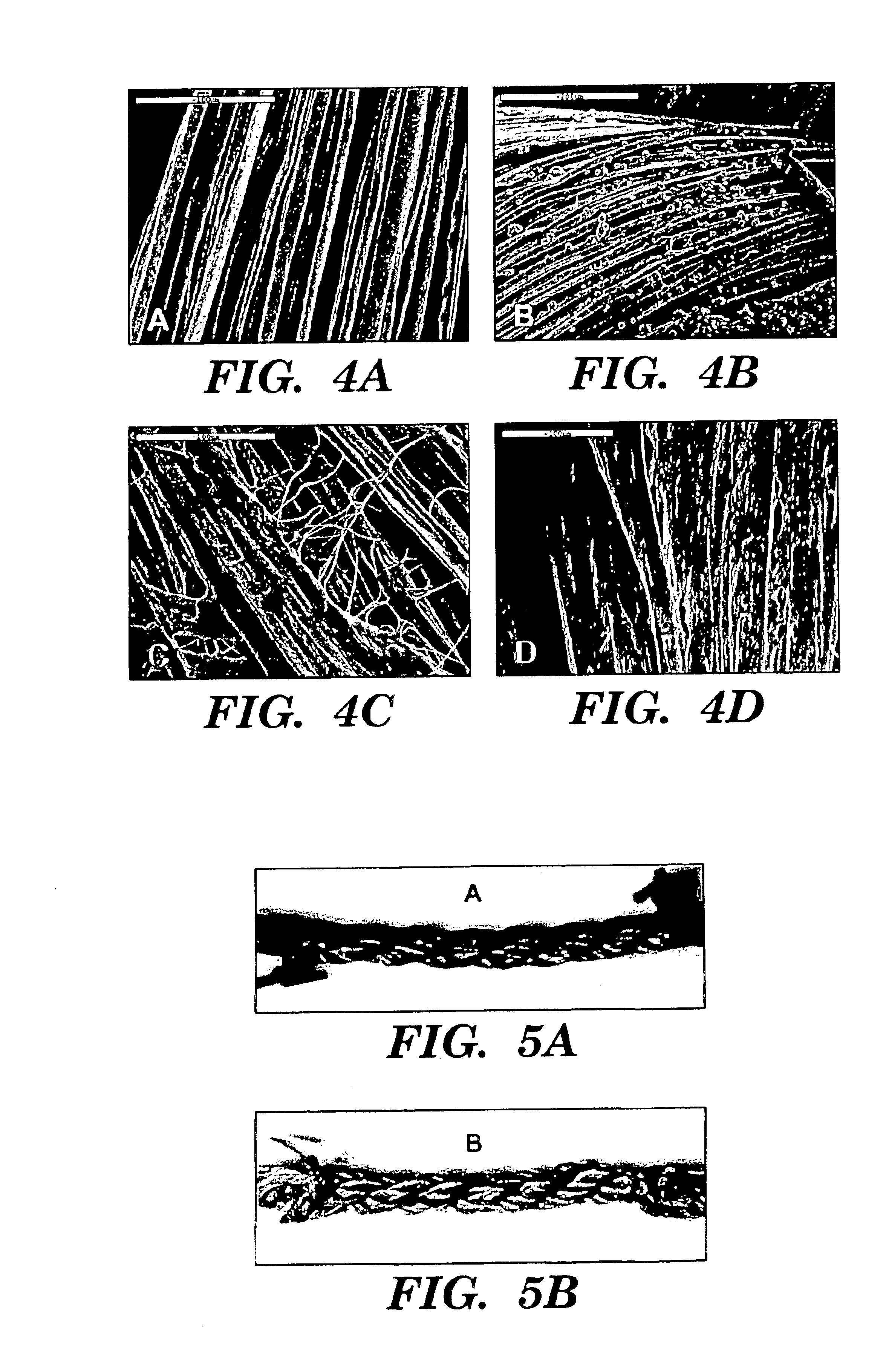
Helically organized silk fibroin fiber bundles for matrices in tissue engineering
InactiveUS6902932B2Immobilised enzymesBioreactor/fermenter combinationsFiber bundleLigament structure
The present invention provides a novel silk-fiber-based matrix having a wire-rope geometry for use in producing a ligament or tendon, particularly an anterior cruciate ligament, ex vivo for implantation into a recipient in need thereof. The invention further provides the novel silk-fiber-based matrix which is seeded with pluripotent cells that proliferate and differentiate on the matrix to form a ligament or tendon ex vivo. Also disclosed is a bioengineered ligament comprising the silk-fiber-based matrix seeded with pluripotent cells that proliferate and differentiate on the matrix to form the ligament or tendon. A method for producing a ligament or tendon ex vivo comprising the novel silk-fiber-based matrix is also disclosed.
Owner:ALLERGAN INC +1
Methods for treating tumors and cancerous tissues
InactiveUS20050214268A1Improve bioavailabilityBiocideGenetic material ingredientsAbnormal tissue growthApoptosis
The invention disclosed herein relates generally to immunotherapy and, more specifically, to therapeutic methods for treating tumors and cancerous tissues by first inducing necrosis or apoptosis (e.g., cryotherapy, chemotherapy, radiation therapy, ultrasound therapy, or a combination thereof applied against at least a portion of the tumor or cancerous tissue), and then delivering one or more se doses of antigen presenting cells (e.g., autologous dendritic cells) intratumourally or proximate to the tumor or cancerous tissue, but only after a selected period of time sufficient for the bioavailablity of liberated cancer-specific antigens (monitored over the selected period of time) resulting from the necrosis or apoptosis to be at or near a maximum value. The present invention provides an alternative strategy to the ex vivo loading of target antigen to antigen presenting cells such as, for example, enriched autologous dendritic cells for purposes of enhancing an immune response.
Owner:SANGRETECH BIOMEDICAL
Immunostimulatory nucleic acid molecules for activating dendritic cells
InactiveUS20070065467A1Th responseEffective adjuvantOrganic active ingredientsSugar derivativesDendritic cellPyrimidine Nucleotides
The present invention relates generally to methods and products for activating dendritic cells. In particular, the invention relates to oligonucleotides which have a specific sequence including at least one unmethylated CpG dinucleotide which are useful for activating dendritic cells. The methods are useful for in vitro, ex-vivo, and in vivo methods such as cancer immunotherapeutics, treatment of infectious disease and treatment of allergic disease.
Owner:UNIV OF IOWA RES FOUND
Biodegradable polyurethane/urea compositions
The present invention relates to biocompatible, biodegradable polyurethane / urea polymeric compositions that are capable of in-vivo curing with low heat generation to form materials suitable for use in scaffolds in tissue engineering applications such as bone and cartilage repair. The polymers are desirably flowable and injectable and can support living biological components to aid in the healing process. They may be cured ex-vivo for invasive surgical repair methods, or alternatively utilized for relatively non-invasive surgical repair methods such as by arthroscope. The invention also relates to prepolymers useful in the preparation of the polymeric compositions, and to methods of treatment of damaged tissue using the polymers of the invention.
Owner:POLYNOVO BIOMATERIALS PTY LTD
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
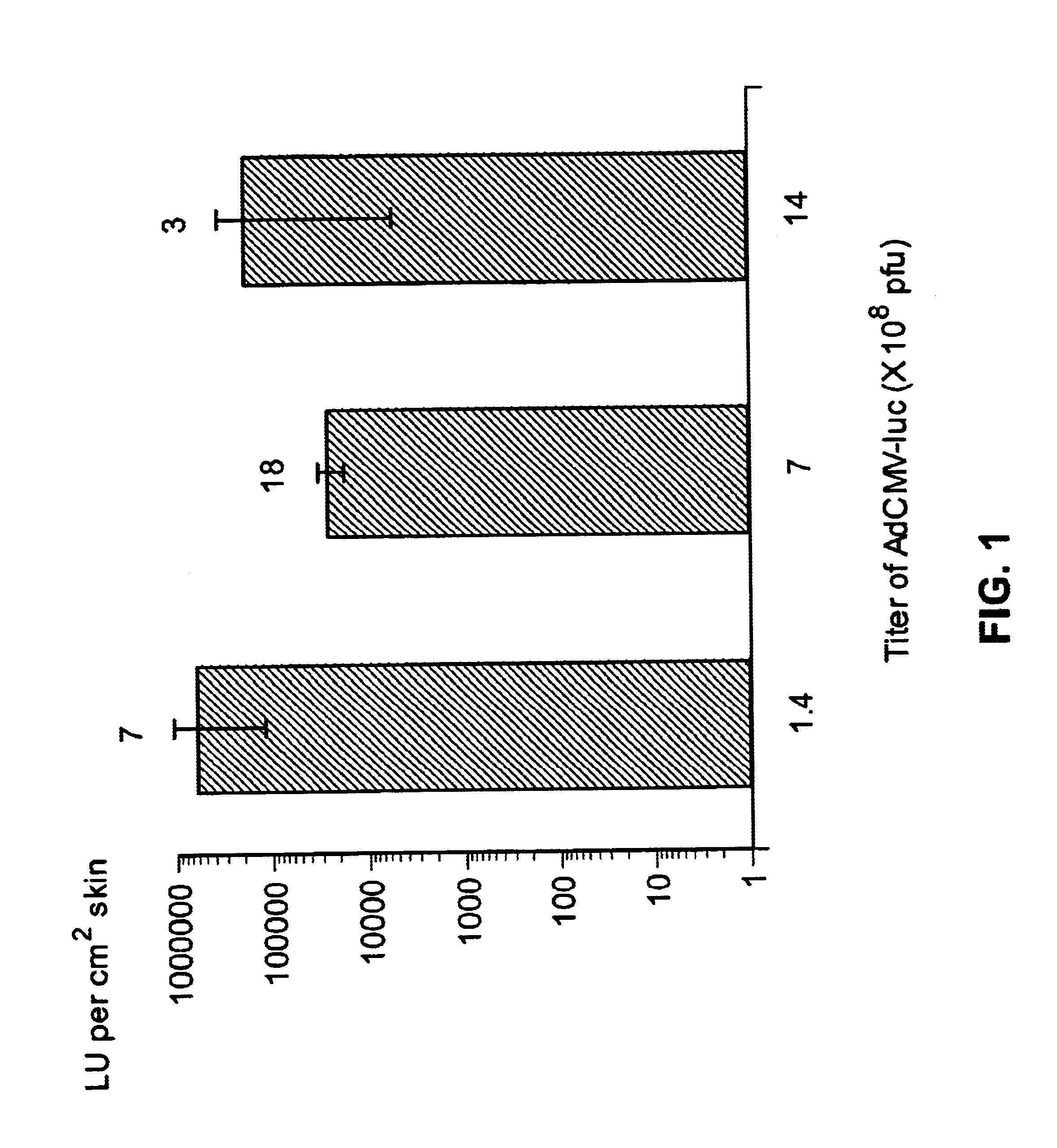
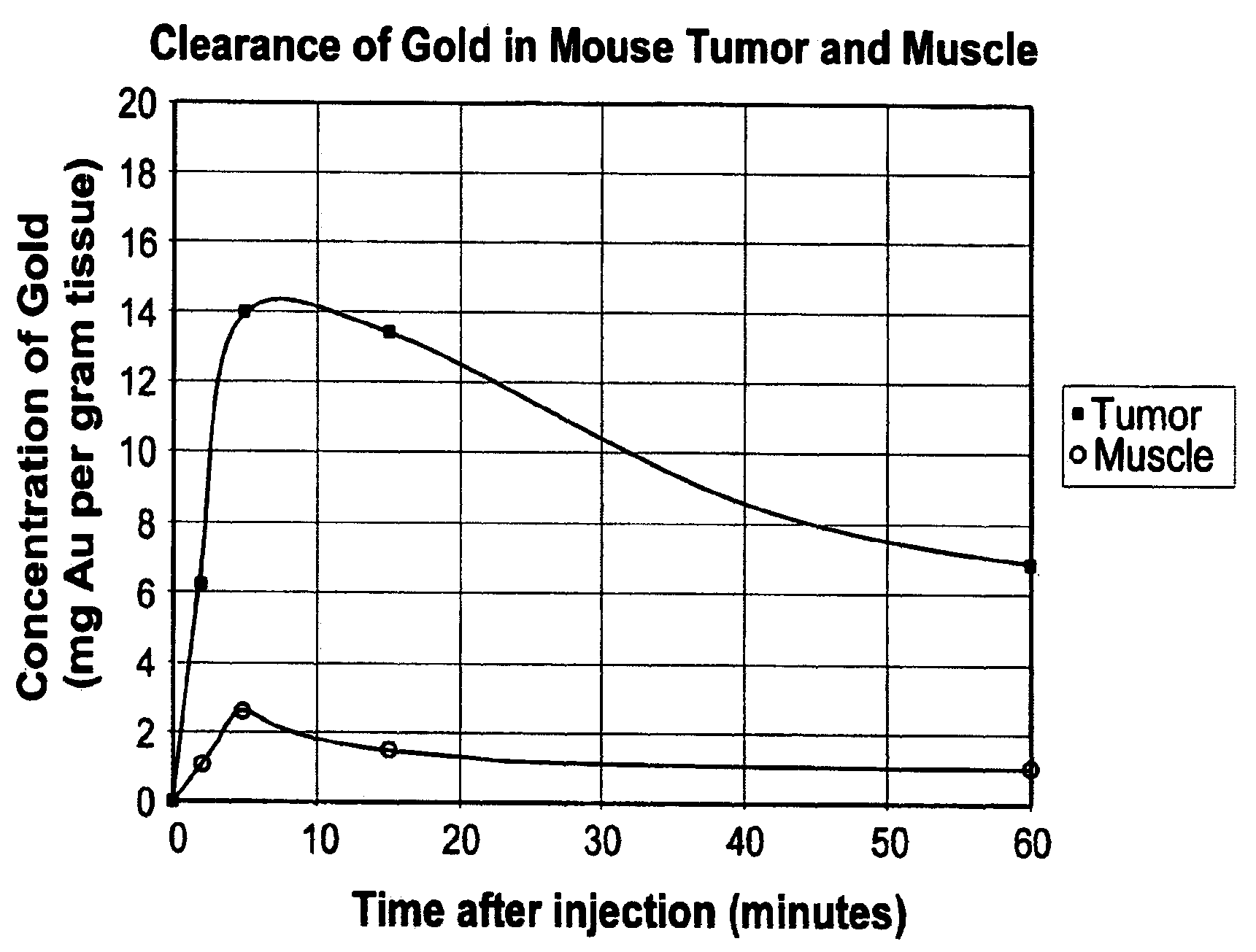
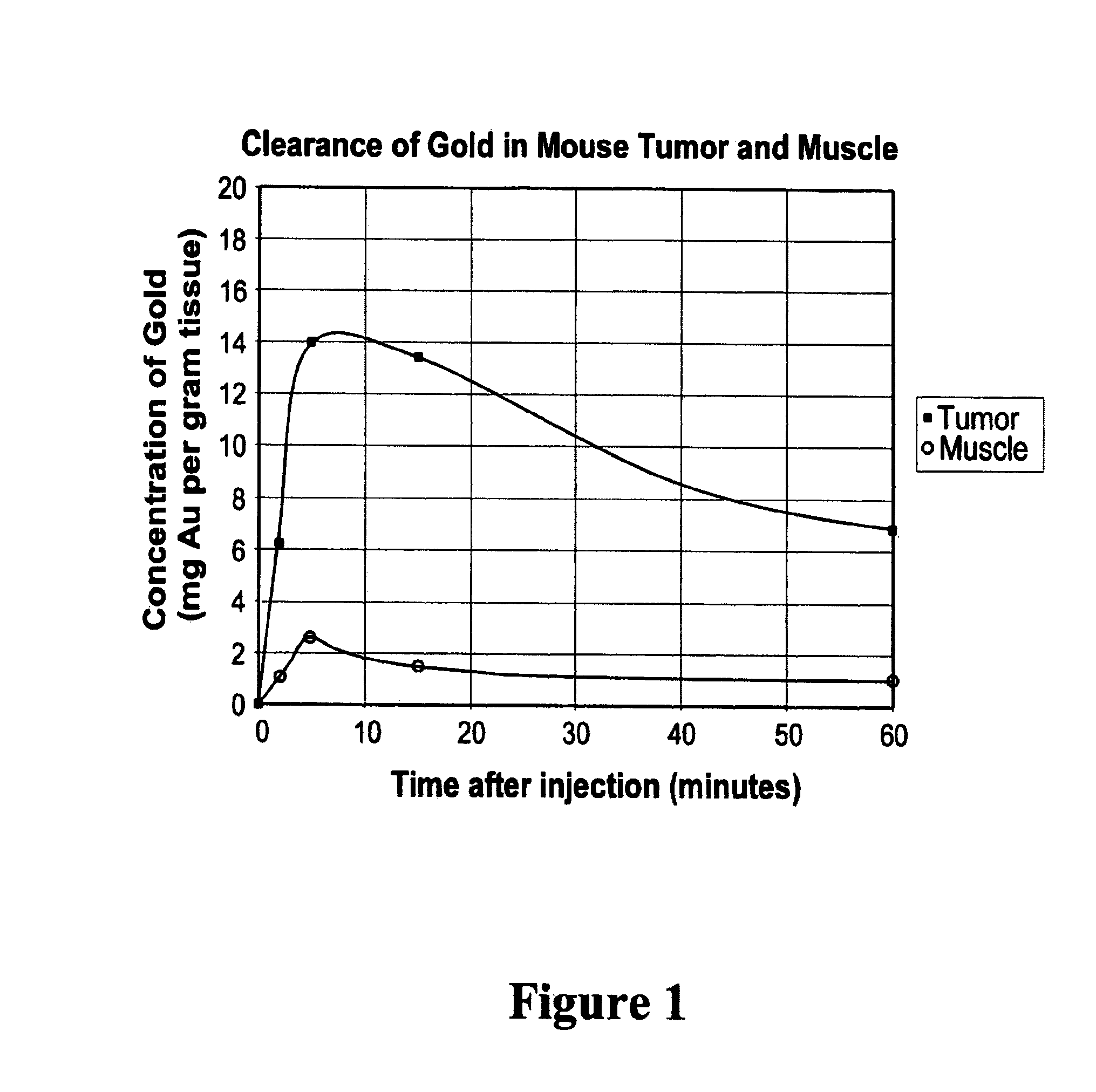
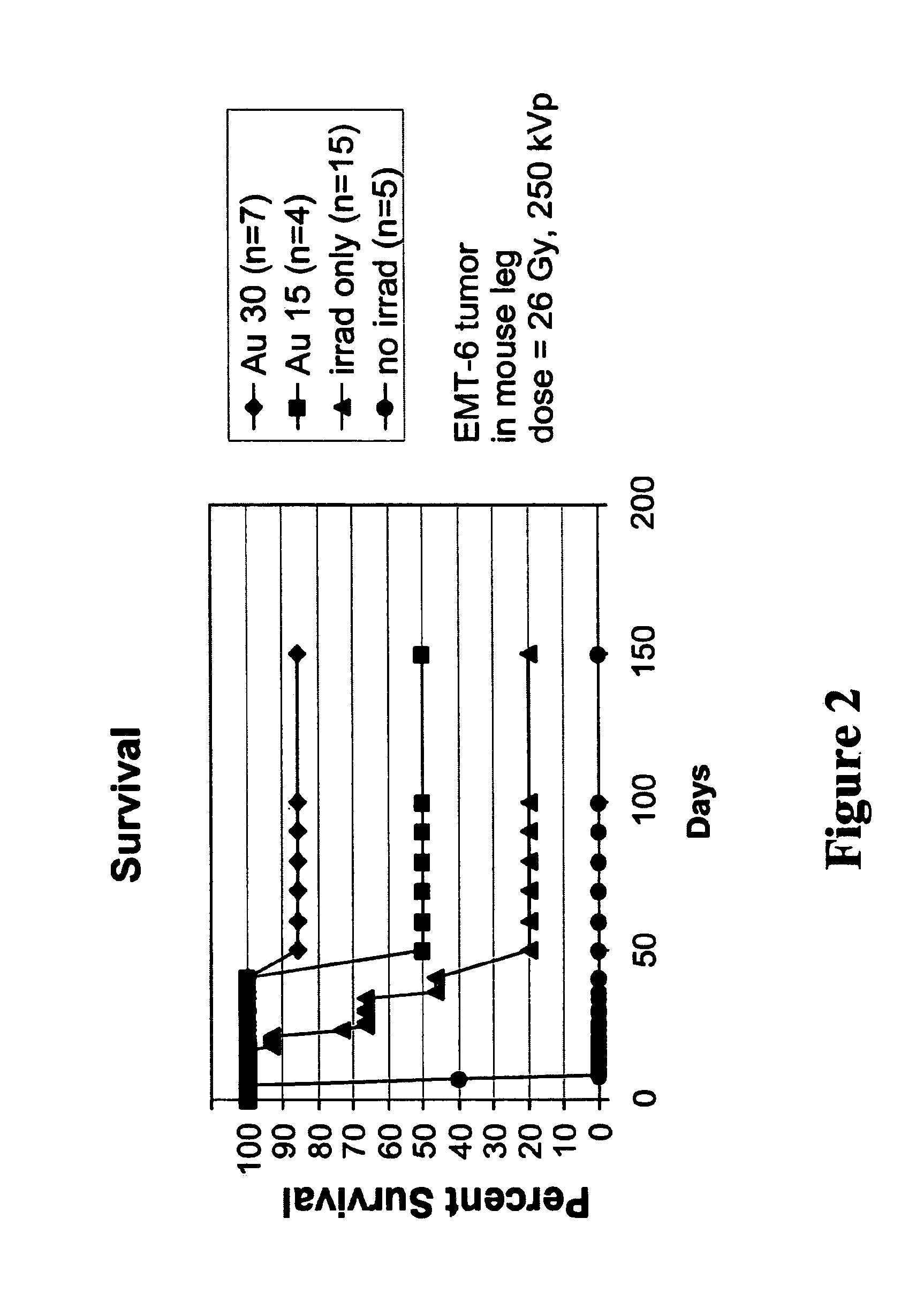
Methods of enhancing radiation effects with metal nanoparticles
Owner:NANOPROBES
Reprogramming compositions and methods of using the same
InactiveUS20120207744A1Improve versatilityIncrease pluripotencyBiocideOrganic active ingredientsRegenerative medicineCell biology
The present invention provides compositions and methods of using the compositions to alter the developmental potency of a cell. The present invention provides in vivo and ex vivo cell reprogramming and programming methods suitable for autologous cell therapy and regenerative medicine.
Owner:FATE THERAPEUTICS
Use of cationic lipids to deliver cas9
ActiveUS20150071903A1Prevent and delay onsetSlow onsetFusion with RNA-binding domainFusion with DNA-binding domainDiseaseLipid formation
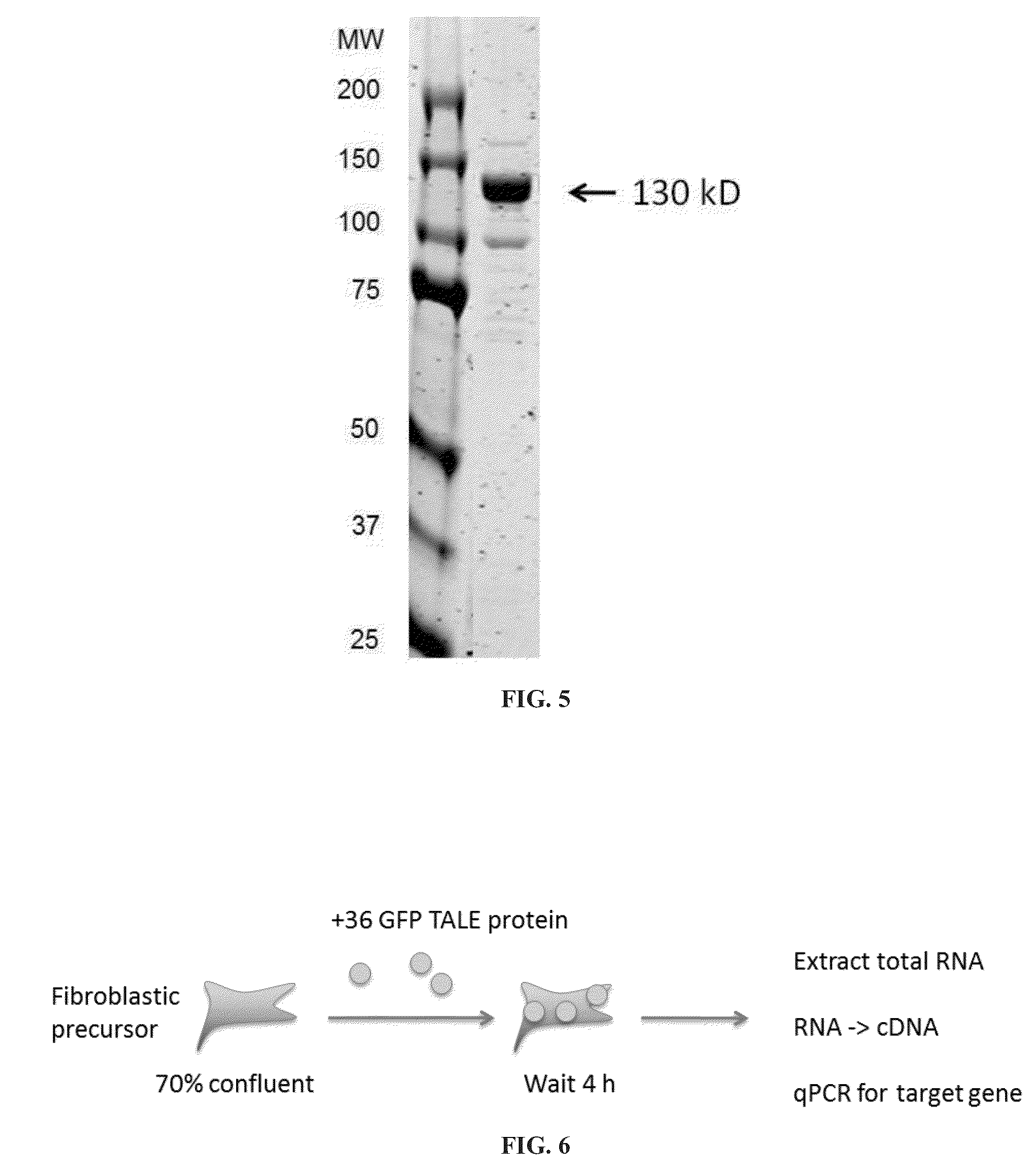
Compositions, methods, strategies, kits, and systems for the supercharged protein-mediated delivery of functional effector proteins into cells in vivo, ex vivo, or in vitro are provided. Compositions, methods, strategies, kits, and systems for delivery of functional effector proteins using cationic lipids and cationic polymers are also provided. Functional effector proteins include, without limitation, transcriptional modulators (e.g., repressors or activators), recombinases, nucleases (e.g., RNA-programmable nucleases, such as Cas9 proteins; TALE nuclease, and zinc finger nucleases), deaminases, and other gene modifying / editing enzymes. Functional effector proteins include TALE effector proteins, e.g., TALE transcriptional activators and repressors, as well as TALE nucleases. Compositions, methods, strategies, and systems for the delivery of functional effector proteins into cells is useful for therapeutic and research purposes, including, but not limited to, the targeted manipulation of a gene associated with disease, the modulation of the expression level of a gene associated with disease, and the programming of cell fate.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Application of nanotechnology and sensor technologies for ex-vivo diagnostics
InactiveUS7052854B2Easy diagnosisQuick checkMicrobiological testing/measurementMaterial analysis by electric/magnetic meansAnalyteBody fluid
Systems and methods for the ex vivo diagnostic analysis of samples of bodily fluids, including exhaled breath and blood. The present invention uses nanostructure-based assemblies in combination with sensor technology to provide an efficient and accurate means for identifying the presence of a target analyte / biomarker in a sample of bodily fluid. In a preferred embodiment, the nanostructure-based assemblies of the present invention include detecting means such as RNA oligonucleotide chains or “apparatus” and releasable surrogate markers such as DMSO.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Endometrial stem cells and methods of making and using same
InactiveUS20090053182A1Rapid rate of cellular divisionBiocidePeptide/protein ingredientsCell lineageEndometrium
The invention provides pluripotent stem cells and methods for making and using pluripotent stem cells. Pluripotent stem cells, among other things, can differentiate into various cell lineages in vitro, ex vivo and in vivo. Pluripotent stem cells, among other things, can also be used to produce conditioned medium.
Owner:MEDISTEM LAB
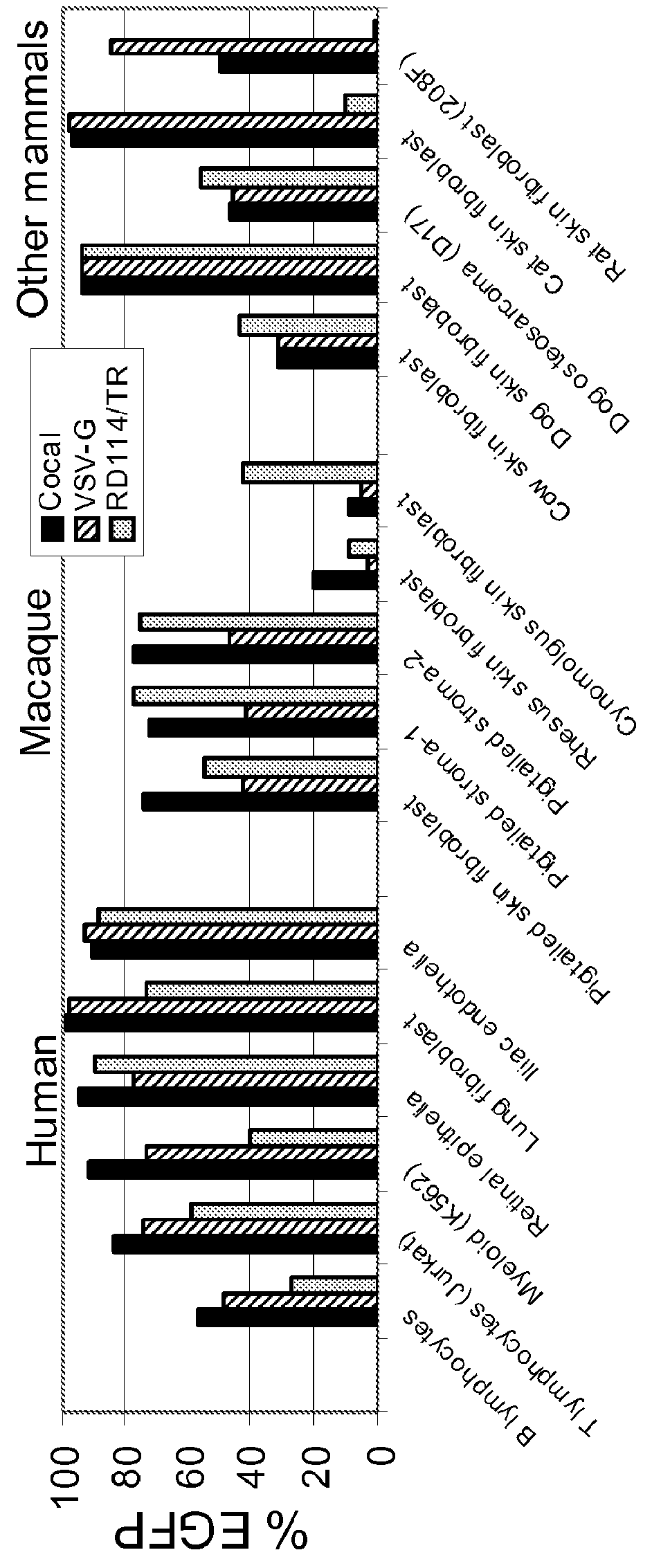
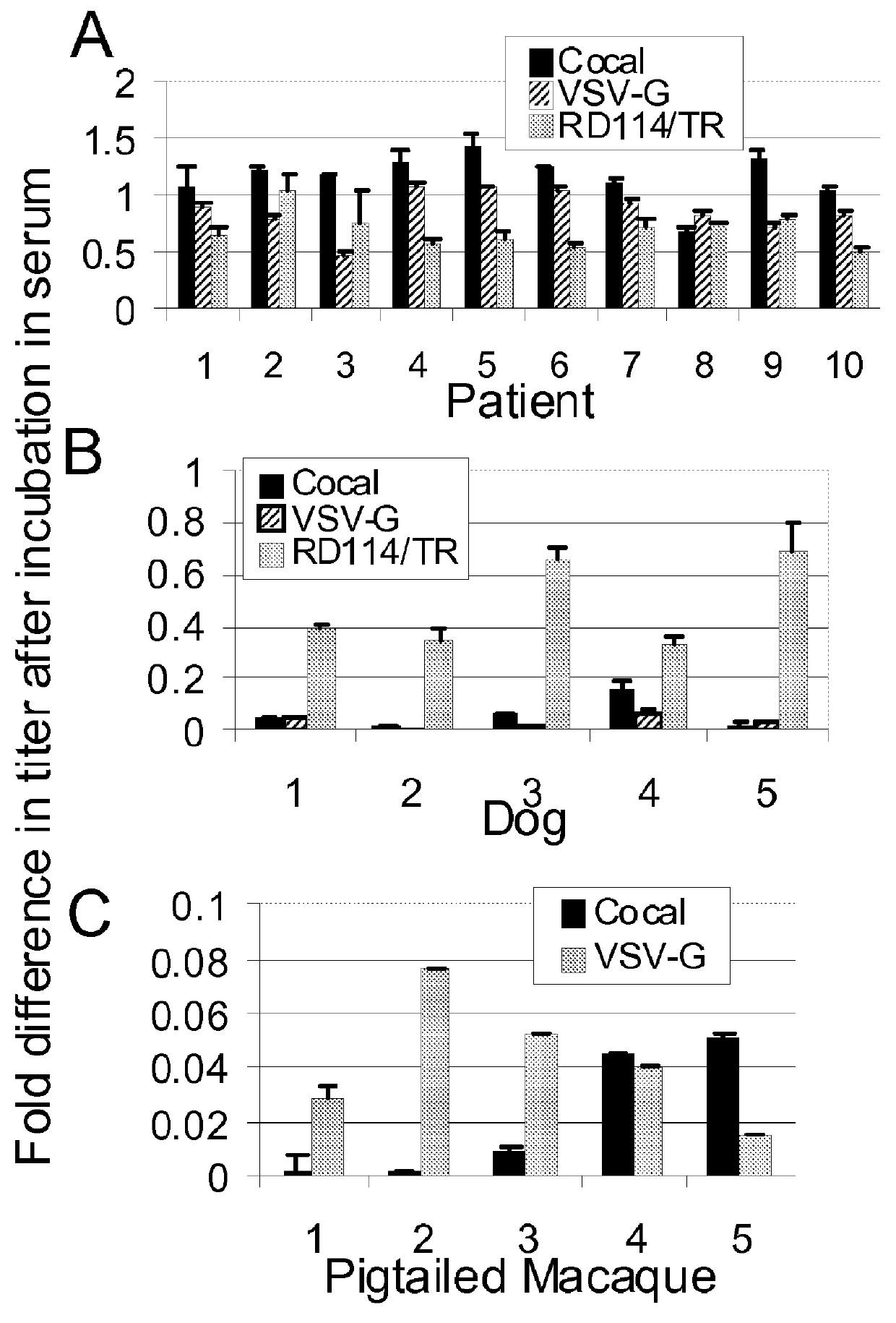
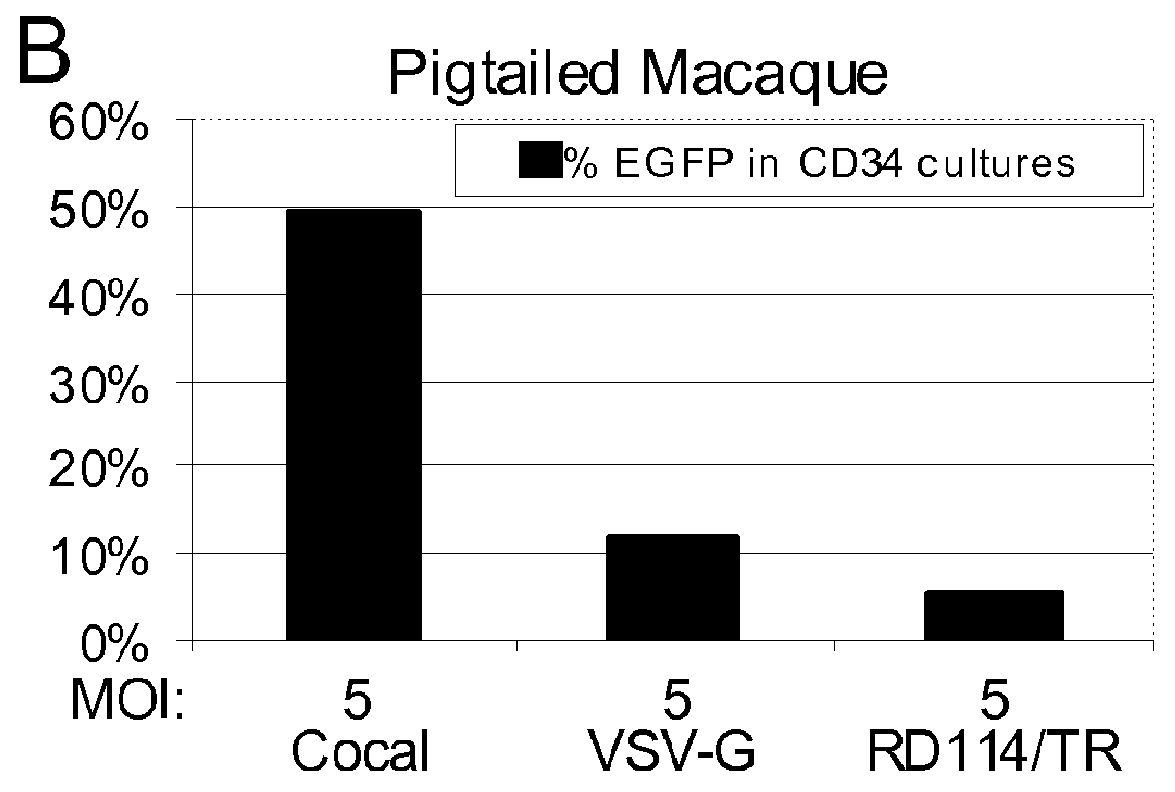
Cocal vesiculovirus envelope pseudotyped retroviral vectors
InactiveUS20120164118A1Increase serum stabilitySsRNA viruses negative-senseBiocideCocal vesiculovirusVirus-Retrovirus
Provided herein are Cocal vesiculovirus envelope pseudotyped retroviral vectors that exhibit high titers, broad species and cell-type tropism, and improved serum stability. Disclosed Cocal vesiculovirus envelope pseudotyped retroviral vectors may be suitably employed for gene therapy applications and, in particular, for the ex vivo and in vivo delivery of a gene of interest to a wide variety of target cells.
Owner:FRED HUTCHINSON CANCER RES CENT
Method and system for mammalian joint resurfacing
Owner:NEXT ORTHOSURGICAL INC
Method and system for mammalian joint resurfacing
A method and system for the creation or modification of the wear surface of orthopedic joints, involving the preparation and use of one or more partially or fully preformed and procured components, adapted for insertion and placement into the body and at the joint site. In a preferred embodiment, component(s) can be partially cured and generally formed ex vivo and further and further formed in vivo at the joint site to enhance conformance and improve long term performance. In another embodiment, a preformed balloon or composite material can be inserted into the joint site and filled with a flowable biomaterial in situ to conform to the joint site. In yet another embodiment, the preformed component(s) can be fully cured and formed ex vivo and optionally further fitted and secured at the joint site. Preformed components can be sufficiently pliant to permit insertion through a minimally invasive portal, yet resilient enough to substantially assume, or tend towards, the desired form in vivo with additional forming there as needed.
Owner:VERTEBRAL TECH INC
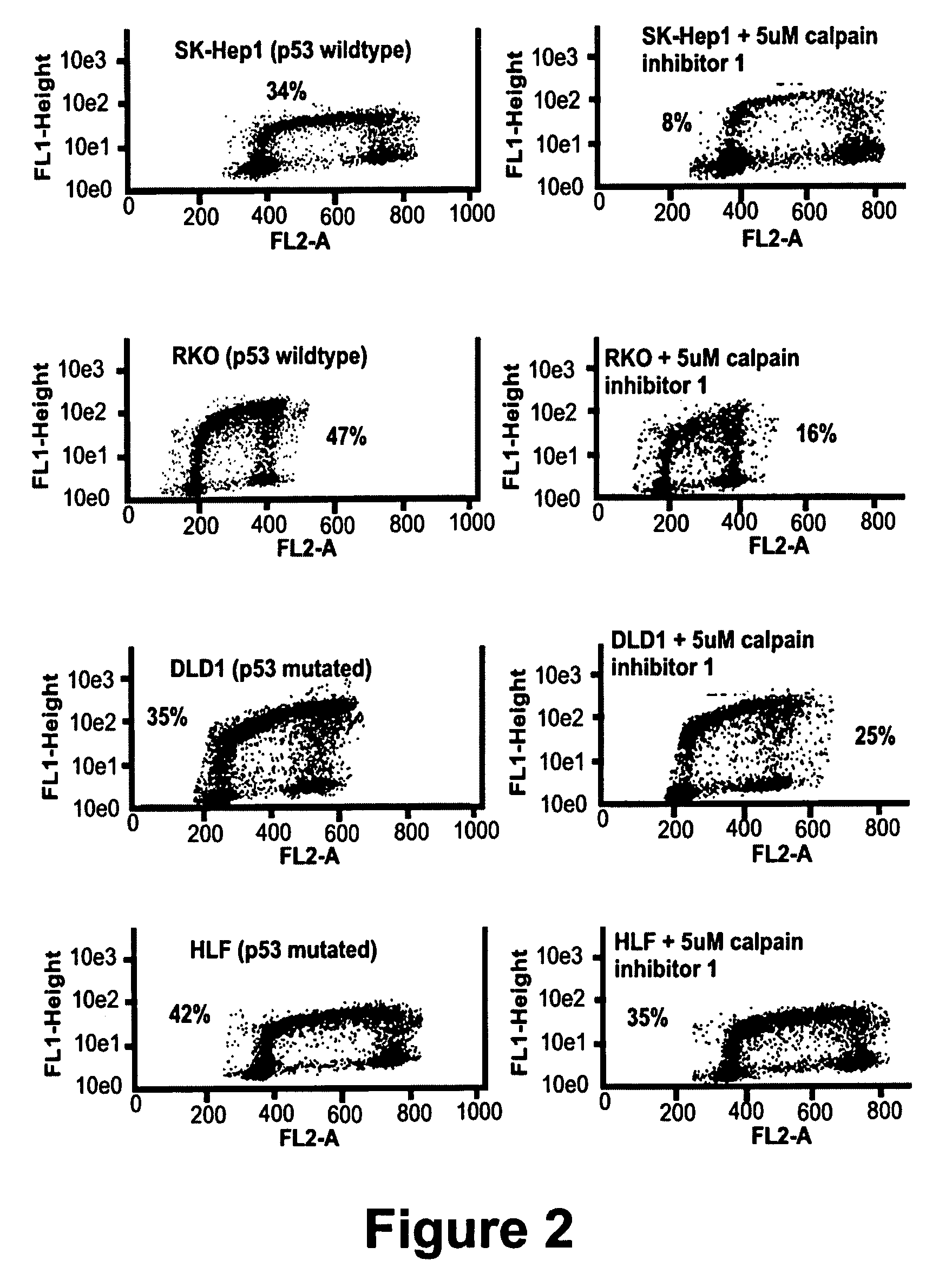
Calpain inhibitors and their applications
InactiveUS7001770B1Enhance p53-mediated apoptosisIncrease infectivityPeptide/protein ingredientsFermentationCo administrationApoptosis
The present invention provides a method to enhance apoptosis in a cell by the administration of p53 in combination with a calpain inhibitor. The present invention provides a method of increasing the infectivity of a cell to a viral vector by treatment of the cell with a calpain inhibitor. the present invention further provides a method of enhancing transciption of a therapeutic transgene from the CMV promoter. The present invention also provides a method of suppress the in vivo CTL response to viral vectors by the use of calpain inhibitors. The present invention further provides a pharmaceutical formulations of p53 and a calpain inhibitor in a pharmaceutically acceptable carrier. The present invention provides a method of ablating neoplastic cells in a mammalian organism in vivo by the co-administration of a calpain inhibitor and p53. The present invention also provides a method of ablating neoplastic cells in a population of normal cells contaminated by said neoplastic cells ex vivo by the administration of a recombinant adenovirus in combination with a calpain inhibitor to said population.
Owner:CANJI
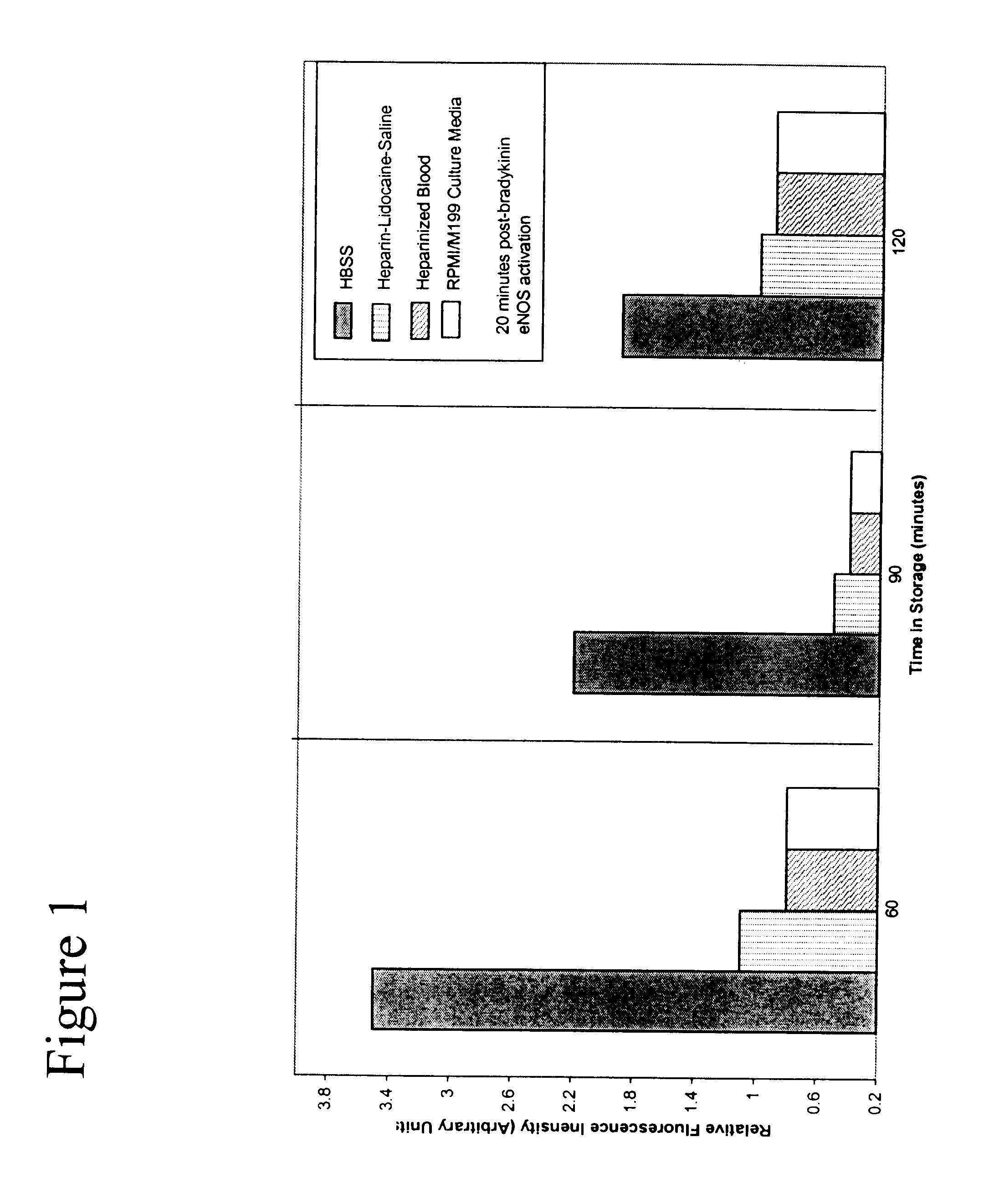
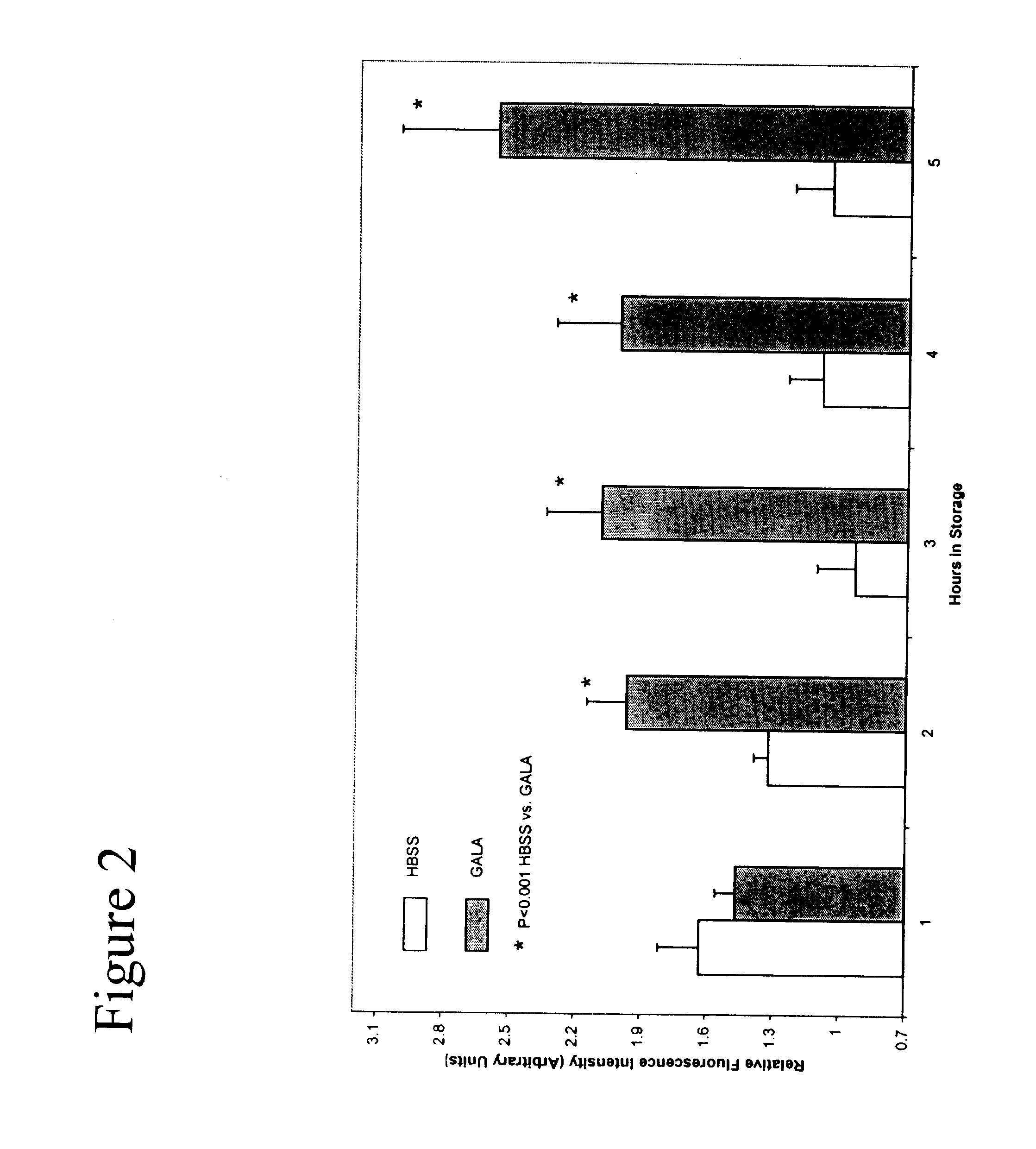
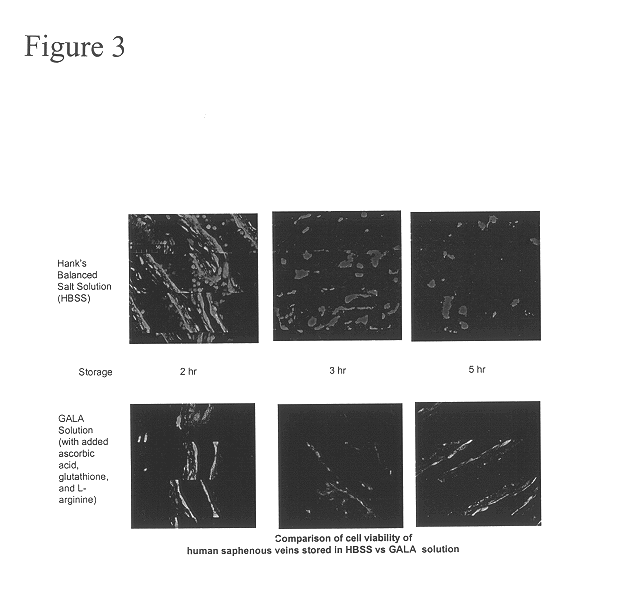
Composition and methods for tissue preservation
The present invention provides for compositions and methods for the preservation of tissues and organs ex vivo and in situ. In addition, the present invention provides for kits that may be used in the preparation of the solutions of the present invention.
Owner:VETERANS AFFAIRS UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF
T-cell therapy formulation
Ex-vivo prepared T-cells are harvested from cell culture conditions and formulated in medium suitable for infusion. The formulation is made by labeling the cells with one or more agents which have reactivity for T-cell surface moieties capable of delivery activation signals upon cross-linking and mixing the labeled cells with biodegradable nanospheres or microspheres coated with a material capable of cross-linking the agents attached to the T-cell surface moieties. Alternatively, the formulation may be made by mixing a population of T-cells with biodegradable nanospheres or microspheres coated with a first material and one or more second materials. The first material binds the second material and the second material has reactivity for surface moieties on the T-cells and the interaction of the second materials with the T-cells causes the activation of the T-cells. In either method, the mixture of T-cells and biodegradable spheres are suspended in a medium suitable for infusion, and the mixture is packaged in a container.
Owner:IMMUNOVATIVE THERAPIES +1
Electrochromic device and photodynamic treatment device comprising such an electrochromic device
InactiveUS20100082081A1Increase the light areaOptimize and tune transmissionLight therapyNon-linear opticsAbnormal tissue growthBladder Infections
Presently, many variations of light treatment are used in health care. Prime examples are the in-vivo or ex-vivo photodynamic treatment (PDT) of skin diseases, cancer / tumors, psoriasis, mood disorders, bladder infections, promoting wound closure, recovering spinal cord injuries, and countering muscle / bone atrophy. PDT is a treatment that uses a drug, called a photo-sensitizer or photosensitizing agent, and a particular type of light. The invention relates to an improved PDT device.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Digital topological analysis of trabecular bone MR images and prediction of osteoporosis fractures
The invention provides method, system and device for determining trabecular bone structure and strength by digital topological analysis, and offers, for the first time, a demonstration of superior associations between vertebral deformity and a number of architectural indices measured in the distal radius, thus permitting reliable and noninvasive detection and determination of the pathogenesis of osteoporosis. A preferred embodiment provides imaging in three dimension of a region of trabecular bone, after which the 3D image is converted into a skeletonized surface representation. Digital topological analysis is applied to the converted image, and each image voxel is identified and classified as a curve, a surface, or a junction; and then associated with microarchitectural indices of trabecular bone to quantitatively characterize the trabecular bone network. The invention is applicable in vivo, particularly on human subjects, or ex vivo.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods of ex vivo progenitor and stem cell expansion by co-culture with mesenchymal cells
Methods of ex-vivo expansion and at the same time inhibiting differentiation of stem cells by co-culture with mesenchymal cells, transplantable populations of renewable progenitor and stem cells expanded thereby, and their uses in therapeutic applications.
Owner:GAMIDA CELL
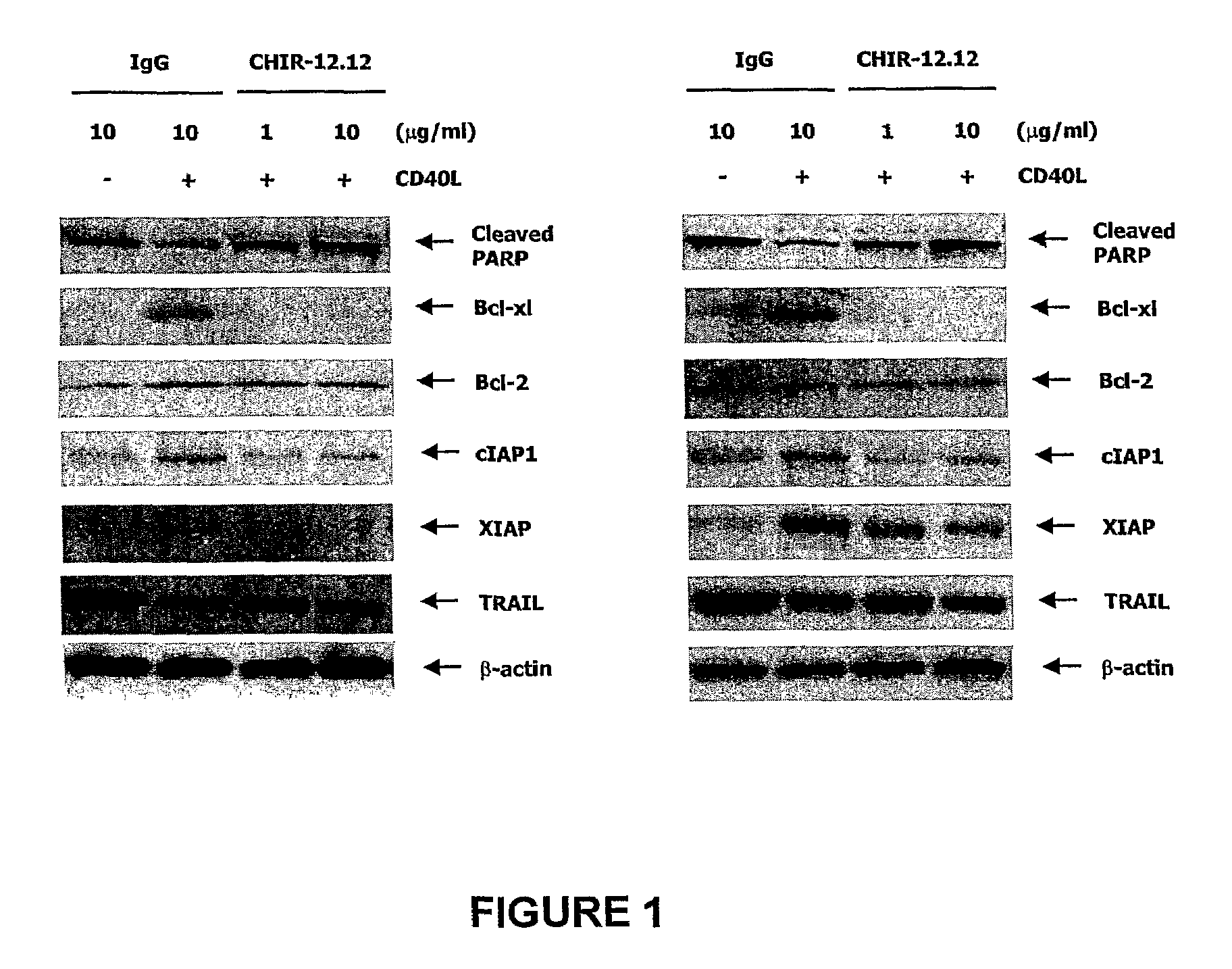
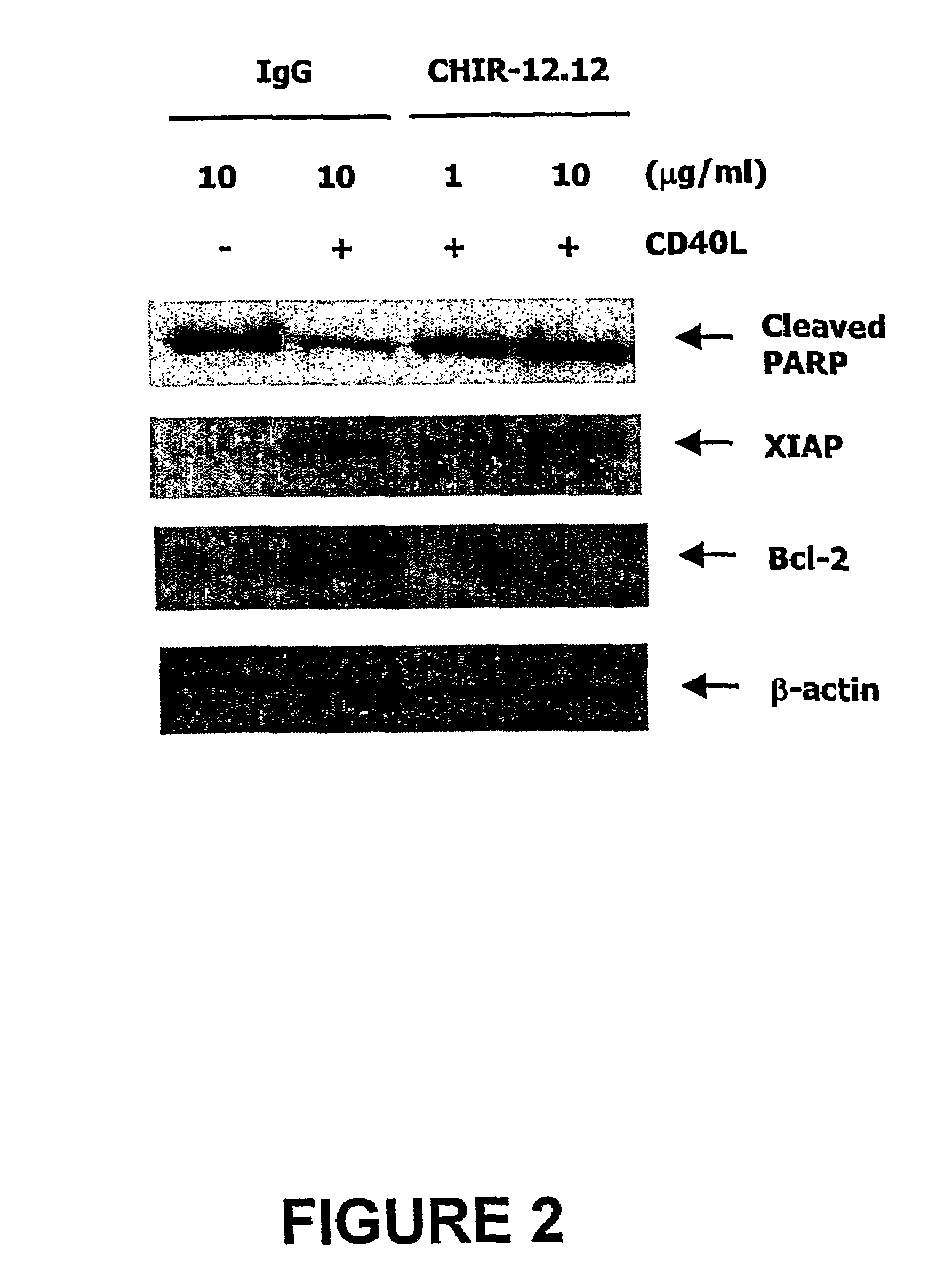
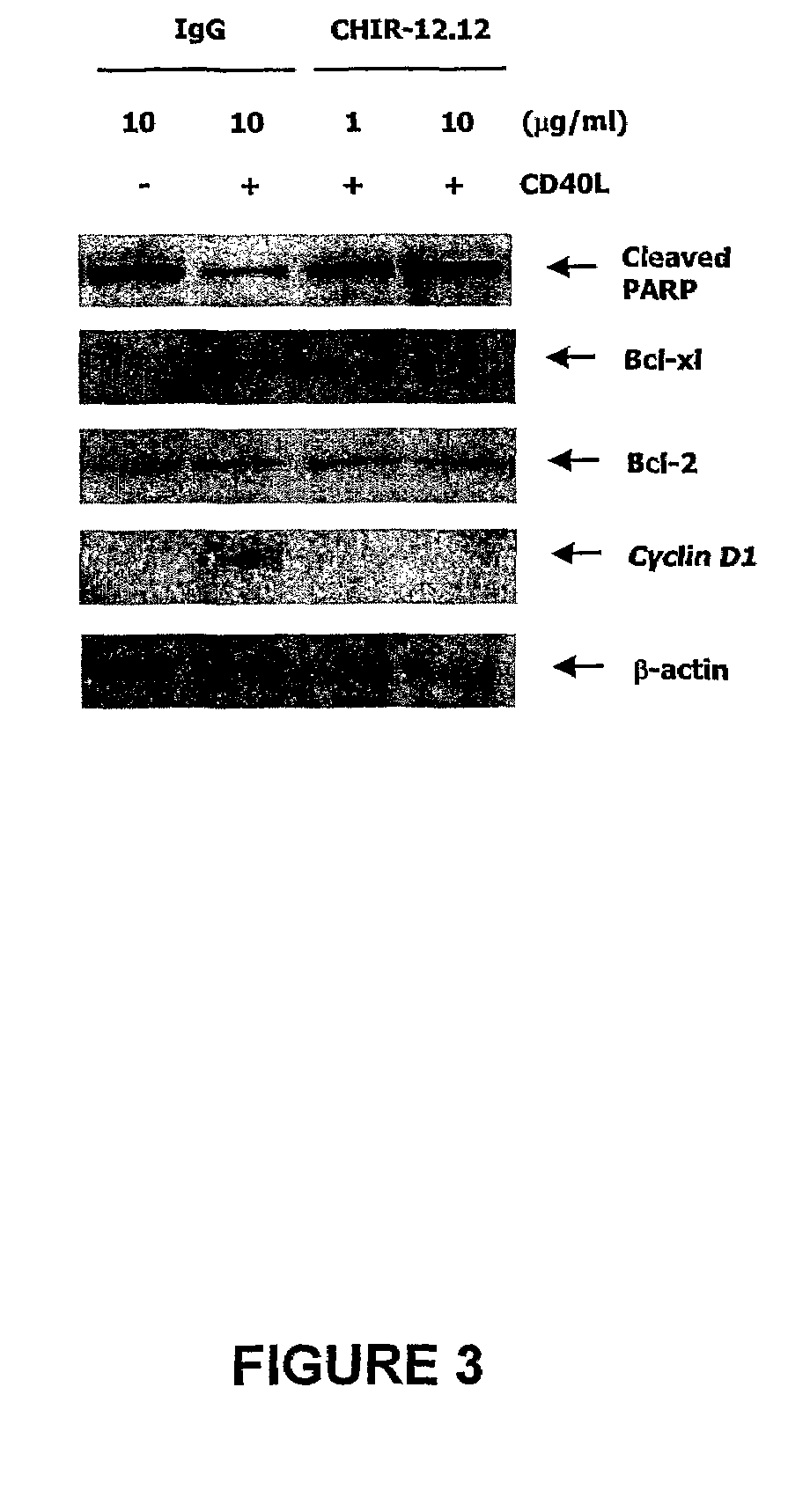
Methods of monitoring the efficacy of anti-CD40 antibodies in treating a subject for a CD40-expressing cancer
InactiveUS8337851B2Microbiological testing/measurementBiological material analysisSignal onApoptosis
Methods for identifying subjects having a cancer or pre-malignant condition that will benefit from anti-CD40 therapeutic agents that modulate CD40L-mediated CD40 signaling are provided. The methods comprise the use of biomarkers of cellular apoptosis, cell proliferation and survival, and CD40 signaling pathways to monitor ex vivo response to one or more anti-CD40 therapeutic agents of interest that modulate CD40 signaling on CD40-expressing neoplastic cells. The ex vivo prognostic assays can be used alone or in conjunction with other prognostic assays to identify candidate subjects who will benefit from treatment with anti-CD40 therapeutic agents. Methods of the invention also comprise the use of these biomarkers to monitor in vivo efficacy of treatment with an anti-CD40 therapeutic agent.
Owner:XOMA TECH LTD
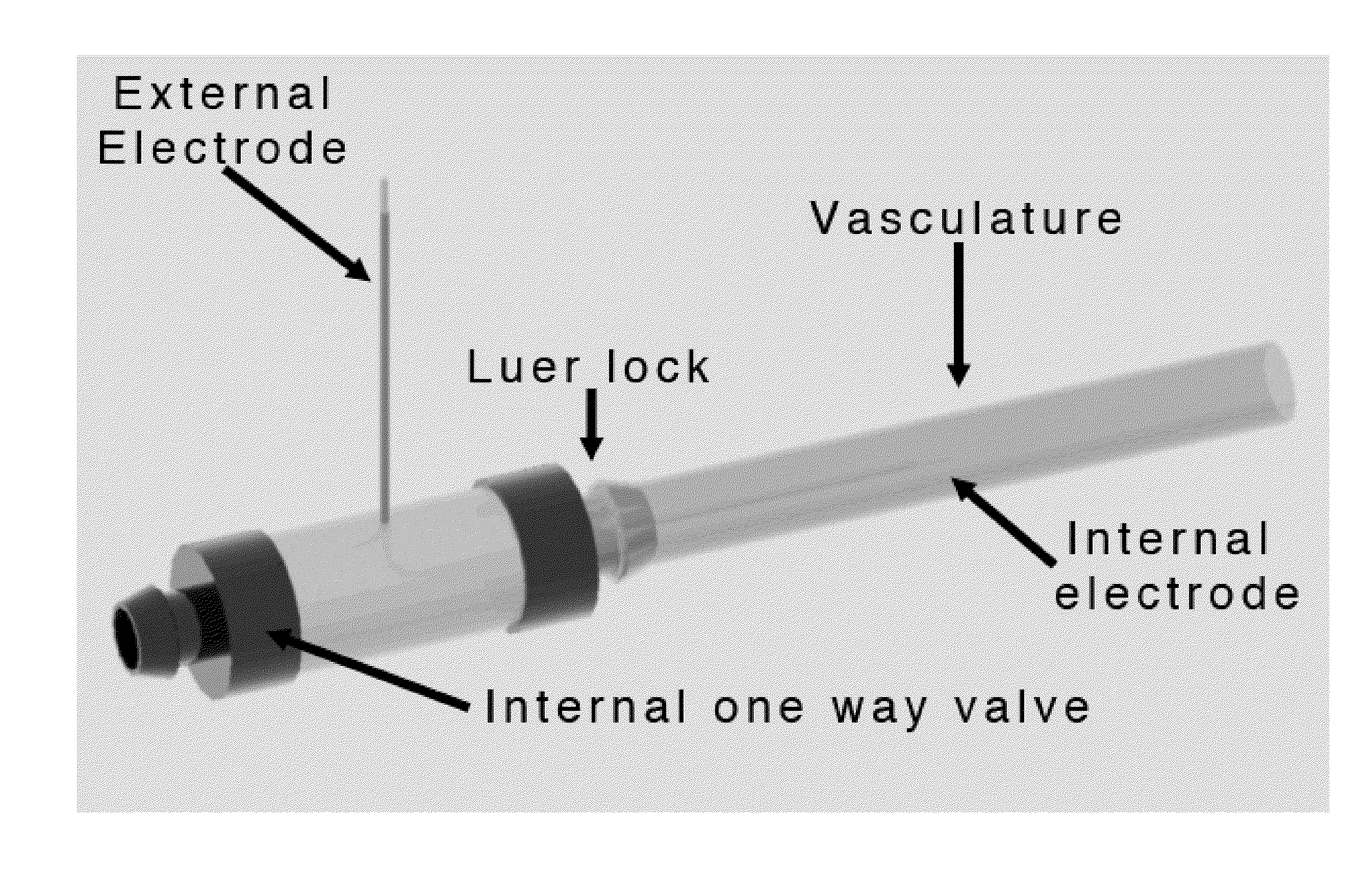
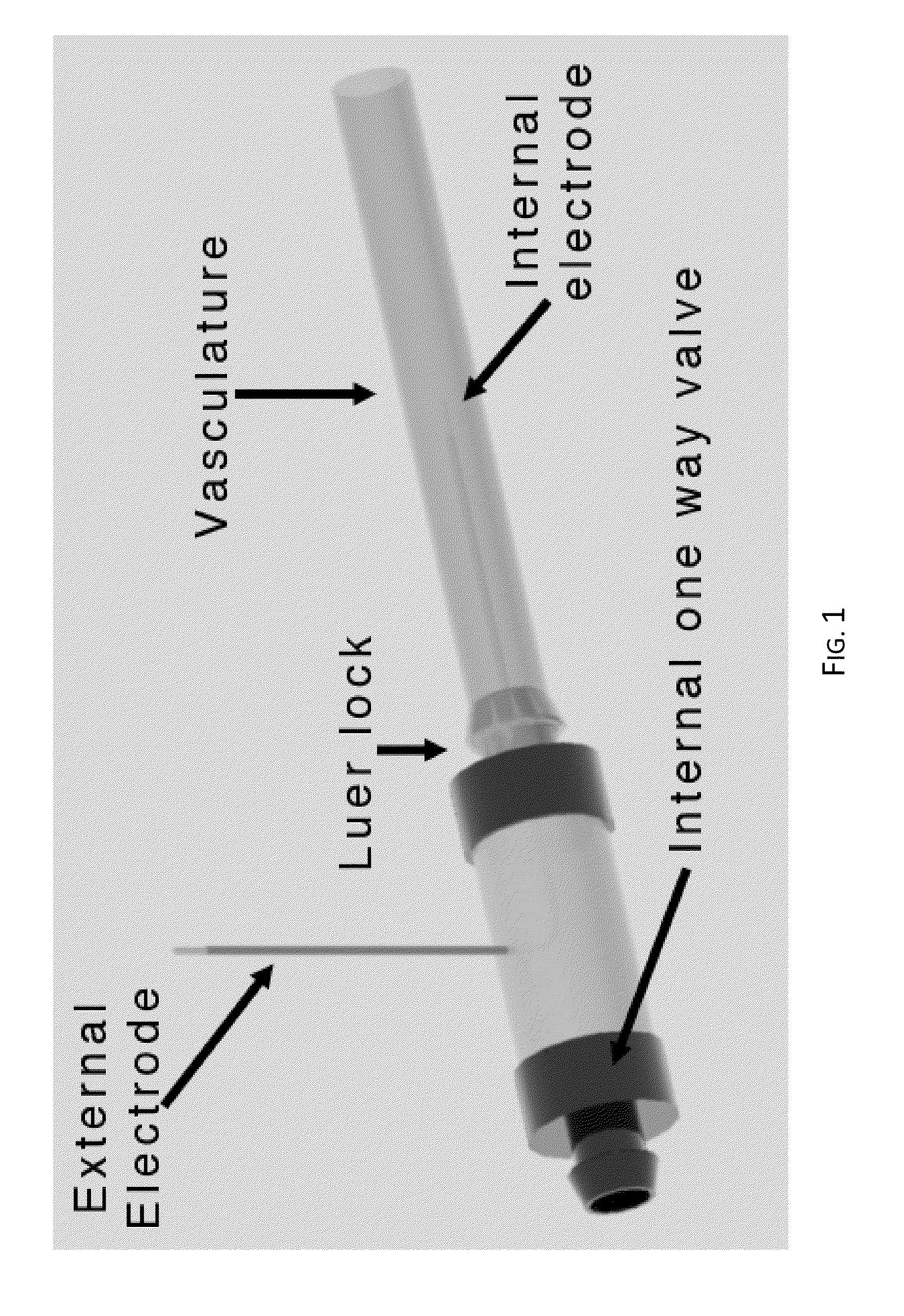
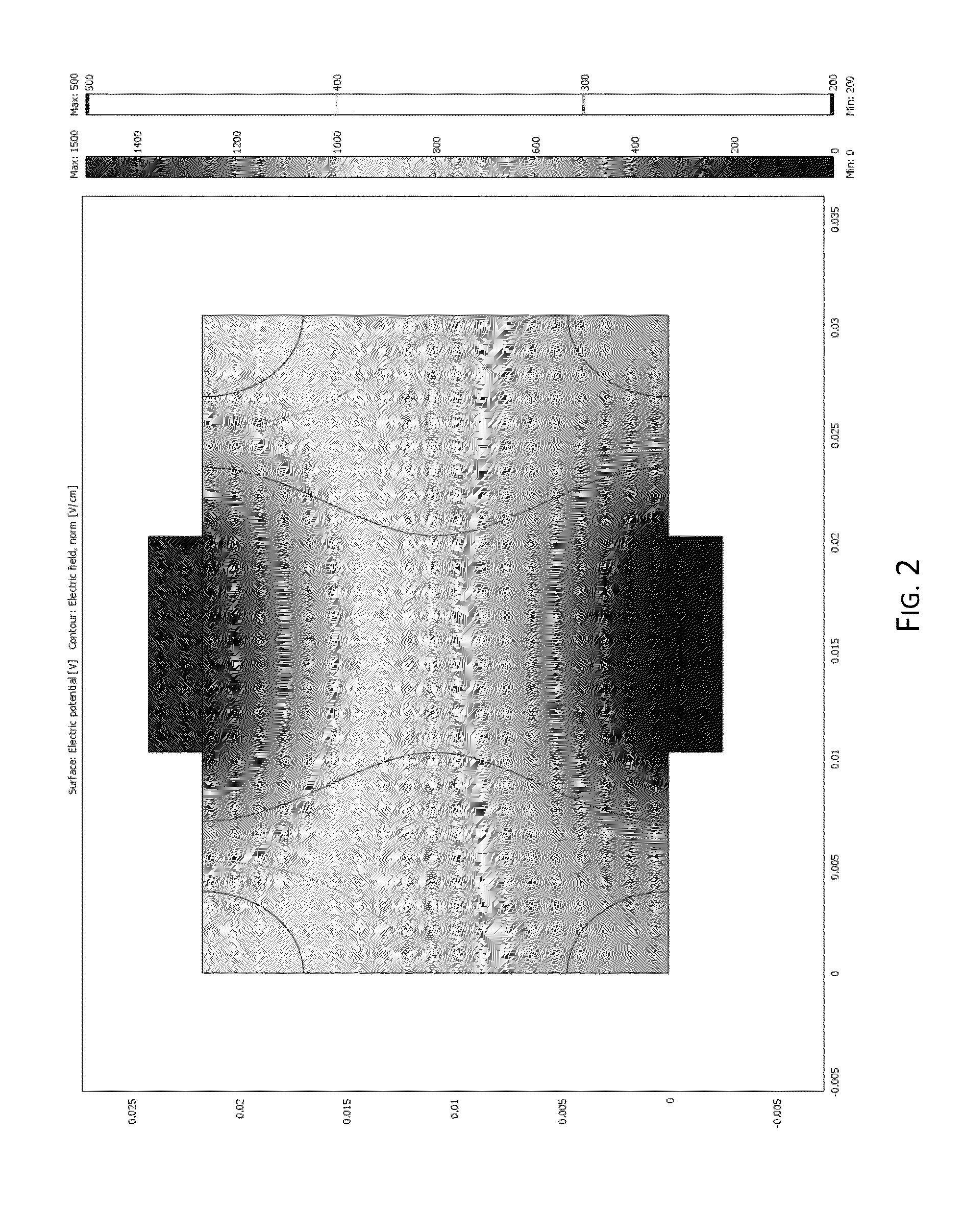
Irreversible electroporation using tissue vasculature to treat aberrant cell masses or create tissue scaffolds
ActiveUS20130253415A1Easy to storeImprove breathabilityElectrotherapyIntravenous devicesDiseaseNatural source
The present invention relates to the field of medical treatment of diseases and disorders, as well as the field of biomedical engineering. Embodiments of the invention relate to the delivery of Irreversible Electroporation (IRE) through the vasculature of organs to treat tumors embedded deep within the tissue or organ, or to decellularize organs to produce a scaffold from existing animal tissue with the existing vasculature intact. In particular, methods of administering non-thermal irreversible electroporation (IRE) in vivo are provided for the treatment of tumors located in vascularized tissues and organs. Embodiments of the invention further provide scaffolds and tissues from natural sources created using IRE ex vivo to remove cellular debris, maximize recellularization potential, and minimize foreign body immune response. The engineered tissues can be used in methods of treating subjects, such as those in need of tissue replacement or augmentation.
Owner:VIRGINIA TECH INTPROP INC
Inhibitors of serine proteases, particularly HCV NS3-NS4A protease
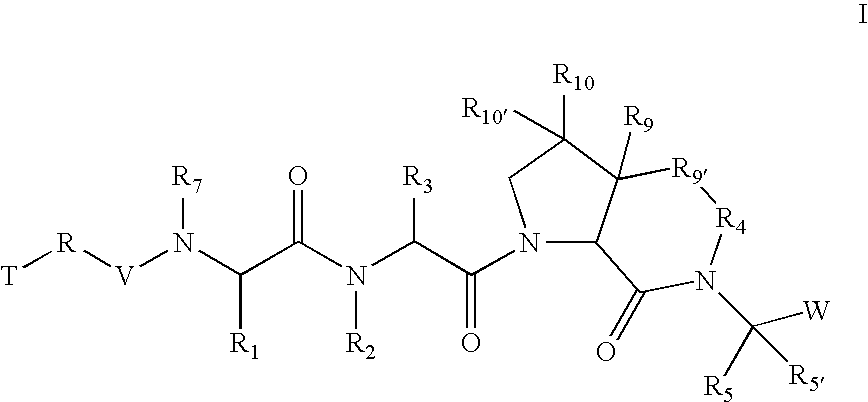
The present invention relates to compounds of formula I: or a pharmaceutically acceptable salt, or mixtures thereof, that inhibit serine protease activity, particularly the activity of hepatitis C virus NS3-NS4A protease. As such, they act by interfering with the life cycle of the hepatitis C virus and are useful as antiviral agents. The invention further relates to pharmaceutically acceptable compositions comprising said compounds either for ex vivo use or for administration to a patient suffering from HCV infection and processes for preparing the compounds. The invention also relates to methods of treating an HCV infection in a patient by administering a pharmaceutical composition comprising a compound of this invention.
Owner:VERTEX PHARMA INC
Reagents and methods for diagnosing, imaging and treating atherosclerotic disease
The invention provides a novel human Mab Fab, cloned by phage display, and its use in diagnostic and therapeutic methods. In particular the invention provides a method for analyzing the OxLDL components of atherosclerotic plaques in vivo and a means to determine their relative pathology. As the method is based on a human Fab rather than a mouse Mab, the progress or regression of the disease may be monitored over time. The antibody may also be used for the analysis of surgical or serum samples ex vivo for the presence of OxLDL. The antibody may also be used to target therapeutic agents to the site of atherosclerotic plaques or may have use as a therapeutic agent itself.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com