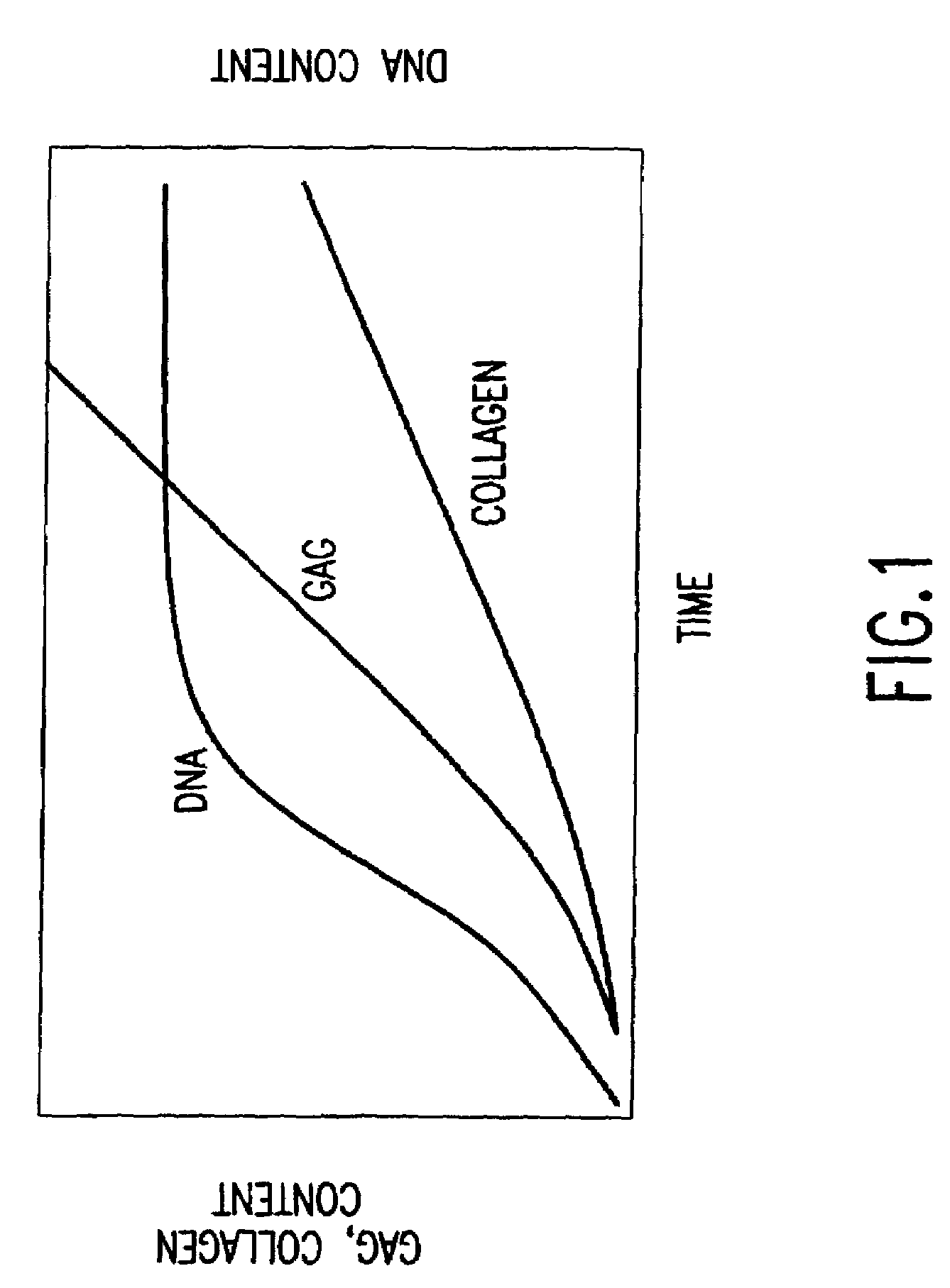
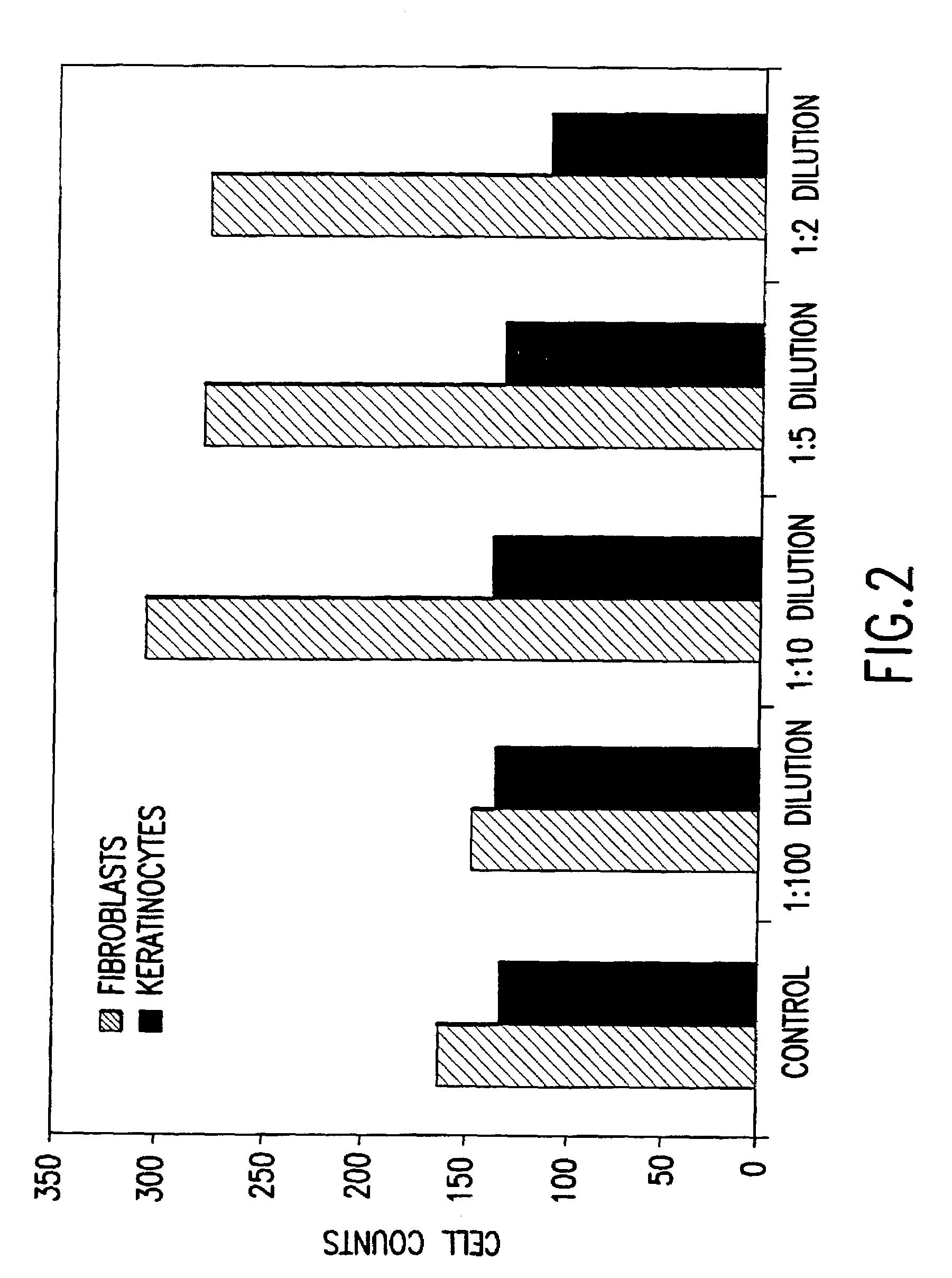
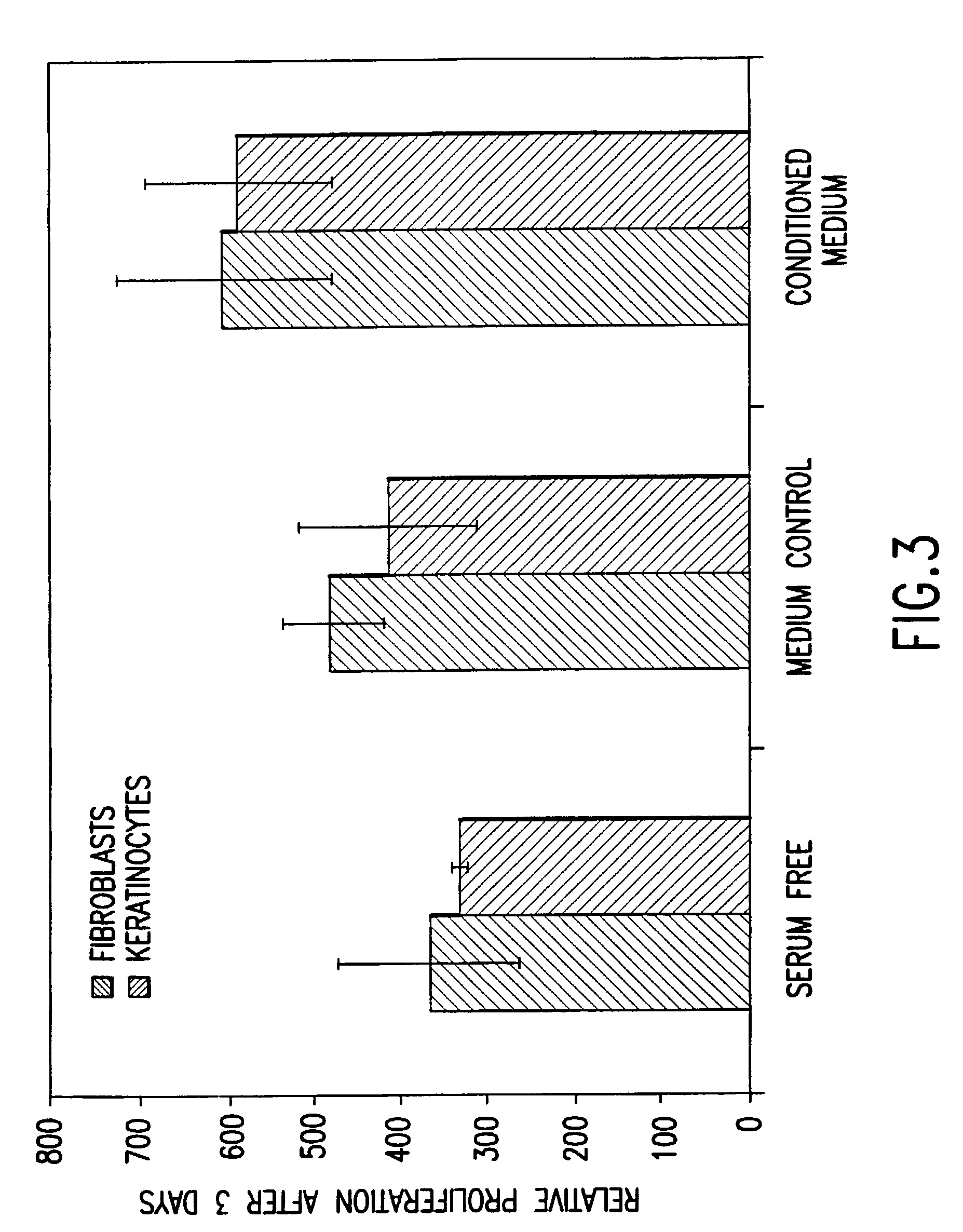
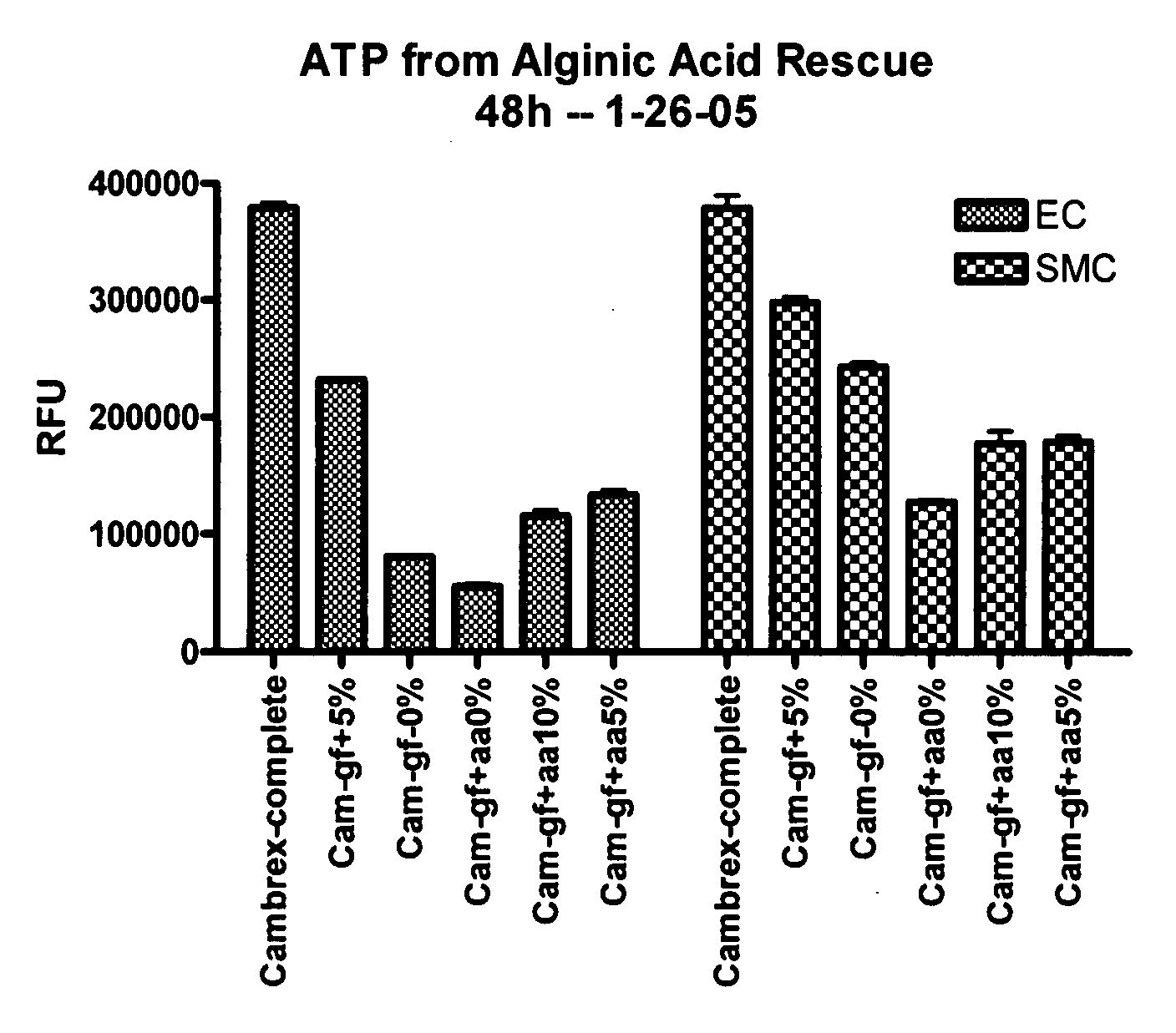
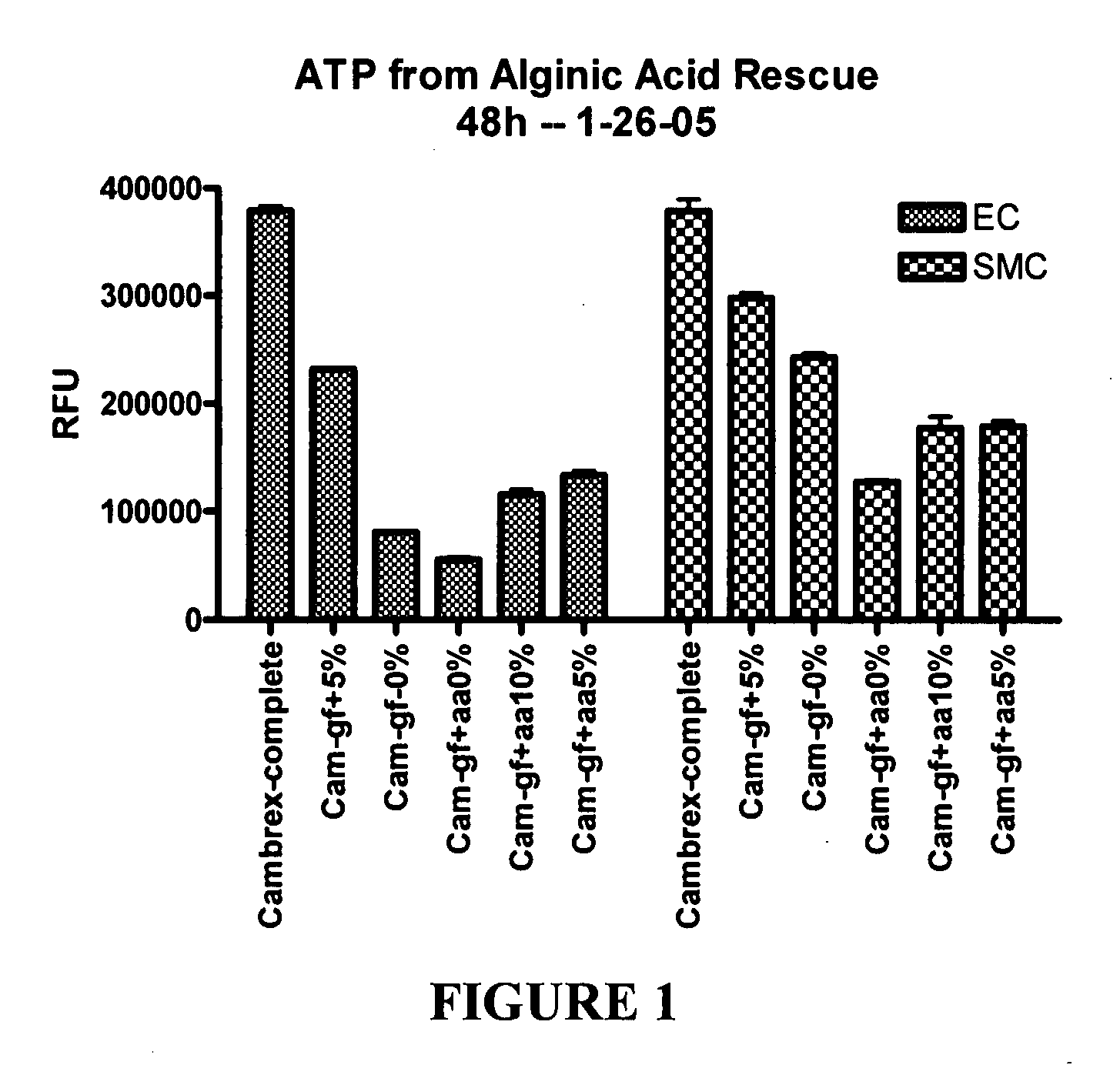
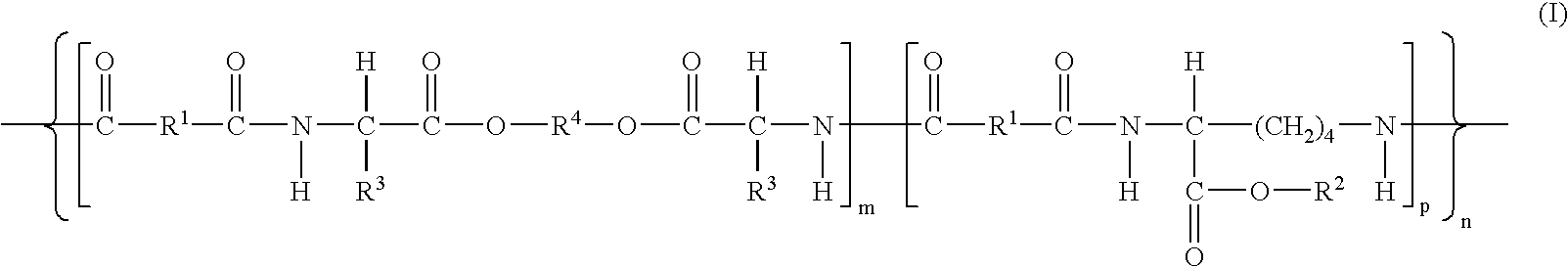
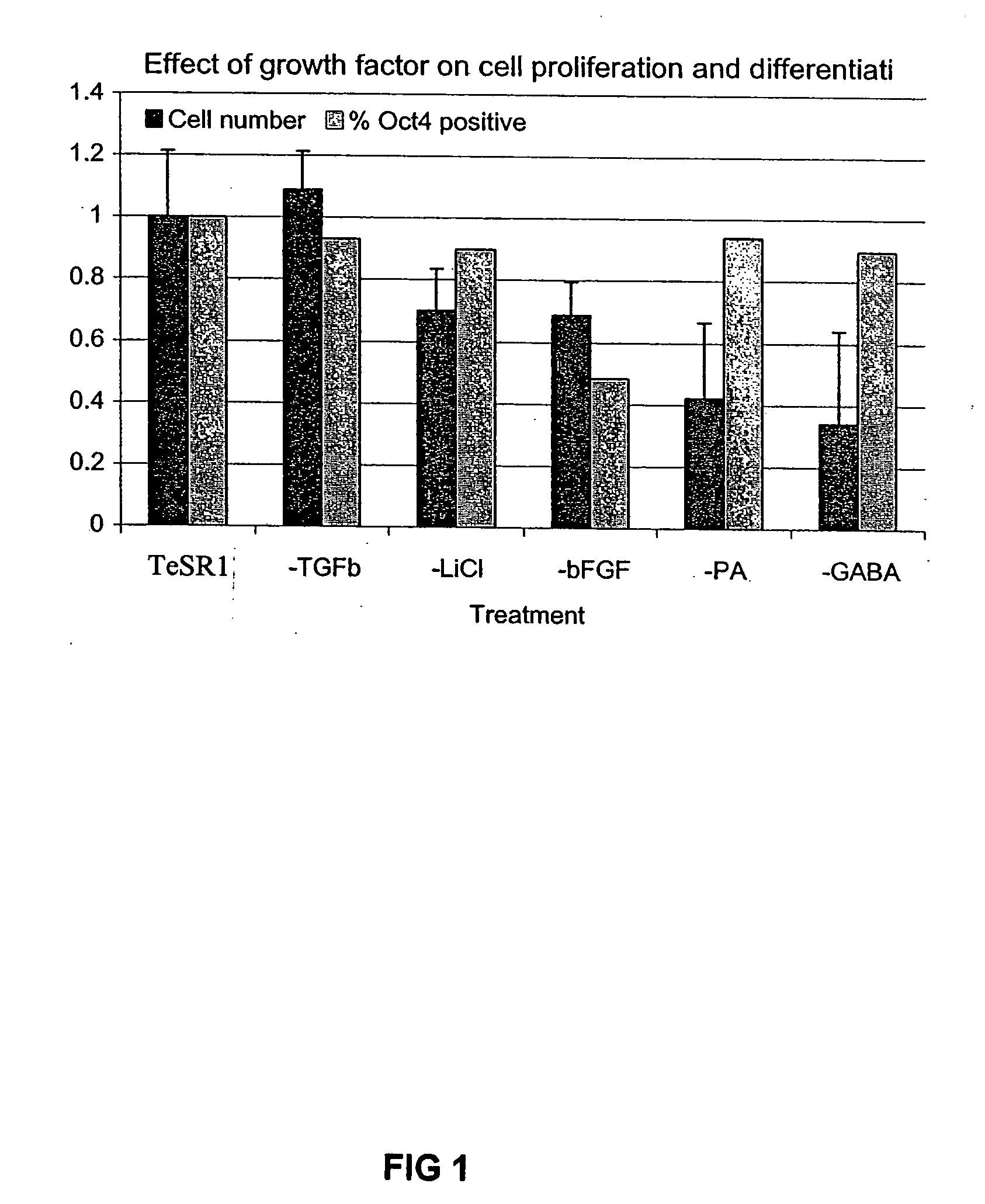
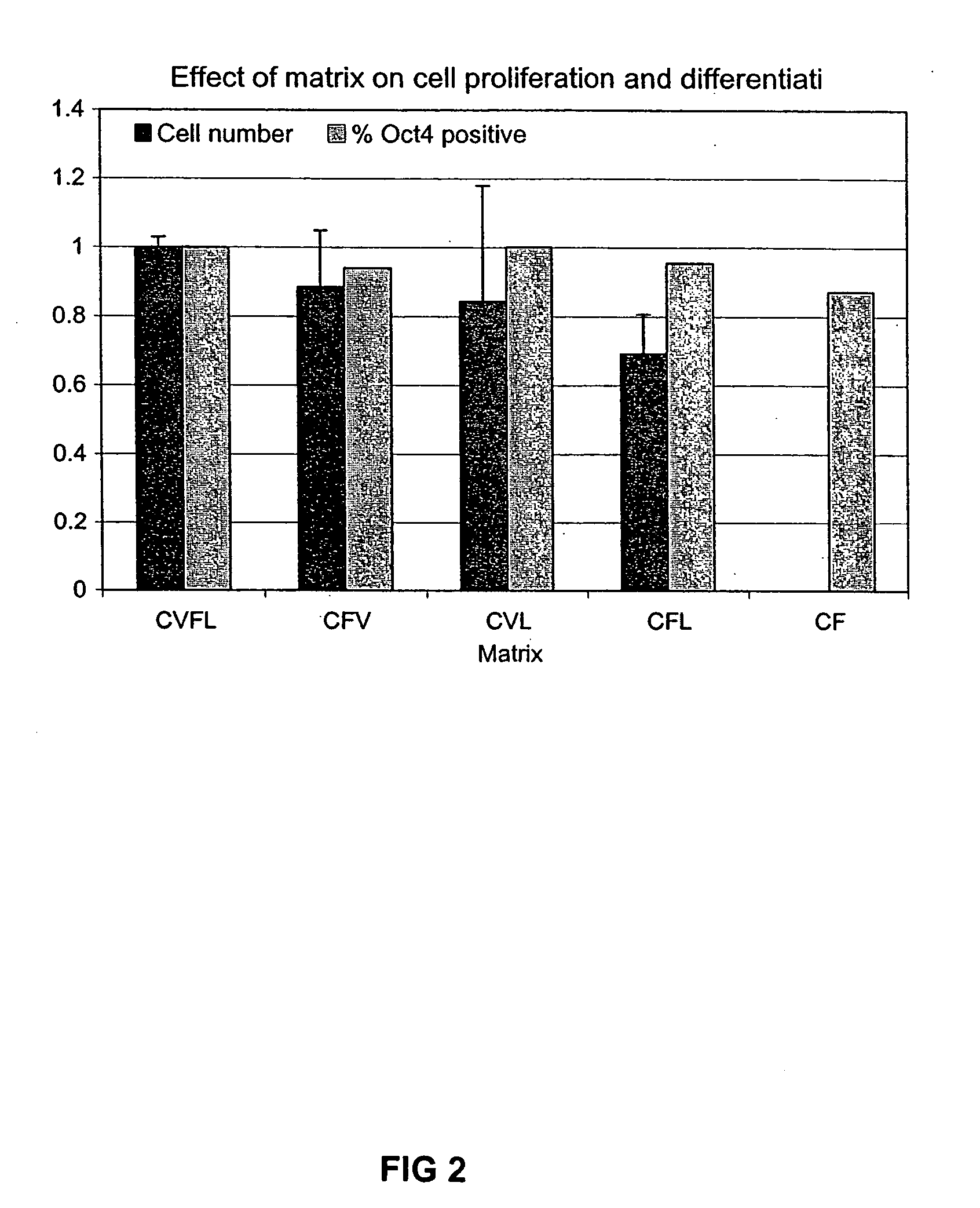
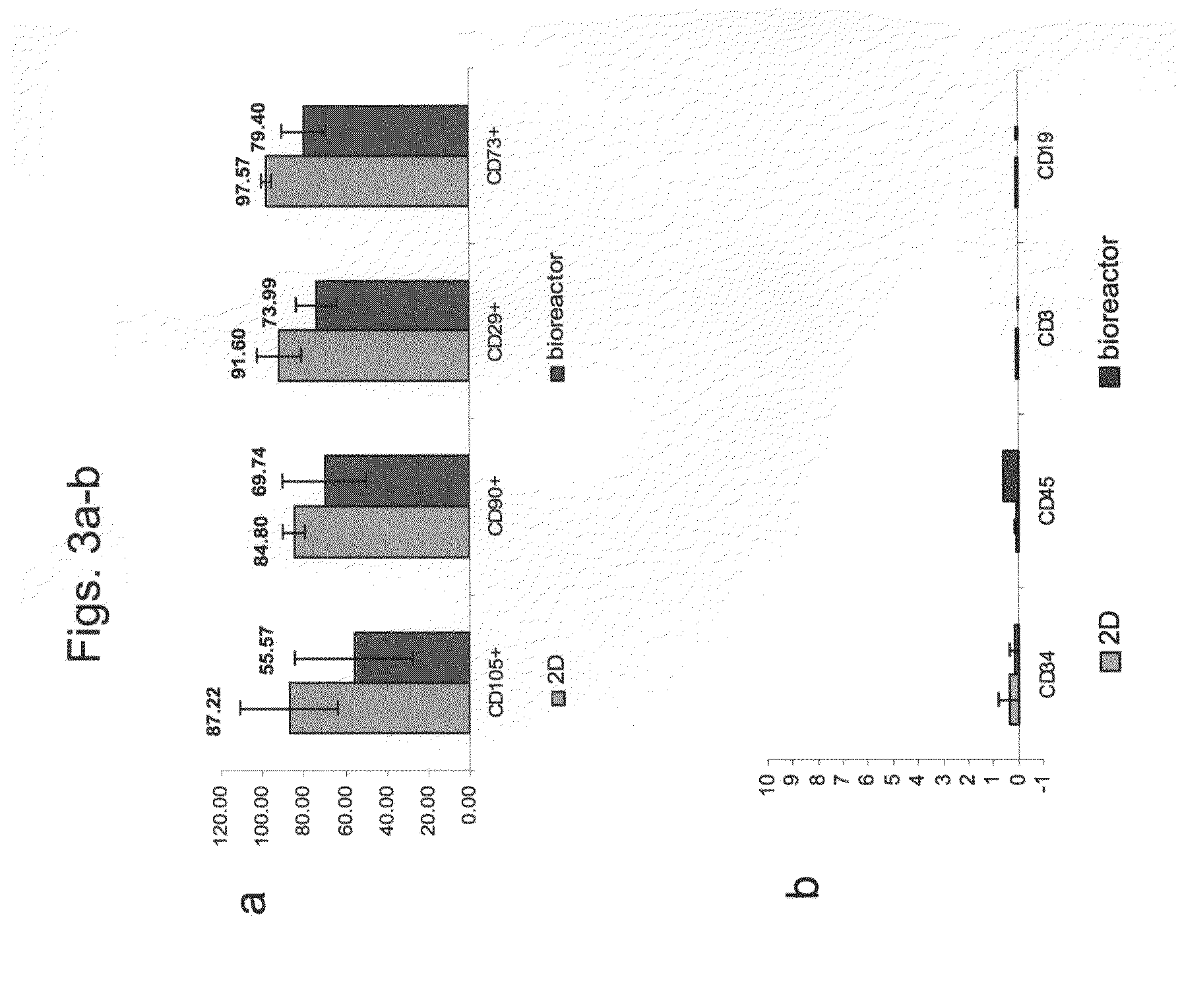
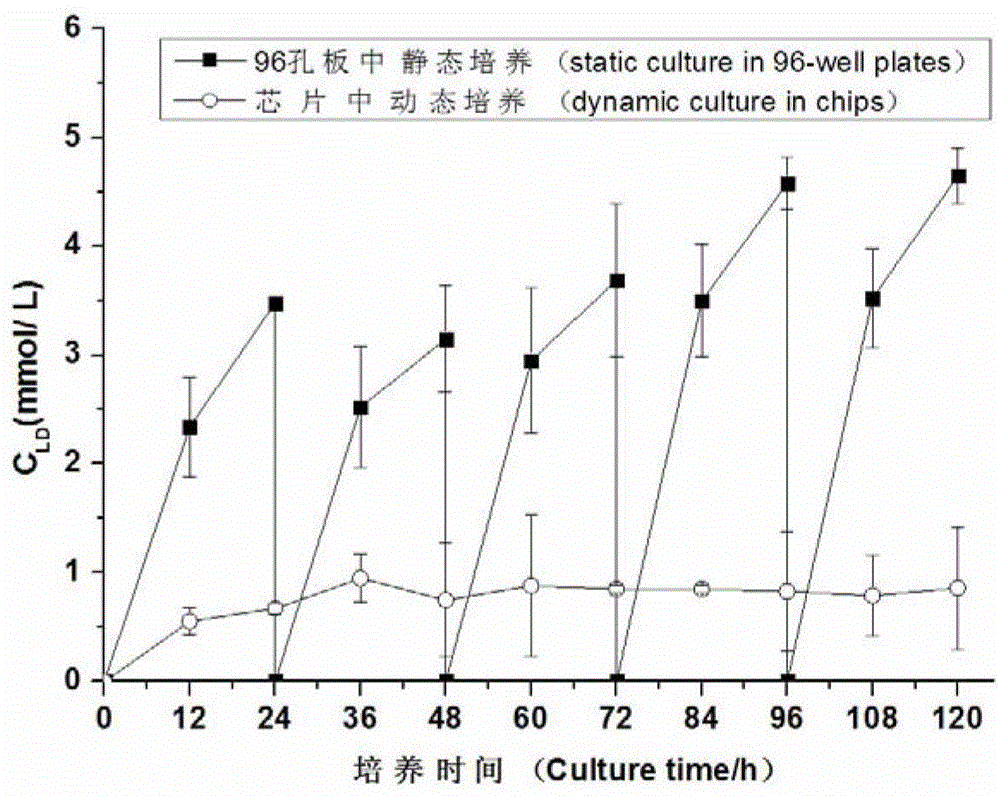
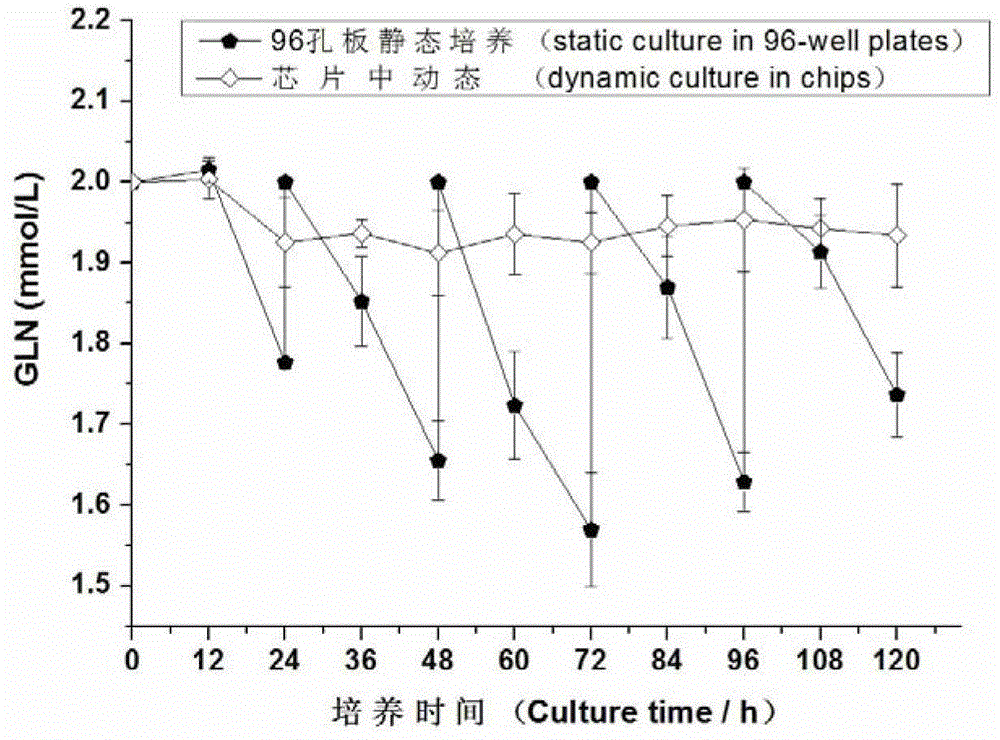
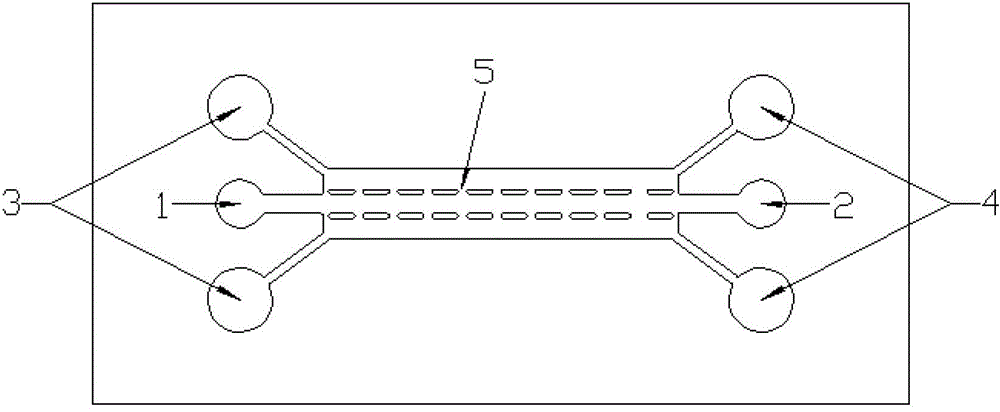
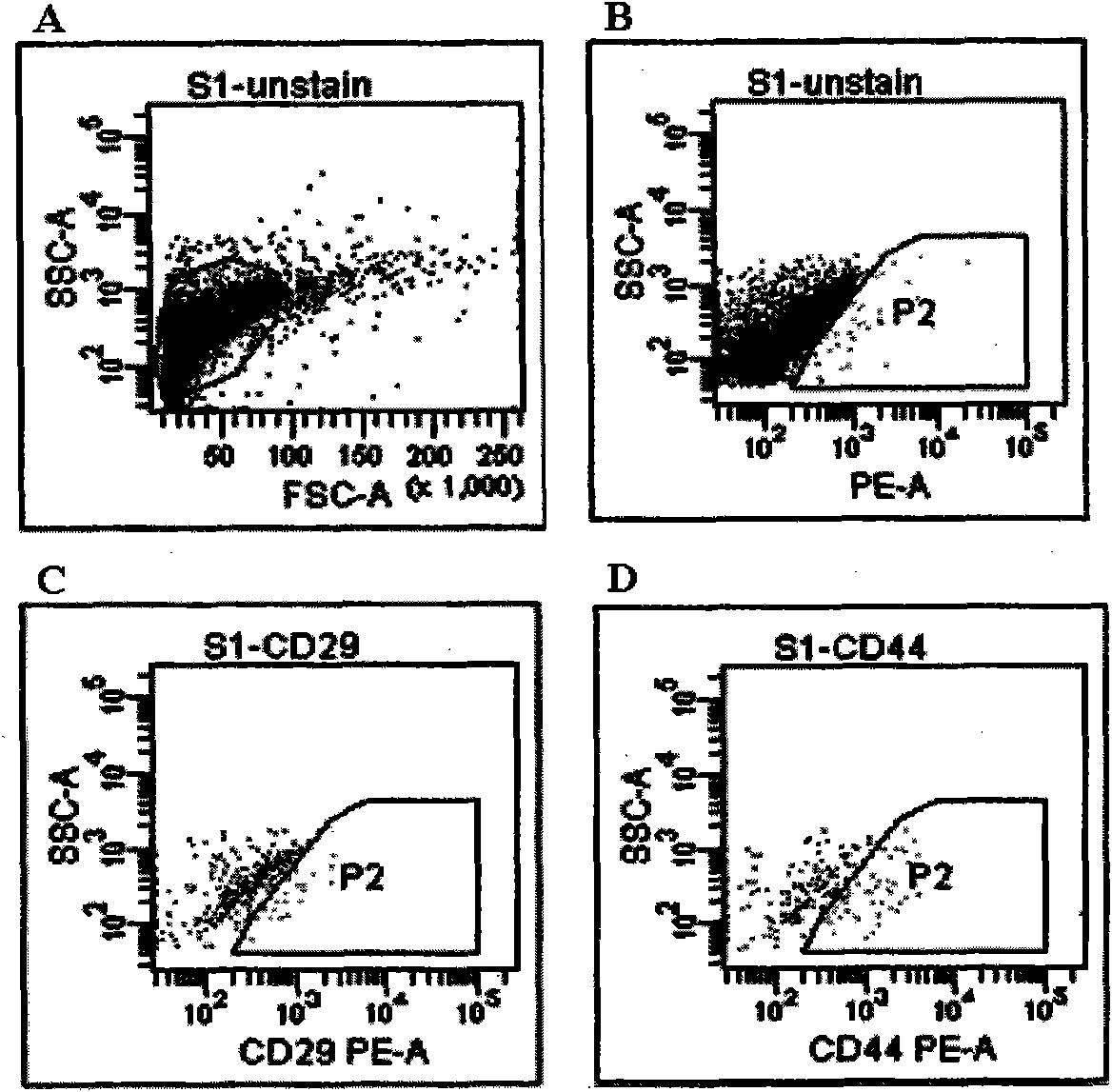
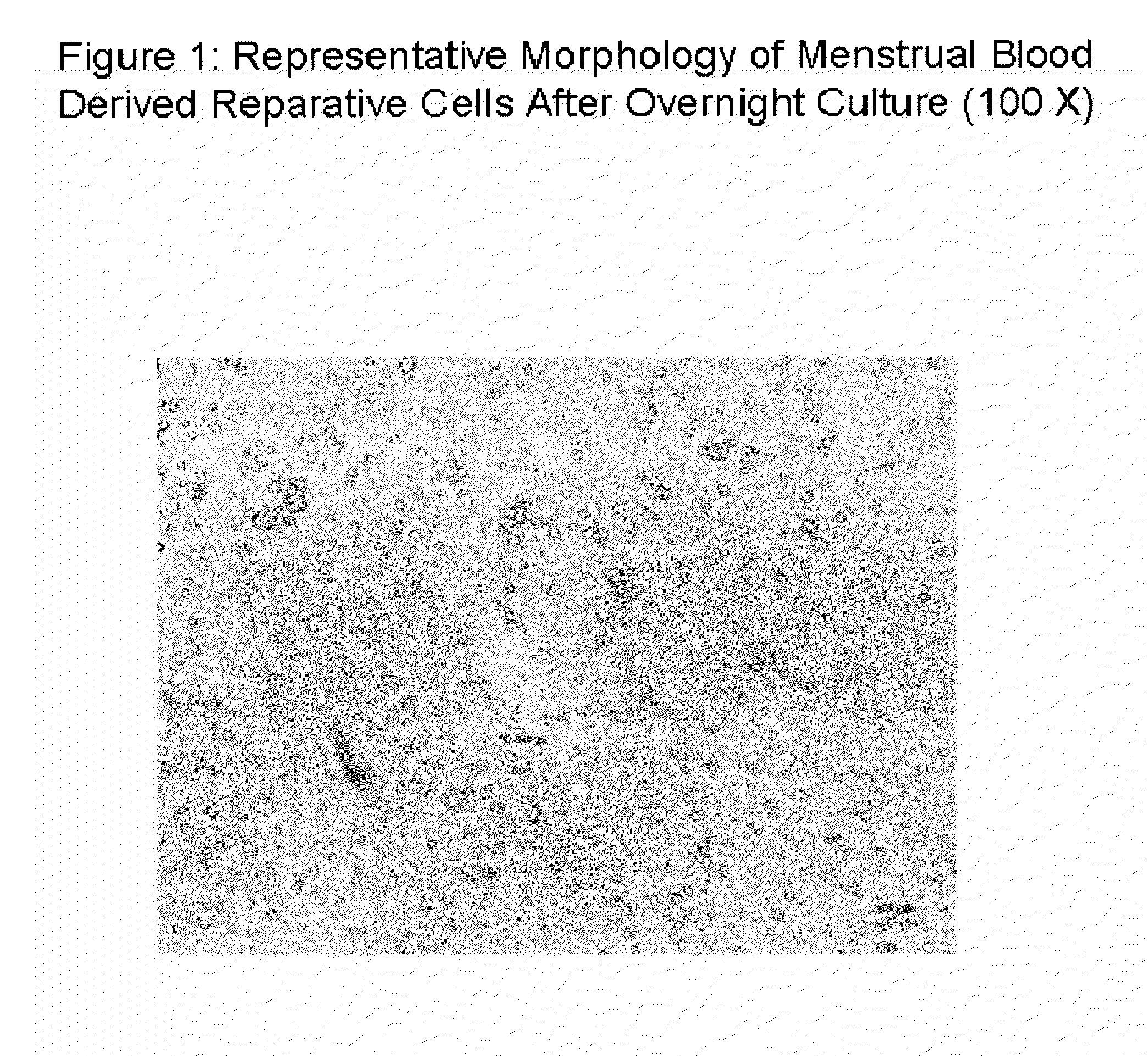
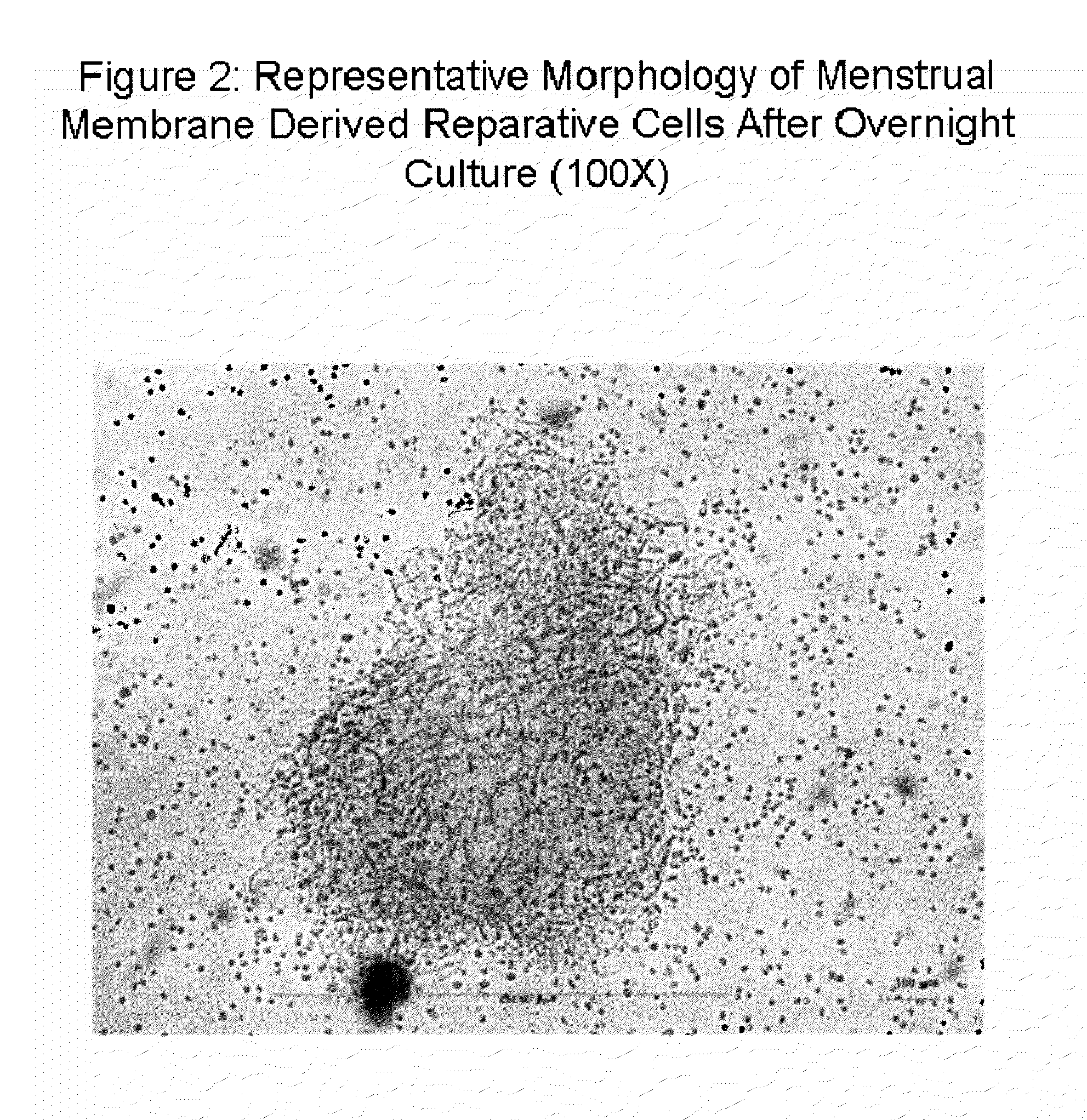
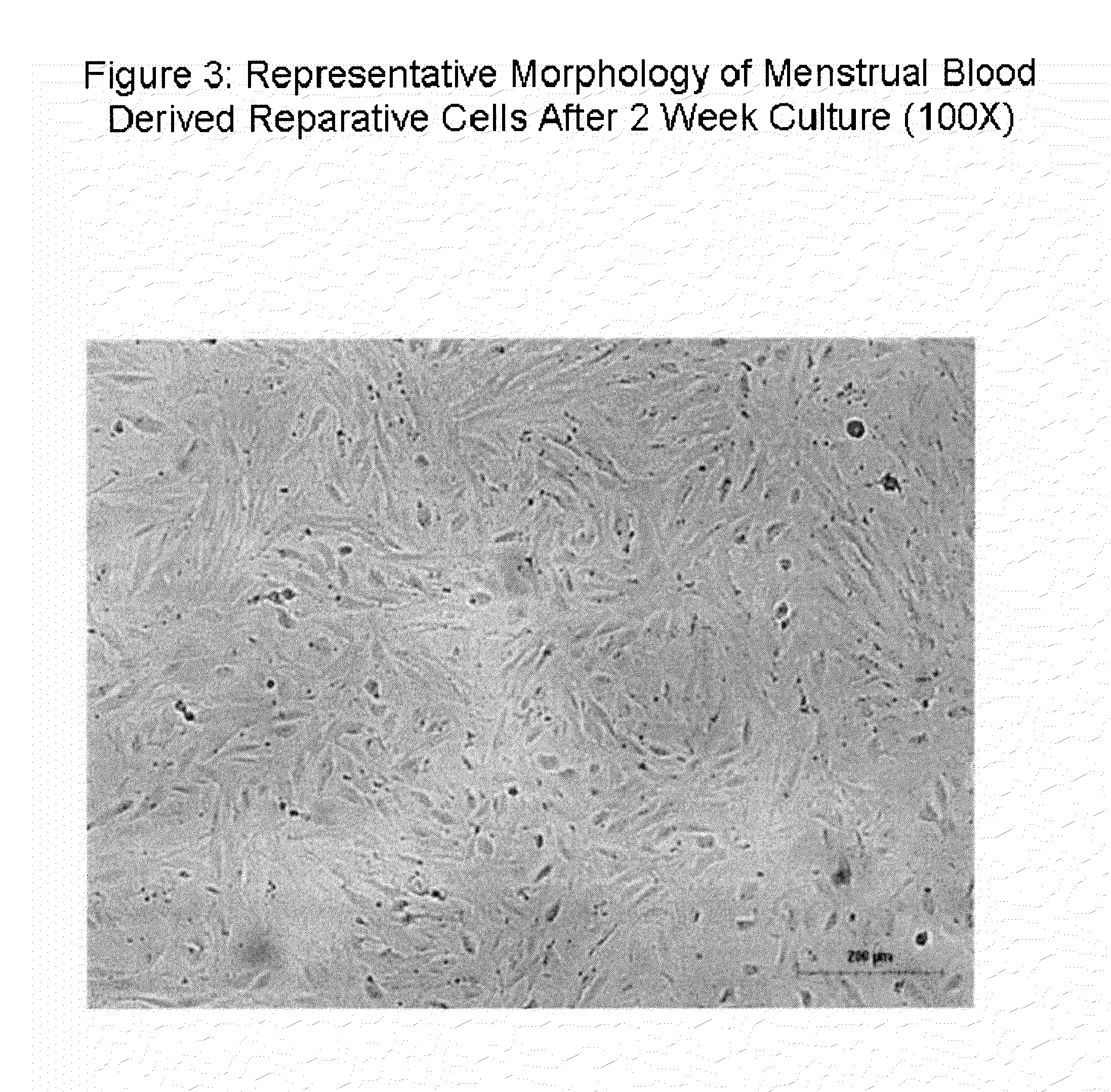
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
464 results about "Conditioned medium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Conditioned medium is spent media harvested from cultured cells. It contains metabolites, growth factors, and extracellular matrix proteins secreted into the medium by the cultured cells.
Methods of making conditioned cell culture medium compositions
InactiveUS6372494B1Eliminate wrinklesEliminate frown lineCosmetic preparationsPeptide/protein ingredientsReserve CellCell culture media
Novel products comprising conditioned cell culture medium compositions and methods of use are described. The conditioned cell medium compositions of the invention may be comprised of any known defined or undefined medium and may be conditioned using any eukaryotic cell type. The medium may be conditioned by stromal cells, parenchymal cells, mesenchymal stem cells, liver reserve cells, neural stem cells, pancreatic stem cells and / or embryonic stem cells. Additionally, the cells may be genetically modified. A three-dimensional tissue construct is preferred. Once the cell medium of the invention is conditioned, it may be used in any state. Physical embodiments of the conditioned medium include, but are not limited to, liquid or solid, frozen, lyophilized or dried into a powder. Additionally, the medium is formulated with a pharmaceutically acceptable carrier as a vehicle for internal administration, applied directly to a food item or product, formulated with a salve or ointment for topical applications, or, for example, made into or added to surgical glue to accelerate healing of sutures following invasive procedures. Also, the medium may be further processed to concentrate or reduce one or more factors or components contained within the medium.
Owner:ALLERGAN INC
Defined media for stem cell culture
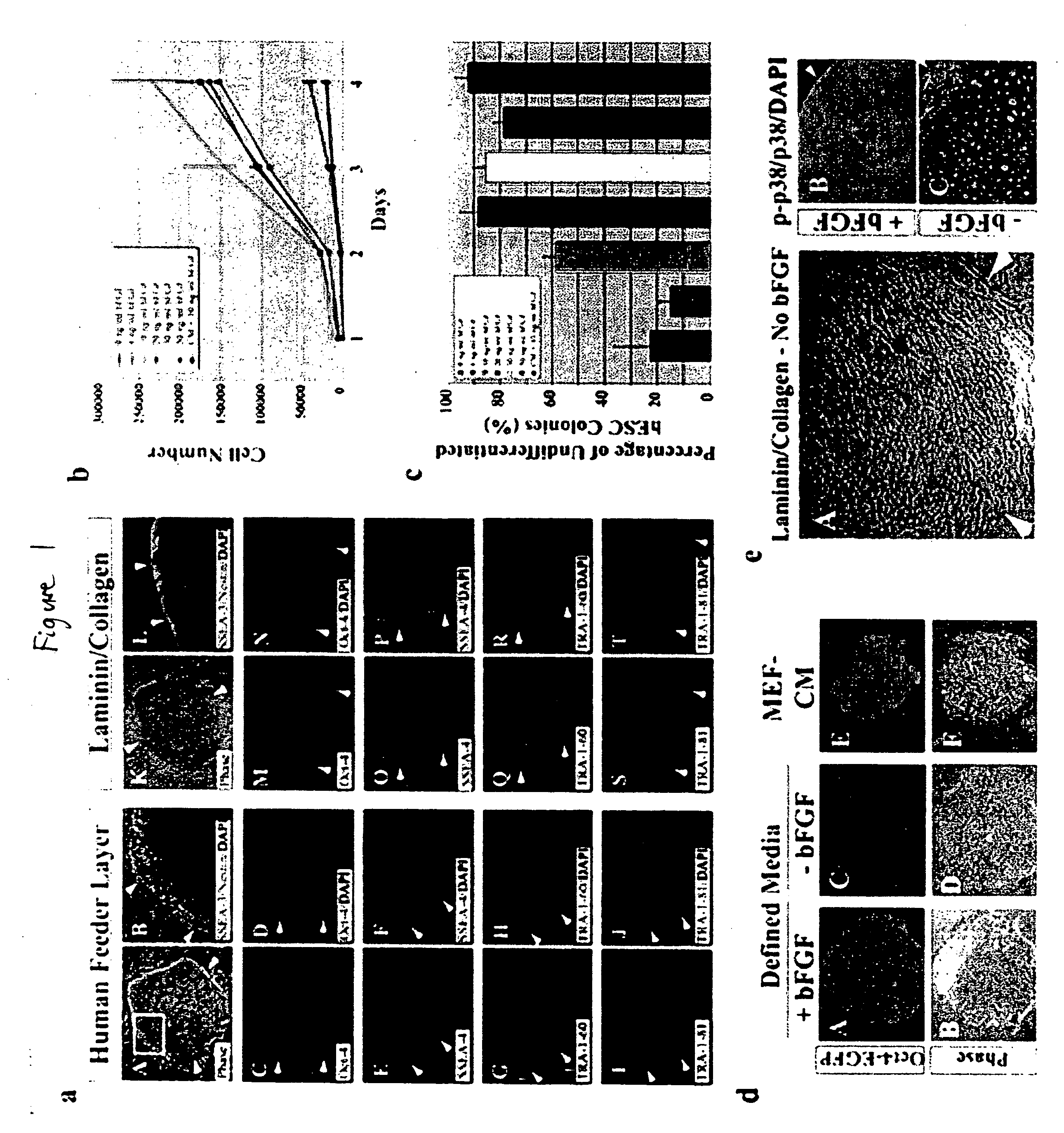
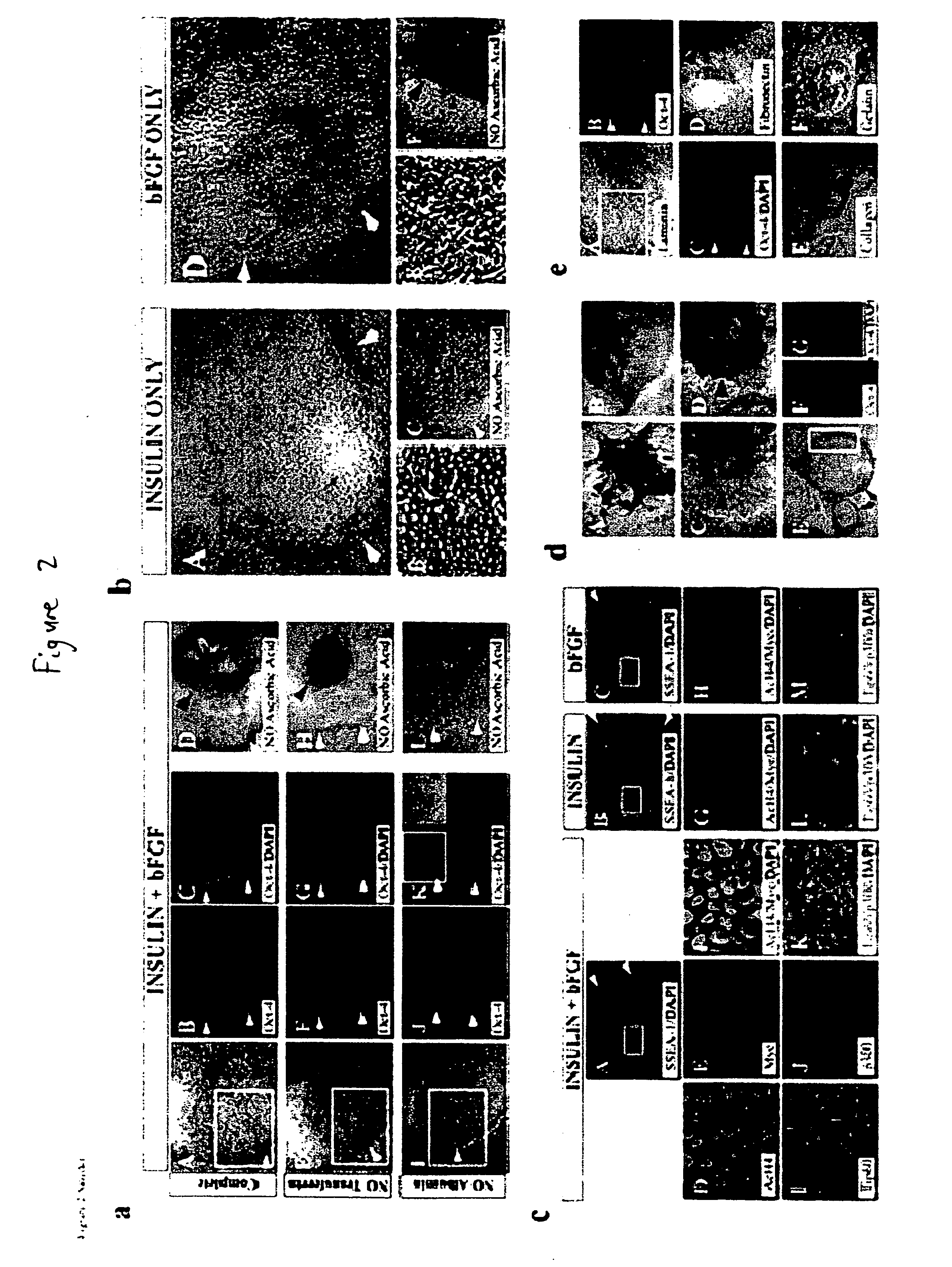
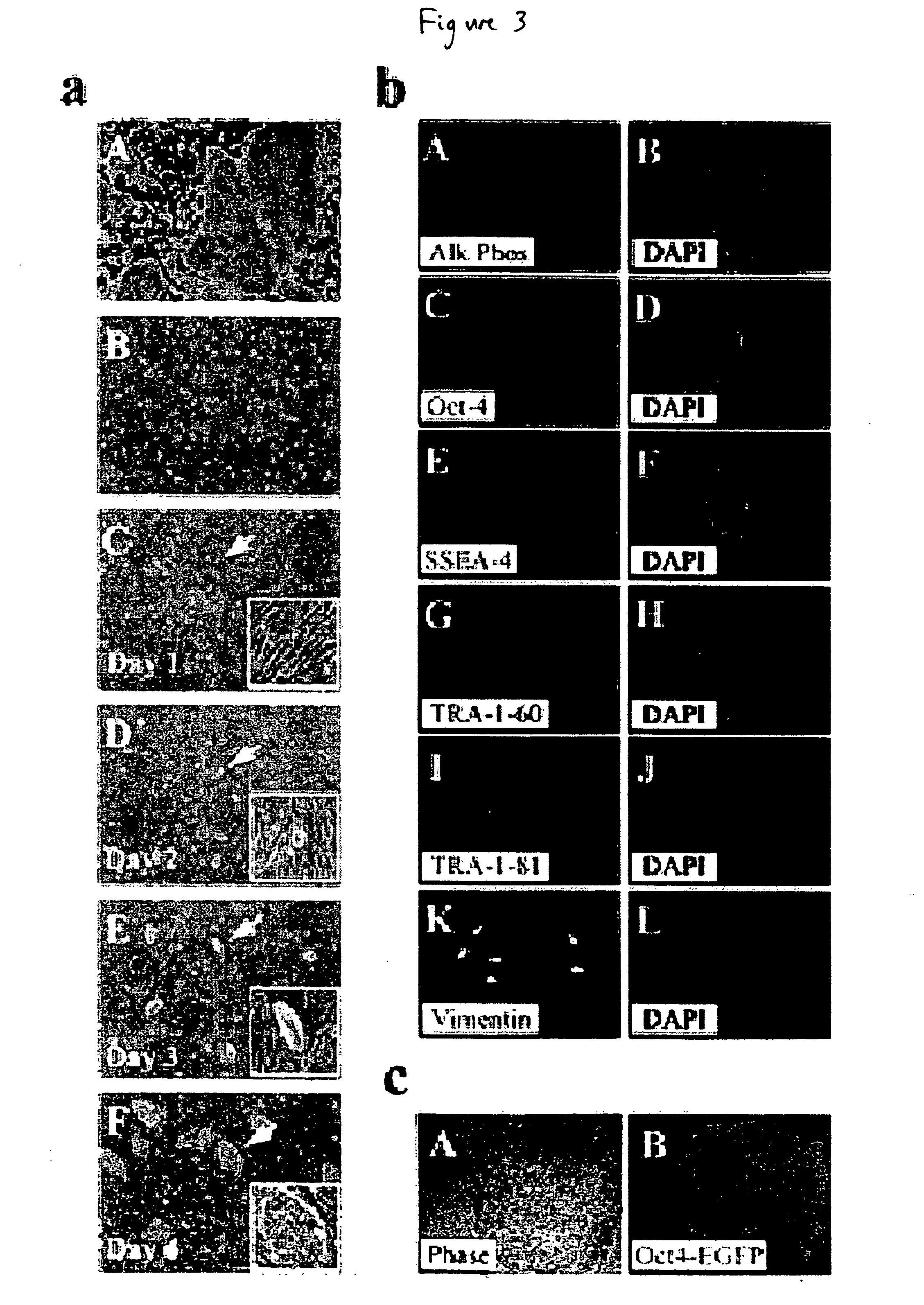
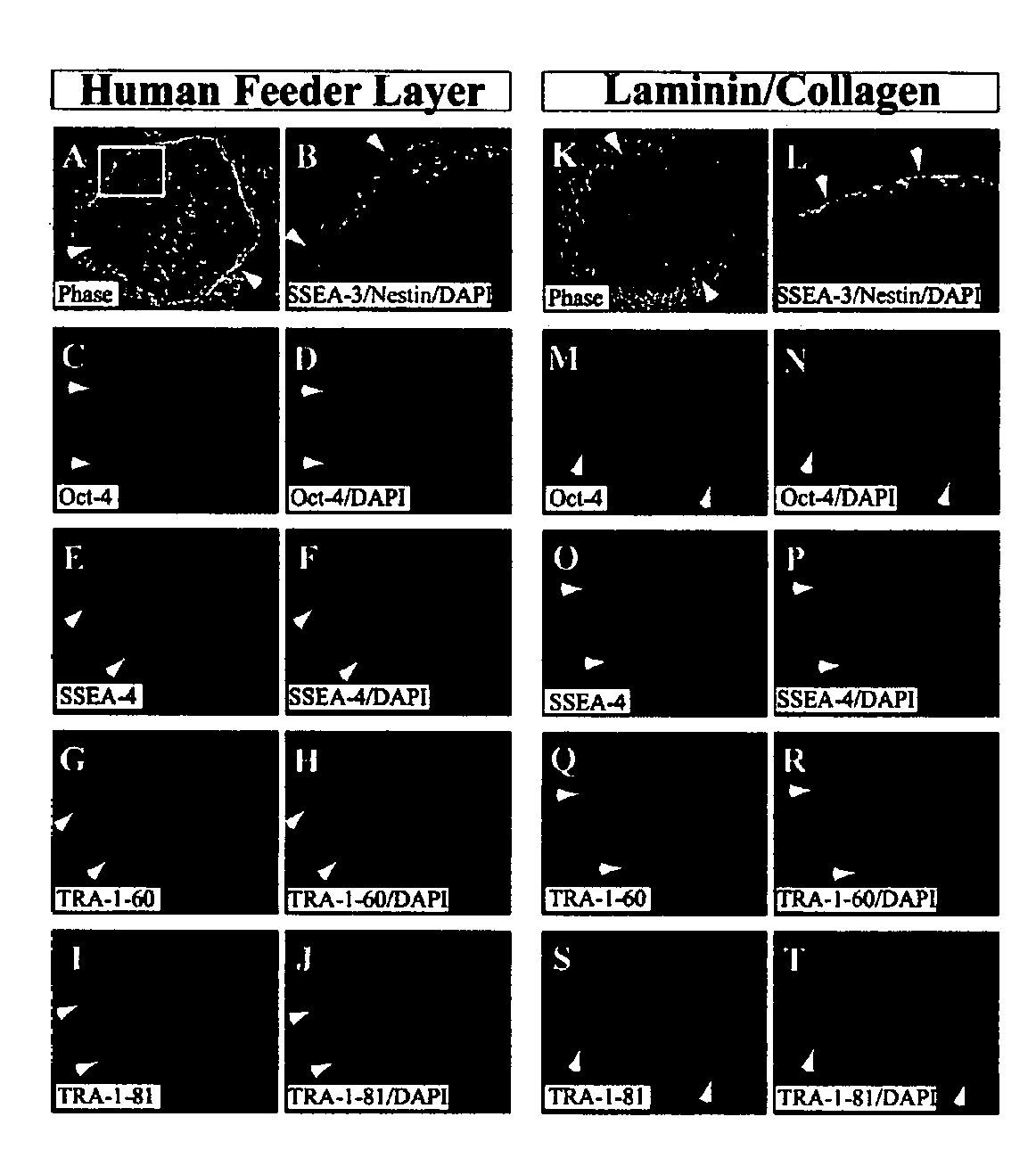
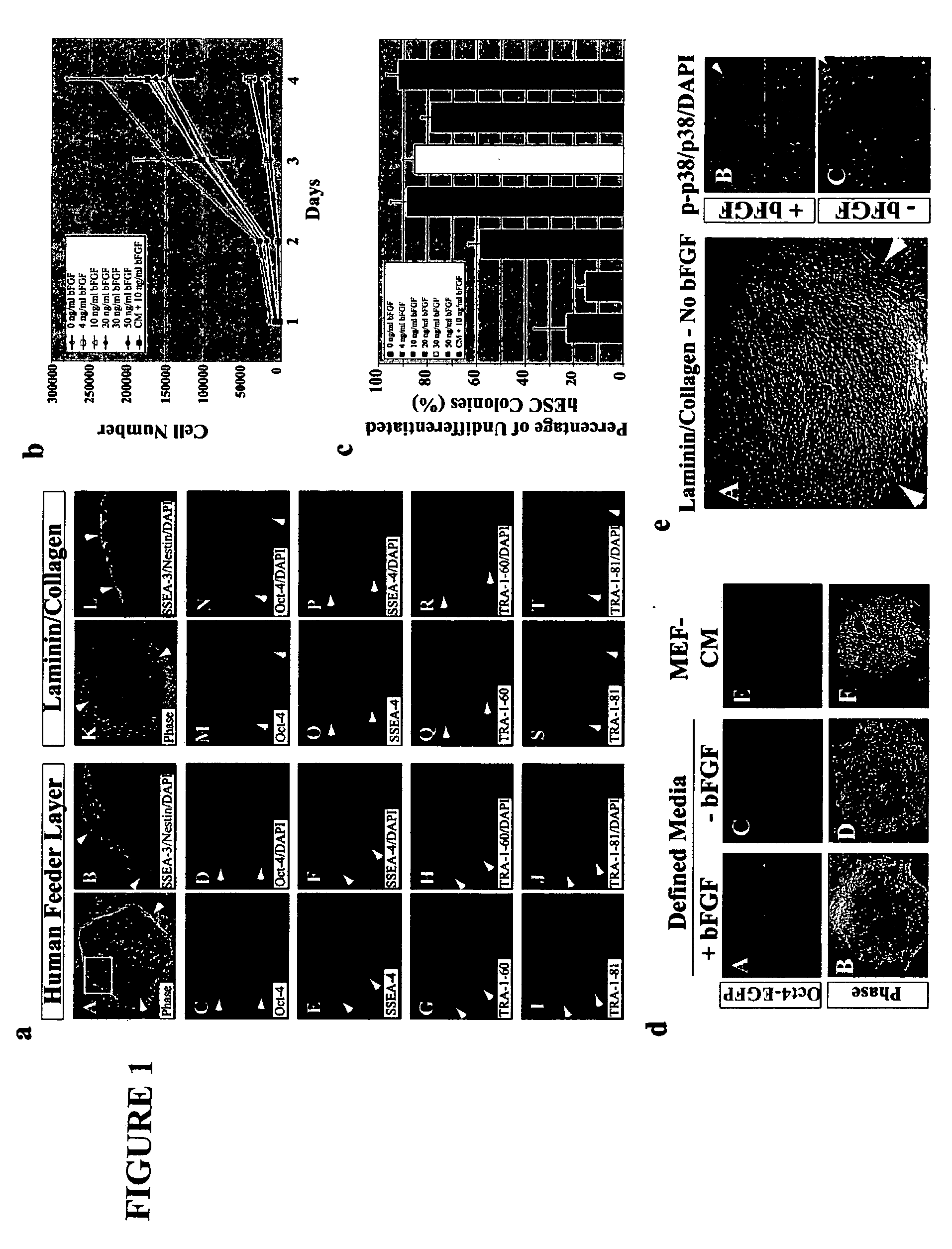
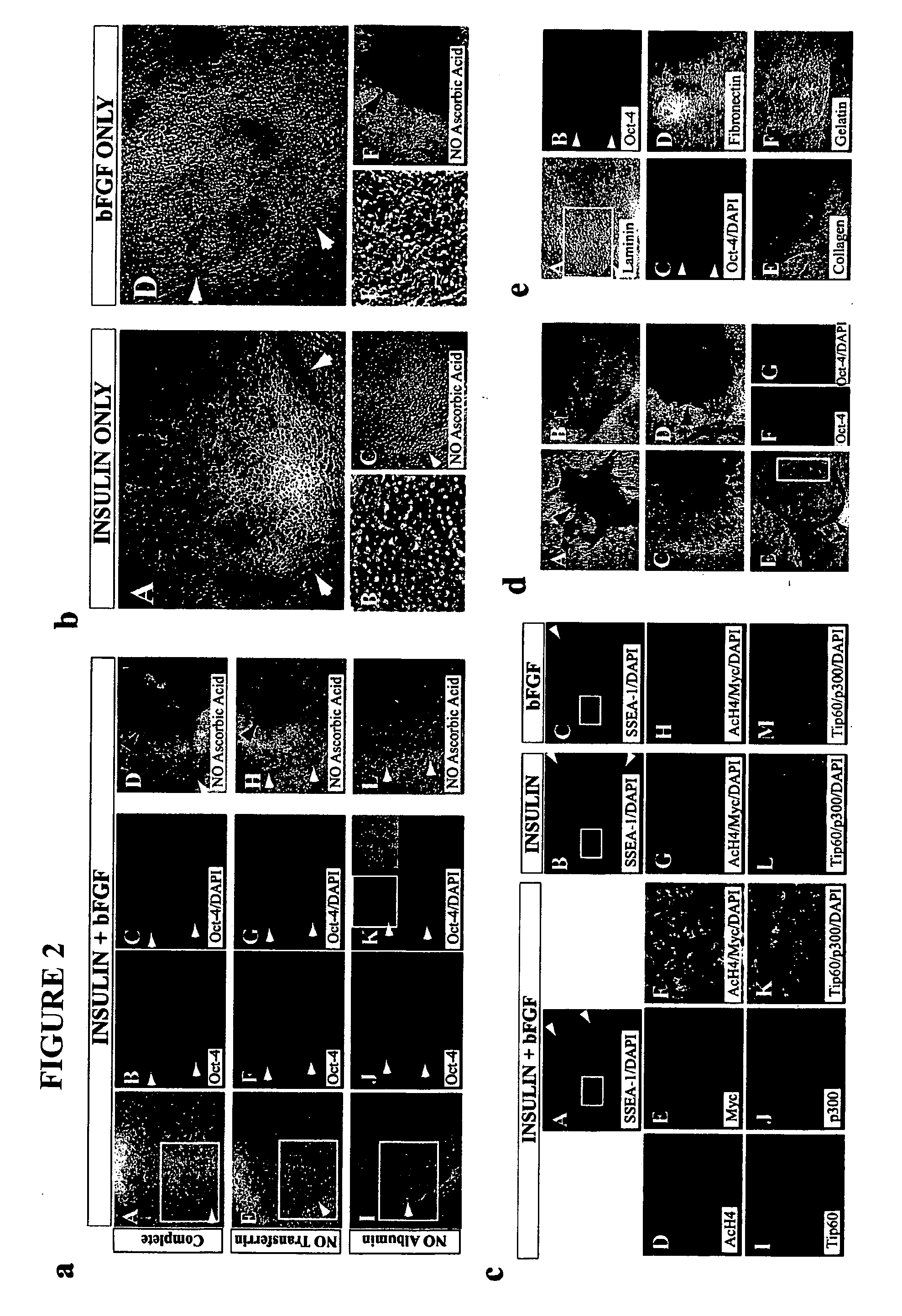
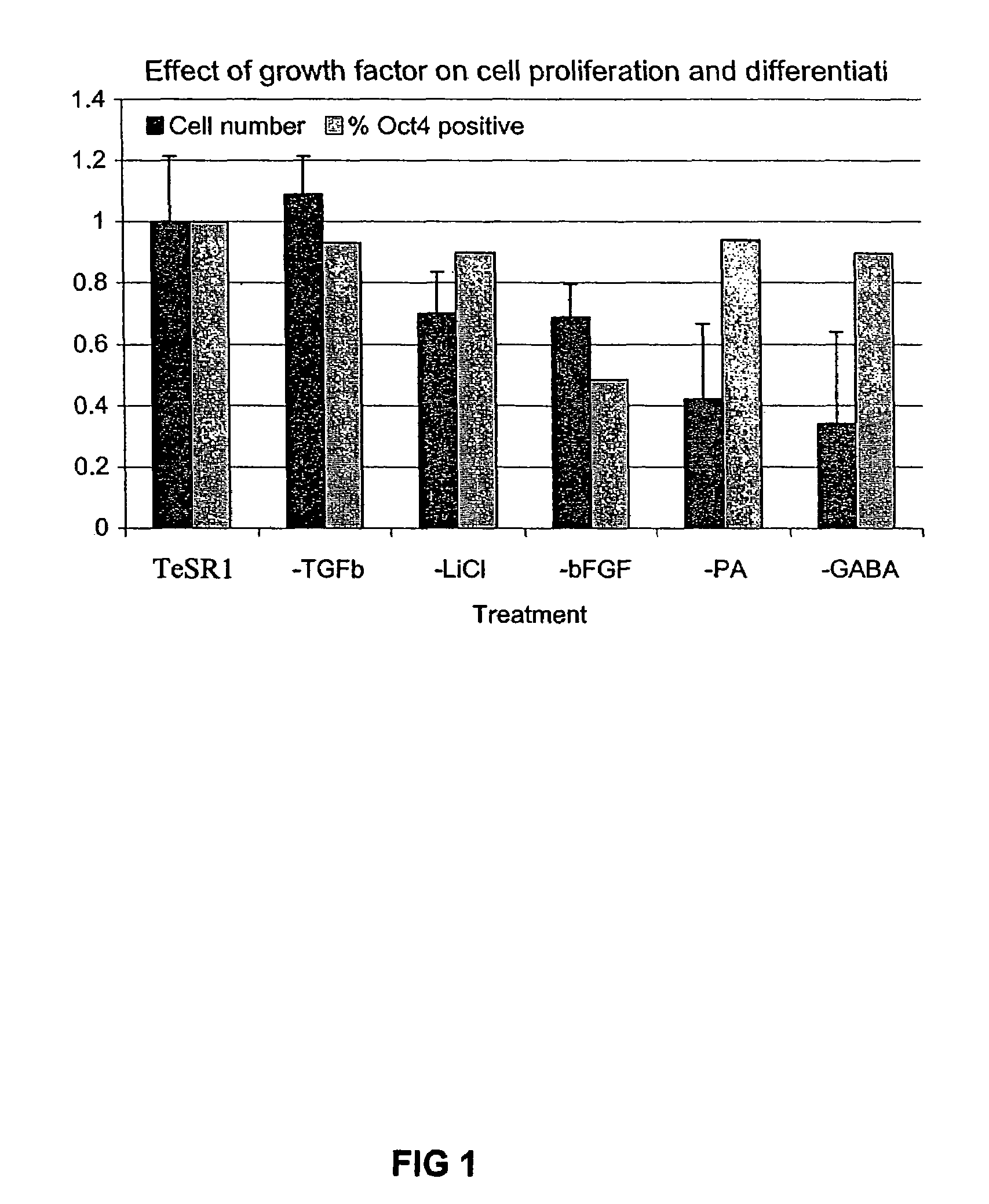
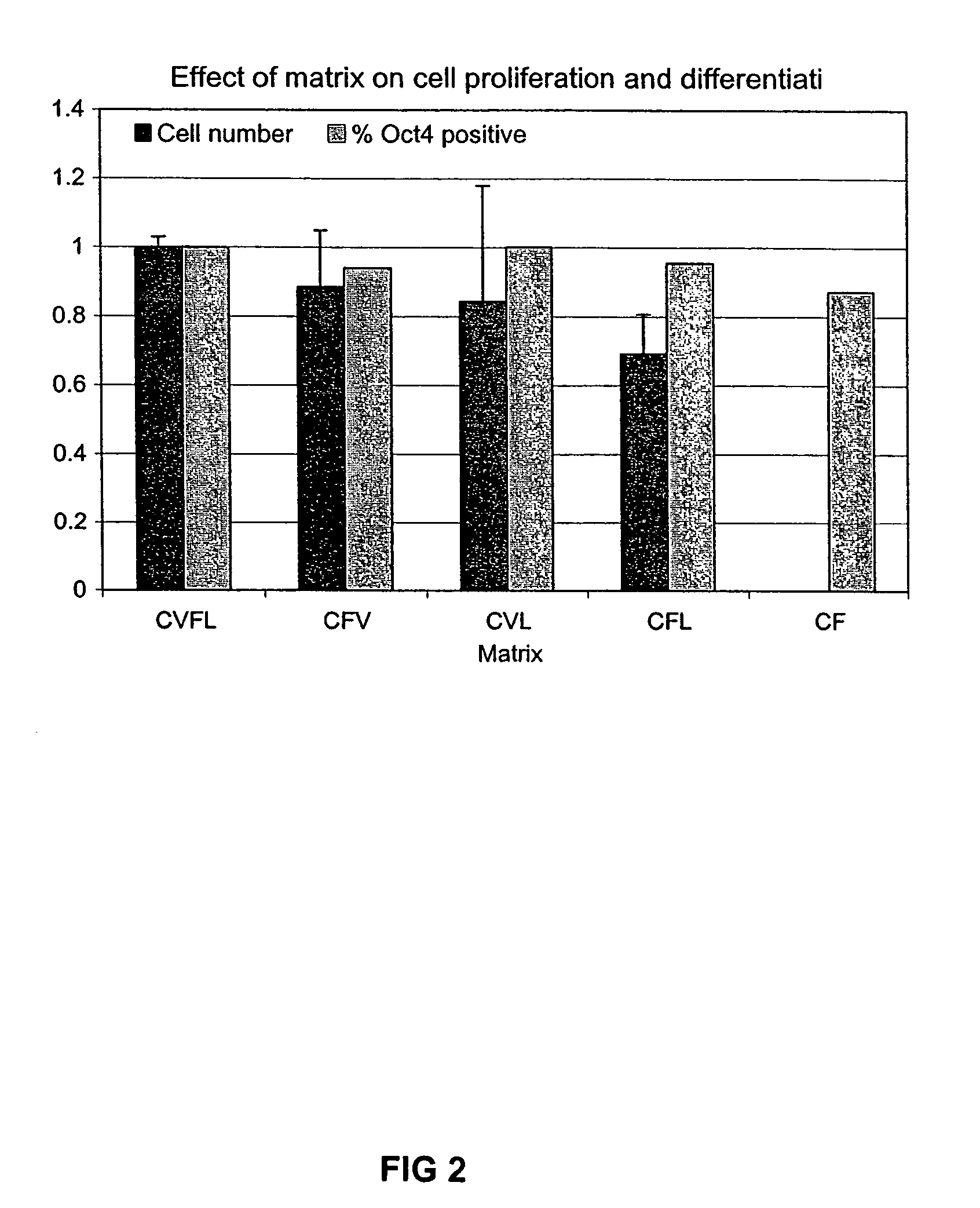
Stem cells, including mammalian, and particularly primate primordial stem cells (pPSCs) such as human embryonic stem cells (hESCs), hold great promise for restoring cell, tissue, and organ function. However, cultivation of stem cells, particularly undifferentiated hESCs, in serum-free, feeder-free, and conditioned-medium-free conditions remains crucial for large-scale, uniform production of pluripotent cells for cell-based therapies, as well as for controlling conditions for efficiently directing their lineage-specific differentiation. This instant invention is based on the discovery of the formulation of minimal essential components necessary for maintaining the long-term growth of pPSCs, particularly undifferentiated hESCs. Basic fibroblast growth factor (bFGF), insulin, ascorbic acid, and laminin were identified to be both sufficient and necessary for maintaining hESCs in a healthy self-renewing undifferentiated state capable of both prolonged propagation and then directed differentiation. Having discerned these minimal molecular requirements, conditions that would permit the substitution of poorly-characterized and unspecified biological additives and substrates were derived and optimized with entirely defined constituents, providing a “biologics”-free (i.e., animal-, feeder-, serum-, and conditioned-medium-free) system for the efficient long-term cultivation of pPSCs, particularly pluripotent hESCs. Such culture systems allow the derivation and large-scale production of stem cells such as pPSCs, particularly pluripotent hESCs, in optimal yet well-defined biologics-free culture conditions from which they can be efficiently directed towards a lineage-specific differentiated fate in vitro, and thus are important, for instance, in connection with clinical applications based on stem cell therapy and in drug discovery processes.
Owner:THE BURNHAM INST
Defined media for pluripotent stem cell culture
Stem cells, including mammalian, and particularly primate primordial stem cells (pPSCs) such as human embryonic stem cells (hESCs), hold great promise for restoring cell, tissue, and organ function. However, cultivation of stem cells, particularly undifferentiated hESCs, in serum-free, feeder-free, and conditioned-medium-free conditions remains crucial for large-scale, uniform production of pluripotent cells for cell-based therapies, as well as for controlling conditions for efficiently directing their lineage-specific differentiation. This instant invention is based on the discovery of the formulation of minimal essential components necessary for maintaining the long-term growth of pPSCs, particularly undifferentiated hESCs. Basic fibroblast growth factor (bFGF), insulin, ascorbic acid, and laminin were identified to be both sufficient and necessary for maintaining hESCs in a healthy self-renewing undifferentiated state capable of both prolonged propagation and then directed differentiation. Having discerned these minimal molecular requirements, conditions that would permit the substitution of poorly-characterized and unspecified biological additives and substrates were derived and optimized with entirely defined constituents, providing a “biologics”-free (i.e., animal-, feeder-, serum-, and conditioned-medium-free) system for the efficient long-term cultivation of pPSCs, particularly pluripotent hESCs. Such culture systems allow the derivation and large-scale production of stem cells such as pPSCs, particularly pluripotent hESCs, in optimal yet well-defined biologics-free culture conditions from which they can be efficiently directed towards a lineage-specific differentiated fate in vitro, and thus are important, for instance, in connection with clinical applications based on stem cell therapy and in drug discovery processes.
Owner:THE BURNHAM INST
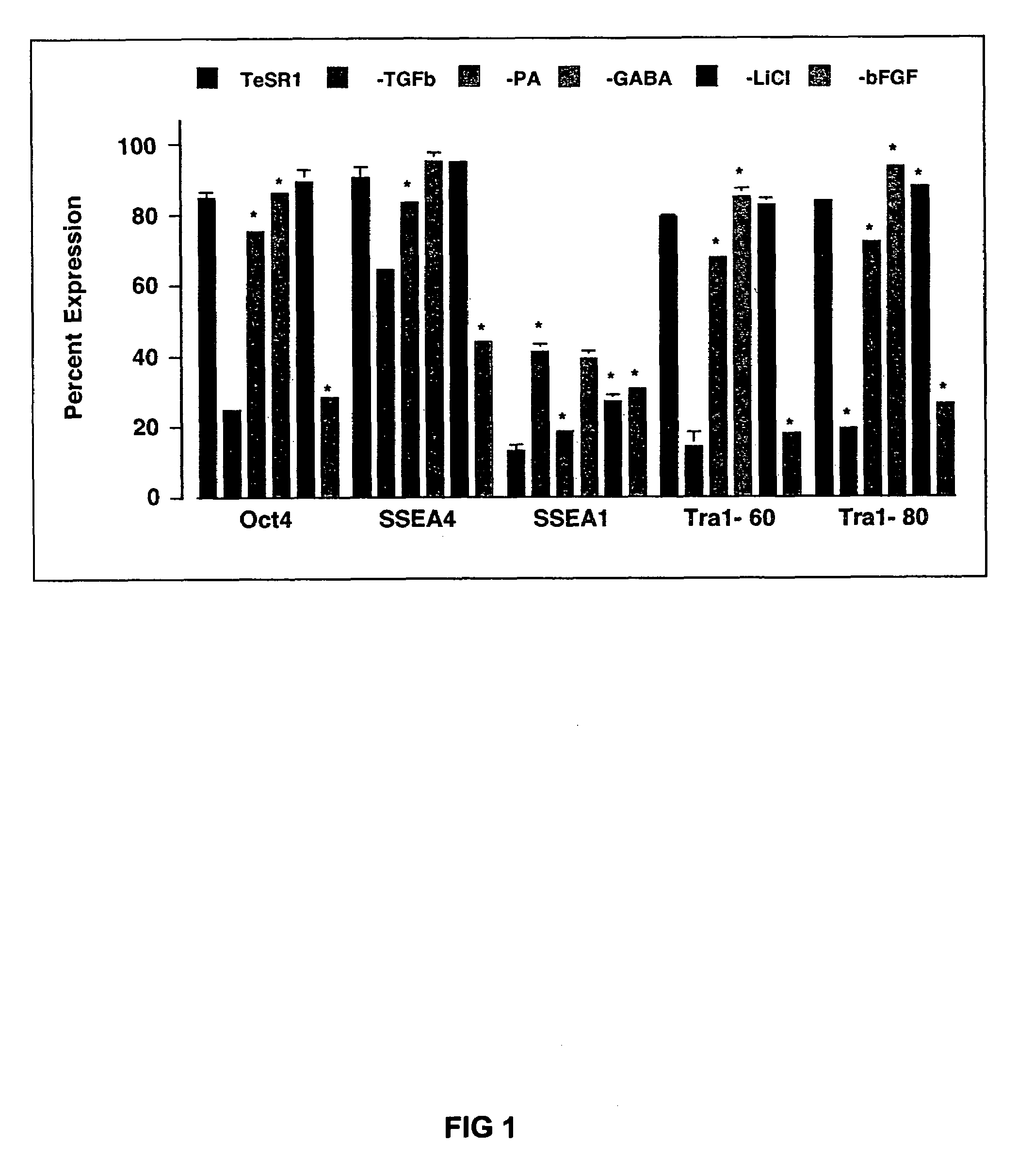
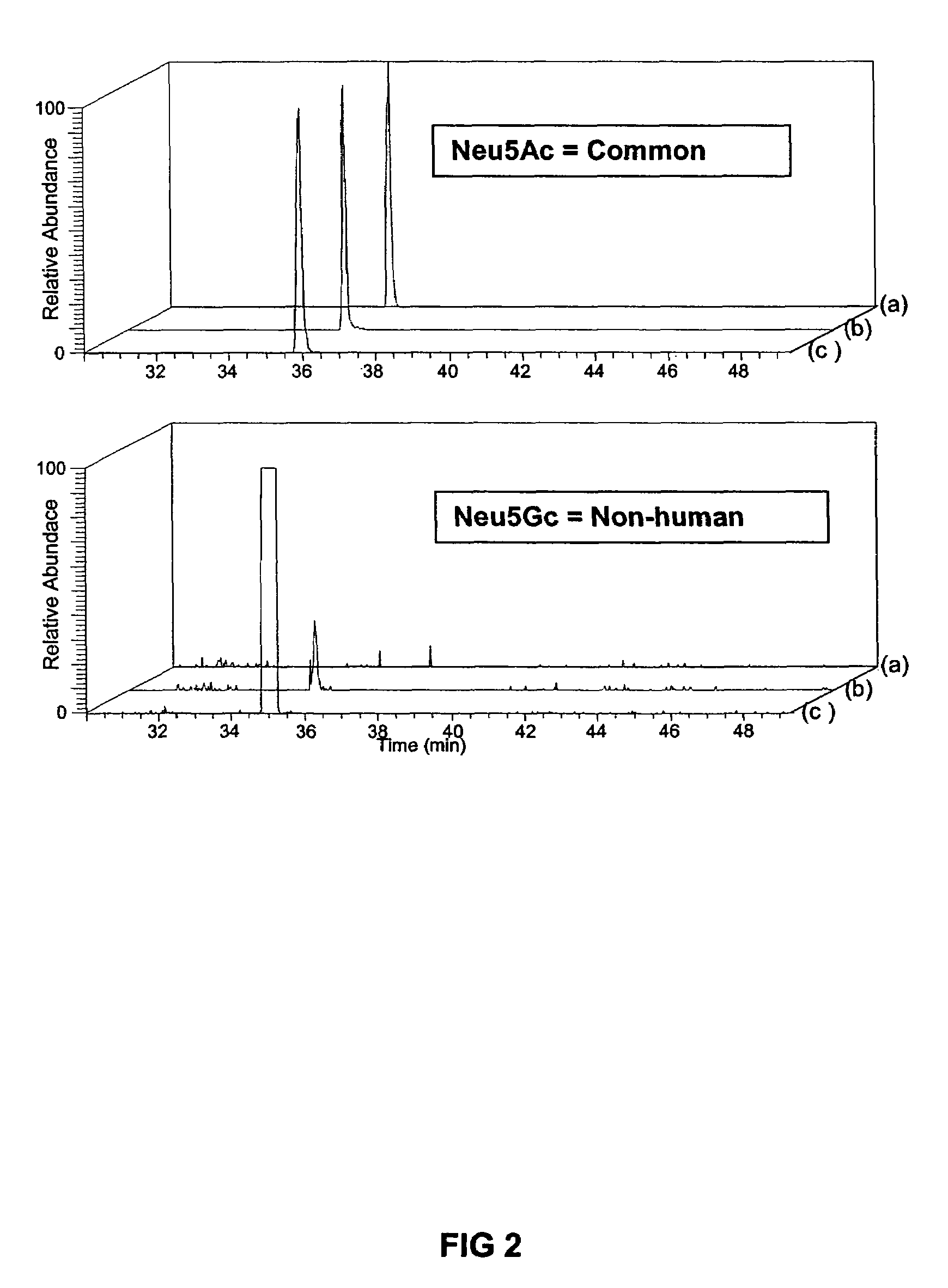
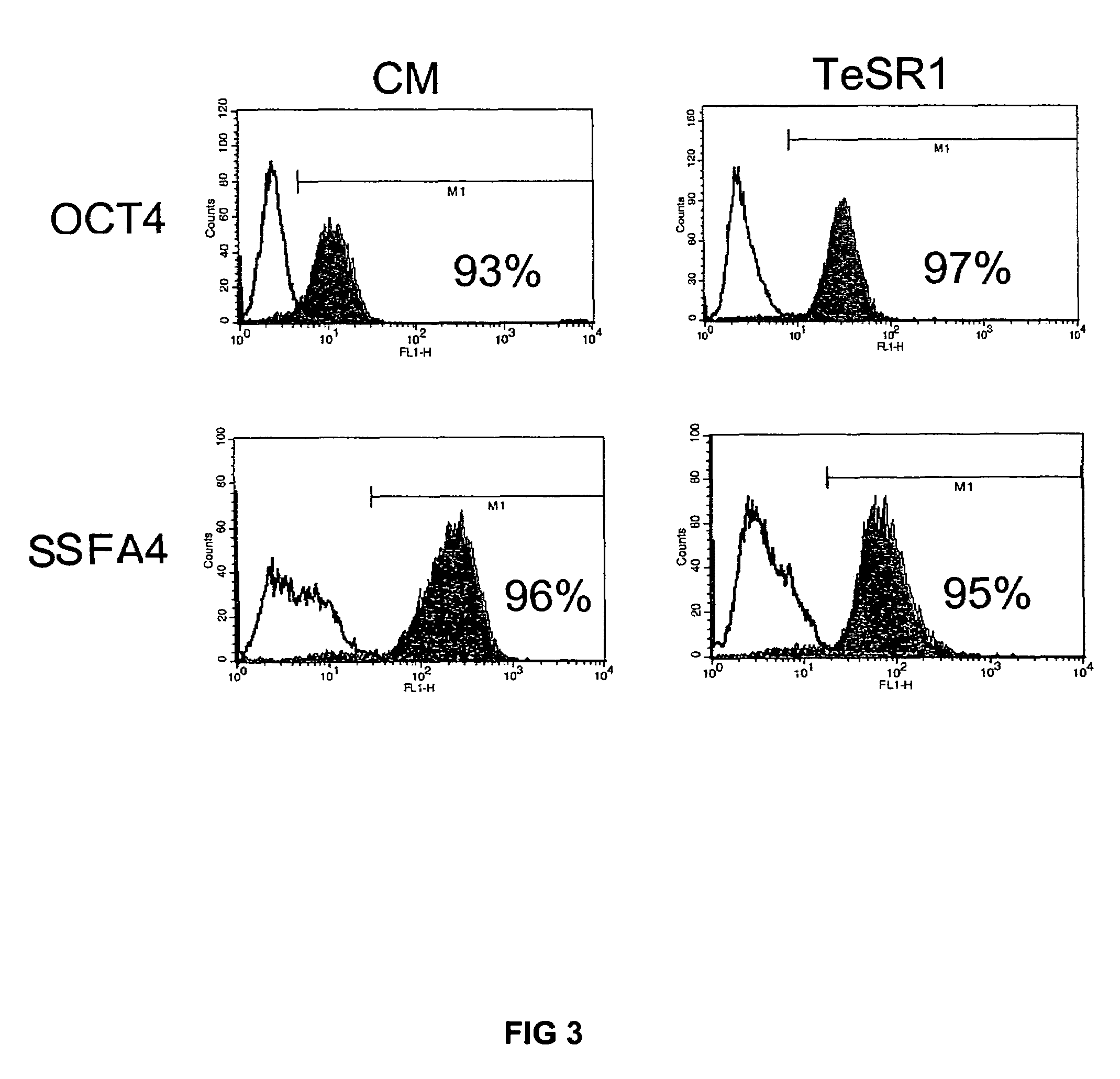
Culturing human embryonic stem cells in medium containing pipecholic acid and gamma amino butyric acid
Previous methods for culturing human embryonic stem cells have required either fibroblast feeder cells or a medium which has been exposed to fibroblast feeder cells in order to maintain the stem cells in an undifferentiated state. It has now been found that if high levels of fibroblast growth factor are used in a medium with gamma amino butyric acid, pipecholic acid, lithium and lipids, the stem cells will remain undifferentiated indefinitely through multiple passages, even without feeder cells or conditioned medium. A humanized matrix of human proteins can be used as a basement matrix to culture the cells. New lines of human embryonic stem cells made using these culture conditions, the medium and the matrix, will never have been exposed to animal cells, animal products, feeder cells or conditioned medium.
Owner:WISCONSIN ALUMNI RES FOUND
Medium containing pipecholic acid and gamma amino butyric acid and culture of embryonic stem cells
Previous methods for culturing human embryonic stem cells have required either fibroblast feeder cells or a medium which has been exposed to fibroblast feeder cells in order to maintain the stem cells in an undifferentiated state. It has now been found that if high levels of fibroblast growth factor, gamma amino butyric acid, pipecholic acid, lithium and transforming growth factor beta are added to the medium in which the stem cells are cultured, the stem cells will remain undifferentiated indefinitely through multiple passages, even without feeder cells or conditioned medium.
Owner:WISCONSIN ALUMNI RES FOUND
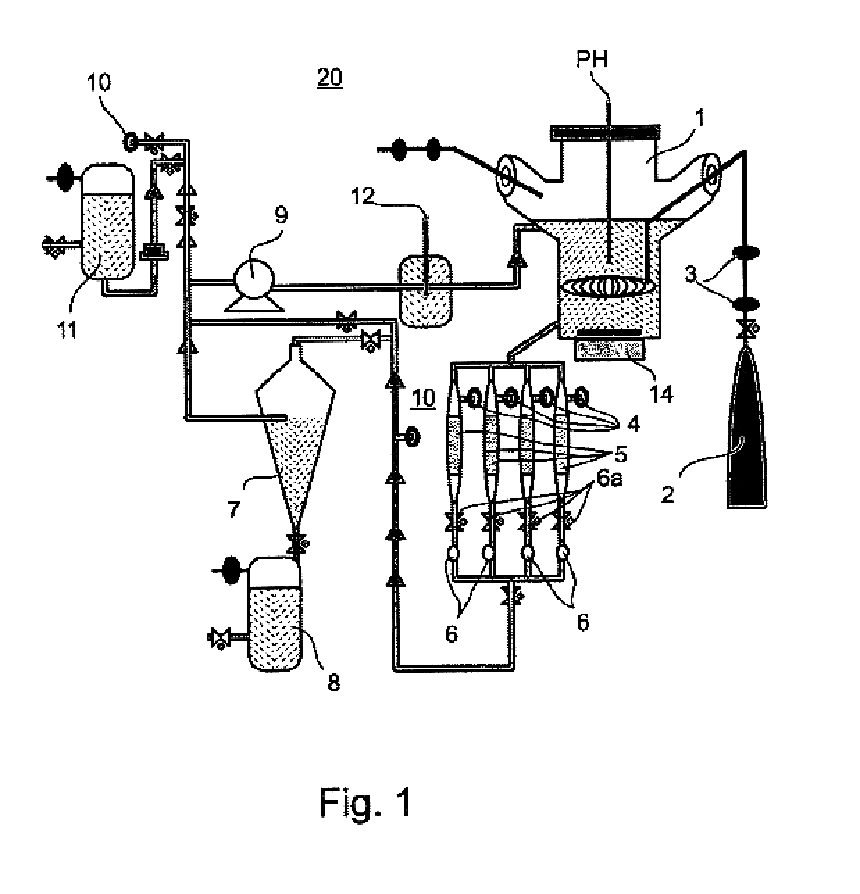
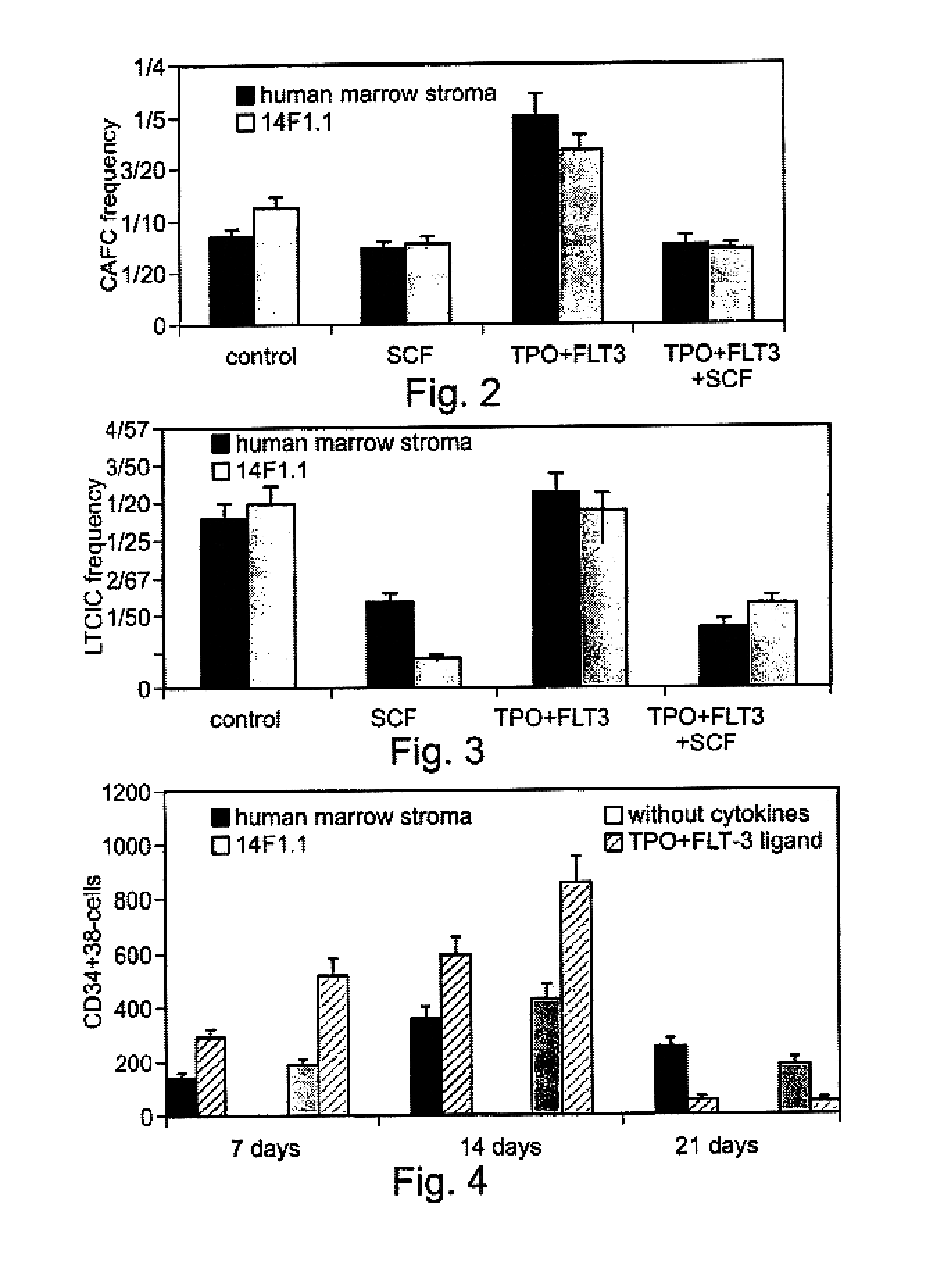
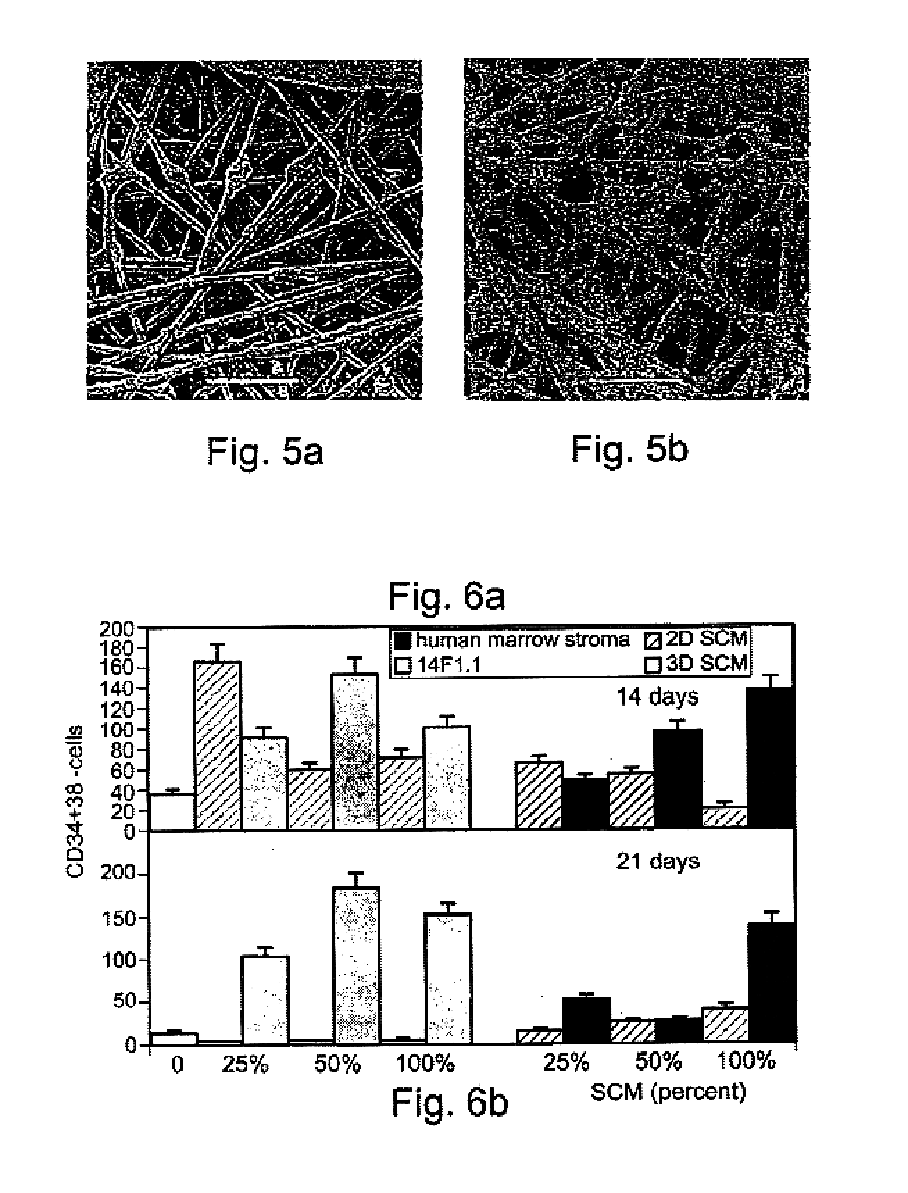
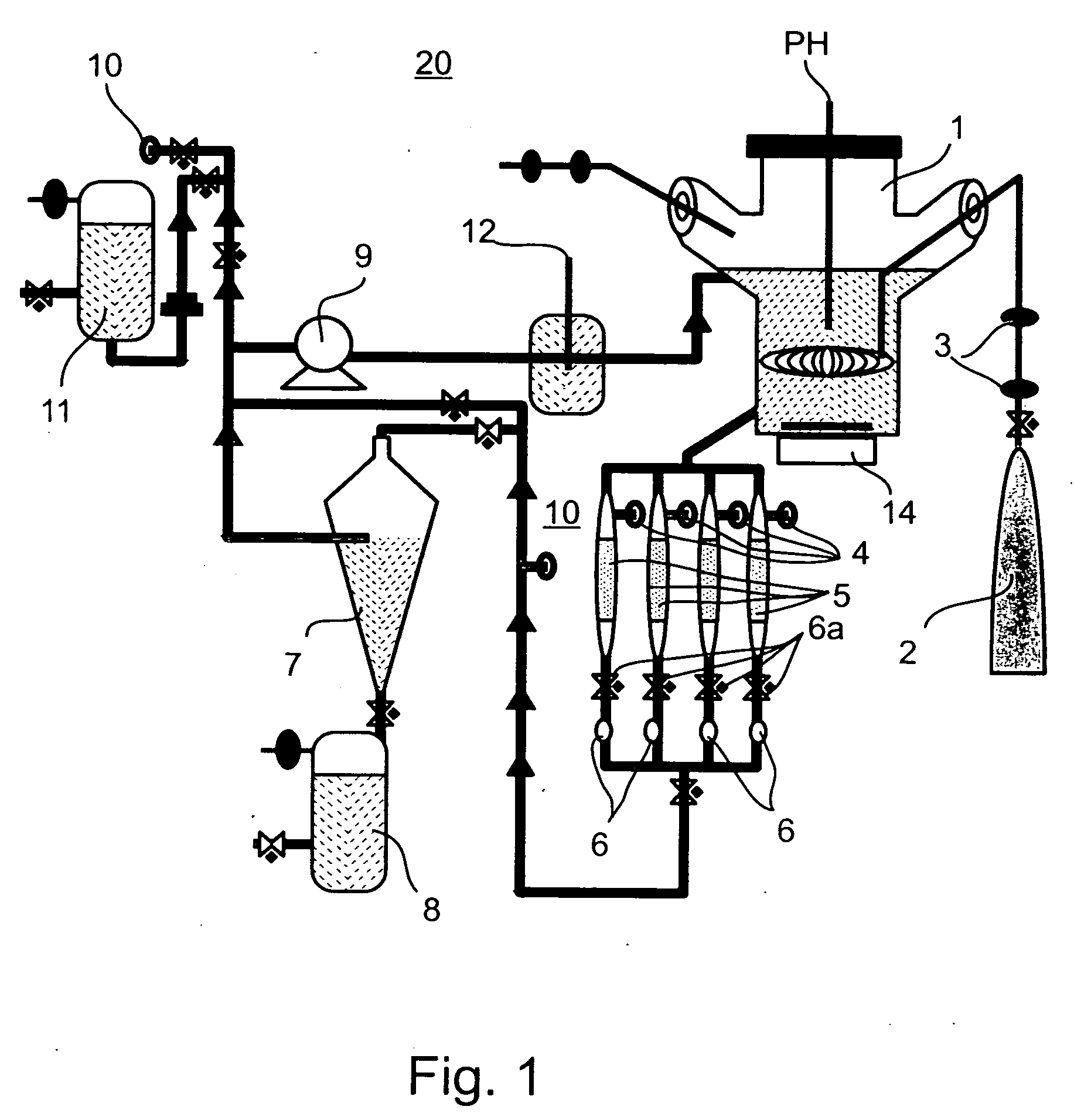
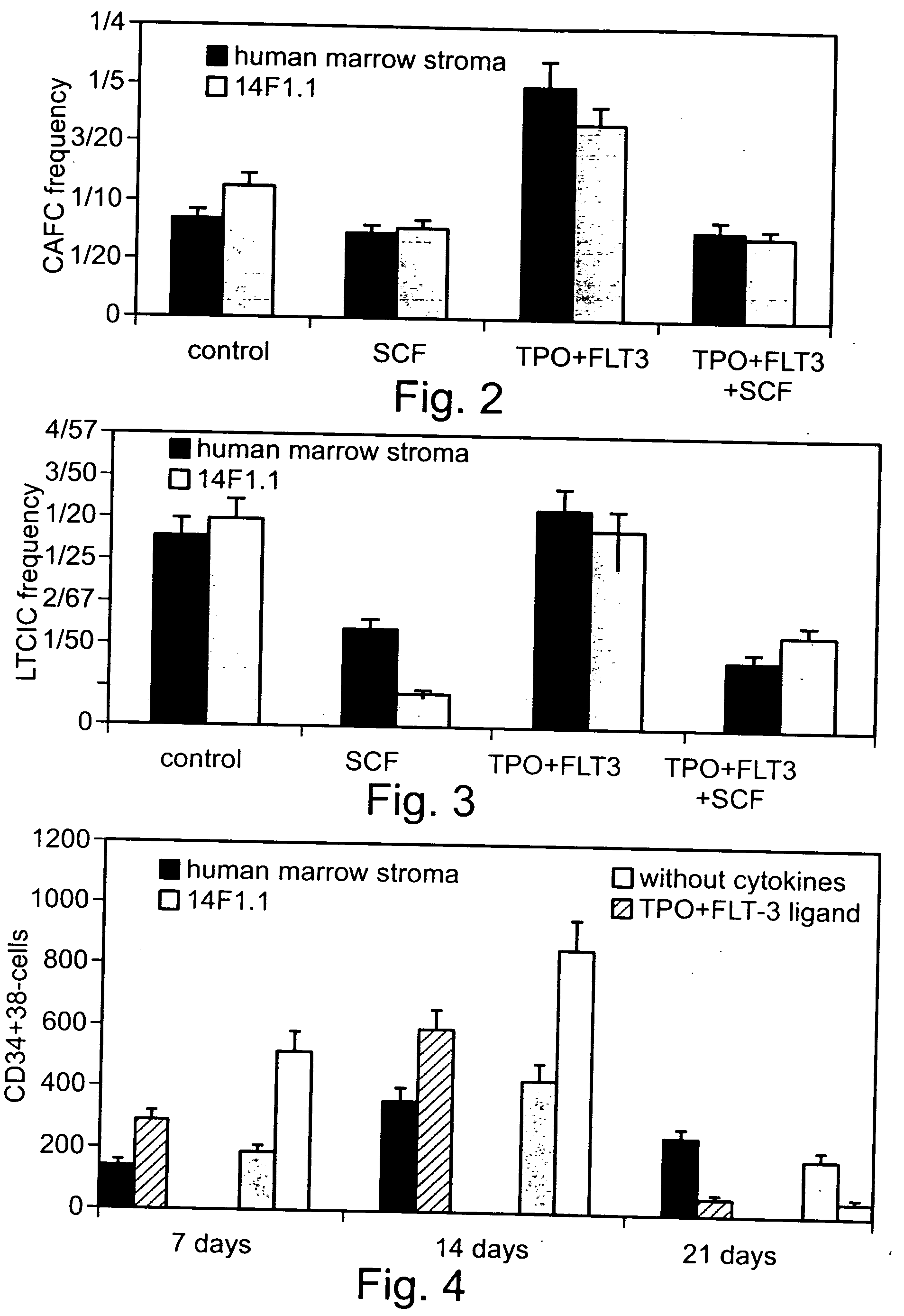
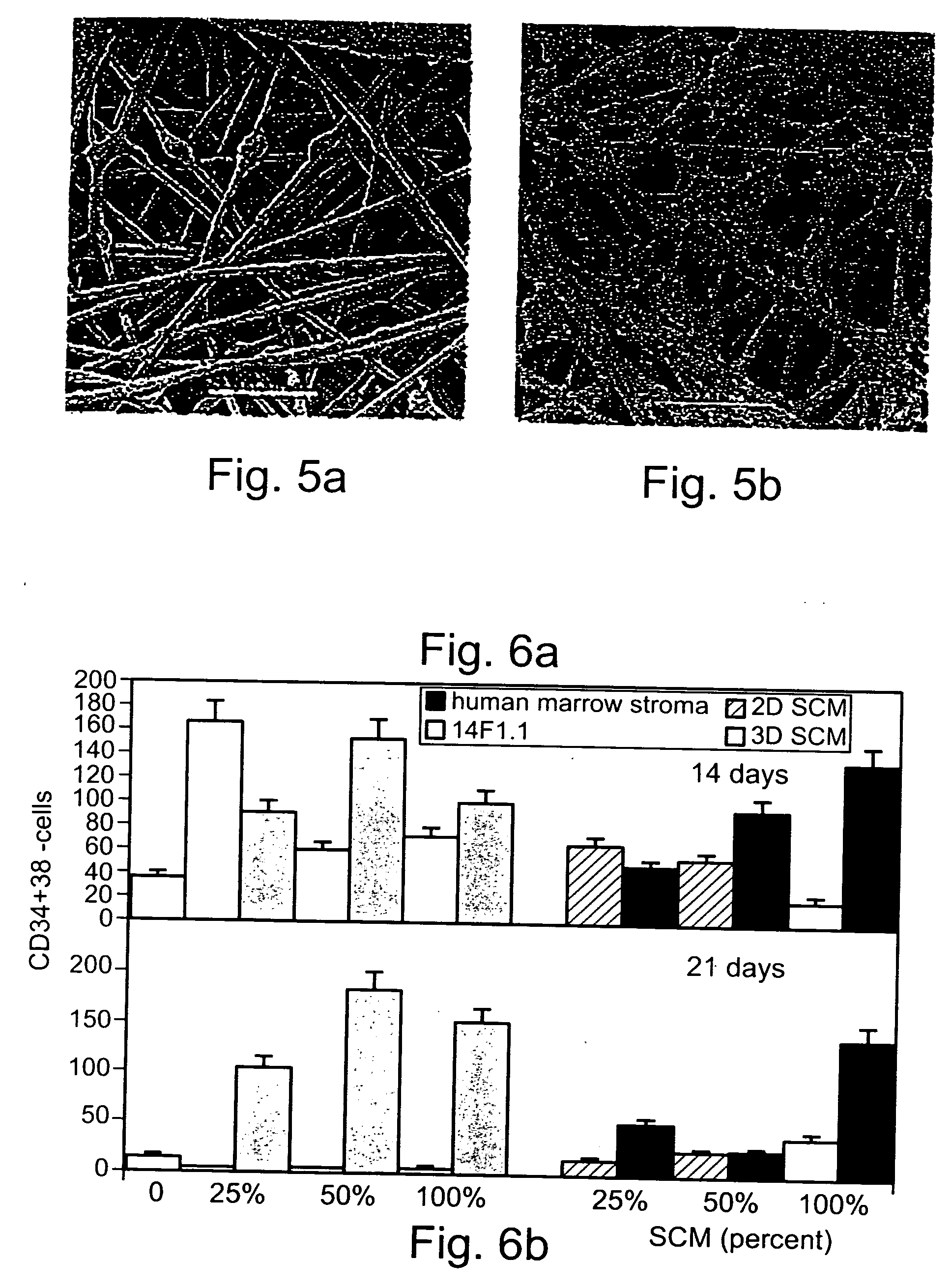
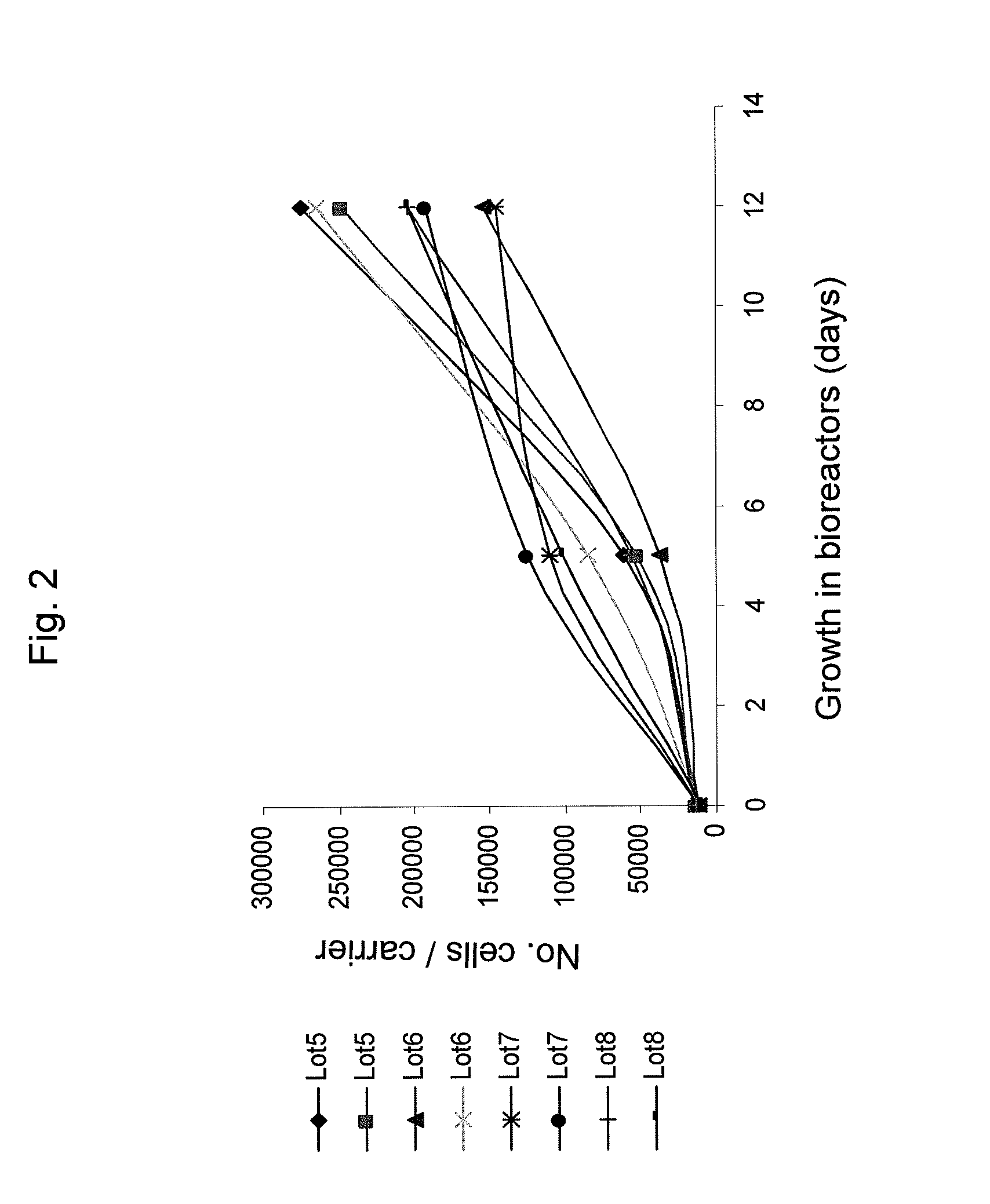
Method of producing undifferentiated hemopoietic stem cells using a stationary phase plug-flow bioreactor
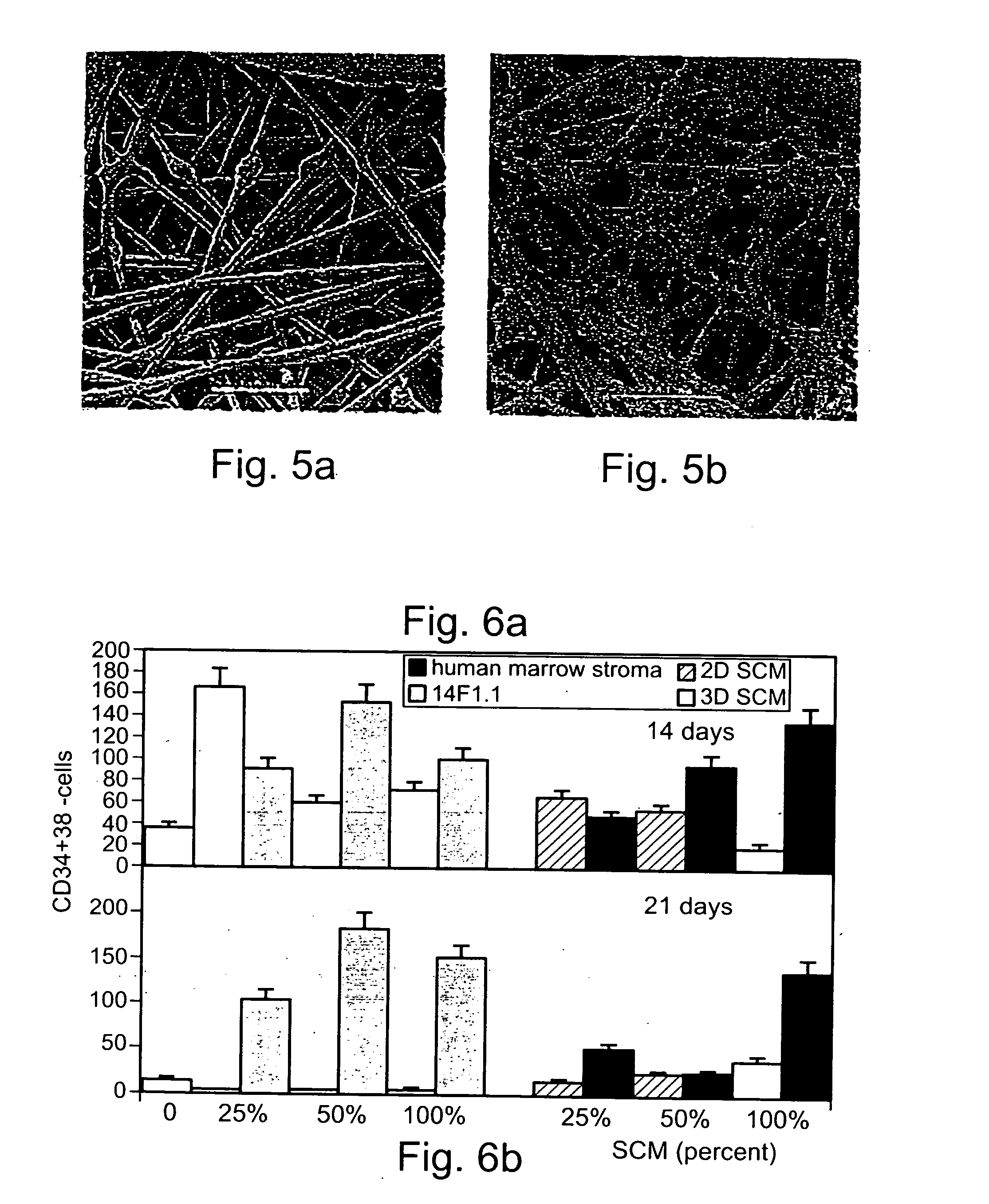
A method of expanding / maintaining undifferentiated hemopoietic stem cells or progenitor cells by obtaining undifferentiated hemopoietic stem cells or progenitor cells; and either seeding the undifferentiated hemopoietic stem cells or progenitor cells into a stationary phase plug-flow bioreactor in which a three-dimensional stromal cell culture has been pre-established on a substrate in the form of a sheet, the substrate including a non-woven fibrous matrix forming a physiologically acceptable three-dimensional network of fibers, thereby expanding / maintaining undifferentiated hemopoietic stem cells or progenitor cells, or culturing the undifferentiated hemopoietic stem cells or progenitor cells in conditioned medium obtained from such a reactor.
Owner:PLURISTEAM LTD +1
Endometrial stem cells and methods of making and using same
InactiveUS20090053182A1Rapid rate of cellular divisionBiocidePeptide/protein ingredientsCell lineageEndometrium
The invention provides pluripotent stem cells and methods for making and using pluripotent stem cells. Pluripotent stem cells, among other things, can differentiate into various cell lineages in vitro, ex vivo and in vivo. Pluripotent stem cells, among other things, can also be used to produce conditioned medium.
Owner:MEDISTEM LAB
Methods for the culture of human embryonic stem cells on human feeder cells
InactiveUS7432104B2Artificial cell constructsMammal material medical ingredientsBone Marrow Stromal CellCell culture media
Methods and cell culture medium for the generation of human pluripotent embryonic stem cells are disclosed. Human embryonic stem cells are cultured with human granulosa feeder cells, muscle cells, Fallopian ductal epithelial cells, bone marrow stromal cells, and skin fibroblasts and the embryonic stem cells maintain their pluripotent phenotype. The human pluripotent embryonic stem cells can be cultured without feeder cells, and in the presence of supplemental growth factors. The human pluripotent embryonic stem cells can be alternatively cultured with conditioned medium obtained from a cell culture capable of maintaining human embryonic stem cells in a pluripotent state, wherein the cell culture is a human granulosa cell culture.
Owner:VIACYTE INC
Alternative compositions and methods for the culture of stem cells
InactiveUS20050037488A1Artificial cell constructsMammal material medical ingredientsBone Marrow Stromal CellCell culture media
Methods and cell culture medium for the generation of human pluripotent embryonic stem cells are disclosed. Human embryonic stem cells are cultured with human granulosa feeder cells, muscle cells, Fallopian ductal epithelial cells, bone marrow stromal cells, and skin fibroblasts and the embryonic stem cells maintain their pluripotent phenotype. The human pluripotent embryonic stem cells can be cultured without feeder cells, and in the presence of supplemental growth factors. The human pluripotent embryonic stem cells can be alternatively cultured with conditioned medium obtained from a cell culture capable of maintaining human embryonic stem cells in a pluripotent state, wherein the cell culture is a human granulosa cell culture.
Owner:VIACYTE INC
Adipose-derived stem cells and lattices
InactiveUS20050282275A1Promote growthFacilitate differentiationGenetic material ingredientsMammal material medical ingredientsCell-Extracellular MatrixConnective tissue fiber
The present invention provides adipose-derived stem cells and lattices. In one aspect, the present invention provides a lipo-derived stem cell substantially free of adipocytes and red blood cells and clonal populations of connective tissue stem cells. The invention also provides a method of isolating stem cells from adipose tissues. The cells can be employed, alone or within biologically-compatible compositions, to generate differentiated tissues and structures, both in vivo and in vitro. Additionally, the cells can be expanded and cultured to produce hormones and to provide conditioned culture media for supporting the growth and expansion of other cell populations. In another aspect, the present invention provides a lipo-derived lattice substantially devoid of cells, which includes extracellular matrix material from adipose tissue. The lattice can be used as a substrate to facilitate the growth and differentiation of cells, whether in vivo or in vitro, into anlagen or even mature tissues or structures.
Owner:UNIVERSITY OF PITTSBURGH +1
Adipose-derived stem cells and lattices
InactiveUS7470537B2Genetic material ingredientsMammal material medical ingredientsCell-Extracellular MatrixECM Protein
The present invention provides adipose-derived stem cells (ADSCs), adipose-derived stem cell-enriched fractions (ADSC-EF) and adipose-derived lattices, alone and combined with the ADSCs of the invention. In one aspect, the present invention provides an ADSC substantially free of adipocytes and red blood cells and clonal populations of connective tissue stem cells. The ADSCs can be employed, alone or within biologically-compatible compositions, to generate differentiated tissues and structures, both in vivo and in vitro. Additionally, the ADSCs can be expanded and cultured to produce molecules such as hormones, and to provide conditioned culture media for supporting the growth and expansion of other cell populations. In another aspect, the present invention provides an adipose-derived lattice substantially devoid of cells, which includes extracellular matrix material from adipose tissue. The lattice can be used as a substrate to facilitate the growth and differentiation of cells, whether in vivo or in vitro, into anlagen or even mature tissues or structures.
Owner:UNIVERSITY OF PITTSBURGH
Conditioned cell culture medium compositions and methods of use
InactiveUS7118746B1Eliminate wrinkles, frown lines, scarringCondition the skinOrganic active ingredientsCosmetic preparationsReserve CellCell culture media
Novel products comprising conditioned cell culture medium compositions and methods of use are described. The conditioned cell medium compositions of the invention may be comprised of any known defined or undefined medium and may be conditioned using any eukaryotic cell type. The medium may be conditioned by stromal cells, parenchymal cells, mesenchymal stem cells, liver reserve cells, neural stem cells, pancreatic stem cells and / or embryonic stem cells. Additionally, the cells may be genetically modified. A three-dimensional tissue construct is preferred. Once the cell medium of the invention is conditioned, it may be used in any state. Physical embodiments of the conditioned medium include, but are not limited to, liquid or solid, frozen, lyophilized or dried into a powder. Additionally, the medium is formulated with a pharmaceutically acceptable carrier as a vehicle for internal administration, applied directly to a food item or product, formulated with a salve or ointment for topical applications, or, for example, made into or added to surgical glue to accelerate healing of sutures following invasive procedures. Also, the medium may be further processed to concentrate or reduce one or more factors or components contained within the medium.
Owner:ALLERGAN INC
Bioactive wound dressings and implantable devices and methods of use
The present invention provides wound dressings, optionally surgically implantable, containing biodegradable, polymers and hydrogels having allogenic or autologous precursor cells, such as stem cells and progenitor cells dispersed within. Alternatively, the wound dressings can have conditioned medium obtained from the precursor cells dispersed within. The wound dressings promote tissue restoration processes at a site of application or implantation. Additional bioactive agents can also be dispersed within the polymer / hydrogel matrix, which can be formulated to biodegrade at a controlled rate by adjusting the composition. Methods are also provided for using such biodegradable wound dressings as a delivery device or carrier for the precursor cells, conditioned medium and bioactive agents, or as coatings on implantable medical devices, to promote tissue restoration at a lesion site.
Owner:MEDIVAS LLC
Culturing human embryonic stem cells
Previous methods for culturing human embryonic stem cells have required either fibroblast feeder cells or a medium which has been exposed to fibroblast feeder cells in order to maintain the stem cells in an undifferentiated state. It has now been found that if high levels of fibroblast growth factor are used in a medium with gamma amino butyric acid, pipecholic acid, lithium and lipids, the stem cells will remain undifferentiated indefinitely through multiple passages, even without feeder cells or conditioned medium. A humanized matrix of human proteins can be used as a basement matrix to culture the cells. New lines of human embryonic stem cells made using these culture conditions, the medium and the matrix, will never have been exposed to animal cells, animal products, feeder cells or conditioned medium.
Owner:WISCONSIN ALUMNI RES FOUND
Novel methods, compositions and devices for inducing neovascularization
The invention provides methods of inducing neovascularization in a subject in need thereof. The invention further provides compositions, devices and implantable products generated from conditioned media, and in particular, from conditioned media from cultured umbilical cord populations. These compositions are useful for inducing neovascularization. The invention also provides methods of distributing compositions, devices and products to health care professionals.
Owner:CASE WESTERN RESERVE UNIV
Methods related to wound healing
ActiveUS20070231297A1Promote formationPromote wound healingBiocideEpidermal cells/skin cellsWound healingCytokine
The invention is directed to methods for the treatment of wounds. Such methods utilize novel compositions, including but not limited to amnion-derived multipotent cells (herein referred to as AMP cells), conditioned media derived therefrom (herein referred to as amnion-derived cellular cytokine suspension or ACCS), cell lysates derived therefrom, cell products derived therefrom, each alone or in combination.
Owner:STEMNION
Cultivation of human embryonic stem cells in the absence of feeder cells or without conditioned medium
InactiveUS7439064B2Maintain normalMaintaining the karyotype of the stem cellsCulture processArtificial cell constructsFeeder LayerStem cell culture
The invention relates to methods for culturing human embryonic stem cells by culturing the stem cells in an environment essentially free of mammalian fetal serum and in a stem cell culture medium including amino acids, vitamins, salts, minerals, transferring, insulin, albumin, and a fibroblast growth factor that is supplied from a source other than just a feeder layer the medium. Also disclosed are compositions capable of supporting the culture and proliferation of human embryonic stem cells without the need for feeder cells or for exposure of the medium to feeder cells.
Owner:WICELL RES INST
Methods for Cell Expansion and Uses of Cells and Conditioned Media Produced Thereby for Therapy
A method of cell expansion is provided. The method comprising culturing adherent cells from placenta or adipose tissue under three-dimensional culturing conditions, which support cell expansion.
Owner:PLURISTEAM LTD
Conditioned medium and uses thereof
InactiveUS20080206171A1Conservation of compositionPreventing and reducing irritant effect of cosmeticBiocideCosmetic preparationsMicroorganismSide effect
The invention relates to compositions containing an agent that has an irritant side effect and a conditioned cell culture medium and / or of an extract thereof, for use, e.g., in the treatment of signs of inflammation and / or of immune disorders, the medium being obtainable by contact with at least one culture of digestive tract cells and at least one probiotic microorganisms. Methods of use.
Owner:LOREAL SA
Method for constructing three-dimensional neural stem cell model in two steps by adopting micro-fluidic technology
ActiveCN103146650AReduce the cost of trainingReduce usageNervous system cellsTissue/virus culture apparatusCollagen iNeurogenesis
The invention relates to a method for constructing a three-dimensional neural stem cell model in two steps by adopting the micro-fluidic technology. The method is characterized in that a rat tail collagen I is used as a three-dimensional support, a micro-pillar array type micro-fluidic chip is used as a culture platform, and a neural stem cell is cultured in two steps, wherein in the early culture stage, a culture medium for promoting the amplification of the neural stem cell is injected into a cell culture chamber, and in the later culture stage, a conditioned medium suitable for the growth of the neural stem cell and the daughter cells thereof is used, and a three-dimensional composite structure which is similar to a nerve tissue is formed by simulating the microenvironment of different neurogenesis stages in the body. The method provided by the invention is good in repeatability and can be used for construction a plurality of groups of samples. The adopted microfluidic culture system is in a microliter volume and can be regulated accurately, thus the amount of various high-cost cell growth factors, immunologic fluorescent antibodies and cell hormones used in the process of culturing the cell can be reduced greatly, and the cell culture cost can be lowered. The three-dimensional neural stem cell model is expected to be a nerve tissue substitute for screening a novel medicament or monitoring an environmental toxin.
Owner:DALIAN UNIV OF TECH
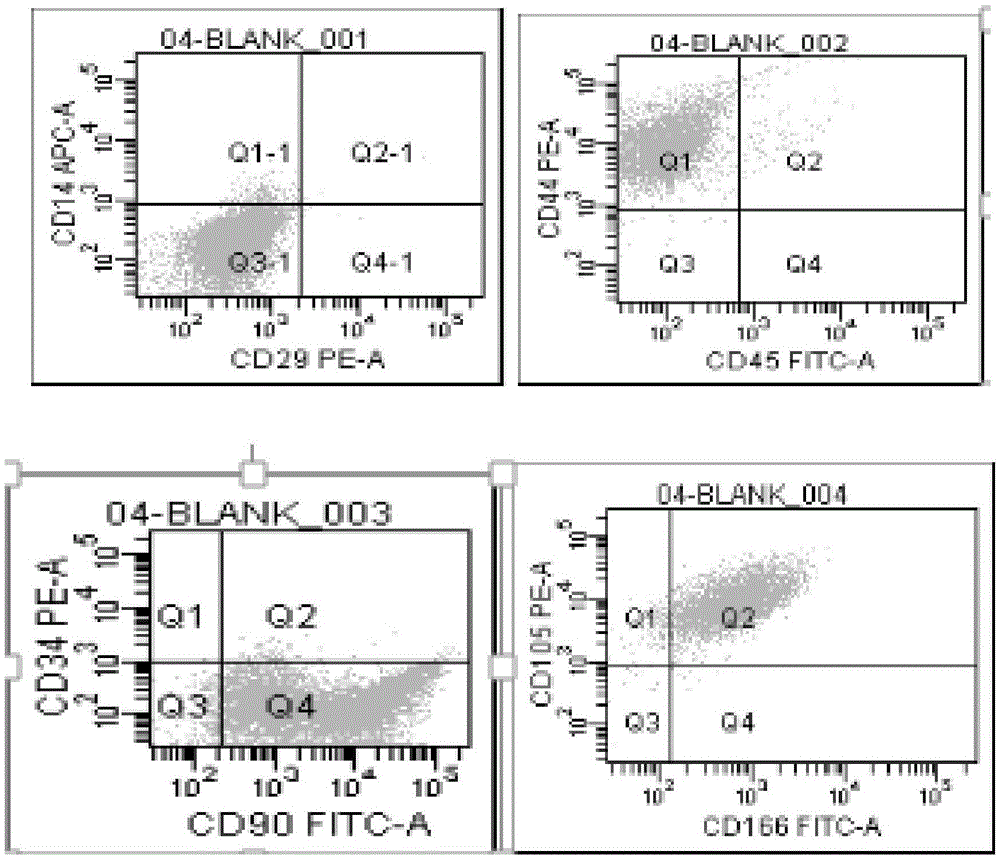
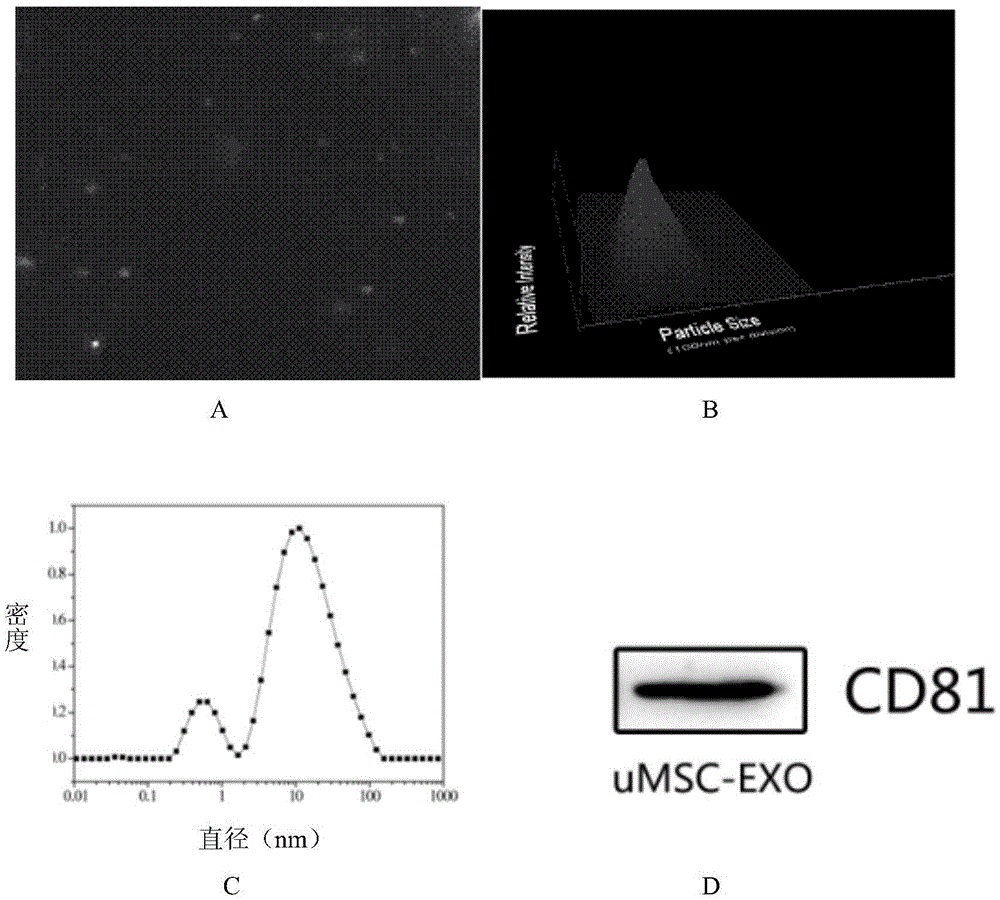
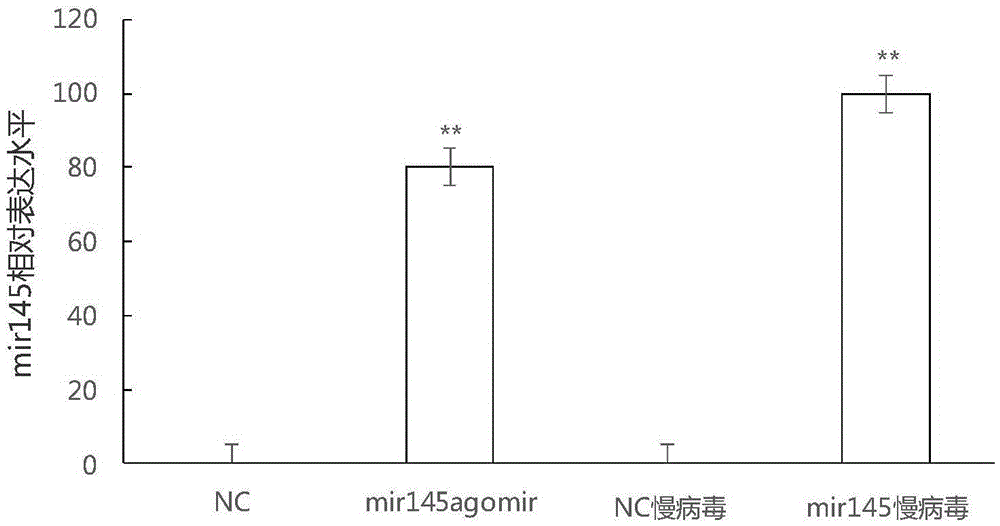
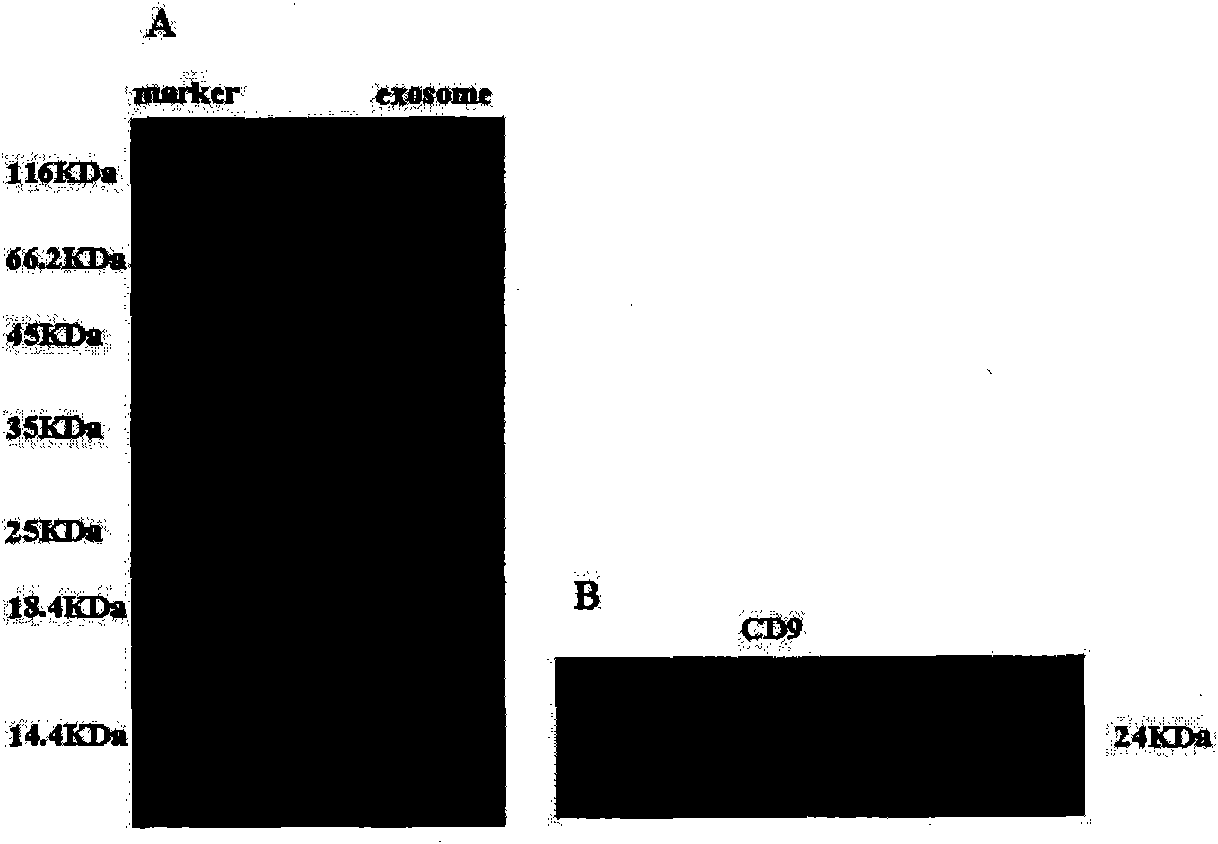
MiRNA145-5p-modified umbilical cord mesenchymal stem cell exosome and preparation and application of miRNA145-5p-modified umbilical cord mesenchymal stem cell exosome
ActiveCN105483081APowerfulTargetedSkeletal/connective tissue cellsUnknown materialsDefect healingMesenchymal stem cell
The invention belongs to the technical field of biology and particularly relates to a miRNA145-5p-modified umbilical cord mesenchymal stem cell exosome and preparation and application of the miRNA145-5p-modified umbilical cord mesenchymal stem cell exosome. The invention provides a preparation method of the highly-miRNA145-5p-expressed umsc-exosome (umbilical cord mesenchymal stem cell exosome) and application of various biological preparations for accelerating full-thickness skin defect healing and antagonizing scar contracture. The preparation method of the exosome includes (1), adopting fresh umbilical cords to culture human umbilical cord mesenchymal stem cells; (2) preparing mesenchymal stem cells highly expressed in miRNA145-5p; (3), storing conditional media; (4) performing exosome extraction and purification. The highly-miRNA145-5p-expressed umbilical cord mesenchymal stem cell exosome is transferred into a full-thickness wound model of rat backs by serving as a biological preparation, so that skin granulation tissue proliferation can be promoted effectively, wound healing is accelerated and scar contracture can be antagonized; novel approaches and novel methods are provided for wound treatment.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Medium and culture of embryonic stem cells
ActiveUS20060084168A1Promotes robust growthCulture processArtificial cell constructsButyric acidFibroblast growth factor
Previous methods for culturing human embryonic stem cells have required either fibroblast feeder cells or a medium which has been exposed to fibroblast feeder cells in order to maintain the stem cells in an undifferentiated state. It has now been found that if high levels of fibroblast growth factor, gamma amino butyric acid, pipecholic acid, lithium and transforming growth factor beta are added to the medium in which the stem cells are cultured, the stem cells will remain undifferentiated indefinitely through multiple passages, even without feeder cells or conditioned medium.
Owner:WISCONSIN ALUMNI RES FOUND
Method and apparatus for maintenance and expansion of hemopoietic stem cells and/or progenitor cells
InactiveUS20050181504A1PromotePromote growthMicroorganism based processesMammal material medical ingredientsFiberProgenitor
A method of preparing a stromal cell conditioned medium useful in expanding undifferentiated hemopoietic stem cells to increase the number of the hemopoietic stem cells is provided. The method comprising: (a) establishing a stromal cell culture in a stationary phase plug-flow bioreactor under continuous flow on a substrate in the form of a sheet, the substrate including a non-woven fibrous matrix forming a physiologically acceptable three-dimensional network of fibers, thereby expanding undifferentiated hemopoietic stem cells; and (b) when a desired stromal cell density has been achieved, collecting medium from the stationary phase plug-flow bioreactor, thereby obtaining the stromal cell conditioned medium useful in expanding the undifferentiated hemopoietic stem cells.
Owner:PLURISTEAM LTD
Human umbilical cordmesenchymal stem cell-derived exosome and application thereof
ActiveCN101890050ASimple technical solution for preparationEasy to operateDigestive systemSkeletal/connective tissue cellsDiseaseLiver and kidney
The invention relates to extraction of an active ingredient exosome from a human umbilical cordmesenchymal stem cell and application thereof in preparation of medicaments for treating liver and kidney injury and skin diseases. The human umbilical cordmesenchymal stem cell-derived exosome is prepared by the following steps of: (1) culturing a human umbilical cordmesenchymal stem cell; (2) preparing a cordmesenchymal stem cell conditioned medium; and (3) separating and purifying the exosome. The experiments for infusing the human umbilical cordmesenchymal stem cell exosome serving as a medicament into a mice acute liver injury model, a rat kidney injury model and a rat skin scald model show that the medicament can improve the liver function, the kidney function and the skin condition, promote the pathological recovery of the liver, the kidney and the skin, reduce the death rate, fulfill the aims of blocking and reversing the development of the liver and kidney injury and open up a new approach for clinical treating of the liver and kidney injury.
Owner:江苏澳洋生物医药研究院有限责任公司
Endometrial stem cells and methods of making and using same
InactiveUS20130156726A1Rapid rate of cellular divisionBiocidePeptide/protein ingredientsCell lineageEx vivo
The invention provides pluripotent stem cells and methods for making and using pluripotent stem cells. Pluripotent stem cells, among other things, can differentiate into various cell lineages in vitro, ex vivo and in vivo. Pluripotent stem cells, among other things, can also be used to produce conditioned medium.
Owner:XON CELLS
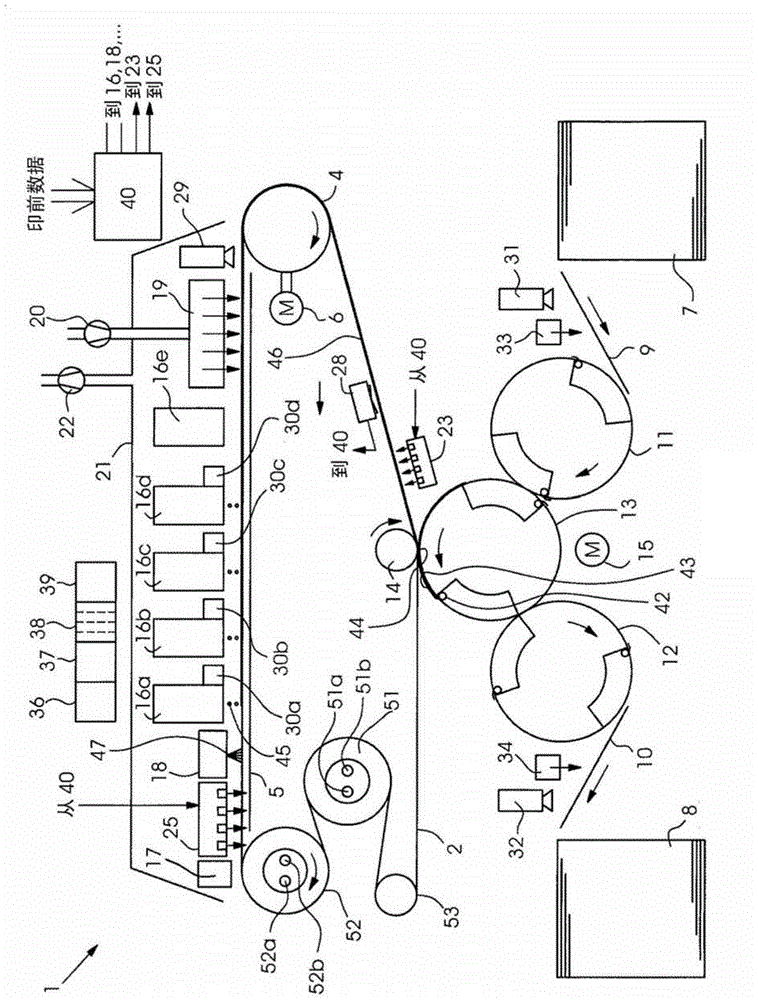
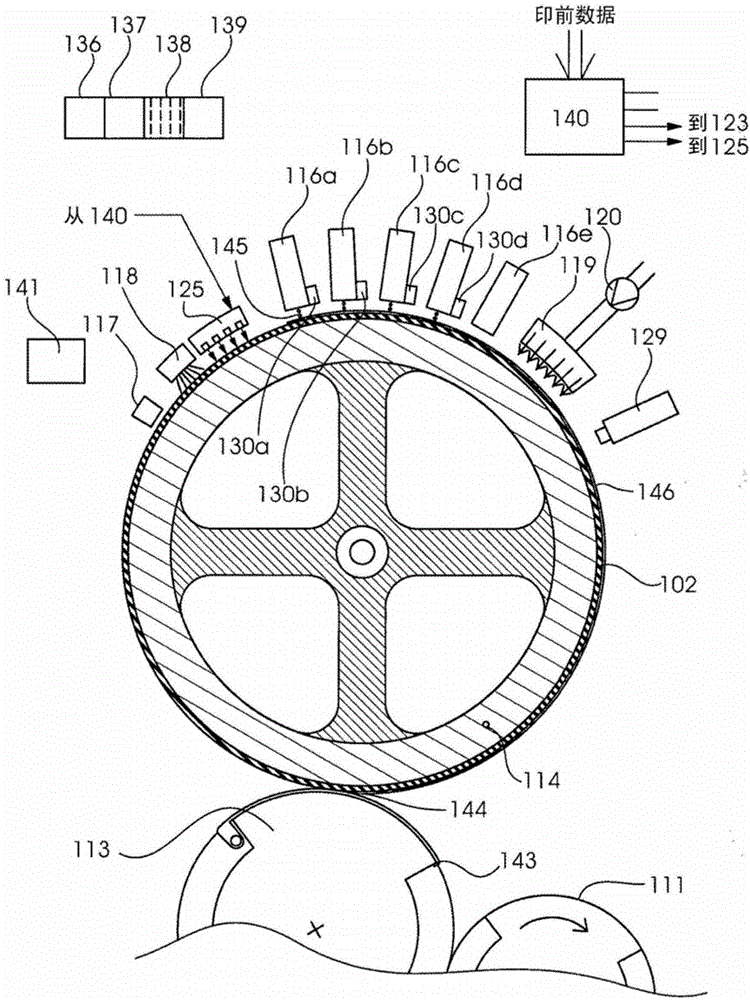
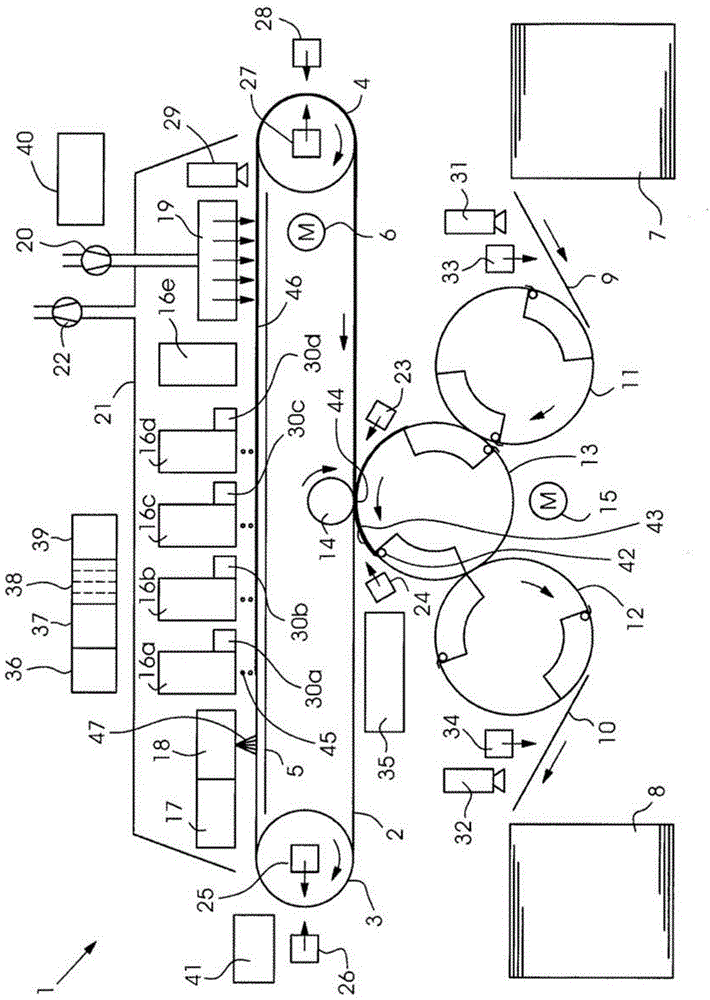
Method for indirectly applying printing liquid to a printing substrate
InactiveCN104661825AFluidityEasy to disengageDuplicating/marking methodsOther printing apparatusEngineeringElectrical and Electronics engineering
A method for the indirect application of printing liquid onto a printing material provides an intermediate carrier, preferably a circulating belt, a liquid conditioning medium including a first substance applied onto the intermediate carrier and a printing liquid, in particular an inkjet ink, including a second substance applied onto the conditioning medium on the intermediate carrier. The printing liquid is situated as droplets or a layer substantially on the conditioning medium and the droplets or the layer form a contact region on their underside with the conditioning medium. The printing liquid is heated, preferably by way of a dryer and the printing liquid is transferred from the intermediate carrier onto the printing material.
Owner:HEIDELBERGER DRUCKMASCHINEN AG
Methods for cell expansion and uses of cells and conditioned media produced thereby for therapy
Methods for treating a subject suffering from a compromised endogenous hematopoietic system are described that comprise administering to the subject a therapeutically effective amount of adherent stromal cells. Methods of preparing adherent stromal cells and pharmaceutical compositions comprising the cells are also described.
Owner:PLURISTEAM LTD
Method and apparatus for maintenance and expansion of hemopoietic stem cells and/or progenitor cells
InactiveUS20050176143A1PromotePromote growthMicroorganism based processesMammal material medical ingredientsFiberProgenitor
A method of expanding undifferentiated hemopoietic stem cells to increase the number of the hemopoietic stem cells is provided. The method comprising: (a) obtaining the undifferentiated hemopoietic stem cells; and (b) culturing the undifferentiated hemopoietic stem cells in a medium containing a stromal cell conditioned medium, the stromal cell conditioned medium being derived from a stationary phase plug-flow bioreactor in which a three dimensional stromal cell culture has been established under continuous flow of a culture medium on a substrate in: the form of a sheet, the substrate including a non-woven fibrous matrix forming a physiologically acceptable three-dimensional network of fibers, thereby expanding the undifferentiated hemopoietic stem cells.
Owner:PLURISTEAM LTD
Mesenchymal stem cell particles
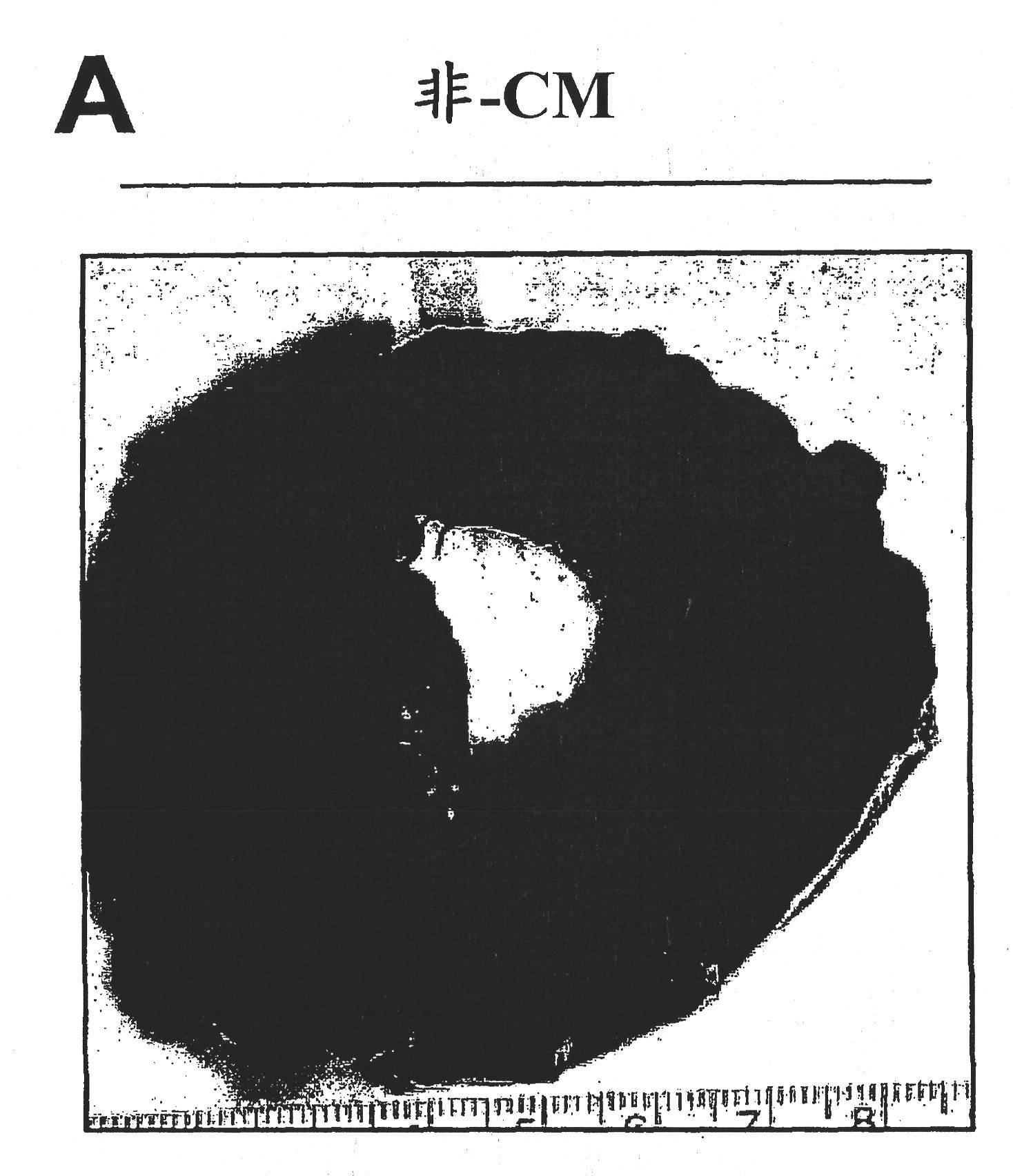
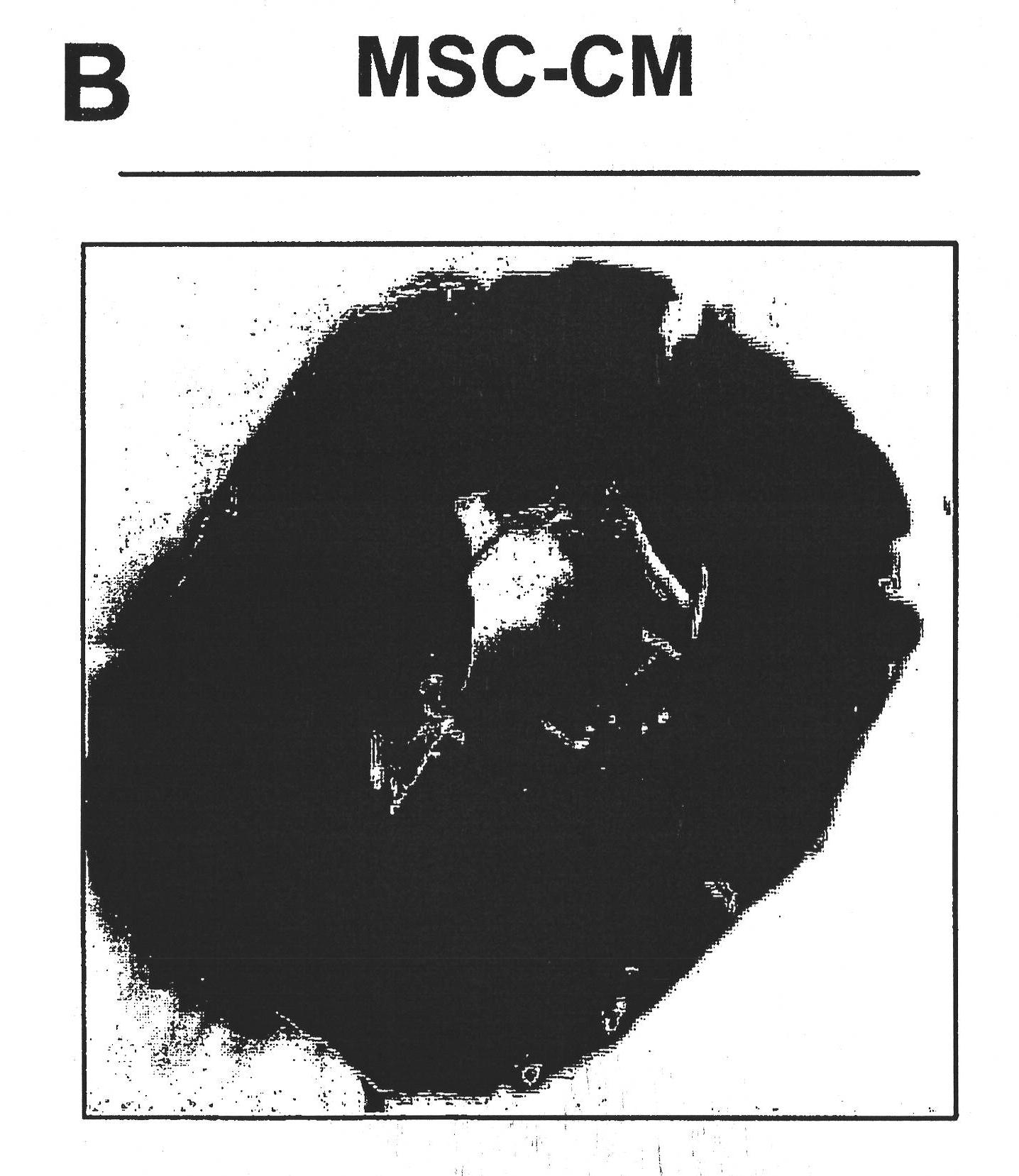
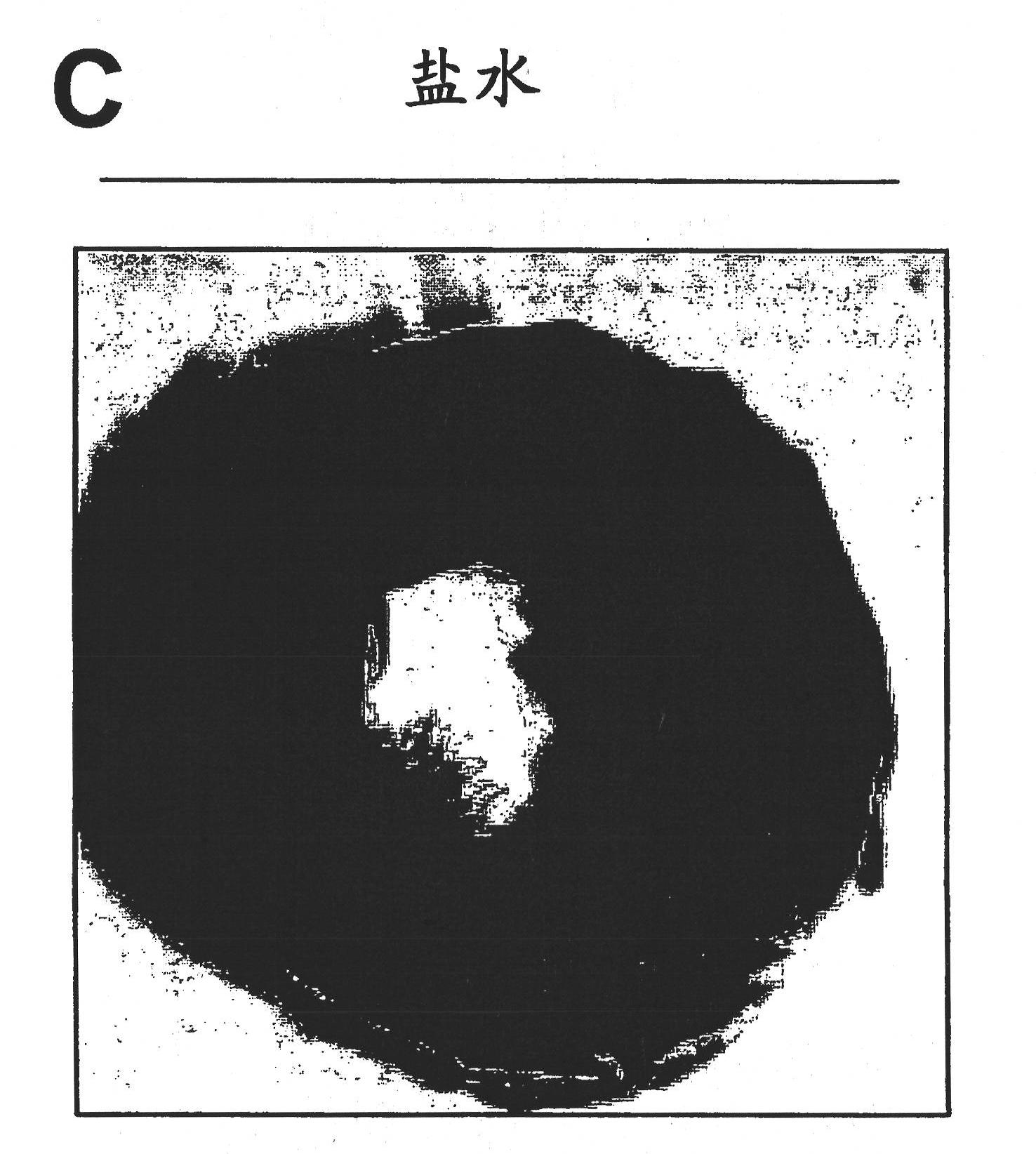
We describe a particle secreted by a mesenchymal stem cell and comprising at least one biological property of a mesenchymal stem cell. The biological property may comprise a biological activity of a mesenchymal stem cell conditioned medium (MSC- CM) such as cardioprotection or reduction of infarct size. The particle may comprise a vesicle or an exosome.
Owner:AGENCY FOR SCI TECH & RES
Methods related to wound healing
ActiveUS20100080779A1Promote formationPromote wound healingOrganic active ingredientsBiocideWound healingCytokine
The invention is directed to methods for the treatment of wounds. Such methods utilize novel compositions, including but not limited to amnion-derived multipotent cells (herein referred to as AMP cells), conditioned media derived therefrom (herein referred to as amnion-derived cellular cytokine suspension or ACCS), cell lysates derived therefrom, cell products derived therefrom, each alone or in combination.
Owner:STEMNION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com