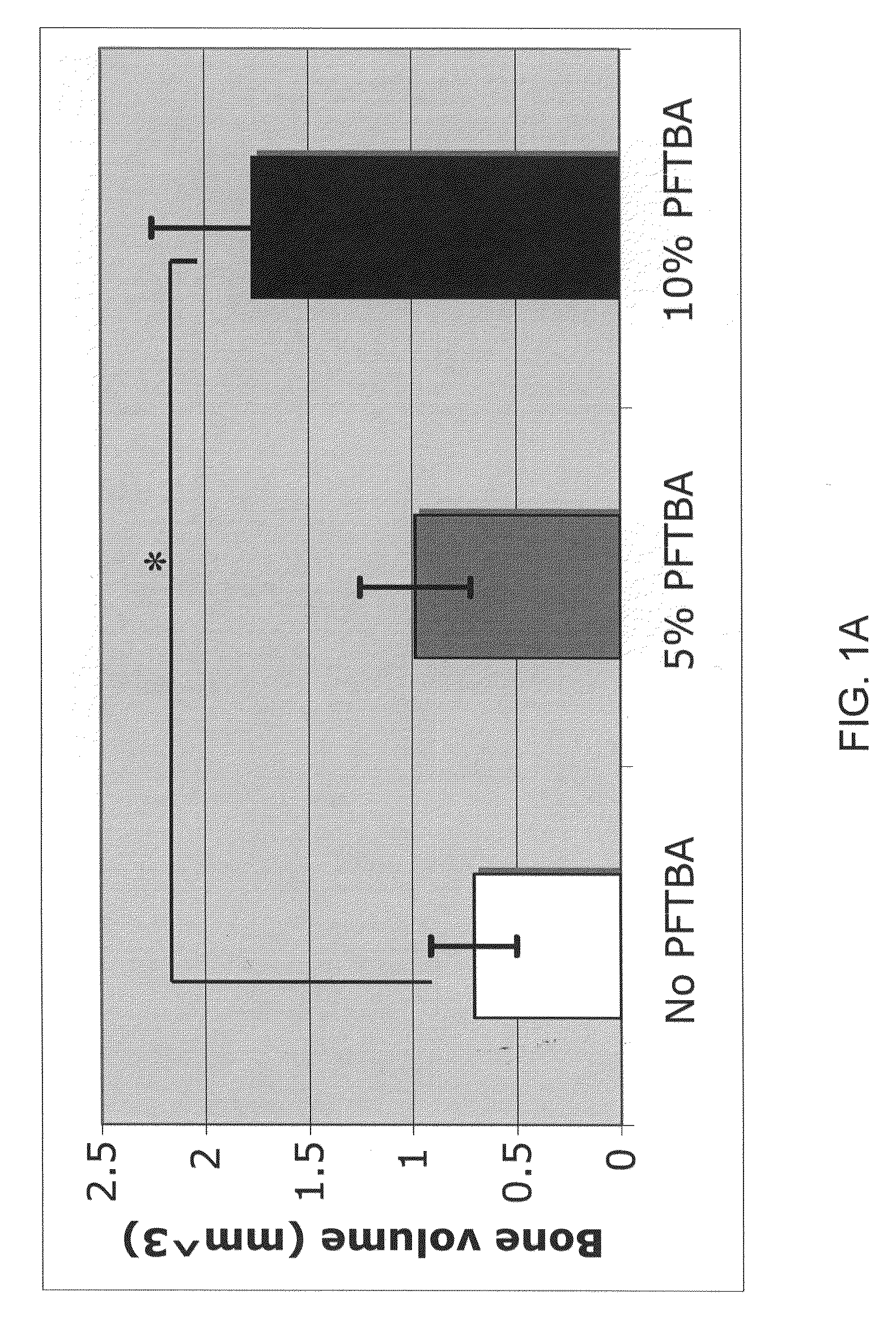
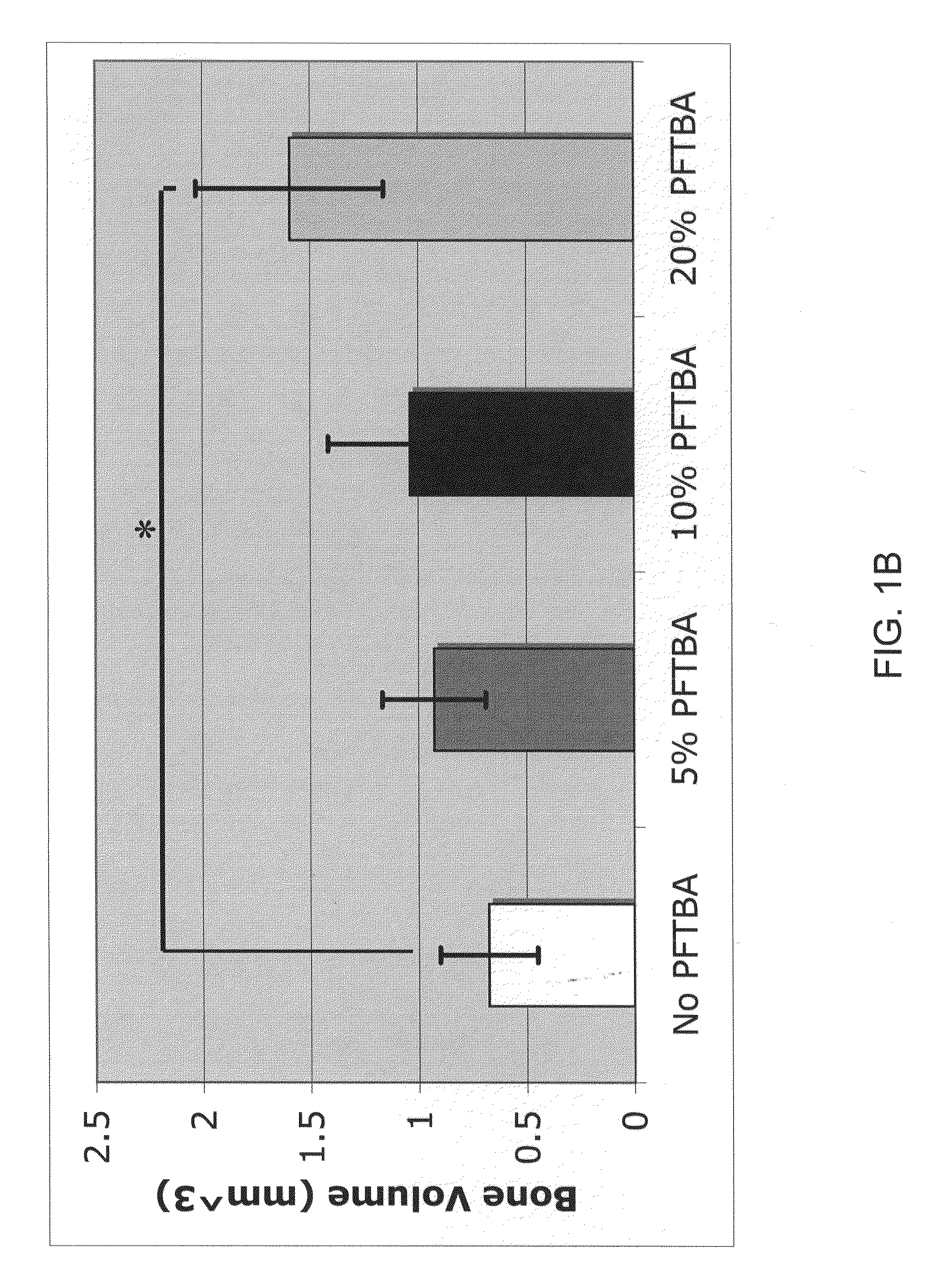
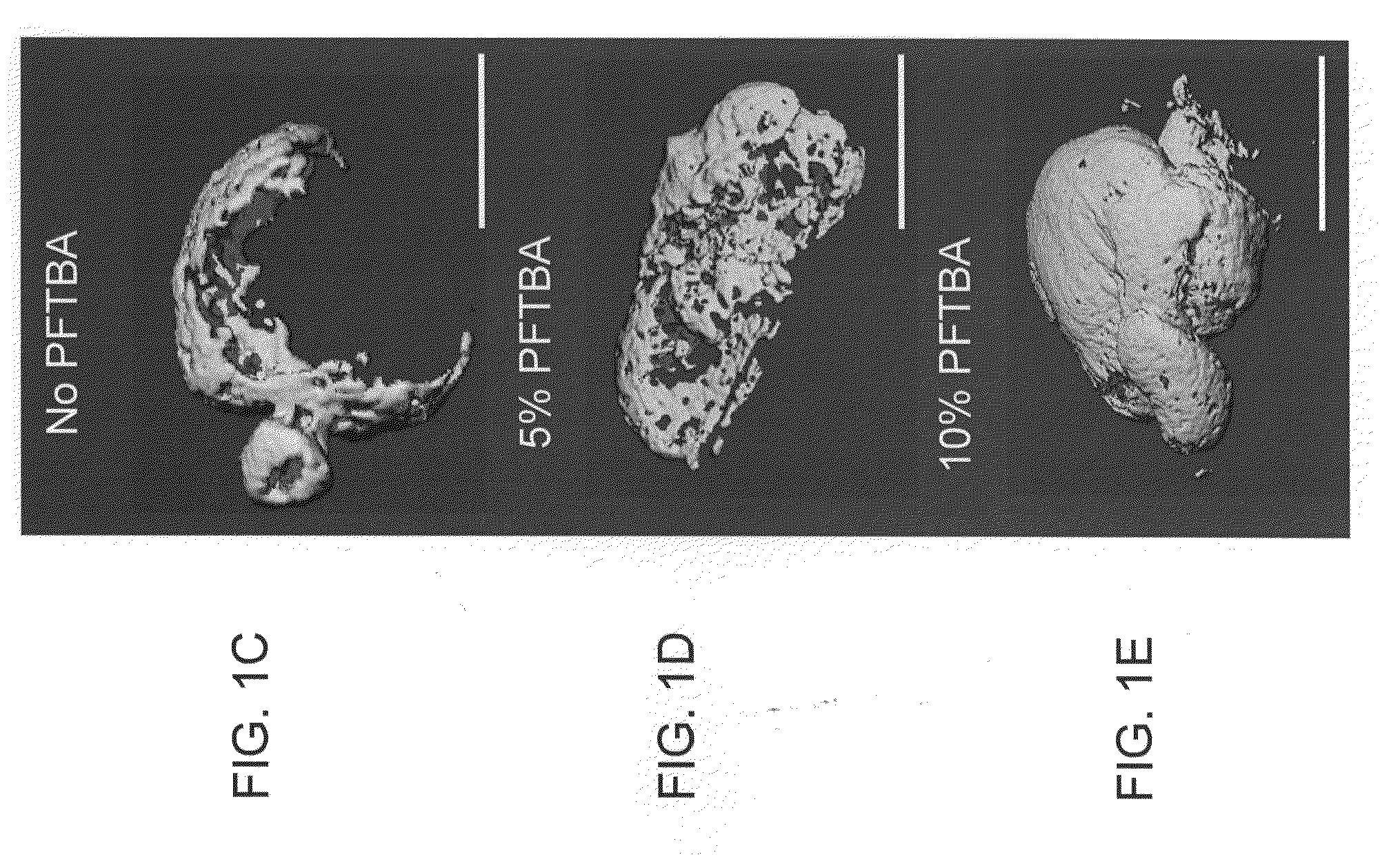
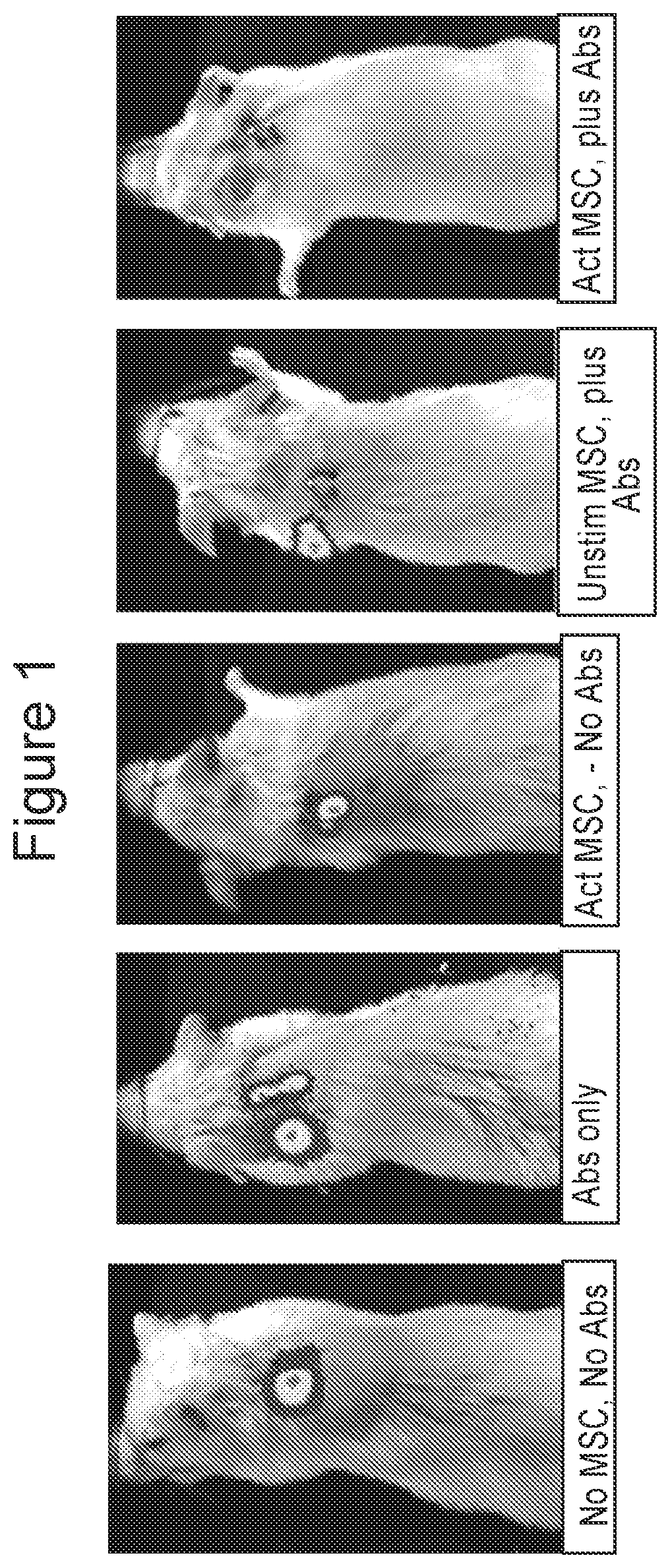
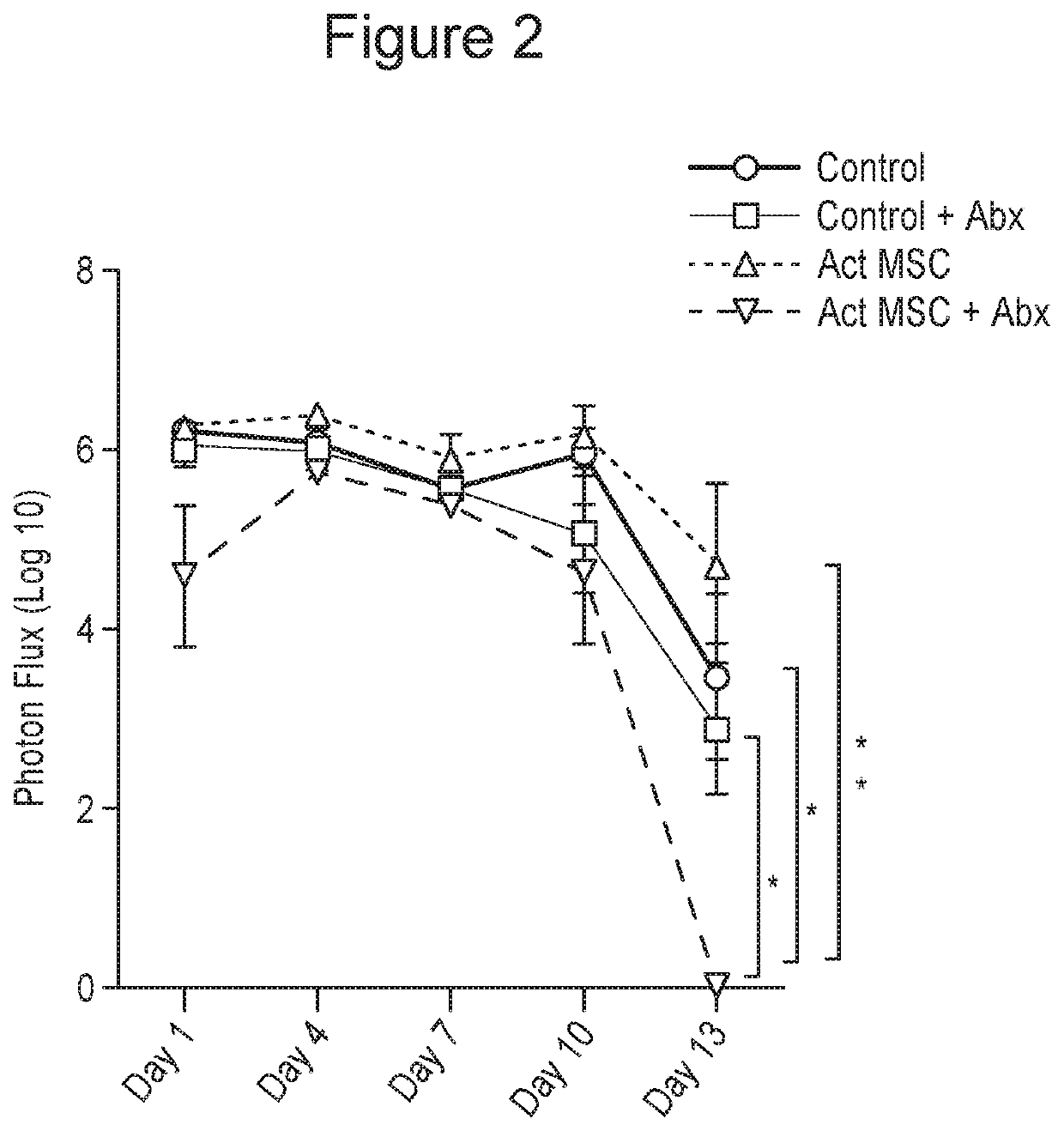
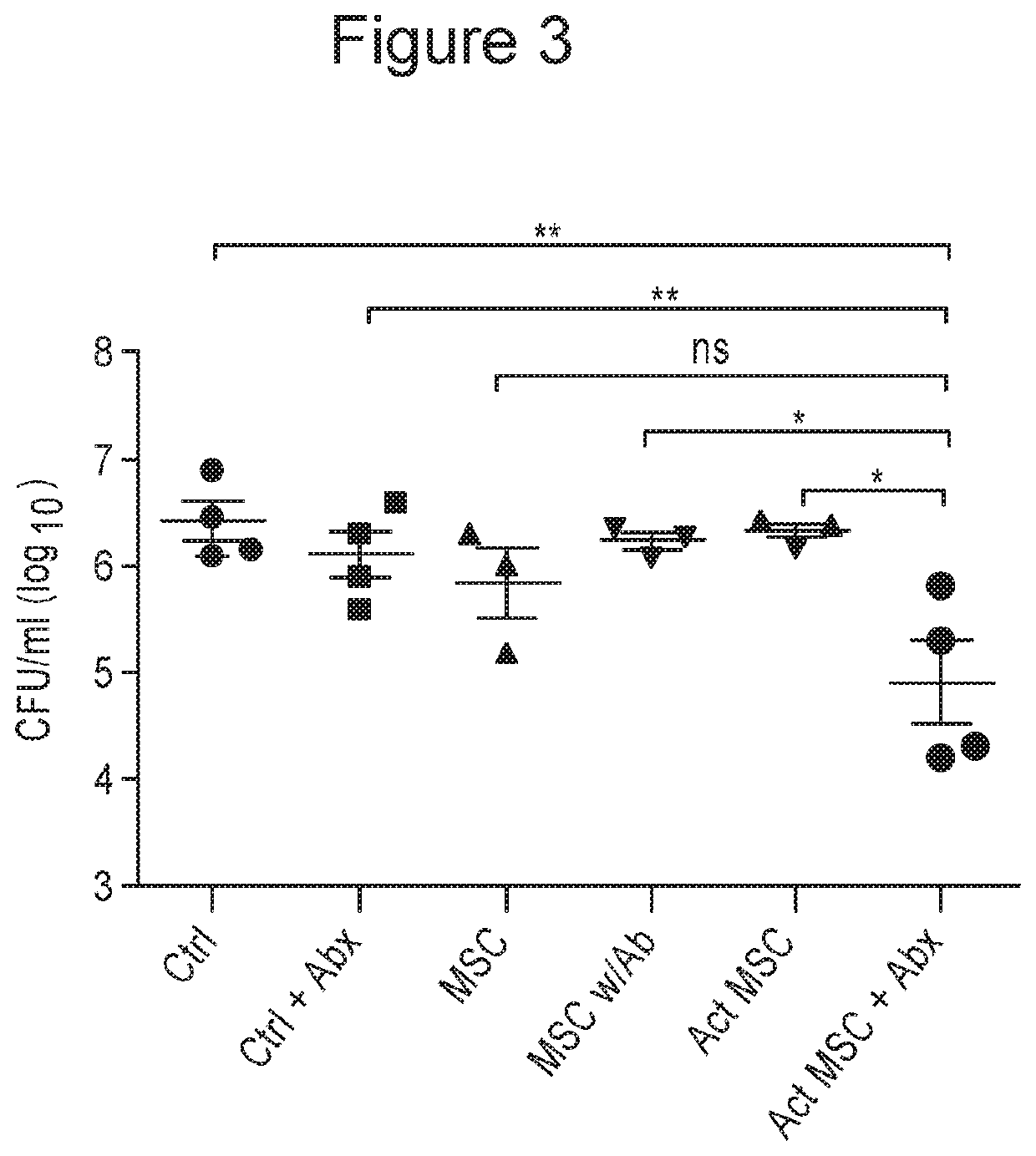
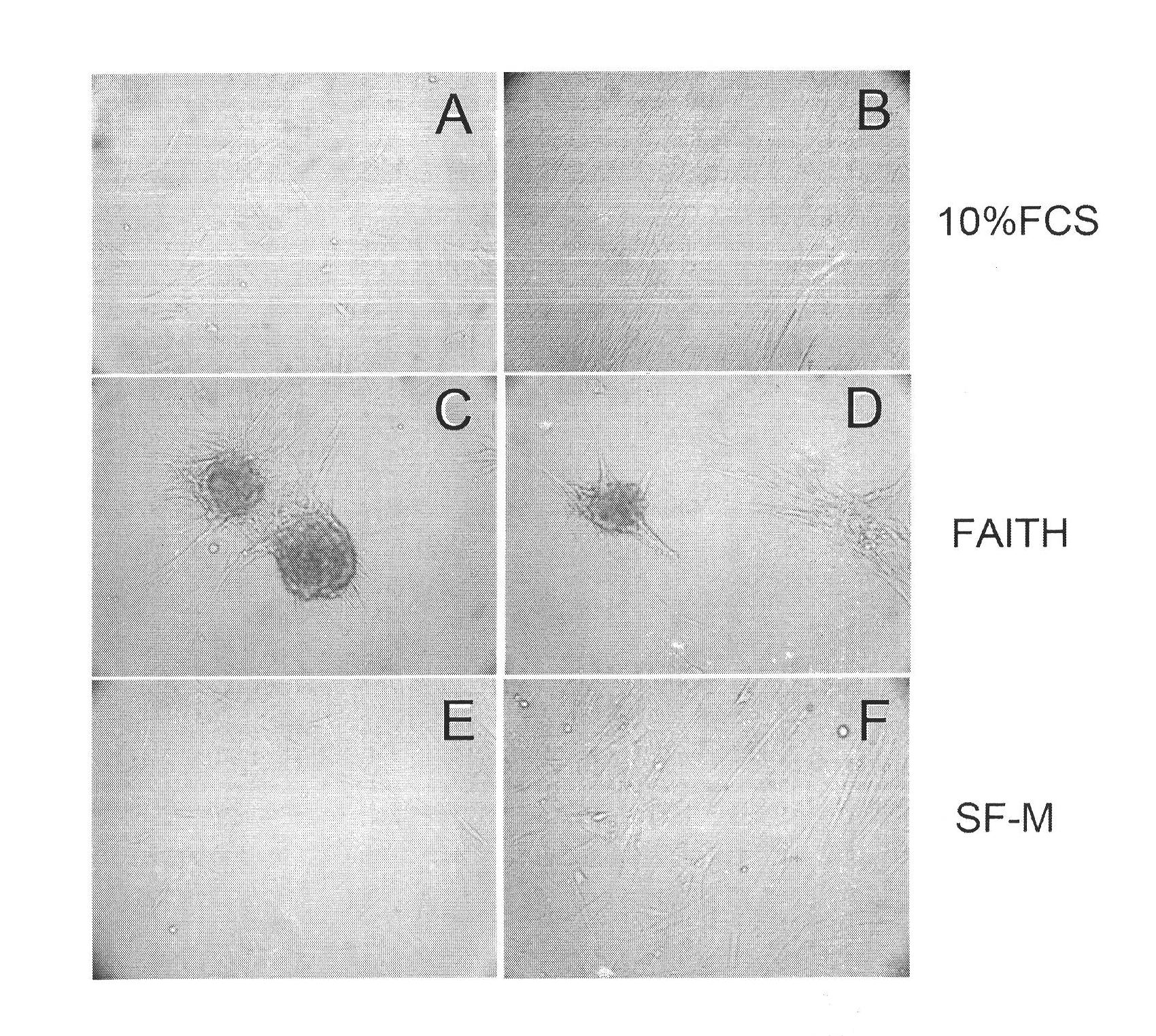
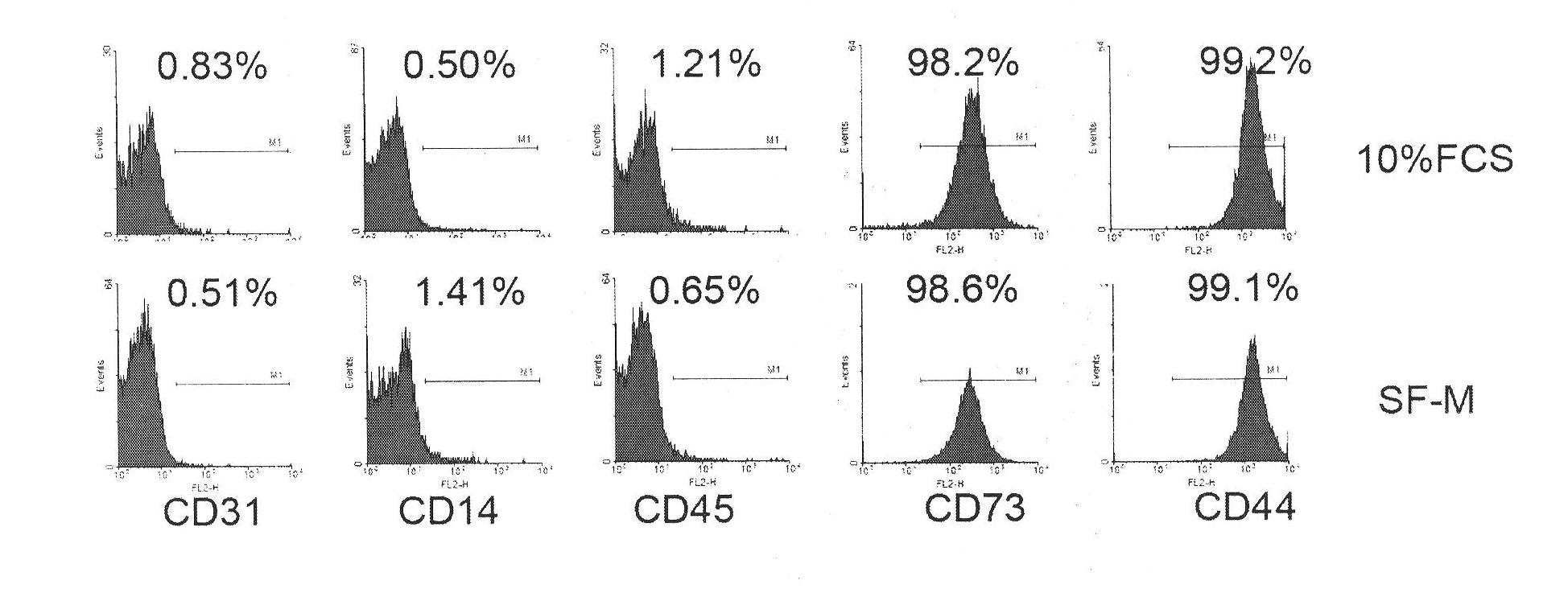
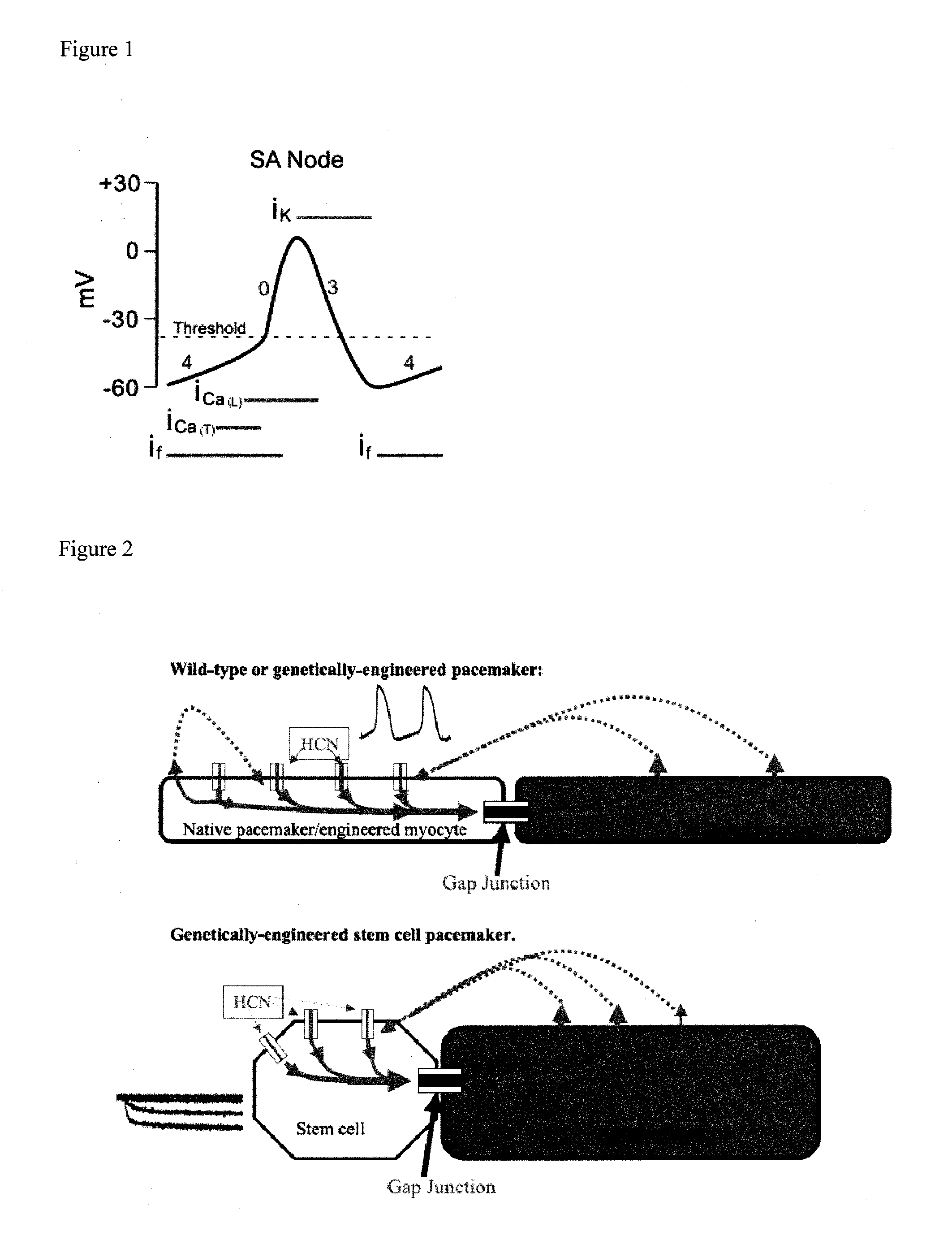
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4146 results about "Mesenchymal stem cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mesenchymal stem cells are multipotent stromal cells that can differentiate into a variety of cell types, including osteoblasts (bone cells), chondrocytes (cartilage cells), myocytes (muscle cells) and adipocytes (fat cells which give rise to marrow adipose tissue).
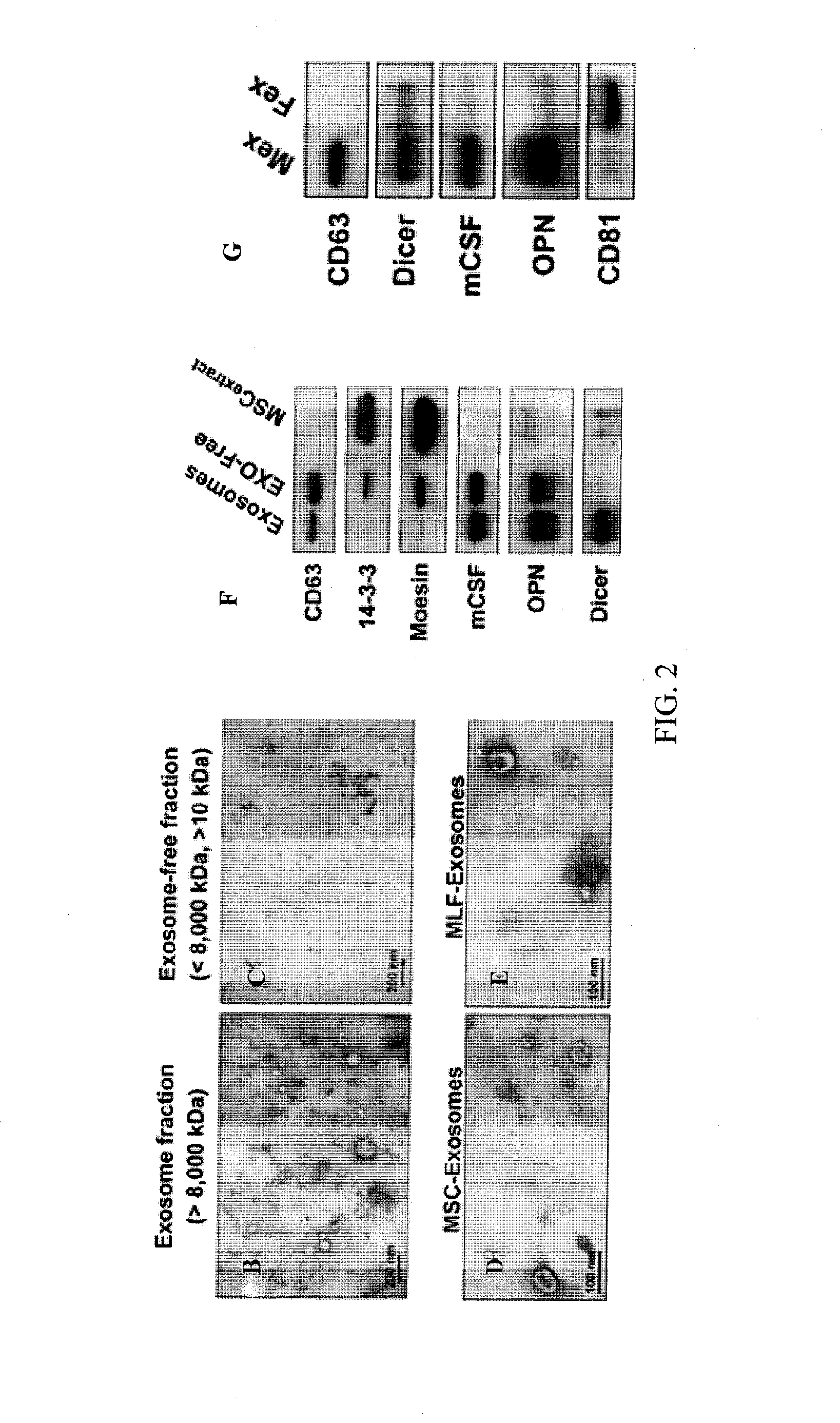
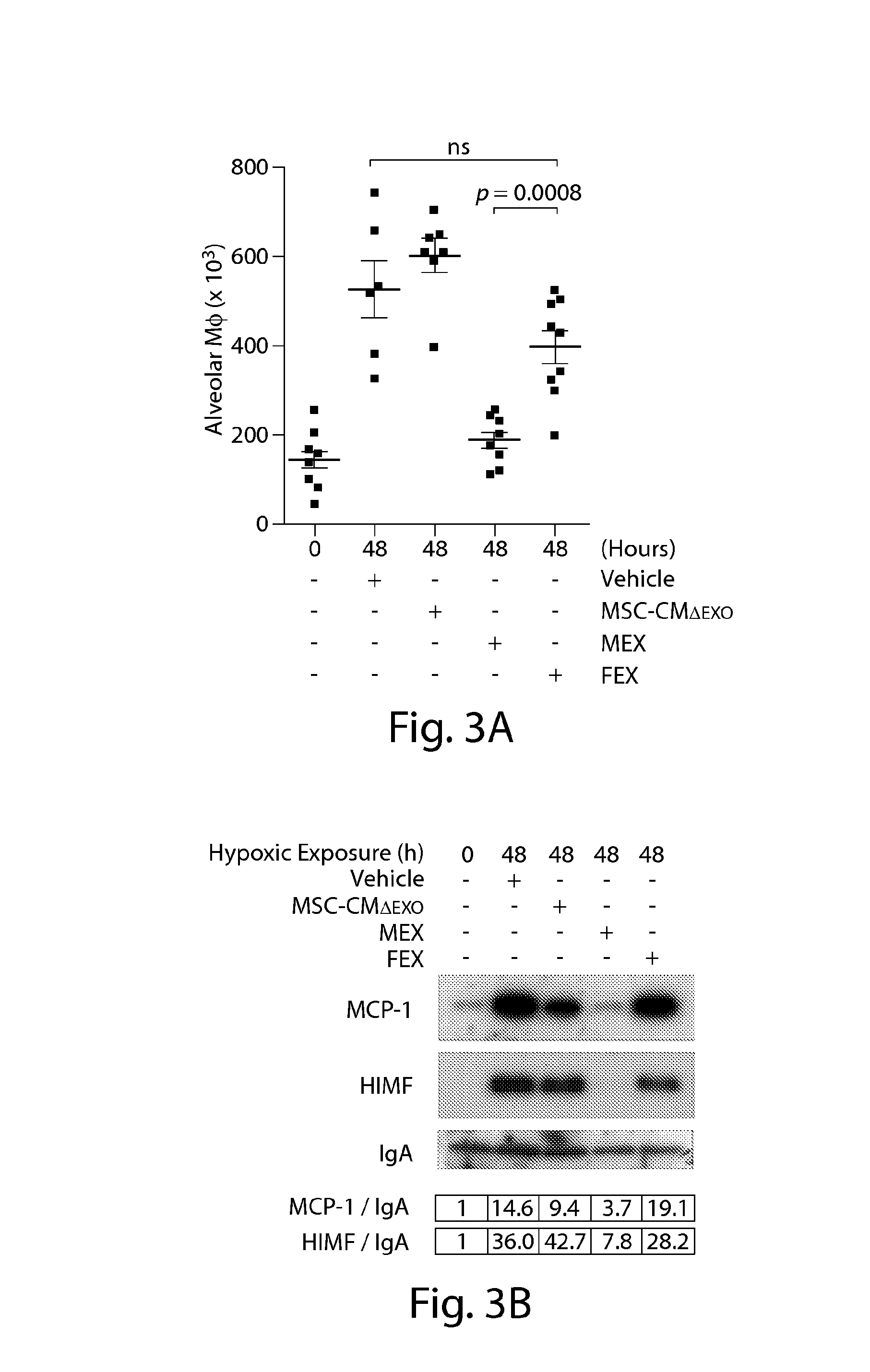
Methods of making conditioned cell culture medium compositions
InactiveUS6372494B1Eliminate wrinklesEliminate frown lineCosmetic preparationsPeptide/protein ingredientsReserve CellCell culture media
Novel products comprising conditioned cell culture medium compositions and methods of use are described. The conditioned cell medium compositions of the invention may be comprised of any known defined or undefined medium and may be conditioned using any eukaryotic cell type. The medium may be conditioned by stromal cells, parenchymal cells, mesenchymal stem cells, liver reserve cells, neural stem cells, pancreatic stem cells and / or embryonic stem cells. Additionally, the cells may be genetically modified. A three-dimensional tissue construct is preferred. Once the cell medium of the invention is conditioned, it may be used in any state. Physical embodiments of the conditioned medium include, but are not limited to, liquid or solid, frozen, lyophilized or dried into a powder. Additionally, the medium is formulated with a pharmaceutically acceptable carrier as a vehicle for internal administration, applied directly to a food item or product, formulated with a salve or ointment for topical applications, or, for example, made into or added to surgical glue to accelerate healing of sutures following invasive procedures. Also, the medium may be further processed to concentrate or reduce one or more factors or components contained within the medium.
Owner:ALLERGAN INC
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
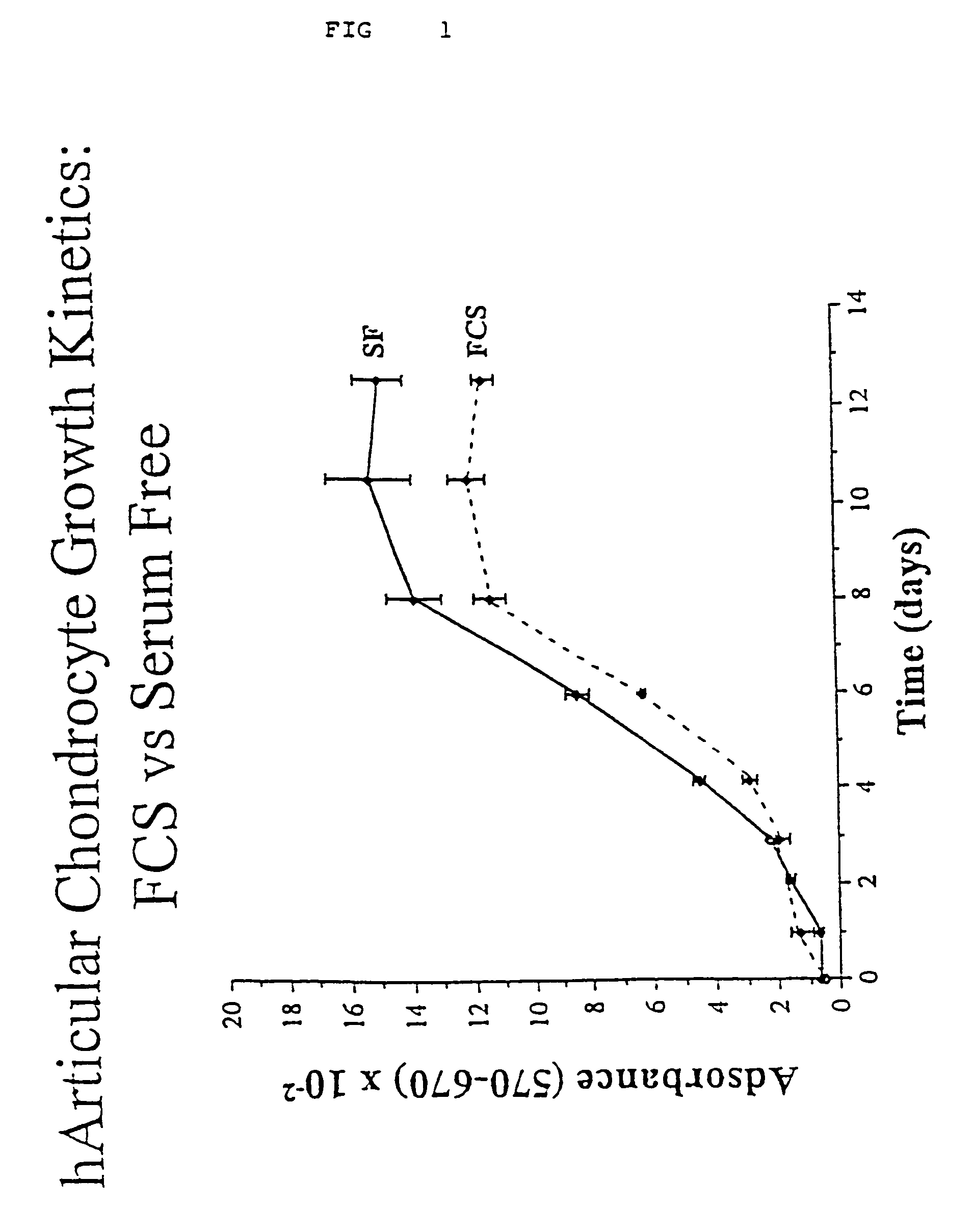
Cell culture environments for the serum-free expansion of mesenchymal stem cells
InactiveUS20050265980A1Promoting mesenchymal stem cell (MSC) expansionBiocideCulture processSerum freeMesenchymal stem cell
Compositions and methods for promoting mesenchymal stem cell expansion while maintaining a pluripotent phenotype are disclosed. Serum-free cell culture systems and kits and methods of use for mesenchymal stem cell expansion are provided. Methods also comprise the use of the expanded mesenchymal stem cells to treat various disorders or diseases, particularly those of the cardiovascular system, bone, or cartilage.
Owner:BECTON DICKINSON & CO
Methods of treatment using electromagnetic field stimulated stem cells
InactiveUS20050084962A1High proliferation ratePromote differentiationMutant preparationArtificial cell constructsElectricityMesenchymal stem cell
Methods of modifying stem cells, in particular mesenchymal stem cells, using electric or electromagnetic fields. In one embodiment, the present invention provides methods of modulating a mesenchymal stem cell activity, the method comprising administering electric stimulation to mesenchymal stem cells in vitro. In another embodiment, the present invention provides methods for the treatment of a human or other mammal subject in need thereof, comprising providing an in vitro culture comprising mesenchymal stem cells, administering an electric stimulation to the in vitro culture, and implanting the mesenchymal stem cells into the mammal subject. In another embodiment, the present invention provides methods for the treatment of a human or other mammal subject, the method comprising implanting mesenchymal stem cells into the mammal subject, and administering an electric stimulation to the mesenchymal stem cells in situ. The present invention also comprises compositions comprising mesenchymal stem cells treated with electric stimulation.
Owner:EUROPEAN BIOINFORMATICS INSTITUTE
Conditioned cell culture medium compositions and methods of use
InactiveUS7118746B1Eliminate wrinkles, frown lines, scarringCondition the skinOrganic active ingredientsCosmetic preparationsReserve CellCell culture media
Novel products comprising conditioned cell culture medium compositions and methods of use are described. The conditioned cell medium compositions of the invention may be comprised of any known defined or undefined medium and may be conditioned using any eukaryotic cell type. The medium may be conditioned by stromal cells, parenchymal cells, mesenchymal stem cells, liver reserve cells, neural stem cells, pancreatic stem cells and / or embryonic stem cells. Additionally, the cells may be genetically modified. A three-dimensional tissue construct is preferred. Once the cell medium of the invention is conditioned, it may be used in any state. Physical embodiments of the conditioned medium include, but are not limited to, liquid or solid, frozen, lyophilized or dried into a powder. Additionally, the medium is formulated with a pharmaceutically acceptable carrier as a vehicle for internal administration, applied directly to a food item or product, formulated with a salve or ointment for topical applications, or, for example, made into or added to surgical glue to accelerate healing of sutures following invasive procedures. Also, the medium may be further processed to concentrate or reduce one or more factors or components contained within the medium.
Owner:ALLERGAN INC
Scaffolds with oxygen carriers, and their use in tissue regeneration
Provided are fibrin or silk matrices comprising an oxygen carrier, and matrices, which comprise an oxygen carrier and mesenchymal stem cells. Also provided are methods of generating and using same for ex vivo or in vivo tissue regeneration and / or repair such as for treating a non-union bone fracture and a condition requiring spinal fusion.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Activated stem cells and systemic treatment methods for infected wounds
ActiveUS11185560B2Promote healingReduce the burden onAntibacterial agentsPharmaceutical delivery mechanismMesenchymal stem cellExcipient
Owner:COLORADO STATE UNIVERSITY
Methods of treatment using electromagnetic field stimulated stem cells
InactiveUS20060057693A1High proliferation ratePromote differentiationBiocideSkeletal disorderElectricityMesenchymal stem cell
Methods of modifying stem cells and compositions of modified stem cells, in particular mesenchymal stem cells, using electric or electromagnetic fields. In various embodiments, the present invention's methods are for the treatment of a human or other mammal subject in need thereof, comprising providing an in vitro culture comprising mesenchymal stem cells, administering an electric stimulation to the in vitro culture, and implanting the mesenchymal stem cells into the subject. Methods also include treatment of a human or other mammal subject, comprising implanting mesenchymal stem cells into the subject, and administering an electric stimulation to the mesenchymal stem cells in situ. Other embodiments include methods of administering electric stimulation in conjunction with growth factors to stem cells.
Owner:EUROPEAN BIOINFORMATICS INSTITUTE
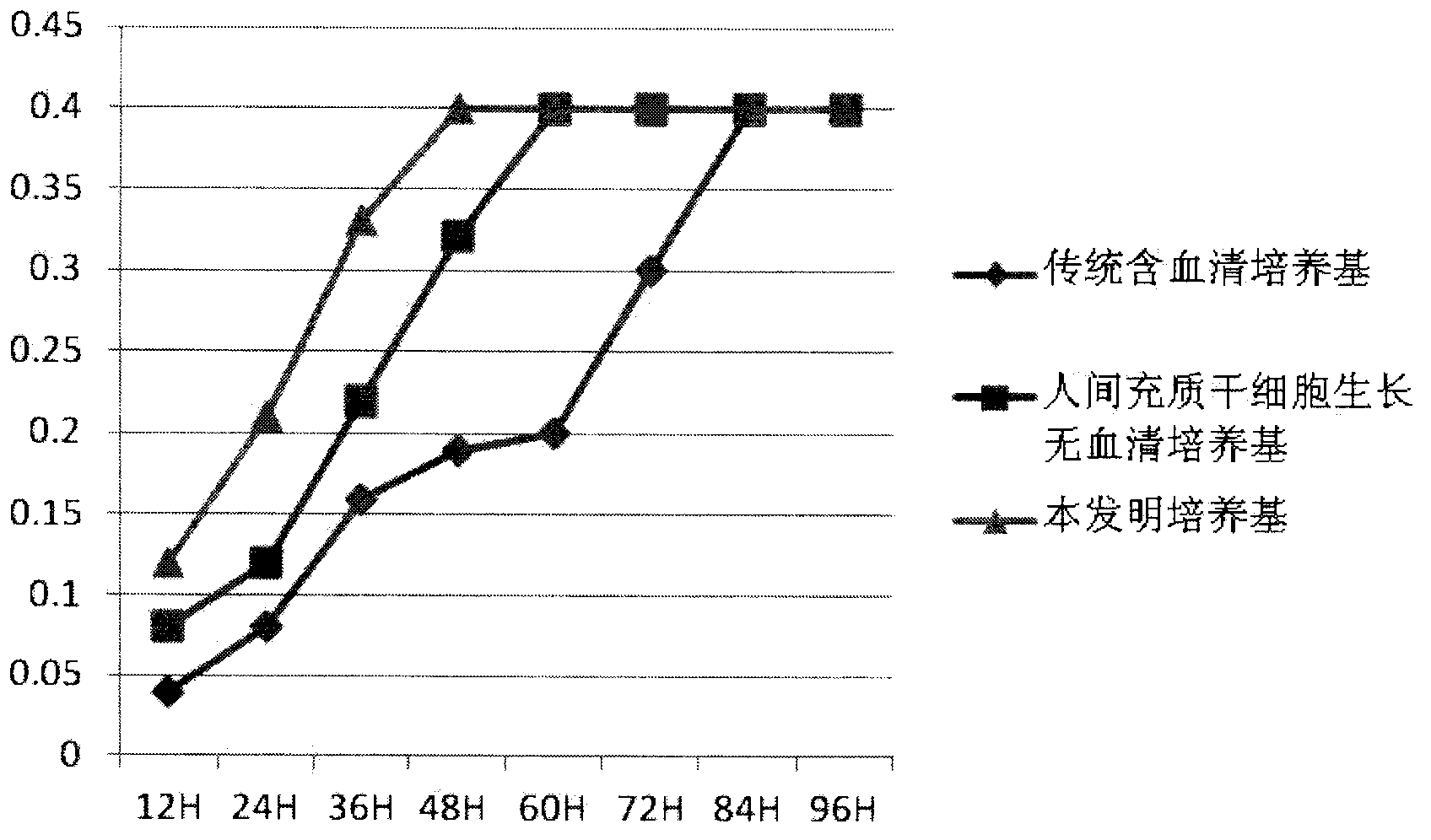
Culture medium for culturing mesenchymal stem cells
InactiveCN101984048AThe chemical composition is clear and clearThe possibility of causing a qualitative differenceSkeletal/connective tissue cellsLipid formationProtein molecules
The invention discloses a culture medium for culturing mesenchymal stem cells. The culture medium comprises improved minimum medium IMDM, cell growth factor combination, insulin, trace elements, lipid substances, vitamin substances, hormone substances, protein molecules, amino acid substances and other compounds. The culture medium provided by the invention does not contain serum or plasma (comprising animal or human serum or plasma), thus, when the system is used for culturing the mesenchymal stem cells, zoonosis caused by the animal serum or plasma in the cell therapy process can be avoided, and the risk of human immunity reaction which is possibly caused in the cell therapy process can be avoided. The culture medium has specific chemical constituent, thus the possibility of different cultured mesenchymal stem cell properties caused by the difference of culture systems can be avoided. The culture medium is not only suitable for culturing the mesenchymal stem cells of human marrow and umbilical cord resources but also suitable for culturing the mesenchymal stem cells of other tissues or other animals, and has the advantage of wide application range.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Layered bio-adhesive compositions and uses thereof
The invention generally provides compositions and methods for promoting and enhancing wound closure and healing. Specifically, the invention provides a biologic composition which comprises a support layer which serves as transport scaffold, for example made of gelatin, which is coated or impregnated with a bio-adhesive molecule such as rose bengal or glyceraldehyde. The composition can also comprise an artificial or biological matrix, optionally processed (i.e. cleaned and coated with extracellular matrix proteins) to enhance cell attachment and survival. The composition can further comprise a monolayer of epithelial, endothelial cells or mesenchymal cells. The invention provides methods for using the compositions for treating wounds due to disease, trauma or surgery. Specific methods for treating ocular wounds are provided.
Owner:UNIV OF LOUISVILLE RES FOUND INC
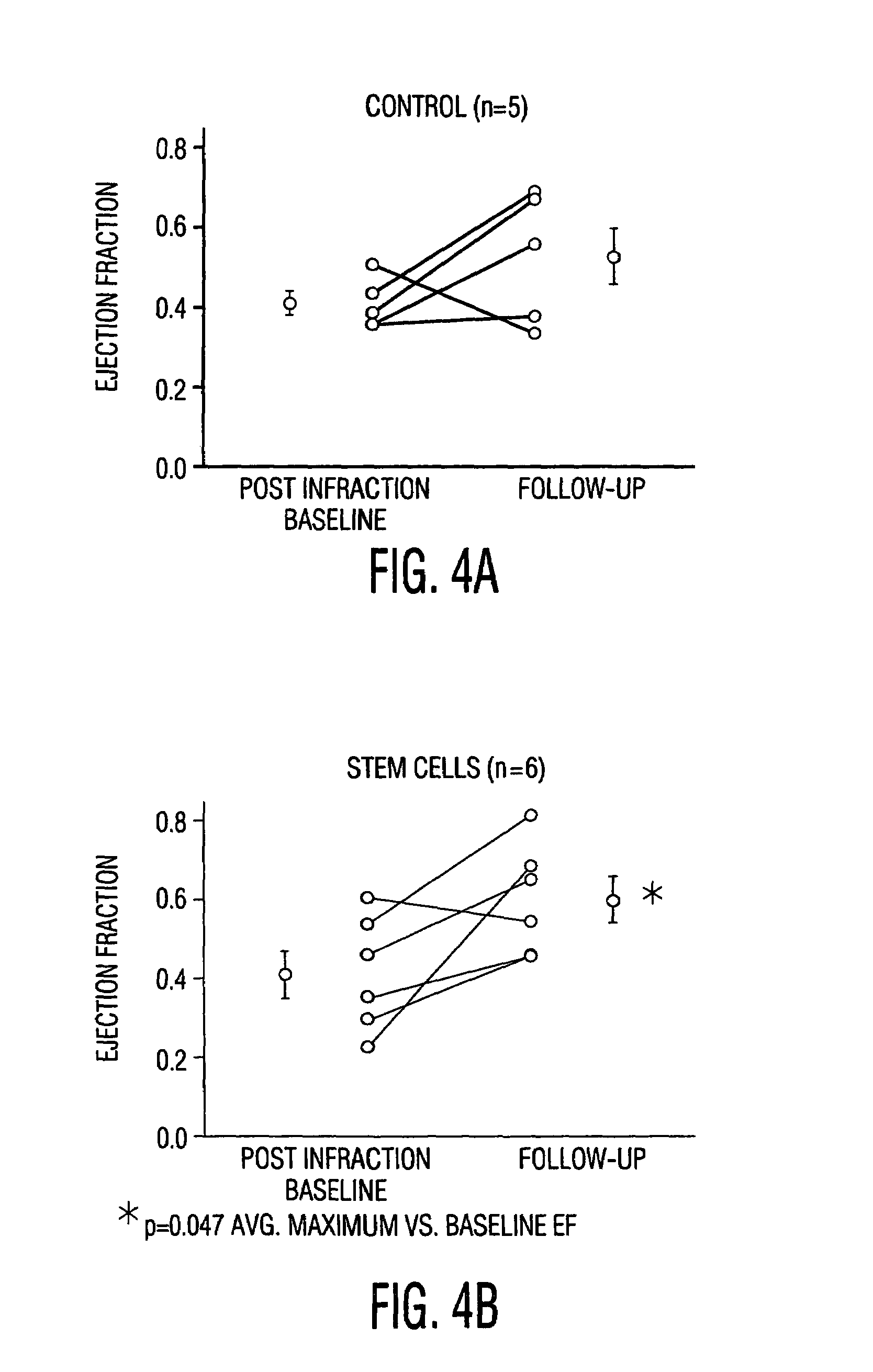
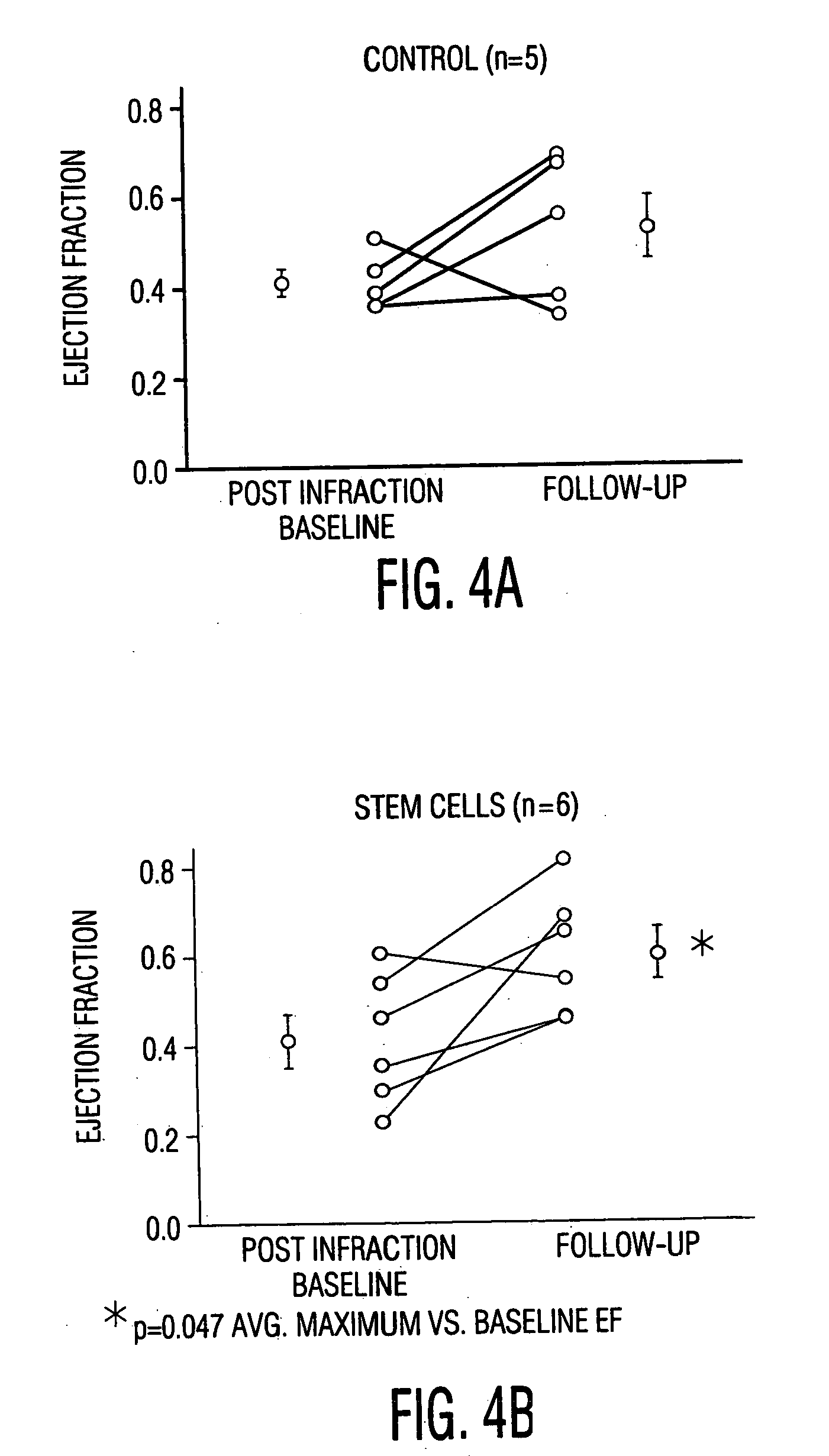
Cardiac muscle regeneration using mesenchymal stem cells
InactiveUS7514074B2Increase opportunitiesShorten the timeBiocideGenetic material ingredientsCardiac muscleMesenchymal stem cell
Disclosed is a method for producing cardiomyocytes in vivo by administering to the heart of an individual a cardiomyocyte producing amount of mesenchymal stem cells. These cells can be administered as a liquid injectible or as a preparation of cells in a matrix which is or becomes solid or semi-solid. The cells can be genetically modified to enhance myocardial differentiation and integration. Also disclosed is a method for replacing cells ex vivo in a heart valve for implantation.
Owner:MESOBLAST INT
Artificial cartilage containing chondrocytes obtained from costal cartilage and preparation process thereof
ActiveUS20090228105A1Ability to useEfficient regenerationBone implantMammal material medical ingredientsMesenchymal stem cellCostal cartilage
The present invention relates to an artificial cartilage containing mesenchymal stem cell (MSC)-like dedifferentiated cells obtained by passage culturing costal chondrocytes, and a preparation process thereof.
Owner:BIO SOLUTION CO LTD +2
Method and device for activating stem cells
InactiveUS20100119492A1Enhance osteoblast phenotypeBioreactor/fermenter combinationsBiocideActive agentOsteoblast
Invention embodiments described herein include methods and devices for stimulating mesenchymal stem cells in a stem cell source to differentiate into osteoblasts capable of forming bone. Devices and methods described include exposing a stem cell source, such as bone marrow aspirate, adipose tissue and / or purified allogenic stem cells, to an active agent, in a manner effective to form activated stem cells.
Owner:DEPUY SYNTHES PROD INC
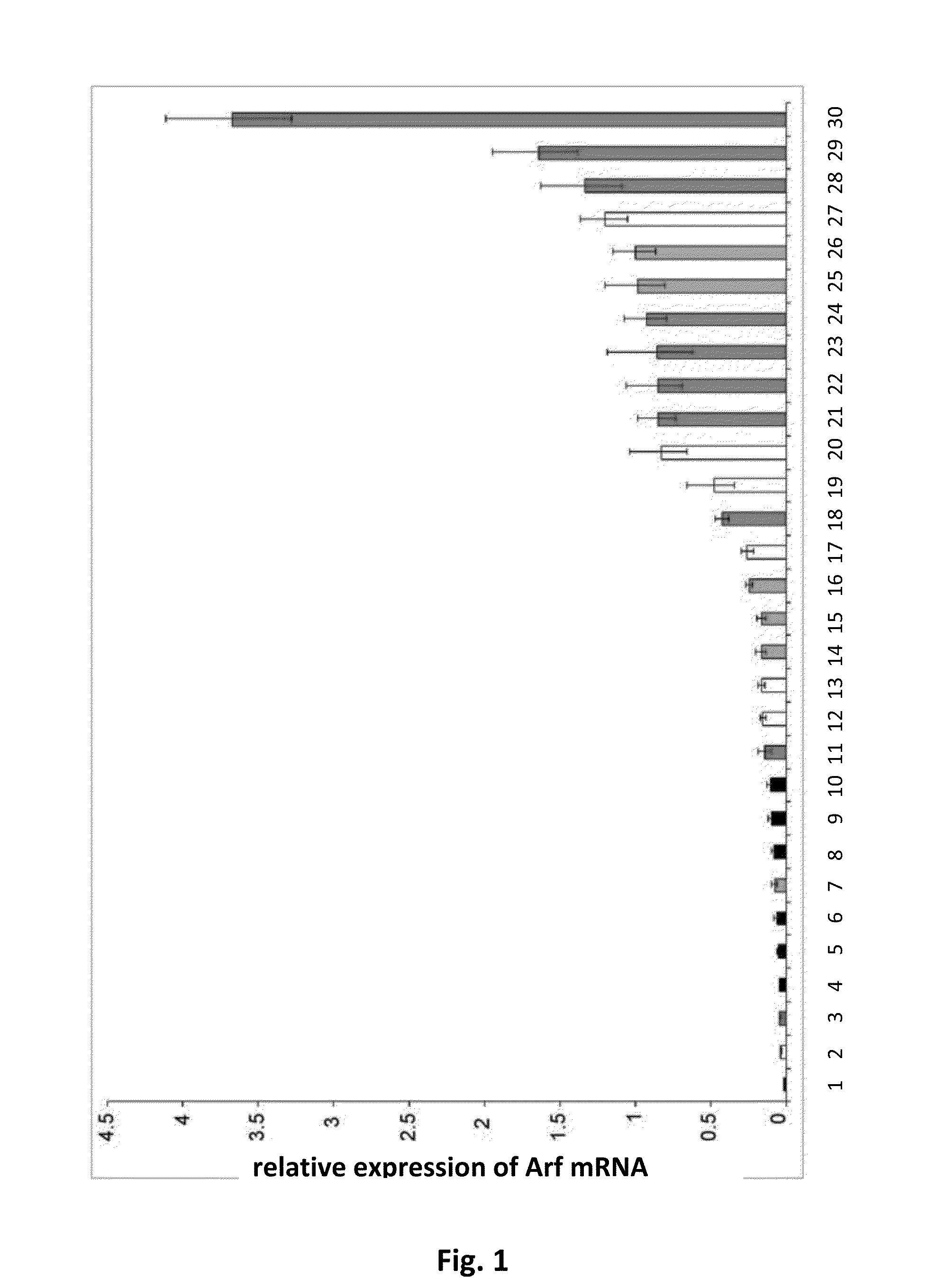
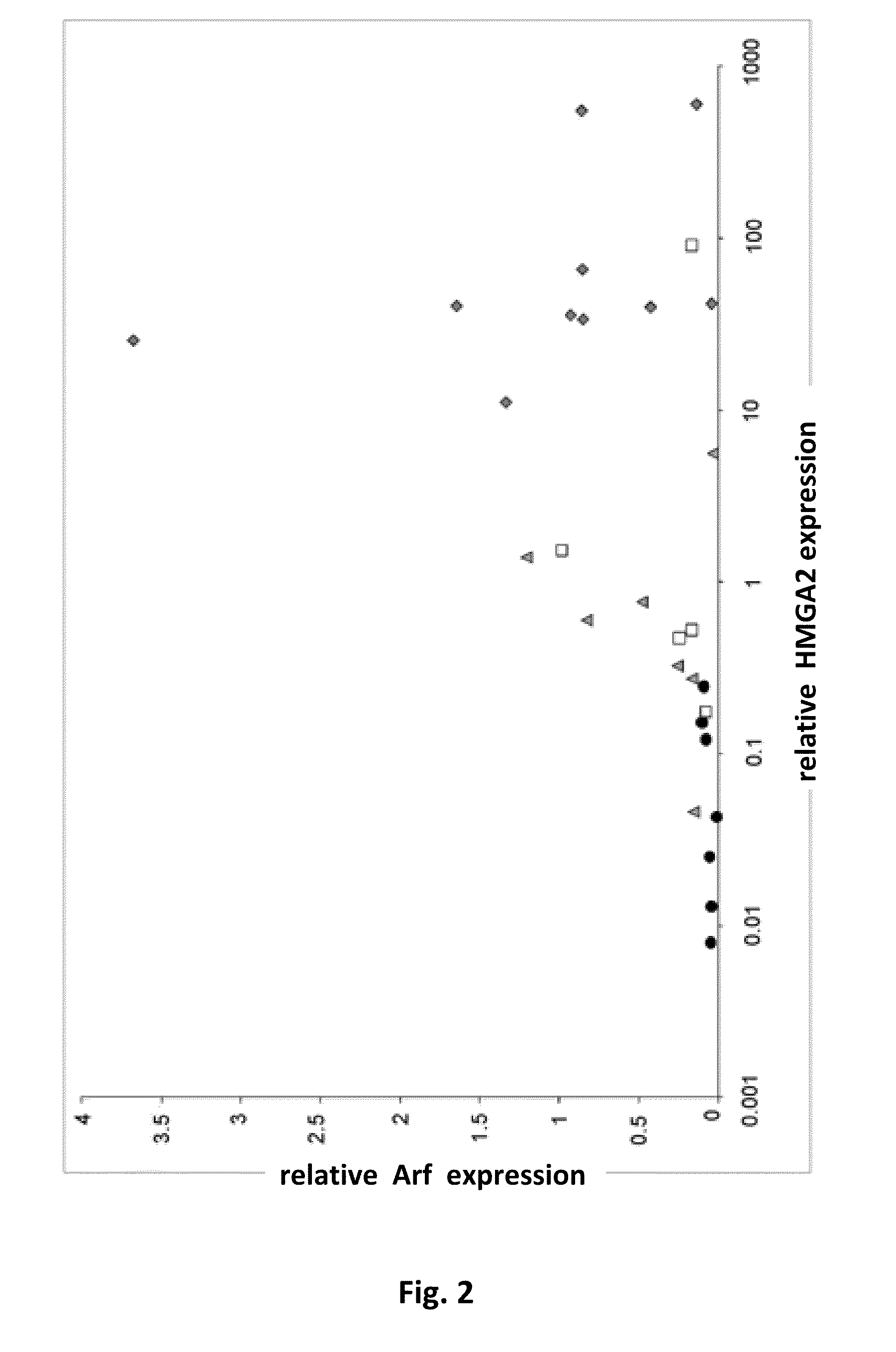
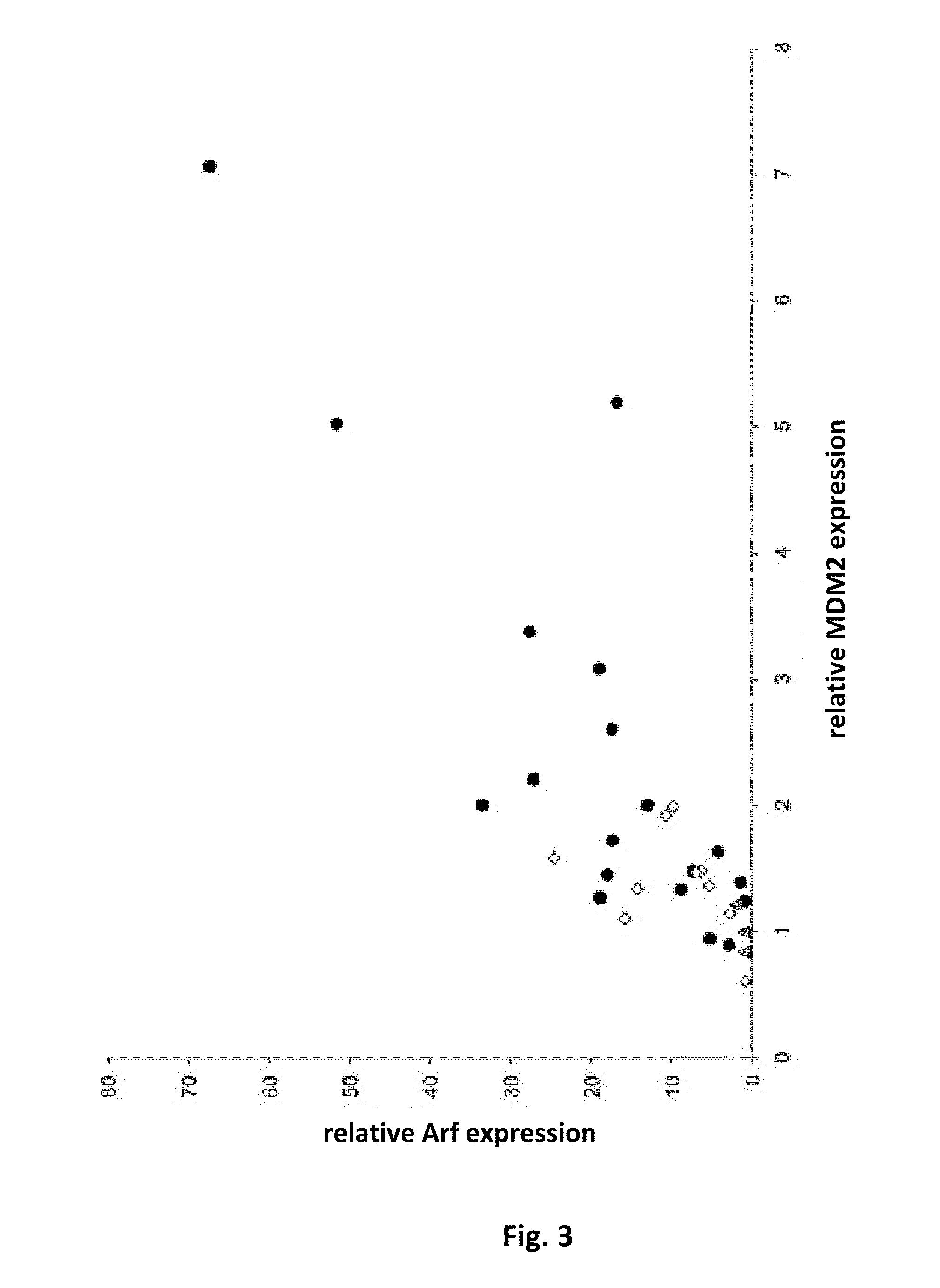
p19Arf, HMGA2 and MDM2 For Use in the Diagnosis and Treatment of Aberrant Cell Growth
InactiveUS20130149314A1Reduced cell capacityOrganic active ingredientsBiocideDiseaseMesenchymal stem cell
Provided are novel methods and compositions for the diagnosis, prognosis and treatment of leiomyomas, in particular uterine leiomyoma (UL). In addition, methods of identifying anti-tumor agents are described. Furthermore, novel methods and compositions are provided for the treatment of diseases characterized by an aberrant growth of mesenchymal stem cells and their descendants and for the treatment of obesity are disclosed.
Owner:UNIV OF BREMEN
Serum-free medium for mesenchymal stem cells
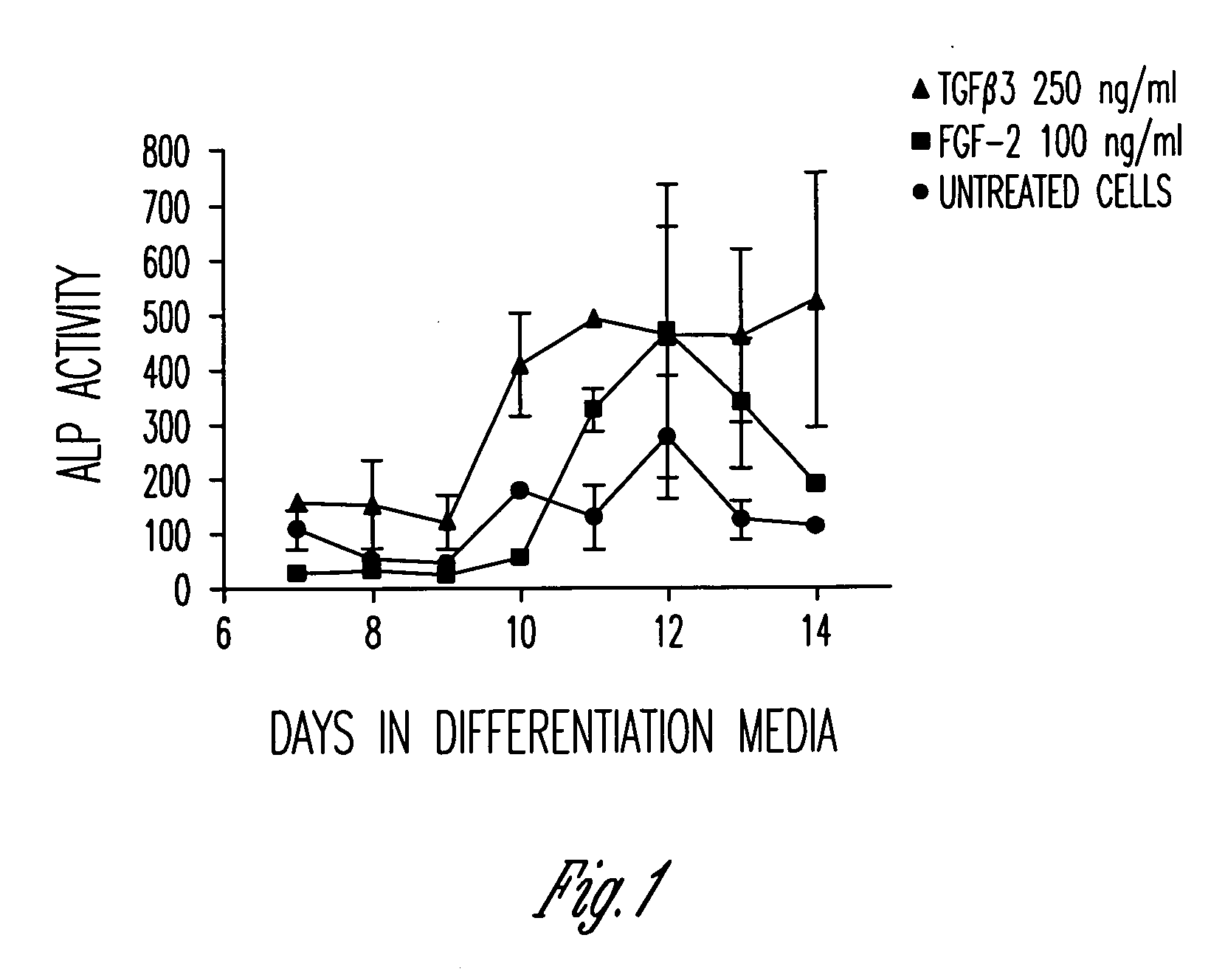
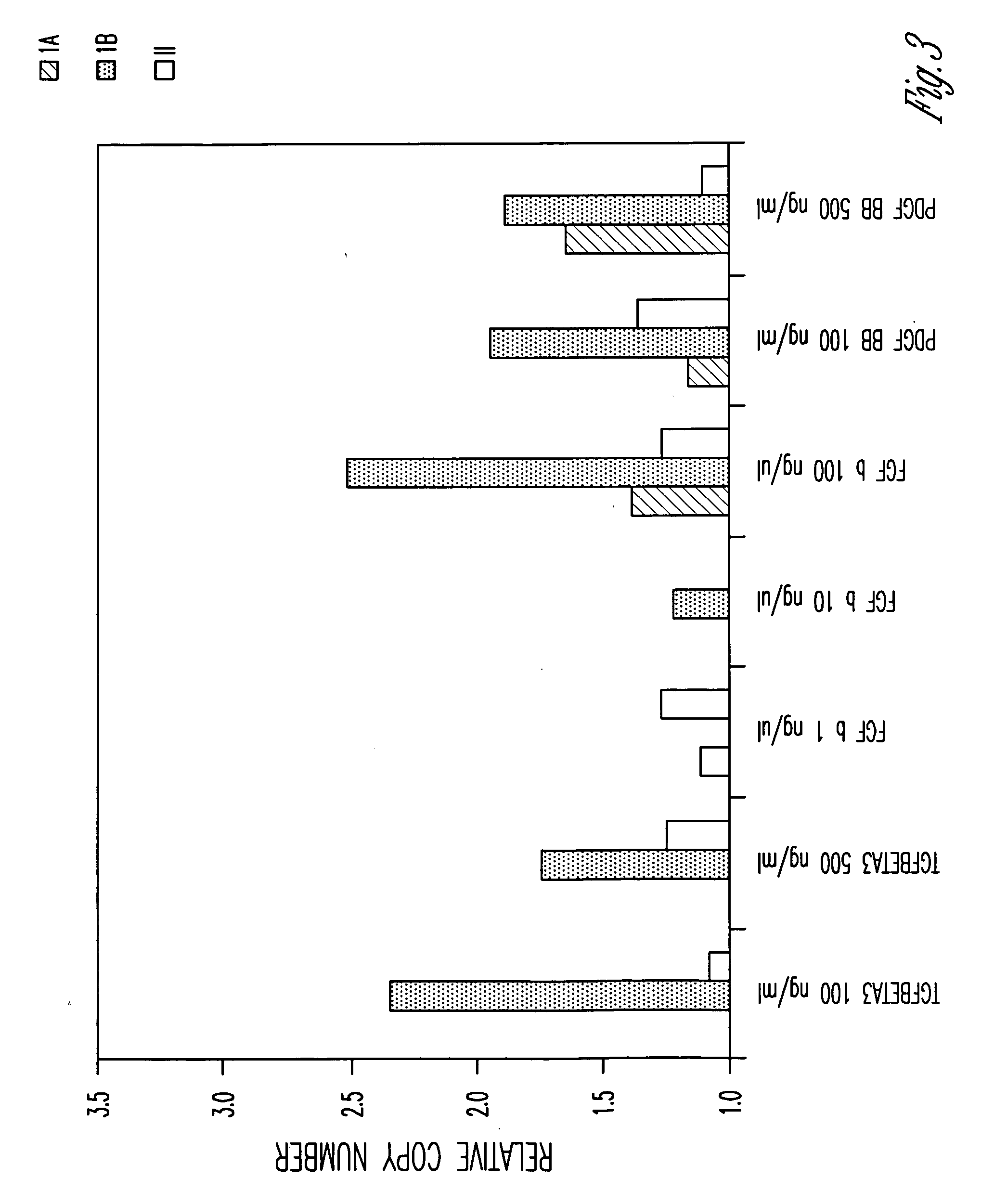
Serum-free media for growth and proliferation of chondrocytes and mesenchymal stem cells in culture are provided. A serum-free medium for growth of chondrocytes includes a serum-free composition comprising FGF-2, linoleic acid, ascorbic acid, B-mercaptoethanol, transferrin and dexamethasone. Further, the composition comprises EGF, PDGFbb, insulin and albumin. A method for growing chondrocytes in a serum free medium comprising the compostion is also provided. Also provided for mesenchymal stem cell growth, is a serum-free medium which includes a composition comprising FGF-2, LIF, SCF, pantotenate, biotin and selenium and method, therefore.
Owner:CONSORZIO PER LA GESTIONE DEL CENT DI BIOTECNOLOGIA AVANZATA +1
Cardiac muscle regeneration using mesenchymal stem cells
InactiveUS20050112104A1Shorten the timeEasy to convertBiocideGenetic material ingredientsCardiac muscleMesenchymal stem cell
Disclosed is a method for producing cardiomyocytes in vivo by administering to the heart of an individual a cardiomyocyte producing amount of mesenchymal stem cells. These cells can be administered as a liquid injectible or as a preparation of cells in a matrix which is or becomes solid or semi-solid. The cells can be genetically modified to enhance myocardial differentiation and integration. Also disclosed is a method for replacing cells ex vivo in a heart valve for implantation.
Owner:MESOBLAST INT
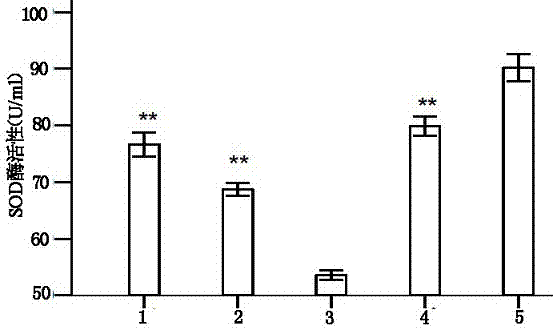
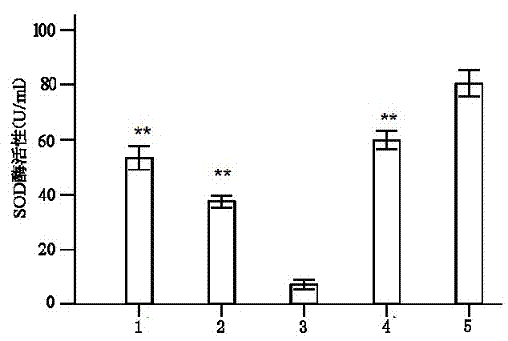
Serum-free culture medium for mesenchymal stem cells
ActiveCN102827807AAvoid instabilityClear chemical compositionSkeletal/connective tissue cellsCell phenotypeSodium bicarbonate
The invention relates to the field of biology, and discloses a serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, Hepes, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, protocatechuic acid, lipid, amino acid, vitamins, trace elements, Pluronic F-68, hydrocortisone, vitamin C, bonding amine or recombinant human fibronectin, progesterone, putrescine, heparin, serotonin, epidermal growth factors (EGFs), b-fibroblast growth factors (FGF), platelet derive growth factor (PDGF)-BB and insulin-like growth factor (IGF)-I. The serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation and avoids the doped animal components and unstable batches, and the results of the cultured mesenchymal stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
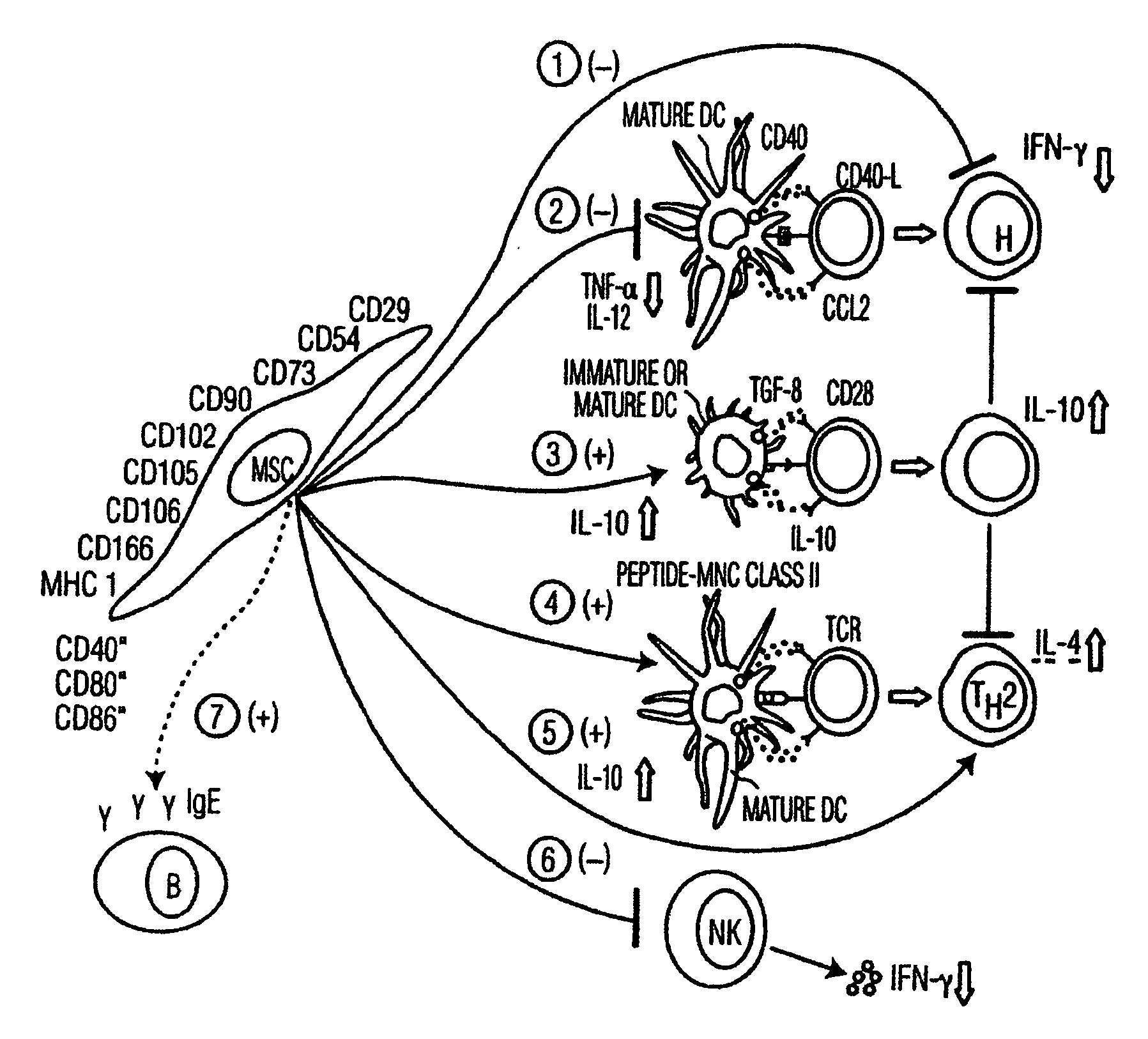
Mesenchymal stem cells and uses therefor
InactiveUS20080095749A1Improve immunityPromotes tumor suppressionBiocideNervous disorderAutoimmune responsesAutoimmune disease
Methods of treating autoimmune diseases, allergic responses, cancer, inflammatory diseases, or fibrosis in an animal, promoting would healing, repairing epithelial damage and promoting angiogenesis in an organ or tissue of an animal by administering to the animal mesenchymal stem cells in an effective amount.
Owner:MESOBLAST INT
Engineered three-dimensional connective tissue constructs and methods of making the same
InactiveUS20140099709A1Improve adaptabilitySuitable for implantationBone implantLigamentsConnective tissue fiberStromal cell
Disclosed are engineered, living, three-dimensional connective tissue constructs comprising connective tissue cells. In some embodiments, the connective tissue cells are derived from multi-potent cells such as mesenchymal stem / stromal cells. In some embodiments, the cells are cohered to one another. In some embodiments, the multi-potent cells have been exposed to one or more differentiation signals to provide a living, three-dimensional connective tissue construct. In some embodiments, the constructs are substantially free of pre-formed scaffold at the time of use. Also disclosed are implants for engraftment, arrays of connective tissue constructs for in vitro experimentation, as well as methods of making the same.
Owner:ORGANOVO
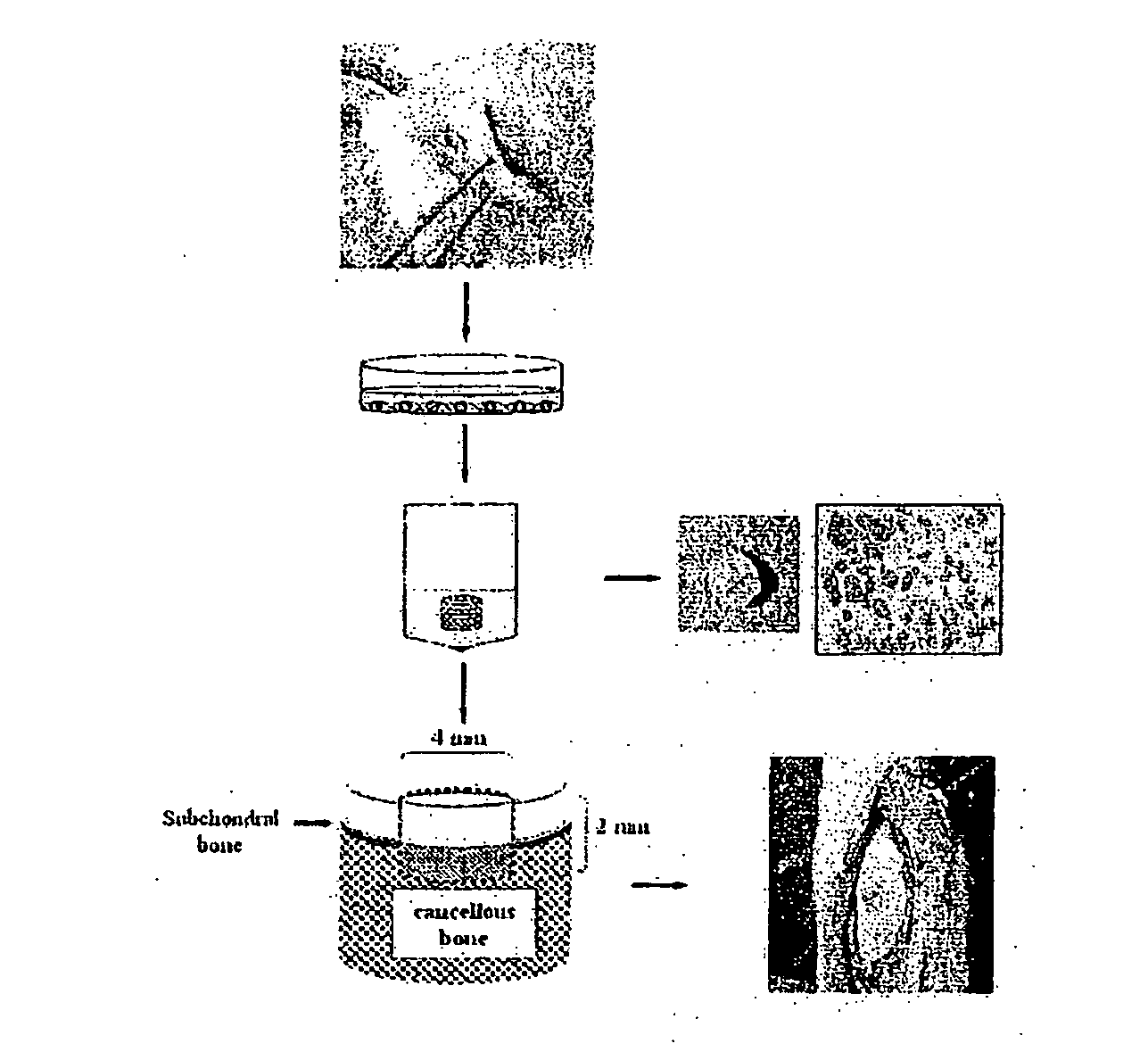
Cartilage repair methods
The present application discloses methods for repairing hyaline cartilage defects. The methods comprise a combination of introducing autologous bone mesenchymal stem cells to a joint, and applying to the joint a membrane comprising a polyester entangled with a polysaccharide. In some aspects, the bone mesenchymal stem cells are mesenchymal stem cells originating in bone underlying the joint. In these aspects, contact between the joint and the mesenchymal stem cells can be effected by introducing apertures through the bone using standard surgical techniques such as microfracture, abrasion, or drilling. Cartilage which forms in response to application of these methods is hyaline cartilage rather than fibrocartilage.
Owner:ISTO TECH II LLC
Compositions and methods for the stimulation or enhancement of bone formation and the self-renewal of cells
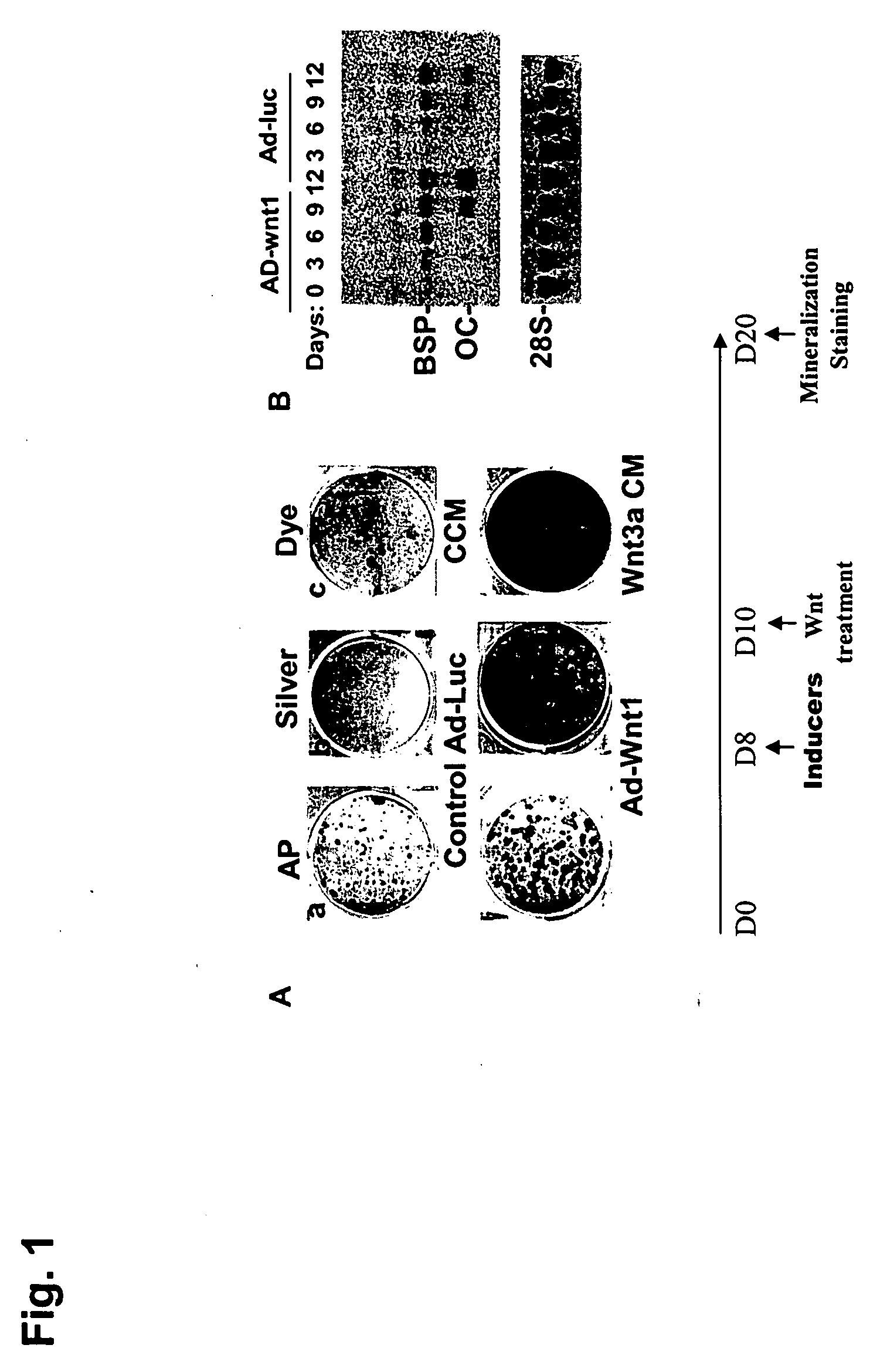
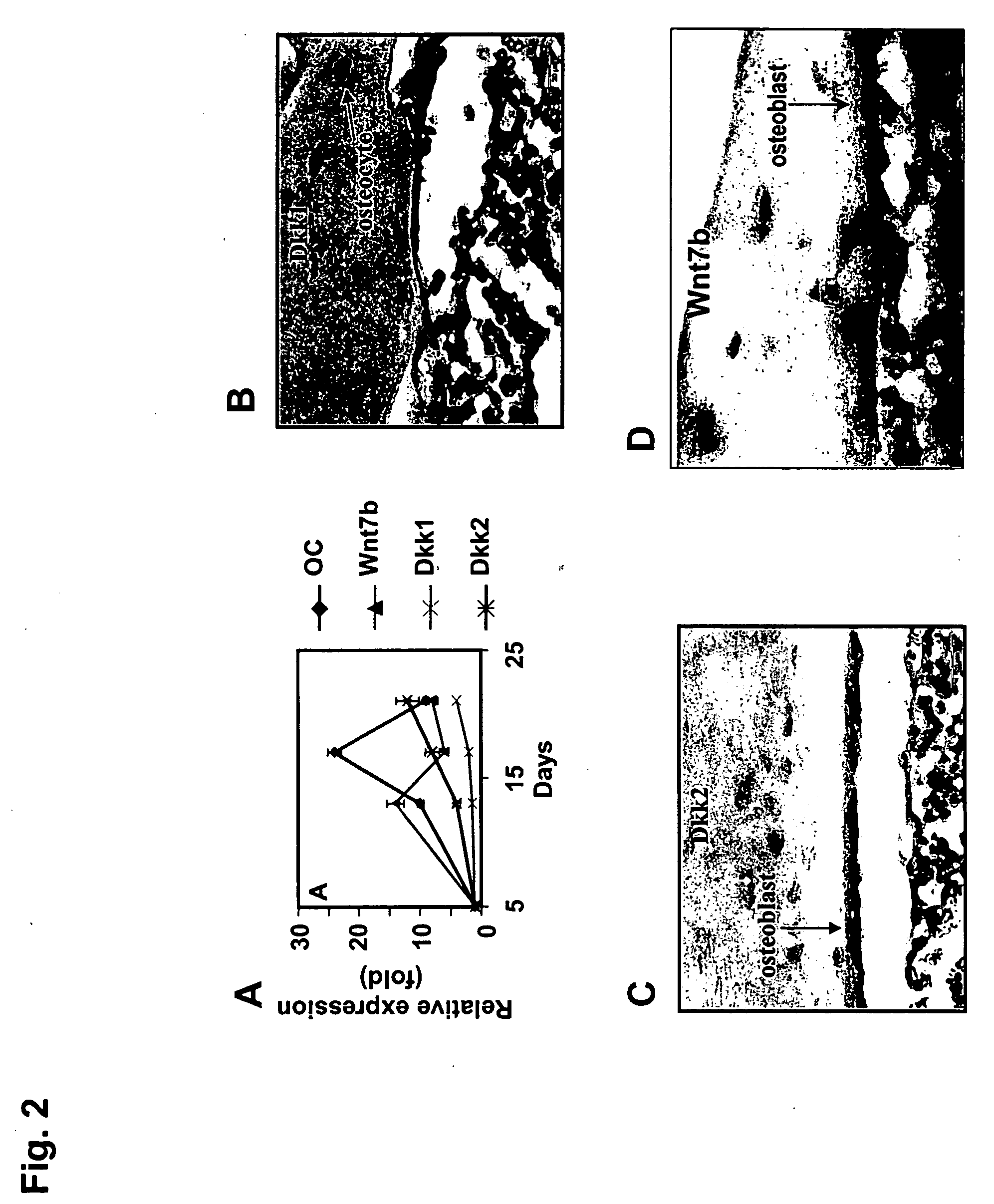
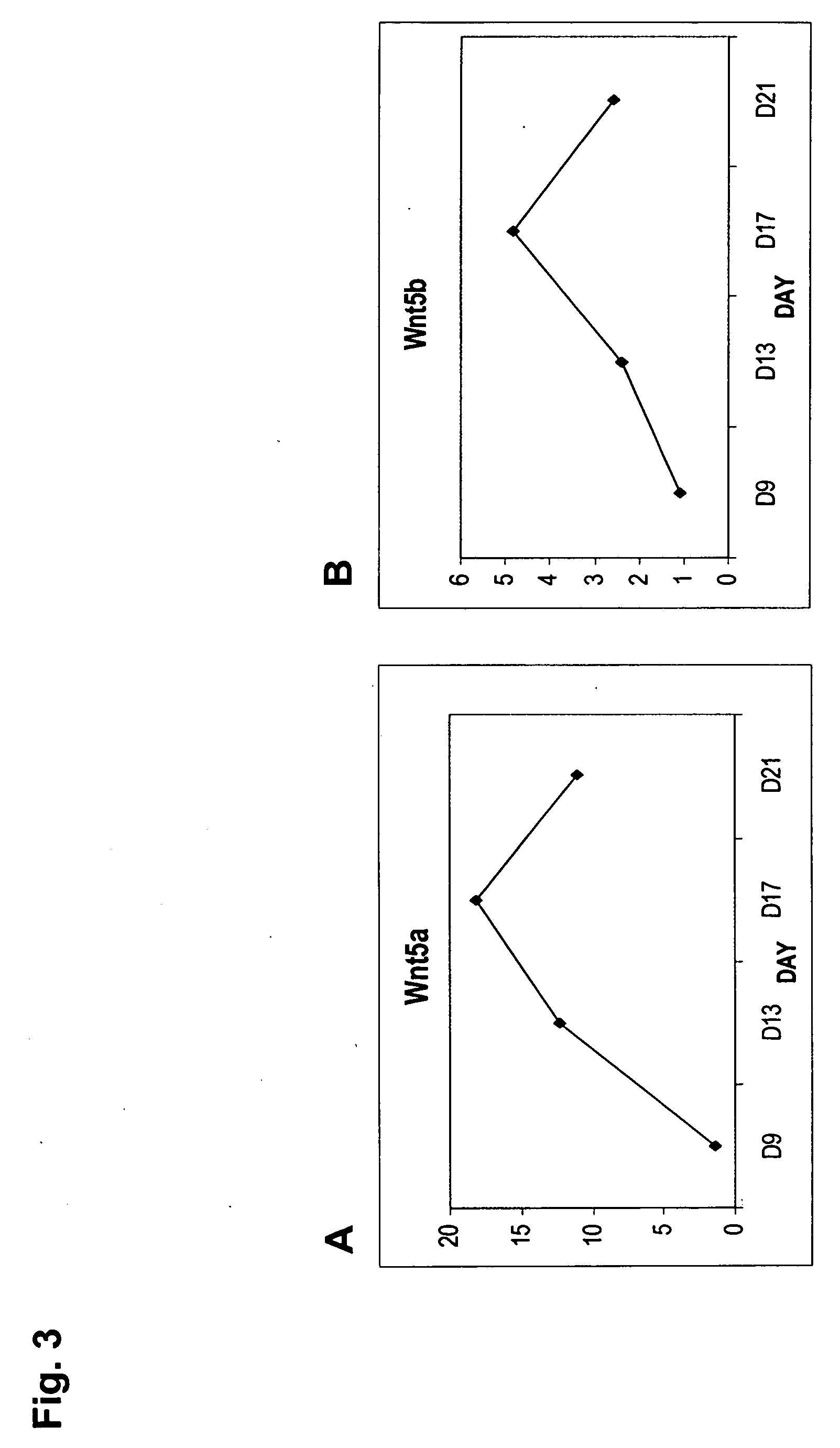
ActiveUS20050261181A1Improve biological activityPromote mineralizationBiocidePeptide/protein ingredientsOsteoblastWnt inhibitor
Compositions and methods for the treatment of bone diseases, bone fractures, bone injuries and other bone abnormalities involving the use of Dkk protein, a Wnt antagonist, a Wnt inhibitor, or any other related protein for the stimulation or enhancement of mineralization and for stimulating the renewal of cells. One Dkk protein, Dickkopf-2 (Dkk-2), acts to stimulate bone formation independently of Wnt proteins which may be inhibited and / or antagonized by Dkk-2. Dkk-2 displayed enhanced specific targeting ability and enhanced biological activity in stimulating or enhancing mineralization. Dkk-2 also played a role in the differentiation and self-renewal of hematopoietic stem cells and mesenchymal stem cells, particularly in osteoblastogenesis and osteoclastogenesis.
Owner:ENZO BIOCHEM
Nanofiber scaffold
ActiveUS20110142804A1Easy to controlEasy to disassembleBiocidePharmaceutical delivery mechanismNanofiber scaffoldMedicine
The invention is directed to a device and method to prevent migration of Human Mesenchymal Stem Cells (hMSCs) from a delivery site while allowing communication between the stem cells and native cardiomyocytes. The device is characterized by scaffold pore size, fiber diameter and biomaterial selection. The invention includes a two part polyurethane scaffold that prevents migration of stem cells, allows gap junction formation through pores and is packaged for minimally invasive delivery.
Owner:BIOSURFACES +1
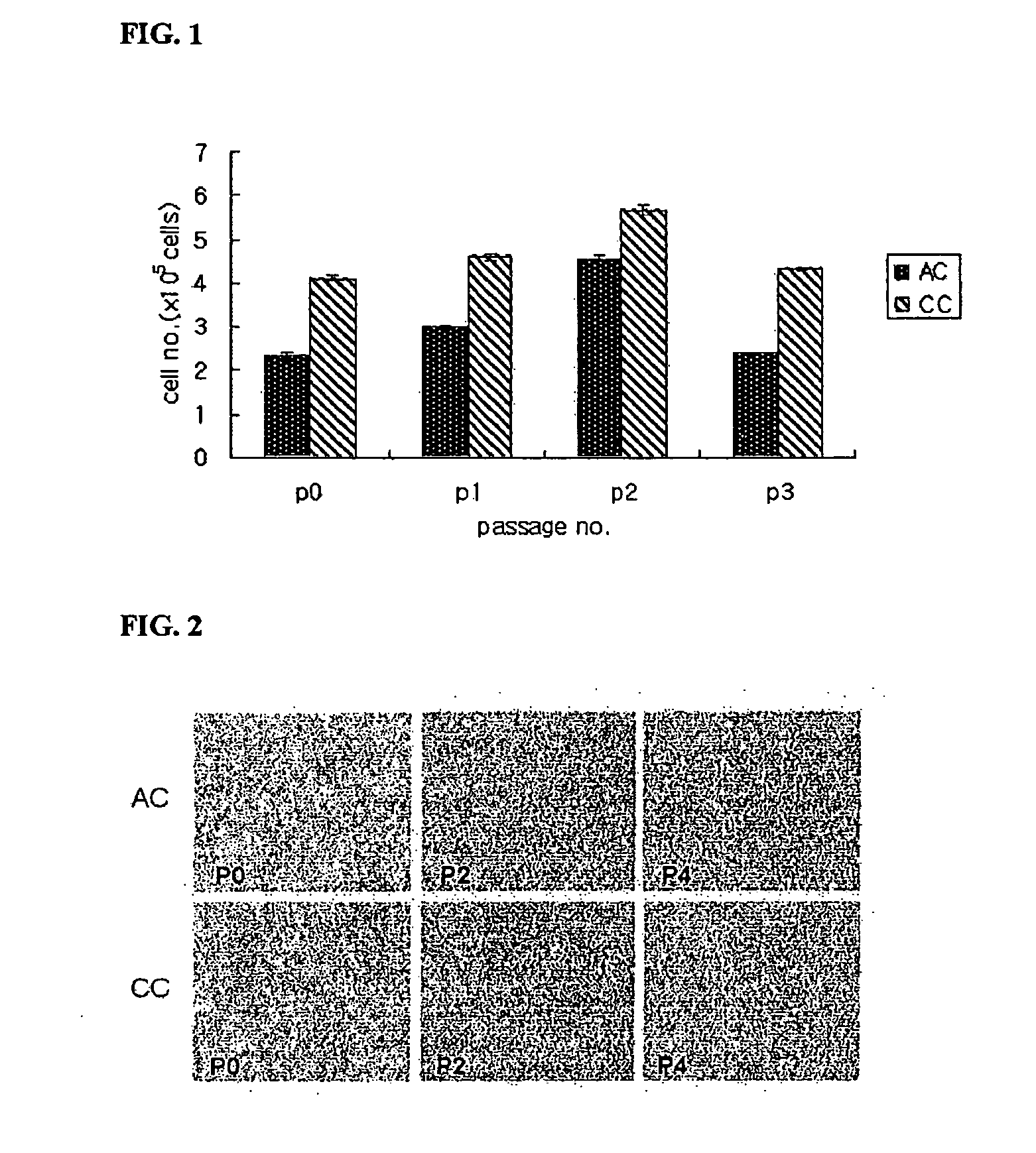
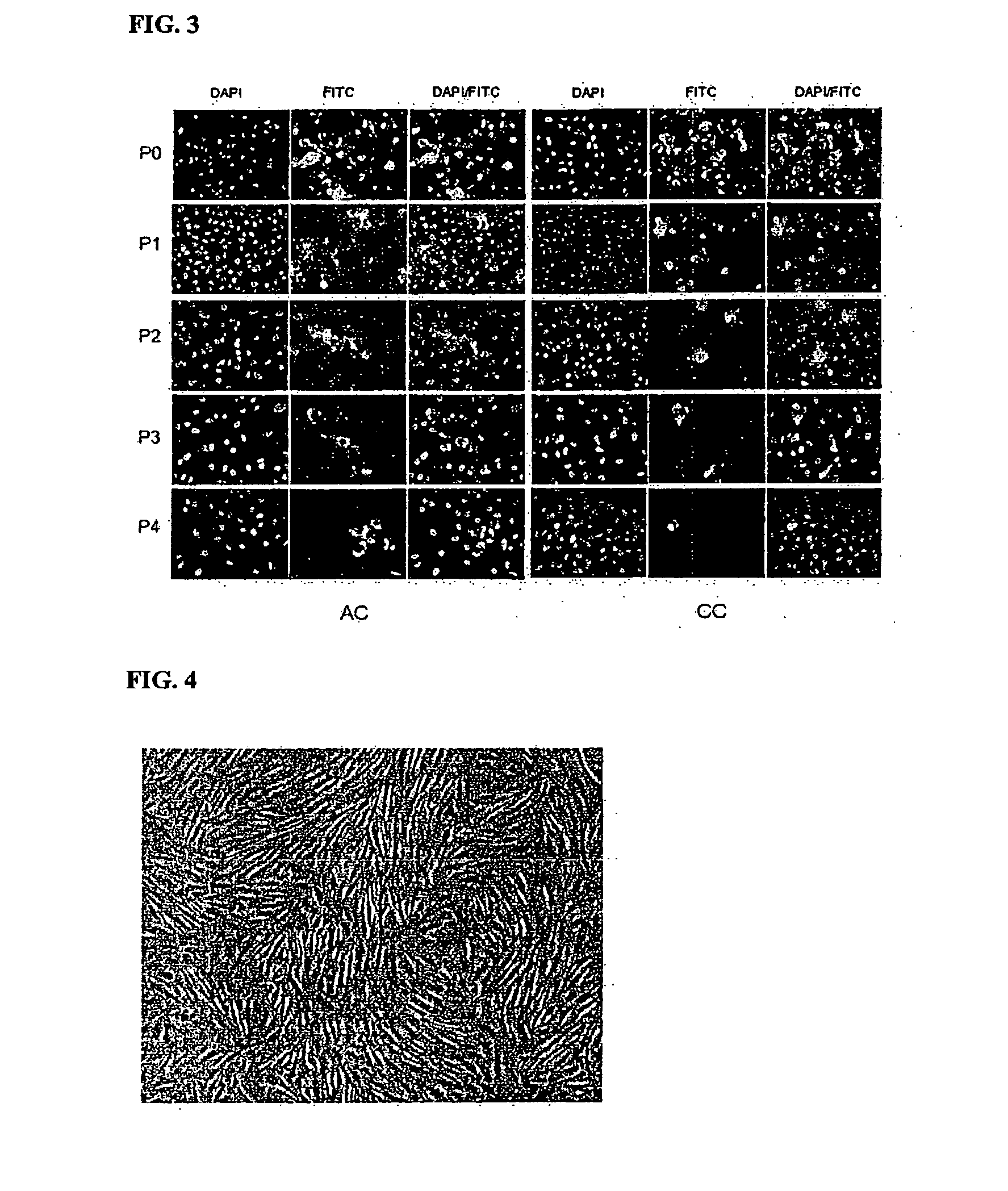
Method for preparing human umbilical cord mesenchymal stem cells
The invention relates to a method for preparing human umbilical cord mesenchymal stem cells, which comprises the steps of collection, transportation, handover, separation, freezing, recovering, primary culture and subculture of umbilical cords. The method has simple process, ensures low production cost, good freezing effect, little cell damage, low toxic side effect and no harm to human body and reduces the residual of the freezing protection liquid in the recovered cells of tissue blocks; and the prepared mesenchymal stem cells can be applied to the clinical treatment of various diseases.
Owner:江苏省北科生物科技有限公司 +1
Stem Cells For Treating Lung Diseases
InactiveUS20090274665A1Optimize allocationGood treatment effectBiocidePeptide/protein ingredientsDiseaseMesenchymal stem cell
The invention is compositions and methods for treating lung diseases and conditions using mesenchymal stem cells. The preferred stem cells are those derived from a human umbilical cord, or from bone marrow.
Owner:CELL THERAPY TECH INC ET AL
Cryopreservation of Adipose Tissue for the Isolation of Mesenchymal Stem Cells
The present invention relates to a method and composition for the cryopreservation of adipose tissue with the intention to use this tissue in the culturing of stem and / or progenitor cells. The method uses a specific cryoprotection medium to prevent damage of the original tissue during the cryopreservation while still maintaining a high viability of the stem and / or progenitor cells obtained from the cryopreserved adipose tissue. Furthermore the cryoprotection medium of the present invention does not contain any kind of xenogeneic sera, a critical factor since it is the intention of that the cryopreserved tissue is used for obtaining stem and / or progenitor cells that can be used in medicine. The cryoprotection medium is characterized in that it is a solution of physiological water comprising glycerol and sucrose and / or trehalose and optionally serum albumin.
Owner:CRYO SAVE
Clinical-grade human mesenchymal stem cell serum-free complete medium
ActiveCN103243071APromote growthLow toxicitySkeletal/connective tissue cellsInsulin-like growth factorCuticle
The invention relates to a human mesenchymal stem cell culture medium. According to the culture medium, the basal culture medium comprises the following components based on the final concentration: 1-2g / L of human serum albumin, 5-10mg / L of transferring, 2-8mg / L of fibronectin, 1-4mg / L of laminin, 50g / L of Fe(NO3)3.9H2O, 417g / L of FeSO4.7H2O, 1-3mu g / L of estradiol, 2-5mu g / L of testosterone, 1-3mu g / L of progesterone, 39.25-117.74 mu g / L of dexamethasone, 5-10mg / L of insulin, 376.36mg / L of riboflavin, 80.96-242.87mg / L of coenzyme A, 4.41-6.17mg / L of butanediamine, 1-2mg / L of taurine, 0.61-1.85mg / L of aminoethanol, 8.81-26.42mg / L of pyruvic acid, 3.78-7.56mu g / L of sodium selenate, 292.3-584.6mg / L of L-glutamine, 2-8mu g / L of vascular endothelial growth factor, 4-10mu g / L of epidermal growth factor, 4-10mu g / L of basic fibroblast growth factor, 1-5mu g / L of leukaemia inhibitory factor, 1-5mu g / L of insulin-like growth factor-I and 2-8mu g / L of stem cell factor. The culture medium does not contain the animal serum, the potential animal endogenous endotoxin or virus of the animal serum is eliminated, and the culture medium is conveniently applied to clinics.
Owner:QINGDAO RESTORE BIOTECHNOLOGY CO LTD
Human fat mesenchymal stem cell extract and lyophilized powder and application thereof
ActiveCN103784474ABiologically activeEasy to prepareCosmetic preparationsToilet preparationsBiotechnologyChemical products
The invention discloses a human fat mesenchymal stem cell extract and lyophilized powder and applications thereof. The human fat mesenchymal stem cell extract is prepared by the steps: collecting subcultured P3-P30 generations of human fat mesenchymal stem cells, culturing with cell growth liquid until 80% of cells are fused, removing culture liquid, washing the cells with a phosphate buffer solution for three times, adding into a serum-free culture solution, continuously culturing for 72 hours, collecting the serum-free culture solution, digesting uncollected cells with a digestion solution, crushing the cells with an ultrasonic crushing device, centrifuging, and then collecting supernatant; and mixing the collected serum-free culture solution and the collected supernatant, filtering, collecting filtrate, concentrating, and sterilizing. The human fat mesenchymal stem cell extract and the lyophilized powder thereof have multiple biological activities, can be used for preparing wound healing or cell repair medicaments and other biopharmaceutical products, and can also be used for preparing whitening products or skin anti-wrinkle products and other fine chemical products.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
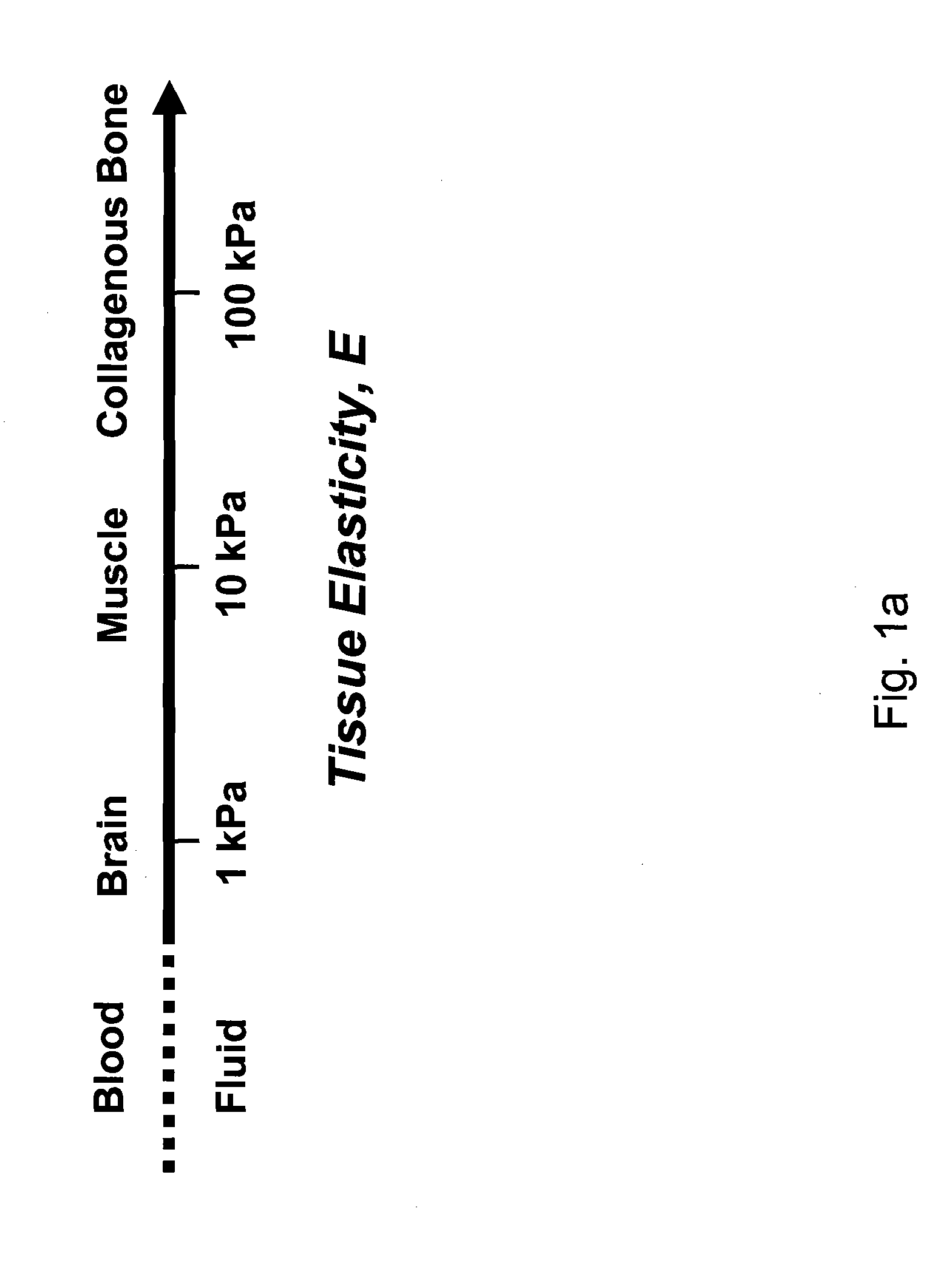
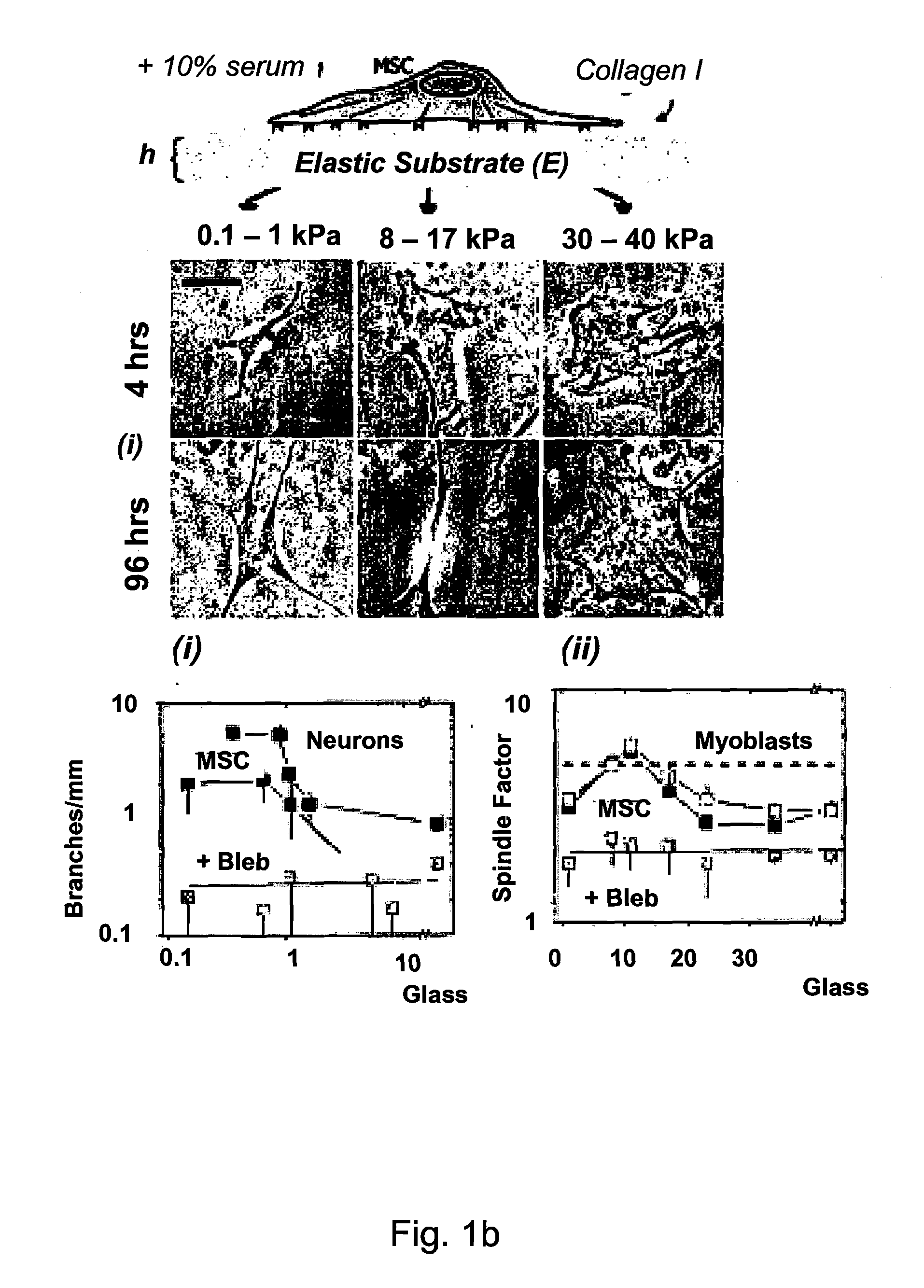
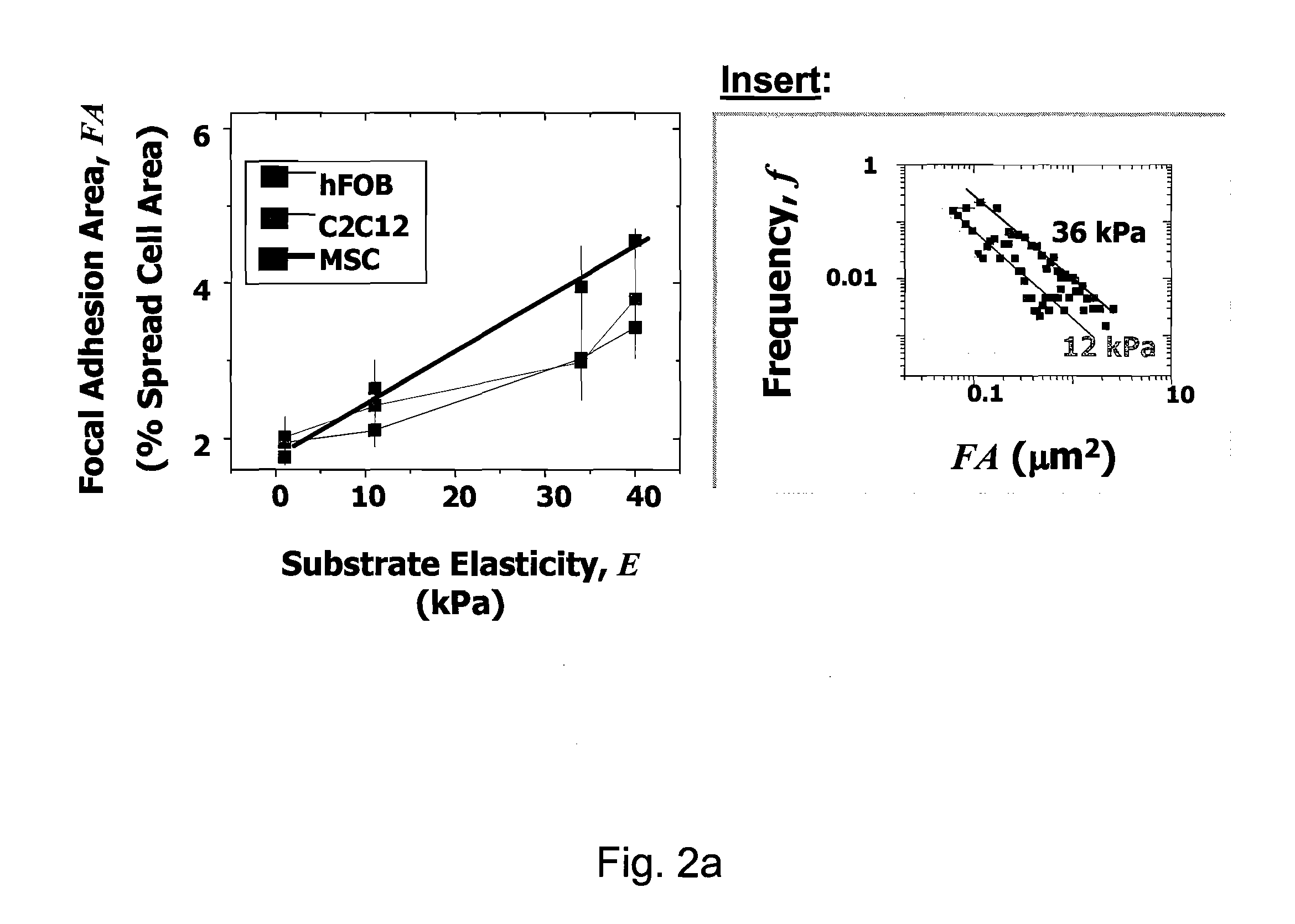
Regulating Stem Cell Differentiation By Controlling 2D and 3D Matrix Elasticity
InactiveUS20100015709A1Cell culture supports/coatingSkeletal/connective tissue cellsMyosinMesenchymal stem cell
Provided are methods for the selection and regulation of the mechanical properties of 2D or 3D biocompatible substrates or tissue microenvironments as a technique to regulate in vitro differentiation, cell shape and / or lineage commitment of anchorage-dependent cells, such as mesenchymal stem cells into, e.g., neurogenic-, myogenic-, and osteogenic-type cells. Substrate mechanical properties include elasticity, tension, adhesion, and myosin-based contractile mechanisms. Inhibitors can be introduced to further regulate differentiation.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
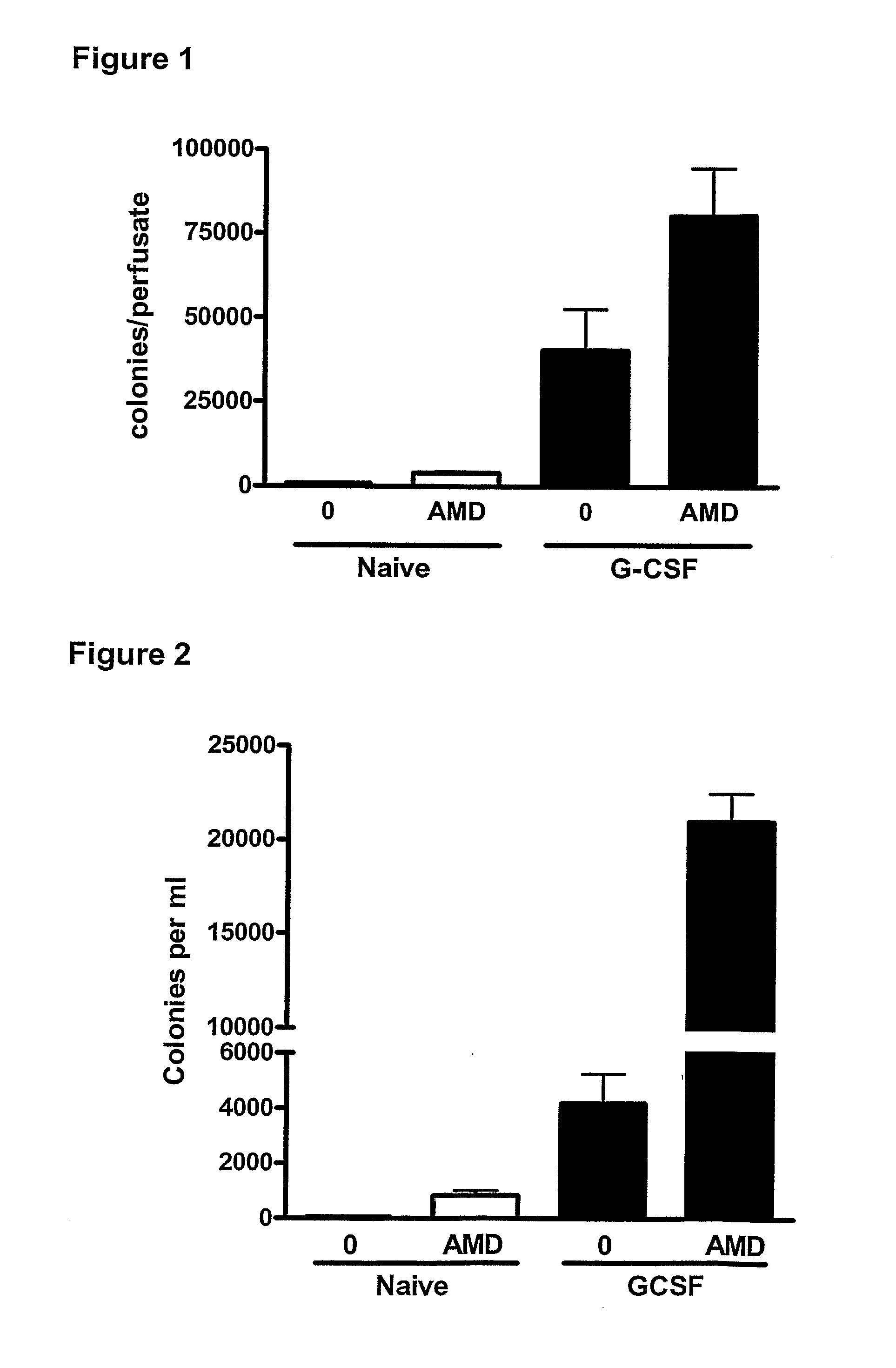
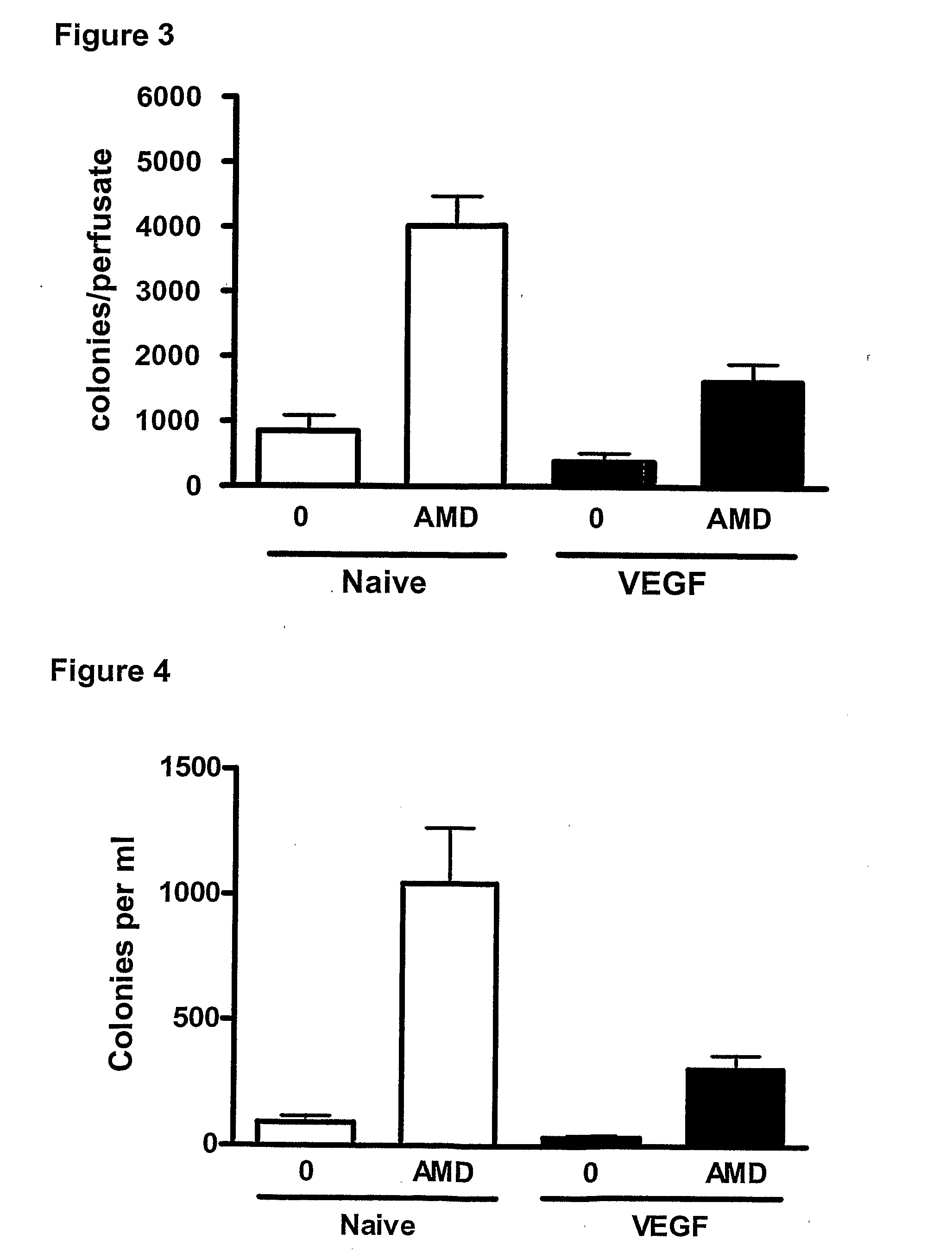
Methods
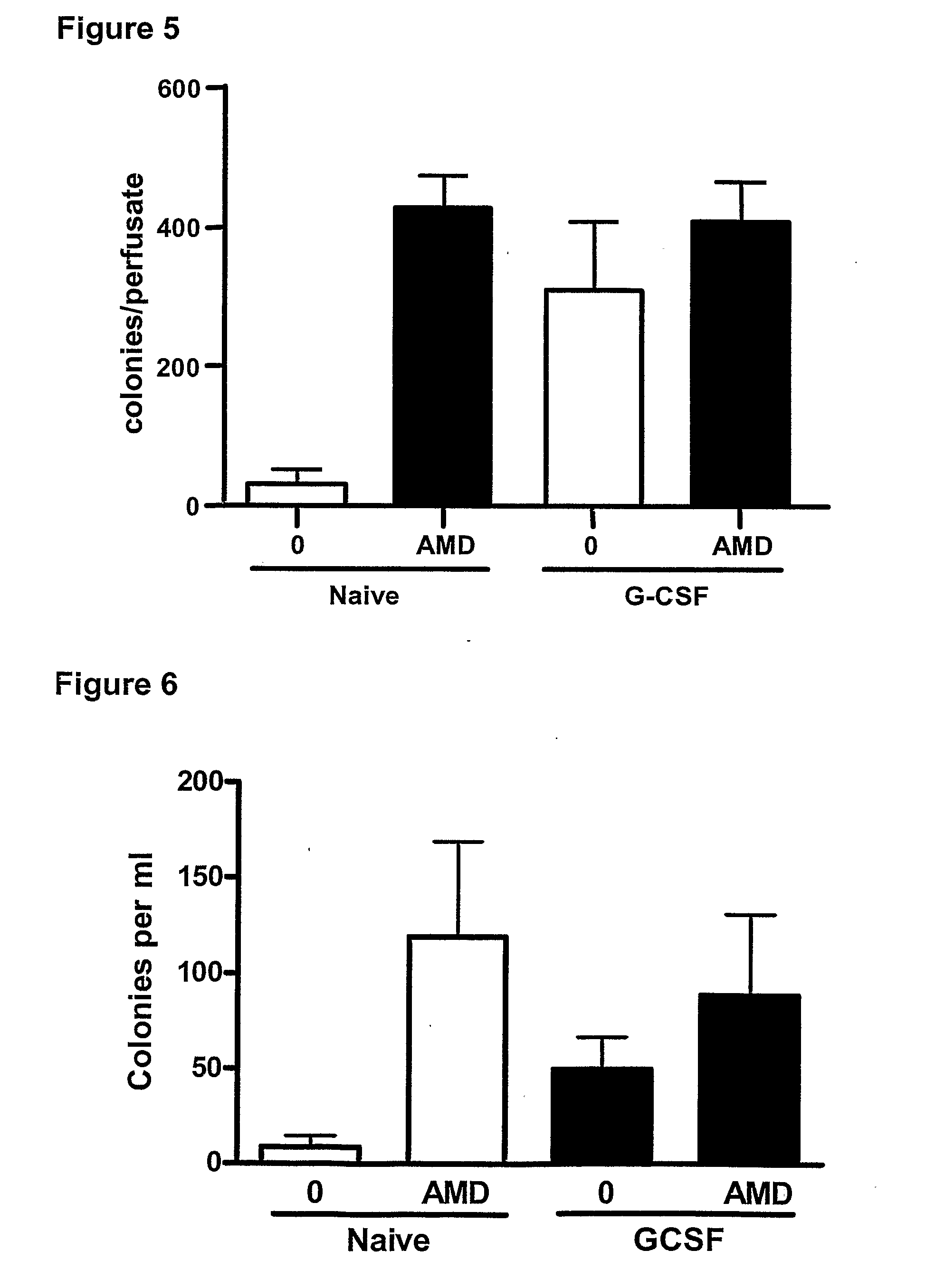
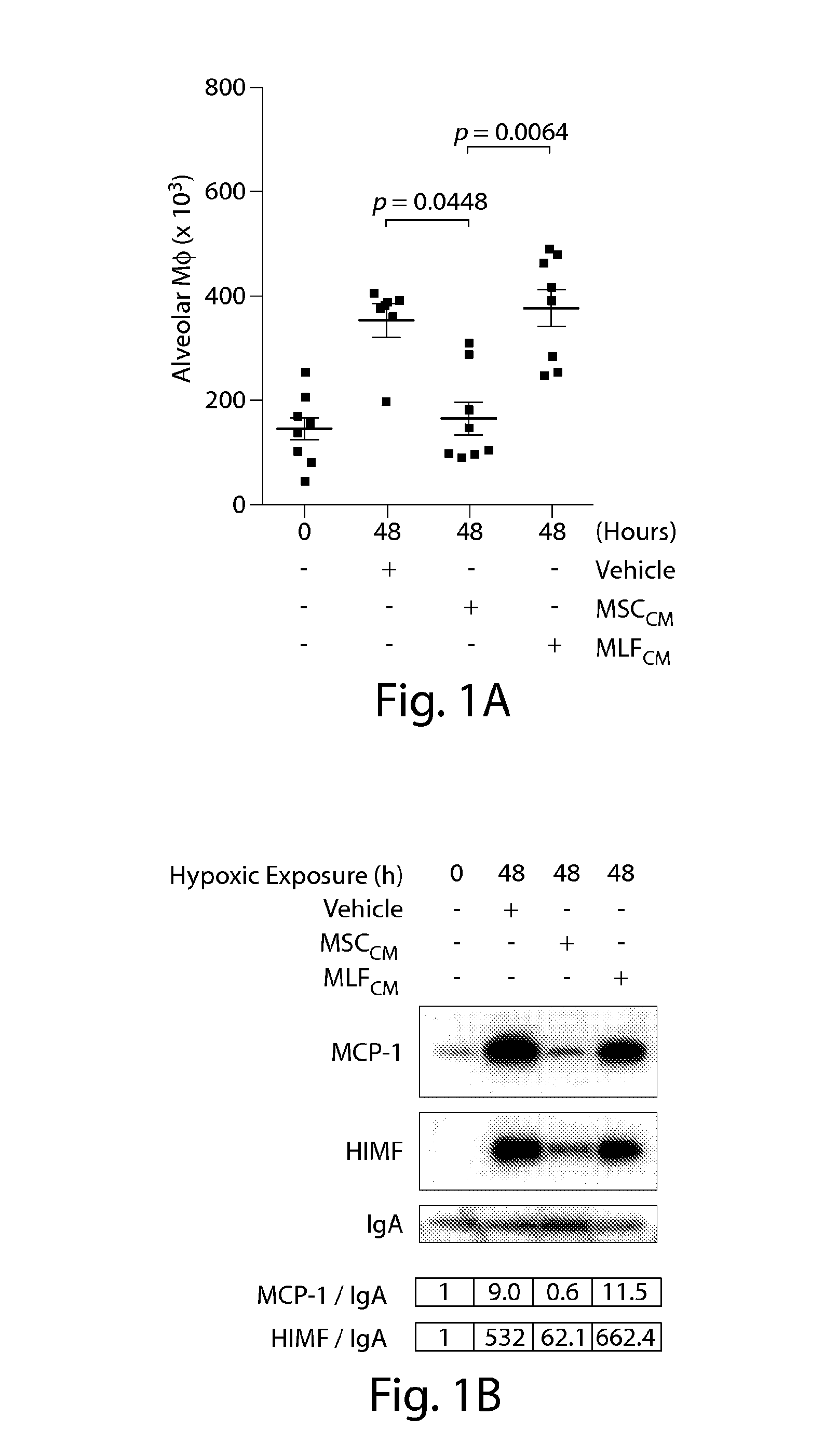
InactiveUS20110044997A1Reduce unwanted side effectPreventing agonism of unnecessary targetsBiocideNervous disorderProgenitorCXCR4
The current invention provides a method for mobilising endothelial progenitor cells (EPC) and / or mesenchymal stem cells (MSC) in a patient, wherein the method comprises the steps of (i) administering a vascular endothelial growth factor receptor (VEGFR) agonist to the patient; and (ii) administering an antagonist of CXCR4 to the patient. Also provided are uses of EPC and MSC harvested using the methods of the invention.
Owner:IMPERIAL INNOVATIONS LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com