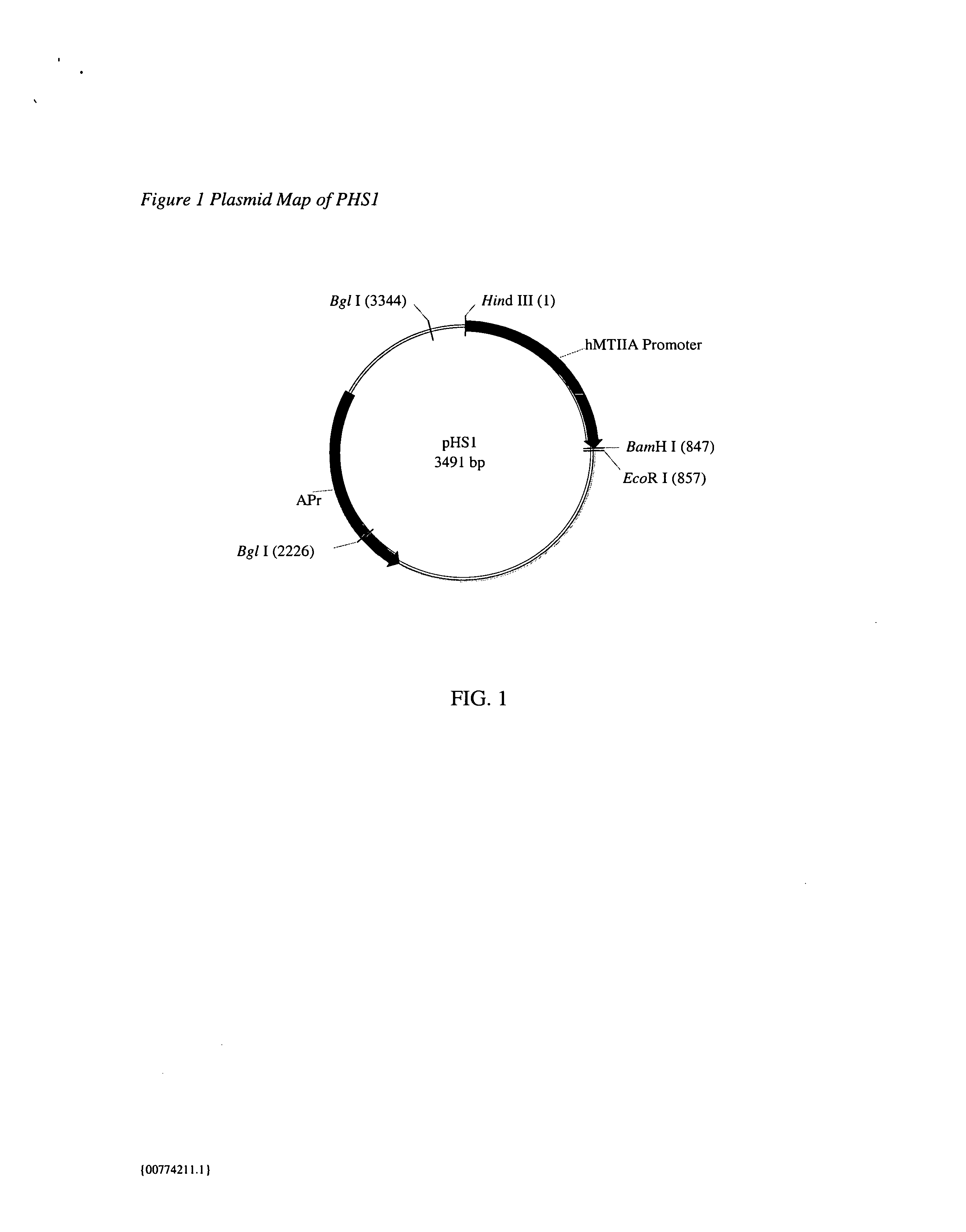
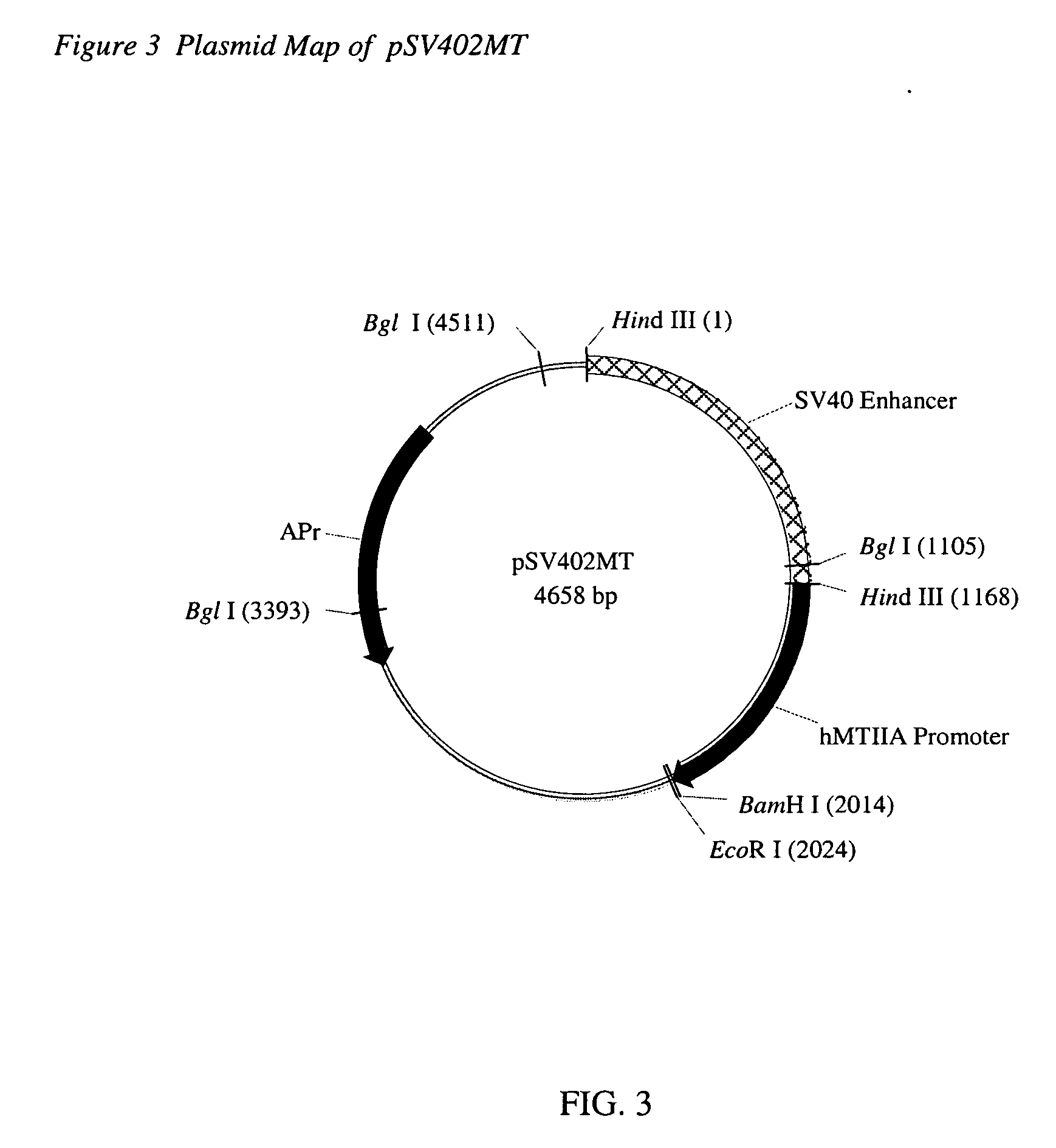
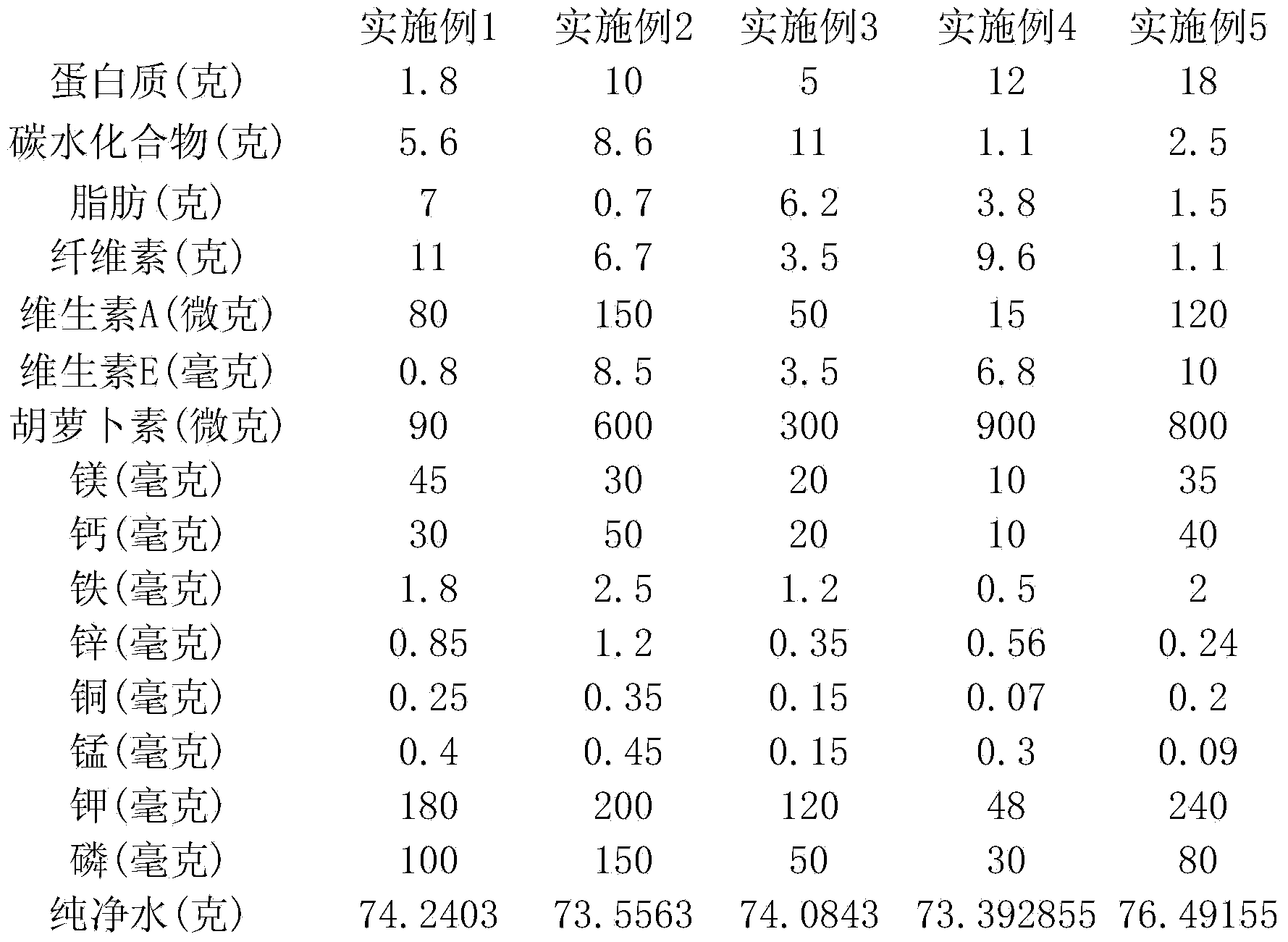
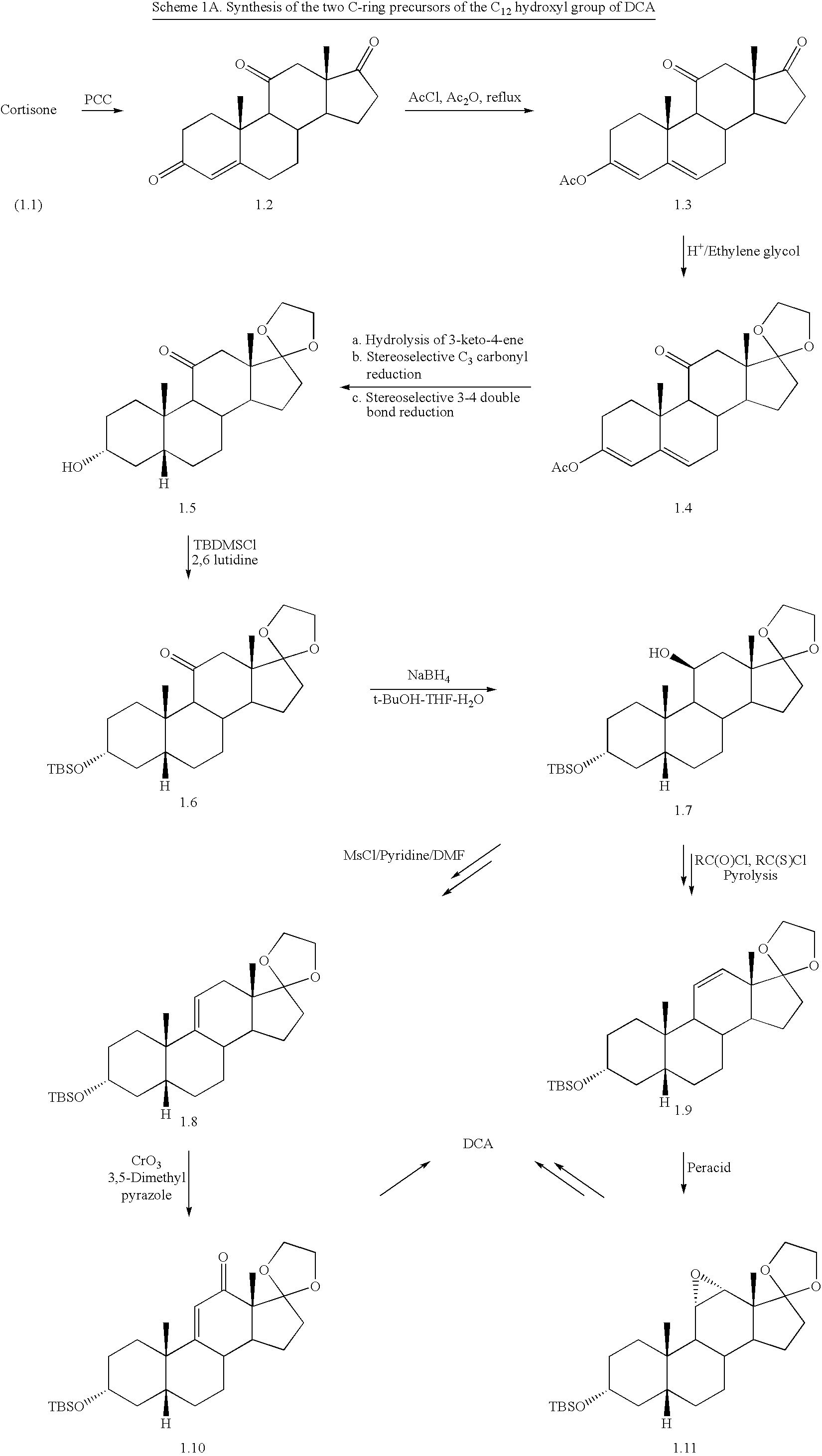
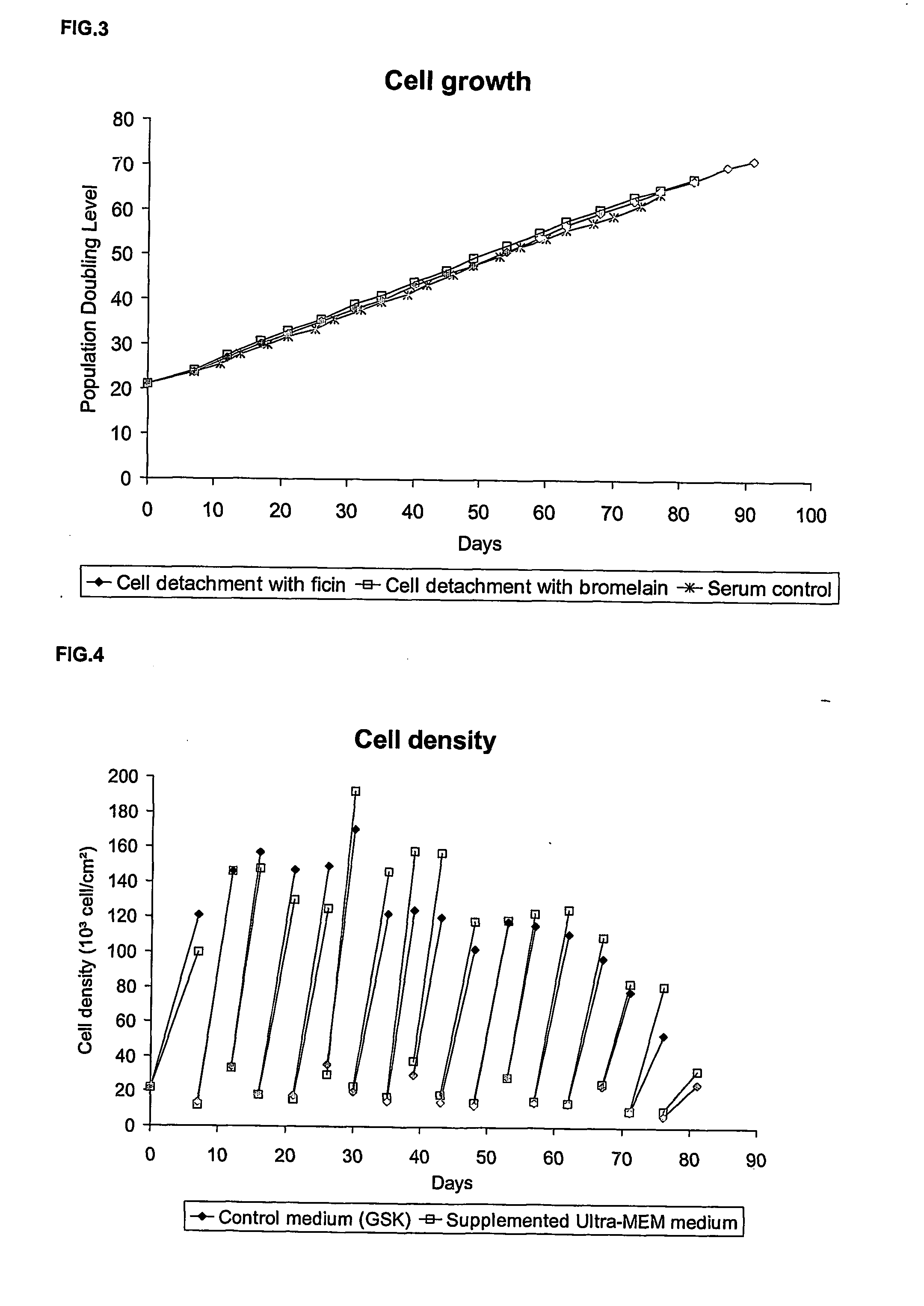
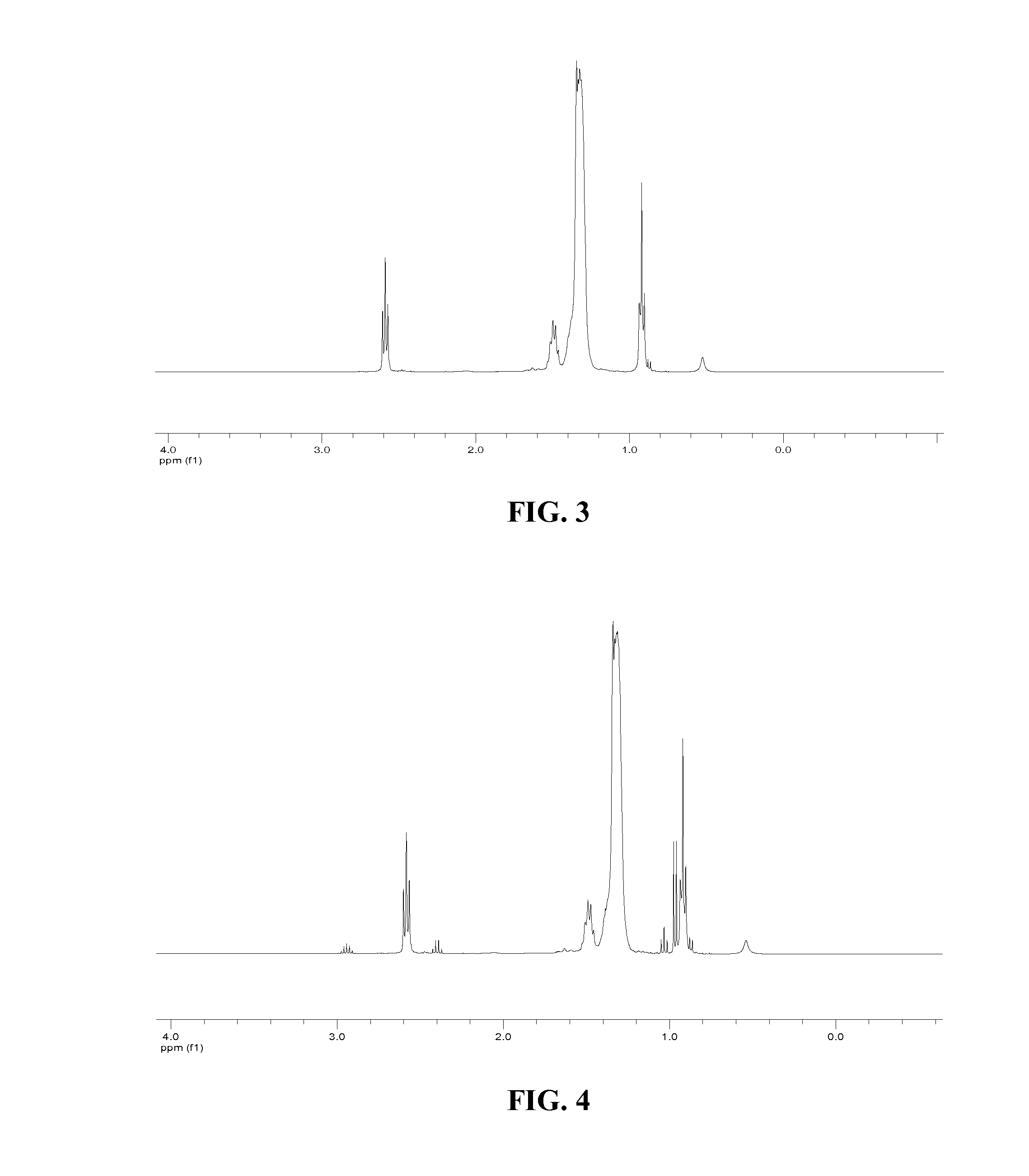
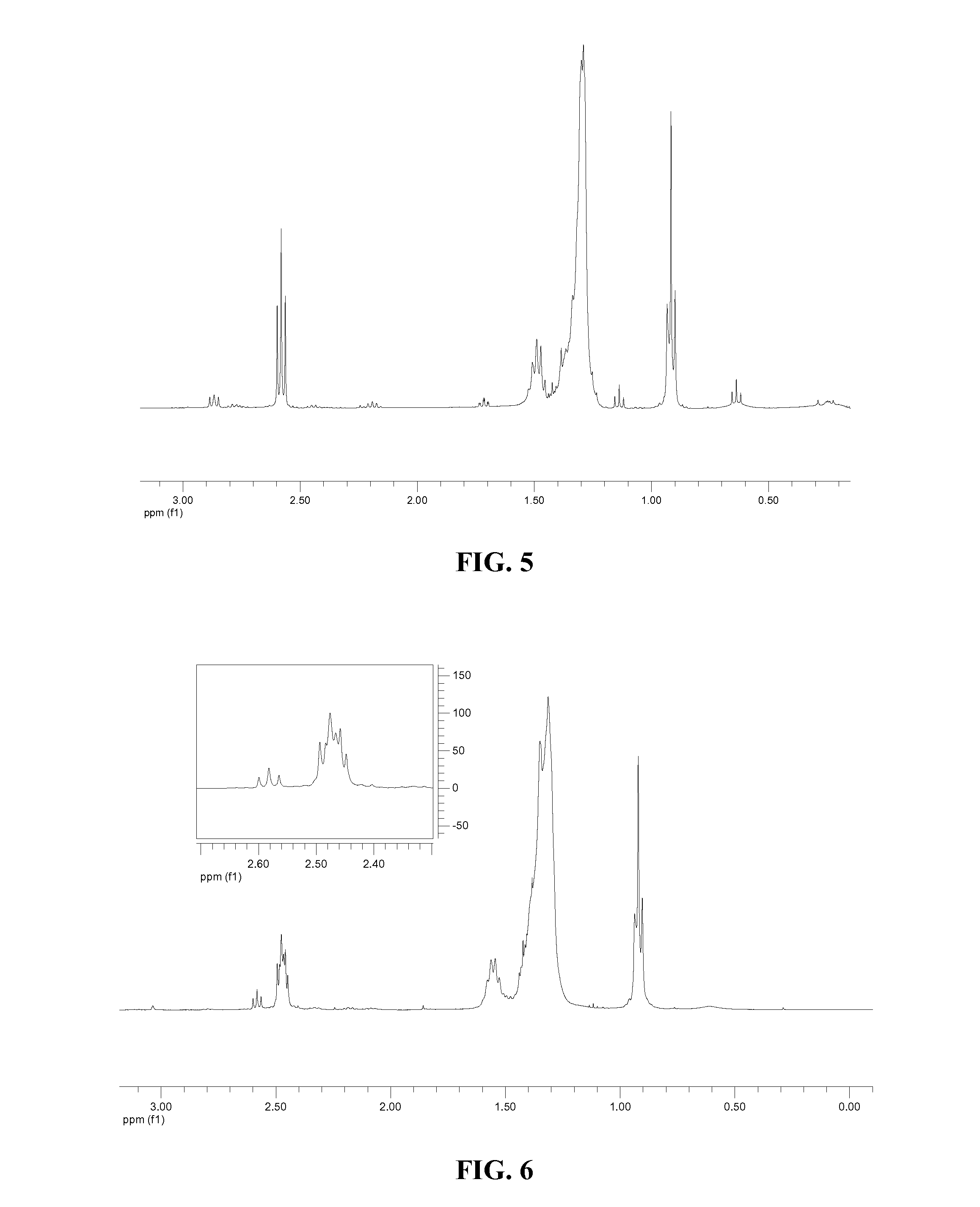
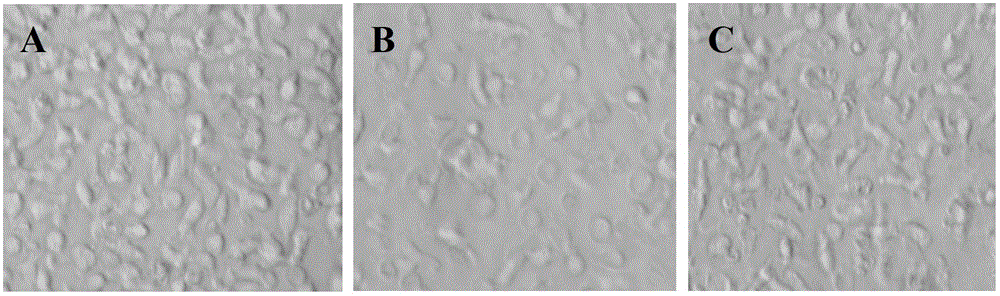
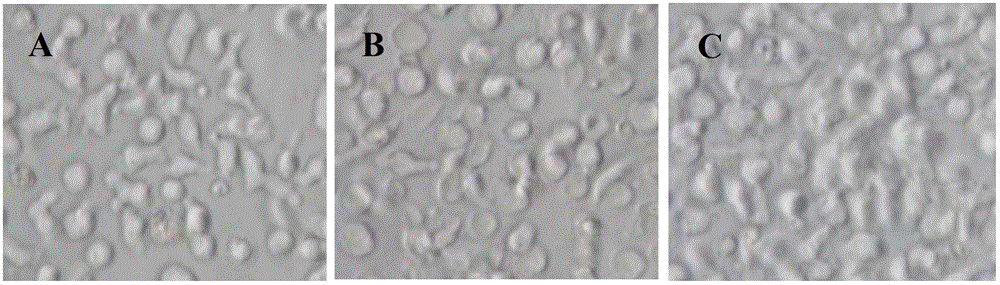
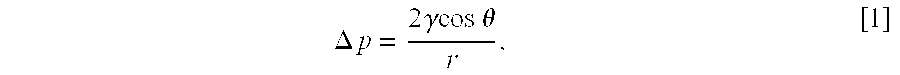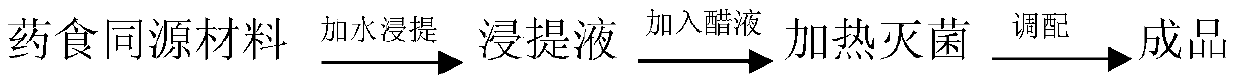Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
449 results about "Animal Sources" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Indicates that a product is derived from an animal.
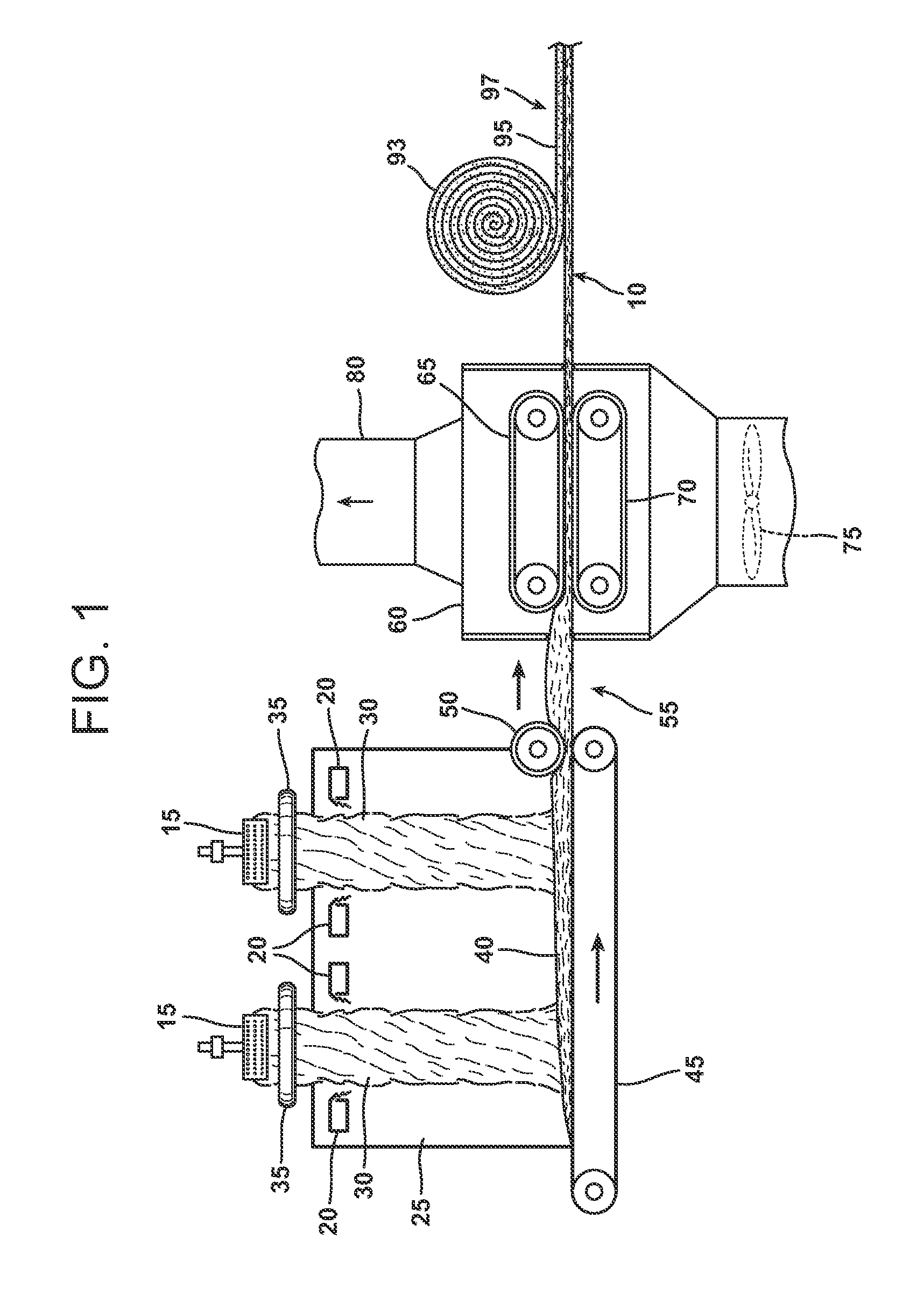
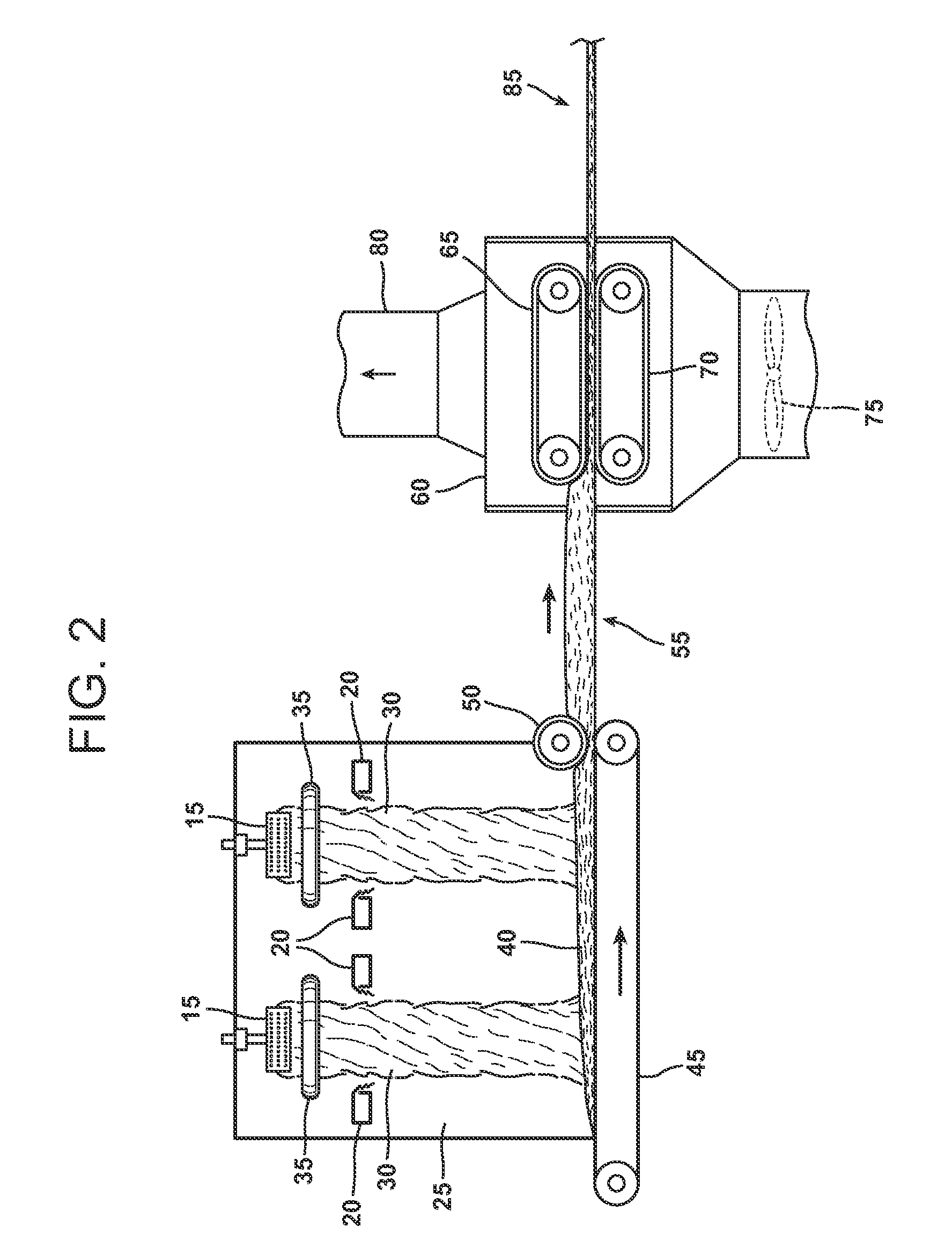
Biomass hydrolysate and uses and production thereof
The present invention includes a palatable, stable composition comprising a biomass hydrolysate emulsion for incorporation, into, or used as, nutritional products, cosmetic products or pharmaceutical products. Preferred sources for biomass are microbial sources, plant sources and animal sources. The present invention also provides methods for making such compositions, specifically, a method for producing a product comprising a nutrient, particularly a long chain polyunsaturated fatty acid, comprising hydrolyzing a biomass comprising the nutrient and emulsifying the hydrolyzed biomass. Such compositions and methods are useful, for example, for increasing intake of nutrients such as omega-3 long chain polyunsaturated fatty acids having 18 or more carbons.
Owner:MARTEK BIOSCIENCES CORP
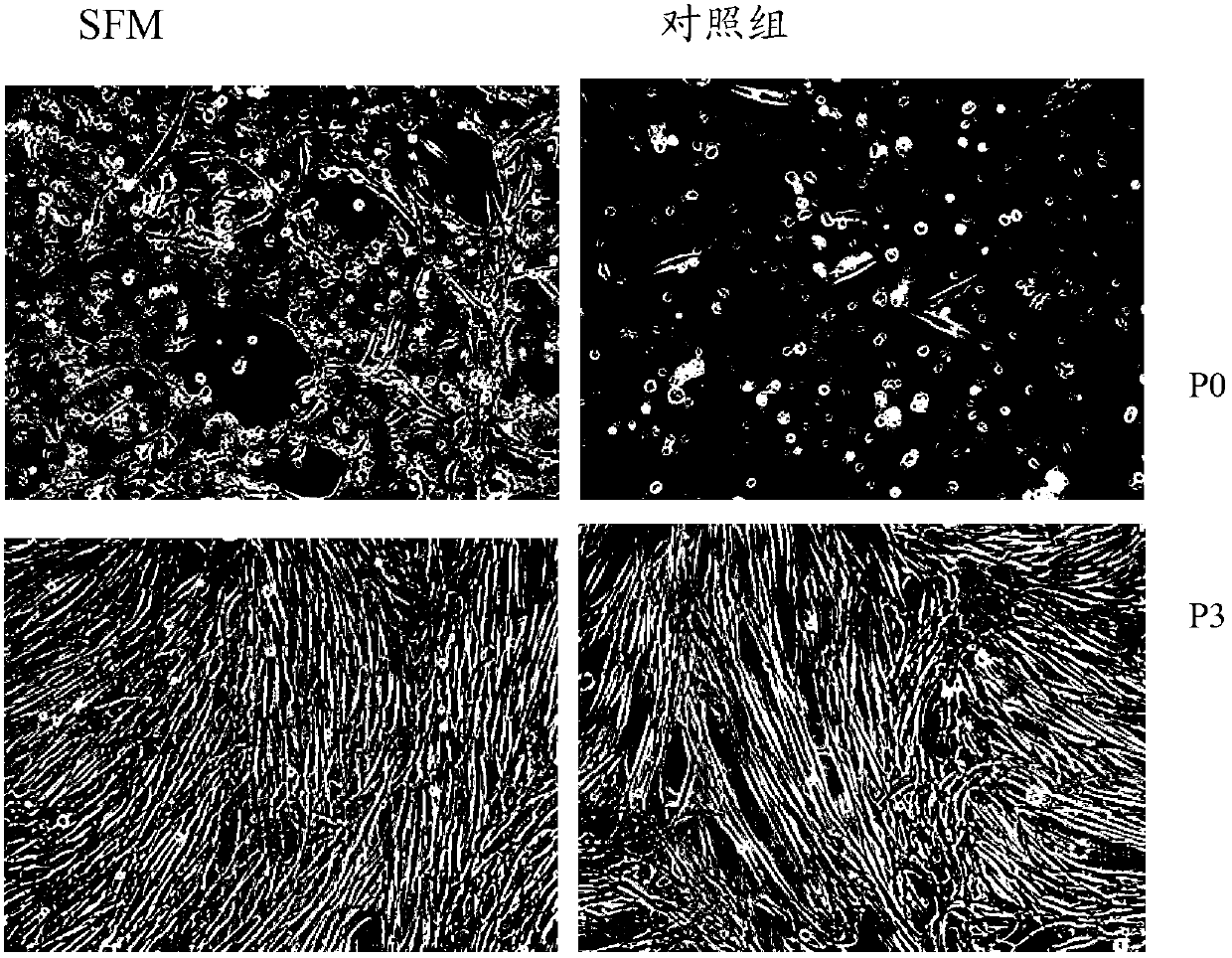
Serum-free culture medium for mesenchymal stem cells
ActiveCN102827807AAvoid instabilityClear chemical compositionSkeletal/connective tissue cellsCell phenotypeSodium bicarbonate
The invention relates to the field of biology, and discloses a serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, Hepes, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, protocatechuic acid, lipid, amino acid, vitamins, trace elements, Pluronic F-68, hydrocortisone, vitamin C, bonding amine or recombinant human fibronectin, progesterone, putrescine, heparin, serotonin, epidermal growth factors (EGFs), b-fibroblast growth factors (FGF), platelet derive growth factor (PDGF)-BB and insulin-like growth factor (IGF)-I. The serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation and avoids the doped animal components and unstable batches, and the results of the cultured mesenchymal stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
Biocompatibility modified starch sponges
ActiveCN101455857AFlexibleActive bleeding is easily controlledAbsorbent padsBandagesFreeze-dryingBiocompatibility Testing
The invention relates to biocompatible modified starch sponge and use of the biocompatible modified starch sponge as a hemostatic material, an anti-adhesion material, a material for promoting tissue healing, a surgical sealant or a wound tissue adhesive. Modified starch is one or a combination of more than one among pre-gelatinized starch, acid modified starch, dextrin, oxidized starch, esterified starch, etherified starch, crosslinked starch, grafted starch and composite modified starch. The sponge is prepared by vacuum freeze drying of the modified starch and other biocompatible hemostatic material, coagulant, plasticizer and so on. The biocompatible modified starch sponge has the advantages that the biocompatible modified starch sponge has flexible form and good biocompatibility, can be directly acted on bloody wound surfaces, avoids the conditions of hypersusceptibility, infection and difficult healing of wounds caused by adoption of hemostatic materials such as animal source / human source collagens, obviously improves the water absorption speed, has larger viscosity, forms a zymoplastic mixture which has good adhesion, calks broken tissues and blood vessels, and is used for hemostasis of active hemorrhage.
Owner:BEIJING UNIVERSAL LIKANG TECH CO LTD
Cell lines for expressing enzyme useful in the preparation of amidated products
InactiveUS20060292672A1High expressionGood for survivalAnimal cellsSugar derivativesPeptidylglycine alpha-amidating monooxygenaseChemical compound
Cell lines are provided for expressing peptidylglycine alpha-amidating monooxygenase (PAM), or one of its two catalytic domains. High levels of enzyme expression are achieved while utilizing a non-animal source, low protein tissue culture medium. A robust two-step downstream purification results in high enzyme purity. Resulting PAM, or its PHM catalytic domain, is used to catalyze the enzymatic conversion of X-Gly to X-alpha-hydroxy-Gly or X—NH2 (X being a peptide or any chemical compound having a carbonyl group to which a glycine group can be covalently attached). Methods of preparing preferred cell lines are also set forth.
Owner:ENTERIS BIOPHARMA
Methods of using natural products as dewatering aids for fine particles
InactiveUS6526675B1Reduce moistureImprove hydrophobicityPigmenting treatmentDrying using combination processesLipid formationSlurry
A method of dewatering fine particulate materials is disclosed. In this method, an aqueous slurry of fine particles is treated with appropriate hydrophobizing reagents so that the particulate material becomes moderately hydrophobic. A lipid of vegetable or animal origin is then added to the slurry in solutions of light hydrocarbon oils and short-chain alcohols, so that the hydrophobic lipid molecules adsorb on the moderately hydrophobic surface and, thereby, greatly enhance its hydrophobicity. By virtue of the enhanced hydrophobicty, the water molecules adhering to the surface are destabilized and more readily removed during the process of mechanical dewatering. The moisture reduction can be further improved using appropriate electrolytes in conjunction with the lipids, spraying surface tension lowering reagents onto the filter cake, subjecting the cake to a suitable vibratory means, and using combinations thereof.
Owner:YOON ROE HOAN
Preparation method of microbial water quality purifying fungicide
ActiveCN103820427AEasy to adaptRapid multiplicationEnergy based wastewater treatmentOn/in inorganic carrierMicroorganismFungicide
The invention discloses a mineralized water purifying agent which loads magnetotactic plant source microorganisms and a preparation method thereof. The preparation method comprises the following steps: acclimating animal source microorganisms into plant source microorganisms by using a plant source culture solution, wherein the animal source microorganisms comprise anaerobic and aerobiotic microorganisms which are in a weight ratio of 1:1; and acclimating the plant source microorganisms into plant source magnetotactic microorganisms, then loading on sandy mineral stone powder to form biological minerals so as to generate a purifying fungicide. According to the invention, purchased animal source microbial strains are acclimated to recover the original wild nature of microorganisms so as to quickly survive, adapt and propagate. The problem that common microbial water quality purifying treatment fungicide splashed just floats on the surface, cannot submerse in water and cement sludge quickly, is easy to wash away by water flows, needs to be input frequently and is high in operating cost is solved. The preparation method can be cooperated with the original comprehensive treatment system and task, so that the treatment difficulty is greatly reduced, the treatment effect is improved, and the preparation method has the price advantage compared with that of conventional dredging manner.
Owner:钟华
Kit for separating genome DNA by using magnetic balls and application thereof
InactiveCN101792757AIncrease productionHigh purityMicrobiological testing/measurementDNA preparationMagnetic beadPhenol
The invention provides a kit for separating genome DNA by using magnetic balls and application thereof. The kit comprises magnetic balls, a magnetic frame, a genome DNA extraction reagent (lysing solution, binding solution, rinsing solution A, rinsing solution B and eluent) and specifications; the main steps of extracting the gene DNA comprise cell lysis, nucleic acid absorption, impurity removing and nucleic acid elution. The application of the kit for extracting the genome DNA does not need to use large-toxicity organic solvents of phenol, chloroform and the like, has good safety, and simple, fast and time-saving operation, and simultaneously can extract a plurality of samples. The kit can extract the genome DNA from materials of animals, plants, bacteria, fungus, blood, virus, animal source feed stuff, samples in the forensic medicine and the like, the DNA has high yield and purity, and the obtained genome DNA can be used for experiments such as PCR amplification, gene cloning, construction of genomic library, sequence measurement, molecular hybridization, molecular marking and the like. The kit can be stored at the temperature of 4 DEG C, also can be placed at room temperature, and is convenient to transport.
Owner:上海鼎国生物技术有限公司
Bio-based aqueous binder for fiberglass insulation materials and non-woven mats
InactiveUS20110003522A1Reduced insulation performanceImprove worker safetyOther chemical processesProtein adhesivesGlass fiberAnimal Sources
An aqueous binder composition is provided that includes a protein-containing biomass and a pH adjuster. Optionally, a crosslinking agent and / or a moisture resistant agent may be included in the binder composition. The protein-containing biomass is natural in origin and may be derived from plant or animal sources. The pH adjuster is used to adjust the pH of the binder to a desired pH and lower the viscosity of the protein-based biomass. In addition, the pH adjuster may act as a crosslinking agent. The crosslinking agent may be any compound suitable for crosslinking the protein-containing biomass and reacting with the moisture resistant agent, when the moisture resistant agent is present in the binder. In addition, the binder has a light color after it has been cured. The environmentally friendly, formaldehyde-free binder may be used in the formation of insulation materials and non-woven chopped strand mats.
Owner:OWENS CORNING INTELLECTUAL CAPITAL LLC
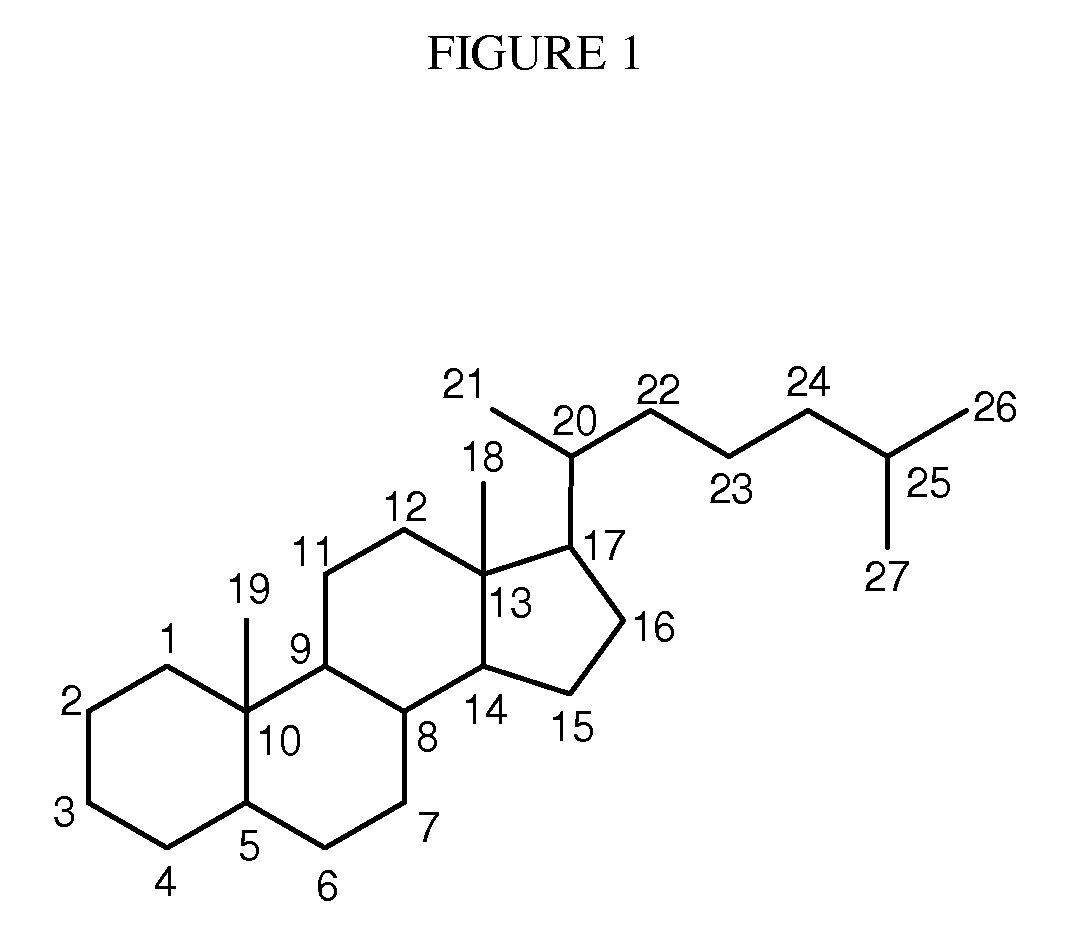
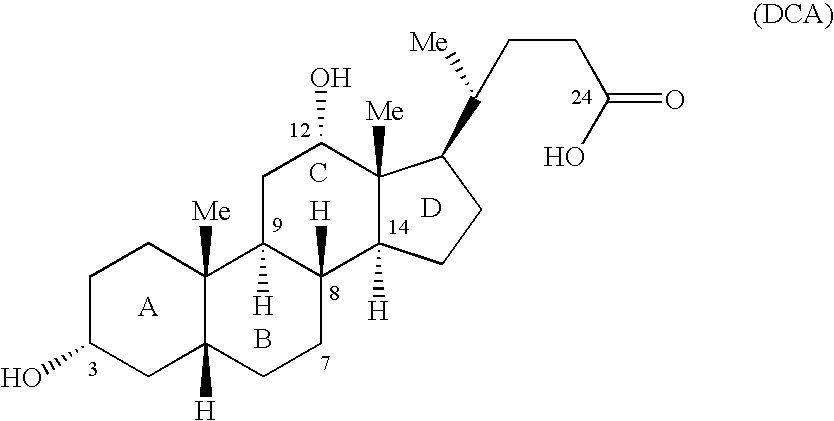
Synthetic bile acid compositions and methods
Bile acids and related compositions and methods of synthesis and use. More specifically, deoxycholic acid and related compositions, said compositions being free of all moieties of animal origin and free of pyrogenic moieties.
Owner:ALLERGAN SALES LLC
Human amnion mesenchymal stem cell serum-free culture medium and culture method thereof
The invention relates to a human amnion mesenchymal stem cell serum-free culture medium and a culture method thereof. The culture medium is formed by adding human serum albumin, human transferrin, human insulin and sodium selenite into a DMEM / F12 basic culture medium. The culture method for the culture medium comprises the following steps of: digesting human amnion by using trypsin, then digesting the human amnion by using collagenase IV and deoxyribonuclease I, and filtering the mixture to obtain single cell suspension; and adding the human serum albumin, the transferrin, the insulin and the sodium selenite into the DMEM / F12 basic culture medium in a ratio of VDMEM to VF12 of 1:1, and putting human amnion mesenchymal stem cells in a 37 DEG C CO2 incubator with saturated humidity and volume fraction of 5 percent under the serum-free condition, wherein culture in vitro and amplification are realized by solution change and transfer of culture, potentiality of multi-direction differentiation is maintained, and the amplified cells can be induced in vitro to form cartilage cells, osteoblasts and adipocytes. The culture medium and the culture method have the characteristics of no other animal sources, wide source and no limitation of ethics.
Owner:辽宁艾米奥干细胞与再生医学研究院有限公司
Methods for producing synthetic surfaces that mimic collagen coated surfaces for cell cultue
ActiveUS20100273261A1Low costReduce issueLiquid surface applicatorsCell culture supports/coatingCoated surfaceSimple Organic Compounds
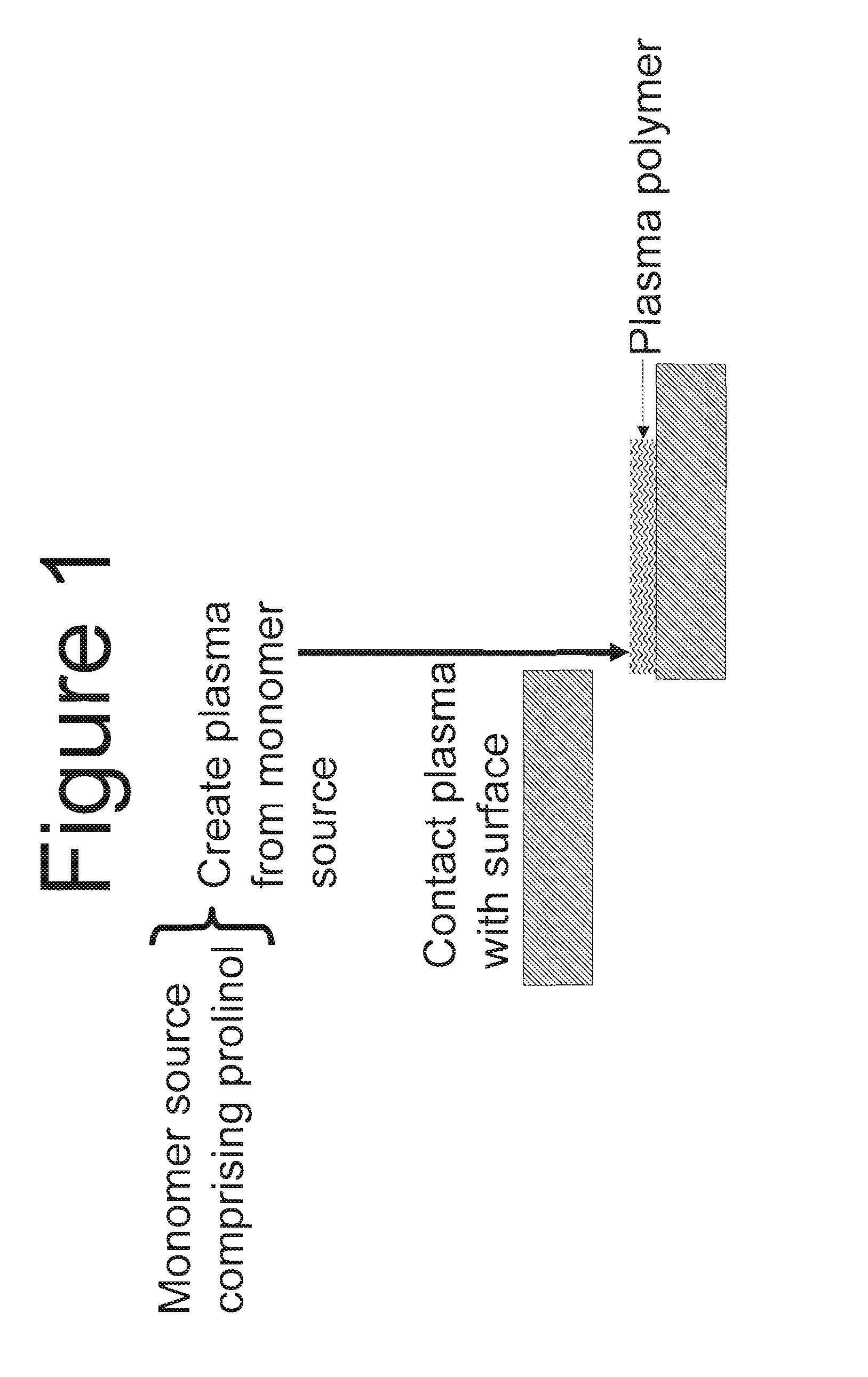
The present invention discloses methods for producing synthetic surfaces that mimic collagen coated surfaces for cell culture comprising: providing a monomer source comprising one or more organic compounds which are capable of polymerization, wherein at least one organic compound is prolinol; creating a plasma of said monomer source; and contacting at least a portion of a surface with the plasma to provide a plasma polymer coated surface. Advantageously, such methods provide an animal-free, synthetic, chemically defined surface that mimics a collagen coated surface for cell culture. Advantageously, such methods not only reduce the cost and / or issues associated with animal-derived collagen but are also amenable to large scale manufacturing.
Owner:CORNING INC
Identification method for animal derived materials
ActiveCN101196463AHigh detection sensitivityLow costMicrobiological testing/measurementMaterial analysis by optical meansFood ComponentAgricultural science
An identification method of animal source character component is provided, which relates to an identification method of food component. The invention overcomes that the current identification method of animal source character component has the defects of low testing sensitivity and unable to test the heat-processed manufactured meat. The animal source character component is identified by the following method: extracting the source character sample DNA of the animal for testing, PCR testing and gel electrophoresis, and then identifying the component of animal source character. The method in the invention has high testing sensitivity, and the identification number limit can be below 1 percent. The identification method in the invention can test the heat-processed manufactured meat, and mix manufactured meat and bone meal product. The identification number limit also can reach below 1 percent. The identification method in the invention doesn't use liquid nitrogen in identification process, which not only lowers the cost, but also improves the safety of the operation.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Readily deinkable toners
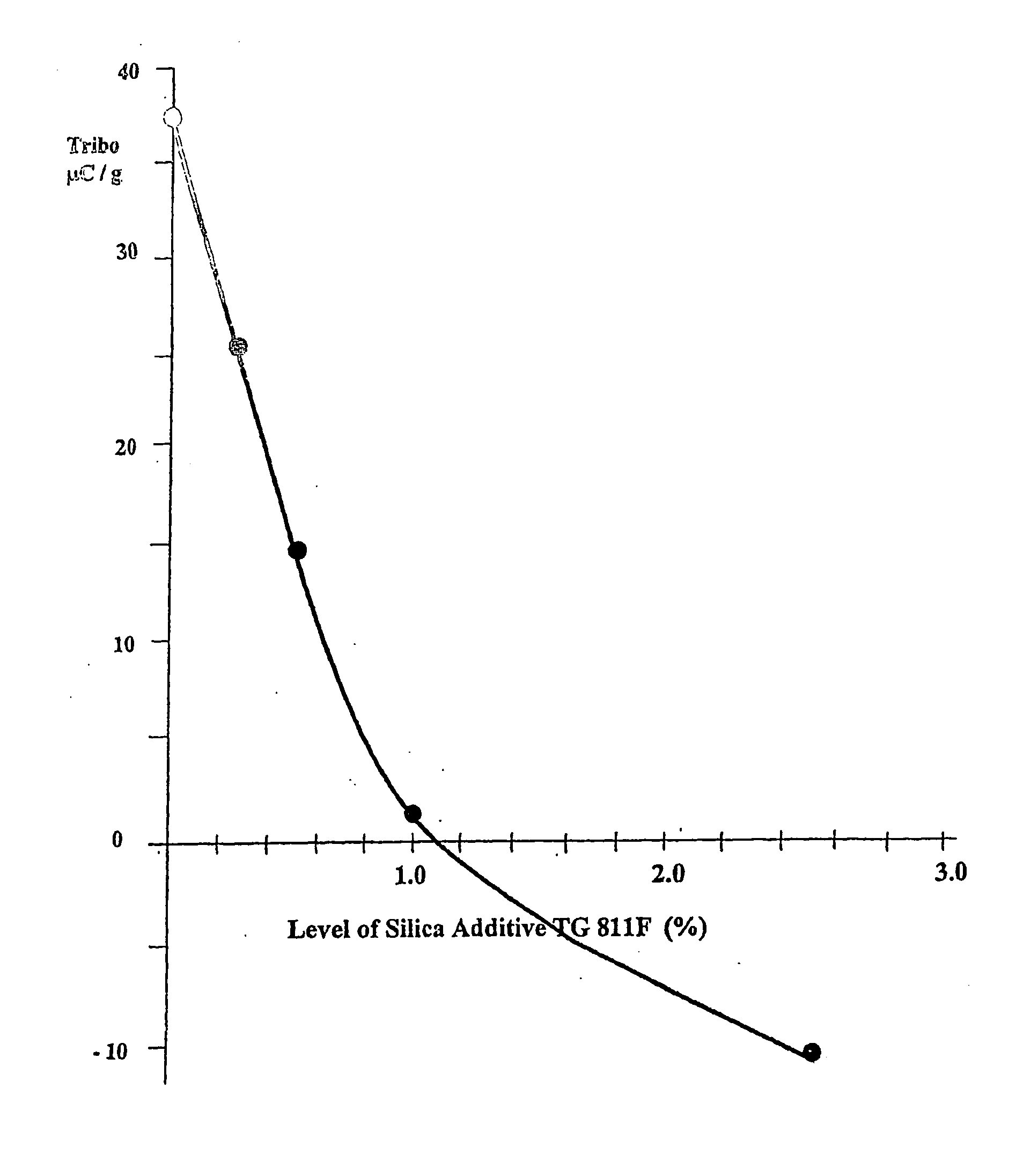
Broadly the invention provides for a deinkable toner composition, an image made with the deinkable toner, and a method for making the toner including a coloring agent; a thermoplastic polymer; and a protein. In another embodiment the toner includes a coloring agent and a thermoplastic polymer where the protein has been incorporated into the polymer itself. In typical embodiments the protein is derived from soybeans but may be from other plant or animal sources. Typically the toner has a positive triboelectric charge of between about 10 to about 40 microCoulomb / g, or a negative triboelectric charge of between about 10 to about 40 microCoulomb / g.
Owner:BATTELLE MEMORIAL INST
Serum-free medium and method for culturing mesenchymal stem cell
InactiveCN106479971AImprove stabilityIncrease success rateCulture processSkeletal/connective tissue cellsCell phenotypeSerum free media
The invention belongs to the technical field of stem cells, and particularly relates to a serum-free medium for culturing a mesenchymal stem cell. According to the serum-free medium, a basal culture medium L-DMEM or DMEM / F12 and multiple additives are combined in a customized manner for MSCs in vitro culture, so that ingredients in the culture medium are synergized; the stability and success rate for culturing MSCs in vitro can be greatly improved especially due to application of DKK-1; and the safety of cultured cells in clinical researches is improved by adopting a formula without animal-sourced ingredient. Compared with the prior art, the serum-free medium has the advantages of greatly improving the value adding efficiency of MSCs in vitro culture and maintaining cell growth form of MSCs and cell phenotype stability thereof, provides a novel efficient, stable and safety culture system to MSCs culture in clinical application, and has high scientific researching and medical application values.
Owner:深圳江淼医疗有限公司
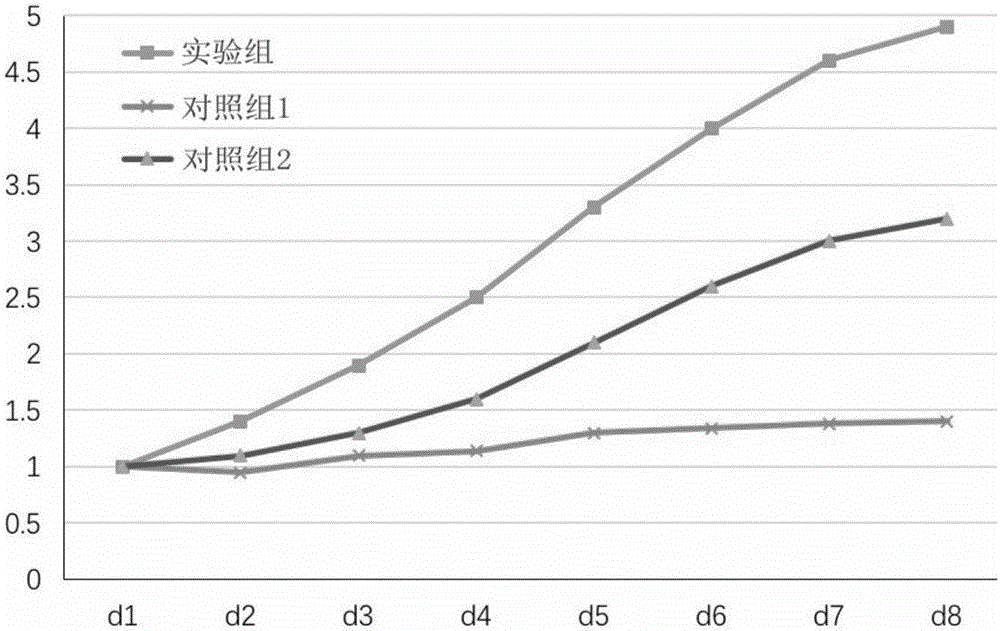
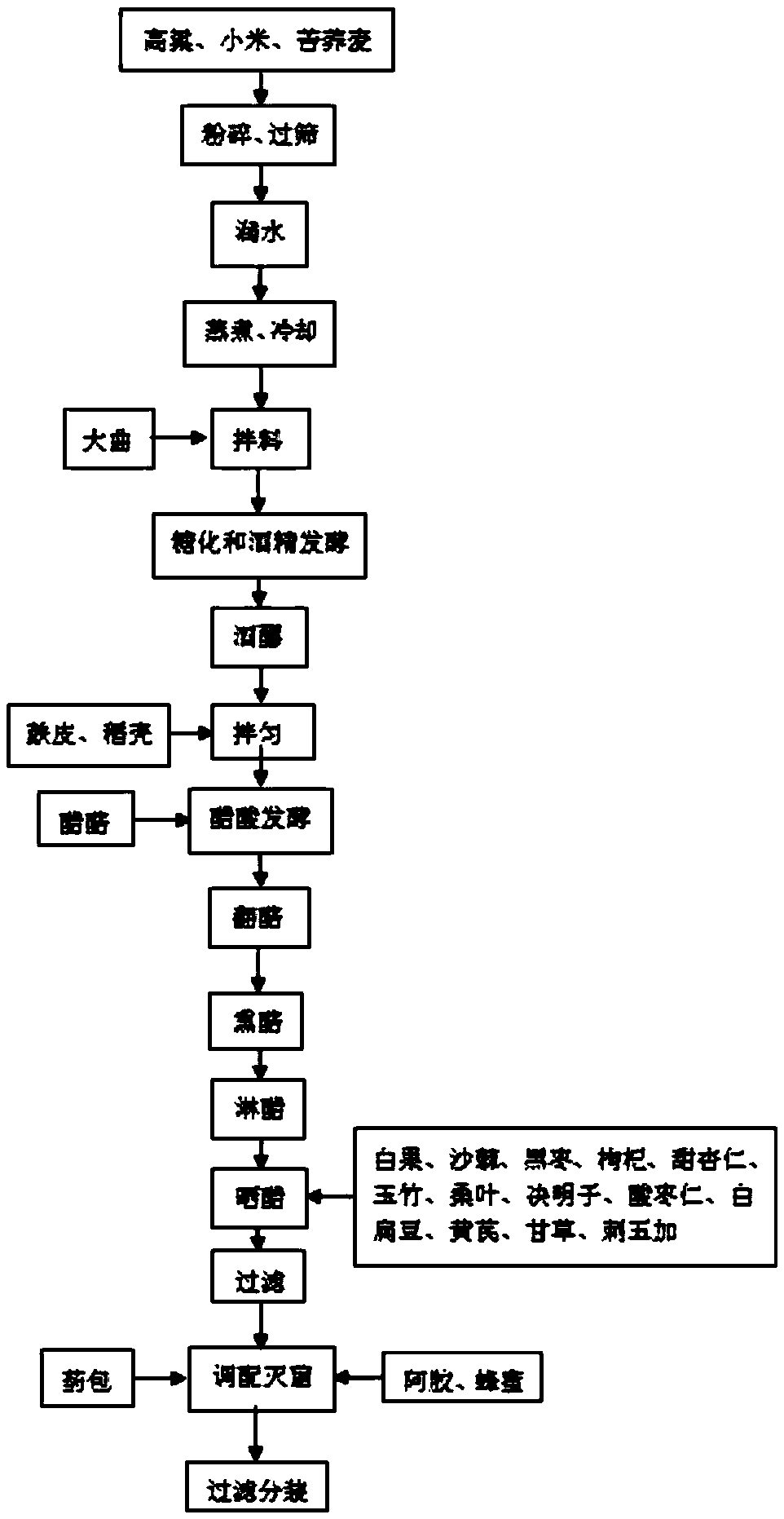
Health care vinegar and brewing process thereof
ActiveCN105505732AIncrease profitIncrease concentrationDigestive systemUnknown materialsAdditive ingredientPlant Sources
The invention provides health care vinegar and a brewing process thereof. The brewing process of the health care vinegar comprises the following steps of material steaming, saccharification, alcoholic fermentation, acetic fermentation, solid-substrate fermentation conducted through fumigation, vinegar spraying, ageing, blending and sterilization. In order to guarantee mellow and normal mouthfeel of the health care vinegar, the solid state fermentation mode is adopted in the production process; medicine-and-food homologous materials which are from different sources are added at different stages of ageing and vinegar decoction separately, and sufficient release and utilization of nutritional ingredients of the medicine-and-food homologous materials are guaranteed. The medicine-and-food homologous materials of the plant source are subjected to smashing treatment, then added at the vinegar sunning and ageing stages and reutilized at the vinegar decoction stage, so that the raw material utilization rate is raised; the materials of the animal source are added at the vinegar decoction stage, and it is guaranteed that efficacy and mouthfeel of the materials are not destroyed. The health care vinegar prepared through the preparation method is suitable for being eaten by people in different physique conditions, the physique conditions can be improved when the vinegar is eaten for a long time, and a good health care function is achieved.
Owner:TIANJIN UNIV OF SCI & TECH
Compositions for topical enzymatic debridement
InactiveUS20050281806A1Facilitates effective dispersionFacilitated releaseBiocideOrganic active ingredientsMedicineLotion
Owner:PRECISION DERMATOLOGY
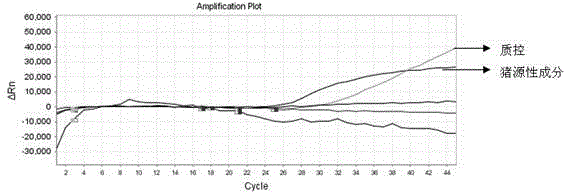
Primer and probe composition for distinguishing various animal sources in colla corii asini, kit and multiple real-time fluorescence quantification PCR detection method
ActiveCN104531884AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationBiotechnologyColla corii asini
The invention discloses a primer and probe composition for distinguishing various animal sources in colla corii asini. The composition is good in specificity and high in sensitivity. The invention further discloses a kit comprising a probe and a primer. The kit can distinguish donkey, horse, cow and pig source ingredients in the colla corii asini at the same time, and is high in accuracy, high in throughput and convenient to use. The invention further discloses a multiple real-time fluorescence quantification PCR detection method for distinguishing various animal sources in the colla corii asini. The method can qualitatively detect donkey, horse, cow and pig source ingredients in the colla corii asini at the same time, and is applicable to animal hide and skin detection, applicable to animal source detection on deep-processed colla corii asini as well, and is convenient and fast to implement.
Owner:BIOTECH RES CENT SHANDONG ACADEMY OF AGRI SCI
Zooblast culture medium dry powder composition, culture medium composition and preparation method thereof
The invention provides a dry powder composition for a zooblast culture medium. Compared with the prior dry powder composition for the zooblast culture medium, the dry powder composition provided by the invention reduces the serum dosage of animals by introduction of recombinant protein or animal origin compositions, the dry powder composition for the zooblast culture medium can be matched with low-content animal serum for use without additional introduction of protein compositions (such as the recombinant protein, plant protein or the animal origin compositions including cytokines and so on), and has the same or better function on promoting the growth of zooblast compared with high-content animal serum, so that the dry powder composition for the zooblast culture medium does not generate side effects along with the protein compositions, and has good safety and low cost. Cells cultured by the dry powder composition for the zooblast culture medium have small difference for different batches, low cost and good safety, are favorable for separating downstream products of the cells, and are suitable to be used for virus hosts, expression vectors and so on of biological products and other biological products.
Owner:BEIJING SKYWING TECH CO LTD
Process for the preparation of lubricants
ActiveUS20070004599A1Short reaction timeFatty oils/acids recovery from wastePhysical/chemical process catalystsAlcoholCyanide
The present invention provides an improved process for the preparation of lubricants from vegetable oil or fat obtained from animal source. The present invention involves a reaction of vegetable oil or fat with an alcohol in the presence of a double metal cyanide catalyst, at a temperature in the range of 150° to 200° C. for a period of 3-6 hrs to obtain the desired bio-lubricant.
Owner:COUNCIL OF SCI & IND RES
Culture medium additive and application thereof
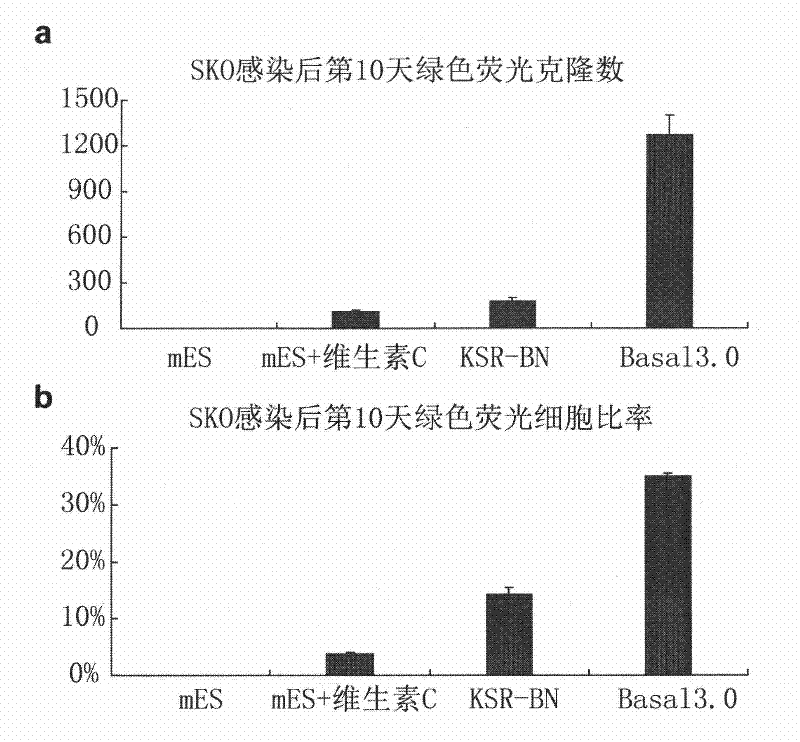
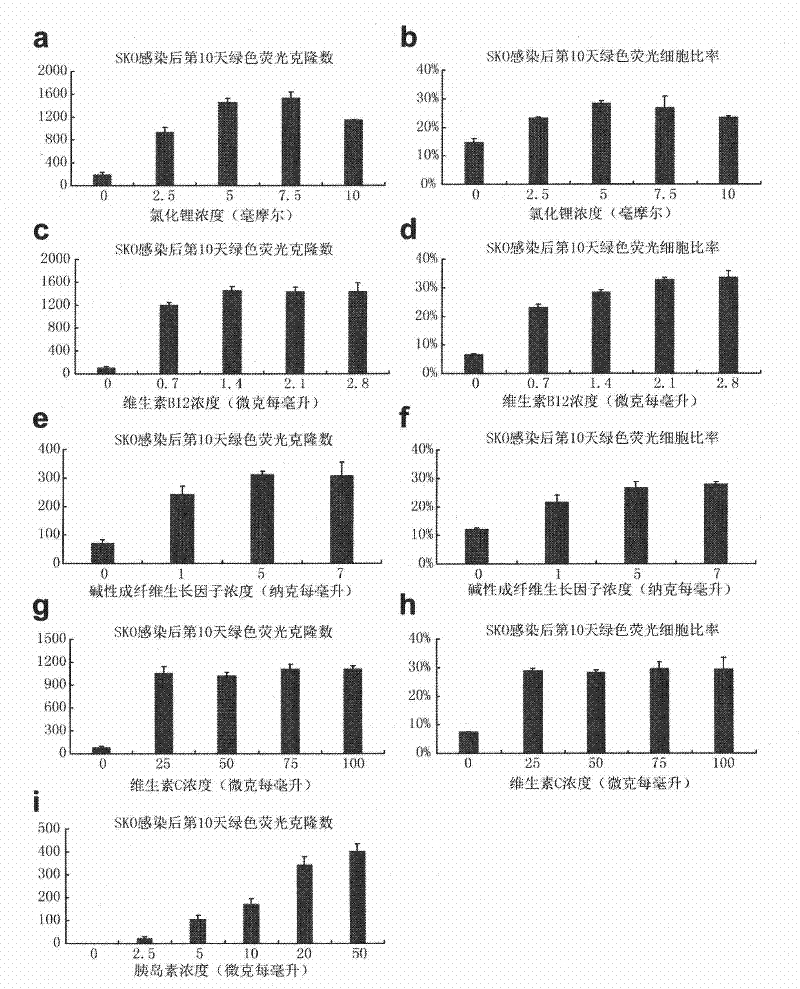
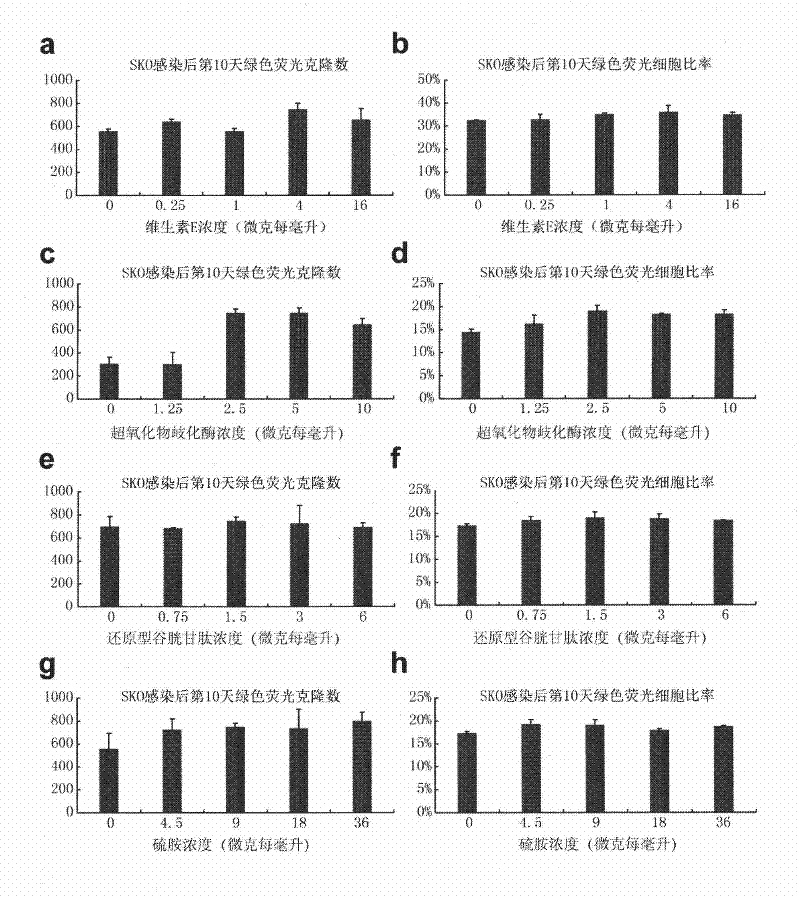
The invention provides a culture system which contains defined components and efficiently acquires induced pluripotent stem cells (iPS cells) from somatic cells. The culture medium additive provided by the invention contains vitamin C, vitamin B12, insulin, glycogen synthase kinase-3 inhibitor, receptor tyrosine kinase and antioxidant. The culture medium additive provided by the invention can also contain a substituted serum cell growth promoter. The invention also provides a complete medium acquired by inducing pluripotent stem cells, which is prepared from one or more of basic culture medium, serum and substituted serum additive and the culture medium additive. The culture system provided by the invention does not have serum, has no animal source pollution, contains defined chemical components, efficiently acquires iPS cells, can maintain cell growth and proliferation in the process of conversion from somatic cells to iPS cells under the condition that feeder cells do not exist, simultaneously accelerates the induction course of the iPS cells obviously, and greatly improves the efficiency of inducing the somatic cells into the iPS cells.
Owner:杭州健崃生物科技有限公司
Algae microbial soil conditioner specially used for saline-alkali soil and preparation method thereof
InactiveCN106588445AImprove salinityThe effect of increasing production is obviousCalcareous fertilisersMagnesium fertilisersAlkali soilPlant Sources
The invention belongs to the field of soil conditioners and relates to an algae microbial soil conditioner specially used for saline-alkali soil. The algae microbial soil conditioner is prepared by blending the raw materials including an acidic raw material, a plant sourced organic substance, an animal sourced organic substance, a composite probiotic agent, an enzymolysed algae extract, and medium and trace elements. The soil conditioner is prepared by combining the probiotics and the algae active extract, so that on one hand, pH value of the saline-alkali soil can be adjusted, saline-alkali degree is reduced, root system growth environment of crops is improved and damage on a root system due to the salt and alkali is reduced; and on the other hand, available phosphorus, immobilized by soil, can be released, so that available microelements in soil are effectively supplied, thereby improving soil fertility. Meanwhile, alginic acid can improve the water retention and fertilizer saving capability of soil, thereby finally improving the soil completely, increasing crop quality and utilization rate of fertilizers, and achieving yield increasing.
Owner:QILU UNIV OF TECH
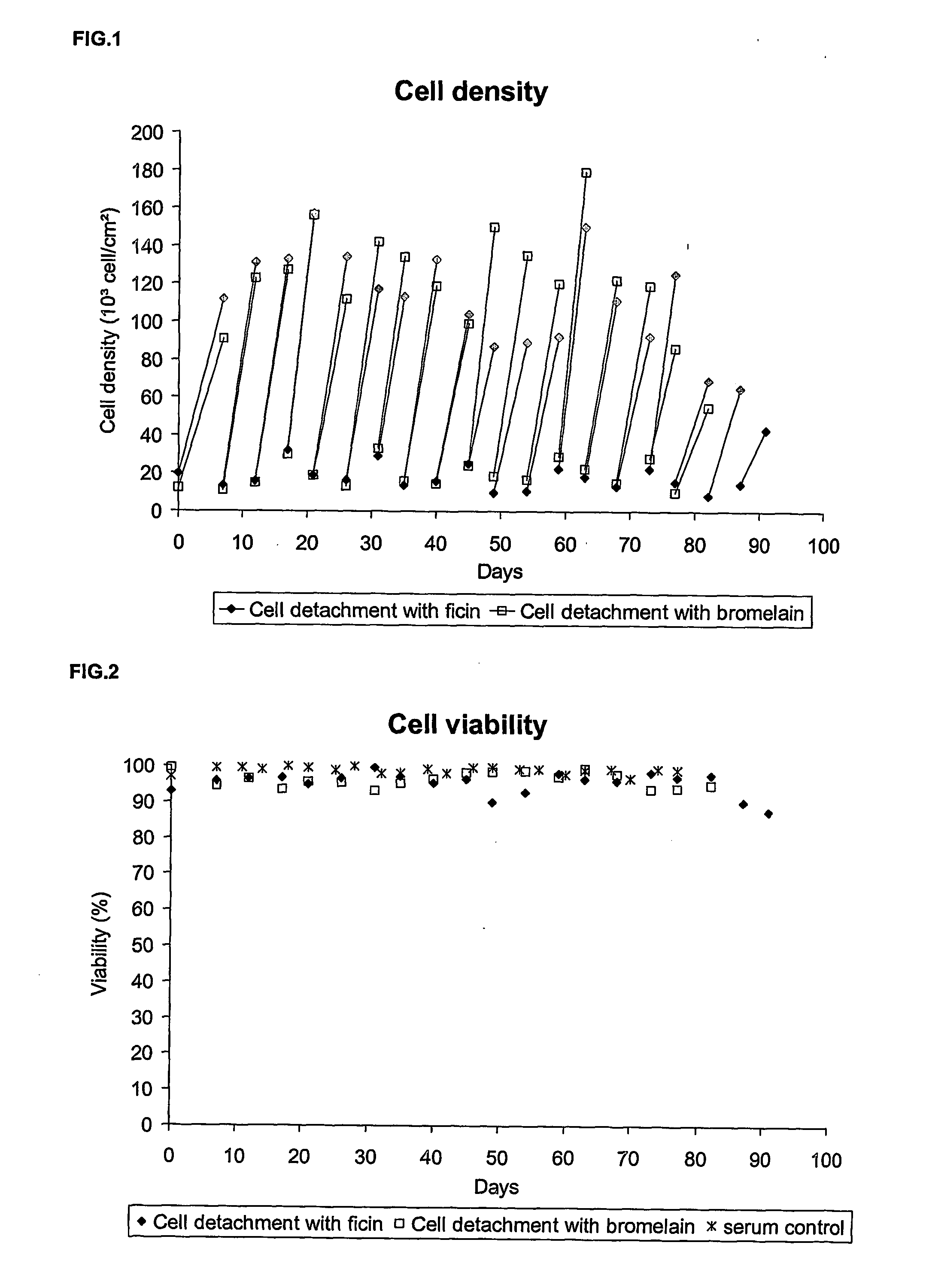
Animal-free cell culture method
InactiveUS20060183224A1Easy to replaceSsRNA viruses positive-senseCell culture mediaBiotechnologyCell culture media
The present invention relates to a process for animal, preferably human, diploid anchorage-dependent cell culture, in the absence of exogenous components of primary animal origin, and to a cell culture medium substantially free of exogenous components of primary animal origin suitable for carrying out said process. In particular the invention concerns a cell culture medium which comprises at least one, more preferably several, exogenous animal-free growth factors. The present invention also relates to a process for cultivating animal, preferably human diploid anchorage-dependent cells in a medium according to the invention, involving the use of a trypsin substitute of non-animal origin for passaging cells. The invention further relates to a process for producing viruses, viral vaccines and the like.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
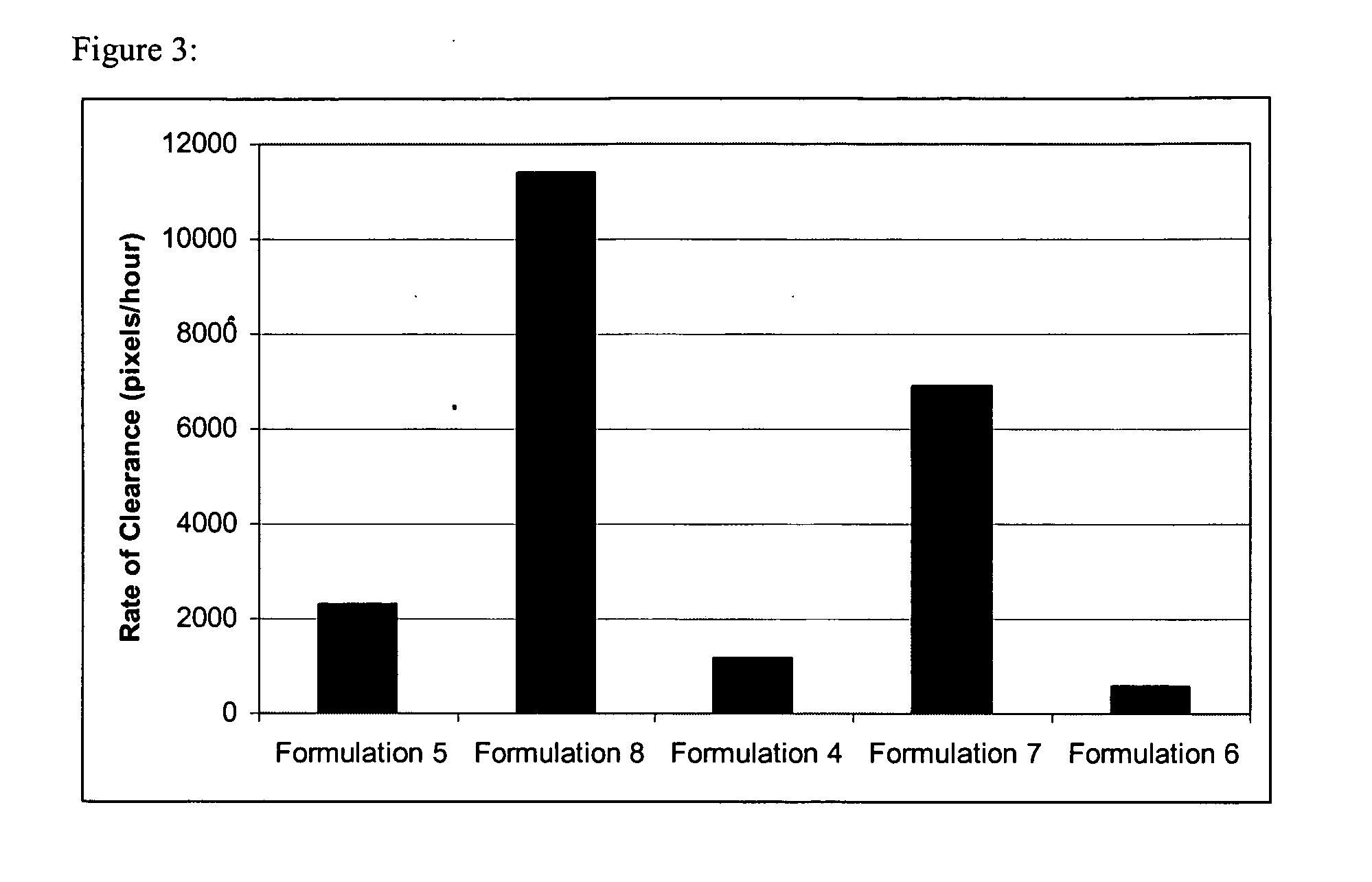
Methods and systems for administering a drug program related to livestock
ActiveUS7606394B2Quality assuranceHigh customer satisfactionData processing applicationsDrug and medicationsBiotechnologyDraft animals
Owner:JBS USA FOOD CO +1
Method for preparing meaty paste essence by controlling natural amino acids and obtained product
The invention discloses a method for preparing meaty paste essence by controlling natural amino acids and an obtained product. The method is characterized by preparing the meaty paste essence through the natural amino acid control technology, and preparing the natural paste essence by combining the amino acids from natural animals with other auxiliary materials through the Maillard reaction technology. The obtained product belongs to the purely natural food condiments without toxic or side effects. The meaty paste essence not only has aroma close to the natural aroma, attractive flavor and good taste, but also has higher nitrogen content, rich nutrition, good safety performance and stable product property.
Owner:TIANNING FLAVOR JIANGSU
Method of making an olefin polymerization catalyst activator
ActiveUS20150203602A1Group 3/13 element organic compoundsPhosphorus organic compoundsSolubilityReducing agent
A method for preparing ammonium tetrakis(pentafluorophenyl)borate salt comprising reacting a secondary amine with an aldehyde to form an iminium ion; hydrogenating the iminium ion by reaction with a reducing agent to form a tertiary amine; reacting the tertiary amine with a mineral acid to form an amine salt; and reacting the amine salt with K[B(C6F5)4], Li[B(C6F5)4], or combinations thereof to form an ammonium tetrakis(pentafluorophenyl)borate salt, wherein the secondary amine is derived from a non-animal source and the aldehyde has seven or more carbon atoms; wherein the ammonium tetrakis(pentafluorophenyl)borate salt is characterized by a solubility at 25° C. in hexane, cyclohexane or methylcyclohexane of at least 10 weight percent; and wherein the tertiary amine has a molecular weight of at least 450 g / mole is provided.
Owner:DOW GLOBAL TECH LLC
Process for the preparation of lubricants
ActiveUS7842653B2Short reaction timeFatty oils/acids recovery from wastePhysical/chemical process catalystsCyanideVegetable oil
The present invention provides an improved process for the preparation of lubricants from vegetable oil or fat obtained from animal source. The present invention involves a reaction of vegetable oil or fat with an alcohol in the presence of a double metal cyanide catalyst, at a temperature in the range of 150° to 200° C. for a period of 3-6 hrs to obtain the desired bio-lubricant.
Owner:COUNCIL OF SCI & IND RES
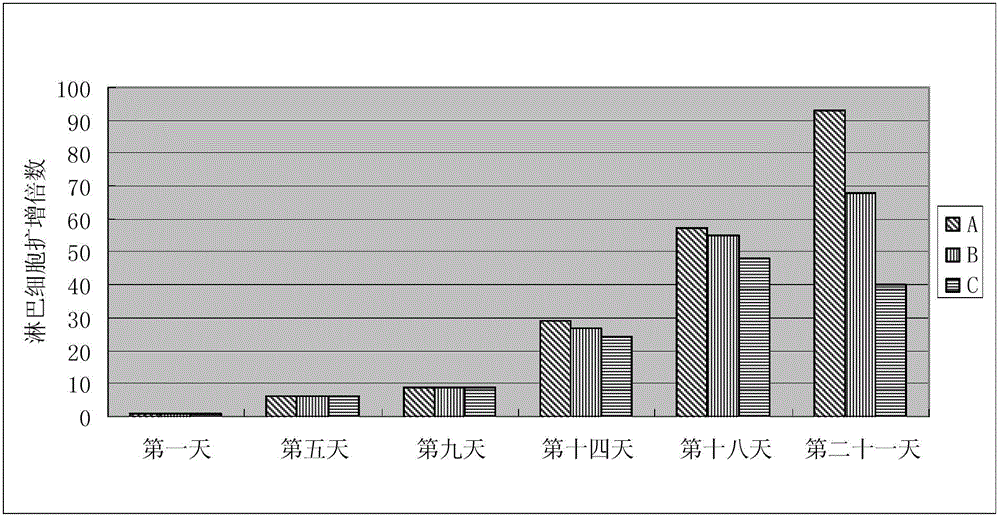
Animal source-free and serum-free culture medium of lymphocyte
InactiveCN103146648AGood amplification factorEnhance cell viabilityBlood/immune system cellsCell phenotypeSodium bicarbonate
The invention relates to the biological field and discloses an animal source-free and serum-free culture medium of lymphocyte. The culture medium disclosed by the invention essentially consists of IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, recombinant human insulin, human transferrin, human serum albumin, 2-mercaptoethanol, N-acetyl-cysteine, lipid, amino acid, vitamin, microelement, ferric citrate, hydrocortisone, cholamine and non-essential amino acid. The serum-free culture medium disclosed by the invention has the advantages of clear chemical components, no animal source, no serum, safe and ideal culture cells; the instability caused by the doping of animal components and batches is avoided; the result of culturing lymphocyte shows that the total number of the cells and the cell phenotypes are normal; and the serum-free culture medium disclosed by the invention has a good industrial application prospect.
Owner:BEIJING JING MENG STEM CELL TECH
Method for preparing retinal pigment epithelia
InactiveCN102618488AAvoid potential risksHigh differentiation efficiencyVertebrate cellsArtificial cell constructsAnimal SourcesBiology
The invention discloses a method for preparing retinal pigment epithelia. According to the method, stem cells are induced by utilizing a liver X receptor (LXR) activating agent to be differentiated into the retinal pigment epithelia. Compared with the prior art, the method for inducing the stem cells to be differentiated into the retinal pigment epithelia by utilizing the LXR receptor activating agent has the advantages that: a feed layer is not used, so that potential risks caused by an animal source feed layer are avoided; and the differentiation efficiency can be improved, and the differentiation time can be shortened.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Biomass hydrolysate and uses and production thereof
The present invention includes a palatable, stable composition comprising a biomass hydrolysate emulsion for incorporation, into, or used as, nutritional products, cosmetic products or pharmaceutical products. Preferred sources for biomass are microbial sources, plant sources and animal sources. The present invention also provides methods for making such compositions, specifically, a method for producing a product comprising a nutrient, particularly a long chain polyunsaturated fatty acid, comprising hydrolyzing a biomass comprising the nutrient and emulsifying the hydrolyzed biomass. Such compositions and methods are useful, for example, for increasing intake of nutrients such as omega-3 long chain polyunsaturated fatty acids having 18 or more carbons.
Owner:MARTEK BIOSCIENCES CORP
Method for making a biofabricated material containing collagen fibrils
ActiveUS20170233834A1Facilitates uniform uptakeSame elasticityMonocomponent protein artificial filamentDomestic upholsteryFiberChemical synthesis
Described herein is a method for producing a biofabricated material from collagen or collagen-like proteins which are recombinantly produced and which contain substantially no 3-hydroxyproline. The collagen or collagen-like proteins are isolated from animal sources, or produced by recombinant DNA techniques or by chemical synthesis. The collagen or collagen-like proteins are fibrillated, crosslinked, dehydrated and lubricated thus forming the biofabricated material having a substantially uniform network of collagen fibrils.
Owner:MODERN MEADOW INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com