Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Surgical sealant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Biocompatible crosslinked polymers
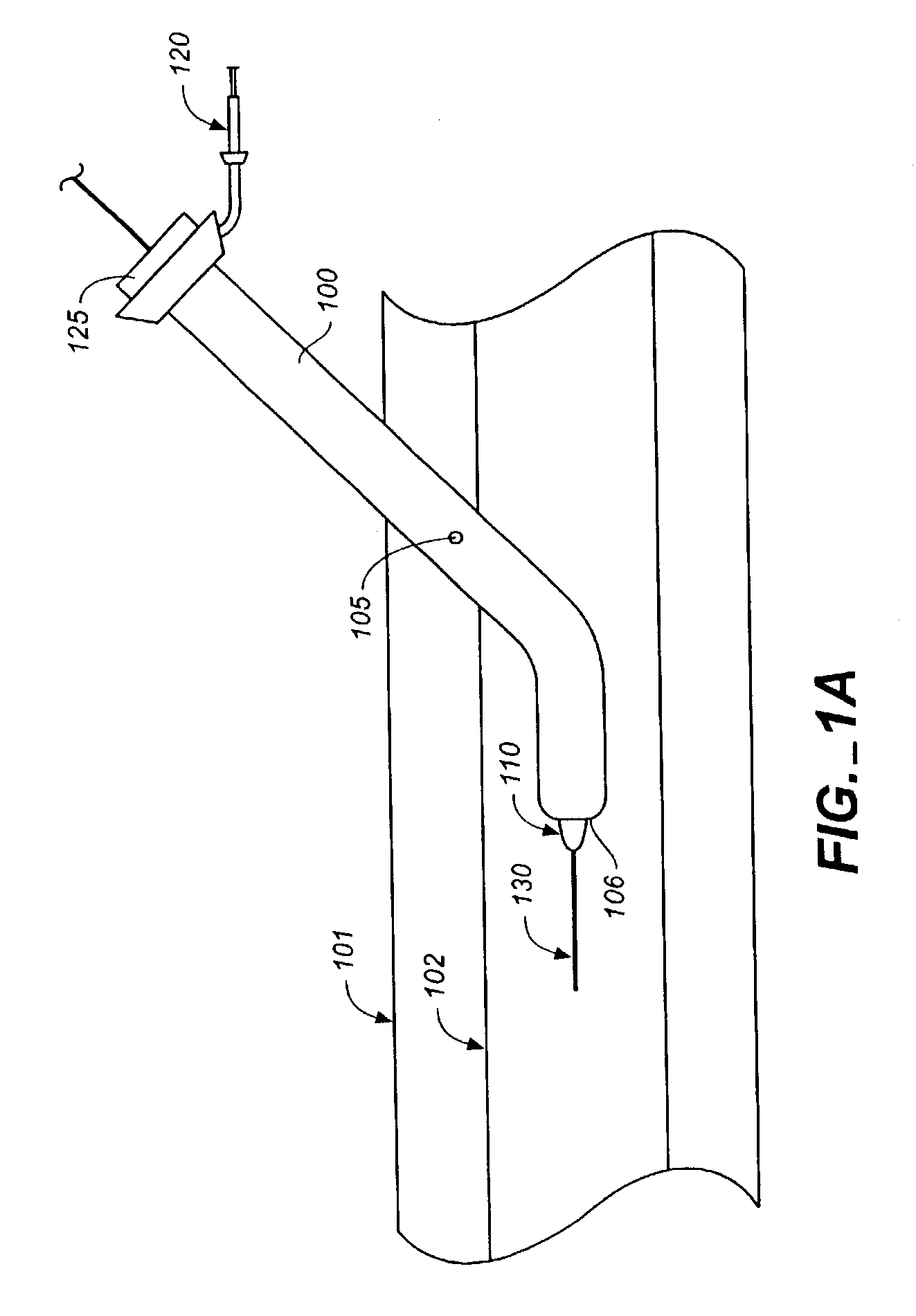
InactiveUS7009034B2Improve performanceImprove visibilityUltrasonic/sonic/infrasonic diagnosticsPowder deliveryWound dressingPost operative
Biocompatible crosslinked polymers, and methods for their preparation and use, are disclosed in which the biocompatible crosslinked polymers are formed from water soluble precursors having electrophilic and nucleophilic functional groups capable of reacting and crosslinking in situ. Methods for making the resulting biocompatible crosslinked polymers biodegradable or not are provided, as are methods for controlling the rate of degradation. The crosslinking reactions may be carried out in situ on organs or tissues or outside the body. Applications for such biocompatible crosslinked polymers and their precursors include controlled delivery of drugs, prevention of post-operative adhesions, coating of medical devices such as vascular grafts, wound dressings and surgical sealants. Visualization agents may be included with the crosslinked polymers.
Owner:INCEPT LLC
Liquid applicator and method of use
An applicator for dispensing uniform thickness layers of liquid to surfaces and especially surgical sealants to surgical sites to create, in situ, a surgical incise drape is disclosed. The applicator employs a supported thin layer of foam which achieves layer thickness which are substantially independent of the pressure applied to the applicator during use. Methods of using the applicator are also disclosed.
Owner:ADVANCED MEDICAL SOLUTIONS PLYMOUTH
Biocompatible crosslinked polymers with visualization agents
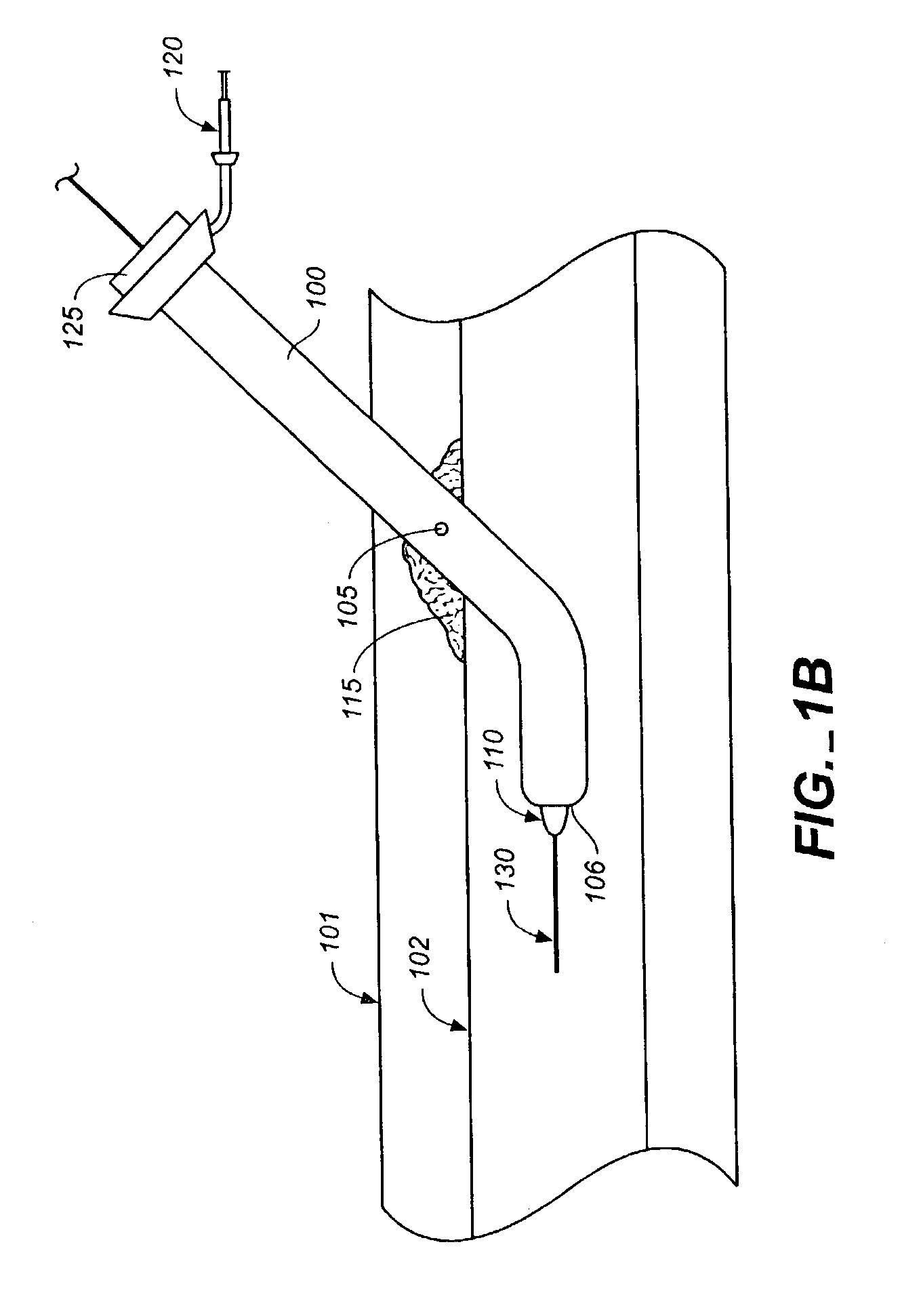
InactiveUS7332566B2Improve performanceEasy to usePowder deliveryFibrinogenWound dressingBlood vessel
Owner:INCEPT LLC
Vascular sealant delivery device and sheath introducer and method
A sheath introducer is inserted through the skin and into a body lumen, e.g., the femoral artery, for a medical procedure. Upon completion of the medical procedure, the sheath introducer is partially withdrawn from the body lumen so that at least one through-wall hole in a body region of the sheath introducer is positioned outside the body lumen, but under the skin. An opening in a distal end tip of the sheath introducer is sealed so that no blood is flowing into the sheath introducer the after sheath introducer is partially withdrawn. A surgical sealant is injected into the sheath introducer and flows out of the at least one through-wall hole and surrounds the access site. After injection of the surgical sealant, the sheath introducer is removed. The surgical sealant seals the puncture and minimizes any blood flow from the body lumen through the puncture.
Owner:MEDTRONIC VASCULAR INC
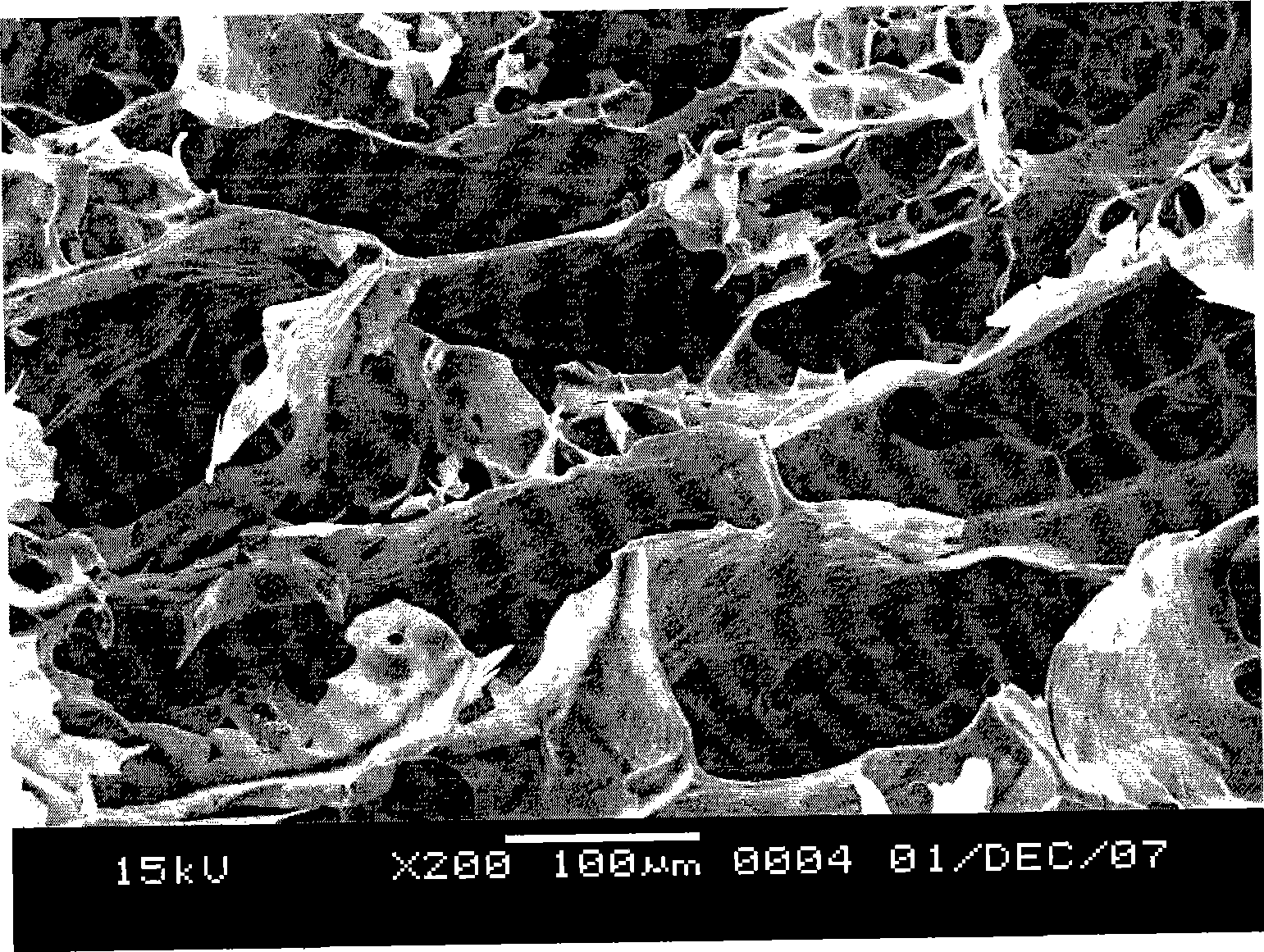
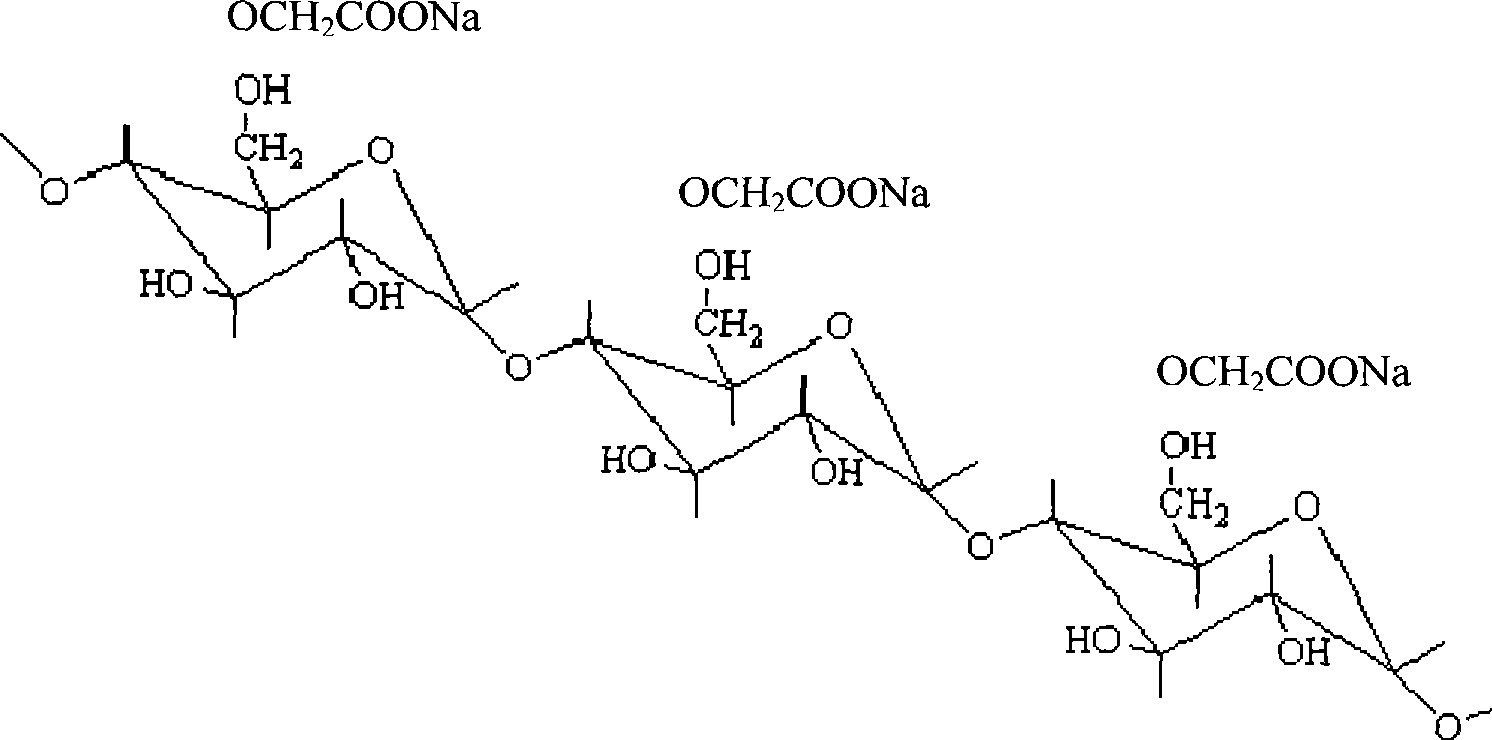
Denaturated starch absorbable hemostatic material and preparation method thereof
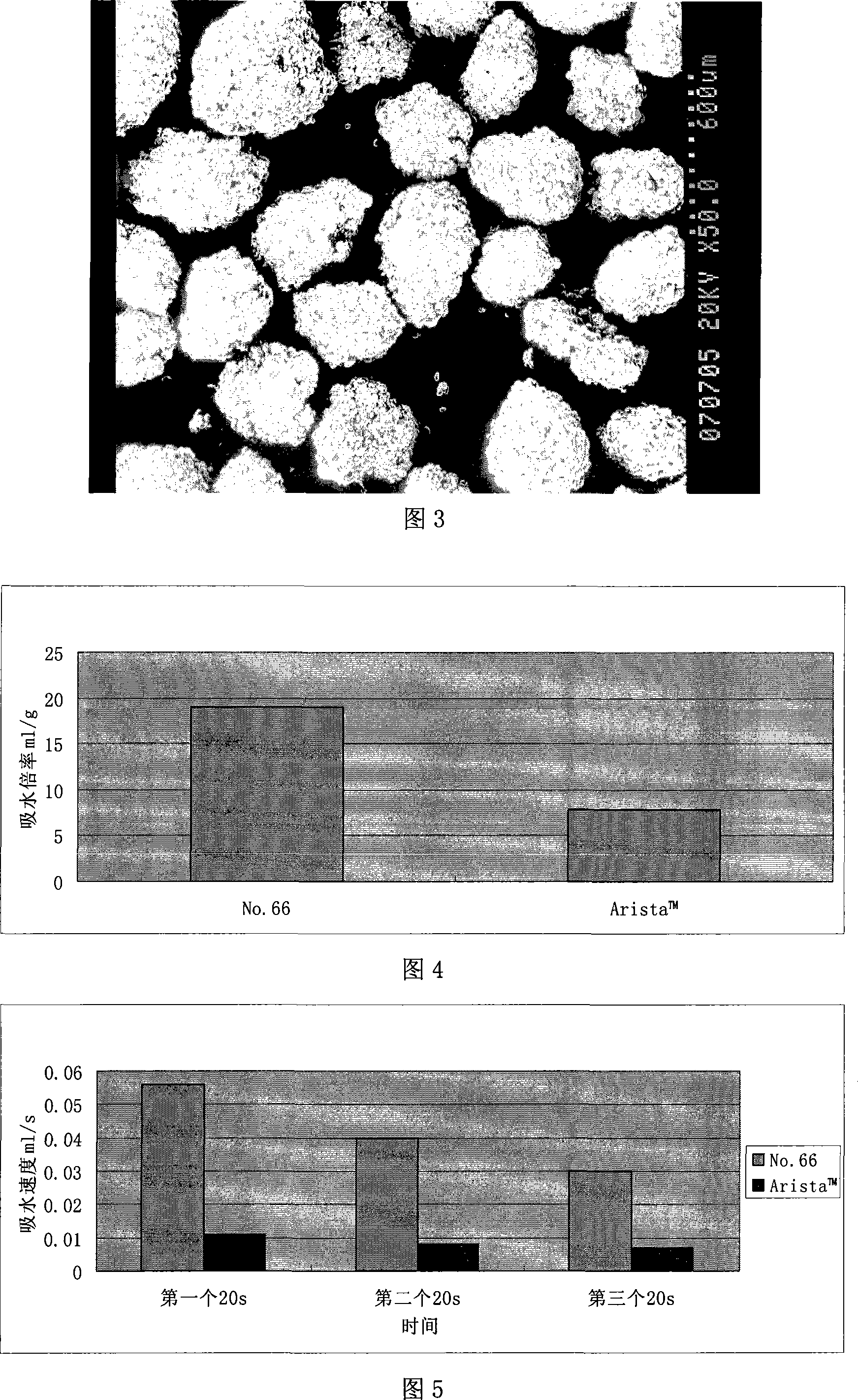
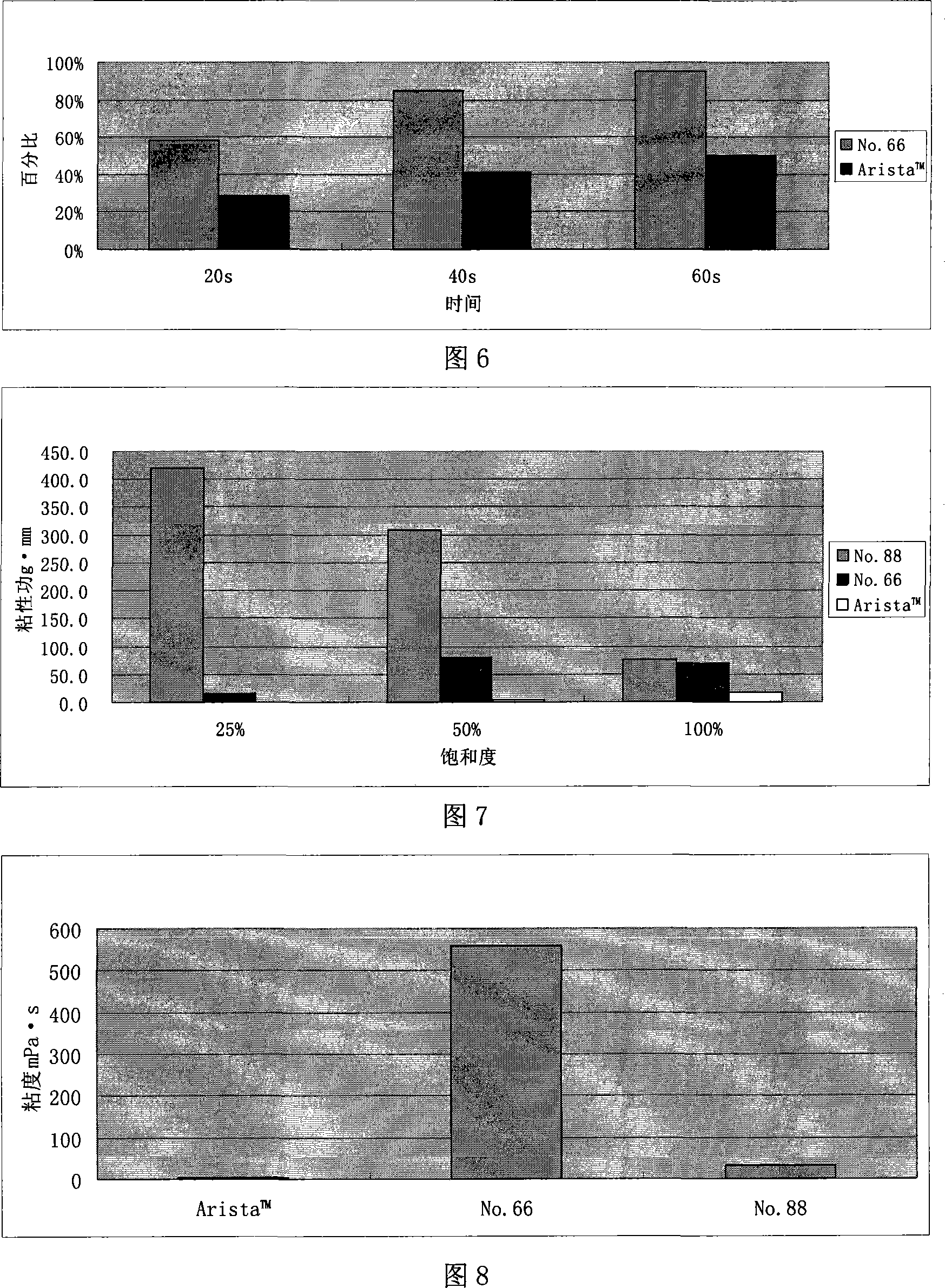
InactiveCN101121041AHigh viscosityImprove water absorption speedSurgical adhesivesPharmaceutical delivery mechanismMedicineBlood plasma
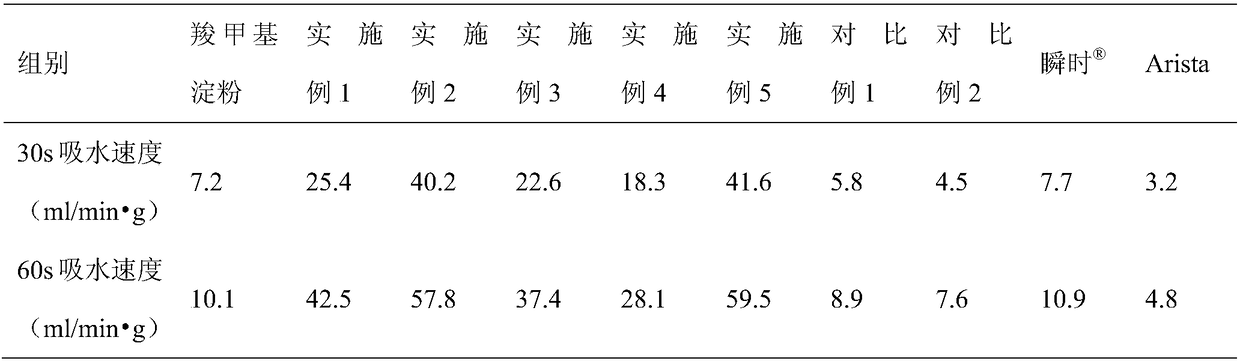
An absorbable modified starch hemostatic material and preparation thereof, wherein the hemostatic material is etherified starch, or a mixture of one or more etherified starches, crosslinked starches. The modified starch has a molecular weight of 15,000 - 10,000,000, a particle size of 10 - 1000µm, and a water absorption rate of 1 - 100. The biocompatible hemostatic material can directly effect on the wound with blood, concentrate the blood quickly to congulate blood; moreover, the gelatiniform mixture formed with blood has high viscosity, which can plug damaged tissue and blood vessel. The biocompatible hemostatic material is easy to swell in the water and easy to be washed so that the residue can be reduced; it is stable, not easy to decompose, and has long shelf life and storage advantage. The biocompatible hemostatic material can also be used as absorbable surgical antisticking material, promoting tissue healing material, surgical sealant and wound no-joint tissue adhesive.
Owner:美国淀粉医疗公司
Biodegradable and biocompatible crosslinked polymer hydrogel prepared from PVA and/or PEG macromer mixtures
InactiveUS20050271727A1High elastic modulusLow elongationPowder deliverySurgical adhesivesWound dressingBlood vessel
Biodegradable and biocompatible polymeric hydrogels based on the mixtures of poly(vinyl alcohol) and poly(ethylene glycol) macromers, and methods for their preparation and use, are disclosed. The polymerization may be carried out in situ on organs or tissues or outside the body. Applications for such biocompatible crosslinked hydrogels include prevention of post-operative adhesions, surgical sealants, embolic therapies, controlled delivery of drugs, coating of medical devices such as vascular grafts, wound dressings and other medical applications.
Owner:CALLISYN PHARMA
Biocompatibility modified starch sponges
ActiveCN101455857AFlexibleActive bleeding is easily controlledAbsorbent padsBandagesFreeze-dryingBiocompatibility Testing
The invention relates to biocompatible modified starch sponge and use of the biocompatible modified starch sponge as a hemostatic material, an anti-adhesion material, a material for promoting tissue healing, a surgical sealant or a wound tissue adhesive. Modified starch is one or a combination of more than one among pre-gelatinized starch, acid modified starch, dextrin, oxidized starch, esterified starch, etherified starch, crosslinked starch, grafted starch and composite modified starch. The sponge is prepared by vacuum freeze drying of the modified starch and other biocompatible hemostatic material, coagulant, plasticizer and so on. The biocompatible modified starch sponge has the advantages that the biocompatible modified starch sponge has flexible form and good biocompatibility, can be directly acted on bloody wound surfaces, avoids the conditions of hypersusceptibility, infection and difficult healing of wounds caused by adoption of hemostatic materials such as animal source / human source collagens, obviously improves the water absorption speed, has larger viscosity, forms a zymoplastic mixture which has good adhesion, calks broken tissues and blood vessels, and is used for hemostasis of active hemorrhage.
Owner:BEIJING UNIVERSAL LIKANG TECH CO LTD
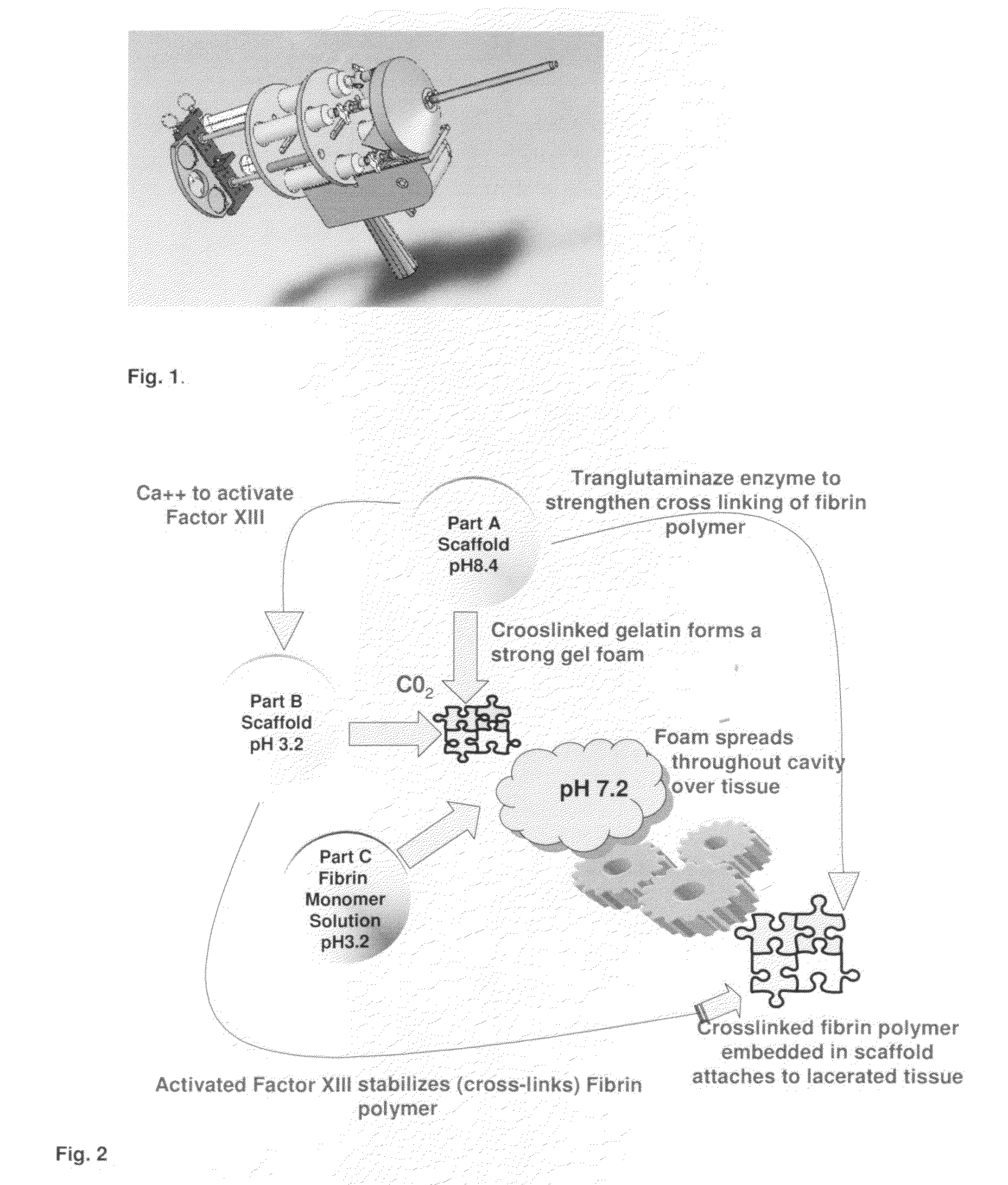
Tissue Sealant for Use in Non Compressible Hemorrhage
InactiveUS20110066182A1Excellent hemostatic agent candidateLeast riskSuture equipmentsPowder deliveryTissue sealantThoracic cavity
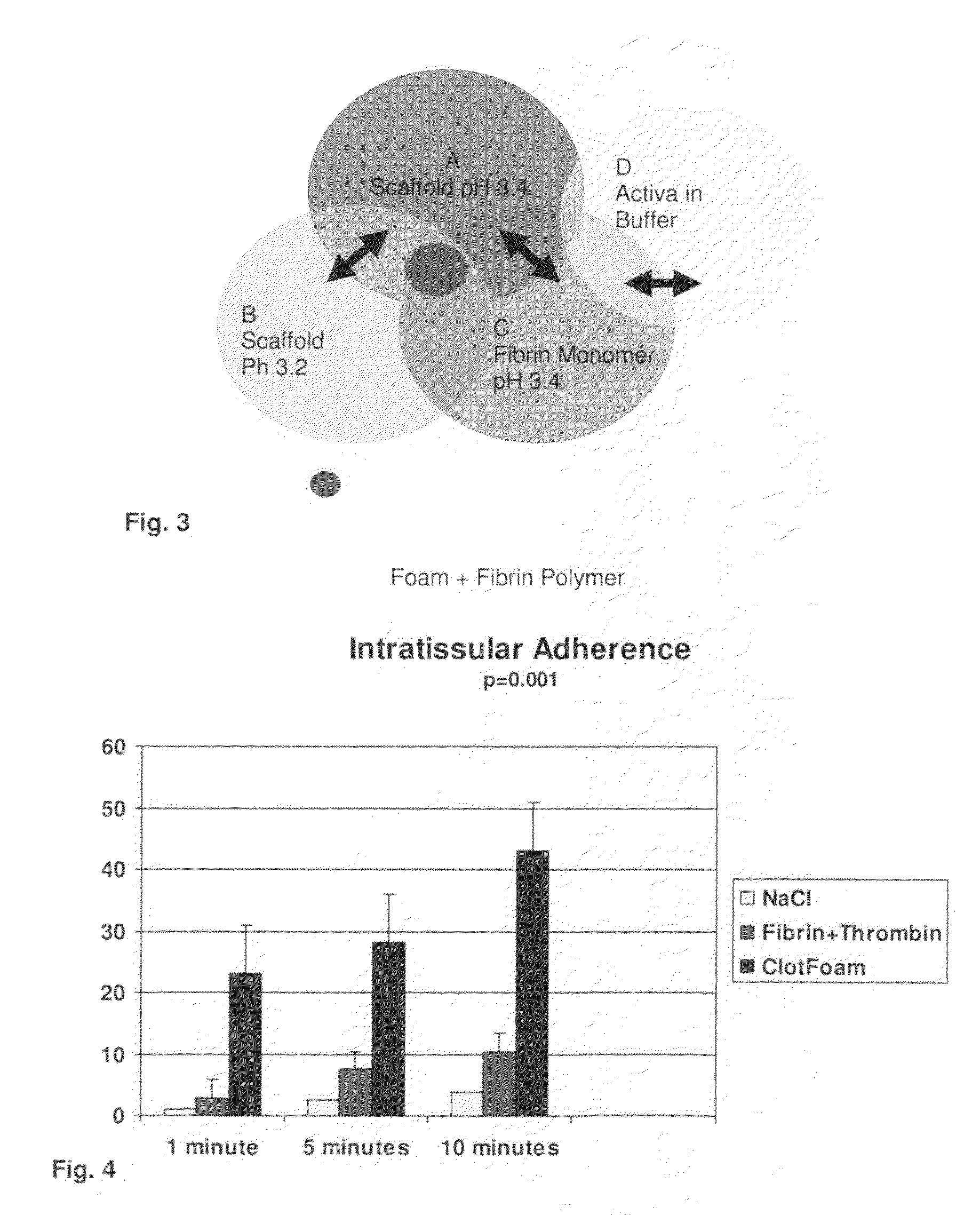
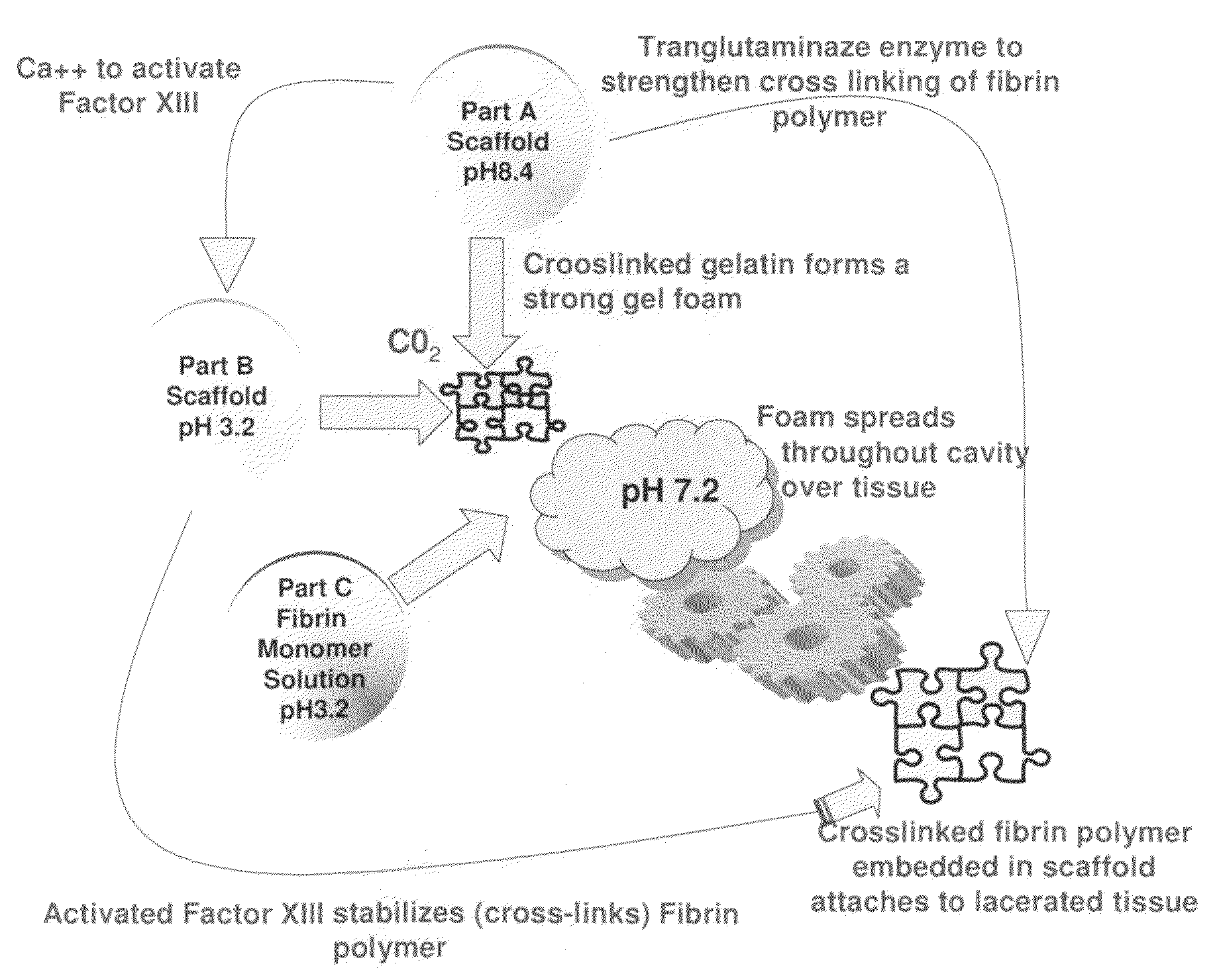
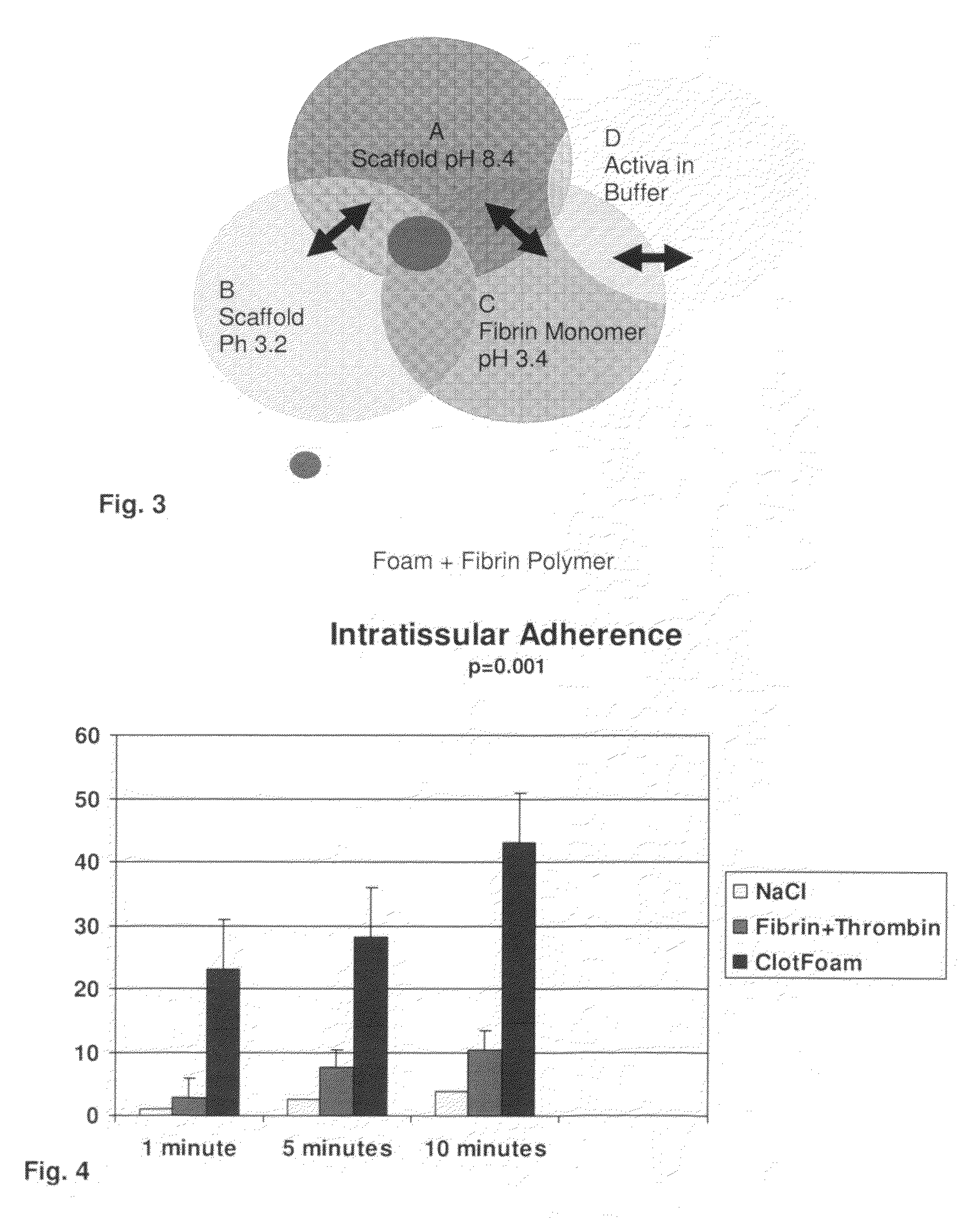
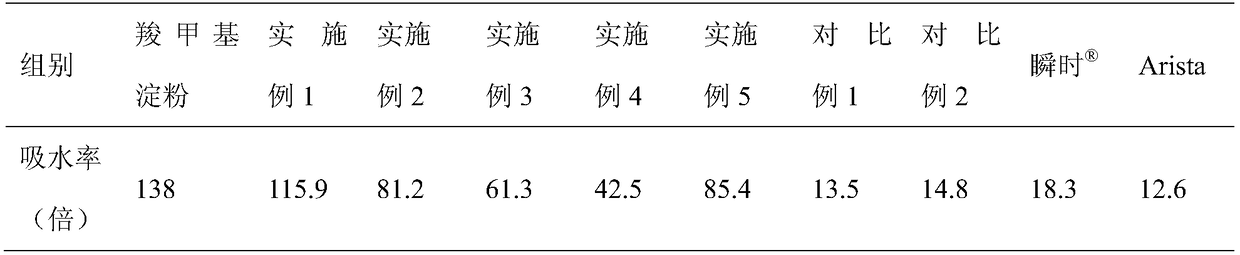
ClotFoam is a surgical sealant and hemostatic agent designed to be used in cases of non-compressible hemorrhage. It can be applied in the operating room through laparoscopic ports, or directly over lacerated tissue in laparotomy procedures or outside the operating room through a mixing needle and / or a spray injection method following abdominal, chest, extremities or other intracavitary severe trauma to promote hemostasis. Its crosslinking technology generates an adhesive three-dimensional polymeric network or scaffold that carries a fibrin sealant required for hemostasis. When mixed, Clotfoam produces a foam that spreads throughout a body cavity reaching the lacerated tissue to seal tissue and promote the coagulation cascade.The viscoelastic attachment properties of the foam as well as the rapid formation of a fibrin clot that ensure that the sealant remains at the site of application without being washed away by blood or displaced by movement of the target tissue .
Owner:FALUS GEORGE D PHD
Biocompatible crosslinked polymers with visualization agents
InactiveUS20060147409A1Improve performanceEasy to usePowder deliveryFibrinogenWound dressingPost operative
Biocompatible crosslinked polymers, and methods for their preparation and use, are disclosed in which the biocompatible crosslinked polymers are formed from water soluble precursors having electrophilic and nucleophilic functional groups capable of reacting and crosslinking in situ. Methods for making the resulting biocompatible crosslinked polymers biodegradable or not are provided, as are methods for controlling the rate of degradation. The crosslinking reactions may be carried out in situ on organs or tissues or outside the body. Applications for such biocompatible crosslinked polymers and their precursors include controlled delivery of drugs, prevention of post-operative adhesions, coating of medical devices such as vascular grafts, wound dressings and surgical sealants. Visualization agents may be included with the crosslinked polymers.
Owner:INCEPT LLC
Biocompatibility pre-gelatinized modified starch and preparation thereof
InactiveCN101497670AAbsorbentSpeed up absorptionSurgical adhesivesFibre treatmentBiocompatibility TestingHigh pressure
The invention relates to a biocompatible pre-gelatinized modified starch. The water absorbency is not less than one time, and the biocompatible pre-gelatinized modified starch is taken as biocompatible hemostatic material, biocompatible anti-blocking material, biocompatible tissue-healing promoting material, biocompatible surgical sealant or biocompatible wound closure tissue glue. The invention has the advantages that the biocompatible pre-gelatinized modified starch is directly acted on the wounded area with blood for immediately stopping bleeding, has obviously increased water absorbency and speed of water absorption and greater viscosity and stickiness and further plays the role in preventing the tissue and the blood vessel from being damaged during the process of stopping bleeding; the modified starch is easy to swell or dissolve in water, and is washed by normal saline after the bleeding stopping so as to reduce the residual in the body, to be favorable for wound healing and to avoid the pain due to tearing the gauze and the bandage out; the pre-gelatinized modified starch has the actions of bacterial resistance and anti-inflammatory; and the pre-gelatinized modified starch is stable, not easy to decompose, long in guarantee period, convenient for storage, resistant at high pressure and low pressure, resistant at high temperature and low temperature and not easy to change the physicochemical characteristics.
Owner:纪欣
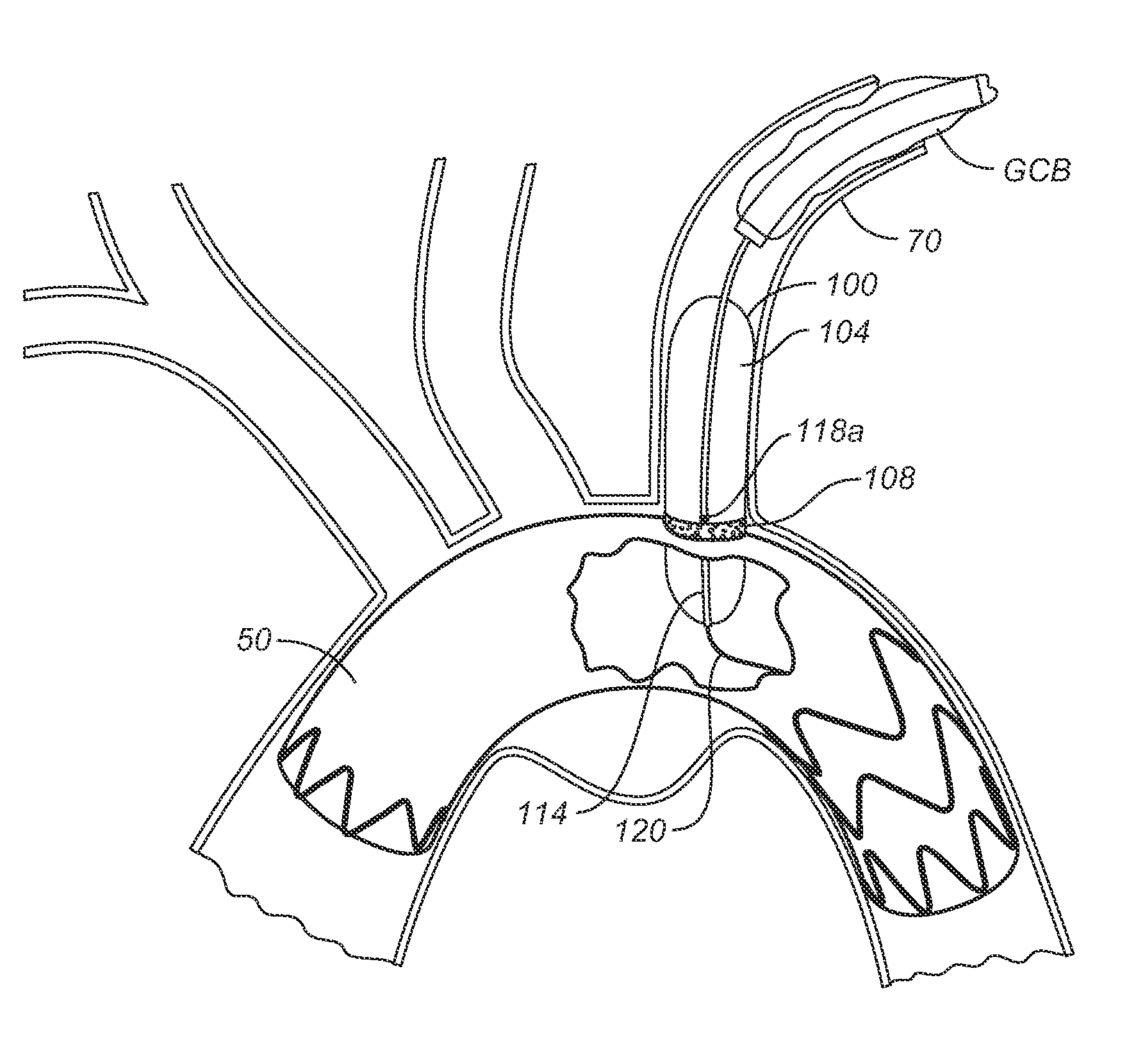
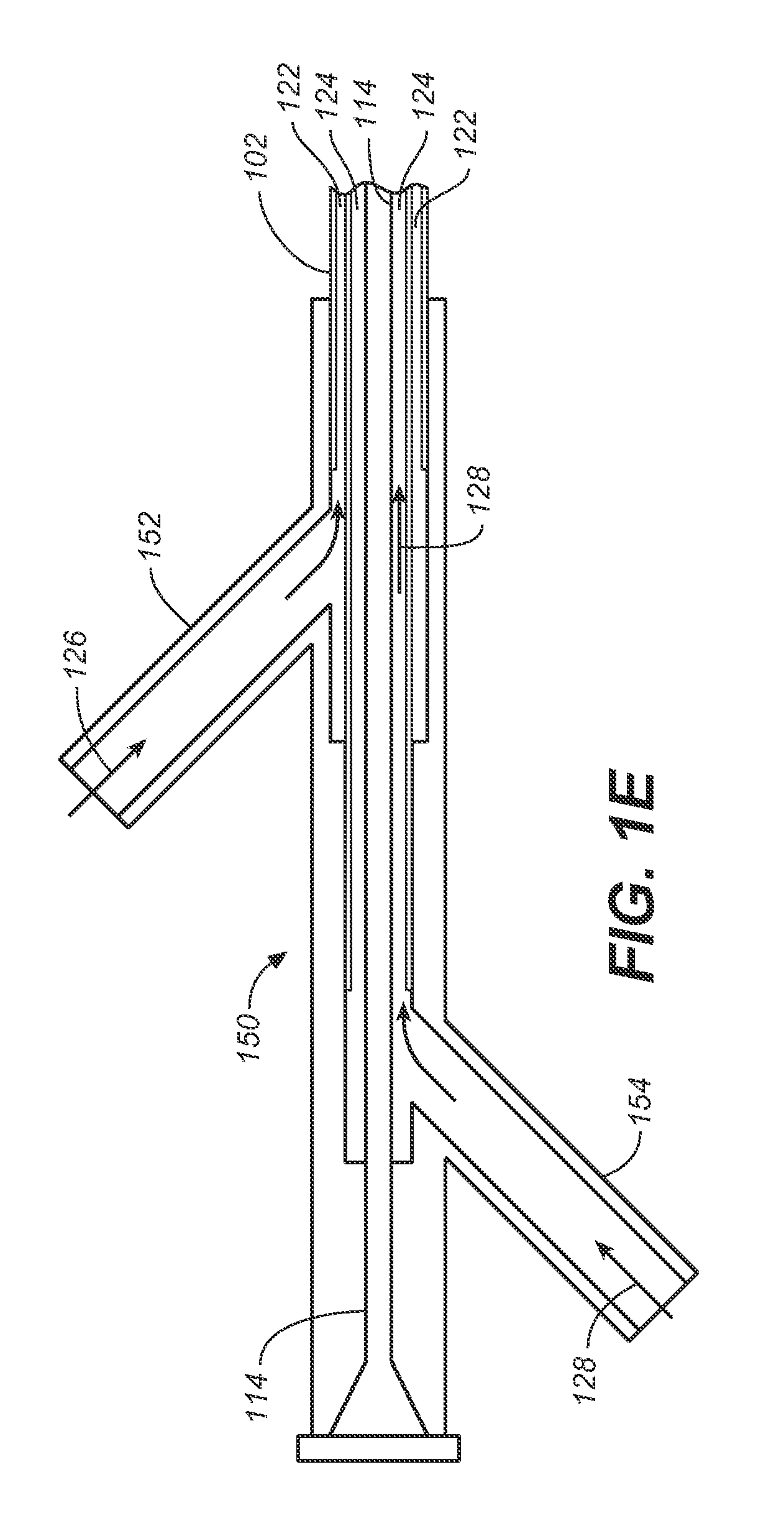
Endolumenal Sealant Delivery Apparatus and Methods
ActiveUS20090264821A1Prevent and minimize riskImprove sealingStentsBalloon catheterStent graftingBiomedical engineering
Endolumenally sealing a zone around the puncture and dilation area of a stent-graft fenestration or sealing the juncture between two lumens with an expandable sealant delivery device. In exemplary embodiments, an expandable sealant delivery device includes a catheter mounted balloon, which has a microporous membrane or a plurality of pores suitable for delivering a surgical sealant.
Owner:MEDTRONIC VASCULAR INC
Biocompatible polymers and hydrogels and methods of use
Owner:INCEPT LLC
Biocompatible polymers and hydrogels and methods of use
Owner:INCEPT LLC
Biocompatible hydrogels made with small molecule precursors
InactiveUS8003705B2Easily degradablePrevent surgical adhesionBiocideFibrinogenWound dressingBlood vessel
Biocompatible crosslinked polymers, and methods for their preparation and use, are disclosed in which the biocompatible crosslinked polymers are formed from water soluble precursors having electrophilic and nucleophilic functional groups capable of reacting and crosslinking in situ. Methods for making the resulting biocompatible crosslinked polymers biodegradable, or not, are provided, as are methods for controlling the rate of degradation. The crosslinking reactions may be carried out in situ on organs or tissues or outside the body. Applications for such biocompatible crosslinked polymers and their precursors include controlled delivery of drugs, prevention of post-operative adhesions, coating of medical devices such as vascular grafts, wound dressings and surgical sealants. Visualization agents may be included with the crosslinked polymers. Embodiments that include hydrogels having isolated hydrolytically degradable esters are set forth. Embodiments including the use of low molecular weight amines to make degradable hydrogels are also set forth.
Owner:INCEPT LLC
Vascular sealant delivery device and sheath introducer and method
A sheath introducer is inserted through the skin and into a body lumen, e.g., the femoral artery, for a medical procedure. Upon completion of the medical procedure, the sheath introducer is partially withdrawn from the body lumen so that at least one through-wall hole in a body region of the sheath introducer is positioned outside the body lumen, but under the skin. An opening in a distal end tip of the sheath introducer is sealed so that no blood is flowing into the sheath introducer the after sheath introducer is partially withdrawn. A surgical sealant is injected into the sheath introducer and flows out of the at least one through-wall hole and surrounds the access site. After injection of the surgical sealant, the sheath introducer is removed. The surgical sealant seals the puncture and minimizes any blood flow from the body lumen through the puncture.
Owner:MEDTRONIC VASCULAR INC
Heart-sealing gel and preparation method thereof
ActiveCN107469135APromote degradationGood biocompatibilitySurgical adhesivesPharmaceutical delivery mechanismPolypyrrolePhosphate
The invention relates to a heart-sealing gel and a preparation method thereof. Specifically speaking, a pyrrole-capped hyperbranched polyaminoester monomer (HPAE-Py) containing dopamine groups and gelatin (Geln) are dissolved into PBS (phosphate buffer solution), the pH value of which is 7.4, so that HPAE-Py / Geln mixed solution is prepared; and aqueous ferric chloride solution is added into the HPAE-Py / Geln mixed solution, so that gel precursor solution is prepared. Ferric chloride can ensue the complexing and self-polymerization of dopamine in the HPAE-Py polymer monomer, so that pyrrole in HPAE-Py is polymerized and complexed with the gelatin, and ultimately, the conductive sticky hydrogel is constructed. At the initial stage of formation, the hydrogel disclosed by the invention has the characteristics of softness and stickiness, and can be applied on a tissue wound, preventing adverse fluid leakage; pyrrole groups are polymerized into polypyrrole, which serves as crosslinking sites to consolidate a gel network structure; the gel has high strength and electrical conductivity; the gel has good biocompatibility, can be degraded, and can be used as a surgical sealant for, for example, wound sealing after cardiac surgery.
Owner:HANGZHOU YAHUI BIOTECH CO LTD
Sealant hydrogel, kit and preparation method of sealant hydrogel
ActiveCN108014365AGood bioadhesionGood biocompatibilitySurgical adhesivesClinical valuePolyethylene glycol
The invention provides sealant hydrogel, a kit and a preparation method of the sealant hydrogel. The preparation method of the sealant hydrogel is simple. On the basis of common two-component hydrogel, a chemically modified polyethylene glycol macromonomer is added, and during use, the hydrogel with certain adhesive force is rapidly produced in situ after mixing. The produced hydrogel has good biocompatibility, bioadhesion, biodegradability and adjustable degradation performance, gelation time of the hydrogel is greatly shortened, important indexes such as gel content, swelling rate and the like are substantially increased, and degradation speed can be adjusted according to needs of application occasions. The sealant hydrogel can have important clinical value in the medical field, especially when used as a surgical sealant.
Owner:沈伟
Tissue sealant for use in non compressible hemorrhage
InactiveUS8314211B2Excellent hemostatic agent candidateLeast riskSuture equipmentsPowder deliveryTissue sealantLaparoscopes
Owner:FALUS GEORGE D PHD
Medical absorbable polysaccharide composite material and application thereof
ActiveCN109498833AGood biocompatibilityNon-cytotoxicSurgical adhesivesPharmaceutical delivery mechanismFreeze-dryingBiocompatibility Testing
The invention discloses a medical absorbable polysaccharide composite material and application thereof. According to the medical absorbable polysaccharide composite material, starch with poor water absorbability is used as one material in the polysaccharide composite material; furthermore, a polysaccharide material with good water absorbability is added; the composite polysaccharide material is obtained through directly mixing, or through adding water, uniformly mixing and then carrying out freeze drying, without the need of cross-linking reaction. The material has the advantages of high waterabsorption rate, rapid water absorption speed, higher gel strength, good biocompatibility and the like, and can be directly used on wound surfaces with blood and for stopping bleeding of body surfaces, tissue organs in inner parts of bodies and in body cavities; the material can be used for rapidly stopping bleeding, can be absorbed by human bodies and has a sticky plugging effect. The material also can be further used as a post-operative anti-adhering material, a tissue healing promoting material, a surgical sealant, a wound suture-free tissue adhesive, a tissue filling material and a tissuedebridement material.
Owner:济南格莱威医疗科技有限公司
Blood products with binding agent
The present invention provides a non-liquid biomaterial that may be used as a surgical sealant, a suture support, a blood flow controller, an adhesion reducing agent, an adhesion preventing agent, a tissue support, a tissue filler, a wound dressing or a combination thereof. The non-liquid biomaterial may comprise a blood derived material such as plasma, platelet poor plasma, platelet rich plasma or a material derived from blood containing tissue aspirate, such as bone marrow aspirate, a protein binding agent and a polymerizing agent. Methods for making and using the non-liquid biomaterial are also provided.
Owner:BIOMET MFG CORP
Dental/Surgical Sealants Including Shapeable Particles
A sealant composition, suitable for surgical or dental use, comprises a sealant and, dispersed therein, one or more particles of a material which is prestressed and / or capable of undergoing expansion or contraction.
Owner:DRFP HLDG LTD
Surgical sealant kit and application thereof in brain and spine surgery
ActiveCN113521376AReduce swellingGood swelling propertiesSurgical adhesivesPharmaceutical delivery mechanismSurgical operationSpinal column
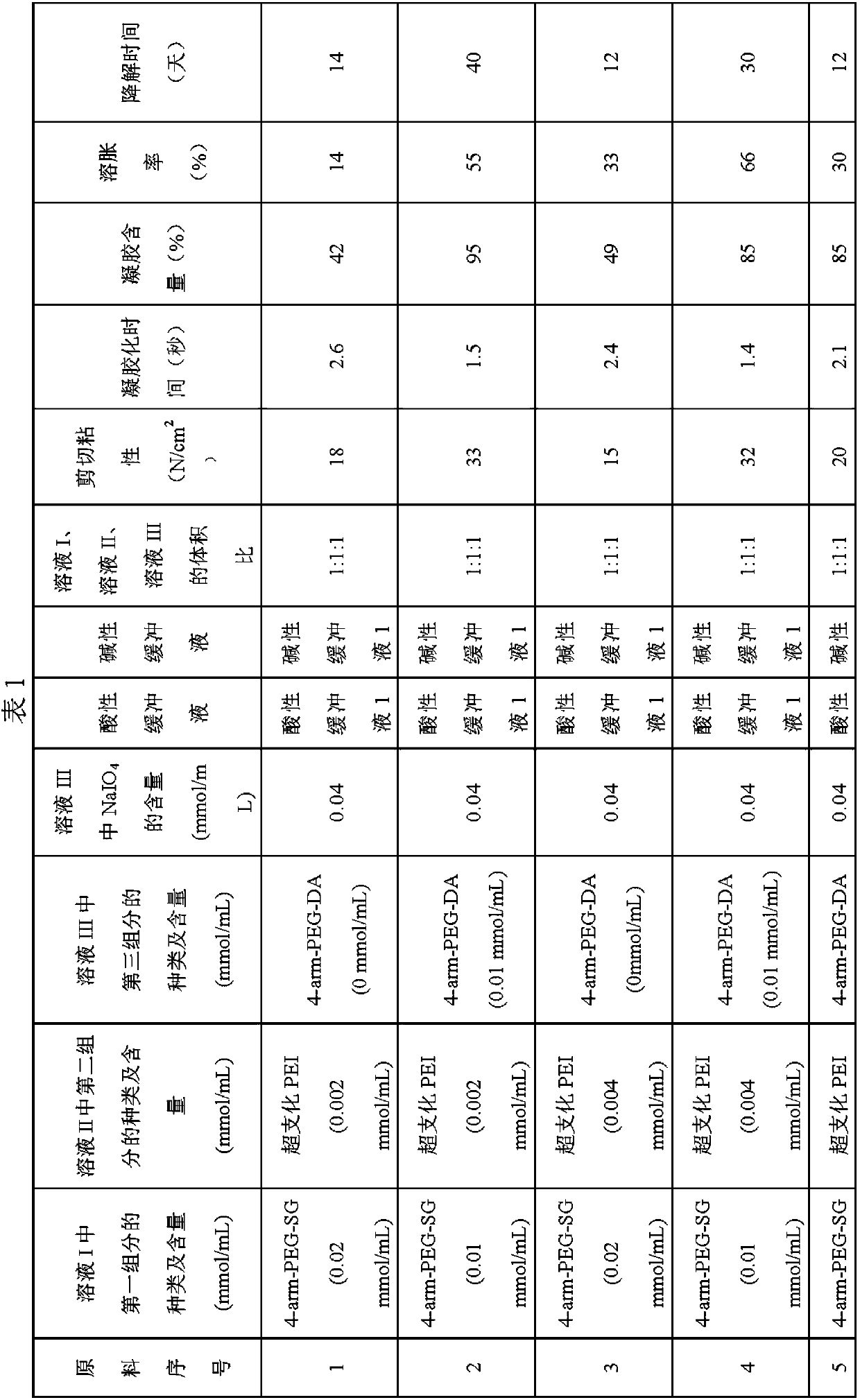
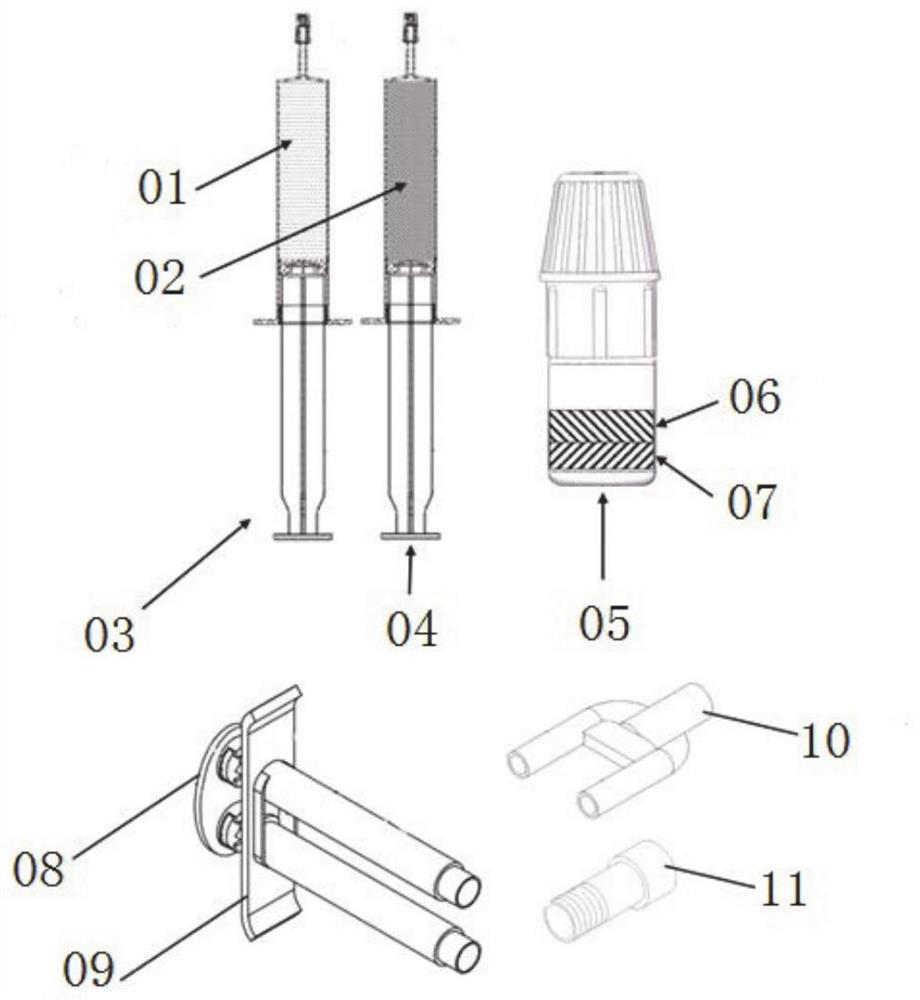
The invention relates to a surgical sealant kit and application thereof in brain and spine surgery. The surgical sealant kit comprises a first injector containing an acidic buffer solution of an amino compound; a second syringe containing an alkaline buffer solution; a powder bottle containing two polyethylene glycol derivatives of different structures and / or molecular weights; and matched tool that comprises a push plate, an injector bracket, a two-way connector, an atomizing nozzle and other parts. The sealant is hydrogel formed by compounding and cross-linking two polyethylene glycol derivatives with different molecular weights and / or structures and an amino compound, meanwhile, the amino compound is dissolved in the acidic buffer solution, so that the swelling ratio of the sealant is further reduced, and the gel forming speed is increased. The kit has the characteristics that the swelling rate is greater than-20% and less than 20%, and the gel forming time is less than 1s, and can be used for auxiliary sealing of surgical parts such as brain and spine.
Owner:SAIKE SAISI BIOTECH CO LTD
Hydrogel forming covalent cross-linking rapidly under mild conditions and preparation method thereof
ActiveCN102206409AStable structureHigh viscoelasticitySurgical adhesivesAbsorbent padsCross-linkPolyethylene glycol
The invention discloses a hydrogel forming covalent cross-linking rapidly under mild conditions and a preparation method thereof. The hydrogel contains two components, i.e. NHS (N-Hydroxy Succinimide) ester of PEG (Polyethylene Glycol) and PEG containing N-terminal group cysteine. The two components are dissolved and mixed to obtain the hydrogel. The hydrogel disclosed by the invention can form covalent cross-linking rapidly under mild conditions, and is particularly suitable for being manufactured into surgical sealants.
Owner:HAINAN JIANKE PHARMA
Liquid applicator and method of use
An applicator for dispensing uniform thickness layers of liquid to surfaces and especially surgical sealants to surgical sites to create, in situ, a surgical incise drape is disclosed. The applicator employs a supported thin layer of foam which achieves layer thickness which are substantially independent of the pressure applied to the applicator during use. Methods of using the applicator are also disclosed.
Owner:ADVANCED MEDICAL SOLUTIONS PLYMOUTH
Endolumenal sealant delivery apparatus and methods
ActiveUS8206430B2Prevent and minimize riskImprove sealingStentsBalloon catheterStent graftingBiomedical engineering
Endolumenally sealing a zone around the puncture and dilation area of a stent-graft fenestration or sealing the juncture between two lumens with an expandable sealant delivery device. In exemplary embodiments, an expandable sealant delivery device includes a catheter mounted balloon, which has a microporous membrane or a plurality of pores suitable for delivering a surgical sealant.
Owner:MEDTRONIC VASCULAR INC
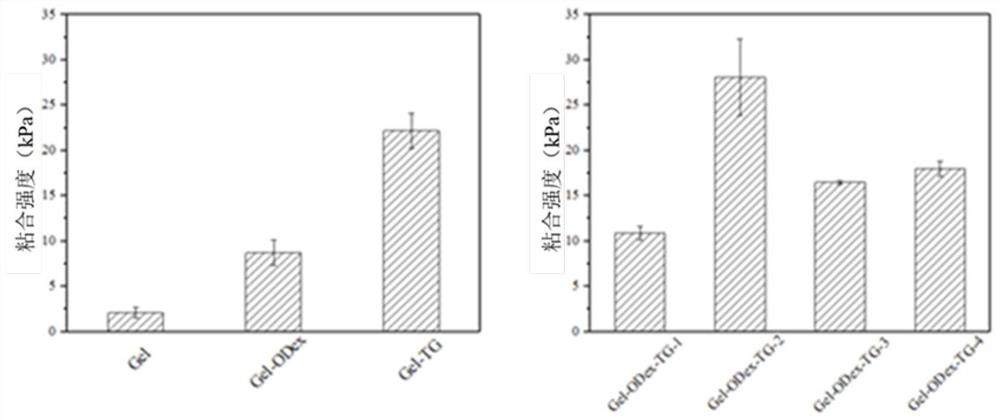
Dual-network adhesive for crosslinking of transglutaminase and preparation method of dual-network adhesive
ActiveCN112494710AGood biocompatibilityHigh strengthSurgical adhesivesPharmaceutical delivery mechanismHuman bodyAdhesive
The invention discloses a dual-network adhesive for crosslinking of transglutaminase and a preparation method of the dual-network adhesive. The preparation method comprises the following steps: simplytreating gelatin, mixing the gelatin with glucan aldehyde, and performing crosslinking under physiological conditions through the promotion action of the transglutaminase to form the dual-network adhesive. According to the double-network characteristic, the strength of the adhesive body is enhanced, and the adhesion performance of the adhesive is improved through introduction of the glucan aldehyde. The preparation method has the characteristics of simple raw material synthesis, injectable adhesive and easiness in application. By utilizing the characteristics, the dual-network adhesive can beused as a surgical sealant, a medical adhesive and the like to be clinically applied, and has a good application prospect in the fields of human body internal wound repair and the like.
Owner:TIANJIN UNIV
Fibrin based glue with functionalized hydrophilic polymer protein binding agent
The present invention provides a non-liquid biomaterial that may be used as a surgical sealant, a suture support, a blood flow controller, an adhesion reducing agent, an adhesion preventing agent, a tissue support, a tissue filler, a wound dressing or a combination thereof. The non-liquid biomaterial may comprise a blood derived material such as plasma, platelet poor plasma, platelet rich plasma or a material derived from blood containing tissue aspirate, such as bone marrow aspirate, a protein binding agent and a polymerizing agent. Methods for making and using the non-liquid biomaterial are also provided.
Owner:BIOMET MFG CORP
Surgical sealant products and method of use
A surgical joint replacement kit including a first joint replacement component and a surgical sealant. The first joint replacement component is capable of being implanted to replace a joint between a first bone and a second bone. The first bone is cut in conjunction with implanting the first joint replacement component. Cutting the first bone causes a bodily fluid to flow from the first bone. The surgical sealant includes an electrospun dextran base and an effective amount of at least one of fibrinogen and thrombin. The at least one of fibrinogen and thrombin is applied to the electrospun dextran base to form the surgical sealant. The surgical sealant is capable of staunching the flow of the bodily fluid from the first bone.
Owner:ST TERESA MEDICAL
Surgical sealant
ActiveUS20180272028A1High film strengthLow adhesion strengthSurgical adhesivesMacromolecular non-active ingredientsPolymer scienceSide chain
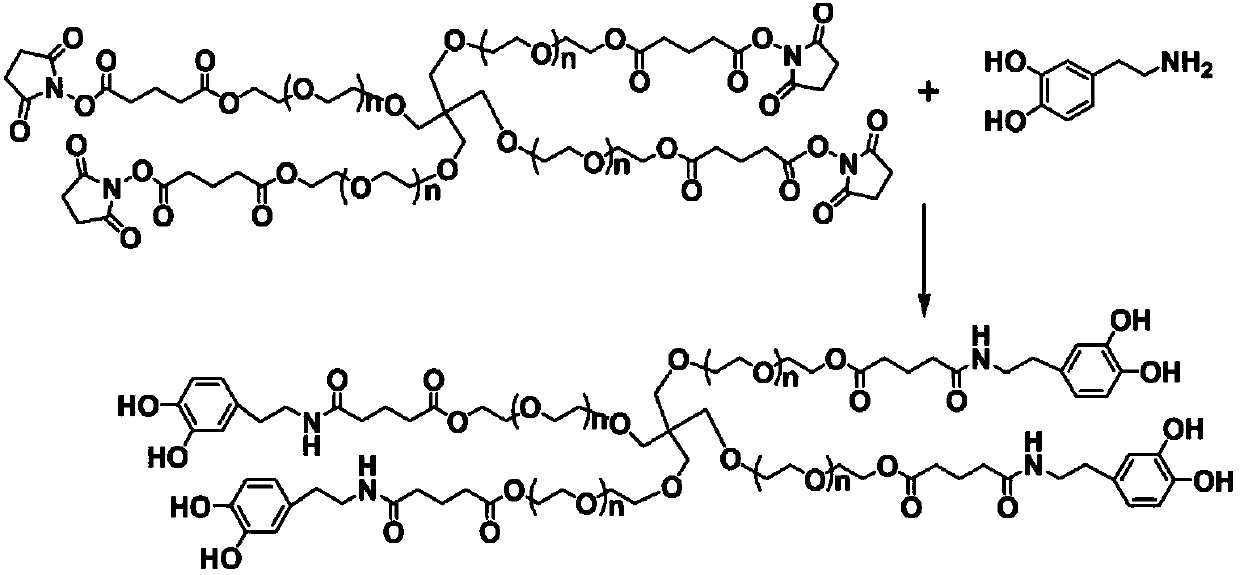
A surgical sealant comprising a first agent containing a hydrophobically-modified gelatin derived from a cold-water fish, and a second agent containing a water-soluble molecule for crosslinking, wherein the water-soluble molecule for crosslinking is at least one kind selected from the group consisting of poly acids and acid anhydrides having two or more active ester groups, and aldehyde compounds having two or more aldehyde groups, the hydrophobically-modified gelatin derived from a cold-water fish is a gelatin in which at least a part of amino groups of side chains of a gelatin derived from a cold-water fish has been substituted by hydrophobic groups, and the hydrophobic groups are linear chain aliphatic groups each having 8 to 18 carbon atoms, with a substitution rate (number of moles of hydrophobic groups / (total number of moles of hydrophobic groups and reactive amino groups in gelatin)×100) of 3 to 20 mol %.
Owner:NAT INST FOR MATERIALS SCI
A composition for promoting regeneration of dental pulp and dentin
ActiveCN104548212BRepair entryEasy injectionPeptide/protein ingredientsDigestive systemFibrin gluePhosphate
Owner:新科沃再生医学(苏州)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com