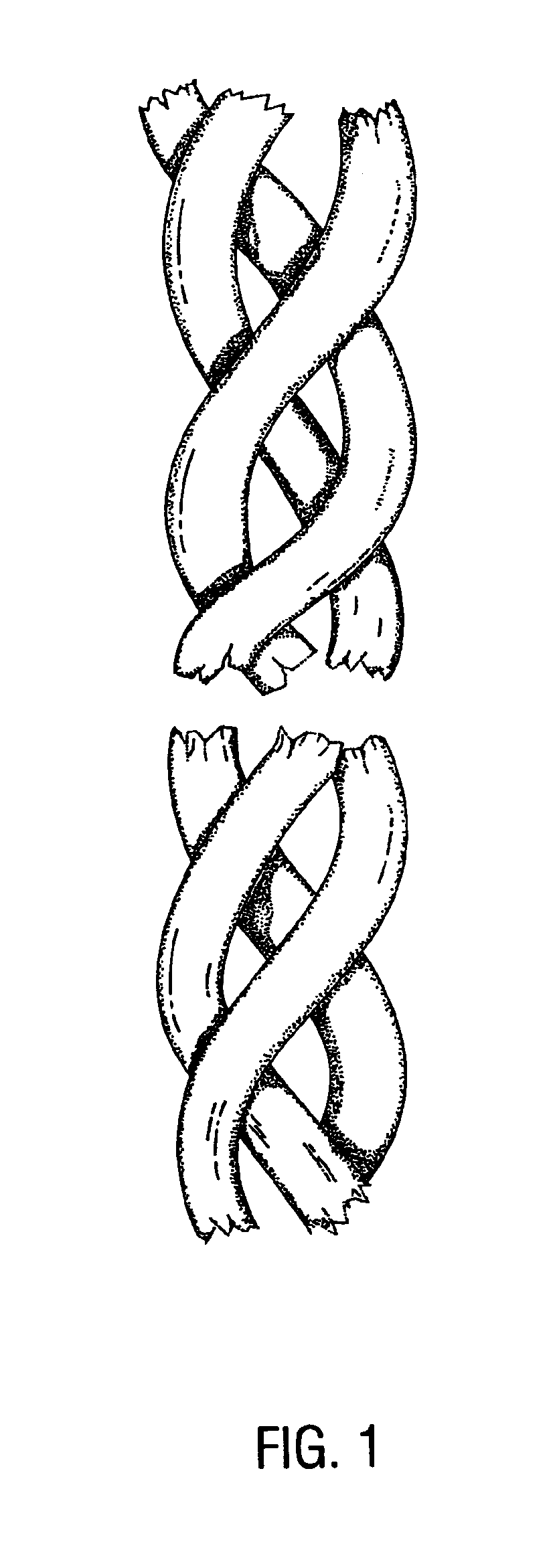Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30647 results about "Freeze-drying" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
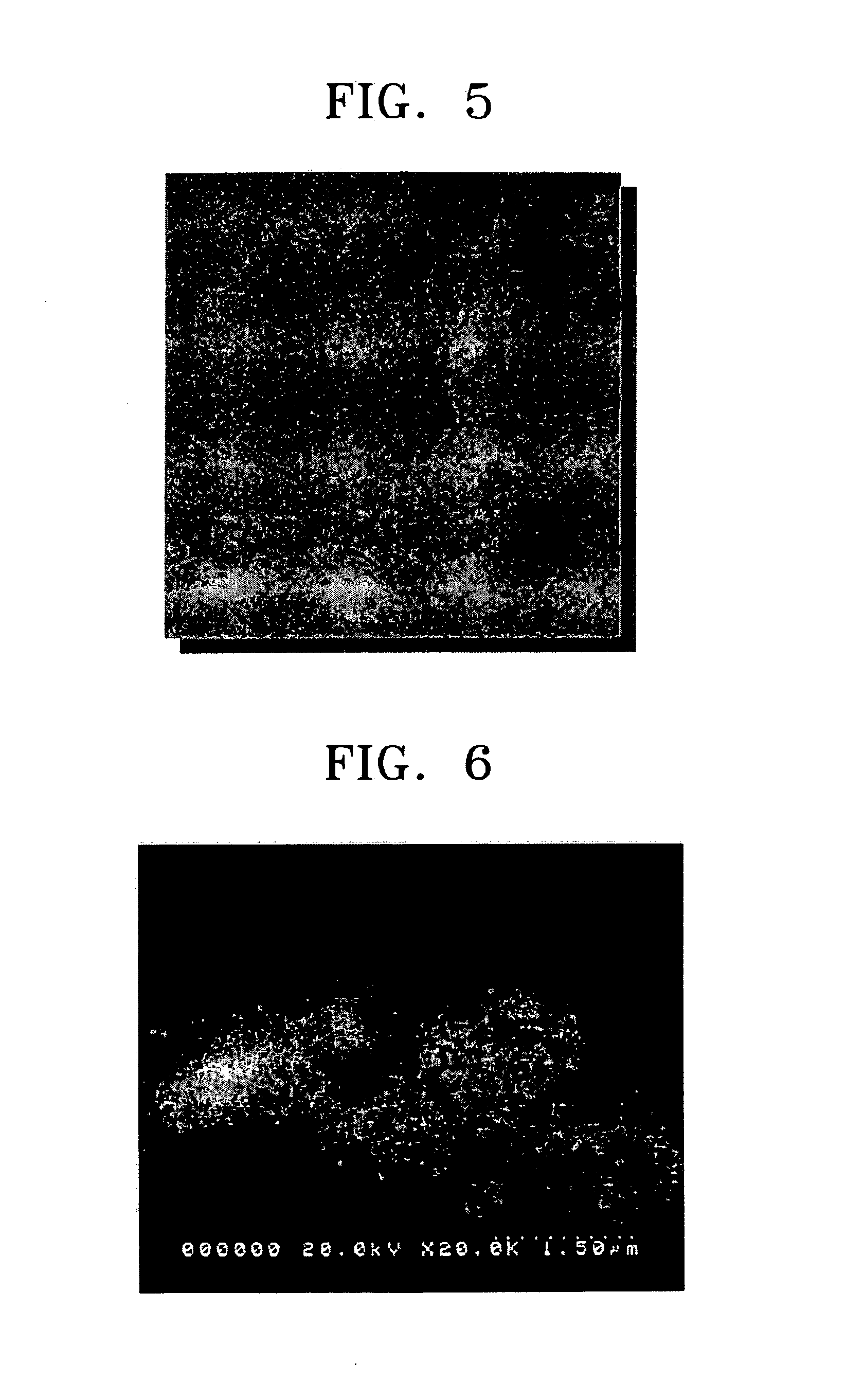
Freeze drying, also known as lyophilisation or cryodesiccation, is a low temperature dehydration process that involves freezing the product, lowering pressure, then removing the ice by sublimation. This is in contrast to dehydration by most conventional methods that evaporate water using heat.
Plasma protein matrices and methods for their preparation
InactiveUS7009039B2Rapid cell growthRapid vascularizationBiocidePeptide/protein ingredientsBiological propertyFreeze-drying
A freeze dried biocompatible matrix comprising plasma proteins, useful as implants for tissue engineering as well as in biotechnology, and methods of producing the matrix are provided. Mechanical and physical parameters can be controlled by use of auxiliary components or additives which may be removed after the matrix is formed in order to improve the biological properties of the matrix. The matrices according to the present invention may be used clinically per se, or as a cell-bearing implant.
Owner:PROCHON BIOTECH
Method of making an absorbent composite
In a method of making an absorbent composite, a porous, stabilized structure is formed and impregnated with a flowable superabsorbent precursor. The flowable superabsorbent precursor is cross-linked to form a superabsorbent material within the stabilized structure. The surface area of one of the flowable superabsorbent precursor impregnated with the stabilized structure and the superabsorbent material formed within the structure is increased. In one embodiment, the surface is increased by freeze drying the impregnated structure.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Method of preparing catalyst for manufacturing carbon nanotubes
InactiveUS20070020167A1Improve uniformityMinimize agglomerationMaterial nanotechnologyNanostructure manufactureFreeze-dryingCarbon nanotube
A novel method of forming catalyst particles, on which carbon nanotubes grow based, on a substrate with increased uniformity, and a method of synthesizing carbon nanotubes having improved uniformity are provided. A catalytic metal precursor solution is applied to a substrate. The applied catalytic metal precursor solution is freeze-dried, and then reduced to catalytic metal. The method of forming catalyst particles can minimize agglomeration and / or recrystallization of catalyst particles when forming the catalyst particles by freeze-drying the catalyst metal precursor solution. The catalyst particles formed by the method has a very uniform particle size and are very uniformly distributed on the substrate.
Owner:SAMSUNG SDI CO LTD
Preparation method of plant enzyme food
The invention discloses a preparation method of plant enzyme food, belonging to the field of food processing technology. The method comprises the following steps of: performing twice fermentation and compounding on the natural plant raw materials of fresh fruits, vegetables, mushrooms, medicines, grains and flowers to obtain the secondary-fermentation stock solution or raw sauce, and storing; filling and sealing the secondary-fermentation stock solution or raw sauce to obtain liquid or sauce-type plant enzyme food or compound plant enzyme food; or performing low-temperature concentration and freeze drying to obtain solid plant enzyme food or compound plant enzyme food. Due the adoption of the preparation method, the obtained natural plant enzyme food has the functions of improving the immunity of human body, activating cells, promoting metabolism and the like.
Owner:ZHEJIANG UNIVERSITY OF SCIENCE AND TECHNOLOGY
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Lyophilization process and products obtained thereby
ActiveUS20070116729A1Dissolve fastSuitable for useBiocidePowder deliveryHigh concentrationFreeze-drying
A lyophilization process which comprises dissolving a material in one or more solvents for said material to form a solution; forcing said material at least partially out of solution by combining the solution and a non-solvent for the material, which non-solvent is miscible with the solvent or solvents used and wherein said non-solvent is volatilizable under freeze-drying conditions. In addition, for hydrophobic and / or lipophilic materials, the anti-solvent can be omitted, and the solution of the material in the solvent can be subjected directly to freeze drying. The lyophilizates can then be reconstituted with typical aqueous diluent in the case of hydrophilic materials. Hydrophobic and or lipophilic materials can be initially reconstituted with propylene glycol and / or polyethyleneglycol to form a high concentration solution therein and this is further diluted for use with a diluent of Intralipid, plasma, serum, or even whole blood.
Owner:SCIDOSE PHARMA +1
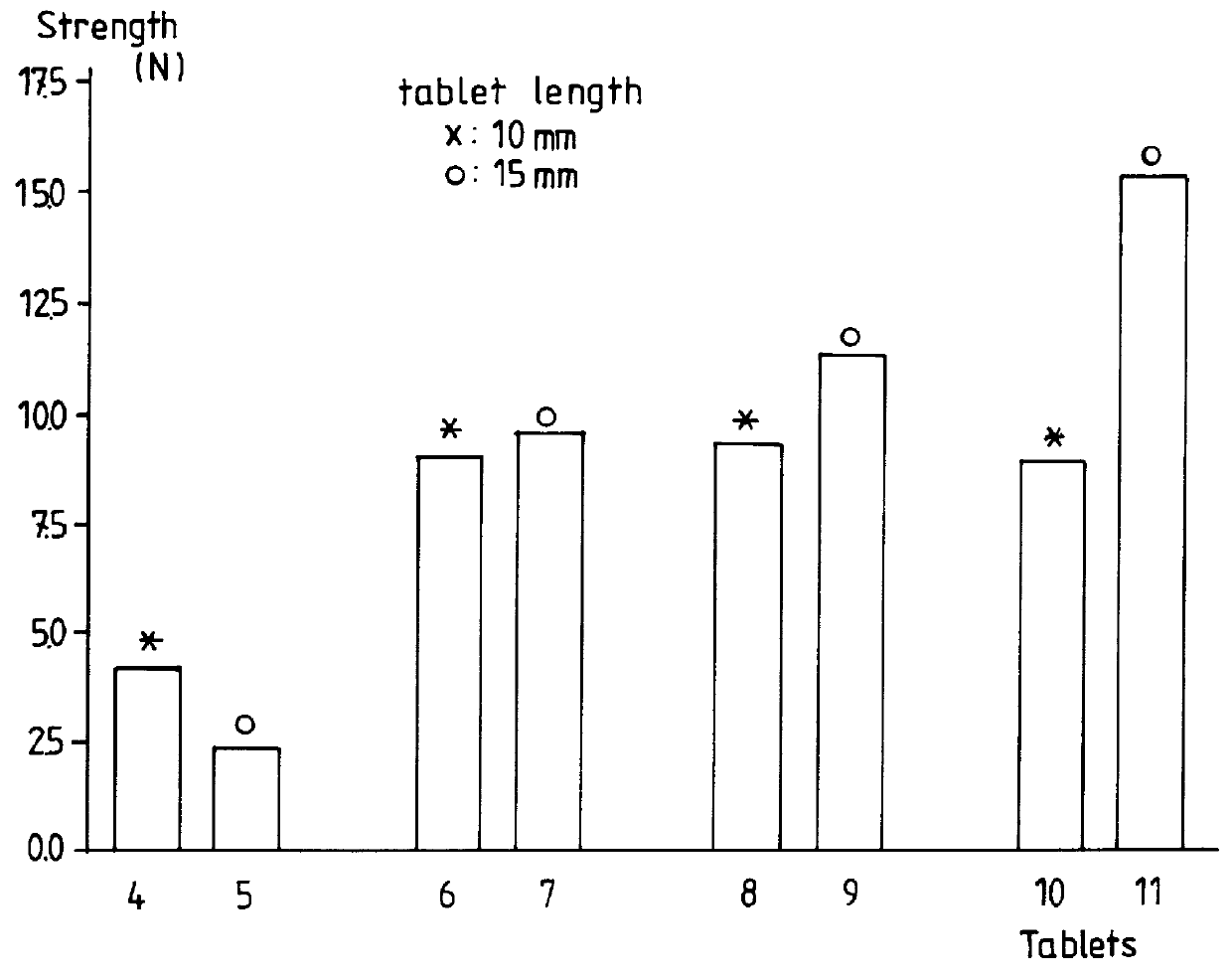
Freeze-dried disintegrating tablets
Freeze-dried disintegrating tablets, said tablets containing at least a therapeutic agent, a matrix forming agent and a binding agent, in which the tablets contain more than 20% by weight of a matrix forming agent selected from the group consisting of maltodextrins having a DE value between 12 and 40, isomalt and mixtures thereof, the weight ratio between said matrix forming agent and the binding agent being comprised between 2:1 and 50:1.
Owner:UNIV GENT
Freeze dried fecal microbiota for use in fecal microbial transplantation
The present invention provides freeze-dried compositions that include an extract of human feces and a cryoprotectant, and methods for making and using such compositions, including methods for replacing or supplementing or modifying a subject's colon microbiota, and methods for treating a disease, pathological condition, and / or iatrogenic condition of the colon.
Owner:RGT UNIV OF MINNESOTA
Freeze-dried preparation of human growth hormone
A readily-soluble freeze-dried solid preparation of hGH with a minimal content of degradation products in terms of deamidation, dimers, polymers, and sulphoxide forms, obtainable by a method comprising a single lyophilization of an aqueous slurry of an amorphous hGH isoprecipitate, the slurry having a pH of from about 4.7 to 5.0 and being essentially free of buffer components other than acetate.
Owner:NOVO NORDISK AS
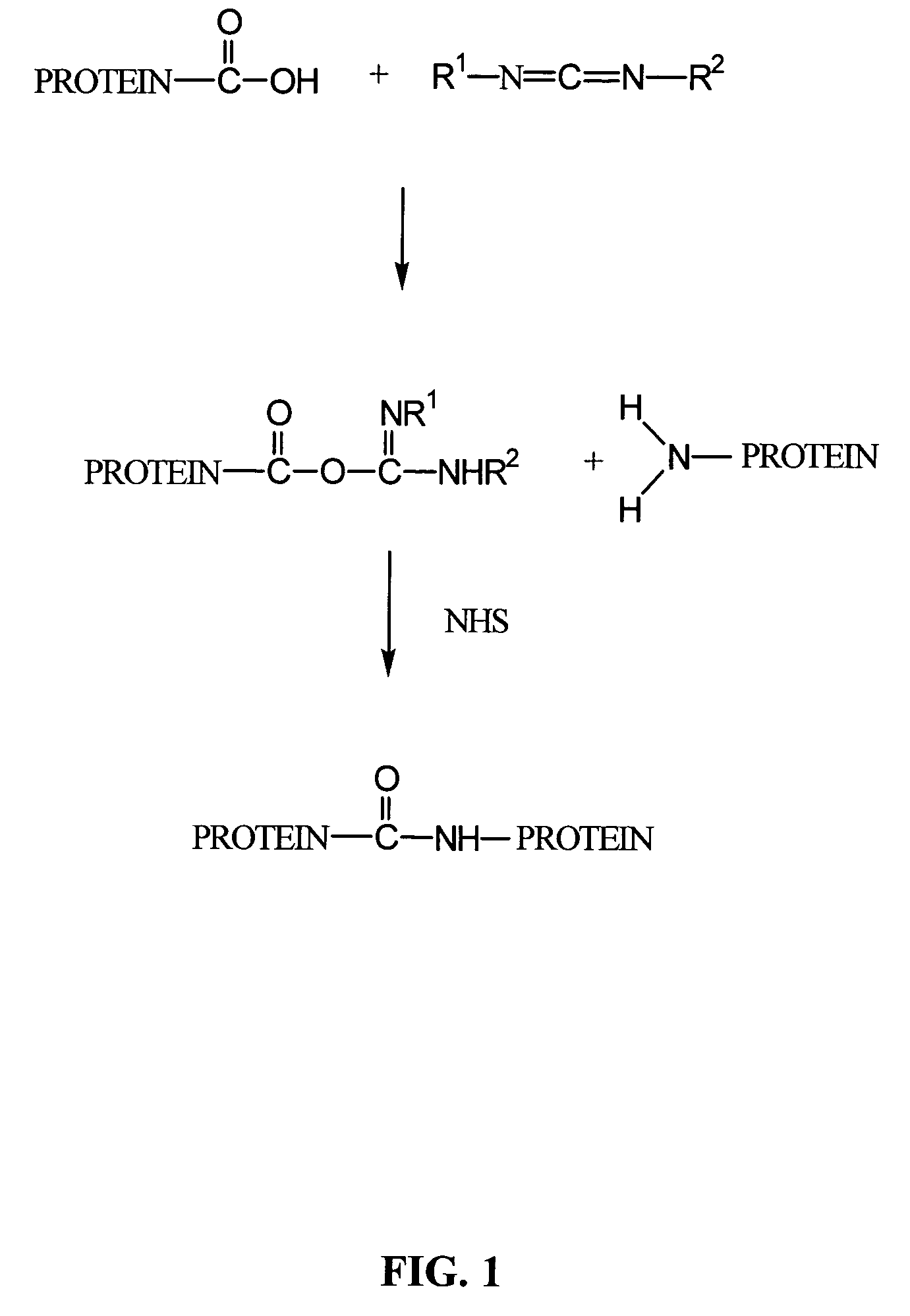
Crosslinked compositions comprising collagen and demineralized bone matrix, methods of making and methods of use
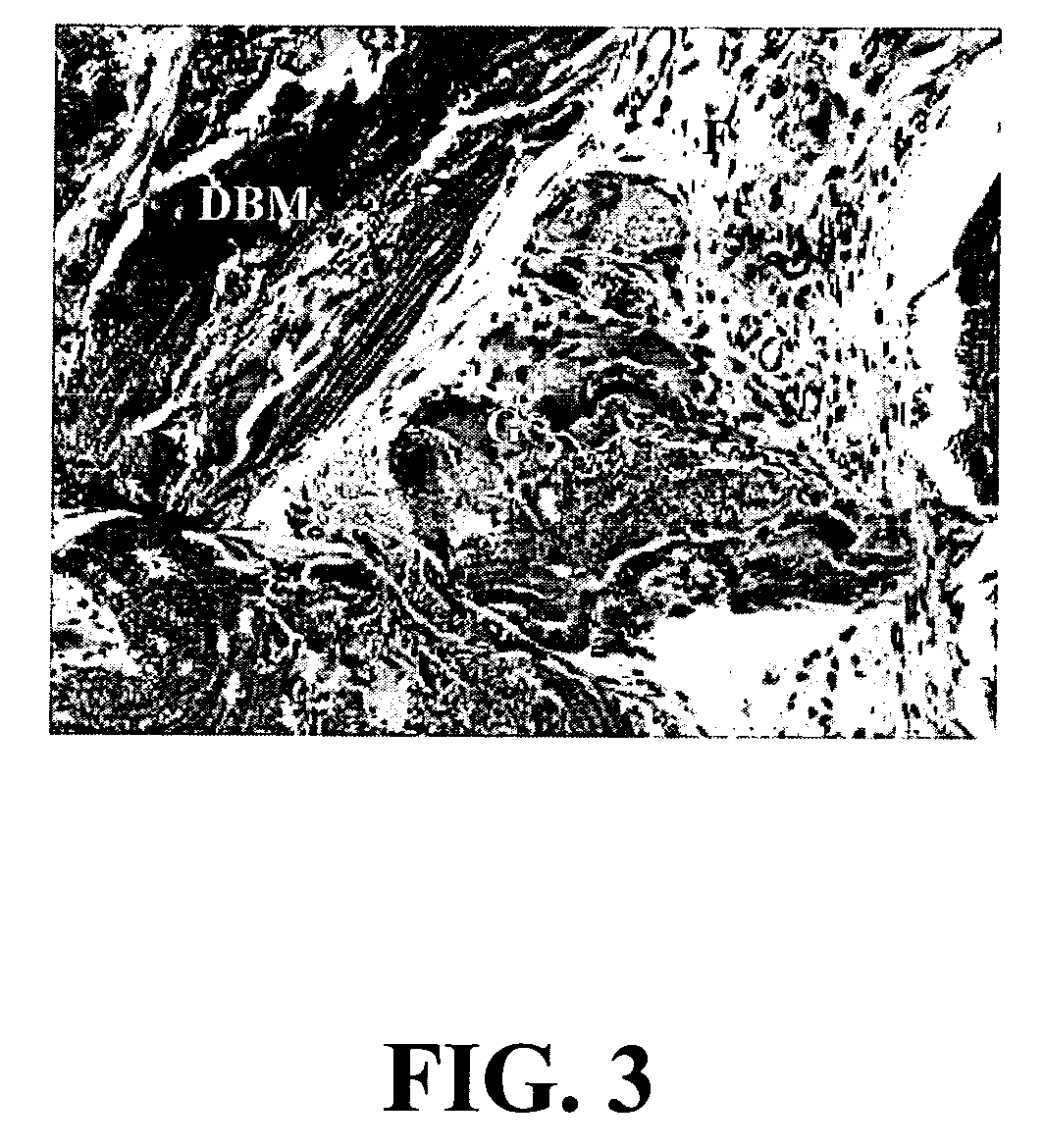
A composition comprising a collagen protein and demineralized bone matrix is described wherein the composition is chemically cross-linked with a carbodiimide such as N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC). The crosslinking reaction can be conducted in the presence of N-hydroxysuccinimide (NHS). The collagen can be in a porous matrix or scaffolding. The DBM can be in the form of particles dispersed in the collagen. A method of making the composition is also described wherein a collagen slurry is cast into the desired shape, freeze dried to form a porous scaffolding and infitrated with a solution comprising the cross-linking agent. The composition can be used as an implant for tissue (e.g., soft tissue or bone) engineering.
Owner:WARSAW ORTHOPEDIC INC
Prevention of molecular weight reduction of the polymer, impurity formation and gelling in polymer compositions
ActiveUS20050042294A1Reduce and eliminate molecular weight reductionReduce molecular weightPowder deliveryBiocideSolubilityActive agent
Polymer and drug containing compositions and method of preparing such compositions are disclosed. The dispersed phase formulation has a polymer, a pharmaceutically or biologically active agent and a small fraction of low pKa acid additive. Stable, filter sterilizable, non-gelling solutions containing GnRH analogues at least at levels typically used in sustained release formulations and a method of increasing solubility of a high level of a GnRH analogue or a freeze-dried antgonist of GnRH in a polymer containing solution are also disclosed. The amount of the acid additive in the polymer solution is such that it is sufficient to increase the solubility of the high level of the GnRH analogue in the polymer solution without affecting the release characteristics of the microspheres prepared therefrom.
Owner:OAKWOOD LAB LLC
Method for preparing graphene powder
The invention relates to a method for preparing graphene powder in large scale, which is characterized by comprising the following steps of: firstly, uniformly peeling graphene oxide into a graphene oxide suspension solution; then, atomizing the graphene oxide solution by using the spray drying technology comprising spray pyrolysis drying and spray freeze drying, and removing a solvent to obtain graphene oxide powder; and finally, oxidizing grapheme by using the non-expansion heat treatment process to obtain non-agglomerative graphene powder. The continuous preparation process of the spray technology and the non-expansion heat treatment process ensure the large-scale preparation of the graphene powder. The prepared graphene powder comprising intermediate product graphene oxide powder does not have agglomeration and has good dispersivity in the solvent. The graphene powder is used as a filling material to prepare high strength composite materials, conductive composite materials, novel air-tight flame-retardant composite materials, novel nanodevices and the like.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Pulmonary insulin crystals
The present invention relates to zinc free insulin crystals having a diameter below 10 mum and to therapeutic powder formulations suitable for pulmonary administration containing such insulin crystals. The crystals of the present invention exhibit a better stability profile than powders of essentially the same composition prepared by spray drying, freeze-drying, vacuum drying and open drying. The therapeutic powder formulations elucidate better flowing properties than corresponding amorphous powder formulations.
Owner:NOVO NORDISK AS
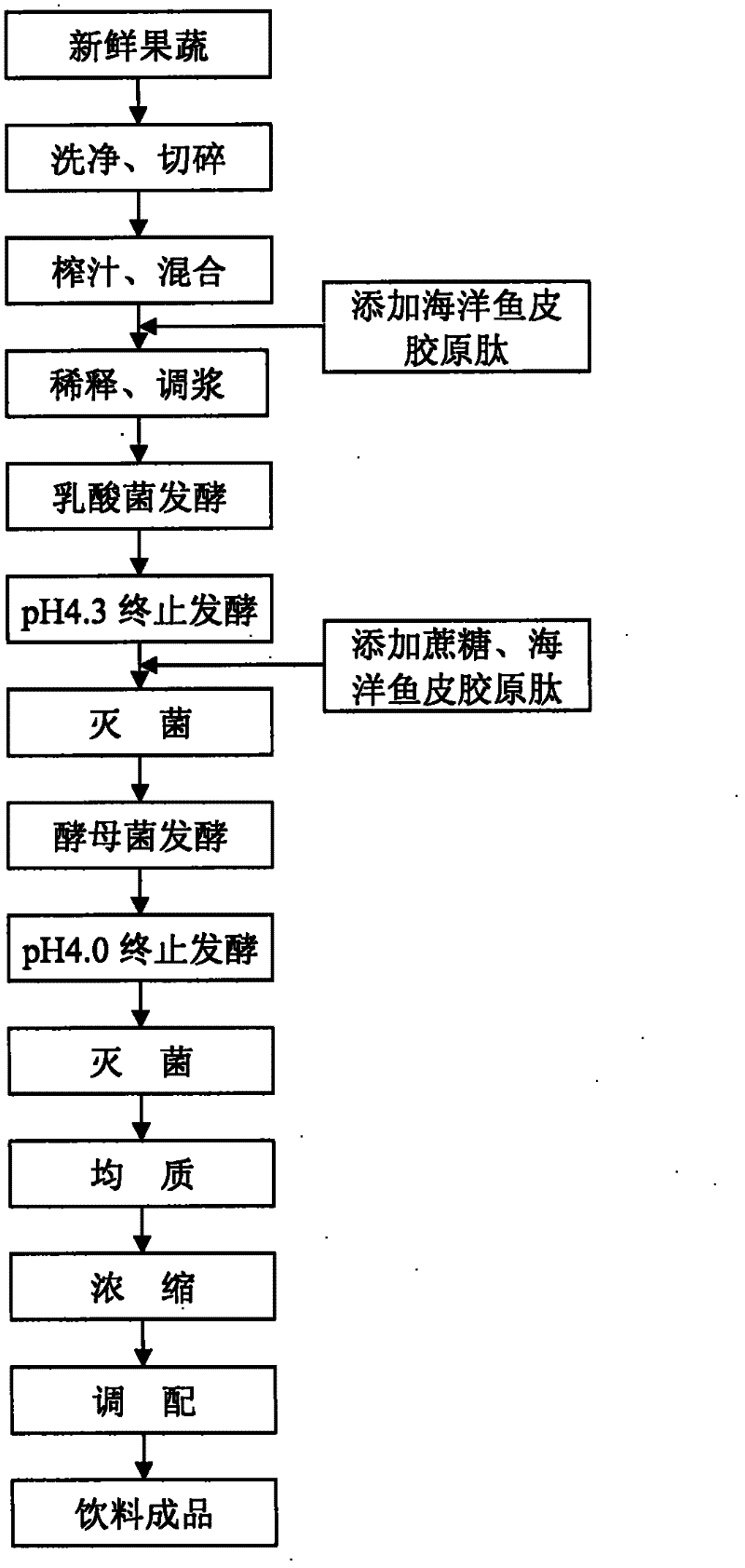
Natural fruit and vegetable ferment beverage, and preparation method thereof
ActiveCN102356899AWith health functionPrevent diseaseYeast food ingredientsLactobacillusMetaboliteFreeze-drying
The present invention discloses a natural fruit and vegetable ferment beverage, and a preparation method thereof. The preparation method for the ferment beverage comprises: adding freshly-squeezed fruit and vegetable juice to marine fish skin collagen peptide; adopting the resulting mixture as a fermentation substrate; carrying out dilution, slurry mixing and sterilization, and then adopting a direct vat and freeze-dried type lactic acid starter to carry out fermentation; stopping the fermentation after 7-11 days until the pH value is 4.3; adding a supplementary feed and carrying out sterilization; then adding high-activity dried yeast to carry out fermentation; stopping the fermentation after 5-7 days until the pH value is 4.0; after storing the fermentation broth at the low temperature, carrying out homogenization, concentration, centrifugation, blending, sterilization and bulking to finally prepare the novel natural fruit and vegetable ferment beverage. According to the ferment beverage of the present invention, the stepwise fermentation is adopted for the freshly-squeezed fruit and vegetable juice by using the lactic acid bacteria and the yeast; the marine fish skin collagen peptide is added to promote the growth and the metabolism of fermentation bacteria, such that metabolic products of nutritional ingredients in the natural fruits and vegetables are rich so as to provide a beneficial nutrition and health effect.
Owner:CHINA NAT RES INST OF FOOD & FERMENTATION IND CO LTD
Composition and method for healing tissues
InactiveUS20050147679A1Enhances chemotactic activityGood healing effectOrganic active ingredientsBiocideAdhesiveAdditive ingredient
The composition and method for healing tissues is a medicinal composition for facilitating the growth, protection and healing of tissues and cells in animals and humans. The composition is formulated as a either a powder, gel, paste, film, fluid injectable, rehydratable freeze-dried paste or sponge, sprayable solution, topically applied patch with adhesive and reservoir system, an intermediate for coatables such as films and bandages, a matrix for membranes, or as a matrix of flexible polymer(s), or delivered as either an orally ingestible liquid, tablet or capsule. The main ingredient of the formulated compositions is hydrolyzed collagen, which can be combined with polysulfated glycosaminoglycans, hyaluronic acid or salts thereof, or a glucosamine salt, and mixtures thereof. The composition may be formulated as an aqueous eye drop solution.
Owner:PETITO GEORGE D +1
Composition for a Tissue Repair Implant and Methods of Making the Same
ActiveUS20100036503A1Stay in shapeHigh affinityBone implantTissue regenerationTissue repairFreeze-drying
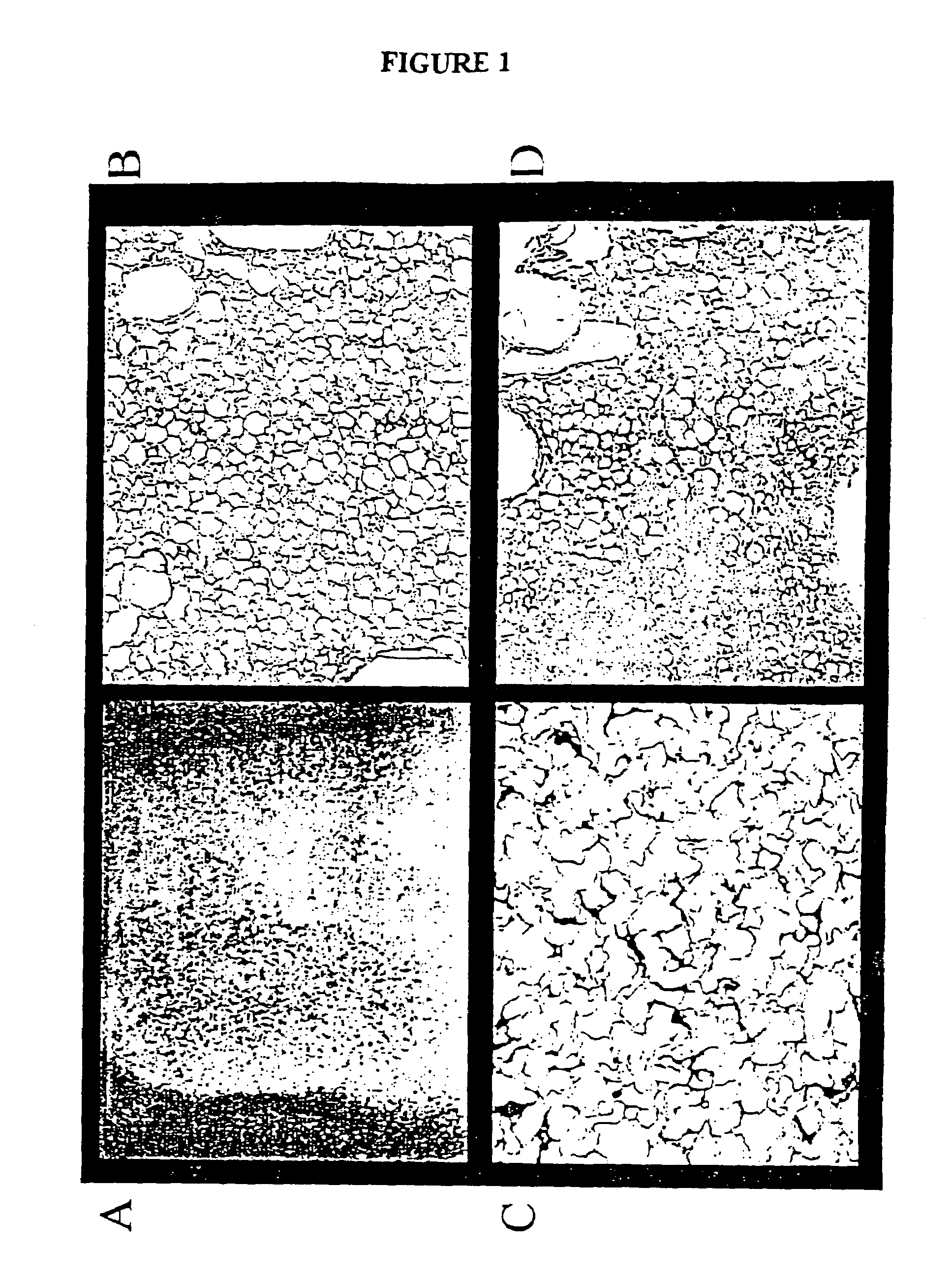
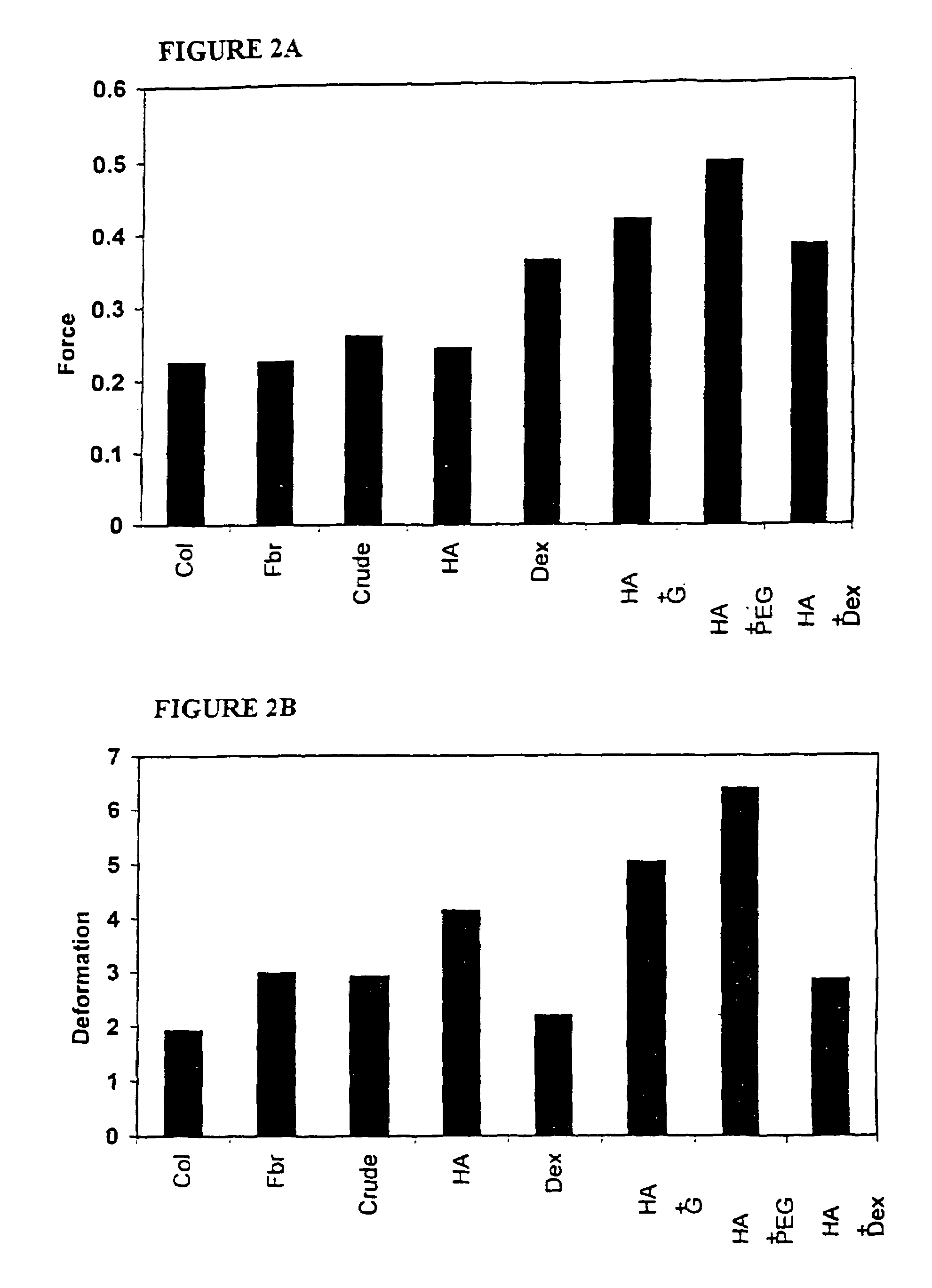
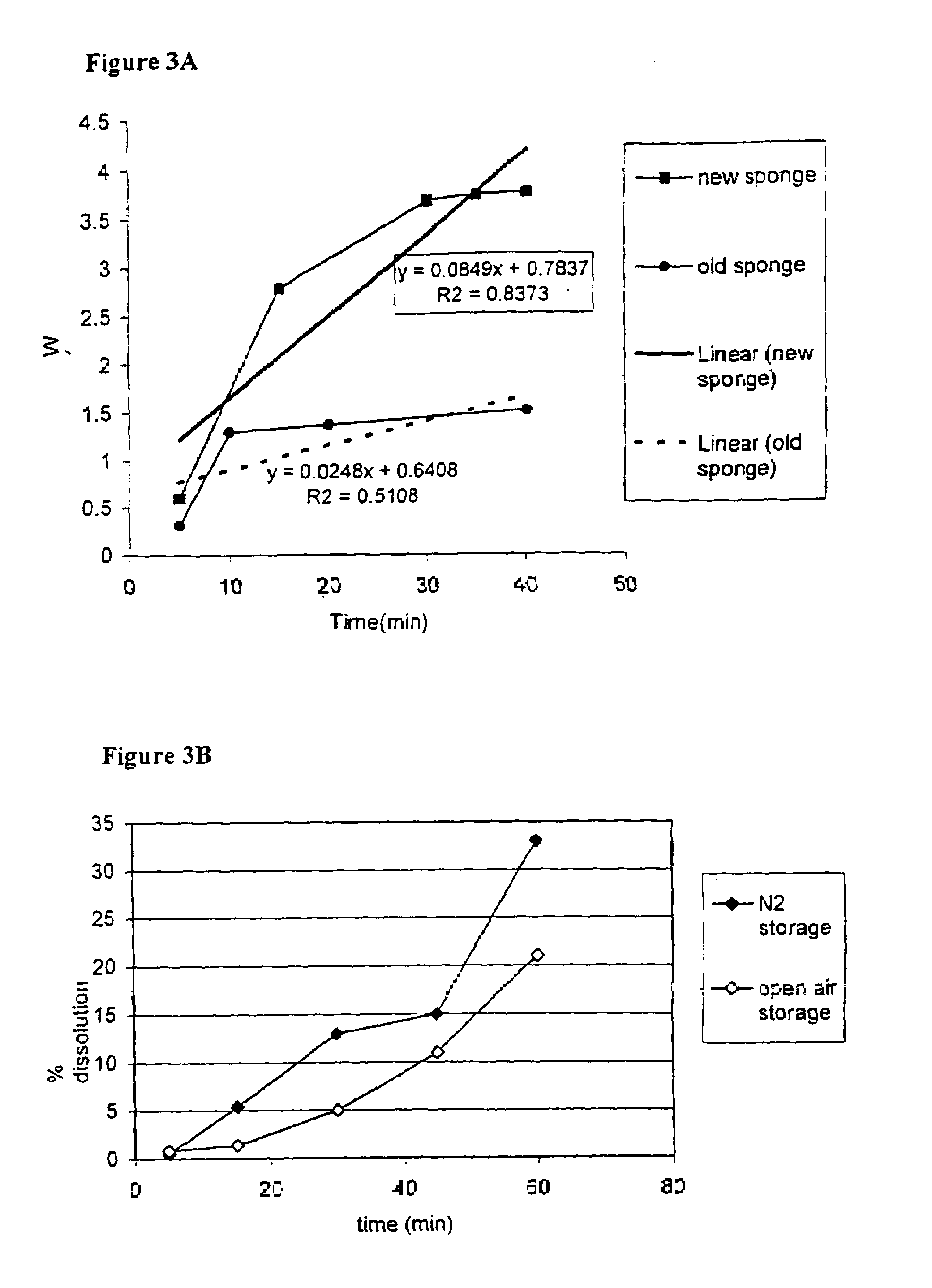
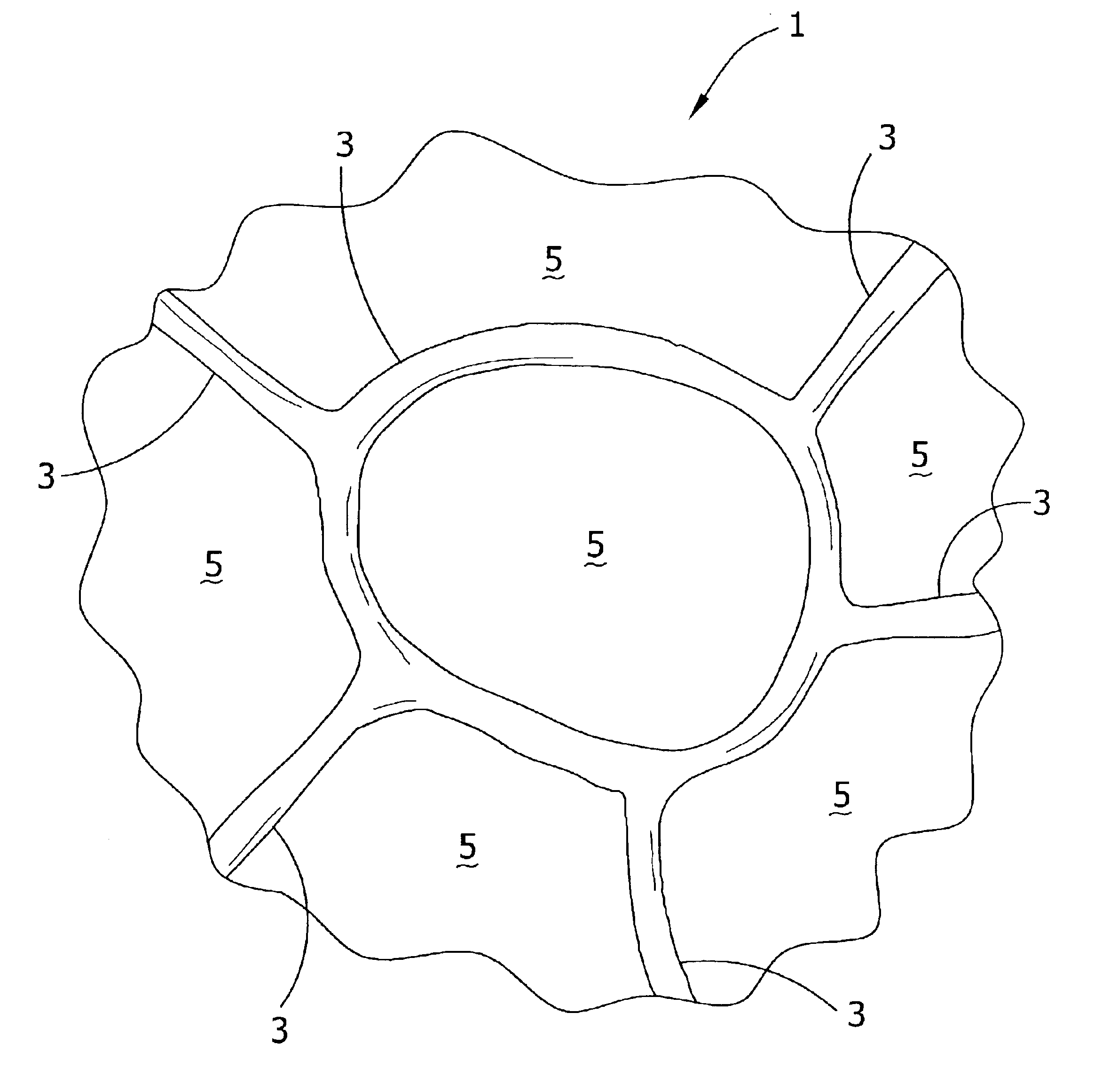
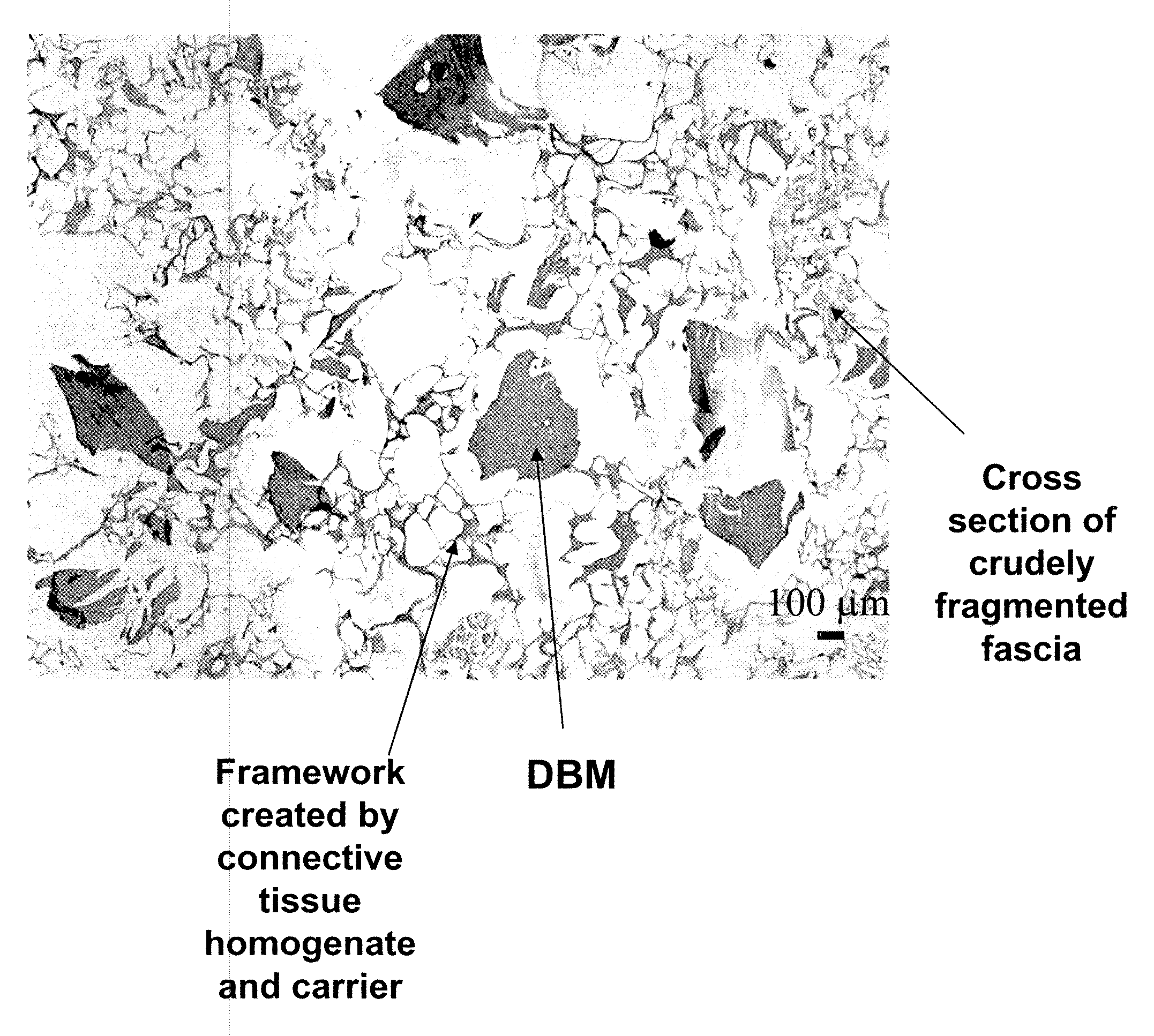
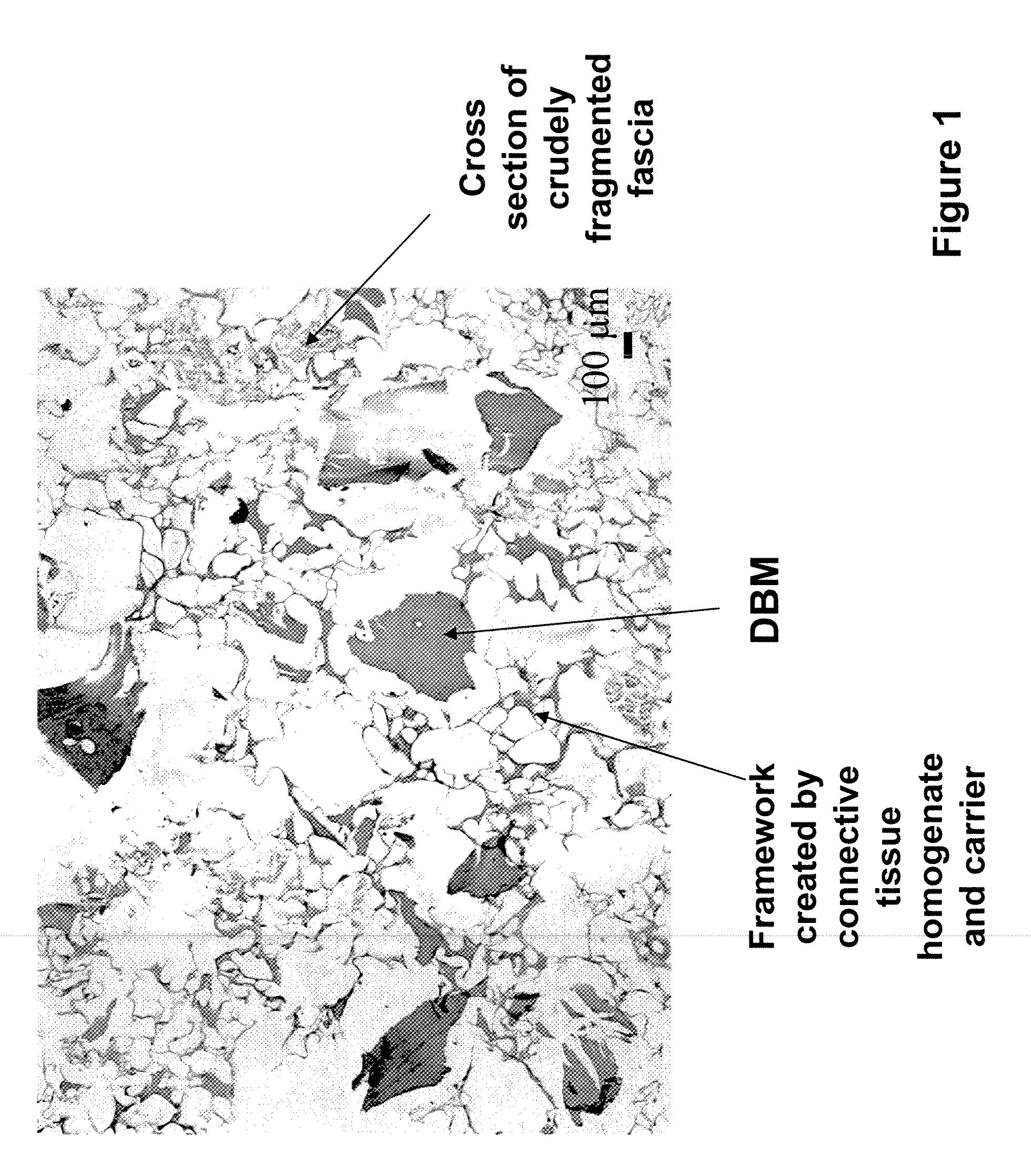
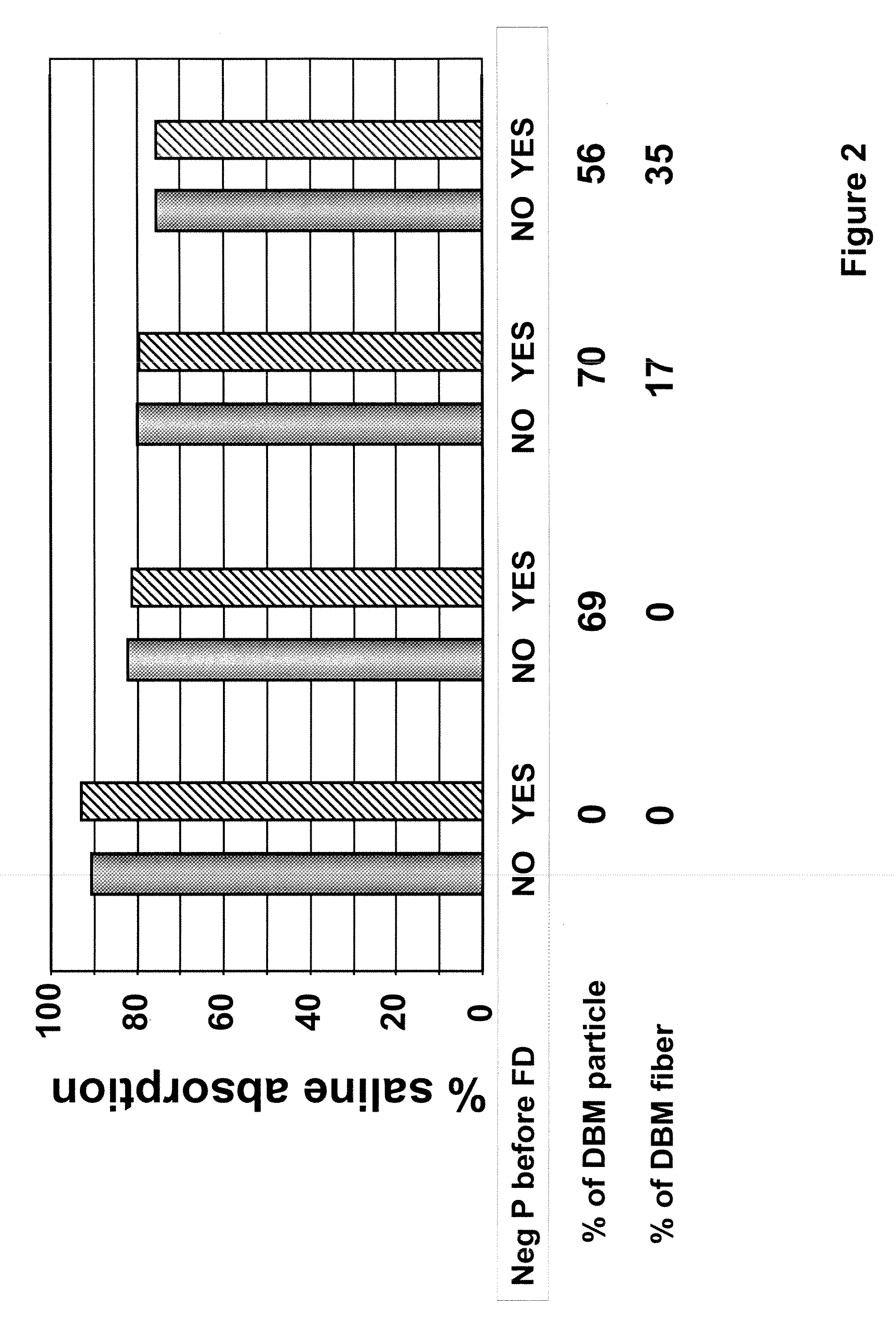
The invention is directed to a process for making a tissue repair implant having a porous sponge-like structure to repair bone, cartilage, or soft tissue defects by producing a connective tissue homogenate from one or more connective tissues; mixing the connective tissue homogenate with a carrier solution to produce a connective tissue carrier; optionally mixing one or more natural or synthetic bone fragements with said connective tissue carrier to produce a tissue repair mixture; freezing or freeze-drying the tissue repair mixture to produce a porous sponge-like structure and create a three-dimensional framework to entrap the natural or synthetic bone fragments, treating the frozen or freeze-dried porous sponge-like structure with one or more treatment solutions to produce a stabilized porous sponge-like structure. A crudely fragmented connective tissue from one or more connective tissues is optionally mixed with the tissue repair mixture before freezing or freeze-drying. The tissue repair implant having a porous sponge-like structure is optionally combined with one or more bioactive supplements or one or more agents that have bioactive supplement binding site(s) to increase the affinity of growth factors, differentiation factor, cytokines, or anti-inflammatory agents to the tissue repair implant. The invention is further directed toward applying such tissue repair implant for tissue repair.
Owner:LIFENET HEALTH
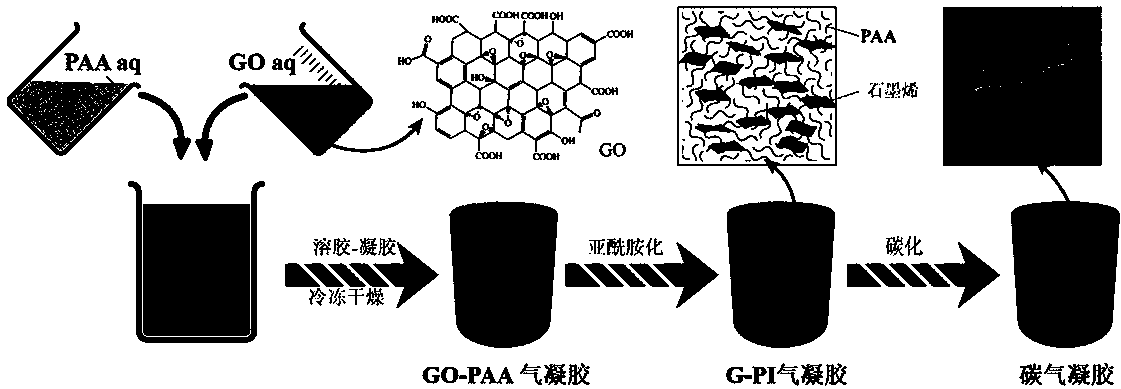
Method for preparing graphene aerogel and graphene/ metallic oxide aerogel
InactiveCN103413689ARich sourcesLow priceHybrid capacitor electrodesGrapheneSupercritical dryingCross-link
The invention discloses a method for preparing graphene aerogel and graphene / metallic oxide aerogel. The method is characterized in that graphite oxide aqueous solution serves as a raw material, alcohol serves as a cross-linking agent, a precursor solution is obtained through the simple mixing and dispersing processes, the hydrothermal method is adopted, the freeze drying method or a supercritical drying method is adopted, and then the graphene aerogel is obtained . The graphene / metallic oxide aerogel can be prepared by adding metallic oxide into the polymeric precursor solution. On one hand, the graphene vinyl aerogel material has the characteristic of the graphene aerogel, and on the other hand, the graphene vinyl aerogel material can have the physicochemical characteristic of the metallic oxide. In addition, the aerogel material has a large specific surface and good electrical conductivity, can directly serve as a supercapacitor electrode, and has large specific capacity, good rate capability and cycling stability. The materials used in the method are low in cost, the industrial process is simple, and the method is friendly to the environment.
Owner:UNIV OF SCI & TECH BEIJING
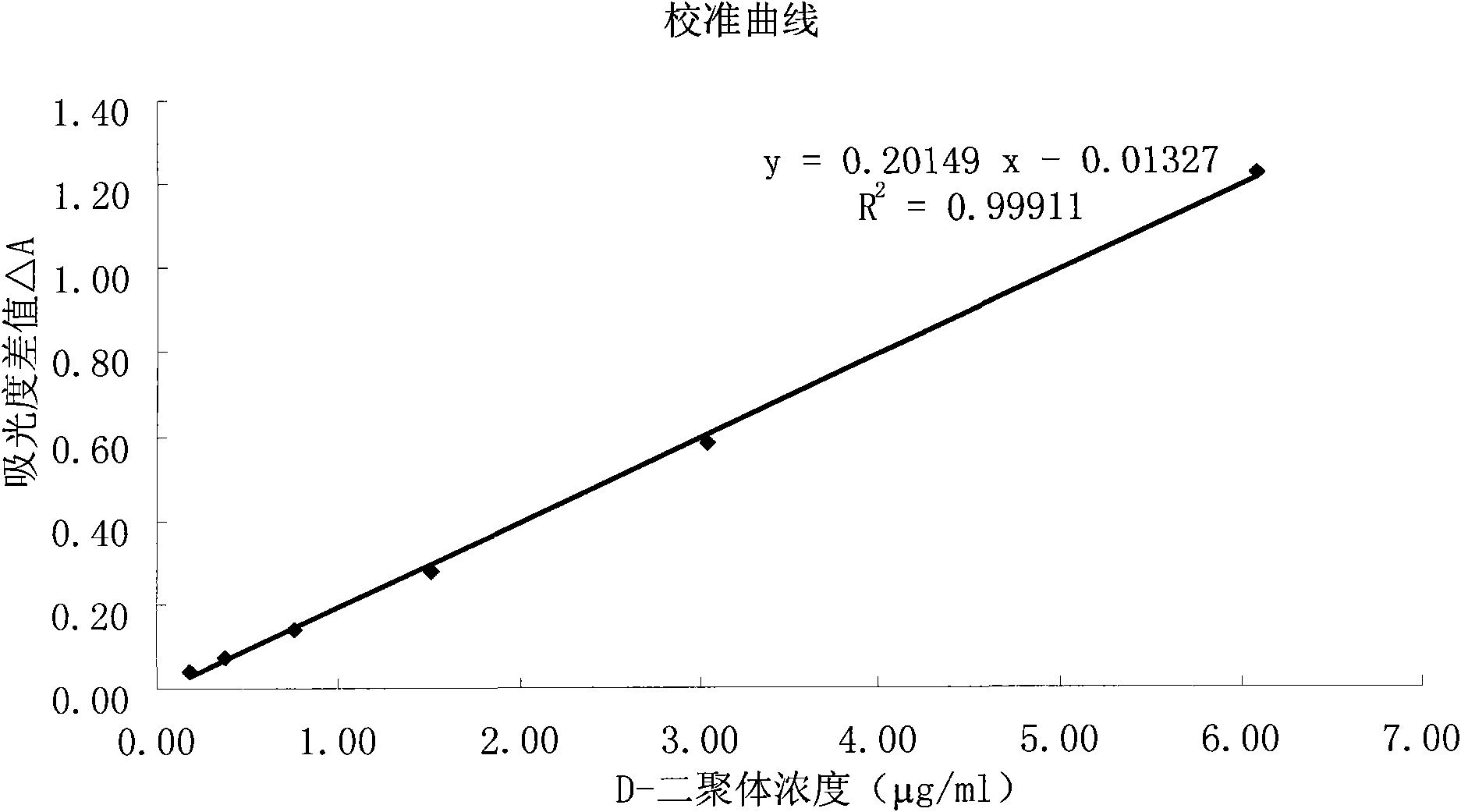
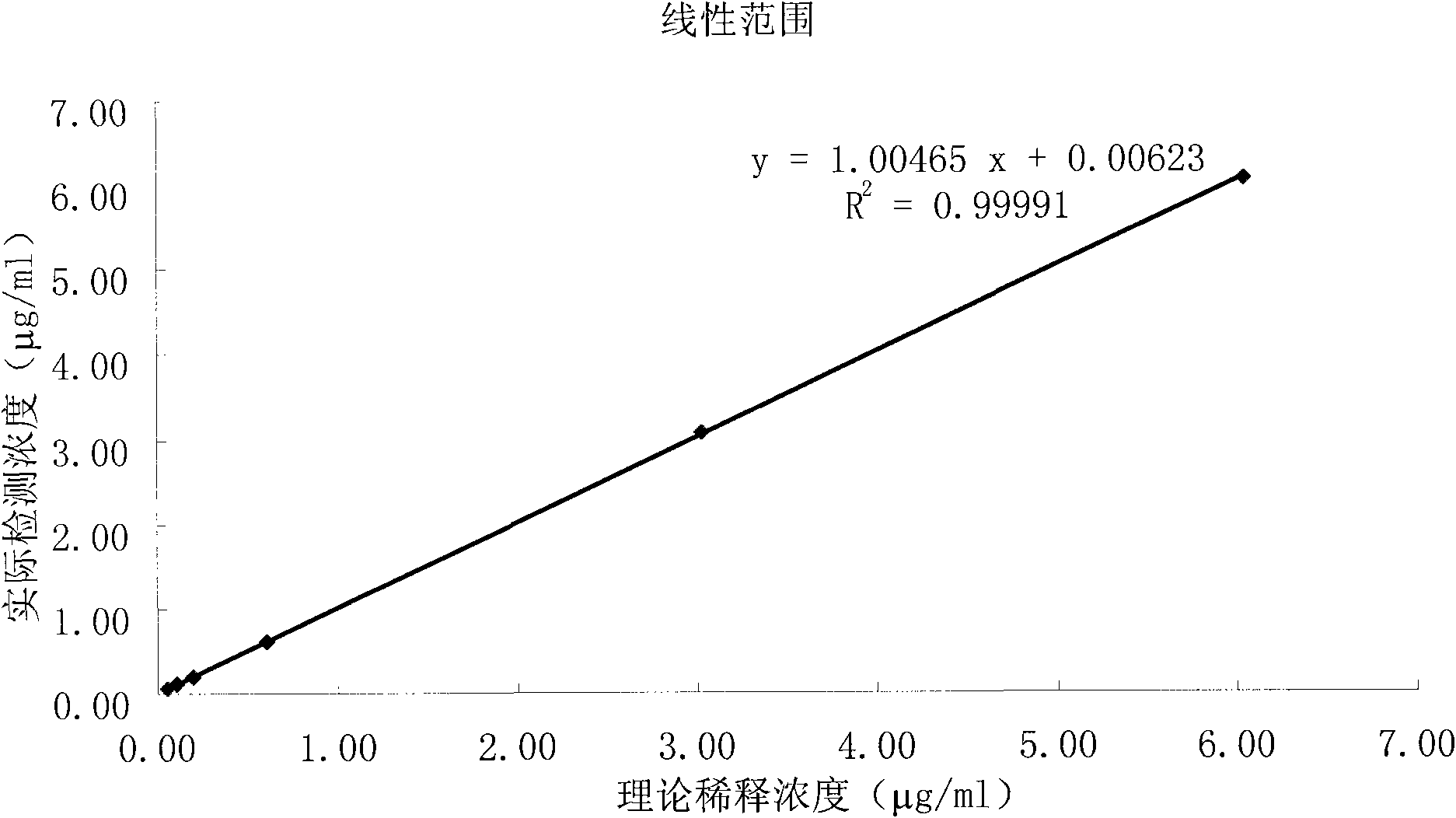
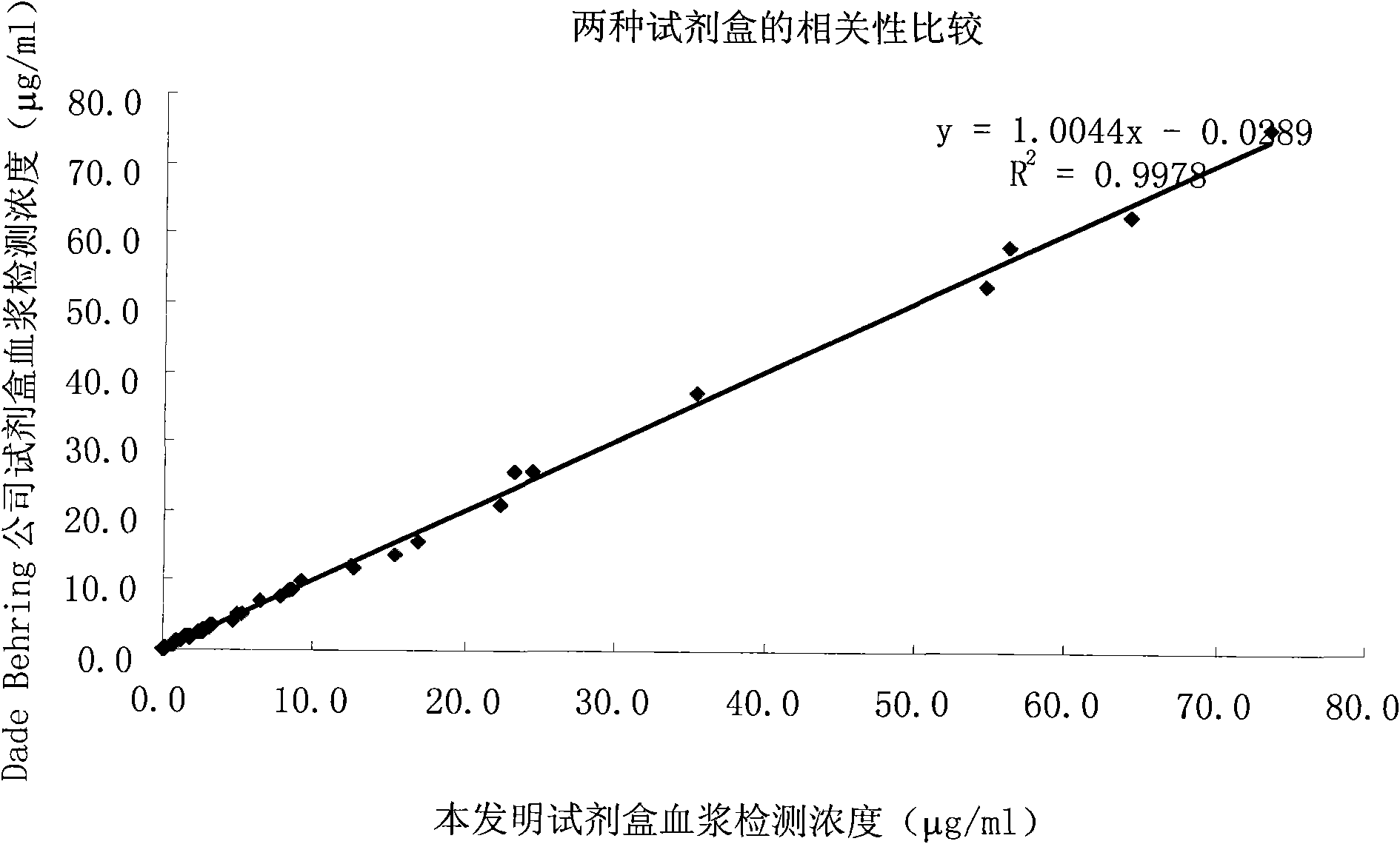
D-Dimer measuring kit (latex immunonephelometry method)
ActiveCN101819202AHigh refractive indexHigh chemical inertnessMaterial analysisFreeze-dryingPreservative
The invention relates to a kit for measuring the D-Dimer content by adopting a latex immunonephelometry method. The kit comprises a D-Dimer reagent R1, a D-Dimer reagent R2, a D-Dimer diluent and a D-Dimer calibration product, wherein the D-Dimer reagent R1 comprises a buffering solution, a stabilizing agent (1), coagulant and preservative; the D-Dimer reagent R2 comprises mouse anti-human D-Dimer monoclonal antibody latex enhanced particles, a buffering solution, a stabilizing agent (2) and preservative; the D-Dimer diluent comprises a buffering solution and preservative; and the D-Dimer calibration product is prepared by subpacking and freeze-drying a solution comprising a fibrin degradation product D-Dimer, a buffering solution, a stabilizing agent (3), excipient and preservative. The kit has the advantages of simple and rapid operation, accurate quantification, high sensitivity, strong specificity, low detection cost and strong instrument compatibility and is suitable for being popularized and used in various big-scale and small-scale hospitals.
Owner:SHANGHAI SUNBIO TECH
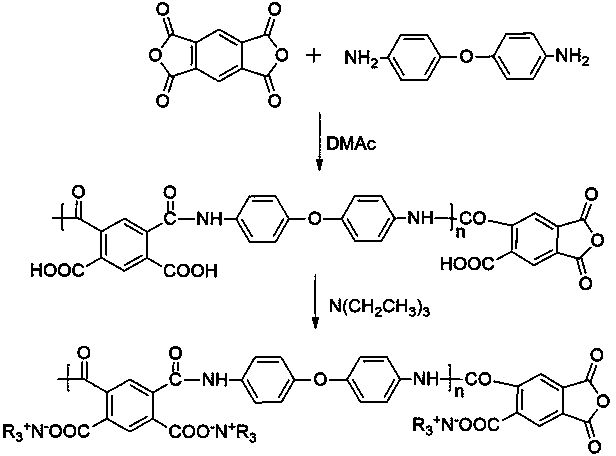
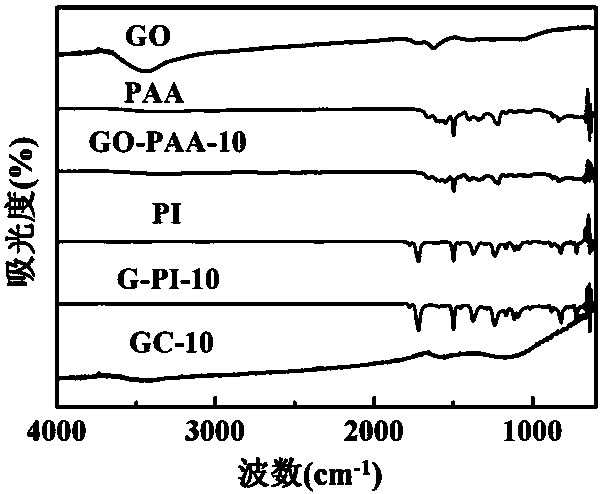
Preparation method of graphene/polyimide-based carbon aerogel
InactiveCN104355302AEasy to makeThe preparation process is environmentally friendlyFreeze-dryingNew energy
The invention belongs to the technical field of nanoporous material, namely carbon aerogel, and particularly relates to graphene oxide-crosslinked polyimide-based carbon aerogel and a preparation method thereof. The invention carbon aerogel is prepared by crosslinking polyamide acid aerogel by graphene oxide, and comprises the components of graphene oxide and one or more water-soluble polyimide precursor-polyamide acids. The preparation method comprises the following steps: mixing an aqueous graphene oxide solution with the water-soluble polyimide precursor-polyamide acid, and preparing graphene oxide / polyamide acid aerogel through sol-gel and freeze-drying processes; preparing the graphene / polyimide-based carbon aerogel through thermal imidization and high temperature carbonization. According to the preparation method, a toxic reagent formaldehyde is not used; the prepared carbon aerogel has a mesoporous, microporous and macroporous three-level three-dimensional network porous structure, high specific surface area, high conductivity and stable physical and chemical properties, and is an ideal electrode material for preparing a supercapacitor and other new energy devices and a high-performance adsorbent material.
Owner:FUDAN UNIV
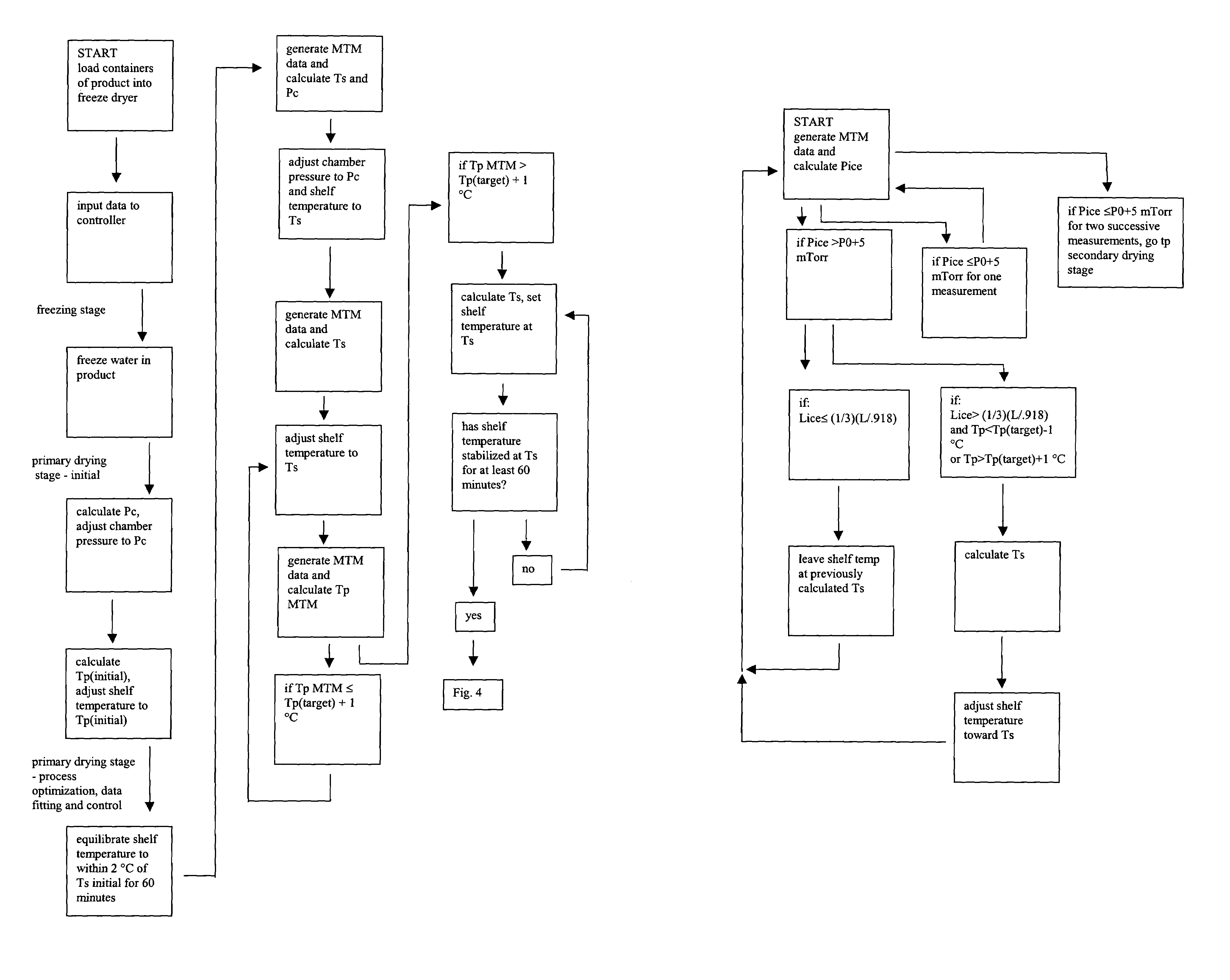
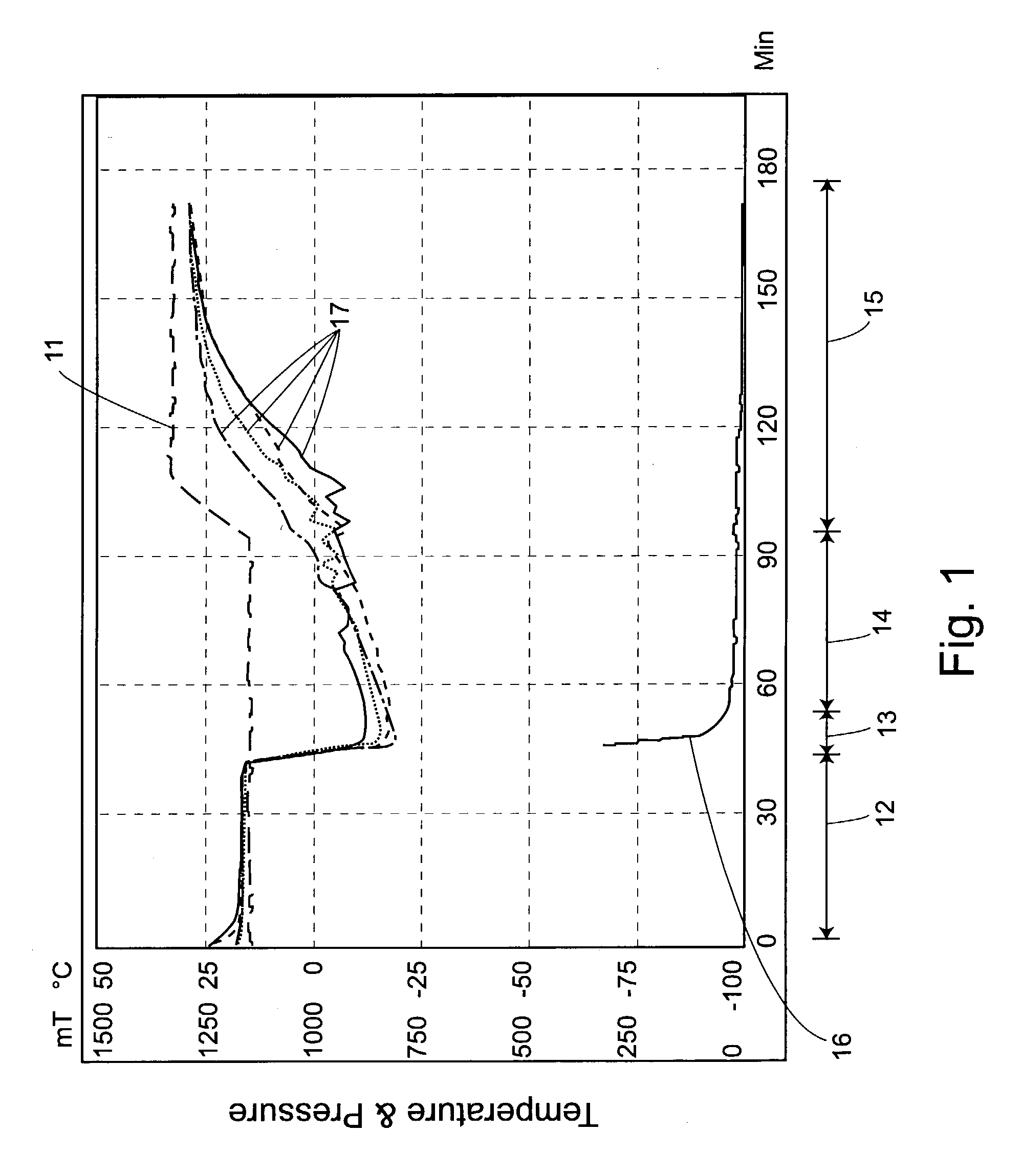
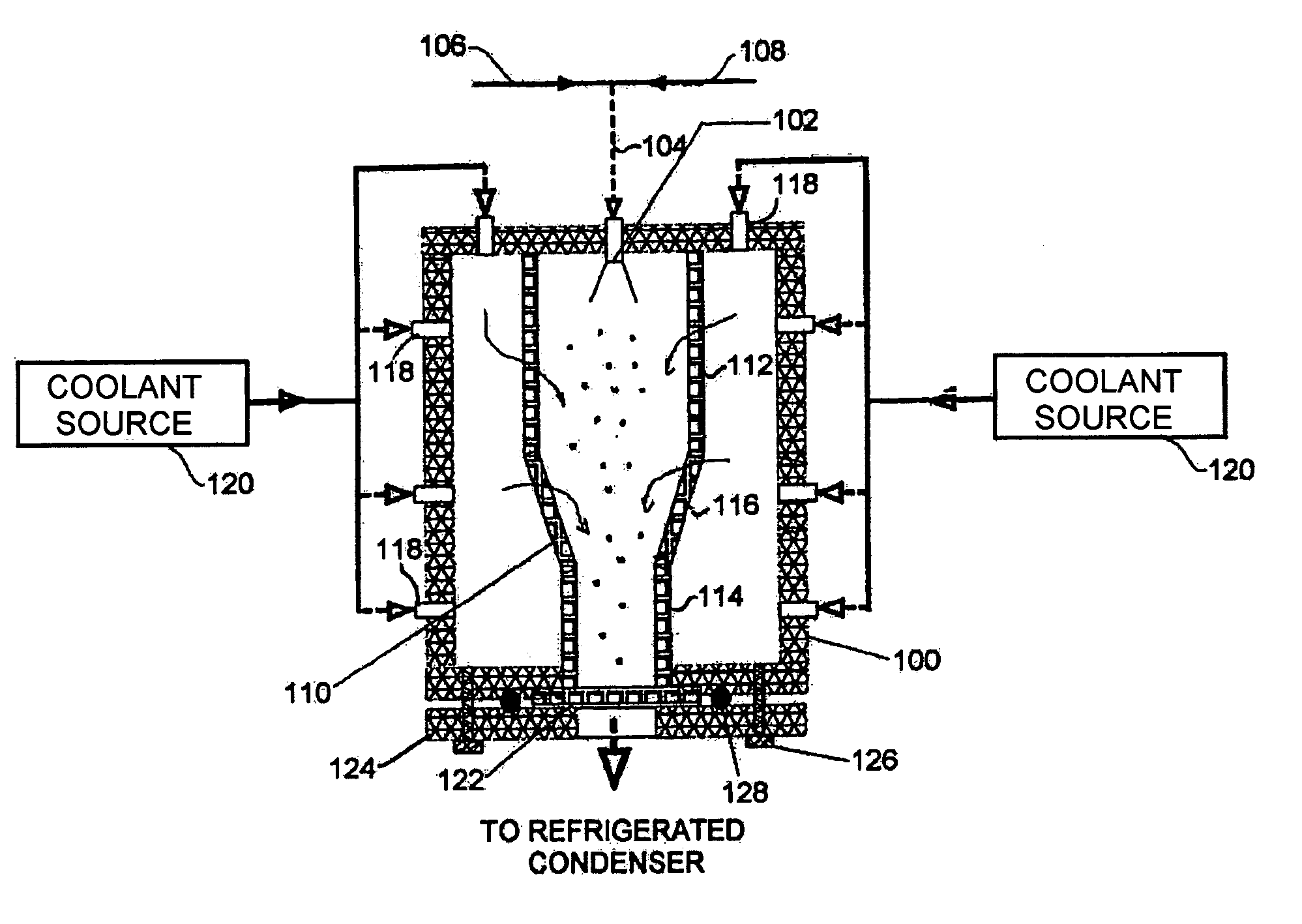
Automated process control using manometric temperature measurement
ActiveUS6971187B1Simple processDrying solid materials without heatDrying machines with progressive movementsFreeze-dryingControl system
Owner:PURDUE RES FOUND INC +1
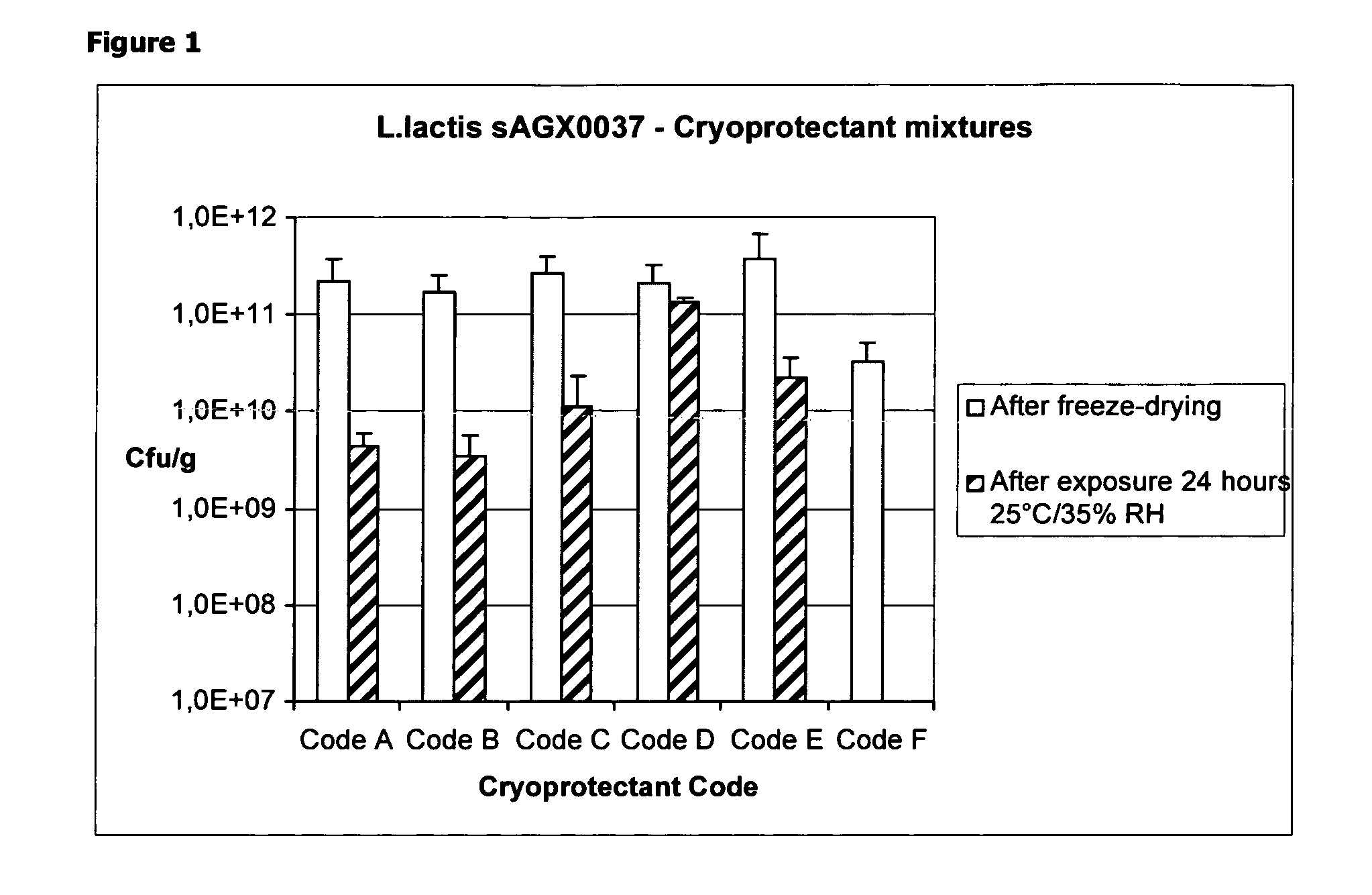
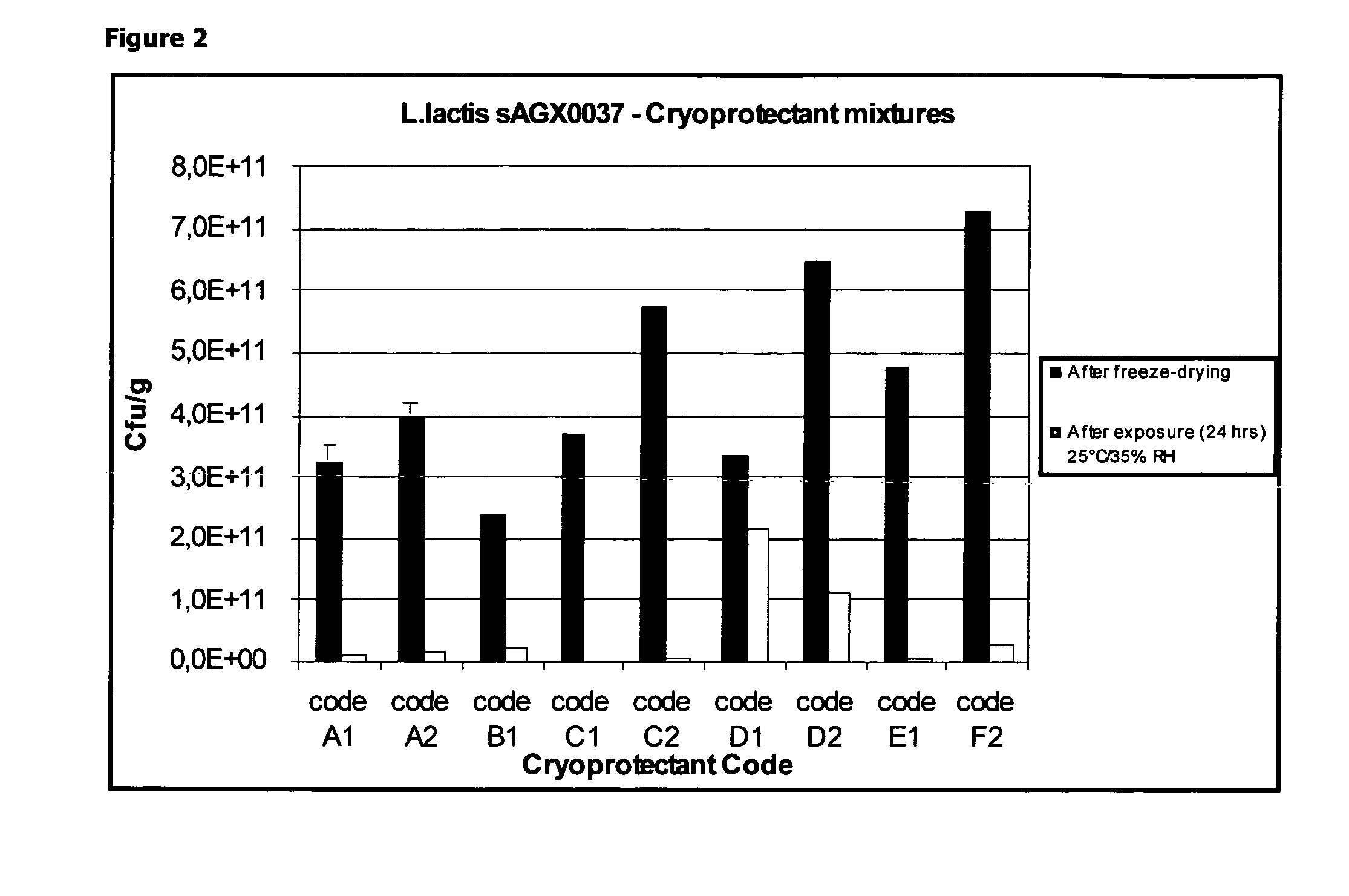
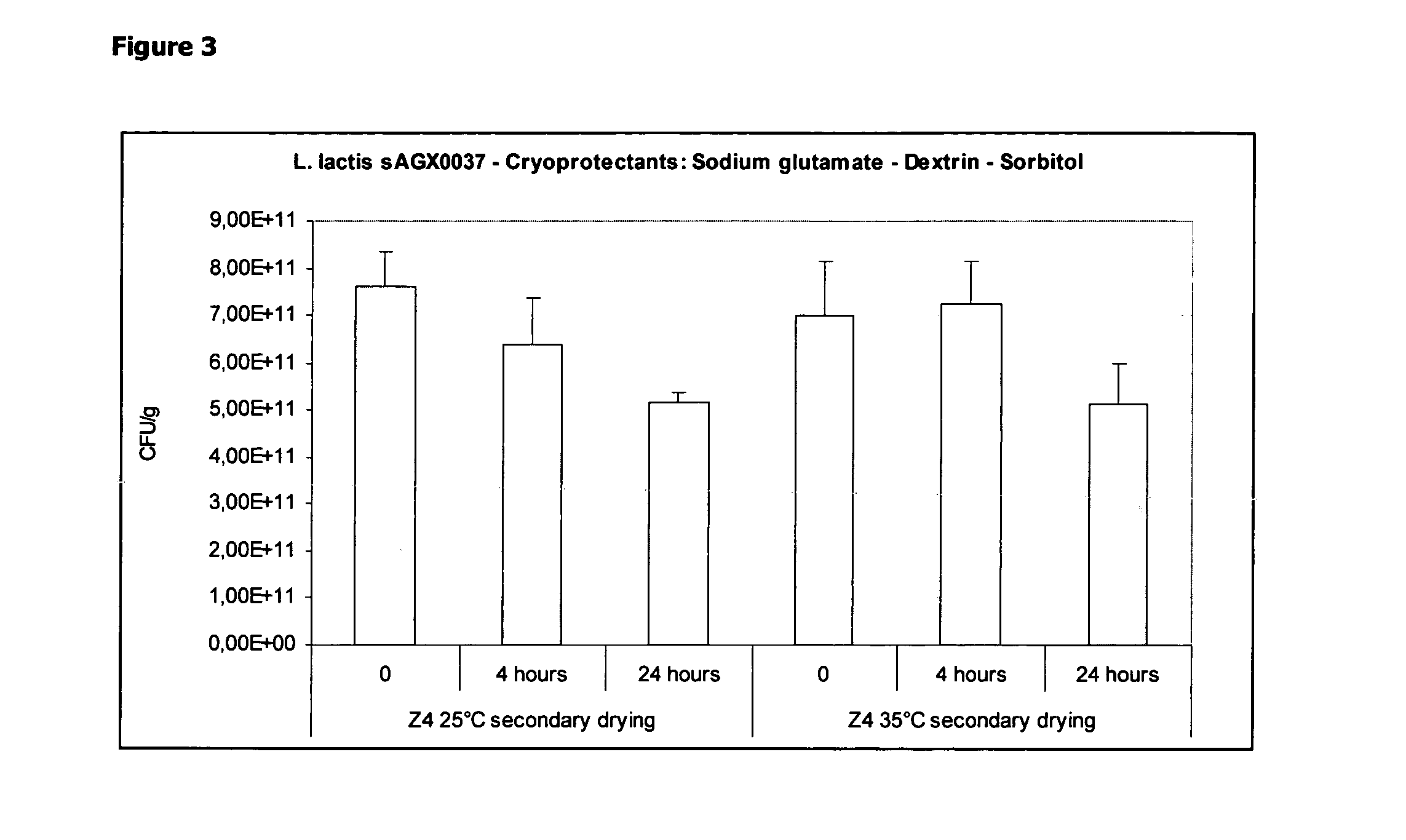
Cryoprotectants for freeze drying of lactic acid bacteria
ActiveUS20120039853A1Improve survivabilityImprove textureBiocideMilk preparationBacteroidesVaccination
The present invention comprises the discovery and development of an effective cryoprotectant composition, without containing skim milk or any other animal-derived compounds, to achieve long-term stability of freeze-dried lactic acid bacteria (LAB), at different temperatures, whereby the retention of viability of the freeze-dried LAB after 6 months of storage, preferably after 9 months of storage, more preferably after 12 months of storage is more than 50%. The invention is in the field of producing freeze dried bacteria, in particular Lactic acid bacteria. More in particular, the invention relates to the use of a novel combination of cryoprotectants for increasing the viability of bacteria after freeze drying, improving the texture of the lyofilized cake for easy grinding and improving the long term stability of the freeze dried bacteria at different temperature conditions. The invention further relates to such freeze dried bacteria for use in food industry or in human or animal health applications. More in particular, the invention relates to the increased viability and long-term storage of recombinant bacteria capable of expressing heterologous proteins or peptides and administered to humans or animals for therapeutic or vaccination purposes.
Owner:INTREXON ACTOBIOTICS NV
Preservation of bioactive materials by freeze dried foam
ActiveUS7135180B2Improve permeabilityReduce residual moistureSsRNA viruses negative-senseOrganic active ingredientsPreservativeFreeze-drying
This invention provides methods and compositions to preserve bioactive materials in a dried foam matrix. Methods provide non-boiling foam generation and penetration of preservative agents at temperatures near the phase transition temperature of the membranes.
Owner:MEDIMMUNE LLC
Method for preparing fibrilia carboxylation cellulose nanowhiskers
The invention provides a method for preparing fibrilia carboxylation cellulose nanowhiskers, which is characterized by comprising the following steps: soaking fibrilia powder in sodium hydroxide for processing, then processing the fibrilia powder by a former treating agent and taking out the fibrilia powder to be dried in a vacuum oven to obtain preprocessed fibrilia powder; and placing the preprocessed fibrilia powder in a TEMPO oxidation system for catalytic oxidation to obtain a stable cellulose nanowhiskers suspending liquid after mechanical processing and freeze drying the suspending liquid to obtain the fibrilia carboxylation cellulose nanowhiskers having grain diameters of 3-10 nm. According to the invention, fibrilia carboxylation and nano fibrillation are realized and surfaces of prepared nanocrystalline celluloses have carboxyl functional groups, thus the surfaces generate negative charges, electrostatic repulsion among the negative charges can avoid the reunion of nanoparticles, so that the nanocrystalline celluloses can be well dispersed in water and the obtained nanocrystalline celluloses have excellent uniformity of grain sizes.
Owner:DONGHUA UNIV
Lactobacillus micro-capsule as well as preparation method and use
ActiveCN101496555AReduce harmHigh viable countBacteria material medical ingredientsAnimal feeding stuffEcological environmentFreeze-drying
The invention relates to a lactobacillus microcapsule, a method for preparing the same, and the use of the same. The lactobacillus microcapsule consists of an outer layer of wall material, a freeze dried protective agent and lactobacilli. The invention also provides the method for preparing the lactobacillus microcapsule and the use of the lactobacillus microcapsule as a feed additive. The lactobacillus microcapsule can effectively protect the lactobacilli in a core material, prolong the survival time of the lactobacilli at room temperature and improve the tolerance of the lactobacilli to the metal ions in the feed; in addition, the lactobacillus microcapsule has good gastric acid resistance and can disintegrate in the intestinal canal quickly to release the lactobacilli, thereby improving the utilization rate of the lactobacilli, balancing the micro-ecological environment in the intestinal canal, suppressing the growth of pathogenic bacteria, protecting the health of the intestinal canals of animals, reducing the incidence rate of the intestinal canals of the animals and the like.
Owner:BEIJING DABEINONG TECH GRP CO LTD +1
Freeze-dried ginseng berry tea
InactiveUS6210738B1Easy to adjustPromote healthCosmetic preparationsHair cosmeticsAdditive ingredientFreeze-drying
The present invention relates to novel compositions of freeze-dried ginseng berry and other natural health promoting ingredients in a mixture suitable for making tea. The compositions and methods of preferred embodiments of the present invention provide a natural vitamin containing composition for making a tea beverage which can be consumed hot or cold and which provides the consumer with a refreshing, delicious, stimulating and healthful experience.
Owner:CHEN JAU FEI
Powder formation by atmospheric spray-freeze drying
ActiveUS7007406B2High emitted doseEasy to storeDrying solid materials without heatDrying machines with progressive movementsFreeze-dryingAtomizer nozzle
A method of manufacturing heat-sensitive pharmaceutical powder is disclosed. The original pharmaceutical substances are dissolved in a solution or suspended in a suspension, which is sprayed through an atomizing nozzle and frozen in a cold gas phase or liquid nitrogen atomized directly in the spray-freeze chamber or gas jacket at the same time (for cooling purposes). The particles are freeze-dried at roughly atmospheric pressure in a down-stream fluid flow with exit filter thereby to remove moisture entrapped on or inside the frozen particles. The system has applicability for forming other powders.
Owner:AEROSOL THERAPEUTICS
Chitosan collagen and calcium alginate compounded spongy biological dressing and its preparation process
The composite spongy biological dressing contains chitosan, collagen and calcium alginate with weigh tmixing ratio o f 0.5-8:0.5-8:0.1-8. Its preparation method includes the following steps: selecting chitosan and collagen type I, adding calcium alginate, compounding and cross-linking, using buffer solution to make neutralization, emulsifying, prefreezing and one-step freeze-drying so as to obtain the invented dressing with good biological compatibility and strong adhesion property. Said invented dressing possesses active function of promoting wound healing and hemostatic action, can be combined with anti-bacterial medicine to obtain gene engineeirng dressing for curing wound surface infection, also can be combined with active growth factor or active cell to form gene engineering dressingfor curing intractable ulcer and burn wound surface.
Owner:JIANGXI RUIJI BIOTECH CO LTD
Plant polysaccharide cigarette filter tip and preparation method thereof
The invention discloses a plant polysaccharide cigarette filter tip and a preparation method thereof. Plant polysaccharide, starch, adsorbent, plasticizer, emulsifier, waterproofing agent and plant protein are blended according to a certain proportion, so that the filter tip is prepared. The preparation method includes the following steps: (a) the starch, water and the plasticizer are blended and stirred, so that modified starch solution is obtained; (b) the plant polysaccharide and the modified starch solution are blended; (c) under certain conditions, the emulsifier, the waterproofing agent and the adsorbent are added into the mixture of the plant polysaccharide and the modified starch solution, and after stirring, the mixture is injected into a long cylindrical mold made of anti-freezing, heat-proofing material and molded; (d) the semifinished product is placed into a freezing environment and frozen, i.e. prefreezing; (e) the semifinished product in step d is placed into a freeze dryer and freeze-dried; (f) the porous composite body is taken out of the mold and deburred, and thereby a filter tip stick is obtained. Material resources are rich, the cost is low, and the three wastes cannot be produced. The performance is good, the filter tip can reduce the nicotine content by 40.2 to 84.1 percent, the tar by 26 to 72.1 percent and the nicotinamide by 20 to 39.2 percent, and after use, waste can be biodegraded.
Owner:WUHAN LICHENG BIOTECH +1
Implantation of micronized allograft tissue over a microfractured defect
Techniques, mixtures, mixing and delivery kits, and improved delivery instruments for implantation of micronized allograft tissue over a microfractured defect. Allograft cartilage tissue is delivered over a cartilage defect that has been debrided and microfractured, without the need for a periosteal covering or separate type of patch sewn over the top. The allograft tissue may be any micronized cartilage particulates obtained by various methods, for example, cartilage delivered in its native form, dehydrated via lyophilization, “freeze-dried,” dehydrated via desiccation, or dehydrated by any other method.
Owner:ARTHREX
Method for producing porous structures
InactiveUS6447701B1Homogeneous structureBioreactor/fermenter combinationsBiological substance pretreatmentsFreeze-dryingMaterials science
Owner:HESCHEL INGO +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com