Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
277 results about "Human growth hormone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Injection syringe including device for preparation of injection
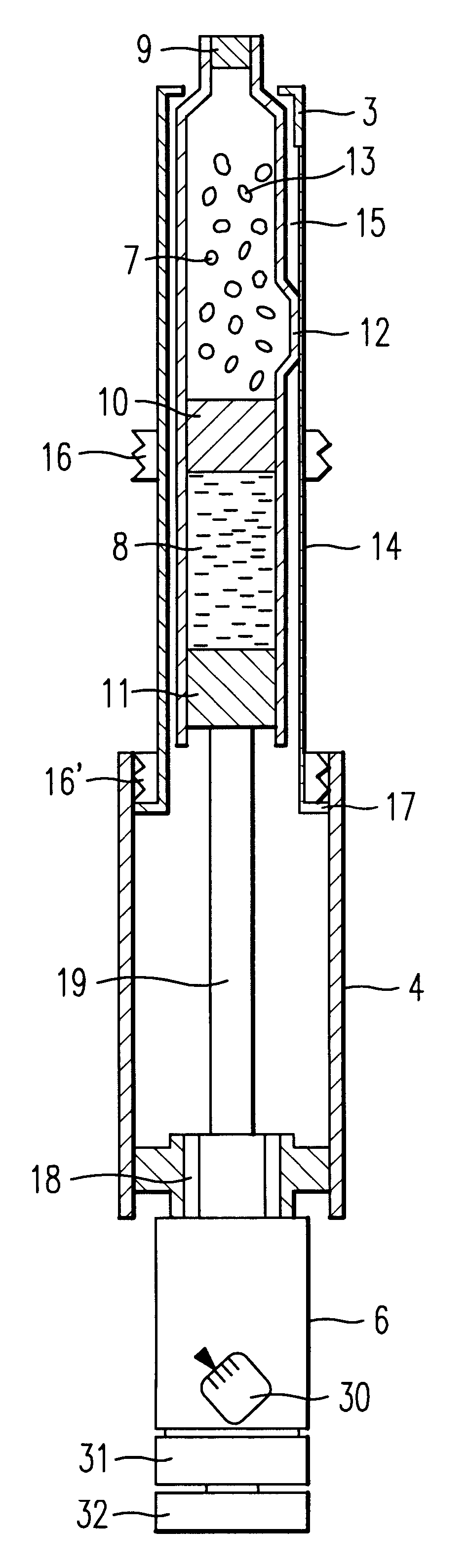
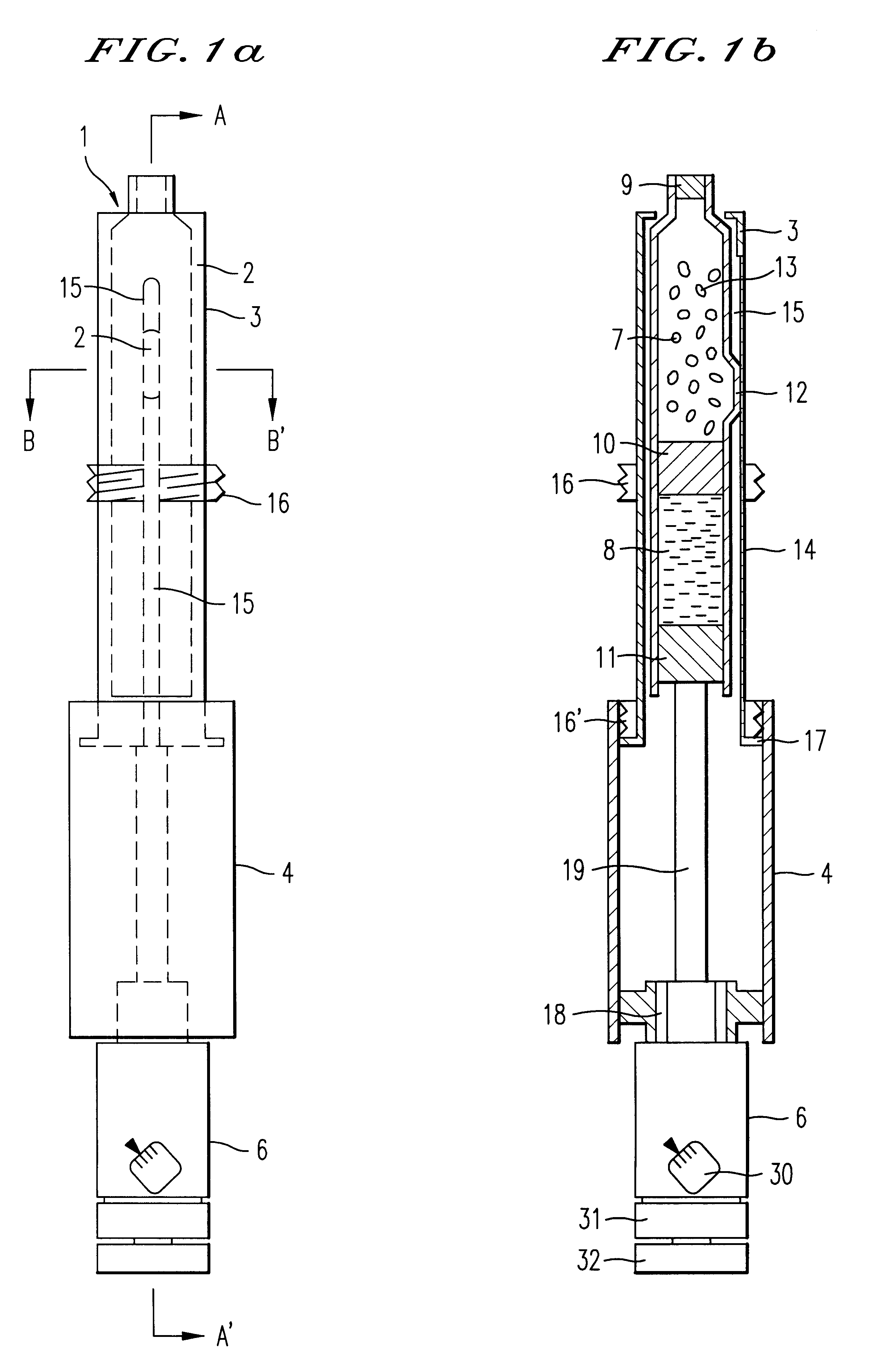
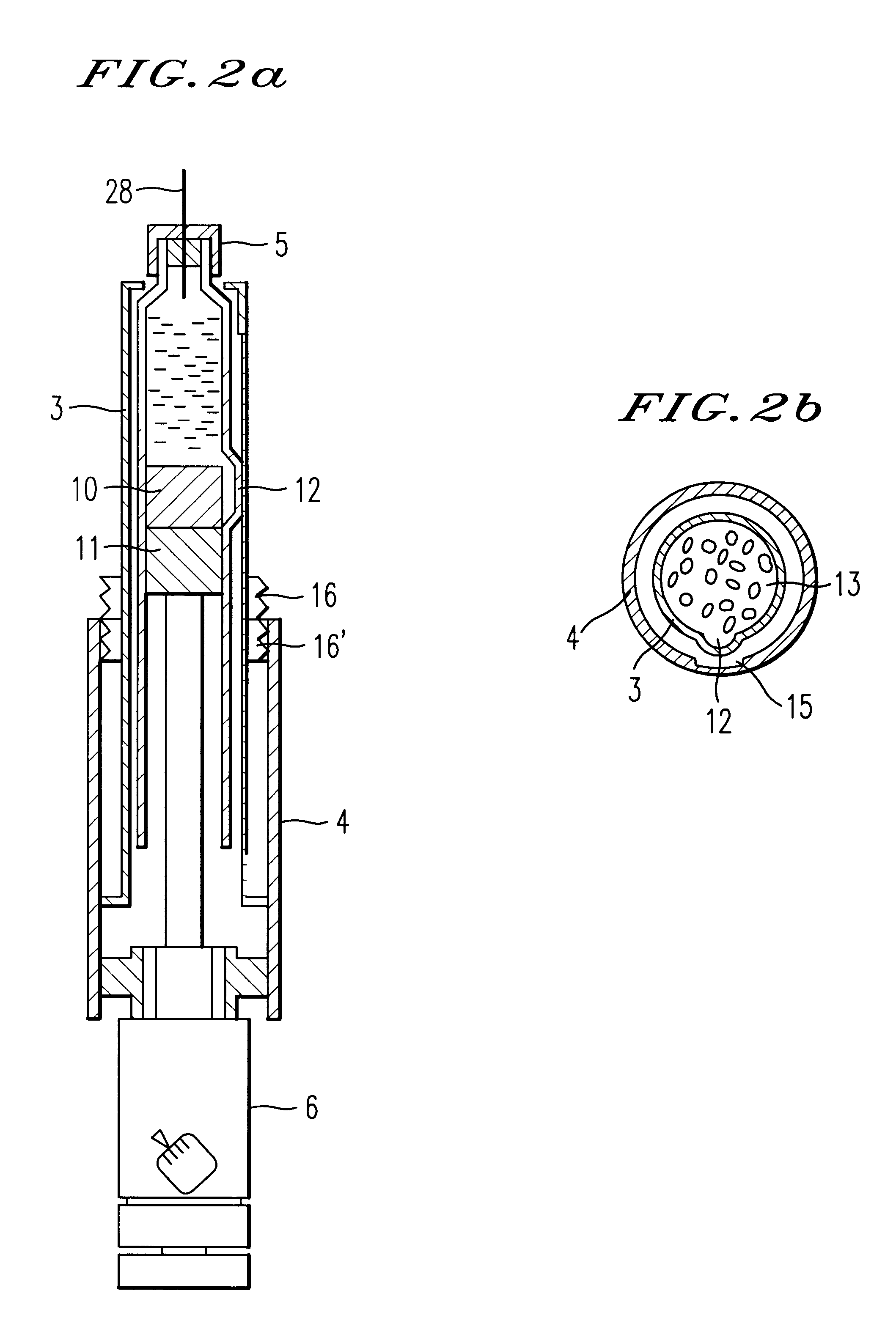
An injection syringe including a device for preparation of an injection in situ, useful to the injection which is liable to suffer chemical changes if left for a long time in the state of solution or dispersion ready to inject. The injection syringe is portable by a patient who may prepare a necessary injection in situ with the use of a device included in the syringe which will automatically perform an injection with a prescribed dosage. The injection syringe includes a device for preparation of an injection, whereby mechanical impacts affecting the medicine and consequently, chemical changes with a medicine would be minimized during the step of dissolving the medicine. The syringe as noted is especially useful for preparation of injection and injection of human growth hormones, interferon and various polypeptides which are of an environmentally sensitive nature and liable to suffer chemical changes if left for a long time in the state of solution or dispersion.
Owner:JCR PHARMA
Freeze-dried preparation of human growth hormone
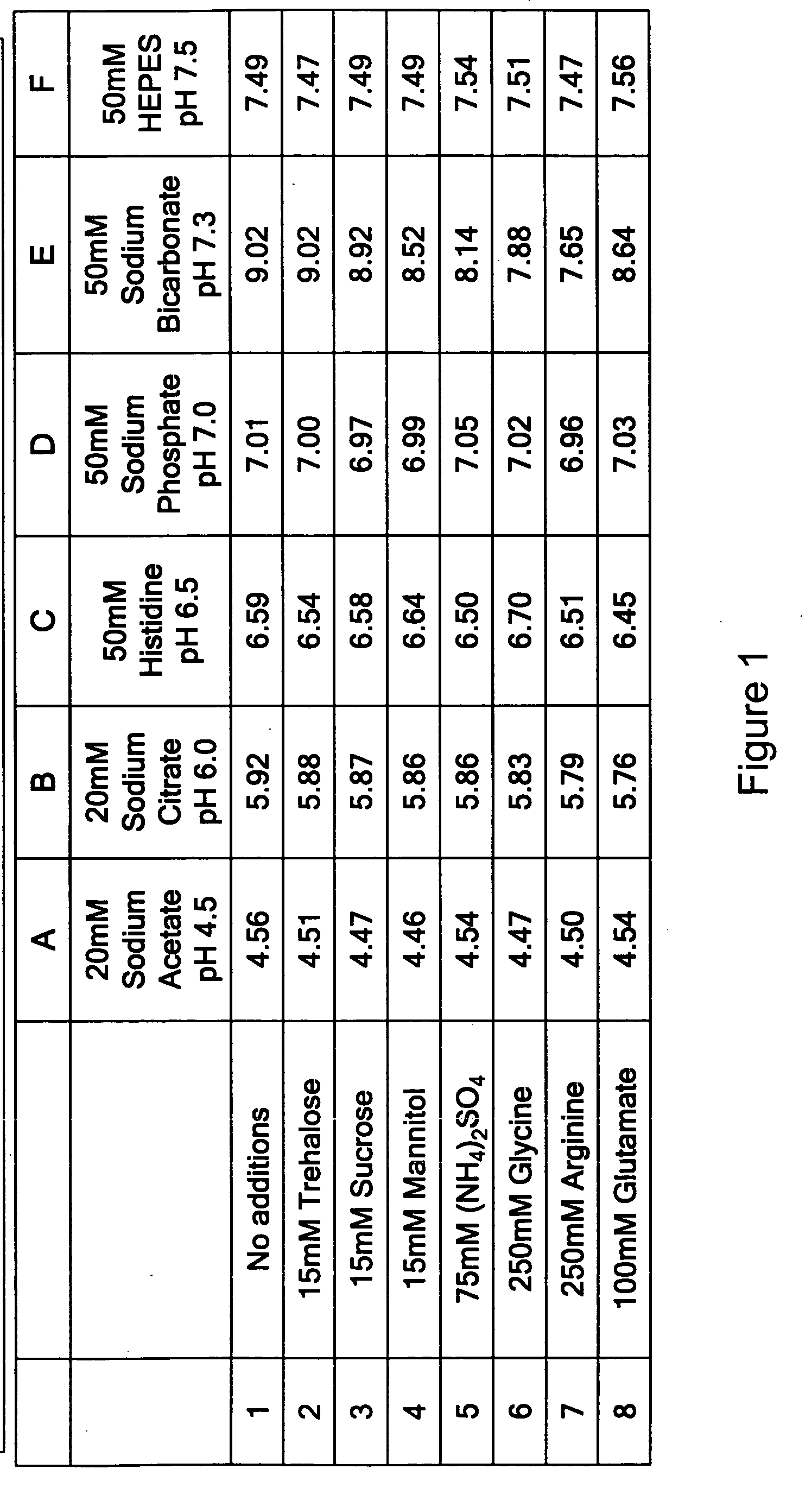
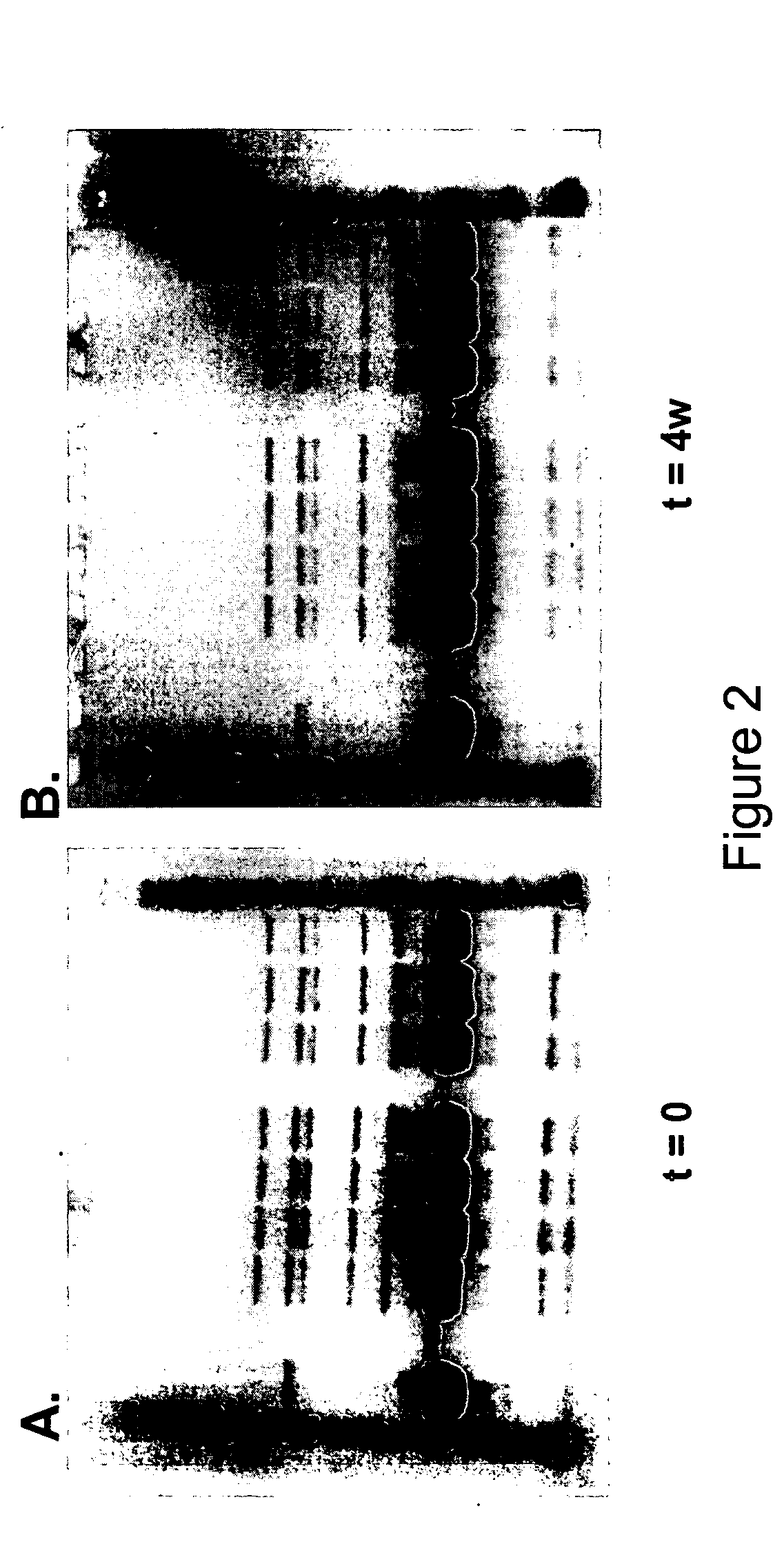
A readily-soluble freeze-dried solid preparation of hGH with a minimal content of degradation products in terms of deamidation, dimers, polymers, and sulphoxide forms, obtainable by a method comprising a single lyophilization of an aqueous slurry of an amorphous hGH isoprecipitate, the slurry having a pH of from about 4.7 to 5.0 and being essentially free of buffer components other than acetate.
Owner:NOVO NORDISK AS
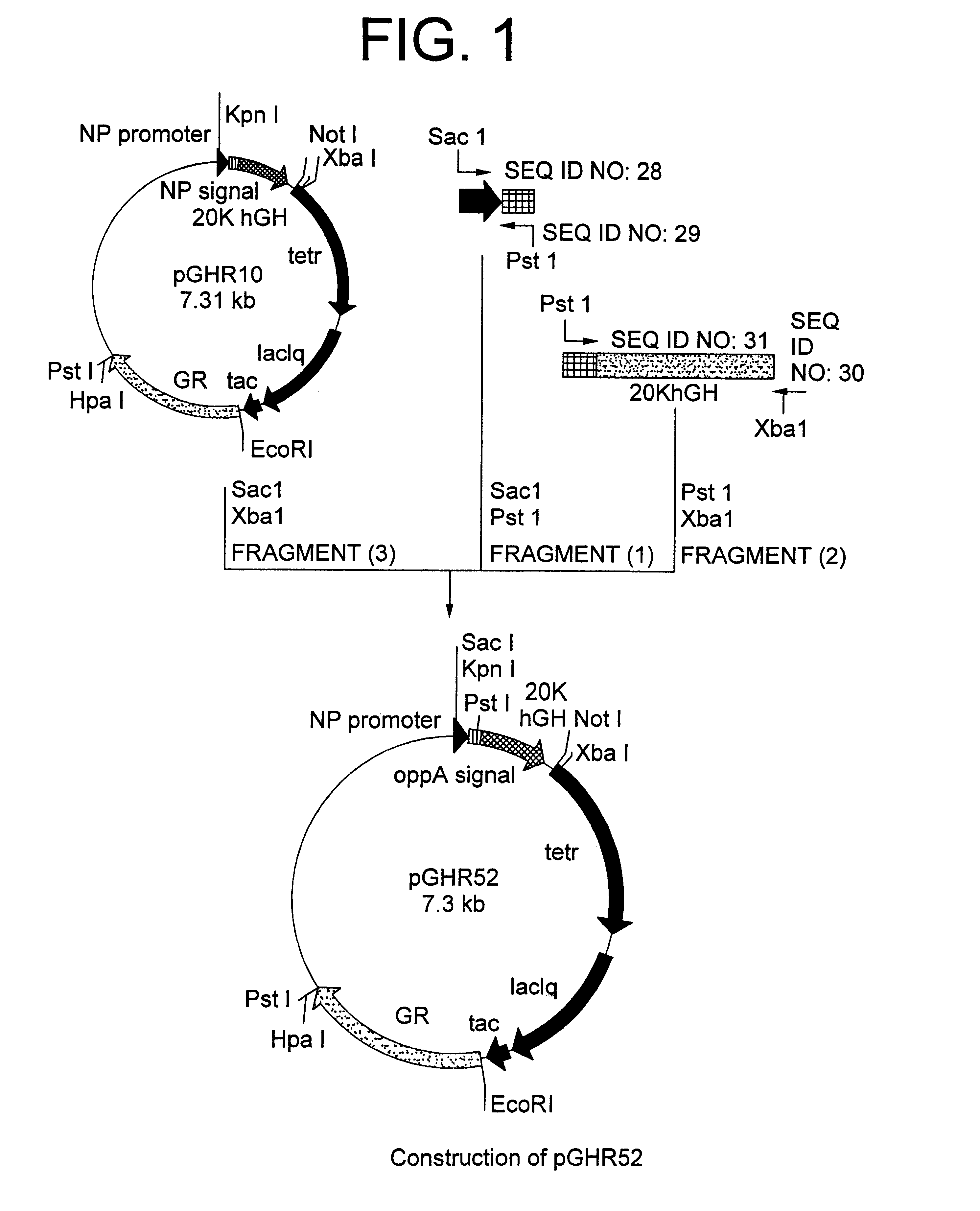
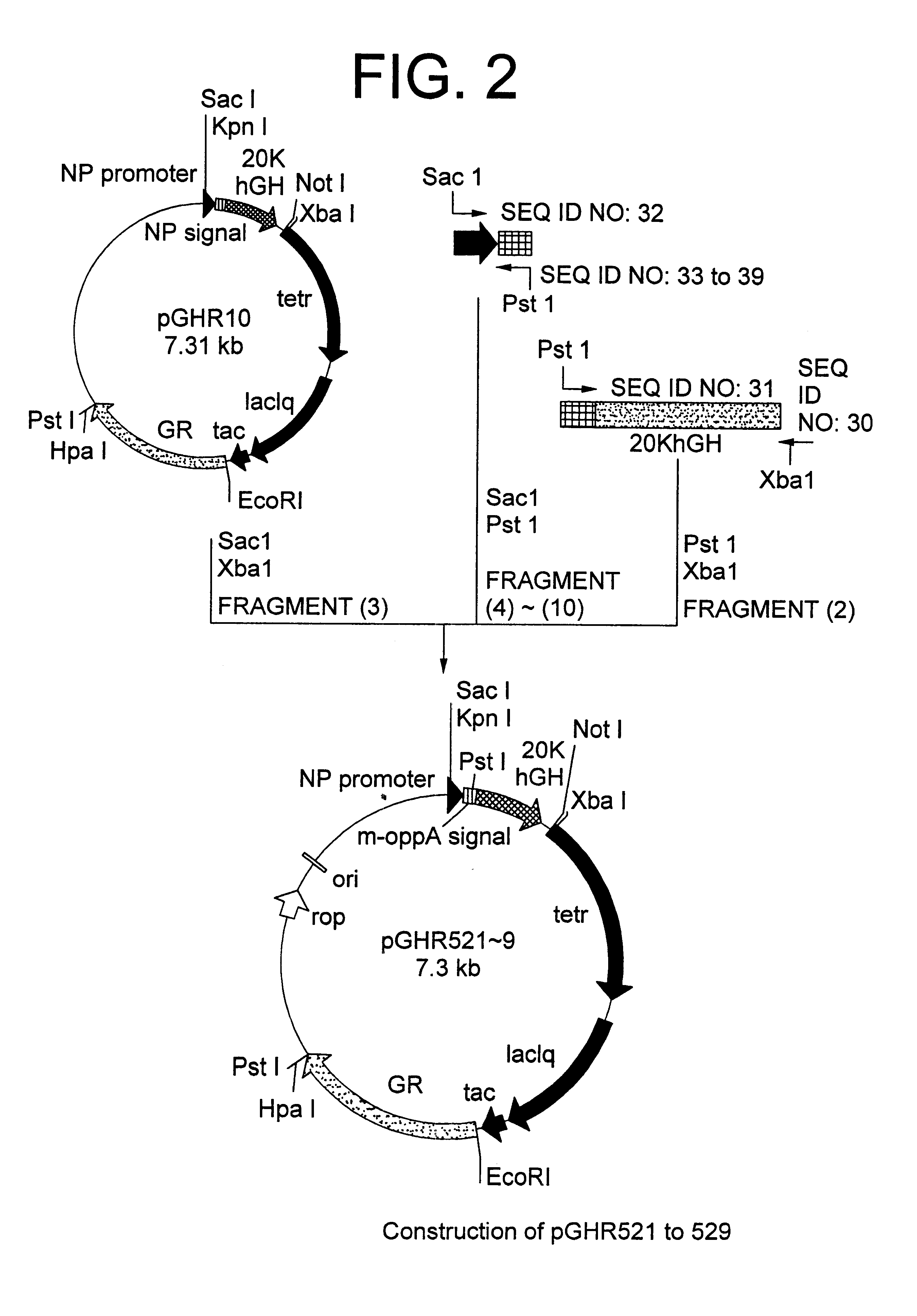
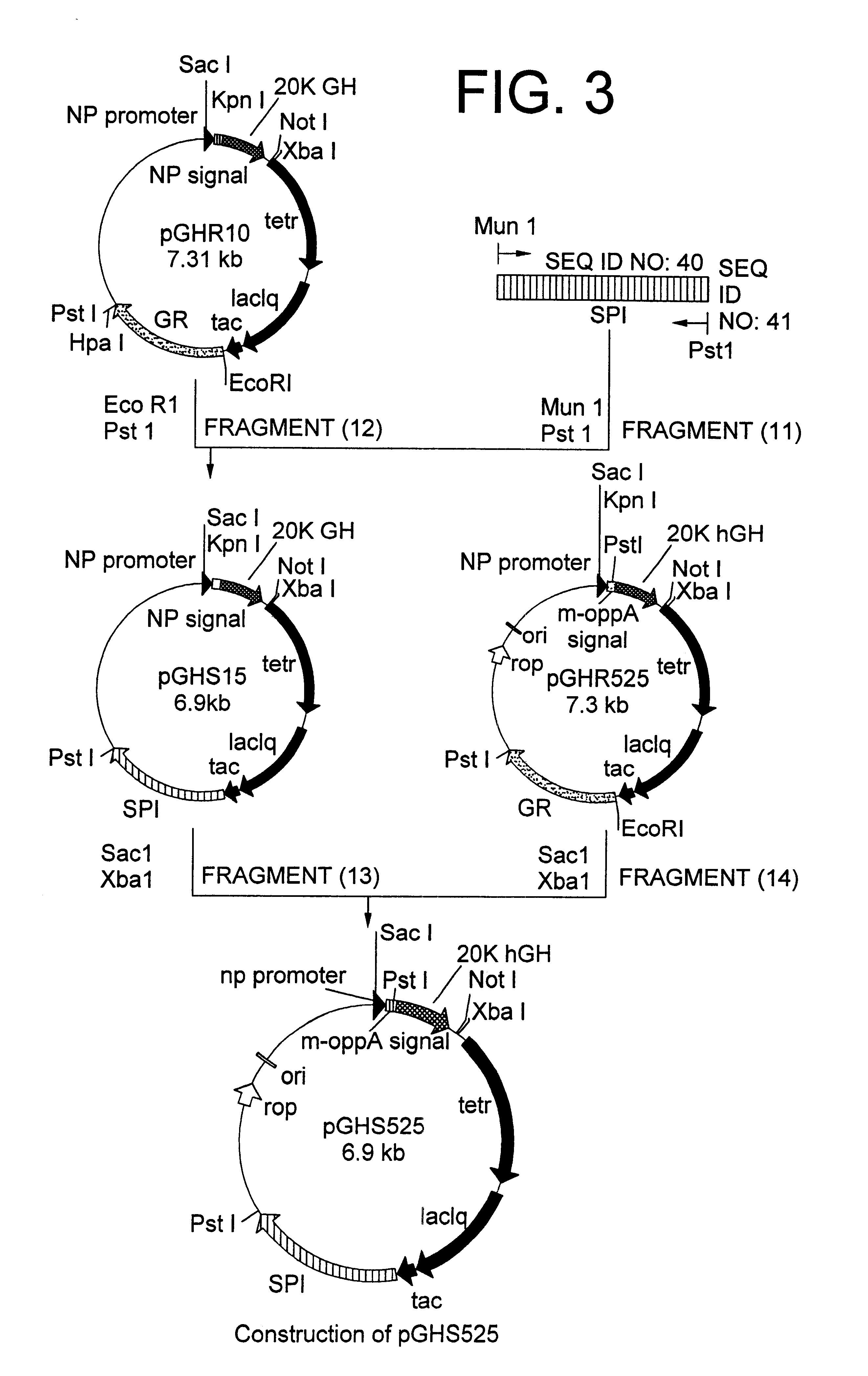
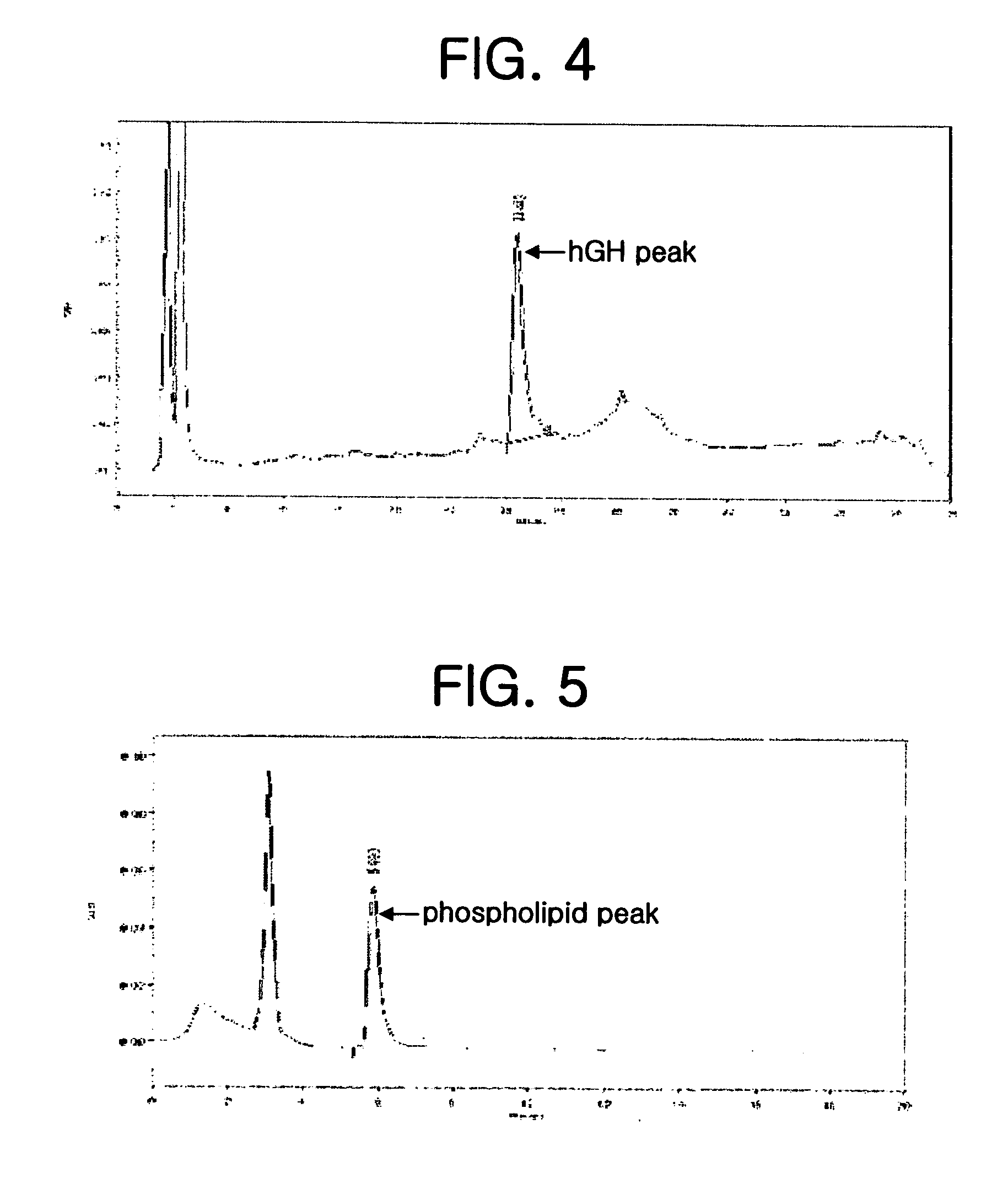
Method for secretory production of human growth hormone
InactiveUS6436674B1Increase productionDecreased tendency for lysisBacteriaHydrolasesHuman growth hormoneA-DNA
A DNA encoding 20K hGH is connected directly to a gene encoding Escherichia coli OppA protein secretion signal, or a modified form thereof, and a DNA encoding signal peptidase 1 to construct a recombinant plasmid, E. coli is transformed by the plasmid and cells of the resulting E. coli transformant strain are cultured for secretory production of the 20K hGH in the E. coli periplasm. This method enables efficient secretory production of 20K hGH and easy isolation and purification of 20K hGH from the periplasm fraction because the level of impure proteins in the E. coli periplasm is low.
Owner:MITSUI CHEM INC
Composition for improving skin conditions comprising human growth hormone as an active ingredient
InactiveUS20070081963A1Improve skin conditionCosmetic preparationsHair cosmeticsWrinkle skinBULK ACTIVE INGREDIENT
Disclosed herein is a skin condition-improving composition for topical application to the skin, comprising human growth hormone as an active ingredient, and a method for improving skin conditions using the same. The disclosed composition exhibits various skin conditioning effects, such as acne treatment, wrinkle improvement, dark spot removal, skin elasticity improvement, hair growth stimulation, skin aging prevention, skin moisturization and the proliferation of skin epidermal stem cells.
Owner:REGERON
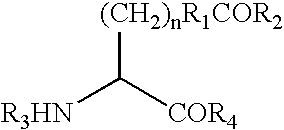
Macromolecular drug complexes having improved stability and therapeutic use of the same
InactiveUS20050147581A1Safe and easy and effective deliveryAlleviate and eliminate and reverse complicationPowder deliveryOrganic active ingredientsDiseaseSomatotropic hormone
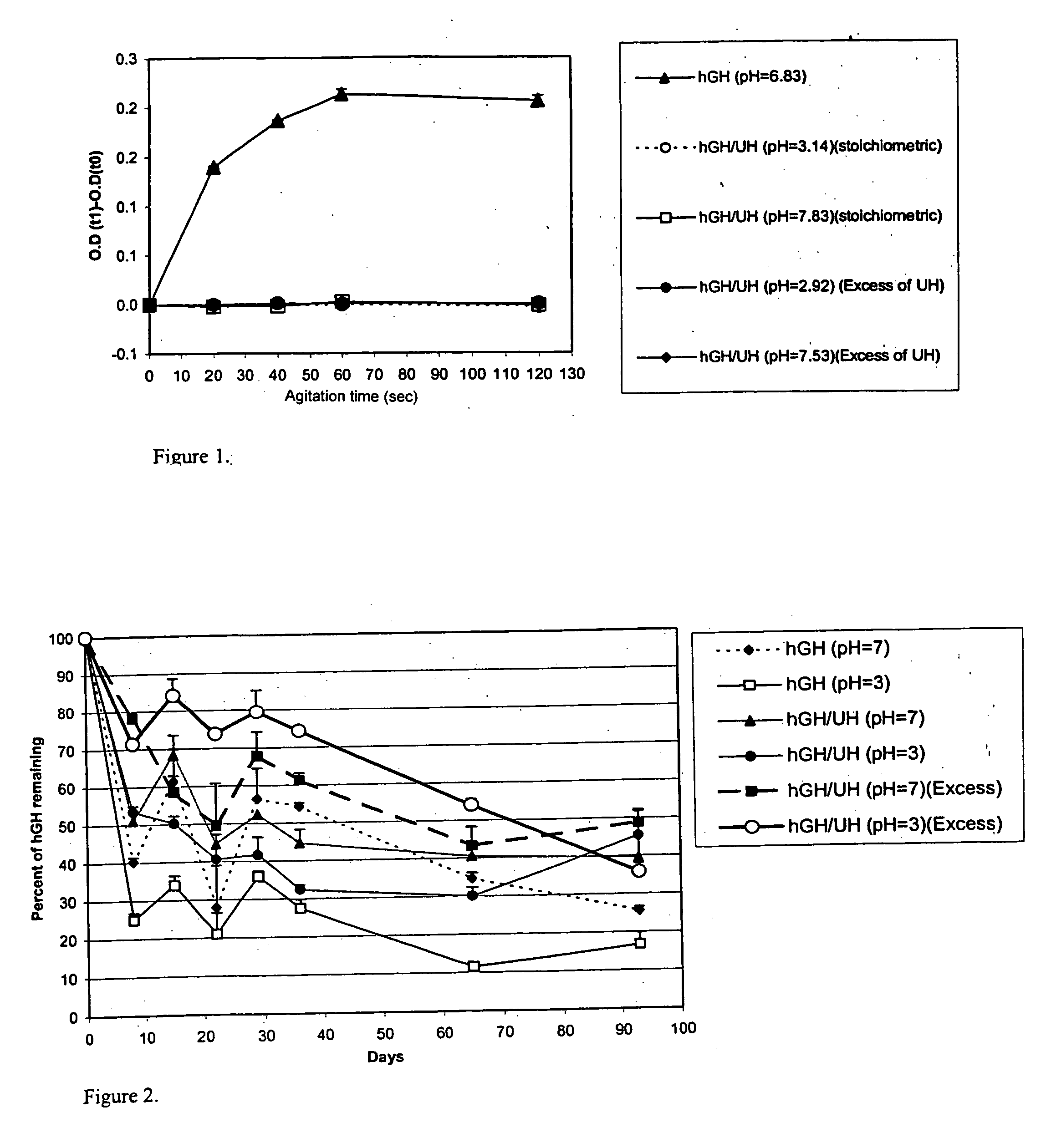
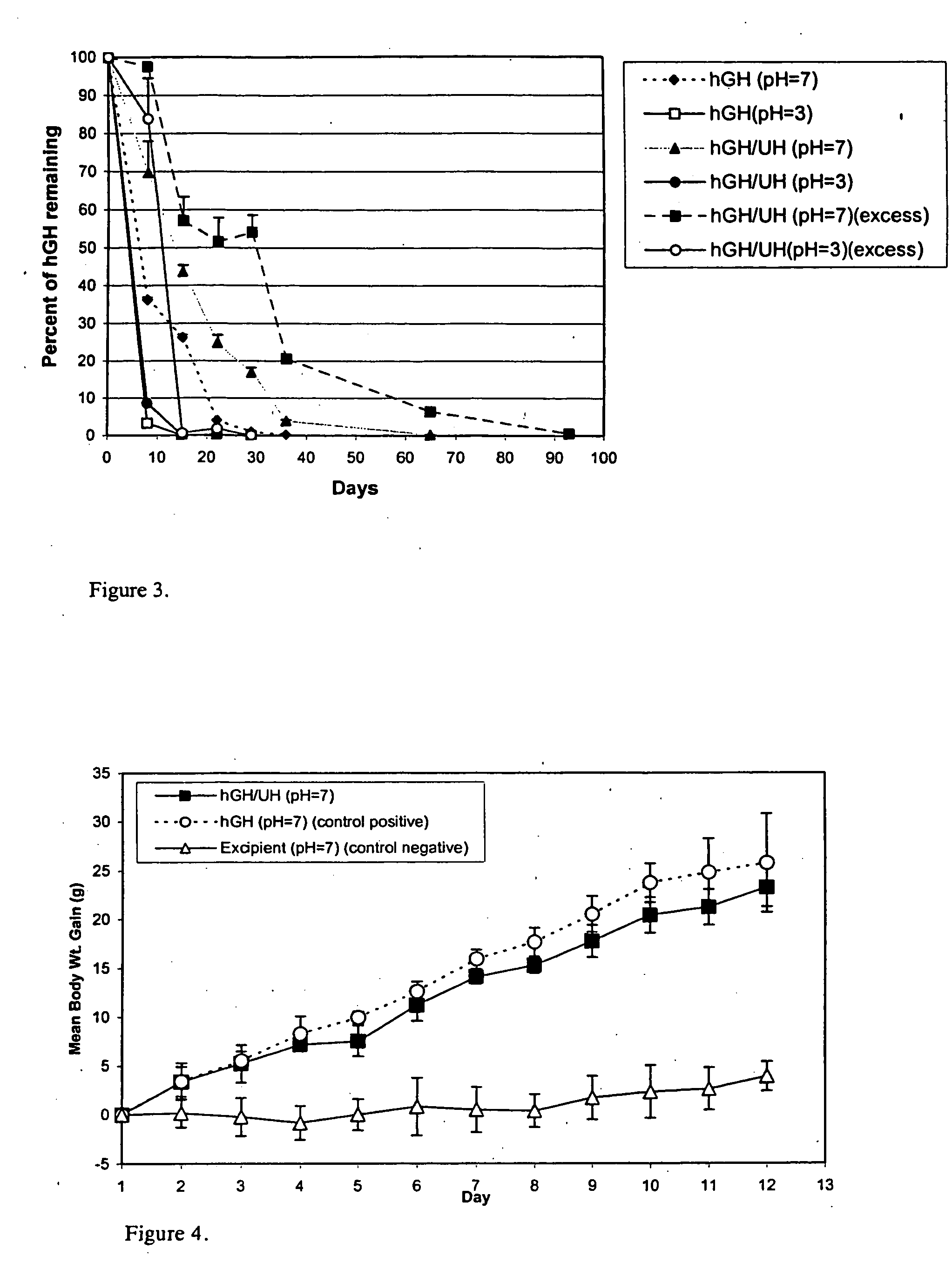
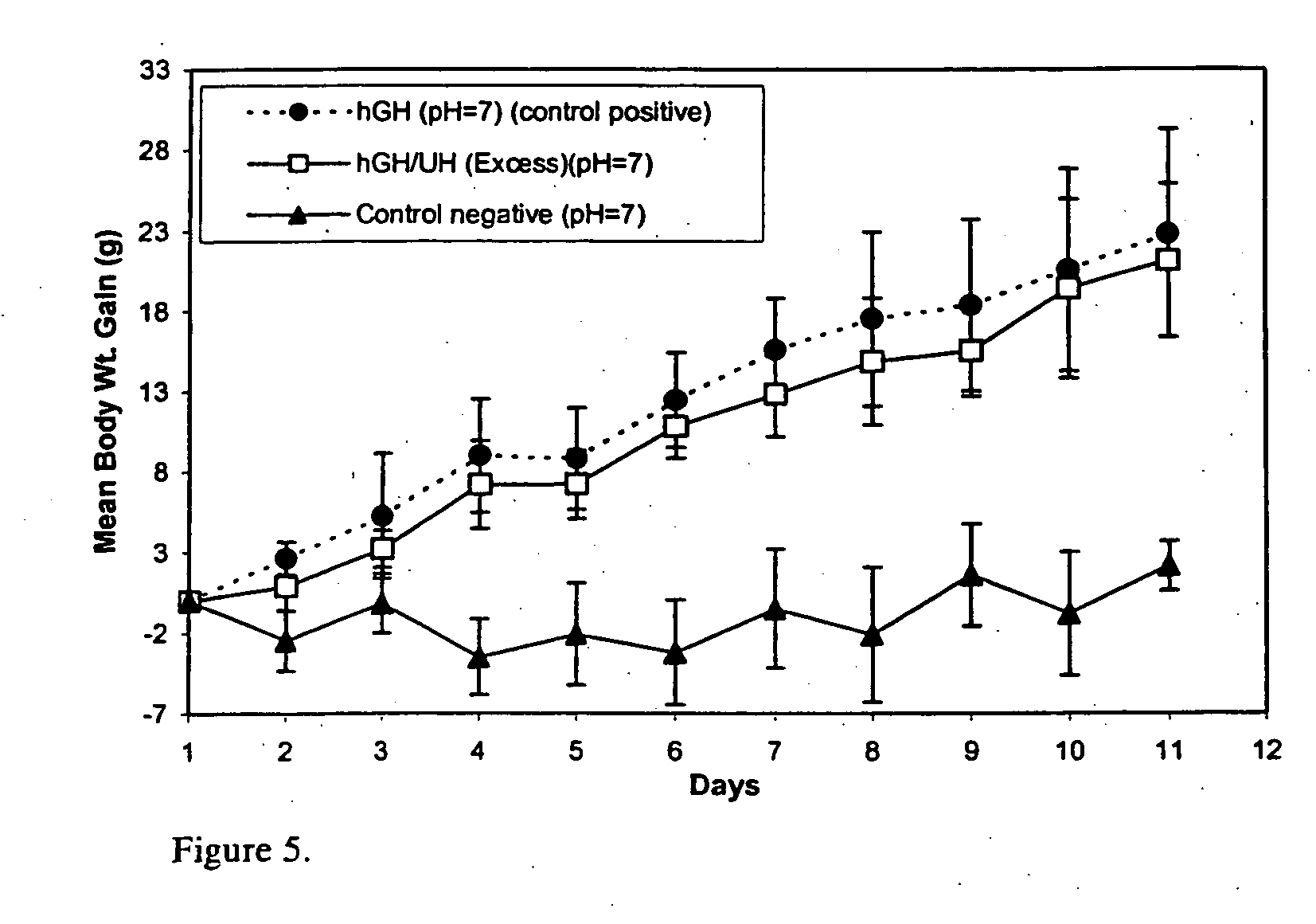
Macromolecular drug complexes containing a protein therapeutic, like human growth hormone, and an excess stoichiometric molar amount of a polymer, like heparin, and compositions containing the same, are disclosed. Compositions containing the macromolecular drug complexes are administered, including via pulmonary delivery, to individuals suffering from a disease or condition, and the complexes release the protein therapeutic, in vivo, to treat the disease or condition.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
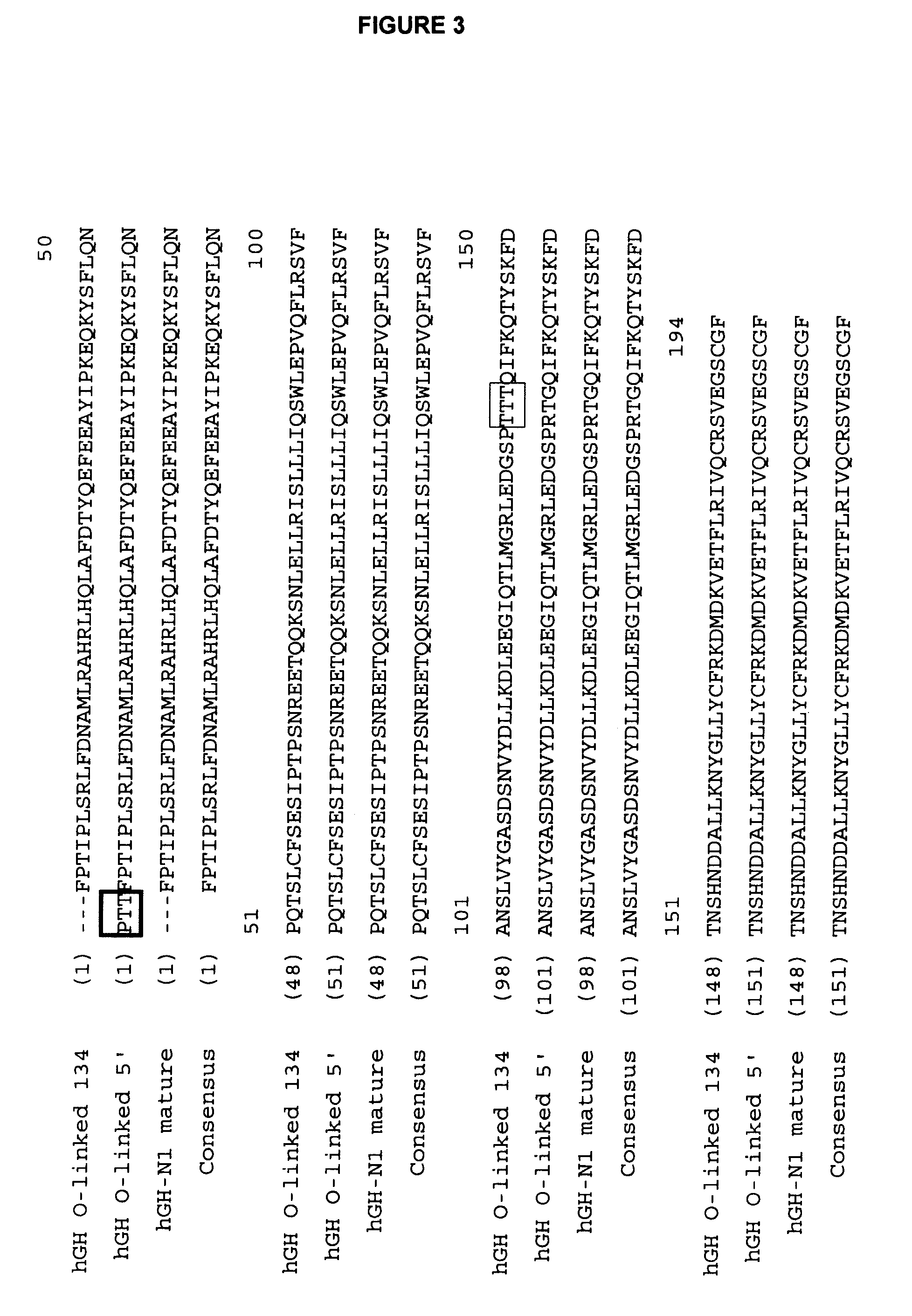
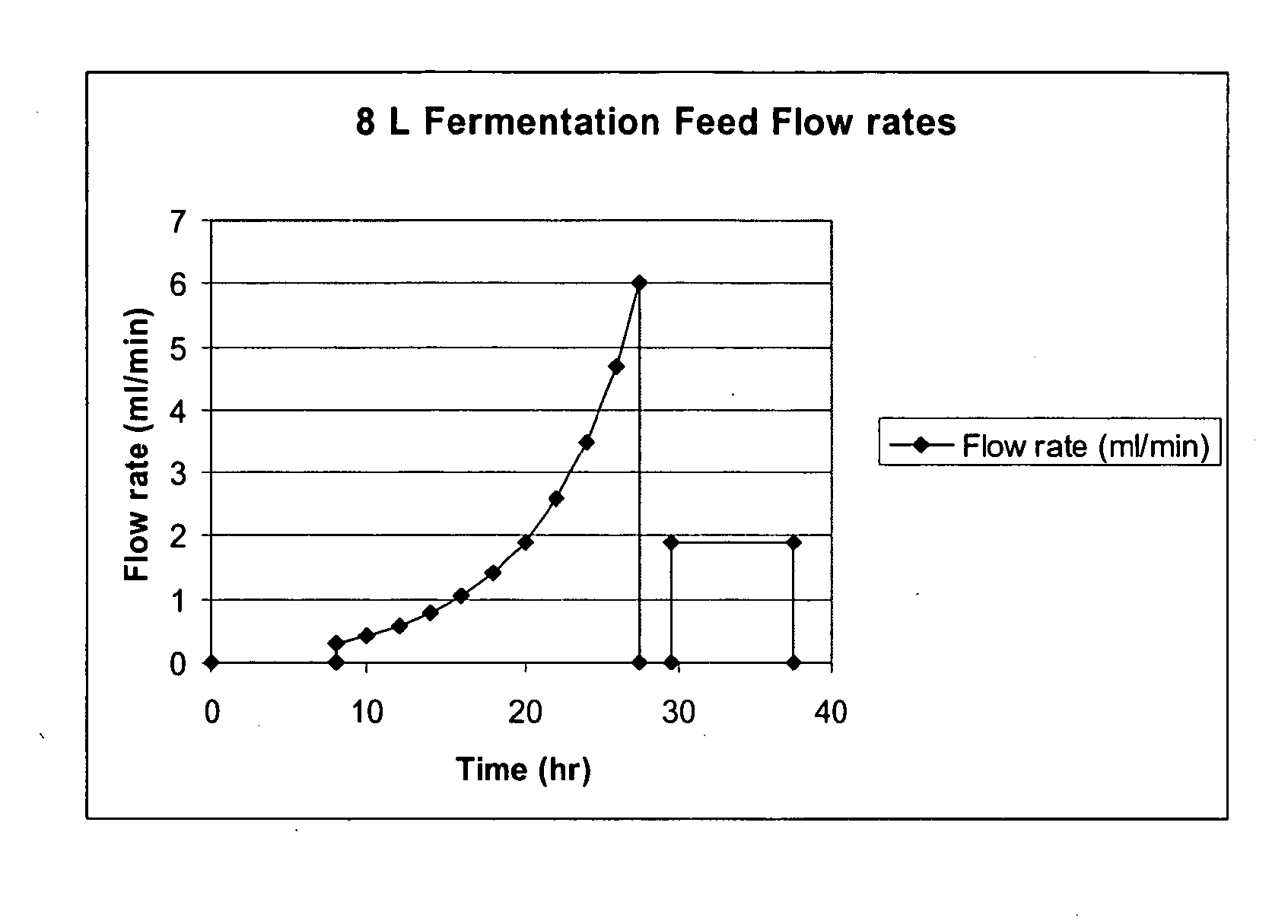
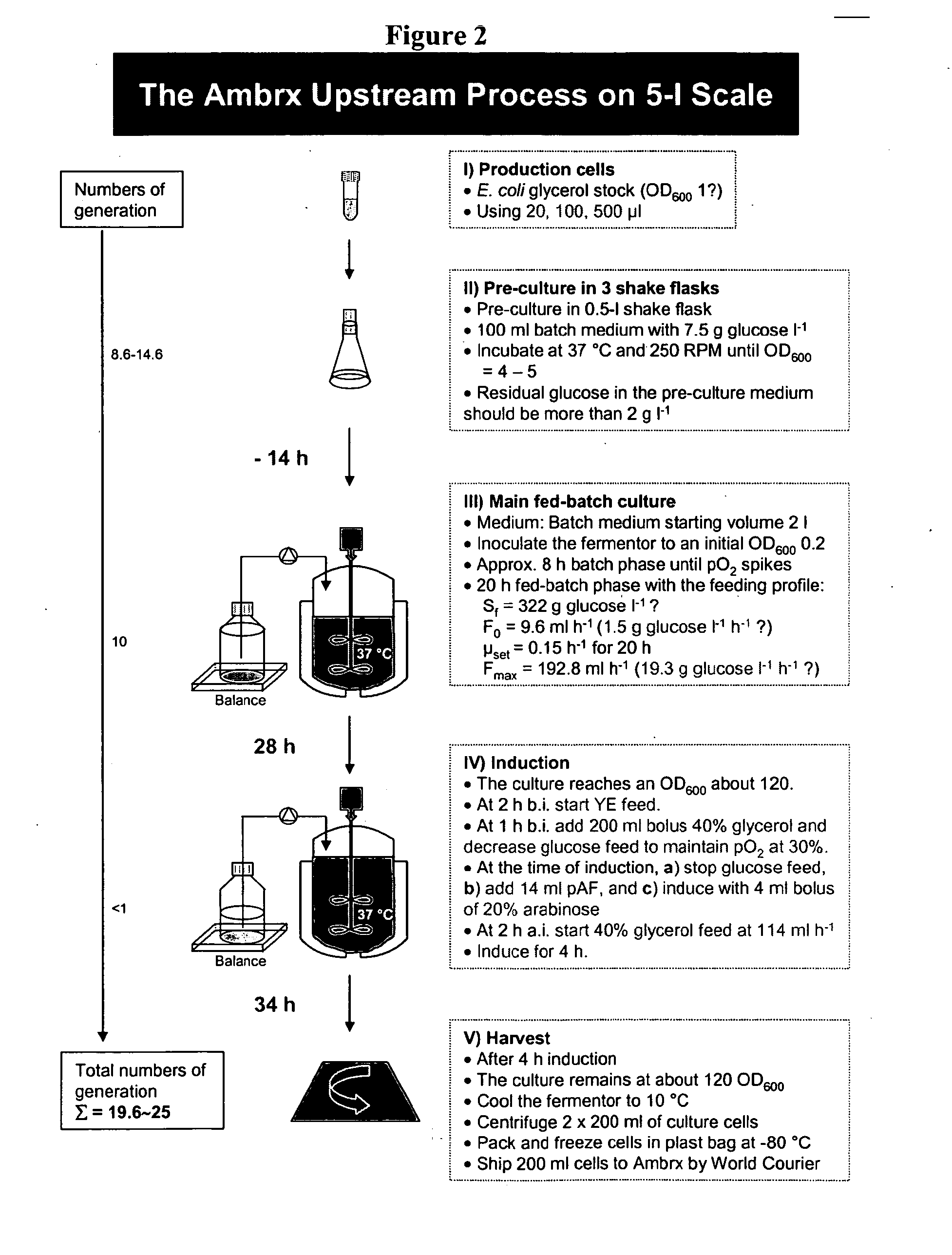
Methods for expression and purification of recombinant human growth hormone
The present invention relates generally to the production, purification, and isolation of human growth hormone (hGH). More particularly, the invention relates to the production, purification, and isolation of substantially purified hGH from recombinant host cells or culture medium including, for example, yeast, insect, mammalian and bacterial host cells. The process of the present invention is also useful for purification of hGH linked to polymers or other molecules.
Owner:AMBRX
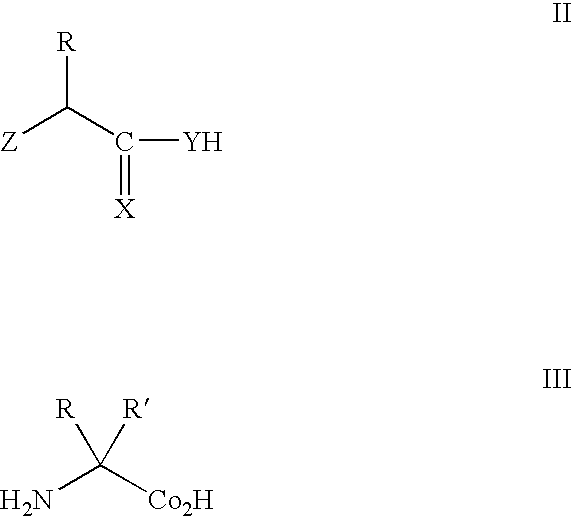
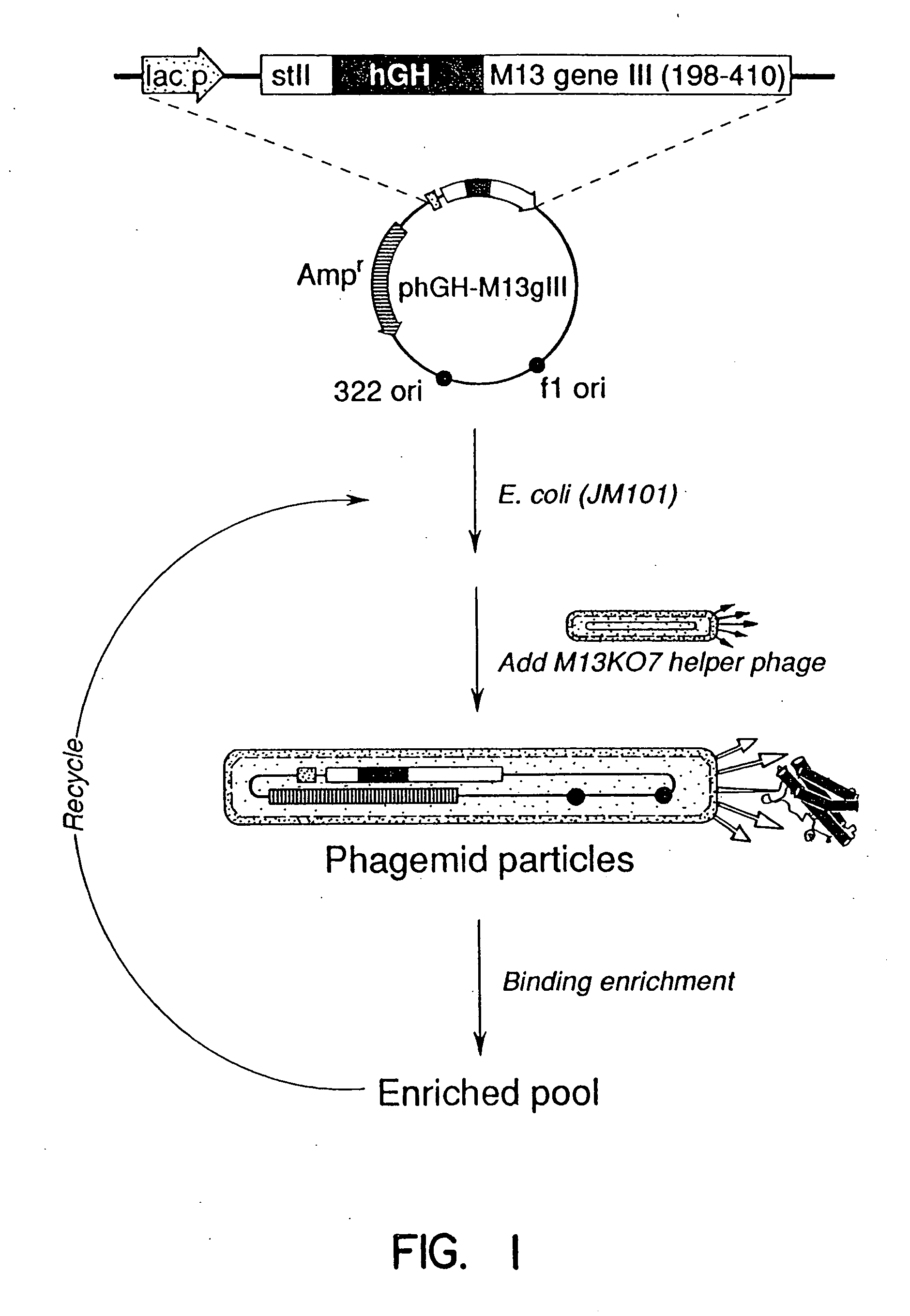
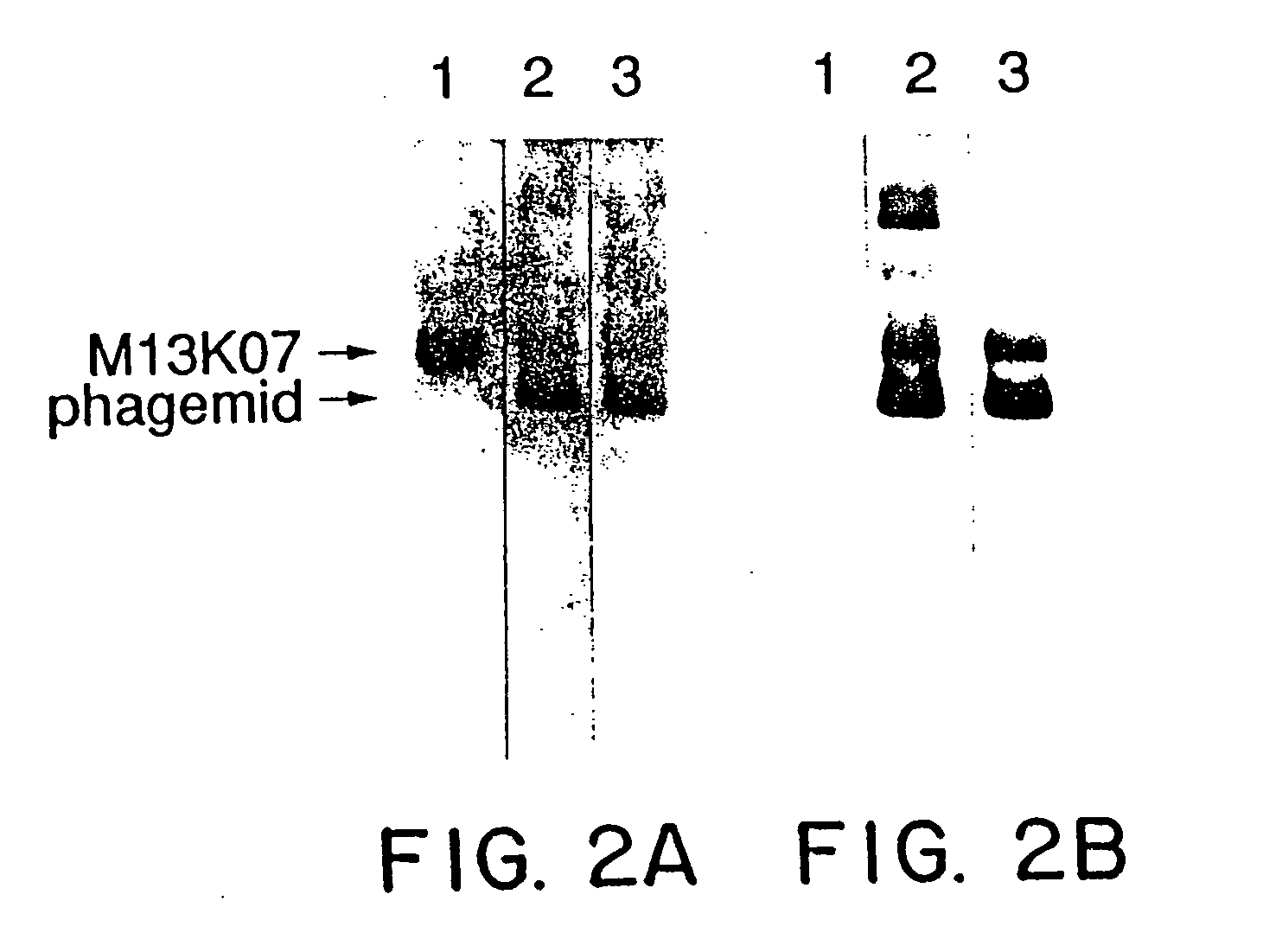
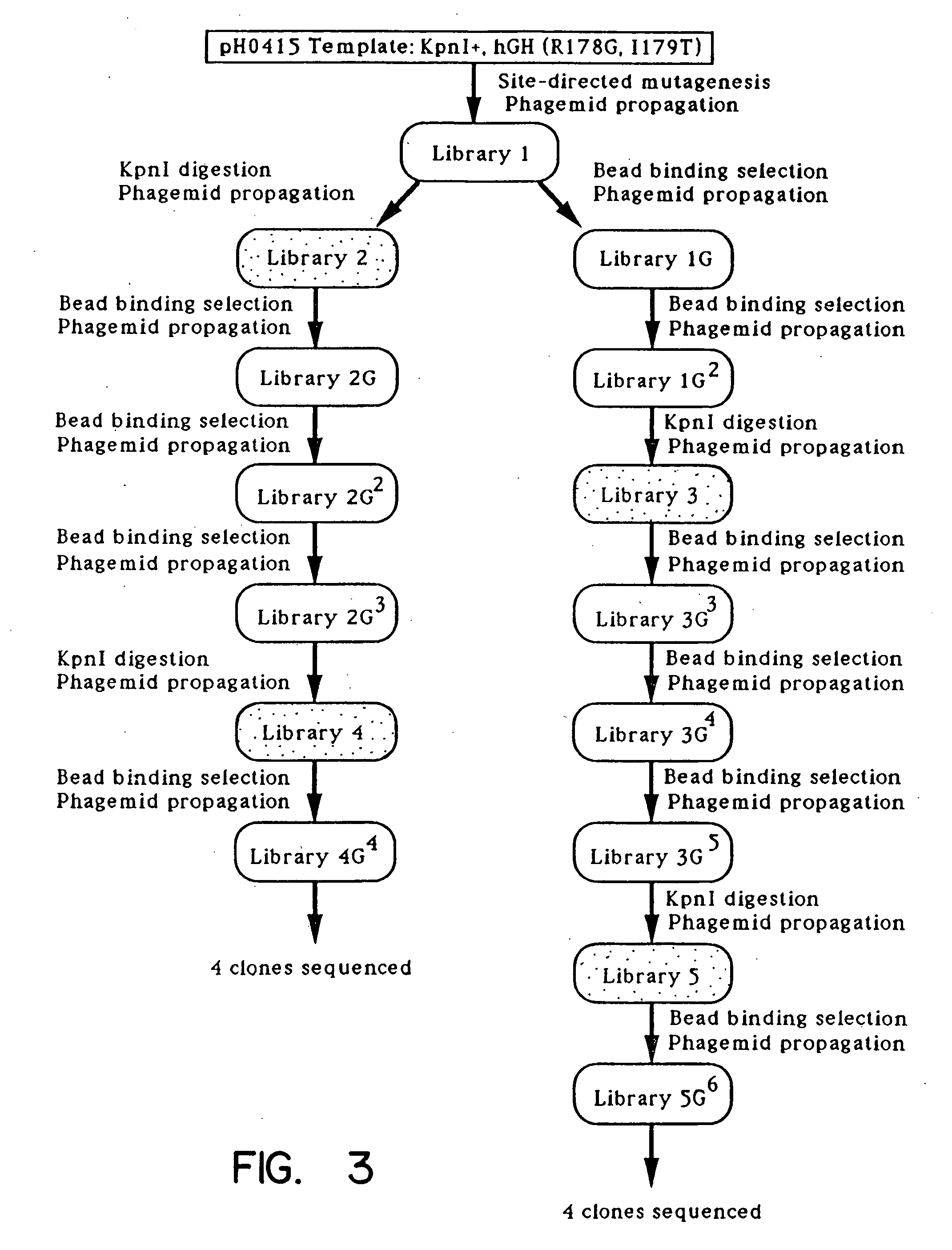
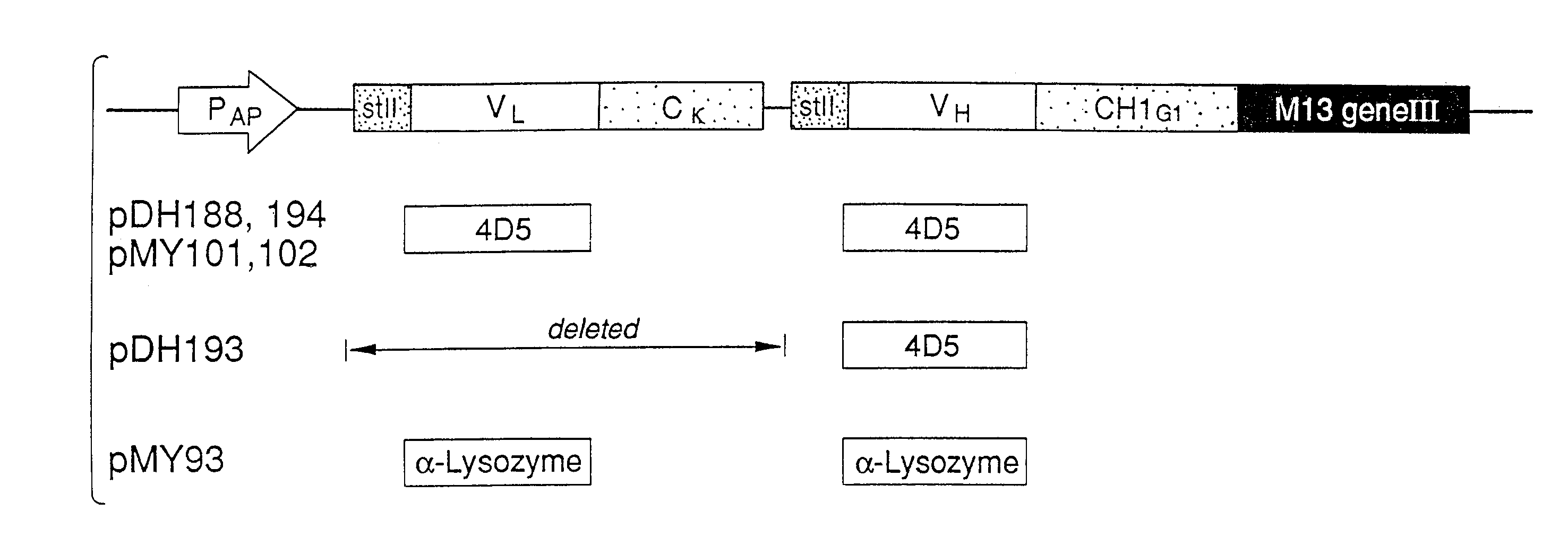
Enrichment method for variant proteins with altered binding properties
InactiveUS20060115874A1Valid choiceHigh affinity bindingVirusesPeptide/protein ingredientsEnrichment methodsAntibody fragments
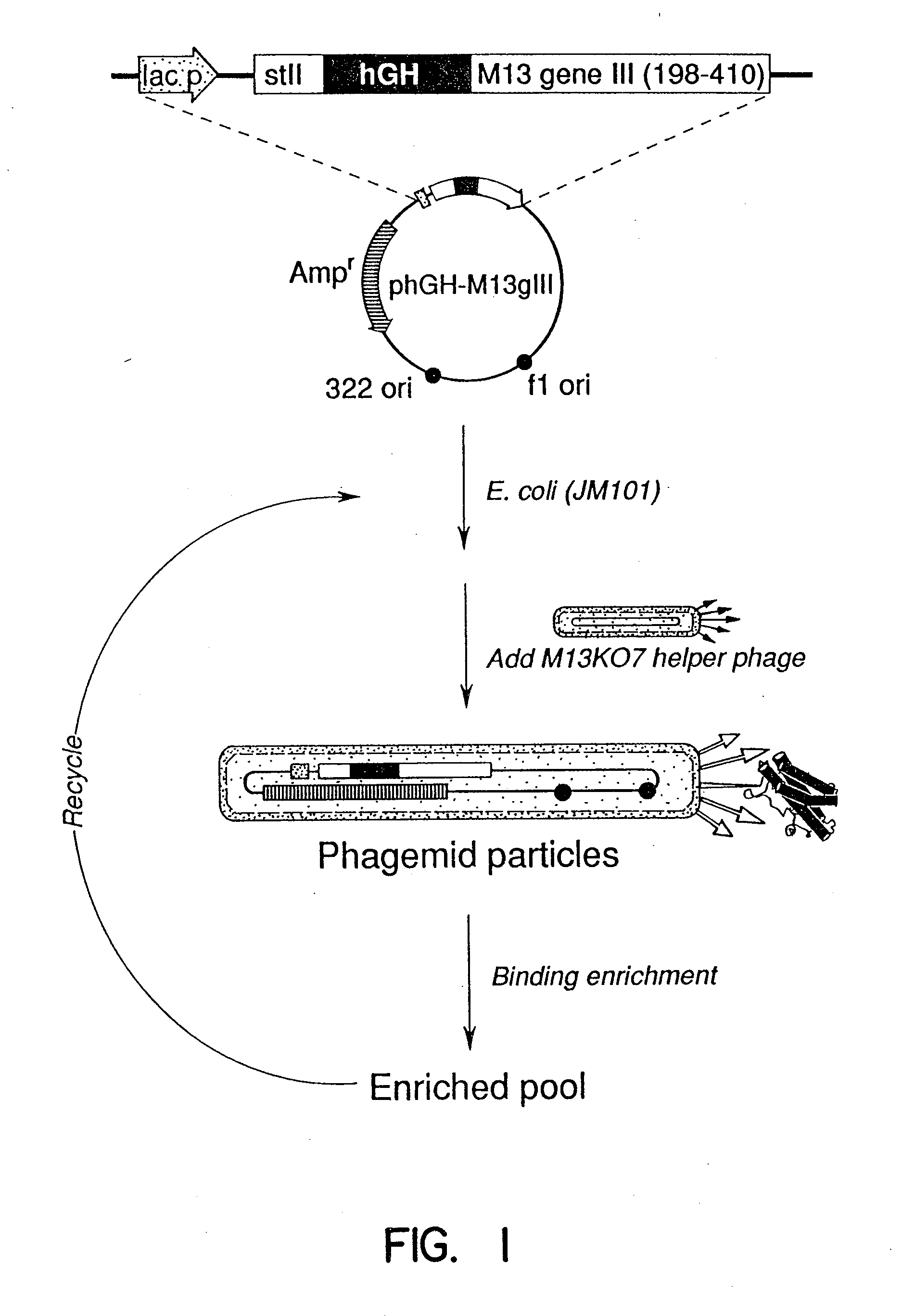
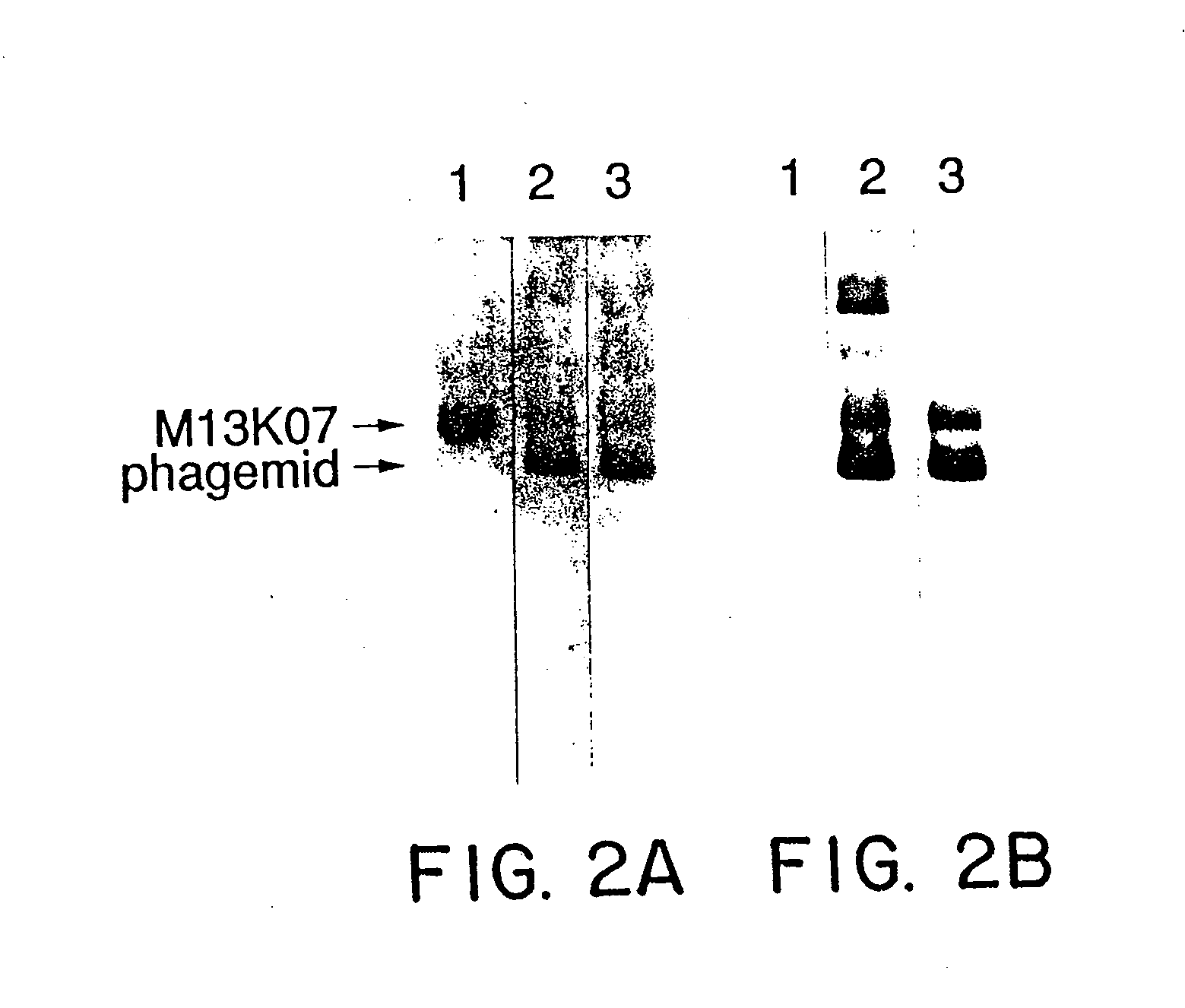
A method for selecting novel proteins such as growth hormone and antibody fragment variants having altered binding properties for their respective receptor molecules is provided. The method comprises fusing a gene encoding a protein of interest to the carboxy terminal domain of the gene III coat protein of the filamentous phage M13. The gene fusion is mutated to form a library of structurally related fusion proteins that are expressed in low quantity on the surface of a phagemid particle. Biological selection and screening are employed to identify novel ligands useful as drug candidates. Disclosed are preferred phagemid expression vectors and selected human growth hormone variants.
Owner:GENENTECH INC
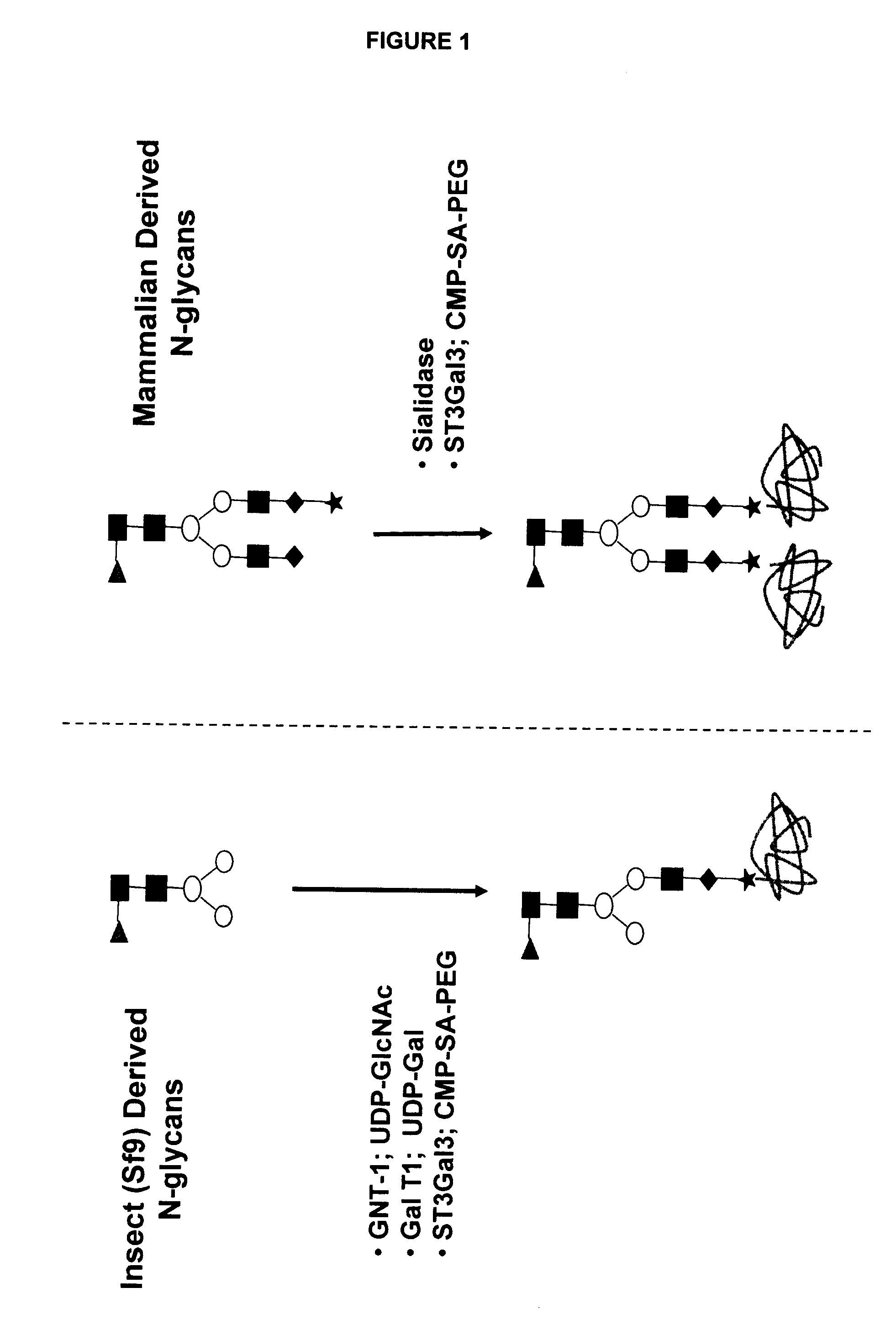
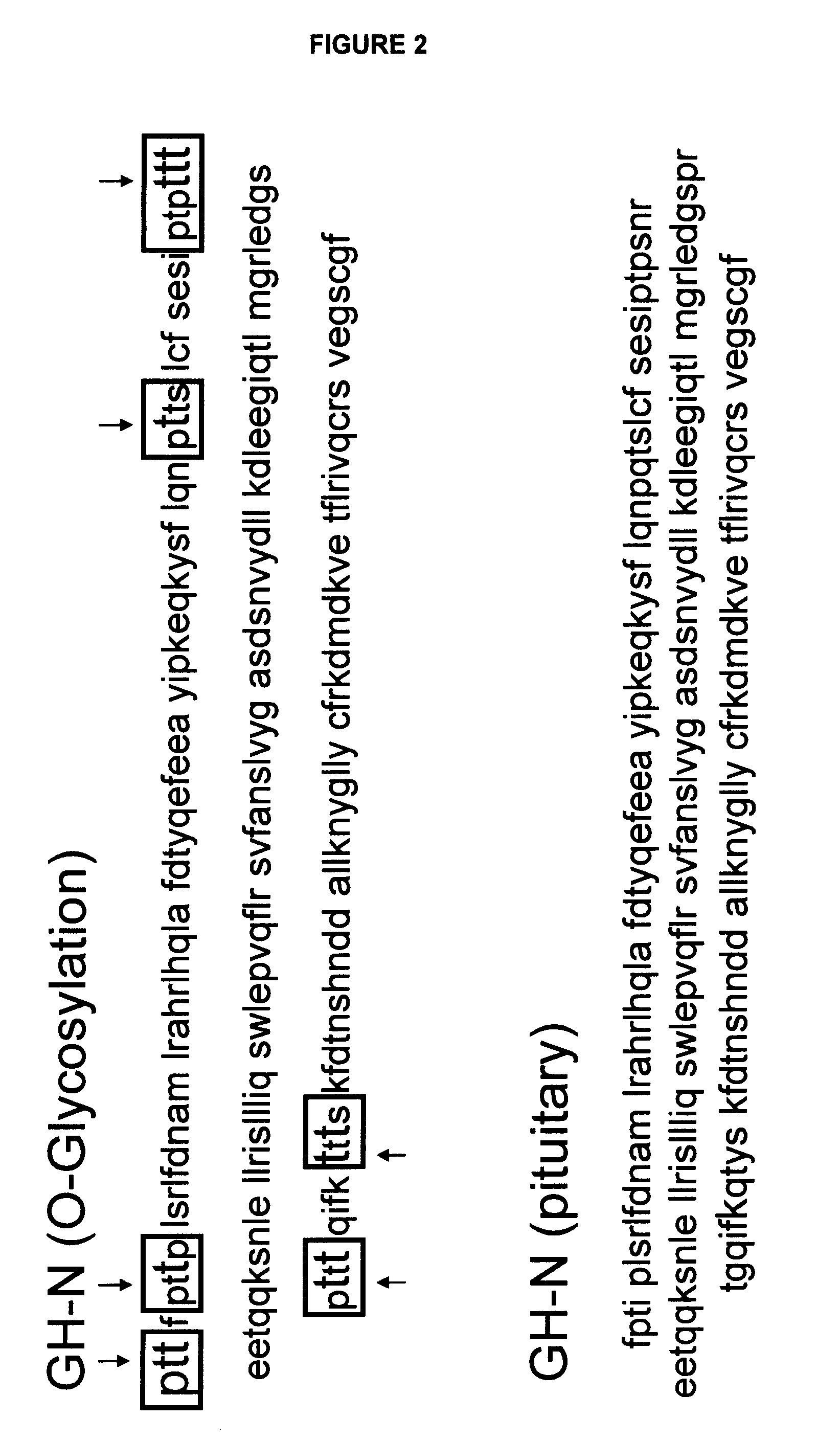
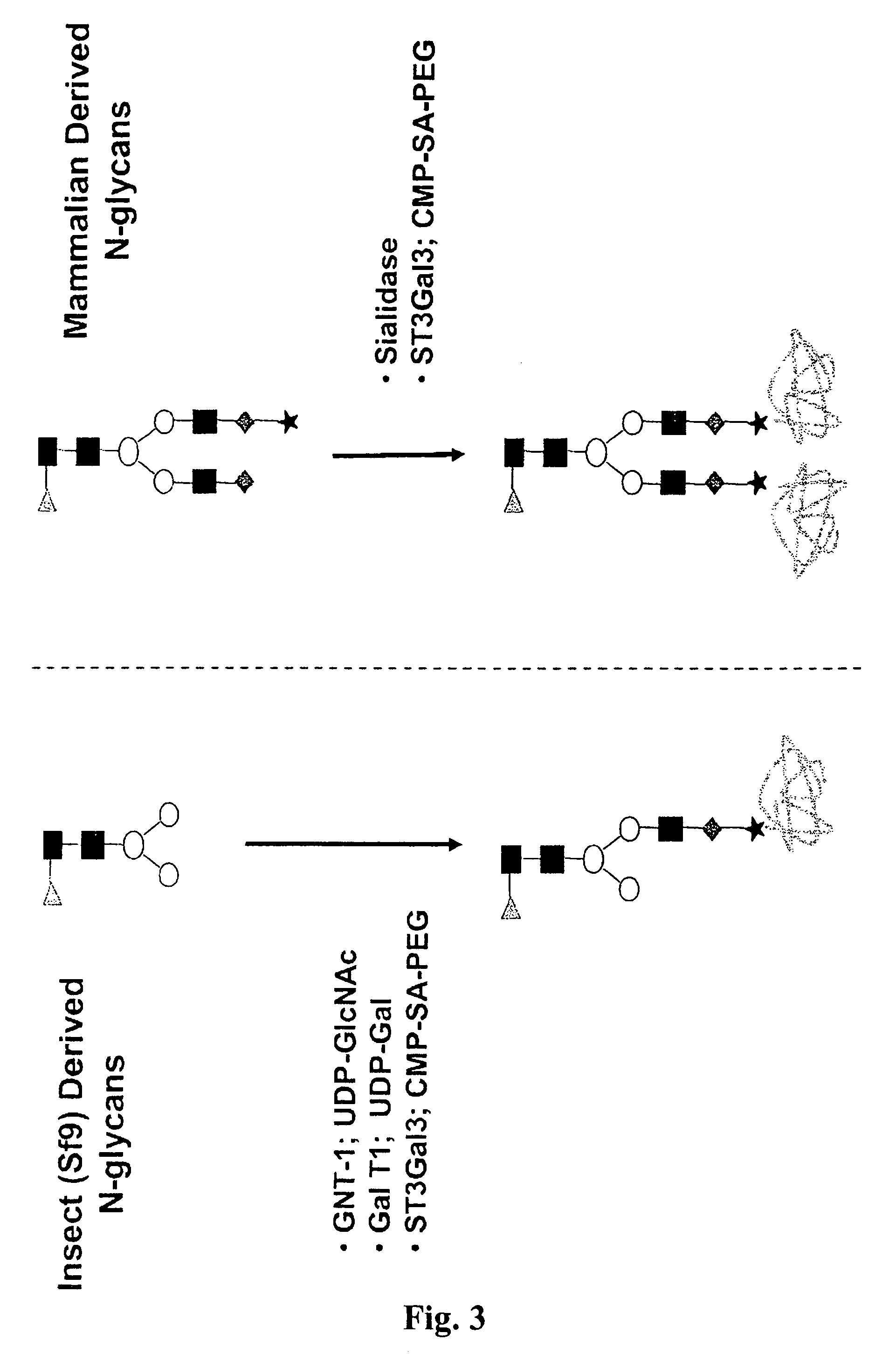
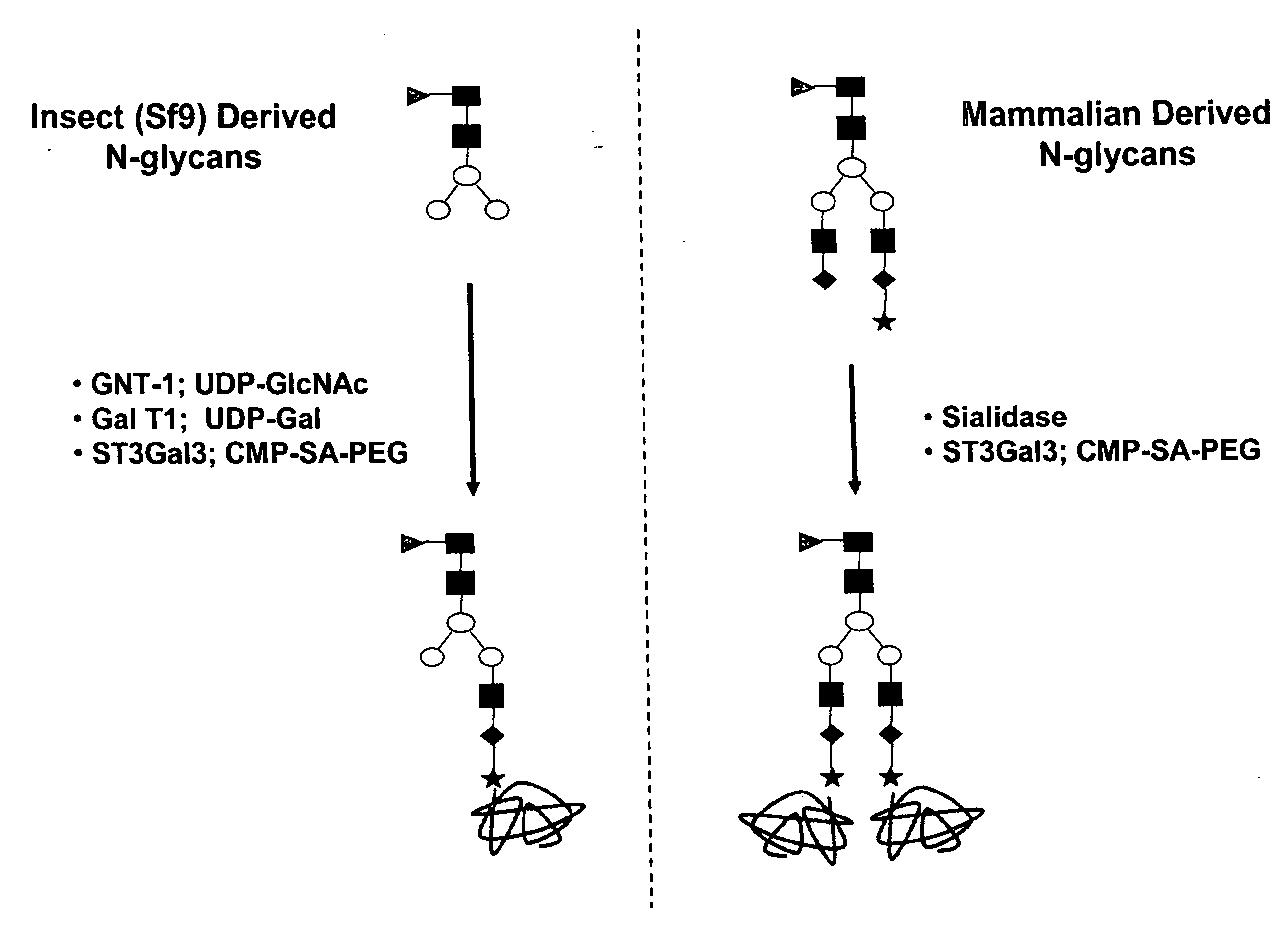
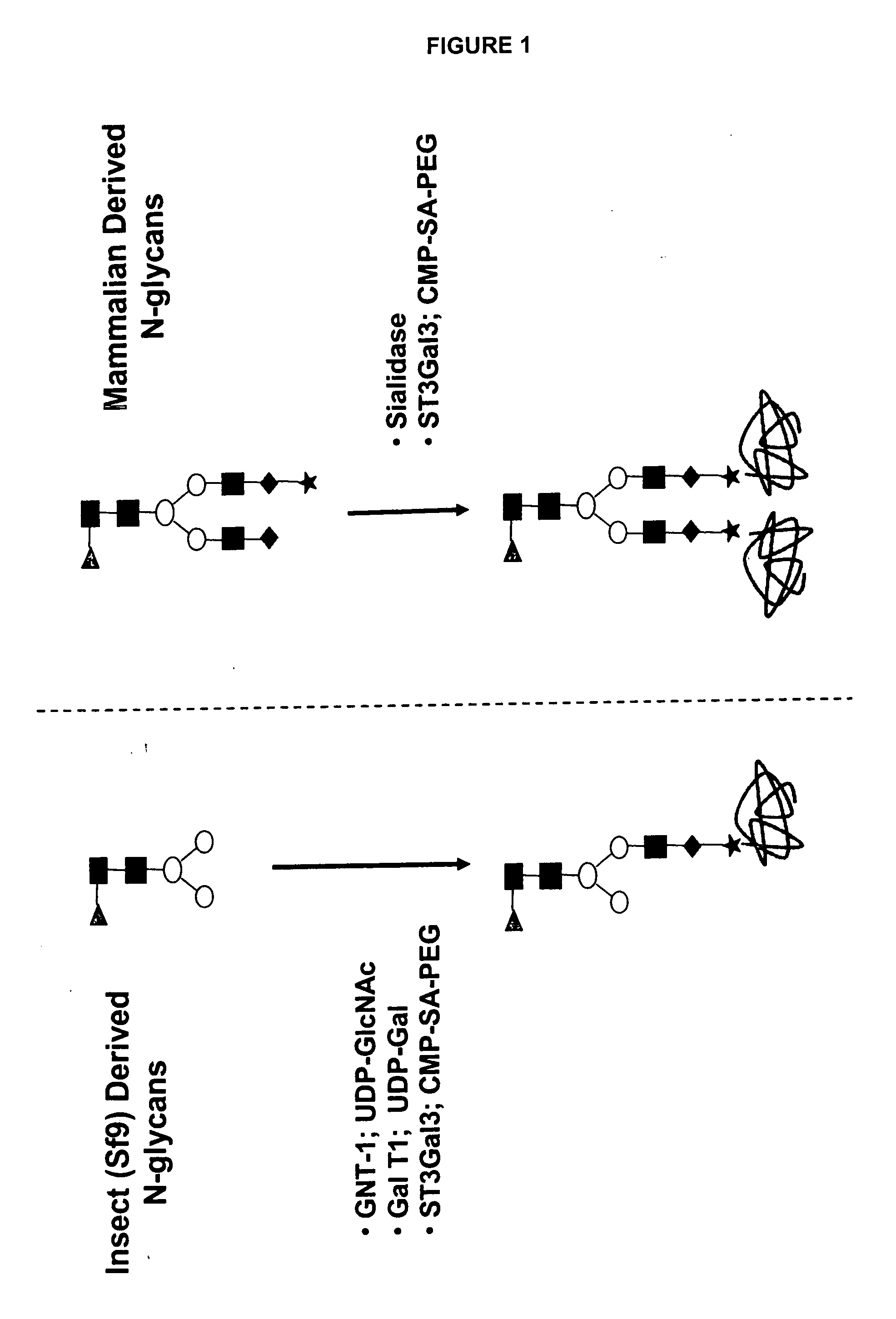
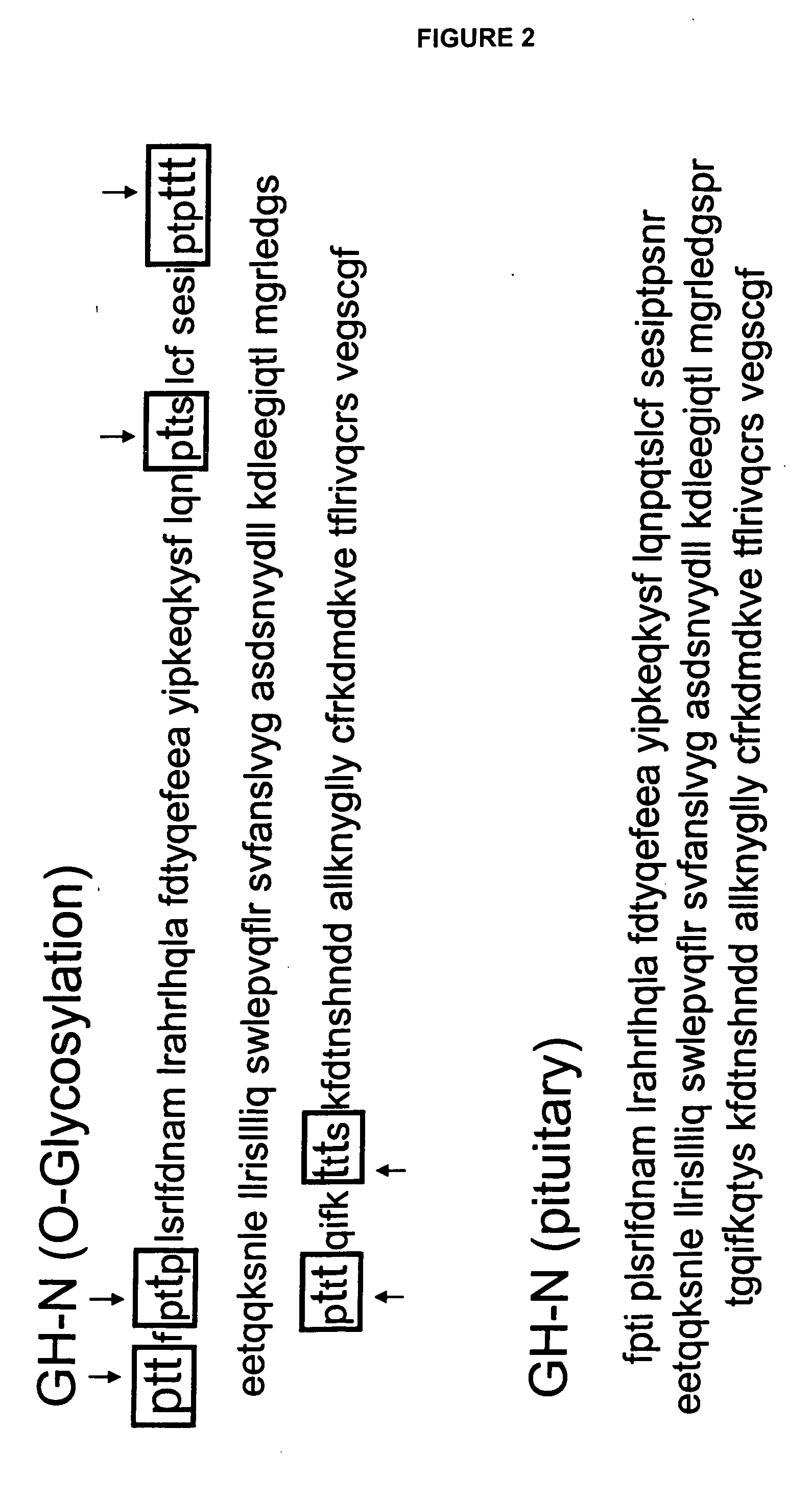
Compositions and methods for the preparation of protease resistant human growth hormone glycosylation mutants
The present invention relates to protease resistant mutants of human growth hormone, which contain newly introduced proteolysis resistant mutations and N-linked or O-linked glycosylation site(s), such that these recombinantly produced polypeptides have glycosylation patterns distinctly different from that of the naturally occurring human growth hormone. The polynucleotide coding sequences for the mutants, expression cassettes comprising the coding sequences, cells expressing the mutants, and methods for producing the mutants are also disclosed. Further disclosed are pharmaceutical compositions comprising the mutants and method for using the mutants.
Owner:NOVO NORDISK AS +1
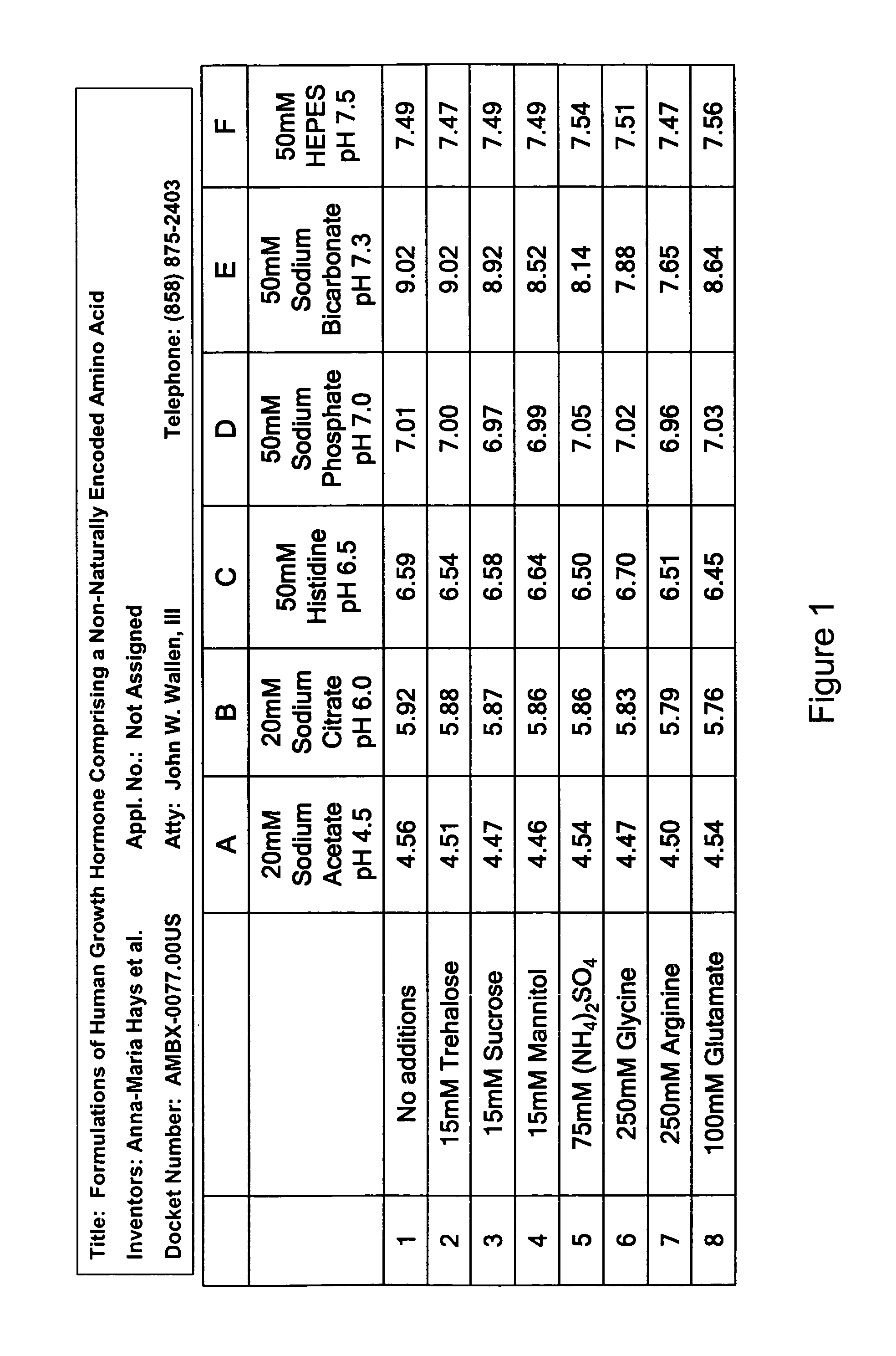
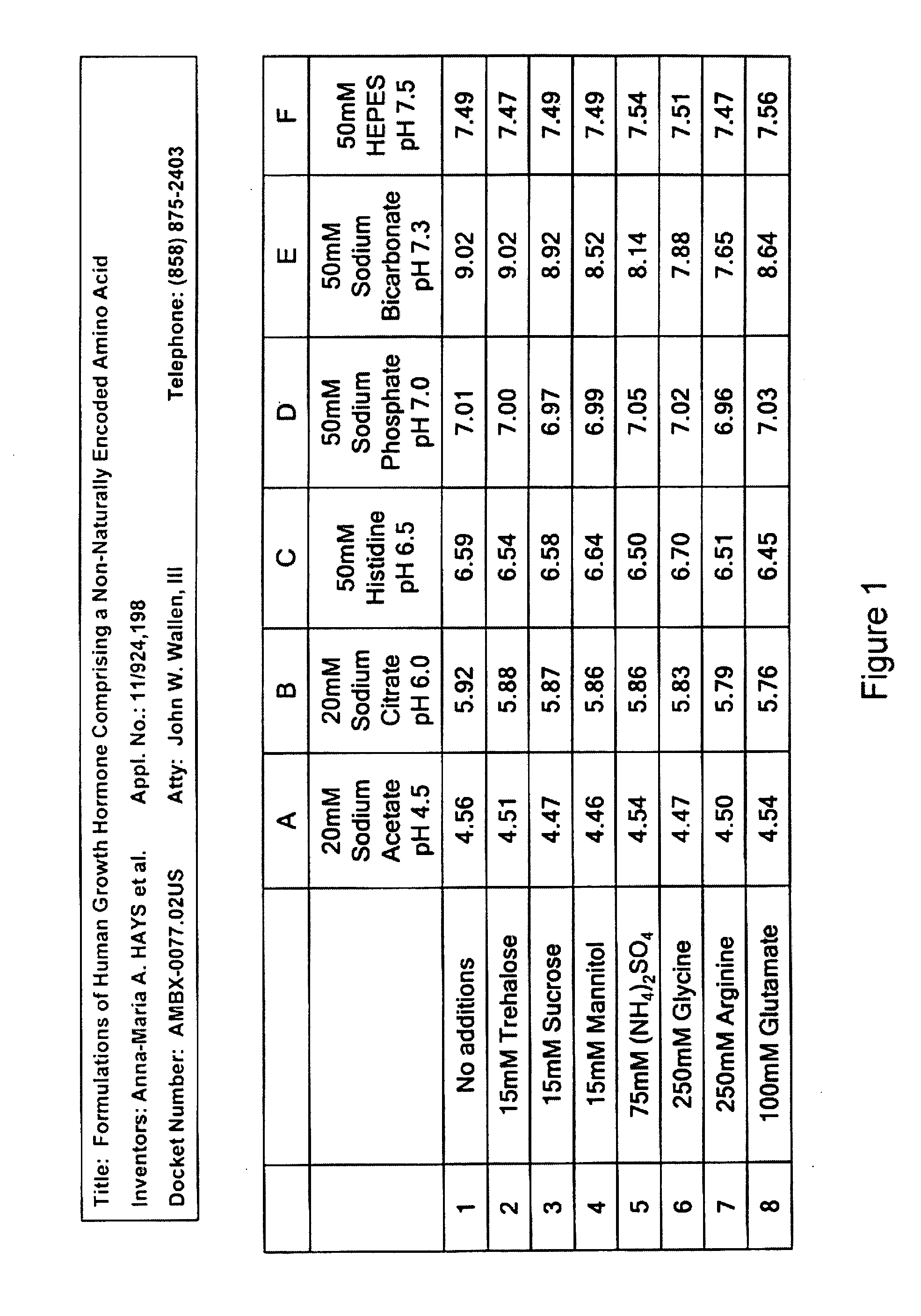
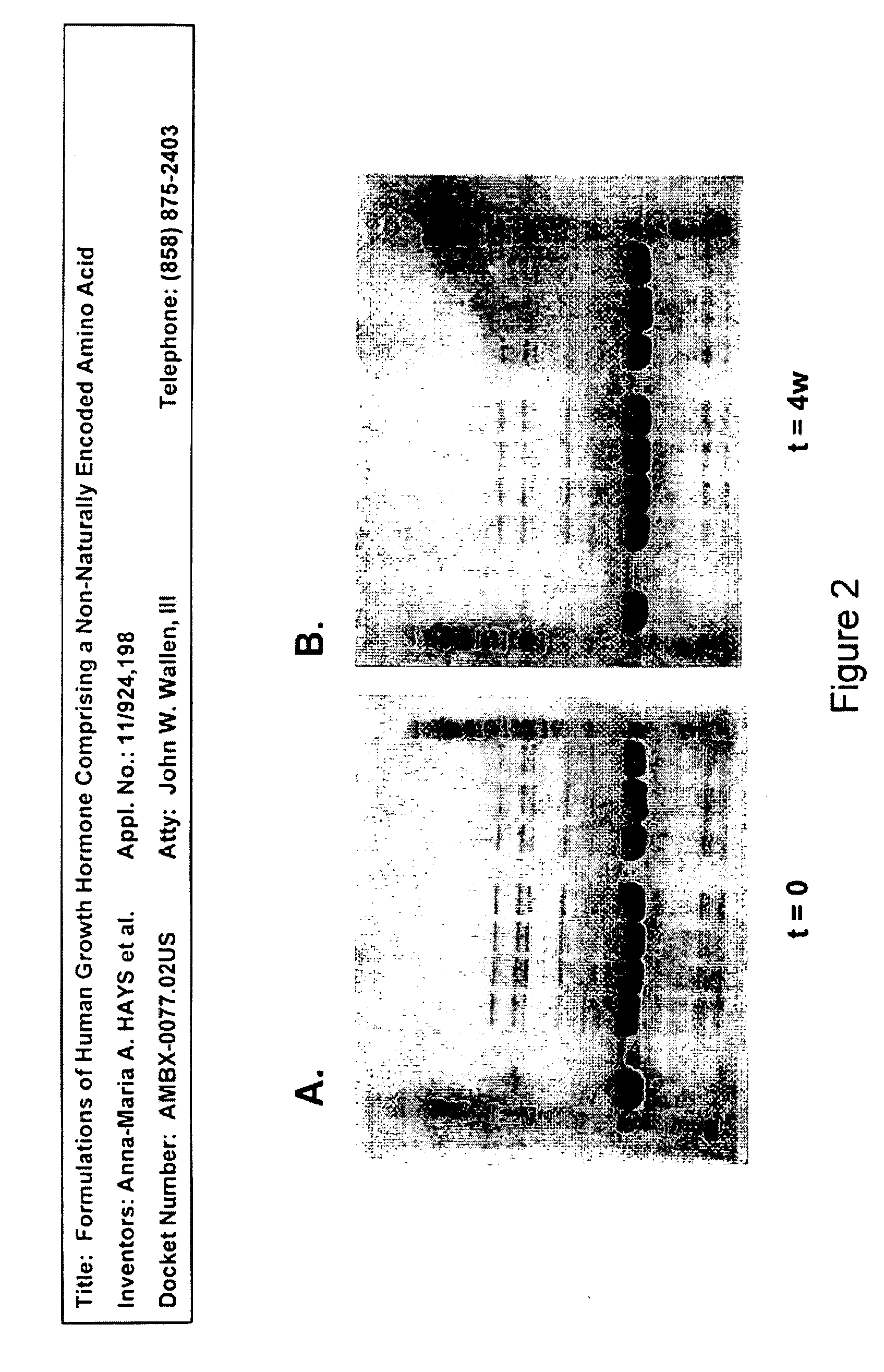
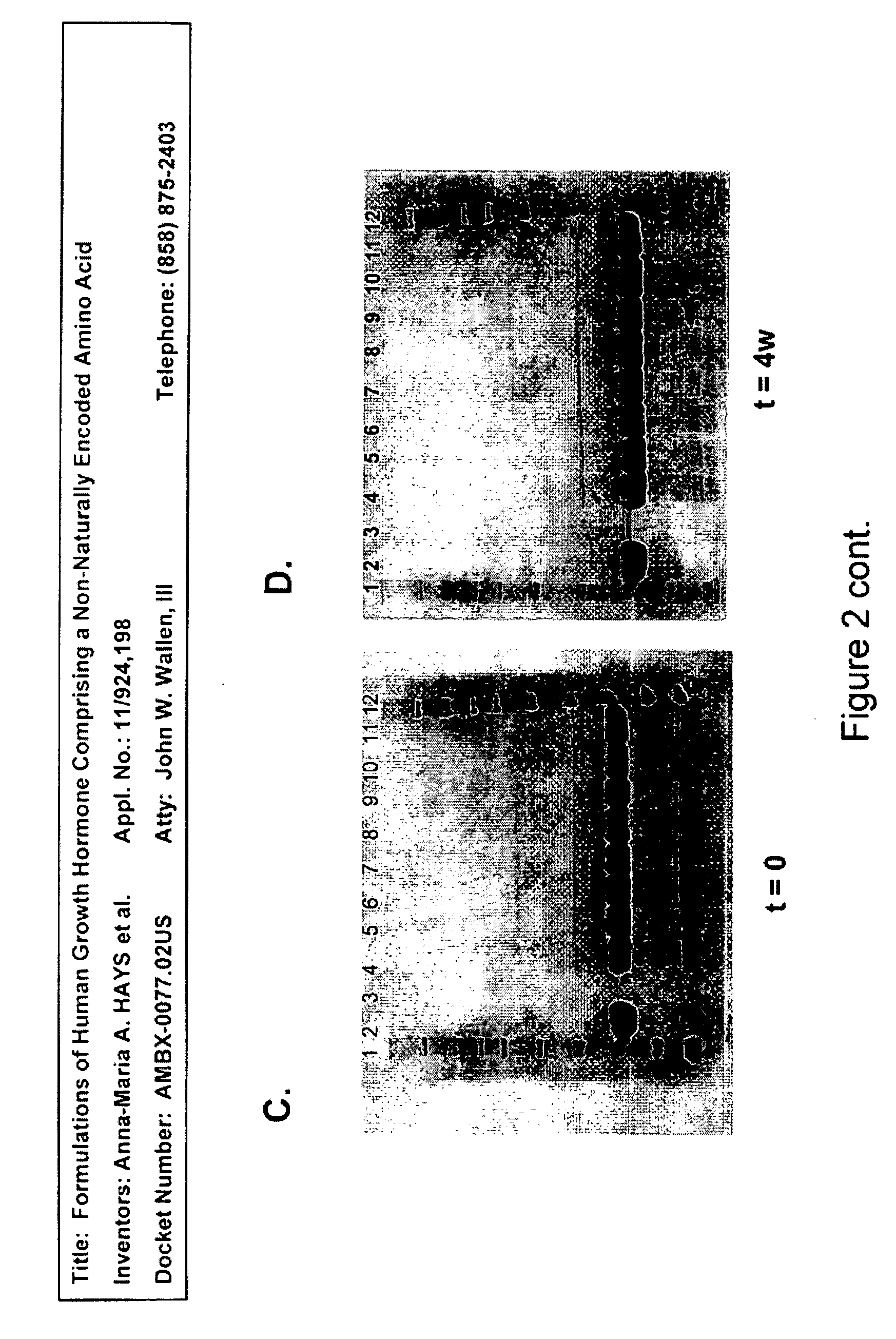
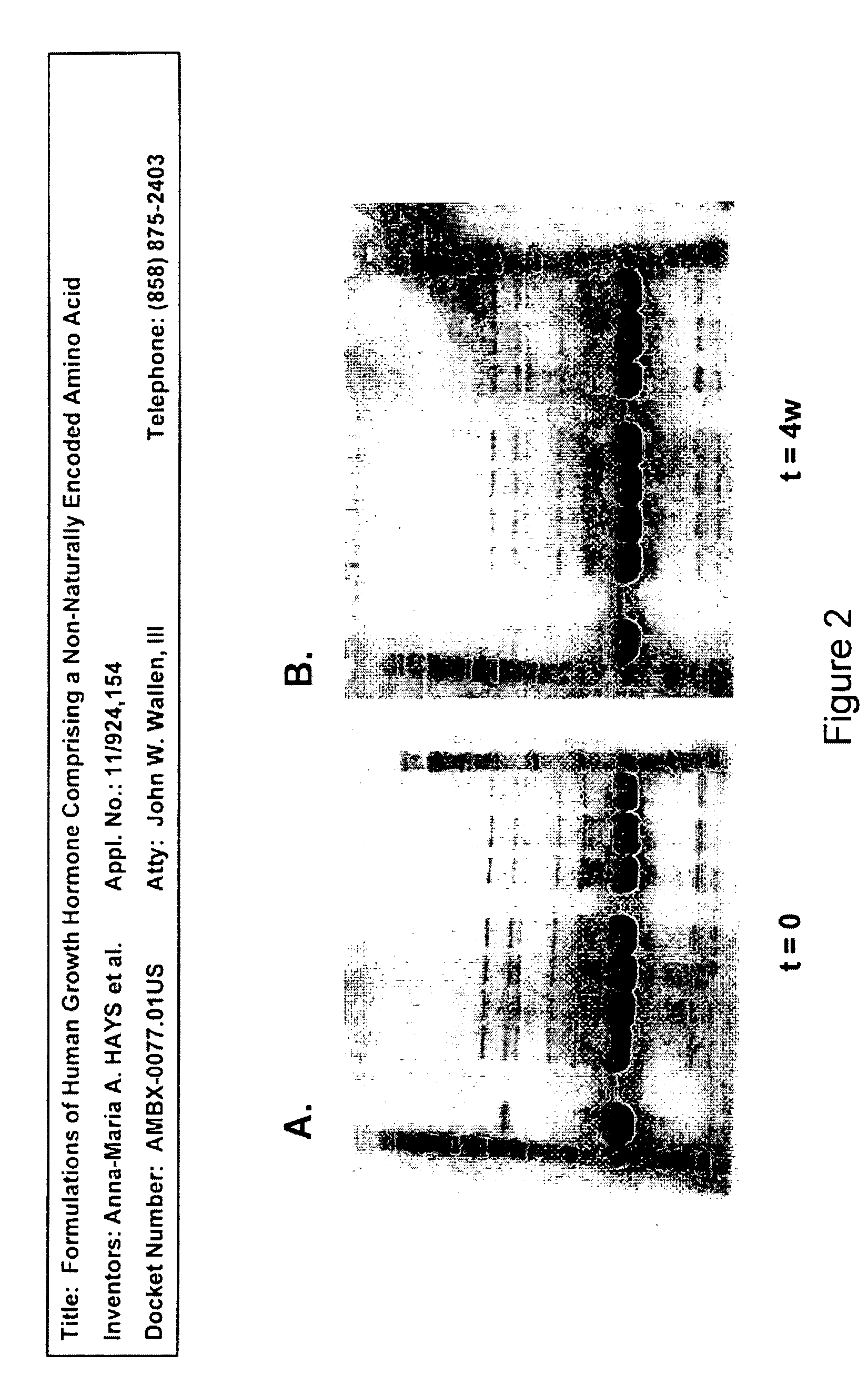
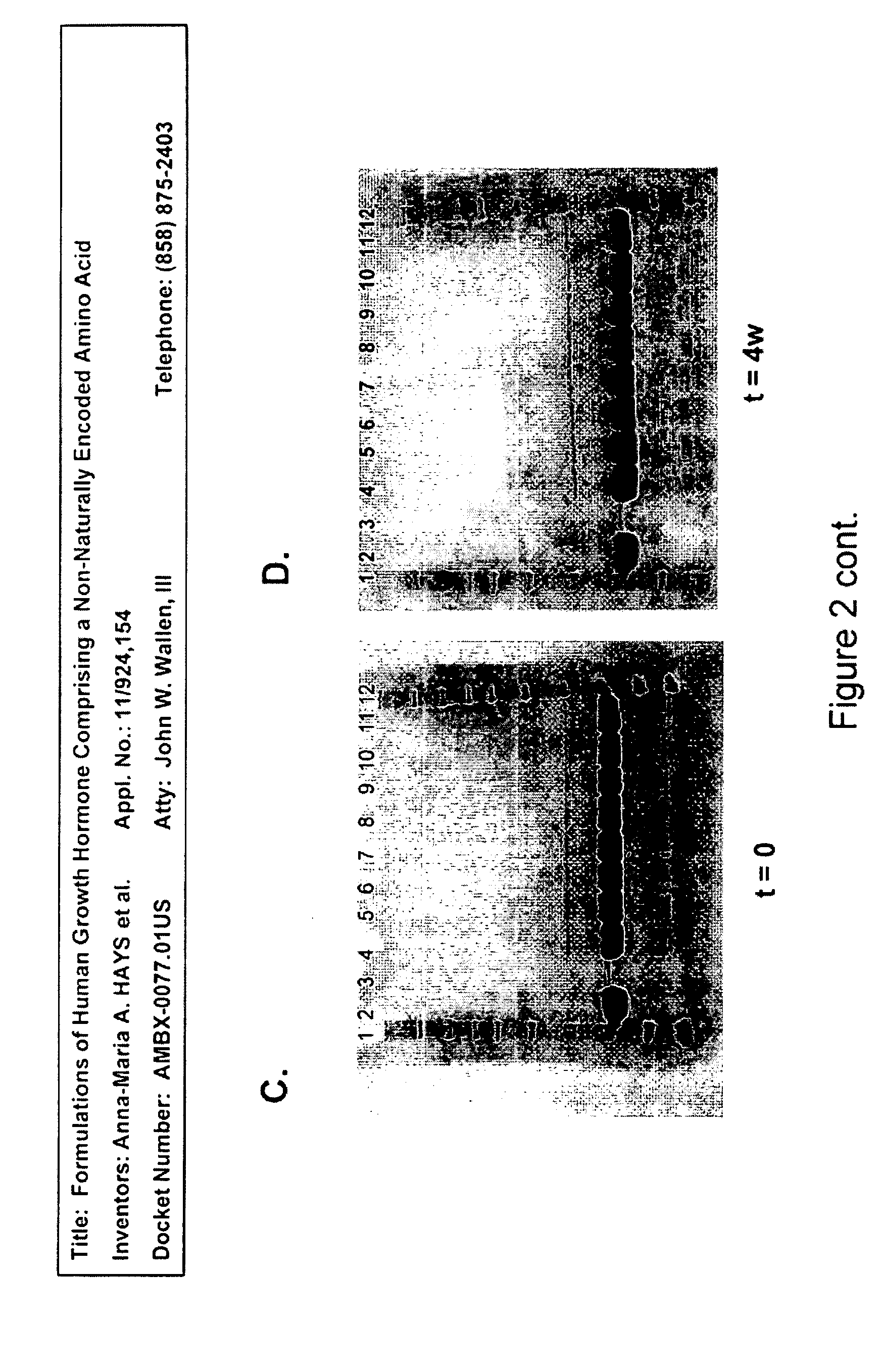
Formulations of human growth hormone comprising a non-naturally encoded amino acid at position 35
InactiveUS7816320B2Minimize formationReduced activityPeptide/protein ingredientsSkeletal disorderSomatotropic hormoneHuman growth hormone
Owner:AMBRX
Compositions and methods for the preparation of human growth hormone glycosylation mutants
InactiveUS7932364B2Peptide/protein ingredientsPeptide preparation methodsSomatotropic hormoneHuman growth hormone
The present invention relates to mutants of human growth hormone, which contain newly introduced N-linked or O-linked glycosylation site(s), such that these recombinantly produced polypeptides have glycosylation patterns distinctly different from that of the naturally occurring human growth hormone. The polynucleotide coding sequences for the mutants, expression cassettes comprising the coding sequences, cells expressing the mutants, and methods for producing the mutants are also disclosed. Further disclosed are pharmaceutical compositions comprising the mutants and method for using the mutants.
Owner:NOVO NORDISK AS
Compositions and methods for the preparation of protease resistant human growth hormone glycosylation mutants
InactiveUS20070154992A1Sugar derivativesPeptide/protein ingredientsHuman growth hormoneProtease resistant
The present invention relates to protease resistant mutants of human growth hormone, which contain newly introduced proteolysis resistant mutations and N-linked or O-linked glycosylation site(s), such that these recombinantly produced polypeptides have glycosylation patterns distinctly different from that of the naturally occurring human growth hormone. The polynucleotide coding sequences for the mutants, expression cassettes comprising the coding sequences, cells expressing the mutants, and methods for producing the mutants are also disclosed. Further disclosed are pharmaceutical compositions comprising the mutants and method for using the mutants.
Owner:NOVO NORDISK AS
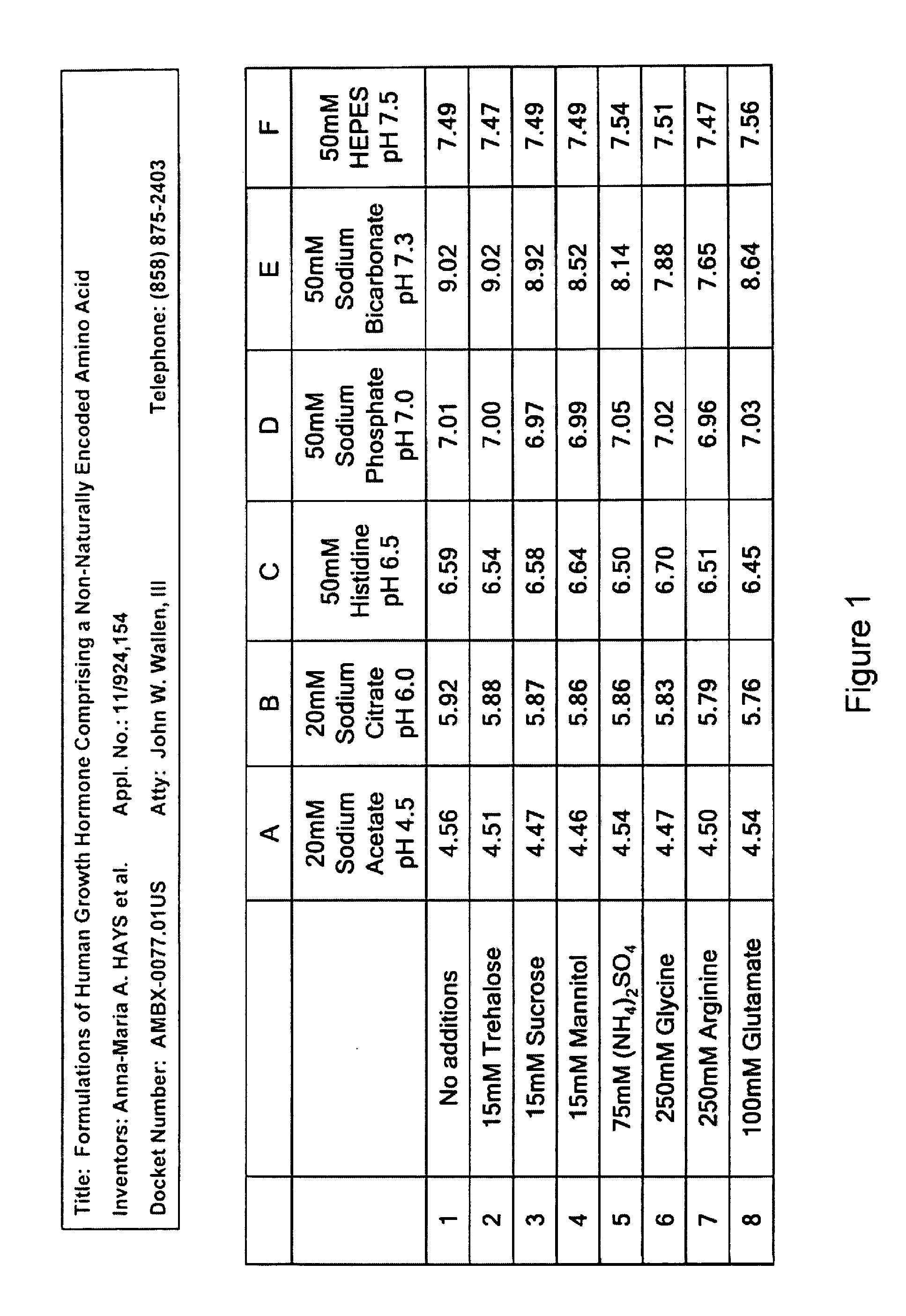
Formulations of human growth hormone comprising a non-naturally encoded amino acid
InactiveUS20060135427A1Minimize formationReduce biological activityPeptide/protein ingredientsSkeletal disorderHuman growth hormoneGrowth hormone preparation
Owner:AMBRX
Modified human growth hormone
InactiveUS20060189529A1Good water solubilityImprove solubilityAntibacterial agentsSugar derivativesHuman growth hormoneSomatropin
Owner:AMBRX
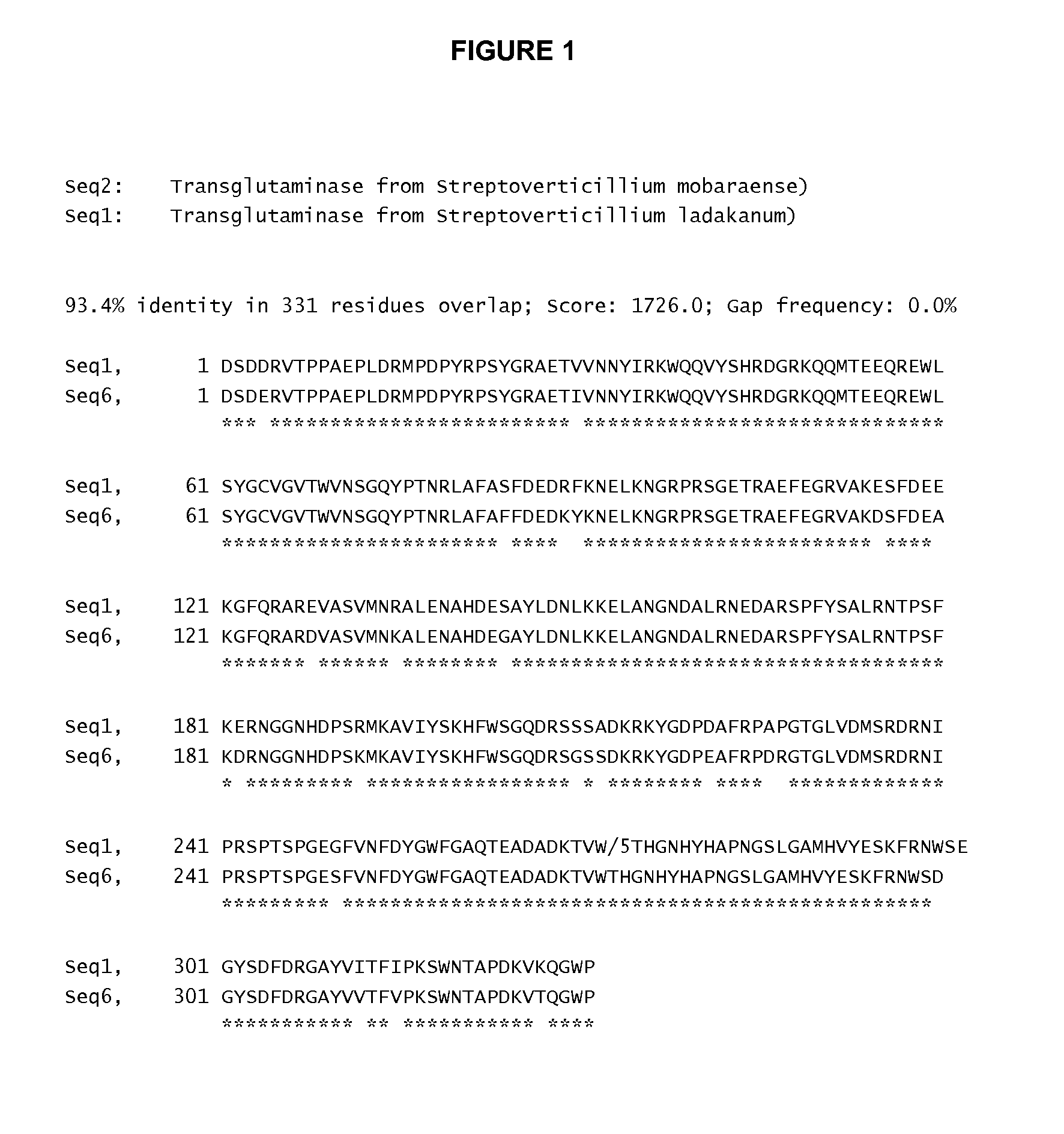
Transglutaminase Variants with Improved Specificity
InactiveUS20100087371A1Enhance site-specificityFungiBacteriaHuman growth hormoneStreptoverticillium ladakanum
Variants of transglutaminase from Streptoverticillium ladakanum, which variants have improved selectivity for Gln-141 of human growth hormone are provided.
Owner:NOVO NORDISK AS
Transglutaminase variants with improved specificity
InactiveUS20090318349A1High site-specificityEnhance site-specificityFungiBacteriaHuman growth hormoneStreptoverticillium ladakanum
Variants of transglutaminase from Streptoverticillium ladakanum, which variants have improved selectivity for Gln-141 of human growth hormone are provided.
Owner:NOVO NORDISK AS
Transglutaminase Variants with Improved Specificity
InactiveUS20100099610A1High site-specificityPeptide/protein ingredientsAntipyreticHuman growth hormoneStreptoverticillium ladakanum
Variants of transglutaminase from Streptoverticillium ladakanum, which variants have improved selectivity for Gln-141 of human growth hormone are provided.
Owner:NOVO NORDISK HEALTH CARE AG
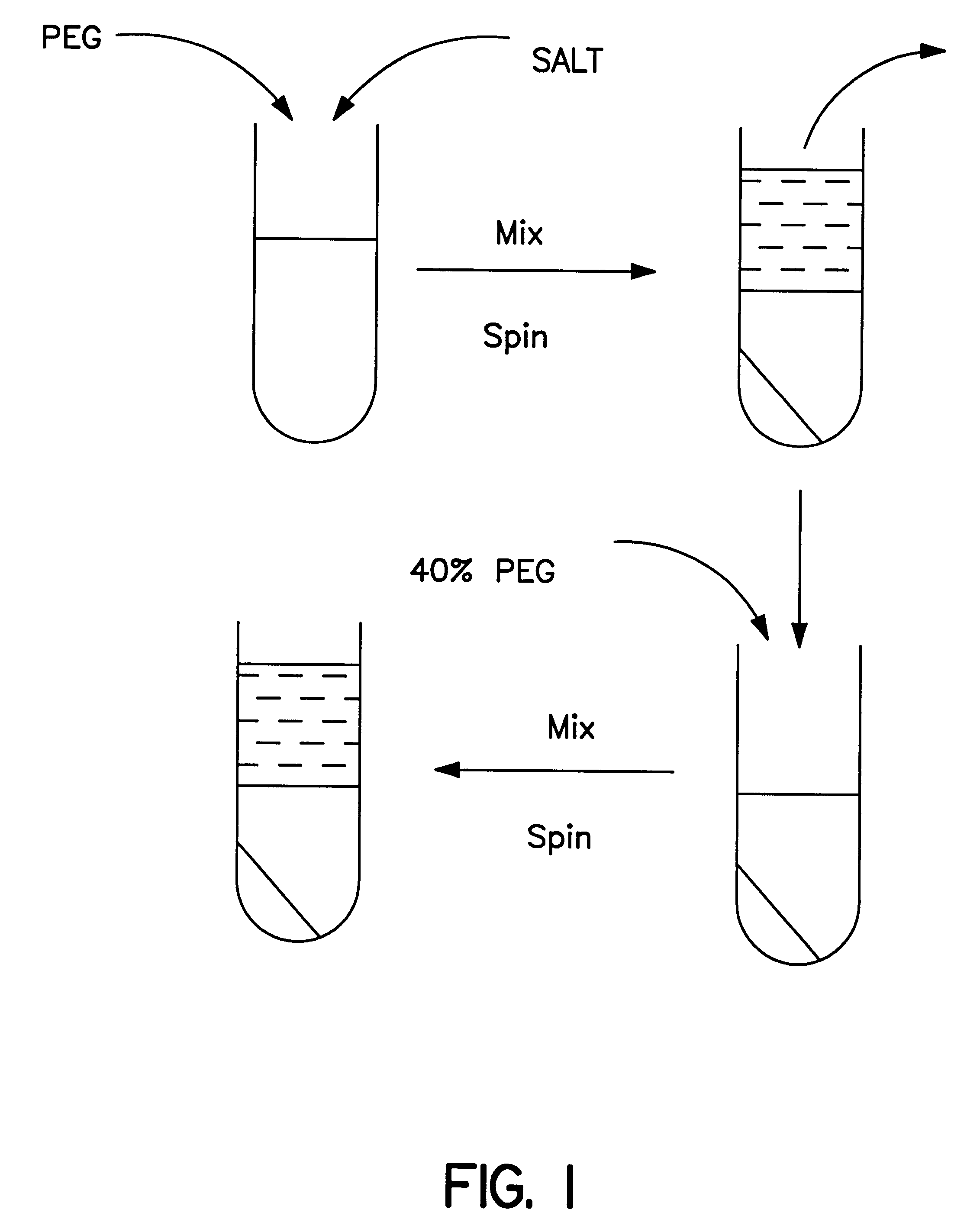
Methods for protein purification using aqueous two-phase extraction
Methods are provided in this invention for the isolation of human growth hormone, growth hormone antagonist, or a homologue of either, from a biological source. The methods of the invention use multi-phase extraction.
Owner:NV ORGANON +1
Modified Human Growth Hormone
InactiveUS20080102124A1Good water solubilityImprove solubilityBiocidePowder deliveryHuman growth hormoneSomatropin
Owner:AMBRX
Pegylated recombinant human growth hormone compounds
ActiveUS20110112021A1Reduce dosing frequencyReduced activityNervous disorderAntipyreticLipoatrophyDisease
A chemically modified human Growth Hormone (rhGH) prepared by attaching a transient linker which comprises a polyethylene glycol. The chemically modified protein may have a much longer lasting rhGH activity than that of the unmodified rhGH, enabling reduced dose and scheduling opportunities and the modified rhGH may not cause lipoatrophy. Also includes methods of use for the treatment and / or prevention of diseases or disorders in which use of growth hormone is beneficial.
Owner:ASCENDIS PHARM AS
Human growth hormone formulations
InactiveUS20080095837A1Organic active ingredientsPeptide/protein ingredientsDiseaseBuccal administration
The present invention relates to dosage forms of human growth hormone, the use of an absorption enhancer to allow absorption of human growth hormone into the systemic circulation in a biologically active form, in particular after oral administration, as well as the use of oral dosage forms comprising human growth hormone and an absorption enhancer for the treatment of human growth hormone deficiencies and disorders associated therewith.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Methods for Expression and Purification of Recombinant Human Growth Hormone
InactiveUS20080050777A1Sugar derivativesPeptide/protein ingredientsSomatotropic hormoneHuman growth hormone
The present invention relates generally to the production, purification, and isolation of human growth hormone (hGH). More particularly, the invention relates to the production, purification, and isolation of substantially purified hGH from recombinant host cells or culture medium including, for example, yeast, insect, mammalian and bacterial host cells. The process of the present invention is also useful for purification of hGH linked to polymers or other molecules.
Owner:AMBRX
Biological modifying and recombinant human growth hormone compound and preparing method thereof
InactiveCN1528787AEasy to maintain biological activityImprove biostabilityCarrier-bound/immobilised peptidesGrowth hormonesMonomethoxypolyethylene glycolHalf-life
The invention is a bio-modified recombinant growth hormone (rhGH) complex and preparing method, making glutamine residue-containing rhGH react with amino donor polyethylene glycol alkyl amine PEG or monomethoxypolyethylene glycol (mPEG) under the catalysis of transglutaminase (mTG), to make acylamide in gamma-bit of the glutamine residue and amino in primary amine bit of the amino donor form an amido bond, thus obtaining it, namely PEG-rhGH or Mpeg-rhGH. It has high biostability and long in vivo half life, applied to prepare permanent injection drugs or develop oral drugs.
Owner:EAST CHINA NORMAL UNIV
Enrichment method for variant proteins with altered binding properties
InactiveUS20080038717A1Integrity of the rigid secondary structuresPreserve integrityVirusesPeptide/protein ingredientsSomatotropic hormoneAntibody fragments
Owner:GENENTECH INC
Long-acting polypeptides and methods of producing and administering same
InactiveUS20150158926A1Peptide/protein ingredientsAntibody mimetics/scaffoldsHuman growth hormonePharmaceutical formulation
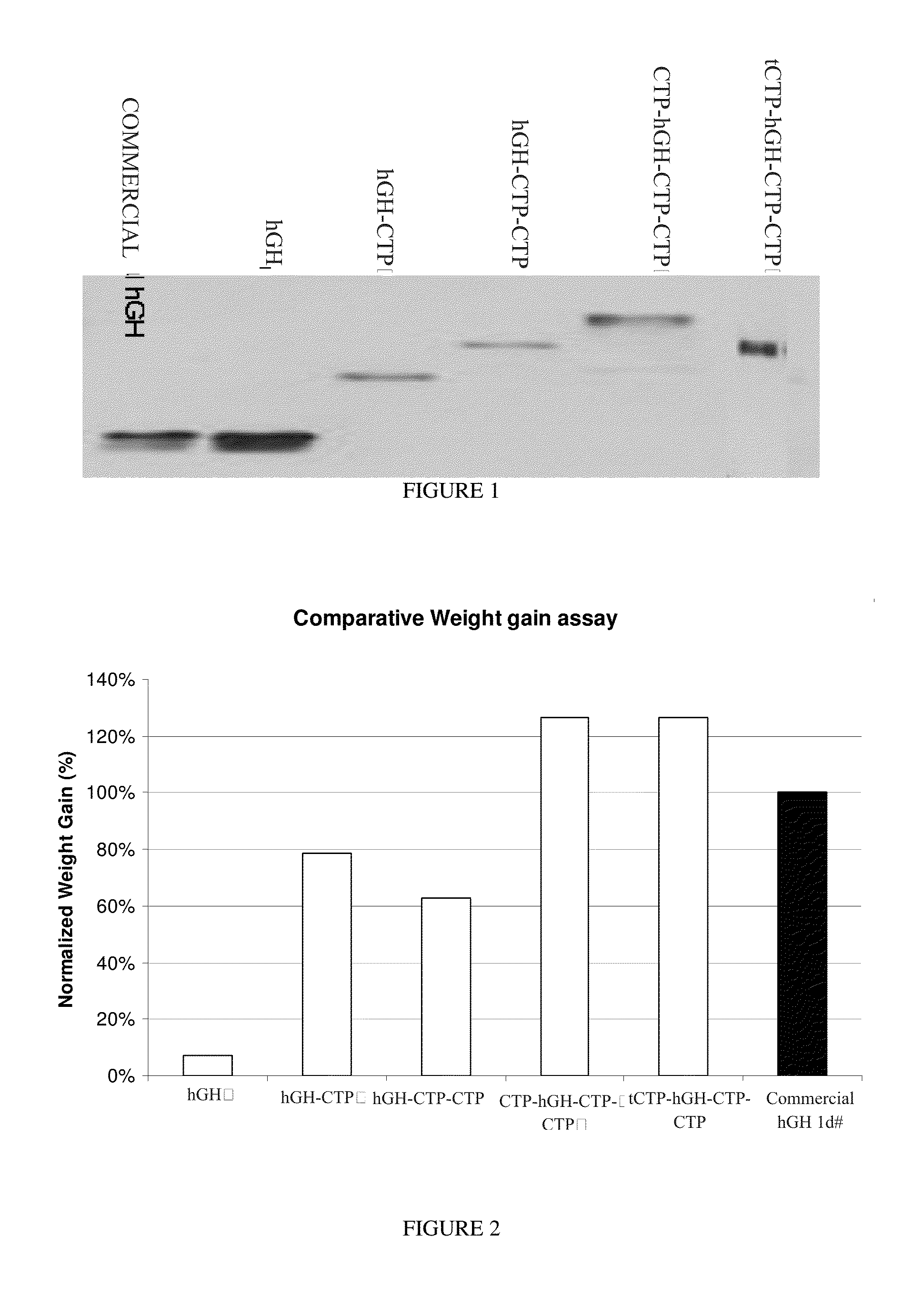
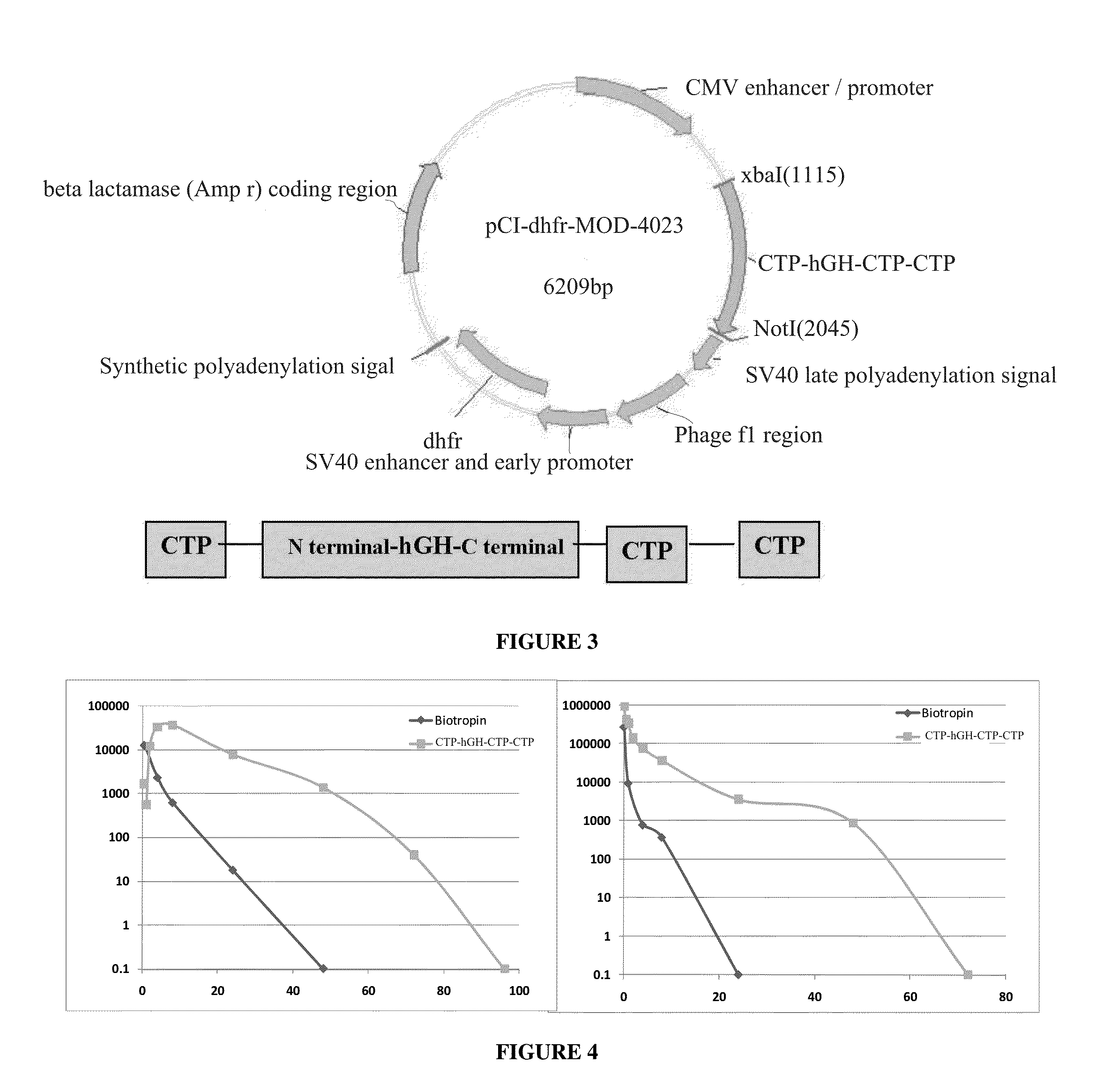
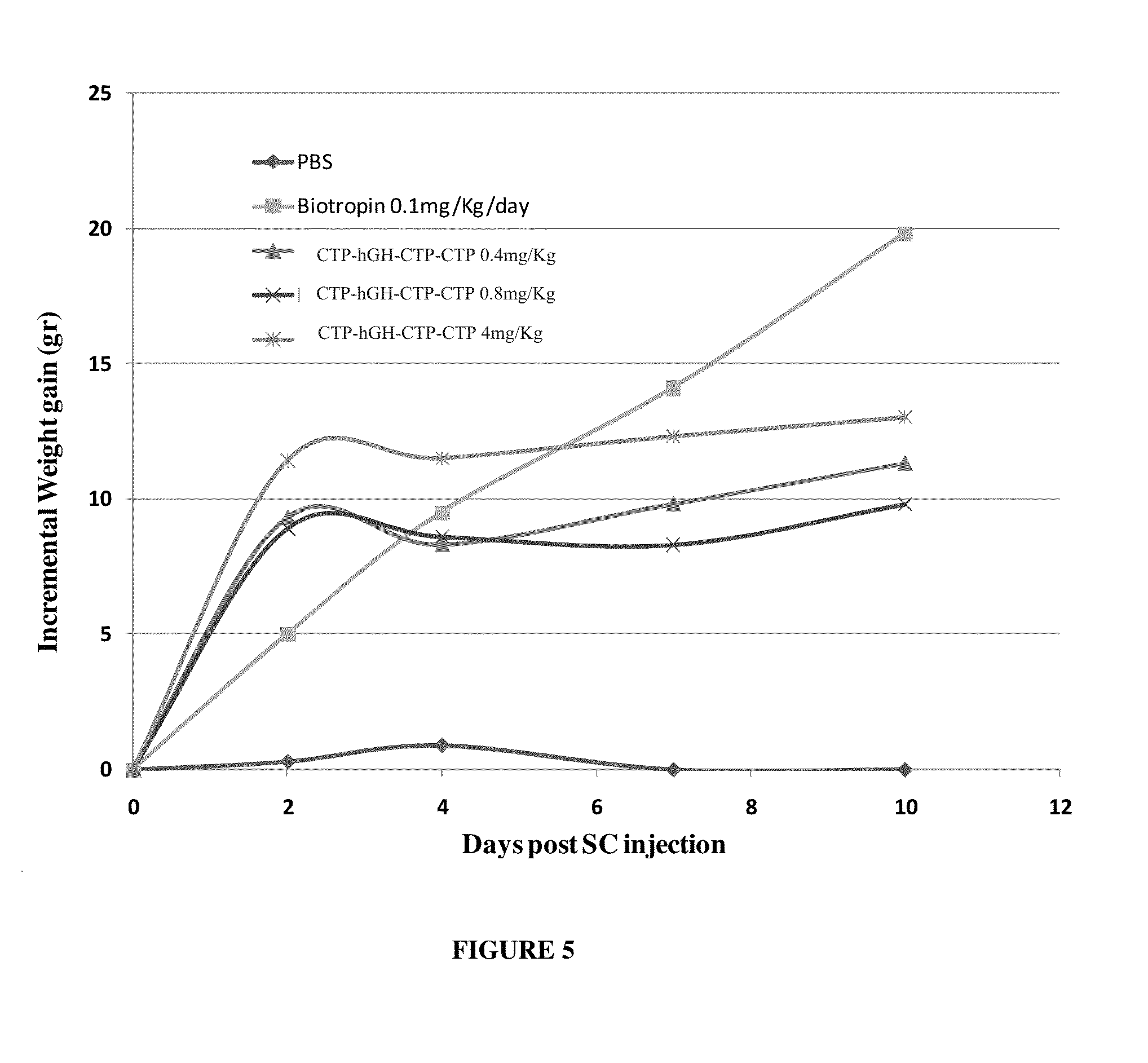
CTP-modified human growth hormone polypeptides and pharmaceutical formulations and pharmaceutical compositions comprising the same and methods of producing, and using the same are disclosed.
Owner:OPKO BIOLOGICS
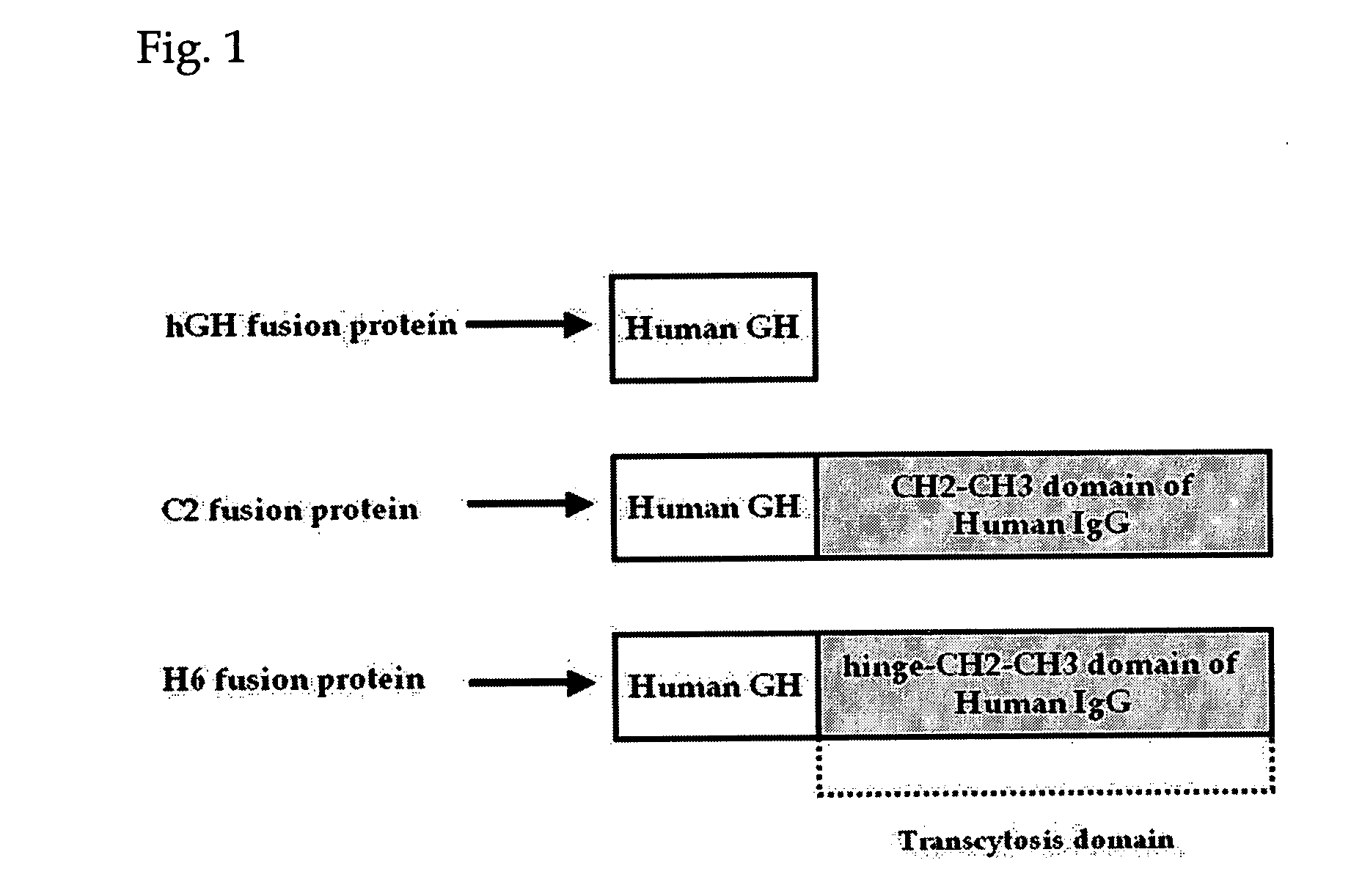
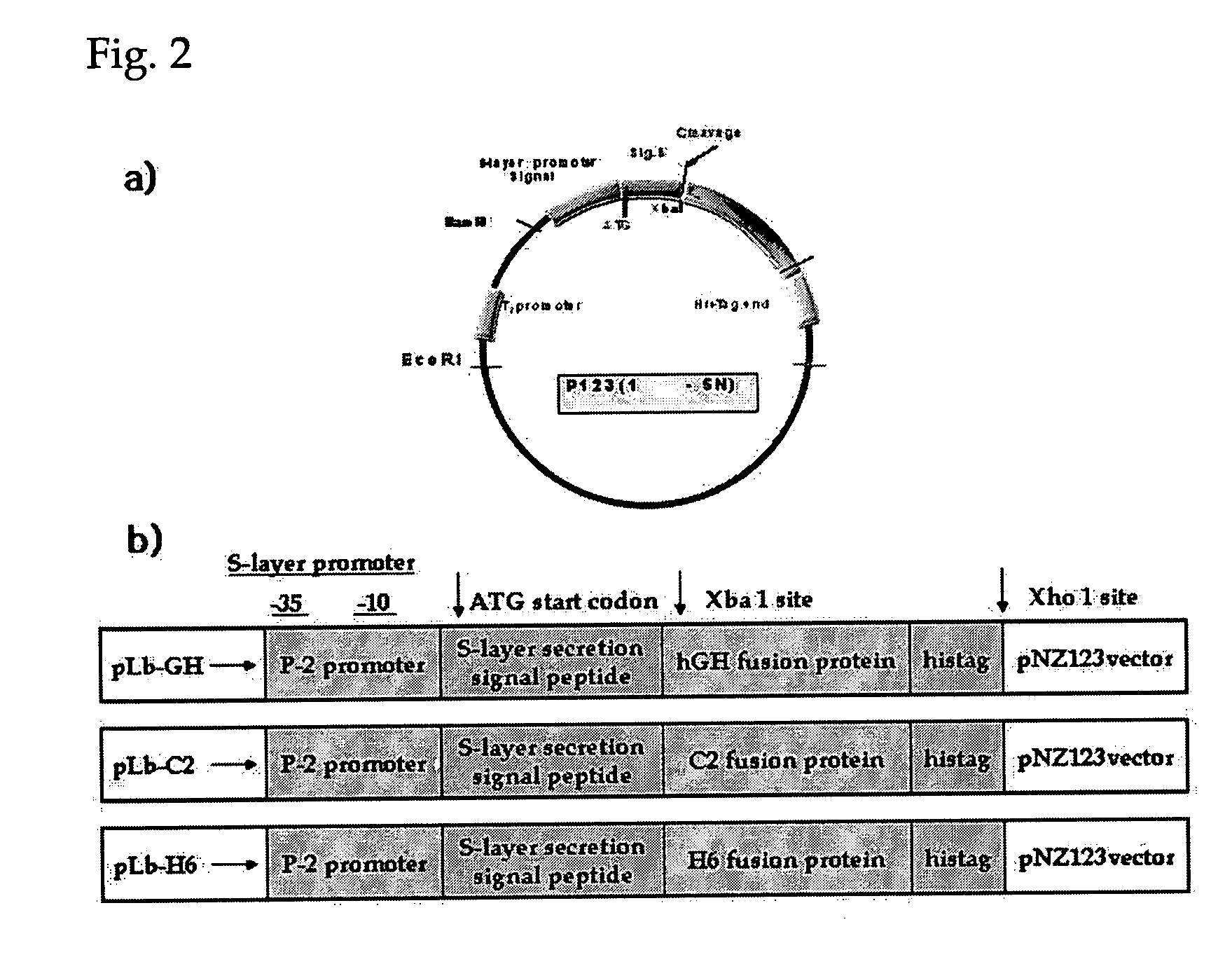
Probiotic microorganisms producing chimeric human growth hormone fused with Fc fragment of human IgG for oral delivery system and methods for producing them
The present invention relates to probiotic microorganisms producing chimeric human growth hormone for oral use and methods for preparing them. The invention provides probiotic Lactobacillus or yeast transformant expressing chimeric protein which is human growth hormone fused with Fc fragment of human IgG, in which the transformants are safely delivered into intestine though oral route. Also, the invention provides a chimeric protein-expressing vector which can induce transcytosis in intestine epithelial cells. Accordingly, the invention demonstrates that the chimeric protein for oral delivery system can be absorbed in intestine, and delivery of the chimeric protein by oral route using Lactobacillus has very excellent efficiency in vivo test in rats. Accordingly, the Lactobacillus of the present invention is an excellent deliverer of protein drugs.
Owner:INSILICO CO LTD
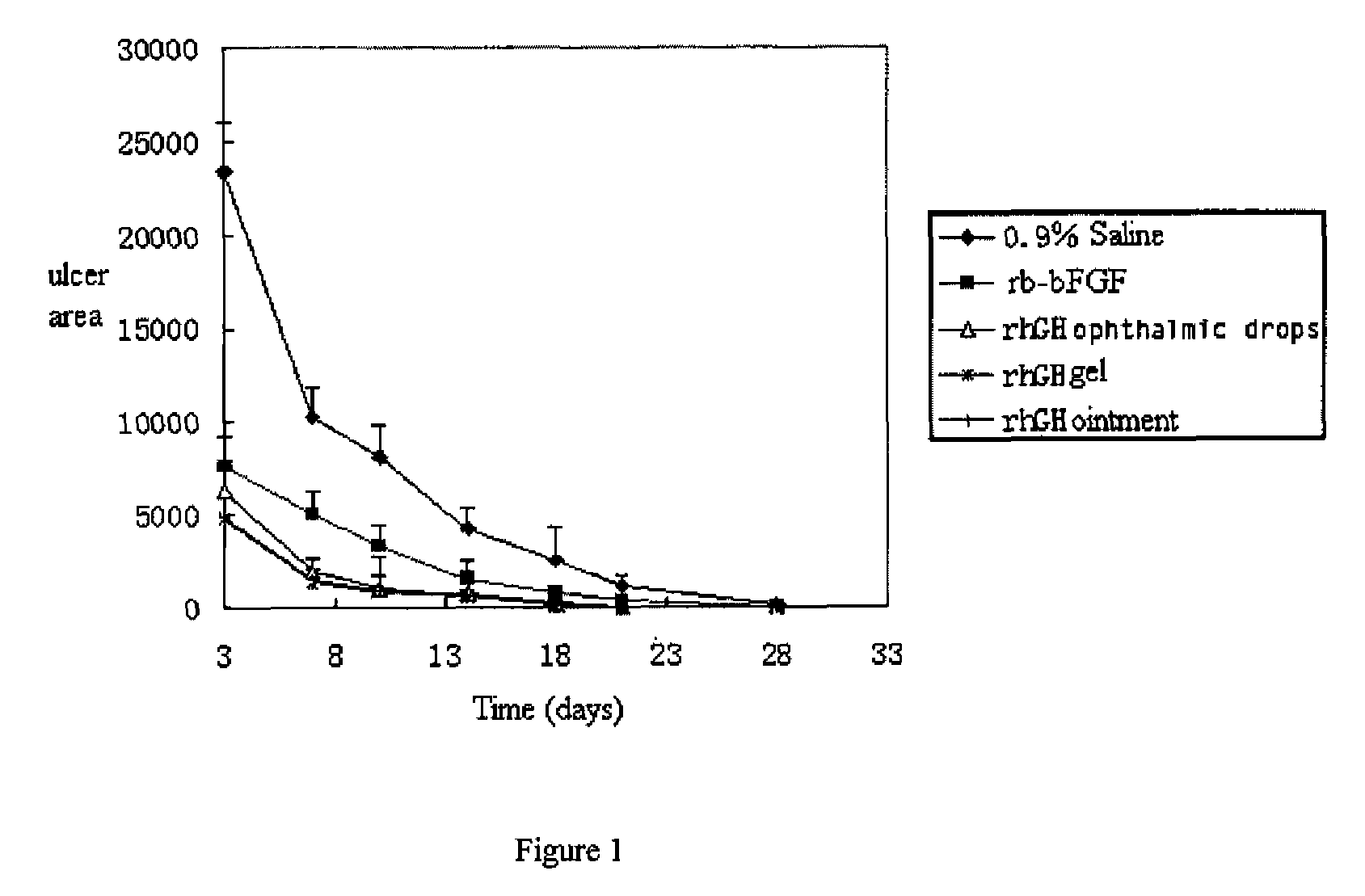
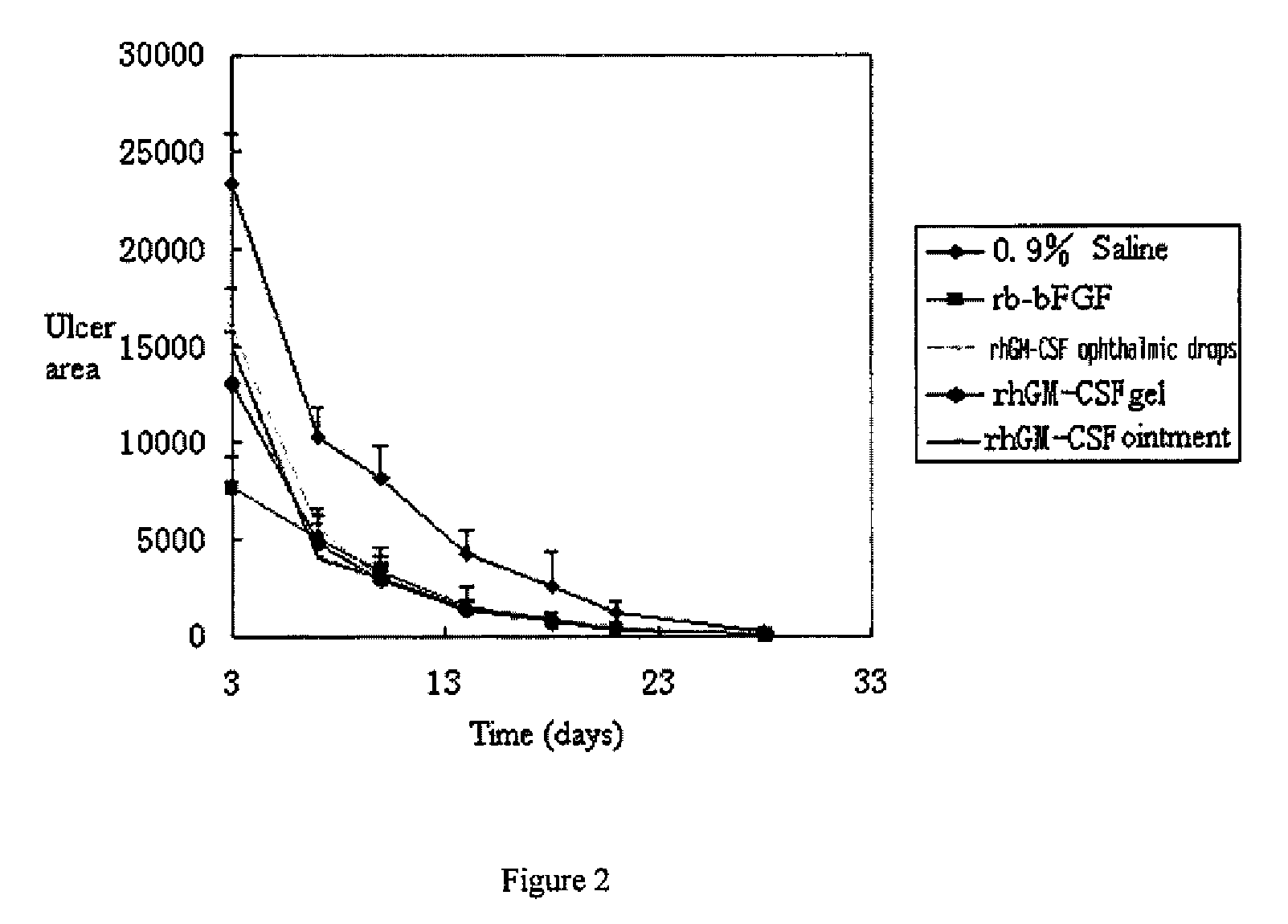
Ophthalmic hGM-CSF preparation
InactiveUS7858582B2Avoid excessive viscosityBenefit to drugSenses disorderPeptide/protein ingredientsGynecologyHuman growth hormone
Owner:CHANGCHUN GENESCIENCE PHARM CO LTD
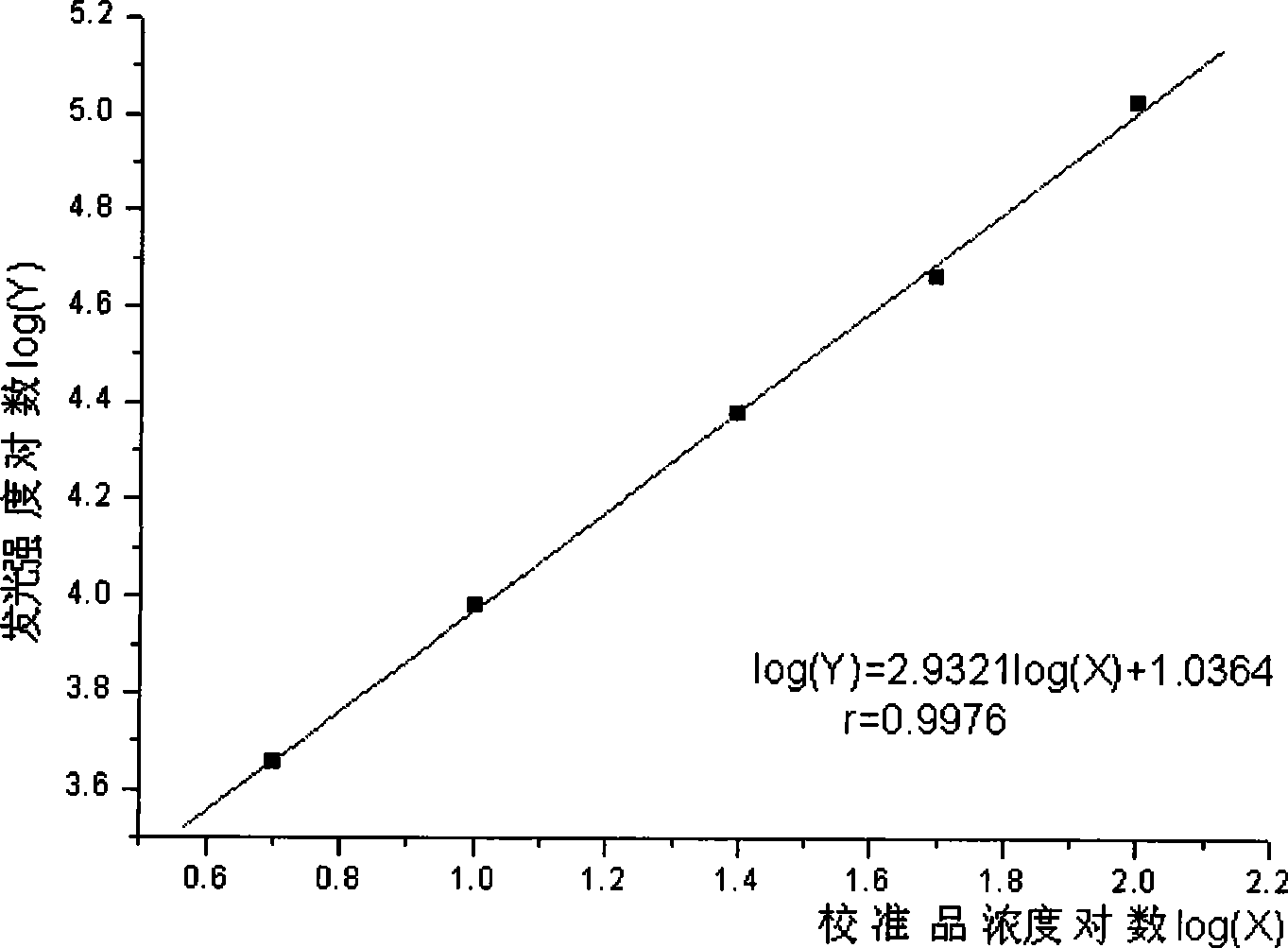
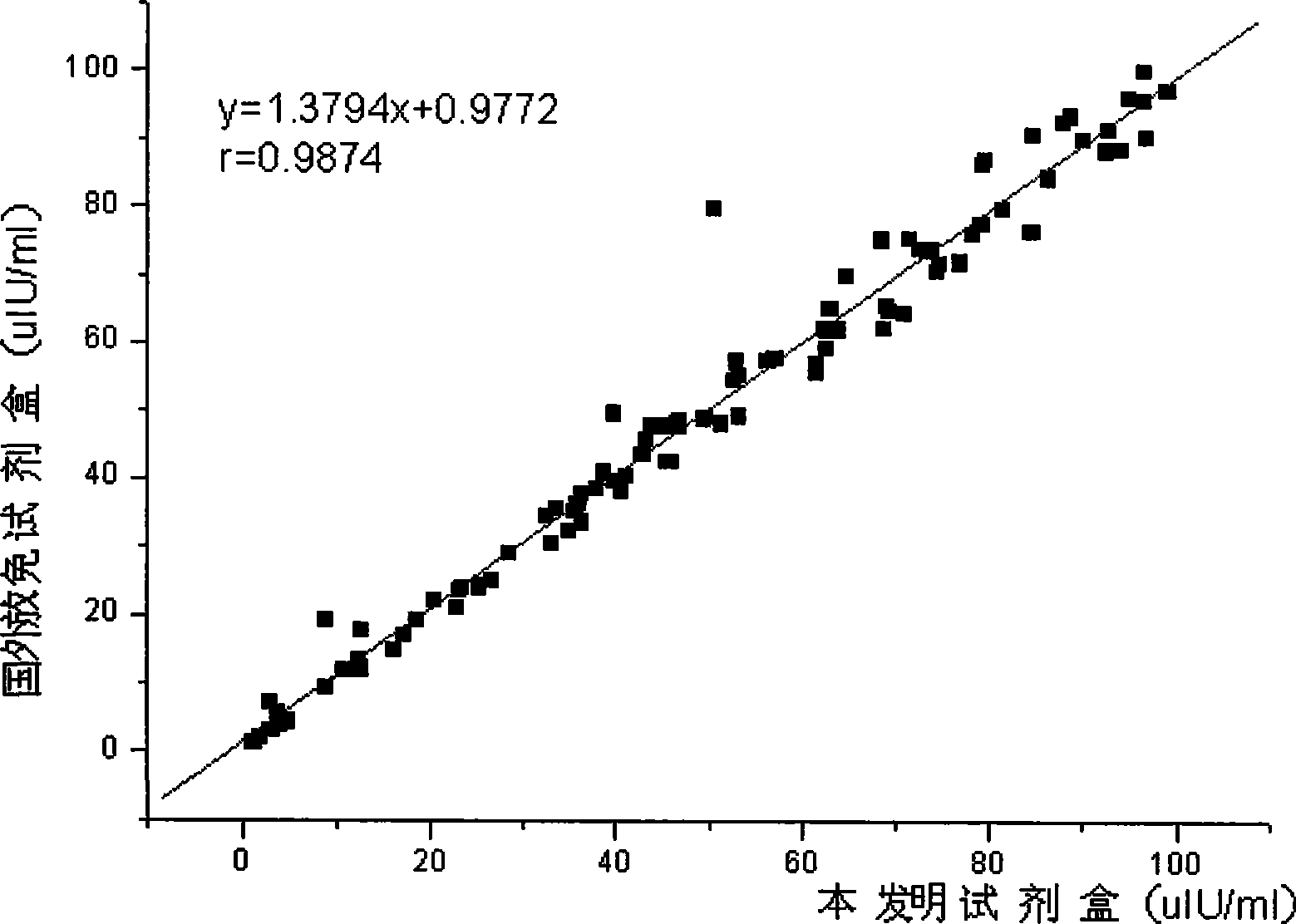
Chemical luminescence immune assay determination reagent kit for detecting human growth hormone
InactiveCN101368973AImprove performanceEasy to operateChemiluminescene/bioluminescenceBiological testingLuminescencePollution
The invention relates to the immunoassay field, in particular discloses a chemiluminescence immunoassay test kit and a preparing method thereof for detecting human growth hormone. The test kit uses principle of enzyme-catalyzed chemiluminescence, and adopts the micro hole plate as the solid-phase carrier so as to achieve batch detection. The monoclonal antibody can be labeled by alkaline phosphatase and horseradish peroxidase. Without radioactive pollution, the test kit has stable performance, simple operation, accurate and sensitive result and swift reaction speed.
Owner:北京科美东雅生物技术有限公司
Formulations of Human Growth Hormone Comprising a Non-Naturally Encoded Amino Acid
InactiveUS20080113913A1Minimize formationReduced activityPeptide/protein ingredientsSkeletal disorderHuman growth hormoneAmino acid
Owner:AMBRX
Formulations of Human Growth Hormone Comprising a Non-Naturally Encoded Amino Acid
InactiveUS20080113912A1Minimize formationReduced activityPeptide/protein ingredientsSkeletal disorderHuman growth hormoneAmino acid
Owner:AMBRX
Drug for recovering renal function
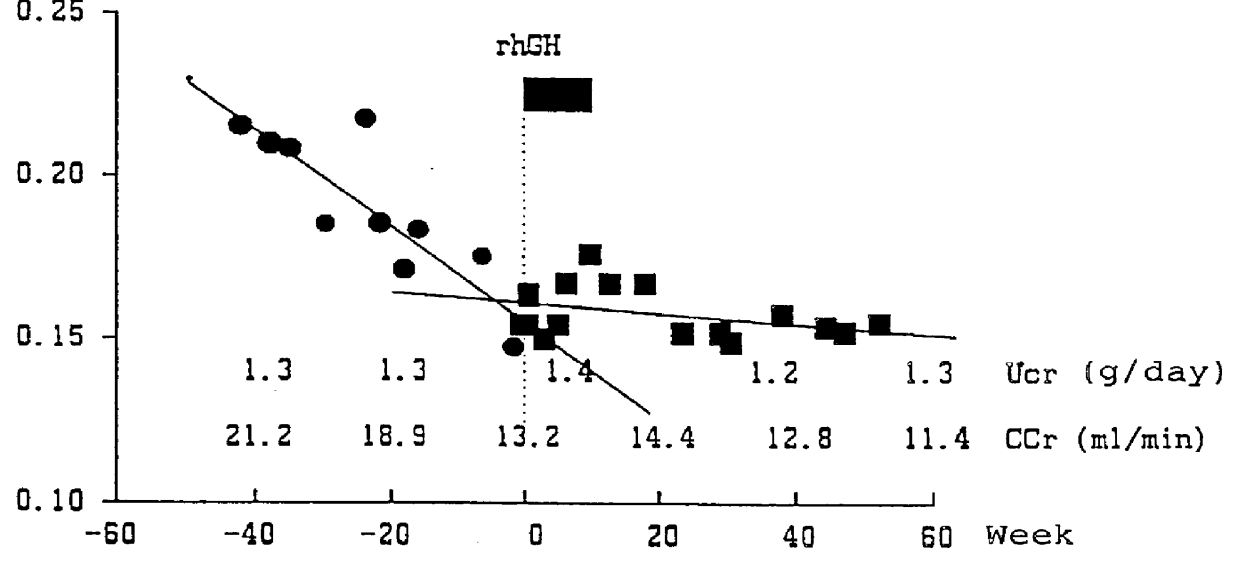
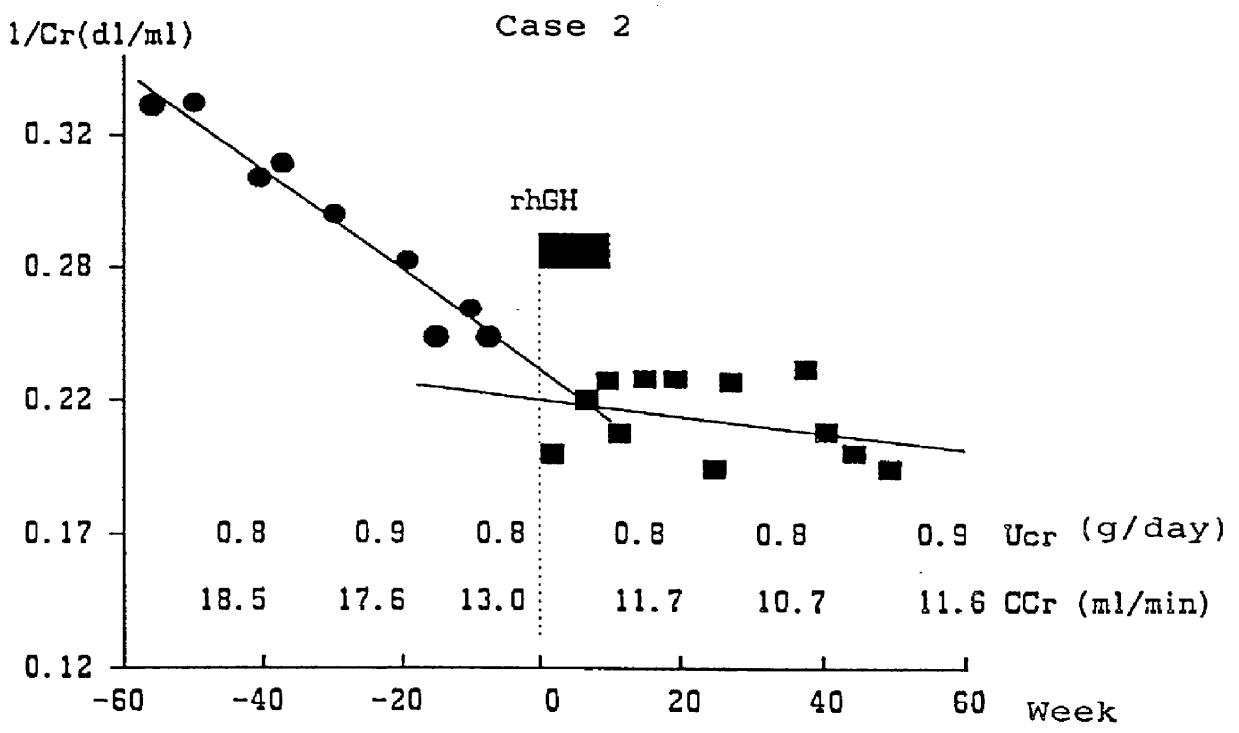
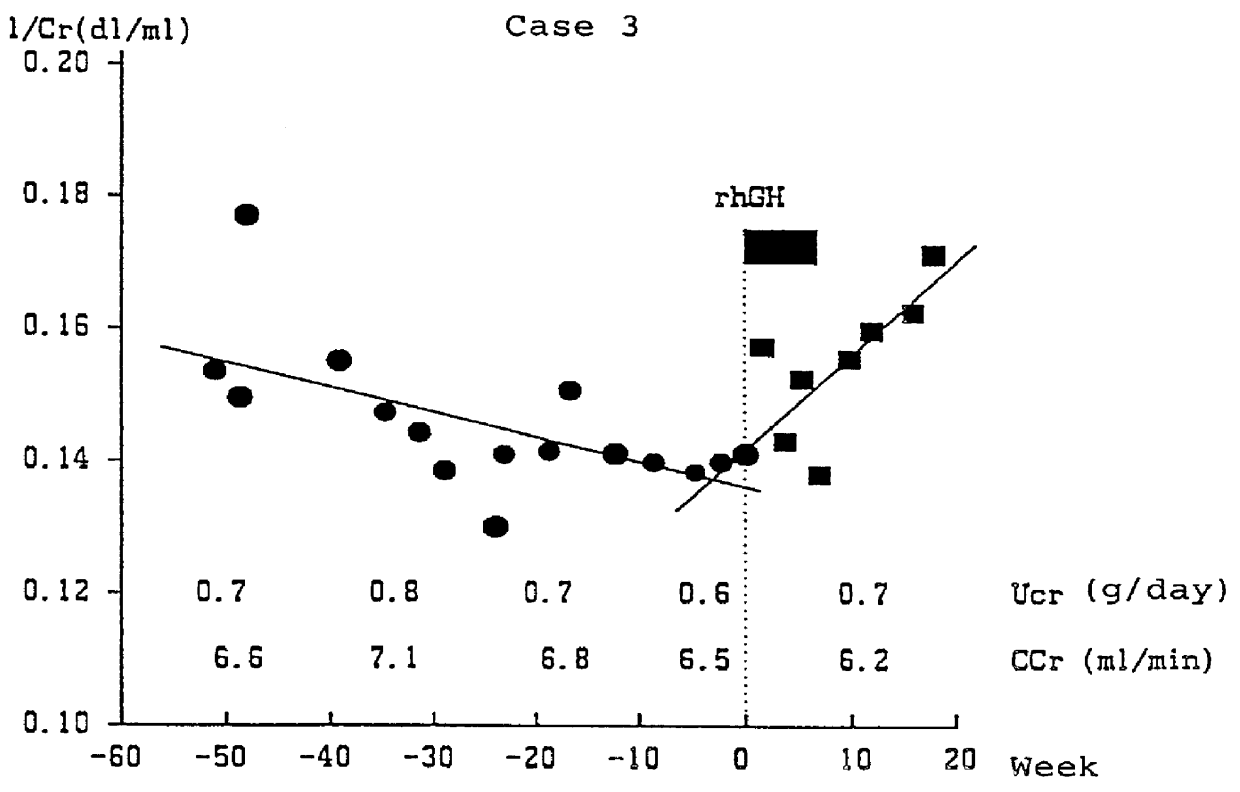
PCT No. PCT / JP96 / 01225 Sec. 371 Date Mar. 27, 1998 Sec. 102(e) Date Mar. 27, 1998 PCT Filed May 9, 1996 PCT Pub. No. WO97 / 12631 PCT Pub. Date Apr. 10, 1997Drug which contains human growth hormones capable of recovering the renal function when administered to a patient who has renal insufficiency but has not yet undergone kidney dialysis. By administrating the drug, the loss of renal function represented by an extreme decrease in creatinine clearance or the reciprocal of blood creatinine level can be prevented and thus it can dispense with the dialytic treatment for patients with renal insufficiency.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com