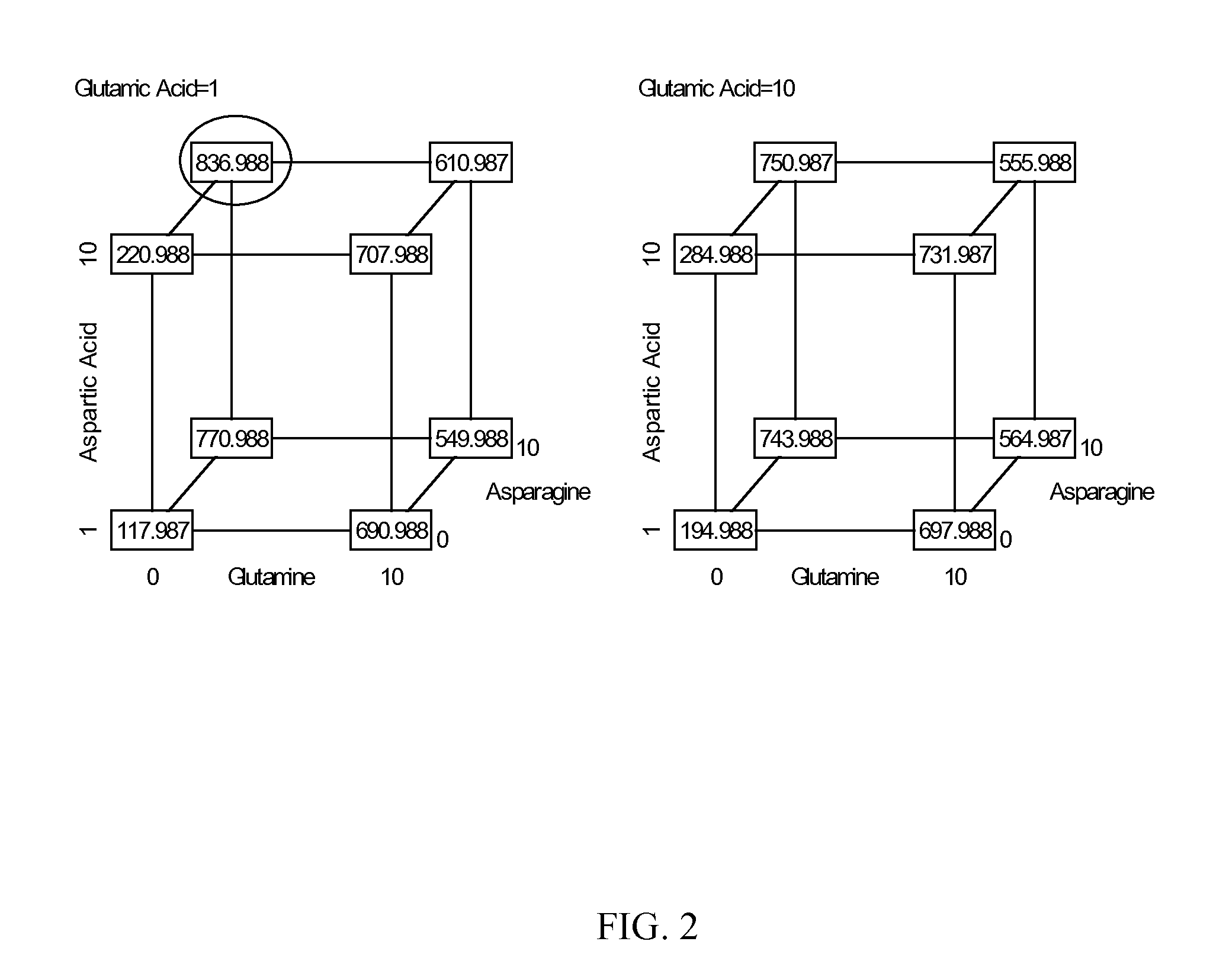
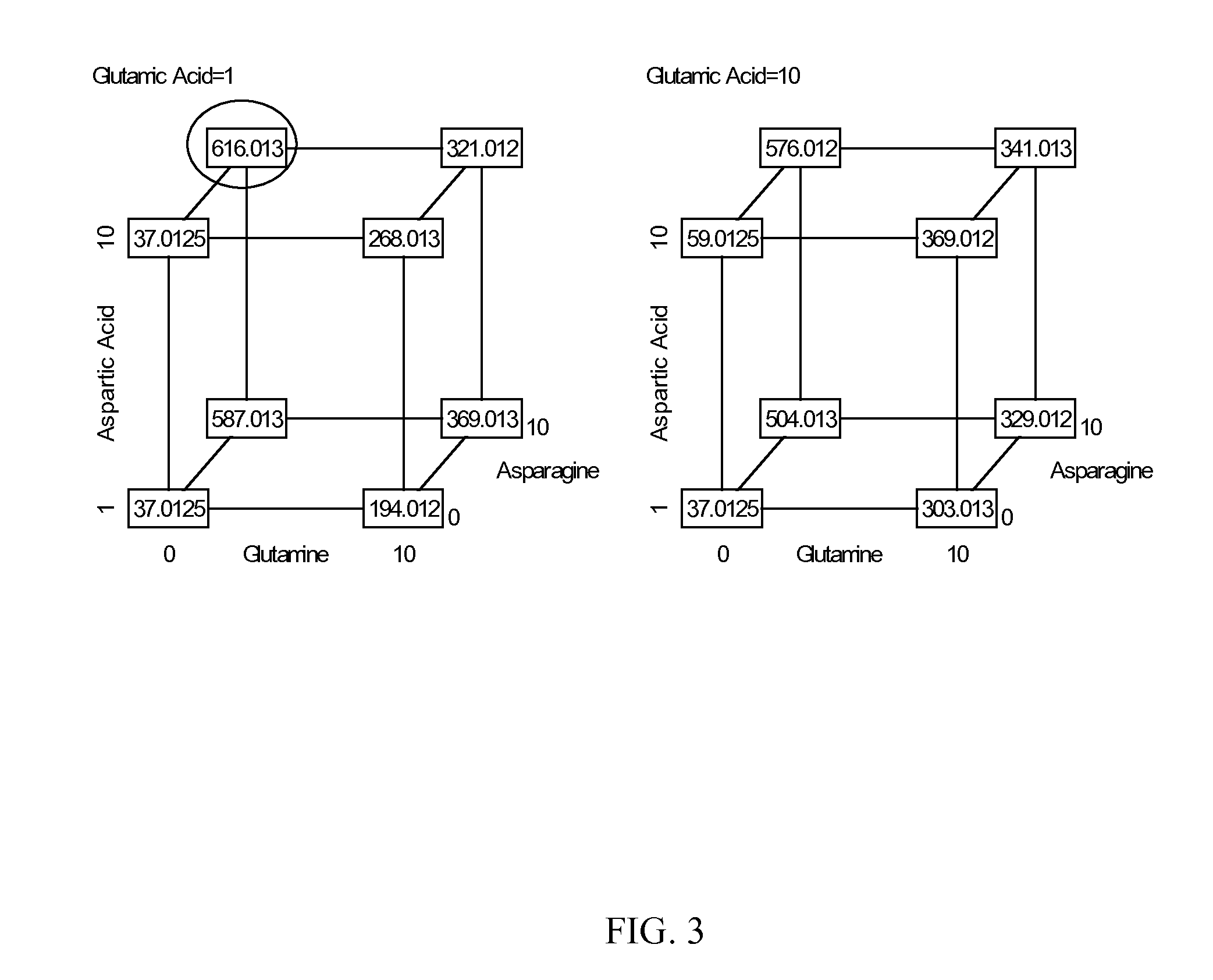
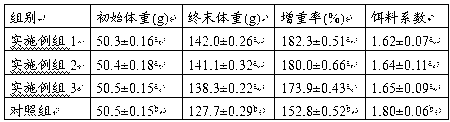
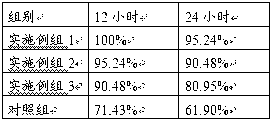
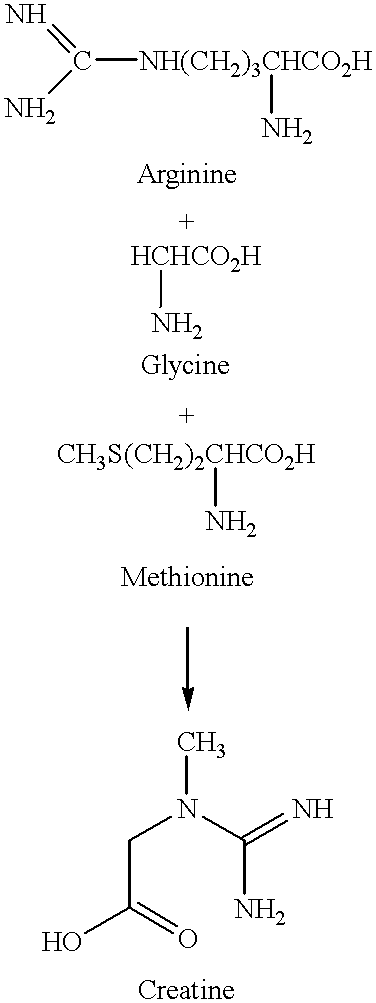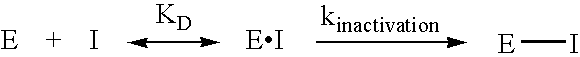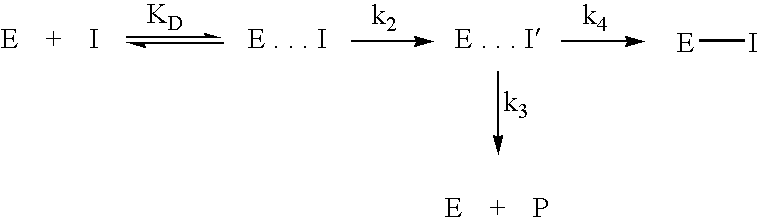Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2678 results about "Glutamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
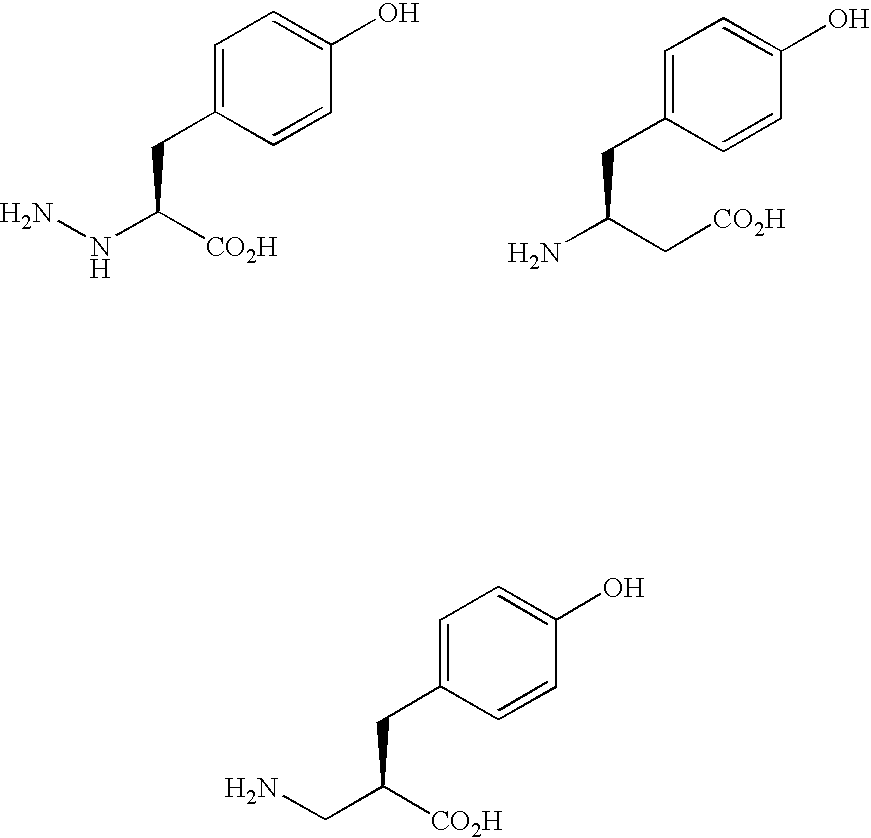
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral, polar amino acid. It is non-essential and conditionally essential in humans, meaning the body can usually synthesize sufficient amounts of it, but in some instances of stress, the body's demand for glutamine increases, and glutamine must be obtained from the diet. It is encoded by the codons CAA and CAG.
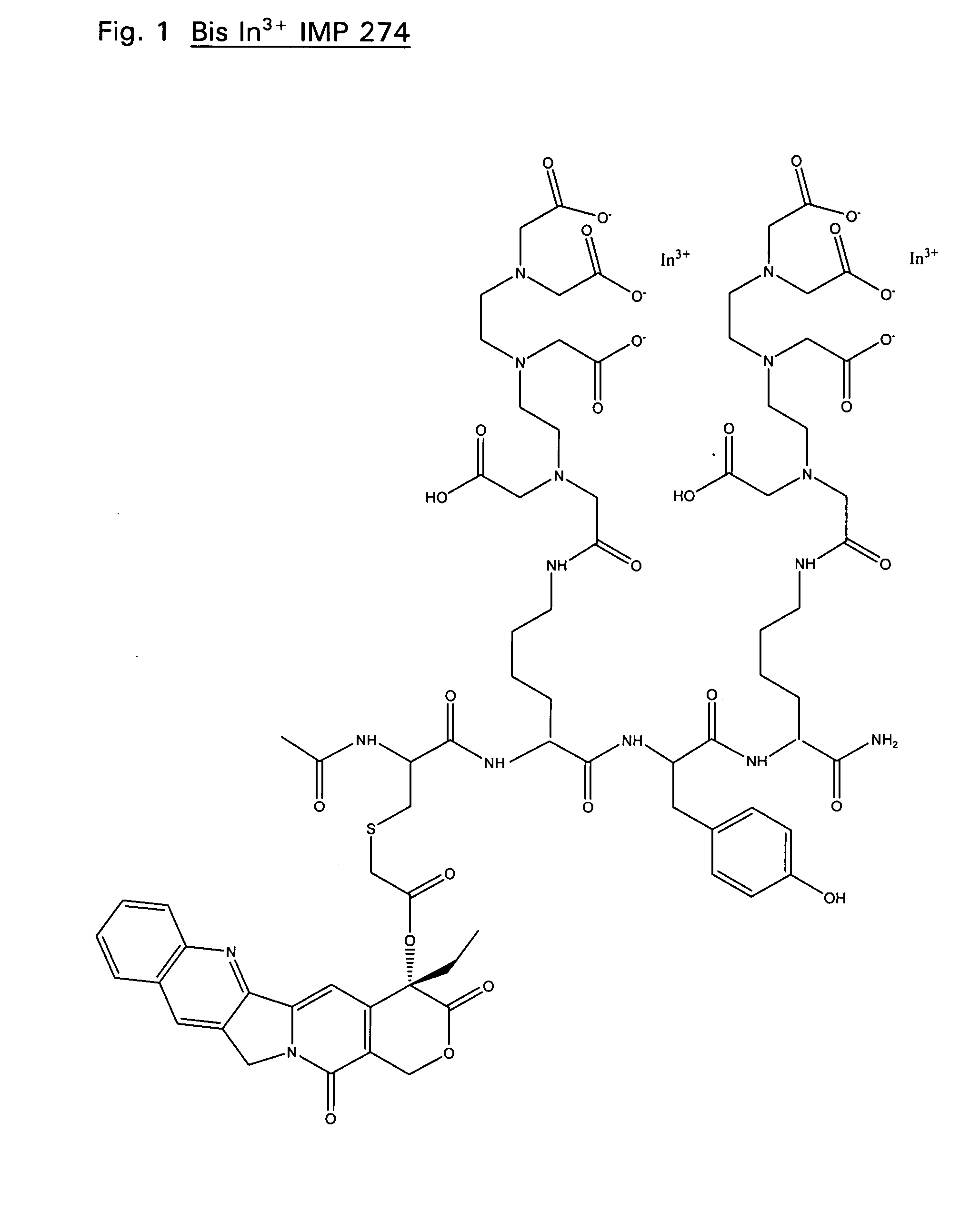
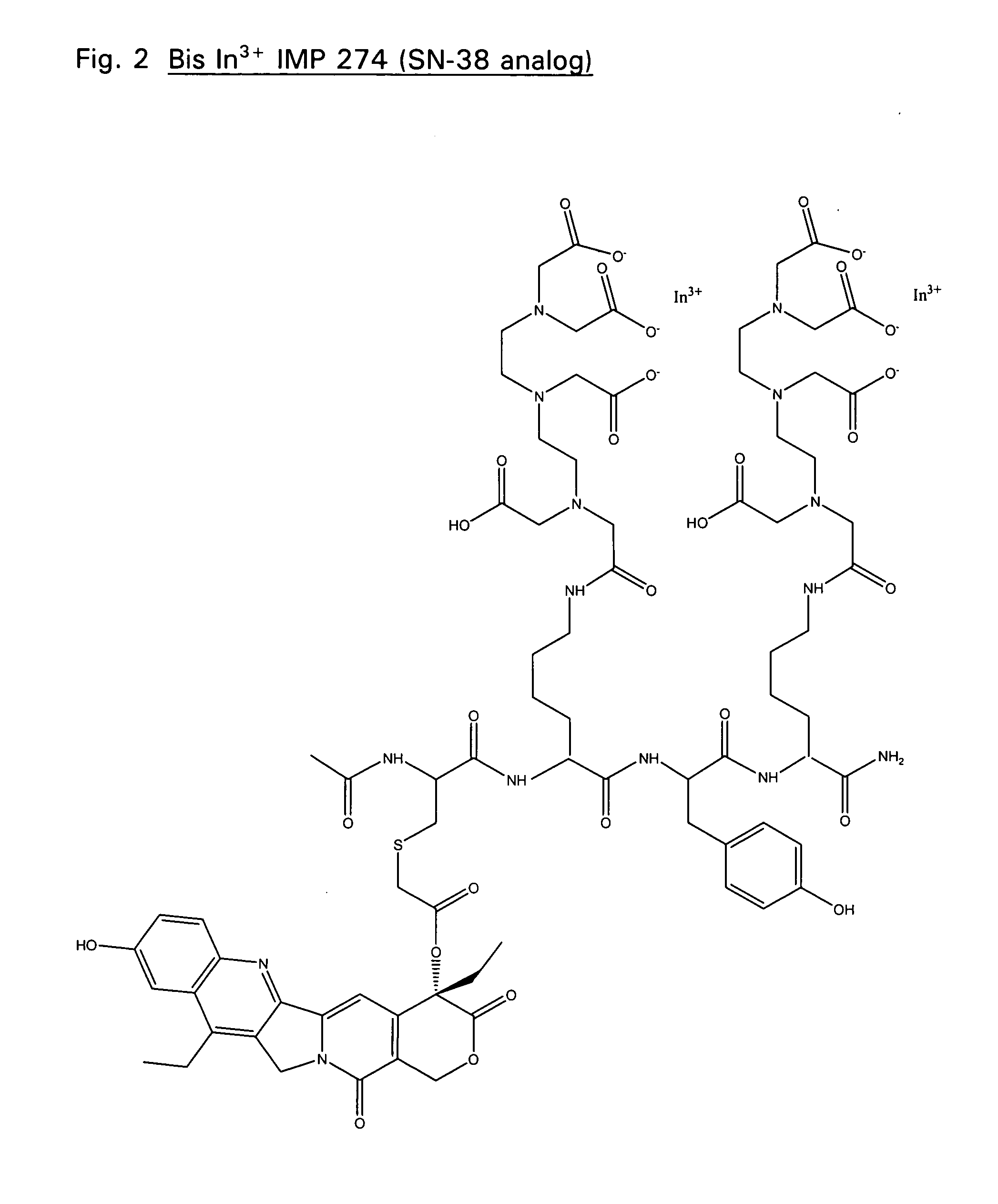
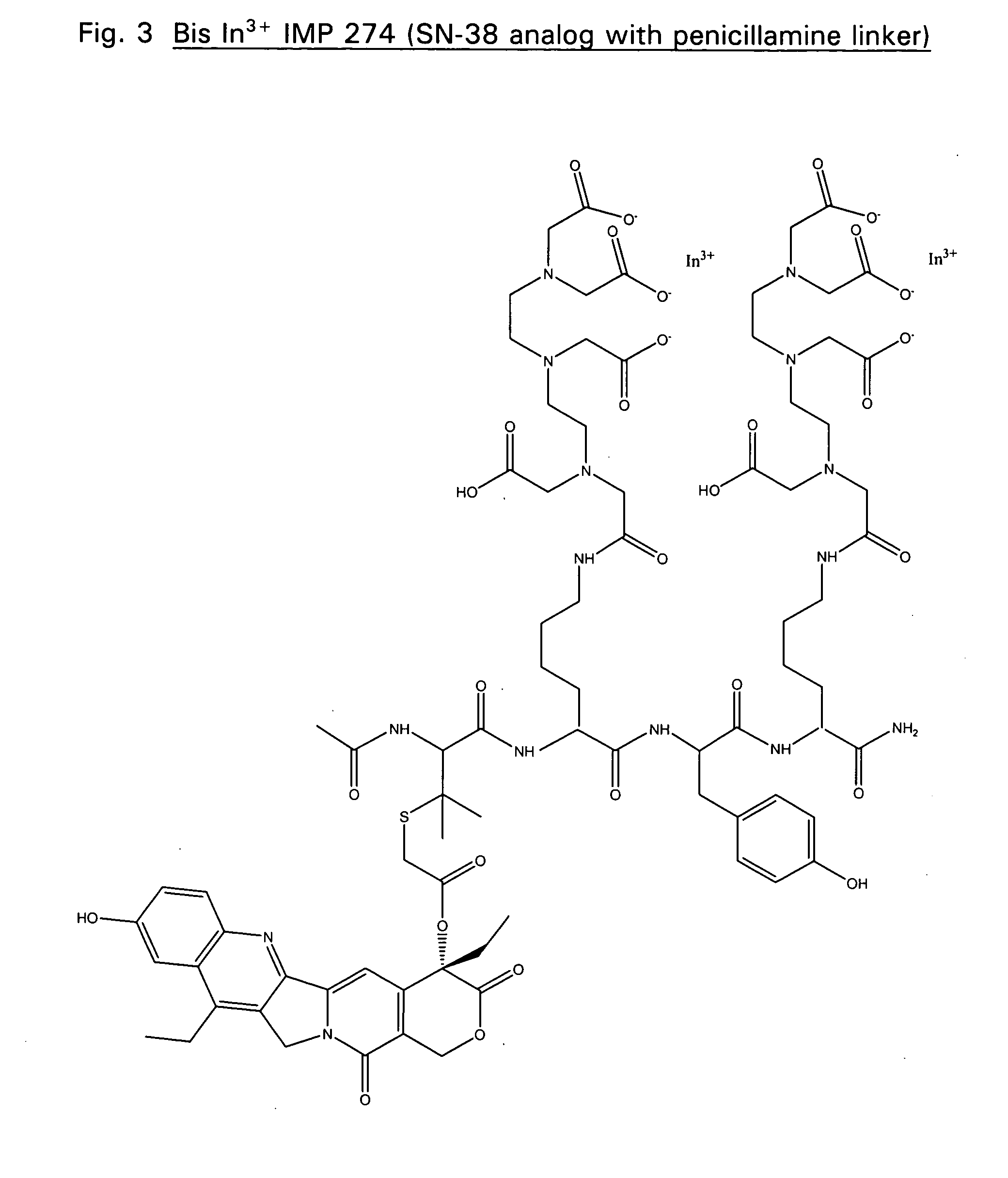
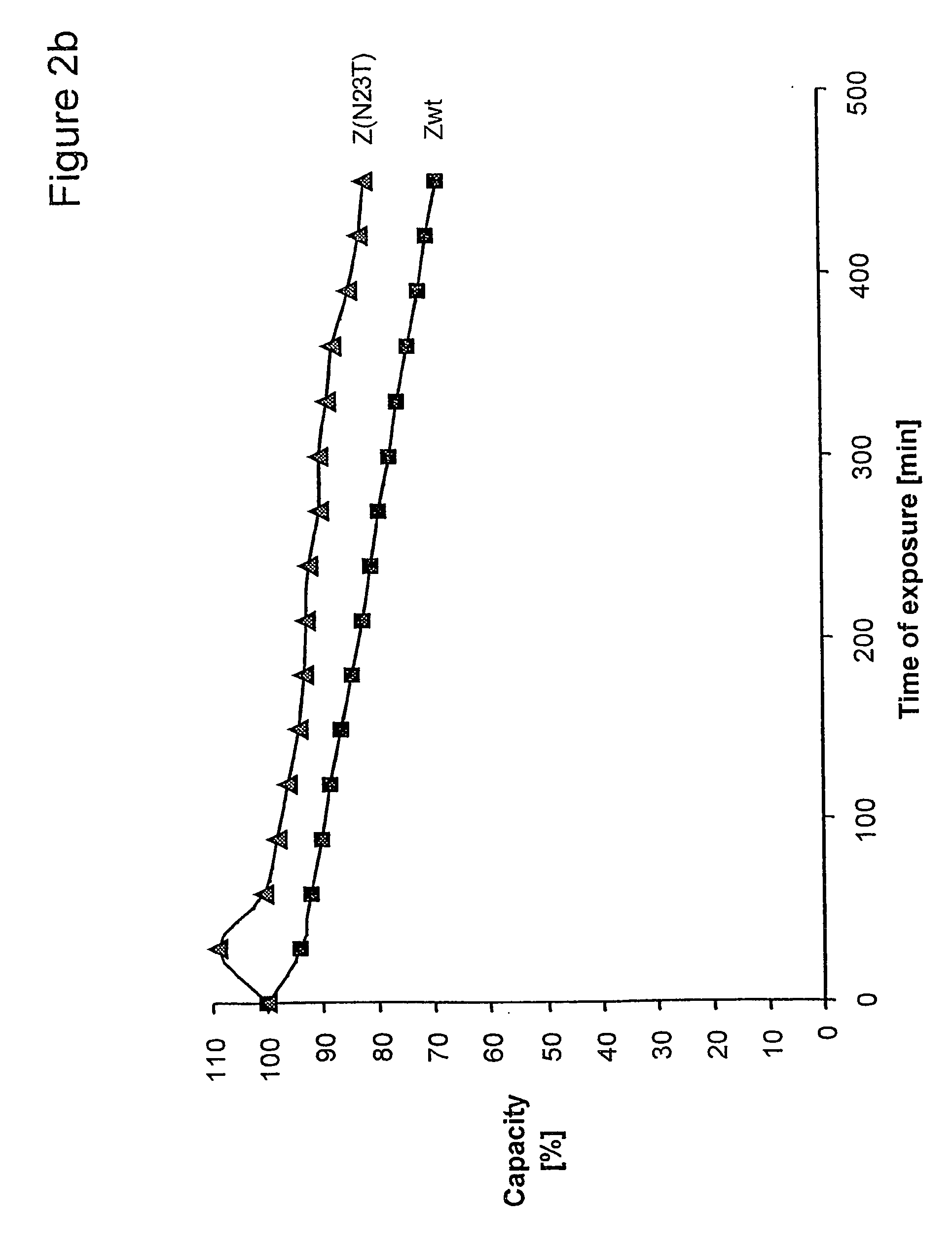
Therapeutic and diagnostic conjugates for use with multispecific antibodies
InactiveUS20050002945A1Low toxicityPromote localizationAntibacterial agentsAntimycoticsSemicarbazoneEther
Disclosed are compounds that include two or more haptens conjugated by a spacer or a carrier. The haptens may include diethylenetriaminepentaacetate (DTPA), histimine-succinyl-glutamine (HSG), or combinations of DTPA and HSG. The compound also includes an effector molecule which may be conjugated to one or more of the haptens, the spacer / carrier, or both. The effector molecule may be conjugated by a number of linkages including an ester linkage, an imino linkage, an amino linkage, a sulfide linkage, a thiosemicarbazone linkage, a semicarbazone linkage, an oxime linkage, an ether linkage, or combinations of these linkages. Also disclosed are methods of synthesizing the compounds and / or precursors of the compounds.
Owner:IMMUNOMEDICS INC
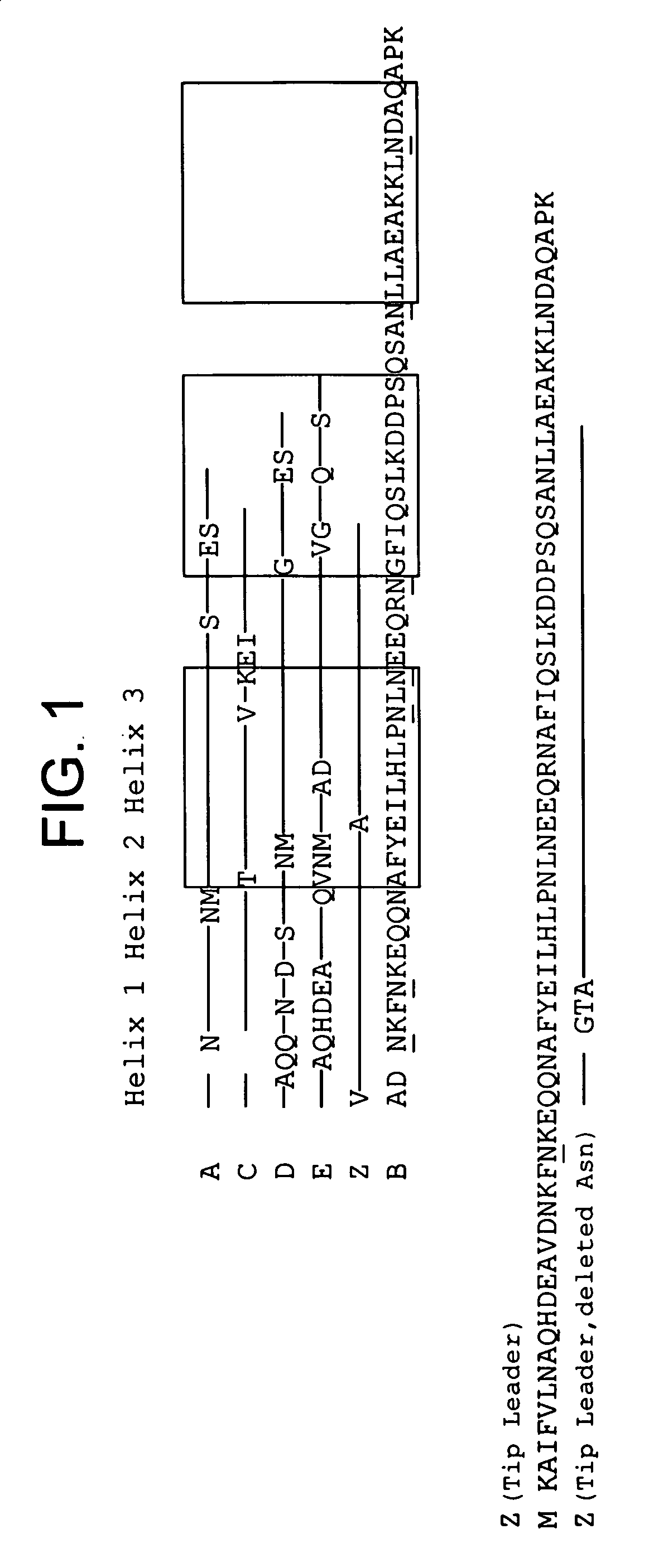
Mutant protein
InactiveUS20060194950A1Improve stabilityIncreased pH-valuesBacteriaSerum immunoglobulinsMutated proteinComplementarity determining region
Owner:GE HEALTHCARE BIO SCI CORP
Mutated immunoglobulin-binding protein
ActiveUS20050143566A1Improve stabilityIncreased pH-valuesSerum immunoglobulinsComponent separationComplementarity determining regionChemical stability
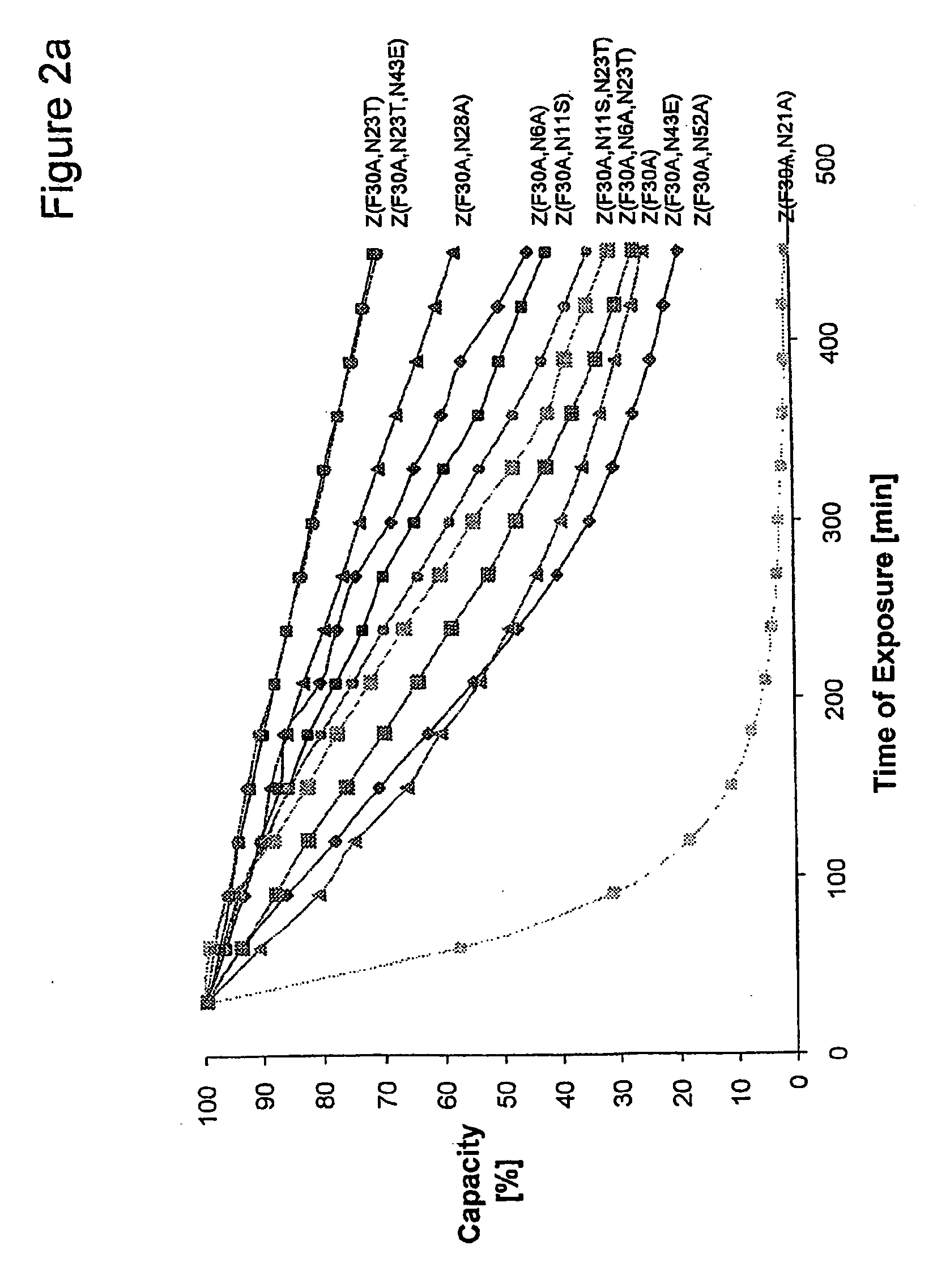
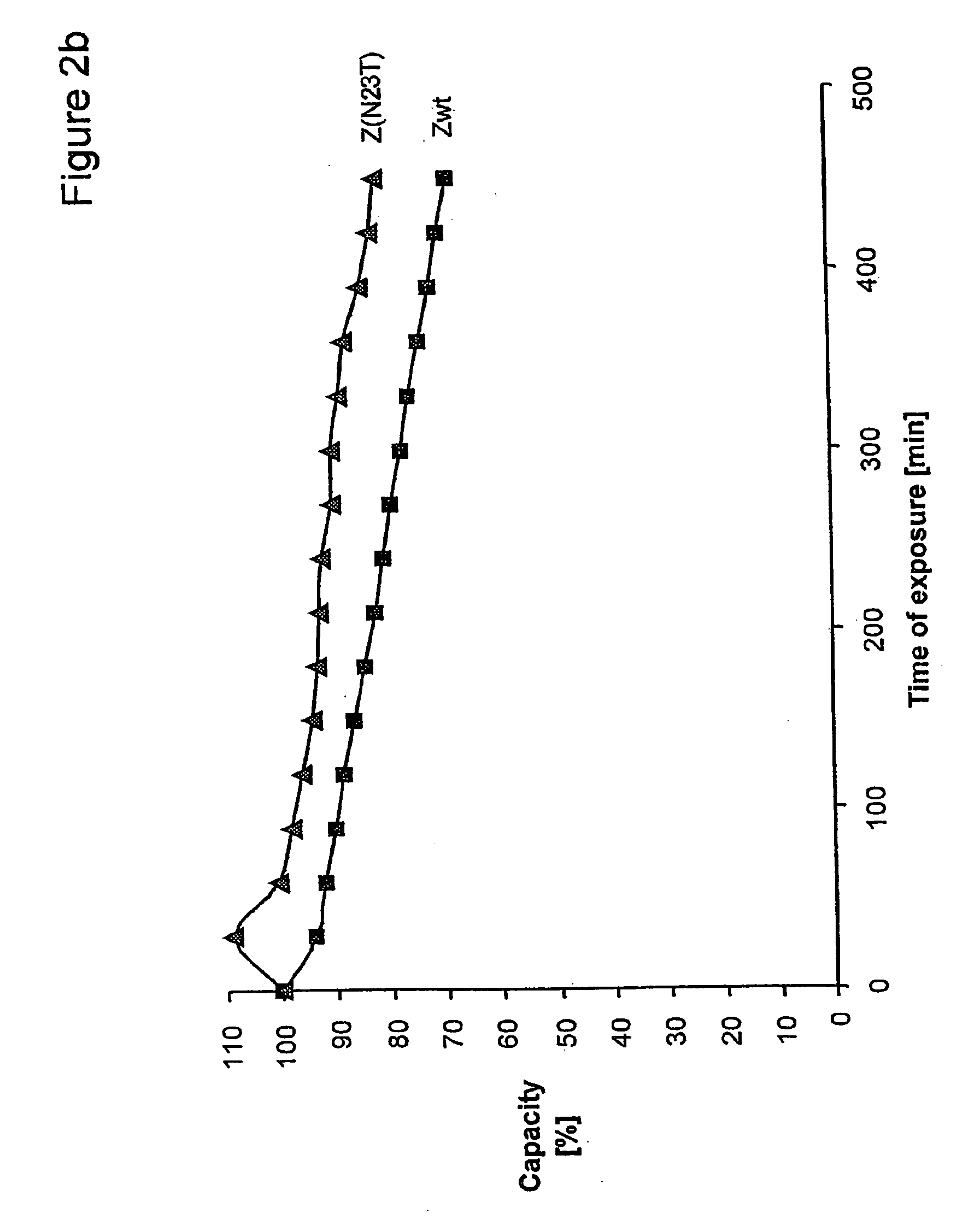
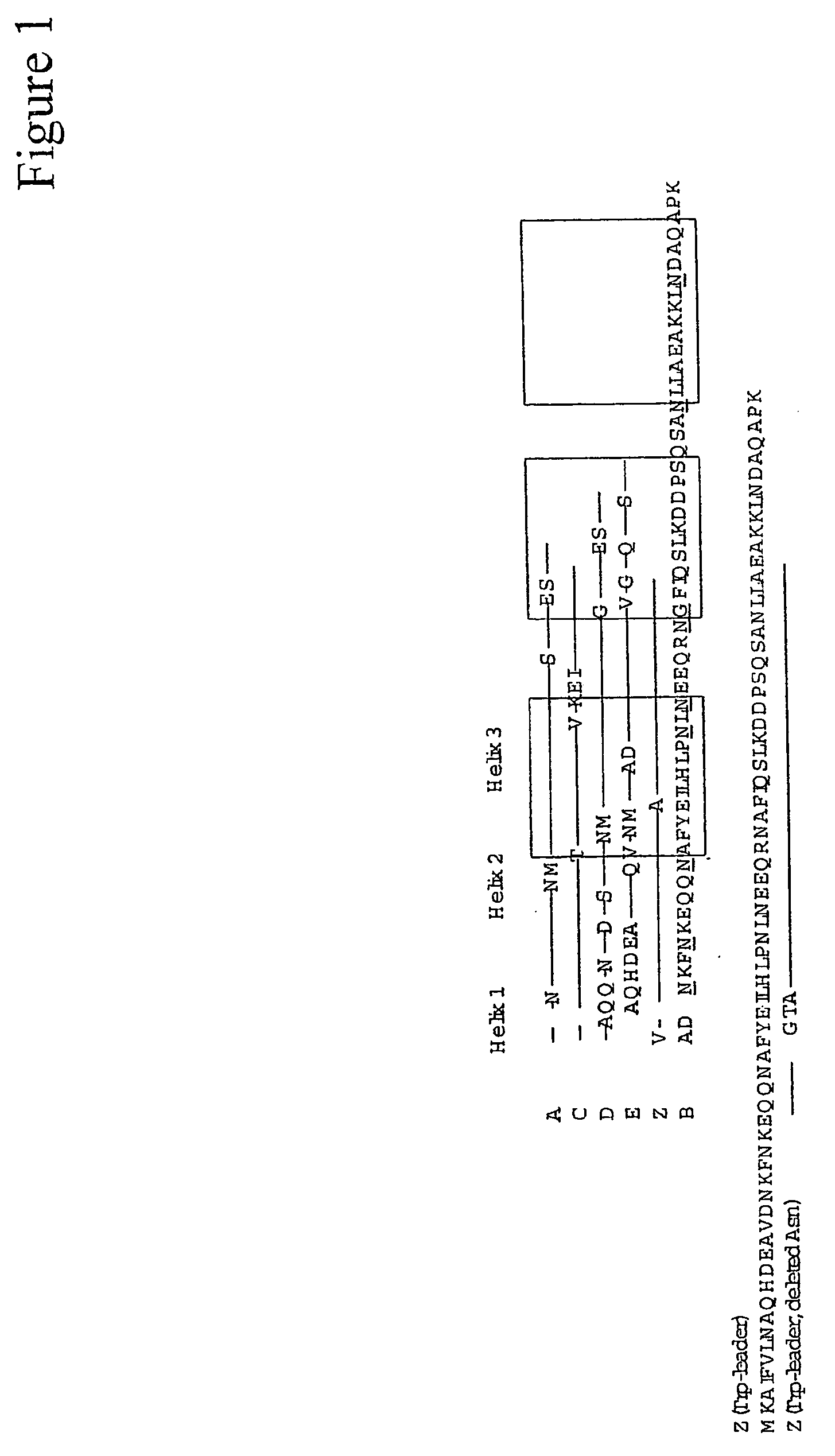
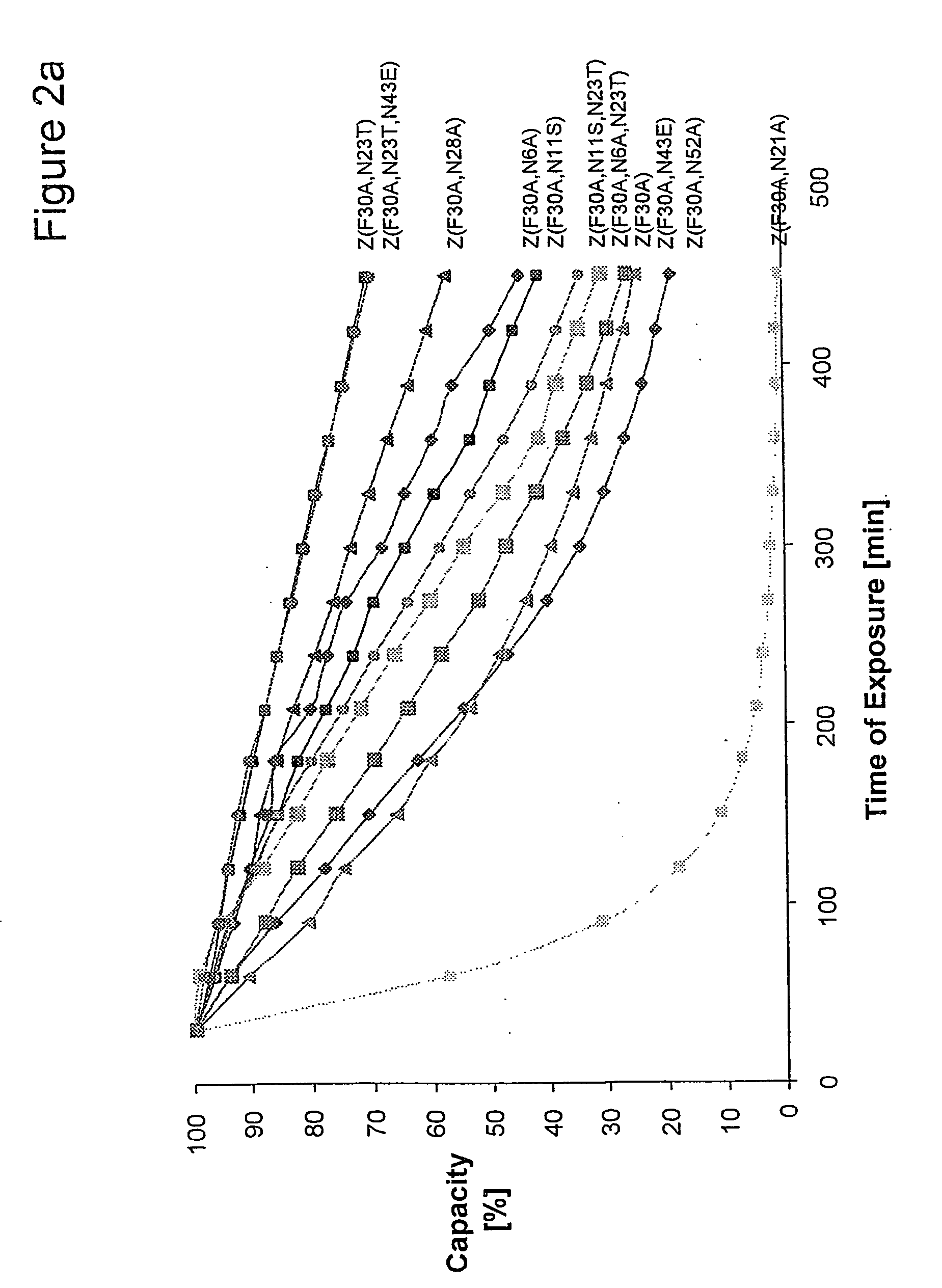
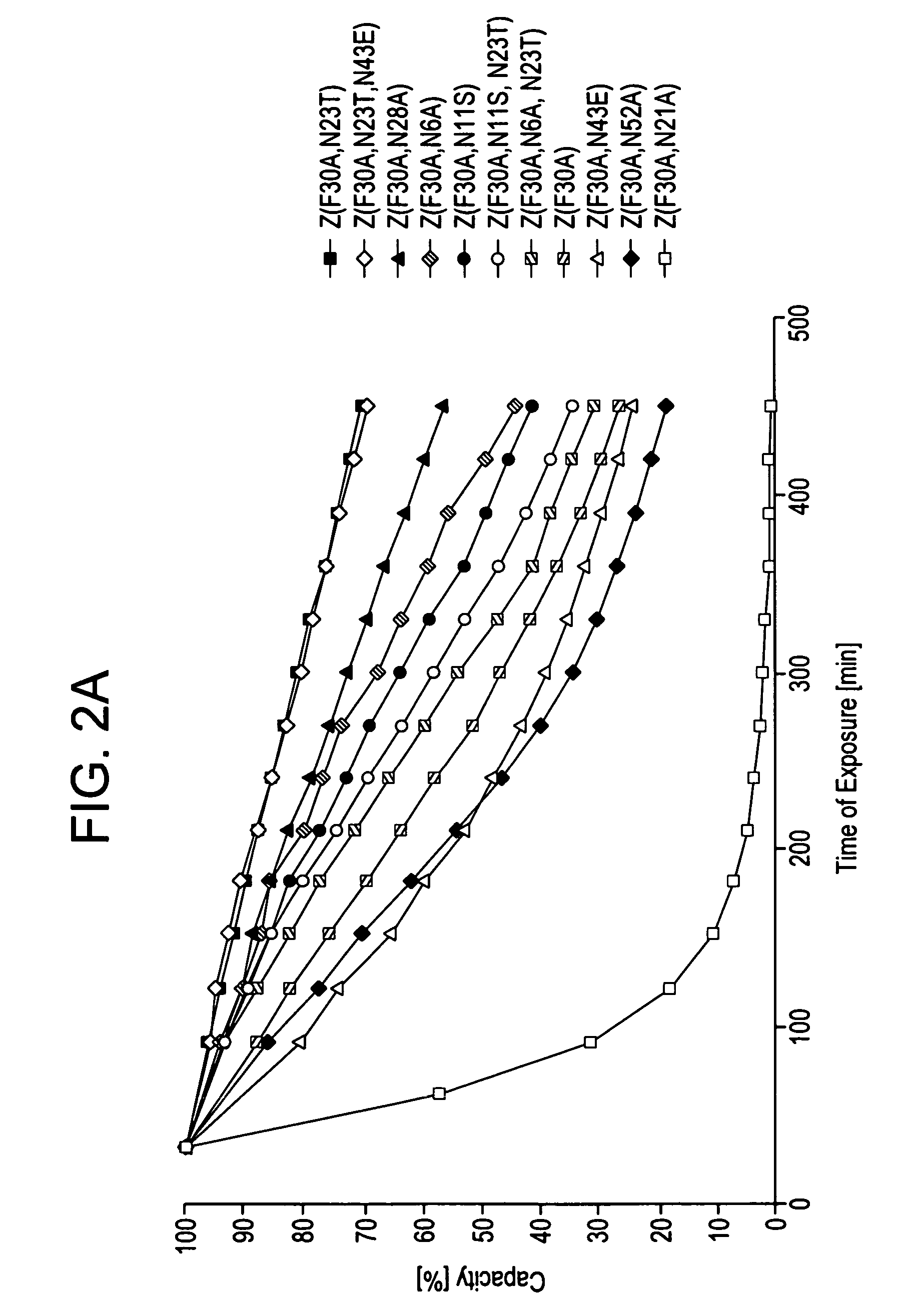
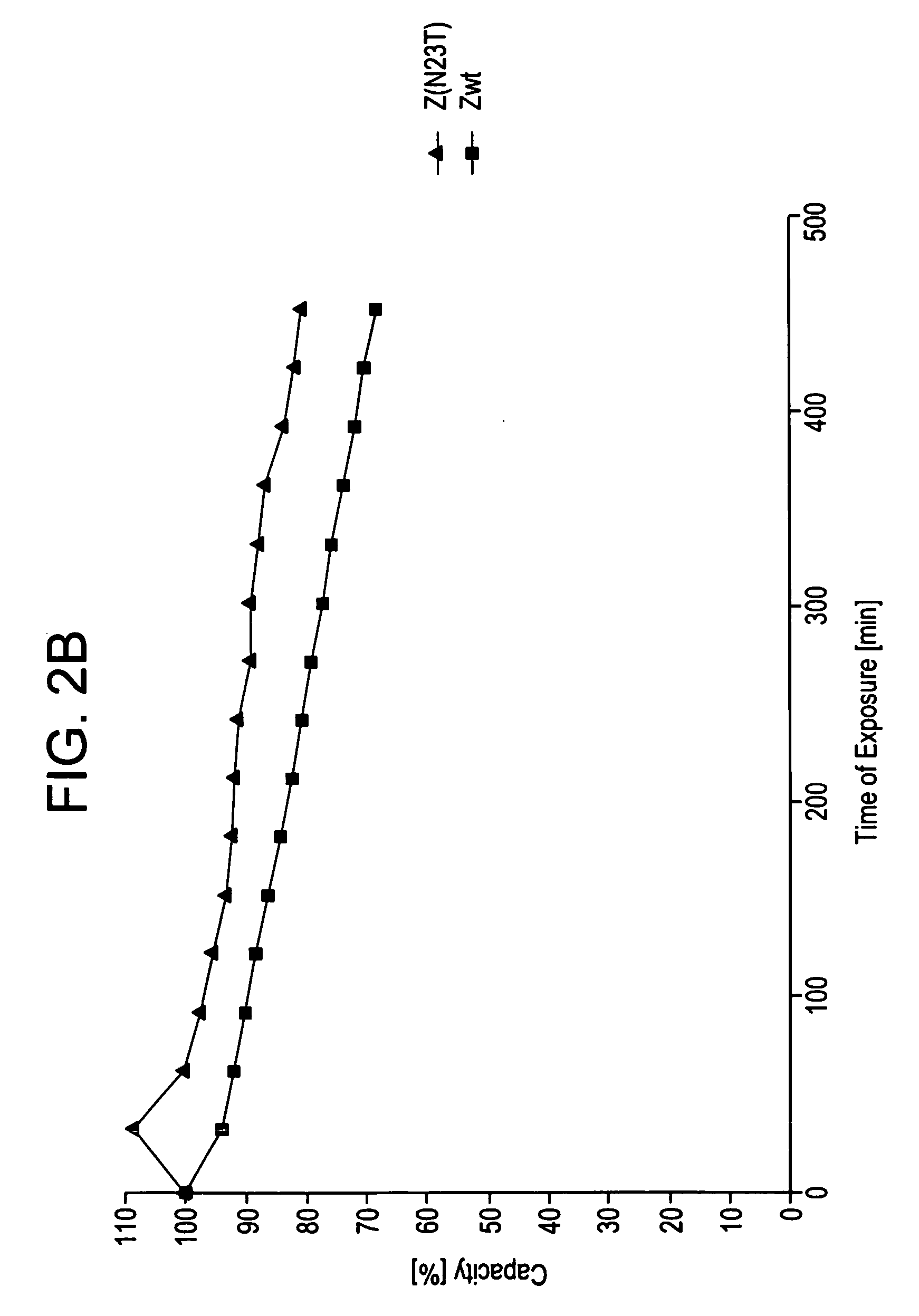
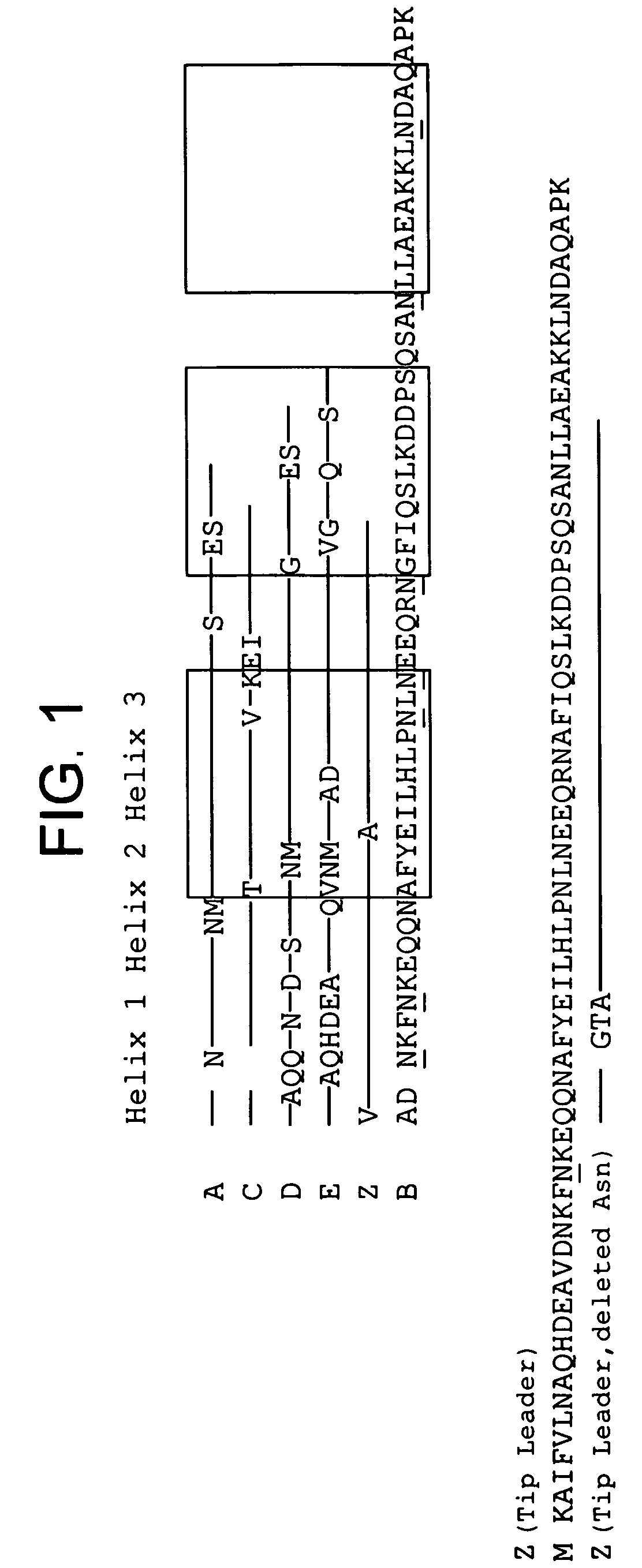
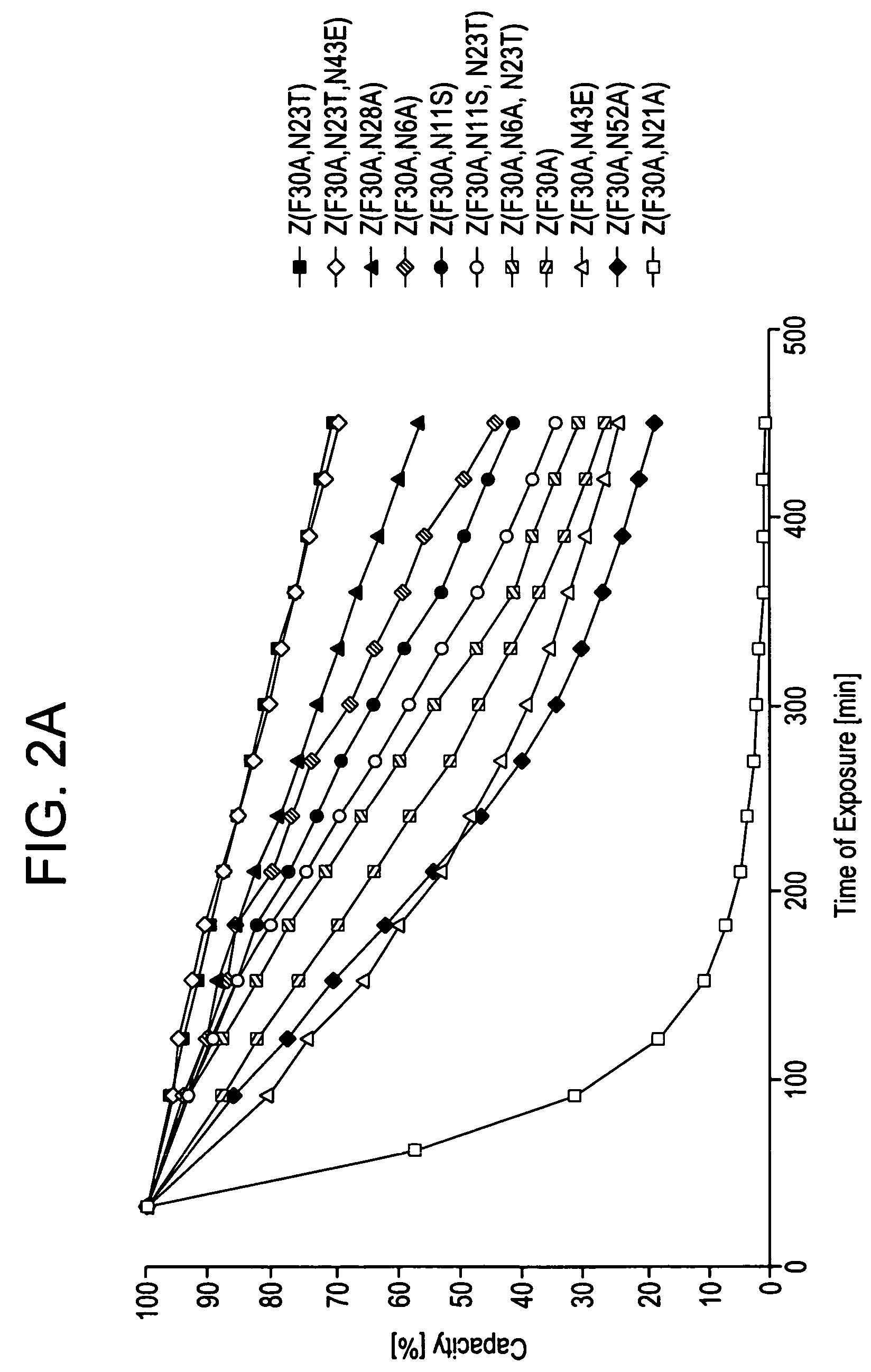
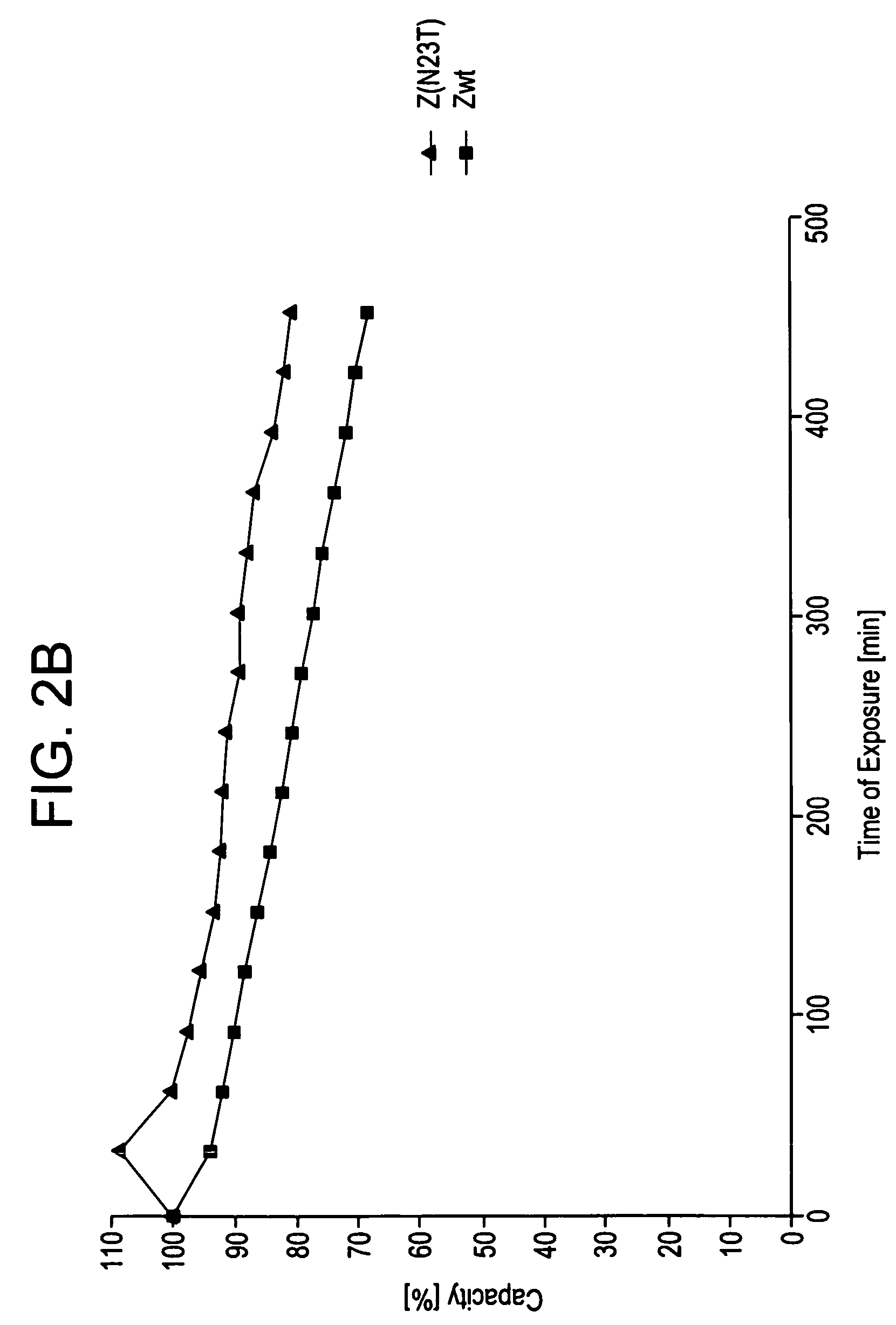
The present invention relates to an immunoglobulin-binding protein, wherein at least one asparagine residue has been mutated to an amino acid other than glutamine or aspartic acid, which mutation confers an increased chemical stability at pH-values of up to about 13-14 compared to the parental molecule. The protein can for example be derived from a protein capable of binding to other regions of the immunoglobulin molecule than the complementarity determining regions (CDR), such as protein A, and preferably the B-domain of Staphylococcal protein A. The invention also relates to a matrix for affinity separation, which comprises an immunoglobulin-binding protein as ligand coupled to a solid support, in which protein ligand at least one asparagine residue has been mutated to an amino acid other than glutamine.
Owner:CYTIVA BIOPROCESS R&D AB
Pharmaceutical composition and method for the transdermal delivery of calcium
InactiveUS20070292493A1Reduce disadvantagesReduce and prevent likelihoodHalogenated hydrocarbon active ingredientsBiocideArginineTryptophan
The present invention relates to a method and transdermal pharmaceutical composition for preventing or reducing the likelihood of calcium deficiency or imbalances caused by calcium deficiency. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of calcium and a pharmaceutically acceptable carrier constituting a pluronic lecithin organogel. In addition to calcium, the transdermal pharmaceutical composition may also contain a therapeutically effective amount of: (1) a pharmaceutically acceptably salt of other minerals such as magnesium, zinc, selenium, manganese, or chromium; (2) a vitamin such as vitamin A, vitamin D, vitamin C, vitamin E or B-complex vitamins, choline, lecithin, inositol, PABA, biotin, or bioflavomoids; (3) a carotenoid such as lycopene or lutein; (4) a hormone such as dehydroepiandrosterone, progesterone, pregnenolone, or melatonin; (5) an amino acid such as arginine, glutamine, lysine, phenylalanine, tyrosine, GABA, tryptophan, carnitine, or acetyl-l-carnitine; (6) a fatty acid such as a fish oil or flax seed oil; (7) a vita-nutrient such as coenzyme Q10; (8) a cartilage building nutrient such as glucosamine, chondroitin, or MSM, (9) a herb such as ginkgo biloba, echinacea, 5-HTP, St. John's wort, or saw palmetto; or (9) any combination thereof. The transdermal pharmaceutical composition may be topically administered to a human to prevent or reduce the likelihood of calcium deficiency or imbalances caused by calcium deficiency such as hypertension, high cholesterol, colon and rectal cancer, osteomalacia, rickets, osteoporosis, cardiovascular disease, preeclampsia, tooth decay, and premenstrual syndrome.
Owner:BRIERRE BARBARA T
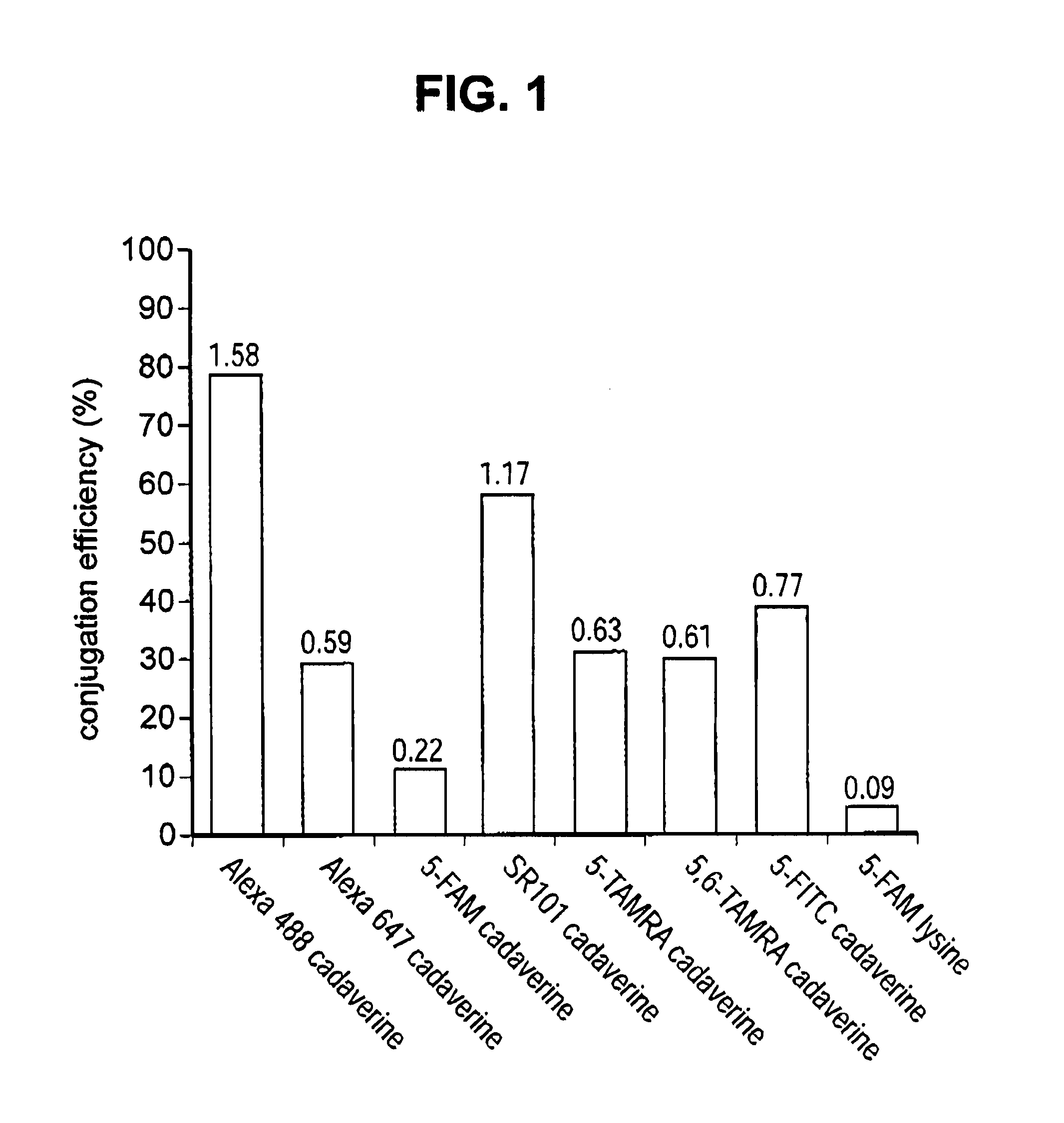
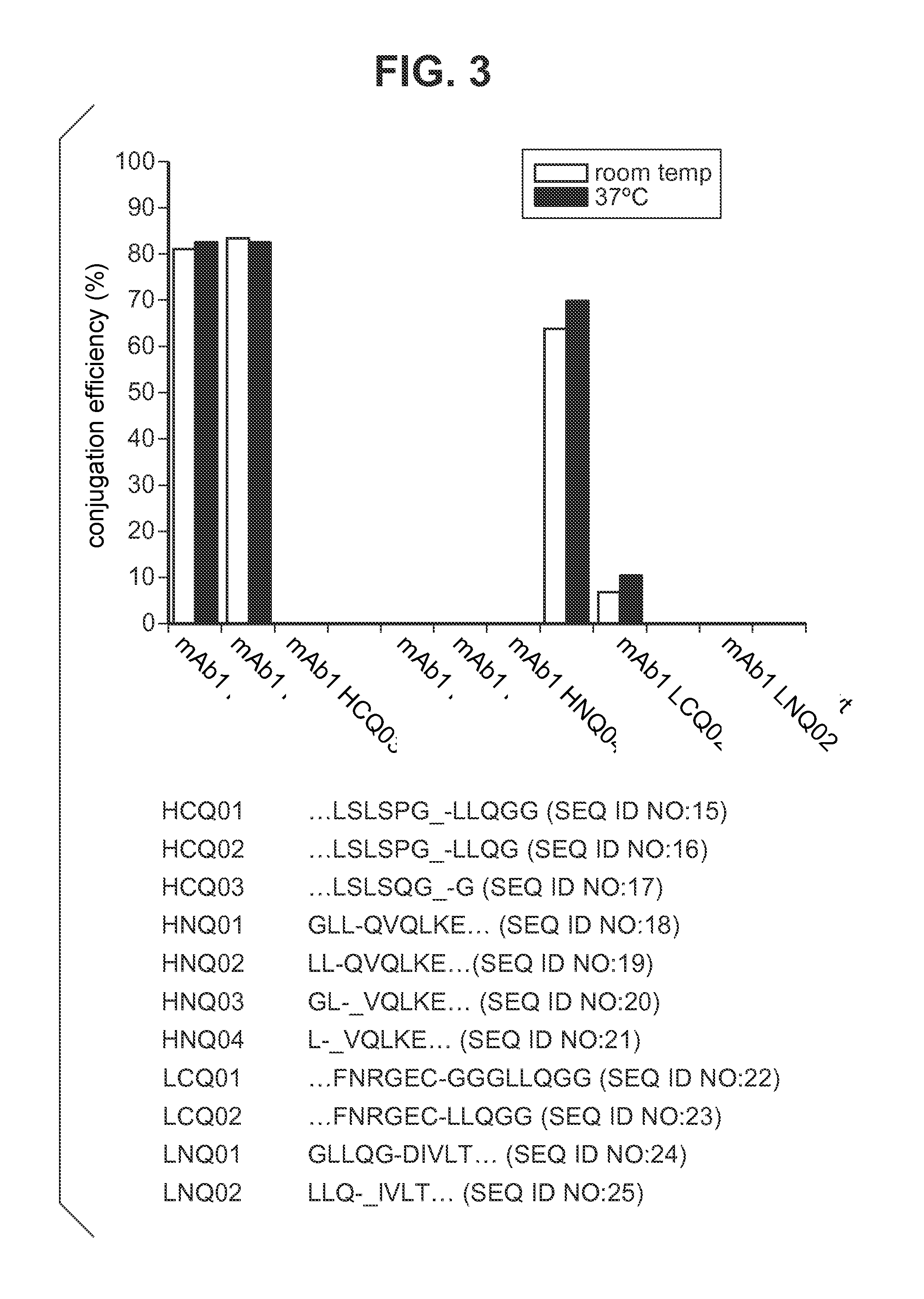
Engineered polypeptide conjugates and methods for making thereof using transglutaminase
ActiveUS20130230543A1Improve bindingImmunoglobulins against cell receptors/antigens/surface-determinantsDrug compositionsBispecific antibodyBiochemistry
The present invention provides engineered polypeptide conjugates (e.g., antibody-drug-conjugates, toxin-(biocompatible polymer) conjugates, antibody-(biocompatible polymer) conjugates, and bispecific antibodies) comprising acyl donor glutamine-containing tags and amine donor agents. In one aspect, the invention provides an engineered Fc-containing polypeptide conjugate comprising the formula (Fc-containing polypeptide)-T-A, wherein T is an acyl donor glutamine-containing tag engineered at a specific site or comprises an endogenous glutamine made reactive by the Fc-containing polypeptide engineering, wherein A is an amine donor agent, and wherein the amine donor agent is site-specifically conjugated to the acyl donor glutamine-containing tag or the endogenous glutamine. The invention also provides methods of making engineered polypeptide conjugates using transglutaminase.
Owner:PFIZER INC RINAT NEUROSCIENCE CORP
Protein ligands
ActiveUS20060194955A1Retention characteristicReduce leakageImmunoglobulins against animals/humansBiological testingComplementarity determining regionStaphylococcus
The present invention relates to the use of an alkali-stable protein, wherein at least one asparagine residue has been mutated to an amino acid other than glutamine or aspartic acid, which mutation confers an increased chemical stability at pH-values of up to about 13-14 compared to the parental molecule. The protein can for example be derived from a protein capable of binding to other regions of the immunoglobulin molecule than the complementarity determining regions (CDR), such as protein A, and preferably the B-domain of Staphylococcal protein A. The invention also relates to a matrix for affinity separation, which comprises an immunoglobulin-binding protein as ligand coupled to a solid support, in which protein ligand at least one asparagine residue has been mutated to an amino acid other than glutamine.
Owner:CYTIVA BIOPROCESS R&D AB
Compositions of orthogonal lysyl-tRNA and aminoacyl-tRNA synthetase pairs and uses thereof
Compositions and methods of producing components of protein biosynthetic machinery that include orthogonal lysyl-tRNAs, orthogonal lysyl-aminoacyl-tRNA synthetases, and orthogonal pairs of lysyl-tRNAs / synthetases, which incorporate homoglutamines into proteins are provided in response to a four base codon. Methods for identifying these orthogonal pairs are also provided along with methods of producing proteins with homoglutamines using these orthogonal pairs.
Owner:THE SCRIPPS RES INST
Production of polypeptides
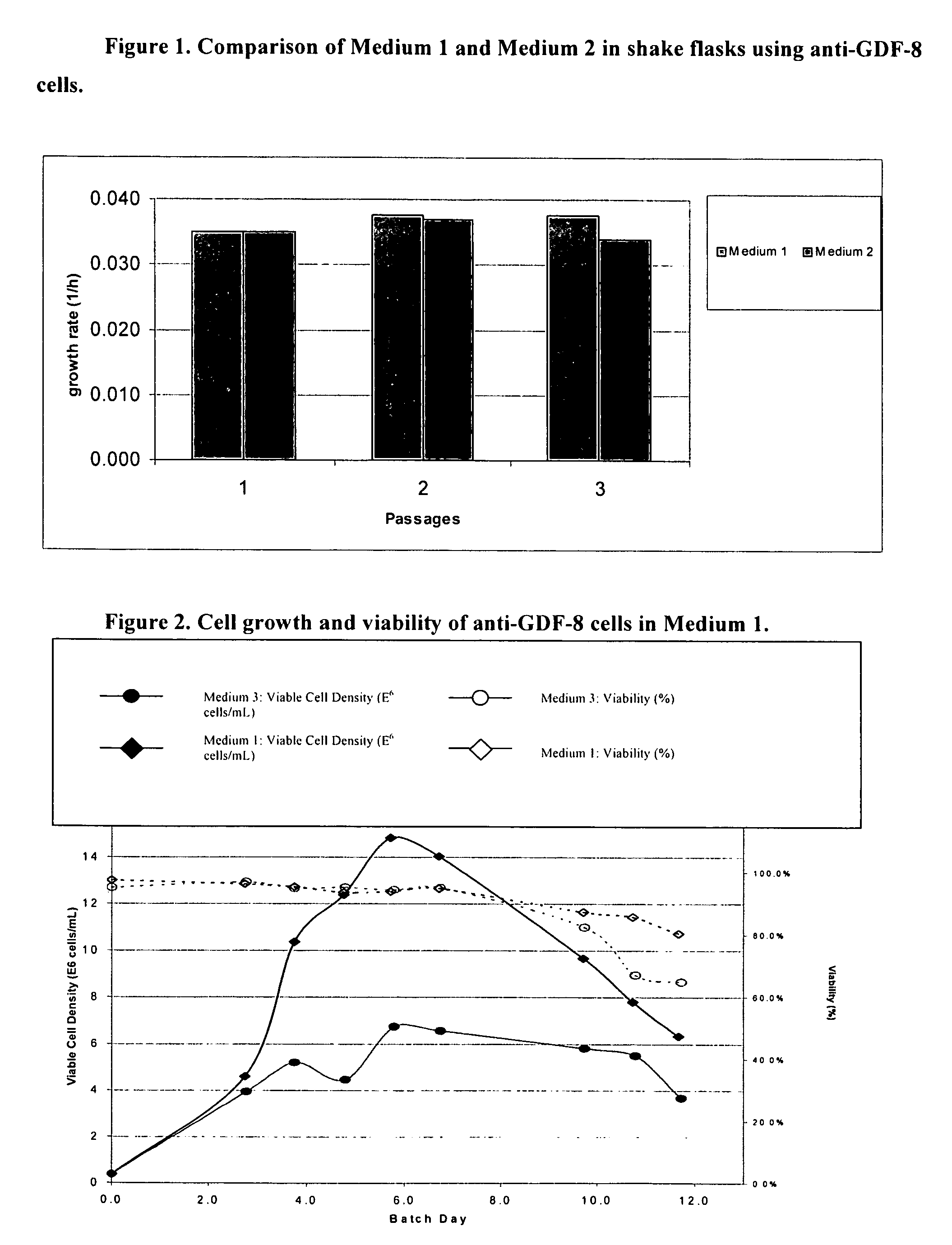
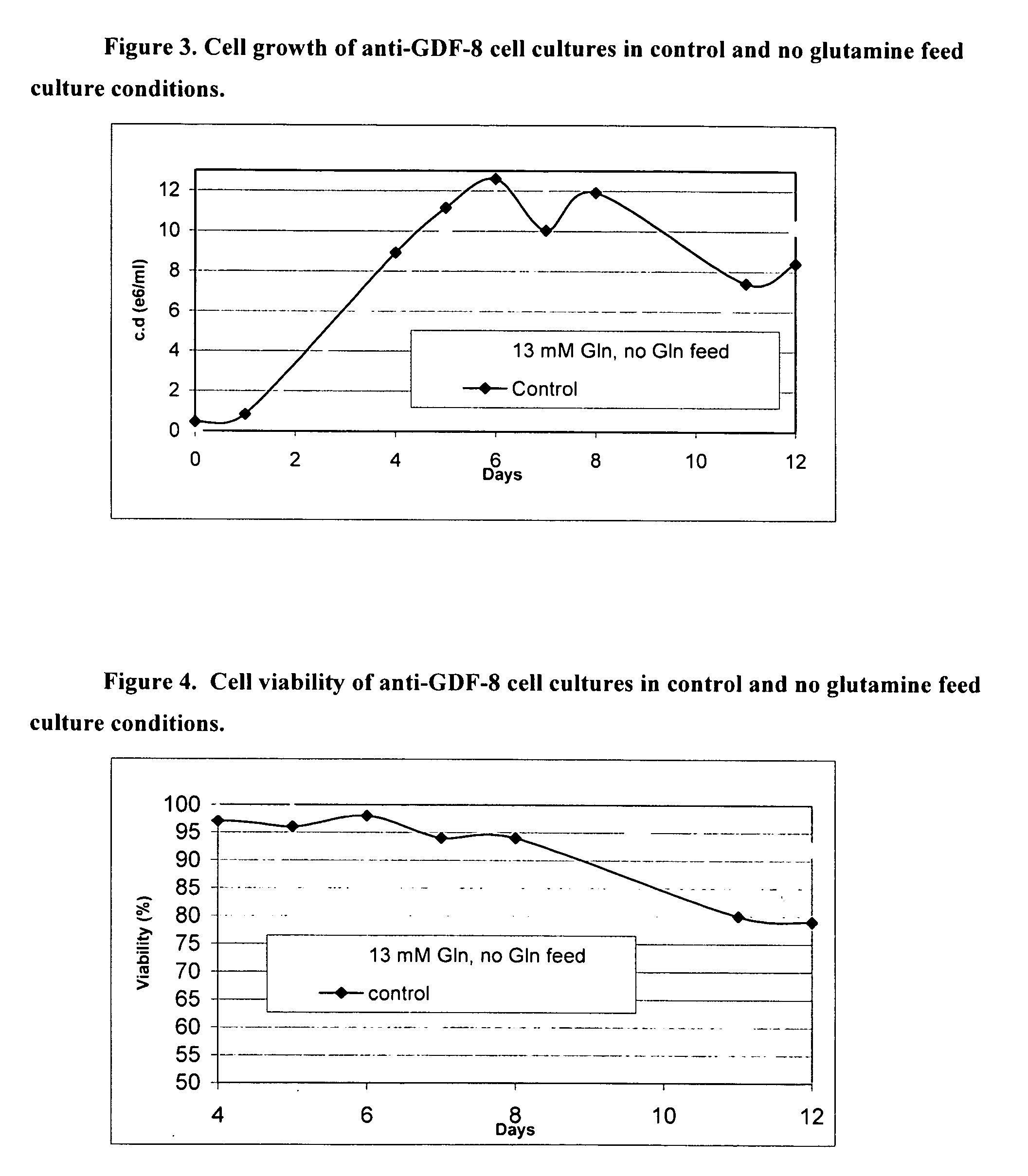
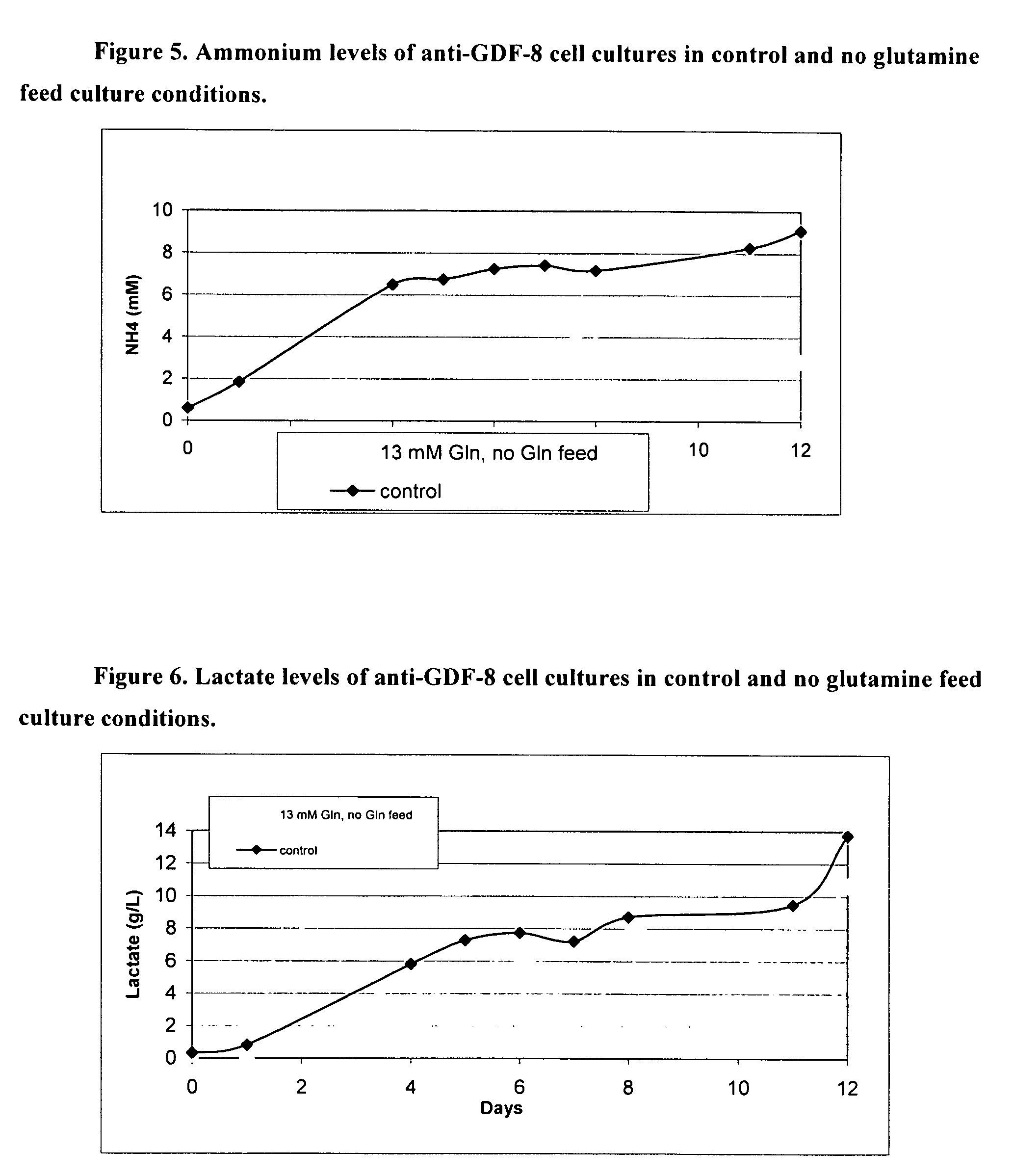
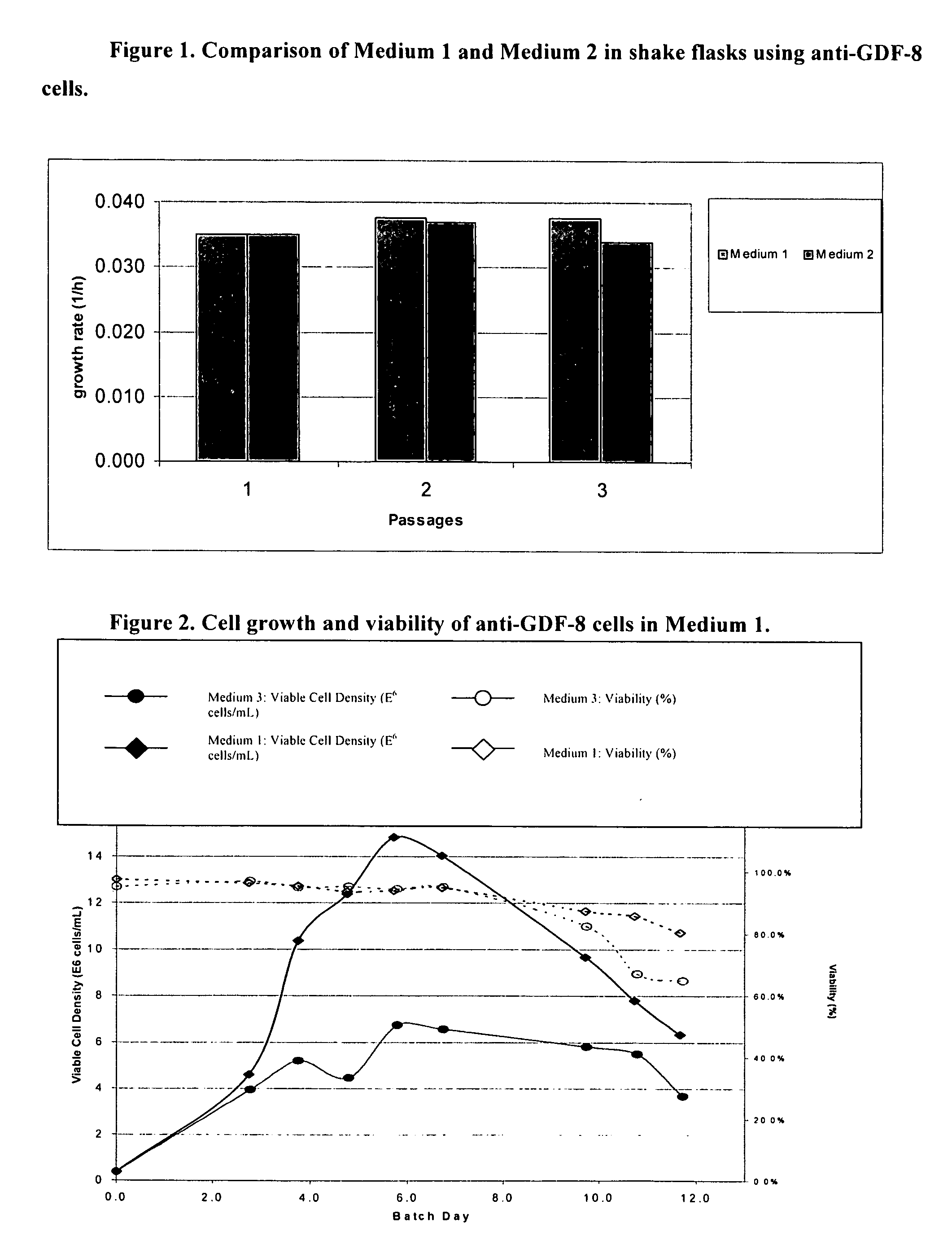
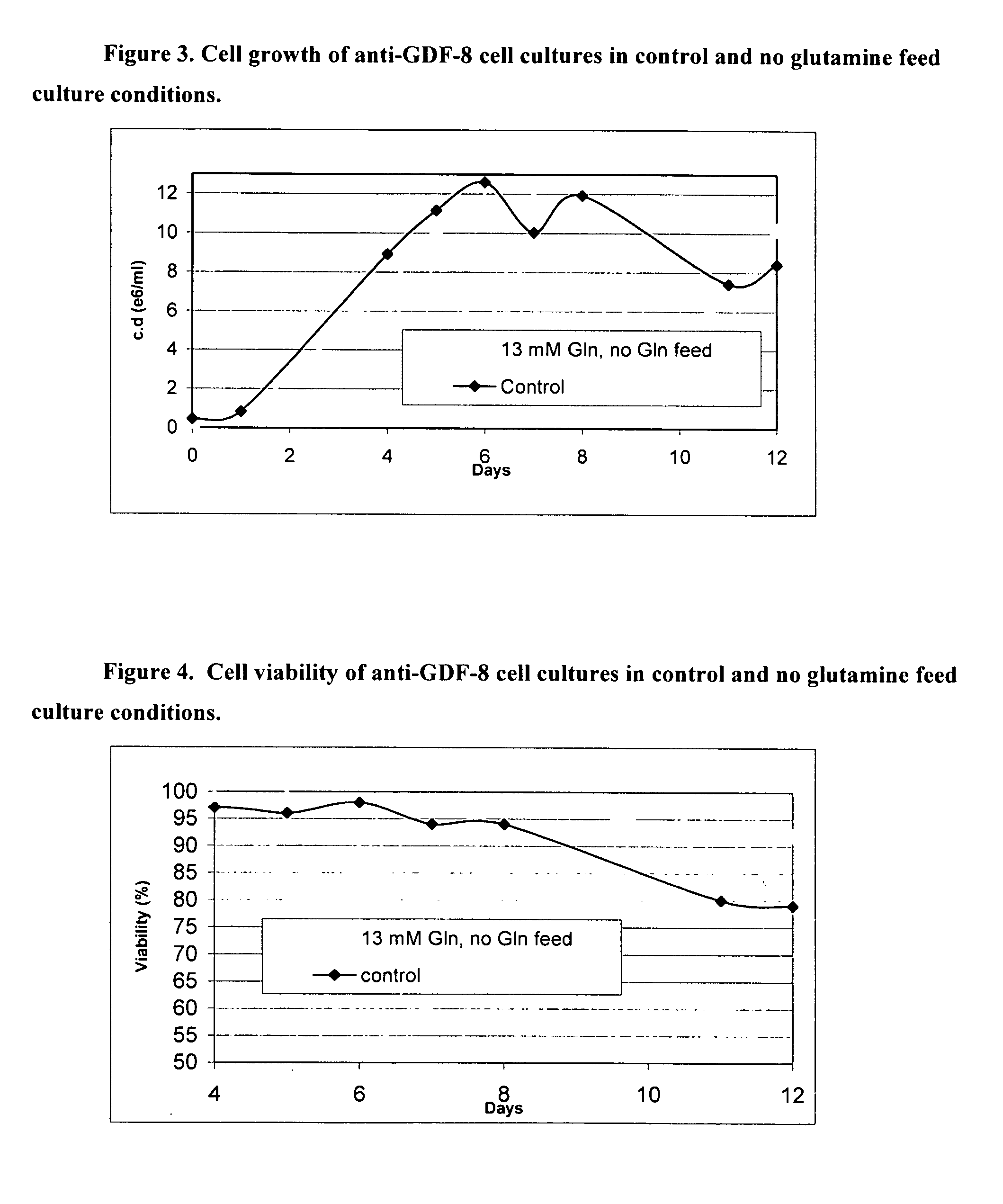
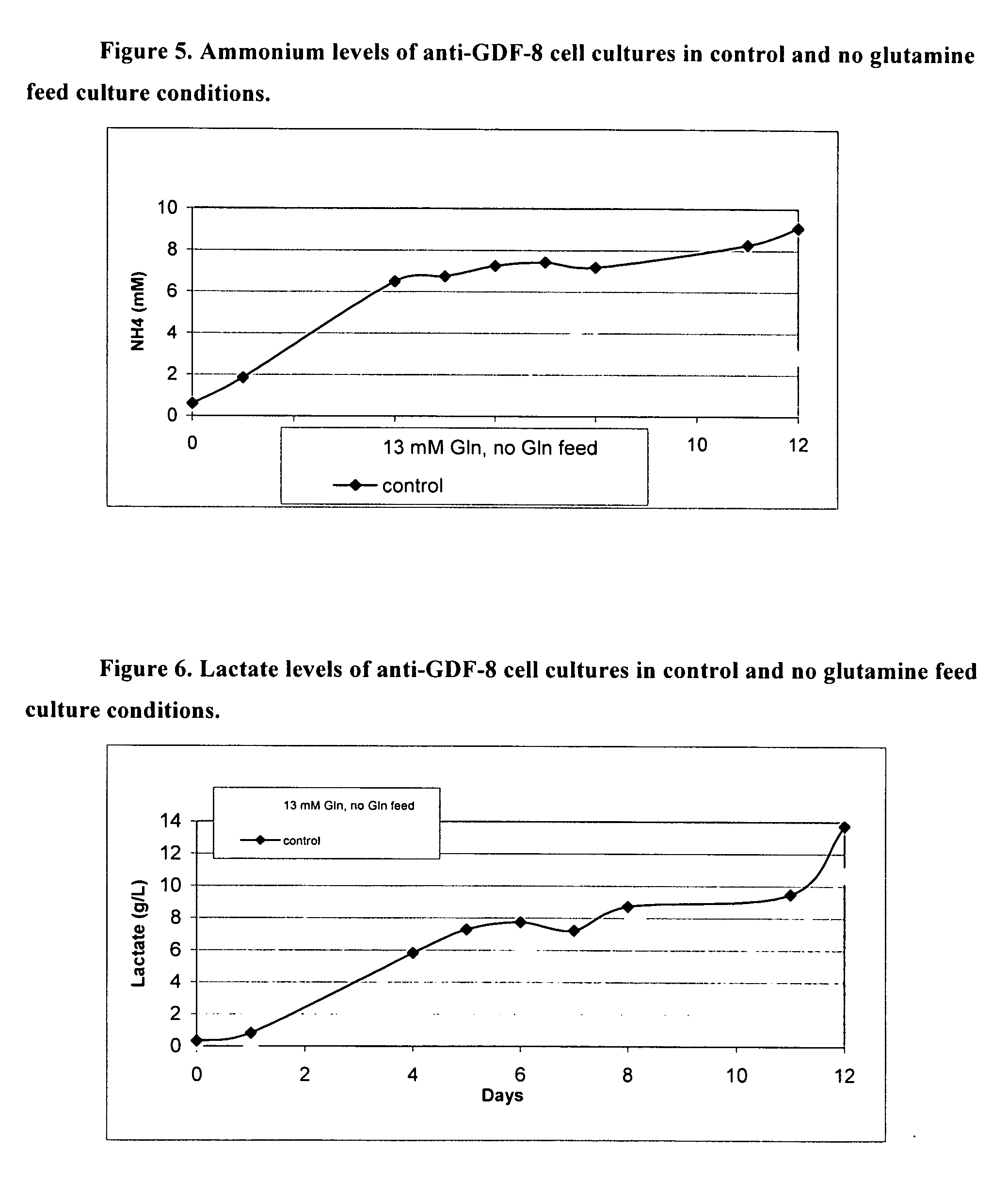
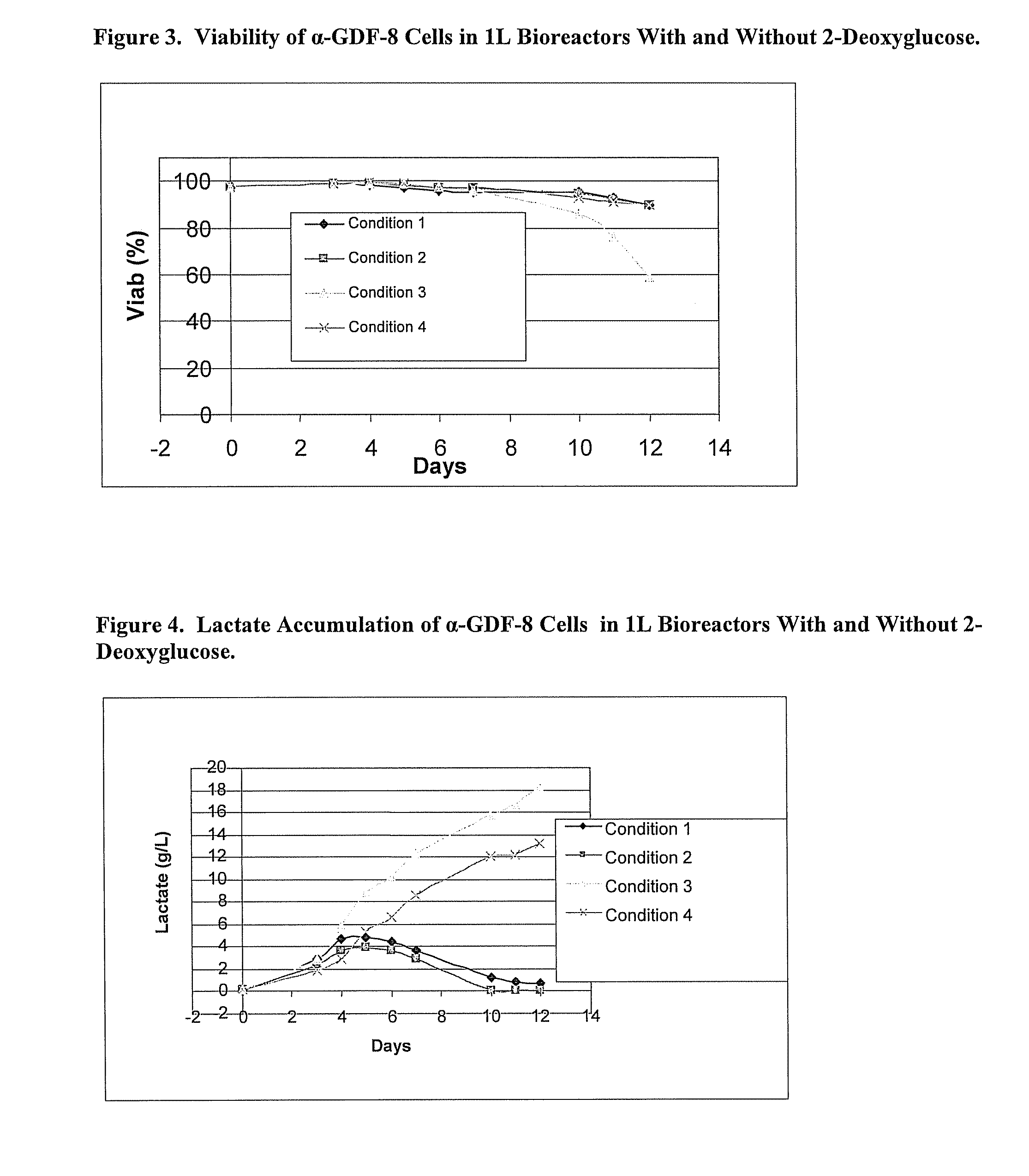
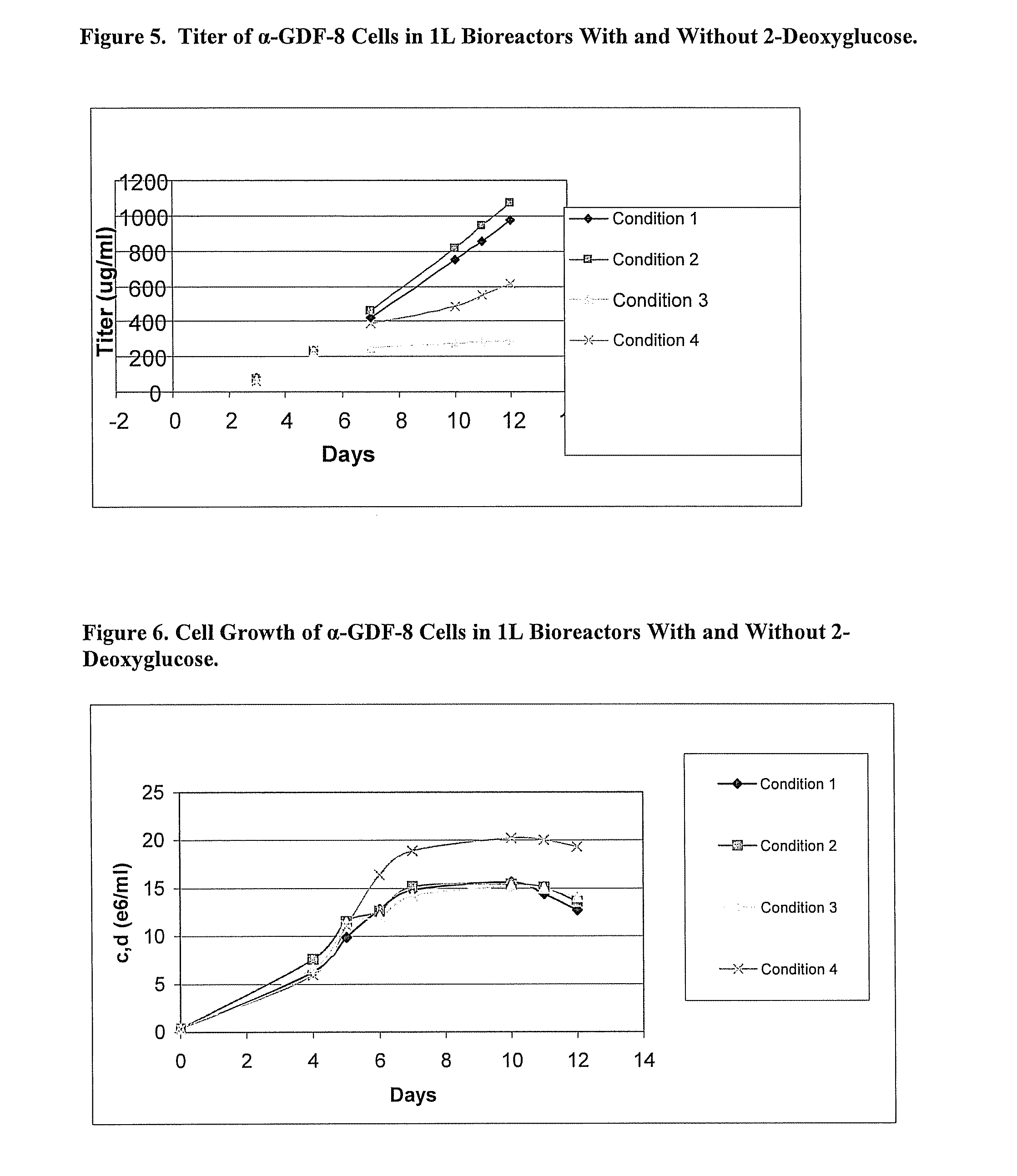
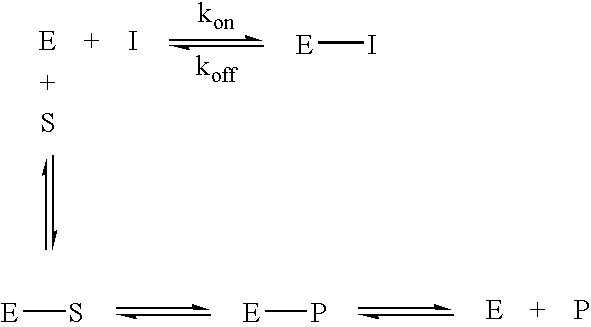
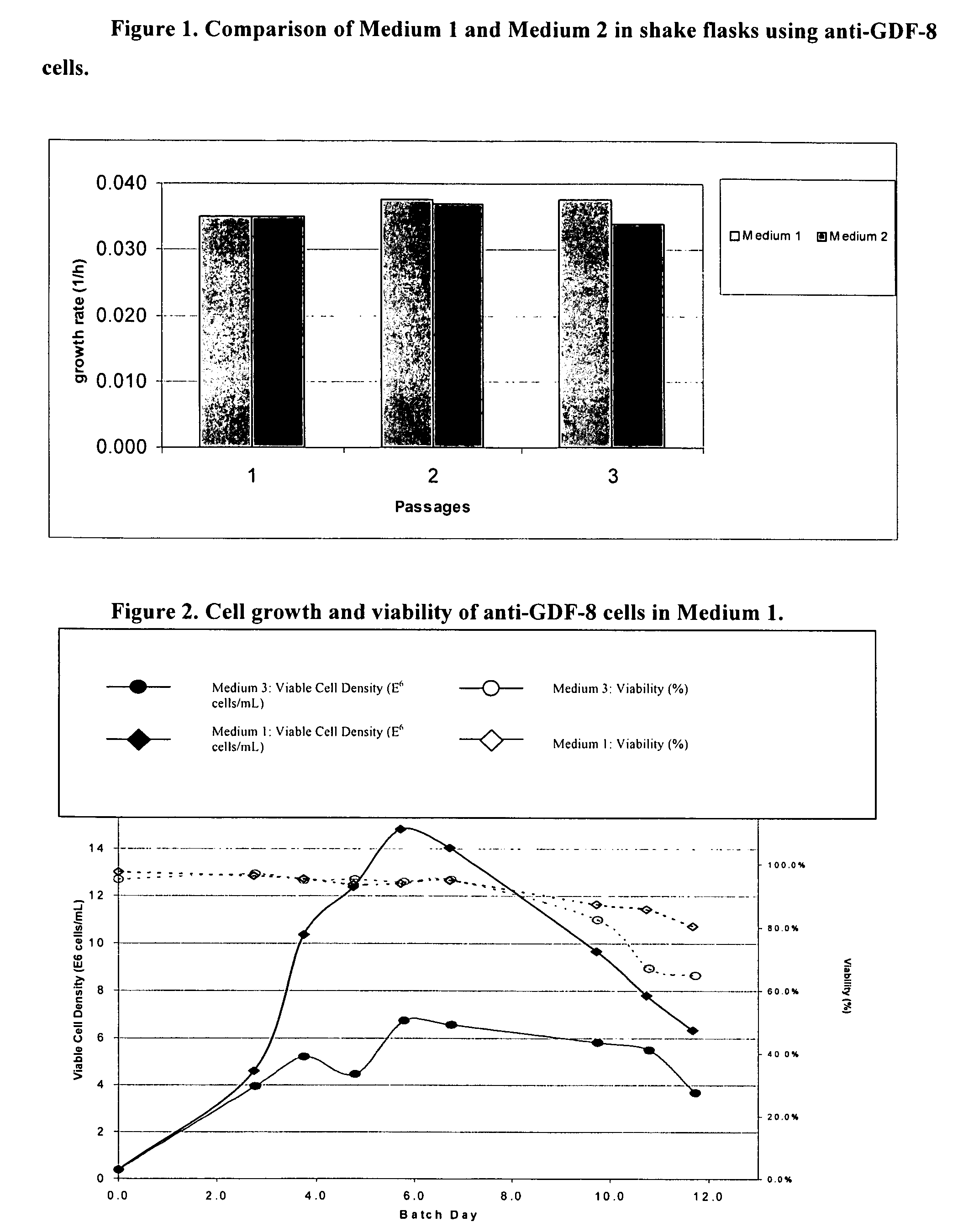
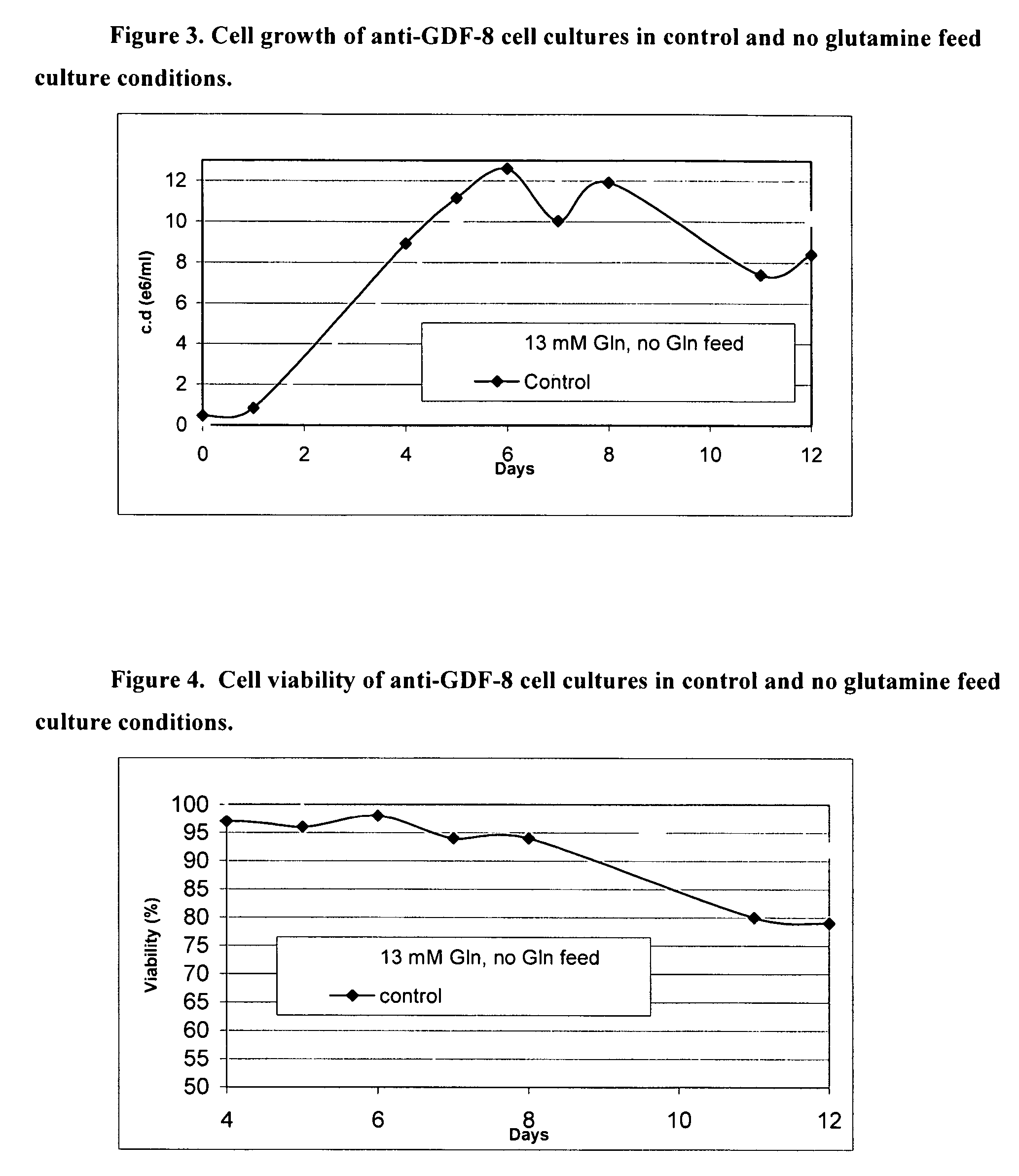
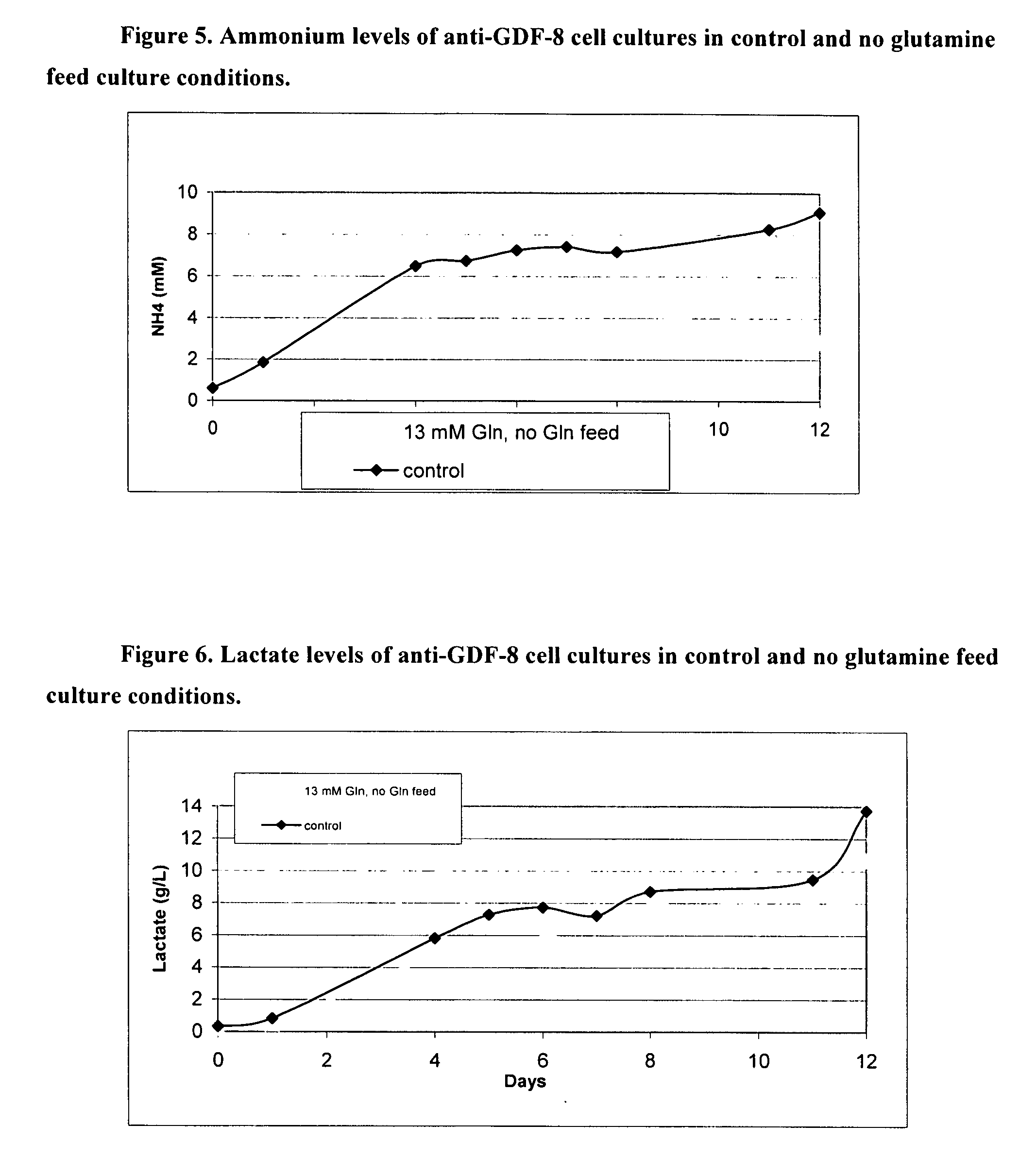
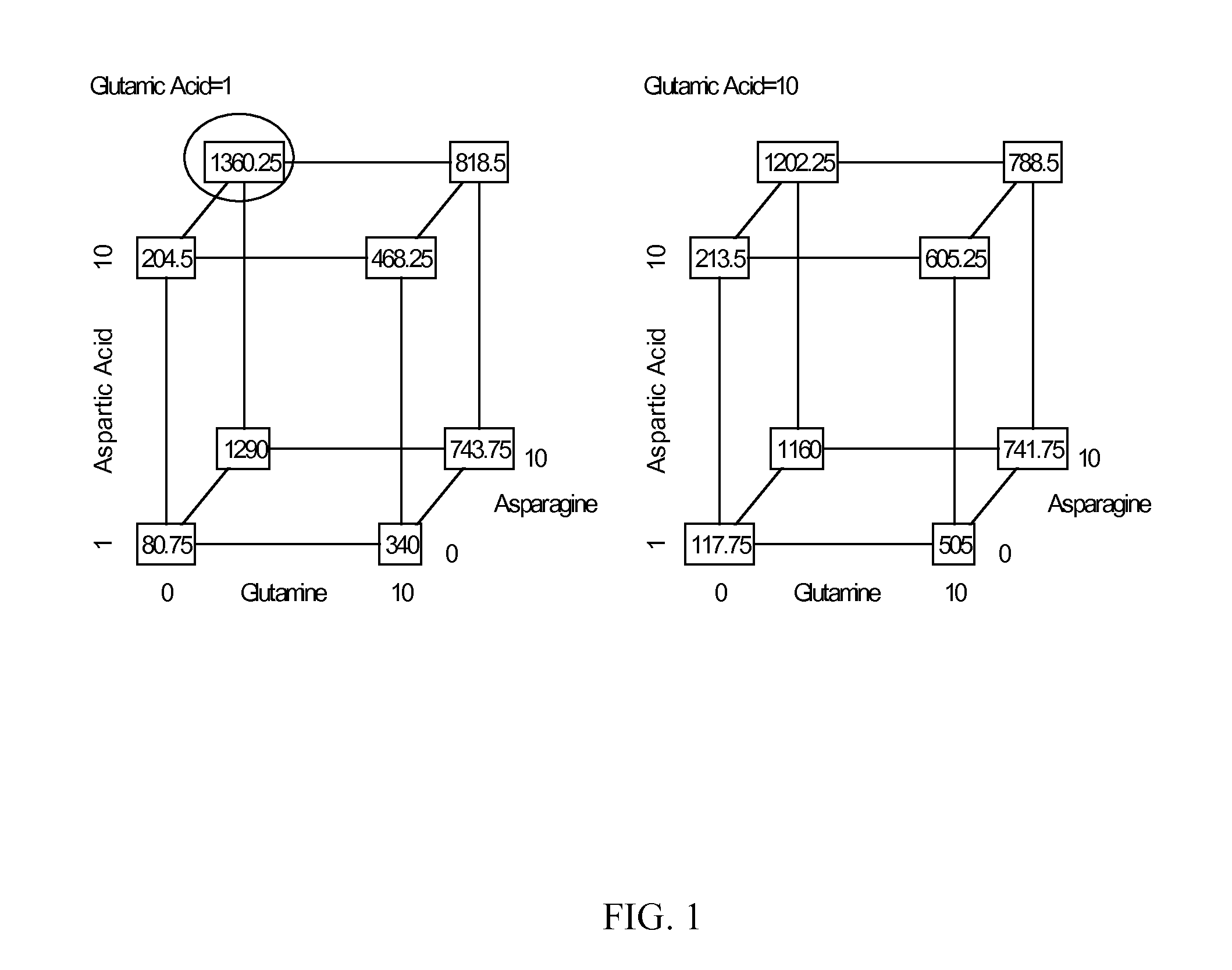
An improved system for large scale production of proteins and / or polypeptides in cell culture, particularly in media characterized by one or more of: i) a cumulative amino acid concentration greater than about 70 mM; ii) a molar cumulative glutamine to cumulative asparagine ratio of less than about 2; iii) a molar cumulative glutamine to cumulative total amino acid ratio of less than about 0.2; iv) a molar cumulative inorganic ion to cumulative total amino acid ratio between about 0.4 to 1; or v) a combined cumulative glutamine and cumulative asparagine concentration between about 16 and 36 mM, is provided. The use of such a system allows high levels of protein production and lessens accumulation of certain undesirable factors such as ammonium and / or lactate. Additionally, culture methods including a temperature shift, typically including a decrease in temperature when the culture has reached about 20-80% of it maximal cell density, are provided. Alternatively or additionally, the present invention provides methods such that, after reaching a peak, lactate and / or ammonium levels in the culture decrease over time.
Owner:PFIZER IRELAND PHARM CORP
Modification of peptide and protein
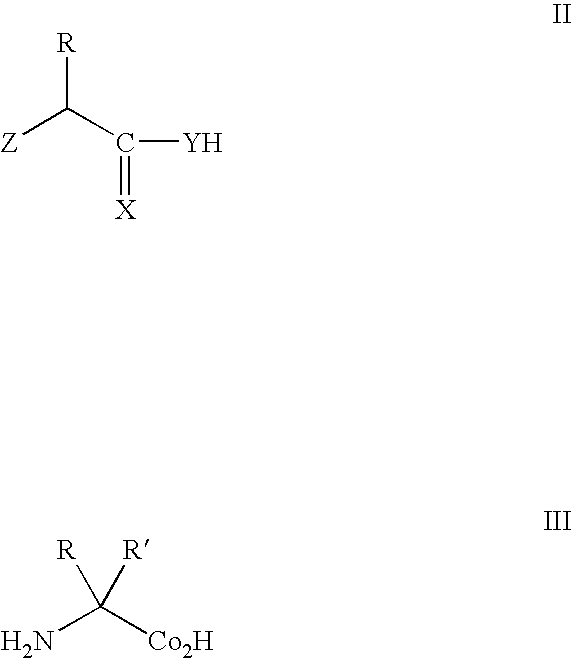
PCT No. PCT / JP95 / 01994 Sec. 371 Date Sep. 8, 1997 Sec. 102(e) Date Sep. 8, 1997 PCT Filed Sep. 29, 1995 PCT Pub. No. WO96 / 10089 PCT Pub. Date Apr. 4, 1996A process for modifying a physiologically active peptide or a physiologically active protein which comprises reacting a physiologically active peptide or a physiologically active protein having at least a glutamine residue with a substance having an amino donor in the presence of a transglutaminase originating in a microorganism to thereby form an acid amide bond at the gamma -position acid amide group of the glutamine residue with the amino group of the amino donor; and the product of modification obtained thereby.
Owner:AJINOMOTO CO INC
Immune enhancing compositions and methods of use thereof
InactiveUS20050271726A1Easy to synthesizeEffective absorptionPowder deliveryOrganic active ingredientsGlycineBlood level
A method of administering parenterally, particularly intramuscularly, glutamine and cystine and glycine plus selenium; or lactalbumin plus selenium; or lactalbumin and glutamine and cystine and glycine plus selenium, through a long-acting pharmaceutically acceptable carrier to a patient. The method comprises injecting a mixture of glutamine, cystine, glycine, lactalbumin and selenium in order to maintain the mixture systemically or locally for a sufficient time period so as to maintain blood levels of glutathione within an improved therapeutic range.
Owner:CRUM ALBERT
Compositions of orthogonal lysyl-trna and aminoacyl-trna synthetase pairs and uses thereof
Compositions and methods of producing components of protein biosynthetic machinery that include orthogonal lysyl-tRNAs, orthogonal lysyl-aminoacyl-tRNA synthetases, and orthogonal pairs of lysyl-tRNAs / synthetases, which incorporate homoglutamines into proteins are provided in response to a four base codon. Methods for identifying these orthogonal pairs are also provided along with methods of producing proteins with homoglutamines using these orthogonal pairs.
Owner:THE SCRIPPS RES INST
Dietary supplement for boosting energy and increasing muscular strength
InactiveUS6193973B1Enhances general energy boostIncrease muscle strengthBiocideOrganic chemistryMuscle strengthDietary supplement
A dietary supplement is provided that comprises creatine combined with ginseng and astragalus and, optionally, glutamine. The dietary supplement enhances the general energy boost and muscular strength increase achieved from the consumption of creatine alone.
Owner:TUTTLE B DAVID
Production of polypeptides
ActiveUS20060121568A1Cell receptors/surface-antigens/surface-determinantsGenetically modified cellsTotal amino acidsInorganic ions
An improved system for large scale production of proteins and / or polypeptides in cell culture, particularly in media characterized by one or more of: i) a cumulative amino acid concentration greater than about 70 mM; ii) a molar cumulative glutamine to cumulative asparagine ratio of less than about 2; iii) a molar cumulative glutamine to cumulative total amino acid ratio of less than about 0.2; iv) a molar cumulative inorganic ion to cumulative total amino acid ratio between about 0.4 to 1; or v) a combined cumulative glutamine and cumulative asparagine concentration between about 16 and 36 mM, is provided. The use of such a system allows high levels of protein production and lessens accumulation of certain undesirable factors such as ammonium and / or lactate. Additionally, culture methods including a temperature shift, typically including a decrease in temperature when the culture has reached about 20-80% of it maximal cell density, are provided. Alternatively or additionally, the present invention provides methods such that, after reaching a peak, lactate and / or ammonium levels in the culture decrease over time.
Owner:PFIZER IRELAND PHARM CORP
Glycolysis-inhibiting substances in cell culture
ActiveUS8192951B2Reduce accumulationHigh levelGenetically modified cellsCulture processMetabolic wastePharmaceutical drug
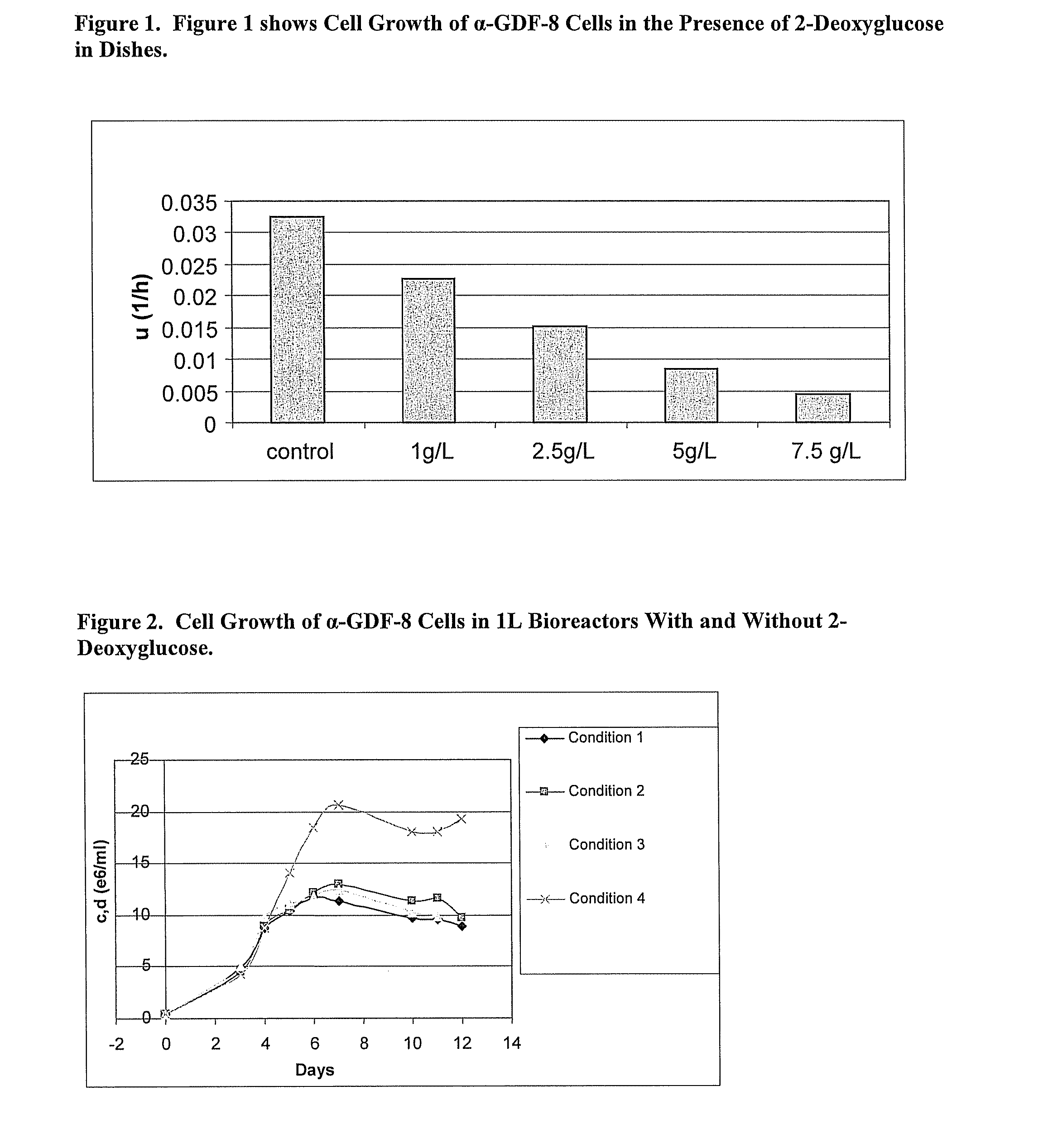
An improved system for large scale production of proteins and / or polypeptides in cell culture is provided. In accordance with the present invention, cells expressing the protein or polypeptide of interest are grown in media that comprise a glycolysis-inhibiting substance. Additionally and / or alternatively, cells expressing the protein or polypeptide of interest are grown in media in which glutamine is limited. The use of such a system allows high levels of protein or polypeptide production and lessens accumulation of undesirable metabolic waste products such as lactate. Proteins and polypeptides expressed in accordance with the present invention may be advantageously used in the preparation of pharmaceutical, immunogenic, agricultural or other commercial compositions.
Owner:WYETH LLC
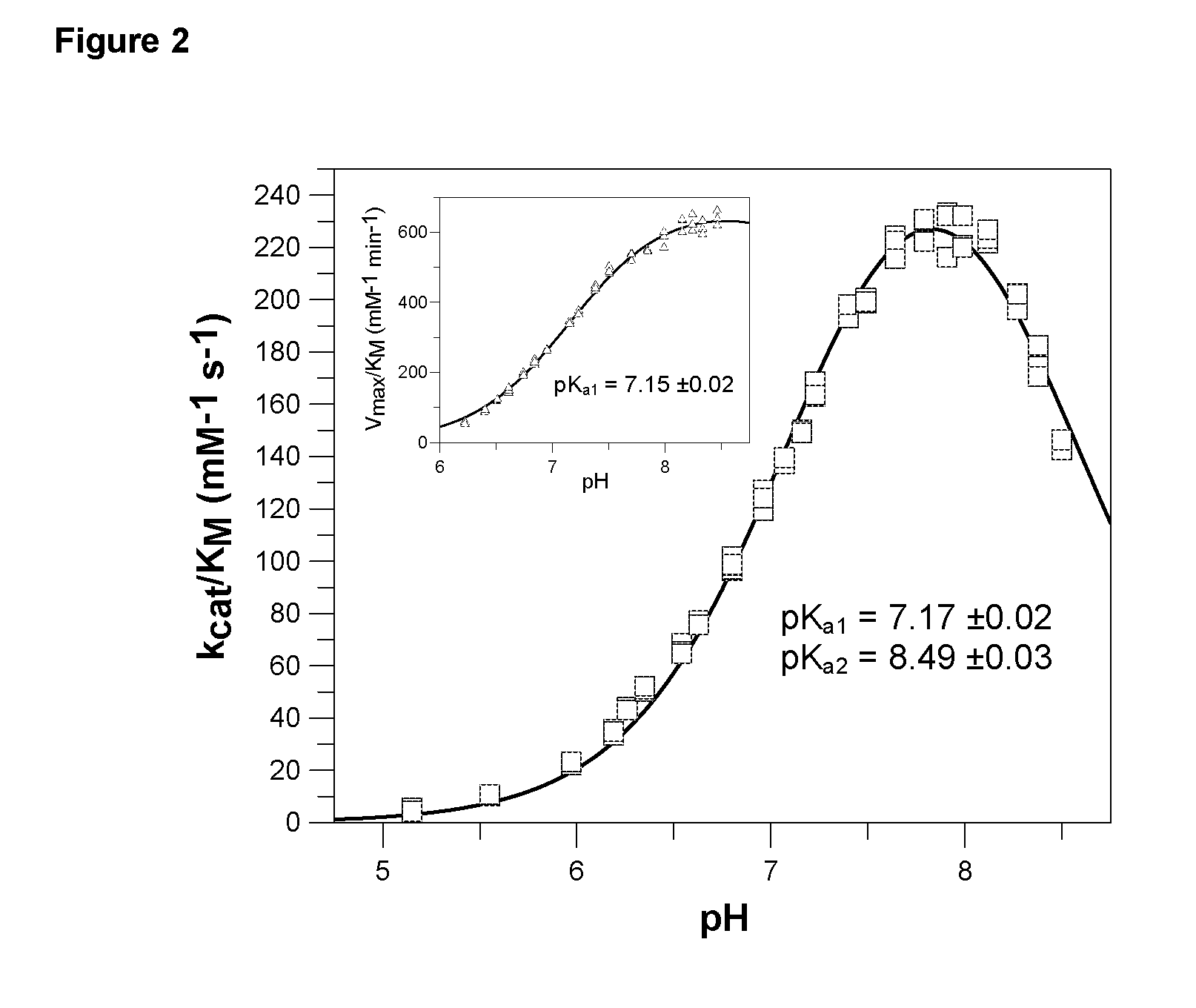
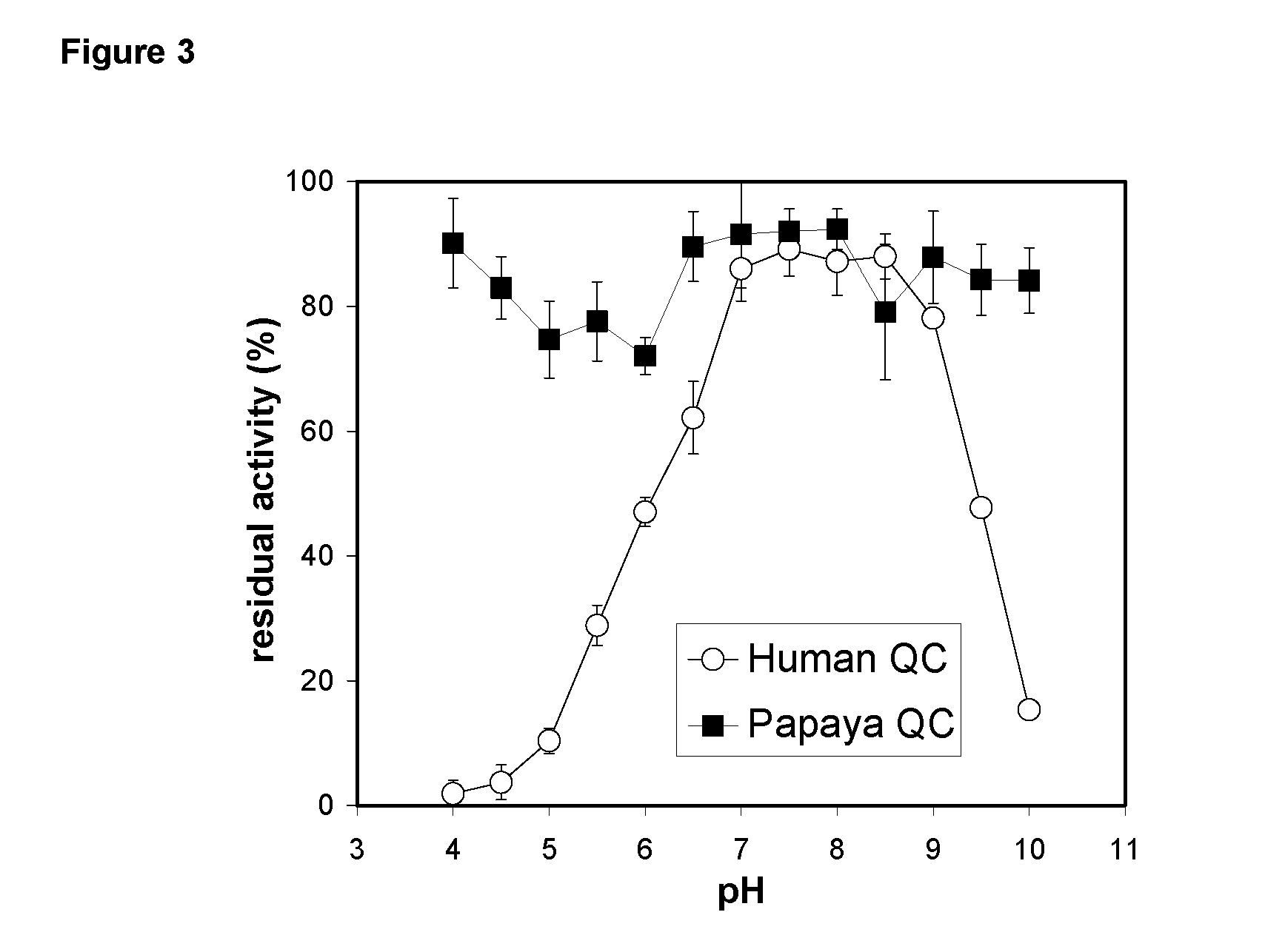
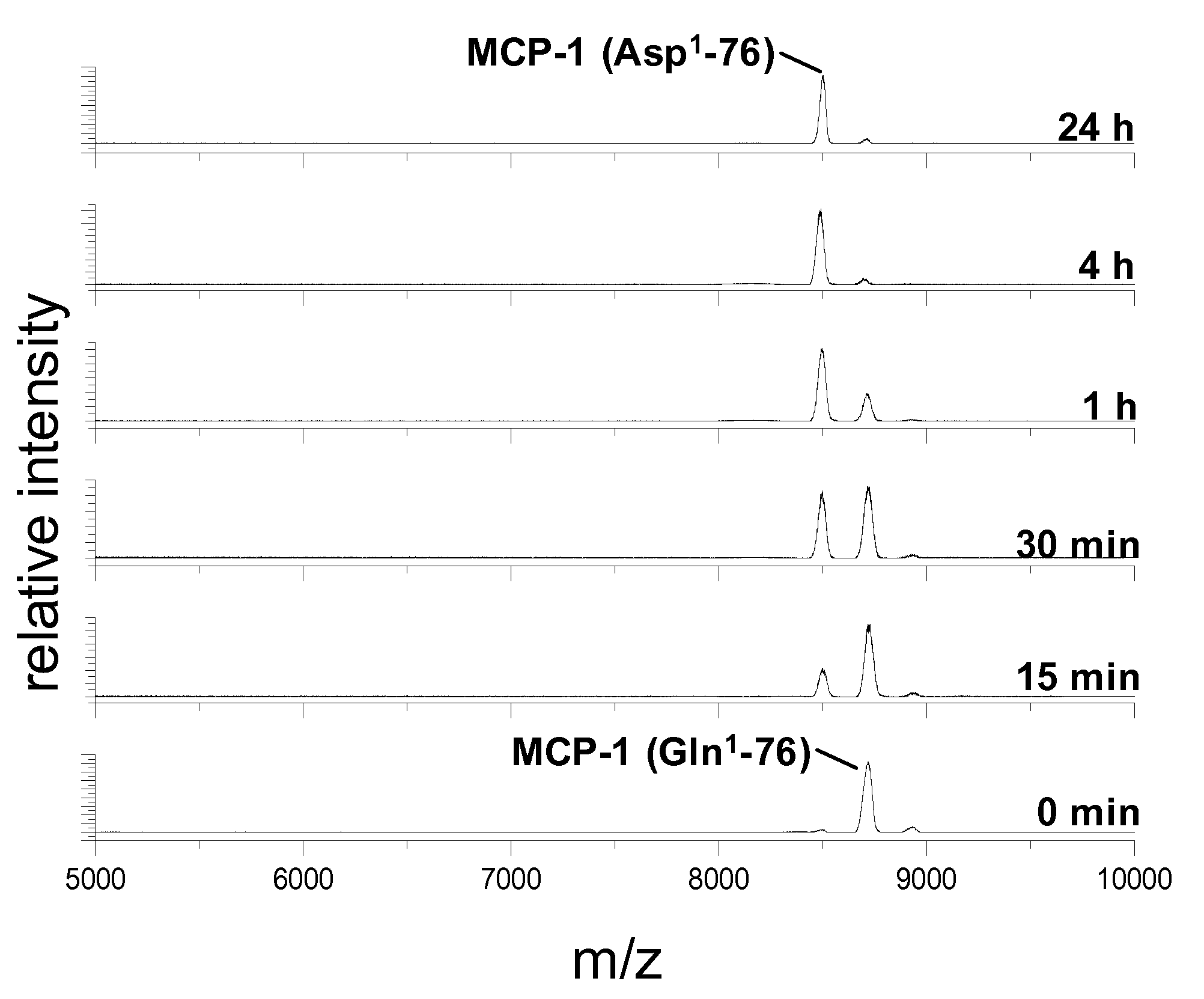
Inhibitors of glutaminyl cyclase
The present invention relates to novel inhibitors of glutaminyl cyclase and combinations thereof for the treatment of neuronal disorders, especially Alzheimer's disease, Down Syndrome, Parkinson disease, Chorea Huntington, pathogenic psychotic conditions, schizophrenia, impaired food intake, sleep-wakefulness, impaired homeostatic regulation of energy metabolism, impaired autonomic function, impaired hormonal balance, impaired regulation, body fluids, hypertension, fever, sleep dysregulation, anorexia, anxiety related disorders including depression, seizures including epilepsy, drug withdrawal and alcoholism, neurodegenerative disorders including cognitive dysfunction and dementia.
Owner:VIVORYON THERAPEUTICS NV
Use of effectors of glutaminyl and glutamate cyclases
ActiveUS20070191366A1Stimulate gastrointestinal tractPrevent proliferationBiocideNervous disorderCyclaseMedicine
The present invention provides novel physiological substrates of mammalian glutaminyl cyclase (QC, EC 2.3.2.5), new effectors of QC, methods for screening for such effectors, and the use of such effectors and pharmaceutical compositions comprising such effectors for the treatment of conditions that can be treated by modulation of QC-activity. Preferred compositions additionally comprise inhibitors of DP IV or DP IV-like enzymes for the treatment or alleviation of conditions that can be treated by modulation of QC- and DP IV-activity.
Owner:VIVORYON THERAPEUTICS NV
Nutritional conjunctive support therapy for recovery in animals following stress or illness
ActiveUS20070009502A1Good treatment effectRapid preventionBiocideBacteria material medical ingredientsHeifer calfFluid replacement
A composition of probiotics, vitamins and minerals, electrolytes with glutamine and glucose along with medium chain triglycerides are provided as a supplement to animals. This is administered to cattle, calves, sheep, pigs, horses, dogs, and cats that are experiencing stress and or are undergoing medical drug therapy to treat conditions of diseases along with pre and post operative surgical conditions. The composition helps correct imbalances in beneficial bacteria, provides energy and helps in rehydration to reduce recovery time from stress or in disease treatment in these animals.
Owner:VETAB PLUS
Use of glutaminyl cyclase inhibitors
ActiveUS20090068699A1BiocideOrganic active ingredientsMild cognitive impairment (MCI)Percent Diameter Stenosis
An inhibitor of a glutaminyl peptide cyclotransferase, and use thereof for the treatment and / or prevention of a disease or disorder selected from the group consisting of inflammatory diseases selected froma. neurodegenerative diseases, e.g. mild cognitive impairment (MCI), Alzheimer's disease, neurodegeneration in Down Syndrome, Familial British Dementia, Familial Danish Dementia, multiple sclerosis,b. chronic and acute inflammations, e.g. rheumatoid arthritis, atherosclerosis, restenosis, pancreatitis,c. fibrosis, e.g. lung fibrosis, liver fibrosis, renal fibrosis,d. cancer, e.g. cancer / hemangioendothelioma proliferation, gastric carcinomas,e. metabolic diseases, e.g. hypertension,f. and other inflammatory diseases, e.g. neuropathic pain, graft rejection / graft failure / graft vasculopathy, HIV infections / AIDS, gestosis, tuberous sclerosis.Additionally disclosed are a respective diagnostic method, assay and kit.
Owner:VIVORYON THERAPEUTICS NV
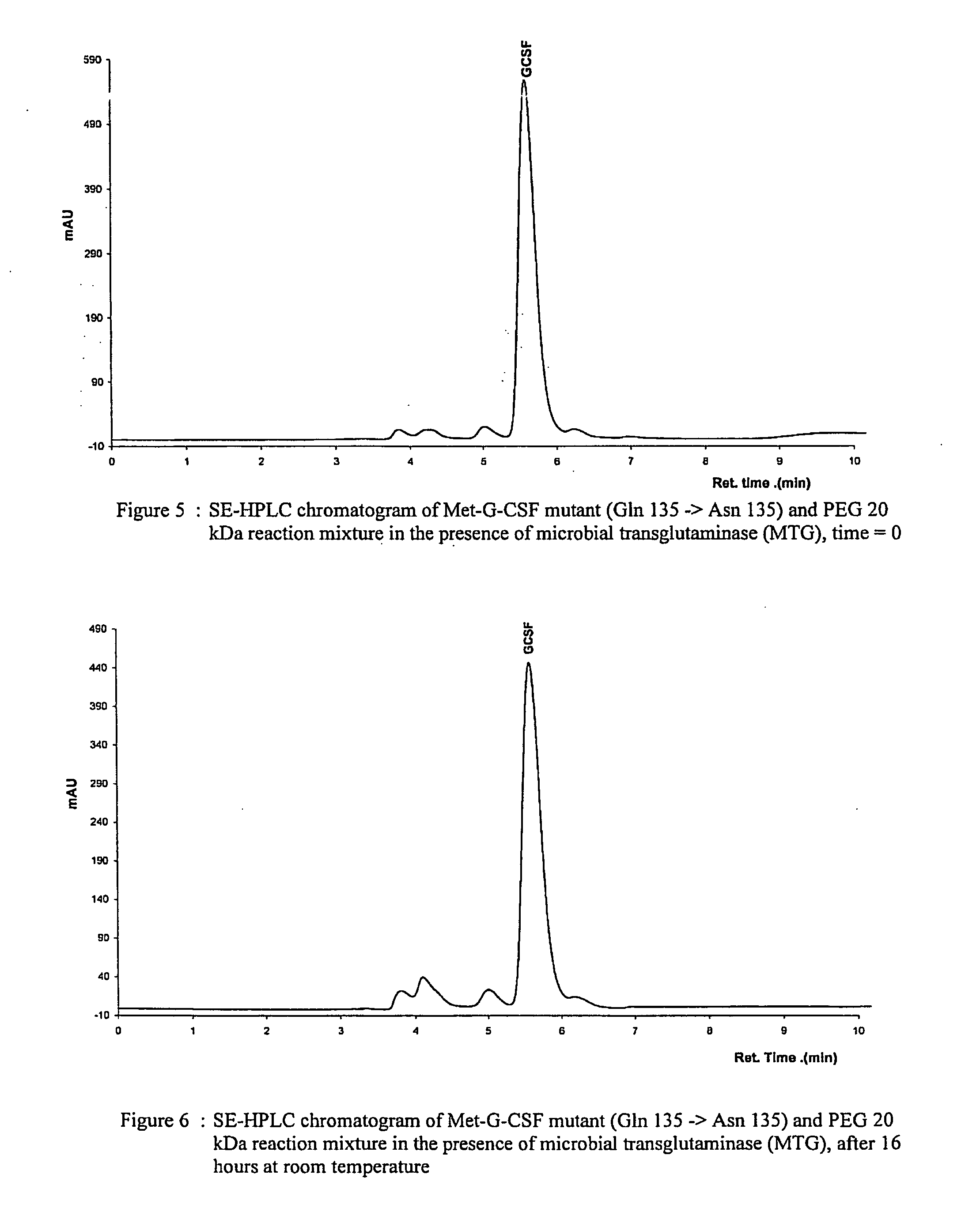
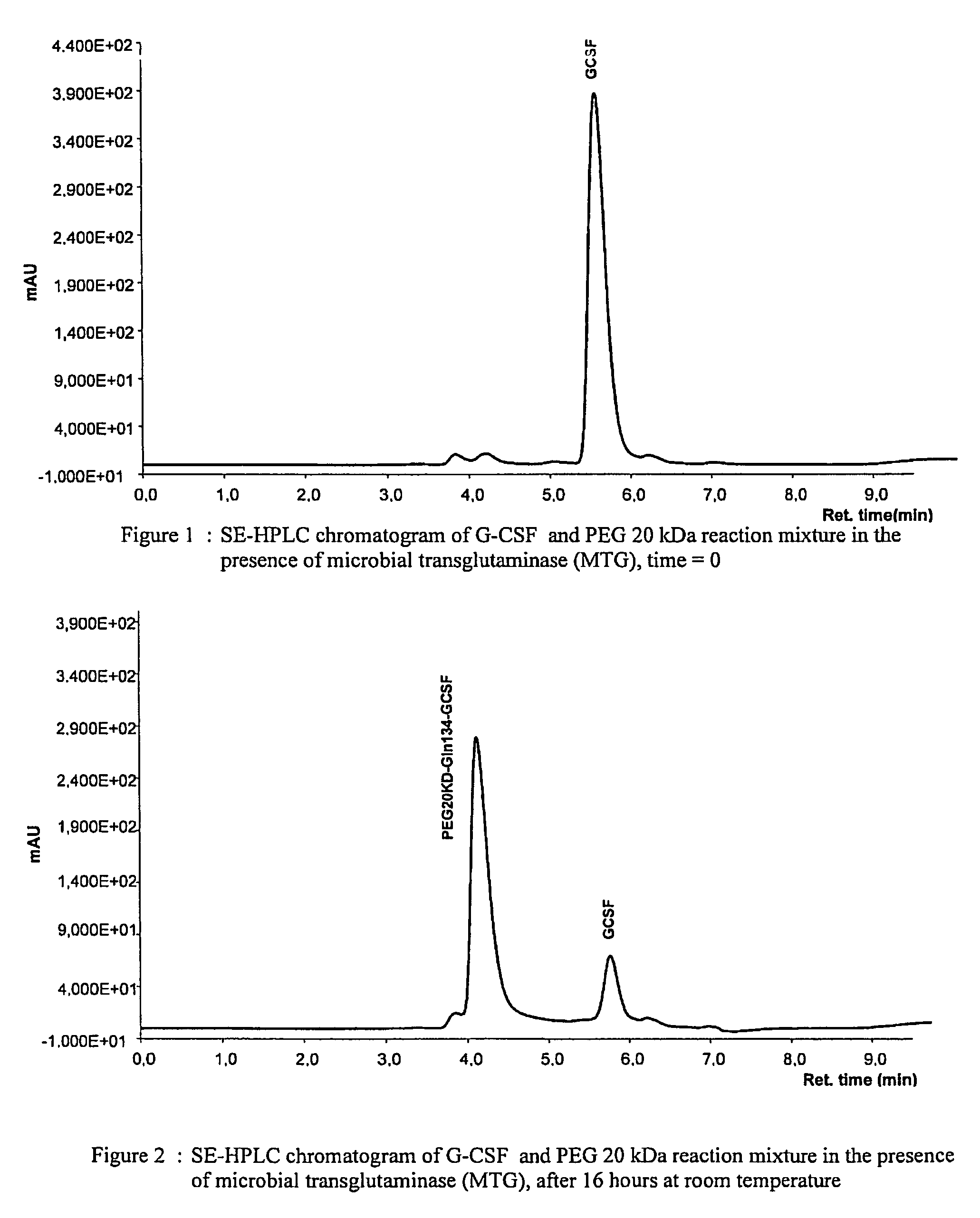
G-csf site-specific mono-conjugates
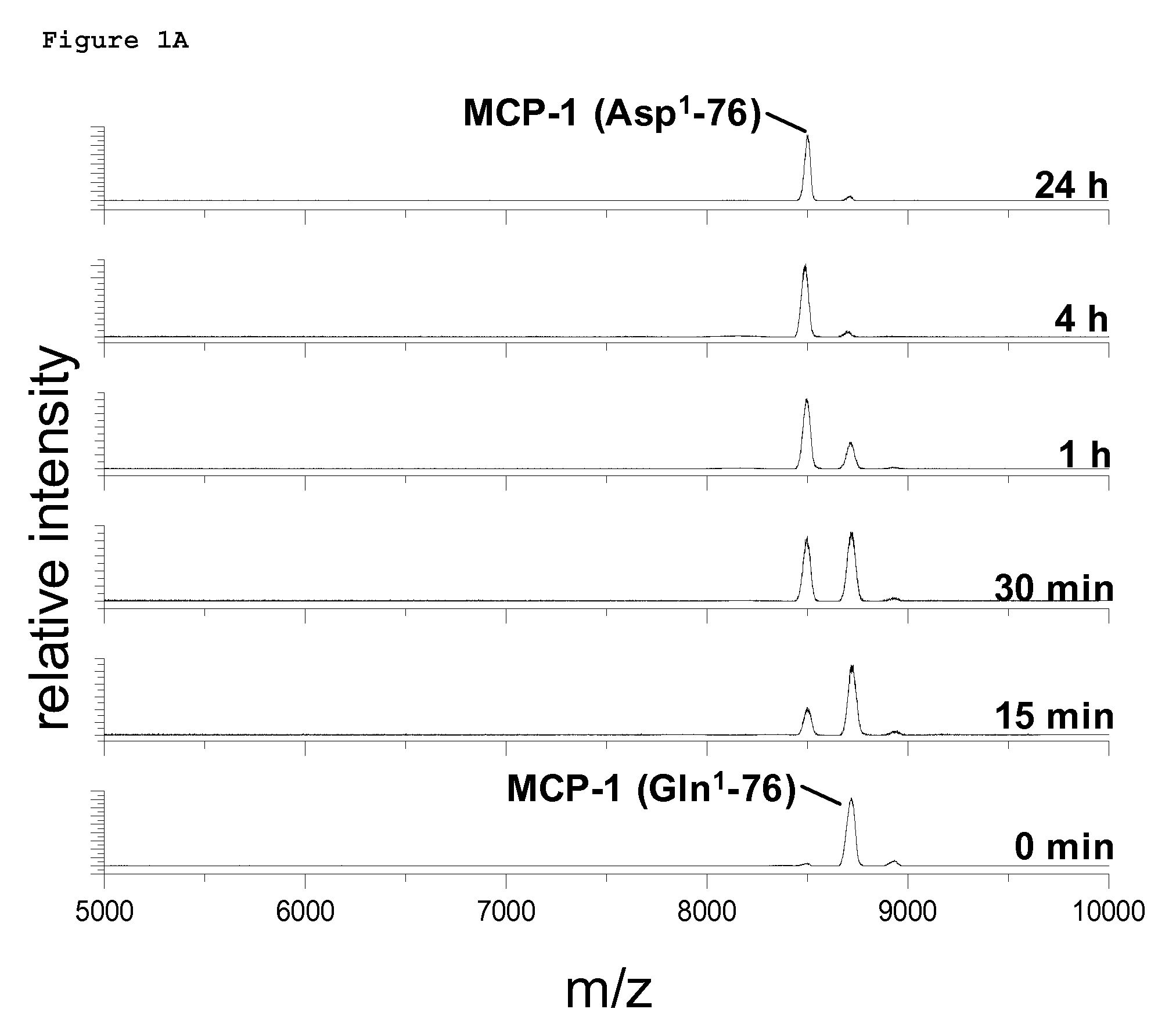
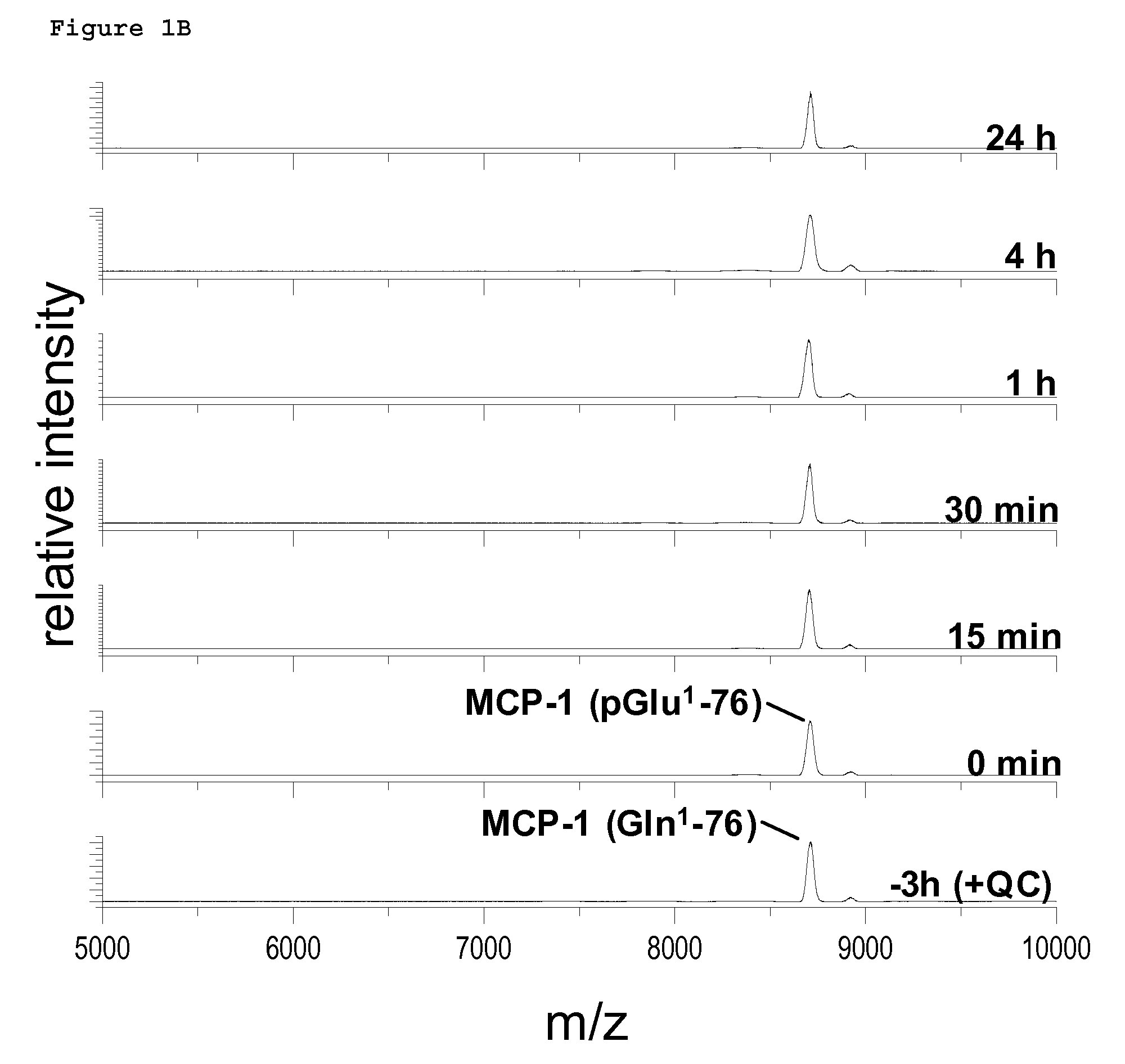
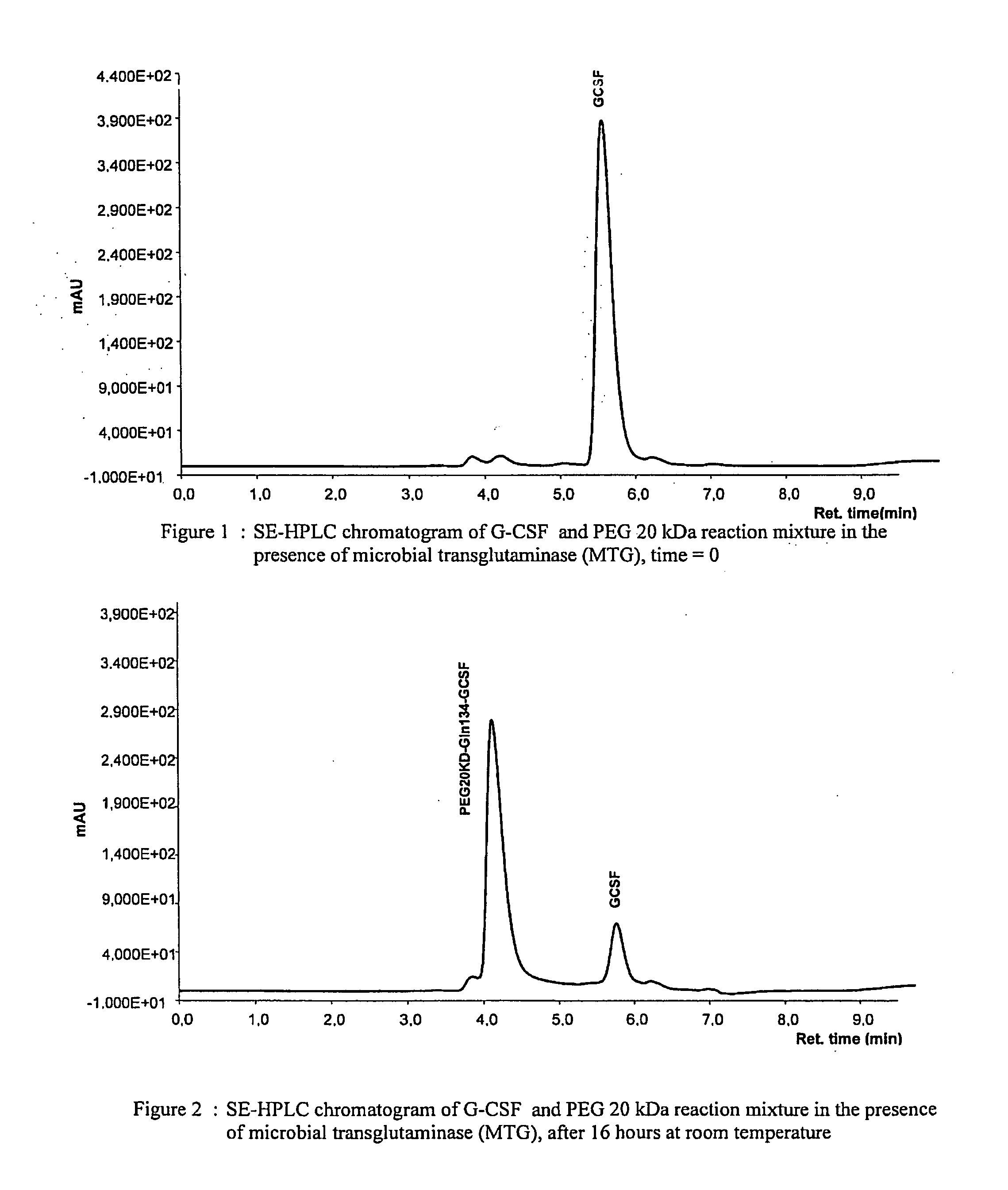
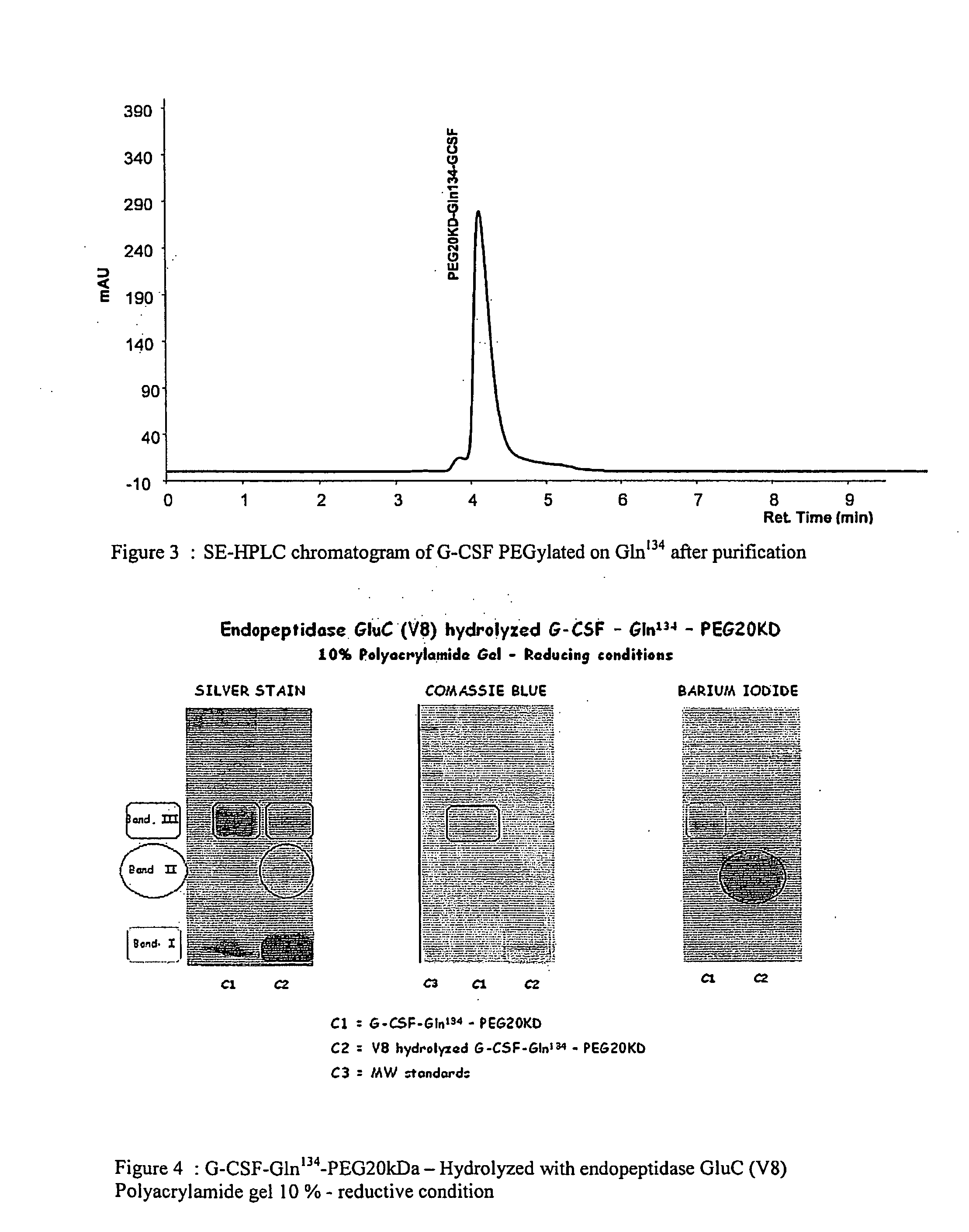
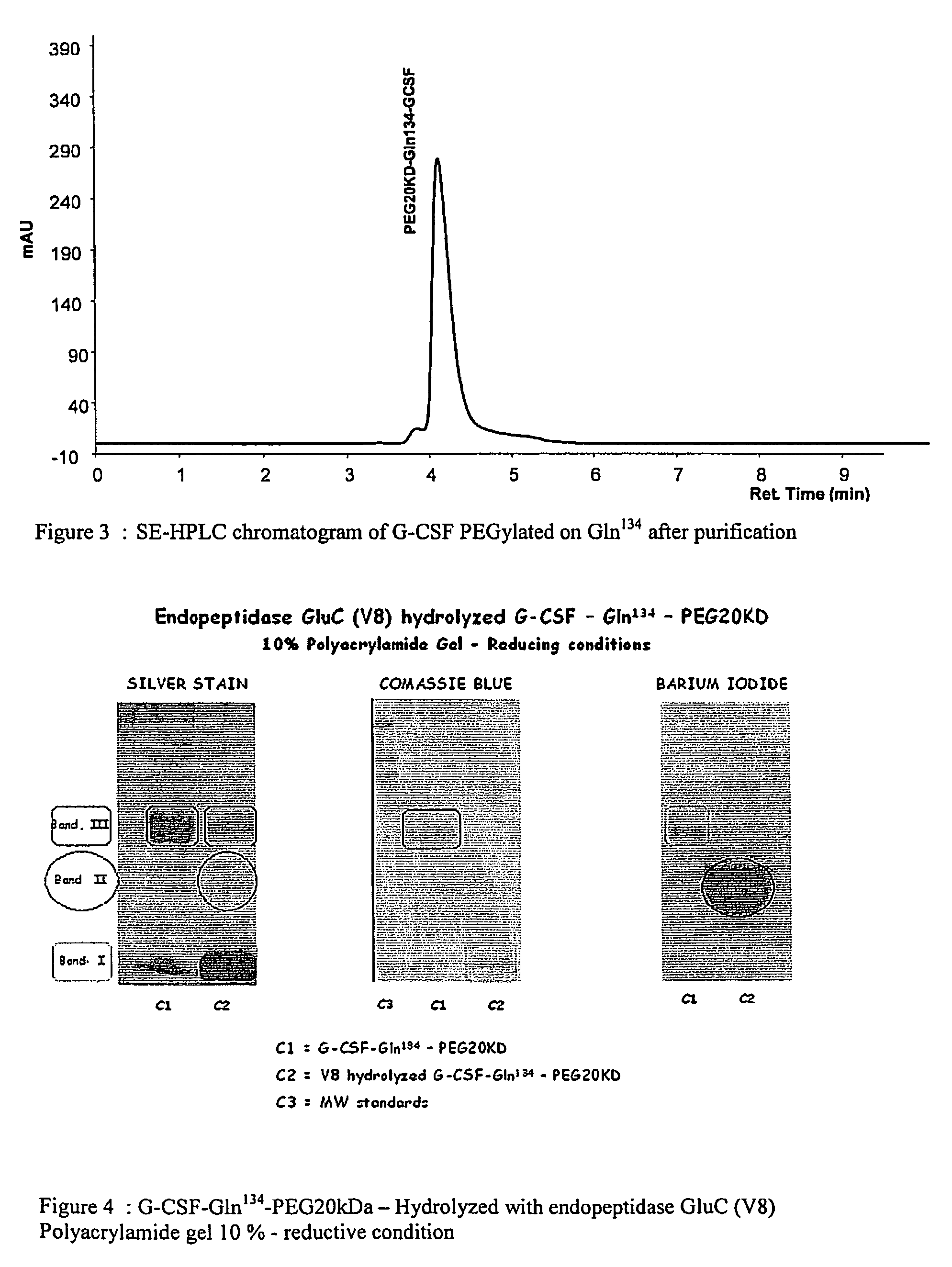
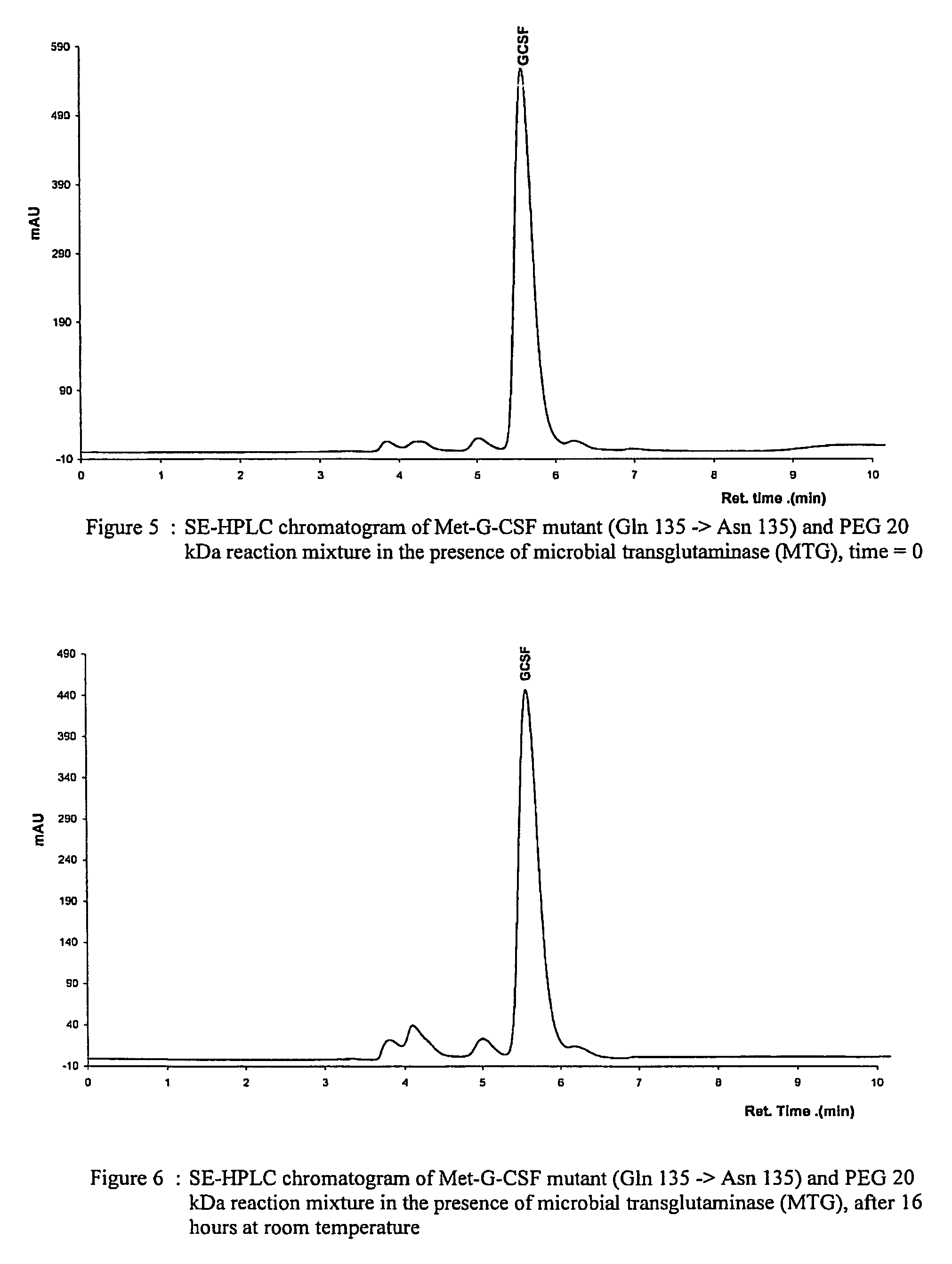
Novel site-specific mono-conjugates of Granulocyte Colony Stimulating Factor (G-CSF) are hereby described, with analogues and derivatives thereof, which stimulate proliferation and differentiation of progenitor cells to mature neutrophiles. These conjugates have been obtained using transglutaminase to covalently and site-specifically bind a hydrophilic, non-immunogenic polymer to a single glutamine residue of the human G-CSF native sequence and analogues thereof. These novel site-specific mono-conjugated derivatives are recommended for therapeutic use since they are stable in solution and exhibit significant biological activity in vitro and a longer bloodstream half-life, as compared to the non-conjugated protein, with a consequent prolonged pharmacological activity.
Owner:BIO KER
Freezing flour-dough improver and uses thereof
ActiveCN101411344AOvercome stabilityOvercome volumeDough treatmentPre-baking dough treatmentYeastVitamin C
The invention discloses a frozen dough modifying agent, which is prepared by evenly mixing an enzyme preparation (including one or a plurality of alpha-amylase, cellulose, hemicellulase, pentosanase, lipase, glucose oxidase, glutamine transaminage and so on), vitamin C, an emulsifying agent, a thickening agent, wheat gluten powder, lecithin and stuffing according to certain proportion. The frozen dough modifying agent has the advantages that the frozen dough modifying agent can effectively improve the stability of dough during fermentation and roasting processes, improve the freezing resistance property of yeasts, increase the volume of finished products, improve the tissue of the finished products, reduce the loss of the vitality of the yeasts during the freezing process, and effectively delay the aging of the finished products.
Owner:ANGELYEAST CO LTD
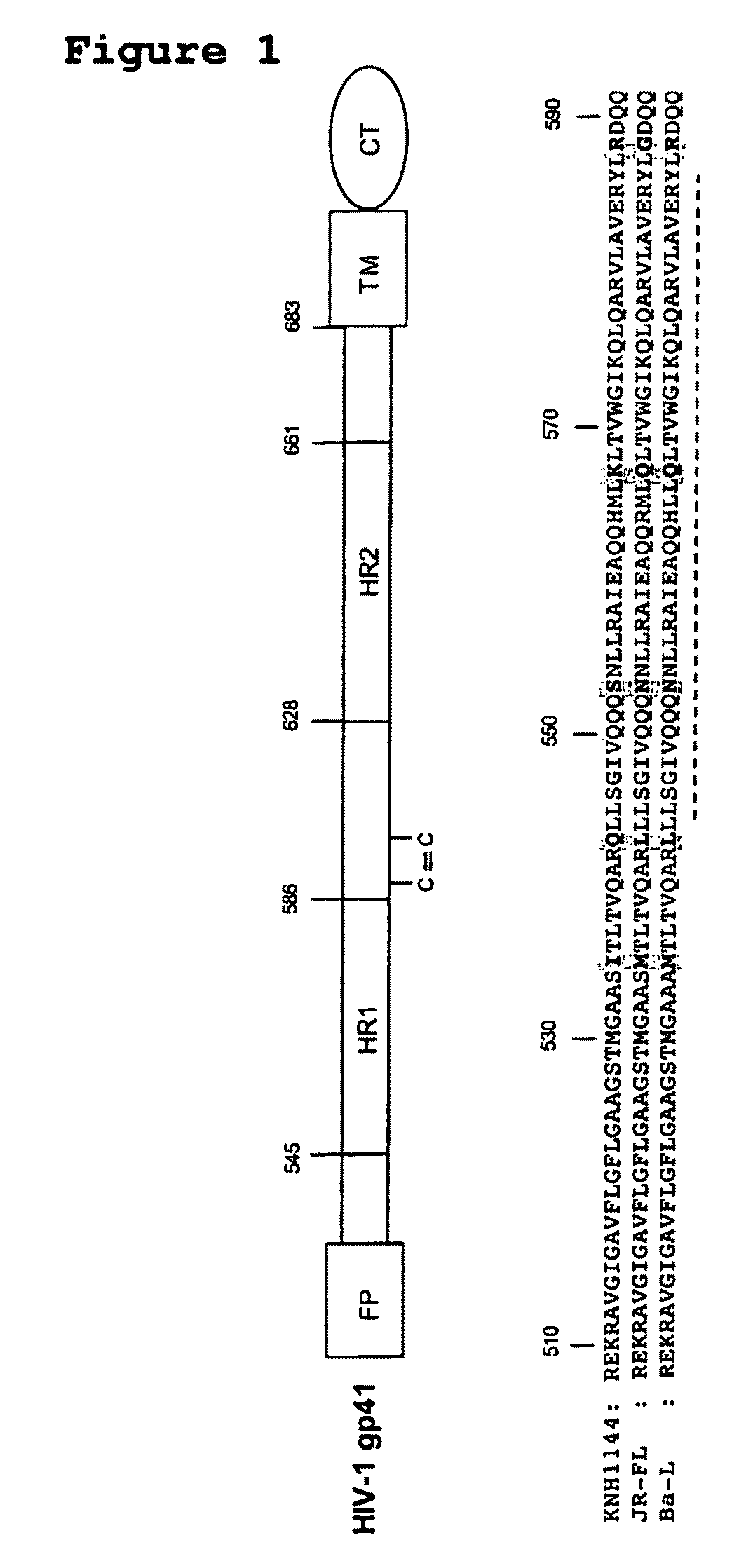
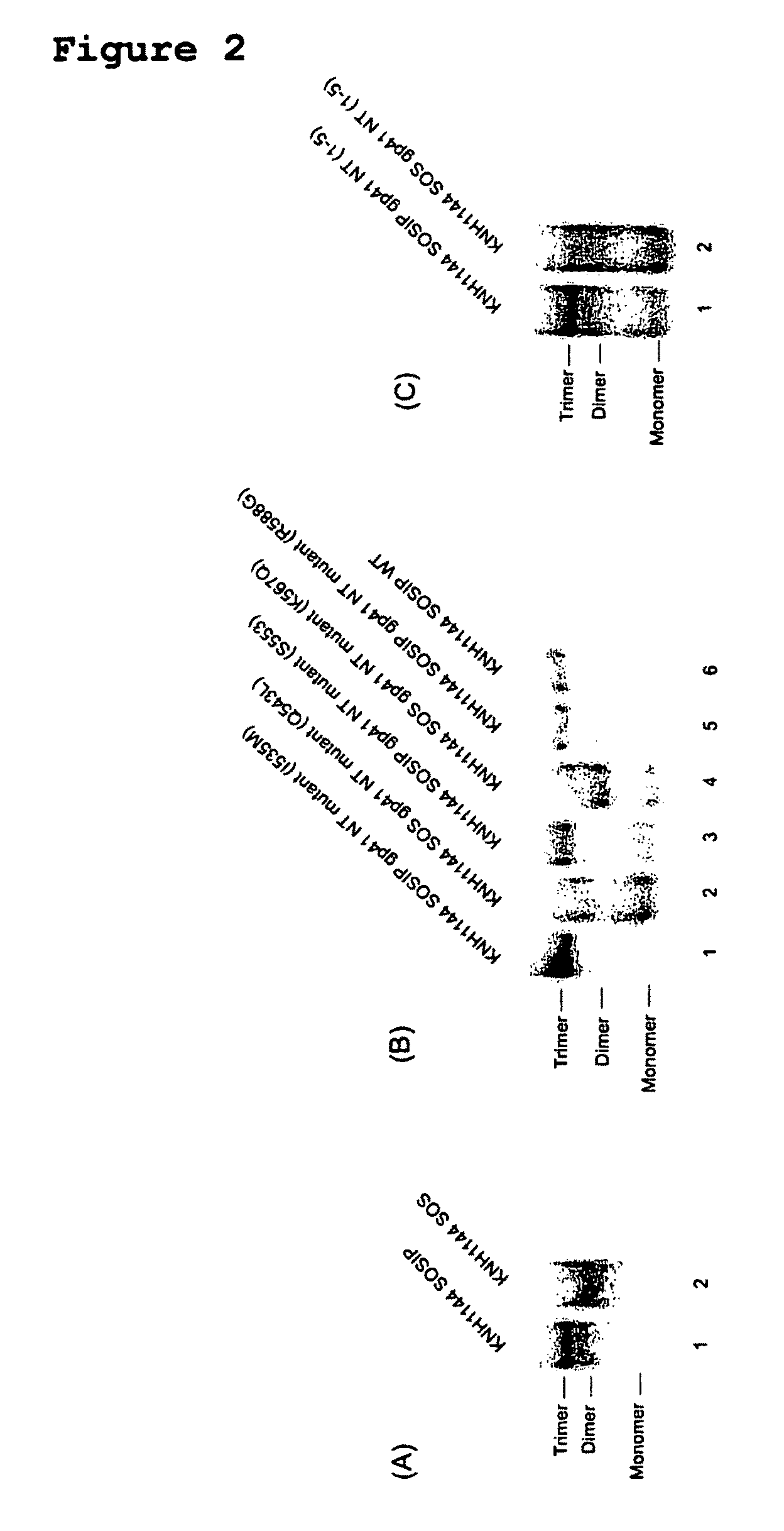
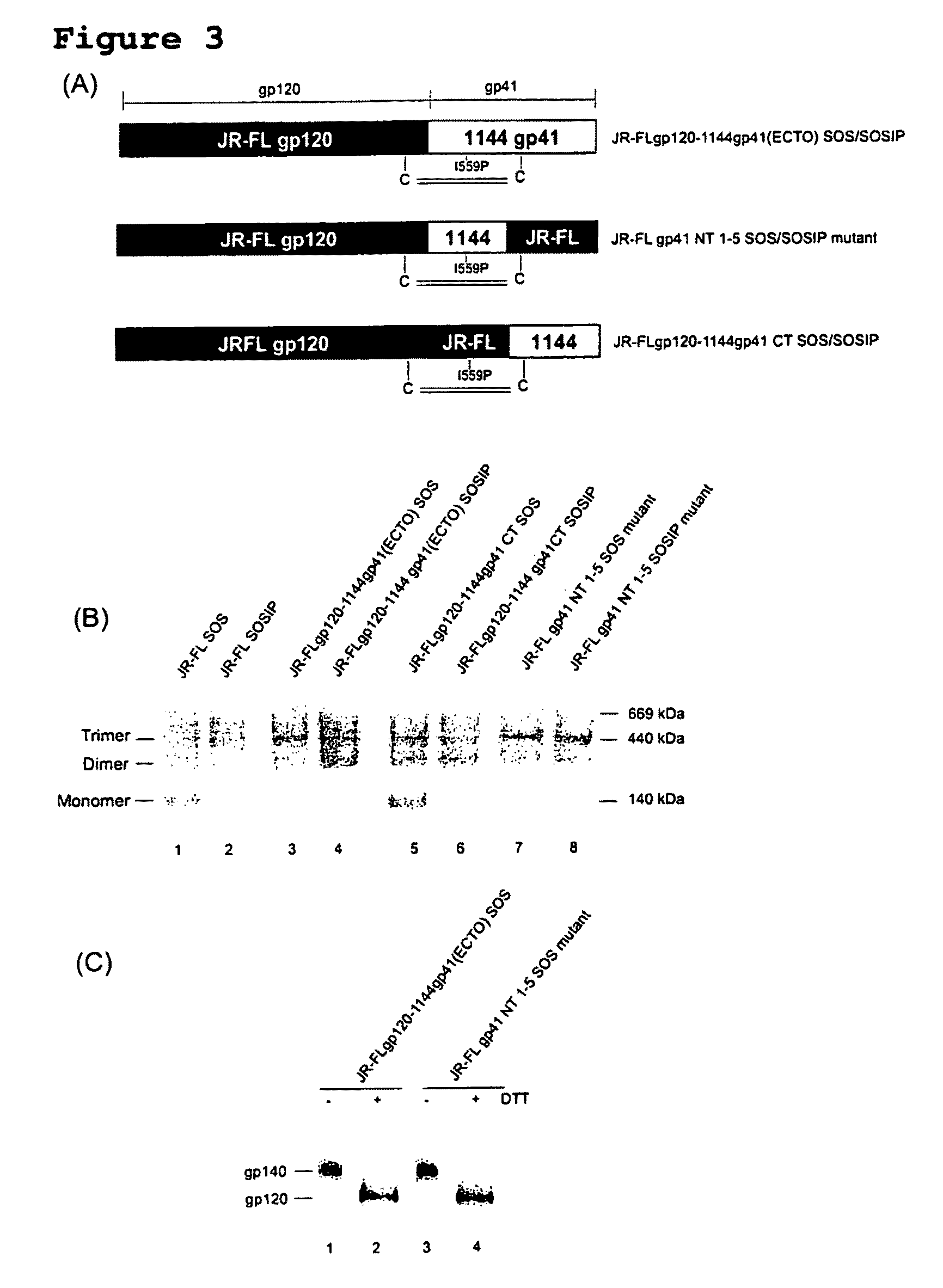
Soluble, stabilized, proteolytically cleaved, trimeric HIV-1 gp140 proteins comprising modifications in the N-terminus of the gp41 ectodomain
InactiveUS7939083B2Improve stabilityOverall antigenic structure of the trimer not adversely affectedViral antigen ingredientsVirus peptidesArginineGlutamine
Owner:CORNELL RES FOUNDATION INC +1
G-CSF site-specific mono-conjugates
Owner:BIO KER SRL
Production of anti-abeta
InactiveUS7335491B2Antibody mimetics/scaffoldsGenetically modified cellsBiotechnologyTotal amino acids
Owner:PFIZER IRELAND PHARM CORP
Compositions containing delta-9-THC-amino acid esters and process of preparation
ActiveUS8809261B2Improve formulation characteristic and bioavailabilityExcellent thermalBiocideSenses disorderDiseaseTyrosine
Compositions of the formulae (I), (II) and (III); where R1, R2 and R3 are residues of amino acids such as, but not limited to, valine, sarcosine, leucine, glutamine, tryptophan, tyrosine, alanine and 4(4-aminophenyl)butyric acid or combination thereof, and salts thereof. Methods of preparation of these compositions and methods of treating any disease condition responsive to THC comprising administration of at least one these compositions in a pharmaceutically acceptable carrier using a pharmaceutically acceptable formulation.
Owner:UNIVERSITY OF MISSISSIPPI
Protein ligands
ActiveUS7709209B2Retention characteristicReduce leakageImmunoglobulins against animals/humansBiological testingComplementarity determining regionChemical stability
Owner:CYTIVA BIOPROCESS R&D AB
Production of proteins in glutamine-free cell culture media
ActiveUS20110091936A1Enhance cell viabilityIncrease culture longevityHydrolasesAntibody mimetics/scaffoldsCell culture mediaMammalian cell
Owner:F HOFFMANN LA ROCHE & CO AG
Nursery pig concentrated feed
ActiveCN101606639AImprove orderImprove immunityFood processingAnimal feeding stuffAdditive ingredientThreonine
The invention relates to a pig feed, in particular to a nursery pig concentrated feed, comprising the following raw materials by weight proportion: 30-50 of sucking pig composite vitamin, 30-50 of low copper mineral element, 60-100 of peeled soybean meal, 100-180 of fermented soybean meal, 100-150 of extruded soybean, 110-180 of steam fish meal, 50-100 of dried porcine soluble, 60-120 of acid whey powder, 80-90 of glucose powder, 5-10 of glutamine, 80-100 of chyle fat powder, 40-49 of calcium carbonate, 12-14 of basic zinc chloride, 4-6 of salt, 55-60 of calcium biphosphate, 6-10 of sodium butyrate, 12-15 of lysine, 6-8 of threonine, 4-6 of TP-100 enzyme, 4-5 of cysteine, 5-7 of se-enriched yeast and 6-10 of copper chloride hydroxide. The nursery pig concentrated feed has the advantages of milk nutrition composition simulation, good palatability, high digestibility, large feed intake because the baby pigs like to eat and improvement of survival rate as well as immunity of the nursery pigs.
Owner:江西大佑农生物科技有限公司
Freshwater fish feed and preparation method thereof
ActiveCN103340334AMeeting nutritional needsImprove immunityFood processingAnimal feeding stuffEgg proteinPhytase
The invention discloses freshwater fish feed and a preparation method thereof. The feed comprises the following materials in percentage by weight: 5%-17% of import fish powder, 8%-22% of flour, 3%-16% of potato starch, 3%-18% of beer yeast, 5%-15% of bean pulps, 5%-15% of peanut pulps, 5%-10% of rape seed cakes, 2%-8% of corn powder, 3%-9% of cooked egg protein, 5%-8% of dried meat floss powder, 1%-2% of liquid phytase, 1%-3% of non-starch polysaccharase, 0.5%-1.9% of glutamine, 1%-2% of microecological preparation and 1%-3% of immune polysaccharides. The preparation process comprises a crushing step, a mixing step, a pelletizing step and a spraying step. The freshwater fish feed has the beneficial effects of being capable of well satisfying the nutrition needs of freshwater fish, improving the immunity of the freshwater fish, prompting intestinal absorption and improving the production performance of the freshwater fish.
Owner:射阳六和饲料有限公司
Recombinant deamidated gliadin antigen
The present invention provides a method for determining whether a subject is suffering from celiac disease by contacting a sample of bodily fluid from the subject, with an antigen formed from a gliadin fusion protein immobilized on a solid support. The gliadin fusion protein of the antigen includes a recombinant deamidated gliadin linked to a tag such as Glutathione-S transferase (GST) protein. The antigen is prepared by immobilizing on the solid support the gliadin fusion protein via the tag. The antigen can further include tissue Transglutaminase (tTG) cross-linked to the gliadin fusion protein. When tTG is present, the tTG and recombinant deamidated gliadin are mixed together prior to immobilization to the solid phase.
Owner:BIO RAD LAB INC
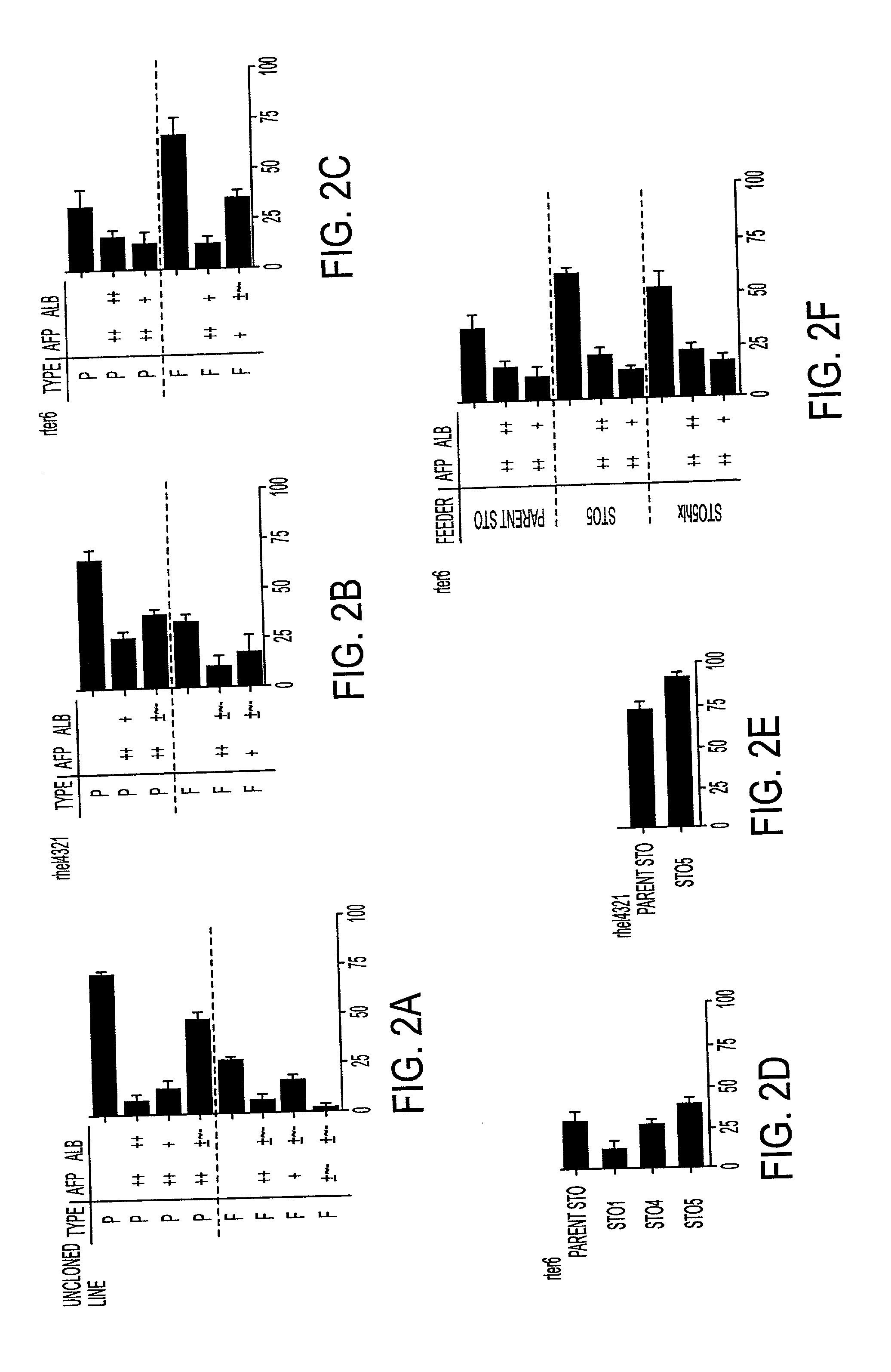
Processes for clonal growth of hepatic progenitor cells
A method of propagating mammalian endodermally derived progenitors such as hepatic progenitors, their progeny, or mixtures thereof is developed which includes culturing mammalian progenitors, their progeny, or mixtures thereof on a layer of embryonic mammalian feeder cells in a culture medium. The culture medium can be supplemented with one or more hormones and other growth agents. These hormones and other growth agents can include insulin, dexamethasone, transferrin, nicotinamide, serum albumin, beta-mercaptoethanol, free fatty acid, glutamine, CUSO4, and H2SeO3. The culture medium can also include antibiotics. Importantly, the culture medium does not include serum. The invention includes means of inducing the differentiation of the progenitors to their adult fates such as the differentiation of hepatic progenitor cells to hepatocytes or biliary cells by adding, or excluding epidermal growth factor, respectively. The method of producing mammalian progenitors is useful in that the progenitors can be used subsequently in one or more of the following processes: identification of growth and differentiation factors, toxicological studies, drug development, antimicrobial studies, or the preparation of an extracorporeal organ such as a bioartificial liver.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com