Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Osteomalacia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Osteomalacia is softening of the bones. It most often occurs because of a problem with vitamin D, which helps your body absorb calcium.
Pharmaceutical composition and method for the transdermal delivery of calcium
InactiveUS20070292493A1Reduce disadvantagesReduce and prevent likelihoodHalogenated hydrocarbon active ingredientsBiocideArginineTryptophan
The present invention relates to a method and transdermal pharmaceutical composition for preventing or reducing the likelihood of calcium deficiency or imbalances caused by calcium deficiency. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of calcium and a pharmaceutically acceptable carrier constituting a pluronic lecithin organogel. In addition to calcium, the transdermal pharmaceutical composition may also contain a therapeutically effective amount of: (1) a pharmaceutically acceptably salt of other minerals such as magnesium, zinc, selenium, manganese, or chromium; (2) a vitamin such as vitamin A, vitamin D, vitamin C, vitamin E or B-complex vitamins, choline, lecithin, inositol, PABA, biotin, or bioflavomoids; (3) a carotenoid such as lycopene or lutein; (4) a hormone such as dehydroepiandrosterone, progesterone, pregnenolone, or melatonin; (5) an amino acid such as arginine, glutamine, lysine, phenylalanine, tyrosine, GABA, tryptophan, carnitine, or acetyl-l-carnitine; (6) a fatty acid such as a fish oil or flax seed oil; (7) a vita-nutrient such as coenzyme Q10; (8) a cartilage building nutrient such as glucosamine, chondroitin, or MSM, (9) a herb such as ginkgo biloba, echinacea, 5-HTP, St. John's wort, or saw palmetto; or (9) any combination thereof. The transdermal pharmaceutical composition may be topically administered to a human to prevent or reduce the likelihood of calcium deficiency or imbalances caused by calcium deficiency such as hypertension, high cholesterol, colon and rectal cancer, osteomalacia, rickets, osteoporosis, cardiovascular disease, preeclampsia, tooth decay, and premenstrual syndrome.
Owner:BRIERRE BARBARA T
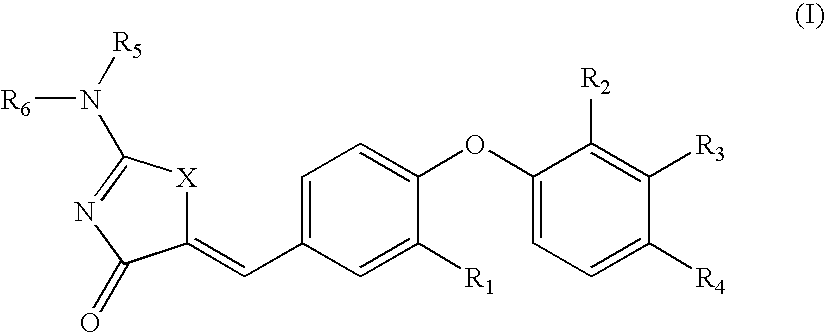
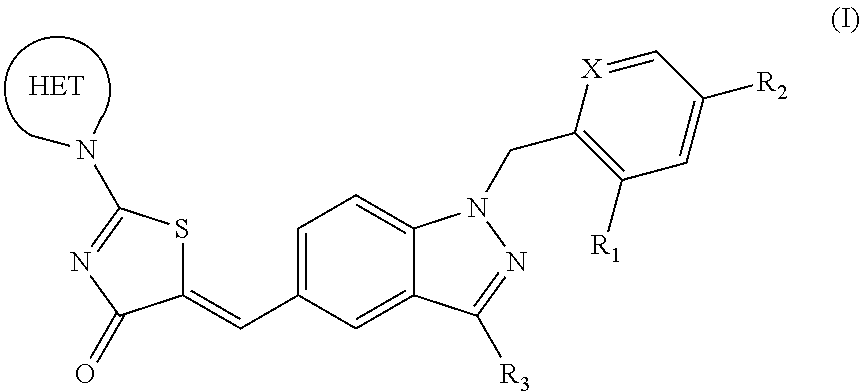
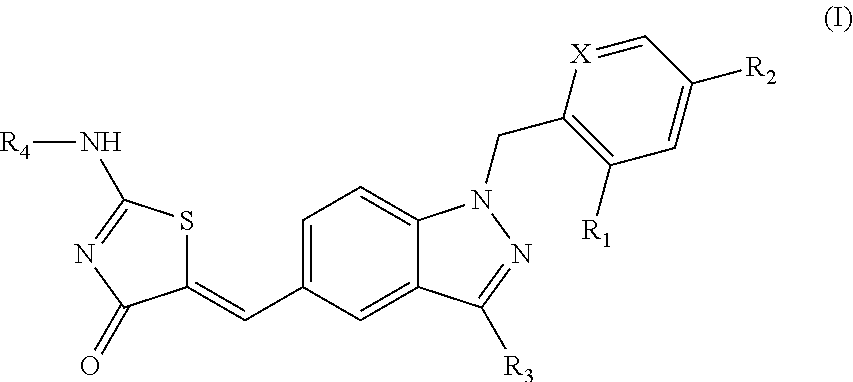
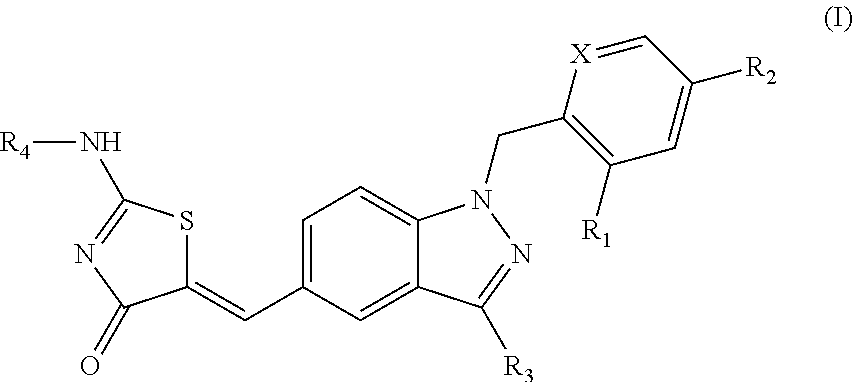
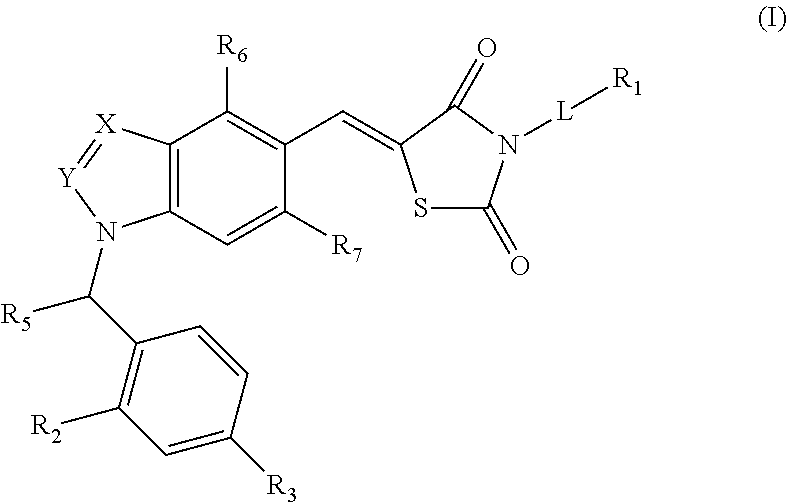
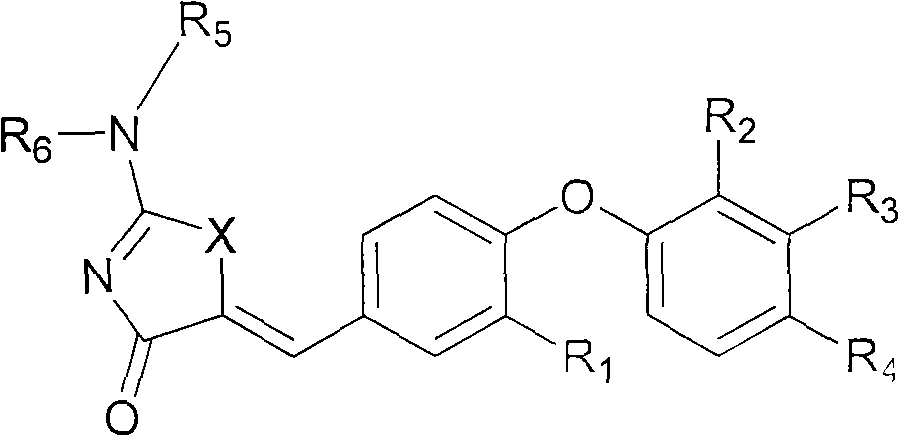
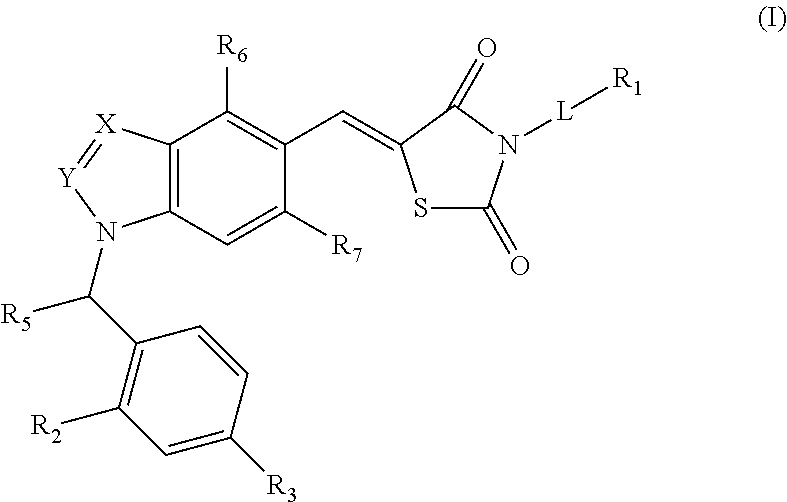
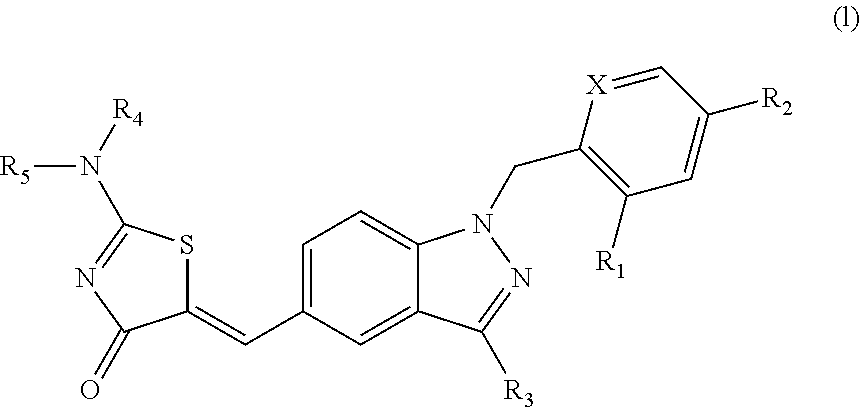
SUBSTITUTED PHENOXY AMINOTHIAZOLONES as estrogen related receptor-alpha modulators
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
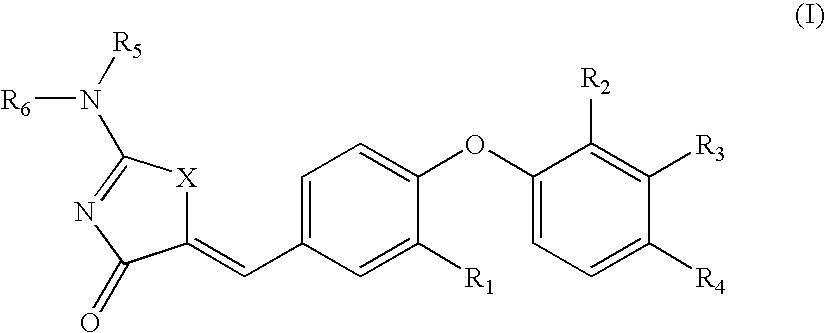
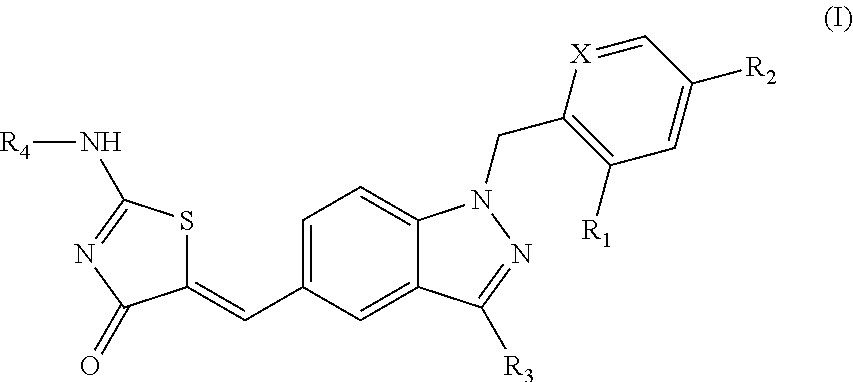
Substituted aminothiazolone indazoles as estrogen related receptor-alpha modulators
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
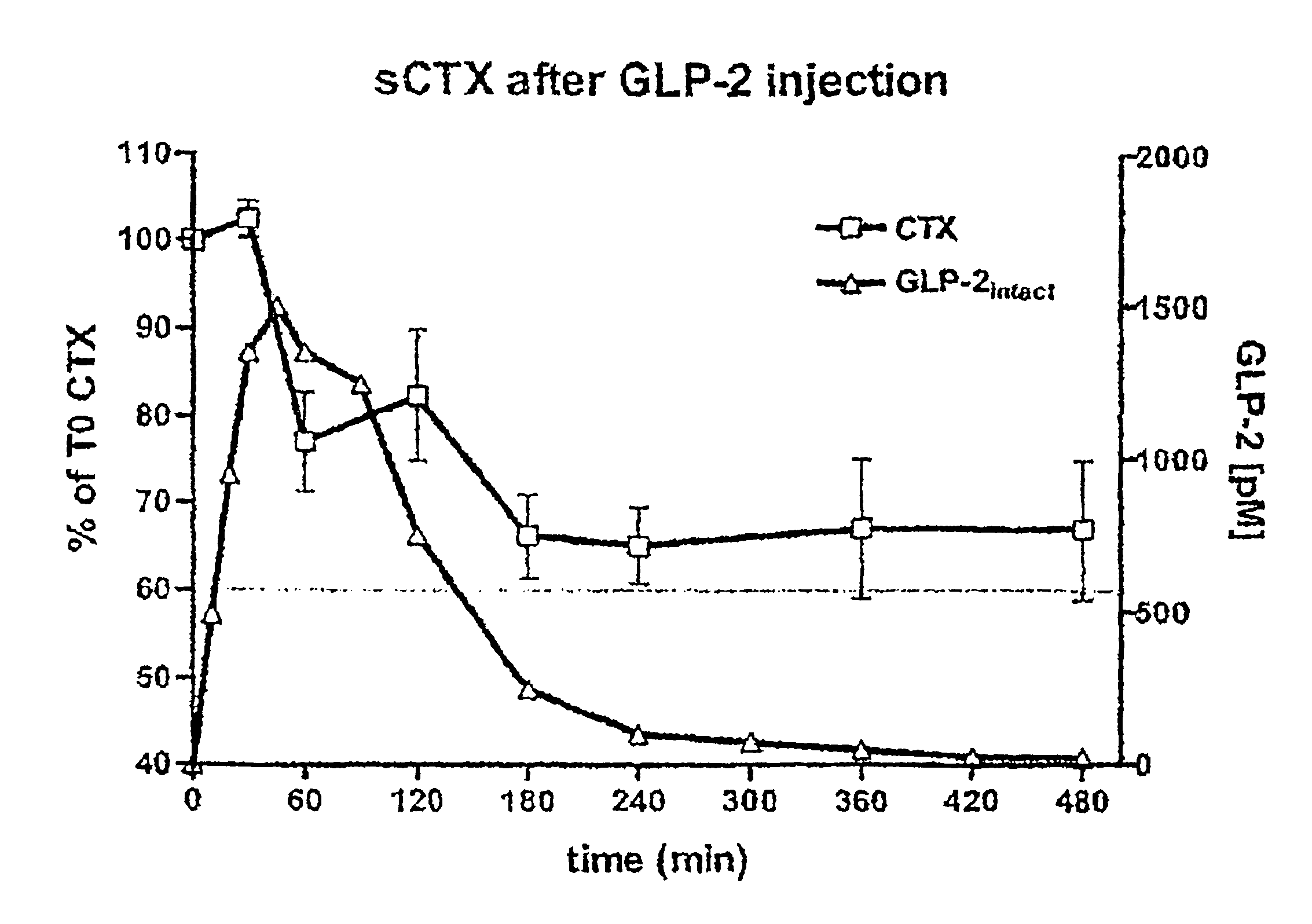
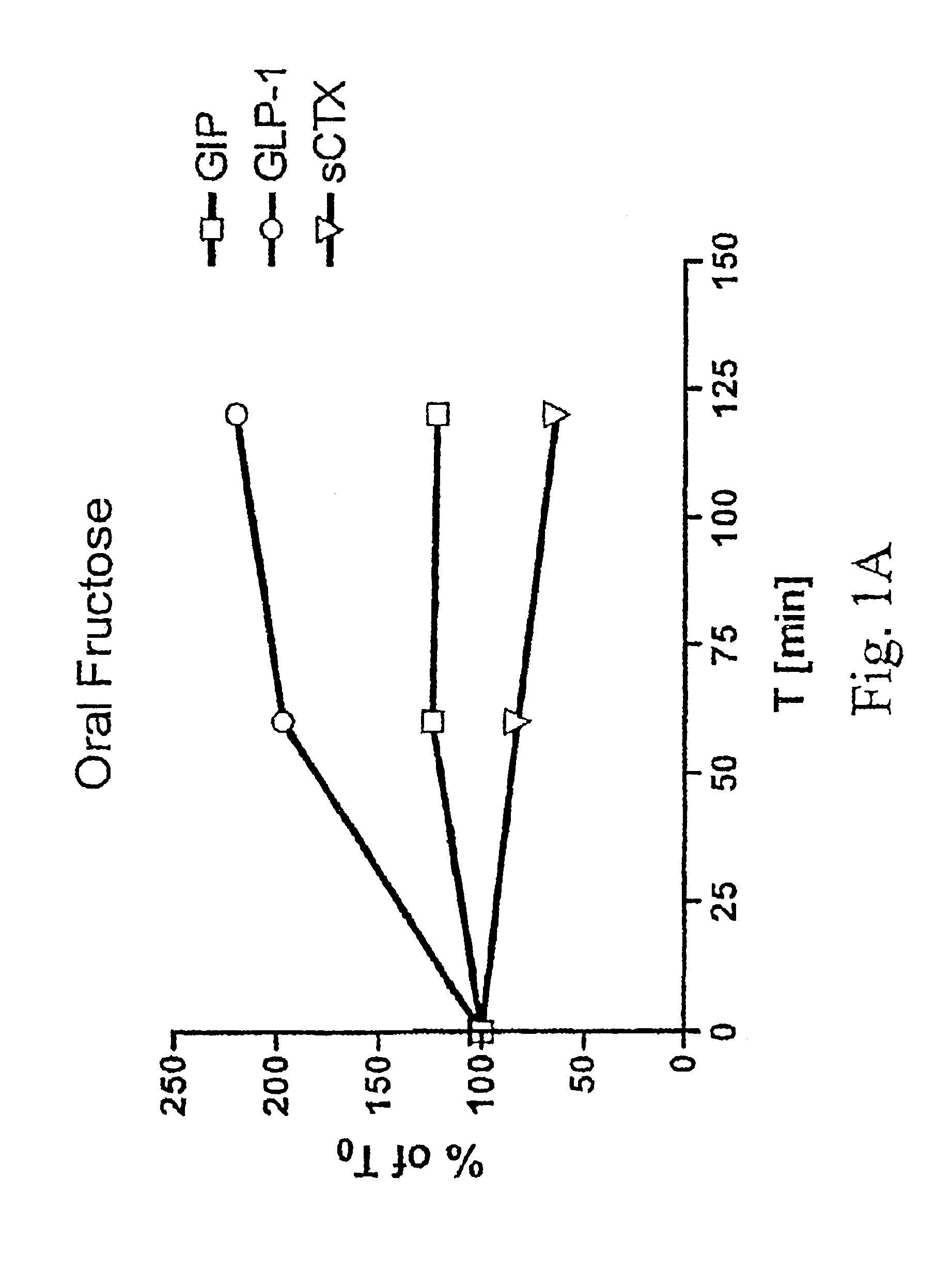
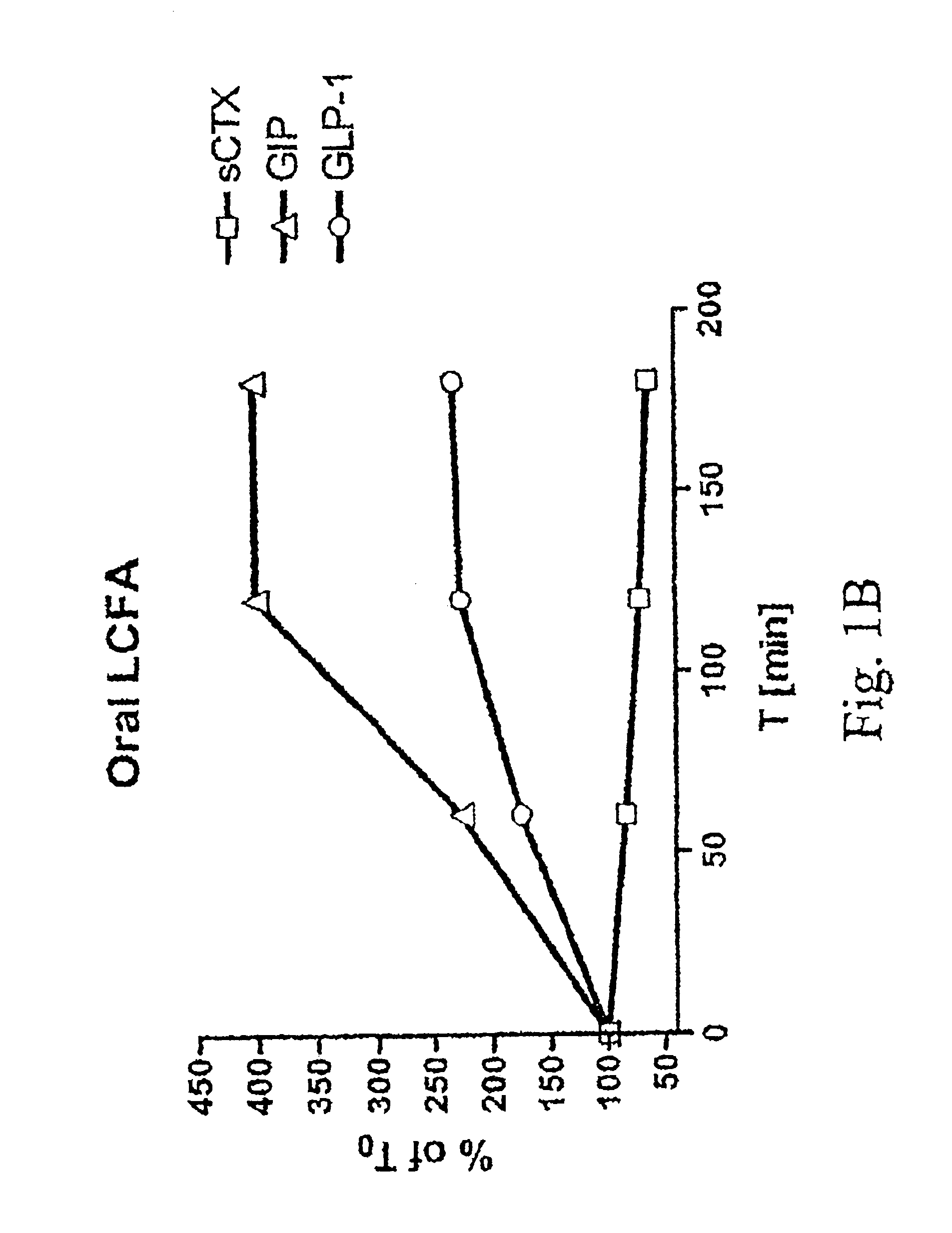
Method of inhibiting bone resorption and/or promoting bone formation using GLP-2 and related compounds
Disclosed are methods of inhibiting bone resorption and / or promoting bone formation using GLP-2 and related Compounds. The invention has a wide spectrum of important uses including hyperparathyroidism, Paget's disease, hypercalcemia of malignancy, osteolytic lesions produced by bone metastasis, bone loss due to immobilisation or sex hormone deficiency, osteomalacia, hyperostosis and osteopetrosis.
Owner:SANOS BIOSCI
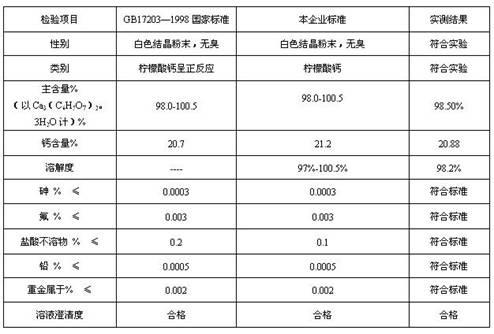
Preparation method of high soluble crystal calcium citrate
InactiveCN102351688AImprove solubilityPromote absorptionCarboxylic acid salt preparationOsteomalaciaChemical reaction
The invention relates to a preparation method of high soluble crystal calcium citrate in the fields of biochemical engineering, medicine and food. The technical scheme of the method comprises the following steps: (1) mixing citric acid, calcium carbonate and water, serving as raw materials, in a ratio of 3.84:3:1; (2) placing the mixture in a reaction tank at 20-50 DEG C after mixing, starting a machine to perform a chemical reaction for 50-60 minutes; (3) stopping the machine for 25-35 minutes to perform a chemical reaction for maturing; (4) starting the machine for 25-35 minutes to discharge high soluble crystal calcium citrate under the crystallization temperature; and (5) filtering, drying and packaging to obtain the finished product. The invention has the following beneficial effects: the molecular formula of the synthesized high soluble crystal calcium citrate is Ca3H3 (C6H5O7)2(HCO3)3, the solubility is 98%, the high solution type calcium citrate is soluble calcium and can be used to supplement calcium and cure osteoporosis and osteomalacia, human and animals can absorb the high soluble crystal calcium citrate easily; and the calcium citrate injection and calcium citrate oral liquid can also be used, and the use of the high soluble crystal calcium citrate is not limited by insoluble calcium citrate.
Owner:SHANDONG HENGTONG BIOTECH
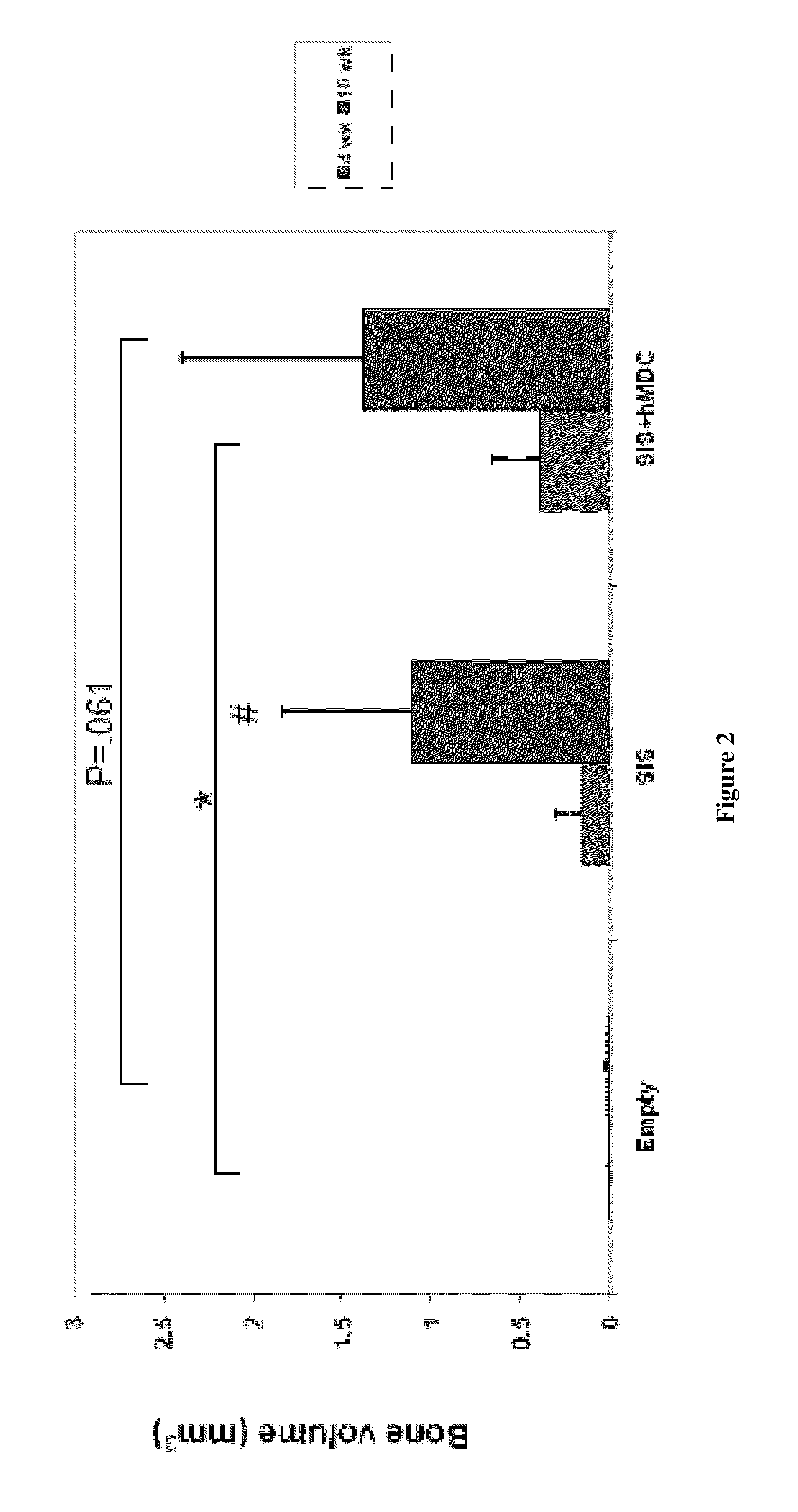
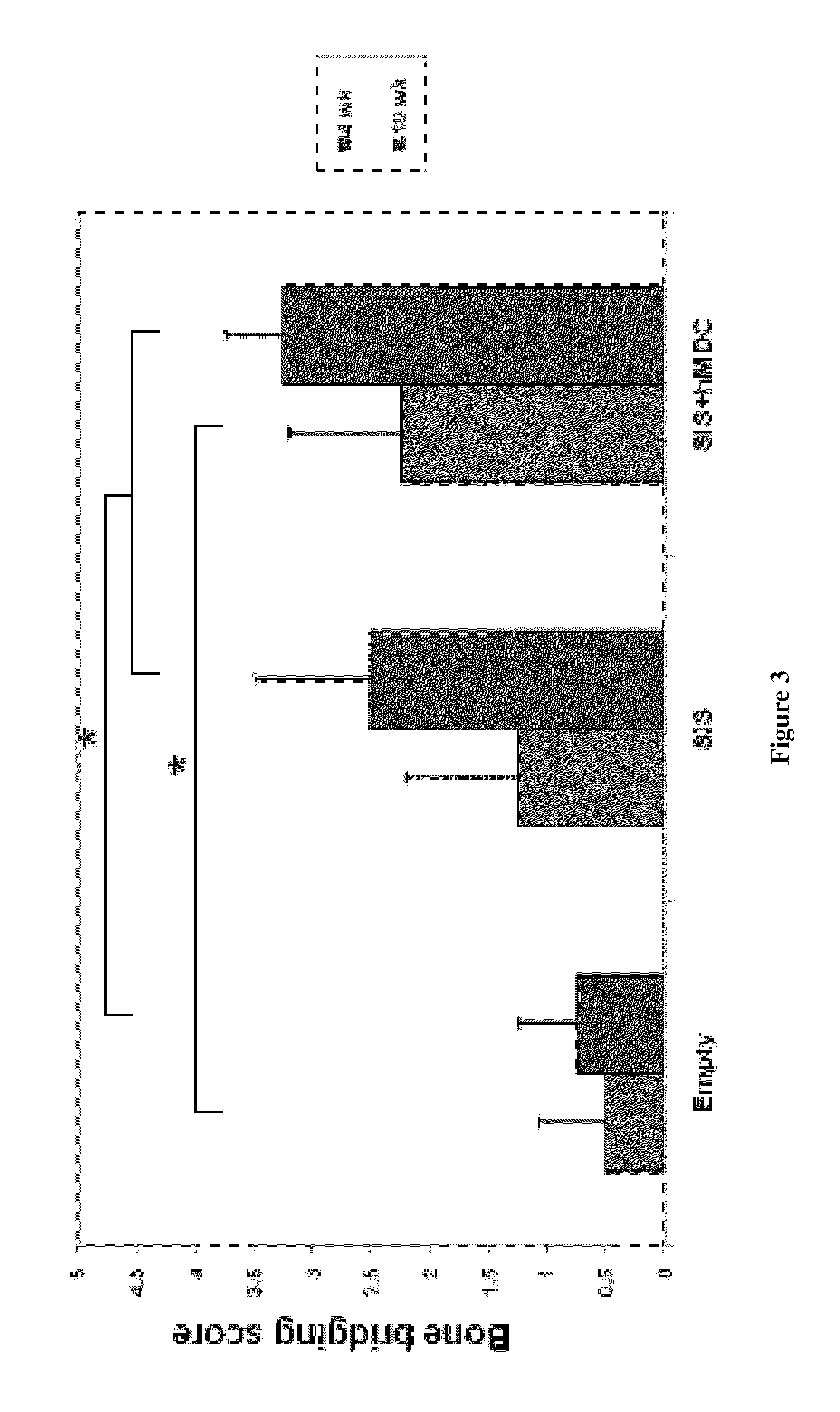
Bone augmentation utilizing muscle-derived progenitor compositions in biocompatible matrix, and treatments thereof
The present invention provides muscle-derived progenitor cells that show long-term survival following transplantation into body tissues and which can augment non-soft tissue following introduction (e.g. via injection, transplantation, or implantation) into a site of non-soft tissue (e.g. bone) when combined with a biocompatible matrix, preferably SIS. The invention further provides methods of using compositions comprising muscle-derived progenitor cells with a biocompatible matrix for the augmentation and bulking of mammalian, including human, bone tissues in the treatment of various functional conditions, including osteoporosis, Paget's Disease, osteogenesis imperfecta, bone fracture, osteomalacia, decrease in bone trabecular strength, decrease in bone cortical strength and decrease in bone density with old age.
Owner:COOK MYOSITE +1
19-nor-vitamin D analogs with 1,2 or 3,2 heterocyclic ring
19-nor-vitamin D analogs having an additional heterocyclic ring connecting the 3β-oxygen and carbon-2 or the 1α-oxygen and carbon-2 of the A-ring of the analog, and pharmaceutical uses therefore, are described. These compounds exhibit significant activity in mobilization of bone, making them therapeutic agents for the treatment or prophylaxis of osteoporosis, osteomalacia, osteopenia, renal osteodystrophy and hypoparathyroidism.
Owner:WISCONSIN ALUMNI RES FOUND
Aminothiazolones as estrogen related receptor-alpha modulators
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
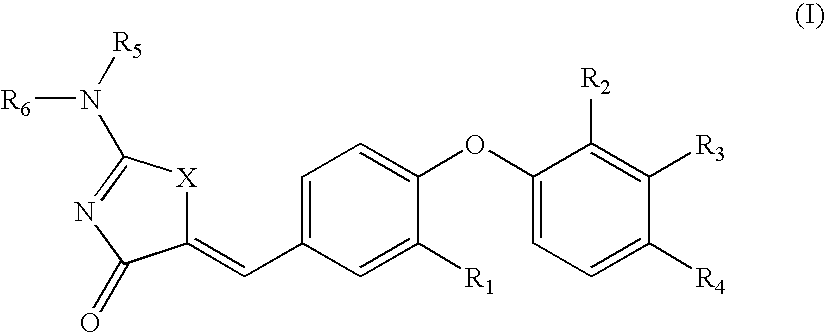
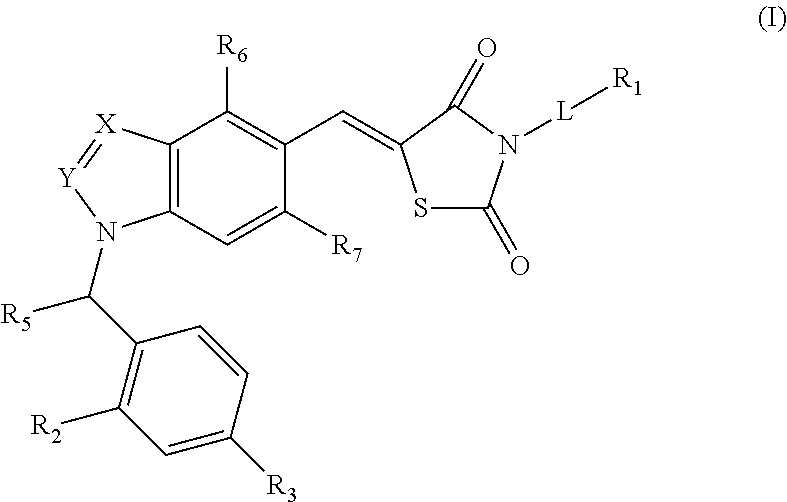
SUBSTITUTED THIAZOLIDINEDIONE INDAZOLES, INDOLES AND BENZOTRIAZOLES AS ESTROGEN-RELATED RECEPTOR-a MODULATORS
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
Preparation method of soluble strontium citrate
InactiveCN102372625ATo promote metabolismPromote growth and developmentCarboxylic acid salt preparationStrontium carbonateOsteomalacia
The invention relates to a preparation method of soluble strontium citrate in the field of biochemical and pharmaceutical foods. The technical scheme comprises the following steps of: uniformly mixing citric acid, strontium carbonate and water according to a mixing ratio; putting the uniformly mixed materials into a reaction kettle at 60-80 DEG C; starting up to carry out chemical reaction for 180-240 minutes; standing for 50-60 minutes until the chemical reaction is mature; stirring the materials in the reaction kettle for 60-70 minutes; discharging soluble strontium citrate at the crystallization temperature; filtering and drying the soluble strontium citrate; and then packaging the soluble strontium citrate to obtain a finished product. The preparation method of soluble strontium citrate, provided by the invention, has the following advantages that: the combined soluble strontium citrate with chemical structure molecular formula of Sr3(C6H5O7)2(H2CO3)3, which is white crystal powder, is a food feed additive medicine capable of treating osteoporosis and osteomalacia, easily absorbed by human and animals and used for preventing soluble strontium citrate injections and soluble strontium citrate oral liquids from being restricted by insoluble strontium citrate.
Owner:SHANDONG HENGTONG BIOTECH
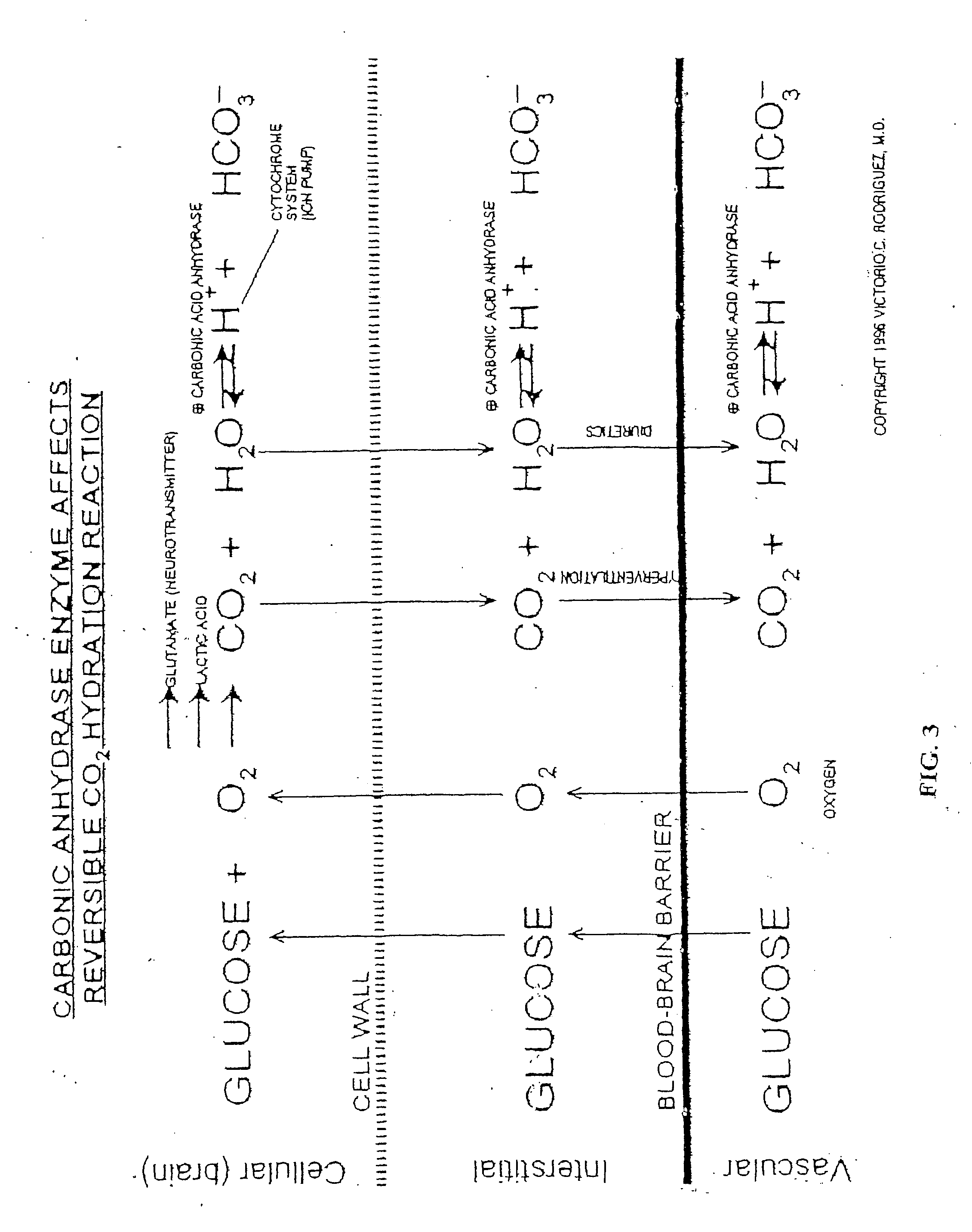
Therapeutic and prophylactic uses of cell specific carbonic anhydrase enzymes in treating aging, disorders of aging, cancer, as growth factors, and as an alternative to stem cell therapy
A method for the treatment and prophylaxis of diseases or conditions associated with aging which includes oxidative stress and cancer. Such diseases or conditions are associated with a decrease or increase presence of one or a combination of one or more Carbonic Anhydrase isozymes I TO VII in the tissue of a subject. These diseases include but are not limited to chronic neurodegenerative diseases such as Alzheimer's disease, parkinson's disease, multiple sclerosis, autism, lou gehrig's disease, huntington's disease, immune disorders which includes systemic lupus erythematosus, diabetes mellitus; musculo-skeletal disorders which includes osteoporosis,arthritis, osteomalacia. Cancer which includes the breast, lung, colon, prostate gland, ovary, esophagus, liver, rectum. The method comprises administering to the patient a pharmaceutically effective non toxic amount of one or a combination of one or more compounds that increases or decreases the level of one or more Cell Specific Carbonic Anhydrase Enzymes whose levels have been increased or decreased in the tissue of the subject. Such compound maybe the Cell Specific Carbonic Anhydrase Enzymes I to VII. A compound that when absorbed reacts or dissociates to form cell specific carbonic enzymes or one or more compounds that when administered promotes the increase or decrease level of one or more cell specific Carbonic anhydrase I,II,III,IV,V,VI,VII. As growth factors of stem cells as an alternative to stem cell therapy. These methods includes the administering of one or a combination of one or more of these compounds over an extended period of time ranging from 6 months until the patient dies.
Owner:RODRIGUEZ VICTORIO C
Medicine for promoting increase of bone substance
InactiveCN1444966AAnalgesic and anti-inflammatoryPromotes pain relief and anti-inflammationSkeletal disorderUnknown materialsBone cellGinseng
A medicine for promoting bone growth and preventing osteomalacia and bone fracture is prepared from calcium hydrogen phosphate, VC, cod-liver oil and 10 Chinese-medicinal materials including ginseng,safflower, notoginseng, etc. Its advantages are high curative effect (98.1% for total effective rate) and no toxic by-effect.
Owner:JILIN CENT HOSPITAL
Feed composition for preventing bovine osteomalacia
InactiveCN103892065AReduce morbidityReduce economic lossFood processingAnimal feeding stuffOsteomalaciaAnimal science
The invention relates to a feed composition for preventing bovine osteomalacia. The feed composition is obtained through the steps of processing Radix Astragali and corn to form 10-40 mesh powder, cutting straws to form segments with the length of below 3cm, and uniformly stirring all above raw materials. The bovine osteomalacia attack rate of cattle using the feed composition is substantially lower than the bovine osteomalacia attack rate of cattle using control feeds, so the economy loss of the cattle farming industry, caused by the bovine osteomalacia, can be reduced.
Owner:邢果花
GDF-9/BMP-15 modulators for the treatment of bone disorders
InactiveUS7790161B2Organic active ingredientsPeptide/protein ingredientsOsteodystrophyDegenerative Disorder
The invention provides methods for treating or preventing bone degenerative disorders. The disorders treated or prevented include, for example, osteopenia, osteomalacia, osteoporosis, osteomyeloma, osteodystrophy, Paget's disease, osteogenesis imperfecta, and bone degenerative disorders associated with chronic renal disease, hyperparathyroidism, and long-term use of corticosteroids. The disclosed therapeutic methods include administering to a mammal an inhibitor of GDF-9 or BMP-15 in an amount effective to: (1) treat or prevent a bone degenerative disorder; (2) slow bone deterioration; (3) restore lost bone; (4) stimulate new bone formation; and / or (5) maintain bone mass and / or bone quality. The invention also provides methods for administering a GDF-9 agonist or a BMP-15 agonist to treat a bone disorder characterized by increased bone density or mass.
Owner:WYETH LLC
Cancellous bone-invigorating formula for treating osteoporosis and preparation technology thereof
ActiveCN103405573APrevent loss of bone densitySignificant effectSkeletal disorderPlant ingredientsBone densityAngelica Sinensis Root
The invention mainly relates to a cancellous bone-invigorating formula for treating osteoporosis and a preparation technology thereof. The formula comprises the following raw materials by weight: 12-18 parts of radix rehmanniae praeparata, 13-17 parts of cornus officinalis, 10-14 parts of angelica sinensis, 14-16 parts of epimedium brevicornum maxim, 10-14 parts of semen cuscutae, 10-14 parts of medlar, 11-13 parts of rhizoma atractylodis macrocephalae and 11-13 parts of lumbricus. The cancellous bone-invigorating formula can treat the primary and secondary osteoporosis and osteomalacia effectively, takes significant curative effects on symptoms such as back, waist or whole body aching and tired, limb weakness, legs and feet cramp, deadlimb, dysphoria and irritability, palpitation and insomnia, sweating, a reddened tongue, yellowish coated tongue, a yellowish coatedtongue with crack and thready rapid pulse and the like due to deficiency of kidney-yin and blood stasis, and achieves remarkable effects of preventing bone mineral density from being reduced, reducing the bone turnover, and improving the balanced capacity. According to the invention, the preparation technology is simple, the production cost is low, and the curative effect of the drug prepared according to the formula is remarkable. Therefore, the formula and the preparation technology thereof are suitable for popularization and application.
Owner:LUOYANG ORTHOPEDIC TRAUMATOLOGICAL HOSPITAL
Formula of Xibixiao tincture and use method thereof
InactiveCN109528842ARelieve painEffective treatmentAntipyreticAnalgesicsCynomorium songaricumMedicine
The invention belongs to the technical field of medical treatment and especially relates to a formula of Xibixiao tincture, which includes: rhizoma corydalis, angelica sinensis, cynomorium songaricum,rhizoma chuanxiong, sappan wood, radix clematidis, and pawpaw. In the invention, the powders of the medicines including the rhizoma corydalis, angelica sinensis, cynomorium songaricum, rhizoma chuanxiong, sappan wood, radix clematidis and pawpaw are matched with baijiu, so that the product is used for tuina therapy on an injury position of a patient so as to absorb the medicine. The Xibixiao tincture is easy to use, has effects of soothing tendon and activating collateral, activating blood and relieving pain, and removing stasis and freeing meridian, can effectively treat diseases such as knee osteoarthritis, synovitis, osteoproliferation, chondromalacia patellae and knee joint sprain, and can effectively the pain of the patients.
Owner:柳倩
Calcifediol soft capsules
ActiveUS10525018B2Improve bioavailabilityHydroxy compound active ingredientsAntipyreticJoint diseaseOsteo arthritis
The present invention relates to calcifediol soft capsules, to their use in the treatment or prevention of diseases related to vitamin D deficiency, such as vitamin D deficiency, demineralization such as hypocalcemia and hypophosphatemia, renal osteodystrophy, rickets, osteoporosis, osteopenia, osteoarthritis, osteoarthrosis, osteomalacia, hypoparathyroidism, and inflammatory bowel disease, and to their process of manufacture.
Owner:FAES FARMA SA
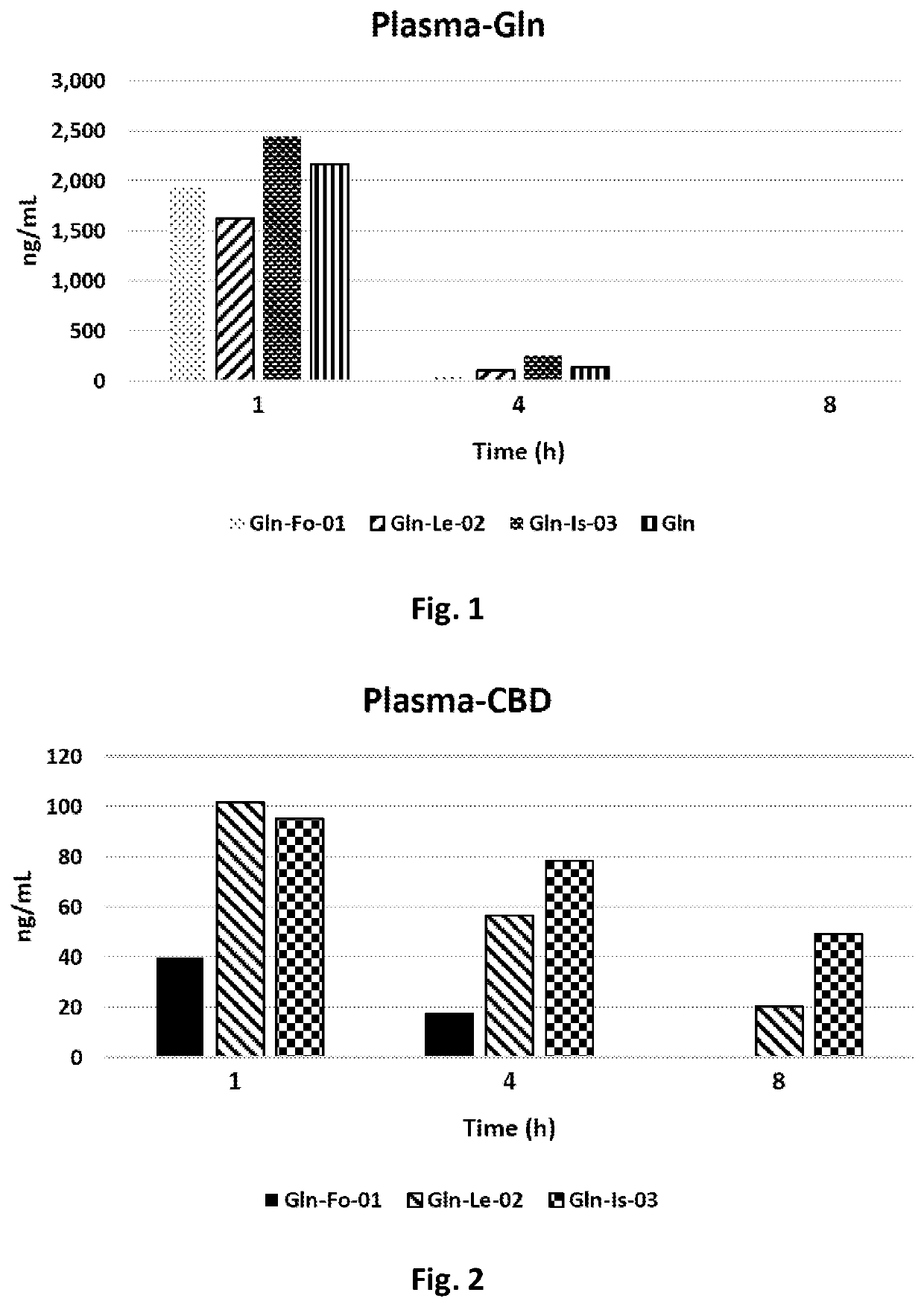
Cannabidiol Combination Compositions
PendingUS20210275556A1Improve bioavailabilityComplementary synergetic effectHydroxy compound active ingredientsSulfur/selenium/tellurium active ingredientsArthritis osteoarthritisPAGET'S BONE DISEASE
Embodiments of the invention are directed to formulations comprising Cannabidiol (CBD) and glucosamine (Gln). Further embodiments of the invention are directed to methods of treating or preventing Arthritis, Osteoarthritis, Rheumatoid arthritis, Osteoporosis, Osteopenia, jaw pain, joint pain, knee pain, back pain, multiple sclerosis, Osteomalacia and Paget's disease of bone, wherein the method comprises administering a formulation comprising Cannabidiol (CBD) and glucosamine (Gln).
Owner:COMPANION SCI LLC
Substituted phenoxy aminothiazolones as estrogen related receptor-alpha modulators
The present invention relates to compounds of formula (I), methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
Method for preparing taurine VC calcium citrate
InactiveCN102329288APromote absorptionSulfonic acids salts preparationCarboxylic acid salt preparationOsteomalaciaChemical reaction
The method relates to a method for preparing taurine VC calcium citrate in the field of biochemistry, medicines and food. The method comprises the following steps: (1) uniformly mixing citric acid, calcium oxide, water, taurine and VC in a ratio of 7.68:4.48:1:5:7.04; (2) after uniform mixing, putting the mixture in a reactor at 20-50 DEG C and starting the reactor to carry out chemical reaction for 50-60 minutes; (3) stopping the reactor for 25-35 minutes to ensure the chemical reaction to be mature; (4) starting the reactor for 25-35 minutes to exhaust taurine VC calcium citrate under crystallization temperature; and (5) carrying out packaging after filtration and drying to obtain the finished product. The method has the following beneficial effects: the synthesized taurine VC calcium citrate has solubility as high as 98%, is soluble calcium, can be used for supplementing calcium and treating osteoporosis and osteomalacia, is easily absorbed by human and animals, can be used in the forms of calcium citrate injections and calcium citrate oral liquid and is not limited by indissolvable calcium citrate.
Owner:SHANDONG HENGTONG BIOTECH
Medicine for promoting increase of bone substance
InactiveCN1185003CAnalgesic and anti-inflammatoryPromotes pain relief and anti-inflammationSkeletal disorderUnknown materialsTherapeutic effectBone growth
Owner:JILIN CENT HOSPITAL
Nano-carrier topical composition with vitamin d3
ActiveUS20220105036A1Reduce deliveryOrganic active ingredientsInorganic non-active ingredientsCholesterolBULK ACTIVE INGREDIENT
The nano-carrier topical composition with Vitamin D3 includes Vitamin D3, Span 40, Span 80, cholesterol, ethanol, almond oil, glycerin, water, aloe vera gel, and sodium hydroxide. The nano-carrier topical composition with Vitamin D3 forms an alkaline composition having nano-carriers with an average diameter of 50.8 μm. The composition can be transported through the stratum corneum so that the composition may be used for fulfilling daily requirements for Vitamin D3, treatment of bone conditions, such as rickets and osteomalacia, and supportive therapy in osteopenia and osteoporosis. Optionally, the composition may include perfume and / or one or more preservatives (e.g., a carbomer, methyl paraben, EDTA, or the like), or other additives that do not affect the active ingredients. The composition is preferably formulated as a cream or emulsion wherein the nano-carriers are niosomes.
Owner:AL ALI SADAT A +1
Traditional Chinese medicine for treating chondromalacia patellae and preparation method of traditional Chinese medicine
InactiveCN105169248ASmall side effectsEasy to useSkeletal disorderPlant ingredientsBerchemia sinicaLiver and kidney
The invention relates to a traditional Chinese medicine for treating chondromalacia patellae. The traditional Chinese medicine is prepared from the following components in parts by weight: 15-25 parts of raspberries, 15-25 parts of radix cynanchi bungei, 15-25 parts of wolfberry fruits, 5-15 parts of Berchemia sinica Schneid., 5-15 parts of herb of Chinese alyxia, 5-15 parts of root or leaf of bigleaf cayratia, 3-9 parts of corydalis tuber, 3-9 parts of radix curcumae and 3-9 parts of ligusticum wallichii. According to the traditional Chinese medicine ointment for treating liver and kidney deficient type chondromalacia patellae, provided by the invention, by taking the theory of traditional Chinese medicine as a basis, the traditional Chinese medicine ointment takes pure traditional Chinese medicine as the raw materials so that the toxic side effect is small; the traditional Chinese medicine ointment is convenient to use and is subjected to ultra-micro crushing so that the pharmacological effect is released to a greater extent; the preparation method is simple and the treatment effect on the liver and kidney deficient type chondromalacia patellae is remarkable.
Owner:肖荣叶
Cannabidiol combination compositions
PendingCN112930183AImprove bioavailabilityHydroxy compound active ingredientsSulfur/selenium/tellurium active ingredientsArthritis osteoarthritisPAGET'S BONE DISEASE
Embodiments of the invention are directed to formulations comprising Cannabidiol (CBD) and glucosamine (Gln). Further embodiments of the invention are directed to methods of treating or preventing Arthritis, Osteoarthritis, Rheumatoid arthritis, Osteoporosis, Osteopenia, jaw pain, joint pain, knee pain, back pain, multiple sclerosis, Osteomalacia and Paget's disease of bone, wherein the method comprises administering a formulation comprising Cannabidiol (CBD) and glucosamine (Gln).
Owner:COMPANION SCI LLC
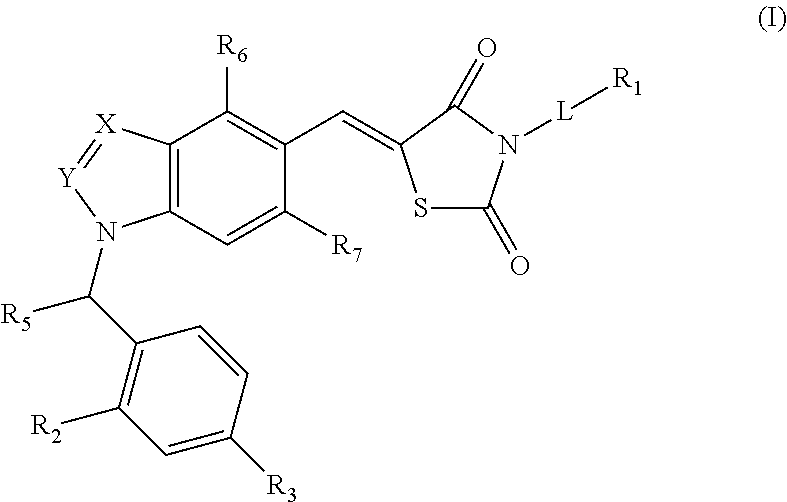
Substituted thiazolidinedione indazoles, indoles and benzotriazoles as estrogen-related receptor-α modulators
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
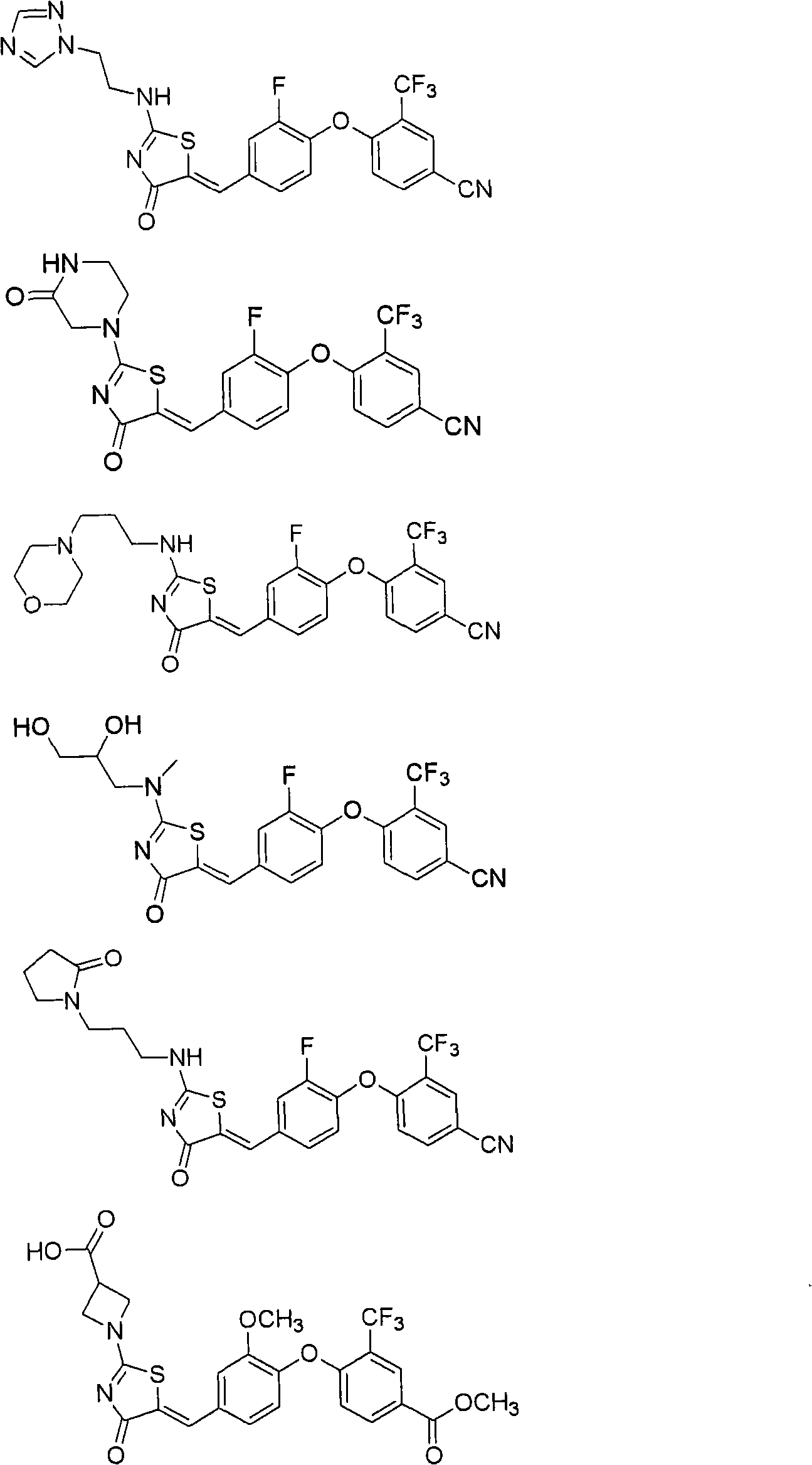
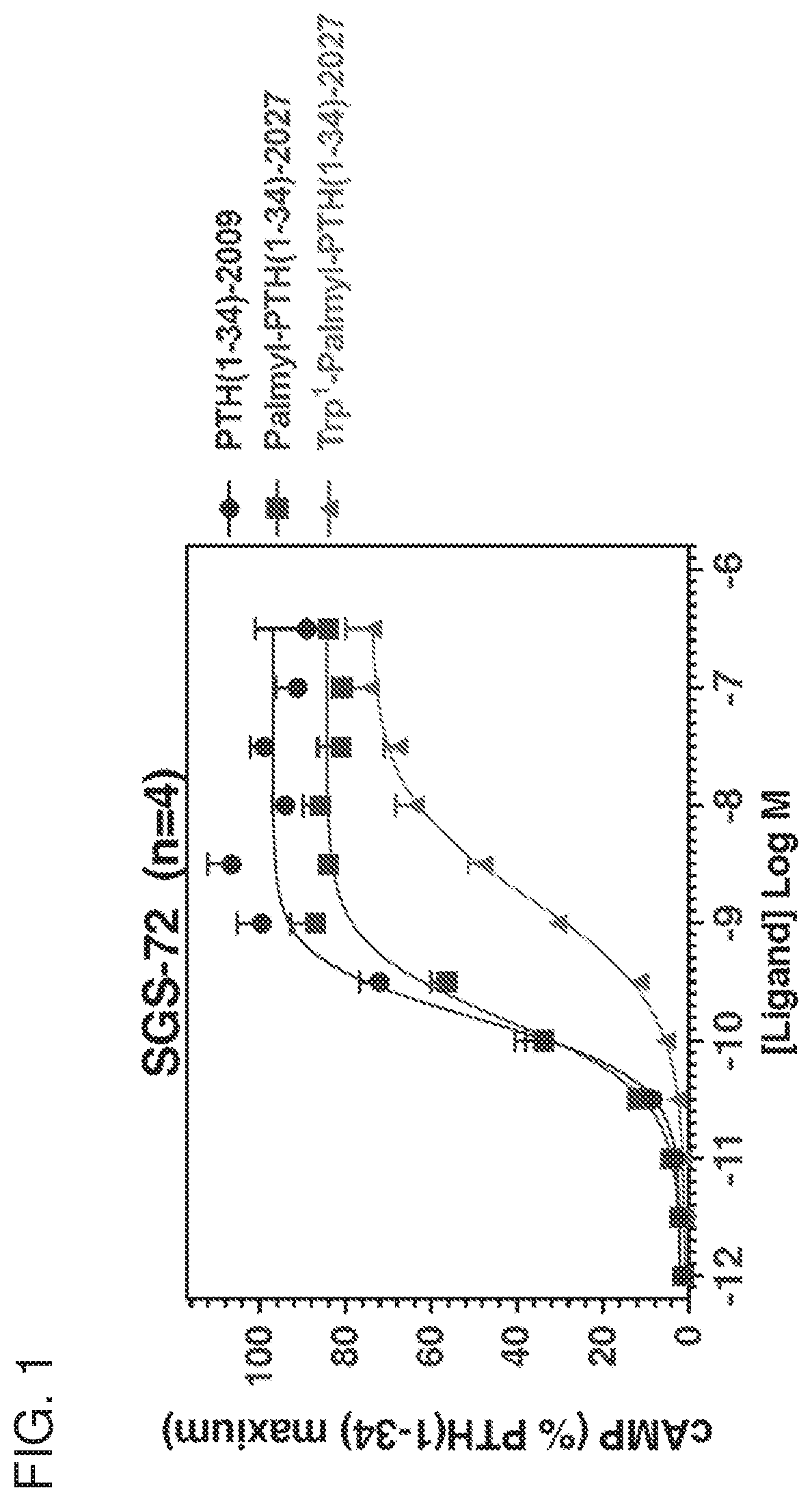
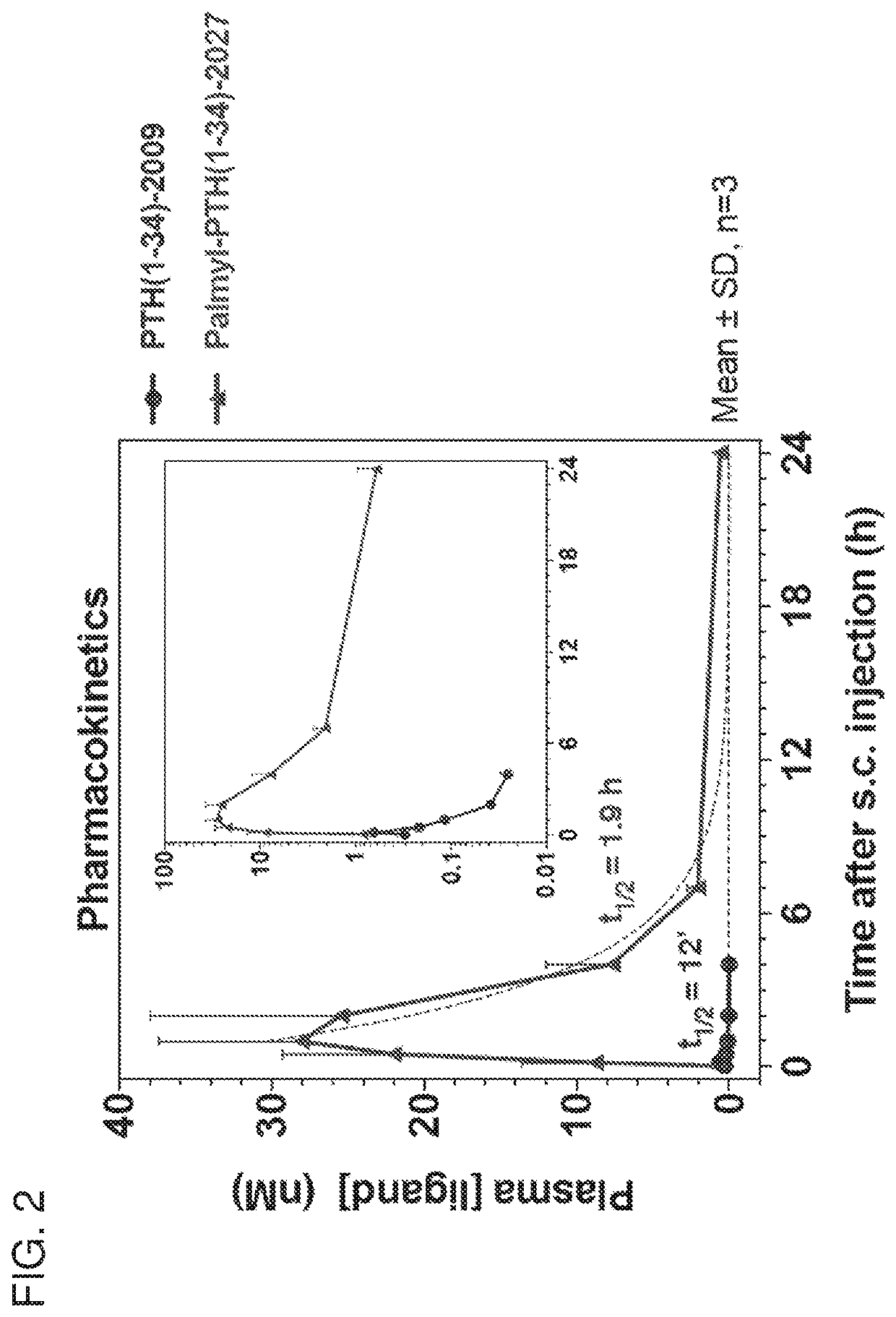
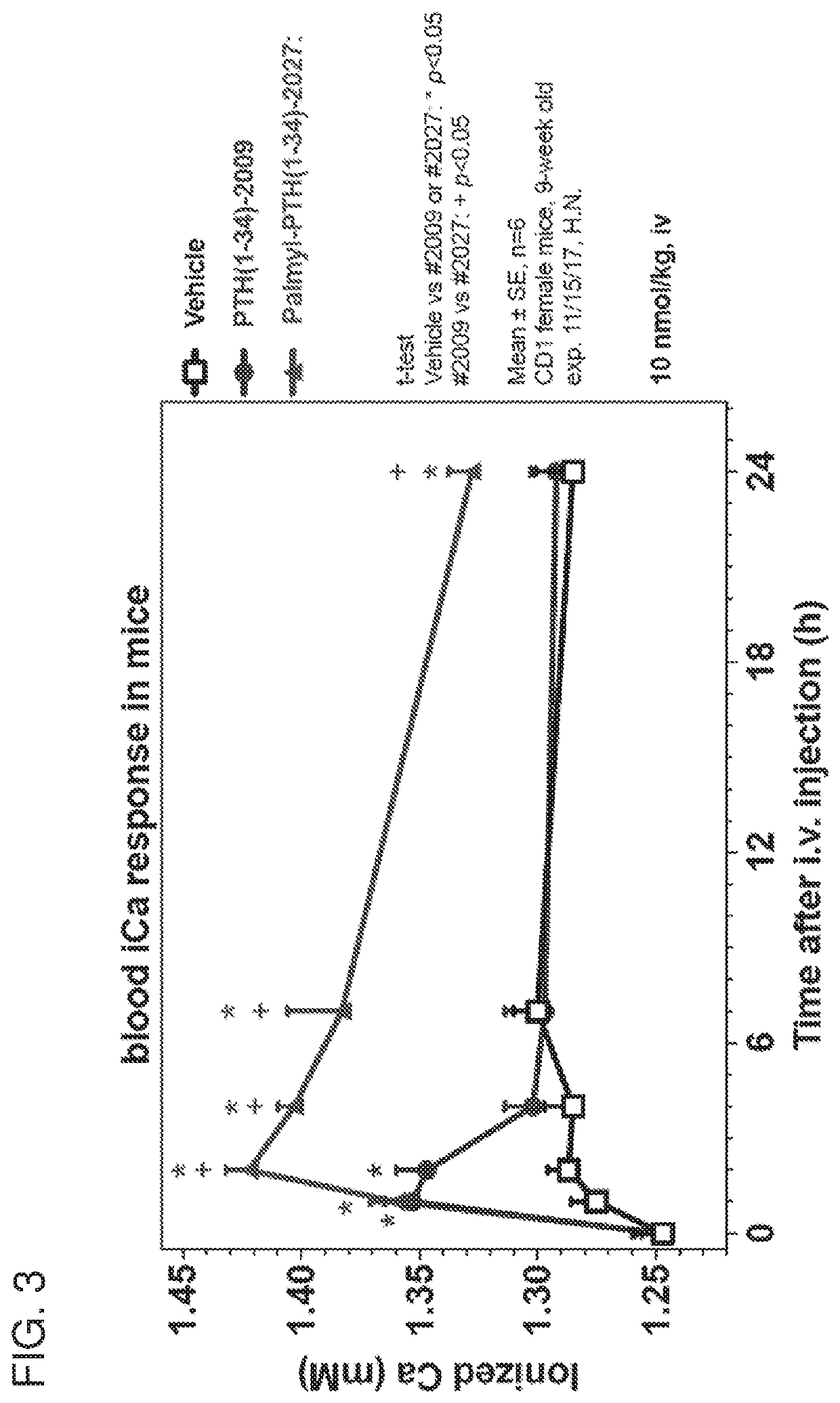
Parathyroid hormone polypeptide conjugates and methods of their use
PendingUS20200405820A1Reducing constitutive activityFacilitated releasePeptide/protein ingredientsPharmaceutical non-active ingredientsArthritisHyperphosphoremia
Disclosed are peptide-fatty acid conjugates, pharmaceutical compositions containing them, and methods of their medical use in the treatment of, e.g., a disease or condition associated with the PTHR1 signaling overactivity (e.g., hypercalcemia, hypophosphatemia, hyperparathyroidism, or Jansen's chondrodysplasia) or deficiency (e.g., hypoparathyroidism, hyperphosphatemia, osteoporosis, fracture repair, osteomalacia, arthritis, or thrombocytopenia).
Owner:THE GENERAL HOSPITAL CORP
Aminothiazolones as estrogen related receptor-alpha modulators
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
Aminothiazolones as estrogen related receptor-alpha modulators
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
Nano-carrier topical composition with vitamin D3
ActiveUS11497714B2Reduce deliveryOrganic active ingredientsInorganic non-active ingredientsCholesterolBULK ACTIVE INGREDIENT
The nano-carrier topical composition with Vitamin D3 includes Vitamin D3, Span 40, Span 80, cholesterol, ethanol, almond oil, glycerin, water, aloe vera gel, and sodium hydroxide. The nano-carrier topical composition with Vitamin D3 forms an alkaline composition having nano-carriers with an average diameter of 50.8 μm. The composition can be transported through the stratum corneum so that the composition may be used for fulfilling daily requirements for Vitamin D3, treatment of bone conditions, such as rickets and osteomalacia, and supportive therapy in osteopenia and osteoporosis. Optionally, the composition may include perfume and / or one or more preservatives (e.g., a carbomer, methyl paraben, EDTA, or the like), or other additives that do not affect the active ingredients. The composition is preferably formulated as a cream or emulsion wherein the nano-carriers are niosomes.
Owner:AL ALI SADAT A +1
Feed-grade calcium hydrogen phosphate and preparation method thereof
InactiveCN111202167AReasonable formulaNutritional balanceFood processingAnimal feeding stuffBiotechnologyAnimal science
The invention discloses improved feed-grade calcium hydrogen phosphate. The improved feed-grade calcium hydrogen phosphate is prepared from the components in parts by weight: 100-200 parts of slaked lime, 30-40 parts of flour, 6-12 parts of rice bran, 1-5 parts of vegetable oil, 6-10 parts of hydrochloric acid, 1-3 parts of yellow mealworm sand, 2-6 parts of pomace and 8-12 parts of nutrition additives. The feed has the characteristics of high phosphorus content, high water solubility and the like, the growth and development of livestock and poultry can be accelerated, the fattening period isshortened, and weight is quickly gained; and the breeding rate and survival rate of the livestock and poultry can be improved, at the same time, the disease and cold resistance of the livestock and poultry is improved, and the preventive and therapeutic effect of osteomalacia, white dysentery and paralysis of the livestock and poultry is provided.
Owner:福泉市胜科饲料有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com