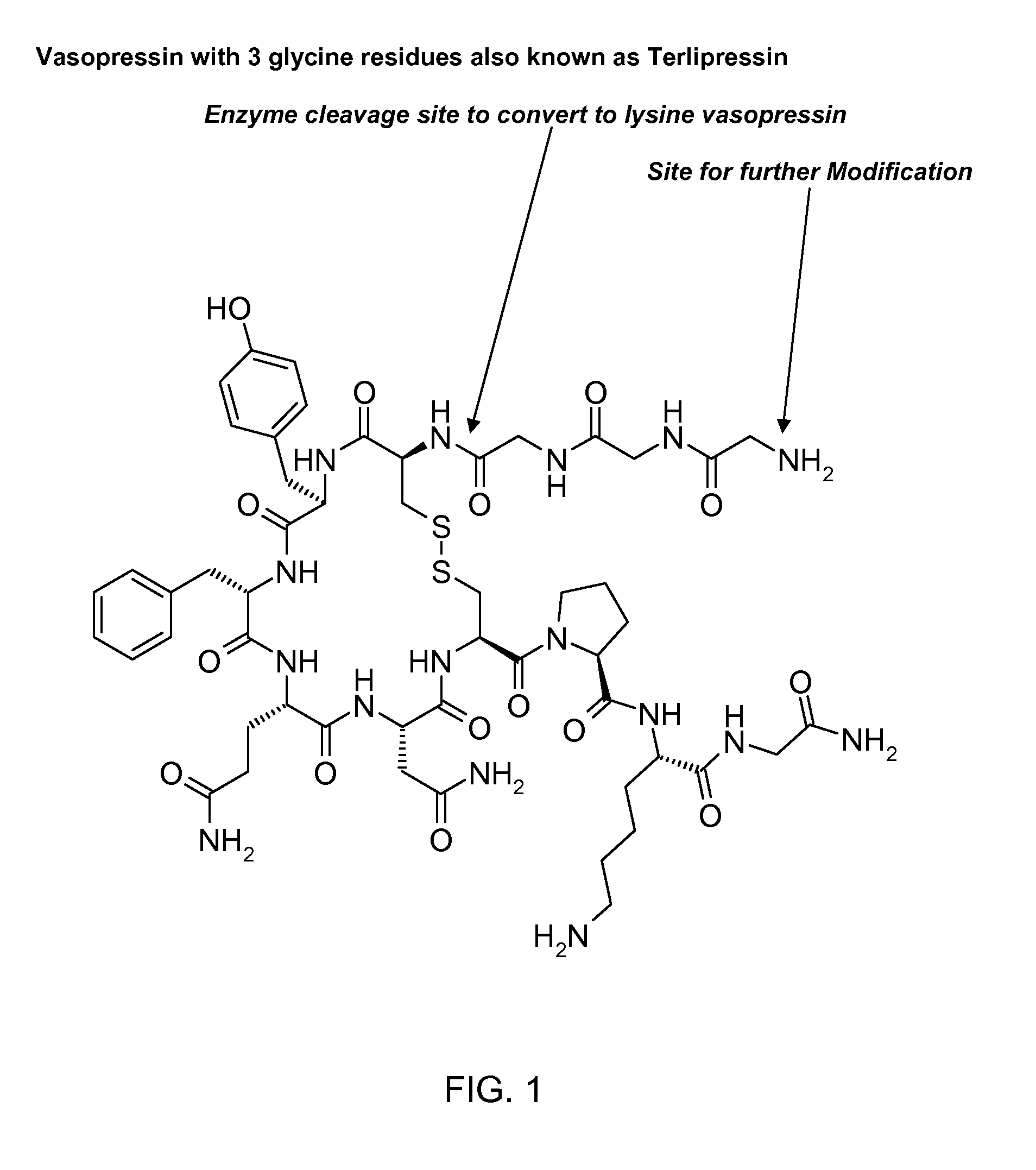
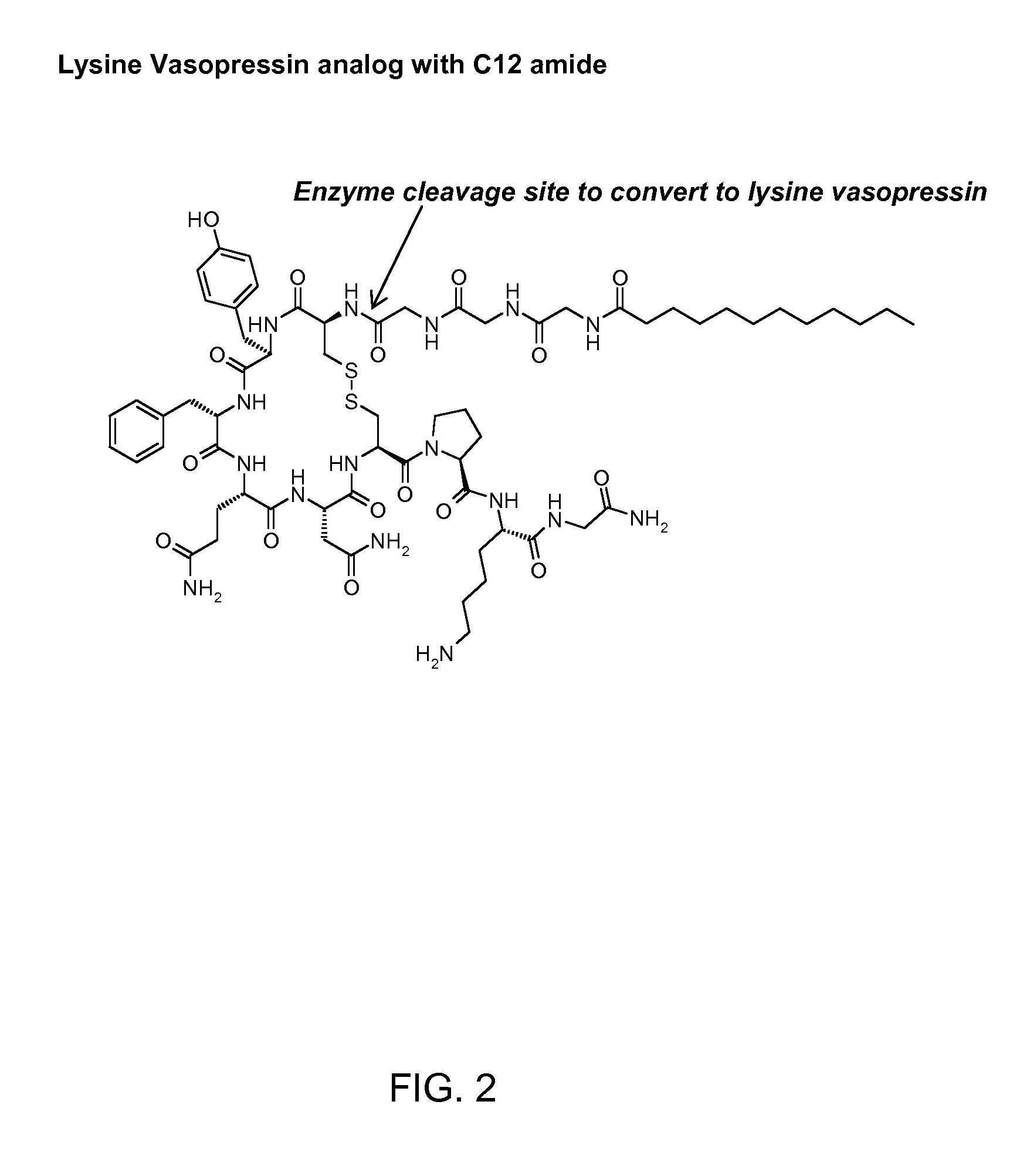
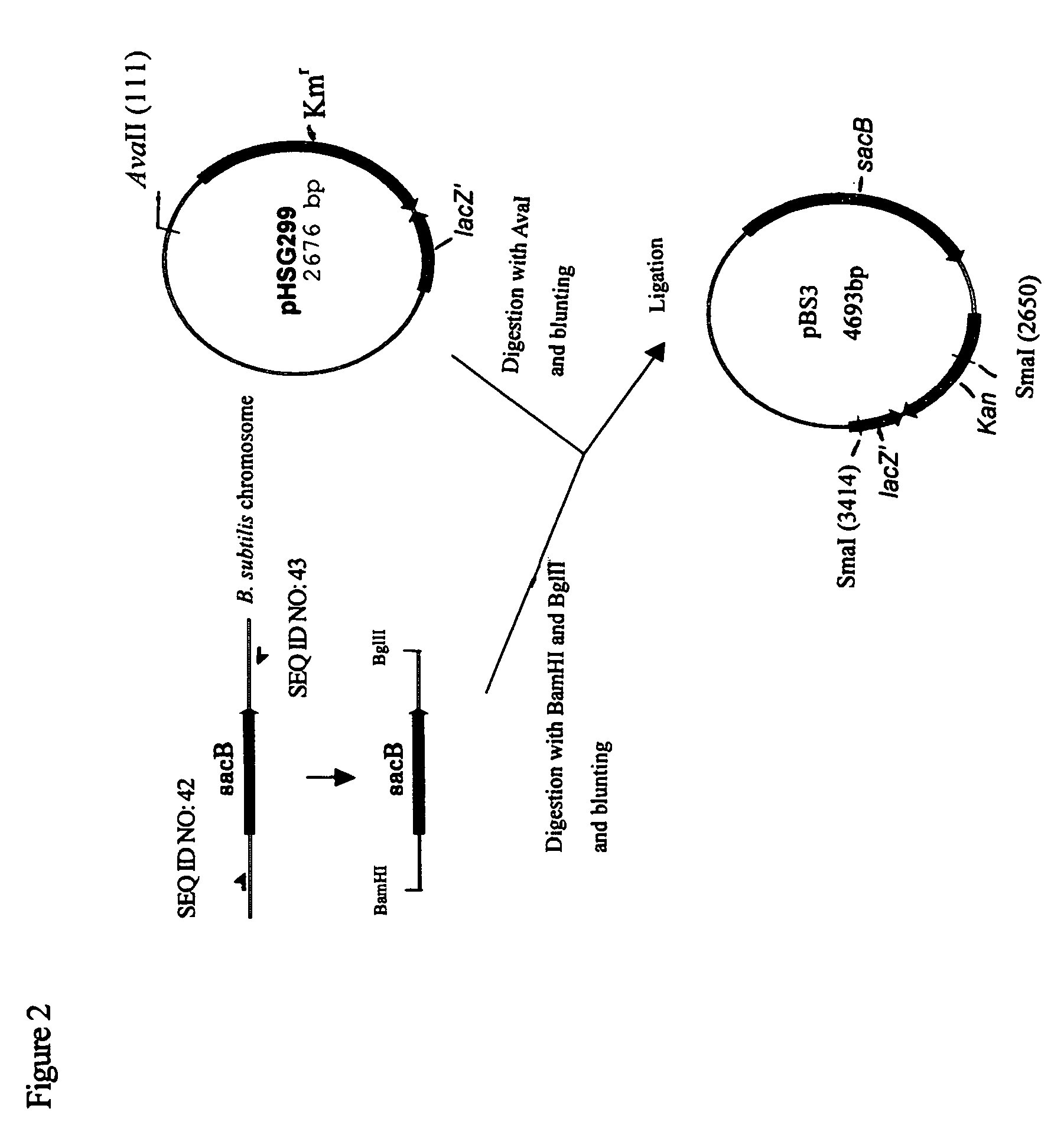
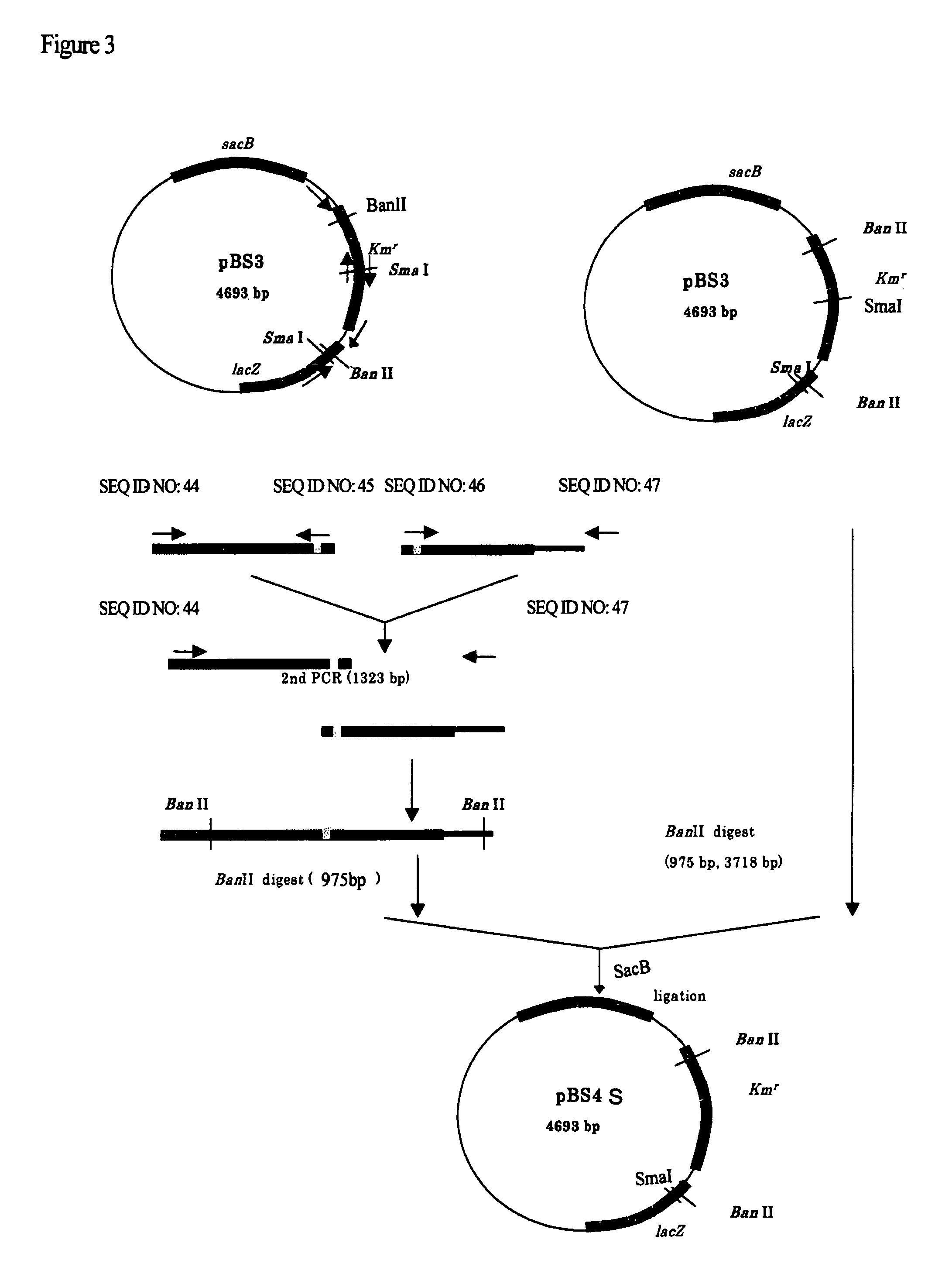
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
5346 results about "Arginine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Arginine, also known as l-arginine (symbol Arg or R), is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, an α-carboxylic acid group, and a side chain consisting of a 3-carbon aliphatic straight chain ending in a guanidino group. At physiological pH, the carboxylic acid is deprotonated (−COO⁻), the amino group is protonated (−NH₃⁺), and the guanidino group is also protonated to give the guanidinium form (-C-(NH₂)₂⁺), making arginine a charged, aliphatic amino acid. It is the precursor for the biosynthesis of nitric oxide. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG.
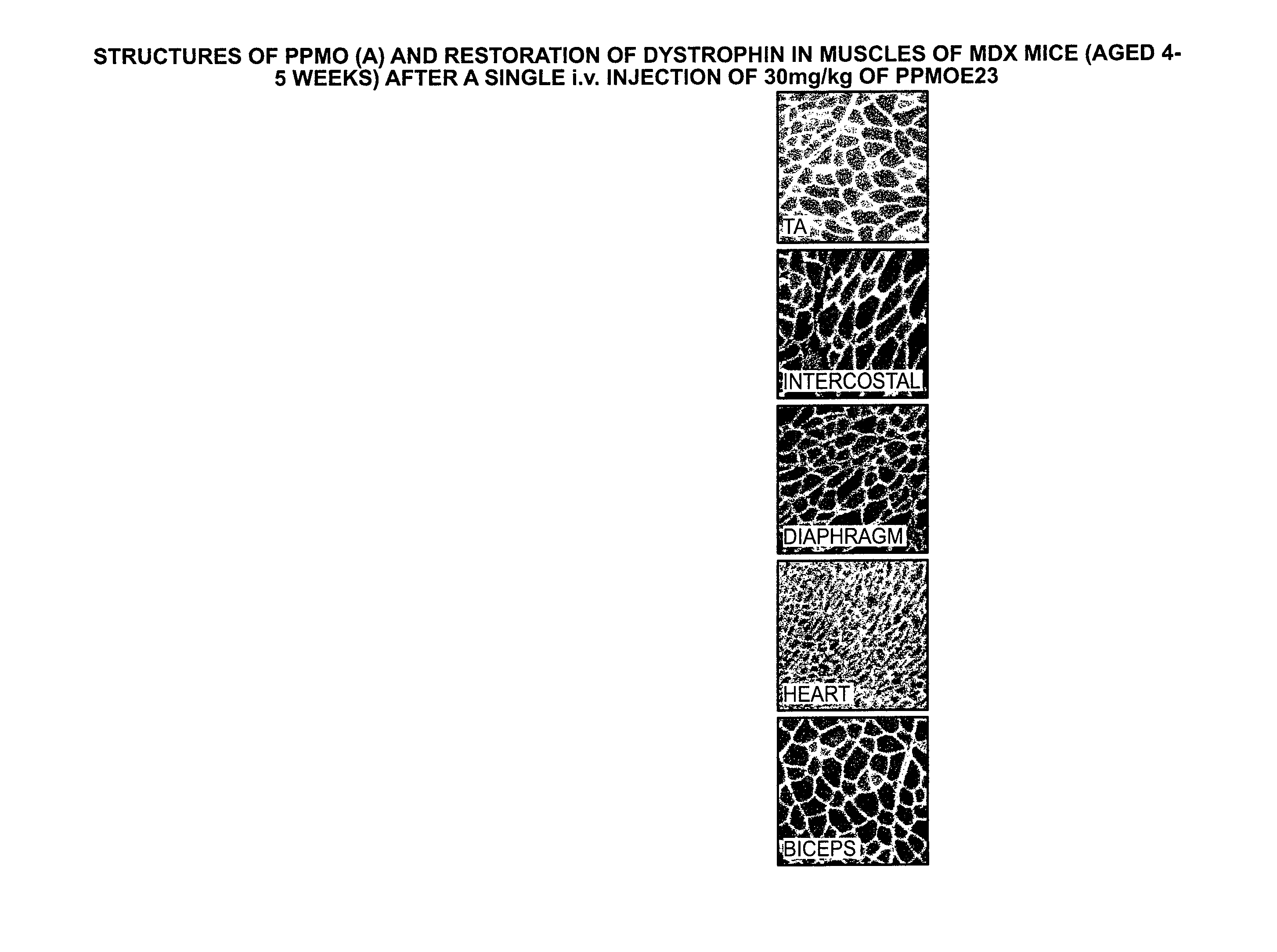
Compound and method for treating myotonic dystrophy
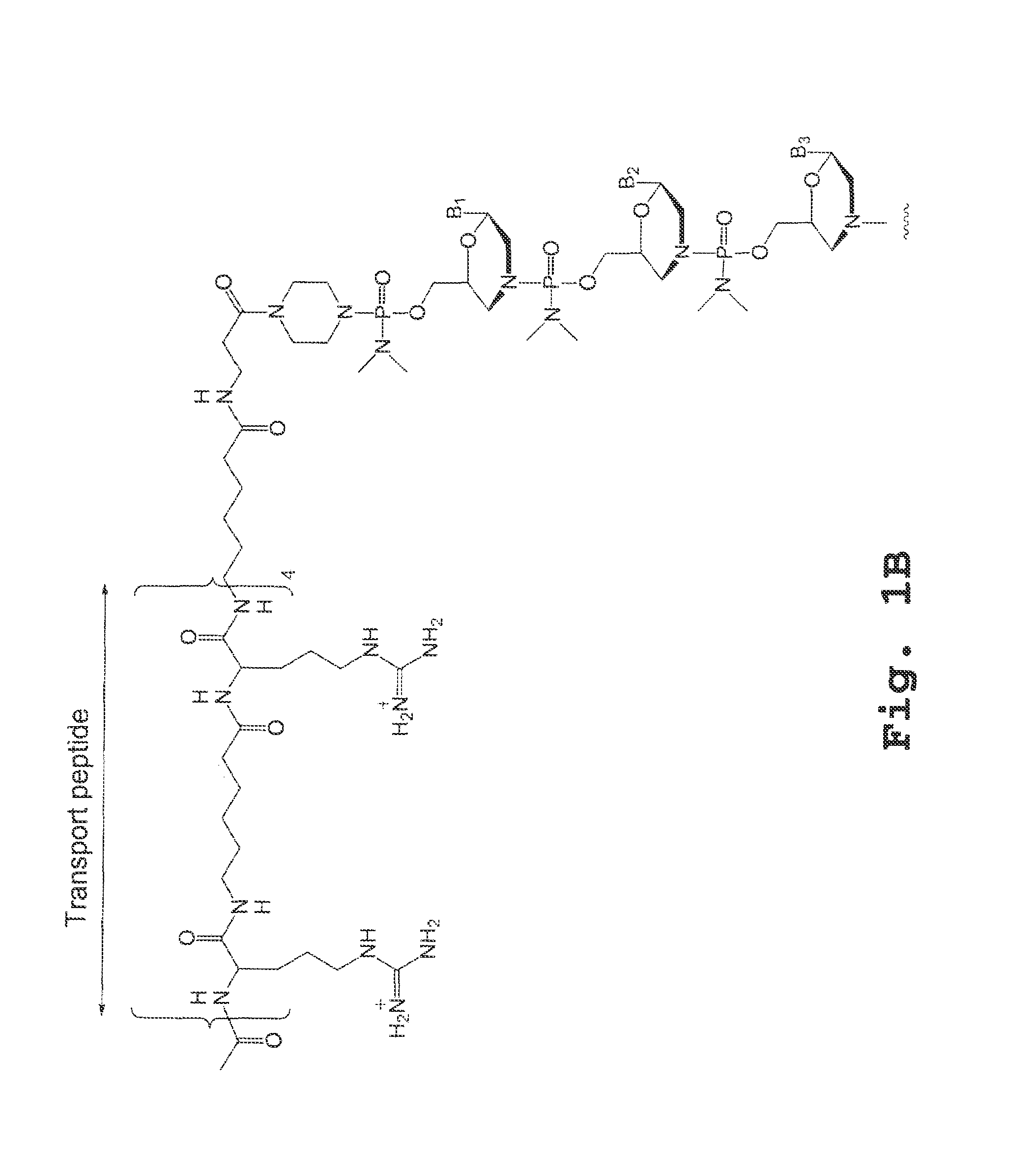
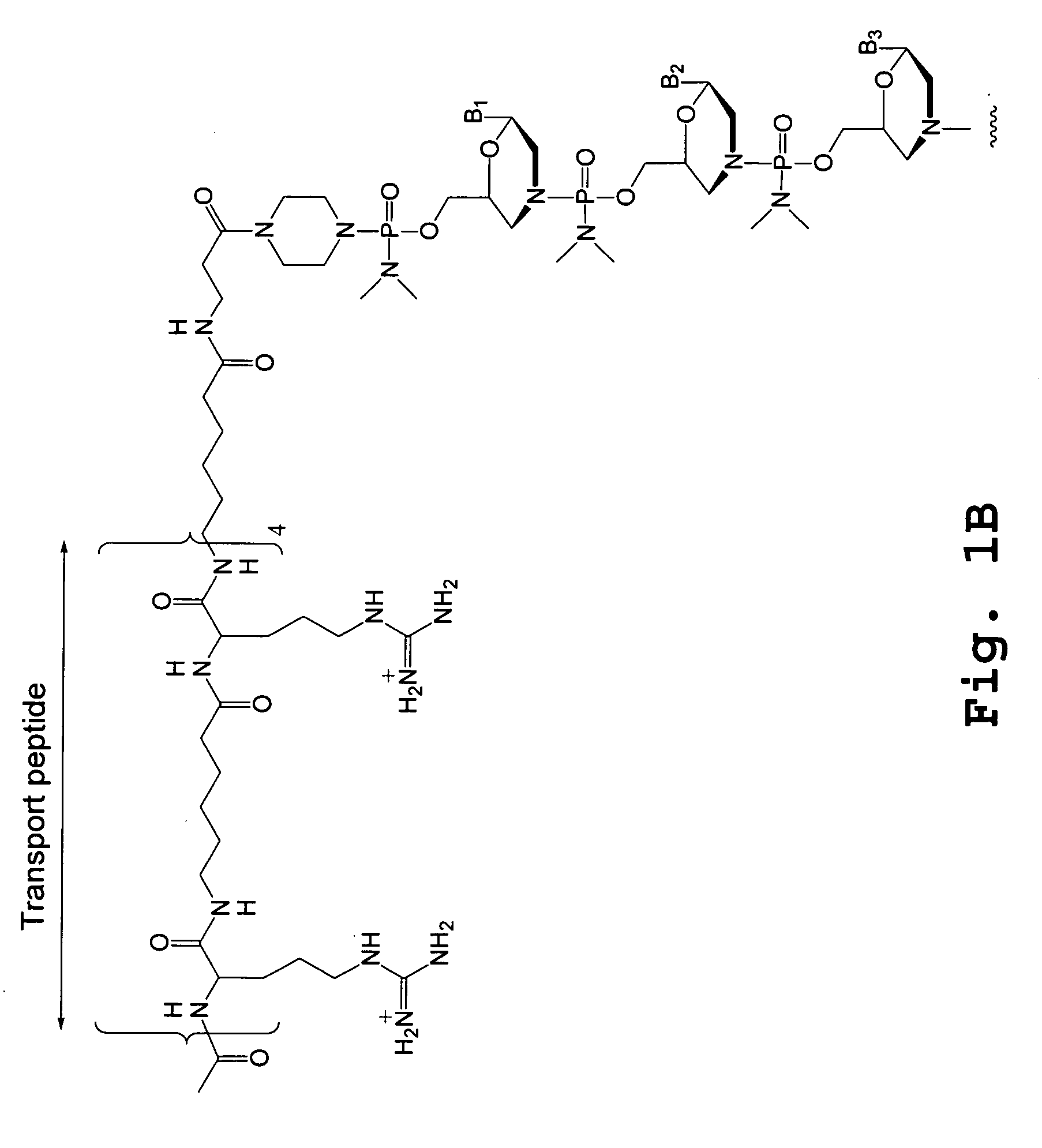
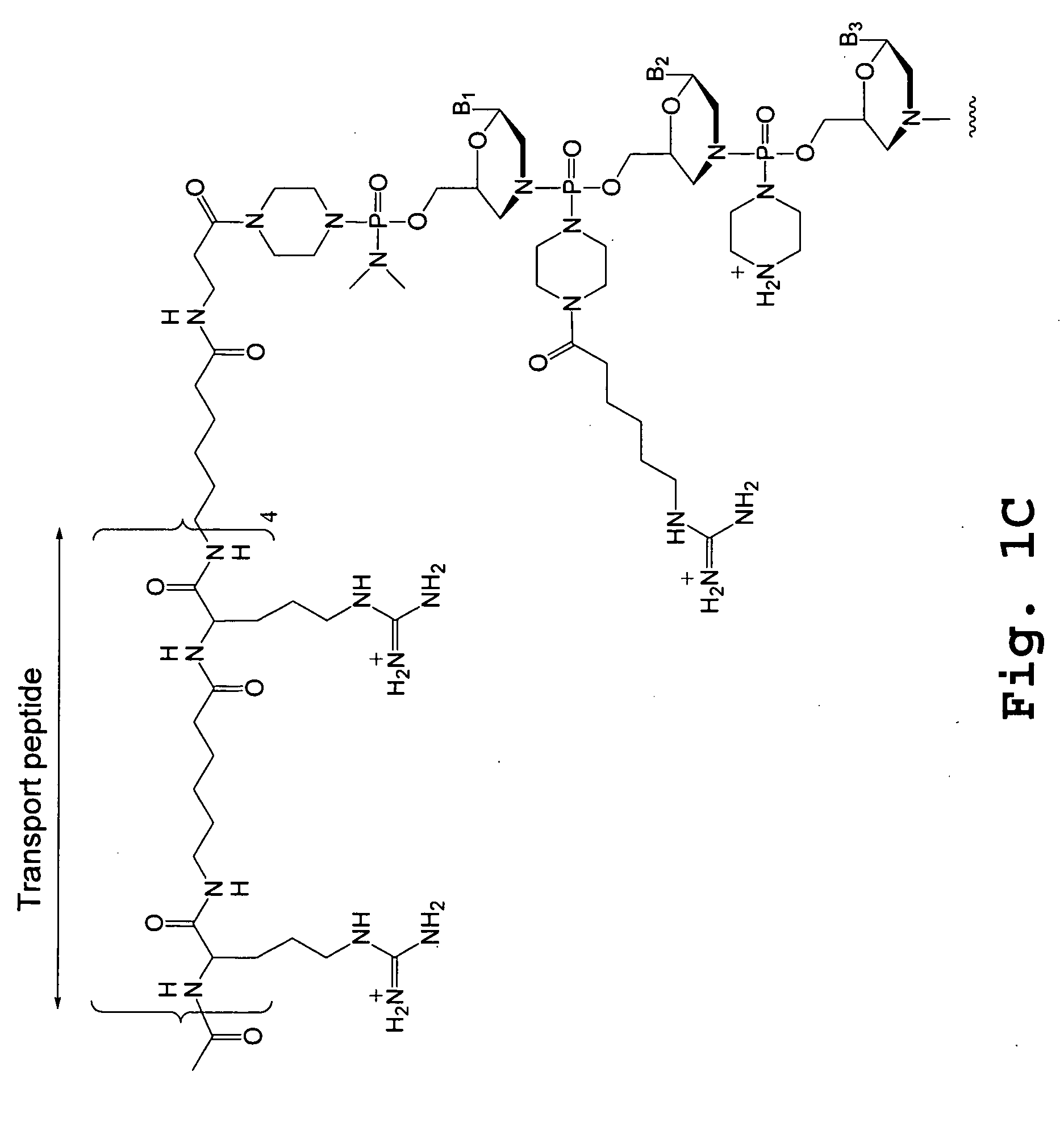
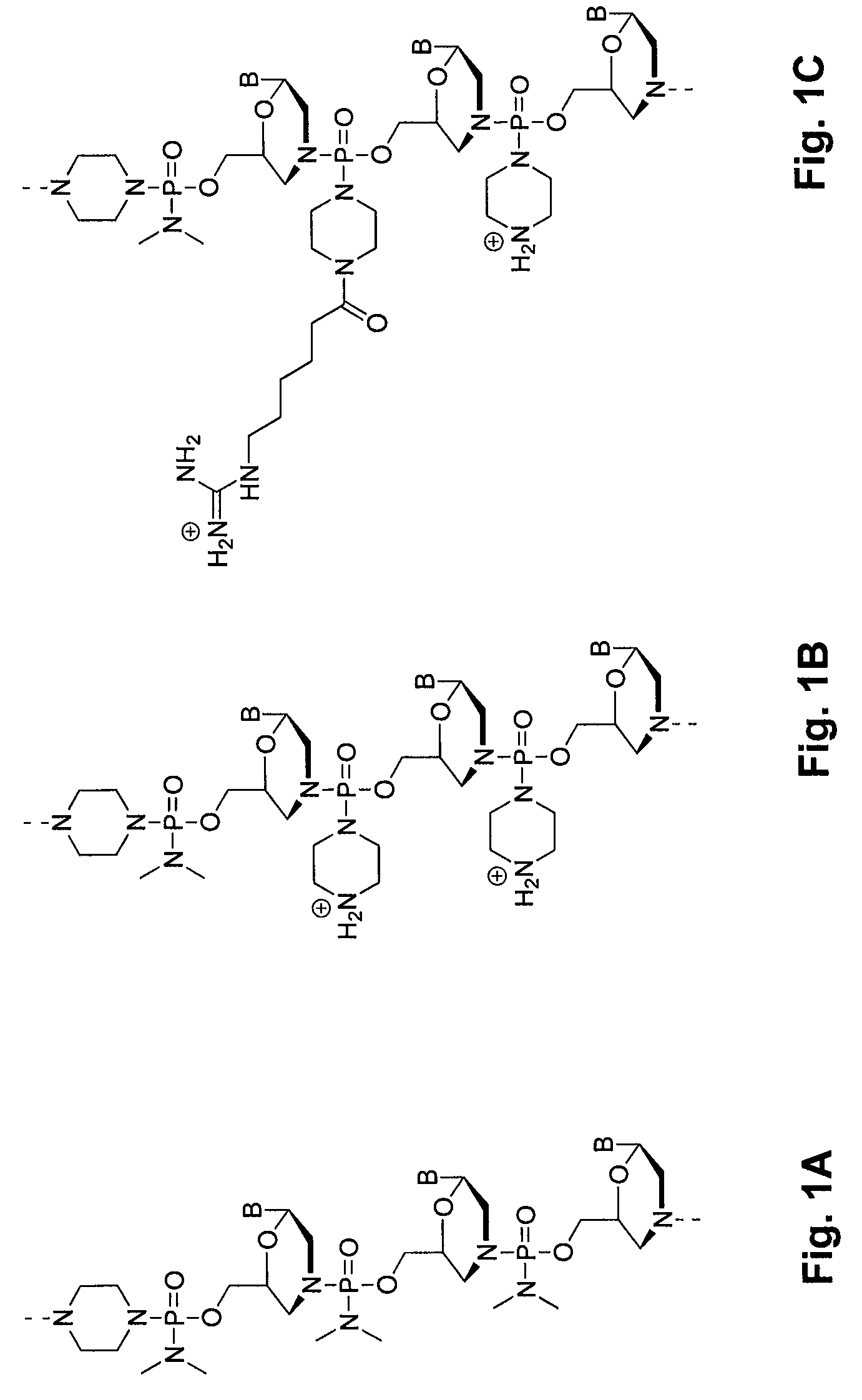
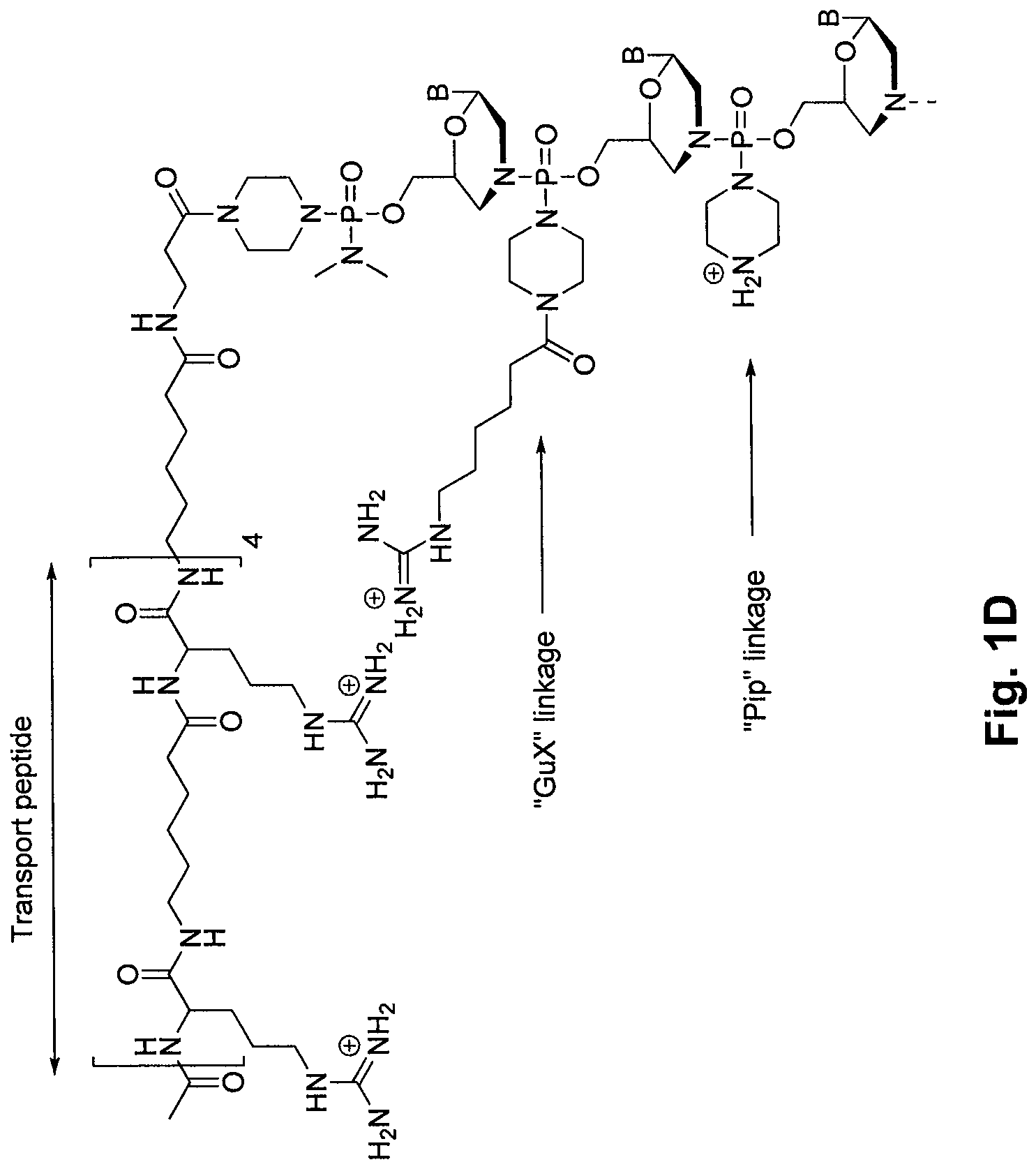
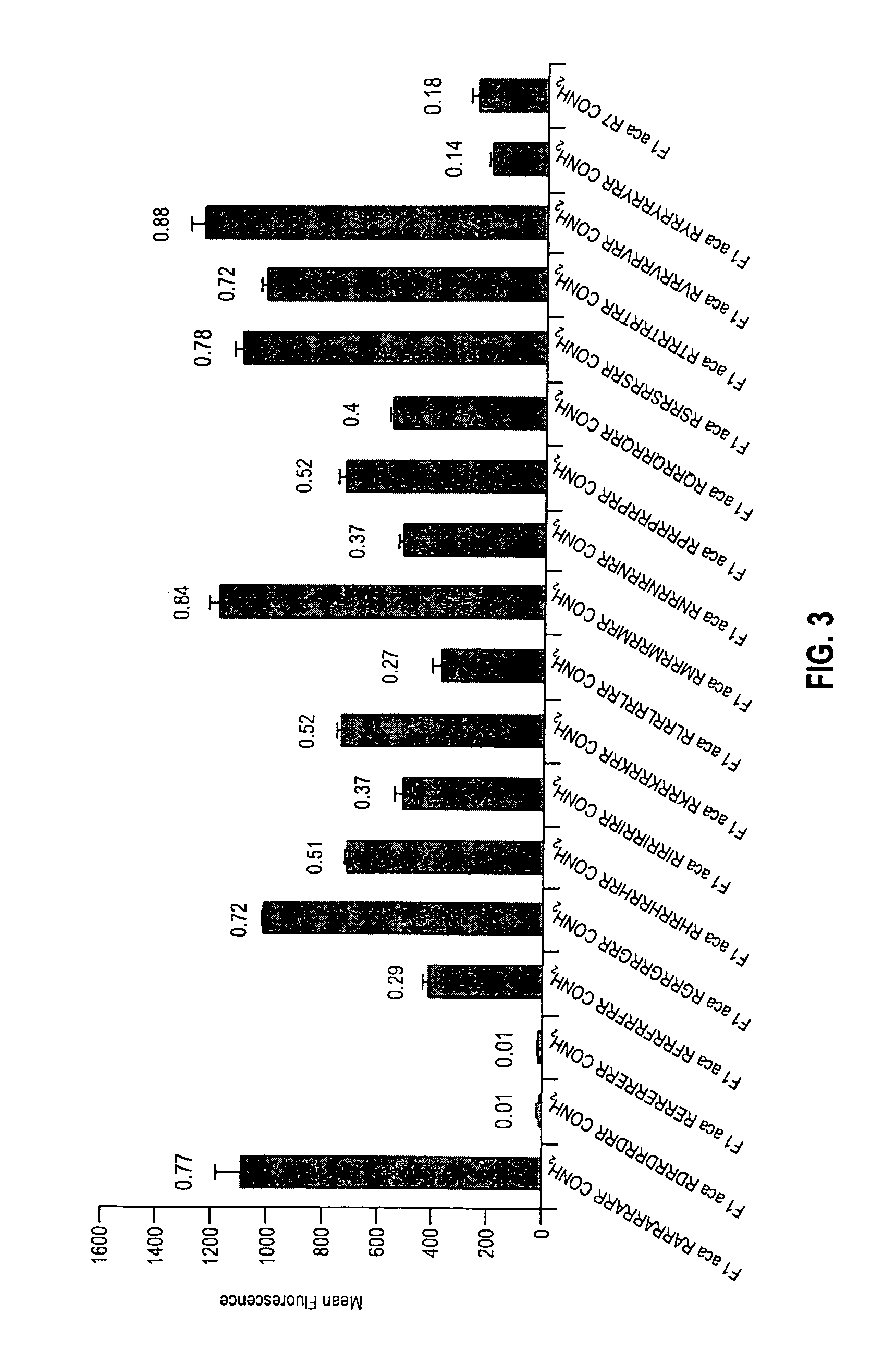
An antisense compound for use in treating myotonic dystrophy DM1 or DM2, a method of enhancing antisense targeting to heart and quadricep muscles, and a method for treating DM1 or DM2 in a mammalian subject are disclosed. The oligonucleotide has 8-30 bases, with at least 8 contiguous bases being complementary to the polyCUG or polyCCUG repeats in the 3′UTR region of dystrophia myotonica protein kinase (DMPK) mRNA in DM1 or DM2, respectively. Conjugated to the oligonucleotide is a cell-penetrating peptide having the sequence (RXRR(B / X)R)2XB, where R is arginine; B is β-alanine; and each X is —C(O)—(CH2)n—NH—, where n is 4-6. The antisense compound is effective to selectively block the sequestration of muscleblind-like 1 protein (MBNL1) and / or CUGBP, in heart and quadricep muscle in a myotonic dystrophy animal model.
Owner:SAREPTA THERAPEUTICS INC
Tissue specific peptide conjugates and methods
Cell-penetrating peptides useful for targeting a therapeutic compound to a selected mammalian tissue, methods for their identification, methods of forming conjugate compounds containing such peptides, and conjugates formed thereby are disclosed. The cell-penetrating peptides are 8 to 30 amino acid residues in length and consist of subsequences selected from the group consisting of RXR, RX, RB, and RBR; where R is arginine, B is β-alanine, and each X is independently —C(O)—(CHR1)n—NH—, where n is 4-6 and each R1 is independently H or methyl, such that at most two R1's are methyl. In one embodiment, X is a 6-aminohexanoic acid residue.
Owner:AVI BIOPHARMA
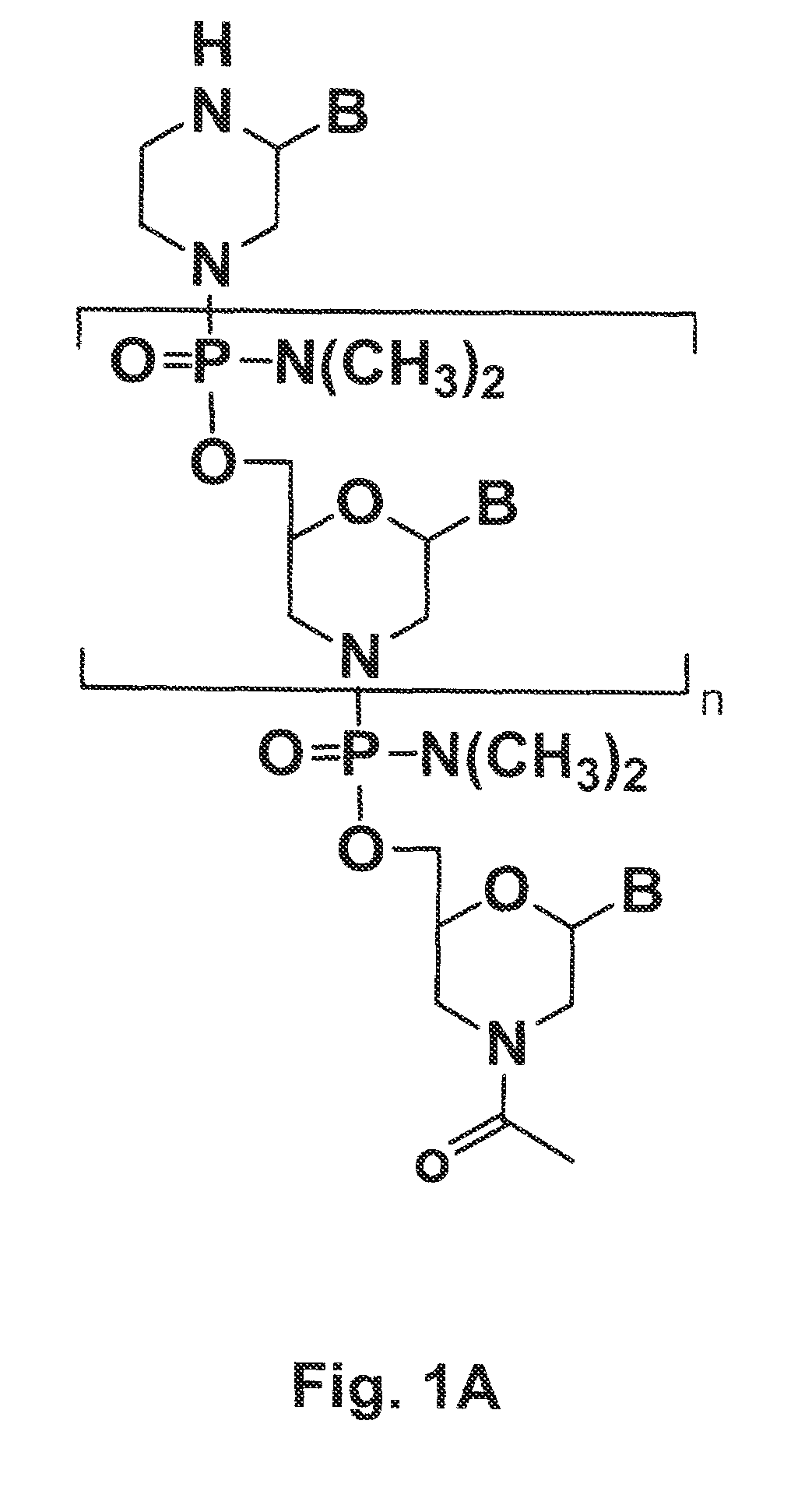
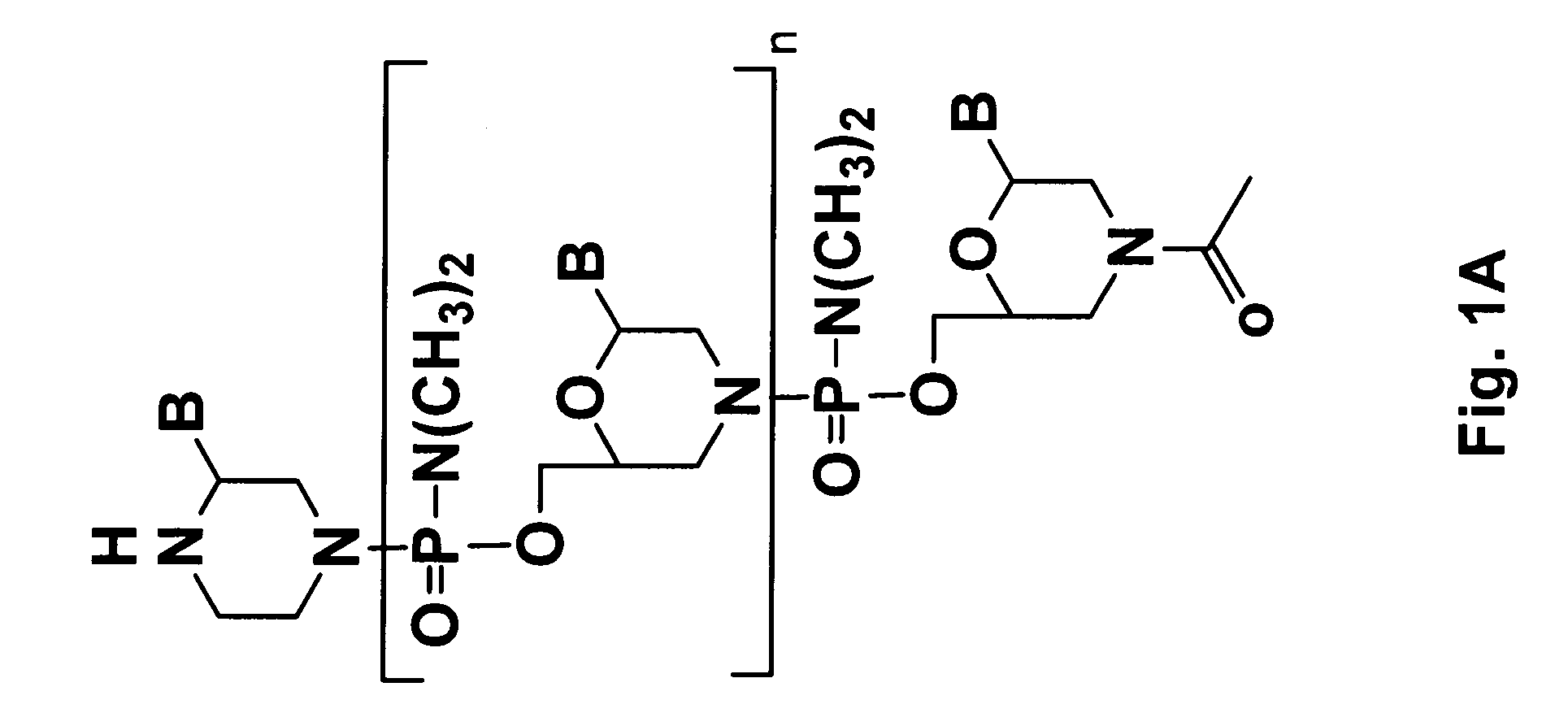
Oligonucleotide analogs having cationic intersubunit linkages
ActiveUS7943762B2High expressionHigh activitySugar derivativesActivity regulationNeutral Amino AcidsOligomer
Owner:AVI BIOPHARMA
Method of coating an intravascular stent with an endothelial cell adhesive five amino acid peptide
InactiveUS6140127APromoting cell attachmentRestore patencyBiocidePeptide/protein ingredientsCell specificArginine
Endothelial cell attachment to an intravascular stent is promoted by coating the stent with an endothelial cell specific adhesion peptide. Coating is preferably carried out by activating the intravascular stent using plasma glow discharge, applying on the stent a layer or plurality of layers of a polymer such as poly(2-hydroxyethylmethacrylate), applying a tresylation solution containing pyridine and tresyl chloride, and applying a five amino acid peptide having the sequence glycine-arginine-glutamic acid-aspartic acid-valine.
Owner:CORDIS CORP
Antibacterial antisense oligonucleotide and method
ActiveUS20080194463A1High activityHigh antibacterial activityAntibacterial agentsActivity regulationArginineAntibacterial activity
A method for enhancing, by at least 10 fold, the antibacterial activity of an antisense oligonucleotide composed of morpholino subunits linked by phosphorus-containing intersubunit linkages. The method includes one or both of: conjugating an arginine-rich carrier to a 3′ or 5′ end of the oligonucleotide and modifying the oligonucleotide to contain 20%-50% intersubunit linkages that are positively charged at physiological pH. Also disclosed is an antisense oligonucleotide having enhanced antibacterial activity by virtue of one or both modifications.
Owner:SAREPTA THERAPEUTICS INC
Thermal treatment process for tobacco materials
ActiveUS20100300463A1Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
Oligonucleotide analogs having cationic intersubunit linkages
ActiveUS20090088562A1Avoid infectionReduce viral infectionSugar derivativesActivity regulationNeutral Amino AcidsOligomer
Morpholino oligomers containing both uncharged and cationic intersubunit linkages are provided. The oligomers are oligonucleotide analogs containing predetermined sequences of base-pairing moieties. The presence of the cationic intersubunit linkages in the oligomers, typically at a level of about 10-50% of total linkages, provides enhanced antisense activity, in various antisense applications, relative to the corresponding uncharged oligomers. Also provided are such oligomers conjugated to peptide transporter moieties, where the transporters are preferably composed of arginine subunits, or arginine dimers, alternating with neutral amino acid subunits.
Owner:AVI BIOPHARMA
Biocompatible carrier containing L-arginine
L-arginine conjugated to a polymeric matrix is disclosed. The matrix can be used as, for example, a coating for an implantable medical device such as a stent.
Owner:ABBOTT CARDIOVASCULAR
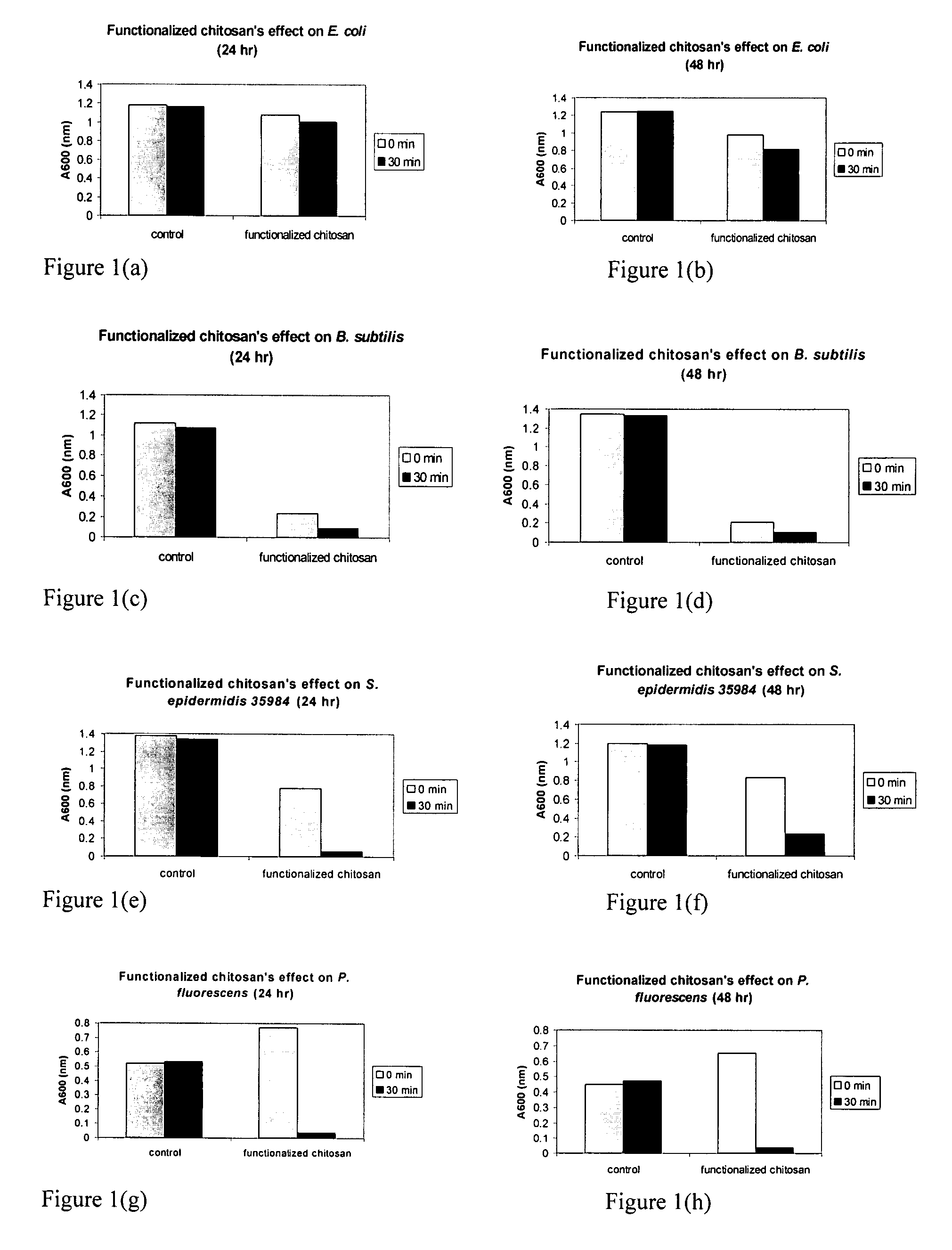
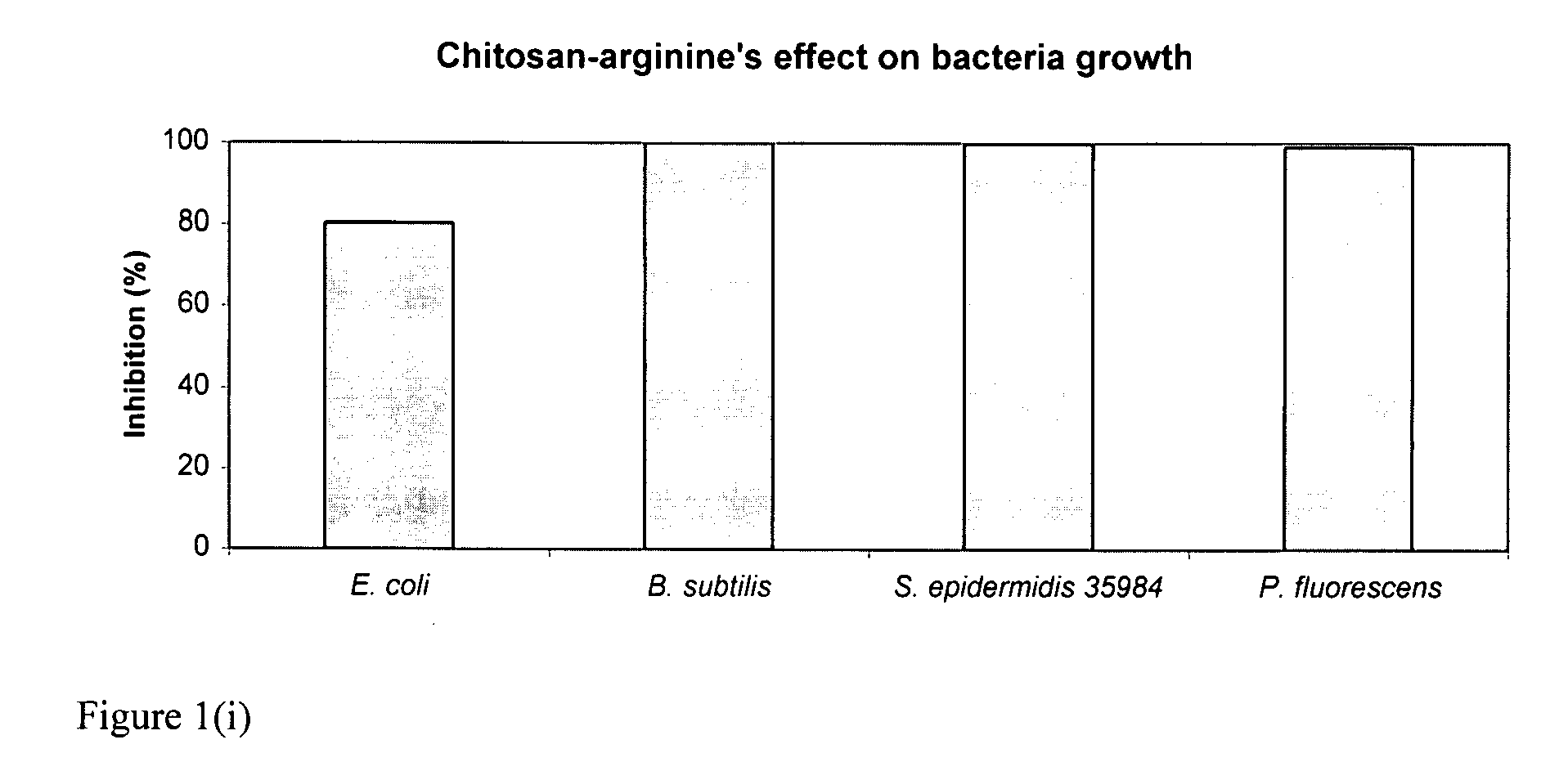
Chitosan-derivative compounds and methods of controlling microbial populations
ActiveUS20070281904A1Inhibit microbial growthControl and treat and prevent growthAntibacterial agentsCosmetic preparationsMicroorganismArginine
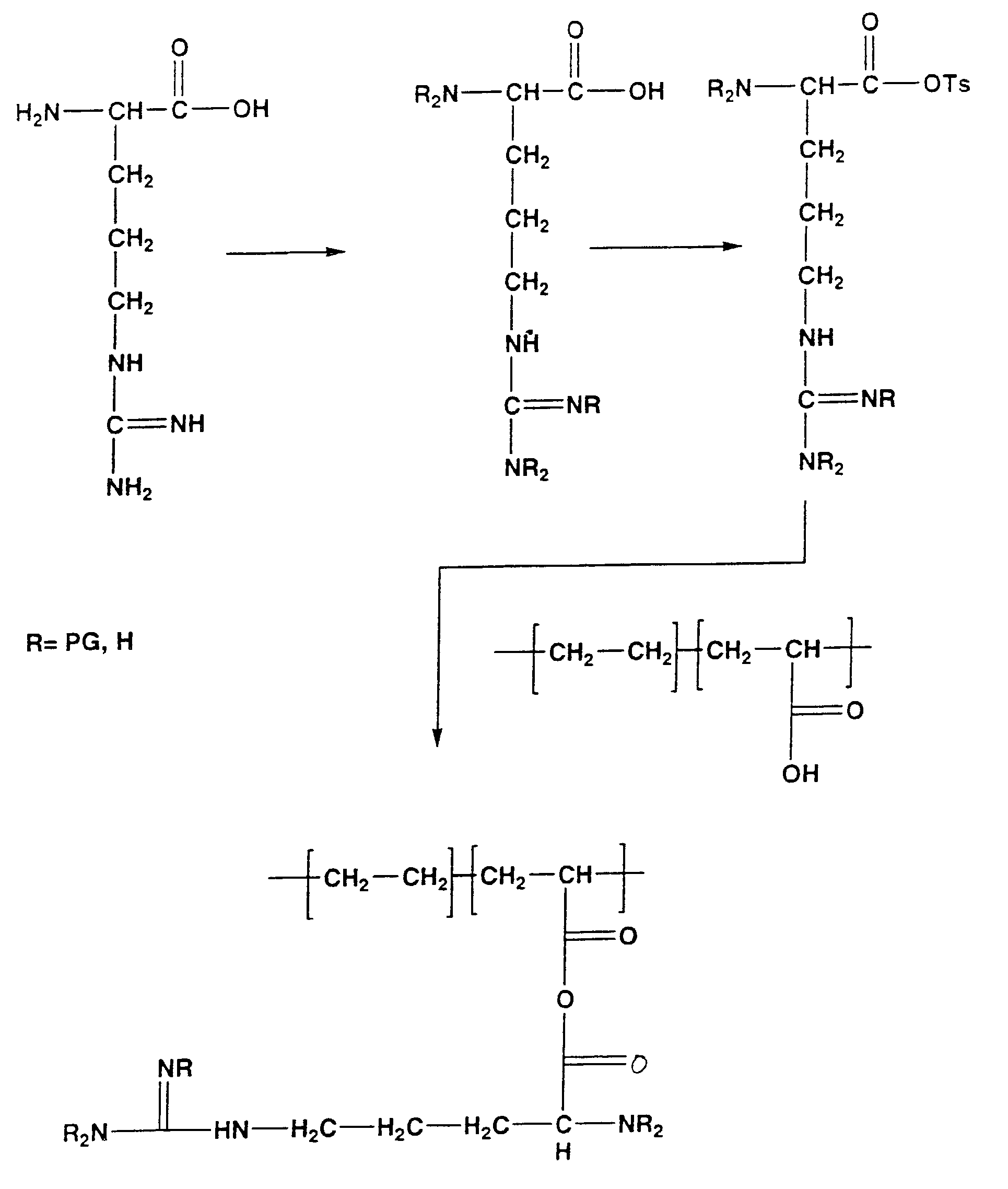
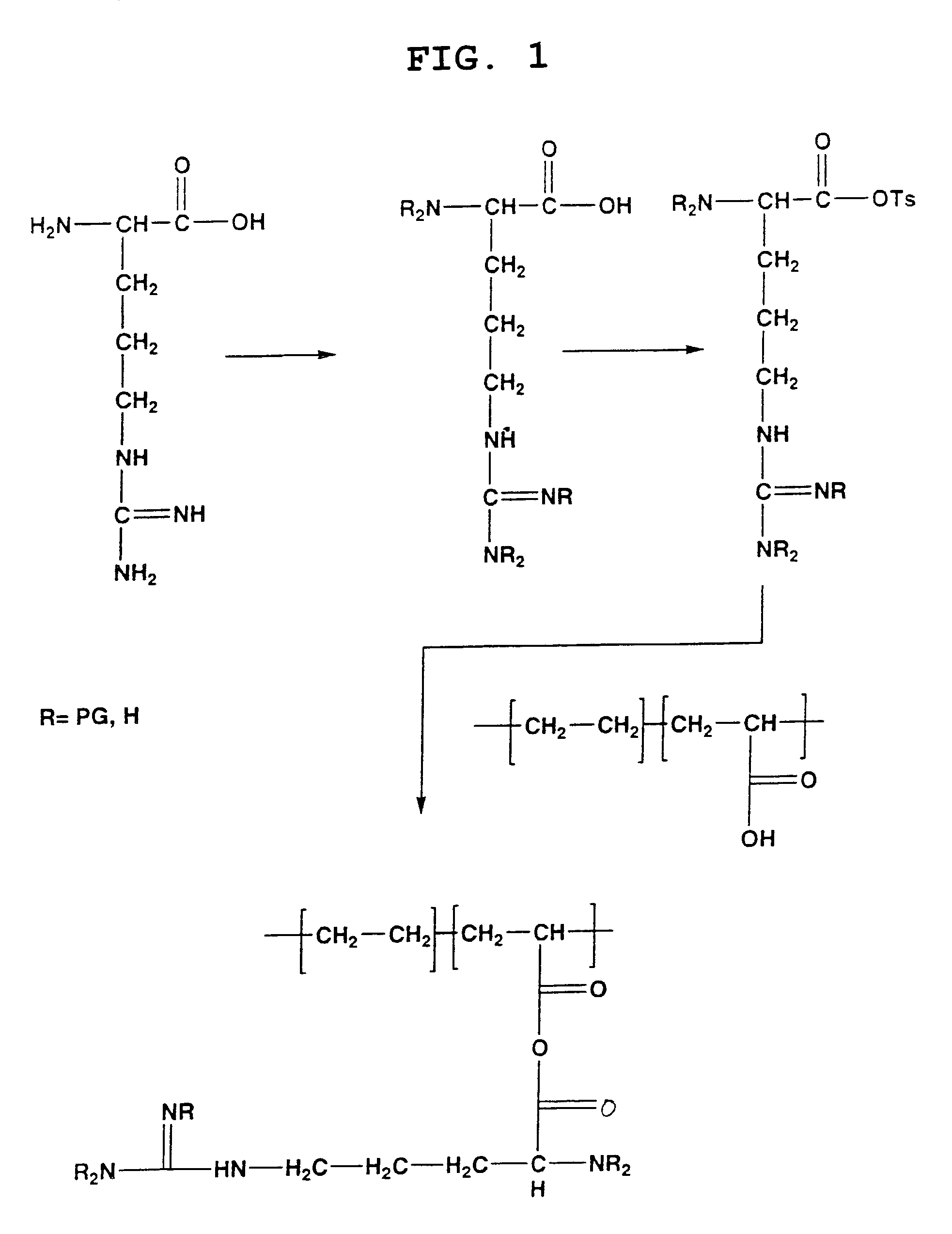
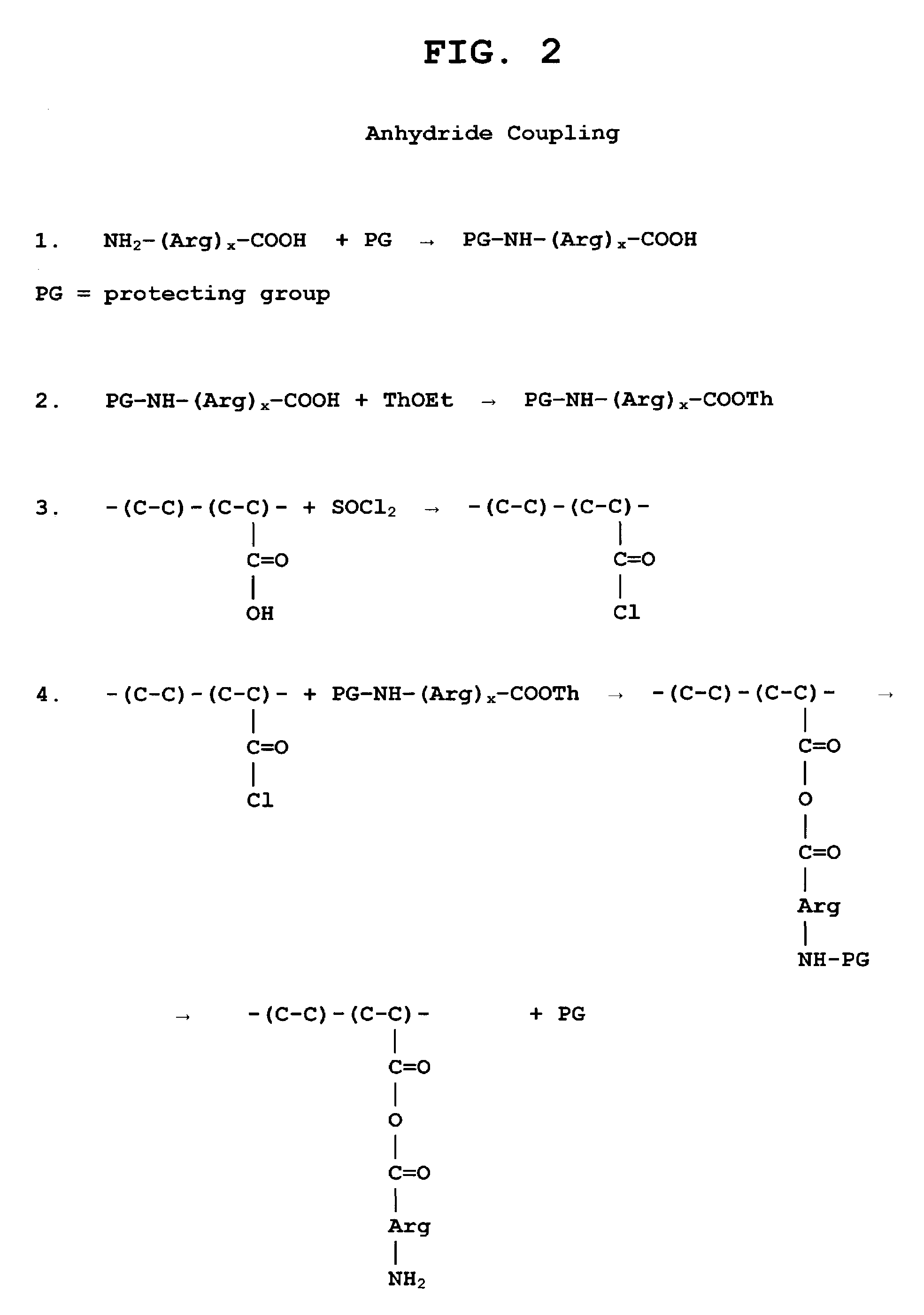
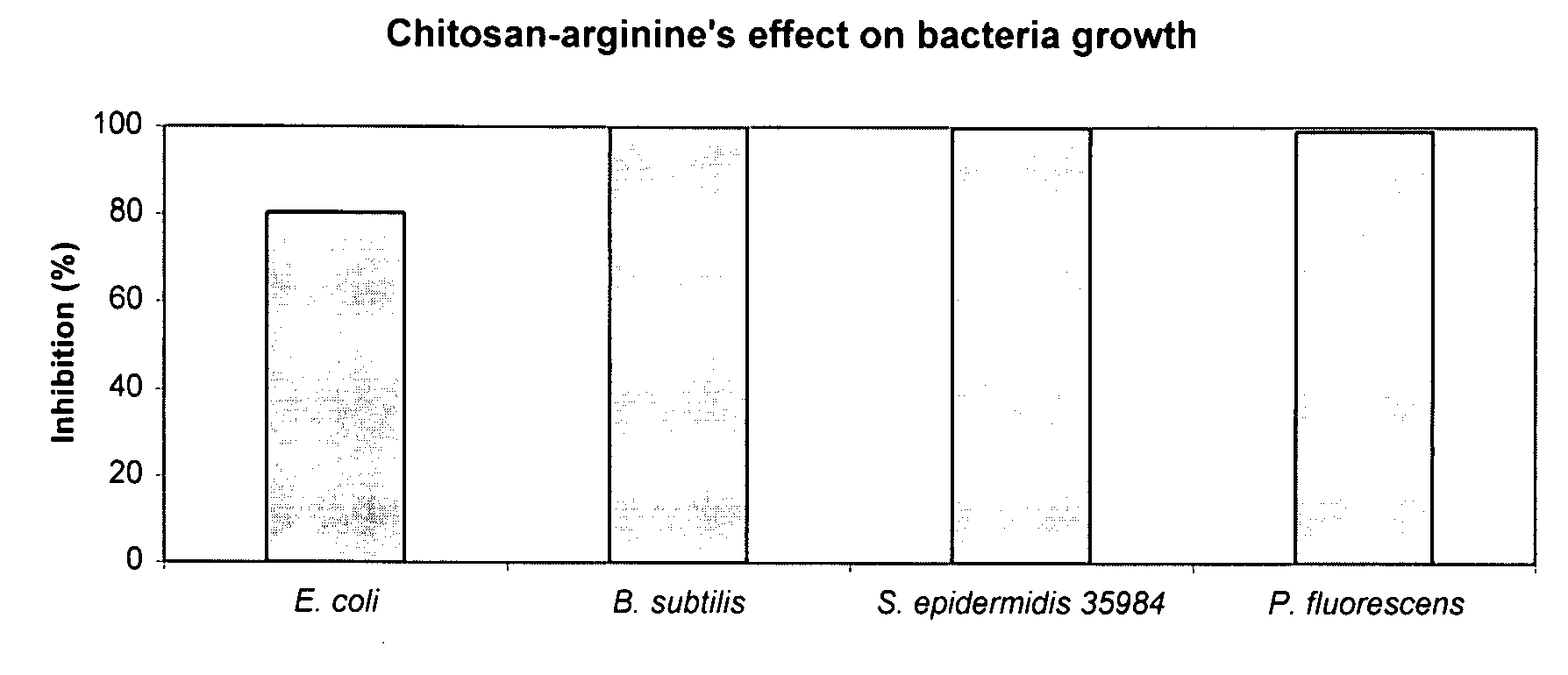
The present invention is directed to chitosan-derivative compounds and structures, methods of making chitosan-derivative compounds and methods for controlling, inhibiting and enhancing microbial populations in a variety of environments. The present invention is also directed to the control, inhibition and enhancement of microbial populations in animals, particularly humans. The microbial populations include bacteria, viruses and other pathogens where control of microbial populations are a necessity. The chitosan-derivative compounds of the present invention include chitosan-arginine compounds, related chitosan-L / D unnatural amino acid compounds, chitosan-acid amine compounds, chitosan-L / D natural amino acid derivative compounds, co-derivatives of the chitosan-derivative compounds, salts of the chitosan derivative compounds, and chitosan-guanidine compounds.
Owner:HAWAII CHITOPURE +1
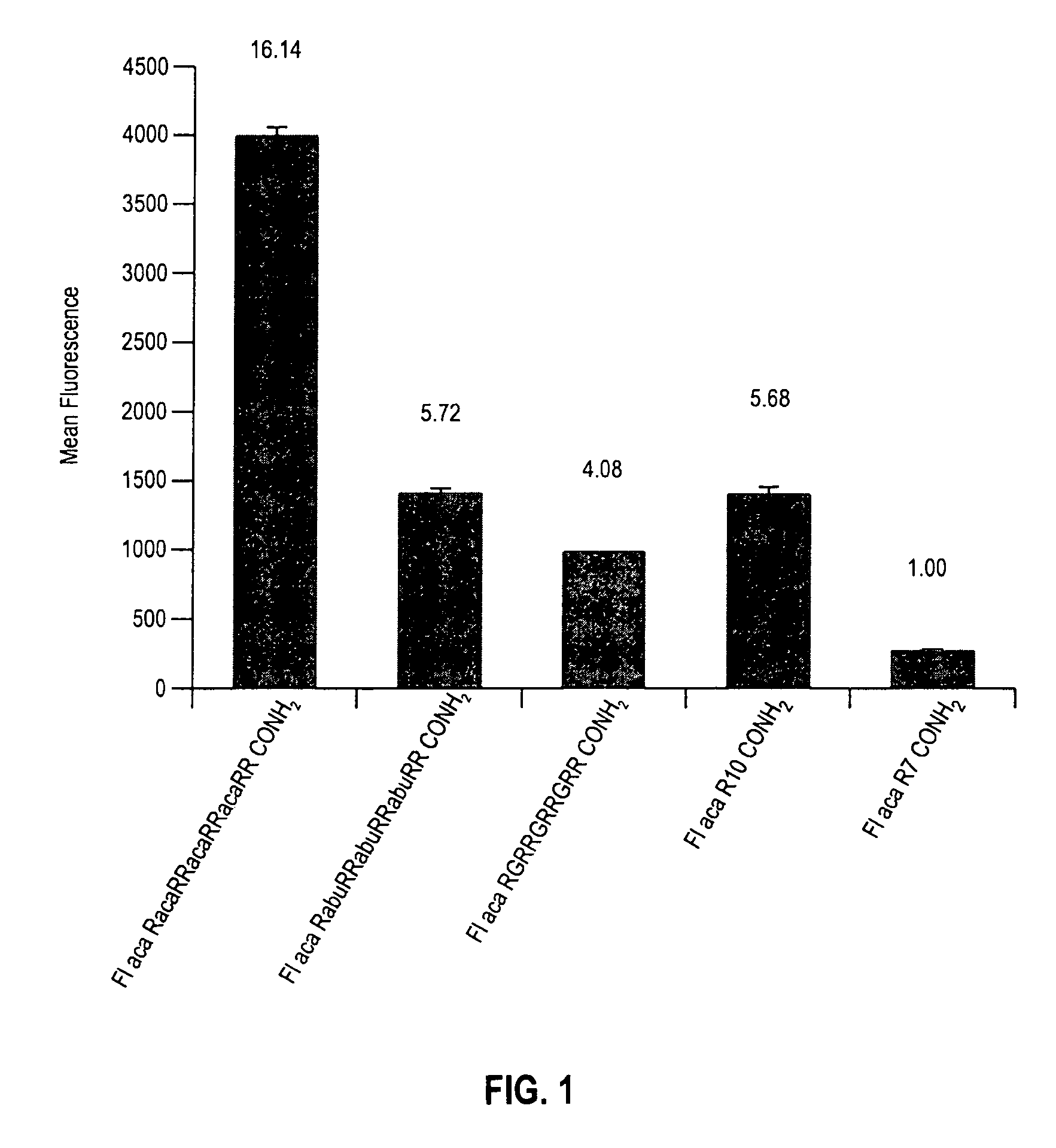
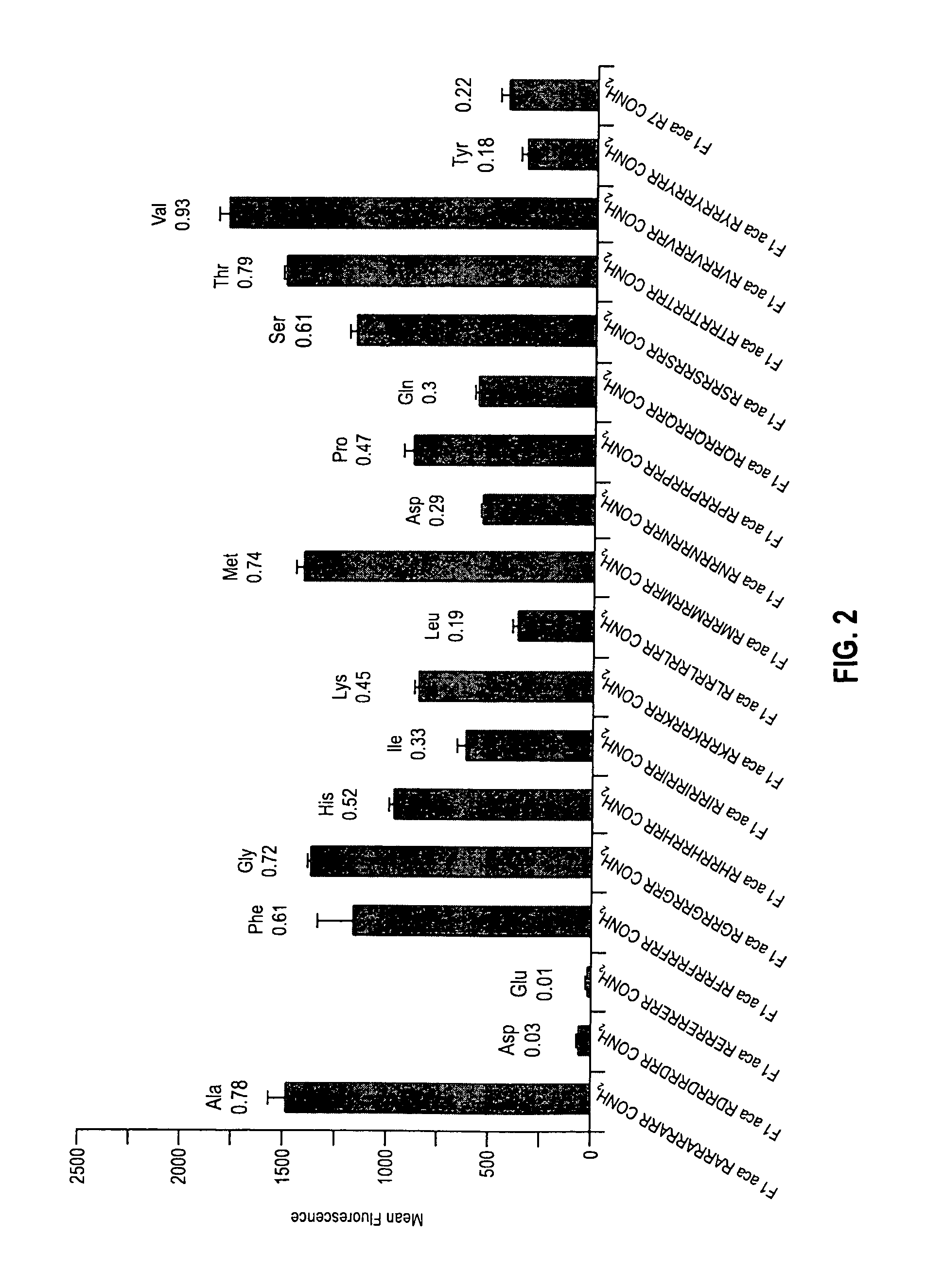
Transporters comprising spaced arginine moieties
The present invention provides compositions and methods for enhancing transport of biologically active compounds across biological membranes and across and into animal epithelial or endothelial tissues. The composition includes a biologically active agent and a transport moiety. The transport moiety includes a structure selected from the group consisting of (ZYZ)nZ, (ZY)nZ, (ZYY)nZ and (ZYYY)nZ. Subunit “Z” is L-arginine or D-arginine, and subunit “Y” is an amino acid that does not comprise an amidino or guanidino moiety. Subscript “n” is an integer ranging from 2 to 10. The method for enhancing transport involves the administration of the aforementioned composition.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Stabilized antibody-containing formulations
ActiveUS20090291076A1Good cakingIncrease of the viscosity of the high-concentration antibody-containing solutionAntibody ingredientsImmunoglobulinsArginineThreonine
The present invention relates to antibody-containing lyophilized formulations free from reducing sugars, non-reducing sugars, sugar alcohols or polysaccharides as excipients and including one or more amino acid selected from the group consisting of arginine, histidine, lysine, serine, proline, glycine, alanine and threonine or a salt thereof.
Owner:CHUGAI PHARMA CO LTD
Novel oral forms of a phosphonic acid derivative
InactiveUS20120190647A1Improved aqueous solubilityIncrease ratingsBiocideOrganic active ingredientsEnprofyllineArginine
Novel solution complexes of zoledronic acid are described which give rise to improved properties of zoledronic acid. The invention includes aqueous solution and molecular complexes of zoledronic acid with and optical isomers of asparagine, histidine, arginine and proline as well as pharmaceutical complexes containing them and methods of treatment using them.
Owner:THAR PHARMA
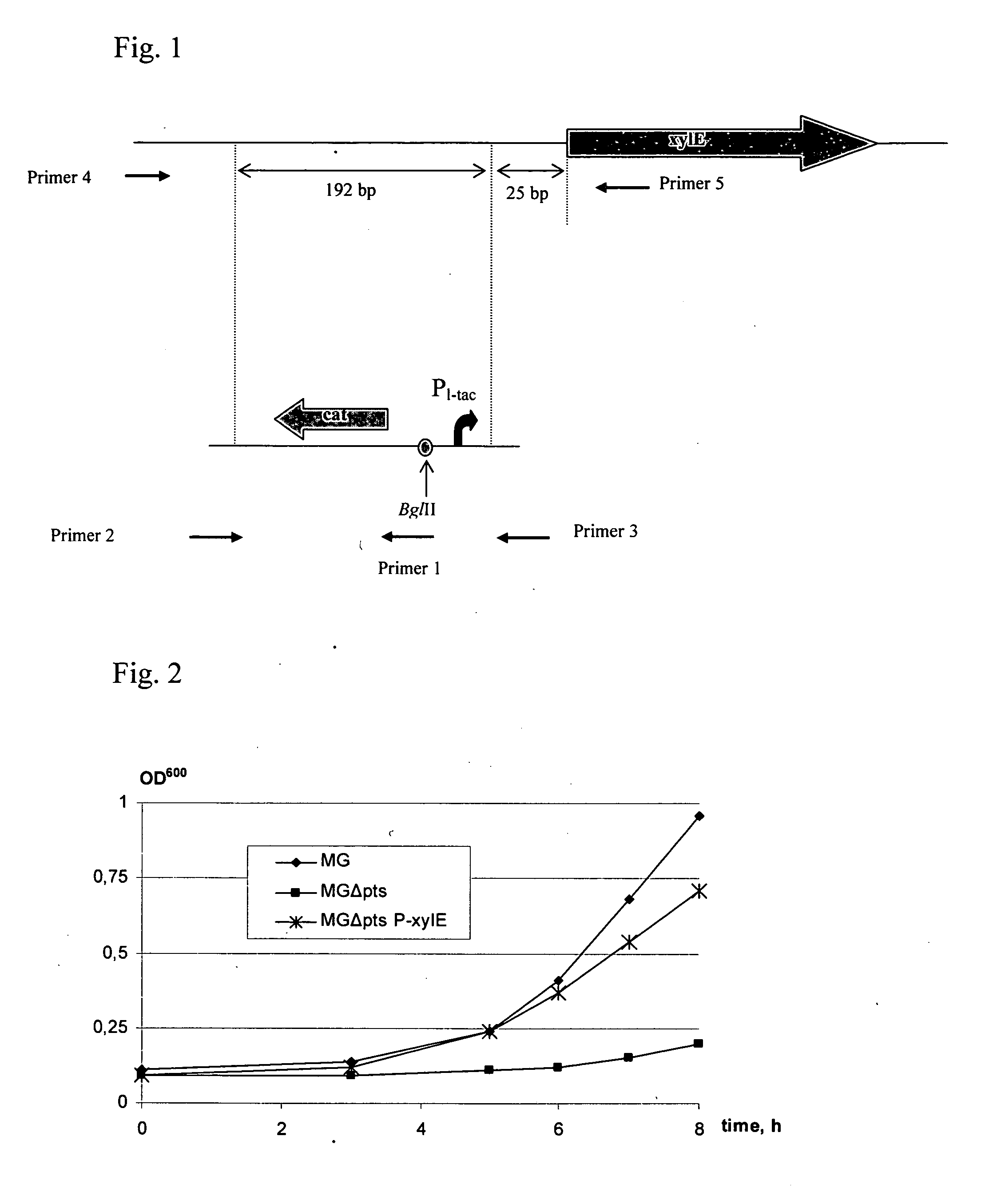
Method for producing L-amino acids using bacteria of the Enterobacteriaceae family
ActiveUS20060088919A1Improve productivityD-xylose permease is enhancedBacteriaDepsipeptidesL-threonineArginine
There is disclosed a method for producing L-amino acid, for example L-threonine, L-lysine, L-histidine, L-phenylalanine, L-arginine or L-glutamic acid, using a bacterium of the Enterobacteriaceae family, wherein the bacterium has been modified to enhance an activity of D-xylose permease.
Owner:AJINOMOTO CO INC
Composition for long-acting peptide analogs
ActiveUS20090088387A1Increase perfusionImprove the level ofAntibacterial agentsPeptide/protein ingredientsHalf-lifeArginine
The invention describes compositions of peptide analogs that are active in blood or cleavable in blood to release an active peptide. The peptide analogs have a general formula: A-(Cm)x-Peptide, wherein A is hydrophobic moiety or a metal binding moiety, e.g., a chemical group or moiety containing 1) an alkyl group having 6 to 36 carbon units, 2) a nitrilotriacetic acid group, 3) an imidodiacetic acid group, or 4) a moiety of formula (ZyHisw)p, wherein Z is any amino acid residue other than histidine, His is histidine, y is an integer from 0-6; w is an integer from 1-6; and p is an integer from 1-6; wherein if A has alkyl group with 6 to 36 carbon units x is greater than 0; and Cm is a cleavable moiety consisting of glycine or alanine or lysine or arginine or N-Arginine or N-lysine, wherein x is an integer between 0-6 and N may be any amino acid or none. The peptide analogs are complexed with polymeric carrier to provide enhanced half-life.
Owner:PHARMAIN CORP
CATIONIC PEPTIDES FOR siRNA INTRACELLULAR DELIVERY
What is described is a composition for delivery of a RNA molecule to a cell, comprising: a double stranded RNA (dsRNA) molecule of about 15 to about 40 base pairs; and a polynucleotide delivery-enhancing peptide, comprising a region of alternating lysine and histidine residues, or of alternating D and L forms of arginine.
Owner:NASTECH PHARMA
Beneficial Effects of Increasing Local Blood Flow
InactiveUS20090105336A1Increase oxygenationImprove tissue nutritionBiocideOrganic active ingredientsTopical creamArginine
The present invention provides a treatment for enhancing the ability of the body to heal wounds. A topical cream is described which improves blood flow by the transdermal delivery of the nitric oxide precursor L-Arginine either alone or with an adjunct, theophylline. The delivery of the active agents is accomplished by use of a vehicle which contains a hostile biophysical environment which is also hostile to hydrogen bond formation.
Owner:STRATEGIC SCI & TECH
Pharmaceutical composition and method for the transdermal delivery of calcium
InactiveUS20070292493A1Reduce disadvantagesReduce and prevent likelihoodHalogenated hydrocarbon active ingredientsBiocideArginineTryptophan
The present invention relates to a method and transdermal pharmaceutical composition for preventing or reducing the likelihood of calcium deficiency or imbalances caused by calcium deficiency. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of calcium and a pharmaceutically acceptable carrier constituting a pluronic lecithin organogel. In addition to calcium, the transdermal pharmaceutical composition may also contain a therapeutically effective amount of: (1) a pharmaceutically acceptably salt of other minerals such as magnesium, zinc, selenium, manganese, or chromium; (2) a vitamin such as vitamin A, vitamin D, vitamin C, vitamin E or B-complex vitamins, choline, lecithin, inositol, PABA, biotin, or bioflavomoids; (3) a carotenoid such as lycopene or lutein; (4) a hormone such as dehydroepiandrosterone, progesterone, pregnenolone, or melatonin; (5) an amino acid such as arginine, glutamine, lysine, phenylalanine, tyrosine, GABA, tryptophan, carnitine, or acetyl-l-carnitine; (6) a fatty acid such as a fish oil or flax seed oil; (7) a vita-nutrient such as coenzyme Q10; (8) a cartilage building nutrient such as glucosamine, chondroitin, or MSM, (9) a herb such as ginkgo biloba, echinacea, 5-HTP, St. John's wort, or saw palmetto; or (9) any combination thereof. The transdermal pharmaceutical composition may be topically administered to a human to prevent or reduce the likelihood of calcium deficiency or imbalances caused by calcium deficiency such as hypertension, high cholesterol, colon and rectal cancer, osteomalacia, rickets, osteoporosis, cardiovascular disease, preeclampsia, tooth decay, and premenstrual syndrome.
Owner:BRIERRE BARBARA T
Use of phosphoketolase for producing useful metabolites
Owner:AJINOMOTO CO INC
Thermal treatment process for tobacco materials
ActiveUS8434496B2Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
Gene products of bacillus licheniformis which form odorous substances and improved biotechnological production methods based thereon
InactiveUS20070190605A1Reduce formationImprove filtering effectBacteriaHydrolasesBacillus licheniformisPropanoic acid
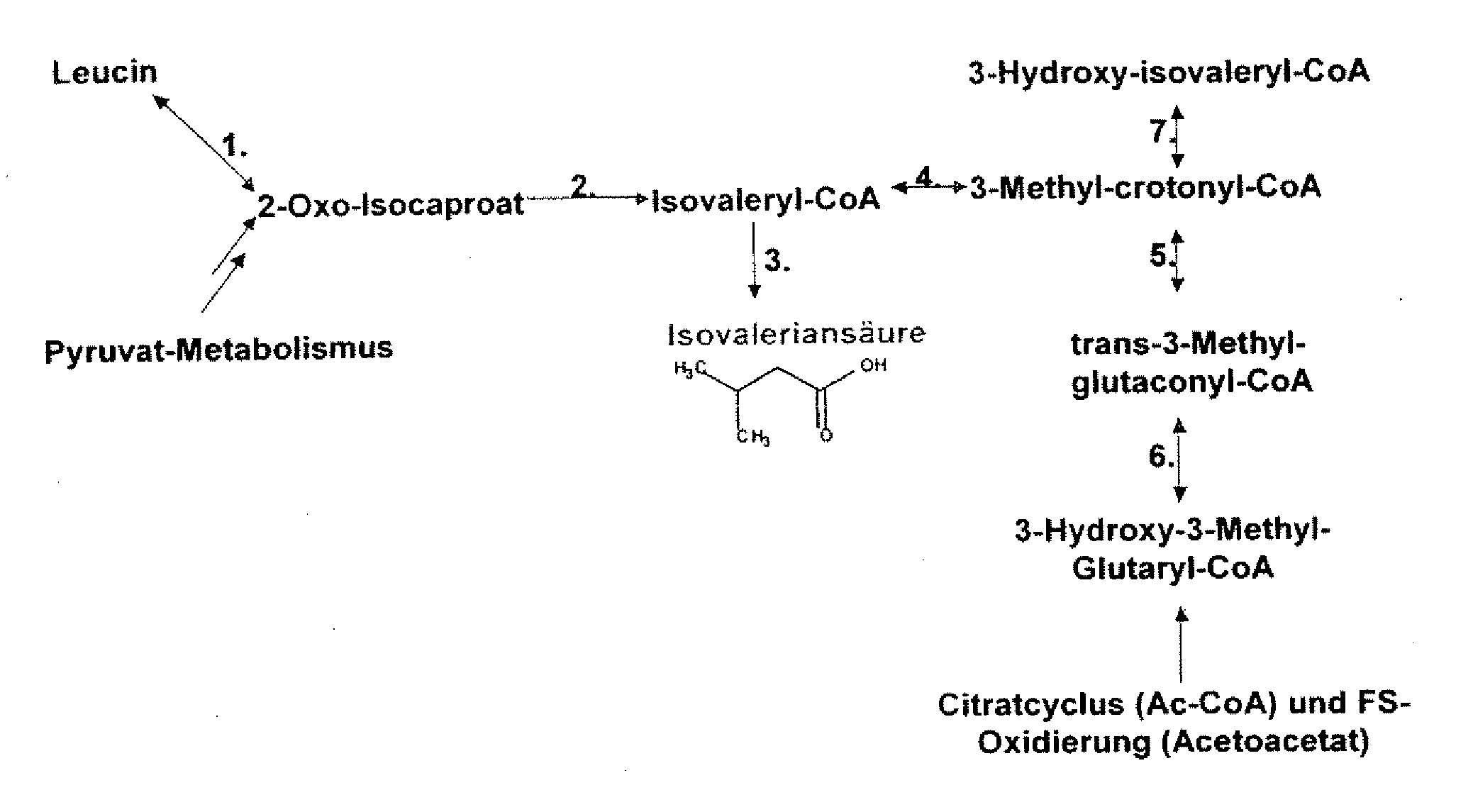
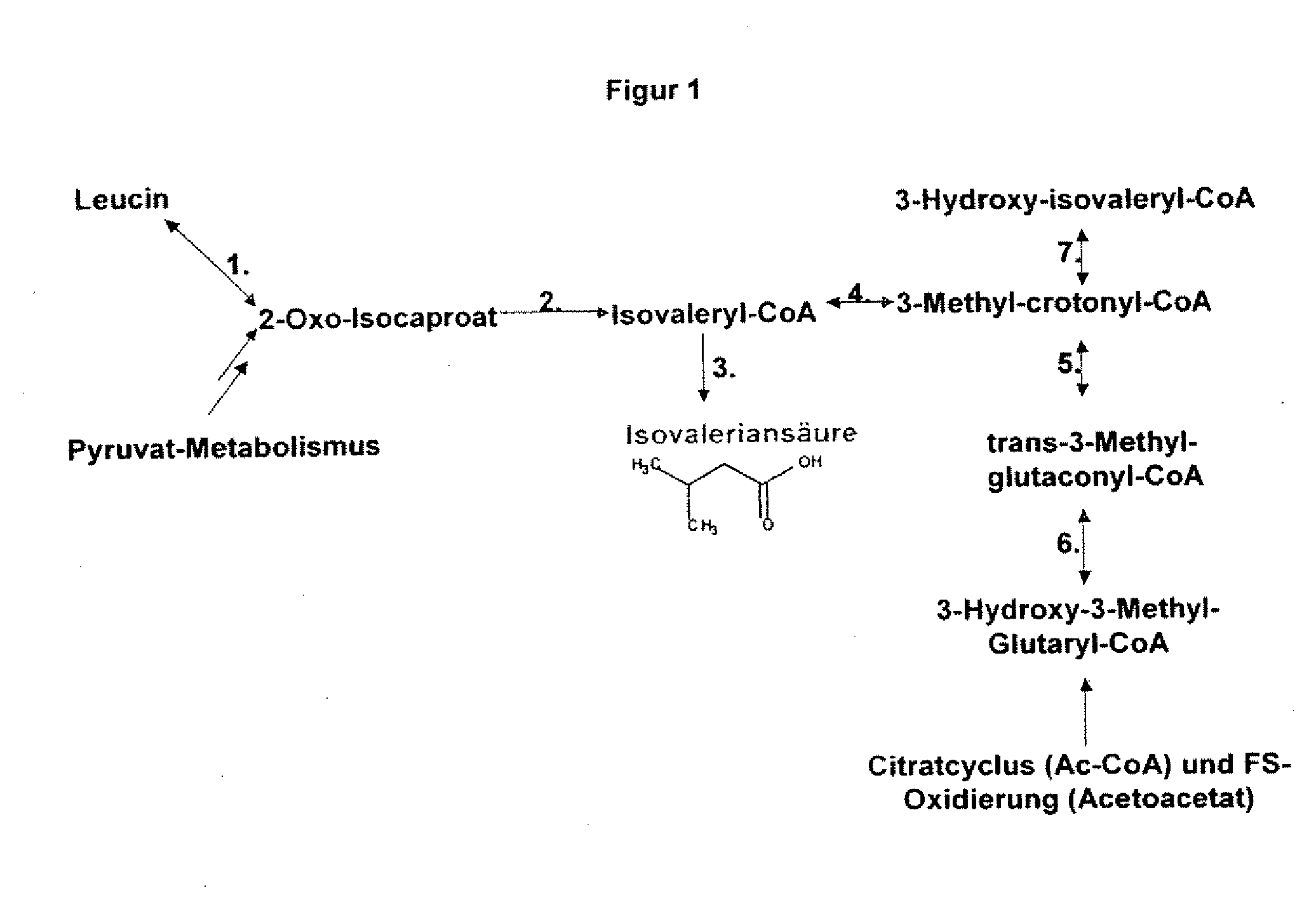
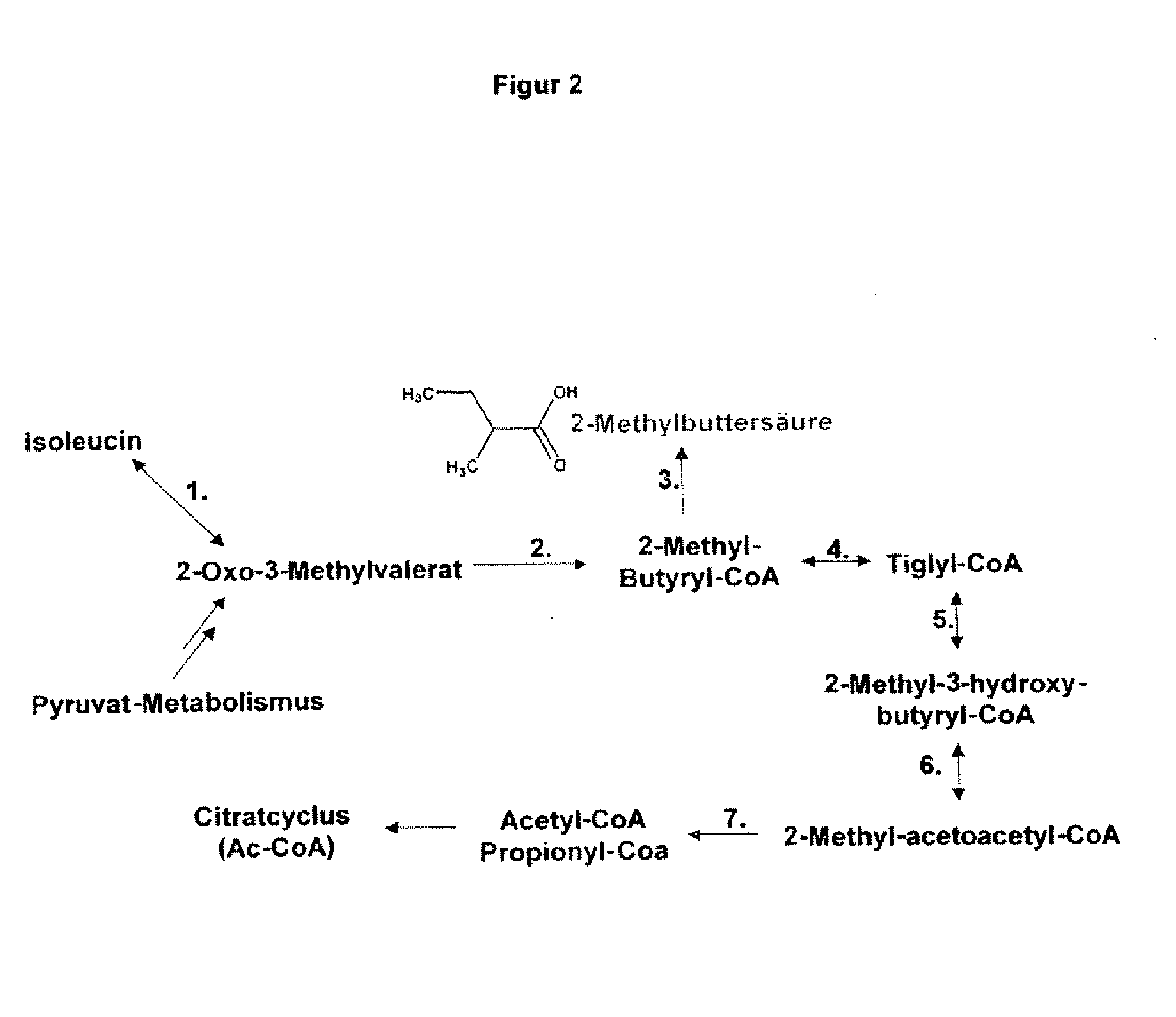
The present invention relates to 25 hitherto undescribed genes of B. licheniformis and gene products derived therefrom and all sufficiently homologous nucleic acids and proteins thereof. They occur in five different metabolic pathways for the formation of odorous substances. The metabolic pathways in question are for the synthesis of: 1) isovalerian acid (as part of the catabolism of leucine), 2) 2-methylbutyric acid and / or isobutyric acid (as part of the catabolism of valine and / or isoleucine), 3) butanol and / or butyric acid (as part of the metabolism of butyric acid), 4) propyl acid (as part of the metabolism of propionate) and / or 5) cadaverine and / or putrescine (as parts of the catabolism of lysine and / or arginine). The identification of these genes allows biotechnological production methods to be developed that are improved to the extent that, to assist these nucleic acids, the formation of the odorous substances synthesized via these metabolic pathways can be reduced by deactivating the corresponding genes in the micro-organism used for the biotechnological production. In addition, these gene products are thus available for preparing reactions or for methods according to their respective biochemical properties.
Owner:BASF AG
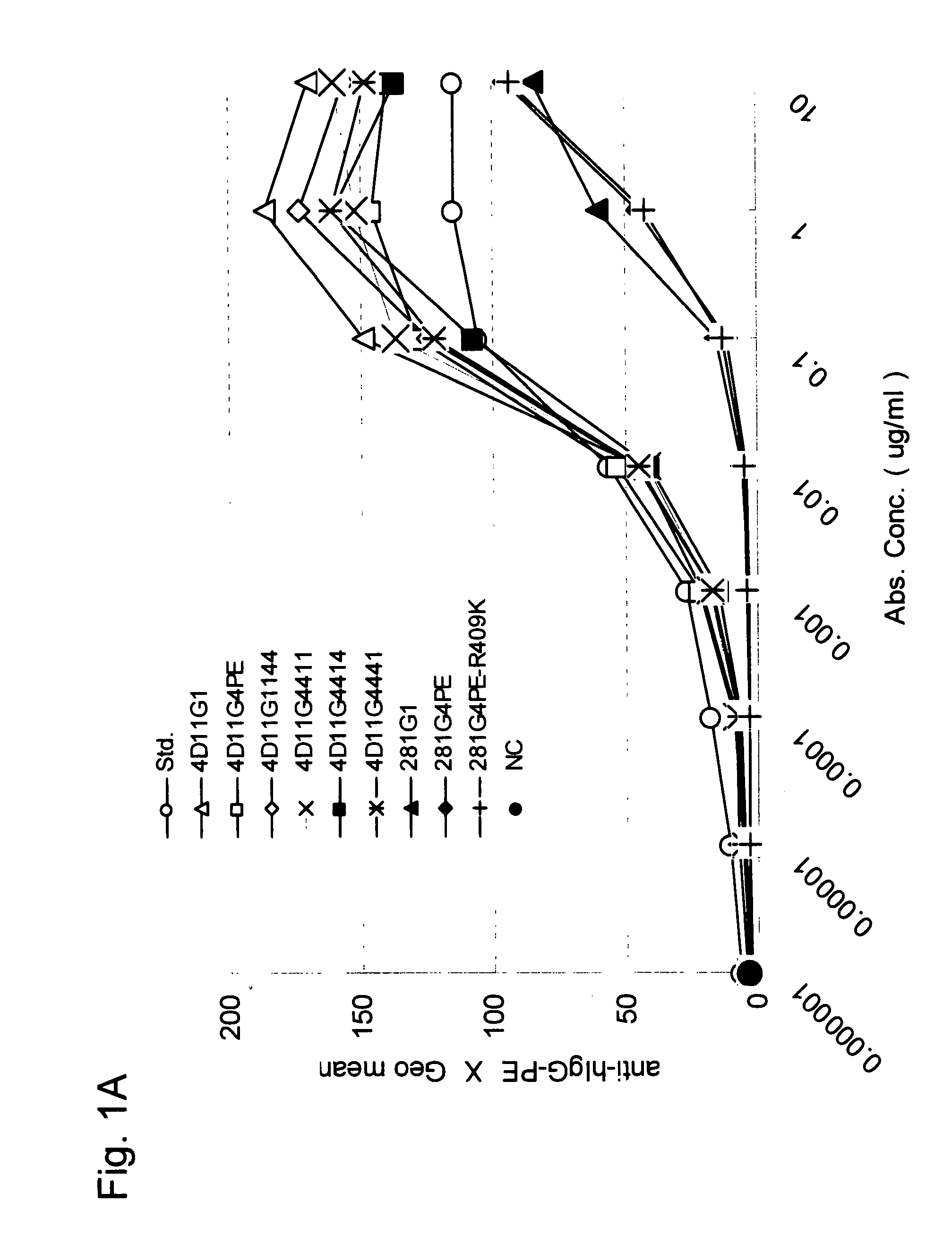
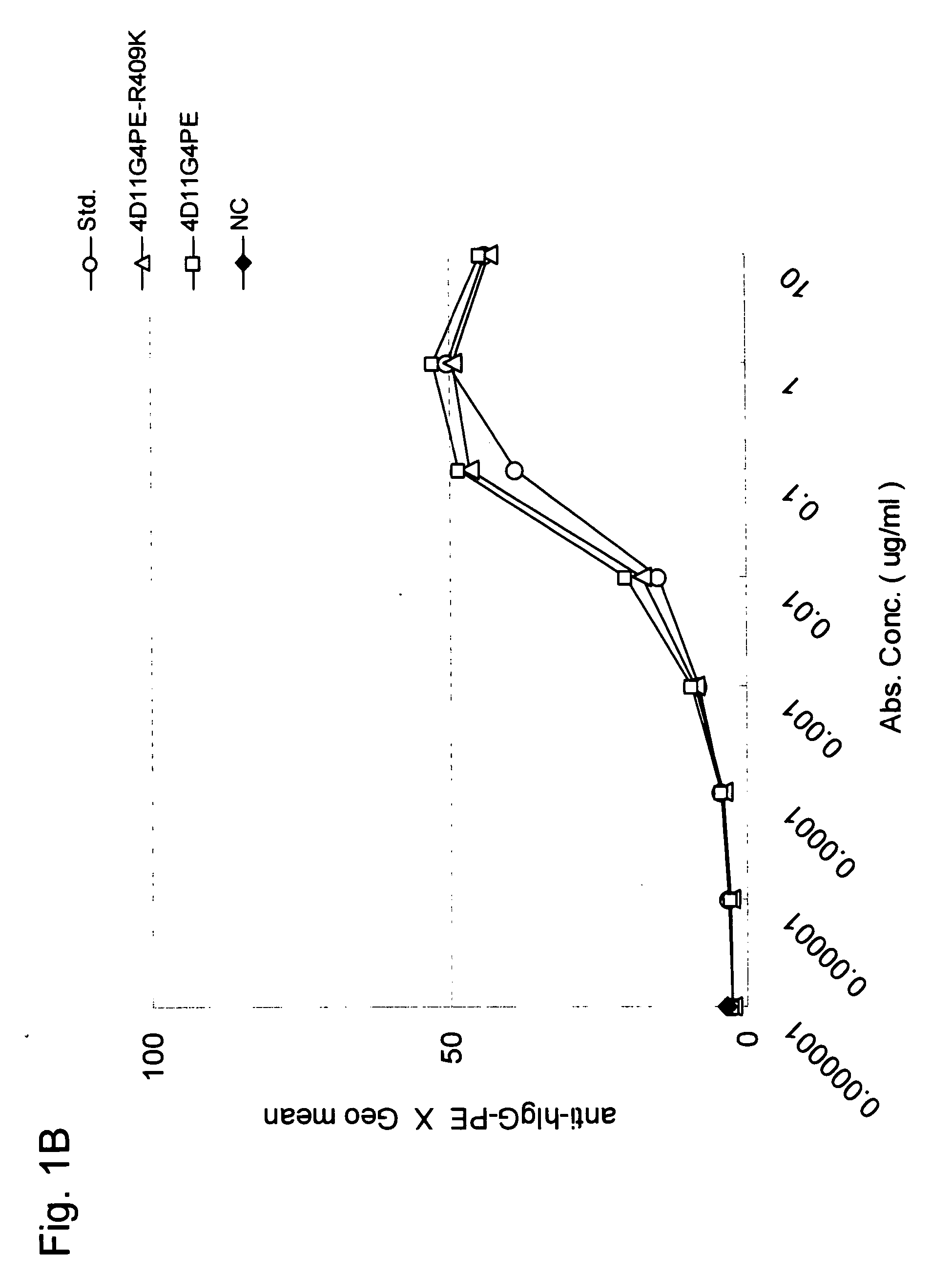
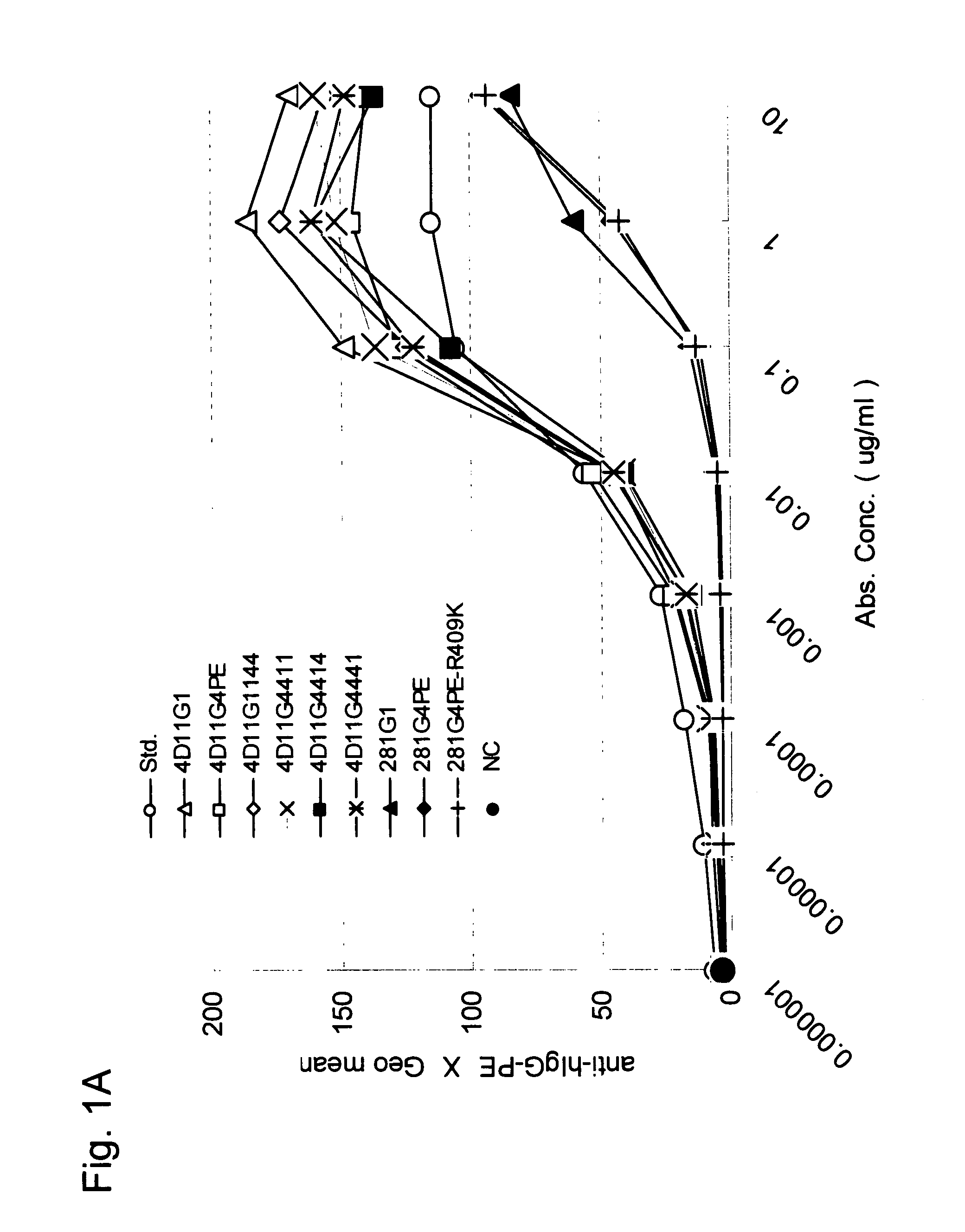
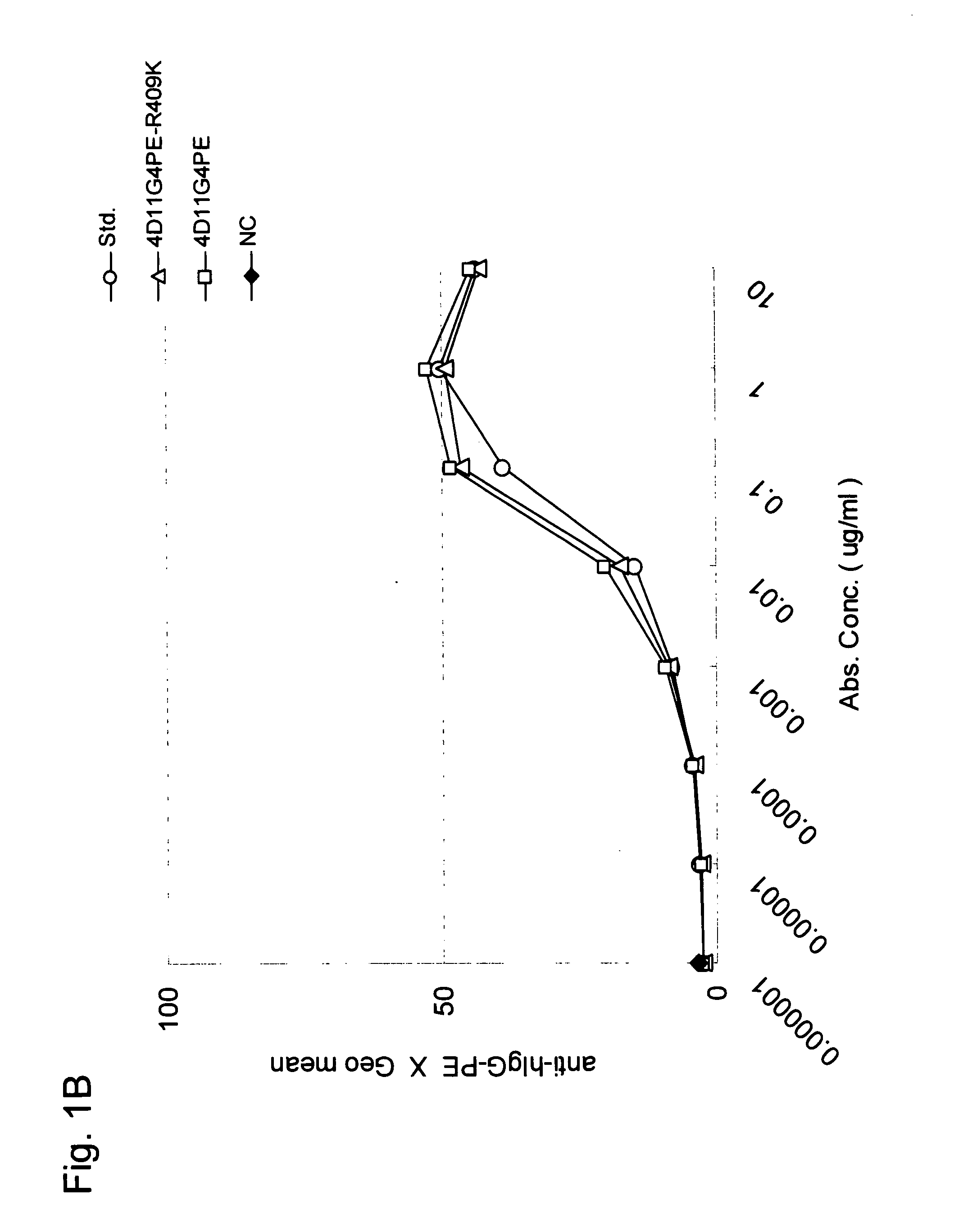
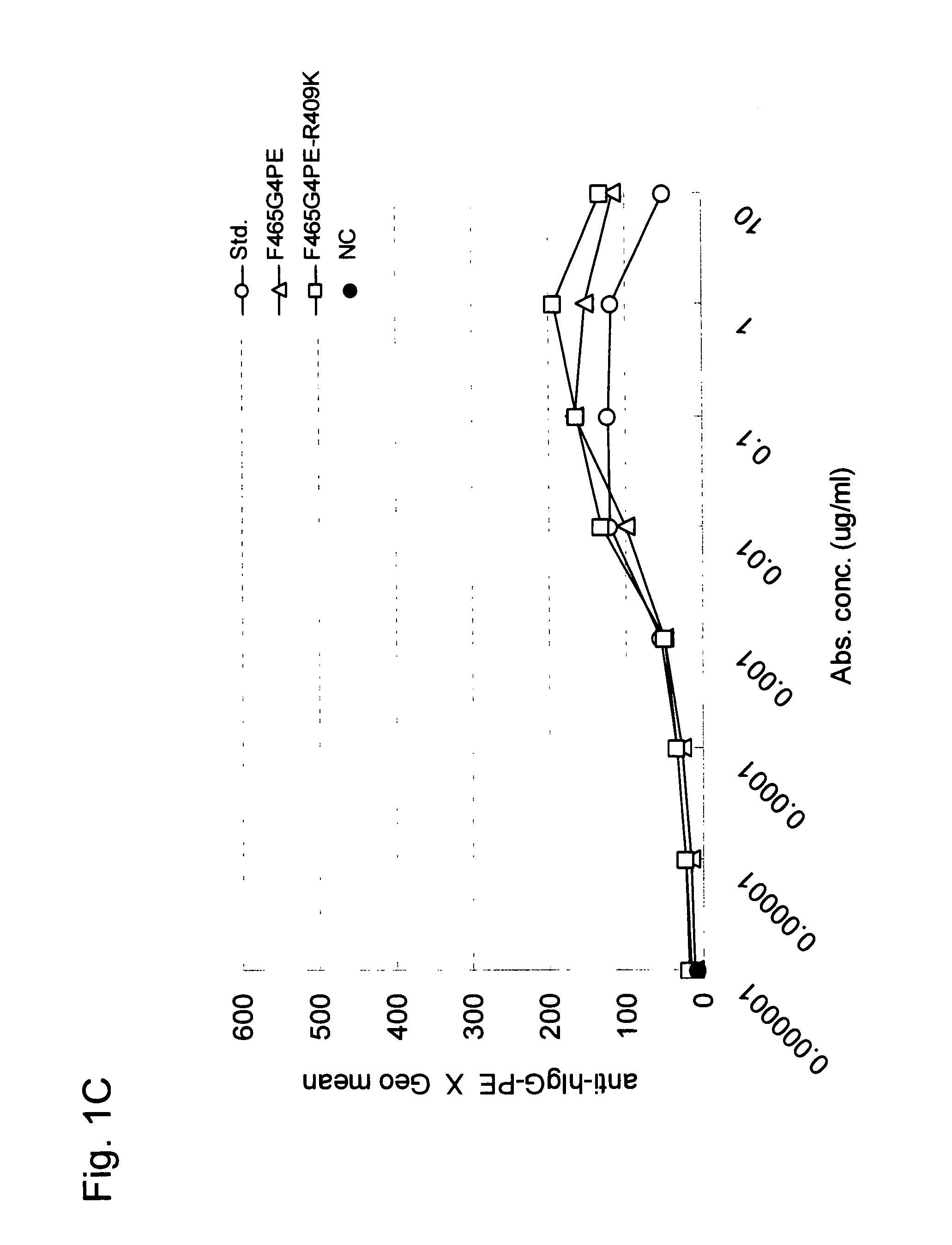
Stabilized Human Igg4 Antibodies
ActiveUS20080063635A1Stable productionReduce adverse effectsNervous disorderAntipyreticArginineMutant
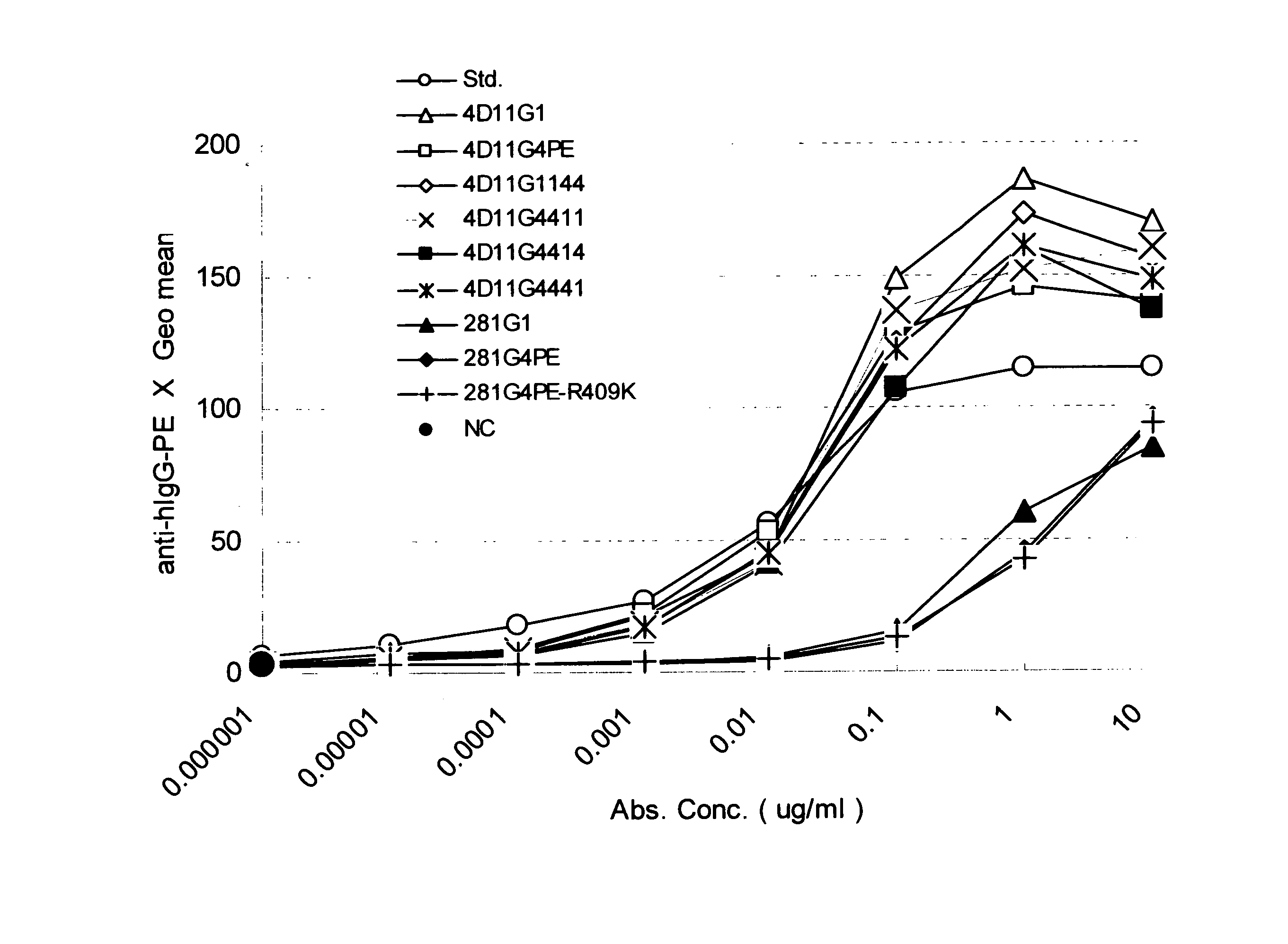
A highly stable mutant of human IgG4 antibody is provided. Such antibody is an antibody in which the CH3 domain of human IgG4 is substituted with the CH3 domain of human IgG1 and which exhibits inhibited aggregate formation, an antibody in which the CH3 and CH2 domains of human IgG4 are substituted with the CH3 and CH2 domains of human IgG1, respectively, or an antibody in which arginine at position 409 indicated in the EU index proposed by Kabat et al. of human IgG4 is substituted with lysine and which exhibits inhibited aggregate formation.
Owner:KYOWA HAKKO KIRIN CO LTD
Stabilized human Igg4 antibodies
A highly stable mutant of human IgG4 antibody is provided. Such antibody is an antibody in which the CH3 domain of human IgG4 is substituted with the CH3 domain of human IgG1 and which exhibits inhibited aggregate formation, an antibody in which the CH3 and CH2 domains of human IgG4 are substituted with the CH3 and CH2 domains of human IgG1, respectively, or an antibody in which arginine at position 409 indicated in the EU index proposed by Kabat et al. of human IgG4 is substituted with lysine and which exhibits inhibited aggregate formation.
Owner:KYOWA HAKKO KIRIN CO LTD
Thermal treatment process for tobacco materials
ActiveUS8991403B2Alter natureAlter characterTobacco preparationTobacco treatmentArginineTobacco product
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
Beneficial effects of increasing local blood flow
InactiveUS20110028548A1Increase oxygenationImprove tissue nutritionBiocidePeptide/protein ingredientsArginineNitric oxide
Owner:STRATEGIC SCI & TECH
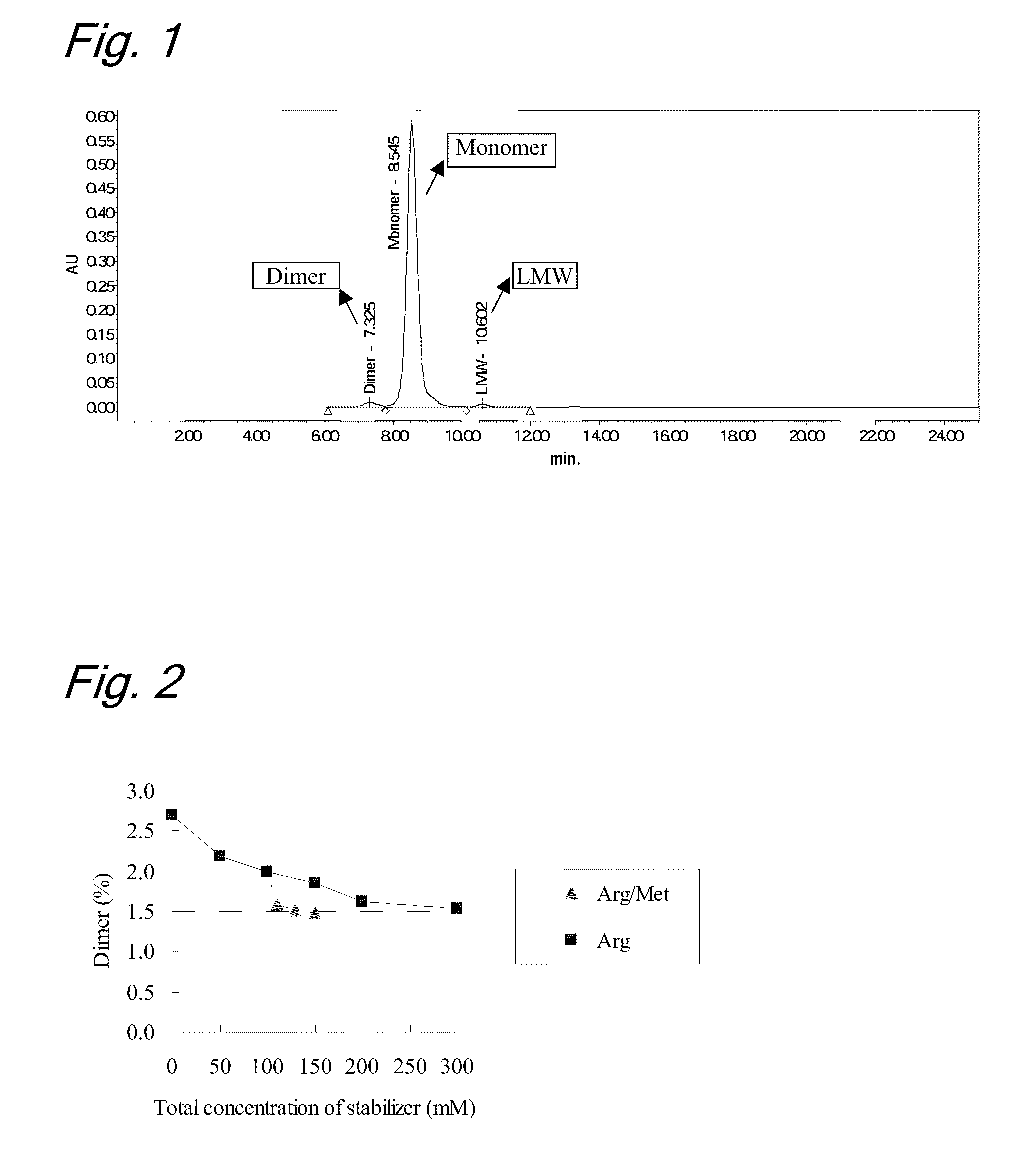
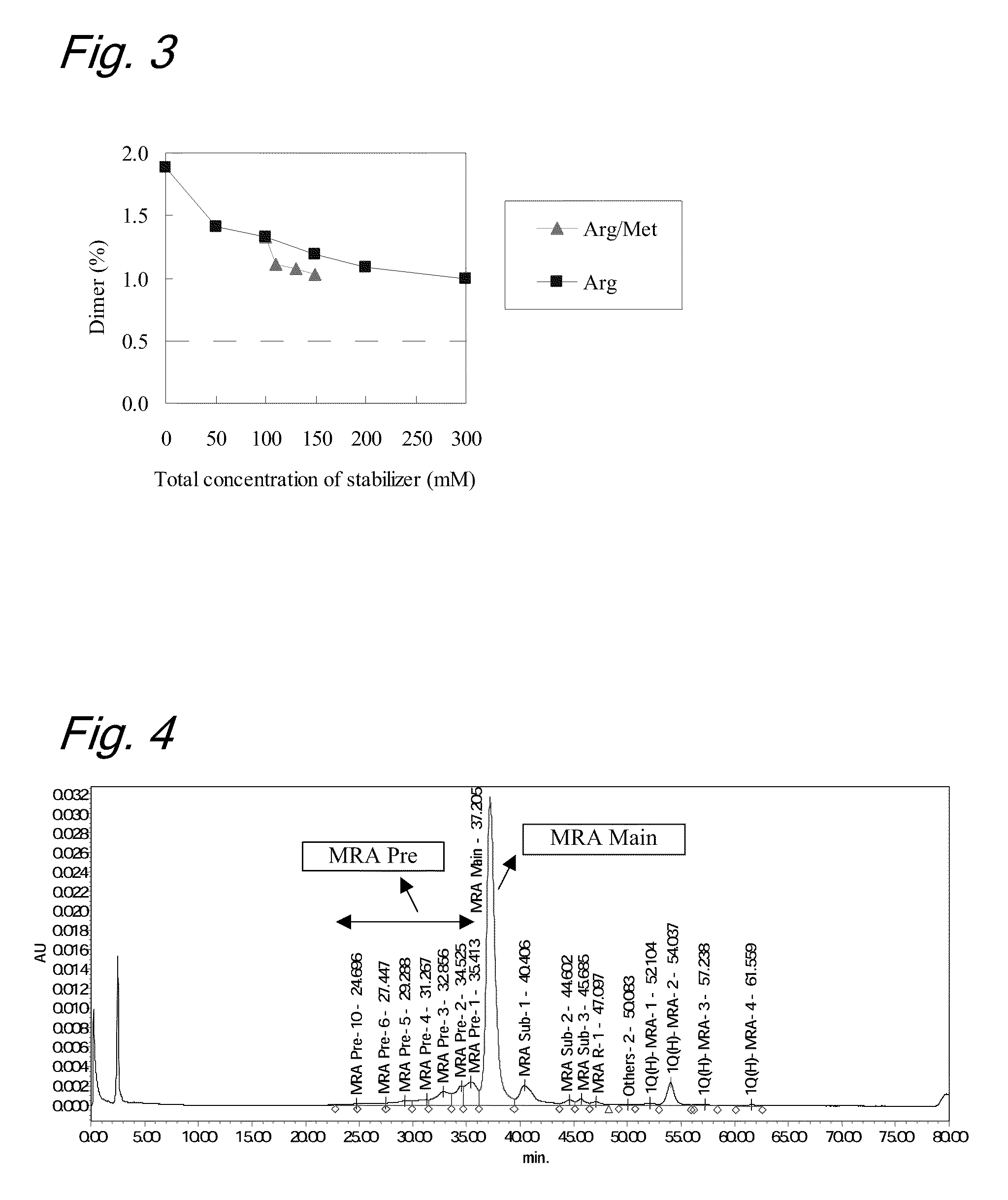
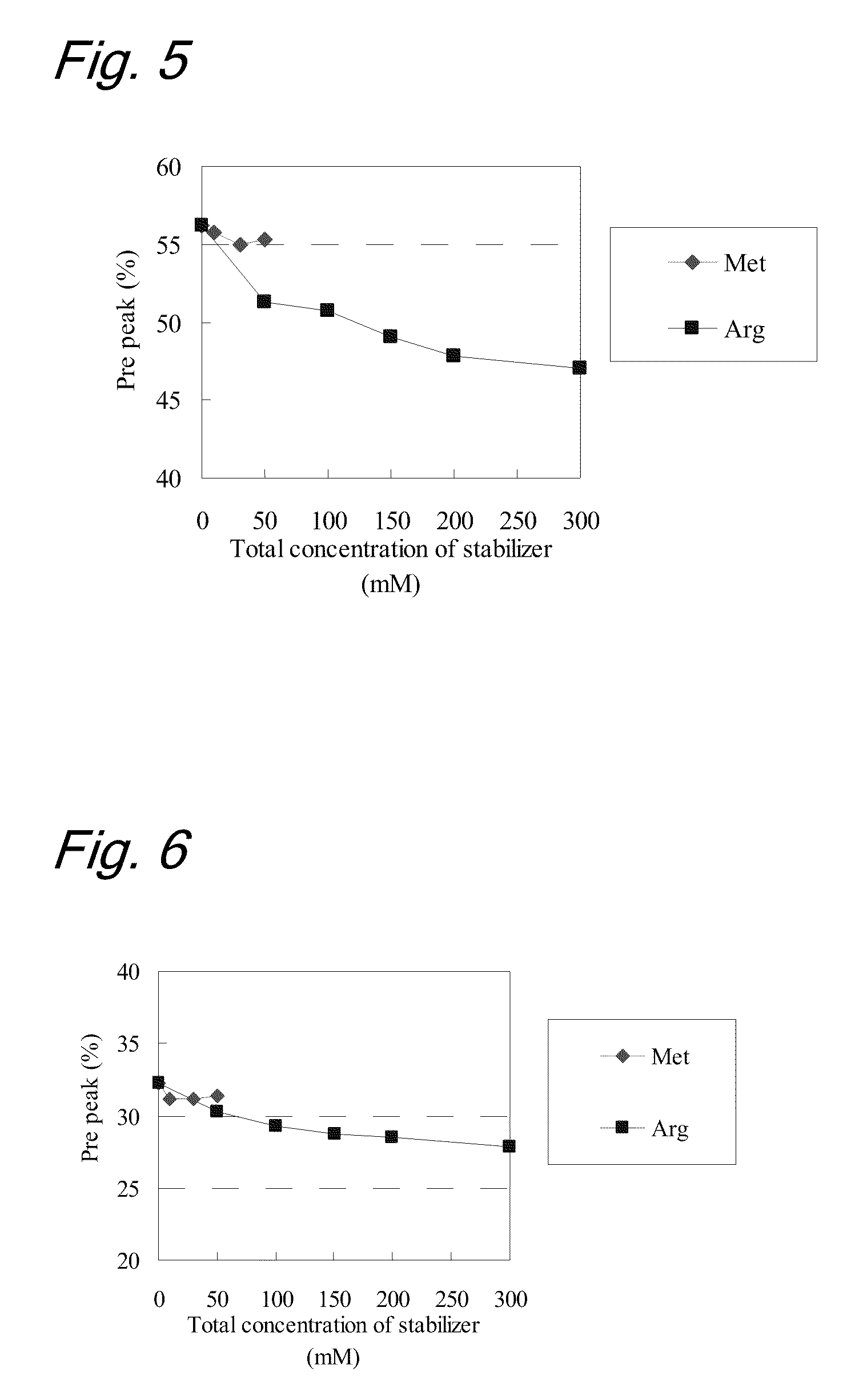
High concentration antibody-containing liquid formulation
ActiveUS20100285011A1Prevent dimerizationPharmaceutical delivery mechanismPeptide preparation methodsHigh concentrationMedicine
The problem to be solved is to provide an antibody-containing formulation which is stable and suited for subcutaneous administration, wherein dimerization and deamidation is prevented during long-term storage. The present application is directed to a stable antibody-containing liquid formulation characterized by containing arginine and methionine.
Owner:CHUGAI PHARMA CO LTD +1
Controlled release nitric oxide producing agents
Disclosed are various controlled release pharmaceutical compositions that include an agent that enhances or modulates the endogenous production of nitric oxide in a mammal. Controlled release pharmaceutical compositions of L-arginine, its salts, peptides, and biological equivalents, together with methods of using the compositions are included. Also included are controlled release pharmaceutical compositions of botanical extracts that modulate or enhance the production of nitric oxide, either alone or in combination with L-arginine or its biological equivalent.
Owner:KUHRTS ERIC H
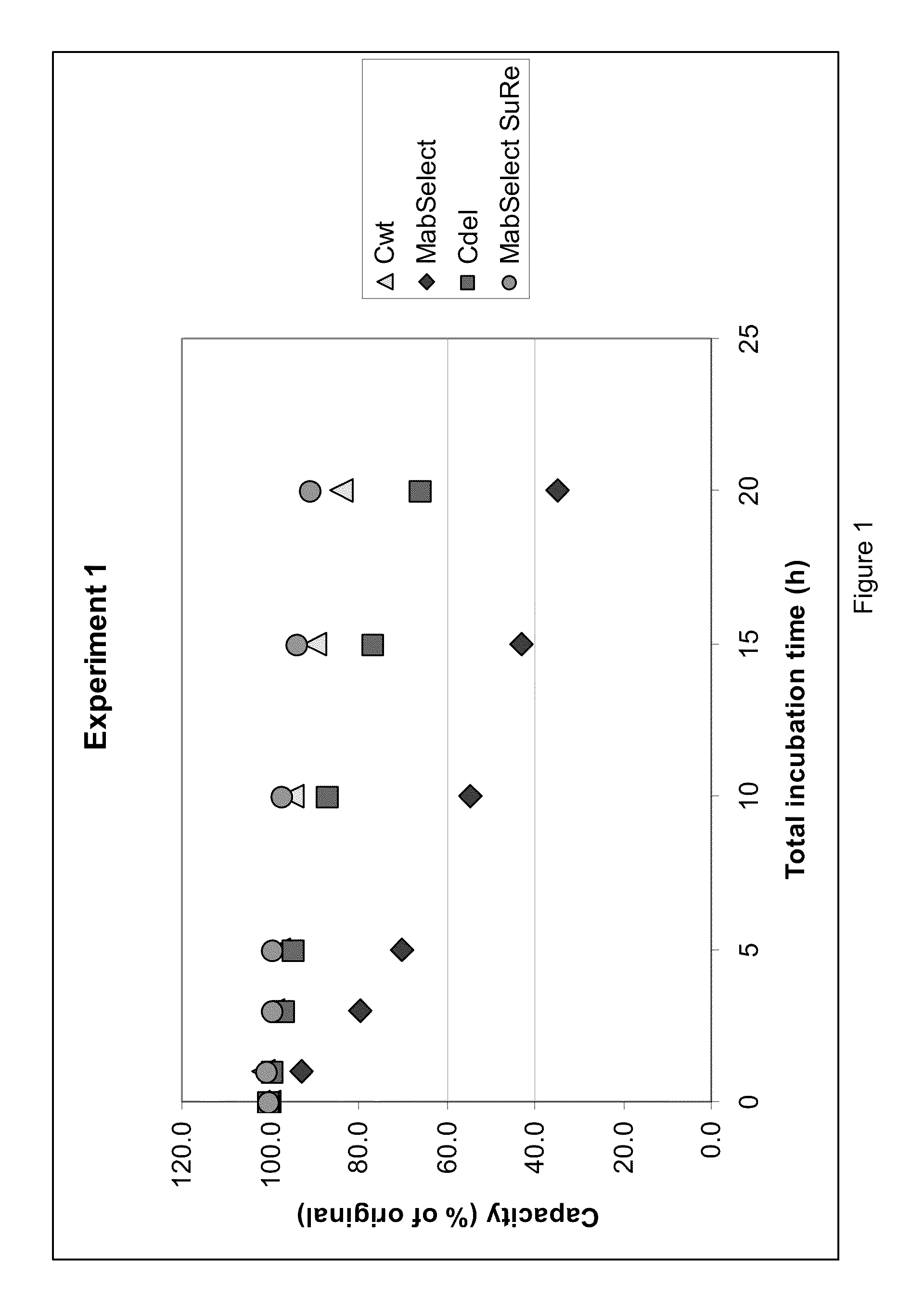
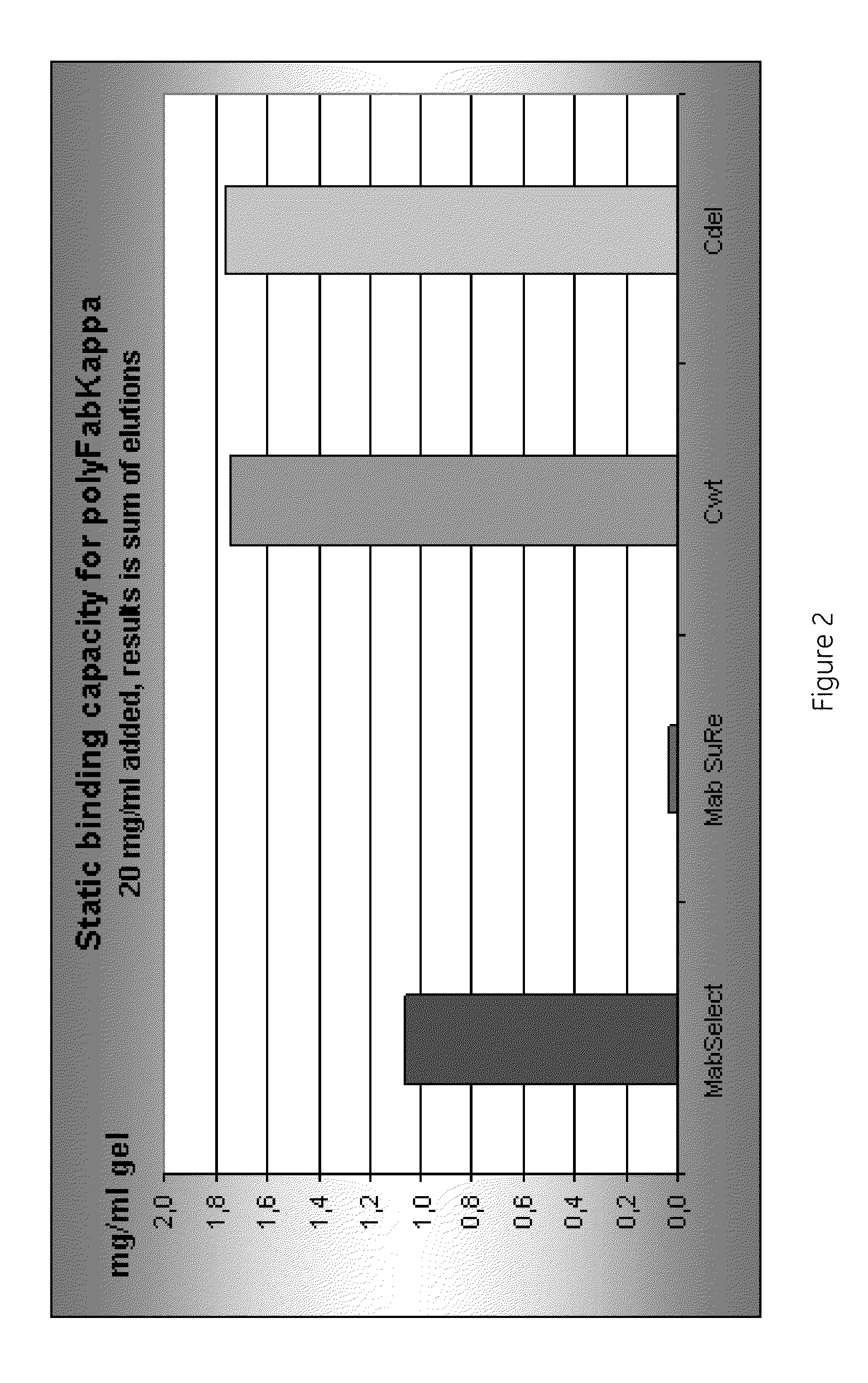
Chromatography ligand comprising domain C from Staphylococcus aureus protein A for antibody isolation
ActiveUS8329860B2Process economyPeptide/protein ingredientsSolid sorbent liquid separationArginineCoupling
The present invention relates to a chromatography ligand, which comprises Domain C from Staphylococcus protein A (SpA), or a functional fragment or variant thereof. The chromatography ligand presents an advantageous capability of withstanding harsh cleaning in place (CIP) conditions, and is capable of binding Fab fragments of antibodies. The ligand may be provided with a terminal coupling group, such as arginine or cysteine, to facilitate its coupling to an insoluble carrier such as beads or a membrane. The invention also relates to a process of using the ligand in isolation of antibodies, and to a purification protocol which may include washing steps and / or regeneration with alkali.
Owner:CYTIVA BIOPROCESS R&D AB
Thermal treatment process for tobacco materials
ActiveUS8944072B2Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of preparing a tobacco material for use in a smoking article is provided, including (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof; (ii) heating the mixture; and (iii) incorporating the heat-treated mixture into a smoking article as a smokable material. A smoking article in the form of a cigarette is also provided that includes a tobacco material pre-treated to inhibit reaction of asparagine to form acrylamide in mainstream smoke. Upon smoking, the smoking article is characterized by an acrylamide content of mainstream smoke that is reduced relative to an untreated control smoking article.
Owner:R J REYNOLDS TOBACCO COMPANY
Compositions and methods for treating diseases
This invention relates to compositions and methods for treatment of vascular conditions. The invention provides arginine polymers and arginine homopolymers for the treatment and / or prevention of glaucoma, pulmonary hypertension, asthma, chronic obstructive pulmonary disease, erectile dysfunction, Raynaud's syndrome, heparin overdose, vulvodynia, and wound healing. The invention also provides arginine polymers and arginine homopolymers for use in organ perfusate and preservation solutions.
Owner:LUMEN THERAPEUTICS
Composition for an in vitro fertilization medium
InactiveUS6130086AImprove stabilityIncrease stimulationCulture processMedical devicesArginineTryptophan
PCT No. PCT / JP96 / 02503 Sec. 371 Date Mar. 2, 1998 Sec. 102(e) Date Mar. 2, 1998 PCT Filed Sep. 4, 1996 PCT Pub. No. WO97 / 08946 PCT Pub. Date Mar. 13, 1997The present invention aims to provide a medium composition for in vitro fertilization, in particular, a composition usable in the culture of ova or early embryos which are fertilized eggs, the preparation or culture of sperm, and the pre-treatment of ova or sperm. The composition comprises, as its essential components, L-phenylalanine, L-tryptophan, L-lysine, L-threonine, L-valine, L-methionine, L-isoleucine, L-leucine, L-proline, glycine, L-alanine, L-tyrosine, L-histidine, L-arginine, L-taurine, L-aspartic acid, L-serine, L-asparagine, L-glutamic acid, L-glutamine and L-cystine, provided that at least a part of the L-cystine may be replaced by L-cysteine.
Owner:FUSO PHARMA INDS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com