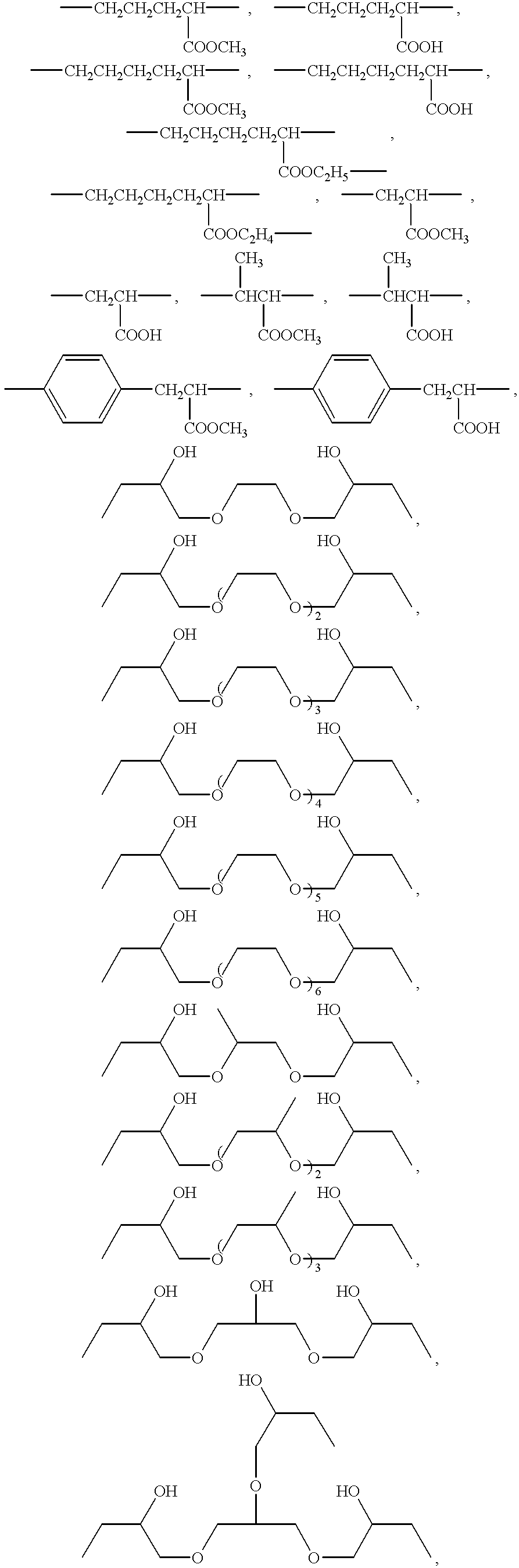
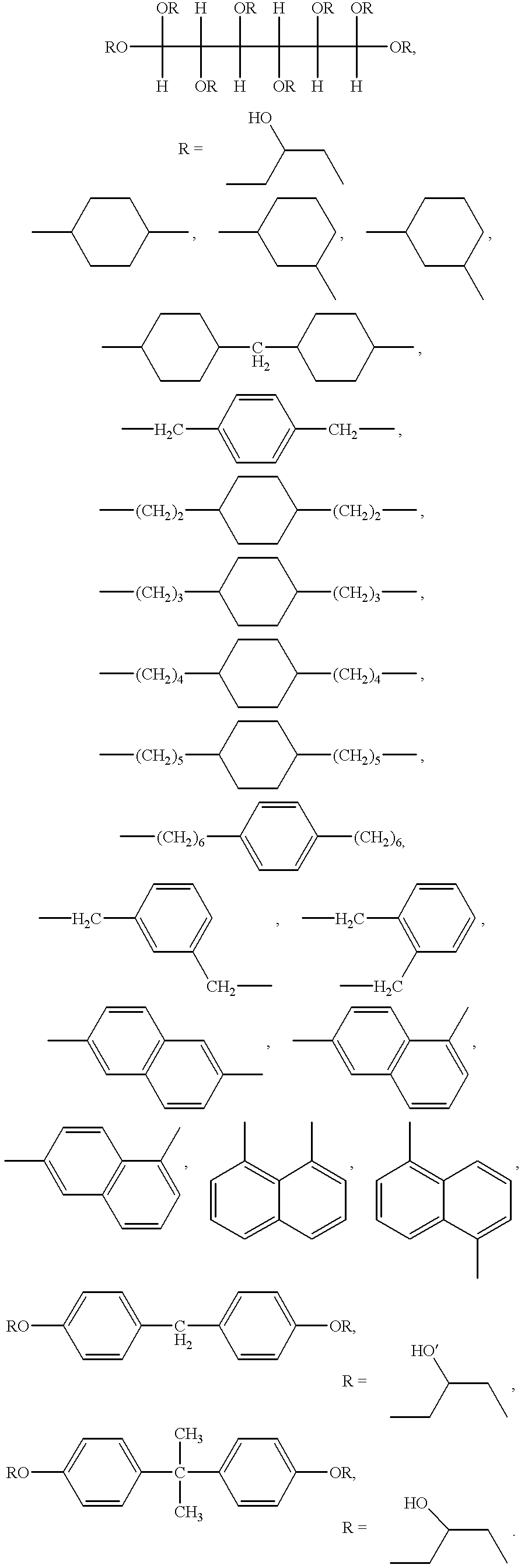
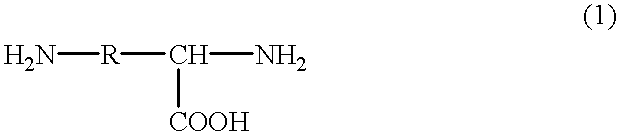Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3355 results about "Aspartic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
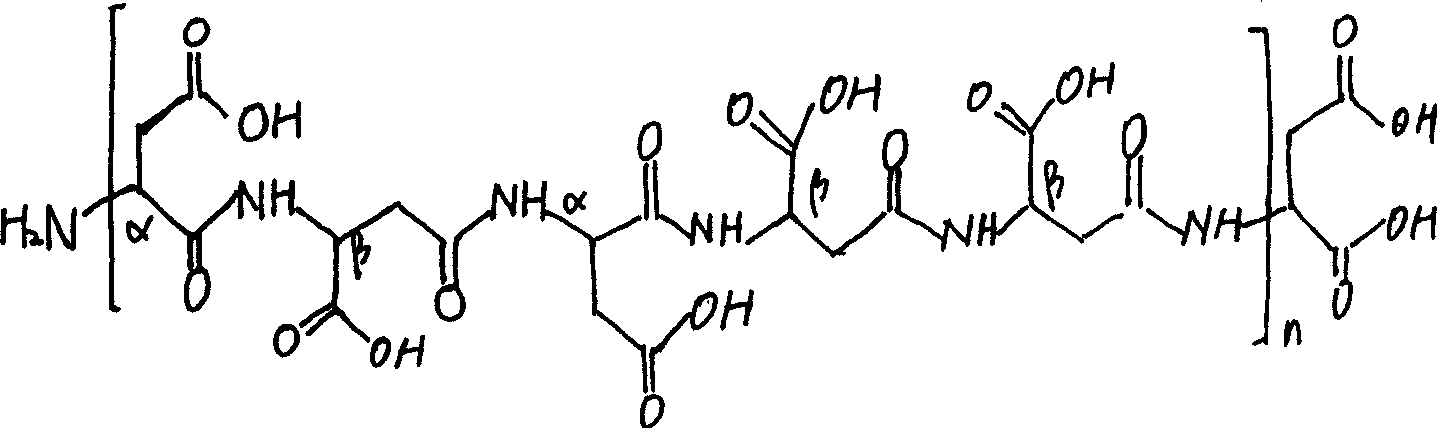
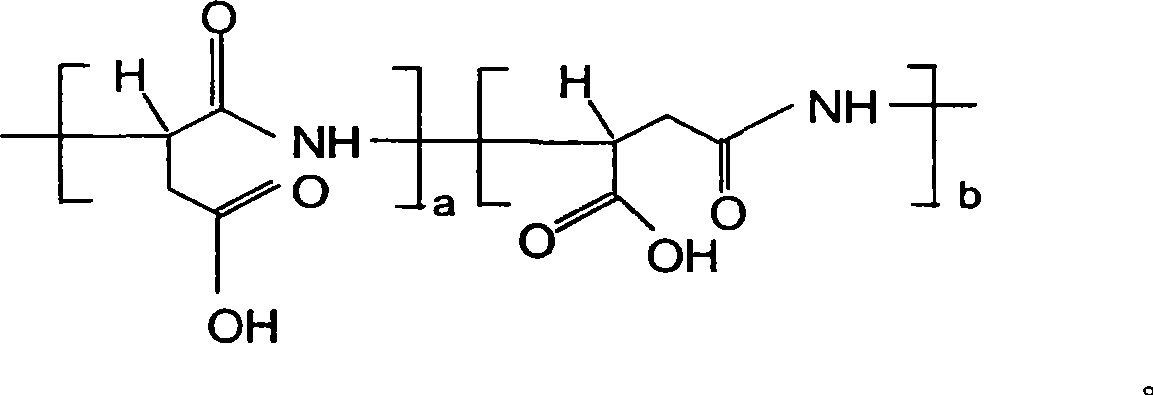
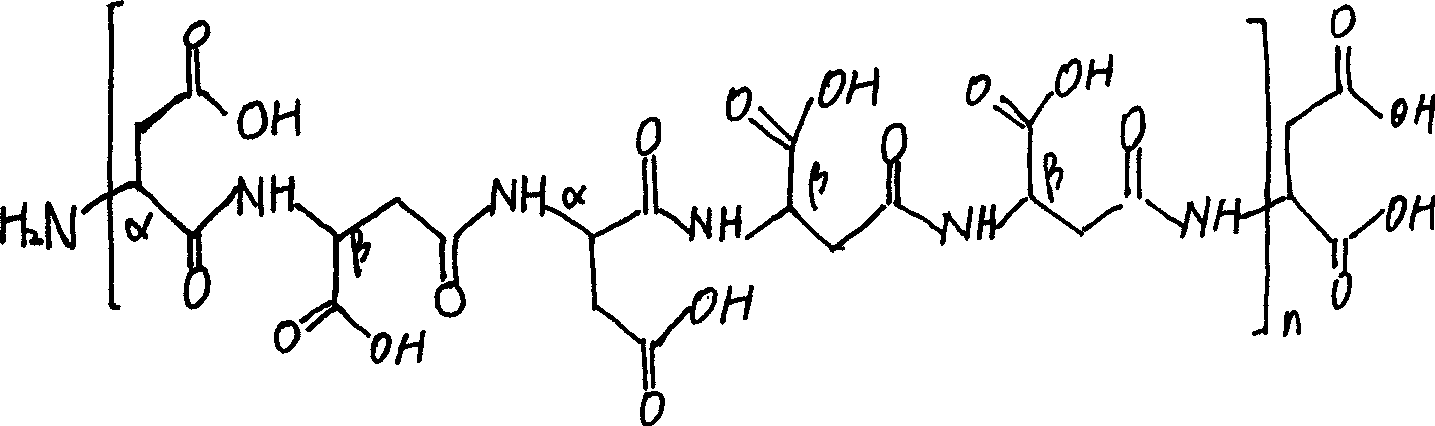
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Similar to all other amino acids it contains an amino group and a carboxylic acid. Its α-amino group is in the protonated –NH⁺₃ form under physiological conditions, while its α-carboxylic acid group is deprotonated −COO⁻ under physiological conditions. Aspartic acid has an acidic side chain (CH₂COOH) which reacts with other amino acids, enzymes and proteins in the body. Under physiological conditions (pH 7.4) in proteins the side chain usually occurs as the negatively charged aspartate form, −COO⁻. It is a non-essential amino acid in humans, meaning the body can synthesize it as needed. It is encoded by all the codons GAU and GAC.
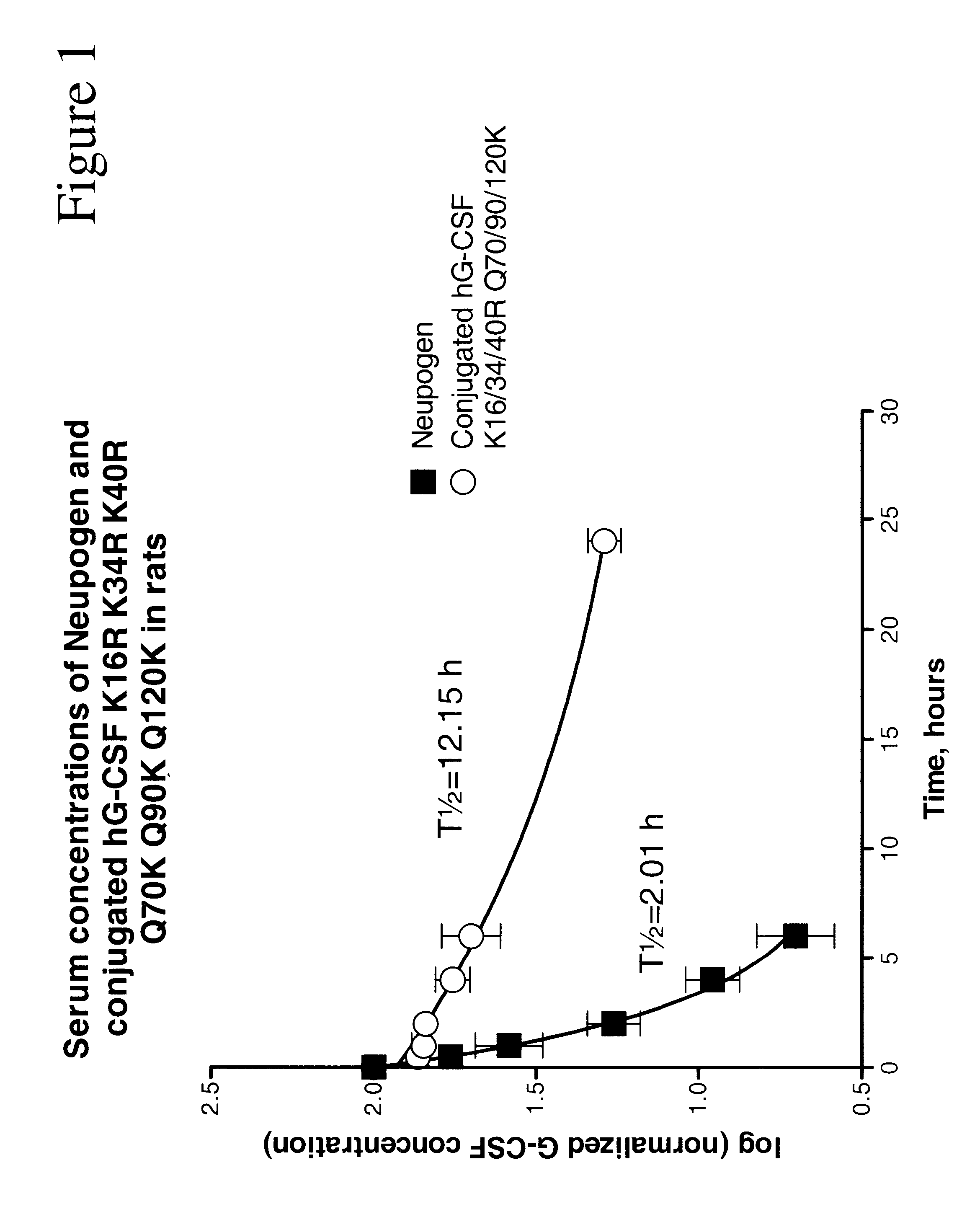
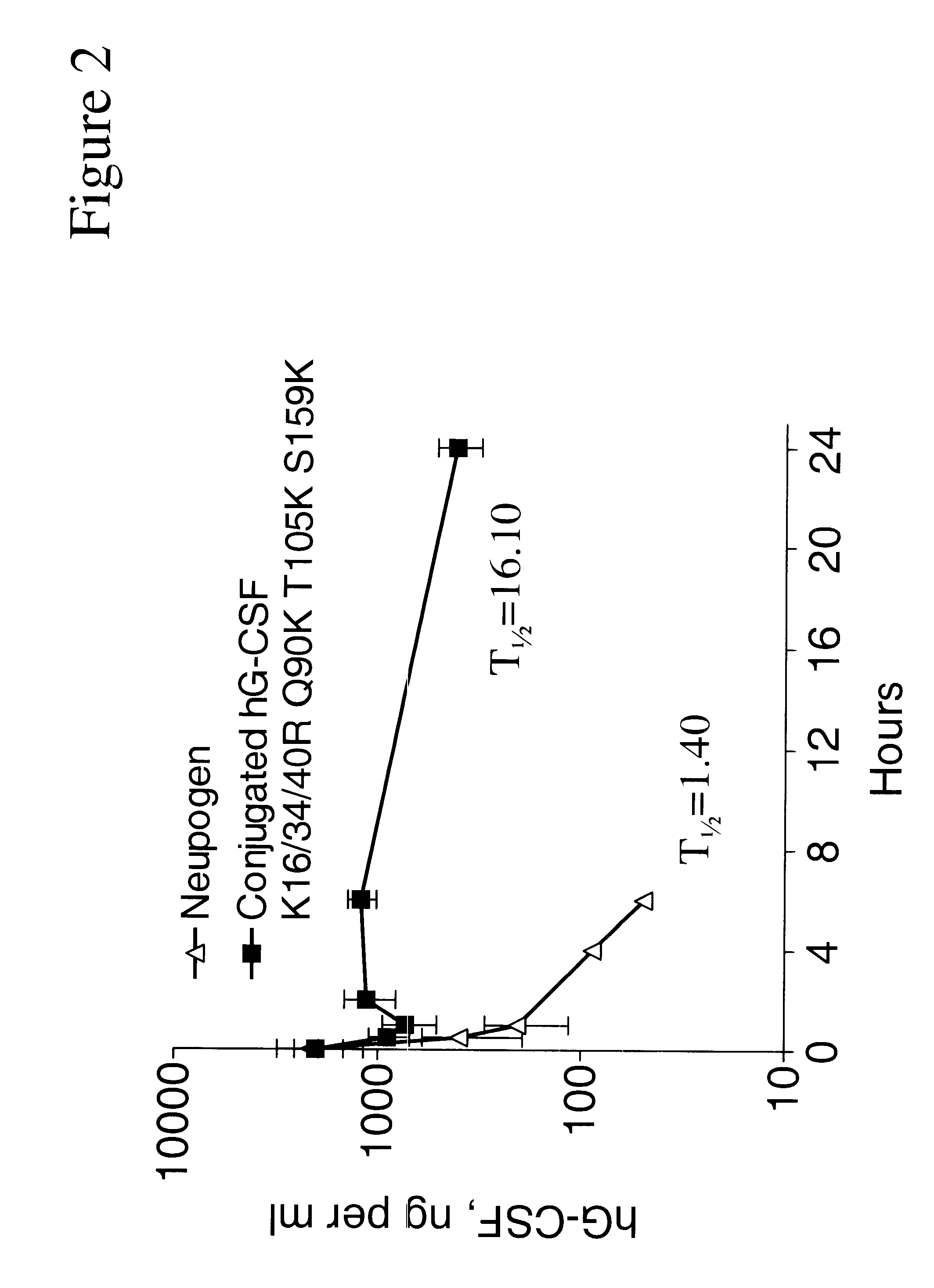
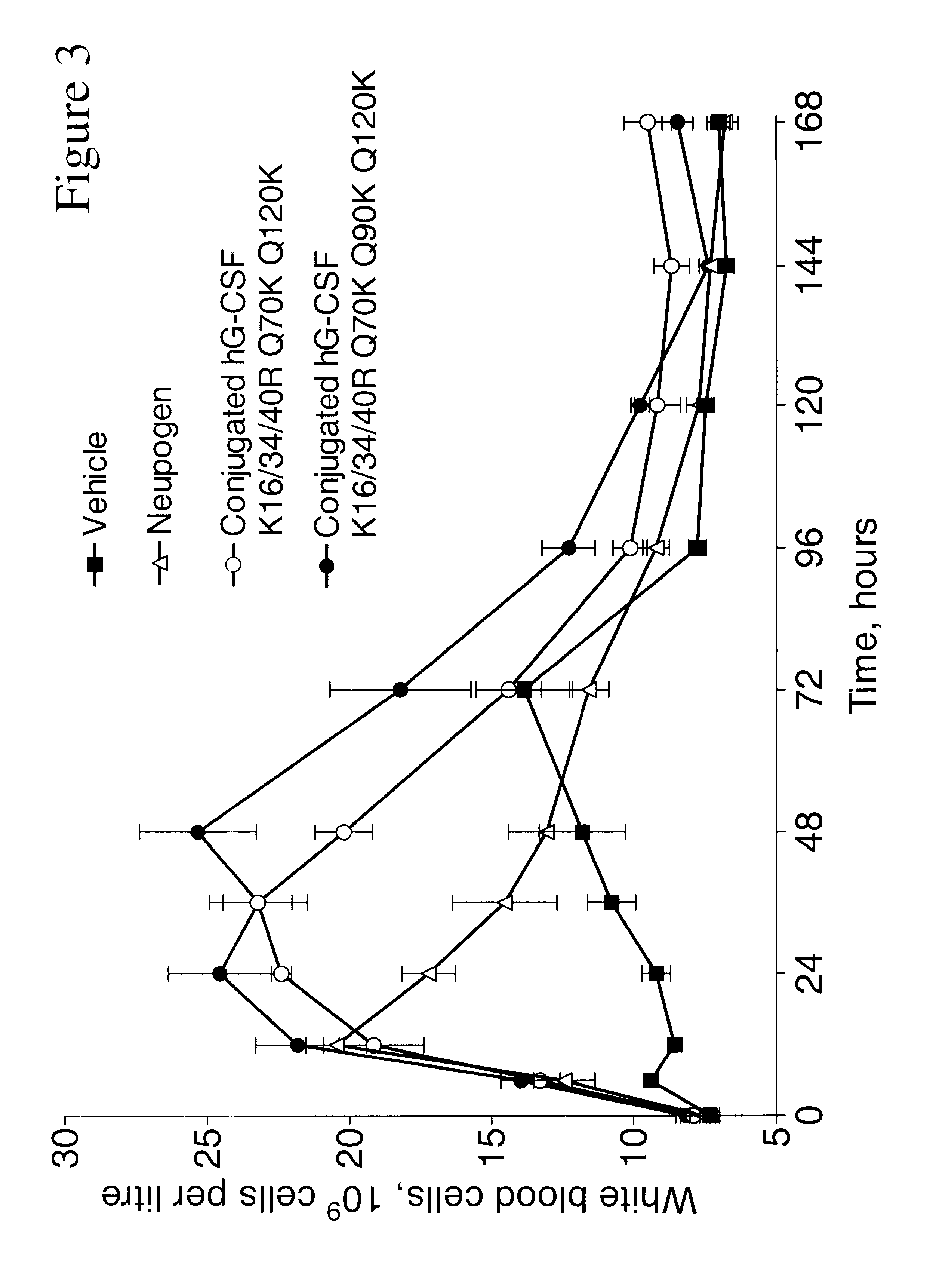
G-CSF conjugates
InactiveUS6555660B2Improved propertyReduced in vitroBiocidePeptide/protein ingredientsHalf-lifePolyethylene glycol
The invention relates to polypeptide conjugates comprising a polypeptide exhibiting G-CSF activity and having an amino acid sequence that differs from the amino acid sequence of human G-CSF in at least one specified introduced and / or removed amino acid residue comprising an attachment group for a non-polypeptide moiety, and having at least one non-polypeptide moiety attached to an attachment group of the polypeptide. The attachment group may e.g. be a lysine, cysteine, aspartic acid or glutamic acid residue or a glycosylation site, and the non-polypeptide moiety may e.g. be a polymer such as polyethylene glycol or an oligosaccharide. The conjugate, which has a reduced in vitro bioactivity compared to hG-CSF, has one or more improved properties such as increased biological half-life and increased stimulation of neutrophils.
Owner:MAXYGEN
Method of coating an intravascular stent with an endothelial cell adhesive five amino acid peptide
InactiveUS6140127APromoting cell attachmentRestore patencyBiocidePeptide/protein ingredientsCell specificArginine
Endothelial cell attachment to an intravascular stent is promoted by coating the stent with an endothelial cell specific adhesion peptide. Coating is preferably carried out by activating the intravascular stent using plasma glow discharge, applying on the stent a layer or plurality of layers of a polymer such as poly(2-hydroxyethylmethacrylate), applying a tresylation solution containing pyridine and tresyl chloride, and applying a five amino acid peptide having the sequence glycine-arginine-glutamic acid-aspartic acid-valine.
Owner:CORDIS CORP
Thermal treatment process for tobacco materials
ActiveUS20100300463A1Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
Method of preparing an aliphatic polyurea spray elastomer system
This invention concerns a method for the preparation of polyurea elastomers, comprising: (a) reacting an amine chain extender with dialkyl maleate to form an aspartic ester, wherein the chain extender has a molar amount of amine groups that is less than the moles of dialkyl maleate; (b) blending the aspartic ester with one or more polyoxyalkyleneamines to prepare a resin blend; (c) contacting the resin blend with an isocyanate under conditions effective to form a polyurea elastomer. This invention also concerns a a polyurea elastomer, comprising the reaction product of (a) a resin blend containing one or more polyoxyalkyleneamine and an aspartic ester and (b) an isocyanate, wherein the aspartic ester in the resin blend comprises a reaction product of an amine chain extender and a dialkyl maleate, wherein the mole ratio of amine functionality in the amine chain extender to dialkyl maleate or fumarate is greater than 1:1.
Owner:JPMORGAN CHASE BANK N A AS COLLATERAL AGENT +1
Mutant cholera holotoxin as an adjuvant
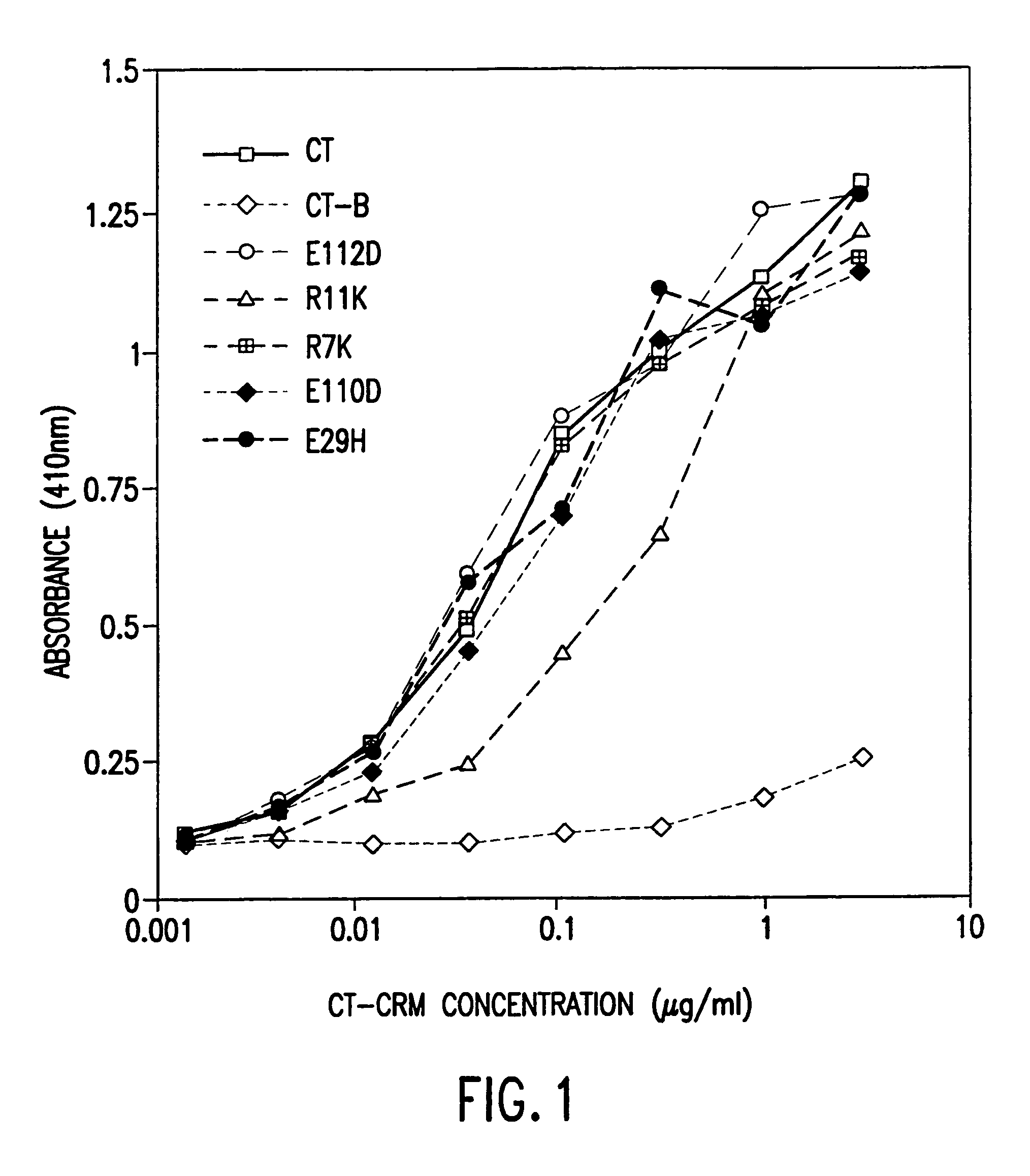
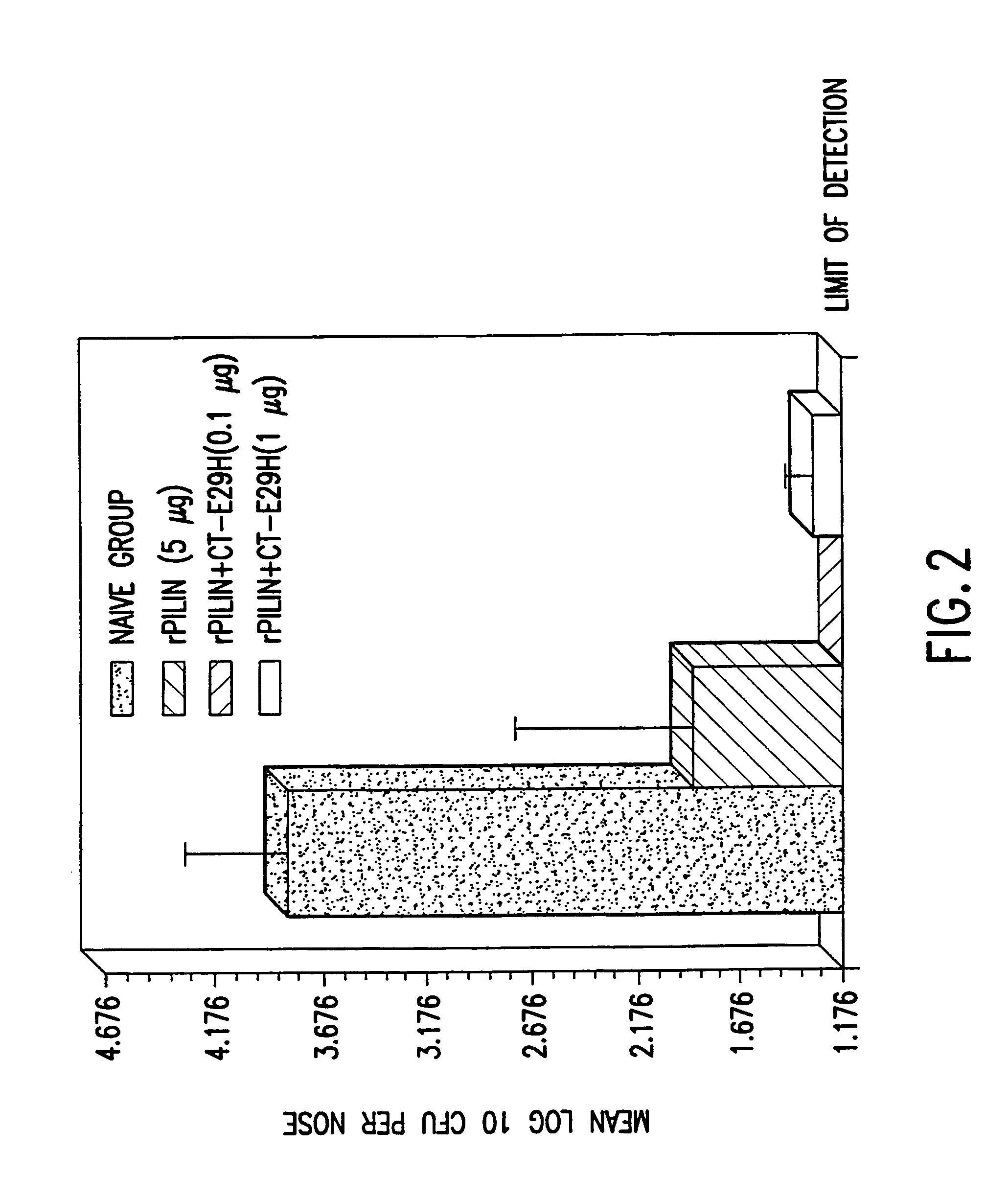
InactiveUS7384640B1Low toxicityEnhance immune responseSsRNA viruses negative-senseBacteriaAntigenAdjuvant
A mutant cholera holotoxin featuring a point mutation at amino acid 29 of the A subunit, wherein the glutamic acid residue is replaced by an amino acid other than aspartic acid, is useful as an adjuvant in an antigenic composition to enhance the immune response in a vertebrate host to a selected antigen from a pathogenic bacterium, virus, fungus or parasite. In a particular embodiment, the amino acid 29 is histidine. The mutant cholera holotoxin may contain at least one additional mutation in the A subunit at a position other than amino acid 29. The antigenic composition may include a second adjuvant in addition to the mutant cholera holotoxin.
Owner:UNIFORMED SERVICES UNIV OF HEALTH SCI UNITED STATES OF AMERICA AS REPRESENTED BY THE +1
Mutant protein
InactiveUS20060194950A1Improve stabilityIncreased pH-valuesBacteriaSerum immunoglobulinsMutated proteinComplementarity determining region
Owner:GE HEALTHCARE BIO SCI CORP
Mutated immunoglobulin-binding protein
ActiveUS20050143566A1Improve stabilityIncreased pH-valuesSerum immunoglobulinsComponent separationComplementarity determining regionChemical stability
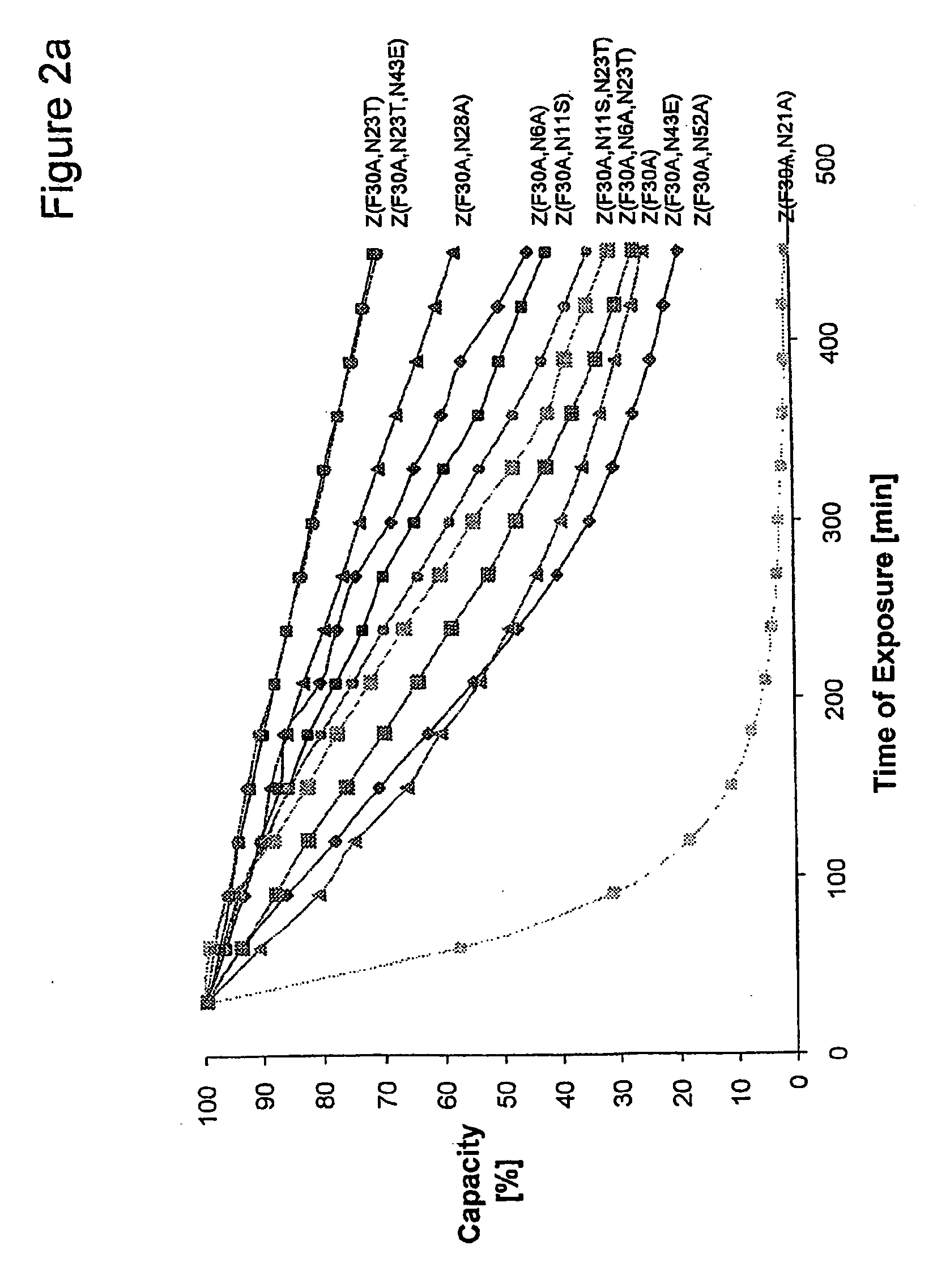
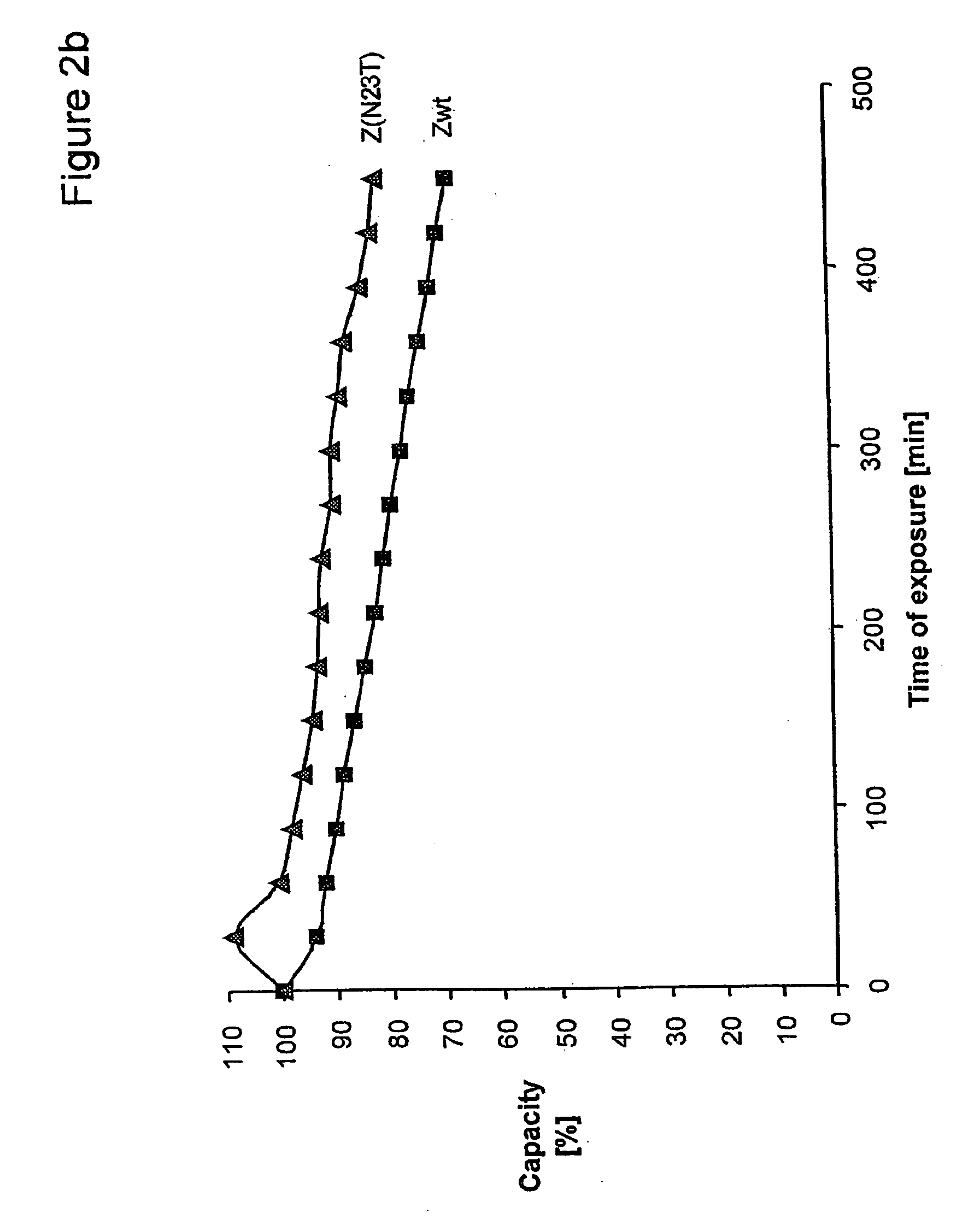
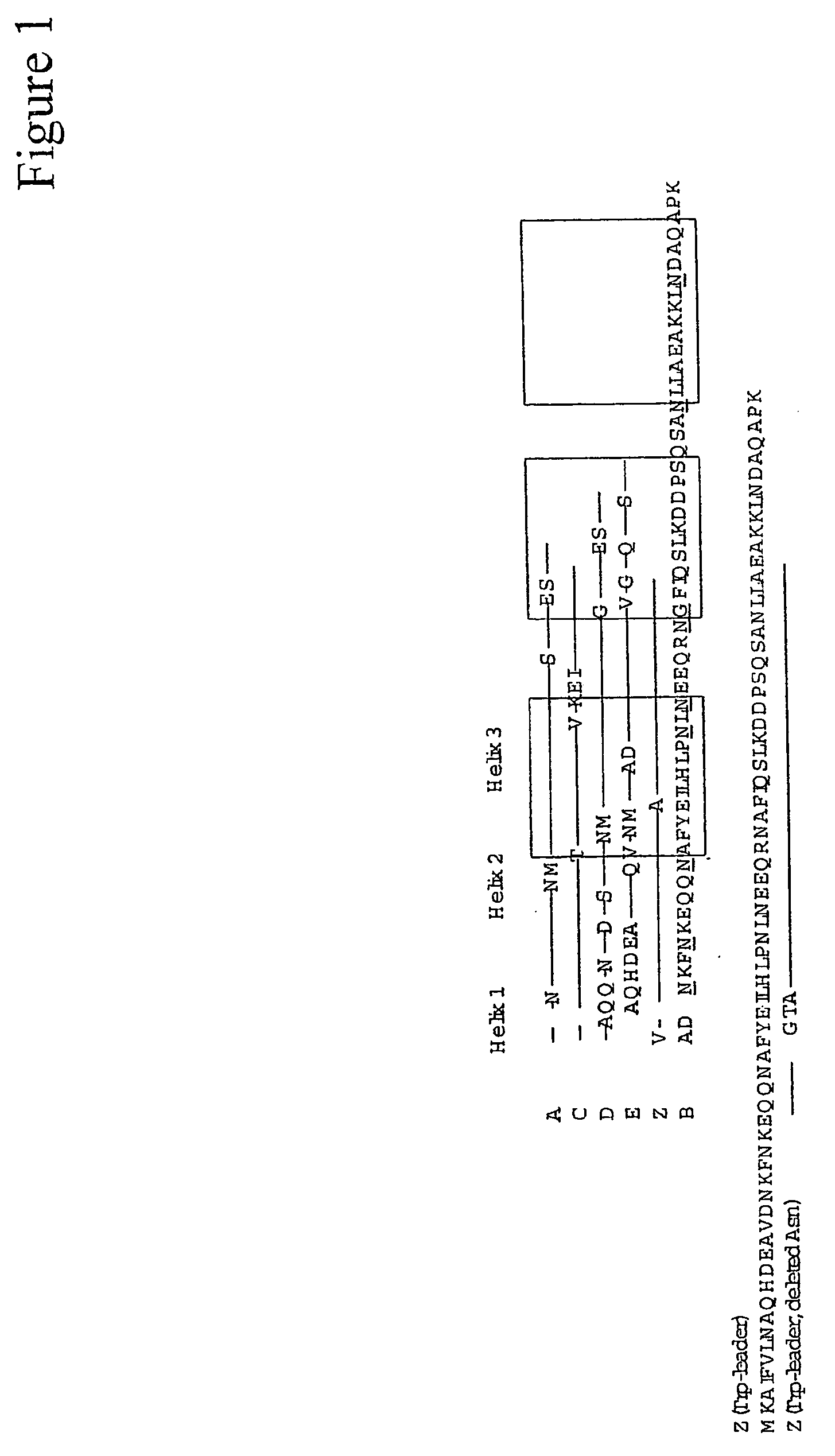
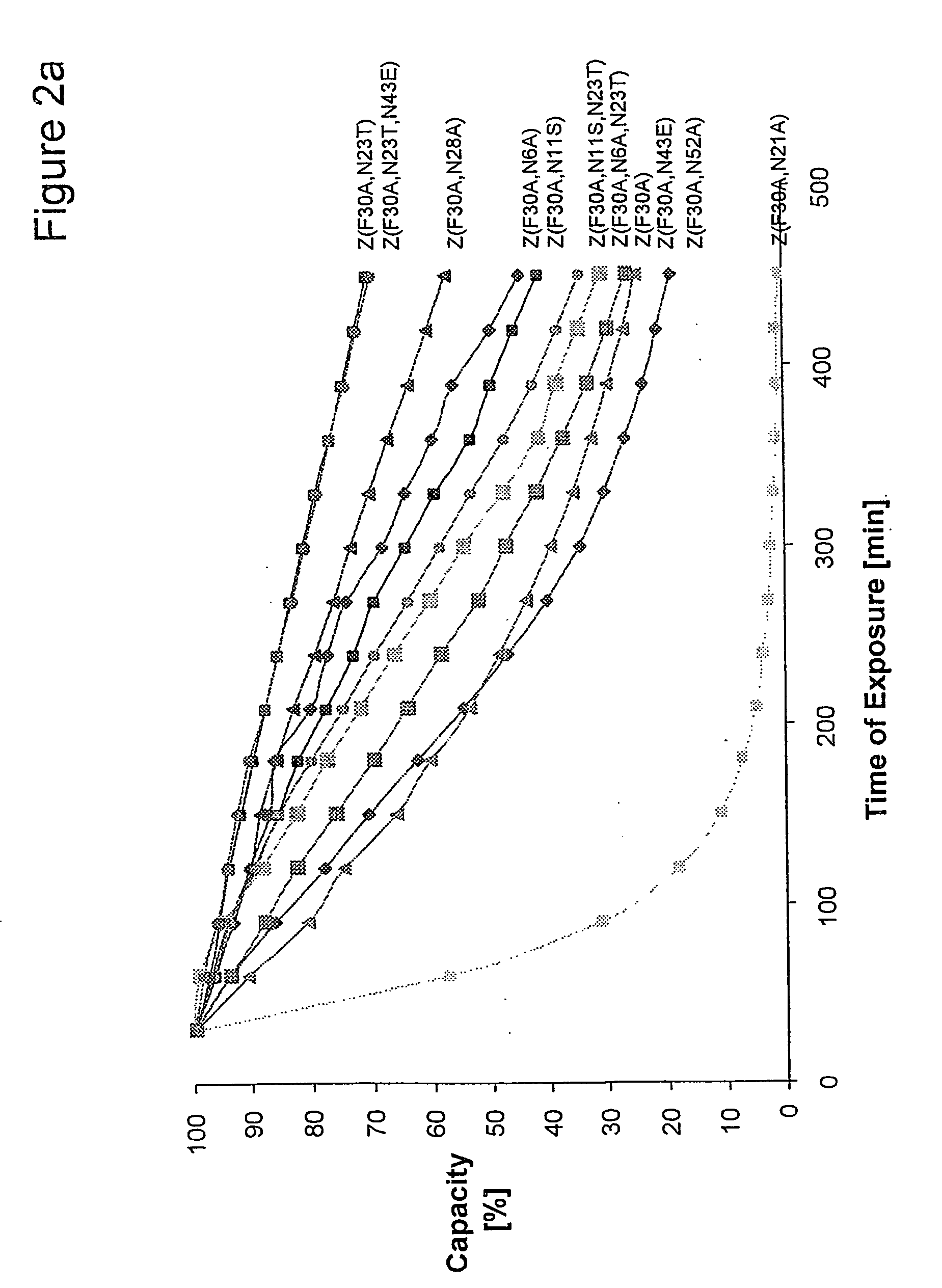
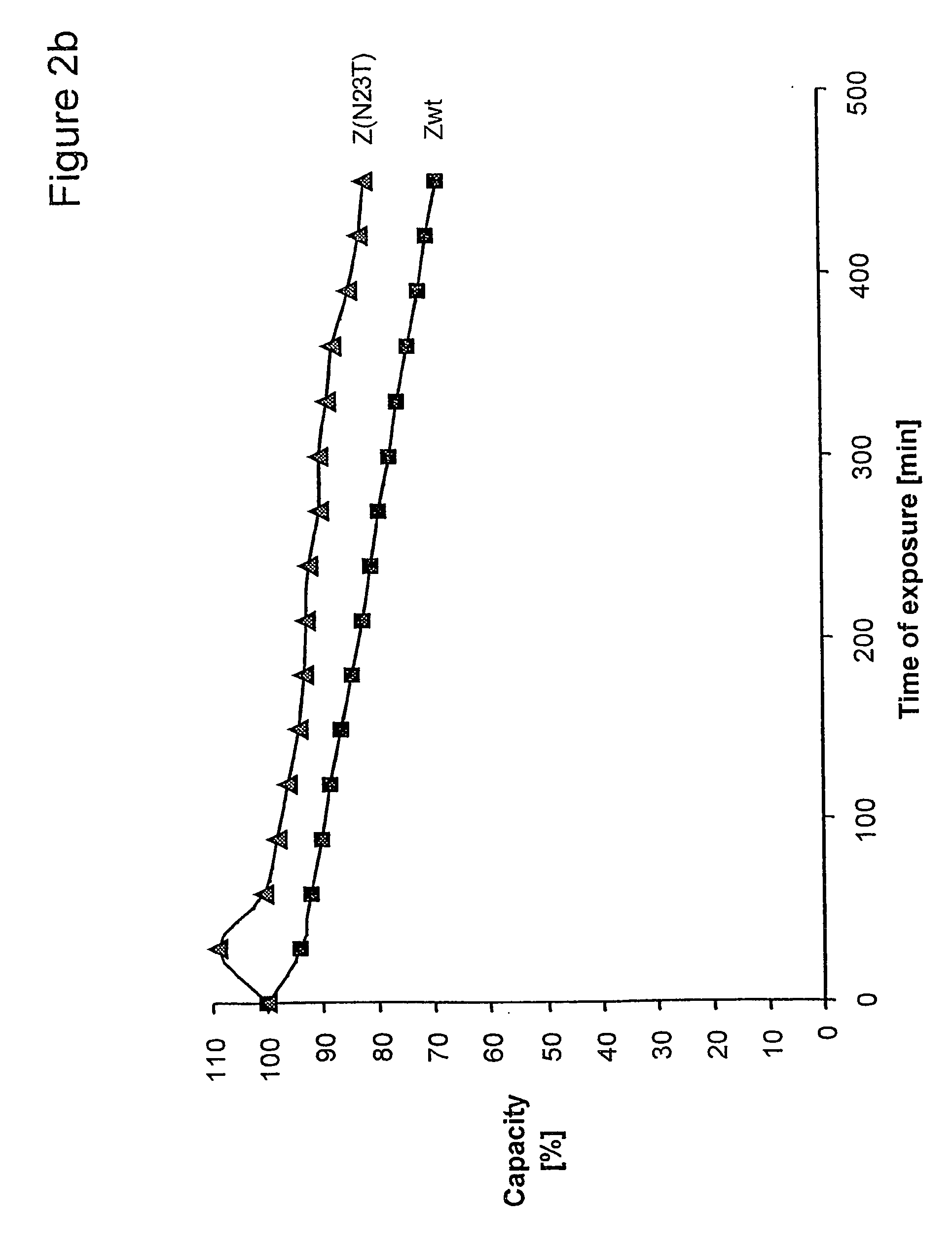
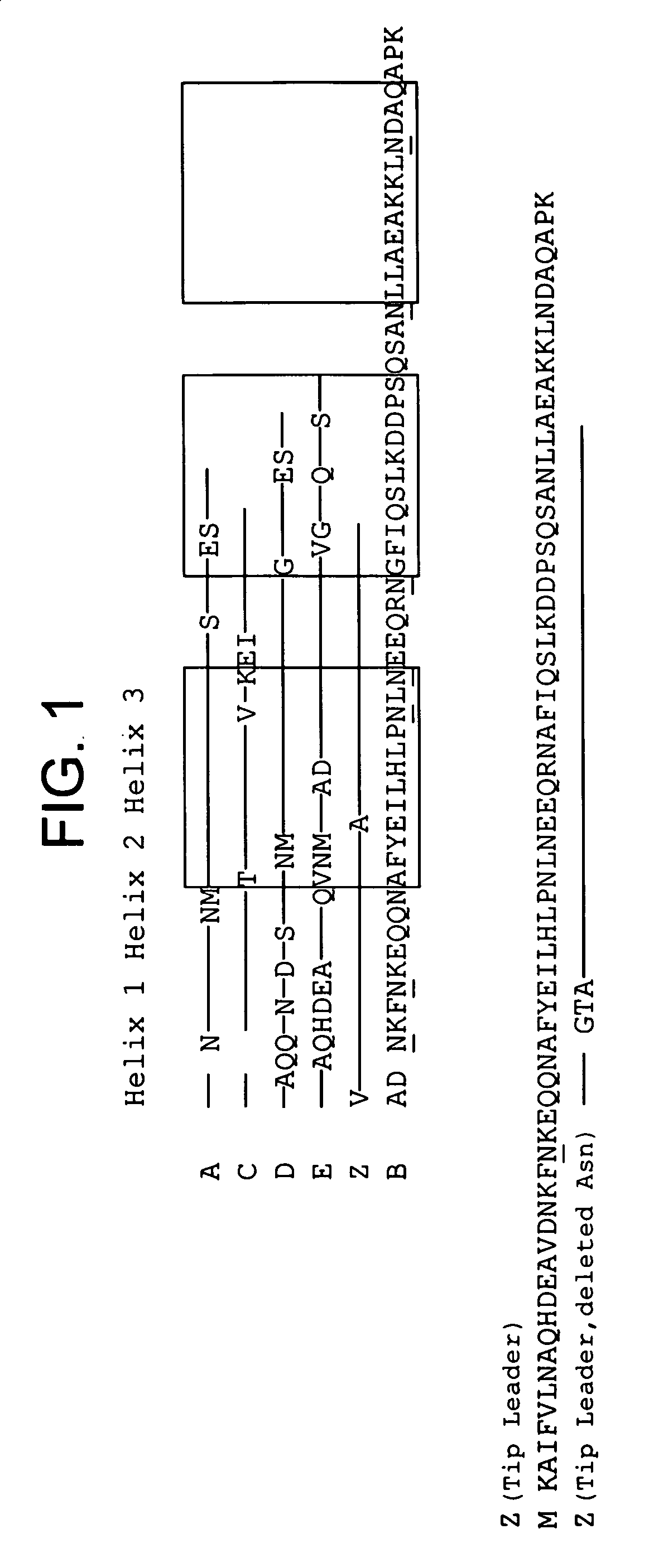
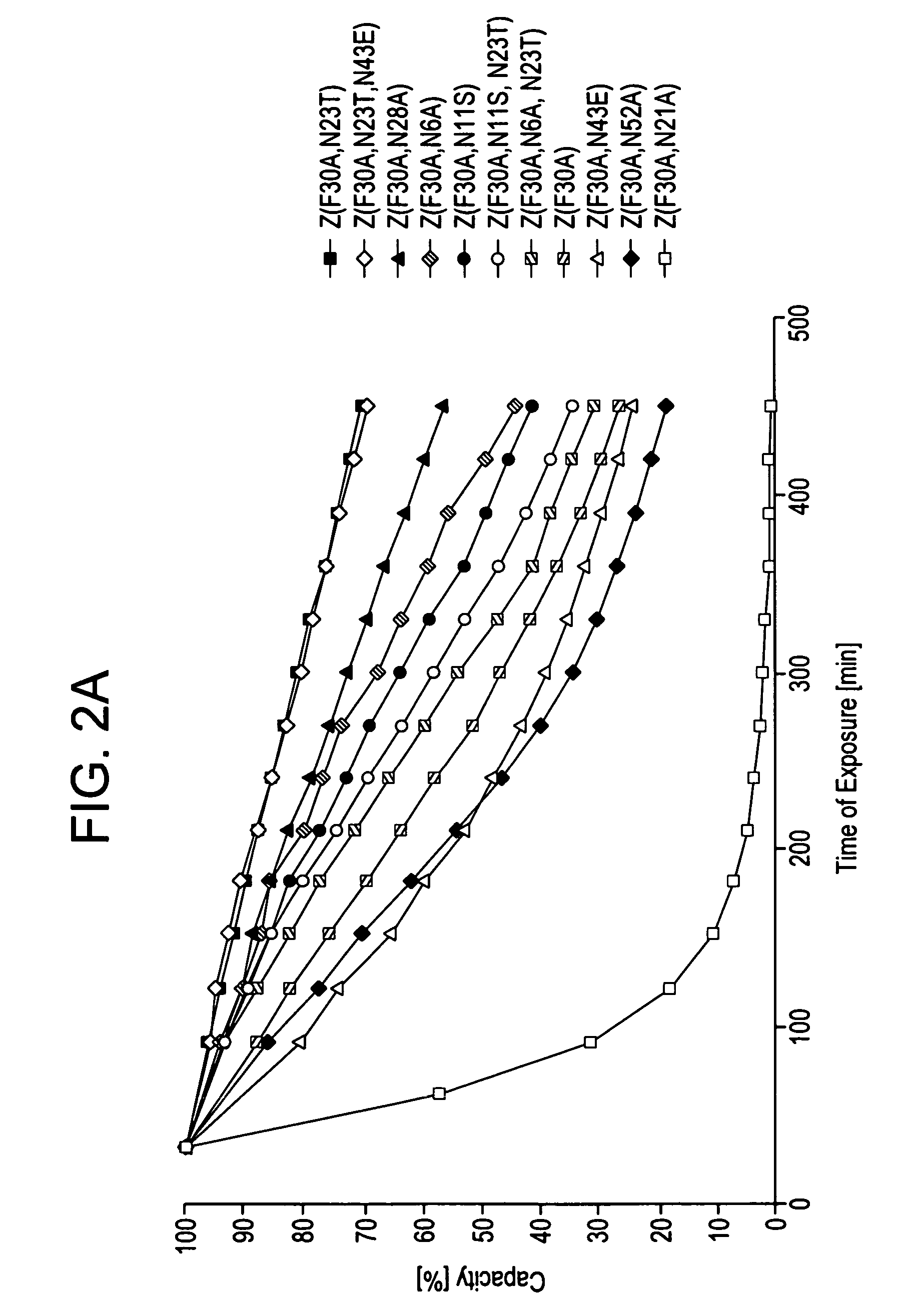
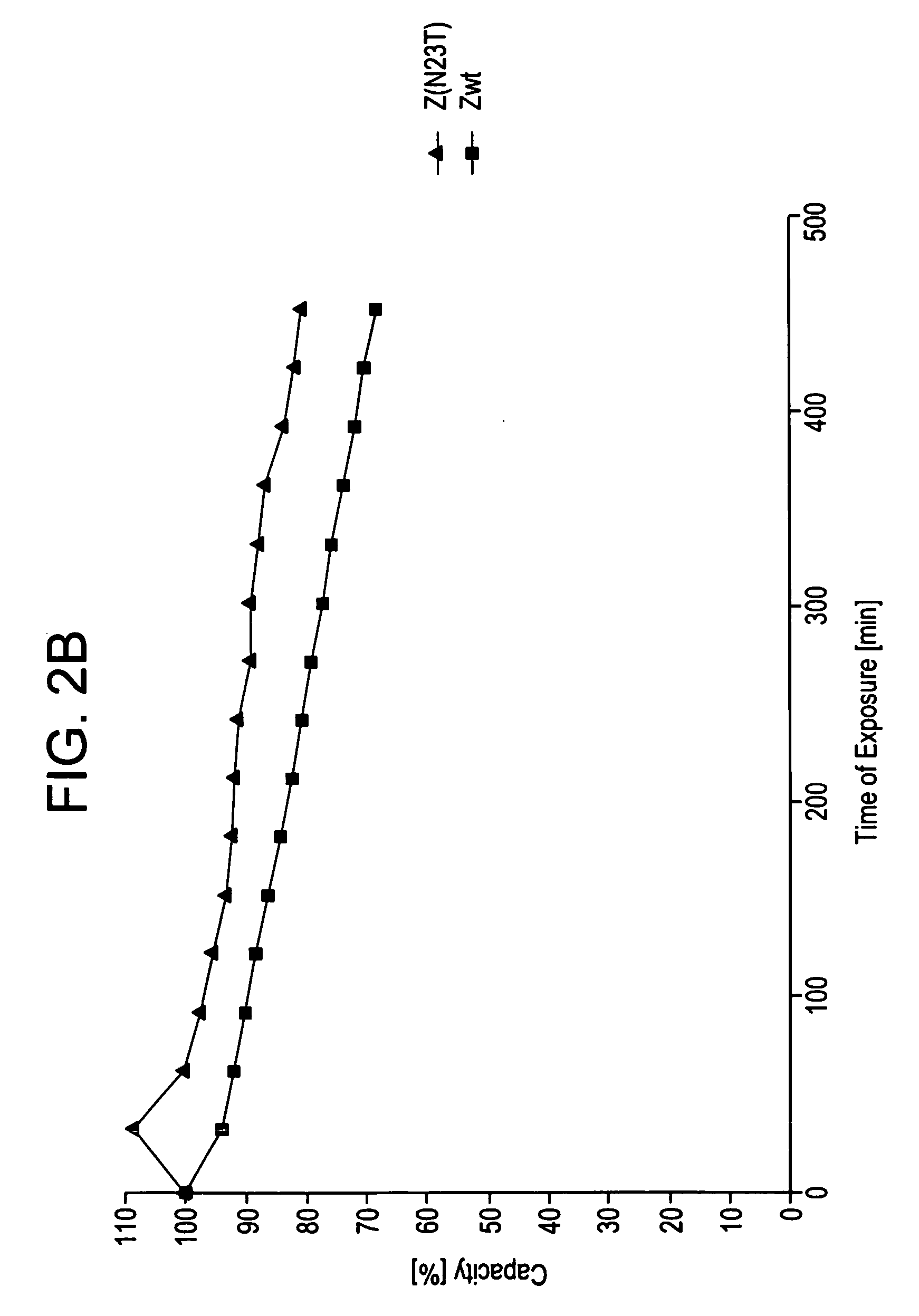
The present invention relates to an immunoglobulin-binding protein, wherein at least one asparagine residue has been mutated to an amino acid other than glutamine or aspartic acid, which mutation confers an increased chemical stability at pH-values of up to about 13-14 compared to the parental molecule. The protein can for example be derived from a protein capable of binding to other regions of the immunoglobulin molecule than the complementarity determining regions (CDR), such as protein A, and preferably the B-domain of Staphylococcal protein A. The invention also relates to a matrix for affinity separation, which comprises an immunoglobulin-binding protein as ligand coupled to a solid support, in which protein ligand at least one asparagine residue has been mutated to an amino acid other than glutamine.
Owner:CYTIVA BIOPROCESS R&D AB
Thermal treatment process for tobacco materials
ActiveUS8434496B2Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
Protein ligands
ActiveUS20060194955A1Retention characteristicReduce leakageImmunoglobulins against animals/humansBiological testingComplementarity determining regionStaphylococcus
The present invention relates to the use of an alkali-stable protein, wherein at least one asparagine residue has been mutated to an amino acid other than glutamine or aspartic acid, which mutation confers an increased chemical stability at pH-values of up to about 13-14 compared to the parental molecule. The protein can for example be derived from a protein capable of binding to other regions of the immunoglobulin molecule than the complementarity determining regions (CDR), such as protein A, and preferably the B-domain of Staphylococcal protein A. The invention also relates to a matrix for affinity separation, which comprises an immunoglobulin-binding protein as ligand coupled to a solid support, in which protein ligand at least one asparagine residue has been mutated to an amino acid other than glutamine.
Owner:CYTIVA BIOPROCESS R&D AB
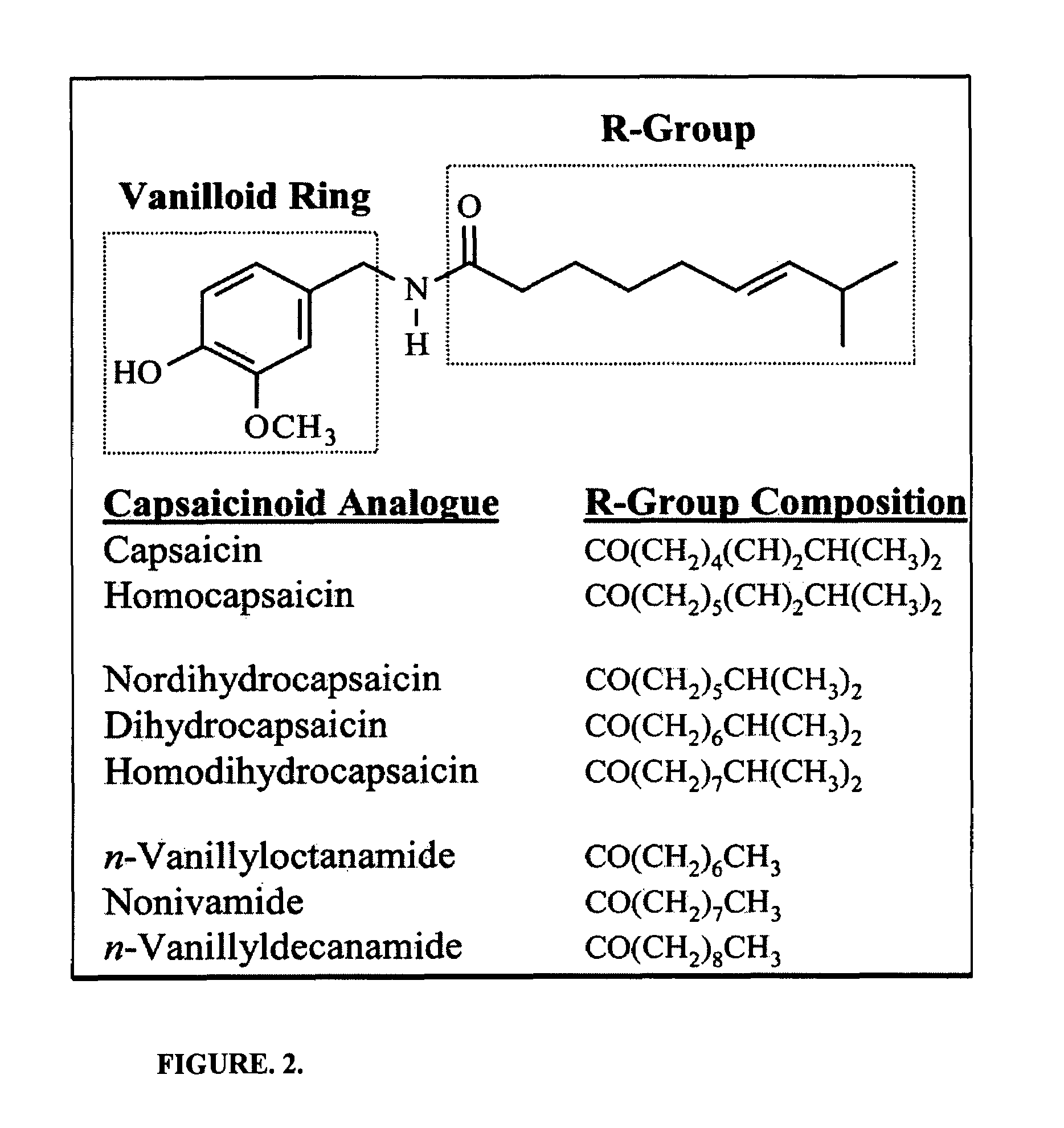
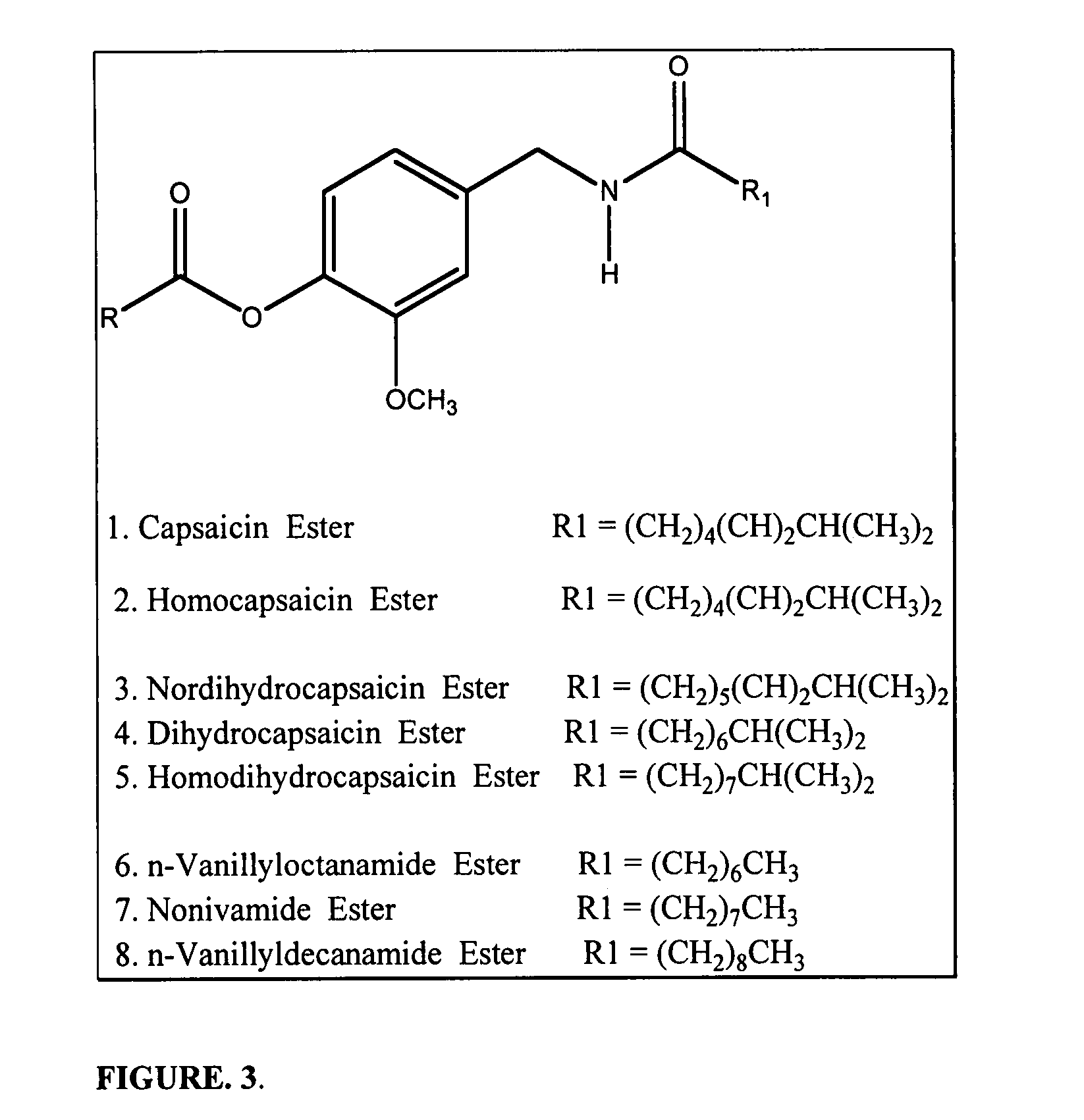
Methods and products which utilize N-acyl-L-aspartic acid
The invention provides therapeutic methods and products for the treatment of inflammation, inflammatory diseases and conditions, and proliferative diseases and conditions. The invention also provides methods and products for inhibiting inflammation in excised cells, tissues and organs. The invention further provides oral care methods and products for the treatment of the tissues of an animal's mouth. Finally, the invention provides personal care methods and products for the treatment of the skin of an animal. All of these methods and products utilize N-acyl-L-aspartic acid or an ester or pharmaceutically-acceptable salt thereof.
Owner:AMPIO PHARMA
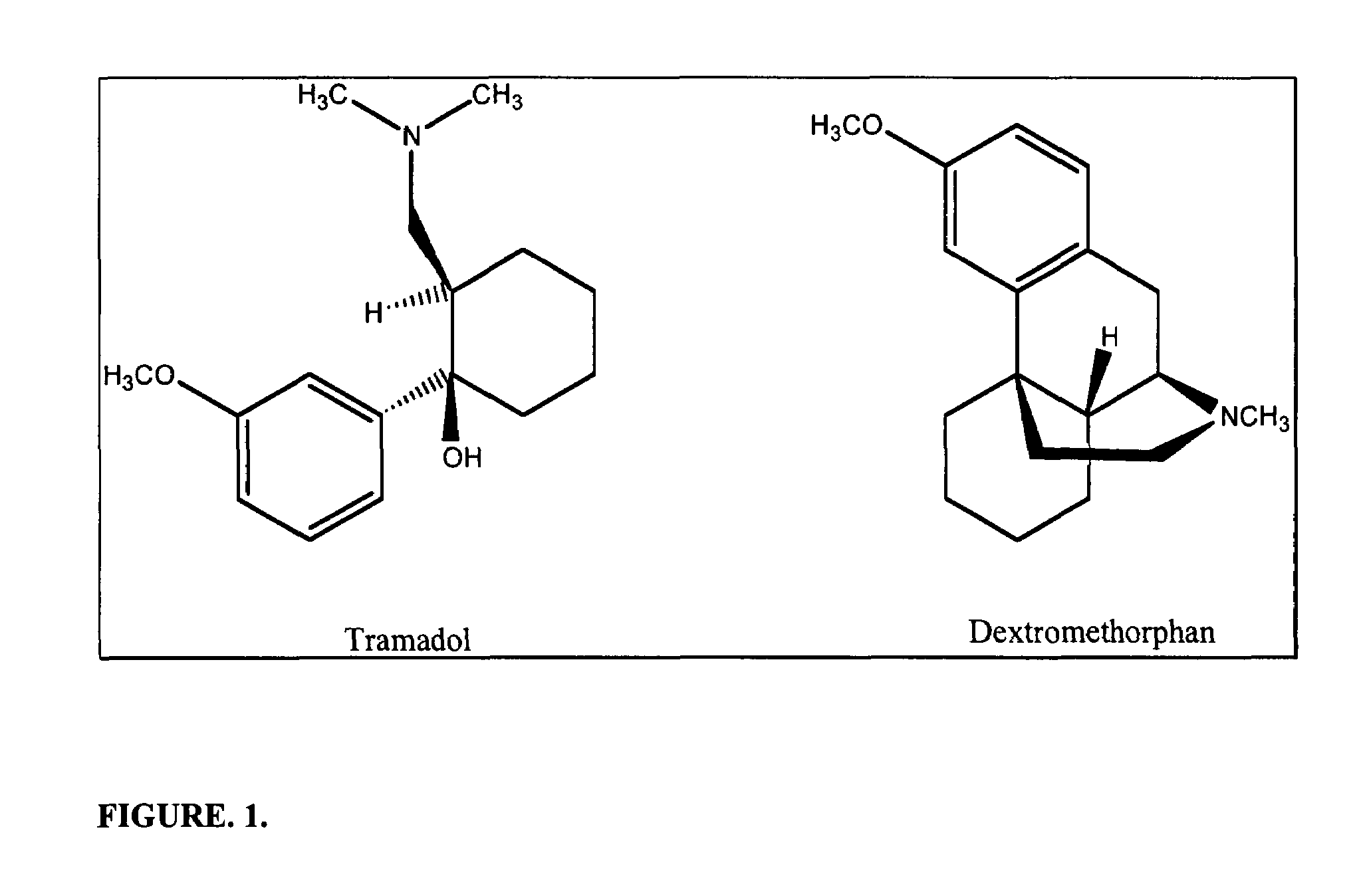
Novel pharmaceutical compositions for treating chronic pain and pain associated with neuropathy
InactiveUS20080058362A1Reduced plasma concentrationEffective pain managementBiocideAmide active ingredientsDextrorphanSustained release drug
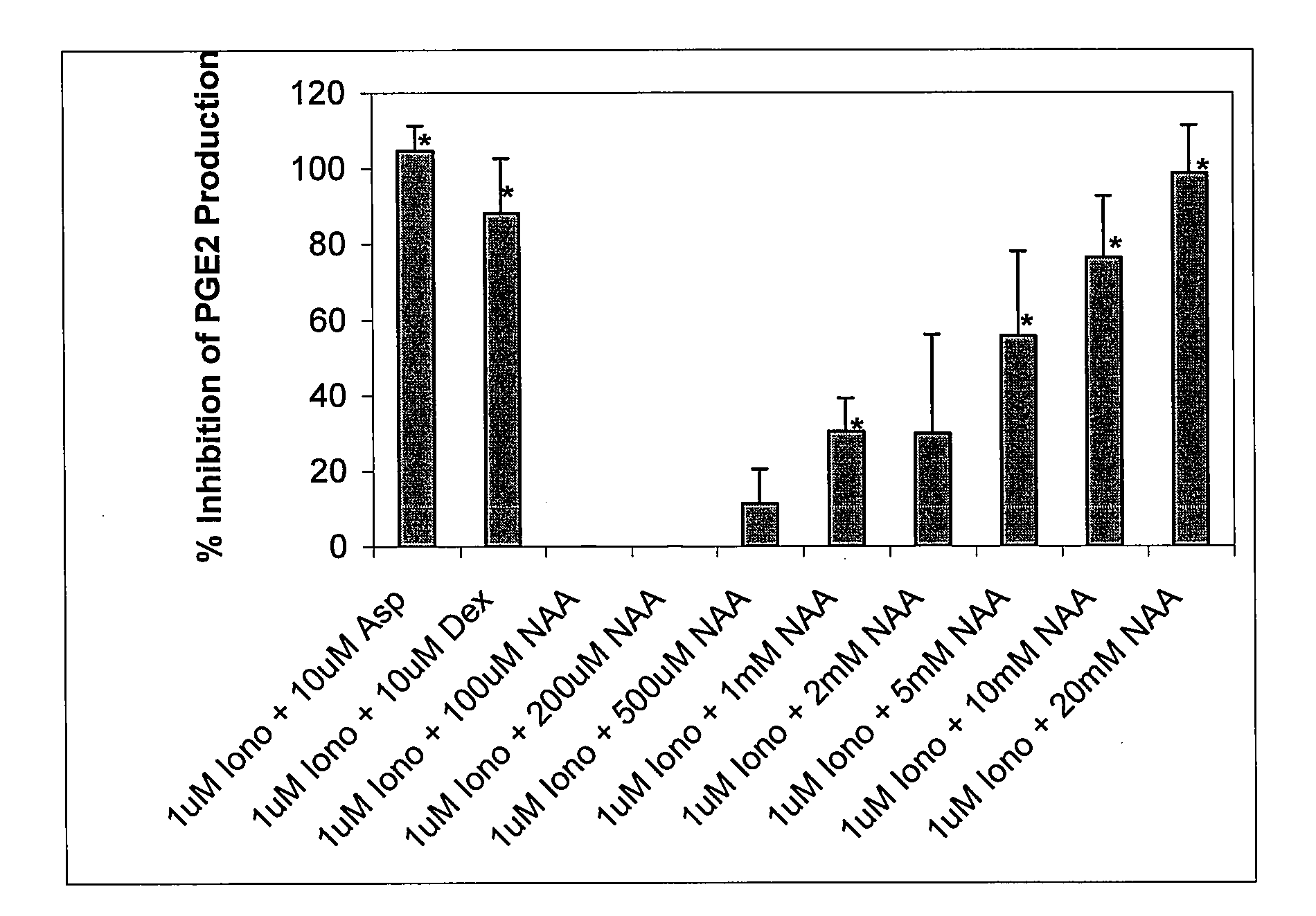
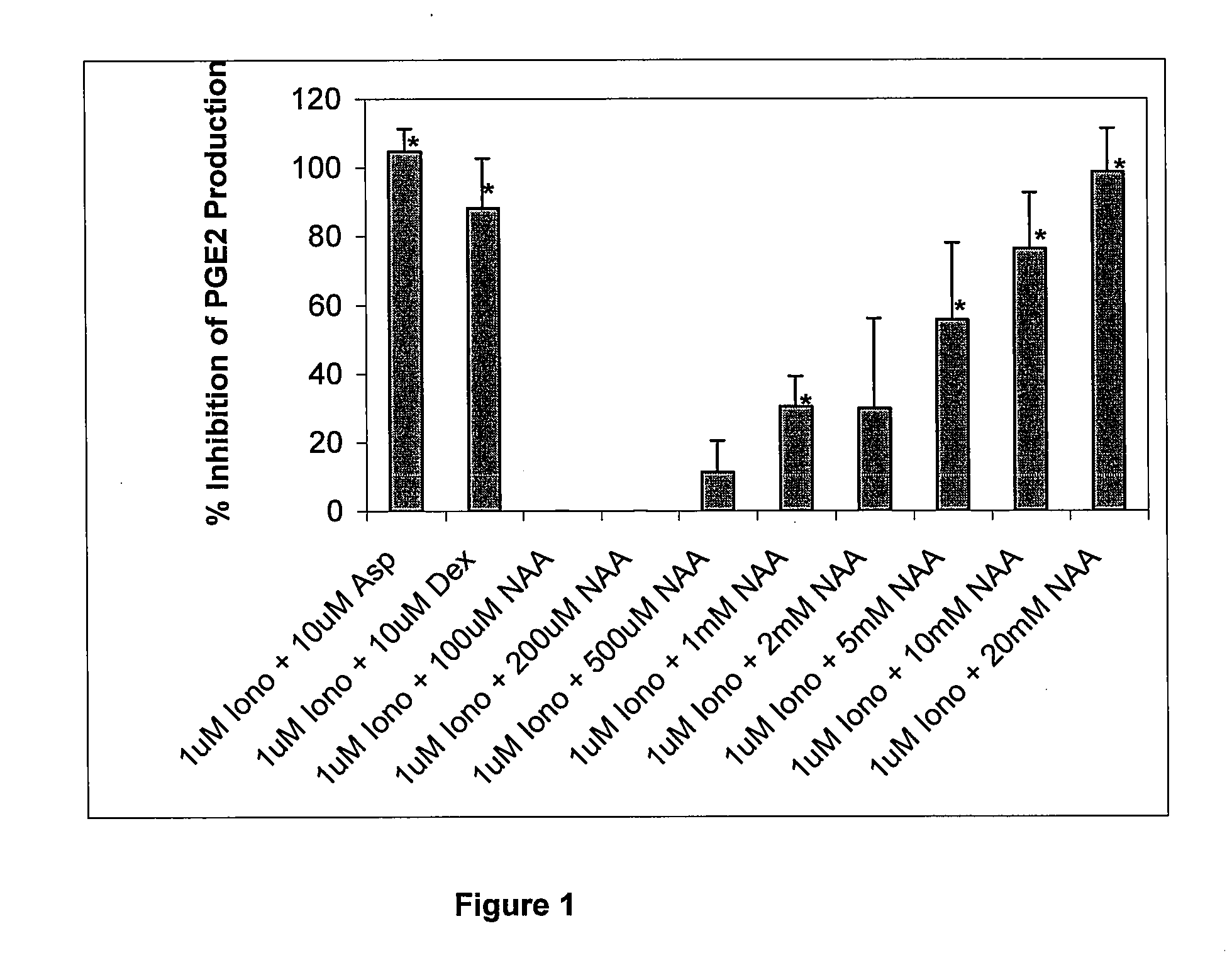
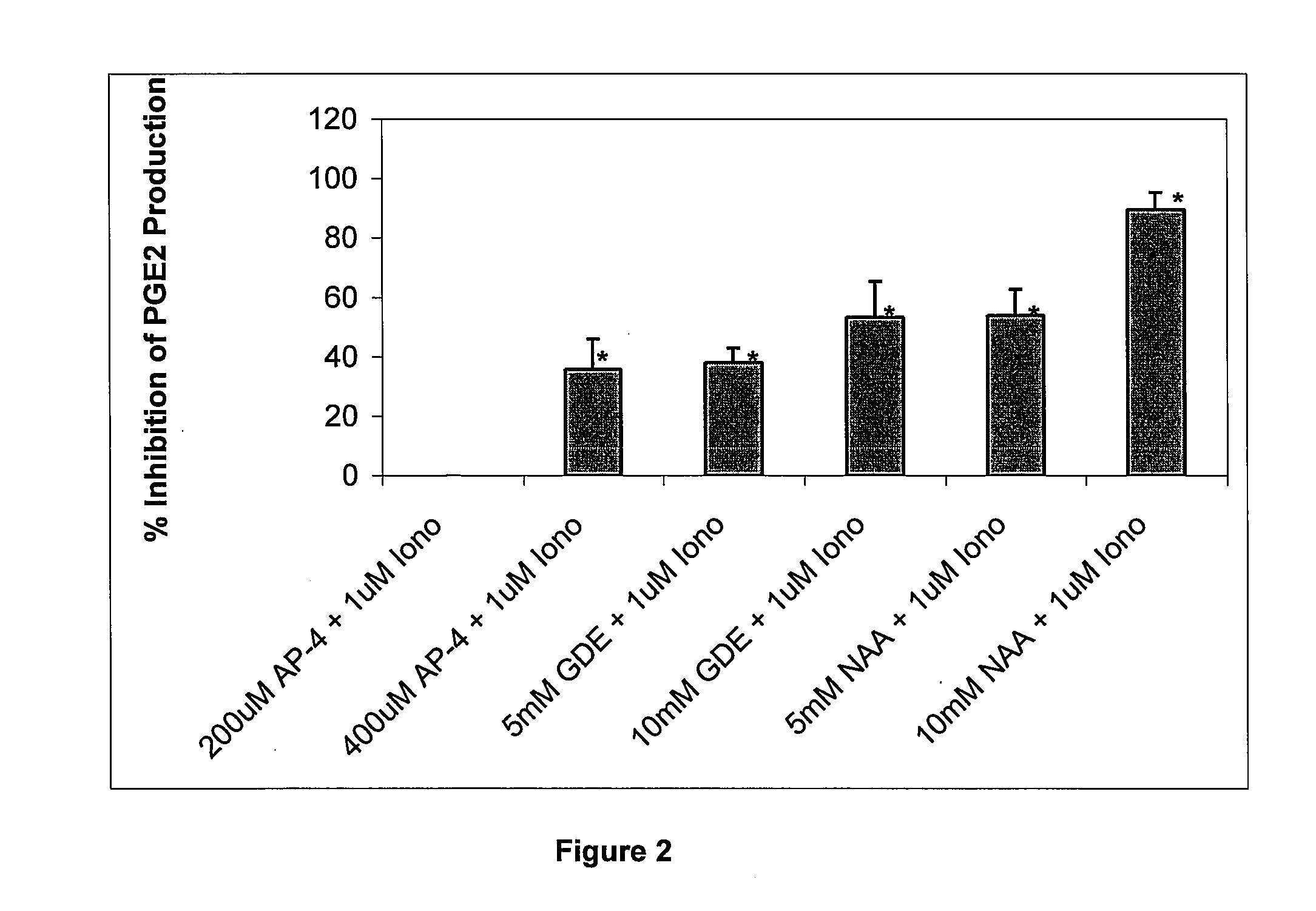
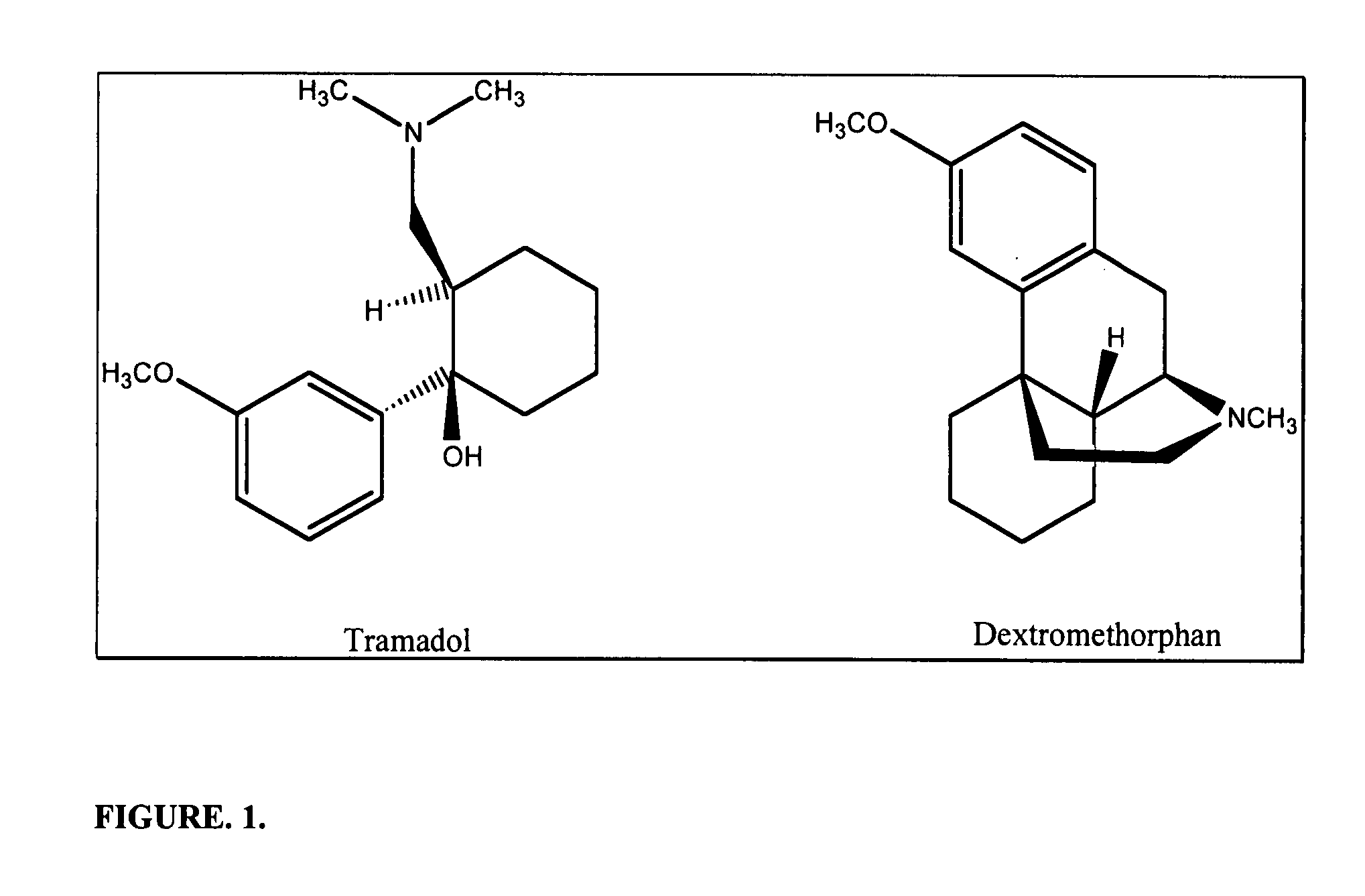
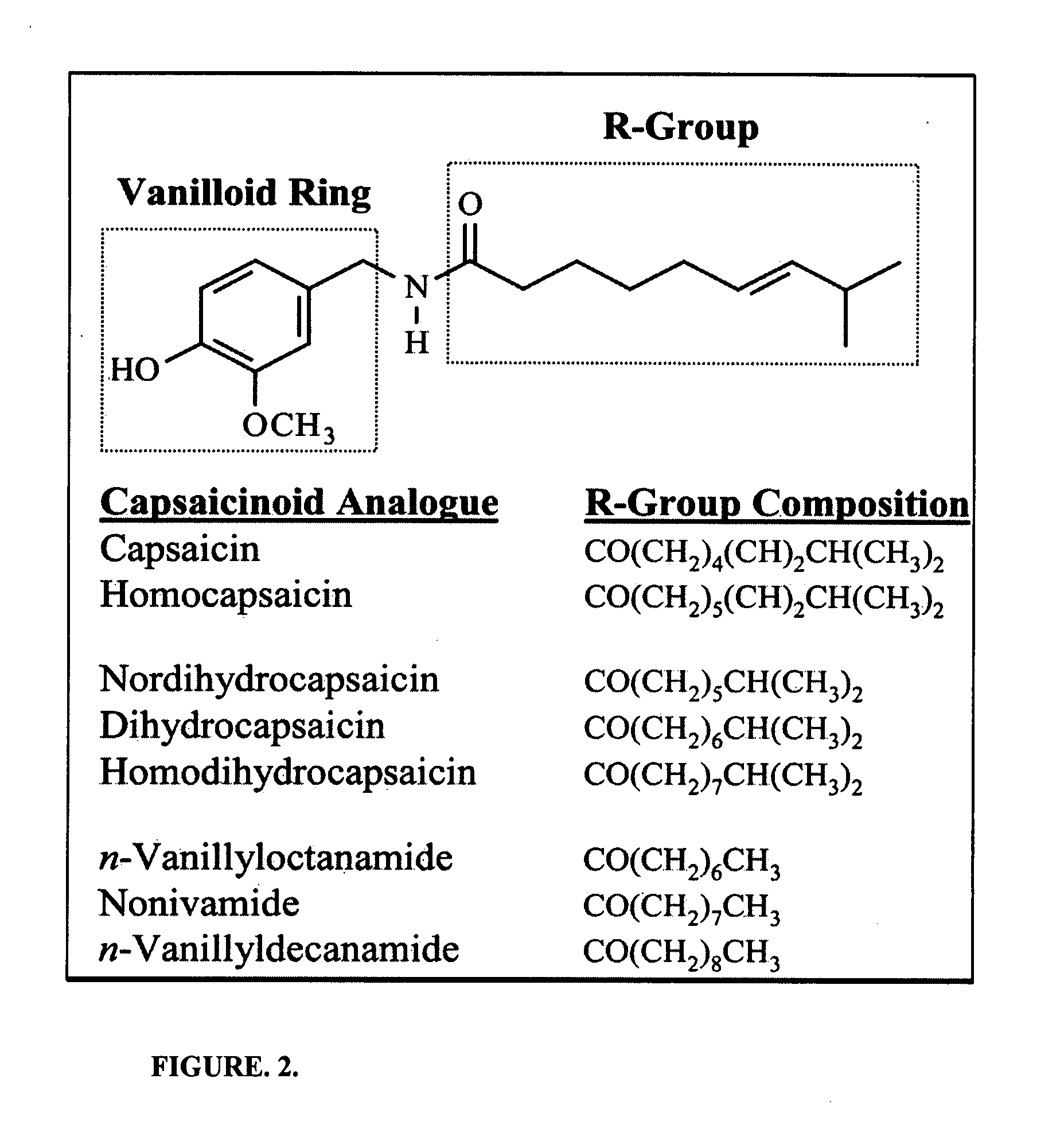
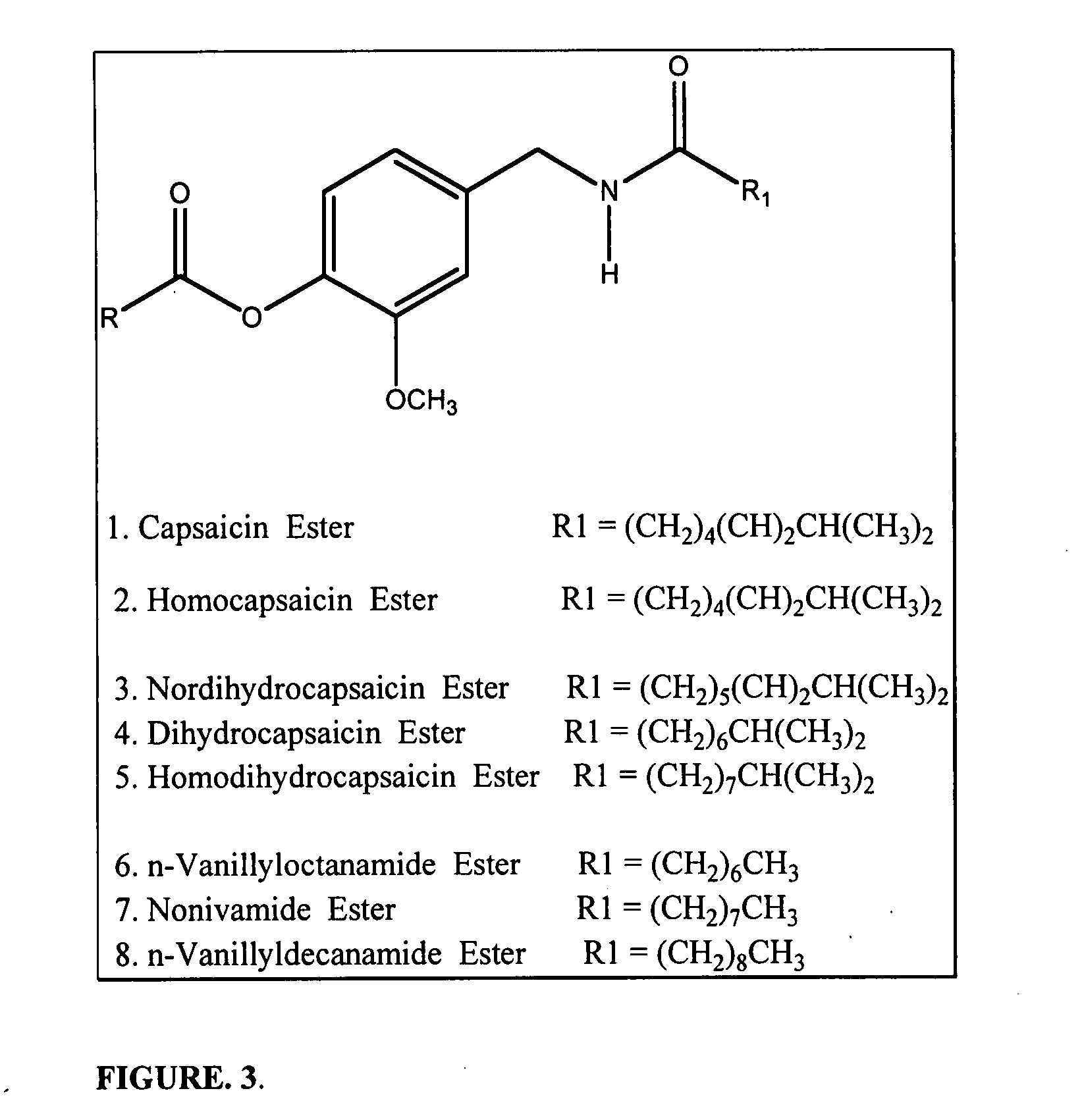
Chronic pain is alleviated in a mammal suffering there from by administering to the mammal a chronic pain alleviating amount of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, in combination with a μ-opiate analgesic such as tramadol or an analogously acting molecular entity, and a capsaicin or an ester of capsaicin, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
Compositions of tocol-soluble therapeutics
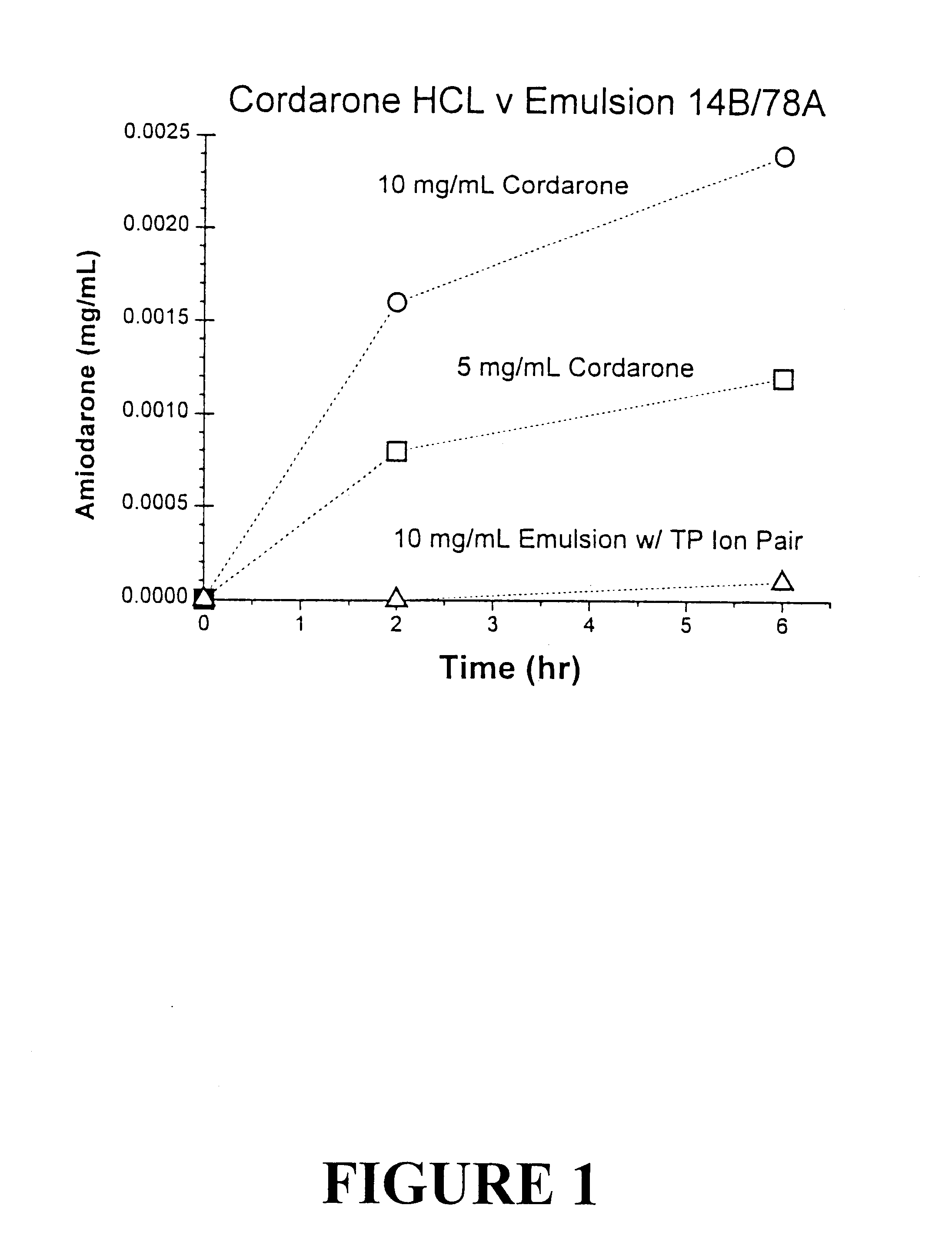
Tocol-based compositions of charged amphiphilic and water soluble pharmaceutically active compounds or their charged precursors are prepared by forming a tocol-soluble ion pair with an oppositely charged ion-pair forming compound capable of forming a tocol-soluble ion-pair with the active compound.Also disclosed are novel compounds tocopherolsuccinate-aspartate and tocopherolsuccinate-glutamate, which are useful as ion-pair forming compounds.
Owner:SONUS PHARM INC
Thermal treatment process for tobacco materials
ActiveUS8991403B2Alter natureAlter characterTobacco preparationTobacco treatmentArginineTobacco product
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
Compositions including iron
Compositions and methods for prevent, stabilize, reverse or treat disorders related to iron deficiency in a human or other animal. In a first embodiment, the composition includes about 10 mg to about 500 mg of one or more forms of iron, wherein at least one form of iron is an aspartic acid-glycine chelate of iron; and about 5 mg to about 500 mg of one or more forms of an organic acid. In another embodiment, the composition includes about 50 to about 150 mg of one or more forms of iron, wherein at least one form of iron is an aspartic acid-glycine chelate of iron; about 50 to about 250 mg of one or more forms of an organic acid; about 150 to about 250 mg of one or more forms of ascorbic acid; about 0.5 mg to about 1.5 mg vitamin B12; about 50 to about 150 mg intrinsic factor; and about 0.5 mg to about 1.5 mg folic acid.
Owner:DRAGTEK CORP
Method for producing L-methionine by fermentation
InactiveUS7611873B1High activityIncrease productivityBacteriaSugar derivativesSerine dehydrogenaseThreonine
L-Methionine is produced by culturing a microorganism which is deficient in repressor of L-methionine biosynthesis system and / or enhanced intracellular homoserine transsuccinylase activity is cultured in a medium so that L-methionine should be produced and accumulated in the medium, and collecting the L-methionine from the medium. The microorganism preferably further exhibits reduced intracellular S-adenosylmethionine synthetase activity, L-threonine auxotrophy, enhanced intracellular cystathionine γ-synthase activity and enhanced intracellular aspartokinase-homoserine dehydrogenase II activity. The present invention enables breeding of L-methionine-producing bacteria, and L-methionine production by fermentation.
Owner:AJINOMOTO CO INC
Thermal treatment process for tobacco materials
ActiveUS8944072B2Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of preparing a tobacco material for use in a smoking article is provided, including (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof; (ii) heating the mixture; and (iii) incorporating the heat-treated mixture into a smoking article as a smokable material. A smoking article in the form of a cigarette is also provided that includes a tobacco material pre-treated to inhibit reaction of asparagine to form acrylamide in mainstream smoke. Upon smoking, the smoking article is characterized by an acrylamide content of mainstream smoke that is reduced relative to an untreated control smoking article.
Owner:R J REYNOLDS TOBACCO COMPANY
Nitrogen fertilizer compound synergist and preparation method
ActiveCN101450880AImprove the absorption and utilization effectIncrease profitAgriculture gas emission reductionFertilizer mixturesTrace element compositionNitrification inhibitors
The invention relates to nitrogen element fertilizer, in particular to a nitrogen element fertilizer composite synergist and a preparation method thereof. The composite synergist consists of a biochemical inhibitor, a synergist, a nitrogen element stabilizer, a carrier and microelements, the weight portion ratio of the materials is 1:0.01-1: 0.01-0.5: 0.5-1: 0-0.05, wherein the biochemical inhibitor can be a nitrification inhibitor and a urease inhibitor, the synergist can be poly-aspartic acid, and the nitrogen element stabilizer can be hydrolyzed amino acid, humic acid, alginic acid or alginic acid water soluble salt. The preparation method comprises the following steps: crushing the raw materials, screening the raw materials with a sieve of 30 to 100 meshes, and stirring and mixing the raw materials according to the proportion. The nitrogen element fertilizer composite synergist is suitable for growing long acting nitrogenous fertilizer taking urea or ammonium nitrogen fertilizer as raw materials and can be applied to various soils together with urea or ammonium nitrogen fertilizer so as to effectively prolong the fertilizer efficiency period of the urea or the ammonium nitrogen fertilizer. The fertilizer efficiency period can reach 100 to 120 days. The nitrogen element fertilizer composite synergist has remarkable resistance to diseases, drought and lodging, effectively improves the capability of soil to reserve nutrients, and has good effects for off-season crops.
Owner:SHENYANG INST OF APPLIED ECOLOGY - CHINESE ACAD OF SCI
Implantable sensor with biocompatible coating for controlling or inhibiting tissue growth
ActiveUS20060008500A1Inhibit growthPrevent formation of tissueMaterial nanotechnologyElectrotherapySteroid CompoundAdhesion process
All or a portion of a surface of an implantable sensor is covered with a biocompatible coating formed at least partially of a biomaterial matrix having properties that promote a substantially even growth of tissue cells over the surface of the coating. Additional materials, such as growth factors, agents that recruit endogenous stem cells, and cell adhesion motif arginine, glycine, aspartic acid may be included in the coating. Autologous cells may be added to the coating prior to implantation. The sensor surface may also be textured, by etching or abrading, in order to promote even tissue growth. Alternatively, the sensor surface may be covered with a coating having properties that inhibit the growth of tissue. These coatings may include a biomaterial, a biomaterial matrix having a drug, such as a sirolimus or a steroid, an active component, or a self assembled monolayer.
Owner:CARDIAC PACEMAKERS INC
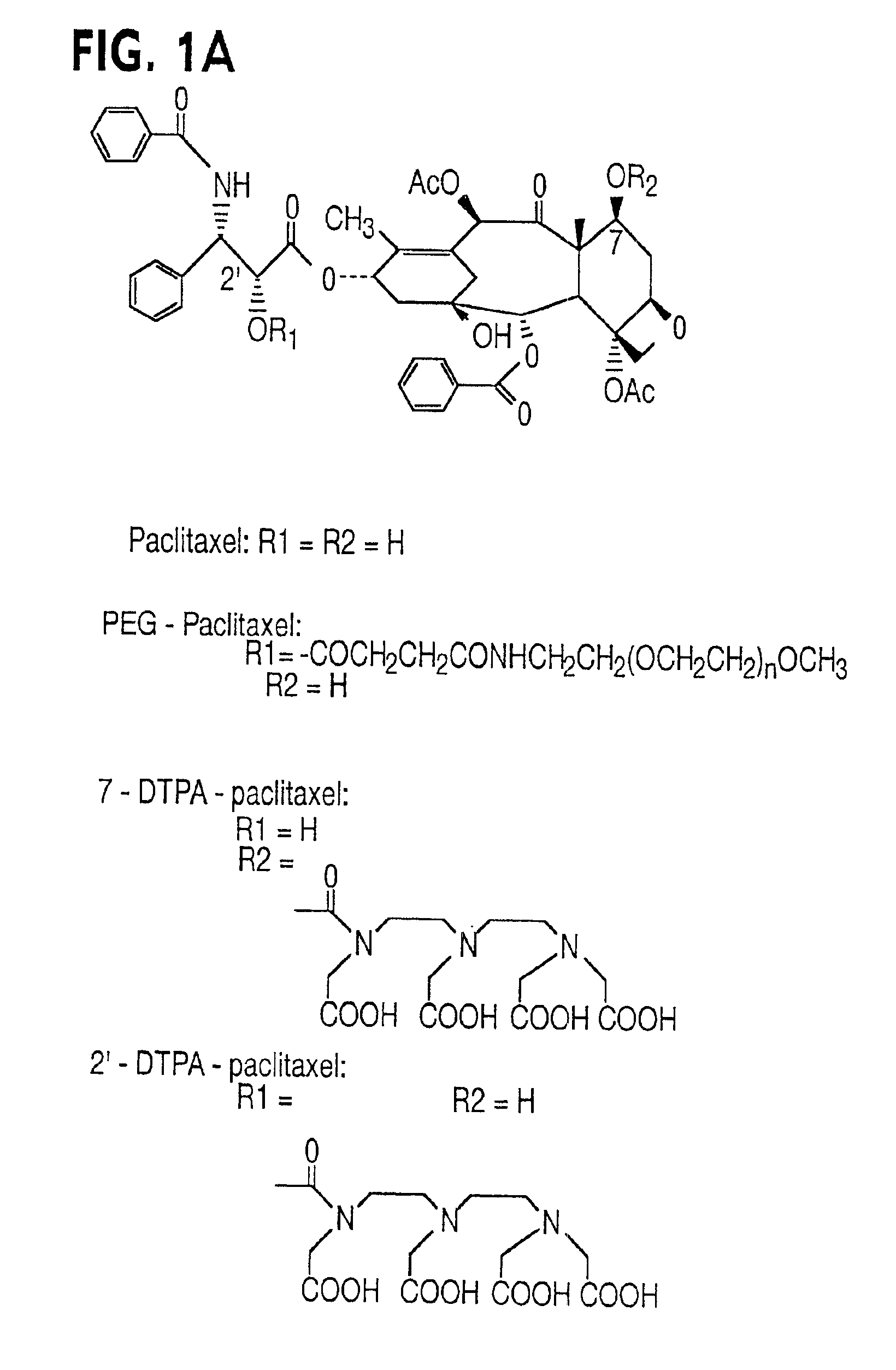
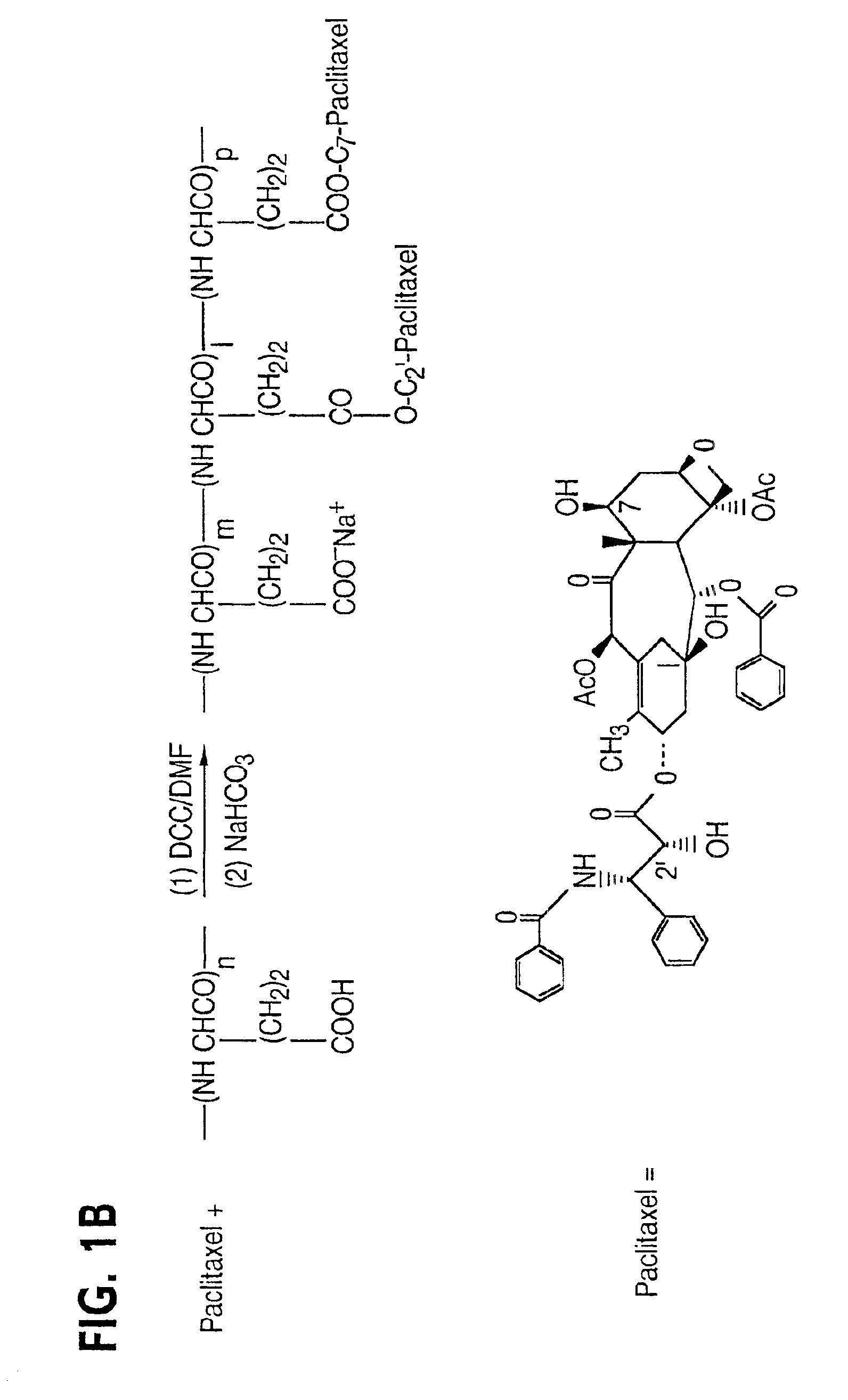
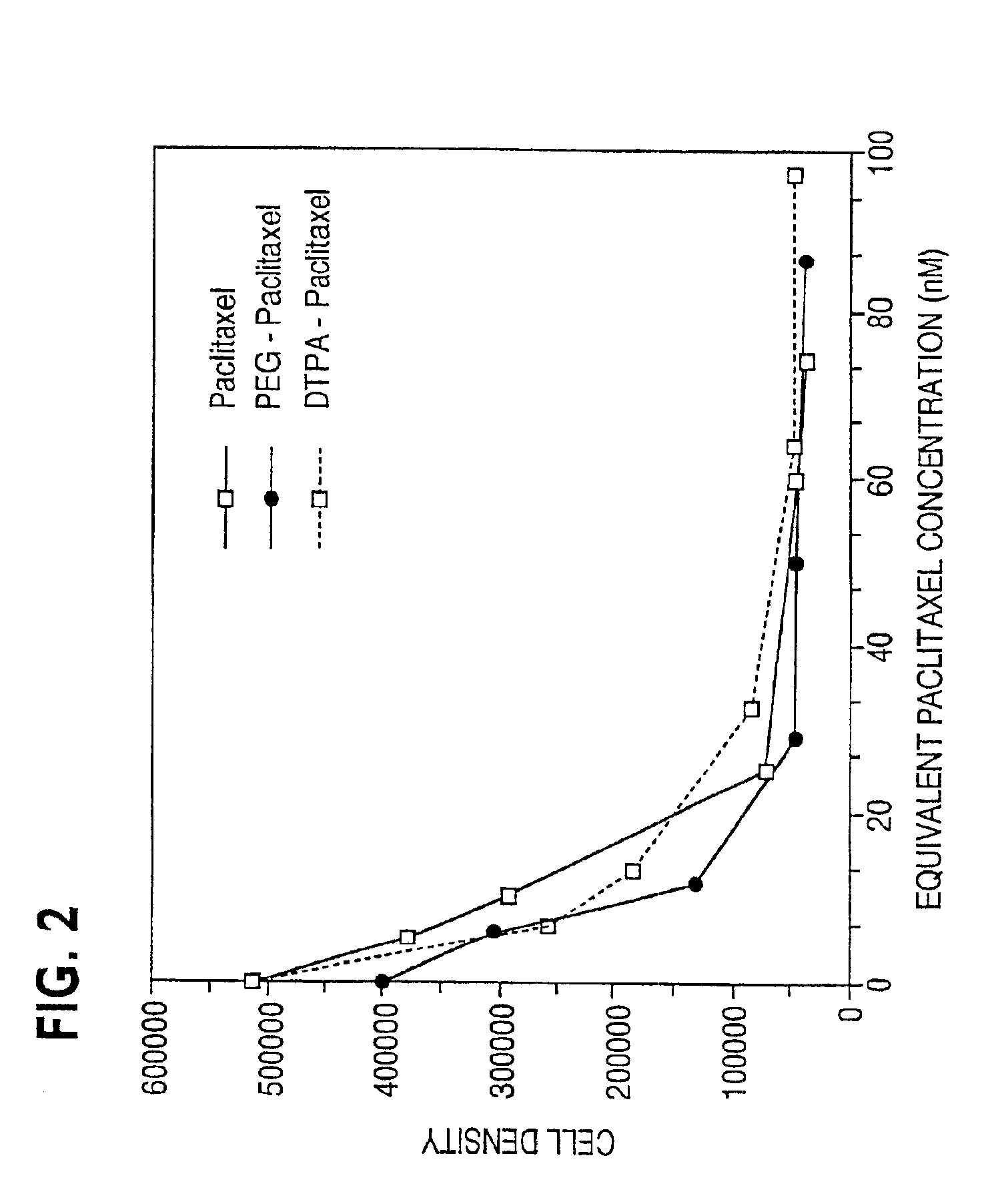
Water soluble paclitaxel derivatives
InactiveUS6884817B2Surprising antitumor activityImprove efficacyHeavy metal active ingredientsBiocideDocetaxel-PNPDocetaxel
Disclosed are water soluble compositions of paclitaxel and docetaxel formed by conjugating the paclitaxel or docetaxel to a water soluble polymer such as poly-glutamic acid, poly-aspartic acid or poly-lysine. Also disclosed are methods of using the compositions for treatment of tumors, auto-immune disorders such as rheumatoid arthritis. Other embodiments include the coating of implantable stents for prevention of restenosis.
Owner:PG TXL COMPANY
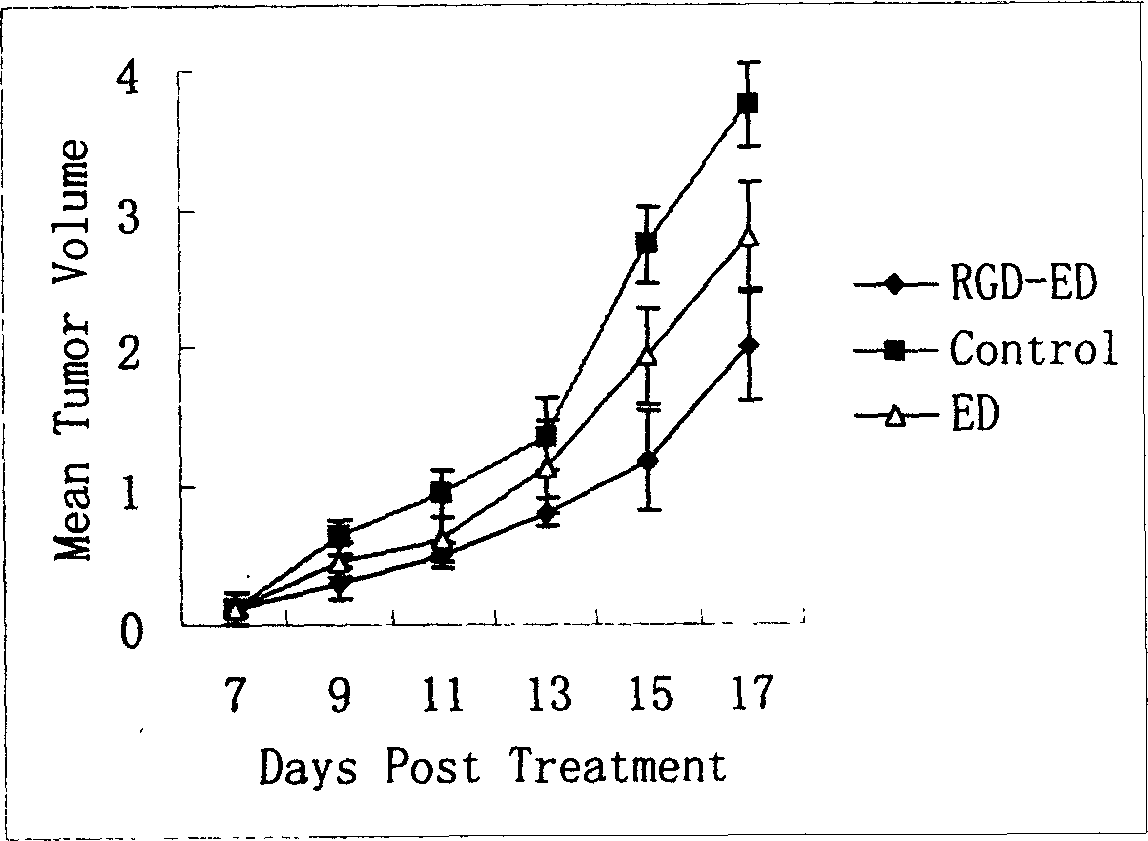
Peptide for high performance inhibition of angiogenesis and method for preparing same and use thereof
ActiveCN1699408AImprove and enhance growthImprove and enhance the anti-tumor effectPeptide/protein ingredientsSkeletal disorderEscherichia coliInclusion bodies
The invention relates to a peptide for high performance inhibition of angiogenesis and method for preparing same and use, wherein high performance blood vessel production inhibiting agent RGD-ED with integration compatibility is designed, the inhibiting agent comprises polypeptide polypeptide-valine-arginine-arginine-alanine-aspartate-arginine-alanine-alanine-valine-praline, its one or two ends are connected with polypeptides containing arginine-glycine-aspartic acid sequence. The RGD-ED provided by the invention can be synthesized. The invention also discloses the expression of one RGD-ED in bacillus coli through gene engineering method, wherein the RGD-ED is prepared through the steps of inclusion body protein segregation, dissolution and renaturation, and ion-exchange chromatography segregation and purification.
Owner:CHINA PHARM UNIV
Pharmaceutical compositions for treating chronic pain and pain associated with neuropathy
InactiveUS7645767B2Reduce concentrationEfficient managementBiocideAmide active ingredientsOpiatePharmaceutical medicine
Chronic pain is alleviated in a mammal suffering there from by administering to the mammal a chronic pain alleviating amount of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, in combination with a μ-opiate analgesic such as tramadol or an analogously acting molecular entity, and a capsaicin or an ester of capsaicin, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
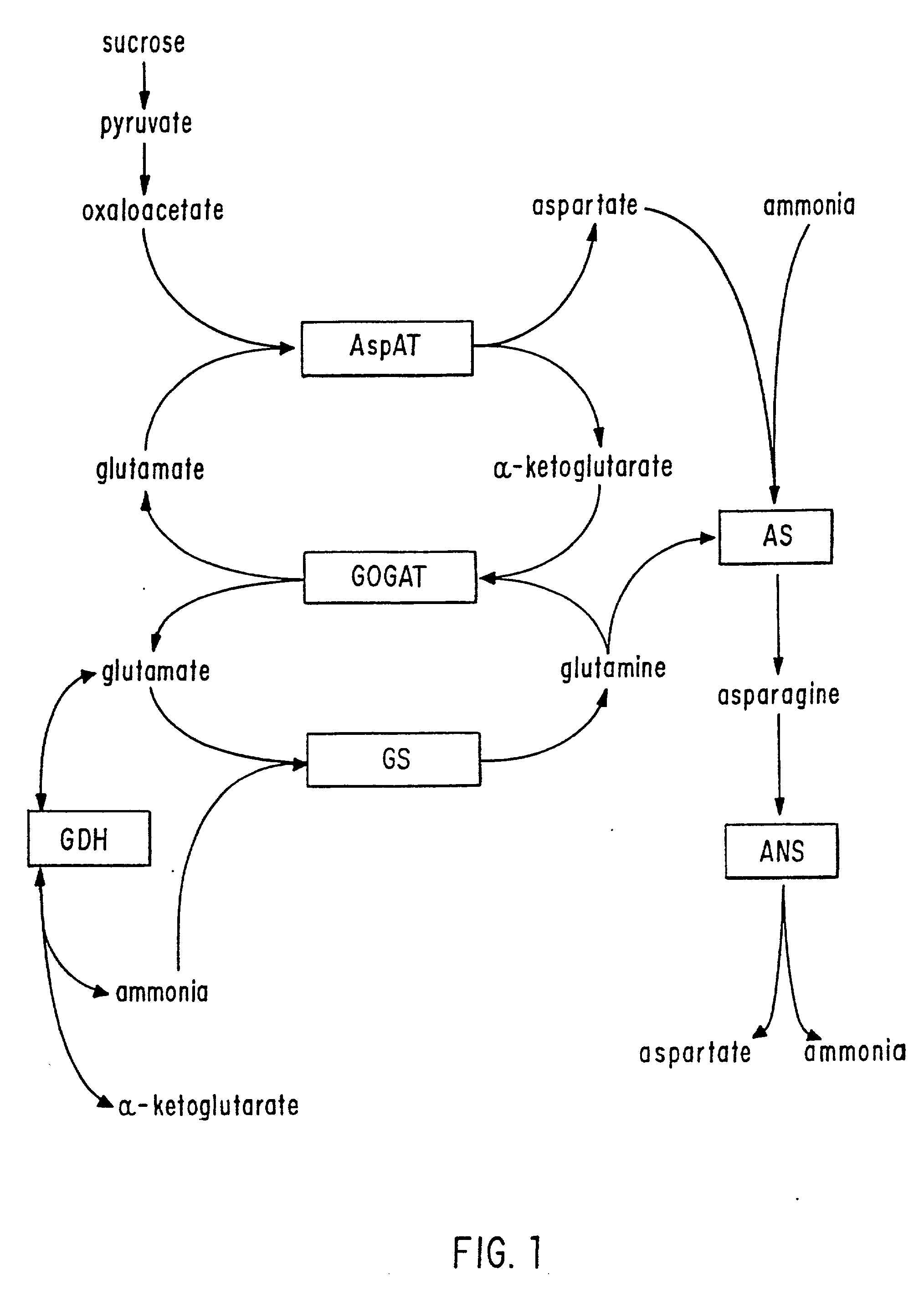
Transgenic plants that exhibit enhanced nitrogen assimilation
InactiveUS20020069430A1Improve growth characteristicsImproved vegetativeClimate change adaptationOther foreign material introduction processesPlant cellThreonine
Transgenic plants containing free amino acids, particularly at least one amino acid selected from among glutamic acid, asparagine, aspartic acid, serine, threonine, alanine and histidine accumulated in a large amount, in edible parts thereof, and a method of producing them are provided. In this method, glutamate dehydrogenase (GDH) gene is introduced into a plant together with a regulator sequence suitable for over expressing the sequence encoding GDH gene in plant cells.
Owner:AJINOMOTO CO INC
Rapid acting and long acting insulin combination formulations
ActiveUS20080039368A1Increase speedReduce the amount of solutionPeptide/protein ingredientsMetabolism disorderBefore BreakfastIntensive insulinotherapy
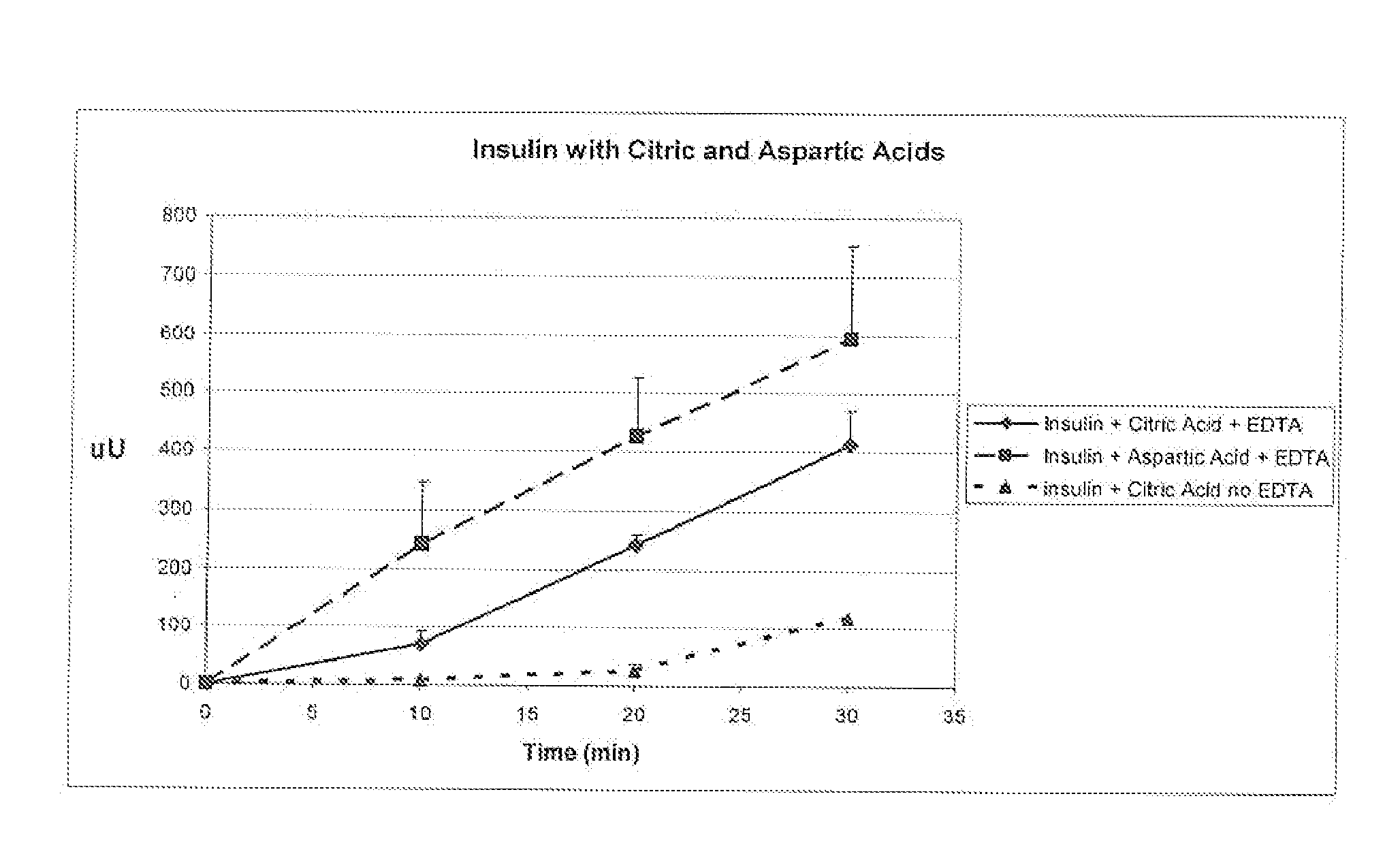
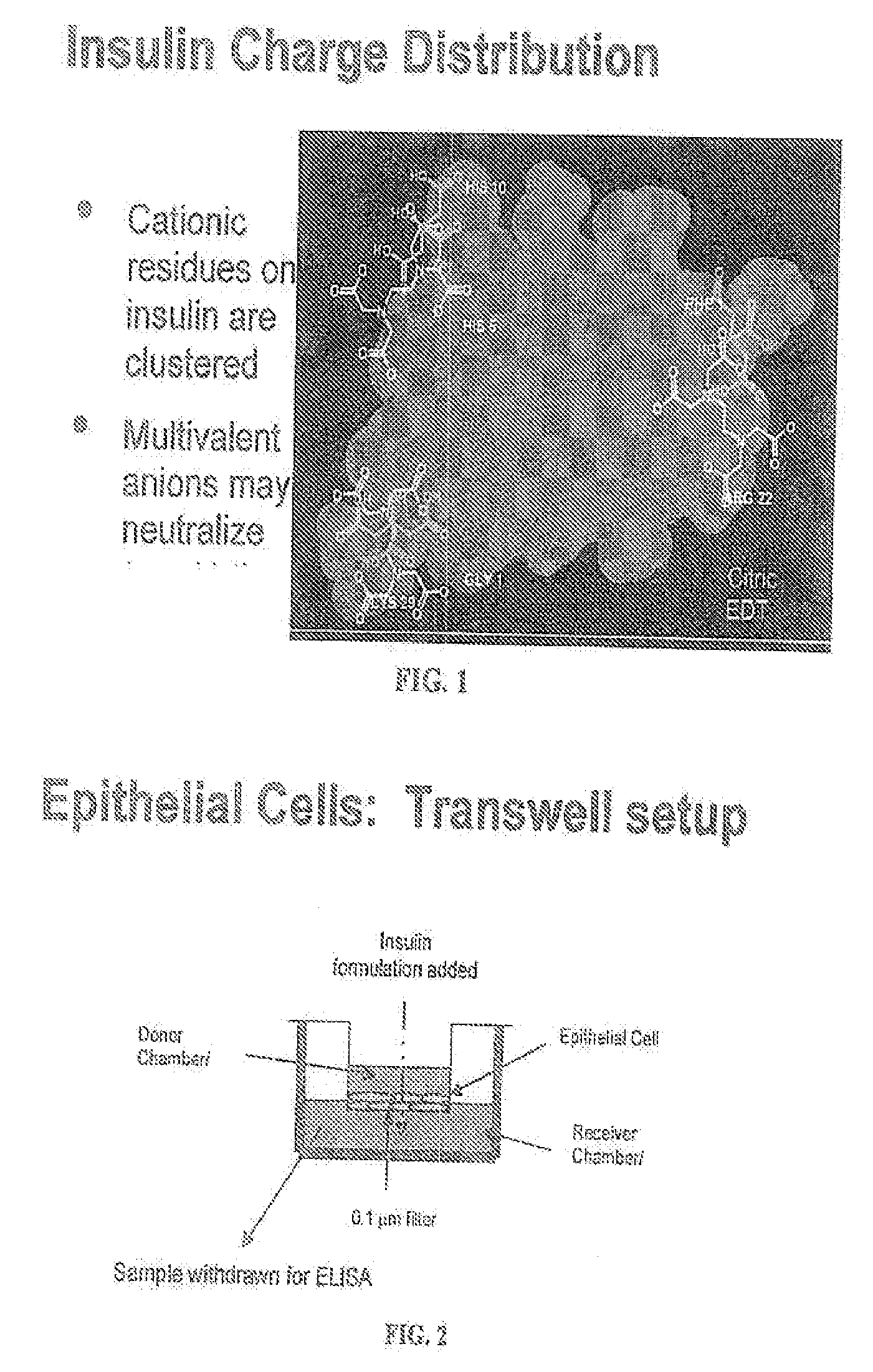
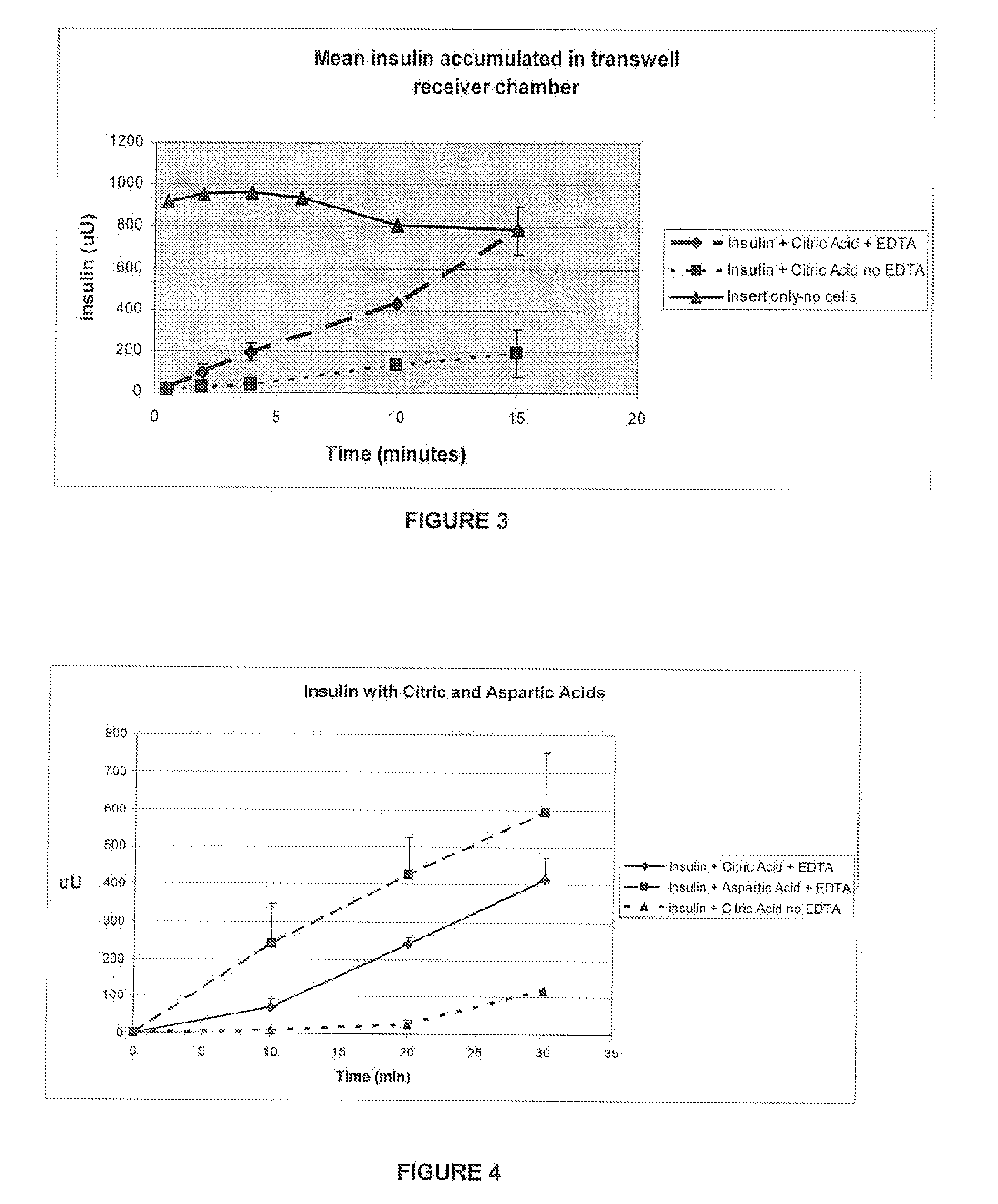
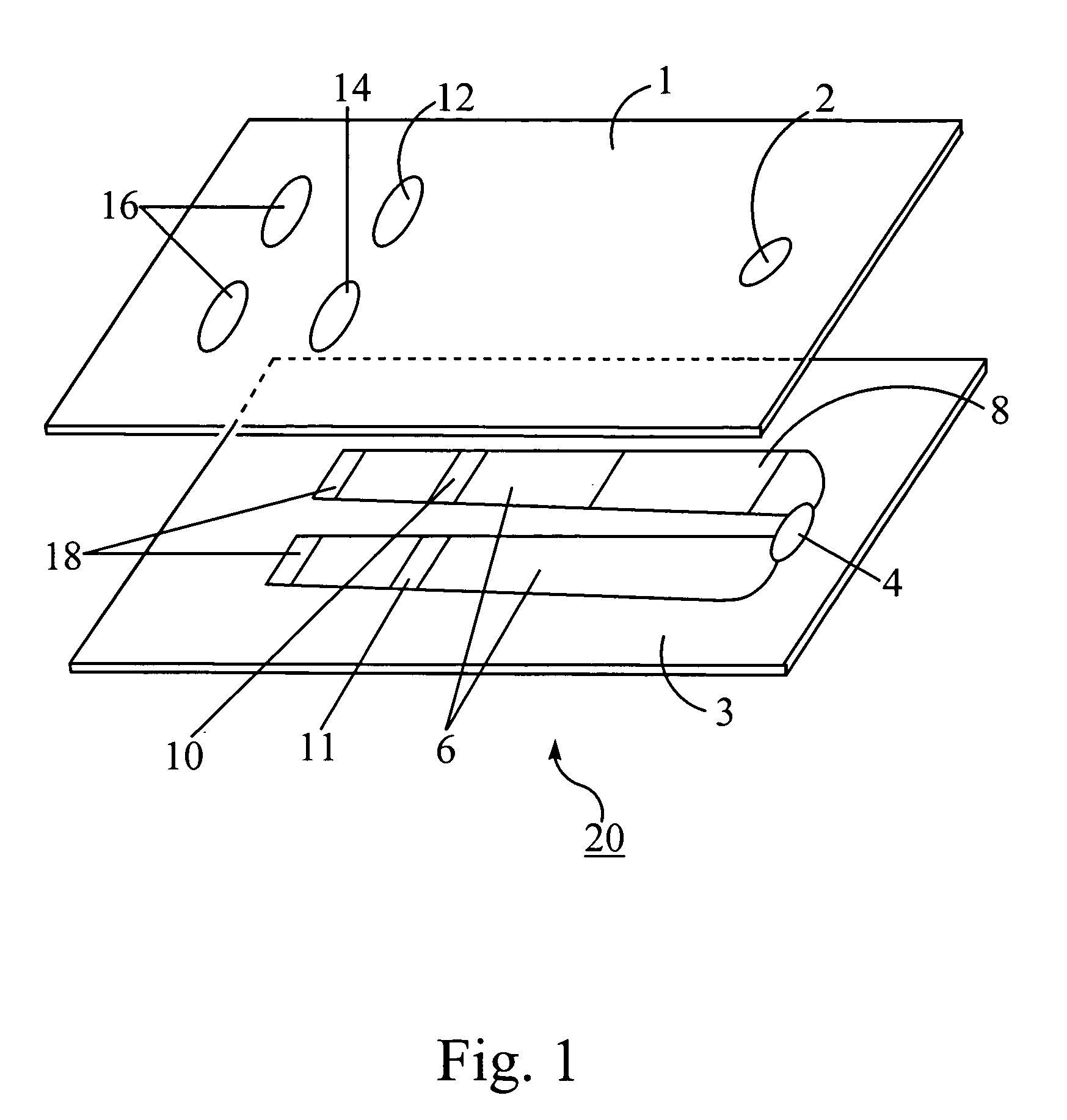
A combined rapid acting-long acting insulin formulation has been developed wherein the pH of the rapid acting insulin is adjusted so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day. Experiments have been performed to demonstrate, the importance of the addition of specific acids to hexameric insulin to enhance speed and amount of absorption and preserve bioactivity following dissociation into the monomeric form by addition of a chelator such as EDTA. As shown by the examples, the preferred acids are aspartic, maleic, succinic, glutamic and citric acid. These are added in addition to a chelator, preferably ethylenediaminetetraacetic acid (EDTA). The results show that the citric acid formulation was more effective at dropping the blood glucose rapidly than the identical rapid acting formulation prepared with HCl in swine. Charge masking by the polyacid appears to be responsible for rapid insulin absorption. EDTA was not effective when used with adipic acid, oxalic acid or HCl at hastening the absorption of insulin. These results confirm the results seen in clinical subjects and patients with diabetes treated with the rapid acting insulin in combination with citric acid and EDTA.
Owner:ELI LILLY & CO
Test for the rapid evaluation of ischemic states and kits
The present invention relates to rapid methods for the detection of ischemic states and to kits for use in such methods. Provided for is a rapid method of testing for and quantifying ischemia based upon methods of detecting and quantifying the existence of an alteration of the serum protein albumin which occurs following an ischemic event; methods for detecting and quantifying this alteration include evaluating and quantifying the cobalt binding capacity of circulating albumin, analysis and measurement of the ability of serum albumin to bind exogenous cobalt, detection and measurement of the presence of endogenous copper in a purified albumin sample and use of an immunological assay specific to the altered form of serum albumin which occurs following an ischemic event. Also taught by the present invention is the detection and measurement of an ischemic event by measuring albumin N-terminal derivatives that arise following an ischemic event, including truncated albumin species lacking one to four N-terminal amino acids or albumin with an acetylated N-terminal Asp residue.
Owner:ISCHEMIA TECH
Nicotinamide agent and preparation method thereof
The present invention is reducing nicotine amide coenzyme as enzyme testing reagent for testing body fluid. The enzyme testing reagent of the present invention includes oxidizing nicotine amide coenzyme, corresponding enzyme and substrate. The oxidizing coenzyme is reduced into reducing nicotine amide coenzyme slowly to test the tested matter. The reagent includes alanine aminotransferase reagent, aspartic acid aminotransferase reagent, urea reagent, ammonia reagent, creatinine reagent, CO2 reagent, etc. Owing to constant creation of nicotine amide coenzyme, the present invention has greatly raised reagent stability and greatly lowered reagent cost.
Owner:TECOM SCI CORP
L-threonine producing bacterium belonging to the genus Escherichia and method for producing L-threonine
Owner:AJINOMOTO CO INC
Production process of cross-linked polysuccinimide resin
Owner:MITSUI CHEM INC
Non-Phosphorus composite anti incrustation eorrosion snhibiter and its application in water treatment
ActiveCN1785853AImprove performanceExcellent resistance to CaCO
<sub>3</sub>
Dirt performanceScale removal and water softeningMolybdatePolyaspartic acid
The present invention relates to a phosphorus-free composite antiincrustation corrosion inhibitor. It includes polyaspartic acid and / or polyepoxysuccinic acid and at least one kind of scale inhibition and dispersion agent, in which the scale inbibition and dispersion agent is polymer containing carboxylic acid group, or it includes polyaspartic acid and / or polyepoxysuccinic acid, zinc salt, molybdate or tungstate and polymer containing carboxylic acid group. Said invention is applicable to treatment of circulating cooling water.
Owner:BEIJING YANHUA PETRO CHEM
Abuse-resistant opioid solid dosage form
InactiveUS20060073102A1Prevent and discourage abuseGood curative effectOrganic active ingredientsPill deliveryN-methyl-D-aspartate Receptor AntagonistsOpioid abuse
The present invention pertains to a solid dosage form comprising an analgesically effective amount of opioid analgesic and an opioid abuse-deterring amount of a nontoxic N-methyl-D-aspartate receptor antagonist contained in a carrier which isolates, or separates, the antagonist from the opioid analgesic. The nontoxic N-methyl-D-aspartate receptor antagonist is released and made available only when the dosage form is misused, as would be the case when the dosage form is crushed or dissolved and thereafter administered in a manner other than that indicated, e.g., by injection or intranasally.
Owner:ENDO PHARMA INC
Gene encoding resistance to acetolactate synthase-inhibiting herbicides
InactiveUS20060130172A1Inhibit growthInhibition of reproductionTransferasesOther foreign material introduction processesAcetolactate synthaseSulfonylurea
A mutant acetolactate synthase (ALS) enzyme that confers cross-resistance to all sulfonylurea, imidazolinone, pyrimidinyloxybenzoate, triazolopyrimidine and sulfonylamino-carbonyl-triazolinone herbicides is provided. The mutant enzyme contains an aspartic acid to glutamic acid substitution mutation at a newly identified conserved region of the ALS enzyme. A gene encoding the enzyme is also provided, as are transgenic plants that have been genetically engineered to contain and express the gene. The transgenic plants are cross-resistant to sulfonylurea, imidazolinone, pyrimidinyloxybenzoate, triazolopyrimidine and sulfonylamino-carbonyl-triazolinone herbicides.
Owner:VIRGINIA TECH INTPROP INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com