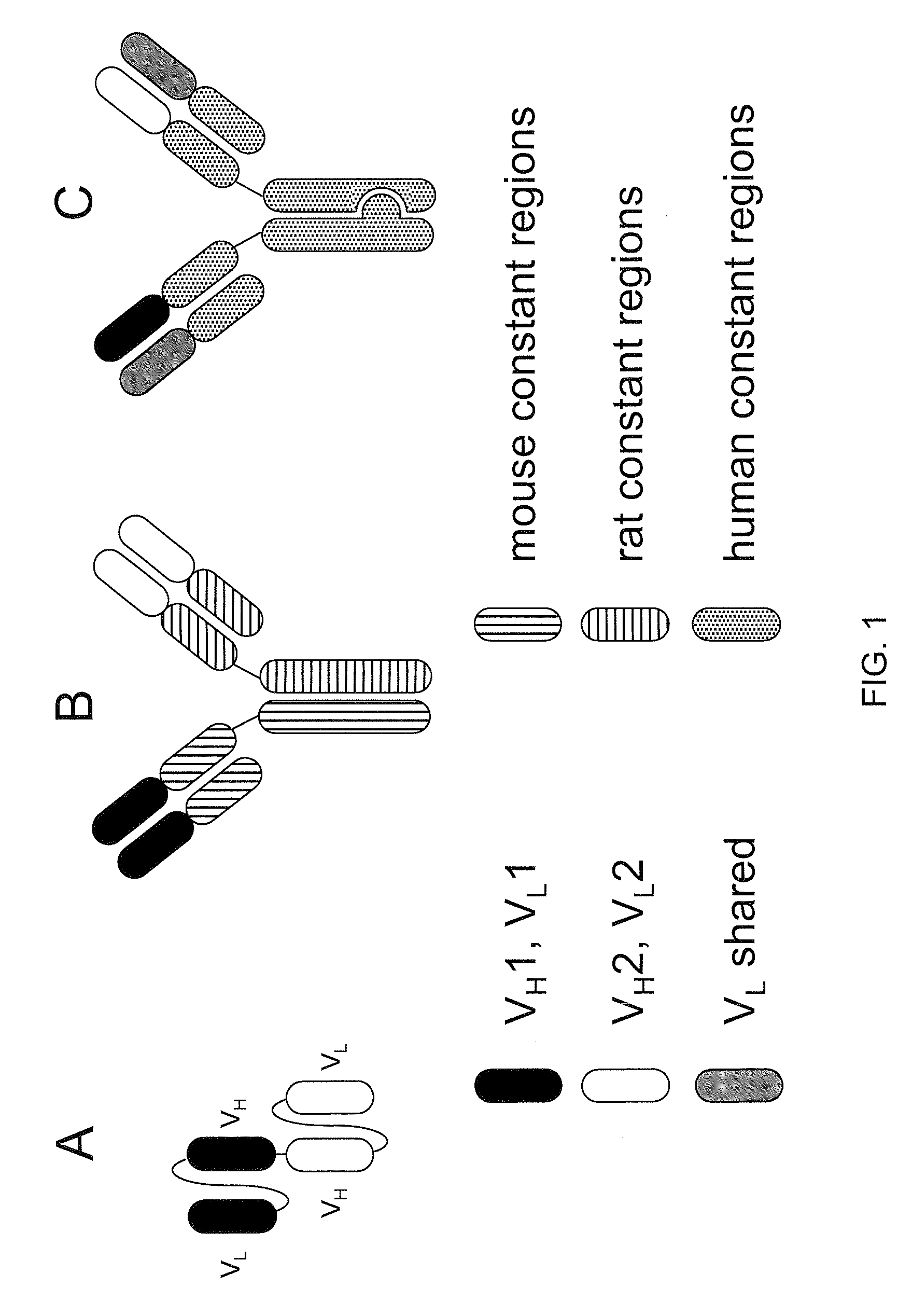
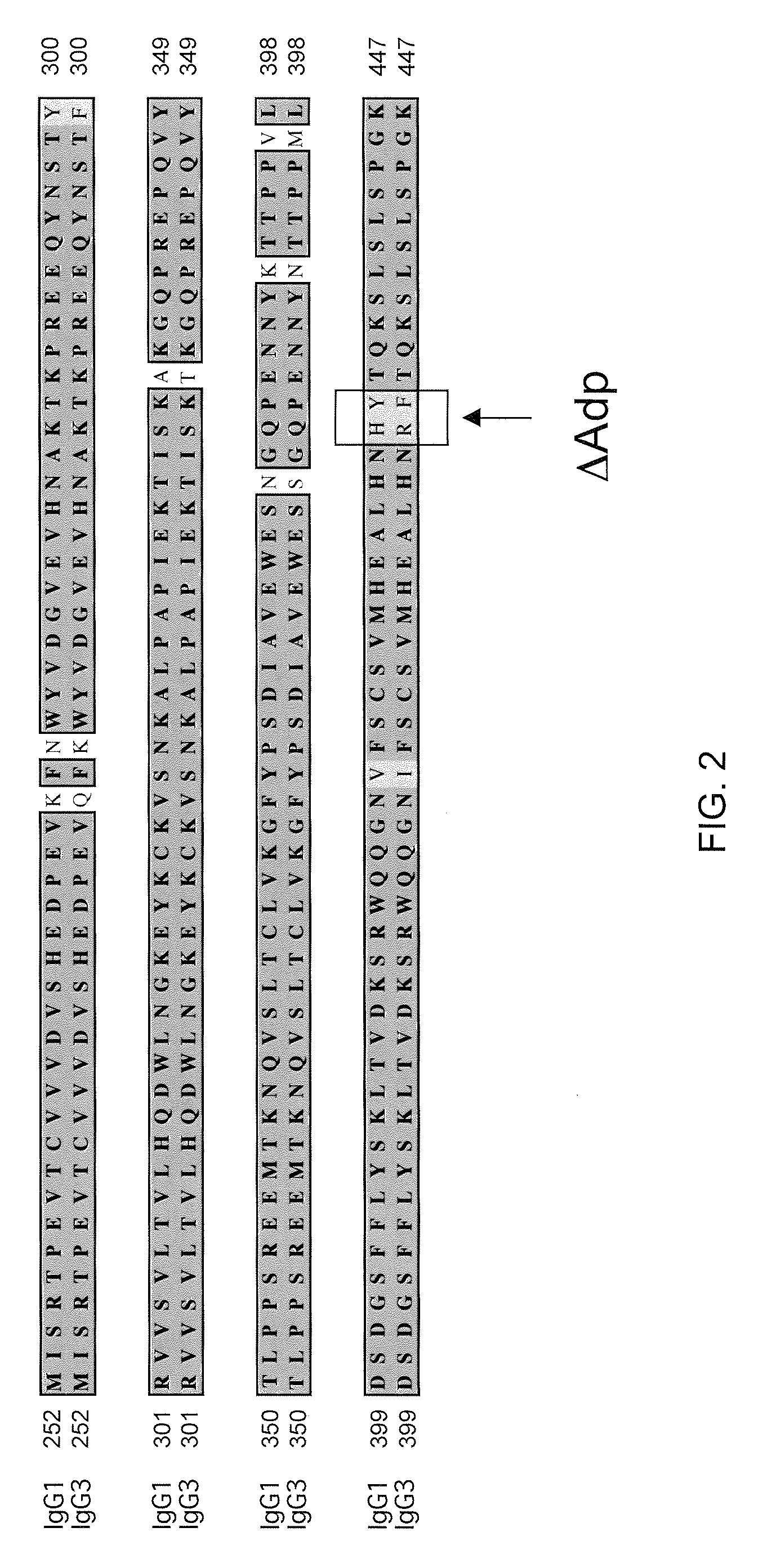
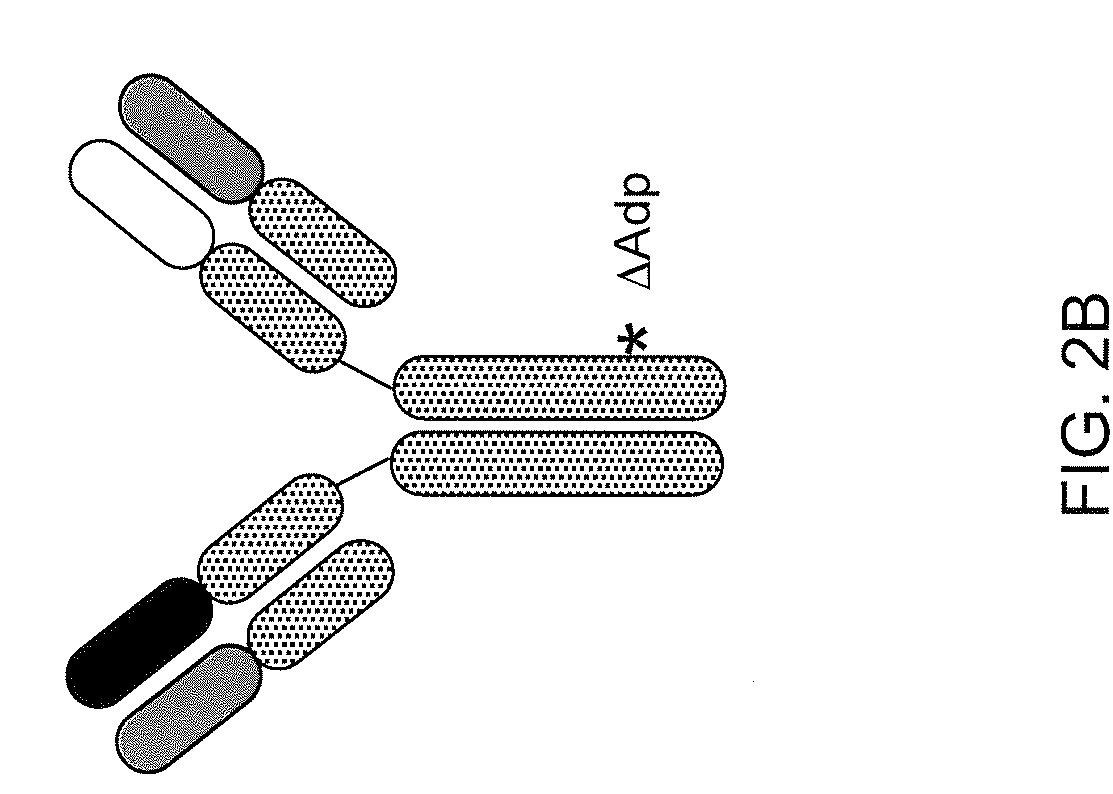
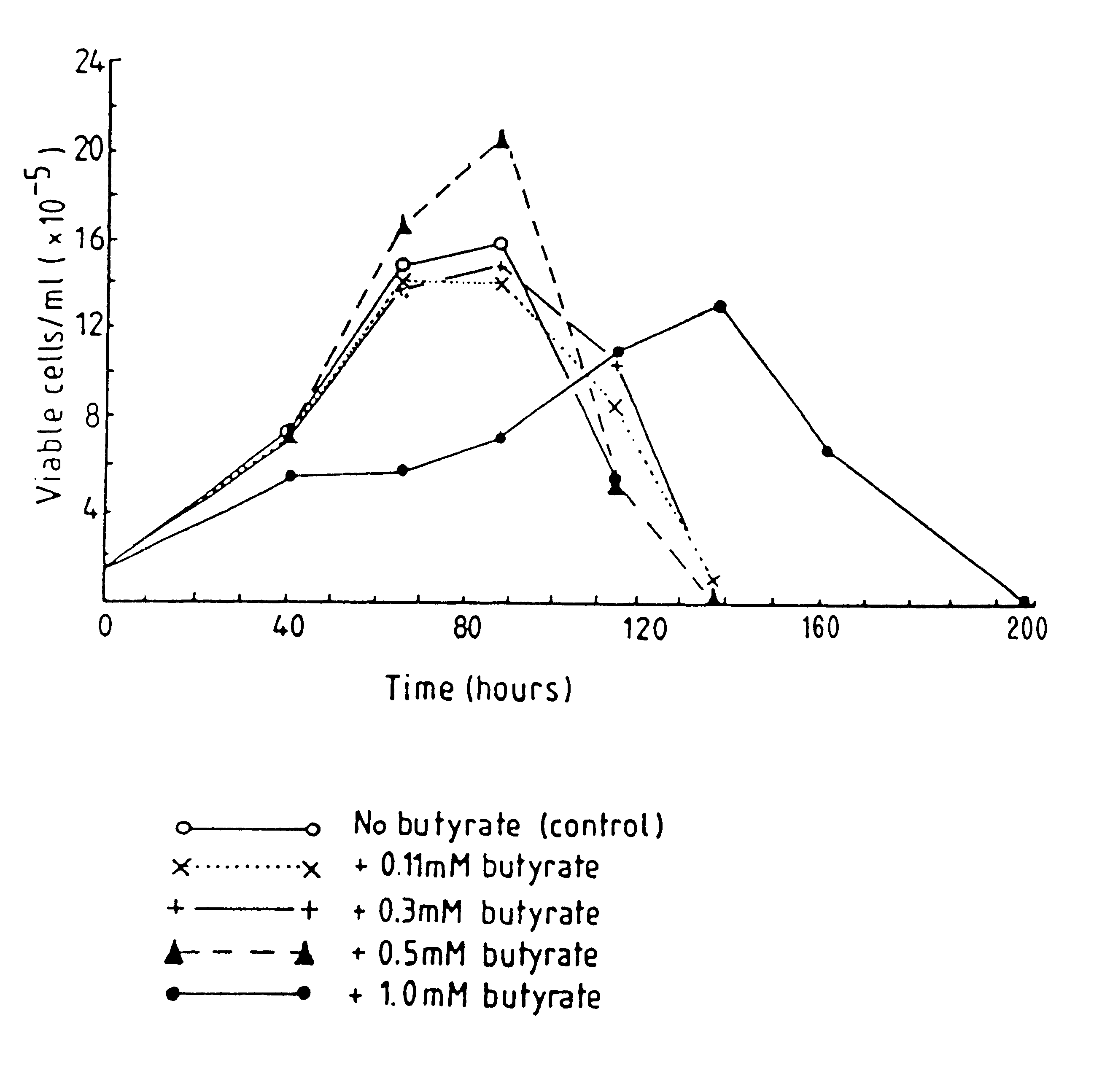
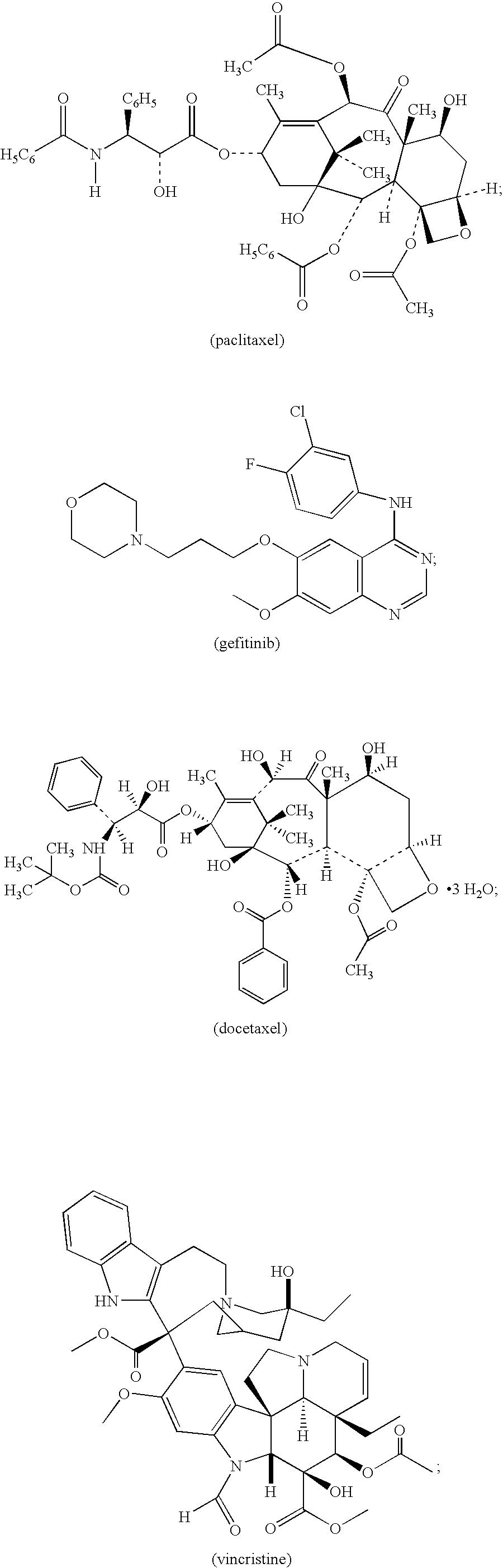
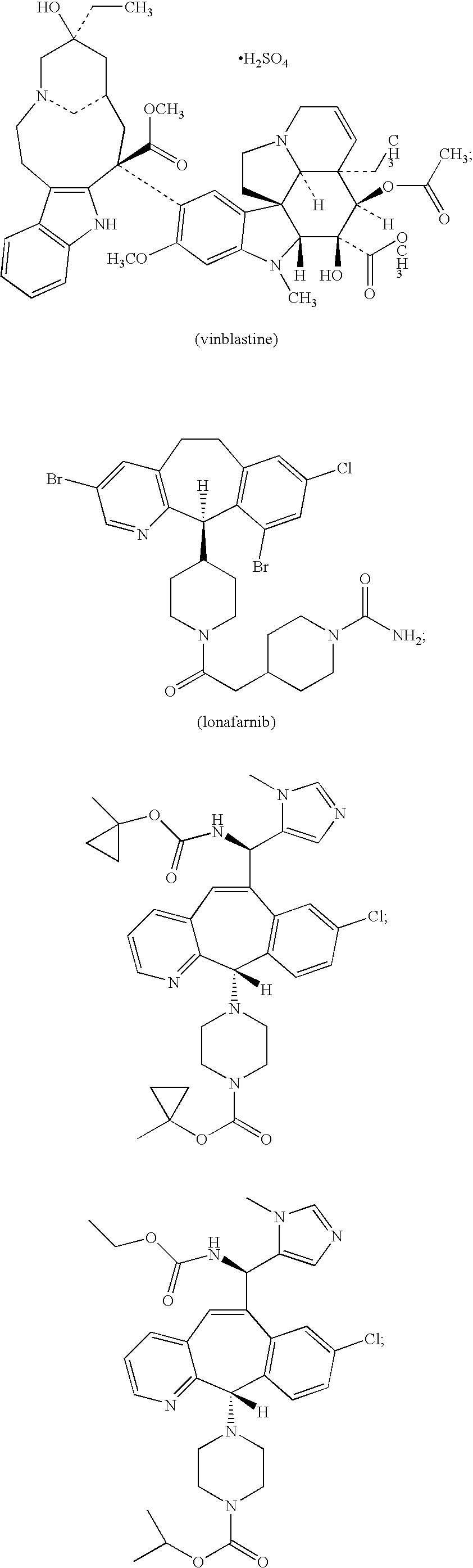
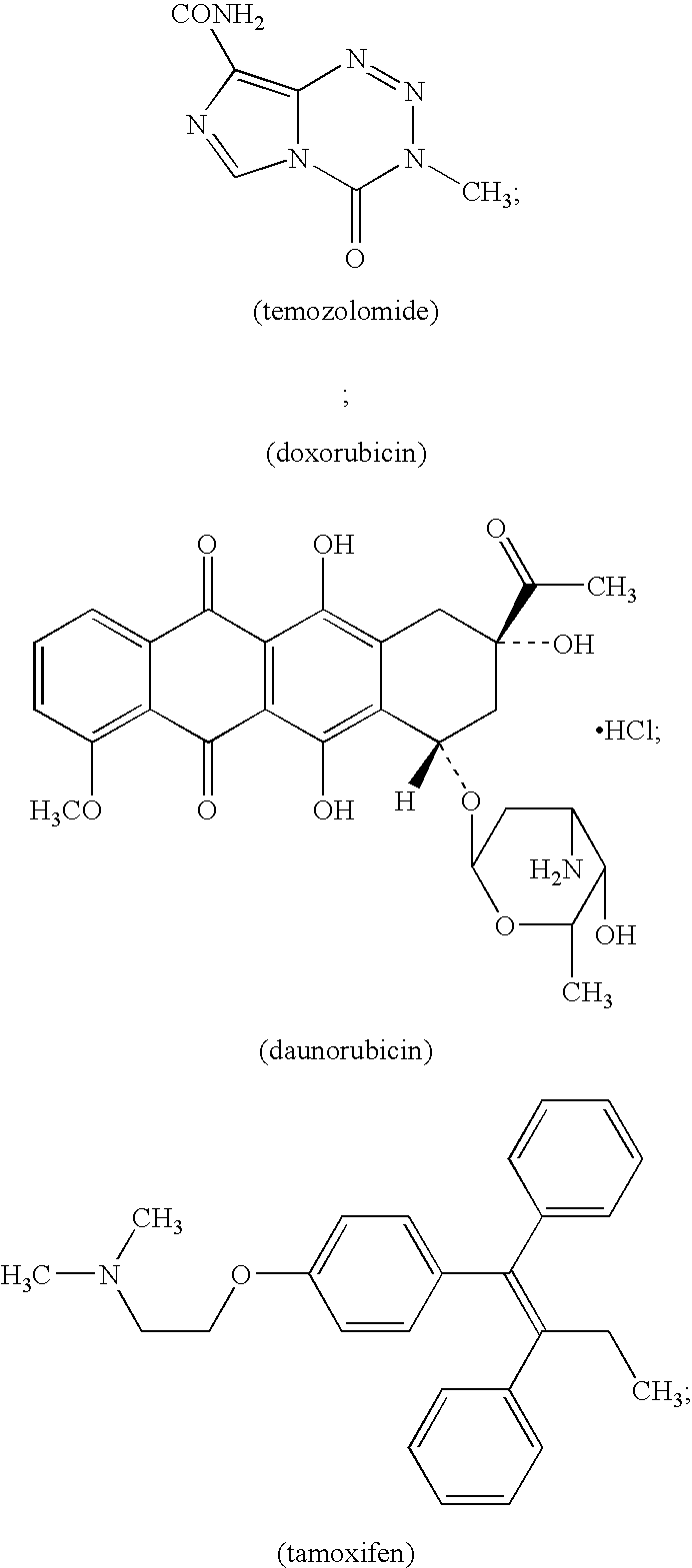
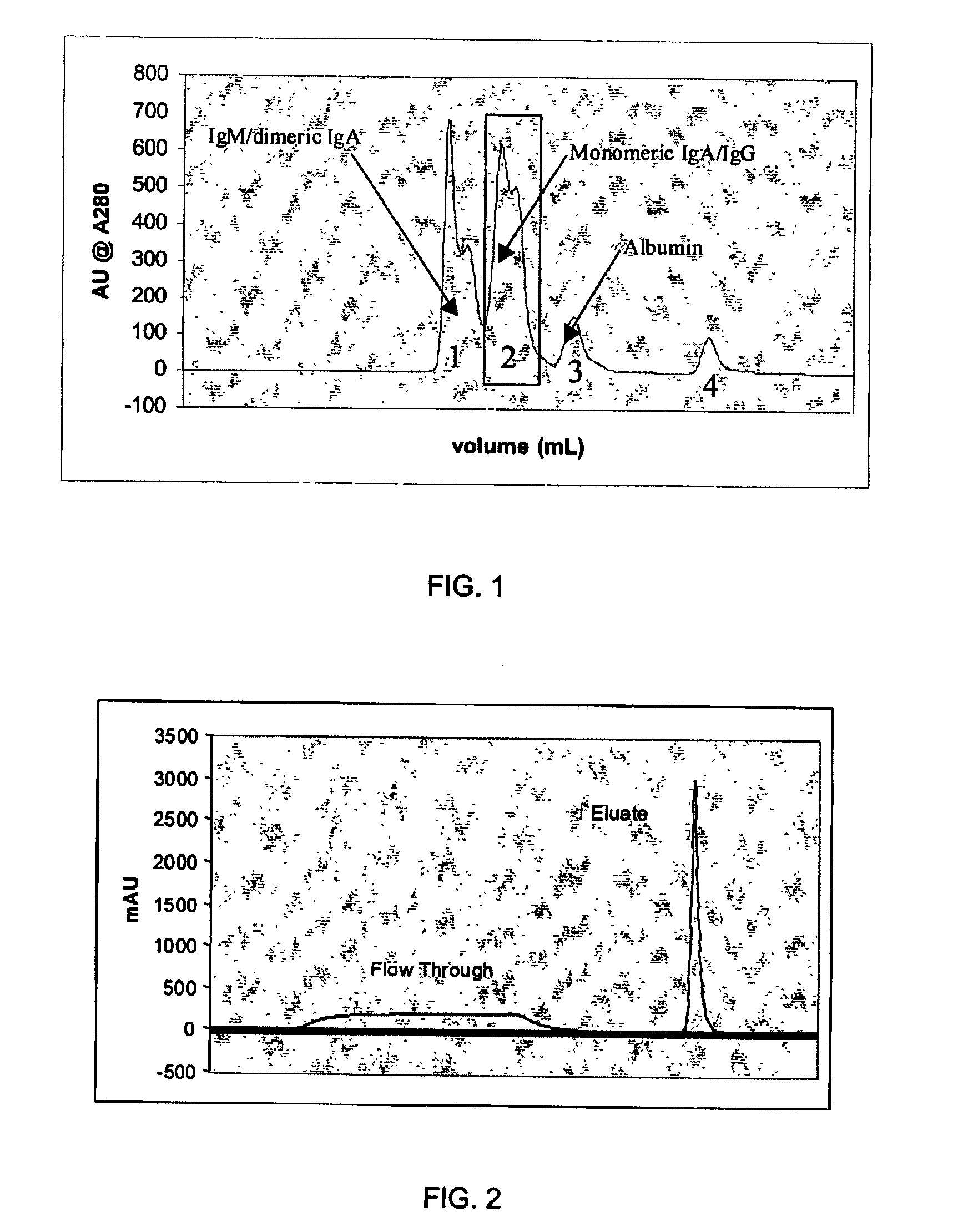
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1617results about "Serum immunoglobulins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
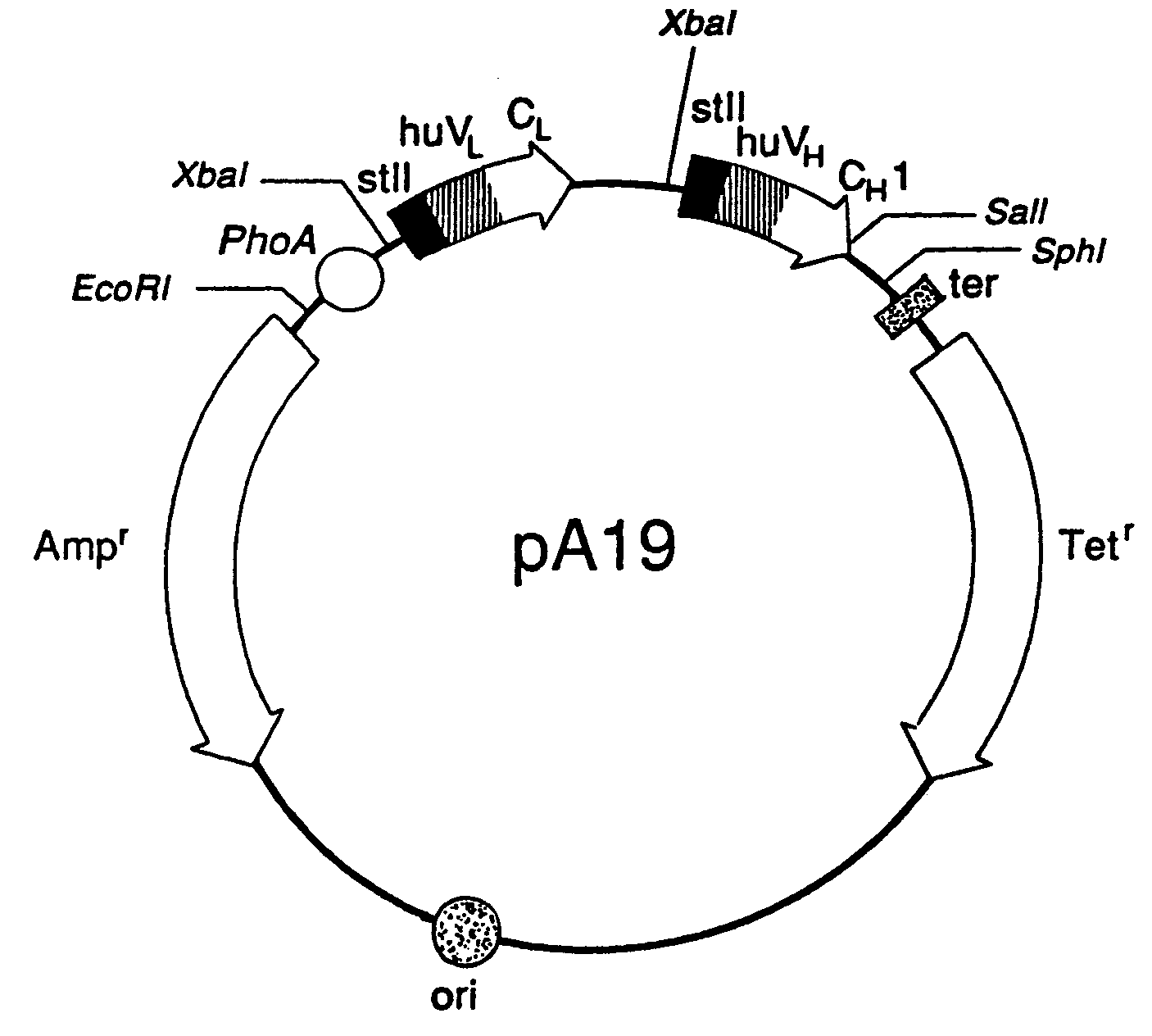
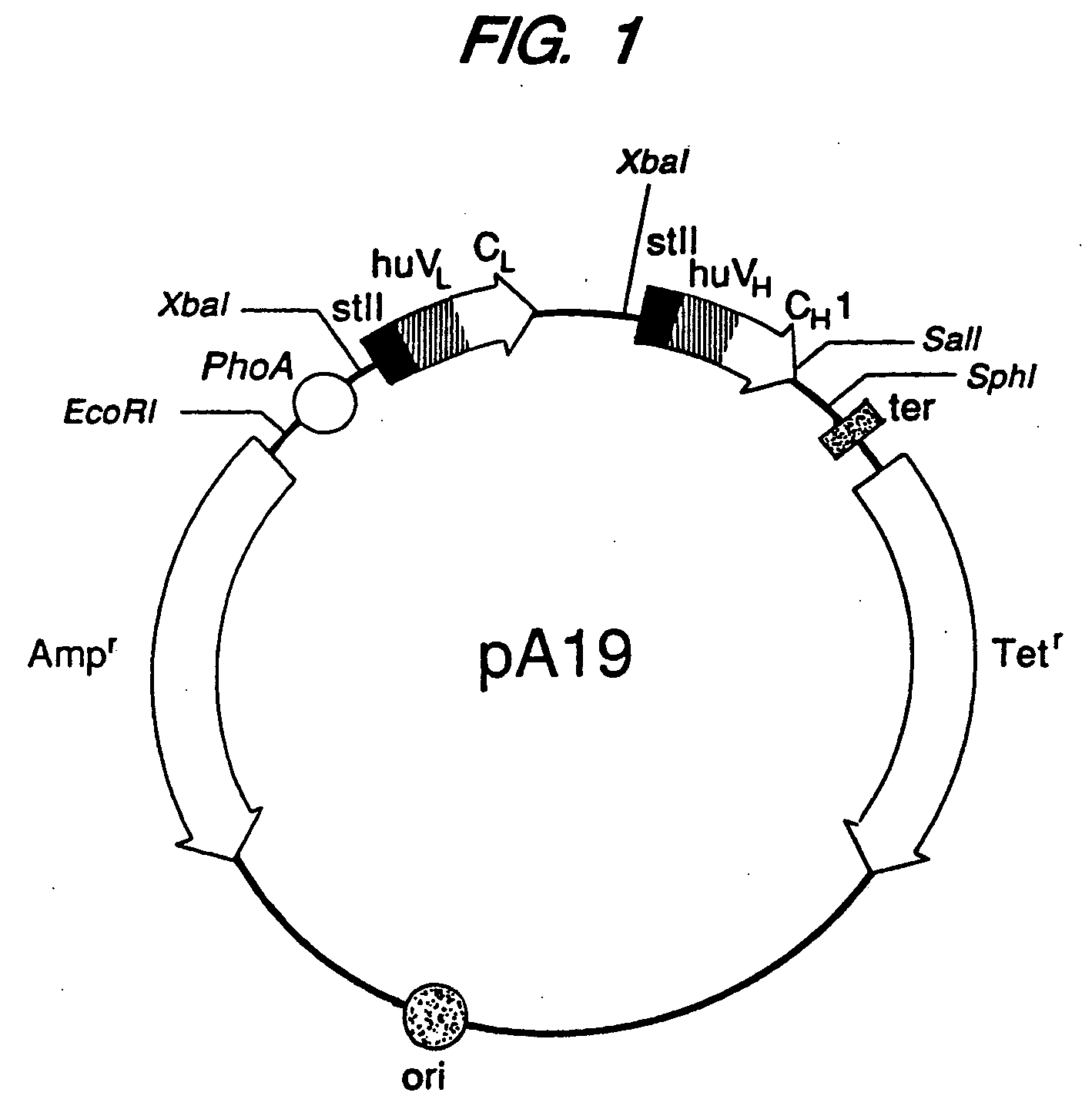
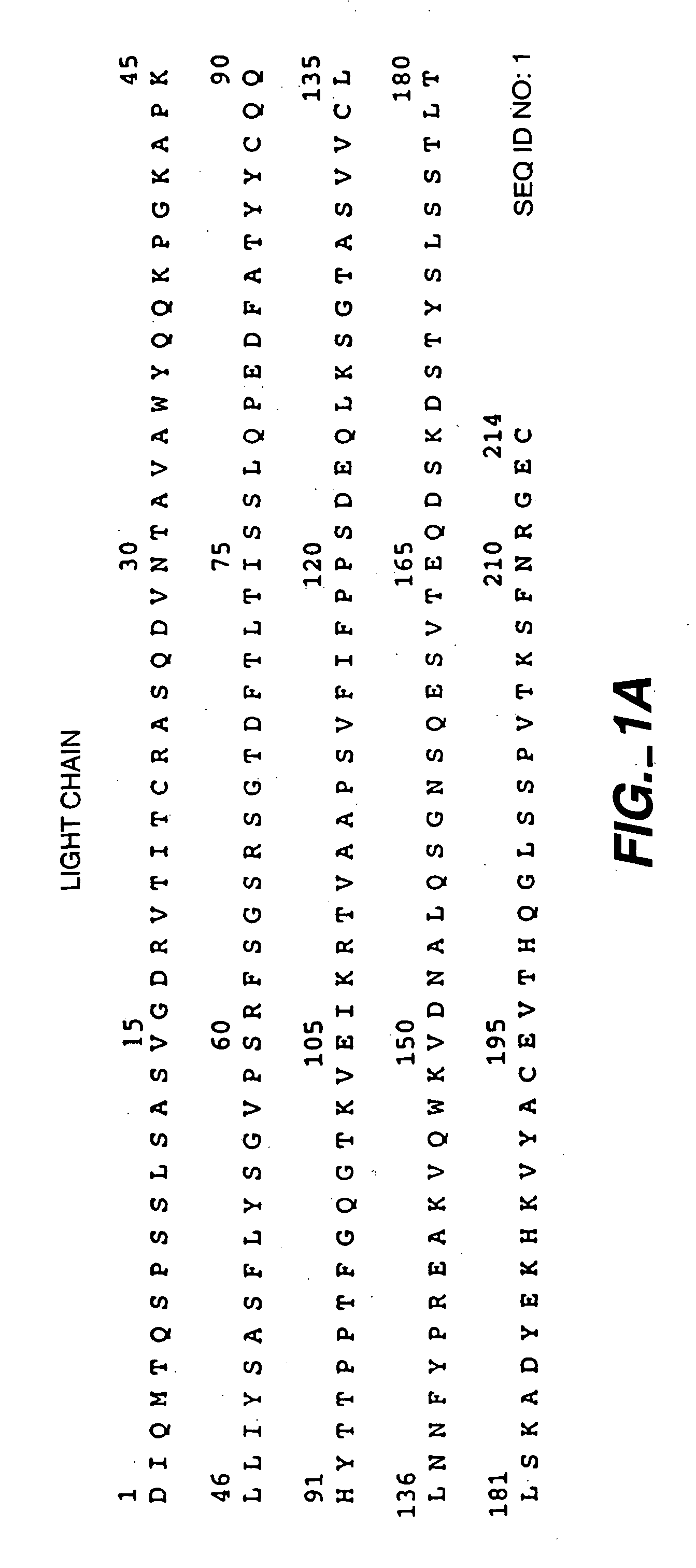
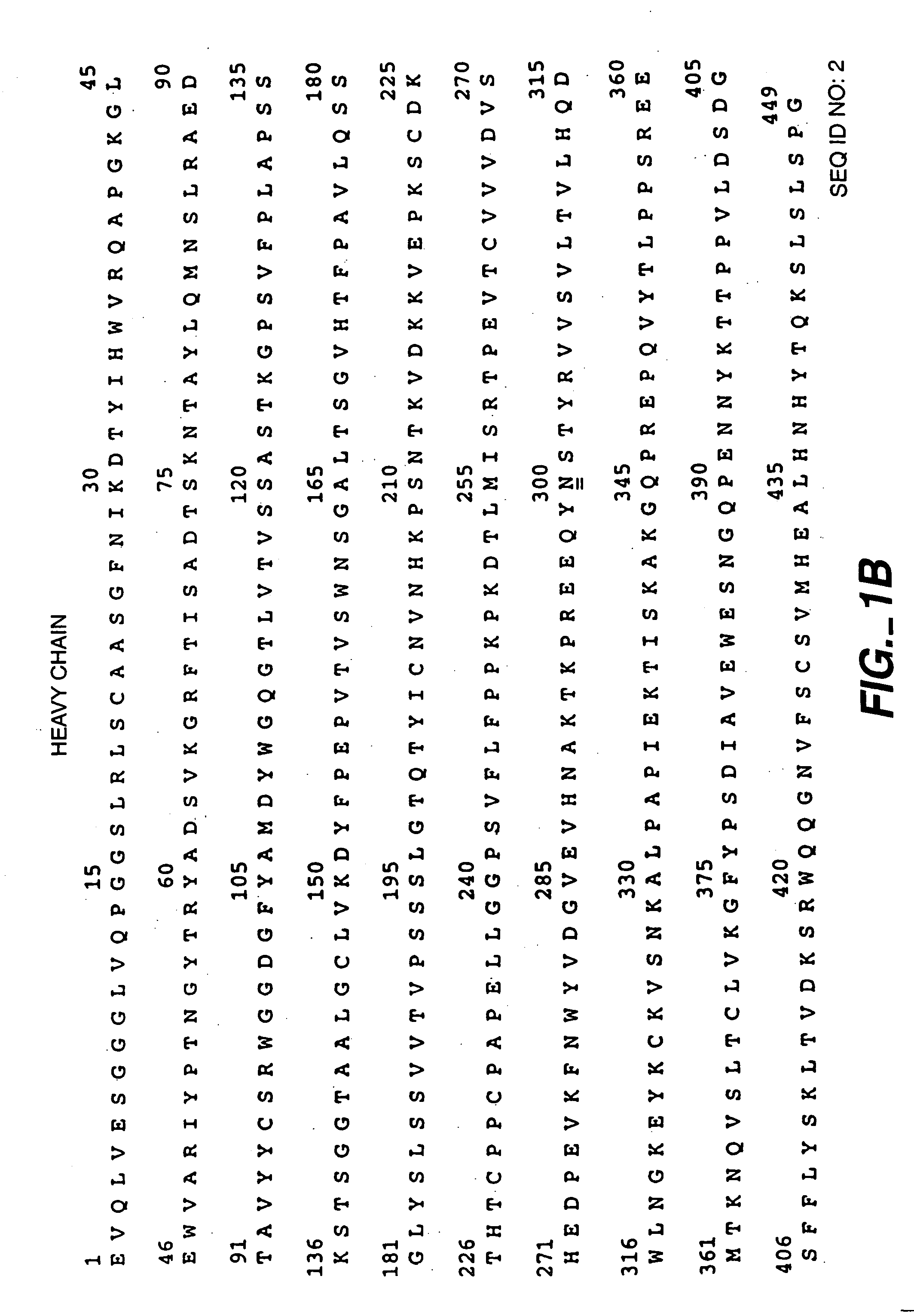
Readily Isolated Bispecific Antibodies with Native Immunoglobulin Format
ActiveUS20100331527A1Improve abilitiesReduces and eliminates bindingHybrid immunoglobulinsSerum immunoglobulinsImmunoglobulin heavy chainHeavy chain
A bispecific antibody format providing ease of isolation is provided, comprising immunoglobulin heavy chain variable domains that are differentially modified in the CH3 domain, wherein the differential modifications are non-immunogenic or substantially non-immunogenic with respect to the CH3 modifications, and at least one of the modifications results in a differential affinity for the bispecific antibody for an affinity reagent such as Protein A, and the bispecific antibody is isolable from a disrupted cell, from medium, or from a mixture of antibodies based on its affinity for Protein A.
Owner:REGENERON PHARM INC
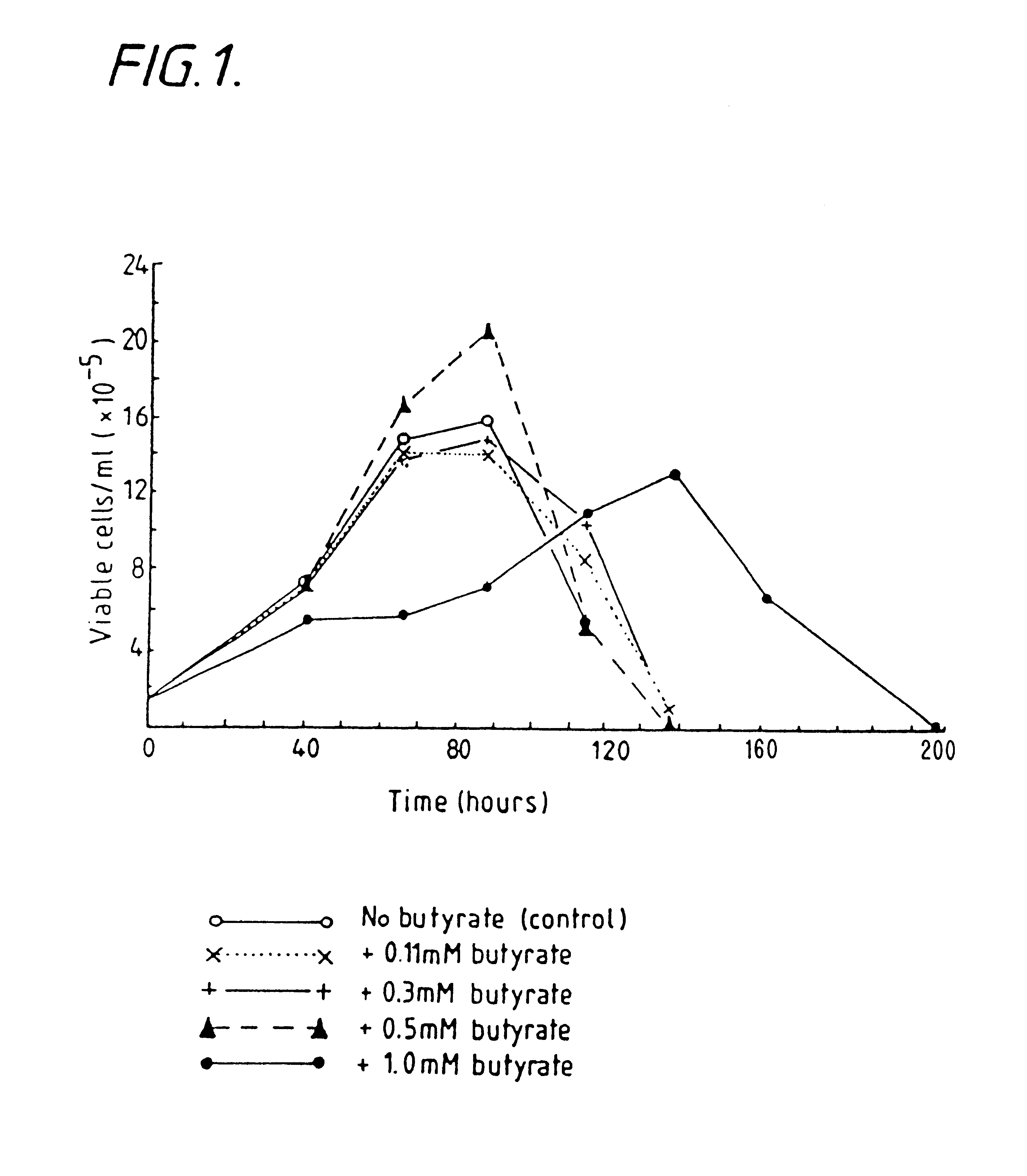
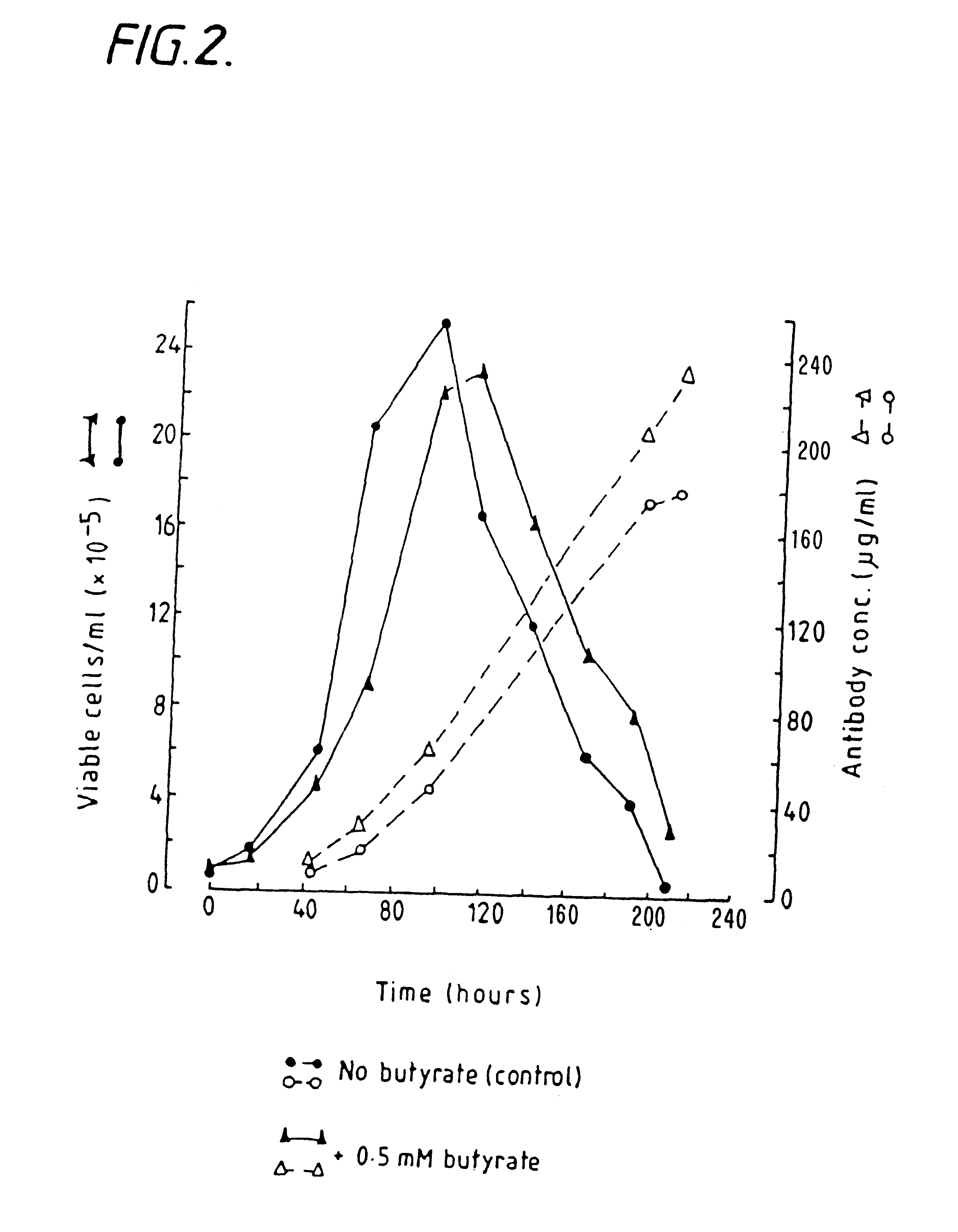
Production of proteins by cell culture
InactiveUS6413746B1High protein yieldReduce cell viabilityImmunoglobulins against blood group antigensPeptide/protein ingredients3D cell cultureBiochemistry
Methods for obtaining a protein by culture of hybridoma cells, wherein said protein is an immunoglobulin, are disclosed. The methods involve culturing animal hybridoma cells in continuous presence of an alkanoic acid or salt thereof, which enhances protein production, wherein said alkanoic acid or salt thereof is present at 2 concentration range of 0.1 mM to 200 mM.
Owner:LONZA LTD
IGG separation medium
A separation medium having a base matrix and matrix-bound groups which exhibit recombinant Protein A containing a cysteine. The groups are of formula:where B is a bridge which binds to the base matrix and X includes a heteroatom N or S from rProtein A-cys. In a preferred embodiment X is a thioether sulphur and / or a secondary amine (-NH-). An alternative embodiment features a variant of Protein A in which the C-terminal residue is cysteine.
Owner:GE HEALTHCARE BIOPROCESS R&D
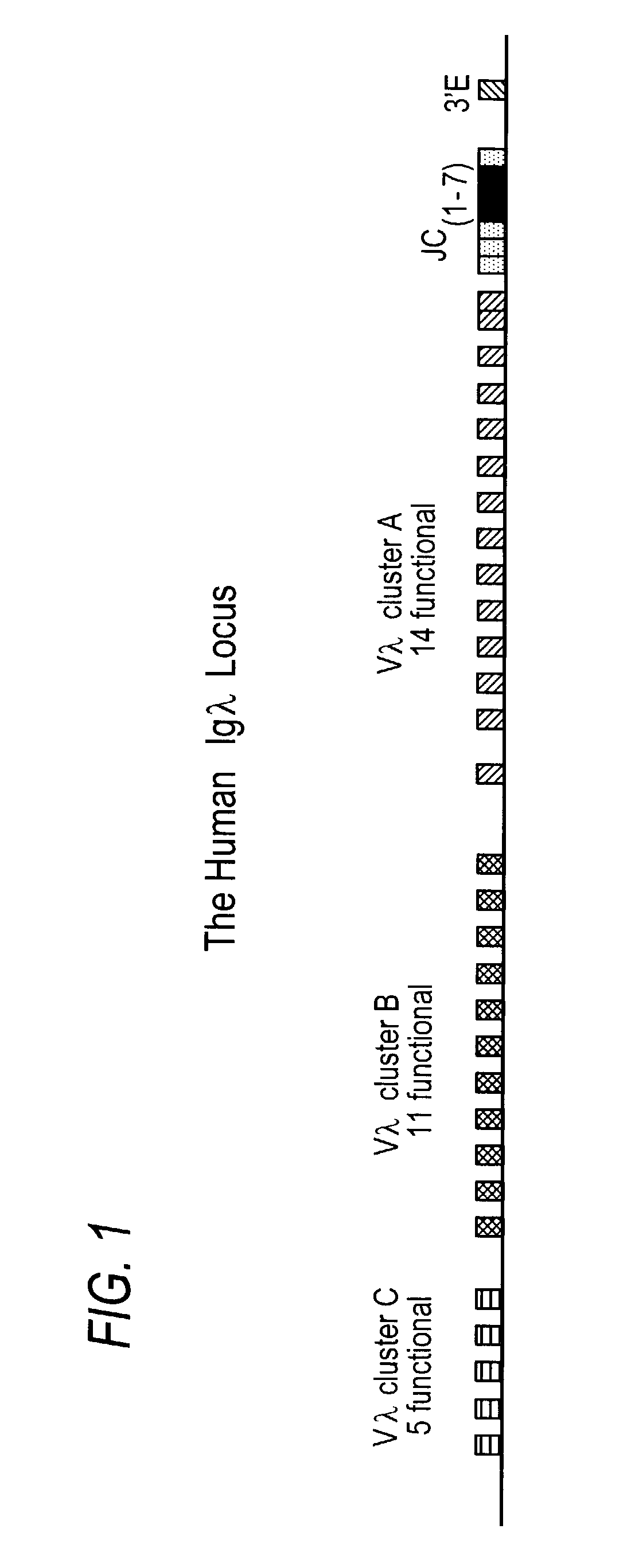
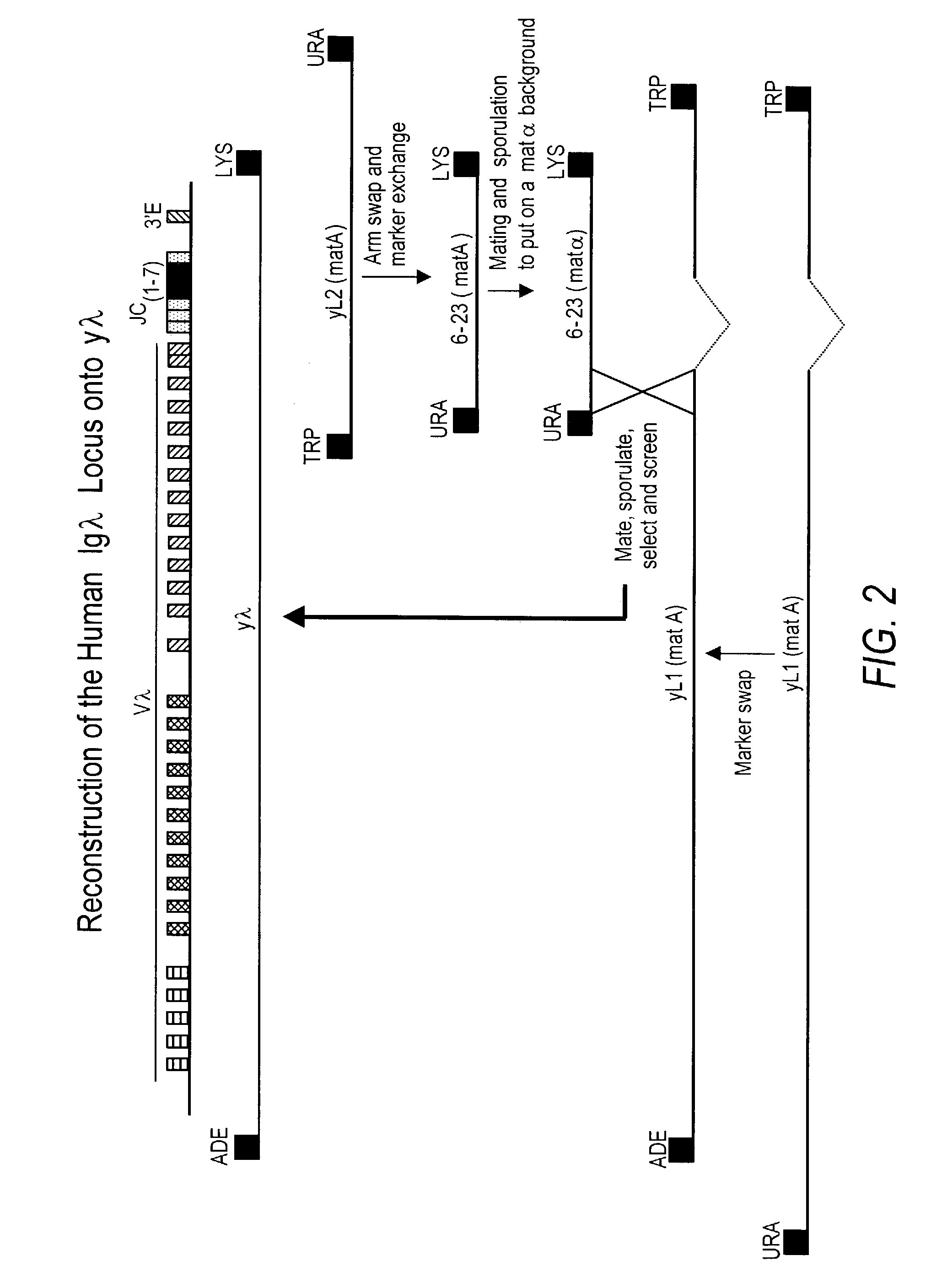
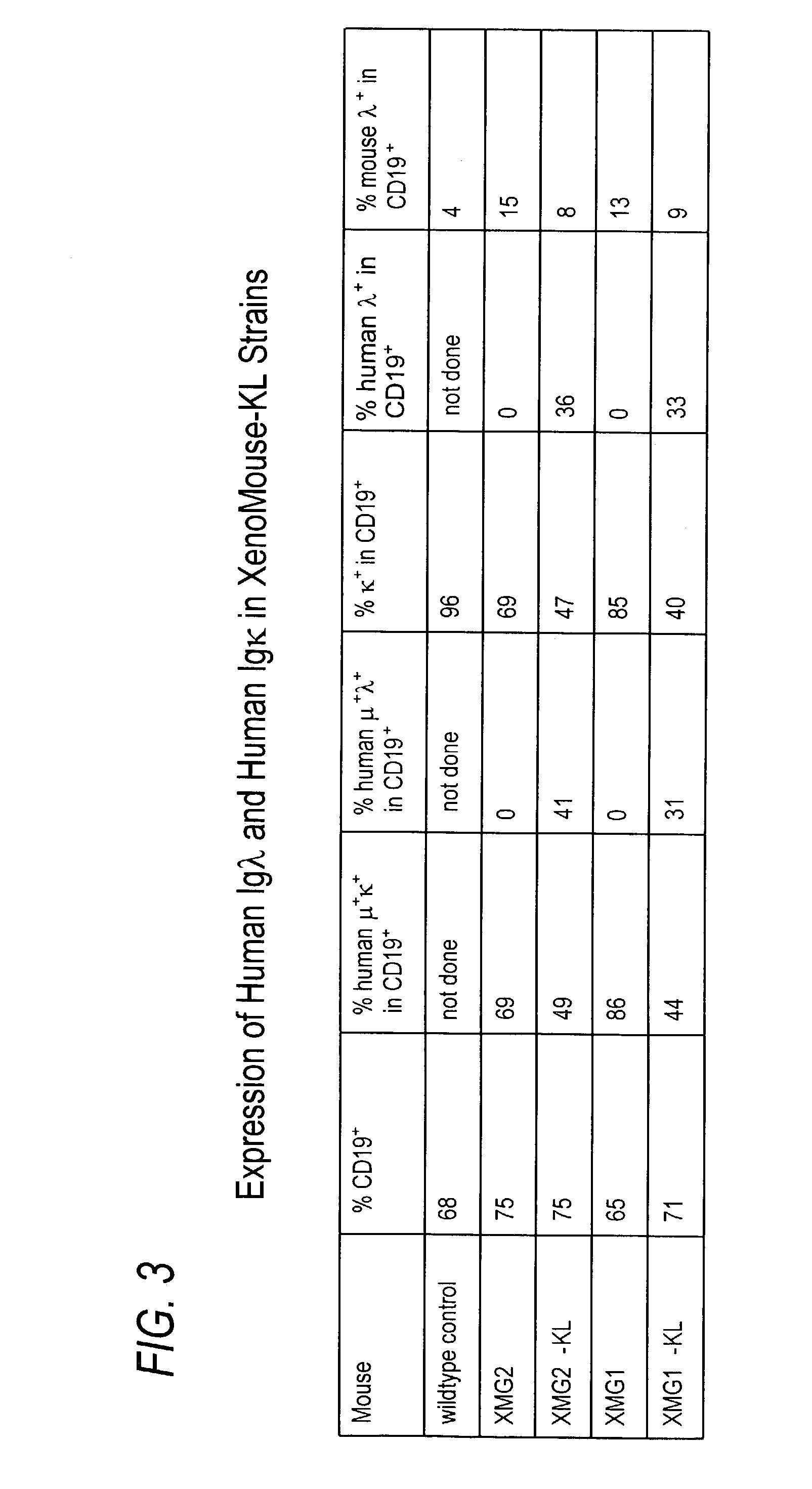
Transgenic animals bearing human Iglambda light chain genes
ActiveUS7435871B2Restore normal B-cell developmentAnimal cellsSerum immunoglobulinsPhysiologyLambda Light Chain Gene
Owner:AMGEN FREMONT INC
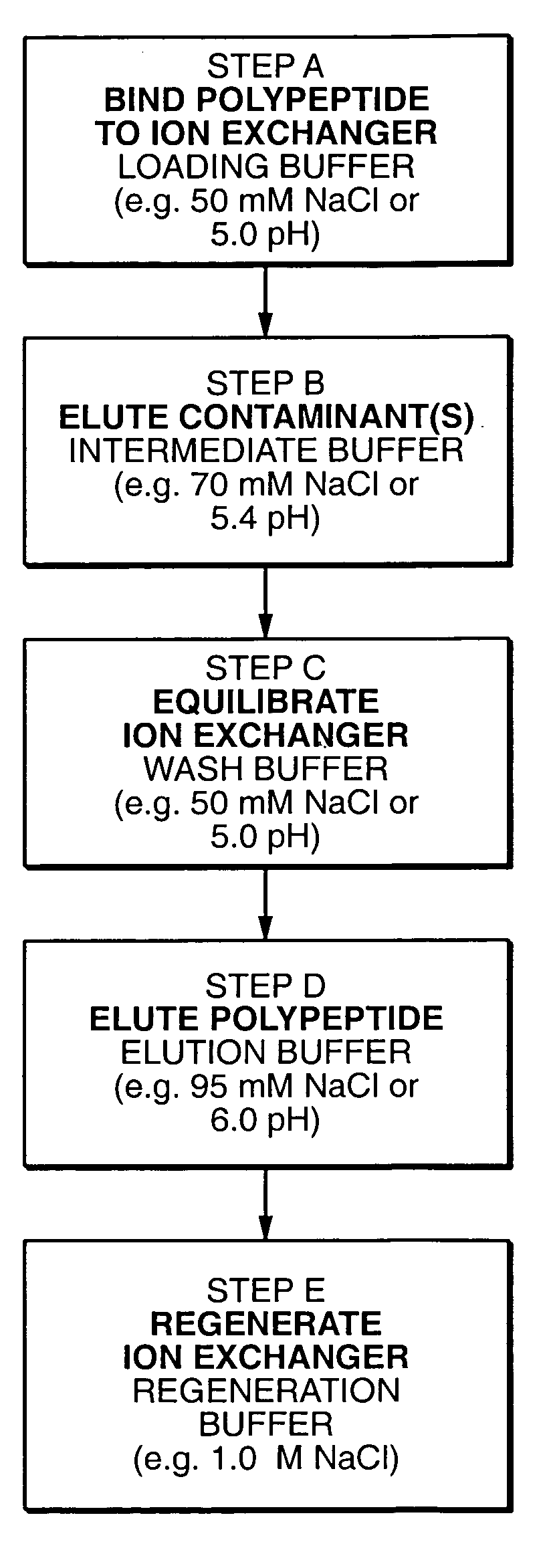
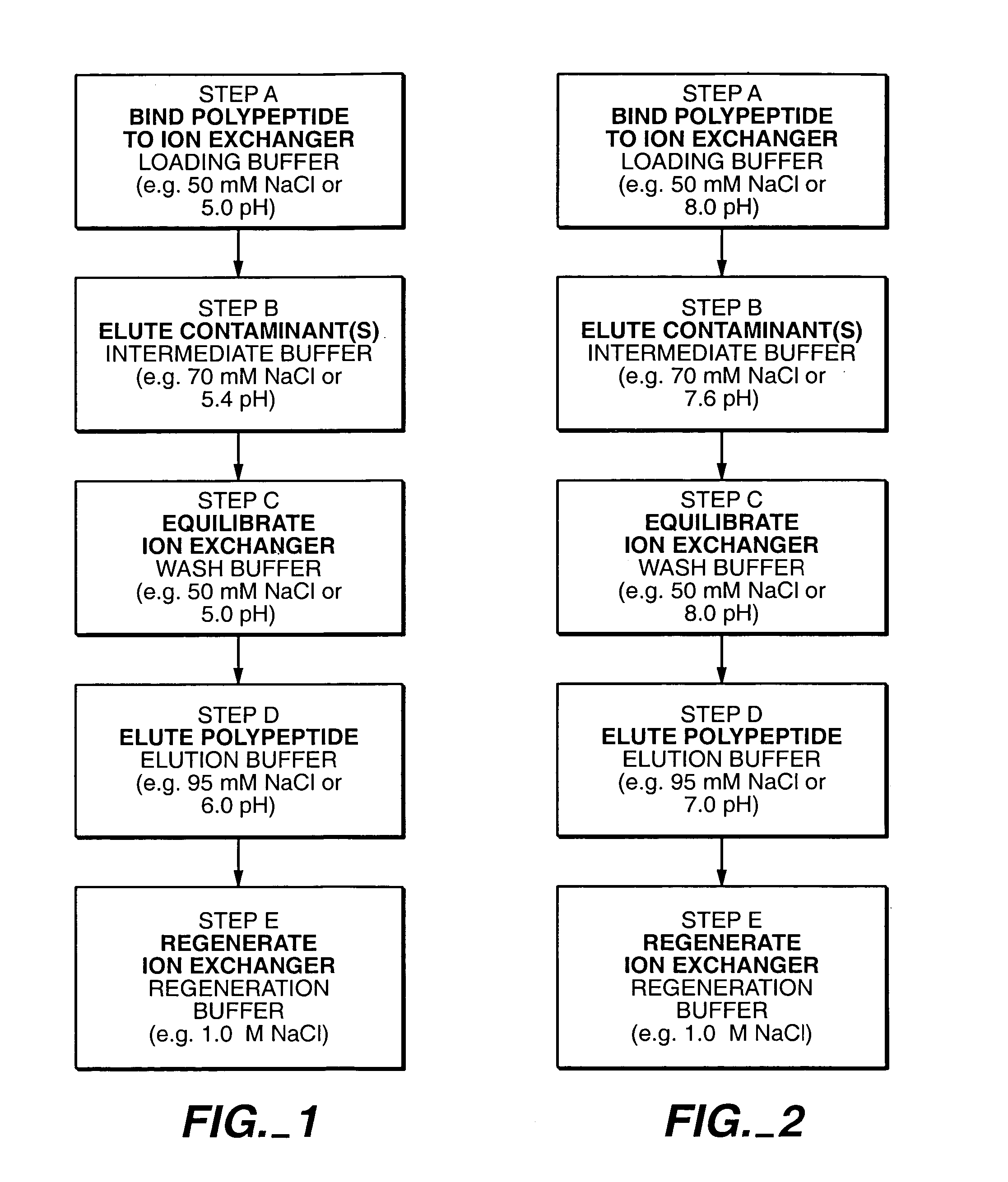
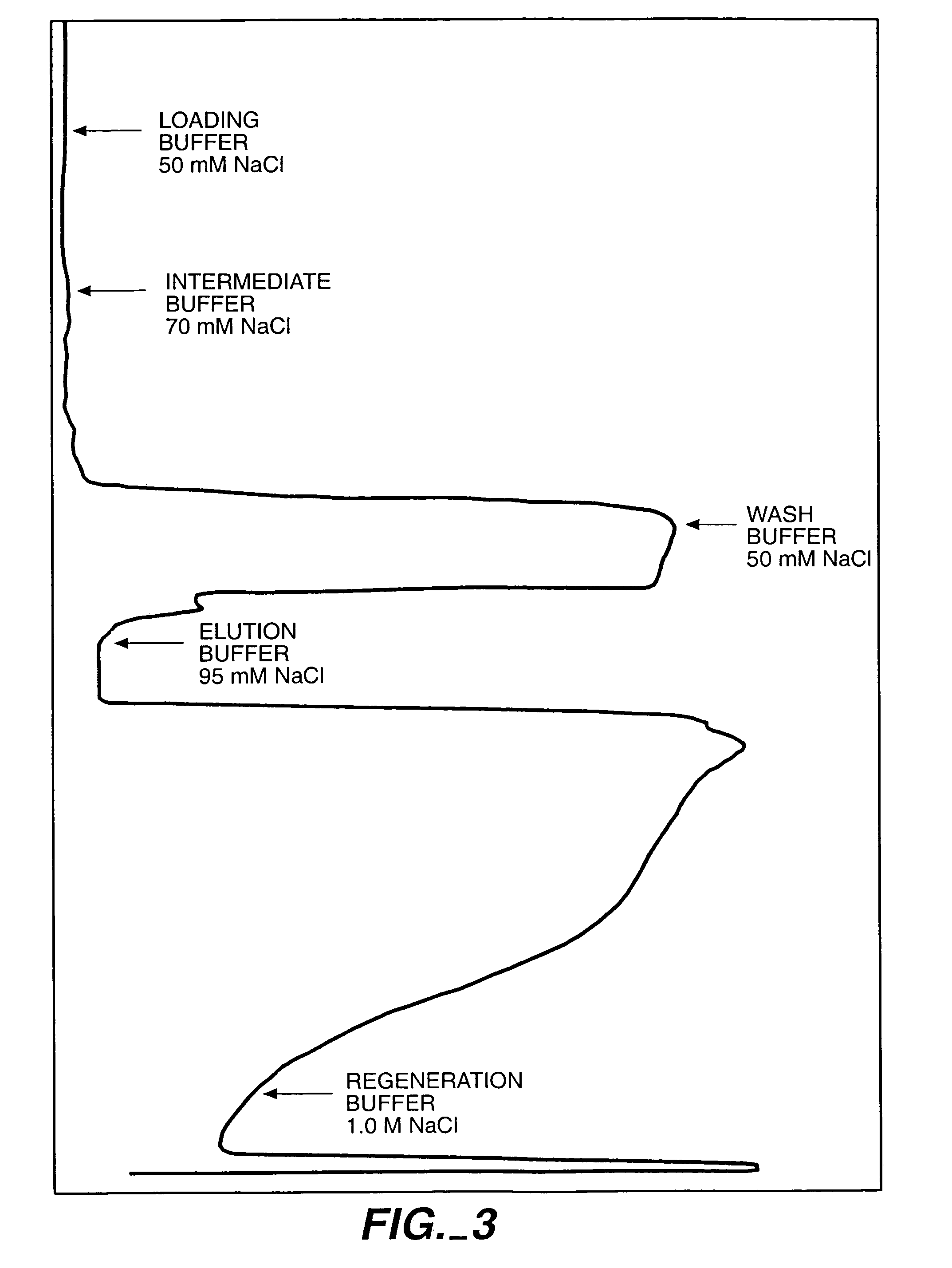
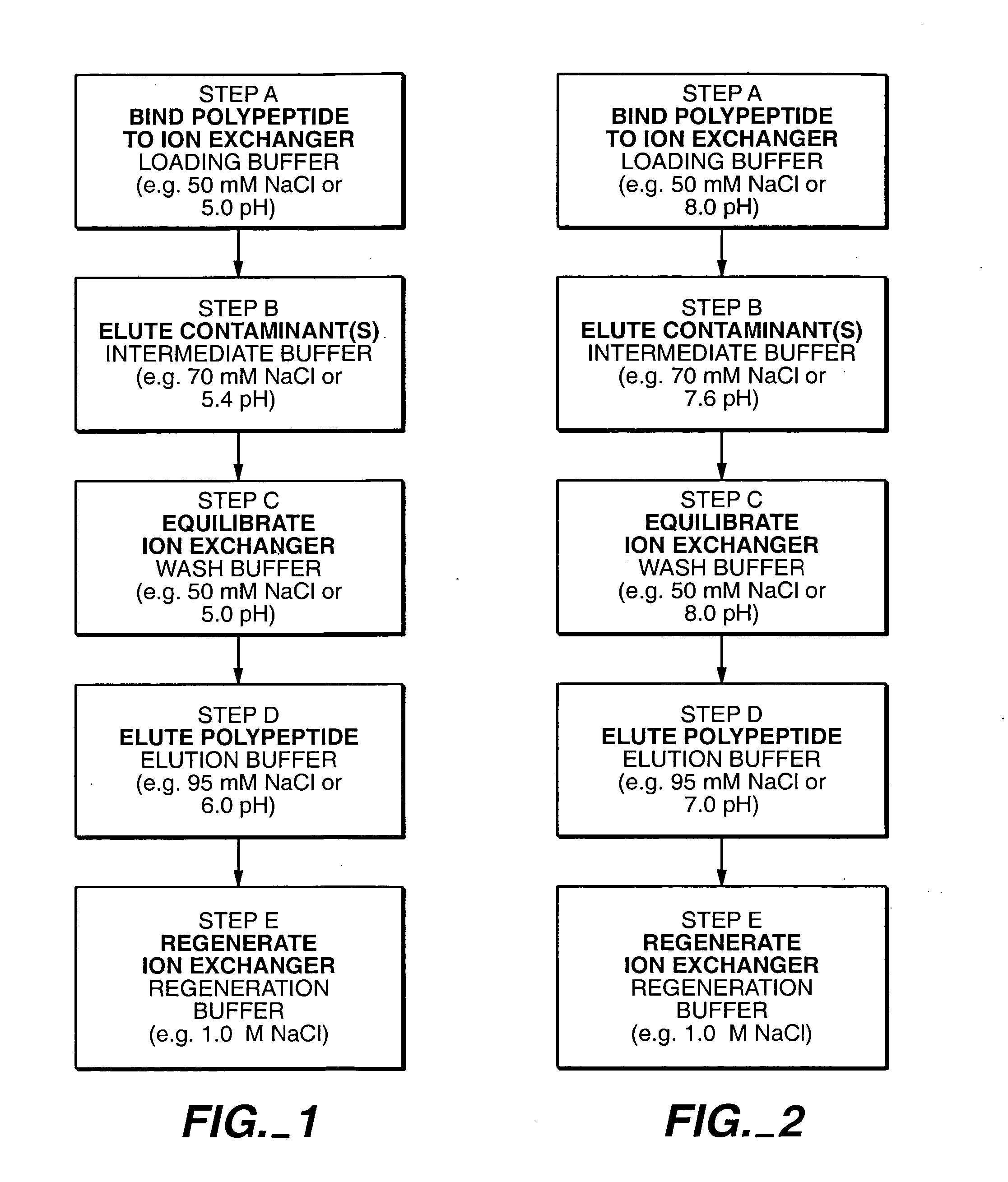
Protein purification
InactiveUS7074404B2High recovery ratePeptide/protein ingredientsSerum immunoglobulinsIon chromatographyMulti pollutant
A method for purifying a polypeptide by ion exchange chromatography is described which involves changing the conductivity and / or pH of buffers in order to resolve a polypeptide of interest from one or more contaminants.
Owner:GENENTECH INC
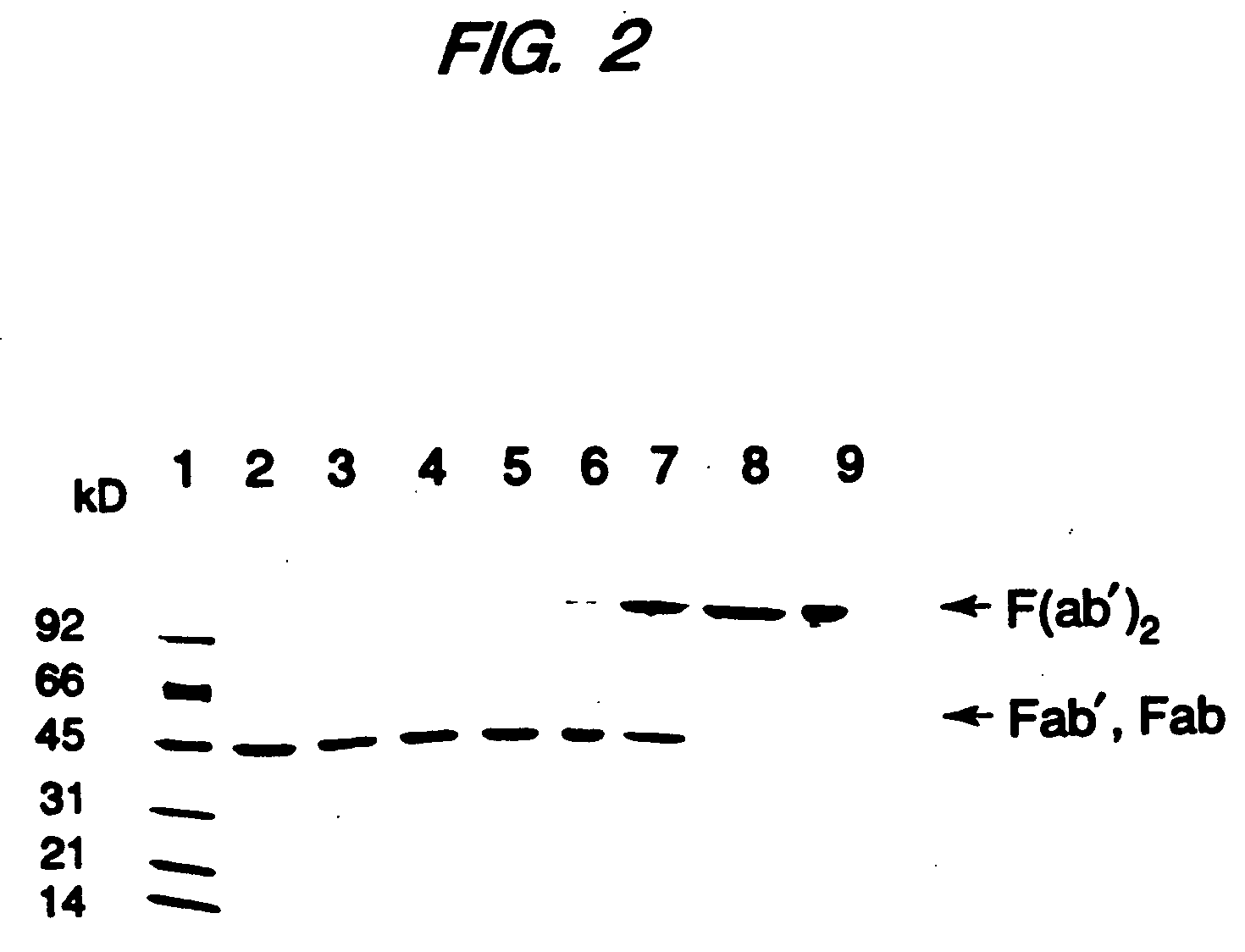
Expression of functional antibody fragments
InactiveUS7018809B1Easy to prepareFacilitated releaseHybrid immunoglobulinsSerum immunoglobulinsMicroorganismHeavy chain
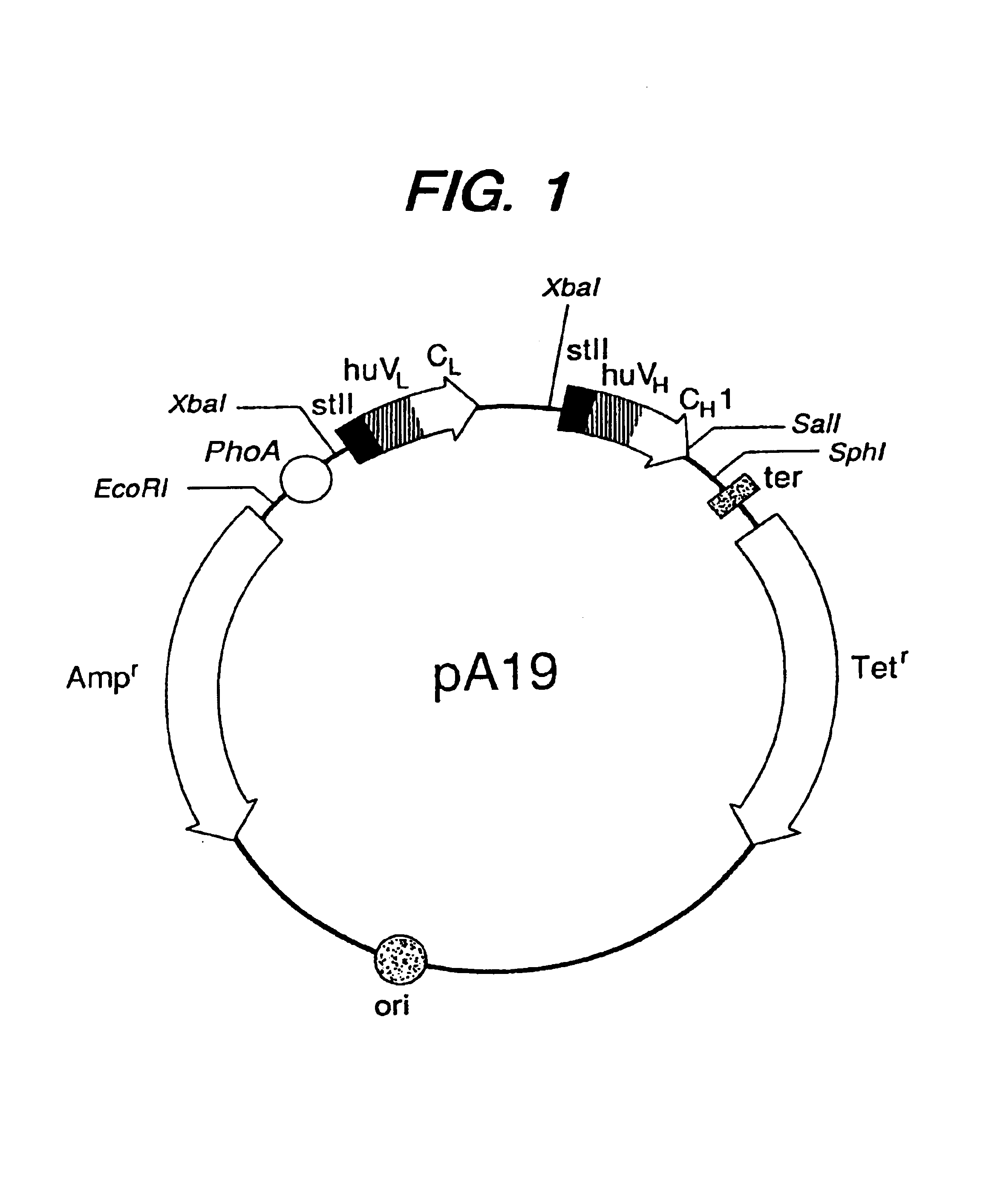
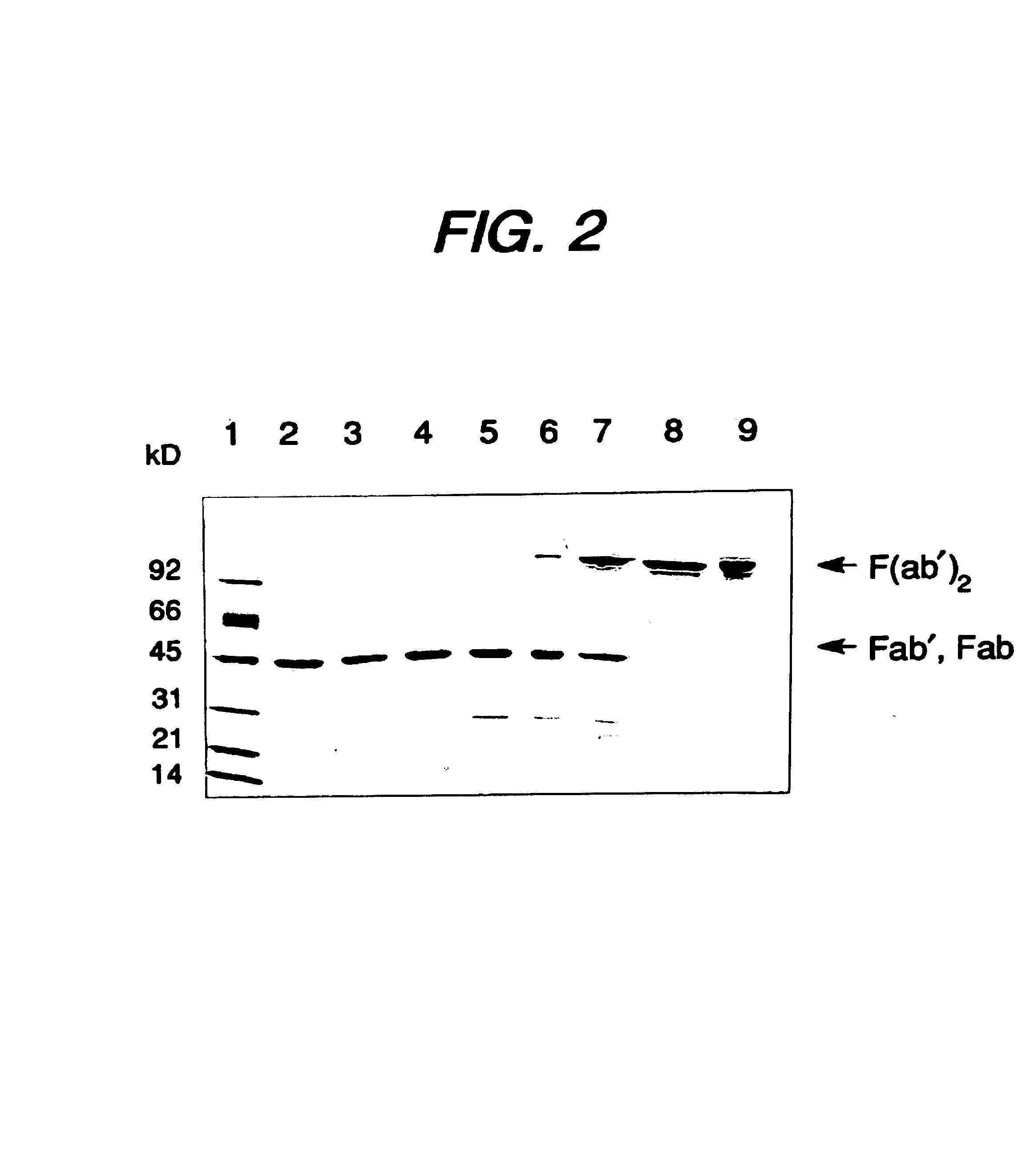
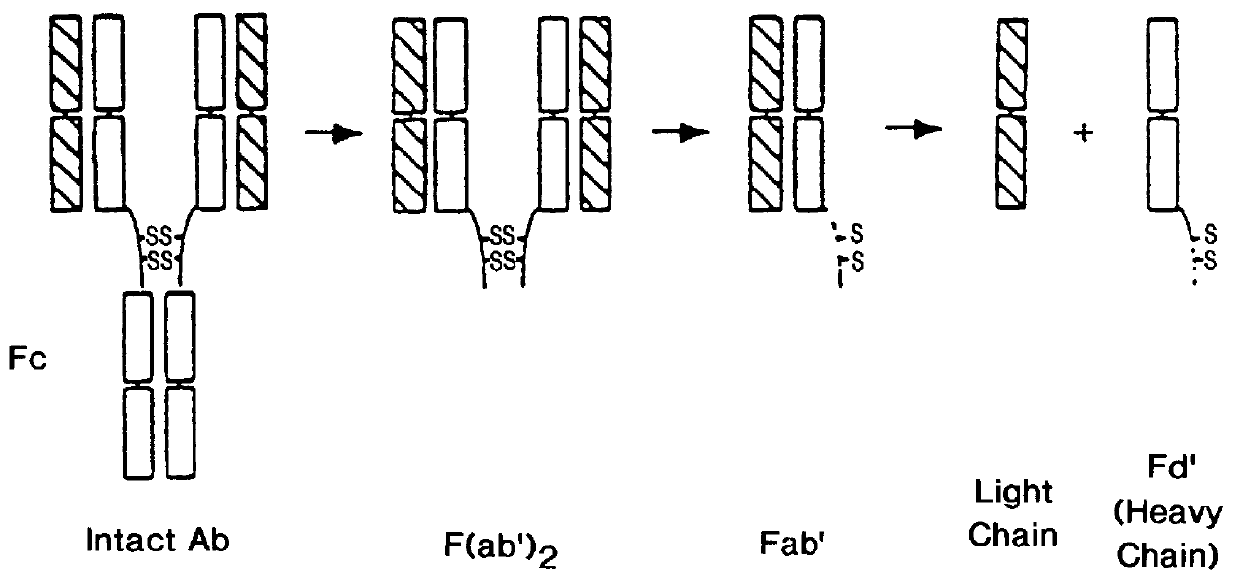
Methods for the high yield production of antibody Fv-containing polypeptides, especially Fab′ and F(ab′)2 antibody fragments are provided. Expression of heavy and light chain Fv in a microbial secretory system is followed by recovery of Fv from the periplasm under conditions that maintain a cysteine residue as a free thiol. The free thiol is reacted with free thiol of an antibody fragment of the same or differing specificity, or with agents such as diagnostic labels or therapeutic moieties. The products offer advantages of homogeneity and purity not available through the use of known methods for preparing such derivatives.
Owner:GENENTECH INC
Process for purifying antibody
InactiveUS7064191B2High ADCC activityHigh puritySerum immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAntigen binding
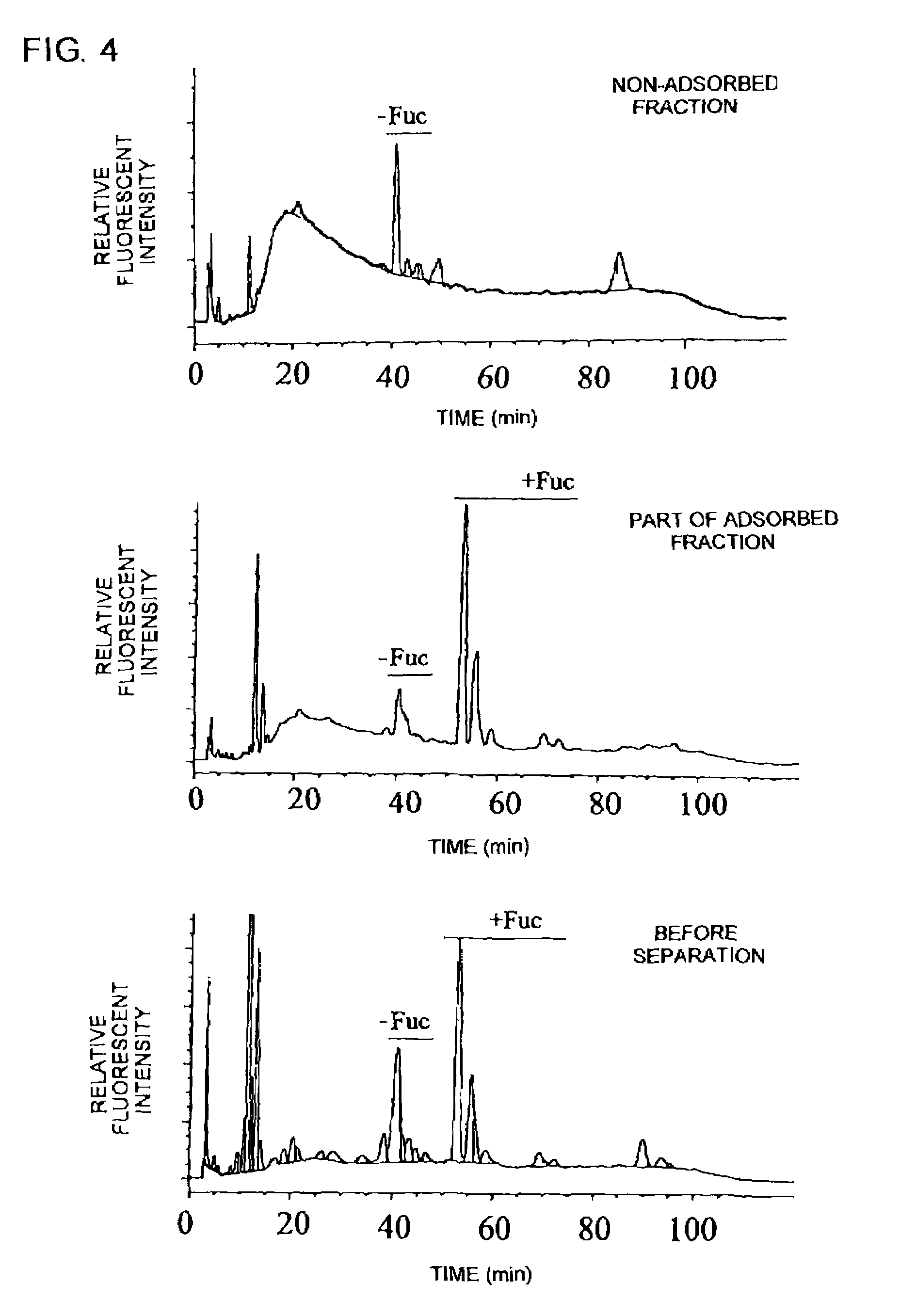
The present invention relates to a process for purifying an antibody having a desired property, which comprises using a substance having an affinity to a carbohydrate binding to the antibody; a medicament comprising, as an active ingredient, the antibody purified by the process; and a method for diagnosing or preventing various diseases, which comprises using a substance having an affinity to a carbohydrate binding to an antibody.
Owner:KYOWA HAKKO KIRIN CO LTD
Mutant protein
InactiveUS20060194950A1Improve stabilityIncreased pH-valuesBacteriaSerum immunoglobulinsMutated proteinComplementarity determining region
Owner:GE HEALTHCARE BIO SCI CORP
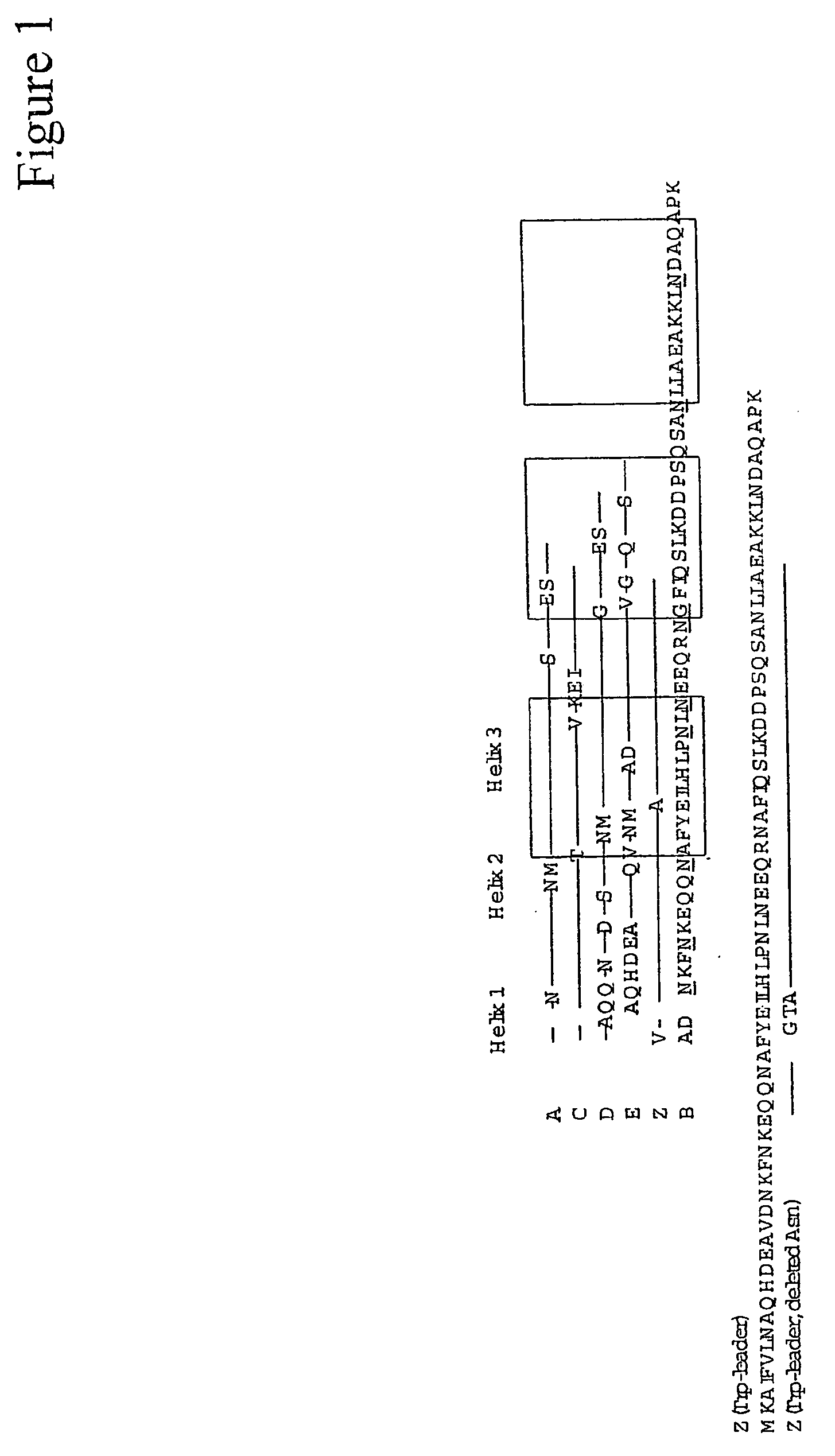
Mutated immunoglobulin-binding protein
ActiveUS20050143566A1Improve stabilityIncreased pH-valuesSerum immunoglobulinsComponent separationComplementarity determining regionChemical stability
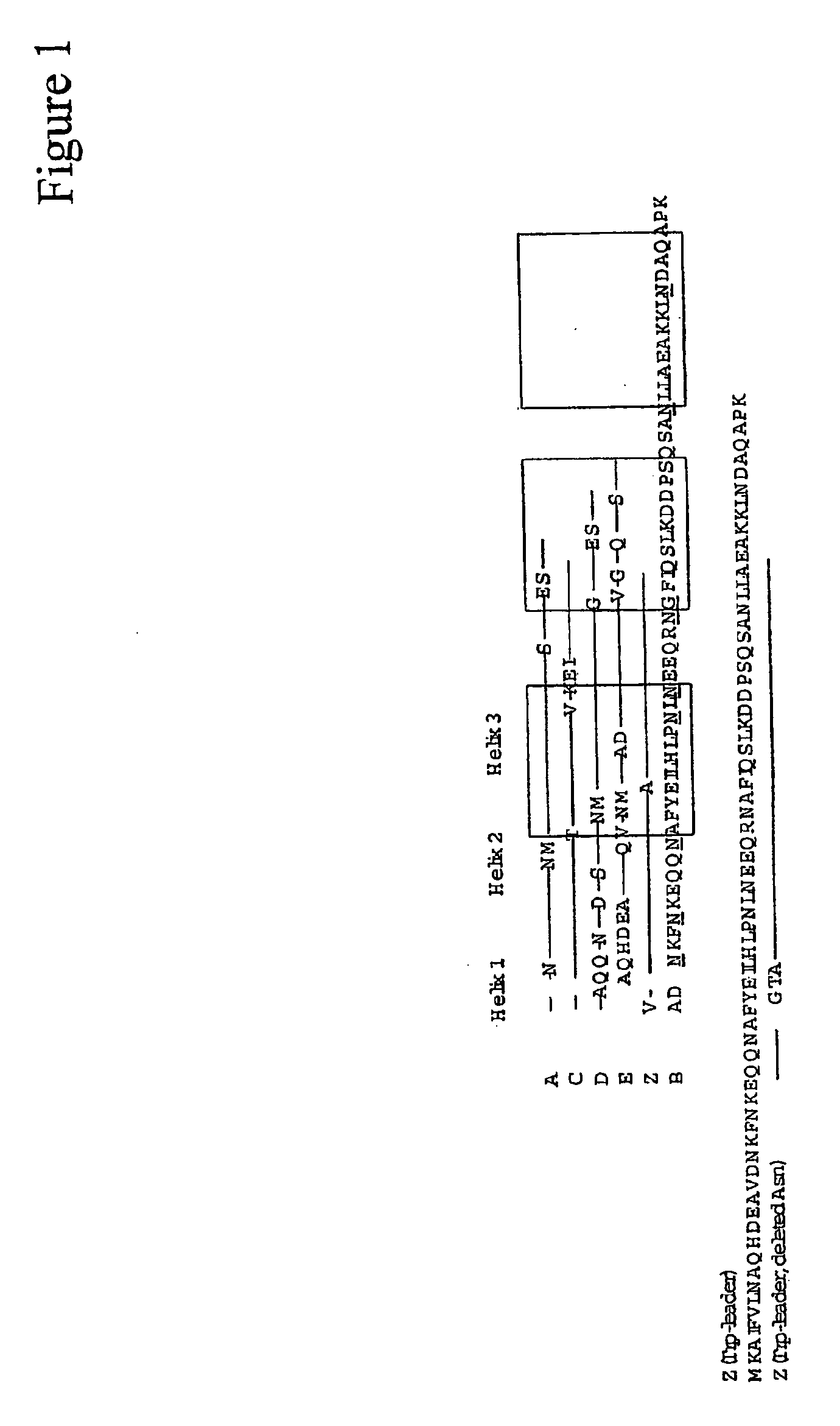
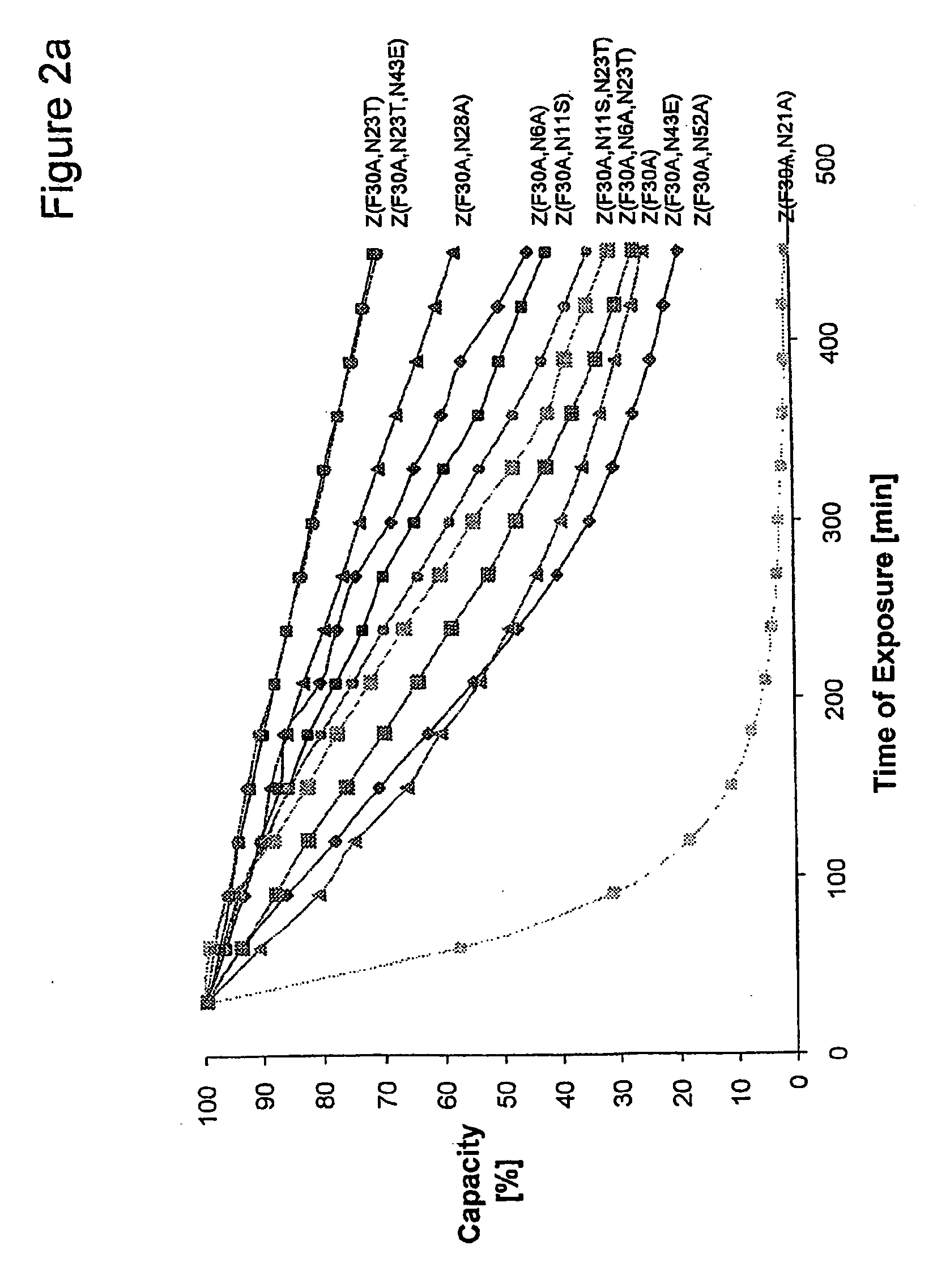
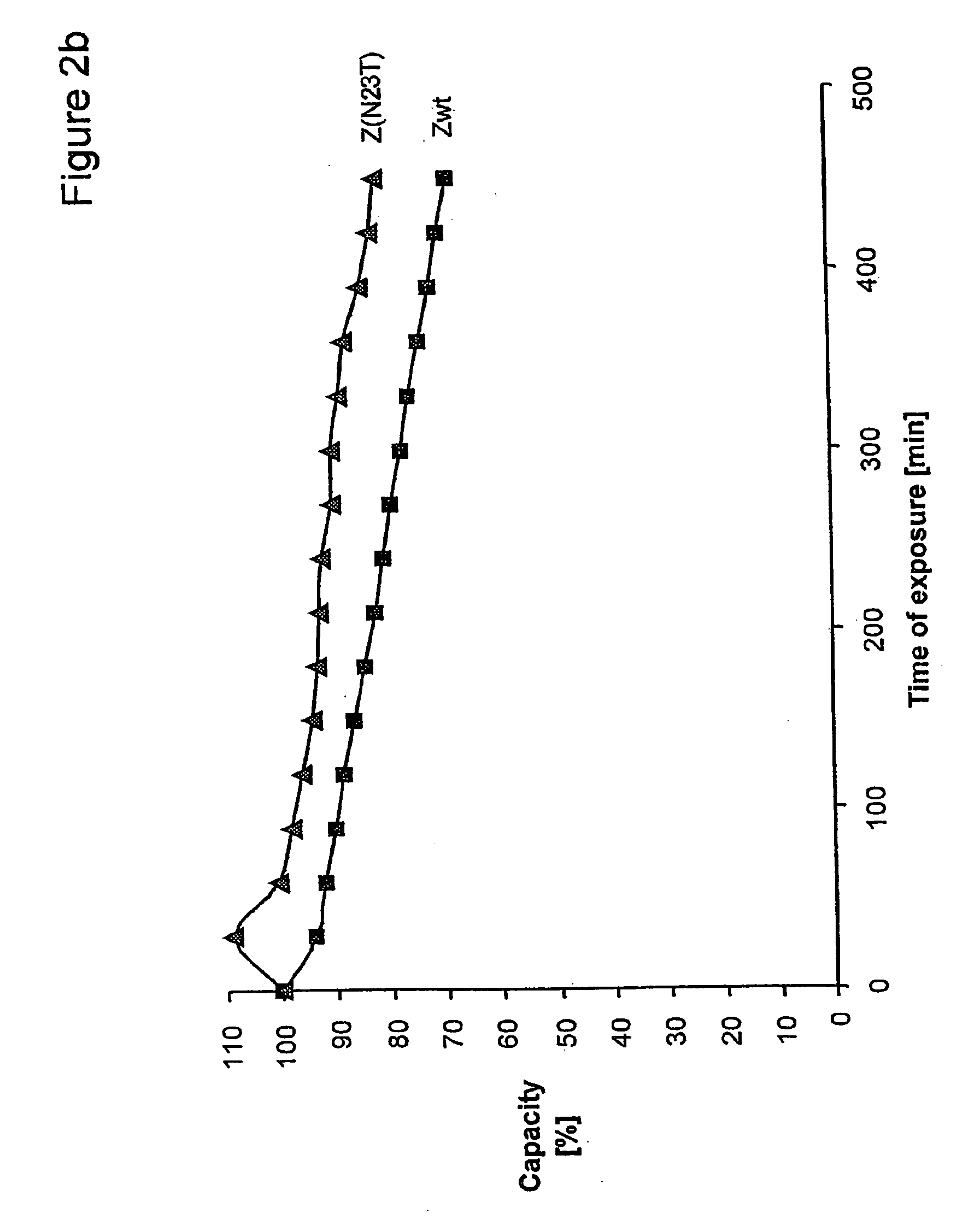
The present invention relates to an immunoglobulin-binding protein, wherein at least one asparagine residue has been mutated to an amino acid other than glutamine or aspartic acid, which mutation confers an increased chemical stability at pH-values of up to about 13-14 compared to the parental molecule. The protein can for example be derived from a protein capable of binding to other regions of the immunoglobulin molecule than the complementarity determining regions (CDR), such as protein A, and preferably the B-domain of Staphylococcal protein A. The invention also relates to a matrix for affinity separation, which comprises an immunoglobulin-binding protein as ligand coupled to a solid support, in which protein ligand at least one asparagine residue has been mutated to an amino acid other than glutamine.
Owner:CYTIVA BIOPROCESS R&D AB
Method of purifying protein
InactiveUS20060142549A1Efficient removalSerum immunoglobulinsColony-stimulating factorActive proteinDNA Contamination
Problems to be Solved: The present invention provides a simpler and less expensive method for purifying physiologically active proteins, especially antibodies, which can ensure removal of impurities such as DNA contaminants and viruses, and which can minimize a loss of physiologically active proteins. Means for Solving the Problems: A method for removing impurities in a physiologically active protein-containing sample, which comprises the following steps: 1) allowing the physiologically active protein-containing sample to be converted into an aqueous solution of low conductivity at a pH below the isoelectric point of the physiologically active protein; and 2) removing the resulting particles.
Owner:CHUGAI PHARMA CO LTD
Method for preparing human immunoglobulin concentrates for therapeutic use
InactiveUS7186410B2Simple processHighly compatibleAntibacterial agentsSerum immunoglobulinsAnion-exchange chromatographyBlood plasma
The invention concerns a method for preparing human immunoglobulin concentrates for therapeutic use, from plasma or a plasma fraction. The method comprises pre-purification and a single anion-exchange chromatography carried out at alkaline pH, thereby enabling the immunoglobulins to be retained on the chromatographic support and fractionated. The method enables to obtain IgG, IgA and IgM concentrates.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
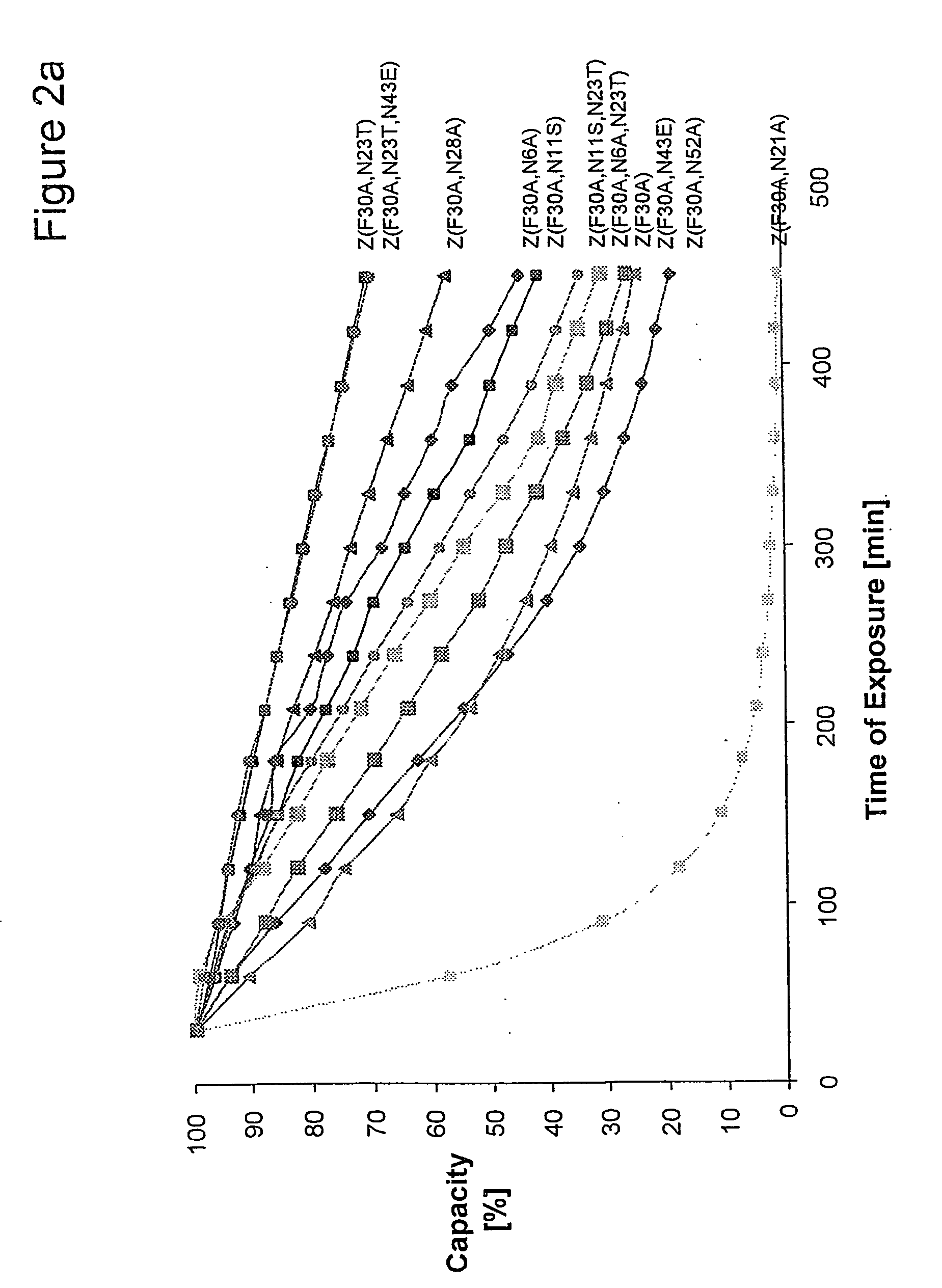
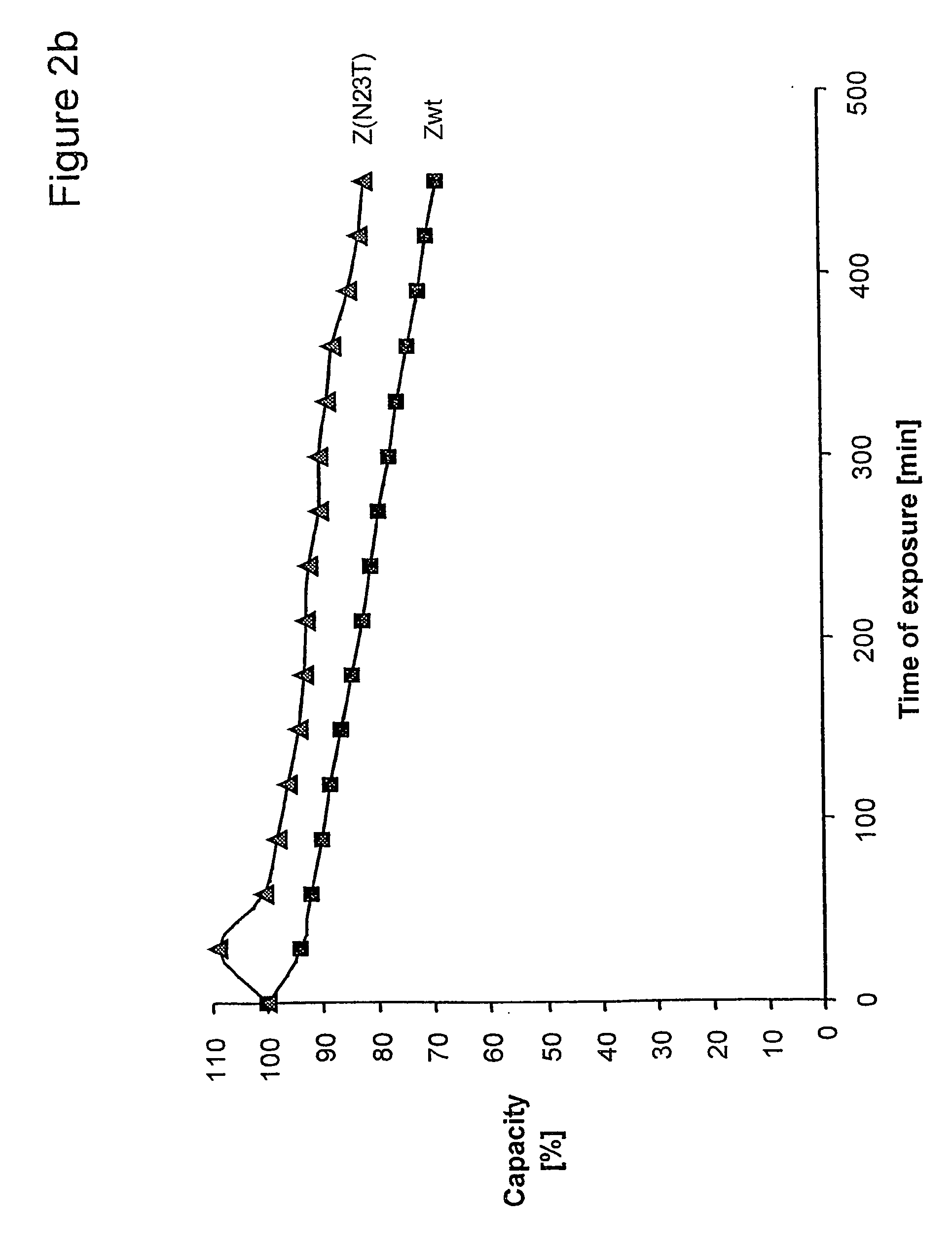
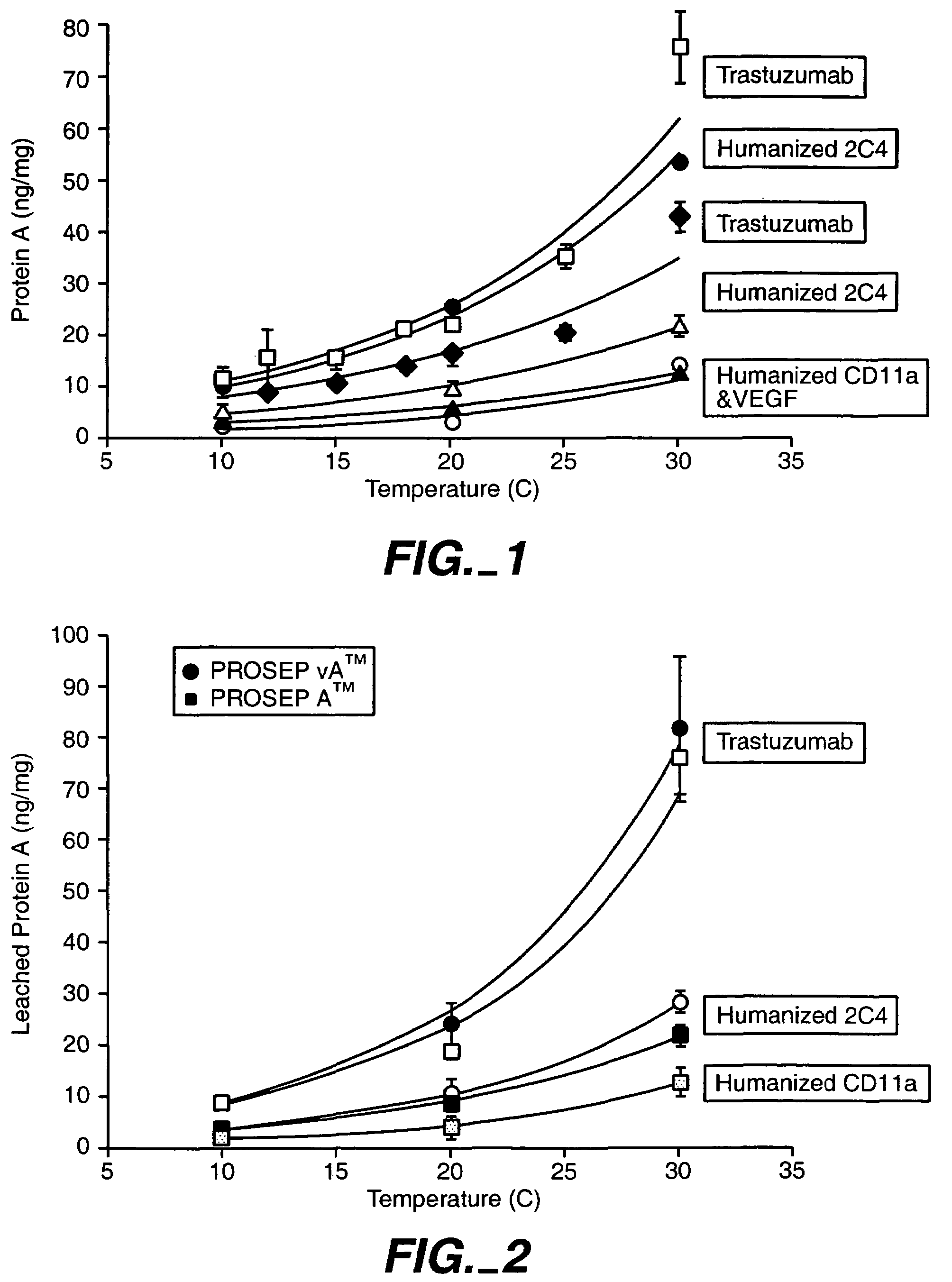
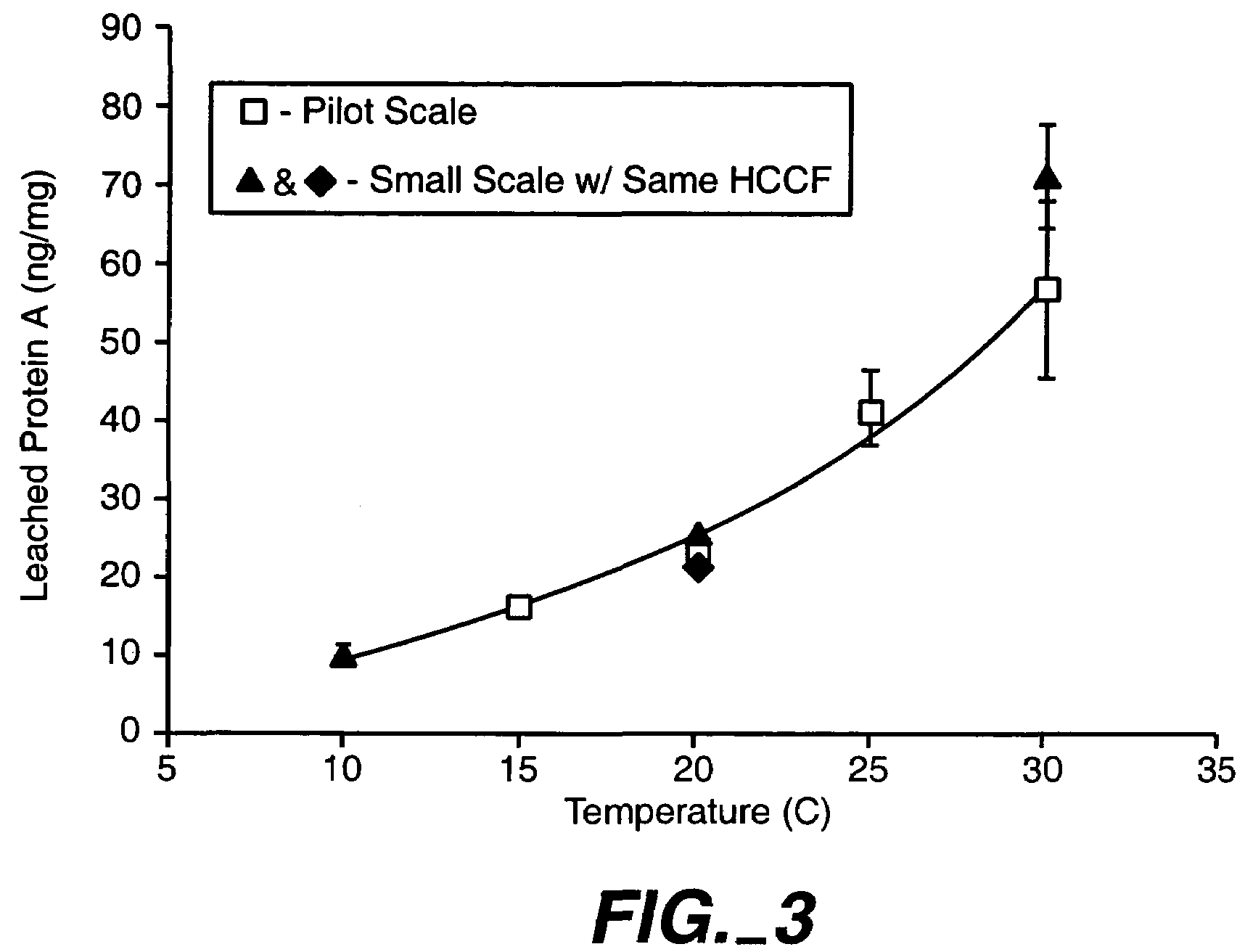
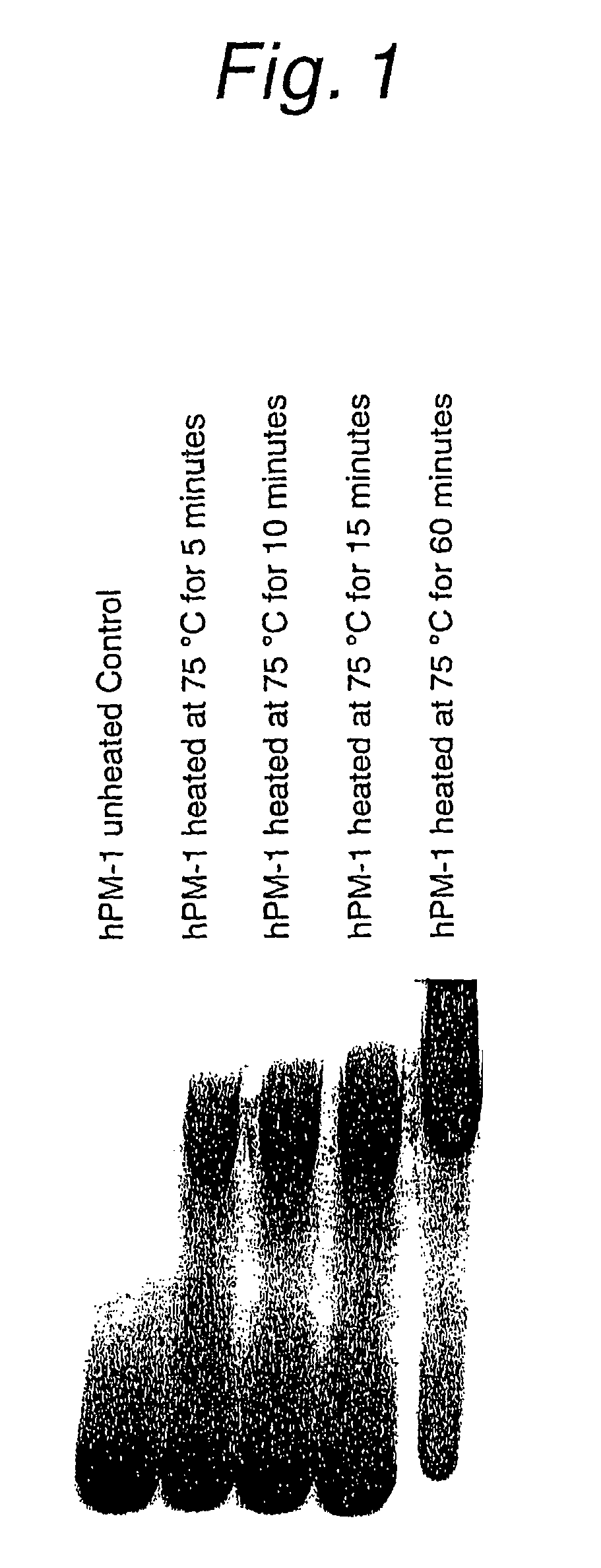
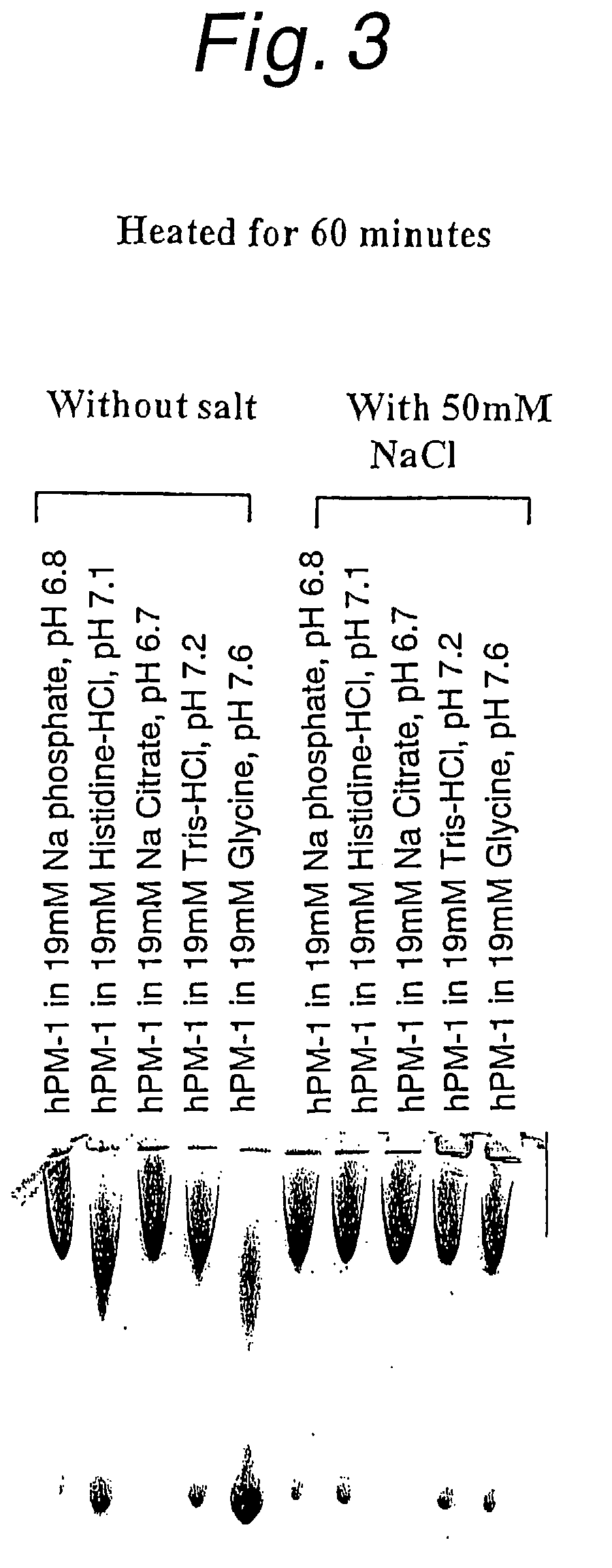
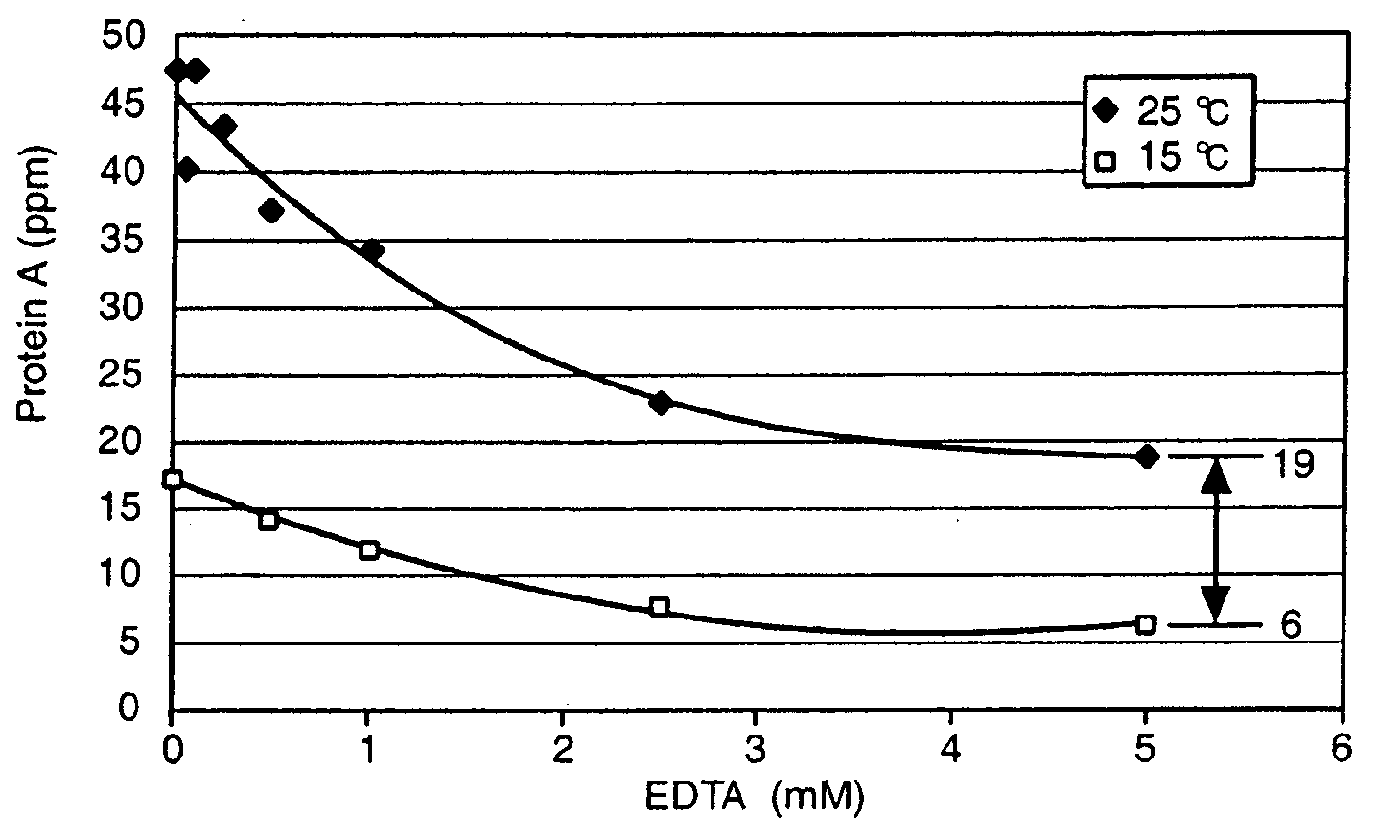
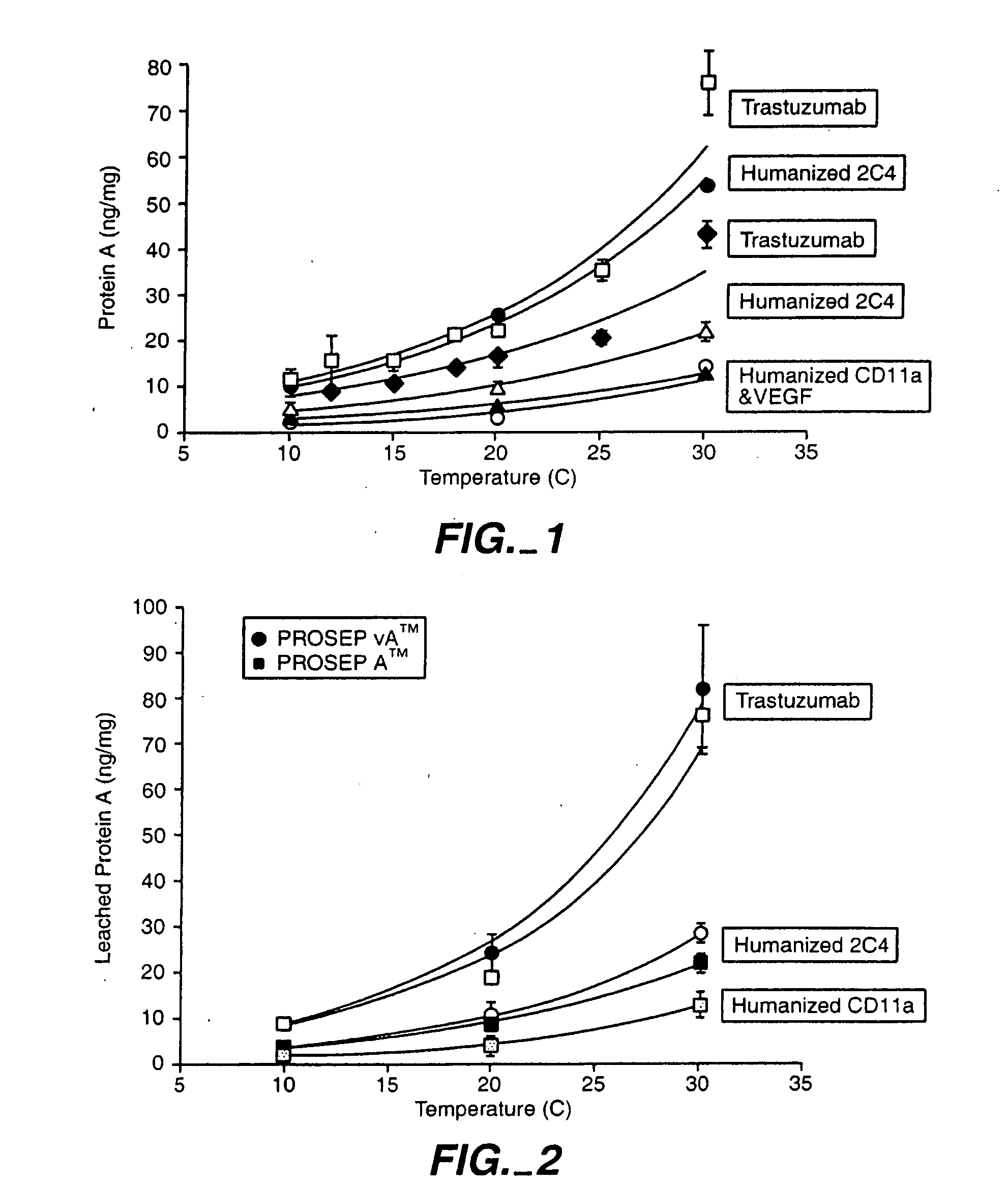
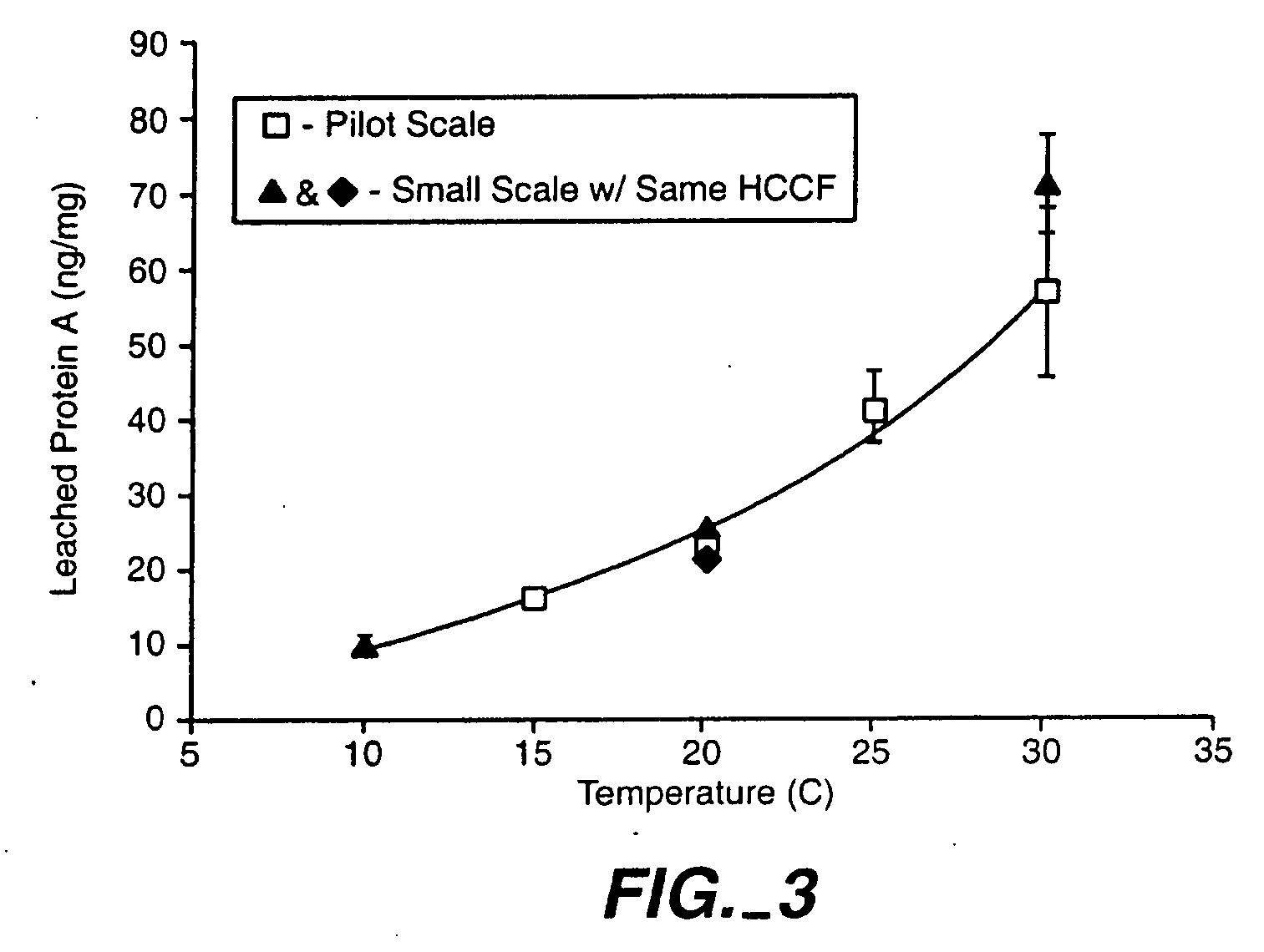
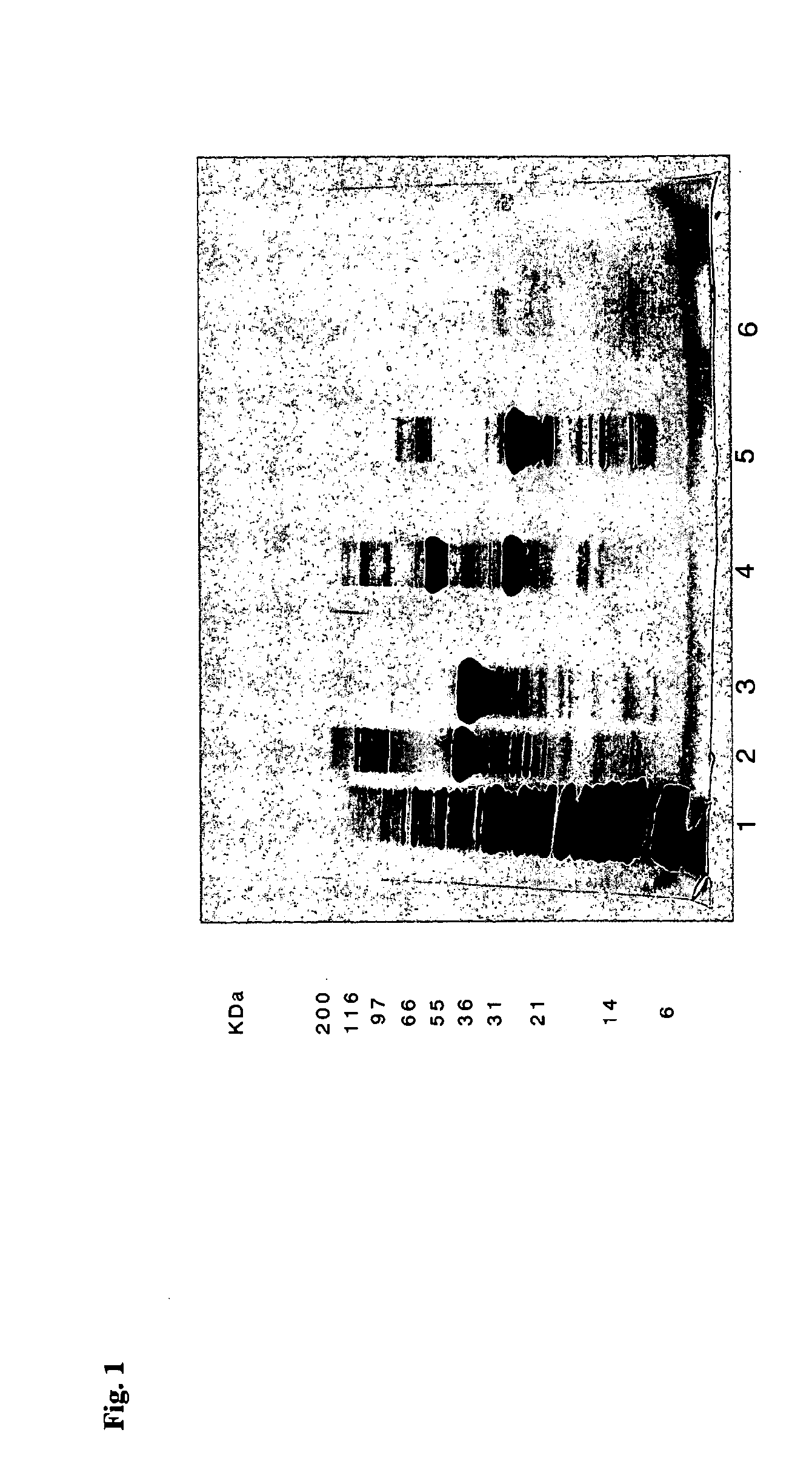
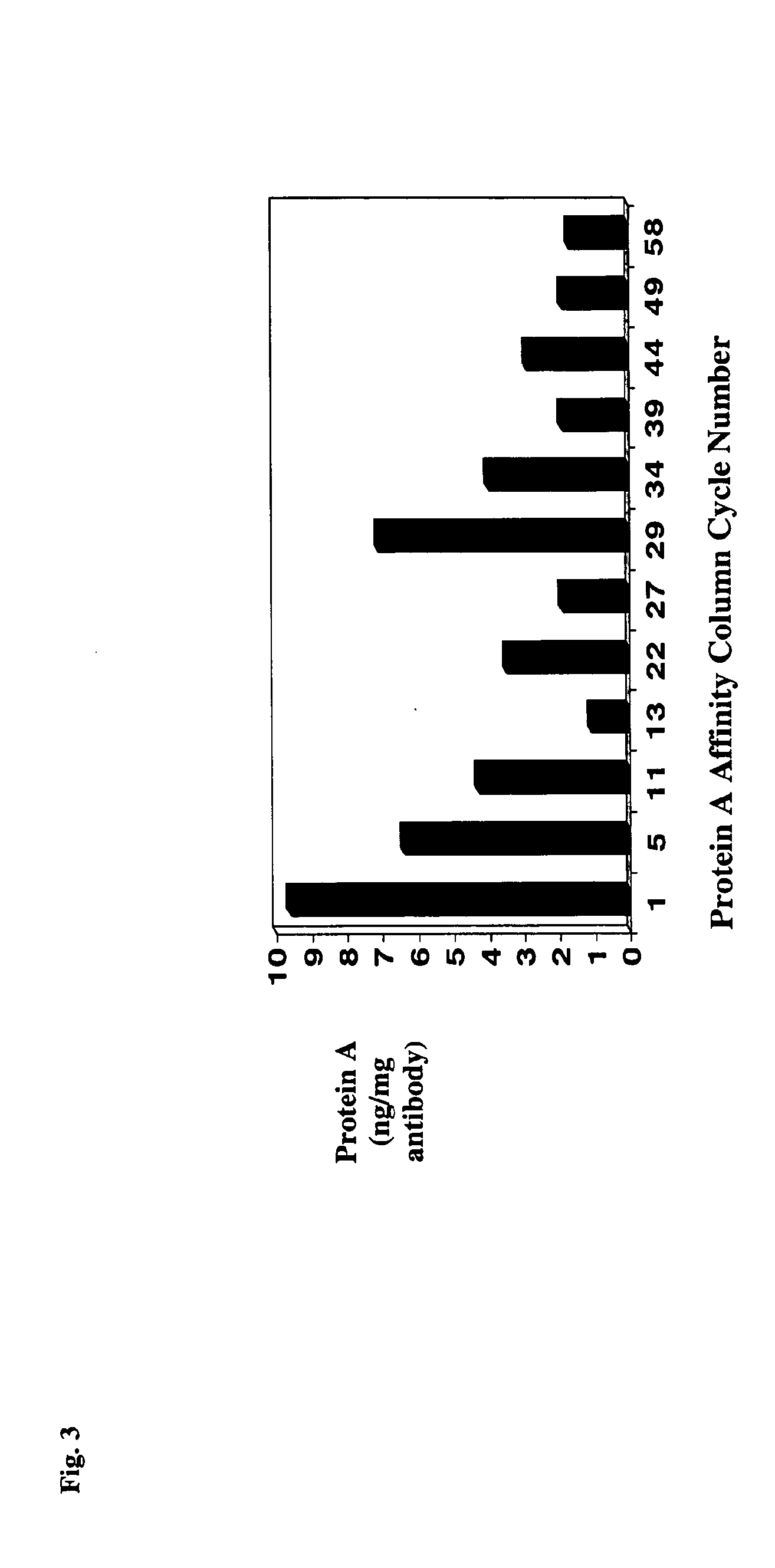
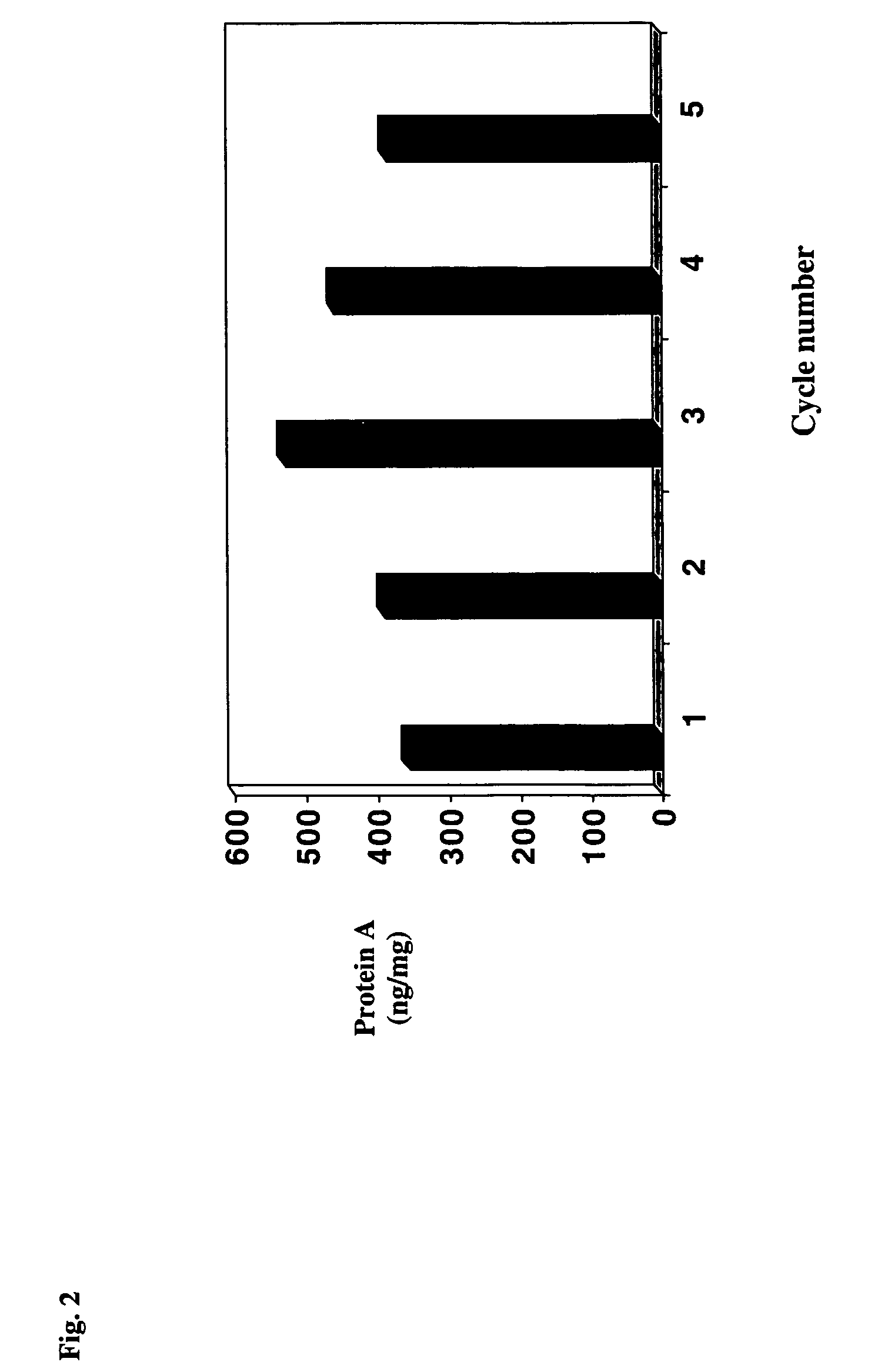
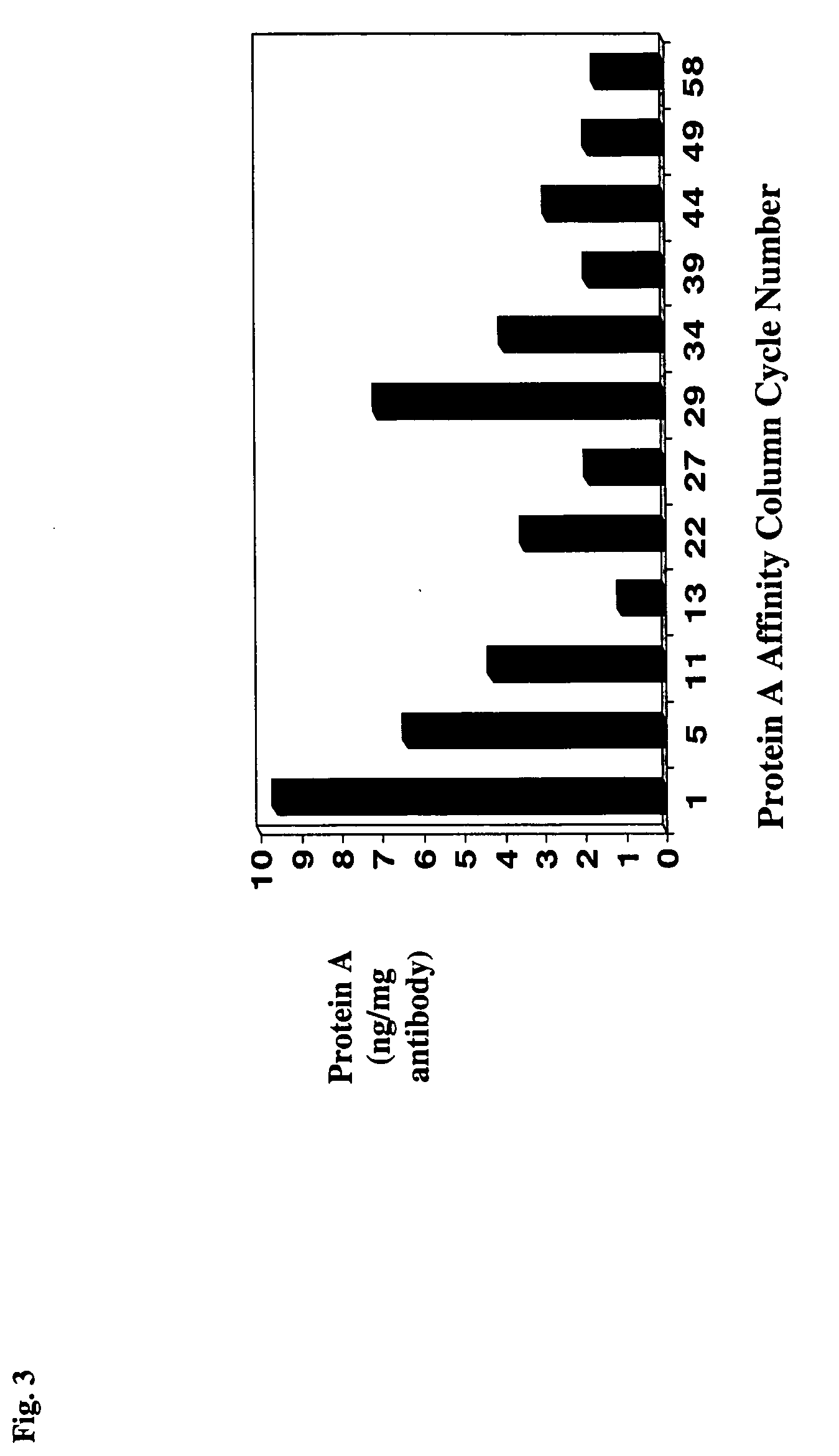
Reducing protein A leaching during protein A affinity chromatography
ActiveUS7485704B2Reduce the temperatureProtein A leaching is reduced.Serum immunoglobulinsSolid sorbent liquid separationProteinase activityAntibody Affinity Chromatography
A method for reducing leaching of protein A during protein A affinity chromatography is described which involves reducing temperature or pH of, or by adding one or more protease inhibitors to, a composition that is subjected to protein A affinity chromatography.
Owner:GENENTECH INC
Stabilized anti-interleukin-6 antibody-containing preparations
InactiveUS8632778B2Reduce aggregationImprove stabilityOrganic active ingredientsBiocideCrystallographyInterleukin 6
The present invention provides stabilized preparations containing an antibody in a glycine buffer and / or a histidine buffer and also provides processes for preparing a protein-containing stabilized preparation, comprising adjusting the pH with a basic amino acid or a basic amino acid derivative or a salt thereof.
Owner:CHUGAI PHARMA CO LTD
Expression of functional antibody fragments
InactiveUS20050244929A1Easy to prepareFacilitated releaseHybrid immunoglobulinsBacteriaMicroorganismHeavy chain
Methods for the high yield production of antibody Fv-containing polypeptides, especially Fab′ and F(ab′)2 antibody fragments are provided. Expression of heavy and light chain Fv in a microbial secretory system is followed by recovery of Fv from the periplasm under conditions that maintain a cysteine residue as a free thiol. The free thiol is reacted with free thiol of an antibody fragment of the same or differing specificity, or with agents such as diagnostic labels or therapeutic moieties. The products offer advantages of homogeneity and purity not available through the use of known methods for preparing such derivatives.
Owner:GENENTECH INC
Reducing protein a leaching during protein a affinity chromatography
ActiveUS20090099344A1Reduce the temperatureProtein A leaching is reducedSerum immunoglobulinsSolid sorbent liquid separationProteinase activityEnzyme inhibitor
A method for reducing leaching of protein A during protein A affinity chromatography is described which involves reducing temperature or pH of, or by adding one or more protease inhibitors to, a composition that is subjected to protein A affinity chromatography.
Owner:GENENTECH INC
Antibody purification by protein a and ion exchange chromatography
ActiveUS20060194953A1Serum immunoglobulinsImmunoglobulins against animals/humansIon exchangeAntibody Affinity Chromatography
A novel method for selectively removing leaked protein A from antibody purified by means of protein A affinity chromatography is disclosed.
Owner:LONZA BIOLOGICS PLC
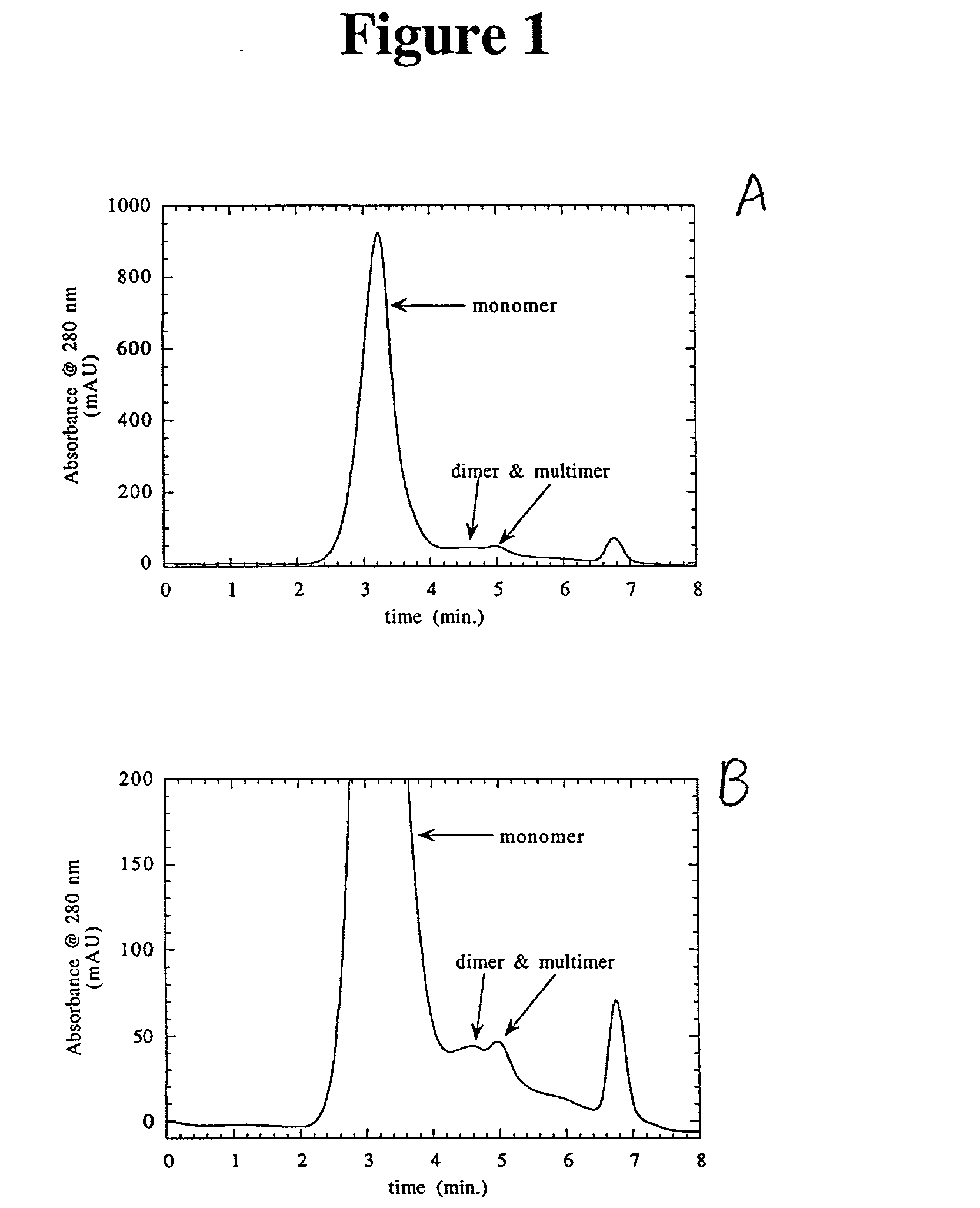
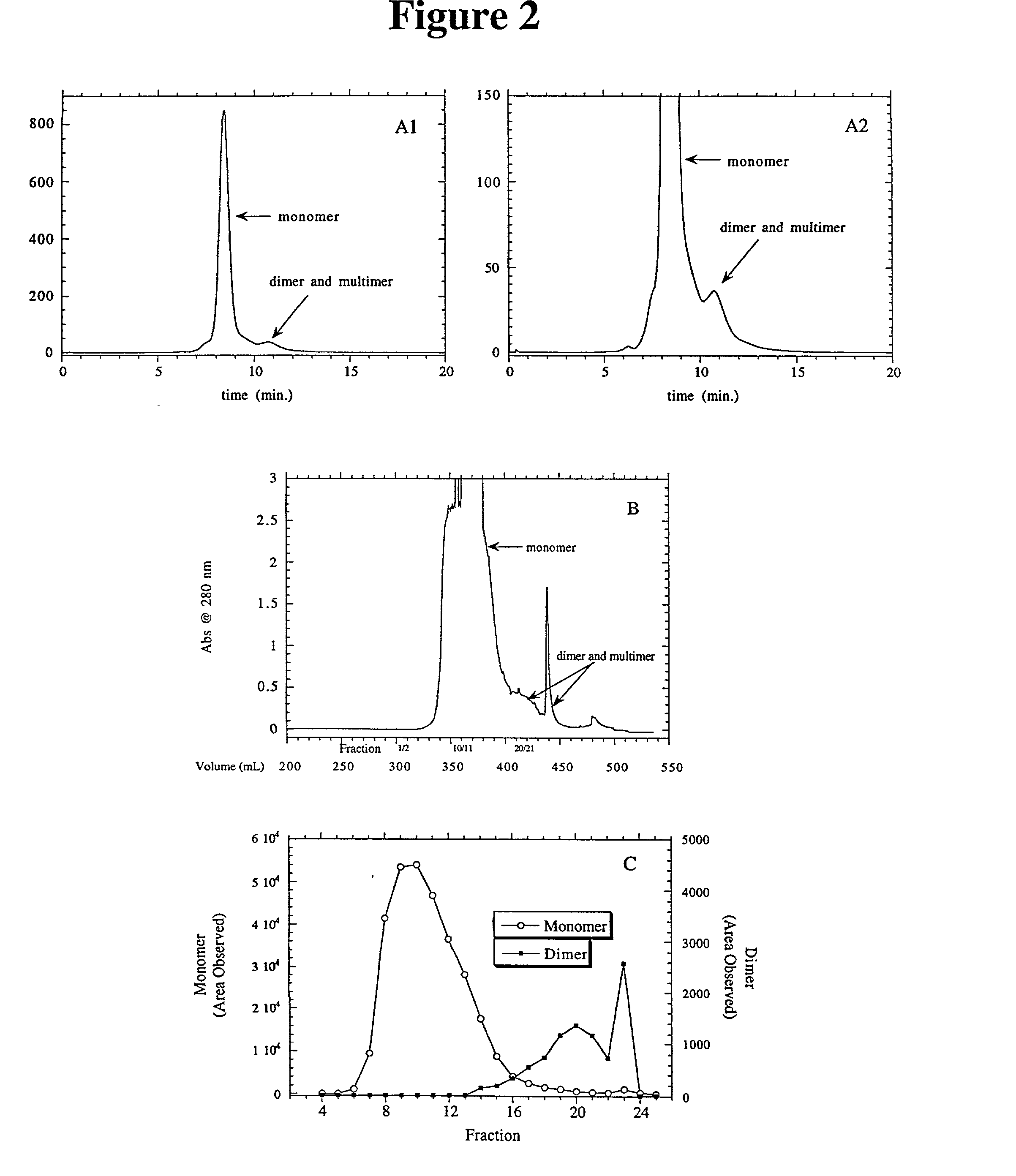
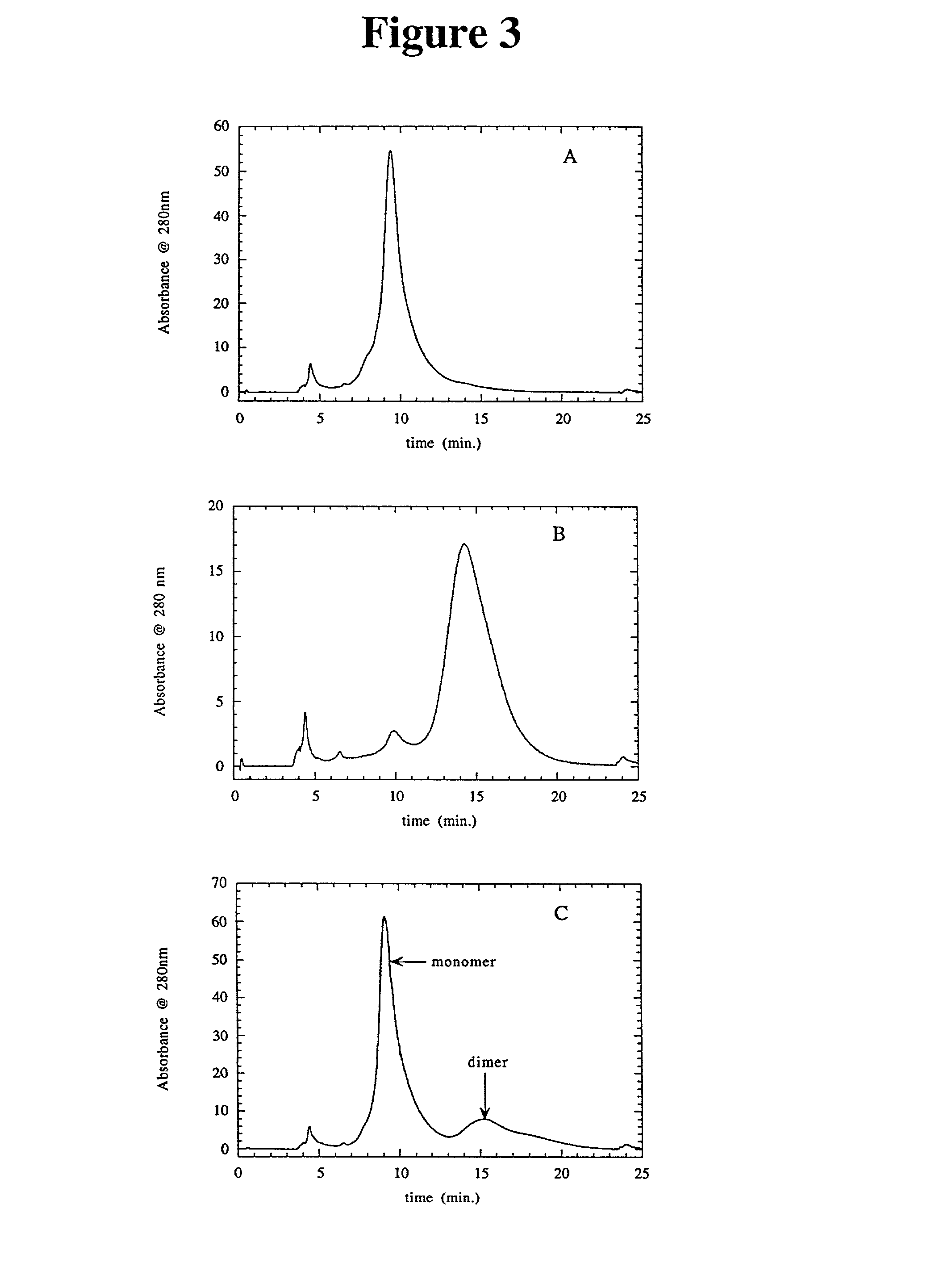
Separation of polypeptide monomers
InactiveUS20020010319A1Easy to separateInexpensiveComponent separationOther chemical processesAnion-exchange chromatographyAnion Exchange Proteins
A method is disclosed for separating a polypeptide monomer from a mixture comprising dimers and / or multimers. The method comprises applying the mixture to either a cation-exchange chromatography resin or an anion-exchange chromatography resin and eluting the mixture at a gradient of about 0-1 M of an elution salt, wherein the monomer is separated from the dimers and / or multimers present in the mixture.
Owner:GENENTECH INC
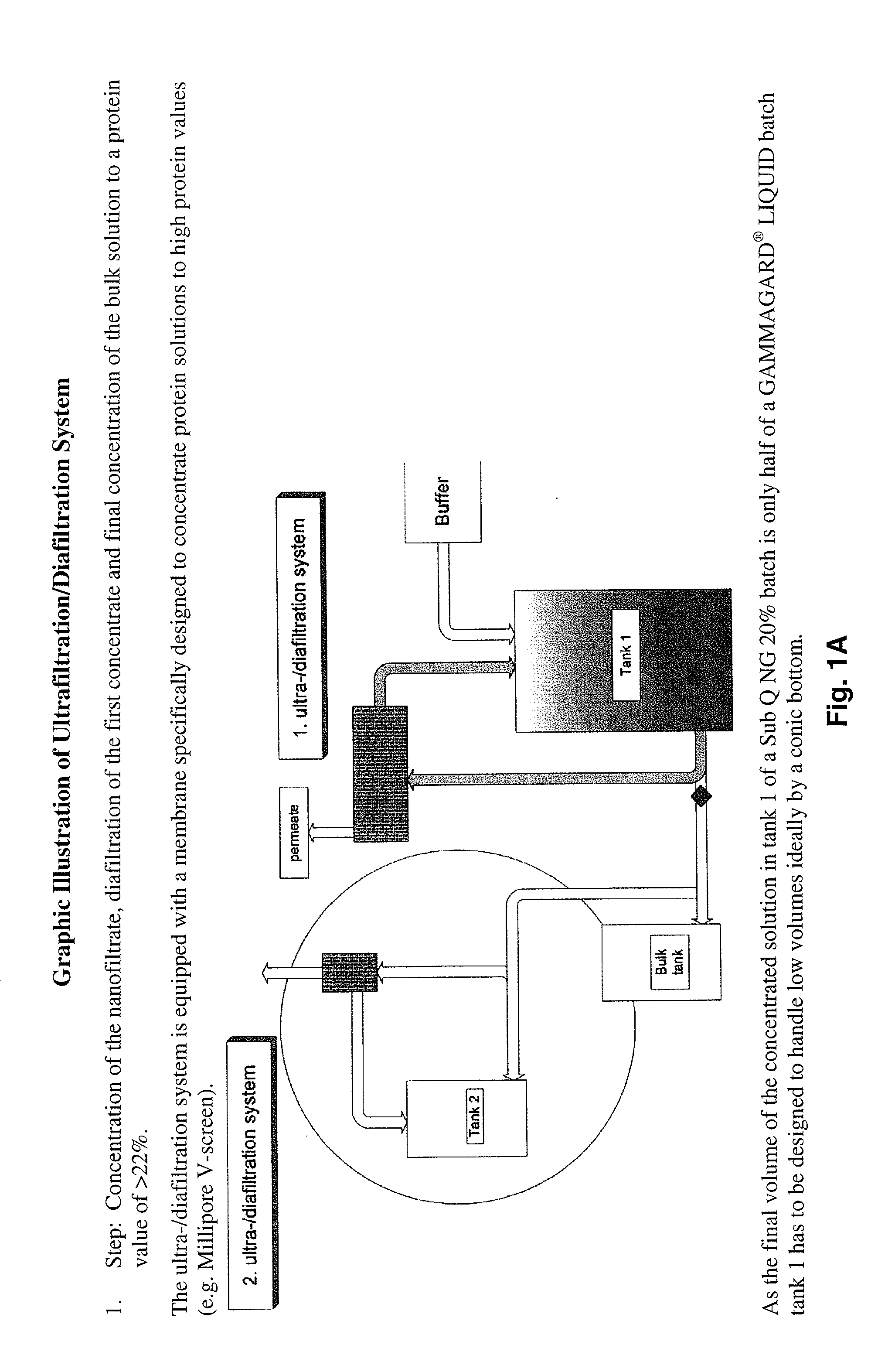
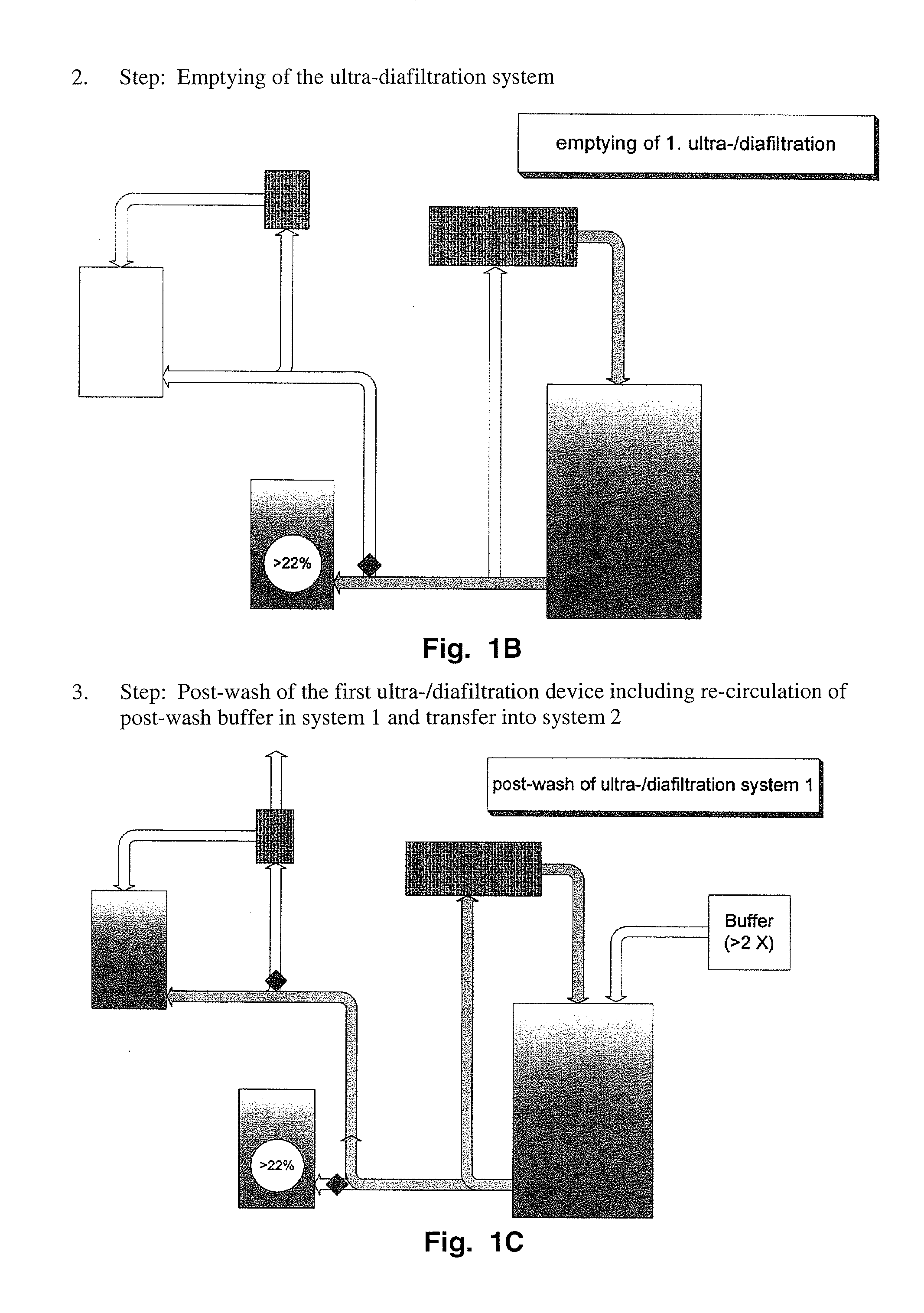
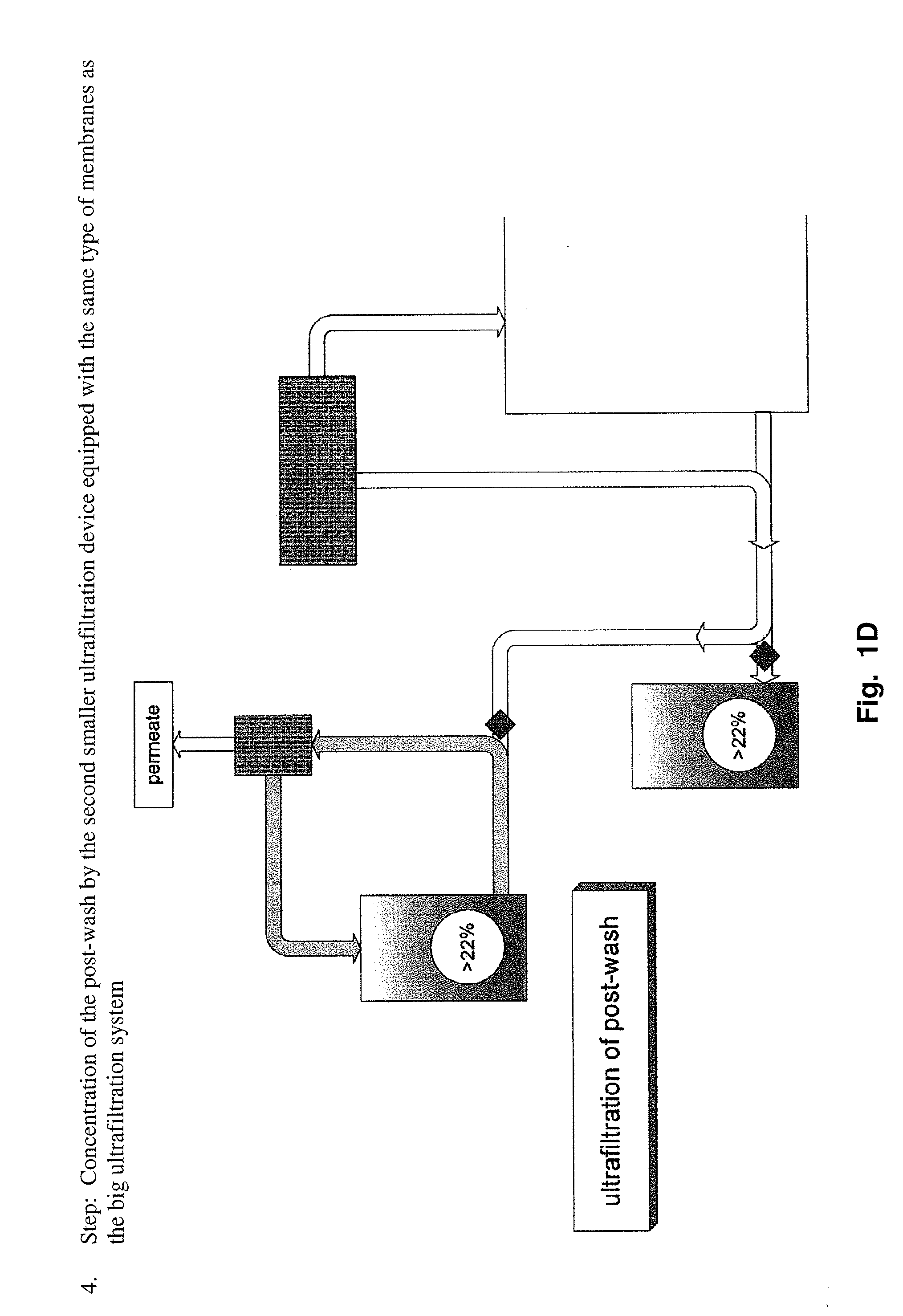
Method to produce a highly concentrated immunoglobulin preparation for subcutaneous use
The present invention relates to a new and improved method for preparing a highly concentrated immunoglobulin composition from pooled plasma for subcutaneous injection. A composition comprising 20% or more immunoglobulin suitable for subcutaneous use is also described.
Owner:TAKEDA PHARMA CO LTD
Protein purification
InactiveUS20050063972A1High recovery ratePeptide/protein ingredientsSerum immunoglobulinsMulti pollutantIon exchange
A method for purifying a polypeptide by ion exchange chromatography is described which involves changing the conductivity and / or pH of buffers in order to resolve a polypeptide of interest from one or more contaminants.
Owner:GENENTECH INC
Animal Models and Therapeutic Molecules
The invention discloses methods for the generation of chimaeric human-non-human antibodies and chimaeric antibody chains, antibodies and antibody chains so produced, and derivatives thereof including fully humanised antibodies; compositions comprising said antibodies, antibody chains and derivatives, as well as cells, non-human mammals and vectors, suitable for use in said methods.
Owner:KIMAB LTD
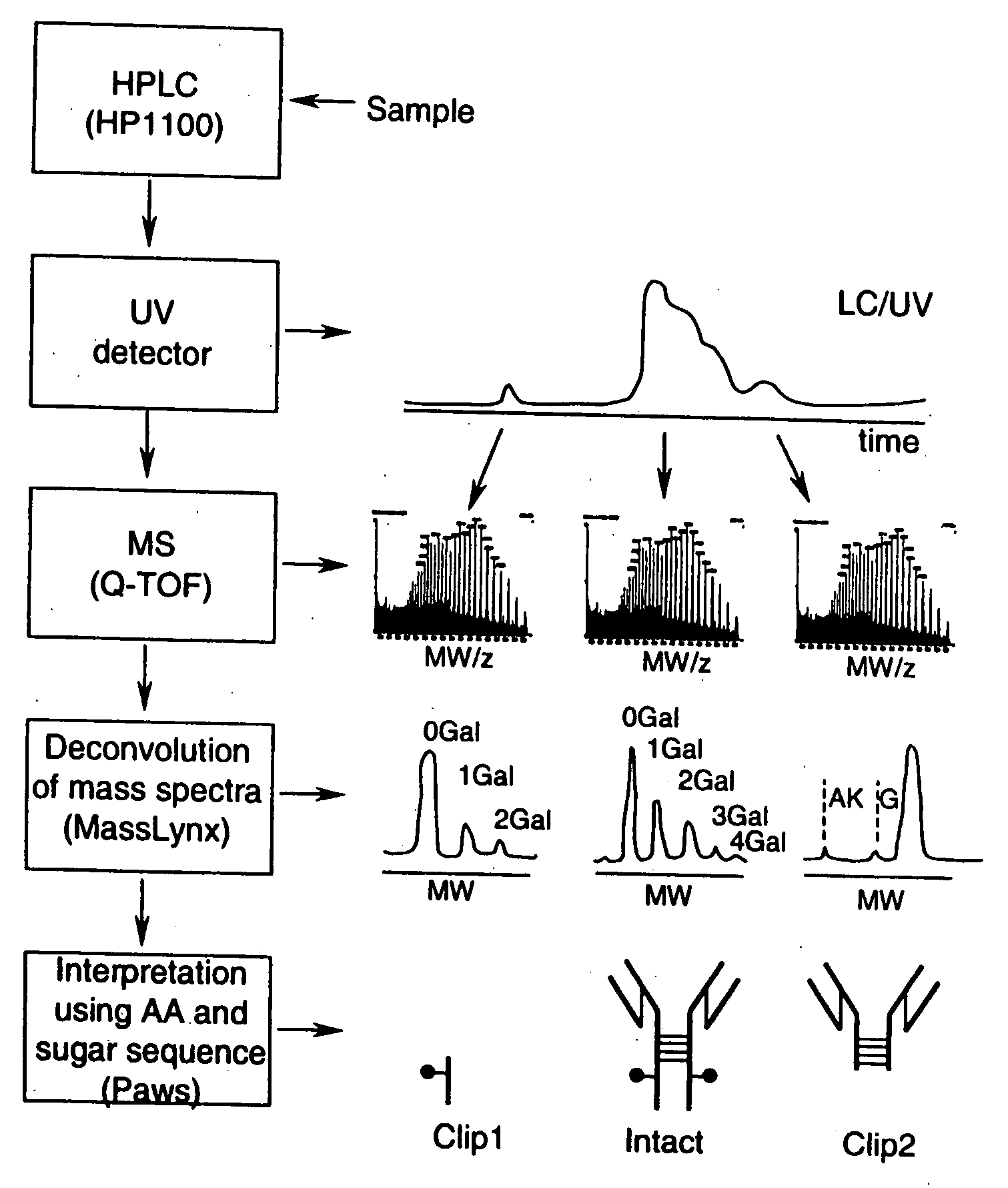
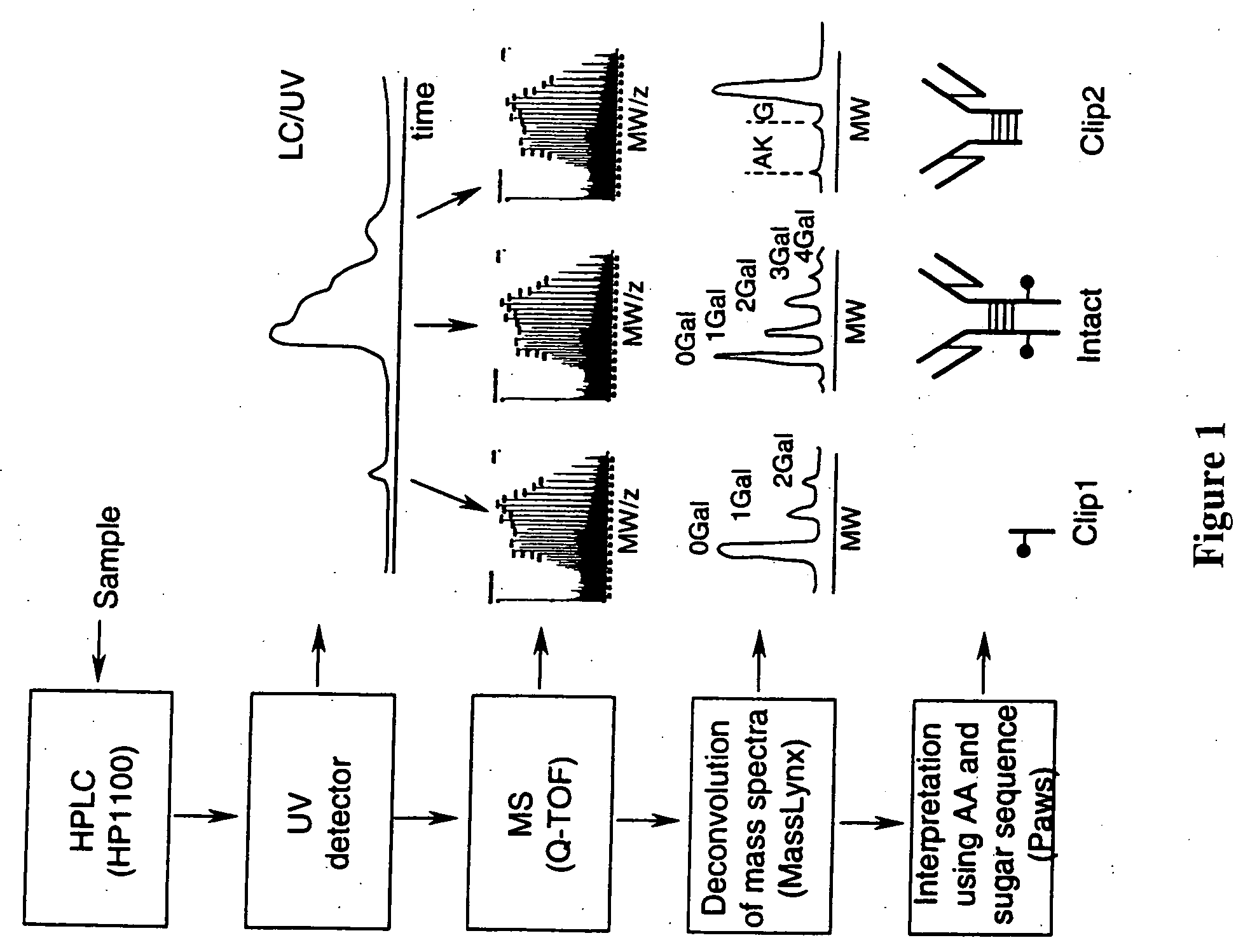
LC/MS method of analyzing high molecular weight proteins
InactiveUS20050161399A1Serum immunoglobulinsComponent separationLiquid chromatography mass spectroscopyBiochemistry
Owner:AMGEN INC
Protein recovery
The invention herein provides a method for recovering a polypeptide comprising exposing a composition comprising a polypeptide to a reagent which binds to, or modifies, the polypeptide, wherein the reagent is immobilized on a solid phase; and then passing the composition through a filter bearing a charge which is opposite to the charge of the reagent in the composition, so as to remove leached reagent from the composition.
Owner:GENENTECH INC
Protein a chromatography
InactiveUS20060030696A1Increase ionic strengthThroughputSerum immunoglobulinsImmunoglobulins against animals/humansProtein AChromatography
A novel method for selectively removing leaked protein A from antibody purified by means of protein A affnitiy chromatography is disclosed.
Owner:LONZA BIOLOGICS PLC
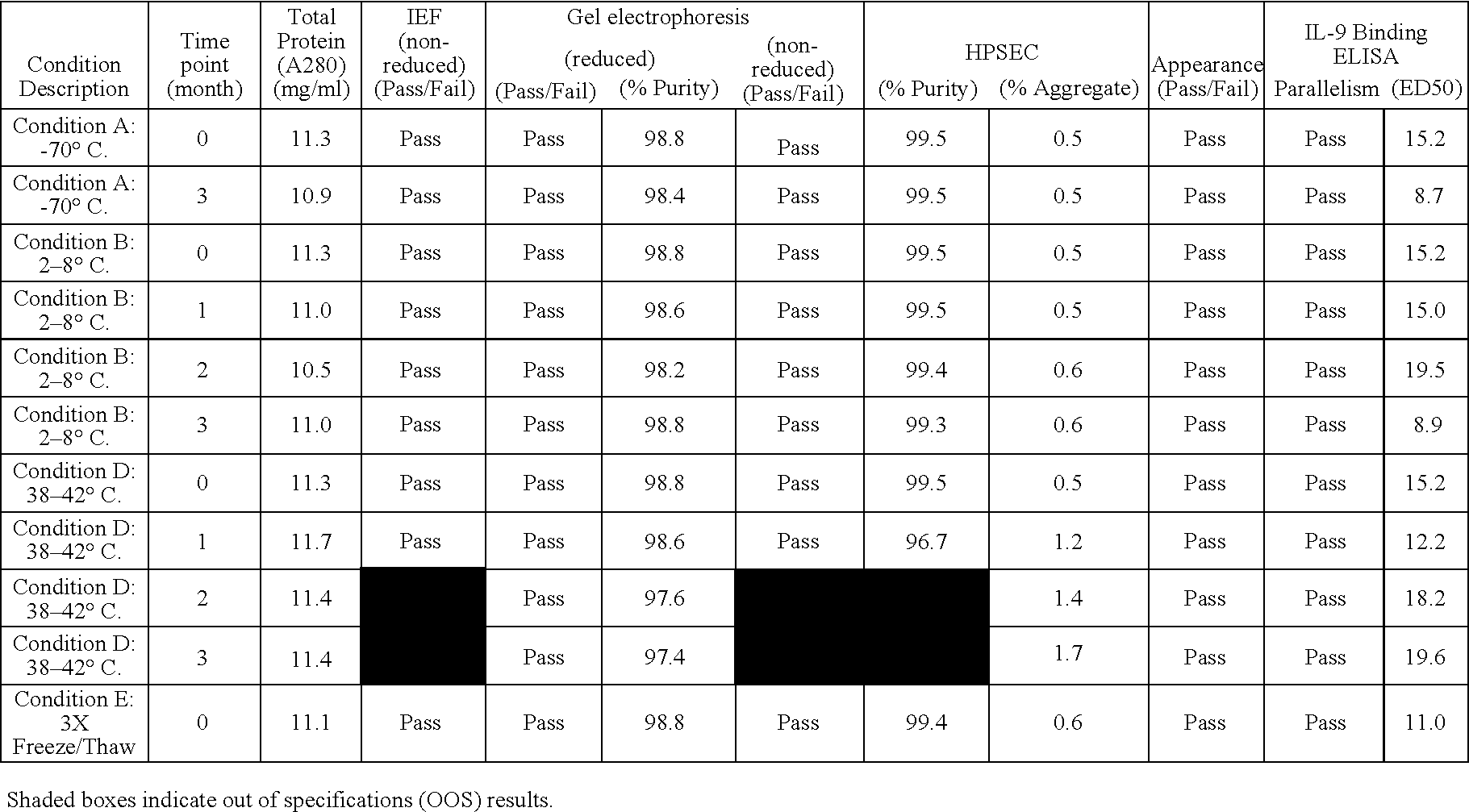
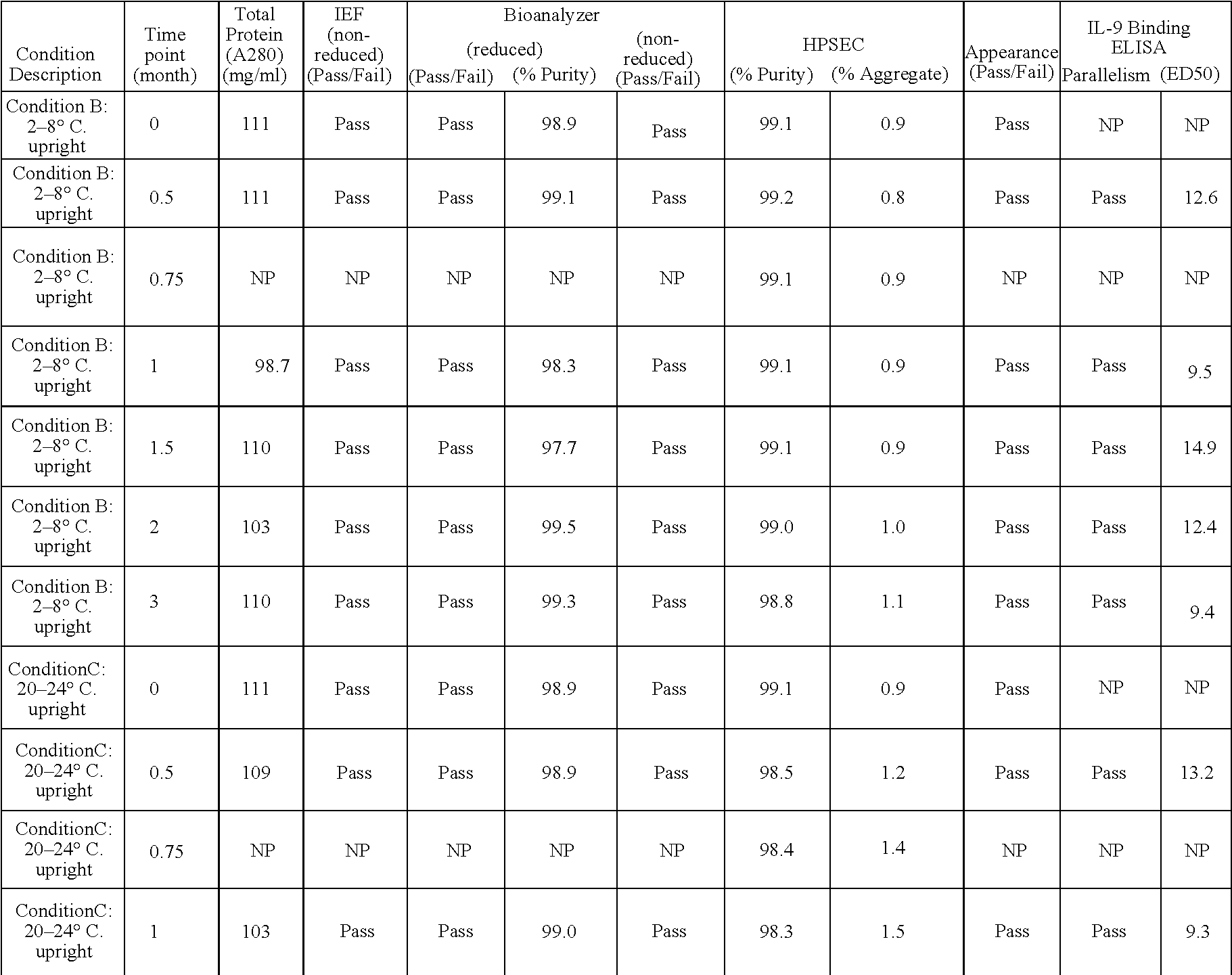
Anti-IL-9 antibody formulations and uses thereof
InactiveUS20050260204A1Low to undetectable levelEasy to manageAntibacterial agentsAntimycoticsDiseaseAntibody fragments
The present invention provides liquid formulations of antibodies or antibody fragments that immunospecifically bind to an IL-9 polypeptide, which formulations exhibit stability, low to undetectable levels of aggregation, and very little to no loss of the biological activities of the antibodies or antibody fragments, even during long periods of storage. In particular, the present invention provides liquid formulations of antibodies or fragments thereof that immunospecifically bind to an IL-9 polypeptide, which formulations are substantially free of surfactants, sugars, sugar alcohols, amino acids other than histidine (preferably with pKa values of less than 5 and above 7), and / or other common excipients. Furthermore, the invention provides methods of preventing, treating or ameliorating a disease or disorder associated with or characterized by aberrant expression and / or activity of an IL-9 polypeptide, a disease or disorder associated with or characterized by aberrant expression and / or activity of the IL-9R or one or more subunits thereof, an autoimmune disease, an inflammatory disease, a proliferative disease, or an infection (preferably, a respiratory infection), or one or more symptoms thereof, utilizing the liquid formulations of the present invention.
Owner:MEDIMMUNE LLC
High stability porous metal oxide spherules used for one-step antibody purifications
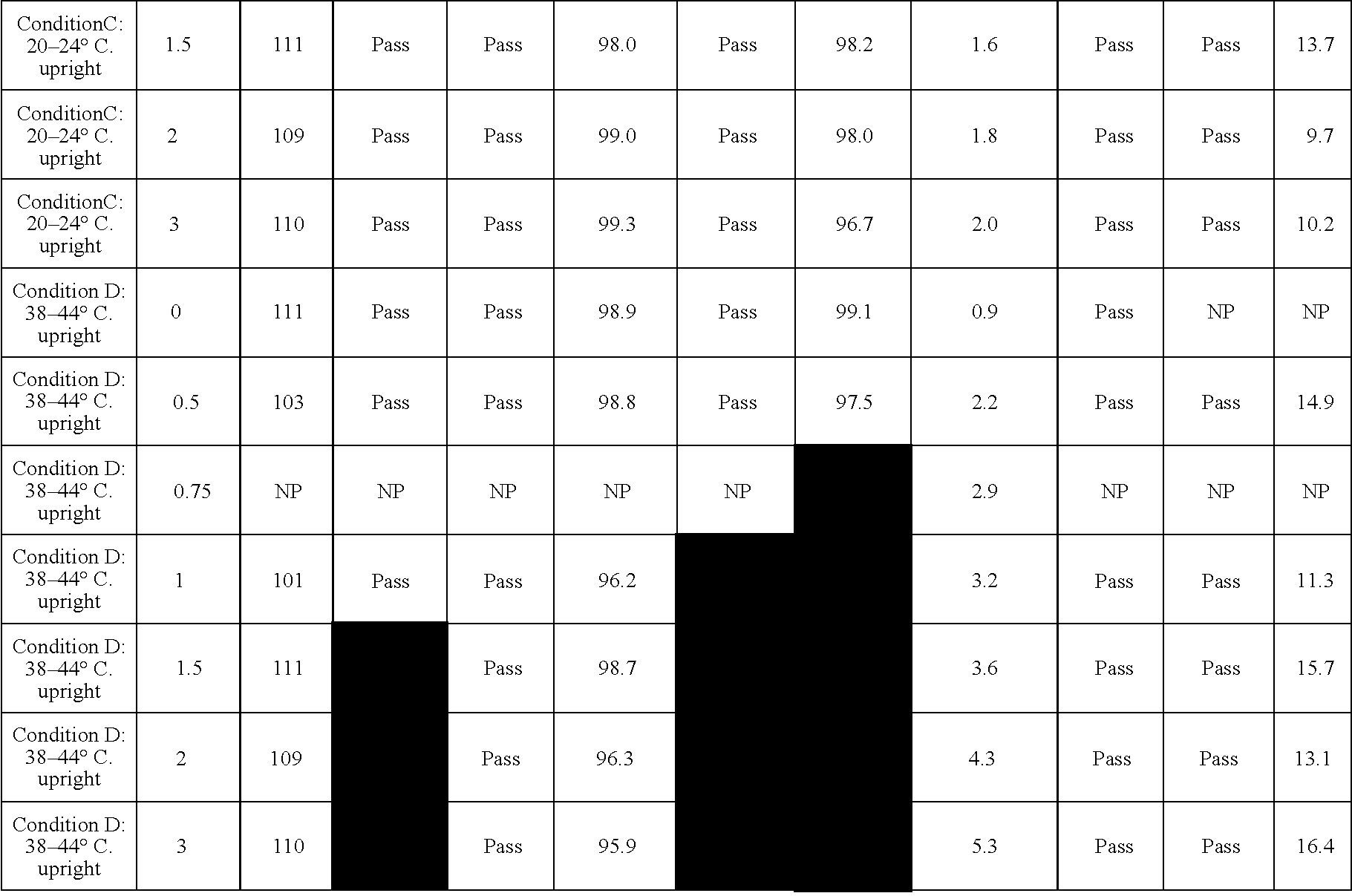
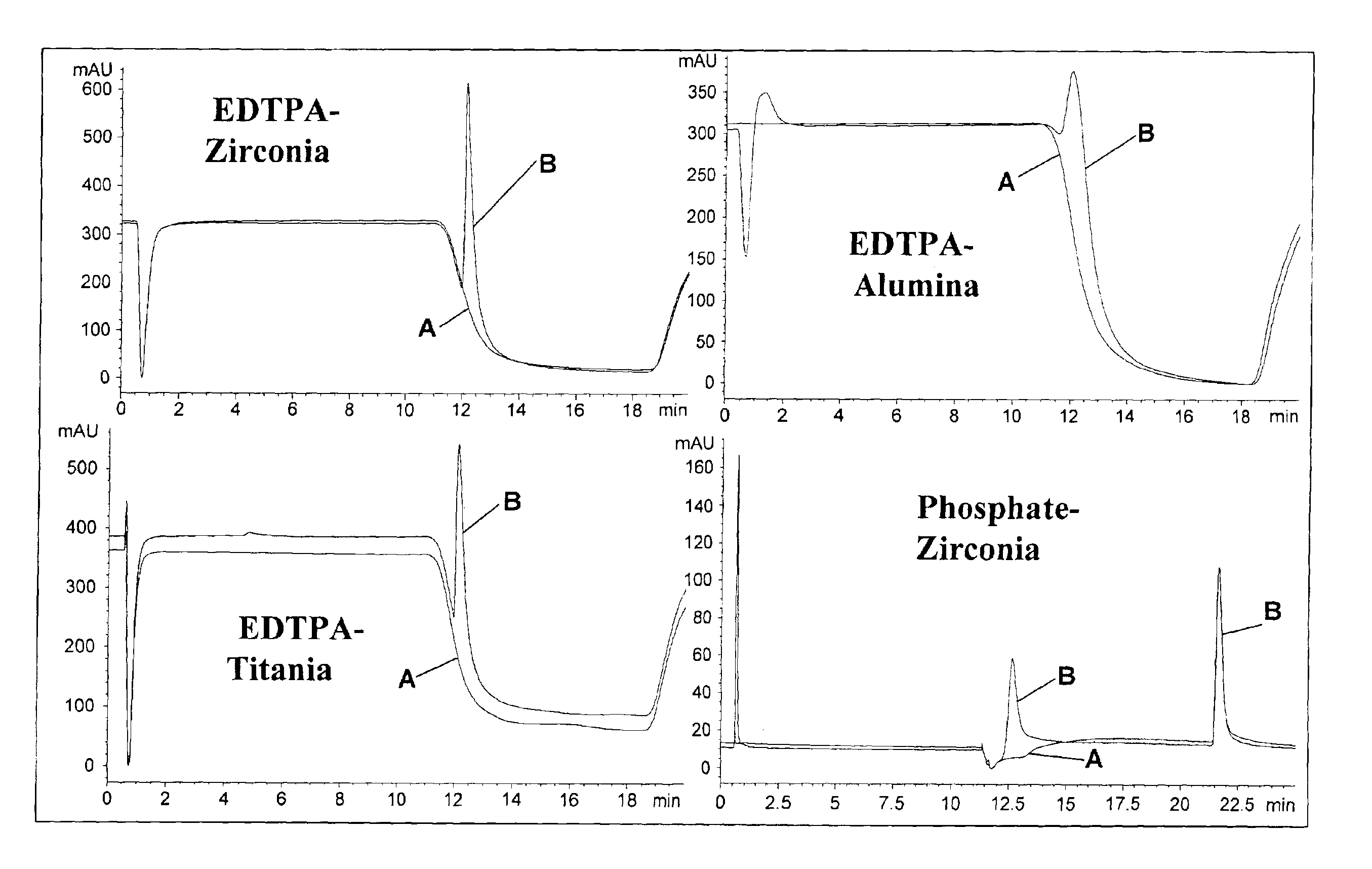
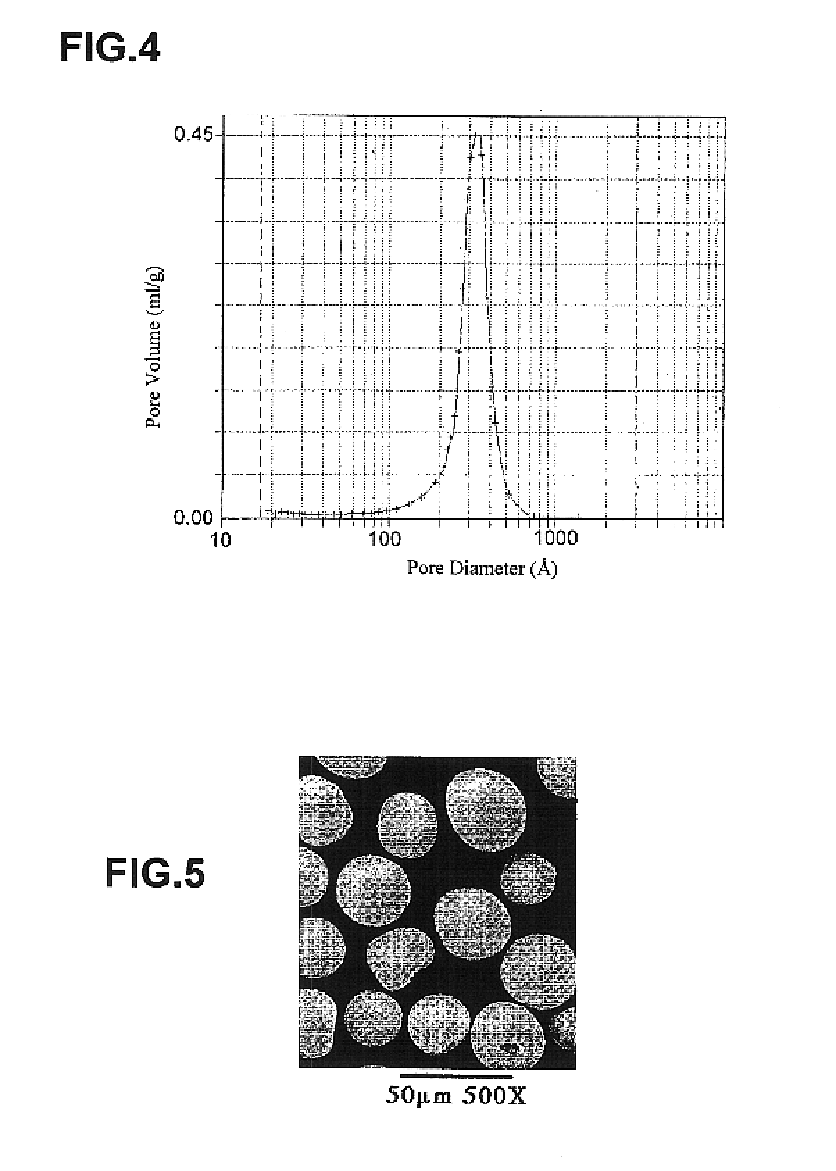
InactiveUS6846410B2Efficient methodIon-exchange process apparatusChromatographic cation exchangersEthylenediamineGram
The present invention describes the use of porous metal oxides for the preparative purification of antibodies and biomolecules within a range of particle physical characteristics. Typically, particles from 15 to 100 microns in average diameter with pores sizes ranging from 400 to 600 angstroms and surface areas from 10 to 50 square meters per gram, and pore volumes from 0.1 to 0.4 mL / gram can be used for purification processes. The metal oxide particles that fall within this range of physical properties show enhanced utility and greater chromatographic capacity for antibodies than materials oxide particles falling outside of this range. Metal oxides such as zirconia, titania and alumina can all be modified with a multi-Lewis base moiety such as an organophosphate ethylenediamine-N,N-tetra(methylenephosphonic) acid (EDTPA), to produce a bio-compatible purification media for, biomolecules.
Owner:ZIRCHROM SEPARATIONS
Stable antibody formulation
The present invention provides a pharmaceutical formulation comprising an antibody or antigen-binding fragment thereof that exhibits high stability; along with methods of use thereof.
Owner:MERCK SHARP & DOHME CORP
Chromatographic method for high yield purification and viral inactivation of antibodies
InactiveUS6955917B2Minimizes post virus treatment manipulationYield maximizationPeptide/protein ingredientsSerum immunoglobulinsLipid formationLow ionic strength
An improved process for the purification of antibodies from human plasma or other sources is disclosed. The process involves suspension of the antibodies at pH 3.8 to 4.5 followed by addition of caprylic acid and a pH shift to pH 5.0 to 5.2. A precipitate of contaminating proteins, lipids and caprylate forms and is removed, while the majority of the antibodies remain in solution. Sodium caprylate is again added to a final concentration of not less than about 15 mM. This solution is incubated for 1 hour at 25° C. to effect viral inactivation. A precipitate (mainly caprylate) is removed and the clear solution is diluted with purified water to reduce ionic strength. Anion exchange chromatography using two different resins is utilized to obtain an exceptionally pure IgG with subclass distribution similar to the starting distribution. The method maximizes yield and produces a gamma globulin with greater than 99% purity. The resin columns used to obtain a high yield of IgG retain IgM and IgA. IgA and IgM may be eluted from these resins in high yield and purity.
Owner:BAYER HEALTHCARE LLC
Protein purification
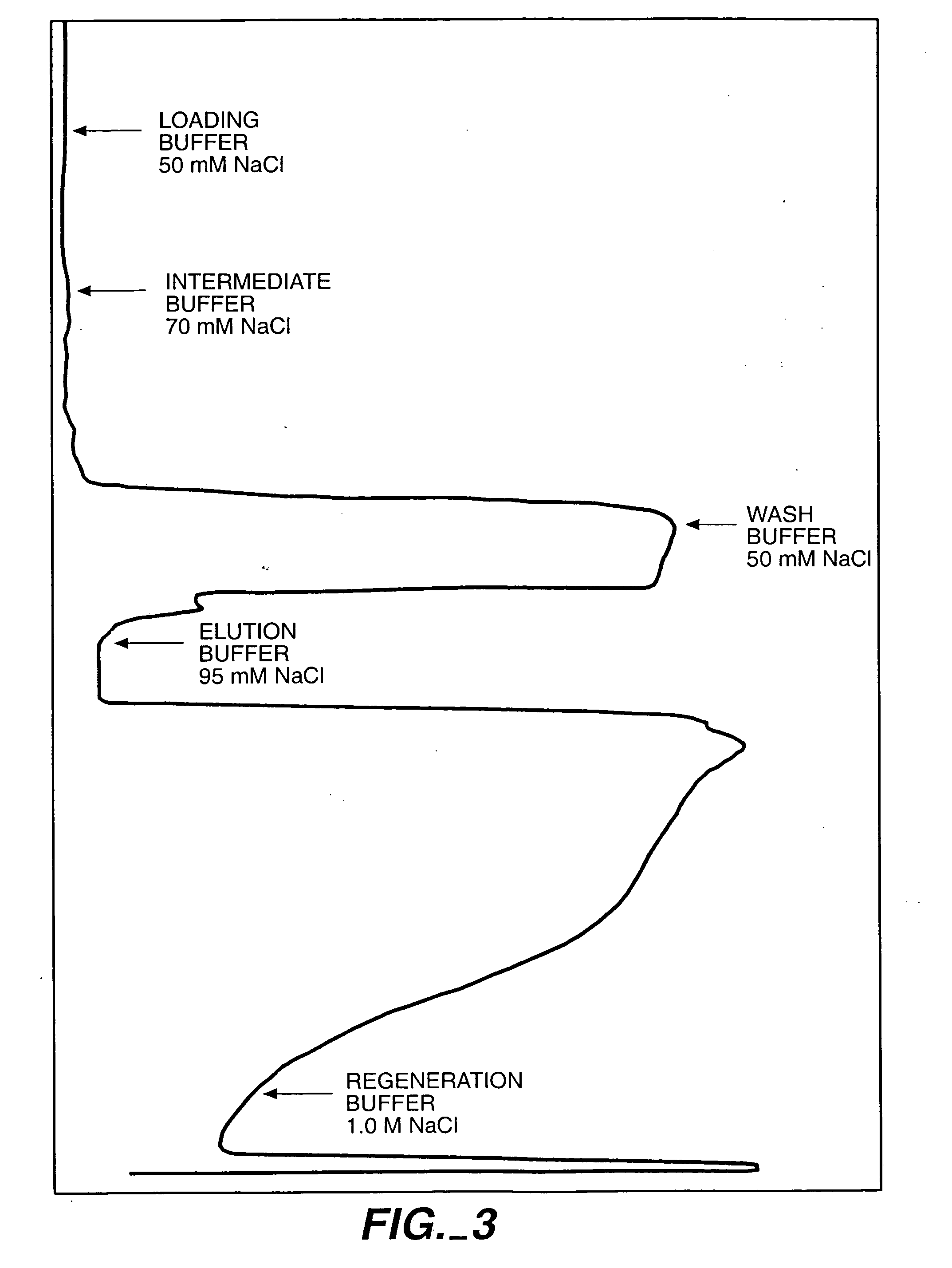
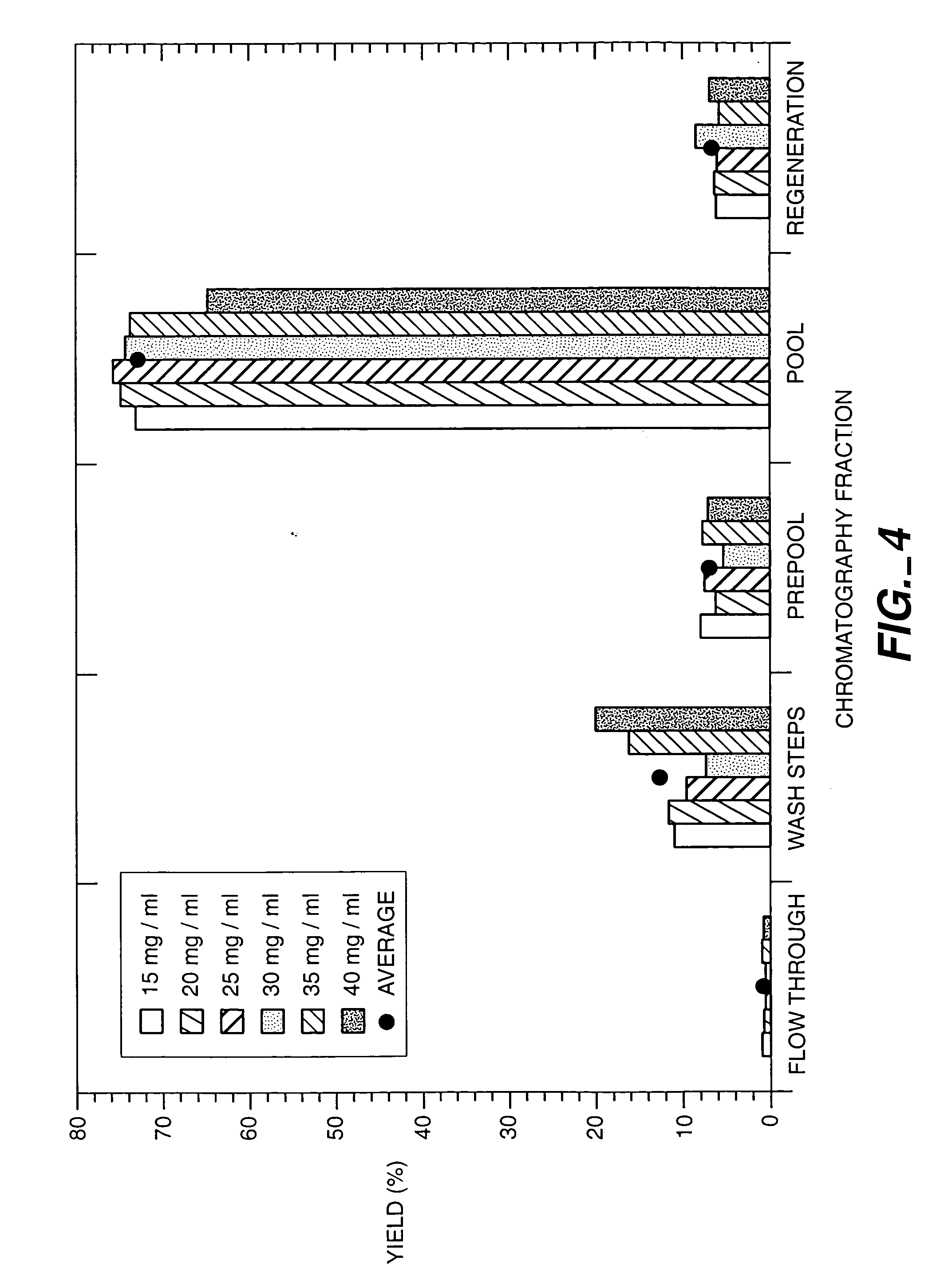
InactiveUS20050215769A1Serum immunoglobulinsImmunoglobulins against cytokines/lymphokines/interferonsProtein purificationSolid phases
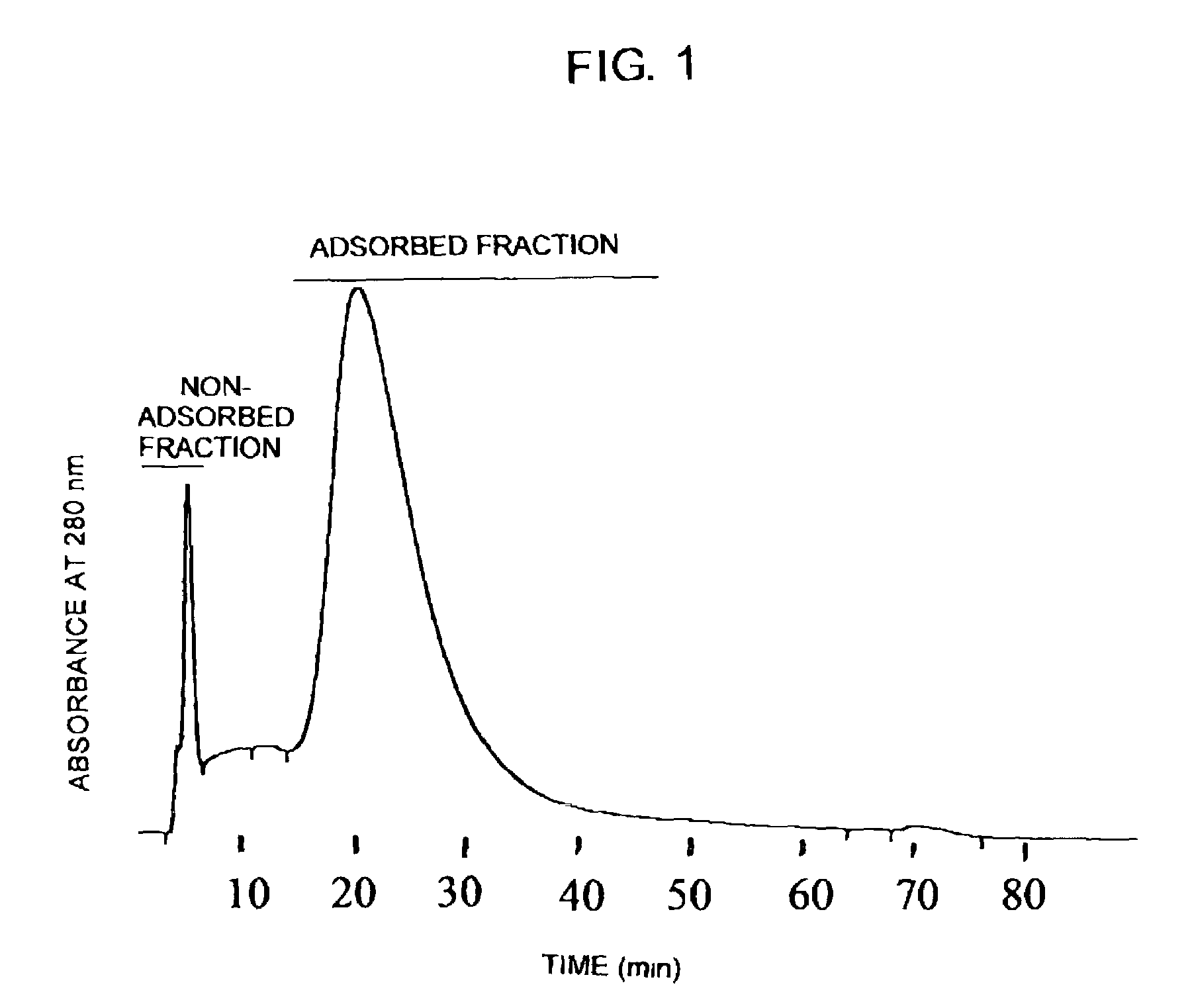
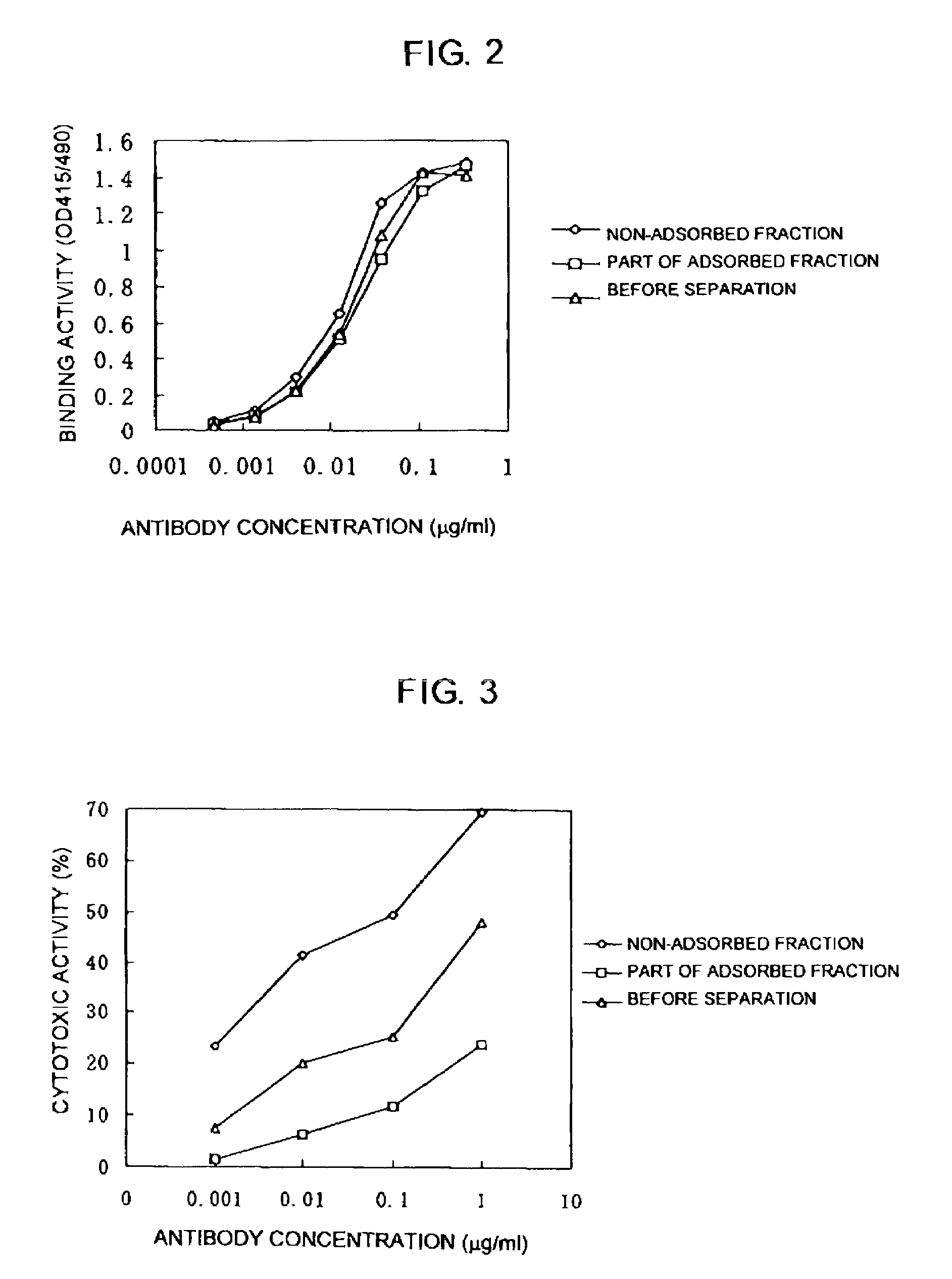
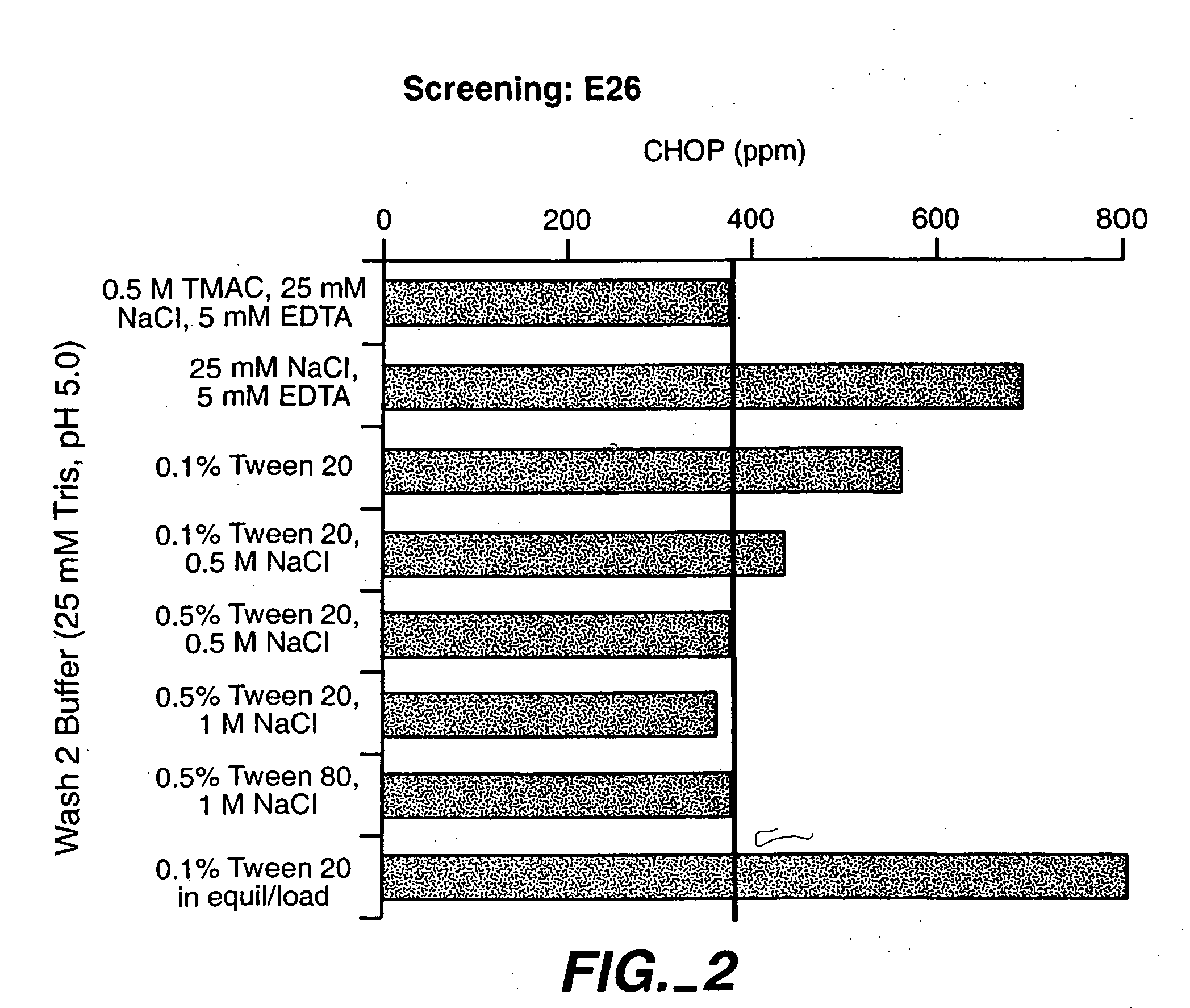
A method for purifying proteins by Protein A chromatography is described which comprises removing contaminants by washing the solid phase with various intermediate wash buffers.
Owner:BREECE TIMOTHY +5
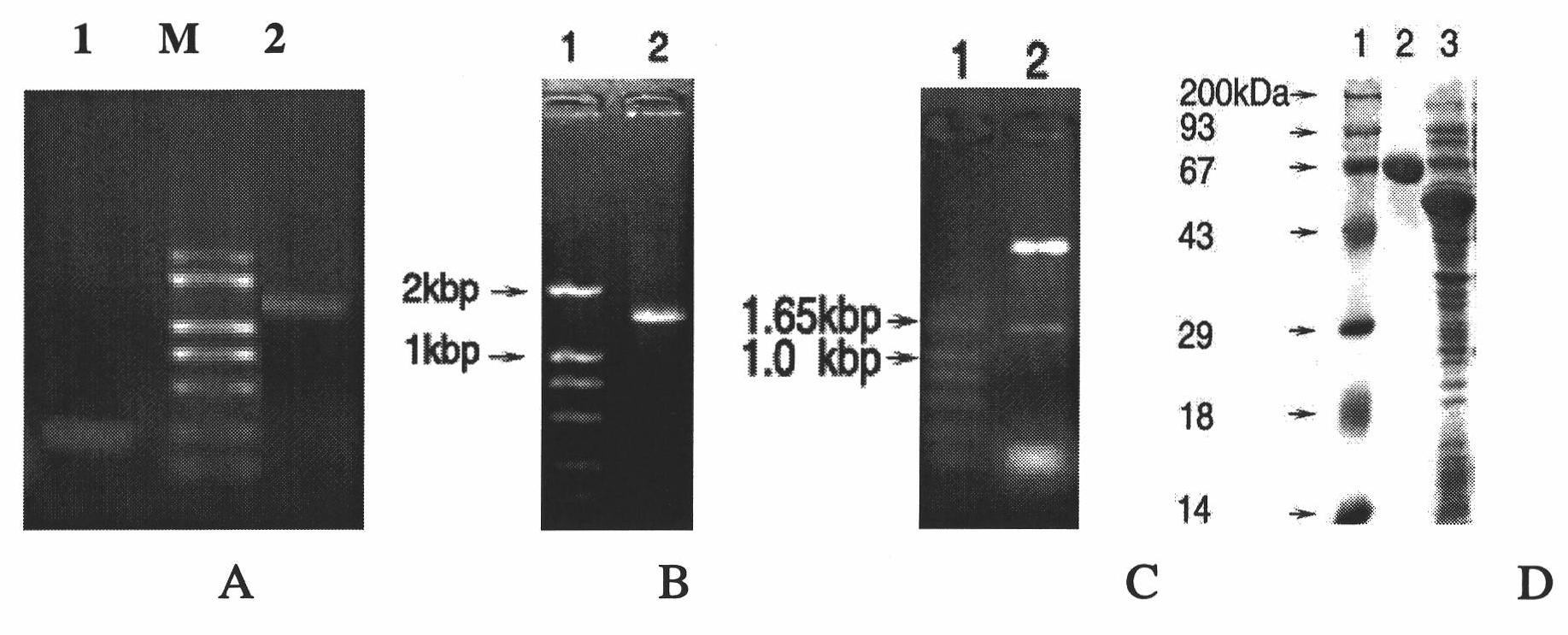
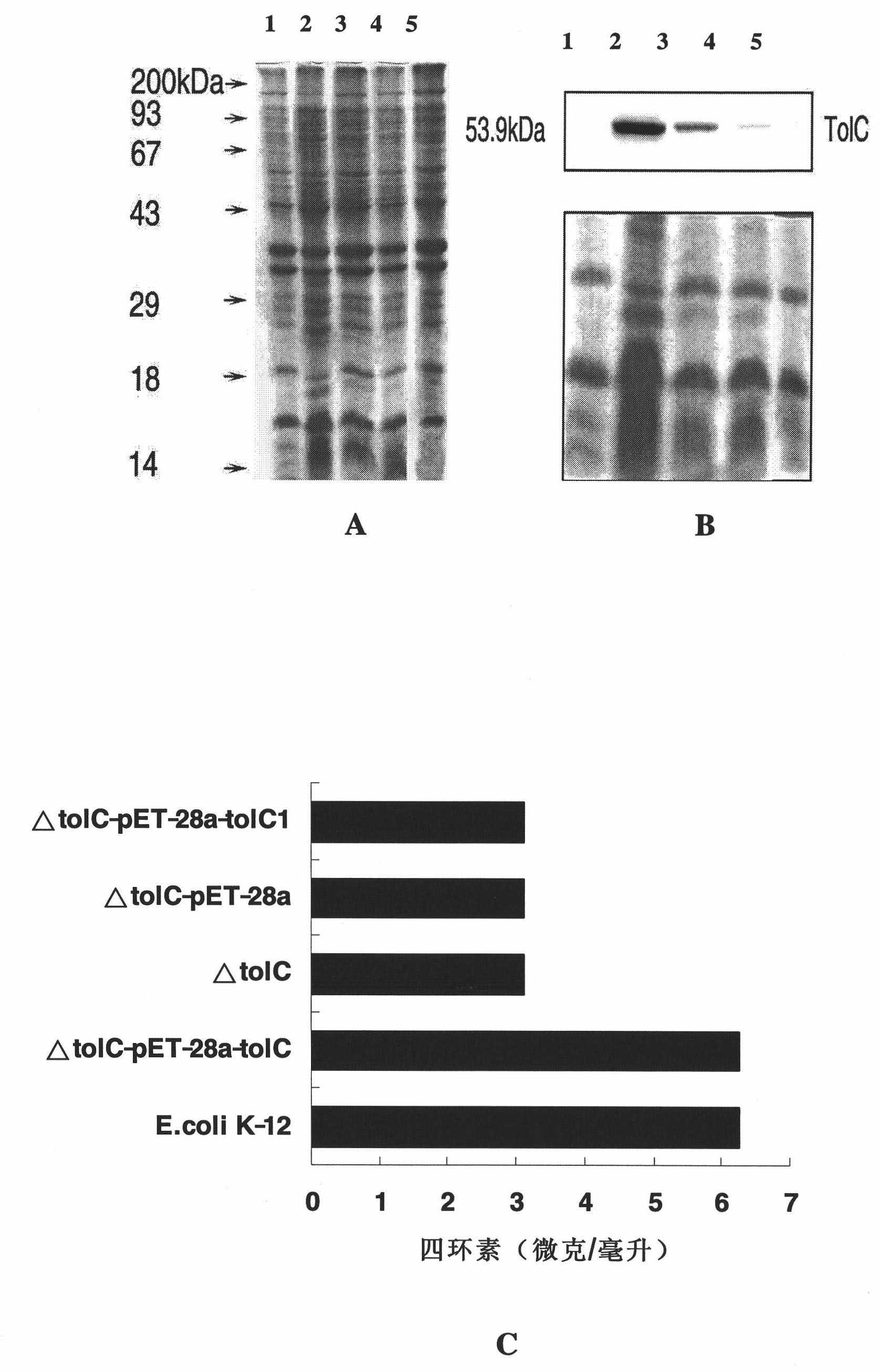
Escherichia coli TolC antigen as well as antibody and application thereof
InactiveCN101974072ASuppress drug resistanceStable targetingAntibacterial agentsSerum immunoglobulinsEscherichia coliAntigen
The invention belongs to the biotechnology field, and in particular relates to escherichia coli TolC antigen and antibody. The antibody has the specificity aiming to a sequence shown by SEQ ID NO:1. The invention also provides a gene sequence for encoding the escherichia coli TolC antigen and application of the antibody to preparing a drug for inhibiting the bacterial resistance. The anti-TolC fragment SNGYRDANGI antibody enhances the sensitivity of bacteria to antibiotic and can specifically and efficiently inhibit the functions of drug resistance outer membrane protein of escherichia coli, thereby solving the problem of the bacterial resistance. Compared with the traditional antibody, the antibody has higher safety and operability on the application of the bacterial resistance drug.
Owner:SUN YAT SEN UNIV
Oral immunotherapy of bacterial overgrowth
InactiveUS6096310AInhibiting and reducingReduce acidityBacterial antigen ingredientsSerum immunoglobulinsDiseaseBacteroides
The object of the present invention is the oral administration of animal immunoglobulins for the treatment of gastrointestinal disorders caused by bacterial and / or yeast overgrowth. Immunoglobulins derived from the blood, plasma or serum of animals, such as cow, goats, sheep and pigs, contain a broad spectrum of antibodies to bacteria and yeast that afflict the gastrointestinal tract of human patients.
Owner:BIER MILAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com