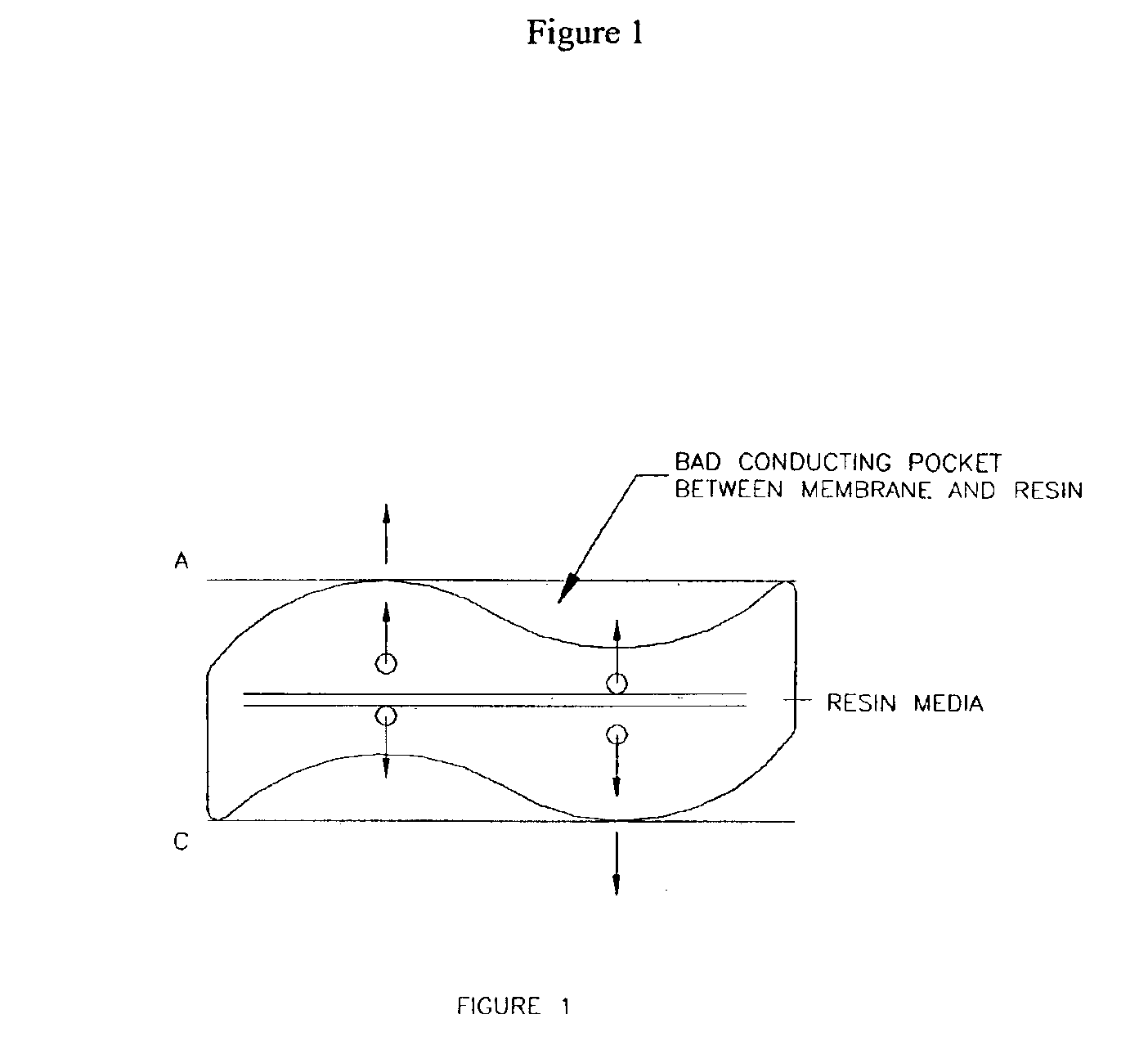
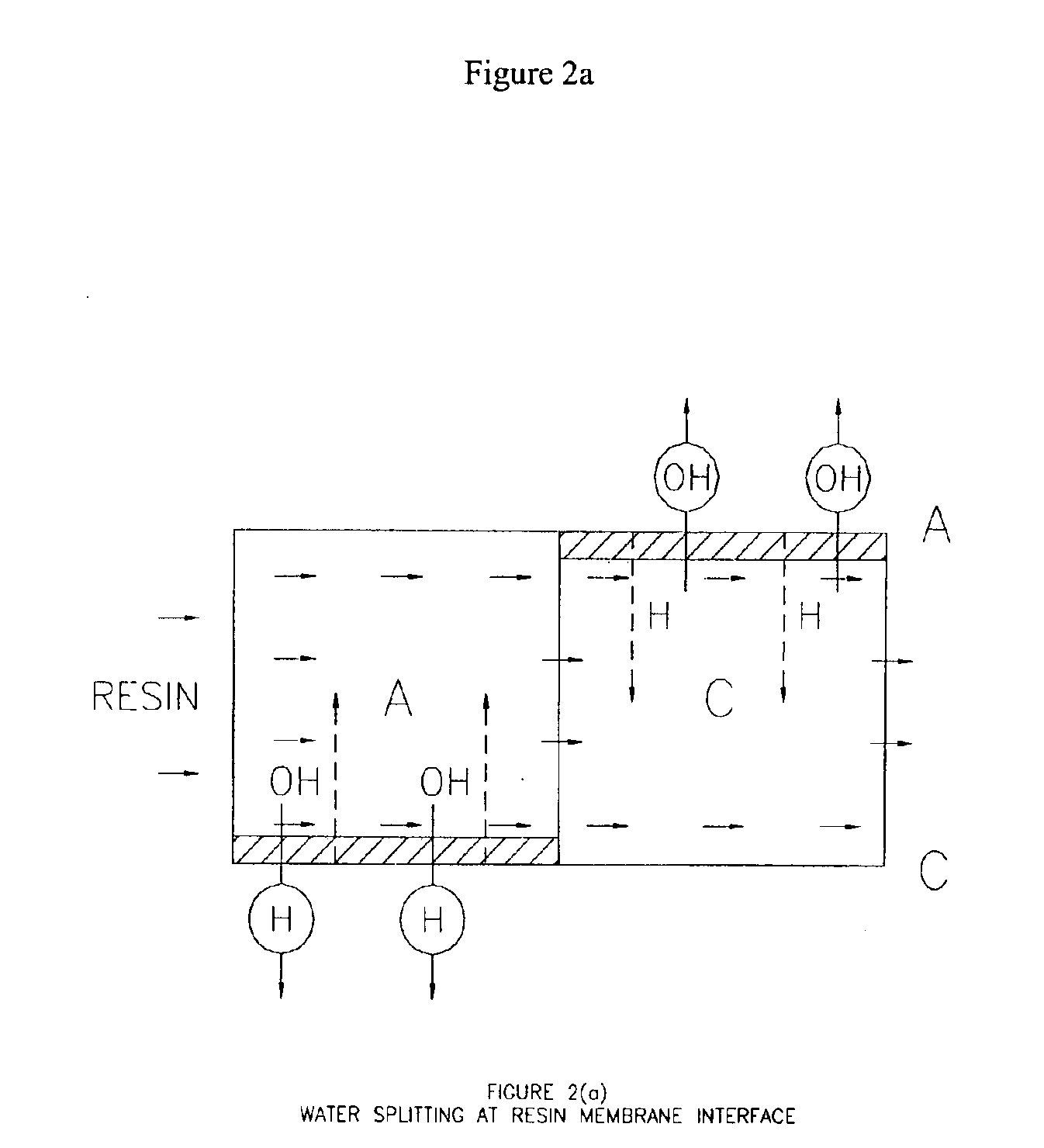
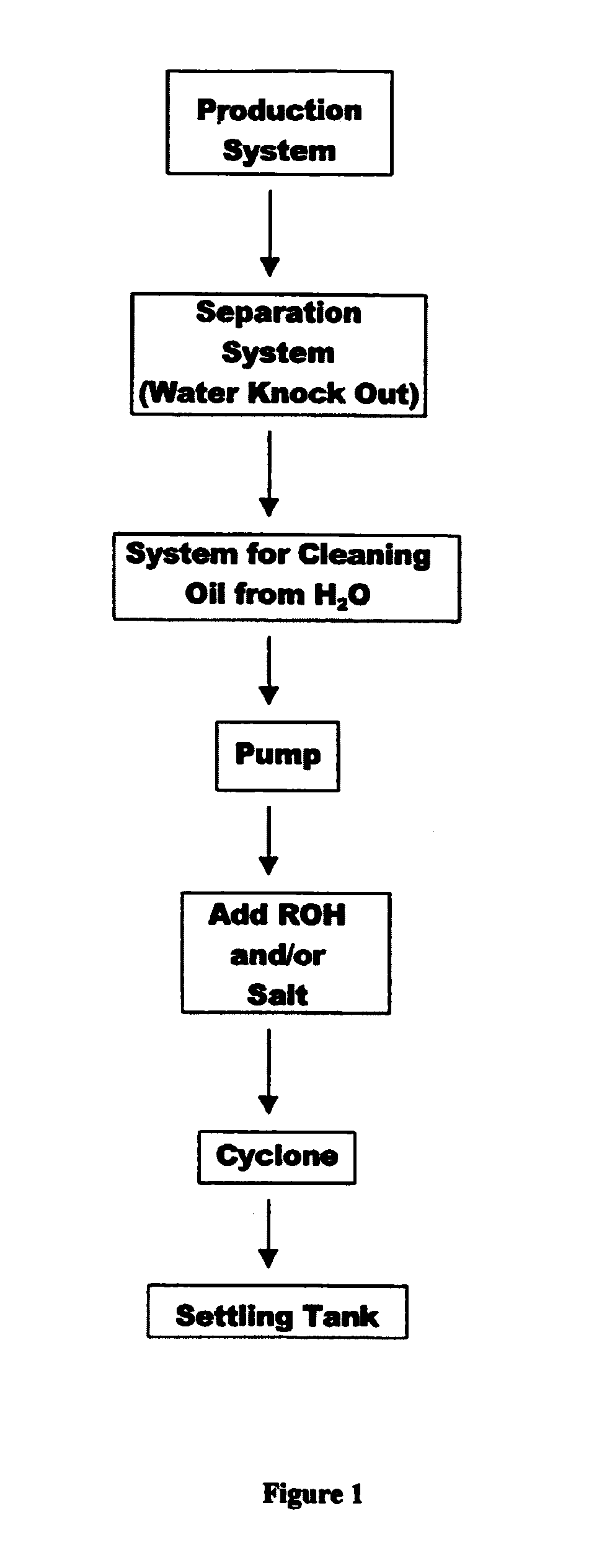
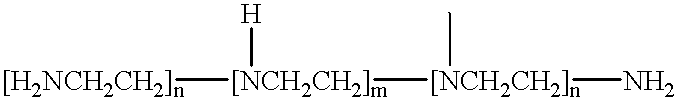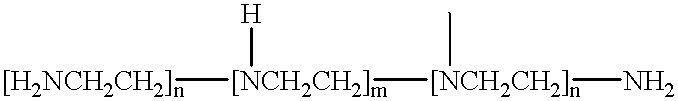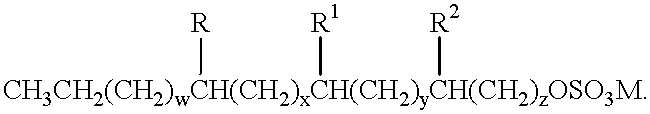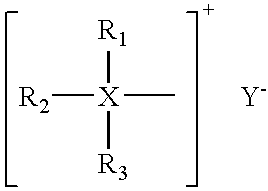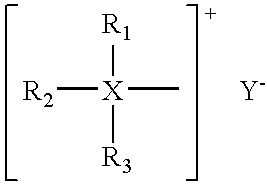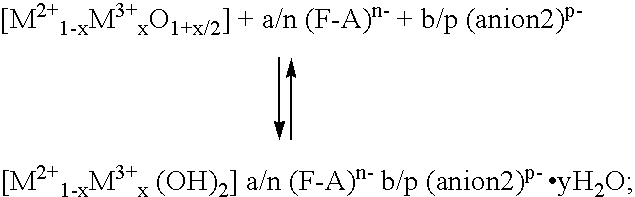Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
616 results about "Ionic strength" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
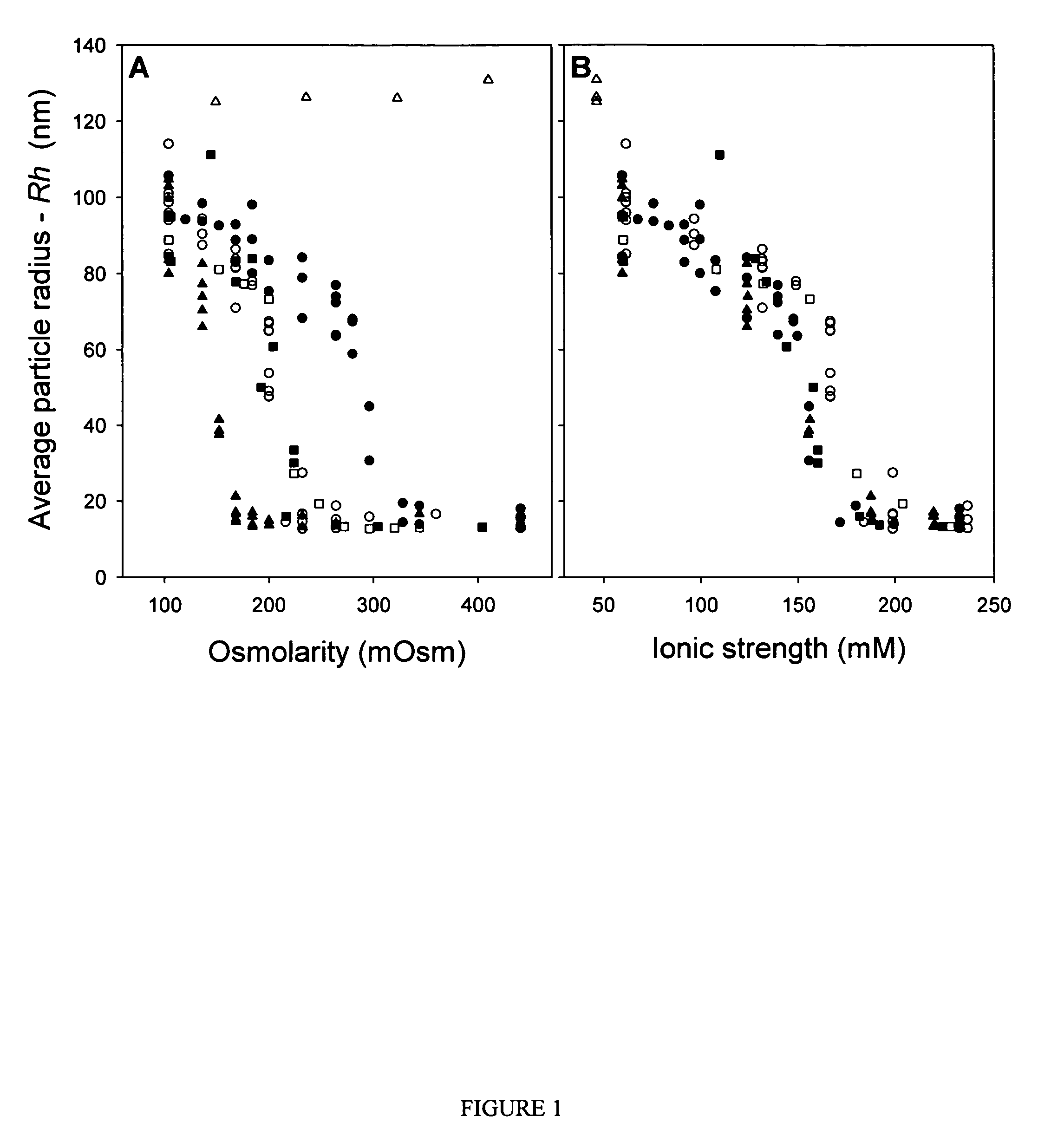
The concept of ionic strength was first introduced by Lewis and Randall in 1921 while describing the activity coefficients of strong electrolytes. The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation constant or the solubility of different salts. One of the main characteristics of a solution with dissolved ions is the ionic strength. Ionic strength can be molar (mol/L) or molal (mol/kg water) and to avoid confusion the units should be stated explicitly.
Buffer solution for electroporation and a method comprising the use of the same
InactiveUS20050064596A1High transfection efficiencyReduce cell deathPeptide/protein ingredientsGenetic material ingredientsElectroporationIon
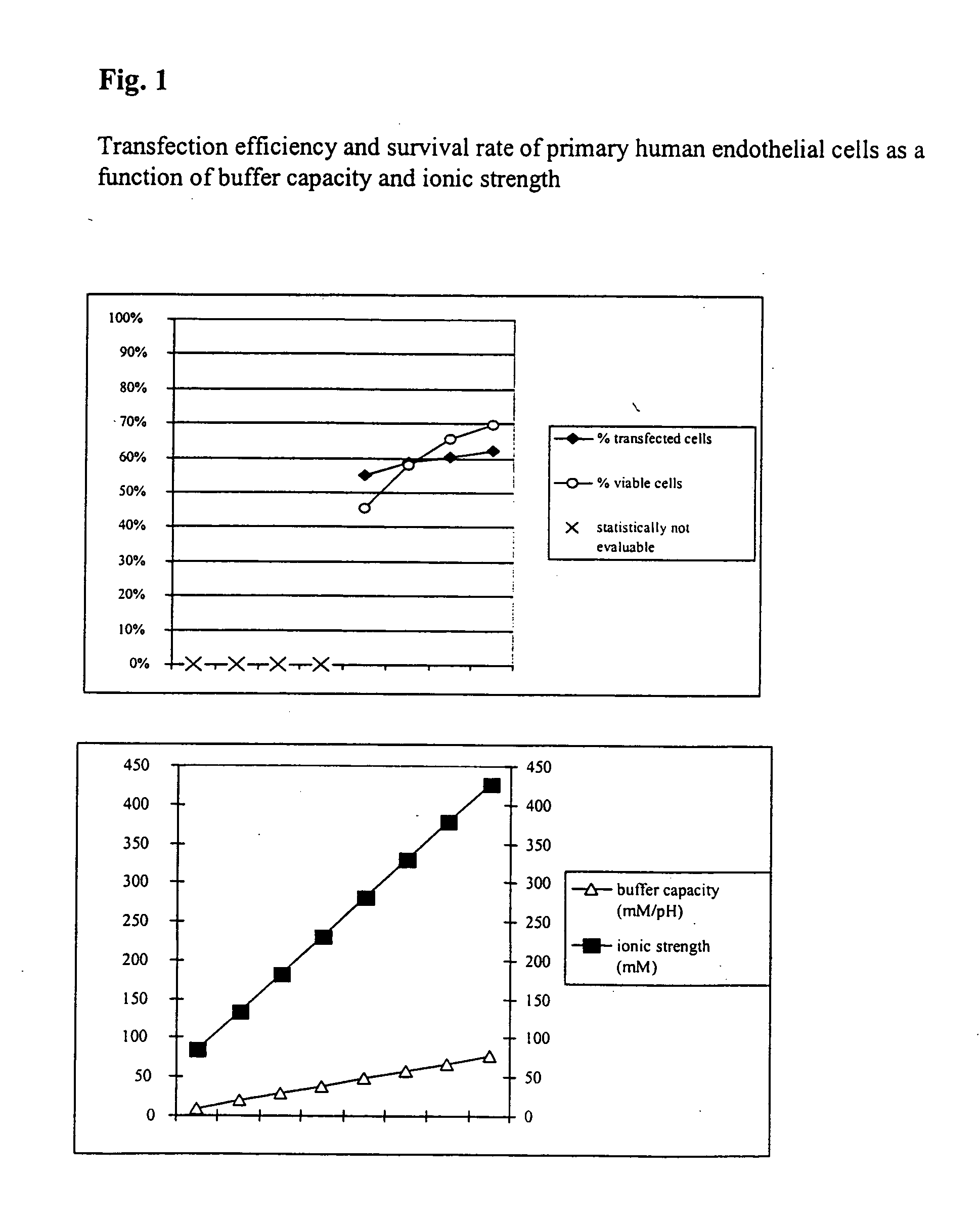
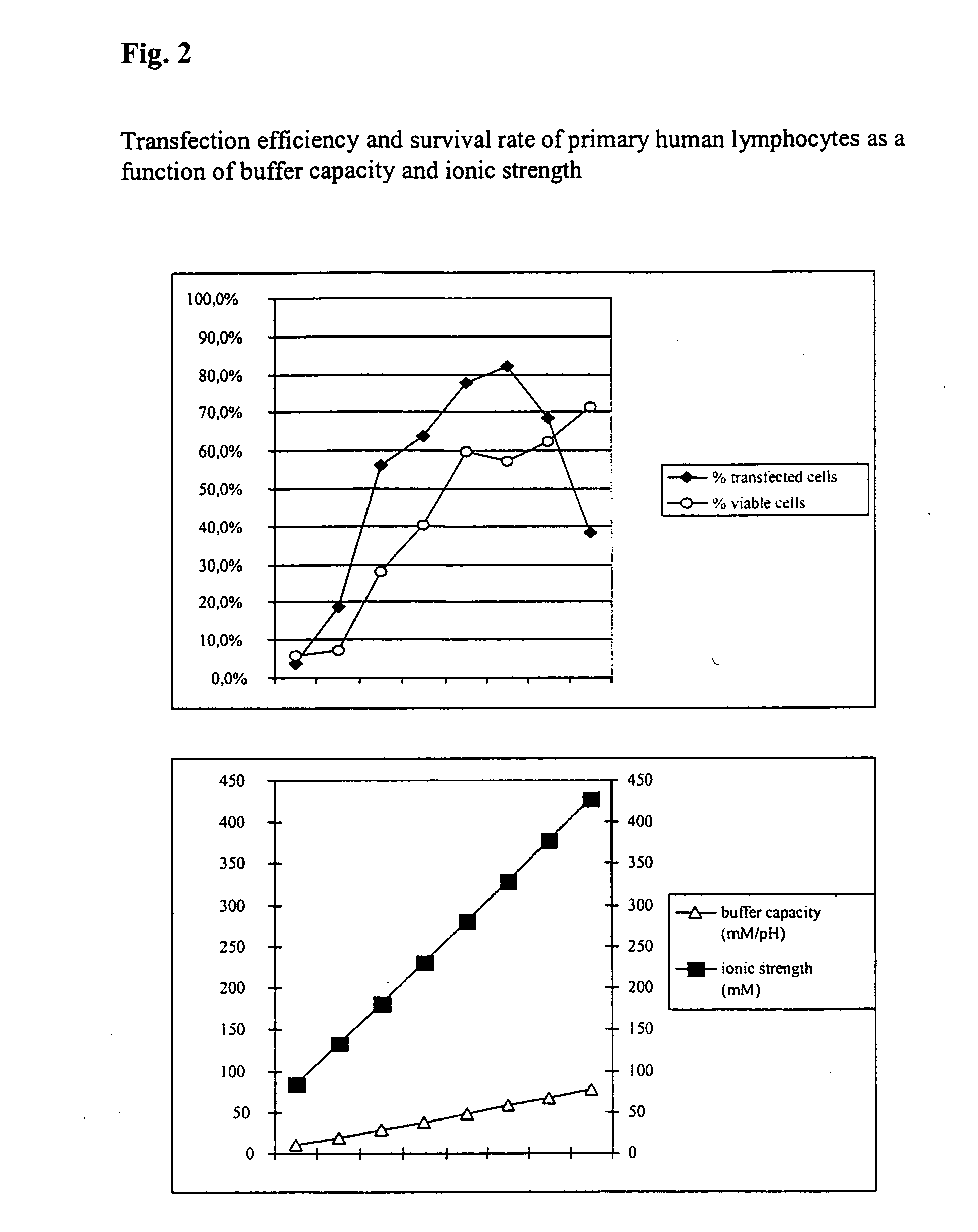
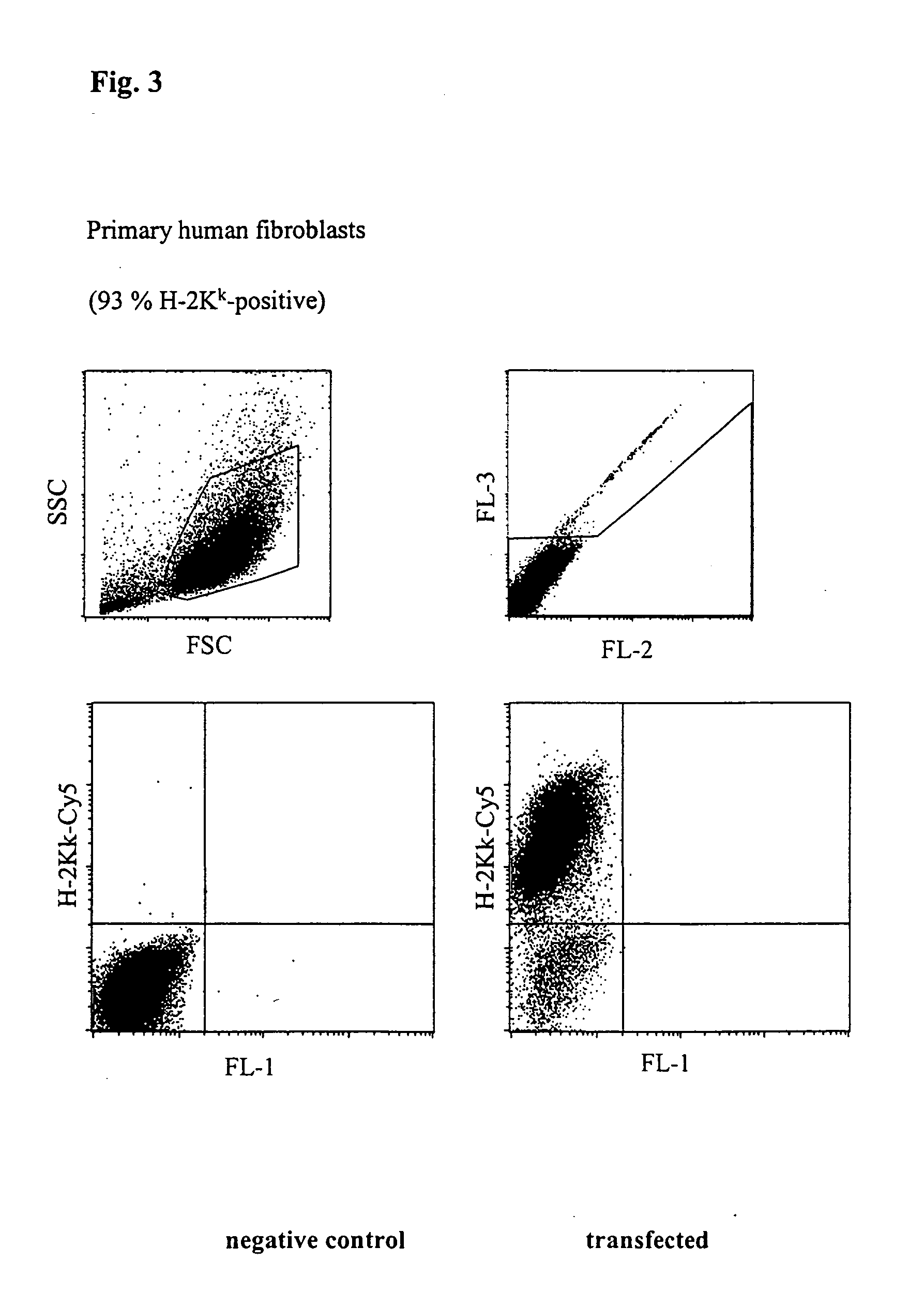
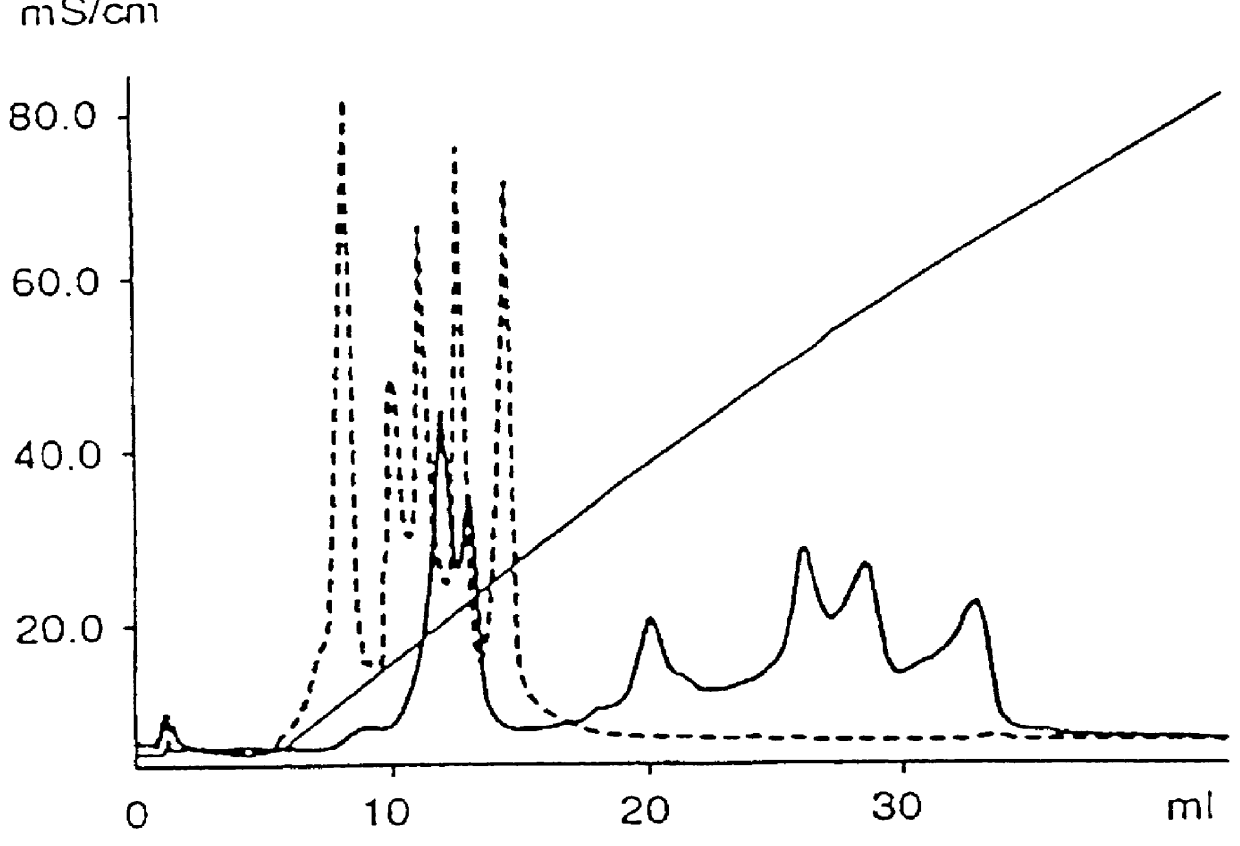
The invention relates to a buffer solution for suspending animal or human cells and for dissolving biologically active molecules in order to introduce said biologically active molecules into the cells using an electric current and to a method for introducing biologically active molecules into animal or human cells using an electric current and a buffer solution. The inventive buffer solution has a buffering capacity of at least 20 mmol*I−1*pH−1 and an ionic strength of at least 200 mmol*I−1 during a change to the pH value from pH 7 to pH 8 and at a temperature of 25° C. The use of a buffer solution of this type in the corresponding method allows biologically active molecules to be introduced into animal and human cells with a high degree of transfection efficiency and at the same time a low cell mortality. Different cell types, in particular dormant and actively dividing cells of low activity, can be successfully transfected in said buffer solution.
Owner:LONZA COLOGNE
Process for chromatographic separation of peptides and nucleic acid, and new high affinity ion exchange matrix
InactiveUS6090288ACation exchanger materialsComponent separationChromatographic separationTransferrin
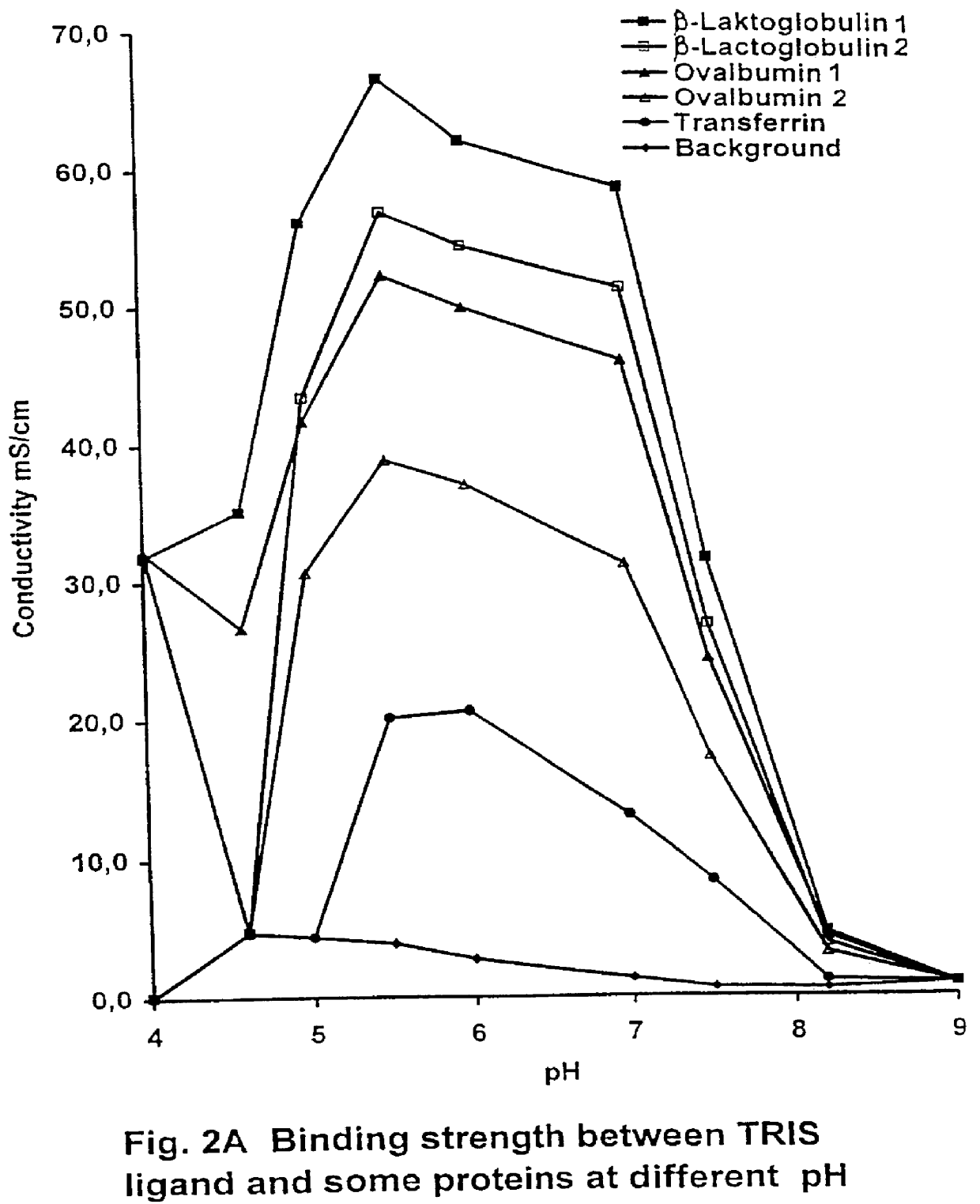
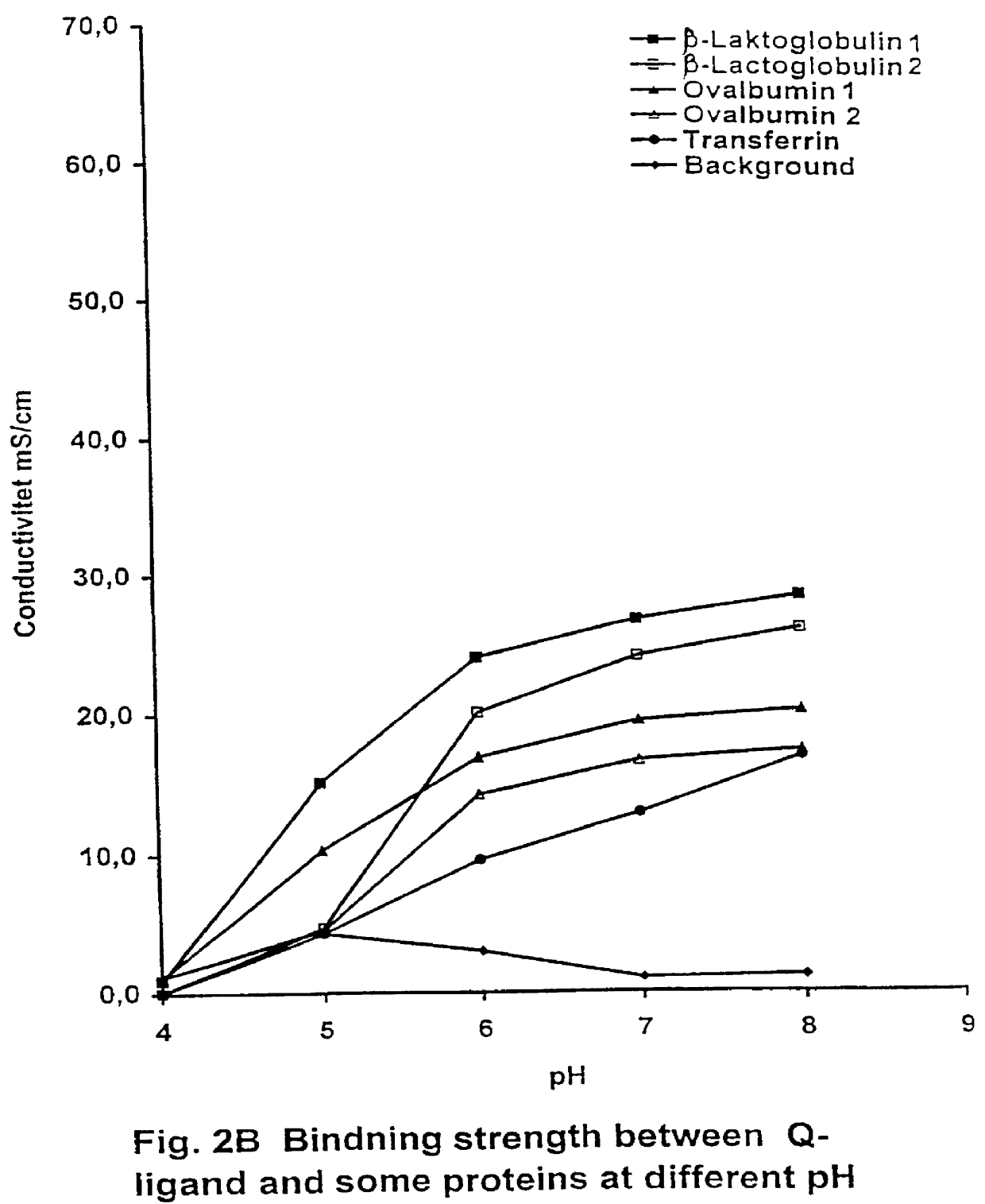
PCT No. PCT / SE97 / 00237 Sec. 371 Date Dec. 29, 1998 Sec. 102(e) Date Dec. 29, 1998 PCT Filed Feb. 14, 1997 PCT Pub. No. WO97 / 29825 PCT Pub. Date Aug. 21, 1997Process for separating off a peptide or a nucleic acid by an anion exchanger (I) characterized in that a) the anion exchanger (I) exhibits ligands, which (i) contain a primary, secondary or tertiary amino group and (ii) are covalently bound to an organic polymer (matrix), b) there on a carbon atom at a distance of 2 or 3 atoms away from an amino nitrogen in the ligands is a hydroxyl group or a primary, secondary or tertiary amino group, and c) the maximum elution ionic strength in the pH range 2-14 for at least one of the proteins transferrin, ovalbumin 1, ovalbumin 2, beta -lactoglobulin 1 and beta -lactoglobulin 2 on the anion exchanger is higher than the elution ionic strength required for a quaternary comparative ion exchanger.
Owner:GE HEALTHCARE BIOPROCESS R&D
Method of completing poorly consolidated formations
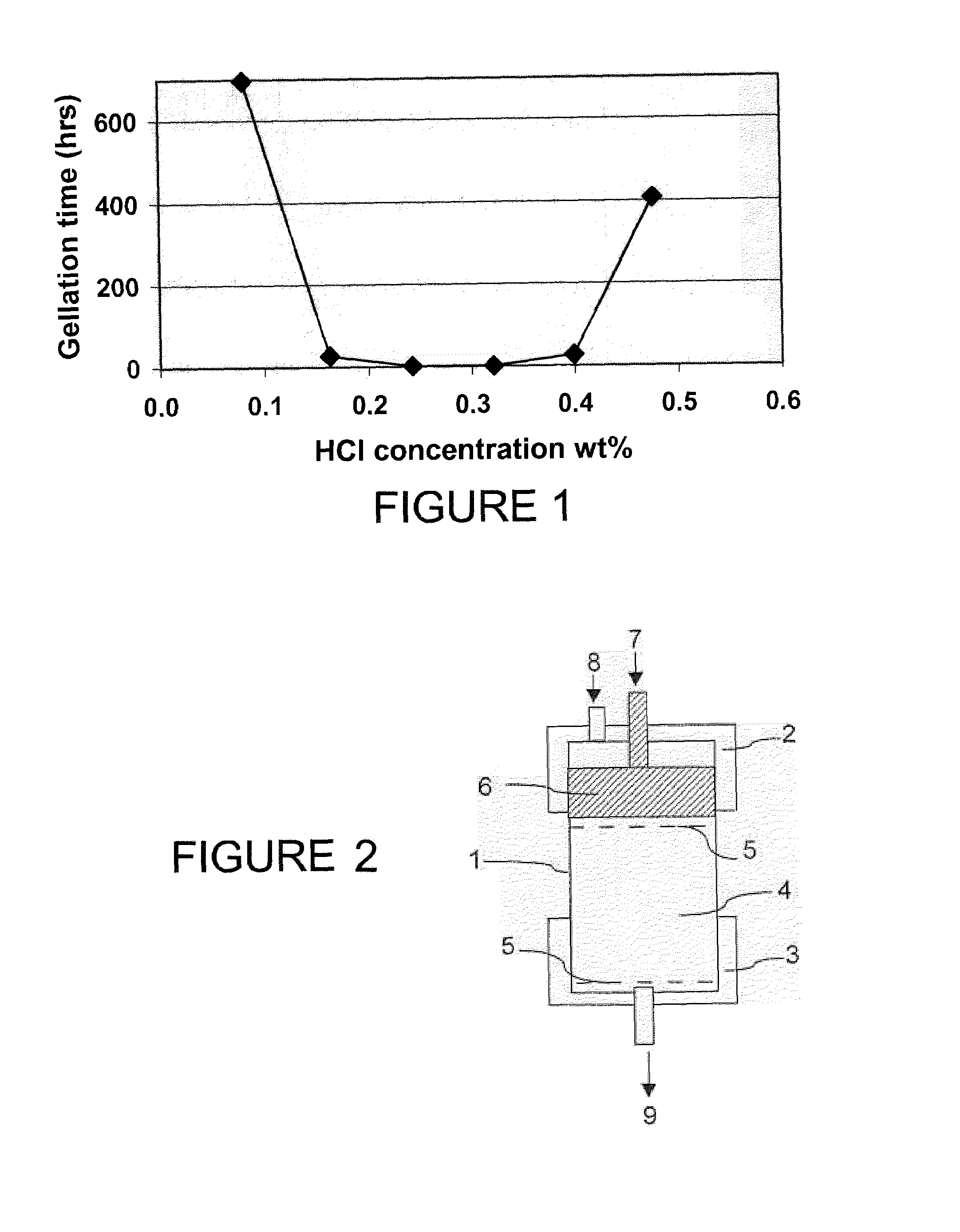
It is proposed a method for completing an unconsolidated interval, including particulates, in a subterranean formation, including a consolidation steps. The consolidation step is performed by injection of an aqueous solution of colloidal particles with a pH modifier and / or an ionic-strength modifier. A hard gel is formed that holds the particulates together. The consolidation is followed by hydraulic fracturing. Diversion towards area of less permeability may be enhanced by the use of micrometric particles.
Owner:SCHLUMBERGER TECH CORP
Novel hydrogels and uses thereof
ActiveUS20060025524A1Easy adhesionRapid isolationDrug photocleavageOintment deliveryIonic strengthUltimate tensile strength
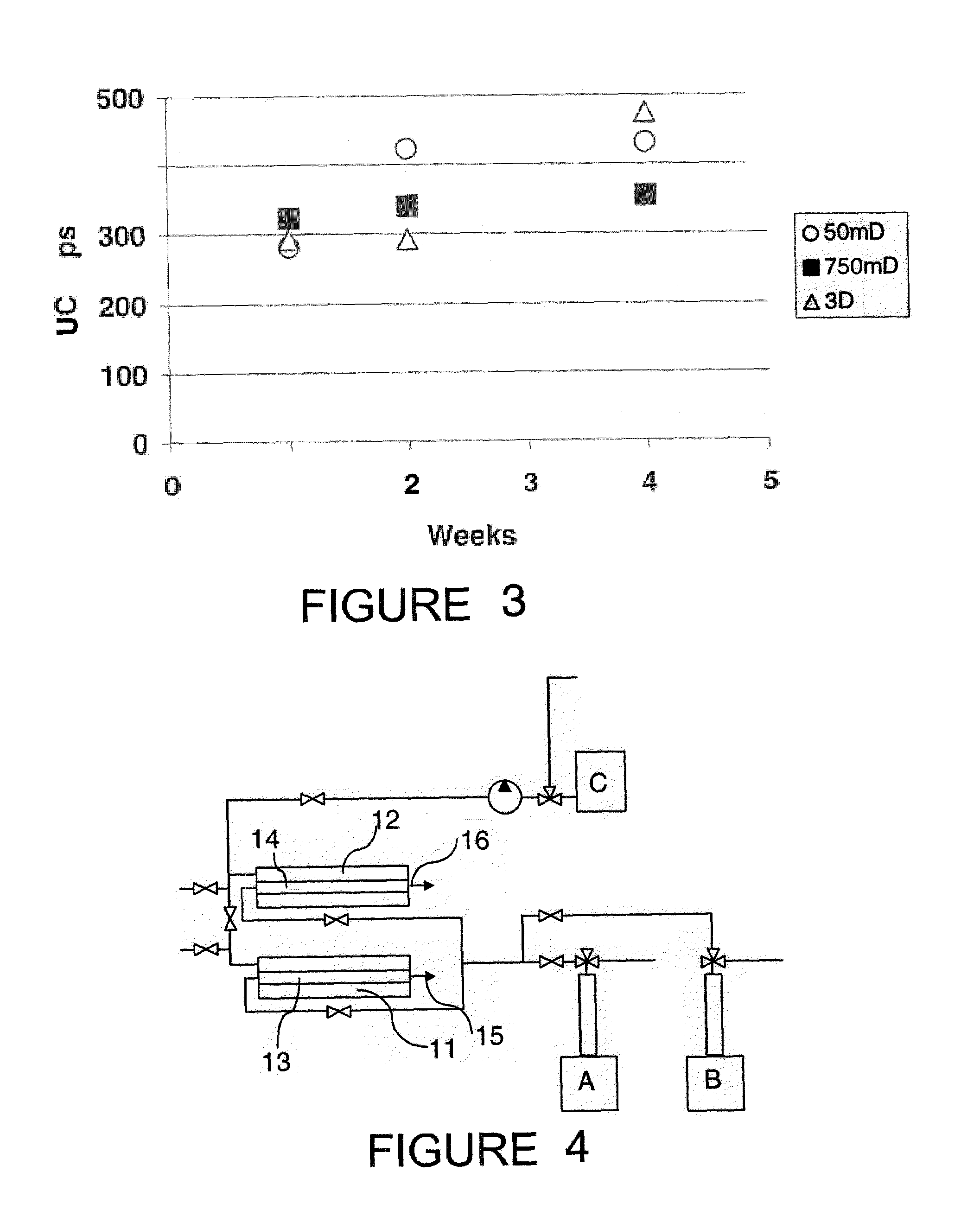
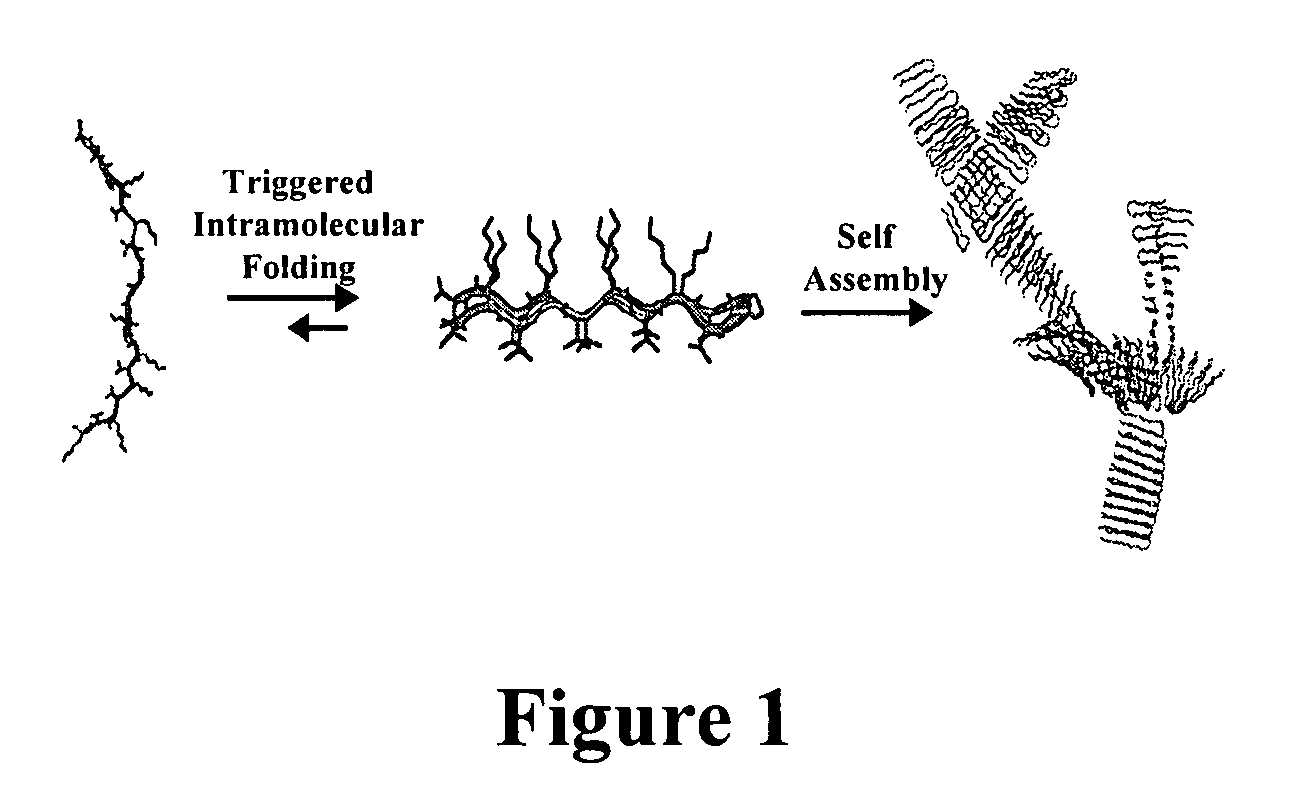
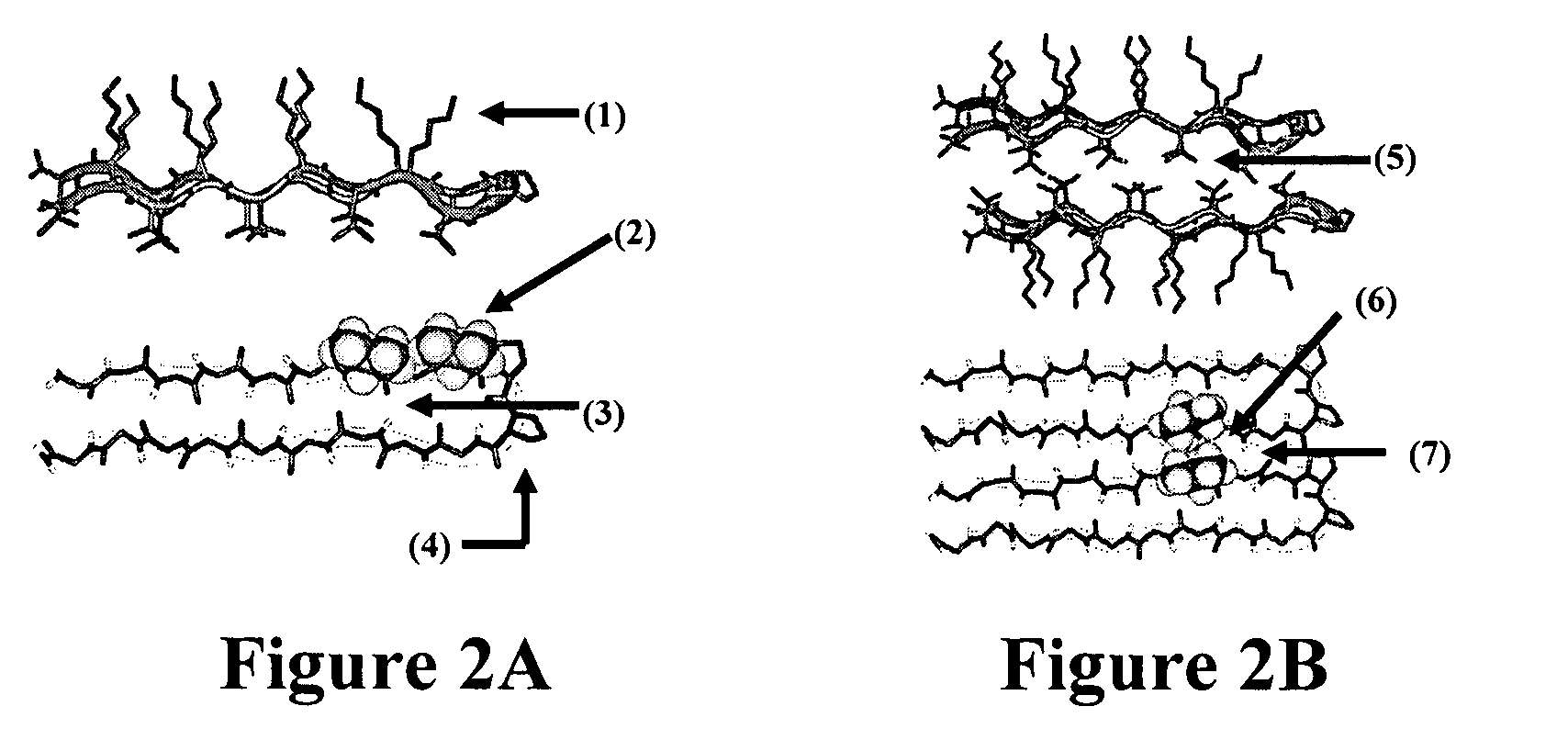
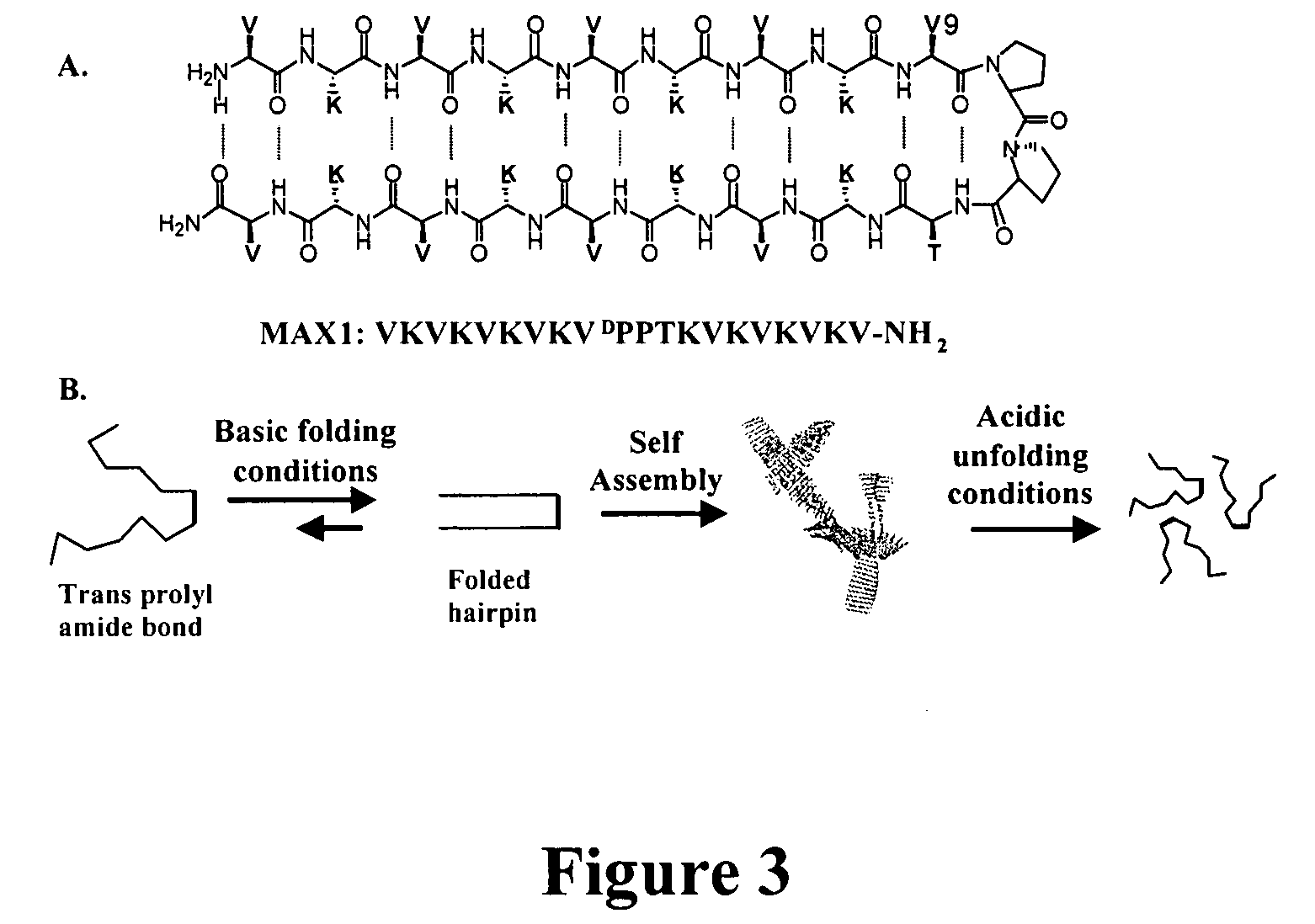
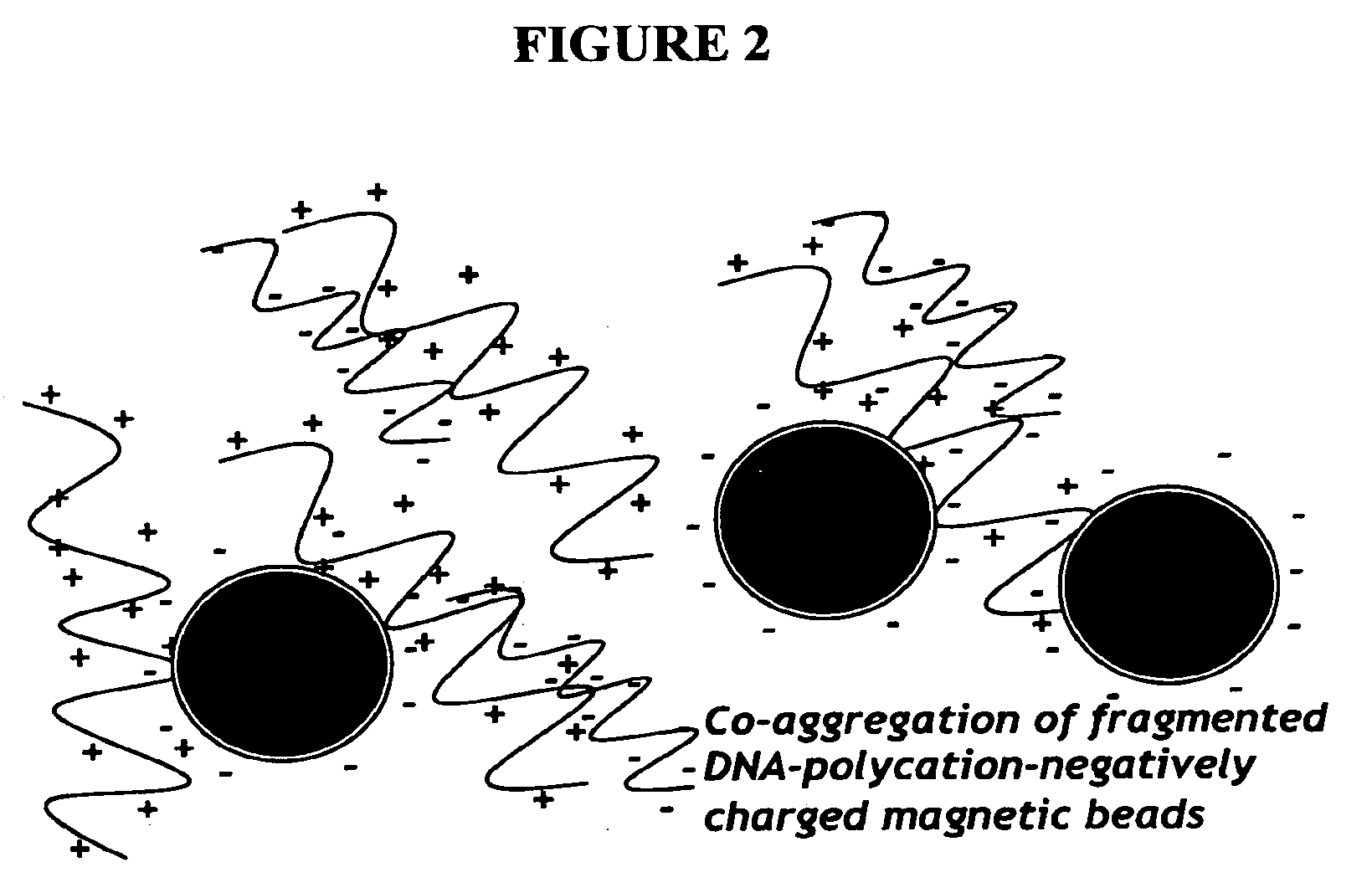
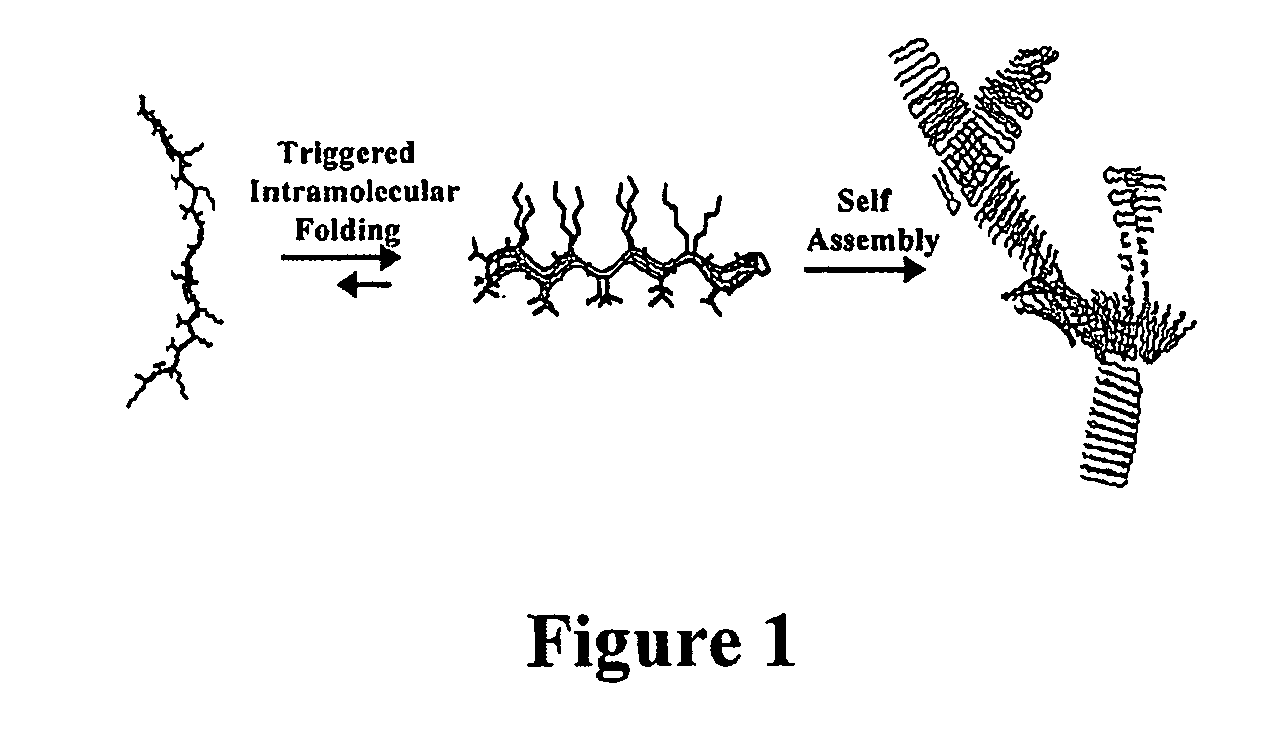
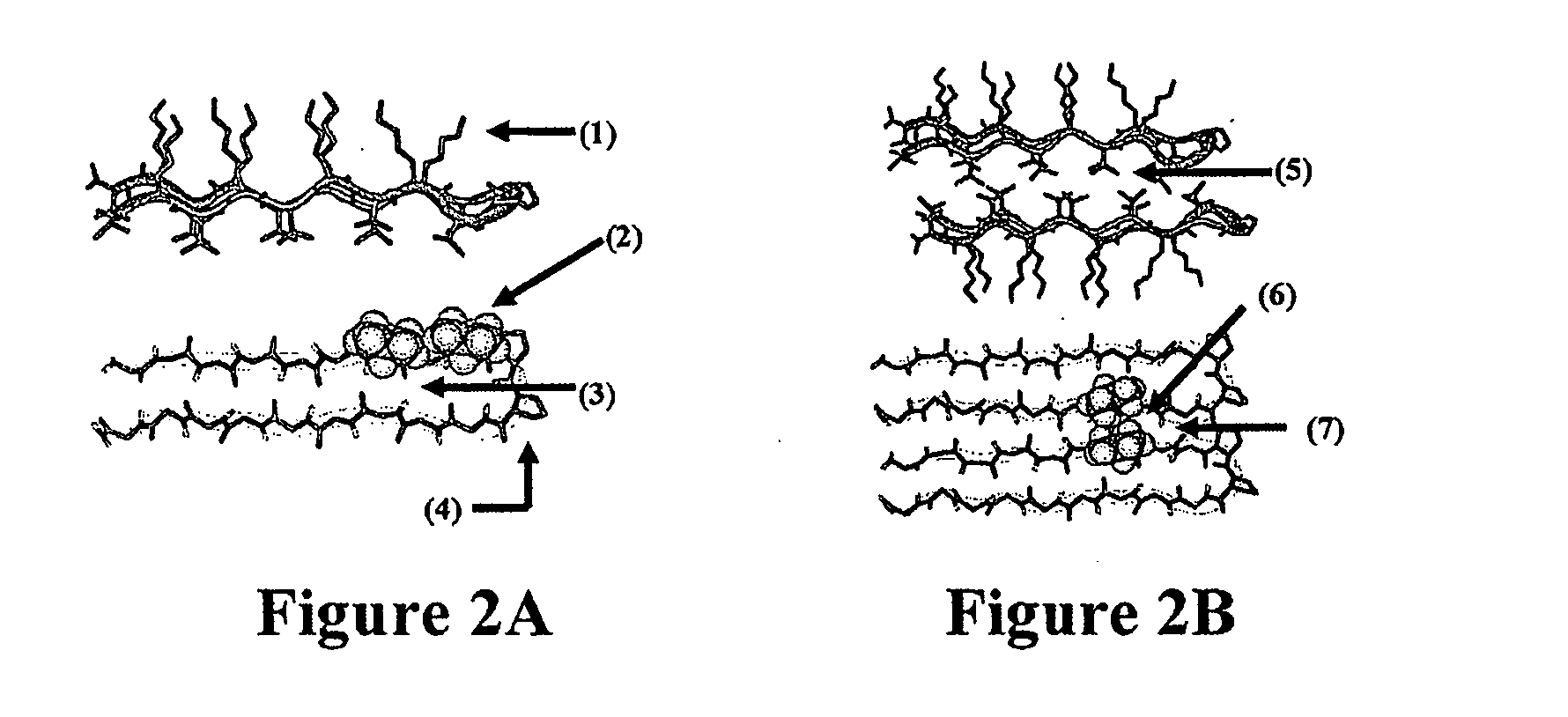
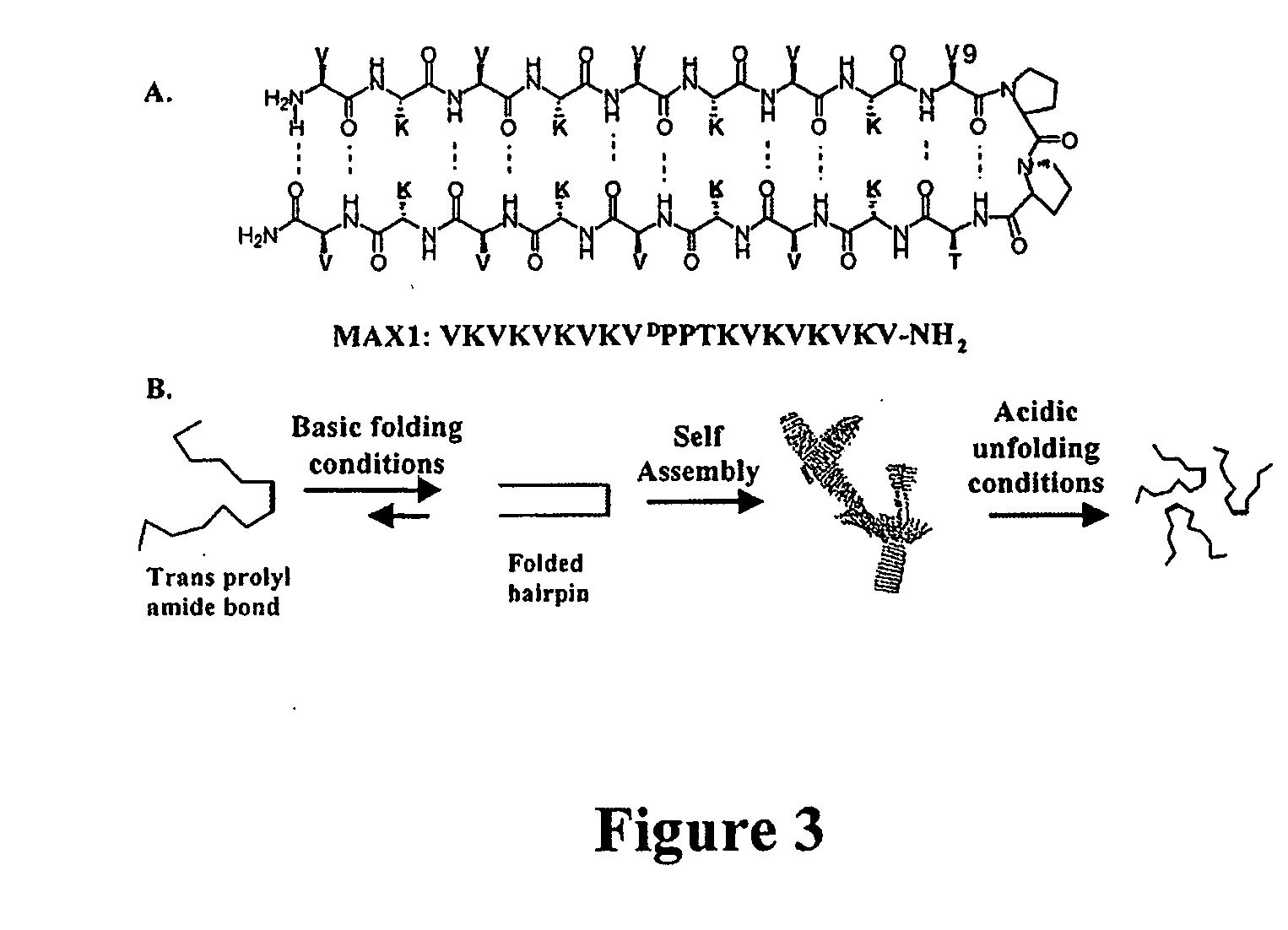
The present invention provides novel hydrogels and methods of making and using such hydrogels. The present invention provides hydrogels that may be formed by the self-assembly of peptides in solution. Such self-assembly may be brought about by a change in one or more characteristics of the solution. Characteristics of the solution that may be changed include pH, ionic strength, temperature, and concentration of one or more specific ions. In addition, hydrogels of the invention may be disassembled by changing one or more characteristic of the hydrogel such as pH, ionic strength, temperature, and concentration of one or more specific ions.
Owner:UNIV OF DELAWARE TECH CORP +1
Beneficial effects of increasing local blood flow
InactiveUS20110028548A1Increase oxygenationImprove tissue nutritionBiocidePeptide/protein ingredientsArginineNitric oxide
Owner:STRATEGIC SCI & TECH
Method and kit for extracting prion protein
InactiveUS6150172AMethod is fastThe testing process is simplePeptide preparation methodsDepsipeptidesIonic strengthSheep brain
A method for extracting prion protein from a biological material, e.g., an animal tissue or product. In a specific example, abnormal prion protein is extracted from homogenized sheep brain with hexafluoro-2-propanol. The hexafluoro-2-propanol is separated from the aqueous brain preparation by increasing the ionic strength of the aqueous solution. Prion protein in the organic extract can be further purified, or the extract can be tested, e.g., by immunoassay, for the presence of prion protein, and more particularly abnormal prion protein. The extraction process permits testing for the presence of abnormal prior protein, e.g., for diagnosis of transmissible spongiform encephalopathies (TSE).
Owner:US SEC AGRI
Compositions containing expandable microspheres and an ionic compound, as well as methods of making and using the same
InactiveUS20070044929A1Inorganic compound additionThin material handlingZeta potentialIonic strength
This invention relates to composition containing expandable microspheres and at least one ionic compound and having a zeta potential that is greater than or equal to zero mV at a pH of about 9.0 or less at an ionic strength of from 10−6 M to 0.1 M., as well as methods of making and using the composition.
Owner:INT PAPER CO
Functional synthetic molecules and macromolecules for gene delivery
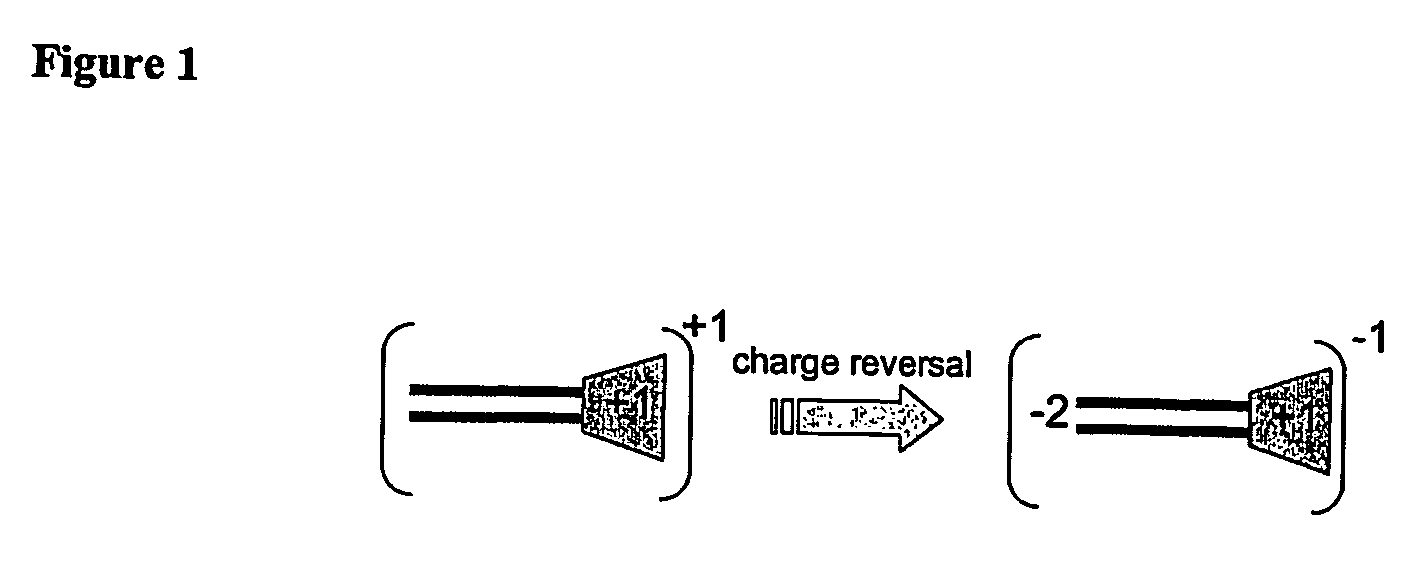
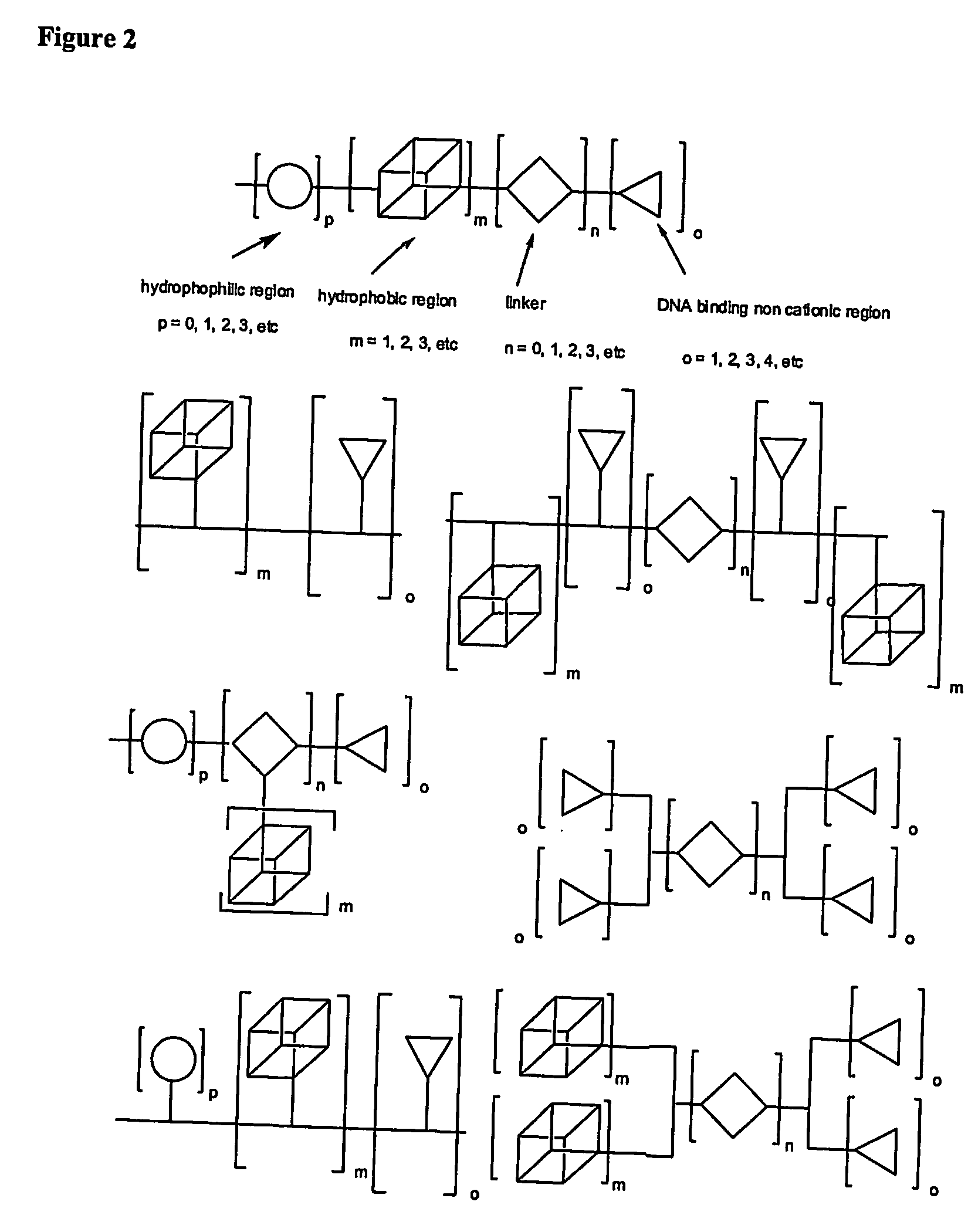
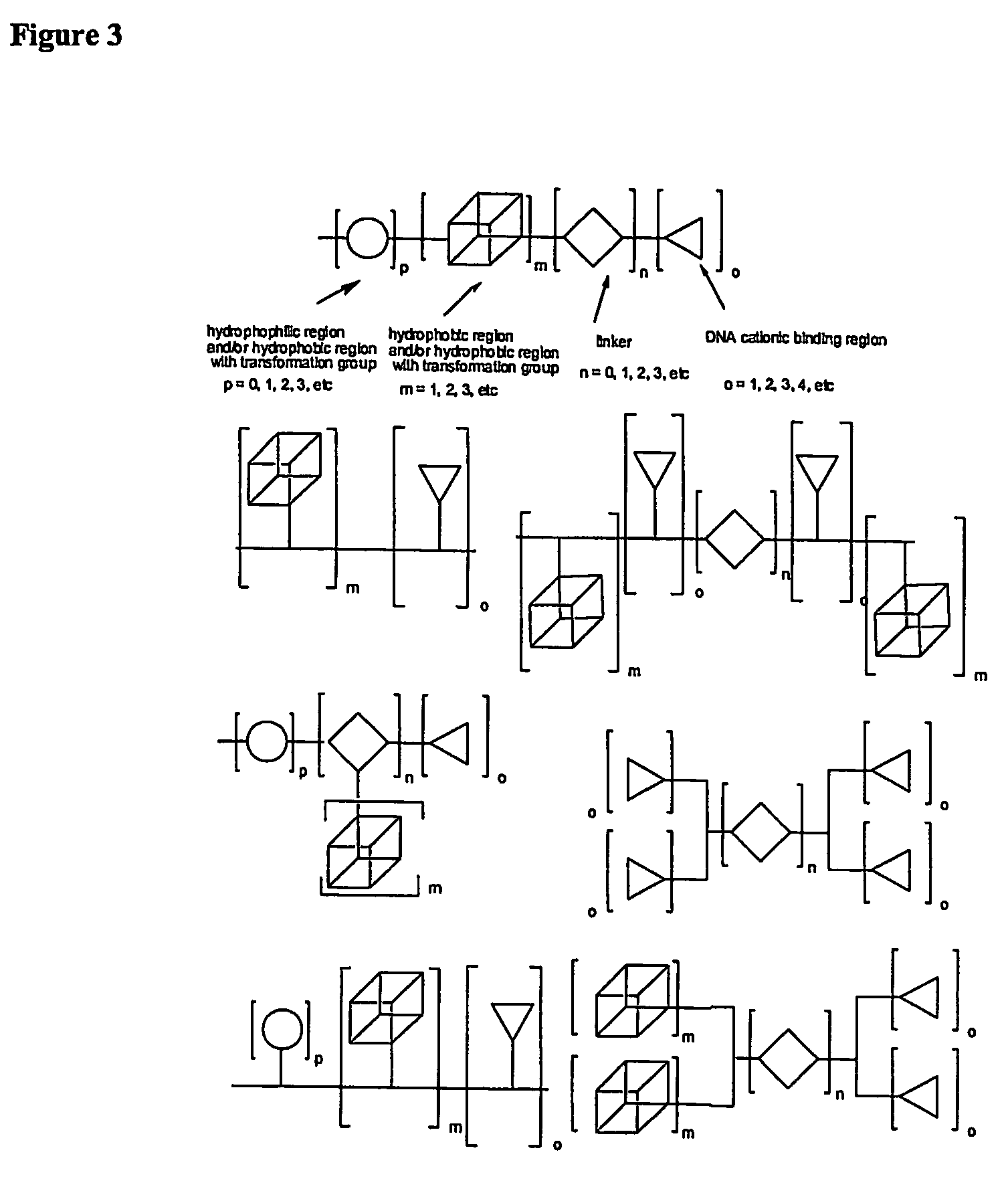
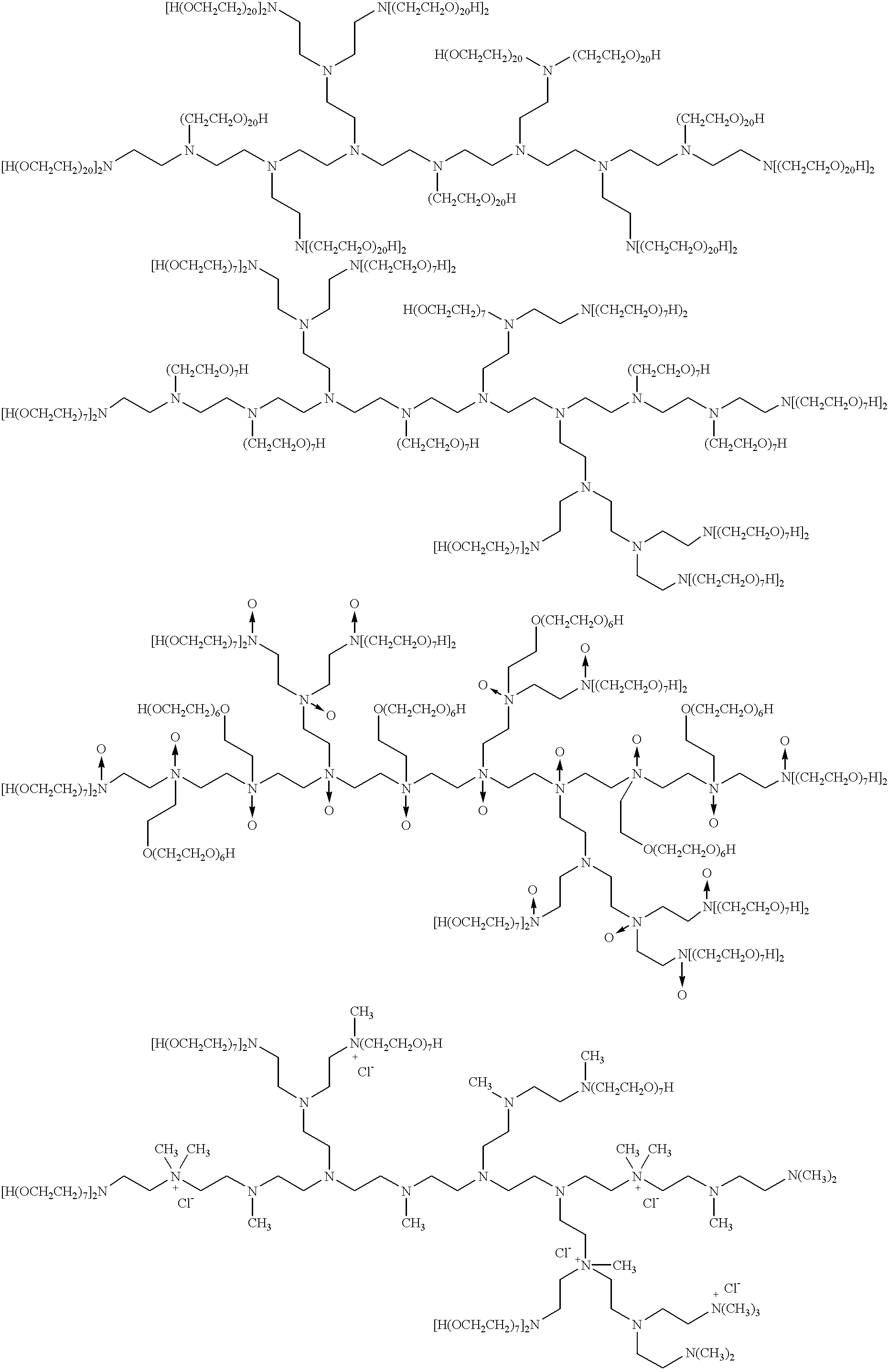
The present invention describes a synthetic non-viral vector composition for gene therapy and the use of such compositions for in vitro, ex vivo and / or in vivo transfer of genetic material. The invention proposes a pharmaceutical composition containing 1) a non-cationic amphiphilic molecule or macromolecule and its use for delivery of nucleic acids or 2) a cationic amphiphilic molecule or macromolecule that transforms from a cationic entity to an anionic, neutral, or zwitterionic entity by a chemical, photochemical, or biological reaction and its use for delivery of nucleic acids. Moreover this invention describes the use of these non-viral vector compositions in conjunction with a surface to mediate the delivery of nucleic acids. An additional embodiment is the formation of a hydrogel with these compositions and the use of this hydrogel for the delivery of genetic material. A further embodiment of this invention is the use of a change in ionic strength for the delivery of genetic material.
Owner:FIFTH BASE
Process for preparing substrates with porous surface
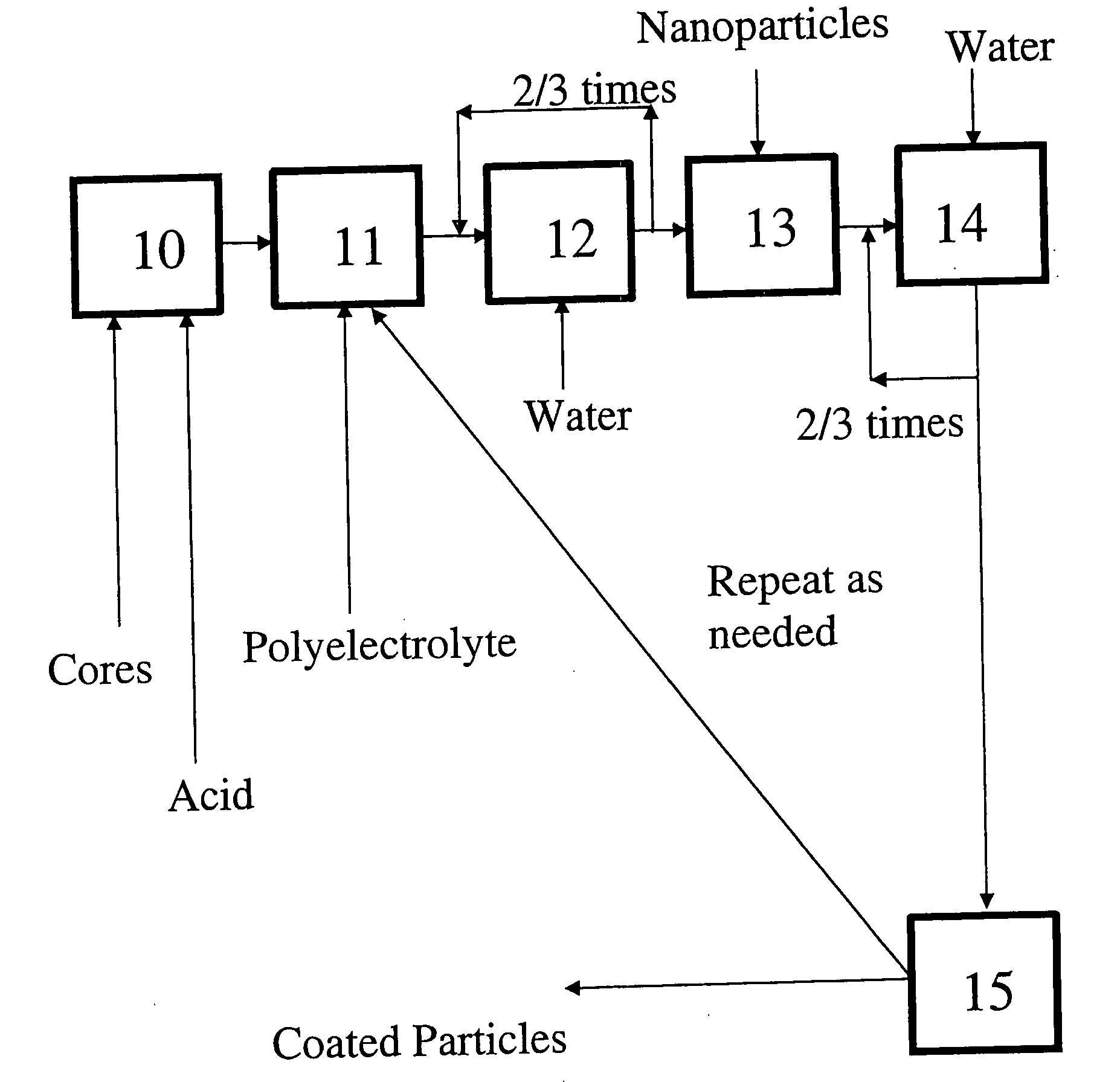
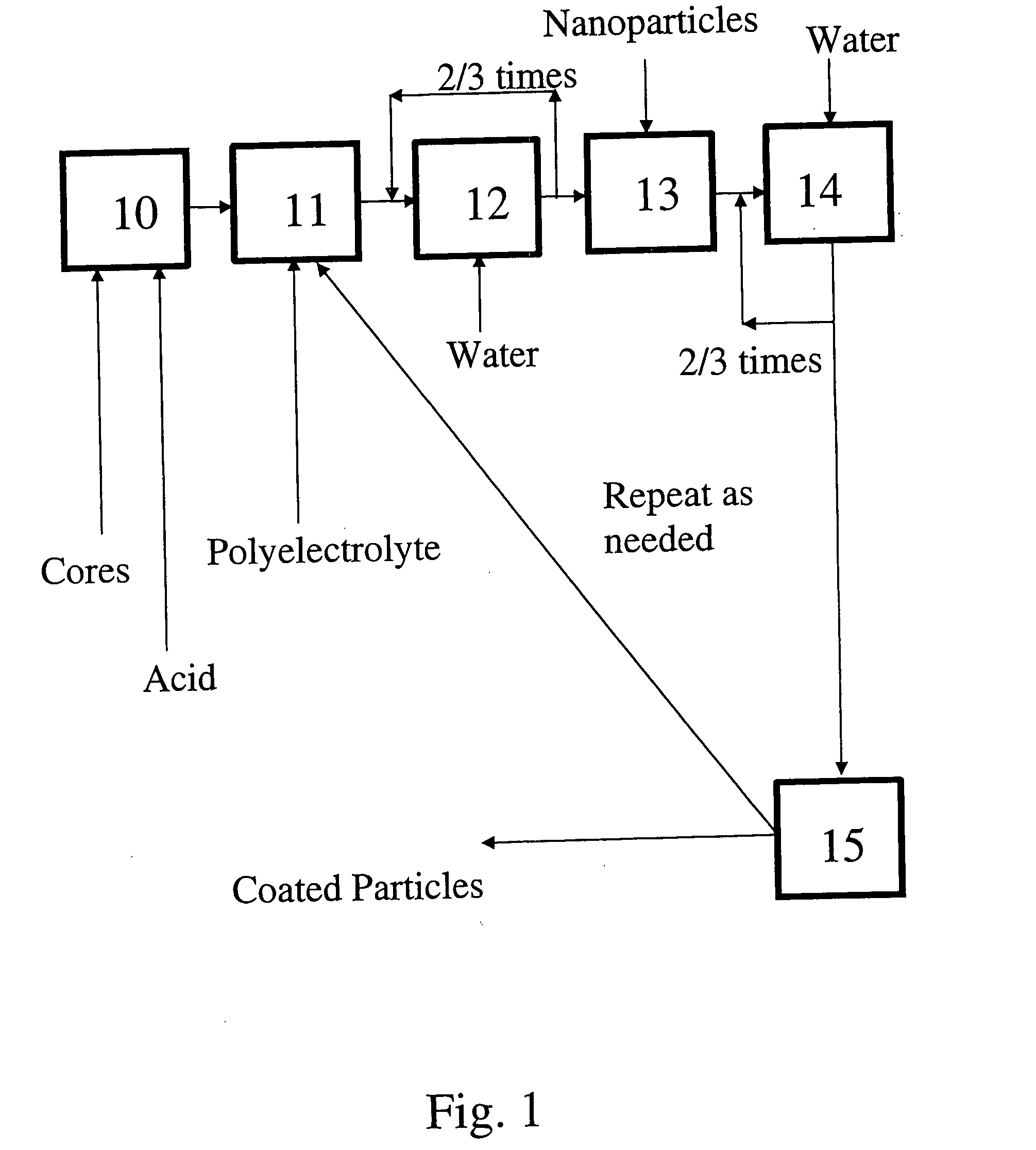
InactiveUS20080277346A1Material nanotechnologyLiquid surface applicatorsCoated surfacePolyelectrolyte
A process for preparing nanoparticle coated surfaces including the steps of electrostatically coating surfaces with polyelectrolyte by exposing the surface to a solution or suspension of polyelectrolyte, removing excess non-bound polyelectrolyte, then further coating the particles with a multi-layer of charged nanoparticles by exposing the polyelectrolyte-coated surface to a fluid dispersion including the charged nanoparticles. The process steps can optionally be repeated thereby adding further layers of polyelectrolyte followed by nanoparticles as many times as desired to produce a second and subsequent layers. The polyelectrolyte has an opposite surface charge to the charged nanoparticles and a molecular weight at the ionic strength of the fluid that is effective so that the first, second, and subsequent layers independently comprise a multiplicity of nanoparticle layers that are thicker than monolayers.
Owner:ADVANCED MATERIALS TECHNOLOGIES
Compositions and methods to prevent AAV vector aggregation
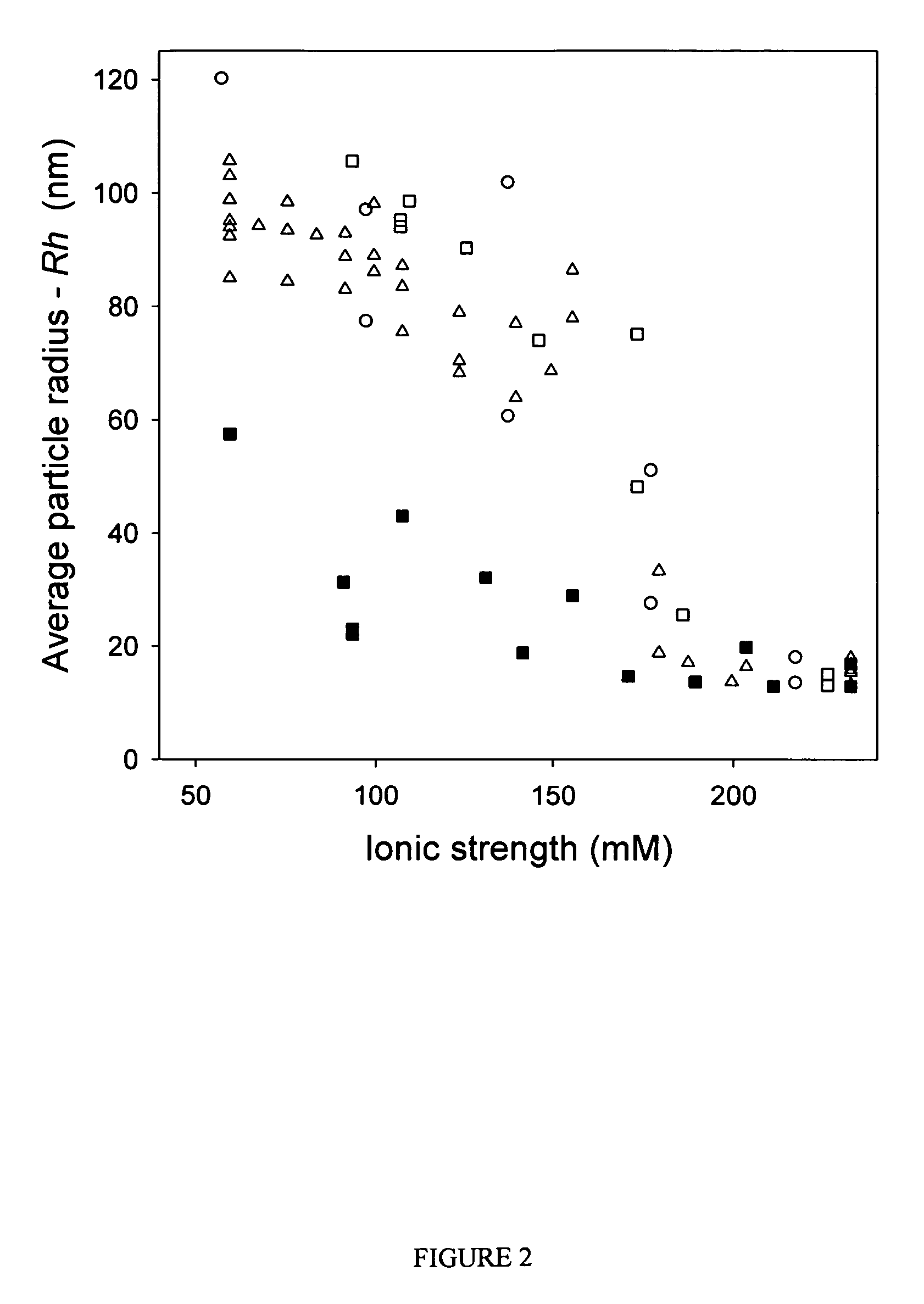
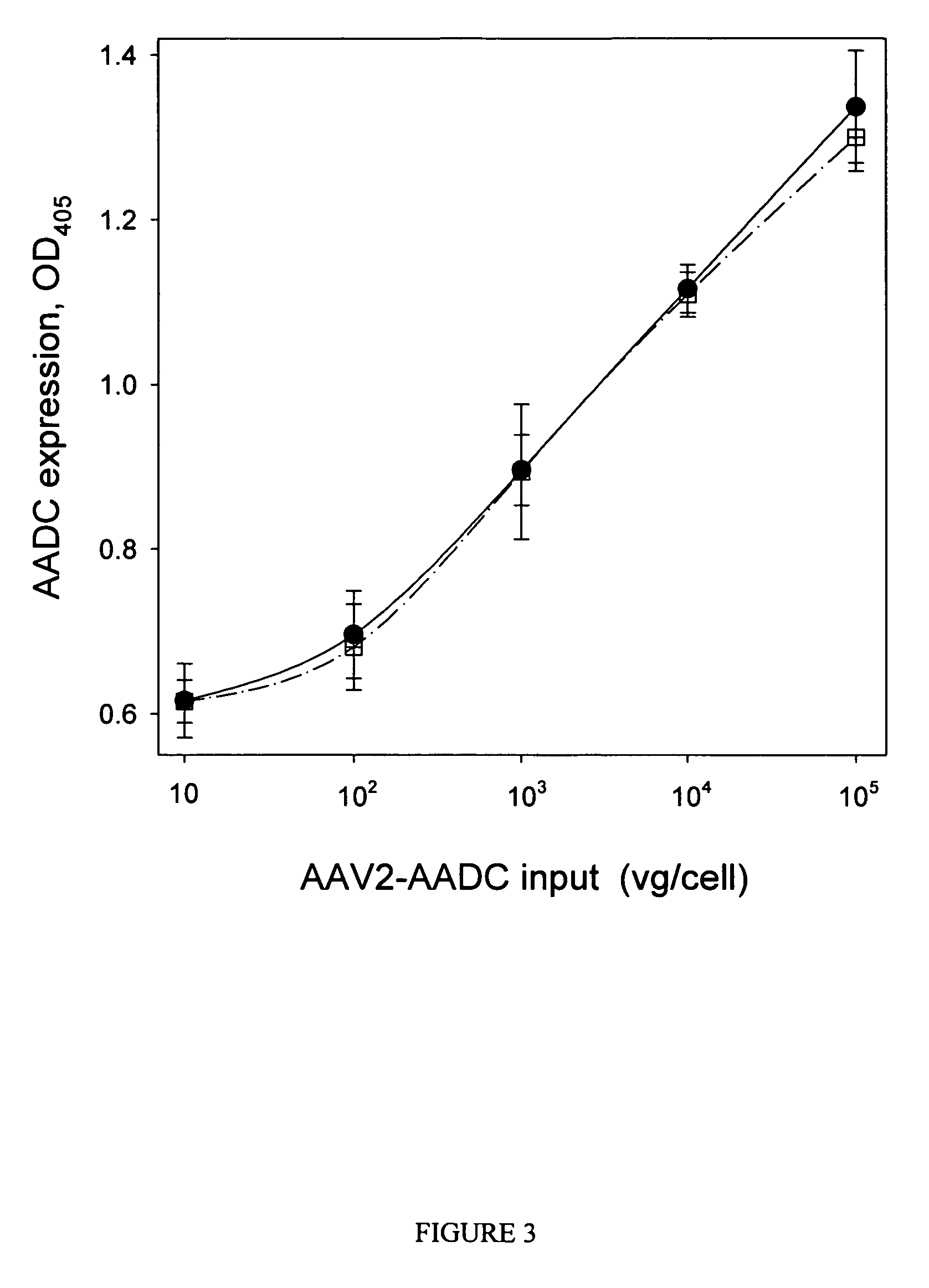
ActiveUS7704721B2Increase ionic strengthImprove efficiencyRecovery/purificationSsDNA virusesFreeze thawingIonic strength
Compositions and methods are provided for preparation of concentrated stock solutions of AAV virions without aggregation. Formulations for AAV preparation and storage are high ionic strength solutions (e.g. μ˜500 mM) that are nonetheless isotonic with the intended target tissue. This combination of high ionic strength and modest osmolarity is achieved using salts of high valency, such as sodium citrate. AAV stock solutions up to 6.4×1013 vg / mL are possible using the formulations of the invention, with no aggregation being observed even after ten freeze-thaw cycles. The surfactant Pluronic® F68 may be added at 0.001% to prevent losses of virions to surfaces during handling. Virion preparations can also be treated with nucleases to eliminate small nucleic acid strands on virions surfaces that exacerbate aggregation.
Owner:GENZYME CORP
Dispersion, alignment and deposition of nanotubes
A dispersible nanocomposite comprising nanotubes associated with nanoplatelets. A method for creating an exfoliated nanotubes solution, aligning nanotubes and depositing them on a substrate or in matrix. In one embodiment, the method includes a nanocomposite of at least one nanotube electrostatically associated with at least one nanoplatelet. The nanoplatelets may be removed from the suspension by altering the ionic strength to create an exfoliated nanotube solution. The exfoliated nanotube solution for injection into microchannel templates and aligned deposition.
Owner:TEXAS A&M UNIVERSITY
Clay control additive for wellbore fluids
ActiveUS20060289164A1Effective and stableRaise the possibilityFluid removalFlushingIonic strengthUltimate tensile strength
A clay stabilizer which is capable of inhibiting swelling in a wide variety of clay types and is also capable of restoring permeability in formations which have previously been damaged by clay swelling. Amine salts of differing molecular weights configurations and ionic strength are combined to provide transport into micropores, mesopores and macropores in the formation and to effect cationic change therein. A poly quaternary amine having a high to very high charge density is added along with lower molecular weight amine salts to substantially permanently exchange cations with the clay in the formation.
Owner:THE LUBRIZOL CORP
Functional synthetic molecules and macromolecules for gene delivery
The present invention describes a synthetic non-viral vector composition for gene therapy and the use of such compositions for in vitro, ex vivo and / or in vivo transfer of genetic material. The invention proposes a pharmaceutical composition containing 1) a non-cationic amphiphilic molecule or macromolecule and its use for delivery of nucleic acids or 2) a cationic amphiphilic molecule or macromolecule that transforms from a cationic entity to an anionic, neutral, or zwitterionic entity by a chemical, photochemical, or biological reaction and its use for delivery of nucleic acids. Moreover this invention describes the use of these non-viral vector compositions in conjunction with a surface to mediate the delivery of nucleic acids. An additional embodiment is the formation of a hydrogel with these compositions and the use of this hydrogel for the delivery of genetic material. A further embodiment of this invention is the use of a change in ionic strength for the delivery of genetic material.
Owner:FIFTH BASE
Purification of proteins
InactiveUS20110020327A1Speed up the processPeptide/protein ingredientsImmunoglobulins against growth factorsParticulatesSolid particle
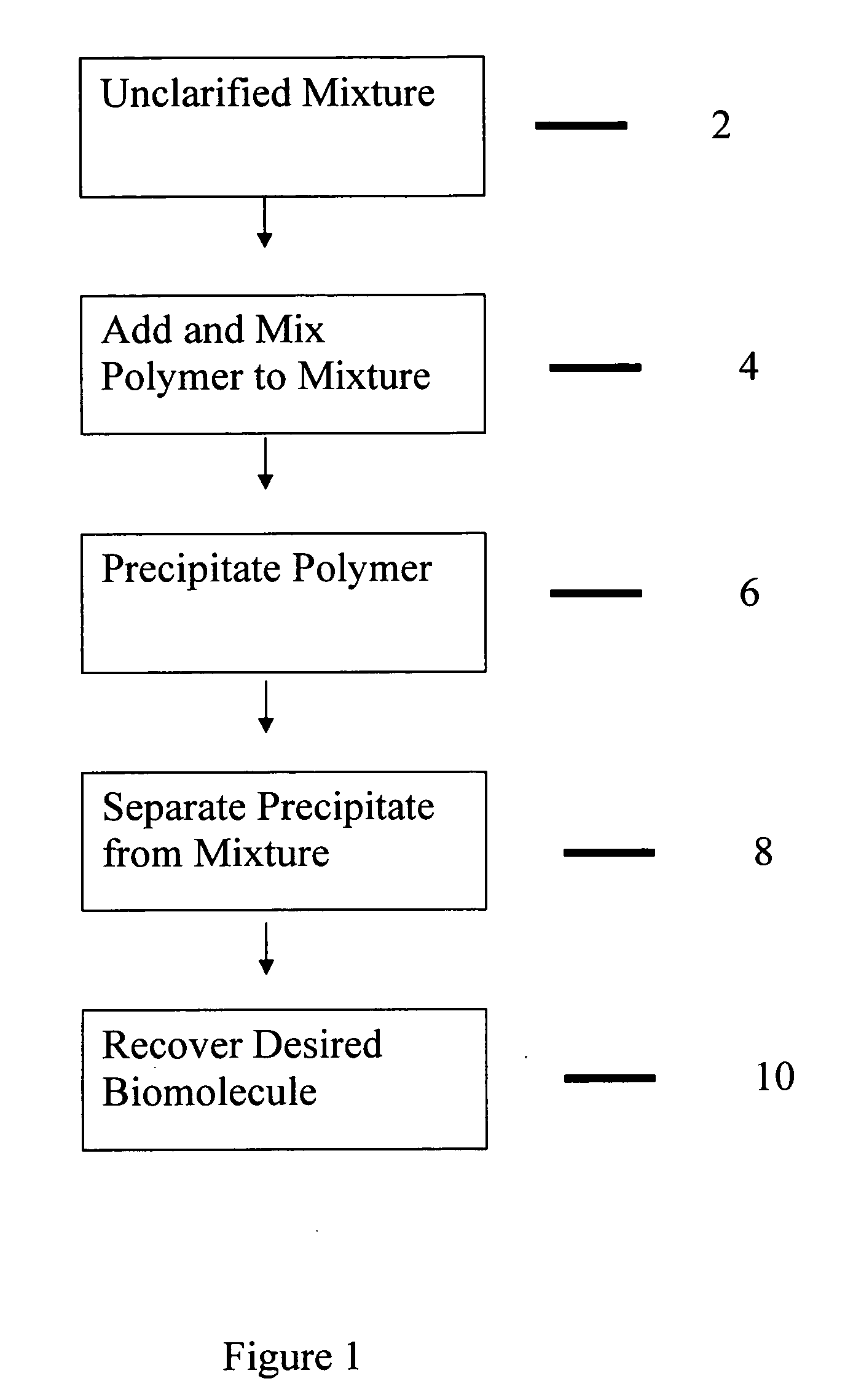
The present invention relates to a bimodal polymer such as a soluble polymer capable of irreversibly binding to insoluble particulates and a subset of soluble impurities and also capable of reversibly binding to one or more desired biomolecules in an unclarified biological material containing stream and the methods of using such a material to purify one or more desired biomolecules from such a stream without the need for prior clarification. Such a polymer comprises domains of charged pendant groups such as primary, secondary, tertiary or quaternary amines, (first mode) and is rendered insoluble and precipitates out of solution simply upon complexing with oppositely charged solid particulates and a fraction of the soluble impurities in an amount sufficient to form an aggregate that can no longer be held in solution. The polymer further comprises other domains of pendant groups that are charged or uncharged, hydrophilic or hydrophobic or have a ligand that is selective for the biomolecule of interest depending on the process conditions such as pH, ionic strength, salts, and the like (second mode). When present in one mode, such as the uncharged form, said pendant groups are capable of binding to one or more desired biomolecules within the stream (protein, polypeptide, etc) in an unclarified cell broth. The precipitate can then be removed from the stream, such as by being filtered out from the remainder of the stream and the desired biomolecule is recovered such as by selective elution.
Owner:MILLIPORE CORP
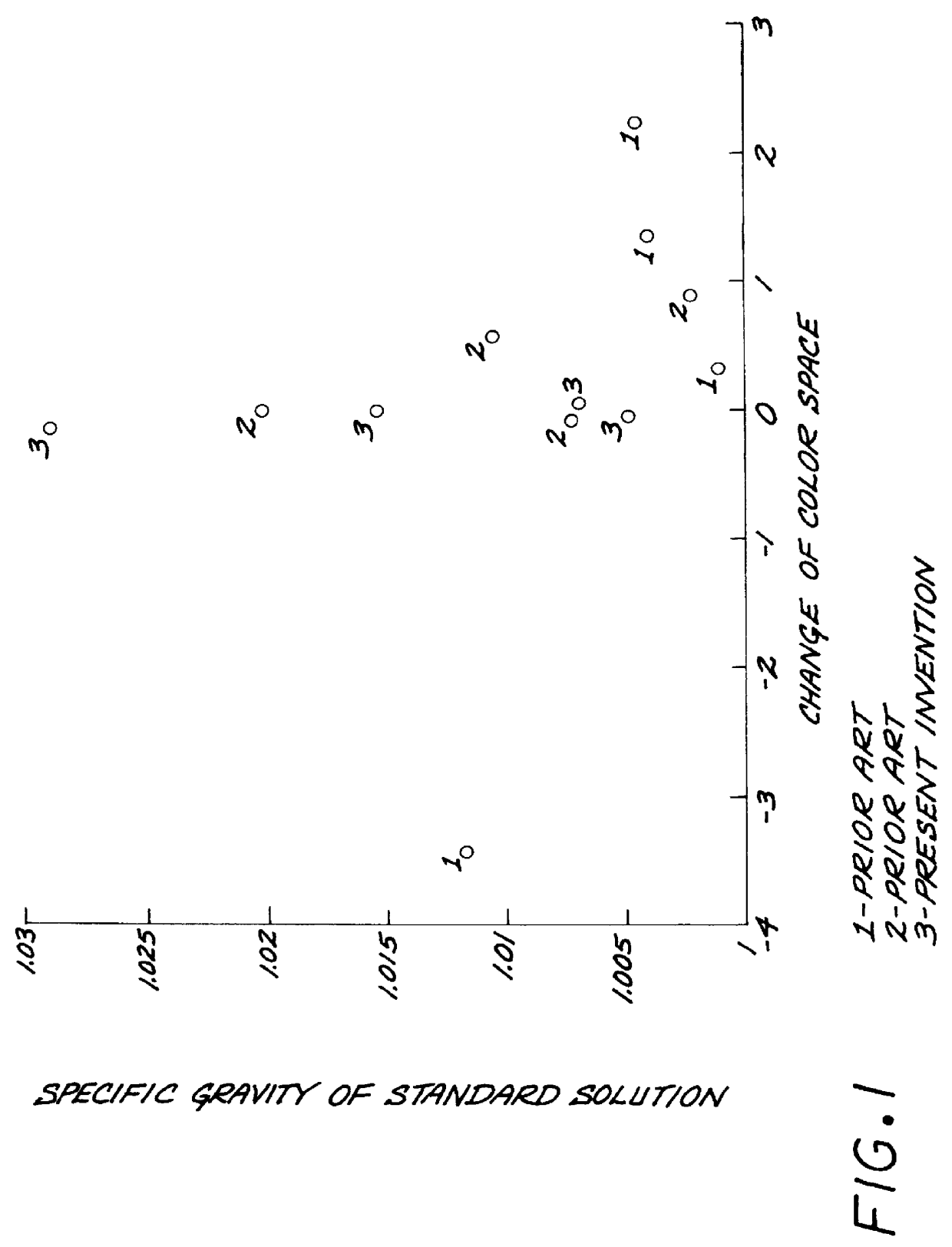
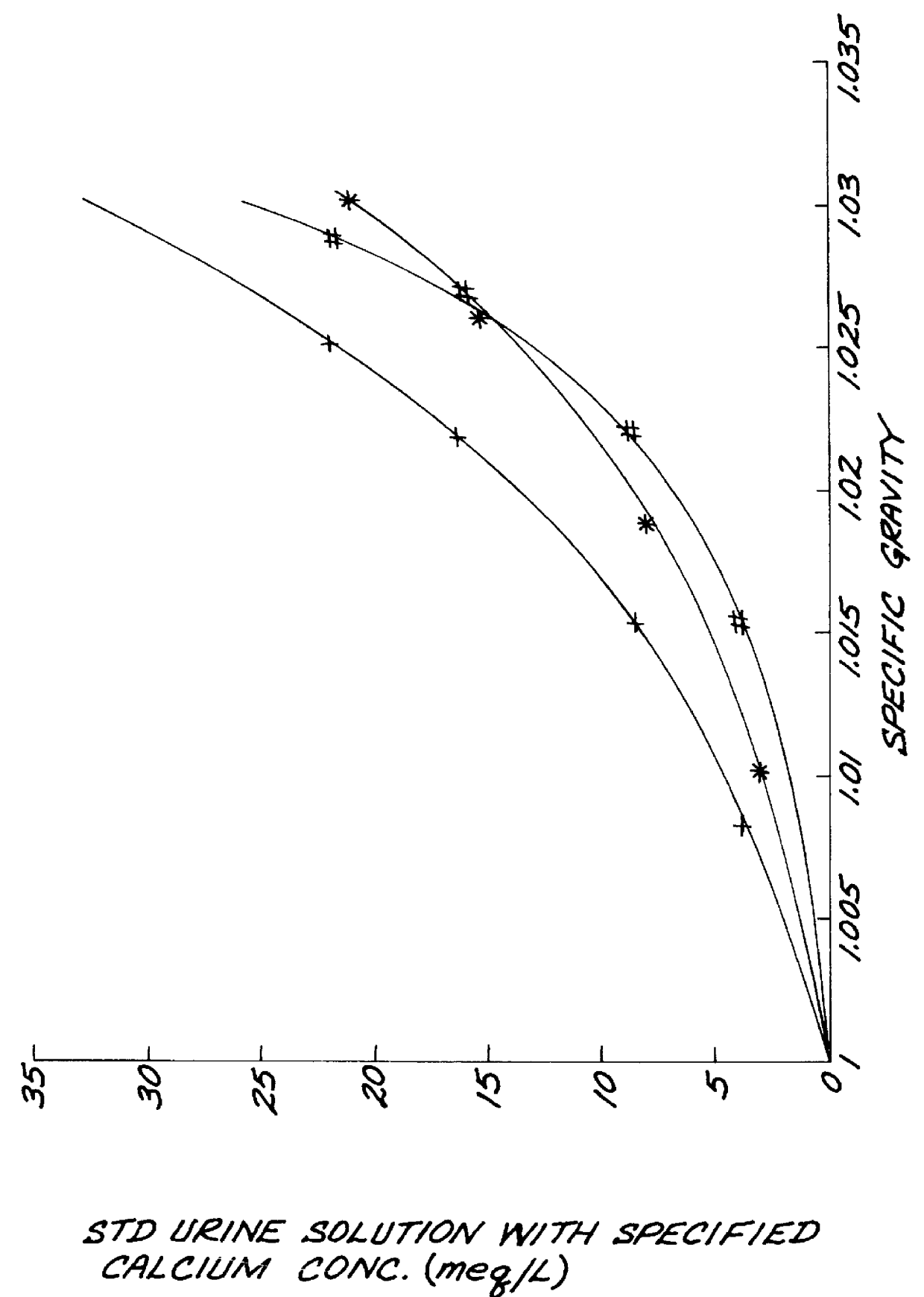
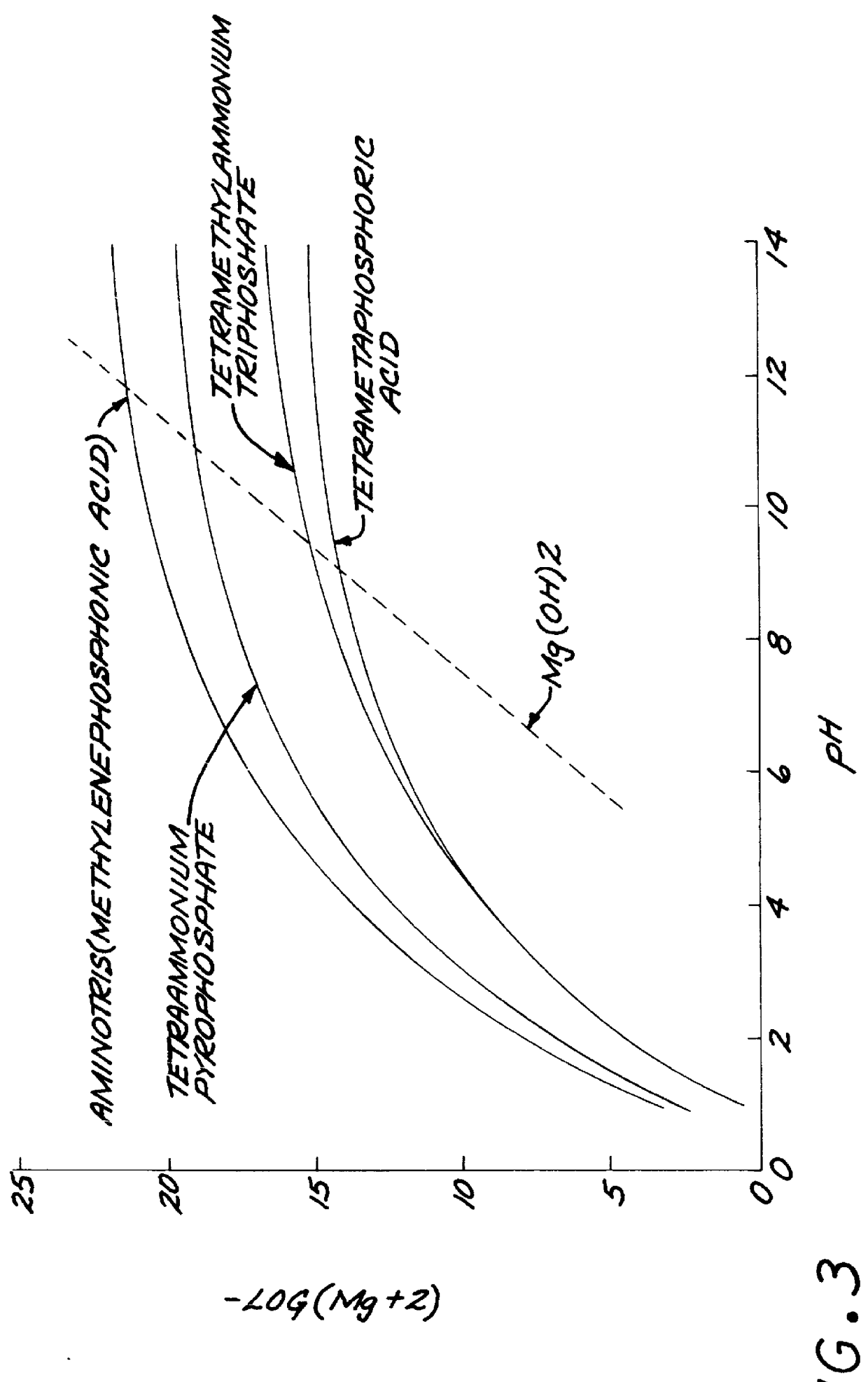
Test strips for the determination of the ionic strength or specific gravity of an aqueous sample
InactiveUS6149865ACarry-out efficiently and reliablyFavorable for determinationAnalysis using chemical indicatorsMaterial analysis by observing effect on chemical indicatorInorganic phosphateHydrogen
The ionic strength or the specific gravity of an aqueous test sample is determined by mixing the sample with a reagent composition on a test strip. The strip includes a bibulous carrier containing an organic phosphonic acid, such as 1-hydroxyethlidene-1,1 -Diphosphonic acid and aminotris(methylenephosphonic acid), or inorganic phosphate having one of the structure formulas, where M stands for one equivalent of an alkali metal, ammonium, lower alkyl ammonium, or hydrogen ion, with the proviso that at least one of the M is hydrogen; x is an integer ranging from zero to two and y is also an integer ranging from zero to one, and serving to buffer the metal ions, and a chromogenic indicator capable of providing a detectable response of the ions being sequestered and coordinated within the complex in the mixture.
Owner:TECO DIAGNOSTICS
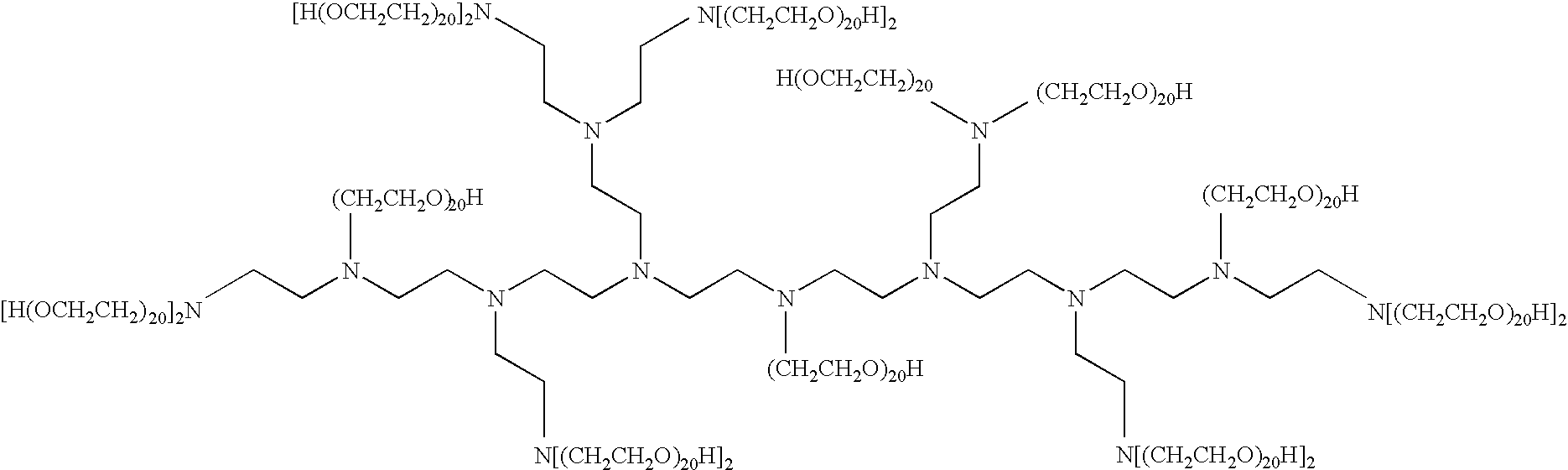
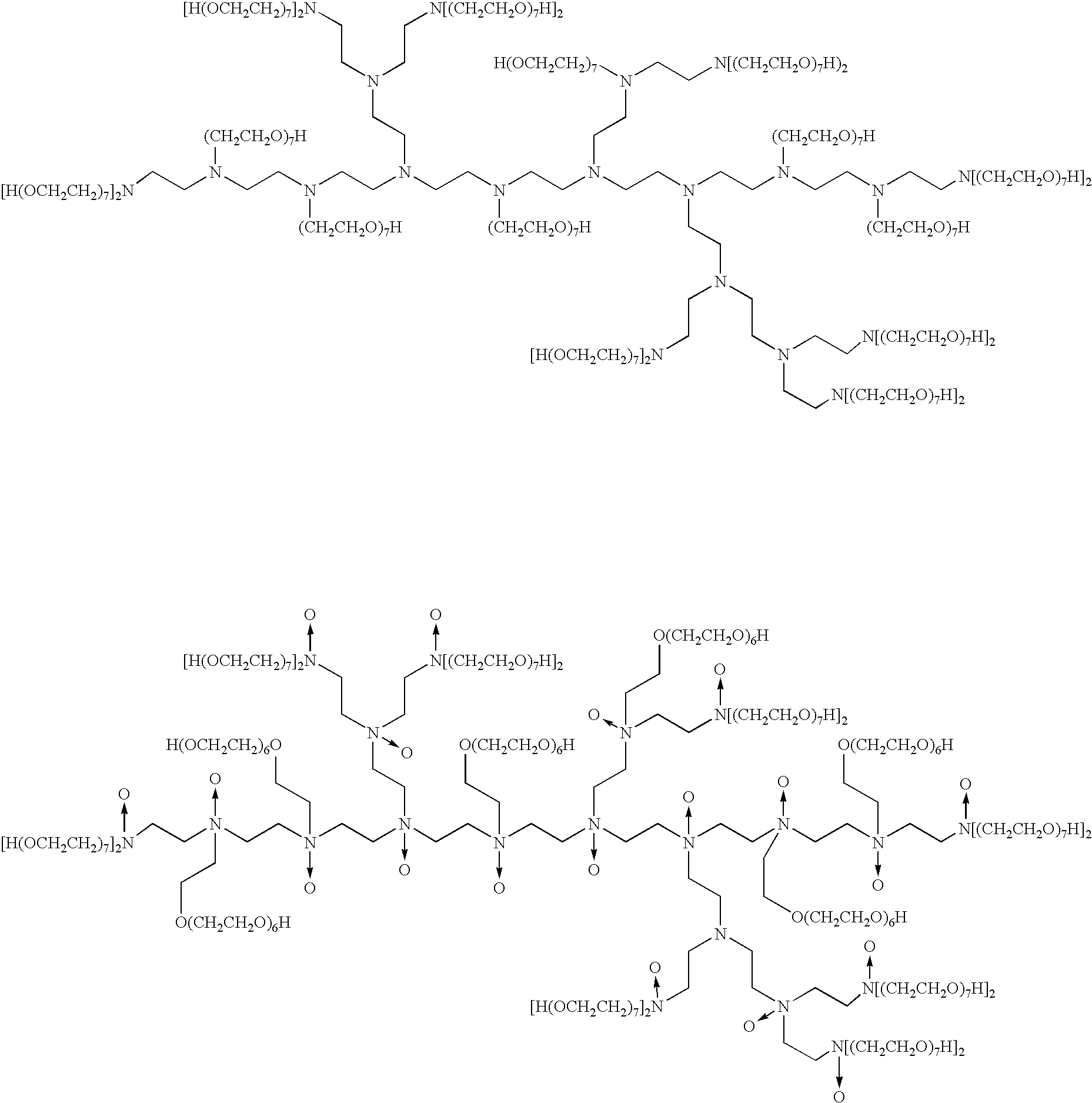
Liquid composition comprising polyalkoxylated polyamines or polyimines
InactiveUS6683038B2Organic detergent compounding agentsNon-surface-active detergent compositionsImideIonic strength
This invention relates to a pouched liquid composition which comprises an alkoxylated amine, imine, amide or imide compound, small amount of water and specific levels high ionic strength chelating agents.
Owner:THE PROCTER & GAMBLE COMPANY
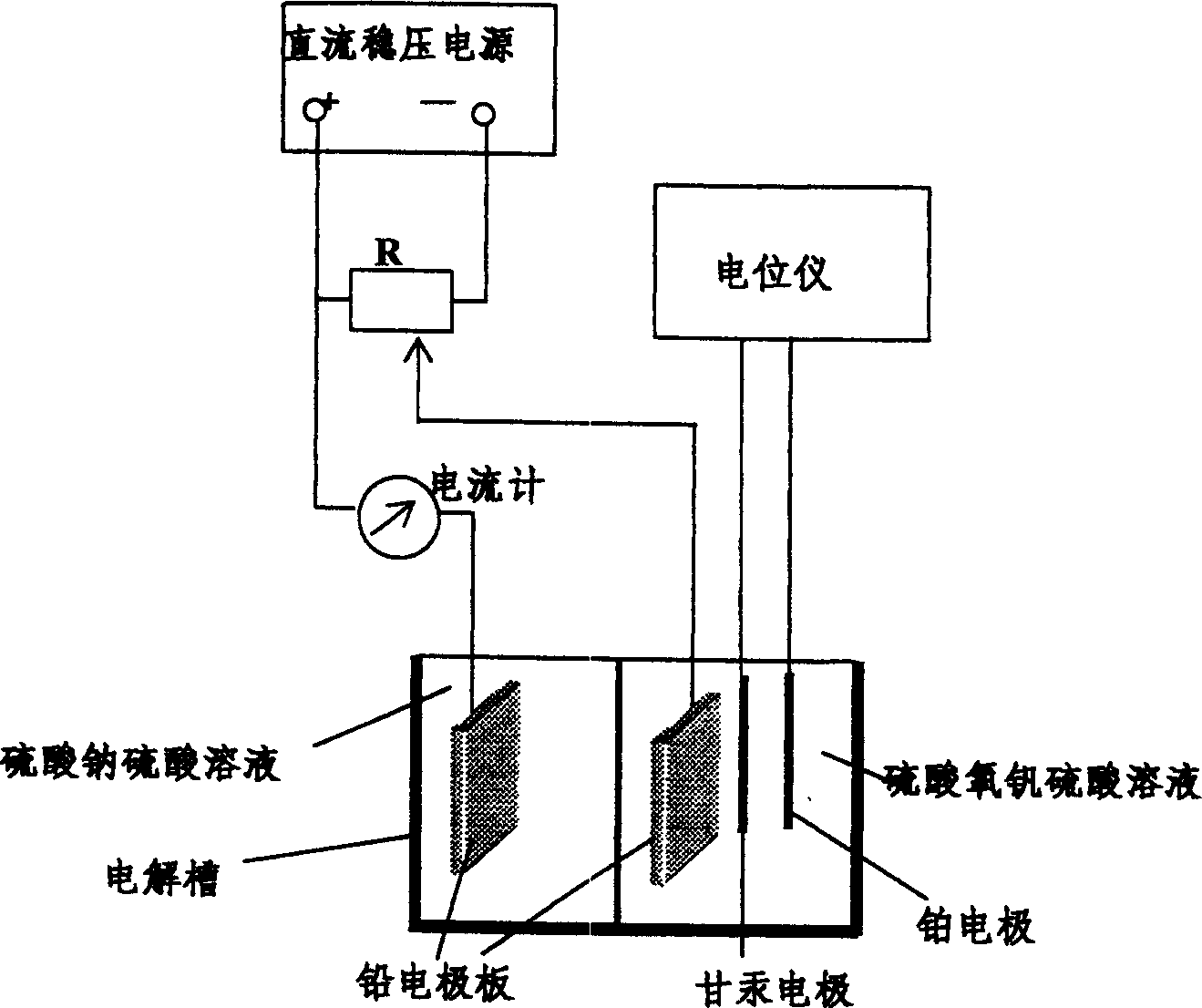
Process for electrolyzing preparing electrolyte of full vanadium ion flow battery
InactiveCN1598063ASimplify the assembly processExtended service lifeElectrolysis componentsLead-acid accumulatorsLiquid cellVanadyl sulfate
The invention relates to the field of the cell manufacturing and especially relates to a method of preparing the electrolyte of the vanadium ion liquid cell, making the 1:1 thin vitriol from the thick vitriol, adding the trioxid bi-vanadium and then the five-trioxid bi-vanadium to get the vitriol oxide vanadium; adding the Na2SO4, emulsification agent OP and other additive, placing the vitriol oxide vanadium at the cathode of the electrolytic cell and the same ion intension at the anticathode and electrolyse, getting the vitriol electrolyte used for vitriol batteries which includes half quadrivalence vanadium and half tervalent vanadium. The invention simplyfy the equipment of vitriol batteries and chemical procedure, enhance the work efficiency, extend the use time of the functional materials of vitriol batteries, such as the electrode and the dissepiment, needn't change the electrolyte of the anode, avoid wasting raw material, avail the producing by large scale.
Owner:攀枝花钢铁有限责任公司钢铁研究院 +1
Liquid composition
InactiveUS20020045559A1Organic detergent compounding agentsSurface-active detergent compositionsImideIonic strength
This invention relates to a pouched liquid composition which comprises an alkoxylated amine, imine, amide or imide compound, small amount of water and specific levels high ionic strength chelating agents.
Owner:THE PROCTER & GAMBLE COMPANY
Nucleic acid separation and purification method based on reversible charge interactions
InactiveUS20090275486A1Increase ionic strengthSugar derivativesMicrobiological testing/measurementIonic strengthPurification methods
The invention provides a method for purifying nucleic acids using a polycationic reagent and an anionic substrate to form a complex with a nucleic acid to be purified. The complex may be separated from other components of a mixture and the nucleic acid eluted from the complex with a high ionic strength solution or an anionic reagent.
Owner:KURN NURITH +1
Novel hydorgels and uses thereof
ActiveUS20070128175A1Promotes adhesion and proliferationRapid isolationPowder deliveryBiocideIonic strengthSelf-assembly
The present invention provides novel hydrogels and methods of making and using such hydrogels. The present invention provides hydrogels that may be formed by the self-assembly of peptides in solution. Such self-assembly may be brought about by a change in one or more characteristics of the solution. Characteristics of the solution that may be changed include pH, ionic strength, temperature, and concentration of one or more specific ions. In addition, hydrogels of the invention may be disassembled by changing one or more characteristic of the hydrogel such as pH, ionic strength, temperature, and concentration of one or more specific ions.
Owner:UNIVERSITY OF DELAWARE
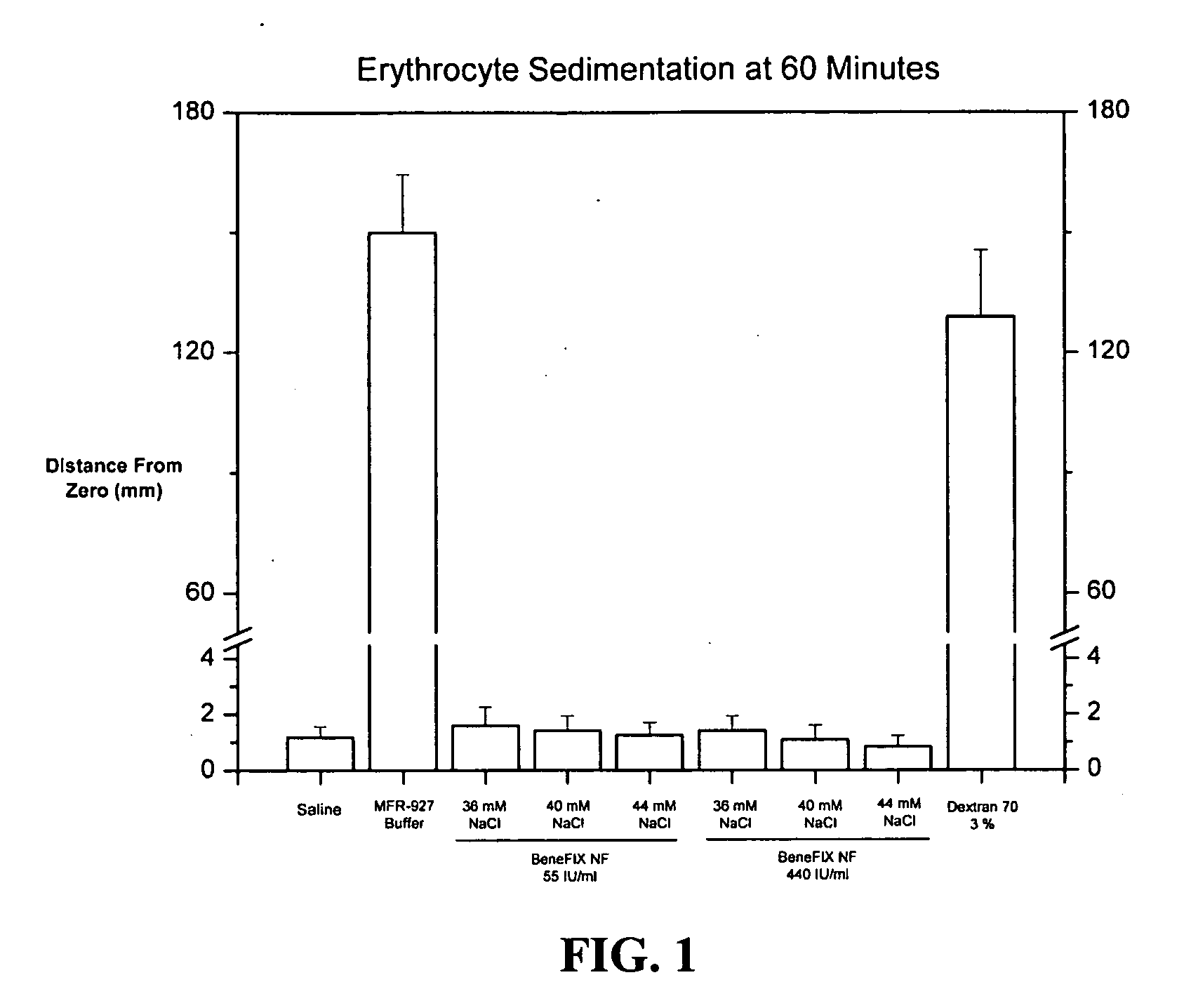
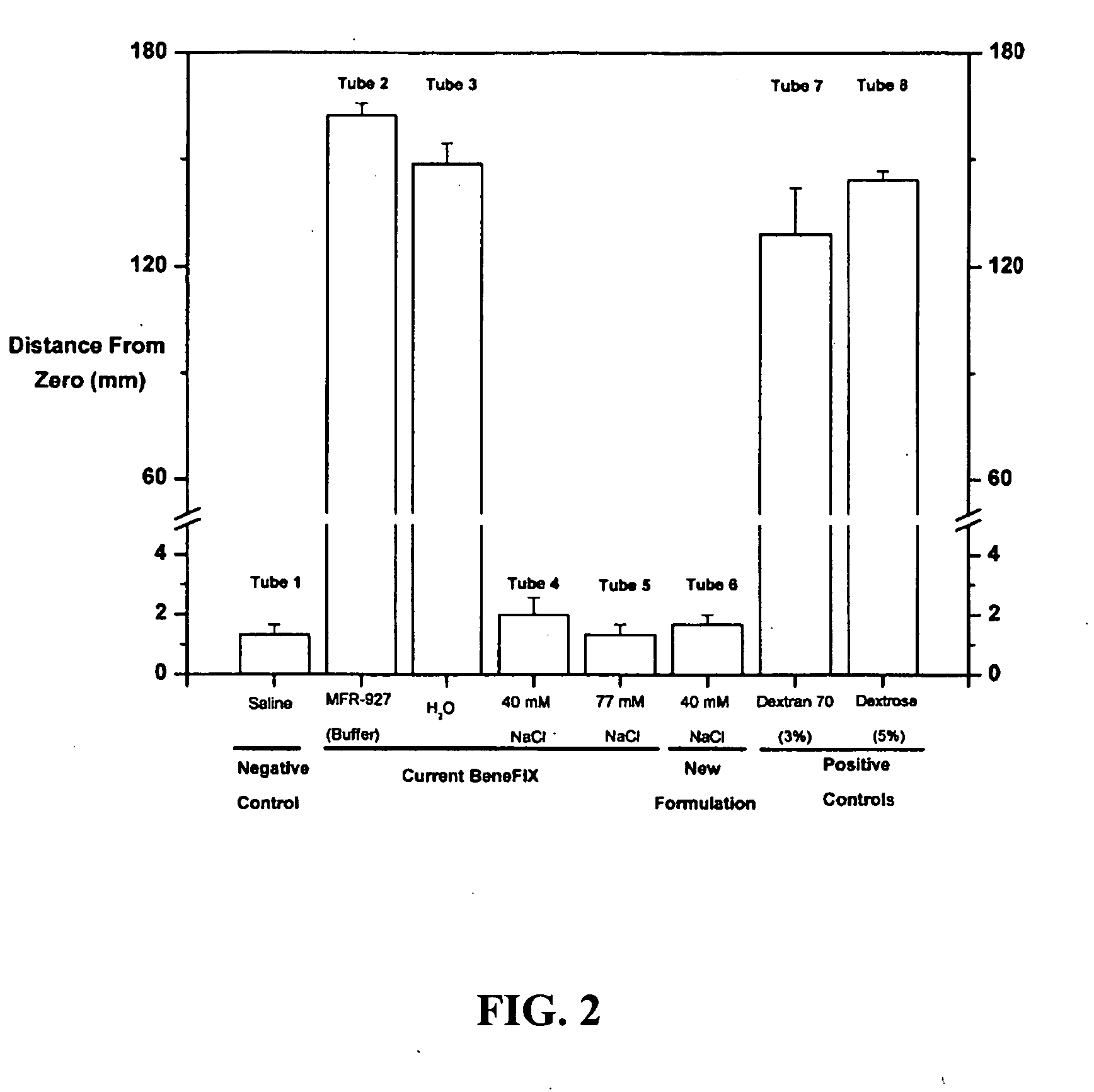
Sodium chloride solution for drug reconstitution or dilution
InactiveUS20070135343A1Prevent agglutinationIncrease ionic strengthBiocidePeptide/protein ingredientsHemolysisPresent method
The invention provides methods for preparing pharmaceutical formulations for injection such that upon injection the formulation does not cause erythrocyte agglutination, hemolysis, and / or cell shrinkage. To prevent agglutination, a pharmaceutical formulation ready for injection needs to have a sufficient ionic strength. To prevent hemolysis or cell shrinkage, a pharmaceutical formulation ready for injection needs to be about isotonic with respect to plasma. The invention provides methods that prepare pharmaceutical formulations for injection that have both the sufficient ionic strength to prevent agglutination and the requisite tonicity to prevent significant hemolysis or cell dehydration or shrinkage. The present methods involve the use of sodium chloride solutions that are about 25 mM to about 150 mM for reconstituting lyophilized cakes (or other non-liquid pharmaceutical formulations) into solution or for diluting pharmaceutical formulation solutions.
Owner:WYETH LLC
Method of Completing Poorly Consolidated Formations
It is proposed a method for completing an unconsolidated interval, including particulates, in a subterranean formation, including a consolidation steps. The consolidation step is performed by injection of an aqueous solution of colloidal particles with a pH modifier and / or an ionic-strength modifier. A hard gel is formed that holds the particulates together. The consolidation is followed by hydraulic fracturing. Diversion towards area of less permeability may be enhanced by the use of micrometric particles.
Owner:SCHLUMBERGER TECH CORP
Underarm products with superabsorbent component
InactiveUS20050095210A1Reduce humidityImprove water absorptionCosmetic preparationsToilet preparationsIonic strengthSuperabsorbent polymer
A stick or soft solid suspension product comprising: (a) 0.01-20 weight % of a polyacrylate superabsorbent polymer (sodium salt), with a salt or ionic strength tolerance under a Baseline Absorption Test sufficient to give at least 25 weight % water absorption; (b) 10-88 weight % of a volatile silicone having a flash point of 100 degrees C. or less; (c) a selected gelling agent; (d) 0-5 weight % of a surfactant with a hydrophilic / lipophilic balance in the range of 3-13; (e) 0-25 weight % of an antiperspirant active or an effective amount of a deodorizing agent which is not an antiperspirant active; (f) 0-20 weight % of a nonvolatile silicone having a flash point greater than 100 degrees C.; and (g) 0-20 weight % of an emollient; provided that the water content is ≦2 weight %.
Owner:COLGATE PALMOLIVE CO
Crosslinking hyaluronic acid sodium gel for injection and preparation method thereof
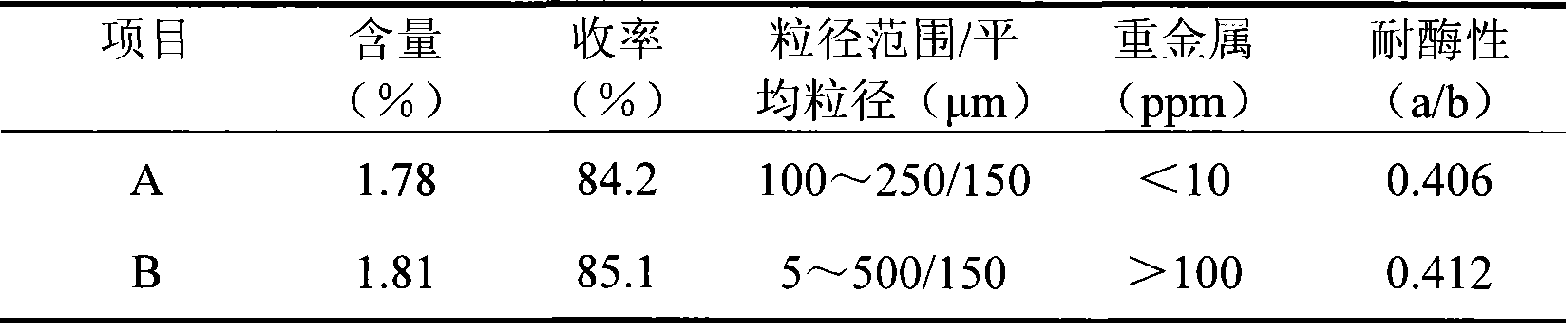
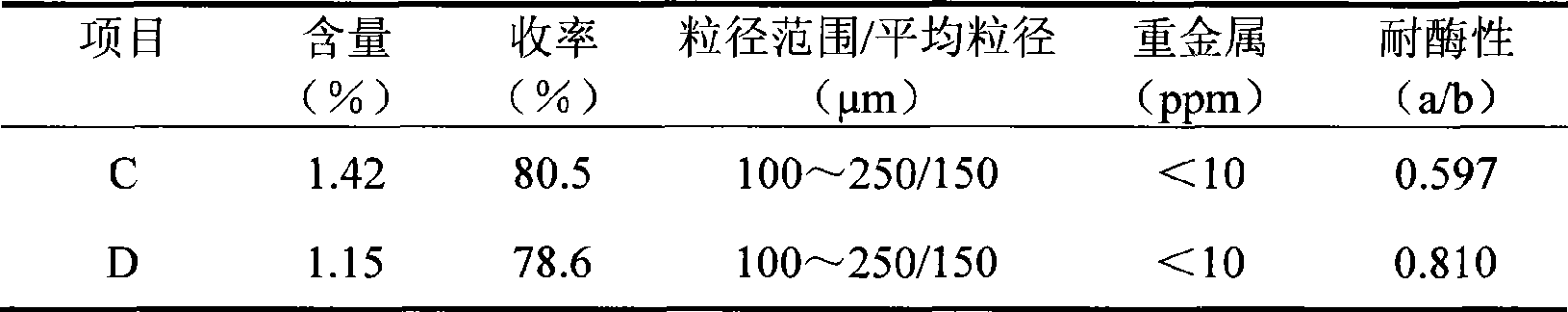
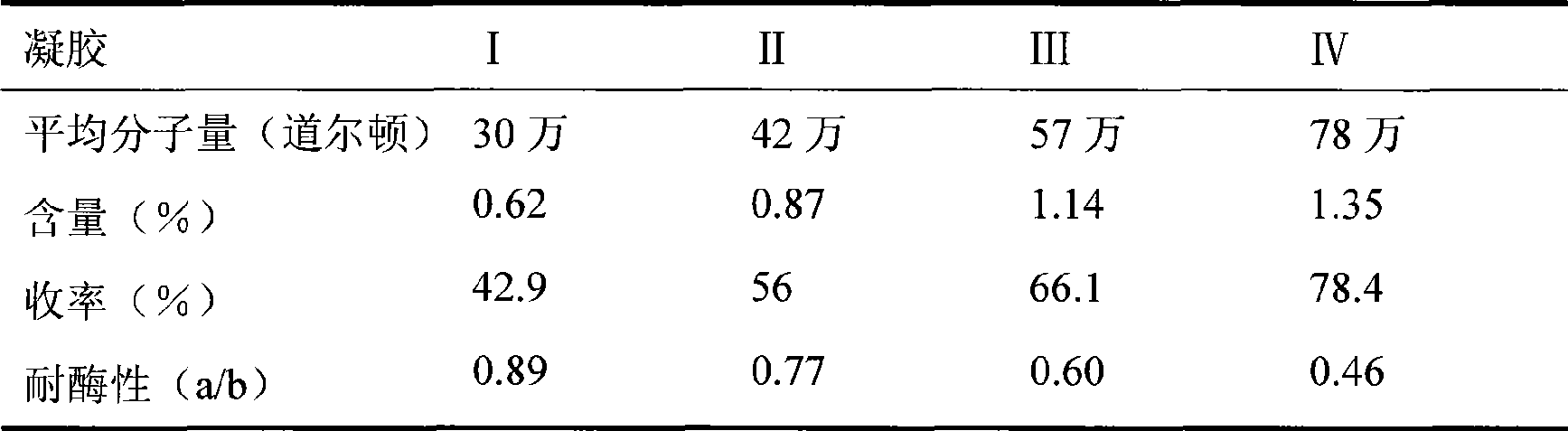
The invention relates to a cross bonding sodium hyaluronate gel for injection and a preparation method thereof. The method is characterized in that sodium hyaluronate and glycidyl ether are mixed and react in alkaline solution to form water-insoluble gel, the gel is purified in deionized water and broken into particles of certain sizes; ionic strength of the deionized water is improved to contract the gel particles. The cross bonding sodium hyaluronate gel features asepsis, apyrogeneity and small and uniform particles; therefore the gel can be applied to prepare injection for beauty care and medical treatment.
Owner:SHANDONG FREDA PHARMA GRP CO LTD +1
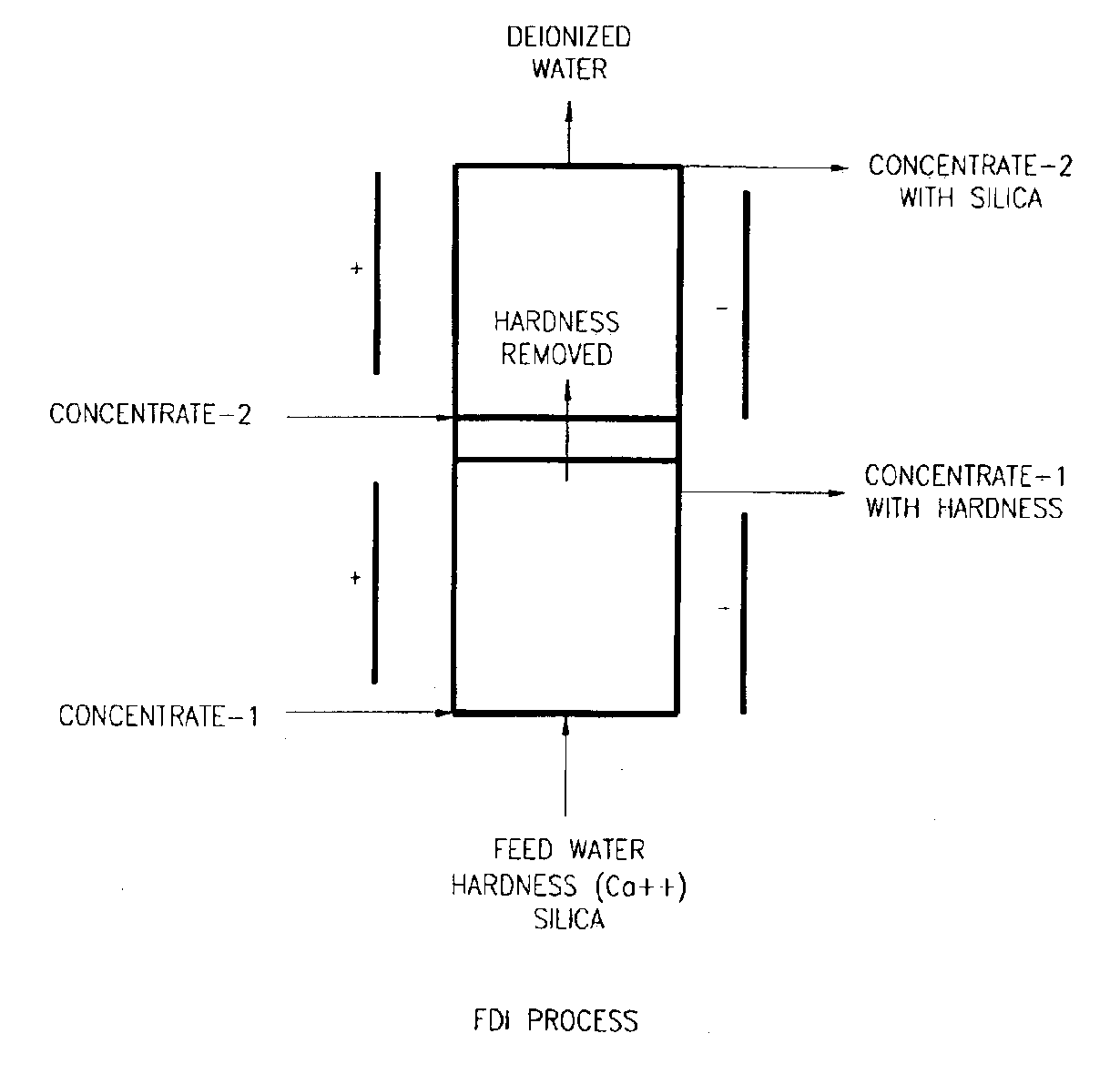
Fractional deionization process
InactiveUS6896814B2Easy to disassembleEfficient removalElectrolysis componentsIon-exchanger regenerationElectricityIonic strength
A liquid treatment process is described for sequential removal of ionic species of progressively decreasing ionic strength without precipitation or “scaling.” An aspect of the invention includes dual electrodeionization operations. The first electrodeionization operation is performed at a voltage calculated to remove strongly ionized species such as calcium and magnesium from the feed water without scaling. The product of the first electrodeionization operation is then subjected to a second electrodeionization operation. The second electrodeionization operation is performed at a voltage greater than the first electrodeionization operation, and is designed to remove more weakly ionized species such as silica and carbon dioxide, preventing scaling. More than two successive electrodeionization operations may be performed if desired. Multiple electrodeionization operations may occur in a single electrodeionization stack or in multiple electrodeionization stacks.
Owner:AQUATECH INT LLC
Method for recovering water soluble surfactants
InactiveUS7033504B1Less polarWater contaminantsSolid sorbent liquid separationGrowth retardantPhosphonium
Disclosed is a method for recovering and re-employing ionic surfactants from solutions, dispersions and emulsions of water and liquid hydrocarbons, by the addition of a second non-aqueous polar phase and an inorganic salt which changes the ionic strength of the surfactant and causes the surfactant to partition principally into the non-aqueous second polar phase. More specifically, the method applies to recovering hydrate growth inhibitor / modifying compounds, with cationic surfactants such as ammonium, phosphonium and sulphonium alkylated compounds from the effluent of hydrocarbon production wells; the effluent including water, hydrate inhibitor compound, at least one additional polar solvent and inorganic salt. Sufficient additional ions of inorganic salt, and if necessary, an alcohol / glycol are added to the effluent to form a second polar phase that is less polar than the aqueous phase, into which the hydrate inhibitor can then dissolve and be separated from the effluent.
Owner:SHELL OIL CO
Composition comprising layered host material with intercalated functional-active organic compound
An aqueous dispersion comprising particles of a layered host material and functional-active organic compound dispersed in an aqueous medium, wherein the weight ratio of layered host material to functional-active compound is less than 20 and at least 50% of the functional-active organic compound in the dispersion is intercalated between layers of the layered host material particles, and further wherein the aqueous medium comprises additional soluble salts or ionized molecules or polymers distinct from the functional-active compound and has an ionic strength of greater than 5 mS / cm. A method of delivering a desired functional-active organic compound to a target system, comprising delivering such an aqueous dispersion to the target system, wherein the aqueous dispersion comprises the desired functional-active organic compound intercalated in a layered host material, and additional soluble salts or ionized molecules or polymers which are distinct from the functional-active compound and which are also present in the target system to which the functional-active compound is delivered.
Owner:EASTMAN KODAK CO
Topical delivery of arginine of cause beneficial effects
A preparation is disclosed for producing enhanced blood flow in tissue thus causing beneficial effects such as promoting hair growth on scalp tissue lacking sufficient hair, restoring normal sexual function in males with erectile dysfunction. Specifically, this is a preparation which provides local delivery of the amino acid L-arginine, an important biological precursor to the main substance which is responsible for relaxation of blood vessels permitting enhancement of blood flow. In the preferred embodiments, the L-arginine is provided so that it can be topically applied to the scalp or penis. The preparation also contains an agent which aids in the transfer of L-arginine into the tissue. In the preferred embodiments this agent overcomes the resistance to transfer caused by the high charge density of L-arginine. In the preferred embodiments this means is high ionic strength created by addition of choline chloride, magnesium chloride and sodium chloride. This preparation when applied nightly to scalp tissue lacking sufficient hair for a period of time causes substantial growth of hair on the scalp. Further, when applied to the penis of a subject with erectile dysfunction causes restoration of normal sexual function.
Owner:STRATEGIC SCI & TECH
Lithium composite compound particles and process for producing the same, and non-aqueous electrolyte secondary battery
ActiveUS20110281168A1Excellent cycle characteristicsExcellent in high-temperature storage propertyFinal product manufactureLi-accumulatorsHigh temperature storageIonic strength
The present invention relates to lithium composite compound particles having a composition represented by the formula: Li1+xNi1-y-zCoyMzO2 (M=B or Al), wherein the lithium composite compound particles have an ionic strength ratio A (LiO− / NiO2−) of not more than 0.3 and an ionic strength ratio B (Li3CO3+ / Ni+) of not more than 20 as measured on a surface of the respective lithium composite compound particles using a time-of-flight secondary ion mass spectrometer. The lithium composite compound particles of the present invention can be used as a positive electrode active substance of a secondary battery which has good cycle characteristics and an excellent high-temperature storage property.
Owner:TODA IND
Dishwashing method
InactiveUS20090082242A1High pHReduce ionic strengthInorganic/elemental detergent compounding agentsOrganic/inorganic per-compounds compounding agentsIonic strengthPhosphate
A method of cleaning a soiled load in an automatic dishwasher comprising the step of contacting the load with a phosphate free wash liquor comprising exfoliated nanoclay, the liquor having a pH of from about 9 to about 12 and an ionic strength of from about 0.001 to about 0.02 moles / l.
Owner:THE PROCTER & GAMBLE COMPANY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com