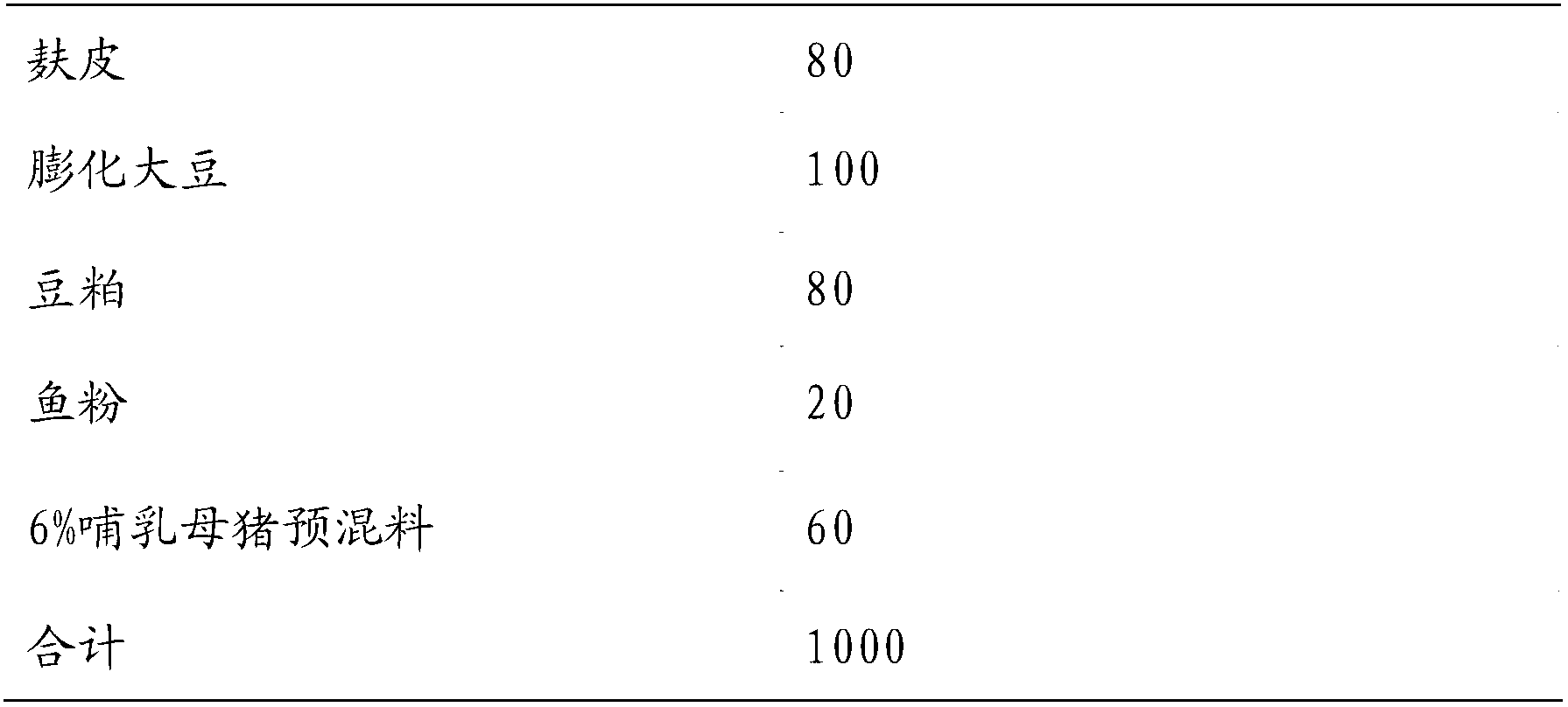Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
5069 results about "Choline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
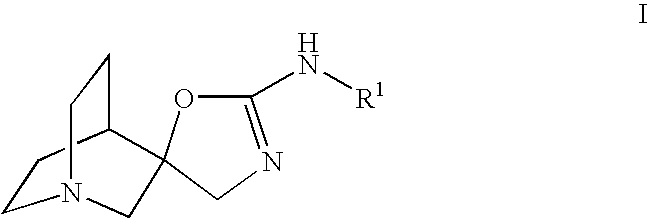
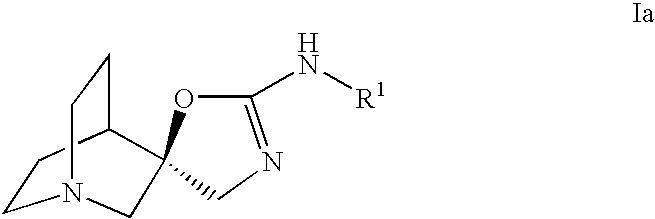
Choline /ˈkoʊliːn/ is a water-soluble vitamin-like essential nutrient. It is a constituent of lecithin, which is present in eggs and in many plants and animal organs. The term cholines refers to the class of quaternary ammonium salts containing the N,N,N-trimethylethanolammonium cation (X⁻ on the right denotes an undefined counteranion).
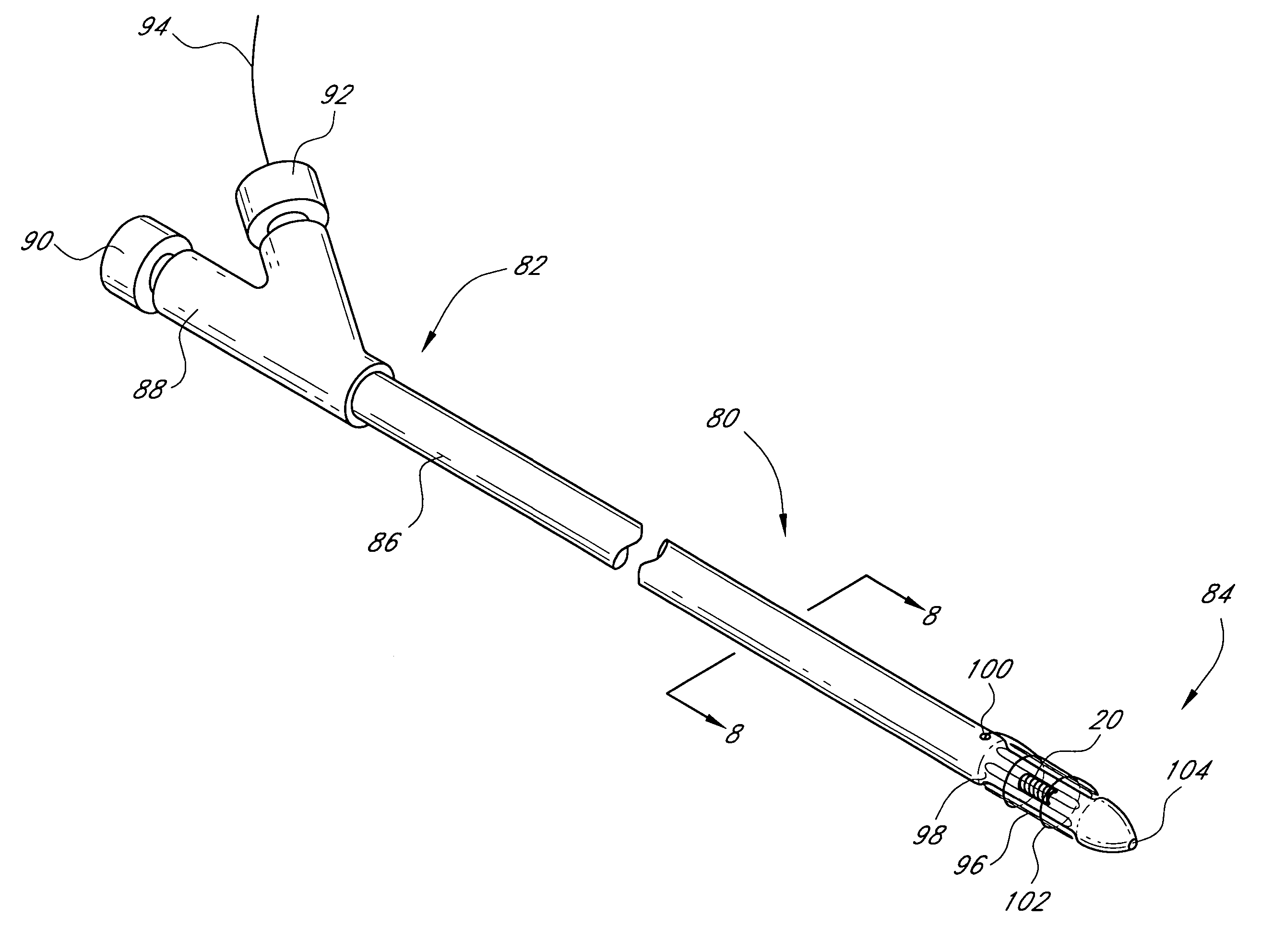
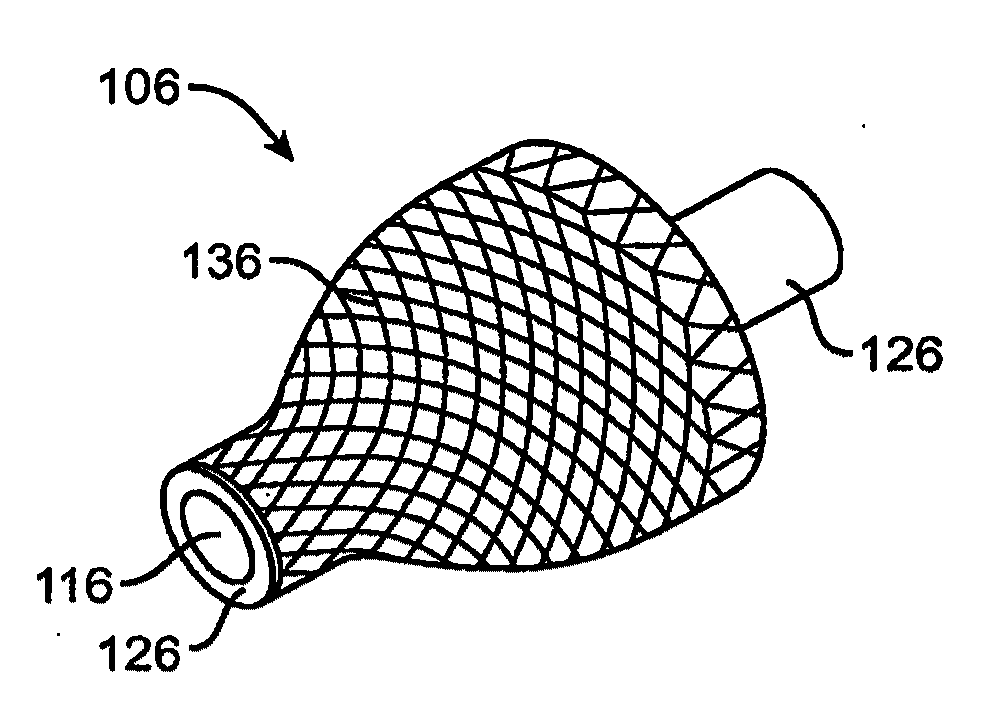
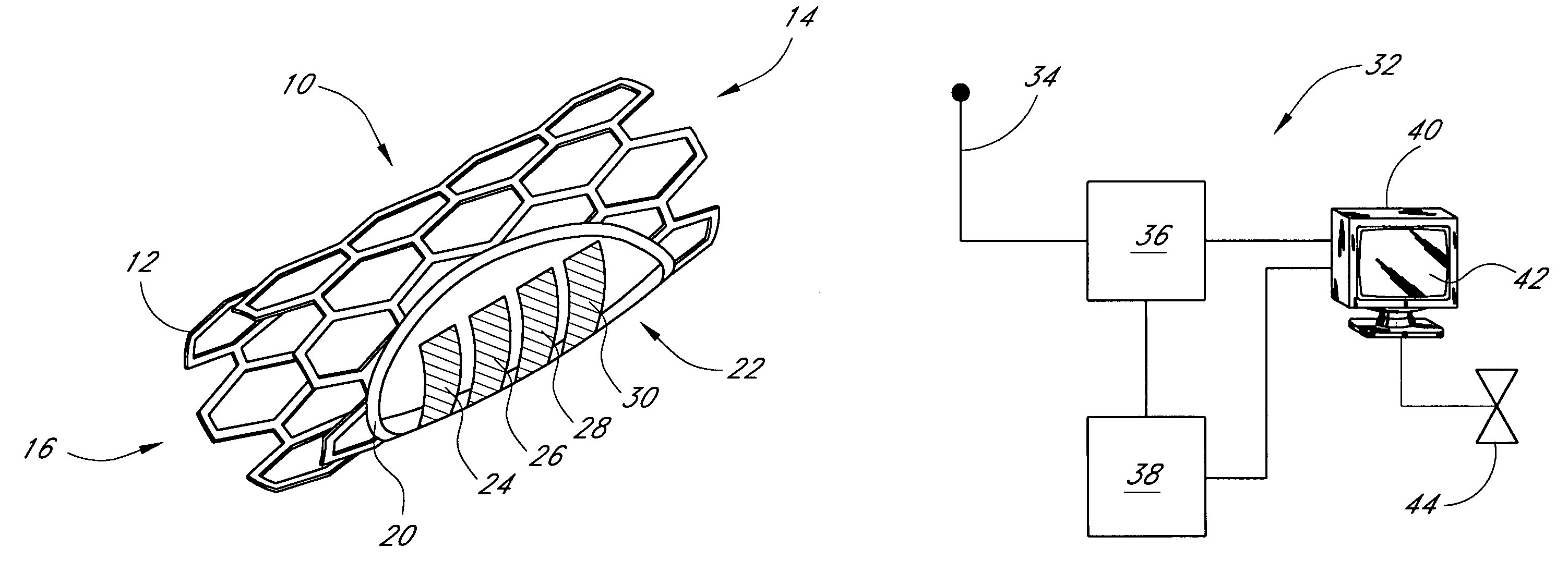
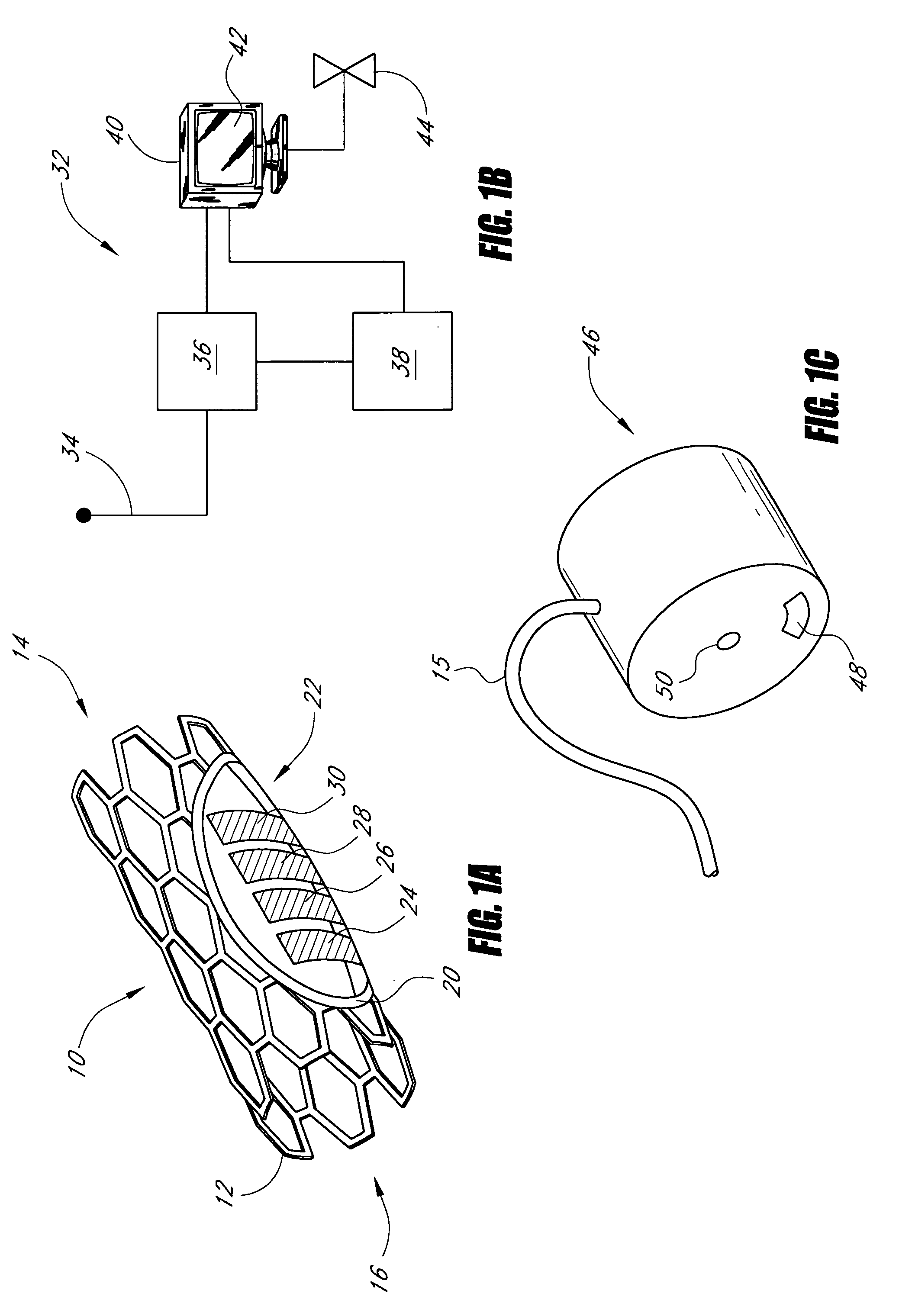
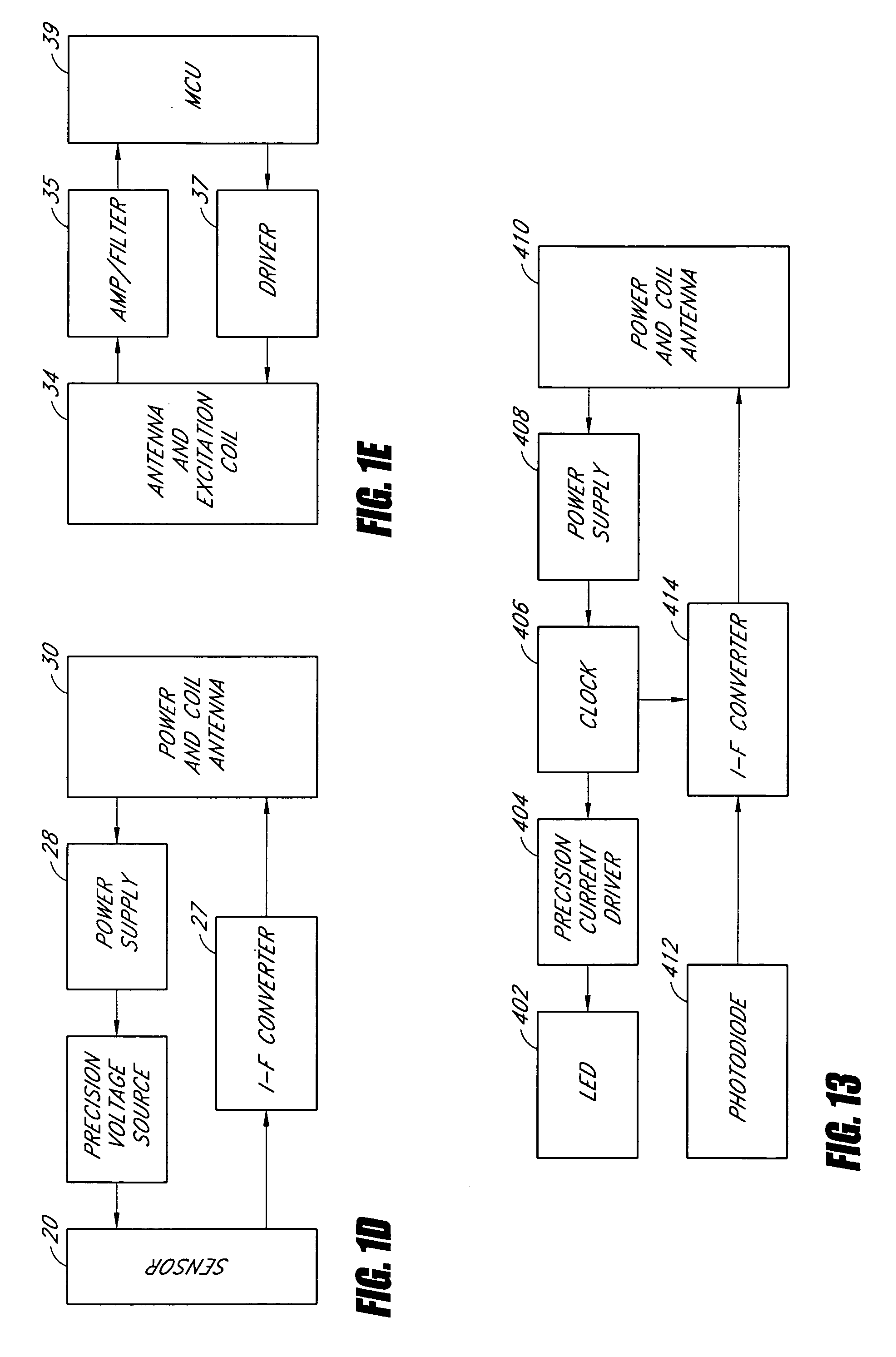
Sensors for detecting substances indicative of stroke, ischemia, or myocardial infarction
ActiveUS20060079740A1Thickness minimizationTransport of glucose to the sensor is not altered over timeStentsCatheterMetaboliteCitrulline
A sensor is disclosed, for implantation within a blood vessel to monitor a substance in or property of blood. In one embodiment, the sensor detects nitric oxide or a nitric oxide metabolite. In another embodiment, other substances such as glutamate, aspartate, arginine, citrulline, acetylcholine, calcium, potassium, or dopamine are monitored. The sensor may be attached to a support structure such as a stent, guidewire, or catheter. In a further embodiment, a catheter is disclosed that extracts patient fluid to a sensor outside the body for monitoring a substance or property of the patient fluid. Methods are also disclosed.
Owner:SILVER JAMES H +1
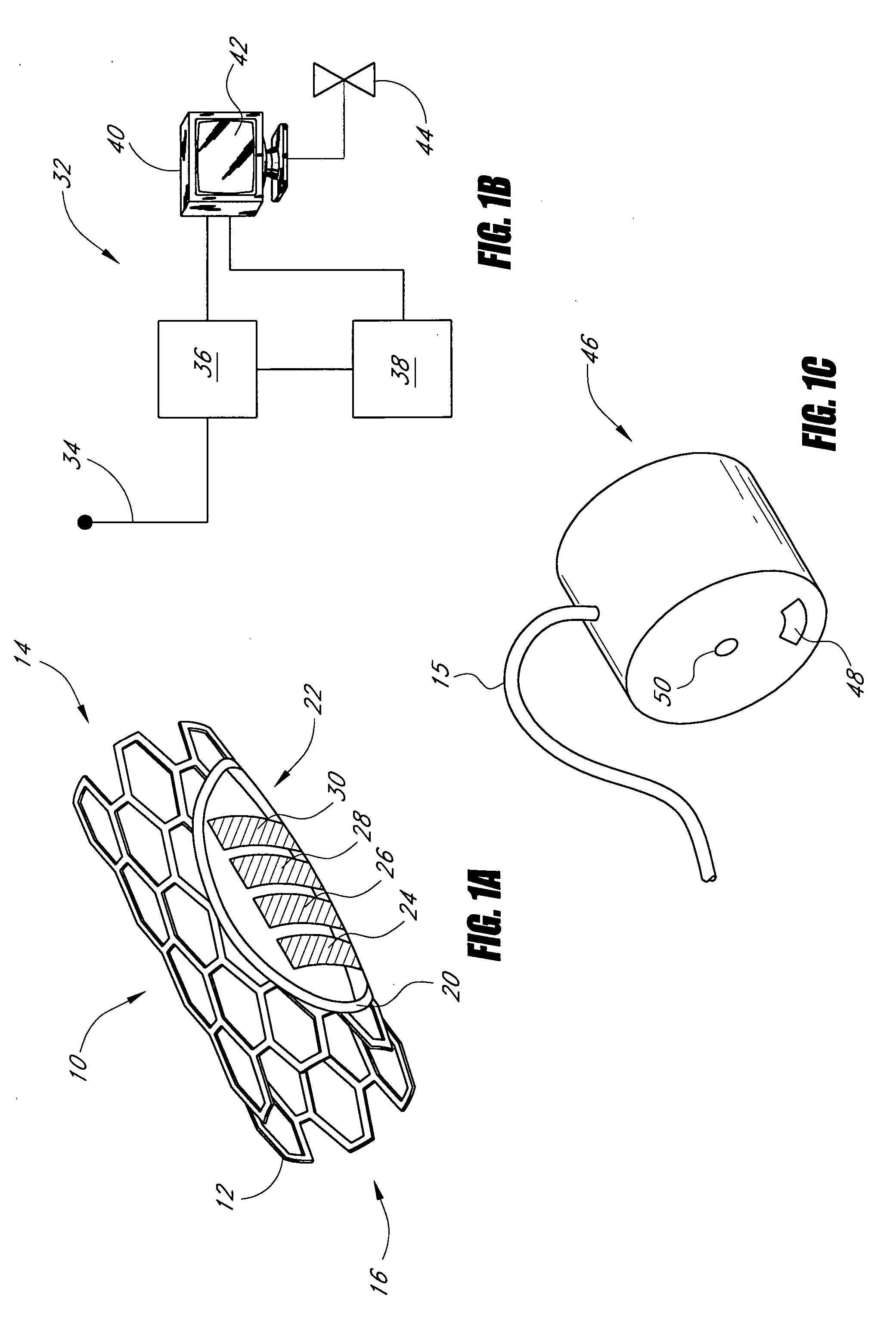
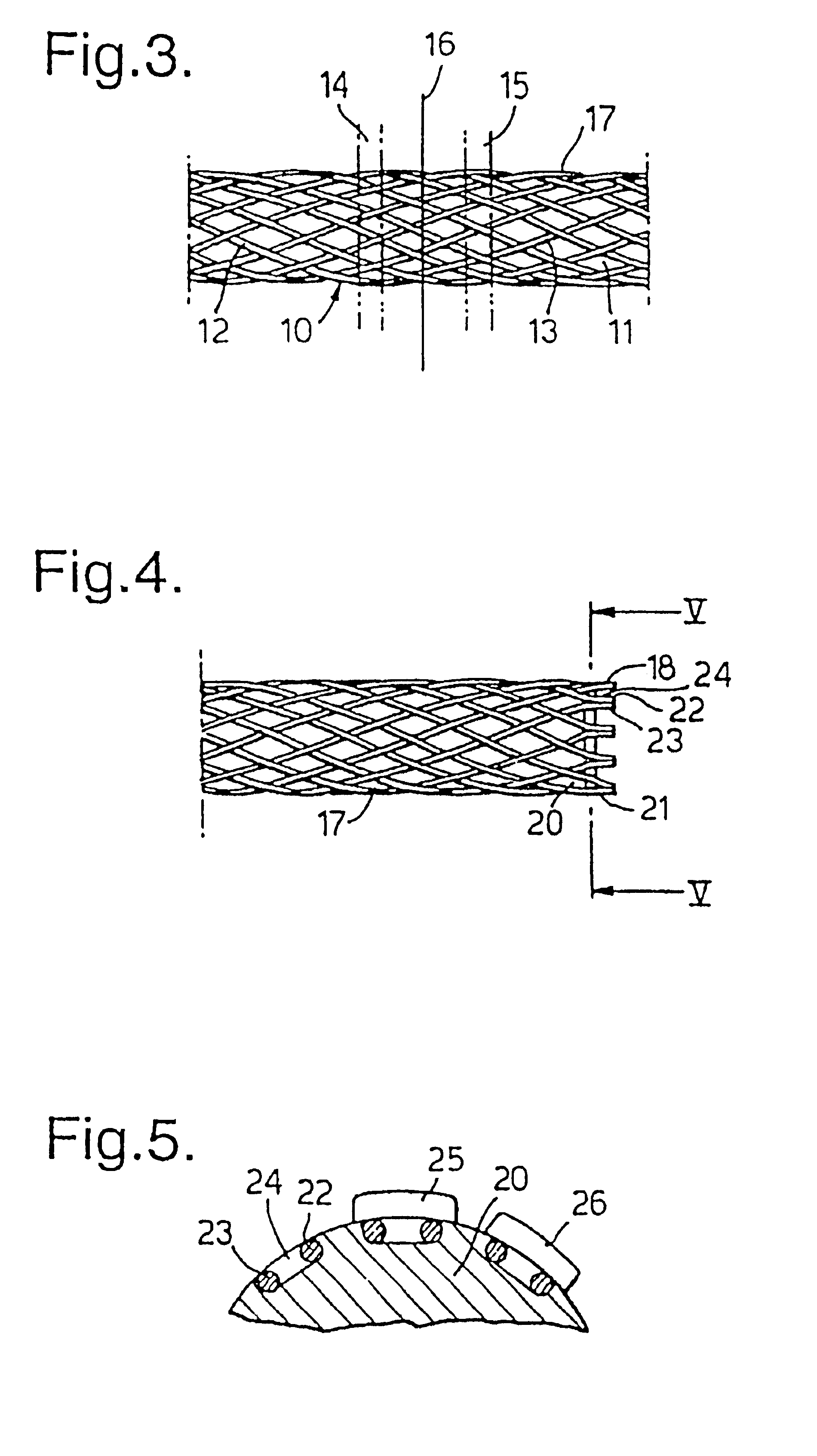
Biocompatible crosslinked coating and crosslinkable coating polymer composition for forming such a coating
A braided stent (1) for transluminal implantation in body lumens is self-expanding and has a radial expanded configuration in which the angle α between filaments is acute. Some or all of filaments (6,7) are welded together in pairs at each end (4,5) of the stent to provide beads (8), thereby strengthening the stent and assisting its deployment from a delivery device. The stent is preferably completely coated using a biocompatible polymeric coating, said polymer preferably having pendant phosphoryl choline groups. A method of making the stent by braiding and welding is described as well as a delivery device for deploying the device.The present invention provides a biocompatible crosslinked coating and a crosslinkable coating polymer composition for forming such a coating. The biocompatible crosslinked coating may be formed by curing a polymer of 23 mole % (methacryloyloxy ethyl)-2-(trimethylammonium ethyl) phosphate inner salt, 47 mole % lauryl methacrylate, 5 mole % γtrimethoxysilyl propyl methacrylate and 25 mole % of hydroxy propyl methacrylate. The crosslinkable coating polymer may include 23 mole % (methacryloyloxy ethyl)-2-(trimethylammonium ethyl) phosphate inner salt, 47 mole % lauryl methacrylate, 5 mole % γtrimethoxysilyl propyl methacrylate and 25 mole % of hydroxy propyl methacrylate.<?insert-end id="INS-S-00001" ?>
Owner:BIOCOMPATIBLES UK LTD
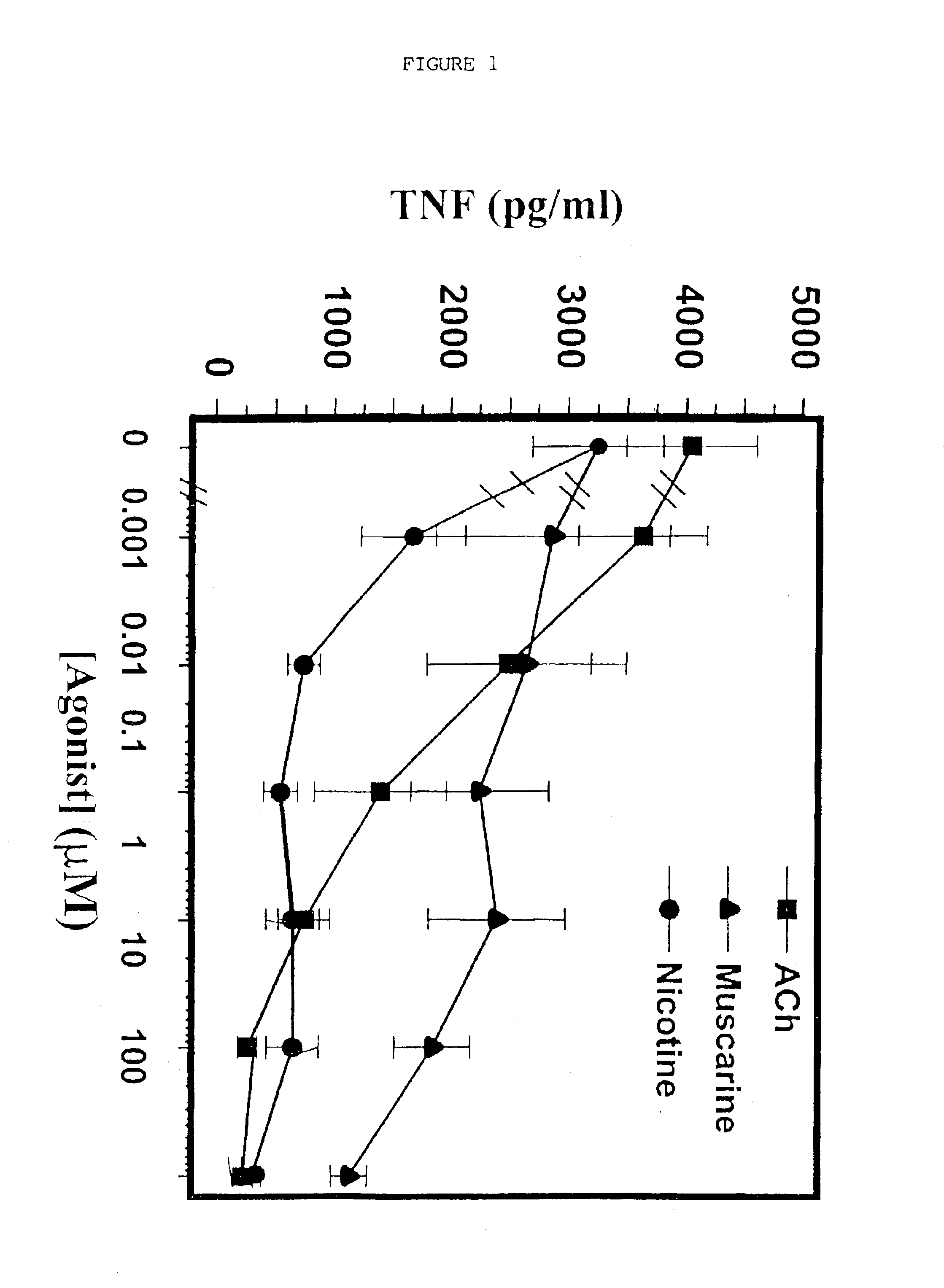
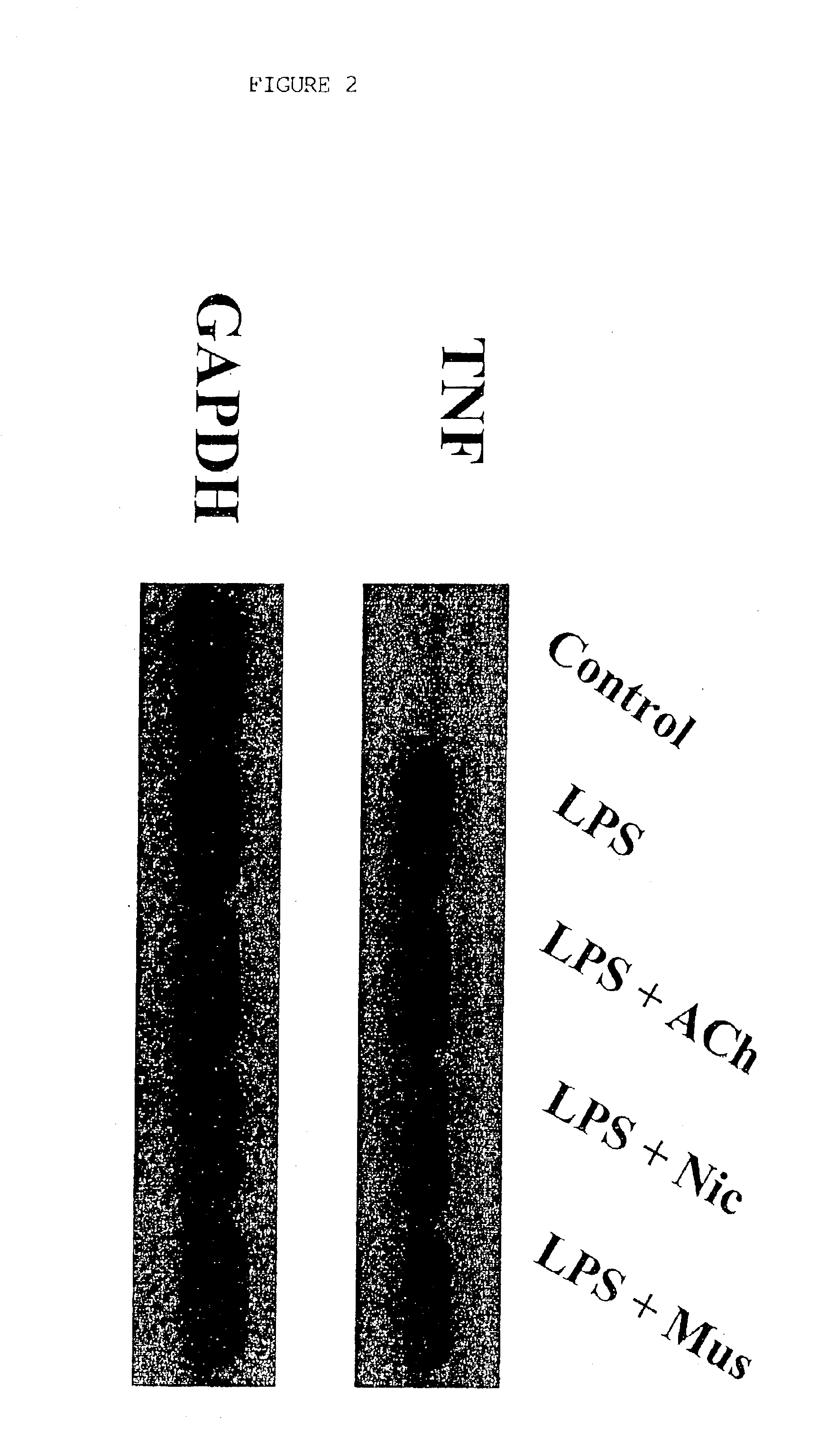
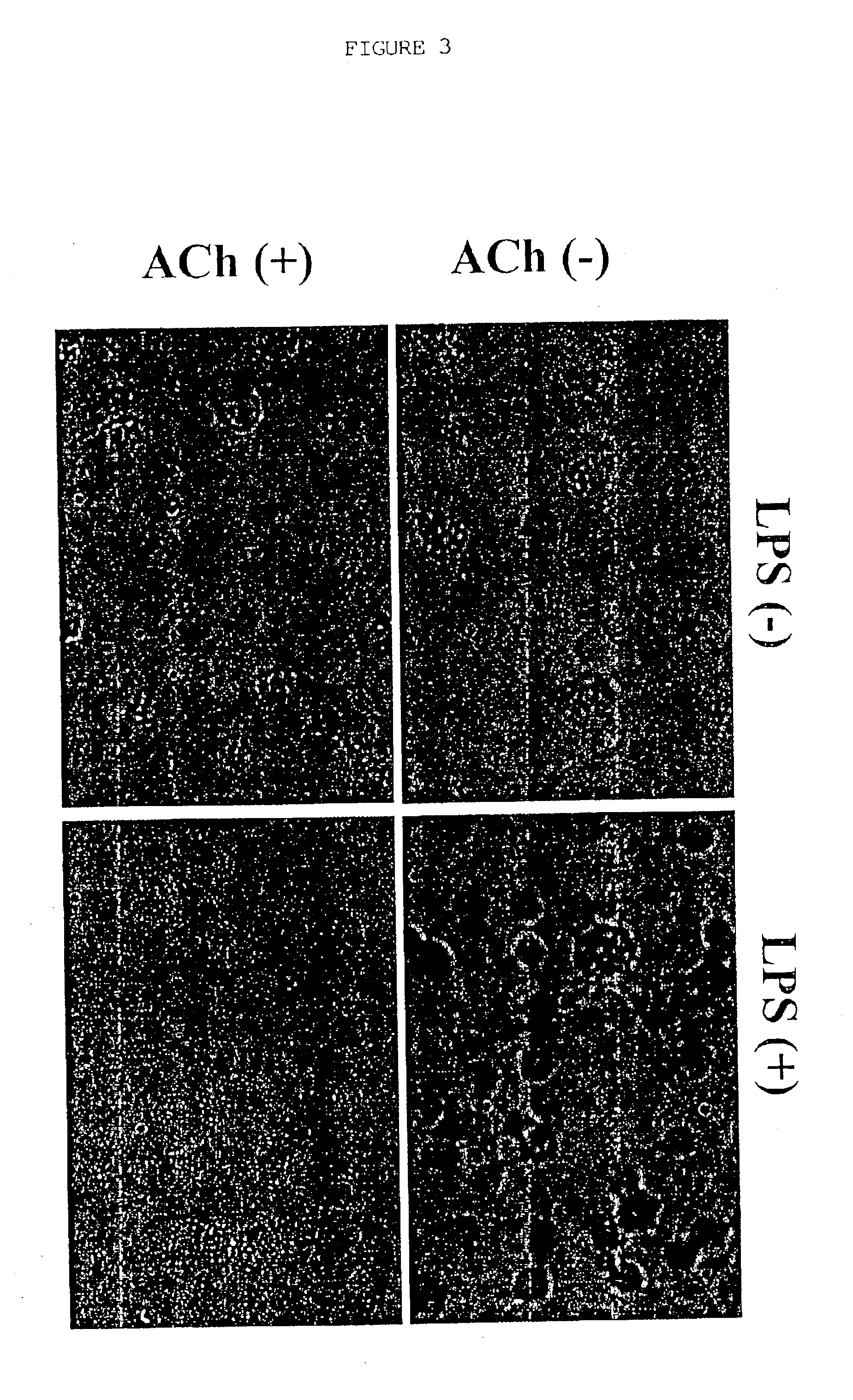
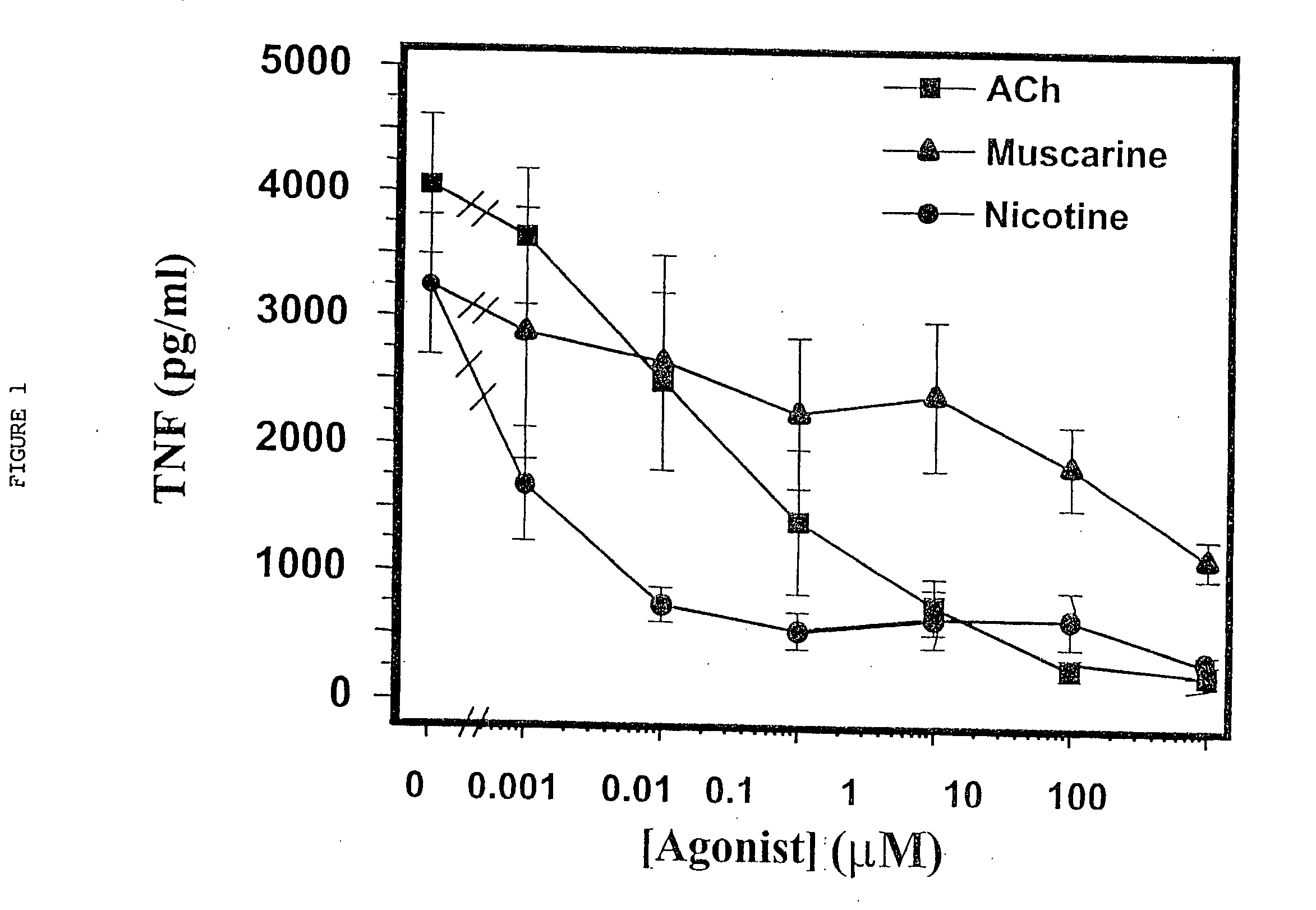
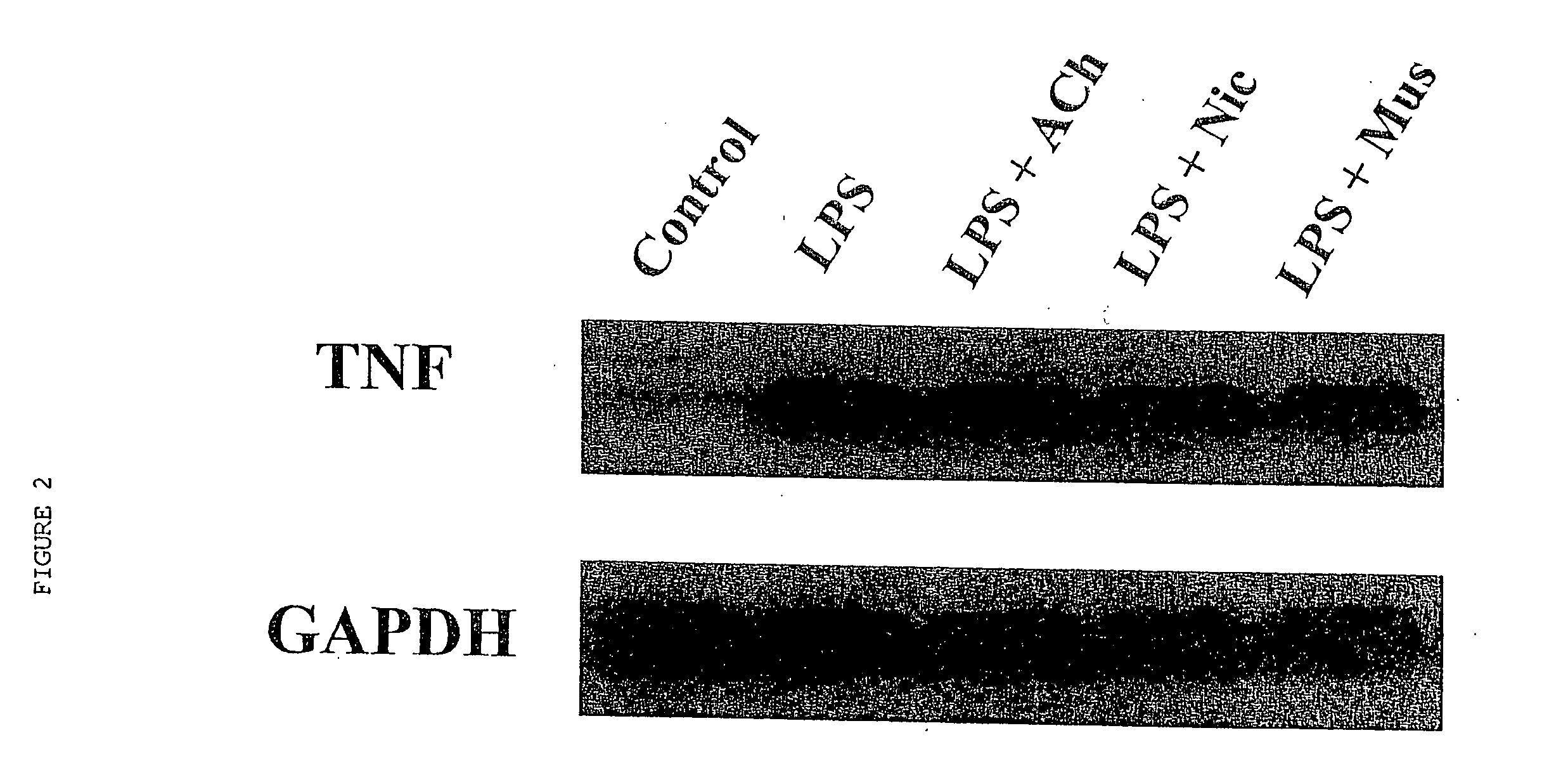
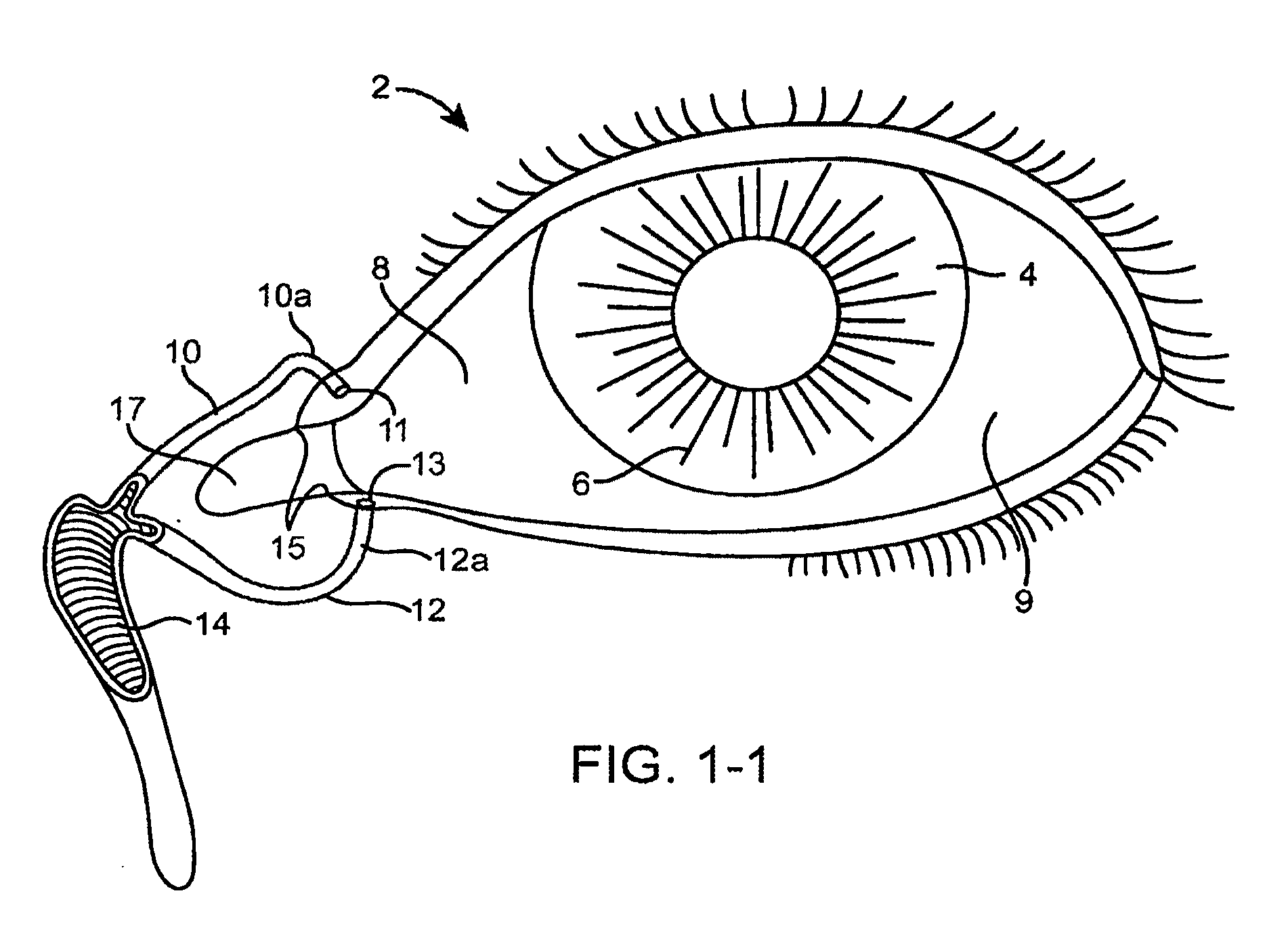
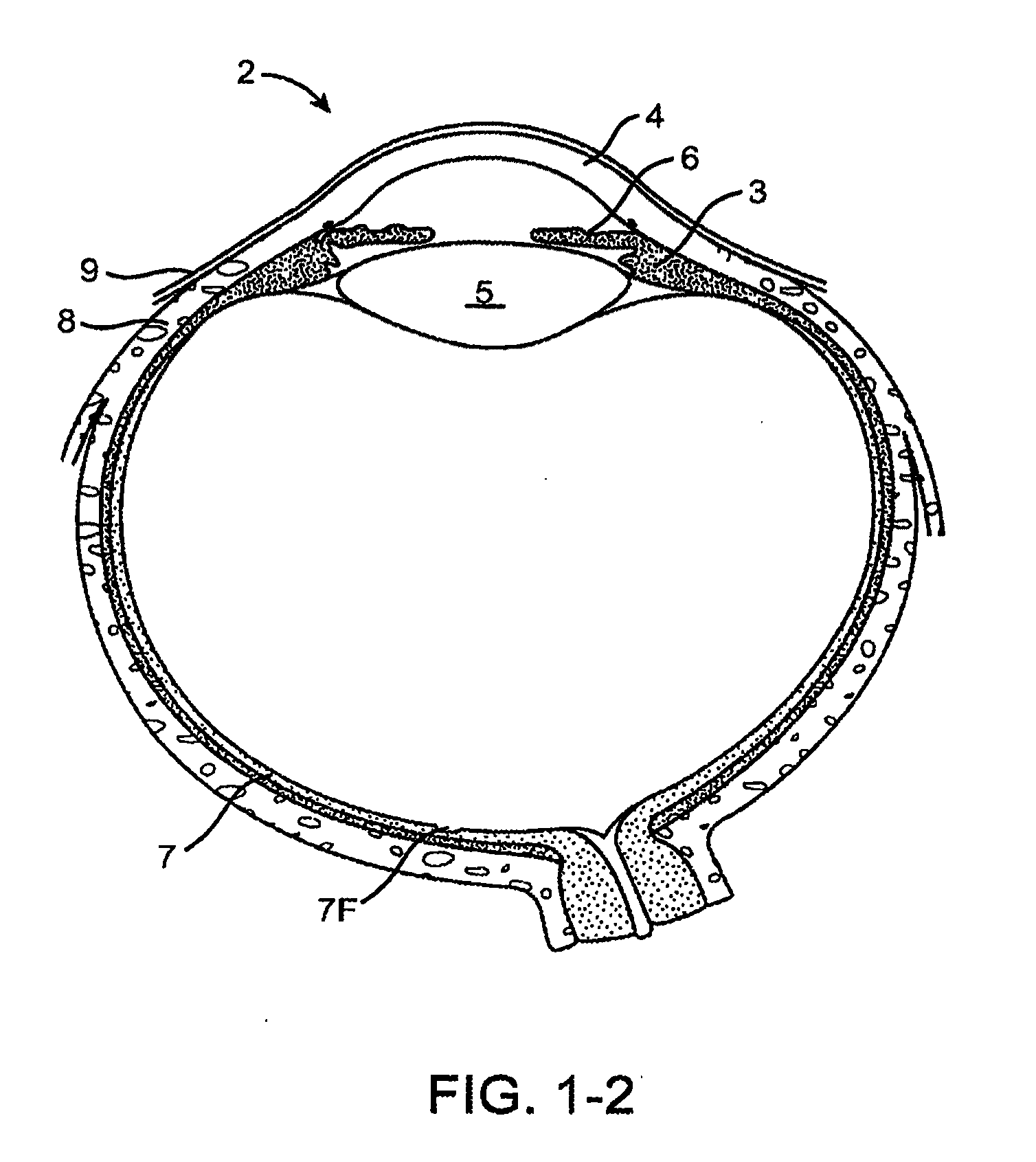
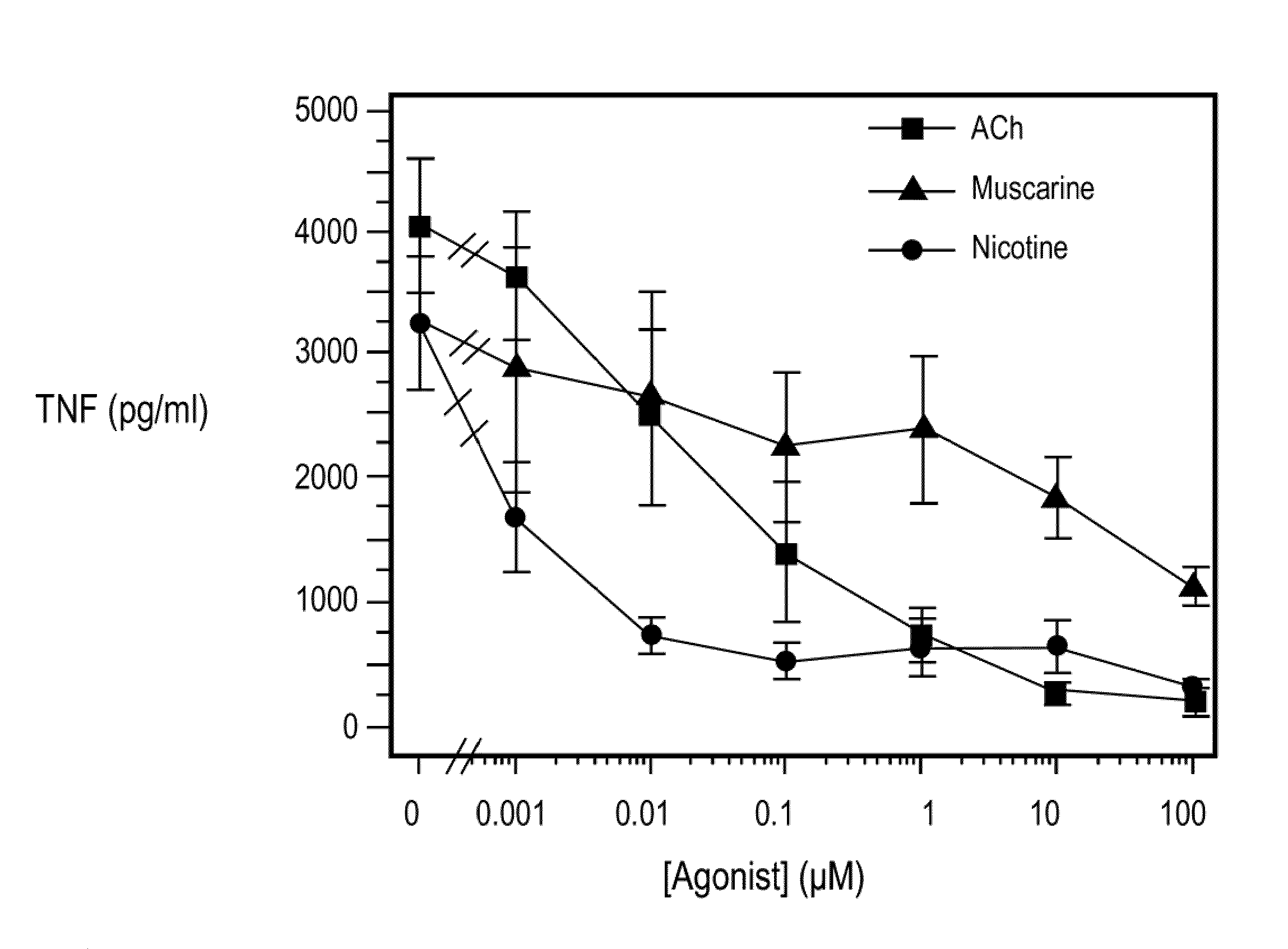
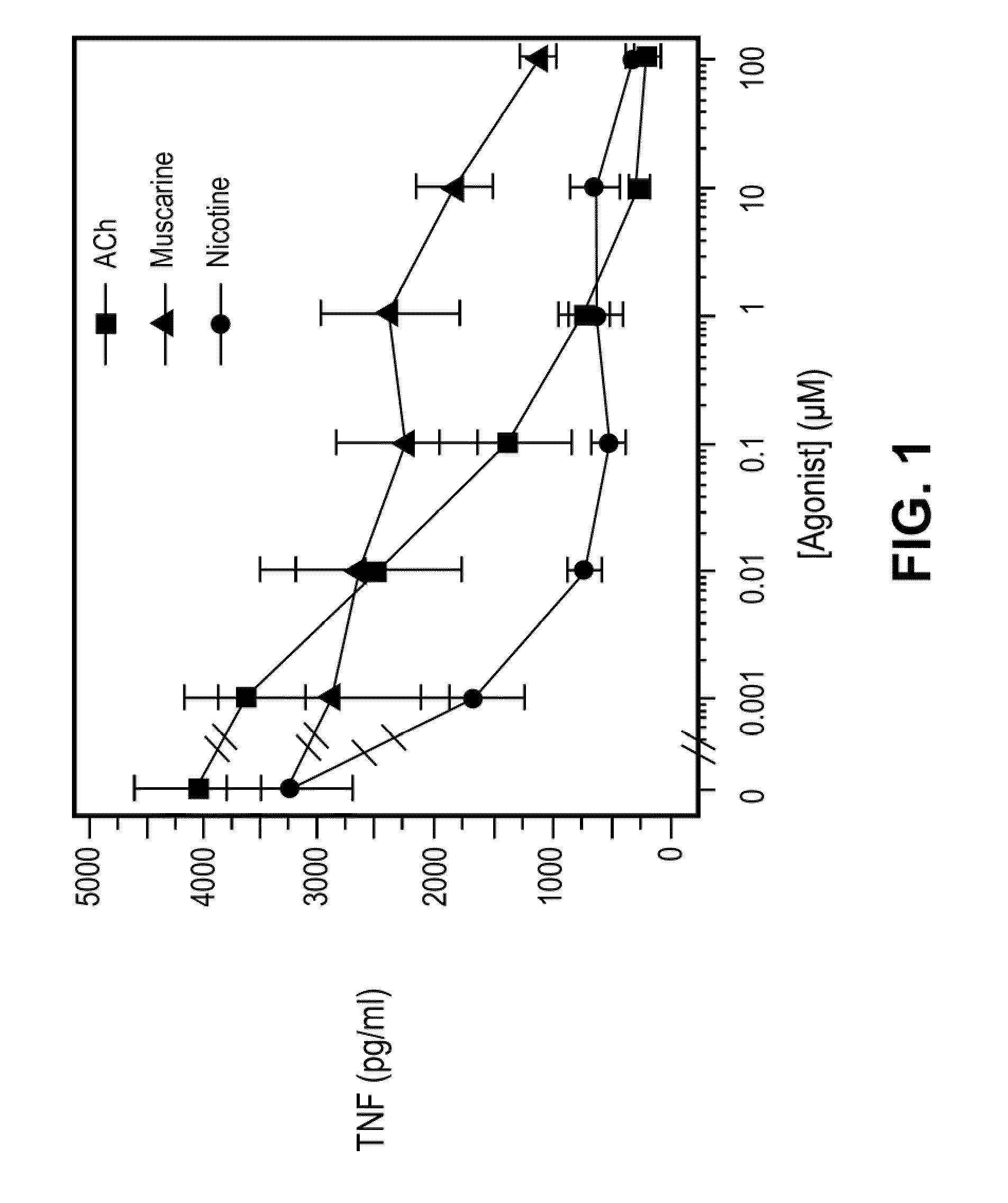
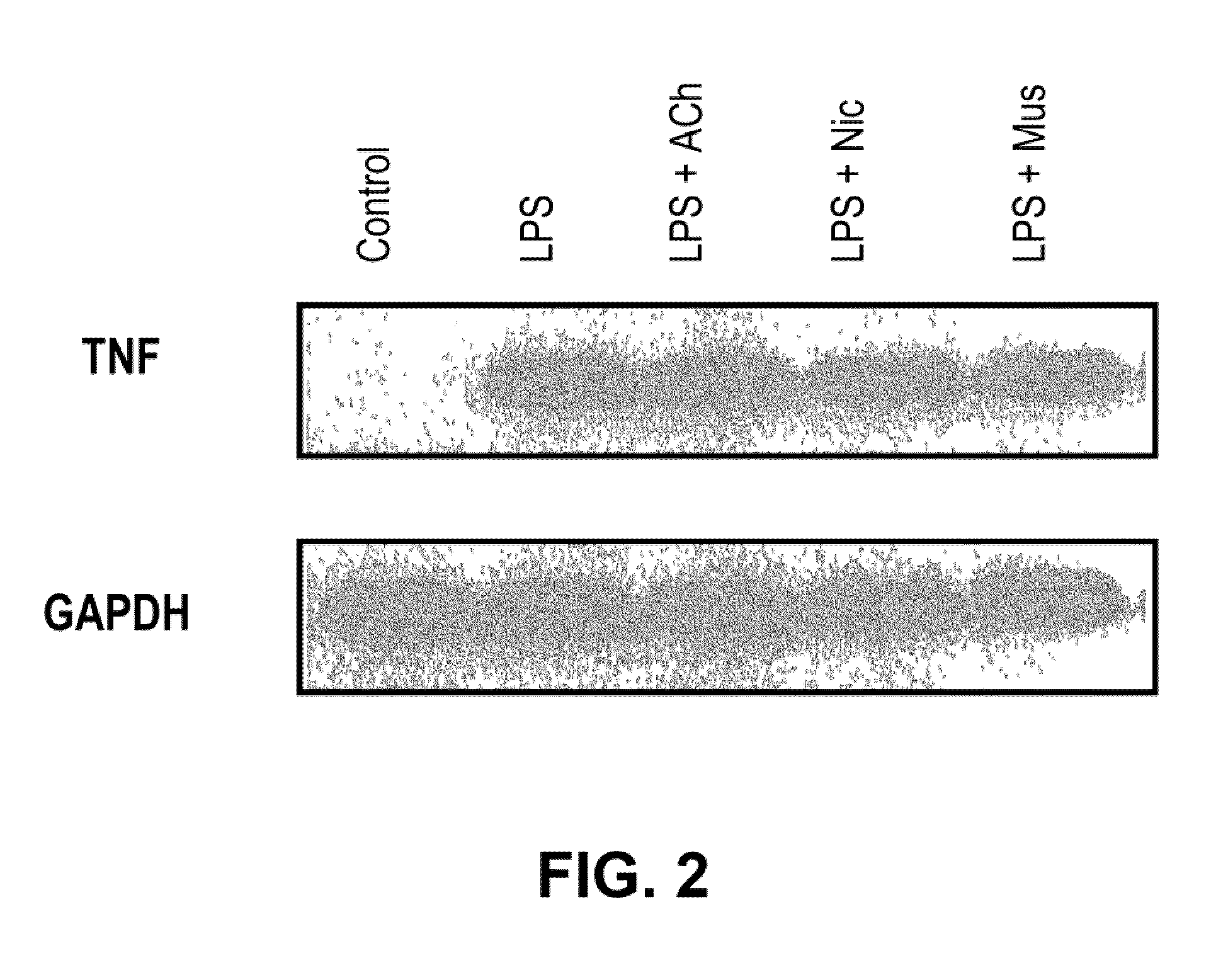
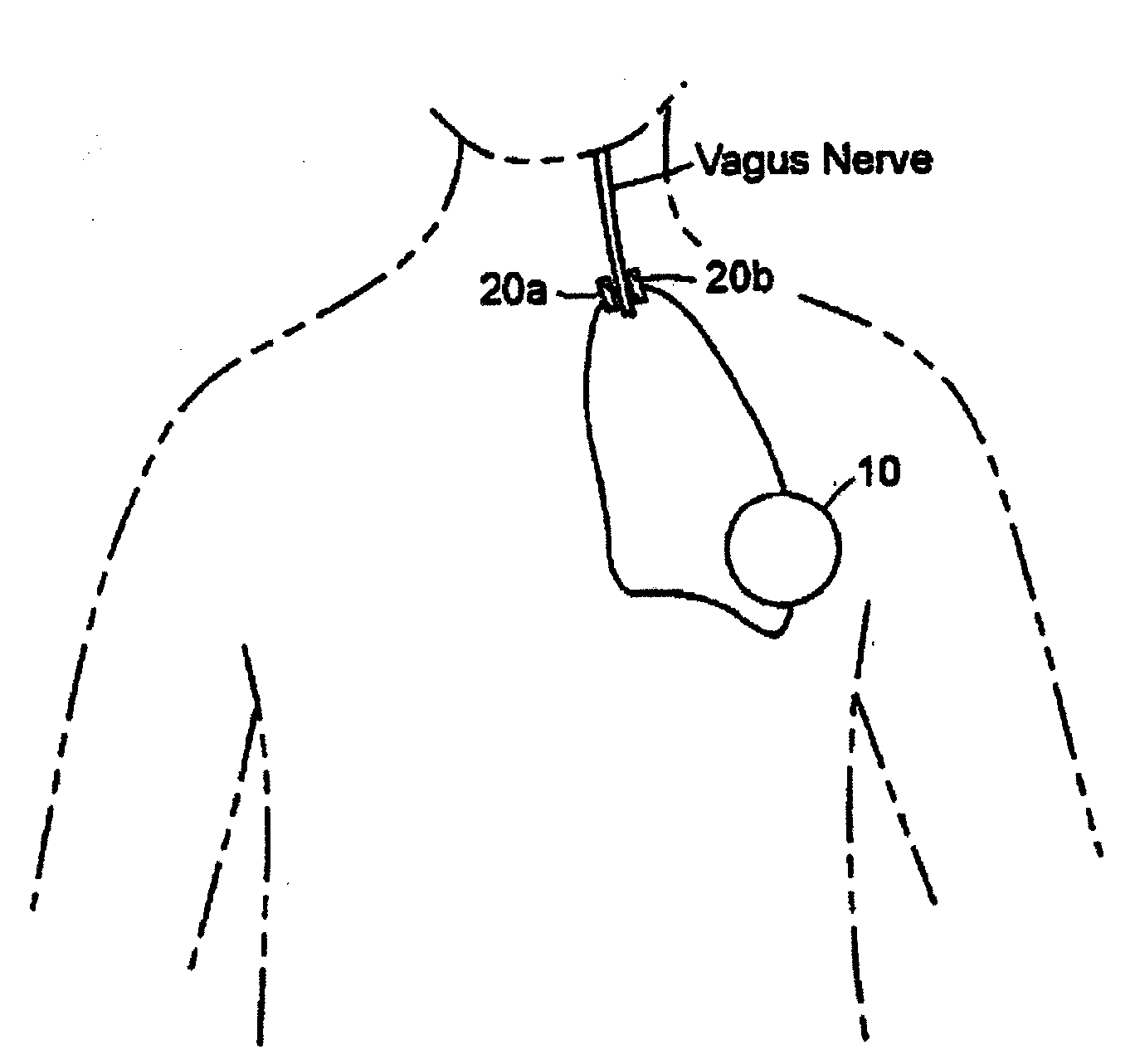
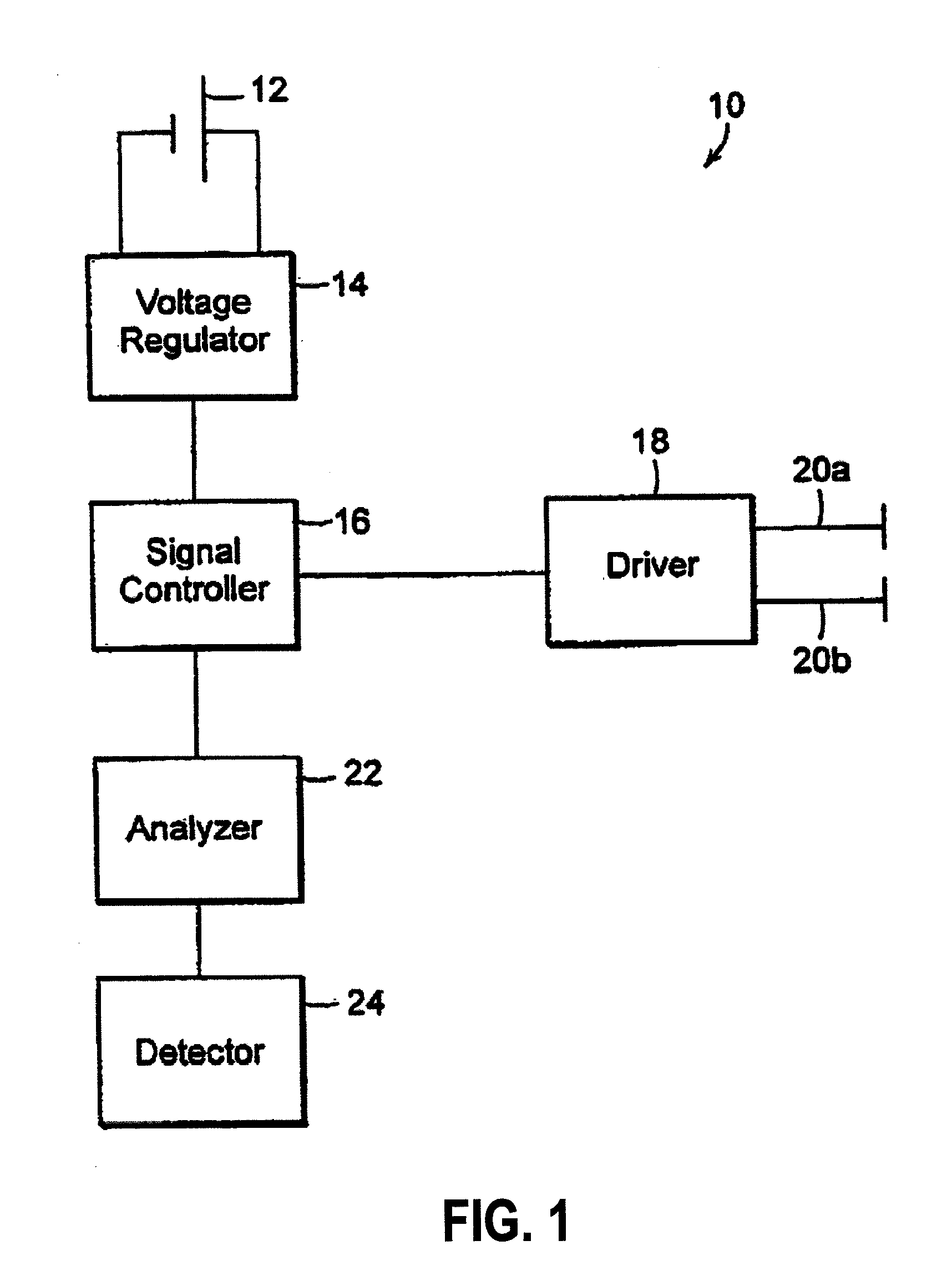
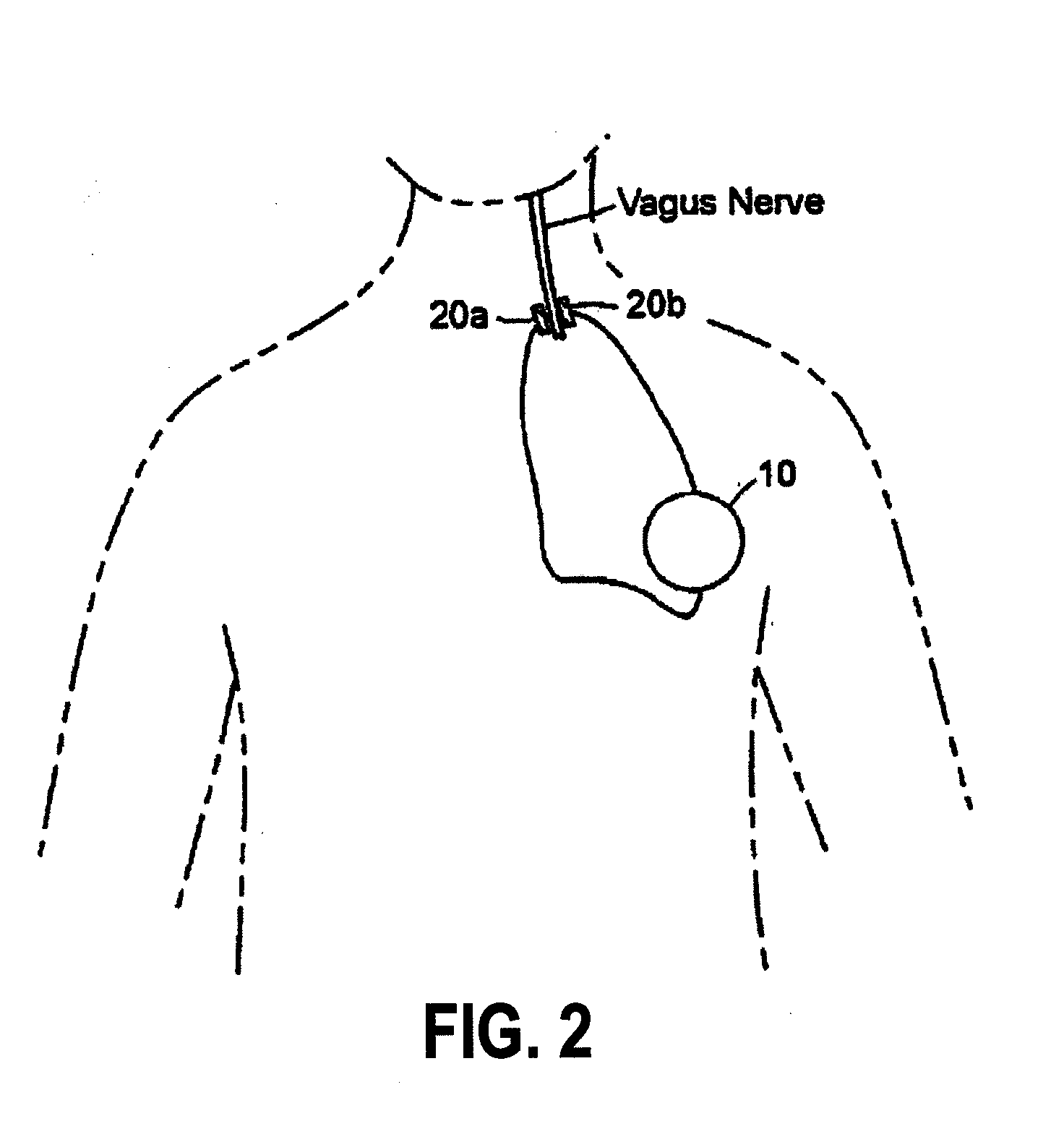
Inhibition of inflammatory cytokine production by cholinergic agonists and vagus nerve stimulation
A method of inhibiting the release of a proinflammatory cytokine in a cell is disclosed. The method comprises treating the cell with a cholinergic agonist. The method is useful in patients at risk for, or suffering from, a condition mediated by an inflammatory cytokine cascade, for example endotoxic shock. The cholinergic agonist treatment can be effected by stimulation of an efferent vagus nerve fiber, or the entire vagus nerve.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Inhibition of inflammatory cytokine production by cholinergic agonists and vagus nerve stimulation
InactiveUS20050125044A1Inhibition releaseImplantable neurostimulatorsHeterocyclic compound active ingredientsEfferentEndotoxic shock
A method of inhibiting the release of a proinflammatory cytokine in a cell is disclosed. The method comprises treating the cell with a cholinergic agonist. The method is useful in patients at risk for, or suffering from, a condition mediated by an inflammatory cytokine cascade, for example endotoxic shock. The cholinergic agonist treatment can be effected by stimulation of an efferent vagus nerve fiber, or the entire vagus nerve.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Proteinic drug delivery system using membrane mimetics
A mixed liposome pharmaceutical formulation with multilamellar vesicles, comprises a proteinic pharmaceutical agent, water, an alkali metal lauryl sulphate in a concentration of from 1 to 10 wt. / wt. %, at least one membrane-mimetic amphiphile and at least one phospholipid. The membrane-mimetic amphiphile is hyaluronic acid, pharmaceutically acceptable salts of hyaluronic acid, lauramidopropyl betain, lauramide monoisopropanolamide, sodium cocoamphopropionate, bishydroxypropyl dihydroxypropyl stearammonium chloride, polyoxyethylene dihydroxypropyl stearammonium chloride, dioctadecyldimethylammonium chloride, sulphosuccinates, stearamide DEA, gamma-linoleic acid, borage oil, evening of primrose oil, monoolein, sodium tauro dihydro fusidate, fusidic acid, alkali metal isostearyl lactylates, alkaline earth metal isostearyl lactylates, panthenyl triacetate, cocamidopropyl phosphatidyl PG-diammonium chloride, stearamidopropyl phosphatidyl PG-diammonium chloride, borage amidopropyl phosphatidyl PG-diammonium chloride, borage amidopropyl phosphatidylcholine, polysiloxy pyrrolidone linoleyl phospholipid, trihydroxy-oxo-cholanylglycine and alkali metal salts thereof, and octylphenoxypolythoxyethanol, polydecanol X-lauryl ether, polydecanol X-oleyl ether, wherein X is from 9 to 20, or combinations thereof. The phospholipid is phospolipid GLA, phosphatidyl serine, phosphatidylethanolamine, inositolphosphatides, dioleoylphosphatidylethanolamine, sphingomyelin, ceramides, cephalin, triolein, lecithin, saturated lecithin and lysolecithin, or a combination thereof. The amount of each membrane mimetic amphiphile and phospholipid is present 1 to 10 wt. / wt. % of the total formulation, and the total concentration of membrane mimetic amphiphiles and phospholipids is less than 50 wt. / wt. % of the formulation.
Owner:GENEREX PHARMA
Drug delivery implants for inhibition of optical defects
InactiveUS20100114309A1Avoid side effectsInhibition releaseSenses disorderEye surgeryFar-sightednessHomatropine
An implant for use with an eye comprises an implantable structure and a therapeutic agent. The therapeutic agent is deliverable from the structure into the eye so as to therapeutically effect and / or stabilize a refractive property of the eye. In many embodiments, the refractive property of the eye may comprise at least one of myopia, hyperopia or astigmatism. The therapeutic agent can comprise a composition that therapeutically effects or stabilizes the refractive property of the eye. The therapeutic agent may comprise at least one of a mydriatic or a cycloplegic drug. For example, the therapeutic agent may include a cycloplegic that comprises at least one of atropine, cyclopentolate, succinylcholine, homatropine, scopolamine, or tropicamide. In many embodiments, a retention element can be attached to the structure to retain the structure along a natural tissue surface.
Owner:MATI THERAPEUTICS
Inhibition of inflammatory cytokine production by cholinergic agonists and vagus nerve stimulation
InactiveUS20090248097A1Implantable neurostimulatorsHeterocyclic compound active ingredientsEfferentEndotoxic shock
A method of inhibiting the release of a proinflammatory cytokine in a cell is disclosed. The method comprises treating the cell with a cholinergic agonist. The method is useful in patients at risk for, or suffering from, a condition mediated by an inflammatory cytokine cascade, for example endotoxic shock. The cholinergic agonist treatment can be effected by stimulation of an efferent vagus nerve fiber, or the entire vagus nerve.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Treating inflammatory disorders by stimulation of the cholinergic Anti-inflammatory pathway
ActiveUS20090143831A1ElectrotherapyArtificial respirationMechanical irritationCholinergic anti-inflammatory pathway
Described herein are methods for treating a subject suffering from or at risk for a condition mediated by an inflammatory cytokine cascade, by electrically or mechanically stimulating vagus nerve activity in an amount sufficient to inhibit the inflammatory cytokine cascade.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Production of hSA-linked butyrylcholinesterases in transgenic mammals
InactiveUS20060253913A1Promote formationImprove stabilityAnimal cellsVectorsMammalButyrylcholinesterase
The present invention provides methods for the large-scale production of recombinant butyrylcholinesterase fused to human serum albumin in cell culture, and in the milk and / or urine of transgenic mammals. The recombinant butyrylcholinesterase-albumin fusion protein of this invention can be used to treat and / or prevent organophosphate pesticide poisoning, nerve gas poisoning, cocaine intoxication, and succinylcholine-induced apnea.
Owner:PHARMATHENE
Pharmaceutical composition and method for the transdermal delivery of calcium
InactiveUS20070292493A1Reduce disadvantagesReduce and prevent likelihoodHalogenated hydrocarbon active ingredientsBiocideArginineTryptophan
The present invention relates to a method and transdermal pharmaceutical composition for preventing or reducing the likelihood of calcium deficiency or imbalances caused by calcium deficiency. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of calcium and a pharmaceutically acceptable carrier constituting a pluronic lecithin organogel. In addition to calcium, the transdermal pharmaceutical composition may also contain a therapeutically effective amount of: (1) a pharmaceutically acceptably salt of other minerals such as magnesium, zinc, selenium, manganese, or chromium; (2) a vitamin such as vitamin A, vitamin D, vitamin C, vitamin E or B-complex vitamins, choline, lecithin, inositol, PABA, biotin, or bioflavomoids; (3) a carotenoid such as lycopene or lutein; (4) a hormone such as dehydroepiandrosterone, progesterone, pregnenolone, or melatonin; (5) an amino acid such as arginine, glutamine, lysine, phenylalanine, tyrosine, GABA, tryptophan, carnitine, or acetyl-l-carnitine; (6) a fatty acid such as a fish oil or flax seed oil; (7) a vita-nutrient such as coenzyme Q10; (8) a cartilage building nutrient such as glucosamine, chondroitin, or MSM, (9) a herb such as ginkgo biloba, echinacea, 5-HTP, St. John's wort, or saw palmetto; or (9) any combination thereof. The transdermal pharmaceutical composition may be topically administered to a human to prevent or reduce the likelihood of calcium deficiency or imbalances caused by calcium deficiency such as hypertension, high cholesterol, colon and rectal cancer, osteomalacia, rickets, osteoporosis, cardiovascular disease, preeclampsia, tooth decay, and premenstrual syndrome.
Owner:BRIERRE BARBARA T
A combination of mitochondrial nutrients for relieving stress, preventing and improving stress-related disorders
InactiveUS20060257502A1Accelerated agingIncreasing oxidative metabolismBiocideCosmetic preparationsAlpha-TocopherolL-Carnosine
A dietary supplement of mitochondrial nutrients is designed for relieving stress, preventing and improving stress-related disorders, such as chronic fatigue syndrome, diabetes, age-associated cognitive dysfunction and diseases (Parkinson's and Alzheimer's disease). The supplement composition has the following nutrients: B vitamins (cyanocobalamin 2-1,000 ug, thiamin 1-1,000 mg, niacin 15-2,000 mg, pyridoxine 1-1,000 mg, Pantothenate 5-150 mg, folic acid 400-40,000 ug), alpha-tocopherol 10-800 mg, ascorbic acid 50-10,000 mg, calcium 20-2,000 mg, vitamin A 200-10,000 ug, alpha-lipoic acid 100-1,000 mg, N-acetyl cysteine 100-3,000 mg, L-carnosine 100-9,000 mg, tyrosine 100-9,000 mg, vanillin 10-100 mg, phosphatidylserine 10-800 mg, resveratrol 10-50 mg, dehydroepiandrosterone 1-50 mg, and melatonin 0.1-3 mg, all of which have been individually used experimentally or clinically for relieving stress, preventing and treating age- and stress-related disorders and diseases but no combination of these compounds has been used. Many embodiments also contain at least one adjunct ingredient such as coenzyme Q 10-200 mg, acetyl-L-carnitine 100-2,000 mg, choline 50-1,000 mg, and creatine 100-2,000 mg.
Owner:LIU JIANKANG
Compositions and methods for treating and preventing memory impairment using citicoline
InactiveUS20030114415A1Low toxicityAvoid damageBiocideCarbohydrate active ingredientsMemory disorderCiticoline
This invention relates to compositions and methods for preventing and treating cognitive dysfunction or memory impairment. The compositions include an effective amount of citicoline, or pharmaceutically-acceptable salts thereof, and one or more of the compounds selected from the group consisting of linoleic acid and linolenic acid. Other compositions of this invention include an effective amount of citicoline, or pharmaceutically-acceptable salt thereof, wherein said citicoline is metabolized to form at least one of cytidine, uridine, and choline. Still other compositions of this invention include effective amounts of choline, cytidine, and / or uridine, or their pharmaceutically-acceptable salts. This invention also encompasses methods for preparing these compositions.
Owner:MASSACHUSETTS INST OF TECH
Synergistic insecticidal mixtures
The invention relates to insecticidal mixtures of spinosyns and agonists or antagonists of nicotinic acetylcholine receptors for protecting plants against attack by pests.
Owner:DOW AGROSCIENCES LLC
Method and synergistic composition for treating attention deficit/hyperactivity disorder
InactiveUS6541043B2Minimize side effectsBiocideHydroxy compound active ingredientsBeta-CaroteneBetaine
A composition and method for treating Attention Deficit / Hyperactivity Disorder (ADHD) is provided which can be used both with and without ethical drugs now used to treat ADHD. The composition contains dimethylaminoethanol (DMAE), omega 3-fatty acids, betaine, oligomeric proanthocyanidins (OPC), folic acid, vitamins C, E, B12, B6, B5 and beta-carotene and minerals (calcium, magnesium, zinc and selenium). Ethical drugs such as amphetamines, methylphenidate HCl and pemoline are known to control ADHD, but each has significant side effects when used in their therapeutic dose. When combining the composition with such ethical drugs, the amount of the ethical drug can be lowered below a level which causes undesirable side effects which is an important feature. Preferred compositions contain one or more of lecithin, choline, 5-hydroxytryptophan, tyrosine, Reishi Extract, Kava Extract, Gingko, Ginseng and St. John's Wort.
Owner:PHILIP C LANG
Alzheimer's disease treatment with multiple therapeutic agents delivered to the olfactory region through a special delivery catheter and iontophoresis
InactiveUS20120323214A1Reduce and preventAvoid destructionNervous disorderHead electrodesApoptosisExcitotoxicity
This invention describes the administration of multiple therapeutic agents with insulin in conjunction with bexarotene, ketamine, monoclonal antibodies Etanercept, IGF-1, and acetylcholine esterase inhibitors physostigmine, for treatment of Alzheimer's disease and other neurodegenerative diseases. Insulin, improves memory; also augments and amplifies the effects of the adjuvant therapeutic agents (paracrine and intracrine effects) and consequently reduces the β amyloid, its soluble precursors, prevents damage to the neuronal skeletal network (taupathy), and blocks glutamate excitotoxicity, reduces brain inflammation, prevents apoptosis, and increases the acetylcholine levels in the neurons and synapses; by using a combination of insulin, bexarotene, ketamine, Etanercept, IGF-1, and physostigmine therapeutic agents. The results are achieved by using the specially designed Iontophoresis incorporated olfactory mucosal delivery (ORE) catheter device located at the olfactory nerves, sphenoid sinus, and adjacent structures described here, to transport the large molecules of therapeutic agents to treat AD delivered to the CNS bypassing BBB from ORE.
Owner:WEDGE THERAPEUTICS
Sensors for detecting substances indicative of stroke, ischemia, or myocardial infarction
InactiveUS7769420B2Thickness minimizationTransport of glucose to the sensor is not altered over timeStentsCatheterMetaboliteCitrulline
Owner:SILVER JAMES H +1
Hen feedstuff for improving egg-laying performance
ActiveCN101181016AIncrease egg productionImprove immunityAnimal feeding stuffAccessory food factorsEggshellVitamin C
The invention relates to a layer foodstuff used for enhancing egg laying performance, which has the components in weight portion as follow: 100 to 160 portions of calcium hydrogen phosphate, 250 to 350 portions of mountain flour, 40 to 70 portions of common salt, 20 to 40 portions of saleratus, 12 to 20 portions of choline, 30 to 40 portions of complex trace elements, 8 to 10 portions of complex vitamins, 1 to 3 portions of vitamin C, 25 to 30 portions of methionine, 50 to 100 portions of fish meal, and 250 to 400 portions of zeolite powder. Such additives as nutritive additive are added into the foodstuff in the invention, which has the advantages of apparently strengthening immunity of the layer, maintaining a long egg laying peak period, good quality of an egg shell, high egg laying rate, low layer culling rate, and obviously improving egg laying performance.
Owner:KUNMING HEMEIHUA FEED
Acryloyloxyethylphosphorylcholine Containing Polymer Conjugates and Their Preparation
InactiveUS20100166700A1Prevents and reduces efficiencyExtended half-lifeFactor VIINervous disorderPolymer sciencePharmaceutical drug
The present invention relates to polymeric reagents and conjugates thereof, methods for synthesizing the polymeric reagents and conjugates, pharmaceutical compositions comprising the conjugates and methods of using the polymer conjugates including therapeutic methods where conjugates are administered to patients.
Owner:KODIAK SCI
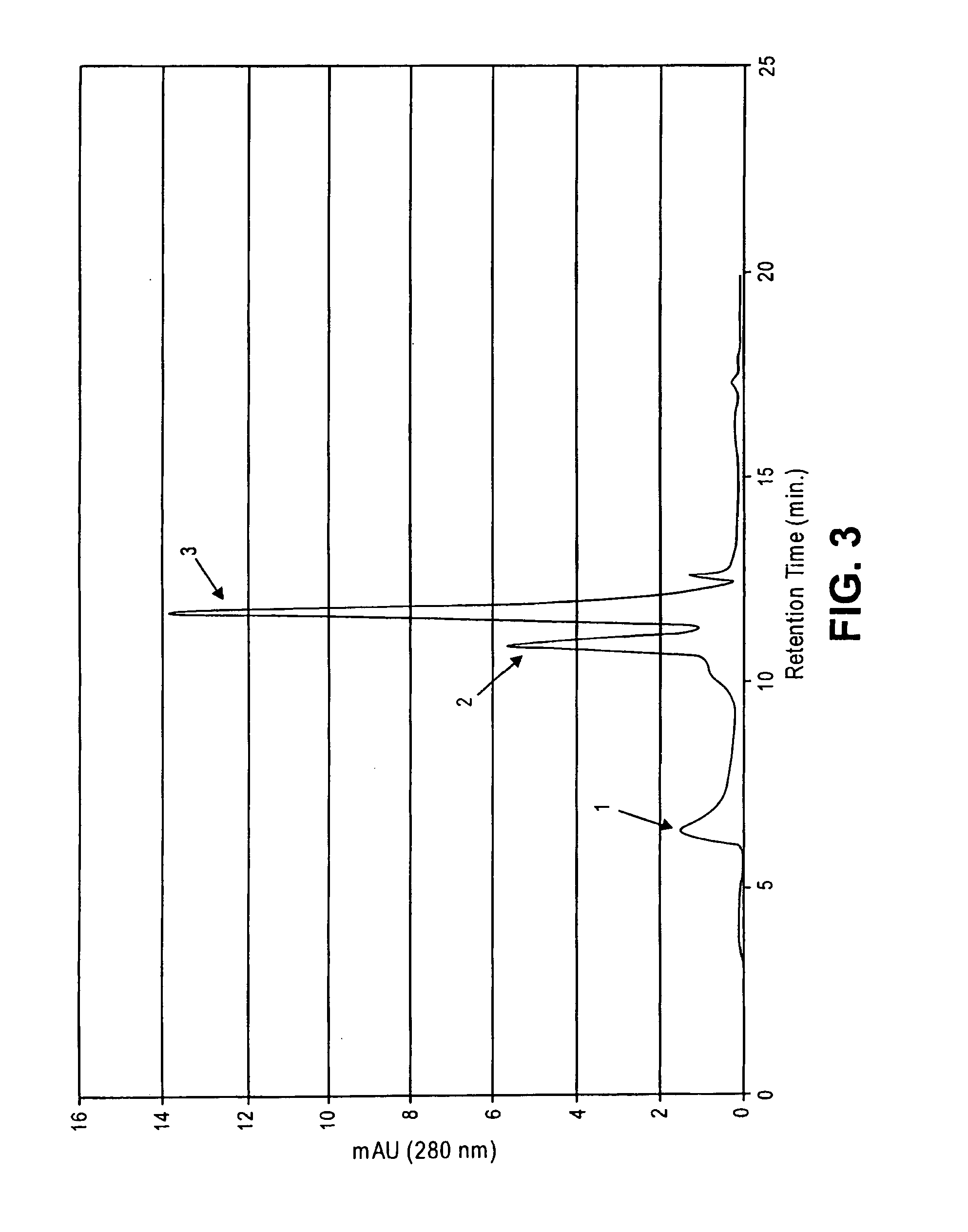
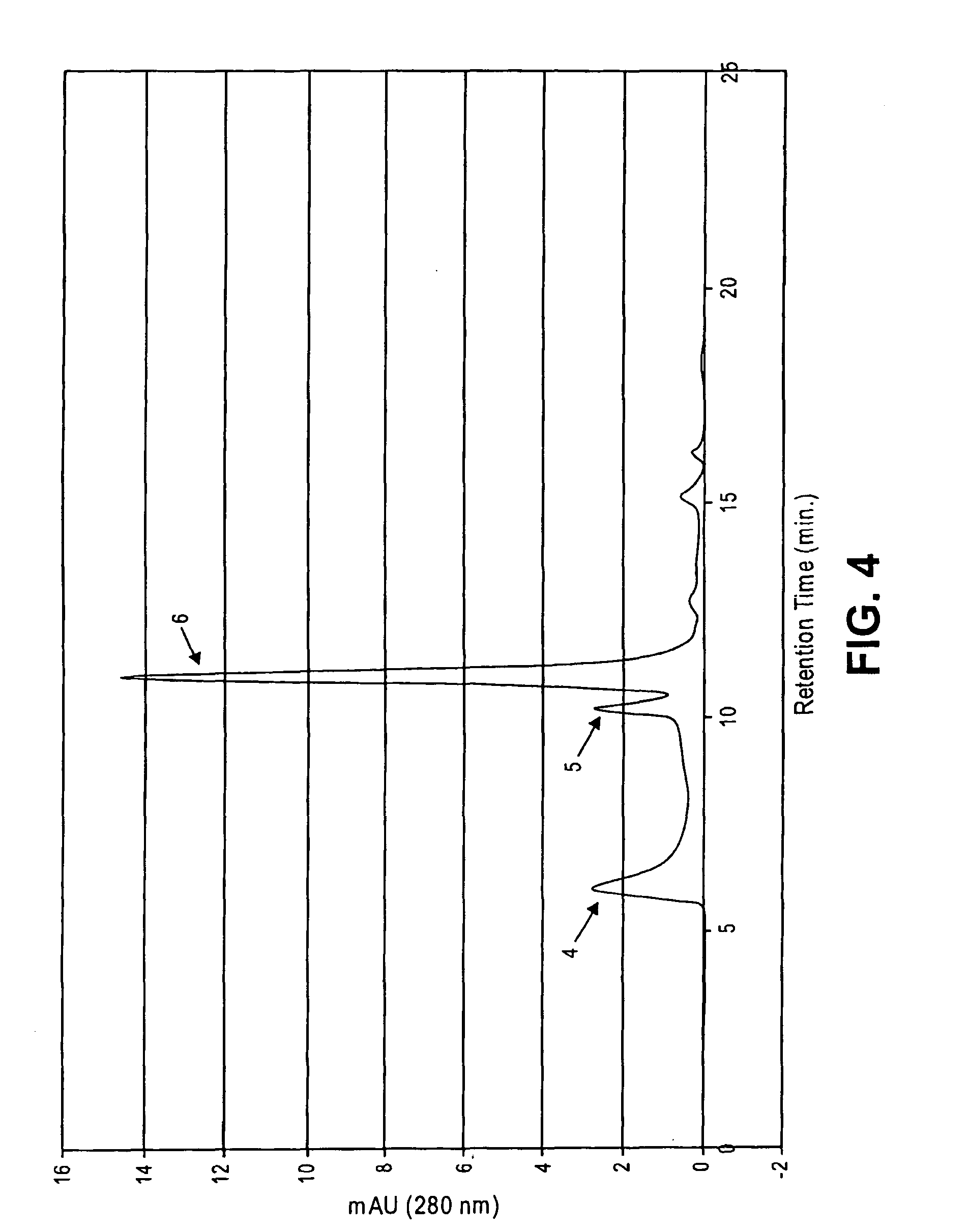
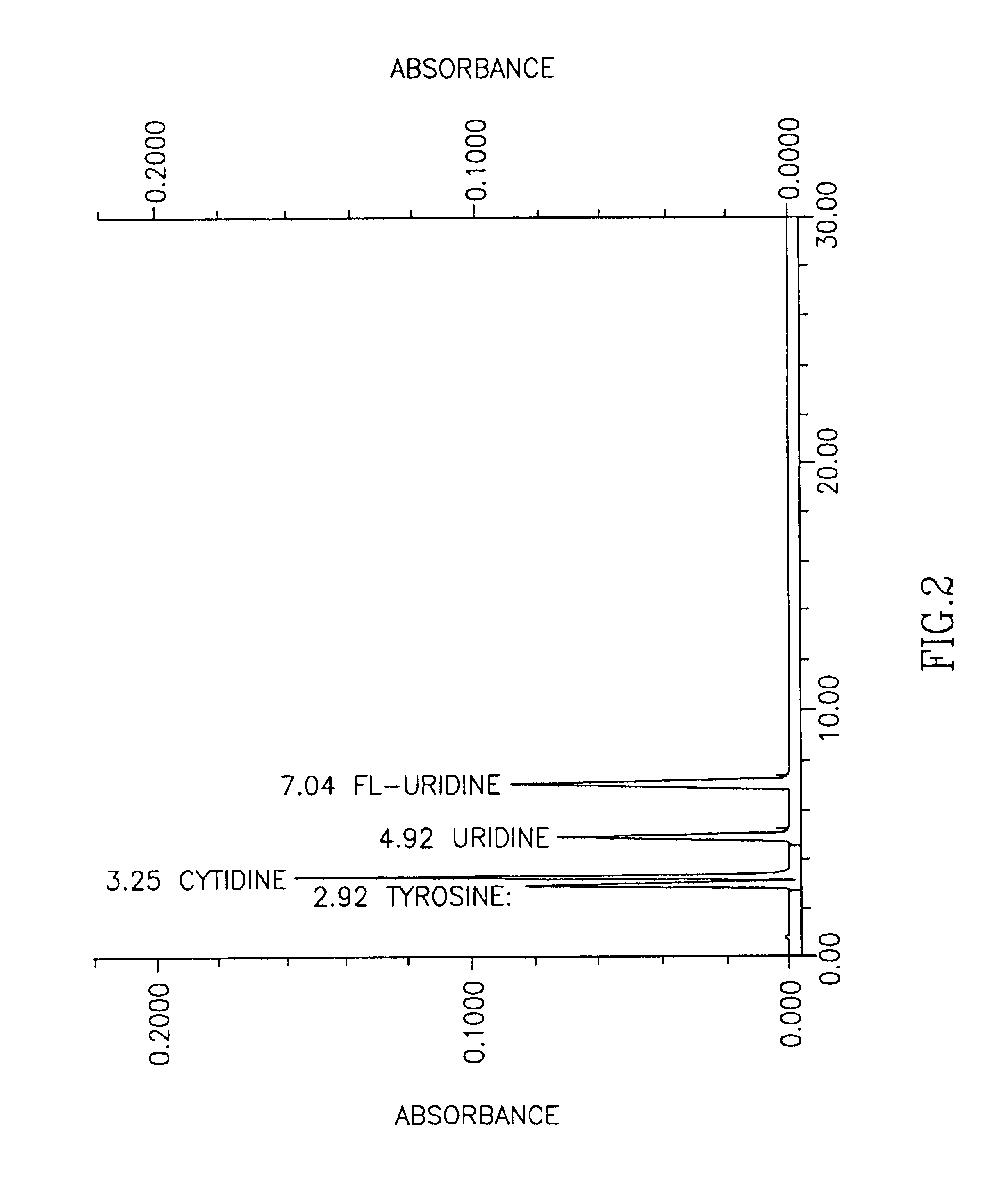
Methods for increasing blood cytidine and/or uridine levels and treating cytidine-dependent human diseases
InactiveUS6989376B2Enhancing uridine bioavailabilityBiocideNervous disorderMedicineUridine Nucleotides
Methods of treating certain neurological diseases using exogenous uridine or a uridine source alone as a precursor of endogenous cytidine, particularly in the human brain, are disclosed. Methods are also disclosed wherein exogenous uridine or a uridine source is combined either with drugs increasing uridine availability or with compounds that serve as a source of choline in phospholipid synthesis.
Owner:MASSACHUSETTS INST OF TECH
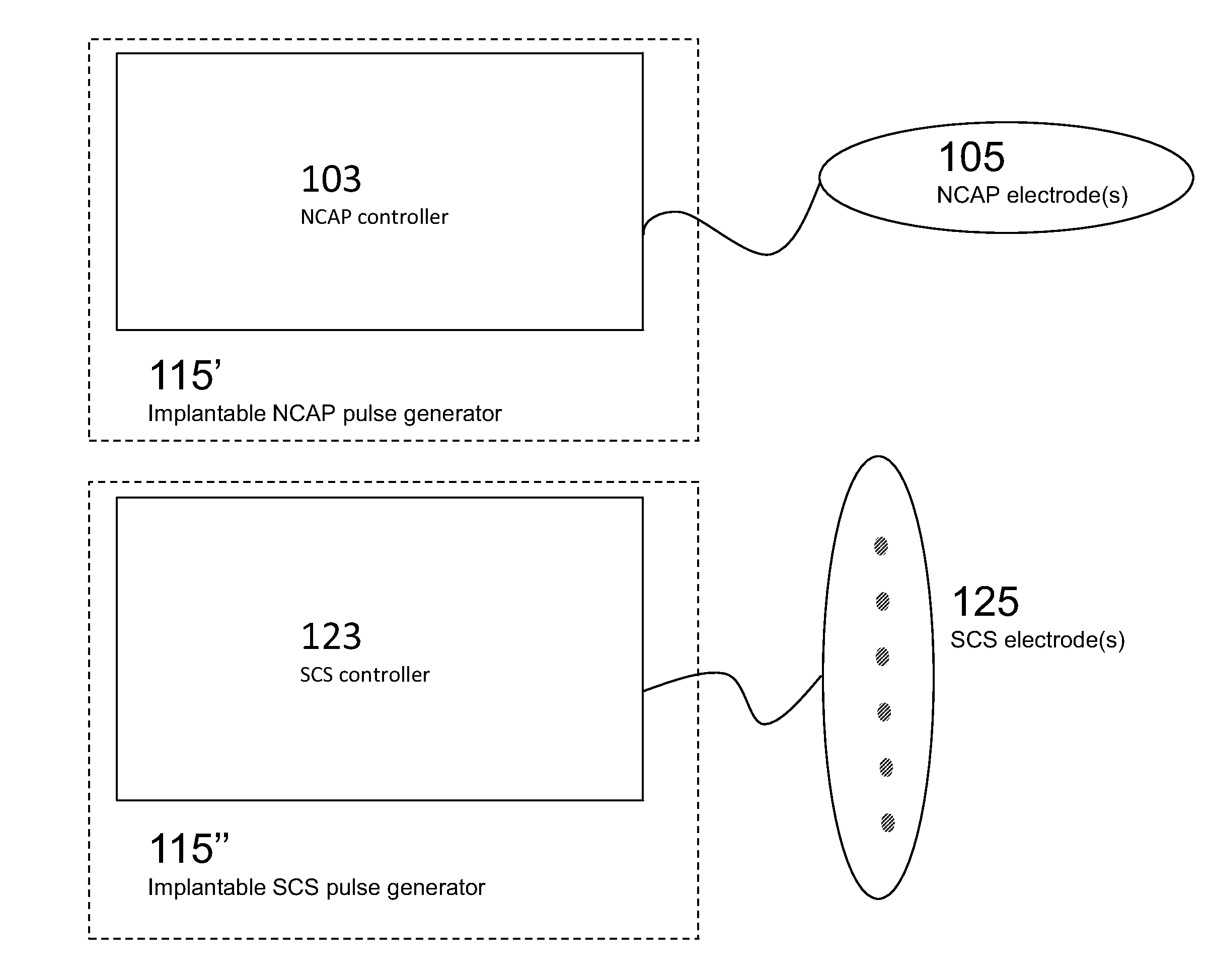
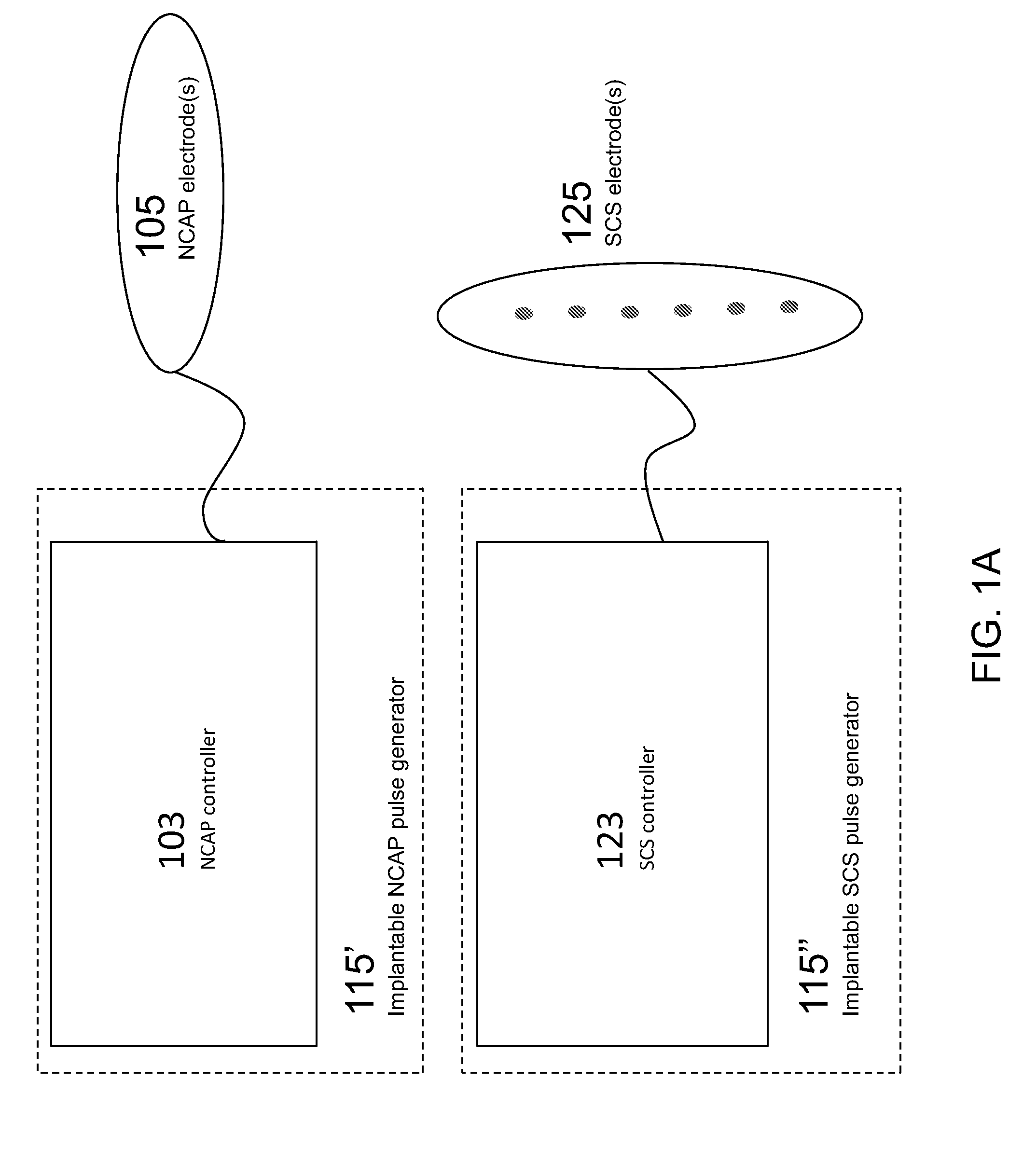
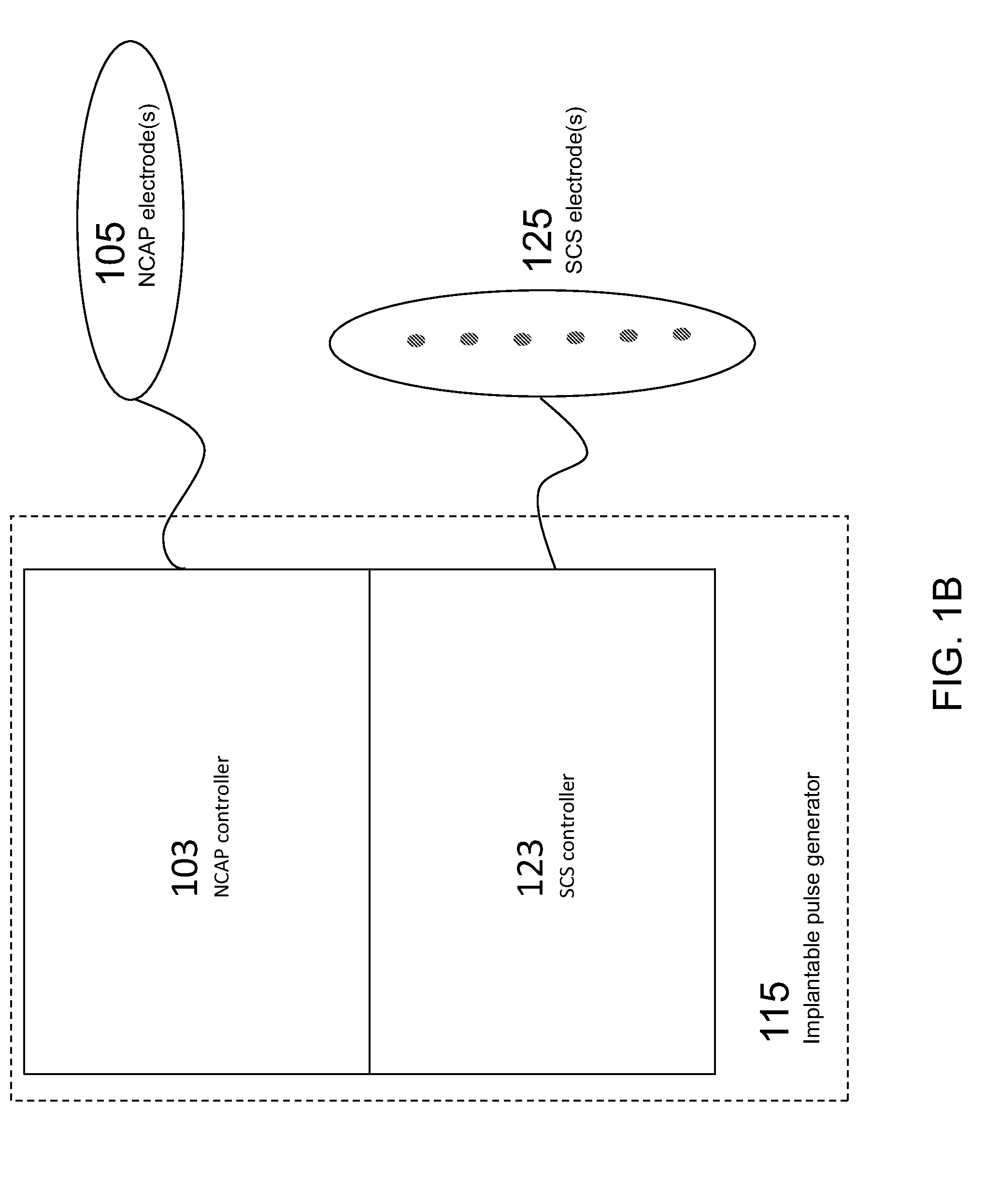
Modulation of the cholinergic Anti-inflammatory pathway to treat pain or addiction
ActiveUS20110106208A1Good effectIncrease stimulationSpinal electrodesChronic painCholinergic anti-inflammatory pathway
Methods and devices for the treatment of chronic pain by modulation of the cholinergic anti-inflammatory pathway. In particular, the methods and systems described herein may be used to enhance chronic pain therapies such as spinal cord stimulation (SCS). Thus, the present invention describes devices and methods for modulation of the cytokine pathway by stimulation of the neuronal cholinergic anti-inflammatory pathway (NCAP) to enhance the treatment of chronic pain by SCS. The use of NCAP in conjunction with SCS may potentiate the effects of SCS and / or prevent the desensitization of the patient to SCS.
Owner:SETPOINT MEDICAL CORP
Hen feedstuff for producing healthy egg
ActiveCN101181018AEnhance physical fitnessAnimal feeding stuffAccessory food factorsPhytaseOMEGA-3 POLYUNSATURATED FATTY ACIDS
The invention relates to a layer foodstuff used for producing health care eggs, consisting of the following components in weight portion: 120 to 160 portions of calcium hydrogen phosphate, 250 to 350 portions of mountain flour, 50 to 60 portions of common salt, 20 to 40 portions of saleratus, 15 to 20 portions of choline, 35 to 40 portions of complex trace elements, 8 to 10 portions of complex vitamins, 10 to 15 portions of lysine, 25 to 30 portions of methionine, 220 to 400 portions of pine needle meal, 1 to 3 portions of bacillus subtilis, 8 to 10 portions of copper sulfate, 2 to 5 portions of phytase, 1 to 5 portions of yeast selenium, 3 to 6 portions of vitamin E, and 250 to 400 portions of Chinese traditional herbal medicine preparation. The invention adds nutritive additives etc. into the foodstuff; therefore the egg laid by the layer contains such beneficial elements as selenium, vitamin E, and is rich in Omega-3 polyunsaturated fatty acid and low in cholesterol.
Owner:KUNMING HEMEIHUA FEED
An ecological soft-shelled turtle complete compound feed
InactiveCN101548727APromote oxidative metabolismReduce depositionClimate change adaptationAnimal feeding stuffMicrobial agentBody colour
An ecological soft-shelled turtle complete compound feed The invention discloses an ecological soft-shelled turtle complete compound feed, including the following material components (each component is in the air-dry substance weight percentage): 30-40% of white fish meal, 15-25% of red fish meal, 4-8 % of fermented soybean mea, 3-6% of extruded soybean, 1-4% of wheat gluten, 4-8% of beer yeast, 3-6% of maize protein meal, 1-3% of marigold flower powder, 18-25% of pre-gelatinized starch, 1-2% of calcium phosphate, 0.1-0.3% of fat metabolism regulator, 0.05-0.3% of enzyme preparation, 0.1-0.4% of microbial agents, 0.1-0.3% of compound multivitamins, 0.5-1.5% of composite multi-ore, 0.2-0.5% of 50% choline chloride. The invention improves the appearance body colour and inherent quality of the artificial breeding soft-shelled turtle which can be comparable wit natural growth wild soft-shelled turtles, thereby enhancing the commodity value of soft-shelled turtles.
Owner:ZHEJIANG JINDADI FEED
Transdermal method and apparatus
Owner:ALDRED KATHERINE M
Six percent-premix for lactating sows and wheat-type daily ration prepared from six percent-premix
ActiveCN103053852AImprove reproductive performanceIncrease feed intakeAnimal feeding stuffComposite electrolytePhytase
The invention belongs to the field of feeds, and particularly relates to a six percent-premix for lactating sows and a wheat-type daily ration prepared from the six percent-premix. The premix comprises multivitamins, composite trace elements, DL-methionine, lysine, threonine, L-arginine, valine, composite electrolyte, compound enzyme, 5000 IU of phytase, functional additives, feed attractants, calcium hydrogen phosphate, stone powder, salt, choline, antioxidants and defatted rice bran. On the whole, the daily ration for the lactating sows is capable of protecting the development of mammary glands of the sows, ensuring plentiful milk with high quality in the lactation period of the sows and increasing the survival rate of suckling piglets, and enables the piglets to have higher weaning weight.
Owner:河南宏展生物科技有限公司
Combination therapy using 1-aminocyclohexane derivatives and acetylcholinesterase and inhibitors
InactiveUS20090124659A1Avoid damageReduce riskBiocideNervous disorderCholinesterase inhibitionDepressant
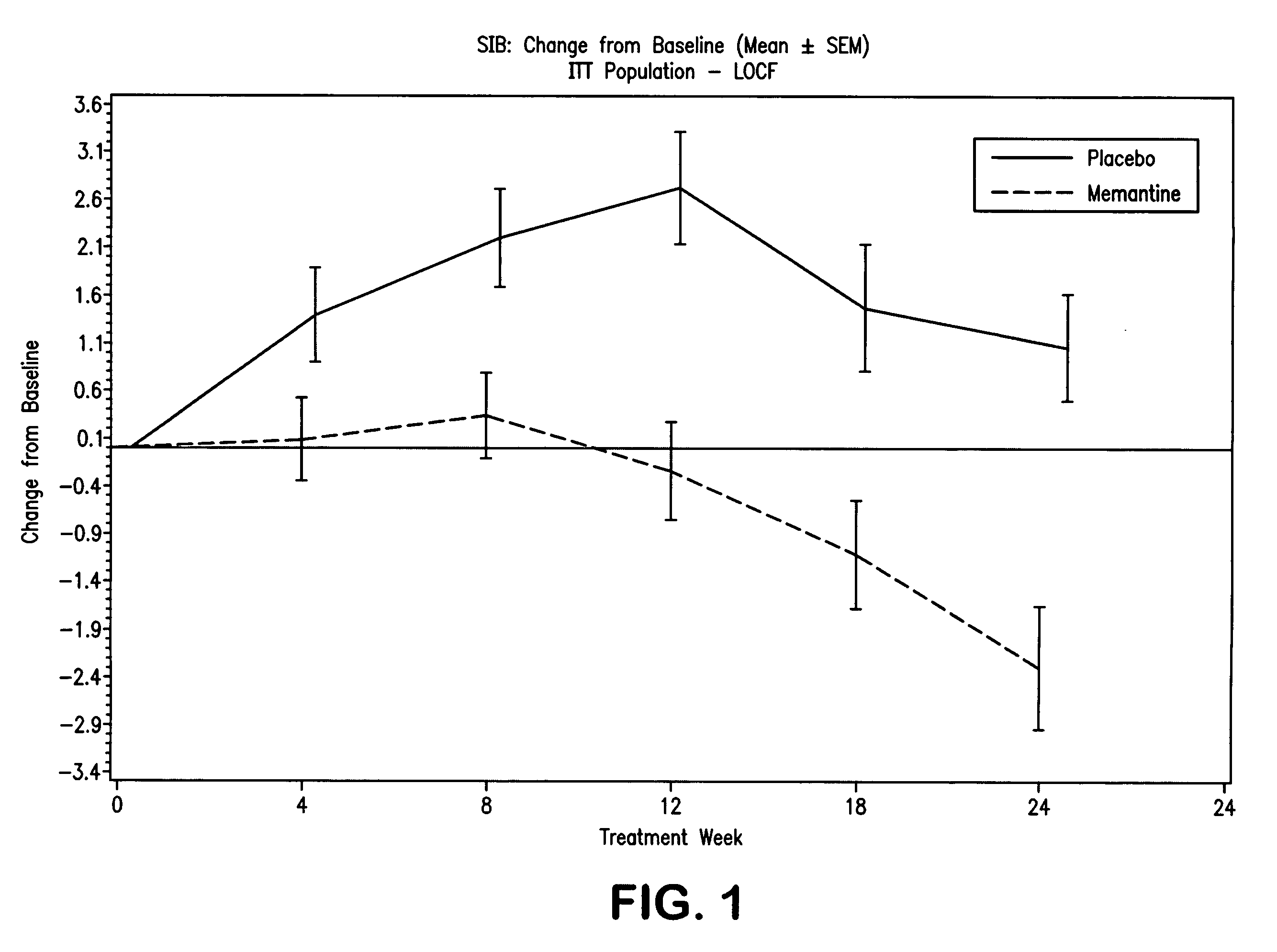
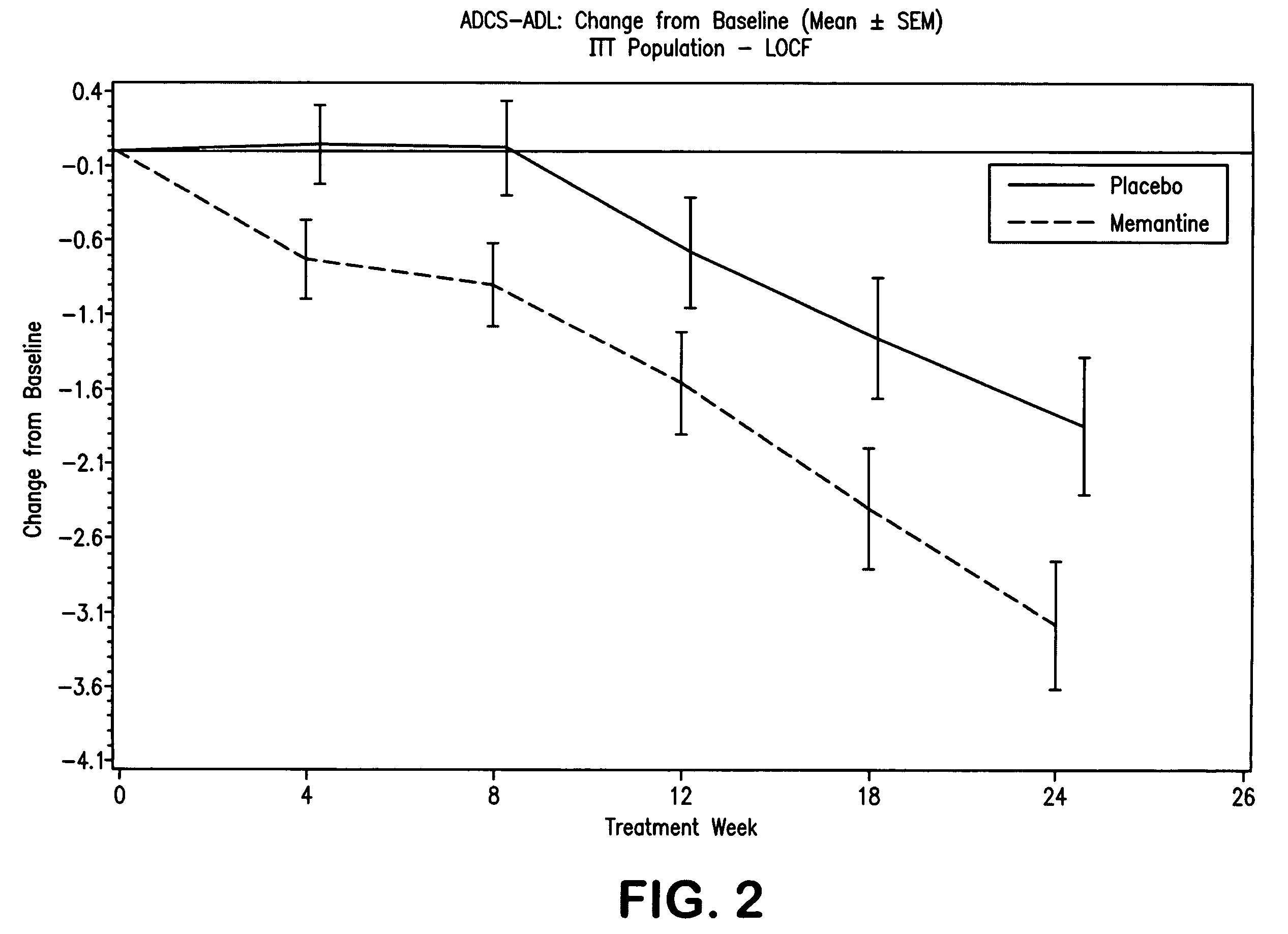
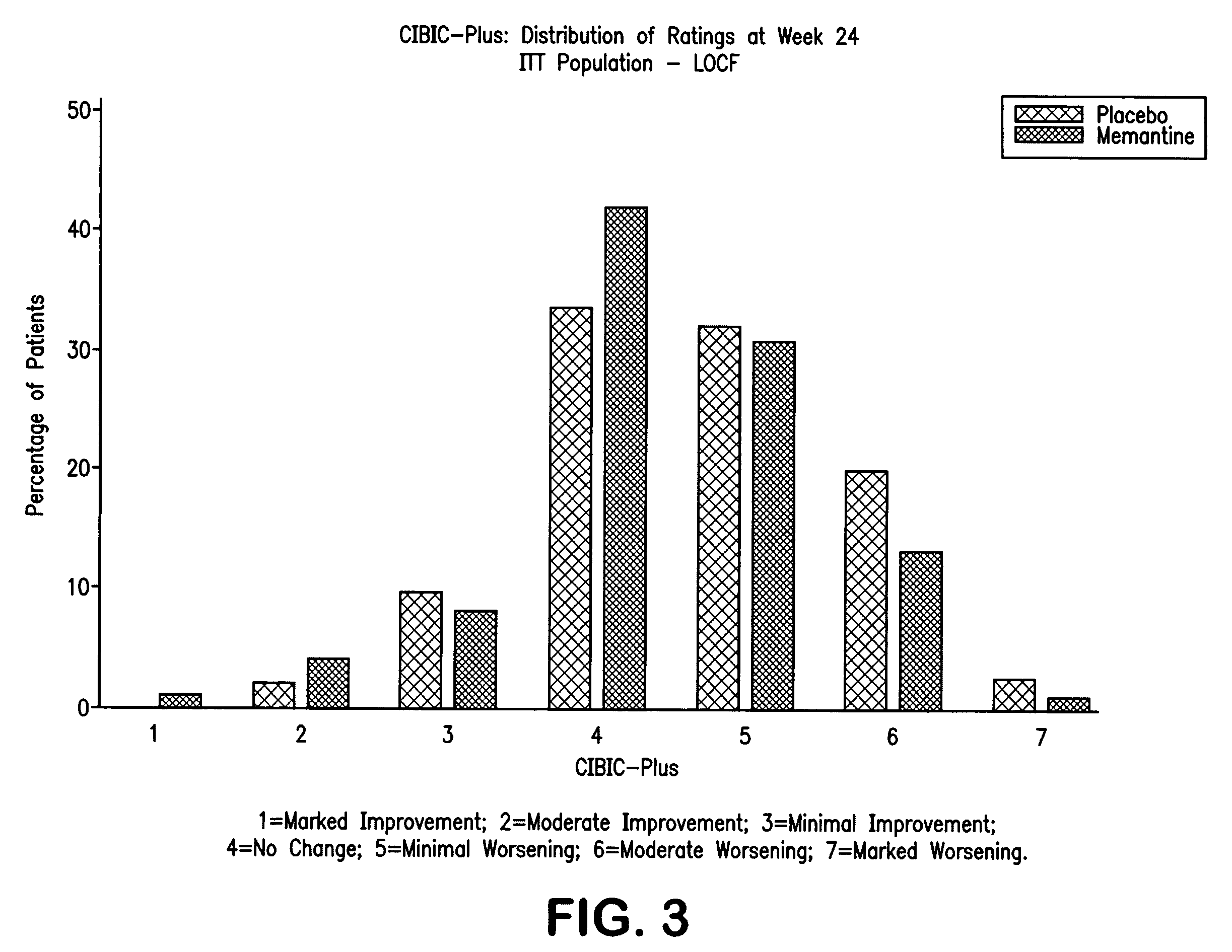
The invention relates to a novel drug combination therapy useful in the treatment of dementia comprising administering an 1-aminocyclohexane derivative such as memantine or neramexane and an acetylcholinesterase inhibitor (AChEI) such as galantamine, tacrine, donepezil, or rivastigmine.
Owner:MERZ PHARMA GMBH & CO KGAA
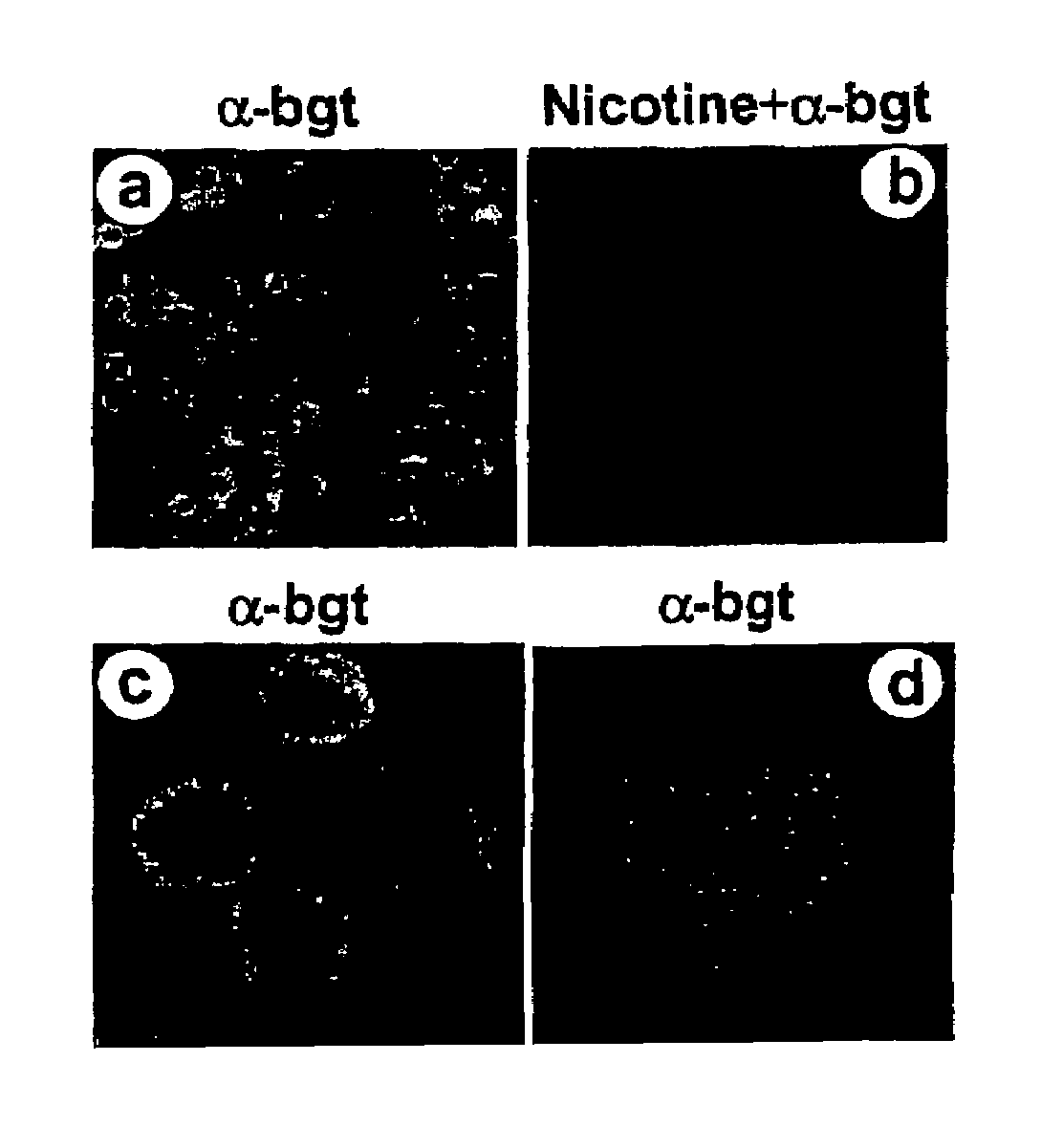
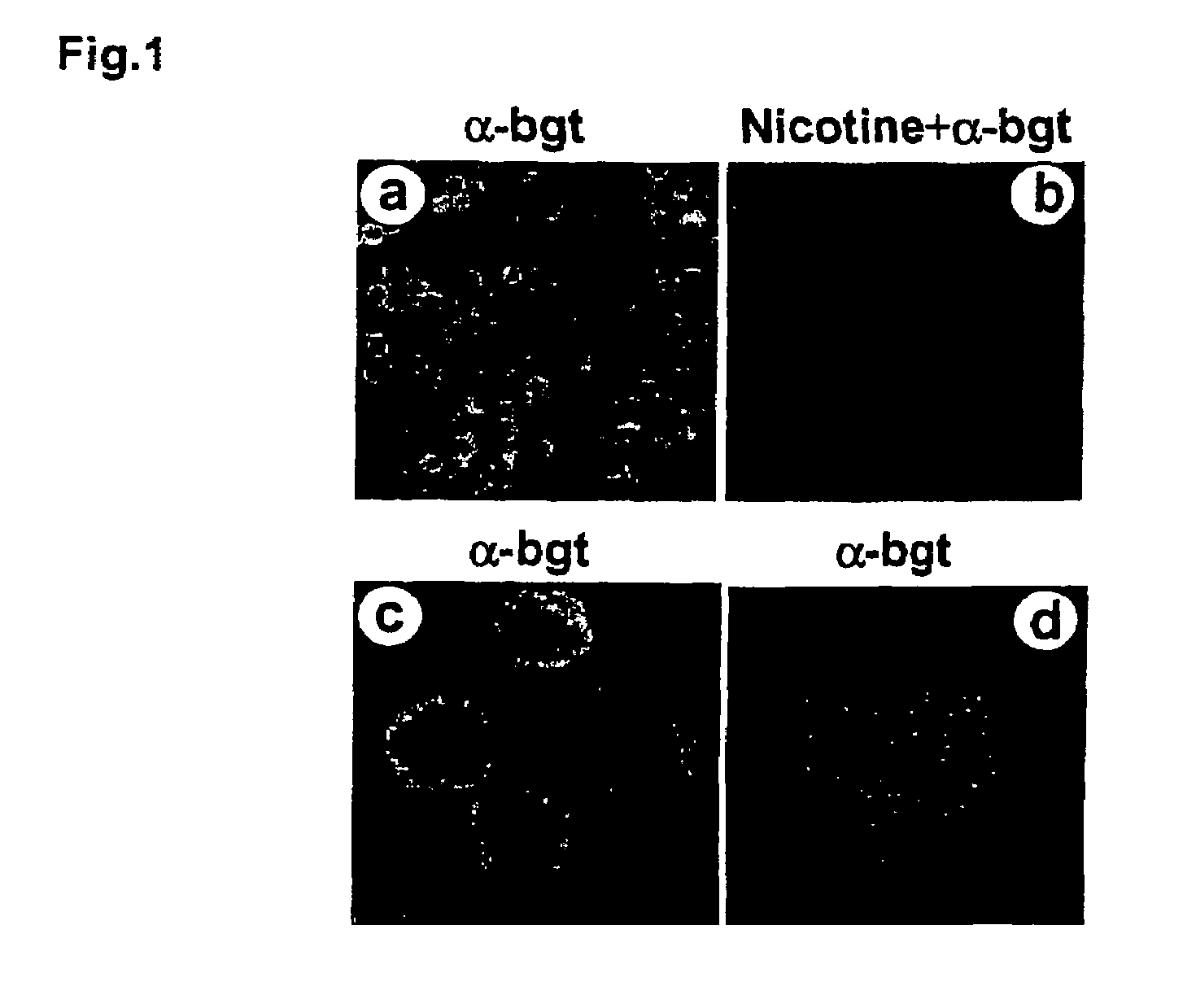
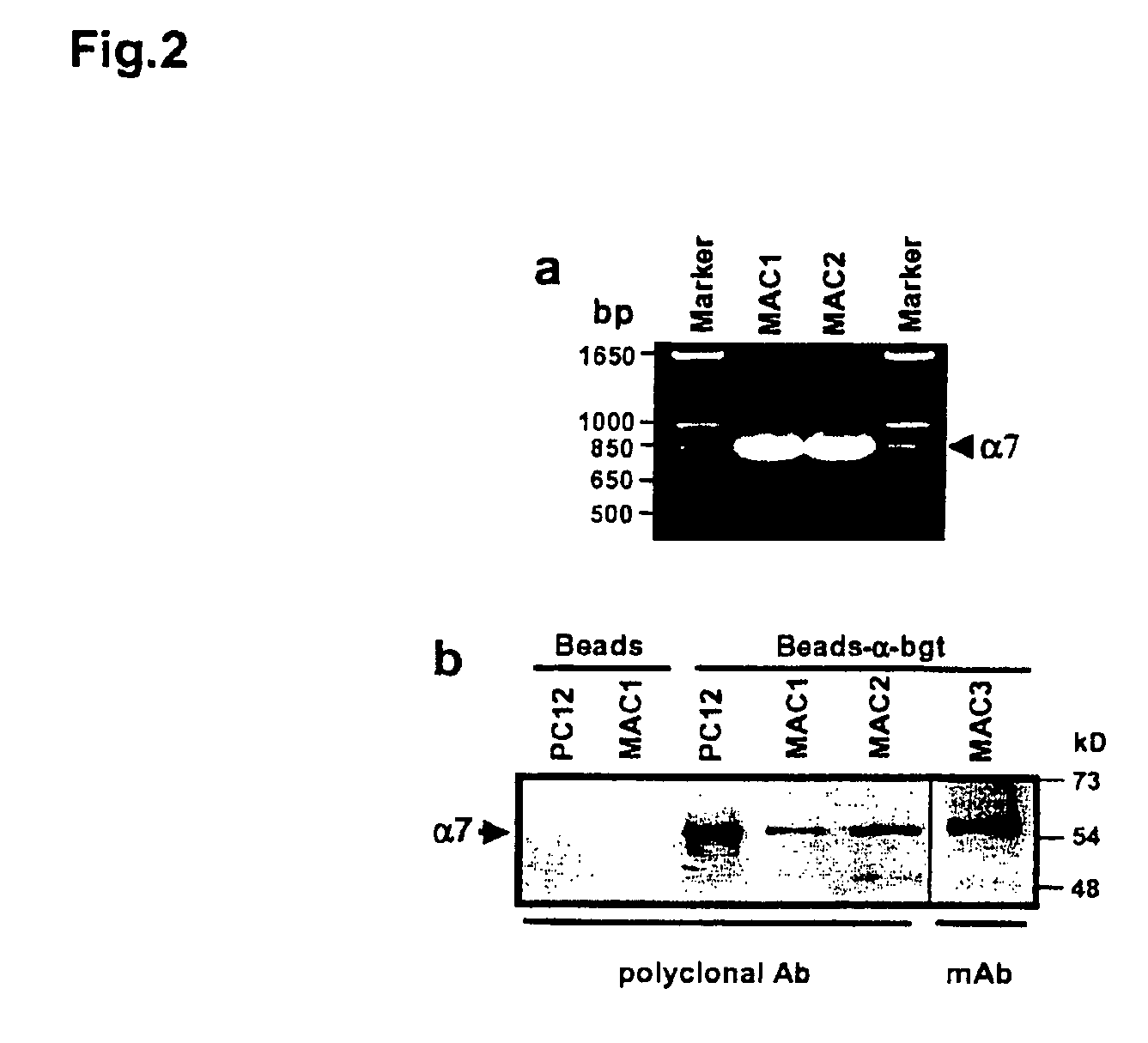
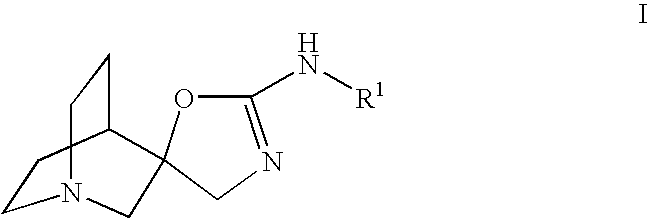
Treatment of pancreatitis using alpha 7 receptor-binding cholinergic agonists
A method of treating a patient suffering from pancreatitis comprising treating said patient with a therapeutically effective amount of a cholinergic agonist selective for an α7 nicotinic receptor in an amount sufficient to decrease the amount of the proinflammatory cytokine that is released from a macrophage wherein said condition is acute pancreatitis. The compounds of the present invention include a quaternary analog of cocaine; (1-aza-bicyclo[2.2.2]oct-3-yl)-carbamic acid 1-(2-fluorophenyl)-ethyl ester; a compound of formula (I), a compound of formula (II), a compound of formula (III), a compound of formula (IV), and an oligonucleotide or mimetic capable of attenuating the symptoms of acute pancreatitis wherein the oligonucleotide or mimetic consists essentially of a sequence greater than 5 nucleotides long that is complementary to an mRNA of an α7 cholinergic receptor. The variables of formulae (I), (II), (III) and (IV) are described herein
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Quinuclidine compounds as alpha-7 nicotinic acetylcholine receptor ligands
The disclosure provides compounds of formula I, including their salts, as well as compositions and methods of using the compounds. The compounds are ligands for the nicotinic α7 receptor and may be useful for the treatment of various disorders of the central nervous system, especially affective and neurodegenerative disorders.
Owner:BRISTOL MYERS SQUIBB CO
An environmentally-friendly soft-shelled turtle compound feed
InactiveCN101548726AImprove utilizationLow nutritional needsFood processingClimate change adaptationMicrobial agentChemistry
The invention discloses an environmentally-friendly soft-shelled turtle compound feed, including the following material components (each component is in the air-dry substance weight percentage): 30-35% of white fish meal, 15-25% of red fish meal, 5-10 % of fermented soybean mea, 3-6% of extruded soybean, 1-4% of wheat gluten, 5-10% of beer yeast, 18-25% of pre-gelatinized starch, 1-2% of calcium phosphate, 0.05-0.3% of enzyme preparation, 0.1-0.4% of microbial agents, 0.1-0.3% of compound multivitamins, 0.5-1.5% of composite multi-ore, 0.2-0.5% of 50% choline chloride. The inventive prescription can reduce the nitrogen and phosphorus content in the compound feed, improve the digestive absorption and deposition rate of the nitrogen and phosphorus in the soft-shelled turtle breeding, and can reduce the output of nitrogen and phosphorus in water to improve the breeding environment.
Owner:ZHEJIANG JINDADI FEED
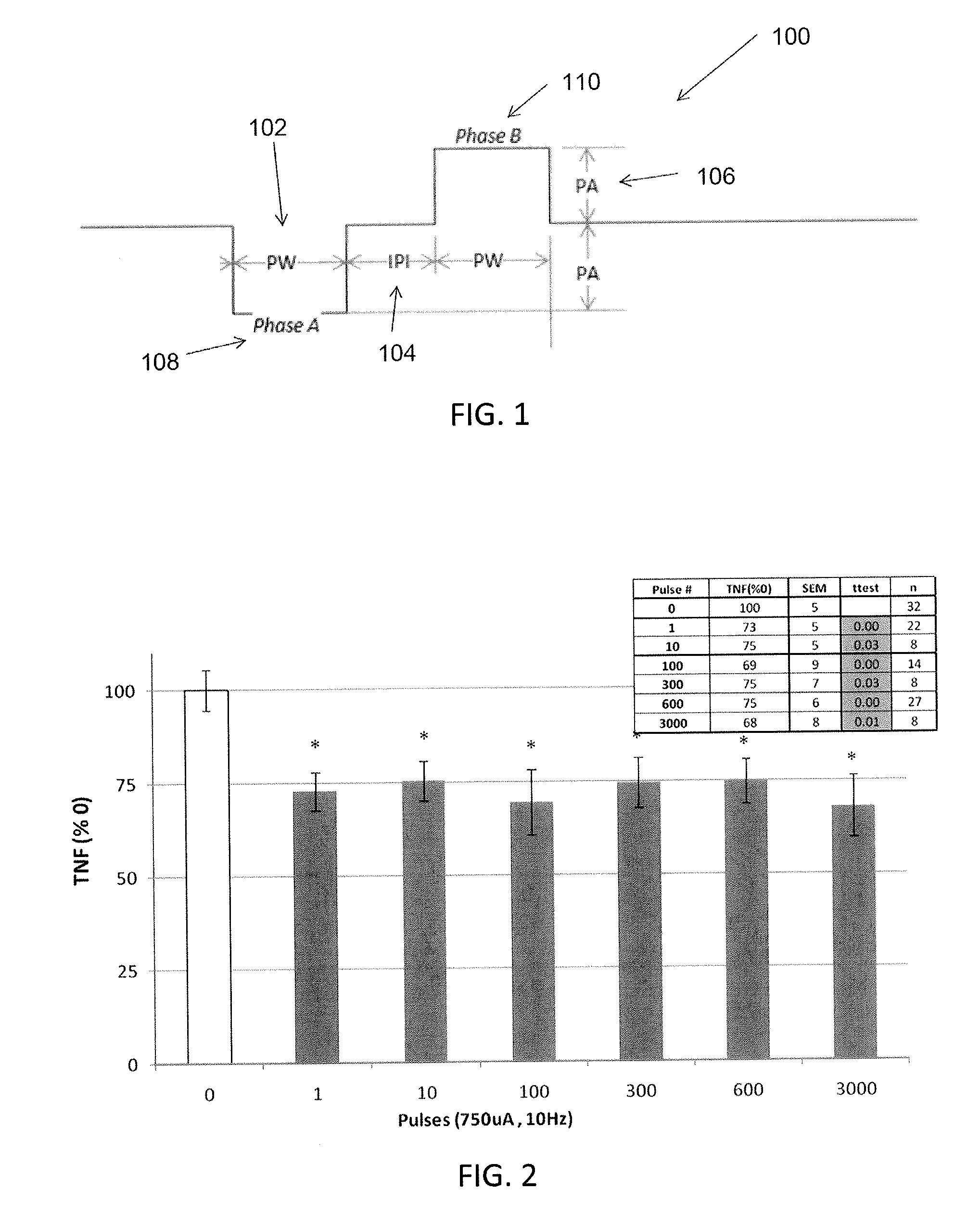
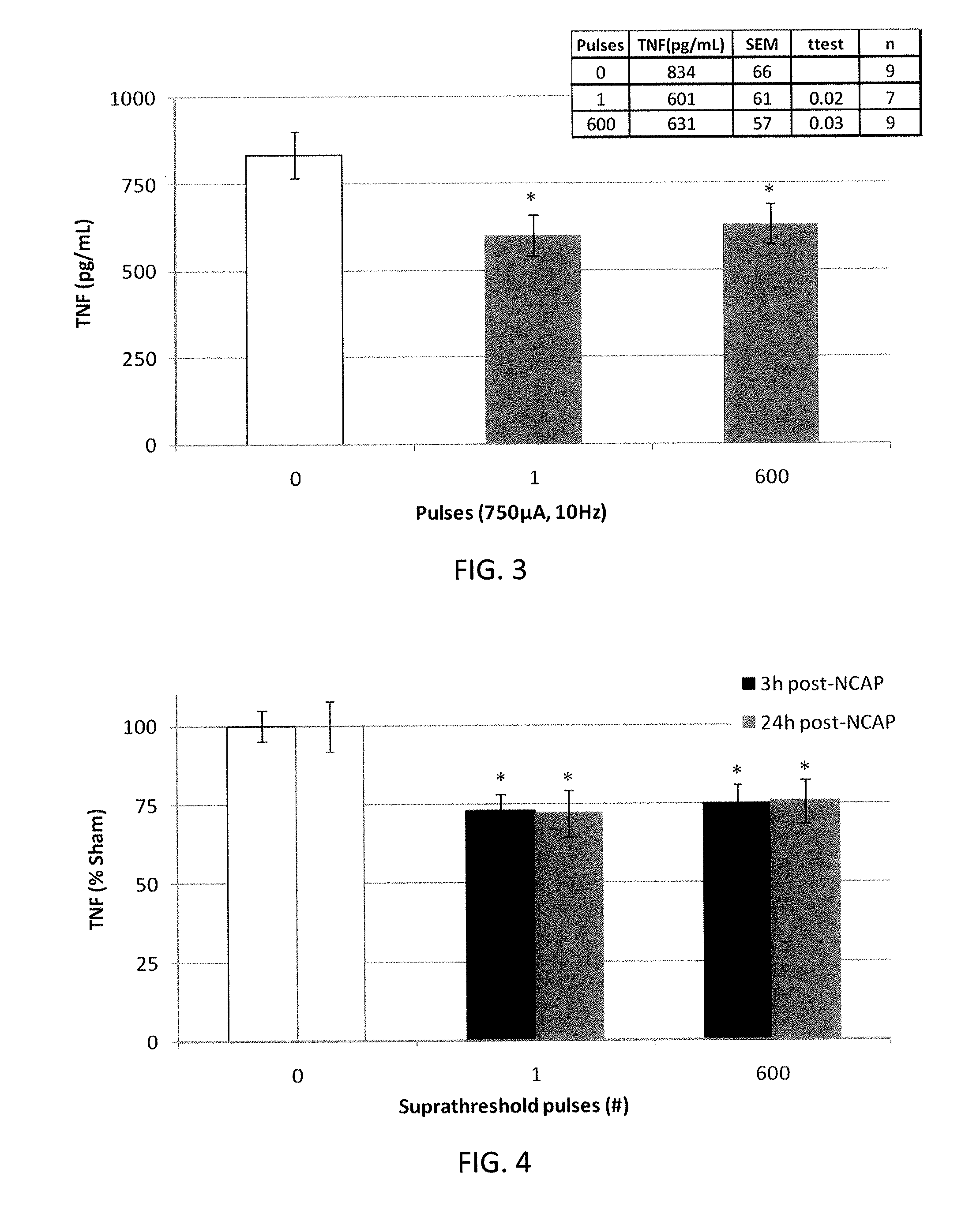
Extremely low duty-cycle activation of the cholinergic anti-inflammatory pathway to treat chronic inflammation
ActiveUS9211410B2Reduce power consumptionMaintain curative effectImplantable neurostimulatorsArtificial respirationInitial doseCholinergic anti-inflammatory pathway
Described herein are systems and methods for applying extremely low duty-cycle stimulation sufficient to treat chronic inflammation with progressively longer delays (off periods) from an initial stimulation. In particular, described herein are supra-threshold pulses of electrical stimulation sufficient to result in a long-lasting (e.g., >48 hours) inhibition of pro-inflammatory cytokines and / or effects of chronic inflammation; the delay between initial doses (which may be single-pulse doses) may be extended for subsequent doses, potentially dramatically enhancing battery and device longevity.
Owner:SETPOINT MEDICAL CORP
Corn type mixed feed for weaned pig and preparation method thereof
ActiveCN103355556AMeeting nutritional needsImprove digestibilityAnimal feeding stuffBiotechnologyNutrition
The invention discloses a corn type mixed feed for a weaned pig and a preparation method thereof. The mixed feed comprises the following raw materials: dried corn, puffed corn, soybean meal, puffed soybean, a wheat germ cake, flour, fish meal, plasma protein powder, whey powder, soya-bean oil, stone flour, calcium hydrogen phosphate, salt, lysine 70%, methionine 99%, threonine 99%, choline chloride 60%, glucose, compound enzyme, a vitamin premix and a trace element premix. The preparation method comprises the following steps: crushing; mixing; and granulation. The invention has the following beneficial effects: nutritional requirements of the weaned pig are met; the mixed feed has good palatability and a high digestive rate and can reduce various discomforts caused by weaning stress, enhance the immunity of the weaned pig, improve food consumption and productivity of the weaned pig, promote rapid and healthy growth of the weaned pig and guarantee the survival rate of the weaned pig.
Owner:黔东南新希望农牧科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com