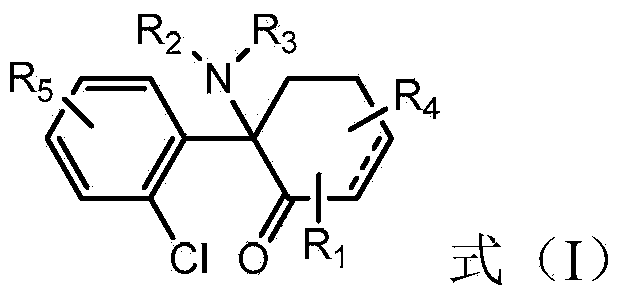
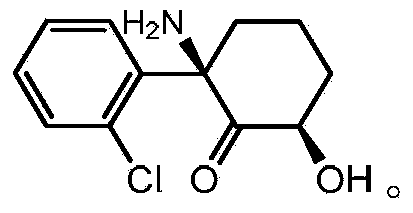
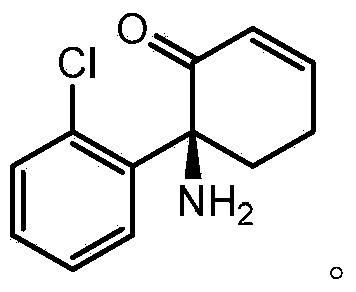
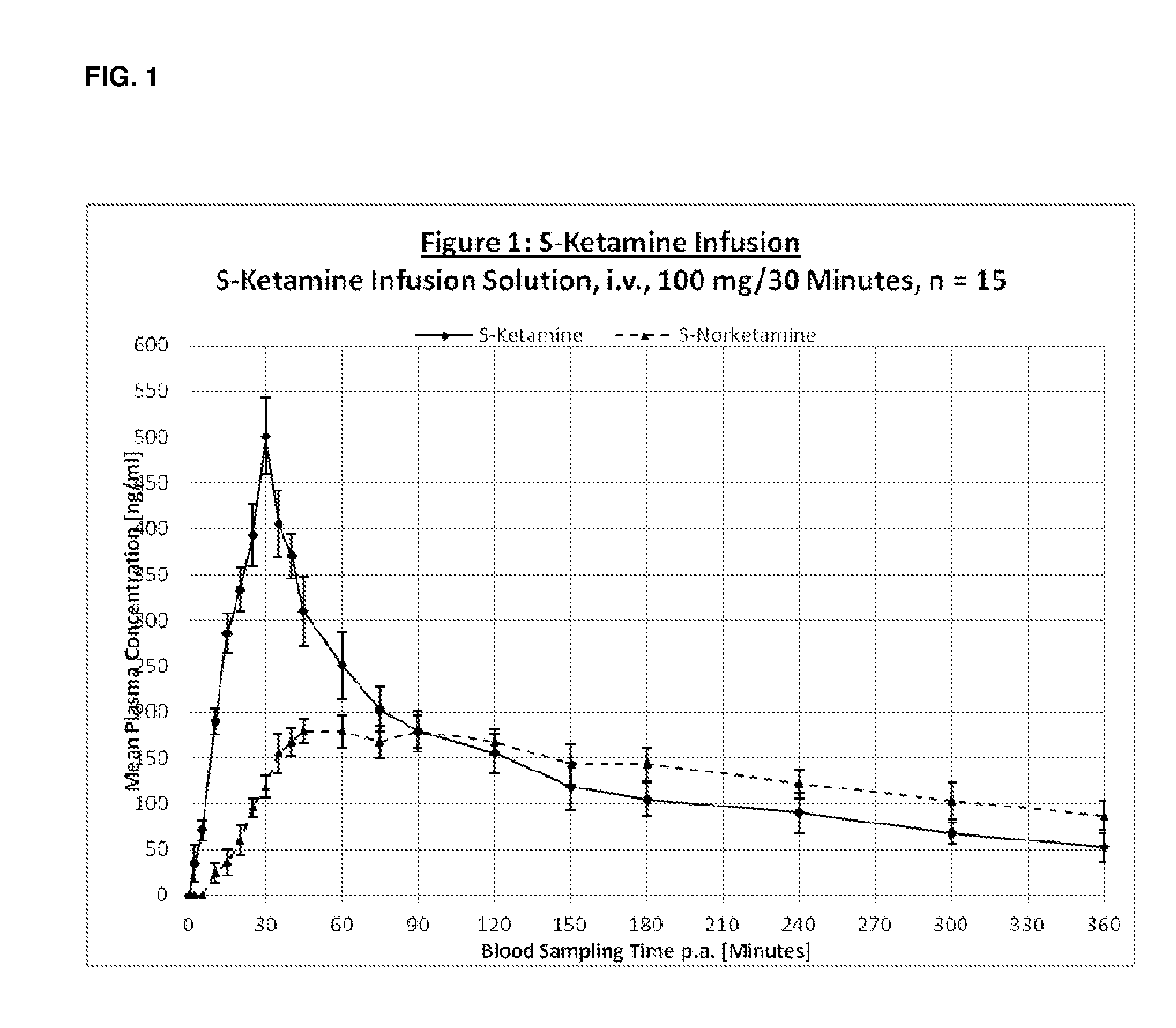
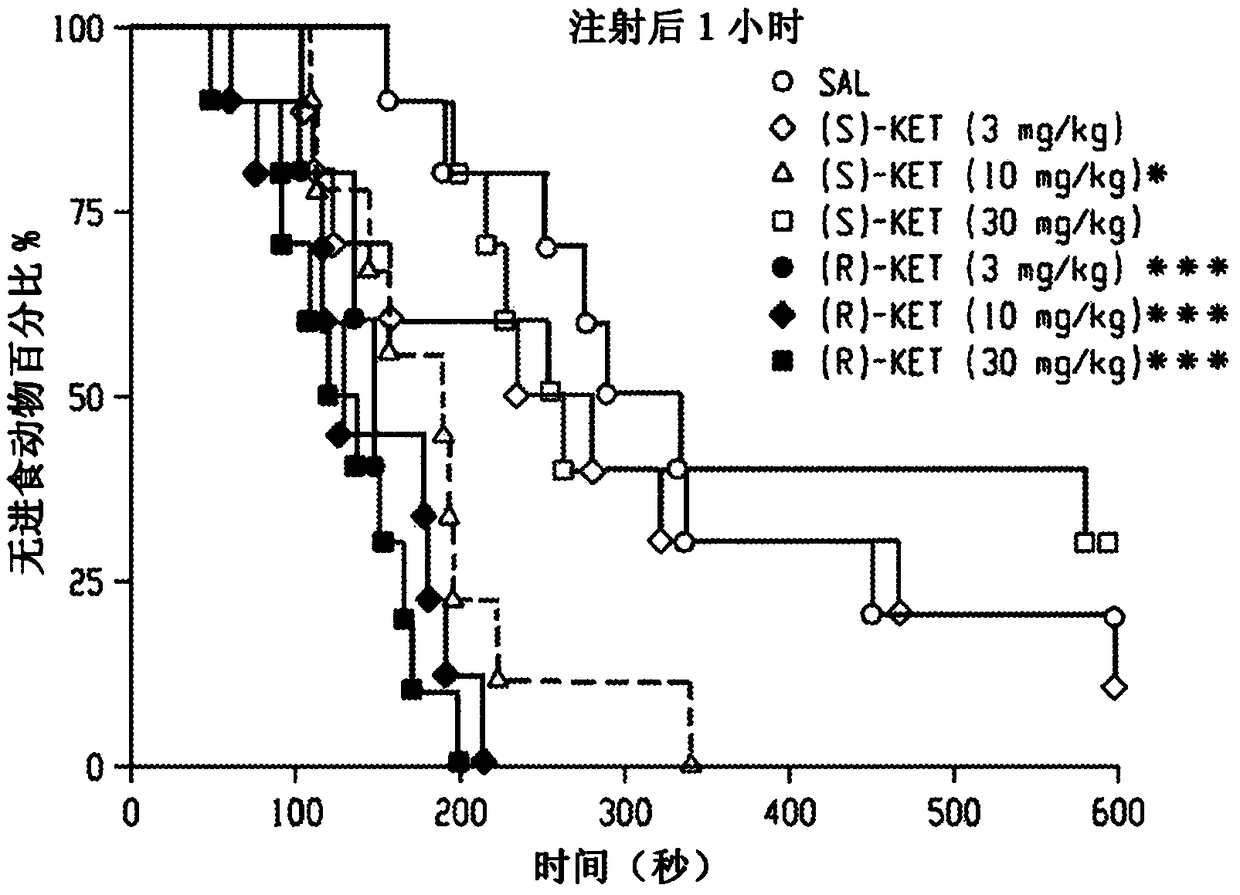
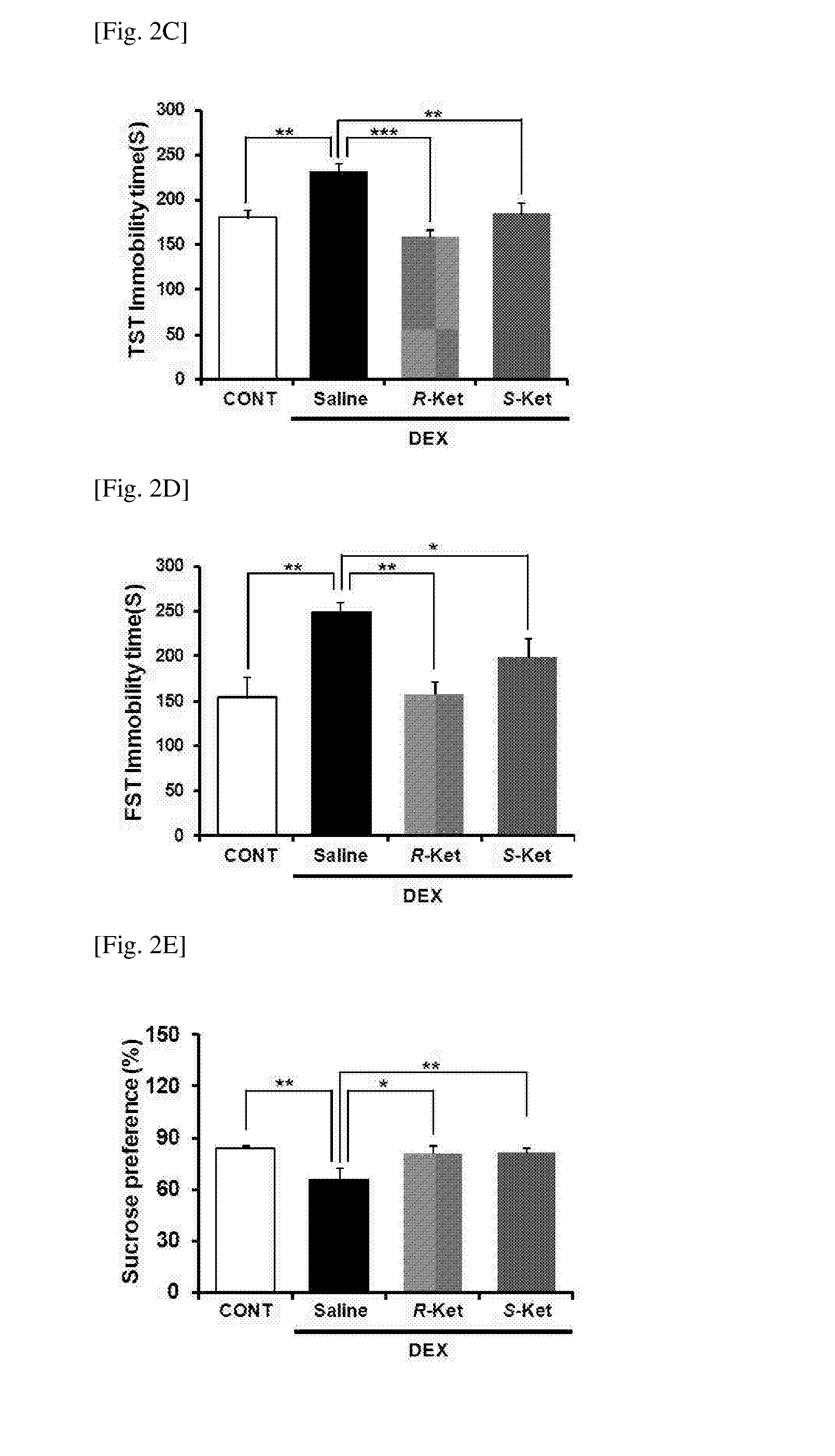
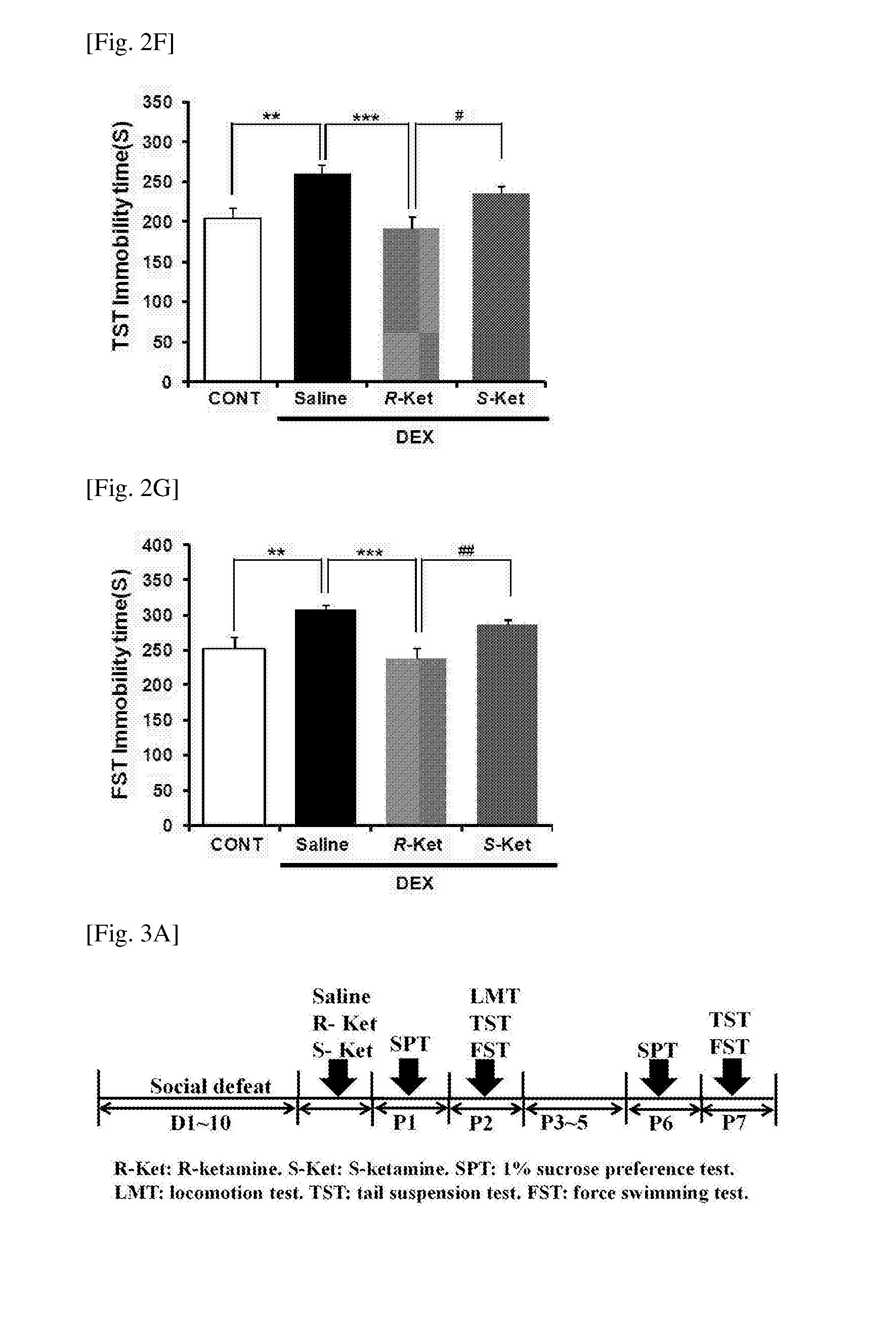
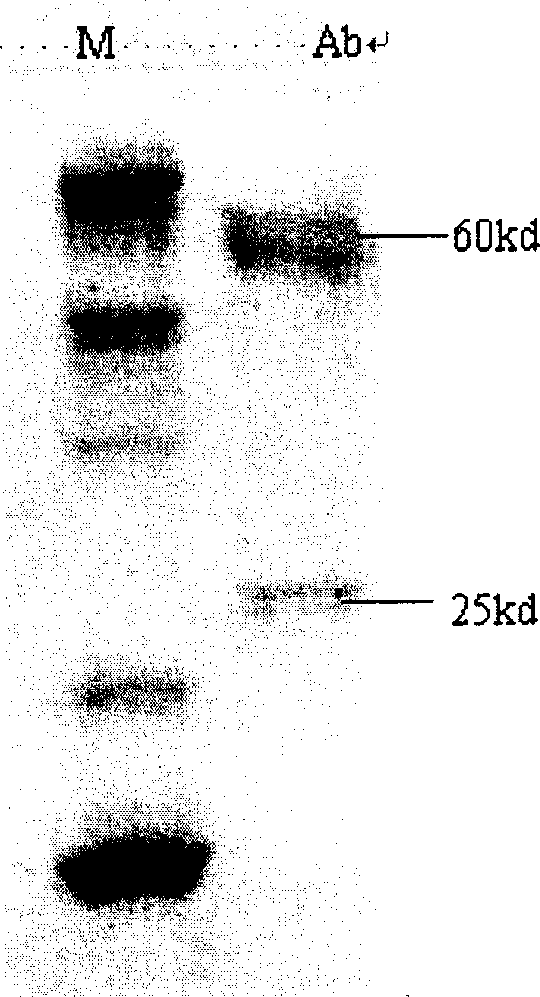Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
178 results about "Ketamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ketamine is a medication mainly used for starting and maintaining anesthesia. It induces a trance-like state while providing pain relief, sedation, and memory loss. Other uses include for chronic pain, sedation in intensive care, and depression. Heart function, breathing, and airway reflexes generally remain functional. Effects typically begin within five minutes when given by injection, and last up to about 25 minutes.
Preparation of topical regional compositions for the relief of pain
Owner:FROME BRUCE M
Novel pharmaceutical compositions for treating chronic pain and pain associated with neuropathy
InactiveUS20080058362A1Reduced plasma concentrationEffective pain managementBiocideAmide active ingredientsDextrorphanSustained release drug
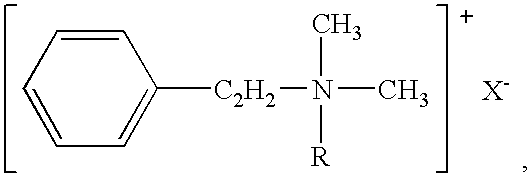
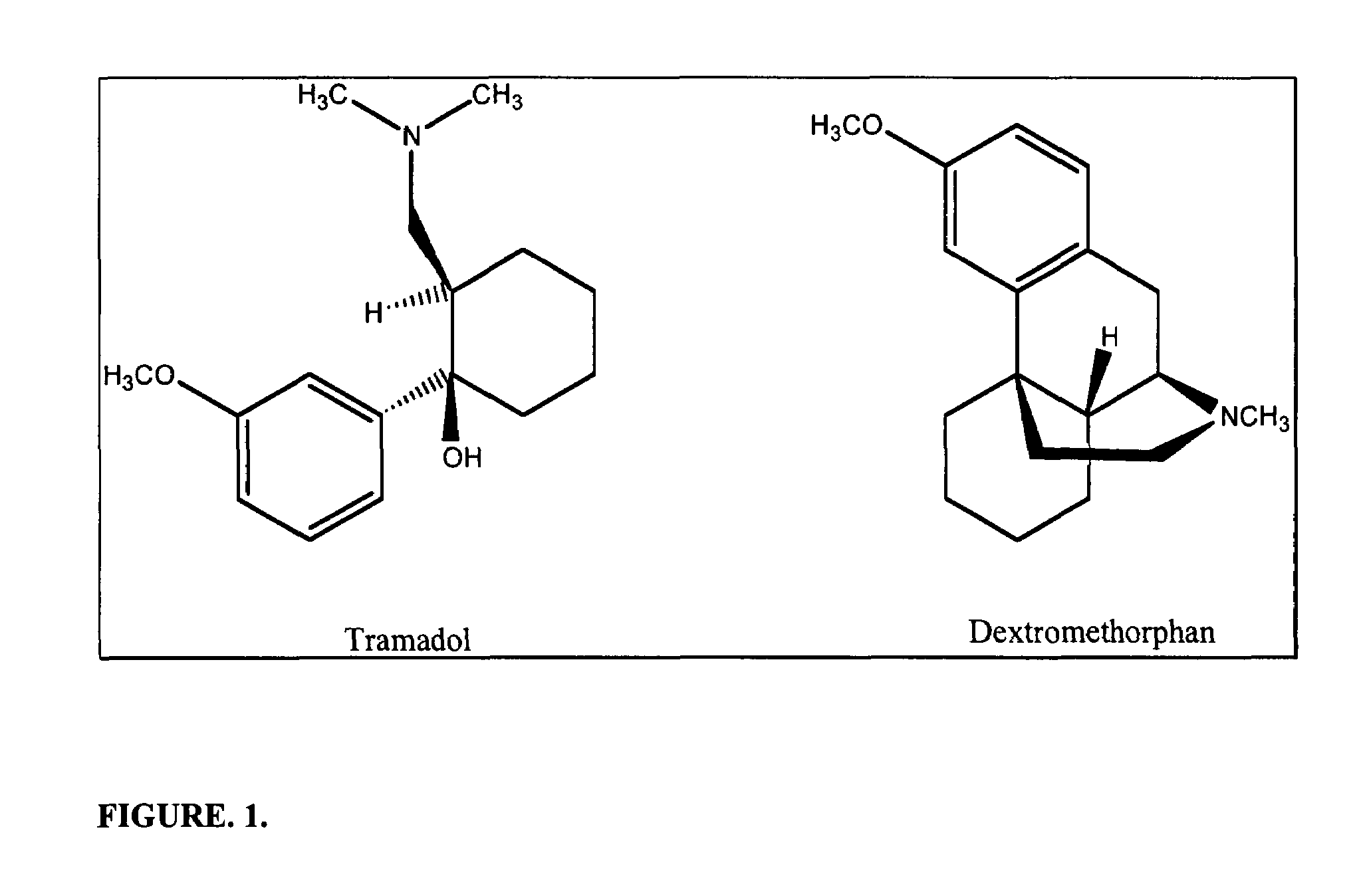
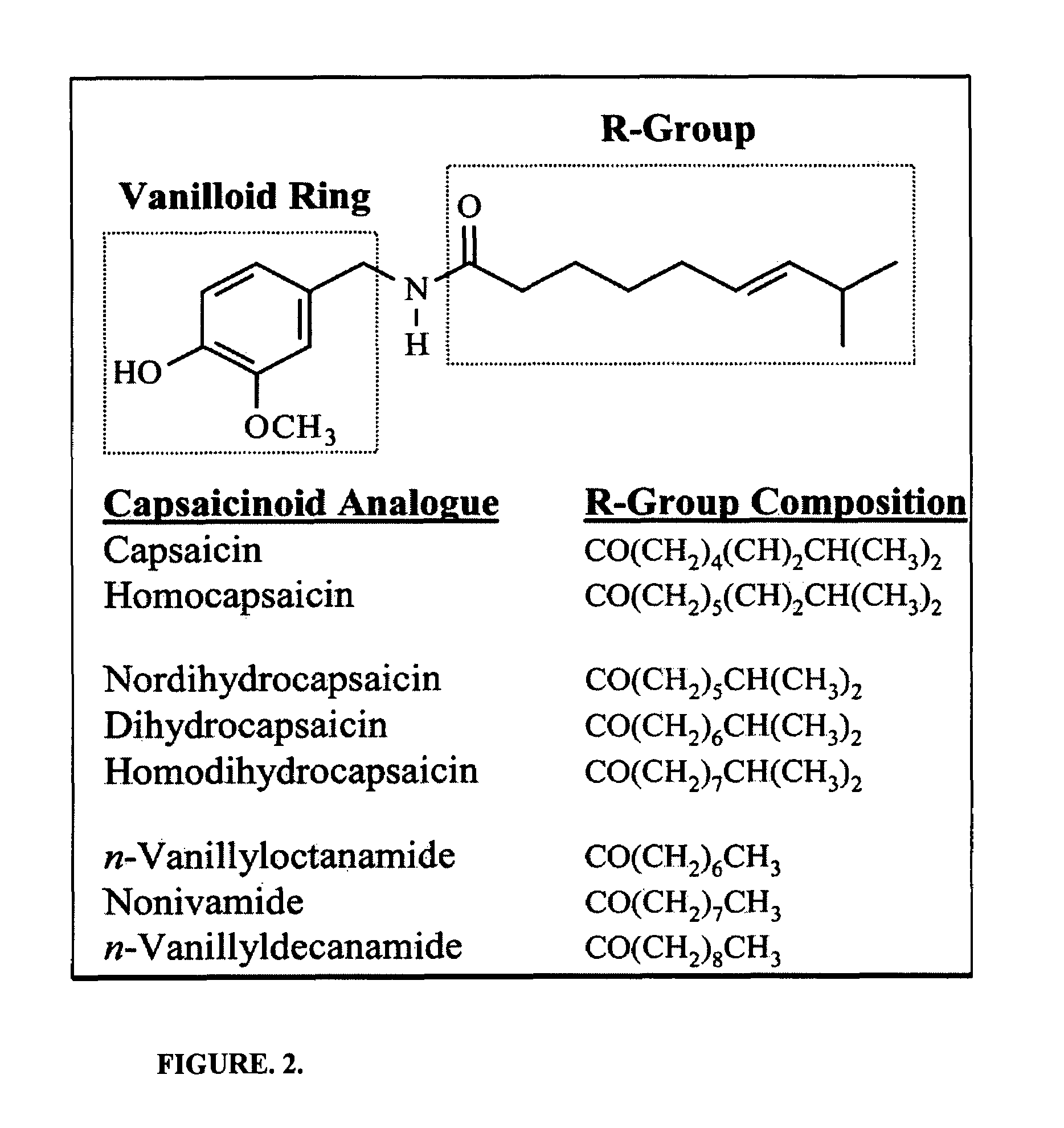
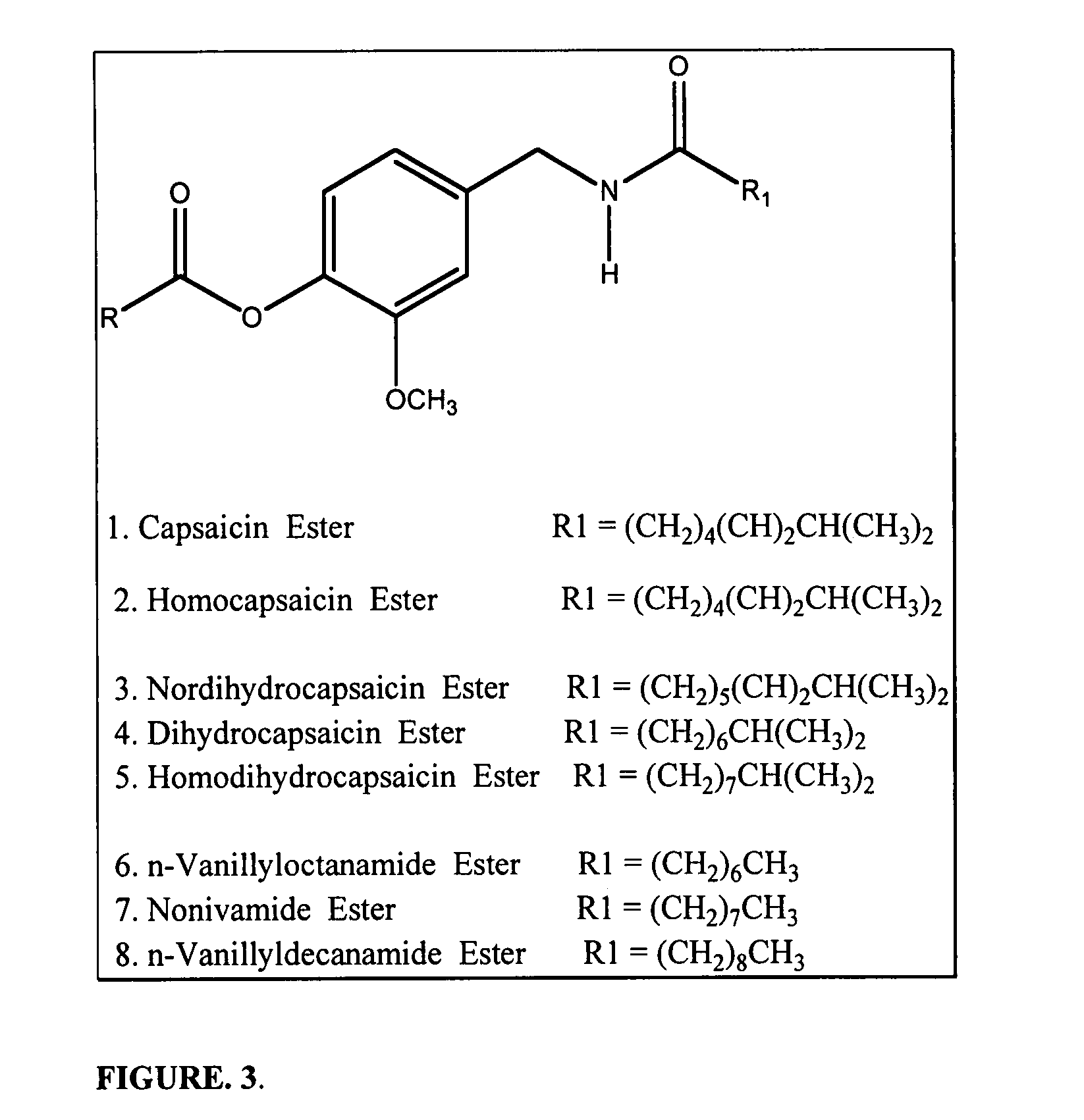
Chronic pain is alleviated in a mammal suffering there from by administering to the mammal a chronic pain alleviating amount of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, in combination with a μ-opiate analgesic such as tramadol or an analogously acting molecular entity, and a capsaicin or an ester of capsaicin, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
Alzheimer's disease treatment with multiple therapeutic agents delivered to the olfactory region through a special delivery catheter and iontophoresis
InactiveUS20120323214A1Reduce and preventAvoid destructionNervous disorderHead electrodesApoptosisExcitotoxicity
This invention describes the administration of multiple therapeutic agents with insulin in conjunction with bexarotene, ketamine, monoclonal antibodies Etanercept, IGF-1, and acetylcholine esterase inhibitors physostigmine, for treatment of Alzheimer's disease and other neurodegenerative diseases. Insulin, improves memory; also augments and amplifies the effects of the adjuvant therapeutic agents (paracrine and intracrine effects) and consequently reduces the β amyloid, its soluble precursors, prevents damage to the neuronal skeletal network (taupathy), and blocks glutamate excitotoxicity, reduces brain inflammation, prevents apoptosis, and increases the acetylcholine levels in the neurons and synapses; by using a combination of insulin, bexarotene, ketamine, Etanercept, IGF-1, and physostigmine therapeutic agents. The results are achieved by using the specially designed Iontophoresis incorporated olfactory mucosal delivery (ORE) catheter device located at the olfactory nerves, sphenoid sinus, and adjacent structures described here, to transport the large molecules of therapeutic agents to treat AD delivered to the CNS bypassing BBB from ORE.
Owner:WEDGE THERAPEUTICS
NMDA receptor antagonist formulation with reduced neurotoxicity
The present invention is directed to pharmaceutical compositions of effective amounts of NMDA receptor antagonists and preservative for the administration to a patient in need of effective analgesia and anesthesia. The compositions of the invention advantageously do not cause any significant neurotoxicity. The preferred NMDA receptor antagonist is ketamine. The preferred preservative is benzalkonium chloride.
Owner:MYRIAD GENETICS INC (US) +4
Pharmaceutical compositions for treating chronic pain and pain associated with neuropathy
InactiveUS7645767B2Reduce concentrationEfficient managementBiocideAmide active ingredientsOpiatePharmaceutical medicine
Chronic pain is alleviated in a mammal suffering there from by administering to the mammal a chronic pain alleviating amount of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, in combination with a μ-opiate analgesic such as tramadol or an analogously acting molecular entity, and a capsaicin or an ester of capsaicin, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
Neuropathy cream
Owner:OZTURK BINNUR +1
Prolonged administration of NMDA antagonist drug and safener drug to create improved stable neural homeostasis
InactiveUS20050222270A1Stable and lasting improved neural homeostasisBiocideOrganic active ingredientsDiseaseNervous system
An NMDA antagonist (such as ketamine) is administered with a safener (such as clonidine) in patients suffering from neurologic disorders other than pain. The ketamine is adminsitered at a dosage that causes slurred speech, for a span of several days. This treatment enables a patient's nervous system to return to a healthy “set point”, also called an improved stable neural homeostasis, in a manner similar to a broken bone healing itself if protected from jostling and reinjury by a cast. In at least some patients, this treatment can ease problems such as addictions to illegal or pain-killing drugs, nicotine, or alcohol, compulsive or criminal behavioral problems, severe depression, obsessive-compulsive disorders, phobias, etc. It may also provide some relief in some patients for problems such as chronic fatigue, chemical sensitivities, allergies, autoimmune disorders, and diabetes.
Owner:OLNEY JOHN W +3
The use of (2r, 6r)-hydroxynorketamine, (s)-dehydronorketamine and other stereoisomeric dehydro and hydroxylated metabolites of (r,s)- ketamine in the treatment of depression and neuropathic pain
InactiveCN104395283AInhibitory concentrationNervous disorderOrganic compound preparationMetaboliteRegional pain
Owner:UNITED STATES OF AMERICA +2
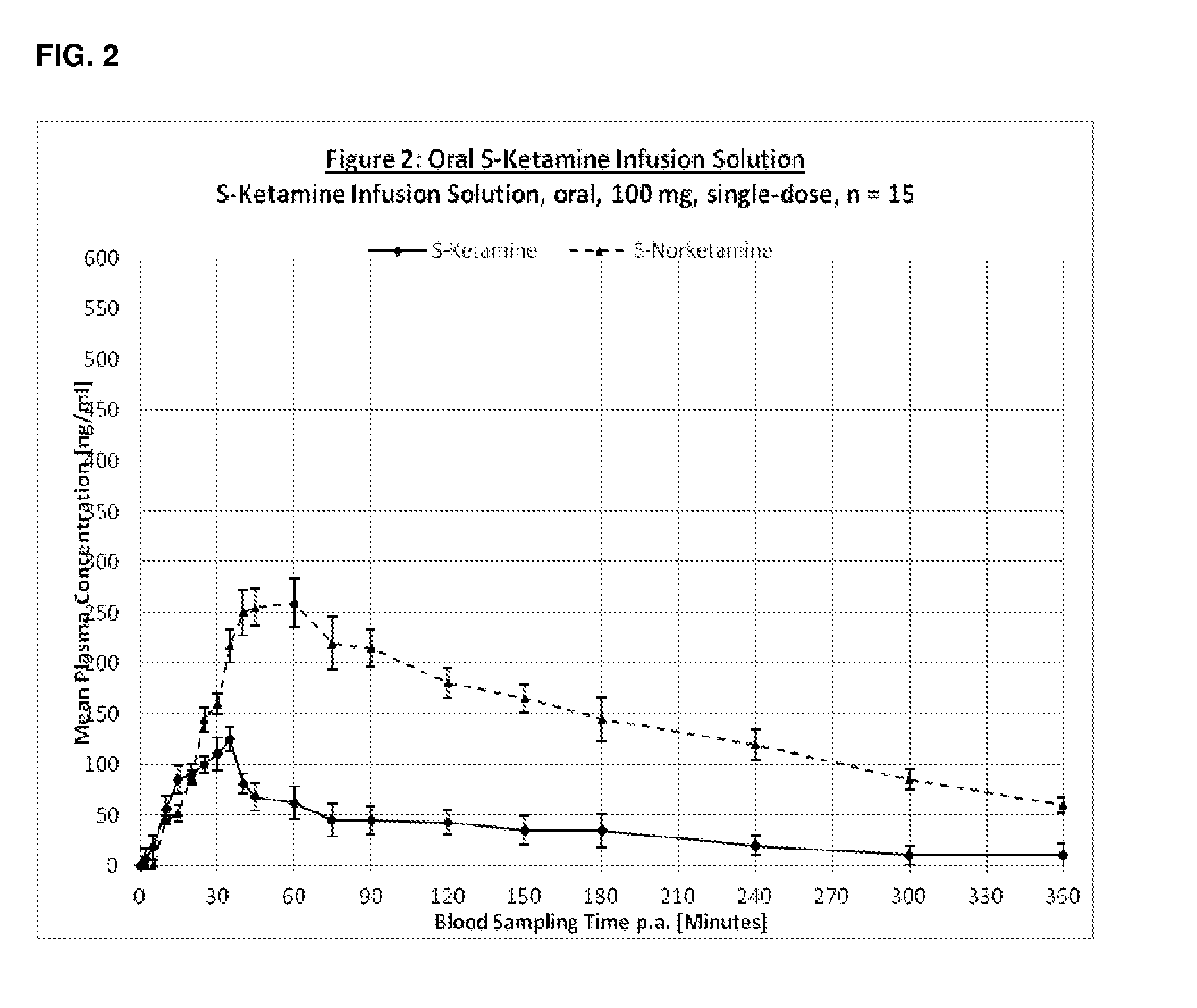
Oral transmucosal adminstration forms of s-ketamine
InactiveUS20140079740A1Increase heart rateHigh energyOrganic active ingredientsBiocideKetaminePharmacology
The present invention relates to methods and compositions for the treatment of pain, in a preferred embodiment relating to the oral transmucosal administration of S-Ketamine, its salts or derivatives.
Owner:CLINPHARM SUPPORT
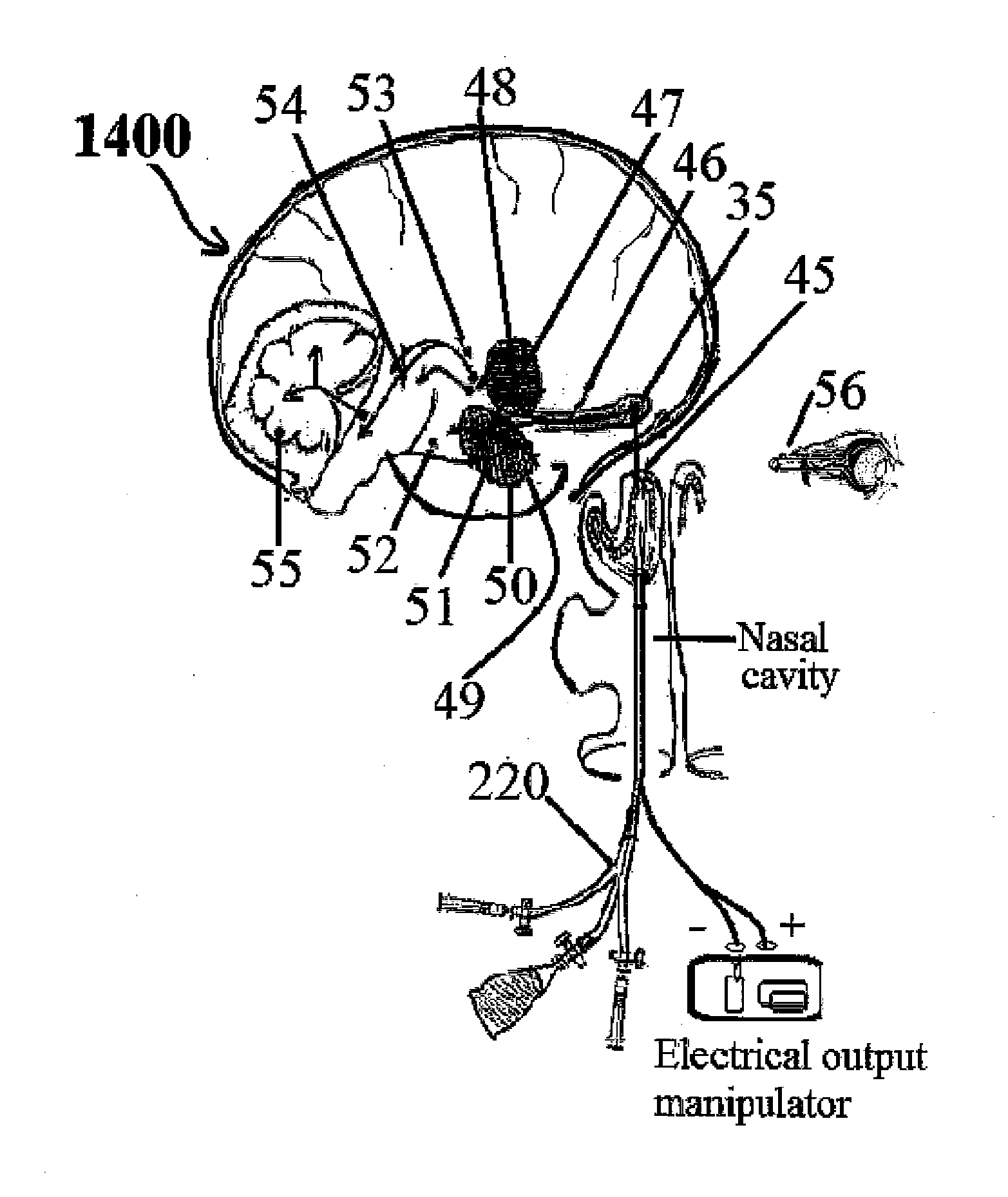
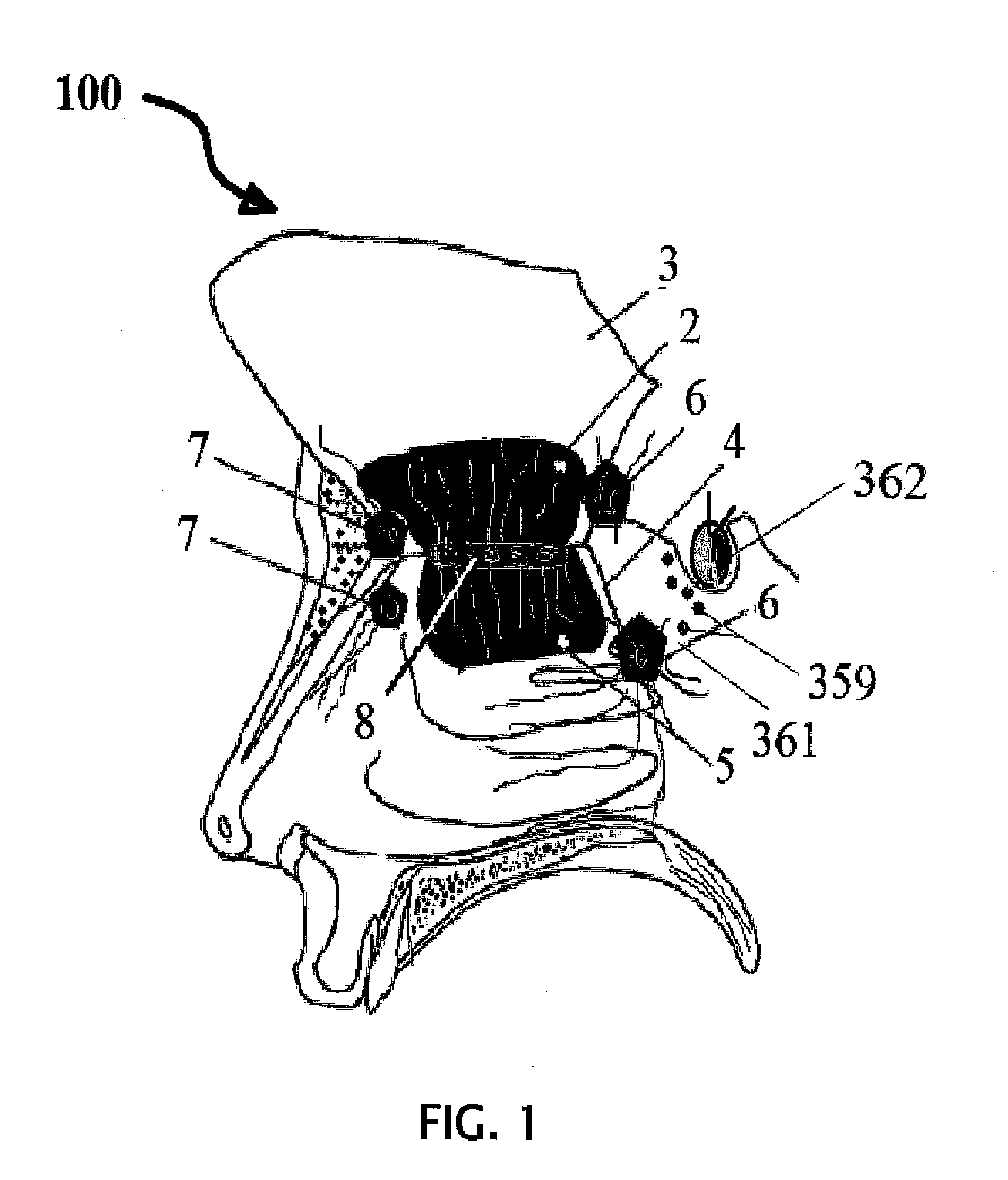
Intranasal administration of ketamine to treat depression
ActiveUS8785500B2Minor adverse side effectAct quicklyBiocideOrganic active ingredientsEnteral administrationKetamine
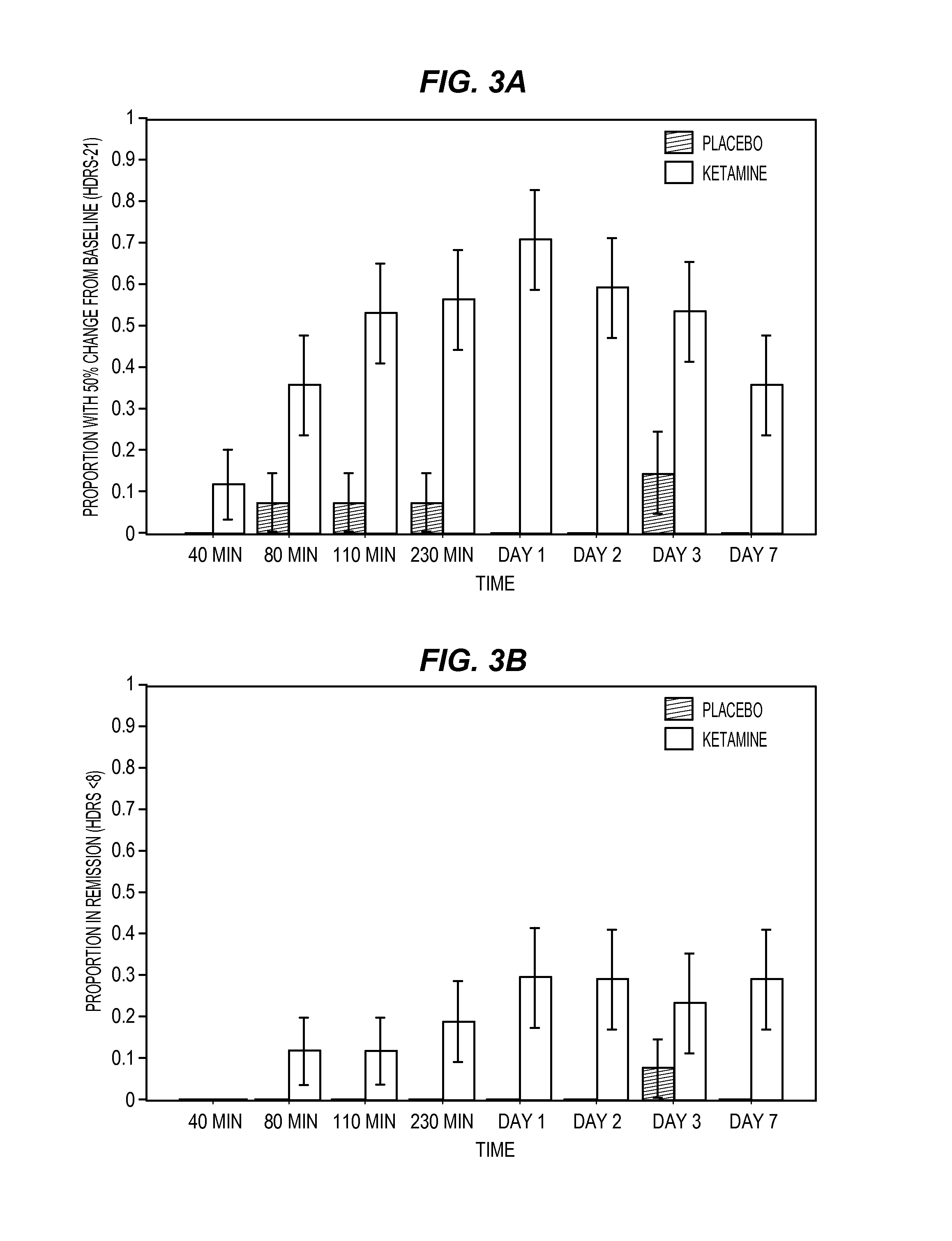
Methods and compositions for the treatment of treatment-resistant depression are described. More specifically, the invention demonstrates that intranasal administration of ketamine is effective to ameliorate the symptoms of depression in a patient who has not responded to an adequate trial of one antidepressant in the current episode and has recurrent or chronic depressive symptoms (>2 years).
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +3
Alzheimer's disease treatment with multiple therapeutic agents delivered to the olfactory region through a special delivery catheter and iontophoresis
InactiveUS20140012182A1Large deliveryAvoid destructionNervous disorderHead electrodesApoptosisExcitotoxicity
This invention describes the administration of multiple therapeutic agents with insulin in conjunction with bexarotene, ketamine, monoclonal antibodies Etanercept, IGF-1, and acetylcholine esterase inhibitors physostigmine, for treatment of Alzheimer's disease and other neurodegenerative diseases. Insulin, improves memory; also augments and amplifies the effects of the adjuvant therapeutic agents (paracrine and intracrine effects) and consequently reduces the β amyloid, its soluble precursors, prevents damage to the neuronal skeletal network (taupathy), and blocks glutamate excitotoxicity, reduces brain inflammation, prevents apoptosis, and increases the acetylcholine levels in the neurons and synapses; by using a combination of insulin, bexarotene, ketamine, Etanercept, IGF-1, and physostigmine therapeutic agents. The results are achieved by using the specially designed Iontophoresis incorporated olfactory mucosal delivery (ORE) catheter device located at the olfactory nerves, sphenoid sinus, and adjacent structures described here, to transport the large molecules of therapeutic agents to treat AD delivered to the CNS bypassing BBB from ORE.
Owner:WEDGE THERAPEUTICS
Prolonged administration of NMDA antagonist and safener drug to alter neuropathic pain condition
InactiveUS20050148673A1Reduce neurotoxic side effectInherent activityBiocideOrganic active ingredientsNR1 NMDA receptorSide effect
A drug that inhibits NMDA receptors (such as ketamine, a surgical anesthetic) is continuously administered to patients suffering from neuropathic pain. Unless the NMDA antagonist drug has inherent safening activity, this treatment requires a “safener” drug to prevent the neurotoxic side effects of NMDA antagonists. One class of safener drugs that increase the efficacy of the treatment include alpha-2 adrenergic agonists, such as clonidine. The treatment lasts for several days and nights, continuously. A maximum tolerated dosage is titered for each patient, such as by observing slurring of speech, and the patient does not lose consciousness except during normal sleep. Magnesium and / or drugs that inhibit ketamine-degrading enzymes can also be used. Patients who suffered for years from chronic intractable pain emerged from this treatment with apparently permanent relief, or with lasting reductions in their levels of pain.
Owner:HARBUT RONALD E +2
Nutrigenomics methods and compositions
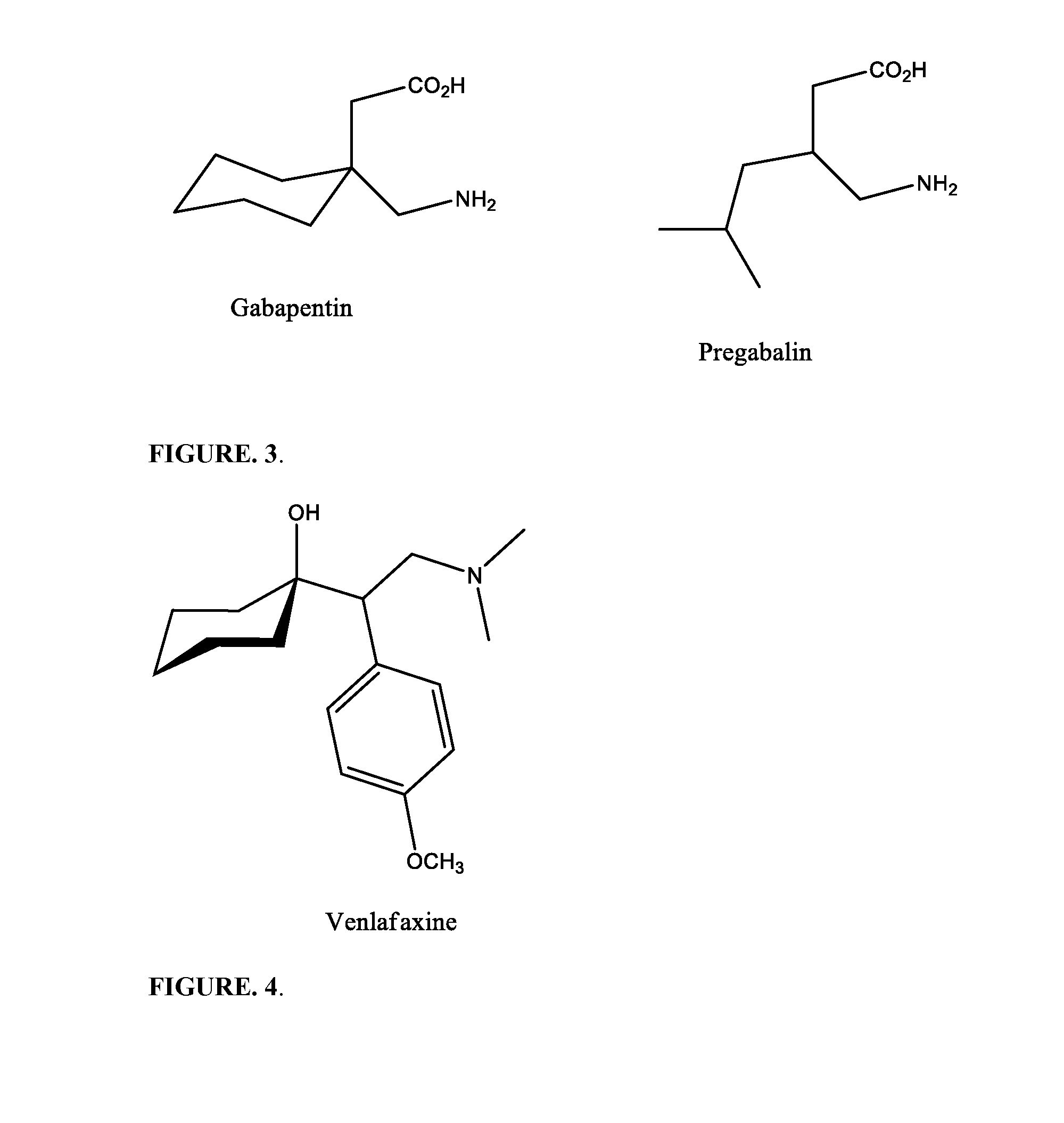
The present invention provides a proprietary compositions and systems to modulate genetic and metabolomic contributing factors affecting disease diagnosis, stratification, and prognosis, as well as the metabolism, efficacy and / or toxicity associated with specific vitamins, minerals, herbal supplements, homeopathic ingredients, and other ingredients for the purposes of customizing a subject's nutritional supplement formulation to optimize specific health outcomes. Specific to this invention the utilization of certain known polymorphic genes associated with Substance Use Disorder (SUD) are analyzed to target certain genetic anomalies that lead to a high risk and predisposition to SUD. The genotypic patterns are then utilized to provide certain nutritional customized solutions especially related to the attenuation of aberrant abuse of physician prescribed narcotic pain medication across all pain conditions. A priority GENOPROFILE is measured and directs the customization of a subsequent nutraceutical to act as a therapeutic modality. Specifically the treatment includes slow attenuation of the pain medication by incorporating orals (shakes, liquid beverages, pills, tablets, troche, ointments etc.), Intramuscular, Intravenous, intra-rectal and any form necessary to deliver a sufficient amount of an anti-craving and anti-stress nutraceutical. Moreover, the invention includes examples of novel analgesic ointments coupling Synaptamine and such analgesic and other anesthetic compounds including but not limited to Gabapentin, Ketamine, Baclofen, Ketoprofen, Amitriptyline, Lidocaine, Cyclobenzapine, Diclofenac, Menthol, Camphor and Capsaicin. The GENOPROFILE will be used to determine pain sensitivity Intolerance.
Owner:BLUM KENNETH +3
Compounded transdermal pain management
The present embodiments relate to topically delivered medication (compounded) for treatment of pain, inflammation, muscle fatigue, spasms, and / or other ailments. A transdermal cream may provide the effective topical administration of multiple medications simultaneously. The transdermal cream may include a salt load of approximately 30% or greater. The transdermal cream may include a unique base composition such that the transdermal cream may be able to remain stable and avoid degradation for six months or more and capable of effective delivery of active ingredient concentrations exceeding approximately 40% or more of the total formulation weight. The active ingredients may include a nerve depressant, NSAID, muscle relaxant, opiate agonist, local anesthetic, NMDA receptor antagonist, and a tricyclic antidepressant. In one embodiment, the transdermal cream may comprise ketamine HCL, gabapentin, clonidine HCL and baclofen. The transdermal cream may deliver an enhanced topical delivery flux of ketamine via a single transdermal application.
Owner:CMPD LICENSING
Analgesic composition for topical use
An analgesic composition, is disclosed which comprises a mixture of piroxicam, dexamethasone, ketamine, lidocaine injection, dimethyl sulfoxide, gabapentin and Vanicream™, preferably in the form of a cream or ointment. The composition is applied topically for the relief of pain of arthritis, neuropathy, post-herpetic (shingles) conditions, sore muscles, tendons and ligaments, and local reactions to insect bites or stings.
Owner:CATHCART CELEVATORON H
Topical formulations for treatment of neuropathy
Topical treatments for neuropathy are described. The treatments include topical formulations of NMDA antagonists and one additional active ingredient. In one example, the formulation includes ketamine and gabapentin for the treatment of a subject's neuropathy. These transdermal or topical compositions provide a surprising degree of effective relief from the symptoms of peripheral neuropathy and can be administered to subjects to treat various neuropathies.
Owner:TARAXOS
Pharmaceutical compositions for treating pain associated with dysmenorrhea
InactiveUS20150313892A1Reduced plasma concentrationEfficient managementBiocidePeptide/protein ingredientsMolecular entityN-methyl-D-aspartate Receptor Antagonists
Pain associated with primary and secondary dysmenorrhea is relieved in a human suffering there from by administering to the human a pain relieving amount of a synergistically acting sub-therapeutic combination of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, magnesium, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, tramadol or its analog such as recemic tramadol or an analogously acting molecular entity or pharmaceutically acceptable salt thereof, and an anticonvulsant and / or a tricyclic anti-depressant or pharmaceutically acceptable salt thereof, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
Anxiolytic composition, formulation and method of use
InactiveUS20140057988A1Prevents and ameliorates and treat anxietyFast onsetBiocideOrganic active ingredientsDental proceduresNMDA receptor
A method for treating, ameliorating or preventing the onset of anxiety in a subject comprises administering to such subject an NMDA receptor antagonist in an amount that is sub-anesthetic and hypo-analgetic. The NMDA receptor antagonist may comprise ketamine and its pharmaceutically acceptable salts, and is administered as a premedication. Instances of use in this manner include administration prior to an anxiety causing event, such as a medical or a dental procedure. The administration of the NMDA receptor antagonist composition is particularly useful as a premedication for adults.
Owner:WEG STUART
Monoclonal antibody for ketamine detection and immune detection board
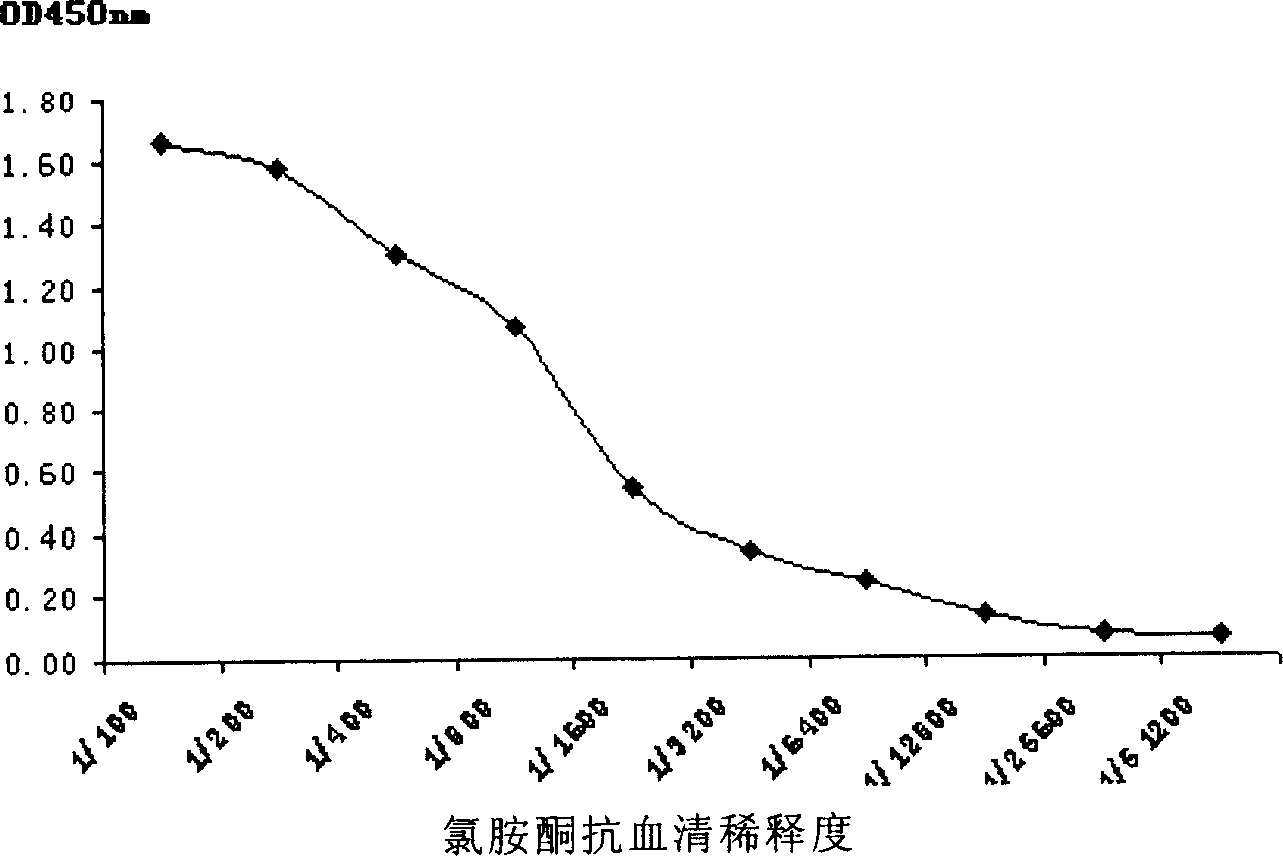
ActiveCN1907953AImproving immunogenicityPreserved immunoreactivitySerum albuminBiological testingHplc methodMetabolite
The invention discloses a hapten and complete antigen used for ketamine detection and antibody preparation. The invention also discloses an anti-ketamine monoclonal antibody prepared by the complete antigen and a colloidal gold-labeled ketamine monoclonal antibody immunoassay plate used for detecting ketamine in drugs or urine, etc., human samples. Compared with HPLC method, the invented detection plate is simple, portable and easy to carry, and can be used for spot detection without need of expensive equipment. The whole detection for ketamine by using the detection plate can be completed in 10 minutes with sensitivity up to 50 ng and with no cross reaction with 39 kinds of common pharmaceuticals, drugs, and ketamine metabolites in vivo.
Owner:SHANGHAI CRIMINAL SCI TECH RES INST +1
Testing strip and dispenser
InactiveUS20070065338A1Prevent undesired ingestingAnalysis using chemical indicatorsAnalysis by subjecting material to chemical reactionChange colorEngineering
A testing strip includes an elongated strip carrier and a reagent on the carrier. The reagent reacts with one of Rohypnol, Ketamine, GHB, GBL, and 1,4-butanediol to change color indicating the presence of Rohypnol, Ketamine, GHB, GBL, and 1,4-butanediol. A dispenser for dispensing testing strips is also disclosed. The dispenser includes a container and at least one testing strip including an elongated strip carrier and a reagent on the carrier that reacts with one of Rohypnol, Ketamine, GHB, GBL, and 1,4-butanediol to change color indicating the presence of Rohypnol, Ketamine, GHB, GBL, and 1,4-butanediol.
Owner:SCHINDLER JERRY +1
Precoated sand used for sand prevention for intermediate and low temperate oil deposit and preparation method thereof
InactiveCN102660246ASimple preparation processReduce dosageDrilling compositionEpoxyAging resistance
The invention relates to precoated sand used for sand prevention for intermediate and low temperate oil deposit and a preparation method thereof. The preparation method comprises the following steps of: 1)evenly mixing 40-50 parts of epoxy resin and 12-25 parts of solvent to prepare solution; 2) adding 2-4 parts of ketamine curing agent into the solution to be evenly mixed; 3) evenly mixing 1.6-2.2 parts of coupling agent and 1000 parts of quartz sand; 4) adding the solution into the quartz sand to be evenly mixed; and 5) dispersing pre-packaged sand, rolling to scatter the sand to be packaged after the sand to be packaged is thoroughly dried, and bagging to be packaged in a sealing mode. The precoated sand prepared with the preparation method disclosed by the invention has the advantages of small use amount of required sand consolidation agent and low medicament cost, and the volatile polyamine curing agent with pungent smell is modified into latent curing agent which is free from peculiar smell and is easy to store. The precoated sand has a simple preparation technology, can be used without being prepared, can be stored for a long time in the dry place, has the advantages of good temperature resistance, ageing resistance and corrosion resistance, high solidified sand core strength and good permeability.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
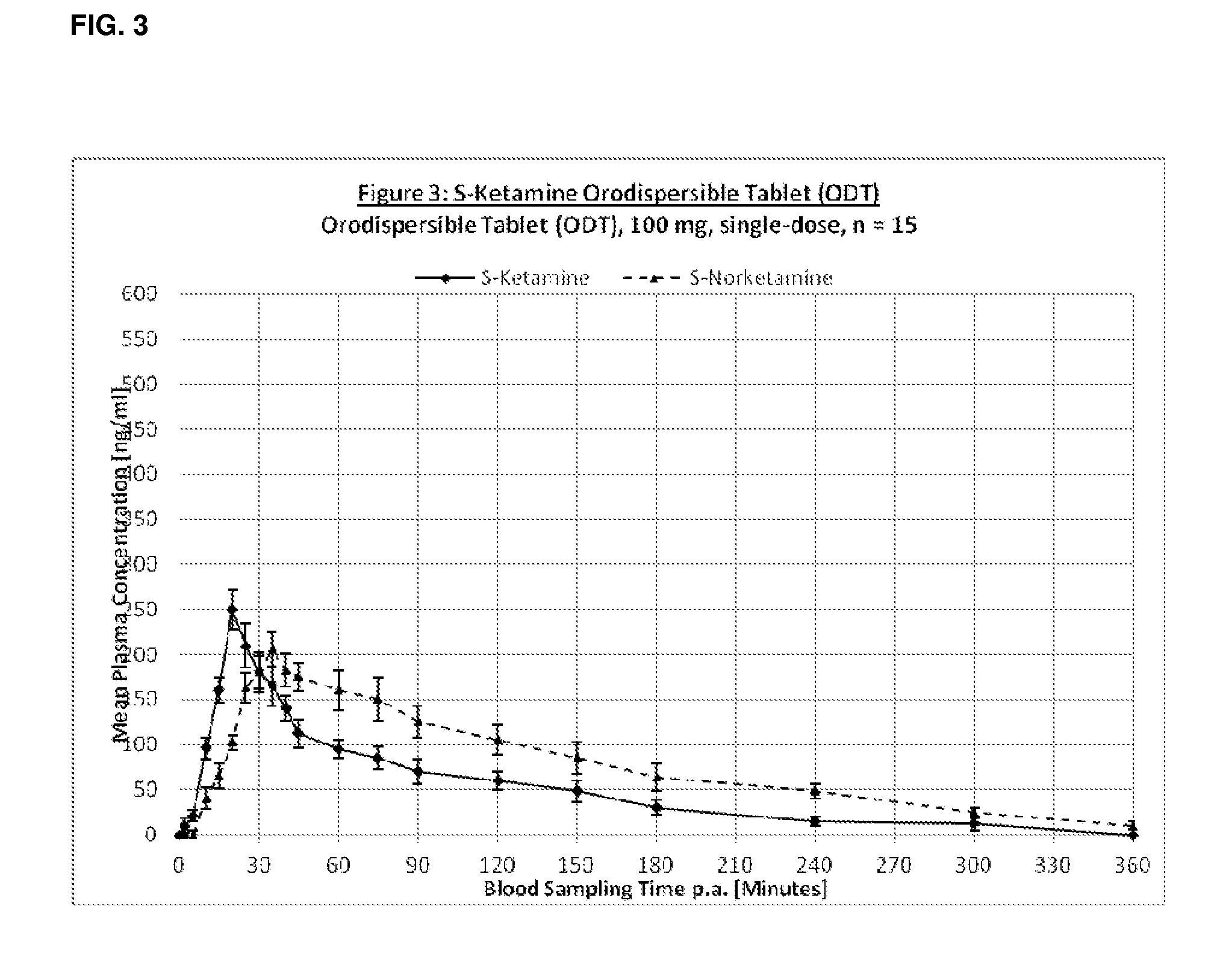
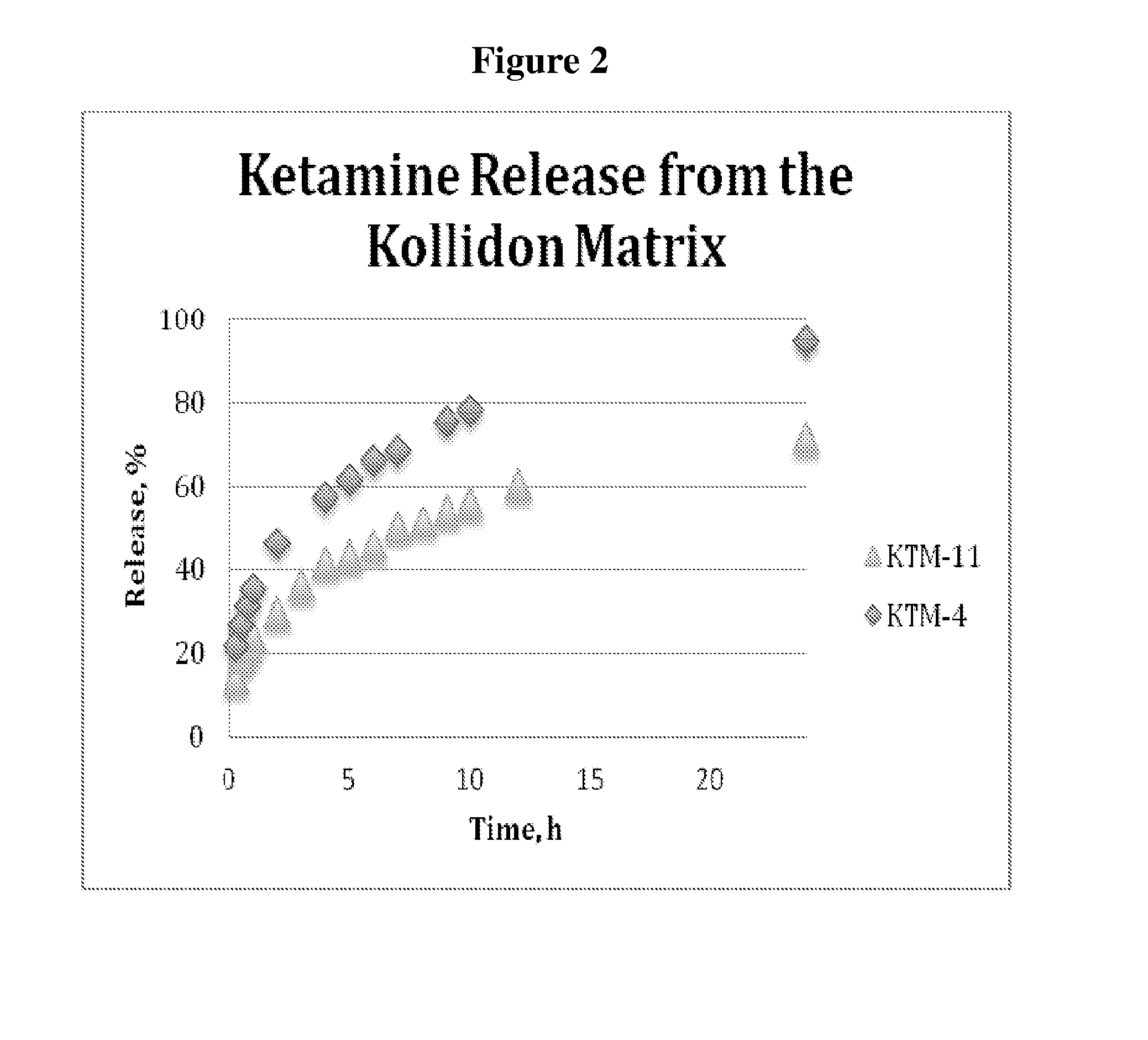
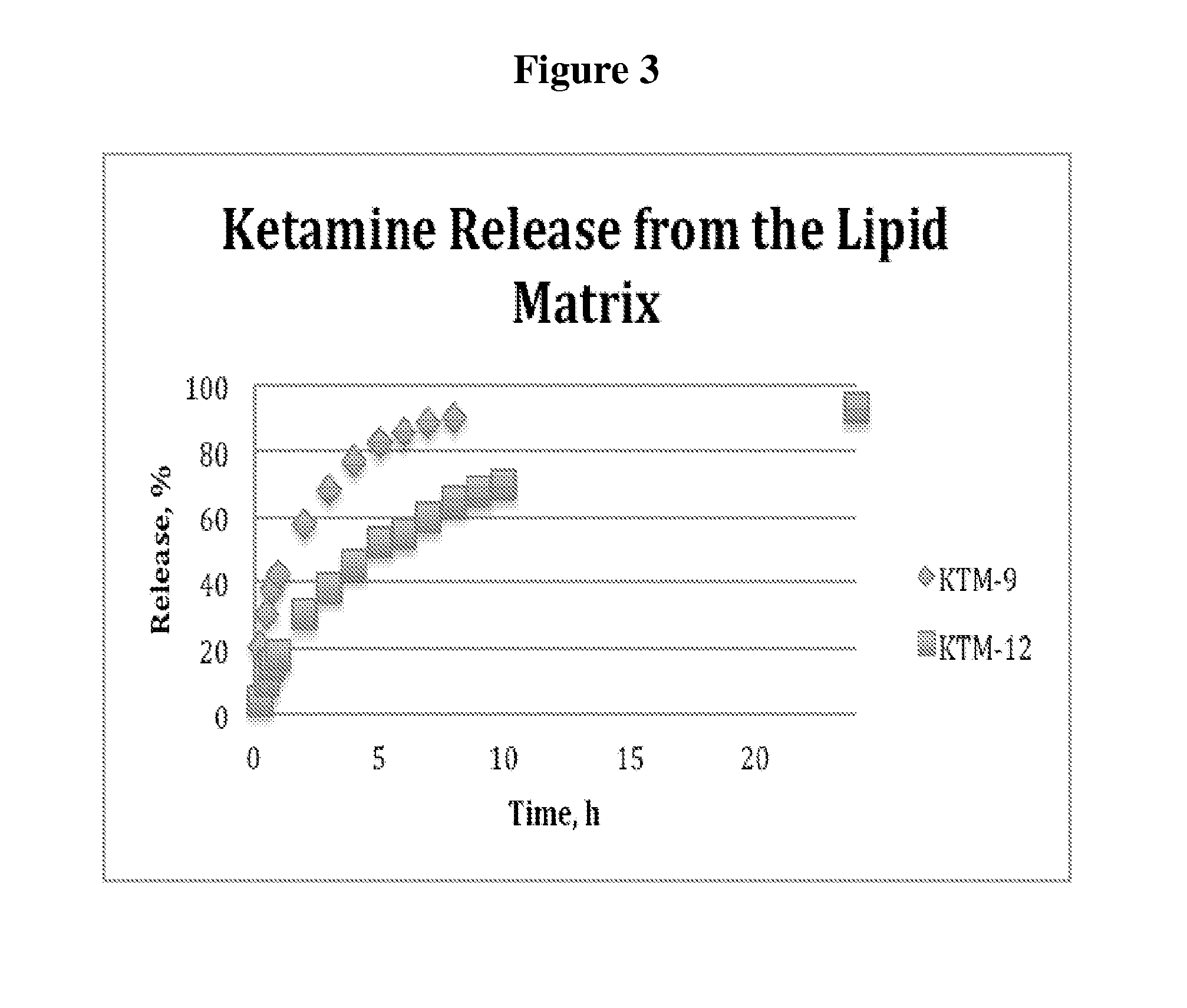
Single-layer oral dose of neuro-attenuating ketamine
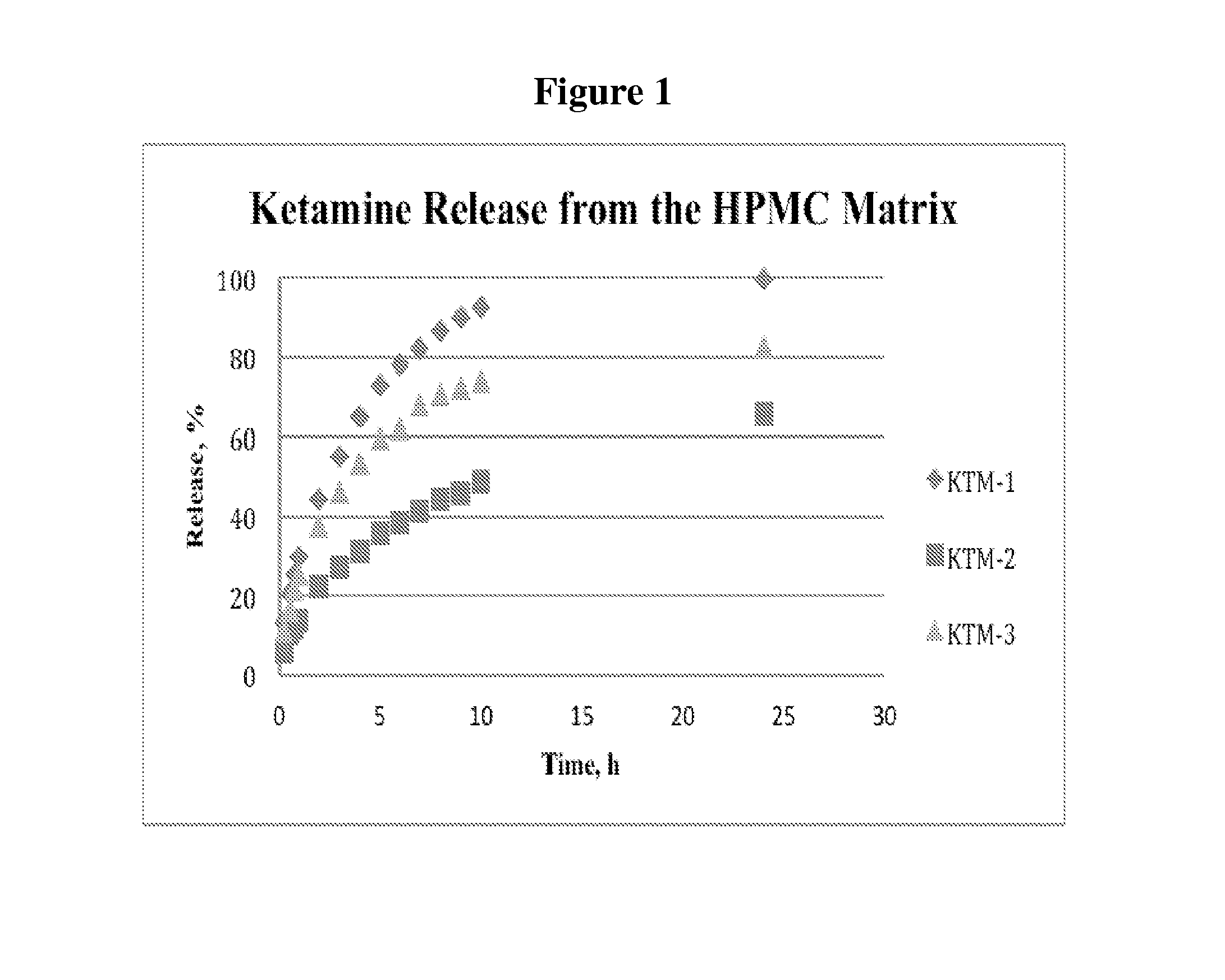
The present invention is directed to oral neuro-attenuating ketamine (NAKET) tablet formulations, and methods of administration, which ensure the steady release of a therapeutically effective concentration of ketamine from an oral tablet without neurologically toxic spikes in ketamine concentration. In particular, the present invention provides single layer oral tablet formulation of NAKET. In a specific embodiment, the NAKET tablet formulation, and methods of administration provide steady administration of NAKET to a subject for 24 hours or greater, for example, up to 36 hours, after a single administration event.
Owner:AMORSA THERAPEUTICS
Age related macular degeneration treatment
InactiveUS20120156202A1Reduce edemaReducing blood cholesterolSenses disorderPharmaceutical delivery mechanismBlood vesselInsulin humulin
A method for treating age related macular degeneration (AMD) using an insulin preparation applied topically to the conjunctival sac of the affected eye. Another aspect of this invention is using antiangiogenic adjuvant therapeutic agents such as bevacizumab, ranibizumab, pegaptanib, etanercept, instilled in to the afflicted eye conjunctival sac with insulin to prevent further formation of new blood vessels, and shrink the existing pathologically formed blood vessels and reduce the edema in wet AMD. This method incorporates putting the patients on low fat diet, aerobic exercise, ketamine-a NMDA blocker, reducing the blood cholesterol using adjuvant therapeutic agents selected from Statins, that are inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A, (i.e. HMG-Co A) reductase which in turn reduce drusen formation that leads to AMD, combined with insulin ophthalmic drops.
Owner:SHANTHA TOTADA R +2
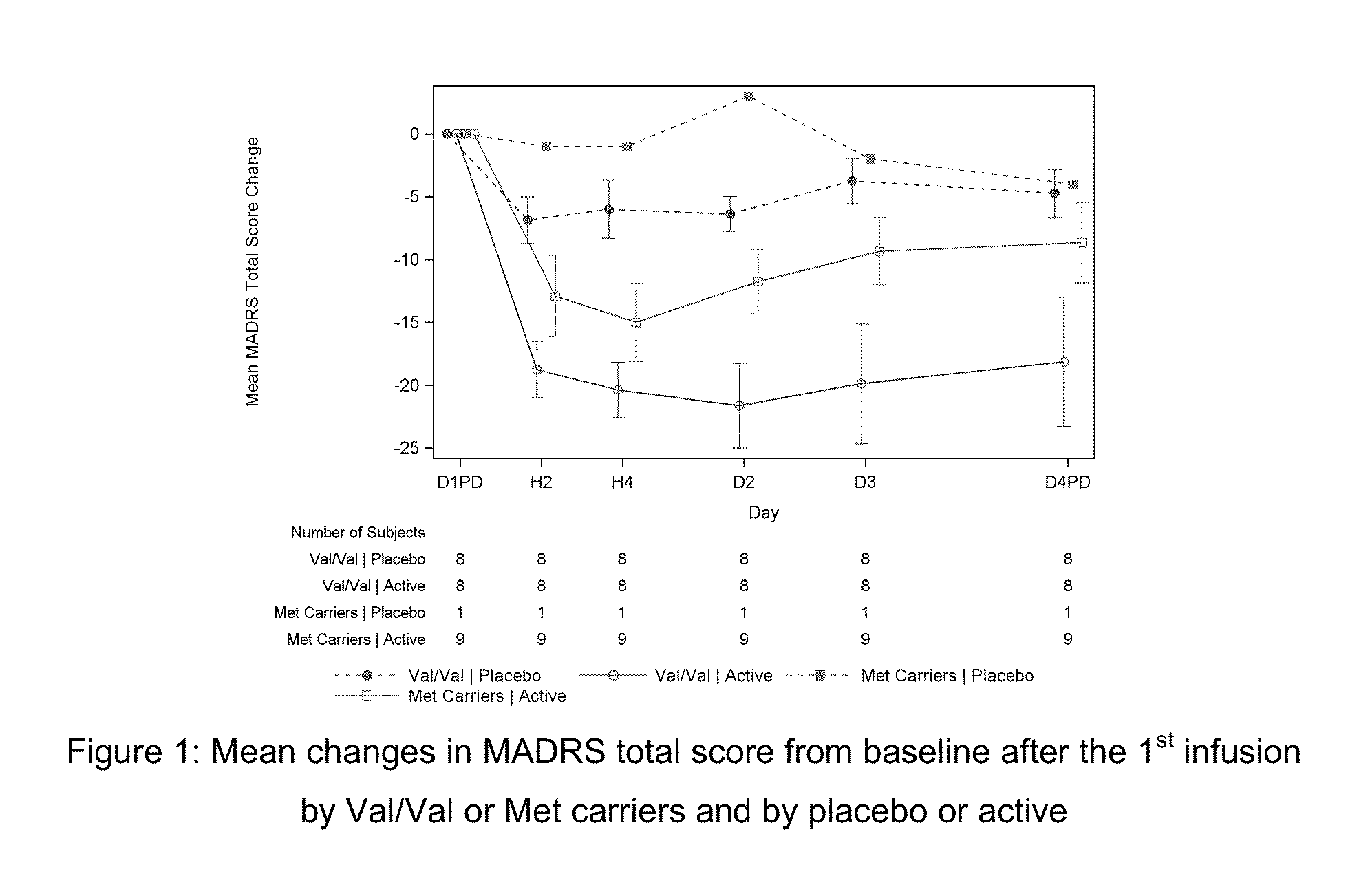
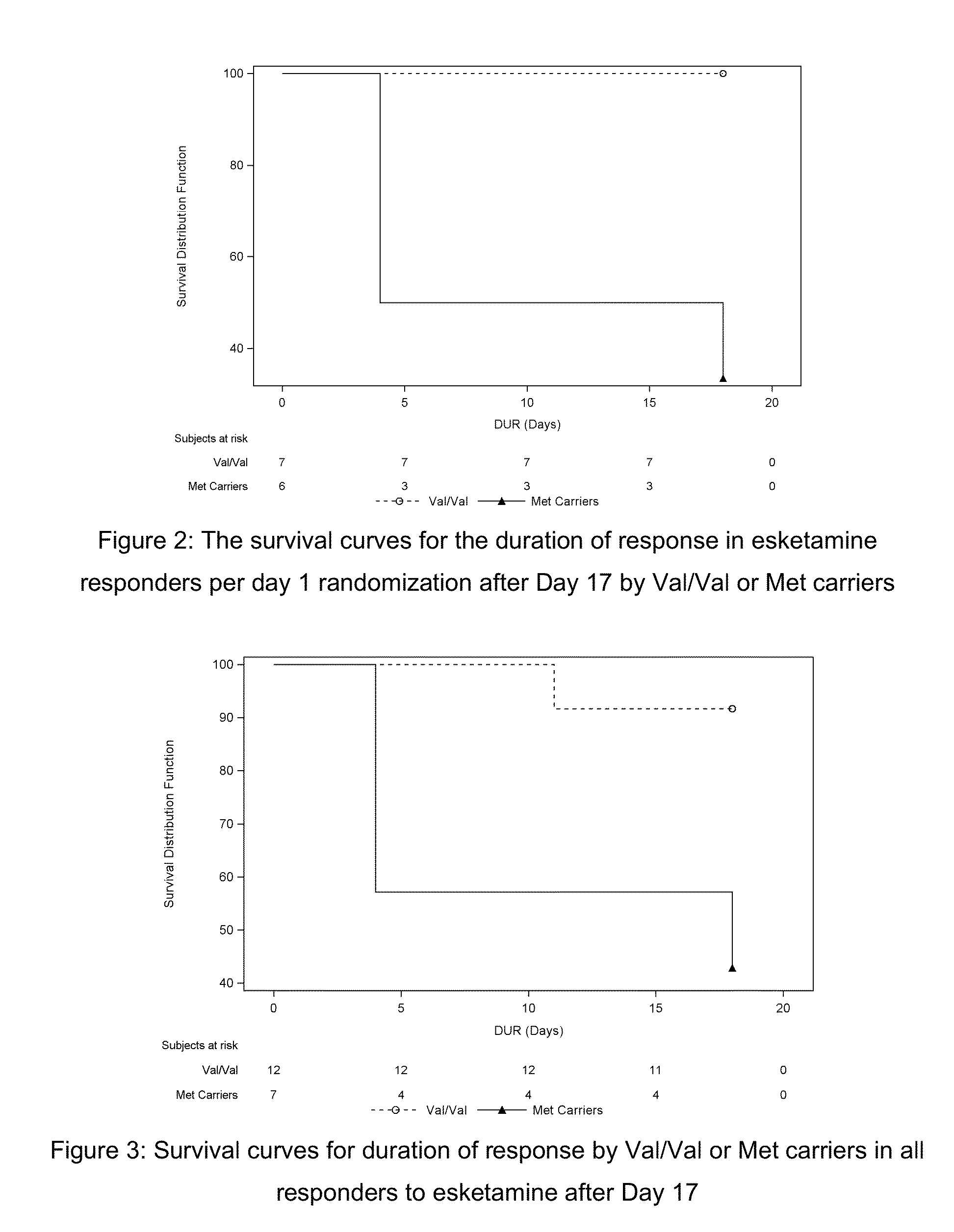
VAL66MET (SNP rs6265) GENOTYPE SPECIFIC DOSING REGIMENS AND METHODS FOR THE TREATMENT OF DEPRESSION
The present invention is directed to methods and dosing regimens for the treatment of depression (preferably, treatment resistant depression), for the treatment of depression in a suicidal patient, and / or for the treatment and / or prevention of suicidality (e.g. suicidal ideations) comprising genotyping a patient to determine their Val66Met rs6265 polymorphism in BDNF and administering a ketamine, preferably esketamine, preferably intranasal esketamine, according to a dosing regimen matched to the patient's genotype.
Owner:JANSSEN PHARMA NV
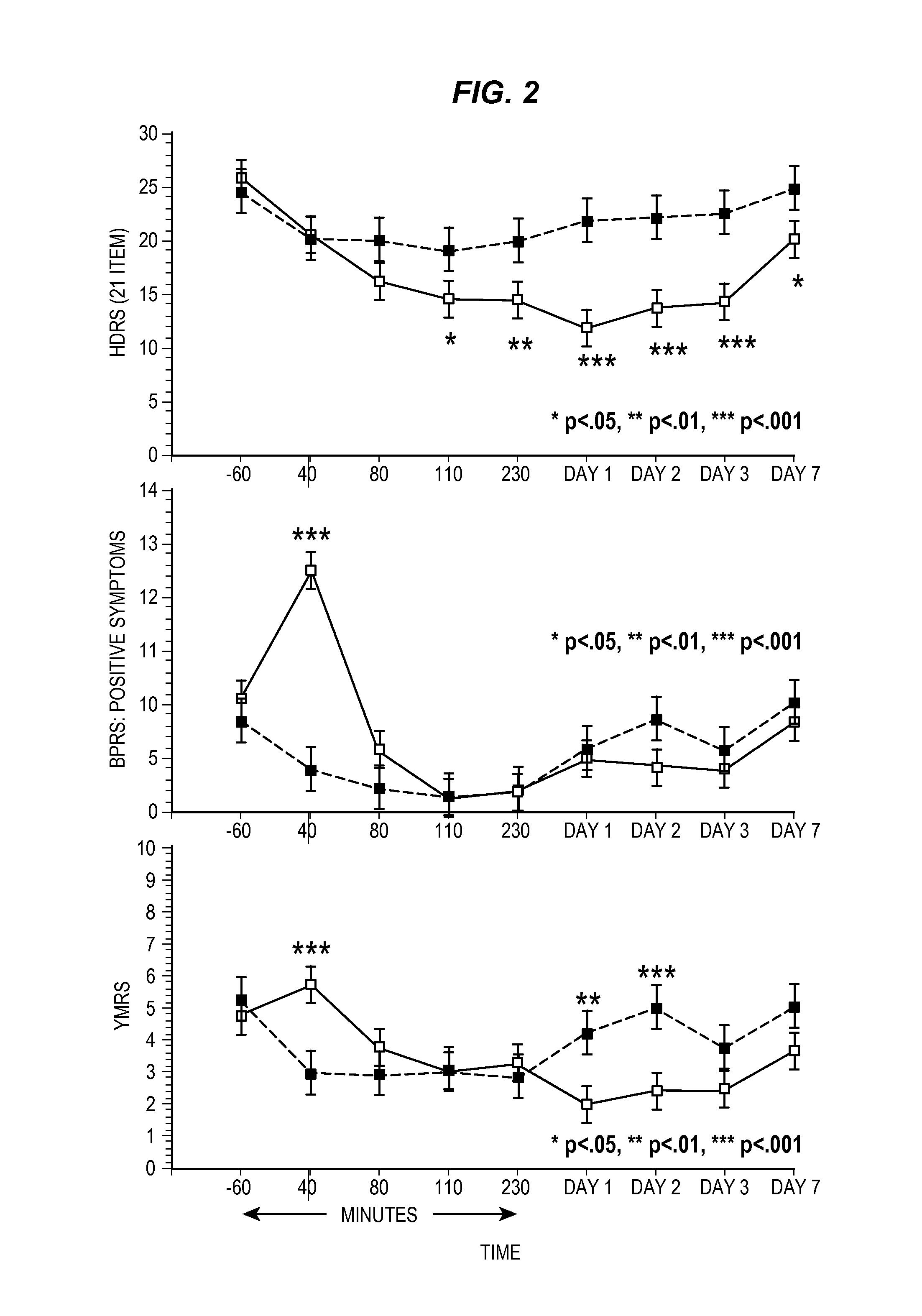
Application of ketamine to treatment of major depressive disorder
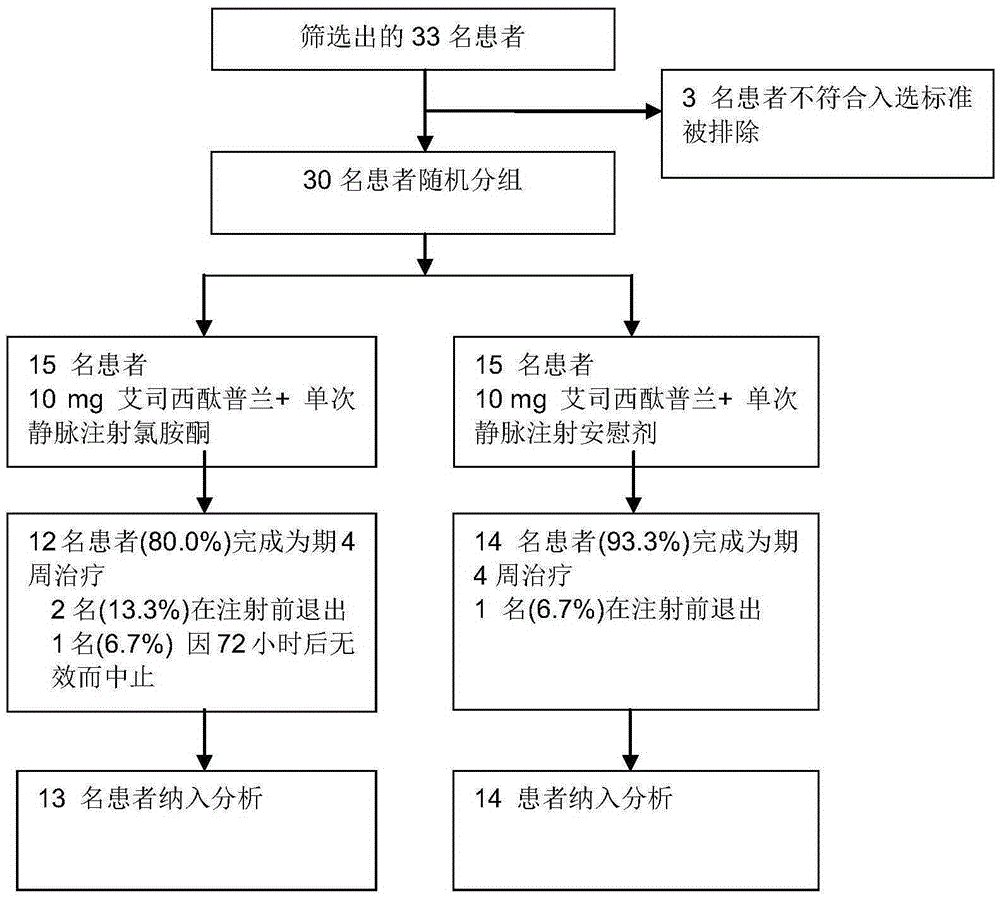
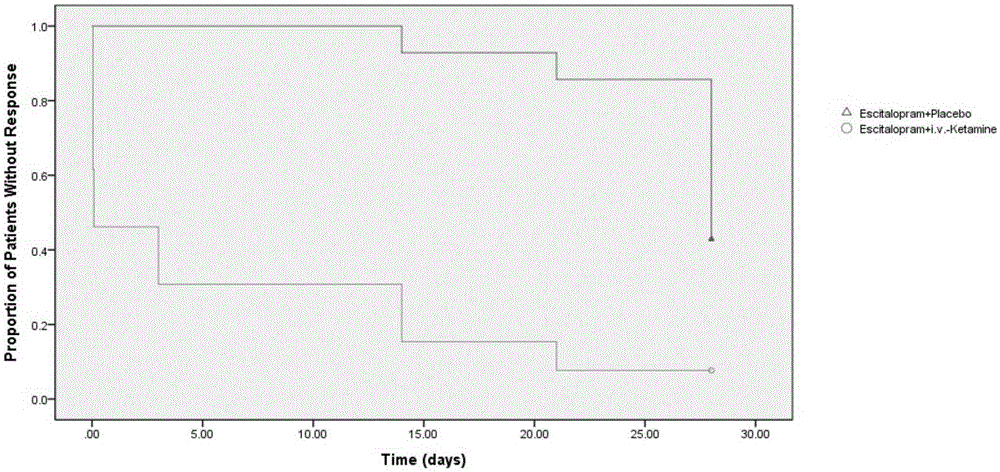
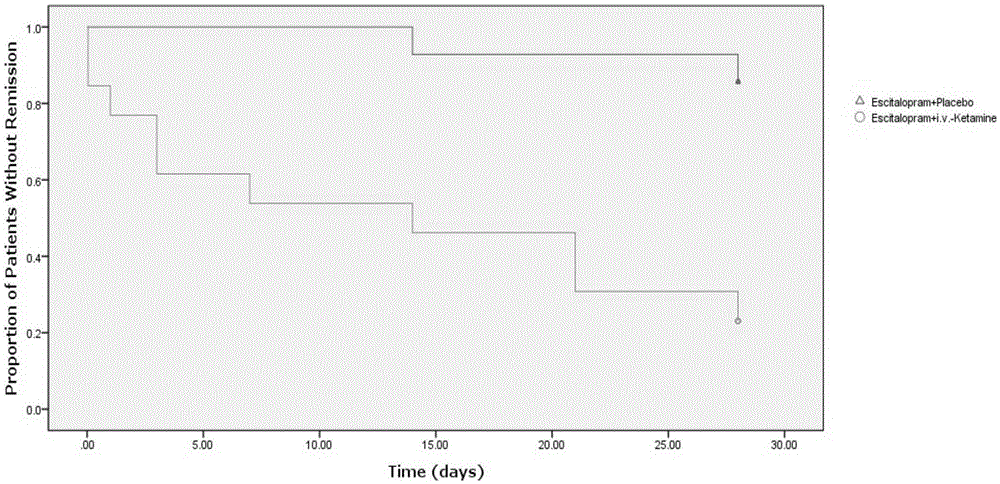
ActiveCN106562952AReduce suicideSignificant antidepressant effectOrganic active ingredientsNervous disorderDisplay lagEscitalopram
The invention provides an application of a combined drug composition of ketamine or S-(+)-ketamine or a pharmaceutically acceptable salt of ketamine or S-(+)-ketamine, and escitalopram or a pharmaceutically acceptable salt of escitalopram, to the preparation of drugs for treating major depressive disorder. The major depressive disorder can be major depressive disorder breaking out for the first time, major depressive disorder breaking out again or refractory depression. The invention furthermore provides an application of ketamine or S-(+)-ketamine or the pharmaceutically acceptable salt of ketamine or S-(+)-ketamine to the preparation of the drugs for treating the major depressive disorder, and a drug preparation containing ketamine or S-(+)-ketamine or the pharmaceutically acceptable salt of ketamine or S-(+)-ketamine, and escitalopram or the pharmaceutically acceptable salt of escitalopram. Ketamine and conventional antidepressants are used together; and a proper amount of ketamine is intravenously injected once at first day of start of treatment, so that the anti-depression effect of oral antidepressants can be effectively improved, the effect display lag period is remarkably shortened, and suicidal actions of patients can be remarkably reduced.
Owner:北京安博睿达医药科技有限公司 +1
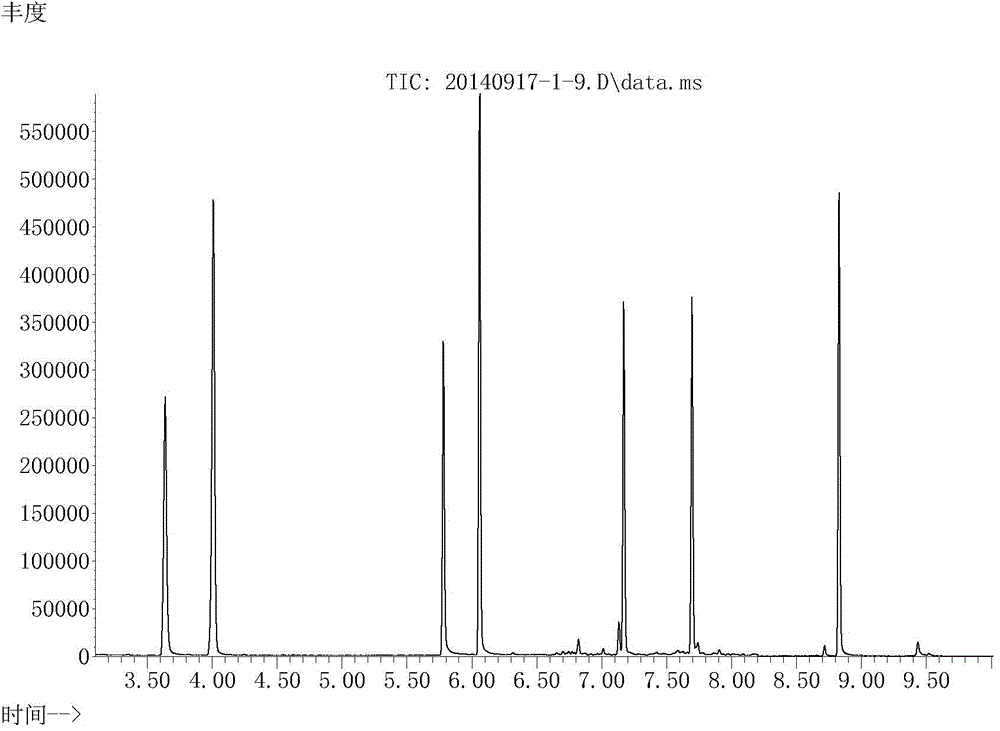
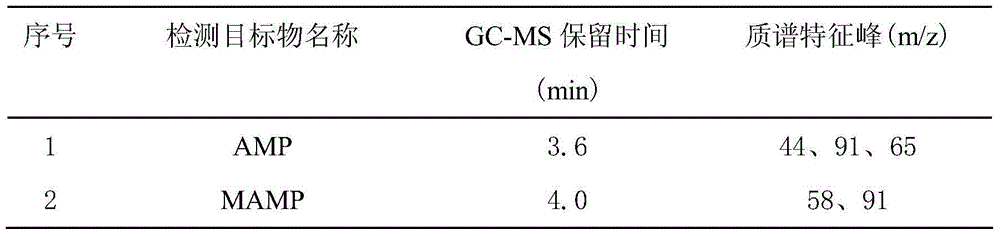
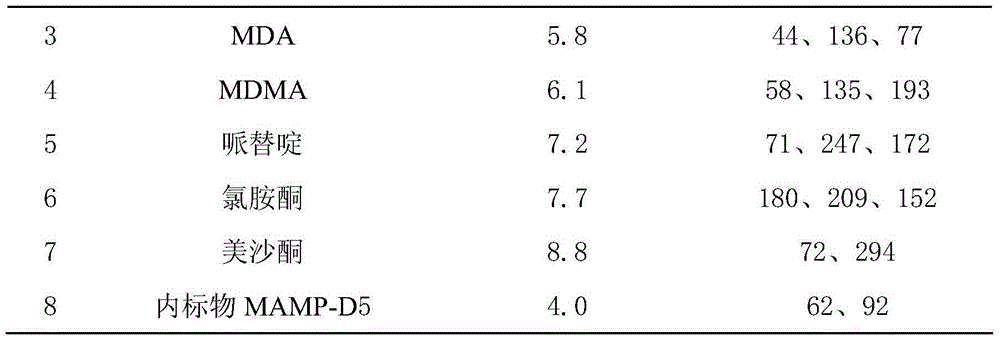
Method for assaying amphetamines, ketamine, pethidine and methadone in blood and urine
The invention belongs to the technical field of analytical chemistry, and relates to a method for rapidly assaying four amphetamines (amphetamine, methamphetamine, methylene-dioxy-amphetamine and methylene-dioxy-methamphetamine), ketamine, pethidine and methadone in blood and urine. The method is high in sensitivity and specificity and wide in linear range, can be used for qualitatively and quantitatively assaying analytes of unknown concentrations in the blood and the urine, and is simple and rapid to operate; according to the method, processes of extracting, and drying by evaporating are not required, and characteristics that a judicial expertise task is urgent and has a high requirement on assaying time can be met; an assaying system, provided by the invention, provides a new technical platform for drug assaying, and is easy to popularize and apply.
Owner:FUDAN UNIV
Xanthiphenyl ketamine or its salt and its preparing process
A process for preparing xanthiphenyl ketoamine or its salt includes such steps as reflux reaction of p-hydroxy benzylidenacetone, N-methyl piperethanamine hydrochloride and polyformaldehyde in absolute alcohol for 6-9.5 hr until fully solidifying, filtering, washing, recrystallizing 1-2 times, then drying to obtain xanthiphenyl ketoamine hydrochloride, neutralizing to obtain xanthiphenyl ketoamine, and reaction on acid to obtain the corresponding salt. Its advantages are simple process, high output rate and high purity.
Owner:WUXI JIMIN KEXIN SHANHE PHARMA
Methods of using (2r, 6r)-hydroxynorketamine and (2s, 6s)-hydroxynorketamine in treatment of depression, anxiety, anhedonia, suicidal ideation, and post traumatic stress disorders
Disclosed is a method of treating Psychotic Depression, Suicidal Ideation, Disruptive Mood Dysregulation Disorder, Persistent Depressive Disorder (Dysthymia), Premenstrual Dysphoric Disorder, Substance / Medication-Induced Depressive Disorder, Depressive Disorder Due to Another Medical Condition, Other Specified Depressive Disorder, Unspecified Depressive Disorder, Separation Anxiety Disorder, Selective Mutism, Specific Phobia, Social Anxiety Disorder (Social Phobia), Panic Disorder, Panic Attack (Specifier), Agoraphobia, Generalized Anxiety Disorder, Substance / Medication-Induced Anxiety Disorder, Anxiety Disorder Due to Another Medical, Other Specified Anxiety Disorder, Unspecified Anxiety Disorder, or fatigue, the method including administering a pharmaceutical composition containing an effective amount of an active agent, wherein the active agent is purified (2R,6R)-hydroxynorketamine, purified (2S,6S)-hydroxynorketamine, or a combination thereof, or a pharmaceutically acceptable saltthereof, together with a pharmaceutically acceptable carrier to a patient in need of such treatment.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
Application Of R-ketamine And Salt Thereof As Pharmaceuticals
ActiveUS20160220513A1Less side effectsQuick effectOrganic active ingredientsNervous disorderDiseaseTherapeutic effect
Provided is a novel compound having rapid and long-lasting therapeutic effects on diseases exhibiting depressive symptoms. Specifically, provided are an agent for prevention and / or treatment of a depressive symptom, consisting of R(−)-ketamine or a pharmacologically acceptable salt thereof, and a pharmaceutical composition for prevention and / or treatment of a depressive symptom, comprising R(−)-ketamine or a pharmacologically acceptable salt thereof in an effective amount for reducing a depressive symptom, and being substantially free of S(+)-ketamine, and a pharmacologically acceptable salt thereof.
Owner:CHIBA UNIVERSITY
Rimsulfuron.haloxyfop-P-methyl water dispersible granule and preparation method thereof
InactiveCN102669140AReduce pollutionAdvanced dosage formBiocideAnimal repellantsWater dispersibleKetamine
The invention discloses a rimsulfuron.haloxyfop-P-methyl water dispersible granule as a weed killer and a preparation method thereof, belonging to the technical field of weeding for plant protection. The rimsulfuron.haloxyfop-P-methyl water dispersible granule comprises the following components in percentage by mass: 10-20% of rimsulfuron, 5-10% of haloxyfop-P-methyl, 3-5% of wetting agent K12, 5-8% of ketamine as a dispersing agent, 5-7% of ammonium sulfate as a disintegrating agent, 5-10% of white carbon black as a carrier and the balance of light calcium carbonate as packing. The rimsulfuron.haloxyfop-P-methyl water dispersible granule can be used for preventing and controlling grassy weeds such as crab grass, moleplant seeds, annual broad-leaved weeds such as crowndaisy chrysanthemum, piemarker and the like and cyperaceae weeds such as cyperus rotundus and the like in a tobacco field. The rimsulfuron.haloxyfop-P-methyl water dispersible granule has the advantages of no need of specific equipment for a process, simplicity in operation, low preparation cost, little pollution to the environment, safety in storage and transportation, stable preventing effect and the like.
Owner:SOUTHWEST UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com