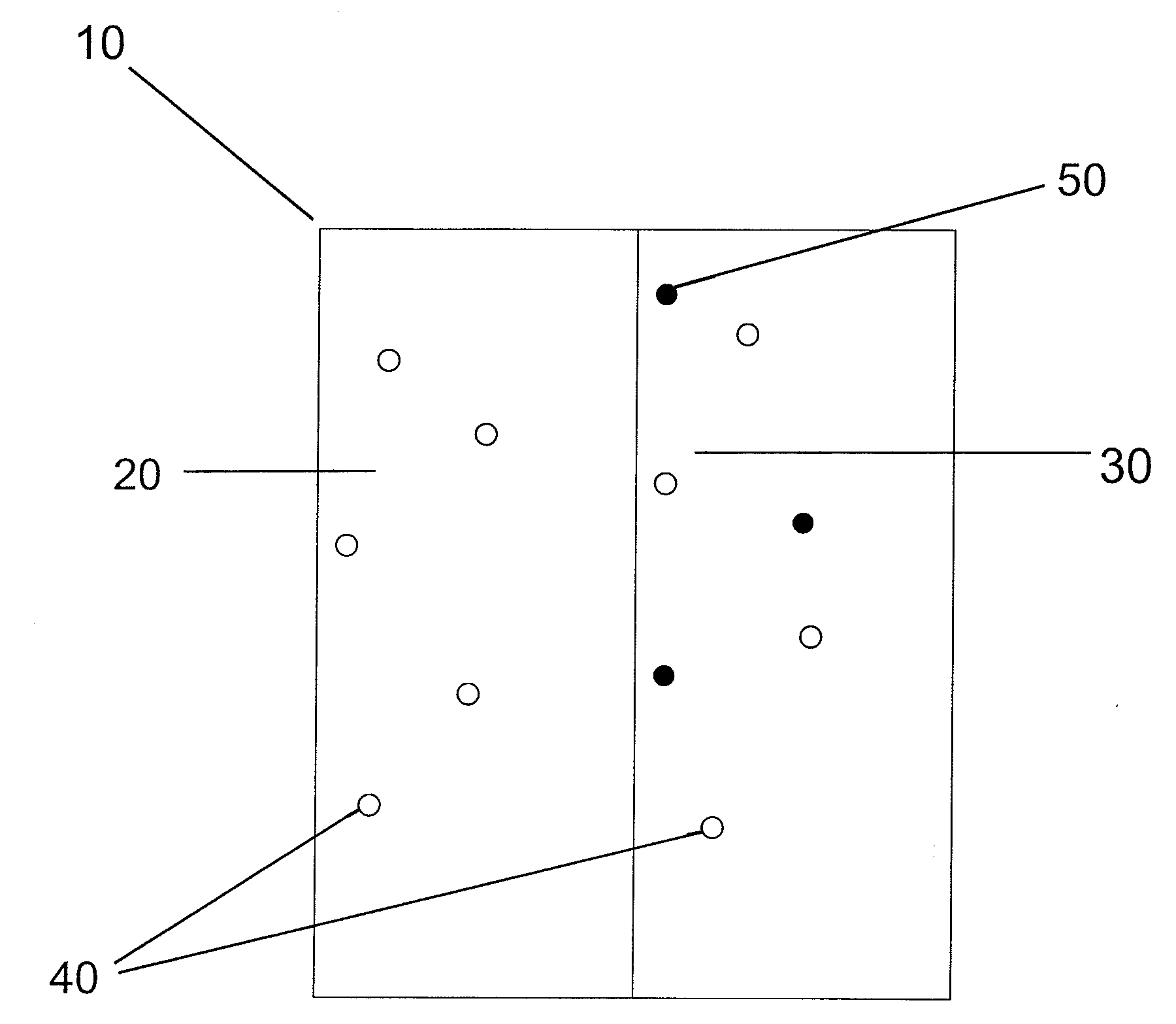
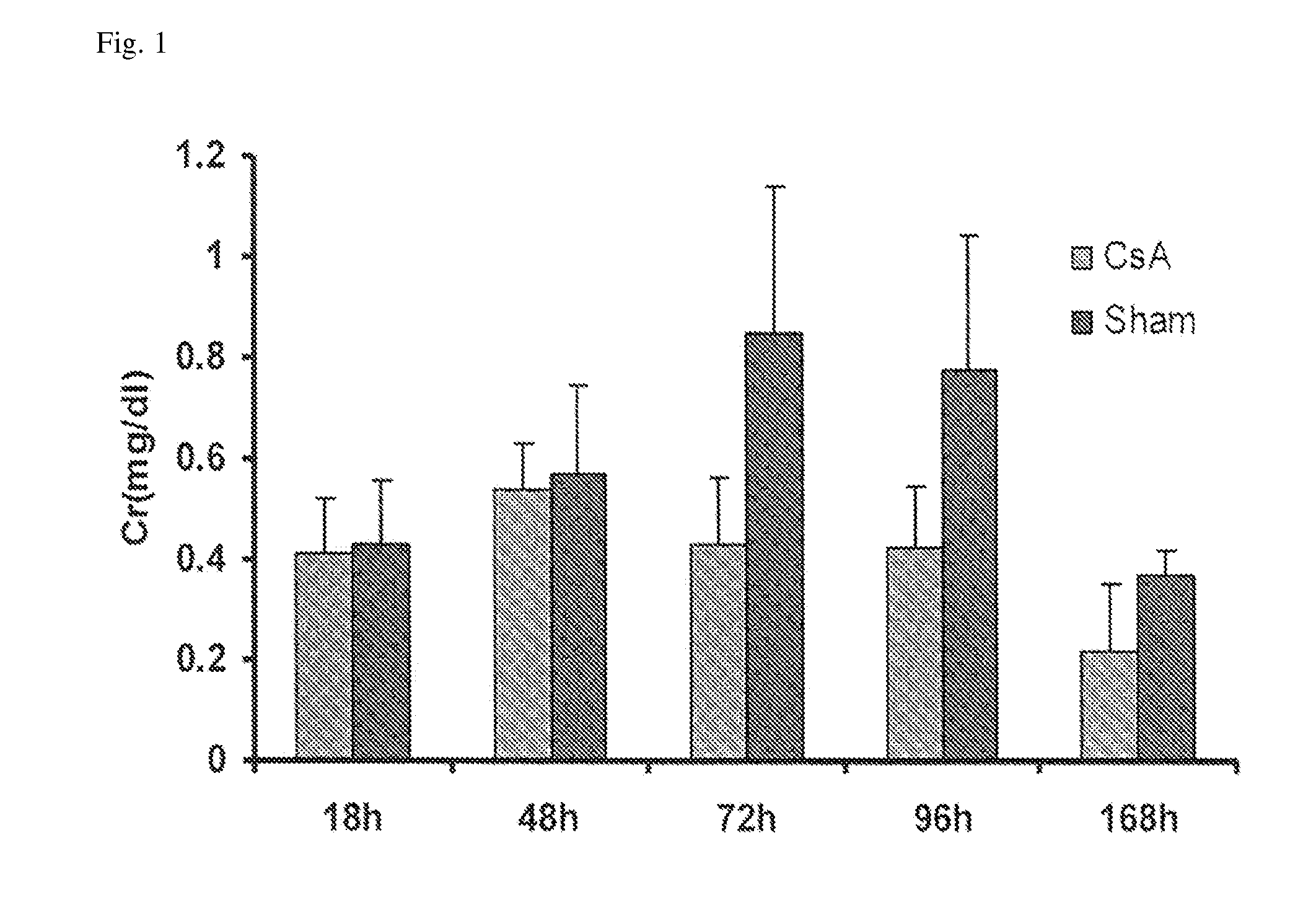
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
158 results about "Single administration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Omega-3 fatty acids and dyslipidemic agent for lipid therapy
InactiveUS20060211762A1Effective treatmentBiocideMetabolism disorderDyslipidemiaCoronary artery disease
A method and composition for blood lipid therapy by administering to the subject an effective amount of a dyslipidemic agent and omega-3 fatty acids. The method utilizes a single administration or a unit dosage of a combination of dyslipidemic agent and omega-3 fatty acids for the treatment of patients with hypertriglyceridemia, hypercholesterolemia, mixed dyslipidemia, coronary heart disease (CHD), vascular disease, artherosclerotic disease and related conditions, and the prevention or reduction of cardiovascular and vascular events.
Owner:RELIANT PHARMACEUTICALS INC +1
Omega-3 Fatty Acids and Dyslipidemic Agent for Lipid Therapy
InactiveUS20090012167A1Effective treatmentBiocideAnimal repellantsDyslipidemiaCoronary artery disease
Owner:RELIANT PHARMACEUTICALS INC +1
Mycoplasma hyopneumoniae bacterin vaccine
The invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration. The composition comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity. In a preferred embodiment, the adjuvant mixture comprises an acrylic acid polymer, most preferably CARBOPOL®, and a mixture of a metabolizable oil such as one or more unsaturated terpene hydrocarbons, preferably squalene or squalane, and a polyoxyethylene-polyoxypropylene block copolymer such as PLURONIC®. The vaccine composition may optionally include a preservative, preferably thimerosol and / or EDTA. In another embodiment, the invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration, and comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant or adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity, in combination with other vaccine components.
Owner:ZOETIS SERVICE LLC
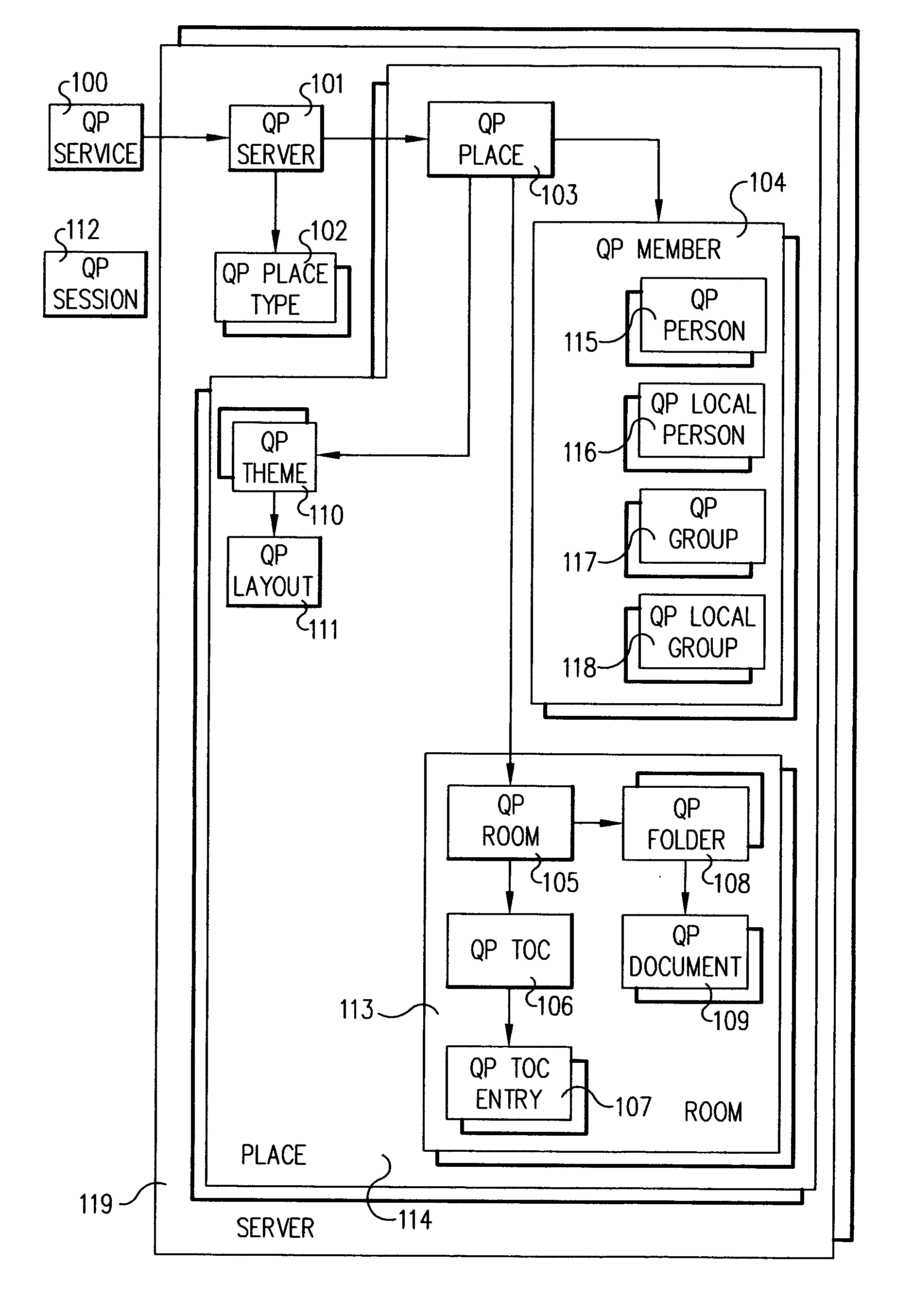
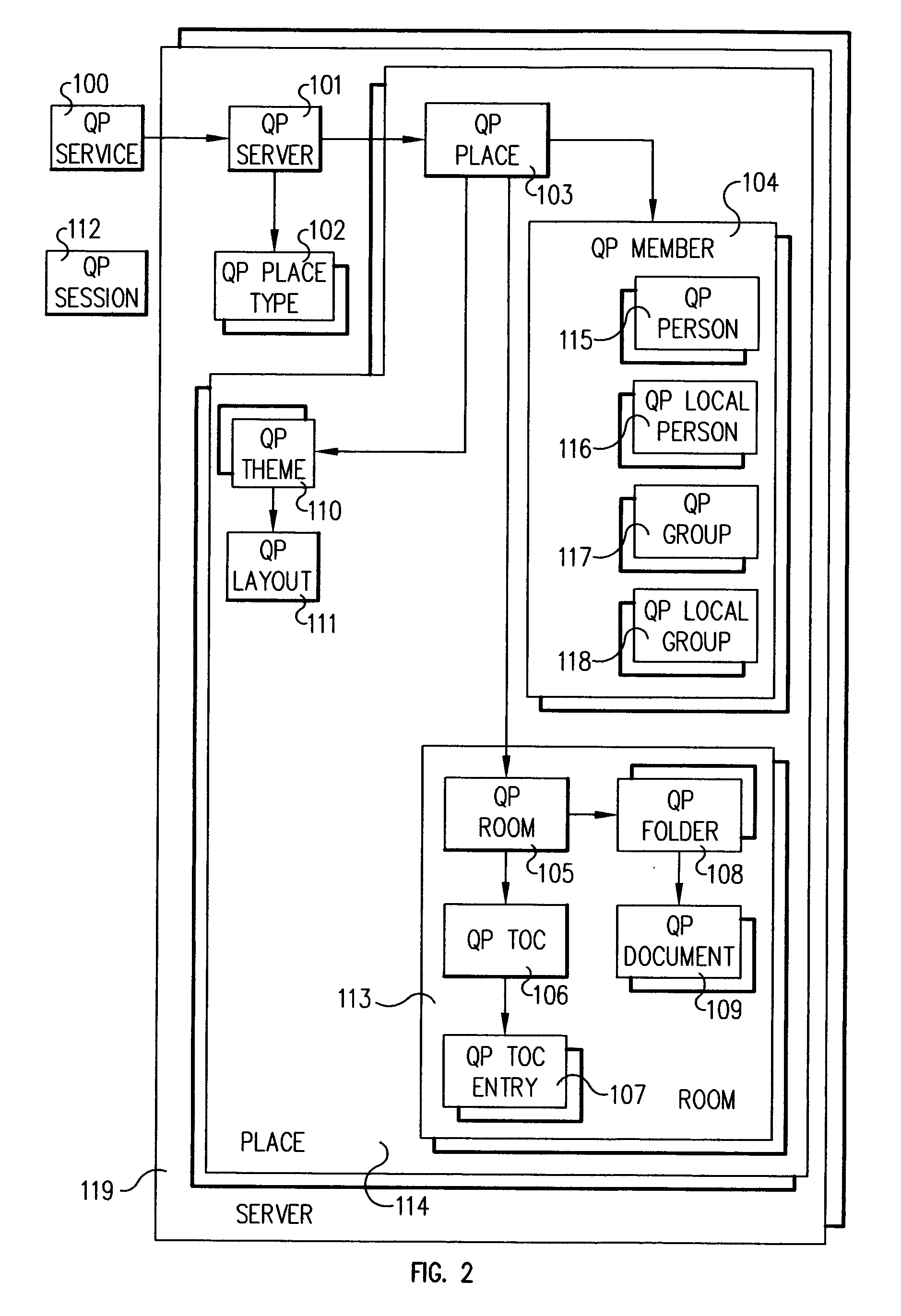
System and method for invoking methods on place objects in a distributed environment
InactiveUS20040128312A1Data processing applicationsDigital data processing detailsAuthentication systemDocumentation
Methods on objects in a distributed environment are accessed and invoked on a system including a plurality of server objects instantiated in the environment in the same address domain, which share a same user directory and authentication system, are on a same user network, and are administered by a single administration team. A service catalog is provided for cataloging a plurality of objects as nodes on a tree, objects including the plurality of servers objects within the distributed environment. A document file is provided for encapsulating cataloged relationships of server objects in the distributed environment and for storing object attributes generated by traversing the tree.
Owner:IBM CORP
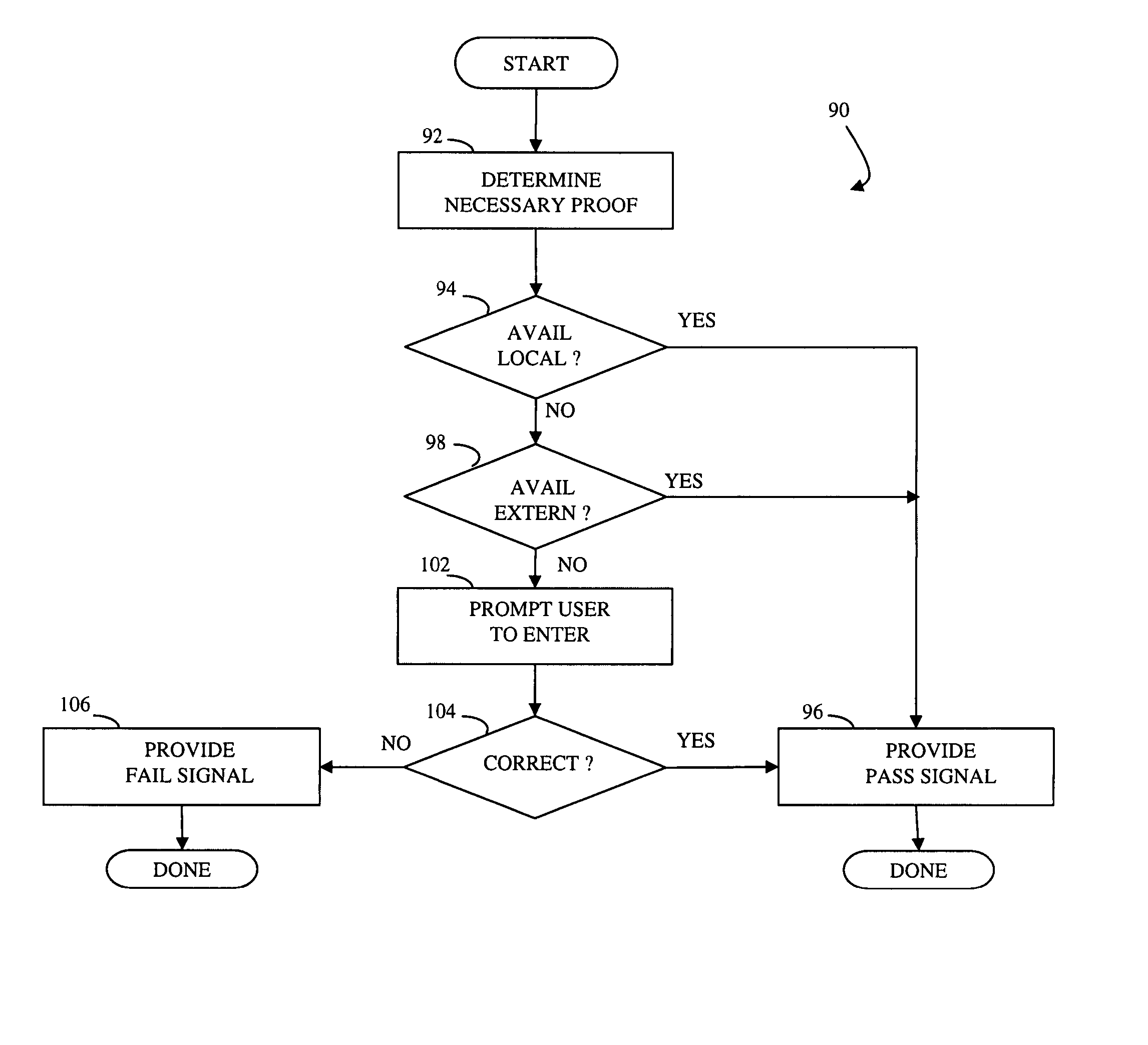
Access control
InactiveUS20050010783A1Promote recoveryLimited accessData processing applicationsDigital data authenticationOne-way functionSingle administration
At least one administration entity controls access to an electronic device by the at least one administration entity generating credentials and a plurality of corresponding proofs for the electronic device, wherein no valid proofs are determinable given only the credentials and values for expired proofs, the electronic device receiving the credentials, if access is authorized at a particular time, the electronic device receiving a proof corresponding to the particular time, and the electronic device confirming the proof using the credentials. The at least one administration entity may generate proofs after generating the credentials. A single administration entity may generate the credentials and generate the proofs. There may be a first administration entity that generates the credentials and other administration entities that generate proofs. The first administration entity may also generate proofs or may not. The credentials may be a digital certificate that includes a final value that is a result of applying a one way function to a first one of the proofs. Each of the proofs may be a result of applying a one way function to a future one of the proofs. The digital certificate may include an identifier for the electronic device.
Owner:ASSA ABLOY AB
Novel clonidine formulation
Owner:TRIS PHARMA
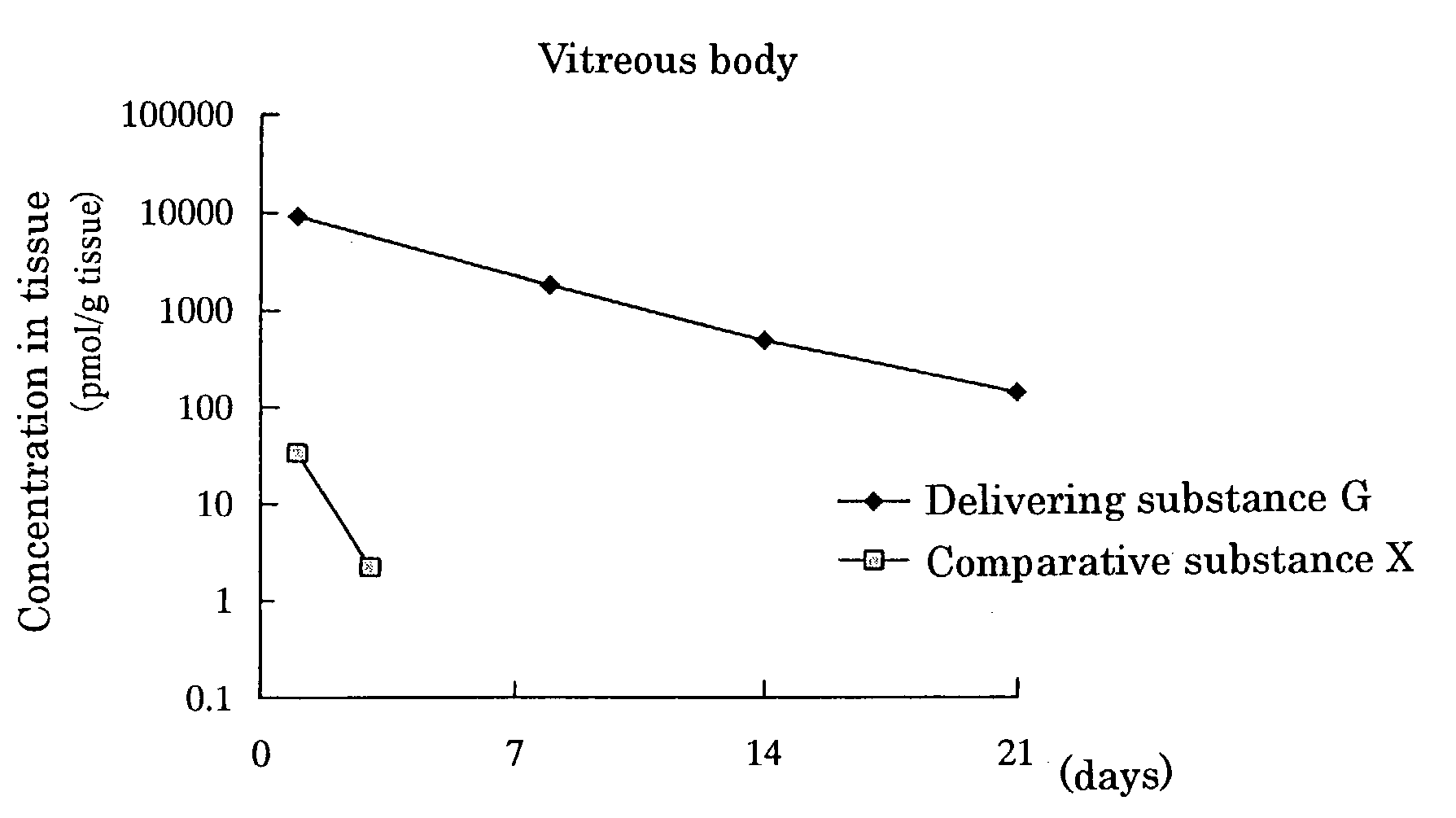
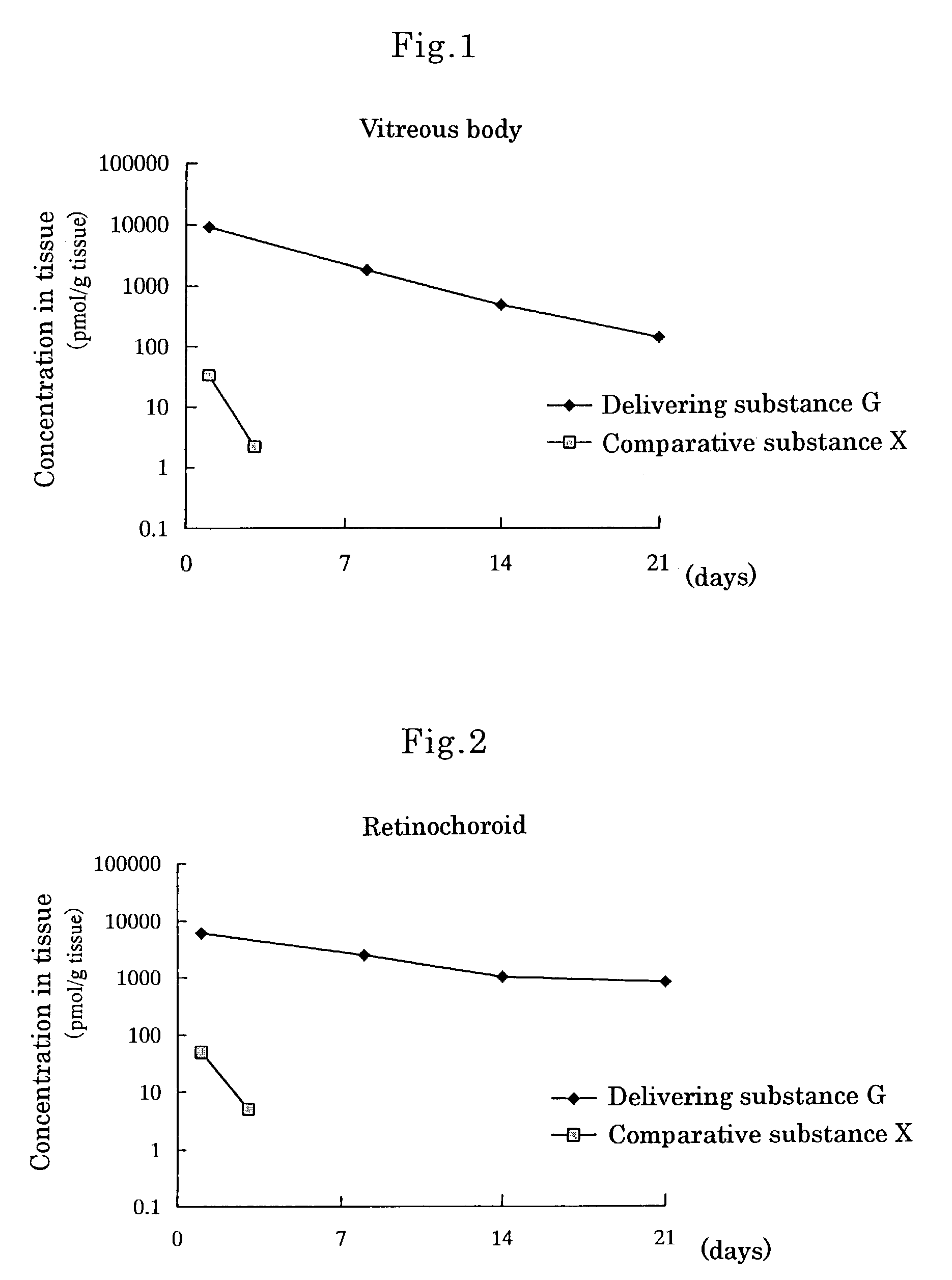
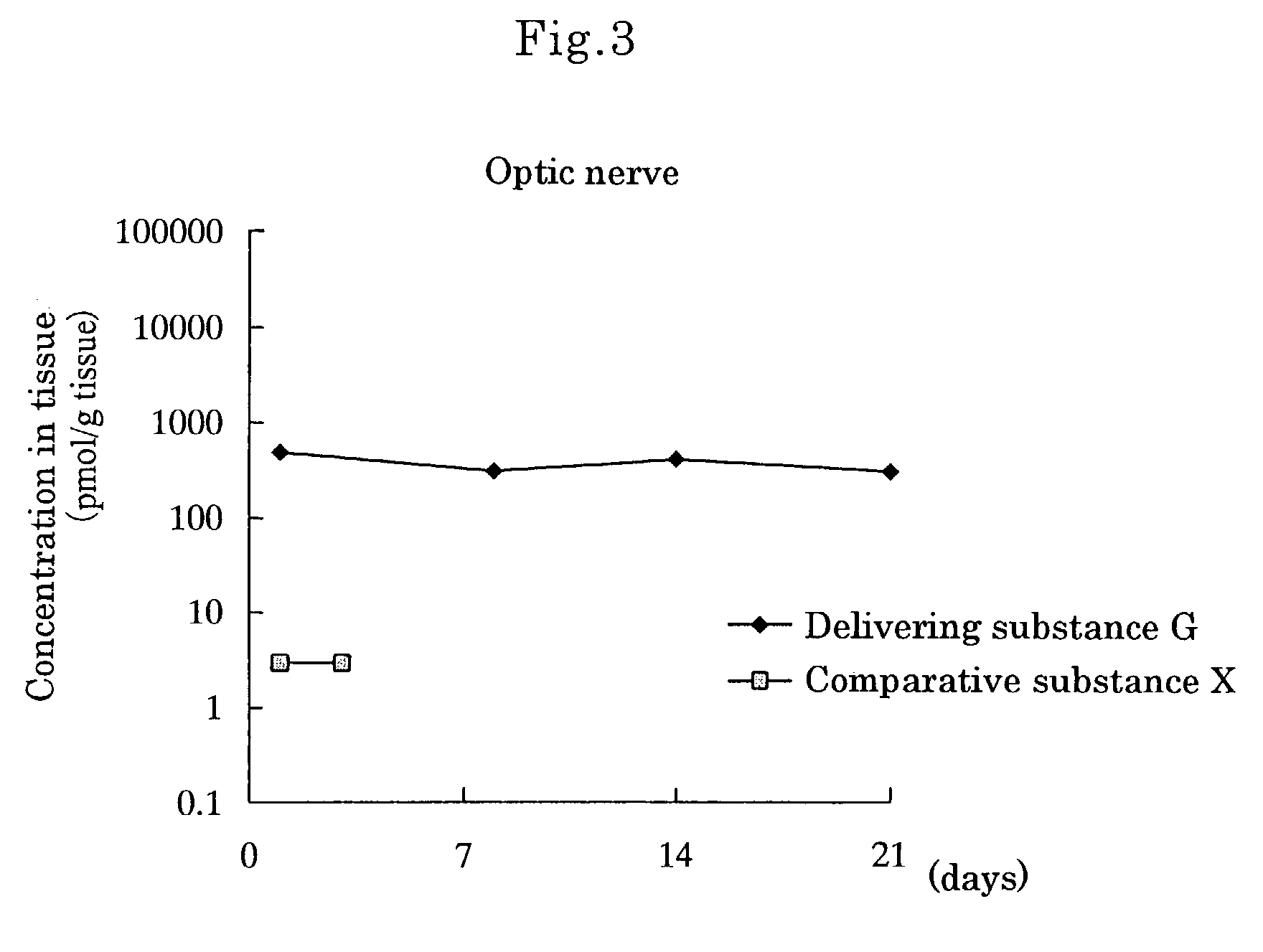
Delivering substance and drug delivery system using the same
InactiveUS7455855B2Extended half-lifeReduce the amount requiredAntibacterial agentsBiocideWhole bodyCurative effect
An object of the present invention is to prepare substances which are excellent in delivery and enable drugs to be retained in a body effectively over a long period and to construct a drug delivery system using the substances. When the delivering substance which is obtained by reacting polyalkylene glycol or a reactive derivative thereof, a phospholipid and a drug with each other to form covalent bonds is administered systemically or topically, the substance is retained at a target site in a body for a long period, thereby making it possible to sustain drug efficacy over a long period by a single administration.
Owner:SANTEN PHARMA CO LTD
Vaccine formulations for intradermal delivery comprising adjuvants and antigenic agents
InactiveUS20060171917A1Improve therapeutic efficacyEnhance immune responseSsRNA viruses negative-senseBacterial antigen ingredientsAdjuvantImmunogenicity
The present invention relates to compositions for intradermal delivery of an antigenic or immunogenic agent in combination with one or more adjuvants. The immunogenic compositions of the invention comprise an antigenic or immunogenic agent and at least one adjuvant, which enhances the immune response to the antigenic or immunogenic agent, once delivered to the intradermal compartment of a subject's skin. The immunogenic compositions of the invention have enhanced efficacy as the adjuvants of the composition promote recruitment of antigen presenting cells to the intradermal compartment and thus enhance presentation and / or availability of the antigenic or immunogenic agent to the antigen presenting cells. The enhanced efficacy of the immunogenic compositions of the invention results in a therapeutically and / or prophylactically effective immune response after a single intradermal dose, with lower doses of adjuvant than conventionally used, achieving therapeutic efficacy from a single administration.
Owner:BECTON DICKINSON & CO
Mycoplasma hyopneumoniae bacterin vaccine
The invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration. The composition comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity. In a preferred embodiment, the adjuvant mixture comprises an acrylic acid polymer, most preferably Carbopol, and a mixture of a metabolizable oil such as one or more unsaturated terpene hydrocarbons, preferably squalene or squalane, and a polyoxyethylene-polypropylene block copolymer such as Pluronic®. The vaccine composition may optionally include a preservative, preferably thimerosol and / or EDTA. In another emodiment, the invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration, and comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant or adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity, in combination with other vaccine components.
Owner:ZOETIS SERVICE LLC
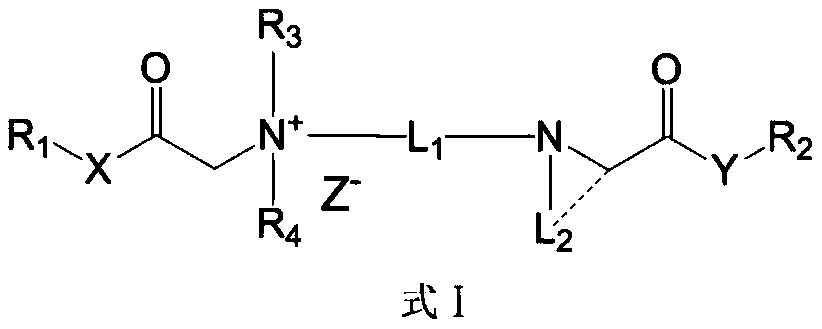
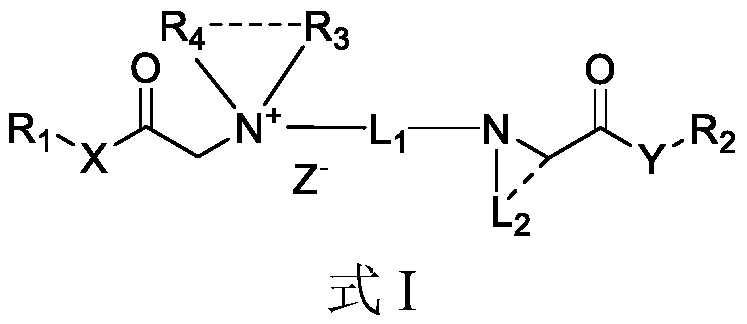
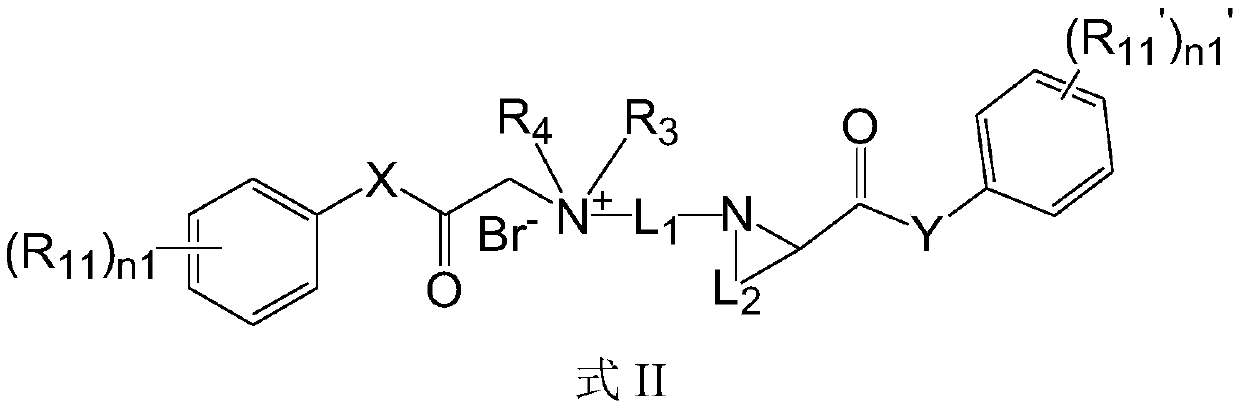
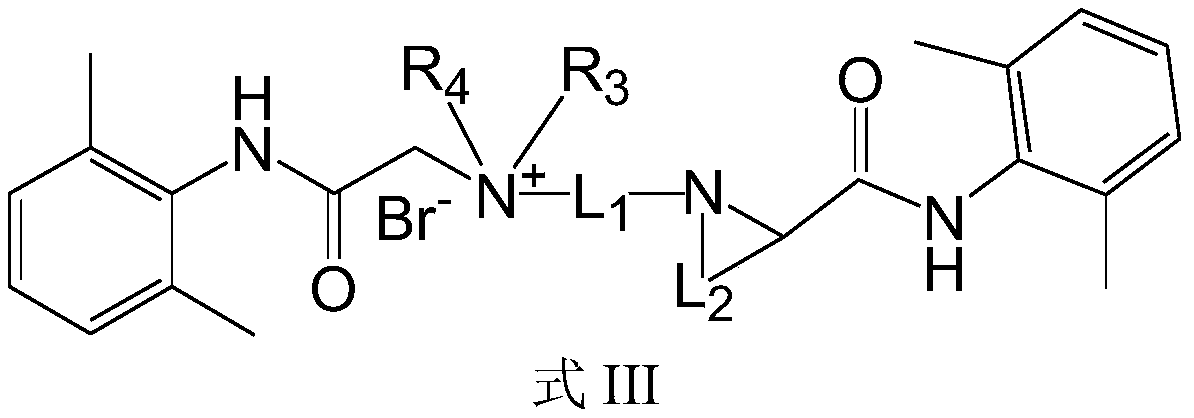
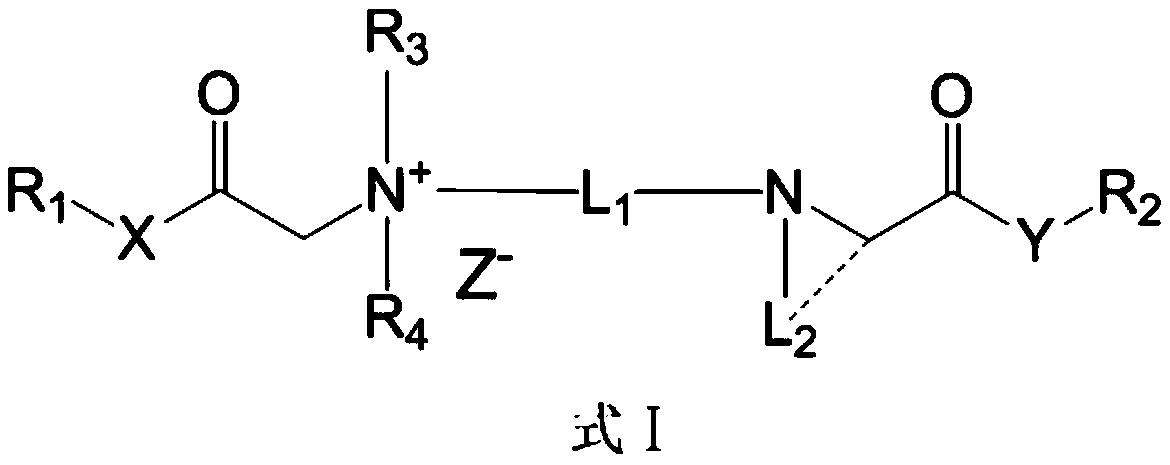
Quaternary ammonium salt compound and preparation method and application thereof
ActiveCN110156665AQuick effectImprove securityOrganic compound preparationAnaestheticsMetaboliteSide effect
The invention discloses a quaternary ammonium salt compound and a preparation method and application thereof. The invention provides a compound shown in a formula I with a novel structure, or a pharmaceutically acceptable salt thereof, or a stereoisomer thereof, or a solvate thereof, or a prodrug thereof, or a metabolite thereof. The compound has the advantages of rapid onset of action, long-termlocal anesthesia effect after single administration, sensory nerve block time greater than motor nerve block time, and both long-term local anesthesia effect and selective local anesthesia effect, remarkably reduces side effects of QX314 and QX314 compositions and quaternary ammonium salt compounds with surfactant structural characteristics, and has better safety. Namely, the compound of formula Iand the pharmaceutically acceptable salt thereof can be used for preparing safe drugs with long-term local anesthesia and selective local anesthesia effects, and have the advantages of long-term local anesthesia, good local anesthesia selectivity, less nerve damage and high safety.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
One dose vaccination against mycoplasma infections of pigs
The present invention provides a one phase, aqueous vaccine composition for immunizing an animal against infection by Mycoplasma hyopneumoniae, comprising: an immunizing amount of a Mycoplasma hyopneumoniae bacterin, an acrylic acid polymer in the concentration range between 0.8 and 1.2 mg / ml, and a pharmaceutically acceptable carrier, and substantially no oil. It is especially useful for immunizing a pig against infection by Mycoplasma hyopneumoniae for at least 20 weeks after a single administration, which effective immunity is reached within 4 weeks after vaccination.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
One dose vaccination against mycoplasma infections of pigs
ActiveUS8444989B1Bacterial antigen ingredientsBacteriaVaccines AdministeredMycoplasma pneumoniae Infections
The present invention provides a one phase, aqueous vaccine composition for immunizing an animal against infection by Mycoplasma hyopneumoniae, comprising: an immunizing amount of a Mycoplasma hyopneumoniae bacterin, an acrylic acid polymer in the concentration range between 0.8 and 1.2 mg / ml, and a pharmaceutically acceptable carrier, and substantially no oil. It is especially useful for immunizing a pig against infection by Mycoplasma hyopneumoniae for at least 20 weeks after a single administration, which effective immunity is reached within 4 weeks after vaccination.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Vaccine formulations for intradermal delivery comprising adjuvants and antigenic agents
InactiveUS20070292386A9Enhances therapeutic efficacy and immune responseSsRNA viruses negative-senseBacterial antigen ingredientsAdjuvantImmunogenicity
The present invention relates to compositions for intradermal delivery of an antigenic or immunogenic agent in combination with one or more adjuvants. The immunogenic compositions of the invention comprise an antigenic or immunogenic agent and at least one adjuvant, which enhances the immune response to the antigenic or immunogenic agent, once delivered to the intradermal compartment of a subject's skin. The immunogenic compositions of the invention have enhanced efficacy as the adjuvants of the composition promote recruitment of antigen presenting cells to the intradermal compartment and thus enhance presentation and / or availability of the antigenic or immunogenic agent to the antigen presenting cells. The enhanced efficacy of the immunogenic compositions of the invention results in a therapeutically and / or prophylactically effective immune response after a single intradermal dose, with lower doses of adjuvant than conventionally used, achieving therapeutic efficacy from a single administration.
Owner:BECTON DICKINSON & CO
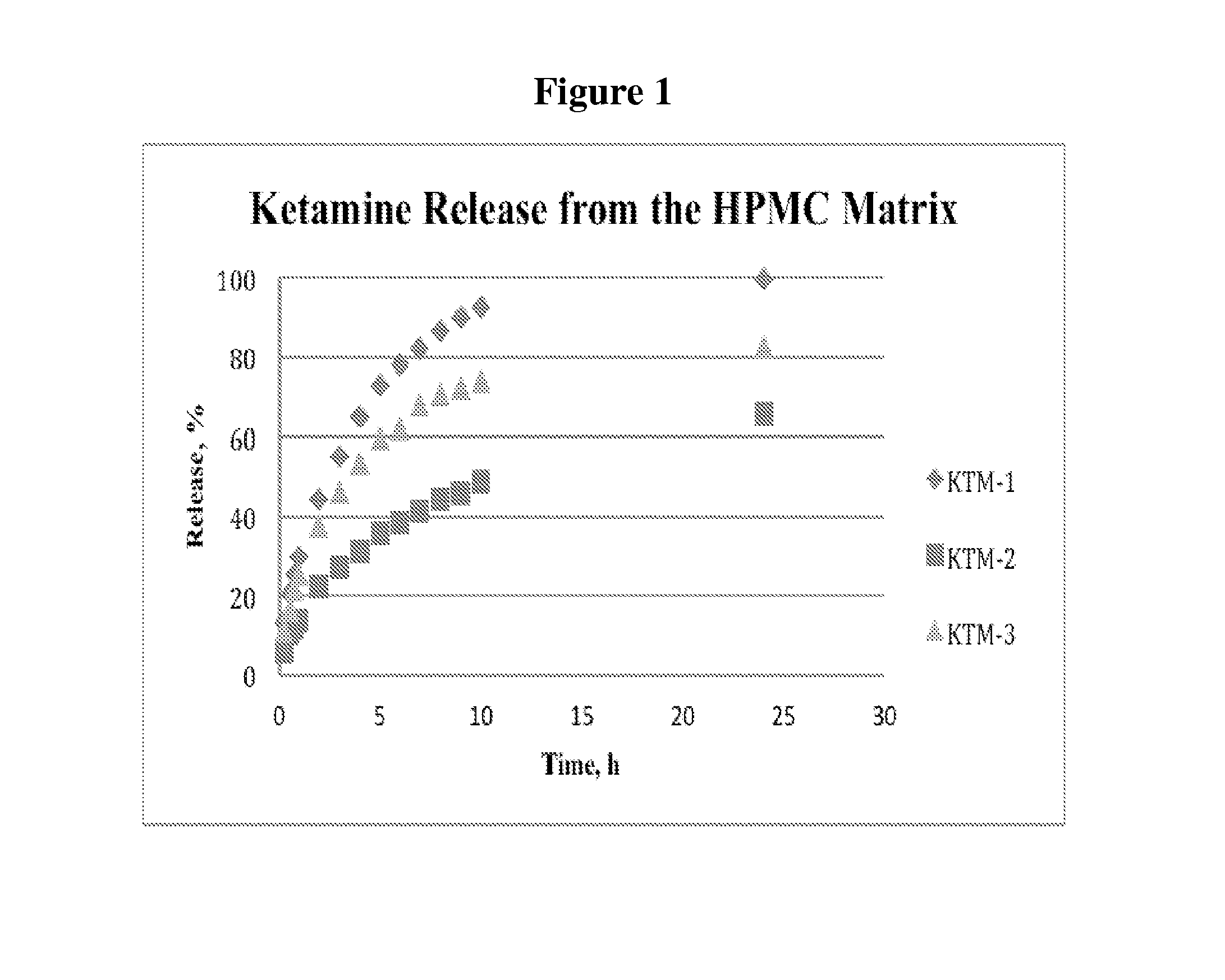
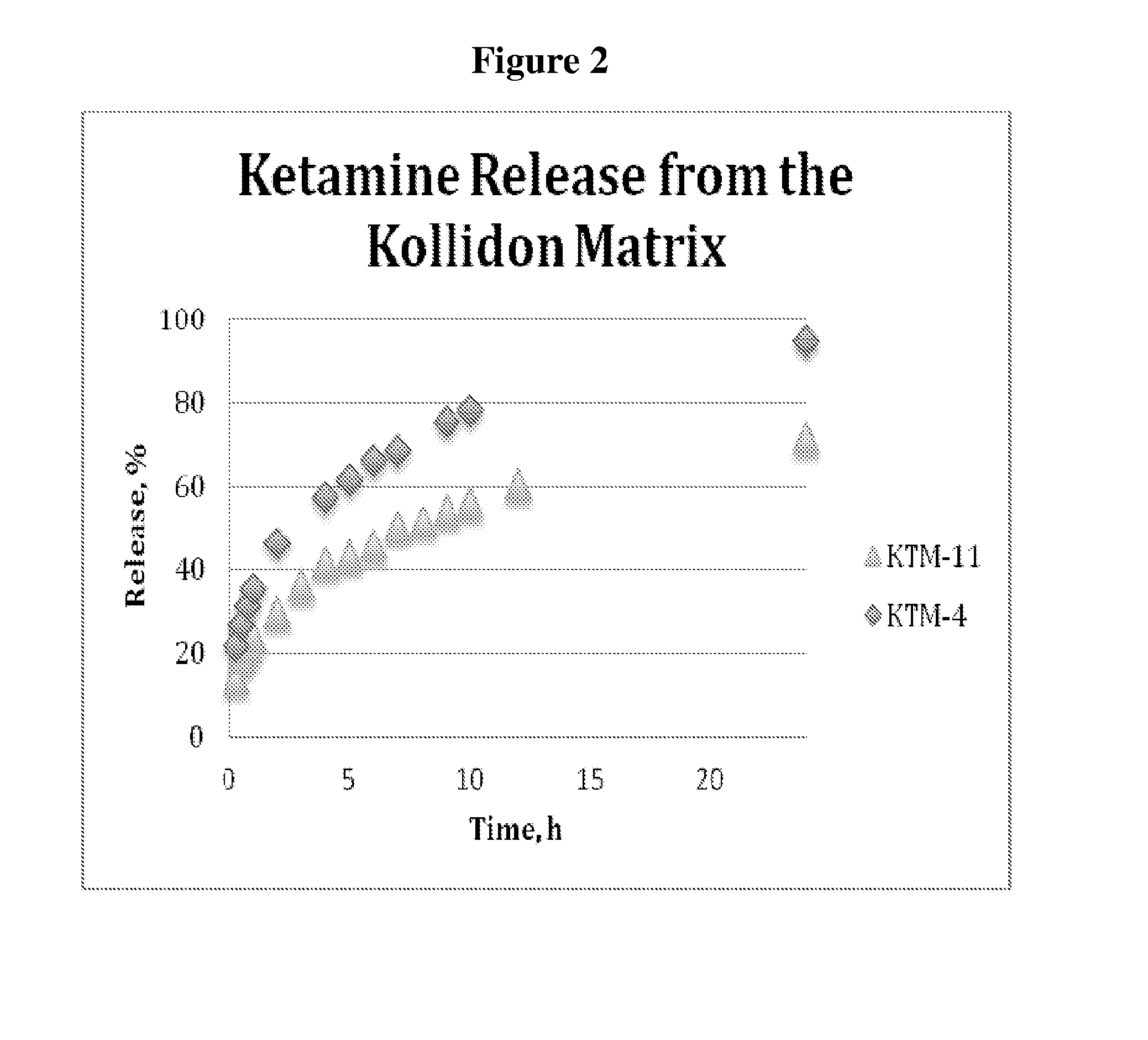
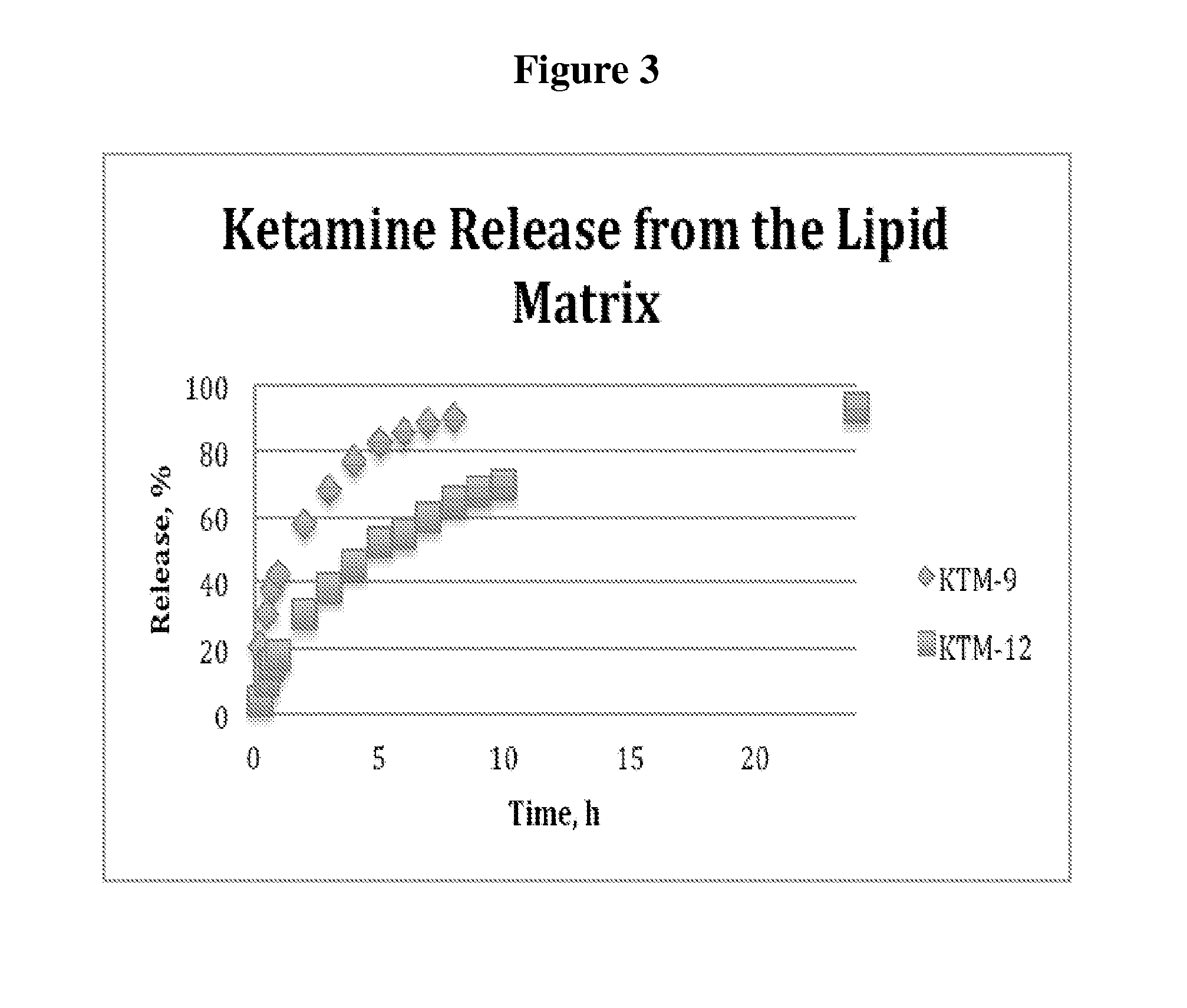
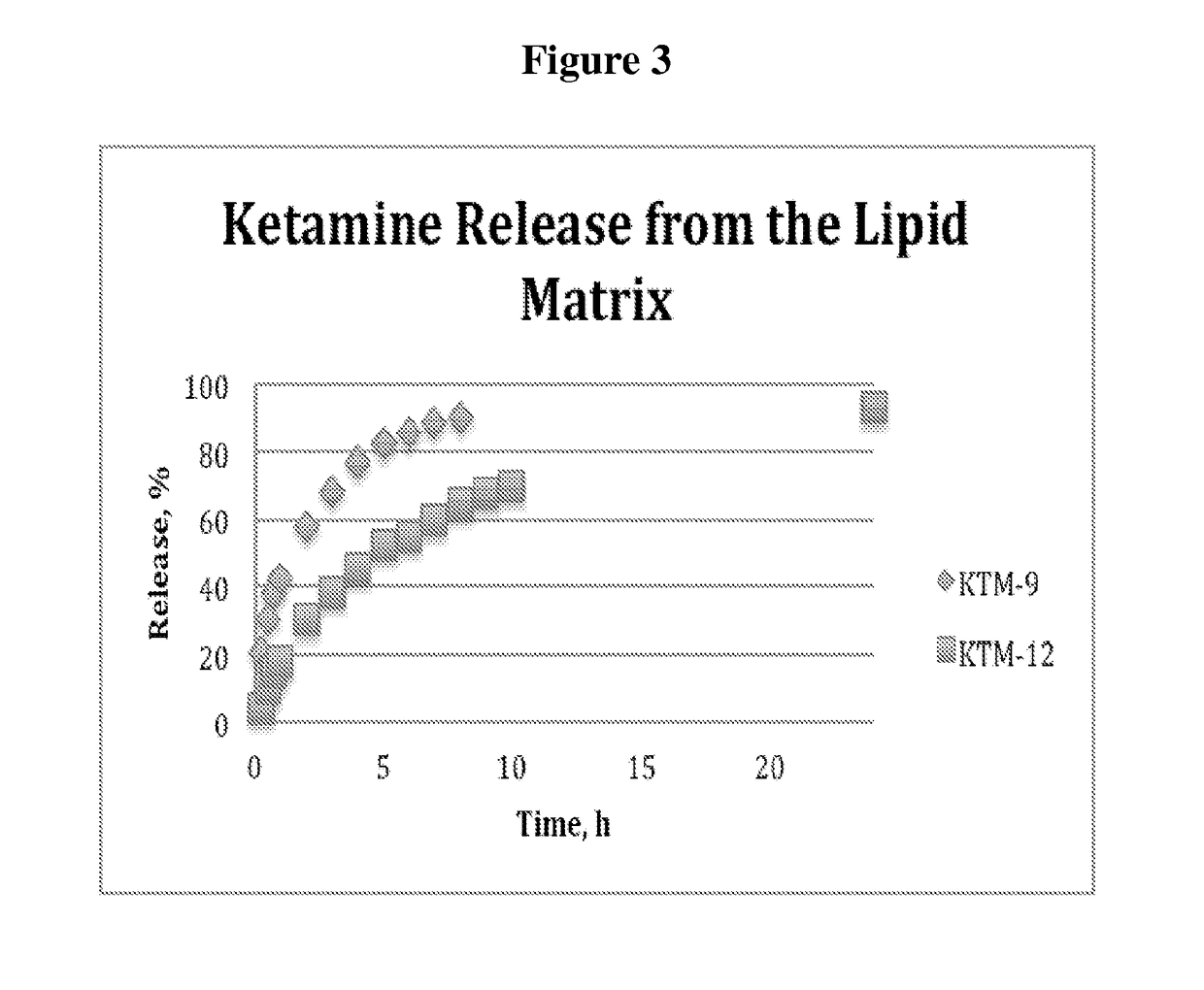
Single-layer oral dose of neuro-attenuating ketamine
The present invention is directed to oral neuro-attenuating ketamine (NAKET) tablet formulations, and methods of administration, which ensure the steady release of a therapeutically effective concentration of ketamine from an oral tablet without neurologically toxic spikes in ketamine concentration. In particular, the present invention provides single layer oral tablet formulation of NAKET. In a specific embodiment, the NAKET tablet formulation, and methods of administration provide steady administration of NAKET to a subject for 24 hours or greater, for example, up to 36 hours, after a single administration event.
Owner:AMORSA THERAPEUTICS
Long-acting colloidal insulin formulation and its preparation
InactiveUS20090110742A1Easy injectionEasy to fillPowder deliveryNanotechTolerabilityLong acting insulin
The invention relates to injectable long-acting insulin formulations for the treatment of types I and II diabetes in humans and animals.The essential object of the invention is to provide an injectable long-acting insulin formulation in the form of a colloidal suspension which is stable, which has a good local tolerance and toxicity compatible with the chronic treatment of diabetics, and which maintains a substantial hypoglycemic effect extending over at least 24 hours after a single administration, e.g. by the subcutaneous route.To achieve this object, the invention relates to a stable aqueous colloidal formulation of insulin-laden nanoparticles of at least one poly(Leu-block-Glu) in which the pH is between 5.8 and 7.0, the osmolarity O (in mOsmol) . . . : 270≦O≦800, and the viscosity v (in mPa.s) is low, namely v≦40. The nanoparticles of poly(Leu-block-Glu) have a mean hydrodynamic diameter Dh such that: 15≦Dh≦40.The invention relates to an antidiabetic drug based on this long-acting insulin formulation and injectable using needles of gauge 29G, 30G or 31G.
Owner:FLAMEL TECHNOLOGIES
Chemical entities that kill senescent cells for use in treating age-related disease
InactiveUS10195213B2Hydroxy compound active ingredientsPharmaceutical delivery mechanismRegimenAge related disease
Owner:UNITY BIOTECHNOLOGY INC
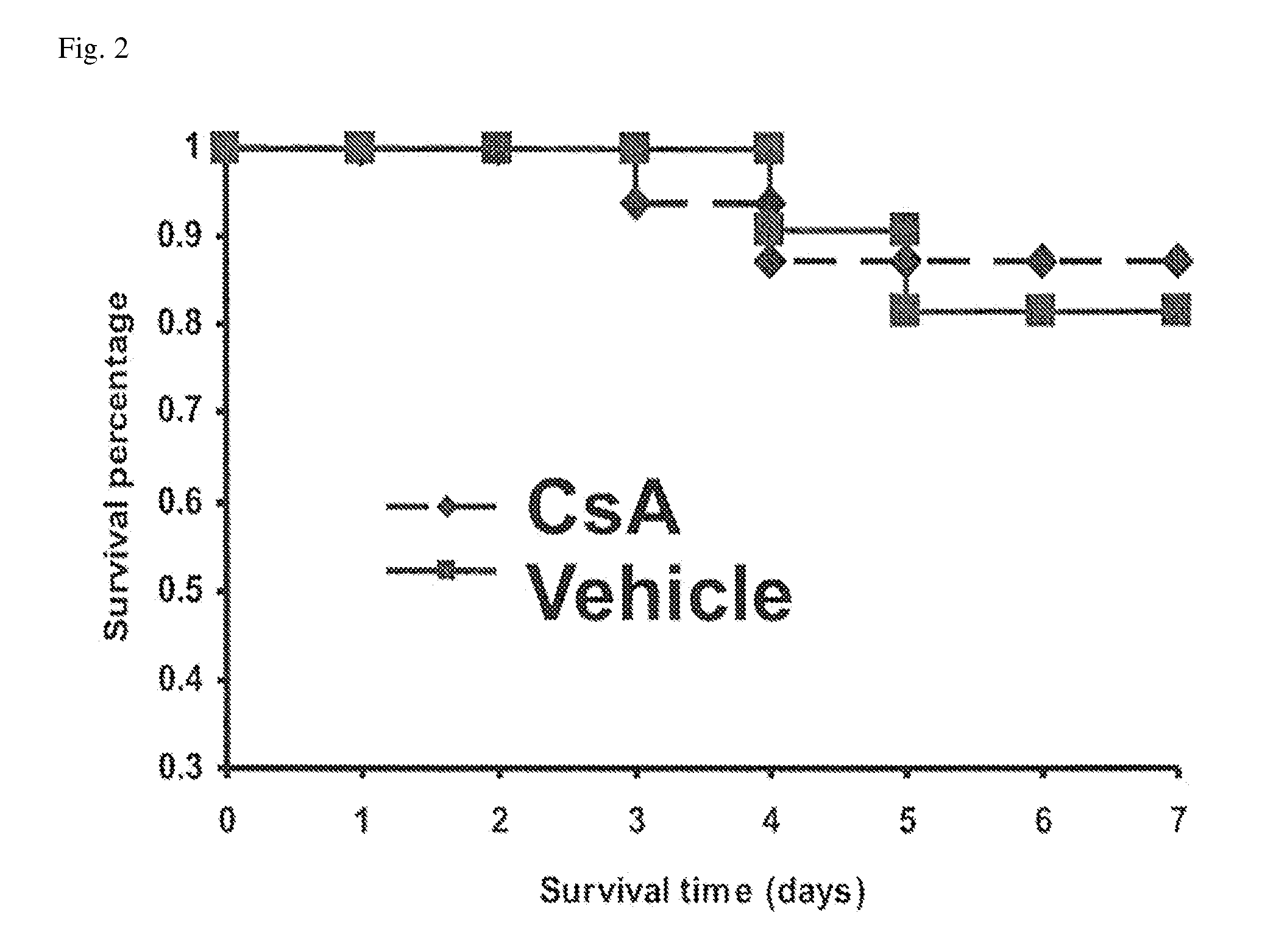
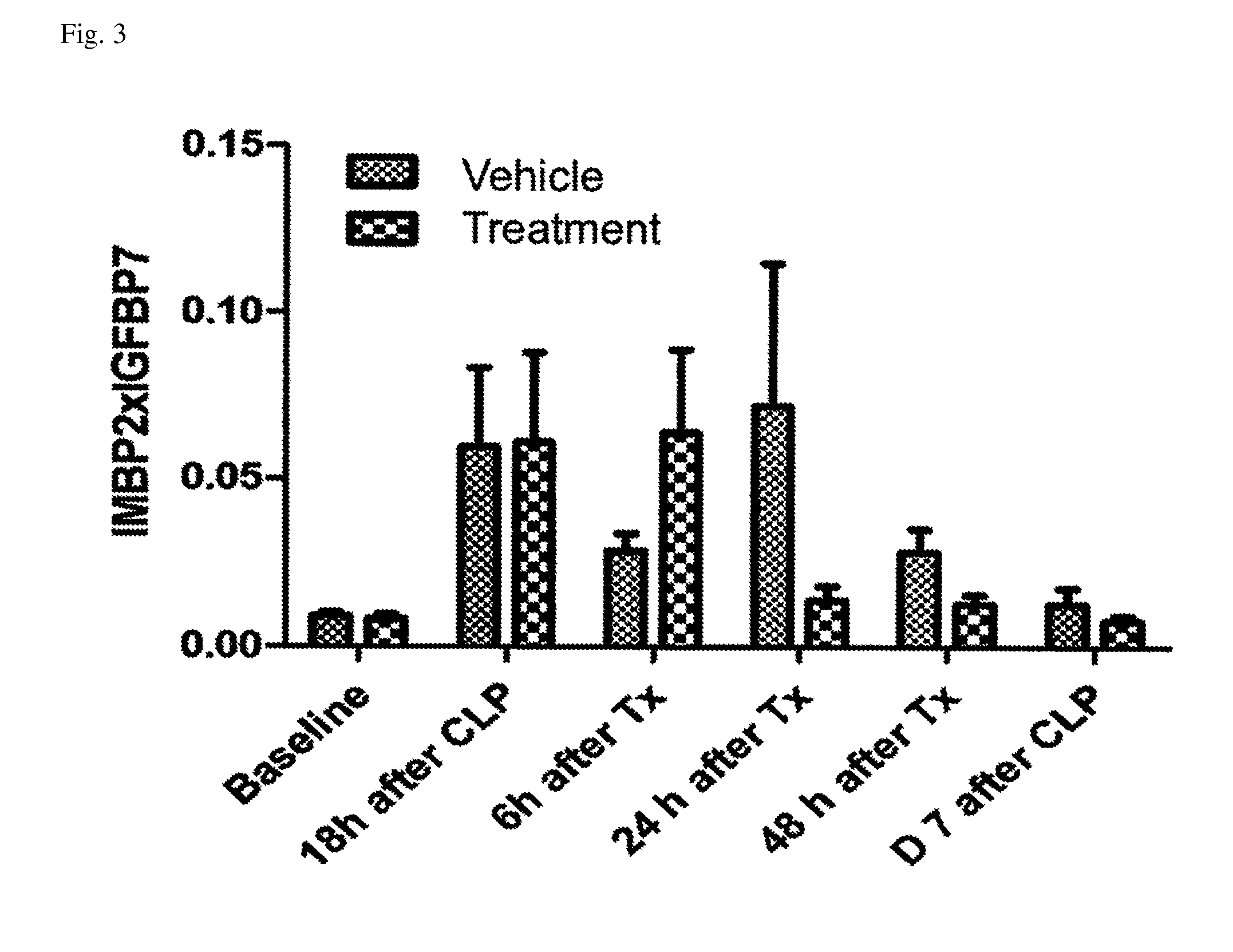
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
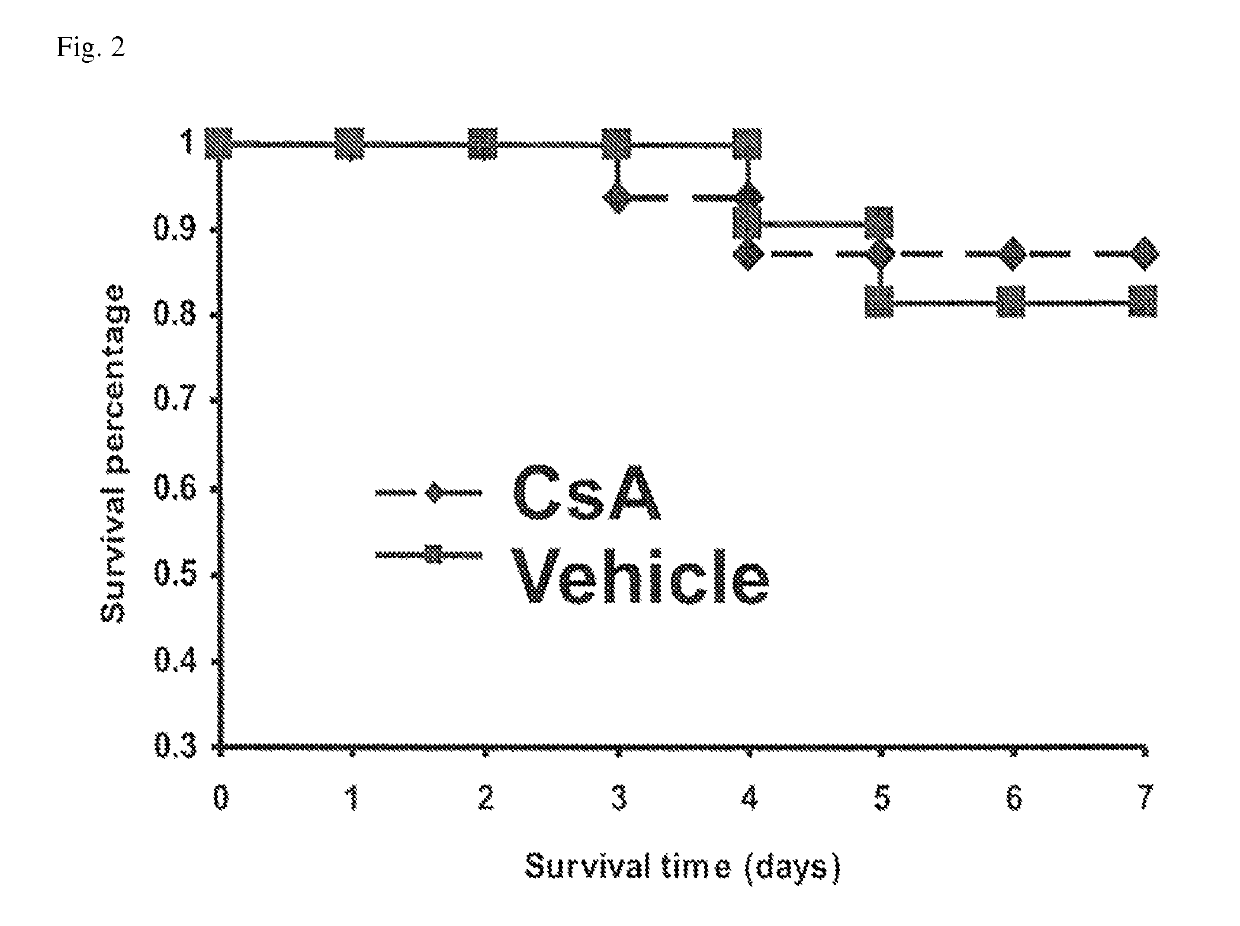
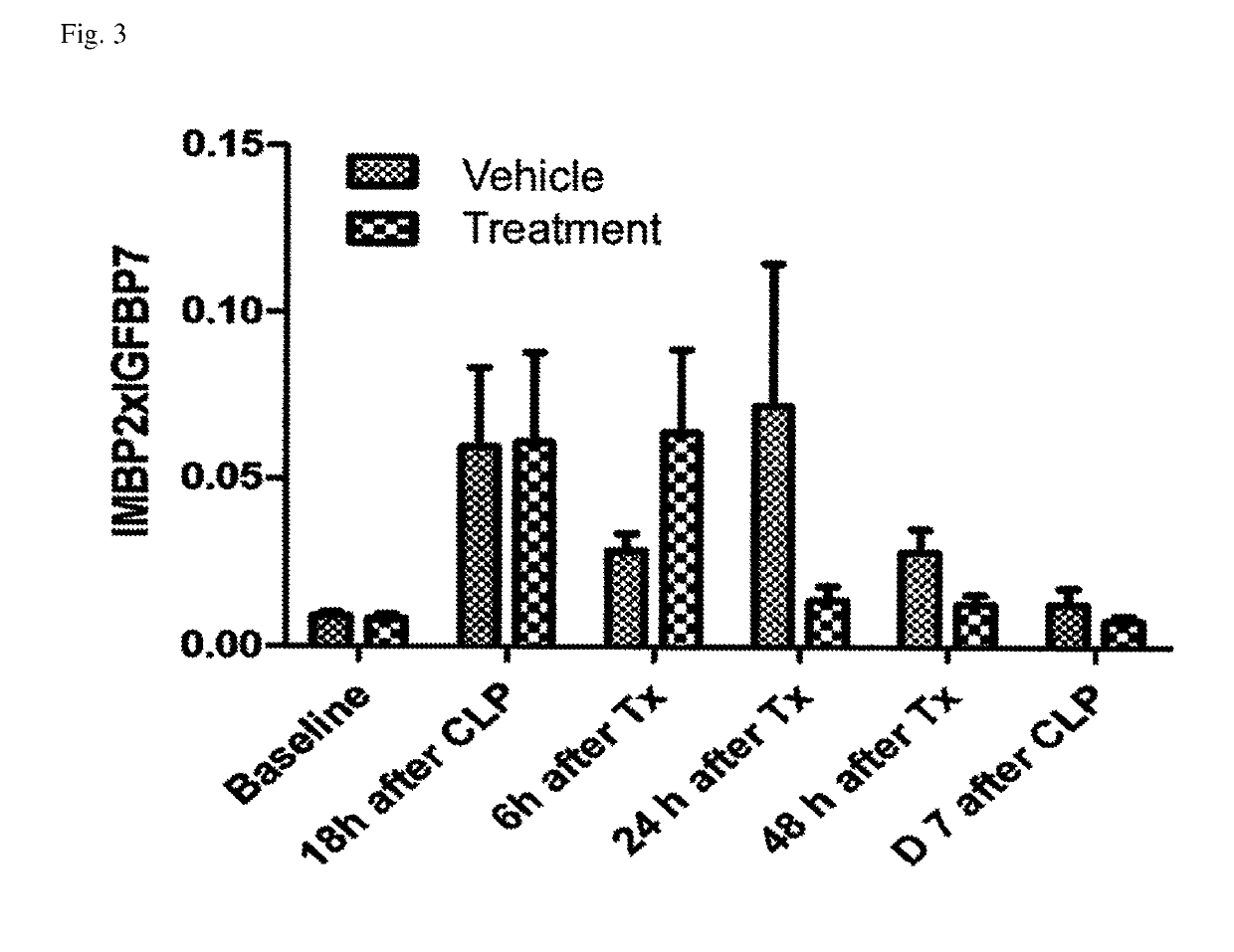
ActiveUS10300108B2Easy to replaceImprovements in renal statusDisease diagnosisCyclic peptide ingredientsRenal FailuresSingle administration
Owner:ASTUTE MEDICAL +1
Fast Release Dosage Forms for Antibiotics
ActiveUS20080050446A1Improve solubilityFast dissolutionAntibacterial agentsBiocideCarrageenanPhosphate
A multiparticulate, pharmaceutical dosage form containing at least one antibiotic which is sparingly wettable with aqueous media or sparingly soluble in aqueous media and a combination of carrageenan and tricalcium phosphate and optionally sucrose ester. Also, an administration system having this dosage form arranged in a drinking straw with at least one barrier device for single administration, optionally together with a conveying liquid.
Owner:DS TECH GMBH
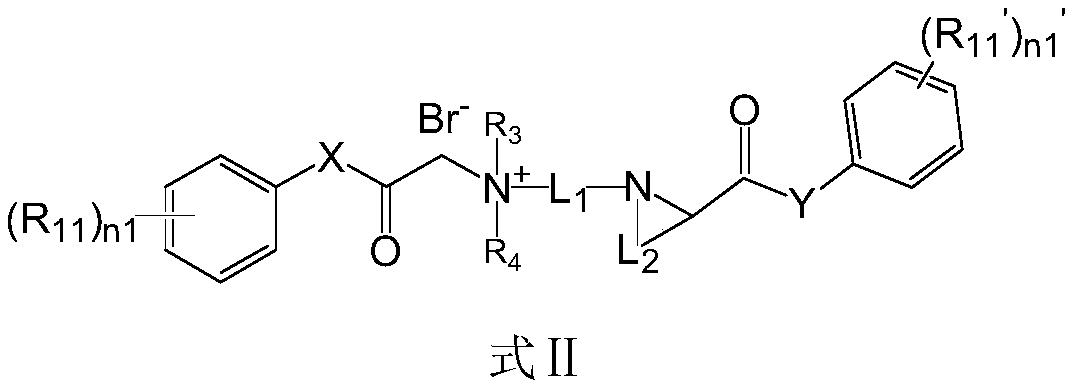
Quaternary ammonium salt compound as well as preparation method and application thereof
The invention discloses a quaternary ammonium salt compound as well as a preparation method and application thereof, and belongs to the field of chemical medicines. The present invention provides a compound represented by a formula I, or a pharmaceutically acceptable salt, a stereoisomer, a solvate, a prodrug, or a metabolite thereof. The compound takes effect fast, a long-time local anesthesia effect is achieved after single administration, the sensory nerve blocking time is longer than the motor nerve blocking time, the long-acting local anesthetic effect and the selective local anesthetic effect are achieved, and meanwhile, the side effect of the quaternary ammonium salt compound with the structural characteristics of a surfactant is obviously reduced. The compound has high safety, namely, the compound shown in the formula I and the pharmaceutically acceptable salt thereof can be used for preparing safe medicines with long-time local anesthesia and selective local anesthesia effects. The compound has the advantages of long local anesthesia action time, good local anesthesia selectivity and high safety.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Pharmaceutical composition taking metformin hydrochloride and vigelegting as active component as well as preparing method and uses thereof
The invention relates to a pharmaceutical composition which takes metformin hydrochloride and vildagliptin as the active ingredients and the preparation method and usage thereof. The invention takes metformin hydrochloride and vildagliptin as the pharmaceutical active ingredients and mixed with pharmaceutically acceptable excipients to form the pharmaceutical composition. The invention can be used for diet control and the treatment of type 2 diabetes can not be controlled appropriately by movements, and the invention can also be used in the type 2 diabetes which can not be controlled by the single administration of metformin hydrochloride or vildagliptin. The contents of the invention take metformin hydrochloride and vildagliptin as the raw materials and are added with given type and proportion of excipients, then the invention is prepared and developed into tablets, capsules, granules, dispersion tablets, chew tablets, buccal tablets, effervescent tablets, effervescent granules and other various oral preparations according to the technical means illustrated by the invention.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Cationic compound and preparation method and application thereof
ActiveCN110156666AQuick effectImprove securityOrganic compound preparationCarboxylic acid amides preparationMetaboliteSide effect
The invention discloses a cationic compound and a preparation method and application thereof. The invention provides a compound shown in a formula I with a novel structure, or a pharmaceutically acceptable salt thereof, or a stereoisomer thereof, or a solvate thereof, or a precursor drug thereof, or a metabolite thereof. The compound has the advantages of quick effect, long-time local anesthesia effect after single administration, sensory nerve blocking time being longer than motor nerve blocking time, and both long-term local anesthesia effect and selective local anesthesia effect, remarkablyreduces side effects of QX314 and QX314 compositions and cationic compounds with surfactant structural characteristics, and has better safety. Namely, the compound of formula I and the pharmaceutically acceptable salt thereof can be used for preparing safe drugs with long-term local anesthesia and selective local anesthesia effects, and have the advantages of long-term local anesthesia, good local anesthesia selectivity, less nerve damage and high safety.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Synthetic peptide-based emergency vaccine against foot and mouth disease (FMD)
ActiveUS20150306203A1Reduce manufacturing costQuality improvementSsRNA viruses positive-sensePeptide/protein ingredientsEpitopeAnimal use
Synthetic FMD peptide immunogens and compositions containing the same are disclosed. Methods for detecting, treating, and preventing an FMD infection in an animal using the synthetic FMD peptide immunogens are also disclosed. In a specific embodiment, a peptide-based emergency vaccine and formulations thereof against Foot and Mouth Disease is described. Various vaccine formulations contain a mixture of peptides derived from FMDV VP1 protein; each peptide containing a B cell FMDV neutralizing / receptor binding epitope sequence linked to an artificial Th epitope to enhance the immunogenicity of each peptide. Disclosed vaccine formulations containing viral immunogens can optionally be supplemented with a mixture of peptides representing the FMDV endogenous Th epitopes derived from FMDV proteins, homologues and functional analogues thereof. Such viral peptide compositions are prepared in an acceptable delivery system as vaccine formulations and can provide protection pigs and cattle from infection upon FMDV challenge with only single administration.
Owner:UNITED BIOMEDICAL INC
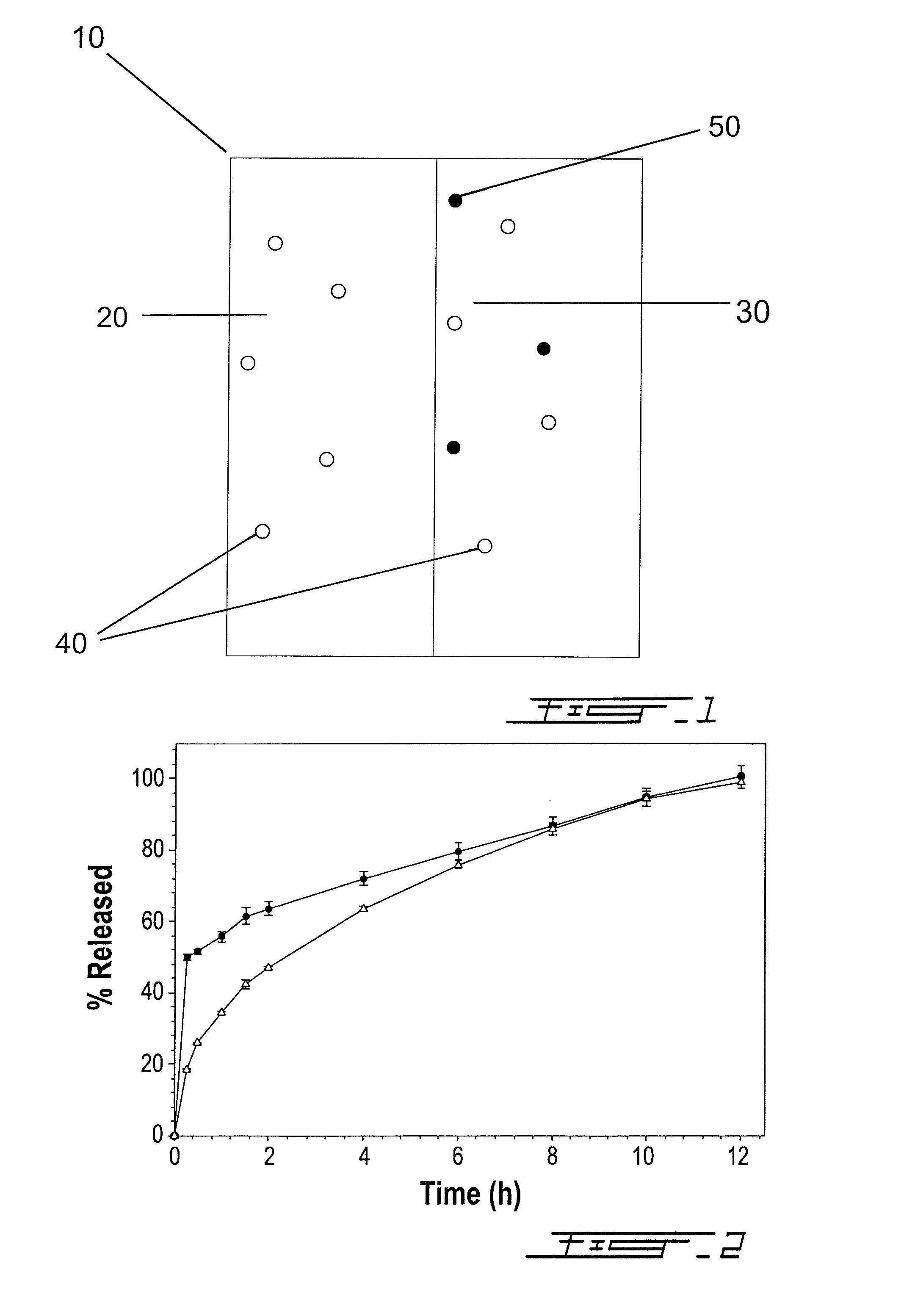
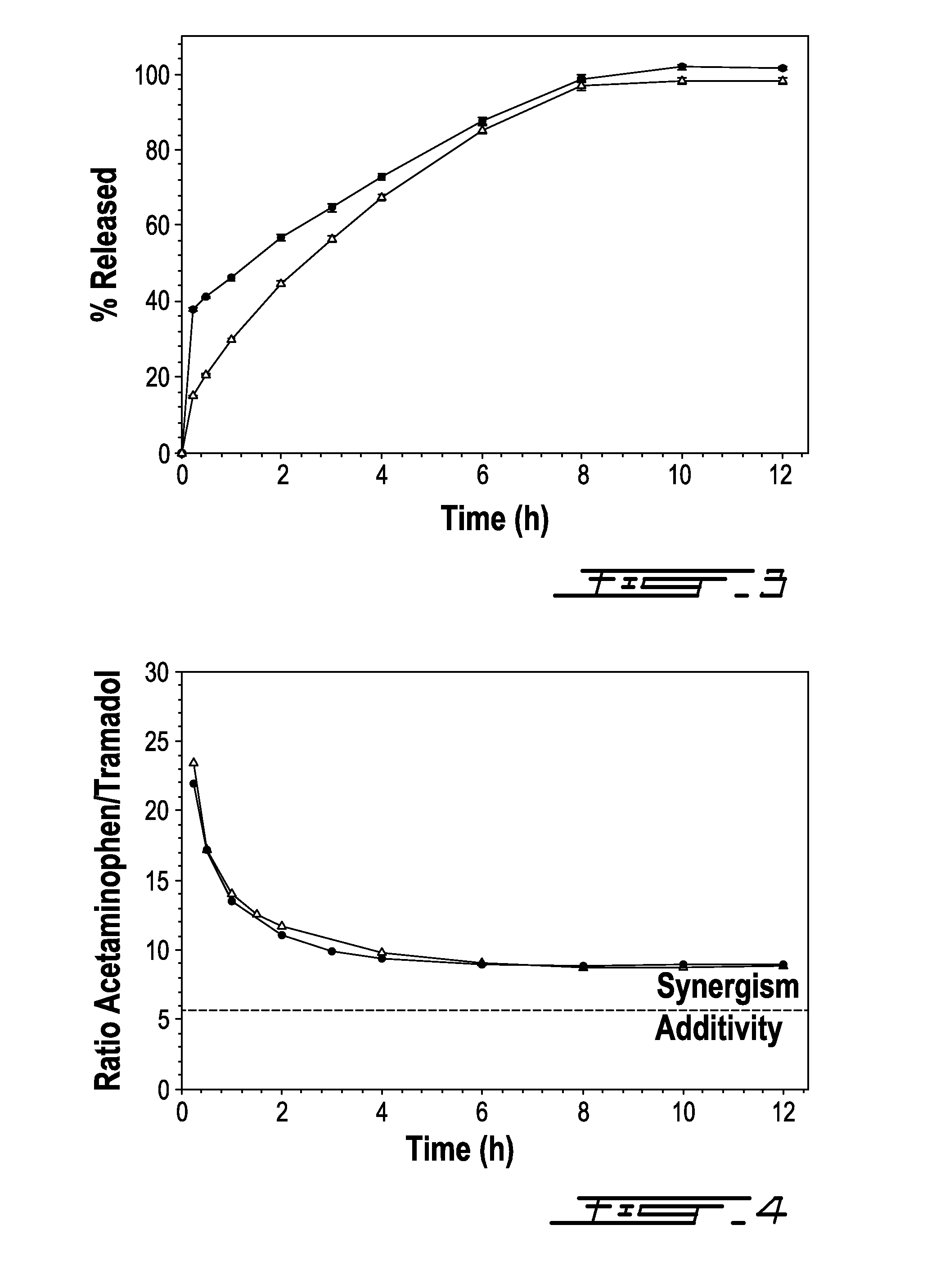
Bilayer composition for the sustained release of acetaminophen and tramadol
ActiveUS8895066B2Rapid and sustained analgesic reliefBiocideNervous disorderAcetamido-CNUMedicinal chemistry
The invention relates to a bilayer composition for the delivery of acetaminophen and tramadol over at least a twelve hour period following initial administration. A single administration of the bilayer composition can provide analgesia starting in less than half an hour to about one hour after initial administration with a duration of at least twelve hours after initial administration.
Owner:LABOPHARM BARBADOS LTD 36646
Bilayer Composition for the Sustained Release of Acetaminophen and Tramadol
ActiveUS20090130183A1Rapid and sustained analgesic reliefBiocideNervous disorderSingle administrationTramadol
The invention relates to a bilayer composition for the delivery of acetaminophen and tramadol over at least a twelve hour period following initial administration. A single administration of the bilayer composition can provide analgesia starting in less than half an hour to about one hour after initial administration with a duration of at least twelve hours after initial administration.
Owner:LABOPHARM BARBADOS LTD 36646
Liquid preparation for oral administration used in CT colonography, and composition for imaging digestive tract
InactiveCN103237563AReduce the burden onEasy to manufactureX-ray constrast preparationsSolution deliveryColonic irrigationIntrathecal
The present invention provides a liquid preparation for oral administration that is capable of reducing the amount of colonic irrigation solution taken during gastrointestinal pretreatment and decreasing the burden on the subject without sacrificing test accuracy and without prolonging the time required for pretreatment, and a composition for gastrointestinal radiography used to prepare the liquid preparation for oral administration. Namely, provided are a liquid preparation for oral administration taken during gastrointestinal imaging by CT colonography that comprises an iodine compound and a water-soluble polymer or saline laxative, wherein 300 mL to 1200 mL are taken on the day before CT colonography in a single administration or divided among several administrations, and a composition for gastrointestinal radiography comprising an iodine compound and a water-soluble polymer or saline laxative, wherein 300 mL to 1200 mL are taken in the form of an aqueous solution, having an iodine content of 3.5 mg / mL to 90 mg / mL prepared by dissolving in water, on the day before CT colonography in a single administration or divided among several administrations.
Owner:EA PHARMA CO LTD
Dry powder inhalant of interferon alpha
ActiveCN102716469APeptide/protein ingredientsPharmaceutical delivery mechanismInterferon alphaAndrology
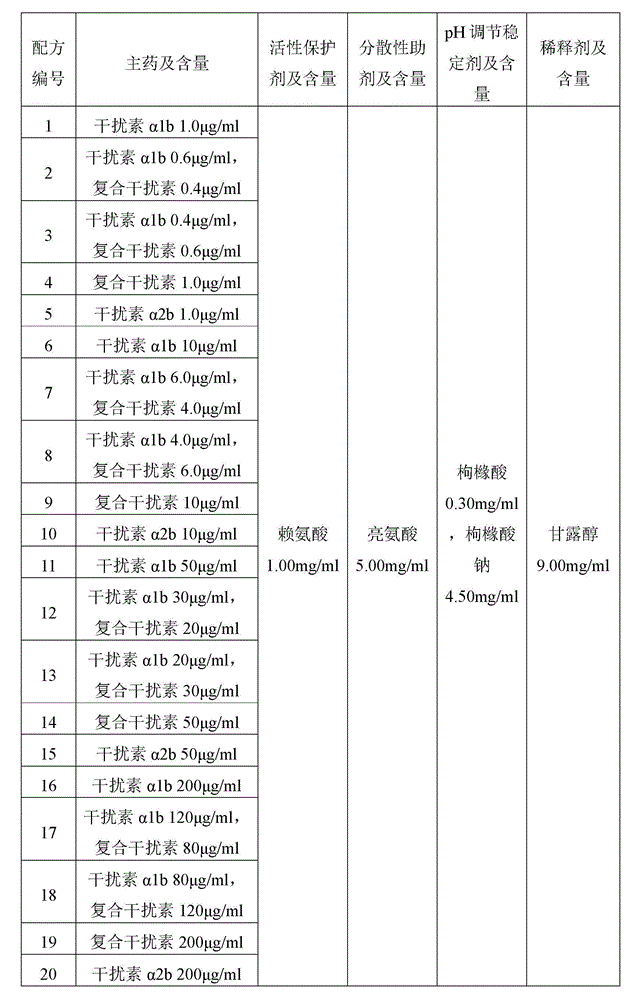
The invention belongs to the field of preparations of protein drugs, and relates to a dry powder inhalant of interferon alpha. The dry powder inhalant contains consensus interferon of treatment effective dose and pharmaceutic adjuvant of the dry powder inhalant of proper dose; the interferon alpha consists of interferon alpha1b and the consensus interferon at a weight ratio of (4:6)-(6:4); and the pharmaceutic adjuvant of the dry powder inhalant comprises one or more of active protective agent, dispersive assistant, pH stability regulator, diluent and / or large-granularity carrier. In an optimized scheme, the dry powder inhalant of interferon alpha in single administration contains 1-200mu g of interferon alpha. The dry powder inhalant of interferon alpha provided by the invention has better stability and safety than the dry powder inhalant of interferon alpha of the prior art.
Owner:BEIJING TRI PRIME GENE PHARMA CO LTD
Mycoplasma hyopneumoniae bacterin vaccine
The invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration. The composition comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity. In a preferred embodiment, the adjuvant mixture comprises an acrylic acid polymer, most preferably CARBOPOL®, and a mixture of a metabolizable oil such as one or more unsaturated terpene hydrocarbons, preferably squalene or squalane, and a polyoxyethylene-polyoxypropylene block copolymer such as PLURONIC®. The vaccine composition may optionally include a preservative, preferably thimerosol and / or EDTA.
Owner:ZOETIS SERVICE LLC
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS20160303187A1Easy to replaceImprovements in renal statusDisease diagnosisCyclic peptide ingredientsRenal FailuresSingle administration
It is an object of the present invention to provide methods and compositions for protection of subjects from acute kidney injury by treating the subject with compounds that modulate the cell cycle. Modulating the cell cycle can comprise inducing G0 / G1 cell cycle arrest, and / or inducing cell cycle progression. As demonstrated below, even a single administration of a compound which induces G0 / G1 cell cycle arrest can protect subjects from AKI, and may be used prophylactically in advance of, or as a treatment following, various treatments or conditions that are known to be injurious to the kidney, followed optionally by release of the arrest. Once AKI is established, cell cycle progression can be induced to increase replacement of lost and damaged cells
Owner:ASTUTE MEDICAL +1
Pharmaceutical composition for relieving pain
ActiveUS20120142629A1Early onsetRapid pain improvement effectOrganic active ingredientsBiocideCross-linkDisease
The present invention relates to a pharmaceutical composition for relieving pain in a joint disease, including a hyaluronic acid and a pharmaceutically acceptable carrier, in which the hyaluronic acid is cross-linked by cyclizing a double bond in the moiety of a cinnamic acid in a partially amidated hyaluronic acid represented by Formula (1): [Ar—CH═CH—COO—(CH2)n—NH-]m-HA, to form a cycloubutane ring, in which Ar represents an optionally substituted phenyl group, n represents an integer of 2 or 3, HA represents a carboxy residue of the hyaluronic acid, and m represents an amidation ratio of the hyaluronic acid to the total carboxyl group and is in the range of 3 to 50% relative to the total carboxyl group.The pharmaceutical composition of the present invention is an intra-articular formulation that exerts rapid analgesic effects after administration, and shows extremely long durable effects for a human joint disease with only a single administration rather than multiple administrations of a conventional way.
Owner:SEIKAGAKU KOGYO CO LTD
Single-layer oral dose of neuro-attenuating ketamine
The present invention is directed to oral neuro-attenuating ketamine (NAKET) tablet formulations, and methods of administration, which ensure the steady release of a therapeutically effective concentration of ketamine from an oral tablet without neurologically toxic spikes in ketamine concentration. In particular, the present invention provides single layer oral tablet formulation of NAKET. In a specific embodiment, the NAKET tablet formulation, and methods of administration provide steady administration of NAKET to a subject for 24 hours or greater, for example, up to 36 hours, after a single administration event.
Owner:AMORSA THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com