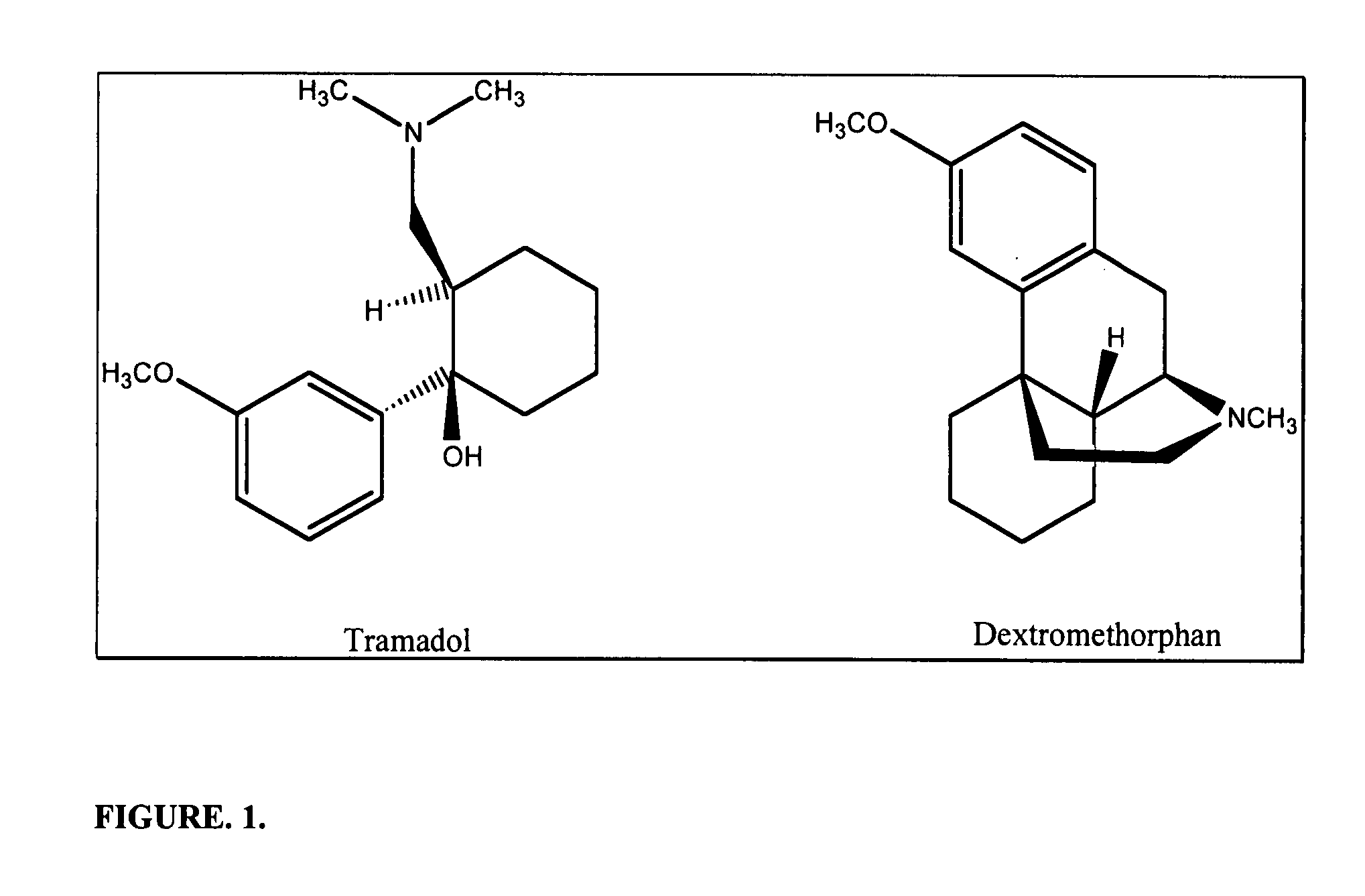
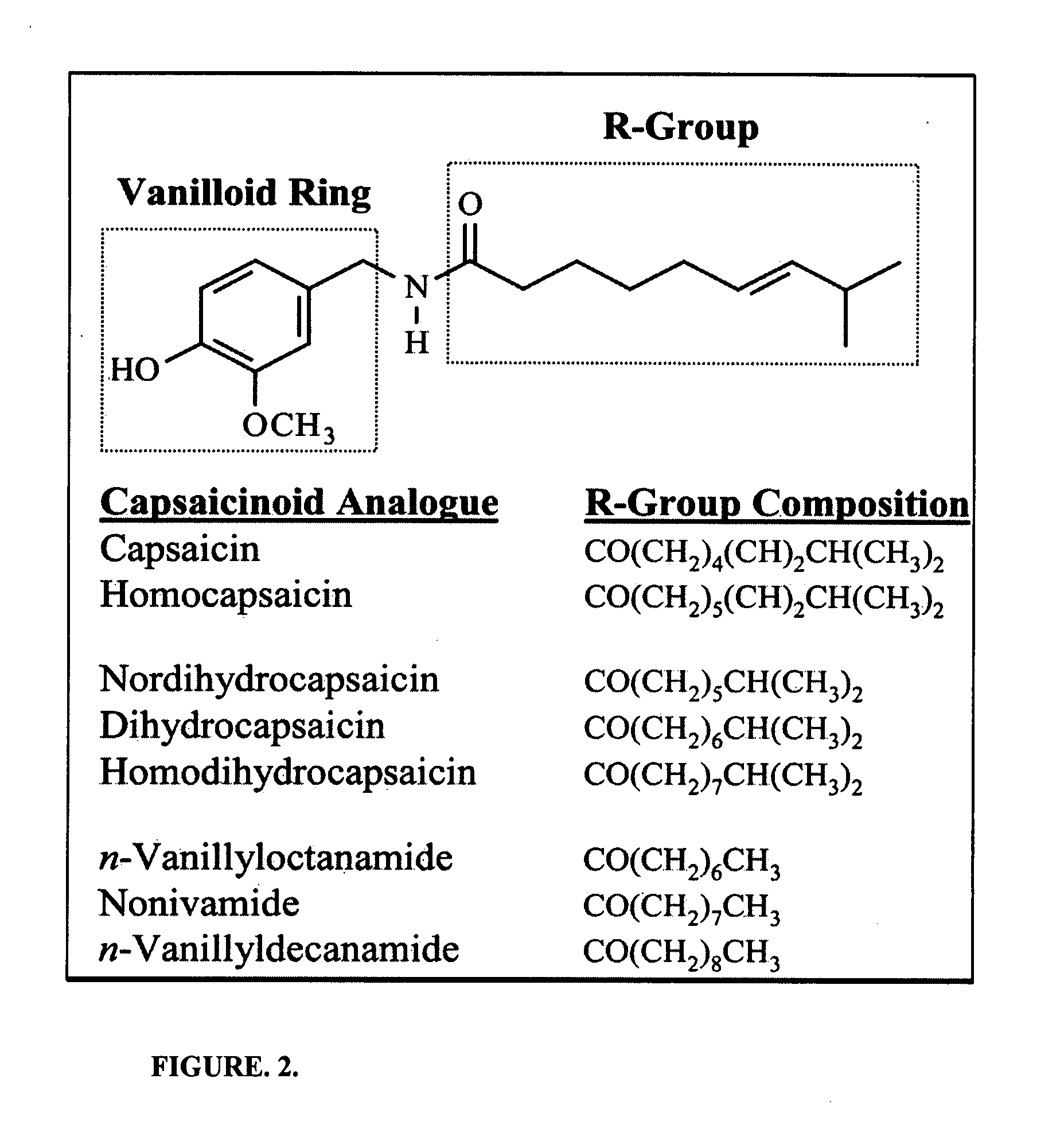
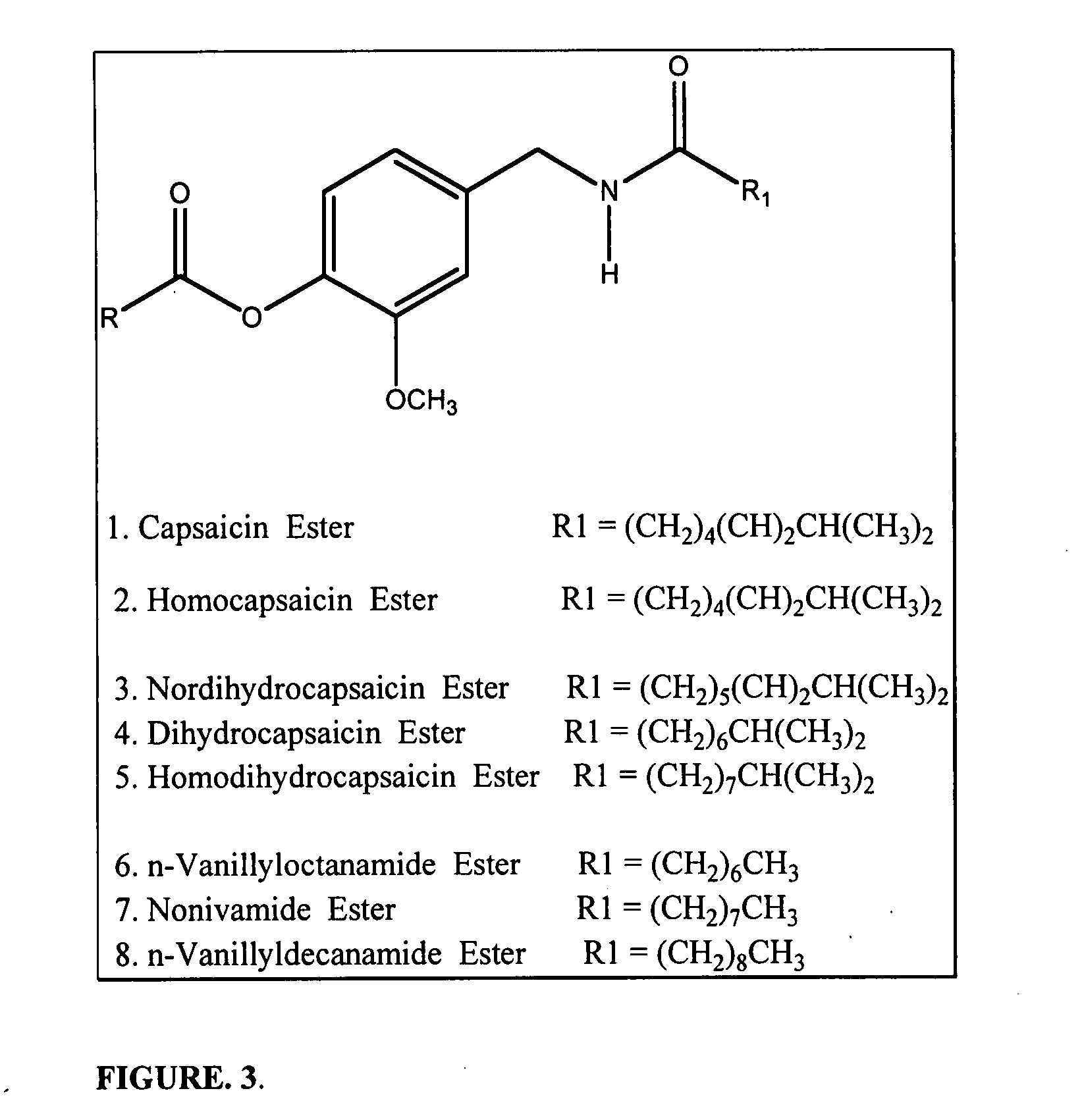
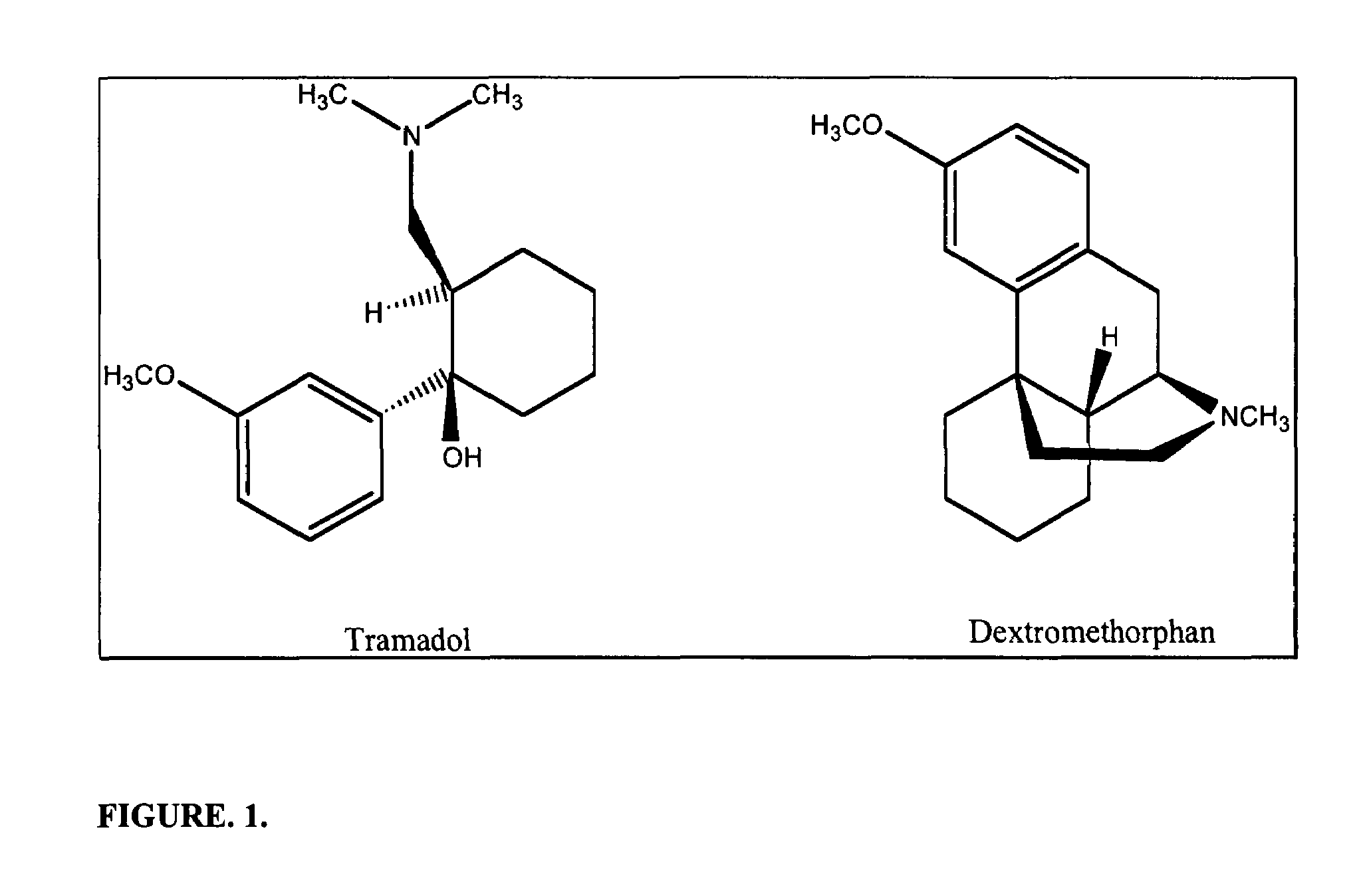
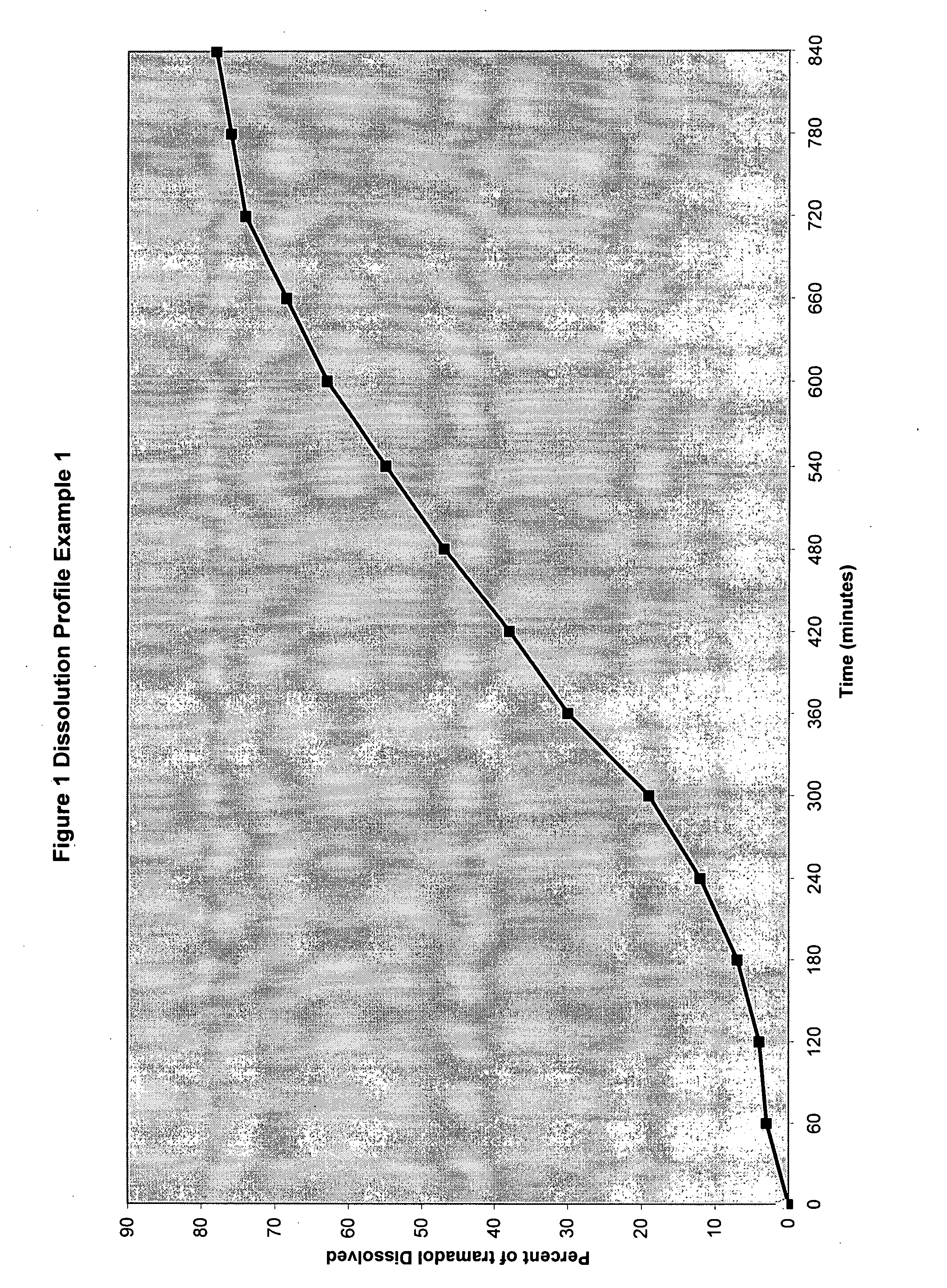
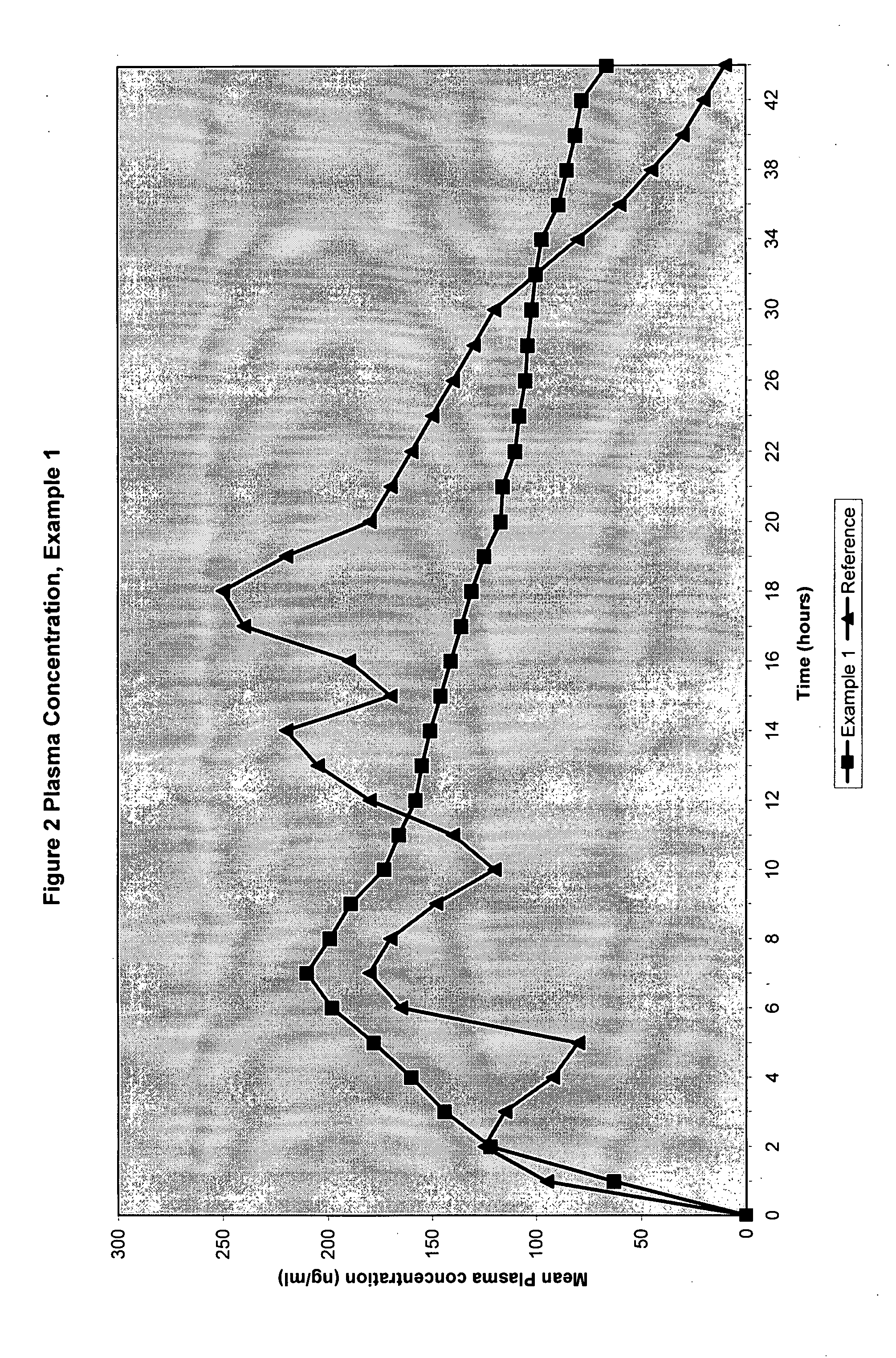
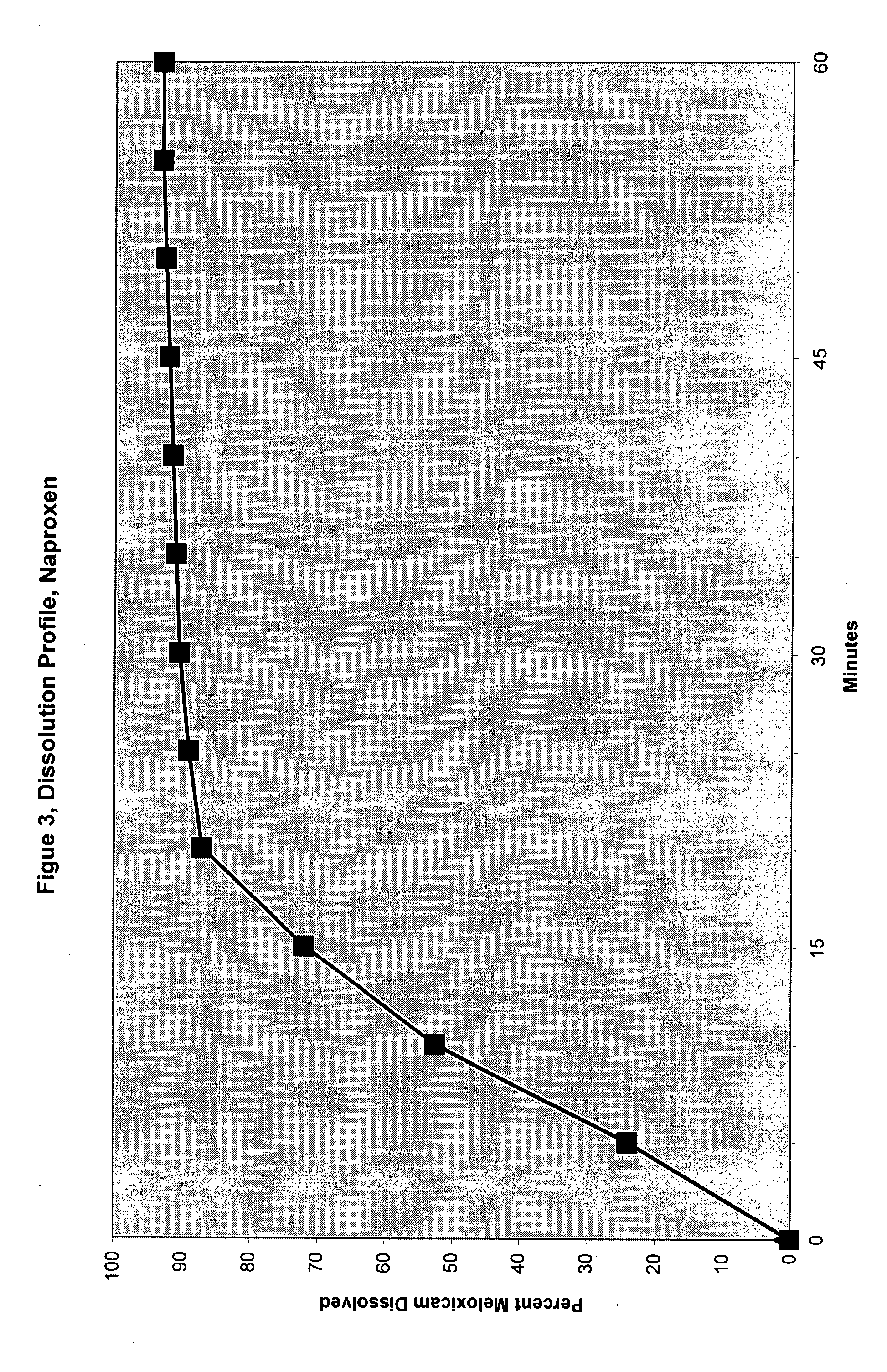
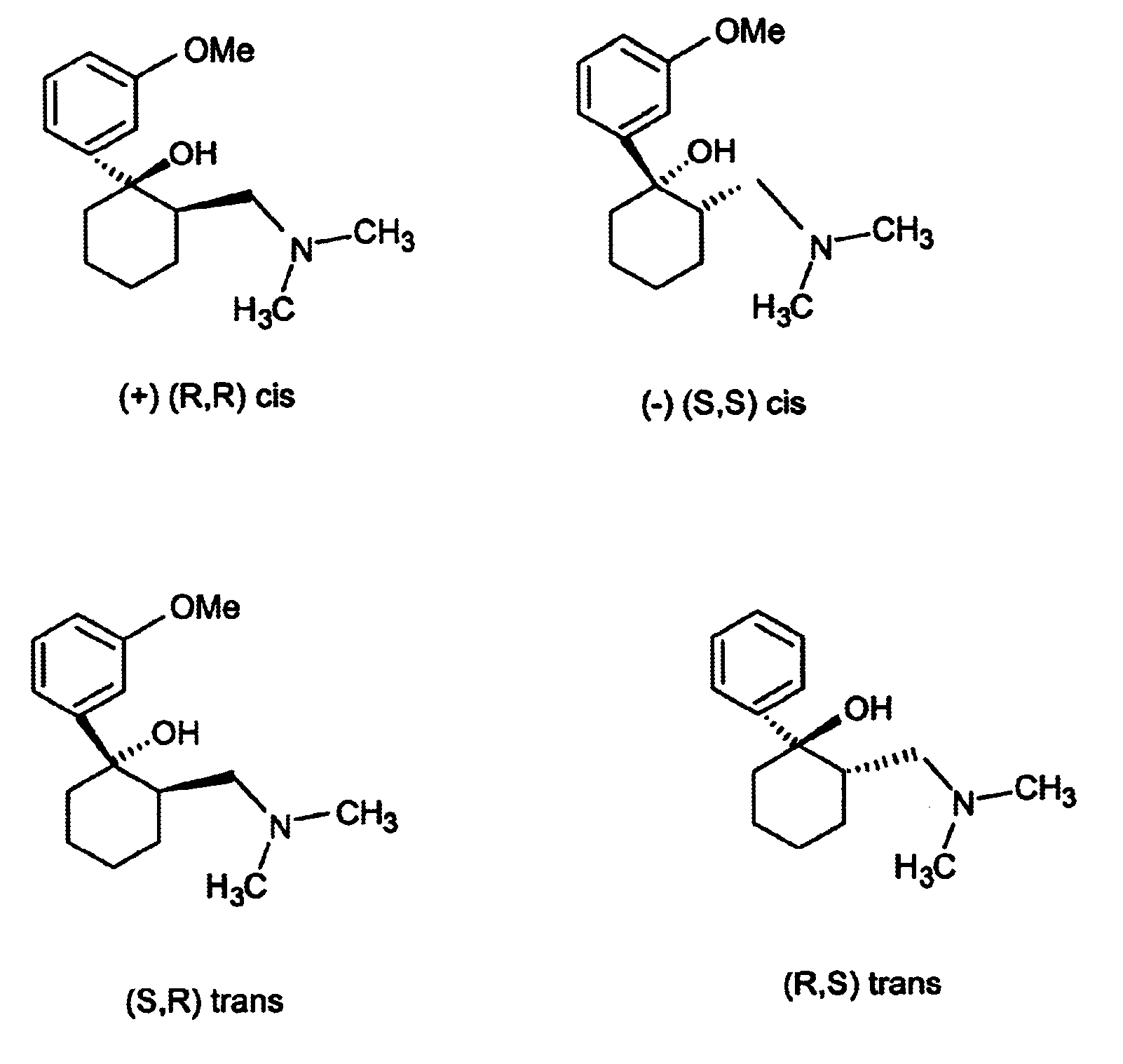
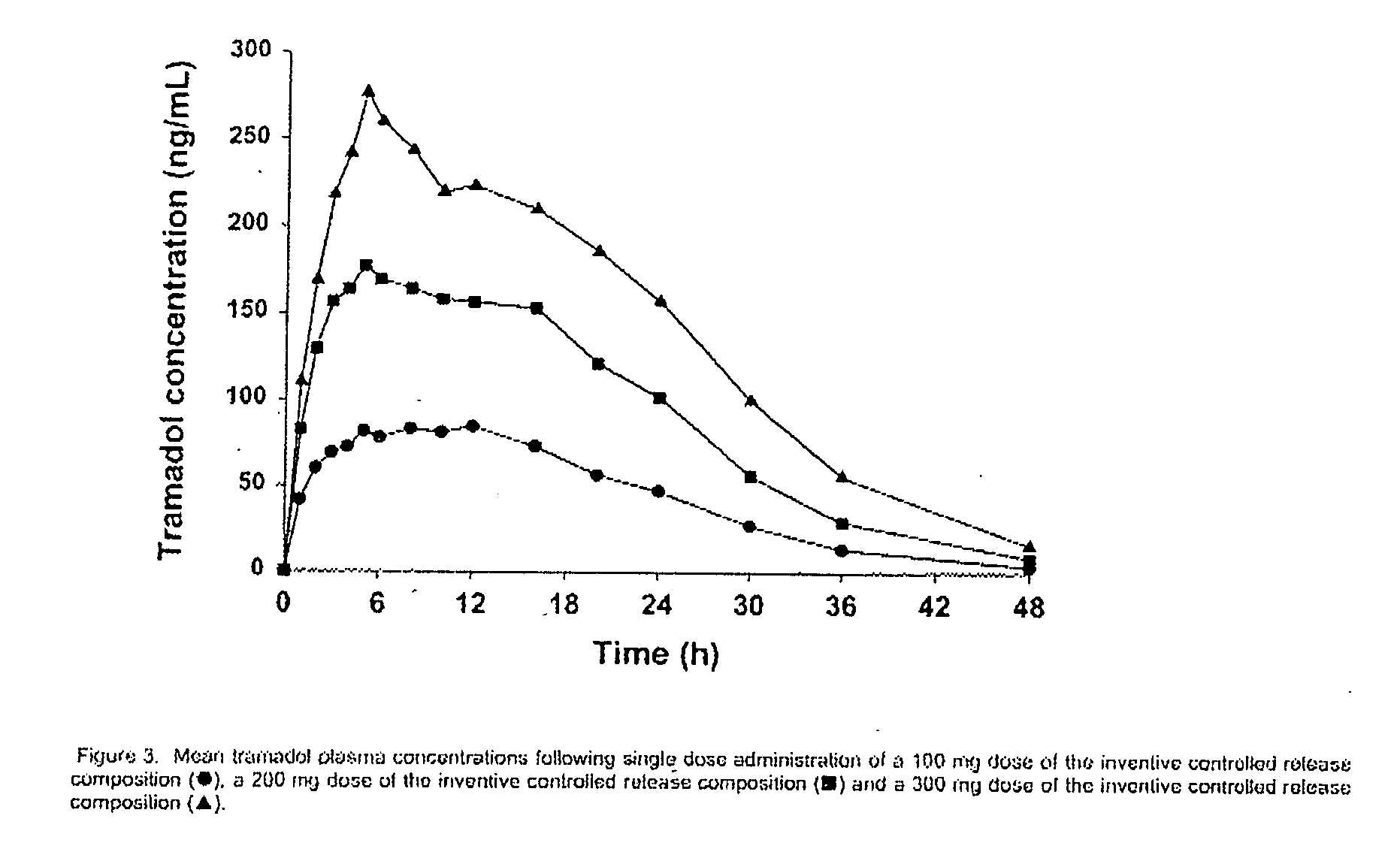
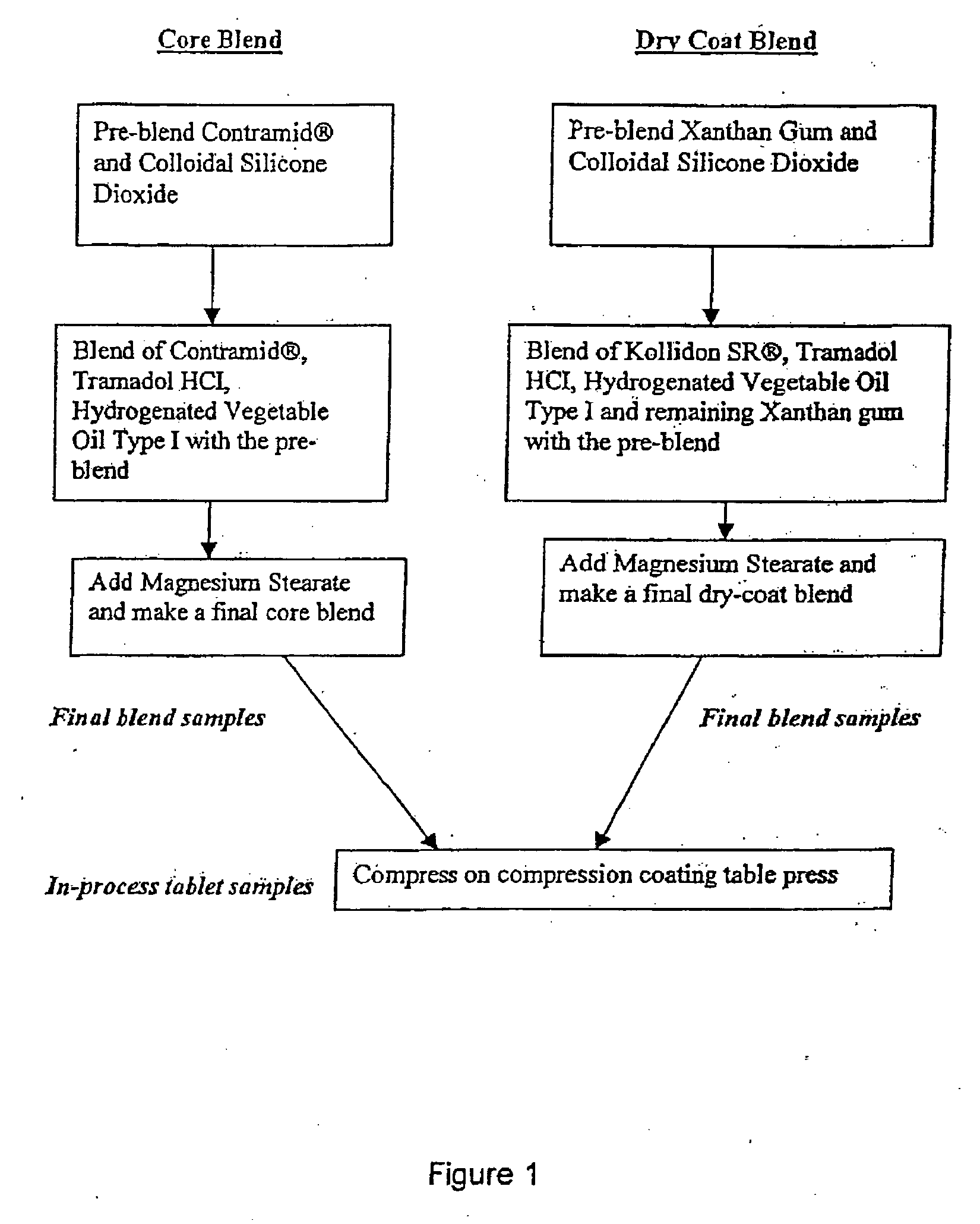
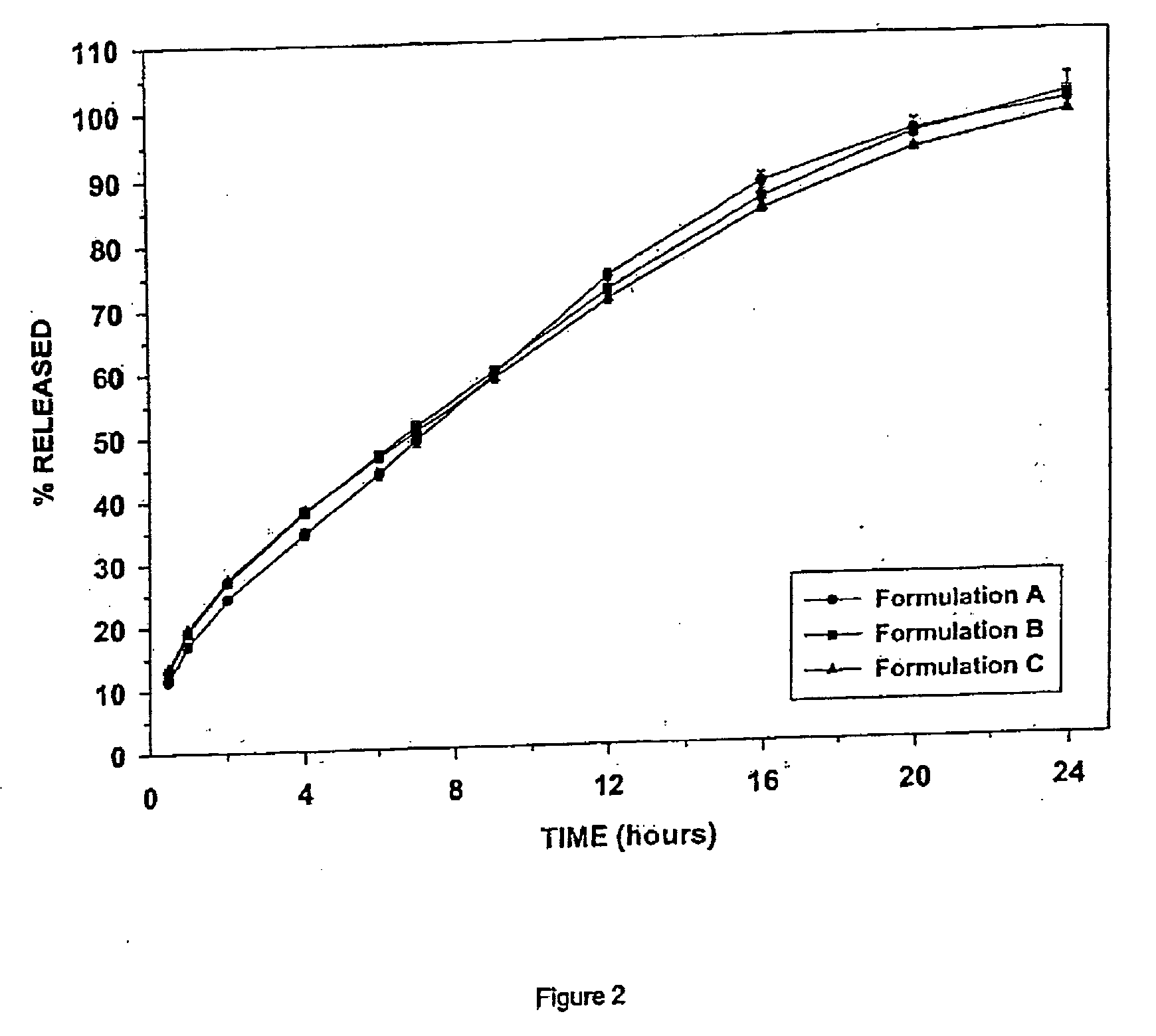
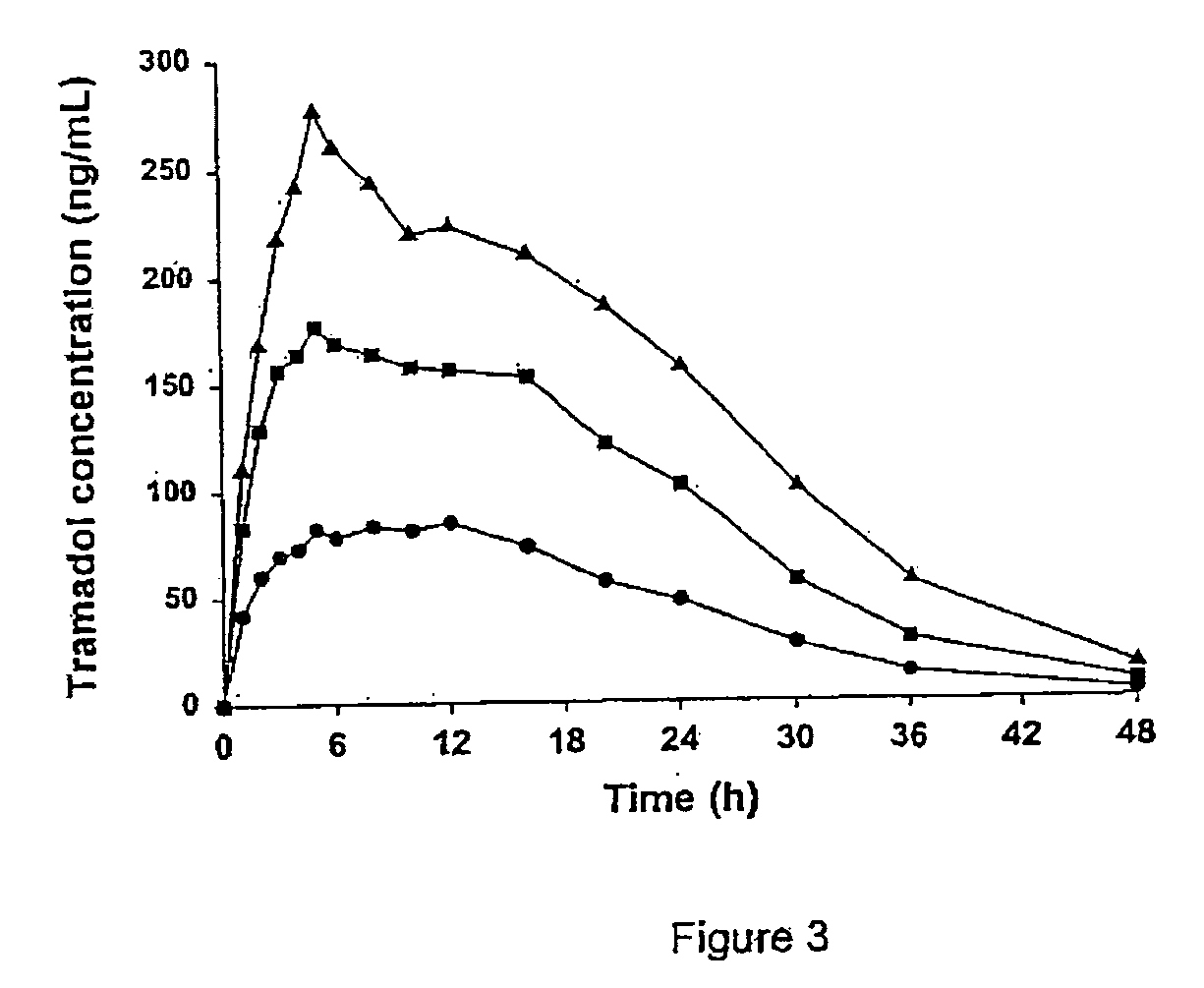
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
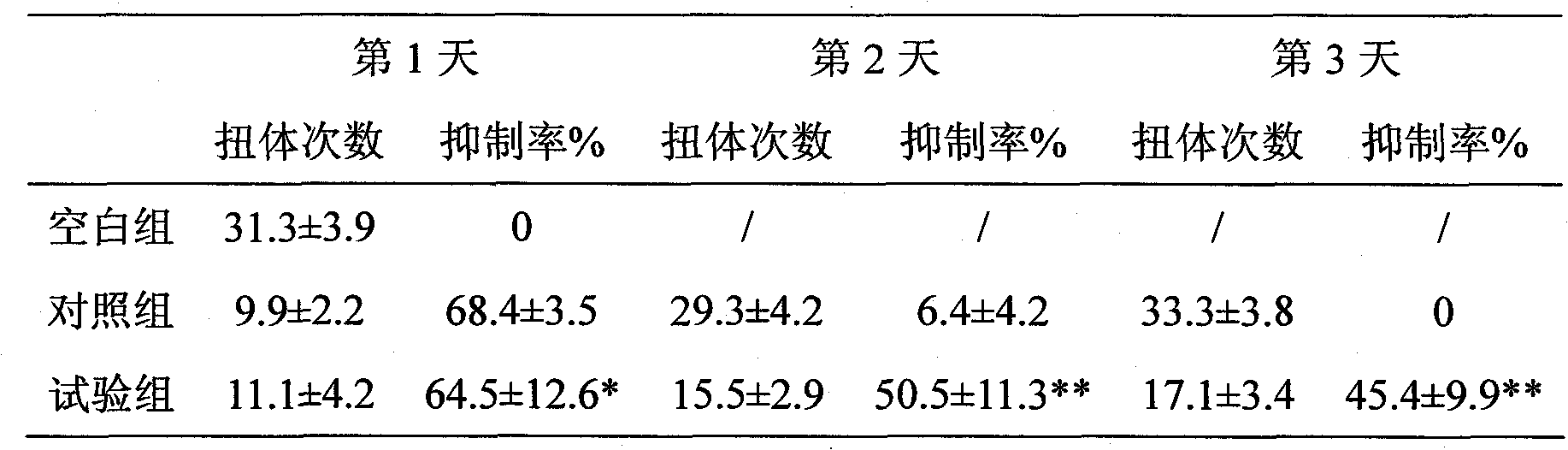
117 results about "Tramadol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to help relieve moderate to moderately severe pain. Tramadol is similar to opioid (narcotic) analgesics. It works in the brain to change how your body feels and responds to pain..
Formulation for intranasal administration
InactiveUS6017963AImprovement in flow property and dispensing accuracyPowder deliveryBiocideInhalationAnalgesic agents
A dosage form for intranasal and / or inhalation administration of an analgesic having low opioid receptor binding activity to a warm blooded animal, that includes a pharmaceutically effective amount of the analgesic. A preferred analgesic is tramadol, a tramadol and / or a pharmaceutically acceptable salt, metabolite or derivative thereof. Methods of making and using the formulation according to the invention are also provided.
Owner:EURO-CELTIQUE SA
Novel pharmaceutical compositions for treating chronic pain and pain associated with neuropathy
InactiveUS20080058362A1Reduced plasma concentrationEffective pain managementBiocideAmide active ingredientsDextrorphanSustained release drug
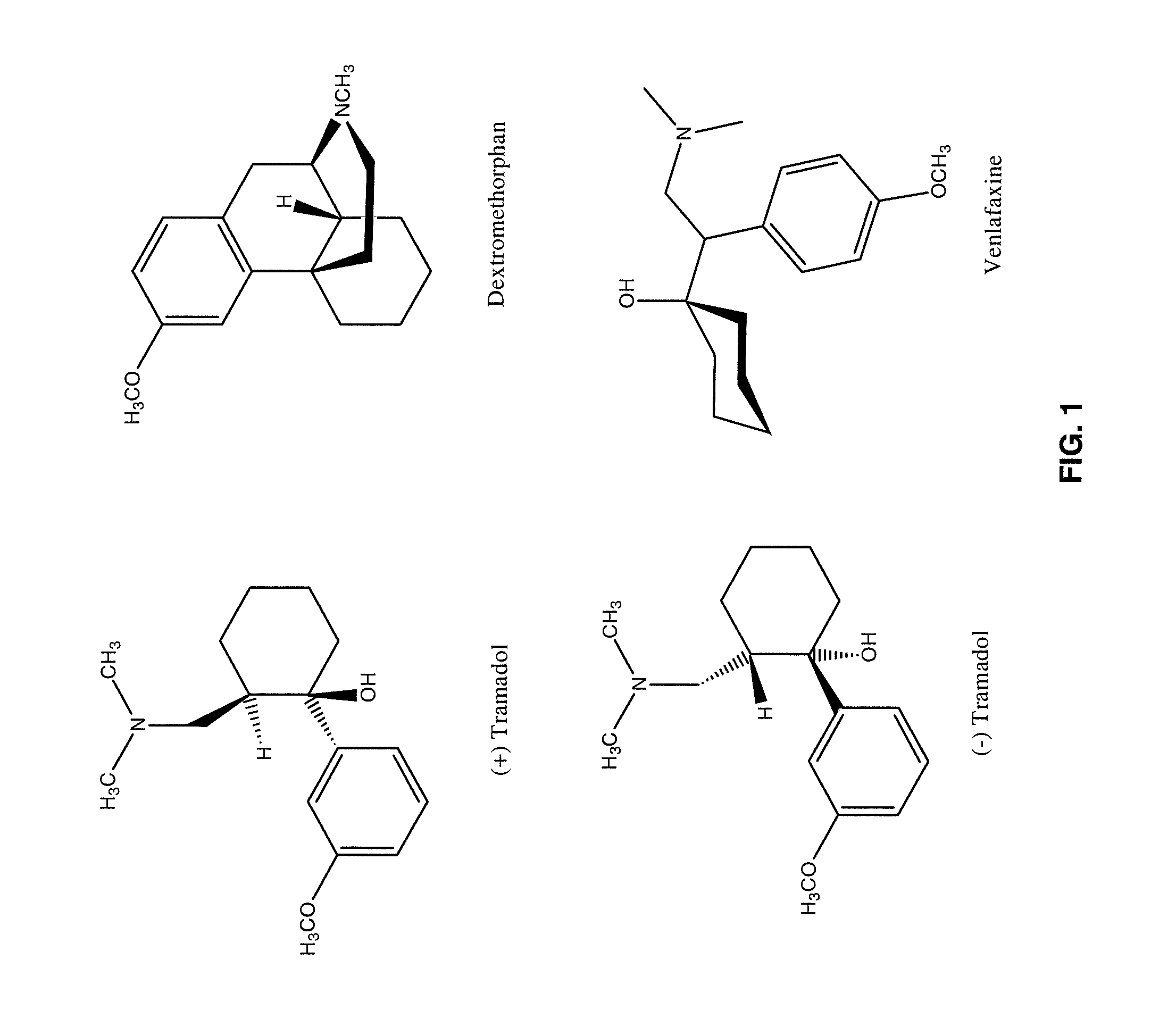
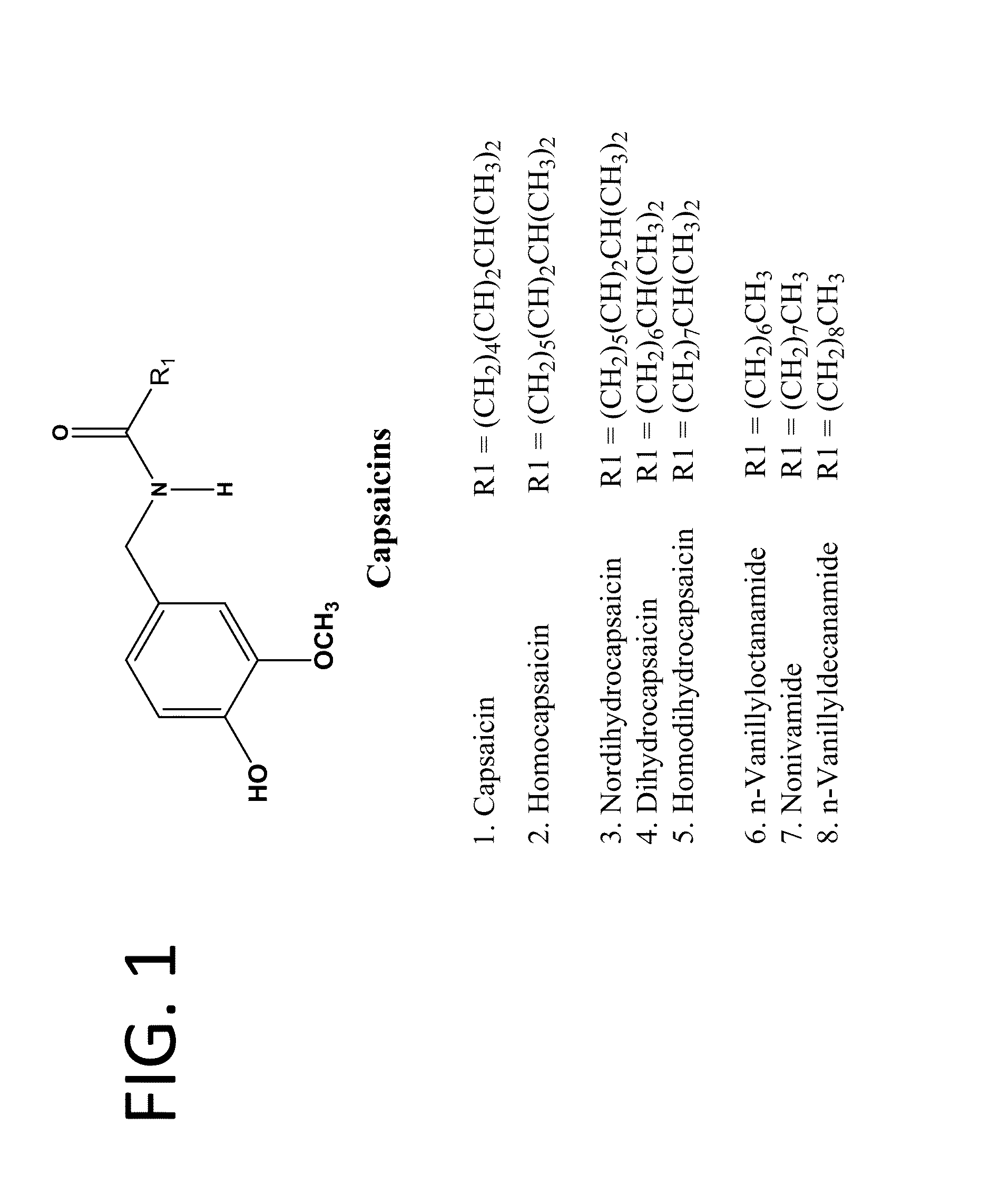
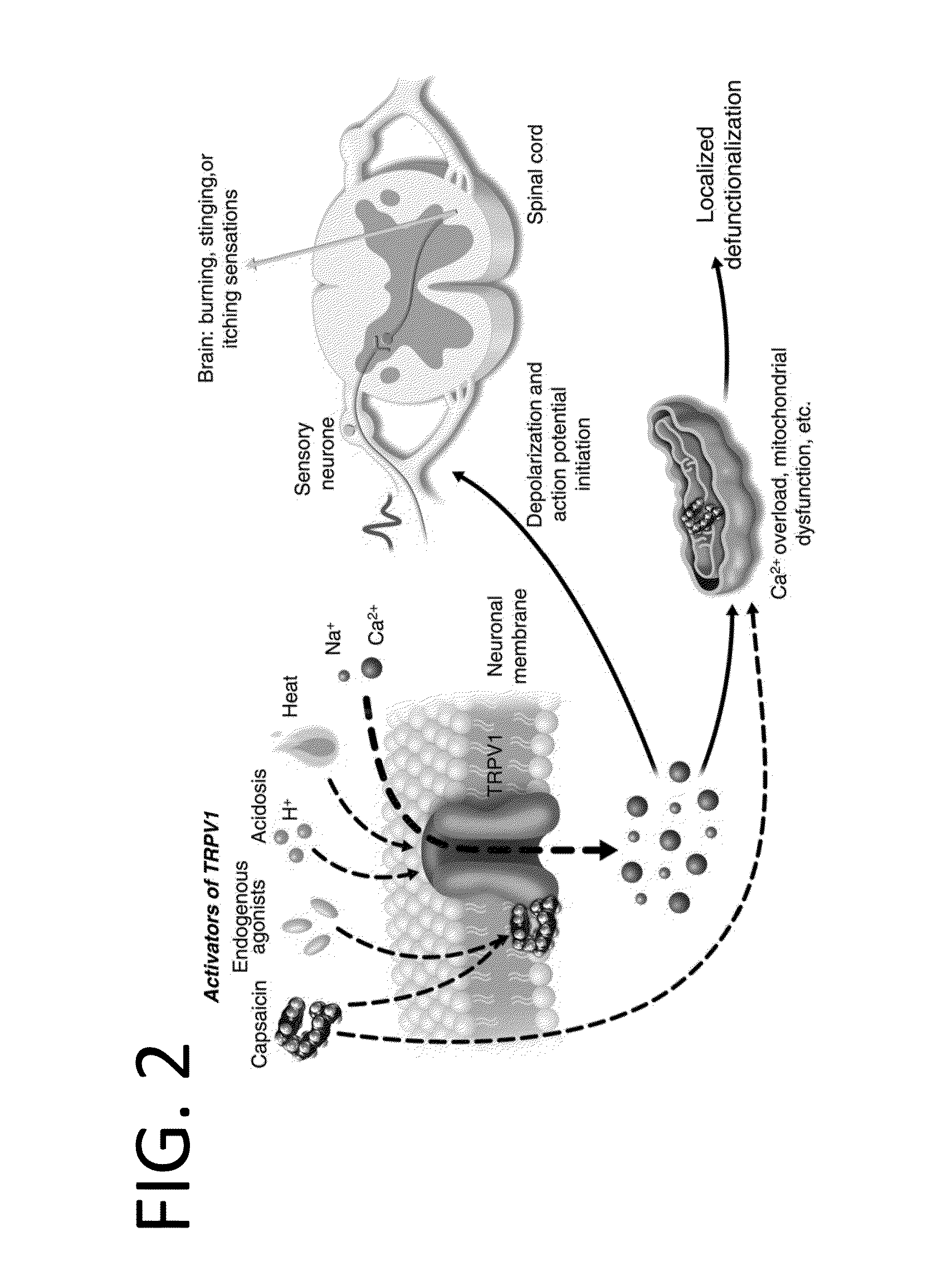
Chronic pain is alleviated in a mammal suffering there from by administering to the mammal a chronic pain alleviating amount of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, in combination with a μ-opiate analgesic such as tramadol or an analogously acting molecular entity, and a capsaicin or an ester of capsaicin, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
Opiod tannate compositions
A composition comprising the tannate of an opioid. Suitable opioids include alfentanil, buprenorphine, butorphanol, carfentanil, cocaine, codeine, dezocine, diacetylmorphine, dihydrocodeine, dihydromorphine, diphenoxylate, diprenorphine, etorphine, fentanyl, heroin, hydrocodone, hydromorphone, beta-hydroxy-3-methylfentanyl, levo-alpha-acetylmethadol, levorphanol, lofentanil, meperidine, methadone, morphine, nalbuphine, nalmefene, o-methylnaltrexone, naloxone, naltrexone, oxycodone, oxymorphone, pentazocine, pethidine, propoxyphene, remifentanil, sufentanil, tilidine and tramadol. The opioid tannate may be readily prepared by reacting an opioid free base with tannic acid, either neat or in the presence of up to about 30 wt. % water, at a temperature of about 60 to about 150° C. and thereafter recovering the resultant opioid tannate. The opioid tannate may also be prepared by an alternative process that involves reacting the opioid free base with water at a temperature such that not more than about 10 wt. % of the opioid tannate will be decomposed and thereafter removing the water by freeze-drying.
Owner:JAME FINE CHEM
Pharmaceutical compositions for treating chronic pain and pain associated with neuropathy
InactiveUS7645767B2Reduce concentrationEfficient managementBiocideAmide active ingredientsOpiatePharmaceutical medicine
Chronic pain is alleviated in a mammal suffering there from by administering to the mammal a chronic pain alleviating amount of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, in combination with a μ-opiate analgesic such as tramadol or an analogously acting molecular entity, and a capsaicin or an ester of capsaicin, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
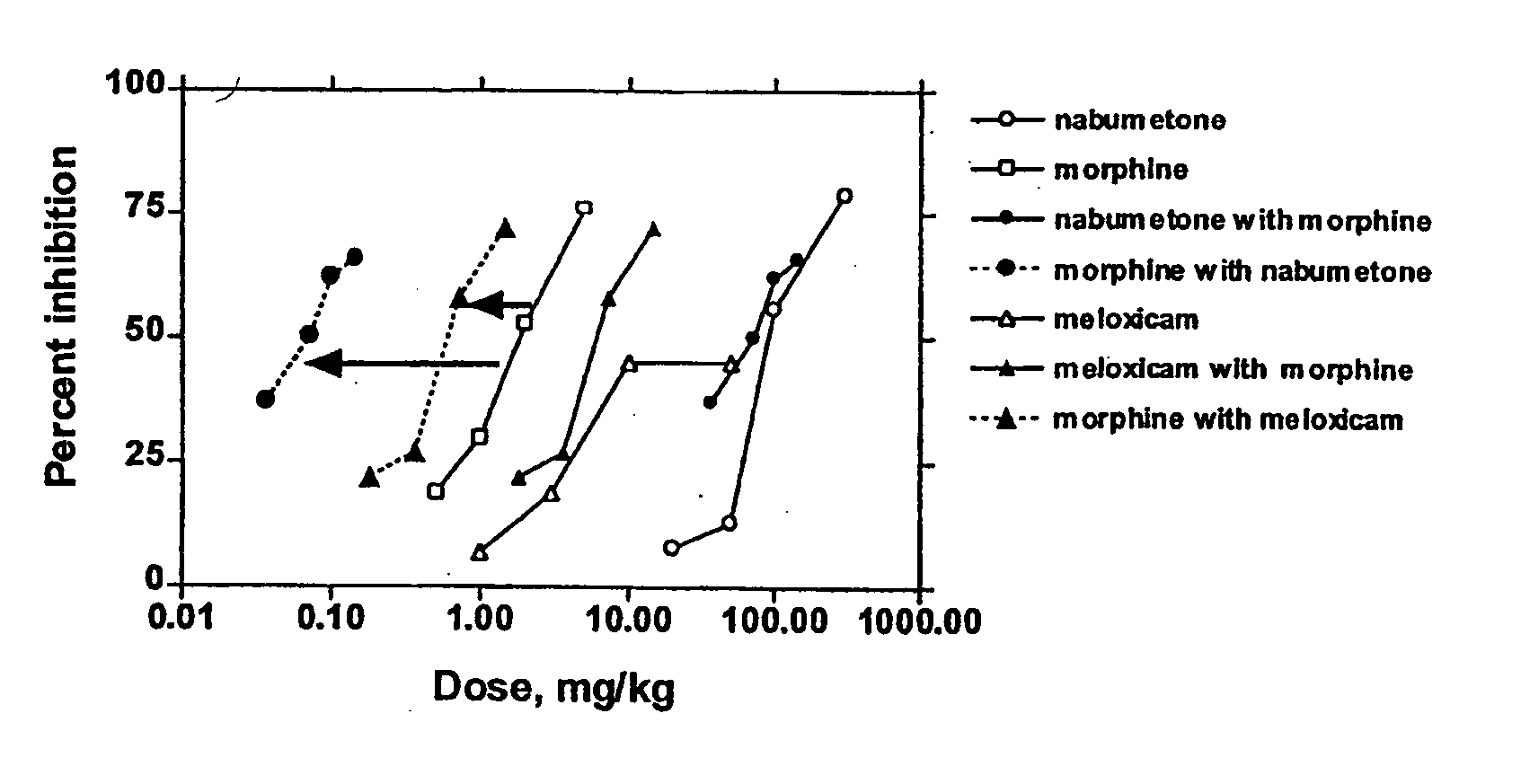
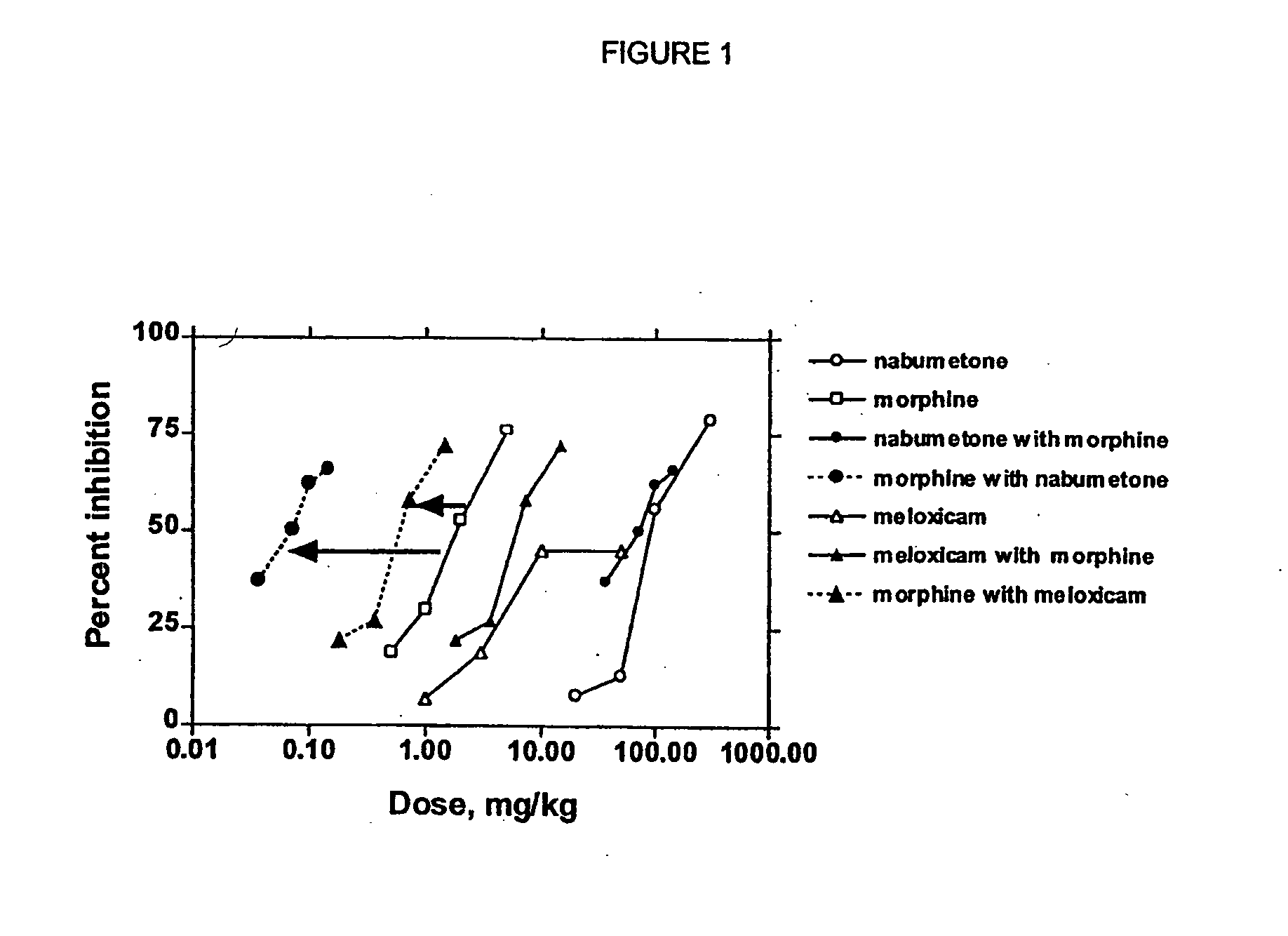
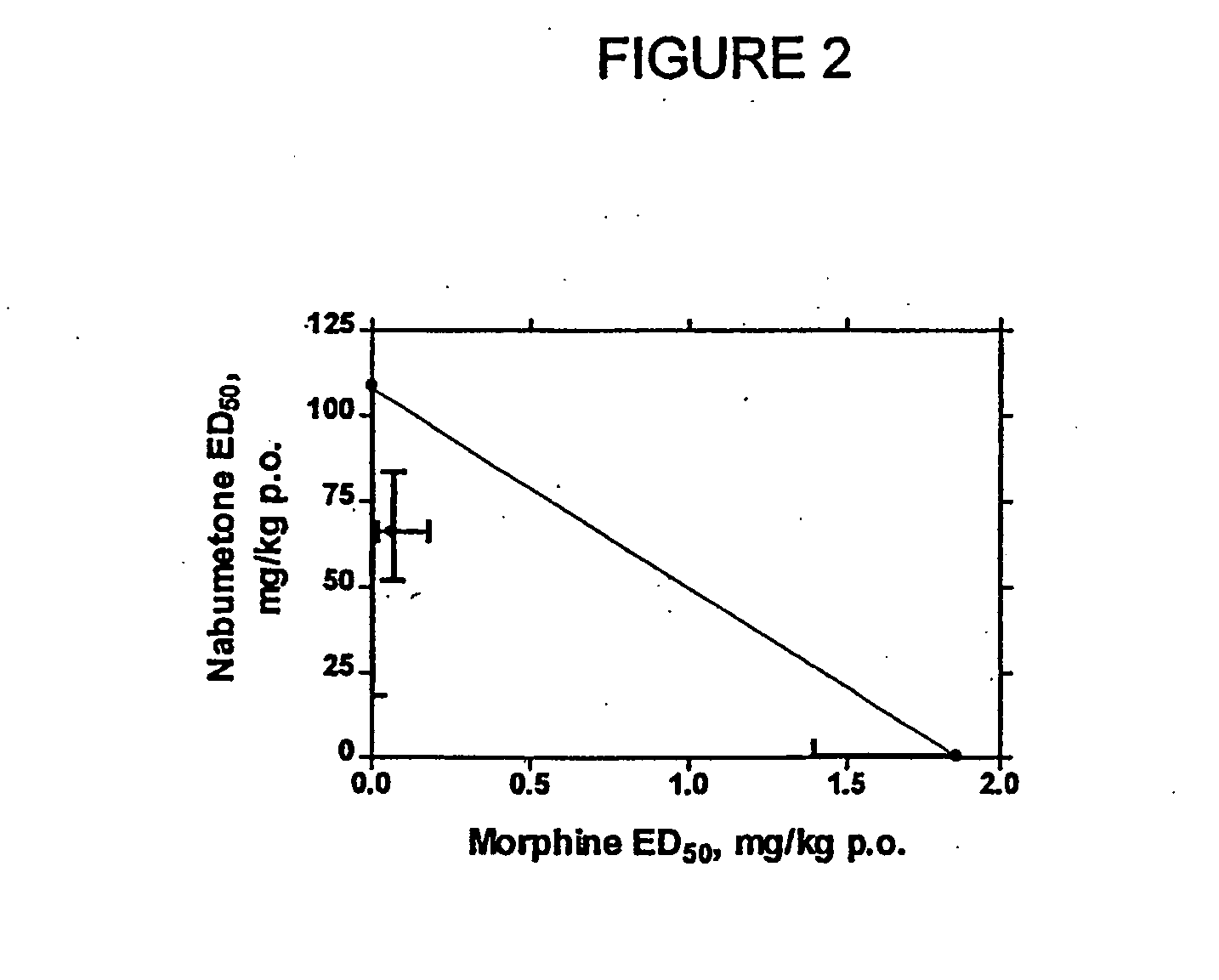
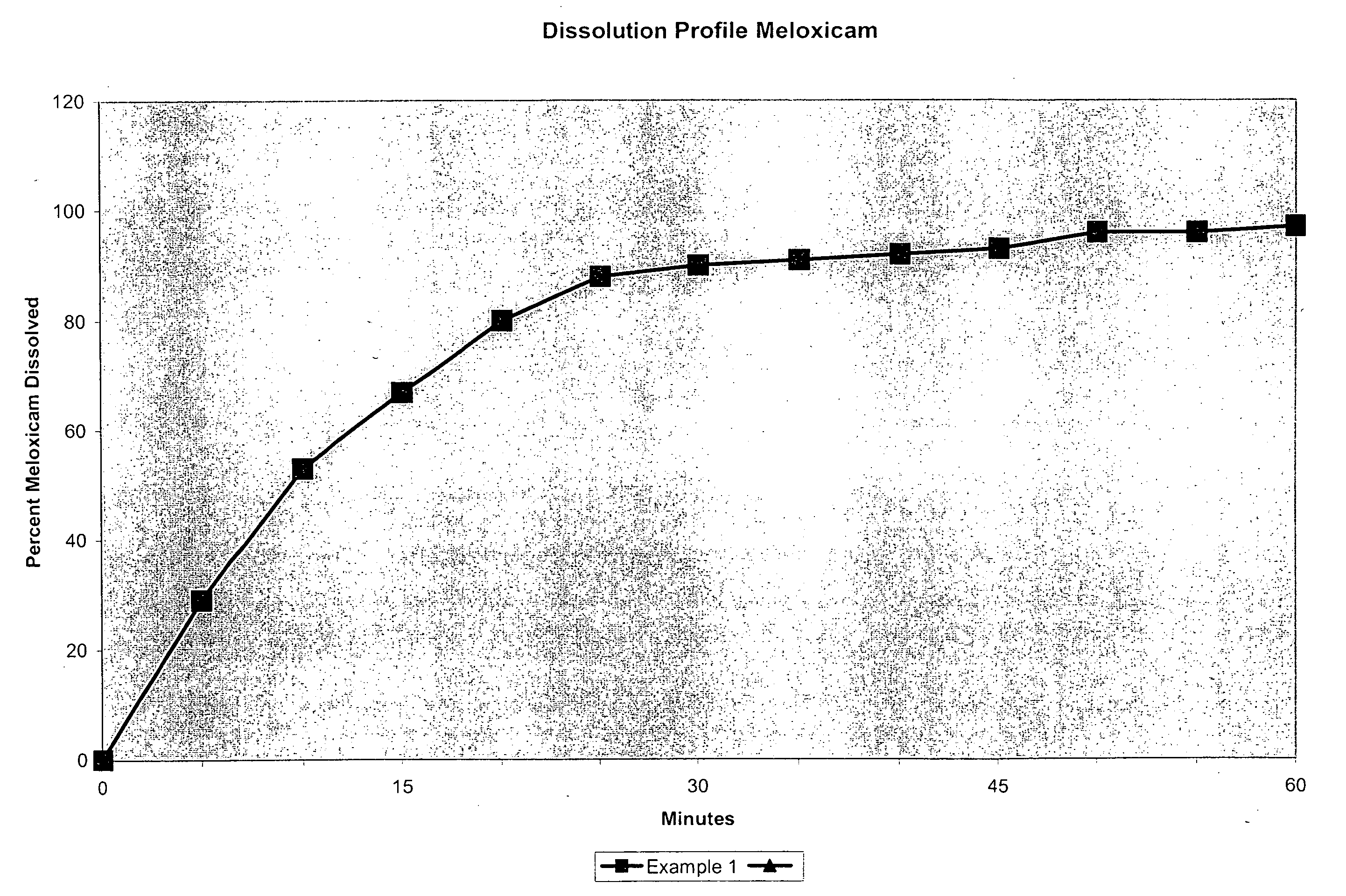
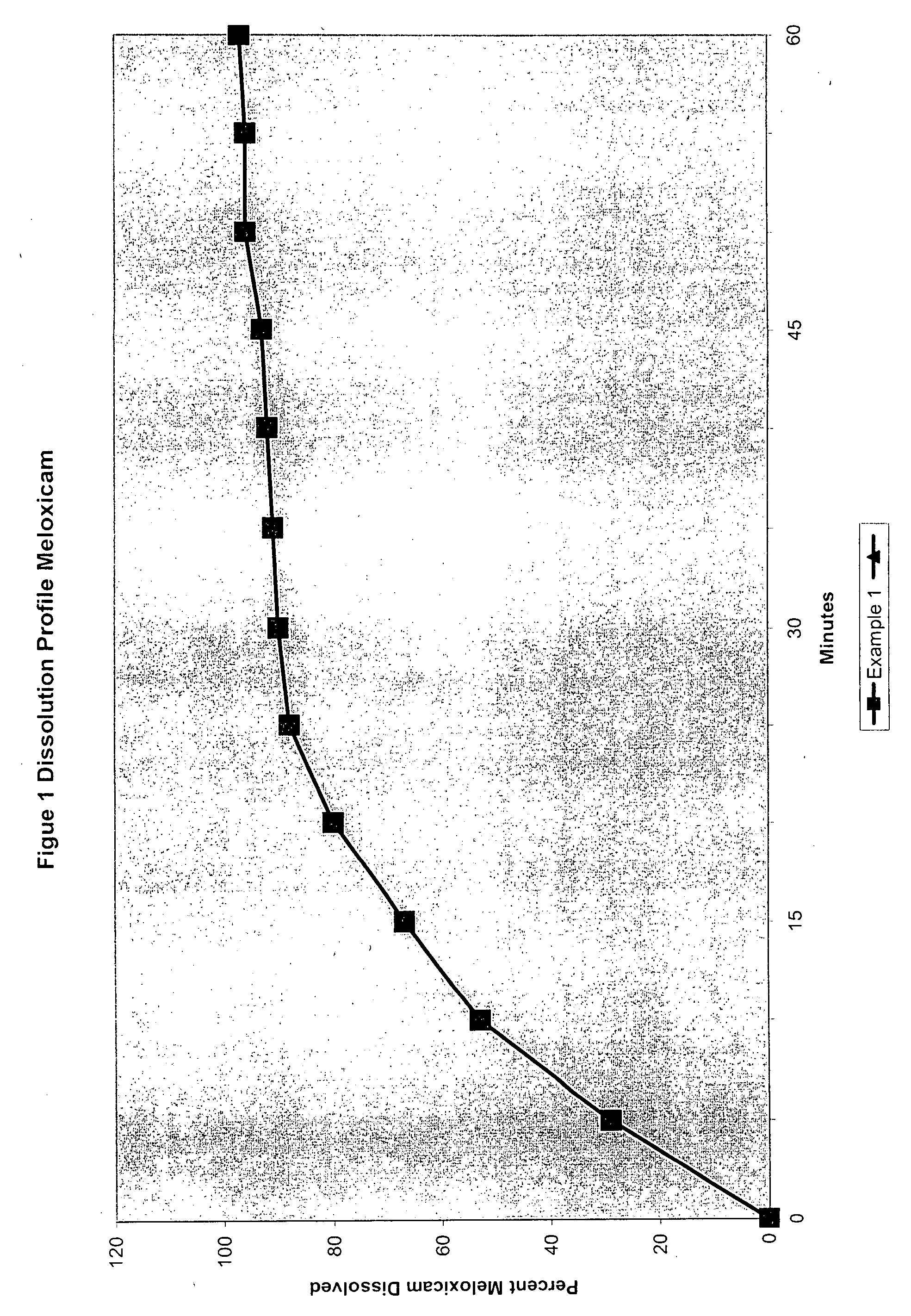
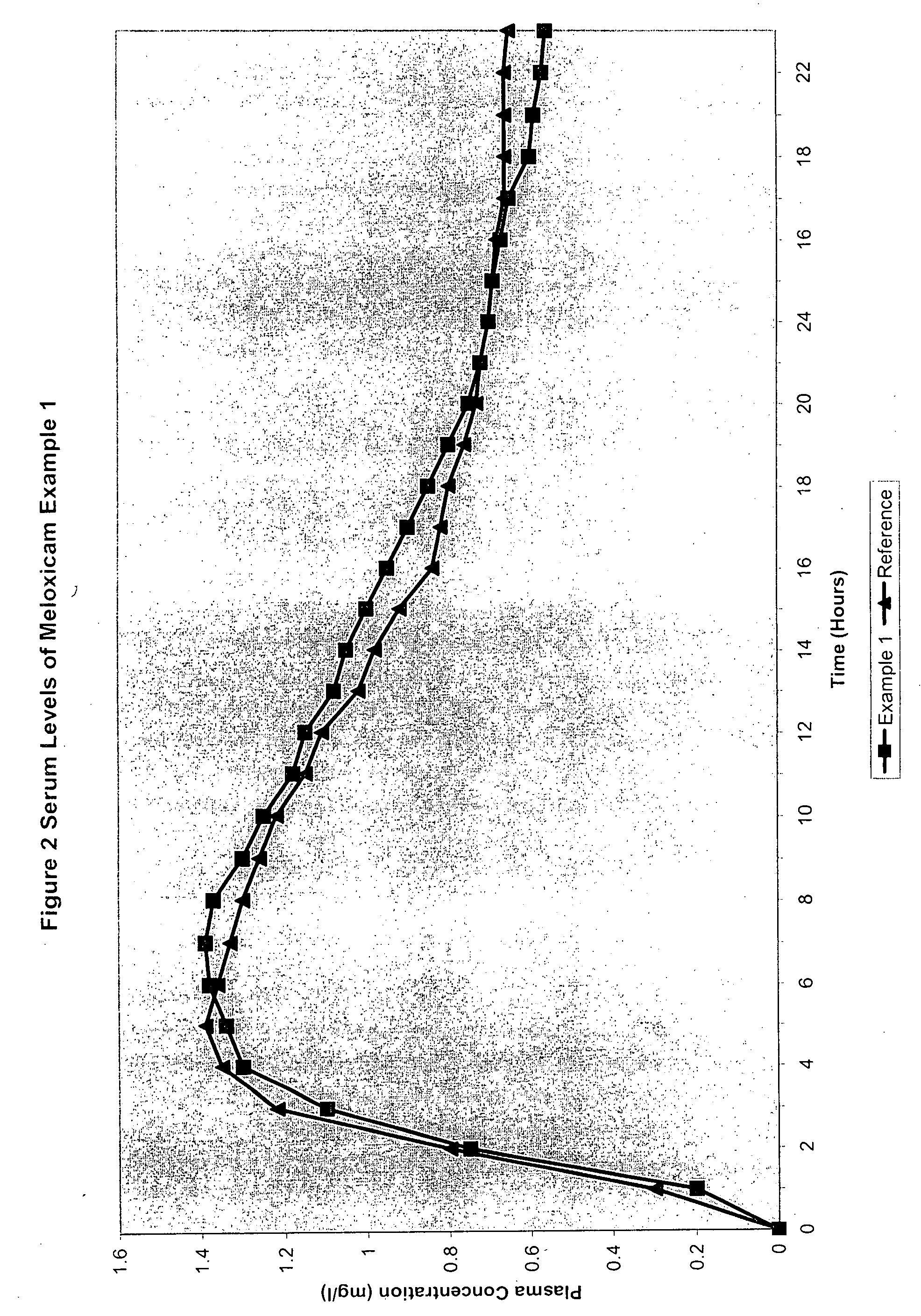
Analgesic combination of tramadol and meloxicam
InactiveUS20080050427A1Reduce concentrationEfficient managementBiocidePowder deliveryMeloxicamPharmaceutical drug
Disclosed is a pharmaceutical composition, comprising a combination of a dose of meloxicam or a pharmaceutically acceptable salt thereof and a dose of oxycodone or a pharmaceutically acceptable salt thereof, said combination in an amount sufficient to provide an analgesic effect in a human patient. Also disclosed is a method of effectively treating pain in humans or other mammals, comprising administering to the patient a combination of a dose of meloxicam or a pharmaceutically acceptable salt thereof and a dose of oxycodone or a pharmaceutically acceptable salt thereof such that the dosing interval of the meloxicam overlaps with the dosing interval of the oxycodone, said combination in an amount sufficient to provide an analgesic effect in a human patient.
Owner:PURDUE PHARMA LP
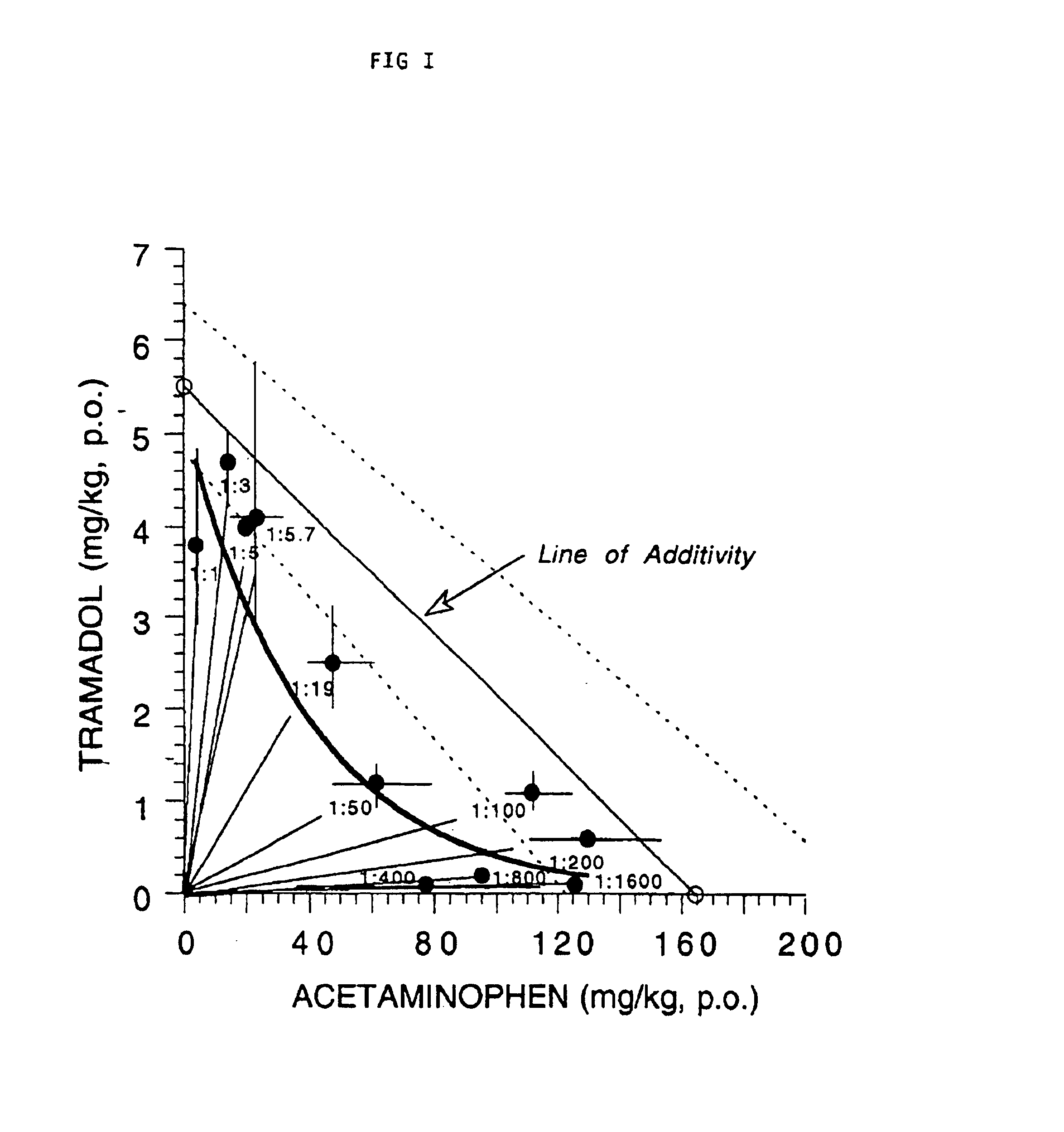
Composition comprising a tramadol material and acetaminophen and its use
This invention relates to a composition comprising a tramadol material and acetaminophen, and its use. As used herein tramadol refers to various forms of tramadol. The compositions are pharmacologically useful in treating pain and tussive conditions. The compositions are also subject to less opioid side-effects such as abuse liability, tolerance, constipation and respiratory depression. Furthermore, where the components of the compositions are within certain ratios the pharmacological effects of the compositions are superadditive (synergistic).
Owner:ORTHO MCNEIL PHARM INC
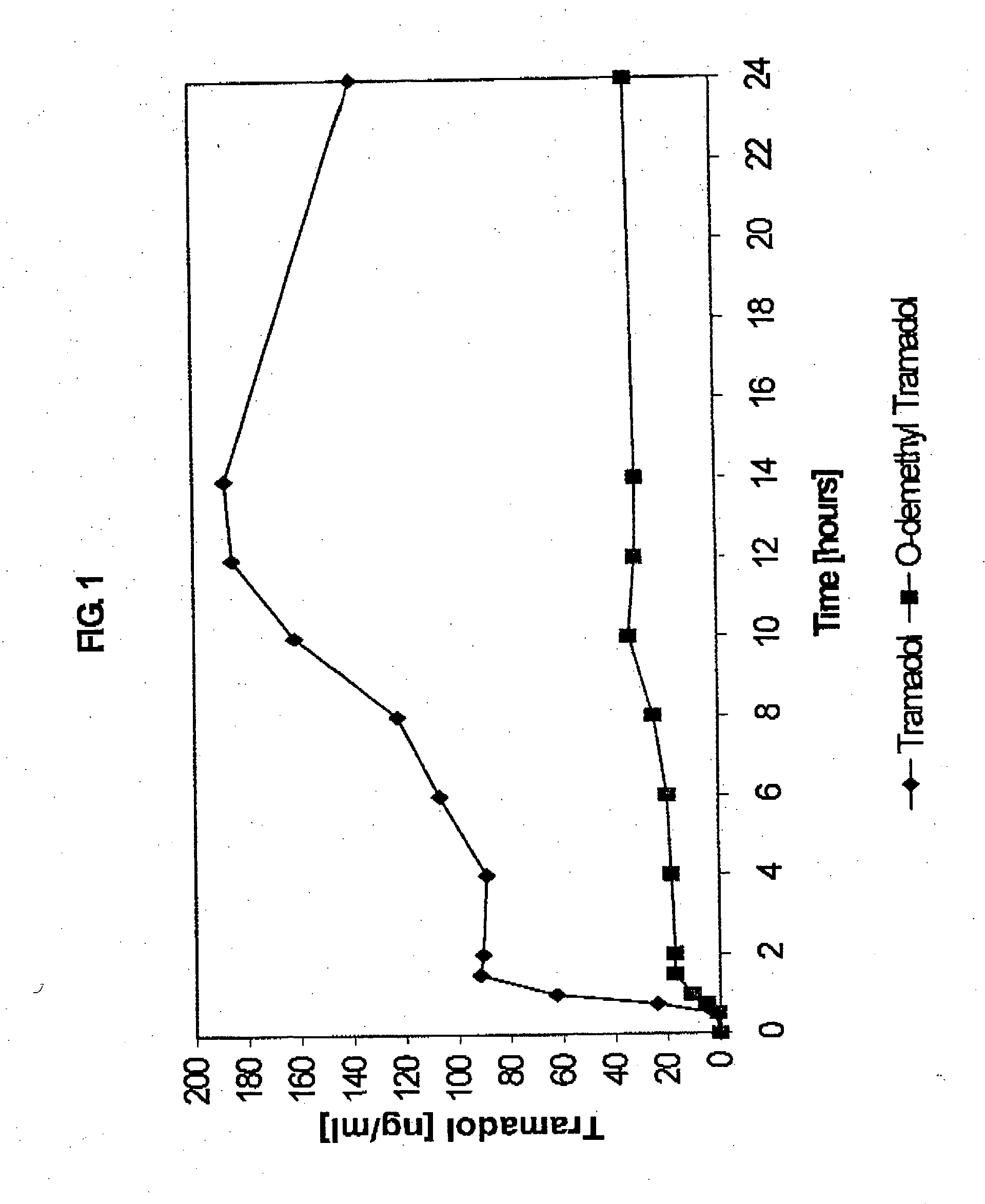
Extended release composition containing Tramadol
InactiveUS20030143270A1Effective controlRelieve painPowder deliveryBiocideBlood concentrationPeak concentration
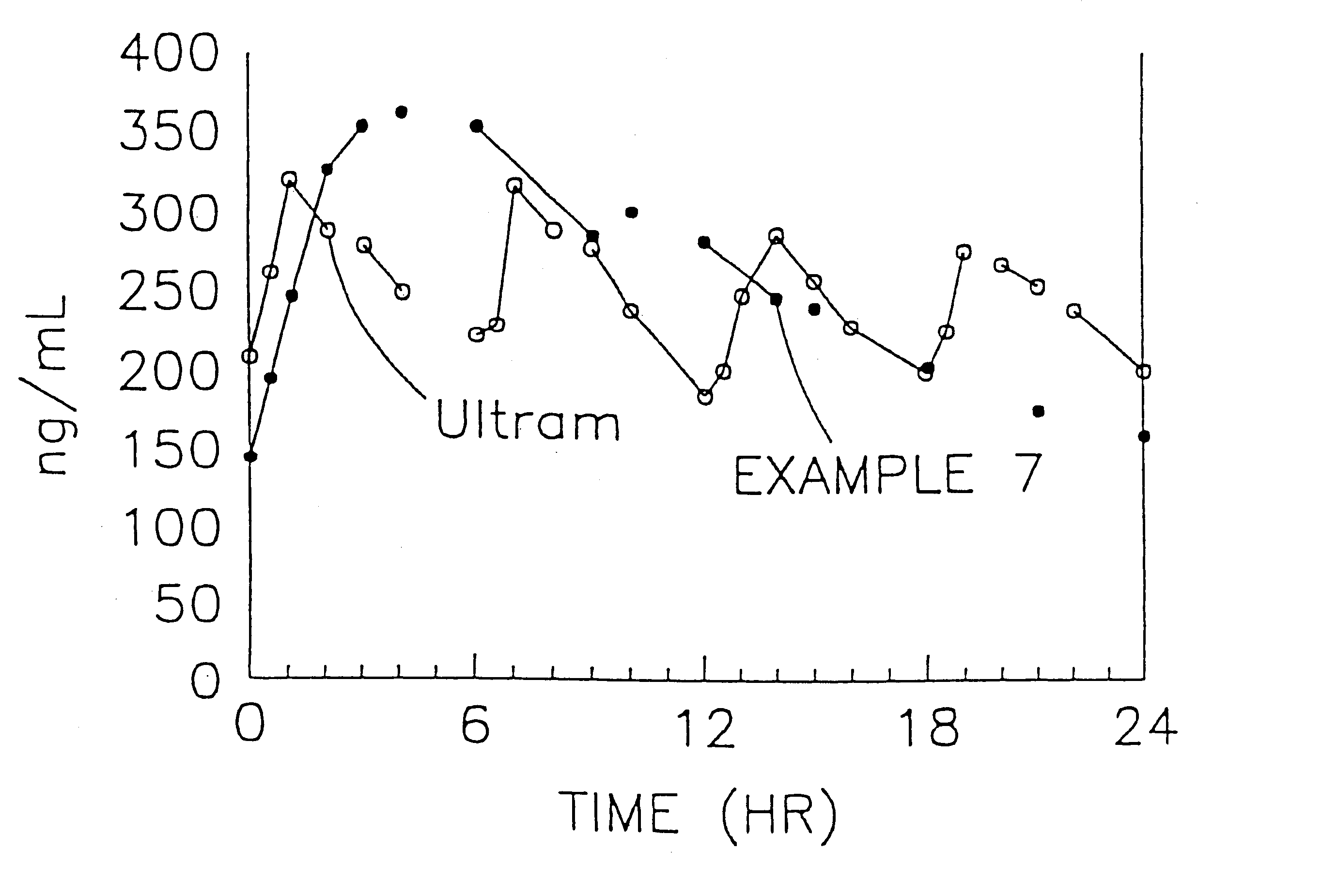
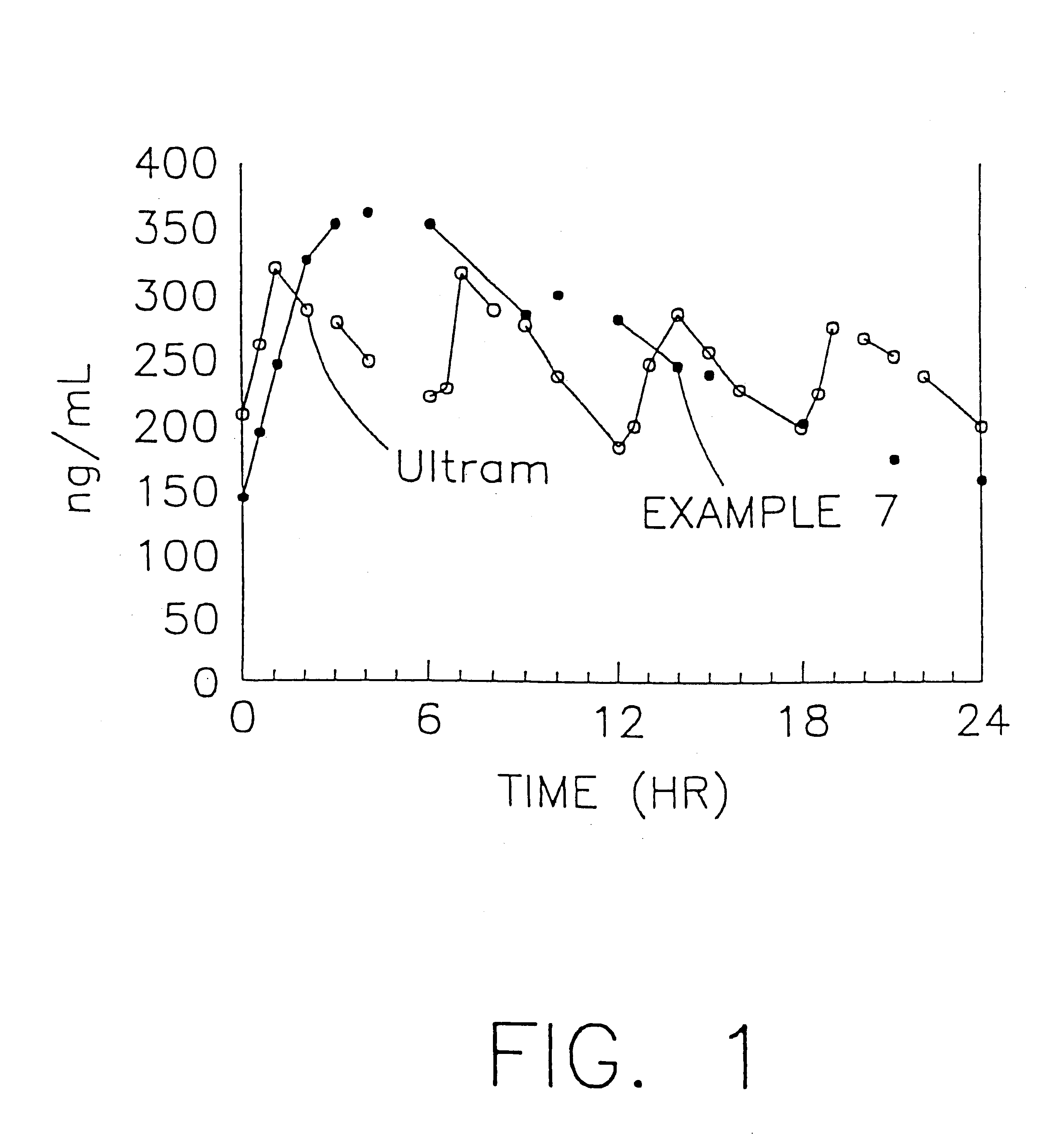
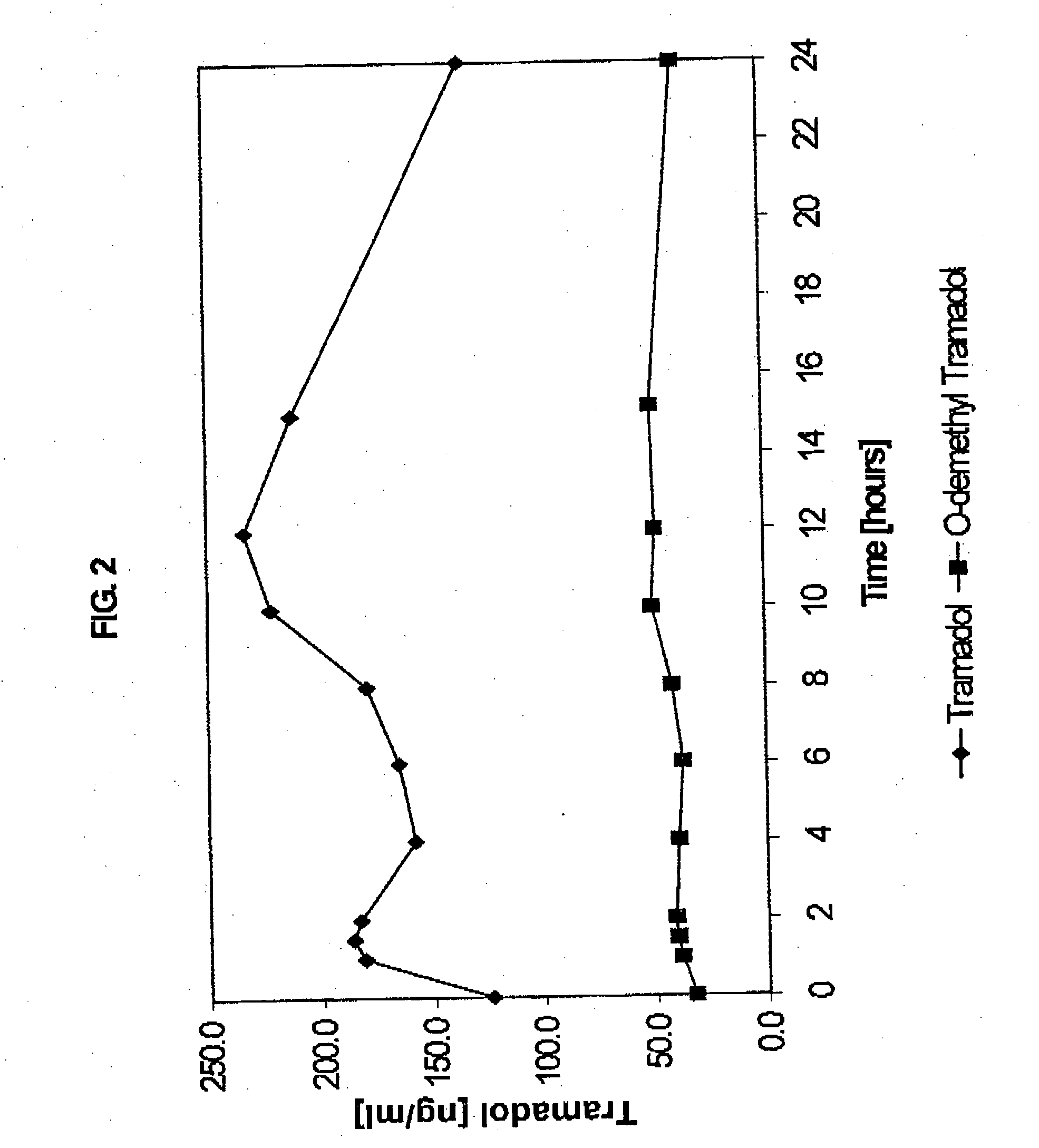
The present invention relates to a once daily extended release pharmaceutical preparation of Tramadol or its acceptable pharmaceutical salts. The preparation provides, effective blood concentration for a period of about 24 hours with reduced peak concentrations. It is characterized that effective Tramadol levels appear within the first hours after administration, the time to maximal Tramadol content Tmax is at least 10 hours and the peak Tramadol concentration is less than three times the concentration obtained after 24 hours of said administration.
Owner:GALEPHAR PHARMA RES
Novel anelgesic combination
The invention discloses a method of administering a pharmaceutical combination comprising an NSAID and a slow release tramadol to a mammal in need of thereof. This invention further discloses an analgesic combination comprising an NSAID and a slow release tramadol for treating pain and pain related conditions.
Owner:NECTID INC
Method of delaying ejaculation
The invention provides a method of delaying ejaculation. The method comprises administering an effective amount of a tramadol material to a human male prior to sexual intercourse. The method is particularly useful for treating premature ejaculation.
Owner:AYTU BIOSCI
Stabilized sustained release tramadol formulations
InactiveUS6645527B2Stable dissolutionAvoid throughputOrganic active ingredientsNervous disorderWaxDissolution
A stabilized sustained release oral solid dosage form which includes an effective amount of tramadol or a pharmaceutically acceptable salt thereof dispersed in a matrix of a hydrophobic material comprising a wax-like substance which was melted or softened during the preparation of the matrix, is cured at a temperature from about 35° C. to about 65° C. for a time period from about 4 to about 72 hours, such that the formulation, when subjected to in-vitro dissolution after exposure to accelerated storage conditions of at least one month at 40° C. / 75% RH, releases an amount of tramadol which does not vary at any given dissolution time point by more than about 20% of the total amount of tramadol released when compared to in-vitro dissolution conducted prior to subjecting the dosage form to the accelerated storage conditions.
Owner:PURDUE PHARMA LP
Titration dosing regimen for controlled release tramadol
ActiveUS7413749B2Reduce adverse side effectsImprove toleranceOrganic active ingredientsNervous disorderDosing regimenControlled release
A titration dosing regimen for the administration of controlled release tramadol analgesic to patients. The titration dosing regimen provides a significant reduction in the occurrence of adverse effects from the introduction of controlled released tramadol dosing, thus increasing patient compliance and medication tolerability.
Owner:PURDUE PHARMA LP
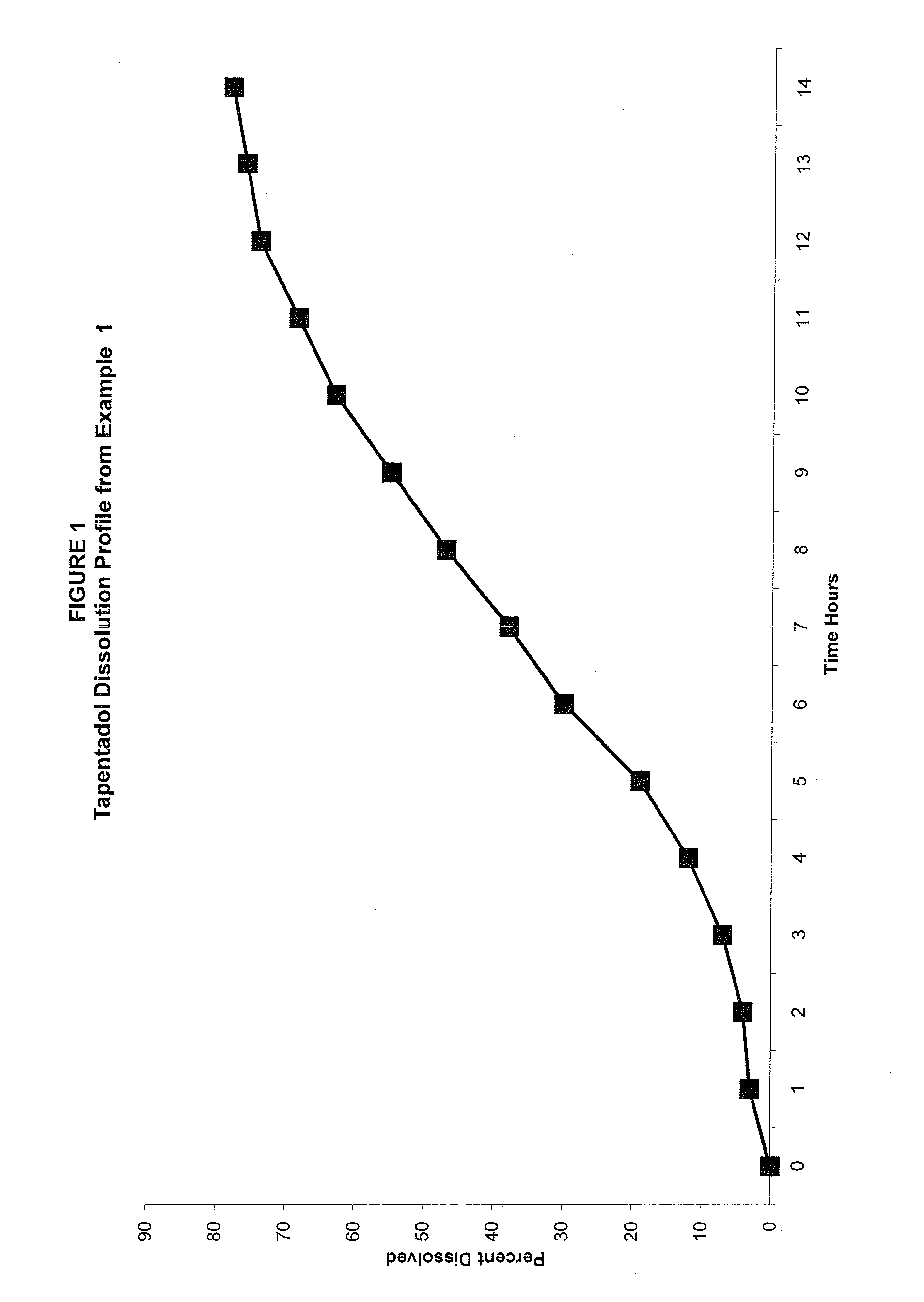
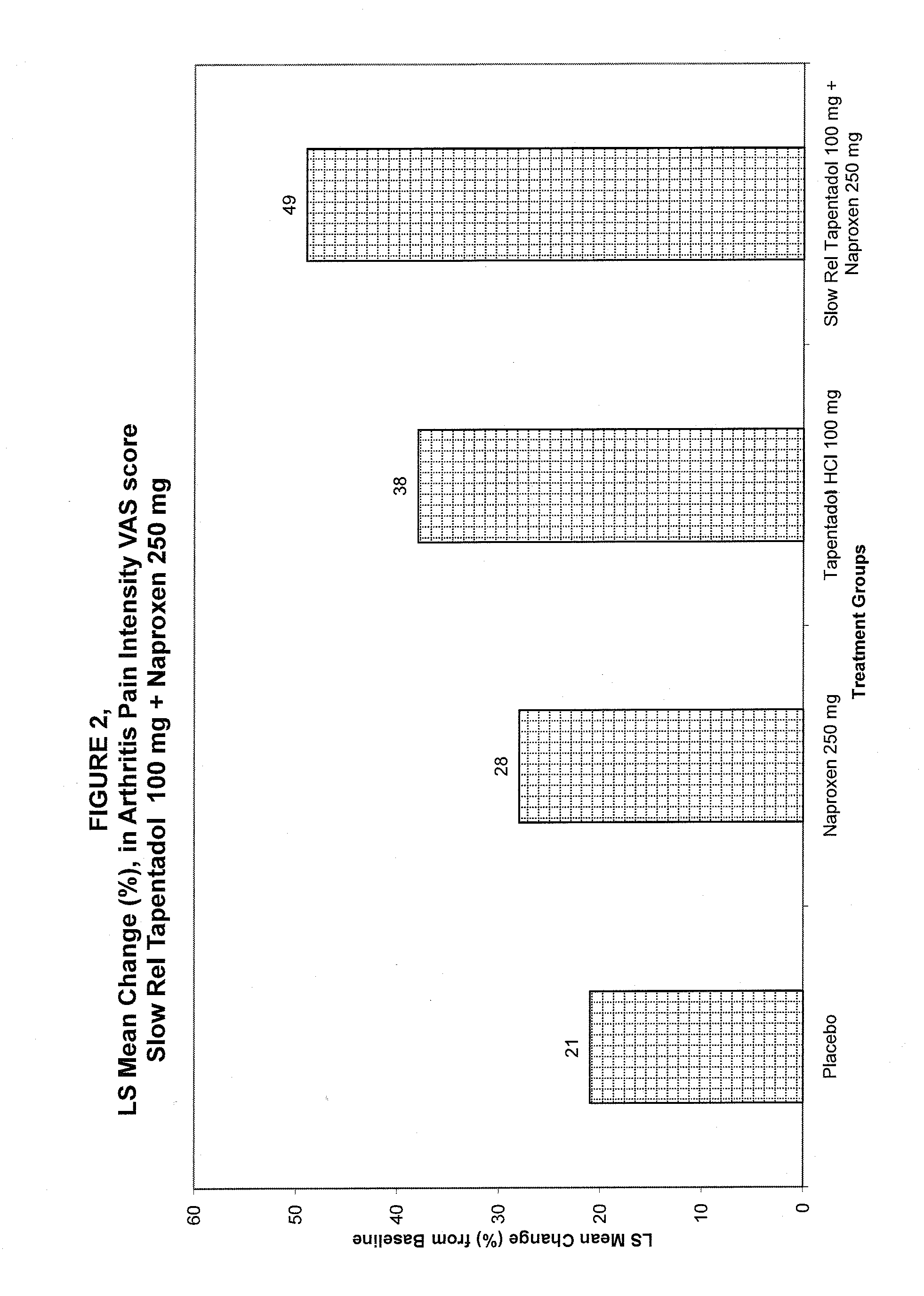
Tapentadol compositions
InactiveUS20100297229A1Reduce doseImprove complianceBiocideNervous disorderGamma-Aminobutyric acidTapentadol Hydrochloride
The present invention provides a method of treating pain and pain related conditions by administering to a patient in need thereof, a therapeutically effective amount of a slow release Tapentadol Hydrochloride and therapeutically effective amount of a second analgesic, wherein the second analgesic is tramadol, gamma-aminobutyric acid (GABA) analogue or an NSAID. The present invention further provides a pharmaceutical composition comprising a therapeutically effective amount of a slow release Tapentadol Hydrochloride and a therapeutically effective amount of a second analgesic, wherein the second analgesic is tramadol, gamma-aminobutyric acid (GABA) analogue or an NSAID.
Owner:GRUNENTHAL GMBH +1
Pharmaceutical drug dosage forms providing different release rates
A pharmaceutical dosage form comprises, in one portion thereof, a substantially single (+)-enantiomer of a chiral drug other than verapamil and, in another, separate portion thereof, a substantially single (-)-enantiomer of the drug wherein, in use, the different enantiomers are released at different rates from the dosage form. The dosage form is useful for administration of chiral drugs where both enantiomers have a valid pharmacological input, and where a clinical benefit may be realised by controlling the release rates of those enantiomers. Examples of such drugs include, in particular, tramadol and warfarin.
Owner:SOSEI R&D LIMITED
Dosage forms
A pharmaceutical dosage form comprises, in one portion thereof, a substantially single (+)-enantiomer of a chiral drug other than verapamil and, in another, separate portion thereof, a substantially single (-)-enantiomer of the drug wherein, in use, the different enantiomers are released at different rates from the dosage form. The dosage form is useful for administration of chiral drugs where both enantiomers have a valid pharmacological input, and where a clinical benefit may be realised by controlling the release rates of those enantiomers. Examples of such drugs include, in particular, tramadol and warfarin.
Owner:SOSEI R&D LIMITED
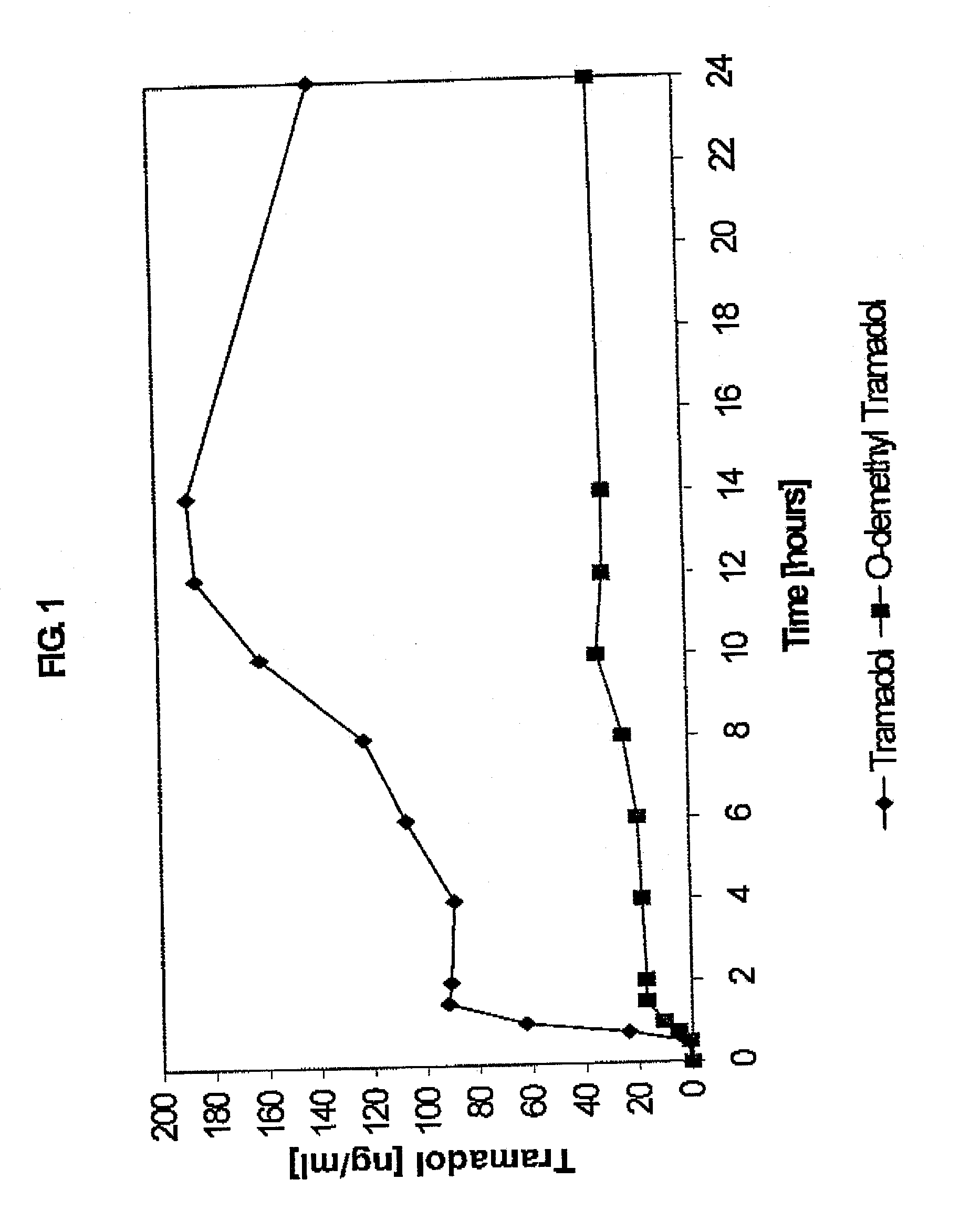
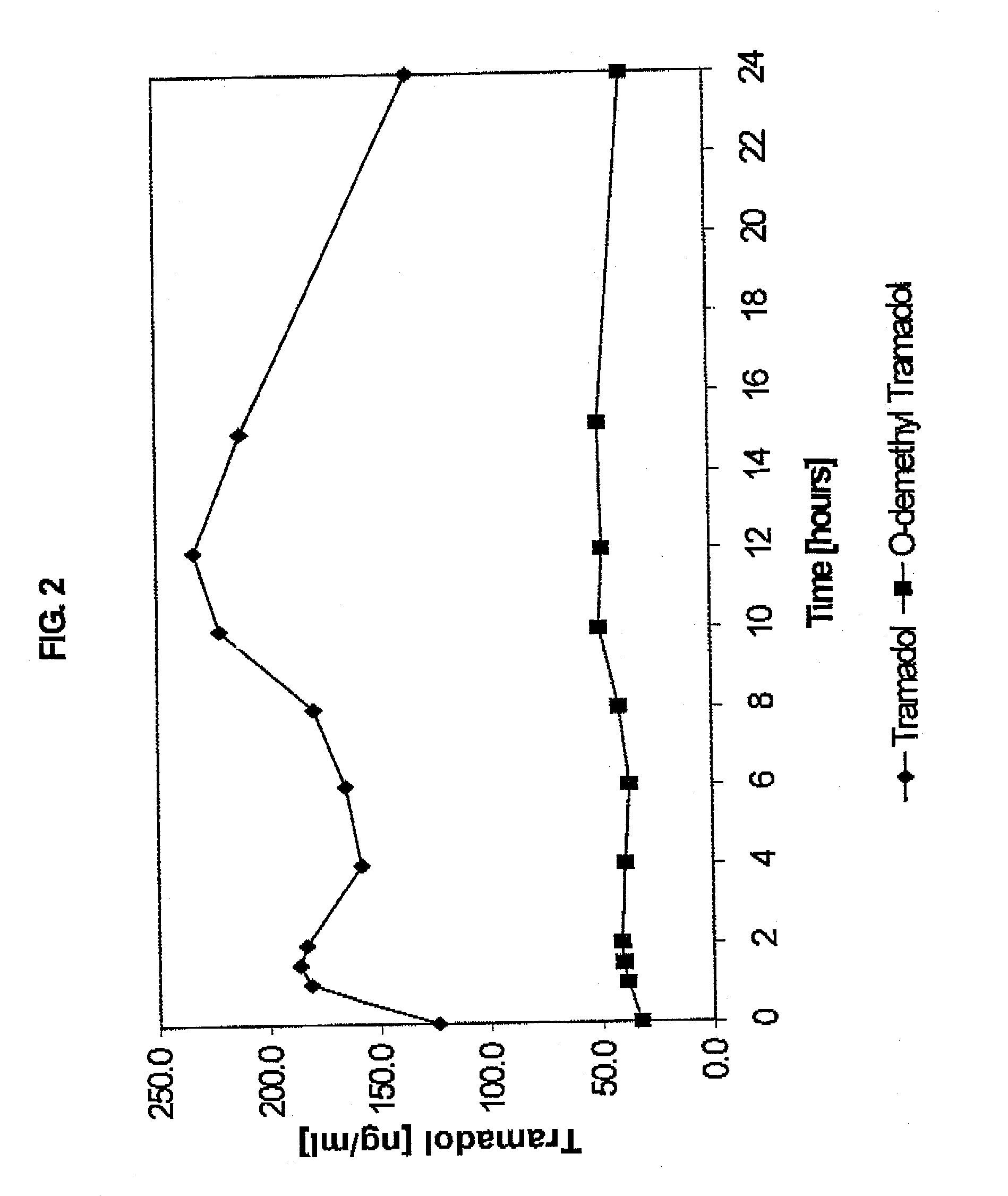
Sustained-release tramadol formulations with 24-hour clinical efficacy
InactiveUS20060172006A1Significant clinical effectOrganic active ingredientsNervous disorderControlled releaseClinical efficacy
There is disclosed a once daily oral pharmaceutical compositon for controlled release of tramadol or a salt thereof, wherein the composition, when ingested orally, provides a clinical effect over 24 hours which is a least as good as the clinical effect over 24 hours of two doses of a twice daily oral pharmaceutical composition for controlled release of tramadol, taken 12 hours apart
Owner:LABOPHARM BARBADOS
Sustained-release tramadol formulations with 24-hour efficacy
A sustained-release tramadol formulation oral administration is provided which, upon initial administration of one dose, provides an analgesic effect within 2 hours, which analgesic effect continues for at least 24 hours after administration
Owner:LABOPHARM BARBADOS LTD 36646
Novel Pharmaceutical Compositions for Treating Chronic Pain and Pain Associated with Neuropathy
InactiveUS20130189354A1Reduced plasma concentrationEfficient managementOrganic active ingredientsBiocideGabapentinChronic pain
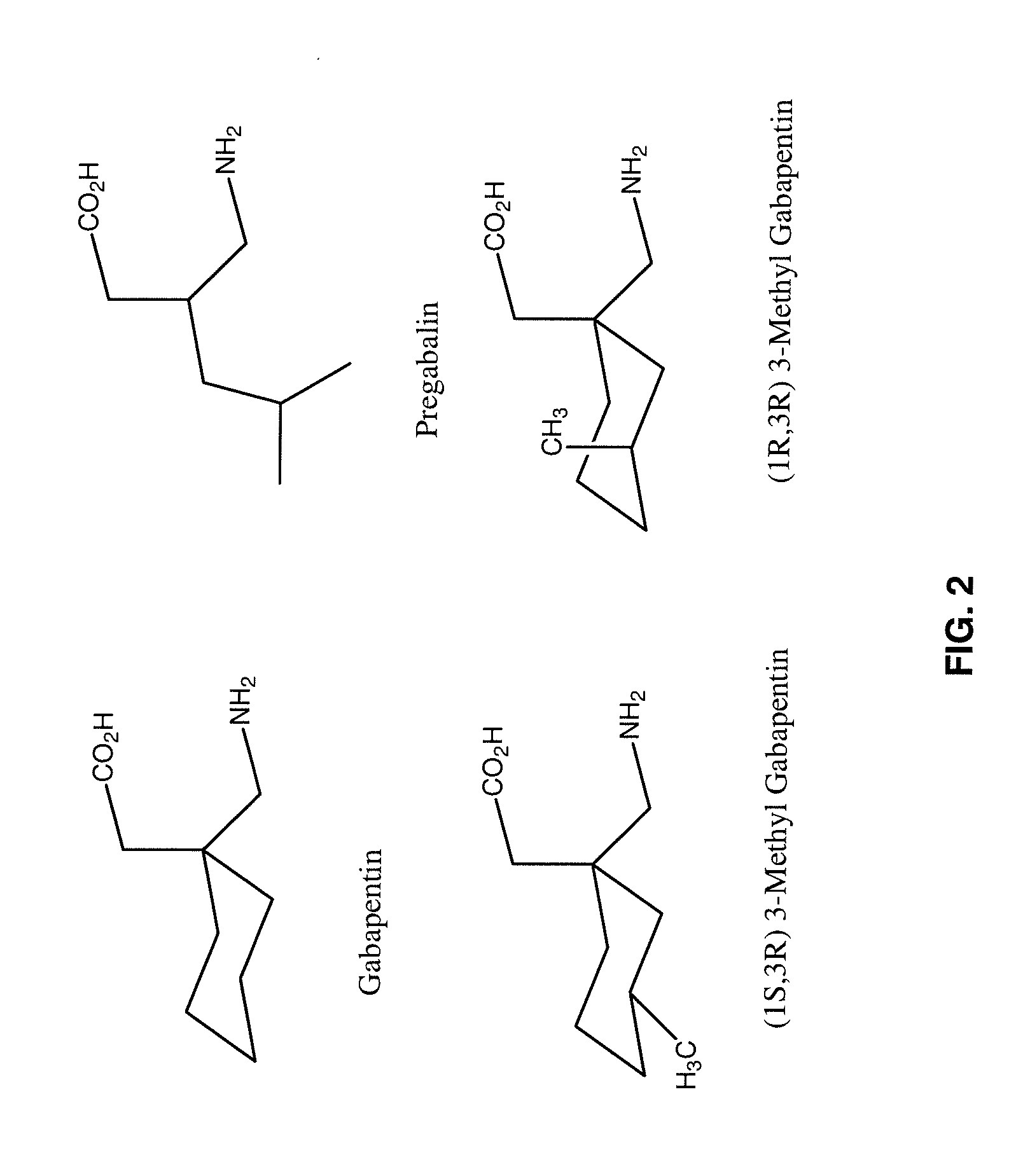
The present invention relates to compositions and methods for treating pain wherein the compositions comprise a combination of tramadol or a pharmaceutically acceptable salt thereof, magnesium or a pharmaceutically acceptable salt thereof; and gabapentin or pregabalin. The therapeutic combination can further contain capsaicin or an ester of capsaicin.
Owner:TRINITY LAB INC
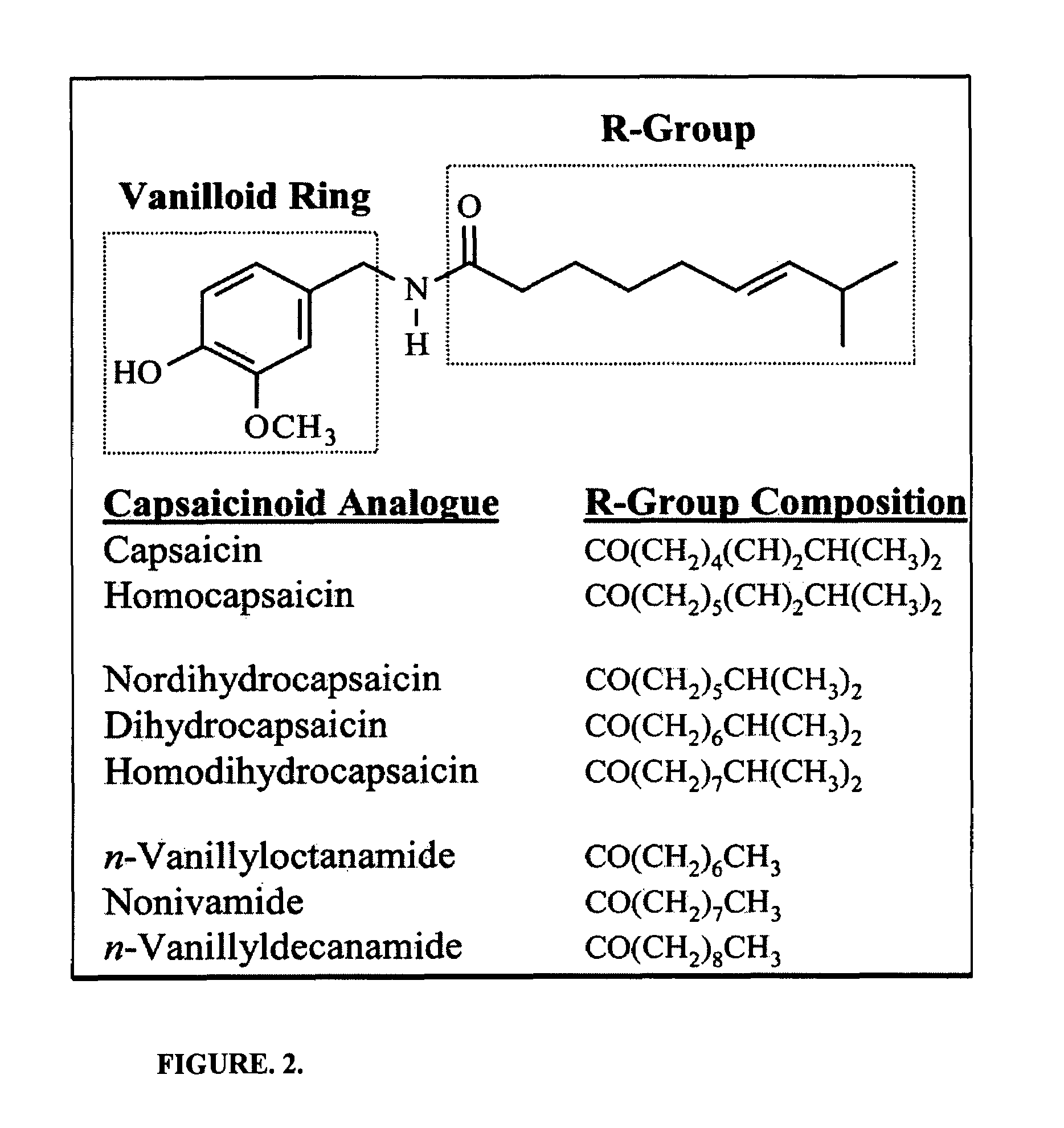
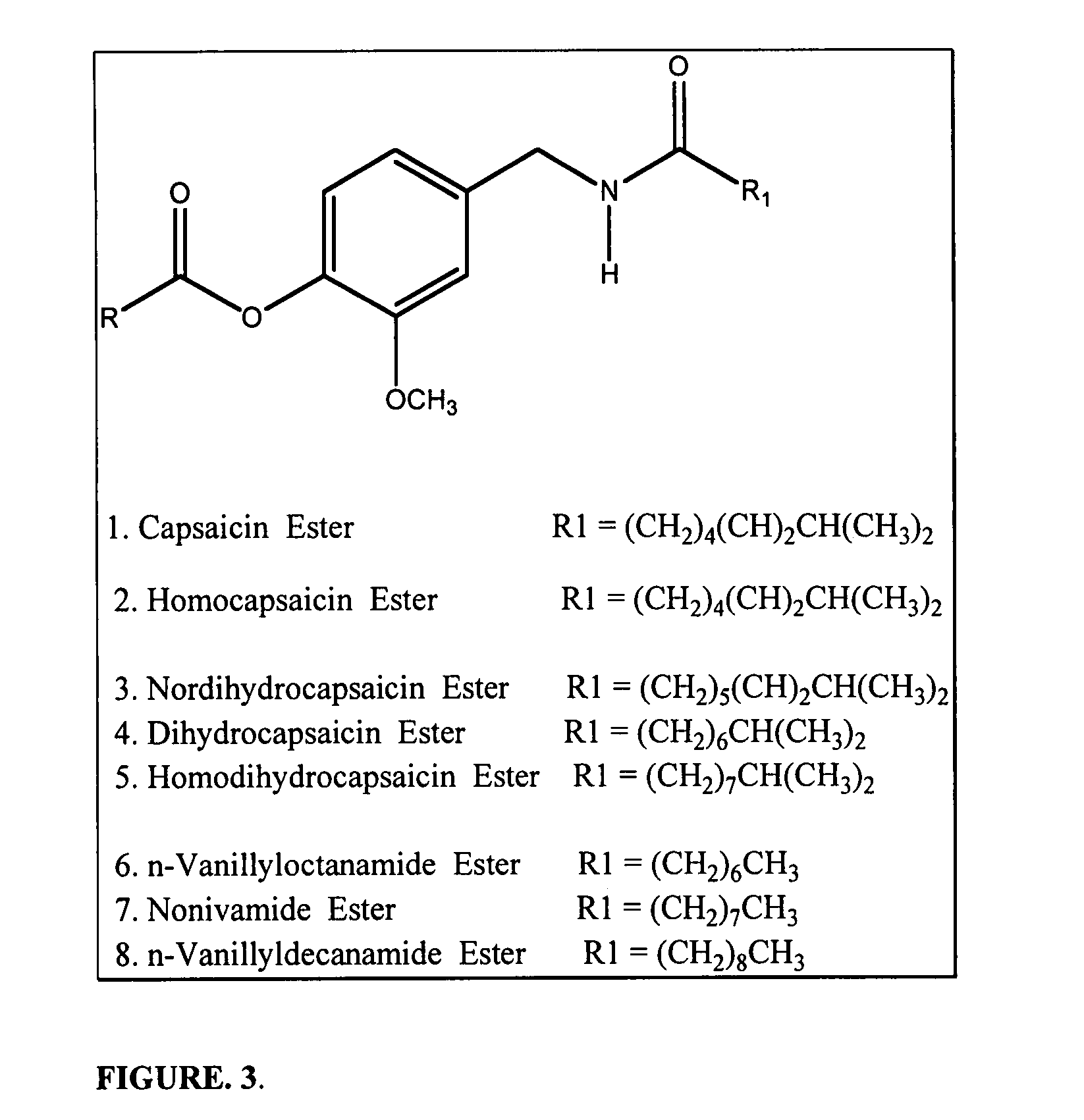
Pharmaceutical Compositions Comprising Capsaicin Esters for Treating Pain and Cold Sores
InactiveUS20140134261A1Increase heart rateGood blood pressureBiocideHydroxy compound active ingredientsDiseaseAmyris
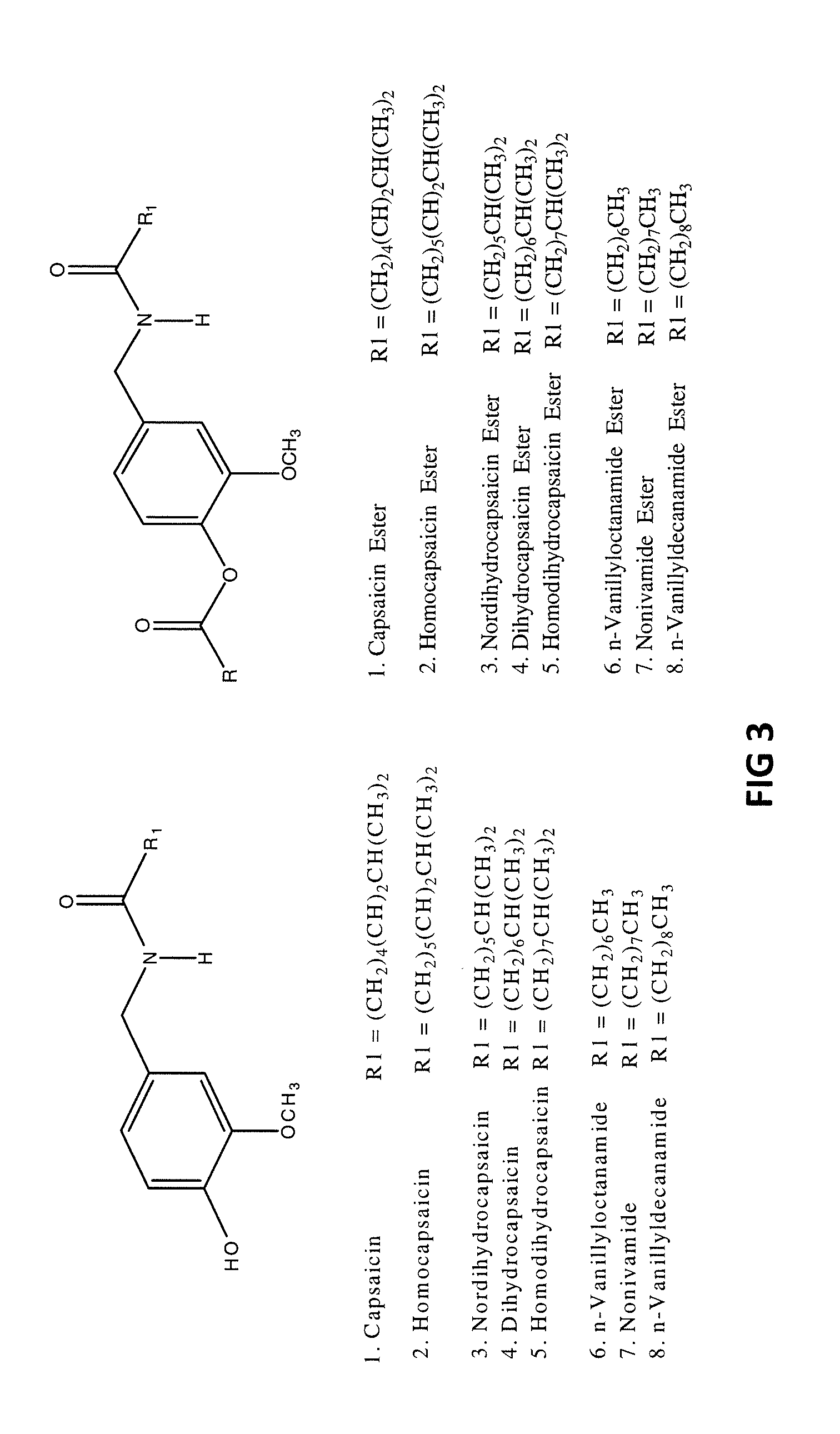
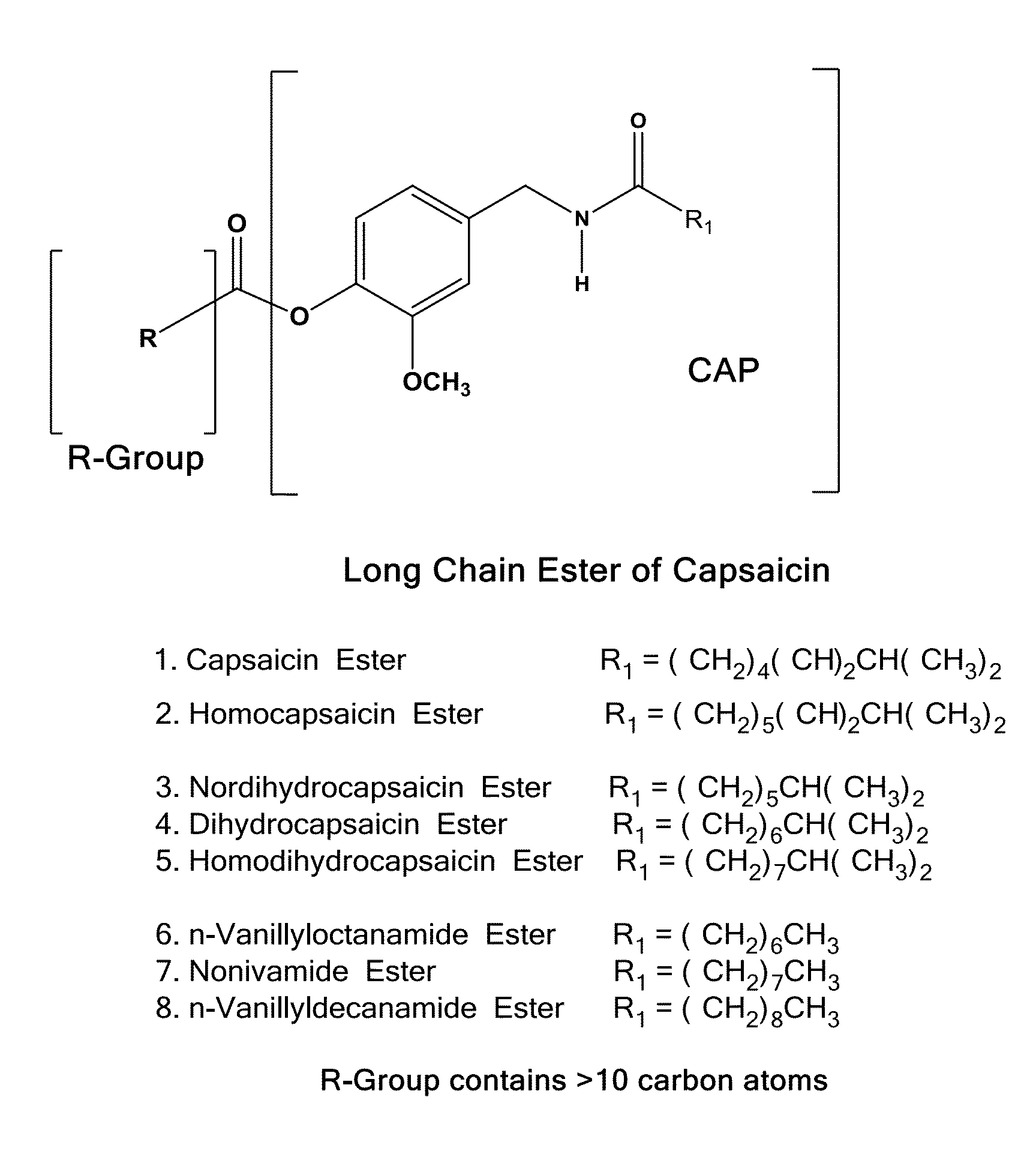
The present invention relates to pharmaceutical compositions comprising ester(s) of capsaicin and at least one other agent selected from salicylates, menthol, boswellic acids, DMSO, methyl sulfonylmethane, NSAIDs, corticosteroids, emu oil, opioid agonists and antagonists, NMDA antagonists, tramadol, hyaluronic acid, α2δ ligands, santalol, santalyl acetate, amyris alcohol, amyris acetate, aloe vera gel and aloe vera juice, for improved therapeutic properties. Further, the present invention relates to pharmaceutical compositions comprising high concentrations of ester(s) of capsaicin. Further, the present invention relates to a method of relieving pain due to various diseases in subjects by administering the pharmaceutical compositions of the invention. Further, the present invention relates to methods of relieving fever blisters due to cold sores in subjects by administering the pharmaceutical compositions comprising an ester of capsaicin and one other agent selected from santalol, santalyl acetate, amyris alcohol and amyris acetate.
Owner:TRINITY LAB INC
Pharmaceutical compositions for treating pain associated with dysmenorrhea
InactiveUS20150313892A1Reduced plasma concentrationEfficient managementBiocidePeptide/protein ingredientsMolecular entityN-methyl-D-aspartate Receptor Antagonists
Pain associated with primary and secondary dysmenorrhea is relieved in a human suffering there from by administering to the human a pain relieving amount of a synergistically acting sub-therapeutic combination of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, magnesium, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, tramadol or its analog such as recemic tramadol or an analogously acting molecular entity or pharmaceutically acceptable salt thereof, and an anticonvulsant and / or a tricyclic anti-depressant or pharmaceutically acceptable salt thereof, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
Sustained-release tramadol formulations with 24-hour efficacy
A sustained-release tramadol formulation oral administration is provided which, upon initial administration of one dose, provides an analgesic effect within 2 hours, which analgesic effect continues for at least 24 hours after administration
Owner:LABOPHARM BARBADOS LTD 36646
Reducing Side Effects of Tramadol
InactiveUS20080261991A1Reduce morbidityOrganic active ingredientsNervous disorderSexual functionSide effect
The invention provides methods of reducing the side effects of tramadol. Accordingly, in one embodiment, the invention provides a method of reducing the incidence of newly-discovered side effects related to sexual function in human males taking a tramadol material. The method comprises administering a phosphodiesterase inhibitor to a male taking the tramadol material. The invention also provides pharmaceutical compositions. In one embodiment, the composition comprises a tramadol material and a phosphodiesterase inhibitor. The invention further provides kits. In one embodiment, the kit comprises a tramadol material and a phosphodiesterase inhibitor.
Owner:AYTU BIOSCI
Novel anelgesic combination
The invention discloses a method of administering a pharmaceutical combination comprising an NSAID and a slow release tramadol to a mammal in need of thereof. This invention further discloses an analgesic combination comprising an NSIAD and a slow release tramadol for treating pain and pain related conditions.
Owner:NECTID INC
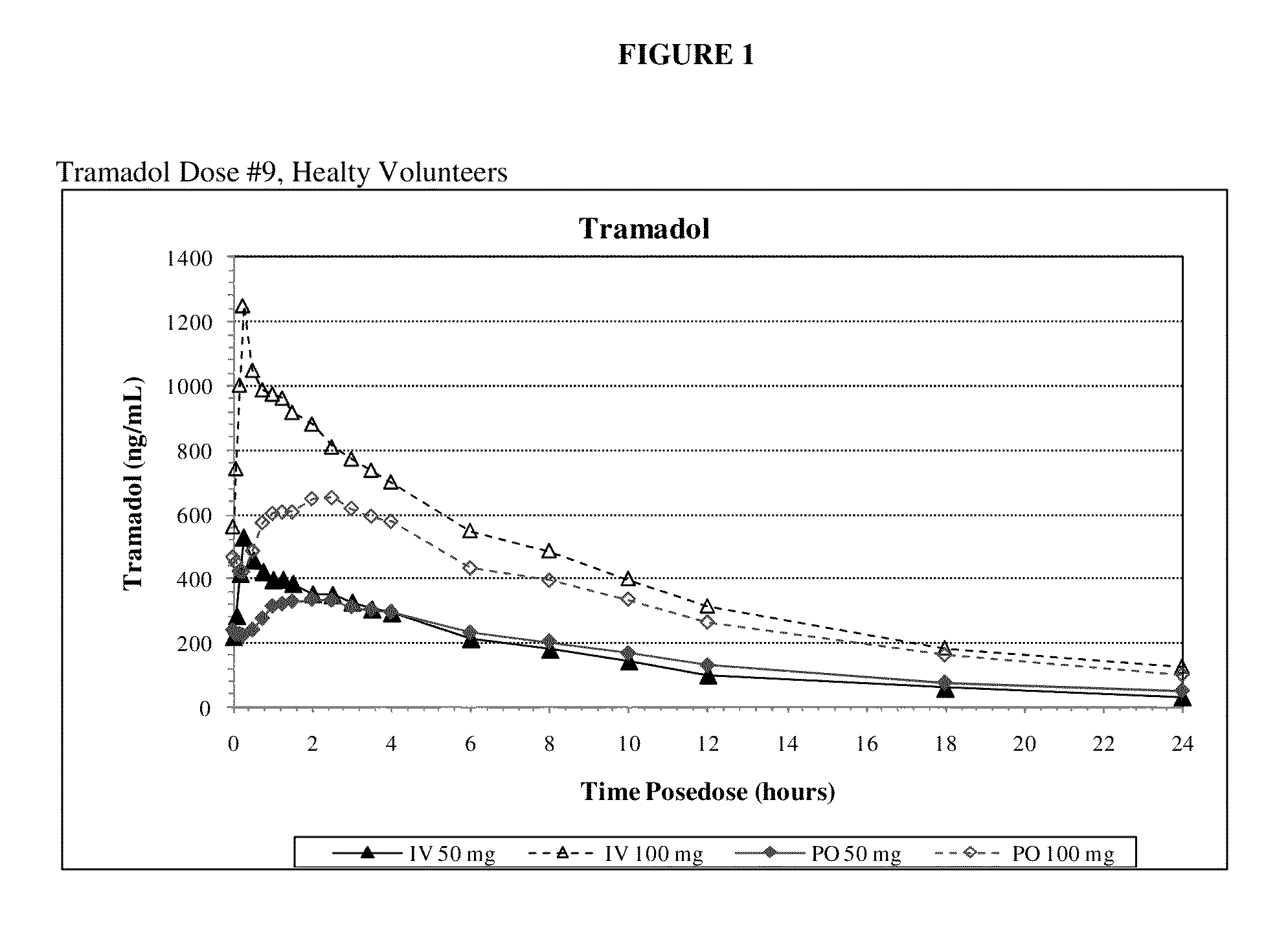
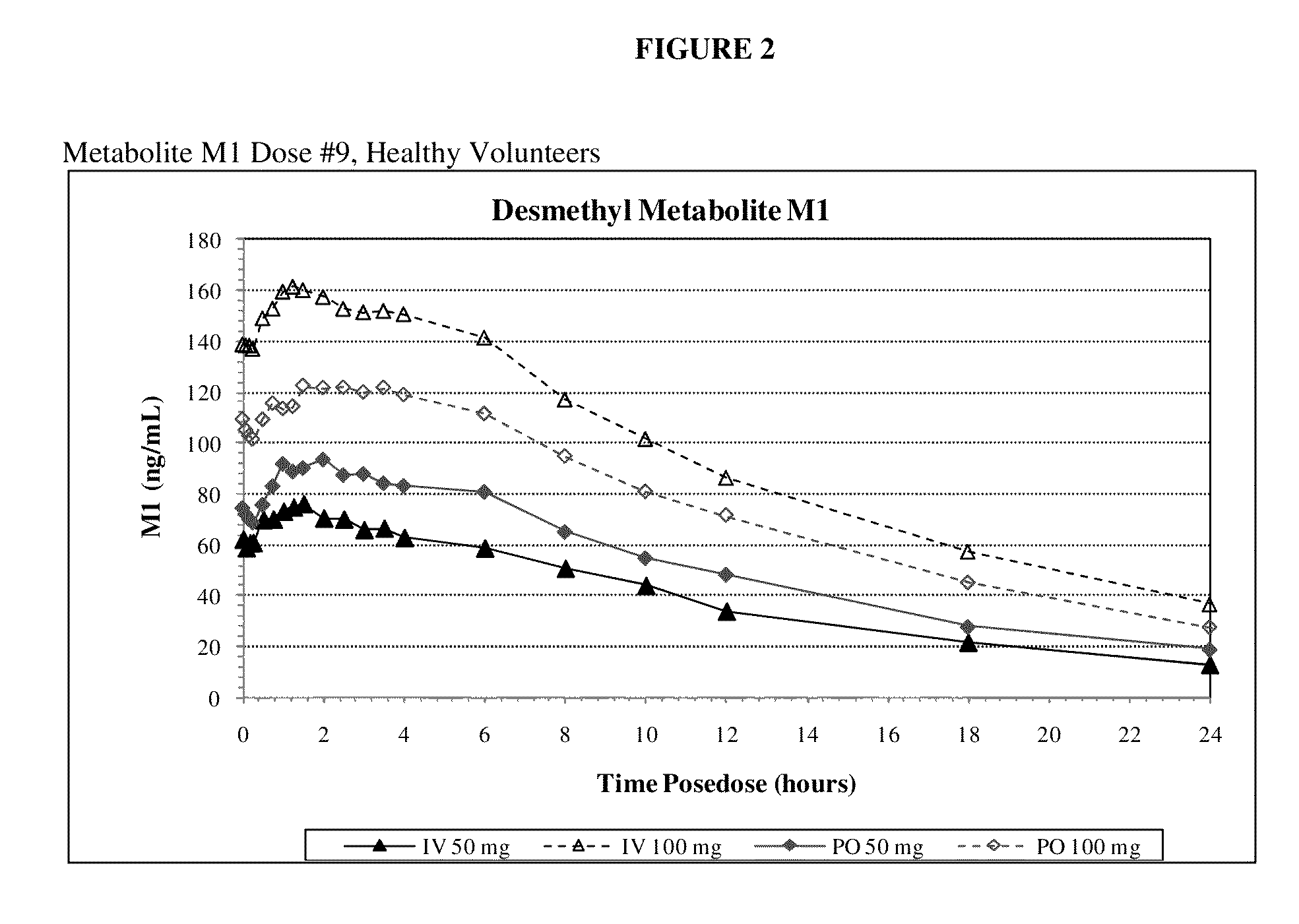
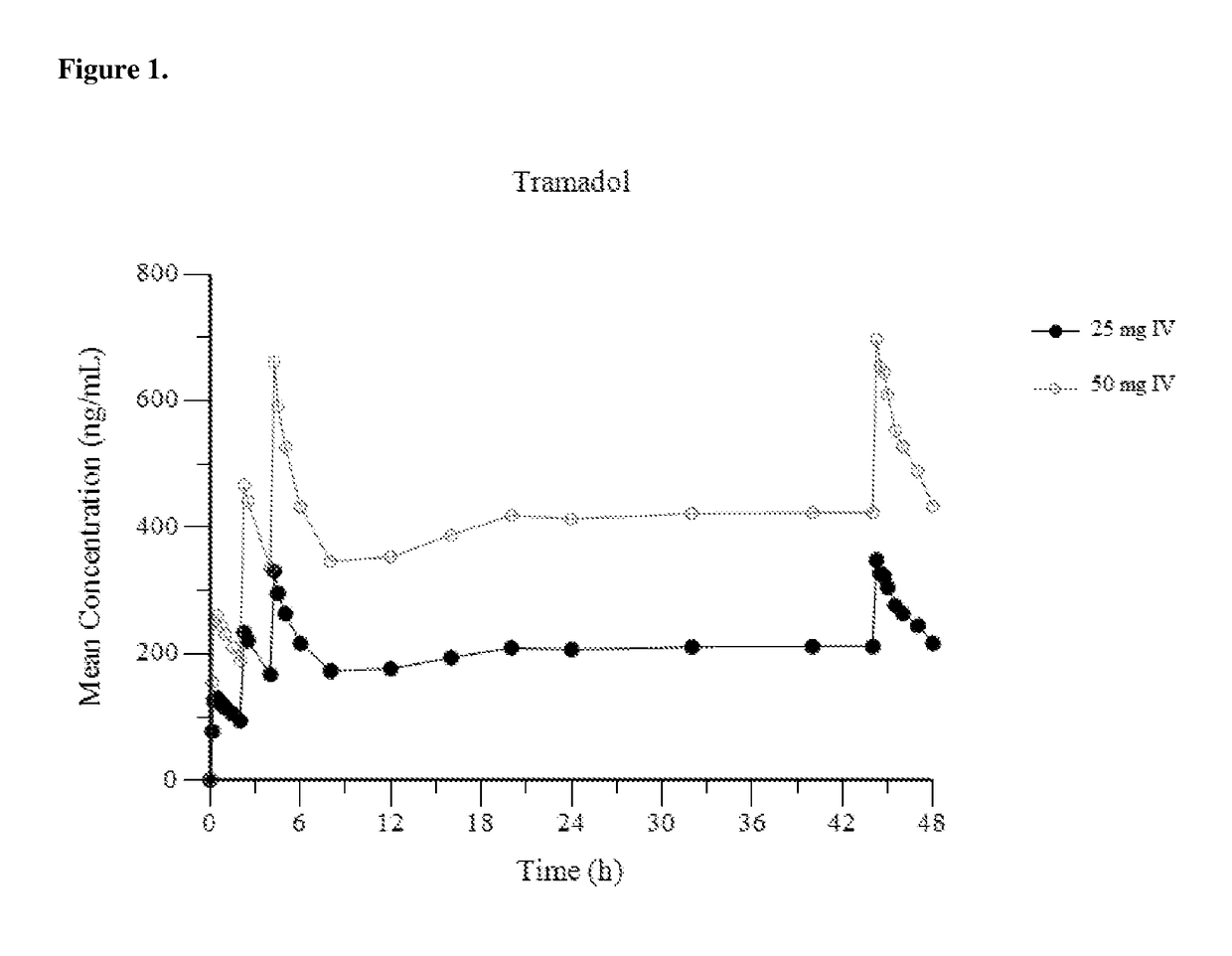
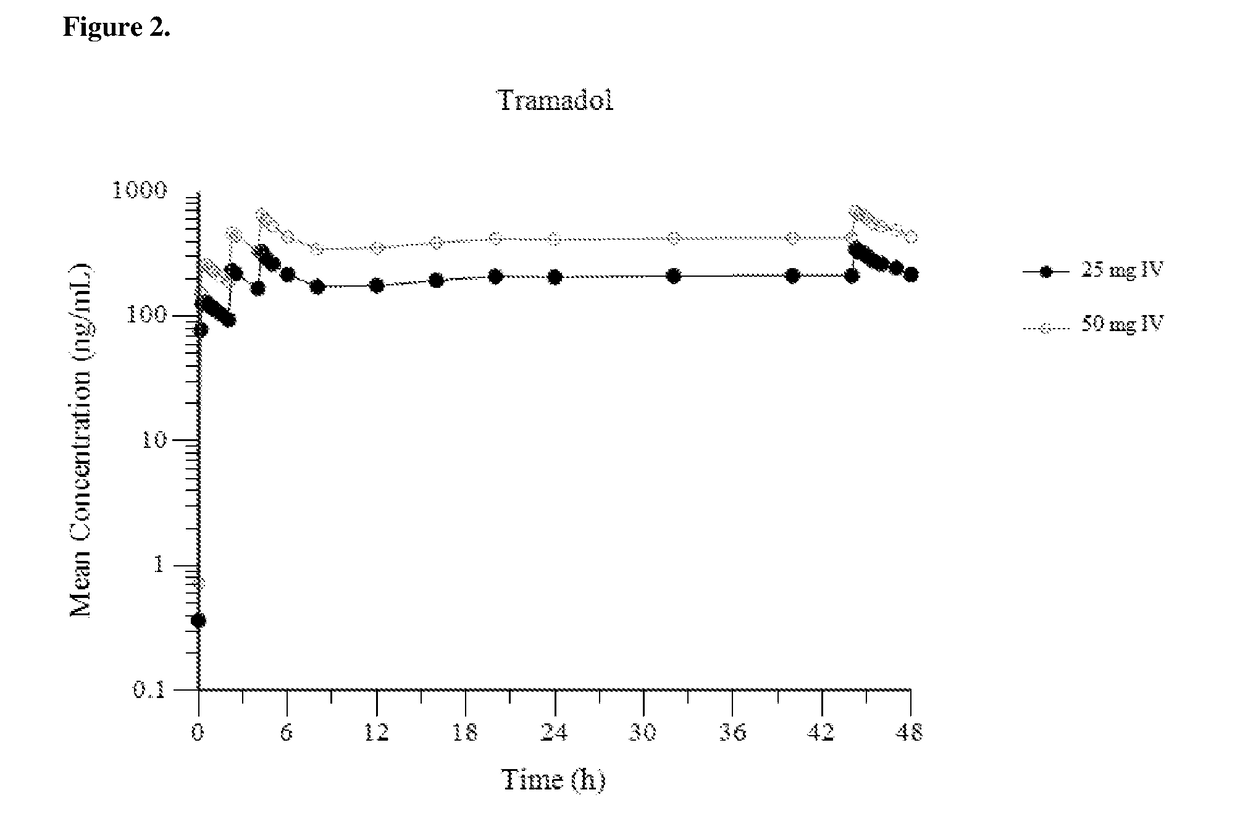
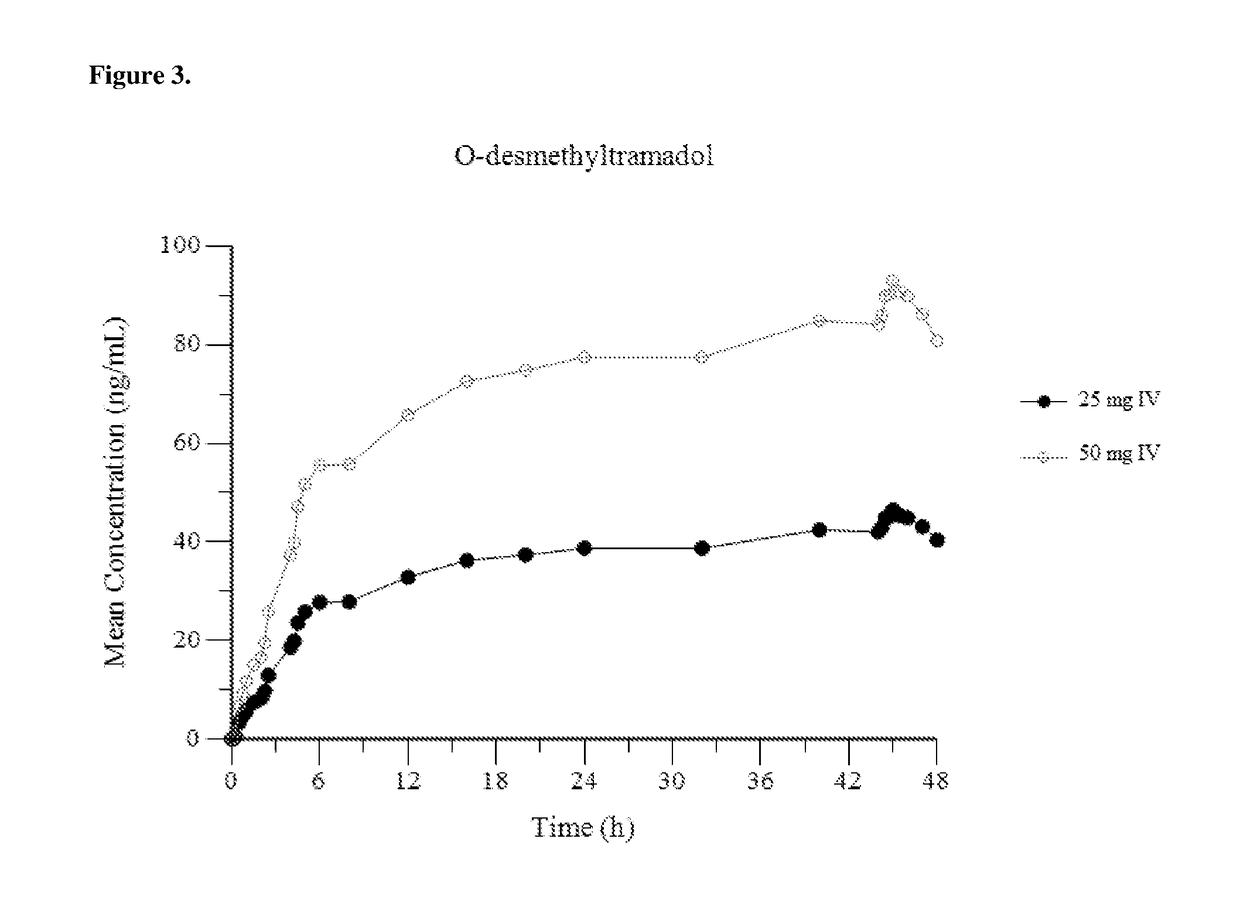
Intravenous administration of tramadol
ActiveUS8895622B2Rapid onsetEliminate side effectsBiocideOrganic active ingredientsIntravenous therapyHuman patient
Owner:REVOGENEX IRELAND
Extended release composition containing tramadol
InactiveUS20070122478A1Efficient ConcentrationBiocideOrganic active ingredientsBlood concentrationPharmaceutical formulation
The present invention relates to a once daily extended release pharmaceutical preparation of Tramadol or its acceptable pharmaceutical salts. The preparation provides, effective blood concentration for a period of about 24 hours with reduced peak concentrations. It is characterized that effective Tramadol levels appears within the first hours after administration, the time to maximal Tramadol content Tmax is at least 10 hours and the peak Tramadol concentration is less than three times the concentration obtained after 24 hours of said administration.
Owner:DEBOECK ARTHUR M +2
Controlled release preparations comprising tramadol and topiramate
This invention relates to an oral pharmaceutical preparation, suitable for dosing every 24 hours, comprising a substrate, which substrate comprises a pharmaceutically effective amount of tramadol or a salt thereof and a pharmaceutically effective amount of topiramate and wherein said substrate may be coated with a controlled release coating; said preparation having a specific dissolution rate in vitro.
Owner:CILAG
Process
InactiveUS6323368B1Organic compound preparationOptically-active compound separationEnantiomerDi-p-toluoyltartaric acid
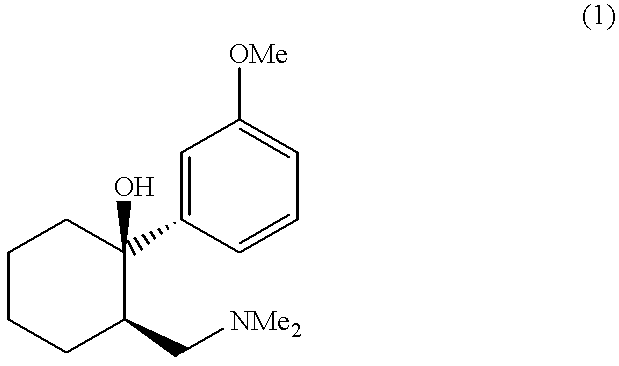
A process for preparing a substantially single enantiomer of tramadol, or a pharmaceutically-acceptable salt thereof, proceeds by means of a classical salt resolution using a substantially single enantiomer of O,O-di-p-toluoyltartaric acid as a resolving agent.
Owner:ARAKIS
Treatment of Depression
InactiveUS20130296437A1Impairment of activityIncreased riskBiocideOrganic active ingredientsPsychiatryTramadol
Owner:E THERAPEUTICS LTD
Sustained-release, oral pharmaceutical formulations and methods of making and using same
A sustained-release, oral pharmaceutical formulation of tramadol comprising a compound formed in situ of tramadol or a tramadol salt and a pharmaceutically acceptable acidic substance. The compound formed in situ has a desired water solubility. Also provided are methods of treatment using the pharmaceutical formulations. Method for preparing such formulations are also provided. The preparation method comprises repeatedly mixing tramadol or its salt with the acidic substance, and moistening the mixture and formulating the mixture under an energy input, such as heat or pressure. Optionally, drying, repeated granulating, extrudation and pelleting may also be included.
Owner:GRUNENTHAL GMBH
Tramadol multivesicular liposome and preparation method thereof
InactiveCN101780039AFirmly connectedHigh encapsulation efficiencyOrganic active ingredientsNervous disorderCholesterolNitrogen gas
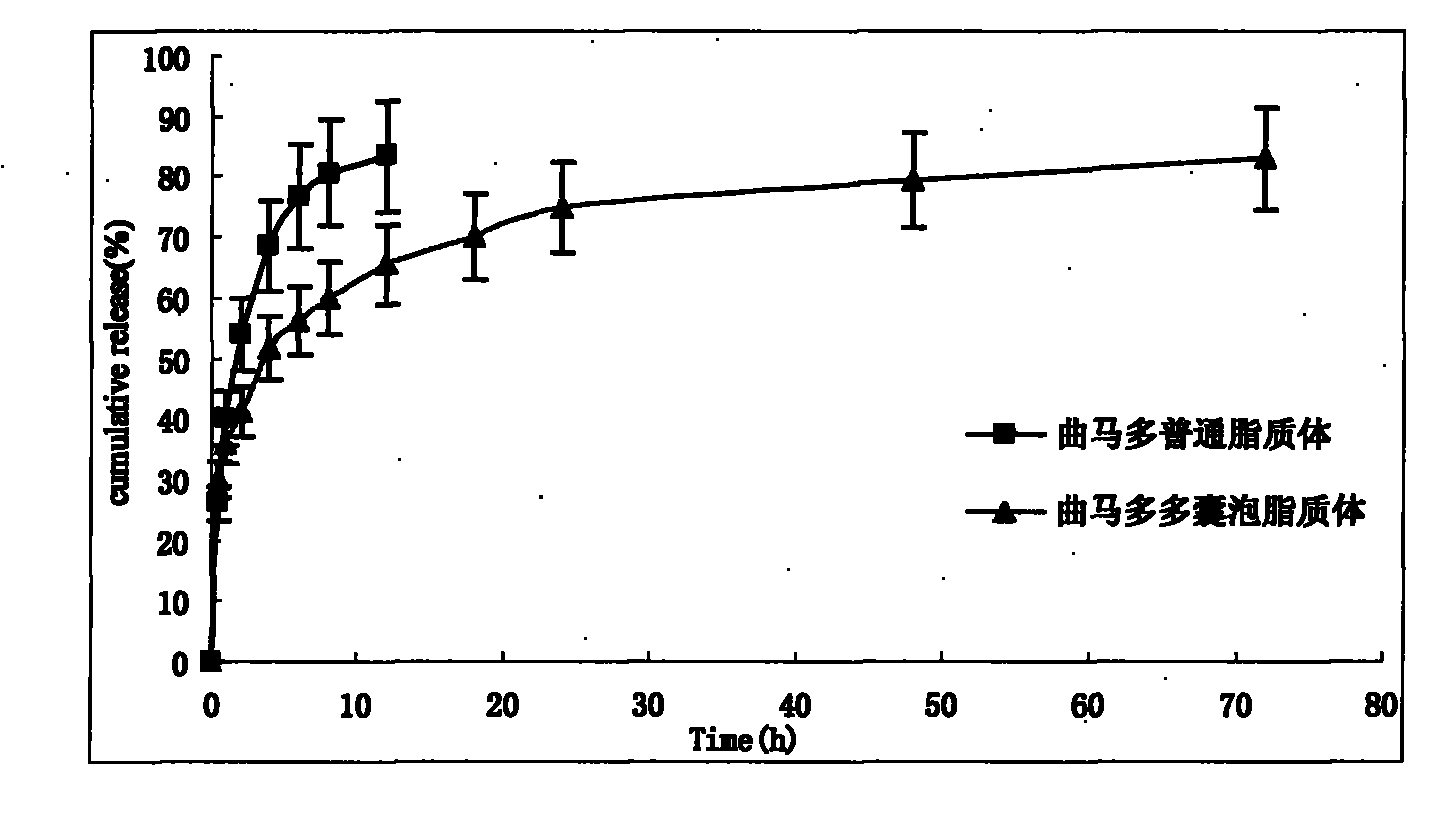
The invention discloses a tramadol multivesicular liposome and a preparation method thereof. The preparation method comprises the following steps of: 1, dissolving phospholipids, cholesterol and neutral lipids into organic solvents to obtain a mixture which serves as an organic phase; 2, preparing 10 to 500mmol / L tramadol solution which serves as an internal water phase; 3, adding the internal water phase with the same volume as that of an organic phase into the organic phase, and mixing and emulsifying the mixture to obtain water-in-oil primary emulsion; 4, preparing an external water phase containing amino acid and osmotic modulators and / or surfactants, and adding the external water phase of which the volume is 2 to 10 times that of the water-in-oil primary emulsion into the water-in-oil primary emulsion, stirring the mixture to form oil-in-water type double emulsion; 5, adding the emulsion into the solution of the amino acid, introducing nitrogen or carbon dioxide into the mixed solution to remove the organic solvent from the emulsion to obtain suspension; 6, dissolving the suspension into the solution of amino acid, centrifuging and taking lower liposome suspension to obtain the tramadol multivesicular liposome. The prepared tramadol multivesicular liposome has the advantages of higher encapsulation efficiency, good slow release effect, and longer analgesic effect.
Owner:NANJING HAILING TRADITIONAL CHINESE MEDICINE RES CO LTD +2
Intravenous administration of tramadol
Owner:REVOGENEX IRELAND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com