Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2375 results about "Blisters" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
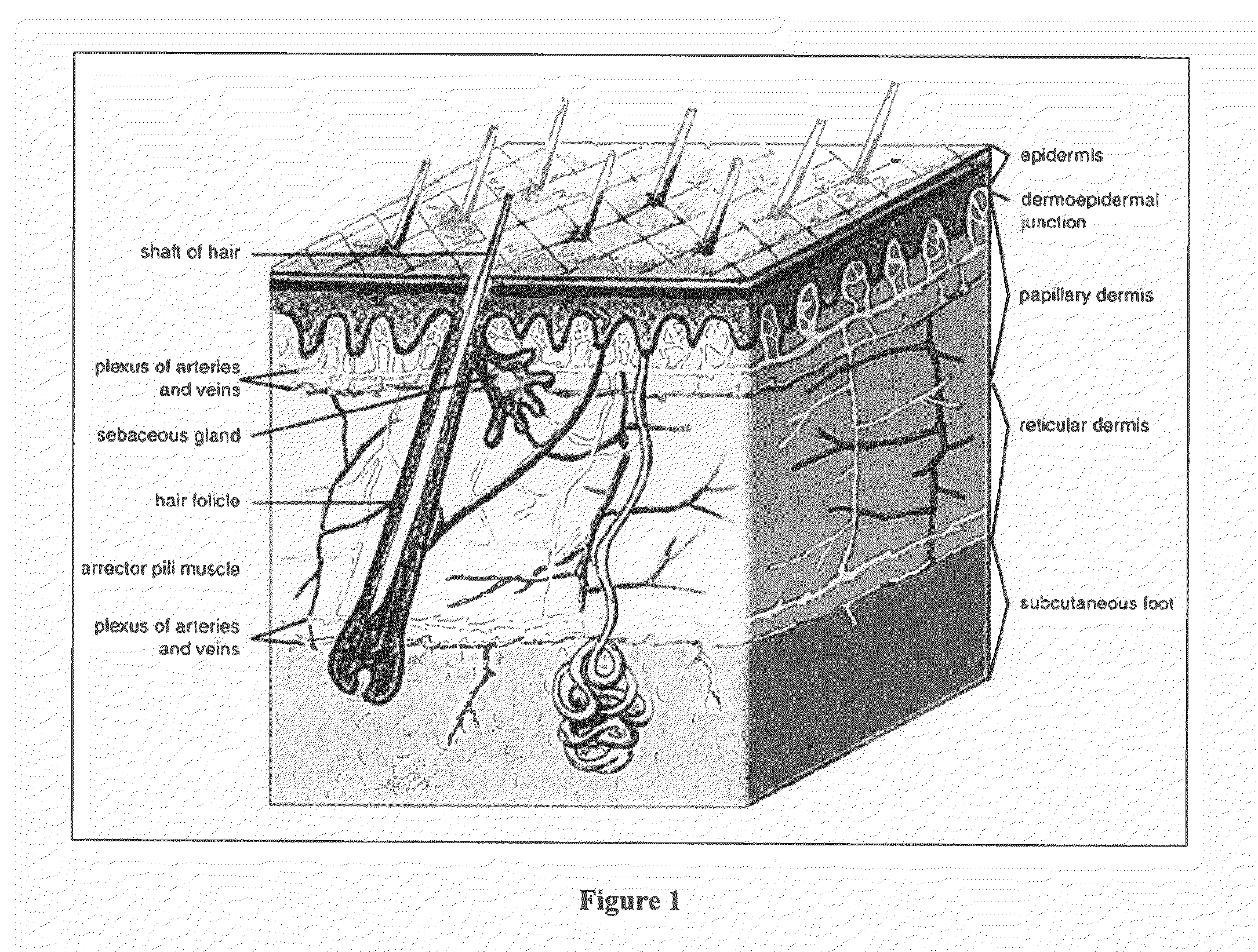
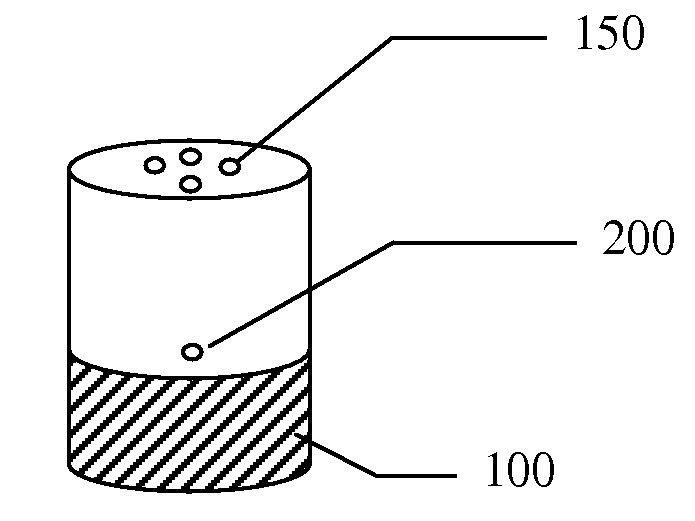
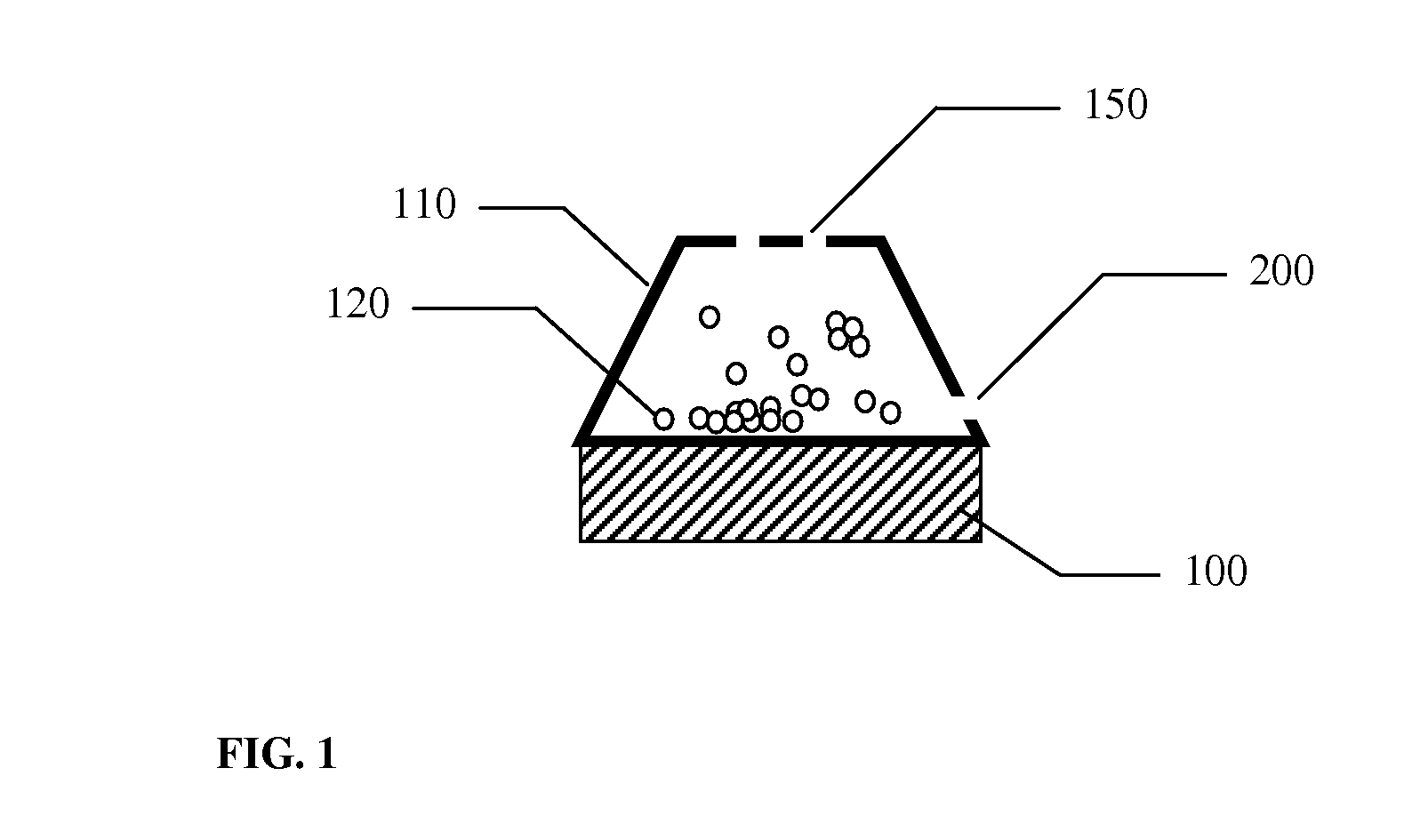
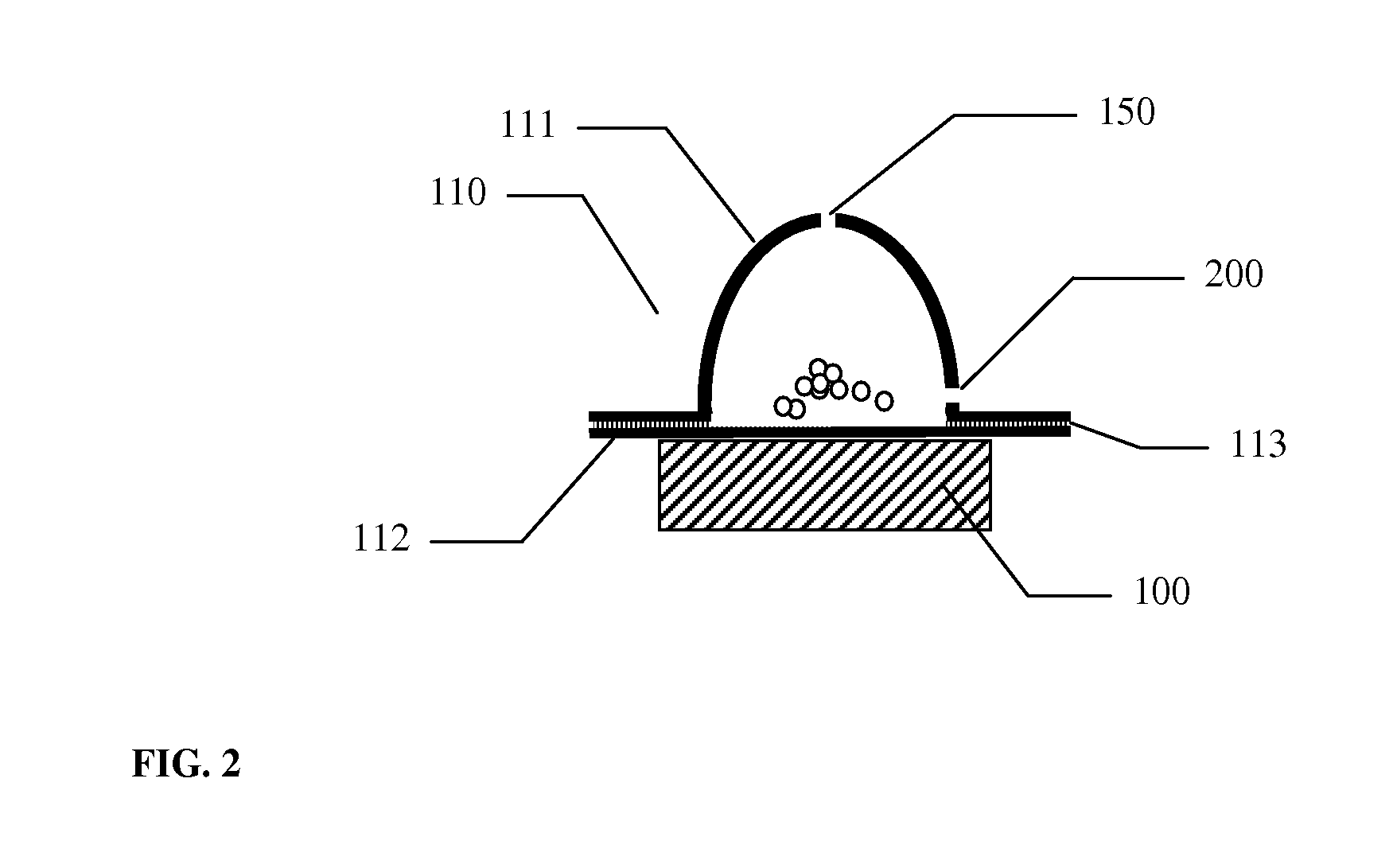
A small pocket of body fluid (serum, blood, or pus) within the upper layers of the skin.
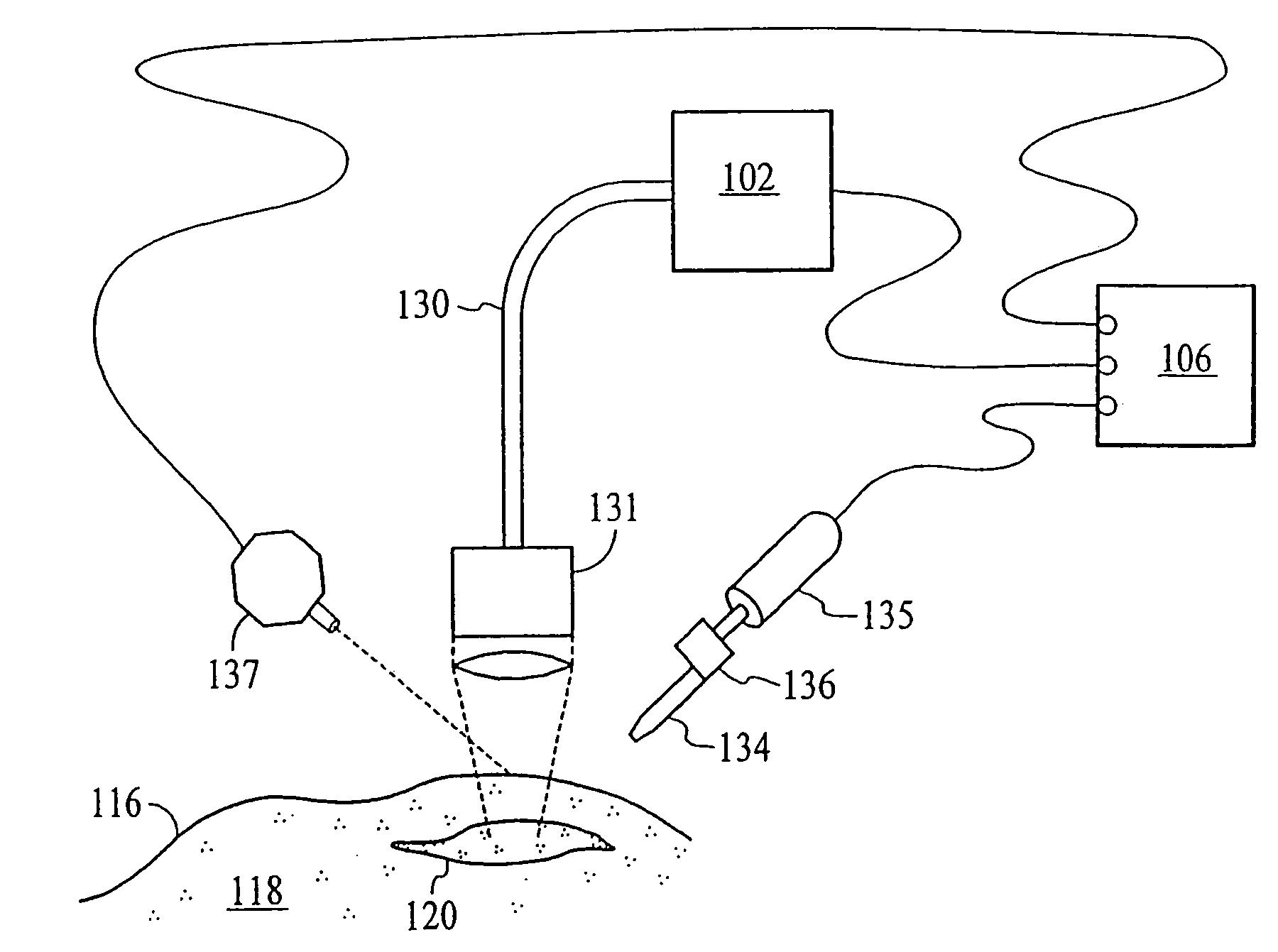
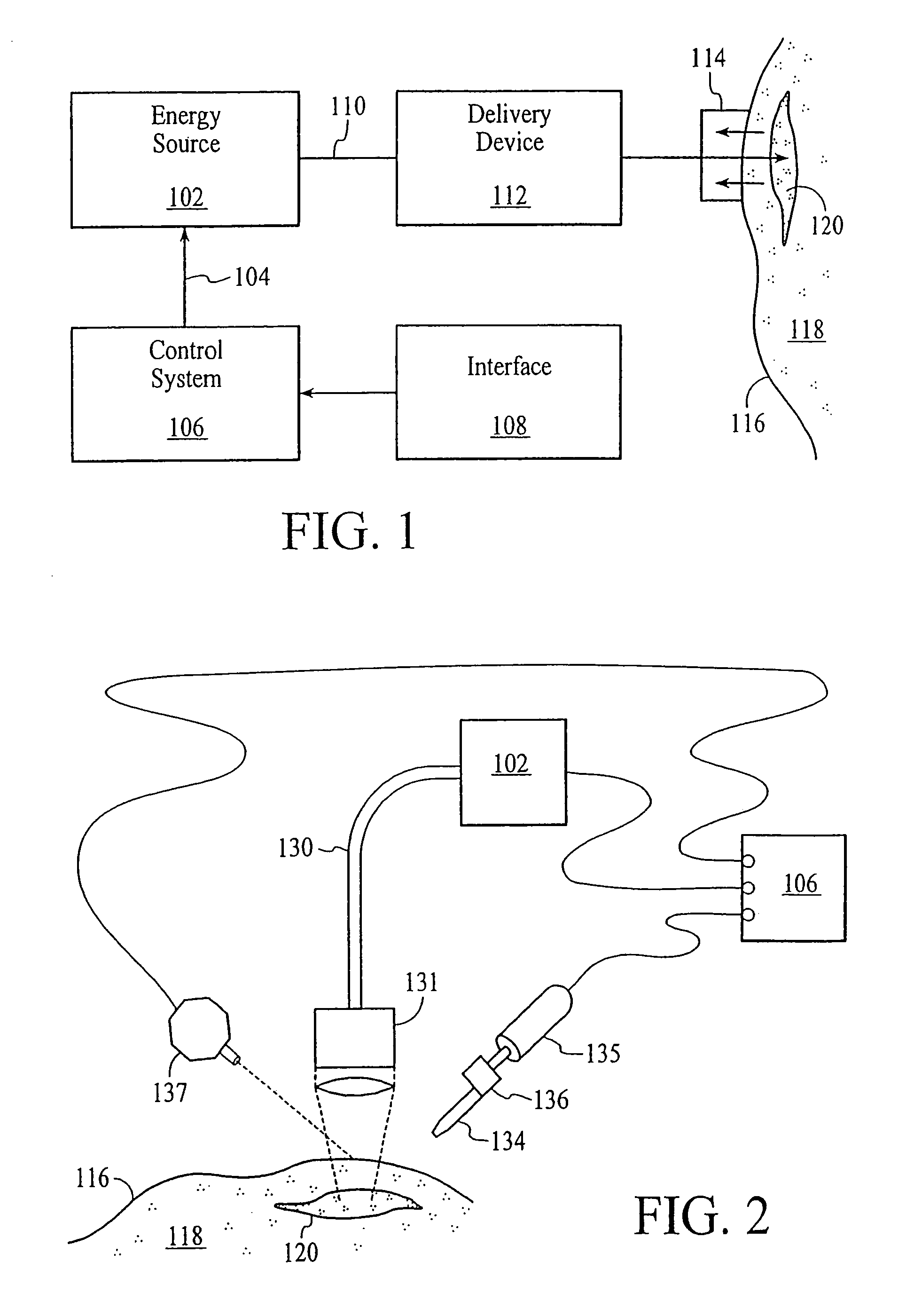
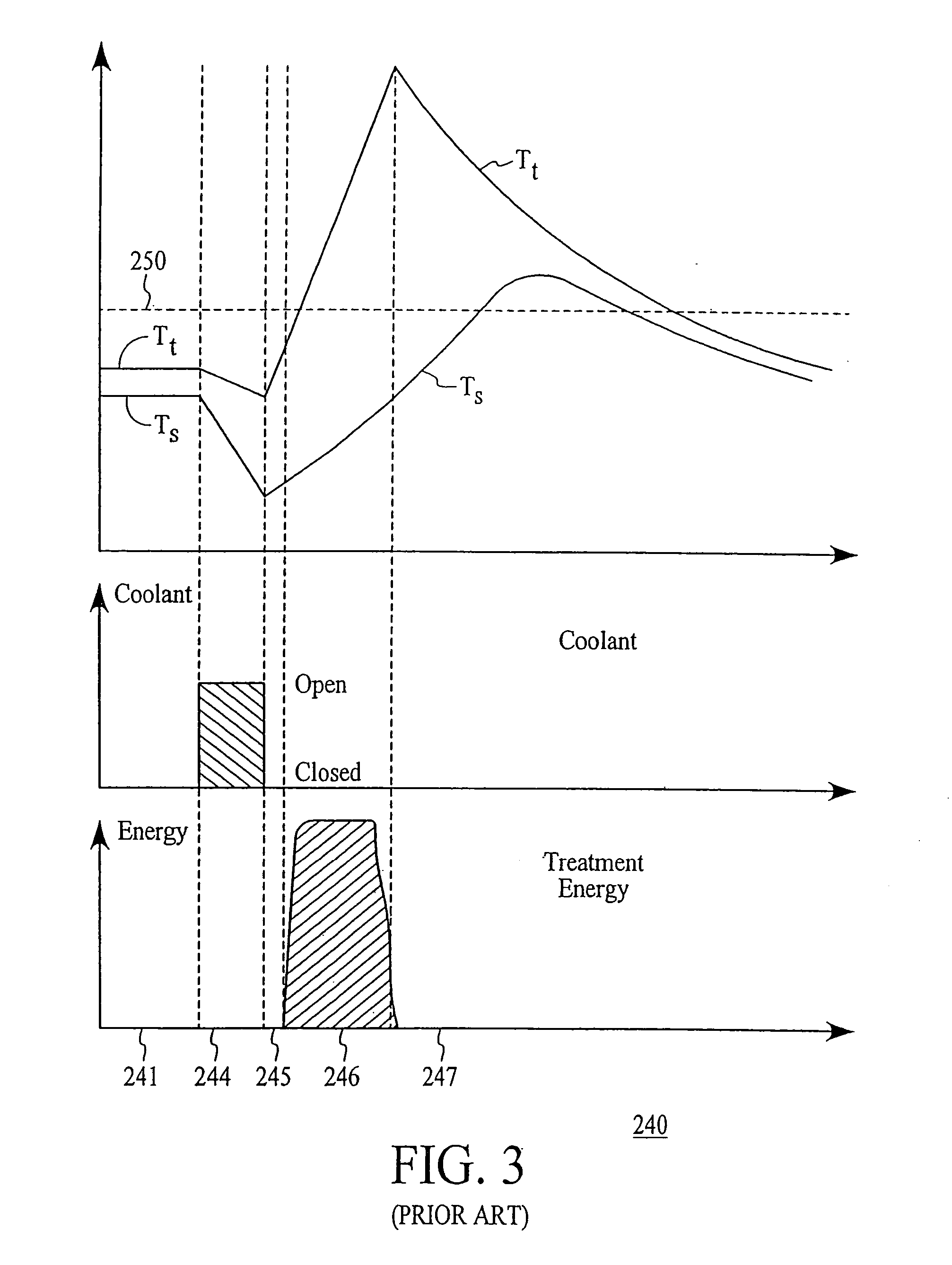
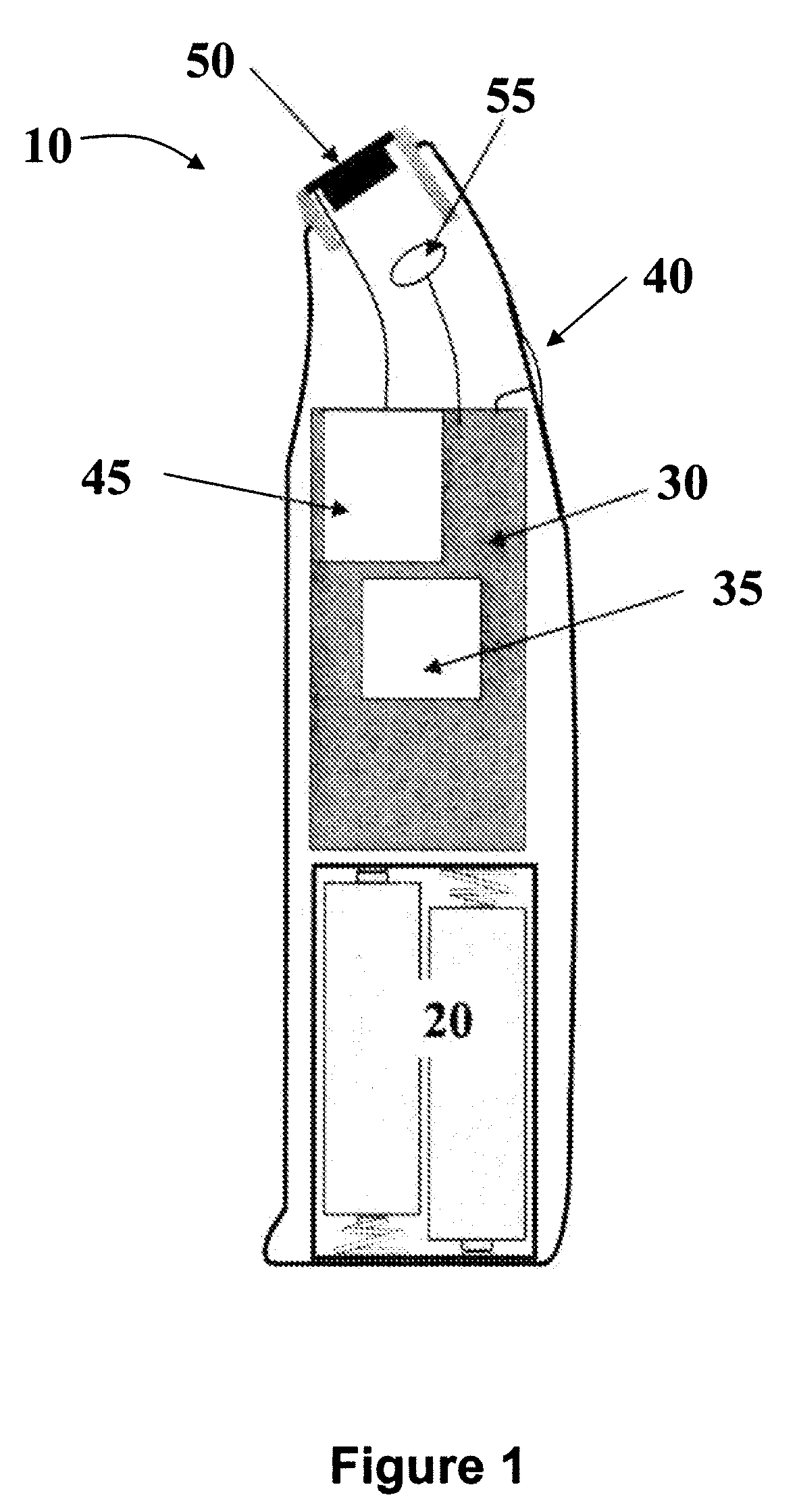
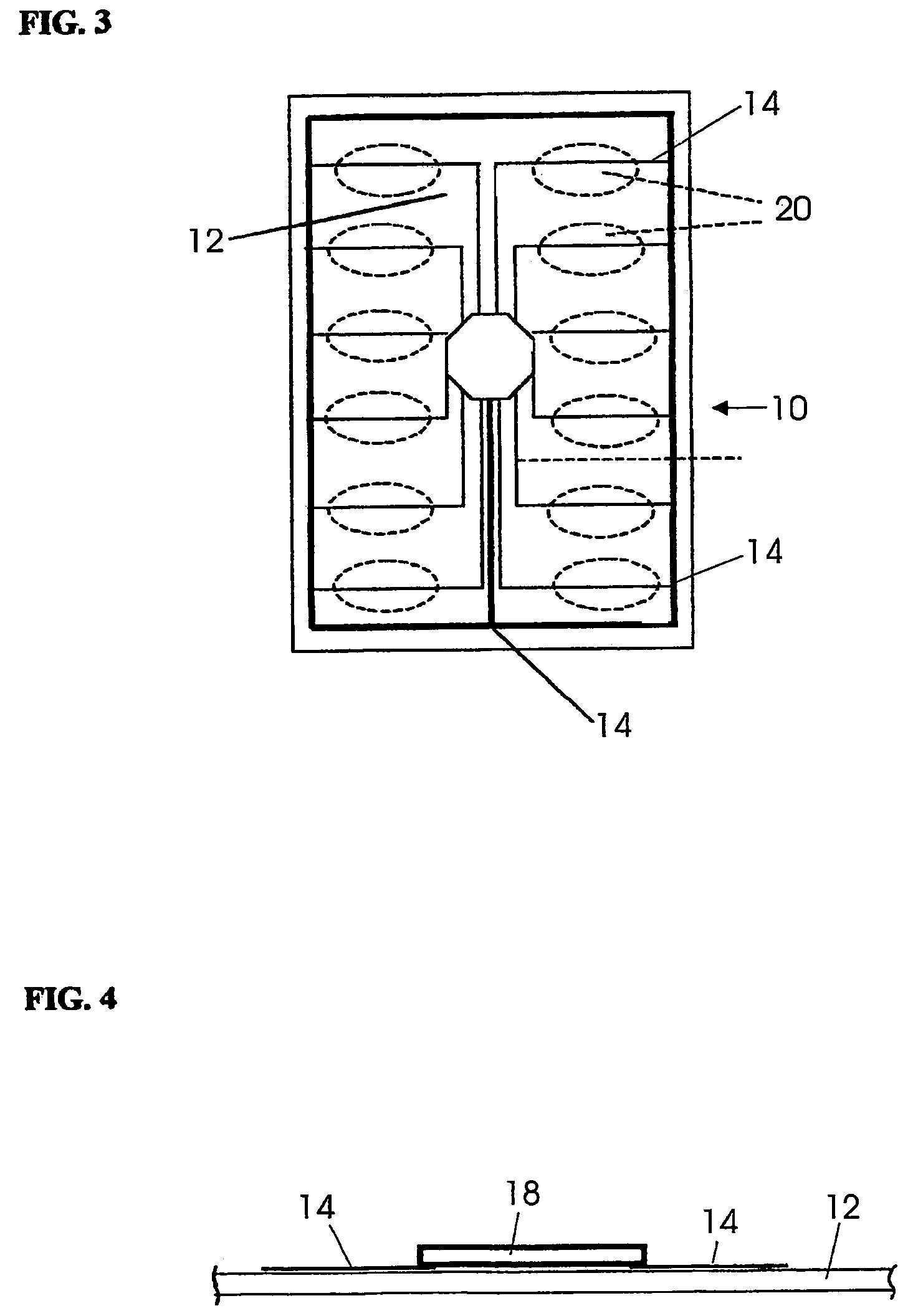
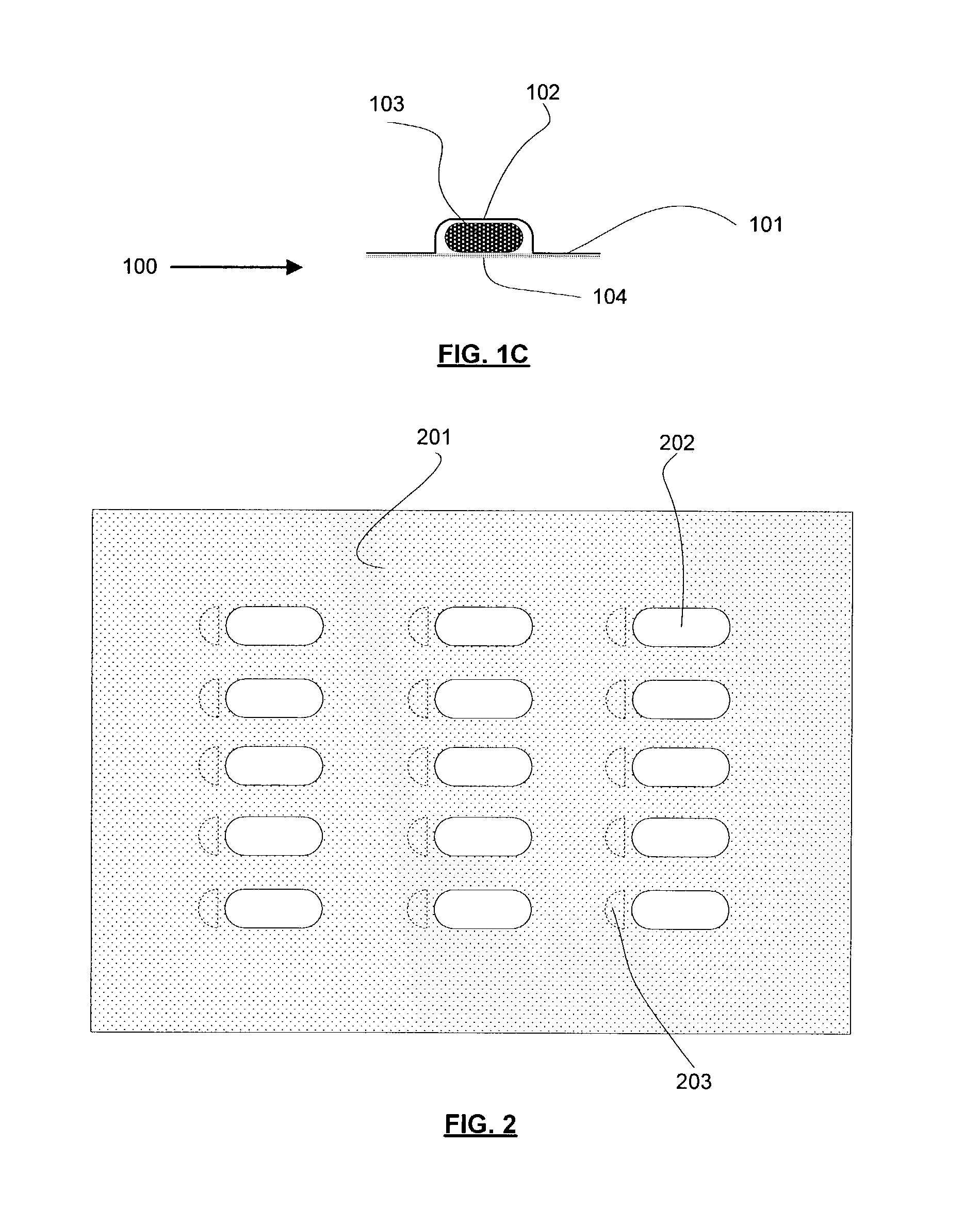
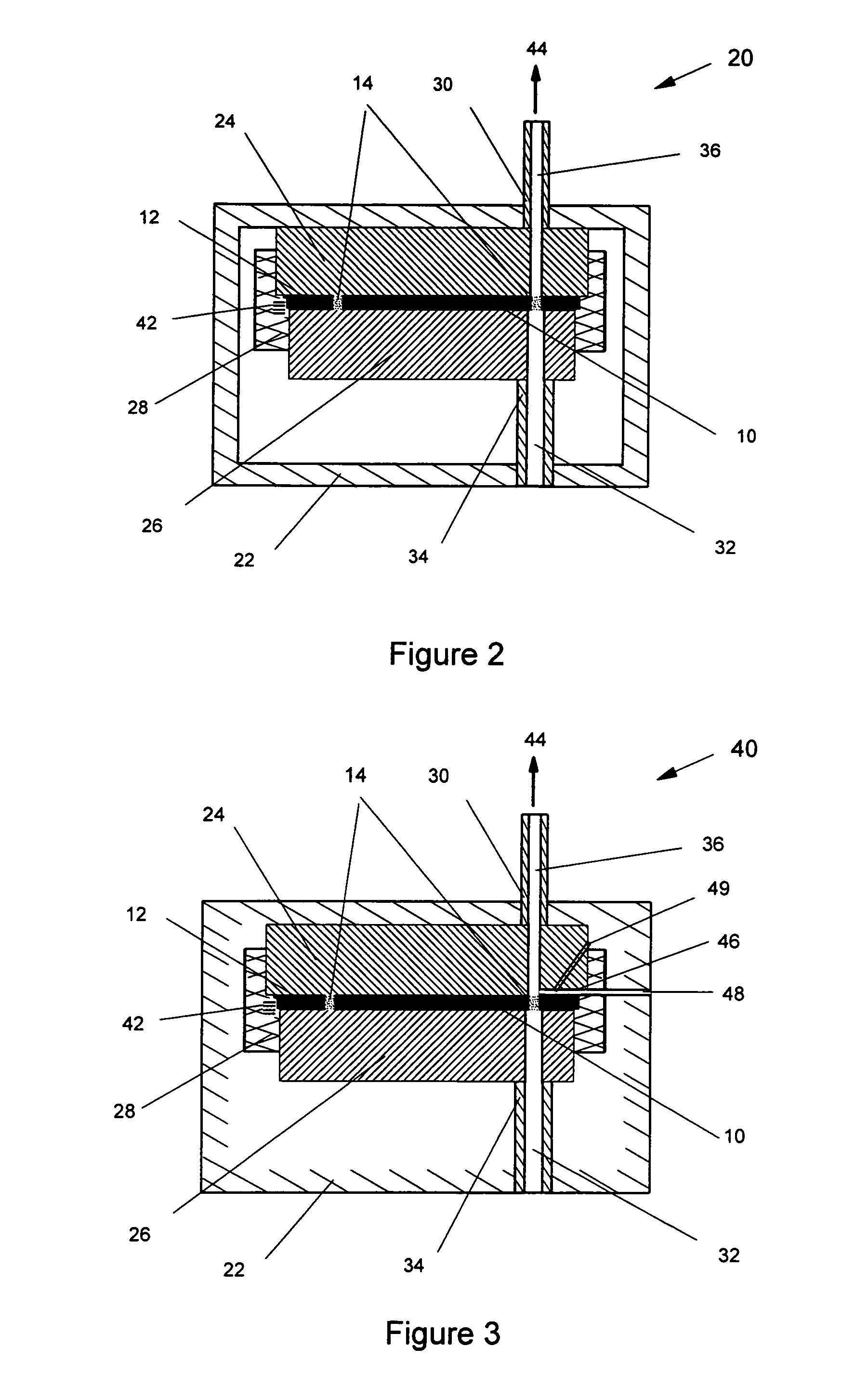
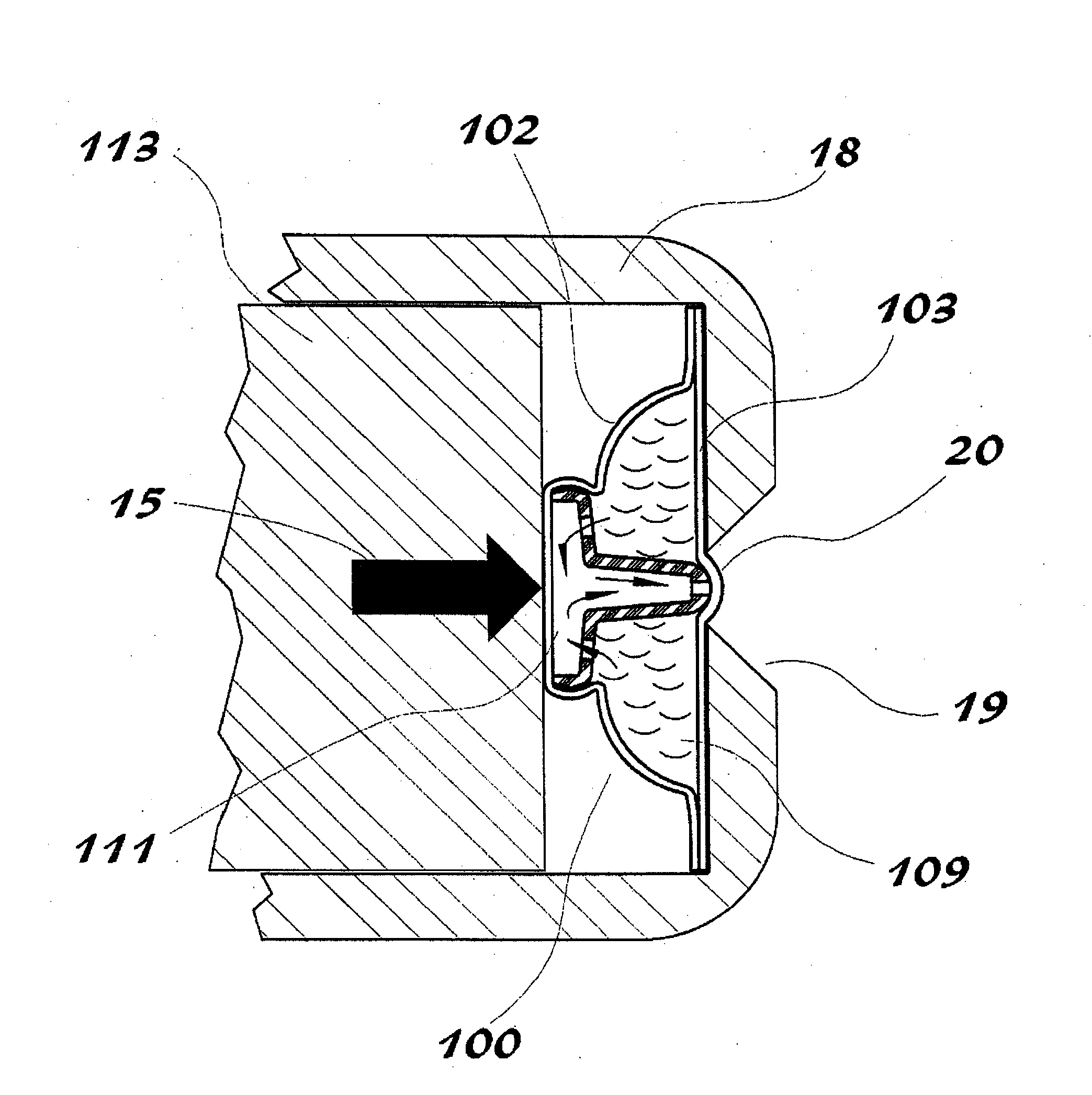
Thermal quenching of tissue
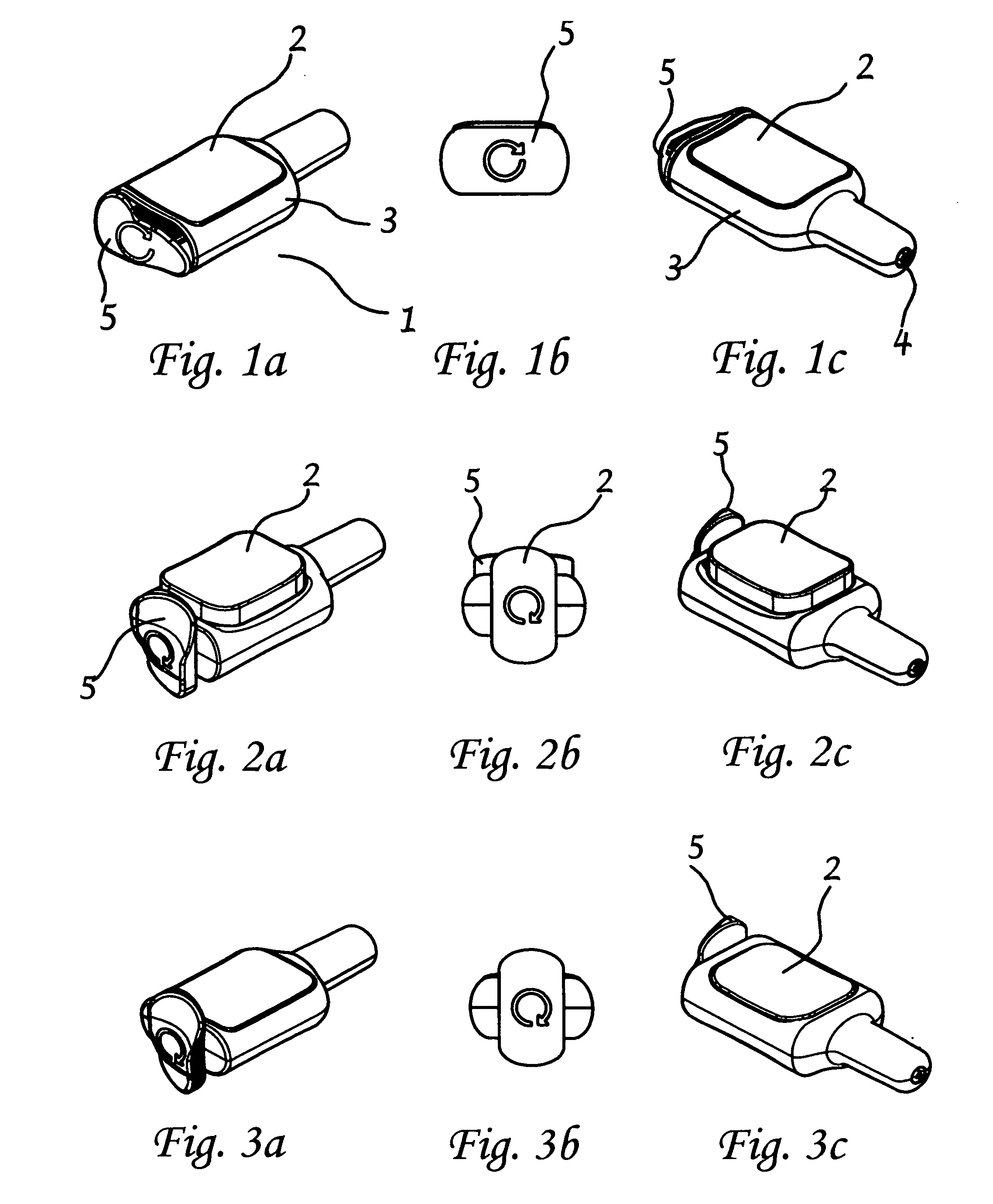
InactiveUS7122029B2Selective heatingHeat buildDiagnosticsSurgical instruments for heatingCvd riskBlisters
Owner:NEW STAR LASERS
Pharmaceutical compositions for lipophilic drugs
InactiveUS7070802B1Good self-emulsifying performanceShelf-stableCyclic peptide ingredientsCapsule deliveryMonoglycerideCyclosporins
Stable solutions of lipophilic drugs, such as cyclosporin, forming a polar lipid self-emulsifying drug delivery system. The solutions can include lipophilic drugs, such as cyclosporin, dissolved in a polar lipid, such as having a C6-C12 fatty acid monoglyceride content of at least about 50%, surfactants and triglycerides. The composition forms a fine emulsion on exposure to water. The encapsulated dosage form of this composition needs neither a hydrophilic component nor air-tight blister packaging, and is particularly suitable for oral administration.
Owner:WATSON LAB INC
Devices and Methods for Treatment of Skin Conditions
InactiveUS20080139974A1Removing fine wrinkleClean skinUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyWrinkle skinHand held
Devices and methods for the treatment of skin conditions and lesions are disclosed herein. A compact hand held device that can be safely used by those suffering from skin conditions, such as acne, warts, cold blisters, blemished skin, or fine wrinkles. The devices employ the application of ultrasound energy and heat for the treatment of skin conditions and lesions. Typically, the peak temperatures employed are about 40° C. to about 70° C. by the devices, are achieved in less than about 20 second, and maintained for less than about 40 second. Ultrasound absorption and thermal conduction transfers heat from the device to the skin and causes a biological response that accelerates acne clearing, treats blemished skin, itching, or fine wrinkles. The total heat transferred is low enough to prevent burns.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
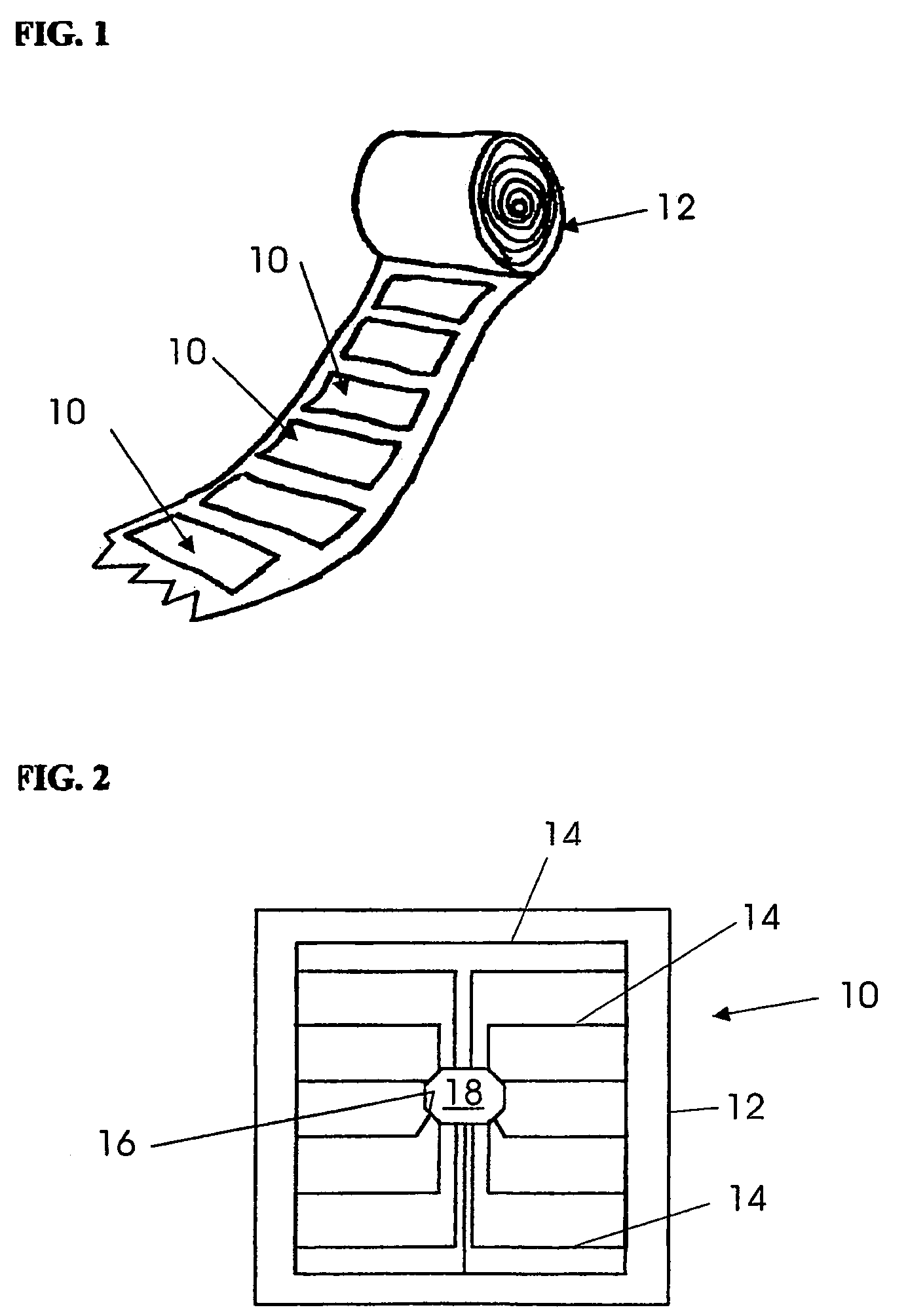
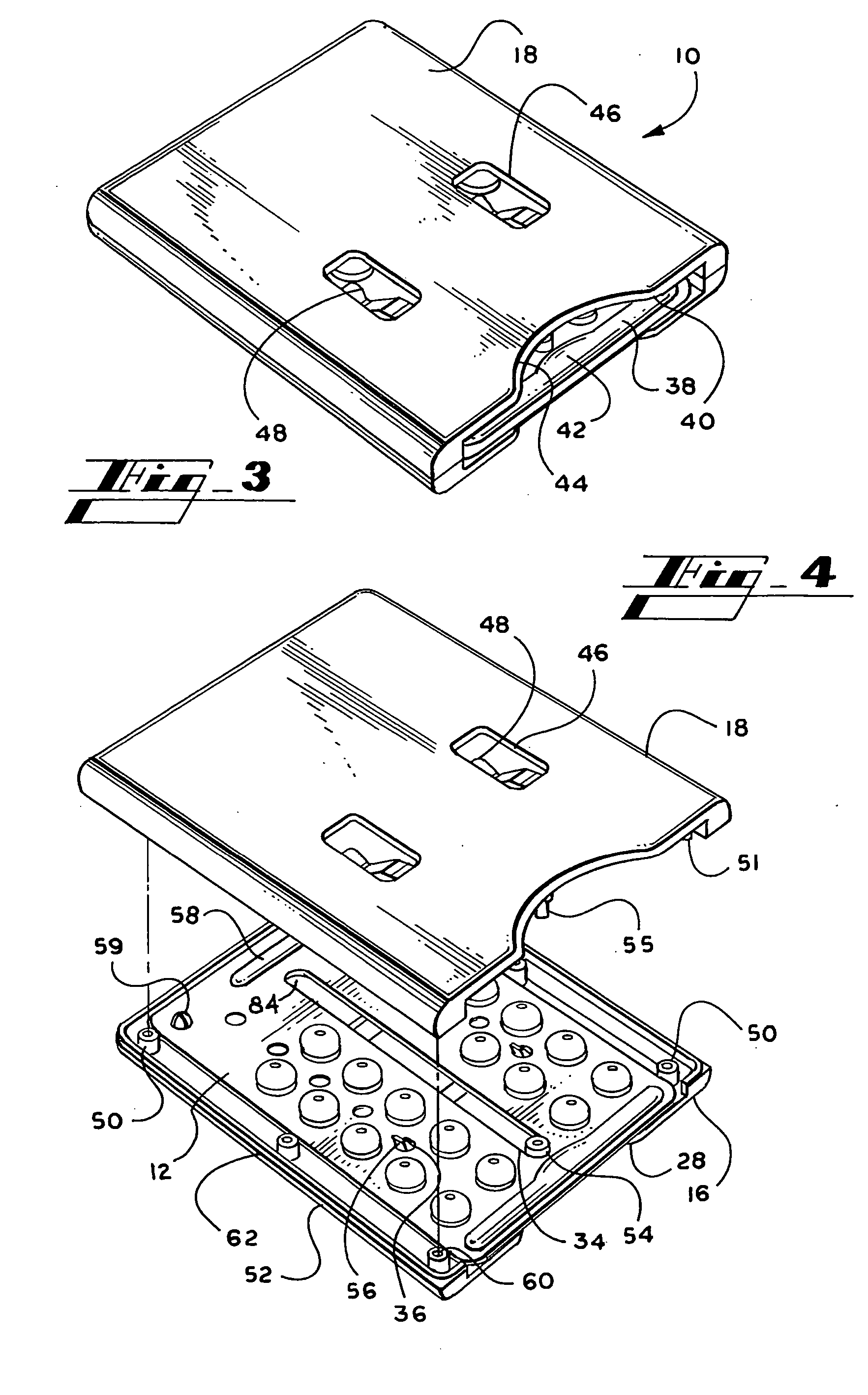
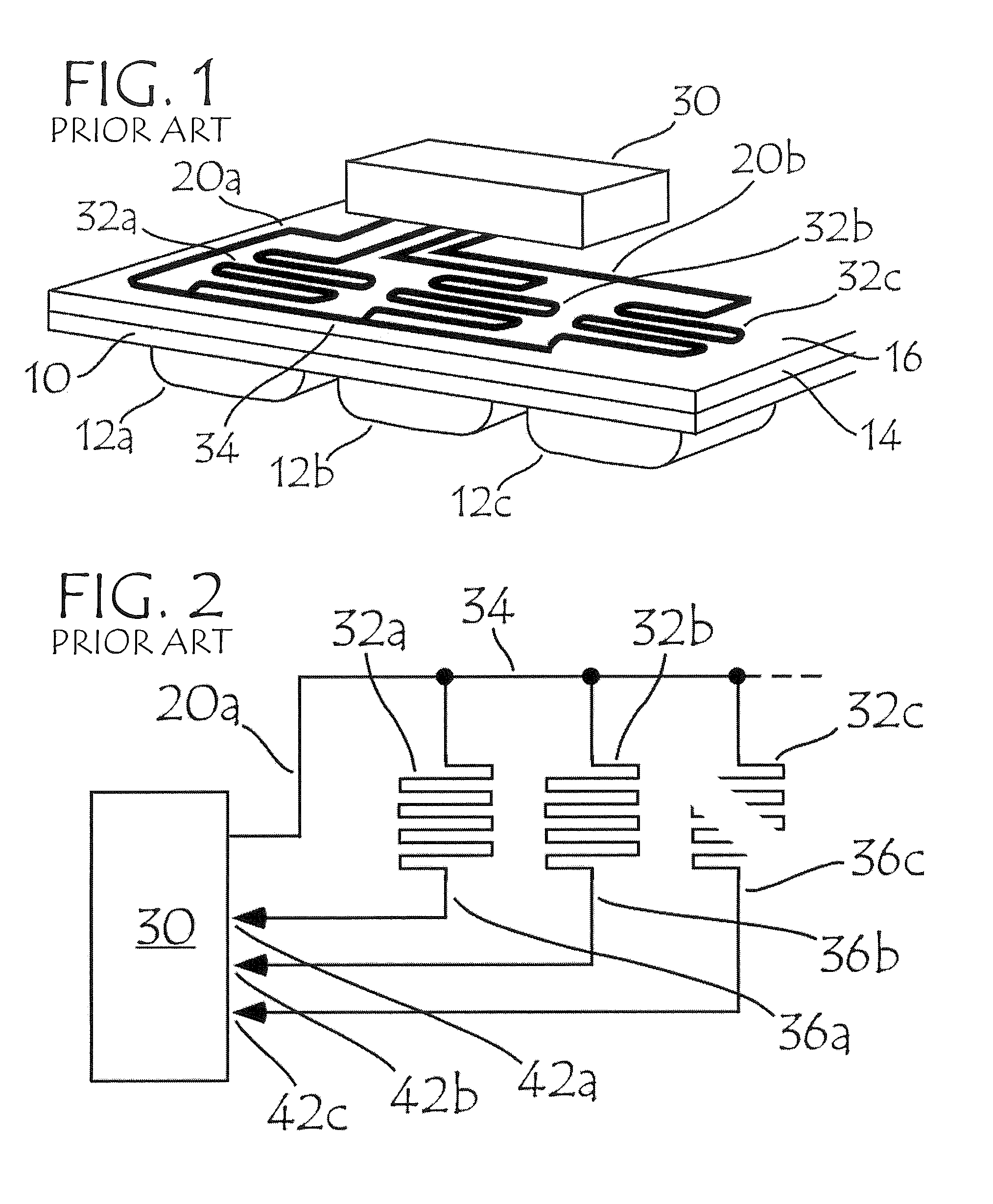
Blister package with electronic content monitoring system
InactiveUS7113101B2Universal applicabilityEfficient operabilityContainer decorationsLevel indicationsMonitoring systemBlister pack
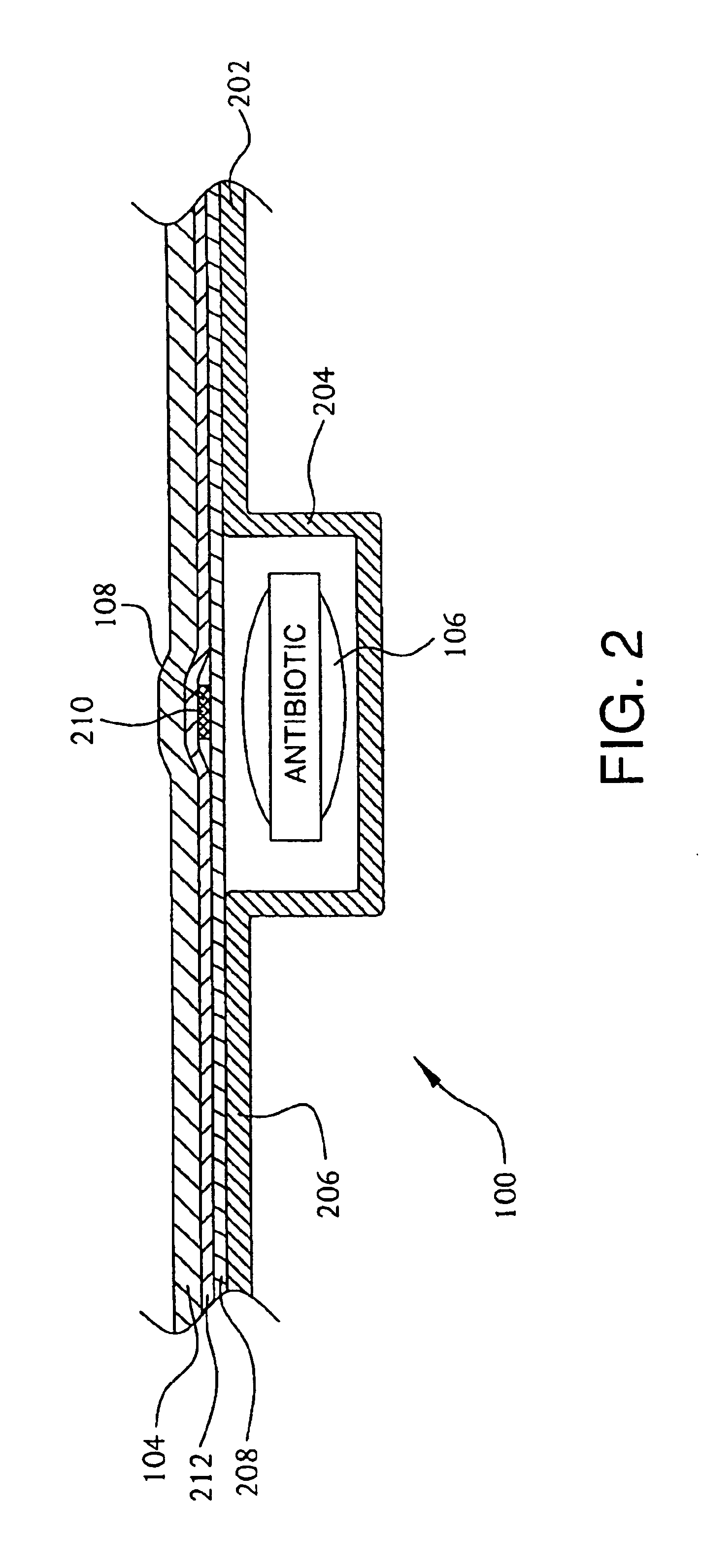
A replicate can be secured to a blister package intended to contain articles, such as pills, and is used to record the removal of individual articles from the blisters. To remove an article from a blister one will usually press against the blister to push the article through a frangible closure seal, breaking the seal in the process. The replicate includes a backing sheet which carries a plurality of traces alignable with corresponding blisters so that when the article is removed from the blister it will not only break the seal but it will also break the corresponding trace. All of the traces are connected to an integrated circuit which may also be formed or provided on the backing sheet, as is a power source for the integrated circuit. The breaking of the trace is an event that is recorded in the integrated circuit for later accessability. The replicate may be secured to the blister package after the package has been produced by conventional form-fill-seal equipment. The individual traces can be formed into a grid of closely spaced traces so that alignment of the traces with the individual blisters is less critical. The replicates may be formed by printing or other conventional methods on a roll of lidstock. After forming the individual replicates are severed from the roll of lidstock for securement to a blister package.
Owner:INTELLIGENT DEVICES SEZC
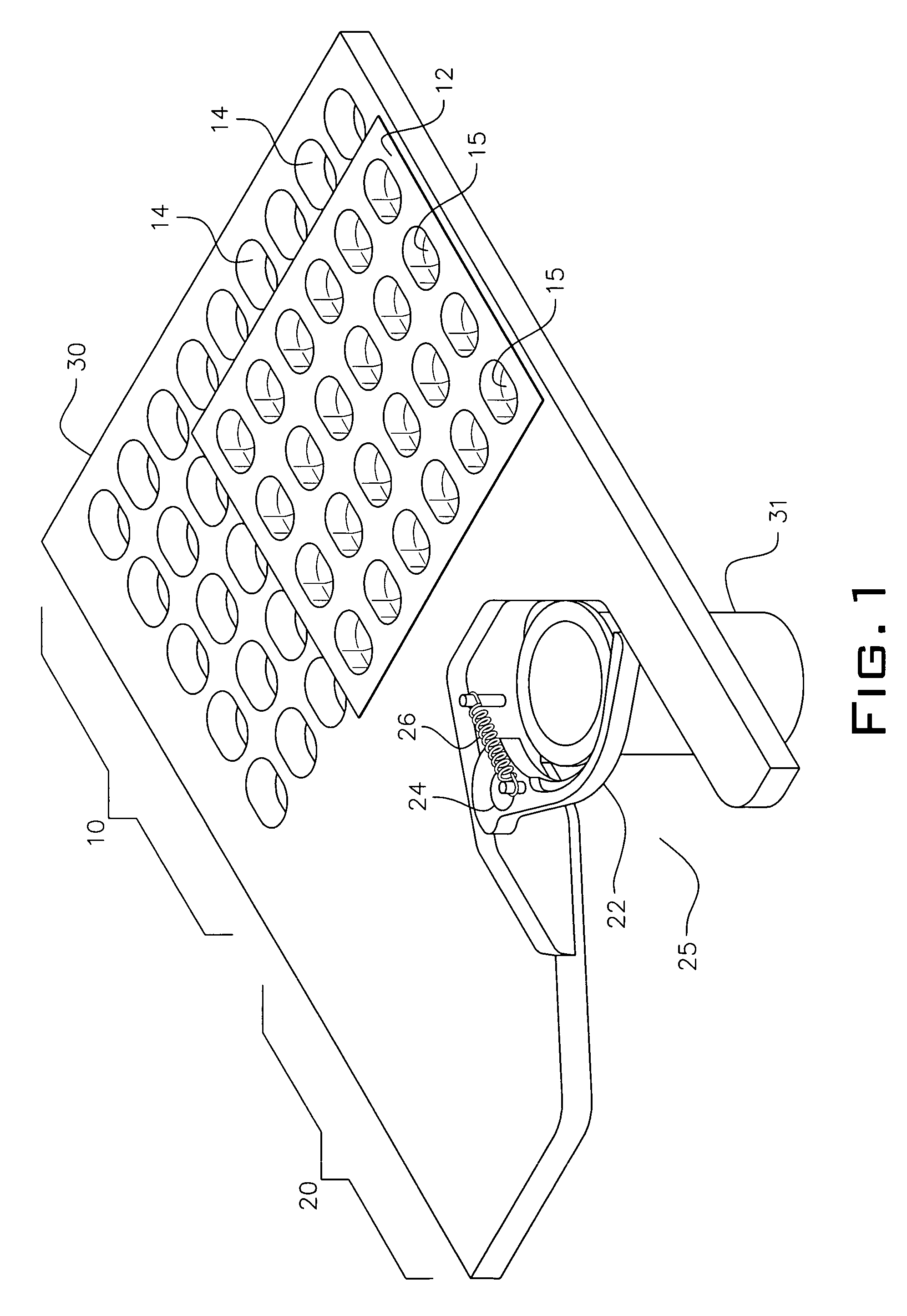
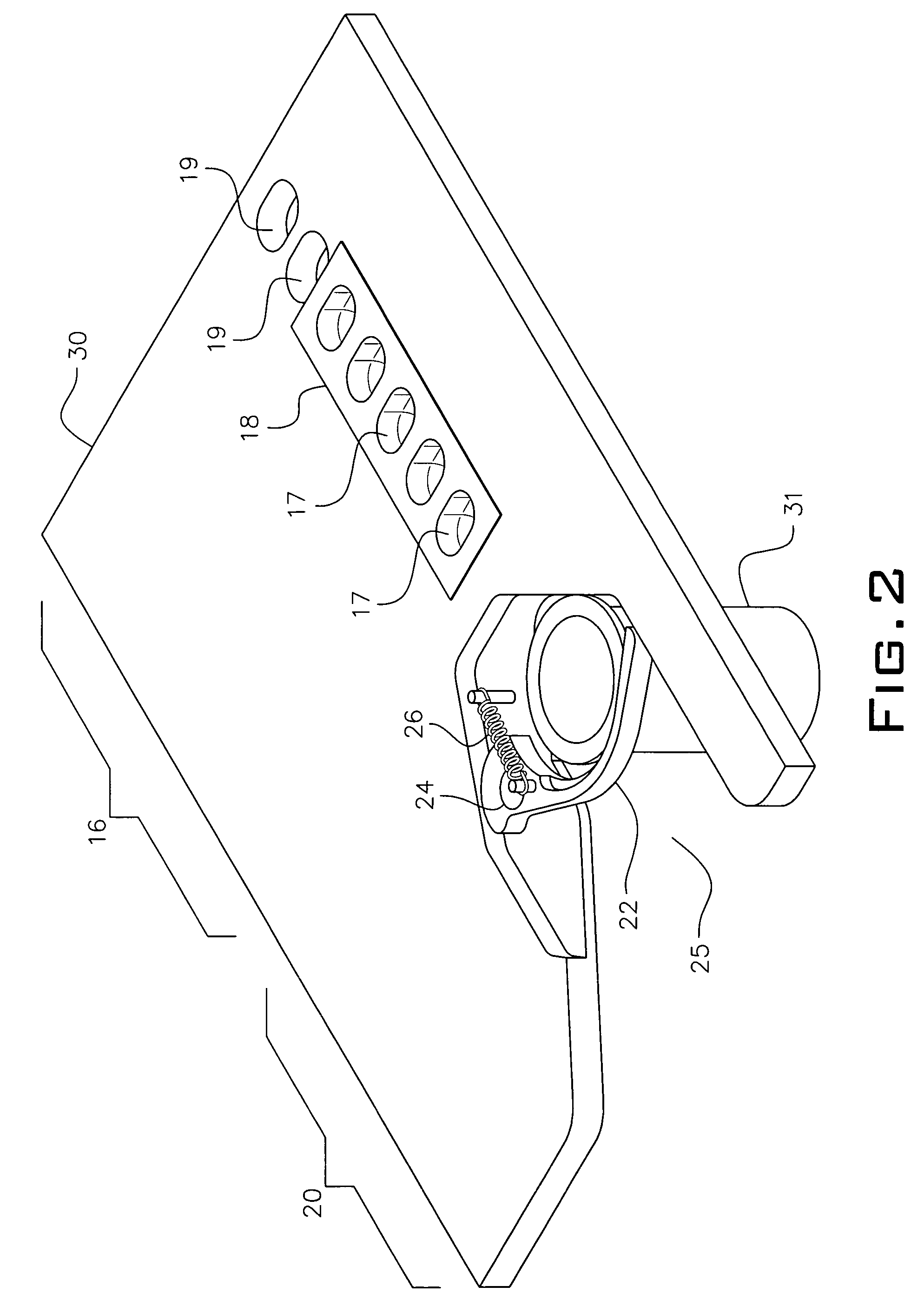
Dry powder inhalers, related blister devices, and associated methods of dispensing dry powder substances and fabricating blister packages
InactiveUS6889690B2Easy to optimizeLimit amount of resistanceSmall article dispensingLiquid surface applicatorsPowder InhalerInhalation
The present invention includes dry powder inhalers and associated multi-dose dry powder packages for holding inhalant formulated dry powder substances and associated fabrication and dispensing methods. The multi-dose package can include a platform body comprising at least one thin piezoelectric polymer material layer defining at least a portion of a plurality of spatially separated discrete elongate dry powder channels having an associated length, width and height; and a metallic material attached to selected portions of the piezoelectric polymer material including each of the regions corresponding to the elongate dry powder channels to, in operation, define active energy releasing vibratory channels. In operation, the elongate channels can be selectively individually activated to vibrate upon exposure to an electrical input.The dry powder inhaler includes an elongate body having opposing first and second outer primary surfaces with a cavity therebetween and having opposing top and bottom end portions and a multi-dose sealed blister package holding a plurality of discrete meted doses of a dry powder inhalable product located in the cavity of the elongate body. The inhaler also includes an inhalation port formed in the bottom end portion of the elongate body, the inhalation port configured to be in fluid communication with at least one of the discrete meted doses during use and a cover member that is pivotably attached to the elongate body so that it remains attached to the body during normal operational periods of use and moves to a first closed position to overlie the inhalation port at the bottom end portion of the body during periods of non-use and moves to a second open position away from the inhalation port during periods of use to allow a user to access the inhalation port.
Owner:ORIEL THERAPEUTICS INC
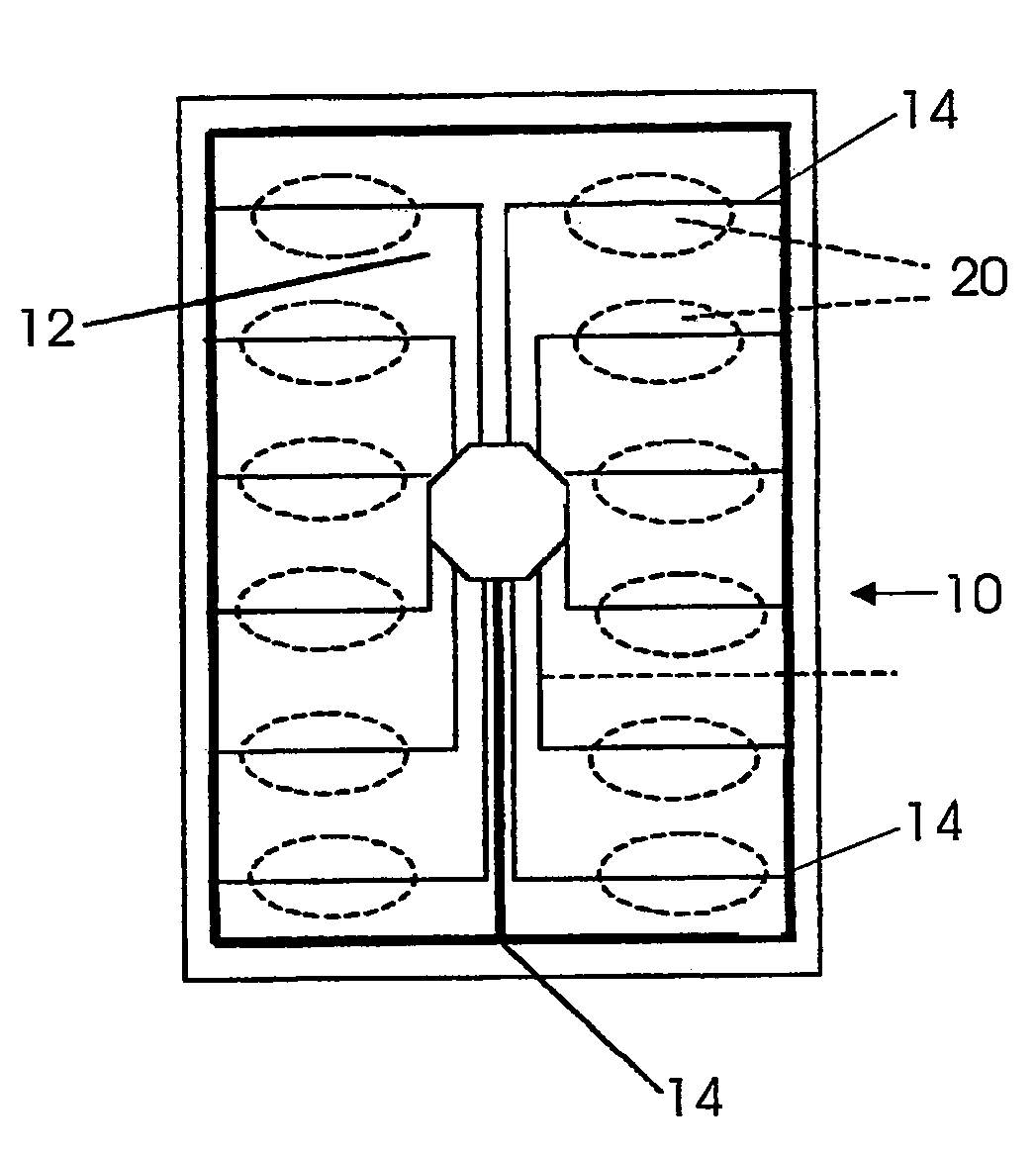
Drug delivery management system
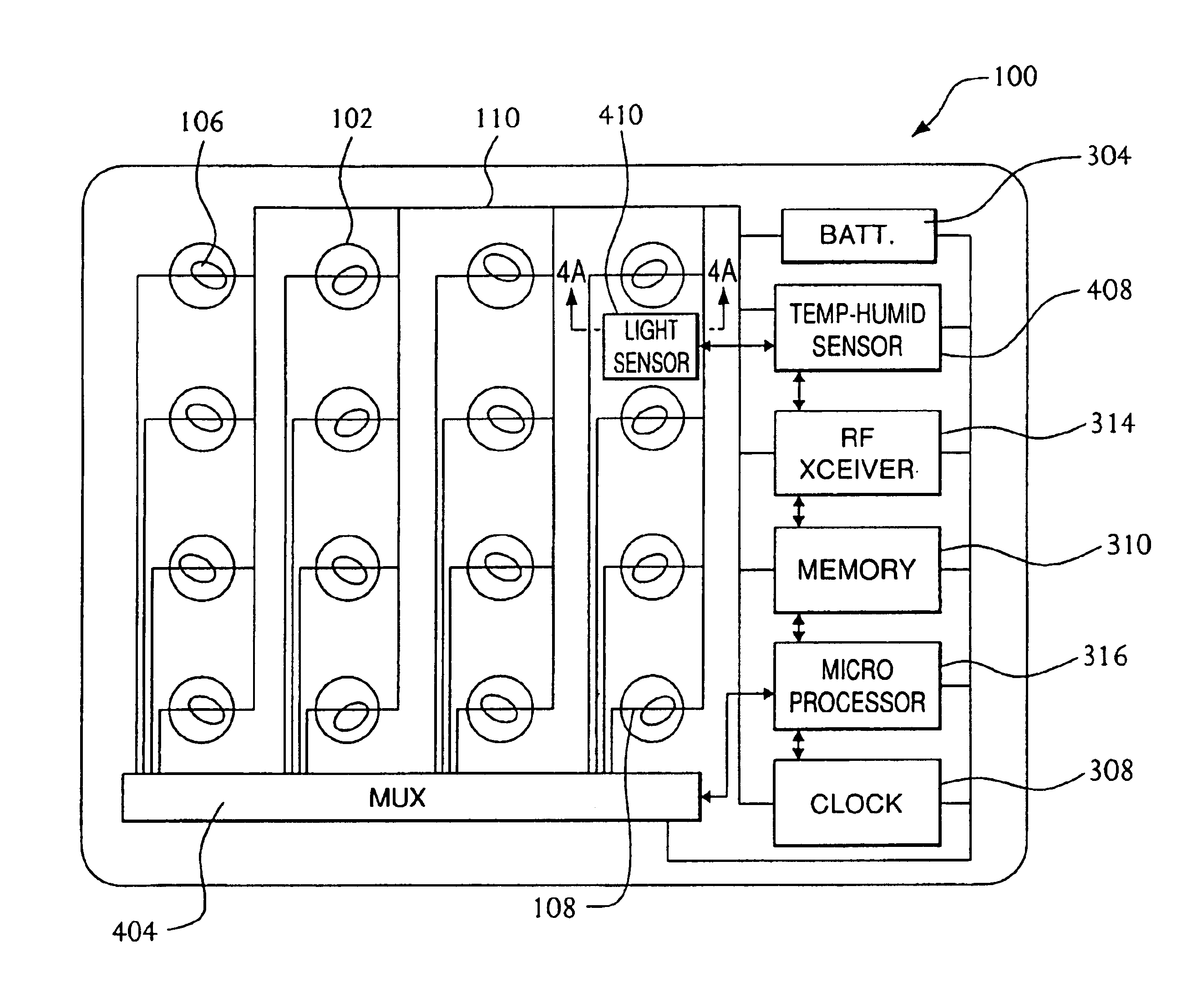
A system for managing delivery of pharmaceutical drugs is formed of a blister package that includes a plurality of cells arranged in a grid. Each of the cells is sealed by a breakable wall and holds a unit-dose of medication. A user gains access to the medication in a given cell by puncturing the breakable wall associated with the given cell. A severable conductor is positioned proximate to each breakable wall. The severable conductor associated with a given cell ruptures upon puncturing of the breakable wall associated with the given cell. A computer chip is electrically connected to the severable conductors. The computer chip senses the puncturing of each cell in the grid by monitoring the rupturing of each of the severable conductors. An RF transmitter is coupled to the computer chip. The RF transmitter sends information corresponding to usage of each of medications stored in the blister package to a remote information transceiver.
Owner:DDMS HLDG
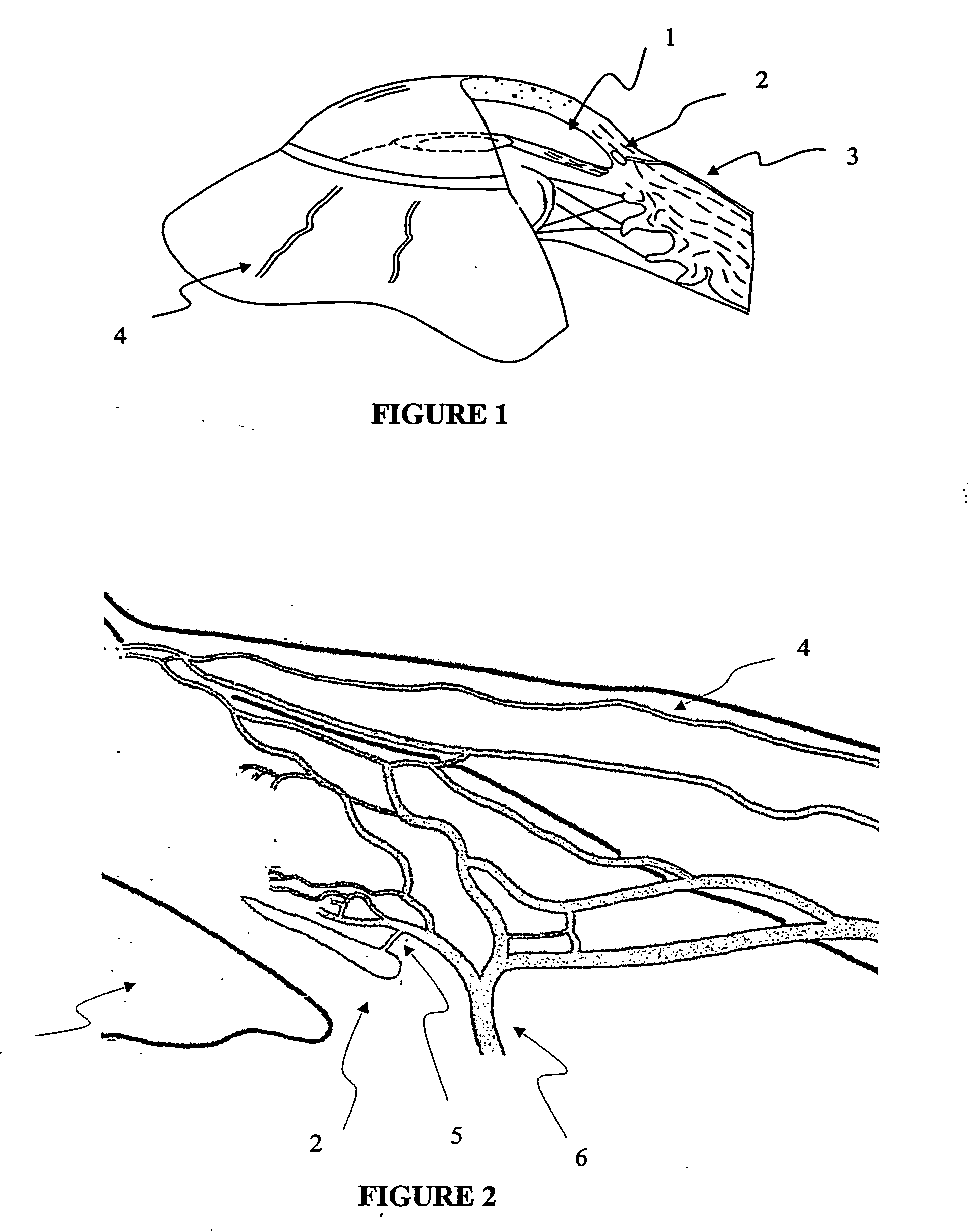
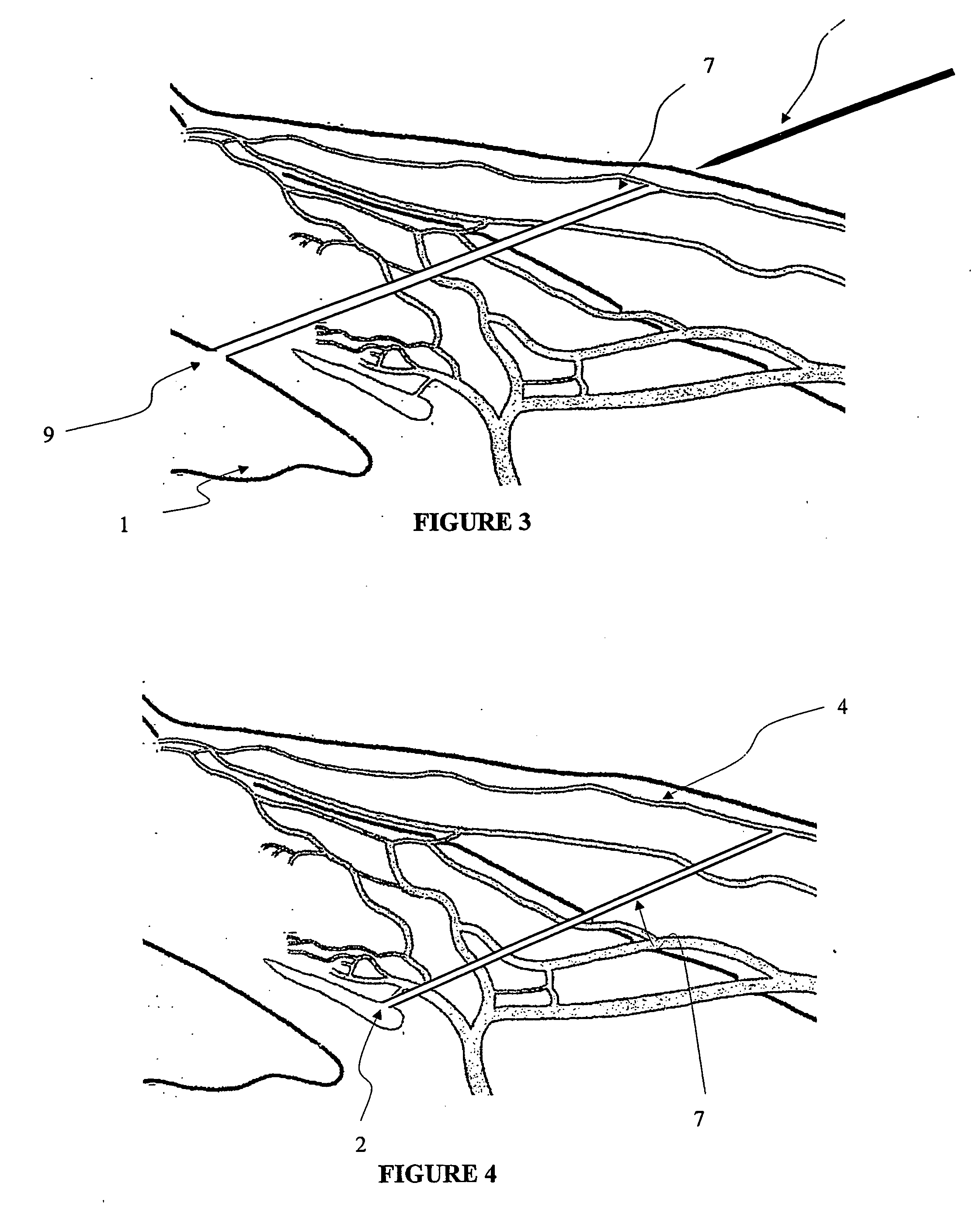
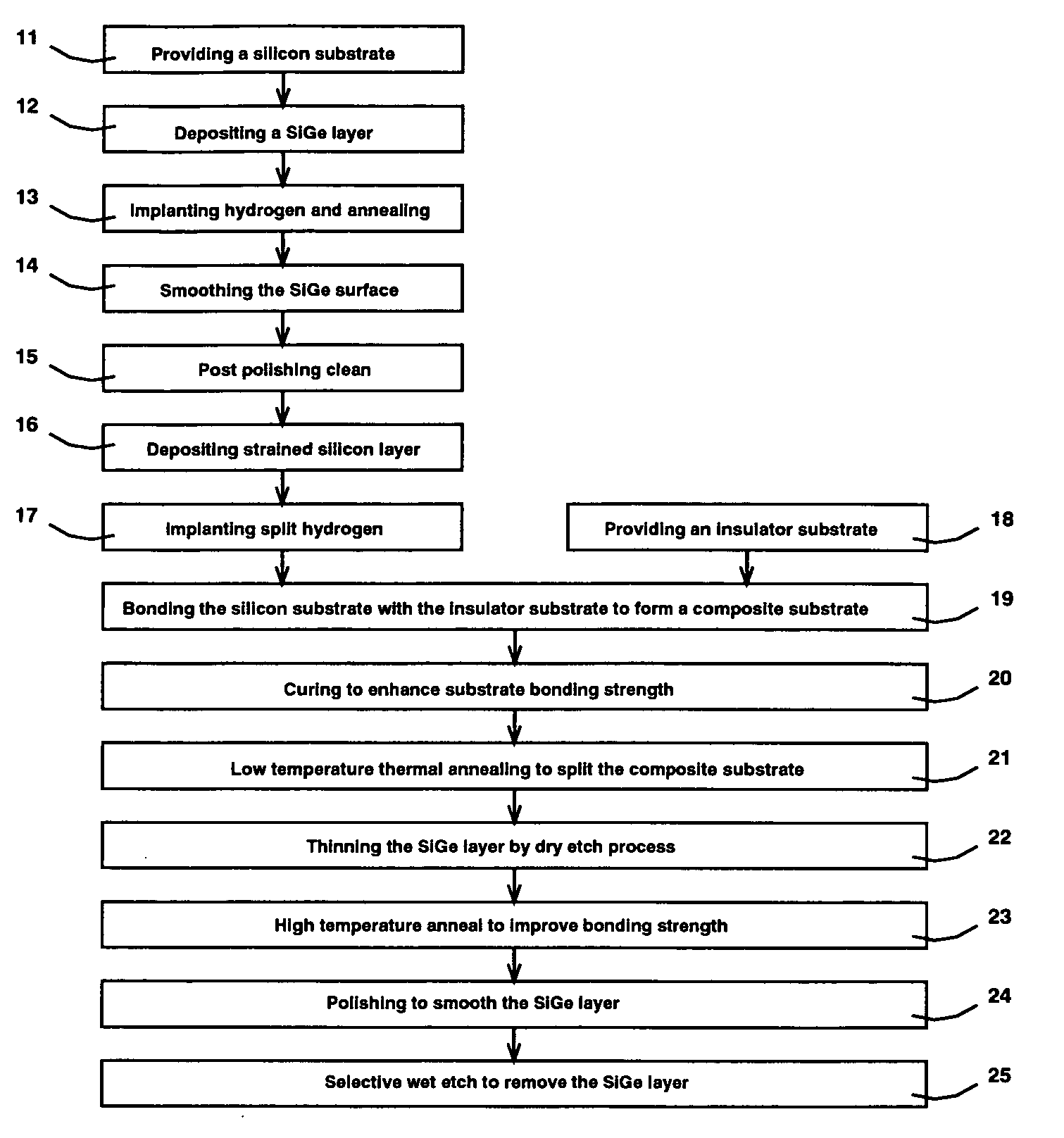
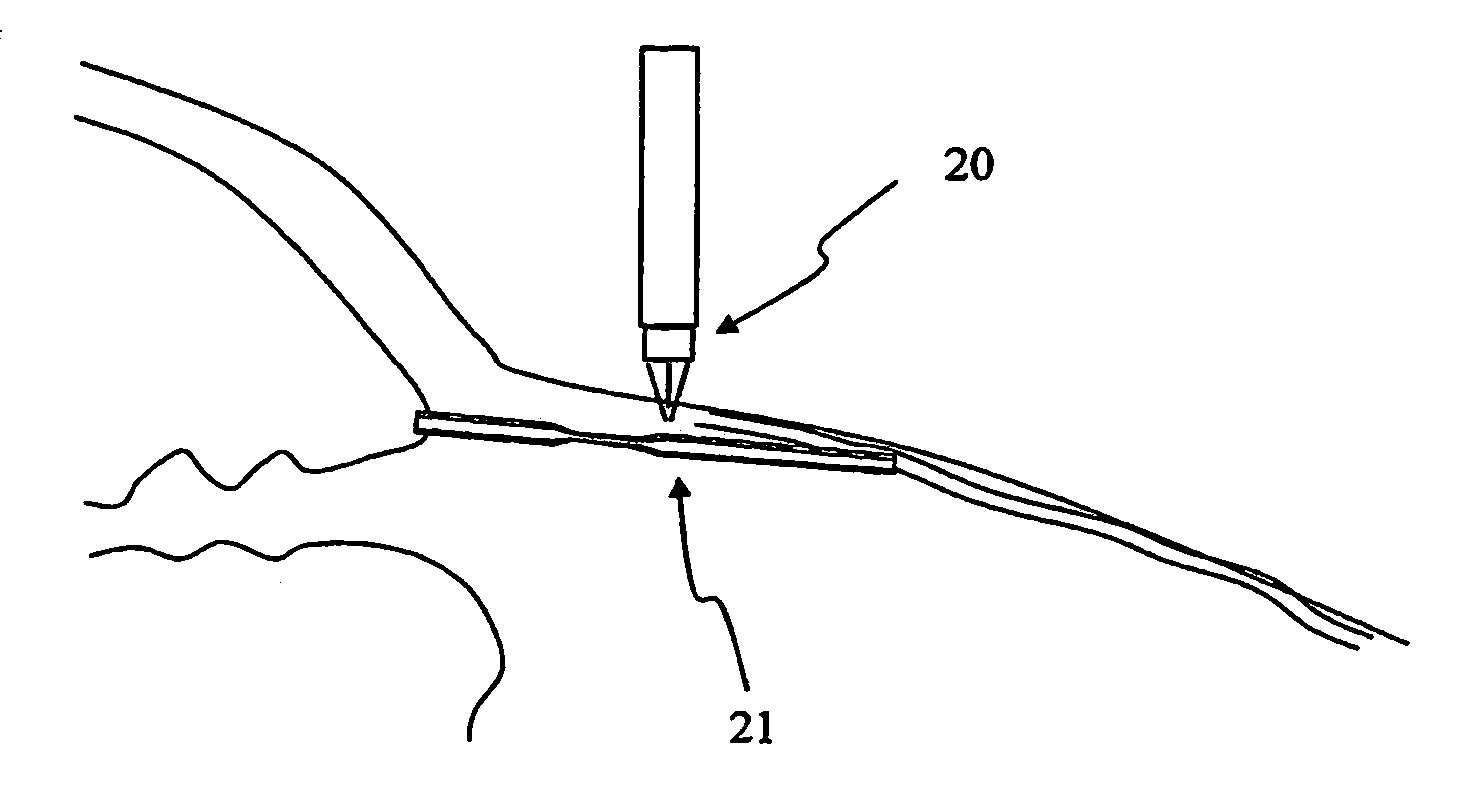
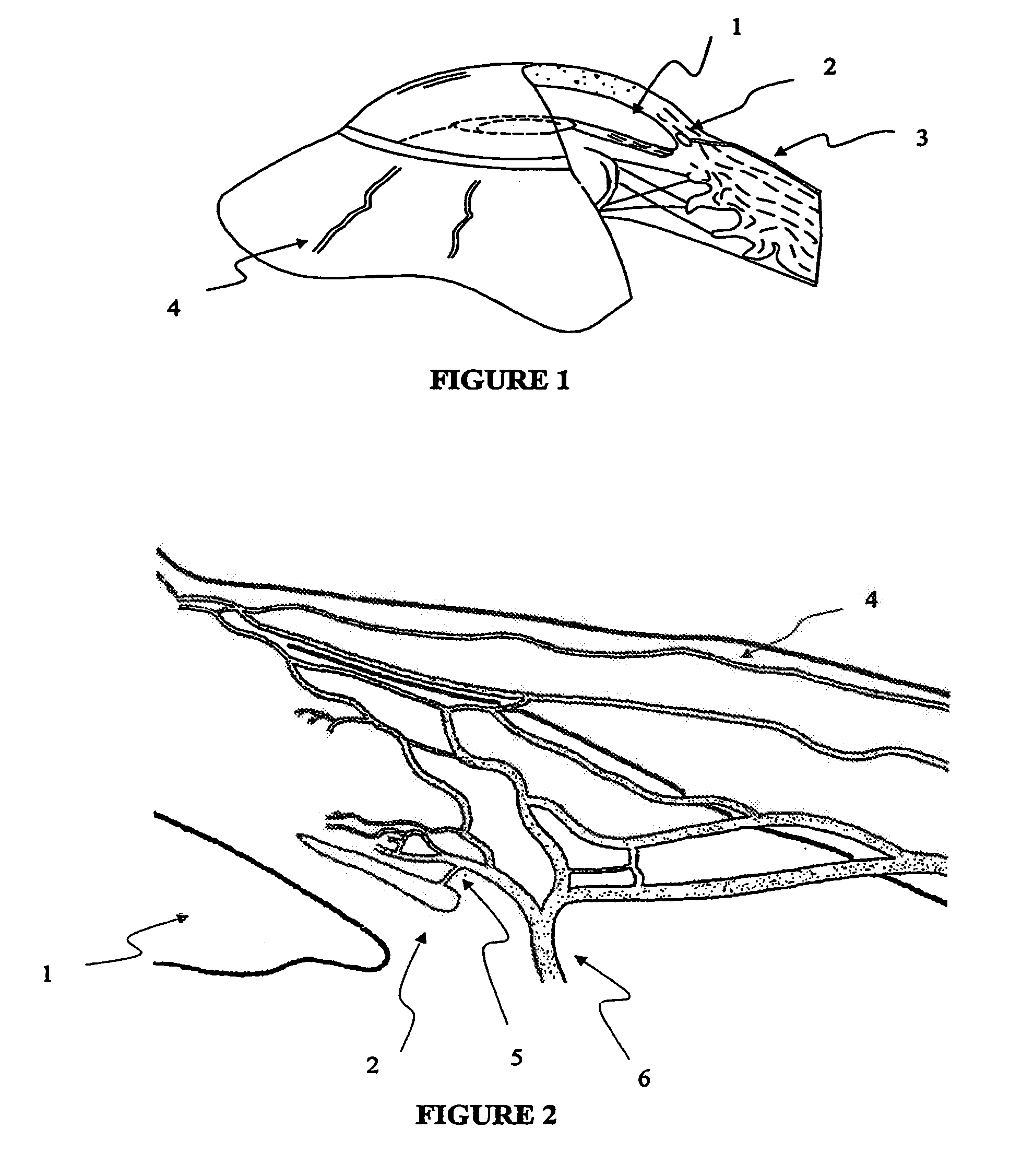
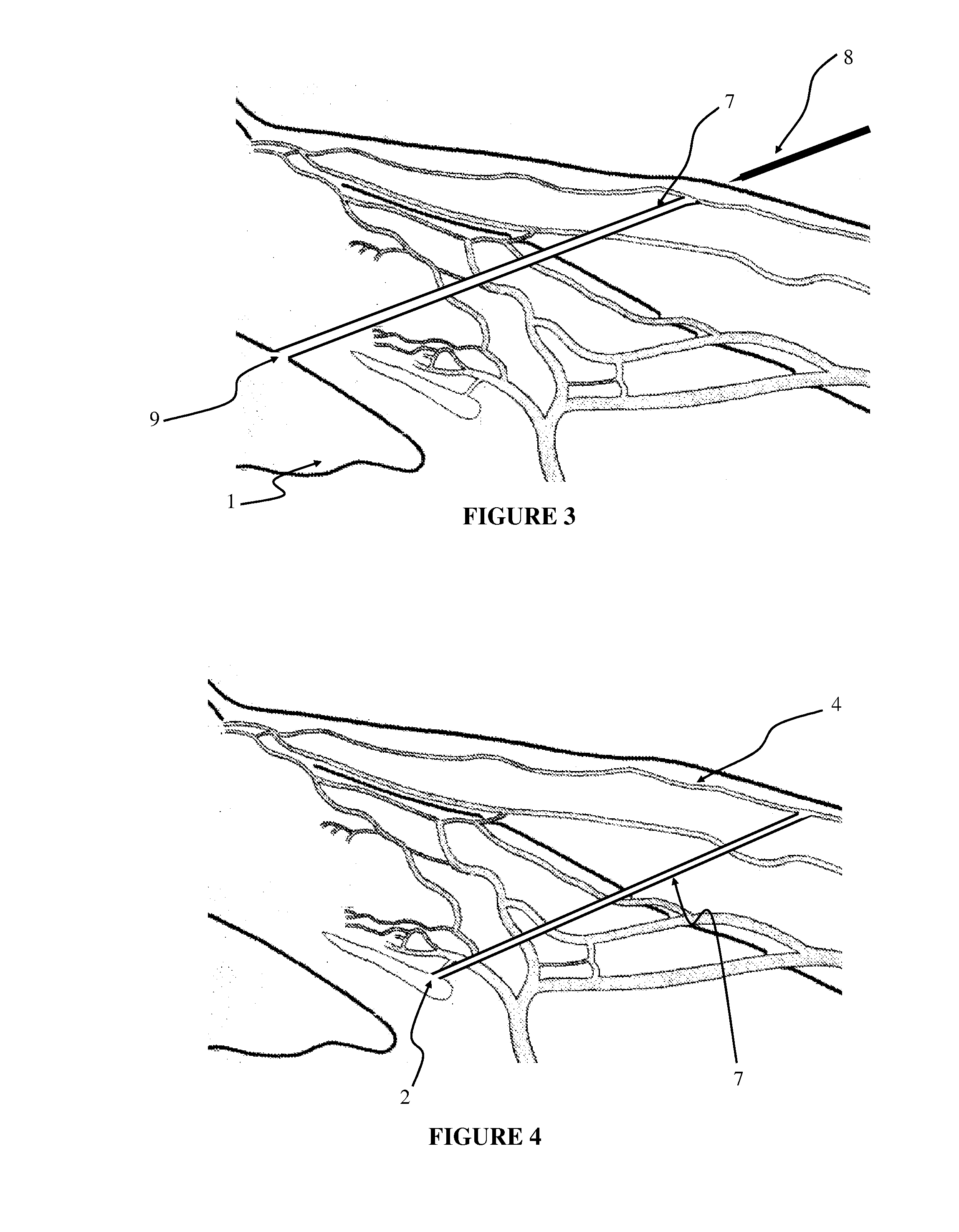
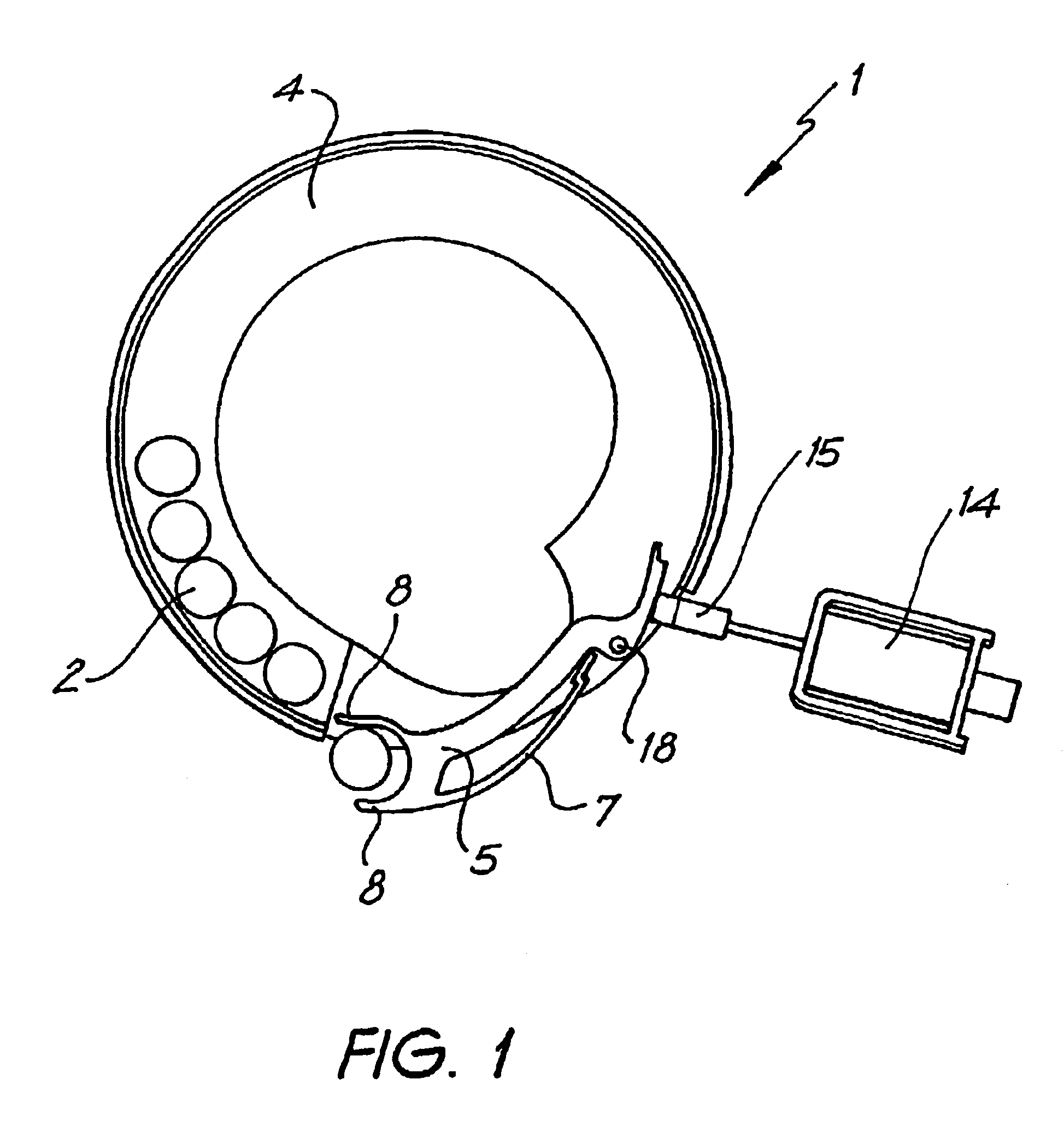
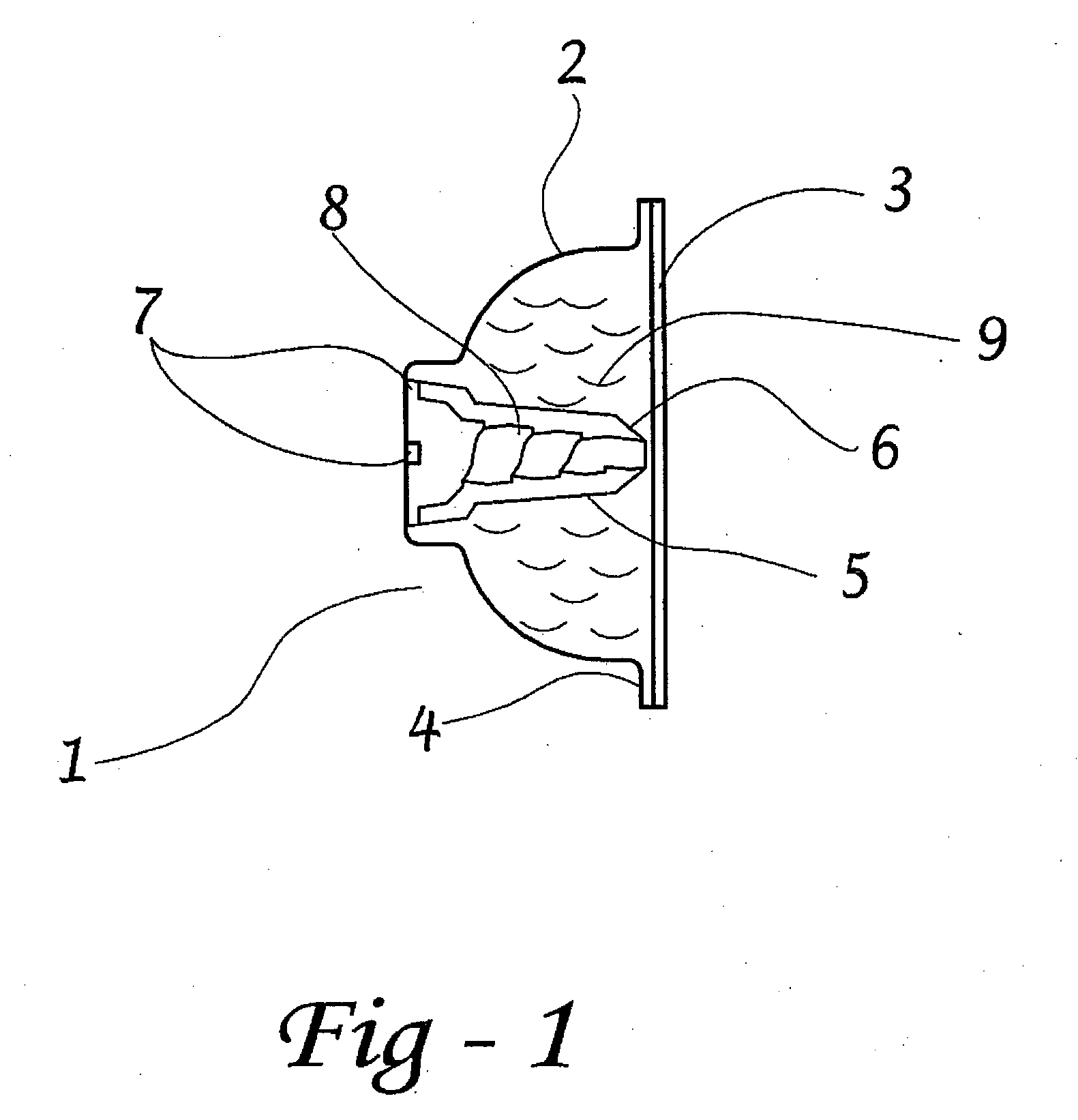
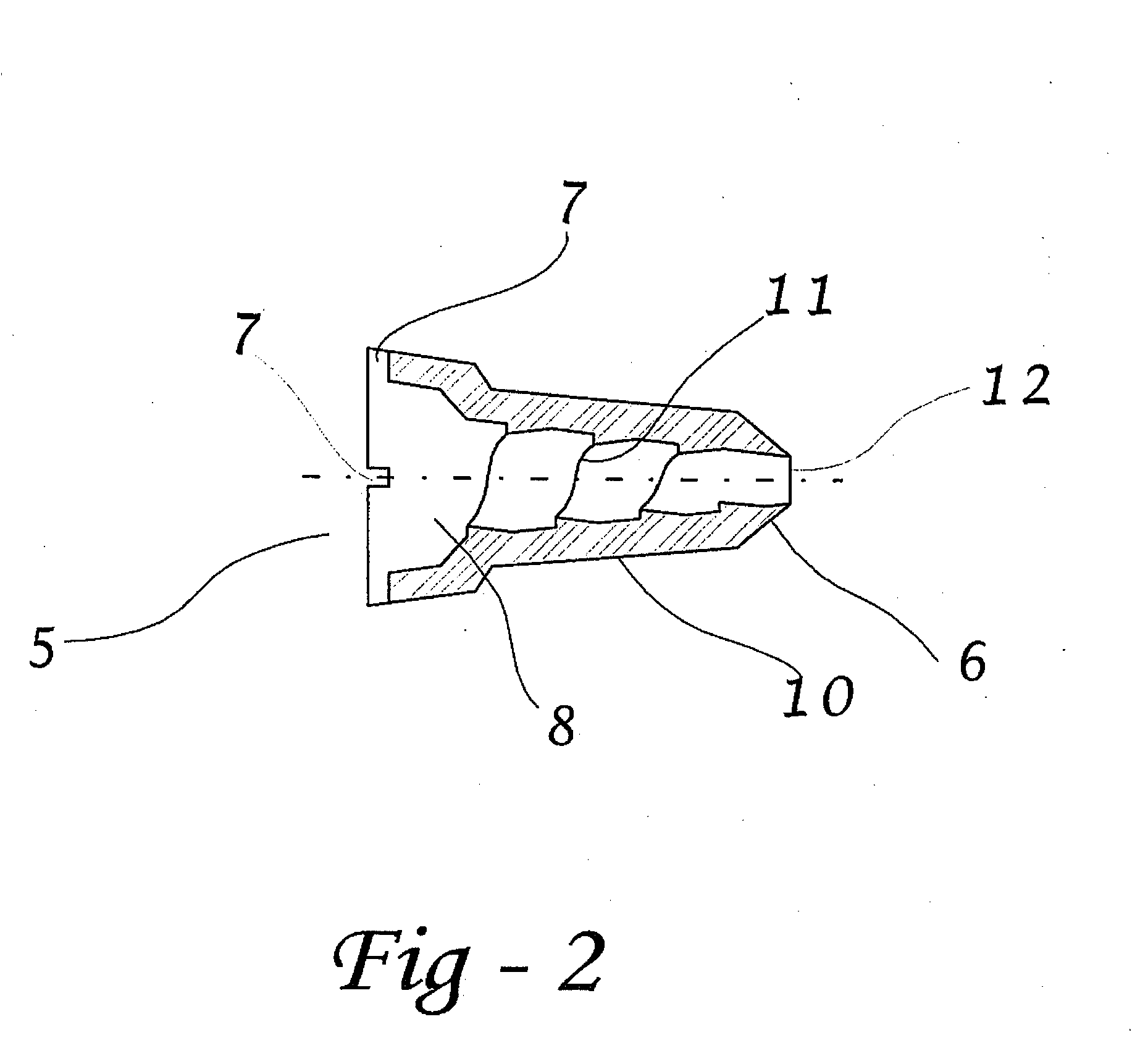
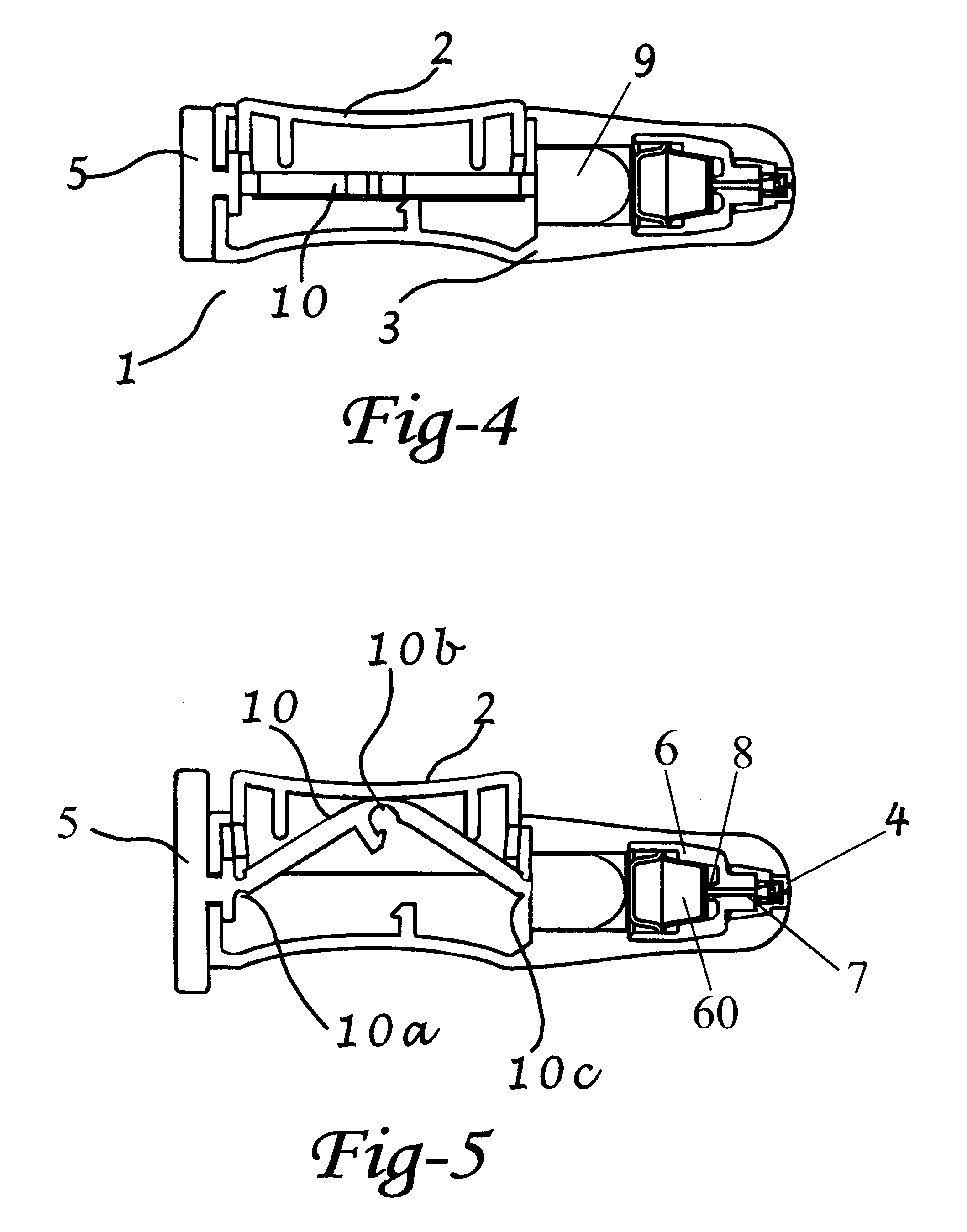
Apparatus and method for surgical bypass of aqueous humor
The invention provides minimally invasive microsurgical tools and methods to form an aqueous humor shunt or bypass for the treatment of glaucoma. The invention enables surgical creation of a tissue tract (7) within the tissues of the eye to directly connect a source of aqueous humor such as the anterior chamber (1), to an ocular vein (4). The tissue tract (7) from the vein (4) may be connected to any source of aqueous humor, including the anterior chamber (1), an aqueous collector channel, Schlemm's canal (2), or a drainage bleb. Since the aqueous humor passes directly into the venous system, the normal drainage process for aqueous humor is restored. Furthermore, the invention discloses devices and materials that can be implanted in the tissue tract to maintain the tissue space and fluid flow.
Owner:ISCI INTERVENTIONAL CORP
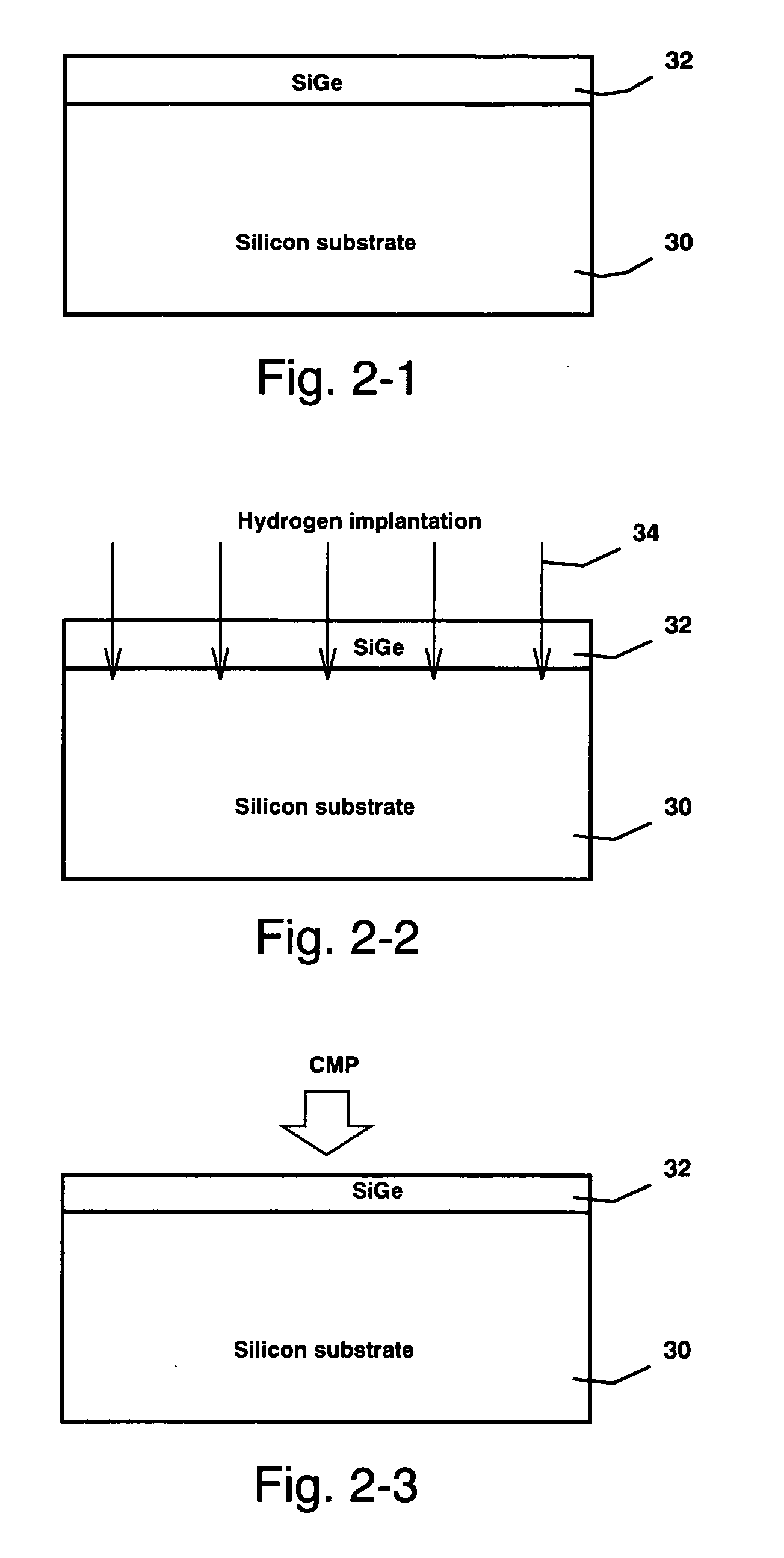
Strained silicon on insulator from film transfer and relaxation by hydrogen implantation
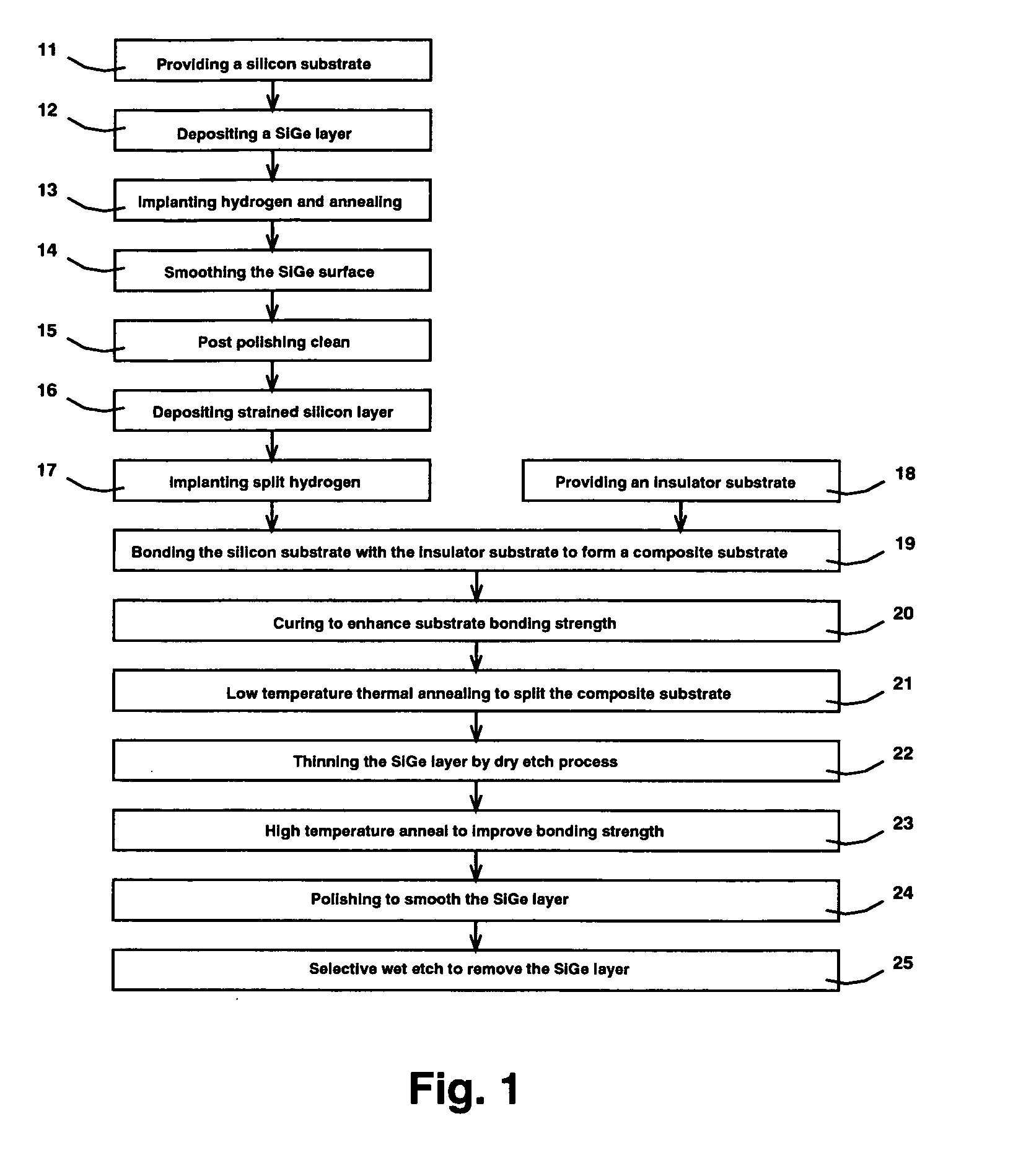
ActiveUS20050153524A1Easy to relaxEasy to integrateSolid-state devicesSemiconductor/solid-state device manufacturingThreading dislocationsHydrogen
Transistors fabricated on SSOI (Strained Silicon On Insulator) substrate, which comprises a strained silicon layer disposed directly on an insulator layer, have enhanced device performance due to the strain-induced band modification of the strained silicon device channel and the limited silicon volume because of the insulator layer. The present invention discloses a SSOI substrate fabrication process comprising various novel approaches. One is the use of a thin relaxed SiGe layer as the strain-induced seed layer to facilitate integration and reduce processing cost. Another is the formation of split implant microcracks deep in the silicon substrate to reduce the number of threading dislocations reaching the strained silicon layer. And lastly is the two step annealing / thinning process for the strained silicon / SiGe multilayer film transfer without blister or flaking formation.
Owner:SHARP KK
Contact lens package
ActiveUS7426993B2Reduced tendency to stick togetherReduce effortOther accessoriesContainer/bottle contructionLiquid mediumEngineering
Owner:COOPERVISION INT LTD
IC interconnect structures and methods for making same
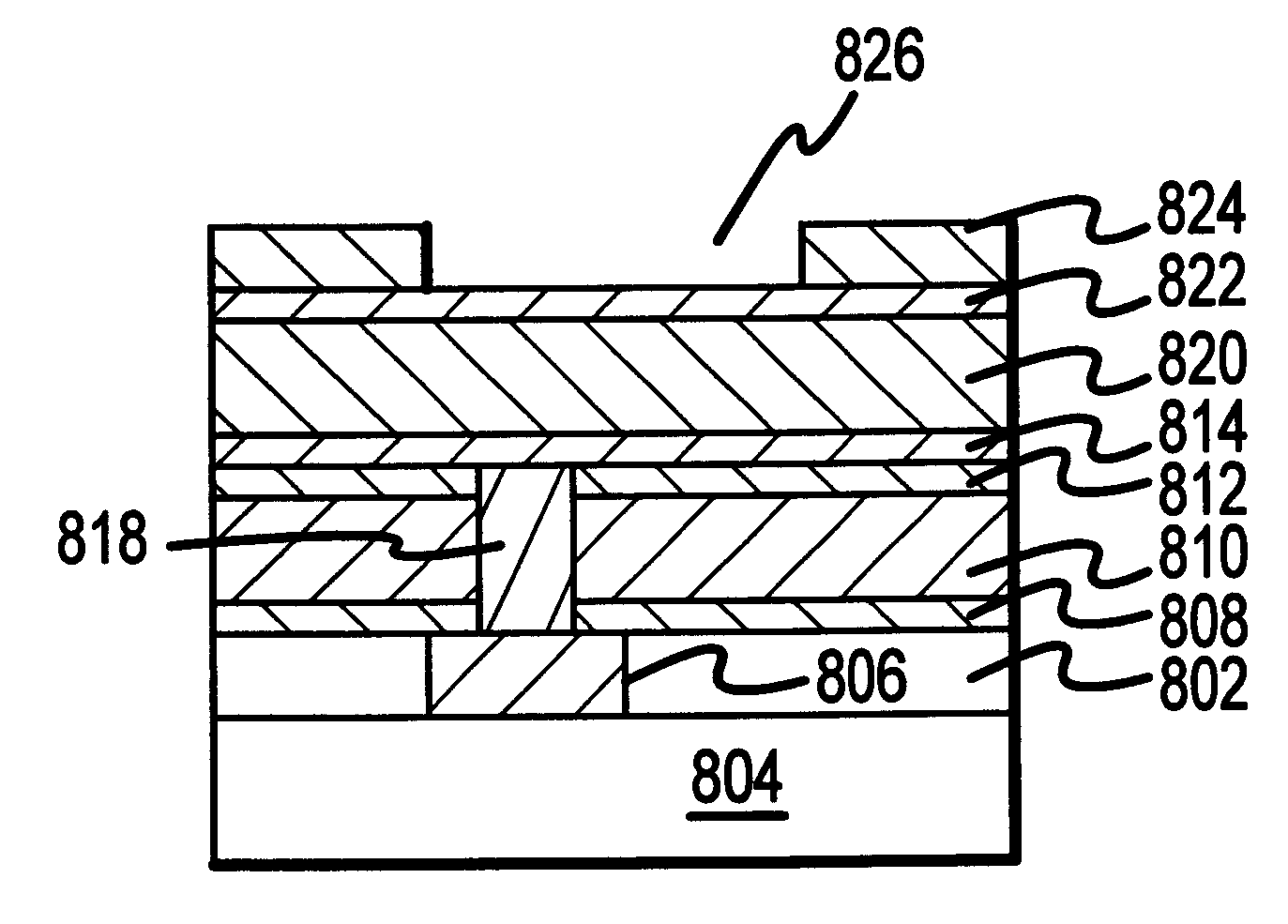
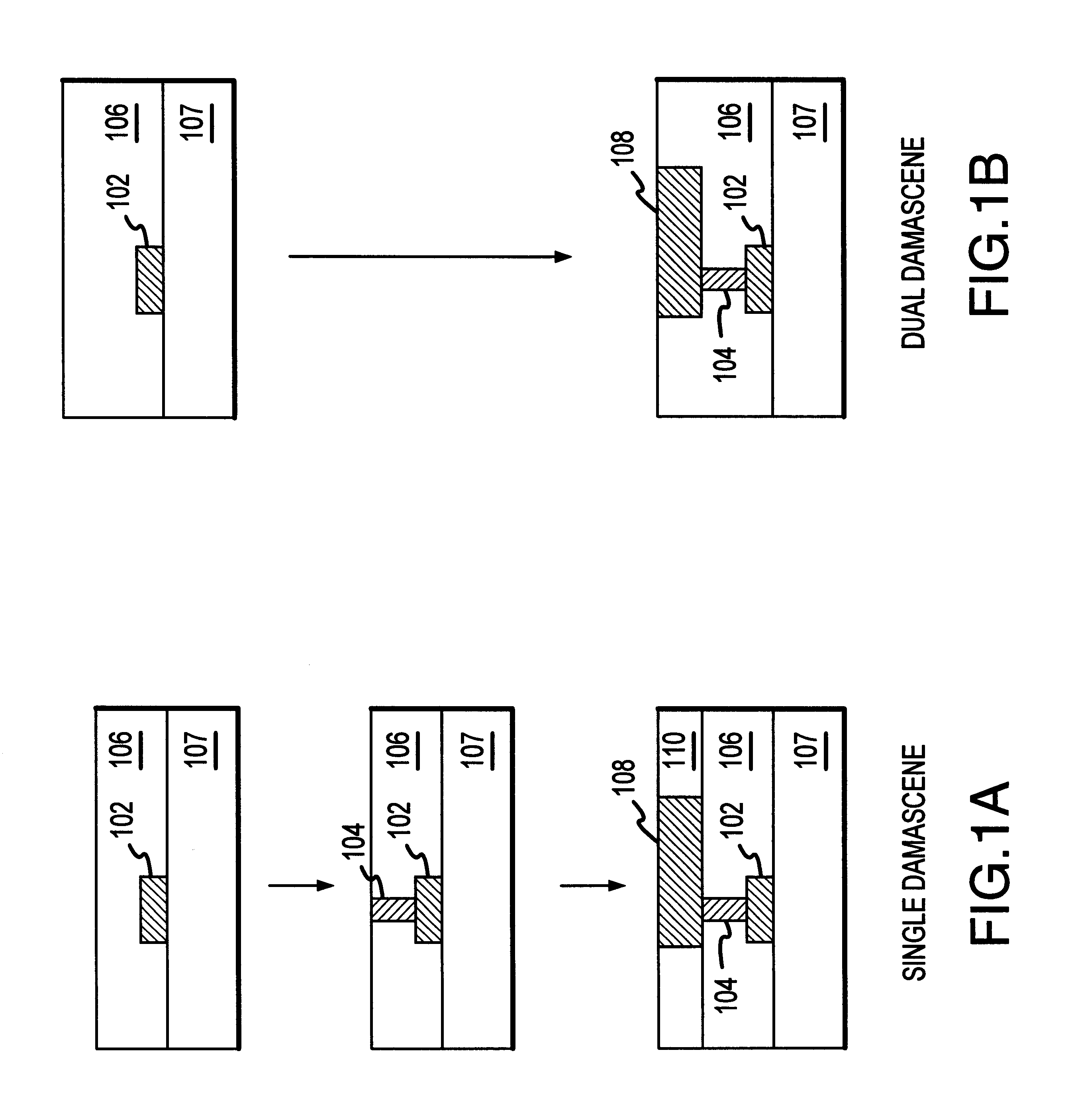
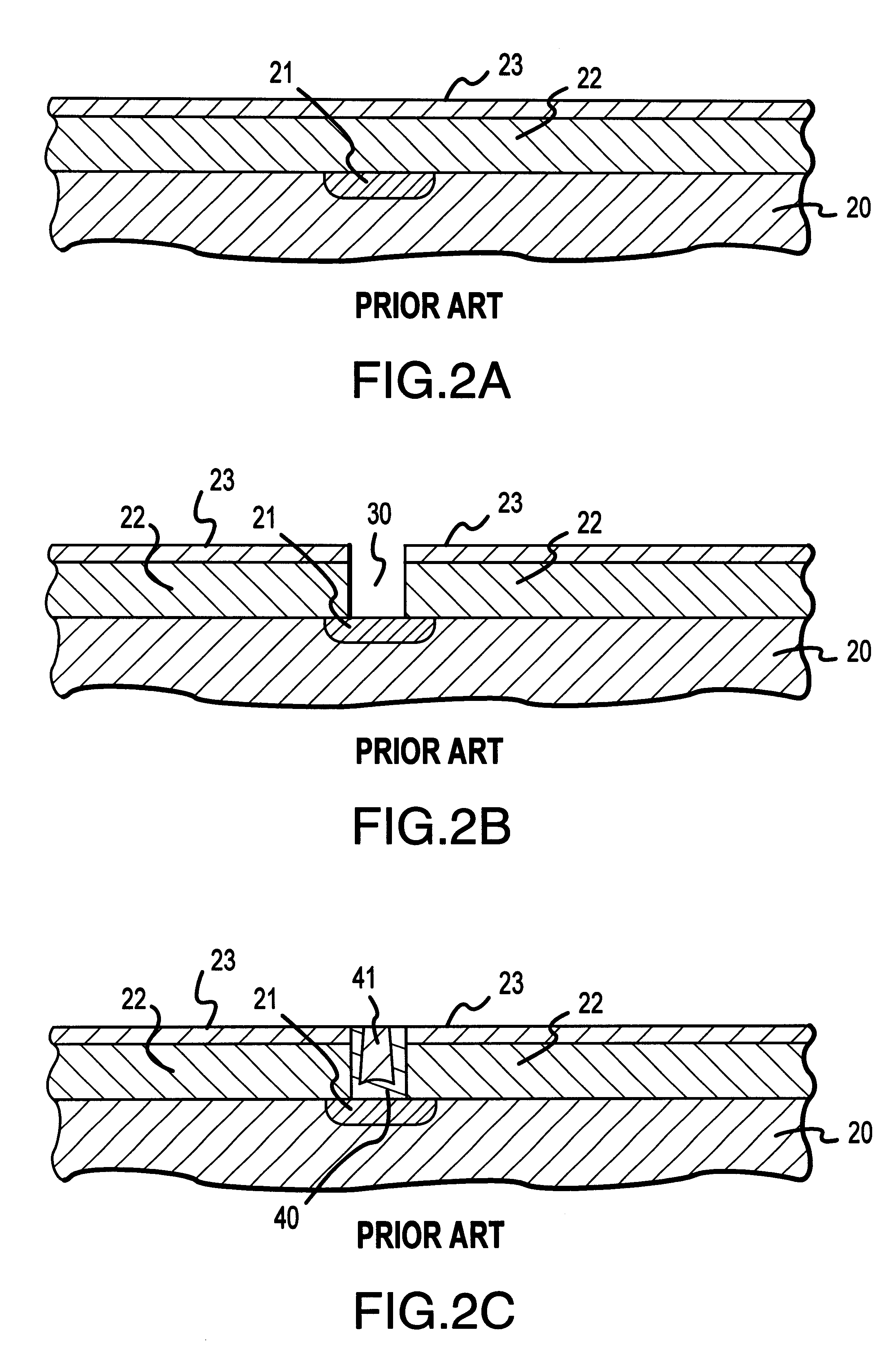
Methods and structures are disclosed for advanced interconnects in sub-micron and sub-half-micron integrated circuit devices fabricated using a single damascene process. a dielectric etch-stop layer (e.g., silicon nitride) is deposited subsequent to rather than prior to CMP processing of the previous metallization layer (e.g., the conductive plug). This scheme effectively eliminates the effect of CMP-induced erosion on the etch-stop layer and therefore allows an extremely thin etch stop to be used. Moreover, a high etch-selectivity can be obtained for the trench etch, and all etch-stop material is removed from beneath the interconnect metal, thereby reducing parasitic effects. A patterned dielectric layer is used as a metal cap in place of the standard blanket silicon nitride layer, thus preventing the formation of blisters and bubbles associated with trapped moisture and gasses, and reducing interconnect capacitance.
Owner:NEWPORT FAB
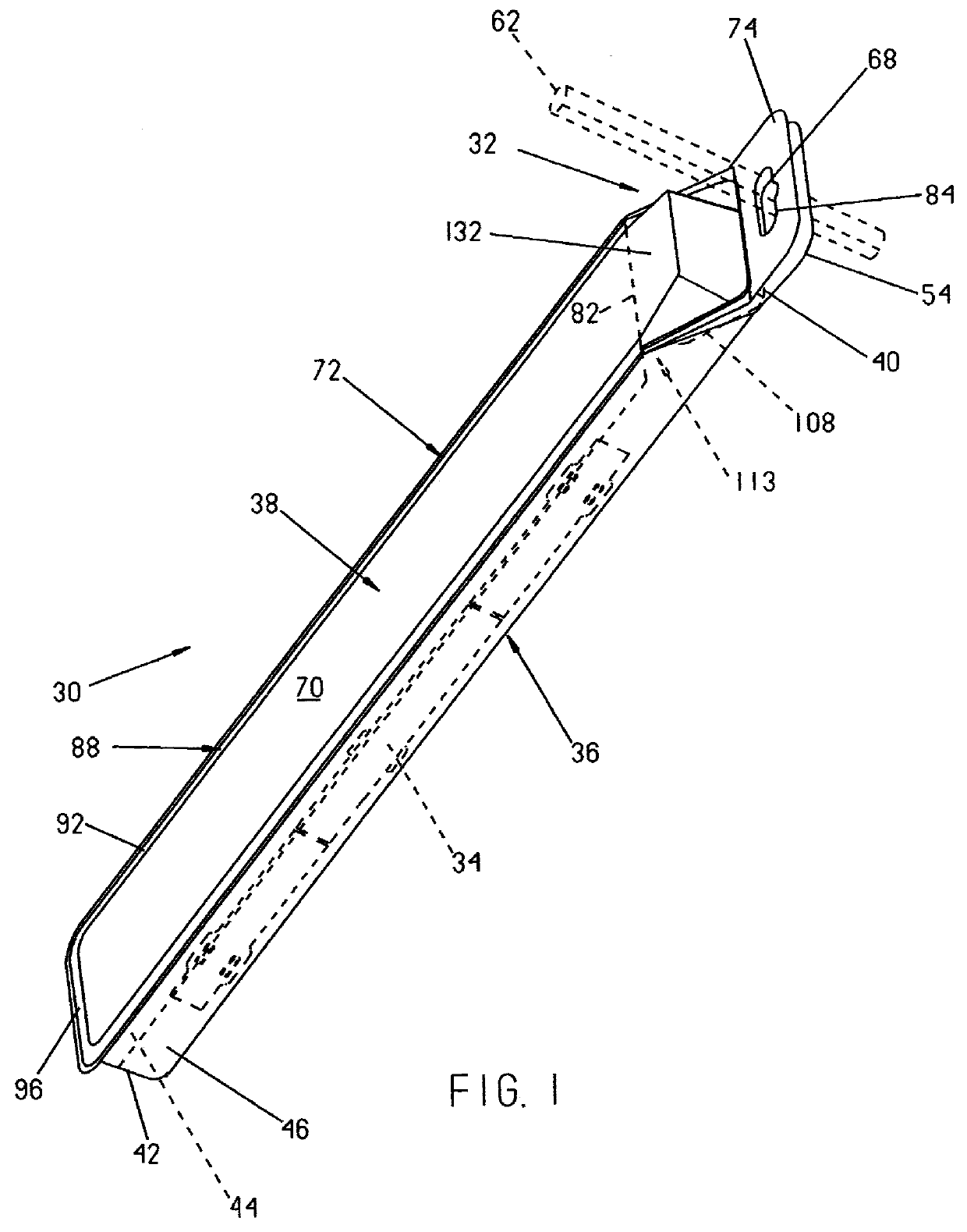
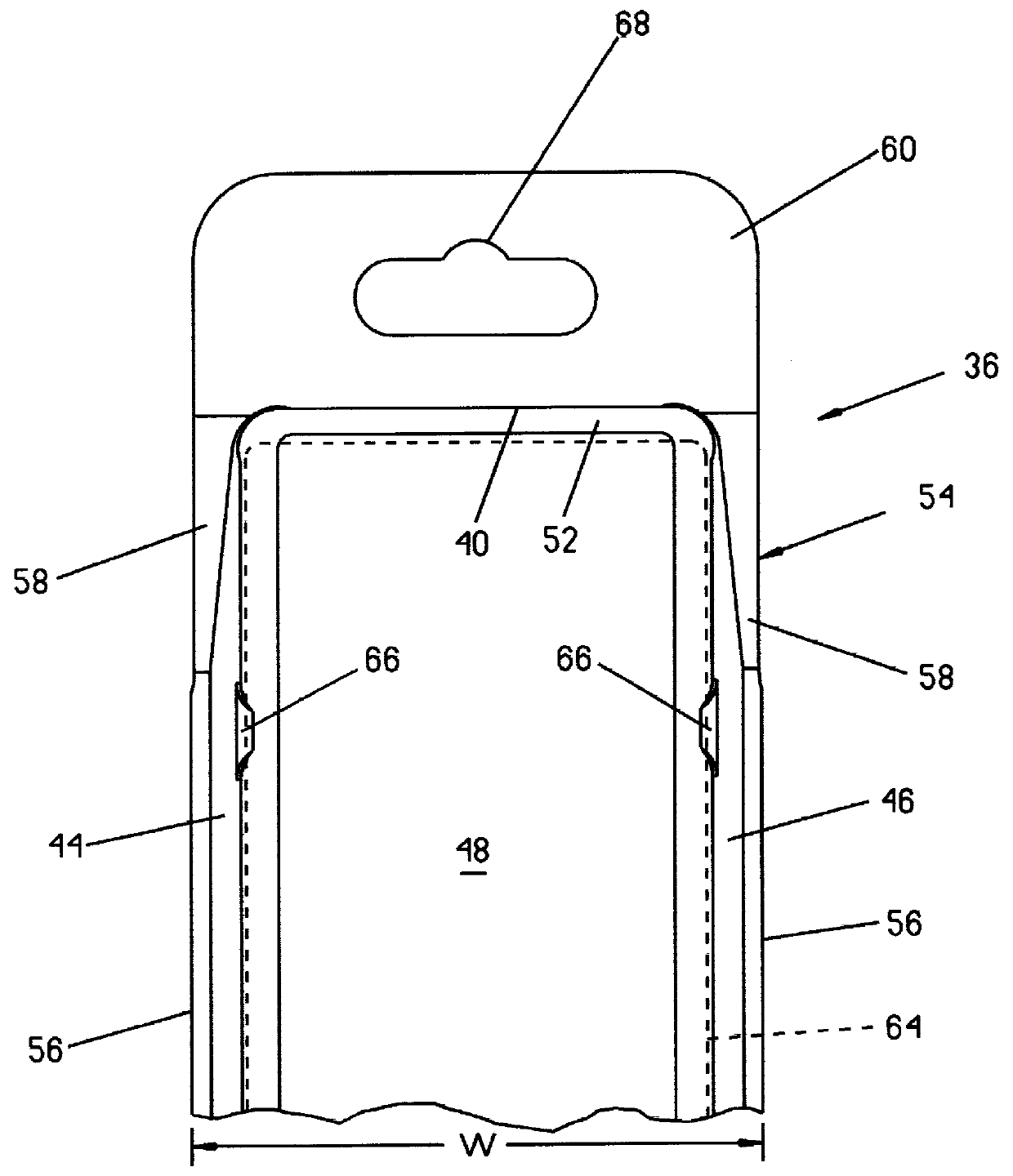
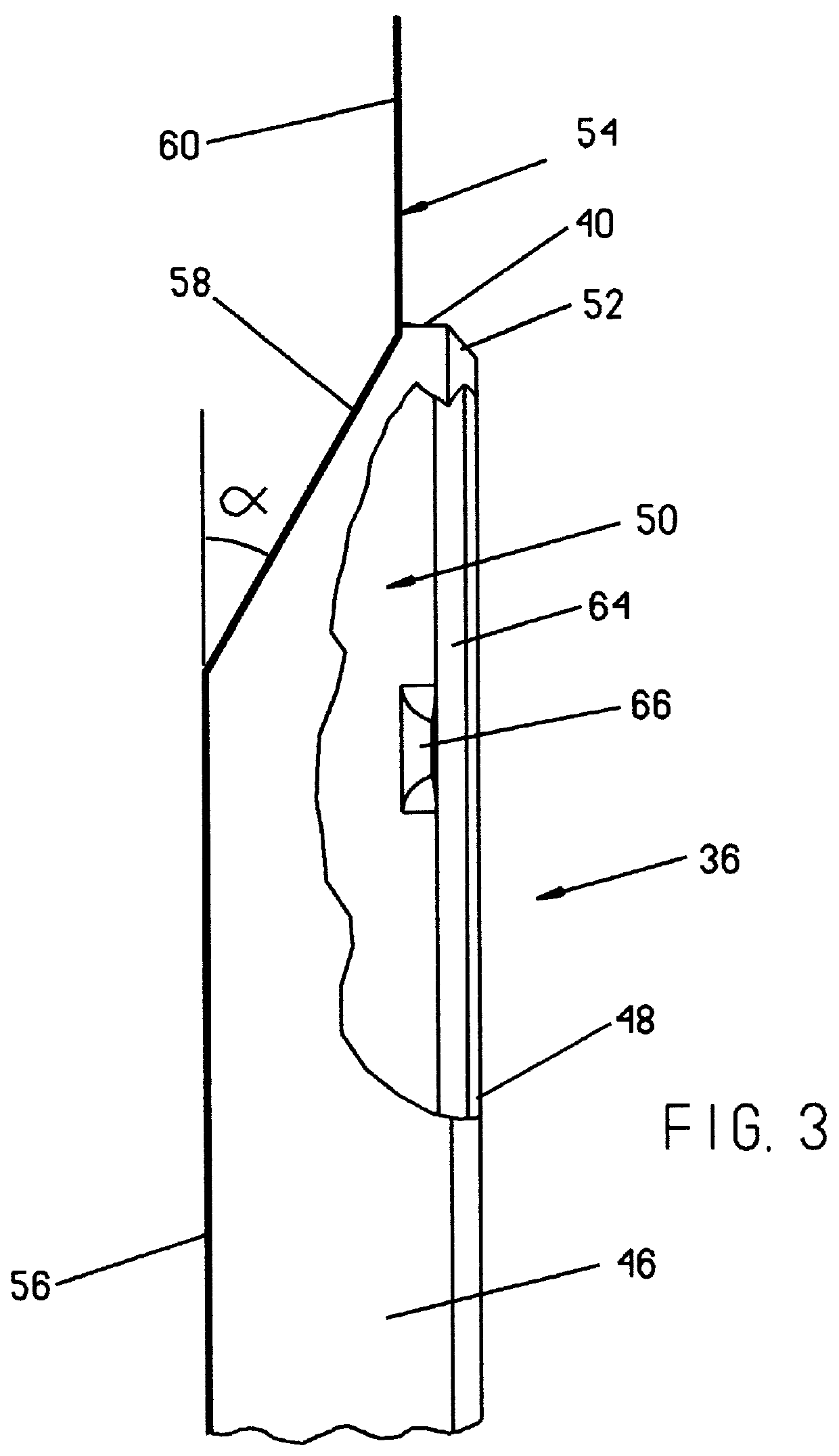
Packaging system for medical devices
Packaging systems for medical devices, such as surgical instruments, implants, and prostheses, are provided herein. In one embodiment, the packaging system comprises a blister tray that is at least semi-transparent and has at least one cavity capable of containing a medical device or portion thereof. The system additionally comprises a card having at least one window, wherein the at least one cavity in the blister tray protrudes through the at least one window in the card. The packaging systems provide a convenient, informative, and preferably sterile means of distributing medical devices.
Owner:WARSAW ORTHOPEDIC INC
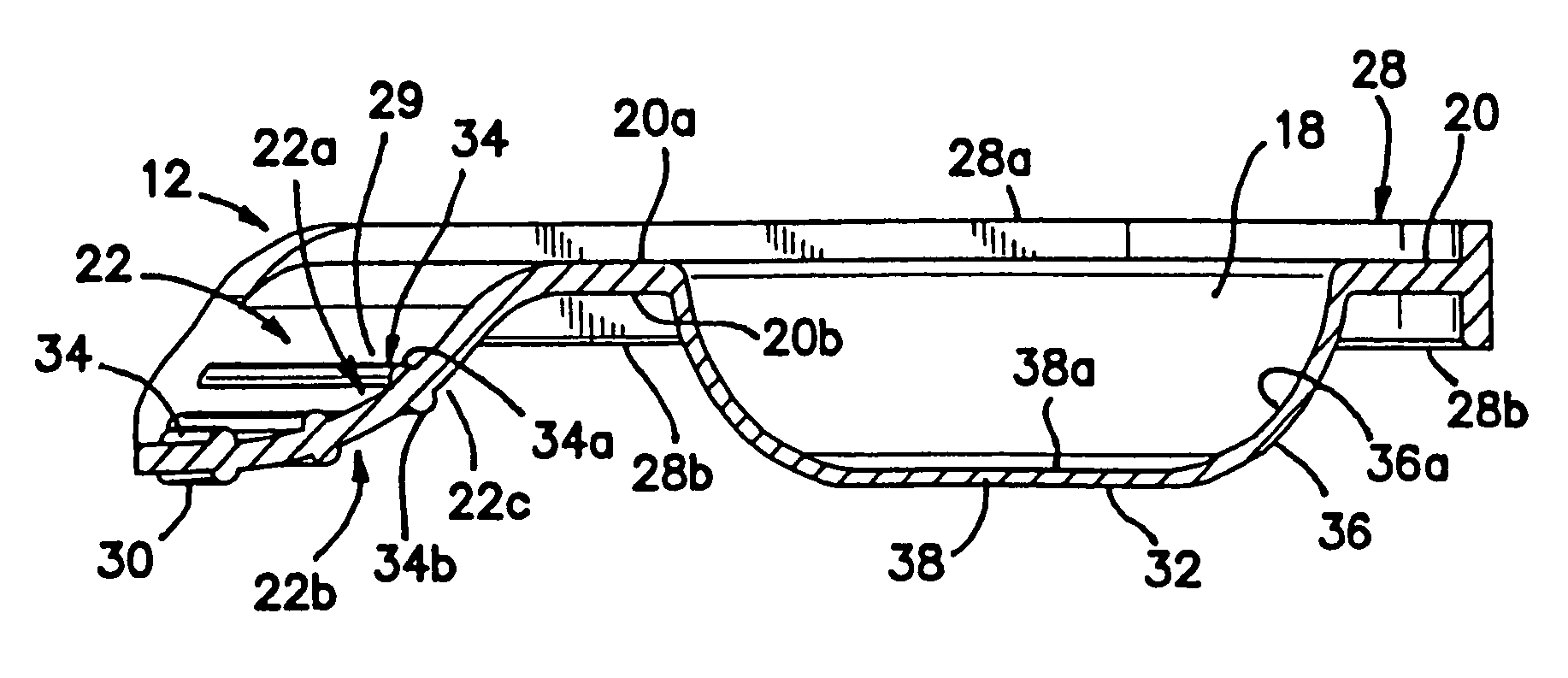
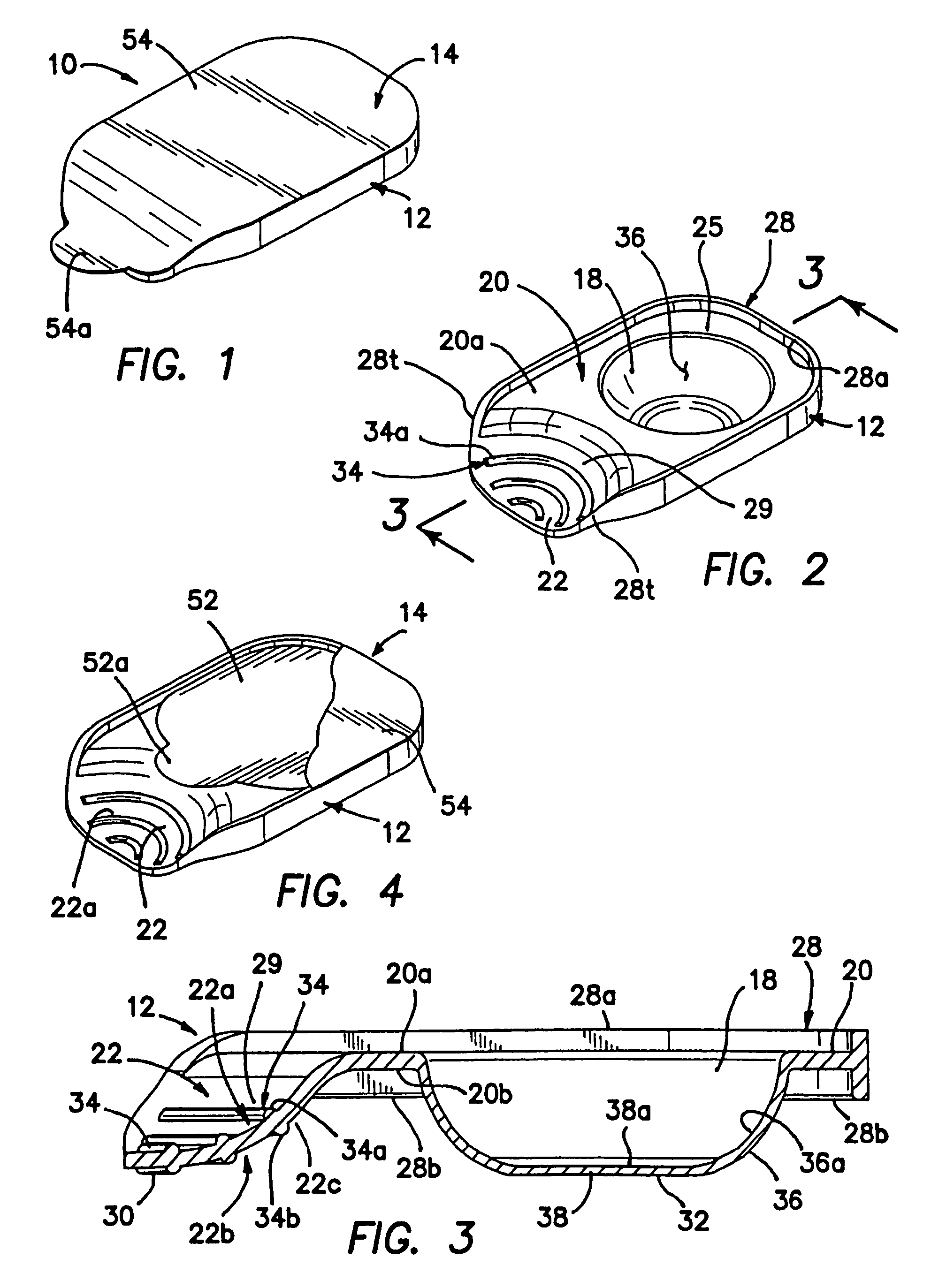
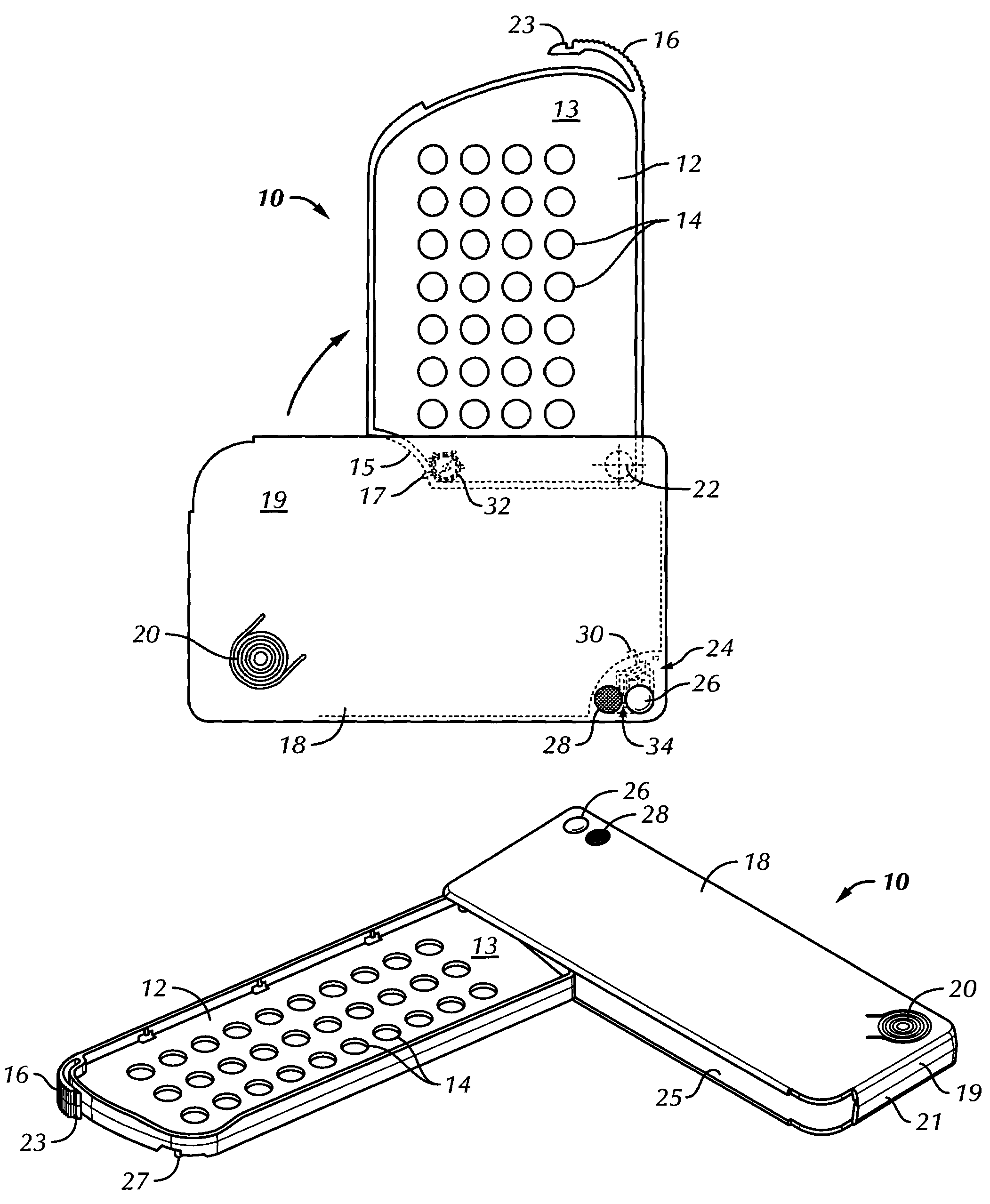
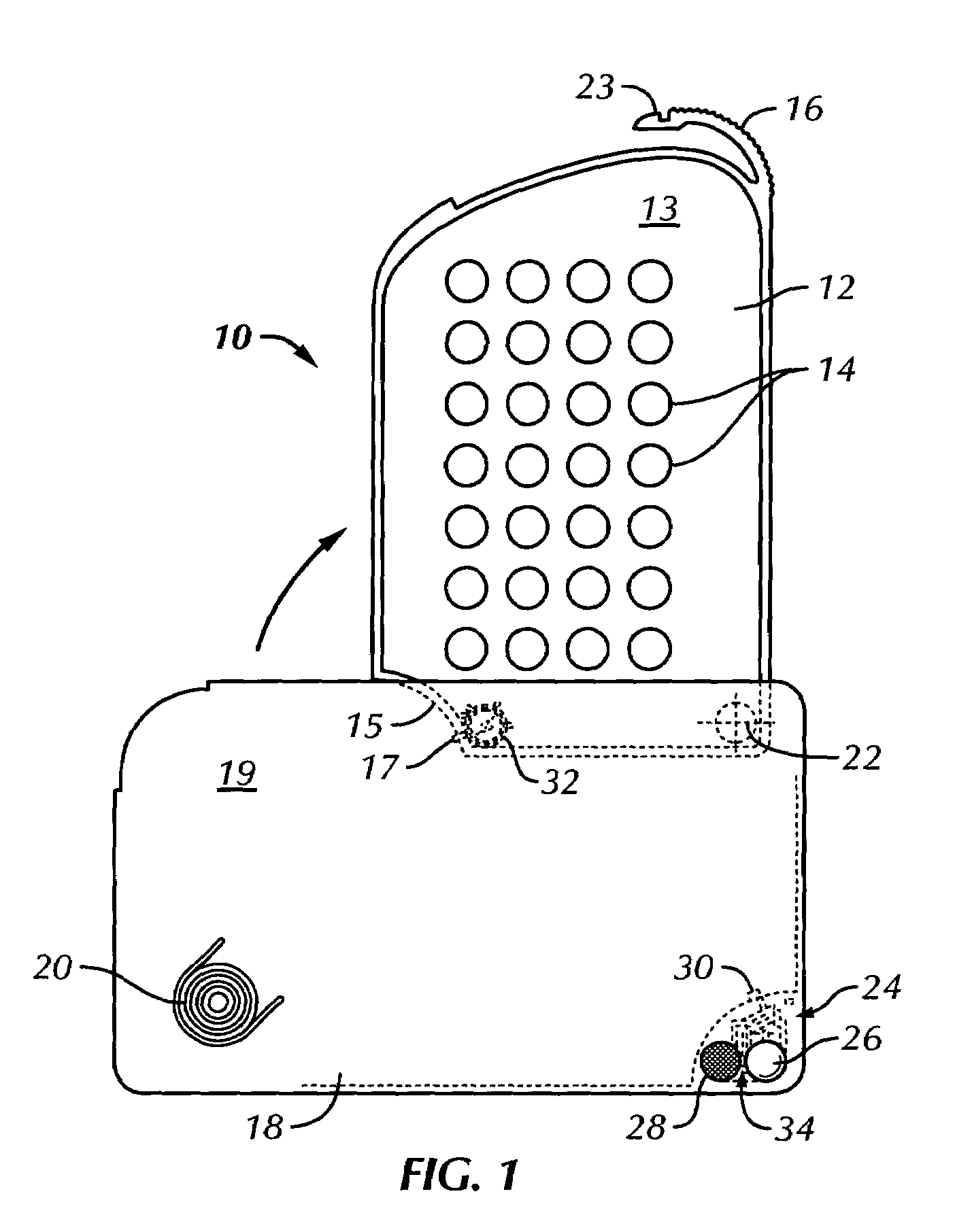
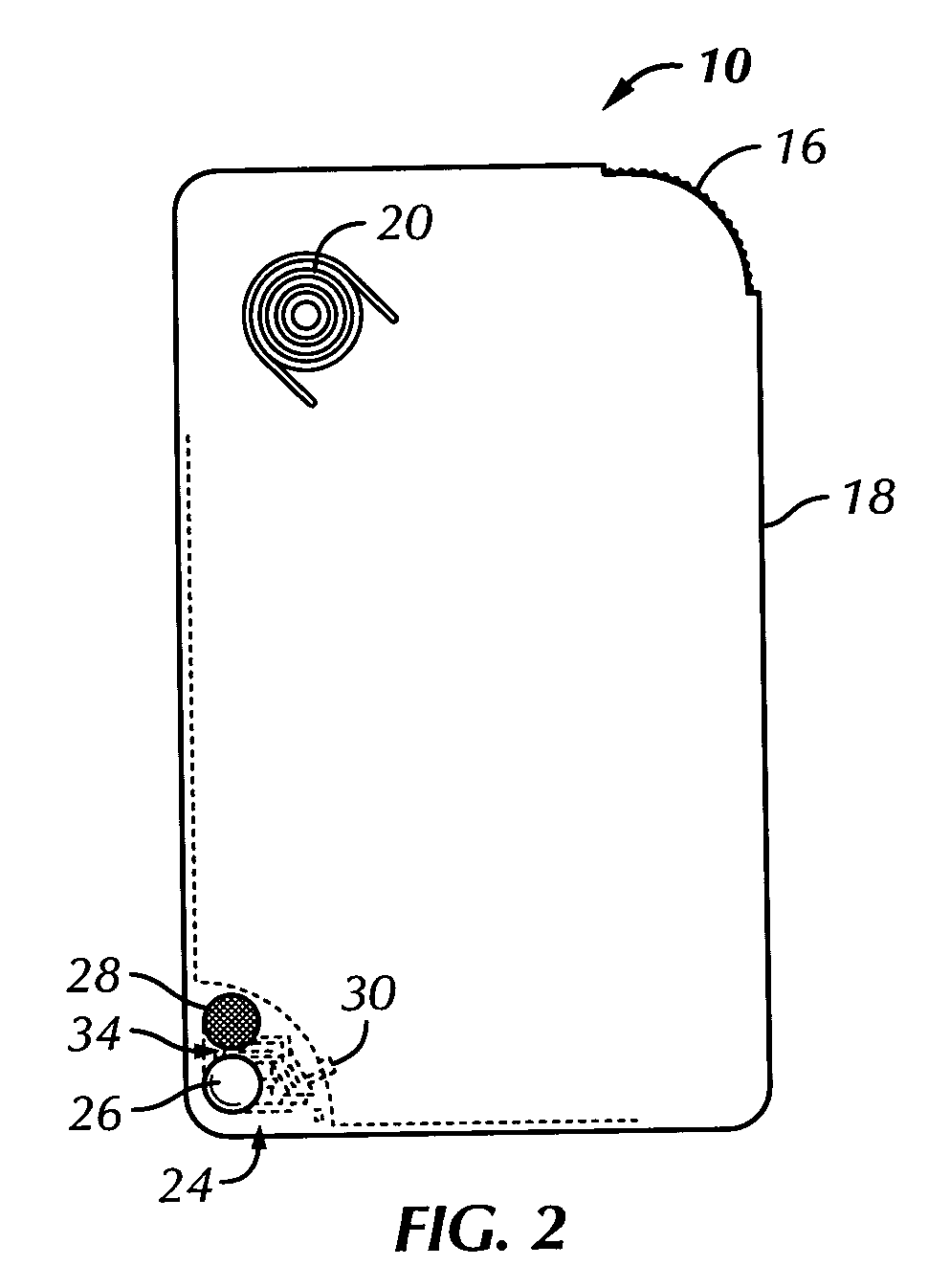
Unit dose container with locking sleeve
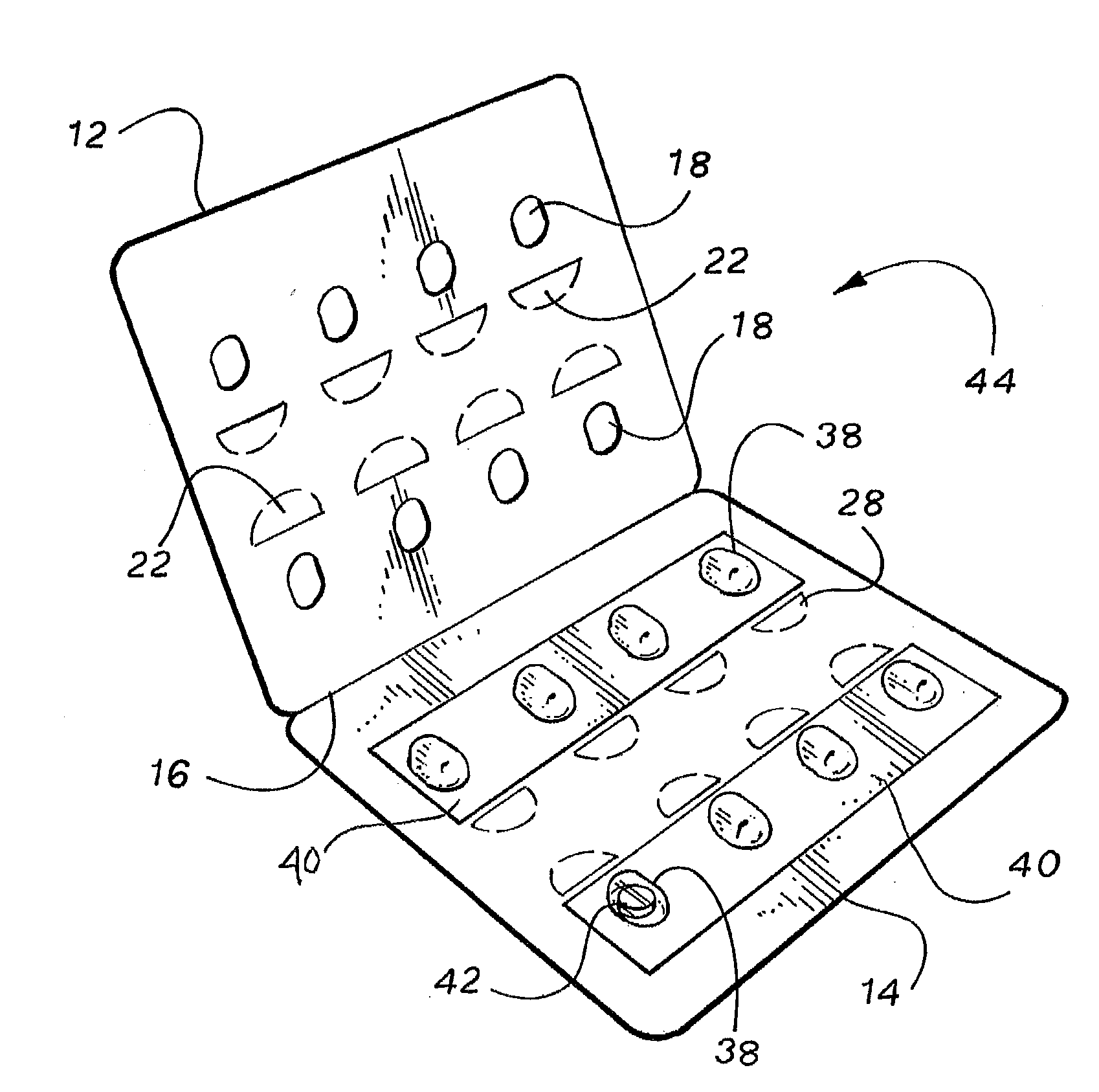
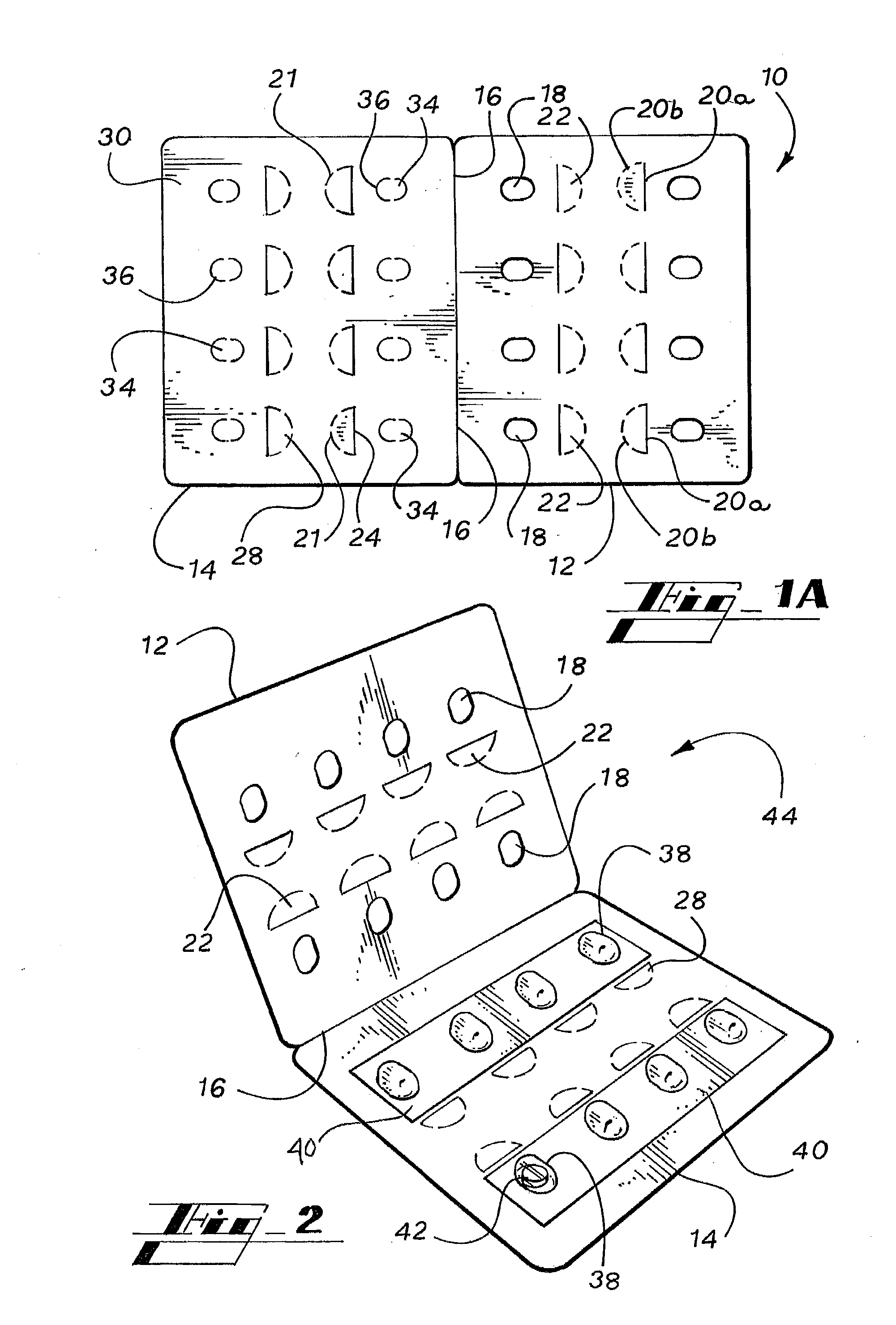
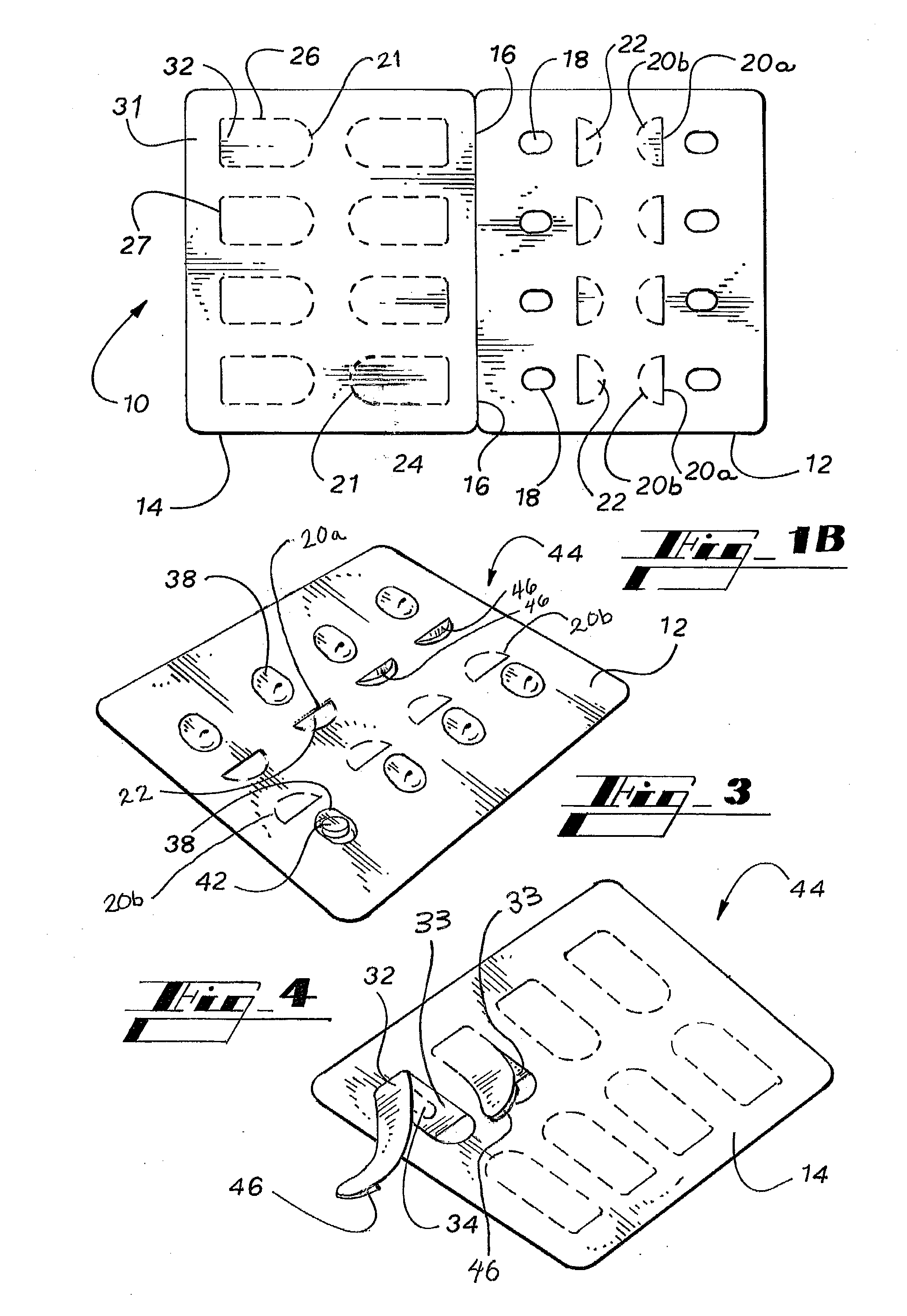
ActiveUS20050183981A1Inexpensive and easy to assemblePrevents and at least frustrates unintentional withdrawSmall article dispensingOther accessoriesDetentBlisters
A container (10) includes a slidable tray (12) including a slot (34) and a locking sleeve (14). The locking sleeve (14) is formed by attaching a base (16) to a top (18). The tray (12) is made from a conventional blister package, with blisters (30) formed in a single layer plastic top for holding items (31). A backing sheet comprising a sealing paper or foil layer is used to secure the items (31) within the blisters (30). The tray (12) is placed on sliding guides (70) of base (16) so that a stop (54) extends through the slot (34) to prevent the tray (12) from sliding out of the sleeve (14). The base (16) includes detents (56) that engage with holes (36) to prevent the tray (12) from sliding. Manipulating a biaser (20), warps the part of the tray (12) between ribs (64) against the force of springs (48) and away from the base (16), which moves holes (36) away from detents (56), thereby allowing the tray (12) to slide within the sleeve (14).
Owner:WESTROCK MWV LLC
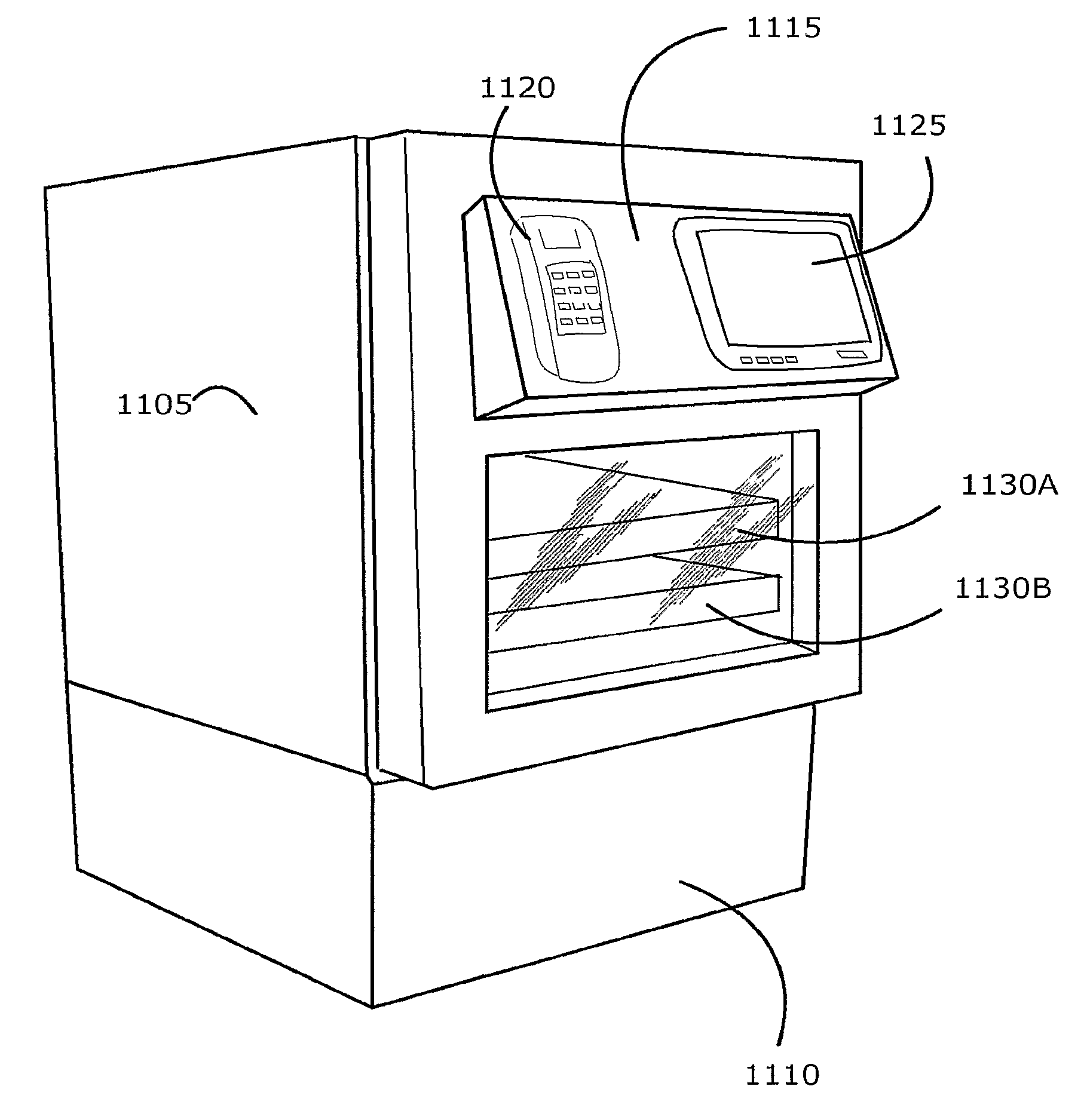
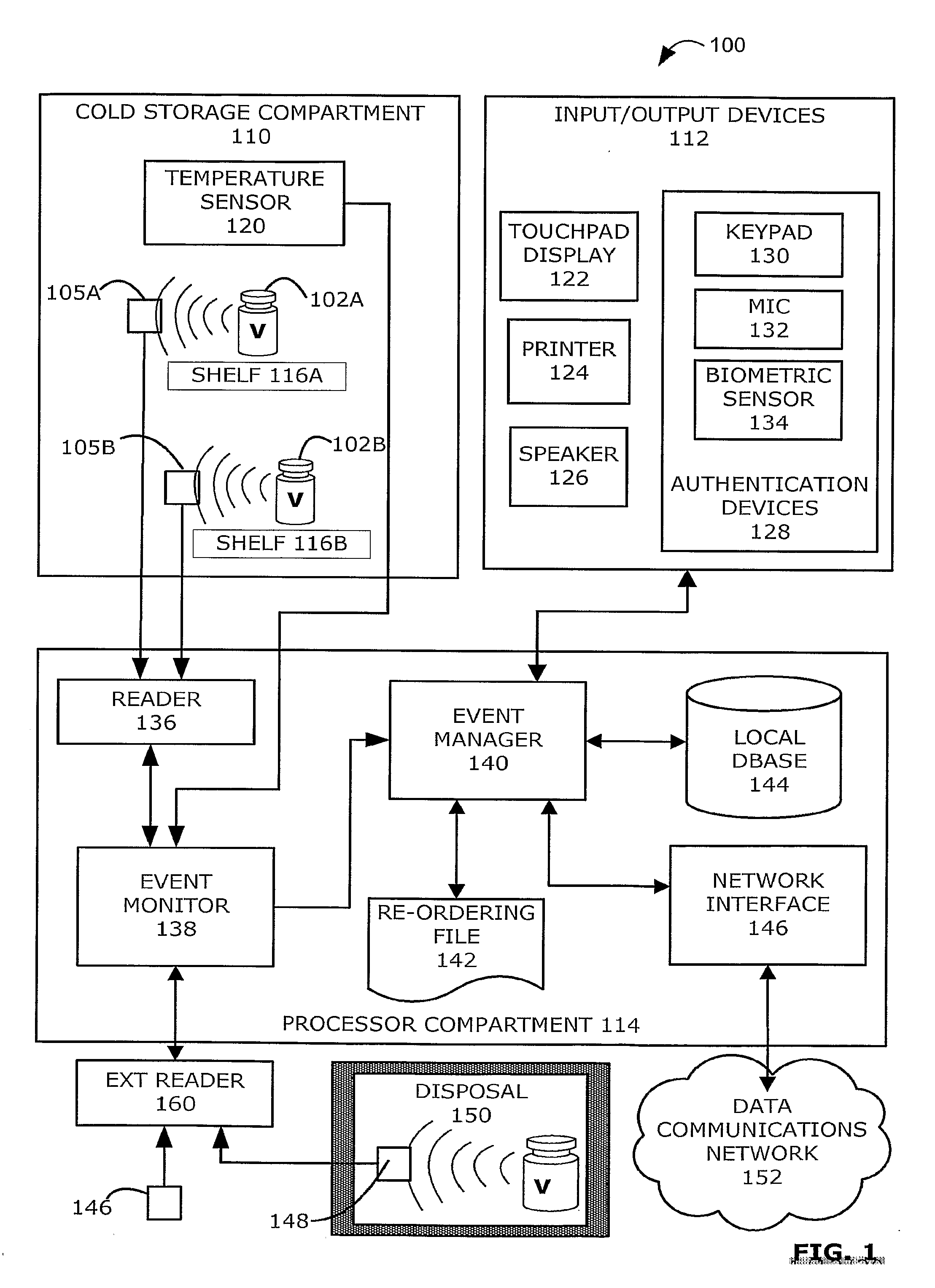
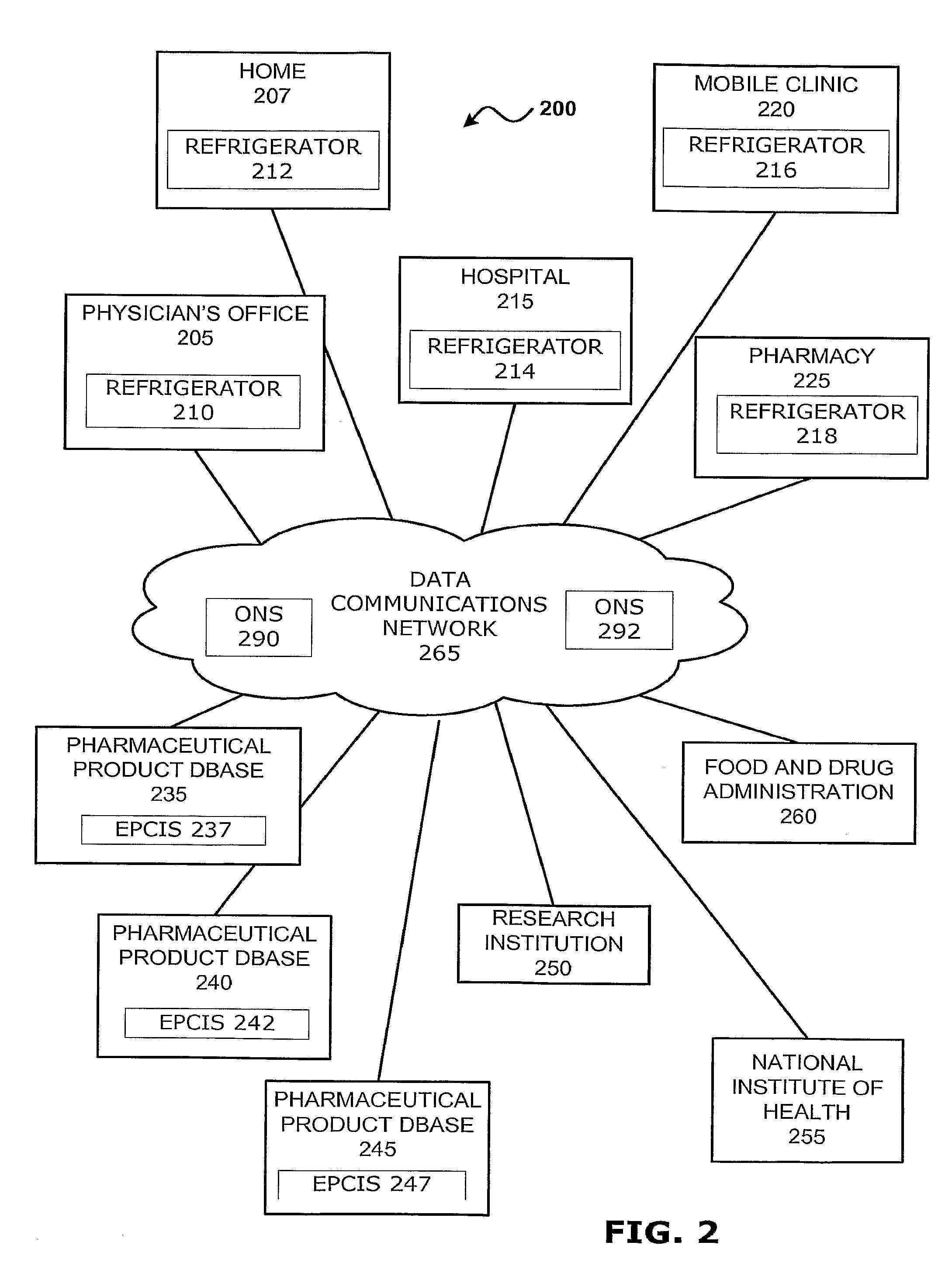
Intelligent Refrigerator for Storing Pharmaceutical Product Containers
Intelligent refrigerator system for storing pharmaceutical product containers, such as vials, ampules, syringes, bottles, medication tubes, blister packs and cartons, at the point of dispensing. Embodiments of the invention use product identification technology, such as radio-frequency identification (RFID) tags and readers, to uniquely identify containers as they are added to or removed from the cold storage compartment of the refrigerator, and automatically retrieve from a local or remote database a variety of details associated with the containers and their contents, such as manufacturing data, expiration dates, time out of refrigeration, inventory levels, safety information, usage statistics, known contraindications and warnings, etc. If the details indicate that there is a problem with a particular pharmaceutical (e.g., that it is counterfeit, expired, suspect, spoiled, recalled or almost depleted), then a message or warning is automatically delivered to a human operator via an attached output device, such as a display screen, speaker or printer. Embodiments of the invention may also be configured to monitor and report temperature faults, power failures and other anomalies associated with the refrigerator or cold storage compartment.
Owner:MERCK SHARP & DOHME LLC
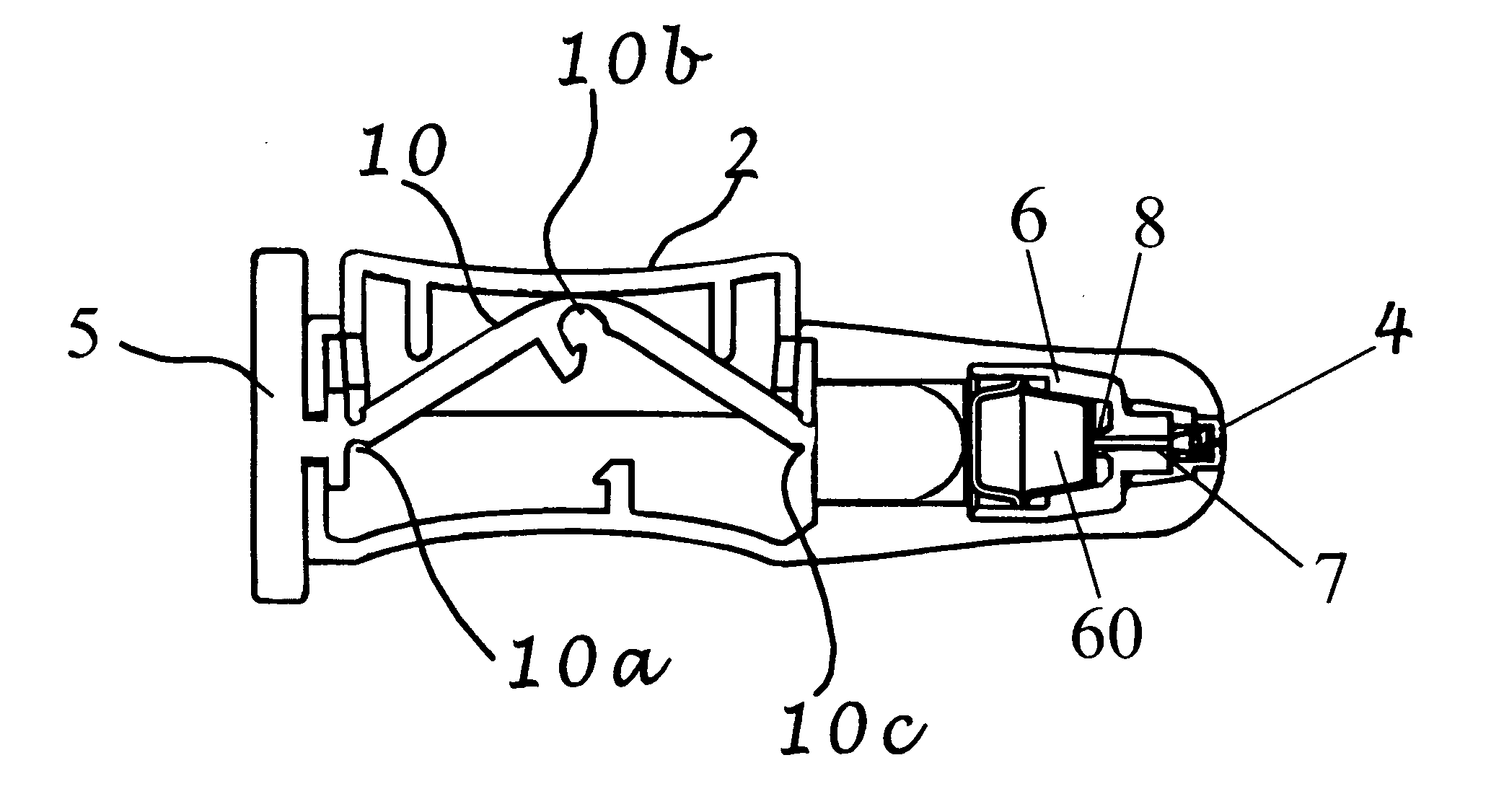
Apparatus and method for surgical bypass of aqueous humor
The invention provides minimally invasive microsurgical tools and methods to form an aqueous humor shunt or bypass for the treatment of glaucoma. The invention enables surgical creation of a tissue tract (7) within the tissues of the eye to directly connect a source of aqueous humor such as the anterior chamber (1), to an ocular vein (4). The tissue tract (7) from the vein (4) may be connected to any source of aqueous humor, including the anterior chamber (1) ), an aqueous collector channel, Schlemm's canal (2), or a drainage bleb. Since the aqueous humor passes directly into the venous system, the normal drainage process for aqueous humor is restored. Furthermore, the invention discloses devices and materials that can be implanted in the tissue tract to maintain the tissue space and fluid flow.
Owner:ISCI INTERVENTIONAL CORP
Machine to automate dispensing of pills
A device having a plurality of cassettes, each filed with a supply of pills and positionable over a target location. The device has a platen beneath the target location with receptacles configured to hold both vials and blister packs. The platen or the cassette is movable so that any blister of the blister pack or the vial can be positioned under the target location to receive a quantity of pills from a cassette.
Owner:QEM INC
Dry powder inhalers, related blister package indexing and opening mechanisms, and associated methods of dispensing dry powder substances
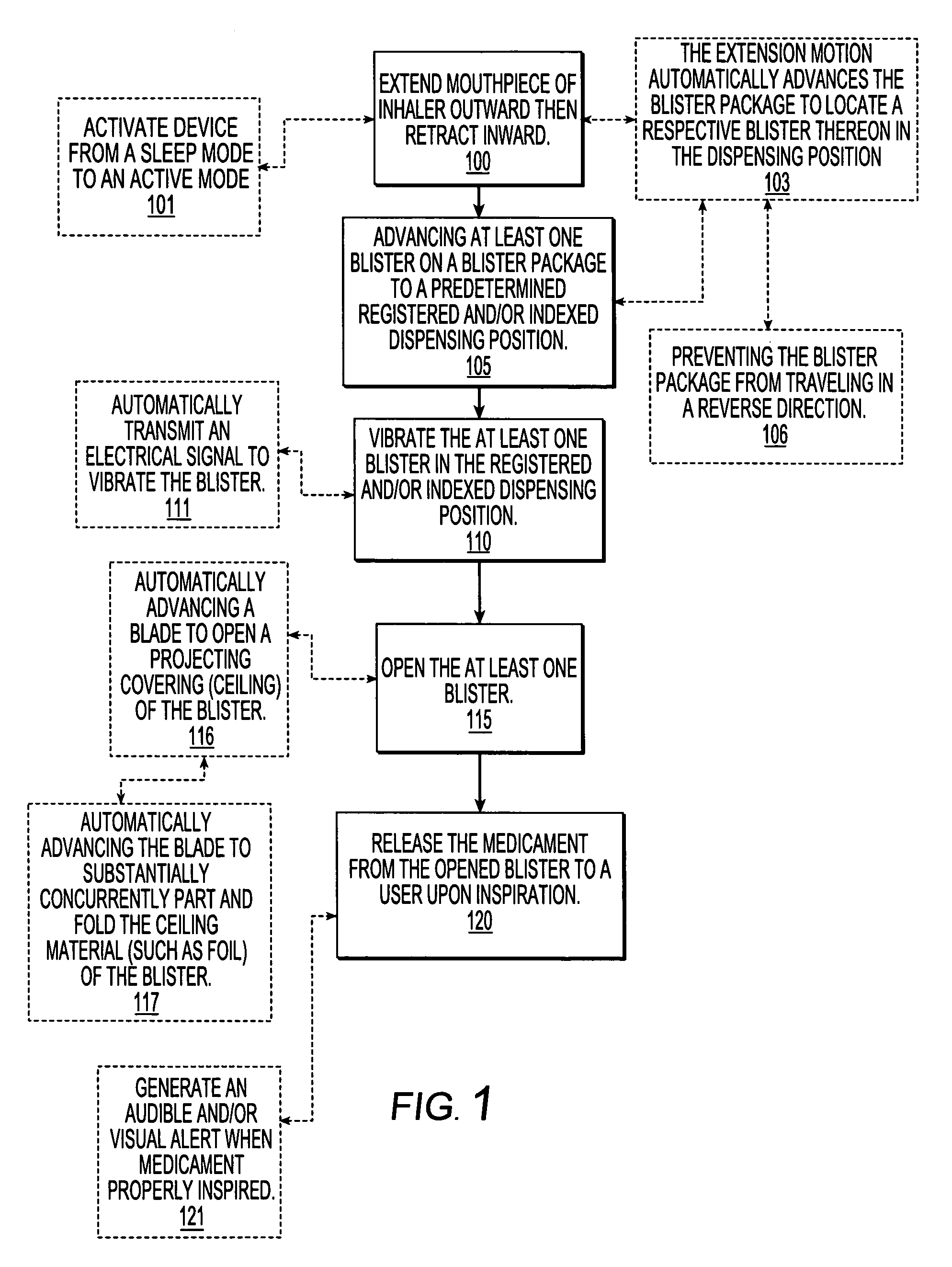
Dry powder inhalers with a multi-dose dry powder package for dispensing pharmaceutical grade formulations of inhalable dry powder, include: (a) a blister package comprising a plurality of spaced apart sealed blisters thereon, each blister having a projecting ceiling and a floor defining a blister channel therebetween, the blister channel comprising a dry powder therein; (b) a movable blade cartridge holding a blade at a forward portion thereof; and (c) an extendable mouthpiece attached to the movable blade cartridge. In operation, a user pulls the mouthpiece outward and then pushes the mouthpiece inward to cause the blister package to advance to position a blister in a selected dispensing position in the inhaler and to cause the blade cartridge to move the blade across a blister ceiling held in the dispensing position in the inhaler to thereby open the blister held in the dispensing position.
Owner:ORIEL THERAPEUTICS INC
Reclosable package and method
InactiveUS6070723AWidth minimizedMaximizing usable package volumePackaging vehiclesContainers for machinesEngineeringBlisters
A reclosable package and method having an integral reclosable door adjacent one end that permits article removal from the end of the package. The package is comprised of a thermoformed blister body joined to a thermoformed backing body. The blister body has sidewalls, endwalls and a peripheral flange broken into a first section adjacent the door where the height of the sidewalls decrease and a second section opposite the door with the first section angled relative to the second section. The backing body carries the door and has an integral peripheral rib inboard of a peripheral flange with the flange having a first section about the door and a second section disposed away from the door. The rib has a pair of longitudinally-extending sections divided by a notch that preferably is a transverse rib that causes the door to bend about a desired fold line that runs generally through or adjacent the ribs or notches when urged away from a closed position. In a preferred method, after performing a multilevel trim operation to trim the multiplanar flanges of one or both the blister body and the backing body, the two bodies are joined at the flange sections about a portion of the periphery to adjacent the fold line using an energy welding process, preferably RF welding, that produces a narrow tear seam that enables finished package flange width to be minimized to thereby also minimize package width.
Owner:PORTAGE PLASTICS
Microblister skin grafting
ActiveUS20130204273A1Reducing patient harm and discomfortAdd featureSurgical instruments for heatingSkin graftingBlisters
Owner:3M INNOVATIVE PROPERTIES CO
Child-resistant and senior-friendly blister card package
Owner:KEY PAK TECH
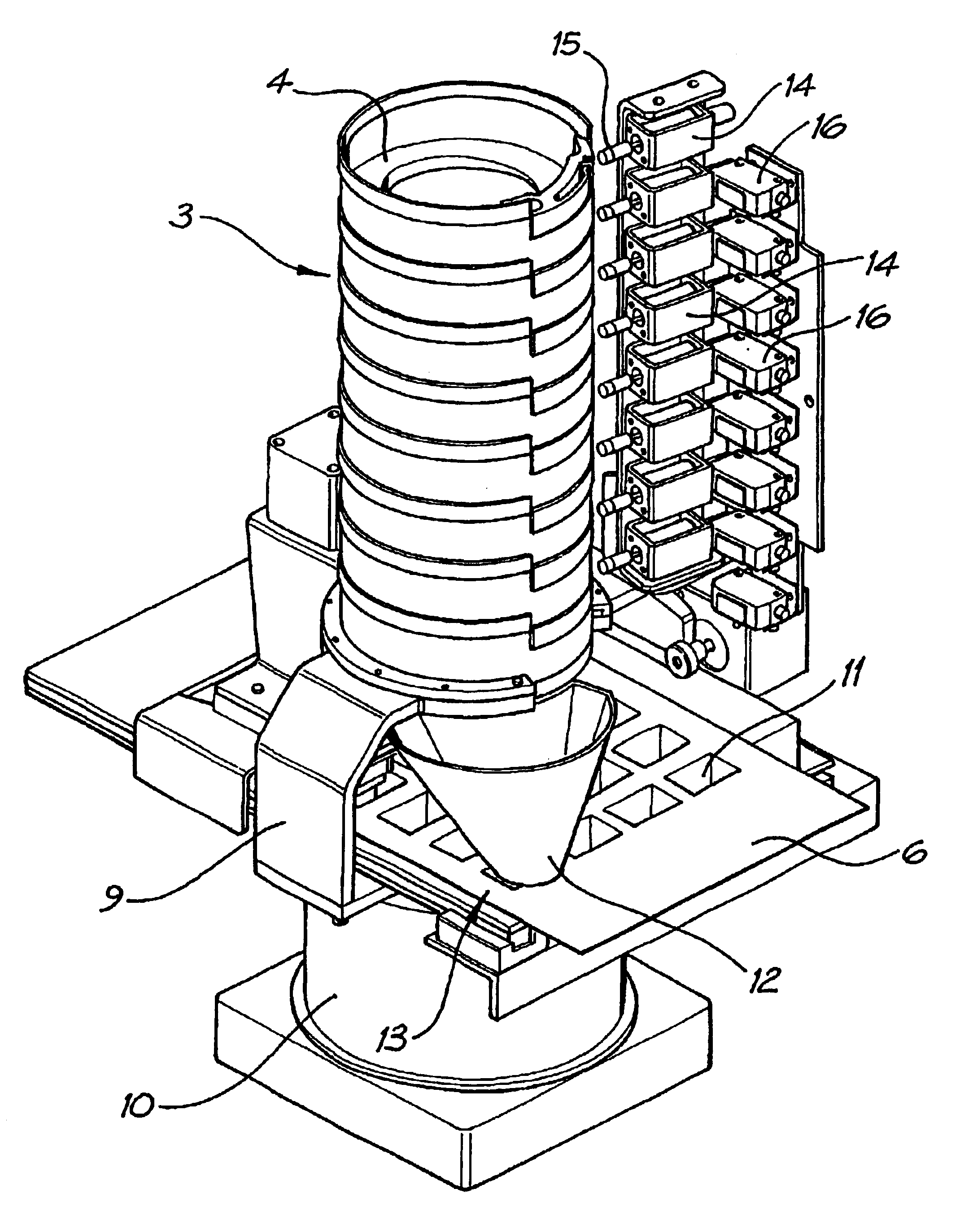
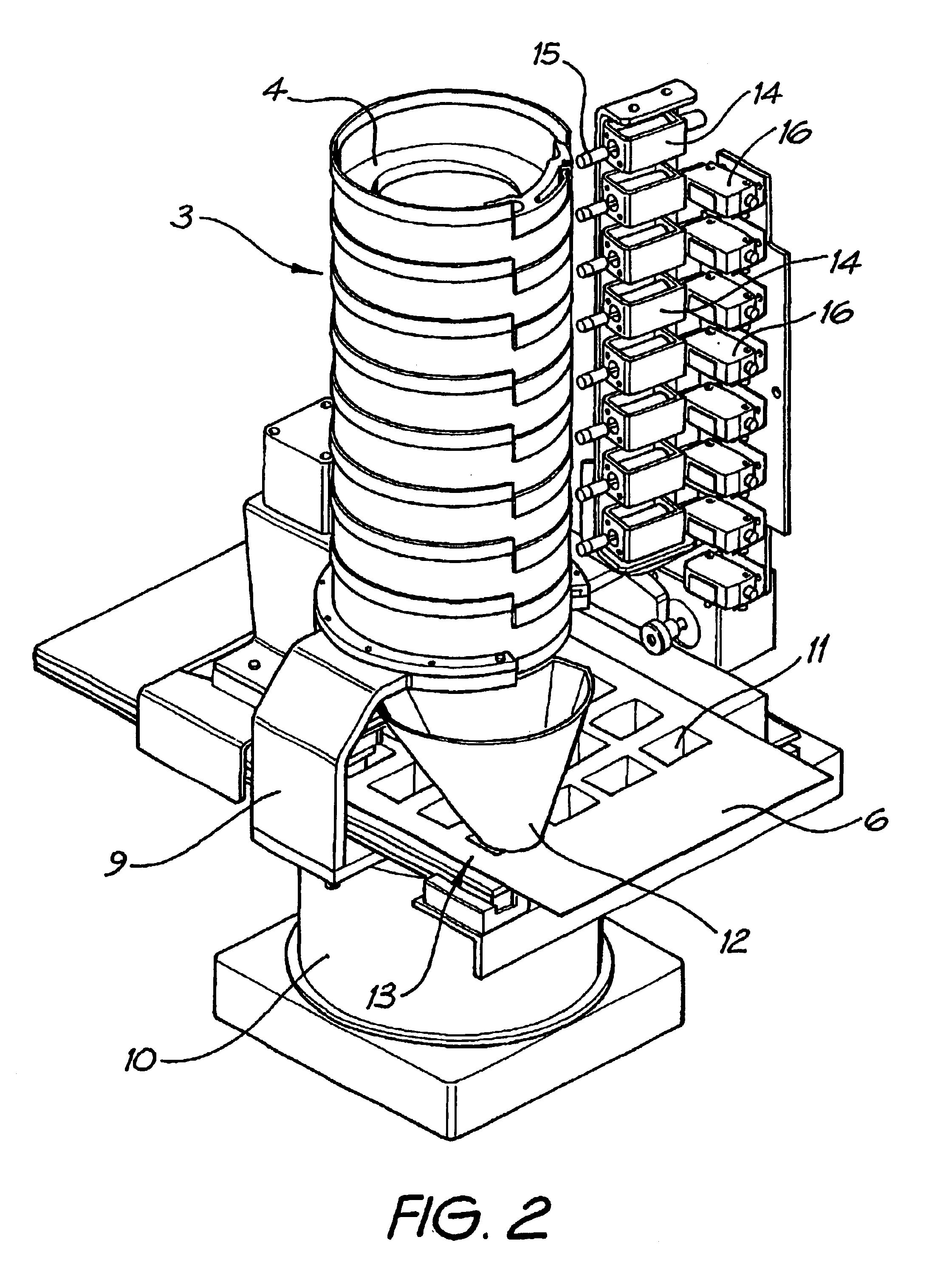
Medication dispenser
InactiveUS6805259B2Coin-freed apparatus detailsOral administration deviceMedication DispenserReciprocating motion
A medication tablet dispenser has an upright casing providing a tower which is subdivided by horizontal partitions into eight compartments which individually contain removable holders each containing a charge of tablets to be dispensed. The holders have framing portions which together provide a funnel opening downwardly into a cavity of a blister sheet. The casing is vibrated back and forth about its vertical axis through a small angle to cause tablets in the holders to progress towards an outlet leading into the funnel and having an associated ejector which discharges selected tablets into the funnel when required by a computer program. Conical vibration of the casing is prevented by a connection located on its vertical axis and held stationary by a fixed arm. The ejectors operate in response to slide-rods individually reciprocated by associated solenoids controlled by the computer program.
Owner:MANREX AUSTRALIA
Blister pack content usage monitoring
ActiveUS8960440B1Save battery powerExtended service lifeSmall article dispensingPharmaceutical containersElectrical resistance and conductanceVoltage ratio
A system is provided for monitoring the removal of blister pack contents. An array of spatially-extended, electrically parallel breakable traces made from electrically resistive material is formed behind a corresponding array of blisters of a blister card. Then this array is connected in series with a reference resistor to form a voltage divider. All resistive traces are formed from the same materials in a single operation. Blister breakage is determined using changes in the ratio of the resistances of the array and the divider. A predictive algorithm is used to adjust the threshold resistance ratio change that signals blister breakage and voltage ratios are used to adjust for battery output changes over time. Breakage events and their time of occurrence are recorded in nonvolatile memory for later retrieval. Additional resistors can be used for activating the system and detecting tampering.
Owner:INTELLIGENT DEVICES SEZC
Child-Resistant Blister Package
ActiveUS20060289328A1InexpensiveEasy to makeSmall article dispensingOther accessoriesEngineeringBlister pack
A package includes a blank having a face panel and a back panel. The face panel includes apertures and face tabs. The back panel includes gates that correspond with apertures, and tab strips that overlap the gates and are adjoined to back tabs. A blister pack is sealed between the face panel and the back panel whereby blisters align over gates and protrude through apertures, and tabs and form a composite pull tab. To remove an item from a blister, the pull tab is pressed out of the panels, the tab strip is peeled from the back panel, and pressure is applied to force the item through the backing sheet of the blister pack and the exposed gate.
Owner:WESTROCK MWV LLC
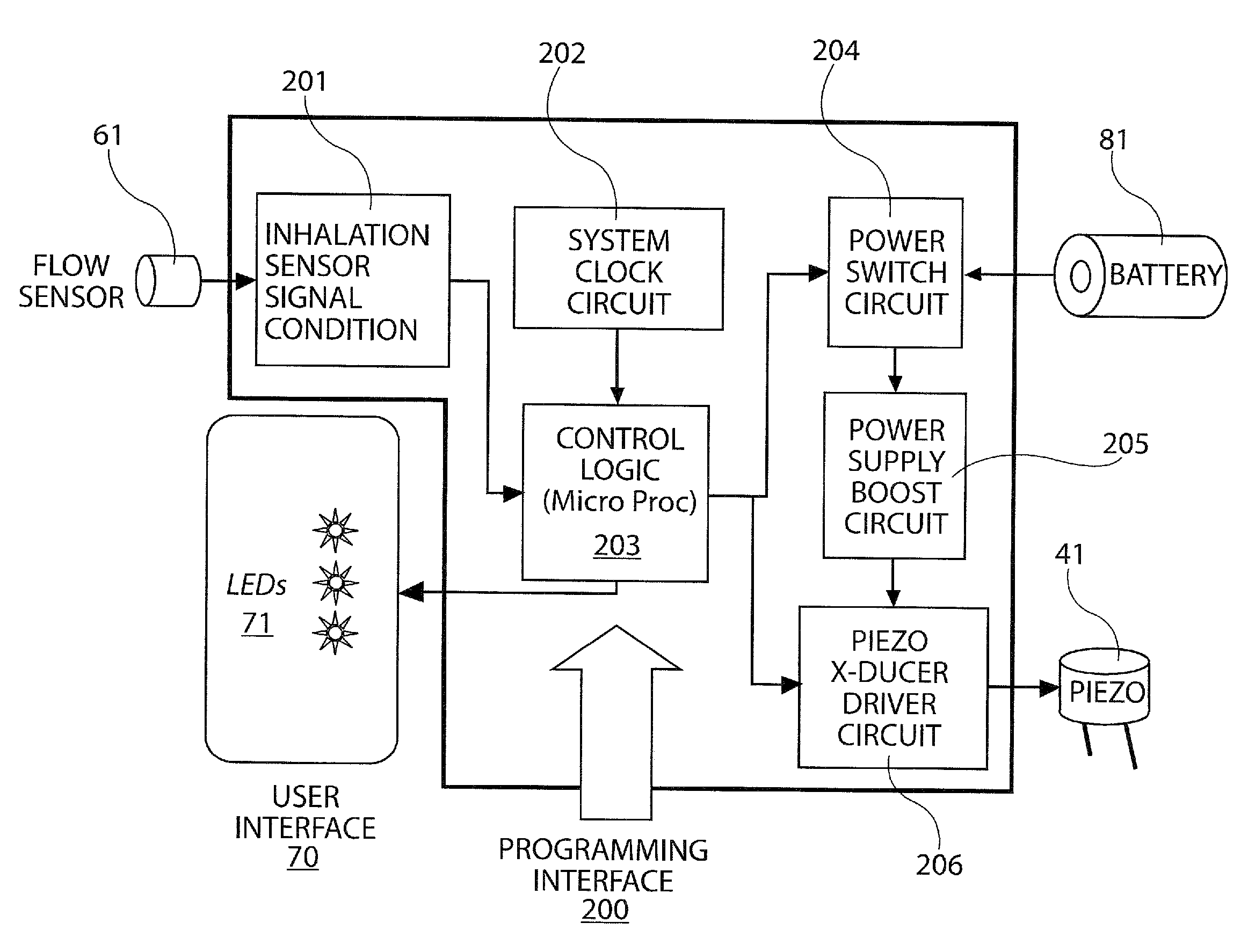
Inhaler
A dry powder inhaler has a vibrator coupled to a blister filled with a dry powder drug substance. One or more of drug ejection apertures in the blister are substantially opposite the vibrator. One or more air intake apertures in the blister are not opposite the vibrator. Upon vibration of the vibrator, the drug substance is deaggregated, aerosolized, and ejected from the drug ejection apertures for inhalation by a patient.
Owner:MICRODOSE THERAPEUTX INC
Rotary cassette system for dry powder inhaler
InactiveUS20100294278A1Simpler and more compact assemblyRespiratorsLiquid surface applicatorsEngineeringBlister pack
The present disclosure provides an inhaler having a vibration element for aerosolizing medicament contained in a blister pack, wherein a plurality of individual blister packs are arranged in a rotary cassette that fits within a housing, and wherein the individual blister packs are dragged up into a clamping position between the vibration element and a piercing element. The motion of the blister pack is controlled by a rotary disk within the housing which further coordinates the movement of the piercing and vibrating elements for the piercing and deaggregation, respectively, of the individual blister packs.
Owner:MICRODOSE THERAPEUTX INC
Dry powder inhaler
ActiveUS8037880B2Small accurate volumePrecise deliveryRespiratorsLiquid surface applicatorsUltra fineExcipient
A new dry powder inhaler is developed as a pulmonary medicine delivery device for dispersing precise tiny dosages (10 μg-50 mg) of pure carrier-free ultra-fine powdered medicament (<5 μm aerodynamics particle size) into a patient's lung. The powder is drawn from the blister cell and dispersed through an outlet tube assisted by two air streams. The first air stream goes through a the blister cell from its upstream side, to significantly fluidize the medicament in the dose to flow upward. The second one extracts the fluidized powder from downstream of the blister cell for further deagglomeration and dispersion of the medicament powder by shear force. The rotating multi-dose blister can hold up to 60 doses, which are pre-metered with pure ultra-fine powdered medicament. So that it has higher drug loading capability in small volumes, compared to most current dry powder inhalers, which usually use some excipient. The inhaler efficiently disperse the aerosolized medicament in the air stream to the deep interior of patient's lung. The fine particle fraction (<4.7 μm) is reported to reach as high as 80% using this inhaler.
Owner:NINGBO INHAL PHARMA CO LTD
Sublingual drug delivery device
A drug delivery device that aerosolizes a dry powder formulation so that it forms a fine coating in the oral cavity and, more specifically, in the sublingual region of the oral cavity is described herein. In the preferred embodiment, the device contains five main parts: (i) a compressed gas canister, (ii) a dispenser body (also referred to herein as the main housing), (iii) a means for storing one or more doses of a drug formulation, (iv) a means for releasing a dose of the drug formulation such as a gas canister or spring piston and (v) a mouthpiece. Preferred configurations include circular, tubular, and rectangular. The means for storing the drug formulation may be configured to separately store one or more materials. In one embodiment, the means for storing the active agent is in the form of one or more drug discs, where the drug discs contain a plurality of blister packs, each storing one dose of the drug formulation. In another embodiment, the means for storing the active agent is a dosage cartridge containing a single dose of the drug formulation. In yet another embodiment, the drug formulation is stored on a ribbon containing a plurality of blister packs, each storing one dose of the drug formulation.
Owner:BIODEL
Combination unit dose dispensing containers
ActiveUS20080283439A1Well mixedHigh vapor barrierSmall article dispensingPowdered material dispensingBlistersDosage form
Owner:MYSTIC PHARMA INC
Intranasal Cartridge Devices
ActiveUS20080177246A1Precise alignmentProvide stabilityAmpoule syringesJet injection syringesNasal passageNasal passages
Intranasal delivery devices include dosage forms containing medical compositions for use in the intranasal devices, and methods of delivering medical compositions to the nasal mucosa of users. The devices dispense a predetermined quantity of fluid into the nasal passage of a user, in which the predetermined quantity of fluid is contained in, or produced in a dosage form or blister that is crushed by a plunger with sufficient force to drive the dosage form against a piercing mechanism, piercing the dosage form and forcing the liquid contents from the dosage form and through a delivery channel into a spray to be directed into the nasal passage of a user. The plunger is connected to a trigger device by a linkage that confers a mechanical advantage to the trigger mechanism.
Owner:MYSTIC PHARMA INC
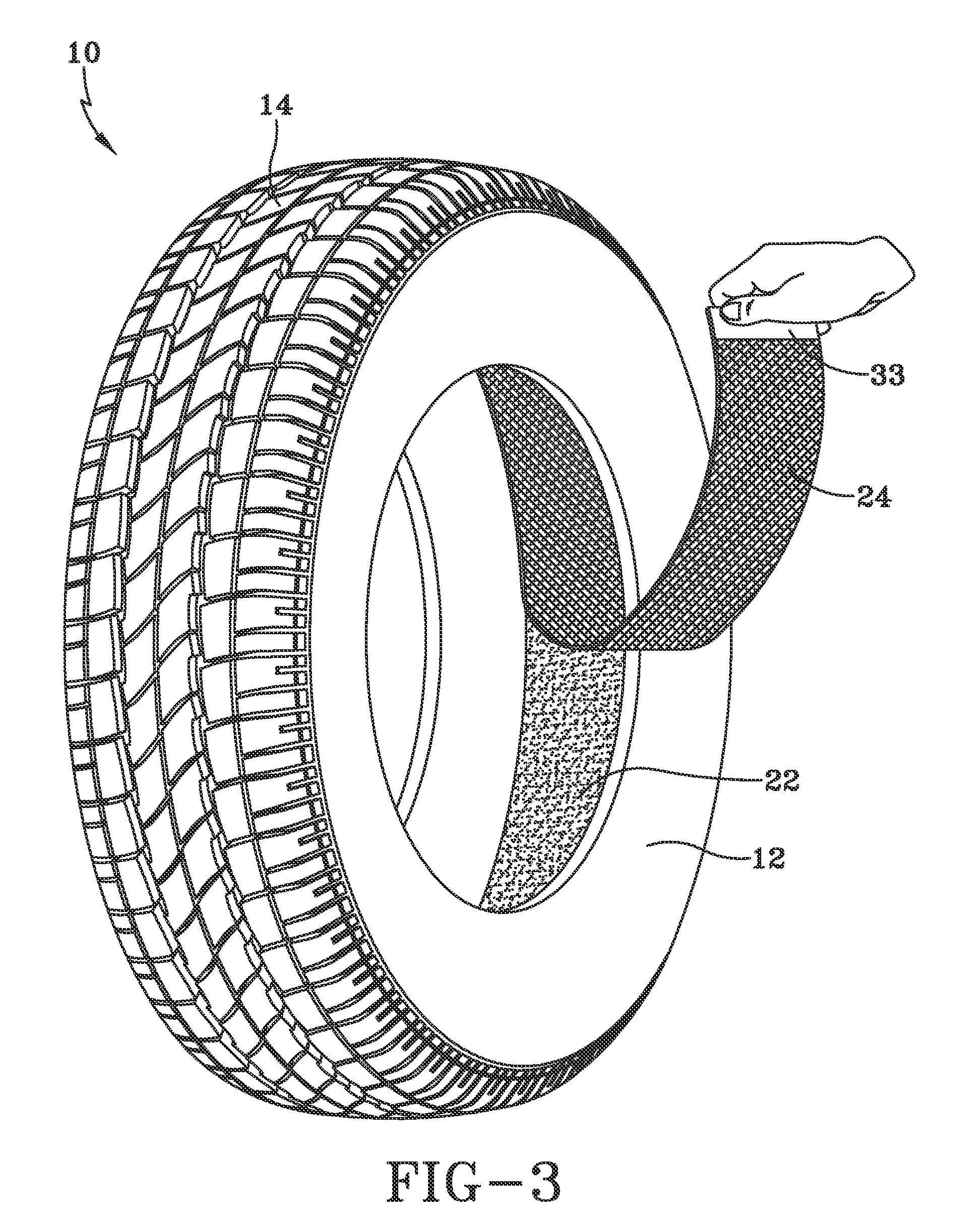
Pneumatic tire having built-In sealant layer and preparation thereof
InactiveUS20090084482A1Eliminate and reduce inner liner blister formationPrevent and reduce of inner liner blister formationWithout separate inflatable insertsTyresEngineeringSealant
A pneumatic tire is provided which includes an outer circumferential rubber tread and a supporting carcass. A rubber inner liner is disposed inwardly from the supporting carcass. A built-in sealant layer is situated adjacent to an innermost removable barrier layer and disposed inwardly from the rubber inner liner. The built-in sealant layer provides self-sealing properties to the pneumatic tire. The pneumatic tire, with its innermost removable barrier layer, keeps the sealant layer from sticking to a tire-building apparatus during tire assembly and, after curing of the assembled tire, is eventually removed to permit gas from the built-in sealant layer to become part of the tire's inflation air to prevent or reduce instances of inner liner blister formation.
Owner:MAJUMDAR RAMENDRA NATH +1
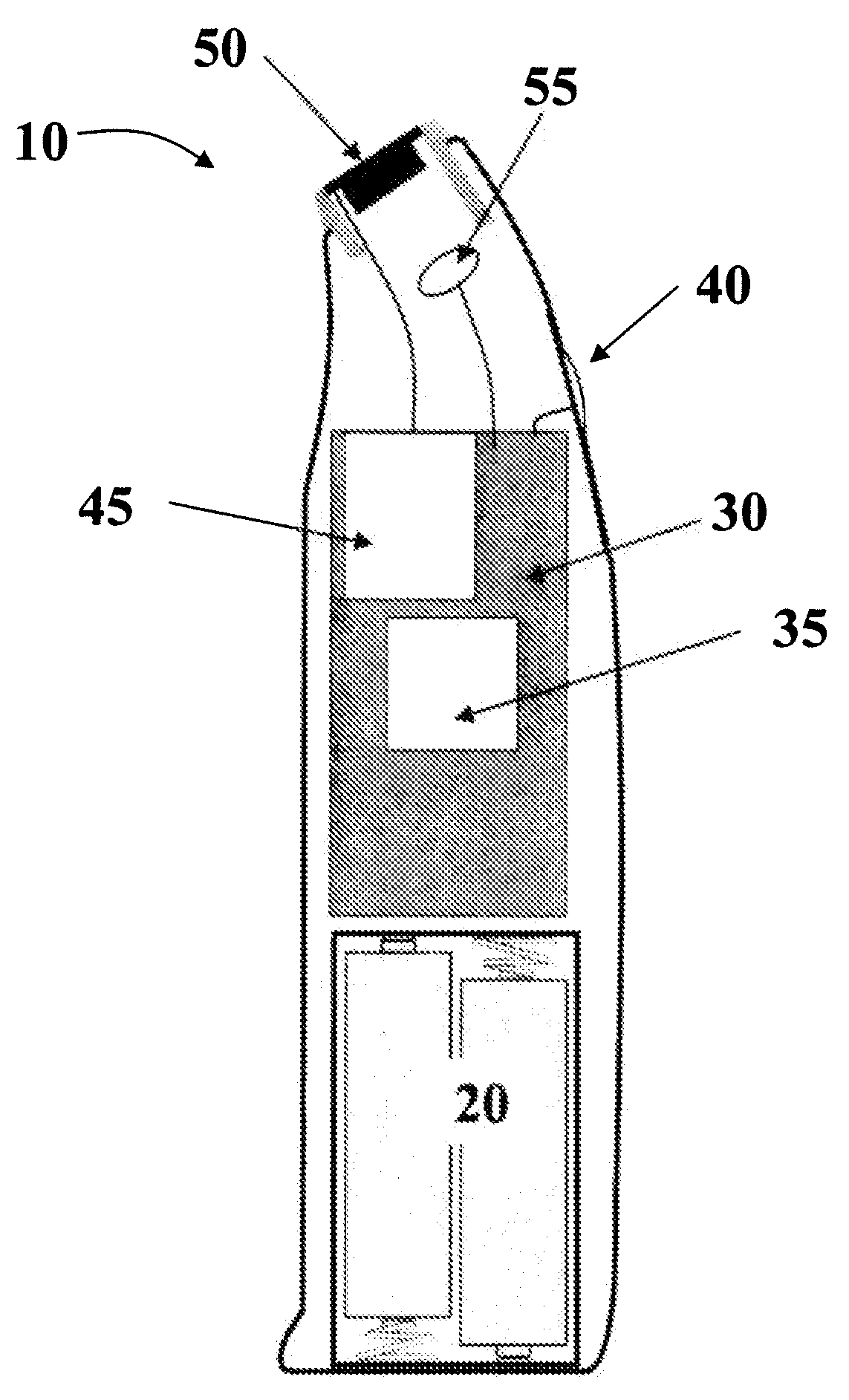
Alarmed tablet dispenser
A tablet dispenser includes a housing formed by first and second shells defining a cavity therebetween. A blister tray configured to receive a plurality of tablets therein is rotatably attached to the housing. The blister tray is moveable between a closed position in which the blister tray is releasably contained within the cavity, and an open position in which at least a portion of the blister tray extends out of the cavity. An alarm unit activates at least one alert signal upon completion of a cycle period. The alarm unit is reset after the blister tray is accessed to dispense at least one tablet.
Owner:WEST PHARM SERVICES INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com